Use these links to rapidly review the document
TABLE OF CONTENTS
TABLE OF CONTENTS 2
Filed Pursuant to Rule 424(b)(4)
Registration No. 333-194342
PROSPECTUS
3,000,000 Shares

Adamas Pharmaceuticals, Inc.
Common Stock
This is the initial public offering of shares of common stock of Adamas Pharmaceuticals, Inc. Prior to this offering, there has been no public market for our common stock. The initial public offering price of the common stock is $16.00 per share. Our common stock has been approved for listing on the NASDAQ Global Market under the symbol "ADMS."
The underwriters have an option to purchase a maximum of 450,000 additional shares to cover over-allotments.
We are an "emerging growth company" as that term is used in the Jumpstart Our Business Startups Act of 2012, and, as such, we have elected to comply with certain reduced public company reporting requirements for this prospectus and future filings.
Investing in our common stock involves a high degree of risk. See "Risk Factors" beginning on page 10.
| |
Price to Public |
Underwriting Discounts and Commissions(1) |
Proceeds to Adamas |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Per Share | $ | 16.00 | $ | 1.12 | $ | 14.88 | ||||
| Total | $ | 48,000,000 | $ | 3,360,000 | $ | 44,640,000 | ||||
- (1)
- See "Underwriting" beginning on page 137 for additional information regarding underwriting compensation.
Certain of our directors and existing stockholders, or their affiliates, are purchasing an aggregate of 362,500 shares of our common stock in this offering. The shares will be offered and sold on the same terms as the other shares that are being offered and sold in this offering to the public.
Delivery of the shares of common stock will be made on or about April 15, 2014.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Credit Suisse | Piper Jaffray | |
William Blair |
Needham & Company |
The date of this prospectus is April 9, 2014
Neither we nor the underwriters have authorized anyone to provide any information or to make any representations other than those contained in this prospectus or in any free writing prospectuses prepared by or on behalf of us or to which we have referred you. We take no responsibility for, and can provide no assurance as to the reliability of, any information that others may give you. This prospectus is an offer to sell only the shares offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this prospectus is accurate only as of its date regardless of the time of delivery of this prospectus or of any sale of common stock.
Through and including May 4, 2014 (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer's obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
Neither we nor the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons who come into possession of this prospectus or any such free writing prospectus in jurisdictions outside the United States are required to inform themselves about and to observe any restrictions as to this offering and the distribution of this prospectus and any such free writing prospectus applicable to that jurisdiction.
This summary highlights information contained elsewhere in this prospectus and does not contain all of the information that you should consider in making your investment decision. Before deciding to invest in our common stock, you should read this entire prospectus carefully, including the sections of this prospectus entitled "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our consolidated financial statements and related notes. Unless the context otherwise requires, references in this prospectus to the "company," "Adamas," "we," "us" and "our" refer to Adamas Pharmaceuticals, Inc.
Adamas Pharmaceuticals, Inc.
We are a specialty pharmaceutical company driven to improve the lives of those affected by chronic disorders of the central nervous system, or CNS. We achieve this by enhancing the pharmacokinetic profiles of approved drugs to create novel therapeutics for use alone and in fixed-dose combination products. We are developing our lead wholly owned product candidate, ADS-5102, for a complication of Parkinson's disease known as levodopa induced dyskinesia, or LID, and as a treatment for chronic behavioral symptoms associated with traumatic brain injury, or TBI. We have successfully completed a Phase 2/3 clinical trial in which patients receiving ADS-5102 had a statistically significant 43% reduction in LID compared to their baseline LID experienced prior to taking ADS-5102, and we intend to initiate a Phase 3 registration trial of ADS-5102 in LID in 2014. Our late-stage therapeutics portfolio also includes an NDA-submitted fixed-dose combination product candidate, MDX-8704, being co-developed with Forest Laboratories, Inc., or Forest, for the treatment of moderate to severe dementia associated with Alzheimer's disease, and an approved controlled-release product, Namenda XR®, which Forest developed and is marketing in the United States under a license from us. We plan to commercialize our wholly owned product candidates, if approved, by developing a specialty CNS sales force to reach high volume prescribing neurologists and psychiatrists in the United States.
Our market opportunity
We estimate that approximately 36 million people in the United States suffer from chronic CNS disorders such as Alzheimer's disease, Parkinson's disease, TBI, epilepsy, psychosis and depression. CNS diseases are frequently treated with multiple medications having different mechanisms of action with the goal of maximizing symptomatic benefits for patients. Existing CNS drugs often require frequent dosing and may have tolerability issues that limit the amount of the drug that can be taken each day. We believe that many CNS disorders could be better treated if the concentrations of existing CNS drugs as a function of time, or the pharmacokinetic profiles, are altered to enhance tolerability and efficacy and if these enhanced drugs are then combined with other existing CNS drugs to improve and streamline the management of these complicated conditions.
Our strategy
Our goal is to build an independent, CNS-focused specialty pharmaceutical company that creates and commercializes novel therapeutics that address significant unmet clinical needs. This goal is supported by a product development strategy that allows us to discover, patent, develop and commercialize novel therapeutics in a capital efficient manner. Our integrated process combines the following elements:
- •
- Market attractiveness. We identify approved products that
are sub-optimally utilized but, with pharmacokinetic enhancements, can significantly improve the treatment of chronic CNS conditions.
- •
- Intellectual property. We seek to discover novel pharmacokinetic and pharmacodynamic relationships and to obtain patent protection for a range of dose strengths, pharmacokinetic
1
- •
- Regulatory pathways. We intend to use the regulatory
pathway provided by Section 505(b)(2) of the U.S. Food, Drug and Cosmetic Act, or FDCA, to pursue approval for novel therapeutics based on existing drugs with less time and expense than are
typically associated with the standard new drug approval pathway.
- •
- Research and development. We have developed a core competency in identifying, formulating and manufacturing controlled-release drug products utilizing coated pellet technology.
profiles, timing of administration and drug combinations as opposed to protecting just specific formulations.
We are implementing our strategy by focusing on the following key objectives:
- •
- Obtain FDA approval of ADS-5102 for LID;
- •
- Develop ADS-5102 for the treatment of additional CNS indications;
- •
- Commercialize ADS-5102 by developing a specialty sales force;
- •
- Develop additional novel therapeutics based on existing CNS drugs; and
- •
- Support Forest in the NDA review and anticipated commercialization of MDX-8704.
Our therapeutics portfolio
Our initial product and product candidates are based on pharmacokinetic enhancements of two approved CNS drugs, amantadine and memantine, which belong to a class of drugs known as aminoadamantanes. We selected aminoadamantanes as our initial area of focus because they have the ability to modulate multiple neurotransmitter systems, which are the molecular pathways that control brain function, and we believe aminoadamantanes potentially have broader therapeutic utility than previously realized.
The following table describes our therapeutics portfolio:
Product and Product Candidates
|
Target Indication(s) | Development Status | Commercial Rights | |||
|---|---|---|---|---|---|---|
| Wholly Owned | ||||||
ADS-5102 |
Levodopa-Induced Dyskinesia |
Phase 3 |
Adamas, worldwide |
|||
| Amantadine | Traumatic Brain Injury | Phase 2/3 ready | Adamas, worldwide | |||
| Undetermined | Phase 2/3 planning | Adamas, worldwide | ||||
ADS-8800 series ADS-5102 based combination therapies |
Undetermined |
Research, Phase 2/3 planning |
Adamas, worldwide |
|||
| Partnered | ||||||
Namenda XR Memantine |
Moderate to severe Alzheimer's dementia |
Marketed |
U.S.-only; licensed to Forest |
|||
MDX-8704 Memantine/Donepezil |
Moderate to severe Alzheimer's dementia |
NDA submitted |
U.S.-only; licensed to Forest |
|||
Wholly owned product candidates. Our most advanced wholly owned product candidate is ADS-5102, a once-nightly, high dose, controlled-release version of amantadine designed to address many of the limitations of immediate-release amantadine. In patients taking ADS-5102, the amantadine plasma concentration achieved from the early morning through mid-day is approximately two-times that reached following administration of immediate-release amantadine, providing substantial benefit to
2
patients as they engage in their daily activities. Further, the lower concentrations occurs in the evening, reducing the negative impact of amantadine's sleep-related side effects. We are developing ADS-5102 initially for treatment of LID. LID is a movement disorder that frequently occurs in patients with Parkinson's disease after long-term treatment with levodopa, the most widely-used drug for Parkinson's disease. Patients with LID suffer from involuntary non-purposeful movements and reduced control over voluntary movements. We estimate that in 2011 approximately 260,000 Parkinson's disease patients in the United States suffered from motor complications as a result of levodopa therapy and approximately 140,000 of these patients suffered from LID. There are no drugs for the treatment of LID that have been approved for marketing in the United States or Europe. As a result, clinicians typically manage LID by decreasing the dose of levodopa, which can exacerbate symptoms of the underlying Parkinson's disease.
We selected LID as the initial indication for ADS-5102 based on results seen in investigator initiated clinical studies of amantadine and in established preclinical models. In our recently completed Phase 2/3 clinical study, ADS-5102 met its primary endpoint, reduction of LID, and several key secondary endpoints. If our anticipated Phase 3 registration trial of ADS-5102 is successful, we anticipate submitting a New Drug Application, or NDA, to the U.S. Food and Drug Administration, or FDA, for ADS-5102 in the first half of 2016. Amantadine has shown promising results in several other CNS indications, and we expect to initiate in late 2014 a Phase 2/3 study of ADS-5102 in a second CNS indication, possibly for the treatment of chronic behavioral symptoms associated with TBI. We anticipate initiating additional Phase 2/3 studies of ADS-5102 in one or more other indications by the end of 2015.
We are investigating and will potentially develop additional products, our ADS-8800 series, based on combining ADS-5102 with approved CNS drugs. Each combination will be designed to provide clinical benefits in specific indications where it appears that including ADS-5102 in combination therapy can address a significant unmet clinical need. Furthermore, we believe our product development strategy is broadly applicable to addressing limitations of CNS drugs whose pharmacokinetic profiles limit dosing, and we intend to initiate additional programs in this area in 2015.
We intend to use the regulatory pathway provided by Section 505(b)(2) of the FDCA to obtain approval for ADS-5102 and our other wholly owned product candidates. Section 505(b)(2) permits the filing of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. We believe this approach will be more time and capital efficient than the Section 505(b)(1) pathway typically used for new chemical entities.
Partnered product and product candidate. Under our license agreement, Forest currently sells one product, Namenda XR, a treatment for moderate to severe dementia associated with Alzheimer's disease. Namenda XR, a controlled-release version of the approved CNS drug memantine, was launched in the United States in June 2013 and is part of Forest's $1.5 billion Namenda franchise. In addition, Forest and we are co-developing MDX-8704, a once-daily fixed-dose combination of Namenda XR and the FDA-approved CNS drug donepezil, for the treatment of moderate to severe dementia associated with Alzheimer's disease. Forest submitted an NDA to the FDA for MDX-8704 in February 2014 and will be responsible for marketing MDX-8704 in the United States if approved. We received from Forest a $65 million upfront payment in November 2012 and two $20 million development milestone payments in the fourth quarter of 2013 related to the completion of studies that support Forest's NDA filing for MDX-8704. We are eligible to receive up to an additional $55 million in payments based on the achievement of certain regulatory milestones prior to and including the first FDA approval of MDX-8704 and royalty payments related to sales of Namenda XR commencing in June 2018 and to sales of MDX-8704 commencing five years after its commercial launch in the United
3
States. Forest has stated that it projects FDA approval and commercial launch of MDX-8704 in the first half of 2015.
Our management team
We are led by a team of executives and directors with significant experience in drug discovery, development and commercialization. In addition to co-founding Adamas, our chief executive officer co-founded CuraGen Corporation (acquired by Celldex Therapeutics, Inc.), and other members of our management team have held senior positions at Syntex, Bayer, Tularik and Elan. Members of our executive team have played leading roles in the development and commercialization of multiple significant drugs in a wide range of therapeutic areas. Our board of directors brings substantial, relevant experience in reimbursement, drug development and commercialization.
Financial overview
We have developed our current portfolio of late stage therapeutics in a capital efficient manner. As of December 31, 2013, we had raised a total of $87 million from equity financings, had received $105 million from our collaboration with Forest, had recognized $5 million in revenue from other sources and had $86 million in cash and cash equivalents and no debt obligations.
Risk factors
Our business is subject to numerous risks, as more fully described in the section entitled "Risk Factors" immediately following this prospectus summary. You should read these risk factors before you invest in our common stock. In particular, these risks include, but are not limited to, the following:
- •
- Our success depends heavily on the approval and successful commercialization of our lead wholly owned product candidate,
ADS-5102, as well as Forest's successful commercialization of Namenda XR and, if approved, MDX-8704;
- •
- Our product candidates and Namenda XR require a complex manufacturing process, and there are risks associated with
scaling up manufacturing and packaging to commercial scale and maintaining commercial production. For example, in November 2013 Forest recalled three packaged lots of Namenda XR when testing revealed
a failure to meet required manufacturing specifications; Namenda XR is one of the components of Forest's fixed-dose combination product candidate MDX-8704;
- •
- We do not directly market any products, expect to incur substantial and increasing losses for the foreseeable future and
had an accumulated deficit as of December 31, 2013 of $20.6 million;
- •
- If clinical studies of our product candidates fail to demonstrate safety and efficacy to the satisfaction of the FDA, we
will be unable to commercialize our product candidates;
- •
- If significant adverse side effects associated with a product or product candidate are identified during development or
after approval, we may need to abandon development of a product candidate or cease marketing a product;
- •
- If we are unable to obtain favorable coverage, reimbursement and formulary placement decisions from third-party payors,
our financial results will be adversely affected;
- •
- Our business will suffer if other manufacturers are able to obtain approval for generic or other competing versions of current and future products in our therapeutic portfolio, such as the possible result of the ten challenges from generic manufacturers relating to Abbreviated New Drug Applications seeking approval for generic versions of Namenda XR;
4
- •
- Our business may be adversely affected if we are unable to obtain and maintain effective intellectual property rights or
others claim that we infringe their intellectual property rights, such as the pending patent infringement lawsuit brought by Teva Pharmaceuticals USA, Inc. and Mayne Pharma against Forest relating to
Namenda XR;
- •
- Our operating results may fluctuate significantly, are difficult to predict and could fall below expectations; and
- •
- We may need additional funds to support our operations, and such funding may not be available on acceptable terms or at all.
Corporate Information
We were incorporated in Delaware in November 2000 under the name NeuroMolecular, Inc. In December 2004, we changed our name to NeuroMolecular Pharmaceuticals, Inc., and in July 2007 we changed our name to Adamas Pharmaceuticals, Inc. Our principal executive offices are located at 2200 Powell Street, Suite 220, Emeryville, California 94608, and our telephone number is (510) 450-3500. Our website address is www.adamaspharma.com. The information contained on our website is not incorporated by reference into this prospectus, and you should not consider any information contained on, or that can be accessed through, our website as part of this prospectus or in deciding whether to purchase our common stock.
Implications of being an emerging growth company
As a company with less than $1 billion in revenue during our last fiscal year, we qualify as an "emerging growth company" as defined in the Jumpstart Our Business Startups Act, or JOBS Act, enacted in April 2012. An emerging growth company may take advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not limited to:
- •
- being permitted to present only two years of audited financial statements and only two years of related Management's
Discussion and Analysis of Financial Condition and Results of Operations in this prospectus;
- •
- not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of
2002, as amended;
- •
- reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration
statements; and
- •
- exemptions from the requirement to hold a nonbinding advisory vote on executive compensation and to obtain stockholder approval of any golden parachute payments not previously approved.
We may use these provisions until the last day of our fiscal year following the fifth anniversary of the closing of this offering. However, if certain events occur prior to the end of such five-year period, including if we become a "large accelerated filer," our annual gross revenue exceeds $1 billion or we issue more than $1 billion of non-convertible debt in any three-year period, we will cease to be an emerging growth company prior to the end of such five-year period.
We have elected to take advantage of certain of the reduced disclosure obligations in the registration statement of which this prospectus is a part and may elect to take advantage of other reduced reporting requirements in future filings. As a result, the information that we provide to our stockholders may be different than you might receive from other public reporting companies in which you hold equity interests.
The JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. We have irrevocably elected not to avail ourselves of this exemption and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
5
Common stock offered |
3,000,000 shares | |
Common stock to be outstanding immediately after this offering |
16,422,992 shares |
|
Underwriters' option to purchase additional shares |
The underwriters have a thirty-day option to purchase up to 450,000 additional shares of common stock as described in "Underwriting." |
|
Use of proceeds |
The net proceeds from the issuance of our common stock in this offering will be approximately $41.5 million, or approximately $48.2 million if the underwriters exercise in full their over-allotment option to purchase additional shares, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. |
|
|
We intend to use all of the net proceeds from this offering, along with our other capital resources, to fund ongoing development of our product candidates, including ADS-5102, for commercialization activities related to any wholly owned, approved product candidate, including developing a specialty CNS sales force, and for working capital and other general corporate purposes. We may also use a portion of the net proceeds to acquire or license other products, businesses or technologies, although we have no present commitments for any such acquisitions or licenses. See "Use of Proceeds" for additional information. |
|
Risk factors |
See "Risk Factors" beginning on page 10 and the other information included in this prospectus for a discussion of factors you should carefully consider before deciding to invest in our common stock. |
|
NASDAQ Global Market symbol |
"ADMS" |
The number of shares of our common stock to be outstanding after this offering is based on 13,422,992 shares of our common stock outstanding as of December 31, 2013 and excludes the following:
- •
- 3,567,858 shares of our common stock issuable upon the exercise of stock options outstanding as of December 31,
2013 at a weighted-average exercise price of $1.445 per share;
- •
- 1,865,000 shares of our common stock issuable upon the exercise of stock options granted after December 31, 2013
with a weighted-average exercise price of $9.47 per share;
- •
- 3,151,940 shares of common stock reserved for future issuance under our 2014 equity incentive plan, or 2014 Plan, which will become effective upon the date the registration statement of which this prospectus forms a part is declared effective (including 1,771,212 shares of common stock reserved for issuance under our 2007 Stock Plan, or 2007 Plan, as of December 31, 2013, which shares were added to the shares reserved under the 2014 Plan) plus up to 3,567,858 additional shares that may be added to the 2014 Plan upon the expiration, termination, forfeiture or other reacquisition of any shares of common stock issuable upon the exercise of stock options outstanding under the 2007 Plan or our 2002 Employee, Director and Consultant
6
- •
- 262,762 shares of our common stock reserved for future issuance under our 2014 Employee Stock Purchase Plan, plus any
automatic increases in the number of shares of common stock reserved for future issuance under the 2014 Employee Stock Purchase Plan; and
- •
- 213,278 shares of our common stock issuable upon the exercise of warrants to purchase common stock outstanding at December 31, 2013 at a weighted-average exercise price of $3.14 per share.
Stock Plan, or 2002 Plan, and any automatic increases in the number of shares of common stock reserved for future issuance under the 2014 Plan;
Unless otherwise indicated, all information in this prospectus reflects and assumes the following:
- •
- the automatic conversion of all outstanding shares of our preferred stock into an aggregate of 3,432,891 shares of our
common stock immediately prior to the closing of this offering;
- •
- the issuance of an aggregate of 474,573 shares of our common stock upon conversion of the Series AA preferred stock
issuable upon the net exercise of warrants to purchase 622,660 shares of Series AA preferred stock with an exercise price of $3.80445 per share and based on a fair market value of $16.00 per
share, the initial public offering price, immediately prior to the closing of this offering;
- •
- the filing of our amended and restated certificate of incorporation and the adoption of our amended and restated bylaws
immediately prior to the closing of this offering;
- •
- no exercise of the underwriters' over-allotment option to purchase additional shares of our common stock; and
- •
- a 2-for-1 forward split of our outstanding common stock and preferred stock effected on March 24, 2014.
7
Summary Consolidated Financial Data
The following tables summarize our consolidated financial data and should be read together with the sections in this prospectus entitled "Selected Consolidated Financial Data" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our consolidated financial statements and related notes included elsewhere in this prospectus.
We have derived the consolidated statements of operations data for the years ended December 31, 2012 and 2013 from our audited consolidated financial statements included elsewhere in this prospectus. Our historical results are not necessarily indicative of the results that should be expected in the future.
| |
Year Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
| |
(in thousands, except per share data) |
||||||
Consolidated Statements of Operations Data: |
|||||||
Revenue |
$ | 37,471 | $ | 71,095 | |||
| | | | | | | | |
Operating expenses |
|||||||
Research and development |
9,192 | 7,410 | |||||
General and administrative |
8,330 | 6,667 | |||||
| | | | | | | | |
Total operating expenses |
17,522 | 14,077 | |||||
| | | | | | | | |
Income from operations |
19,949 | 57,018 | |||||
Interest and other income (expense), net |
(1,537 | ) | (4,818 | ) | |||
Interest expense |
(376 | ) | (88 | ) | |||
| | | | | | | | |
Income before income taxes |
18,036 | 52,112 | |||||
Income tax expense |
(300 | ) | (1,191 | ) | |||
| | | | | | | | |
Net income |
$ | 17,736 | $ | 50,921 | |||
| | | | | | | | |
| | | | | | | | |
Net income attributable to common stockholders(1) |
|||||||
Basic |
$ | 11,441 | $ | 33,068 | |||
| | | | | | | | |
| | | | | | | | |
Diluted |
$ | 11,596 | $ | 35,353 | |||
| | | | | | | | |
| | | | | | | | |
Net income per share attributable to common stockholders(1) |
|||||||
Basic |
$ | 1.21 | $ | 3.48 | |||
| | | | | | | | |
| | | | | | | | |
Diluted |
$ | 1.17 | $ | 3.00 | |||
| | | | | | | | |
| | | | | | | | |
Weighted-average number of shares used in computing net income attributable to common stockholders(1) |
|||||||
Basic |
9,488 | 9,506 | |||||
| | | | | | | | |
| | | | | | | | |
Diluted |
9,924 | 11,806 | |||||
| | | | | | | | |
| | | | | | | | |
Pro forma net income per share attributable to common stockholders (unaudited)(1) |
|||||||
Basic |
$ | 4.13 | |||||
| | | | | | | | |
| | | | | | | | |
Diluted |
$ | 3.51 | |||||
| | | | | | | | |
| | | | | | | | |
Pro forma weighted average number of shares used in computing net income attributable to common stockholders (unaudited)(1) |
|||||||
Basic |
13,414 | ||||||
| | | | | | | | |
| | | | | | | | |
Diluted |
15,790 | ||||||
| | | | | | | | |
| | | | | | | | |
- (1)
- See Notes 2 and 14 to our consolidated financial statements for a description of the method used to compute basic and diluted net income per share.
8
| |
December 31, 2013 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Actual | Pro Forma(1) |
Pro Forma As Adjusted(2) |
|||||||
| |
(in thousands) |
|||||||||
| |
(unaudited) |
|||||||||
Balance sheet data: |
||||||||||
Cash and cash equivalents |
$ | 85,612 | $ | 85,612 | $ | 127,152 | ||||
Working capital |
81,790 | 81,790 | 123,330 | |||||||
Total assets |
86,216 | 86,216 | 127,756 | |||||||
Warrant liability |
6,232 | — | — | |||||||
Convertible preferred stock |
19,149 | — | — | |||||||
Total stockholders' equity |
$ | 56,605 | $ | 81,986 | $ | 123,526 | ||||
- (1)
- The
pro forma column reflects the filing of our amended and restated certificate of incorporation, the automatic conversion of all outstanding shares of our
preferred stock into an aggregate of 3,432,891 shares of common stock immediately prior to the closing of this offering and the reclassification to additional paid-in capital of our preferred stock
warrant liability in connection with the automatic net exercise of our outstanding warrants to purchase shares of preferred stock, and subsequent conversion of preferred stock into common stock
immediately prior to the closing of this offering.
- (2)
- The pro forma as adjusted column reflects the pro forma adjustments described in footnote (1) above and the sale by us of 3,000,000 shares of common stock in this offering, after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
9
Investing in our common stock involves a high degree of risk. You should consider carefully the following risks, together with all the other information in this prospectus, including our financial statements and notes thereto, before you invest in our common stock. If any of the following risks actually materializes, our operating results, financial condition and liquidity could be materially adversely affected. As a result, the trading price of our common stock could decline and you could lose part or all of your investment.
Risks related to our financial condition and need for additional capital
Although we reported net income for 2012 and 2013, we incurred significant losses in prior years and expect to incur substantial losses in the future.
We are a clinical-stage specialty pharmaceutical company and do not currently directly market any products. We currently exclusively license U.S. patent rights for one approved product, Namenda XR, to a wholly owned subsidiary of Forest Laboratories, Inc., or Forest, and Forest markets Namenda XR in the United States, but we do not currently receive royalties on the sales of that product. We continue to incur significant research and development and general and administrative expenses related to our product candidates and our operations. Although we reported net income for 2012 and 2013, this was almost entirely due to milestone payments we received pursuant to our license agreement with Forest. We incurred significant operating losses in 2011 and prior years and expect to incur substantial and increasing losses for the foreseeable future. As of December 31, 2013, we had an accumulated deficit of $20.6 million.
To date, we have financed our operations primarily through private placements of our convertible preferred stock, our collaboration with Forest and, to a lesser extent, government grants, venture debt and with the benefit of tax credits made available under a federal stimulus program supporting drug development. We have devoted substantially all of our efforts to research and development, including clinical studies, but have not completed development of any product candidates. We anticipate that our expenses will increase substantially as we:
- •
- initiate a Phase 3 registration trial of our lead wholly owned product candidate, ADS-5102 in levodopa induced
dyskinesia, or LID;
- •
- develop ADS-5102 for treatment of other indications in addition to LID and develop additional product candidates;
- •
- seek regulatory approvals for our product candidates that successfully complete clinical studies;
- •
- establish a specialty CNS sales force and improve our distribution and marketing capabilities to commercialize products
for which we may obtain regulatory approval;
- •
- enhance operational, financial and information management systems and hire more personnel, including personnel to support
development of our product candidates and, if a product candidate is approved, our commercialization efforts;
- •
- continue the research and development of our current product candidates; and
- •
- seek to discover or in-license additional product candidates.
To be profitable in the future, we or our current and future potential collaboration partners must succeed in developing and commercializing products with significant market potential. This will require us or our partners to be successful in a range of activities, including advancing product candidates, completing clinical studies of product candidates, obtaining regulatory approval for those product candidates and manufacturing, marketing and selling those products for which regulatory approval is obtained. We or our partners may not succeed in these activities and, as a result, we may never generate revenue that is sufficient to be profitable in the future. In the near term, our only anticipated
10
source of significant revenue is from certain milestone payments under our license agreement with Forest. We will not be entitled to receive any royalty payments with respect to sales of Namenda XR until June 2018, and with respect to sales of our partnered product candidate, MDX-8704, until five years after its commercial launch in the United States, assuming it is approved and launched.
Although we reported net income for 2012 and 2013, this was primarily due to the recognition of revenue pursuant to our license agreement with Forest. Even if we attain profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to achieve sustained profitability would depress the value of our stock and could impair our ability to raise capital, expand our business, diversify our product candidates, market our product candidates, if approved, or continue our operations.
Our operating results may fluctuate significantly, which makes our future operating results difficult to predict and could cause our operating results to fall below expectations or our guidance.
Our quarterly and annual operating results may fluctuate significantly in the future, which makes it difficult for us to predict our future operating results. Our sole anticipated source of significant revenue in the near term is from certain milestone payments under our license agreement with Forest. Accordingly, our revenue will depend on the achievement of these milestones as well as any potential future collaboration and license agreements and sales of our product candidates, if approved. Upfront and milestone payments may vary significantly from period to period and any such variance could cause a significant fluctuation in our operating results from one period to the next. Furthermore, our operating results may fluctuate due to a variety of other factors, many of which are outside of our control and may be difficult to predict, including:
- •
- the timing and cost of, and level of investment in, research and development activities relating to our product
candidates, which may change from time to time;
- •
- cost and reimbursement policies with respect to our products candidates, if approved, and existing and potential future
drugs that compete with our product candidates;
- •
- the cost of manufacturing our product candidates, which may vary depending on the quantity of production and the terms of
our agreements with manufacturers;
- •
- expenditures that we will or may incur to acquire or develop additional product candidates and technologies;
- •
- the level of demand for our products, should any of our product candidates receive approval, which may vary significantly;
- •
- future accounting pronouncements or changes in our accounting policies;
- •
- the timing and success or failure of clinical studies for our product candidates or competing product candidates, or any
other change in the competitive landscape of our industry, including consolidation among our competitors or partners; and
- •
- the changing and volatile U.S., European and global economic environments.
The cumulative effects of these factors could result in large fluctuations and unpredictability in our quarterly and annual operating results. As a result, comparing our operating results on a period-to-period basis may not be meaningful. Investors should not rely on our past results as an indication of our future performance. This variability and unpredictability could also result in our failing to meet the expectations of industry or financial analysts or investors for any period. If our revenue or operating results fall below the expectations of analysts or investors or below any forecasts we may provide to the market, or if the forecasts we provide to the market are below the expectations of analysts or investors, the price of our common stock could decline substantially. Such a stock price
11
decline could occur even when we have met any previously publicly stated revenue and/or earnings guidance we may provide.
We may need additional funds and, if we cannot raise additional capital when needed, we may have to curtail or cease operations.
We are seeking to advance multiple product candidates through the research and clinical development process. The completion of the development and the potential commercialization of our product candidates, should they receive approval, will require substantial funds. As of December 31, 2013, we had approximately $85.6 million in cash and cash equivalents. We believe that our available cash and cash equivalents will be sufficient to fund our anticipated level of operations for at least the next 12 months, but there can be no assurance that this will be the case. Our future financing requirements will depend on many factors, some of which are beyond our control, including:
- •
- the rate of progress and cost of our clinical studies;
- •
- the timing of, and costs involved in, seeking and obtaining approvals from the U.S. Food and Drug Administration, or FDA,
and potentially other regulatory authorities;
- •
- the costs of commercialization activities if any of our product candidates is approved, including expanding our sales,
marketing and distribution activities;
- •
- the degree and rate of market acceptance of any products launched by us, Forest or any future partners;
- •
- the coverage of our products by third-party payors and the formulary tier in which health plans and other payors place our
products, if approved, and the rate at which the products are reimbursed;
- •
- our ability to enter into additional collaboration, licensing, commercialization or other arrangements and the terms and
timing of such arrangements; and
- •
- the emergence of competing technologies or other adverse market developments.
We do not have any committed external source of funds or other support for our development efforts other than our license agreement with Forest which may be terminated by Forest upon delivery of notice. Until we can generate sufficient product and royalty revenue to finance our cash requirements, which we may never do, we expect to finance future cash needs through a combination of public or private equity offerings, debt financings, collaborations, strategic alliances, licensing arrangements and other marketing and distribution arrangements. Additional financing may not be available to us when we need it or it may not be available on favorable terms. If we raise additional capital through marketing and distribution arrangements or other collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish certain valuable rights to our product candidates, technologies, future revenue streams or research programs or grant licenses on terms that may not be favorable to us. If we raise additional capital through public or private equity offerings, the ownership interest of our existing stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect our stockholders' rights. If we raise additional capital through debt financing, we may be subject to covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we are unable to obtain adequate financing when needed, we may have to delay, reduce the scope of, or suspend one or more of our clinical studies or research and development programs or our commercialization efforts.
12
Risks related to the development and commercialization of our current and future products
Our success depends heavily on the approval and successful commercialization of ADS-5102, the U.S. approval and successful U.S. commercialization by Forest of MDX-8704 and the successful U.S. commercialization by Forest of Namenda XR. If we are unable to successfully commercialize ADS-5102 or Forest is unable to successfully commercialize MDX-8704 or Namenda XR in the U.S., or either we or Forest experience significant delays in doing so, our business will be materially harmed.
We have invested a significant portion of our efforts and financial resources into the development of ADS-5102, an oral once-nightly controlled-release version of the FDA-approved drug amantadine, and MDX-8704, a fixed-dose combination of the FDA-approved drugs memantine and donepezil. MDX-8704 has been exclusively licensed to Forest in the United States. In addition, we have granted Forest a royalty-bearing license under certain of our patents to commercialize Namenda XR, a controlled-release version of memantine, in the United States. Our ability to generate product and royalty revenue will depend heavily on the successful development, regulatory approval and eventual commercialization of ADS-5102 and MDX-8704 and successful commercialization of Namenda XR. Under the terms of our license agreement with Forest, we will not be entitled to receive royalty payments on the sale of Namenda XR until June 2018 or on the sale of MDX-8704 until five years after it is launched, assuming it is approved and launched. The success of these drugs will depend on numerous factors, including:
- •
- successfully completing clinical studies for ADS-5102;
- •
- receiving marketing approvals from the FDA and, to a lesser extent, similar regulatory authorities outside the United
States for our product candidates;
- •
- establishing commercial manufacturing arrangements with third parties;
- •
- launching commercial sales of any of the product candidates that may be approved;
- •
- the medical community and patients accepting any approved product;
- •
- the placement of any approved products on payors' formulary tiers and the reimbursement rates for the approved products;
- •
- effectively competing with other therapies;
- •
- any approved products continuing to have an acceptable safety profile following approval; and
- •
- obtaining, maintaining, enforcing and defending intellectual property rights and claims.
If we or Forest do not achieve one or more of these factors in a timely manner or at all, we could experience significant delays or an inability to successfully commercialize our product candidates, which would materially harm our business.
If clinical studies of our product candidates fail to demonstrate sufficient safety and efficacy to the satisfaction of the FDA or similar regulatory authorities outside the United States or do not otherwise produce positive results, we may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of our product candidates.
Before obtaining regulatory approval for the sale of our product candidates, we must conduct extensive clinical studies to demonstrate the safety and efficacy of our product candidates in humans. Clinical studies are expensive, are difficult to design and implement, can take many years to complete and are uncertain as to outcome. A failure of one or more of our clinical studies could occur at any stage of testing. The outcome of preclinical testing and early clinical studies may not be predictive of the success of later clinical studies, and interim results of a clinical study do not necessarily predict final results. For example, the successful result of our Phase 2/3 study of ADS-5102 for the treatment
13
of LID, including the lack of difference from placebo in the incidence of sleep-related adverse events, may not be repeated in our anticipated Phase 3 registration trial.
We may experience numerous unforeseen events during, or as a result of, clinical studies that could delay or prevent our ability to receive regulatory approval or commercialize our product candidates, including that:
- •
- clinical studies of our product candidates may produce negative or inconclusive results, and we may decide, or regulators
may require us, to conduct additional clinical studies or abandon product development programs;
- •
- the number of patients required for clinical studies of our product candidates may be larger than we anticipate,
enrollment in these clinical studies may be insufficient or slower than we anticipate or patients may drop out of these clinical studies at a higher rate than we anticipate;
- •
- the cost of clinical studies of our product candidates may be greater than we anticipate;
- •
- our third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in
a timely manner, or at all;
- •
- we might have to suspend or terminate clinical studies of our product candidates for various reasons, including a finding
that our product candidates have unanticipated serious side effects or other unexpected characteristics or that the patients are being exposed to unacceptable health risks;
- •
- regulators may not approve our proposed clinical development plans or may require costly modifications to such plans;
- •
- regulators or institutional review boards may not authorize us or our investigators to commence a clinical study or
conduct a clinical study at a prospective study site;
- •
- regulators or institutional review boards may require that we or our investigators suspend or terminate clinical research
for various reasons, including noncompliance with regulatory requirements; and
- •
- the supply or quality of our product candidates or other materials necessary to conduct clinical studies of our product candidates may be insufficient or inadequate.
If we are required to conduct additional clinical studies or other testing of our product candidates beyond those that we currently contemplate, if we are unable to successfully complete clinical studies or other testing of our product candidates, if the results of these studies or tests are not positive or are only modestly positive or if there are safety concerns, we may:
- •
- be delayed in obtaining marketing approval for our product candidates;
- •
- not obtain marketing approval at all;
- •
- obtain approval for indications that are not as broad as intended;
- •
- have the product removed from the market after obtaining marketing approval;
- •
- be subject to additional post-marketing testing requirements; or
- •
- be subject to restrictions on how the product is distributed or used.
Our product development costs will increase if we experience delays in testing or approvals. Significant clinical study delays also could shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do, which would impair our ability to commercialize our product candidates and harm our business and results of operations.
14
Even if clinical studies demonstrate statistically significant efficacy and acceptable safety for a product, the FDA or similar regulatory authorities outside the United States may not approve it for marketing.
Forest has completed the clinical trials that it and we believe are necessary to support the submission to the FDA of a New Drug Application, or NDA, for MDX-8704 for the treatment of moderate to severe dementia in Alzheimer's disease patients. Forest submitted an NDA to the FDA for MDX-8704 in February 2014. We believe those trials indicated that a single dose of MDX-8704 is bioequivalent to separate doses of Namenda XR and donepezil and that MDX-8704 exhibits the same bioavailability whether administered after fasting, after a meal or when sprinkled on apple sauce. We expect to initiate a Phase 3 registration trial of ADS-5102 in 2014 for LID and, if the trial is successful, intend to submit an NDA for ADS-5102 in that indication. It is possible that the FDA may not consider the results of these studies to be sufficient for approval of the product candidates in their proposed indications. If the FDA were to require Forest or us to conduct additional studies of MDX-8704 or ADS-5102 to obtain approval for the product candidates in their currently contemplated indications, our business and financial results would be materially adversely affected.
Our product candidates have never been manufactured on a commercial scale, and there are risks associated with developing manufacturing and packaging processes and scaling them up to commercial scale.
Our product candidates have never been manufactured on a commercial scale, and there are risks associated with developing manufacturing and packaging processes and scaling them up to commercial scale including, among others, cost overruns, potential problems with process scale-up, process reproducibility, stability issues, lot consistency and timely availability of raw materials. These risks can adversely affect regulatory approval of a product candidate. In addition, even if we could otherwise obtain regulatory approval for any product candidate, there is no assurance that our manufacturer will be able to manufacture the approved product to specifications acceptable to the FDA or other regulatory authorities, to produce it in sufficient quantities to meet the requirements for the potential launch of the product or to meet potential future demand. If our manufacturer is unable to produce sufficient quantities of the approved product, our regulatory approval or commercialization efforts would be impaired, which would have an adverse effect on our business, financial condition, results of operations and growth prospects.
Our product candidates and Namenda XR are complex to manufacture, and manufacturing disruptions may occur.
Our product candidates and Namenda XR all include controlled-released versions of existing drugs, and some are combinations of existing drugs. The manufacture and packaging of controlled-release versions of existing drugs or combinations of existing drugs are substantially more complex than the manufacture and packaging of the immediate-release version of a drug alone. Even after the manufacturing process for a controlled-release or combination product has been scaled to commercial levels and numerous commercial lots have been produced, manufacturing disruptions may occur. Such problems may prevent the production of lots that meet the specifications required for sale of the product and may be difficult and expensive to resolve. For example, in November 2013, Forest recalled three packaged lots of Namenda XR because Forest's dissolution testing revealed a failure to meet specification throughout shelf life. Namenda XR is one of the components of Forest's fixed-dose combination product candidate MDX-8704. If any such issues were to arise with respect to our product candidates or future products, if any, or if Forest's sales of Namenda XR or the regulatory approval or Forest's sales of MDX-8704 were to be negatively impacted by such issues our business, financial results or stock price could be adversely affected.
15
If generic manufacturers use litigation and regulatory means to obtain approval for generic versions of products on which our future revenue depends, our business will suffer.
Under the U.S. Food, Drug and Cosmetic Act, or FDCA, the FDA can approve an Abbreviated New Drug Application, or ANDA, for a generic version of a branded drug without the ANDA applicant undertaking the clinical testing necessary to obtain approval to market a new drug. In place of such clinical studies, an ANDA applicant usually needs only to submit data demonstrating that its product has the same active ingredient(s) and is bioequivalent to the branded product, in addition to any data necessary to establish that any difference in strength, dosage form, inactive ingredients, or delivery mechanism does not result in different safety or efficacy profiles, as compared to the reference drug.
The FDCA requires that an applicant for approval of a generic form of a branded drug certify either that its generic product does not infringe any of the patents listed by the owner of the branded drug in the Approved Drug Products with Therapeutic Equivalence Evaluations, also known as the Orange Book, or that those patents are not enforceable. This process is known as a paragraph IV challenge. Upon receipt of the paragraph IV notice, the owner has 45 days to bring a patent infringement suit in federal district court against the company seeking ANDA approval of a product covered by one of the owner's patents. The discovery, trial and appeals process in such suits can take several years. If this type of suit is commenced, the FDCA provides a 30-month stay on the FDA's approval of the competitor's application. This type of litigation is often time-consuming and costly and may result in generic competition if the patents at issue are not upheld or if the generic competitor is found not to infringe the owner's patents. If the litigation is resolved in favor of the ANDA applicant or the challenged patent expires during the 30-month stay period, the stay is lifted and the FDA may thereafter approve the application based on the standards for approval of ANDAs.
For example, as of March 25, 2014, we had received notice that ten companies had submitted ANDAs to the FDA requesting permission to manufacture and market generic versions of Namenda XR, on which we are entitled to receive royalties from Forest beginning in June 2018. In the notices, these companies allege that the patents associated with Namenda XR, one of which is owned by Forest, one of which is exclusively licensed to Forest by Merz Pharma GmbH & Co. KGaA and others of which are owned by us and licensed by us exclusively to Forest in the United States, are invalid, unenforceable or will not be infringed by the companies' manufacture, use or sale of generic versions of Namenda XR. In January and February 2014, we, Forest, Forest Laboratories Holdings Ltd., Merz Pharma GmbH & Co. KGaA and Merz Pharmaceuticals GmbH filed lawsuits for infringement of the relevant patents against the eight of these companies that had then submitted ANDAs. Because these lawsuits were filed within the requisite 45-day period provided in the FDCA, there are stays preventing FDA approval of the ANDAs for 30 months or until a court decision adverse to the patents. The 30-month stay for these ANDAs will expire beginning in June 2016.
For various strategic and commercial reasons, manufacturers of generic medications frequently file ANDAs shortly after FDA approval of a branded drug regardless of the perceived strength and validity of the patents associated with such product. Based on these past practices, we believe it is likely that one or more such generic manufacturers will file ANDAs with respect to MDX-8704 and ADS-5102, if they are approved by the FDA, prior to the expiration of the patents related to those compounds.
The filing of an ANDA as described above with respect to any of our products could have an adverse impact on our stock price. Moreover, if any such ANDAs were to be approved and the patents covering the relevant products were not upheld in litigation, or if a generic competitor is found not to infringe these patents, the resulting generic competition would negatively affect our business, financial condition and results of operations.
16
Any product candidate that we are able to commercialize may become subject to unfavorable pricing regulations, third-party coverage or reimbursement practices or healthcare reform initiatives, thereby harming our business.
The regulations that govern marketing approvals, pricing, coverage and reimbursement for new therapeutic products vary widely from country to country. Some countries require approval of the sale price of a product before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we might obtain regulatory approval for a product in a particular country, but then be subject to price regulations that delay our commercial launch of the product and negatively impact the revenue we are able to generate from the sale of the product in that country. In particular, in many countries, including many major European markets, therapies that are based on existing generic drugs, such as Namenda XR (memantine) and ADS-5102 (amantadine), or combinations of existing generic drugs, such as MDX-8704, generally are not well-reimbursed. As a result, we anticipate that the commercial success of Namenda XR, ADS-5102 and MDX-8704 will be largely dependent on success in the U.S. market.
