Exhibit 99.1

Targeting Pruritus with Novel Peripherally - Restricted Kappa Agonist Therapeutics December 2019

Forward Looking Statements 2 This presentation contains certain forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. In some cases, you can identify forward - looking statements by the words “anticipate,” “believe,” “continue,” “estimate,” “expect,” “objective,” “ongoing,” “plan,” “propose,” “potential,” “projected”, or “up - coming” and/or the negative of these terms , or other comparable terminology intended to identify statements about the future. Examples of these forward - looking statements in this presentation include, among other things, statements concerning plans, strategies and expectations for the future, inclu din g statements regarding the expected timing of our planned clinical trials and regulatory submissions; the potential results of ong oing and planned clinical trials; future regulatory and development milestones for the Company's product candidates; the size of t he potential markets that are potentially addressable for the Company’s product candidates, including the pruritus market and th e potential commercialization of Korsuva™. These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels o f activity, performance or achievements to be materially different from the information expressed or implied by these forward - look ing statements. Although we believe that we have a reasonable basis for each forward - looking statement contained in this presentati on, we caution you that these statements are based on a combination of facts and factors currently known by us and our expectatio ns of the future, about which we cannot be certain. Factors that may cause actual results to differ materially from any future resu lts expressed or implied by any forward - looking statements include the risks described in the “Risk Factors” section of the Company’ s Annual Report on Form 10 - K for the year ended December 31, 2018, as well as those set forth from time to time in the Company’s other SEC filings, available at http://www.sec.gov. Any forward - looking statements speak only as of the date of this presentati on. The Company undertakes no obligation to publicly update any forward - looking statements, whether as a result of new information, future events or otherwise except as required by law.

The FDA has conditionally accepted KORSUVA™ as the trade name for CR845 / difelikefalin for pruritic indications. CR845 / dif eli kefalin is an investigational drug product, and its safety and efficacy have not been fully evaluated by any regulatory authority. ^ Commercialization rights to CR845 in defined indications - Japan: Maruishi Pharma; South Korea: CKD Pharma ** Breakthrough Designation for IV CR845 for Pruritus CKD - HD # VFMCRP and Cara have rights to promote in Fresenius Medical Care dialysis clinics in the US under a profit share agreement CKD - HD : Chronic Kidney Disease - Hemodialysis; CLD : Chronic Liver Disease STAGE OF DEVELOPMENT Program Indication Preclinical Phase 1 Phase 2 Phase 3 Commercial Rights (ex - Japan and S. Korea)^ KORSUVA™ Injection Pruritus CKD - HD ** US - Cara EU/Other - VFMCRP # Oral KORSUVA™ Pruritus CKD (III - V) Cara Oral KORSUVA™ Pruritus CLD Cara Oral KORSUVA™ Pruritus Atopic Dermatitis Cara 3 Development Pipeline: Chronic Pruritus
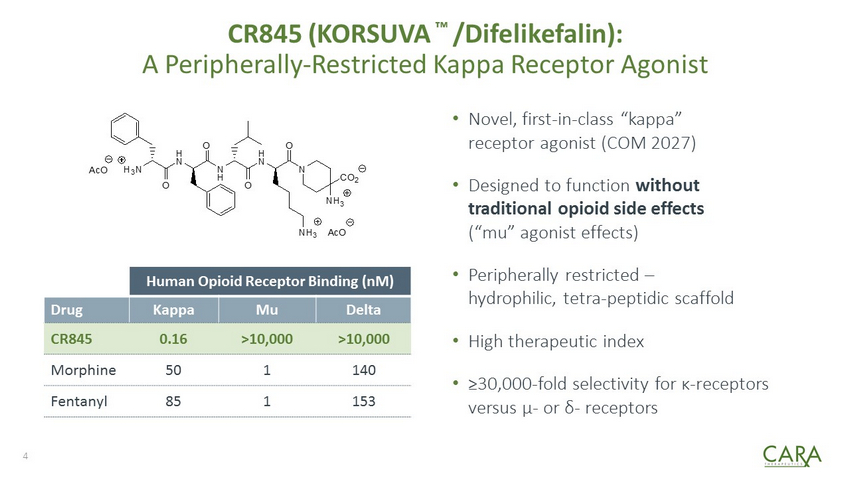
CR845 (KORSUVA ™ / Difelikefalin ): A Peripherally - Restricted Kappa Receptor Agonist • Novel, first - in - class “kappa” receptor agonist (COM 2027) • Designed to function without traditional opioid side effects (“mu” agonist effects) • Peripherally restricted – hydrophilic, tetra - peptidic scaffold • High therapeutic index • ≥30,000 - fold selectivity for κ - receptors versus μ - or δ - receptors Human Opioid Receptor Binding (nM) Drug Kappa Mu Delta CR845 0.16 >10,000 >10,000 Morphine 50 1 140 Fentanyl 85 1 153 N O H N O N H NH 3 O H N O H 3 N NH 3 CO 2 AcO AcO 4

