UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C.
20549
___________
FORM 20-F/A
(Amendment No.1)
___________
(Mark One)
[_] Registration statement pursuant to Section 12(b) or 12(g) of the Securities Exchange Act of 1934
or
[X] Annual report pursuant to Section 13 or
15(d) of the Securities Exchange Act of 1934
For the fiscal year ended December
31, 2011
or
[_] Transition report pursuant to Section 13 or
15(d) of the Securities Exchange Act of 1934
For the transition period from _____ to ______
or
[_] Shell company report pursuant to Section 13
or 15(d) of the Securities Exchange Act of 1934
Date of event requiring this shell company report
Commission file number 001-33295
_______________
3SBio Inc.
(Exact Name
of Registrant as Specified in Its Charter)
Cayman Islands
(Jurisdiction of Incorporation or
Organization)
No. 3 A1, Road 10
Shenyang Economy & Technology
Development Zone
Shenyang 110027
People’s Republic of
China
(Address of Principal Executive Offices)
Bo Tan, Chief Financial Officer
No. 3 A1, Road
10
Shenyang Economy & Technology Development Zone
Shenyang
110027
People’s Republic of China
www.3sbio.com
Telephone (China): 8624-25811820
Email:
ir@3sbio.com
(Name, Telephone, E-mail
and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Name of Each Exchange on Which Registered |
| American Depositary Shares | The NASDAQ Stock Market LLC |
| (Each representing seven ordinary shares, par value US$0.0001 per share) |
Securities registered or to be registered pursuant to Section 12(g) of the Act:
None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
None
________________________
Indicate the number of outstanding shares of each of the Issuer’s classes of capital or common stock as of the close of the period covered by the annual report.
154,473,159 ordinary shares, par value US$0.0001, as of December 31, 2011
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes [_] No [X]
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
Yes [_] No [X]
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes [X] No [_]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer [_] | Accelerated filer [X] | Non-accelerated filer |
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| U.S. GAAP [X] |
International Financial Reporting Standards as issued by the International Accounting Standards Board [_] |
Other [_] |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
Item 17 [_] Item 18 [_]
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Securities Exchange Act of 1934).
Yes[_] No [X]
Explanatory Note
This Amendment No.1 on Form 20-F/A ("Amendment No. 1") to the Company's Annual Report on Form 20-F for the year ended December 31, 2011, originally filed with the Securities and Exchange Commission on April 30, 2012, is to add the audited financial statements and accompanying notes, which were inadvertently omitted in the original filing.
No other changes have been made to the original filing. Except as specifically and expressly disclosed herein, this Amendment No. 1 does not, and does not purport to, amend, update or restate any information in any Items or sections of the original filing, or reflect any events having occurred after the original filing date.
3SBIO INC.
FORM 20-F ANNUAL REPORT
FISCAL YEAR
ENDED DECEMBER 31, 2011
TABLE OF CONTENTS
| INTRODUCTION | ||
| PART I | 1 | |
| ITEM 1. | IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS | 1 |
| ITEM 2. | OFFER STATISTICS AND EXPECTED TIMETABLE | 1 |
| ITEM 3. | KEY INFORMATION | 1 |
| ITEM 4. | INFORMATION ON THE COMPANY | 21 |
| ITEM 4A. | UNRESOLVED STAFF COMMENTS | 48 |
| ITEM 5. | OPERATING AND FINANCIAL REVIEW AND PROSPECTS | 49 |
| ITEM 6. | DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES | 62 |
| ITEM 7. | MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS | 68 |
| ITEM 8. | FINANCIAL INFORMATION | 72 |
| ITEM 9. | THE OFFER AND LISTING | 73 |
| ITEM 10. | ADDITIONAL INFORMATION | 75 |
| ITEM 11. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK | 82 |
| ITEM 12. | DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES | 83 |
| PART II | 85 | |
| ITEM 13. | DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES | 85 |
| ITEM 14. | MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS | 85 |
| ITEM 15. | CONTROLS AND PROCEDURES | 85 |
| ITEM 16A. | AUDIT COMMITTEE FINANCIAL EXPERT | 86 |
| ITEM 16B. | CODE OF ETHICS | 86 |
| ITEM 16C. | PRINCIPAL ACCOUNTANT FEES AND SERVICES | 86 |
| ITEM 16D. | EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES | 86 |
| ITEM 16E. | PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS | 87 |
| ITEM 16F. | CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT | 87 |
| ITEM 16G. | CORPORATE GOVERNANCE | 87 |
| ITEM 16H. | MINE SAFETY DISCLOSURE | 87 |
| PART III | 88 | |
| ITEM 17. | FINANCIAL STATEMENTS | 88 |
| ITEM 18. | FINANCIAL STATEMENTS | 88 |
| ITEM 19. | EXHIBITS | 88 |
INTRODUCTION
Conventions
In this annual report on Form 20-F, unless otherwise indicated:
| • |
The term “our company”, “the Company”, “we”, “us”, or “our”, or any like terms, refer to 3SBio Inc. and its subsidiaries and consolidated variable interest entities, unless the context requires otherwise; | |
| • |
“Share(s)”or “ordinary share(s)” refer to our ordinary share(s), with par value US$0.0001 per share; | |
| • |
“ADS(s)” refer to our American depositary share(s), each of which represents seven ordinary shares; | |
| • |
“ADR(s)” refer to our American depositary receipt(s) that evidence our ADSs; | |
| • |
References to “China” or “PRC” are to the People’s Republic of China, excluding for the purposes of this annual report Hong Kong, Taiwan and Macau; | |
| • |
References to “province(s)” of China are to one or more, as applicable, of the provinces and provincial-level municipalities and autonomous regions of China; | |
| • |
All references to “RMB” or “Renminbi” are to the legal currency of China; | |
| • |
All references to “U.S. dollars”, “USD” or “US$” are to the legal currency of the United States of America; | |
| • |
“U.S. GAAP” refer to the generally accepted accounting principles in the United States of America; | |
| • |
“SFDA” refers to the State Food and Drug Administration of PRC; | |
| • |
The “Commission” or “SEC” refers to the Securities and Exchange Commission of the United States; | |
| • |
Heading, titles, or numbering, or presentation aids of similar nature, other than required captions and numbering applicable to each Item provided on Form 20-F, are only for convenience of reference, for the benefit of readers, and shall be with no substance; | |
| • |
Any website addresses are not intended to function as hyperlinks, and the information contained in such websites is not a part of this filing; and | |
| • |
To the extent available and not at unreasonable costs to us, if determined to facilitate better shareholder communications, at our sole discretion, we may from time to time choose to discuss certain events, developments, or issues after the end of the subject year, or any information not required to disclose, in the annual report on Form 20- F, and may not do so, with respect to other than selected information, or in any future periods. |
Cautionary Statement concerning Forward-looking Statements:
Statutory Safe Harbor
This annual report on Form 20−F contains certain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, which are intended to be covered by the safe harbors created by those laws.
What are Forward-looking Statements
Certain statements, other than statements of historical facts, included or incorporated by reference in this report, may address activities, events, conditions, or developments that we currently expect or anticipate may occur in the future, including, but not limited to, such things as or as related to, business growth and prospects, operation objectives, market size, as may be indicated by patient numbers or disease prevalence estimates, our business strategy, competitive strengths, future government policies and regulations and impact, product pricing trends, regulatory review, approval, and certification progress, product development and launch, research and development progress, prospect for partnerships or collaborations and the outcome thereof, capital expense estimate, future operations and strategies. Such statements may be considered forward-looking statements. References to future successes of our company are usually forward-looking statements. Forward-looking statements include information about possible or assumed future financial condition and results of operations of the Company.
Forward-looking statements include, but are not limited to, those statements using words such as “believe,” “expect,” “plans,” “strategy,” “potential”, “prospects,” “forecast,” “estimate,” “project,” “anticipate,” “aim,” “will” or “would”, “may” or “might”, and words, phrases, expressions, and usages of similar meaning or substance or the negative of such words, phrases, expressions and usages.
Forward-looking statements usually are based on management’s current assumptions, beliefs, expectations, and projections, in light of the information currently available to it.
Subject to Risks, Uncertainties, and Assumptions
Numerous risks and uncertainties attend or impact on the matters addressed by forward-looking statements, any of which could negatively and materially affect our operation results, performance or financial conditions. Therefore you should not place undue reliance on forward-looking statements.
Our actual operation results, performance or financial conditions could differ materially from those contained in forward-looking statements due to a number of factors, including: regulatory actions such as adverse drug pricing adjustments; competition from other domestic and foreign pharmaceutical companies and their products; changes in China's healthcare insurance system, particularly in various government sponsored programs, as to coverage and reimbursement limits and otherwise; changes in the government medical procurement and the competitive bidding system; changes in other Chinese government policies and regulations; risks and uncertainties related to collaboration, joint venture, and partnerships, including deal consummation risks; our ability to enhance existing products and develop, obtain government approvals for, and market new indications or future generations of our existing products and new products; progress of our clinical trials; receipt and timing of regulatory approvals for new products and indications; the segment market growth for our products; market acceptance of our products and hospital or patient demand for our products; our ability to improve and expand our production, sales and distribution network and other aspects of our operations; our ability to diversify our product portfolio; our ability to effectively protect our intellectual property; our ability to identify and acquire new medical technologies, products and product candidates; other changes in the healthcare industry in China ; and fluctuations in general economic and business conditions in China.
We describe in this report some of these risks and uncertainties in greater detail under “Item 3. Key Information—Risk Factors”. Important information regarding risks and uncertainties may also appear elsewhere in this annual report, including but not limited to “Item 4. Information on the Company,” “Item 5. Operating and Financial Review and Prospects,” the Company’s consolidated financial statements referenced in “Item 18. Financial Statements,” and “Item 11. Quantitative and Qualitative Disclosures about Market Risk”.
Although we believe that the assumptions underlying our forward-looking statements are reasonable, any of these assumptions, and therefore, also the forward-looking statements based on these assumptions, could prove to be inaccurate or incomplete.
In light of the significant risks and uncertainties inherent in the forward-looking statements that are included or incorporated by reference in this report, our inclusion or incorporation of such information is not a representation by us or any other person that any possibility, estimation, particular course of action, or prediction will come to fruition or materialize, or our objectives and plans will be achieved.
We urge you to consider and evaluate all forward-looking statements always in light of possible risks, uncertainties, and limitations.
Date of Relevance; No Update
Our forward-looking statements speak only as of the date made, and, for those contained in this annual report, only as of the date of this annual report. We will not update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except in very limited circumstance where the securities laws require us to do so.
Applicable to other Disclosures
From time to time, oral or written forward-looking statements may also be included in other materials of the Company released to the public, including, but not limited to, other SEC filings, news releases, discussions at earnings calls, investor presentations, and website information. All the discussions under this heading “Cautionary Statement concerning Forward-looking Statements” apply to such other oral or written forward-looking statements.
PART I
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
Not applicable.
ITEM 2. OFFER STATISTICS AND EXPECTED TIMETABLE
Not applicable.
ITEM 3. KEY INFORMATION
A. Selected Financial Data
The selected consolidated financial data presented below for the years ended December 31, 2009, 2010 and 2011 are derived from our audited consolidated financial statements (“Financial Statements”) for such periods, which are included elsewhere in this annual report. The selected consolidated financial data presented below for the years ended December 31, 2007 and 2008, and the balance sheet, dated as of December 31, 2007, 2008 and 2009 are derived from our audited consolidated financial statements for such periods, but not included in this annual report.
Our consolidated financial statements are prepared in accordance with U.S. GAAP. Our historical results do not necessarily indicate results expected for any future periods. The selected financial data should be read in conjunction with, and are qualified in their entirety by reference to, our audited consolidated financial statements and related notes and “Item 5. Operating and Financial Review and Prospects” in this annual report.
| Year ended December 31, | ||||||||||||||||||
| 2007 | 2008 | 2009 | 2010 | 2011 | 2011 | |||||||||||||
| RMB | RMB | RMB | RMB | RMB | US$ | |||||||||||||
| (in thousands, except per share, share, per ADS and ADS data) | ||||||||||||||||||
| Selected Statement of Income Data: | ||||||||||||||||||
| Net revenue (1): | 180,173 | 243,245 | 316,920 | 418,628 | 541,614 | 86,054 | ||||||||||||
| Cost of revenue | (17,427 | ) | (21,741 | ) | (25,236 | ) | (41,650 | ) | (58,073 | ) | (9,227 | ) | ||||||
| Gross profit | 162,746 | 221,504 | 291,684 | 376,978 | 483,541 | 76,827 | ||||||||||||
| Total operating expenses | (113,134 | ) | (173,325 | ) | (206,627 | ) | (288,880 | ) | (361,895 | ) | (57,499 | ) | ||||||
| Income from operations | 49,612 | 48,179 | 85,057 | 88,098 | 121,646 | 19,328 | ||||||||||||
| Total other income, net | 35,395 | 2,463 | 10,114 | 14,960 | 14,724 | 2,339 | ||||||||||||
| Income before income tax expenses | 85,007 | 50,642 | 95,171 | 103,058 | 136,370 | 21,667 | ||||||||||||
| Net income attributable to 3SBio Inc. | 81,513 | 39,542 | 83,435 | 81,286 | 108,573 | 17,251 | ||||||||||||
| Basic weighted average number of shares outstanding | 146,646,049 | 151,655,631 | 150,606,317 | 151,241,036 | 153,310,128 | 153,310,128 | ||||||||||||
| Diluted weighted average number of shares outstanding | 146,715,042 | 151,712,749 | 151,034,192 | 154,131,768 | 157,148,685 | 157,148,685 | ||||||||||||
| Net income attributable to 3SBio Inc. per share, basic | 0.56 | 0.26 | 0.55 | 0.54 | 0.71 | 0.11 | ||||||||||||
| Net income attributable to 3SBio Inc. per share, diluted | 0.56 | 0.26 | 0.55 | 0.53 | 0.69 | 0.11 | ||||||||||||
| Net income attributable to 3SBio Inc. per ADS, basic | 3.89 | 1.82 | 3.87 | 3.76 | 4.96 | 0.79 | ||||||||||||
| Net income attributable to 3SBio Inc. per ADS, diluted | 3.89 | 1.82 | 3.87 | 3.69 | 4.84 | 0.77 | ||||||||||||
1
| As of December 31, | ||||||||||||||||||
| 2007 | 2008 | 2009 | 2010 | 2011 | 2011 | |||||||||||||
| RMB | RMB | RMB | RMB | RMB | USD | |||||||||||||
| Selected Balance Sheet Data: | ||||||||||||||||||
| Cash and cash equivalents | 811,026 | 439,237 | 262,767 | 153,250 | 245,813 | 39,056 | ||||||||||||
| Restricted cash | - | - | 9,300 | 1,662 | 665 | 106 | ||||||||||||
| Time deposit with financial institutions | - | 293,809 | 468,451 | 498,405 | 519,201 | 82,493 | ||||||||||||
| Total assets | 973,475 | 952,918 | 1,053,461 | 1,186,397 | 1,311,110 | 208,315 | ||||||||||||
| Total liabilities | 29,134 | 32,523 | 41,223 | 50,344 | 65,904 | 10,471 | ||||||||||||
| Capital stock | 122 | 121 | 121 | 123 | 124 | 20 | ||||||||||||
| Total shareholder’s equity | 944,341 | 920,395 | 1,012,238 | 1,136,053 | 1,245,206 | 197,844 | ||||||||||||
| Selected Cash Flow Data: | ||||||||||||||||||
| Net cash provided by operating activities | 68,762 | 60,468 | 88,638 | 58,133 | 137,111 | 21,785 | ||||||||||||
| Net cash used in investing activities | (36,491 | ) | (368,889 | ) | (266,114 | ) | (165,234 | ) | (52,322 | ) | (8,313 | ) | ||||||
| Net cash provided by/(used in) financing activities | 801,745 | (14,467 | ) | 132 | 6,763 | 15,963 | 2,536 | |||||||||||
| (1) |
Net revenue consists of the invoiced value of goods sold, net of: value-added taxes, or VAT; discretionary sales returns; and trade discounts and allowances. |
Exchange Rate Information
Our business is primarily conducted in China, and all of our revenue and operating expenses are denominated in Renminbi. However, for the convenience of readers, periodic reports made to shareholders include current period amounts translated into U.S. dollars using the current exchange rate. Unless otherwise indicated, all translations from Renminbi to U.S. dollars for financial data have been made at a rate of RMB6.2939 to US$1.00, the exchange rate as set forth in the H.10 statistical release of the Federal Reserve Bank of New York on December 30, 2011.
The following table sets forth information concerning exchange rates between the RMB and the U.S. dollar for the periods indicated. For all periods prior to January 1, 2009, the exchange rate refers to the noon buying rate for U.S. dollars in The City of New York for cable transfers of RMB as certified for customs purposes by the Federal Reserve Bank of New York. For periods beginning on or after January 1, 2009, the exchange rate refers to the exchange rate as set forth in the H.10 statistical release of the Federal Reserve Bank. On March 30, 2012, the latest practicable date, the exchange rate set forth in the H.10 statistical release of the Federal Reserve Board was RMB6.2975 to $1.00.
| Noon Buying Rate | ||||||||||||
| Period End | Average(1) | High | Low | |||||||||
| (RMB per US$1.00) | ||||||||||||
| Period | ||||||||||||
| 2007 | 7.2946 | 7.5806 | 7.2946 | 7.8127 | ||||||||
| 2008 | 6.8225 | 6.9477 | 6.7800 | 7.2946 | ||||||||
| 2009 | 6.8259 | 6.8295 | 6.8176 | 6.8470 | ||||||||
| 2010 | 6.6000 | 6.7689 | 6.8330 | 6.6000 | ||||||||
| 2011 | ||||||||||||
| October | 6.3547 | 6.3710 | 6.3825 | 6.3534 | ||||||||
| November | 6.3765 | 6.3564 | 6.3839 | 6.3400 | ||||||||
| December | 6.2939 | 6.3482 | 6.3733 | 6.2939 | ||||||||
| 2012 | ||||||||||||
| January | 6.3080 | 6.3119 | 6.3330 | 6.2940 | ||||||||
| February | 6.2935 | 6.2997 | 6.3120 | 6.2935 | ||||||||
| March | 6.2975 | 6.3125 | 6.3315 | 6.2975 | ||||||||
_________________
| (1) |
Annual averages are calculated from month-end rates. Monthly averages are calculated from the daily rates during the month. |
2
We make no representation that any Renminbi or U.S. dollar amounts could have been, or could be, converted into U.S. dollars or Renminbi, as the case may be, at any particular rate, the rates stated above, or at all. The PRC government imposes control over its foreign currency reserves in part through direct regulation of the conversion of Renminbi into foreign exchange and through restrictions on foreign trade.
B. Capitalization and Indebtedness
Not applicable.
C. Reasons for the Offer and Use of Proceeds
Not applicable.
D. Risk Factors
You should consider carefully all of the information in this annual report, including the risks described below. If any risk disclosed below or elsewhere in this annual report actually occurs, our business, financial condition or results of operations could be harmed. In such an event, the trading price of our ADS could decline and you might lose all or part of your investment. Some information in this Item 3.D may be a summary of more detailed discussions contained elsewhere in this annual report.
The risks described below are not the only ones concerning us. Our business is also subject to the risks not specifically addressed below that affect many other companies, such as employment relations, general economic conditions, geopolitical events, etc. Further, additional risks not currently known to us or that we currently believe are immaterial also may impact our business, operations, financial condition and ADS price materially and adversely.
3.D.1 Risks Related To Our Company
We are currently dependent on our flagship product, EPIAO. A reduction in revenues of EPIAO would cause our total revenues to decline and could materially harm our business.
We are largely dependent on sales of our erythropoietin, or EPO, product, which we market under the name of EPIAO. We began marketing and selling EPIAO in 1998, and it has been our top-selling product since 2002. Revenues from sales of EPIAO accounted for 61.9%, 59.9% and 58.7% of our total revenues for the years ended December 31, 2009, 2010 and 2011, respectively. We have continued our efforts to promote EPO products in 2012 and expect that sales of EPIAO will continue to comprise a substantial portion of our revenues in the future.
Any reduction in revenues from EPIAO will have a direct negative impact on our business, financial condition and results of operations. Our EPO franchise and associated revenues could be adversely affected by a variety of factors, including, but not limited to:
-
increased competition;
-
pricing pressure caused by competition, government policies, or otherwise;
-
new product introductions;
-
intellectual property issues;
-
problems with raw materials supply;
-
disruptions in manufacturing or distribution;
-
the inability to market effectively our products;
-
newly discovered safety, side effects or other product issues; and
-
negative perception or insufficient recognition of EPIAO by the medical community or other key parties such as third party payers.
3
Beginning in 2006, there have been over-usage and safety concerns in the U.S. and some other regions in regard to EPO products, and there have been ongoing review and scrutiny by the US FDA. We are not aware of similar developments in China. However, if such developments occur, our business may be negatively affected.
Despite our efforts, we may be unable to develop or acquire new products that would enable us to diversify our business and reduce our dependence on EPIAO products, or to do so in such manner as competitively required.
The commercial success of our products depends upon the degree of market acceptance among the medical community. Failure to attain market acceptance among the medical community would have an adverse impact on our operations and profitability.
The commercial success of our products, including in-licensed products, depends upon the degree of market acceptance they achieve among the medical community, particularly physicians and hospitals. Physicians may not prescribe or recommend our products to patients, and procurement departments of hospitals may not purchase our products. The acceptance of any of our products among the medical community will depend upon several factors, including:
-
the safety and effectiveness of the product;
-
the effectiveness of our efforts to market our products to hospitals and physicians;
-
the product’s cost effectiveness;
-
the prevalence and severity of side effects; and
-
the product’s perceived advantages and disadvantages relative to competing products or treatments.
If our products fail to attain market acceptance among the medical community, our operations and profitability would be adversely affected.
The selling prices of our products tend to decline over time. Our success depends on our ability to successfully develop and commercialize additional pharmaceutical products. Our product development efforts may not result in commercially viable products.
As is typical in the Chinese pharmaceutical industry, the average selling prices of our products tend to decline significantly over the life of the product. These declines principally result from increased competition and changes in government policies.
We must therefore constantly identify product candidates that can be developed into cost-effective therapeutic products. We plan to continue to search for in-license and acquisition opportunities and invest in research and development; however, successful product development in our industry is highly uncertain, and relatively few research and development projects produce commercially viable products. If we cannot offset any decline in revenues and margins of our marketed products with new product introductions, our overall results of operations will be materially and adversely affected.
Our products face substantial competition, and we may not be able to compete effectively against current and future competitors.
We operate in a highly competitive environment. Our products compete with other products or treatments for diseases for which our products may be indicated.
-
EPIAO competes with both existing EPO drugs and new drug candidates. In China, EPO drugs are offered by established international companies such as Kirin Brewery Company Limited, or Kirin, and F. Hoffmann-La Roche, Ltd., or Roche, and domestic pharmaceutical companies such as Harbin Pharmaceuticals, Beijing Sihuan, Shanghai Kelong, Chengdu Di’ao Jiuhong Pharmaceutical Factory and others.
-
Competitors for Iron Sucrose Supplement include Beijing Novartis Pharmaceutical Co., Ltd., Nanjing Hencer Pharmaceutical Co., Ltd. and others.
-
While we believe TPIAO is the only recombinant human thrombopoietin, or TPO-based therapeutic, available in the Chinese market to date, other pharmaceutical companies may enter this market and manufacture and market their TPO products.
Certain of our competitors, including biotechnology and pharmaceutical companies, are actively engaged in research and development in areas where we have products or where we are developing product candidates or new indications for existing products. Other companies may discover, develop, acquire or commercialize products before or more successfully than we do.
In the future, we expect that our products will compete with new drugs currently in development, drugs approved for other indications that may be approved for the same indications as those of our products and drugs approved for other indications that are used off-label. Furthermore, our products may compete against products that have lower prices, superior performance, greater ease of administration or other advantages compared to our products. We do not currently have patents of any commercial significance covering EPIAO, our legacy products or several of our product candidates with which to protect these products from direct competition. Our inability to compete effectively could reduce sales or margins, which could have a material adverse effect on our results of operations.
4
As we expand our product portfolio by adding new products and indications, as well as developing second-generation versions of existing products with the same or overlapping labels, some of our products may be used as a substitute for our other products in the same end markets.
An increasing number of foreign pharmaceutical companies have introduced their products into the Chinese market. Subsequent to the reduction of import tariffs pursuant to China’s WTO obligations, the selling prices in China of imported pharmaceuticals have become more competitive. Also, foreign pharmaceutical manufacturers have set up domestic production bases in China leading to increasing direct competition.
Large Chinese state-owned and privately-owned pharmaceutical companies, and foreign pharmaceutical companies, may have greater clinical, research, regulatory, manufacturing, marketing, financial and human resources than we do. In addition, some of our competitors may have technical or competitive advantages over us for the development of technologies and processes. These resources may make it difficult for us to compete with them to successfully discover, develop and market new products and for our current products to compete with new products or new product indications that these competitors may bring to market. There may also be significant consolidation in the pharmaceutical industry among our competitors, or alliances may develop among competitors and these alliances may rapidly acquire significant market share.
In order to gain market share in China, competitors may significantly increase their advertising expenditures and promotional activities, or even engage in irrational or predatory pricing behavior. In addition, our competitors may engage in improper or unfair conducts, or even illegal acts. Third parties may actively engage in activities designed to undermine our brand name or to influence customer confidence in our products.
For these and other possible reasons, we may not be able to compete effectively against current and future competitors. Increased competition may result in price reductions, reduced margins and loss of market share, any of which could have a material adverse effect on our profit margins.
Our competitors may have the ability to manufacture pharmaceutical products substantially similar to ours.
Our ability to compete against our competitors is, to a significant extent, dependent upon our ability to distinguish our products from those of our competitors by providing high quality products at reasonable prices that are perceived to be safe and effective by doctors and patients. Many of our competitors may have been in business longer than we have, may have substantially greater financial and other resources than we have and may be better established in our markets. Our competitors in any particular market may also benefit from raw material sources or production facilities that are closer to such markets, which may provide them with competitive advantages in terms of cost and proximity to consumers.
Our TPIAO product had market exclusivity under a regulatory monitoring period through May 2010, during which other pharmaceutical companies were prohibited from manufacturing or importing similar drugs. Since the monitoring period has expired, other pharmaceutical companies in China may apply for approval of the SFDA to manufacture similar drugs using similar formulae or production techniques.
With respect to TPIAO or any other current or future product of ours, if other manufacturers introduce products substantially similar to ours, we will face more competitive pressure in the market and our sales and profit margin may be adversely affected.
Our business depends on our Shenyang Sunshine and EPIAO brands, and if we are not able to maintain and enhance our brands to sustain our competitive advantage, our reputation, business and operating results may be harmed.
We believe that market awareness of our Shenyang Sunshine and EPIAO brands has contributed significantly to the success of our business. We also believe that maintaining and enhancing the Shenyang Sunshine and EPIAO brands is critical to our competitive advantage.
In order to further penetrate the markets and launch new products, we must expand our manufacturing and sales and marketing efforts. Maintaining quality and cost-effectiveness may be more difficult to achieve.
While our sales and marketing staff will continue to promote our brand to remain competitive, we may not be successful. If we are unable to further enhance our brand recognition and increase awareness of our products, or if we incur excessive marketing and promotion expenses, our business and results of operations may be materially and adversely affected.
We may pursue collaborations, in-licensing arrangements, joint ventures, acquisitions, strategic alliances, partnerships, or other strategic initiatives or arrangements, which may fail to produce anticipated benefits, or adversely affect or disrupt our business.
As part of our business strategy, we continually pursue opportunities of collaboration, in-licensing, joint ventures, acquisitions of products, assets, technologies, or businesses, strategic alliances, or partnerships that we believe would be complementary to or promote our existing business.
Proposing, negotiating and implementing collaborations, in-licensing arrangements, joint ventures, acquisitions, or other strategic arrangements may be a lengthy and complex process. Other companies, including those with substantially greater financial, marketing, sales, technology, or other business resources, may compete with us for these opportunities or arrangements. We may not identify, secure, or complete any such transactions or arrangements in a timely manner, on a cost-effective basis, on acceptable terms, or at all.
5
We have limited experience with respect to these business development activities. Management of a license arrangement, collaboration, joint venture, or other strategic arrangement or integration of acquired assets or businesses may adversely affect or disrupt our current business, decrease our profitability, or cause us to incur significant expenses, or divert management resources that otherwise would be available for our existing business. We may not realize the anticipated benefits of any such transaction or arrangement.
Furthermore, partners, collaborators, or other parties to such transactions or arrangements may fail to fully perform their obligations or meet our expectations or cooperate with us satisfactorily for various reasons, including, due to risks or uncertainties related to their business and operations. There may be conflicts or other collaboration failures and inefficiencies between us and the other parties.
Such transactions or arrangements may also require or stand in need of actions, consents, approvals, waivers, participation or involvement of various degrees from third parties, such as regulators, government authorities, creditors, license grantors or grantees, related individuals, suppliers, distributors, shareholders, or other stakeholders or interested parties. We may not obtain such required or desired actions, consent, approval, waiver, participation or involvement on a timely basis, on acceptable terms, or at all.
We may not achieve one or more of our projected goals, objectives, milestones or targets as to various aspects of our business and operations, in the time frames we announce and expect, or at all.
We may set forth goals, milestones or targets in public announcements, releases and disclosures, regarding timing of the accomplishment of objectives material to our success, such as the commencement and completion of clinical trials, anticipated regulatory submission and approval dates, and timing of product launches. As a public company listed in the United States, we make additional announcements in our public reports, such as this annual report, and in press releases regarding these events, from time to time. The actual timing of these events can vary dramatically due to factors beyond our control, such as delays or failures in our clinical trials, the uncertainties inherent in the regulatory approval process, and delays in achieving manufacturing or marketing arrangements sufficient to commercialize our products. There can be no assurance that our clinical trials will be completed, that we will make regulatory submissions or receive regulatory approvals as planned or that we will be able to adhere to our current schedule for the launch of any of our products. If we fail to achieve one or more of these objectives, goals, milestones or targets as planned, the price of our shares could decline.
We may be classified as a passive foreign investment company, which would result in adverse U.S. federal income tax consequences to U.S. investors in our ADSs or ordinary shares.
We may be classified as a passive foreign investment company, or a PFIC, for United States federal income tax purposes, particularly for the taxable year ended December 31, 2008 (“Year 2008”) and the taxable year ended December 31, 2009 (“Year 2009”).
PFIC status is a factual determination made for each taxable year ending December 31, after the close of such year, on the basis of the composition of our income and our “active” versus “passive” assets for such year. Under the PFIC rules, we will generally be classified as a PFIC if, in the case of any particular taxable year, 75% or more of our gross income consists of certain types of “passive income” (the “income test”) or 50% or more of the value of our assets consists of “passive assets” (the “asset test”). For this purpose, cash and other liquid assets are generally classified as passive assets and goodwill and other unbooked intangibles may generally be classified as active assets. The overall level of our passive assets will be significantly affected by the amount and time-frame within which we deploy the cash raised in our initial public offering, other liquid assets that we presently hold, and the operation cash inflow. In addition, the overall level of our active assets will depend, in great measure, on the valuation of our goodwill and other unbooked intangibles as implied by our market capitalization which may fluctuate.
We believe we were not a PFIC for U.S. federal income tax purposes for our taxable year ended December 31, 2011. For the taxable year ending December 31, 2012, the PFIC status cannot be determined until after the end of the year. Because the PFIC determination is highly fact intensive, there can be no assurance that we will not be classified as a PFIC for any particular taxable year or that the Internal Revenue Service will not challenge any determination concerning our PFIC status for any particular taxable year. With respect to prior years, the financial market disruptions from late 2008 and early 2009 may have materially depressed our market valuation for Year 2008 and Year 2009, and , to our knowledge, there is a lack of guidance from United States authorities regarding how such disruptions should be taken into account in applying the asset test. In particular, it is possible that the disruptions could lead to our being classified as a PFIC under the asset test for Year 2008 and Year 2009. If we were to be or become classified as a PFIC for any particular taxable year during which a U.S. investor holds our ADSs or ordinary shares, such U.S. investor may incur a significantly increased United States income tax liability on gain recognized on the sale or other disposition of our ADSs or ordinary shares and on the receipt of distributions on our ADSs or ordinary shares; and, we generally would continue to be treated as a PFIC for all succeeding years during which such U.S. investor holds our ADSs or ordinary shares. See “Item10.E --- Taxation - United States Federal Income Tax Considerations for U.S. Persons” for more detailed discussions on PFIC matters.
6
Notwithstanding our belief as discussed and any information we provide solely for the convenience of our investors, we are not providing any U.S. tax opinion or advice to U.S. investors concerning the PFIC status of our company, and U.S. investors should consult their own tax advisors concerning the implication of the PFIC rules in his, her or its particular circumstance and determine his, her, or its own tax position as to our PFIC status for a particular taxable year, including, as applicable, for Year 2008 and Year 2009.
Certain of our raw materials, medical devices and components are single-sourced from third parties; third-party supply failures could adversely affect our ability to supply our products.
Certain raw materials necessary for commercial manufacturing and formulation of our products are provided by single-source unaffiliated third-party suppliers. Also, certain medical devices and components necessary for formulation, fill, and finish of our products are provided by single-source unaffiliated third-party suppliers, including the EPO Elisa Kit by R&D Systems Inc., GIBCO cell culture medium by Invitrogen Inc., Pharmacia EPO chromatography purification medium by GE Healthcare, a division of GE, and Disc, a microcarrier for cell cultures, by New Brunswick Scientific Inc. For more details, see “4.B.8 Manufacturing.” Certain of these raw materials, medical devices and components are the proprietary products of these unaffiliated third-party suppliers.
We would be unable to obtain these raw materials, medical devices or components for an indeterminate period of time if these third-party single-source suppliers were to cease or interrupt production or otherwise fail to supply these materials or products to us for any reason, including due to regulatory requirements or action, due to adverse financial developments at or affecting the suppliers, and/or due to unexpected demand, labor shortages or disputes. We would also be unable to obtain these materials, devices and components for an indeterminate period of time if such supply was subsequently found to not be in compliance with our quality standards or resulted in quality failures or product contamination and/or recall when used to manufacture, formulate, fill or finish our products. These events could adversely affect our ability to satisfy demand for our products, which could materially and adversely affect our product sales and operating results.
For example, we have occasionally experienced shortages in certain components necessary for the formulation, fill and finish of certain of our products in our Shenyang facility without impact on our ability to supply these products. However, we may experience shortages in the future resulting in delayed shipments, supply constraints, stock-outs and/or recalls of our products, which could result in interruptions to our production.
We depend on our distributors for sales of our products.
We rely on our network of distributors to distribute our own and our in-licensed products. Our distributors do not sell our products on an exclusive basis. As a result, our products face competition from similar products sold by our distributors.
Our success will depend in part on our ability to form relationships with and manage a changing number of distributors. If our distribution network in China suffers a disruption, our financial condition and results of operations may be adversely affected.
While we have long-standing business relationships with most of our distributors, we do not have long-term contracts with any distributor except for in our export sales, which in the past 5 years represents approximately 5% or less of our total net revenue. Moreover, a significant amount of our revenue is generated by product sales to relatively few distributors, whose mix changes from year to year. In each of the past three years, sales to our top five distributors accounted for 30% to 40% of our total net revenue.
For more information, see “4.B.5 Marketing, Sales and Distribution.”
If any large distributor was to voluntarily or involuntarily suspend or terminate product purchases from us, we would need to divert product sales to other distributors, which could cause disruptions to our revenue and profitability.
We are highly dependent on senior management and key research and development personnel.
We are highly dependent on our senior management to manage our business and operations and our key research and development personnel for the development of new technologies and applications and the enhancement of our existing products. In particular, we rely substantially on our chairman and chief executive officer, Dr. Jing Lou, to manage our operations. We also depend on our key research personnel such as Dongmei Su, our vice president of research and development (“R&D”) and chief technology officer. In addition, we also rely on sales personnel and other personnel with industry knowledge, to market and sell our products. We do not maintain key man life insurance on any of our senior management or key personnel. The loss of any one of them, in particular Dr. Lou, would have a material adverse effect on our business and operations. In addition, although Dr. Lou and Dr. Su have each signed a non-competition agreement with us, we cannot assure you that we will be able to successfully enforce these provisions in the event of a dispute.
7
Competition for senior management and research and development personnel is intense, and the pool of suitable candidates is limited. We may be unable to locate a suitable replacement for any senior management or key research and development personnel that we lose. We compete for qualified personnel with other pharmaceutical companies, universities and research institutions. Intense competition for these personnel could cause our compensation costs to increase significantly, which could have a material adverse effect on our results of operations. Our future success and ability to grow our business will depend in part on the continued service of these individuals and our ability to identify, hire and retain additional qualified personnel. If we are unable to attract and retain qualified employees, we may be unable to meet our business and financial goals.
If we are unable to protect our products through intellectual property rights, our competitors may compete directly against us.
Our success depends, in part, on our ability to protect our products from competition by establishing, maintaining and enforcing intellectual property rights. We try to protect the products and technology that we consider important to our business by filing PRC patent applications, securing pharmaceutical regulatory protection, establishing and enforcing confidentiality contractual obligations, relying on trade secrets, or employing a combination of these methods.
We do not have any patent protection of commercial significance relating to EPIAO. We have patents and patent applications relating to TPIAO and certain of our other products, product candidates and technologies. For more details, see “4.B.7—Intellectual Property.” However, the process of seeking patent protection in the PRC can be lengthy and expensive, and we cannot assure you that these patent applications, or any patent applications we may make in the future in respect of other products, will result in patents being issued, or that any patents issued in the future will be able to provide us with meaningful protection or commercial advantage. Any patent issued to us may be challenged, invalidated or circumvented.
In addition to patents, we rely on trade secrets and proprietary know-how to protect our intellectual property. We have entered into confidentiality agreements with many of our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors. These agreements may not provide meaningful protection or adequate remedies in the event of unauthorized use or disclosure of our proprietary information. In addition, it is possible that third parties could independently develop proprietary information and techniques substantially similar to ours or otherwise gain access to our proprietary information.
We may become involved in patent litigation against third parties to enforce our patent rights, to invalidate patents held by such third parties, or to defend against intellectual property claims. The cost to us of any patent litigation or similar proceeding could be substantial, and it may absorb significant management resources. We do not maintain insurance to cover intellectual property infringement.
Intellectual property rights and confidentiality protections in China may not be as effective as in the United States or other countries, due to, among other causes, lack of procedural rules for discovery and evidence, low damage awards and lack of judicial independence. Implementation and enforcement of PRC intellectual property-related laws have historically been deficient and ineffective and may be hampered by corruption and local protectionism. Policing unauthorized use of proprietary technology is difficult and expensive, and we might need to resort to litigation to enforce or defend patents issued to us or to determine the enforceability, scope and validity of our proprietary rights or those of others. The experience and capabilities of PRC courts in handling intellectual property litigation varies, and outcomes are unpredictable. Further, such litigation may require significant expenditure of cash and management efforts and could harm our business, financial condition and results of operations. An adverse determination in any such litigation could materially impair our intellectual property rights and may harm our business, prospects and reputation.
For more details on the process for applying for and obtaining intellectual property protection in the PRC, see “4.B.11-c Intellectual Property.”
If our products infringe the intellectual property rights of third parties, we may incur substantial liabilities, and we may be unable to sell these products.
Our commercial success depends significantly on our ability to operate without infringing the patents and other proprietary rights of third parties. Under the PRC Patent Law promulgated by the People’s Congress in March 1984, as amended, patent applications are maintained in confidence until their publication at the end of 18 months from the filing date. The publication of discoveries in the scientific or patent literature frequently occurs substantially later than the date on which the underlying discoveries were made and the date on which patent applications are filed. China adopts the first-to-file system under which whoever first files a patent application (instead of the one who makes first actual discoveries) will be awarded the patent. By contrast, the United States patent law endorses the first-to-invent system under which whoever makes the first actual discovery will be awarded the patent. Under the first-to-file system, even after reasonable investigation we may not know with certainty whether we have infringed a third party’s patent because such third party may have filed a patent application without our knowledge while we are still developing that product. We are aware of intellectual property rights held by third parties that relate to products or technologies we are developing. For example, we are aware of a patent held by a third party that may relate to our TPIAO product. We believe, as to each claim in this patent, that we either do not infringe the claim of the patent or that the claim is invalid. While the validity of issued patents, patentability of pending patent applications and applicability of any of them to our programs are uncertain, if asserted against us, any related patent rights could adversely affect our ability to commercialize our products.
8
If a third party claims that we infringe its proprietary rights, one or more of the following may occur:
-
we may become involved in time-consuming and expensive litigation, even if the claim is without merit;
-
we may become liable for substantial damages for past infringement if a court decides that our technology infringes a third party’s patent;
-
a court may prohibit us from selling or licensing our product without a license from the patent holder, which may not be available on commercially acceptable terms, if at all, or which may require us to pay substantial royalties or grant cross licenses to our patents; or
-
we may have to reformulate our product so that it does not infringe patent rights of others, which may not be possible or could be very expensive and time-consuming.
Although to date we have not experienced any of the circumstances listed above, if any of these events occurs, our business will be adversely affected.
Power shortages, natural disasters, terrorist acts or other calamities could disrupt our production and have a material adverse effect on our business, financial position and results of operations.
EPIAO, TPIAO and our legacy products are produced at our manufacturing facilities in Shenyang. A significant disruption at that facility, even on a short-term basis, could impair our ability to produce and ship products on a timely basis, which could have a material adverse effect on our business, financial position and results of operations.
Our Shenyang manufacturing operations are vulnerable to interruption and damage from natural and other types of disasters, including earthquake, fire, floods, environmental accidents, power shortage, interruptions in electricity supply, communications failures and similar events. If any disaster were to occur, our ability to operate our business at our facilities would be seriously impaired. In addition, the nature of our production and research activities could require significant delays in our programs and make it difficult for us to recover from a disaster. We do not maintain business interruption insurance. Accordingly, unexpected business interruptions resulting from disasters could disrupt our operations and thereby result in substantial costs and diversion of resources.
We may experience significant period-to-period quarterly and annual fluctuations in our revenue and operating results, which may result in volatility in our stock price.
We may experience significant period-to-period fluctuations in revenues and operating results. Our revenues and operating results in some quarters may fall below the estimates of securities research analysts, which may cause the value of our ordinary shares and ADSs to decline.
We typically generate higher levels of revenues during the second half of the year, primarily because of the tendency of hospitals to place more orders prior to the year-end holiday season and the fact that more people visit hospitals in the second half of the year, resulting in more prescriptions by physicians during this period.
Generally, our quarterly and annual operating results are affected by a number of factors, such as:
-
seasonal spending patterns of Chinese consumers, including hospitals, dialysis centers and clinics;
-
changes in pricing policies by us, our competitors or the government;
-
the timing and market acceptance of new products and product enhancements by us or our competitors;
-
the loss of key sales personnel or distributors;
-
our involvement in litigation;
-
changes in government policies or regulations; and
-
a downturn in general economic conditions in China.
While certain of the factors identified above, including seasonal spending patterns, changes in pricing policies, market acceptance of new products and changes in government policies, have in the past caused fluctuations in our quarterly financial results, we have not suffered any material and adverse consequences from these fluctuations in the last three years. However, many of these factors are beyond our control, and you should not rely on our results of operations for prior quarters as an indication of our results in any future period. As our revenues vary significantly from quarter to quarter, our business could be difficult to predict and manage and our quarterly results could fall below investor expectations, which could cause our ADS price to decline.
We have previously operated as a private Chinese company and have limited experience in complying with requirements applicable to public companies in the United States. Continued compliance with U.S. GAAP requirements will require significant management resources, financial and other costs.
Historically, we have primarily prepared unaudited financial statements in accordance with PRC GAAP for the purpose of tax reporting and determining the level of statutory reserves until our initial public offering, and have limited resources and a small finance and accounting team. Although we have since invested additional resources and hired additional staff, we expect to continue to encounter substantial difficulty attracting qualified staff with requisite experience due to the high level of competition for experienced financial professionals. We have been providing training for our staff with respect to U.S. GAAP. Our training may not be sufficient or effective.
9
We continue to face increased legal, accounting, administrative and other costs and expenses as a public company that we did not incur as a private company. Compliance with the U.S. Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, as well as other rules of the SEC, the Public Company Accounting Oversight Board and the NASDAQ Stock Market LLC, has resulted in significant legal, audit and financial compliance costs, including costs related to our compliance with Section 404 of the Sarbanes-Oxley Act.
We may fail to achieve and maintain effective internal controls.
We are subject to the reporting obligations under the United States securities laws. We are required by the SEC, as directed by Section 404 of the Sarbanes-Oxley Act, to include a report of management on the effectiveness of our internal control over financial reporting. In addition, an independent registered public accounting firm must attest to and report on the effectiveness of our internal control over financial reporting.
Our management concluded that our internal control over financial reporting was effective as of December 31, 2011, and our independent registered public accounting firm has issued an attestation report, which has concluded that we maintained, in all material respects, effective internal control over financial reporting as of December 31, 2011. See “Item 15. Controls and Procedures.”
Our management may not conclude that our internal controls are effective in the future. Moreover, even if our management concludes that our internal control over financial reporting is effective, our independent public registered accounting firm may disagree. If our independent public registered accounting firm is not satisfied with our internal control over financial reporting or the level at which our controls are designed, operated, documented or reviewed, it then may issue an adverse opinion on the effectiveness of our internal control over financial reporting. Any of these possible outcomes could result in the loss of investor confidence in the reliability of our financial statements.
Furthermore, we have expended, and anticipate that we will continue to expend, considerable costs, management time, and other resources, in an effort to comply with Section 404 and other requirements of the Sarbanes-Oxley Act.
Our export sales may expose us to risks, uncertainties, and liabilities related to the destination countries and international operations. We have very limited experience in operating internationally.
We export our products outside China and plan to continually expand our exports. We had previously operated solely within China until 2003, and had very limited experience in operating internationally and in any particular destination country.
The exports may expose us to risks, uncertainties, and liabilities in or related to the destination countries and international operations. In new markets we may fail to anticipate competitive conditions that are different from those in our existing markets. In a particular destination country, our brands may be less recognized. Our selected distributors may not be suitable for selling our products. We expect that pharmaceutical products are heavily regulated and the local laws and regulations may impose substantial costs and burdens on us. We may be unable to effectively protect or enforce our intellectual property rights; may become involved in disputes or litigations over intellectual property rights; or may encounter other issues in the area of intellectual property.
Other risks and uncertainties may include: particular local forms of products liabilities and regulations with respect to pharmaceutical products; obligations to comply with a wide variety of foreign laws and other regulatory requirements; political instability; economic instability and recessions; changes in tariffs; difficulties of managing foreign operations generally; increased risk of exposure to terrorist activities; financial condition, expertise and performance of our international distributors; export license requirements; unauthorized re-export of our products; potentially adverse tax consequences; and inability to effectively enforce contractual or legal rights; and language and cultural conflicts.
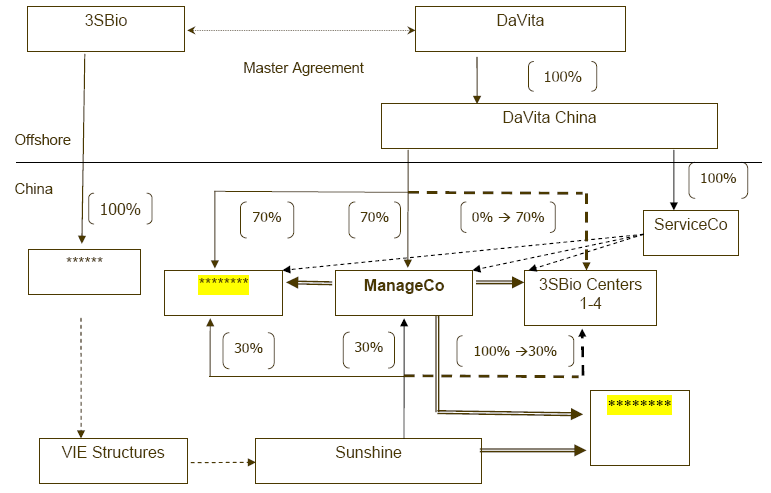
There are certain operational, legal and other risks associated with our contractual arrangements with Liaoning Sunshine and its shareholders that are intended to effect our control over Liaoning Sunshine.
We rely on contractual arrangements with our affiliated variable interest entity, Liaoning Sunshine Bio-Pharmaceutical Company Limited, or Liaoning Sunshine, and its shareholder, Mr. Dan Lou, to maintain control over the business and operations of Liaoning Sunshine. Mr. Dan Lou is our former chairman and the father of Mr. Jing Lou, our chairman and CEO. Liaoning Sunshine holds the rights to our in-licensed product, Iron Sucrose Supplement; in addition, Liaoning Sunshine Science and Technology Development Co. Limited (“LSSTD”), a wholly-owned subsidiary of Liaoning Sunshine, is the principal party in our collaboration with DaVita Inc. (“DaVita”) to provide kidney care services in two Chinese provinces, as set forth under Item 8 and Exhibit 4.10.
10
These contractual control arrangements may not be as effective in providing control over Liaoning Sunshine as direct ownership. If Liaoning Sunshine or its sole shareholder, Mr. Dan Lou, refuses to perform any contractual obligations to us, we could be affected. Dr. Jing Lou is the CEO and a director of LSSTD. While Dr Lou as our Chairman and CEO has a fiduciary duty of loyalty and care to us under Cayman Islands law, the potential exists for conflicts of interests between his duties to us and his duties towards Liaoning Sunshine. We are party to a purchase agreement, pursuant to which we may acquire 100% of the equity interests in Liaoning Sunshine from Mr. Dan Lou, if and when PRC law permits. We may not receive any required regulatory approval on a timely basis, or at all.
In addition, although we believe we comply with current PRC regulations, we cannot assure you that the PRC authorities would agree that our arrangements with Liaoning Sunshine comply with PRC licensing, registration, tax or other regulatory requirements, with existing policies or with requirements or policies that may be adopted in the future.
Any of these risks may affect adversely our economic and voting control over Liaoning Sunshine, which could in turn materially and adversely affect our business, results of operations, and financial condition. For more information, please see “4.C Organizational Structure” and “7.B Related Party Transactions – Transactions with Liaoning Sunshine”.
3.D.2 Risks Related To Our Industry
The pharmaceutical industry in China is highly regulated, and future government regulation may place additional burdens on our business.
The pharmaceutical industry in China is subject to extensive government regulation and supervision. The regulatory framework addresses all aspects of operating in the pharmaceutical industry, including approval, production, licensing and certification requirements and procedures, periodic renewal and reassessment processes, registration of new drugs and environmental protection. Violation of applicable laws and regulations may materially adversely affect our business.
In order to manufacture pharmaceutical products in China, we are required to apply for and obtain a pharmaceutical manufacturing permit from the provincial level food and drug administrative authority. In addition, in order to manufacture and market any drug in China, we are required to apply for and obtain permits and certificates from the SFDA, including the new drug certificate, drug registration certificate (which includes the issuance of a drug approval number) and GMP certificate. (Chinese GMP refers to guidelines and regulations issued from time to time pursuant to PRC laws as part of quality assurance to ensure that products subject to those guidelines and regulations are consistently produced and controlled to the quality and standards appropriate for their intended use.) We are required to renew the pharmaceutical manufacturing permits, drug registration certificates and GMP certificates periodically, generally every five years. China SFDA and other Chinese regulatory agencies operate under considerable time and resources constraints. There may be various factors and risks that impact on the process of obtaining or renewal of these certificates, permits, or similar regulatory approvals. If we are unable to obtain or renew such permits or certificates or any other regulatory approvals required for our operation on a timely basis, or at all, we may be prevented from engaging in the manufacture of our products, or suffer other possible negative consequences, and our business may be adversely affected.
Changes in compliance standards, laws, regulations and regulatory practices may prohibit or render more restrictive certain business activities, increase compliance costs or otherwise adversely impact our operations, profitability or financial position.
For more information, please refer to “4.B.11—Regulations”.
New product development in the pharmaceutical industry is both costly and labor-intensive and has a low rate of successful commercialization.
Our success will depend in part on our ability to enhance our existing products and to develop new products. The development process for pharmaceutical products is complex and uncertain, as well as time-consuming and costly. Relatively few research and development programs produce a commercial product. A product candidate that appears promising in the early phases of development may fail to reach the market for a number of reasons, such as:
-
the failure to demonstrate safety and efficacy in preclinical and clinical trials;
-
the failure to obtain approvals for intended use from relevant regulatory bodies, such as the SFDA;
-
our inability to manufacture and commercialize sufficient quantities of the product economically; and
-
proprietary rights, such as patent rights, held by others related to our product candidate and their refusal to sell or license such rights to us on reasonable terms, or at all.
In addition, product development requires the accurate assessment of market trends. We cannot assure you that:
-
our new product research and development efforts will be successfully and timely completed;
-
the SFDA or other regulatory bodies will grant necessary regulatory clearances or approvals on a timely basis, or at all; or
-
any product we develop will be commercialized or achieve market acceptance.
11
Delays in any part of the development process or our inability to obtain regulatory approval of our products could adversely affect our operating results by restricting or delaying our introduction of new products.
Even if we successfully commercialize new products, these products may address markets that are currently being served by the off-label use of others of our mature products and inadvertently result in a reduction in the sales volume of our mature products or vice versa.
Failure to develop, obtain necessary regulatory clearances or approvals for or successfully commercialize or market potential new products or technologies could have a material adverse effect on our financial condition and results of operations.
We will not be able to commercialize our product candidates if our preclinical studies do not produce successful results or our clinical trials do not demonstrate safety and efficacy in humans.
Before obtaining regulatory approvals for the manufacturing and sale of our product candidates, we must conduct, at our own expense, extensive preclinical tests and clinical trials to demonstrate the safety and efficacy in humans of our product candidates. Preclinical and clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome. Success in preclinical testing and early clinical trials does not ensure that later clinical trials will be successful, and interim results of a clinical trial do not necessarily predict final results. A failure of one or more of our clinical trials can occur at any stage of testing. We may experience numerous unforeseen events during, or as a result of, preclinical testing and the clinical trial process that could delay or prevent our ability to receive regulatory approval or commercialize our product candidates, including:
-
our preclinical tests or clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional preclinical testing or clinical trials, or we may abandon projects that we expect to be promising;
-
we might have to suspend or terminate our clinical trials if the participating patients are being exposed to unacceptable health risks;
-
regulators may require that we hold, suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or safety concerns;
-
the time or cost of our clinical trials may be greater than we currently anticipate;
-
any regulatory approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the product not commercially viable; and
-
our product candidates may produce undesirable side effects or may have other unexpected characteristics.
If we are required to conduct additional clinical trials or other testing of our product candidates beyond those that we currently contemplate, if we are unable to successfully complete our clinical trials or other testing or if the results of these trials or tests are not positive or are only modestly positive, we may:
-
be delayed in obtaining marketing approval for our product candidates;
-
not be able to obtain marketing approval; or
-
obtain approval for indications that are not as broad as intended.
Our product development costs will also increase if we experience delays in testing or approvals. We do not know whether planned clinical trials will begin as planned, will need to be restructured or will be completed on schedule, if at all. Significant clinical trial delays also could allow our competitors to bring products to market before we do and impair our ability to commercialize our products or product candidates.
We may not be able to obtain regulatory approval for any of the products resulting from our development, or may do so only with sufferance of delays, limitations, increased costs or other issues.