Our ability to commercialize any products successfully in the United States will depend in part on the extent to which coverage and reimbursement for these products becomes available from third-party payors, including government health administration authorities, such as those that administer the Medicare and Medicaid programs, and private health insurers. Third-party payors decide which medications they will cover and establish reimbursement levels. A primary trend in the U.S. healthcare industry is cost containment. Third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. Increasingly, third-party payors are requiring that companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. We cannot assure you that coverage and reimbursement will be available for any product that we commercialize and, if reimbursement is available, what the level of reimbursement will be. Coverage and reimbursement may impact the demand for, or the price of, any product for which we obtain marketing approval. If coverage and reimbursement is not available or is available only to limited levels, we may not be able to successfully commercialize any product candidate that we successfully develop and Forest may be unable to successfully market Namenda XR or MDX-8704.
There may be significant delays in obtaining coverage and reimbursement for approved products, and coverage may be more limited than the purposes for which the product is approved by the FDA. Moreover, eligibility for reimbursement does not imply that any product will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Interim payments for new products, if applicable, may also not be sufficient to cover our costs and may not be made permanent. Payment rates may vary according to the use of the product and the clinical setting in which it is used, may be based on payments allowed for lower cost products that are already reimbursed and may be incorporated into existing payments for other services. Net prices for products may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of products from countries where they may be sold at lower prices than in the United States. In the United States, private third-party payors often rely upon Medicare coverage and reimbursement policies and payment limitations in setting their own coverage and reimbursement policies. Our inability to promptly obtain coverage, reimbursement and profitable payment rates from both government funded and private payors for new products that we develop, or products developed or marketed by Forest under our license agreement, could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products and our overall financial condition.
17
If serious adverse side effects are identified during the development of ADS-5102 or any other product candidates, we may need to abandon our development of that product candidate.
Our product candidate ADS-5102, along with our other earlier stage product candidates, are still in clinical or pre-clinical development. The risk of failure during development is high. It is impossible to predict when or if any of our product candidates will prove safe and tolerable enough to receive regulatory approval. For example, amantadine, the active pharmaceutical ingredient in ADS-5102, carries the risk of blurred vision, dizziness, lightheadedness, faintness, trouble sleeping, depression or anxiety, hallucinations, swelling of the hands, legs, or feet, difficulty urinating, shortness of breath and rash. These side effects may be the cause of the relatively low rate of acceptance of amantadine by physicians and patients. Although we believe our controlled-release version of amantadine has reduced the risks of these side effects thereby enabling higher doses, there can be no assurance that our proposed Phase 3 registration trial or future studies in other indications will not fail due to safety or tolerability issues. In such an event, we might need to abandon development of ADS-5102 entirely or for certain indications. If we are forced to abandon development of our product candidates, our business, results of operations and financial condition will be harmed.
Safety issues with Namenda XR, MDX-8704 or ADS-5102, or the parent drugs or other components of Namenda XR, MDX-8704 or ADS-5102, or with approved products of third parties that are similar to Namenda XR, MDX-8704 or ADS-5102, could decrease the potential sales of Namenda XR, MDX-8704 or ADS-5102 or give rise to delays in the regulatory approval process, restrictions on labeling or product withdrawal.
Discovery of previously unknown problems, or increased focus on a known problem, with an approved product may result in restrictions on its permissible uses, including withdrawal of the medicine from the market. The label for Namenda XR lists potential side effects such as headache, diarrhea and dizziness, and side effects have been observed in clinical trial subjects taking MDX-8704 and ADS-5102, such as constipation, dizziness, hallucination, dry mouth, fall, confusion, headache, nausea and weakness in the case of ADS-5102 and dizziness, headache and diarrhea in the case of MDX-8704.
If we or others identify additional undesirable side effects caused by Namenda XR, or by MDX-8704 and ADS-5102 after approval:
- •
- regulatory authorities may require the addition of labeling statements, specific warnings, contraindications or field
alerts to physicians and pharmacies;
- •
- regulatory authorities may withdraw their approval of the product and require us to take our approved drug off the market;
- •
- we may be required to change the way the product is administered, conduct additional clinical trials, change the labeling
of the product or implement a Risk Evaluation and Mitigation Strategy;
- •
- we may have limitations on how we promote our drugs;
- •
- third-party payors may limit coverage or reimbursement for our drugs;
- •
- sales of products may decrease significantly;
- •
- we may be subject to litigation or product liability claims; and
- •
- our reputation may suffer.
Any of these events could prevent us from achieving or maintaining market acceptance of the affected product and could substantially increase our commercialization costs and expenses, which in turn could delay or prevent us from generating significant revenue from its sale.
Namenda XR, MDX-8704 or ADS-5102 may also be affected by the safety and tolerability of their parent drugs or drugs with similar mechanisms of action. Although memantine, which is a component
18
of Namenda XR and MDX-8704, donepezil, which is a component MDX-8704, and amantadine, which is a component of ADS-5102, have been used in patients for many years, newly observed toxicities or worsening of known toxicities, in preclinical studies of, or in patients receiving, memantine, donepezil, or amantadine, or reconsideration of known toxicities of compounds in the setting of new indications, could result in increased regulatory scrutiny of our products and product candidates. The FDA has substantial discretion in the NDA approval process and may refuse to approve any application if the FDA concludes that the risk/benefit analysis of a potential drug treatment for a specific indication does not warrant approval. Thus, although the parent drug for, or a drug related to, one of our product candidates may be approved by the FDA in a particular indication, the FDA may conclude that our product candidate's risk/benefit profile does not warrant approval in a different indication, and the FDA may refuse to approve our product candidate. Such conclusion and refusal would prevent us from developing and commercializing our product candidates and severely harm our business and financial condition.
Following consumption, Namenda XR, MDX-8704 and ADS-5102 first are broken down by the body's natural metabolic processes and then release the active drug and other breakdown substances. While these breakdown substances are generally regarded as safe, it is possible that there could be unexpected toxicity associated with them that will cause Namenda XR, MDX-8704 or ADS-5102 to be poorly tolerated by, or toxic to, humans. Any unexpected toxicity of, or suboptimal tolerance to, the product or product candidates could reduce their sales of approved products and delay or prevent commercialization of our product candidates.
In addition, problems with approved products marketed by third parties that utilize the same therapeutic target or that belong to the same therapeutic class as memantine, amantadine or donepezil could adversely affect the commercialization of Namenda XR, MDX-8704 and ADS-5102. For example, the product withdrawals of Vioxx from Merck and Bextra from Pfizer due to safety issues have caused other drugs that have the same therapeutic target, such as Celebrex from Pfizer, to receive additional scrutiny from regulatory authorities.
The marketing of ADS-5102 and MDX-8704, if approved, will be limited to use for the treatment of specific indications, and if we or Forest want to expand the indications for which these product candidates may be marketed, additional regulatory approvals will need to be obtained, which may not be granted.
We are currently seeking regulatory approval of ADS-5102 for the treatment of LID, and Forest is seeking regulatory approval of MDX-8704 for the treatment of moderate to severe dementia related to Alzheimer's disease. If these product candidates are approved, the FDA will restrict our and Forest's ability to market or advertise the products for other indications, which could limit physician and patient adoption. We or Forest may attempt to develop, promote and commercialize new treatment indications and protocols for the products in the future, but we cannot predict when or if the clearances required to do so will be received. In addition, we would be required to conduct additional clinical trials or studies to support approvals for additional indications for ADS-5102, which would be time consuming and expensive, and may produce results that do not support regulatory approvals. If we do not obtain additional regulatory approvals, our ability to expand our business will be limited.
If our product candidates are approved for marketing, and we are found to have improperly promoted off-label uses, or if physicians misuse our products or use our products off-label, we may become subject to prohibitions on the sale or marketing of our products, significant fines, penalties, and sanctions, product liability claims, and our image and reputation within the industry and marketplace could be harmed.
The FDA and other regulatory agencies strictly regulate the marketing and promotional claims that are made about drug products, such as ADS-5102 and MDX-8704, if approved. In particular, a product may not be promoted for uses or indications that are not approved by the FDA or such other regulatory agencies as reflected in the product's approved labeling. For example, if we receive marketing approval for ADS-5102 for the treatment of LID, the first indication we are pursuing, we
19
cannot prevent physicians from using our ADS-5102 products on their patients in a manner that is inconsistent with the approved label. If we are found to have promoted such off-label uses prior to FDA approval for an additional indication, we may receive warning letters and become subject to significant liability, which would materially harm our business. The federal government has levied large civil and criminal fines against companies for alleged improper promotion and has enjoined several companies from engaging in off-label promotion. If we become the target of such an investigation or prosecution based on our marketing and promotional practices, we could face similar sanctions, which would materially harm our business. In addition, management's attention could be diverted from our business operations, significant legal expenses could be incurred, and our reputation could be damaged. The FDA has also requested that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed. If we are deemed by the FDA to have engaged in the promotion of our products for off-label use, we could be subject to FDA prohibitions on the sale or marketing of our products or significant fines and penalties, and the imposition of these sanctions could also affect our reputation and position within the industry.
Physicians may also misuse our products, potentially leading to adverse results, side effects or injury, which may lead to product liability claims. If our products are misused, we may become subject to costly litigation by our customers or their patients. Product liability claims could divert management's attention from our core business, be expensive to defend and result in sizable damage awards against us that may not be covered by insurance. Furthermore, the use of our products for indications other than those cleared by the FDA may not effectively treat such conditions, which could harm our reputation in the marketplace among physicians and patients. Any of these events could harm our business and results of operations and cause our stock price to decline.
We currently have no sales or distribution personnel and only limited marketing capabilities. If we are unable to develop a sales and marketing and distribution capability, we will not be successful in commercializing ADS-5102 or other future approved products.
We do not have a significant sales or marketing infrastructure and have no experience in the sale, marketing or distribution of therapeutic products. To achieve commercial success for any approved product, we must either develop a sales and marketing organization or outsource these functions to third parties. We expect that the primary focus of our commercialization efforts will be the United States, and we intend to develop our own sales force to commercialize ADS-5102 and our other wholly-owned future approved products in the United States. Commercialization of ADS-5102 and other future approved products outside of the United States, to the extent pursued, is likely to require collaboration with a third party.
There are risks involved with both establishing our own sales and marketing capabilities and entering into arrangements with third parties to perform these services. For example, recruiting and training a sales force is expensive and time-consuming and could delay any product launch. If the commercial launch of a product candidate for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel.
In addition, our existing arrangements for the commercialization of Namenda XR and MDX-8704 may not be successful and we also may not be successful entering into new arrangements with third parties to sell and market our future approved products or may be unable to do so on terms that are favorable to us. We have and will in the future be likely to have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively and could damage our reputation. If we underestimate the size of sales force required to market our products, our commercialization efforts will be adversely affected. If we do not establish sales and marketing capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our future approved products.
20
Our future products may fail to achieve the degree of market acceptance by physicians, patients, healthcare payors and others in the medical community necessary for commercial success.
Our future products may fail to gain sufficient market acceptance by physicians, hospital administrators, patients, healthcare payors and others in the medical community. The degree of market acceptance of our products, after being approved for commercial sale, will depend on a number of factors, including:
- •
- the prevalence and severity of any side effects;
- •
- efficacy, duration of response and potential advantages compared to alternative treatments;
- •
- the price we charge;
- •
- the willingness of physicians to change their current treatment practices;
- •
- convenience and ease of administration compared to alternative treatments;
- •
- the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies;
- •
- the strength of marketing and distribution support; and
- •
- the availability of third-party coverage or reimbursement.
For example, the absence of approved therapeutics to treat LID may require us to educate healthcare providers and patients about LID.
Delays in the enrollment of patients in any of our clinical trials could increase our development costs and delay completion of the study.
We may not be able to initiate or continue clinical studies for our product candidates if we are unable to locate and enroll a sufficient number of eligible patients to participate in these studies as required by the FDA or other regulatory authorities. Location and enrollment of eligible patients may be adversely affected by, for example, our inability to locate and activate clinical study sites at a satisfactory pace to meet our planned timetables. Even if we are able to enroll a sufficient number of patients in our clinical studies, if the pace of enrollment is slower than we expect, the development costs for our product candidates may increase and the completion of our studies may be delayed or our studies could become too expensive to complete. The study design for our Phase 3 trial of ADS-5102 for the treatment of LID is placebo controlled, meaning that a portion of patients will not receive treatment that may help control the symptoms of their Parkinson's disease. Because these symptoms are uncomfortable, a relatively long study period may make it more difficult to enroll and retain patients in the trial.
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do.
The development and commercialization of new therapeutic products is highly competitive. We face competition with respect to our current product candidates, and will face competition with respect to any products that we may seek to develop or commercialize in the future, from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide. For example, in the market for Alzheimer's disease treatments, Namenda XR and MDX-8704 compete or will compete with generic products such as galatamine, rivastigmine and donepezil as well as branded products such as the Exelon patch (Novartis) and Aricept 23 mg (Eisai). ADS-5102, if approved, may face competition from various drugs approved to treatment Parkinson's disease, though not LID, such as Azilect (Teva), Requip XL (GlaxoSmithKline), Mirapex ER (Boehringer Ingelheim), Neupro Patch (UCB), Comtan (Novartis) and Stalevo (Novartis). ADS-5102
21
may also face competition from generic versions of amantadine and from other controlled-release versions of amantadine that may be in development. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization. Many of these competitors are attempting to develop therapeutics for our target indications. In addition, many of our competitors are large pharmaceutical companies that will have a greater ability to reduce prices for their competing drugs in an effort to gain market share and undermine the value proposition that we might otherwise be able to offer to payors.
Many of our competitors, including a number of large pharmaceutical companies that compete directly with us, have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical, biotechnology and diagnostic industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical study sites and patient registration for clinical studies, as well as in acquiring technologies and products complementary to, or necessary for, our programs.
Product liability lawsuits against us could cause us to incur substantial liabilities and limit commercialization of any products that we may develop.
We face an inherent risk of product liability exposure related to the testing of our product candidates in human clinical studies and will face an even greater risk upon commercial sale of any products that are ultimately approved. If we cannot successfully defend ourselves against claims that our product candidates or products caused injuries, we will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
- •
- decreased demand for any product candidates or products that we may develop;
- •
- the inability to commercialize any products that we may develop;
- •
- injury to our reputation and significant negative media attention;
- •
- withdrawal of patients from clinical studies or cancellation of studies;
- •
- significant costs to defend the related litigation;
- •
- substantial monetary awards to patients; and
- •
- loss of revenue.
We currently hold $10.0 million in product liability insurance coverage, which may not be adequate to cover all liabilities that we may incur. Insurance coverage is increasingly expensive. We may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability or associated costs that may arise.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we focus on research programs and product candidates for specific indications. As a result, we may forego or delay pursuit of opportunities with other product candidates or other indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial
22
products or profitable market opportunities. Our spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable products.
If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been advantageous for us to retain sole development and commercialization rights.
Risks related to our reliance on third parties
We have entered into a license agreement with Forest with respect to MDX-8704 and Namenda XR, and may enter into additional license or collaboration agreements. These arrangements may place the development of these product candidates outside our control, may require us to relinquish important rights or may otherwise be on terms unfavorable to us, and if our collaborations are not successful, these product candidates may not reach their full market potential.
In November 2012, we entered into a license agreement with Forest pursuant to which we granted Forest a co-exclusive right to develop and an exclusive right to commercialize fixed-dose memantine-donepezil products, such as MDX-8704, in the United States, and granted Forest a license covering controlled-release versions of memantine, such as Namenda XR. Under the terms of the license agreement, Forest substantially controls the commercialization of these products. Collaborations involving our current or future products, such as our agreement with Forest, are subject to numerous risks, which may include that:
- •
- collaborators have significant discretion in determining the efforts and resources that they will apply to collaborations;
- •
- collaborators may not pursue development and commercialization of our product candidates or may elect not to continue or
renew development or commercialization programs based on clinical study results, changes in their strategic focus due to the acquisition of competitive products, availability of funding or other
external factors, such as a business combination that diverts resources or creates competing priorities;
- •
- collaborators may delay clinical studies, provide insufficient funding for a clinical study program, stop a clinical
study, abandon a product candidate, repeat or conduct new clinical studies or require a new formulation of a product candidate for clinical testing;
- •
- collaborators could independently develop, or develop with third parties, products that compete directly or indirectly
with our products or product candidates;
- •
- a collaborator with marketing, manufacturing and distribution rights to one or more products may not commit sufficient
resources to or otherwise not perform satisfactorily in carrying out these activities;
- •
- we could grant exclusive rights to our collaborators that would prevent us from collaborating with others;
- •
- collaborators may not properly maintain or defend our intellectual property rights or may use our intellectual property or
proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential
liability;
- •
- collaborators may not aggressively or adequately pursue litigation against ANDA filers or may settle such litigation on unfavorable terms;
23
- •
- disputes may arise between us and a collaborator that causes the delay or termination of the research, development or
commercialization of our current or future products or that results in costly litigation or arbitration that diverts management attention and resources;
- •
- collaborations may be terminated, sometimes at-will, without penalty, such as with Forest, and, if terminated, may result
in a need for additional capital to pursue further development or commercialization of the applicable current or future products;
- •
- collaborators may own or co-own intellectual property covering our products that results from our collaborating with them,
and in such cases, we would not have the exclusive right to commercialize such intellectual property; and
- •
- a collaborator's sales and marketing activities or other operations may not be in compliance with applicable laws resulting in civil or criminal proceedings.
On February 18, 2014, Actavis plc and Forest announced the acquisition of Forest by Actavis. We cannot predict whether this acquisition will have an impact on our business or on the license agreement with Forest.
We rely on third parties to conduct our clinical trials, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of these trials.
We do not independently conduct clinical studies of our product candidates. We rely on third parties, such as contract research organizations, or CROs, clinical data management organizations, medical institutions and clinical investigators to perform this function. Our reliance on these third parties for clinical development activities reduces our control over these activities but does not relieve us of our responsibilities. For example, the FDA requires us to comply with standards, commonly referred to as good clinical practices, for conducting, recording and reporting the results of clinical studies to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of patients in clinical studies are protected, even though we are not in control of these processes. These third parties may also have relationships with other entities, some of which may be our competitors. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our clinical studies in accordance with regulatory requirements or our stated protocols, we will not be able to obtain, or may be delayed in obtaining, regulatory approvals for our product candidates and will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates.
We also rely on other third parties to store and distribute supplies for our clinical studies. Any performance failure on the part of our existing or future distributors could delay clinical development or regulatory approval of our product candidates or commercialization of our products, producing additional losses and depriving us of potential product revenue.
We rely on third-party contract manufacturing organizations to manufacture and supply our product candidates for us. If one of our suppliers or manufacturers fails to perform adequately or fulfill our needs, we may be required to incur significant costs and devote significant efforts to find new suppliers or manufacturers. We may also face delays in the development and commercialization of our product candidates.
We currently have limited experience in, and we do not own facilities for, clinical-scale manufacturing of our product candidates and we rely upon third-party contract manufacturing organizations to manufacture and supply drug product for our clinical studies. The manufacture of pharmaceutical products in compliance with the FDA's current good manufacturing practices, or cGMPs, requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Manufacturers of pharmaceutical products often encounter difficulties in production, including difficulties with production costs and yields, quality
24
control, including stability of the product candidate and quality assurance testing, shortages of qualified personnel, as well as compliance with strictly enforced cGMP requirements, other federal and state regulatory requirements and foreign regulations. If our manufacturers were to encounter any of these difficulties or otherwise fail to comply with their obligations to us or under applicable regulations, our ability to provide study drugs in our clinical trials would be jeopardized. Any delay or interruption in the supply of clinical study materials could delay the completion of our clinical studies, increase the costs associated with maintaining our clinical study programs and, depending upon the period of delay, require us to commence new studies at significant additional expense or terminate the studies completely.
All manufacturers of our product candidates must comply with cGMP requirements enforced by the FDA through its facilities inspection program. These requirements include, among other things, quality control, quality assurance and the maintenance of records and documentation. Manufacturers of our product candidates may be unable to comply with these cGMP requirements and with other FDA, state and foreign regulatory requirements. The FDA or similar foreign regulatory agencies may also implement new standards at any time, or change their interpretation and enforcement of existing standards for manufacture, packaging or testing of products. We have little control over our manufacturers' compliance with these regulations and standards. A failure to comply with these requirements may result in fines and civil penalties, suspension of production, suspension or delay in product approval, product seizure or recall or withdrawal of product approval. If the safety of any product supplied is compromised due to our manufacturers' failure to adhere to applicable laws or for other reasons, we may not be able to obtain regulatory approval for or successfully commercialize our products and we may be held liable for any injuries sustained as a result. Any of these factors could cause a delay of clinical studies, regulatory submissions, approvals or commercialization of our product candidates, entail higher costs or impair our reputation.
We currently rely on single source suppliers for each of our product candidates under a development agreement. We do not have a long-term supply agreement in place. Although we believe alternative sources of supply exist, the number of third-party suppliers with the necessary manufacturing and regulatory expertise and facilities is limited, and it could be expensive and take a significant amount of time to arrange for alternative suppliers, which would adversely affect our business. New suppliers of any product candidate would be required to qualify under applicable regulatory requirements and would need to have sufficient rights under applicable intellectual property laws to the method of manufacturing the product candidate. Obtaining the necessary FDA approvals or other qualifications under applicable regulatory requirements and ensuring non-infringement of third-party intellectual property rights could result in a significant interruption of supply and could require the new manufacturer to bear significant additional costs which may be passed on to us.
Risks related to the operation of our business
Our future success depends on our ability to retain our chief executive officer and other key executives and to attract, retain and motivate qualified personnel.
We are highly dependent on our chief executive officer and the other members of our executive and scientific teams. Our executives may terminate their employment with us at any time. The loss of the services of any of these people could impede the achievement of our research, development and commercialization objectives. We maintain "key person" insurance for our chief executive officer but not for any other executives or employees. Any insurance proceeds we may receive under this "key person" insurance would not adequately compensate us for the loss of our chief executive officer's services.
Recruiting and retaining qualified scientific, clinical, manufacturing and sales and marketing personnel will also be critical to our success. We may not be able to attract and retain these personnel
25
on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us.
We expect to expand our development, regulatory and sales and marketing capabilities, and, as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
As of December 31, 2013, we had 22 employees. Over the next several years, we expect to experience significant growth in the number of our employees and the scope of our operations, particularly in sales and marketing. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The physical expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.
We are an "emerging growth company," and we cannot be certain whether the reduced reporting requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an "emerging growth company," as defined in the Jumpstart Our Business Startups Act, or the JOBS Act, which was enacted in April 2012. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We could be an emerging growth company for up to five years, although circumstances could cause us to lose that status earlier. We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of this offering, (b) in which we have total annual gross revenue of at least $1.0 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common stock that is held by non-affiliates exceeds $700 million as of the prior June 30th, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt securities during the prior three-year period. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may suffer or be more volatile.
Business disruptions could seriously harm our future revenue and financial condition and increase our costs and expenses.
Our operations could be subject to earthquakes, power shortages, telecommunications failures, floods, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics and other natural or manmade disasters or business interruptions. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses. Our corporate headquarters is located in California and certain clinical sites for our product candidates,
26
operations of our existing and future partners and suppliers are or will be located in California near major earthquake faults and fire zones. The ultimate impact on us, our significant partners, suppliers and our general infrastructure of being located near major earthquake faults and fire zones and being consolidated in certain geographical areas is unknown, but our operations and financial condition could suffer in the event of a major earthquake, fire or other natural or manmade disaster.
If we obtain approval to commercialize any approved products outside of the United States, a variety of risks associated with international operations could materially adversely affect our business. If any product candidates that we may develop are approved for commercialization outside the United States, we will be subject to additional risks related to entering into international business relationships, including:
- •
- different regulatory requirements for drug approvals in foreign countries;
- •
- reduced protection for intellectual property rights;
- •
- unexpected changes in tariffs, trade barriers and regulatory requirements;
- •
- economic weakness, including inflation or political instability in particular foreign economies and markets;
- •
- difficulties in assuring compliance with foreign corrupt practices laws;
- •
- compliance with tax, employment, immigration and labor laws for employees living or traveling abroad;
- •
- foreign taxes, including withholding of payroll taxes;
- •
- foreign currency fluctuations, which could result in increased operating expenses and reduced revenue, and other
obligations incident to doing business in another country;
- •
- workforce uncertainty in countries where labor unrest is more common than in the United States;
- •
- production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and
- •
- business interruptions resulting from geopolitical actions, including war and terrorism, or natural disasters including earthquakes, typhoons, floods and fires.
Our internal computer systems, or those of our CROs or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of our drug development programs.
Despite the implementation of security measures, our internal computer systems and those of our CROs and other contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. While we have not experienced any such system failure, accident or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our drug development programs. For example, the loss of clinical study data from completed or ongoing clinical studies for any of our product candidates could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development of our product candidates could be delayed.
27
Risks related to intellectual property
Our ability to successfully commercialize our technology and products may be materially adversely affected if we are unable to obtain and maintain effective intellectual property rights for our technologies and product candidates.
Our success depends in large part on our ability to obtain and maintain patent and other intellectual property protection in the United States and in other countries with respect to our proprietary technology and products. In some circumstances, we may not have the right to control the preparation, filing and prosecution of patent applications, or to maintain or enforce the patents, covering technology or products that we license to third parties or that we may license from third parties. Therefore, we cannot be certain that these patents and applications will be prosecuted and enforced in a manner consistent with the best interests of our business. In addition, if third parties who license patents to us or from us fail to maintain such patents, or lose rights to those patents, the rights we have licensed may be reduced or eliminated.
We have sought to protect our proprietary position by filing patent applications in the United States and abroad related to our novel technologies and products that are important to our business. This process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. In addition, we may not pursue or obtain patent protection in all relevant markets. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. Our pending and future patent applications may not result in patents being issued which protect our technology or products, in whole or in part. In addition, our existing patents and any future patents we obtain may not be sufficiently broad to prevent others from using our technologies or from developing competing products and technologies.
The patent position of specialty pharmaceutical and biotechnology companies generally is highly uncertain and involves complex legal and factual questions for which many legal principles remain unresolved. In recent years patent rights have been the subject of significant litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain. Our pending and future patent applications may not result in patents being issued in the United States or in other jurisdictions which protect our technology or products or which effectively prevent others from commercializing competitive technologies and products. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection. In addition, the laws of foreign countries may not protect our rights to the same extent as the laws of the United States. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot be certain that we were the first to make the inventions claimed in our patents or pending patent applications, or that we were the first to file for patent protection of such inventions. In addition, the United States Patent and Trademark Office, or USPTO, might require that the term of a patent issuing from a pending patent application be disclaimed and limited to the term of another patent that is commonly owned or names a common inventor. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights is highly uncertain.
Recent or future patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents. In March 2013, under the recently enacted Leahy-Smith America Invents Act, or America Invents Act, the United States moved from a "first to invent" to a "first-to-file" system. Under a "first-to-file" system, assuming the other requirements for patentability are met, the first inventor to file a patent application generally will be entitled to a patent on the invention regardless of whether another inventor had made the invention earlier. The America Invents Act includes a number of other significant changes to U.S.
28
patent law, including provisions that affect the way patent applications are prosecuted, redefine prior art and establish a new post-grant review system. The effects of these changes are currently unclear as the USPTO only recently developed new regulations and procedures in connection with the America Invents Act and many of the substantive changes to patent law, including the "first-to-file" provisions, only became effective in March 2013. In addition, the courts have yet to address any of these provisions and the applicability of the act and new regulations on specific patents discussed herein have not been determined and would need to be reviewed. However, the America Invents Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business and financial condition. We may become involved in opposition, interference, derivation, inter partes review or other proceedings challenging our patent rights or the patent rights of others, and the outcome of any proceedings are highly uncertain. An adverse determination in any such proceeding could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights.
Even if our patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our owned or licensed patents by developing similar or alternative technologies or products in a non-infringing manner. The issuance of a patent is not conclusive as to its scope, validity or enforceability, and our owned and licensed patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in the patent claims of our owned or licensed patents being narrowed, invalidated or held unenforceable, which could limit our ability to stop or prevent us from stopping others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours or otherwise provide us with a competitive advantage.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on all of our product candidates throughout the world would be prohibitively expensive. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection but where enforcement is not as strong as in the United States. These products may compete with our product candidates in jurisdictions where we do not have any issued patents and our patent claims or other intellectual property rights may not be effective or sufficient to prevent them from so competing. Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biopharmaceuticals, which could make it difficult for us to stop the infringement of our patents or marketing of competing products against third parties in violation of our proprietary rights generally. The initiation of proceedings by third parties to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
29
Obtaining and maintaining our patent protection depends upon compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent prosecution process and following the issuance of a patent. Our failure to comply with such requirements could result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case if our patent were in force.
We may become involved in lawsuits or other proceedings to protect or enforce our patents or other intellectual property, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe or otherwise violate our patents, trademarks, copyrights or other intellectual property. To counter infringement or unauthorized use, we or our licensees may be required to file infringement claims, which can be expensive and time-consuming. For example, in January and February 2014, we, Forest, Forest Laboratories Holdings Ltd., Merz Pharma GmbH & Co. KGaA and Merz Pharmaceuticals GmbH filed patent infringement lawsuits, under Forest's patents and patents owned by us and licensed to Forest, against eight manufacturers of generic pharmaceuticals that have filed ANDAs with the FDA seeking approval to manufacture and sell generic versions of Namenda XR. We anticipate that the prosecution of the lawsuits will require a significant amount of time and attention of our chief executive officer and other senior executives. In addition, in a patent infringement proceeding, a court may decide that a patent of ours is invalid or unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in the Forest litigation or any other litigation or proceeding could put one or more of our patents at risk of being invalidated or interpreted narrowly. Such a result could limit our ability to prevent others from using or commercializing similar or identical technology and products, limit our ability to prevent others from launching generic versions of our products and could limit the duration of patent protection for our products, all of which could have a material adverse effect on our business. A successful challenge to our patents could reduce or eliminate our right to receive royalties from Forest. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation.
Third parties may initiate legal proceedings alleging that we or our collaborators are infringing their intellectual property rights, the outcome of which would be uncertain and could have a material adverse effect on the success of our business.
Our commercial success depends upon our ability and the ability of our collaborators to develop, manufacture, market and sell our product candidates and to use our proprietary technologies without infringing, misappropriating or otherwise violating the proprietary rights or intellectual property of third parties. We or our collaborators may become party to, or be threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our products and technology, including interference, derivation, re-examination, inter partes review, post-grant review, opposition or similar proceedings before the USPTO and its foreign counterparts. The costs of these proceedings could be substantial, and the proceedings may result in a loss of such intellectual property rights. Some of our competitors may be able to sustain the costs of complex patent disputes and litigation more effectively than we can because they have substantially greater resources. In addition, any uncertainties resulting from the initiation and continuation of any disputes or litigation could adversely affect our ability to raise the funds necessary to continue our operations. Third parties may assert infringement claims against us or our collaborators based on existing patents or patents that may
30
be granted in the future. For example, in December 2013 Teva Pharmaceuticals USA and Mayne Pharma International jointly initiated a lawsuit against Forest alleging that the manufacture and commercialization of Namenda XR by Forest infringes the plaintiffs' U.S. patent. Under our license agreement with Forest we are obliged to indemnify Forest under certain circumstances and our royalty entitlements may also be reduced. Our indemnification obligation to Forest, while subject to customary limitations, has no monetary cap, and our right to receive royalties from Forest may be eliminated in any calendar quarter in which certain third party generic competition exists. If we or our collaborators are found to infringe a third-party's intellectual property rights, we could be required to obtain a license from such third-party to continue developing and marketing our products and technology. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. We could be forced, including by court order, to cease commercializing the infringing technology or product. In addition, we could be found liable for monetary damages. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
We may be unable to protect the confidentiality of our trade secrets, thus harming our business and competitive position.
In addition to our patented technology and products, we rely upon trade secrets, including unpatented know-how, technology and other proprietary information, to develop and maintain our competitive position, which we seek to protect, in part, by confidentiality agreements with our employees, our collaborators and consultants. We also have agreements with our employees and selected consultants that obligate them to assign their inventions to us. However, while it is our policy to require our employees and contractors who may be involved in the conception or development of intellectual property to execute such agreements, we may be unsuccessful in executing such an agreement with each party who in fact conceives or develops intellectual property that we regard as our own. In addition, it is possible that technology relevant to our business will be independently developed by a person that is not a party to such an agreement. While to our knowledge the confidentiality of our trade secrets has not been compromised, if the employees, consultants or collaborators that are parties to these agreements breach or violate the terms of these agreements, we may not have adequate remedies for any such breach or violation, and we could lose our trade secrets through such breaches or violations. Further, our trade secrets could be disclosed, misappropriated or otherwise become known or be independently discovered by our competitors. In addition, intellectual property laws in foreign countries may not protect our intellectual property to the same extent as the laws of the United States. If our trade secrets are disclosed or misappropriated, it would harm our ability to protect our rights and adversely affect our business.
We may be subject to claims that our employees have wrongfully used or disclosed intellectual property of their former employers. Intellectual property litigation or proceedings could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for us, and we have no knowledge of any instances of wrongful use or disclosure by our employees to date, we may be subject to claims that we or these employees have used or disclosed intellectual property, including trade secrets or other proprietary information, of an employee's former employer. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant
31
expenses, and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. This type of litigation or proceeding could substantially increase our operating losses and reduce our resources available for development activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other intellectual property related proceedings could adversely affect our ability to compete in the marketplace.
Risks related to government regulation
The regulatory approval process is expensive, time consuming and uncertain and may prevent us or our collaboration partners from obtaining approvals for the commercialization of some or all of our product candidates.
The research, testing, manufacturing, labeling, approval, selling, import, export, marketing and distribution of drug products are subject to extensive regulation by the FDA and other regulatory authorities in the United States and other countries, which regulations differ from country to country. Neither we nor our collaboration partners are permitted to market our product candidates in the United States until we receive FDA approval of an NDA. We have not submitted an application or received marketing approval for any of our product candidates. Obtaining approval of an NDA can be a lengthy, expensive and uncertain process. In addition, failure to comply with FDA and other applicable U.S. and foreign regulatory requirements may subject us to administrative or judicially imposed sanctions, including:
- •
- warning letters;
- •
- civil or criminal penalties and fines;
- •
- injunctions;
- •
- suspension or withdrawal of regulatory approval;
- •
- suspension of any ongoing clinical studies;
- •
- voluntary or mandatory product recalls and publicity requirements;
- •
- refusal to accept or approve applications for marketing approval of new drugs or biologics or supplements to approved
applications filed by us;
- •
- restrictions on operations, including costly new manufacturing requirements; or
- •
- seizure or detention of our products or import bans.
Prior to receiving approval to commercialize any of our product candidates in the United States or abroad, we and our collaboration partners must demonstrate with substantial evidence from well-controlled clinical studies, and to the satisfaction of the FDA and other regulatory authorities abroad, that such product candidates are safe and effective for their intended uses. Results from preclinical studies and clinical studies can be interpreted in different ways. Even if we and our collaboration partners believe the preclinical or clinical data for our product candidates are promising, such data may not be sufficient to support approval by the FDA and other regulatory authorities. Administering any of our product candidates to humans may produce undesirable side effects, which could interrupt, delay or cause suspension of clinical studies of our product candidates and result in the FDA or other regulatory authorities denying approval of our product candidates for any or all targeted indications.
32
FDA approval of an NDA is not guaranteed, and the approval process is expensive and may take several years. The FDA also has substantial discretion in the approval process. Despite the time and expense exerted, failure can occur at any stage, and we could encounter problems that cause us to abandon or repeat clinical studies, or perform additional preclinical studies and clinical studies. The number of preclinical studies and clinical studies that will be required for FDA approval varies depending on the product candidate, the disease or condition that the product candidate is designed to address and the regulations applicable to any particular product candidate. The FDA can delay, limit or deny approval of a product candidate for many reasons, including that:
- •
- a product candidate may not be deemed safe or effective;
- •
- FDA officials may not find the data from preclinical studies and clinical studies sufficient;
- •
- the FDA might not approve our or our third-party manufacturer's processes or facilities; or
- •
- the FDA may change its approval policies or adopt new regulations.
If any of our product candidates fails to demonstrate safety and efficacy in clinical studies or does not gain regulatory approval, our business and results of operations will be materially and adversely harmed.
If the FDA does not conclude our product candidates satisfy the requirements for approval under the Section 505(b)(2) regulatory approval pathway, or if the requirements for approval under Section 505(b)(2) are not as we expect, the approval pathway for our products will likely take significantly longer, cost significantly more and entail significantly greater complications and risks than anticipated, and in any case may not be successful.
We are developing our current and future product candidates, including ADS-5102, with the expectation that they will be eligible for approval through the Section 505(b)(2) regulatory pathway. Section 505(b)(2) of the FDCA would allow an NDA to rely in part on data in the public domain or the FDA's prior conclusions regarding the safety and effectiveness of an approved drug product, which could expedite the development program for our product candidates by potentially decreasing the amount of clinical data that would need to be generated in order to obtain FDA approval. If the FDA does not allow us to pursue the Section 505(b)(2) regulatory pathway as anticipated, we or Forest may need to conduct additional clinical trials, provide additional data and information, and meet additional standards for product approval. If this were to occur, the time and financial resources required to obtain FDA approval for our product candidates, and complications and risks associated with regulatory approval of would likely substantially increase. Moreover, inability to pursue the Section 505(b)(2) regulatory pathway may result in new competitive products reaching the market more quickly than our product candidates, which would adversely impact our competitive position and prospects. Even if we are able to utilize the Section 505(b)(2) regulatory pathway, there is no guarantee this will ultimately lead to accelerated product development or earlier approval for MDX-8704, ADS-5102 or any other product candidate.
Even if we receive regulatory approval for a particular product candidate, we will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense and subject us to penalties if we fail to comply with applicable regulatory requirements.
Once regulatory approval has been granted for a particular product candidate, the approved product and its manufacturer are subject to continual review by the FDA and/or non-U.S. regulatory authorities. Any regulatory approval that we or our collaboration partners receive for our product candidates may be subject to limitations on the indicated uses for which the product may be marketed or contain requirements for potentially costly post-marketing follow-up studies to monitor the safety and efficacy of the product. In addition, if the FDA and/or non-U.S. regulatory authorities approve any
33
of our product candidates, we will be subject to extensive and ongoing regulatory requirements by the FDA and other regulatory authorities with regard to the labeling, packaging, adverse event reporting, storage, advertising, promotion, tracking and recordkeeping for our products. Further, manufacturers of our drug products are required to comply with cGMP regulations, which include requirements related to quality control and quality assurance as well as the corresponding maintenance of records and documentation. Additionally, regulatory authorities must approve these manufacturing facilities before they can be used to manufacture our drug products, and these facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with cGMP regulations. If we or a third party discover previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory authority may impose restrictions on that product, the manufacturer or us, including requiring withdrawal of the product from the market or suspension of manufacturing. If we, our product candidates or the manufacturing facilities for our product candidates fail to comply with regulatory requirements of the FDA and/or other non-U.S. regulatory authorities, we could be subject to administrative or judicially imposed sanctions, including:
- •
- warning letters;
- •
- civil or criminal penalties and fines;
- •
- injunctions;
- •
- suspension or withdrawal of regulatory approval;
- •
- suspension of any ongoing clinical studies;
- •
- voluntary or mandatory product recalls and publicity requirements;
- •
- refusal to approve pending applications for marketing approval of new drugs or supplements to approved applications filed
by us;
- •
- restrictions on operations, including costly new manufacturing requirements; or
- •
- seizure or detention of our products or import bans.
The regulatory requirements and policies may change and additional government regulations may be enacted for which we may also be required to comply. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or in other countries. If we are not able to maintain regulatory compliance, we may not be permitted to market our future products and our business may suffer.
Failure to obtain regulatory approvals in foreign jurisdictions will prevent us from marketing our products internationally.
We may decide to commercialize ADS-5102, ADS-8704 and other future product candidates outside of the United States. To market our future products in the European Economic Area, or EEA, and many other foreign jurisdictions, we must obtain separate regulatory approvals. Specifically, in the EEA, medicinal products can only be commercialized after obtaining a Marketing Authorization, or MA.
Before granting an MA, the European Medicines Agency or the competent authorities of the member states of the EEA make an assessment of the risk-benefit balance of the product on the basis of scientific criteria concerning its quality, safety and efficacy.
We have had limited interactions with foreign regulatory authorities. The approval procedures vary among countries and can involve additional clinical testing, and the time required to obtain approval may differ from that required to obtain FDA approval. Clinical studies conducted in one country may
34
not be accepted by regulatory authorities in other countries. Approval by the FDA does not ensure approval by regulatory authorities in other countries, and approval by one or more foreign regulatory authorities does not ensure approval by regulatory authorities in other foreign countries or by the FDA. However, a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in others. The foreign regulatory approval process may include all of the risks associated with obtaining FDA approval. We may not obtain foreign regulatory approvals on a timely basis, if at all. We may not be able to file for regulatory approvals and even if we file we may not receive necessary approvals to commercialize our products in any market. If we are unable to obtain non-U.S. regulatory approval to market our product candidates in other countries, we may not be able to achieve the financial results we project and our stock price could decline.
Healthcare reform measures could hinder or prevent our product candidates' commercial success.
In the United States, there have been and we expect there will continue to be a number of legislative and regulatory changes to the healthcare system that could affect our future revenue and profitability and the future revenue and profitability of our potential customers. Federal and state lawmakers regularly propose and, at times, enact legislation that would result in significant changes to the healthcare system, some of which are intended to contain or reduce the costs of medical products and services. For example, one of the most significant healthcare reform measures in decades, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or, collectively, the PPACA, was enacted in 2010. The PPACA contains a number of provisions, including those governing enrollment in federal healthcare programs, reimbursement changes and fraud and abuse measures, all of which will impact existing government healthcare programs and will result in the development of new programs. The PPACA, among other things:
- •
- imposes a non-deductible annual fee on entities that manufacture or import certain branded prescription drugs; increases
the minimum level of Medicaid rebates payable by manufacturers of brand-name drugs from 15.1% to 23.1%;
- •
- requires collection of rebates for drugs paid by Medicaid managed care organizations; and
- •
- provides for a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices of applicable branded drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer's outpatient drugs to be covered under Medicare Part D.
While the U.S. Supreme Court upheld the constitutionality of most elements of the PPACA in June 2012, other legal challenges are still pending final adjudication in several jurisdictions. In addition, Congress has also proposed a number of legislative initiatives, including possible repeal of the PPACA. At this time, it remains unclear whether there will be any changes made to the PPACA, whether to certain provisions or its entirety. We can provide no assurance that the PPACA, as currently enacted or as amended in the future, will not adversely affect our business and financial results, and we cannot predict how future federal or state legislative or administrative changes relating to healthcare reform will affect our business.
The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare services to contain or reduce costs of healthcare may, among other things, adversely affect:
- •
- our ability to set a price we believe is fair for our products;
- •
- our ability to generate revenue and achieve or maintain profitability; and
- •
- the availability of capital.
35
If we fail to comply with healthcare regulations, we could face substantial penalties and our business, operations and financial condition could be adversely affected.