KORSUVA ™ Acts on Neuronal and Inflammatory Targets in Pruritus Pathway Source: Paus et al.,J Clin Inv, 2006 - KORSUVA target receptor 5

KORSUVA ™ Injection for Dialysis Patients 6

Pisoni RL et al. Nephrol Dial Transplant 2006 ; Rayner et al., Clin J Am Soc Nephrol 2017 ; Fishbane et al. NDT 2001; Ramakrishnan et al. International Journal of Nephrology and Renovascular Disease 2014 ; Narita et al 2006; Shirazian et al. Int J Nephrol Renovasc Dis. 2017 ; Mathur et al., Clin J Am Soc Nephrol 2010; Szepietowski et al., Neprol Dial Transplant 2004; CKD - associated Pruritus (CKD - aP ) in Hemodialysis (HD) Patients Courtesy of Dr. Gil Yosipovitch Serious itching condition directly related to kidney failure • Reported by ~60% to 70% of HD patients - 30% to 40% patients with moderate to severe itch intensity • In contrast to dermatological pruritus, primary skin lesions are not observed - Superimposed complications of itching may include excoriations with impetigo, linear crusts, papules, ulcerations, and less commonly prurigo nodularis Itching severity associated with worsening Quality of Life (QoL) [social, emotional and physical] • Sleep disturbance, depressed mood/anxiety, socialization • Increased mortality risk 7

US Market Opportunity for KORSUVA™ Injection in Dialysis Patients Per NKF, >500K patients undergoing dialysis in the US 1 • ~60% have some form of pruritus 2,3 • Itching severity associated with worsening Quality of Life (QoL) Sleep disturbance, depressed mood/anxiety, socialization • Increased mortality risk 8 KORSUVA™ granted Breakthrough Therapy Designation for CKD - aP • Significant unmet need • No FDA approved therapies Per Nov. 2018 CMS rule: within the ESRD Prospective Payment System all new dialysis drugs eligible for reimbursement at ASP for 2 yrs under TDAPA, effective Jan. 1, 2020 4 1. National Kidney Foundation 2. Pisoni RL, Wikstrom B, Elder SJ, et al. Nephrol Dial Transplant. 2006;21:3495 - 3505. 3. Ramakrishnan et al. International Journal of Nephrology and Renovascular Disease. 2014:7 1 – 12 4. https://www.govinfo.gov/content/pkg/FR - 2018 - 11 - 14/pdf/2018 - 24238.pdf >500K patients on dialysis ~40% have moderate to severe pruritus 60% of ESRD patients have pruritus 2,3

All Doses of KORSUVA (3x/Wk) Maintained Receptor - Saturating Plasma Concentrations 9 KORSUVA Injection: Convenient Dosing After Each Dialysis Session Human Kappa Receptor K d = 140 nM = 96 pg/mL 320 pg/mL >75% occupancy 2000 4000 6000 8000 10000 Mean CR845 ( pg /mL) 1.0 mcg/kg 0.5 mcg/kg 300 DAY 1 DAY 5

Endpoints: Week 12 Primary • Proportion of subjects achieving ≥3 point improvement from baseline in weekly mean of daily worst itching intensity NRS (WI - NRS) Secondary • Proportion of subjects achieving ≥4 point improvement in WI - NRS • Change from baseline in itch - related Quality of Life as measured by 5 - D Itch and Skindex - 10 questionnaires 10 KALM - 1 Phase 3 Pivotal Study Design 12 Weeks TREATMENT RUN - IN 7 Days END OF TREATMENT 1:1 RANDOMIZATION SCREEN Placebo after each hemodialysis session KORSUVA 0.5 mcg/kg after each hemodialysis session 52 Week Open - Label Extension Ongoing