New pharmaceutical products must be approved by the SFDA before they can be marketed and sold in China. The SFDA requires successful completion of clinical trials and demonstrated manufacturing capability before the grant of approval. Clinical trials are expensive and their results are uncertain. It often takes multiple years before a medicine can be ultimately approved by the SFDA. In addition, the SFDA and other regulatory authorities may apply new standards for safety, manufacturing, packaging, and distribution of future product candidates. Complying with such standards may be time-consuming and expensive and could result in delays in obtaining SFDA approval for our product candidates, or possibly preclude us from obtaining SFDA approval. Furthermore, our future products may not be effective or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude us from obtaining regulatory approval or prevent or limit commercial use. The SFDA and other regulatory authorities operate under considerable time and resource constraints. There may be various factors and risks that impact on the process of regulatory review and approvals. The SFDA and other regulatory authorities may not approve the products that we develop, and even if we do obtain regulatory approvals, such regulatory approvals may take longer than expected or desired, or may be subject to limitations on the indicated uses for which we may market a product, which may limit the size of the market for such product.
12
The pricing of all of our products is subject to government approval, controls, and other regulations. Price-related government actions or measures may limit our profitability or cause us to stop marketing in a region or even stop manufacturing certain products.
Pursuant to the implementing rules of the Drug Administration Law, we are required to seek pricing approval for all our products from the National Development and Reform Commission of the PRC, or the NDRC, and the price administration authorities of the relevant provinces of the PRC in which our pharmaceutical products are manufactured. We have in the past been able to successfully obtain pricing-related approvals.
In addition, in order to access certain local or provincial-level markets, we enter into government-sponsored competitive tendering processes for EPIAO, TPIAO and our legacy products every year or every few years, or as otherwise required by the relevant government authorities, with a designated price range. The competitive bidding in effect sets price ceilings for our products, thereby limiting our profitability. In some instances, if the price range designated by any local government falls below our costs, we may stop marketing such products in that region or even stop manufacturing such products. See “4.B.11-b.3 Distribution.”
Pharmaceutical products included in certain government sponsored insurance programs are subject to price controls in the form of fixed retail prices or retail price ceilings. In addition, the maximum retail prices of such products are also subject to periodic downward adjustments as the PRC government authorities aim to make pharmaceuticals more affordable to the general public. Currently, the pricings with respect to all of our products are being reviewed by the NDRC and the NDRC may impose price cuts on any one or more of these products, in which event our business, revenues and profitability may be adversely and materially affected.
The emphasis of China's health care reform on affordability of medical care may continue to exert price pressure on drug prices; and the uncertainties related to the reform and any adverse developments may further impact on the pricing of our products. For example, our core products EPIAO and TPIAO are currently not included in the National Essential Drugs Registry (see Item 4.B.11-b.5 "- National Essential Drugs Registry"), which inclusion would likely to exert more downward pricing pressure.
Rapid changes in the pharmaceutical industry may render our products obsolete.
The pharmaceutical industry is characterized by rapid changes in technology, constant enhancement of industrial knowhow and frequent emergence of new products. Future technological improvements and continual product developments in the pharmaceutical market may render our existing products obsolete or affect our viability and competitiveness. Therefore, our future success will largely depend on our ability to:
-
improve our existing products;
-
diversify our product portfolio; and
-
develop new and competitively priced products which meet the requirements of the constantly changing market.
If we fail to respond to this environment by improving our existing products or developing new products in a timely fashion, or if our new or improved products do not achieve adequate market acceptance, our business and profitability may be materially and adversely affected.
Anti-corruption measures taken by the government authorities to correct corruptive practices in the pharmaceutical industry, and laws and regulations aiming at combating corruption, could adversely affect us.
The PRC government has from time to time undertaken anti-corruption measures to correct corrupt practices. In the pharmaceutical industry, such practices include, among others, acceptance of kickbacks, bribery or other illegal gains or benefits by the hospitals and medical practitioners from pharmaceutical distributors in connection with the prescription of a certain drug. Nearly all of our sales to our ultimate customers are conducted through third-party distributors. We have no control over our third-party distributors, who may engage in corrupt practices to promote our products. While we maintain strict anti-corruption policies applicable to our internal sales force and third-party distributors, these policies may not be effective. If our affiliated variable interest entity, Liaoning Sunshine, or any of our third-party distributors engage in such practices and the government takes enforcement action, our products may be seized and our own practices, and involvement in the distributors’ practices, investigated. If this occurs, our sales and reputation may be materially and adversely affected.
In addition, government-sponsored anti-corruption campaigns from time to time could have an adverse effect on our efforts to reach new hospital customers. Our sales representatives primarily rely on hospital visits to better educate physicians on our products and promote our brand awareness. In the past, there have been occasions on which our sales representatives were denied access to hospitals in order to avoid the perception of corruption. If this attitude becomes widespread among our potential customers, our ability to promote our products will be adversely affected.
13
Furthermore, we are subject to the U.S. Foreign Corrupt Practices Act, or FCPA, which has been more proactively enforced by the U.S. government in recent years. Even though we have adopted a Code of Ethics that applies to all our employees, which, among other requirements, prohibits improper payments and gifts in our business, and enforces compliance with applicable laws and regulations, including FCPA, we cannot assure you that our employees will comply with this Code or that the increased level of FCPA investigative and enforcement actions will not adversely affect us.
We are subject to environmental regulations and may be exposed to liability and potential costs for environmental compliance.
We are subject to PRC laws and regulations concerning the discharge of effluent water and solid waste during our manufacturing processes. We are required to obtain clearances and authorizations from government authorities for the treatment and disposal of such discharge. We may not at all times comply fully with environmental regulations. Any violation of these regulations may result in substantial fines, criminal sanctions, revocations of operating permits, shutdown of our facilities and obligation to take corrective measures. Our cost of complying with current and future environmental protection laws and regulations and our liabilities which may potentially arise from the discharge of effluent water and solid waste may materially and adversely affect our business, financial condition and results of operations.
The government may take steps toward the adoption of more stringent environmental regulations. Due to the possibility of unanticipated regulatory or other developments, the amount and timing of future environmental expenditures may vary substantially from those currently anticipated. If there is any unanticipated change in the environmental regulations, we may need to incur substantial capital expenditures to install, replace, upgrade or supplement our pollution control equipment or make operational changes to limit any adverse impact or potential adverse impact on the environment in order to comply with new environmental protection laws and regulations. If such costs become prohibitively expensive, we may be forced to cease certain of our business operations.
We may be required to defend lawsuits or pay damages for product liability claims. We do not have any liability or business disruption insurance, and, a claim against us, or an interruption in our business, could adversely affect our reputation and our financial results.
The development and commercialization of pharmaceutical products entails an inherent risk of harm to the patient and, therefore, product liability. If a product liability claim is brought against us, it may, regardless of merit or eventual outcome, result in damage to our reputation, breach of contract with our customers, decreased demand for our products, costly litigation, product recalls, loss of revenue and the inability to commercialize some products. We currently are not aware of any existing or anticipated product liability claims with respect to our products.
Existing PRC laws and regulations do not require us to, nor do we, maintain liability insurance to cover product liability claims. The insurance industry in China is still at an early stage of development. Insurance companies in China offer limited business insurance products. As a result, we do not have business liability, or in particular, product liability or disruption insurance coverage for our operations. Any business disruption, litigation or natural disaster might result in substantial costs and diversion of resources. Any product liability insurance for clinical trials, when obtained, may be prohibitively expensive, or may not fully cover our potential liabilities. The inability to obtain sufficient insurance coverage at an acceptable cost or otherwise to protect against potential product liability claims could prevent or inhibit the commercialization of products that we or our collaborators develop.
3.D.3 Risks Related To Doing Business In China
Adverse changes in political, economic and other policies of the Chinese government could have a material adverse effect on the overall economic growth of China, which could reduce the demand for our products; and could otherwise materially and adversely affect our business, operations or competitive position. Developments in China’s healthcare policy may adversely affect us. Changes and developments in China’s economic, political and social conditions could adversely affect our financial condition and results of operations.
All of our operations are located in China, and substantially all of our sales are made in China. Accordingly, our business, financial condition, results of operations and prospects are affected significantly by economic, political and legal developments in China.
The Chinese economy differs from the economies of most developed countries in many respects, including, but not limited to:
-
the extent of government involvement;
-
the level of development;
-
the growth rate;
-
the control of foreign exchange;
-
the allocation of resources;
-
an evolving regulatory system; and
-
the level of transparency in the regulatory process.
14
While the Chinese economy has experienced significant growth in the past 20 years, growth has been uneven, both geographically, among various sectors of the economy, and during different periods. The Chinese economy may not continue to grow, and if there is growth, such growth may not be steady and uniform; and if there is a slowdown, such a slowdown may have a material negative effect on us.
The Chinese government implements various measures intended to encourage economic growth and guide the allocation of resources. These measures may include differential policies towards specific groups of pharmaceutical companies, such as promotion of traditional medicines or state-owned companies, or investments in biopharmaceutical companies competing against us, which may have an adverse effect on us. Our financial condition and results of operations may be adversely affected by government control over capital investments or changes in tax regulations that are applicable to us. Further, any adverse change in the economic conditions or government policies in China could have a material adverse effect on overall economic growth and the level of healthcare investments and expenditures in China, which in turn could lead to a reduction in demand for our products and consequently have a material adverse effect on our businesses.
The Chinese economy has been transitioning from a planned economy to a more market-oriented economy. Although the Chinese government has implemented reform measures allowing more free play of market forces, the reduction of state ownership of productive assets and the establishment of sound corporate governance in business enterprises, a substantial portion of the productive assets in China is still owned by the Chinese government. The continued control of these assets and other aspects of the national economy by the Chinese government could materially and adversely affect our business. The Chinese government also exercises significant control over Chinese economic growth through the allocation of resources, controlling payment of foreign currency- denominated obligations, setting monetary policy and providing preferential treatment to particular industries or companies.
The Chinese government has implemented a comprehensive healthcare reform from 2009. The reform may not achieve the intended goals in one or more aspects, and consequently, any possible positive benefits in regard to our business and operations flowing from the reform measures, such as greater market demand, may not materialize. We are limited in our ability to predict the current and future effects of the healthcare reform, which could be materially adverse in one or more ways to our business, financial condition or results of operations.
Changes and developments in China’s economic, political and social conditions could adversely affect our financial condition and results of operations. In particular, the pharmaceutical market may grow at a slower pace than expected. An outbreak of avian flu, SARS, swine influenza or other epidemics in China could adversely affect our business, financial condition or results of operations.
Moreover, the political relationship between the United States, Europe, or other Asian nations and China is subject to sudden fluctuation and periodic tension. Changes in political conditions in China and changes in the state of foreign relations are difficult to predict and could adversely affect our operations.
Future changes in laws, regulations or enforcement policies in China could adversely affect our business.
Laws, regulations or enforcement policies in China, including those regulating healthcare and the pharmaceutical industry, are evolving and subject to frequent changes. Further, regulatory agencies in China may periodically, and sometimes abruptly, change their enforcement practices. Therefore, prior enforcement activity, or lack of enforcement activity, is not necessarily predictive of future actions. Any enforcement actions against us could have a material adverse effect on us. Any litigation or governmental investigation or enforcement proceedings in China may be protracted and may result in substantial cost and diversion of resources and management attention, negative publicity, and damage to reputation. In addition, such changes may be applied retroactively and thus subject our business and operations to increased uncertainties and risks.
There are significant uncertainties under the Enterprise Income Tax (“EIT”) law of the PRC, with respect to our PRC enterprise income tax liabilities, and with respect to possible PRC withholding tax upon our shareholders and ADS holders.
There are significant uncertainties under the PRC Enterprise Income Tax Law and accompanying regulations and rules, or the EIT law.
Under the EIT law, if we are deemed a PRC resident enterprise, we could be subject to the EIT at 25%, or any preferential rate, if obtained, on our global income, except that the dividends we receive from our PRC subsidiaries may be exempt from the EIT to the extent such dividends constitute “dividends among qualified PRC resident enterprises.” It is, however, unclear what type of enterprises would be deemed a “qualified resident enterprise” for such purposes. If we are deemed a resident enterprise and determined to have earned income other than exempted dividends from our PRC subsidiaries, the EIT on our global income could significantly increase our tax burden and adversely affect our cash flow and profitability.
15
Further, if we are deemed a PRC resident enterprise under the EIT law, our shareholders and ADS holders that are nonresident could be subject to the withholding income tax, or WHT, upon the dividends payable by us or upon any gains realized from the transfer of our shares or ADSs, if such income is deemed derived from China. The WHT rate is generally 10% to enterprises and 20% to individuals. It is unclear whether, if we are deemed a PRC resident enterprise, our shareholders and ADS holders might be able to claim the benefit of income tax treaties entered into between China and other countries.
Alternatively, if we, as to the holding entities outside China, are deemed non-resident enterprise, the WHT may apply to the dividends (and interests on intercompany loans) paid by our PRC subsidiaries to the holding entities outside China. For the information regarding our holding structure, please refer to “4.C Organization Structure”.
We have received various preferential tax treatments under the EIT law and its predecessor laws with respect to our operations and business. Shenyang Sunshine Pharmaceutical Company Limited, or Shenyang Sunshine, our wholly-owned PRC subsidiary, has been certified as a “New and High Technology Enterprise” that entitled it to a preferential EIT rate of 15% effective from January 1, 2011, to December 31, 2013. This and other past or future preferential tax treatments may expire, or be repealed, discontinued or otherwise modified, such that our tax burden could be materially increased.
For more information, please refer to “4.B.11-d PRC Enterprise Income Tax”.
Restrictions on currency exchange may limit our ability to receive and use our revenue effectively.
We receive nearly all of our revenue in Renminbi, which currently is not a freely convertible currency. A portion of our revenue may be converted into other currencies to meet our foreign currency obligations, including, among others, payment of dividends declared, if any, in respect of our ordinary shares. Under China’s existing foreign exchange regulations, we are able to pay dividends in foreign currencies without prior approval from the State Administration of Foreign Exchange, or the SAFE, by complying with certain procedural requirements. However, the PRC government may take measures to restrict access to foreign currencies for current account transactions.
Our ability to obtain foreign exchange is subject to significant foreign exchange controls which in the case of amounts under the capital account, requires the approval of and/or registration with PRC government authorities, including the SAFE. In particular, if Shenyang Sunshine obtains foreign currency loans from foreign lenders, it must do so within approved limits that satisfy its approval documentation and PRC debt to equity ratio requirements. Further, such loans must be registered with the SAFE. These limitations could affect the ability of Shenyang Sunshine to obtain capital through offshore debt or equity financing.
Exchange rate volatility may adversely affect our competitive position, financial position, operations, or otherwise.
On July 21, 2005, the exchange rate of U.S. dollar to Renminbi ceased to peg the Renminbi to the U.S. dollar. Instead, the Renminbi has been pegged since that date to a basket of currencies, which components are adjusted based on changes in market demand and supply under a set of systematic principles. Since 2005, the Chinese government has widened the daily trading band for Renminbi against non-US dollar currencies from 0.15% to 1% to improve the flexibility of the new foreign exchange system. On June 20, 2010, the People’s Bank of China announced that the PRC government will pursue further reform and enhance the Renminbi exchange rate flexibility. It is difficult to predict how this new policy may impact the Renminbi exchange rate going forward. The Renminbi may be revalued further against the U.S. dollar or other currencies, or may be permitted to enter into a full or limited free float, which may result in an appreciation or depreciation in the value of the Renminbi against the U.S. dollar or other currencies, any of which could give rise to uncertainties in our financial condition and results of operations. The Renminbi appreciated by approximately 31.4% against the U.S. dollar from July 21, 2005 to December 31, 2011.
Any appreciation of the Renminbi may subject us to increased competition from imports; and any devaluation of the Renminbi may adversely affect the value of our net assets, earnings and financial results, and declared dividends in foreign currency terms, as well as our ability to import raw materials and equipment and service our foreign currency obligations. For example, to the extent that we need to convert our U.S. dollars holdings into the Renminbi for our operations, appreciation of the Renminbi against the U.S. dollar would have an adverse effect on the Renminbi amount we receive from the conversion. Conversely, if we decide to convert our Renminbi into U.S. dollars for the purpose of making payments for dividends on our ordinary shares or ADSs, purchasing equipment and raw materials from overseas, or other business purposes, appreciation of the U.S. dollar against the Renminbi would have a negative effect on the U.S. dollar amount available to us.
There are limited hedging tools available to reduce our exposure to exchange rate fluctuations. While we may decide to enter into hedging transactions, the availability and effectiveness of these hedges may be limited and we may not be able to successfully hedge our exposure, if at all. In addition, any currency exchange losses may be magnified by PRC exchange control regulations that restrict our ability to convert RMB into U.S. Dollars.
16
Our operations are subject to the uncertainties and particularities associated with the legal system in China, which could adversely affect our business, or limit the legal protection available to us or to existing or potential investors.
We conduct our business through our operating subsidiaries in China, which are governed by PRC law. China is a civil law jurisdiction based on written codes and statutes. Unlike common law jurisdictions, prior court decisions may be cited as persuasive authority but do not have legally binding force. The PRC government has promulgated laws and regulations in relation to economic matters in general, such as foreign investment, corporate organization and governance, commerce, taxation and trade, with a view to establishing a comprehensive legal system conducive to investment activities. However, the implementation, interpretation and enforcement of these laws and regulations may involve greater uncertainty compared to those in the common law jurisdictions due to a relatively short legislative history, limited volume of court cases and their non-binding nature. Furthermore, many laws, regulations and legal requirements have only recently been adopted by the central or local government agencies, and their implementation, interpretation and enforcement may involve uncertainty due to the lack of established practice available for reference. PRC administrative and court authorities also have significant discretion in interpreting and enforcing statutory and contractual terms. It thus may be more difficult to evaluate the outcome of administrative and court proceedings and the level of legal protection available than in more developed legal systems. These uncertainties may also impede our ability to enforce the contracts we have entered into with our business partners, customers and suppliers. Vis-à-vis our competitors, depending on the government agency or how an application or a case is presented to such agency or other factors, we may receive less favorable application of law. In addition, any litigation or legal proceeding in China may be protracted and result in substantial legal costs and diversion of resources and management attention. We cannot predict the effect of future legal developments in China, including promulgation of new laws, changes to existing laws or the interpretation or enforcement thereof, the preemption of local rules and regulations by national law, the overturn or modification of the lower-level authority’s decisions at the higher level, or the changes in judiciary and administrative practices. As a result, there is substantial uncertainty as to the legal protection available to us or to our investors.
There may be difficulties in effecting services of process and seeking recognition and enforcement of foreign judgments in China.
Substantially all of our assets are located in China, and most of our senior management members and directors reside in China. However, China has not entered into treaties or arrangements providing for the recognition and enforcement of judgments made by the courts of the United States or most other jurisdictions. As a result, it may be difficult or impossible for investors to effect service of process or enforce court judgments against our PRC subsidiaries, our assets, senior management members or directors in China.
Changes in PRC government policy on foreign investment in China may adversely affect our business and results of operations.
We are subject to restrictions on foreign investment imposed by PRC law from time to time. For instance, under the Foreign Investment Industrial Guidance Catalogue, the latest version ("Catalogue") of which became effective on January 30, 2012, some industries are categorized as sectors which are encouraged, restricted or prohibited for foreign investment.
We believe our business as structured is in due compliance with the Catalogue and other currently effective Chinese law and regulations. Kidney care services currently allows foreign ownership of up to 70%; and LSSTD is the principal party in our collaboration with DaVita, with us only exercising control over LSSTD through contractual arrangements with Liaoning Sunshine, LSSTD's parent. As the Catalogue is updated every few years, there can be no assurance that the PRC government will not change its policies in a manner that would cause part or all of our businesses to fall within the restricted or prohibited categories. If any of our businesses becomes prohibited or if we cannot obtain approval from relevant approval authorities to engage in businesses which become restricted for foreign investors, we may be forced to sell or restructure our businesses which have become restricted or prohibited for foreign investment. If we are forced to adjust our corporate structure or business line as a result of changes in government policy on foreign investment, our business, financial condition and results of operations may be materially and adversely affected.
Our auditor, like other independent registered public accounting firms operating in China, is not permitted to be subject to inspection by Public Company Accounting Oversight Board, and as such, investors may be deprived of the benefits of such inspection.
Our independent registered public accounting firm that issues the audit reports included in our annual reports filed with the SEC, Ernst & Young Hua Ming, as an auditor of the companies that are traded publicly in the United States and a firm registered with the Public Company Accounting Oversight Board (United States), or PCAOB, is required by the laws of the United States to undergo regular inspections by PCAOB to assess its compliance with the laws of the United States and professional standards. Because our auditor is located in China, a jurisdiction where PCAOB is currently unable to conduct inspections without the approval of the PRC authorities, our auditor, like other independent registered public accounting firms operating in China, is currently not inspected by PCAOB.
Inspection of other firms that PCAOB has conducted outside of China have identified deficiencies in those firms’ audit procedures and quality control procedures, which may be addressed as part of the inspection process to improve future auditor quality. The inability of PCAOB to conduct inspections of independent registered public accounting firms operating in China makes it more difficult to evaluate the effectiveness of our auditor’s audit procedures or quality control procedure. As a result, investors may be deprived of the benefits of PCAOB inspections.
17
3.D.4 Risks Related To Our ADSs and Other Risks
The market price for our ADSs may be volatile.
The market price for our ADSs may be highly volatile and subject to wide fluctuations in response to various factors, as discussed under this Item 3.D and elsewhere in this annual report on Form 20-F, including, but not limited to, the following:
-
actual or anticipated fluctuations in our quarterly operating results and changes or revisions of our expected results;
-
changes in financial estimates by securities research analysts;
-
announcements of studies and reports relating to the effectiveness or safety of our products or those of our competitors;
-
announcements of technological or competitive developments;
-
any litigation, governmental investigation or enforcement proceedings brought against us by authorities and industry regulators in China or elsewhere;
-
announcements regarding patent litigation or the issuance of patents to us or our competitors;
-
addition or departure of our senior management and key research and development personnel;
-
changes in the economic performance or market valuations of other pharmaceutical or health care companies;
-
economic, regulatory or political developments in China; and
-
sales of additional ordinary shares or ADSs, or increased supplies of ordinary shares or ADS, or the perception that such sales or supplies might occur.
In addition, the securities market has from time to time experienced significant price and volume fluctuations that are not related to the operating performance of particular companies. These market fluctuations may also have a material adverse effect on the market price of our ADSs.
There have been a recent surge of lawsuit filings, regulatory actions, and hostile market manipulations against US-listed Chinese companies, based on allegations of accounting fraud, inadequate disclosure and improper transactions between related parties, and other issues. This development may adversely affect the market price of our ADSs and may affect us in other ways.
Dr. Jing Lou, our chairman and chief executive officer, and his father, Mr. Dan Lou, control a number of shares sufficient to influence corporate actions.
Dr. Jing Lou, our chairman and chief executive officer, and his father, Mr. Dan Lou, together owned or controlled approximately 9.71% of our outstanding ordinary shares as of December 31, 2011. The interests of the Lou family may differ from those of our other shareholders, and they may take actions that advance their interests to the detriment of our other shareholders. Acting together, they would have voting power to influence the outcome of corporate actions submitted to the shareholders for approval and could otherwise influence our management and affairs, including the election of our board of directors. Chairman Lou is not required to stand for election at any meeting of our shareholders, and therefore serves for an undetermined period of time. In addition, this concentration of ownership may prevent attempts to remove or replace senior management.
Our articles of association contain anti-takeover provisions that could adversely affect the rights of holders of our ordinary shares and ADSs.
Our amended and restated articles of association include provisions that could limit the ability of others to acquire control of our company or cause us to engage in change-of-control transactions. These provisions could have the effect of depriving our shareholders of an opportunity to sell their shares at a premium over prevailing market prices by discouraging third parties from seeking to obtain control of our company in a tender offer or similar transaction. These provisions could also serve to entrench our existing board of directors and management. For example, our board of directors has the authority, without further action by our shareholders, to issue preferred shares in one or more series and to fix their designations, powers, preferences, privileges, and relative participating, optional or special rights and the qualifications, limitations or restrictions, including dividend rights, conversion rights, voting rights, terms of redemption and liquidation preferences, any or all of which may be greater than the rights associated with our ordinary shares and ADS. Preferred shares could be issued quickly with terms calculated to delay or prevent a change in control of our company or make removal of management more difficult. If our board of directors decides to issue preferred shares, the voting and other rights of the holders of our ordinary shares and ADSs may be adversely affected. In addition, our articles of association provide that only a third of our board members must stand for re-election at any annual meeting.
18
Holders of ADSs have fewer or less rights than shareholders and must act through the depositary to exercise those rights.
Holders of ADSs do not have the same rights as holders of our ordinary shares and ADS holders only have such rights as are specified in the deposit agreement, which generally are more restricted than the rights of holders of ordinary shares.
Under the deposit agreement, if the vote is by show of hands, the depositary will vote the deposited securities in accordance with the voting instructions received from a majority of holders of ADSs that provided timely voting instructions. If the vote is by poll, the depositary will vote the deposited securities in accordance with the voting instructions it timely receives from ADS holders. In the event of poll voting, deposited securities for which no instructions are received will not be voted.
Under our articles of association, the minimum notice period required to convene a general meeting is seven days. When a general meeting is convened, you may not receive sufficient notice of a shareholders’ meeting to permit you to withdraw your ordinary shares to allow you to cast your vote with respect to any specific matter.
In addition, the depositary and its agents may not be able to send voting instructions to you or carry out your voting instructions in a timely manner.
We will make all reasonable efforts to cause the depositary to extend voting rights to you in a timely manner, but we cannot assure you that you will receive the voting materials in time to ensure that you can instruct the depositary to vote your shares. Furthermore, the depositary and its agents will not be responsible for any failure to carry out any instructions to vote, for the manner in which any vote is cast or for the effect of any such vote.
As a result, you may not be able to exercise your right to vote and you may lack recourse if your ordinary shares are not voted as you requested.
In addition, in your capacity as an ADS holder, you will not be able to call a shareholder meeting.
You may be subject to limitations on transfers of your ADSs; your right to participate in any future rights offerings may be limited; and you may not receive cash dividends or other distributions in certain cases.
Your ADSs are transferable on the books of the depositary. However, the depositary may close its transfer books at any time or from time to time when it deems expedient in connection with the performance of its duties. In addition, the depositary may refuse to deliver, transfer or register transfers of ADSs generally when our books or the books of the depositary are closed, or at any time if we or the depositary deem it advisable to do so because of any requirement of law or of any government or governmental body, or under any provision of the deposit agreement, or for any other reason.
Your right to participate in any future rights offerings may be limited, if we are not able to register in the United States the rights and the securities to which the rights relate under the Securities Act of 1933, as amended, or the Securities Act, or to establish an exemption from the registration requirements, which may cause dilution to your holdings.
In addition, the depositary may, at its discretion, decide that it is inequitable or impractical to make a distribution of cash dividends or other distributions available to any holders of ADSs. For example, the depositary may determine that it is not practicable to distribute certain property through the mail, or that the value of certain distributions may be less than the cost of mailing them. In these cases, the depositary may decide not to distribute such property, in which event you would not receive such distribution.
Since we are a Cayman Islands company, you may have less protection of your shareholder rights than you would as an investor in a U.S. company.
Our corporate affairs are governed by our amended and restated memorandum and articles of association, the Cayman Islands Companies Law and the common law of the Cayman Islands. The rights of shareholders to take action against the directors, the rights of minority shareholders to institute actions, and the fiduciary responsibilities of our directors to us under Cayman Islands law are to a large extent governed by the common law of the Cayman Islands. The common law of the Cayman Islands is derived in part from comparatively limited judicial precedent in the Cayman Islands as well as from English common law, the latter of which has persuasive, but not binding, authority on a court in the Cayman Islands. The rights of our shareholders and the fiduciary responsibilities of our directors under Cayman Islands law are not as clearly established as they would be under statutes or judicial precedent in some jurisdictions in the United States. In particular, the Cayman Islands has a less developed body of securities laws than the United States; and, some states in the United States, such as Delaware, have more developed and judicially interpreted bodies of corporate law than the Cayman Islands.
There is uncertainty regarding whether Cayman Islands courts would:
-
recognize or enforce against us or our directors or officers judgments of courts of the United States predicated upon certain civil liability provisions of United States securities laws; or
-
impose liability against us, in original actions brought in the Cayman Islands, based on certain civil liability provisions of United States securities laws or laws of any state in the United States
19
Cayman Islands companies may not have standing to initiate a shareholder derivative action before the federal courts of the United States.
As a result of all of the above, our public shareholders may have more difficulty in protecting their interests in the face of actions taken by our management, directors or any controlling shareholder than they would as public shareholders of a United States company.
In addition, as a foreign private issuer, we are not required to follow all the NASDAQ’s corporate governance requirements and certain other NASDAQ rules. As a result, holders of our ADSs may not have the same protection afforded to the shareholders of the companies that are subject to additional NASDAQ corporate governance requirements and rules. Please refer to “Item 16G Corporate Governance” for more information.
Your ability to bring an action against us or against our directors and officers, or to enforce a judgment against us or them, will be limited, because, we are incorporated in the Cayman Islands, we conduct substantially all of our operations in China and the majority of our directors and officers reside outside of the United States.
We are incorporated in the Cayman Islands, and we conduct substantially all of our operations in China through our PRC subsidiaries and affiliated entities. Most of our directors and officers reside, and substantially all of the assets of those persons may be located, outside the United States. As a result, it may be difficult or impossible for you to bring an action in the United States against us or against these individuals in the event that you believe that your rights have been violated under United States securities laws or otherwise, including, that, it may be difficult for you to effect service of process within the United States upon these persons. Even if you are successful in bringing an action of this kind, the laws of the Cayman Islands or China may render you unable to enforce a judgment against our assets or the assets of our directors and officers.
20
ITEM 4. INFORMATION ON THE COMPANY
4.A. History and Development of the Company
4.A.1 Corporate Information
3SBio Inc. is an exempted company limited by shares in the Cayman Islands, incorporated in August 2006, under the Cayman Islands Companies Law (2011 Revision).
Our principal executive offices are located at No. 3 A1, Road 10, Shenyang Economy & Technology Development Zone, Shenyang 110027. Our telephone number at this address is (86-24) 2538-6000.
We commenced business operations in 1993 through Shenyang Sunshine Pharmaceutical Company Limited, or Shenyang Sunshine, a limited liability company established in China, which is now our main PRC operating subsidiary. In anticipation of our initial public offering in February 2007, we established a holding company structure through the following series of corporate reorganization transactions:
-
We formed Collected Mind Limited, or Collected Mind, a British Virgin Islands company, in July 2006;
-
Collected Mind acquired 100% of the equity interests of Shenyang Sunshine, which was reorganized as a wholly foreign owned enterprise, or WFOE, in July 2006; and
-
We incorporated 3SBio Inc., a Cayman Islands company, in August 2006, which acquired 100% equity interest in Collected Mind in September 2006.
In addition, please refer to “4.C Organization Structure” for information concerning our affiliated entity, Liaoning Sunshine, whose equity interests we divested as part of the corporate reorganization in November 2006. Please also see Note 1 of the Financial Statements for additional information on our corporate organization.
4.A.2 Principal Capital Expenditures and Divestures
In each of the fiscal years 2009, 2010 and 2011, our capital expenditures, or cash outflow to purchase property, plant and equipment and intangible assets, to acquire equity interests in non-consolidated affiliates, and other investment payments were RMB95.8 million, RMB85.2 million and RMB56.9 million (US$9.0 million), respectively. Historically, capital expenditures were incurred for constructing manufacturing facilities, purchases of production and office equipment, and research laboratory and facilities renovations. In May 2011, we acquired a patent for consideration of RMB8.1 million (US$1.3 million). In December 2011, we paid RMB9.8 million (US$1.6 million) to acquire a land use right with a useful life of 50 years. Barring any new developments, at this time our capital expenditures in 2012 are expected to be approximately USD5 million to USD10 million, a major portion of which is expected to relate to payments for procurement of manufacturing equipment for our recently completed plant and construction of facilities for dialysis related business. We plan to finance these expenditures with a combination of cash generated from our operating activities and existing funds.
4.B Business Overview
Overview
We are a leading biotechnology company in China with fully integrated research and development, manufacturing, and marketing capabilities focusing on bio-pharmaceutical products. Our recombinant, or genetically engineered, protein-based products and product candidates are designed to address large markets with significant unmet medical needs in nephrology, oncology, supportive cancer care, inflammation and infectious diseases. We began operations in 1993.
Our principal products are EPIAO and TPIAO. In addition, we have two legacy products, Intefen and Inleusin; and an in-licensed product, Iron Sucrose Supplement. Substantially all of our revenues and profits are derived from these five products.
For the years ended December 31, 2009, 2010 and 2011, EPIAO generated approximately 61.9%, 59.9% and 58.7% of our total revenue, respectively. According to data derived from IMS Health and internally, EPIAO continued to be the market leader in China both in terms of revenues and sales volume, with market shares of 42.7% and 27.4%, respectively, at the year end of 2011. In 2007, we received from the SFDA licenses to produce and sell pre-filled syringe EPIAO in 2,000 IU, 3,000 IU, 4,000 IU, and 10,000 IU strengths. We launched these EPIAO formats in June 2007. The pre-filled syringe EPIAO has been an important addition to our product portfolio because of its increased safety, ease of use and the flexibility to self-administer the medication at home. In 2011, we received from SFDA the license to produce and sell EPIAO in 36,000 IU strength, the only dosage form of this kind available in China. The 36,000 IU dosage is comparable to the standardized dose used globally for chemotherapy-induced anemia, allowing for less frequent administration than lower dosage forms, expected to provide greater convenience for both patients and caregivers.
21
Our proprietary product, TPIAO, was launched in January 2006 and has continued to gain market acceptance, ending in each of the fiscal years 2009, 2010 and 2011 as our second largest revenue contributor, accounting for 28.3% and 30.7%, and 30.4% of our revenues for 2009, 2010 and 2011, respectively. We will continue to promote TPIAO products to diversify our revenue sources. A new indication for our TPIAO to treat immune thrombocytopenia (ITP) was approved for manufacturing by SFDA in 2011, which may further enhance TPIAO's revenue contribution.
For the three years ended December 31, 2009, 2010 and 2011, approximately 2.2%, 1.8% and 1.5%, respectively, of total revenue were generated by sales of our legacy products Inleusin and Intefen (excluding export sales of Inleusin and Intefen). Beginning in 2004, we began to reduce focus on these products due to increased competition and pricing pressure.
We in-licensed a prescription iron sucrose supplement product, which we began to market in August 2006. Liaoning Sunshine holds the license to Iron Sucrose Supplement. For the three years ended December 31, 2009, 2010 and 2011, approximately 3.4%, 4.1% and 4.6%, respectively, of our total revenue were generated by sales of Iron Sucrose Supplement.
We are actively pursuing eight product candidates in late-, mid- and early-stage clinical and pre-clinical development. These product candidates include: (1) Feraheme® (ferumoxytol), an in-licensed intravenous iron replacement therapeutic agent used to treat iron deficiency anemia in chronic kidney disease (CKD) patients and in patients requiring hemodialysis; (2) Voclosporin, an in-licensed next generation calcineurin inhibitor being developed for use in the prevention of organ rejection following transplantation and the treatment of autoimmune diseases; (3) Pegsiticase (Uricase-PEG 20), a modified pegylated recombinant uricase derived from Candida utilis, being developed for the treatment of refractory gout and tumor lysis syndrome; (4) NuPIAO, our second-generation EPIAO; (5) a HPV vaccine for the prevention of cervical cancer; and, (6) an anti-TNF monoclonal antibody product candidate for treating rheumatoid arthritis, psoriasis, and potentially other inflammatory diseases. In addition, we have a targeted cancer therapeutic development program through collaboration, focusing on programmed cell death, or apoptosis, which at this time includes (1) a pan-Bcl-2 inhibitor for treating non-small cell lung cancer (NSCLC), the most common form of lung cancer; and (2) a multi-IAP inhibitor (including XIAP, cIAP1, cIAP2, ML-IAP) with a broad range of potential indications encompassing solid tumors and leukemia.
We sell our products primarily in China. We also export a small portion of our products to certain developing countries, where we have been approved to sell these products in compliance with local laws and regulations. While exports still constituted a small percentage of our total sales, at 4.4% in 2011, we plan to expand exports because of growing opportunities, particularly, in the global market for biosimilar products, which are new versions of biopharmaceutical products whose patents have expired.
4.B.1 Products
4.B.1.1 Principal Marketed Products
EPIAO
Launched in 1998, EPIAO is an injectable recombinant human erythropoietin, or EPO, that is used to stimulate the production of red blood cells in patients with anemia and to reduce the need for blood transfusions. Anemia is a condition in which insufficient oxygen is delivered to the body’s organs and tissues. EPIAO is a protein-based therapeutic comparable in structure and function to Amgen Inc.’s Epogen and Kirin’s ESPO.
According to IMS Health, an independent research firm, revenues from all EPO drug sales in China were estimated at approximately RMB897.1 million (US$142.5 million) in 2011, representing an approximately 19.7% compound annual growth rate, or CAGR, from 2003. EPIAO, as tracked by IMS Health, has been ranked as the number one EPO drug since 2002 in terms of both units sold and revenues among the foreign and domestic biopharmaceutical companies marketing EPO drugs in China, with market shares of 27.4% and 42.7%, respectively, at the year end of 2011. We have sold over 22.0 million vials of EPIAO since 1999.
EPIAO is approved by the SFDA for three distinct indications: anemia associated with chronic renal failure; red blood cell mobilization, which is the process in which red blood cells are stimulated to proliferate, before, during, and after surgery; and anemia associated with chemotherapy in cancer patients with non-myeloid malignancies, which are cancers that do not originate in the bone marrow or involve myeloid cells, or non-lymphocyte white blood cells found in the bone marrow. With respect to the last indication, EPIAO continues to be the only product of its kind in China.
In 2007, we received from the SFDA licenses to produce and sell pre-filled syringe EPIAO in 2,000 IU, 3,000 IU, 4,000 IU and 10,000 IU strengths. The pre-filled syringe EPIAO is an important addition to our product portfolio because of its increased safety, ease of use and the flexibility to self-administer the medication at home. We launched our pre-filled syringe EPIAO format in June 2007. In As announced in February 2010, we submitted the application to SFDA for a Phase I clinical trial for NuPIAO, our second-generation EPIAO product candidate. NuPIAO is designed to have a longer half-life relative to our first-generation EPIAO.
22
In 2011, we received from SFDA the license to produce and sell EPIAO in 36,000 IU strength, the only dosage form of this kind available in China. The 36,000 IU dosage is comparable to the standardized dose used globally for chemotherapy-induced anemia, allowing for less frequent administration than lower dosage forms, which in turn is expected to provide greater convenience for both patients and caregivers.
TPIAO
We launched TPIAO, our internally developed protein-based therapeutic product, in January 2006. This product is a recombinant human thrombopoietin, or TPO, for the treatment of chemotherapy-induced thrombocytopenia, a deficiency of platelets. Platelets are disc-shaped cells in the blood that assist in coagulation and the arrest of bleeding by repairing the walls of blood vessels. TPIAO represents the first protein-based therapeutic of its type approved for thrombocytopenia in China. In 2011, our total revenues from TPIAO sales were RMB164.8 million (US$26.2 million), accounting for 30.4% of our overall revenues for the year. With respect to TPIAO for the treatment of immune thrombocytopenia, or ITP, an immune system disorder in which the body perceives platelets as foreign and destroys them, we have received the manufacturing approval for this indication from SFDA, as announced in January 2011.
4.B.1.2 Legacy Products
In addition to EPIAO and TPIAO, we market two other protein-based therapeutics that had historically been significant contributors to our overall revenues. Due to unfavorable pricing and increased competition, we refocused our sales and marketing efforts in early 2004, and our legacy products are now marketed primarily by distributors.
Intefen. Intefen is our recombinant interferon alpha-2a product. Intefen is indicated for the treatment of carcinomas of the lymphatic or hematopoietic system, such as lymphoma and leukemia, and viral infectious diseases, such as hepatitis C. We launched Intefen in the Chinese market in 1995.
Inleusin. Inleusin is our recombinant human interleukin-2, or IL-2, product. Inleusin is indicated for the treatment of renal cell carcinoma, the most common form of kidney cancer, metastatic melanoma, a type of skin cancer, and thoratic fluid build-up caused by cancer and tuberculosis. Inleusin is designed to stimulate the immune system in order to fight cancer and infectious diseases. We launched Inleusin in the Chinese market in 1996.
4.B.1.3 In-Licensed Products
Iron Sucrose Supplement. Iron Sucrose Supplement, an intravenously administered prescription drug that is designed to treat anemia associated with iron deficiency, is indicated for patients with end-stage renal disease requiring iron replacement therapy. This product was launched in China in 2005 by Shenyang Borui Pharmaceutical Company Limited, or Borui. We in-license this product from Borui.
4.B.1.4 Three-Year Net Revenues Breakdown by Product
The following table sets forth our net revenues by product and as a percentage of our total net revenues for the years indicated.
| For the years ended December 31, | |||||||||||||||||||||||||||
| 2009 | 2010 | 2011 | 2011 vs 2010 | ||||||||||||||||||||||||
| RMB'000 | % | RMB'000 | % | RMB'000 | US$'000 | % | RMB'000 | % | |||||||||||||||||||
| Domestics sales | |||||||||||||||||||||||||||
| EPIAO | 196,080 | 61.9% | 250,854 | 59.9% | 317,889 | 50,507 | 58.7% | 67,035 | 26.7% | ||||||||||||||||||
| TPIAO | 89,679 | 28.3% | 128,717 | 30.7% | 164,839 | 26,190 | 30.4% | 36,122 | 28.1% | ||||||||||||||||||
| Intefen | 5,522 | 1.7% | 5,358 | 1.3% | 5,229 | 831 | 1.0% | (129 | ) | -2.4% | |||||||||||||||||
| Inleusin | 1,607 | 0.5% | 2,041 | 0.5% | 2,788 | 443 | 0.5% | 747 | 36.6% | ||||||||||||||||||
| Iron Sucrose Supplement | 10,715 | 3.4% | 17,187 | 4.1% | 24,859 | 3,950 | 4.6% | 7,672 | 44.6% | ||||||||||||||||||
| Export sales | 13,216 | 4.2% | 12,211 | 2.9% | 23,890 | 3,796 | 4.4% | 11,679 | 95.6% | ||||||||||||||||||
| Other | 101 | 0.0% | 2,260 | 0.6% | 2,120 | 337 | 0.4% | (140 | ) | -6.2% | |||||||||||||||||
| Total | 316,920 | 100.0% | 418,628 | 100.0% | 541,614 | 86,054 | 100% | 122,986 | 29.4% |
23
4.B.1.5 Product Pipeline
We focus our research and development efforts on both novel and validated protein-based therapeutics for the treatment of diseases in the areas of nephrology, oncology, supportive cancer care, inflammation and infectious diseases, and other selected areas. Our product pipeline, which we expect will be a key contributor to our future growth, consists of eight product candidates in various stages of development. We employ a market-driven approach to our research and development efforts, and our team utilizes the latest molecular biology and biochemical techniques and technologies to develop promising product candidates. Our diversified product pipeline includes: (1) Feraheme® (ferumoxytol), an in-licensed intravenous iron replacement therapeutic agent used to treat iron deficiency anemia in chronic kidney disease (CKD) patients and in patients requiring hemodialysis; (2) Voclosporin, an in-licensed next generation calcineurin inhibitor being developed for use in the prevention of organ rejection following transplantation and the treatment of autoimmune diseases; (3) Pegsiticase (Uricase-PEG 20), a modified pegylated recombinant uricase derived from Candida utilis, being developed for the treatment of refractory gout and tumor lysis syndrome; (4) NuPIAO, our second-generation EPIAO; (5) a human papilloma virus, or HPV, vaccine for the prevention of cervical cancer; and, (6) an anti-TNF monoclonal antibody product candidate for treating rheumatoid arthritis, psoriasis, and potentially other inflammatory diseases. In addition, we have a targeted cancer therapeutic development program through collaboration, focusing on programmed cell death, or apoptosis, which at this time includes (1) a pan-Bcl-2 inhibitor for treating non-small cell lung cancer (NSCLC), the most common form of lung cancer; and (2) a multi-IAP inhibitor (including XIAP, cIAP1, cIAP2, ML-IAP) with a broad range of potential indications encompassing solid tumors and leukemia.
We believe that each of these product candidates, if successfully developed or licensed and approved, would address significant market opportunities. We are required to conduct extensive preclinical and clinical trials in order to generate safety and efficacy data to support a filing for approval by the SFDA.
We are currently expanding the indications for our marketed products, developing next generation, enhanced versions of our marketed products, and bringing novel protein-based therapeutics to market.
The table below** summarizes the respective point of development in China for our product candidates as of December 31, 2011:
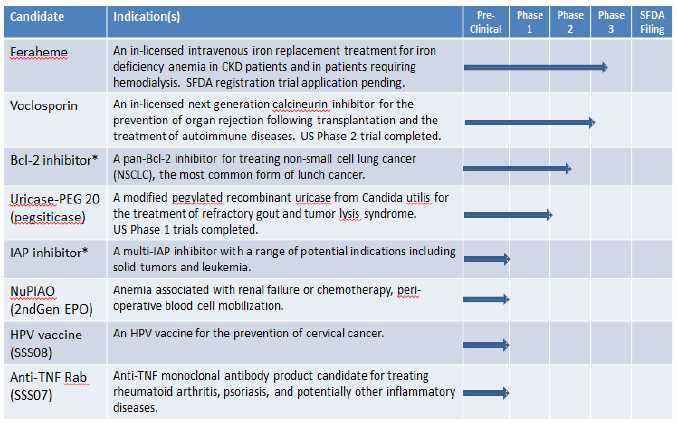
*Developed by Ascentage Shanghai Pharmaceutical Co., Ltd
**The development and commercialization of our product candidates are
subject to numerous risks and uncertainties, as noted in Item 3.D “Risk
Factors”.
24
Phases I, II and III described in the table above are comparable to the similar phases of clinical trials involved in obtaining marketing approval from the U.S. Food and Drug Administration, or US FDA. Under the Administrative Measures on the Registration of Pharmaceutical Products promulgated by the SFDA, the three phases refer to: “Phase I”, evaluation of safety; “Phase II”, evaluation of safety, dosing and efficacy; “Phase III”, larger scale evaluation of safety and efficacy.
4.B.1.6 Expanded Discussion of Products
Our Principal Products
EPIAO
EPIAO, our flagship product, is an injectable recombinant human erythropoietin (EPO) that stimulates the production of red blood cells, cells that transport oxygen from the lungs to all cells of the body. EPO is a naturally occurring growth factor that is normally produced in healthy kidneys and regulates production of red blood cells. Adequate amounts of EPO are required to produce a sufficient number of red blood cells. A significant reduction in the number of circulating red blood cells results in anemia, a condition in which insufficient oxygen is delivered to the body’s organs and tissues. For example, patients with chronic renal failure suffer from anemia because they do not produce sufficient amounts of EPO. Anemia can also be a side effect of chemotherapy treatments for patients, sometimes forcing patients to discontinue chemotherapy.
According to IMS Health, revenues from all EPO drug sales in China were estimated at approximately RMB897.1 million (US$142.5 million) in 2011, representing a compound annual growth rate (CAGR) of approximately 19.7% from 2003 to 2011.
EPIAO is utilized as a replacement protein therapy to restore EPO to normal or physiological levels, stimulate red blood cell production and relieve the symptoms of anemia, thereby improving the quality of life for patients with renal disease and cancer in addition to reducing the need for continuous blood transfusions. Our EPIAO products are available in five different strengths (2,000 IU/vial, 3,000 IU/vial, 4,000 IU/vial, 10,000 IU/vial and 36,000IU/vial) and approved for three distinct medical indications. To date, no other EPO product has been approved in China for all three indications.
-
In 1998, we received approval for the first indication, anemia associated with chronic renal failure;
-
In 2000, we received approval for the use of EPIAO to mobilize red blood cell in patients before, during and after surgery to facilitate a quicker recovery and reduce the need for blood transfusion; and
-
In 2001, we received approval for anemia associated with chemotherapy in cancer patients with non-myeloid malignancies. These patients become anemic as a side effect of chemotherapy and as a general complication associated with cancer.
We were among the first biotechnology companies to introduce a recombinant form of EPO in China. According to IMS Health, our EPIAO was the most widely used recombinant EPO in China in each of the 40 consecutive quarters ending with the first quarter of 2012. EPIAO is on the State Basic Medical Insurance, Work Injury Insurance, and Maternity Insurance Drug Catalogue as a Category B drug, for the treatment of anemia associated with chronic renal failure, and on the provincial medicine catalog for the treatment of anemia associated with chemotherapy in non-myeloid malignancies in certain provinces and cities such as Hubei Province and Shanghai Municipality. (See “4.B.11-b.5 Consumer --- Urban personnel basic medical insurance program.”) According to data from IMS Health, our EPIAO has been the market leader in China since 2002 both in terms of revenues and sales volume, with market shares of 42.7% and 27.4% respectively at the year end of 2011.
In 2007, we received from the SFDA licenses to produce and sell pre-filled syringe EPIAO in 2,000 IU, 3,000 IU, 4,000 IU, 10,000 IU strengths. We launched these EPIAO formats in June 2007. The pre-filled syringe EPIAO has been an important addition to our product portfolio because of its increased safety, ease of use and the flexibility to self-administer the medication at home.
In 2010, we submitted the application for a Phase I clinical trial to SFDA for NuPIAO, our second-generation EPIAO. NuPIAO is a highly glycosylated ESA (erythropoiesis-stimulating agent) with extended half-life and increased biologic activity. We believe that it is comparable to Amgen’s Aranesp. We have already filed a patent application in China for NuPIAO.
In 2011, we received from SFDA the license to produce and sell EPIAO in 36,000 IU strength, the only dosage form of this kind available in China. The 36,000 IU dosage is comparable to the standardized dose used globally for chemotherapy-induced anemia (such treatment option available since 2004 in the United States and 2006 in the United Kingdom), allowing for less frequent administration than lower dosage forms, which in turn is expected to provide greater convenience for both patients and caregivers, for the rapid restoration of hemoglobin to normal levels.
25
Competitive Advantages:
We believe EPIAO’s competitive advantages include:
Focus on quality. We implemented our proprietary process technology and developed quality assurance and control system in compliance with the Chinese GMP standards. Since 2004, we have applied the European Pharmacopoeia (2002 version) standards, which we believe are higher production quality standards than those required for domestic pharmaceutical manufacturers.
Broadest applications and specifications. Since 2002, our EPIAO products have been the only EPO products approved for each of the following three indications: anemia associated with chronic renal failure; red blood cell mobilization; and chemotherapy induced anemia in cancer patients. We provide our EPIAO products in five dosage forms to meet the needs of our patients and their doctors, with the new 36,000 IU strength dosage being the highest dosage formulation of an EPO drug on the Chinese market.
Competitive pricing. Our economy of scale, favorable cost structure and experience in manufacturing afford us the opportunity to competitively price EPIAO while still maintaining attractive margins. In order to secure and maintain a large customer base, we have been pricing our EPIAO products substantially lower than the comparable products produced overseas.
Strong brand awareness. We believe our sustained marketing efforts with medical practitioners and our focus on pre-sale and post-sale services have established EPIAO and Shenyang Sunshine as well-recognized and well-received brands. We have implemented a focused marketing strategy since launching the product in 1998. As part of this strategy, our sales representatives promote our products to doctors by visiting hospitals to better educate physicians and develop brand awareness. We continue to expand our dedicated sales team which focuses specifically on the oncology areas to supplement our existing focus in nephrology.
Clinical Trials:
In 1997, we completed a multicenter, randomized clinical trial with EPIAO in 194 end-stage renal disease patients. This trial compared the efficacy of our product to that of Epogen, Amgen’s recombinant EPO. The data demonstrated that our product was biologically equivalent to Epogen, as demonstrated by comparable restoration of hematocrit and hemoglobin levels, indicators of red blood cell reemergence. Additionally we did not observe any severe adverse reactions. We obtained approval from a branch of the Ministry of Health, or the MOH, in November 1997 and launched EPIAO in China in August 1998.
In 2001, we completed a multicenter, randomized, double blind, placebo-controlled clinical extension trial to evaluate the efficacy of EPIAO in 121 cancer patients with anemia resulting from their chemotherapy regimens. The primary endpoint of this clinical trial was restoration of hemoglobin and hematocrit levels. Patients received either EPIAO or a placebo three times a week for 12 weeks. The results demonstrated that EPIAO was effective in restoring hemoglobin and hematocrit levels in this patient setting. We obtained SFDA approval for this indication in September 2001 and launched EPIAO for this indication in December 2002.
In 2000, we completed a multicenter, randomized placebo controlled extension trial in 105 patients undergoing major orthopedic surgery to evaluate the efficacy of EPIAO in mobilization of red blood cells in patients undergoing surgery. Typically, these patients require allogeneic blood transfusion, where blood comes from a donor instead of the patient himself, thereby exposing the patients to the risk of contracting blood-borne diseases. The primary endpoint of this clinical trial was also restoration of hemoglobin and hematocrit levels. Compared with placebo-controlled patients, patients who received EPIAO before, during and after surgery had higher levels of hemoglobin and hematocrit. We received SFDA approval for this indication in 2000 and launched EPIAO for this indication in December 2002.
A study sponsored by Amgen and published in September 2008 by the Journal of Pharmaceutical Sciences, “Biochemical Assessment Of Erythropoietin Products From Asia Versus US Epoetin Alfa Manufactured By Amgen”, has pointed to EPIAO as the product in Asia most similar to Epogen, the original proprietary EPO developed by Amgen.
TPIAO
TPIAO is our recombinant human thrombopoietin product and we were the first company in the world to research, develop and commercialize a recombinant human thrombopoietin product. TPIAO was launched in January 2006 for the treatment of chemotherapy-induced thrombocytopenia, or platelet deficiency, and represents the first such protein-based therapeutic approved and launched for this indication in China. TPO is a hemopoietic, or blood or blood cell-related, growth factor protein found in plasma. TPO naturally stimulates production of megakaryoctyes, cells with a mutilobed nucleus in the bone marrow and liver, thereby releasing mature platelets and raising the circulating platelet count without increasing the platelet function. Patients undergoing blood stem cell transplantation or cancer chemotherapy, with late-stage liver diseases, or for unknown pathological reasons may suffer from platelet deficiency. We market TPIAO in two strengths: 7,500 IU/vial and 15,000 IU/vial. We began marketing our TPIAO products at the end of 2005 on a trial basis and recorded revenues of RMB89.7 million, RMB128.7 million and RMB164.8 million (US$26.2 million) for the years ended December 31, 2009, 2010 and 2011, respectively. TPIAO is on the State Basic Medical Insurance, Work Injury Insurance, and Maternity Insurance Drug Catalogue as a Category B drug for work injury insurance, and is recently added in the provincial catalogue of Shanghai, Heilongjiang, Shan'xi, and Hainan to treat chemotherapy-induced thrombocytopenia.
26
We began our research and development efforts on TPIAO in 1995 as part of the national “863” scientific program. After successful discovery and pre-clinical studies, we initiated clinical trials in 1999. Phase I and Phase II clinical trials were completed in 2001 and 2002, respectively, which demonstrated TPIAO’s safety and efficacy.
In 2003, we completed a multicenter, randomized, double-blind placebo self-controlled Phase III trial in 305 patients, 223 of whom were cancer patients receiving chemotherapy and 82 of whom were ITP patients who had failed to respond to any other form of treatment. Patients were treated with TPIAO within six to 24 hours after the first chemotherapy cycle daily for 14 days. The primary endpoints were platelet counts and reduction in the number of transfusions. In this trial, TPIAO treatment resulted in a higher platelet count in approximately 78% of the cancer patients and approximately 85% of the ITP patients. There were 18 cases of mild adverse reactions relating to TPIAO treatment, including transient flu-like symptoms, but patients spontaneously recovered without discontinuing treatment.