Certain federal and state healthcare laws and regulations pertaining to fraud and abuse and patients' rights are and will be applicable to our business. We could be subject to healthcare fraud and abuse and patient privacy regulation by both the federal government and the states in which we conduct our business. The regulations that may affect our ability to operate include:
- •
- the federal healthcare program Anti-Kickback Statute, which prohibits knowingly and willfully offering, soliciting,
receiving or providing any remuneration (including any kickback, bribe or rebate), directly or indirectly, overtly or covertly, in cash or in kind, in exchange for or to induce either the referral of
an individual for, or the purchase, order, lease or recommendation of, any good, facility, item or service for which payment may be made, in whole or in part, under federal healthcare programs, such
as the Medicare and Medicaid programs;
- •
- the federal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities
from knowingly presenting, or causing to be presented, false or fraudulent claims for payment or approval, or knowingly using false statements, to obtain payment from the federal government, and which
may apply to entities like us which provide coding and billing advice to customers;
- •
- the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created new federal criminal
statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or obtain, by means of false or fraudulent pretenses,
representations or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the payor (e.g., public or private) and
knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false statements in connection with the delivery of, or payment for,
healthcare benefits, items or services relating to healthcare matters;
- •
- the federal physician self-referral law, commonly known as the Stark Law, which prohibits a physician from making a
referral to an entity for certain designated health services reimbursed by Medicare or Medicaid if the physician or a member of the physician's family has a financial relationship with the entity, and
which also prohibits the submission of any claims for reimbursement for designated health services furnished pursuant to a prohibited referral;
- •
- the federal transparency requirements under the PPACA require manufacturers of drugs, devices, biologicals and medical
supplies for which payment is available under Medicare, Medicaid or the Children's Health Insurance Program (with certain exceptions) to report annually to the U.S. Department of Health and Human
Services, or HHS, information related to physician payments and other transfers of value made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and
teaching hospitals, as well as certain ownership and investment interests held by physicians and their immediate family members;
- •
- HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their
respective implementing regulations, which governs the conduct of certain electronic healthcare transactions and protects the security and privacy of protected health information; and
- •
- state law equivalents of each of the above federal laws, such as anti-kickback, false claims and transparency laws which may be broader in scope and apply to items or services reimbursed by any third-party payor, including commercial insurers.
36
The PPACA, among other things, amended the intent standard of the federal Anti-Kickback Statute and criminal healthcare fraud statutes to a stricter standard such that a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the PPACA codified case law that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil, criminal and/or administrative penalties, damages, fines, disgorgement, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our financial results. Any action against us for violation of these or other laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management's attention from the operation of our business. Moreover, achieving and sustaining compliance with applicable federal and state privacy, security and fraud laws may prove costly.
Risks related to this offering and ownership of our common stock
Our stock price may be volatile, and purchasers of our common stock could incur substantial losses.
Our stock price is likely to be volatile. The stock market in general and the market for securities of specialty pharmaceutical and biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, investors may not be able to sell their common stock at or above the initial public offering price. In addition, the clinical development stage of our operations may make it difficult for investors to evaluate the success of our business to date and to assess our future viability. The market price for our common stock may be influenced by many factors, including:
- •
- the success of competitive products or technologies;
- •
- results of clinical studies of our product candidates or those of our competitors;
- •
- introductions and announcements of new products and product candidates by us, our commercialization partners, or our
competitors, and the timing of these introductions or announcements;
- •
- actions taken by regulatory agencies with respect to our products, product candidates, clinical studies, manufacturing
process or sales and marketing terms;
- •
- variations in our financial results or those of companies that are perceived to be similar to us;
- •
- the success of our efforts to acquire or in-license additional products or product candidates;
- •
- developments concerning our collaborations, including but not limited to those with our sources of manufacturing and our
commercialization partners;
- •
- announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital
commitments;
- •
- developments or disputes concerning patents or other proprietary rights, including patents, litigation matters and our
ability to obtain patent protection for our current or future products;
- •
- our ability or inability to raise additional capital and the terms on which we raise it;
- •
- the recruitment or departure of key personnel;
37
- •
- changes in the structure of healthcare reimbursement systems;
- •
- regulatory or legal developments in the United States and other countries, especially changes in laws or regulations
applicable to our current or future products;
- •
- market conditions in the pharmaceutical and biotechnology sectors;
- •
- actual or anticipated changes in earnings estimates or changes in stock market analyst recommendations regarding our
common stock, other comparable companies or our industry generally;
- •
- trading volume of our common stock;
- •
- sales of our common stock by us or our stockholders;
- •
- general economic, industry and market conditions; and
- •
- the other risks described in this "Risk Factors" section.
These broad market and industry factors may seriously harm the market price of our common stock, regardless of our operating performance. Additionally, following periods of volatility in the market, securities class-action litigation has often been instituted against companies. Such litigation, if instituted against us, could result in substantial costs and diversion of management's attention and resources, which could materially and adversely affect our business, financial condition, results of operations and growth prospects.
A significant portion of our total outstanding shares are restricted from immediate resale but may be sold into the market in the near future. This could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could result in a decrease in the market price of our common stock. Immediately after this offering, we will have outstanding 16,422,992 shares of common stock based on the number of shares outstanding as of December 31, 2013. This includes the shares that we are selling in this offering, which may be resold in the public market immediately without restriction, unless purchased by our affiliates. Of the remaining shares, 13,422,992 shares are currently restricted as a result of securities laws or lock-up agreements but will be able to be sold after the offering as described in the section of this prospectus entitled "Shares Eligible for Future Sale." Moreover, immediately after this offering, holders of an aggregate of 9,936,991 shares of our common stock, including shares of our common stock issuable upon the exercise or, in certain cases, net exercise of outstanding warrants, will have rights, subject to some conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders as described in the section of this prospectus entitled "Description of Capital Stock—Registration rights". We also intend to register all shares of common stock that we may issue under our equity compensation plans. Once we register these shares, they can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates and the lock-up agreements described in the "Underwriting" section of this prospectus.
If you purchase our common stock in this offering, you will incur immediate and substantial dilution in the book value of your shares.
Investors purchasing common stock in this offering will pay a price per share that substantially exceeds the pro forma as adjusted book value per share of our tangible assets after subtracting our liabilities. As a result, investors purchasing common stock in this offering will incur immediate dilution of $8.48 per share, based on an initial public offering price of $16.00 per share and our pro forma as
38
adjusted net tangible book value as of December 31, 2013. For more information on the dilution you may suffer as a result of investing in this offering, see the section entitled "Dilution."
This dilution is due to the substantially lower price paid by our investors who purchased shares prior to this offering as compared to the price offered to the public in this offering and the exercise of stock options granted to our employees. The exercise of any of these options would result in additional dilution. As a result of the dilution to investors purchasing shares in this offering, investors may receive significantly less than the purchase price paid in this offering, if anything, in the event of our liquidation.
After this offering, our executive officers, directors and principal stockholders will maintain the ability to control or significantly influence all matters submitted to stockholders for approval.
Upon the closing of this offering, our executive officers, directors and stockholders who owned more than 5% of our outstanding common stock before this offering will, in the aggregate, beneficially own approximately 65.4% of our common stock, including the effect of the purchase by certain of our directors, holders of 5% or more of our capital stock and their respective affiliates, of an aggregate of 362,500 shares of common stock in this offering. These stockholders, acting together, will continue to be able to significantly influence all matters requiring stockholder approval, including the election and removal of directors and any merger or other significant corporate transactions. The interests of this group of stockholders may not coincide with the interests of other stockholders. As a result, if these stockholders were to choose to act together, they would be able to control or significantly influence all matters submitted to our stockholders for approval, as well as our management and affairs. For example, these stockholders, if they choose to act together, will control or significantly influence the election of directors and approval of any merger, consolidation or sale of all or substantially all of our assets. This concentration of voting power could delay or prevent an acquisition of our company on terms that other stockholders may desire.
We will incur significant increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives.
As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, the Sarbanes-Oxley Act, and rules of the SEC and those of the NASDAQ Global Market have imposed various requirements on public companies including requiring establishment and maintenance of effective disclosure and financial controls. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to incur substantial costs to maintain the same or similar coverage. We estimate the additional costs we will incur as a result of being a public company to be approximately $2 million annually.
The Sarbanes-Oxley Act requires, among other things, that we maintain effective internal control over financial reporting and disclosure controls and procedures. In particular, we must perform system and process evaluation and testing of our internal control over financial reporting to allow management to report on the effectiveness of our internal control over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act, beginning with our annual report on Form 10-K for the fiscal year ended December 31, 2014. In addition, we will be required to have our independent registered public accounting firm attest to the effectiveness of our internal control over financial reporting beginning with our annual report on Form 10-K following the date on which we are no longer an emerging growth company. Our compliance with Section 404 of the Sarbanes-Oxley Act will require that we incur substantial accounting expense and expend significant management efforts. We currently
39
do not have an internal audit group, and we will need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge. If we are not able to comply with the requirements of Section 404 in a timely manner, or if we or our independent registered public accounting firm identify deficiencies in our internal control over financial reporting that are deemed to be material weaknesses, the market price of our stock could decline and we could be subject to sanctions or investigations by the NASDAQ Global Market, the SEC or other regulatory authorities, which would require additional financial and management resources.
Our ability to successfully implement our business plan and comply with Section 404 requires us to be able to prepare timely and accurate financial statements. We expect that we will need to continue to improve existing, and implement new operational and financial systems, procedures and controls to manage our business effectively. Any delay in the implementation of, or disruption in the transition to, new or enhanced systems, procedures or controls, may cause our operations to suffer and we may be unable to conclude that our internal control over financial reporting is effective and to obtain an unqualified report on internal controls from our auditors as required under Section 404 of the Sarbanes-Oxley Act. This, in turn, could have an adverse impact on trading prices for our common stock, and could adversely affect our ability to access the capital markets.
An active trading market for our common stock may not develop.
Prior to this offering, there has been no public market for our common stock. The initial public offering price for our common stock will be determined through negotiations with the underwriters. An active trading market for our shares may never develop or be sustained following this offering. If an active market for our common stock does not develop, it may be difficult for our stockholders to sell shares purchased in this offering without depressing the market price for the shares or at all, and any such sales may be at a loss.
If securities or industry analysts do not publish research, or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend, in part, on the research and reports that securities or industry analysts publish about us or our business. Securities and industry analysts do not currently, and may never, publish research on our company. If no securities or industry analysts commence coverage of our company, the trading price for our stock would likely be negatively impacted. In the event securities or industry analysts initiate coverage, if one or more of the analysts who cover us downgrade our stock or publish inaccurate or unfavorable research about our business, our stock price would likely decline. In addition, if our operating results fail to meet the forecast of analysts, our stock price would likely decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, demand for our stock could decrease, which might cause our stock price and trading volume to decline.
We have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our management will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our common stock. The failure by our management to apply these funds effectively could result in financial losses that could adversely affect our business, cause the price of our common stock to decline and delay the development of our product candidates. Pending their use, we may invest the net proceeds from this offering in a manner that does not produce income or that loses value.
40
Provisions in our corporate charter documents and under Delaware law could make an acquisition of us more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our corporate charter and our bylaws that will become effective upon the closing of this offering may discourage, delay or prevent a merger, acquisition or other change in control of us that stockholders may consider favorable, including transactions in which stockholders might otherwise receive a premium for their shares. These provisions could also limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors. Because our board of directors is responsible for appointing the members of our management team, these provisions could in turn affect any attempt by our stockholders to replace current members of our management team. Among others, these provisions include that:
- •
- our board of directors is divided into three classes with staggered three-year terms which may delay or prevent a change
of our management or a change in control;
- •
- our board of directors has the right to expand the size of our board of directors and to elect directors to fill a vacancy
created by the expansion of the board of directors or the resignation, death or removal of a director, which prevents stockholders from being able to fill vacancies on our board of directors;
- •
- our stockholders may not act by written consent or call special stockholders' meetings; as a result, a holder, or holders,
controlling a majority of our capital stock would not be able to take certain actions other than at annual stockholders' meetings or special stockholders' meetings called by the board of directors,
the chairman of the board, the chief executive officer or the president;
- •
- our certificate of incorporation prohibits cumulative voting in the election of directors, which limits the ability of
minority stockholders to elect director candidates;
- •
- stockholders must provide advance notice and additional disclosures in order to nominate individuals for election to the
board of directors or to propose matters that can be acted upon at a stockholders' meeting, which may discourage or deter a potential acquiror from conducting a solicitation of proxies to elect the
acquiror's own slate of directors or otherwise attempting to obtain control of our company; and
- •
- our board of directors may issue, without stockholder approval, shares of undesignated preferred stock; the ability to issue undesignated preferred stock makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to acquire us.
Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner.
Because we do not anticipate paying any cash dividends on our common stock in the foreseeable future, capital appreciation, if any, will be our stockholders' sole source of gain.
We have never declared or paid cash dividends on our common stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. In addition, the terms of existing or any future debt agreements may preclude us from paying dividends. As a result, capital appreciation, if any, of our common stock will be our stockholders' sole source of gain for the foreseeable future.
41
CAUTIONARY STATEMENT CONCERNING FORWARD-LOOKING STATEMENTS
This prospectus, including the sections titled "Prospectus Summary," "Risk Factors," "Use of Proceeds," "Management's Discussion and Analysis of Financial Condition and Results of Operations," "Market, Industry and Other Data," "Business" and "Shares Eligible for Future Sale," contains forward-looking statements. In some cases you can identify these statements by forward-looking words, such as "believe," "may," "will," "estimate," "continue," "anticipate," "intend," "could," "would," "project," "plan," "potential," "seek," "expect," "goal," or the negative or plural of these words or similar expressions. These forward-looking statements include, but are not limited to, statements concerning the following:
- •
- our ability to obtain and maintain regulatory approval of our product candidates;
- •
- our ability to successfully commercialize any of our products that are approved;
- •
- the rate and degree of market acceptance of our products;
- •
- our estimates of our expenses, ongoing losses, future revenue, capital requirements and our needs for or ability to obtain
additional financing;
- •
- our expected uses of the net proceeds to us from this offering;
- •
- our expectation that our existing capital resources and the net proceeds from this offering will be sufficient to enable
us to complete our ongoing clinical studies;
- •
- our ability to obtain and maintain intellectual property protection for our products and product candidates;
- •
- the ability to scale up manufacturing of our product candidates to commercial scale;
- •
- our reliance on our collaboration partners' performance, over which we do not have control;
- •
- the actual receipt and timing of any milestone payments or royalties from our collaborators;
- •
- our ability to successfully establish and successfully maintain appropriate collaborations and derive significant revenue
from those collaborations;
- •
- our reliance on third parties to conduct our clinical studies;
- •
- our reliance on third-party contract manufacturers to manufacture and supply our product candidates for us;
- •
- our ability to identify, develop, acquire and in-license new products and product candidates;
- •
- our ability to enroll patients in our clinical studies at the pace that we project;
- •
- our ability to retain and recruit key personnel;
- •
- our expectations regarding the time during which we will be an emerging growth company under the Jumpstart Our Business
Startups Act;
- •
- our financial performance; and
- •
- developments and projections relating to our competitors or our industry.
These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described in "Risk Factors." Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this prospectus may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements.
42
You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. Moreover, except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this prospectus to conform these statements to actual results or to changes in our expectations.
You should read this prospectus and the documents that we reference in this prospectus and have filed with the Securities and Exchange Commission, or the SEC, as exhibits to the registration statement of which this prospectus is a part with the understanding that our actual future results, levels of activity, performance and events and circumstances may be materially different from what we expect. We qualify all of the forward-looking statements in this prospectus by these cautionary statements.
43
MARKET, INDUSTRY AND OTHER DATA
We obtained the industry, market and other data throughout this prospectus from our own internal estimates and research, and from industry publications and research, surveys and studies conducted by other third parties. These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates.
Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances that are assumed in this information. In some cases, we do not expressly refer to the sources from which this data is derived. In that regard, when we refer to one or more sources of this type of data in any paragraph, you should assume that other data of this type appearing in the same paragraph is derived from the same sources, unless otherwise expressly stated or the context otherwise requires.
44
The net proceeds from our issuance and sale of 3,000,000 shares of our common stock in this offering will be approximately $41.5 million, or approximately $48.2 million if the underwriters exercise in full their over-allotment option to purchase additional shares, after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
As of December 31, 2013, we had cash and cash equivalents of approximately $85.6 million. We intend to use all of the net proceeds from this offering along with our other capital resources, to fund ongoing development of our product candidates, including ADS-5102, for commercialization activities related to any wholly owned approved product, including developing a specialized CNS sales force, and for working capital and other general corporate purposes. We currently estimate that we will use the net proceeds from this offering, together with our existing cash and cash equivalents, as follows:
- •
- approximately $30 million to fund our Phase 3 registration trial of ADS-5102 in LID and related activities;
- •
- approximately $30 million to fund a Phase 2/3 study of ADS-5102 in a second CNS indication and related
activities;
- •
- approximately $30 million to fund additional product development, including clinical trials; and
- •
- the remainder for working capital, capital expenditures and other general corporate purposes, which may include acquiring or licensing products, businesses or technologies, although we have no present commitments for any such acquisitions or licenses.
This expected use of the net proceeds from this offering and our existing cash and cash equivalents represents our intentions based upon our current plans and business conditions. The amounts and timing of our actual expenditures may vary significantly depending on numerous factors, including the progress of our development and commercialization efforts and the status of and results from clinical studies, as well as any collaborations that we may enter into with third parties for our product candidates and any unforeseen cash needs. As a result, our management will retain broad discretion over the allocation of the net proceeds from this offering.
Pending our use of the net proceeds from this offering, we intend to invest the net proceeds in a variety of capital preservation investments, including short-term, investment grade, interest bearing instruments and U.S. government securities.
Based on our planned use of the net proceeds from this offering and our existing cash and cash equivalents described above, we expect that such funds will be sufficient to enable us to fund the development of ADS-5102 through commercialization in its first indication. However, it is possible that we will not achieve the progress that we expect because the actual costs and timing of drug development, particularly clinical studies, are difficult to predict, subject to substantial risks and delays and often vary depending on the particular indication and development strategy. We do not expect that the net proceeds from this offering and our existing cash and cash equivalents will be sufficient to enable us to fund substantial development of our other product candidates.
45
We have never declared or paid, and do not anticipate declaring or paying in the foreseeable future, any cash dividends on our capital stock. Future determination as to the declaration and payment of dividends, if any, will be at the discretion of our board of directors and will depend on then existing conditions, including our operating results, financial condition, contractual restrictions, capital requirements, business prospects and other factors our board of directors may deem relevant. Our future ability to pay cash dividends on our capital stock may also be limited by the terms of any future debt or preferred securities.
46
The following table sets forth our cash and cash equivalents and capitalization as of December 31, 2013:
- •
- on an actual basis;
- •
- on a pro forma basis to reflect (i) the filing of our amended and restated certificate of incorporation and the automatic
conversion of all outstanding shares of our convertible preferred stock into an aggregate of 3,432,891 shares of our common stock immediately prior to the closing of this offering, (ii) the
reclassification of our convertible preferred stock warrant liability of $6.2 million to additional paid-in capital and (iii) the issuance of an aggregate of 474,573 shares of our common stock upon
conversion of our Series AA preferred stock to be issued upon the net exercise of warrants to purchase shares of Series AA preferred stock with an exercise price of $3.80445 per share and based on a
fair market value of $16.00 per share, the initial public offering price, immediately prior to the closing of this offering; and
- •
- on a pro forma as adjusted basis to further reflect the sale by us of 3,000,000 shares of common stock in this offering, after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
You should read this table together with the sections in this prospectus entitled "Selected Consolidated Financial Data," and "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our consolidated financial statements and related notes included elsewhere in this prospectus.
Share and per share amounts have been adjusted to give effect to a 2-for-1 forward split of our outstanding common stock and preferred stock effected on March 24, 2014.
| |
As of December 31, 2013 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Actual | Pro Forma | Pro Forma As Adjusted |
|||||||
| |
(in thousands, except share and per share data) (unaudited) |
|||||||||
Cash and cash equivalents |
$ | 85,612 | $ | 85,612 | $ | 127,152 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
Covertible preferred stock warrant liability |
6,232 | — | — | |||||||
Convertible preferred stock, $0.001 par value; 6,700,000 shares authorized and 4,719,174 shares issued and outstanding actual; no shares authorized and no shares issued and outstanding pro forma and pro forma as adjusted |
19,149 | — | — | |||||||
Total stockholders' equity: |
||||||||||
Preferred stock, $0.001 par value; no shares authorized, issued or outstanding, actual; 5,000,000 shares authorized, no shares issued or outstanding, pro forma and pro forma as adjusted |
— | — | — | |||||||
Common stock, $0.001 par value; 100,000,000 shares authorized, 9,515,528 shares issued and outstanding; 100,000,000 shares authorized pro forma and pro forma as adjusted; 13,422,992 shares issued and outstanding pro forma; 16,422,992 shares issued and outstanding pro forma as adjusted |
14 | 14 | 17 | |||||||
Additional paid-in capital |
77,163 | 102,544 | 144,081 | |||||||
Accumulated deficit |
(20,572 | ) | (20,572 | ) | (20,572 | ) | ||||
| | | | | | | | | | | |
Total stockholders' equity |
56,605 | 81,986 | 123,526 | |||||||
| | | | | | | | | | | |
Total capitalization |
$ | 81,986 | $ | 81,986 | $ | 123,526 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
47
The outstanding share information in the table above is based on 13,422,992 shares of our common stock outstanding as of December 31, 2013 and excludes the following:
- •
- 3,567,858 shares of our common stock issuable upon the exercise of stock options outstanding as of December 31,
2013 at a weighted-average exercise price of $1.445 per share;
- •
- 1,865,000 shares of our common stock issuable upon the exercise of stock options granted after December 31, 2013
with a weighted-average exercise price of $9.47 per share;
- •
- 3,151,940 shares of common stock reserved for future issuance under our 2014 Plan, (including 1,771,212 shares of common
stock reserved for issuance under our 2007 Plan, as of December 31, 2013, which shares were added to the shares reserved under the 2014 Plan) plus up to 3,567,858 additional shares that may be
added to the 2014 Plan upon the expiration, termination, forfeiture or other reacquisition of any shares of common stock issuable upon the exercise of stock options outstanding under the 2007 Plan or
our 2002 Plan, and any automatic increases in the number of shares of common stock reserved for future issuance under the 2014 Plan);
- •
- 262,762 shares of our common stock reserved for future issuance under our 2014 Employee Stock Purchase Plan, which will
become effective upon the date the registration statement of which this prospectus forms a part is declared effective, plus any automatic increases in the number of shares of our common stock reserved
for issuance under the 2014 Employee Stock Purchase Plan; and
- •
- 213,278 shares of our common stock issuable upon the exercise of warrants to purchase common stock outstanding at December 31, 2013 at a weighted-average exercise price of $3.14 per share.
48
If you invest in our common stock in this offering, your ownership interest will be immediately diluted to the extent of the difference between the initial public offering price per share and the pro forma as adjusted net tangible book value per share of our common stock after this offering.
Our historical net tangible book value as of December 31, 2013 was approximately $56.6 million, or $5.95 per share of common stock. Our historical net tangible book value is the amount of our total tangible assets less our liabilities and preferred stock that is not included within equity. Net historical tangible book value per share is our historical net tangible book value divided by the number of shares of common stock outstanding as of December 31, 2013. The pro forma net tangible book value of our common stock as of December 31, 2013 was approximately $82.0 million, or $6.11 per share. Pro forma net tangible book value per share represents our total tangible assets less our total liabilities, divided by the number of outstanding shares of common stock, after giving effect to the pro forma adjustments referenced under "Capitalization."
After giving effect to (i) the pro forma adjustments referenced under "Capitalization" and (ii) receipt of the net proceeds from our sale of 3,000,000 shares of common stock, after deducting underwriting discounts and commissions and estimated offering expenses payable by us, our pro forma as adjusted net tangible book value as of December 31, 2013 would have been approximately $123.5 million, or $7.52 per share. This represents an immediate increase in pro forma as adjusted net tangible book value of $1.42 per share to our existing stockholders and an immediate dilution of $8.48 per share to investors purchasing common stock in this offering.
The following table illustrates this dilution on a per share basis:
Initial public offering price per share |
$ | 16.00 | |||||
Historical net tangible book value per share as of December 31, 2013 |
$ | 5.95 | |||||
Pro forma increase in net tangible book value per share as of December 31, 2013 attributable to the conversion of preferred stock |
$ | 0.16 | |||||
Pro forma net tangible book value per share as of December 31, 2013, before giving effect to this offering |
$ |
6.11 |
|||||
Pro forma increase in net tangible book value per share attributable to new investors purchasing shares in this offering |
$ | 1.42 | |||||
| | | | | | | | |
Pro forma as adjusted net tangible book value per share after giving effect to this offering |
7.52 | ||||||
| | | | | | | | |
Dilution in pro forma net tangible book value per share to new investors in this offering |
$ | 8.48 | |||||
| | | | | | | | |
| | | | | | | | |
If the underwriters' over-allotment option to purchase additional shares in this offering is exercised in full, the pro forma as adjusted net tangible book value, after giving effect to this offering, would be $7.72 per share and the dilution to new investors would be $8.28 per share.
The table below summarizes, as of December 31, 2013, on the pro forma as adjusted basis described above, the number of shares of our common stock, the total consideration, and the average price per share (i) paid to us by our existing stockholders and (ii) to be paid by new investors
49
purchasing our common stock in this offering, before deducting underwriting discounts and commissions and estimated offering expenses payable by us.
| |
Shares Purchased | Total Consideration | |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Average Price |
|||||||||||||||
| |
Number | Percent | Amount | Percent | ||||||||||||
Existing stockholders |
13,422,992 | 81.7 | % | $ | 90,038,192 | 65.2 | % | $ | 6.71 | |||||||
New investors |
3,000,000 | 18.3 | 48,000,000 | 34.8 | $ | 16.00 | ||||||||||
| | | | | | | | | | | | | | | | | |
Total |
16,422,992 | 100.0 | % | $ | 138,038,192 | 100.0 | % | |||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
The number of shares of our common stock reflected in the discussion and tables above is based on 13,422,992 shares of our common stock outstanding as of December 31, 2013, and excludes:
- •
- 3,567,858 shares of our common stock issuable upon the exercise of stock options outstanding as of December 31,
2013, at a weighted-average exercise price of $1.445 per share;
- •
- 1,865,000 shares of our common stock issuable upon the exercise of stock options granted after December 31, 2013
with a weighted-average exercise price of $9.47 per share;
- •
- 3,151,940 shares of common stock reserved for future issuance under our 2014 Plan, (including 1,771,212 shares of common
stock reserved for issuance under our 2007 Stock Plan, or 2007 Plan, as of December 31, 2013, which shares were added to the shares reserved under the 2014 Plan) plus up to 3,567,858 additional
shares that may be added to the 2014 Plan upon the expiration, termination, forfeiture or other reacquisition of any shares of common stock issuable upon the exercise of stock options outstanding
under the 2007 Plan or our 2002 Plan, and any automatic increases in the number of shares of common stock reserved for future issuance under the 2014 Plan;
- •
- 262,762 shares of our common stock reserved for future issuance under our 2014 Employee Stock Purchase Plan, plus any
automatic increases in the number of shares of common stock reserved for issuance under the 2014 Employee Stock Purchase Plan; and
- •
- 213,278 shares of our common stock issuable upon the exercise of warrants to purchase common stock outstanding at December 31, 2013 at a weighted-average exercise price of $3.14 per share.
Effective on the date of this prospectus, an aggregate of 7,191,053 shares of our common stock were reserved for issuance under the 2014 Plan. Furthermore, we may choose to raise additional capital through the sale of equity or convertible debt securities due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that any of our options are exercised, new options are issued under our equity incentive plans or we issue additional shares of common stock or other equity or convertible debt securities in the future, there will be further dilution to investors participating in this offering.
50
SELECTED CONSOLIDATED FINANCIAL DATA
You should read the following selected consolidated financial data together with the "Management's Discussion and Analysis of Financial Condition and Results of Operations" section of this prospectus and our consolidated financial statements and the accompanying notes appearing elsewhere in this prospectus. We have derived the consolidated statements of operations data for the years ended December 31, 2012 and 2013 and the consolidated balance sheet data as of December 31, 2012 and 2013 from our audited consolidated financial statements and related notes appearing elsewhere in this prospectus. Our historical results for any prior period are not necessarily indicative of results to be expected in any future period.
51
| |
Year Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
| |
(in thousands, except per share data) |
||||||
Consolidated statements of operations data: |
|||||||
Revenue |
$ | 37,471 | $ | 71,095 | |||
| | | | | | | | |
Operating expenses |
|||||||
Research and development |
9,192 | 7,410 | |||||
General and administrative |
8,330 | 6,667 | |||||
| | | | | | | | |
Total operating expenses |
17,522 | 14,077 | |||||
| | | | | | | | |
Income from operations |
19,949 | 57,018 | |||||
Interest and other income (expense), net |
(1,537 | ) | (4,818 | ) | |||
Interest expense |
(376 | ) | (88 | ) | |||
| | | | | | | | |
Income before income taxes |
18,036 | 52,112 | |||||
Income tax expense |
(300 | ) | (1,191 | ) | |||
| | | | | | | | |
Net income |
$ | 17,736 | $ | 50,921 | |||
| | | | | | | | |
| | | | | | | | |
Net income attributable to common stockholders(1) |
|||||||
Basic |
$ | 11,441 | $ | 33,068 | |||
| | | | | | | | |
| | | | | | | | |
Diluted |
$ | 11,596 | $ | 35,353 | |||
| | | | | | | | |
| | | | | | | | |
Net income per share attributable to common stockholders(1) |
|||||||
Basic |
$ | 1.21 | $ | 3.48 | |||
| | | | | | | | |
| | | | | | | | |
Diluted |
$ | 1.17 | $ | 3.00 | |||
| | | | | | | | |
| | | | | | | | |
Weighted-average shares number of shares used in computing net income net income attributable to common stockholders(1) |
|||||||
Basic |
9,488 | 9,506 | |||||
| | | | | | | | |
| | | | | | | | |
Diluted |
9,924 | 11,806 | |||||
| | | | | | | | |
| | | | | | | | |
Pro forma net income per share attributable to common stockholders (unaudited)(1) |
|||||||
Basic |
$ | 4.13 | |||||
| | | | | | | | |
| | | | | | | | |
Diluted |
$ | 3.51 | |||||
| | | | | | | | |
| | | | | | | | |
Pro forma weighted average number of shares used in computing net income attributable to common stockholders (unaudited)(1) |
|||||||
Basic |
13,414 | ||||||
| | | | | | | | |
| | | | | | | | |
Diluted |
15,790 | ||||||
| | | | | | | | |
| | | | | | | | |
- (1)
- See Notes 2 and 14 to our consolidated financial statements for a description of the method used to compute basic and diluted net income per share.
| |
Year Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
| |
(in thousands) |
||||||
Balance sheet data: |
|||||||
Cash and cash equivalents |
$ | 62,957 | $ | 85,612 | |||
Working capital |
25,715 | 81,790 | |||||
Total assets |
64,303 | 86,216 | |||||
Warrant liability |
1,706 | 6,232 | |||||
Total liabilities |
40,186 | 10,462 | |||||
Convertible preferred stock |
19,149 | 19,149 | |||||
Total stockholders' equity |
4,968 | 56,605 | |||||
52
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
The following discussion and analysis should be read in conjunction with our selected consolidated financial data and the consolidated financial statements and related notes included elsewhere in this prospectus. This discussion and other parts of the prospectus contain forward-looking statements based upon current expectations that involve risks and uncertainties. Our actual results and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of several factors, including those set forth under "Risk Factors" and elsewhere in this prospectus.
Overview
We are a specialty pharmaceutical company driven to improve the lives of those affected by chronic disorders of the central nervous system, or CNS. We achieve this by enhancing the pharmacokinetic profiles of proven drugs to create novel therapeutics for use alone and in fixed-dose combination products. We are developing our lead wholly owned product candidate, ADS-5102, for a complication of Parkinson's disease known as levodopa induced dyskinesia, or LID, and as a treatment for chronic behavioral symptoms associated with traumatic brain injury. We have successfully completed a Phase 2/3 clinical study in LID and intend to initiate a Phase 3 registration trial in 2014. Our late-stage therapeutics portfolio also includes an NDA-submitted product candidate, MDX-8704, being co-developed with Forest Laboratories, Inc., or Forest, and an approved product, Namenda XR, which Forest developed and is marketing in the United States under a license from us.
Prior to November 2012, we were developing ADS-8704, a fixed-dose combination of controlled-release memantine and donepezil. Pursuant to our license agreement with Forest, we exclusively licensed to Forest certain U.S. intellectual property rights relating to controlled-release memantine and therapies including memantine. Forest has continued the ADS-8704 program under the name MDX-8704. Under our license agreement with Forest, we received a $65 million upfront payment in November 2012 and two $20 million milestone payments in the fourth quarter of 2013. We are eligible to receive up to an additional $55 million in payments based upon the achievement of certain regulatory milestones prior to and including the first FDA approval of MDX-8704.
Financial operations overview
Summary
Our revenue to date has been generated primarily from license and development revenue pursuant to our license agreement with Forest. We have not generated any commercial product revenue. As of December 31, 2013, we had an accumulated deficit of $20.6 million. Although we reported net income for 2012 and 2013, this was primarily due to the recognition of revenue pursuant to our license agreement with Forest. We incurred significant losses prior to 2012 and expect to incur significant and increasing losses in the foreseeable future as we advance our product candidates into later stages of development and, if approved, commercialization. We cannot assure you that we will receive additional collaboration revenue in the future.
In 2010, we suspended further activities on our influenza product candidate, ADS-8902, due to the expected length of the clinical trial and a change in our strategic focus. At the same time, we entered into an agreement with the National Institute of Allergy and Infectious Diseases, a division of the National Institutes of Health, or NIH, and its subcontractor under which we provided clinical trials supply, protocols, and operational support for further clinical development. We retained the rights to any clinical study data generated by the NIH with respect to clinical studies conducted by the NIH. We have continued to supply clinical operations support through a subcontract with the independent third-party subcontractor.
53
We expect our research and development expenses to increase as we continue to advance our product candidates through clinical development. In addition, if any of our product candidates receive regulatory approval for commercial sale, we expect to incur significant expenses associated with the establishment of a specialty sales force in the United States. Because of the numerous risks and uncertainties associated with drug development, we are unable to predict the timing or amount of expenses incurred or when, or if, we will be able to achieve sustained profitability.
To date, we have raised an aggregate of $87.2 million through the sale of convertible preferred stock. Under our agreement with Forest we received a non-refundable upfront license fee $65.0 million in 2012, $40.0 million in development milestone fees in 2013 and may receive up to an additional $55.0 million in future regulatory milestone fees. Beginning in 2018 we will be entitled to receive royalties in the low to mid-single digits from Forest for sales of Namenda XR in the United States and, five years after commercial launch, in the low double digits to the mid-teens for sales of MDX-8704 in the United States, if approved. As of December 31, 2013, we had cash and cash equivalents of $85.6 million.
Revenue
We have not generated any revenue from commercial product sales to date. Our revenue to date has been generated primarily from non-refundable upfront license payments and reimbursements for research and development expenses under our license agreement with Forest. In addition to upfront license payments, we are also entitled to receive milestone and other contingent payments upon the occurrence of specific events. As of December 31, 2013, we had received $40.0 million in development milestone payments.
The following table summarizes the sources of our revenue for the years ended December 31, 2012 and 2013 (in thousands):
| |
Year Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Forest: |
|||||||
Recognition of upfront license fee |
$ | 35,389 | $ | 29,611 | |||
Recognition of milestone payments |
— | 40,000 | |||||
Reimbursement of development expenses |
725 | 1,093 | |||||
| | | | | | | | |
Forest total |
36,114 | 70,704 | |||||
NIH contracts |
1,207 |
200 |
|||||
Government grants |
150 | 191 | |||||
| | | | | | | | |
Total revenue |
$ | 37,471 | $ | 71,095 | |||
| | | | | | | | |
| | | | | | | | |
We recognized collaboration revenue of $35.4 million and $69.6 million in 2012 and 2013, respectively, pursuant to our license agreement with Forest. We also recognized revenue from Forest of approximately $0.7 million and $1.1 million in development funding in 2012 and 2013, respectively. We expect that our revenue will continue to fluctuate in future periods.
Research and development expenses
Research and development expenses represent costs incurred to conduct research, such as the discovery and development of our wholly owned product candidates, as well as the development of product candidates pursuant to our agreement with Forest. We recognize all research and development costs as they are incurred. We began tracking our external costs by project beginning January 1, 2006.
54
Research and development expenses consist of:
- •
- fees paid to clinical consultants, clinical trial sites and vendors, including clinical research organizations, or CROs,
in conjunction with implementing and monitoring our clinical trials and acquiring and evaluating clinical trial data, including all related fees, such as for investigator grants, patient screening
fees, laboratory work and statistical compilation and analysis;
- •
- expenses related to production of clinical supplies, including fees paid to contracts manufacturing organizations, or
CMOs;
- •
- other consulting fees paid to third parties; and
- •
- employee-related expenses, which include salaries, benefits and stock-based compensation.
We anticipate our research and development expenses will increase as we initiate a Phase 3 registration trial for ADS-5102, expected in 2014.
The following table summarizes our research and development expenses incurred during the years ended December 31, 2012 and 2013 and for the period from January 1, 2006, when we began tracking these expenses by project, to December 31, 2013 (in thousands):
| |
Year Ended December 31, |
For the Period from January 1, 2006 to December 31, 2013 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | ||||||||
Product candidate |
||||||||||
ADS-5102 |
$ | 3,241 | $ | 2,497 | $ | 14,724 | ||||
ADS-8704(1) |
3,604 | 1,306 | 13,255 | |||||||
ADS-8902(2) |
949 | 240 | 19,925 | |||||||
Unallocated research and development expenses(3) |
1,398 | 3,367 | 22,468 | |||||||
| | | | | | | | | | | |
Total research and development expenses |
$ | 9,192 | $ | 7,410 | $ | 70,372 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
- (1)
- ADS-8704
includes program costs that we incurred related to the fixed-dose combination drug that was licensed to Forest. Subsequent to the execution of the
license agreement Forest assigned the name MDX-8704 to the program in the United States.
- (2)
- ADS-8902
includes program costs related to our influenza program that was suspended in 2010. The NIH assumed financial responsibility for the ongoing
clinical activites through an independent third-party subcontractor. We incur the expenses reflected in the above table in providing clinical operations support to an independent third-party
subcontractor for which we are reimbursed.
- (3)
- Unallocated costs include research and development not allocated to a specific program. In 2013, no employee-related expenses were allocated to ADS-5102.
The program-specific expenses summarized in the table above include costs directly attributable to our product candidates. We allocate research and development salaries, benefits, stock-based compensation and indirect costs to our product candidates on a program-specific basis, and we include these costs in the program-specific expenses. The largest component of our total operating expenses has historically been our investment in research and development activities, including the clinical development of our product candidates. We expect our research and development expenses to increase in the future. The process of conducting the necessary clinical research to obtain FDA approval is costly and time consuming. We consider the active management and development of our clinical pipeline to be crucial to our long-term success. The actual probability of success for each product candidate and clinical program may be affected by a variety of factors including but not limited to: the quality of the product candidate, early clinical data, investment in the program, competition,
55
manufacturing capability and commercial viability. Furthermore, we have entered into collaborations with CROs and academic third parties to participate in the development and commercialization of our product candidates, and we may enter into additional collaborations in the future. In situations in which third parties have control over the clinical development of a product candidate, the estimated completion dates are largely under the control of such third parties and not under our control. We cannot forecast with any degree of certainty which of our product candidates, if any, will be subject to future collaborations or how such arrangements would affect our development plans or capital requirements. As a result of the uncertainties discussed above, we are unable to determine the duration and completion costs of our research and development projects or when and to what extent we will generate revenue from the commercialization and sale of any of our product candidates.
General and administrative expenses
General and administrative expenses consist primarily of personnel costs, facilities costs and other expenses for outside professional services, including legal, intellectual property, human resources, board of directors, audit and accounting services. Personnel costs consist of salaries, benefits and stock-based compensation. We expect to incur additional expenses as a result of operating as a public company, including expenses related to compliance with the rules and regulations of the SEC, and those of any national securities exchange on which our securities are traded, additional insurance expenses, investor relations activities and other administration and professional services.
Interest and other income, net
Interest and other income, net consists primarily of interest received on our cash and cash equivalents and gains and losses resulting from the remeasurement of our convertible preferred stock warrant liability. We will continue to record adjustments to the estimated fair value of the convertible preferred stock warrants until they are exercised, expired or converted into warrants to purchase shares of our common stock upon the closing of our initial public offering. At that time, we will reclassify the convertible preferred stock warrant liability as additional paid-in capital and we will no longer record any related periodic fair value adjustments.
Interest expense
Interest expense consists primarily of interest costs related to our borrowings. We had no outstanding borrowings as of December 31, 2013.
Critical accounting policies and significant judgments and estimates
Our management's discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported revenue generated and expenses incurred during the reporting periods. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. We believe that the accounting policies discussed below are critical to understanding our historical and future performance, as these policies relate to the more significant areas involving management's judgments and estimates.
56
Revenue recognition
We generate revenue from collaboration and license agreements for the development and commercialization of our product candidates. Collaboration and license agreements may include non-refundable upfront payments, partial or complete reimbursement of research and development costs, contingent payments based on the occurrence of specified events under our collaboration arrangements, and royalties on sales of product candidates if they are successfully approved and commercialized. Our performance obligations under the collaborations may include the transfer or license of intellectual property rights, provision of research and development services and related materials and participation on certain development and/or commercialization committees with the collaboration partners. We make judgments that affect the periods over which we recognize revenue. We periodically review our estimated periods of performance based on the progress under each arrangement and account for the impact of any changes in estimated periods of performance on a prospective basis.
On January 1, 2011, we adopted an accounting standards update that provides guidance on revenue recognition using the milestone method. Payments that are contingent upon achievement of a substantive milestone are recognized in their entirety in the period in which the milestone is achieved. Milestones are defined events that can only be achieved based on our partner's performance and there is substantive uncertainty about whether the event will be achieved at the inception of the arrangement. Events that are contingent only on the passage of time or only on counterparty performance are not considered milestones subject to this guidance.
Amounts related to research and development funding are recognized as the related services or activities are performed, in accordance with the contract terms. Payments to us are based on the number of full-time equivalent researchers assigned to the collaboration project and the related research and development expenses incurred.
Accrued research and development expenses
As part of the process of preparing financial statements, we are required to estimate and accrue expenses, the largest of which are research and development expenses. This process involves:
- •
- communicating with our applicable personnel to identify services that have been performed on our behalf and estimating the
level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of actual cost;
- •
- estimating and accruing expenses in our financial statements as of each balance sheet date based on facts and
circumstances known to us at the time; and
- •
- periodically confirming the accuracy of our estimates with selected service providers and making adjustments, if necessary.
Examples of estimated research and development expenses that we accrue include:
- •
- fees paid to CROs in connection with nonclinical studies and clinical studies;
- •
- fees paid to investigative sites in connection with clinical studies;
- •
- fees paid to CMOs in connection with the production of clinical study materials; and
- •
- professional service fees for consulting and related services.