Total Randomized (N=378) 11 KALM - 1: Patient Disposition *1 subject was randomized to Placebo but did not receive study drug Completed 170 (90%) Discontinued 18 (10%) Adverse event 9 Subject withdrew consent 6 Subject non - compliance 1 Eligibility 2 Other 0 Completed 162 (86%) Discontinued 27 (14%) Adverse event 14 Subject withdrew consent 8 Subject non - compliance 1 Eligibility 1 Other 3 Placebo (N=188*) KORSUVA (N=189)

NRS: Numeric Rating Scale (0 to 10) where 0 = no itch and 10 = worst itching imaginable 5 - D Itch score ranges from 0 to 25 (lower scores indicate better QoL and reduced itch symptoms) Skindex - 10 scale ranges from 0 to 60 (lower scores indicate better QoL) KALM - 1: Key Baseline Disease Characteristics Baseline Characteristic Mean (SD) or % Placebo N = 188 KORSUVA N = 189 Years Undergoing Hemodialysis 4.7 (4.22) 4.4 (3.98) Years of Pruritus 3.5 (3.37) 3.2 (3.24) Use of Anti - Itch Medication 41.5 % 38.1 % Baseline Worst Itching Intensity NRS 7.3 (1.61) 7.1 (1.44) Baseline 5 - D Itch Total Score 17.9 (3.47) 16.9 (3.47) Baseline Skindex - 10 Total Score 38.3 (15.40) 36.2 (14.36) 12

KALM - 1 Phase 3 Primary Endpoint: ≥3 point improvement WI - NRS 28% 51% 0% 20% 40% 60% Placebo (N = 189) KORSUVA (N = 189) 13 % of Subjects Estimated percentage & P - value based on a logistic regression model with terms for treatment group, baseline WI - NRS score, and s trata Missing data imputed using multiple imputation (MI) under missing at random (MAR) assumption Odd Ratio: 2.72 TOP - LINE RESULTS: KORSUVA subjects >2.5 times more likely to experience ≥3 point improvement P = 0.000019

Estimated percentage & P - value based on a logistic regression model with terms for treatment group, baseline WI - NRS score, and s trata Missing data imputed using multiple imputation MI) under missing at random (MAR) assumption Odd Ratio: 2.9 Secondary Endpoint: ≥4 point improvement WI - NRS TOP - LINE RESULTS: KORSUVA subjects ~3 times more likely to experience ≥4 point improvement 14 18% 39% 0% 10% 20% 30% 40% Placebo (N = 189) KORSUVA (N = 189) % of Subjects P = 0.000032

* P < 0.05, ** P < 0.001 -4 -3 -2 -1 0 ** ** ** ** ** ** ** ** ** * * ** Change in Worst Itching Intensity NRS Over Time LS Means from MMRM with terms for treatment group, week, week by treatment interaction, baseline score and strata Missing data imputed using multiple imputation (MI) under missing at random (MAR) assumption 15 TOP - LINE RESULTS: Significant differences observed in WI - NRS starting at week 1 LS Means over time Weeks in Double - blind Treatment Period Change from Baseline Baseline 1 2 3 4 5 6 7 8 9 10 11 12 Placebo (N=189) KORSUVA (N=189)

Placebo KORSUVA 0 2 4 6 Change form Baseline 5 - D Placebo KORSUVA 0 5 10 15 20 Change form Baseline Skindex - 10 Secondary Endpoints: 5D - Itch and Skindex - 10 LS Mean, standard error & P - value based on ANCOVA with terms for treatment group, baseline score, and strata Missing values imputed using multiple imputation (MI) under MAR assumption 16 TOP - LINE RESULTS: Significant improvements in itch - related QoL measures 35% improvement over placebo 43% improvement over placebo P = 0.0009 P = 0.0004

KALM - 1 Phase 3 Pivotal Top - line Results Summary Study met primary and all secondary endpoints Endpoints at Week 12 KORSUVA 0.5 mcg/kg vs placebo P Value Primary Proportion subjects with ≥3 point improvement in weekly mean of daily WI - NRS 0.000019 Secondary 1) Proportion subjects ≥4 point improvement in weekly mean of daily WI - NRS 2) Change from baseline in 5 - D Itch score 3) Change from baseline in total Skindex - 10 score 0.000032 0.0009 0.0004 17