Although we obtained SFDA approval for TPIAO in May 2005, the SFDA has required us to conduct a Phase IV post-marketing trial on TPIAO as a supportive cancer care product. The three-arm trial included approximately 1,000 cancer patients being treated with chemotherapy. The primary endpoint was the time to recovery of platelet count to normal levels. We initiated this trial in the fourth quarter of 2006. We completed this trial with satisfactory results and submitted the results report to the Food and Drug Administration of Liaoning Province, or Liaoning FDA, in April 2010, which fulfilled SFDA’s requirement.
TPIAO was approved in May 2005 for treatment of chemotherapy-induced thrombocytopenia with a monitoring period of five years, which expired in May 2010. During the monitoring period, other pharmaceutical companies generally were prohibited from manufacturing or importing a similar drug. Since the monitoring period has now expired, other manufacturers in China may apply for approval of the SFDA to conduct clinical trials and then to manufacture and market their TPO products. However, we are not aware of any clinical trial application for TPO products submitted to the SFDA, which must be completed prior to the application for manufacturing the product, and the SFDA review process usually spans multiple years. In addition, we expect that our technology know-how in respect to TPIAO provide us with significant competitive advantage. TPIAO is a unique molecule, the molecular structure of which is heavily glycosylated and presents a manufacturing barrier. We also believe that any new entrants into the TPO market could contribute to the overall market growth for this product.
We have two issued patents in relation to TPIAO.
TPIAO for the treatment of ITP
ITP is characterized by thrombocytopenia that results in bruising and bleeding that can be severe. In certain ITP patients, the immune system malfunctions, perceiving the body’s platelets as foreign and destroying them, potentially resulting in dangerously low platelet counts. Platelets are disc-shaped cells in blood that assist in the clotting process to stop bleeding. TPIAO is being explored as a new approach to treat ITP by stimulating the TPO receptor, directly increasing platelet production to outpace platelet destruction by the immune system.
In the Phase III trial that we completed, which was a multicenter, randomized, placebo-controlled study in 120 ITP patients, all patients were administered Danazol, a synthetic steroid hormone drug routinely used to treat ITP. The treatment group was administered an additional 1.0 μg (or microgram)/kg TPIAO once daily for 14 days. The primary endpoint of this trial was the measurement of platelet counts during the 14 day treatment.
We have received SFDA approval for manufacturing as announced in January 2011.
Legacy Products
In addition to EPIAO and TPIAO, we market two protein-based therapeutics, Intefen and Inleusin, which used to be significant contributors to our overall revenue. Due to unfavorable pricing and increased competition, we refocused our sales and marketing efforts since early 2004 and no longer heavily market these products with our own sales force.
Intefen
Intefen is our recombinant human interferon alpha-2a product for the treatment of malignancies of the lymphatic or hematopoietic system, and viral infectious diseases, including adult chronic hepatitis B, acute and chronic hepatitis C, and genital warts. Interferon is a protein that occurs naturally in the body in very small amounts and comes in three main types: alpha, beta and gamma. When exposed to viruses, certain cells produce interferon, which is released into the bloodstream or intercellular fluid to induce healthy cells to produce enzymes and proteins that counter and combat the infection. The anti-cellular or immuno-regulatory functions of IFN can enable interferon products to play a central role against certain tumor and auto-immune diseases. Interferon alpha has been manufactured by pharmaceutical companies for therapeutic use against hairy-cell leukemia, hepatitis C and hepatitis B, as well as for treatment of genital warts and some rare cancers of blood and bone marrow.
27
We obtained SFDA approval to market Intefen in April 1995 and launched the product in December 1995. Our Intefen is available in lyophilized powder and injectable solution. Both the powder and solution come in three strengths: 1 MIU (million international unit)/vial, 3 MIU/vial and 5 MIU/vial. Revenues from our Intefen products were RMB5.2 million (US$0.8 million) in 2011, representing a decrease of 2.4% over 2010.
In 1994, we completed a multicenter, randomized Phase III clinical trial with Intefen in 127 patients with hepatitis C virus, or HCV. The primary endpoints of the trial were the change in Alanine Amino Transferase recovery rates, or ALT, a measurement of liver function and HCV RNA clearance rates, a measure of the number of viruses present in the patient. The data indicated that Intefen was comparable to Schering Plough’s Intron A when measured with these two tests. The observed side effects were flu-like symptoms that were relatively minor and reversed upon discontinuation of the therapy.
Inleusin
Inleusin is our recombinant human interleukin-2, or IL-2, product that is structurally and functionally the same to naturally occurring IL-2, a body immune regulator. Inleusin is indicated for treatment of renal cell carcinoma, melanoma, thoratic fluid build-up caused by cancer, and tuberculosis. IL-2 is a natural part of the body’s immune response to microbial infection. IL-2 promotes the proliferation and maturation of, among others, T-cells and natural killer cells, both of which are capable of destroying cancer cells directly. IL-2 plays a role in the development of white blood cells or lymphocytes, and anti-inflammatory reactions which are part of the regulation of the immune response.
We obtained SFDA approval to market Inleusin in the Chinese market in June 1995 and launched the product in March 1996. It was the first interleukin product introduced into the Chinese market. Our Inleusin products come in four strengths: 0.1 MIU/vial, 0.2 MIU/vial, 0.5 MIU/vial and 1 MIU/vial. Revenues from our Inleusin products were RMB2.8 million (US$0.4 million) in 2011, representing an increase of 36.6% over 2010.
In 2002, we completed a multicenter, randomized, placebo-controlled extension study in 209 patients with tuberculosis. The primary endpoint was the reduction of severity of infection as measured by thoracic X-Ray imaging the reduction in the number of tuberculosis bacilli, an infectious organism, in a sputum smear. The data demonstrated that Inleusin effectively reduced the severity of tuberculosis in these patients.
In-Licensed Products
Iron Sucrose Supplement. Iron Sucrose Supplement is a complex of polynuclear iron (III)-hydroxide, a bioavailable form of iron, in a sucrose solution for intravenous use. It is indicated for the treatment of iron deficiency anemia in patients with end-stage renal disease and generally considered to have a superior safety profile compared to other forms of iron supplements. Iron sucrose supplement is on the State Basic Medical Insurance, Work Injury Insurance, and Maternity Insurance Drug Catalogue as a Category B drug.
This Iron Sucrose Supplement was launched in China in 2005 by Shenyang Borui Pharmaceutical Company Limited (“Borui”). Through Liaoning Sunshine, we in-licensed a five-year exclusive PRC distribution right for this product from Borui in May 2006. We began to generate revenues from the sale of Iron Sucrose Supplement in the beginning of 2007. We believe this product is complementary to our EPIAO product lines as we have a strong brand reputation in the nephrology market in China, and Iron Sucrose Supplement is designed to treat anemia associated with iron deficiency for patients with renal disease.
In 2008, we through Liaoning Sunshine entered into various agreements with Borui and Chengdu Tiantaishan Pharmaceutical Co. Ltd., or Tiantaishan, the manufacturer for this product, to acquire additional rights to the Iron Sucrose Supplement. Pursuant to these agreements, we hold the exclusive national distribution rights to this product until 2013.
Our Product Candidates
Ferumoxytol (Feraheme® )
As announced in May 2008, we entered into a development and commercialization agreement with AMAG Pharmaceuticals, Inc. (“AMAG”) (NASDAQ:AMAG), a US biopharmaceutical company, for ferumoxytol, an intravenous iron replacement therapeutic agent being developed to treat iron deficiency anemia in CKD patients and in patients requiring hemodialysis.
Under the terms of the agreement, AMAG grants us exclusive rights to develop and commercialize ferumoxytol in the PRC, initially for CKD, and with an option to expand into additional indications. We are responsible for the clinical development, registration, and commercialization in the PRC, and we and AMAG have formed a joint steering committee that oversees and guides the development and commercialization of ferumoxytol in PRC. The agreement has an initial duration of 13 years and will be automatically renewed for a set term if minimum sales thresholds are achieved. AMAG retains all manufacturing rights for ferumoxytol and will provide, under a separate agreement, commercial supply to us at a predetermined supply price.
28
Ferumoxytol was approved in June 2009 by the U.S. Food and Drug Administration to treat iron deficiency anemia in CKD patients and launched commercially in the U.S. by AMAG in July 2009. We have submitted the application for a registrational clinical trial for Ferumoxytol to SFDA as of January 2010. Once approved by the SFDA, we will commence a multi-center randomized efficacy and safety study in China with approximately 200 CKD patients, measuring the mean change in hemoglobin from baseline at Day 35 after first dose.
Voclosporin
We entered into a development and commercialization agreement with Isotechnika Pharma Inc. (“Isotechnika”) (TSX: ISA), a Canada-based biopharmaceutical company focused on the discovery and development of immune modulating therapeutics, for voclosporin, a next generation calcineurin inhibitor being developed for use in the prevention of organ rejection following transplantation and the treatment of autoimmune diseases, as announced in August 2010.
Under the terms of the agreement, Isotechnika grants us exclusive rights to all transplant and autoimmune indications of voclosporin in China, including Hong Kong and Taiwan, excluding ophthalmic indications and medical devices which were previously licensed to Lux Biosciences, Inc. and Atrium Medical Corporation, respectively. We are responsible for the clinical development, registration and commercialization of voclosporin in China. Isotechnika will provide, under a separate agreement, commercial supply to us on a cost-plus basis. We have made an upfront non-refundable licensing payment of US$1.5 million to Isotechnika in December 2010. We may also pay milestone payments when certain criteria are met, as well as ongoing royalties based on our sales of voclosporin. In addition, we invested US$4.5 million in Isotechnika through a three-year convertible debenture with interest rate of 7% per annum, which were fully converted into common equity of Isotechnika at a conversion price of Canada $0.155 per share by November 2010. As of December 31, 2011, we held a 17.6% equity interest in Isotechnika and nominated one representative of ours to Isotechnika’s six-member Board of Directors.
In April 2010, voclosporin completed a phase IIb North American trial for the prevention of kidney rejection following transplantation. In the U.S., New Onset Diabetes after Transplant (“NODAT”) is common and has become a major concern in transplantation of all organs, and, in kidney transplantation, it is shown to be associated with inferior long term graft and patient survival while increasing the cost of patient management. Voclosporin appears to have not only demonstrated equal efficacy with improvements in safety, but to be easy to use for clinicians. We are ready to participate in the global phase III multi-center trial sponsored by Isotechnika, which, if successful, may considerably benefit regulatory review and approval process in China. It is estimated China has approximately 100,000 transplant recipients, a population which is growing by about 10,000 new patients per year.
On March 29, 2011 we submitted an application to the SFDA to conduct the multi-center phase III trial of voclosporin in China.
Apoptosis Collaboration Program
In March 2010, we entered into a strategic alliance with Ascentage Pharma Group Corporation, Ltd. (“APGC”), a Hong Kong based therapeutic research company, to research, develop and commercialize best-in-class targeted cancer therapeutics focusing on programmed cell death, or apoptosis. Apoptosis-targeted small molecules have the potential to play a key role in the next generation of highly effective targeted cancer drugs.
Under the terms of the agreements between us and APGC, and between us and APGC’s PRC affiliated entity, Ascentage Shanghai Pharmaceutical Co., Ltd (“Ascentage SH”), we paid a total consideration of approximately US$3 million, to fund APGC’s apoptosis related R&D programs (“APGC programs”), and for acquiring 40% equity interests in both APGC and Ascentage SH, and the exclusive right to develop and commercialize in mainland China the cancer therapeutics that are developed through APGC programs.
The alliance leverages APGC's expertise in structure-based small molecule design, leads optimization and preclinical development with our proven drug development and commercialization capabilities in China. This investment allows us to gain access to one of the best external science and small molecule drug discovery platforms in China.
Ascentage SH is a spin-off of Ascenta (Shanghai) R&D Center, a wholly owned subsidiary of Ascenta Therapeutics, Inc. (“Ascenta”). Ascentage SH has an established track record in late stage discovery, preclinical and IND enabling work, working with both China's SFDA and the US FDA. Ascentage SH focuses on research and development in oncology new chemical entity (NCE) drug using cutting edge apoptosis technology, building on molecules and compounds licensed from the University of Michigan. It aims to develop best-in-class targeted therapeutics for the unmet medical needs of both China and global markets.
29
APGC has acquired certain rights to Ascenta’s clinical stage, apoptosis-triggering small molecules, including the exclusive rights to an IAP inhibitor in mainland China, Hong Kong, Taiwan and Macau, as well as exclusive rights to a Bcl-2 inhibitor in all regions worldwide outside USA, Canada and Europe. The Bcl-2 inhibitor is a pan-Bcl-2 inhibitor in Phase II development for treating non-small cell lung cancer (NSCLC), the most common form of lung cancer. The IAP inhibitor, currently in pre-clinical development, is a multi-IAP inhibitor (including XIAP, cIAP1, cIAP2, ML-IAP) with a broad range of potential indications encompassing solid tumors and leukemia. Pursuant to our agreements as outlined above, we have the exclusive right to develop and commercialize in mainland China the Bcl-2 inhibitor and the IAP inhibitor through the APGC programs.
Pegsiticase
We have acquired worldwide rights, exclusive of Taiwan, of pegsiticase for all indications from EnzymeRx LLC (“EnzymeRX”), a U.S. based biotechnology company, for a total consideration of US$6.25 million, as announced in November 2010.
Pegsiticase (Uricase-PEG 20) is a pegylated recombinant uricase derived from Candida utilis, modified by the attachment of multiple 20 kilodalton molecules of polyethylene glycol (PEG), and is being developed for the treatment of refractory gout and tumor lysis syndrome. It has been shown to profoundly lower uric acid when administered as by intravenous infusion and intramuscular injection, and was safe and well-tolerated in a pair of phase I clinical studies sponsored by EnzymeRx. Pegsiticase has received Orphan Drug designation from U.S. FDA for refractory gout, tumor lysis syndrome and Lesch-Nyhan syndrome.
We intend to develop Pegsiticase in China and will seek partnerships for its development outside of China. Gout is a common rheumatic disease in China, with prevalence of gout and hyperuricemia estimated to be 0.22% –0.43% and 12.1% –25.2% respectively, according to Rheumatol Int ((2009) 29:481–490). The number of patients in China suffering from gout and hyperuricemia is expected to continue to grow rapidly due to changes in diet and lifestyle. While currently focusing on gout and hyperuricemia, we may choose to explore and develop the other indications in the future.
NuPIAO
As announced in February 2010, we submitted the application for a Phase I clinical trial to SFDA for NuPIAO, our second-generation EPIAO that is designed to have a longer half life and is expected to be comparable in structure to Amgen’s Aranesp. Aranesp (darbepoietin alpha) is a novel erythropoiesis-stimulating protein with a longer circulating half-life than EPO. By using molecular biology and recombinant DNA techniques, we synthesized a series of novel erythropoiesis-stimulating proteins and identified NuPIAO through an activity screening assay. Preliminary testing of NuPIAO has demonstrated an enhanced half-life comparable to the half-life of darbepoietin alpha.
The effect of extended half-life and increased biologic activity as compared with EPIAO would allow for less frequent administration, which is more convenient for both the patient and the caregiver. We have already filed a patent application in China for NuPIAO.
NuPIAO will be investigated to treat anemia associated with both chronic kidney disease and cancer.
SSS08 (HPV Vaccine)
HPV is a common virus that is often passed on through genital contact, typically during sexual contact. At least 50% of sexually active people will get HPV at some time in their lives. About 40 types of HPV can infect the genital areas of men and women. While most strains of HPV cause no symptoms and resolve without treatment, some strains of HPV can cause cervical cancer in women.
Worldwide, cervical cancer is one of the most common cancers in women, and causes significant numbers of deaths in both developed and developing countries. In 1995, scientists from Merck & Co., Inc. and MedImmune Inc. separately demonstrated that expression of the papilloma virus major capsid gene L1 alone, or together with the minor capsid protein L2, is sufficient for the generation of virus-like particles, or VLPs. VLPs mimic in some aspects the infection with virions and induce virus-neutralizing antibodies, making them an attractive candidate for developing a prophylactic vaccine against HPV infections. In June 2006, Merck received approval from the US FDA for its vaccine targeting four strains of HPV that cause approximately 70% of cervical cancers and approximately 90% of genital warts. Our HPV vaccine candidate also targets VLPs of HPV-16 and HPV-18.
SSS07 (Rheumatoid arthritis and other autoimmune diseases)
SSS07 is our anti-TNF monoclonal antibody product candidate that we are developing in collaboration with Epitomics, Inc., a United States-based biotechnology company that is recognized for its proprietary high-affinity rabbit monoclonal antibody technology. Tumor necrosis factor α (TNF) is one of the key chemical messengers that help regulate the inflammatory process and plays an important role in the underlying mechanisms of conditions such as rheumatoid arthritis, psoriasis, and many other inflammatory disorders. When the body produces too much TNF, it overwhelms the immune system’s ability to control inflammation of the joints or of psoriasis-affected skin areas. The TNF inhibitors are molecules that disrupt the TNF function by blocking the binding of TNF to the TNF receptors. Blockage of these receptors can result in a significant reduction in inflammatory activity and reduce symptoms, inhibit the progression of structural damage, and improve physical function in patients with moderate to severe rheumatoid arthritis. SSS07 is a genetically engineered anti-TNF humanized monoclonal antibody designed to bind and deactivate certain TNF molecules. By binding to the native TNF molecule, SSS07 is designed to prevent activation of the inflammation signalling cascade. Several TNF inhibitors developed by other companies have been approved by the US FDA, including Enbrel (entanercept), Remicade (infliximab) and Humira (adalimumab).
30
In March 2006, we entered into a collaboration agreement with Epitomics Inc., under which we are granted an exclusive, royalty bearing, non-transferable and perpetual license in the field of therapeutic usage in order to develop and conduct clinical trials to obtain SFDA approval for the humanized rabbit anti-TNF alpha monoclonal antibody compounds. Under the agreement, we are also granted the right to manufacture, sell, market and distribute (including distributions by engaging third parties) in China anti-TNF alpha monoclonal antibody therapeutics covered under the intellectual property rights owned by Epitomics. We were required to pay an upfront lump sum fee and a royalty payment equivalent to a certain percentage of the net sales of therapeutic products should they reach market. Under the agreement, we grants Epitomics the right to use all intellectual property generated during the development of the therapeutic product. However, if Epitomics uses this intellectual property for the development of the same therapeutic product outside of China, Epitomics shall pay us a royalty equivalent to a certain percentage of the financial benefit from licenses and product sales. If any new developments or agents are created as a result of the collaboration agreement, we will grant to Epitomics a “right of first refusal” with regard to the commercialization of such agents. The details of a development program to commercialize the therapeutic products and the mechanism for calculating the royalties will be determined between Epitomics and us at a later date.
4.B.2 Competition and Principal Markets
In China, our EPIAO competes primarily with Kirin’s ESPO, Roche’s Recormon and the EPO products offered by domestic companies including Harbin Pharmaceuticals, Beijing Sihuan, Shanghai Kelong, Di’ao Group Chengdu Di’ao Jiuhong Pharmaceutical Factory and others. According to IMS Health data, for the past 40 consecutive quarters, EPIAO has been the China market leader, with, in terms of value, 42.7% of market share for 2011, and our closest competitor's market share at 12.5% . According to information of SFDA, EPIAO is also the only EPO approved in the treatment of chemotherapy related anemia; and according to our market data, we are also the only EPO vendor with 10,000 IU and 36,000 IU strength in China.
Competitors for the Iron Sucrose Supplement in China include Beijing Novartis Pharmaceutical Co., Ltd. and Nanjing Hencer Pharmaceutical Co., Ltd., and others.
While we believe TPIAO, our proprietary, internally developed protein-based therapeutic product, is the only recombinant human thrombopoietin, or TPO-based therapeutic, available in the Chinese market to date, the monitoring period for our TPIAO product expired as of May 2010, and other companies may, subject to the review and approval of the SFDA, conduct clinical trials and manufacture and market their TPO products. We are currently not aware of any clinical trial application for TPO products submitted to the SFDA, which must be completed prior to the application for manufacturing the product, and the SFDA review process usually spans multiple years. In addition, we expect that our technology know-hows in respect to TPIAO provide us with significant competitive advantage. TPIAO is a unique molecule, the molecular structure of which is heavily glycosylated and presents a manufacturing barrier. We also believe that any new entrants into the TPO market could contribute to the overall market growth for this product.
We believe that our leadership position in our industry is based on managerial and technological superiority, and the ability to identify and exploit commercially viable products. Other factors affecting our competitive position include time to market, patent position, product efficacy, safety, convenience, reliability, availability and pricing. We believe we are well positioned to compete in the fast-developing Chinese pharmaceutical market with our strong Shenyang Sunshine brand, diverse product portfolio, proven research and development capabilities, established sales and marketing network and favorable cost structure.
Please also see Item 3.D.1 -- "Our products face substantial competition, and we may not be able to compete effectively against current and future competitors" and -- "Our competitors may have the ability to manufacture pharmaceutical products substantially similar to ours".
4.B.2.1 Export Sales
In addition to our domestic sales, we currently export a part of our products to certain developing countries, where we have been approved to sell these products in compliance with local laws and regulations. We initiated our export business in 2003 to a country in the Middle East through an export company in China. Increasingly in subsequent years, we have worked with local agents in countries in the Middle East, South America and Southeast Asia. Our export growth is driven by our compelling value proposition of world-class biological medicines at competitive prices.
31
Export sales accounted for 4.2%, 2.9% and 4.4% of our total revenues for the years ended December 31, 2009, 2010 and 2011, respectively.
| For the Years ended December 31, | |||||||||
| 2009 | 2010 | 2011 | |||||||
| (in thousands, RMB) | |||||||||
| Revenues by geography | |||||||||
| Egypt | 1,210 | 1,611 | 10,145 | ||||||
| South Korea | 1,524 | 1,542 | 1,242 | ||||||
| Brazil | 2,375 | 568 | 1,153 | ||||||
| Pakistan | 3,108 | 1,699 | 2,084 | ||||||
| Thailand | 3,379 | 3,842 | 5,573 | ||||||
| Bangladesh | 1,004 | 1,403 | 1,192 | ||||||
| Rest of World | 616 | 1,546 | 2,501 | ||||||
| Total revenues | 13,216 | 12,211 | 23,890 | ||||||
EPIAO is registered in 17 countries and applications are in progress in other 8 countries, including Chile, Malaysia, Morocco, Russia, South Korea, Taiwan, Turkey, and Venezuela. TPIAO has been registered in Philippines and Dominica. Applications are in progress in Pakistan and Thailand. Intefen has been registered in 9 countries.
We believe market opportunities exist in Europe and other selected regulated markets for new versions of biopharmaceutical products whose patents have expired (known as biosimilar products), with respect to which we are preparing for the process of seeking requisite international certification and regulatory approvals.
4.B.3 Seasonality of Business
Not applicable.
4.B.4 Supply of Raw Materials
Our top five suppliers and the single largest supplier as a percentage of our total inventories purchased were 40.0% and 11.0%, respectively, for the year ended December 31, 2011. We primarily source our raw materials from a variety of international suppliers through their local distributors. We do not anticipate any significant fluctuations in price or any significant disruptions in the supply of our raw materials in the near future. It is our current belief that our switching cost for our suppliers is not high because alternative suppliers are readily available.
4.B.5 Marketing, Sales and Distribution
Our sales force in China benefits from over ten years of experience in marketing protein-based therapeutics. As a result of our history as a provider of therapeutics to the Chinese market, we believe our Shenyang Sunshine brand is widely recognized throughout the PRC medical community for quality and reliability.
We maintain a sales and marketing force in 31 provinces and major cities in China, including the municipalities of Beijing and Shanghai and the city of Guangzhou. As of December 31, 2011, our principal products EPIAO and TPIAO were marketed by our 461 sales and marketing professionals and sold by our network of approximately 110 distributors to healthcare providers including, based on our internal estimates, approximately 3,535 hospitals, clinics and dialysis centers.
Our internal sales and marketing staff details our products to physicians and hospital administrators, and as required by PRC laws, our distributors are engaged to contract with our customers for the sale of our products to physicians and hospitals. In addition, our legacy products Intefen and Inleusin are marketed and sold by distributors.
Our network of approximately 110 distributors distributes our own and our in-licensed products. We select our distributors based on their reputation, market coverage and sales experience. We conduct credit assessments of each of our distributor customers before we enter into a purchase agreement. We do not have an exclusive distribution arrangement with any of our distributors, and some of our distributors market competing brands. For every calendar year, we enter into a distribution agreement with each distributor which provides general terms for the distribution arrangement, such as the designated sales area, place and method for delivery, targets for annual sales volume and receivable collection. Under our standard distribution agreement, a distributor cannot sell our products outside the designated geographical area without first obtaining our written consent. The term of a distributor contract is typically for one year, reflecting the prevailing pricing arrangement under the local competitive bidding process. The price under the agreement may be adjusted by a number of factors, such as the outcome of the competitive bidding process, or regulatory changes during the calendar year.
32
Hospitals in China typically purchase medical products on credit and sometimes do not make payment until more than a year after the purchase. By contrast, the average credit terms that we give to our distributors range from 30 days to 90 days, with the majority averaging around 83 days from the date of our delivery, regardless of whether payments from the hospital to the distributors are received. We believe by selling our products to distributors, we achieve an improved collection rate on our accounts receivable and reduce bad debt expense.
We have a sales department centered in Beijing. We also have a marketing department located in Shanghai.
We have established relationships with many hospital administrators at prominent hospitals and other leading medical institutions, many of whom we believe are advocates for our products. We believe our relationships with these major hospitals and medical institutions raise our profile, enhance awareness of our products in the medical community, medical equipment and supplies industry and among patients, provide us with valuable clinical data to improve our products and keep us abreast of industry trends and developments, all of which in turn helps us market and sell our products.
In export sales, we market our products through distribution agreements with local agents.
A significant amount of our revenue is generated by product sales to relatively few distributors, whose mix changes from year to year. For in each of the past three years, sales to our top five distributors accounted for approximately 30% to 40% of our revenue. Sales to our top ten distributors accounted for approximately 54%, 54%, and 51% of our total revenues for the years ended December 31, 2009, 2010 and 2011, respectively.
4.B.6 Research and Development
As of the date of December 31, 2011, our research and development team consisted of 30 research personnel and medical professionals, including three PhDs, one MD, and fifteen holders of master’s degrees, many of whom have experience in the healthcare and biotechnology research fields, including experience working in research institutions and hospitals and in proceeding through the SFDA drug approval process.
We conduct research and development activities at our Shenyang facilities. To date, our primary sources of new clinical products have been our internal research and development activities and the licensing of compounds from third parties. We believe by complementing our internal research and development efforts with a disciplined strategy of entering into collaborative relationships, we can build a pipeline of diversified pharmaceuticals to drive sustainable revenue growth. For a detailed description of our product pipeline, see “4.B.1.5 Product Pipeline” and “4.B.1.6 Expanded Discussion of Products --- Our Product Candidates” above.
Our research and development expenses for the years ended December 31, 2009, 2010 and 2011 were RMB19.4 million, RMB39.4 million, and RMB41.8 million (US$6.6 million), respectively.
4.B.7 Intellectual Property
We expect that we may, in the future, rely more on patents to protect our proprietary technology. While we have previously sought patent protection only in China, we have acquired intellectual property outside China and may opportunistically seek patents to protect our innovations in jurisdictions outside China in the future.
In China, patents relating to pharmaceutical inventions are effective for 20 years from the date the patent application is filed.
We currently own seven issued PRC patents relating to:
-
the composition of matter of TPIAO, expiring in 2015;
-
a method of manufacturing TPIAO, expiring in 2020;
-
a method and application thereof relating to our manufacturing processes, expiring in 2021;
-
the composition of matter of pegylated-uricase expiring in 2022;
-
a formulation of EPIAO, expiring in 2023;
-
a patent related to certain protein, expiring in 2015; and
-
a method of manufacturing interleukin analogue, expiring in 2026.
33
We have filed two pending PRC patent applications related to, respectively, the manufacturing of a novel erythropoiesis stimulating protein analogue, and formulation and application of human papillomavirus vaccine. In addition, we have acquired a United State patent related to the composition of matter of pegylated-uricase expiring in 2021, and licensed PRC, Hong Kong and Taiwan patents related to voclosporin expiring in 2022.
We are aware of intellectual property rights held by third parties that relate to products or technologies we are developing. For example, we are aware of certain patents held by some third parties that may relate to our TPIAO product and pegylated uricase. We believe, as to any claim in the respective patents, that we either do not infringe the claim of the patent or that the claim is invalid. While the validity of issued patents, patentability of pending patent applications and applicability of any of them to our programs are uncertain, if asserted against us, any related patent rights could adversely affect our ability to commercialize our products.
We own 11 registered trademarks relating to EPIAO, TPIAO, Intefen and Inleusin.
We also rely on trade secrets, proprietary know-how and continuing technological innovation to develop and maintain a competitive position in our product areas. We generally require our employees, consultants and advisors to enter into confidentiality agreements. These agreements provide that all confidential information developed or made known to the individual during the course of the individual’s relationship with us is to be kept confidential and not disclosed to third parties except under specific circumstances. In the case of our employees, the agreements provide that all of the technology which is conceived by the individual during the course of employment is our exclusive property. The development of our technology and many of our processes are dependent upon the knowledge, experience and skills of key scientific and technical personnel. Further, as a matter of company policy, all scientific and technical employees have entered into agreements that generally require disclosure and assignment to us of ideas, developments, discoveries and inventions made by them. However, these agreements may not effectively prevent disclosure of our confidential information or provide meaningful protection for our confidential information if there is unauthorized use or disclosure.
The research, development and commercialization of a biopharmaceutical often involve alternative development and optimization routes, which are presented at various stages in the development process. The preferred routes cannot be predicted at the outset of a research and development program because they will depend upon subsequent discoveries and test results. There are numerous third-party patents in our field, and it is possible that to pursue the preferred development route of one or more of our products we will need to obtain a license to a patent, which would decrease the ultimate profitability of the applicable product. If we cannot negotiate a license, we might have to pursue a less desirable development route or terminate the program altogether. PRC patent and trademark laws are discussed in greater detail in 4.B.11-c.1 “PRC patent law” and 4.B.11-c.2 “Trademarks”.
We cannot assure you that any of our intellectual properties will be able to provide us with meaningful protection or commercial advantages. Despite any measures we take to protect our intellectual property, no assurance can be made that unauthorized parties will not attempt to copy aspects of our products, manufacturing processes or our proprietary technology or to otherwise obtain and use information that we regard as proprietary. The protection of intellectual property rights and proprietary information in China may not be as effective as in the United States or other countries. For example, implementation and enforcement of PRC intellectual property-related laws have historically been deficient and ineffective and may be hampered by corruption and local protectionism. Policing unauthorized use of proprietary technology is difficult and expensive, and we might need to resort to litigation to enforce or defend patents issued to us or to determine the enforceability, scope and validity of our proprietary rights or those of others. The experience and capabilities of PRC courts in handling intellectual property litigation varies, and outcomes are unpredictable. Further, such litigation may require significant expenditure of cash and management efforts and could harm our business, financial condition and results of operations.
To date, we have not been involved in any significant intellectual property disputes or encountered major difficulties in enforcing our intellectual property rights in China.
See “4.B.11-c Intellectual Property” for general discussions on the intellectual property regulations in the Chinese pharmaceutical industry.
Our license agreement with Panacor Bioscience Limited (“Panacor”), a Taiwanese biopharmaceutical company, grants us a license to the patent rights and know-how of ferric compounds or Nephoxil®, as announced in early 2010; in connection, we agreed to make an upfront equity investment in Panacor, which, however, did not receive the approval from relevant regulatory authorities .
4.B.8 Manufacturing
Our Shenyang-based manufacturing operations consist of bulk manufacturing and formulation, fill and finish activities for the production of EPIAO, TPIAO, Intefen and Inleusin for both clinical and commercial purposes. We also manufacture our product candidates for clinical trials at this facility. All fill, finish and packaging activities in relation to our domestic sales are conducted at our Shenyang facility. A portion of our exported products are packaged in our Shenyang facility, and the rest is shipped overseas in bulk format as concentrated solutions of recombinant human erythropoietin, interferon alpha-2a or interleukin-2 and finished and packaged locally. Our Shenyang facilities are certified in accordance with Chinese GMP, and the GMP certificates are currently valid through 2015. We specialize in manufacturing proteins with mammalian expression systems, although we are capable of manufacturing with bacterial expression systems.
34
We generally produce our products based on quarterly order forecasts and anticipated additional orders that we are reasonably confident will be obtained. Lead times for raw materials and components vary and depend on the specific supplier and the availability and demand for the raw materials. Raw materials and supplies are generally available from various suppliers in quantities adequate to meet our needs. However, we have single-source suppliers for some components and value-added steps, including EPO Elisa Kit by R&D systems Inc, GIBCO cell culture medium by Invitrogen Inc., Pharmacia chromatography purification medium by GE Healthcare, a division of GE, and Disc, a microcarrier for cell cultures, by New Brunswick Scientific Inc.
We have not experienced any disruptions in the supply of these raw materials in the past. Unlike in the United States, we do not need SFDA approval to change suppliers. In the event that any one of these supply arrangements or agreements is terminated or the ability of any one of these suppliers to perform under our agreements were to be materially adversely affected, we believe that we will be able to locate, qualify and enter into an agreement with a new supplier on a timely basis. We maintain long-term relationships with most of our suppliers and place orders from these suppliers from time to time on an as-needed basis.
We expect that our existing manufacturing facilities and outside sources will allow us to meet manufacturing needs for our commercial products and product candidates that are in clinical trials.
As part of our overall strategy to increase our manufacturing capacity, we have completed construction of additional manufacturing facilities in early 2010. Our new facilities will support the sustained growth of EPIAO and TPIAO in China, and serve as a key step towards exploring global biosimilar opportunities.
4.B.8.1 Quality Control and Assurance
We have our own independent quality control system and devote significant attention to quality control for the designing, manufacturing and testing of our products. We have established a quality control system in accordance with SFDA regulations. Our laboratories fully comply with the Chinese GMP guidelines and are staffed with highly educated and skilled technicians to ensure quality of all batches of product release.
We implemented European Pharmacopoeia 2002 version on quality control in 2004. European Pharmacopeia of the Council of Europe is a wide range of active substances and excipients used to prepare pharmaceutical products in Europe. In September 2011, the SFDA approved our voluntary upgrade of manufacturing specifications to align EPIAO product quality with European Pharmacopoeia standards. Thus, we believe we are the only Chinese manufacturer with an approved product quality that meets both Chinese Pharmacopoeia and European Pharmacopoeia standards.
Our quality assurance team is also responsible for ensuring that we are in compliance with all applicable regulations, standards and internal policies. Our senior management team is actively involved in setting quality policies and managing internal and external quality performance.
4.B.9 Facilities
Our state-of-art manufacturing facilities are located in the Shenyang Economy & Technology Development Zone, where, after commissioning the additional facilities in 2010, we own four buildings with an aggregate of approximately 28,692 square meters of office, research and development, and manufacturing space. Our facilities in Shenyang consist of three separate divisions capable of producing bulk products, including bacterial expressed proteins and mammalian expressed proteins, and formulating final products. Our manufacturing facilities are equipped with state-of-art and top-line branded equipment, such as bioreactors, centrifuges, chromatography systems and lyophilizers. We own all of our manufacturing facilities in Shenyang. Please refer to “4.B.8 Manufacturing” for productive capacity, products, and other information. Our manufacturing facilities are compliant with Chinese environmental regulations.
In early 2010, we completed building additional manufacturing facilities in Shenyang. We commenced construction in November 2007, and have expended, including contracted amounts that are not yet paid, approximately RMB163 million as of December 31, 2011 for this construction project, inclusive of related machinery and equipment. The new facilities are estimated to increase our manufacturing capacity, subject to actual operating conditions and parameters, by approximately four times. We have funded such capital expenditures with a combination of cash generated from operating activities and the proceeds from our initial public offering in February 2007.
We have leased office space used for local sales and services in various cities of China.
The following table contains information concerning our significant real properties, all owned by us through Shenyang Sunshine:
35
| Location | General Character, Size and Use of Property |
| Shenyang, China | approximately 28,692 square meters, used for office, manufacturing, and research and development. |
| Shenyang, China | 400 square meters, used for general corporate purposes. |
| Beijing, China | 1,000 square meters, used for sales and marketing and corporate purposes |
We believe that our facilities and equipment are in good working condition.
4.B.10 Employees
We had 550,718 and 810 employees as of December 31, 2009, 2010 and 2011, respectively. The following table sets forth the number of our employees categorized by function as of December 31, 2011:
| As of December 31, 2011 | |||
| Manufacturing and services | 209 | ||
| Research and development | 30 | ||
| General and administration | 110 | ||
| Marketing and sales | 461 | ||
| Total | 810 |
From time to time, we may also employ independent contractors to support our marketing and sales and clinical support and research. We may hire additional employees for marketing and sales, customer service, manufacturing and assembly as we grow our business. We have a labor union in accordance with Chinese law and practice, and we consider our relationship with our employees to be good.
In accordance with applicable regulations in the PRC, we participate in a pension contribution plan, a medical insurance plan, an unemployment insurance plan and a personal injury insurance plan for our employees. We have made adequate provisions in accordance with applicable regulations, amounting to RMB3.9 million, RMB5.2 million and RMB8.2 million (US$1.3million) for the years ended December 31, 2009, 2010 and 2011 , respectively.
Also, in accordance with PRC regulations, we make annual contributions towards a housing fund, a supplemental medical insurance fund and a maternity fund.
4.B.11 Regulations
The pharmaceutical industry is heavily regulated in the PRC. This section summarizes the principal PRC regulations related to our business.
4.B.11-a Regulatory Authorities
In the PRC, the SFDA, is the authority that monitors and supervises the administration of pharmaceutical products, medical appliances and equipment as well as food, health food and cosmetics. The SFDA’s predecessor, the State Drug Administration, or the SDA, was established in 1998 as an organization under the State Council to assume the responsibilities previously handled by the MOH, the State Pharmaceutical Administration Bureau of the PRC and the State Administration of Traditional Chinese Medicine of the PRC. The SFDA was founded in March 2003 to replace the SDA and reported to the State Council until the MOH was reorganized and assumed administrative responsibility for the SFDA in March 2008.
The primary responsibilities of the SFDA include:
-
monitoring and supervising the administration of pharmaceutical products, medical appliances and equipment as well as food, health food and cosmetics in the PRC;
-
formulating administrative rules and policies concerning the supervision and administration of food, health food, cosmetics and the pharmaceutical industry;
-
evaluating, registering and approving of new drugs, generic drugs, imported drugs and traditional Chinese medicine;
-
approving and issuing permits for the manufacture and export/import of pharmaceutical products, medical appliances and equipment and approving the establishment of enterprises to engage in the manufacture and distribution of pharmaceutical products; and
-
examining and evaluating the safety of food, health food and cosmetics and handling significant accidents involving these products.
36
The MOH is a ministerial-level authority under the State Council and is primarily responsible for national public health. Following the establishment of the SFDA in 2003, the MOH was put in charge of the overall administration of the national health system in the PRC excluding the pharmaceutical industry. In March 2008, the MOH was reorganized and assumed administrative responsibility for the SFDA. The MOH performs a variety of tasks in relation to the health industry such as establishing social medical institutes, promulgating national regulations, and producing professional codes of ethics for public medical personnel. The MOH is also responsible for international issues, such as those pertinent to foreign companies and governments, and NGOs.
Other than SFDA and MOH, certain aspects of our operations may also come under the jurisdiction of other government authorities at various levels such as NDRC.
4.B.11-b.1 Drug Administration General
Drug administration laws and regulations
The PRC Drug Administration Law (amendment effective December 1, 2001) applies to entities and individuals engaged in the development, production, trade, application, supervision and administration of pharmaceutical products. It regulates and prescribes a framework for the administration of pharmaceutical manufacturers, pharmaceutical trading companies, medicinal preparations of medical institutions and the research, development, manufacturing, packaging, distribution, pricing and advertisements of pharmaceutical products.
The PRC Drug Administration Implementation Regulations promulgated by the State Council, effective on September 15, 2002, provides detailed implementation regulations for the revised PRC Drug Administration Law.
4.B.11-b.2 Medicine Approval and Manufacturing
Examination and approval of new medicines
The SFDA promulgated the Administrative Measures on the Registration of Pharmaceutical Products on October 1, 2007, superseding prior regulations. Under the current regulations, the approval of new medicines requires the following steps:
-
Upon completion of the pre-clinical research of the new medicine, application for registration of the new medicine shall be submitted to the drug regulatory authorities at the provincial level for review. After completion of their review, such drug regulatory authorities shall submit their opinion and report to the SFDA for review;
-
If all the requirements are complied with, the provincial FDA will issue a notice of acceptance of application and proceed with its assessment on whether or not to grant the approval for conducting the clinical research on the new medicine;
-
After obtaining the SFDA’s approval for conducting the clinical research, the applicant may proceed with the relevant clinical research (which is generally conducted in three phases for a new medicine, with Phase IV post- marketing study applicable in certain instances) at institutions with appropriate qualification:
-
Phase I refers to the preliminary clinical trial for clinical pharmacology and body safety. It is conducted to observe the human body tolerance for new medicine and pharmacokinetics, so as to provide a basis for determining the prescription plan;
-
Phase II refers to the stage of preliminary evaluation of clinical effectiveness. The purpose is to preliminarily evaluate the clinical effectiveness and safety of the medicine used on patients with targeted indication, as well as to provide a basis for determining the Phase III clinical trial research plan and the dosage under the prescription plan; and
-
Phase III is a clinical trial stage to verify the clinical effectiveness. The purpose is to test and determine the clinical effectiveness and safety of the medicine used on patients with targeted indication, to evaluate the benefits and risks thereof, and, eventually, to provide sufficient basis for review of the medicine registration application.
-
Phase IV is the clinical study after the new medicine is approved and enters into the market, targeting to investigate the efficacy and any adverse reactions of the drug under the conditions of mass use, and to evaluate the benefits and risks of its use among common and special patient groups and to improve drug dosage.
37
-
After completion of the relevant clinical research, the applicant shall submit its clinical research report together with the relevant supporting documents to the drug regulatory authorities at the provincial level and shall provide raw materials of the standard products to the PRC National Institutes for Food and Drug Control;
-
The drug regulatory authorities at the provincial level shall then review the relevant documents, conduct site inspections and sample examinations and thereafter submit their opinion, inspection report and other application materials to the SFDA for review;
-
The PRC National Institutes for Food and Drug Control will arrange for the examination of the sample new drug supplied by the relevant medicine examination institutes and will then issue the examination result report to the SFDA; and
-
If all the regulatory requirements are satisfied, the SFDA will grant a new drug certificate and a pharmaceutical approval number (assuming the applicant has a valid pharmaceutical manufacturing permit and satisfies the requisite production conditions for the new medicine).
Permits and licenses for manufacturing and registration of drugs
Production License. To manufacture pharmaceutical products in the PRC, a pharmaceutical manufacturing enterprise must first obtain a pharmaceutical manufacturing permit issued by the relevant pharmaceutical administrative authorities at the provincial level where the enterprise is located.
Each pharmaceutical manufacturing permit issued to a pharmaceutical manufacturing enterprise is effective for a period of five years. Any enterprise holding a pharmaceutical manufacturing permit is subject to review by the relevant regulatory authorities on an annual basis. The enterprise is required to apply for renewal of such permit within six months prior to its expiry and will be subject to reassessment by the issuing authorities in accordance with the then prevailing legal and regulatory requirements for the purposes of such renewal.
Business Licenses. In addition to a pharmaceutical manufacturing permit, the manufacturing enterprise must also obtain a business license from the administrative bureau of industry and commerce at the local level after it has obtained the requisite pharmaceutical manufacturing permit.
Registration of Pharmaceutical Products. All pharmaceutical products that are produced in the PRC must bear a registered number issued by the SFDA, with the exception of Chinese herbs and Chinese herbal medicines in soluble form. The pharmaceutical manufacturing enterprises must obtain the medicine registration number before manufacturing any medicine.
GMP Certificates. The World Health Organization encourages the adoption of GMP standards in pharmaceutical production in order to minimize the risks involved in any pharmaceutical production that cannot be eliminated through testing the final products.
China’s Guidelines on Good Manufacturing Practices for Pharmaceuticals, as amended in 2010, or the Guidelines, took effect on March 1, 2011 and set the basic standards for the manufacture of pharmaceuticals. The Guidelines cover issues such as production facilities, qualification of staff at the management level, production plant and facilities, documentation, material packaging and labeling, inspection, production management, sales and return of products and customers’ complaints. On August 2, 2011, the SFDA issued the Administrative Measures on the Certification and Regulation of Good Manufacturing Practice for Pharmaceuticals, which stipulates the procedures of GMP accreditation, including documents and materials to be submitted to the SFDA or to the relevant drug regulatory authorities at the provincial level and on-site and follow-up examinations to be conducted by the relevant SFDA agencies. The application for GMP certificate renewal must be submitted six months prior to its expiration date.
4.B.11-b.3 Distribution
Distribution of pharmaceutical products
According to the PRC Drug Administration Law and its implementing regulations and the Measures for Supervision and Administration of Distribution of Pharmaceutical Products, a manufacturer of pharmaceutical products in the PRC can only engage in the trading of the pharmaceutical products that the manufacturer has produced itself. In addition, such manufacturer can only sell its products to:
-
wholesalers and retailers holding pharmaceutical trading permits;
-
other holders of pharmaceutical manufacturing permits; or
-
medical practitioners holding medical institution practice permits.
A pharmaceutical manufacturer in the PRC is prohibited from selling its products to end-users, or any individuals or entities other than holders of pharmaceutical trading permits, pharmaceutical manufacturing permits or medical institution practice permits.
38
The granting of a pharmaceutical trading permit to wholesalers shall be subject to approval of the relevant drug regulatory authorities at the provincial level, while the granting of pharmaceutical trading permit to retailers shall be subject to the approval of the relevant drug regulatory authorities above the county level. Unless otherwise expressly approved, no pharmaceutical wholesaler may engage in the retail of pharmaceutical products, and neither may pharmaceutical retailers engage in wholesale distribution.
A pharmaceutical distributor (including wholesalers and retailers) shall satisfy the following requirements:
-
retaining qualified personnel with pharmaceutical expertise as required by the law;
-
operating in business site, facilities, warehousing and sanitary environment compatible to the distributed pharmaceutical products;
-
engaging quality management system and personnel compatible to the distributed pharmaceutical products; and
-
complying with relevant rules and regulations to ensure the quality of the distributed pharmaceutical products.
Operations of pharmaceutical distributors shall be conducted in accordance with the Pharmaceutical Operation Quality Management Rules and shall be granted a certificate under such rules by the SFDA.
Pharmaceutical distributors must keep true and complete records of any pharmaceutical products purchased, distributed or sold with the generic name of such products, specification, approval code, term, manufacturer, purchasing or selling party, price and date of purchase or sale. A pharmaceutical distributor must keep such record until one year after the expiry date of such products and in any case, such record must be kept for no less than three years. Penalties may be imposed for any violation of record-keeping.
Pharmaceutical distributors can only distribute pharmaceutical products obtained from those with a pharmaceutical manufacturing permit and a pharmaceutical trading permit.
Restrictions on foreign ownership in pharmaceutical wholesale and retail businesses
Under Foreign Investment Industrial Guidance Catalogue, as amended, foreign investment in wholesale and retail sales and distribution of pharmaceutical products is not restricted.
The Administration Rules on Foreign Investment in Sales and Distribution and related administrative pronouncements permit foreign companies to establish or invest in wholly-foreign-owned enterprises or joint ventures that engage in wholesale or retail sales of pharmaceutical products in China, subject to approvals from the SFDA and MOFCOM or the respective provincial level delegate agencies and certain review and filing requirements.
Price control
The administration of price control of pharmaceutical products is vested in the national and provincial price administration authorities. Depending on the categories of pharmaceutical products in question, the prices of pharmaceutical products listed in the State Basic Medical Insurance, Work Injury Insurance, and Maternity Insurance Drug Catalogue, as amended, or Catalogue, drugs with patents and other drugs whose production or trading may constitute monopolies are subject to the control of the National Development and Reform Commission of the PRC and the relevant provincial or local price administration authorities. In respect of pharmaceutical products manufactured in the PRC, the national price administration authority from time to time publishes price control lists setting out the names of pharmaceutical products and their respective price ceilings. The provincial price administration authorities also publish price control lists in respect of the pharmaceutical products which are manufactured within their respective areas. The main purpose of the price control policy is to set an upper limit to the prices of pharmaceutical products to prevent excessive increases in the prices of such products. Pursuant to the Measures for Medicine Pricing by the Government, the price ceiling is determined mainly by reference to the quality of the product, whether it is a newly developed product, and the GMP compliance status.
The prices of pharmaceutical products included in the price control lists are subject to adjustment upon approval by the price administration authorities from time to time. Pharmaceutical enterprises in the PRC are required to submit cost related information such as raw material prices regularly to the relevant price administration authorities, so that the authorities could take into account the market conditions when setting the prices. The price administration authorities may approve adjustments to the prices upon request if material changes in production costs or significant changes in demand for these pharmaceutical products are recognized.
Competitive bidding
In each province where we market our products, we participate in a government-sponsored competitive bidding process every year or every few years for procurement by state-owned hospitals of medicines included in the provincial medicine catalogs. A government-appointed committee reviews bids submitted and selects one or more medicines for treatment of a particular medical condition. The selection is based on a number of factors, including bid price, quality of the product and manufacturer’s reputation and service. The bid price of the selected medicine will become the purchase price of that medicine to be paid by all state-owned hospitals in the province or local district in question. This bidding mechanism was first instituted in 2004 and has been adopted across China, with provincial variations.
39
4.B.11-b.4 Key Certificates and Permits
Shenyang Sunshine’s current pharmaceutical manufacturing permit was issued by Liaoning FDA on January 1, 2011 and will expire on December 31, 2015. Shenyang Sunshine’s current GMP certificate in relation to TPIAO was issued by the SFDA on July 12, 2010 and will expire on July 11, 2015. Shenyang Sunshine’s current GMP certificate in relation to EPIAO and our legacy products was issued by the SFDA on February 10, 2010 and will expire on February 9, 2015. Liaoning Sunshine’s current pharmaceutical trading permit was issued by Liaoning FDA on December 30, 2009 and will expire on December 29, 2014. Liaoning Sunshine’s current certificate for good operation management practice, or GSP certificate, in relation to pharmaceutical wholesales was issued by Liaoning FDA on December 8, 2008 and will expire on December 7, 2013.
4.B.11-b.5 Consumer and Government Insurance Reimbursement
Product liability
In addition to the strict new drug approval process, certain PRC laws have been promulgated to protect the rights of consumers and to strengthen the control of medical products in the PRC.
Under current PRC law, manufacturers and vendors of defective products in the PRC may incur liability for loss and injury caused by such products. Pursuant to the General Principles of the Civil Law of the PRC, or the PRC Civil Law, promulgated on April 12, 1986, as amended in 2009, a defective product which causes property damage or physical injury to any person may subject the manufacturer or vendor of such product to civil liability for such damage or injury.
On February 22, 1993, the Product Quality Law of the PRC, or the Product Quality Law, was promulgated to supplement the PRC Civil Law aiming to protect the legitimate rights and interests of the end-users and consumers and to strengthen the supervision and control of the quality of products. The Product Quality Law was revised by the Ninth National People’s Congress on July 8, 2000. Pursuant to the Product Quality Law, manufacturers who produce defective products may be subject to civil or criminal liability and have their business licenses revoked.
On October 31, 1993, the PRC Law on the Protection of the Rights and Interests of Consumers, or the Consumers Protection Law, was promulgated, which provides further protection to the legal rights and interests of consumers in connection with the purchase or use of goods and services. Under this law, in addition to other damages and compensations, any company engaged in fraudulent conduct in connection with services and goods supplied, at the request of the aggrieved consumer, may be liable for an incremental damage equal to the amount paid for such service or goods. At present, all business operations must observe and comply with the Consumers Protection Law when they provide their goods and/or consumer services.
On December 26, 2009, the PRC Tort Liability Law, or the Tort Liability Law, was promulgated, and took effect on July 1, 2010. The Tort Liability Law provides basis for a variety of tort claims resulted from defective products, motor accidents, medical malpractice, environmental pollution, and highly dangerous activities and animals. In particular, according to the Tort Liability Law, where any producer or seller knowingly produces or sells defective products that cause death or serious injury to the health of others, the injured party may claim appropriate punitive damages.
Government Medical Insurance Coverage
China has a complex government-sponsored medical insurance system that is currently undergoing reform. Generally, once those individuals covered by medical insurance have paid for medical services, they may seek available reimbursement according to applicable medical insurance programs in which they participate. For public servants and others covered by the 1989 Administrative Measure on State Provision of Healthcare, the PRC government currently either fully or partially reimburses medical expenses for certain approved treatment services. Urban residents in China that are not covered by the 1989 Administrative Measure on State Provision of Healthcare are generally covered by one of two nationwide public medical insurance schemes, which are the Urban personnel basic medical insurance program launched in 1998 and the Urban Residents Basic Medical Insurance Program launched in 2007. Rural residents in China are generally covered under a New Rural Cooperative Medical Program launched in 2003.
The Urban personnel basic medical insurance program which mainly covers employed urban residents, fully or partially reimburses urban workers for certain approved treatments services. The Urban personnel basic medical insurance program is funded by the mandatory medical insurance contribution by employees and their employers and the contribution rates vary based on the economic development status and individual income level of different regions. All of the employee’s contribution and a small portion of the employer’s contribution are allocated to the individual’s reimbursement account, and the remaining portion of the employer’s contribution is aggregated into a social medical expense pool. Participants of the Urban personnel basic medical insurance program may seek reimbursement from both the individual account and the social medical expense pool up to the stipulated reimbursement caps.
40
Urban residents who are not covered by the Urban personnel basic medical insurance program such as students, children and other non-employees, may voluntarily participate in the Urban Residents Basic Medical Insurance Program on a per family’s basis. The Urban Residents Basic Medical Insurance Program is mainly funded by the monthly contributions of each participating family and a fixed amount of annual government subsidies for each individual participant, all of which can be aggregated into a social medical expense pool. There is no specific requirement or guidance from the central government. Local governments separately determine the respective reimbursement policy. Individual participants of the Urban Residents Basic Medical Insurance Program may seek reimbursement from the social medical expense pool up to the stipulated reimbursement cap.
Rural residents can voluntarily participate in the New Rural Cooperative Medical Program on a per family basis. The New Rural Cooperative Medical Program is mainly funded by the monthly contribution of each participating family and a fixed amount of government subsidies for each individual participant, all of which can be aggregated into a social medical expense pool. The individual participants of the New Rural Cooperative Medical Program may seek reimbursement from the social medical expense pool up to the stipulated reimbursement cap.
In general, the reimbursement levels for covered medical expenses for urban non-employees and rural residents, which vary widely from region to region and treatment to treatment, are generally lower than those for urban employees in the same region. These government insurance programs usually maintain reimbursement lists of covered drugs. As part of the healthcare reform, the Chinese government has expanded the coverage of medical insurance programs, including extending coverage to 95% of the national population, increasing the government subsidies for the Urban Residents Basic Medical Insurance Program and the New Rural Cooperative Medical Program, and increasing the reimbursement caps for the social medical expense pools of the three medical insurance programs.