We base our expense accruals related to clinical studies on our estimates of the services received and efforts expended pursuant to contracts with multiple research institutions and clinical research organizations that conduct and manage clinical studies on our behalf. The financial terms of these agreements vary from contract to contract and may result in uneven payment flows. Payments under
57
some of these contracts depend on factors, such as the successful enrollment of patients and the completion of clinical study milestones. Our service providers invoice us monthly in arrears for services performed. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If we do not identify costs that we have begun to incur or if we underestimate or overestimate the level of services performed or the costs of these services, our actual expenses could differ from our estimates.
To date, we have not experienced significant changes in our estimates of accrued research and development expenses after a reporting period. However, due to the nature of estimates, we cannot assure you that we will not make changes to our estimates in the future as we become aware of additional information about the status or conduct of our clinical studies and other research activities.
Estimated fair value of convertible preferred warrants
Freestanding warrants and other similar instruments related to shares that are redeemable are accounted for in accordance with Accounting Standards Codification, or ASC, 480-10, "Distinguishing Liabilities from Equity." Under ASC 480-10, the freestanding warrants that are related to our redeemable convertible preferred stock are classified as liabilities on the balance sheet. The warrants are subject to remeasurement at each balance sheet date and any change in fair value is recognized as a component of other income or expense.
We adjust the liability for changes in fair value until the earlier of the exercise or expiration of the warrants or the completion of a liquidation event, including the completion of an initial public offering, at which time all preferred stock warrants will be converted into warrants to purchase common stock and, accordingly, the liability will be reclassified to equity.
Stock-based compensation
Stock-based compensation cost is measured at the date of grant based on the estimated fair value of the award, net of estimated forfeitures. We estimate the grant date fair value and the resulting stock-based compensation expense using the Black Scholes option pricing model. The grant date fair value of a stock-based award is recognized as an expense over the requisite service period of the award on a straight-line basis.
We account for stock-based compensation arrangements with non-employees using a fair value approach. The fair value of these options is measured using the Black-Scholes option pricing model reflecting an expected life that is assumed to be the remaining contractual life of the option. The compensation costs of these arrangements are subject to remeasurement over the vesting terms as earned.
We recorded total non-cash stock-based compensation expense of $0.8 million and $0.6 million for the years ended December 31, 2012 and 2013, respectively. At December 31, 2013, we had $5.0 million of total unrecognized employee stock-based compensation expense, net of estimated forfeitures, related to stock option grants. This amount will be recognized as expense over a weighted-average period of 4.55 years. We expect to continue to grant stock options in the future, and, to the extent that we do, our actual stock-based compensation expense recognized in future periods will likely increase. The stock-based compensation expense that we recognized in 2013 was increased, and the stock-based compensation expense that we recognize in the first quarter of 2014 and each quarter thereafter through 2019 will be increased, as a result of our determination to calculate that expense based on deemed fair values of our common stock that are higher than the exercise prices of certain stock options granted prior to this offering.
The intrinsic value of all outstanding options as of March 25, 2014 was approximately $51.9 million based on an initial public offering price of $16.00 per share, of which approximately $33.7 million related to vested options and approximately $18.2 million related to unvested options.
58
Determining fair value of stock options
We determine the fair value of each grant of stock options using the estimated fair value of our common stock and the assumptions set forth below. Each of these inputs is subjective and generally requires significant judgment.
The fair value of employee stock options was estimated using the following assumptions:
| |
Year Ended December 31, | |||
|---|---|---|---|---|
| |
2012 | 2013 | ||
Expected volatility |
91% - 92% | 89% - 100% | ||
Risk-free interest rate |
1.15% - 1.41% | 1.45% - 2.48% | ||
Dividend yield |
— | — | ||
Expected term (in years) |
7.00 | 7.25 | ||
The fair value of non-employee stock options was estimated using the following assumptions:
| |
Year Ended December 31, | |||
|---|---|---|---|---|
| |
2012 | 2013 | ||
Expected volatility |
89% - 92% | 88% - 98% | ||
Risk-free interest rate |
0.87% - 1.93% | 1.02% - 2.72% | ||
Dividend yield |
— | — | ||
Contractual life (in years) |
6.25 - 10.00 | 5.25 - 10.00 | ||
Our board of directors intends all options granted to be exercisable at a price per share not less than the per share fair value of our common stock underlying those options on the date of grant. The estimated fair value of our common stock was determined at each valuation date in accordance with the guidelines outlined in the American Institute of Certified Public Accountants Practice Aid, Valuation of Privately-Held-Company Equity Securities Issued as Compensation. Our board of directors, with the assistance of management, developed these valuations using significant judgment and taking into account numerous factors, including developments at our company, market conditions and contemporaneous independent third-party valuations as of December 31, 2012, October 31, 2013, and December 31, 2013.
For all option grant dates through December 31, 2013, the enterprise value was determined based on a Probability Weighted Expected Return Method, or PWERM, Discounted Cash Flow method, or the OPM backsolve method. The allocation of these enterprise values to each part of our capital structure, including our common stock, was done based on the Option Pricing Method, or OPM. OPM treats the rights of the holders of preferred and common shares as equivalent to call options on any value of the enterprise above certain break points of value based upon the liquidation preferences of the holders of preferred shares, as well as their rights to participation and conversion. Thus, the estimated value of the common stock can be determined by estimating the value of its portion of each of these call option rights. The OPM backsolve method derives the implied equity value of a company from a recent transaction involving the company's own securities issued on an arms-length basis. The Discounted Cash Flow method estimates value based on the expectation of future net cash flows, which are then discounted back to the present using a rate of return derived from alternative companies of similar type and risk profile. Under the PWERM the value is estimated based upon analysis of future values for the enterprise under varying scenarios, probabilities are ascribed to these scenarios based on expected future outcomes. Following the closing of this offering, the fair value of our common stock will be determined based on the closing price of our common stock on the NASDAQ Global Market.
59
Income taxes
We file income tax returns in the U.S. federal jurisdiction, California and India. We file U.S. federal income tax returns and California state income tax returns. To date, we have not been audited by the Internal Revenue Service or any state income tax authority.
We use the liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial reporting and the tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse.
We assess the likelihood that the resulting deferred tax assets will be realized. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized.
As of December 31, 2013, our total deferred tax assets were $9.2 million. The deferred tax assets were primarily comprised of federal and state tax net operating losses and tax credit carryforwards. As of December 31, 2013 we had federal net operating loss carryforwards of approximately $3.5 million available to reduce future taxable income. We also had state net operating loss carryforwards of approximately $73.4 million. The federal net operating loss carryforward begins expiring in 2022, and the state net operating loss carryforward began expiring in 2012. Due to uncertainties surrounding our ability to generate future taxable income to realize these tax assets, a full valuation allowance has been established to offset our deferred tax assets. Utilization of net operating loss carryforward may also be subject to an annual limitation due to the ownership change limitations. These annual limitations may result in the expiration of the net operating loss carryforwards before utilization.
At December 31, 2013, we also had federal research and development tax credit carryforwards of approximately $1.4 million that will begin expiring in 2030 and state research and development credit carryforwards of approximately $2.2 million that do not expire.
Results of operations
Comparison of the years ended December 31, 2012 and 2013
The following table summarizes our results of operations for the years ended December 31, 2012 and 2013 (in thousands, except percentages):
| |
Year Ended December 31, |
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Increase/ (Decrease) |
% Increase/ (Decrease) |
|||||||||||
| |
2012 | 2013 | |||||||||||
Revenue |
$ | 37,471 | $ | 71,095 | $ | 33,624 | 90 | % | |||||
Research and development |
9,192 | 7,410 | (1,782 | ) | (19 | )% | |||||||
General and administrative |
8,330 | 6,667 | (1,663 | ) | (20 | )% | |||||||
Interest and other income (expense), net |
(1,537 | ) | (4,818 | ) | 3,281 | 213 | % | ||||||
Interest expense |
(376 | ) | (88 | ) | (288 | ) | NM | ||||||
Revenue
Revenue increased by $33.6 million, to $71.1 million for the year ended December 31, 2013 from $37.5 million for the year ended December 31, 2012. The increase in revenue was due to the upfront license payment, receipt of milestone payments and development funding recognized with respect to our license agreement with Forest. We recognized upfront license and development milestone revenue of $35.4 million and $69.6 million in 2012 and 2013, respectively. Reimbursement of development expenses increased by $0.4 million to $1.1 million for the year ended December 31, 2013 from $0.7 million for the year ended December 31, 2012. The increase was offset by a decrease of $1.0 million in NIH contract revenue for the year ended December 31, 2013.
60
Research and development expenses
Research and development expenses decreased $1.8 million, or 19%, to $7.4 million for the year ended December 31, 2013 from $9.2 million for the year ended December 31, 2012. The decrease in research and development expenses was due to decreased program costs of $2.3 million related to ADS-8704 which we incurred prior to licensing the U.S. rights to the program to Forest in the fourth quarter of 2012. In addition, program expenses for ADS-5102 decreased by $0.7 million due to the completion of a Phase 2/3 clinical study in 2013. Program expenses for ADS-8902 decreased by $0.7 million for the year ended December 31, 2013 due to a reduction in the scope of work under our contract with the NIH. These decreases were partially offset by an increase of $1.9 million of expenses that were not unallocated to a specific program.
General and administrative expenses
General and administrative expenses decreased by $1.7 million, or 20%, to $6.7 million for the year ended December 31, 2013 from $8.3 million for the year ended December 31, 2012. The decrease was primarily related to financial advisory services fees of $1.9 million in relation to the license agreement with Forest incurred in 2012.
Interest and other income (expense), net
Interest and other income (expense), net increased by $3.3 million or 214%, to $4.8 million for the year ended December 31, 2013 from $1.5 million for the year ended December 31, 2012. The increase was primarily attributed to the remeasurement of preferred stock warrants in 2013 and recognition of the change in fair value.
Interest expense
Interest expenses decreased by $0.3 million, to $0.1 million for the year ended December 31, 2013 from $0.4 million for the year ended December 31, 2012. Interest expense consists of interest accrued on convertible notes that were issued as part of the March 2012 Series AA Preferred Stock and Notes Payable Financing. This financing is more fully described in Note 5 to our consolidated financial statements.
Liquidity, capital resources and plan of operation
Since our inception in November 2000, we have funded our operations primarily through proceeds from the sale of convertible preferred stock and warrants, bank debt and the issuance of convertible debt. We have not generated any revenue from the sale of any products. We have incurred losses and generated negative cash flows from operations since inception through 2011. In 2012 and 2013 we recognized a profit and positive cash flow as a result of our license agreement with Forest. As of December 31, 2012 and December 31, 2013, our principal sources of liquidity were our cash and cash equivalents, which totaled $63.0 million and $85.6 million, respectively.
From inception through December 31, 2013, we have received net proceeds of $87.2 million from the sale of convertible preferred stock and $5.0 million from the issuance of convertible notes and under a term loan. The convertible notes and accrued interest thereon were repaid during 2013.
In March 2012, we entered into a convertible note, Series AA preferred stock and warrant purchase agreement, or the Purchase Agreement, with various investors, raising proceeds of $9.3 million in a series of closings between March 2012 and November 2012. This financing is more fully described in Note 5 to our consolidated financial statements.
We believe our existing cash and cash equivalents will be sufficient to fund our projected operating requirements for at least the next 12 months, including operations related to the development of
61
ADS-5102 for LID. However, it is possible that we will not achieve the progress that we expect because the actual costs and timing of drug development, particularly clinical studies, are difficult to predict, subject to substantial risks and delays and often vary depending on the particular indication and development strategy.
The following table summarizes our cash flows for the periods indicated (in thousands):
| |
Year Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Net cash (used in) provided by: |
|||||||
Operating activities |
$ | 51,961 | $ | 26,801 | |||
Investing activities |
(24 | ) | (167 | ) | |||
Financing activities |
7,903 | (3,979 | ) | ||||
Net increase in cash and cash equivalents |
$ | 59,840 | $ | 22,655 | |||
Net cash provided by operating activities was $52.0 million for the year ended December 31, 2012 and was primarily attributable to cash received in the fourth quarter of 2012 under our license agreement with Forest. Net cash provided by operating activities was $26.8 million for the year ended December 31, 2013. The primary use of cash was to fund the ongoing clinical study and product development activities related to ADS-5102.
Net cash used in investing activities amounted to approximately $24,000 and $167,000 for the years ended December 31, 2012 and 2013, respectively, which consisted mainly of property and equipment purchases.
Net cash provided by financing activities for the year ended December 31, 2012 was approximately $7.9 million consisting of approximately $3.9 million from the sale of Series AA convertible preferred stock and $4.0 million of convertible notes. Net cash used in financing activities amounted to $4.0 million for the year ended December 31, 2013 and consisted primarily of repayment of principal of outstanding convertible notes.
Off-balance sheet arrangements
Since our inception, we have not engaged in any off-balance sheet arrangements, including the use of structured finance, special purpose entities or variable interest entities.
Contractual obligations
Our future contractual obligations at December 31, 2013 were as follows (in thousands):
| |
Payments due by period | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Less than 1 year |
1 to 3 years |
3 to 5 years |
More than 5 years |
Total | |||||||||||
Contractual obligations: |
||||||||||||||||
Operating lease obligations |
$ | 228 | $ | 274 | $ | — | $ | — | $ | 502 | ||||||
| | | | | | | | | | | | | | | | | |
Total contractual obligations |
$ | 228 | $ | 274 | $ | — | $ | — | $ | 502 | ||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
JOBS Act accounting election
We are an "emerging growth company," as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this
62
exemption from new or revised accounting standards, and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
Newly adopted accounting pronouncements
In February 2013, the FASB issued guidance which addresses the presentation of amounts reclassified from accumulated other comprehensive income. This guidance does not change current financial reporting requirements, instead an entity is required to cross-reference to other required disclosures that provide additional detail about amounts reclassified out of accumulated other comprehensive income. In addition, the guidance requires an entity to present significant amounts reclassified out of accumulated other comprehensive income by line item of net income if the amount reclassified is required to be reclassified to net income in its entirety in the same reporting period. Adoption of this standard is required for periods beginning after December 15, 2012 for public companies. The amended guidance became effective for us in the first quarter of fiscal year 2013. The adoption of this guidance did not have a material impact on our consolidated financial statements.
Quantitative and qualitative disclosures about market risk
The primary objective of our investment activities is to preserve our capital to fund our operations. We also seek to maximize income from our investments without assuming significant risk. To achieve our objectives, we maintain a portfolio of cash and cash equivalents in a variety of securities of high credit quality. As of December 31, 2013, we had cash and cash equivalents of $85.6 million consisting of cash and liquid investments deposited in highly rated financial institutions in the United States. A portion of our investments may be subject to interest rate risk and could fall in value if market interest rates increase. However, because our investments are primarily short-term in duration, we believe that our exposure to interest rate risk is not significant and a 1% movement in market interest rates would not have a significant impact on the total value of our portfolio. We actively monitor changes in interest rates.
63
Overview
We are a specialty pharmaceutical company driven to improve the lives of those affected by chronic disorders of the central nervous system, or CNS. We achieve this by enhancing the pharmacokinetic profiles of approved drugs to create novel therapeutics for use alone and in fixed-dose combination products. We are developing our lead wholly owned product candidate, ADS-5102, for a complication of Parkinson's disease known as levodopa induced dyskinesia, or LID, and as a treatment for chronic behavioral symptoms associated with traumatic brain injury, or TBI. We have successfully completed a Phase 2/3 clinical trial in which patients receiving ADS-5102 had a statistically significant 43% reduction in LID compared to their baseline LID experienced prior to taking ADS-5102, and we intend to initiate a Phase 3 registration trial of ADS-5102 in LID in 2014. Our late-stage therapeutics portfolio also includes an NDA-submitted fixed-dose combination product candidate, MDX-8704, being co-developed with Forest Laboratories, Inc., or Forest, and an approved controlled-release product, Namenda XR, which Forest developed and is marketing in the United States under a license from us. We plan to commercialize our wholly owned product candidates, if approved, by developing a specialty CNS sales force to reach high volume prescribing neurologists and psychiatrists in the United States.
We estimate that approximately 36 million people in the United States suffer from chronic CNS disorders, such as Alzheimer's disease, Parkinson's disease, TBI, epilepsy, psychosis and depression. We believe that many of these disorders could be better treated if existing CNS drugs were pharmacokinetically enhanced and were used alone or in fixed-dose combinations with other existing CNS drugs. Our initial development efforts have yielded a series of patent-protected, controlled-release therapies based on either amantadine or memantine, approved CNS drugs that are part of a class of molecules called aminoadamantanes. We initially focused on aminoadamantanes because they modulate multiple neurotransmitter systems and we believed that by applying our innovative product development strategy we could develop aminoadamantane-based products with broad therapeutic utility. We are implementing this strategy to develop additional product candidates based on ADS-5102, a controlled-release version amantadine. We also intend to develop product candidates based on approved CNS therapeutics outside the aminoadamantane class.
Our most advanced wholly owned product candidate is ADS-5102, a once-nightly, high dose, controlled-release version of amantadine that we are developing for the treatment of LID. LID is a movement disorder that frequently occurs in patients with Parkinson's disease after long-term treatment with levodopa, the most widely-used drug for Parkinson's disease. Patients with LID suffer from involuntary non-purposeful movements and reduced control over voluntary movements. We estimate that in 2011 approximately 260,000 Parkinson's disease patients in the United States suffered from motor complications as a result of levodopa therapy and approximately 140,000 of these patients suffered from LID. There are no FDA or EMA approved drugs for the treatment of LID. Clinicians typically manage LID by decreasing the dose of levodopa, which can exacerbate symptoms of the underlying Parkinson's disease.
We selected LID as the initial indication for ADS-5102 based on results seen in investigator initiated clinical studies of amantadine and in established preclinical models. In our recently completed Phase 2/3 clinical study, ADS-5102 met its primary endpoint, reduction of LID, and several key secondary endpoints. If our anticipated Phase 3 registration trial of ADS-5102 is successful, we anticipate submitting a New Drug Application, or NDA, to the U.S. Food and Drug Administration, or FDA, for ADS-5102 in the first half of 2016. Amantadine has shown promising results in multiple other CNS indications, and we expect to initiate in 2014 a Phase 2/3 study of ADS-5102 in a second CNS indication, possibly for the treatment of behavioral symptoms associated with TBI, such as irritability and aggression. We anticipate initiating additional Phase 2/3 studies of ADS-5102 in one or more other indications by the end of 2015.
64
Our memantine-based therapeutics are being developed and commercialized in the United States through our partnership with Forest. Forest currently sells one product that is subject to our license agreement, Namenda XR, a treatment of moderate to severe dementia associated with Alzheimer's disease. Namenda XR, a controlled-release version of the approved CNS drug memantine, was launched in the United States in June of 2013 and is part of Forest's $1.5 billion Namenda franchise. In addition, Forest and we are co-developing MDX-8704, a once-daily fixed-dose combination of Namenda XR and the approved CNS drug donepezil, for the treatment of moderate to severe dementia related to Alzheimer's disease. Forest submitted an NDA to the FDA for MDX-8704 in February 2014 and will be responsible for marketing MDX-8704 in the United States if approved. Under our license agreement with Forest, we received a $65 million upfront payment in November 2012 and two $20 million milestone payments in the fourth quarter of 2013. We are eligible to receive up to an additional $55 million in payments based on the achievement of certain regulatory milestones prior to and including the first FDA approval of MDX-8704. Forest has stated that it projects FDA approval and commercial launch of MDX-8704 in the first half of 2015.
We are led by a team of executives and directors with significant experience in drug discovery, development and commercialization. In addition to co-founding Adamas, our CEO co-founded CuraGen Corporation (acquired by Celldex Therapeutics, Inc.), and other members of our management team have held senior positions at Syntex, Bayer, Tularik, and Elan. Members of our executive team have played leading roles in the development and commercialization of multiple significant drugs in a wide range of therapeutic areas. Our board brings substantial, relevant experience in reimbursement, drug development and commercialization.
We have developed our current portfolio of late-stage therapeutics in a capital efficient manner. As of December 31, 2013, we had raised a total of $87 million from equity financings, had received $105 million from our partnership with Forest, had recognized approximately $5 million in revenue from other sources and had $86 million in cash and cash equivalents and no debt obligations. The proceeds from this offering are expected to increase our operational flexibility and enable us to further ADS-5102 in other indications and extend our product development efforts beyond aminoadamantanes.
Our strategy
Our goal is to build an independent, CNS-focused, specialty pharmaceutical company that improves the lives of patients affected by chronic CNS disorders by enhancing the pharmacokinetic profiles of proven drugs to create novel therapeutics that address significant unmet clinical needs. We intend to achieve this goal by leveraging our existing product development process and focusing on key development objectives.
Product development strategy
Our strategy is supported by a product development process that allows us to discover, patent, develop and commercialize novel therapeutics in a capital efficient manner. Our integrated process combines a number of capabilities that together allow us to identify, enhance, patent and then develop proprietary controlled-release and fixed-dose combination products. These capabilities include in-depth knowledge of CNS markets and unmet medical needs, pharmacokinetic and pharmacodynamic competencies and regulatory expertise. Our goal is to develop products that are clinically differentiated
65
from approved drugs that in turn create significant benefits for patients, caregivers, physicians and payors.

The key elements of this strategy are:
- •
- Market attractiveness. We identify approved products that
are sub-optimally utilized due to tolerability issues driven by factors other than the peak concentration of the drug in the blood stream. We believe that these products, with pharmacokinetic
enhancements, can significantly improve the treatment of chronic CNS conditions. For products to be successful, this improvement must be recognized by patients, caregivers, physicians and payors. A
key element of this strategy is targeting conditions treated by a concentrated prescriber base.
- •
- Intellectual property. We seek to discover novel
pharmacokinetic and pharmacodynamic relationships and to obtain patent protection for a range of dose strengths, pharmacokinetic profiles, timing of administration and drug combinations as opposed to
protecting just specific formulations. Pharmacokinetics refers to the manner in which a drug is absorbed, distributed, metabolized and excreted by the body. Pharmacodynamics refers to the biochemical
and physiological effects of a drug on the body.
- •
- Regulatory pathways. We intend to use the regulatory pathway provided by Section 505(b)(2) of the U.S. Food, Drug and Cosmetic Act, or FDCA, to obtain approval for innovative therapeutics based on existing drugs in a manner that we believe will be more time and capital efficient than the standard Section 505(b)(1) pathway used for new chemical entities. While the Section 505(b)(2) pathway is commonly used to obtain approval for fairly simple reformulations of existing drugs, it can also be used to obtain approval for new versions of a drug that enhance the efficacy or tolerability of the drug or that allow the drug to be used in a new indication, as well as for a novel fixed-dose combination. By using the Section 505(b)(2) pathway in this way, we are able to pursue approval for novel therapeutics that we believe have improved clinical utility as compared to the existing drug with less time and expense than are typically associated with the Section 505(b)(1) pathway.
66
- •
- Research and development. We have developed a core competency in identifying, formulating and manufacturing controlled-release drug products based on coated pellet capsule technology. We believe this expertise will enable us to first develop the controlled-release drug and then leverage this experience to further develop this drug in fixed-dose combinations with other CNS therapeutics.
Strategic focus
We are implementing our strategy by focusing on the following key objectives:
- •
- Obtain FDA approval of ADS-5102 for
LID. Based on discussions with the FDA, we believe we need to conduct a single additional safety and efficacy study of ADS-5102 in LID
in order to support the submission of an NDA. We intend to initiate this Phase 3 registration trial in the second half of 2014 and, if the study is successful, submit an NDA for ADS-5102 for
the treatment of LID in the first half of 2016.
- •
- Develop ADS-5102 for the treatment of additional CNS indications, such
as behavioral symptoms associated with TBI. We intend to increase the number of potential indications for ADS-5102 by initiating a Phase
2/3 trial to study its effectiveness in an additional CNS indication, such as chronic irritability and aggression associated with TBI, near the end of 2014 and by initiating additional Phase 2/3
trials in one or more other CNS indications in 2015.
- •
- Commercialize ADS-5102 by developing a specialty sales
force. Assuming ADS-5102 is approved for the treatment of LID, we would expect to commercialize it in the United States by developing a
30 to 60 person sales force that would target the approximately 4,000 neurologists and movement disorder specialists who treat over 60% of late stage Parkinson's disease patients. If ADS-5102 is
approved in a second indication, this sales force could be expanded to target the specialist physicians who focus on patients with those conditions. Furthermore we also believe a targeted sales force
will allow us to more effectively compete for future acquisitions and in-licensing opportunities.
- •
- Develop additional novel therapeutics based on existing CNS
drugs. We have identified several areas of significant unmet clinical need that we believe could be addressed by fixed-dose combination
products incorporating ADS-5102 and another existing CNS drug, and intend to initiate development efforts in these areas in 2014. We also intend to apply our product development approach to other CNS
drugs with pharmacokinetic profiles that limit their dosing and efficacy.
- •
- Support Forest in the NDA review and anticipated commercialization of MDX-8704. Forest submitted an NDA to the FDA for MDX-8704 in February 2014. If approved, MDX-8704 will be marketed for management of moderate to severe dementia associated with Alzheimer's disease, an under-penetrated market we estimate at 2.5 million patients in the United States.
Our market opportunity
We estimate that approximately 36 million people in the United States suffer from chronic CNS disorders, such as Alzheimer's disease, Parkinson's disease, TBI, epilepsy, psychosis and depression. We believe that many of these disorders could be better treated if the pharmacokinetic profiles of existing CNS drugs were altered to enhance tolerability and efficacy and if these enhanced drugs were combined with other existing CNS drugs to improve and streamline the management of these complicated conditions.
CNS diseases are frequently treated with multiple medications having different mechanisms of action with the goal of maximizing symptomatic benefits for patients. Existing CNS drugs often require frequent dosing and may have tolerability issues that limit the amount of the drug that can be taken each day. Onerous side effects due to sub-optimal pharmacokinetic/pharmacodynamic profiles of CNS
67
drugs are also common. Several novel controlled-release CNS drugs that address these effects have been introduced, such as Adderall XR (Shire Specialty Pharmaceuticals), Concerta (Janssen Pharmaceuticals), and Wellbutrin XL (GlaxoSmithKline), and we believe many additional opportunities exist. Further, over the past decade, combination therapies have been introduced in a number of non-CNS therapeutic areas, easing the burden associated with complex medical regimens. The New England Journal of Medicine reported in 2011 that sophisticated public health models of adherence to complex medical regimens have validated the clinical relevance of combination therapies in multiple therapeutic areas. We believe there are significant opportunities to develop new fixed-dose combinations of approved CNS medications that address adverse pharmacokinetic/pharmacodynamic effects, improve efficacy and support greater adherence to the complex medical regimens faced by many CNS patients.
Therapeutic approach and portfolio
We have developed a portfolio of CNS therapeutics addressing significant unmet clinical needs.
Our initial therapeutic approach
Our initial product and product candidates are based upon pharmacokinetic enhancements of two approved CNS drugs, amantadine and memantine, which belong to a class of drugs known as aminoadamantanes. We selected aminoadamantanes as our initial area of focus because they have the ability to modulate multiple neurotransmitter systems and we believe they potentially have broader therapeutic utility than previously realized. Our pharmacokinetic enhancement strategy demands a deep understanding of the relationship between blood level changes and both efficacy and side effects of these drugs. These insights supported the development of a series of novel controlled-release aminoadamantane product candidates that contain significantly higher dose strengths than immediate-release formulations of the same active pharmaceutical ingredients and can be given once daily, as opposed to multiple times daily.
Our Therapeutics Portfolio
Product and Product Candidates
|
Target Indication(s) | Development Status | Commercial Rights | |||
|---|---|---|---|---|---|---|
| Wholly Owned | ||||||
ADS-5102 |
Levodopa-Induced Dyskinesia |
Phase 3 |
Adamas, worldwide |
|||
| Amantadine | Traumatic Brain Injury | Phase 2/3 ready | Adamas, worldwide | |||
| Undetermined | Phase 2/3 planning | Adamas, worldwide | ||||
ADS-8800 series ADS-5102 based combination therapies |
Undetermined |
Research, Phase 2/3 planning |
Adamas, worldwide |
|||
| Partnered | ||||||
Namenda XR Memantine |
Moderate to severe Alzheimer's dementia |
Marketed |
US-only; licensed to Forest |
|||
MDX-8704 Memantine/Donepezil |
Moderate to severe Alzheimer's dementia |
NDA submitted |
US-only; licensed to Forest |
|||
68
Our wholly owned product candidates
We have a number of wholly owned product candidates that are either in late stage clinical trials or being prepared to begin clinical development using the 505(b)(2) regulatory pathway. The most advanced of these are based on the approved drug amantadine. We also anticipate developing and commercializing product candidates based upon other approved CNS therapies.
ADS-5102
ADS-5102 is a controlled-release version of the approved drug amantadine that we are developing initially for LID. We selected LID from an extensive list of potential indications supported by the peer review literature based on results seen in both investigator initiated clinical studies and in established preclinical models. Further there is no FDA or EMA approved drug for treating LID despite significant investment by the pharmaceutical industry.
Overview of Parkinson's disease and LID
Parkinson's disease is a chronic, progressive motor disorder that causes tremors, rigidity, slowed movements and postural instability. The Parkinson's Disease Foundation estimates that there were approximately one million people living with Parkinson's disease in the United States in 2011. We estimate that approximately 687,000 people in the United States have been diagnosed and are being treated for Parkinson's disease. Prevalence of Parkinson's disease increases with age, with approximately 1.6% of people 65 years old or older having the disease compared with 0.3% of people in the general population. As the U.S. population ages the number of people living with Parkinson's disease in the United States is expected to grow at over 3% per year.
The most commonly prescribed treatments for Parkinson's disease are levodopa-based therapies. Levodopa is converted to dopamine to replace the dopamine loss caused by the disease. It is generally effective in providing at least partial relief from the symptoms of Parkinson's disease but fails to modify the underlying disease process. Patients initially take levodopa therapy approximately three times daily and receive relief from symptoms of Parkinson's disease for much of the day; this period of relief is known as "ON" time. As the effects of levodopa wear off, the symptoms of Parkinson's disease return; this is known as "OFF" time. By properly managing the timing of levodopa administration, patients with early stage Parkinson's disease can largely avoid "OFF" time during the day.
69
The table below defines the various terms that are used to describe the fluctuating symptoms of Parkinson's disease.
| Term | Definition | |
|---|---|---|
| "ON" time | "ON" time refers to periods of adequate control of Parkinson's disease symptoms. | |
"OFF" time |
"OFF" time refers to periods of the day when medication is not working well, causing worsening of Parkinson's disease symptoms. |
|
Dyskinesia |
Involuntary twisting, turning movements and loss of control of voluntary movements. |
|
LID |
Levodopa induced dyskinesia, which is a side effect of administration of levodopa and occurs during "ON" time. |
|
Troublesome LID |
LID that interferes with the patient's daily function or causes meaningful discomfort. |
|
"ON" with troublesome LID |
Periods of adequate control of Parkinson's disease symptoms but with troublesome LID. |
|
"ON" without troublesome LID |
Periods of adequate control of Parkinson's disease symptoms without troublesome LID. |
Over time, as Parkinson's disease progresses and dopaminergic neurons further degenerate, most patients require increasing doses of levodopa to achieve equivalent therapeutic benefit. Even with increased doses of levodopa, patients may begin to exhibit unpredictable "OFF" episodes throughout the day. In the later stages of the disease, many patients will suffer from LID. Patients with LID suffer from involuntary non-purposeful movements and reduced control over voluntary movements. The cause of LID is unknown, but it is associated with the pulsatile administration of levodopa treatment, degeneration of key brain structures, the duration of levodopa treatment, total levodopa exposure and other factors. LID can become severely disabling, rendering patients unable to perform routine daily tasks and increasing their risk of falling and social isolation. As Parkinson's disease progresses, the symptoms of LID worsen in frequency and severity. Eventually the total time that a patient spends either "OFF" or "ON" with troublesome LID can become a majority of his or her day. In addition, many Parkinson's disease patients at this stage have difficulty swallowing solid food or pills.
The chart below illustrates the fluctuating symptoms that an early and a late stage Parkinson's disease patient may experience during a two-dose cycle of levodopa taken during a portion of the waking hours.

70
LID can be managed by decreasing the amount of levodopa administered to a patient, but this change can result in an increase in "OFF" time and a decrease in "ON" time. Many patients would rather endure periodic episodes of LID than face unpredictable "OFF" episodes. As a result, these patients will choose to maintain their dose of levodopa even though they will experience times when they are "ON" but suffering from troublesome LID. We estimate that in 2011, approximately 260,000 Parkinson's disease patients in the United States suffered from significant "OFF" time, and approximately 140,000 of these patients suffered from LID.
Limitations of existing approaches to LID
There are currently no medications for the treatment of LID that are approved for marketing in the United States or Europe. As a result, clinicians often attempt to manage late stage Parkinson's disease symptoms with various approved Parkinson's disease products that are not indicated for LID. These include Azilect (Teva), Requip XL (GSK), Mirapex ER (BI), Neupro Patch (UCB) and Comtan (Novartis). These therapies produce clinically relevant reductions in "OFF" time ranging from 0.7-1.8 hours but not significant increases in "ON" time without troublesome LID. In addition, none of these products reduce LID and some actually increase LID.
Physicians may also use the immediate-release form of amantadine, which is approved for the treatment of Parkinson's disease, to treat LID. This approach is supported by a number of investigator initiated clinical studies and case studies, which suggest that it may be effective for the treatment of LID. However, these studies were not well-controlled clinical trials that meet evidence-based clinical or regulatory standards.
In addition to the limited data regarding its effectiveness, we believe that the use of amantadine to treat LID has also been limited by potential side effects at dose levels considered to be effective. The majority of Parkinson's disease patients tolerate twice-daily dosing of 100 mg of amantadine, but often this dosing regimen is insufficient to provide adequate symptom relief. The available literature on amantadine for the treatment of LID indicates that higher doses of amantadine produce a greater reduction in LID symptoms. However, the increased frequency of adverse events at higher doses, in particular CNS events and sleep disturbances, generally limits the use of amantadine at doses greater than 200 mg per day. Immediate-release versions of amantadine are absorbed relatively rapidly by the body with peak concentrations in the blood being reached two to four hours after administration. We believe that the side effects associated with immediate-release amantadine are associated with this rapid rise in concentration within a few hours after dosing.
The Adamas Solution—ADS-5102
ADS-5102 is a controlled-release version of amantadine that addresses many of the limitations of immediate-release amantadine by allowing higher daily doses of amantadine to be administered once-nightly without a significant increase in side effects. In patients taking ADS-5102, the amantadine plasma concentration achieved in the early morning through mid-day is approximately two-times that reached following administration of immediate-release amantadine, providing symptomatic relief to patients as they engage in their daily activities. Further, the lower concentrations occur in the evening, reducing the potential negative impact of amantadine's sleep-related side effects. In addition, ADS-5102 capsules can be opened to sprinkle the contents on food for use by Parkinson's disease patients who have difficulty swallowing due to their illness.
In our Phase 2/3 EASED trial, ADS-5102 demonstrated statistically significant improvements when compared to placebo. At the chosen 340 mg dose, the benefits included a 3.8-hour increase in "ON" time without troublesome LID, a 43% reduction in troublesome LID compared to baseline LID experienced prior to taking ADS-5102, a reduction in the functional impact of LID, no worsening of Parkinson's disease symptoms and a trend towards reduction in "OFF" time. Notably, there was no
71
difference from placebo in the incidence of sleep-related adverse events. By both increasing "ON" time without troublesome LID and reducing LID, ADS-5102 provides a combination of significant clinical benefits that we believe cannot be achieved with other drugs for Parkinson's disease. While there are a number of approved drugs and certain drug candidates that have been demonstrated to reduce "OFF" time, none have been demonstrated to reduce LID and in most cases actually increase LID. For currently marketed products that increase dopamine, including Azilect, Mirapex ER, Requip XL, Neupro and Comtan, the increase in "ON" time without dyskinesia is reported as 0.8-1.5 hours vs. placebo, the "OFF" time reduction is reported as 0.7-1.8 hours versus placebo and the increase in dyskinesia is reported as 7-10% vs. placebo. For levodopa formulation development products, including Rytary and Duodopa, the increase in "ON" time without dyskinesia is reported as 1.0-1.9 hours versus placebo and the reduction in "OFF" time is reported as 1.1-1.9 hours versus placebo, with no change in LID reported.
Commercialization plan for ADS-5102 in LID
We intend to commercialize ADS-5102 in the United States, subject to FDA approval, by developing our own sales force and in other markets through distribution agreements and collaborations with CNS-focused pharmaceutical companies. We plan to focus our commercial efforts on the approximately 4,000 neurologists and movement disorder specialists in the United States who are responsible for the treatment of greater than 60% of the patients with late stage Parkinson's disease. As these physician specialists are heavily concentrated in major urban markets, we believe a 30 to 60 member specialty sales force will provide adequate reach and frequency of communication for successful commercialization.
We will be responsible for negotiating coverage, reimbursement and formulary placement decisions for ADS-5102 in the United States. We believe that if ADS-5102 is approved as the first product indicated in the United States for the treatment of LID, most payors are likely to extend coverage to it and that its placement on payor formularies and the amount of reimbursement will be influenced by the availability and pricing of branded treatments for symptoms of Parkinson's disease, branded treatments for other forms of dyskinesia, generic amantadine and surgical treatments for symptoms of Parkinson's disease.
ADS-5102 development plan for LID
In December 2013 after completion of our Phase 2/3 study, we had a written interaction with the FDA to discuss the remaining clinical studies required to support the submission of an NDA for ADS-5102 for the treatment of LID. Based on the minutes from that interaction, we believe that we need to conduct additional clinical studies prior to submitting an NDA: a Phase 3 efficacy and safety study and additional single dose/multi-dose relative bioavailability studies in healthy volunteers.
The Phase 3 study will be a multi-center, randomized, double-blind, placebo-controlled, two-arm parallel group trial. The primary objective of the Phase 3 study will be to evaluate the efficacy of a once-nightly 340 mg dose of ADS-5102 for the treatment of LID in subjects with Parkinson's disease. Secondary objectives will be to evaluate the potential and the safety and tolerability of ADS-5102 in the study population. Secondary outcome measures include "ON" time without troublesome dyskinesia and "OFF" time based on home diaries. We are in the process of finalizing the details of this study with the FDA.
Prior to completing the Phase 3 study, we intend to hold a pre-NDA meeting with the FDA to determine the contents of the NDA submission for ADS-5102. In addition, prior to submitting the NDA, we intend to meet with regulators in certain markets outside the United States to determine the regulatory pathways for access to those markets.
72
ADS-5102 Phase 1 data—pharmacokinetic profile
Our development of ADS-5102 was driven by the discovery that the side effects of amantadine are not caused primarily by the absolute levels of amantadine in the blood but by the speed at which the maximum concentrations are reached. Immediate-release amantadine is rapidly absorbed by the body with its maximum concentration in the blood being reached in two to four hours. This rapid increase in blood concentration levels is associated with an increased level of CNS side effects. In contrast, the same amount of ADS-5102 is absorbed more slowly with the maximum concentration being achieved many hours later. This slower increase in blood concentration levels is associated with fewer CNS side effects than a more rapid one.
Because of this improved tolerability due to the novel pharmacokinetic profile, we were able to investigate ADS-5102 in clinical studies at dose strengths from 1.3 to 2.1 times greater than the 100 mg twice-daily dose typically used with immediate-release amantadine.
Based on our clinical experience, we are developing a 340 mg dose of ADS-5102 to be taken once-nightly at bedtime. The amantadine plasma concentration for this dose of ADS-5102 is expected to occur 12 to 14 hours after being taken and is approximately two times higher than that of a 100 mg dose of immediate-release amantadine taken twice daily. With this regimen, amantadine plasma concentration would be achieved from the early morning through mid-day, providing relief to patients as they engage in their daily activities, and the lowest concentrations would occur in the evening, reducing the potential for sleep-related side effects. The once-nightly dosing regimen may also provide enhanced convenience and compliance as compared to a twice-daily dosing regimen.
We have completed five Phase 1 pharmacokinetic studies in healthy subjects with two controlled-release versions of amantadine having slightly different release rates. The most frequently occurring adverse events reported in the Phase 1 studies were headache, fatigue, and dizziness, occurring in 5-10% of subjects, and the majority of adverse events were categorized as mild.
ADS-5102 Phase 2/3 data
In 2013, we completed a successful Phase 2/3 clinical trial of ADS-5102 for the treatment of LID. The EASED trial was designed to investigate the safety and efficacy of three dose levels of ADS-5102 administered once-nightly at bedtime for the treatment of LID in Parkinson's disease. The study enrolled 83 Parkinson's disease patients who were randomized in a 1:1:1:1 ratio to the four treatment groups: placebo, 260 mg ADS-5102, 340 mg ADS-5102 and 420 mg ADS-5102. The table below summarizes the change from baseline compared to placebo for the key efficacy endpoints measured in the study. In the two charts and discussion below relating to ADS-5102, only results with a p-value of 0.05 or less are considered to be statistically significant.
73
| Outcome Measure | 260 mg ADS-5102 N=19 |
340 mg ADS-5102 N=20 |
420 mg ADS-5102 N=19 |
|||
|---|---|---|---|---|---|---|
| |
LS Mean Treatment Difference vs. Placebo (95% CI) |
|||||
UDysRS Total Score |
-5.6 (-13.4, 2.2) p=0.159 |
-11.3 (-19.1, -3.5) p=0.005 |
-10.0 (-17.8, -2.2) p=0.013 |
|||
ON Time w/o Troublesome LID, hours |
3.3 (1.1, 5.5) |
3.0 (0.8, 5.2) |
2.7 (0.5, 5.0) |
|||
OFF Time, hours |
-1.3 (-2.7, 0.1) |
-0.9 (-2.3, 0.5) |
0.1 (-1.4, 1.5) |
|||
MDS-UPDRS (Part I, II, III) |
1.2 (-7.7, 10.1) |
-2.2 (-11.2, 6.9) |
1.7 (-7.2, 10.6) |
|||
MDS-UPDRS (Part IV, Item 4.2) |
-0.8 (-1.4, -0.2) |
-1.0 (-1.6, -0.4) |
-1.3 (-2.0, -0.7) |
|||
The chart below shows the change in the Unified Dyskinesia Rating Scale, or UDysRS, score for each group in the EASED study after 8 weeks:
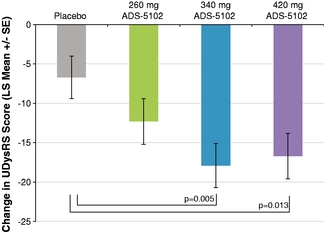
Both the 340 mg and 420 mg dose levels significantly reduced LID as measured by the change in the UDysRS total score over eight weeks versus placebo, meeting the primary endpoint for the clinical study (p=0.005 and p=0.013, respectively). The magnitude of the change for the 340 mg ADS-5102 group was a 43% reduction versus baseline and a 27% reduction versus placebo.
In addition, ADS-5102 significantly increased "ON" time without troublesome LID at the 260 mg, 340 mg and 420 mg dose levels from baseline to week eight relative to placebo by 3.3, 3.0 and 2.7 hours per day, respectively, as measured by patient diaries after eight weeks of treatment (Least square means, p=0.004, p=0.008 and p=0.018, respectively). At the 340 mg dose level, "ON" time with troublesome LID was reduced by 3.0 hours per day from baseline relative to placebo (p=0.008) and "OFF" time was reduced by 0.9 hours per day from baseline relative to placebo after 8 weeks of treatment, though this latter result was not statistically significant (p=0.199).