KALM - 1 Most Commonly Reported TEAEs (Top - Line Data) Treatment - emergent Adverse Events at ≥5% frequency Placebo N = 188; n (%) KORSUVA N = 189; n (%) Diarrhea 7 (3.7) 18 (9.5) Dizziness 2 (1.1) 13 (6.9) Vomiting 6 (3.2) 10 (5.3) Nasopharyngitis 10 (5.3) 6 (3.2) 18


20 20 KORSUVA Injection in CKD - HD: Phase 3 Program KALM - 1 trial (US): Top - line results • Met Primary and all Secondary Endpoints • Generally well - tolerated and safety findings consistent with previous trials KALM - 2 trial (Global): Fully Enrolled • Includes centers in the US, Europe and Asia Pac regions • Interim Assessment Complete • Full Enrollment: Q4, 2019 Open label safety studies: ongoing • US SAFETY STUDY: up to 52 weeks Enrollment Complete - >185 patients completed 6 months - >100 patients have completed 1 year - Safety findings consistent with the Ph 2 trial - no new safety signals observed • GLOBAL SAFETY STUDY: up to 12 weeks treatment and up to 250 patients - Initiated in 2Q, 2019

Vifor Fresenius Medical Care Renal Pharma (VFMCRP) 21 Ex - US License Agreement KORSUVA INJECTION ( difelikefalin ) for the prevention, inhibition or treatment of itch associated with pruritus in hemodialysis/ peritoneal dialysis patients Financials • $70M upfront ($50M cash + $20M in Cara equity at premium) • Up to $470 million in regulatory and commercial milestones • Tiered double - digit royalty based on net sales in licensed territory Licensed Territory • Worldwide, excluding US, Japan & South Korea VFMCRP & Cara co - promotion and profit share arrangement in US Fresenius Medical Care clinics • Cara has sole promotion and profit retention in all non - Fresenius US dialysis clinics

Development Programs for Oral KORSUVA ™ 22 Phase 2 Trial CKD - aP (Stage III - V) Phase 2 Trial Atopic Dermatitis Phase 2 Trial Chronic Liver Disease Pruritus ~30% experience pruritus ~87% to 100% experience pruritus ~30% experience pruritus

Oral KORSUVA ™ for CKD - associated Pruritus: Phase 2 Topline Results 23 A Multicenter , Double - Blind, Randomized, Placebo - Controlled Study to Evaluate the Safety and Efficacy of Oral KORSUVA ™ (CR845, Difelikefalin) in Chronic Kidney Disease Patients with Moderate - to - Severe Pruritus

Per NKF, CKD is a big under - recognized US public health issue • ~30 million people affected (causes more deaths than breast/ prostate cancer) 24 No FDA approved therapies – large unmet medical need • Commonly used medications: anti - histamines, corticosteroids, gabapentin, anti - depressants etc. Oral KORSUVA™, if approved for pre - dialysis patients, would not fall under ESRD bundle payment system ~7.3 million diagnosed with CKD (IQVIA est) 33% receive pruritus tx US Market Opportunity in CKD - aP : Non - Dialysis

Executive Summary • CR845 - 210301 Phase 2 study of Oral KORSUVA™ met the primary endpoint* • A positive dose - related trend was observed for all secondary endpoints. • Oral KORSUVA was generally well tolerated with the safety profile consistent with prior studies. • Oral KORSUVA 1mg was identified as the efficacious and safe dose to be studied in Phase 3. The primary endpoint was defined as the Change from baseline in weekly mean of daily Worst Itching Intensity NRS (WI - NRS) score and the study would be considered positive if at least one safe and efficacious dose was identified.