Urban personnel basic medical insurance program
According to the State Council Decision on the Establishment of the Basic Medical Insurance System of Personnel in Cities and Townships promulgated by the State Council in December 1998, the Ministry of Labor and Social Security assumed the responsibilities for the reform of the medical insurance system. As part of the reform of the state basic medical insurance system for employees in the urban areas, the Ministry of Labor and Social Security, the MOH, the SDFA and various other governmental departments jointly issued the State Basic Medical Insurance, Work Injury Insurance, and Maternity Insurance Drug Catalogue, or the Catalogue, with a view to enhancing the management of the use of drugs under the medical insurance system. The drugs listed in the Catalogue are covered by the Urban Personnel Basic Medical Insurance Program, or Program.
--- Reimbursement under the urban personnel basic medical insurance program
The Ministry of Labor and Social Security, together with other government authorities, determines which medicines are to be included in or removed from the Catalogue for the Program, and under which category a medicine should fall, both of which affect the amounts reimbursable to program participants for their purchases of those medicines. These determinations are based on a number of factors, including whether the medicine is consumed in large quantities and commonly prescribed for clinical use, and whether the medicine is considered to be important in meeting the basic healthcare needs of the general public. A program participant can be reimbursed for the full cost of a Category A medicine and generally 80% to 90% of the cost of a Category B medicine. Although it is designated as a national program, the implementation of the Program is delegated to various provincial governments, each of which has established its own medicine catalogue. A provincial government must include all Category A medicines listed in the Catalogue in its provincial medicine catalogue, but may use its discretion based on its own selection criteria to add other medicines to, or exclude Category B medicines listed in the Catalogue from, its provincial medicine catalogue, so long as the combined numbers of the medicines added and excluded do not exceed 15% of the number of the Category B medicines listed in the Catalogue. In addition, provincial governments may not downgrade a nationally classified Category A medicine to Category B.
According to the national guideline as issued by the State Council, the total amount of reimbursement for the cost of prescription and over-the-counter medicines, in addition to other medical expenses, for an individual program participant in a calendar year is generally capped at four times the local average annual salary level; all the costs below the reimbursement threshold and a percentage above the threshold need to be paid from the individual account, with the other portions paid from the social medical expense pool. The amount in a participant’s account varies, depending upon the amount of contributions made by the participant and his or her employer. Generally, program participants who are from relatively wealthier eastern parts of China or metropolitan areas have greater amounts in their accounts than those from less developed areas.
While inclusion of a medicine in the Catalogue or the provincial medical catalogues may improve the sales volume of the medicine, a selected medicine generally is subject to various price controls, including fixed retail price or retail price ceiling, and periodical government-imposed price adjustments.
National Essential Drugs Registry
41
As part of the healthcare reform, the PRC Ministry of Health has established the National Essential Drugs Registry, or Registry, which includes medicines selected with a view towards meeting the basic requirements of medical care in China. None of our products is included in the Registry. The Administration Measures for National Essential Drugs Registry (Tentative Implementation) was promulgated to accompany the Registry. Eligible participants in the government-sponsored medical insurance programs are entitled to reimbursement for varying percentages of the cost of the medicines that are included in the Registry. All the medicines in the Registry are subject to the government price control.
Prescription regulations
As announced by Ministry of Health, the Prescription Administrative Measures, took effect on May 1, 2007, which stipulates that doctors may only use the generic names of drugs in their prescriptions instead of brand names and that medical institutions may offer patients the same type of drug from no more than two separate pharmaceutical companies. The purpose of this regulation is to combat the practice of doctors receiving kickbacks from pharmaceutical companies for prescribing higher priced, or even unneeded, drugs to patients.
4.B.11-c Intellectual Property
4.B.11-c.1 PRC patent law
The PRC government first allowed patents for the protection of proprietary rights, as set forth in the 1985 China Patent Law (revised on December 27, 2008, effective as of October 1, 2009). Pharmaceutical inventions were not patentable under the China Patent Law until 1994. Patents relating to pharmaceutical inventions are effective for 20 years from the date the patent application is filed.
Patent prosecution
The Chinese patent prosecution system is different from the United States system in a number of ways. The Chinese patent system, like most countries other than the United States, adopts the principle of “first to file.” This means that, where more than one person files a patent application for the same invention, a patent will be granted to the person who first filed the application. The United States uses a principle of first to discover to determine the granting of patents. In addition, the PRC requires absolute novelty in order for an invention to be patentable. Pursuant to this requirement, any prior written or oral publication, demonstration or use before filing the patent application prevents an invention from being patented in the PRC. Conversely, inventors in the United States have a one year grace period after publication of the invention in which they may file a patent. Patents issued in the PRC are not enforceable in Hong Kong, Taiwan or Macau, each of which has independent patent systems. Patents are filed at the State Intellectual Property Office, or SIPO, in Beijing.
Patent enforcement
A patent holder who believes the patent is being infringed may either file a civil legal suit or file an administrative complaint with a provincial or municipal office of SIPO. A PRC court may issue a preliminary injunction upon the patent holder’s or an interested party’s request before instituting any legal proceedings or during the proceedings. Damages for infringement are calculated as: (1) the loss actually suffered by the patent holder arising from the infringement; (2) if such actual loss cannot be ascertained, the benefit gained by the infringing party from the infringement; (3) if such actual loss or benefit cannot be ascertained, a reasonable amount by reference to certain times of the patent’s license fees; or (4) if damages cannot be established by method (1) through (3), court-ordered statutory damages in the range from RMB10,000 to RMB1,000,000. The reasonable costs expended by the patent holder to cause infringement to cease are required to be added to the amount calculated under (1) through (3) above.
Generally, as in other jurisdictions, the patent holder in the PRC has the burden of proving that the patent is being infringed. However, if the holder of a manufacturing process patent alleges infringement of such patent, the alleged infringing party has the burden of proving that there has been no infringement.
Compulsory license
Pursuant to the PRC Patent Law, as amended, the SIPO may, on the basis of the application of any entity or individual that is qualified to exploit an invention patent or utility model patent, grant a compulsory license to any such entity or individual if: (1) where, within three years of the date on which the patent right is granted and within four years of the date of patent application, the patent owner has not exploited the patent, or not done so adequately, without any reasonable justification; or (2) where, the patent owner's act of exploitation of the patent is held in accordance with law to be monopolistic and it is necessary to grant the compulsory license to remove or reduce any anti-competitive and adverse effect.
Additionally, under the PRC Patent Law as amended, for public health purposes, the SIPO may grant a compulsory license for manufacturing patented medicines and exporting them to countries or regions which comply with the provisions of relevant international treaties acceded to by the PRC.
A compulsory license can also be granted where a national emergency or any extraordinary state of affairs occurs or where the public interest so requires. We believe no compulsory license has yet been granted by the SIPO.
42
International patent treaties
The PRC is also a signatory to all major intellectual property conventions, including Paris Convention for the Protection of Industrial Property, Madrid Agreement on the International Registration of Marks and Madrid Protocol, Patent Cooperation Treaty, Budapest Treaty on the International Recognition of the Deposit of Microorganisms for the Purposes of Patent Procedure and the Agreement on Trade-Related Aspects of Intellectual Property Rights, or TRIPs.
Although patent rights are national rights, there is also a large degree of international cooperation under the Patent Cooperation Treaty, or the PCT, to which China is a signatory. Under the PCT, applicants in one country can seek patent protection for an invention simultaneously in a number of other member countries by filing a single international patent application. The fact that a patent application is pending is no guarantee that a patent will be granted, and even if granted, the scope of a patent may not be as broad as the subject of the initial application.
4.B.11-c.2 Trademarks
The PRC Trademark Law was promulgated in 1982, followed by the PRC Trademark Implementing Regulations in 1988, and was amended on October 27, 2001. As noted above, the PRC is signatory to the Madrid Agreement and the Madrid Protocol. These agreements provide a mechanism whereby an international registration produces the same effects as an application for registration of the mark made in each of the countries designated by the applicant.
The PRC Trademark Office is responsible for the registration and administration of trademarks throughout the country. Like patents, the PRC has adopted a “first-to-file” principle with respect to trademarks.
PRC law provides that the following acts constitute infringement of the exclusive right to use a registered trademark:
-
use of a trademark that is identical with or similar to a registered trademark in respect of the same or similar commodities without the authorization of the trademark registrant;
-
sale of commodities infringing upon the exclusive right to use the trademark;
-
counterfeiting or making, without authorization, representations of a registered trademark of another person, or sale of such representations of a registered trademark as were counterfeited, or made without authorization;
-
altering a registered trademark and putting commodities on which the altered registered trademark is used into the market without the consent of the trademark registrant; and
-
otherwise infringing upon the exclusive right of another person to use a registered trademark.
In the PRC, a trademark owner who believes the trademark is being infringed has three options:
-
The trademark owner can provide his trademark registration certificate and other relevant evidence to the State or local administrative bureau of industry and commerce (“AIC”) which can, at its discretion, launch an investigation. The AIC may take such actions as order the infringer to immediately cease the infringing behavior, seize and destroy the representations of the trademark in question and impose a fine. If the trademark owner is dissatisfied with the AIC’s decision, he may apply to have the decision reconsidered.
-
The trademark owner may institute civil proceedings directly with a court. Civil redress for trademark infringement includes:
-
injunctions;
-
requiring the infringer to take steps to mitigate the damage (i.e., print notices in newspapers); and
-
damages (i.e; compensation for the economic loss and injury to reputation as a result of trademark infringement suffered by the trademark holder).
-
The amount of compensation is calculated according to either the gains earned by the infringer from the infringement or the loss suffered by the trademark owner, including expenses incurred by the trademark holder to deter such infringement. If it is difficult to determine the gains earned by the infringer from the infringement, or the loss suffered by the trademark owner, the court may elect to award compensation up to RMB500,000.
-
If the case is so serious as to constitute a crime, the trademark owner may lodge a complaint with the relevant public security organ.
4.B.11-c.3 Administrative protection and monitoring periods for new drugs
According to the Administrative Measures on the Registration of Pharmaceutical Products (promulgated first in 2002, revised in 2005 and 2007), with a view to protecting public health, the SFDA may provide for administrative monitoring periods of up to five years for new drugs approved to be manufactured, to continually monitor the safety of those new drugs. The key element in determining the availability and duration of the monitoring period is the safety of the new drug. The SFDA will consider, among other things, whether the new drug has been previously launched domestically or overseas, what type of new drug it is and what process and technology are involved in the production of the new drug.
43
During the monitoring period of a new drug, the SFDA will not approve any other enterprise’s application to manufacture or import a similar new drug. After a new drug enters the monitoring period, for an application whose clinical trial has already been approved by SFDA, the application shall continue in the regular review process, and SFDA may approve the production or import of an application in compliance with the requirements, as well as monitor the new drug produced by the pharmaceutical manufacturer within the territory of China.
Any applicant objecting to the SFDA’s decision can appeal within 60 days after its receipt of the SFDA’s decision. If the applicant is dissatisfied with the result of the appeal, it may apply for an administrative review with a special committee consisting of senior officials of the SFDA or file an administrative lawsuit with a court in China.
EPIAO for anemia associated with chemotherapy in cancer patients with non-myeloid malignancies held a 6-year administrative protection period till September 2007. TPIAO for the treatment of chemotherapy-induced thrombocytopenia held a 5-year administrative protection period till May 2010.
4.B.11-d PRC Enterprise Income Tax
On March 16, 2007, the PRC Enterprise Income Tax Law, or the EIT Law, was enacted. Under the EIT Law, effective January 1, 2008, China adopted a uniform tax rate of 25% for all enterprises (including foreign-invested enterprises) and revoked the then current tax exemption, reduction and preferential treatments applicable to foreign-invested enterprises.
There is a transition period for enterprises, whether foreign-invested or domestic, that were receiving preferential tax treatments granted by relevant tax authorities at the time the EIT Law became effective. Enterprises that are subject to an enterprise income tax, or EIT, rate lower than 25% may continue to enjoy the lower rate and gradually transition to the new tax rate within five years after the effective date of the EIT Law. Enterprises that are currently entitled to exemptions or reductions from the standard income tax rate for a fixed term may continue to enjoy such treatment until the fixed term expires.
Preferential tax treatments will continue to be granted to industries and projects that are strongly supported and encouraged by the state, and enterprises otherwise classified as such “encouraged” high-tech enterprises will be entitled to a preferential 15% EIT rate. On April 14, 2008, the Measures for the Recognition and Administration of New and High-tech Enterprises, or the Measures, were promulgated jointly by the Ministry of Science and Technology, the Ministry of Finance and the State Administration of Taxation and became retroactively effective from January 1, 2008. Under the Measures, the term "new- and high-tech enterprise" is defined as a resident enterprise that has been registered in the PRC (excluding Hong Kong, Macao or Taiwan) for more than one year, conducts business in the new and high-tech fields encouraged by government as listed in an appendix to the Measures, continuously undertakes research and development and technology conversion, and relies on proprietary intellectual property rights as the basis of its business operation. Such new and high-tech enterprises may apply for tax benefits.
On February 15, 2012, Shenyang Sunshine received the “New and High Technology Enterprise” renewal certification that entitled it to a reduced 15% EIT rate, effective retroactively from January 1, 2011 to December 31, 2013. Shenyang Sunshine also held that certification with the 15% EIT rate for 2008 to 2010.
Under the EIT law and the implementation rules issued by the State Council, PRC income tax is applicable to all dividends payable to: subject to any applicable tax treaty, (1) investors that are “non-resident enterprises”, which do not have an establishment or place of business in the PRC, or which have such establishment or place of business but the relevant income is not effectively connected with the establishment or place of business, generally at the rate of 10%, and (2) non-resident individual investors, at the individual rate of 20%, to the extent such dividends have their sources within the PRC. Similarly, any gain realized on the transfer of ADSs or ordinary shares by such investors is also subject to PRC income tax at the same rate as such gain is treated as income derived from sources within the PRC. Such taxes are subject to WHT.
Under the EIT Law and related regulations, an enterprise established outside of the PRC with “de facto management bodies” within the PRC is deemed a PRC resident enterprise and is subject to the EIT at the 25% statutory rate or any rate applicable to such resident enterprise on its worldwide income. The related regulations define the term “de facto management bodies” as “establishments that carry out substantial and overall management and control over the manufacturing and business operations, personnel, accounting, properties, etc. of an enterprise.” In April 2009, the PRC State Administration of Taxation, or the SAT, issued Circular No. 82 “Issues concerning the Determination of Companies that are Organized outside China but controlled by Chinese-Nationals as Resident Enterprise pursuant to the Standard of De Facto Management Bodies.” This Circular limits the applicability of the tax residency determination employing the de facto management bodies standard to such enterprises incorporated outside China whose principal control investors are enterprises or enterprise groups within China. In July 2011, the SAT issued "Administrative Measures on Income Tax of Chinese-controlled Resident Enterprises Incorporated Overseas (Trial)", or Circular 45, to supplement Circular 82 and other tax laws and regulations. Circular 45 clarifies certain issues relating to resident status determination. While substantially all of our management functions are currently based in China, and will likely remain in China for the foreseeable future, we do not have any enterprise or enterprise group within China as our principal control investors. Please see Item 7.A “Major Shareholders”. We cannot assure you, however, that the SAT will not in future apply or enforce the tax residence tests set forth in Circular 82 and Circular 45 beyond those controlled by enterprise or enterprise group within China, to including those controlled by PRC individuals or foreign persons like us.
44
We have not been taxed as a resident enterprise at any time since the promulgation of the EIT Law. It remains, however, unclear whether PRC tax authorities would require or permit us to be treated as a PRC resident enterprise.
Starting from December 2010, Shenyang Sunshine has begun to be subject to municipal construction tax and education surcharges, at 12% of VAT payable and business tax payable, as a result of the change in the China tax regulation effective from such time. Previously Shenyang Sunshine as a wholly foreign-owned enterprise was not subject to those taxes.
Risk and uncertainties as to tax residency
Notwithstanding the discussions above, there are substantial uncertainties regarding the interpretation, application and enforcement of PRC laws and regulations, including, that PRC National Tax Administration and local and other authorities may change their views on the tax residency issue in future. See “3.D.3 — Our operations are subject to the uncertainty associated with the legal system in China, which could adversely affect our business, or limit the legal protection available to us or to existing or potential investors.”
If we were deemed a PRC resident enterprise, we could be subject to the EIT at 25%, or any preferential rate, if obtained, on our global income, except that the dividends we receive from our PRC subsidiaries may be exempt from the EIT to the extent such dividends constitute “dividends among qualified PRC resident enterprises.” It is, however, unclear what type of enterprises would be deemed a “qualified PRC resident enterprise” for such purposes. If we were considered a resident enterprise and determined to have earned income other than exempted dividends from our PRC subsidiaries, the EIT on our global income could significantly increase our tax burden and materially and adversely affect our cash flow and profitability.
Further, If we were deemed a PRC resident enterprise under the EIT Law, our shareholders and ADS holders who are deemed non-resident enterprises could be subject to the WHT upon the dividends payable by us or upon any gains realized from the transfer of our shares or ADSs, if such income is deemed derived from China, provided that (i) such non-resident enterprise investor has no establishment or premises in China, or (ii) it has establishment or premises in China but its income derived from China has no real connection with such establishment or premises; and our non-resident individual shareholders and ADS holders could be subject to the WHT upon the dividends payable by us or upon any gains realized from the transfer of our shares or ADSs, if such income is deemed derived from China. It is unclear whether, if we were deemed a PRC resident enterprise, our shareholders and ADS holders might be able to claim the benefit of income tax treaties entered into between China and other countries. If we were required under the EIT Law to withhold PRC income tax on our dividends payable to our non-resident shareholders and ADS holders, or if any gains realized from the transfer of our shares or ADSs by our non-resident shareholders and ADS holders were subject to the WHT, your investment in our shares or ADSs could be materially and adversely affected.
Alternatively, if our holding entities outside China, as applicable, are deemed non-resident enterprises, the WHT may apply to the dividends (and interests on intra-company loans) paid by our PRC subsidiaries to such holding entities outside China. For the information regarding our holding structure, please refer to “4.C Organization Structure”.
4.B.11-e Regulation of foreign currency exchange, dividend distribution and overseas listing
Foreign currency exchange
Foreign currency exchange regulation in China is primarily governed by the following rules:
-
the Foreign Currency Administration Rules (1996), as amended, or the Exchange Rules; and
-
the Administration Rules of the Settlement, Sale and Payment of Foreign Exchange (1996), or the Administration Rules.
Under the Exchange Rules, the Renminbi is convertible for current account items, including the distribution of dividends, interest payments, trade and service-related foreign exchange transactions. Conversion of Renminbi for capital account items, such as direct investment, loan, security investment and repatriation of investment, however, is still subject to the approval of the SAFE.
Under the Administration Rules, foreign-invested enterprises in China, such as Shenyang Sunshine, may only buy, sell and/or remit foreign currencies at those banks authorized to conduct foreign exchange business after providing valid commercial documents and, in the case of capital account item transactions, obtaining approval from the SAFE. Capital investments by foreign-invested enterprises outside of China are also subject to limitations, which include but not limited to registration with approvals by the SAFE, MOFCOM and other relevant government authorities.
45
Dividend distribution
The principal regulations governing distribution of dividends paid by wholly foreign owned enterprises include:
-
the Wholly Foreign Owned Enterprise Law (1986), as amended; and
-
the Wholly Foreign Owned Enterprise Law Implementation Rules (1990), as amended.
Under these regulations, foreign-invested enterprises in China may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. In addition, foreign-invested enterprises in China are required to set aside certain amounts out of their accumulated profits each year, if any, to fund certain reserve funds, bonus and welfare funds. These funds are not distributable as cash dividends.
Regulation of foreign exchange in certain onshore and offshore transactions
On October 21, 2005, the SAFE issued the Notice on Issues Relating to the Administration of Foreign Exchange in Fund-raising and Reverse Investment Activities of Domestic Residents Conducted via Offshore Special Purpose Companies, or Notice No. 75, which became effective as of November 1, 2005.
Pursuant to Notice No.75, prior to establishing or assuming control of an offshore company for the purpose of financing that offshore company with assets or equity interests in an onshore enterprise in the PRC, each PRC resident who is an ultimate control person, whether a natural or legal person, must complete the overseas investment foreign exchange registration procedures with the applicable local SAFE branch. An amendment to the registration with the local SAFE branch is required to be filed by any PRC resident that directly or indirectly holds interests in that offshore company upon either the injection of equity interests or assets of an onshore enterprise to the offshore company, or the completion of any overseas fundraising by such offshore company. An amendment to the registration with the local SAFE branch is also required to be filed by such PRC resident when there is any material change involving a change in the capital of the offshore company, such as (a) an increase or decrease in its capital, (b) a transfer or swap of shares, (c) a merger or division, (d) a long-term equity or debt investment or (e) the provision of a guarantee to third parties.
Under Notice No. 75, failure to comply with the registration procedures set forth in Notice 75 may result in restrictions being imposed on the foreign exchange activities of the relevant onshore company, including the payment of dividends and other distributions to its offshore parent or affiliate and the capital inflow from the offshore entity, and may also subject the relevant PRC residents to penalties under PRC foreign exchange administration regulations.
4.B.11-f Other national and provincial level laws and regulations
We are subject to changing regulations under many other laws and regulations administered by governmental authorities at the national, provincial and municipal levels, some of which are or may become applicable to our business. Our hospital customers are also subject to a wide variety of laws and regulations that could affect the nature and scope of their relationships with us.
For example, regulations control the confidentiality of patients’ medical information and the circumstances under which patient medical information may be released for inclusion in our databases, or released by us to third parties. These laws and regulations governing both the disclosure and the use of confidential patient medical information may become more restrictive in the future.
We also comply with numerous additional state and local laws relating to matters such as safe working conditions, environmental protection and fire hazard control. We believe that we are currently in compliance with these laws and regulations; however, we may be required to incur significant costs to comply with these laws and regulations in the future. Unanticipated changes in existing regulatory requirements or adoption of new requirements could therefore have a material adverse effect on our business, results of operations and financial condition.
46
4.C Organizational Structure
The following is a list of our subsidiaries and consolidated affiliated entities as of February 29, 2012, the latest practicable date:
|
|
Place of | |||||
|
Name |
Time of Formation | Formation | Relationship | |||
|
|
||||||
|
Collected Mind Limited |
July 2006 | British Virgin Islands | Wholly-owned subsidiary of 3SBio Inc. | |||
|
--- China Sansheng Medical Limited* |
November 2009 | Hong Kong | Wholly-owned subsidiary of Collected Mind | |||
|
--- Shenyang Sunshine Pharmaceutical Co., Limited |
January 1993 | PRC | Wholly-owned subsidiary of Collected Mind | |||
|
--- Jiangsu Sunshine Pharmaceutical Technology Company Limited |
December 2010 | PRC | 95% owned by Shenyang Sunshine and 5% owned by Liaoning Sunshine | |||
|
--- Taizhou Huan Sheng Investment Management Company Limited |
December 2010 | PRC | Wholly-owned subsidiary of Shenyang Sunshine | |||
|
--- 80% Limited Partner interest in Taizhou Huan Sheng Healthcare Industry Investment Center, LLP ("3SBio Ventures") |
May 2011 | PRC | 80% Limited Partner interest owned by Shenyang Sunshine | |||
|
--- IP Assets Series of 3SBio, LLC* (Note1) |
November 2010 | USA | Shenyang Sunshine as the sole series member | |||
|
US Assets Series of 3SBio, LLC* (Note1) |
November 2010 | USA | 3SBio Inc. as the sole series member | |||
|
Liaoning Sunshine Bio-Pharmaceutical Company Limited (“Liaoning Sunshine”) |
February 2000 | PRC | Consolidated affiliated entity | |||
|
--- Liaoning Sunshine Science and Technology Development Co. Limited |
December 2009 | PRC | Wholly-owned subsidiary of Liaoning Sunshine, consolidated affiliated entity |
*As of December 31, 2011, not involved in any business activities.
Note 1: each assets series is a segregated assets series, as provided under the Delaware Limited Liability Company Act, of 3SBio, LLC, a limited liability company formed in Delaware in November 2010. For the year ended December 31, 2011, IP Assets Series has no U.S. taxable income; and, with respect to US Assets Series, we had submitted an application for the extension of filing federal taxes return before the tax filing deadline.
4.C.1 Affiliated Entities
Historically, we conducted our manufacturing and marketing activities through our wholly owned subsidiary, Shenyang Sunshine, and certain distribution and logistics activities through Beijing Sunshine (now dissolved) and Liaoning Sunshine.
We had held equity interests in Liaoning Sunshine and Beijing Sunshine, and divested these interests as part of our corporate reorganization in 2006.
Liaoning Sunshine is primarily engaged in the distribution of our in-licensed products, currently comprising Iron Sucrose Supplement, as Shenyang Sunshine, our principal subsidiary, may only engage in the trading of the pharmaceutical products that it has produced itself. Liaoning Sunshine is also a party to our agreement with DaVita as described under Item 8.B and Exhibit 4.10.
At December 31, 2011, Liaoning Sunshine had total assets of RMB28.8million (US$4.6million) . For the three years ended December 31, 2009, 2010 and 2011, Liaoning Sunshine’s revenue represented approximately 4.5%, 5.4% and 5.3% of our total net revenue, respectively.
4.C.2 Economic and Control Arrangements
Shenyang Sunshine entered into a series of contractual arrangements with Liaoning Sunshine and its 100% shareholder, Mr. Dan Lou, to enable us to maintain control over it, including: (1) a business cooperation agreement; (2) a purchase agreements for the acquisition of equity interest in Liaoning Sunshine; (3) a voting agreement; and (4) an equity pledge agreement. Please see “7.B Related Party Transactions - Transactions with Liaoning Sunshine” for more details about these contractual arrangements. Liaoning Sunshine has been included in our consolidated financial statements as a variable interest entity (“VIE”). VIEs are those entities of which a company, through contractual and/or other arrangements, bears the risks of and enjoys the rewards normally associated with ownership, and of which therefore the company is the primary beneficiary.
47
4.D. Property, Plant and Equipment
Please refer to “4.B.9 Facilities” for a discussion of our property, plant and equipment.
ITEM 4A. UNRESOLVED STAFF COMMENTS
Not applicable.
48
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
You should read the following in conjunction with our audited consolidated financial statements for the periods covered by this report, together with the accompanying notes, all included elsewhere in this annual report. This discussion contains forward-looking statements and other information and statements that are subject to risks, uncertainties, assumptions and limitations. Our actual results could differ materially from such information and statements. In evaluating our business, you should carefully consider the information provided under the caption “Item 3. Key Information—D. Risk Factors” and other information about risks, uncertainties, assumption in this report and our other disclosures. Please also read the information under the heading “Cautionary Statement concerning Forward-looking Statement” at beginning of this annual report.
5.A Operating Results
5.A.1 Significant Trends, Factors and Developments
Certain significant trends, factors, and developments, as discussed below, have historically affected or had a material effect on our results of operations, or, are reasonably likely to affect or have a material effect on our results of operations in future periods.
Significant Market Potential and Government Policies
With its growing economy, healthcare expenditures in China are rising rapidly. According to the PRC Ministry of Health, total healthcare expenditures are projected to grow from 2008 to 2016 at a CAGR of 13%; notably, in US dollar amounts, China’s per capita healthcare expenditures in 2009 were only US$192, compared to the United States’ per capita healthcare expenditures in 2009 of US$6,815. The economic growth enhances the ability to pay. Urbanization and aging population in China are expected to continue to contribute to the growth of healthcare spending.
China's healthcare reform started from 2009 aims at healthcare accessibility and affordability, with significant commitments of government funding for healthcare expenditures. The coverage expansion now takes over 95% of China's population, or 1.3 billion people, under different health insurance schemes (See Item 4.B.11-b.5 “Consumer and Government Insurance Reimbursement”), bringing about significant growth in the number of patients receiving care, with increased reimbursement limits. The hospital system reforms are expected to remove bottlenecks in the system. The improvement in basic health services, a priority of the reform, particularly in urban community healthcare centers and clinics, of those in lower-tier cities, as well as in rural clinics, may result in more patients being properly diagnosed with conditions that can be treated using our products.
Our year-on-year revenues increase for 2009, 2010 and 2011, in RMB terms, are 30.3%, 32.1%, and 29.4%, respectively. We are serving a market sector that is greatly under-penetrated. For example, in the US, approximately 80% of patients with end-stage renal diseases are on dialysis, while this figure is estimated to be at 10-15% or less in China. The majority of patients on dialysis receive EPO treatment, our EPIAO being the leading product in China's EPO market for the past 39 consecutive quarters and accounting for 42.7% of total EPO sale revenues in China as of year-end 2011. The central government recently identifies dialysis services as a key healthcare objective1. Additional impetus comes from that the government encourages private and foreign companies entering into healthcare services2, as well as other positive regulatory developments. We believe the number of dialysis clinics and services could see considerable growth. Over 70% of our EPIAO sales come from the dialysis segment. We are also continually developing our marketing and sales.
In addition, we expect a favorable policy environment for the biopharmaceutical industry and especially for firms like us who are innovation-oriented with strong R&D and market capabilities, as indicated by a series of official pronouncements, including the Decision on Speeding up the Development and Promotion of Strategic New Industries (October 2010, by the State Council); Biotech Development – 12th Five-year Plan (November 2011, by Ministry of Science and Technology), and Biopharma Development -- Twelfth Five-year Plan (submitted to the State Council in early 2012, by NDRC).
There are, however, also uncertainties and challenges in
various respects of our operating environment, such as the ongoing hospital reform and the bidding system as evolved in
provincial and local variations
___________________
| 1 |
2012 Government Report: “III. 2012 Priority Objectives – (6) Provision, Protection and Improvement for the Life of People: ... promote the support and security on a comprehensive basis for CKD and other 7 catastrophic diseases; ...” |
| 2 |
See 2012 Government Report, delivered by Premier Jiabao Wen to the 2012 (5th ) Session, 11th National People's Congress on March 5th , 2012: “ III. 2012 Priority Objectives – (6) Provision, Protection and Improvement for the Life of People: ... encourage and direct social capital to invest in and operate hospitals, and speed up the forming of a medical service industry set-up with participation of multiple actors that opens up internationally ...” |
49
Continuing Pricing Pressure
The selling prices of some of our products have declined over time due to increased pricing pressure from industry peers and various government price control measures and the bidding mechanism in China. Please see under Item 4.B.11-b.3 “Distribution” “--- Price control” and “--- Competitive bidding.” On the other hand, price decreases may to a certain extent be compensated by improved sales volumes.
The average selling prices of our legacy products and lower dosage EPIAO products have declined over time. We were able to maintain relatively stable overall average selling prices for our TPIAO and higher dosage EPIAO products over the past few years. We remain focused on our strategy of growing sales for our high-dosage products and increasing sales volumes in order to maintain healthy margins for our EPIAO products. In addition, the stable average prices of our TPIAO products, combined with their market acceptance since launch, have contributed to our revenue growth and favorable overall margins.
We believe our ability to continue to grow our revenue and remain profitable in the face of downward pricing pressure is primarily dependent on the following factors:
Enhancing Product Mix. We are continually enhancing our product mix through the introduction of new products, such as the 36,000 IU dosage EPIAO, our proprietary drug, TPIAO, with the new indication for ITP, our pre-filled syringe EPIAO and our Iron Sucrose supplement. We believe the product candidates in our pipeline will further contribute to an enhanced product mix. The new introductions generally can maintain higher pricing due to potential status as innovative drugs or first-to-market generics, which (1) would often be accompanied by patents or the administratively enforced exclusivity in the form of monitoring periods; (2) may come with inherent technology barriers to competitors; (3) if not included in any government medical insurance programs, may allow more freedom in setting the price with less regulatory intervention; or (4) may be eligible for premium pricing in any regulatory reviews such as conducted by the NDRC.
Realigning Sales and Marketing Resources. As pricing pressures drive down the average selling price of a product, we may focus our internal sales force on other more profitable products, thereby improving our overall product mix and sales force efficiency. For example, since 2004 we have devoted a decreasing amount of resources to the marketing of our two legacy products, Intefen and Inleusin, as we strategically realigned our internal focus on EPIAO, and, in particular, its higher dosage forms, and TPIAO. At the same time, we began outsourcing the sales efforts for the two legacy products to distributors who manage their own sales forces.
Export Sales. While export sales accounted for a small percentage of our total revenues in past periods, as we are seeking to expand internationally and pursue global biosimilar opportunities, future growth in export sales may assist us in offsetting pricing pressures in our domestic market. See “4.B.2.1 Export Sales.”
The NDRC has instituted price cuts affecting many drugs over the past few years. We expect further drug price cuts in this year to adversely affect us, while the timing and magnitude of any impact is uncertain at this point.
Government Insurance Reimbursement and Competitive Bidding
Eligible participants in the government-sponsored medical insurance programs in China are entitled to reimbursement for varying percentages of the cost for any medicines that are included in the Registry and applicable reimbursement lists. See the relevant sections under 4.B.11-b.5. Factors that affect the inclusion of medicines in the Registry and any applicable reimbursement list may include whether the medicine is consumed in large volumes and commonly prescribed for clinical use in China and whether it is considered to be important in meeting the basic healthcare needs of the general public. The inclusion of a medicine in the Registry and any applicable reimbursement list can substantially improve the sales volume of the medicine due to the availability of third-party reimbursements; while, on the other hand, subjects it to price controls in the form of fixed retail prices or retail price ceilings, as well as periodical price adjustments by the regulatory authorities. All of our products are included in the Catalogue.
In each province where we market our products, we participate in a government-sponsored competitive bidding process every year or every few years for procurement by state-owned hospitals of medicines included in the provincial medicine catalogs. A government-appointed committee reviews bids submitted and selects one or more medicines for treatment of a particular medical condition. The selection is based on a number of factors, including bid price, quality of the product and manufacturer’s reputation and service. The bid price of the selected medicine will become the purchase price of that medicine to be paid by all state-owned hospitals in the applicable region. The competitive bidding in effect sets price ceilings for our products, thereby limiting our profitability; and, if we are not selected in these competitive biddings, we may lose market share to our competitors, and our business may be adversely affected. Furthermore, the bidding system is evolving and the provincial and local variations may produce uncertainties that can impact drug makers like us.
50
International Expansion
We plan to expand exports because of growing opportunities in the global market. Particularly, we believe market opportunities exist in Europe and other selected regulated markets for biosimilar products. We are searching for international partners to assist us for this expansion.
In 2010, we completed construction of additional manufacturing facilities in Shenyang, PRC, which have been certified with the Chinese current GMP. The new facilities are also designed to be compliant with the regulations of European Medicines Agency, or EMEA, and other major international regulatory guidelines. Similar to the US FDA, the EMEA is a European agency for the evaluation of medicinal products.
In September 2011, the SFDA approved our voluntary upgrade of manufacturing specifications to align EPIAO product quality with European Pharmacopoeia standards. Thus, we believe we are the only Chinese manufacturer with an approved product quality that meets both Chinese Pharmacopoeia and European Pharmacopoeia standards.
We are preparing for the process of seeking requisite international certification and regulatory approvals. We plan to first seek international recognition through the Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme (jointly referred to as PIC/S). PIC/S are two international instruments between countries and pharmaceutical inspection authorities, which provide together an active and constructive co-operation in the field of GMP. There are now 39 participating authorities of 33 countries, including the regulatory agencies of many European countries, Australia, Canada, and others. Approval by a participant country will facilitate the review process by other participants. After PIC/S recognition, we plan then to initiate EMEA application. The process and the timing of events, however, may be impacted by many factors that are not within our control.
Substantial capital and management resources will be required in order to secure regional partnerships, seek regulatory certifications and approvals and promote our brand. We may be unable to successfully execute the expansion plan due to inability to obtain suitable partners to assist us, changing competitive dynamics of the PIC/S and European biosimilar markets or inability to obtain required certification and other approvals for selling our products in those regions.
3SBio Ventures
As announced in August 2011, we formed an investment partnership (“3SBio Ventures”) with Taizhou Oriental CMC Limited ("Taizhou Oriental"), the investment arm of Taizhou China Medicine City Company (“CMC”). CMC, located in Jiangsu province, is a national level high-tech industrial park dedicated to particularly biopharmaceutical innovation, and is supported directly by the Ministry of Health, Ministry of Science and Technology and the SFDA. The investment partnership is managed by Taizhou Huan Sheng Investment Management Company Limited, a wholly-owned subsidiary of ours. We and Taizhou Oriental have committed RMB200 million and RMB50 million respectively to 3SBio Ventures. The initial contribution is 20% of the total commitment with the balance to be drawn down as required. 3SBio Ventures will seek investments that support our strategic interests and has an investment horizon of 8 years with an option to extend. The setup of this platform is consistent with, and will advance, our standing strategy of pursuing collaborations and in-licenses, and will play a key role in our future pipeline development.
5.A.2 Significant Components of Revenue and Expenses
Net revenues
Net revenues consist of the invoiced value of goods sold, net of VAT, trade discounts and allowances, and, in very rare circumstances, discretionary sales returns. In the PRC, VAT on the invoice amount is collected on behalf of tax authorities in respect of the sales of goods. Revenue is stated net of VAT. VAT collected from customers is offset by VAT paid for purchases, with the net amount recorded as a liability in the consolidated balance sheet until it is paid to the authorities.
We sell our products primarily to distributors, who resell them to healthcare providers, including hospitals and dialysis centers. With respect to our principal products, EPIAO and TPIAO, we rely on our own sales force to promote them to the hospitals and other customers. Because of PRC regulations governing the distribution of pharmaceuticals by manufacturers, we direct our customers to purchase our products from designated distributors. We generally sell our products to these distributors at a discount of approximately 8% off the wholesale prices to healthcare providers such as hospitals and dialysis centers. With respect to legacy products, Intefen and Inleusin, we primarily rely on distributors to market, as well as sell, our products with their own sales force. We reimburse the related costs of these distributors’ sales efforts in the form of a negotiated discount.
51
We generally recognize revenue at the time our products are delivered and the customers take ownership and assume risk of loss, collection of the relevant receivable is probable, persuasive evidence of an arrangement exists and the sales price is fixed or determinable. We are a party to binding purchase agreements with our distributors each time we make a sale of our products. Under these purchase agreements, our distributors agree to pay a fixed amount of money per unit of our products over a period of time. Our distributors take ownership of our products when they accept our products.
Allowance for doubtful accounts
We maintain allowances for doubtful accounts for estimated losses resulting from the failure of our customers to make required payments. We review the accounts receivable on a periodic basis and make general and specific allowances when there is doubt as to the collectability of individual balances. In evaluating the collectability of our customers’ balances, we consider many factors, including the aging of the receivables, the customers’ past payment history and current credit-worthiness. We are subject to stringent requirements mandated by scrutiny of the local Chinese tax authorities on write-offs of bad debts. For example, bad debts must be aged over three years and sufficient evidence must be provided to prove customers’ inability to make payments. As a result, this creates a substantial time lag between the time when our bad debt provision is made and the removal of such doubtful debt from our books. We made an allowance for doubtful accounts of RMB2,542,000 (US$404,000) as of December 31, 2011. As of December 31, 2011, there were no material amounts of receivables outstanding for more than one year that was not fully reserved.
VAT
Our revenues are recorded net of VAT. VAT is charged based on the selling price of our products at a general rate of 17%. In China, pharmaceutical companies are accustomed to having the market sales data referenced to revenue inclusive of the VAT, which is referred to as “gross revenue”. Gross revenue is mainly presented for non-financial purpose. For example, the data quoted by IMS Health throughout this annual report are quoted on gross revenue.
Cost of revenue
Our cost of revenue includes costs of raw materials, packaging, labor costs and manufacturing overhead. Our manufacturing overhead is primarily comprised of factory staff costs, allocated utilities and depreciation of our production facilities. We believe the relatively low cost of labor in the PRC provides us with a significant competitive advantage compared to international competitors that are not producing products in the PRC. We expect that, after we commence operations in our new manufacturing facilities, depreciation charges will increase on a per unit basis in early years before the full deployment of the capacity of our new manufacturing facilities.
Operating expenses
Our operating expenses include research and development costs, or R&D costs, sales, marketing and distribution expenses and general and administrative expenses. The key components of our operating expenses are described below.
Research and development costs. Our research and development costs are related to activities such as preclinical studies and clinical trials and consist primarily of:
-
Direct and allocated salaries and related expenses for research and development personnel;
-
Fees paid to consultants and clinical research organizations in conjunction with their monitoring our clinical trials and acquiring and evaluating data in conjunction with our clinical trials;
-
Direct cost of materials used in research and development;
-
Direct cost of equipment that lacks an alternative future use;
-
Fees paid to independent research organizations in conjunction with preclinical animal studies;
-
Allocated depreciation of research equipment and laboratory facilities;
-
Manufacturing costs of our clinical trial supply quantities for our product candidates;
-
Non-refundable upfront payments paid and/or milestone payments related to our in-licensing agreements;
-
Costs associated with other clinical development such as seminar hosting and process optimization; and
-
Costs for seeking EMEA approval so that we may enter the European market for biosimilar products.
52
We expense both internal and external research and development costs as incurred, other than laboratory equipment with alternative future uses, which we capitalize.
The following table shows the research and development costs that have been incurred for our two principal products, EPIAO and TPIAO, and others during each of the years indicated.
| Year ended December 31, | ||||||||||||
| 2009 | 2010 | 2011 | 2011 | |||||||||
| RMB | RMB | RMB | US$ | |||||||||
| (in thousands) | ||||||||||||
| Research and development costs by product | ||||||||||||
| EPIAO | 2,394 | 6,004 | 7,615 | 1,210 | ||||||||
| TPIAO | 5,293 | 1,700 | 5,635 | 895 | ||||||||
| Others | 11,740 | 31,705 | 28,555 | 4,537 | ||||||||
| 19,427 | 39,409 | 41,805 | 6,642 | |||||||||
The table above contains research and development costs attributable to the ongoing clinical trials for expanded indications of our marketed products, as well as preclinical and clinical trials for our product candidates.
Sales, marketing and distribution expenses. Our sales, marketing and distribution expenses primarily consist of salaries, employee benefits, bonuses and related expenses, including share-based compensation for our sales and marketing staff. They also include the direct costs attributable to our sales and marketing activities, such as conferences and seminar hosting and attendance, travel, entertainment and advertising expenses. We expect our sales, marketing and distribution expenses to increase in absolute dollar amounts in the future as we continue to expand the portfolio of our products.
General and administrative expenses. Our general and administrative expenses primarily consist of salaries and employee benefits, including share-based compensation for our administrative staff, as well as depreciation charges of office premises and equipment. We expect our general and administrative expenses to increase in absolute monetary amounts in line with our continued growth.
5.A.3 Critical Accounting Policies and Estimates
The discussion and analysis of our operating results and financial position are primarily based on our audited financial statements, which have been prepared in accordance with U.S. GAAP. The reporting of our operating results and consolidated financial position are sensitive to accounting methods, assumptions and estimates that underlie the preparation of our financial statements. We base our assumptions and estimates on historical experience and on various other assumptions that we believe to be reasonable and which form the basis for making judgments about matters that are not readily apparent from other sources. Our management evaluates these estimates on an ongoing basis. Actual results may differ from these estimates as facts, circumstances and conditions change or as a result of different assumptions.
The selection of critical accounting policies, the judgments and other uncertainties affecting application of those policies, and the sensitivity of reported results to changes in conditions and assumptions are factors to be considered when reviewing our financial statements. Our principal accounting policies are set forth in detail in Note 2 to our consolidated financial statements included elsewhere in this annual report. We believe the following critical accounting policies involve the most significant judgments and estimates used in the preparation of our financial statements.
Consolidation
The consolidated financial statements include the financial statements of the Company and its subsidiaries, including the VIE of which the Company is the primary beneficiary. All significant intercompany balances and transactions between the Company, its subsidiaries and consolidated VIE have been eliminated upon consolidation.
Revenue recognition
Sales of pharmaceutical products represent the invoiced value of goods, net of VAT, sales returns, trade discounts and allowances. We recognize revenue when products are delivered and the customer takes ownership and assumes risk of loss, collection of the relevant receivable is probable, persuasive evidence of an arrangement exists and the sales price is fixed or determinable. Shipping and handling costs that we pay on shipment and recover from our customers are included in revenue and sales, marketing and distribution expenses.
53
In the PRC, VAT at a general rate of 17% on invoice amount is collected on behalf of tax authorities in respect of the sales of goods. Revenue is stated net of VAT. VAT collected from customers is offset with VAT paid by us for purchases, with the net amount recorded as a liability in the consolidated balance sheets until it is paid to the tax authorities.
Determination of other-than-temporary impairment of investment securities
A decline in the market value of any investment security, including those that are available-for-sale that is deemed to be other-than-temporary, results in an impairment to reduce the carrying amount to fair value. The impairment is charged to earnings and a new cost basis for the security is established. To determine whether an impairment is other-than-temporary, we consider whether we have the ability and intent to hold the investment until a market price recovery and consider whether evidence indicating the cost of the investment is recoverable outweighs evidence to the contrary. Evidence considered in this assessment includes the reasons for the impairment, the severity and duration of the impairment, changes in value subsequent to year-end, forecasted performance of the investee, and the general market condition in the geographic area or industry the investee operates in.
Research and development costs
We critically identify any research and development activities that could be objectively measured and recognized and are aimed at the discovery of new products, indications, or betterment of processes. We capitalize the costs of tangible and intangible assets used for research and development purpose and having future alternative uses. We amortize these assets over their useful lives. Research and development costs are expensed as incurred. Research and development costs consist primarily of the remuneration of research and development staff, depreciation, material, clinical trial costs as well as amortization of acquired technology and know-how used in research and development with alternative future uses. Research and development expenses also include costs associated with collaborative research and development and in-licensing arrangements, including upfront fees paid to collaboration partners in connection with technologies which have not reached technological feasibility and did not have an alternative future use. Reimbursement of research and development costs for arrangements with our collaboration partners is recognized when the obligations are incurred. Expenses relating to new products are charged to the statement of income until such time that we obtain the new medicine certificate. The costs of research and development services such as pre-clinical tests outsourced or contract consulting services for upgrading our facilities are also included in research and development costs. Upfront and milestone payments made to third parties in connection with particular research and development collaborations with no alternative uses are expensed as incurred. The determination of alternative use requires judgment about the application of developed processes and know-how as well as market acceptance of related products. Different judgments regarding alternative uses could result in a change in the timing and amount of costs capitalized as materials and equipment.
Estimated useful lives and impairment of long-lived assets
We make estimates of the useful lives of property, plant and equipment with finite useful lives, in order to determine the amount of depreciation expense to be recorded during any reporting period. Our total depreciation expense for the years ended December 31, 2009, 2010 and 2011 was RMB7.0 million, RMB13.9 and RMB20.0 million (US$3.2 million), respectively. The useful lives are estimated at the time the assets are acquired and are based on historical experience with similar assets as well as anticipated technological or other changes. If technological changes were to occur more rapidly than anticipated or in a different form than anticipated, the useful lives assigned to these assets may need to be shortened, resulting in the recognition of increased depreciation expense in future periods. We make estimates of useful lives of the intangible assets based on the estimation of the period that the intangible assets can generate expected future cash flows for us. Amortization expense for the years ended December 31, 2009, 2010 and 2011 of RMB1.1 million, RMB1.1 million and RMB2.8 million (US$0.4 million) respectively, was included in cost of revenue. In progress research and development (“IPR&D”) assets with an indefinite useful life are not amortized.
Long-lived assets, including property, plant and equipment and intangible assets with finite useful lives, are reviewed for impairment whenever events or changes in circumstances indicate, in management’s judgment, that the carrying amount of an asset may not be recoverable. Recoverability of long-lived assets to be held and used is measured by a comparison of the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future undiscounted cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the asset. Fair value is measured by the asset’s discounted cash flows or market value, if readily determinable.
If intangible assets with indefinite use lives are subsequently determined to have finite useful lives, amortization will be provided prospectively over their estimated remaining useful lives and will be accounted for in the same manner as other intangible assets that are subject to amortization. Intangible assets with indefinite useful lives are tested for impairment annually or more frequently if events or changes in circumstances indicate that they might be impaired. There have been no impairment charges on the Company's intangible assets with indefinite useful lives.
54
Allowance for doubtful accounts
We evaluate the recoverability of our account receivables based on a combination of factors. We regularly analyze our significant customer accounts, and, when we become aware of a specific customer’s inability to meet its financial obligations to us, such as in the case of bankruptcy filings or deterioration in the customer’s operating results or financial position, we record a specific reserve for bad debt to reduce the related receivable to the amount we reasonably believe is collectible. We also record reserves for bad debt for all other customers based on a variety of factors, including the length of time the receivables are past due, the financial health of the customer and historical experience. If circumstances related to specific customers change, our estimates of the recoverability of receivables could be further adjusted. In the event that our account receivables become uncollectible, we record additional adjustments to receivables to reflect the amounts at net realizable value. The accounting effect of this entry would be a charge to income. We believe that the current charges to income are sufficient to reflect the recoverability of our accounts receivable.
Inventories
We state all inventories at the lower of cost or market value. Cost is determined using the weighted average cost method. Write-down on inventories is made when conditions indicate that the selling price could be less than cost due to physical deterioration, usage, obsolescence and reductions in estimated future demand. We balance the need to maintain strategic inventory levels with the risk of obsolescence due to varying customer demand levels and changing technology, although this rarely happens. Unfavorable changes in market conditions may result in additional inventory reserves that could adversely impact our gross margins. Conversely, favorable changes in demand could result in higher gross margins when we sell products.
Share-based compensation
We record share-based compensation at fair value and expenses at fair value over underlying service period, which is presumed to be the vesting period. We estimate the fair value of each stock option grant using an option-pricing model, which requires us to make certain assumptions related to volatility, expected life, dividend yield and interest-free interest rate.
Deferred tax assets and valuation allowance
We account for income tax using the liability method and deferred tax assets and liabilities are recognized for temporary differences. In assessing the realization of deferred tax assets, we have considered whether it is more likely than not based on all sources of positive and negative evidence that some or all of the deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Based on the level of projected future taxable income over the periods in which the deferred tax assets are deductible, we have provided a valuation allowance to reduce the amount of deferred tax assets when it is more likely than not that we will not be able to realize the benefits of certain deductible differences.
Uncertain tax positions
We evaluate our income tax positions and recognize in the financial statements the effects of tax positions when it is more likely than not these positions will be sustained upon examination. For tax positions meeting the more likely than not threshold, the amount recognized in the financial statements is the largest benefit that has a greater than 50 percent likelihood of being realized upon ultimate settlement with the relevant tax authority. We accrue interest, and penalties if necessary, related to our balance of unrecognized tax benefits.
5.A.4 Results of Operations
The table below sets forth selected results of operations data expressed as a percentage of total revenues, for the years indicated. Our historical results of operations are not necessarily indicative of the results for any future period.
| Year ended December 31, | |||||||||
| 2009 | 2010 | 2011 | |||||||
| % | % | % | |||||||
| Selected Statement of Income Data: | |||||||||
| Net revenue: | |||||||||
| EPIAO | 61.9 | 59.9 | 58.7 | ||||||
| TPIAO | 28.3 | 30.7 | 30.4 | ||||||
| Intefen | 1.7 | 1.3 | 1.0 | ||||||
| Inleusin | 0.5 | 0.5 | 0.5 | ||||||
| Iron Sucrose Supplement | 3.4 | 4.1 | 4.6 | ||||||
| Export | 4.2 | 2.9 | 4.4 | ||||||
| Others | 0.0 | 0.6 | 0.4 | ||||||
| Total net revenue | 100 | 100 | 100 | ||||||
| Cost of revenue | (8.0 | ) | (10.0 | ) | (10.7 | ) | |||
| Gross margin | 92.0 | 90.0 | 89.3 | ||||||
| Operating expenses: | |||||||||
| Research and development | (6.1 | ) | (9.4 | ) | (7.7 | ) | |||
| Sales, marketing and distribution | (47.9 | ) | (46.6 | ) | (47.0 | ) | |||
| General and administrative | (11.4 | ) | (13.3 | ) | (12.4 | ) | |||
| Grant income | 0.2 | 0.3 | 0.3 | ||||||
| Income from operations | 26.8 | 21.0 | 22.5 | ||||||
| Other income/(expense),net | |||||||||
| Interest income | 3.7 | 3.0 | 3.4 | ||||||
| Net realized gain on available-for-sale securities | 0.5 | - | - | ||||||
| Impairment loss on available-for-sale securities | (1.5 | ) | - | (0.2 | ) | ||||
| Others | 0.5 | 0.6 | (0.5 | ) | |||||
| Total other income, net | 3.2 | 3.6 | 2.7 | ||||||
| Income before income tax expense | 30.0 | 24.6 | 25.2 | ||||||
| Income tax expense | (3.7 | ) | (5.2 | ) | (5.1 | ) | |||
| Net income | 26.3 | 19.4 | 20.1 | ||||||
| Net loss attributable to non-controlling interests | - | - | 0.1 | ||||||
| Net income attributable to 3SBio Inc. | 26.3 | 19.4 | 20.2 |
55
Year ended December 31, 2011 compared with year ended December 31, 2010
Net revenue. Our net revenue increased by 29.4%, from RMB418.6 million in 2010 to RMB541.6 million (US$86.1 million) in 2011. This increase was primarily attributable to the continued strong sales of EPIAO and TPIAO, which grew 26.7% and 28.1%, respectively, over the same period in 2010. Revenue from our leading EPIAO products increased from RMB250.9 million in 2010 to RMB317.9 million (US$50.5 million) in 2011.TPIAO remained our second largest revenue contributor in 2011, accounting for 30.4% of total net revenues. The EPIAO and TPIAO growth was primarily due to the continued strong demand in the oncology and nephrology markets.
Cost of revenue. Our cost of revenue was RMB41.7 million and RMB58.1 million (US$9.2 million) for the years ended December 31, 2010 and 2011, respectively. The increase of 39.4% was primarily due to the increase in sales volume, and the RMB12.1 million in depreciation charges related to the new plant as compared to RMB6.8 million of depreciation charges in 2010. Cost of revenue as a percentage of net revenue remained relatively stable at around 10% for both 2010 and 2011 due to the enhanced capacity utilization and improved production efficiency of the new plant.
Operating expenses. Our total operating expenses increased by 25.3% from RMB288.9 million in 2010 to RMB361.9 million (US$57.5 million) in 2011.
-
Research and development costs. R&D costs increased by 6.1% to RMB41.8 million (US$6.6 million) in 2011 from RMB39.4 million in 2010, which was in line with the R&D progress of current pipeline products;
-
Sales, marketing and distribution (SMD) expenses. Our SMD expenses increased by 30.7% to RMB254.8 million (US$40.5 million) in 2011 from RMB194.9 million in 2010. The increase in SMD is broadly in line with overall sales growth. SMD expenses as a percentage of net revenue increased slightly from 46.6% in 2010 to 47.0% in 2011. The increase was primarily attributable to the expanded sales and marketing activities, including new product launch initiatives for TPIAO for ITP as well as the municipal construction tax and education surcharges levied as a result of the change in the China tax regulation effective from December 2010.
-
General and administrative expenses. Our general and administrative expenses increased by 19.8% from RMB55.9 million in 2010 to RMB66.9 million (US$10.6 million) in 2011. The increase was primarily attributable to employee compensation.
Other income, net. We had other income of RMB14.7 million (US$2.3 million) in 2011, compared to other income of RMB15.0 million in 2010.
56
Income before income tax expense. As a result of the foregoing, our income before income tax expense increased by 32.3% from RMB103.1 million in 2010 to RMB136.4 million (US$21.7 million) in 2011.
Income tax expense. Our income tax expense increased by 29.6% from RMB21.8 million in 2010 to RMB28.2 million (US$4.5 million) in 2011. The increase in income tax expense was in line with the improved income before income tax expense.