Based on analysis of the pharmacokinetic, safety and efficacy data from the EASED study, we have selected 340 mg ADS-5102 taken once-nightly as the recommended dose regimen and anticipate using that dose in our Phase 3 trial. We believe that this dose offers the best benefit/risk ratio of the doses we have studied.
74
The chart below shows the average levels of "ON" time without troublesome LID, "ON" time with troublesome LID, "OFF" time, and sleep, recorded by patients in the 340 mg dose group and the placebo group at baseline and after eight weeks of treatment.

Treatment with ADS-5102 did not result in worsening of Parkinson's disease symptoms, as measured by the MDS-UPDRS combined score, a standard measurement of Parkinson's disease related disability. The adverse events reported in this study were typically mild to moderate in severity and consistent with Parkinson's disease and the known amantadine adverse event profile. Five patients had serious adverse events. The most common adverse events, occurring in more than 10% of the subjects or by more than two subjects in any ADS-5102 group, were constipation, dizziness, hallucination, dry mouth, fall, confusional state, headache, nausea and asthenia. Notably, there was no difference from placebo in the incidence of sleep-related adverse events.
ADS-5102 for behavioral symptoms associated with traumatic brain injury
We are currently evaluating the development of ADS-5102 for the treatment of chronic irritability and aggression arising from traumatic brain injury. Approximately 1.7 million people in the United States experience a TBI annually, and over 3.2 million are estimated to have chronic symptoms associated with TBI. The chronic manifestations of TBI include long-term impairment of cognitive and behavioral functions. In particular, irritability and aggression are highly prevalent in individuals with TBI contributing to social isolation, increased burden of care, disrupted interpersonal relationships and difficulties with full community integration. The diagnosis and management of these long-term effects of TBI are extremely challenging, and there are no FDA-approved treatments for any of the chronic behavioral symptoms associated with TBI.
A recently completed independent, investigator-initiated, placebo-controlled study indicates that amantadine may reduce irritability and aggression in individuals with TBI. This study, which was led by Dr. Flora Hammond at Indiana University School of Medicine, enrolled seventy-six subjects in a parallel-group, randomized, double-blind, controlled trial of a 100 mg twice daily dose of amantadine (n=38) versus placebo (n=38). In this study, patients receiving amantadine had a statistically significant reduction in irritability and aggression of approximately 40% as compared to patients receiving placebo. Dr. Hammond is in the process of completing a second study on amantadine expected to be released in the first half of 2014.
75
We believe that ADS-5102's characteristics, including once-nightly dosing and possibly a higher tolerated dose, have the potential to make it a better option than immediate-release amantadine for the treatment of chronic irritability and aggression arising from TBI.
Additional indications for ADS-5102
We intend to continue to review the results of preclinical studies, clinical trials and case reports published in peer reviewed medical journals to evaluate additional potential CNS indications for ADS-5102, including post-concussive syndrome, multiple sclerosis fatigue, Attention Deficit Hyperactivity Disorder, depression, hyperkinetic movement disorders and antipsychotic-induced weight gain. We anticipate that by using the 505(b)(2) regulatory pathway we will be able to initiate the clinical development of ADS-5102 in new indications with a Phase 2/3 study and will not typically need to conduct any Phase 1 studies prior to initiating Phase 2/3 studies. As a result, we retain substantial flexibility in our development plans and are able to respond to new clinical data and changes in the commercial environment. We currently expect to initiate Phase 2/3 studies of ADS-5102 in additional CNS indications by the end of 2014.
ADS-8800 series (ADS-5102-based combination products)
Using the product development strategy we employed with memantine, we are investigating and will potentially develop additional combination products based upon combining ADS-5102 with second agents. We have identified certain approved CNS drugs that we believe have the potential to be combined with ADS-5102 to treat one or more chronic CNS conditions such as Alzheimer's disease, Parkinson's disease, TBI, epilepsy, psychosis or depression. Each combination will be designed to provide clinical benefits in specific indications where it appears that combination therapy including ADS-5102 can address a significant unmet clinical need. We believe we will be able to use the 505(b)(2) regulatory pathway to initiate clinical development of these product candidates. Additional drug-drug interaction studies to assess the potential for interaction between ADS-5102 and the second agent may be required unless the two agents have been previously studied. We anticipate progressing into Phase 2/3 studies in combination therapies with minimal additional work.
Additional programs
We believe our product development strategy is broadly applicable to addressing limitations of other CNS drugs whose pharmacokinetic profiles limit dosing and intend to initiate additional programs in 2015. We are currently evaluating several different approved CNS drugs for potential use in a range of CNS indications.
Other wholly owned product candidates
ADS-8704 (outside of the United States only)
We have retained the rights to develop fixed-dose combinations of controlled-release memantine and donepezil outside of the United States. We are currently evaluating potential development and commercialization pathways for ADS-8704, a fixed-dose combination of our proprietary controlled-release version of memantine and donepezil for the treatment of moderate to severe dementia related to Alzheimer's disease in various non-U.S. markets.
ADS-8902 for severe influenza
We developed ADS-8902, a triple combination antiviral drug therapy for influenza, which is designed to inhibit viral replication at multiple points in the virus proliferation pathway. ADS-8902 is a proprietary fixed-dose combination product containing three FDA approved products, amantadine, oseltamivir, and ribavirin. The National Institutes of Health, or NIH, is currently conducting a multi-
76
center, 520 patient Phase 2/3 trial of amantadine, oseltamivir, and ribavirin for the treatment of severe influenza. The trial was initiated in 2011 and as of February 2014 it had randomized 98 patients. As the rate of enrollment in the trial is heavily dependent on the prevalence and severity of seasonal influenza each year, we have not projected an anticipated completion date for the trial. If the NIH trial is successful, we may seek to license rights to ADS-8902 to pharmaceutical companies for which the treatment of influenza is a commercial focus. In 2010, we suspended further activities on ADS-8902, due to the expected length of the clinical trial and a change in our strategic focus.
Our partnered product and product candidate
Our memantine-based therapeutics are being developed and commercialized in the United States through our partnership with Forest for the treatment of dementia associated with moderate to severe Alzheimer's disease.
Namenda XR and MDX-8704
Namenda XR is a controlled-release version of memantine approved in the United States in 2010 for the treatment of moderate to severe dementia related to Alzheimer's disease and is marketed in the United States by our partner Forest. Pursuant to our agreement, we have exclusively licensed to Forest multiple U.S. patents covering Namenda XR.
MDX-8704 is a once-daily fixed-dose combination of the approved drugs Namenda XR and donepezil that we are co-developing with Forest for the treatment of moderate to severe dementia related to Alzheimer's disease in the United States.
Overview of Alzheimer's disease dementia
Alzheimer's disease dementia is a progressive neurodegenerative condition that affects over 5 million people in the United States. There is no known cure for Alzheimer's disease or any of the other conditions that cause dementia. Existing pharmaceutical therapies are approved for the treatment of symptoms of the disease; do not alter disease progression. Even if disease modifying therapies are developed and approved, we believe it is likely that there will be a continuing need for symptomatic treatments. In 2013, approximately 3.4 million people in the United States were treated for Alzheimer's disease dementia, an increase of approximately 4% from 2009, and in U.S. sales of pharmaceutical treatments for Alzheimer's disease for the 12 months ended November 30, 2013 were approximately $2.7 billion. We believe that the number of people treated for Alzheimer's disease will continue to increase as the number of elderly people in the United States increases, diagnosis of dementia becomes more common and health care reform improves access to treatments.
Existing treatments for Alzheimer's disease dementia
The only two classes of drugs approved for the treatment of Alzheimer's disease dementia are acetylcholinesterase inhibitors, or AChEIs, and NMDA receptor antagonists. Donepezil is the leading AChEI and forms of memantine are the only NMDA receptor antagonists approved for Alzheimer's disease. Memantine is currently marketed by Forest in the United States in an immediate-release version under the brand name Namenda and in an controlled-release version under the brand name Namenda XR. Donepezil is sold by Pfizer and Eisai under the brand name Aricept and as a generic drug by a number of manufacturers. Namenda XR is approved for the treatment of moderate to severe dementia related to Alzheimer's disease, and donepezil is approved for the treatment of dementia in patients with mild to severe Alzheimer's disease. In February 2014, Forest announced that effective as of August 15, 2014 it would no longer supply immediate-release Namenda tablets, and that it will focus on Namenda XR and MDX-8704.
77
Both memantine and donepezil are considered to be generally safe and well tolerated. The most common side effects of memantine are headache, diarrhea and dizziness. The most common side effects of donepezil are nausea, diarrhea, not sleeping well, vomiting, muscle cramps, feeling tired and not wanting to eat.
Treatment of moderate to severe Alzheimer's disease dementia with combination therapy
The concurrent use of memantine and donepezil is a well-established treatment option for patients with moderate to severe dementia related to Alzheimer's disease. The current treatment recommendations from the American Association of Geriatric Psychiatry encourage the use of an AChEI for the treatment of mild Alzheimer's disease and then to add memantine when patients progress to the moderate phase of the disease. Despite this, only approximately 1,400,000 out of 2,500,000 patients with moderate to severe Alzheimer's disease are treated with combination therapy, as shown below.

Concurrent use of memantine and donepezil is supported by clinical data which shows that in patients with moderate to severe Alzheimer's disease, combination therapy resulted in a statistically significant improvement in the Severe-Impairment-Battery, or SIB, a commonly used outcome measure, as compared to treatment with donepezil alone. A second study demonstrated that concurrent use of Namenda XR and an AChEI also demonstrated a statistically significant improvement in the SIB as compared to treatment with donepezil alone.
Concurrent treatment with memantine and donepezil is generally safe and well tolerated with the most common side effects seen in clinical trials being dizziness, headache and diarrhea. Of these side effects, incidence of dizziness with concurrent treatment is 5% as compared with 1% for treatment with donepezil alone.
The MDX-8704 solution
We developed MDX-8704, a once-daily fixed-dose combination of Namenda XR and donepezil, to simplify the co-administration of these drugs by a patient or caregiver with the goal of increasing compliance and adherence to the prescribed regimen. Namenda XR exhibits a much lower initial rise in plasma concentration when compared to immediate-release memantine, which we believe is central to its dosing protocol of once-daily administration and at a higher daily dose as compared to immediate-release memantine. By improving the tolerability and formulating a once-daily preparation of memantine, we have enabled a once-daily fixed-dose combination of memantine with donepezil. In addition, MDX-8704 capsules can be opened to sprinkle the contents on apple sauce. Forest plans to make MDX-8704 available in two dose strengths, initially, a combination of 28 mg memantine ER and
78
10 mg donepezil and a combination of 14 mg memantine ER and 10 mg donepezil. We believe that MDX-8704 has the potential to be adopted by patients already taking combination therapy as well as moderate to severe patients currently taking donepezil alone.
MDX-8704 development pathway
Based on formal meetings with the FDA in October 2011 and June 2013, we believe that MDX-8704 can be approved using the shortened development pathway provided by Section 505(b)(2) of the FDCA. The FDA indicated that two additional clinical studies would be required for approval of MDX-8704, including a bioequivalence study and a food-effect/sprinkle study. These studies were conducted by our partner Forest and they demonstrated that MDX-8704 both is bioequivalent to separate doses of Namenda XR and donepezil, and exhibits the same bioavailability whether administered after fasting, after a meal or when sprinkled on apple sauce. Based on these studies, Forest submitted an NDA in February 2014 for MDX-8704 for the treatment of patients with moderate to severe Alzheimer's disease who are already being co-administered memantine, as either Namenda or Namenda XR, and 10 mg donepezil.
Subject to MDX-8704 receiving this initial approval, Forest anticipates submitting a supplemental application to expand the indication for MDX-8704 to include patients who are on a stable dose of 10 mg donepezil and are ready to initiate treatment with MDX-8704. This supplemental application will include manufacturing information to support two additional MDX-8704 doses, a fixed-dose combination of 7 mg Namenda XR/10 mg donepezil and a fixed-dose combination of 21 mg Namenda XR/10 mg donepezil. These additional dose combinations will allow patients who are receiving a stable dose of 10 mg donepezil but are naïve to Namenda XR to initiate treatment with MDX-8704 while utilizing the same three-step titration that is currently approved for Namenda XR.
Intellectual property
Our success will significantly depend upon our ability to obtain and maintain patent and other intellectual property and proprietary protection for our drug candidates, including usage, pharmacokinetic, composition-of-matter and formulation patents, as well as patent and other intellectual property and proprietary protection for our novel discoveries and other important technology inventions and know-how. In addition to patents, we rely upon unpatented trade secrets, know-how and continuing technological innovation to develop and maintain our competitive position.
We seek to protect our proprietary information, in part, by using confidentiality agreements with our commercial partners, collaborators, employees and consultants and invention assignment agreements with our employees and selected consultants. Despite these measures, any of our intellectual property and proprietary rights could be challenged, invalidated, circumvented, infringed or misappropriated, or such intellectual property and proprietary rights may not be sufficient to permit us to take advantage of current market trends or otherwise to provide competitive advantages. For more information, please see "Risk factors—Risks related to intellectual property."
Our current products and product candidates are based on novel discoveries related to the clinical implications of the timing of administration of drugs and pharmacokinetic and pharmacodynamic relationships. These discoveries led us to modify the pharmacokinetic profile of existing drugs in a manner that enables increased tolerability of higher doses as compared to immediate-release versions. We are able to apply these pharmacokinetic and pharmacodynamic insights to the development of novel fixed-dose combination therapeutics, potentially yielding significant clinical benefits. As such, our intellectual property covers the novel pharmacokinetic properties of our formulations and combinations and their methods of use.
79
As of March 25, 2014, we owned 16 issued U.S. patents, eight U.S. patent applications and additional patents and patent applications in other jurisdictions. The patent portfolios for Namenda XR, MDX-8704/ADS-8704, and ADS-5102 as of March 25, 2014 are summarized below:
Namenda XR, MDX-8704 and ADS-8704
Namenda XR and MDX-8704 are covered by a total of 13 of our issued U.S. patents containing method and compositions claims relating to their pharmacokinetic profile and method claims relating to dosing of memantine. These patents expire as late as 2029 and are exclusively licensed to Forest. We also own additional foreign patents and patent applications covering Namenda XR and ADS-8704.
ADS-5102
ADS-5102 is currently covered by one issued U.S. method of use patent with claims relating to its pharmacokinetic profile which expires in 2027 and an additional five U.S. patent applications. These patents and patent applications are wholly owned by us and are not subject to any license agreements. We also own additional foreign patent applications covering ADS-5102.
Sales and marketing
We intend to commercialize ADS-5102 in the United States, subject to FDA approval, by developing our own sales force and in other markets through distribution agreements and collaborations with CNS-focused pharmaceutical companies. We plan to focus our commercial efforts on the approximately 4,000 neurologists and movement disorder specialists who are responsible for the treatment of approximately 60% of the patients in the United States with LID. As these physician specialists are heavily concentrated in major urban markets, we believe a 30 to 60 member specialty sales force will provide adequate reach and frequency of communication for successful commercialization. We intend that the members of our specialty salesforce will have proven experience and be able to effectively communicate the clinical value and pharmacoeconomic advantage of ADS-5102. To complement the specialty sales force, we will recruit experienced sales management, medical marketing and reimbursement professionals to support our commercialization efforts. We believe a targeted sales force will allow us to more effectively compete for future acquisitions and in-licensing opportunities.
License agreement with Forest
In November 2012, we entered in a license agreement with a wholly owned subsidiary of Forest. Subject to the terms of the license agreement, we granted Forest an exclusive license, with the right to sublicense, under the relevant elements of our intellectual property, to commercialize human therapeutics containing memantine in the United States; a co-exclusive license along with us, with the right to sublicense, to develop and manufacture such products in the United States; and a non-exclusive license, with a right to sublicense, to develop and manufacture (but not commercialize) such products outside of the United States solely in support of the development or commercialization of such products within the United States. The license agreement establishes a joint development committee consisting of representatives from us and Forest to oversee the development of a fixed-dose memantine-donepezil product, such as MDX-8704, in the United States with Forest having final decision making authority with certain restrictions. Forest is required to use commercially reasonable efforts to develop such a product in accordance with development and regulatory plans that we and Forest have mutually agreed upon that may be modified by the joint development committee or by Forest pursuant to the terms of the agreement. Forest is responsible for paying all costs associated with such development and reimburses us on a cost-plus basis for work performed by us at its request in support of the development. In addition, Forest is required at its expense to use commercially reasonable efforts to commercialize fixed-dose memantine-donepezil product in the United States.
80
In connection with the execution of the license agreement, Forest made a non-refundable, upfront payment to us of $65 million. In the fourth quarter of 2013, Forest made two payments to us of $20 million each relating to the satisfaction of certain development milestones. In addition, Forest is required to make aggregate milestone payments to us of up to $55 million upon the occurrence of certain regulatory milestones, including up to $25 million upon FDA acceptance of an NDA for a fixed-dose memantine-donepezil product covered by the license agreement and up to $30 million upon FDA approval of such a product. Commencing five years after the initial launch of a fixed-dose memantine-donepezil product in the United States, we are entitled to receive royalties at rates ranging from the low double digits to the mid-teens on the net sales by Forest, its affiliates and any sublicensees of such products in the United States. In addition, commencing in June of 2018, we are entitled to receive low to mid single digit royalties on net sales in the United States by Forest, its affiliates or any of its sublicensees of controlled-release versions of memantine, such as Namenda XR, or any other product covered by the terms of the license agreement. Forest's obligation to pay royalties with respect to fixed-dose memantine-donepezil products continues until the later of (i) 15 years after the commercial launch of the first fixed-dose memantine-donepezil product by Forest in the United States or (ii) the expiration of the Orange Book listed patents for which Forest obtained rights from us covering such product. Forest's obligations to pay royalties with respect to controlled-release versions of memantine or any other product covered by the agreement continue until the expiration of our Orange Book listed patents covering such product. Forest's obligations to pay royalties are subject to reduction in certain circumstances. In addition, Forest shall have no obligation to pay any royalty with respect to any product covered by the license agreement in any quarter in which there is significant competition from generic products, as defined in the agreement, in the United States. If we or our affiliates develop or commercialize the licensed products outside of the United States (other than in Japan) or otherwise enable a third party to do so, and such development or commercialization requires the use of or reference to certain data generated pursuant to the development plan, we will be obligated to make certain payments to Forest.
The license agreement terminates on a product by product basis upon the expiration of all royalty obligations with respect to each product and terminates in its entirety upon the expiration of all royalty obligations with respect to all products covered by the license agreement. Upon expiration of the license agreement with respect to a product, all licenses and other rights granted to Forest by us with respect to that product become fully paid up and irrevocable. In addition, Forest may terminate the license agreement with respect to fixed-dose memantine-donepezil products by delivering to us notice of its intent to cease development and commercialization of such products.
If Forest fails to make certain milestone payments to us by specified dates and within five business days after receiving notice from us of such failure again fails to make the required payment, then the license agreement automatically terminates with respect to fixed-dose memantine-donepezil products. Prior to Forest's payment of all of the milestones payments that may become due under the license agreement, we can seek to terminate the license agreement with respect to fixed-dose memantine-donepezil products in the event that Forest has materially breached its obligation to use commercially reasonable efforts to develop those products, subject to customary notice provisions. Otherwise, our remedy for any breach of the license agreement by Forest is to seek damages or equitable relief, not termination of the license agreement. Furthermore, we have no right to terminate the license agreement with respect to controlled-release version of memantine or other products that are not fixed-dose memantine-donepezil products.
Competition
Our industry is highly competitive and subject to rapid and significant technological change. While we believe that our development experience and scientific knowledge provide us with competitive advantages, we may face competition from large pharmaceutical and biotechnology companies, smaller
81
pharmaceutical and biotechnology companies, specialty pharmaceutical companies, generic drug companies, academic institutions, government agencies and research institutions and others.
Many of our competitors may have significantly greater financial, technical and human resources than we have. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Our commercial opportunity could be reduced or eliminated if our competitors develop or market products or other novel technologies that are more effective, safer or less costly than any that will be commercialized by us, or obtain regulatory approval for their products more rapidly than we may obtain approval for ours. Our success will be based in part on our ability to identify, develop and manage a portfolio of drugs that are safer, more efficacious and/or more cost-effective than alternative therapies.
ADS-5102
Currently there are no FDA or EMA drug therapies approved for the treatment of LID. While a number of major pharmaceutical companies, including Merck and Novartis, as well as smaller biopharmaceutical companies have had programs aimed at developing treatments for LID, we believe ADS-5102 is one of the most advanced. Other products in late stage development for Parkinson's disease, include candidates from Impax, Kyowa Hakko, Abbvie, Civitas and other companies. Products approved to treat late stage Parkinson's disease include Azilect (Teva), Requip XL (GlaxoSmithKline), Mirapex ER (Boehringer Ingelheim), Neupro Patch (UCB), Comtan (Novartis) and Stalevo (Novartis) and generic versions of amantadine and other drugs. Physicians may use these drugs to attempt to manage LID. In selective cases for late stage patients, physicians and patients/caregivers will consider neurosurgical intervention such as deep brain stimulation.
Namenda XR/MDX-8704
In the market for Alzheimer's disease treatments, Namenda XR and MDX-8704 compete or will compete with generic products such as galatamine, rivastigmine and donepezil as well as branded products such as the Exelon patch (Novartis) and Aricept 23 mg (Eisai). In addition, Forest currently markets Namenda, the immediate-release version of memantine, which physicians and patients may favor instead of Namenda XR, the controlled-release version. In addition, generic versions of Namenda may be available in 2015. Several generic manufacturers are currently seeking regulatory approval to market generic versions of Namenda XR and, if MDX-8704 is approved, they may seek to market generic versions of MDX-8704. We are also aware that Lundbeck and other biopharmaceutical companies are developing treatments for Alzheimer's disease that may compete with Namenda XR and MDX-8704. In February 2014, Forest announced that, effective as of August 15, 2014, it would no longer supply immediate-release Namenda tablets, and that it will focus on Namenda XR and MDX-8704.
Third-party reimbursement
Sales of pharmaceutical products depend in significant part on the availability of coverage and adequate reimbursement by third-party payors, such as state and federal governmental authorities, including those that administer the Medicare and Medicaid programs, managed care organizations and private insurers. Decisions regarding the extent of coverage and amount of reimbursement to be provided for Namenda XR, MDX-8704 and ADS-5102 are or will be made on a plan by plan basis. Each plan determines whether or not it will provide coverage for a drug, what amount it will pay the manufacturer for the drug, and on what tier of its formulary the drug will be placed. The position of a drug on the formulary generally determines the co-payment that a patient will need to make to obtain the drug and can strongly influence the adoption of a drug by patients and physicians. Patients who are prescribed treatments for their conditions and providers performing the prescribed services generally
82
rely on third-party payors to reimburse all or part of the associated healthcare costs. Patients are unlikely to use our products unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our products. Additionally, a third-party payor's decision to provide coverage for a drug does not imply that an adequate reimbursement rate will be approved. Also, third-party payors are developing increasingly sophisticated methods of controlling healthcare costs. As a result, coverage, reimbursement and placement determinations are complex and are often the subject of extensive negotiations between the payor and the owner of the drug.
Forest is responsible for obtaining coverage and negotiating reimbursement amounts and formulary placement for Namenda XR and MDX-8704. Under our agreement with Forest, we will be entitled to receive payments from Forest based on future net sales of these products. The amount of revenue we will receive under the agreement is therefore significantly dependent on the extent to which Forest is able to obtain favorable coverage, reimbursement and placement decisions from payors.
We will be responsible for negotiating coverage, reimbursement and placement decisions for ADS-5102 if approved. Coverage, reimbursements and placement decisions for a new product are based on many factors including the coverage, reimbursement and placement of already marketed branded drugs for the same or similar indications, the safety and efficacy of the new product, availability of generics for similar indications, and the clinical need for the new product. Currently, there are no drugs approved for the treatment of LID, and generic amantadine is not approved for this indication.
We have had preliminary discussions regarding the potential coverage, reimbursement and placement of ADS-5102 with consultants and representatives of payors, but have not begun formal negotiations with any payors. Based on these discussions, we believe that if ADS-5102 is approved as the first product indicated for the treatment of LID, most payors are likely to extend coverage to it and that its placement on payor formularies and the amount of reimbursement will be influenced by the aforementioned products, generic amantadine and generic and branded treatments for symptoms of Parkinson's disease. Within the Medicare program, as self-administered drugs, MDX-8704 and ADS-5102 would be, and Namenda XR is, reimbursed under the expanded prescription drug benefit, known as Medicare Part D. This program is a voluntary Medicare benefit administered by private plans that operate under contracts with the federal government. These Part D plans negotiate discounts with drug manufacturers, which are passed on to each of the plan's enrollees. Historically, Part D beneficiaries have been exposed to significant out-of-pocket costs after they surpass an annual coverage limit and until they reach a catastrophic coverage threshold. However, changes made by recent legislation will reduce this patient coverage gap, known as the "donut hole", by transitioning patient responsibility in that coverage range from 100% in 2010 to only 25% in 2020. To help achieve this reduction, beginning in 2011, pharmaceutical manufacturers are required to pay quarterly discounts of 50% off the negotiated price of branded drugs issued to Medicare Part D patients in the donut hole.
If a drug product is reimbursed by Medicare or Medicaid, pricing and rebate programs must comply with, as applicable, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 as well as the Medicaid rebate requirements of the Omnibus Budget Reconciliation Act of 1990, or the OBRA, and the Veterans Health Care Act of 1992, or the VHCA, each as amended. Among other things, the OBRA requires drug manufacturers to pay rebates on prescription drugs to state Medicaid programs and empowers states to negotiate rebates on pharmaceutical prices, which may result in prices for our future products that will likely be lower than the prices we might otherwise obtain. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply.
An ongoing trend has been for third-party payors, including the U.S. government, to apply downward pressure on the reimbursement of pharmaceutical products. Also, the trend towards managed health care in the United States and the concurrent growth of organizations such as health maintenance organizations may result in lower reimbursement for pharmaceutical products. We expect that these trends will continue as these payors implement various proposals or regulatory policies, including various provisions of the recent health reform legislation that affects reimbursement of these products. There are currently, and we expect that there will continue to be, a number of federal and state proposals to implement controls on reimbursement and pricing, directly and indirectly.
83
Manufacturing
We currently have no manufacturing facilities and limited personnel with manufacturing experience. We rely on third-party manufacturers to produce bulk drug substance and drug products required for our clinical trials of ADS-5102. We plan to continue to rely upon contract manufacturers and to manufacture commercial quantities of our ADS-5102 and other product candidates if and when we receive approval for marketing by the applicable regulatory authorities.
Our current products and product candidates are based upon controlled-release coated pellet products that are quite difficult to manufacture. As shown below, these products consist of an inert core, a drug layer, an optional seal coating and controlled-release coatings. Our products are made in a fluidized bed coating machine in sequential steps. At each step, the intermediate product is assayed and released if it meets the particular specification for that step. Once the extended or controlled-release coating is applied, the assay includes a step to insure that the desired dissolution rate is achieved. These coatings are relatively thin, and susceptible to changes in raw materials, temperature, humidity and other manufacturing process parameters. We have invested significant time and money to understand and manipulate drug release, and will continue to do so.

Forest is responsible for all manufacturing related to Namenda XR and MDX-8704. We have clinical supplies of ADS-5102 manufactured for us by a contract manufacturing organization under a development agreement and do not have a long-term contract in place. We are currently seeking to qualify an additional manufacturer to include in our anticipated NDA for ADS-5102. Contract manufacturers often encounter difficulties involving production yields, quality control and quality assurance, as well as shortages of qualified personnel. Qualifying manufacturers and providers of packaging services is a lengthy process; if at any time, one or more of our qualified contract organizations were not able to manufacture our drug substance or provide the requisite services, our business and financial condition would be materially adversely affected.
Our third-party manufacturers, their facilities and all lots of drug substance and drug products used in our clinical trials are required to be in compliance with current Good Manufacturing Practices, or cGMP. The cGMP regulations include requirements relating to organization of personnel, buildings and facilities, equipment, control of components and drug product containers and closures, production and process controls, packaging and labeling controls, holding and distribution, laboratory controls, records and reports, and returned or salvaged products. The manufacturing facilities for our products must meet cGMP requirements and FDA satisfaction before any product is approved and we can manufacture commercial products. Our third-party manufacturers are also subject to periodic inspections of facilities by the FDA and other authorities, including procedures and operations used in the testing and manufacture of our products to assess our compliance with applicable regulations.
84
Failure to comply with statutory and regulatory requirements subjects a manufacturer to possible legal or regulatory action, including warning letters, the seizure or recall of products, injunctions, consent decrees placing significant restrictions on or suspending manufacturing operations and civil and criminal penalties. These actions could have a material impact on the availability of our products.
Government regulation
The FDA and comparable regulatory agencies in state and local jurisdictions and in foreign countries impose substantial requirements upon the clinical development, manufacture and marketing of pharmaceutical products. These agencies and other federal, state and local entities regulate research and development activities and the testing, manufacture, quality control, safety, effectiveness, labeling, storage, recordkeeping, tracking, approval, import, export, advertising and promotion of our products.
The process required by the FDA before product candidates may be marketed in the United States generally involves the following:
- •
- nonclinical laboratory and animal tests including some that must be conducted in accordance with Good Laboratory
Practices;
- •
- submission of an IND, which must become effective before clinical trials may begin;
- •
- adequate and well-controlled human clinical trials to establish the safety and efficacy of the proposed drug candidate for
its intended use;
- •
- pre-approval inspection of manufacturing facilities and selected clinical investigators for their compliance with Good
Manufacturing Practices, or cGMP, and Good Clinical Practices; and
- •
- FDA approval of an NDA to permit commercial marketing for particular indications for use.
The testing and approval process requires substantial time, effort and financial resources. Prior to commencing the first clinical trial with a product candidate, we must submit an IND to the FDA. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, raises safety concerns or questions about the conduct of the clinical trial by imposing a clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. Submission of an IND may not result in FDA authorization to commence a clinical trial. A separate submission to the existing IND must be made for each successive clinical trial conducted during product development. Further, an independent institutional review board for each medical center proposing to conduct the clinical trial must review and approve the plan for any clinical trial and its informed consent form before the clinical trial commences at that center. Regulatory authorities or an institutional review board or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk. Some studies also include a data safety monitoring board, which receives special access to unblinded data during the clinical trial and may halt the clinical trial if it determines that there is an unacceptable safety risk for subjects or other grounds, such as no demonstration of efficacy.
In general, for purposes of NDA approval, human clinical trials are typically conducted in three sequential phases that may overlap.
- •
- Phase 1—Studies are initially conducted to test the product candidate for
safety, dosage tolerance, absorption, metabolism, distribution and excretion in healthy volunteers or patients.
- •
- Phase 2—Studies are conducted with groups of patients with a specified disease or condition to provide enough data to evaluate the preliminary efficacy, optimal dosages and dosing schedule and expanded evidence of safety. Multiple Phase 2 clinical trials may be conducted to obtain information prior to beginning larger and more expensive Phase 3 clinical trials.
85
- •
- Phase 3—These clinical trials are undertaken in larger patient populations to further evaluate dosage, to provide statistically significant evidence of clinical efficacy and to further test for safety in an expanded patient population at multiple clinical trial sites. These clinical trials are intended to establish the overall risk/benefit ratio of the product and provide an adequate basis for product labeling. These trials may be done globally to support global registrations.
Our product development strategy relies on using Phase 2/3 studies as a central element of our clinical development plans. Typically these studies involve the testing of two or more doses of a product candidate, as is characteristic of a Phase 2 study, and also include a sufficient number of patients so that statistically significant evidence of efficacy can be obtained, as is characteristic of a Phase 3 study. In addition, we conduct the studies in a manner that we believe is consistent with the requirements for a Phase 3 study. We believe this approach has the potential to significantly shorten the time frame required for clinical development. The FDA generally requires that sponsors successfully complete two Phase 3 studies to obtain approval for a new drug though in certain circumstances a single Phase 3 study is sufficient. We design and conduct our Phase 2/3 studies in a manner that is intended to allow the study to qualify as a Phase 3 study for the purposes of approval. The FDA has broad discretion in determining whether or not a completed Phase 2/3 study will be considered the equivalent of a Phase 3 study for the purposes of approval, and there can be no assurance that the FDA will agree with our assessment that the design, conduct and results of a Phase 2/3 study are such that the study should be treated as a Phase 3 study.
The FDA may require, or companies may pursue, additional clinical trials after a product is approved. These so-called Phase 4 studies may be made a condition to be satisfied after approval. The results of Phase 4 studies can confirm the effectiveness of a product candidate and can provide important safety information.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the product candidate as well as finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, must develop methods for testing the identity, strength, quality and purity of the final product. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
ANDA approval process
The Drug Price Competition and Patent Term Restoration Act of 1984, commonly known as the Hatch-Waxman Act, established abbreviated FDA approval procedures for drugs that are shown to be equivalent to proprietary drugs previously approved by the FDA through its NDA process. Approval to market and distribute these drugs is obtained by filing an abbreviated NDA, or ANDA, with the FDA. An ANDA is a comprehensive submission that contains, among other things, data and information pertaining to the active pharmaceutical ingredient, drug product formulation, specifications and stability of the generic drug, as well as analytical methods, manufacturing process validation data and quality control procedures. Premarket applications for generic drugs are termed abbreviated because they generally do not include preclinical and clinical data to demonstrate safety and effectiveness. Instead, a generic applicant must demonstrate that its product is bioequivalent to the innovator drug. In certain situations, an applicant may obtain ANDA approval of a generic product with a strength or dosage form that differs from a referenced innovator drug pursuant to the filing and approval of an ANDA suitability petition. The FDA will approve the generic product as suitable for an ANDA application if it finds that the generic product does not raise new questions of safety and effectiveness as compared to the innovator product. A product is not eligible for ANDA approval if the FDA determines that it is not equivalent to the referenced innovator drug, if it is intended for a different use, or if it is not
86
subject to an approved suitability petition. However, such a product might be approved under an NDA, with supportive data from clinical trials.
505(b)(2) approval process
Section 505(b)(2) of the FDCA provides an alternate regulatory pathway to FDA approval for new or improved formulations or new uses of previously approved drug products. Specifically, Section 505(b)(2) permits the filing of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. The applicant may rely upon the FDA's findings of safety and effectiveness for an approved product that acts as the Reference Listed Drug, or RLD. The FDA may also require 505(b)(2) applicants to perform additional studies or measurements to support the change from the RLD. The FDA may then approve the new product candidate for all or some of the labeled indications for which the referenced product has been approved, as well as for any new indication sought by the Section 505(b)(2) applicant.
Our current and anticipated product candidates based upon ADS-5102 are or will be based on already approved active pharmaceutical ingredients, or APIs, rather than new chemical entities, and a formulation that has been through Phase 1 studies. Accordingly, we expect to be able to rely on information from previously conducted studies involving our ADS-5102 formulation in our clinical development plans and our NDA submissions. For product candidates that involve novel fixed-dose combinations of existing drugs or for studies of an existing product or product candidate in a new indication, we expect that we will generally be able to initiate Phase 2/3 studies without conducting any new non-clinical or Phase 1 studies. In those instances where our product candidate is a pharmacokinetically enhanced version of an approved API, we will need to conduct certain non-clinical and Phase 1 studies to confirm the pharmacokinetic profile of the product candidate prior to conducting Phase 2/3 studies.
Orange Book listing
In seeking approval for a drug through an NDA, including a 505(b)(2) NDA, applicants are required to list with the FDA certain patents whose claims cover the applicant's product. Upon approval of an NDA, each of the patents listed in the application for the drug is then published in Approved Drug Products with Therapeutic Equivalence Evaluations, also known as the Orange Book. Any applicant who files an ANDA seeking approval of a generic equivalent version of a drug listed in the Orange Book or a 505(b)(2) NDA referencing a drug listed in the Orange Book must certify to the FDA that (1) no patent information on the drug product that is the subject of the application has been submitted to the FDA; (2) such patent has expired; (3) the date on which such patent expires; or (4) such patent is invalid or will not be infringed upon by the manufacture, use or sale of the drug product for which the application is submitted. This last certification is known as a Paragraph IV certification. If the competitor has provided a Paragraph IV certification to the FDA, the competitor must also send notice of the Paragraph IV certification to the holder of the NDA for the RLD and the patent owner once the application has been accepted for filing by the FDA. The NDA holder or patent owner may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days of the receipt of a Paragraph IV certification prevents the FDA from approving the application until the earlier of 30 months from the date of the lawsuit, expiration of the patent, settlement of the lawsuit or a decision in the infringement case that is favorable to the applicant. The applicant may also elect to submit a "Section VIII" statement certifying that its proposed label does not contain, or carves out, any language regarding the patented method-of-use rather than certify to a listed method-of-use patent. We and Forest have received notices of ANDAs submitted to the FDA requesting permission to manufacture and market generic versions of Namenda XR, and we, Forest, Forest Laboratories Holdings Ltd., Merz Pharma
87
GmbH & Co. KGaA and Merz Pharmaceuticals GmbH are currently in litigation with the notifying parties. For further information, see "—Legal proceedings."
NDA submission and review by the FDA
The results of product development, nonclinical studies and clinical trials are submitted to the FDA as part of an NDA. The submission of an NDA requires payment of a substantial user fee to the FDA. The FDA may convene an advisory committee to provide clinical insight on application review questions. The FDA reviews applications to determine, among other things, whether a product is safe and effective for its intended use and whether the manufacturing controls are adequate to assure and preserve the product's identity, strength, quality and purity. Before approving an NDA, the FDA will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Once the NDA submission has been accepted for filing, which occurs, if at all, within 60 days after submission of the NDA, the FDA typically takes ten months to review the application and respond to the applicant, which can take the form of either a Complete Response Letter or Approval. The review process is often significantly extended by FDA requests for additional information or clarification. The FDA may delay or refuse approval of an NDA if applicable regulatory criteria are not satisfied, require additional testing or information and/or require post-marketing testing and surveillance to monitor safety or efficacy of a product. FDA approval of any NDA submitted by us will be at a time the FDA chooses. Also, if regulatory approval of a product is granted, such approval may entail limitations on the indicated uses for which such product may be marketed. Once approved, the FDA may withdraw the product approval if compliance with pre- and post-marketing regulatory standards is not maintained or if problems occur after the product reaches the marketplace. In addition, the FDA may require Phase 4 post-marketing studies to monitor the effect of approved products, and may limit further marketing of the product based on the results of these post-marketing studies.
Post-approval requirements
Any products manufactured or distributed by us pursuant to FDA approvals are subject to continuing regulation by the FDA, including recordkeeping requirements and reporting of adverse experiences. Drug and biologic manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP, which impose certain procedural and documentation requirements upon us and our third-party manufacturers. We cannot be certain that we or our present or future suppliers will be able to comply with the cGMP regulations and other FDA regulatory requirements. If our present or future suppliers are not able to comply with these requirements, the FDA may halt our clinical trials, require us to recall a product from distribution, or withdraw approval of the NDA.
The FDA closely regulates the marketing and promotion of drugs. A company can make only those claims relating to safety and efficacy, purity and potency that are approved by the FDA. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties. Physicians may prescribe legally available products for uses that are not described in the product's labeling and that differ from those tested by us and approved by the FDA. Such off-label uses are common across medical specialties. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, restrict manufacturer's communications on the subject of off-label use.
88
Moreover, the recently enacted Drug Supply Chain Security Act imposes new obligations on manufacturers of pharmaceutical products related to product and tracking and tracing. Among the requirements of this new legislation, manufacturers will be required to provide certain information regarding the drug products to individuals and entities to which product ownership is transferred, label drug product with a product identifier, and keep certain records regarding the drug product. The transfer of information to subsequent product owners by manufacturers will eventually be required to be done electronically. Manufacturers will also be required to verify that purchasers of the manufacturers' products are appropriately licensed. Further, under this new legislation, manufactures will have drug product investigation, quarantine, disposition, and notification responsibilities related to counterfeit, diverted, stolen, and intentionally adulterated products, as well as products that are the subject of fraudulent transactions or which are otherwise unfit for distribution such that they would be reasonably likely to result in serious health consequences or death.
Other healthcare regulations
Our business activities, including but not limited to, research, sales, promotion, distribution, medical education and other activities following product approval will be subject to regulation by numerous regulatory and law enforcement authorities in the United States in addition to the FDA, including potentially the Department of Justice, the Department of Health and Human Services and its various divisions, including the Centers for Medicare and Medicaid Services, and state and local governments. Our business activities must comply with numerous healthcare laws, including but not limited to, the federal Anti-Kickback Statute, the False Claims Act, the Veterans Health Care Act and similar state laws.
The federal Anti-Kickback Statute prohibits, among other things, any person or entity, from knowingly and wilfully offering, paying, soliciting or receiving any remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any item or service reimbursable under Medicare, Medicaid or other federal healthcare programs. The term remuneration has been interpreted broadly to include anything of value. There are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution. The exceptions and safe harbors are drawn narrowly and practices that involve remuneration that may be alleged to be intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exception or safe harbor. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the Anti-Kickback Statute. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all of its facts and circumstances.
The federal False Claims Act prohibits, among other things, any person or entity from knowingly presenting, or causing to be presented, a false claim for payment to, or approval by, the federal government or knowingly making, using, or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government.
We, and our business activities, are subject to the civil monetary penalties statute which imposes penalties against any person or entity who, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
Additionally, the federal Physician Payments Sunshine Act within the Patient Protection and Affordable Care Act, or PPACA, and its implementing regulations, require certain manufacturers of drugs, devices, biological and medical supplies for which payment is available under Medicare, Medicaid or the Children's Health Insurance Program (with certain exceptions) to report information related to certain payments or other transfers of value made or distributed to physicians and teaching
89
hospitals, or to entities or individuals at the request of, or designated on behalf of, the physicians and teaching hospitals and to report annually certain ownership and investment interests held by physicians and their immediate family members.
In addition, we may be subject to data privacy and security regulation by both the federal government and the states in which we conduct our business. The Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and its implementing regulations, imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information. Among other things, HITECH makes HIPAA's privacy and security standards directly applicable to business associates—independent contractors or agents of covered entities that receive or obtain protected health information in connection with providing a service on behalf of a covered entity. HITECH also created four new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable business associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys' fees and costs associated with pursuing federal civil actions. In addition, state laws govern the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
The Veterans Health Care Act of 1992 requires manufacturers of "covered drugs" to offer those drugs for sale to certain federal agencies, including but not limited to, the Department of Veterans Affairs, on the Federal Supply Schedule, which requires compliance with applicable federal procurement laws.
Depending on the circumstances, failure to comply with these laws can result in penalties, including criminal, civil and/or administrative criminal penalties, damages, fines, disgorgement, exclusion of products from reimbursement under government programs, "qui tam" actions brought by individual whistleblowers in the name of the government, refusal to allow us to enter into supply contracts, including government contracts, reputational harm, diminished profits and future earnings and the curtailment or restructuring of our operations, any of which could adversely affect our business.
The United States and some foreign jurisdictions are considering or have enacted a number of legislative and regulatory proposals designed to change the healthcare system in ways that could affect our ability to sell our products profitably. Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives.
For example, in March 2010, the PPACA was passed, which has the potential to substantially change health care financing by both governmental and private insurers, and to significantly impact the U.S. pharmaceutical industry. The PPACA, among other things, revised the methodology by which rebates owed by manufacturers to the state and federal government for covered outpatient drugs under the Medicaid Drug Rebate Program are calculated, increased the minimum Medicaid rebates owed by most manufacturers under the Medicaid Drug Rebate Program, extended the Medicaid Drug Rebate program to utilization of prescriptions of individuals enrolled in Medicaid managed care organizations, subjected manufacturers to new annual fees and taxes for certain branded prescription drugs, and provided incentives to programs that increase the federal government's comparative effectiveness research.