• Phase 2 dose ranging study to assess safety and efficacy of 3 dose levels of oral KORSUVA on itch severity and itch - related QoL compared to placebo across diverse CKD population • Enrolled Stage 3 to 5 CKD patients (non - dialysis and dialysis) with chronic moderate to severe pruritus • Stratified to treatment based on renal disease status: ▪ Stage 3 CKD non - dialysis ▪ Stage 4 or 5 CKD non - dialysis ▪ Stage 4 or 5 CKD on hemodialysis (20% enrollment cap) • The study to be considered positive if at least one safe and efficacious dose is identified. 26 Oral KORSUVA ™ for CKD - aP

Oral KORSUVA ™ for CKD - aP: Ph 2 Trial Design 27 12 Weeks TREATMENT (Once Daily) RUN - IN 7 Days END OF TREATMENT RANDOMIZE (N = 240; 1:1:1:1) SCREEN Placebo Difelikefalin : 0.25 mg Baseline Mean WI - NRS > 5 Mean WI - NRS at Week 12 Difelikefalin : 0.5 mg Difelikefalin : 1.0 mg Endpoints: Week 12 Primary • Change from baseline in weekly mean of daily Worst Itching Intensity NRS (WI - NRS) score Secondary • Change from baseline in itch - related QoL x Skindex - 10 x 5 - D Itch • Proportion of subjects achieving >3 points improvement from baseline in weekly mean of daily WI - NRS score

Total Dosed (N=269) 28 Oral KORSUVA ™ for CKD - aP : Patient Disposition Completed 57 (85%) Discontinued 10 (15%) Adverse event 4 Subject withdrew consent 3 Subject non - compliance 0 Eligibility 0 Lost to follow - up 0 Other 3 Completed 60 (87%) Discontinued 9 (13%) Adverse event 3 Subject withdrew consent 2 Subject non - compliance 2 Eligibility 0 Lost to follow - up 2 Other 0 Placebo (N=67) Difelikefalin 0.25 mg (N=69) Difelikefalin 0.5 mg (N=66) Difelikefalin 1.0 mg (N=67) Completed 57 (86%) Discontinued 9 (14%) Adverse event 6 Subject withdrew consent 2 Subject non - compliance 0 Eligibility 0 Lost to follow - up 0 Other 1 Completed 54 (81%) Discontinued 13 (19%) Adverse event 8 Subject withdrew consent 3 Subject non - compliance 0 Eligibility 0 Lost to follow - up 0 Other 2

Oral KORSUVA ™ for CKD - aP : Demographics Demographic Characteristic Placebo Difelikefalin N (%) N = 67 0.25 mg N = 69 0.5 mg N = 66 1.0 mg N = 67 Males 37 (55) 34 (49) 33 (50) 35 (52) Age - Mean (SD) 66 (12) 66 (11) 69 (12) 68 (11) Hispanic or Latino 34 (51) 30 (44) 31 (47) 33 (49) White 47 (70) 49 (71) 49 (74) 48 (72) Black 17 (25) 17 (25) 12 (18) 15 (22) Asian 2 (3) 1 (1) 5 (8) 4 (6) 29

Oral KORSUVA ™ for CKD - aP : Baseline Disease Characteristics Baseline Characteristics Placebo Difelikefalin N (%) N = 67 0.25 mg N = 69 0.5 mg N = 66 1.0 mg N = 67 Stage 3 CKD Non - Dialysis (30 ≤ eGFR <60mL/min/1.73m 2 ) 40 (60) 41 (59) 38 (58) 40 (60) Stage 4 or 5 CKD Non - Dialysis (eGFR <30 mL/min/1.73m 2 ) 15 (22) 16 (23) 16 (24) 15 (22) Stage 4 or 5 CKD on Hemodialysis (eGFR <30 mL/min/1.73m 2 ) 12 (18) 12 (17) 12 (18) 12 (18) History of Diabetes 51 (76) 46 (67) 45 (68) 48 (72) History of Hypertension 66 (99) 63 (91) 61 (92) 61 (91) 30

Oral KORSUVA ™ for CKD - aP : Baseline Itch Characteristics Baseline Itch Characteristics Placebo Difelikefalin Mean (SD) N = 67 0.25 mg N = 69 0.5 mg N = 66 1.0 mg N = 67 Baseline Worst Itching Intensity NRS 6.98 (1.10) 7.24 (1.17) 7.04 (1.20) 7.04 (1.27) Baseline Skindex - 10 Total Score 34.9 (14.3) 36.5 (13.3) 33.1(14.3) 35.7(13.9) Baseline 5 - D Itch Total Score 16.8 (3.1) 16.2 (3.6) 16.2 (3.1) 16.4 (2.7) 31 NRS: Numeric Rating Scale (0 to 10) where 0 = no itch and 10 = worst itching imaginable 5 - D Itch score ranges from 5 to 25 (lower scores indicate better QoL and reduced itch symptoms) Skindex - 10 scale ranges from 0 to 60 (lower scores indicate better QoL)