Net income attributable to 3SBio Inc. As a result of the foregoing, our net income increased by 33.6% from RMB81.3 million in 2010 to RMB108.6 million (US$17.2 million) in 2011.
Year ended December 31, 2010 compared with year ended December 31, 2009
Net revenue. Our net revenue increased by 32.1%, from RMB316.9 million in 2009 to RMB418.6 million in 2010. This increase was primarily attributable to the rapid market adoption of our TPIAO products, which grew 43.5% to RMB128.7 million in 2010, compared to RMB89.7 million in 2009. TPIAO was our second largest revenue contributor in both 2009 and 2010, accounting for 28.3% and 30.7% of total revenue, respectively. The increase was also attributable to greater revenue from our leading EPIAO products, which increased by 27.9%, from RMB196.1 million in 2009 to RMB250.9 million in 2010. The EPIAO growth was primarily due to the expansion of our oncology sales force and concurrent increase in market share.
Cost of revenue. Our cost of revenue was RMB25.2 million and RMB41.7 million, for the years ended December 31, 2009 and 2010, respectively. The increase of 65.5% was primarily due to the increase in sales volume and the RMB6.8 million in depreciation charges related to the new plant. Cost of revenue as a percentage of net revenue remained relatively stable at approximately 8% to 10% for 2009 and 2010 due to enhancement of cost controls and improvement of our raw material utilization rates.
Operating expenses. Our total operating expenses increased by 39.8% from RMB206.6 million in 2009 to RMB288.9 million in 2010.
-
Research and development costs. R&D costs increased by 102.9% to RMB39.4 million in 2010 from RMB19.4 million in 2009, which was primarily due to: (1) the expensing in 2010 of the US$1.5 million up-front payment to acquire exclusive rights to all transplant and autoimmune indications of voclosporin in mainland China, Hong Kong and Taiwan from Isotechnika; and (2) expensing of RMB5.0 million of the RMB17.0 million prepayment made to Ascentage Pharma pursuant to the research and development contracts, based on research and development activities preformed to date. We incurred no such expenses in 2009.
-
Sales, marketing and distribution (SMD) expenses. Our SMD expenses increased by 28.5% to RMB194.9 million in 2010 from RMB151.7 million in 2009. The increase in SMD was broadly in line with overall sales growth. SMD expenses as a percentage of net revenue decreased slightly from 47.9% in 2009 to 46.6% in 2010.
-
General and administrative expenses. Our general and administrative expenses increased by 54.3% from RMB36.2 million in 2009 to RMB55.9 million in 2010. The increase was primarily attributable to employee compensation, legal, auditing and office renovation expenses.
Other income, net. We had other income of RMB15.0 million in 2010, compared to other income of RMB10.1 million in 2009, primarily due to the fact that we incurred a net realized loss on disposal of available-for-sale securities of RMB4.6 million and a net realized gain on available-for-sale securities of RMB1.6 million in 2009. However, no such losses were incurred in 2010.
Income before income tax expense. As a result of the foregoing, our income before income tax expense increased by 8.3% from RMB95.2 million in 2009 to RMB103.1 million in 2010.
Income tax expense. Our income tax expense increased by 85.5% from RMB11.7 million in 2009 to RMB21.8 million in 2010. The increase in income tax expense is primarily due to a one-off uncollectible accounts receivable of RMB18.3million being deducted before income tax in 2009, which led to a lower level of income tax expense, as well as China income tax at rate of 10% for interest income Collected Mind earned in mainland China
Net income attributable to 3SBio Inc. As a result of the foregoing, our net income decreased by 2.6% from RMB83.4 million in 2009 to RMB81.3 million in 2010.
57
5.B Liquidity and Capital Resources
Overview
As of December 31, 2011, we had cash, cash equivalents, restricted cash and time deposits of RMB765.7 million (US$121.7 million) and working capital of RMB928.9 million (US$147.6 million). For the year ended December 31, 2011, our primary sources of funding for both our working capital and our long-term funding needs have been the net proceeds from our initial public offering in 2007 and cash flows from operating activities. Our primary uses of funds in 2011 have been for upgrading of existing facilities, procurement of new manufacturing equipment, procurement of land use right, acquisition of a patent and investment in dialysis related business. As of December 31, 2011, our cash and time deposits position denominated in RMB totaled RMB598.8 million (US$95.1 million) and our cash and time deposits position denominated in foreign currencies (mainly US$) totaled US$26.5 million. As of December 31, 2011, we had cash and cash equivalents of RMB245.8 million (US$39.1 million), restricted cash of RMB0.7 million (US$0.1 million) and time deposits of RMB519.2 million (US$82.5 million). As of December 31, 2011, we had RMB33.7 million (US$5.4 million) invested in equity securities and investment-grade bond securities.
The following table summarizes the sources and uses of our cash flows for the years indicated:
| Year ended December 31, | ||||||||||||
| 2009 | 2010 | 2011 | 2011 | |||||||||
| RMB | RMB | RMB | US$ | |||||||||
| (in thousands) | ||||||||||||
| Net cash provided by operating activities | 88,638 | 58,133 | 137,111 | 21,785 | ||||||||
| Net cash used in investing activities | (266,114 | ) | (165,234 | ) | (52,322 | ) | (8,313 | ) | ||||
| Net cash provided by financing activities | 132 | 6,763 | 15,963 | 2,536 | ||||||||
Net cash provided by operating activities
Our net cash provided by operating activities was RMB137.1 million (US$21.8 million) in 2011 compared to RMB58.1 million in 2010. The increase of RMB78.9 million in cash provided by operating activities in 2011 from 2010 reflects increase in net income of RMB27.3 million and in net operating receivables of RMB51.6 million, including accounts and notes receivables, inventories, prepaid expenses and other receivables, income tax payables, accrued expenses and prepayment to related parties.
Our net cash provided by operating activities was RMB58.1 million in 2010 compared to RMB88.6 million in 2009. The decrease of RMB30.5 million in cash provided by operating activities in 2010 from 2009 reflects decrease in net income of RMB2.1 million and in net operating receivables of RMB54.4 million, including accounts and notes receivables, other receivables, inventories, accounts payable, other payables, income tax payables, accrued expenses, long-term receivable, deferred grant income and prepayment to related parties, partially offset by RMB17.9 million increase in share-based compensation.
Net cash used in investing activities
Our net cash used in investing activities was RMB52.3 million (US$8.3 million) in 2011, compared to net cash used in investing activities of RMB165.2 million in 2010. The cash outlay in investing activities in 2011 consisted primarily of RMB31.5 million for renovation of existing facilities and procurement of new manufacturing equipment; RMB8.1 million for acquiring a patent; RMB9.8 million for acquiring a land use right; RMB20.8 million for net purchasing of time deposits with financial institutions; and RMB7.5 million for investment in our dialysis business; partially offset by RMB24.4 million for repayment of a loan receivable and RMB1.0 million from the release of restricted cash.
Our net cash used in investing activities was RMB165.2 million in 2010, compared to net cash used in investing activities of RMB266.1 million in 2009. The cash outlay in investing activities in 2010 consisted primarily of RMB40.8 million for renovation of existing facilities and procurement of new manufacturing equipment; RMB32.0 million for purchasing available-for-sale securities; RMB41.3 million for acquiring Pegsiticase related tangible and intangible assets; RMB30.0 million for net purchase of time deposits with financial institutions; RMB26.4 million for purchasing a loan receivable; and RMB3.1 million for acquiring a 40% equity investments in APGC and Ascentage SH, partially offset by RMB9.3 million from the release of restricted cash.
58
Net cash provided by financing activities
Net cash provided by financing activities in 2011 was RMB16.0 million (US$2.5 million), compared to net cash provided by financing activities of RMB6.8 million in 2010. The increase was attributable to the RMB11.3million (US$1.8 million) investment into 3SBio Ventures by CMC, and the gross proceeds of RMB4.7 million (US$0.7million) in 2011 from issuance of ordinary shares upon grantee exercise of share options under our share-based compensation arrangements.
Net cash provided by financing activities in 2010 was RMB6.8 million, compared to net cash provided by financing activities of RMB0.1 million in 2009. The increase is caused by greater gross proceeds in 2010 from issuance of ordinary shares upon grantees’ exercise of share options granted through our share-based compensation arrangements than in 2009.
We believe that our current cash and anticipated cash flow from operations will be sufficient to meet our anticipated cash needs, including planned capital expenditures for upgrading existing facilities and building new manufacturing facilities in Shenyang, other working capital needs for investing in research and development and increasing sales and marketing efforts, at least for the next two years.
Our indebtedness as of December 31, 2009, 2010 and 2011 was nil.
We do not expect to pay dividends in the near future as we plan to use our resources for our growth. However, should we decide to pay dividends, our wholly-owned subsidiary’s ability to pay dividends to us is subject to various restrictions, including legal restriction in the PRC, which permits payment of dividends only out of net income determined in accordance with PRC accounting standards and regulations. In addition, under PRC law, our China-based subsidiaries and consolidated VIE is required to set aside 10% of its net income as reserve funds, until such reserves have reached at least 50% of its respective registered capital. These reserves are not distributable as cash dividends to us for use by us to satisfy our obligations, such as debt incurred at the holding company level. Such dividends may be subject to EIT, as discussed under Paragraph 4.B.11-d “PRC Enterprise Income Tax”. Furthermore, PRC law also imposes restrictions on our China-based consolidated entities with respect to transferring certain of their net assets to us in the form of loans and advances. Amounts so restricted as described in all of the foregoing include paid-up capital and statutory reserves funds of our PRC-based subsidiaries, and the net assets of the consolidated VIE, in which we have no legal ownership, totaling RMB610,693,000 (US$97,029,000) as of December 31, 2011. These restrictions have not had, and we do not currently expect these restrictions to, in foreseeable future, have any material impact on our ability to meet our cash obligations.
Capital Expenditures
Historically, most of our capital expenditures were incurred for the construction of our new manufacturing facility, purchases of production and office equipment, upgrade of our research laboratory, and upgrades for our plant and office renovation.
In the year ended December 31, 2011, our capital expenditures of RMB56.9 million (US$9.0 million) related primarily to renovation of existing facilities, procurement of new manufacturing equipment, procurement of land use right, acquisition of a patent and investment in dialysis business.
In the year ended December 31, 2010, our capital expenditures of RMB85.2 million related primarily to renovation of existing facilities, procurement of new manufacturing equipment, and acquisition of Pegsiticase related tangible and intangible assets.
In the year ended December 31, 2009, our capital expenditures of RMB95.8 million related primarily to construction of our new manufacturing facilities, renovation of existing facilities and procurement of new manufacturing equipment.
Our future capital requirements may include, but are not limited to: upgrading our existing facilities for our development and production, potential investments in our dialysis business and other projects, and building new facilities. We are not obligated to meet any absolute minimum dollar spending requirements, and our future capital requirements will depend on many factors. The major components of our capital expenditures for 2012 may include the upgrading of existing manufacturing facilities and procurement of new manufacturing equipment.
We expect to fund our capital expenditure needs with a combination of cash generated from operating activities and our existing cash, cash equivalents and time deposits. We do not anticipate that we will require debt financing to fund our capital expenditures in the near term, although this may change in future periods.
59
5.A-B.1 Recently Issued Accounting Pronouncements
ASU 2011-04
In May 2011, the Financial Accounting Standards Board (“FASB”) issued Accounting Standard Update (“ASU”) No. 2011-04, Fair Value Measurement (“Topic 820”), Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRSs (“ASU 2011-04”) which amends the fair value measurement guidance and includes some enhanced disclosure requirements. The most significant change in disclosures is an expansion of the information required for Level 3 measurements based on unobservable inputs. The standard is effective for fiscal years beginning after December 15, 2011. We will adopt ASU 2011-04 beginning January 1, 2012 and do not expect the adoption to have a material impact on our consolidated financial statements and disclosures.
ASU 2011-05
In June 2011, the FASB issued ASU 2011-05, Comprehensive income (Topic 220), Presentation of Comprehensive Income. ASU 2011-05 increases the prominence of other comprehensive income in financial statements. Under this ASU, an entity will have the option to present the components of net income and comprehensive income in either one or two consecutive financial statements. The ASU eliminates the option in U.S. GAAP to present other comprehensive income in the statement of changes in equity. An entity should apply the ASU retrospectively. For a public entity, the ASU is effective for fiscal years, and interim periods within those years, beginning after December 15, 2011. We early adopted ASU 2011-05 in the year ended December 31, 2011.
5.A-B.2 Inflation
According to the National Bureau of Statistics of China, the change of consumer price index in China was -0.7%, 3.3%, and 5.4% in 2009, 2010 and 2011, respectively. We can be affected by high rates of inflation in China. For example, certain operating costs and expenses, such as employee compensation and office operating expenses may increase as a result of higher inflation. Additionally, because a substantial portion of our assets consists of cash and cash equivalents and short-term investments, high inflation could significantly reduce the value and purchasing power of these assets. We are not able to hedge our exposure to higher inflation in China.
5.C Research and Development
See “4.B.6 Research and Development”
5.D Trend Information
Other than as disclosed elsewhere in this annual report on Form 20-F, as of the date of this annual report, we are not aware of any significant recent trends in production, sales and inventory, the state of the order book and costs and selling prices since fiscal year 2011.
For fiscal year 2012, other than as disclosed elsewhere in this annual report on Form 20-F, as of the date of this report, we are not aware of any known trends, uncertainties, demands, commitments, or events that are reasonably likely to have a material effect on our net revenue, income from continuing operations, profitability, liquidity or capital resources, or that would cause reported financial information not necessarily to be indicative of future operating results or financial condition.
5.E Off-Balance Sheet Arrangements
We do not have any outstanding derivative financial instruments, interest rate swap transactions, foreign currency forward contracts, or other off-balance sheet arrangements.
60
5.F Tabular Disclosure of Contractual Obligations
The following table sets forth our contractual obligations and commercial commitments as of December 31, 2011.
| Payment due by period | |||||||||||||||
| less than | after 5 | ||||||||||||||
| Total | 1 year | 1-3 years | 3-5 years | years | |||||||||||
| (RMB in thousands) | |||||||||||||||
| Operating Lease Obligations | 585 | 552 | 33 | — | — | ||||||||||
| Capital Commitments | 12,112 | 12,112 | — | — | — | ||||||||||
| Total Obligation and commitments | 12,697 | 12,664 | 33 | — | — | ||||||||||
Milestone, Royalty and Other Payments (not included in the table above)
Under our development and commercialization agreement with AMAG for ferumoxytol, AMAG received an upfront payment of US$1 million from us and is eligible to receive additional milestone payments upon regulatory approval of ferumoxytol in China for CKD and other specified indications developed in China. AMAG is also entitled to receive tiered, double-digit (up to 25%) royalties based on sales of ferumoxytol by us.
Under the terms of the agreements between us and APGC and Ascentage SH, we paid a total consideration of approximately US$3 million to acquire 40% equity interests in both APGC and Ascentage SH, and to fund APGC research and development programs, and acquired the exclusive right to develop and commercialize in mainland China the cancer therapeutics that are developed through APGC research and development programs. We may also make future milestone payments when certain criteria are met, and may make royalty payments from our future product sales in China.
Under our collaboration agreement with Epitomics Inc., we will be required to pay a royalty payment equivalent to a certain percentage of the net sales of therapeutic products developed through the collaboration, should such products reach market. The details of a development program to commercialize the therapeutic products and the mechanism for calculating the royalties will be determined between Epitomics and us at a later date.
Cautionary Statement:
Not to affect the general applicability of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 to this or other parts of this annual report, as more fully discussed in “Cautionary Statement concerning Forward-looking Statements” at the beginning of this annual report, the information contained in above table and descriptions regarding future milestone, royalty and other payments constitutes, as provided under Form 20-F Item 5.G, “forward-looking statements” within the meaning of those laws, and are subject to various risks and uncertainties, such as changes in the circumstances surrounding the subject contracts or commitments.
61
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
6.A Executive Officers and Directors
The following table sets forth our executive officers and directors, their ages as of December 31, 2011 and the positions held by them. The business address for each of our executive officers and directors is Shenyang Sunshine Pharmaceutical Co. Ltd., No. 3 A1, Road 10, Economic & Technology Development Zone, Shenyang 110027, the People’s Republic of China.
| Name | Age | Position |
| Dr. Jing Lou(2) | 49 | Chairman of the board of directors and Chief Executive Officer, Director |
| Peiguo Cong | 57 | Independent Director |
| Bin Huang | 51 | Vice President of Human Resources and Director |
| Bo Tan | 39 | Chief Financial Officer (principal financial and accounting officer) |
| Lawrence S. Wizel(1)(3) | 69 | Independent Director |
| Mingde Yu(1)(2)(3) | 66 | Independent Director |
| Moujia Qi(1)(2)(3) | 79 | Independent Director |
| Dr. Dongmei Su | 42 | Director, Vice President, R&D, and Chief Technology Officer |
| Ke Li | 44 | Vice President and Corporate Secretary |
| (1) |
Member of the audit committee |
| (2) |
Member of the nominating committee |
| (3) |
Member of the compensation committee |
Dr. Jing Lou is our co-founder, chairman of the board of directors and chief executive officer. He has served as the chief executive officer of Shenyang Sunshine since 2000. He joined Shenyang Sunshine as director of research and development in 1995, leading the manufacturing process development for EPIAO and TPIAO in the United States. Dr. Lou completed his post-doctoral study at the United States National Institute of Health in 1995. He received his Ph.D. in molecular and cell Biology in 1993 from Fordham University, where he researched interferon gene regulation, and received his medical doctor degree in 1985 from Shanghai Second Military Medical University. Dr. Lou also received an EMBA degree in 2007 from China Europe International Business School, a business school ranked in the Financial Times’ world top-25 business school list.
Bin Huang is our vice president of human resources and a director. Mr. Huang has served as vice president in charge of human resources and legal matters since joining Shenyang Sunshine in 1993. Before that, he acted as office manager for the Shenyang Army Medical Research Center from 1976 to 1993. He received his master’s degree in Business Administration from Qinghua University in 2002 and a bachelor’s degree in engineering from Northeast University in 1987.
Peiguo Cong has served as a member of our board of directors since March 2010. He has been the managing partner of Beijing Jun You Law Firm since 1994. Mr. Cong also currently serves as a member on the Finance and Securities Committee of the China National Lawyers Association, a member of the All China Federation of Industry and Commerce, a director of the Chinese Society of Mergers and Acquisitions, and an independent committee member of the Credit Committee of the National Development Bank. He teaches as a part-time professor at Peking University. In addition, Mr. Cong served as an independent director of China Southern Fund Management Company (2009-2012) and Tianjin Lishen Battery Joint-Stock Co., Ltd. and Haining China Leather city stock Co., Ltd. (2009-2011). Mr. Cong obtained his bachelor's degree in law and master's degree in economics law from Peking University in 1982 and 1984, respectively. He was an associate professor at Peking University, a visiting professor at UCLA, and a visiting scholar at University of Michigan and other U.S. universities.
Bo Tan has served as our CFO since February 2009. He has extensive experience within the financial and pharmaceutical industries, having worked across private equity, equity research and commercial practice. Previously, he served as the Executive Director and a member of Investment Committee for Bohai Industrial Fund Management Company, a private equity fund in China. Earlier in his career, he spent six years in the pharmaceutical industry with Eli Lilly & Company and EMD Pharmaceuticals, Inc. in North America and went on to serve as a China healthcare and consumer analyst at Lehman Brothers Asia and Macquarie Securities in Hong Kong. He received his MBA degree from Thunderbird School of International Management, an MA degree in economics from the University of Connecticut and a BA degree in economics from Renmin University of China. Mr. Tan is also a Chartered Financial Analyst.
Lawrence S. Wizel has served as a director since September 2006. Since 2006, Mr. Wizel has served on the board of directors of American Oriental Bioengineering, Inc. (NYSE: AOB), a Chinese company focused on certain prescription and OTC pharmaceutical products and nutraceutical products. From 2007 to 2012, Mr. Wizel was a member of the board of directors of Puda Coal, Inc. (PINK:PUDA), a supplier of metallurgical coking coal to the industrial sector in China. He was a partner at Deloitte & Touche LLP from 1980 to June 2006. He also served as a deputy professional practice director and northeast region China service group leader of Deloitte & Touche LLP from 2002 to 2006. He has extensive experience serving a diverse client base of both publicly-held and private companies and has also assisted numerous Chinese companies with their filings with the Securities and Exchange Commission. He is a member of the American Institute of Certified Public Accountants and the New York State Society of Certified Public Accountants. He received his bachelor’s degree in science from Michigan State University.
62
Mingde Yu has served as a director since February 2008. He has extensive experience in manufacturing and distribution management in the pharmaceutical industry in China. He has held a number of senior positions both in the government and the private sectors, including as the Chief Technology Officer and the Head of Manufacturing at both Liaoning Fuxi Pharmaceutical Co. and Fuxi Traditional Chinese Medicine Co. (from 1978 to 1983), Bureau Chief of the Fuxi City Food & Drug Administration from 1983 to 1991, Bureau Chief of the Liaoning Provincial Food & Drug Administration from 1991 to 1997, Drug Department Chief of the Economic Operations Bureau of the State Economic and Trade Committee from 1997 to 1998 and Vice-Director of Economic Operations Department of NDRC from 1998 to 2003. Mr. Yu is currently the honorary Chairman and Director of the Beijing Pharmaceutical Group and is the Chair for the China Pharmaceutical Enterprises Administrative Association since 2004 and China Medical Entrepreneur Association. Mr. Yu also holds senior consultancy roles with the China Chemical Drug Association and the National Pharmaceutical Industry & Commerce Association. In addition, Mr. Yu currently serves as an independent member on the board of directors for the following companies: China Nuokang Bio-Pharmaceutical Inc. (NASDAQ:NKBP); North China Pharmaceuticals; Wohua Biologicals Co.; and Qingtao Huaren Pharmaceuticals, the last three listed on Chinese stock exchanges. Mr. Yu graduated from the Macromolecule Materials and Engineering Department, Chemical Technology School at Dalian University of Technology.
Moujia Qi has served as one of our directors since August 2006. He currently serves as an independent member on the board of directors of China Pharmaceutical Group Limited (Hong Kong Stock Exchange: 1093), and as an independent member on the supervisory board of Wuhan Humanwell Hi-Tech Industry Co., LTD (Shanghai Stock Exchange: 600079). He has also served as the Chairman of the China Starch Industry Association for the past five years. He was the deputy director and chief engineer of Huabei Pharmaceutical Factory and has held several management positions in various state-owned companies in the pharmaceutical industry. He has also served as the deputy director and chief director of the State Medicine Administration of the PRC before he retired in 1994.
Dr. Dongmei Su is our vice president and chief technology officer responsible for research and development and manufacturing process engineering, and has served as a director since April 2012. She is the named co-inventor for four of our patents. Ms. Su joined Shenyang Sunshine in 1993. She served as director of research and development and manufacturing since 1997. She received her bachelor’s degree in biochemical engineering from Jilin University in 1992, and her master’s and doctor’s degree in microbiology and pharmacology from Shenyang Pharmaceutical University in 2001 and 2010, respectively.
Ke Li, our vice president and corporate secretary, is responsible for all corporate and government regulatory matters. Mr. Li has served as the corporate secretary of Shenyang Sunshine since 1996. He joined us in 1993 and held various administrative positions at Shenyang Sunshine before he was appointed corporate secretary. Mr. Li received his master’s degree in Business Administration from Liaoning University in 2001 and his bachelor’s degree in engineering from Jilin University in 1988.
6.B Compensation
Our executive officers receive compensation in the form of annual salaries and bonuses. Our board of directors determines management compensation based on the recommendation of the compensation committee.
While we do not have a specific bonus plan setting the calculation of our annual bonuses, each executive officer is entitled to receive an annual discretionary bonus based upon his or her performance. Our non-executive directors receive annual cash compensation and share-based awards for their board services.
In 2011, the aggregate cash compensation we paid to our directors and executive officers was approximately RMB13.2 million (US$2.1 million); and we also granted restricted stock to our directors and executive officers, as set forth in “6.E -- Equity Grant to Directors and Senior Officers”.
Other than described in the foregoing, our executive officers may participate in compensatory or benefits plans or arrangements that is available by its terms to employees, officers or directors generally, the operation of which uses the same method to allocate benefits to management and non-management participants.
63
6.C Board Practices
Our board of directors currently consists of seven members, among whom, as determined by our board of directors, Mr. Lawrence S. Wizel, Mr. Mingde Yu, Mr. Moujia Qi, and Mr. Peiguo Cong are independent directors within the meaning of Rule 5605(a)(2) of the NASDAQ Marketplace Rules as amended from time to time.
6.C.1 Audit Committee
Our audit committee of the board of directors, currently consisting of Mr. Lawrence S. Wizel (Chair), Mr. Mingde Yu, and Mr. Moujia Qi, was established in October 2006. Since there are no specific requirements under Cayman Islands law on the composition of our audit committee, our practice was established by our board of directors by reference to similarly situated issuers. Our practice is in line with Rule 5605(c) of the NASDAQ Marketplace Rules that requires the audit committees of United States companies to have a minimum of three independent directors. All members of our audit committee satisfy the “independence” requirements of each of the NASDAQ Marketplace Rules and Section 10A(m)(3)(B)(i) of the Securities Exchange Act of 1934, as amended, or the Exchange Act. In addition, our board of directors has determined that Mr. Lawrence S. Wizel qualifies as an audit committee financial expert as defined in Item 16A of Form 20-F. The audit committee is responsible for overseeing the accounting and financial reporting processes of our company and audits of our financial statements, including the appointment, compensation and oversight of the work of our independent auditors.
6.C.2 Compensation Committee
Our compensation committee of the board of directors, currently consisting of Mr. Mingde Yu (Chair), Mr. Lawrence S. Wizel and Mr. Moujia Qi, was established in January 2009. All members of our compensation committee satisfy the “independence” requirements of the NASDAQ Marketplace Rules. The compensation committee is responsible for assisting the board of directors to oversee our compensation and benefits programs and to determine the compensation package of chief executive officer and other executive officers.
6.C.3 Nominating Committee
Our nominating committee of the board of directors, currently consisting of Mr. Jing Lou (Chair), Mr. Mingde Yu, and Mr. Moujia Qi, was established in February 2010. Mr. Yu and Mr. Qi satisfy the “independence” requirements of the NASDAQ Marketplace Rules. The nominating committee is responsible for assisting the board of directors in selecting nominees for election to the board of directors and monitoring the composition of the Board and other governance matters.
6.C.4 Investment Committee
Our investment committee is appointed by the board of directors to assist the board in fulfilling its responsibility to oversee our investment policy. The investment committee currently consists of the four independent directors, our chief executive officer and chief financial officer. In carrying out its responsibilities, the investment committee evaluates our investment policy periodically, approves any exceptions to the existing investment policy, and, recommends appropriate material policy changes to our board of directors.
6.C.5 Terms of Directors and Executive Officers
Our articles of association provide for a staggered board of directors. At each annual general meeting of our shareholders, one third of our directors (or, if their number is not a multiple of three, the number nearest to but not greater than one-third) are required to stand for reelection. The Chairman shall not be required to stand for reelection at any annual meeting, and will serve for an indefinite term. In addition, the Chairman will not be taken into account in determining the number of directors who must stand for reelection in each year.
The particular directors that must stand for reelection at each annual general meeting are determined according to a rotation. Should a director choose not to offer himself or herself for reelection at an annual meeting, the number of directors who must stand for reelection at that meeting is reduced accordingly. Directors who have been longest in office since their last re-election or appointment shall stand for reelection at the annual meeting, provided that, in the case of directors who were last elected on the same day, which director must stand for reelection shall (unless they otherwise agree among themselves) be determined by lot.
Any director appointed to fill a vacancy shall serve only until the next annual meeting, and shall not be taken into account in determining which particular directors or the number of directors who will stand for reelection at such annual meeting.
64
Our executive officers are appointed by and serve at the discretion of our board of directors.
6.C.6 Employment and Management Agreements
We have entered into employment agreements with all of our executive officers. Under these agreements, each of our executive officers is employed for a specified time period. We may terminate his or her employment for cause at any time, without notice or remuneration, for certain acts of the employee, including but not limited to a conviction or plea of guilty to a felony or to an act of fraud, misappropriation or embezzlement, negligence or dishonest act to the detriment of the company, or misconduct of the employee and failure to perform his or her agreed-to duties after a reasonable opportunity to cure the failure. Furthermore, we may terminate the employment without cause at any time, in which case we will pay the employee severance amount equal to one month of his or her salary for each year of his or her service for our company. An executive officer may terminate the employment at any time upon one-month advance written notice.
Each executive officer has agreed to hold, both during and subsequent to the term of the employment agreement, our confidential information in strict confidence and not to disclose such information to anyone except our other employees who have a need to know such information in connection with our business. The executive officers have also agreed to assign to us all rights, titles and interests to or in any inventions that they may conceive or develop during the period of employment, including any copyrights, patents, mark work rights, trade secrets or other intellectual property rights pertaining to such inventions. Specifically, each executive officer has agreed not to, while employed by us and for a period of three years following the termination or expiration of the employment agreement, (i) approach our clients, customers or contacts or other persons or entities introduced to the executive officer for the purposes of doing business with such persons or entities, and will not interfere with the business relationship between us and such persons and/or entities; (ii) assume employment with or provide services as a director or otherwise for any of our competitors, or engage, whether as principal, partner, licensor or otherwise, in any business which is in direct or indirect competition with our business; or (iii) seek directly or indirectly, to solicit the services of any of our employees employed by us at or after the date of the executive officer’s termination, or in the year preceding such termination.
Such employment agreement is the only agreement between us and our employee directors with respect to their services to us.
Each of our independent directors has received an appointment letter from us, which contains customary terms and conditions, including advance notice of termination, service compensation, confidentiality obligations, directorship termination, and indemnification. The appointment letters do not provide for any benefit upon termination of appointment.
In connection with the retirement of certain of our directors and officers, we may enter into retirement agreements with any of them, with benefits in due recognition of the retiree’s contributions to the Company, our compensation practice, the pre-retirement level of compensation, and any post-retirement obligations and services to us.
6.D Employees
See “4.B.10 Employees.”
6.E Share Ownership
Directors and Senior Officers Share Ownership
Please refer to the applicable part under Item 7.A “Major Shareholders”.
Employee Participation in the Company Capital: 2006 Stock Plan
The following is a summary description of our 2006 stock plan, as amended, which is subject to and qualified in its entirety by reference to the full text thereof as contained in Exhibits 4.1 and 4.2 to this annual report.
We adopted our 2006 stock plan in September 2006, as amended in April 2010, and it provides for the grant of stock, stock options, restricted stock and restricted stock units, each of which we refer to as “awards.” The purpose of the plan is to provide additional incentive to those officers, employees, directors, consultants and other service providers whose contributions are essential to the growth and success of our business, in order to strengthen the commitment of such persons to us and motivate such persons to faithfully and diligently perform their responsibilities and attract and retain competent and dedicated persons whose efforts will result in our long-term growth and profitability.
65
Not more than 10,000,000 ordinary shares plus a number of shares equal to 10% of any additional shares of the Company issued following the date of the adoption of the 2006 stock plan by the board of directors may be issued under this plan. The 2006 stock plan provides for the grant to our employees, directors, and consultants or any other participants that the board of directors shall decide in good faith. The board of directors has complete discretion to select the grantees and to establish the terms and conditions of the grants, subject to the provisions of the 2006 stock plan.
Plan Administration. Our 2006 stock plan is administered by the board of directors or the compensation committee of the board of directors. With respect to the grant of the awards to employees or consultants who are neither directors nor officers, the board of directors may authorize one or more officers to grant such awards.
Award Agreement. Awards to be granted under our 2006 stock plan are evidenced by an award agreement that sets forth the terms and conditions for each award grant, which may include, among other things, the vesting schedule, exercise price, type of option and expiration date of each award grant.
Eligibility. We may grant awards to an officer, director, employee, consultant, advisor or a service provider of our company or any of our parent or subsidiaries, provided that directors of our company or any of our parent or subsidiary who are not also employees of our company or any of our parent or subsidiaries, and consultants or advisors to our company or any of our parent or subsidiaries may not be granted incentive stock options.
Option Term. The term of each option to be granted under the 2006 stock plan may not exceed five years from the date of grant.
Exercise Price. In the case of non-qualified stock options, the per share exercise price of shares purchasable under an option shall be determined by the plan administrator in the administrator’s sole discretion at the time of grant. In the case of incentive stock options, the per share exercise price of shares purchasable under an option shall not be less than 100% of the fair market value per share at the time of grant. However, if we grant an incentive stock option to an employee, who at the time of that grant owns shares representing more than 10% of the voting power of all classes of our share capital, the exercise price cannot be less than 110% of the fair market value of our ordinary shares on the date of that grant.
Amendment and Termination. Subject to certain exceptions, our board of directors may amend, suspend or terminate the 2006 stock plan at any time and for any reason. No such termination or amendment shall affect any shares previously issued or any option previously granted. Subject to extension by amendment, the 2006 stock plan will terminate automatically 10 years after the later of (i) its adoption by the board of directors or (ii) the most recent increase in the number of shares reserved that was approved by our shareholders.
Employee Participation in the Company Capital: 2010 Equity Incentive Plan
The following is a summary description of our 2010 equity incentive plan, which is subject to and qualified in its entirety by reference to the full text thereof as contained in Exhibit 4.3 to this annual report.
We adopted our 2010 equity incentive plan in March 2010, and it provides for the grant of share options, stock appreciation rights, dividend equivalent rights, shares, restricted shares, and restricted share units, each of which we refer to as “awards”. The purpose of the plan is to attract and retain the best available personnel, to provide additional incentives to employees, directors and consultants and to promote the success of the company’s business. Subject to annual adjustments equal to 15% of new issuances of shares, the maximum aggregate number of shares that may be issued pursuant to awards is 22,500,000 ordinary shares. The 2010 equity incentive plan provides for the grant of awards to our employees, directors, and consultants.
Plan Administration. The 2010 equity incentive plan is administered by the board of directors or the compensation committee of the board of directors. With respect to the grant of awards to employees or consultants who are neither directors nor officers, the board of directors may authorize one or more officers to grant such awards.
Award Agreement. Awards granted under our 2010 equity incentive plan are evidenced by an award agreement that sets forth the terms and conditions for each award grant, which may include, among other things, types of awards; vesting schedule; exercisability; exercise price; repurchase; right of first refusal; forfeiture; transferability of awards; form of payment; payment contingencies; satisfaction of any performance criteria upon termination of employment; and term of award.
Eligibility. We may grant awards to employees, directors, and consultants of us, any parent corporation or subsidiary corporation of ours, or any variable interest entities of them, except that special rules apply to the grant of incentive stock options.
66
Exercise or Purchase Price. The exercise or purchase price of an award is generally established in reference to the fair market value per share on the date of grant.
Amendment and Termination. Our board of directors may at any time terminate, suspend, or amend the 2010 equity incentive plan in any respect, provided that no termination, suspension or amendment may adversely affect any award previously granted. The 2010 equity incentive plan shall continue in effect for a term of ten (10) years unless sooner terminated.
Equity Grants to Directors and Senior Officers
The following table summarizes, as of December 31, 2011, the latest practicable date, options, restricted stock and restricted share units (or RSUs) that we granted to several of our directors and executive officers under our stock plans:
| Number of ADSs | ||||
| to be issued upon | ||||
| exercise of options (or | Per ordinary | |||
| upon settlement or | share | |||
| vesting of restricted | exercise | |||
| shares and RSUs) | price (US$) | Grant date | Date of Expiration | |
| 50,000 | 0.779 | March 20, 2009 | April 1, 2014 | |
| Dr. Jing Lou | 300,000** | n/a | November 9, 2009 | n/a |
| 252,500*** | n/a | April 8, 2010 | n/a | |
| 230,000*** | n/a | March 11, 2011 | n/a | |
| Bin Huang | 12,000 | 0.779 | March 20, 2009 | April 1, 2014 |
| 10,000** | n/a | November 9, 2009 | n/a | |
| 10,000*** | n/a | April 8, 2010 | n/a | |
| 10,000*** | n/a | March 11, 2011 | n/a | |
| * | 0.779 | March 20, 2009 | April 1, 2014 | |
| Bo Tan | * | n/a | November 9, 2009 | n/a |
| * | n/a | April 8, 2010 | n/a | |
| * | n/a | March 11, 2011 | n/a | |
| Lawrence S.Wizel | * | n/a | January 8, 2009 | n/a |
| Mingde Yu | * | n/a | January 8, 2009 | n/a |
| * | n/a | April 8, 2010 | n/a | |
| * | n/a | June 9, 2010 | n/a | |
| Moujia Qi | * | n/a | January 8, 2009 | n/a |
| * | n/a | April 8, 2010 | n/a | |
| * | n/a | June 9, 2010 | n/a | |
| Dongmei Su | * | n/a | November 9, 2009 | n/a |
| * | n/a | April 8, 2010 | n/a | |
| * | n/a | March 11, 2011 | n/a | |
| Ke Li | * | n/a | November 9, 2009 | n/a |
| * | n/a | April 8, 2010 | n/a | |
| * | n/a | March 11, 2011 | n/a |
| * |
Upon exercise, vesting or settlement of all equity grants exercisable or vesting within 60 days of December 31, 2011, such person would beneficially own less than 1% of our outstanding ordinary shares. |
| ** |
RSU grant: RSUs granted in 2009 have a four-year graded vesting period. The RSUs cannot be settled in cash and one RSU will be settled with one ADS upon vesting. There is no other transferability restriction to the RSUs except for the graded vesting schedule. |
| *** |
Restricted stock grant: All restricted stock granted in 2010 and 2011 vest at the end of a four-year vesting period. |
67
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
A. Major Shareholders
The following table sets forth information with respect to beneficial ownership of our ordinary shares as of December 31, 2011, the latest practicable date, by:
-
each of our executive officers and directors; and
-
each person known by us to beneficially own 5% or more of our ordinary shares.
| Ordinary Shares beneficially | ||||||
| owned(1)(2) | ||||||
| Number of Ordinary | ||||||
| Name | Shares | % | ||||
| Directors and executive officers: | ||||||
| Dr. Jing Lou(3) | 5,164,748 | 3.34 | ||||
| Lawrence S. Wizel | * | * | ||||
| Mingde Yu | * | * | ||||
| Bo Tan | * | * | ||||
| Moujia Qi | * | * | ||||
| Bin Huang(4) | 1,267,117 | 0.82 | ||||
| Dr. Dongmei Su | * | * | ||||
| Ke Li | * | * | ||||
| Principal shareholders: | ||||||
| FMR LLC (5) | 16,915,934 | 10.95 | ||||
| Lan’s Holdings Limited(6) | 8,232,219 | 5.33 | ||||
| Samuel D. Isaly(7) | 15,211,700 | 9.85 | ||||
| Dan Lou(8) | 9,834,017 | 6.37 | ||||
| Sectoral Asset Management Inc.(9) | 9,806,391 | 6.35 | ||||
*Upon exercise, vesting or settling of all equity grants exercisable or vesting within 60 days of December 31, 2011 such person beneficially would own less than 1% of our outstanding ordinary shares.
| (1) |
Beneficial ownership is determined in accordance with Rule 13d-3 of the General Rules and Regulations under the Exchange Act, and includes voting or investment power with respect to the ordinary shares. |
| (2) |
In calculating the percentages of beneficial ownership for each listed person, (i) the options exercisable by such person, and (ii) the RSUs or restricted stock awarded to such person that are vested, or will vest, in each case of (i) to (ii) within 60 days of December 31, 2011, shall be treated as exercised, vested, and, or, settled in ordinary shares, as the case may be. |
| (3) |
Dr. Jing Lou's total holdings, as reported here, included 3,886,310 ordinary shares held in trust on his behalf by Achieve Well International Limited, and 1,278,438 ordinary shares underlying equity grants vested as of December 31, 2011. |
| (4) |
Mr. Bin Huang’s holdings, as reported here, included 1,146,745 ordinary shares held indirectly through his wholly- owned company, Known Virtue International Limited, and 85,372 ordinary shares underlying options exercisable by Mr. Bin Huang as well as 35,000 ordinary shares underlying RSUs vested as of December 31, 2011. |
| (5) |
FMR LLC is a Delaware limited liability company. Its business address is at 82 Devonshire Street, Boston, Massachusetts 02109, U.S.A. All the information relating to this shareholder is based on a report on Schedule 13G filed with the Securities and Exchange Commission on February 13, 2012. |
| (6) |
Lan’s Holdings Limited is a British Virgin Islands international business company owned by Mr. Xiaobing Liu (49%) and Ms. Ying Luan (51%), each of whom has both voting and investment power over the ordinary shares reported for this shareholder. Its business address is at Room 22B, No.969 Beijing Road West, Jianan District, Shanghai, PRC. All the information relating to this shareholder is based on a report on Schedule 13G filed with the Securities and Exchange Commission on February 12, 2010. |
| (7) |
Samuel D. Isaly, a U.S. citizen, is the control person with respect to OrbiMed Advisors LLC and OrbiMed Capital LLC, both investment advisors, who together hold a total of 15,211,700 ordinary shares on behalf of certain persons. Their business address is at 601 Lexington Avenue, 54th Floor, New York, New York 10022, U.S.A. All the information relating to this shareholder is based on a report on Schedule 13G filed with the Securities and Exchange Commission on February 14, 2012. |
68
| (8) |
Mr. Dan Lou's total holdings, as reported here, included 9,834,017 ordinary shares held indirectly through his wholly-owned company Hero Grand Management Limited, a British Virgin Islands international business company. Mr. Dan Lou is our former chairman of the board of directors, with business address at our corporate address. |
| (9) |
Sectoral Asset Management Inc., a Canadian corporation, held as the investment advisor on behalf of various investment advisory clients a total of 9,806,391 ordinary shares, of which 9,082,094 ordinary shares were beneficially owned by one of its investment advisory client, Pictet Funds – Generics, a Luxemburg investment company. Jérôme G. Pfund and Michael L. Sjöström, both Swiss citizens, together held the majority of shares of Sectoral Asset Management Inc. The principal business address of Sectoral Asset Management Inc. is 2120-1000 Sherbrooke St., West Montreal, PQ H3A 3G4, Canada. All the information relating to this shareholder is based on a report on Schedule 13G filed with the Securities and Exchange Commission on February 17, 2012. |
The persons listed above do not have different voting rights. We do not have information as to the portion of our securities held in the U.S. and the number of record holders in the U.S. We are not aware of any arrangement that may, at a subsequent date, result in a change of control of our company.
Changes in the Ownership of Major Shareholders:
Except as set forth below, we are not aware of any significant change in the percentage ownership of our outstanding ordinary shares held by any major shareholders during the past three years:
| Percentage Ownership (as of the date of the column) | |||
| Major Shareholders | 12/31/2009 | 12/31/2010 | 12/31/2011 |
| Achieve Well International Limited | 2.58%* | No change | No change |
| Starry Investments Limited | 5.62% | 4.37% | Not reported |
| W&W International Investments Limited | 5.13% | 4.49% | Not reported. |
| FMR LLC | 7.29% | 11.9% | 10.95% |
| Samuel D. Isaly | Not reported | 5.11%** | 9.85% |
| Happyview Finance Ltd. | 8.37% | 5.50% | 4.53% |
| Sectoral Asset Management Inc. | Not reported | Not reported | 6.35% |
*Compared to approximately 16.52% as of December 31, 2008.
**As of 3/31/2011.
Information in the above table is derived from certain reports on Schedule 13G filed with the Securities and Exchange Commission. More information regarding any major shareholder may be found under this Item in our annual report on Form 20-F for any particular year at the end of which such shareholder's percentage ownership was 5% or more and in such reports on Schedule 13G as filed.
B. Related Party Transactions
Pursuant to the Article 121 of our Articles of Association, the audit committee of our board of directors conducts a review of all related party transactions on an ongoing basis and review and approve transactions with potential conflicts of interest. Set forth below is the description of certain related party transactions for the period from the beginning of 2011 up to February 29, 2012, the latest practicable date.
Transactions with Liaoning Sunshine
Sales from Shenyang Sunshine to Liaoning Sunshine were RMB0.6 million, RMB0.4 million and RMB0.4 million (US$0.07 million) for the years ended December 31, 2009, 2010 and 2011, respectively.
69
To enable us to maintain economic and voting control over Liaoning Sunshine, Shenyang Sunshine entered into a series of contractual arrangements with Liaoning Sunshine and its 100% shareholder, Mr. Dan Lou, both of whom are related parties to us, as Mr. Dan Lou is the father of Dr. Jing Lou, our chairman and CEO. As a result, Liaoning Sunshine has been included in our consolidated financial statements as a variable interest entity. These contractual arrangements include:
(1) Business cooperation agreement
Pursuant to the business cooperation agreement among Shenyang Sunshine, Liaoning Sunshine and us, Shenyang Sunshine provides technology support services and market development and consulting services to Liaoning Sunshine, and, as consideration, receives 70% of Liaoning Sunshine’s annual sales revenues as service fees. In addition, Liaoning Sunshine has agreed that, without the prior written consent of a majority of our independent directors, it will not increase or decrease its registered capital, declare dividends or make similar payments, make any investment, incur any indebtedness, mortgage or dispose of its material assets, or consolidate or merge with any other entity.
(2) Purchase agreements for the acquisition of equity interest in Liaoning Sunshine
Pursuant to the purchase agreement between Shenyang Sunshine and Mr. Dan Lou, as amended, Mr. Dan Lou granted Shenyang Sunshine an exclusive right to purchase, to the extent permissible under PRC law, his 100% equity interest in Liaoning Sunshine for a purchase price of RMB13.5 million, immediately after Shenyang Sunshine obtains the requisite PRC government approval for it to acquire Liaoning Sunshine. The full purchase price pursuant to the purchase agreement was prepaid by Shenyang Sunshine to Mr. Lou shortly after the signing of this agreement in December 2006.
(3) Voting agreement
Pursuant to the voting agreement among Shenyang Sunshine, Mr. Dan Lou and Liaoning Sunshine, Mr. Dan Lou is required to consult with and follow any lawful instruction of Shenyang Sunshine whenever he exercises his rights as Liaoning Sunshine’s shareholder.
(4) Equity pledge agreement
Pursuant to the equity pledge agreement between Shenyang Sunshine and Mr. Dan Lou, Mr. Dan Lou pledged all of his equity interests in Liaoning Sunshine to Shenyang Sunshine to guarantee his obligations under the purchase agreement and the voting agreement.
Transactions with Liaoning Sunshine Science and Technology Development Co. Limited
Liaoning Sunshine Science and Technology Development Co. Limited is a related party to us, as it is a wholly-owned subsidiary of Liaoning Sunshine. LSSTD is the principal party in our collaboration with DaVita to provide kidney care services in two Chinese provinces, and Shenyang Sunshine may supply DaVita with anemia management drugs throughout China under a supply agreement that is under negotiation, all as set forth in the Master Agreement (see Exhibit 4.10 attached hereto), to which both we and LSSTD are parties.
Transactions with APGC and Ascentage SH
APGC and Ascentage SH may be deemed related parties to us as we acquired and own a 40% equity interest in each of them pursuant to the agreements with them. Pursuant to the same agreements, we paid RMB17 million to fund the apoptosis related R&D program in 2010. Before entering into the agreements, APGC and Ascentage SH were not related to us.
Management and Compensatory Contracts
See Item 6.C.6 “Employment and Management Agreements” for a description of the employment and management agreements we enter into with our directors and officers.
Equity Grants to Directors and Officers
See Item 6E --- “Equity Grant to Directors and Senior Officers” for a description of share-based compensation awards we have granted to our directors and senior officers.
70
C. Interests of Experts and Counsel
Not Applicable.
71
ITEM 8. FINANCIAL INFORMATION
A. Consolidated Statements and Other Financial Information
Consolidated Financial Statements
See “Item18. Financial Statements”.
Legal Proceedings
We are not aware of any legal or arbitration proceedings, or any governmental proceedings pending or known to be contemplated, including those relating to bankruptcy, receivership or similar proceedings and those involving any third party, which may have, or have had in the recent past, significant effects on the company’s financial position or profitability. Neither are we aware of any material proceeding in which any of our directors, any member of our senior management, or any of our affiliates is either a party adverse to us or any of our subsidiaries or has a material interest adverse to us or any of our subsidiaries.
From time to time, we may be subject to various claims and legal actions arising in the ordinary course of business.
Our intellectual property may be subject to theft and other unauthorized use, and our ability to protect our intellectual property is limited. In addition, we may in the future be subject to claims that we have infringed the intellectual property rights of others.
Please refer to discussions under “Item 3.D Risk Factors” with respect to significant regulatory and legal risks.
Dividend Policy
Since the incorporation of our company in 2006, we have never declared or paid any cash dividends on our ordinary shares. However, if we declare dividends in the future, we may be subject to currency exchange and other restriction. See “3.D.3—Restrictions on currency exchange may limit our ability to receive and use our revenues effectively.”
The timing, amount and form of future dividends, if any, will depend, among other things, on our future results of operations and cash flow, our future prospects, our capital requirements and surplus, the amount of distributions, if any, received by us from our subsidiaries and our affiliated entities, and other factors deemed relevant by our board of directors. Any future dividends on our ordinary shares would be declared by and subject to the discretion of our board of directors.
Holders of ADSs will be entitled to receive dividends, if any, subject to the terms of the deposit agreement, to the same extent as holders of ordinary shares, less the fees and expenses payable under the deposit agreement, and after deduction of any applicable taxes.
B. Significant Changes
Collaboration with DaVita
In March 2012, we entered a collaboration agreement with DaVita Inc. (NYSE: DVA, "DaVita"), a leading provider of kidney care services for those diagnosed with chronic kidney disease, to provide kidney care services in Jilin and Liaoning, two provinces in northeastern China. The total investment is US$20 million with DaVita and us contributing 70% and 30%, respectively. In addition, we and DaVita have also agreed to enter into a supply agreement for us to provide DaVita with anemia management drugs throughout China. This supply agreement and certain other related agreements are still under negotiation. Further, while we believe these arrangements are in compliance with current Chinese law and regulations, and the government policy is promoting foreign capital entering in health service sector, there may be uncertainties, in terms of review, approval and other regulatory requirements, associated with foreign investment in owning, operating and managing dialysis clinics as a new area of regulation in China. Please refer to Item 3.D "Risks Factors" for discussions on relevant risks and uncertainties, such as related to collaboration and strategic arrangements, and the legal and regulatory environment in China.
Except as disclosed above and elsewhere in this annual report, we are not aware of any significant changes since the date of the financial statements included in this annual report.
72
ITEM 9. THE OFFER AND LISTING
A. Offer and Listing Details
Our ADSs have been listed on the NASDAQ Global Market of the NASDAQ Stock Market LLC under the symbol “SSRX” since February 7, 2007. The outstanding ADSs are identified by the CUSIP number 885754105. Each of our ADSs represents seven ordinary shares.
The following table provides the highest and lowest closing prices for our ADSs on the NASDAQ Global Market for (1) the full financial years ended December 31, 2009, 2010, and 2011, (2) each full fiscal quarter of the financial years ended December 31, 2010 and 2011, and (3) each of the most recent six months.
| Closing Price | ||
| Per ADS | ||
| Highest | Lowest | |
| US$ | US$ | |
| Annual | ||
| 2009 | 14.20 | 4.51 |
| 2010 | 16.25 | 10.01 |
| 2011 | 19.99 | 9.60 |
| Quarterly | ||
| First Quarter of 2010 | 13.50 | 10.01 |
| Second Quarter of 2010 | 14.33 | 10.02 |
| Third Quarter of 2010 | 15.05 | 11.31 |
| Fourth Quarter of 2010 | 16.25 | 12.65 |
| First Quarter of 2011 | 17.29 | 14.78 |
| Second Quarter of 2011 | 19.99 | 15.14 |
| Third Quarter of 2011 | 18.69 | 11.93 |
| Fourth Quarter of 2011 | 13.24 | 9.60 |
| First Quarter of 2012 | 15.03 | 9.47 |
| Most recent six months | ||
| October 2011 | 12.48 | 10.31 |
| November 2011 | 13.24 | 11.13 |
| December 2011 | 13.17 | 9.60 |
| January 2012 | 11.84 | 9.47 |
| February 2012 | 12.02 | 10.71 |
| March 2012 | 15.03 | 11.75 |
Source: Bloomberg
73
B. Plan of Distribution
Not applicable.
C. Markets
Our ADSs, each representing seven ordinary shares, have been listed on the NASDAQ since February 7, 2007 under the symbol “SSRX.”
D. Selling Shareholder
Not applicable.
E. Dilution
Not applicable.
F. Expenses of The Issue
Not applicable.
74
ITEM 10. ADDITIONAL INFORMATION
A. Share Capital
Not applicable.
B. Memorandum and Articles of Association
We incorporate by reference into this annual report such information, as required by Item 10.B of Form 20-F, of the description of our amended and restated memorandum and articles of association contained in our F-1 registration statement (File No. 333-140099), as amended, initially filed with the Commission on January 19, 2007, except, that the entirety of the section under the heading “Differences in Corporate Law - Mergers and Similar Arrangements description” shall be replaced by the contents below. Our shareholders adopted our amended and restated memorandum and articles of association by unanimous resolutions on September 5, 2006. See also Item 19 - Exhibit 1.2.
Cayman Islands Law - Mergers and Similar Arrangements
Set forth below is a summary of the provisions of the Companies Law of the Cayman Islands regarding mergers and similar arrangements, as applicable to us, that may be deemed significantly different from the laws applicable to companies incorporated in the United States and their shareholders, and the effects of such Cayman Islands law:
(1) Merger and Consolidation
A merger of two or more constituent companies under Cayman Islands law requires a plan of merger or consolidation (“Plan”) to be approved by the directors of each constituent company and authorization by (a) a special resolution of the members of each constituent company and (b) such other authorization as may be specified in such constituent company’s articles of association.
Shareholders’ approval is not required in a merger between a Cayman Islands parent company and a Cayman Islands subsidiary with at least 90% of its issued shares entitled to vote owned by the parent company.
The consent of each holder of a fixed or floating security interest over a constituent company is required unless this requirement is waived by a court in the Cayman Islands.
The Plan must be filed with the Registrar of Companies together with supporting documents including certain declarations.
A dissenting shareholder of a Cayman Islands constituent company is, following the specified procedures, entitled to payment of the fair value of his shares, as mutually agreed upon or determined by the Cayman Islands court, upon dissenting to a merger or consolidation, except, generally, where such person's shares are listed on a recognized stock exchange or such qualified trading market or the transaction consideration consists of securities of such nature. The exercise of appraisal rights will preclude the exercise of any other rights save for the right to seek relief on the grounds that the merger or consolidation is void or unlawful.
Court approval is not required for a merger or consolidation effected in compliance with these statutory procedures.
(2) Reconstruction and Amalgamation and Takeover Offer
In addition, there are statutory provisions that facilitate the reconstruction and amalgamation of companies, provided that the arrangement is approved by a majority in number of each class of shareholders and creditors with whom the arrangement is to be made, and who must in addition represent three-fourths in value of each such class of shareholders or creditors, as the case may be, that are present and voting either in person or by proxy at a meeting, or meetings, convened for that purpose. The convening of the meetings and subsequently the arrangement must be sanctioned by the Grand Court of the Cayman Islands. While a dissenting shareholder has the right to express to the court the view that the transaction ought not to be approved, the court can be expected to approve the arrangement if it determines that:
-
the statutory provisions as to the required vote have been met;
-
the shareholders have been fairly represented at the meeting in question and the statutory majority are acting bona fide without coercion of the minority to promote interests adverse to those of the class;
-
the arrangement is such that may be reasonably approved by an intelligent and honest man of that class acting in respect of his interest; and
-
the arrangement is not one that would more properly be sanctioned under some other provision of the Companies Law.