90
The Foreign Corrupt Practices Act
The Foreign Corrupt Practices Act, or FCPA, prohibits any U.S. individual or business from paying, offering or authorizing payment or offering of anything of value, directly or indirectly, to any foreign official, political party or candidate for the purpose of influencing any act or decision of the foreign entity in order to assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the United States to comply with accounting provisions requiring the company to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations.
Federal laws providing for patent term extensions and data exclusivity
Provisions of various federal laws may allow a company to extend market exclusivity for a product beyond the expiration dates of the patents covering the product by either extending the term of the patents or limiting the right of a competitor to reference the company's data in a regulatory submission. These laws include the Hatch-Waxman Act and the Best Pharmaceuticals for Children Act of 2002. We do not anticipate materially benefiting from these provisions.
Foreign regulation
In addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products to the extent we choose to develop or sell any products outside of the United States. The approval process varies from country to country and the time may be longer or shorter than that required to obtain FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country.
Employees
As of December 31, 2013, we had 22 full-time employees. Of these employees, 12 are engaged in research and development. Our employees are not represented by labor unions or covered by collective bargaining agreements. We consider our relationship with our employees to be good.
Facilities
We lease approximately 7,700 square feet of office space in Emeryville, California under a lease that expires in February 2016. We believe that our existing facilities and other available properties will be sufficient for our needs for the foreseeable future.
Legal proceedings
As of March 25, 2014, we had received notice that ten companies had submitted ANDAs to the FDA requesting permission to manufacture and market generic versions of Namenda XR, on which we are entitled to receive royalties from Forest beginning in June 2018. In the notices, these companies allege that the patents associated with Namenda XR, some of which are owned by Forest or licensed by Forest from Merz Pharma GmbH & Co. KGaA and others of which are owned by us and licensed by us exclusively to Forest in the United States, are invalid, unenforceable or will not be infringed by the companies' manufacture, use or sale of generic versions of Namenda XR. In January and February 2014, we, Forest, Forest Laboratories Holdings Ltd., Merz Pharma GmbH & Co. KGaA and Merz Pharmaceuticals GmbH filed lawsuits in the U.S. District Court for the District of Delaware for infringement of the relevant patents against the eight of these companies that had then submitted ANDAs. We are seeking judgment that (i) the defendants have infringed the patents at issue, (ii) that the effective date of any approval of the defendants' ANDAs shall not be earlier than the expiration
91
date of the last to expire of the relevant patents, including any extensions or exclusivities, (iii) that the defendants be enjoined from commercially manufacturing, using, offering for sale, or selling in the United States, or importing into the United States any products that infringe or induce or contribute to the infringement of the patents at issue prior to the expiration date of the last to expire of the patents, including extensions and exclusivities, and (iv) that we, Forest, Forest Laboratories Holdings Ltd., Merz Pharma GmbH & Co. KGaA and Merz Pharmaceuticals GmbH be awarded monetary relief, in addition to any attorney's fees, costs and expenses relating to the actions. Because these lawsuits were filed within the requisite 45-day period provided in the FDCA, there are stays preventing FDA approval of the ANDAs for 30 months or until a court decision adverse to the patents. The 30-month stay for these ANDAs will begin to expire in June 2016.
92
Executive officers and directors
The following table sets forth information regarding our executive officers and directors as of December 31, 2013:
Name
|
Age | Position | ||
|---|---|---|---|---|
Gregory Went, Ph.D. |
50 | Co-Founder, Chief Executive Officer and Chairman of the board of directors | ||
Anthony Rimac |
50 | Chief Financial Officer | ||
Natalie McClure, Ph.D. |
61 | Senior Vice President, Product Development | ||
Michael Coffee |
67 | Senior Vice President, Strategy & Planning | ||
Jeffrey Knapp(8) |
48 | Chief Commercial Officer | ||
Richard Booth(1)(5) |
66 | Director | ||
Martha Demski(2)(6) |
61 | Director | ||
William Ericson(1)(3) |
55 | Director | ||
Sara Grootwassink Lewis(2)(3)(7) |
46 | Director | ||
Ivan Lieberburg, M.D., Ph.D. |
64 | Director | ||
John MacPhee, MPH(2) |
46 | Director | ||
David Mahoney(1)(3) |
59 | Director | ||
George Rehm(4) |
65 | Director |
- (1)
- Member
of the compensation committee
- (2)
- Member
of the audit committee
- (3)
- Member
of the nominating and corporate governance committee
- (4)
- Mr. Rehm
has resigned from our board of directors as of the date of this prospectus.
- (5)
- Mr. Booth
was elected to our board of directors on January 28, 2014.
- (6)
- Ms. Demski
was elected to our board of directors on March 4, 2014.
- (7)
- Ms. Grootwassink
Lewis was elected to our board of directors on March 4, 2014.
- (8)
- Mr. Knapp joined us on February 24, 2014 as our Chief Commercial Officer.
Gregory Went, Ph.D. Dr. Went has served as our Chief Executive Officer and Chairman of our board of directors since our inception in 2000. Previously, Dr. Went co-founded CuraGen Corporation in 1992, where he served as an Executive Vice President and Director from 1996 to 1999. Dr. Went also has served as a Director of Angelica Therapeutics, Inc., a biotechnology company, since 2006. Dr. Went holds a Ph.D. in Chemical Engineering from the University of California, Berkeley and a B.S. in Chemical Engineering from Carnegie Mellon University. We believe Dr. Went's extensive knowledge of our company, the pharmaceutical industry and our competitors qualifies him to serve on our board of directors.
Anthony Rimac. Mr. Rimac has served as our Chief Financial Officer since July 2011. From 2005 to 2011, Mr. Rimac served as Chief Financial Officer of Aerovance, Inc., a biopharmaceutical company. From 2001 to 2005, Mr. Rimac served as Vice President—Finance at Artemis Medical, Inc., a medical device company that was sold to Johnson & Johnson in 2004. Mr. Rimac holds a B.A. in Business Economics from the University of California, Santa Barbara, and an M.B.A. from Santa Clara University.
Natalie McClure, Ph.D. Dr. McClure has served as our Senior Vice President, Product Development since 2013 and served as our Vice President, Regulatory Affairs from 2009 to 2013. From 2008 through February 2011, Dr. McClure served as a consultant for DevRx Consulting, which
93
specializes in pharmaceutical development consulting. From 2005 to 2008, Dr. McClure served as VP, Regulatory Affairs of Cerimon Pharmaceuticals, Inc., a biopharmaceutical company. Dr. McClure holds a Ph.D. in Organic Chemistry from Stanford University and a B.S. in Chemistry from the University of Michigan.
Michael Coffee. Mr. Coffee has served as our Senior Vice President, Strategy & Planning since November 2013 and served as our Senior Vice President Sales & Marketing from May 2009 to February 2010. From June 2010 to July 2013, Mr. Coffee served as Chief Business Officer of MediciNova, Inc., a pharmaceutical company. From February 2005 to March 2009, Mr. Coffee served as the Chief Business Officer of Avigen, Inc., a pharmaceutical company. Mr. Coffee holds a B.S. in Biology and Chemistry from Siena College and is a graduate of the advanced management program at Amos Tuck School of Business.
Jeffrey H. Knapp. Mr. Knapp has served as our Chief Commercial Officer since February 2014. From July 2006 to March 2013, Mr. Knapp served as Chief Commercial Officer of Affymax, Inc., a biopharmaceutical company. From November 2005 to April 2006, Mr. Knapp served as Senior Vice President, Sales and Marketing at Abgenix, Inc., a biopharmaceutical company. From October 2004 to July 2005, Mr. Knapp served as Vice President, Sales and Marketing, North America at Pharmion Corporation, a pharmaceutical company. From November 2001 to October 2004, Mr. Knapp served as Vice President, U.S. sales and marketing at EMD Pharmaceuticals, a division of Merck KGaA, a pharmaceutical company. He has also held sales, marketing and business development positions at Eli Lilly and Company and Schering-Plough Corporation, both pharmaceutical companies. Mr. Knapp holds a B.A. from Wittenberg University.
Richard Booth. Mr. Booth has served as a member of our board of directors since January 2014. Mr. Booth serves on the board of directors of The Hanover Insurance Group, Inc., a property and casualty insurance company, and Sun Life Financial Inc., an insurance and financial services company. He is also a trustee of Northeast Utilities, a utility company, and serves on the boards of directors of several privately-held organizations. Since July 2009, Mr. Booth has served as the Vice Chairman of Guy Carpenter & Company, LLC, a global risk management and reinsurance specialist and a wholly owned subsidiary of Marsh & McLennan Companies, Inc. From June 2008 to March 2009, Mr. Booth served as a corporate officer, and from October 2008 to March 2009, as Vice Chairman, Transition Planning and Chief Administrative Officer, of American International Group, Inc., an insurance and financial services company. From 2000 to 2009, Mr. Booth served as Chairman of HSB Group, Inc., a specialty insurer and reinsurer, also serving as its President and Chief Executive Officer from 2000 to 2007. Mr. Booth is a senior adviser to Century Capital Management. From 2004 to 2008, Mr. Booth was a member of the Financial Accounting Standards Advisory Council. Mr. Booth is a member of the American Institute of Certified Public Accountants. Mr. Booth received B.S. and M.S. degrees from the University of Hartford. We believe Mr. Booth's extensive experience in business and management, including, in particular, strategic planning, capital and financial markets, accounting and financial reporting, qualifies him to serve on our board of directors.
Martha Demski. Ms. Demski has served as a member of our board of directors since March 2014. Since April 2013, Ms. Demski has served as Senior Vice President, Chief Financial Officer and Corporate Secretary of Ajinomoto Althea, Inc., a fully-integrated contract development and manufacturing organization. From August 2011 to April 2013, Ms. Demski served as Senior Vice President and Chief Financial Officer of Althea Technologies, Inc. From July 2008 to December 2010, Ms. Demski served as the Interim Chief Operating Officer and Chief Financial Officer of the Sidney Kimmel Cancer Center (SKCC), a non-profit corporation engaged in biomedical research, which voluntarily filed for Chapter 11 bankruptcy in 2009. From April 2006 to May 2008, Ms. Demski served as Senior Vice President of U.S. Trust. Ms. Demski currently serves as a member of the board of directors of Chimerix, Inc., a publicly traded biopharmaceutical company, where she serves as chair of
94
the audit committee. From 2005 to July 2008, Ms. Demski served on the Board of Trustees at SKCC, as well as Chair of the Audit Committee and Chair of the Governance and Nominating Committee. From December 1988 to June 2004, Ms. Demski served as Vice President, Chief Financial Officer, Treasurer and Secretary of Vical Incorporated, a publicly traded biopharmaceutical company. Ms. Demski holds a B.A. from Michigan State University and M.B.A. from The University of Chicago Booth School of Business. We believe that Ms. Demski's more than 30 years' experience in the fields of finance and biotechnology as well her experience as a chief financial officer of a publicly traded company and her experience in conducting financing transactions qualifies her to serve on our board of directors.
William Ericson. Mr. Ericson has served as a member of our board of directors since 2005. Mr. Ericson has been a General Partner at Mohr Davidow Ventures LP, or MDV, a venture capital firm, since 2000, and has served as Managing Partner since 2008. Prior to joining MDV, Mr. Ericson founded and operated Venture Law Group LLP's Seattle office from 1996 to 2000. Mr. Ericson currently serves as a member of the board of directors of Pacific Biosciences of California, Inc., a publicly traded gene sequencing company, Northwestern University School of Law and a number of MDV's privately held portfolio companies. Mr. Ericson holds a B.S.F.S. from the School of Foreign Service at Georgetown University and a J.D. from Northwestern University School of Law. We believe Mr. Ericson's experience with companies in the life sciences industry qualifies him to serve on our board of directors.
Sara Grootwassink Lewis. Ms. Grootwassink Lewis has served as a member of our board of directors since March 2014. She is a private investor and Chief Executive Officer of Lewis Corporate Advisors, LLC, a capital markets and board advisory firm. From May 2002 to February 2009, Ms. Grootwassink Lewis served as the Chief Financial Officer of Washington Real Estate Investment Trust, and from December 2001 to May 2002 she served as Managing Director, Finance and Capital Markets. Ms. Grootwassink Lewis currently serves as a member of the board of directors of CapitalSource Inc., a publicly traded commercial finance company, where she serves as the chairman of the audit committee, chairman of the nominating and corporate governance committee and a member of the compensation committee. Ms. Grootwassink Lewis currently serves on the board of directors and serves as the chairman of the audit committee and serves on the nominating/corporate governance committee of PS Business Parks, Inc., a publicly traded owner, operator and developer of commercial properties. Ms. Grootwassink Lewis currently serves as a member of the board of directors and on the audit committee of Plum Creek Timber Company, Inc., a publicly traded company and one of the largest landowners in the nation. Ms. Grootwassink Lewis holds a B.S. in Finance from the University of Illinois, Urbana-Champaign. We believe that Ms. Grootwassink Lewis' experience as a Chief Financial Officer of a publicly traded company, qualification as a chartered financial analyst, extensive experience in corporate finance and strong strategic planning and accounting skills qualifies her to serve on our board of directors.
Ivan Lieberburg, M.D., Ph.D. Dr. Lieberburg has served as a member of our board of directors since 2004. Dr. Lieberburg has been a member of the Tavistock Group, a private equity firm, since 2009 where he concentrates on health care and life sciences investment opportunities. From 1987 to 2009, Dr. Lieberburg was employed by Elan Pharmaceuticals, Inc. (formerly Athena Neurosciences, Inc.) where his most recent roles were as Executive Vice President, Corporate Office of Technology and Chief Medical Officer. Dr. Lieberburg holds an A.B. in Biology from Cornell University, a Ph.D. in Neurobiology from The Rockefeller University and an M.D. from the University of Miami Leonard M. Miller School of Medicine. Dr. Lieberburg is board certified in internal medicine and endocrinology/metabolism. We believe Dr. Lieberburg's executive experience in the life sciences industry and his medical training qualifies him to serve on our board of directors.
John MacPhee, MPH. Mr. MacPhee has served as a member of our board of directors since May 2013 and provided consulting services to us from March 2011 to May 2013. Since 2011, Mr. MacPhee
95
has served as the Executive Director and CEO of The Jed Foundation, a non-profit organization. From 2005 to 2011, Mr. MacPhee served as Executive Vice President of Par Pharmaceutical, Inc. and President of Par's Strativa Pharmaceuticals division, where he oversaw commercial operations, clinical development, medical affairs, alliance management and business development. Previously, Mr. MacPhee worked at Forest Laboratories, Inc., where he led the launches of Celexa, Lexapro and Namenda. Mr. MacPhee also serves as a board member for Bottom Line, a nonprofit organization. Mr. MacPhee holds a B.A. from Columbia College, an M.B.A. from New York University and an MPH from Columbia University. We believe Mr. MacPhee's extensive experience building successful specialty pharmaceutical companies and commercializing drug products qualifies him to serve on our board of directors.
David Mahoney. Mr. Mahoney has served as a member of our board of directors since 2009. Mr. Mahoney has served on the board of directors of Symantec Corporation, a publicly-traded software technology company since 2003, including as a member of the Compensation and Nominating and Governance Committees. Mr. Mahoney also served as a member of the Audit Committee of Symantec from 2003 to 2011. Mr. Mahoney has served on the board of directors of Corcept Therapeutics Incorporated, a pharmaceutical company, since 2011. He also serves on the boards of directors of several privately-held organizations including San Francisco Museum of Modern Art and Mercy Corps and is a Trustee of the Schwab/Landis Family of Funds. Mr. Mahoney also serves on the board of directors of Northern California Public Broadcasting, Inc., a non-profit public television and radio operator. From 1999 to 2001, Mr. Mahoney served as co-CEO of McKesson HBOC, Inc., a healthcare supply management and information technology company and as CEO of McKesson LLC, a healthcare management and connectivity company. He joined McKesson Corporation in 1990 as Vice President for Strategic Planning. Prior to joining McKesson, Mr. Mahoney was a principal with McKinsey & Company, a management consulting firm, where he worked from 1981 to 1990. Mr. Mahoney holds a B.A. from Princeton University and an M.B.A. from Harvard University. We believe Mr. Mahoney's extensive experience in pharmaceutical distribution, fiscal management and in operating and advising technology companies qualifies him to serve on our board of directors.
George Rehm. Mr. Rehm has served as a member of our board of directors since 2008. Mr. Rehm has served as Managing Partner of aeris CAPITAL AG in Switzerland since 2006 where he holds board seats in numerous portfolio companies. Mr. Rehm remains of counsel to the Munich, Heidelberg and Berlin law firm of Weitnauer Partners. Mr. Rehm holds a B.S.F.S. from Georgetown University and a J.D. from the University of California, Hastings College of Law. We believe Mr. Rehm's extensive experience in fiscal management and in operating and advising in the IT, semiconductor, health care and medical device industries qualifies him to serve on our board of directors.
Each of our executive officers serves at the discretion of our board of directors and holds office until his or her successor is duly elected and qualified or until his or her earlier resignation or removal. There are no family relationships among any of our directors or executive officers.
Board composition
Our business and affairs are managed under the direction of our board of directors, which currently consists of 9 members. The members of our board of directors were elected in compliance with the provisions of our amended and restated certificate of incorporation, as amended, and a voting agreement among certain of our stockholders, as amended. The voting agreement will terminate upon the closing of this offering and none of our stockholders will have any special rights regarding the election or designation of members of our board of directors.
Our board of directors will consist of 8 members upon the closing of this offering. In accordance with our amended and restated certificate of incorporation to be filed in connection with this offering, immediately after this offering, our board of directors will be divided into three classes with staggered
96
three-year terms. At each annual general meeting of stockholders, the successors to directors whose terms then expire will be elected to serve from the time of election and qualification until the third annual meeting following election. Our directors will be divided among the three classes as follows:
- •
- The Class I directors will be William Ericson, Martha Demski and Ivan Lieberburg, and their terms will expire at
our annual meeting of stockholders to be held in 2015;
- •
- The Class II directors will be Gregory Went, Sara Grootwassink Lewis and Richard Booth, and their terms will expire
at our annual meeting of stockholders to be held in 2016; and
- •
- The Class III directors will be David Mahoney and John MacPhee, and their terms will expire at our annual meeting of stockholders to be held in 2017.
We expect that additional directorships resulting from an increase in the number of directors will be distributed among the three classes so that, as nearly as possible, each class will consist of one-third of the directors. The division of our board of directors into three classes with staggered three-year terms may delay or prevent a change of our management or a change in control.
Director independence
Under the listing requirements and rules of The NASDAQ Global Market, independent directors must comprise a majority of a listed company's board of directors within a specified period of time after this offering.
Our board of directors has undertaken a review of its composition, the composition of its committees, and the independence of each director. Based upon information requested from and provided by each director concerning his or her background, employment, and affiliations, including family relationships, our board of directors has determined that all of our board of directors except Dr. Went do not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each of these directors is "independent" as that term is defined under the applicable rules and regulations of the SEC, and the listing requirements and rules of The NASDAQ Global Market. In making this determination, our board of directors considered the current and prior relationships that each non-employee director has with our company and all other facts and circumstances our board of directors deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director.
Board leadership structure
Our board of directors is currently led by a combined chairman of the board and chief executive officer. Our board of directors believes that this leadership structure is the most effective for us at this time. Because our chief executive officer is closest to the many facets of our business, our board of directors believes that the chief executive officer is in the best position to lead our board of directors most effectively and, accordingly, to serve in the critical role of chairman of the board. In addition, as the chief executive officer is directly involved in managing the company, having a chairman who also serves as chief executive officer facilitates timely communication with the board on critical business matters. Furthermore, we believe that this combined leadership structure is appropriate for our company because (i) our chairman and chief executive officer conveys a singular, cohesive message to our stockholders, employees, industry partners and the investment community and (ii) this structure eliminates any ambiguity as to who is accountable for the company's performance. Our directors and management team engage frequently and directly in the flow of information and ideas and we believe our combined leadership structure facilitates the quality, quantity and timeliness of the information flow and communication. Our board of directors believes that there is a well-functioning and effective balance between strong company leadership and oversight by active, independent directors.
97
Lead independent director
Our board of directors has appointed Mr. Mahoney to serve as our lead independent director. As lead independent director, Mr. Mahoney presides over periodic meetings of our independent directors, serves as a liaison between our Chairman and the independent directors and performs such additional duties as our board of directors may otherwise determine and delegate.
Board committees
Our board of directors has established an audit committee, a compensation committee and a nominating and corporate governance committee. Our board of directors may establish other committees to facilitate the management of our business. The composition and functions of each committee are described below. Members serve on these committees until their resignation or until otherwise determined by our board of directors.
Audit committee
Our audit committee consists of Ms. Demski, Ms. Grootwassink Lewis and Mr. MacPhee. Our board of directors has determined that Ms. Demski, Ms. Grootwassink Lewis and Mr. MacPhee are independent under the NASDAQ listing standards and Rule 10A-3(b)(1) of the Securities Exchange Act of 1934, as amended, or Exchange Act. The chair of our audit committee is Ms. Demski. Our board of directors has determined that each of Ms. Demski and Ms. Grootwassink Lewis is an "audit committee financial expert" within the meaning of the SEC regulations. Our board of directors has also determined that each member of our audit committee can read and understand fundamental financial statements in accordance with applicable requirements. In arriving at these determinations, the board of directors has examined each audit committee member's scope of experience and the nature of their employment in the corporate finance sector. The functions of this committee include:
- •
- selecting a qualified firm to serve as the independent registered public accounting firm to audit our financial
statements;
- •
- helping to ensure the independence and performance of the independent registered public accounting firm;
- •
- discussing the scope and results of the audit with the independent registered public accounting firm, and reviewing, with
management and the independent accountants, our interim and year-end operating results;
- •
- developing procedures for employees to submit concerns anonymously about questionable accounting or audit matters;
- •
- reviewing our policies on risk assessment and risk management;
- •
- reviewing related party transactions;
- •
- obtaining and reviewing a report by the independent registered public accounting firm at least annually, that describes
our internal quality-control procedures, any material issues with such procedures, and any steps taken to deal with such issues when required by applicable law; and
- •
- approving (or, as permitted, pre-approving) all audit and all permissible non-audit services, other than de minimis non-audit services, to be performed by the independent registered public accounting firm.
Compensation committee
Our compensation committee consists of Mr. Mahoney, Mr. Ericson and Mr. Booth. Our board of directors has determined that each of Mr. Mahoney, Mr. Ericson and Mr. Booth is independent under
98
the NASDAQ listing standards, is a "non-employee director" as defined in Rule 16b-3 promulgated under the Exchange Act and is an "outside director" as that term is defined in Section 162(m) of the U.S. Internal Revenue Code of 1986, as amended, or Section 162(m). The chair of our compensation committee is Mr. Booth. The functions of this committee include:
- •
- reviewing and approving, or recommending that our board of directors approve, the compensation of our executive officers;
- •
- reviewing and recommending, at least every two years and after consultation with our nominating and corporate governance
committee, to our board of directors the compensation of our directors;
- •
- reviewing and approving, or recommending that our board of directors approve, the terms of compensatory arrangements with
our executive officers;
- •
- administering our stock and equity incentive plans;
- •
- selecting independent compensation consultants and assessing conflict of interest compensation advisers;
- •
- reviewing with our chief executive officer the plans for succession of our executive officers and to make recommendations
to our board of directors with respect to the selection of appropriate individuals to succeed such positions;
- •
- reviewing and approving, or recommending that our board of directors approve, incentive compensation and equity plans; and
- •
- reviewing and establishing general policies relating to compensation and benefits of our employees and reviewing our overall compensation philosophy.
Nominating and corporate governance committee
Our nominating and corporate governance committee consists of Mr. Mahoney, Mr. Ericson and Ms. Grootwassink Lewis. Our board of directors has determined that each of Mr. Mahoney, Mr. Ericson and Ms. Grootwassink Lewis are independent under the NASDAQ listing standards. The chair of our nominating and corporate governance committee is Mr. Mahoney. The functions of this committee include:
- •
- identifying, evaluating and selecting, or recommending that our board of directors approve, nominees for election to our
board of directors and its committees;
- •
- evaluating the performance of our board of directors and of individual directors;
- •
- considering and making recommendations to our board of directors regarding the composition of our board of directors and
its committees;
- •
- reviewing developments in corporate governance practices;
- •
- evaluating the adequacy of our corporate governance practices and reporting;
- •
- reviewing management succession plans;
- •
- developing and making recommendations to our board of directors regarding corporate governance guidelines and matters and
a code of business conduct and ethics; and
- •
- overseeing an annual evaluation of the board of directors' performance.
99
Role of the board in risk oversight
The audit committee of the board of directors is primarily responsible for overseeing our risk management processes on behalf of the board of directors. Going forward, we expect that the audit committee will receive reports from management at least quarterly regarding our assessment of risks. In addition, the audit committee reports regularly to the board of directors, which also considers our risk profile. The audit committee and the board of directors focus on the most significant risks we face and our general risk management strategies. While the board of directors oversees our risk management, management is responsible for day-to-day risk management processes. Our board of directors expects management to consider risk and risk management in each business decision, to proactively develop and monitor risk management strategies and processes for day-to-day activities and to effectively implement risk management strategies adopted by the audit committee and the board of directors. We believe this division of responsibilities is the most effective approach for addressing the risks we face and that our board of directors leadership structure, which also emphasizes the independence of the board of directors in its oversight of its business and affairs, supports this approach.
Code of business conduct and ethics
Effective upon the closing of this offering, we have adopted a code of business conduct and ethics that applies to all of our employees, officers and directors, including those officers responsible for financial reporting. Following the closing of this offering, the code of business conduct and ethics will be available on our website at www.adamaspharma.com. We intend to disclose any amendments to the code, or any waivers of its requirements, on our website to the extent required by the applicable rules and exchange requirements. The inclusion of our website address in this prospectus does not incorporate by reference the information on or accessible through our website into this prospectus.
Compensation committee interlocks and insider participation
None of the members of our compensation committee has ever been an officer or employee of the company. None of our executive officers serve, or have served during the last fiscal year, as a member of the board of directors, compensation committee or other board committee performing equivalent functions of any entity that has one or more executive officers serving as one of our directors or on our compensation committee.
Director compensation
We currently provide cash compensation to certain of our non-employee directors. We have a policy of reimbursing our directors for their reasonable out-of-pocket expenses in connection with attending board of directors and committee meetings. From time to time, we have granted stock options to certain of our non-employee directors as compensation for their services. In addition, Mr. MacPhee and Mr. Mahoney are paid $24,000 per year for their service on our board of directors and Dr. Lieberburg is paid $6,000 per year for his service on our board of directors. Mr. Went, who is also an employee, is compensated for his service as an employee and does not receive any additional compensation for his service on our board of directors.
The following table sets forth information regarding compensation earned by our non-employee directors during the fiscal year ended December 31, 2013. Mr. Booth, Ms. Demski and
100
Ms. Grootwassink Lewis did not serve on our board of directors in 2013 and are therefore not included in the table below.
Name
|
Cash Compensation ($) |
Option Awards ($)(1) |
All Other Compensation ($) |
Total ($) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
William Ericson |
— | — | — | — | |||||||||
Ivan Lieberburg, M.D., Ph.D. |
6,000 | — | — | 6,000 | |||||||||
George Rehm |
— | — | — | — | |||||||||
David Mahoney |
24,000 | — | — | 24,000 | |||||||||
John MacPhee, MPH |
14,571 | 196,305 | 15,694 | (2) | 226,570 | ||||||||
- (1)
- The
amounts in this column reflect the aggregate grant date fair value of each option award granted during the fiscal year, computed in accordance with FASB
ASC Topic 718. The valuation assumptions used in determining such amounts are described in Note 12 to our financial statements included in this prospectus. The table below lists the aggregate
number of shares and additional information with respect to the outstanding option awards held by each of our non-employee directors.
- (2)
- Represents cash compensation paid to Mr. MacPhee for consulting services from January 2013 to May 2013.
Name
|
Date of Grant |
Number of Shares Underlying Option |
Exercise Price |
Option Expiration Date |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
William Ericson |
— | — | — | — | |||||||||
Ivan Lieberburg, M.D., Ph.D. |
— | — | — | — | |||||||||
George Rehm |
— | — | — | — | |||||||||
David Mahoney |
— | — | — | — | |||||||||
John MacPhee, MPH |
12/13/2013 | 24,000 | $ | 3.305 | 12/12/2023 | ||||||||
Compensation policy for non-employee directors
Our board of directors has adopted a compensation policy for our non-employee directors, to be effective upon the closing of this offering. Under this policy, each of our non-employee directors will be paid $30,000 as an annual cash retainer, and our lead independent director will receive an additional annual cash payment of $15,000. Annual fees will also be paid to our directors who chair our board committees and to committee members, as follows:
Committee
|
Annual Chair Fee | Annual Member Fee | |||||
|---|---|---|---|---|---|---|---|
Audit Committee |
$ | 15,000 | $ | 7,500 | |||
Compensation Committee |
$ | 10,000 | $ | 5,000 | |||
Nominating and Governance Committee |
$ | 7,000 | $ | 3,500 | |||
Our non-employee directors will also receive an initial equity award upon commencement of service as a board member; thereafter, each non-employee director will receive an annual equity retainer. Currently, the form of award in each case will be a non-qualified stock option. The initial award will be an option to purchase 30,000 shares of our common stock that will vest annually over three years of service. Each subsequent annual award will be an option to purchase 15,000 shares of our common stock that will vest after one year of service. Additionally, upon the closing of a change of control, the vesting of all outstanding equity awards held by our non-employee directors will accelerate in full.
101
Summary compensation table
The following table provides information regarding the compensation of our principal executive officer and each of our two other most highly compensated executive officers during the fiscal year ended December 31, 2013. We refer to these executive officers in this prospectus as our named executive officers.
Name and Principal Position
|
Year | Salary ($) |
Bonus ($) |
Option Awards ($)(1) |
All Other Compensation ($)(2) |
Total ($) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Gregory Went, Ph.D. |
2013 | 400,000 | 328,000 | — | — | 728,000 | |||||||||||||
Anthony Rimac |
2013 |
275,000 |
147,906 |
— |
965 |
423,871 |
|||||||||||||
Natalie McClure, Ph.D. |
2013 |
325,000 |
236,453 |
410,250 |
15 |
971,718 |
|||||||||||||
- (1)
- The
amounts in this column reflect the aggregate grant date fair value of each option award granted during the fiscal year, computed in accordance with ASC
Topic 718. The valuation assumptions used in determining such amounts are described in Note 12 to our financial statements included in this prospectus.
- (2)
- The amounts in this column reflect the net premiums we paid on behalf of the employee for life insurance, short-term and long-term disability insurance.
Outstanding equity awards at fiscal year-end
The following table provides information regarding outstanding equity awards held by our named executive officers as of December 31, 2013.
| |
Option Awards | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Number of Securities Underlying Unexercised Options(1) |
|
|
||||||||||
| |
Option Exercise Price |
Option Expiration Date |
|||||||||||
Name
|
Exercisable | Unexercisable | |||||||||||
Gregory Went, Ph.D. |
250,000 | (2) | — | $ | 1.875 | 9/11/2016 | |||||||
|
250,000 | (3) | — | $ | 0.665 | 11/15/2021 | |||||||
|
60,000 | (4) | — | $ | 0.665 | 2/21/2022 | |||||||
Anthony Rimac |
200,000 |
(3) |
— |
$ |
0.665 |
11/15/2021 |
|||||||
|
60,000 | (4) | — | $ | 0.665 | 2/21/2022 | |||||||
Natalie McClure, Ph.D. |
46,666 |
(5) |
— |
$ |
1.755 |
3/2/2020 |
|||||||
|
40,000 | (3) | — | $ | 0.665 | 11/15/2021 | |||||||
|
60,000 | (4) | — | $ | 0.665 | 2/21/2022 | |||||||
|
50,000 | (6) | — | $ | 3.305 | 12/23/2023 | |||||||
- (1)
- The
options listed are fully vested or are subject to an early exercise right and may be exercised in full prior to vesting of the shares underlying such
options. Vesting of all options is subject to continued service on the applicable vesting date.
- (2)
- The shares subject to this option are fully vested.
102
- (3)
- 50%
of the shares subject to this option were vested as of December 31, 2013 and the remainder vest in approximately equal increments on a monthly
basis thereafter through July 1, 2016.
- (4)
- 36.67%
of the shares subject to this option were vested as of December 31, 2013 and the remainder vest in approximately equal increments on a monthly
basis thereafter through March 1, 2017.
- (5)
- 78.33%
of the shares subject to this option were vested as of December 31, 2013 and the remainder vest in approximately equal increments on a monthly
basis thereafter through February 1, 2015.
- (6)
- None of the shares subject to this option were vested as of December 31, 2013. All shares will vest according to the following schedule, provided that the optionee remains in continuous service on each such date: the first 20% of the option shares shall vest upon the completion of twelve months of service measured from the vesting commencement date of December 23, 2013 and (ii) an additional 1/60th of the option shares shall vest upon the optionee's completion of each of the next forty-eight months of service thereafter on the first day of each month. This option will be fully vested on January 1, 2019.
Employment agreements, Executive Severance Plan and Transaction Bonus Plan
Offer letters
We currently do not have employment agreements with any of our executive officers. All of our executive officers are employed on an "at will" basis, with no fixed term of employment, pursuant to the terms of their respective offer letters, each of which is described below. Each offer letter also contains standard terms related to vacation and participation in our employee benefit plans, and in addition, requires execution of our form of confidential information and proprietary information agreement.
Gregory Went. Dr. Went is a party to an offer letter dated with us dated March 8, 2006. These terms superseded in their entirety the terms of an employment agreement originally entered into between Dr. Went and us in 2002, and subsequently amended. Pursuant to the offer letter, Dr. Went agreed to continue to serve as the company's chief executive officer, at a then-agreed base salary of $250,000, which has been subsequently increased to $400,000. In connection with entering into the offer letter, Dr. Went was granted an option to purchase 250,000 shares of common stock with an exercise price of $1.875 per share and is fully vested as of 2007. In addition, pursuant to the offer letter Dr. Went was eligible to receive an additional option to purchase 300,000 shares of common stock following our attainment of a designated sales milestone related to certain of our commercial products which milestone was not achieved.
At a meeting of the board of directors held in May 2013, the board adopted a bonus plan to provide for the payment of annual cash awards for the achievement of certain corporate and individual objectives. Under the bonus plan, target bonus levels were set at 50% of base salary for the CEO.
Anthony Rimac. Mr. Rimac is a party to an offer letter with us dated June 8, 2011 to serve as our Chief Financial Officer. Pursuant to the offer letter, we agreed to pay Mr. Rimac an annual base salary of $275,000, $25,000 of which was agreed to be deferred until the closing of a financing with proceeds to us in excess of $2 million subsequent to the closing of our 2011 Series AA preferred stock financing, which subsequent financing occurred in 2012. In addition, the offer letter provided that Mr. Rimac would be eligible for additional stock and cash bonus awards based on achievement of milestones to be determined in the future. Based upon the recommendation of Dr. Went, our board subsequently approved an annual bonus target for Mr. Rimac at 35% of his annual base salary.
Pursuant to the offer letter, Mr. Rimac was granted an option to purchase 200,000 shares of our common stock with an exercise price of $0.665 per share. On the first anniversary of Mr. Rimac's
103
employment by us, 20% of the shares subject to this option vested, and the remainder of the shares subject to the option vest thereafter as to 1/48th of the award per month of Mr. Rimac's service with us.
Natalie McClure. Dr. McClure is a party to an offer letter with us dated December 17, 2009 to serve as our Vice President, Regulatory Affairs. By letter of February 18, 2011, Dr. McClure agreed to assume a full-time work schedule at an annual base salary of $325,000. Pursuant to her 2009 offer letter, in March of 2010 Dr. McClure was granted an option to purchase 46,666 shares of common stock at an exercise price of $1.755 per share, with vesting to occur as to 20% of the option on the first anniversary of her employment commencement date, with the remainder of the option to vest thereafter as to 1/48th of the award per month. Based upon the recommendation of Dr. Went, our board subsequently approved an annual bonus target for Dr. McClure at 25% of her annual base salary.
Michael Coffee. Mr. Coffee is a party to an offer letter with us dated November 27, 2013 to serve as our Senior Vice President, Strategy and Planning. Previously, Mr. Coffee served as a consultant to us under a consulting agreement originally entered into in February 2010, which terminated upon his becoming an employee with us. Pursuant to the offer letter, we agreed to pay Mr. Coffee an annual base salary of $265,000, with an initial target bonus of 25% of that base salary based on achievement of milestones to be determined in the future. Pursuant to the offer letter Mr. Coffee was granted an option to purchase 110,000 shares of common stock at an exercise price of $3.305 per share, with vesting to occur as to 20% of the option on the first anniversary of his employment commencement date, with the remainder of the option to vest thereafter as to 1/48th of the award per month.
Jeffrey Knapp. Mr. Knapp is party to an offer letter with us dated February 24, 2014 to serve as our Chief Commercial Officer. Pursuant to the offer letter, we agreed to pay Mr. Knapp an annual base salary of $340,000, with an initial target bonus of 30% of that base salary based on achievement of milestones to be determined in the future. In addition, we granted to Mr. Knapp an option to purchase 260,000 shares of our common stock at an exercise price of $11.225 per share. The Option will vest as to 20% of the shares underlying the option on the first anniversary of Mr. Knapp's employment commencement date, with the remainder to vest thereafter as to 1/48th of the balance per month.
Executive Severance Plan
Our board of directors has adopted our Executive Severance Plan to be effective upon the closing of this offering, to provide our executives with severance benefits in the event their employment with us terminates without cause or, in case of a termination of employment that occurs in connection with our undergoing a change in control, either without cause or for for good reason. To be considered to have occurred in connection with our undergoing a change in control, the termination (or resignation) must occur at or within 12 months after our consummating the change in control.
To be eligible for benefits under our Executive Severance Plan, the executive must hold the title of vice president or above on or within 90 days before the date of either the termination or the date of a change in control.
Benefits under the Executive Severance Plan include both a cash portion, determined by reference to monthly base salary, and eligibility for group medical coverage (COBRA). Additionally, if the triggering termination or resignation occurs in connection with a change in control, the cash portion of the severance is determined by reference to both base pay and target bonus, the executive's equity awards that were outstanding at the time of the change in control will become fully vested and the post-termination period to exercise options will be extended up to the earlier of 12 months after the termination or the expiration date of the option.
The amount and timing of cash severance payments and the duration of COBRA coverage are determined by the executive's title, length of service and whether the termination is in connection with
104
a change in control. If the termination is not in connection with a change in control, cash severance is paid as salary continuation. If the termination is in connection with a change in control, cash severance is paid in a lump sum.
The following table set forth the cash severance and COBRA benefits under our Executive Severance Plan:
| |
Other than in connection with a change in control | In connection with a change in control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Title
|
Cash Severance (multiple of monthly base pay) |
COBRA Period (number of months) |
Cash Severance (multiple of monthly base pay plus pro-rated target bonus) |
COBRA Period (number of months) |
|||||||
Chief Executive Officer |
12 |
12 |
18 | 18 | |||||||
All other participants |
Lesser of 9, and 1/2 the executive's full months of service |
Same as length of salary continuation period |
12 |
12 |
|||||||
Under the Executive Severance Plan, participants are eligible for severance benefits if we or our successor terminates the executive's employment other than due to (i) the commission of or indictment for a felony, or a misdemeanor involving fraud or moral turpitude, (ii) an act which constitutes gross negligence, willful misconduct or insubordination in the course of employment, or (iii) the continued failure of the executive to perform the essential duties and responsibilities of his or her position, after having received notice of the deficiencies and having had 30 days to cure such defects in performance.
Under the Executive Severance Plan, participants are also eligible for severance benefits at or within 12 months following a change in control if the executive resigns for any of the following "good reasons": (i) a decrease in base salary or target bonus by more than 10% (other than a comparable decrease in compensation for all of our executives), (ii) a material decrease in the executive's duties or responsibilities (but excluding a change in title or reporting relationship), (iii) a relocation of the executive's primary work location by more than 50 miles, and (iv) our failure to obtain an agreement from our successor to continue this Executive Severance Plan, in each case without the executive's consent. In the case of items (ii) and (iii), the executive must provide us (or our successor) with notice within 30 days of the triggering event, and we (or our successor) will have 30 days thereafter to remedy the situation.
To receive benefits under the Executive Severance Plan, in addition to having a termination or resignation that qualifies for benefits, the executive will be required to execute a full release of claims in a form satisfactory to us, within certain designated timeframes. This release of claims may include certain post-employment restrictive covenants, including confidentiality and non-solicitation clauses.
Transaction Bonus Plan
Our board of directors has adopted our Transaction Bonus Plan to provide key employees with additional incentives in connection with our licensing agreement with Forest.
Under the Transaction Bonus Plan, one percent of certain payments, or Covered Payments, that we expect to receive under the Forest license agreement will be designated for award under the Transaction Bonus Plan, with aggregate payments not to exceed $1,600,000. Covered Payments include both the initial licensing payment as well as milestone payments, but do not include royalty or certain other payments pursuant to the Forest license agreement. Any non-cash consideration received under the Forest license agreement that qualifies as a Covered Payment will be valued in good faith by our board of directors.
Employees will be selected to participate in the Transaction Bonus Plan by our board of directors upon recommendation of our chief executive officer. From time to time, our board of directors will
105
allocate some or all of the available pool among eligible participants, designated as either fixed dollar amounts or as a percentage of the total pool. Bonus payments generally will be awarded within 60 days after the date that a Covered Payment is made to us, and a participant is eligible to receive an allocation with respect to that Covered Payment if he or she (a) remains employed with us through the date of payment, and (b) if required by our board of directors, signs a general release of claims within the designated timeframe.
If our board of directors determines that payments to be made under the Transaction Bonus Plan might result in our loss of a deduction under Section 280G of the Code, or imposition of an excise tax under Section 4999 of the Code on the participant, with respect to such payments, our board of directors may determine to submit the payments to stockholders for approval; if holders of less than 75% of our outstanding stock approve the payments, then the payments will not be paid. In addition, a participant's bonus will be reduced if and to the extent that, as a result of the application of the excise tax under Code Section 4999, his or her after-tax proceeds would be greater than if no reduction had occurred.
The Transaction Bonus Plan may not be amended or terminated without the consent of any participant who may be adversely affected.
We have paid an aggregate of $650,000 and $400,000 of bonuses, including to our named executive officers, in 2012 and 2013, respectively, pursuant to the Transaction Bonus Plan.
Employee benefit and stock plans
The principal features of our equity incentive plans and our 401(k) plan are summarized below. These summaries are qualified in their entirety by reference to the actual text of the plans, which, other than the 401(k) plan, are filed as exhibits to the registration statement of which this prospectus is a part.
2002 Employee, Director and Consultant Stock Plan
Our board of directors adopted and our stockholders approved our 2002 Employee, Director and Consultant Stock Plan, or 2002 Plan, in October 2002. Our 2002 Plan was amended and restated most recently in April 2012. Our 2002 Plan terminated in October 2012 and no new awards have been granted since that date, but outstanding awards remain subject to the terms of our 2002 Plan and applicable award agreements. Our 2002 Plan provided for the grant of incentive stock options, or ISOs, within the meaning of Section 422 of the Code, to our employees, and for the grant of nonstatutory stock options, or NSOs, and restricted stock awards to our employees, directors and consultants.