Primary Endpoint: Change from Baseline to Week 12 for WI - NRS 32 LS Mean from MMRM with terms for treatment group, week, week by treatment interaction as fixed effects; baseline score and st rat a as covariates; patient as a repeated measures Missing data imputed using multiple imputation (MI) under missing at random (MAR) assumption Significant difference in WI - NRS in patients treated with 1 mg oral KORSUVA ™ compared to placebo -5 -4 -3 -2 -1 0 Change from Baseline p=0.018 Placebo 0.25 mg 0.50 mg 1.00 mg Difelikefalin

* P < 0.05, ** P < 0.01 -5 -4 -3 -2 -1 0 Placebo (N = 67) DFK 1.00 mg (N = 67) ** * ** ** * * * * ** * LS Means over time Weeks in Double - blind Treatment Period Change from Baseline Change in Worst Itching Intensity NRS Over Time LS Mean from MMRM with terms for treatment group, week, week by treatment interaction as fixed effects; baseline score and st rat a as covariates; patient as a repeated measures Missing data imputed using multiple imputation (MI) under missing at random (MAR) assumption 33 Significant differences between 1mg oral KORSUVA and placebo observed in WI - NRS starting at week 2

Secondary Endpoint: ≥ 3 point improvement in WI - NRS at week 12 34 Estimated percentage; P - values; and Odds ratios are based on a logistic regression model with terms for treatment group, baselin e WI - NRS score, and renal disease status Missing data imputed using multiple imputation (MI) under missing at random (MAR) assumption 72% of Oral KORSUVA 1.0 mg subjects experienced ≥ 3 point improvement from baseline 0% 25% 50% 75% % of Subjects Placebo 0.25 mg 0.50 mg 1.00 mg Difelikefalin p=0.110

Proportion of subjects with ≥ 3 point improvement in WI - NRS over time 4.50 17.1 22.1 25.3 30.4 32.8 40.7 47.5 48.5 50.4 53.5 57.9 5.9 29 51.8 50.9 50.2 57.4 60.5 57.9 61.8 66.9 72 72.1 0.00 10.00 20.00 30.00 40.00 50.00 60.00 70.00 80.00 1 2 3 4 5 6 7 8 9 10 11 12 Weeks in Double - blind Treatment Period Model adjusted percent of subjects with ≥ 3 point improvement in WI - NRS Placebo (n = 67) KORSUVA (n = 67) ** ** * * ** * Estimated percentage & P - value based on a logistic regression model with terms for treatment group, baseline WI - NRS score, and r enal disease status Missing data imputed using multiple imputation (MI) under missing at random (MAR) assumption * P < .05, ** P < .01 % of Subjects

Additional Pre - specified Endpoints 36 Estimated percentage and P - values are based on a logistic regression model with terms for treatment group, baseline WI - NRS score, and renal disease status Missing data imputed using multiple imputation (MI) under missing at random (MAR) assumption Placebo 0.25 mg 0.5 mg 1.0 mg 0 10 20 30 40 50 % o f S u b j e c t s Difelikefalin p=0.006 p=0.037p=0.027 Placebo 0.25 mg 0.5 mg 1.0 mg 0 20 40 60 80 100 % o f S u b j e c t s Difelikefalin p=0.001 p=0.007 NRS Complete Responder* Patient Global Impression of Change # *80% of NRS scores at Week 12 equal to 0 or 1. # ‘Much Improved’ or ‘Very Much Improved’ at Week 12.

Oral KORSUVA ™ for CKD - aP : Summary of Adverse Events Placebo Difelikefalin N (%) N = 67 0.25 mg N = 69 0.5 mg N = 66 1.0 mg N = 67 Subjects with at least one TEAE 34 (51) 35 (51) 34 (52) 39 (58) Subjects with at least one serious TEAE 5 (7.5) 9 (13.0) 9 (13.6) 9 (13.4) Deaths 3 0 0 1 Non - fatal SAEs 2 9 9 8 Subjects with TEAE resulting in treatment discontinuation 5 (7.5) 2 (2.9) 5 (7.6) 9 (13.4) 37 Reasons for death include acute respiratory failure (Placebo = 2), coronary arterial disease (DFK 1mg = 1) and cardiac arrest (P lacebo = 1).