75
When a take-over offer is made and accepted by holders of 90.0% of the shares within four months, the offeror may, within a two month period commencing on the expiration of such four month period, require the holders of the remaining shares to transfer such shares on the terms of the offer. An objection can be made to the Grand Court of the Cayman Islands but this is unlikely to succeed in the case of an offer which has been so approved unless there is evidence of fraud, bad faith or breach of the Companies Law.
If an arrangement and reconstruction or take-over offer is approved or accepted, the dissenting shareholder(s) are unlikely to have any rights comparable to appraisal rights, which would otherwise ordinarily be available to dissenting shareholders of United States corporations, providing rights to receive payment in cash for the judicially determined value of the shares.
C. Material Contracts
We have not entered into any material contracts to which we or any of our subsidiaries or affiliated entities is a party for the two years immediately preceding publication of this annual report, other than in the ordinary course of business, or other than those listed in the Exhibits hereto or discussed elsewhere in this annual report.
D. Exchange Controls
There are no exchange control regulations or currency restrictions in the Cayman Islands.
For exchange control in China, please refer to “4.B.11-e Regulation of foreign currency exchange, dividend distribution, and overseas listing”.
E. Taxation
The following summary of certain material Cayman Islands, PRC, and U.S. federal income tax matters with respect to an investment in our ADSs or ordinary shares is based upon laws and relevant interpretations thereof in effect as of the date of this annual report, all of which are subject to change, possibly with retroactive effect. This summary does not deal with all possible tax consequences relating to an investment in our ADSs or ordinary shares, such as the tax consequences under laws of any jurisdictions other than the jurisdictions addressed herein, as well as any provincial, state and local tax laws.
We urge any prospective purchaser or current holder of our ADSs or ordinary shares to consult your own tax advisor regarding your particular circumstances and the U.S. federal income and estate tax consequences to you of acquiring, owning and disposing of ADSs or ordinary shares, as well as any tax consequences arising under the laws of PRC, any state, local or foreign or other tax jurisdiction and the possible effects of changes in U.S. federal or other tax laws.
Cayman Islands Taxation
The Cayman Islands currently levies no taxes on individuals or corporations based upon profits, income, gains or appreciation and there is no taxation in the nature of inheritance tax or estate duty. There are no other taxes likely to be material to us levied by the Government of the Cayman Islands except for stamp duties which may be applicable on instruments executed in, or after execution brought within the jurisdiction of the Cayman Islands.
The Cayman Islands is not party to any double tax treaties that are applicable to any payments made to or by our company.
PRC Taxation
If the PRC tax authorities determine that we are a “resident enterprise” for PRC enterprise income tax purposes, a withholding tax of 10% for certain U.S. shareholder and ADS holders that are enterprises, and, an individual income tax of 20% for individual U.S. shareholders and ADS holders, may be imposed on dividends they receive from us and on gains realized on their sale or other disposition of shares or ADSs that are deemed income derived from sources within the PRC. It is unclear whether then U.S. shareholder and ADS holders might be eligible for the benefits of the income tax treaty between the United States and the PRC.
76
For more information, please see “4.B.11-d PRC Enterprise Income Tax”.
United States Federal Income Tax Considerations for U.S. Persons
The following is a summary of the material United States federal income tax considerations relating to the acquisition, ownership, and disposition of ADSs or ordinary shares by U.S. Holders (as defined below) that will hold their ADSs or ordinary shares as “capital assets” (generally, property held for investment) under the United States Internal Revenue Code (the “Code”). This summary is based upon existing United States federal income tax law, which is subject to differing interpretations or change, possibly with retroactive effect. You should note that no rulings have been or are expected to be sought from the U.S. Internal Revenue Service (the “IRS”), nor have we sought an opinion of counsel, with respect to any U.S. federal income tax matters described below, and we cannot assure you that the IRS or a court will not take contrary positions, which may result in material adverse tax consequences to U.S. Holders.
This summary does not discuss all aspects of United States federal income taxation that may be important to particular investors in light of their individual circumstances, and does not deal with the tax consequences to investors subject to special tax rules (for example, financial institutions, insurance companies, broker-dealers, regulated investment companies, real estate investment trusts, certain U.S. expatriates, and tax-exempt organizations (including private foundations), traders that elect to mark to market, persons liable for alternative minimum tax, governments or agencies or instrumentalities thereof), holders who are not U.S. Holders, holders who own (directly, indirectly, or constructively) 10% or more of our voting stock, investors that will hold ADSs or ordinary shares as part of a straddle, hedge, conversion, constructive sale, or other integrated transaction for United States federal income tax purposes, or U.S. Holders that have a functional currency other than the United States dollar, all of whom may be subject to tax rules that differ significantly from those summarized below.
Please note this description does not address (i) alternative minimum tax consequences or (ii) any other U.S. federal tax (such as estate or gift tax) consequences.
This discussion is not a comprehensive description of all of the U.S. federal tax consequences that may be relevant with respect to the acquisition, ownership and disposition of ADSs or ordinary shares.
The discussion below assumes that the representations contained in the deposit agreement are true and that the obligations in the deposit agreement and any related agreement have been and will be complied with in accordance with the terms. If you hold ADSs, you should be treated as the holder of the underlying ordinary shares represented by those ADSs for U.S. federal income tax purposes.
We urge you to consult your own tax advisor regarding your particular circumstances and the U.S. federal income and estate tax consequences to you of owning and disposing of ADSs or ordinary shares, as well as any tax consequences arising under the laws of any state, local or foreign or other tax jurisdiction and the possible effects of changes in U.S. federal or other tax laws.
U.S. Holder
For purposes of this summary, a “U.S. Holder” is a beneficial owner of ADSs or ordinary shares that is, for United States federal income tax purposes, (i) an individual who is a citizen or resident of the United States; (ii) a corporation or other entity taxable as a corporation for United States federal income tax purposes, created in, or organized under the law of, the United States or any state thereof or the District of Columbia; (iii) an estate, the income of which is includible in gross income for United States federal income tax purposes regardless of its source; or (iv) a trust (A) the administration of which is subject to the primary supervision of a United States court and which has one or more United States persons who have the authority to control all substantial decisions of the trust or (B) that has otherwise validly elected to be treated as a United States person under the Code.
If a partnership or other entity or arrangement classified as a partnership for United States federal income tax purposes is a beneficial owner of our ADSs or ordinary shares, the United States federal income tax treatment of a partner in the partnership will generally depend upon the status of the partner and the activities of the partnership. This summary does not address the tax consequences of any such partner. If you are a partner of a partnership holding shares or ADSs, you should consult your tax advisors.
For United States federal income tax purposes, U.S. Holders of ADSs will be treated as the beneficial owners of the underlying shares represented by the ADSs. Accordingly, deposits or withdrawal of shares for ADSs will not be subject to United States federal income tax.
The U.S. Treasury has expressed concerns that parties to whom American depositary shares are pre-released before shares are delivered to the depositary, or intermediaries in the chain of ownership between holders of American depositary shares and the issuer of the security underlying the American depositary shares, may be taking actions that are inconsistent with the claiming of foreign tax credits by holders of American depositary shares. These actions would also be inconsistent with the claiming of the reduced rate of tax, described below, applicable to dividends received by certain non-corporate holders. Accordingly, the creditability of any PRC taxes, and the availability of the reduced tax rate for dividends received by certain non-corporate U.S. Holders, each described below, could be affected by actions taken by such parties or intermediaries.
77
Threshold PFIC Classification Matters
A non-United States corporation, such as the company, will be treated as a “passive foreign investment company” (a “PFIC”), for United States federal income tax purposes, if 75% or more of its gross income consists of certain types of “passive” income (the “income test”) or 50% or more of its assets (based on an average of the quarterly values during a taxable year) are classified as assets that either produce passive income or are held for the production of passive income (the “asset test”). “Passive income” includes, for example, dividends, interest, certain rents and royalties, certain gains from the sale of stock and securities, and certain gains from commodities transactions.
Certain “look through” rules apply for purposes of the income and asset tests described above. If we own, directly or indirectly, 25% or more of the total value of the outstanding shares of another foreign corporation, we will be treated as if we held directly a proportionate share of the other corporation’s assets and received directly a proportionate share of the other corporation’s income. In applying this rule, however, it is not clear whether the contractual arrangements between us and our affiliated VIE entities will be treated as ownership of stock.
The determination of whether we are, or will become, classified as a PFIC is a fact intensive determination that is made annually based on the composition and amounts of income that we earn and the composition and valuation of our assets, all of which are subject to change. For this purpose, cash is categorized as a passive asset and the company’s unbooked intangibles are taken into account.
We believe we were not a PFIC for U.S. federal income tax purposes for our taxable year ended December 31, 2011. Based on our current income and assets, it is uncertain whether we will be classified as a PFIC for United States federal income tax purposes for our current taxable year ending December 31, 2012, a determination that can only be made after the close of the taxable year, or for our future taxable years. The overall level of our passive assets will be significantly affected by the amount and time-frame within which we deploy the cash raised in our initial public offering, other liquid assets that we presently hold, and the operation cash inflow.
Because the PFIC determination is highly fact intensive, there can be no assurance that we will not be classified as a PFIC for any particular taxable year or that the Internal Revenue Service will not challenge any determination concerning our PFIC status for any particular taxable year. With respect to prior years, the financial market disruptions from late 2008 and early 2009 may have materially depressed our market valuation for the 2008 and 2009 taxable years, and, to our knowledge, there is a lack of guidance from United States authorities regarding how such disruptions should be taken into account in applying the asset test. In particular, it is possible that the disruptions could lead to our being classified as a PFIC under the asset test for the 2008 and 2009 taxable years.
If we are classified as a PFIC for any year during which a U.S. Holder holds our ADSs or ordinary shares, we generally will continue to be treated as a PFIC for all succeeding years during which such U.S. Holder holds our ADSs or ordinary shares.
Furthermore, because PFIC status is a fact intensive determination made on an annual basis, no assurance can be given that we are not or will not become classified as a PFIC and will depend upon, in great measure, the timing of our capital expenditures and the value of our unbooked intangibles.
Notwithstanding our belief as discussed here and these information we provide solely for the convenience of our investors, we are not providing any U.S. tax opinion or advice to U.S. investors concerning the PFIC status of our company, and U.S. investors should consult their own tax advisors concerning the implication of the PFIC rules in his, her or its particular circumstance and determine his, her, or its own tax position as to our PFIC status for a particular taxable year, including, as applicable, for the 2008 and 2009 taxable years.
The discussion below under “Distributions on ADSs or Ordinary Shares” and “Disposition of ADSs or Ordinary Shares” is written on the basis that we will not be classified as a PFIC for United States federal income tax purposes. The discussion further below under “Passive Foreign Investment Company” summarizes the PFIC rules that would be applicable to an investment in our ADSs or ordinary shares if we were to be or become classified as a PFIC.
Distributions on ADSs or Ordinary Shares
We do not currently intend to pay dividends in the foreseeable future. Subject to the PFIC rules discussed below, any actual or constructive distributions paid by the company on ADSs or ordinary shares out of our current or accumulated earnings and profits, as determined under United States federal income tax principles, generally will be includible in the gross income of a U.S. Holder as dividend income on the day such distributions are actually or constructively received. Any distributions in excess of current and accumulated earnings and profits will be treated as a non-taxable return of capital to the extent of the U.S. Holder’s adjusted tax basis in its ADSs or ordinary shares and thereafter as gain from the sale or exchange of a capital asset. However, we do not intend to determine our earnings and profits on the basis of United States federal income tax principles. U.S. Holders should therefore assume that any distribution by the company with respect to the shares or ADSs will constitute dividend income. Dividends received on the ADSs or ordinary shares will not be eligible for the dividends-received deduction to corporate U.S. Holders.
78
With respect to non-corporate U.S. Holders (including individual U.S. Holders) for taxable years beginning before January 1, 2013, dividends may be taxed at the lower applicable capital gains rate (“qualified dividend income”) provided that (1) the ADSs or ordinary shares are readily tradable on an established securities market in the United States or we are eligible for the benefit of any applicable income tax treaty with the United States, (2) we are not a passive foreign investment company (as discussed below) for either our taxable year in which the dividend was paid or the preceding taxable year, (3) certain holding period requirements are met, and (4) such non-corporate U.S. Holders are not under an obligation to make related payments with respect to positions in substantially similar or related property. For this purpose, ADSs listed with the Nasdaq Stock Market LLC will generally be considered to be readily tradable on an established securities market in the United States. You should consult your tax advisor regarding the availability of the lower rate for dividends paid with respect to our ADSs or ordinary shares.
The amount of any distribution paid by us in Renminbi will be determined by the spot rate of exchange on the date when the distribution is actually or constructively received by a U.S. Holder, regardless of whether the Renminbi is actually converted into United States dollars at that time. If the Renminbi received as a dividend distribution are not converted into United States dollars on the date of receipt, a U.S. Holder may realize exchange gain or loss on a subsequent conversion of such Renminbi into United States dollars. The amount of any gain or loss realized in connection with a subsequent conversion will be treated as ordinary income or loss, and generally will be treated as U.S. source income or loss for United States foreign tax credit purposes.
A U.S. Holder may be eligible, subject to a number of complex limitations, to claim a foreign tax credit in respect of any non-U.S. withholding taxes imposed on dividends received on ADSs or ordinary shares. Dividends generally will be treated as income from foreign sources for United States foreign tax credit purposes. The foreign tax credit limitation is calculated separately with respect to specific classes of income. For this purpose, distributions characterized as dividends distributed by us will generally constitute “passive category income” or, in the case of certain U.S. Holders, “general category income.” A U.S. Holder who does not elect to claim a foreign tax credit for such withholding taxes, may instead claim a deduction, for United States federal income tax purposes, in respect of such withholdings, but only for a year in which such holder elects to do so for all creditable foreign income taxes.
If PRC withholding taxes apply to dividends paid to you with respect to the ADSs or ordinary shares, it is unclear whether you may be able to obtain a reduced rate of PRC withholding taxes under the income tax treaty between the United States and the PRC if certain requirements are met.
Dispositions of ADSs or Ordinary Shares
Subject to the PFIC rules discussed below, you generally will recognize taxable gain or loss realized on the sale, exchange, or other taxable disposition of ADSs or ordinary shares equal to the difference between (i) the amount realized on the disposition (i.e., the amount of cash plus the fair market value of any property received), and (ii) your adjusted tax basis in the ADSs or ordinary shares. Such gain or loss will generally be a capital gain or loss. If you are a non-corporate U.S. Holder, including an individual U.S. Holder, who has held the ADS or ordinary share for more than one year, you will generally be eligible for reduced tax rates. The deductibility of capital losses is subject to limitations. Generally, any gain or loss recognized will not give rise to foreign source income for U.S. foreign tax credit purposes.
If we are treated as a “resident enterprise” for PRC tax purposes, it is unclear whether you may be eligible for the benefits of the income tax treaty between the United States and the PRC. If PRC tax were to be imposed on any gain from the disposition of the ADSs or ordinary shares, a U.S. Holder that is eligible for the benefits of the income tax treaty between the United States and the PRC may elect to treat the gain as PRC source income.
A U.S. holder that receives currency other than United States dollar upon the sale, exchange or other taxable disposition of ADSs or ordinary shares will realize an amount equal to the United States dollar value of the foreign currency on the date of sale, exchange or other taxable disposition (or, if ADSs are traded on an established securities market, in the case of cash basis U.S. Holders and electing accrual basis U.S. holders, the settlement date). A U.S. holder will have a tax basis in the foreign currency received equal to the United States dollar amount realized. The amount of any gain or loss realized in connection with a subsequent conversion will be treated as ordinary income or loss, and generally will be treated as U.S. source income or loss for United States foreign tax credit calculation purposes.
You should also consult your own tax advisor regarding the U.S. federal income tax consequences if you receive currency other than U.S. dollars upon the disposition of ADSs or ordinary shares.
79
Passive Foreign Investment Company
If we are or were to become classified as a PFIC for any taxable year during which you hold our ADSs or ordinary shares, unless you make a “mark-to-market” election (as described below), you would be subject to special rules with respect to (i) any gain realized on the sale or other disposition of ADSs or ordinary shares, and (ii) any “excess distribution” made by us on ADSs or ordinary shares (generally, any distributions paid to you in respect of ADSs or ordinary shares during a single taxable year that are greater than 125% of the average annual distributions received by you during the three preceding taxable years or, if shorter, your holding period for such ADSs or ordinary shares).
Under the PFIC rules:
-
the gain or excess distribution would be allocated ratably over your holding period for ADSs or ordinary shares;
-
the amount allocated to the taxable year in which the gain or excess distribution was realized, and any taxable year prior to the first taxable year that you held ADSs or ordinary shares in which we are classified as a PFIC (a “pre-PFIC year”), would be taxable as ordinary income; and
-
the amount allocated to each prior year, other than the current year and any pre-PFIC year, would be subject to tax at the highest tax rate in effect for that year, and an interest charge generally applicable to underpayments of tax would be imposed on the resulting tax for each such year for the period it had been deferred.
In addition, notwithstanding any election you may make, dividends that you receive from us will not be eligible for the preferential tax rates applicable to “qualified dividend income” (as discussed above in “Distributions on ADSs or Ordinary Shares”).
As an alternative to the foregoing rules, a holder of “marketable stock” in a PFIC may make a mark-to-market election, provided that the shares are “regularly traded” on a “qualified exchange,” such as the NASDAQ Stock Market LLC. Stock is “regularly traded” for any calendar year if such stock is traded in other than de minimis quantities on at least 15 days during each calendar quarter. While the company anticipates that the ADSs will qualify as being “regularly traded” on the NASDAQ Stock Market LLC, no assurances may be given that the ADSs will qualify as being regularly traded on such exchange. If you make a valid mark-to-market election, you will generally (i) include as ordinary income for each taxable year the excess, if any, of the fair market value of your ADSs as determined at the end of the taxable year over the adjusted tax basis of such ADSs and (ii) deduct as an ordinary loss the excess, if any, of the adjusted tax basis of your ADSs over the fair market value of such ADSs as determined at the end of the taxable year, but only to the extent of the amount previously included in income as a result of the mark-to-market election. Your adjusted tax basis in your ADSs would be adjusted to reflect any income or loss resulting from the mark-to-market election.
Alternatively, if you make an election to treat the company as a “qualified electing fund” (“QEF Election”) you generally will not be subject to the special rules discussed above. Instead, you will be subject to current U.S. federal income tax on your pro rata share of our ordinary earnings and net capital gain, regardless of whether such amounts are actually distributed to you by us. However, you can make a QEF Election only if we agree to furnish you annually with certain tax information and we currently do not intend to prepare or provide such information.
Under recently enacted legislation, unless otherwise provided by the U.S. Department of the Treasury, each U.S. Holder of a PFIC is required to file an annual report containing such information as the Department of the Treasury may require. Prior to such legislation, a U.S. Holder of a PFIC was required to file IRS Form 8621 only for each taxable year in which such shareholder received distributions from the PFIC, recognized gain on a disposition of the PFIC stock, or made a “reportable election.” U.S. Holders are urged to consult their own tax advisors regarding any reporting requirements related to PFIC status that may apply to them.
You are urged to consult your tax advisor concerning the United States federal income tax consequences of acquiring, holding, and disposing of ADSs or ordinary shares if we are or become classified as a PFIC, including the possibility of making a mark-to-market election.
Information Reporting and Backup Withholding
Generally, information reporting requirements will apply to distributions on ADSs or ordinary shares or proceeds on the disposition of ADSs or ordinary shares paid within the United States (and, in certain cases, outside the United States) to U.S. Holders other than certain exempt recipients, such as corporations. Furthermore, backup withholding may apply to such amounts if the U.S. Holder fails to (i) provide a correct taxpayer identification number, (ii) report interest and dividends required to be shown on its U.S. federal income tax return, or (iii) make other appropriate certifications in the required manner. U.S. Holders who are required to establish their exempt status generally must provide such certification on IRS Form W-9.
Backup withholding is not an additional tax. Amounts withheld as backup withholding from a payment to you may be credited against your U.S. federal income tax liability and you may obtain a refund of any excess amounts withheld by filing the appropriate claim for refund with the IRS and furnishing any required information in a timely manner.
80
An individual U.S. Holder and certain entities may be required to submit to the IRS information with respect to his or her beneficial ownership of the ADSs or ordinary shares, if such ADSs or ordinary shares are not held on his or her behalf by a U.S. financial institution. You are urged to consult your tax advisor regarding the effects, if any, of this requirement on your ownership and disposition of our ADSs or ordinary shares.
F. Dividends and Paying Agents
Not applicable.
G. Statement by Experts
Not applicable.
H. Documents on Display
We have previously filed with the Commission our registration statement on Form F-1, as amended.
We are subject to the periodic reporting and other informational requirements of the Exchange Act. Under the Exchange Act, we are required to file reports and other information with the Securities and Exchange Commission. Specifically, we are required to file annually a report on Form 20-F no later than four months after the close of our fiscal year, which is December 31. Copies of reports and other information, when so filed, may be inspected without charge and may be obtained at prescribed rates at the public reference facilities maintained by the Securities and Exchange Commission at 100 F Street, N.E., Washington, D.C. 20549. The public may obtain information regarding the Public Reference Room by calling the Commission at 1-800-SEC-0330 or (202) 551-8090. The Securities and Exchange Commission also maintains a web site at www.sec.gov that contains reports, proxy and information statements, and other information regarding registrants like us that make electronic filings with the Securities and Exchange Commission using its EDGAR system.
As a foreign private issuer, we are exempt from the rules under the Exchange Act prescribing the furnishing and content of quarterly reports and proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act.
As permitted under NASDAQ Stock Market Rule 5250(d)(1)(C), we post our annual reports filed with the SEC on our web site at http://www.3sbio.com. We will not furnish hard copies of our annual reports to holders of our ordinary shares and ADSs unless we receive a request in writing by a holder. Upon receipt of such a request in writing, we will provide hard copies of our annual reports to the requesting holder free of charge.
81
ITEM 11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Cautionary Statement:
Not to affect the general applicability of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 to this or other parts of this annual report, as more fully discussed in “Cautionary Statement concerning Forward-looking Statements” at the beginning of this annual report, all the information contained under this Item, as provided under Form 20-F Item 11(d), constitutes “forward looking statement” within the meaning of those laws and are subject to various risks and uncertainties, except for information regarding how our primary market risk exposures are managed and certain historical facts.
Interest Rate Risk
Interest-earning instruments carry a degree of interest rate risk. Our exposure to interest rate risk primarily relates to the interest income generated by cash invested in demand and time deposits from our initial public offering proceeds and cash inflow from our operations. We have not used any derivative financial instruments to manage our interest risk exposure. If market interest rates for such deposits decrease in the near future, such decrease may cause the amount of our interest income to fall. A hypothetical 10% decrease in the annual average applicable interest rate in fiscal year 2012 would result in a decrease of approximately RMB1.9 million in interest income on our balance of such demand and time deposits as of December 31, 2011. In comparison, a hypothetical 10% decrease in the annual average applicable interest rate in fiscal year 2011 would have resulted in a decrease of approximately RMB1.3 million in interest income on our balance of such demand and time deposits as of December 31, 2010.
Foreign Currency Risk
On July 21, 2005, the PRC government changed its decade-old policy of pegging the value of the RMB to the U.S. dollar. Under the current policy, the RMB is permitted to fluctuate within a narrow and managed band against a basket of certain foreign currencies. This change in policy has resulted in substantial appreciation of the RMB against the U.S. dollar since then. While the international reaction to the RMB revaluation has generally been positive, there remains significant international pressure on the PRC government to adopt an even more flexible currency policy, which could result in a further and more significant appreciation of the RMB against the U.S. dollar.
Substantially all of our revenue and costs are denominated in RMB, while a significant portion of our financial assets are denominated in U.S. dollars, to which our exposure to foreign exchange risk principally relates. We have not hedged exposures denominated in foreign currencies.
To the extent that we need to convert the balance of U.S. dollars we hold into Renminbi for our operations, fluctuation in the exchange rate between the Renminbi and U.S. dollar would impact on the Renminbi amount we receive from the conversion. Our cash, cash equivalents, restricted cash and time deposits with original maturities over three months denominated in U.S. dollars was US$24.5 million as of December 31, 2011, compared to US$25.8 million as of December 31, 2010. If we had converted such balance from US dollar to RMB at December 31, 2011, the converted amount would have been RMB154.2 million. If RMB were to appreciate by 5% against U.S. dollar over the December 31, 2011 exchange rate, the converted amount would be RMB146.5 million. Please also see “3.D.3 - Exchange rate volatility may adversely affect our competitive position, financial position, operations, or otherwise”.
82
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
Fees and Charges Payable by ADS Holders
The table below summarizes the fees and charges that a holder of our ADSs may have to pay, directly or indirectly, to our ADS depositary, JPMorgan Chase Bank, or JPMorgan, pursuant to the Deposit Agreement, which was filed as Exhibit 99.A to our Registration Statement on Form F-6 filed with the SEC on January 22, 2007, and the types of services and the amount of the fees or charges paid therefor. The contents under this heading “Fees and Charges Payable by ADS Holders” are subject to and qualified in its entirety by reference to the full text of the Deposit Agreement.
| Category | Depositary actions | Associated Fee |
| Depositing or substituting underlying shares | Issuances against or in respect to or pursuant
to: —Deposits of shares —Share distributions, rights and other distributions —Stock dividend or split declared by us —Merger, exchange of securities or any other transaction or event or other distribution affecting the ADSs or the deposited shares |
$5.00 for each 100 ADSs (or portion thereof)
issued or delivered (The Depositary may sell, by public or private sale, sufficient securities and property received in respect of share distributions, rights and other distributions prior to such deposit to pay such charge.) |
| Withdrawing underlying shares | —ADSs surrendered for withdrawal of deposited shares or ADS cancelled or reduced for any other reason | $5.00 for each 100 ADSs (or portion thereof) reduced, cancelled or surrendered |
| Transferring, splitting or grouping receipts | -- Transfers | $1.50 per ADR |
| General depositary services |
— Services performed by the Depositary in administering the ADRs —Conversion of foreign currency into U.S. dollars |
— Up to $0.03 per ADS (or portion thereof) in each calendar year for
services performed by the Depositary in administering the ADRs (which fee
shall be assessed against ADR holders as of the record date or dates set
by the Depositary not more than once each calendar year and shall be
payable at the sole discretion of the Depositary by billing such ADR
holders or by deducting such charge from one or more cash
dividends or other cash distributions). —Expenses of the Depositary in connection with the conversion of
foreign currency into U.S. dollars are paid out of such foreign currency,
|
| Receiving or distributing dividends | -Distribution of cash, shares, rights, and other properties. | -$0.02 or less per ADS for distribution of cash
or net proceeds of sale. -Distribution of shares and securities (treated as shares), the fee being in an amount equal to the fee for the execution and delivery of ADSs which would have been charged as a result of the deposit of shares, $5.00 for each 100 ADSs (or portion thereof). |
In addition, an ADS holder may also be liable for: (i) stock transfer or other taxes and other governmental charges; (ii) cable, telex and facsimile transmission and delivery charges incurred at the request of persons depositing, or ADR holders delivering Shares, ADRs or deposited securities; (iii) transfer or registration fees for the registration or transfer of deposited securities on any applicable register in connection with the deposit or withdrawal of deposited securities.
83
Fees Payable by the Depositary to the Issuer
JPMorgan, as our depositary, has agreed to reimburse us for certain expenses related to our ADS program which we incur each year. For 2011, we received US$0.3 million in reimbursements from JPMorgan, primarily related to accountant fees and investor relations expenses.
84
PART II
ITEM 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
Not Applicable.
ITEM 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
None.
ITEM 15. CONTROLS AND PROCEDURES
Evaluation of disclosure controls and procedures
Our management, with the participation of our chief executive officer and chief financial officer, has performed an evaluation of the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act) as of the end of the period covered by this report, as required by Rule 13a-15(b) under the Exchange Act.
Based upon that evaluation, our management has concluded that, as of December 31, 2011, our disclosure controls and procedures were effective in ensuring that the information required to be disclosed by us in the reports that we file and furnish under the Exchange Act was recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms, and that the information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to our management, including our chief executive officer and chief financial officer, to allow timely decisions regarding required disclosure.
Report of Management on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act, for our company. Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with U.S. GAAP. Included in our internal control over financial reporting are policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP, and that our receipts and expenditures are being made only in accordance with authorizations from our management and directors; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on our financial statements.
As required by Section 404 of the Sarbanes-Oxley Act of 2002 and related rules as promulgated by the Securities and Exchange Commission, our management has assessed the effectiveness of our internal control over financial reporting as of December 31, 2011 based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this assessment, our management has concluded that our internal control over financial reporting was effective as of December 31, 2011.
Because of its inherent limitations, a system of internal control over financial reporting may not prevent or detect all misstatements. In addition, projections of any evaluation of effectiveness of our internal control over financial reporting to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our independent registered public accounting firm, Ernst & Young Hua Ming, has audited the effectiveness of our internal control over financial reporting as of December 31, 2011, as stated in its report, which appears on page F-2 of this annual report on Form 20-F.
Changes in Internal Controls
There were no changes in our internal control over financial reporting identified in connection with the evaluation required by paragraph (d) of Rules 13a-15 and 15d-15 in the Exchange Act that occurred during the period covered by this annual report that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
85
ITEM 16A. AUDIT COMMITTEE FINANCIAL EXPERT
Our board of directors has determined that Mr. Lawrence S. Wizel qualifies as an audit committee financial expert as defined in Item 16A of Form 20-F. Each of the members of our Audit Committee is an “independent director” as defined under Rule 10A-3 of the Securities Exchange Act of 1934 and Rule 5605 of the NASDAQ Stock Market Rules.
ITEM 16B. CODE OF ETHICS
Our board of directors has adopted a Code of Ethics (See Exhibit 11.1), which is applicable to all of our directors, officers and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions.
We have filed our code of ethics as an exhibit to our annual report on Form 20-F for the year ended December 31, 2006 and have posted the code on our website www.3sbio.com. We hereby undertake to provide to any person, without charge, a copy of our code of ethics within ten working days after we receive such person’s written request.
ITEM 16C. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The following table sets forth the aggregate fees by categories specified below in connection with certain professional services rendered by our principal external auditors, for each of the last two fiscal years. We did not pay any other fees to our principal external auditors during such periods.
| Year ended December 31, | |||||||||
| 2010 | 2011 | 2011 | |||||||
| RMB | RMB | US$ | |||||||
| (in thousands) | |||||||||
| Audit Fees(1) | 4,410 | 4,764 | 757 | ||||||
| Audit-related Fees | - | - | - | ||||||
| Tax Fees(2) | - | 260 | 41 | ||||||
| Other Fees | - | - | - | ||||||
(1) “Audit fees” means the aggregate fees billed in each of the
fiscal years listed for professional services rendered by our principal external
auditors for the integrated audit, including the financial audit and the audit
pursuant to Section 404 of the Sarbanes-Oxley Act of 2002;
(2) “Tax fees”
means the aggregate fees billed in each of the fiscal years listed for
professional services rendered by our principal external auditors for tax
compliance, tax advice, and tax planning.
The pre-approval policy and procedures for accounting fees, as described in paragraph (c)(7)(i) of Rule 2-01 of Regulation S-X, are contained in the charter of the audit committee of our board of directors, and summarized below:
-
Prior to formally appointing or re-appointing the principal external auditor, our audit committee reviews and assesses the independence of the principal external auditor, including but not limited to any relationships with us, or any other entity that may impair the principal external auditor’s judgment or independence in respect to us.
-
Our audit committee discusses with the principal external auditor the overall scope of the external audit, including identified risk areas and any additional agreed-upon procedures.
-
Our audit committee reviews the principal external auditor’s compensation to ensure that an effective, comprehensive and complete audit can be conducted for the agreed compensation level.
All non-audit services were pre-approved by our audit committee.
ITEM 16D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
None.
86
ITEM 16E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
None.
ITEM 16F. CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT
Not applicable.
ITEM 16G. CORPORATE GOVERNANCE
As permitted by NASDAQ, in lieu of the NASDAQ corporate governance rules, but subject to certain exceptions, we may follow home country practice.
Composition of the Nominating Committee
The nominating committee of our board of directors currently has one member that is not an independent director within the meaning of Rule 5605(a)(2) of the NASDAQ Marketplace Rules, as amended from time to time. This practice of ours differs from Rule 5605(e) of the NASDAQ Marketplace Rules. There is no specific requirement under Cayman Islands law on a nominating committee of the board of directors comprised solely of independent directors.
Shareholder Approval of Equity Compensation Arrangements
We are not required to obtain shareholder approval when a stock option or purchase plan is to be established or materially amended or other equity compensation arrangement made or materially amended, pursuant to which stock may be acquired by officers, directors, employees, or consultants. This practice of ours differs from Rule 5635(c) of the NASDAQ Marketplace Rules. There is no specific requirement under Cayman Islands law on shareholder approval with respect to equity compensation arrangements. The amendment to our 2006 stock plan and the establishment of our 2010 equity incentive plan were adopted by our board of directors without shareholder approval.
ITEM 16H. MINE SAFETY DISCLOSURE
Not applicable.
87
PART III
ITEM 17. FINANCIAL STATEMENTS
The registrant has elected to provide the consolidated financial statements and related information specified in Item 18 in lieu of Item 17.
ITEM 18. FINANCIAL STATEMENTS
The audited consolidated financial statements and the reports of our principal external auditors are included in this annual report beginning on page F-1.
ITEM 19. EXHIBITS
| Exhibit Number | Description of Exhibits | |
| 1.1 | Amended and Restated Memorandum of Association of 3SBio Inc. (incorporated by reference to Exhibit 3.1 from our F- 1 registration statement (File No. 333-140099), as amended, initially filed with the Commission on January 19, 2007) | |
| 1.2 | Amended and Restated Articles of Association of 3SBio Inc. (incorporated by reference to Exhibit 3.2 from our F-1 registration statement (File No. 333-140099), as amended, initially filed with the Commission on January 19, 2007, except that Article 61(2) shall now read:“At any general meeting of the Company, two or more Members entitled to vote and present in person or by proxy or, in the case of a Member being a corporation, by its duly authorised representative representing not less than one-third in nominal value of the total issued voting shares in the Company throughout the meeting shall form a quorum for all purposes.” The amendment to Article 61(2) was approved at the 2010 Annual General Meeting by a special resolution on October 29, 2010.) | |
| 2.1 | Form of Share Certificate of 3SBio Inc. (incorporated by reference to Exhibit 4.1 from our F-1 registration statement (File No. 333-140099), as amended, initially filed with the Commission on January 19, 2007) | |
| 2.2 | Form of Deposit Agreement, including Form of ADR, of 3SBio Inc. (incorporated by reference to Exhibit 4.2 from our F-1 registration statement (File No. 333-140099), as amended, initially filed with the Commission on January 19, 2007) | |
| 4.1 | 2006 Stock Plan adopted by 3SBio Inc., dated as of September 5, 2006 (incorporated by reference to Exhibit 10.1 from our F-1 registration statement (File No. 333-140099), as amended, initially filed with the Commission on January 19, 2007) | |
| 4.2 | First Amendment to 2006 Stock Plan, effective April 8, 2010 (incorporated by reference to Exhibit 4.2 from our annual report on Form 20-F for the year ended December 31, 2009, filed with the Commission on June 25, 2010) | |
| 4.3 | 2010 Equity Incentive Plan, effective April 8, 2010 (incorporated by reference to Exhibit 4.3 from our annual report on Form 20-F for the year ended December 31, 2009, filed with the Commission on June 25, 2010) | |
| 4.4 | Form Purchase Contract for FCS between Shenyang Sunshine Pharmaceutical Company Limited and Shanghai Weike Biochemical Reagent Co., Ltd. (incorporated by reference to Exhibit 4.2 from our annual report on Form 20-F for the year ended December 31, 2008, filed with the Commission on April 21, 2009) | |
| 4.5 | Form Purchase Agreement for BPT-6 culture medium between Shenyang Sunshine Pharmaceutical Company Limited and Invitrogen Trading (Shanghai) Company Limited (incorporated by reference to Exhibit 4.5 from our annual report on Form 20-F for the year ended December 31, 2009, filed with the Commission on June 25, 2010) | |
| 4.6 | Form of Distribution Agreement (incorporated by reference to Exhibit 10.15 from our F-1 registration statement (File No. 333-140099), as amended, initially filed with the Commission on January 19, 2007) | |
| 4.7 | Sanitary Piping System Contract between Shenyang Sunshine Pharmaceutical Company Limited and Shanghai Macroprocess Technology Co. Ltd., dated March30, 2009 (incorporated by reference to Exhibit 4.7 from Amendment No. 1 to our annual report on Form 20-F for the year ended December 31, 2009, filed with the Commission on November 3, 2011) | |
| 4.8 | Installation Contract for the Core Cleanroom Area of the New Plant between Shenyang Sunshine Pharmaceutical Company Limited, and Suntec Cleanroom & HVAC Engineering Co. Ltd. and Suntec (Suzhou) Cleanroom System Co. Ltd., dated July 3, 2009 (incorporated by reference to Exhibit 4.8 from Amendments No. 2 and No. 3 to our annual report on Form 20-F for the year ended December 31, 2009, filed with the Commission on November 3, 2011 ) | |
| 4.9* | Partnership Agreement among Shenyang Sunshine Pharmaceutical Co., Limited, Taizhou Huan Sheng Investment Management Company Limited, and Taizhou Oriental CMC Limited, dated April 25, 2011 |
| 4.10* | Master Agreement among DaVita Care PTE. LTD., DaVita China PTE. LTD., 3SBio, Inc., and Liaoning Sunshine Science and Technology Development Co. Limited, made as of February 21, 2012 (portions of this exhibit have been omitted and filed separately with the Commission pursuant to a request for confidential treatment) |
88
| Exhibit Number | Description of Exhibits | |
| 8.1* | List of Subsidiaries of 3SBio Inc. | |
| 11.1 | Code of Ethics (incorporated by reference to Exhibit 11.1 from our annual report on Form 20-F for the year ended December 31, 2006, filed with the Commission on June 29, 2007) | |
| 12.1* | CEO Certification Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 12.2* | CFO Certification Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 13.1* | CEO Certification Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 13.2* | CFO Certification Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 15.1* | Consent of Ernst & Young Hua Ming |
* Filed herewith.
89
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
3SBio Inc.
By: /s/ Dr. Jing
Lou
Name: Dr. Jing Lou
Title: Chief Executive Officer
Date: April 30, 2012
INDEX TO THE CONSOLIDATED FINANCIAL STATEMENTS
| Consolidated Financial Statements of 3SBio Inc. | Page |
| Reports of Independent Registered Public Accounting Firm | F-1 |
| Consolidated Balance Sheets as of December 31, 2010 and 2011 | F-3 |
| Consolidated Statements of Income for the years ended December 31, 2009, 2010 and 2011 | F-4 |
| Consolidated Statements of Comprehensive Income for the years ended December 31, 2009, 2010 and 2011 | F-5 |
| Consolidated Statements of Shareholders’ Equity for the years ended December 31, 2009, 2010 and 2011 | F-6 |
| Consolidated Statements of Cash Flows for the years ended December 31, 2009, 2010 and 2011 | F-7 |
| Notes to Consolidated Financial Statements | F-8 |
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of 3SBio Inc.
We have audited the accompanying consolidated balance sheets of 3SBio Inc. as of December 31, 2011 and 2010, and the related consolidated statements of income, comprehensive income, shareholders’ equity, and cash flows for each of the three years in the period ended December 31, 2011. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of 3SBio Inc. at December 31, 2011 and 2010, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2011, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), 3SBio Inc.’s internal control over financial reporting as of December 31, 2011, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated April 30, 2012 expressed an unqualified opinion thereon.
/s/ Ernst & Young Hua Ming
Beijing, People’s Republic
of China
April 30, 2012
F-1
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of 3SBio Inc.
We have audited 3SBio Inc.’s internal control over financial reporting as of December 31, 2011, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). 3SBio Inc.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, 3SBio Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2011, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of 3SBio Inc. as of December 31, 2011 and 2010, and the related consolidated statements of income, comprehensive income, shareholders’ equity and cash flows for each of the three years in the period ended December 31, 2011 of 3SBio Inc. and our report dated April 30, 2012 expressed an unqualified opinion thereon.
/s/ Ernst & Young Hua Ming
Beijing, People’s Republic
of China
April 30, 2012
F-2
| 3SBio Inc. |
| Consolidated Balance Sheets |
| At December 31, 2010 and 2011 |
| (RMB and US$ expressed in thousands, except share data) |
|
|
December 31, | |||||||||||
| Note | ||||||||||||
|
|
2010 | 2011 | 2011 | |||||||||
|
|
RMB | RMB | US$ | |||||||||
|
ASSETS |
||||||||||||
|
Current assets |
||||||||||||
|
Cash and cash equivalents |
153,250 | 245,813 | 39,056 | |||||||||
|
Restricted cash |
1,662 | 665 | 106 | |||||||||
|
Time deposits with financial institutions |
378,405 | 499,201 | 79,315 | |||||||||
|
Accounts receivable, less allowance for doubtful accounts: |
3 | 78,500 | 113,949 | 18,105 | ||||||||
|
December 31, 2010: RMB2,663; December 31, 2011: RMB2,542 (US$404) |
||||||||||||
|
Notes receivable |
55,646 | 47,243 | 7,506 | |||||||||
|
Inventories |
4 | 21,718 | 27,604 | 4,386 | ||||||||
|
Prepaid expenses and other receivables |
5 | 39,390 | 26,672 | 4,238 | ||||||||
|
Available-for-sale securities |
6 | 50,667 | 22,830 | 3,627 | ||||||||
|
Prepayment to related parties |
21 | 12,000 | 6,000 | 953 | ||||||||
|
Deferred tax assets |
17(a) | 2,198 | 2,750 | 437 | ||||||||
|
Total current assets |
793,436 | 992,727 | 157,729 | |||||||||
|
|
||||||||||||
|
Time deposits with financial institutions |
120,000 | 20,000 | 3,178 | |||||||||
|
Available-for-sale securities |
6 | 12,697 | 10,848 | 1,724 | ||||||||
|
Investment in non-consolidated affiliates |
7 | 3,835 | 2,245 | 357 | ||||||||
|
Property, plant and equipment, net |
8 | 199,456 | 198,053 | 31,467 | ||||||||
|
Prepaid land use rights |
9 | 8,188 | 17,448 | 2,772 | ||||||||
|
Prepayment and non-current deposits |
10 | 1,555 | 16,801 | 2,669 | ||||||||
|
Intangible assets, net |
11 | 44,299 | 49,615 | 7,883 | ||||||||
|
Long-term receivables |
19 | 2,558 | 3,111 | 494 | ||||||||
|
Deferred tax assets |
17(a) | 373 | 262 | 42 | ||||||||
|
Total assets |
1,186,397 | 1,311,110 | 208,315 | |||||||||
|
|
||||||||||||
|
LIABILITIES AND SHAREHOLDERS’ EQUITY |
||||||||||||
|
|
||||||||||||
|
Current liabilities |
||||||||||||
|
|
||||||||||||
|
Accounts payable |
5,030 | 6,218 | 988 | |||||||||
|
Deferred grant income |
12 | 1,374 | 374 | 59 | ||||||||
|
Accrued expenses and other payables |
13 | 39,552 | 48,389 | 7,689 | ||||||||
|
Income tax payable |
1,986 | 8,894 | 1,413 | |||||||||
|
|
||||||||||||
|
Total current liabilities |
47,942 | 63,875 | 10,149 | |||||||||
|
|
||||||||||||
|
Deferred grant income |
12 | 2,402 | 2,029 | 322 | ||||||||
|
|
||||||||||||
|
Total liabilities |
50,344 | 65,904 | 10,471 | |||||||||
|
|
||||||||||||
|
Commitments and contingencies |
22 | |||||||||||
|
|
||||||||||||
|
Shareholders’ equity |
||||||||||||
|
Share capital – ordinary shares US$0.0001 par value, 500,000,000 shares authorized, |
14 | 123 | 124 | 20 | ||||||||
|
152,654,148 and 154,473,159 issued and outstanding as of December 31, 2010 and 2011 respectively |
||||||||||||
|
Additional paid-in capital |
14 | 946,717 | 973,218 | 154,629 | ||||||||
|
Accumulated other comprehensive loss |
23 | (89,531 | ) | (126,290 | ) | (20,065 | ) | |||||
|
Retained earnings |
278,744 | 387,317 | 61,538 | |||||||||
|
Total shareholders’ equity attributable to 3SBio Inc. |
1,136,053 | 1,234,369 | 196,122 | |||||||||
|
Non-controlling interest |
- | 10,837 | 1,722 | |||||||||
|
Total shareholders’ equity |
1,136,053 | 1,245,206 | 197,844 | |||||||||
|
|
||||||||||||
|
Total liabilities and shareholders’ equity |
1,186,397 | 1,311,110 | 208,315 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
| 3SBio Inc. |
| Consolidated Statements of Income |
| For the years ended December 31, 2009, 2010 and 2011 |
| (RMB and US$ expressed in thousands, except share data) |
| Year ended December 31, | |||||||||||||||
| Note | 2009 | 2010 | 2011 | 2011 | |||||||||||
| RMB | RMB | RMB | US$ | ||||||||||||
|
Net revenue |
15 | 316,920 | 418,628 | 541,614 | 86,054 | ||||||||||
|
Cost of revenue |
(25,236 | ) | (41,650 | ) | (58,073 | ) | (9,227 | ) | |||||||
|
Gross profit |
291,684 | 376,978 | 483,541 | 76,827 | |||||||||||
|
|
|||||||||||||||
|
Operating expenses |
|||||||||||||||
|
|
|||||||||||||||
|
Research and development costs |
(19,427 | ) | (39,409 | ) | (41,805 | ) | (6,642 | ) | |||||||
|
Sales, marketing and distribution expenses |
(151,679 | ) | (194,877 | ) | (254,767 | ) | (40,478 | ) | |||||||
|
General and administrative expenses |
(36,195 | ) | (55,850 | ) | (66,908 | ) | (10,631 | ) | |||||||
|
Grant income |
12 | 674 | 1,256 | 1,585 | 252 | ||||||||||
|
Income from operations |
85,057 | 88,098 | 121,646 | 19,328 | |||||||||||
|
|
|||||||||||||||
|
Interest income |
11,532 | 12,950 | 18,499 | 2,939 | |||||||||||
|
Net realized gain on available-for-sale securities |
6(a) | 1,611 | - | - | - | ||||||||||
|
Impairment loss on available-for-sale securities |
6(b) | (4,624 | ) | - | (1,334 | ) | (212 | ) | |||||||
|
Share of gain/(loss) in non-consolidated affiliates |
7 | - | 704 | (1,590 | ) | (253 | ) | ||||||||
|
Other income/(loss), net |
20 | 1,595 | 1,306 | (851 | ) | (135 | ) | ||||||||
|
Total other income, net |
10,114 | 14,960 | 14,724 | 2,339 | |||||||||||
|
Income before income tax expense |
95,171 | 103,058 | 136,370 | 21,667 | |||||||||||
|
Income tax expense |
17(a) | (11,736 | ) | (21,772 | ) | (28,210 | ) | (4,482 | ) | ||||||
|
Net income |
83,435 | 81,286 | 108,160 | 17,185 | |||||||||||
|
Less: net loss attributable to non-controlling interest, net of tax |
- | - | (413 | ) | (66 | ) | |||||||||
|
|
|||||||||||||||
|
Net income attributable to 3SBio Inc. |
83,435 | 81,286 | 108,573 | 17,251 | |||||||||||
|
|
|||||||||||||||
|
Net income attributable to 3SBio Inc. per share: |
|||||||||||||||
|
- Basic |
0.55 | 0.54 | 0.71 | 0.11 | |||||||||||
|
- Diluted |
0.55 | 0.53 | 0.69 | 0.11 | |||||||||||
|
|
|||||||||||||||
|
Weighted average shares: |
|||||||||||||||
|
- Basic |
150,606,317 | 151,241,036 | 153,310,128 | 153,310,128 | |||||||||||
|
- Diluted |
151,034,192 | 154,131,768 | 157,148,685 | 157,148,685 | |||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
| 3SBio Inc. |
| Consolidated Statements of Comprehensive Income |
| For the years ended December 31, 2009, 2010 and 2011 |
| (RMB and US$ expressed in thousands, except share data) |
|
|
Year ended December 31, | ||||||||||||||
|
|
Note | 2009 | 2010 | 2011 | 2011 | ||||||||||
|
|
RMB | RMB | RMB | US$ | |||||||||||
|
|
|||||||||||||||
|
Net income |
83,435 | 81,286 | 108,160 | 17,185 | |||||||||||
|
|
|||||||||||||||
|
Other comprehensive income/(loss), net of tax: |
|||||||||||||||
|
Net unrealized gain/(loss) in available-for-sale securities, net of nil tax |
23 | 1,205 | 21,335 | (26,545 | ) | (4,218 | ) | ||||||||
|
Foreign currency translation adjustments, net of nil tax |
23 | 313 | (10,258 | ) | (10,214 | ) | (1,623 | ) | |||||||
|
|
23 | 1,518 | 11,077 | (36,759 | ) | (5,841 | ) | ||||||||
|
Comprehensive income |
84,953 | 92,363 | 71,401 | 11,344 | |||||||||||
|
|
|||||||||||||||
|
Less: comprehensive loss attributable to non-controlling interest |
- | - | (413 | ) | (66 | ) | |||||||||
|
Comprehensive income attributable to 3SBio Inc. |
84,953 | 92,363 | 71,814 | 11,410 | |||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
| 3SBio Inc. |
| Consolidated Statements of Shareholders’ Equity |
| For the years ended December 31, 2009, 2010 and 2011 |
| (RMB and US$ expressed in thousands, except share data) |
| Additional | Accumulated | Non- | Total share- | |||||||||||||||||||||
| Share capital | paid-in | other | Retained | controlling | holders’ | |||||||||||||||||||
| Note | No. of shares | Amount | capital | comprehen- | earnings | interest | equity | |||||||||||||||||
| sive loss | ||||||||||||||||||||||||
| RMB | RMB | RMB | RMB | RMB | RMB | |||||||||||||||||||
|
Balance as of January 1, 2009 |
150,575,955 | 121 | 908,377 | (102,126 | ) | 114,023 | - | 920,395 | ||||||||||||||||
|
Share-based compensation, net of nil tax |
||||||||||||||||||||||||
|
-Shares issued upon vesting of shares/RSUs granted to independent directors and executives |
18(a) | 48,734 | - | 3,642 | - | - | - | 3,642 | ||||||||||||||||
|
-Share options granted to executives and employees |
18(b) | - | - | 3,116 | - | - | - | 3,116 | ||||||||||||||||
|
-Shares issued pursuant to exercise of vested options |
18(b) | 16,772 | - | 132 | - | - | - | 132 | ||||||||||||||||
|
Net income |
- | - | - | - | 83,435 | - | 83,435 | |||||||||||||||||
|
Net unrealized loss on available-for-sale securities, net of nil tax |
6(b) | - | - | - | 1,205 | - | - | 1,205 | ||||||||||||||||
|
Foreign currency translation adjustments, net of nil tax |
- | - | - | 313 | - | - | 313 | |||||||||||||||||
|
Balance as of December 31, 2009 |
150,641,461 | 121 | 915,267 | (100,608 | ) | 197,458 | - | 1,012,238 | ||||||||||||||||
|
Share-based compensation, net of nil tax |
||||||||||||||||||||||||
|
-Shares issued upon vesting of shares/RSUs granted to independent directors and executives |
18(a) | 1,103,499 | 1 | 22,538 | - | - | - | 22,539 | ||||||||||||||||
|
-Share options granted to executives and employees |
18(b) | - | - | 2,149 | - | - | - | 2,149 | ||||||||||||||||
|
-Shares issued pursuant to exercise of vested options |
18(b) | 909,188 | 1 | 6,763 | - | - | - | 6,764 | ||||||||||||||||
|
Net income |
- | - | - | - | 81,286 | - | 81,286 | |||||||||||||||||
|
Net unrealized gain on available-for-sale securities, net of nil tax |
6(b) | - | - | - | 21,335 | - | - | 21,335 | ||||||||||||||||
|
Foreign currency translation adjustments, net of nil tax |
- | - | - | (10,258 | ) | - | - | (10,258 | ) | |||||||||||||||
|
Balance as of December 31, 2010 |
152,654,148 | 123 | 946,717 | (89,531 | ) | 278,744 | - | 1,136,053 | ||||||||||||||||
|
Share-based compensation, net of nil tax |
||||||||||||||||||||||||
|
-Shares issued upon vesting of shares/RSUs granted to independent directors and executives |
18(a) | 1,062,220 | 1 | 21,128 | - | - | - | 21,129 | ||||||||||||||||
|
-Share options granted to executives and employees |
18(b) | - | - | 660 | - | - | - | 660 | ||||||||||||||||
|
-Shares issued pursuant to exercise of vested options |
18(b) | 756,791 | - | 4,713 | - | - | - | 4,713 | ||||||||||||||||
|
Capital injection from non-controlling interest shareholder |
- | - | - | - | - | 11,250 | 11,250 | |||||||||||||||||
|
Net income attributable to 3SBio Inc. |
- | - | - | - | 108,573 | - | 108,573 | |||||||||||||||||
|
Net income attributable to non-controlling interest |
- | - | - | - | - | (413 | ) | (413 | ) | |||||||||||||||
|
Net unrealized loss on available-for-sale securities, net of nil tax |
6(b) | - | - | - | (26,545 | ) | - | - | (26,545 | ) | ||||||||||||||
|
Foreign currency translation adjustments, net of nil tax |
- | - | - | (10,214 | ) | - | - | (10,214 | ) | |||||||||||||||
|
Balance as of December 31, 2011 |
154,473,159 | 124 | 973,218 | (126,290 | ) | 387,317 | 10,837 | 1,245,206 | ||||||||||||||||
|
Balance as of December 31, 2011 (US$) |
20 | 154,629 | (20,065 | ) | 61,538 | 1,722 | 197,844 | |||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
| 3SBio Inc. |
| Consolidated Statements of Cash Flows |
| For the years ended December 31, 2009, 2010 and 2011 |
| (RMB and US$ expressed in thousands) |
|
|
Year ended December 31, | ||||||||||||||
|
|
Note | 2009 | 2010 | 2011 | 2011 | ||||||||||
|
|
RMB | RMB | RMB | US$ | |||||||||||
|
Cash flow from operating activities |
|||||||||||||||
|
Net income |
83,435 | 81,286 | 108,573 | 17,251 | |||||||||||
|
Adjustments to reconcile net income to net cash provided by operating activities: |
|||||||||||||||
|
Loss/(gain) on disposal of property, plant and equipment |
402 | (371 | ) | 2,226 | 354 | ||||||||||
|
Allowance for/(reversal of) doubtful accounts |
(1,588 | ) | 624 | 71 | 11 | ||||||||||
|
Depreciation of property, plant and equipment |
8 | 7,009 | 13,906 | 20,002 | 3,178 | ||||||||||
|
Amortization of prepaid land use rights |
9 | 353 | 353 | 369 | 59 | ||||||||||
|
Amortization of intangible assets |
11 | 1,100 | 1,103 | 2,781 | 442 | ||||||||||
|
Amortization of deferred grant income |
12 | (374 | ) | (374 | ) | (1,374 | ) | (218 | ) | ||||||
|
Share-based compensation expenses |
18(c) | 6,560 | 24,498 | 21,193 | 3,367 | ||||||||||
|
Non-controlling interest |
- | - | (413 | ) | (66 | ) | |||||||||
|
Realized gain on available-for-sale securities |
6(a) | (1,611 | ) | - | - | - | |||||||||
|
Impairment of available-for-sale securities |
6(b) | 4,624 | - | 1,334 | 212 | ||||||||||
|
Foreign currency exchange (gain)/loss, net |
(596 | ) | 249 | (176 | ) | (28 | ) | ||||||||
|
Deferred income tax expense/(benefit) |
17(a) | (1,591 | ) | 1,075 | (441 | ) | (70 | ) | |||||||
|
Share of (gain)/loss in non-consolidated affiliates |
7 | - | (704 | ) | 1,590 | 253 | |||||||||
|
Changes in operating assets and liabilities: |
|||||||||||||||
|
Accounts receivable |
(4,146 | ) | (23,605 | ) | (35,560 | ) | (5,651 | ) | |||||||
|
Notes receivable |
(6,425 | ) | (24,381 | ) | 8,403 | 1,335 | |||||||||
|
Inventories |
(7,658 | ) | (6,312 | ) | (5,609 | ) | (891 | ) | |||||||
|
Prepaid expenses and other receivables |
(459 | ) | (5,265 | ) | (11,529 | ) | (1,832 | ) | |||||||
|
Prepayment and non-current deposits |
- | - | 16 | 3 | |||||||||||
|
Accounts payable |
797 | 2,294 | 1,188 | 189 | |||||||||||
|
Accrued expenses and other payables |
8,148 | 4,026 | 12,072 | 1,918 | |||||||||||
|
Income tax payable |
658 | 72 | 6,908 | 1,098 | |||||||||||
|
Long-term receivables |
- | 659 | (513 | ) | (82 | ) | |||||||||
|
Deferred grant income |
- | 1,000 | - | - | |||||||||||
|
Prepayment to related parties |
- | (12,000 | ) | 6,000 | 953 | ||||||||||
|
Net cash provided by operating activities |
88,638 | 58,133 | 137,111 | 21,785 | |||||||||||
|
|
|||||||||||||||
|
Cash flow from investing activities |
|||||||||||||||
|
Purchase of property, plant and equipment |
(95,802 | ) | (40,821 | ) | (34,883 | ) | (5,542 | ) | |||||||
|
Proceeds from disposal of property, plant and equipment |
111 | - | 14 | 2 | |||||||||||
|
Purchase of available-for-sale securities |
(3,475 | ) | (31,951 | ) | - | - | |||||||||
|
Proceeds from sale of available-for-sale securities |
16,995 | - | - | - | |||||||||||
|
Issuance of short-term loan |
- | (26,400 | ) | (2,000 | ) | (318 | ) | ||||||||
|
Repayment of short-term loan |
- | - | 26,400 | 4,195 | |||||||||||
|
Purchase of intangible assets |
11 | - | (41,277 | ) | (8,097 | ) | (1,286 | ) | |||||||
|
Prepaid land use rights |
9 | - | - | (9,826 | ) | (1,561 | ) | ||||||||
|
Purchase of time deposits |
(727,355 | ) | (463,405 | ) | (399,201 | ) | (63,427 | ) | |||||||
|
Proceeds from maturity of time deposits |
552,712 | 433,451 | 378,405 | 60,122 | |||||||||||
|
Restricted cash for investment in a subsidiary |
(9,300 | ) | (1,000 | ) | - | - | |||||||||
|
Release of restricted cash for investment in a subsidiary |
- | 9,300 | 1,000 | 159 | |||||||||||
|
Investment in non-consolidated affiliates |
- | (3,131 | ) | - | - | ||||||||||
|
Prepayment for investment |
- | - | (4,134 | ) | (657 | ) | |||||||||
|
Net cash used in investing activities |
(266,114 | ) | (165,234 | ) | (52,322 | ) | (8,313 | ) | |||||||
|
|
|||||||||||||||
|
Cash flow from financing activities |
|||||||||||||||
|
Gross proceeds from exercise of stock options |
132 | 6,763 | 4,713 | 749 | |||||||||||
|
Proceeds from investment by non-controlling interest |
- | - | 11,250 | 1,787 | |||||||||||
|
Net cash provided by financing activities |
132 | 6,763 | 15,963 | 2,536 | |||||||||||
|
|
|||||||||||||||
|
Effect of foreign currency exchange rate change on cash |
874 | (9,179 | ) | (8,189 | ) | (1,301 | ) | ||||||||
|
Net (decrease)/ increase in cash and cash equivalents |
(176,470 | ) | (109,517 | ) | 92,563 | 14,707 | |||||||||
|
|
|||||||||||||||
|
Cash and cash equivalents, at beginning of year |
439,237 | 262,767 | 153,250 | 24,349 | |||||||||||
|
Cash and cash equivalents, at end of year |
262,767 | 153,250 | 245,813 | 39,056 | |||||||||||
|
Supplemental disclosures of cash flow information: |
|||||||||||||||
|
Income taxes paid |
(12,669 | ) | (20,279 | ) | (21,264 | ) | (3,379 | ) | |||||||
|
Non-cash transactions: |
|||||||||||||||
|
Acquisition of property, plant and equipment included in accrued expenses and other payables |
(5,385 | ) | (6,923 | ) | (3,680 | ) | (585 | ) | |||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
1. Principal activities, organization and basis of presentation
Principal activities
3SBio Inc. (the “Company”) and subsidiaries (collectively the “Group”) are principally engaged in the research, development, manufacture and distribution of pharmaceutical products in the People’s Republic of China (the “PRC”). The Group currently manufactures and/or distributes five types of biopharmaceutical products, which include the following:
- EPIAO, an injectable recombinant human erythropoietin that is used to stimulate the production of red blood cells in patients with anemia and to reduce the need for blood transfusions;
- TPIAO, a recombinant human thrombopoietin indicated for the treatment of chemotherapy- induced thrombocytopenia;
- Two legacy products, including Intefen, a recombinant interferon alpha-2a product indicated for the treatment of carcinoma of the lymphatic or hematopoietic system and viral infectious diseases; and Inleusin, a recombinant human IL-2 product indicated for the treatment of renal cell carcinoma, metastatic melanoma, thoratic fluid build-up caused by cancer and tuberculosis; and
- Iron Sucrose Supplement (“Iron”), an intravenously administered prescription drug that is designed to treat anemia associated with iron deficiency, is indicated for patients with end-stage renal disease requiring iron replacement therapy.