Authorized shares. The maximum number of shares of our common stock that could be issued under our 2002 Plan was 4,000,000. Shares subject to stock awards granted under our 2002 Plan that expire or terminate without being exercised in full or are settled in cash do not reduce the number of shares available for issuance under our 2002 Plan. Additionally, shares issued pursuant to stock awards under our 2002 Plan that we repurchased become available for future grant under our 2002 Plan.
Plan administration. Our board of directors or a duly authorized committee of our board of directors administers our 2002 Plan and the stock awards granted under it. Under our 2002 Plan, our board of directors has the authority to determine and amend the terms of awards, including recipients, the exercise, purchase or strike price of stock awards, if any, the number of shares subject to each stock award, the vesting schedule applicable to the awards, and the form of consideration, if any, payable upon exercise or settlement of the award and the terms of the award agreements for use under our 2002 Plan.
106
Corporate transactions. Our 2002 Plan provides that in the event of certain specified corporate transactions, outstanding awards may be assumed or continued by a surviving or acquiring corporation's parent company, or similar stock awards may be substituted for outstanding awards.
Under our 2002 Plan, our board of directors may provide for accelerated vesting of unvested options or stock awards, may, following notice to participants, require exercise or acceptance of stock awards within a certain period, and may also provide for payment in lieu of exercise to holders of stock awards that would otherwise terminate if not exercised before the effective date of a corporate transaction, with the amount of payment equal to the value the holder would receive upon exercise.
In the event of a change in control of our company, awards granted under the 2002 Plan will not receive automatic acceleration of vesting and exercisability, although this treatment may be provided for by the administrator or in an award agreement.
Transferability. Under our 2002 Plan, awards are generally not transferable other than by will or the laws of descent and distribution, except as otherwise determined by the administrator or as provided under our 2002 Plan.
2007 Stock Plan
Our board of directors adopted and our stockholders approved our 2007 Stock Plan, or 2007 Plan, in December 2007. Our 2007 Plan was amended in September 2013. Our 2007 Plan provides for the grant of ISOs to our employees, and for the grant of NSOs and restricted stock awards our employees, directors and consultants.
Our 2014 Equity Incentive Plan, or 2014 Plan, will become effective on the date the registration statement of which this prospectus forms a part is declared effective by the SEC. As a result, we do not expect to grant any additional awards under the 2007 Plan following that date, although any awards granted under the 2007 Plan will remain subject to the terms of our 2007 Plan and applicable award agreements, until such outstanding awards that are stock options are exercised, or until they terminate or expire by their terms, and until any restricted stock awards become vested, terminate or are forfeited.
Authorized shares. The maximum number of shares of our common stock that may be issued under our 2007 Plan is 5,274,854; this is also the maximum number of shares that may be issued upon the exercise of ISOs.
Shares subject to stock awards granted under our 2007 Plan that expire or terminate without being exercised in full do not reduce the number of shares available for issuance under our 2007 Plan. Additionally, shares issued pursuant to stock awards under our 2007 Plan that we repurchase or that are forfeited become available for future grant under our 2007 Plan.
Plan administration. Our board of directors or a duly authorized committee of our board of directors administers our 2007 Plan and the stock awards granted under it. Under the 2007 Plan, our board of directors has the authority to determine and amend the terms of awards and award agreements, including recipients, the exercise or purchase of stock awards, the number of shares subject to each stock award, the vesting schedule applicable to the awards, and the form of consideration, if any, payable upon exercise or settlement of the award.
Corporate transactions. Our 2007 Plan provides that in the event our company is a party to a merger or consolidation, all shares acquired under the 2007 Plan and all options shall be subject to the agreement of merger or consolidation. In this circumstance, outstanding stock options may be assumed or continued by a surviving or acquiring corporation's parent company, or similar stock option awards may be substituted for outstanding awards.
107
If outstanding stock option awards are not assumed, continued or substituted in connection with a corporate transaction, our board of directors may provide for cancellation of such options without the payment of any consideration, and may also provide for payment in lieu of exercise to holders of stock awards that would otherwise terminate if not exercised before the effective date of a corporate transaction, with the amount of payment equal to the value the holder would receive upon exercise, either as to all options or only the portion that had previously vested under its terms. Our board of directors may also accelerate the vesting of option awards, contingent upon the closing of such merger or consolidation.
Transferability. Under our 2007 Plan, our board of directors may provide for restrictions on the transferability of awards, as specified in the applicable award agreement. These restrictions may include special forfeiture provisions, repurchase rights, rights of first refusal and other transfer restrictions, as determined by our board of directors.
Plan amendment or termination. Our board of directors has the authority to amend, suspend, or terminate our 2007 Plan, although certain material amendments require the approval of our stockholders, and amendments that would impair the rights of any participant require the consent of that participant.
2014 Equity Incentive Plan
Our board of directors adopted our 2014 Plan in February 2014, and our stockholders approved the 2014 Plan in March 2014. The 2014 Plan will be the successor to our 2007 Plan. From the date the registration statement of which this prospectus forms a part is declared effective, no further grants will be made under our 2007 Plan. Our 2014 Plan provides for the grant of ISOs to our employees and for the grant of NSOs, stock appreciation rights, restricted stock awards, restricted stock unit awards, performance stock awards, performance cash awards, and other forms of equity compensation to our employees, directors and consultants.
Authorized shares. The maximum number of shares of our common stock that may be issued under our 2014 Plan is 7,191,053. Additionally, the number of shares of our common stock reserved for issuance under our 2014 Plan will automatically increase on the first day of each fiscal year for a period of up to 10 years, commencing on the first day of the fiscal year following the year in which the 2014 Plan becomes effective, in an amount equal to 4% of the total number of shares of our capital stock outstanding on the last day of the preceding fiscal year, or a lesser number of shares determined by our board of directors. The maximum number of shares of our common stock that may be issued upon the exercise of ISOs under our 2014 Plan is 13,600,000.
Shares subject to stock awards granted under our 2014 Plan that expire or terminate without being exercised in full, or that are paid out in cash rather than in shares, do not reduce the number of shares available for issuance under our 2014 Plan. Additionally, shares issued pursuant to stock awards under our 2014 Plan that we repurchase or that are forfeited, as well as shares used to pay the exercise price of a stock award or to satisfy the tax withholding obligations related to a stock award, become available for future grant under our 2014 Plan.
Plan administration. Our board of directors, or a duly authorized committee of our board of directors, will administer our 2014 Plan. Our board of directors may also delegate to one or more of our officers the authority (1) to designate employees (other than officers) to receive specified stock awards, and (2) to determine the number of shares subject to such stock awards. Subject to the terms of our 2014 Plan, the board of directors has the authority to determine the terms of awards, including recipients, the exercise, purchase or strike price of stock awards, if any, the number of shares subject to each stock award, the fair market value of a share of our common stock, the vesting schedule applicable to the awards, together with any vesting acceleration, the form of consideration, if any,
108
payable upon exercise or settlement of the award and the terms of the award agreements for use under our 2014 Plan.
The board of directors has the power to modify outstanding awards under our 2014 Plan. The board of directors has the authority to reprice any outstanding option or stock appreciation right, cancel any outstanding stock award in exchange for new stock awards, cash or other consideration, or take any other action that is treated as a repricing under generally accepted accounting principles, with the consent of any adversely affected participant.
Section 162(m) limits. At such time as necessary for compliance with Section 162(m) of the Code, no participant may be granted stock awards that are intended to comply with Section 162(m) of the Code covering more than 1,000,000 shares of our common stock under our 2014 Plan during any calendar year pursuant to stock options, stock appreciation rights and other stock awards whose value is determined by reference to an increase over an exercise price or strike price of at least 100% of the fair market value of our common stock on the date of grant. Additionally, no participant may be granted in a calendar year a performance stock award covering more than 1,000,000 shares of our common stock or a performance cash award having a maximum value in excess of $1,000,000 under our 2014 Plan. These limitations are intended to give us the flexibility to grant compensation that will not be subject to the $1,000,000 annual limitation on the income tax deductibility imposed by Section 162(m) of the Code.
Performance awards. We believe our 2014 Plan permits the grant of performance-based stock and cash awards that may qualify as performance-based compensation that is not subject to the $1,000,000 limitation on the income tax deductibility imposed by Section 162(m) of the Code. Our compensation committee may structure awards so that the stock or cash will be issued or paid only following the achievement of certain pre-established performance goals during a designated performance period.
Our compensation committee may establish performance goals by selecting from one or more of the following performance criteria: (1) earnings (including earnings per share and net earnings); (2) earnings before interest, taxes and depreciation; (3) earnings before interest, taxes, depreciation and amortization; (4) earnings before interest, taxes, depreciation, amortization and legal settlements; (5) earnings before interest, taxes, depreciation, amortization, legal settlements and other income (expense); (6) earnings before interest, taxes, depreciation, amortization, legal settlements, other income (expense) and stock-based compensation; (7) earnings before interest, taxes, depreciation, amortization, legal settlements, other income (expense), stock-based compensation and changes in deferred revenue; (8) total stockholder return; (9) return on equity or average stockholder's equity; (10) return on assets, investment or capital employed; (11) stock price; (12) margin (including gross margin); (13) income (before or after taxes); (14) operating income; (15) operating income after taxes; (16) pre-tax profit; (17) operating cash flow; (18) sales or revenue targets; (19) increases in revenue or product revenue; (20) expenses and cost reduction goals; (21) improvement in or attainment of working capital levels; (22) economic value added (or an equivalent metric); (23) market share; (24) cash flow; (25) cash flow per share; (26) share price performance; (27) debt reduction; (28) implementation or completion of projects or processes; (29) user satisfaction; (30) stockholders' equity; (31) capital expenditures; (32) debt levels; (33) operating profit or net operating profit; (34) workforce diversity; (35) growth of net income or operating income; (36) billings; (37) bookings; (38) the number of users, including but not limited to unique users; (39) employee retention; and (40) to the extent that an award is not intended to comply with Section 162(m) of the Code, other measures of performance selected by the board of directors.
Our compensation committee may establish performance goals on a company-wide basis, with respect to one or more business units, divisions, affiliates, or business segments, and in either absolute terms or relative to the performance of one or more comparable companies or the performance of one or more relevant indices. Unless otherwise specified by our board of directors (i) in the award agreement at the time the award is granted or (ii) in such other document setting forth the
109
performance goals at the time the performance goals are established, our compensation committee will appropriately make adjustments in the method of calculating the attainment of the performance goals as follows: (1) to exclude restructuring and other nonrecurring charges; (2) to exclude exchange rate effects; (3) to exclude the effects of changes to generally accepted accounting principles; (4) to exclude the effects of any statutory adjustments to corporate tax rates; (5) to exclude the effects of any "extraordinary items" as determined under generally accepted accounting principles; (6) to exclude the dilutive effects of acquisitions or joint ventures; (7) to assume that any business divested by the Company achieved performance objectives at targeted levels during the balance of a performance period following such divestiture; (8) to exclude the effect of any change in the outstanding shares of our common stock by reason of any stock dividend or split, stock repurchase, reorganization, recapitalization, merger, consolidation, spin-off, combination or exchange of shares or other similar corporate change or any distributions to common stockholders other than regular cash dividends; (9) to exclude the effects of stock-based compensation and the award of bonuses under our bonus plans; (10) to exclude costs incurred in connection with potential acquisitions or divestitures that are required to be expensed under generally accepted accounting principles; (11) to exclude the goodwill and intangible asset impairment charges that are required to be recorded under generally accepted accounting principles; and (12) to exclude the effect of any other unusual, non-recurring gain or loss or other extraordinary item.
Corporate transactions. Our 2014 Plan provides that in the event of certain specified significant corporate transactions, as defined under our 2014 Plan, each outstanding award will be treated as the administrator determines. The administrator may (1) arrange for the assumption, continuation or substitution of a stock award by a successor corporation; (2) arrange for the assignment of any reacquisition or repurchase rights held by us to a successor corporation; (3) accelerate the vesting, in whole or in part, of the stock award and provide for its termination prior to the transaction; (4) arrange for the lapse, in whole or in part, of any reacquisition or repurchase rights held by us; or (5) cancel or arrange for the cancellation of the stock award prior to the transaction in exchange for a cash payment, if any, determined by the board. The plan administrator is not obligated to treat all stock awards or portions of stock awards in the same manner, even those that are of the same award type.
Transferability. A participant may not transfer stock awards under our 2014 Plan other than by will, the laws of descent and distribution or as otherwise provided under our 2014 Plan.
Plan amendment or termination. Our board of directors has the authority to amend, suspend or terminate our 2014 Plan, provided that such action does not materially impair the existing rights of any participant without such participant's written consent. No awards may be granted after the tenth anniversary of the date our board of directors adopted our 2014 Plan. No stock awards may be granted under our 2014 Plan while it is suspended or after it is terminated.
2014 Employee Stock Purchase Plan
Our board of directors adopted the 2014 Employee Stock Purchase Plan, or ESPP, in February 2014, and our stockholders approved the ESPP in March 2014. The ESPP will become effective upon the date the registration statement of which this prospectus forms a part is declared effective. The purpose of the ESPP is to secure the services of new employees, to retain the services of existing employees and to provide incentives for such individuals to exert maximum efforts toward our success and that of our affiliates. The ESPP is intended to qualify as an "employee stock purchase plan" within the meaning of Section 423 of the Code.
Share reserve. Following this offering, the ESPP authorizes the issuance of 262,762 shares of our common stock pursuant to purchase rights granted to our employees or to employees of any of our designated affiliates. The number of shares of our common stock reserved for issuance will
110
automatically increase on January 1 of each calendar year, from January 1, 2015 (assuming the ESPP becomes effective in 2014) through January 1, 2024, by the least of (1) 1% of the total number of shares of our common stock outstanding on December 31 of the preceding calendar year, and (2) 520,000 shares; provided, that prior to the date of any such increase, our board of directors may determine that such increase will be less than the amount set forth in clauses (1) and (2). As of the date hereof, no shares of our common stock have been purchased under the ESPP.
Administration. Our board of directors has the authority administer the ESPP and may delegate this authority to the compensation committee. The ESPP is implemented through a series of offerings under which eligible employees are granted purchase rights to purchase shares of our common stock on specified dates during such offerings. Under the ESPP, we may specify offerings with durations of not more than 27 months, and may specify shorter purchase periods within each offering. Each offering will have one or more purchase dates on which shares of our common stock will be purchased for employees participating in the offering. We currently intend to have six month offerings with one purchase period (of approximately six months in duration) per offering, except that the first purchase period under our first offering may be shorter or longer than six months, depending on the date on which the underwriting agreement relating to this offering becomes effective. An offering under the ESPP may be terminated under certain circumstances.
Payroll deductions. Generally, all regular employees, including executive officers, employed by us or by any of our designated affiliates, may participate in the ESPP and may contribute, normally through payroll deductions, up to 15% of their earnings (as defined in the ESPP) for the purchase of our common stock under the ESPP. Unless otherwise determined by our board of directors, common stock will be purchased for the accounts of employees participating in the ESPP at a price per share equal to the lower of (a) 85% of the fair market value of a share of our common stock on the first date of an offering or (b) 85% of the fair market value of a share of our common stock on the date of purchase. For the initial offering, which we expect will commence upon the execution and delivery of the underwriting agreement relating to this offering, the fair market value on the first day of the offering period will be the price at which shares are first sold to the public.
Limitations. Employees may have to satisfy one or more of the following service requirements before participating in the ESPP, as determined by our board of directors, including: (1) being customarily employed for more than 20 hours per week; (2) being customarily employed for more than five months per calendar year; or (3) continuous employment with us or one of our affiliates for a period of time (not to exceed two years). No employee may purchase shares under the ESPP at a rate in excess of $25,000 worth of our common stock based on the fair market value per share of our common stock at the beginning of an offering for each year such a purchase right is outstanding and the maximum number of shares an employee may purchase during a single purchase period is 2,500. Finally, no employee will be eligible for the grant of any purchase rights under the ESPP if immediately after such rights are granted, such employee has voting power over 5% or more of our outstanding capital stock measured by vote or value pursuant to Section 424(d) of the Code.
Changes to capital structure. In the event that there occurs a change in our capital structure through such actions as a stock split, merger, consolidation, reorganization, recapitalization, reincorporation, stock dividend, dividend in property other than cash, large nonrecurring cash dividend, liquidating dividend, combination of shares, exchange of shares, change in corporate structure or similar transaction, the board of directors will make appropriate adjustments to (1) the number of shares reserved under the ESPP, (2) the maximum number of shares by which the share reserve may increase automatically each year, (3) the number of shares and purchase price of all outstanding purchase rights, and (4) the number of shares that are subject to purchase limits under ongoing offerings.
111
Corporate transactions. In the event of certain significant corporate transactions, including: (1) a sale of all or substantially all of our assets; (2) the sale or disposition of 90% of our outstanding securities; (3) the consummation of a merger or consolidation where we do not survive the transaction; and (4) the consummation of a merger or consolidation where we do survive the transaction but the shares of our common stock outstanding immediately prior to such transaction are converted or exchanged into other property by virtue of the transaction, any then-outstanding rights to purchase our stock under the ESPP may be assumed, continued or substituted for by any surviving or acquiring entity (or its parent company). If the surviving or acquiring entity (or its parent company) elects not to assume, continue or substitute for such purchase right, then the participants' accumulated payroll contributions will be used to purchase shares of our common stock within 10 business days prior to such corporate transaction, and such purchase rights will terminate immediately.
ESPP amendment; termination. Our board of directors has the authority to amend or terminate our ESPP, provided that except in certain circumstances any such amendment or termination may not materially impair any outstanding purchase rights without the holder's consent. We will obtain stockholder approval of any amendment to our ESPP as required by applicable law or listing requirements.
401(k) plan
We maintain a tax-qualified retirement plan that provides eligible U.S. employees with an opportunity to save for retirement on a tax advantaged basis. Eligible employees are able to defer eligible compensation up to certain Code limits, which are updated annually. We have the ability to make matching and discretionary contributions to the 401(k) plan but have not done so to date. Employee contributions are allocated to each participant's individual account and are then invested in selected investment alternatives according to the participants' directions. Employees are immediately and fully vested in their own contributions. The 401(k) plan is intended to be qualified under Section 401(a) of the Code, with the related trust intended to be tax exempt under Section 501(a) of the Code. As a tax-qualified retirement plan, contributions to the 401(k) plan are deductible by us when made, and contributions and earnings on those amounts are not taxable to the employees until withdrawn or distributed from the 401(k) plan.
Pension benefits
Our named executive officers did not participate in, or otherwise receive any benefits under, any pension or retirement plan sponsored by the company during 2013.
Nonqualified deferred compensation
Our named executive officers did not participate in, or earn any benefits under, a nonqualified deferred compensation plan during 2013.
Limitation on liability and indemnification matters
Upon the closing of this offering, our amended and restated certificate of incorporation will contain provisions that limit the liability of our current and former directors for monetary damages to the fullest extent permitted by Delaware law. Delaware law provides that directors of a corporation will not be personally liable for monetary damages for any breach of fiduciary duties as directors, except liability for:
- •
- any breach of the director's duty of loyalty to the corporation or its stockholders;
- •
- any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law;
112
- •
- unlawful payments of dividends or unlawful stock repurchases or redemptions; or
- •
- any transaction from which the director derived an improper personal benefit.
Such limitation of liability does not apply to liabilities arising under federal securities laws and does not affect the availability of equitable remedies, such as injunctive relief or rescission.
Our amended and restated certificate of incorporation and our amended and restated bylaws will provide that we are required to indemnify our directors to the fullest extent permitted by Delaware law. Our amended and restated bylaws will also provide that, upon satisfaction of certain conditions, we shall advance expenses incurred by a director in advance of the final disposition of any action or proceeding, and permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in that capacity regardless of whether we would otherwise be permitted to indemnify him or her under the provisions of Delaware law. Our amended and restated certificate of incorporation and amended and restated bylaws will also provide our board of directors with discretion to indemnify our officers and employees when determined appropriate by the board. We have entered and expect to continue to enter into agreements to indemnify our directors and executive officers. With certain exceptions, these agreements provide for indemnification for related expenses including, among other things, attorneys' fees, judgments, fines and settlement amounts incurred by any of these individuals in any action or proceeding. We believe that these bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors and officers. We also maintain customary directors' and officers' liability insurance.
The limitation of liability and indemnification provisions in our amended and restated certificate of incorporation and amended and restated bylaws may discourage stockholders from bringing a lawsuit against our directors for breach of their fiduciary duty. These provisions may also reduce the likelihood of derivative litigation against our directors and officers, even though an action, if successful, might benefit us and other stockholders. Further, a stockholder's investment may be adversely affected to the extent that we pay the costs of settlement and damage awards against directors and officers as required by these indemnification provisions. At present, there is no pending litigation or proceeding involving any of our directors, officers or employees for which indemnification is sought and we are not aware of any threatened litigation that may result in claims for indemnification.
Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, or the Securities Act, may be permitted for directors, executive officers or persons controlling us, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Rule 10b5-1 sales plans
Our directors and executive officers may adopt written plans, known as Rule 10b5-1 plans, pursuant to which, if adopted, they would contract with a broker to buy or sell shares of our common stock on a periodic basis. Under a Rule 10b5-1 plan, a broker executes trades pursuant to parameters established by the director or officer when entering into the plan, without further direction from them. The director or officer may amend a Rule 10b5-1 plan in some circumstances and may terminate a plan at any time. Our directors and executive officers also may buy or sell additional shares outside of a Rule 10b5-1 plan when they are not in possession of material nonpublic information subject to compliance with the terms of our insider trading policy. Prior to 180 days after the date of this offering (subject to early termination), the sale of any shares under such plan would be subject to the lock-up agreement that the director or officer has entered into with the underwriters.
113
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Other than the compensation arrangements described elsewhere in this prospectus, we describe below transactions and series of similar transactions, since January 1, 2011, to which we were a party or will be a party, in which:
- •
- the amounts involved exceeded or will exceed $120,000; and
- •
- any of our directors, executive officers, or holders of more than 5% of our capital stock, or any member of the immediate family of the foregoing persons, had or will have a direct or indirect material interest.
Compensation arrangements for our directors and named executive officers are described elsewhere in this prospectus. Share and per share amounts have been adjusted to give effect to a 2-for-1 forward split of our outstanding common stock and preferred stock effected on March 24, 2014.
Sale of Series AA Preferred Stock and issuance of Secured Convertible Promissory Notes and Warrants to purchase preferred stock
In March 2012 we entered into a Series AA Preferred Stock and Secured Note and Warrant Purchase Agreement, or the 2012 Purchase Agreement, with certain investors, including entities affiliated with our directors and beneficial owners of more than 5% of our capital stock. Pursuant to the 2012 Purchase Agreement, we issued (i) an aggregate of 1,051,342 shares of our Series AA preferred stock at a purchase price of $3.80445 per share for an aggregate purchase price of approximately $4 million (ii) approximately $4 million of secured convertible promissory notes, convertible into shares of our preferred stock, or the 2012 Notes and (iii) warrants to purchase preferred stock, or the 2012 Warrants. The 2012 Notes carried an interest rate of 10% per annum and had a maturity date of March 23, 2013. From March 2013 to August 2013, we repaid the outstanding principal balance and all accrued interest of the 2012 Notes. The 2012 Warrants are exercisable for shares of preferred stock issued in a subsequent financing or, if no subsequent financing occurs prior to the closing of our initial public offering, they are exercisable for shares of Series AA preferred stock. Except to the extent earlier exercised, the 2012 Warrants will automatically net exercise immediately prior to the closing of this offering pursuant to their terms.
The following table summarizes the purchase of Series AA preferred stock by holders of more than 5% of our capital stock pursuant to the 2012 Purchase Agreement.
Participants
|
Number of shares of Series AA preferred stock |
|||
|---|---|---|---|---|
Entities affiliated with Mohr Davidow Ventures(1) |
226,098 | |||
aeris CAPITAL Equity Investments, L.P.(2) |
452,208 | |||
Entities affiliated with DAG Ventures(3) |
113,032 | |||
- (1)
- Consists
of 226,098 shares of Series AA preferred stock held of record by MDV IX, L.P. as nominee for MDV IX, L.P. and MDV ENF
IX, L.P. William Ericson, a member of our board of directors, is a General Partner of Mohr Davidow Ventures.
- (2)
- George
Rehm, a member of our board of directors, is a Managing Partner of aeris CAPITAL AG, the investment advisor to aeris CAPITAL Equity
Investments LP and has an ownership interest in aeris CAPITAL Equity Ltd., the general partner of aeris CAPITAL Equity Investments, LP.
- (3)
- Consists of (i) 103,234 shares held of record by DAG Ventures III-QP, L.P., (ii) 9,700 shares held of record by DAG Ventures III, L.P., and (iii) 98 shares held of record by
114
DAG Ventures GP Fund III, LLC. DAG Ventures Management III, LLC is the sole general partner of each of DAG Ventures III-QP, L.P. and DAG Ventures III L.P, and is the sole managing member of DAG Ventures GP Fund III LLC. John Caddedu and R. Thomas Goodrich are the managing members of DAG Ventures Management III, LLC, and share voting and investment power over these shares.
The following table summarizes purchases of the 2012 Notes by holders of more than 5% of our capital stock pursuant to the 2012 Purchase Agreement.
Participants
|
Aggregate Principal Amount of 2012 Notes |
|||
|---|---|---|---|---|
Entities affiliated with Mohr Davidow Ventures(1) |
$ | 860,239 | ||
aeris CAPITAL Equity Investments, L.P.(2) |
$ | 1,720,433 | ||
Entities affiliated with DAG Ventures(3) |
$ | 430,240 | ||
- (1)
- Consists
of $860,239 aggregate principal amount of notes issued to MDV IX, L.P. as nominee for MDV IX, L.P. and MDV ENF IX, L.P.
William Ericson, a member of our board of directors, is a General Partner of Mohr Davidow Ventures.
- (2)
- George
Rehm, a member of our board of directors, is a Managing Partner of aeris CAPITAL AG, the investment advisor to aeris CAPITAL Equity
Investments LP and has an ownership interest in aeris CAPITAL Equity Ltd., the general partner of aeris CAPITAL Equity Investments, LP.
- (3)
- Consists of (i) $392,800 aggregate principal amount of notes issued to DAG Ventures III-QP, L.P., (ii) $36,986 aggregate principal amount of notes issued to DAG Ventures III, L.P., and (iii) $454 aggregate principal amount of notes issued to DAG Ventures GP Fund III, LLC.
The following table summarizes 2012 Warrants issued to holders of more than 5% of our capital stock pursuant to the 2012 Purchase Agreement, assuming such 2012 Warrants are exercisable for shares of Series AA preferred stock:
Participants
|
Shares of Series AA Preferred Stock Issuable upon exercise of 2012 Warrants |
|||
|---|---|---|---|---|
Entities affiliated with Mohr Davidow Ventures(1) |
22,382 | |||
aeris CAPITAL Equity Investments, L.P.(2) |
44,766 | |||
Entities affiliated with DAG Ventures(3) |
11,174 | |||
- (1)
- Consists
of warrants to purchase 22,382 shares of Series AA preferred stock held of record by MDV IX, L.P., as nominee for MDV IX, L.P.
and MDV ENF IX, L.P. William Ericson, a member of our board of directors, is a General Partner of MDV.
- (2)
- George
Rehm, a member of our board of directors, is a Managing Partner of aeris CAPITAL AG, the investment advisor to aeris CAPITAL Equity
Investments LP and has an ownership interest in aeris CAPITAL Equity Ltd., the general partner of aeris CAPITAL Equity Investments, LP.
- (3)
- Consists of warrants to purchase (i) 10,212 shares of Series AA preferred stock held of record by DAG Ventures III-QP, L.P., (ii) 960 shares of Series AA preferred stock held of
115
record by DAG Ventures III, L.P., and (iii) 2 shares of Series AA preferred stock held of record by DAG Ventures GP Fund III, LLC. DAG Ventures Management III, LLC is the sole general partner of each of DAG Ventures III-QP, L.P. and DAG Ventures III L.P, and is the sole managing member of DAG Ventures GP Fund III LLC. John Caddedu and R. Thomas Goodrich are the managing members of DAG Ventures Management III, LLC, and share voting and investment power over these shares.
Sale of Series AA preferred stock, Series AA-1 preferred stock and Warrants to purchase Series AA preferred stock
From June 2011 and until December 2011 we sold an aggregate of 1,814,010 shares of Series AA preferred stock at a purchase price of $3.80445 per share and issued 566,268 shares of Series AA preferred stock upon conversion of convertible promissory notes. In connection with those sales, we issued (i) 1,287,554 shares of Series AA-1 preferred stock and (ii) warrants to purchase up to 462,762 shares of Series AA preferred stock, or the June 2011 Warrants, all pursuant to a Series AA Preferred Stock Purchase Agreement, or the AA Purchase Agreement. The purchasers in this transaction included certain of our officers and directors and entities affiliated with our directors and beneficial owners of more than 5% of our capital stock. The June 2011 Warrants are exercisable for shares of Series AA Preferred Stock. Except to the extent earlier exercised, the June 2011 Warrants will automatically net exercise immediately prior to the closing of this offering pursuant to their terms.
The following table summarizes the number of shares of Series AA preferred stock and Series AA-1 preferred stock issued to such purchasers.
Participants
|
Number of shares of Series AA preferred stock |
Number of shares of Series AA-1 preferred stock |
|||||
|---|---|---|---|---|---|---|---|
Entities affiliated with Mohr Davidow Ventures(1) |
1,055,068 | 468,500 | |||||
aeris CAPITAL Equity Investments, L.P.(2) |
527,534 | 300,000 | |||||
Entities affiliated with DAG Ventures(3) |
527,526 | 250,002 | |||||
NCD Investors, a Delaware Multiple Series LLC |
59,140 | 80,008 | |||||
David Mahoney |
13,144 | 3,000 | |||||
Gregory Went |
33,380 | 8,160 | |||||
- (1)
- Consists
of (i) 468,500 shares of Series AA-1 preferred stock and 792,220 shares of Series AA preferred stock held of record by MDV
VII, L.P. as nominee for MDV VII, L.P., MDV VII Leaders' Fund, L.P., MDV ENF VII(A), L.P. and MDV ENF VII(B), L.P. (ii) 262,848 shares of Series AA
preferred stock held of record by MDV IX, L.P. as nominee for MDV IX, L.P. and MDV ENF IX, L.P. William Ericson and Jonathan Feiber are Managing Members of Ninth MDV Partners,
L.L.C., or Ninth MDV, the general partner of MDV IX, L.P. Each of William Ericson, Jonathan Feiber and Ninth MDV may be deemed to share voting and dispositive power over the shares held by MDV
IX. Seventh MDV Partners, L.L.C. is the general partner of MDV VII, L.P. and has sole voting and investment power over the shares. Nancy Schoendorf and Mr. Feiber, as managing members of
Seventh MDV Partners, L.L.C., share such power. William Ericson, a member of our board of directors, is a general partner with Mohr Davidow Ventures.
- (2)
- George Rehm, a member of our board of directors, is a Managing Partner of aeris CAPITAL AG, the investment advisor to aeris CAPITAL Equity Investments LP and has an ownership interest in aeris CAPITAL Equity Ltd., the general partner of aeris CAPITAL Equity Investments, LP.
116
- (3)
- Consists of (i) 228,302 shares of Series AA-1 preferred stock and 481,744 shares of Series AA preferred stock held of record by DAG Ventures III-QP, L.P., (ii) 21,476 shares of Series AA-1 preferred stock and 45,312 shares of Series AA preferred stock held of record by DAG Ventures III, L.P., and (iii) 224 shares of Series AA-1 preferred stock and 470 shares of Series AA preferred stock held of record by DAG Ventures GP Fund III, LLC. DAG Ventures Management III, LLC is the sole general partner of each of DAG Ventures III-QP, L.P. and DAG Ventures III L.P, and is the sole managing member of DAG Ventures GP Fund III LLC. John Caddedu and R. Thomas Goodrich are the managing members of DAG Ventures Management III, LLC, and share voting and investment power over these shares.
The following table summarizes the June 2011 Warrants issued to holders of more than 5% of our capital stock and our directors pursuant to the AA Purchase Agreement, assuming such June 2011 Warrants are exercisable for shares of Series AA preferred stock:
Participants
|
Shares of Series AA Preferred Stock Issuable upon exercise of June 2011 Warrants |
|||
|---|---|---|---|---|
Entities affiliated with Mohr Davidow Ventures(1) |
211,012 | |||
aeris CAPITAL Equity Investments, L.P.(2) |
105,506 | |||
Entities affiliated with DAG Ventures(3) |
105,504 | |||
David Mahoney |
2,628 | |||
Gregory Went |
6,674 | |||
- (1)
- Consists
of warrants to purchase 211,012 shares of Series AA preferred stock held of record by MDV VII, L.P. as nominee for MDV VII
Leaders' Fund, L.P., MDV ENF VII(A), L.P. and MDV ENF VII(B), L.P. William Ericson, a member of our board of directors, is a General Partner with Mohr Davidow Ventures.
- (2)
- George
Rehm, a member of our board of directors, is a Managing Partner of aeris CAPITAL AG, the investment advisor to aeris CAPITAL Equity
Investments LP and has an ownership interest in aeris CAPITAL Equity Ltd., the general partner of aeris CAPITAL Equity Investments, LP.
- (3)
- Consists of warrants to purchase (i) 96,348 shares of Series AA preferred stock held of record by DAG Ventures III-QP, L.P., (ii) 9,062 shares of Series AA preferred stock held of record by DAG Ventures III, L.P., and (iii) 94 shares of Series AA preferred stock held of record by DAG Ventures GP Fund III, LLC. DAG Ventures Management III, LLC is the sole general partner of each of DAG Ventures III-QP, L.P. and DAG Ventures III L.P, and is the sole managing member of DAG Ventures GP Fund III LLC. John Caddedu and R. Thomas Goodrich are the managing members of DAG Ventures Management III, LLC, and share voting and investment power over these shares.
Issuance of convertible promissory notes and warrants to purchase preferred stock
In April 2011 we entered into a Note and Warrant Purchase Agreement, or the 2011 Purchase Agreement, with certain investors, including entities affiliated with our directors and beneficial owners of more than 5% of our capital stock. Pursuant to the 2011 Purchase Agreement, we issued (i) approximately $2,125,000 of convertible promissory notes, convertible into shares of our preferred stock, or the 2011 Notes and (ii) warrants to purchase preferred stock, or the April 2011 Warrants. The 2011 Notes carried an interest rate of 6% per annum and had a maturity date of January 6, 2012. In June 2011, the outstanding principal balance and all accrued interest of the 2011 Notes converted into
117
shares of Series AA preferred stock. The April 2011 Warrants are exercisable for shares of Series AA Preferred Stock. Except to the extent earlier exercised, the April 2011 Warrants will automatically net exercise immediately prior to the closing of this offering pursuant to their terms.
The following table summarizes purchases of the 2011 Notes by holders of more than 5% of our capital stock pursuant to the 2011 Purchase Agreement.
Participants
|
Aggregate Principal Amount of 2011 Notes |
|||
|---|---|---|---|---|
Entities affiliated with Mohr Davidow Ventures(1) |
$ | 1,000,000 | ||
aeris CAPITAL Equity Investments, L.P.(2) |
$ | 500,000 | ||
Entities affiliated with DAG Ventures(3) |
$ | 500,000 | ||
- (1)
- Consists
of $1,000,000 aggregate principal amount of notes issued to MDV VII, L.P. as nominee for MDV VII, L.P., MDV VII Leaders' Fund,
L.P., MDV ENF VII(A), L.P. and MDV ENF VII(B), L.P. William Ericson, a member of our board of directors, is a General Partner with Mohr Davidow Ventures.
- (2)
- George
Rehm, a member of our board of directors, is a Managing Partner of aeris CAPITAL AG, the investment advisor to aeris CAPITAL Equity
Investments LP and has an ownership interest in aeris CAPITAL Equity Ltd., the general partner of aeris CAPITAL Equity Investments, LP.
- (3)
- Consists of (i) $456,600 aggregate principal amount of notes issued to DAG Ventures III-QP, L.P., (ii) $42,950 aggregate principal amount of notes issued to DAG Ventures III, L.P., and (iii) $450 aggregate principal amount of notes issued to DAG Ventures GP Fund III, LLC.
The following table summarizes April 2011 Warrants issued to holders of more than 5% of our capital stock pursuant to the 2011 Purchase Agreement, assuming such April 2011 Warrants are exercisable for shares of Series AA preferred stock:
Participants
|
Shares of Series AA Preferred Stock Issuable upon exercise of April 2011 Warrants |
|||
|---|---|---|---|---|
Entities affiliated with Mohr Davidow Ventures(1) |
26,284 | |||
aeris CAPITAL Equity Investments, L.P.(2) |
13,142 | |||
Entities affiliated with DAG Ventures(3) |
13,138 | |||
- (1)
- Consists
of warrants to purchase 26,284 shares of Series AA preferred stock held of record by MDV VII, L.P. as nominee for MDV VII,
L.P., MDV VII Leaders' Fund, L.P., MDV ENF VII(A), L.P. and MDV ENF VII(B), L.P. William Ericson, a member of our board of directors, is a General Partner of Mohr Davidow
Ventures.
- (2)
- George
Rehm, a member of our board of directors, is a Managing Partner of aeris CAPITAL AG, the investment advisor to aeris CAPITAL Equity
Investments LP and has an ownership interest in aeris CAPITAL Equity Ltd., the general partner of aeris CAPITAL Equity Investments, LP.
- (3)
- Consists of warrants to purchase (i) 12,000 shares of Series AA preferred stock held of record by DAG Ventures III-QP, L.P., (ii) 1,128 shares of Series AA preferred stock held of record by DAG Ventures III, L.P., and (iii) 10 shares of Series AA preferred stock held
118
of record by DAG Ventures GP Fund III, LLC. DAG Ventures Management III, LLC is the sole general partner of each of DAG Ventures III-QP, L.P. and DAG Ventures III L.P, and is the sole managing member of DAG Ventures GP Fund III LLC. John Caddedu and R. Thomas Goodrich are the managing members of DAG Ventures Management III, LLC, and share voting and investment power over these shares.
Warrant Exercises
On March 28, 2014, entities affiliated with Mohr Davidow Ventures exercised all of their 2012 Warrants, June 2011 Warrants, April 2011 Warrants and common stock warrants to acquire an aggregate of 259,678 shares of Series AA preferred stock and an aggregate of 13,332 shares of common stock. On April 4, 2014, entities affiliated with DAG Ventures exercised their 2012 Warrants, their June 2011 Warrants and their April 2011 Warrants to acquire an aggregate of 129,816 shares of Series AA preferred stock.
Stockholders Agreement
We are party to a stockholders agreement under which certain holders of our capital stock, including certain holders of 5% of our capital stock, our chief executive officer and one of our directors, have agreed to vote in a certain way on certain matters, including with respect to the election of directors or a sale of the company. The stockholders agreement also provides for a right of first refusal in favor of certain holders of our stock with regard to certain issuances of capital stock and provides certain rights of first refusal and co-sale in respect of certain sales of securities by the parties to the stockholders agreement. The agreement also provides for a put right in the event that a party to the agreement becomes a Howard Hughes Medical Institute Investigator. Upon the closing of this offering, the stockholders agreement will terminate and none of our stockholders will have any special rights regarding the election or designation of members of our board of directors. The rights of first refusal will not apply to with respect to this offering.
Consulting Agreement
In March 2011, we entered into a consulting agreement with John MacPhee. Pursuant to the consulting agreement, from April 2011 until May 2013 we paid Mr. MacPhee aggregate consulting fees of $80,797 and, in November 2011, we granted Mr. MacPhee an option to purchase 192,000 shares of our common stock, with an exercise price of $0.665 per share, in consideration for his consulting services provided in assisting with corporate development activities. The consulting agreement terminated upon Mr. MacPhee's appointment to our board of directors in May 2013.
Investor Rights Agreement
We are party to an investor rights agreement that provides holders of our preferred stock, including certain holders of 5% of our capital stock and entities affiliated with certain of our directors, with certain registration rights, including the right to demand that we file a registration statement or request that their shares be covered by a registration statement that we are otherwise filing. The investor rights agreement also provides for a right of first refusal in favor of certain holders of our stock with regard to certain issuances of our capital stock and the parties thereto have agreed to vote in a certain way on certain matters, including with respect to sales of the company. The rights of first refusal will not apply to, and will terminate upon, closing of this offering. For a more detailed description of these registration rights, see the section of this prospectus entitled "Description of Capital Stock—Registration Rights."
119
Voting Agreement
We are party to a voting agreement under which certain holders of our capital stock, including certain holders of 5% of our capital stock and entities affiliated with certain of our directors, have agreed to vote in a certain way on certain matters, including with respect to the election of directors. Pursuant to the voting agreement, each of MDV VII, L.P. and aeris CAPITAL Equity Investments, L.P. was granted the right to designate one member of our board of directors. William Ericson and George Rehm were designated by MDV and aeris, respectively, under the voting agreement. Upon the closing of this offering, the voting agreement will terminate and none of our stockholders will have any special rights regarding the election or designation of members of our board of directors.
Right of First Refusal and Co-Sale Agreement
We are party to a right of first refusal and co-sale agreement with holders of our preferred stock and our founders, including certain holders of 5% of our capital stock and entities affiliated with certain of our directors, pursuant to which the holders of preferred stock have a right of first refusal and co-sale in respect of certain sales of securities by our founders. Upon the closing of this offering, the right of first refusal and co-sale agreement will terminate.
Indemnification agreements
Our amended and restated certificate of incorporation, which will be effective upon the closing of this offering, will contain provisions limiting the liability of directors, and our amended and restated bylaws will provide that we will indemnify each of our directors to the fullest extent permitted under Delaware law. Our amended and restated certificate of incorporation and amended and restated bylaws will also provide our board of directors with discretion to indemnify our officers and employees when determined appropriate by the board. In addition, we have entered and expect to continue to enter into agreements to indemnify our directors and executive officers. For more information regarding these agreements, see the section of this prospectus entitled "Executive Compensation—Limitations on liability and indemnification matters."
Participation in this Offering
Certain of our directors and existing stockholders, or their affiliates, are purchasing an aggregate of 362,500 shares of our common stock in this offering. The shares will be offered and sold on the same terms as the other shares that are being offered and sold in this offering to the public.
Employment arrangements
We have extended an offer letter to our executive officers in connection with their employment, and have adopted an Executive Severance Plan and a Transaction Bonus Plan in which our executive officers are participants as described in greater detail in the section of this prospectus titled "Executive Compensation—Employment agreements, Executive Severance Plan and Transaction Bonus Plan."
Policies and procedures for related party transactions
Our board of directors have adopted a policy, effective upon the closing of this offering, that our executive officers, directors, nominees for election as a director, beneficial owners of more than 5% of any class of our common stock and any members of the immediate family of any of the foregoing persons are not permitted to enter into a related person transaction with us without the prior consent of our audit committee. Any request for us to enter into a transaction with an executive officer, director, nominee for election as a director, beneficial owner of more than 5% of any class of our common stock or any member of the immediate family of any of the foregoing persons in which the amount involved exceeds $25,000 and such person would have a direct or indirect interest must first be
120
presented to our audit committee for review, consideration and approval. In approving or rejecting any such proposal, our audit committee is to consider the material facts of the transaction, including, but not limited to, whether the transaction is on terms no less favorable than terms generally available to an unaffiliated third party under the same or similar circumstances and the extent of the related person's interest in the transaction. We did not have a formal review and approval policy for related party transactions at the time of any of the transactions described above. However, all of the transactions described above under "Certain Relationships And Related Party Transactions" were entered into after presentation, consideration and approval by our board of directors.