Oral KORSUVA ™ for CKD - aP : Most Commonly Reported TEAEs Placebo Difelikefalin N (%) N = 67 0.25 mg N = 69 0.5 mg N = 66 1.0 mg N = 67 Dizziness 0 0 2 (3.0) 5 (7.5) Fall 0 0 3 (4.5) 4 (6.0) Constipation 2 (3.0) 2 (2.9) 2 (3.0) 4 (6.0) Diarrhea 1 (1.5) 2 (2.9) 3 (4.5) 4 (6.0) Fatigue 1 (1.5) 4 (5.8) 1 (1.5) 3 (4.5) Urinary tract infection 0 4 (5.8) 2 (3.0) 3 (4.5) Hypertension 1 (1.5) 4 (5.8) 0 1 (1.5) Gastrooesophageal reflux disease 0 0 4 (6.1) 0 38 Most common TEAE = incidence ≥ 5% in at least one treatment group and strictly greater than placebo

Conclusions • CR845 210301 Phase 2 study of Oral KORSUVA™ met the primary endpoint • Oral KORSUVA™ was generally well tolerated with a safety profile consistent with prior studies • Oral KORSUVA™ 1mg was identified as the efficacious and safe dose to be advanced into Phase 3 • Aim to initiate Phase 3 development program in 2020

Oral KORSUVA ™ : Additional Development Programs 40 Atopic Dermatitis Chronic Liver Disease

Atopic Dermatitis Associated Pruritus: Ph 2 Trial Ongoing 41 Study Double blind, randomized, PBO - controlled study in adult subjects with AD and moderate to severe pruritus Primary Endpoint : • Change from baseline in the weekly mean of the daily 24 - hour I - NRS score at Week 12 Secondary Endpoints: • Change in itch related QoL: Skindex - 10, 5 - D Itch scales & Sleep Quality Assessment at week 12 • Responder analysis (Week 12): Change from baseline in I - NRS score of > 4 points 12 Weeks TREATMENT RUN - IN 7 Days END OF TREATMENT RANDOMIZE (N=240) SCREEN Placebo: BID CR845 : 0.25 mg BID Baseline: Mean NRS > 5 NRS at Week 12 CR845 : 0.5 mg BID CR845 : 1 mg BID END OF EXTENSION 4 weeks EXTENSION

Pruritus Associated with Primary Biliary Cholangitis (PBC): Phase 2 42 Study A 16 - week, double blind, randomized, PBO - controlled study in PBC patients with moderate to severe pruritus Primary Endpoint : • Change from baseline in the weekly mean of the daily 24 - hour WI - NRS score at week 16 Secondary Endpoints: • Change in itch related QoL: Skindex - 10 & 5 - D Itch scales at week 16 • Responder analysis (Week 16): Change from baseline in weekly main of daily worst NRS score of > 3 points 16 Weeks TREATMENT RUN - IN 7 Days END OF TREATMENT 1:1 RANDOMIZATION SCREEN Placebo BID (N=30) Oral KORSUVA 1 mg BID (N=30)

*p<0.001 vs pentazocine (n=39) 43 Human Abuse Liability Study: Comparator Schedule IV CR845 Exhibited No “Drug Liking” Over 8 - Hour Test Session Mixed - model repeated measures analysis 0 to 100 point bipolar VAS scale Overall drug l iki n g V AS mean +1 SE 50.9 51.8 0 25 50 75 100 Placebo CR845 5 μg/kg CR845 15 μg/kg Pentazocine 0.5 mg/kg * * Strong disliking Neither like nor dislike Strong liking 49.2 73.3 *

44 Projected Clinical Milestones – 2019/ 2020 Pruritus / KORSUVA™ Injection Pruritus / Oral KORSUVA™ 4Q, 2019 Top - line data from Phase 2 Trial CKD - aP (Stage III - V) 2020 Top - line data from Global Ph 3 trial, KALM - 2 (CKD - aP in dialysis pts) Top - line data from Phase 2 Trial in AD & PBC patients with pruritus 2H, 2020 NDA Submission

Financial Highlights 45 Pro forma Cash and marketable securities (SEPTEMBER 30, 2019) $249.1M Net loss (SEPTEMBER 30, 2019) ($32.8M) Shares outstanding (POST - JULY OFFERING) ~46.4M