Organization
The Company was incorporated in the Cayman Islands in August 2006 under the Cayman Islands Companies Law as an exempted company with limited liability.
On February 7, 2007, the Company’s shares were listed on the Nasdaq Global Market following the completion of its initial public offering (“IPO”). The Company does not conduct any substantive operations of its own but conducts its primary business operations through its subsidiaries and variable interest entities (“VIEs”).
The Company was incorporated as part of the reorganization of Shenyang Sunshine Pharmaceutical Company Limited (“Shenyang Sunshine”) and its consolidated entities (the “Reorganization”) in preparation of the Company’s IPO. In connection with the Reorganization, and pursuant to a shareholders’ agreement amongst the existing beneficial shareholders of Shenyang Sunshine, an ultimate beneficial shareholder of Shenyang Sunshine established Collected Mind Limited (“Collected Mind”) in July 2006 in the British Virgin Islands and held the interest in Collected Mind on behalf of all the ultimate beneficial shareholders of Shenyang Sunshine or their nominees, in proportion to their respective effective share ownership in Shenyang Sunshine. Collected Mind acquired all of the equity interest in Shenyang Sunshine in August 2006. Shortly after this acquisition, the Company was established as the ultimate holding company. In September 2006, in consideration of the Company’s issuance of shares to the ultimate beneficial shareholders of Collected Mind or their nominees in proportion to each of their beneficial interest in Collected Mind, the entire equity interest in Collected Mind was acquired by the Company. Upon completion of the Reorganization, Collected Mind and the Company became Shenyang Sunshine’s immediate and ultimate holding companies, respectively. The proportionate ownership of the Company immediately after the Reorganization is substantially the same.
F-8
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
Shenyang Sunshine was established in the PRC in January 1993 as a Sino-foreign joint stock limited company. In connection with the Reorganization, Shenyang Sunshine became a wholly foreign-owned enterprise in the PRC.
Prior to the Reorganization, Shenyang Sunshine consolidated both Liaoning Bio-Pharmaceutical Company Limited (“Liaoning Sunshine”) and Beijing Sunshine Bio-Product Sales Company Limited (“Beijing Sunshine”) as it held a 90% controlling interest in Liaoning Sunshine and it was the primary beneficiary of Beijing Sunshine, which was deemed to be a VIE in accordance with Accounting Standards Codification (“ASC”) subtopic 810-10, Consolidation: Overall (“ASC 810-10”).
In connection with the Reorganization and for purposes of compliance with certain PRC rules and regulations related to the distribution of pharmaceutical products, Shenyang Sunshine transferred its 45% equity interest in Beijing Sunshine to an executive officer in October 2006 and its 90% equity interest in Liaoning Sunshine to a director in November 2006. In December 2006 and January 2007, Shenyang Sunshine entered into certain contractual arrangements with Beijing Sunshine and Liaoning Sunshine, the two VIEs, respectively, including business operation agreements, equity interest acquisition agreement, voting agreement, equity interest pledge agreement and supplementary arrangements attached to these agreements, such that:
- Shenyang Sunshine is entitled to a majority of the revenues of the VIEs which are derived in the form of technology support, market development and consulting services fees;
- Shenyang Sunshine is granted an exclusive right to acquire, to the extent permissible under PRC law, the equity interests in the VIEs;
- the equity holders of the VIEs are required to consult with and follow any lawful instruction of Shenyang Sunshine whenever they exercise their rights as equity holders; and
- the equity interests in the VIEs are pledged to Shenyang Sunshine to guarantee the obligations of the equity holders under the above contractual arrangements.
As a result of these contractual arrangements, Shenyang Sunshine consolidates the VIEs (as required by ASC 810-10) because it is the primary beneficiary of the VIEs. Through the aforementioned agreements, Shenyang Sunshine is obligated to absorb substantially all of the profits and all of the expected losses from the VIEs’ activities.
However, uncertainties in the PRC legal system could cause the Group’s current ownership structure to be found in violation of future PRC laws or regulations and could limit the Company’s ability, through Shenyang Sunshine, the primary beneficiary, to enforce its rights under these contractual arrangements. Furthermore, shareholders of the VIEs may have interests that are different than those of the Company, which could potentially increase the risk that they would seek to act contrary to the terms of the above aforementioned agreements. In such case, the Company may not be able to operate or control the VIEs, or do so to the extent as our business requires, which may result in deconsolidation of the VIEs.
Beijing Sunshine was dissolved during 2008. Liaoning Sunshine is a domestic limited liability company incorporated in the PRC primarily for the purpose of distributing products manufactured by Shenyang Sunshine. Liaoning Sunshine has also been involved in distributing one third-party franchised pharmaceutical product since 2008. In 2009, through supplementary agreements, Shenyang Sunshine is deemed to have acquired the 10% non-controlling interest in Liaoning Sunshine. The aggregate carrying amounts of the total assets and total liabilities of Liaoning Sunshine as of December 31, 2011 were RMB28,823,000 (US$4,579,000) and RMB32,416,000 (US$5,150,000), respectively, including current assets of RMB11,974,000 (US$1,902,000), non-current assets of RMB16,849,000 (US$2,677,000), current liabilities of RMB2,416,000 (US$383,000) and non-current liabilities of RMB30,000,000 (US$4,767,000). There is no pledge or collateralization of its assets. Creditors of Liaoning Sunshine have no recourse to the general credit of Shenyang Sunshine, which is the primary beneficiary of Liaoning Sunshine. Liaoning Sunshine’s net income for the year ended December 31, 2011 was RMB715,000 (US$114,000). For the years ended December 31, 2009, 2010 and 2011, the Group’s transactions with Liaoning Sunshine were all pursuant to the terms of our agreements with Liaoning Sunshine, as described above.
F-9
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
Shenyang Sunshine Baiao Pharmaceutical Co. Ltd. (“Shenyang Baiao”) was a wholly-owned subsidiary of Collected Mind, which was established in July 2007 and dissolved in April 2009. Throughout its existence, Shenyang Baiao was not involved in any significant business activities and there was no significant gain or loss upon its dissolution.
China Sansheng Medical Limited (“HK Sunshine”) was established in Hong Kong in November 2009 as a wholly-owned subsidiary of Collected Mind and will engage in merger and acquisition efforts, as well as import and export transactions. As of December 31, 2011, HK Sunshine has not been involved in any business activities.
Liaoning Sunshine Technology & Development Co., Ltd. (“Benxi Sunshine”) was established as a wholly-owned subsidiary of Liaoning Sunshine in December 2009 and had engaged in medical technology development, transfer of technology and technical consulting activities.
Pursuant to the strategic alliance entered between the Group and Ascentage Pharma Group Corporation, Ltd. (“APGC”) in February 2010, the Group acquired a 40% equity interest in both APGC and Ascentage Shanghai Pharmaceutical Co., Ltd. (“Ascentage SH”, a PRC domestic company under common control with APGC), in December 2010 and June 2010, respectively. APGC and Ascentage SH are involved in pharmaceutical products IP rights holding and maintenance, as well as research and development (“R&D”) activities execution. Refer to Note 7 and Note 16(b) for details of this arrangement.
On November 4, 2010, the Group entered into an assets acquisition agreement with EnzymeRX LLC (“EnzymeRX”) to purchase certain R&D related assets. Related to the assets acquisition arrangement, the Group formed 3SBio, LLC, a limited liability company in Delaware, USA, on November 30, 2010. Two LLC series under 3SBio, LLC, 3SBio IP Assets Series (“IP Series”) and 3SBio US Assets Series (“Assets Series”), were established to hold the assets acquired from EnzymeRX. Each assets series is a segregated assets series with liabilities insulation between the distinct series, as provided under the Delaware Limited Liability Company Act. The members of IP Series and Assets Series are Shenyang Sunshine and the Company, respectively. As of December 31, 2011, neither of these two LLC series has been involved in any business activities.
In December 2010, Jiangsu Sunshine Pharmaceutical Technology Company Limited (“Jiangsu Sunshine”) was established as wholly-owned subsidiaries of the Company in the PRC, with paid-in capital of RMB30,000,000 (US$4,500,000). Jiangsu Sunshine will be engaged in seeking medical technology development and other R&D opportunities in southern part of the PRC. As of December 31, 2011, Jiangsu Sunshine has not been involved in any business activities.
In December 2010, Taizhou Huan Sheng Investment Management Company Limited (“Tai Zhou Huan Sheng”) was established as a wholly-owned subsidiary of the Company in the PRC, with paid-in capital of RMB1,000,000 (US$152,000). Tai Zhou Huan Sheng was established to cooperate with other investors to invest in local R&D projects.
In May 2011, Taizhou Huan Sheng Healthcare Industry Investment Center, LLP (“3SBio Ventures”) was established in the PRC with a total investment amount of RMB250,000,000 (US$39,700,000). Shenyang Sunshine and Taizhou China Medicine City Company (“CMC”) owned 80% and 20% limited partner interest in 3SBio Ventures, respectively. By the end of 2011, the investment of RMB56,300,000 (US$8,900,000) has been injected by the two parties. The investment partnership is managed by Tai Zhou Huan Sheng, the general partner of 3SBio Ventures. 3SBio Ventures will be engaged in seeking investments in the life science sector that support the Group’s strategic development. 3SBio Ventures will have an investment horizon of eight years with an option to extend.
F-10
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
Basis of presentation
The accompanying consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”).
2. Summary of significant accounting policies and practices
Principles of consolidation
The consolidated financial statements include the financial statements of the Company and its subsidiaries, including the VIE that the Company is the primary beneficiary. All significant intercompany balances and transactions between the Company, its subsidiaries and consolidated VIE have been eliminated upon consolidation.
Currency translation for financial statement presentation
Solely for the convenience of the readers, certain 2011 RMB amounts included in the accompanying consolidated financial statements have been translated into U.S. dollars at the rate of US$1.00 = RMB6.2939, being the noon buying rate for U.S. dollars in effect on December 30, 2011 for cable transfers in RMB per U.S. dollar as certified for custom purposes by the Federal Reserve Bank of New York. No representation is made that the RMB amounts could have been, or could be, converted into U.S. dollars at that rate or at any other rate on December 31, 2011, or on any other date.
Foreign currency transactions
The reporting currency of the Group is Renminbi (“RMB”).The functional currency of the Company’s PRC-based subsidiaries and consolidated VIE is RMB, whereas the functional currency of the Company and its non-PRC-based subsidiaries is the U.S. dollar (“US$”).
Transactions of PRC-based subsidiaries and VIE denominated in currencies other than RMB are remeasured into RMB at the exchange rates quoted by the People’s Bank of China (the “PBOC”) prevailing at the dates of the transactions. Monetary assets and liabilities denominated in foreign currencies are remeasured into RMB using the applicable exchange rates quoted by the PBOC at the balance sheet dates. The resulting exchange differences are recorded in “other income, net” in the consolidated statements of income.
Assets and liabilities of the Company and non-PRC-based subsidiaries are translated into RMB using the exchange rate on the balance sheet dates. Income and expenses are translated at average exchange rates prevailing during the year. The gains and losses resulting from translation of financial statements of the Company and the Company’s non-PRC-based subsidiaries are recorded as foreign currency translation adjustments, a separate component of accumulated other comprehensive income/(loss) within shareholders’ equity.
Comparative figures
Certain items in prior years’ consolidated financial statements have been reclassified to conform to the current year’s presentation.
Use of estimates
The preparation of the consolidated financial statements in accordance with U.S. GAAP requires management of the Company to make a number of estimates and assumptions relating to the reported amount of assets and liabilities and the disclosure of contingent assets and liabilities on the date of the consolidated financial statements and the reported amounts of revenue and expenses during the reporting period. Actual financial results could differ significantly from those estimates. On an ongoing basis, management reviews its estimates and assumptions including, but not limited to, those related to the recoverability of the carrying amount and estimated useful lives of long-lived assets; valuation and other-than-temporary impairment of investments; realizability of inventories; allowance for doubtful accounts receivables; provision for sales rebates; valuation allowance for deferred tax assets; and the fair value of share-based compensation. Changes in facts and circumstances may result in revised estimates.
F-11
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
Concentrations of risks
Concentration of credit risk
Cash and cash equivalents, restricted cash, time deposits, accounts receivable and notes receivable are potentially subject to concentration of credit risk. Cash and cash equivalents, restricted cash and time deposits are placed with financial institutions that management believes are of high credit quality. Accounts receivable are derived from revenue earned from customers located primarily in the PRC. The Company maintains an allowance for doubtful accounts based upon historical experience and the age of receivables, as well as taking into consideration economic and regulatory conditions. Determining appropriate allowances for these losses is an inherently uncertain process, and ultimate losses may vary from the current estimates, resulting in a material impact to future statements of income or cash flows. Due to the relatively small dollar amount of individual accounts receivable, the Company generally does not require collateral on these balances. The allowance for doubtful accounts was RMB2,663,000 and RMB2,542,000 (US$404,000) as of December 31, 2010 and 2011, respectively.
As of December 31, 2010, one customer accounted for more than 10% of outstanding net accounts receivable. As of December 31, 2011, no single customer accounted for more than 10% of outstanding net accounts receivable. Details are listed below:
|
|
December 31, | |||||||||||||||||
|
|
2010 | 2011 | 2011 | |||||||||||||||
|
|
RMB’000 | % | RMB’000 | % | US$’000 | % | ||||||||||||
|
Beijing Tianxingpuxin Bio-Medical Co., Ltd. |
9,162 | 12% | 10,588 | 9% | 1,682 | 9% | ||||||||||||
Currency convertibility risk
RMB is not a fully convertible currency. The PRC State Administration for Foreign Exchange, under the authority of the People’s Bank of China (“PBOC”), controls the conversion of RMB into foreign currencies. The value of the RMB is subject to changes in central government policies and to international economic and political developments affecting supply and demand in the PRC foreign exchange trading system market. All foreign exchange transactions involving RMB must take place either through the PBOC or other institutions authorized to buy and sell foreign exchange. Commencing from July 21, 2005, the PRC government moved the RMB into a managed floating exchange rate regime based on market supply and demand with reference to a basket of currencies. The exchange rate has continued to fluctuate since that time.
Business and economic risks
The Company participates in a fast-growing and highly competitive industry. The Company believes that changes in any of the following areas could have a material adverse effect on the Company’s future financial position, results of operations or cash flows: government regulations and policies; product pricing pressures; government sponsored insurance programs and government medical procurement; competition; demand for our major products; marketing and distribution network; product development; operation enhancement; and outside collaboration. There are other risks and uncertainties that could affect the Company materially.
F-12
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
Sales to the Company’s top five distributors accounted for 30% to 40% of total net revenue for the years ended December 31, 2009, 2010 and 2011. The following is sales to the customer that individually comprises 10% or more of net revenues:
|
|
Year ended December 31, | |||||||||||||||||||||||
|
|
2009 | 2010 | 2011 | 2011 | ||||||||||||||||||||
|
|
RMB’000 | % | RMB’000 | % | RMB’000 | % | US$’000 | % | ||||||||||||||||
|
Beijing Tianxingpuxin Bio-Medical Co., Ltd. |
33,408 | 10% | 46,762 | 11% | 57,382 | 11% | 9,117 | 11% | ||||||||||||||||
Purchases from the Company’s top five suppliers accounted for 30% to 60% of total inventories purchased for the years ended December 31, 2009, 2010 and 2011. The following are inventory purchases from suppliers that individually comprise 10% or more of total inventories purchased in any of the years ended December 31, 2009, 2010 and 2011:
|
|
Year ended December 31, | |||||||||||||||||||||||
|
|
2009 | 2010 | 2011 | 2011 | ||||||||||||||||||||
|
|
RMB’000 | % | RMB’000 | % | RMB’000 | % | US$’000 | % | ||||||||||||||||
|
Chengdu Tiantaishan Pharmaceutial Co., Ltd. |
1,638 | 7% | 1,622 | 7% | 3,553 | 11% | 565 | 11% | ||||||||||||||||
|
Invitrogen Hong Kong Ltd. |
2,294 | 10% | 1,325 | 5% | 2,974 | 9% | 473 | 9% | ||||||||||||||||
|
Nanjing Lifenergy R&D Co., Ltd. |
2,839 | 13% | 2,426 | 10% | 2,156 | 6% | 343 | 6% | ||||||||||||||||
|
Zhenjiang Shuangfeng Pharmaceutical Packing Co.,Ltd. |
3,759 | 17% | 1,563 | 6% | 1,336 | 4% | 212 | 4% | ||||||||||||||||
|
Shanghai Weike Biochemical Reagent Co.,Ltd. |
2,314 | 10% | 925 | 4% | 1,379 | 4% | 219 | 4% | ||||||||||||||||
|
Total |
12,844 | 57% | 7,861 | 32% | 11,398 | 34% | 1,812 | 34% | ||||||||||||||||
The Company’s operations could be adversely affected by significant political, economic and social uncertainties in the PRC.
Foreign currency exchange rate risk
The Company’s exposure to foreign currency exchange rate risk primarily relates to cash and cash equivalents, time deposits and accounts receivables denominated in US$, Australian Dollar (AUD) and Hong Kong Dollar (HKD). The functional currency of the Company is US$, and the reporting currency is RMB. Since July 21, 2005, the RMB has been permitted to fluctuate within a narrow and managed band against a basket of certain foreign currencies. The depreciation of the US$ against RMB was approximately 4.6% in 2011. Any significant revaluation of RMB may materially and adversely affect the cash flows, revenues, earnings and financial position, and the value of, and any dividends payable on, the ADS in U.S. dollars. As a result, an appreciation of RMB against the U.S. dollar would result in foreign currency translation losses when translating the net assets of the Company from the U.S. dollar into RMB.
Fair value measurements of financial instruments
The carrying amounts of the Group’s cash and cash equivalents, time deposits, restricted cash, accounts receivable, notes receivable and accounts payables approximate their fair value as of the balance sheet dates because of their short maturity. Fair values for investment securities that are classified as available-for-sale securities are determined by using quoted market prices and valuation methodologies, primarily discounted cash flow analysis.
F-13
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
Cash and cash equivalents, time deposits and restricted cash
Cash and cash equivalents consist of cash on hand, interest-bearing deposits placed with banks and short-term, highly liquid investments with original maturities of three months or less. Interest-bearing time deposits with financial institutions with original maturities in excess of three months are excluded from cash and cash equivalents and classified as time deposits. Time deposits with remaining maturities longer than one year are classified as non-current assets. The interest rates of the time deposits ranged from 1.00% to 5.3% per annum with a weighted-average original maturity of 1.3 years.
Restricted cash as of December 31, 2011 comprises RMB665,000 (US$106,000) housing maintenance enhancement fund. The maintenance enhancement fund can only be used upon employees’ application and the related government authority’s approval.
As of December 31, 2010 and 2011, the amount of cash and cash equivalents, restricted cash and time deposits with original maturities over three months held in financial institutions by geographic location is as follows:
| December 31, | |||||||||
| 2010 | 2011 | 2011 | |||||||
| RMB’000 | RMB’000 | US$’000 | |||||||
| - the PRC | 597,678 | 675,083 | 107,260 | ||||||
| - Hong Kong | 55,639 | 90,596 | 14,395 | ||||||
| 653,317 | 765,679 | 121,655 | |||||||
As of December 31, 2010 and 2011, the Group’s cash and cash equivalents, restricted cash and time deposits with original maturities over three months denominated in U.S. dollars, Renminbi and other foreign currencies are as follows:
| December 31, | ||||||
| 2010 | 2011 | |||||
| ’000 | ’000 | |||||
| U.S. dollars | 25,761 | 24,516 | ||||
| Renminbi | 470,151 | 598,810 | ||||
| HKD | 8,177 | 7,752 | ||||
| AUD | 542 | 974 | ||||
The group holds its cash, cash equivalents, restricted cash and time deposits in major financial institutions that management believes are of high credit quality.
Accounts receivable and notes receivable
Accounts receivable are stated at the invoiced amount after deduction of trade discounts and allowances, if any, and is non-interest bearing. The allowance for doubtful accounts is the Group’s best estimate of the amount of credit losses in the Group’s existing accounts receivable. The Group determines the allowance based on historical write-off experience, customer specific facts and economic conditions.
F-14
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
Outstanding account balances are reviewed individually for collectability. Account balances are written off after all means of collection have been exhausted and the potential for recovery is considered remote. Shenyang Sunshine and its consolidated entities are required to comply with local tax requirements on the write-offs of doubtful accounts, which allow for such write-offs only when sufficient evidence is available to prove the debtor’s inability to make payments. The Group does not have any off-balance-sheet credit exposure related to its customers.
No notes receivable provision is considered necessary as the notes are issued by reputable commercial banks in the PRC, which means risk of non-payment by customers rests with the banks.
Inventories
Inventories are stated at the lower of cost or market. Cost is determined using the weighted average cost method. Cost of work-in-progress and finished goods comprise direct materials, direct production cost and an allocated proportion of production overheads based on normal operating capacity. Adjustments are made to write down excess or obsolete inventories to their estimated realizable values.
Available-for-sale securities
Available-for-sale securities consist of debt and equity marketable securities and a convertible promissory note. Available-for-sale securities considered available for use in current operations are classified as current assets.
Available-for-sale securities are recorded at fair value. Unrealized holding gains and losses, net of the related tax effect, if any, are reported as a separate component of accumulated other comprehensive income until realized. Realized gains and losses from the sale or disposal of available-for-sale securities are determined on a specific-identification basis.
A decline in the market value of any available-for-sale security that is deemed to be other-than-temporary results in an impairment to the carrying amount to fair value. The impairment is charged to earnings and a new cost basis for the security is established. To determine whether impairment is other-than-temporary, the Group considers whether it has the ability and intent to hold the investment until a market price recovery and considers whether evidence indicating the cost of the investment is recoverable outweighs evidence to the contrary. Evidence considered in this assessment includes the reasons for the impairment, the severity and duration of the impairment, changes in value subsequent to year-end, forecasted performance of the investee, and the general market condition in the geographic area or industry the investee operates in.
Interest income from available-for-sale securities is recognized in other income when earned.
Investments in non-consolidated affiliates
Pursuant to ASC 323-10: Investments – Equity Method and Joint Ventures – Overall, investments in companies in which the Group can exercise significant influence but does not own a majority equity interest or control are accounted for using the equity method of accounting. Significant influence is generally presumed to exist when the Group owns between 20% and 50% of the investee, holds substantial management rights or holds an interest of less than 20%, but the investee is a limited liability partnership or limited liability corporation that is treated as a flow-through entity.
Under the equity method of accounting, only the Group’s investment in and amounts due to and from the equity investee are included in the consolidated balance sheets. The Group initially records its investment at cost and adjusts the carrying amount of the investment to recognize the Group’s proportionate share of each equity investee’s net income or loss in the consolidated statements of income after the date of acquisition. The difference between the cost of the equity investee and the amount of the underlying equity in the net assets of the equity investee is recognized as equity method goodwill included in equity method investment on the consolidated balance sheets.
F-15
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
Impairment of investments in non-consolidated affiliates
For investments accounted for using equity method of accounting, the Group determines impairment by assessing whether an other-than-temporary decline in value of the investment has been sustained. If it has been determined that an investment has sustained an other-than-temporary decline in its value, the investment is written down to its fair value by a charge to earnings. This evaluation is dependent on the specific facts and circumstances. The Group evaluates information such as the investee’s budgets, business plans and financial statements in addition to quoted market prices, if any, in determining whether an other-than-temporary decline in value exists. Factors indicative of an other-than-temporary decline include recurring operating losses, credit defaults and subsequent rounds of financing at an amount below the cost basis of the Group’s investment. This list is not all-inclusive and the Group weighs all known quantitative and qualitative factors.
Property, plant and equipment
Property, plant and equipment are stated at cost less accumulated depreciation.
Depreciation on property, plant and equipment is calculated based on the straight-line method (after taking into account their respective estimated residual values) over the estimated useful lives of the assets as follows:
| Buildings | 10 - 20 years |
| Plant and machinery | 5 - 10 years |
| Motor vehicles | 4 - 5 years |
| Furniture, fixtures and computer equipment | 3 - 10 years |
No depreciation is provided in respect of construction-in-progress.
Depreciation of property, plant and equipment attributable to manufacturing activities is capitalized as part of inventory, and expensed to cost of revenues when inventory is sold.
Expenditures for maintenance and repairs are expensed as incurred.
The gain or loss on the disposal of property, plant and equipment is the difference between the net sales proceeds and the carrying amount of the relevant assets and is recognized in the consolidated statements of income.
Intangible assets
Intangible assets mainly include an exclusive distribution right of Iron Sucrose in the PRC, a patent acquired from an unrelated third party, as well as acquired Pegsiticase related global intellectual property (“IP”) rights and related in-progress R&D (“IPR&D”), which were determined to have alternative future use. Intangible assets other than IPR&D held by the Group have finite lives, are amortized over their estimated useful lives, ranging from 3.7 to 20 years (weighted average life of 7.8 years) and are carried at cost less accumulated amortization with no residual value. Amortization expense was recognized based on the straight-line method over the estimated useful lives. IPR&D is identified as having an indefinite useful life, which is not subject to amortization.
If intangible assets with indefinite use lives are subsequently determined to have finite useful lives, amortization will be provided prospectively over their estimated remaining useful lives and will be accounted for in the same manner as other intangible assets that are subject to amortization.
F-16
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
Impairment of long-lived assets
Long-lived assets, including property, plant and equipment and intangible assets with finite useful lives, are reviewed for impairment whenever events or changes in circumstances indicate, in management’s judgement, that the carrying amount of an asset may not be recoverable.
Recoverability of long-lived assets to be held and used is measured by a comparison of the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future undiscounted cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the asset. Fair value is measured by the asset’s discounted cash flows or market value, if readily determinable.
Intangible assets with indefinite useful lives are tested for impairment annually or more frequently if events or changes in circumstances indicate that they might be impaired.
Prepaid land use rights
Prepaid land use rights represent the cost of land use rights in the PRC. Land use rights are carried at cost and recognized as expense on a straight-line basis over the respective periods of the rights of 30 to 50 years.
The current portion of prepaid land use rights represents the amount expected to be recognized as expense within one year from the balance sheet date, which has been included in “Prepaid expenses and other receivables” in the consolidated balance sheets.
Revenue recognition
Sales of pharmaceutical products represent the invoiced value of goods, net of value added taxes (“VAT”), sales returns, trade discounts and allowances. In accordance with ASC 605-10: Revenue recognition – Overall, the Group recognizes revenue when products are delivered and the customer takes ownership and assumes risk of loss, collection of the relevant receivable is probable, persuasive evidence of an arrangement exists and the sales price is fixed or determinable. Shipping and handling costs paid by the Group and recovered from customers are included in revenue and sales, marketing and distribution expenses.
In the PRC, VAT at a general rate of 17% on invoice amount is collected on behalf of tax authorities in respect of the sales of goods. Revenue is stated net of VAT. VAT collected from customers is offset with VAT paid by the Group for purchases, with the net amount recorded as a liability in the consolidated balance sheets until it is paid to the tax authorities.
Government grants
Government grants received from PRC government agencies are recognized as deferred grant income and recognized in the consolidated statement of income as and when they are incurred for the specific research and development projects or general operation purpose for which these grants are received.
Government grants relating to the domestic acquisition of property, plant and equipment are recognized in the consolidated balance sheets as deferred income when there is reasonable assurance that it will be received and amortized as other income over the weighted average useful life of the assets purchased under the related subsidized capital project.
Retirement and other post-retirement benefits
Contributions to retirement schemes (which are defined contribution plans) are charged to the consolidated statements of income as and when the related employee service is provided.
F-17
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
Pursuant to the relevant PRC regulations, the Company’s PRC consolidated entities is required to make contributions for each employee at a rate of approximately 20% on a standard salary base as determined by the local Social Security Bureau, to a defined contribution retirement scheme organized by the local Social Security Bureau in respect of the retirement benefits for the Group’s employees in the PRC. The total amount of contributions of RMB2,560,000, RMB3,367,000 and RMB5,046,000 (US$802,000) for the years ended December 31, 2009, 2010 and 2011, respectively, was charged to expense in the consolidated statements of income. The Group has no other obligation to make payments in respect of retirement benefits of the employees.
Income taxes
Income taxes are accounted for under the liability method. Under this method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases, as well as operating loss and tax credit carry forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. A valuation allowance is provided to reduce the amount of deferred tax assets if it is considered more likely than not that some portion or all of the deferred tax assets will not be realized. The effect on deferred tax assets and liabilities of a change in tax rate is recognized in the consolidated statements of income in the period that includes the enactment date.
The Group applies ASC 740-10: Income Taxes: Overall (“ASC 740-10”) to account for uncertainty in income taxes. ASC 740-10 requires that an entity recognizes in the consolidated financial statements the impact of a tax position, if that position is not more likely than not to be sustained upon examination, based on the technical merits of the position. Recognized income tax positions are measured at the largest amount that is greater than 50% likely of being realized on a cumulative probability basis. Changes in recognition or measurement are reflected in the period in which the change in judgement occurs. The Group has elected to classify interest and penalties related to unrecognized tax benefits, if and when required, as part of income tax expense in the consolidated statements of income. As of and for the years ended December 31, 2009, 2010 and 2011, no unrecognized tax benefits or interest and penalties associated with uncertainty in income taxes have been recognized.
Advertising costs
Advertising costs are expensed as incurred. Advertising costs included in sales, marketing and distribution expenses were RMB3,011,000, RMB1,204,000 and RMB858,000 (US$136,000) for the years ended December 31, 2009, 2010 and 2011, respectively.
R&D costs
R&D costs are expensed as incurred. R&D costs consist primarily of the remuneration of R&D staff, depreciation, materials, clinical trial costs as well as amortization of acquired technology and know-how used in R&D with alternative future uses. R&D costs also include costs associated with collaborative R&D and in-licensing arrangements, including upfront fees paid to collaboration partners in connection with technologies which have not reached technological feasibility and did not have an alternative future use. Reimbursement of R&D costs for arrangements with collaboration partners is recognized when the obligations are incurred.
Commitments and contingencies
In the normal course of business, the Group is subject to contingencies, including legal proceedings and claims arising out of the business that relate to a wide range of matters, including among others, product liability. The Group records accruals for such contingency based upon the assessment of the probability of occurrence and, where determinable, an estimate of the liability. The Group may consider many factors in making these assessments including past history and the specifics of each matter. As the Group has not become aware of any product liability claim since operations commenced, the Group has not recognized a liability for product liability claims.
F-18
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
Earnings per share (“EPS”)
In accordance with ASC 260-10: Earnings Per Share: Overall, basic net income attributable to 3SBio Inc. per share is computed by dividing net income attributable to 3SBio Inc. by the weighted average number of ordinary shares outstanding. Diluted net income attributable to 3SBio Inc. per share is calculated by dividing net income attributable to 3SBio Inc. by the weighted average number of ordinary and dilutive potential ordinary shares outstanding during the period. Potential ordinary shares consist of the incremental ordinary shares issuable upon the exercise of stock options, as well as vesting of nonvested shares and restricted share units (“RSUs”) that will be settled in the Company’s stock. The dilutive effect of outstanding stock options, nonvested shares and RSUs are reflected in diluted earnings attributable to 3SBio Inc. per share by application of the treasury stock method. The calculation of diluted net income attributable to 3SBio Inc. per share excludes all anti-dilutive shares.
The following table sets forth the computation of basic and diluted net income attributable to 3SBio Inc. per share:
| Year ended December 31, | ||||||||||||
| 2009 | 2010 | 2011 | 2011 | |||||||||
| RMB’000 | RMB’000 | RMB’000 | US$’000 | |||||||||
|
Numerator for basic and diluted net income attributable to 3SBio Inc. per share: |
||||||||||||
|
- Net income attributable to 3SBio Inc. |
83,435 | 81,286 | 108,573 | 17,251 | ||||||||
|
Denominator: |
||||||||||||
|
- Basic weighted average shares |
150,606,317 | 151,241,036 | 153,310,128 | 153,310,128 | ||||||||
|
- Effect of dilutive potential ordinary shares |
||||||||||||
|
RSUs |
31,485 | 1,183,002 | 1,487,696 | 1,487,696 | ||||||||
|
Nonvested shares |
355,540 | 543,719 | 1,448,590 | 1,448,590 | ||||||||
|
Options |
40,850 | 1,164,011 | 902,271 | 902,271 | ||||||||
| 427,875 | 2,890,732 | 3,838,557 | 3,838,557 | |||||||||
|
Diluted weighted average shares |
151,034,192 | 154,131,768 | 157,148,685 | 157,148,685 | ||||||||
|
Basic net income attributable to 3SBio Inc. per share |
0.55 | 0.54 | 0.71 | 0.11 | ||||||||
|
Diluted net income attributable to 3SBio Inc. per share |
0.55 | 0.53 | 0.69 | 0.11 | ||||||||
The diluted net income attributable to 3SBio Inc. per share calculation for the years ended December 31, 2009, 2010 and 2011 did not include the effect of share options to purchase a total of 585,100, nil and nil ordinary shares, respectively, because to do so would have been anti-dilutive.
F-19
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
Comprehensive Income
Comprehensive income is defined to include all changes in equity except those resulting from investment by owners and distributions to owners. The Group’s components of comprehensive income during the years ended December 31, 2009, 2010 and 2011 were net income, net unrealized gains and losses on available-for-sale securities and foreign currency translation adjustments.
Share-based payments
The Group adopts ASC 718-10: Compensation – Stock Compensation: Overall (“ASC 718-10”) for all share-based payments made by the Group. ASC 718-10 requires that all compensation cost related to employee share options or similar equity instruments be measured at grant date fair values of the awards. The Group recognizes compensation cost on a straight-line basis over the requisite service period, which is generally the same as the vesting period. The Group adopts a lattice option-pricing model to determine the fair value of share options granted and forfeitures are estimated based on historical experience and are periodically reviewed.
The Group accounts for equity instruments issued to each member of the board of directors who is not an employee of the Group in accordance with the provisions of ASC 718-10. As those non-employee directors were all appointed to a board position that will be filled by shareholder election when the existing term expires and the awards granted to these directors are only for their services as directors, awards granted to these non-employee directors are treated in the same way as share-based payment to employees.
Segment reporting
The Group uses the management approach in determining operating segments. The management approach considers the internal organization and reporting used by the Group’s chief operating decision maker for making operating decisions, allocating resources and assessing performance as the source of determining the Group’s reportable segments. Management, including the chief operating decision maker, reviews operating results solely by monthly revenue and operating results of the Group and, as such, the Group has determined that the Group has a single operating segment as defined by ASC 280-10: Segment Reporting: Overall, which is the research, development, manufacture and distribution of pharmaceutical products in the PRC.
Recently issued accounting standards
In May 2011, the Financial Accounting Standards Board (“FASB”) issued Accounting Standard Update (“ASU”) No. 2011-04, Fair Value Measurement (“Topic 820”), Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRSs (“ASU 2011-04”) which amends the fair value measurement guidance and includes some enhanced disclosure requirements. The most significant change in disclosures is an expansion of the information required for Level 3 measurements based on unobservable inputs. The standard is effective for fiscal years beginning after December 15, 2011. The Group will adopt ASU 2011-04 beginning January 1, 2012 and does not expect the adoption to have a material impact on its consolidated financial statements and disclosures.
In June 2011, the FASB issued Accounting Standard Update (“ASU”) 2011-05, Comprehensive income (Topic 220), Presentation of Comprehensive Income. ASU 2011-05 increases the prominence of other comprehensive income in financial statements. Under this ASU, an entity will have the option to present the components of net income and comprehensive income in either one or two consecutive financial statements. The ASU eliminates the option in U.S. GAAP to present other comprehensive income in the statement of changes in equity. An entity should apply the ASU retrospectively. For a public entity, the ASU is effective for fiscal years, and interim periods within those years, beginning after December 15, 2011. The Group early adopted ASU 2011-05 in the year ended December 31, 2011.
F-20
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
3. Accounts receivable, net
Accounts receivable, net, consists of the following:
|
|
December 31, | ||||||||
|
|
2010 | 2011 | 2011 | ||||||
|
|
RMB’000 | RMB’000 | US$’000 | ||||||
|
|
|||||||||
|
Accounts receivable |
81,163 | 116,491 | 18,509 | ||||||
|
Less: Allowance for doubtful accounts |
(2,663 | ) | (2,542 | ) | (404 | ) | |||
|
Total accounts receivable, net |
78,500 | 113,949 | 18,105 | ||||||
An analysis of the allowance for doubtful accounts is as follows:
|
|
December 31, | |||||||||||
|
|
2009 | 2010 | 2011 | 2011 | ||||||||
|
|
RMB’000 | RMB’000 | RMB’000 | US$’000 | ||||||||
|
|
||||||||||||
|
Balance at beginning of year |
4,503 | 2,915 | 2,663 | 423 | ||||||||
|
Credited to income |
(1,588 | ) | (234 | ) | (111 | ) | (17 | ) | ||||
|
Written off against the allowance |
- | (18 | ) | (10 | ) | (2 | ) | |||||
|
|
||||||||||||
|
Balance at end of year |
2,915 | 2,663 | 2,542 | 404 | ||||||||
The Group’s accounts receivable balances are all related to third-party customers. The Group has a credit policy in place and the exposure to credit risk is monitored on an ongoing basis. The Group does not obtain collateral from customers.
4. Inventories
Inventories consist of the following:
|
|
December 31, | ||||||||
|
|
2010 | 2011 | 2011 | ||||||
|
|
RMB’000 | RMB’000 | US$’000 | ||||||
|
|
|||||||||
|
Raw materials |
2,571 | 3,391 | 539 | ||||||
|
Work-in-progress |
12,524 | 15,223 | 2,419 | ||||||
|
Finished goods |
4,790 | 6,682 | 1,062 | ||||||
|
Consumables and packaging materials |
1,833 | 2,308 | 366 | ||||||
|
|
|||||||||
|
Total inventories |
21,718 | 27,604 | 4,386 | ||||||
5. Prepaid expenses and other receivables
Prepaid expenses and other receivables consist of the following:
F-21
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
|
|
December 31, | ||||||||
|
|
2010 | 2011 | 2011 | ||||||
|
|
RMB’000 | RMB’000 | US$’000 | ||||||
|
|
|||||||||
|
Prepayments and other deposits |
4,358 | 7,444 | 1,183 | ||||||
|
Loan receivable |
26,400 | 2,000 | 318 | ||||||
|
Other receivables |
8,632 | 17,228 | 2,737 | ||||||
|
|
|||||||||
|
Total prepaid expenses and other receivables |
39,390 | 26,672 | 4,238 | ||||||
Prepayments and other deposits as of December 31, 2010 and 2011 include RMB353,000 and RMB550,000 (US$87,000), respectively, current portion of prepaid land use rights (Note 9); and RMB3,379,000 and RMB3,245,000 (US$516,000) advances to employees.
Loan receivable as of December 31, 2011 was a short-term loan made to a third party. It is an uncollateralized three-month loan with interest rate mirrored to prevailing market rate. This loan was fully repaid in March 2012.
Other receivables mainly include interest receivable and other miscellaneous receivables.
6. Available-for-sale securities
At December 31, 2010 and 2011, the fair values of available-for-sale securities held by the Group are as follows:
|
|
December 31, 2010 | ||||||||||||||
|
|
|||||||||||||||
|
|
Foreign | ||||||||||||||
|
|
currency | Gross | |||||||||||||
|
|
translation | unrealized | Impairment | ||||||||||||
|
|
Cost | adjustments | gain | loss | Fair value | ||||||||||
|
|
RMB’000 | RMB’000 | RMB’000 | RMB’000 | RMB’000 | ||||||||||
|
Current portion: |
|||||||||||||||
|
Equity securities |
30,192 | (492 | ) | 20,967 | - | 50,667 | |||||||||
|
|
|||||||||||||||
|
Non-current portion: |
|||||||||||||||
|
Convertible promissory note |
1,334 | (14 | ) | - | - | 1,320 | |||||||||
|
Corporate debt securities |
11,360 | (951 | ) | 968 | - | 11,377 | |||||||||
|
|
12,694 | (965 | ) | 968 | - | 12,697 | |||||||||
|
Total available-for-sale securities |
42,886 | (1,457 | ) | 21,935 | - | 63,364 | |||||||||
F-22
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
|
|
December 31, 2011 | |||||||||||||||||
|
|
||||||||||||||||||
| Foreign | ||||||||||||||||||
|
|
currency | Gross | ||||||||||||||||
|
|
translation | unrealized | Impairment | |||||||||||||||
|
|
Cost | adjustments | gain/(loss) | loss | Fair value | Fair value | ||||||||||||
|
|
RMB’000 | RMB’000 | RMB’000 | RMB’000 | RMB’000 | US$’000 | ||||||||||||
|
Current portion: |
||||||||||||||||||
|
Equity securities |
30,192 | (1,869 | ) | (5,493 | ) | - | 22,830 | 3,627 | ||||||||||
|
|
||||||||||||||||||
|
Non-current portion: |
||||||||||||||||||
|
Convertible promissory note |
1,334 | - | - | (1,334 | ) | - | - | |||||||||||
|
Corporate debt securities |
11,360 | (1,395 | ) | 883 | - | 10,848 | 1,724 | |||||||||||
|
|
12,694 | (1,395 | ) | 883 | (1,334 | ) | 10,848 | 1,724 | ||||||||||
|
Total available-for-sale securities |
42,886 | (3,264 | ) | (4,610 | ) | (1,334 | ) | 33,678 | 5,351 | |||||||||
The fair values of equity securities and corporate debt securities are based on quoted market prices at each reporting date. The fair value of the convertible promissory note was determined using the income approach.
The corporate debt securities held by the Group as of December 31, 2011 are interest-bearing at 4.625 - 7.5% per annum and are publicly traded outside the PRC.
On August 23, 2010, the Group purchased a US$4,500,000 (RMB29,700,000) convertible debenture from Isotechnika Pharma Inc. (“Isotechnika”) with interest rate of 7% per annum. The convertible debenture carries a three-year maturity from issuance and the Group can convert the debenture into Isotechnika’s common shares anytime before maturity at a fixed rate of Canadian Dollar (“CAD”) 0.155 per share. As of December 31, 2010, the Group had fully converted the debenture into 30,734,877 common shares and recorded the converted shares as equity securities in available-for-sale securities. As of December 31, 2011, the Group held a 17.6% equity investment in Isotechnika and nominated one member to Isotechnika’s six-member board of directors. The fair market price of Isotechnika’s common shares decreased from CAD0.155 as of the conversion date to CAD0.12 as of December 31, 2011, representing a 22.5% decrease compared to the conversion date. As such, the investment in Isotechnika’s common shares was in an unrealised loss position with unrealized loss of US$873,000 (RMB5,493,000) as of December 31, 2011. Based on the relatively short duration of time in which the investment cost exceeded the fair market price and considering that the Group has the ability and intent to hold the investment until the cost is recovered, the Group does not believe there is other-than-temporary impairment of its investment in Isotechnika as of December 31, 2011.
On November 30, 2010, the Group purchased a convertible promissory note (the “Note”) with par value of US$200,000 (RMB1,334,000) and an annual interest rate of 7.5% compounded on a quarterly basis. As of December 31, 2011, the Group had terminated its contract with the issuer as the further financing was not completed. As such, the Group had recorded an impairment loss in respect of the Note equal to its face value of US$200,000 (RMB1,334,000). Refer to Note 24 for details.
(a) Net realized gain/(loss) on available-for-sale securities
There was no net realized gain/(loss) on available-for-sale securities for the year ended December 31, 2010 and 2011, as the Group did not dispose of any available-for-sale securities.
F-23
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
In May 2009, the Group disposed of the Lehman Brothers’ corporate debt securities at a price of US$382,000 (RMB2,616,000) recognizing a gain of US$123,000 (RMB840,000) during the year ended December 31, 2009. The Group also realized a net gain of RMB771,000 (US$112,000), which was from disposals of available-for-sale securities with proceeds of RMB14,379,000 (US$2,105,000) in 2009. The latter includes the disposal of the impaired available-for-sale securities described in 6(b) below.
(b) Impairment loss on available-for-sale securities
The Group evaluates whether unrealized losses on available-for-sale securities indicate other-than-temporary impairment.
Other than the impairment loss recorded for the investment in the Note, there were no indicators of other-than-temporary impairment of the Group’s available-for-sale securities held as of December 31, 2011.
During the year ended December 31, 2008, the Group acquired perpetual securities issued by Lloyds totalling US$2,000,000. As of December 31, 2008, the fair value of the perpetual securities was US$1,356,000 (RMB9,254,000) based on quoted market price and the Group recognized an other-than-temporary impairment loss of US$644,000 (RMB4,391,000) at that time. The perpetual securities were exchanged for preference shares in January 2009 at the election of Lloyds. On the date of exchange, the fair value of the preference shares was lower than the carrying value of the perpetual securities, resulting in a further impairment loss of US$676,000 (RMB4,624,000) being recognized for the year ended December 31, 2009. In May 2009, the Group disposed of the preference shares at a price of US$820,000 and a gain on disposal of RMB962,000 (US$140,000) was recognized.
An unrealized holding gain of RMB1,205,000, unrealized holding gain of RMB21,335,000 and unrealized holding loss of RMB26,545,000 (US$4,218,000) on available-for-sale securities was recognized in accumulated other comprehensive income for the years ended December 31, 2009, 2010 and 2011, respectively.
Other-than-temporary impairment of available-for-sale securities is analyzed as follows:
|
|
Year ended December 31, | |||||||||||
|
|
2009 | 2010 | 2011 | 2011 | ||||||||
|
|
RMB’000 | RMB’000 | RMB’000 | US$’000 | ||||||||
|
Balance at beginning of year |
4,391 | - | - | - | ||||||||
|
Addition during the year |
4,624 | - | 1,334 | 212 | ||||||||
|
Realized upon disposal |
(9,015 | ) | - | - | - | |||||||
|
Balance at end of year |
- | - | 1,334 | 212 | ||||||||
(c) Maturity information of available-for-sale securities at year-end
Maturities of the Group’s corporate debt securities classified as non-current available-for-sale are as follows:
| Year ended December 31, | ||||||
| 2011 | 2011 | |||||
| RMB’000 | US$’000 | |||||
| Due in one through five years | 5,342 | 849 | ||||
| Due after five years through ten years | 5,506 | 875 | ||||
| Total | 10,848 | 1,724 | ||||
F-24
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
7. Investment in non-consolidated affiliates
|
|
Year ended December 31, | |||||||||||
|
|
Ownership | 2010 | 2011 | 2011 | ||||||||
|
|
Percentage | RMB’000 | RMB’000 | US$’000 | ||||||||
|
Equity method investments |
||||||||||||
|
- Ascentage SH |
40% | 3,041 | 2,245 | 357 | ||||||||
|
- APGC |
40% | 794 | - | - | ||||||||
|
Total |
3,835 | 2,245 | 357 | |||||||||
The Group acquired 40% equity interests in APGC and Ascentage SH at a cost of RMB1,131,000 and RMB2,000,000 in December 2010 and June 2010, respectively. APGC and Ascentage SH are controlled by the same group of shareholders. APGC was incorporated in Hong Kong in June 2009, while Ascentage SH was incorporated in the PRC in June 2009. APGC and Ascentage SH are involved in pharmaceutical products IP rights holding and maintenance, as well as R&D activities. The Group accounts for their investment in APGC and Ascentage SH under the equity method.
For the year ended December 31, 2010, the Group recognized its share of equity-method investment loss from APGC of RMB337,000, and its share of equity-method investment gain from Ascentage SH of RMB1,041,000, respectively.
For the year ended December 31, 2011, the Group recognized its share of equity-method investment loss from APGC of RMB794,000 (US$126,000), and its share of equity-method investment loss from Ascentage SH of RMB796,000 (US$127,000), respectively.
8. Property, plant and equipment, net
Property, plant and equipment consist of the following:
|
|
December 31, | ||||||||
|
|
2010 | 2011 | 2011 | ||||||
|
|
RMB’000 | RMB’000 | US$’000 | ||||||
|
|
|||||||||
|
Buildings |
91,452 | 102,864 | 16,343 | ||||||
|
Plant and machinery |
115,112 | 118,923 | 18,895 | ||||||
|
Motor vehicles |
6,569 | 5,953 | 946 | ||||||
|
Furniture, fixtures and computer equipment |
41,365 | 45,588 | 7,243 | ||||||
|
Construction-in-progress |
16,501 | 10,919 | 1,735 | ||||||
|
|
|||||||||
|
|
270,999 | 284,247 | 45,162 | ||||||
|
Less: Accumulated depreciation |
(71,543 | ) | (86,194 | ) | (13,695 | ) | |||
|
|
|||||||||
|
Total property, plant and equipment |
199,456 | 198,053 | 31,467 | ||||||
F-25
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
The Company completed the construction of its new manufacturing facility in Shenyang province in February 2010. Construction-in-progress as of December 31, 2011 included machinery and equipment not ready for intended use and renovation of old facilities.
The depreciation of property, plant and equipment is calculated using the straight-line method over their respective estimated useful life. Depreciation expense was RMB7,009,000, RMB13,906,000 and RMB20,002,000 (US$3,178,000) for the years ended December 31, 2009, 2010 and 2011, respectively.
All of the Group’s buildings are located in the PRC.
9. Prepaid land use rights
Prepaid land use rights represent the land use rights of the Group and are analyzed as follows:
|
|
December 31, | ||||||||
|
|
2010 | 2011 | 2011 | ||||||
|
|
RMB’000 | RMB’000 | US$’000 | ||||||
|
|
|||||||||
|
Prepaid land use rights |
10,593 | 20,419 | 3,244 | ||||||
|
Less: Accumulated amortization |
(2,052 | ) | (2,421 | ) | (385 | ) | |||
|
Balance as of December 31, 2011 |
8,541 | 17,998 | 2,859 | ||||||
|
|
|||||||||
|
Including: |
|||||||||
|
Non-current portion |
8,188 | 17,448 | 2,772 | ||||||
|
Current portion |
353 | 550 | 87 | ||||||
The estimated amortization expense of prepaid land use rights for the next five years and thereafter is as follows:
| RMB’000 | US$’000 | |||||
| 2012 | 550 | 87 | ||||
| 2013 | 550 | 87 | ||||
| 2014 | 550 | 87 | ||||
| 2015 | 550 | 87 | ||||
| 2016 | 550 | 87 | ||||
| Thereafter | 15,248 | 2,424 | ||||
| Total | 17,998 | 2,859 |
In 2005, the Group paid RMB9,924,000 for a land use right for a term of 30 years in respect of land located beside the existing plant of the Group. In 2011, the Group paid RMB9,826,000 (US$1,561,000) for a land use right for a term of 50 years in respect of land located in Taizhou City, Jiangsu Province, the PRC and the certificate has not been issued at the balance sheet date due to administrative processing reasons. The prepayment is charged to expense on a straight line basis over the lease term of 50 years.
Land lease expense for the years ended December 31, 2009, 2010 and 2011 were RMB353,000, RMB353,000 and RMB369,000 (US$59,000), respectively.
10. Prepayment and non-current deposits
F-26
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
Included in prepayment and non-current deposits as of December 31, 2010 and 2011 are prepayment for the acquisition of property, plant and equipment and other deposits of RMB1,555,000 and RMB16,801,000 (US$2,669,000), respectively, which are not expected to be utilized or recovered within one year.
11. Intangible assets, net
|
|
December 31, | ||||||||
|
|
2010 | 2011 | 2011 | ||||||
|
|
RMB’000 | RMB’000 | US$’000 | ||||||
|
|
|||||||||
|
Cost: |
|||||||||
|
Exclusive distribution right |
5,500 | 5,500 | 874 | ||||||
|
China IP rights |
1,782 | 1,782 | 283 | ||||||
|
U.S. IP rights |
4,047 | 4,047 | 643 | ||||||
|
Patent |
- | 8,097 | 1,286 | ||||||
|
Others |
219 | 219 | 35 | ||||||
|
|
11,548 | 19,645 | 3,121 | ||||||
|
Accumulated amortization: |
|||||||||
|
Exclusive distribution right |
2,475 | 3,575 | 568 | ||||||
|
China IP rights |
- | 89 | 14 | ||||||
|
U.S. IP rights |
- | 289 | 46 | ||||||
|
Patent |
- | 1,288 | 205 | ||||||
|
Others |
3 | 18 | 2 | ||||||
|
|
(2,478 | ) | (5,259 | ) | (835 | ) | |||
|
Intangible assets subject to amortization, net |
9,070 | 14,386 | 2,286 | ||||||
|
IPR&D |
35,229 | 35,229 | 5,597 | ||||||
|
Total intangible assets, net |
44,299 | 49,615 | 7,883 | ||||||
The exclusive distribution right is for distributing Iron in the PRC. The Group purchased the 5-year right from an unrelated third party in 2008, based on which the useful life was determined.
The China and U.S. IP rights and IPR&D were acquired in an assets acquisition. The initial carrying values for these assets were determined by allocating the total cost of the acquisition to the individual assets on a relative fair value basis. Useful lives of the China and U.S. IP rights were determined based on the estimated period for which the IP rights are expected to generate future cash flows for the Group. IPR&D represents the acquisition cost of the test results and data related to the pre-clinical and phase-I clinical trials and were capitalized because they have alternative future uses. IPR&D will not be subject to amortization until the R&D project is substantially completed.
The Group acquired a patent from an unrelated third party in 2011 with a valid period of 20 years from February 1995. This is capitalized and amortized over the remaining period of the patent.
Amortization expense of RMB1,100,000, RMB1,103,000 and RMB2,781,000 (US$442,000) for the years ended December 31, 2009, 2010 and 2011, respectively, was included in cost of revenue.
The estimated amortization expense of intangible assets for the next five years and thereafter is as follows:
F-27
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
| RMB’000 | US$’000 | |||||
| 2012 | 3,701 | 588 | ||||
| 2013 | 3,426 | 544 | ||||
| 2014 | 2,601 | 413 | ||||
| 2015 | 577 | 92 | ||||
| 2016 | 393 | 62 | ||||
| Thereafter | 3,688 | 587 | ||||
| Total | 14,386 | 2,286 |
12. Deferred grant income
The Group’s deferred grant income represents the unamortized portion of government grants.
In August 1999, the Group received a government grant of RMB15,000,000, approved by the State Development Planning Committee of the PRC, for the purchase of plant and equipment costing a total of RMB45,029,000.
In December 2010, the Group received government grant of RMB1,000,000, approved by a PRC provincial government for a hemodialysis project. The grant was recorded in deferred grant income as at December 31, 2010.
Amortization of deferred grant income was RMB374,000, RMB374,000 and RMB1,374,000 (US$218,000) for the years ended December 31, 2009, 2010 and 2011, respectively. Deferred grant income as of December 31, 2011 is expected to be amortized as follows:
| RMB’000 | US$’000 | |||||
| 2012 | 374 | 59 | ||||
| 2013 | 374 | 59 | ||||
| 2014 | 374 | 59 | ||||
| 2015 | 374 | 59 | ||||
| 2016 | 374 | 59 | ||||
| Thereafter | 533 | 86 | ||||
| Total | 2,403 | 381 |
The Group’s grant income is analyzed as follows:
|
|
Year ended December 31, | |||||||||||
|
|
2009 | 2010 | 2011 | 2011 | ||||||||
|
|
RMB’000 | RMB’000 | RMB’000 | US$’000 | ||||||||
|
|
||||||||||||
|
Blood treatment project |
300 | - | - | - | ||||||||
|
R&D activities support |
- | 882 | 211 | 34 | ||||||||
|
Amortization of deferred grant income |
374 | 374 | 1,374 | 218 | ||||||||
|
Total grant income |
674 | 1,256 | 1,585 | 252 | ||||||||
F-28
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
13. Accrued expenses and other payables
Accrued expenses and other payables consist of the following:
|
|
December 31, | ||||||||
|
|
2010 | 2011 | 2011 | ||||||
|
|
RMB’000 | RMB’000 | US$’000 | ||||||
|
|
|||||||||
|
Accrued selling and marketing expenses |
16,924 | 19,069 | 3,030 | ||||||
|
Accrued salaries, bonus and welfare expenses |
8,227 | 11,391 | 1,810 | ||||||
|
Other taxes payable |
5,124 | 10,422 | 1,656 | ||||||
|
Payable to plant, property and equipment vendors |
6,923 | 3,680 | 585 | ||||||
|
Payable to R&D service contractors |
33 | 1,535 | 244 | ||||||
|
Advances from customers |
547 | 733 | 116 | ||||||
|
Other accrued expenses |
1,774 | 1,559 | 248 | ||||||
|
|
|||||||||
|
Total accrued expenses and other payables |
39,552 | 48,389 | 7,689 | ||||||
14. Shareholders’ equity
(a) Share capital
The Company is authorized to issue up to 500 million shares with par value of $0.0001 per share, of which 152,654,148 and 154,473,159 shares were issued and outstanding as of December 31, 2010 and 2011, respectively. As of December 31, 2010 and 2011, no preferred shares have been issued, nor does the Company have current plans to issue preferred shares.
During the year ended December 31, 2009, 16,772 ordinary shares were issued when vested options were exercised at a consideration of US$1.15 per share (US$8.05 per ADS, each ADS represents seven ordinary shares of the Company). In addition, 48,734 shares were issued upon vesting of nonvested shares.
During the year ended December 31, 2010, a total of 909,188 ordinary shares were issued when vested options were exercised, which are related to three different grants of options. Among the newly issued shares pursuant to the exercises, 99,995 shares were issued at a consideration of US$1.60 per share (US$11.20 per ADS), 563,213 shares at US$1.15 per share (US$8.05 per ADS), and the remaining 245,980 shares at US$0.78 per share (US$5.45 per ADS). In addition, 1,103,499 shares were issued upon vesting of nonvested shares and RSUs.
During the year ended December 31, 2011, a total of 756,791 ordinary shares were issued when vested options were exercised, which are related to three different grants of options. Among the newly issued shares pursuant to the exercises, 376,978 shares were issued at US$1.15 per share (US$8.05 per ADS), and the remaining 379,813 shares were issued at US$0.78 per share (US$5.45 per ADS). In addition, 1,062,220 shares were issued upon vesting of nonvested shares and RSUs.
(b) Retained earnings
The Company’s PRC-based subsidiaries and the consolidated VIE are either foreign invested enterprises established in the PRC, or PRC domestic companies. Therefore, these PRC-based consolidated entities are required under PRC rules and regulations, as well as their respective Articles of Association, to make appropriations from retained earnings to statutory reserves. The appropriation requires transferring 10% of after-tax profits, as determined in accordance with PRC GAAP, to a statutory surplus reserve until the reserve balance reaches 50% of these PRC-based consolidated entities’ respective registered capital. The transfer to this reserve must be made before distribution of dividends to the equity holders can be made, and such appropriations are required to be approved by these PRC-based consolidated entities’ respective boards of directors.
F-29
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
Transfers of RMB9,466,000, RMB11,587,000 and RMB15,613,000 (US$2,481,000) have been made to the statutory surplus reserve for the years ended December 31, 2009, 2010 and 2011, respectively.
The accumulated balances of this statutory surplus reserve maintained by the Company’s PRC consolidated entities as of December 31, 2009, 2010 and 2011 were RMB23,742,000, RMB35,329,000 and RMB50,942,000 (US$8,094,000), respectively.
The statutory surplus reserve is non-distributable but can be used to offset against previous years’ losses, if any, and may be converted into issued capital in proportion to the respective equity holding of the equity holders, provided that the balance of the reserve after such conversion is not less than 25% of the registered capital.
15. Revenues
The Group’s revenues are primarily derived from the distribution of self-manufactured pharmaceutical products and one franchised product.
The Group operates and manages its business solely in the PRC and sales were predominately made to customers located in the PRC.
The Group’s products are subject to price control by the PRC government. The maximum prices of the products are published by the price administration authorities from time to time.
The Group’s revenues are analyzed as follows:
| Year ended December 31, | ||||||||||||
| 2009 | 2010 | 2011 | 2011 | |||||||||
| RMB’000 | RMB’000 | RMB’000 | US$’000 | |||||||||
| Domestic sales: | ||||||||||||
| - EPIAO | 196,080 | 250,854 | 317,889 | 50,507 | ||||||||
| - TPIAO | 89,679 | 128,717 | 164,839 | 26,190 | ||||||||
| - Intefen | 5,522 | 5,358 | 5,229 | 831 | ||||||||
| - Inleusin | 1,607 | 2,041 | 2,788 | 443 | ||||||||
| - Iron | 10,715 | 17,187 | 24,859 | 3,950 | ||||||||
| Export sales | 13,216 | 12,211 | 23,890 | 3,796 | ||||||||
| Others | 101 | 2,260 | 2,120 | 337 | ||||||||
| Total revenues | 316,920 | 418,628 | 541,614 | 86,054 | ||||||||
16. Collaborations
(a) AMAG Pharmaceuticals, Inc. (“AMAG”)
During the year ended December 31, 2008, the Company entered into a development and commercialization agreement with AMAG for ferumoxytol, an intravenous iron replacement therapeutic agent being developed to treat iron deficiency anemia in chronic kidney disease (“CKD”) patients.
F-30
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
Under the terms of the agreement, AMAG grants the Company exclusive rights to develop and commercialize ferumoxytol in the PRC, initially for CKD, and with an option to explore into further co-development indications. The Company is responsible for the clinical development, registration, and commercialization of ferumoxytol in the PRC. The agreement has an initial duration of thirteen years and will be automatically renewed for a set term if minimum sales thresholds are achieved. AMAG will retain all manufacturing rights for ferumoxytol and will provide, under a separate agreement, commercial supply to the Company at a predetermined supply price.
In 2008, the Company paid a non-refundable payment of US$1,000,000 to AMAG for the licensing right to commercialize and market ferumoxytol in the PRC which was immediately expensed as it had no other alternative use. The Company is required to make milestone payments of US$1,500,000 upon obtaining the regulatory approval from the China State Food and Drug Administration (“SFDA”) for the commercialization and marketing of ferumoxytol within the PRC and upon any other co-developed indications being approved by the SFDA for their commercialization and marketing within the PRC. The Company is also required to pay a royalty fees based on the future sales of ferumoxytol.
In January 2010, the Company submitted an application for a registration clinical trial to SFDA in the PRC for ferumoxytol.
(b) APGC
In March 2010, the Group entered into a strategic alliance with APGC, a Hong Kong based therapeutic research company, to research, develop and commercialize best-in-class targeted cancer therapeutics focusing on programmed cell death, or apoptosis. Apoptosis targeted small molecules have the potential to play a key role in the next generation of highly effective targeted cancer drugs. The arrangement also involves Ascentage SH, a PRC domestic company under common control with APGC (Note 7).
Under the terms of the agreements between the Group and APGC, and the Group and Ascentage SH, the Group paid consideration of RMB17,000,000 (US$2,526,000) in funding APGC apoptosis related R&D programs (“APGC R&D programs”). A joint steering committee with members from the Group and APGC has also been set up to monitor the progress of the APGC R&D programs. In return, the Group has been granted the exclusive right in mainland China to develop and commercialize the cancer therapeutics that are developed through APGC R&D programs, while APGC will retain the rights in Hong Kong, Taiwan and Macau. RMB5,000,000 and RMB11,000,000 (US$1,748,000) of the RMB17,000,000 prepayment has been utilized and expensed as R&D costs as of December 31, 2010 and 2011, respectively. The Group may be required to pay future milestone payments and royalty payments to APGC after the commercialization of apoptosis. Such terms are still under negotiation.
As of December 31, 2011, the Group has determined that APGC and Ascentage SH are VIEs but the Group is not the primary beneficiary as the Group does not have the power to direct or control the operational activities that most significantly impact the economic performance of the VIEs. As such, the Group accounts for their investment in APGC and Ascentage SH as equity method investments. As required by ASC 810-10, the Group will continually assess whether it is the primary beneficiary of APGC and Ascentage SH.
A tabular comparison of the carrying amount of the Group’s investment in both APGC and Ascentage SH, by category, as at December 31, 2011 and the maximum exposure to loss, is listed below:
| Carrying value of investment in VIE | Maximum | |||||||||||
| Equity | R&D | exposure to | ||||||||||
| investment | prepayment | Total | loss | |||||||||
| RMB ’000 | RMB ’000 | RMB ’000 | RMB ’000 | |||||||||
| APGC | - | 1,000 | 1,000 | 1,000 | ||||||||
| Ascentage SH | 2,245 | 5,000 | 7,245 | 7,245 | ||||||||
F-31
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
As APGC and Ascentage SH are both incorporated as limited liability companies, the Group’s maximum loss is limited to its investment cost as the Group is not obligated to fund losses of APGC and Ascentage SH.
(c) Isotechnika
In August 2010, the Group entered into a licensing, development and commercialization agreement with Isotechnika, a Canada-based company focused on the discovery and development of immune modulating therapeutics, for voclosporin, a next generation calcineurin inhibitor being developed for use in the prevention of organ rejection following transplantation and the treatment of autoimmune diseases.
Under the terms of the agreement, the Group obtained exclusive rights to all transplant and autoimmune indications of voclosporin, exclusive of ophthalmic indications and medical devices which were previously licensed to others, in the greater PRC, including Hong Kong and Taiwan. Under a separate commercial supply agreement, Isotechnika will provide supply to the Group on a cost-plus basis.
The Group made an upfront non-refundable licensing payment of US$1,500,000 in December 2010 and may be required to pay milestone payments when certain criteria are met, as well as ongoing royalties based on sales of voclosporin. The upfront licensing payment was expensed as R&D costs for the year ended December 31, 2010 as it was determined that it had no alternative use.
17. Taxation
(a) Income taxes
Cayman Islands and British Virgin Islands Taxes
Under the current laws of the Cayman Islands and British Virgin Islands, the Company and Collected Mind, are not subject to tax on income or capital gains. In addition, upon payments of dividends by the Company or Collected Mind, no Cayman Islands or British Virgin Islands withholding tax will be imposed.
U.S. Tax
IP Series and Assets Series, as part of 3SBio, LLC, are subject to U.S. taxes. For the year ended December 31, 2010, both IP Series and Assets Series had no taxable income. For the year ended December 31, 2011, IP Series has no U.S. taxable income and Assets Series was assessing its U.S. income tax obligation.
Hong Kong Tax
Hong Kong profits tax has not been assessed on HK Sunshine as the Group had no assessable profits arising in Hong Kong during the year ended December 31, 2010 and 2011.
PRC Tax
Under the current PRC Enterprise Income Tax (“EIT”) Law which has been effective since 2008, domestic enterprises and foreign investment enterprises (the "FIE") are subject to, with limited exceptions, a unified 25% enterprise income tax rate, except for certain entities that enjoyed the tax holidays.
F-32
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
According to the EIT law, entities that qualify as High and New Technology Enterprise (“HNTE”) are entitled to the preferential EIT rate of 15%. Shenyang Sunshine qualified as HNTE from 2009 to 2011. In February 2012, Shenyang Sunshine successfully renewed the HNTE certificate which was effective retroactively from January 1, 2011 to December 31, 2013. Other PRC entities are subject to EIT rate of 25% effective from January 1, 2008.
Under the EIT Law, an enterprise established outside of the PRC with effective management located in the PRC is considered a resident enterprise and will be subject to the EIT on its worldwide income. The Implementation Regulations of the EIT Law further defines effective management as substantial and overall management having control over production and business operations, personnel, accounting and properties of an enterprise. The Company, if considered a PRC resident enterprise for tax purposes, would be subject to the applicable PRC EIT rate on its worldwide income. The Company believe the current administrative practice and interpretation do not apply the concept of “place of effective management” to us. The Company has not accrued for PRC tax on such basis. Management will continue to monitor the related development and application and its tax position.
Furthermore, under the current EIT Law, dividends paid by a foreign investment enterprise to any of its foreign non-resident enterprise investors are subject to a 10% withholding tax. A lower tax rate will be applied if such foreign non-resident enterprise investor’s jurisdiction of incorporation has signed a tax treaty or arrangement for the avoidance of double taxation and the prevention of fiscal evasion with respect to taxes on income with China. The subsequent circular issued in January 2008 stipulates that no income tax will be withheld on the distribution of earnings of foreign invested enterprises where the relevant earnings were generated prior to January 1, 2008 with dividends distribution declared in 2008 and beyond. However, the income tax withholding rate will be 10% or the lower treaty rate on earnings generated after December 31, 2007.
Shenyang Sunshine is entitled to an extra 50% reduction on qualified R&D expenditures according to the EIT law.
Income tax expense/(benefit) represents PRC income tax as follows:
| Year ended December 31, | ||||||||||||
| 2009 | 2010 | 2011 | 2011 | |||||||||
| RMB’000 | RMB’000 | RMB’000 | US$’000 | |||||||||
| Current | 13,327 | 20,697 | 28,651 | 4,552 | ||||||||
| Deferred | (1,591 | ) | 1,075 | (441 | ) | (70 | ) | |||||
| Total | 11,736 | 21,772 | 28,210 | 4,482 | ||||||||
The components of income/(losses) before income tax expense are as follows:
| Year ended December 31, | ||||||||||||
| 2009 | 2010 | 2011 | 2011 | |||||||||
| RMB’000 | RMB’000 | RMB’000 | US$’000 | |||||||||
| PRC | 105,039 | 137,141 | 176,302 | 28,012 | ||||||||
| Other Countries | (9,868 | ) | (34,083 | ) | (39,932 | ) | (6,345 | ) | ||||
| 95,171 | 103,058 | 136,370 | 21,667 | |||||||||
F-33
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
The income before tax expense for Other Countries refers to the Company and Collected Mind located outside the PRC.
Following is a reconciliation of total income tax expense to the amounts computed by applying the PRC income tax rate of 25% for the years ended December 31, 2009, 2010 and 2011:
|
|
Year ended December 31, | |||||||||||
|
|
2009 | 2010 | 2011 | 2011 | ||||||||
|
|
RMB’000 | RMB’000 | RMB’000 | US$’000 | ||||||||
|
|
||||||||||||
|
Income before income tax expense |
95,171 | 103,058 | 136,370 | 21,667 | ||||||||
|
|
||||||||||||
|
Computed tax expense at statutory rate of 25% |
23,793 | 25,765 | 34,093 | 5,417 | ||||||||
|
|
||||||||||||
|
Increase/(reduction) in income taxes: |
||||||||||||
|
International rate differences |
2,467 | 5,277 | 8,613 | 1,368 | ||||||||
|
PRC preferential tax rates |
(10,476 | ) | (11,523 | ) | (18,043 | ) | (2,867 | ) | ||||
|
Non-deductible expenses |
269 | 847 | 2,653 | 422 | ||||||||
|
Non-taxable income |
(3,160 | ) | (56 | ) | (116 | ) | (18 | ) | ||||
|
Change in valuation allowance on deferred tax assets |
(247 | ) | 1,177 | 1,167 | 185 | |||||||
|
Tax concession for R&D costs |
(852 | ) | (1,706 | ) | (1,743 | ) | (277 | ) | ||||
|
Withholding income tax |
- | 2,163 | 1,421 | 226 | ||||||||
|
Others |
(58 | ) | (172 | ) | 165 | 26 | ||||||
|
|
||||||||||||
|
Income tax expense |
11,736 | 21,772 | 28,210 | 4,482 | ||||||||
|
Effective tax rate % |
12.3% | 21.1% | 20.7% | 20.7% | ||||||||
As of December 31, 2011, the Group has unused tax losses of RMB9,981,000 (US$1,586,000). The tax losses will expire as follows if unutilized by:
| RMB’000 | US$’000 | |||||
| 2012 | - | - | ||||
| 2013 | 233 | 37 | ||||
| 2014 | 313 | 50 | ||||
| 2015 | 2,922 | 464 | ||||
| 2016 | 6,513 | 1,035 | ||||
| 9,981 | 1,586 |
The tax effects of temporary differences that give rise to significant portions of the deferred tax assets are presented below:
F-34
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
|
|
December 31, | ||||||||
|
|
2010 | 2011 | 2011 | ||||||
|
|
RMB’000 | RMB’000 | US$’000 | ||||||
|
|
|||||||||
|
Property, plant and equipment |
281 | 312 | 50 | ||||||
|
Accounts receivable |
530 | 525 | 83 | ||||||
|
Long-term receivable |
75 | 56 | 9 | ||||||
|
Inventories |
98 | 149 | 24 | ||||||
|
Accrued expenses |
2,372 | 2,630 | 418 | ||||||
|
Deferral of R&D costs for tax purposes |
167 | 56 | 9 | ||||||
|
Tax loss carry forwards |
866 | 2,269 | 360 | ||||||
|
|
|||||||||
|
Total deferred tax assets |
4,389 | 5,997 | 953 | ||||||
|
|
|||||||||
|
Less: valuation allowance |
(1,818 | ) | (2,985 | ) | (474 | ) | |||
|
|
|||||||||
|
Net deferred tax assets |
2,571 | 3,012 | 479 | ||||||
|
|
|||||||||
|
Consolidated balance sheet classification: |
|||||||||
|
|
|||||||||
|
Current |
2,198 | 2,750 | 437 | ||||||
|
Non-current |
373 | 262 | 42 | ||||||
|
Total |
2,571 | 3,012 | 479 | ||||||
An analysis of the valuation allowance is as follows:
|
|
Year ended December 31, | |||||||||||
|
|
2009 | 2010 | 2011 | 2011 | ||||||||
|
|
RMB’000 | RMB’000 | RMB’000 | US$’000 | ||||||||
|
|
||||||||||||
|
Balance at beginning of year |
1,252 | 641 | 1,818 | 289 | ||||||||
|
(Credited)/charged to income |
(247 | ) | 1,177 | 1,167 | 185 | |||||||
|
Tax losses expired during the year |
(364 | ) | - | - | - | |||||||
|
|
||||||||||||
|
Balance at end of year |
641 | 1,818 | 2,985 | 474 | ||||||||
In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which the temporary differences become deductible or utilized. The Group considers projected future taxable income and tax planning strategies in making this assessment. Based upon an assessment of the level of historical taxable income and projections for future taxable income over the periods in which the deferred tax assets are deductible or can be utilized, management provided valuation allowances of RMB1,818,000 and RMB2,985,000 (US$474,000) as of December 31, 2010 and 2011, respectively.
F-35
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
As of December 31, 2011, the Company intends to indefinitely reinvest the earnings of its foreign subsidiaries. Determination of the amount of the unrecognized deferred tax liability related to distributing these earnings is not practicable. Further, the Company has not recorded deferred tax on the outside basis of Liaoning Sunshine, the VIE of the Group, as Liaoning Sunshine is currently in an accumulated loss position.
The Company has evaluated its income tax uncertainty under ASC 740-10. ASC 740-10 clarifies the accounting for uncertainty in income taxes by prescribing the recognition threshold a tax position is required to meet before being recognized in the financial statements. The Company has elected to classify interest and penalties related to an uncertain tax position, if and when required, as part of income tax expense in the consolidated statements of income.
In accordance with ASC 740-10, the Group did not have any significant unrecognized tax benefits for the years ended December 31, 2010 and 2011. In addition, the Company does not expect that the amount of unrecognized tax benefits will change significantly within the next twelve months.
The income tax returns of the Group’s operating entities in the PRC for the years from 2007 to 2011 are open to examination by the PRC state and local tax authorities.
(b) PRC value added tax (“VAT”)
According to the value-added tax policy from the relevant tax authorities, the sale of pharmaceutical equipment and products by the Group’s PRC-based subsidiaries and consolidated VIE are subject to an output VAT of 17%, while the purchase of products by these entities is subject to an input VAT tax rate of 17%. VAT payable or receivable is the net difference between periodic output VAT and deductible input VAT.
18. Share-based compensation
On September 5, 2006, the Company adopted the 2006 stock incentive plan (the “2006 Plan”) pursuant to which the shares, share options, restricted shares and restricted share units (“RSU”) of the Company can be granted to directors and employees upon the approval by the board of directors or the compensation committee of the board of directors. Under the 2006 Plan, the Company is authorized to issue up to 10,000,000 shares plus a number of shares equal to 10% of any additional shares of the Company issued following the date of the adoption of the 2006 Plan by the board of directors. The 2006 Plan will remain in effect for ten years from the date of adoption, unless otherwise extended. The term of each option granted under the 2006 Plan may not exceed five years from the date of grant.
On March 31, 2010, the Company adopted the 2010 equity incentive plan (the “2010 Plan”) which provides for the grant of share options, share appreciation rights, dividend equivalent rights, shares, restricted shares and restricted share units of the Company to employees, directors and consultants. Under the 2010 Plan, the Company is authorized to issue up to 22,500,000 ordinary shares, subject to possible adjustments. The 2010 Plan is administered by the board of directors and the compensation committee of the board of directors. With respect to the grant of awards to employees or consultants who are neither directors nor officers, the board of directors may authorize one or more officers to grant such awards. The purpose of the plan is to attract and retain the best available personnel, to provide additional incentives to employees, directors and consultants and to promote the success of the company’s business. The 2010 Plan became effective in April 2010. It will continue to be in effect for a term of ten years unless terminated sooner.
(a) Nonvested Shares and Restricted Share Units (“RSUs”)
In March 2011, the Company granted 63,910 and 2,279,200 shares to independent directors and employees, respectively. There are no transferability restrictions attached to the shares. Grants to independent directors will vest evenly every six months in the next year from the grant date; while grants to the executive management will vest on the fourth anniversary of the grant date.
F-36
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
A summary of the status of the Company’s nonvested shares and RSUs as of December 31, 2010 and the changes during the year ended December 31, 2011, is presented below:
|
|
Weighted | Weighted | ||||||||||
|
|
average | average | ||||||||||
|
|
Number of | grant-date | grant-date | |||||||||
|
|
nonvested | fair value | Number of | fair value | ||||||||
|
|
shares | (per share) | RSUs | (per RSU) | ||||||||
|
|
US$ | US$ | ||||||||||
|
Nonvested at |
||||||||||||
|
December 31, 2010 |
2,686,056 | 1.56 | 2,848,125 | 1.55 | ||||||||
|
Awarded |
2,343,110 | 2.20 | - | - | ||||||||
|
Vested |
(112,845 | ) | 1.49 | (949,375 | ) | 1.55 | ||||||
|
Forfeited |
- | - | - | - | ||||||||
|
Nonvested at |
||||||||||||
|
December 31, 2011 |
4,916,321 | 1.86 | 1,898,750 | 1.55 |
As of December 31, 2011, total unrecognized compensation costs related to nonvested shares and RSUs amounted to RMB32,099,000 (US$5,100,000) and RMB5,690,000 (US$904,000), respectively, which is expected to be recognized over a weighted-average period of 2.0 years and 1.3 years, respectively. The total fair value of vested shares during the years ended December 31, 2009, 2010 and 2011, was RMB365,000, RMB11,024,000 and RMB10,341,000 (US$1,643,000), respectively. To the extent the actual forfeiture rate is different from the original estimate, actual share-based compensation costs related to these awards may be different from the expectation. The aggregate intrinsic value of nonvested shares and RSUs as of December 31, 2011 was RMB62,624,000 (US$9,950,000).
(b) Share Options
The Company uses a lattice-based model to estimate the fair value of options awarded.
The historical volatility of the Company’s shares and the volatility of a combination of peer companies of similar nature and size were used to estimate the expected volatility of the Company’s shares. The risk-free rate for periods within the expected term of the options is based on the U.S. government bond in effect at the time of grant. Expected dividend yields are based on historical dividends.
The following table presents the assumptions used to estimate the fair values of the share options granted in the periods presented:
|
|
Year ended December 31, | ||||||||
|
|
2009 | 2010 | 2011 | ||||||
|
Expected volatility |
75% | - | - | ||||||
|
Weighted average expected volatility |
75% | - | - | ||||||
|
Expected dividends |
0.0 | - | - | ||||||
|
Sub-optimal early exercise factor |
2 times | - | - | ||||||
|
Risk-free interest rate |
1.77% | - | - | ||||||
F-37
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
No share options were granted in 2010 and 2011.
In addition, the Company applies an expected forfeiture rate in determining the grant date fair value of the share option grants. The estimation of the forfeiture rate was based primarily upon historical experience of employee turnover. To the extent the Company revises this estimate in the future, compensation cost could be materially impacted.
The following table summarizes the share option activity for the year ended December 31, 2011:
|
|
Weighted | |||||||||||
|
|
Weighted | average | ||||||||||
|
|
average | remaining | Aggregate | |||||||||
|
|
Number of | exercise | contractual | intrinsic | ||||||||
|
|
options | price | life | value | ||||||||
|
|
US$ | Years | US$ | |||||||||
|
Exercisable as at December 31, 2010 |
2,128,624 | 0.90 | 2.43 | 2,692,000 | ||||||||
|
Granted |
- | - | ||||||||||
|
Expired |
(317,184 | ) | 1.17 | |||||||||
|
Forfeited |
(12,845 | ) | 0.78 | |||||||||
|
Exercised |
(756,791 | ) | 0.96 | |||||||||
|
|
||||||||||||
|
Outstanding, December 31, 2011 |
1,041,804 | 0.78 | 2.25 | 708,427 | ||||||||
|
Vested and expected to vest as at December 31, 2011 |
995,137 | 0.78 | 2.25 | 676,693 | ||||||||
|
Exercisable as at December 31, 2011 |
458,466 | 0.78 | 2.25 | 311,757 |
The aggregate intrinsic value in the table above represents the value of the Company’s closing stock price on the last trading day in 2011 in excess of the exercise price of share options. Total intrinsic value of share options exercised for the three years ended December 31, 2009, 2010 and 2011 was US$5,000, US$706,000 and US$875,000 (RMB5,507,000), respectively.
As of December 31, 2011, total unrecognized compensation costs related to unvested share options amounted to RMB120,000 (US$19,000), which is expected to be recognized over a weighted-average period of 0.25 years. To the extent the actual forfeiture rate is different from the original estimate; actual share-based compensation costs related to these awards may be different from the expectation.
The table below summarizes the weighted average fair value and exercise price of share options granted:
|
|
Year ended December 31, | ||||||||
|
|
2009 | 2010 | 2011 | ||||||
|
Weighted average grant-day fair value of share options granted during the year: |
|||||||||
|
Where exercise price is lower than market price |
- | - | - | ||||||
|
|
|||||||||
|
Where exercise price is equal to market price |
US$0.41 per share option | - | - | ||||||
|
Weighted average exercise price of share options granted during the year |
|||||||||
|
Where exercise price is lower than market price |
- | - | - | ||||||
|
Where exercise price is equal to market price |
US$0.78 per share option | - | - |
F-38
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
The total fair value of share options vested during the years ended December 31, 2009, 2010 and 2011 was RMB2,637,000, RMB3,281,000 and RMB1,542,000 (US$245,000), respectively.
(c) Compensation cost
Total compensation cost (for shares, RSUs and share options) recognized is as follows:
|
|
Year ended December 31, | |||||||||||
|
|
2009 | 2010 | 2011 | 2011 | ||||||||
|
|
RMB’000 | RMB’000 | RMB’000 | US$’000 | ||||||||
|
|
||||||||||||
|
Cost of revenues |
557 | 1,007 | 423 | 67 | ||||||||
|
Research and development costs |
1,216 | 6,864 | 7,316 | 1,163 | ||||||||
|
Sales, marketing and distribution expenses |
577 | 907 | 815 | 129 | ||||||||
|
General and administrative expenses |
4,210 | 15,720 | 12,639 | 2,008 | ||||||||
|
|
||||||||||||
|
Charged to income statement |
6,560 | 24,498 | 21,193 | 3,367 | ||||||||
|
Capitalized as property, plant and equipment |
198 | 189 | 319 | 51 | ||||||||
|
Capitalized as inventory |
- | - | 277 | 44 | ||||||||
|
Total share-based compensation cost |
6,758 | 24,687 | 21,789 | 3,462 | ||||||||
19. Long-term receivables
Long-term receivables, net, consist of the following:
|
|
December 31, | ||||||||
|
|
2010 | 2011 | 2011 | ||||||
|
|
RMB’000 | RMB’000 | US$’000 | ||||||
|
|
|||||||||
|
Long-term receivables |
3,416 | 3,929 | 624 | ||||||
|
Less: Allowance for doubtful accounts |
(858 | ) | (818 | ) | (130 | ) | |||
|
Total long-term receivables, net |
2,558 | 3,111 | 494 | ||||||
The allowance for doubtful accounts was charged to the consolidated statements of income during 2011. The Group’s long-term receivables are due from third-party customers, and are to be repaid in monthly instalments over the next 2.5 to 4.3 years. The Group has a credit policy in place and the exposure to credit risk is monitored on an ongoing basis. The Group does not obtain collateral from customers.
F-39
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
20. Other income/(loss), net
The Group’s other income, net, consists of the following:
|
|
Year ended December 31, | |||||||||||
|
|
2009 | 2010 | 2011 | 2011 | ||||||||
|
|
RMB’000 | RMB’000 | RMB’000 | US$’000 | ||||||||
|
|
||||||||||||
|
Recovery of investor relationship fees |
1,159 | 2,065 | 1,895 | 301 | ||||||||
|
Exchange gain/(loss) |
596 | (249 | ) | 176 | 28 | |||||||
|
Gain/(loss) on disposal of property, plant and equipment |
(320 | ) | 257 | (2,226 | ) | (354 | ) | |||||
|
Withholding business tax |
- | (1,082 | ) | (874 | ) | (139 | ) | |||||
|
Others |
160 | 315 | 178 | 29 | ||||||||
|
|
||||||||||||
|
Total other income/(loss), net |
1,595 | 1,306 | (851 | ) | (135 | ) | ||||||
21. Related party transactions
The Company’s related party transactions are all incurred in the normal course of conducting its business, and are all with its equity investees, APGC and its wholly owned subsidiary, Ascentage Jiangsu, as well as Ascentage SH. The related party transactions represent the R&D expenses incurred on APGC R&D programs, and the prepayments to related parties represents the upfront R&D funding for the APGC R&D programs.
A summary of these transactions for the years ended December 31, 2009, 2010 and 2011 is as follows:
| Year ended December 31, | ||||||||||||
| 2009 | 2010 | 2011 | 2011 | |||||||||
| RMB’000 | RMB’000 | RMB’000 | US$’000 | |||||||||
| R&D costs | - | 5,000 | 6,000 | 953 | ||||||||
The prepayment to related parties balances as of December 31, 2009, 2010 and 2011 are as follows:
| As at December 31, | ||||||||||||
| 2009 | 2010 | 2011 | 2011 | |||||||||
| RMB’000 | RMB’000 | RMB’000 | US$’000 | |||||||||
| Prepayment of R&D costs | - | 12,000 | 6,000 | 953 | ||||||||
22. Commitments and contingencies
(a) Operating lease commitments
The Group leases land from the PRC government under operating leases (Note 9). Operating lease charges are prepaid in full at the inception of the lease. The Group also leases staff quarters and motor vehicles under operating leases. The leases typically run for a period of one year.
F-40
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
For the years ended December 31, 2009, 2010 and 2011, total rental expenses for operating leases, including land lease expense, were RMB1,183,000, RMB885,000 and RMB2,282,000 (US$363,000), respectively.
As of December 31, 2011, total future minimum lease payments under non-cancellable operating leases were as follows:
| RMB’000 | US$’000 | |||||
| 2012 | 552 | 88 | ||||
| 2013 | 33 | 5 | ||||
| 2014 | - | - | ||||
| Total | 585 | 93 |
(b) Capital commitments
Capital commitments for purchase, installation and construction of property, plant and equipment as of December 31, 2011 were RMB12,112,000 (US$1,924,000). They are all due within the next year.
23. Accumulated balances related to each component of other comprehensive income
|
|
Net unrealized | Accumulated | |||||||
|
|
gain/(loss) on | Foreign currency | other | ||||||
|
|
available-for-sale | translation | comprehensive | ||||||
|
|
securities | adjustments | loss | ||||||
|
|
RMB’000 | RMB’000 | RMB’000 | ||||||
|
Balance as of January 1, 2009 |
(605 | ) | (101,521 | ) | (102,126 | ) | |||
|
Current year other comprehensive income |
1,205 | 313 | 1,518 | ||||||
|
Balance as of December 31, 2009 |
600 | (101,208 | ) | (100,608 | ) | ||||
|
Current year other comprehensive income/(loss) |
21,335 | (10,258 | ) | 11,077 | |||||
|
Balance as of December 31, 2010 |
21,935 | (111,466 | ) | (89,531 | ) | ||||
|
Current year other comprehensive loss |
(26,545 | ) | (10,214 | ) | (36,759 | ) | |||
|
Balance as of December 31, 2011 |
(4,610 | ) | (121,680 | ) | (126,290 | ) | |||
|
Balance as of December 31, 2011 (US$’000) |
(732 | ) | (19,333 | ) | (20,065 | ) |
The net unrealized holding gain/(loss) on available-for-sale securities arising during the year and the amount of gains and losses reclassified out of accumulated other comprehensive income into earnings and recognized in other comprehensive income for the years ended December 31, 2009, 2010 and 2011 are as follows:
|
|
Year ended December 31, | |||||||||||
|
|
2009 | 2010 | 2011 | 2011 | ||||||||
|
|
RMB’000 | RMB’000 | RMB’000 | US$’000 | ||||||||
|
Net unrealized (loss)/gain in available-for-sale securities arising during the year |
(1,808 | ) | 21,335 | (27,879 | ) | (4,430 | ) | |||||
|
Less: reclassification adjustment for realized gain included in net income on disposal of available-for-sale securities |
1,611 | - | - | - | ||||||||
|
|
||||||||||||
|
Less: reclassification adjustment for realized loss included in net income on providing impairment on available-for-sale securities |
(4,624 | ) | - | (1,334 | ) | (212 | ) | |||||
|
|
||||||||||||
|
Net unrealized gain/(loss) in available-for-sale securities recognized in other comprehensive income |
1,205 | 21,335 | (26,545 | ) | (4,218 | ) |
F-41
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
24. Fair value measurement
ASC 820-10: Fair Value Measurements and Disclosures: Overall (“ASC 820-10”), establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
Level 1 — Observable inputs that reflect quoted prices (unadjusted) for identical assets or liabilities in active markets;
Level 2 — Include other inputs that are directly or indirectly observable in the marketplace; and
Level 3 — Unobservable inputs which are supported by little or no market activity.
ASC 820-10 describes three main approaches to measuring the fair value of assets and liabilities: (1) market approach; (2) income approach and (3) cost approach. The market approach uses prices and other relevant information generated from market transactions involving identical or comparable assets or liabilities. The income approach uses valuation techniques to convert future amounts to a single present value amount. The measurement is based on the value indicated by current market expectations about those future amounts. The cost approach is based on the amount that would currently be required to replace an asset.
Assets and liabilities measured at fair value on a recurring basis
In accordance with ASC 820-10, the Group measures cash and cash equivalents, restricted cash, time deposits with financial institutions and available-for-sale securities, at fair value. Cash and cash equivalents, restricted cash, time deposits with financial institutions and available-for-sale debt and equity securities are valued using quoted market prices. The convertible promissory note issued by Uricase was initially recognized at the purchase price. During 2011, the Note was written down to zero value and an impairment loss of US$200,000 (RMB1,334,000) was recorded.
Assets measured at fair value on a recurring basis are summarized below:
|
|
As of December 31, 2010 | |||||||||||
|
|
Quoted prices in | Significant | Total fair value | |||||||||
|
|
active market for | unobservable | ||||||||||
|
|
identical assets | inputs | ||||||||||
|
|
(Level1 | ) | (Level 3 | ) | ||||||||
|
|
(RMB’000 | ) | (RMB’000 | ) | (RMB’000 | ) | (US’000 | ) | ||||
|
|
||||||||||||
|
Cash and cash equivalents |
153,250 | - | 153,250 | 23,220 | ||||||||
|
Restricted cash |
1,662 | - | 1,662 | 252 | ||||||||
|
Time deposits with financial institutions |
498,405 | - | 498,405 | 75,516 | ||||||||
|
Available-for-sale securities |
62,044 | 1,320 | 63,364 | 9,601 | ||||||||
| 715,361 | 1,320 | 716,681 | 108,589 |
F-42
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
| As of December 31, 2011 | ||||||||||||
| Quoted prices in | Significant | Total fair value | ||||||||||
| active market for | unobservable | |||||||||||
| identical assets | inputs | |||||||||||
| (Level1 | ) | (Level 3 | ) | |||||||||
| (RMB’000 | ) | (RMB’000 | ) | (RMB’000 | ) | (US’000 | ) | |||||
|
Cash and cash equivalents |
245,813 | - | 245,813 | 39,056 | ||||||||
|
Restricted cash |
665 | - | 665 | 106 | ||||||||
|
Time deposits with financial institutions |
519,201 | - | 519,201 | 82,492 | ||||||||
|
Available-for-sale securities |
33,678 | - | 33,678 | 5,351 | ||||||||
|
|
||||||||||||
|
Total |
799,357 | - | 799,357 | 127,005 | ||||||||
The following table presents the reconciliation for the Group’s assets measured and recorded at fair value on a recurring basis, using significant unobservable inputs (Level 3) for the year ended December 31, 2011:
|
|
Significant Unobservable Inputs (Level 3) | |||||
|
|
(RMB’000 | ) | (US$’000 | ) | ||
|
|
||||||
|
Balance at December 31, 2010 |
1,320 | 210 | ||||
|
Purchases |
- | - | ||||
|
Foreign currency translation adjustment previously included in other comprehensive income |
14 | 2 | ||||
|
Impairment loss |
(1,334 | ) | (212 | ) | ||
|
|
||||||
|
Balance at December 31, 2011 |
- | - | ||||
Assets and liabilities measured at fair value on a nonrecurring basis
The Group measures certain financial assets, including equity method investments, at fair value on a nonrecurring basis only if an impairment charge were to be recognized. The Group’s non-financial assets, such as intangible assets, including IPR&D, and fixed assets, would be measured at fair value only if they were determined to be other-than-temporarily impaired.
For the year ended December 31, 2011 the Group recorded an impairment loss of US$200,000 (RMB 1,334,000) in respect of the investment in the Note issued by Uricase (Note 6).
F-43
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
25. Subsequent events
(a) Grant of nonvested shares
On January 5, 2012, the Company granted nonvested shares to employees and independent directors pursuant to the 2010 Plan. A total of 2,450,000 nonvested shares (equal to 350,000 ADSs) were awarded. The nonvested shares granted to employees will either cliff vest on the seventh anniversary or vest in four equal tranches on the first, second, third and fourth anniversary from the date of grant. The nonvested shares granted to the independent directors will vest as follows: 50% six months after the date of grant and another 50% one year after the date of grant.
(b) Collaboration with DaVita Inc.
On March 9, 2012, the Group announced a collaboration agreement to establish a company in the PRC with DaVita Inc., a leading provider of kidney care services for those diagnosed with chronic kidney disease, to provide kidney care services in Jilin and Liaoning provinces in northeastern China. The Group will invest US$6 million for a 30% share of equity interest in the PRC company. In addition, DaVita and 3SBio have also agreed to enter into a nationwide supply agreement for anemia management drugs.
26. Condensed financial information of the Company
As of December 31, 2011, the restricted net assets held by the Group’s consolidated PRC subsidiaries and VIE exceeded 25% of the Group’s consolidated net assets.
Under PRC law, there are certain restrictions on the Company’s PRC subsidiaries and VIE with respect to transferring certain of their net assets to the Company either in the form of dividends, loans, or advances. Amounts restricted include paid-in capital and statutory surplus reserve of the Company’s PRC subsidiary and the net assets of the VIE in which the Company has no legal ownership, totaling approximately RMB610,693,000 (US$97,029,000) as of December 31, 2011. Foreign exchange and other regulation in the PRC may further restrict the Company’s PRC subsidiaries and VIE from transferring funds to the Company in the form of dividends, loans or advances. The Company has approximately RMB597,678,000 and RMB675,083,000 of cash and cash equivalents, restricted cash and time deposits with original maturities over three months in the PRC as of December 31, 2010 and 2011, respectively, of which approximately RMB470,151,000 and RMB598,809,000 are denominated in RMB as of December 31, 2010 and 2011 respectively.
The Company did not have significant capital and other commitments, long-term obligations, or guarantees as of December 31, 2010 and 2011.
F-44
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
26. Condensed Financial Information of the Company (continued)
Condensed Balance Sheets of the Company
(RMB and US$
expressed in thousands)
|
|
December 31, | ||||||||
|
|
2010 | 2011 | 2011 | ||||||
|
|
RMB | RMB | US$ | ||||||
|
ASSETS |
|||||||||
|
Current assets |
|||||||||
|
Cash and cash equivalents |
6,624 | 1,529 | 243 | ||||||
|
Time deposits with financial institutions |
96,159 | 44,667 | 7,097 | ||||||
|
Prepaid expenses and other receivables |
1,338 | 701 | 111 | ||||||
|
Available-for-sale securities |
50,667 | 22,830 | 3,627 | ||||||
|
Amounts due from subsidiaries |
600,134 | 631,028 | 100,260 | ||||||
|
|
|||||||||
|
Total current assets |
754,922 | 700,755 | 111,338 | ||||||
|
|
|||||||||
|
Available-for-sale securities |
12,697 | 10,848 | 1,724 | ||||||
|
Investment in subsidiaries |
376,737 | 530,884 | 84,350 | ||||||
|
|
|||||||||
|
Total assets |
1,144,356 | 1,242,487 | 197,412 | ||||||
|
|
|||||||||
|
LIABILITIES AND SHAREHOLDERS' EQUITY |
|||||||||
|
Current liabilities |
|||||||||
|
|
|||||||||
|
Accrued expenses and other payables |
508 | 323 | 51 | ||||||
|
Amounts due to subsidiaries |
7,795 | 7,795 | 1,239 | ||||||
|
|
|||||||||
|
Total current liabilities |
8,303 | 8,118 | 1,290 | ||||||
|
|
|||||||||
|
Total liabilities |
8,303 | 8,118 | 1,290 | ||||||
|
|
|||||||||
|
Shareholders’ equity |
|||||||||
|
Share capital – ordinary shares US$0.0001 par value, 500,000,000 shares authorized, |
123 | 124 | 20 | ||||||
|
152,654,148 and 154,473,159 issued and outstanding as of December 31, 2010 and 2011 respectively |
|||||||||
|
Additional paid-in capital |
946,717 | 973,218 | 154,629 | ||||||
|
Accumulated other comprehensive loss |
(89,531 | ) | (126,290 | ) | (20,065 | ) | |||
|
Retained earnings |
278,744 | 387,317 | 61,538 | ||||||
|
Total shareholders’ equity |
1,136,053 | 1,234,369 | 196,122 | ||||||
|
Total liabilities and shareholders’ equity |
1,144,356 | 1,242,487 | 197,412 | ||||||
F-45
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
26. Condensed Financial Information of the Company (continued)
Condensed Statements of Income of the Company
(RMB and
US$ expressed in thousands)
|
|
Year ended December 31, | |||||||||||
|
|
2009 | 2010 | 2011 | 2011 | ||||||||
|
|
RMB | RMB | RMB | US$ | ||||||||
|
|
||||||||||||
|
Operation expenses |
||||||||||||
|
General and administrative expenses |
(6,952 | ) | (24,790 | ) | (21,865 | ) | (3,474 | ) | ||||
|
Loss from operations |
(6,952 | ) | (24,790 | ) | (21,865 | ) | (3,474 | ) | ||||
|
|
||||||||||||
|
Interest income |
6,650 | 2,638 | 1,536 | 244 | ||||||||
|
Net realized gain on available-for-sale securities |
1,611 | - | - | - | ||||||||
|
Impairment loss on available-for-sale securities |
(4,624 | ) | - | (1,334 | ) | (212 | ) | |||||
|
Other income, net |
955 | 228 | 538 | 86 | ||||||||
|
Total other income, net |
4,592 | 2,866 | 740 | 118 | ||||||||
|
|
||||||||||||
|
Loss before share of net income of subsidiaries and VIE |
(2,360 | ) | (21,924 | ) | (21,125 | ) | (3,356 | ) | ||||
|
|
||||||||||||
|
Share of net income of subsidiaries and VIE, net of tax |
85,795 | 103,210 | 129,698 | 20,607 | ||||||||
|
|
||||||||||||
|
Net income |
83,435 | 81,286 | 108,573 | 17,251 | ||||||||
F-46
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
26. Condensed Financial Information of the Company (continued)
Condensed Statements of Comprehensive Income of the Company
(RMB and US$ expressed in thousands)
|
|
Year ended December 31, | |||||||||||
|
|
2009 | 2010 | 2011 | 2011 | ||||||||
|
|
RMB | RMB | RMB | US$ | ||||||||
|
|
||||||||||||
|
Net income |
83,435 | 81,286 | 108,573 | 17,251 | ||||||||
|
|
||||||||||||
|
Other comprehensive income/(loss), net of tax: |
||||||||||||
|
Net unrealized gain/(loss) in available-for-sale securities, net of nil tax |
1,205 | 21,335 | (26,545 | ) | (4,218 | ) | ||||||
|
Foreign currency translation adjustments, net of nil tax |
392 | (26,683 | ) | (34,663 | ) | (5,507 | ) | |||||
|
Foreign currency translation adjustments related to subsidiaries and VIE, net of tax |
(79 | ) | 16,425 | 24,449 | 3,884 | |||||||
|
|
1,518 | 11,077 | (36,759 | ) | (5,841 | ) | ||||||
|
|
||||||||||||
|
Comprehensive income |
84,953 | 92,363 | 71,814 | 11,410 | ||||||||
F-47
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
26. Condensed Financial Information of the Company (continued)
Condensed Statements of Cash Flows of the Company
(RMB
and US$ expressed in thousands)
|
|
Year ended December 31, | |||||||||||
|
|
2009 | 2010 | 2011 | 2011 | ||||||||
|
|
RMB | RMB | RMB | US$ | ||||||||
|
Cash flow from operating activities |
||||||||||||
|
Net income |
83,435 | 81,286 | 108,573 | 17,251 | ||||||||
|
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
|
Share-based compensation expenses |
6,560 | 24,498 | 21,193 | 3,367 | ||||||||
|
Realized gain on available-for-sale securities |
(1,611 | ) | - | - | - | |||||||
|
Impairment of available-for-sale securities |
4,624 | - | 1,334 | 212 | ||||||||
|
Share of net income of subsidiaries and VIE |
(85,795 | ) | (103,210 | ) | (129,698 | ) | (20,607 | ) | ||||
|
Changes in operating assets and liabilities: |
||||||||||||
|
Amounts due from subsidiaries |
(157,218 | ) | (49,821 | ) | (55,343 | ) | (8,793 | ) | ||||
|
Prepaid expenses and other receivables |
434 | 960 | 596 | 94 | ||||||||
|
Accrued expenses and other payables |
7 | 501 | (185 | ) | (29 | ) | ||||||
|
Net cash used in operating activities |
(149,564 | ) | (45,786 | ) | (53,530 | ) | (8,505 | ) | ||||
|
|
||||||||||||
|
Cash flow from investing activities |
||||||||||||
|
Purchase of available-for-sale securities |
(3,475 | ) | (31,951 | ) | - | - | ||||||
|
Proceeds from sale of available-for-sale securities |
16,995 | - | - | - | ||||||||
|
Purchase of time deposits |
(143,544 | ) | (96,159 | ) | (44,667 | ) | (7,097 | ) | ||||
|
Proceeds from maturity of time deposits |
191,471 | 143,544 | 96,159 | 15,278 | ||||||||
|
Net cash provided by investing activities |
61,447 | 15,434 | 51,492 | 8,181 | ||||||||
|
|
||||||||||||
|
Cash flow from financing activities |
||||||||||||
|
Gross proceeds from exercise of stock options |
132 | 6,763 | 4,713 | 749 | ||||||||
|
Net cash provided by financing activities |
132 | 6,763 | 4,713 | 749 | ||||||||
|
|
||||||||||||
|
Effect of foreign currency exchange rate change on cash |
554 | (8,739 | ) | (7,770 | ) | (1,234 | ) | |||||
|
Net decrease in cash and cash equivalents |
(87,431 | ) | (32,328 | ) | (5,095 | ) | (809 | ) | ||||
|
|
||||||||||||
|
Cash and cash equivalents, at beginning of year |
126,383 | 38,952 | 6,624 | 1,052 | ||||||||
|
Cash and cash equivalents, at end of year |
38,952 | 6,624 | 1,529 | 243 | ||||||||
F-48
| 3SBio Inc. |
| Notes to Consolidated Financial Statements |
| For the years ended December 31, 2009, 2010 and 2011 |
26. Condensed Financial Information of the Company (continued)
Note to the Condensed Financial statements
(a) Basis of presentation
The condensed financial information of the Company has been prepared using the same accounting policies as set out in the Company’s consolidated financial statements except that the Company used the equity method to account for investment in its subsidiaries and VIE. The Company records its investment in its subsidiaries and VIE under the equity method of accounting. The subsidiaries and VIE did not pay any dividends to the Company for the years presented. Certain information and footnote disclosures normally included in financial statements prepared in accordance with U.S. GAAP have been condensed or omitted by reference to the consolidated financial statements.
F-49