121
The following table sets forth information regarding the beneficial ownership of our common stock as of February 28, 2014 by:
- •
- each of our directors and named executive officers;
- •
- all of our directors and executive officers as a group; and
- •
- each person, or group of affiliated persons, who is known by us to beneficially own more than 5% of our common stock.
Beneficial ownership is determined according to the rules of the SEC and generally means that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power of that security, including options and warrants that are currently exercisable or exercisable within 60 days after February 28, 2014. Shares of our common stock issuable pursuant to stock options and warrants are deemed outstanding for computing the percentage of the person holding such options or warrants and the percentage of any group of which the person is a member but are not deemed outstanding for computing the percentage of any other person. Except as indicated by the footnotes below, we believe, based on the information furnished to us, that the persons named in the table below have sole voting and investment power with respect to all shares of common stock shown that they beneficially own, subject to community property laws where applicable. The information does not necessarily indicate beneficial ownership for any other purpose, including for purposes of Sections 13(d) and 13(g) of the Exchange Act.
Our calculation of the percentage of beneficial ownership prior to this offering is based on 13,443,824 shares of common stock outstanding as of February 28, 2014, assuming the conversion of all outstanding shares of our preferred stock into common stock immediately upon the closing of this offering, as if this conversion had occurred as of February 28, 2014. Our calculation of the percentage of beneficial ownership after this offering is based on 16,443,824 shares of common stock outstanding immediately after the closing of this offering and assumes:
- •
- the issuance of an aggregate of 474,573 shares of our common stock upon conversion of the Series AA preferred stock
issuable upon the net exercise of warrants to purchase 622,660 shares of Series AA preferred stock with an exercise price of $3.80445 and based on a fair market value of $16.00 per
share, immediately prior to the closing of this offering;
- •
- a 2-for-1 forward split of our outstanding common stock and preferred stock effected on March 24, 2014; and
- •
- no exercise of the underwriters' over-allotment option to purchase additional shares of our common stock.
Certain of our directors and existing stockholders, or their affiliates, are purchasing an aggregate of 362,500 shares of our common stock in this offering.
122
Unless otherwise indicated in the footnotes to the following table, the address of each beneficial owner listed in the table below is c/o Adamas Pharmaceuticals, Inc., 2200 Powell Street, Suite 220, Emeryville, California 94608.
| |
Number of Shares Beneficially Owned |
Percentage of Shares Beneficially Owned |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Name of Beneficial Owner
|
Before the Offering |
After the Offering |
Before the Offering |
After the Offering |
|||||||||
5% Stockholders: |
|||||||||||||
Entities affiliated with Mohr Davidow Ventures(1) |
4,222,263 |
4,522,263 |
30.9 |
% |
27.2 |
% |
|||||||
aeris CAPITAL Equity Investments, L.P.(2) |
2,172,128 | 2,172,128 | 16.0 | % | 13.1 | % | |||||||
Entities affiliated with DAG Ventures(3) |
1,629,351 | 1,629,351 | 12.0 | % | 9.8 | % | |||||||
Entities affiliated with NCD Investors, a Delaware Multiple Series LLC(4) |
854,120 | 916,620 | 6.4 | % | 5.6 | % | |||||||
Named executive officers and directors: |
|||||||||||||
Gregory Went, Ph.D.(5) |
2,020,201 | 2,020,201 | 13.6 | % | 11.3 | % | |||||||
Natalie McClure, Ph.D(6) |
396,666 | 396,666 | 2.9 | % | 2.4 | % | |||||||
Anthony Rimac(7) |
260,000 | 260,000 | 1.9 | % | 1.6 | % | |||||||
William Ericson(8) |
4,252,263 | 4,552,263 | 31.1 | % | 27.3 | % | |||||||
Ivan Lieberburg, M.D., Ph.D.(9) |
178,000 | 178,000 | 1.3 | % | 1.1 | % | |||||||
George Rehm(10) |
2,172,128 | 2,172,128 | 16.0 | % | 13.1 | % | |||||||
David Mahoney(11) |
137,156 | 137,156 | 1.0 | % | * | ||||||||
John MacPhee, MPH(12) |
216,000 | 216,000 | 1.6 | % | 1.3 | % | |||||||
Richard Booth(13) |
83,778 | 83,778 | * | * | |||||||||
Martha Demski(14) |
30,000 | 30,000 | * | * | |||||||||
Sara Grootwassink Lewis(15) |
30,000 | 30,000 | * | * | |||||||||
All executive officers and directors as a group (13 persons)(16) |
10,211,608 | 10,511,608 | 60.5 | % | 52.9 | % | |||||||
- *
- Less
than 1% of the outstanding shares of common stock
- (1)
- Includes (i) 3,522,058 shares held of record by MDV VII, L.P., as nominee for MDV VII, L.P., MDV VII Leaders' Fund, L.P., MDV ENF VII(A), L.P., and MDV ENF VII(B), L.P., (ii) 488,946 shares held of record by MDV IX, L.P., as nominee for MDV IX, L.P., and MDV ENF IX, L.P., (iii) an aggregate of 17,056 shares of common stock, on an as converted basis, issuable upon the net exercise of Series AA warrants held by MDV IX, L.P., as nominee for MDV IX, L.P., and MDV ENF IX, L.P., (iv) an aggregate of 180,871 shares of common stock, on an as converted basis, issuable upon the net exercise of Series AA warrants held by MDV VII, L.P., as nominee for MDV VII, L.P., MDV VII Leaders' Fund, L.P., MDV ENF VII(A), L.P., and MDV ENF VII(B), L.P., and (v) an aggregate of 13,332 shares of common stock issuable upon exercise of common stock warrants held by MDV VII, L.P., as nominee for MDV VII, L.P., MDV VII Leaders' Fund, L.P., MDV ENF VII(A), L.P., and MDV ENF VII(B), L.P. William Ericson and Jonathan Feiber are Managing Members of Ninth MDV Partners, L.L.C., the general partner of MDV IX, L.P., as nominee for MDV IX, L.P., and MDV ENF IX, L.P. Each of William Ericson, Jonathan Feiber and Ninth MDV Partners, L.L.C. may be deemed to share voting and dispositive power over the shares held by MDV IX, L.P., as nominee for MDV IX, L.P., and MDV ENF IX, L.P. Seventh MDV Partners, L.L.C. is the general partner of MDV VII, L.P., as nominee for MDV VII, L.P., MDV VII Leaders' Fund, L.P., MDV ENF VII(A), L.P., and MDV ENF VII(B), L.P. and has sole voting and investment power over the shares. Nancy Schoendorf and Mr. Feiber, as managing members of Seventh MDV Partners, L.L.C., share such power. William Ericson, a general partner with Mohr Davidow Ventures and a member of our board of directors,
123
may be deemed to indirectly beneficially own the shares affiliated with Mohr Davidow Ventures. Gregory Went, our Chief Executive Officer and Chairman of our board of directors, is a limited partner of MDV ENF IX, L.P. but has no voting or dispositive power with respect to the shares held by MDV IX, L.P. The address of these entities is c/o Mohr Davidow Ventures, 3000 Sand Hill Road, Building 3, Suite 290, Menlo Park, CA 94025. The number of shares beneficially owned after the offering includes 265,150 shares of common stock purchased in this offering by MDV VII, L.P., 6,043 shares of common stock purchased in this offering by MDV ENF VII(A), L.P., 3,146 shares of common stock purchased in this offering by MDV ENF VII(B), L.P., and 25,661 shares of common stock purchased in this offering by MDV VII Leaders' Fund, L.P.
- (2)
- Represents
2,047,574 shares held directly by aeris CAPITAL Equity Investments, L.P., or the aeris LP, and an aggregate of 124,554 shares of
common stock, on an as converted basis, issuable upon the net exercise of Series AA warrants. George Rehm is a member of our board of directors and is a Managing Partner of aeris CAPITAL AG,
the investment advisor to the aeris LP, and has an economic interest in aeris CAPITAL Equity Ltd., the general partner of the aeris LP, or the aeris GP. By virtue of his
economic interest in the aeris GP, Mr. Rehm may be deemed to indirectly beneficially own the shares affiliated with aeris CAPITAL Equity Investments, L.P. The address of aeris
CAPITAL Equity Investments, L.P. is Walker House, 87 Mary Street, Grand Cayman KY 1-9002 Cayman Islands.
- (3)
- Includes
(i) 1,397,596 shares held of record by DAG Ventures III-QP, L.P. and an aggregate of 90,364 shares of common stock, on an as
converted basis, issuable upon the net exercise of Series AA warrants, (ii) 131,449 shares held of record by DAG Ventures III, L.P., (iii) an aggregate of 8,495 shares of
common stock, on an as converted basis, issuable upon the net exercise of Series AA warrants held by DAG Ventures III, L.P., (iv) 1,368 shares held of record by DAG
Ventures GP Fund III, LLC and (v) an aggregate of 79 shares of common stock, on an as converted basis, issuable upon the net exercise of Series AA warrants held by DAG
Ventures GP Fund III, LLC. DAG Ventures Management III, LLC is the sole general partner of each of DAG Ventures III—QP, L.P. and DAG Ventures III L.P.,
and is the sole managing member of DAG Ventures GP Fund III LLC. John Caddedu and R. Thomas Goodrich are the managing members of DAG Ventures Management III, LLC, and share voting
and investment power over these shares. The address for the entities affiliated with DAG Ventures is 251 Lytton Avenue, Suite 200, Palo Alto, CA 94301.
- (4)
- Represents
854,120 shares held directly by NCD Investors, a Delaware Multiple Series LLC. Redstone Management, LLC is the managing member of
NCD Investors, a Delaware Multiple Series LLC. Brent Jones, Mark Harris, Jared Stone, Thomas Vardell and Hosein Khajeh-Hosseiny, the managing members of Redstone Management, LLC, may be
deemed to share voting and investment power with respect to these shares. The address for NCD Investors, a Delaware Multiple Series LLC is 649 San Ramon Valley Road, Danville, CA 94526. The
number of shares beneficially owned after the offering includes 62,500 shares of common stock purchased in this offering by Northgate Capital FBO NCD Investors, a Delaware Multiple Series LLC.
- (5)
- Represents
(i) 563,996 shares held in irrevocable trusts for which Dr. Went and his wife are trustees or custodial arrangements for which Dr. Went is
custodian for the benefit of Dr. Went's children, (ii)(a) 8 shares held directly by Dr. Went, (b) an aggregate of 28,197 shares of common stock, on an as converted basis, issuable
upon the exercise of common stock and net exercise of Series AA warrants and 1,428,000 shares issuable pursuant to stock options exercisable within 60 days after February 28,
2014.
- (6)
- Represents 396,666 shares issuable pursuant to stock options exercisable within 60 days after February 28, 2014.
124
- (7)
- Represents
260,000 shares issuable pursuant to stock options exercisable within 60 days after February 28, 2014.
- (8)
- Includes
30,000 shares issuable pursuant to stock options exercisable within 60 days after February 28, 2014. William Ericson is a
general partner with Mohr Davidow Ventures and may be deemed to indirectly beneficially own the shares affiliated with Mohr Davidow Ventures. Mr. Ericson disclaims beneficial ownership thereof
except to the extent of his respective proportionate pecuniary interest in such shares.
- (9)
- Represents
16,000 shares held by Ivan Lieberburg and Janice Kirsch as Community Property with Right of Survivorship, over which Dr. Lieberburg shares
voting and dispositive power, 84,000 shares held directly by Dr. Lieberburg and 78,000 shares issuable pursuant to stock options exercisable within 60 days after February 28,
2014.
- (10)
- Represents
2,047,574 shares held directly by aeris CAPITAL Equity Investments, L.P., or the aeris LP, and an aggregate of 124,554 shares of
common stock, on an as converted basis, issuable upon the net exercise of Series AA warrants. George Rehm is a member of our board of directors and is a Managing Partner of aeris CAPITAL AG,
the investment advisor to the aeris LP, and has an economic interest in aeris CAPITAL Equity Ltd., the general partner of the aeris LP, or the aeris GP. By virtue of his
economic interest in the aeris GP, Mr. Rehm may be deemed to indirectly beneficially own the shares affiliated with aeris CAPITAL Equity Investments, L.P.
- (11)
- Represents
23,821 shares held directly by Mr. Mahoney, an aggregate of 2,003 shares of common stock, on an as converted basis, issuable upon the net
exercise of Series AA warrants and 111,332 shares issuable pursuant to stock options exercisable within 60 days after February 28, 2014.
- (12)
- Represents
216,000 shares issuable pursuant to stock options exercisable within 60 days after February 28, 2014.
- (13)
- Represents
36,000 shares of common stock and 17,778 shares of common stock issuable upon the exercise of common stock warrants and 30,000 shares issuable
pursuant to stock options exercisable within 60 days after February 28, 2014.
- (14)
- Represents
30,000 shares issuable pursuant to stock options exercisable within 60 days after February 28, 2014. Ms. Demski was elected
to our board of directors on March 4, 2014.
- (15)
- Represents
30,000 shares issuable pursuant to stock options exercisable within 60 days after February 28, 2014. Ms. Grootwassink Lewis
was elected to our board of directors on March 4, 2014.
- (16)
- Represents 723,825 shares held by our current directors and executive officers, 28,197 shares of common stock, on an as converted basis, issuable upon the exercise of common stock warrants and net exercise of Series AA warrants held by an executive officer, 3,045,414 shares issuable pursuant to stock options exercisable within 60 days after February 28, 2014, 6,058,578 shares held by entities affiliated with certain of our directors and 355,594 shares of common stock, on an as converted basis, issuable upon the exercise of common stock warrants and net exercise of Series AA warrants held by two of our directors and entities affiliated with certain of our directors.
125
General
The following description of our capital stock and certain provisions of our amended and restated certificate of incorporation and amended and restated bylaws are summaries and are qualified by reference to the amended and restated certificate of incorporation and the amended and restated bylaws that will be in effect upon the closing of this offering. Copies of these documents will be filed with the SEC as exhibits to our registration statement, of which this prospectus forms a part. The descriptions of our common stock and preferred stock reflect changes to our capital structure that will occur upon the closing of this offering.
Upon the closing of this offering, our amended and restated certificate of incorporation will provide for common stock and will authorize shares of undesignated preferred stock, the rights, preferences and privileges of which may be designated from time to time by our board of directors.
Upon the closing of this offering, our authorized capital stock will consist of 105,000,000 shares, all with a par value of $0.001 per share, of which:
- •
- 100,000,000 shares are designated as common stock; and
- •
- 5,000,000 shares are designated as preferred stock.
As of December 31, 2013, we had outstanding 9,515,528 shares of common stock and 4,719,174 shares of preferred stock. Immediately prior to the closing of this offering all outstanding shares of preferred stock will convert into an aggregate of 3,432,891 shares of our common stock. Our outstanding capital stock was held by 94 stockholders of record as of December 31, 2013. As of December 31, 2013, we had outstanding options to acquire an aggregate of 3,567,858 shares of common stock pursuant to our 2002 Plan and 2007 Plan. As of December 31, 2013, we also had outstanding warrants to purchase an aggregate of 213,278 shares of common stock and warrants to purchase an aggregate of 622,660 shares of preferred stock, which preferred stock warrants will automatically net exercise immediately prior to the closing of this offering, to the extent not exercised prior to such time.
Common stock
Voting
Each holder of our common stock is entitled to one vote for each share of common stock held on all matters submitted to a vote of stockholders, except as otherwise expressly provided in our amended and restated certificate of incorporation or required by applicable law. Our amended and restated certificate of incorporation does not provide for cumulative voting for the election of directors, which means that the holders of a majority of the then-outstanding shares of our common stock can elect all of the directors then standing for election.
Dividends and distributions
Subject to preferences that may apply to any shares of preferred stock outstanding at the time, the holders of outstanding shares of our common stock are entitled to receive dividends, if any, out of funds legally available at the times and in the amounts that our board of directors may determine.
Liquidation
Upon our liquidation, dissolution or winding-up, the assets legally available for distribution to our stockholders would be distributable ratably among the holders of our common stock and any participating preferred stock outstanding at that time after payment of liquidation preferences, on any outstanding shares of preferred stock and payment of other claims of creditors.
126
Rights and preferences
The rights, preferences, and privileges of holders of our common stock are subject to, and may be adversely affected by, the rights of holders of shares of any series of preferred stock that we may designate and issue in the future.
Preemptive or similar rights
Holders of our common stock have no preemptive, conversion, subscription or other similar rights, and there are no redemption or sinking fund provisions applicable to our common stock.
Fully paid and nonassessable
All outstanding shares of our common stock are fully paid and nonassessable, and all of the shares of common stock to be issued upon the closing of this offering will be fully paid and nonassessable.
Preferred stock
As of December 31, 2013, there were 4,719,174 shares of our preferred stock outstanding. Immediately prior to the closing of this offering, all outstanding shares of our preferred stock will convert into an aggregate of 3,432,891 shares of our common stock.
Upon the closing of this offering, our board of directors may, without further action by our stockholders, fix the rights, preferences, privileges and restrictions of up to an aggregate of 5,000,000 shares of preferred stock in one or more series and authorize their issuance. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms and the number of shares constituting any series or the designation of such series, any or all of which may be greater than the rights of our common stock. The issuance of our preferred stock could adversely affect the voting power of holders of our common stock and the likelihood that such holders will receive dividend payments and payments upon liquidation. In addition, the issuance of preferred stock could have the effect of delaying, deferring or preventing a change of control or other corporate action. Upon the closing of this offering, no shares of preferred stock will be outstanding, and we have no present plan to issue any shares of preferred stock.
Warrants
As of December 31, 2013, we had outstanding warrants to purchase an aggregate of 622,660 shares of our Series AA preferred stock with an exercise price of $3.80445 per share. Each of these warrants has a net exercise provision under which the holder, in lieu of payment of the exercise price in cash, can surrender the warrant and receive a net number of shares of our common stock based on the fair market value of such stock at the time of exercise of the warrant after deduction of the aggregate exercise price. Except to the extent earlier exercised, all of these warrants will automatically net exercise immediately prior to the closing of this offering pursuant to their terms.
As of December 31, 2013, we also had outstanding warrants to purchase an aggregate of 213,278 shares of our common stock with exercise prices ranging from $0.525 to $14.05 per share. Each of these warrants has a net exercise provision under which the holder, in lieu of payment of the exercise price in cash, can surrender the warrant and receive a net number of shares of our common stock based on the fair market value of such stock at the time of exercise of the warrant after deduction of the aggregate exercise price. Unless earlier exercised, warrants to purchase an aggregate of 88,884 shares of common stock will expire on September 25, 2014, warrants to purchase an aggregate of 38,562 shares of common stock will expire on the earlier of (i) a sale of the company for cash or (ii) ten years from the date of issuance, and warrants to purchase an aggregate of 85,832 shares of common stock will expire on November 26, 2014.
127
Registration rights
We are party to an investor rights agreement that provides that holders of our preferred stock and certain holders of common stock that received the common stock upon conversion of preferred stock have certain registration rights. This investor rights agreement was entered into in June 2011 and has been amended and/or restated from time to time in connection with our preferred stock financings. The registration of shares of our common stock pursuant to the exercise of registration rights described below would enable the holders who have these rights to sell these shares without restriction under the Securities Act when the applicable registration statement is declared effective. We will pay the registration expenses, other than underwriting discounts and commissions, of the shares registered pursuant to the demand, piggyback and Form S-3 registrations described below.
Generally, in an underwritten offering, the managing underwriter, if any, has the right, subject to specified conditions and limitations, to limit the number of shares the registration rights holders participating in any offering may include in any particular registration. The demand, piggyback and Form S-3 registration rights described below will expire on the earlier of (i) the date that is five years after the closing of this offering or (ii) with respect to each stockholder following the closing of this offering, at such time as (A) such stockholder holds less than 0.5% of the company's common stock on an as-converted, fully diluted basis and (B) such stockholder is entitled to sell all of its shares pursuant to Rule 144 of the Securities Act during any 90-day period.
Demand registration rights. The holders of an aggregate of 9,936,991 shares of our common stock (including shares previously issued upon conversion of preferred stock, shares issuable upon conversion of outstanding preferred stock and shares issuable upon conversion of shares of preferred stock issuable upon the exercise or, in certain cases, net exercise of outstanding warrants) are entitled to certain demand registration rights. At any time beginning sixth months after the closing of this offering, the holders of not less than 30% of these shares may, on not more than two occasions, request that we file a registration statement having an aggregate offering price to the public of not less than $10,000,000 to register at least 30% of their shares.
Piggyback registration rights. In connection with this offering, the holders of an aggregate of 9,936,991 shares of our common stock previously issued upon conversion of preferred stock and common stock issuable upon conversion of outstanding preferred stock and shares of preferred stock issuable upon the exercise or, in certain cases, net exercise of outstanding warrants, were entitled to, and the necessary percentage of holders waived, rights to include their shares of registrable securities in this offering. In the event that we propose to register any of our securities under the Securities Act, either for our own account or for the account of other security holders, the holders of these shares will be entitled to certain "piggyback" registration rights allowing them to include their shares in such registration, subject to certain marketing and other limitations. As a result, whenever we propose to file a registration statement under the Securities Act other than with respect to a demand registration or a registration statement on Form S-3, S-4 or S-8, the holders of these shares are entitled to notice of the registration and have the right, subject to limitations that the underwriters may impose on the number of shares included in the registration, to include their shares in the registration.
Form S-3 registration rights. The holders of an aggregate of 9,936,991 shares of our common stock previously issued upon conversion of preferred stock and common stock issuable upon conversion of outstanding preferred stock and shares of preferred stock issuable upon the exercise or, in certain cases, net exercise of outstanding warrants will be entitled to certain Form S-3 registration rights. Such holders may make a request that we register their shares on Form S-3 if we are qualified to file a registration statement on Form S-3. Such request for registration on Form S-3 must cover securities the aggregate offering price of which, after payment of underwriting discounts and commissions, is at least $3,000,000.
128
Anti-takeover provisions
Section 203 of the Delaware General Corporation Law
We are subject to Section 203 of the Delaware General Corporation Law, which prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years after the date that such stockholder became an interested stockholder, with the following exceptions:
- •
- before such date, the board of directors of the corporation approved either the business combination or the transaction
that resulted in the stockholder becoming an interested stockholder;
- •
- upon closing of the transaction that resulted in the stockholder becoming an interested stockholder, the interested
stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction began, excluding for purposes of determining the voting stock outstanding (but not the
outstanding voting stock owned by the interested stockholder) those shares owned by (i) persons who are directors and also officers and (ii) employee stock plans in which employee
participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer;
or
- •
- on or after such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of the stockholders, and not by written consent, by the affirmative vote of at least 662/3% of the outstanding voting stock that is not owned by the interested stockholder.
In general, Section 203 defines business combination to include:
- •
- any merger or consolidation involving the corporation and the interested stockholder;
- •
- any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested
stockholder;
- •
- subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of
the corporation to the interested stockholder;
- •
- any transaction involving the corporation that has the effect of increasing the proportionate share of the stock or any
class or series of the corporation beneficially owned by the interested stockholder; or
- •
- the receipt by the interested stockholder of the benefit of any loss, advances, guarantees, pledges or other financial benefits by or through the corporation.
In general, Section 203 defines an "interested stockholder" as an entity or person who, together with the person's affiliates and associates, beneficially owns, or within three years prior to the time of determination of interested stockholder status did own, 15% or more of the outstanding voting stock of the corporation. The statute could prohibit or delay mergers or other takeover or change in control attempts and, accordingly, may discourage attempts to acquire us even though such a transaction may offer our stockholders the opportunity to sell their stock at a price above the prevailing market price.
Certificate of incorporation and bylaws to be in effect upon the closing of this offering
Our amended and restated certificate of incorporation to be in effect upon the closing of this offering will provide for our board of directors to be divided into three classes with staggered three- year terms. Only one class of directors will be elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms. Because our stockholders do not have cumulative voting rights, our stockholders holding a majority of the voting power of our shares of common stock outstanding will be able to elect all of our directors. The directors may be removed by the stockholders only for cause and upon the vote of holders of a
129
majority of the shares then entitled to vote at an election of directors. Furthermore, the authorized number of directors may be changed only by resolution of our board of directors, and vacancies and newly created directorships on our board of directors may, except as otherwise required by law or determined by our board, only be filled by a majority vote of the directors then serving on the board, even though less than a quorum.
Our amended and restated certificate of incorporation and amended and restated bylaws also will provide that all stockholder actions must be effected at a duly called meeting of stockholders and not by a consent in writing. A special meeting of stockholders may be called only by a majority of our whole board of directors, the chair of our board of directors, our chief executive officer or our president. Our amended and restated bylaws will also provide that stockholders seeking to present proposals before a meeting of stockholders to nominate candidates for election as directors at a meeting of stockholders must provide timely advance notice in writing, and will specify requirements as to the form and content of a stockholder's notice.
Our amended and restated certificate of incorporation will further provide that, immediately after this offering, the affirmative vote of holders of at least 662/3% of the voting power of all of the then-outstanding shares of voting stock, voting as a single class, will be required to amend certain provisions of our certificate of incorporation, including provisions relating to the structure of our board of directors, the size of the board, removal of directors, special meetings of stockholders, actions by written consent and cumulative voting. The affirmative vote of holders of at least 662/3% of the voting power of all of the then-outstanding shares of voting stock, voting as a single class, will be required to amend or repeal our bylaws, although our bylaws may be amended by a simple majority vote of our board of directors.
The foregoing provisions will make it more difficult for our stockholders to replace our board of directors as well as for another party to obtain control of the company by replacing our board of directors. Since our board of directors has the power to retain and discharge our officers, these provisions could also make it more difficult for our stockholders or another party to effect a change in management. In addition, the authorization of undesignated preferred stock makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change the control of the company.
These provisions are intended to enhance the likelihood of continued stability in the composition of our board of directors and its policies. These provisions are also designed to reduce our vulnerability to an unsolicited acquisition proposal and to discourage certain tactics that may be used in proxy fights. However, such provisions could have the effect of discouraging others from making tender offers for our shares and may have the effect of deterring hostile takeovers or delaying changes in control of the company or our management. As a consequence, these provisions also may inhibit fluctuations in the market price of our stock that could result from actual or rumored takeover attempts.
Limitations of liability and indemnification
See the section of this prospectus entitled "Executive Compensation—Limitation on liability and indemnification matters."
Listing
Our common stock has been approved for listing on The NASDAQ Global Market under the trading symbol "ADMS."
Transfer agent and registrar
Upon the closing of this offering, the transfer agent and registrar for our common stock will be American Stock Transfer & Trust Company, LLC.
130
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, no public market for our common stock existed, and a liquid trading market for our common stock may not develop or be sustained after this offering. Future sales of substantial amounts of our common stock in the public market, including shares issued upon exercise of outstanding options and warrants, or the anticipation of such sales, could adversely affect prevailing market prices of our common stock from time to time and could impair our future ability to raise equity capital in the future. Furthermore, because only a limited number of shares of our common stock will be available for sale shortly after this offering due to certain contractual and legal restrictions on resale described below, sales of substantial amounts of our common stock in the public market after such restrictions lapse, or the anticipation of such sales, could adversely affect the prevailing market price of our common stock and our ability to raise equity capital in the future.
Based on the number of shares outstanding as of December 31, 2013, upon the closing of this offering, 16,422,992 shares of our common stock will be outstanding, assuming no exercise of the underwriters' over-allotment option to purchase additional shares of common stock and no exercise of outstanding options or warrants. Of the outstanding shares, all of the shares sold in this offering will be freely tradable, except that any shares held by our affiliates, as that term is defined in Rule 144 under the Securities Act, may only be sold in compliance with the limitations described below.
The remaining shares of our common stock outstanding after this offering are restricted securities as such term is defined in Rule 144 under the Securities Act, or are subject to lock-up agreements with us as described below. In addition, any shares sold in this offering to our existing stockholders that are our affiliates will be subject to lock-up agreements. Following the expiration of the lock-up period, restricted securities may be sold in the public market only if registered or if they qualify for an exemption from registration such as by reason of Rule 144 or 701 promulgated under the Securities Act, as amended, described in greater detail below.
Rule 144
In general, a person who has beneficially owned restricted shares of our common stock for at least six months would be entitled to sell their securities provided that (i) such person is not deemed to have been one of our affiliates at the time of, or at any time during the 90 days preceding a sale and (ii) we are subject to the periodic reporting requirements of the Exchange Act, for at least 90 days before the sale. Persons who have beneficially owned restricted or unrestricted shares of our common stock for at least six months but who are our affiliates at the time of, or any time during the 90 days preceding, a sale, would be subject to additional restrictions, by which such person would be entitled to sell within any three-month period only a number of securities that does not exceed the greater of either of the following:
- •
- 1% of the number of shares of our common stock outstanding after this offering, which will equal approximately 164,230
shares immediately after the closing of this offering, based on the number of common shares outstanding as of December 31, 2013 and assuming no exercise of the underwriters' over-allotment
option to purchase additional shares of our common stock; or
- •
- the average weekly trading volume of our common stock on The NASDAQ Global Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale;
provided, in each case, that we are subject to the periodic reporting requirements of the Exchange Act, for at least 90 days before the sale. Such sales both by affiliates and by non-affiliates must also comply with current public information provisions and sales by affiliates must comply with manner of sale and notice provisions of Rule 144.
131
Rule 701
Rule 701 under the Securities Act, as in effect on the date of this prospectus, permits resales of shares in reliance upon Rule 144 but without compliance with certain restrictions of Rule 144, including the holding period requirement. Most of our employees, executive officers, directors or consultants who purchased shares under a written compensatory plan or contract may be entitled to rely on the resale provisions of Rule 701, but all holders of Rule 701 shares are required to wait until 90 days after the date of this prospectus before selling their shares. However, substantially all Rule 701 shares are subject to lock-up agreements as described below and under the section of this prospectus entitled "Underwriting" and will become eligible for sale at the expiration of those agreements.
Lock-up agreements
We, our directors and officers, and substantially all of our stockholders, optionholders and warrant holders have agreed with the underwriters that for a period of 180 days following the date of this prospectus, we will not offer, sell, assign, transfer, pledge, contract to sell or otherwise dispose of or hedge any shares of our common stock or any securities convertible into or exchangeable for shares of our common stock, subject to specified exceptions. Credit Suisse Securities (USA) LLC and Piper Jaffray & Co. may, in their sole discretion, at any time, release all or any portion of the shares from the restrictions in this agreement.
Registration rights
The holders of our convertible preferred stock and warrants to purchase shares of our convertible preferred stock, or their transferees, are entitled to certain rights with respect to the registration of those shares under the Securities Act. For a description of these registration rights, see the section of this prospectus entitled "Description of Capital Stock—Registration rights." If these shares are registered, they will be freely tradable without restriction under the Securities Act, except for shares held by affiliates.
Equity incentive plans
As soon as practicable after the closing of this offering, we intend to file a Form S-8 registration statement under the Securities Act, to register shares of our common stock issued or reserved for issuance under our equity compensation plans and agreements. This registration statement will become effective immediately upon filing, and shares covered by this registration statement will thereupon be eligible for sale in the public markets, subject to vesting restrictions, the lock-up agreements described above and Rule 144 limitations applicable to affiliates. For a more complete discussion of our equity compensation plans, see the section of this prospectus entitled "Executive Compensation—Employee benefit and stock plans."
132
MATERIAL UNITED STATES FEDERAL INCOME
TAX CONSEQUENCES TO NON-U.S. HOLDERS
The following summary describes the material U.S. federal income and estate tax consequences of the acquisition, ownership and disposition of our common stock acquired in this offering by Non-U.S. Holders (as defined below). This discussion does not address all aspects of U.S. federal income and estate taxes and does not deal with foreign, state and local consequences that may be relevant to Non-U.S. Holders in light of their particular circumstances, nor does it address U.S. federal tax consequences other than income and estate taxes. Special rules different from those described below may apply to certain Non-U.S. Holders that are subject to special treatment under the Code, such as financial institutions, insurance companies, tax-exempt organizations, broker-dealers and traders in securities, U.S. expatriates, "controlled foreign corporations," "passive foreign investment companies," corporations that accumulate earnings to avoid U.S. federal income tax, persons that hold our common stock as part of a "straddle," "hedge," "conversion transaction," "synthetic security" or integrated investment or other risk reduction strategy, persons subject to the alternative minimum tax or Medicare contribution tax, partnerships and other pass-through entities, and investors in such pass-through entities. Such Non-U.S. Holders are urged to consult their own tax advisors to determine the U.S. federal, state, local and other tax consequences that may be relevant to them. Furthermore, the discussion below is based upon the provisions of the Code, and Treasury regulations, rulings and judicial decisions thereunder as of the date hereof, and such authorities may be repealed, revoked or modified, perhaps retroactively, so as to result in U.S. federal income and estate tax consequences different from those discussed below. We have not requested a ruling from the U.S. Internal Revenue Service, or IRS, with respect to the statements made and the conclusions reached in the following summary, and there can be no assurance that the IRS will agree with such statements and conclusions. This discussion assumes that the Non-U.S. Holder holds our common stock as a "capital asset" within the meaning of Section 1221 of the Code (generally, property held for investment).
Persons considering the purchase of our common stock pursuant to this offering should consult their own tax advisors concerning the U.S. federal income and estate tax consequences of acquiring, owning and disposing of our common stock in light of their particular situations as well as any consequences arising under the laws of any other taxing jurisdiction, including any state, local or foreign tax consequences.
For the purposes of this discussion, a "Non-U.S. Holder" is, for U.S. federal income tax purposes, a beneficial owner of common stock that is neither a U.S. Holder, nor a partnership (or other entity treated as a partnership for U.S. federal income tax purposes regardless of its place of organization or formation). A "U.S. Holder" means a beneficial owner of our common stock that is for U.S. federal income tax purposes (a) an individual who is a citizen or resident of the U.S., (b) a corporation or other entity treated as a corporation created or organized in or under the laws of the U.S., any state thereof or the District of Columbia, (c) an estate the income of which is subject to U.S. federal income taxation regardless of its source or (d) a trust if it (1) is subject to the primary supervision of a court within the U.S. and one or more U.S. persons have the authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person.
Distributions
Subject to the discussion below, distributions, if any, made on our common stock to a Non-U.S. Holder of our common stock to the extent made out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles) generally will constitute dividends for U.S. tax purposes and will be subject to withholding tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. To obtain a reduced rate of withholding under a treaty, a Non-U.S. Holder generally will be required to provide us with a properly executed IRS Form W-8BEN,
133
or other appropriate form, certifying the Non-U.S. Holder's entitlement to benefits under that treaty. In the case of a Non-U.S. Holder that is an entity, Treasury Regulations and the relevant tax treaty provide rules to determine whether, for purposes of determining the applicability of a tax treaty, dividends will be treated as paid to the entity or to those holding an interest in that entity. If a Non-U.S. Holder holds stock through a financial institution or other agent acting on the holder's behalf, the holder will be required to provide appropriate documentation to such agent. The holder's agent will then be required to provide certification to us or our paying agent, either directly or through other intermediaries. If you are eligible for a reduced rate of U.S. federal withholding tax under an income tax treaty, you may be able to obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim for a refund with the IRS.
We generally are not required to withhold tax on dividends paid to a Non-U.S. Holder that are effectively connected with the Non-U.S. Holder's conduct of a trade or business within the U.S. (and, if required by an applicable income tax treaty, are attributable to a permanent establishment that such holder maintains in the U.S.) if a properly executed IRS Form W-8ECI, stating that the dividends are so connected, is furnished to us (or, if stock is held through a financial institution or other agent, to such agent). In general, such effectively connected dividends will be subject to U.S. federal income tax, on a net income basis at the regular graduated rates. A corporate Non-U.S. Holder receiving effectively connected dividends may also be subject to an additional "branch profits tax," which is imposed, under certain circumstances, at a rate of 30% (or such lower rate as may be specified by an applicable treaty) on the corporate Non-U.S. Holder's effectively connected earnings and profits, subject to certain adjustments.
To the extent distributions on our common stock, if any, exceed our current and accumulated earnings and profits, they will first reduce the Non-U.S. Holder's adjusted basis in our common stock, but not below zero, and then will be treated as gain to the extent of any excess, and taxed in the same manner as gain realized from a sale or other disposition of common stock as described in the next section.
Gain on disposition of our common stock
Subject to the discussion below regarding backup withholding and foreign accounts, a Non-U.S. Holder generally will not be subject to U.S. federal income tax with respect to gain realized on a sale or other disposition of our common stock unless (a) the gain is effectively connected with a trade or business of such holder in the U.S. (and, if required by an applicable income tax treaty, is attributable to a permanent establishment that such holder maintains in the U.S.), (b) the Non-U.S. Holder is a nonresident alien individual and is present in the U.S. for 183 or more days in the taxable year of the disposition and certain other conditions are met or (c) we are or have been a "United States real property holding corporation" within the meaning of Code Section 897(c)(2) at any time within the shorter of the five-year period preceding such disposition or such holder's holding period. In general, we would be a U.S. real property holding corporation if interests in U.S. real estate comprised (by fair market value) at least half of our business assets. We believe that we are not, and do not anticipate becoming, a U.S. real property holding corporation. Even if we are treated as a U.S. real property holding corporation, gain realized by a Non-U.S. Holder on a disposition of our common stock will not be subject to U.S. federal income tax so long as (1) the Non-U.S. Holder owned, directly, indirectly and constructively, no more than five percent of our common stock at all times within the shorter of (i) the five-year period preceding the disposition or (ii) the holder's holding period and (2) our common stock is regularly traded on an established securities market. There can be no assurance that our common stock will qualify as regularly traded on an established securities market.
If you are a Non-U.S. Holder described in (a) above, you will be required to pay tax on the net gain derived from the sale at regular graduated U.S. federal income tax rates, and corporate Non-U.S. Holders described in (a) above may be subject to the additional branch profits tax at a 30% rate or
134
such lower rate as may be specified by an applicable income tax treaty. If you are an individual Non-U.S. Holder described in (b) above, you will be required to pay a flat 30% tax on the gain derived from the sale, which gain may be offset by U.S. source capital losses (even though you are not considered a resident of the U.S.).
Information reporting requirements and backup withholding
Generally, we must report information to the IRS with respect to any dividends we pay on our common stock including the amount of any such dividends, the name and address of the recipient, and the amount, if any, of tax withheld. A similar report is sent to the holder to whom any such dividends are paid. Pursuant to tax treaties or certain other agreements, the IRS may make its reports available to tax authorities in the recipient's country of residence.
Dividends paid by us (or our paying agents) to a Non-U.S. Holder may also be subject to U.S. backup withholding. U.S. backup withholding generally will not apply to a Non-U.S. Holder who provides a properly executed IRS Form W-8BEN or otherwise establishes an exemption.
Under current U.S. federal income tax law, U.S. information reporting and backup withholding requirements generally will apply to the proceeds of a disposition of our common stock effected by or through a U.S. office of any broker, U.S. or foreign, except that information reporting and such requirements may be avoided if the holder provides a properly executed IRS Form W-8BEN or otherwise meets documentary evidence requirements for establishing Non-U.S. Holder status or otherwise establishes an exemption. Generally, U.S. information reporting and backup withholding requirements will not apply to a payment of disposition proceeds to a Non-U.S. Holder where the transaction is effected outside the U.S. through a non-U.S. office of a non-U.S. broker. Information reporting and backup withholding requirements may, however, apply to a payment of disposition proceeds if the broker has actual knowledge, or reason to know, that the holder is, in fact, a U.S. person. For information reporting purposes, certain brokers with substantial U.S. ownership or operations will generally be treated in a manner similar to U.S. brokers.
Any amounts of tax withheld under the backup withholding rules may be credited against the tax liability of persons subject to backup withholding, provided that the required information is timely furnished to the IRS.
Foreign accounts
A U.S. federal withholding tax of 30% may apply on dividends on and the gross proceeds of a disposition of our common stock paid to a foreign financial institution (as specifically defined by applicable rules) unless such institution enters into an agreement with the U.S. government to withhold on certain payments and to collect and provide to the U.S. tax authorities substantial information regarding U.S. account holders of such institution (which includes certain equity holders of such institution, as well as certain account holders that are foreign entities with U.S. owners). This U.S. federal withholding tax of 30% will also apply on dividends on and the gross proceeds of a disposition of our common stock to a non-financial foreign entity unless such entity provides the withholding agent with either a certification that it does not have any substantial direct or indirect U.S. owners or provides information regarding substantial direct and indirect U.S. owners of the entity. The withholding tax described above will not apply if the foreign financial institution or non-financial foreign entity otherwise qualifies for an exemption from the rules. Under certain circumstances, a Non-U.S. Holder might be eligible for refunds or credits of such taxes. Holders are encouraged to consult with their own tax advisors regarding the possible implications of the legislation on these rules for their investment in our common stock.
135
The IRS has issued guidance providing that the withholding provisions described above will generally apply to payments of dividends made on or after July 1, 2014 and to payments of gross proceeds from a sale or other disposition of common stock on or after January 1, 2017.
Federal estate tax
An individual Non-U.S. Holder who is treated as the owner of, or has made certain lifetime transfers of, an interest in our common stock will be required to include the value thereof in his or her gross estate for U.S. federal estate tax purposes, and may be subject to U.S. federal estate tax unless an applicable estate tax treaty provides otherwise, even though such individual was not a citizen or resident of the U.S. at the time of his or her death.
EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS OWN TAX ADVISOR REGARDING THE TAX CONSEQUENCES OF PURCHASING, HOLDING AND DISPOSING OF OUR COMMON STOCK, INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAW.
136
Under the terms and subject to the conditions contained in an underwriting agreement dated April 9, 2014, we have agreed to sell to the underwriters named below, for whom Credit Suisse Securities (USA) LLC and Piper Jaffray & Co. are acting as representatives, the following respective numbers of shares of common stock:
Underwriter
|
Number of Shares |
|||
|---|---|---|---|---|
Credit Suisse Securities (USA) LLC |
1,275,000 | |||
Piper Jaffray & Co. |
975,000 | |||
William Blair & Company, L.L.C. |
375,000 | |||
Needham & Company, LLC |
375,000 | |||
| | | | | |
Total |
3,000,000 | |||
| | | | | |
| | | | | |
The underwriting agreement provides that the underwriters are obligated to purchase all the shares of common stock in the offering if any are purchased, other than those shares covered by the over-allotment option described below. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may be increased or the offering may be terminated.
We have granted to the underwriters a 30-day over-allotment option to purchase on a pro rata basis up to 450,000 additional shares at the initial public offering price less the underwriting discounts and commissions. The option may be exercised only to cover any over-allotments of our common stock in this offering.
The underwriters are offering the shares, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel including the validity of the shares, and subject to other conditions contained in the underwriting agreement, such as the receipt by the underwriters of officer's certificates and legal opinions. The offering of the shares by the underwriters is also subject to the underwriters' right to reject any order in whole or in part.
Certain of our directors and existing stockholders, or their affiliates, are purchasing an aggregate of 362,500 shares of common stock in this offering. The shares will be offered and sold on the same terms as the other shares that are being offered and sold in this offering to the public.
The underwriters propose to offer the shares of common stock initially at the public offering price on the cover page of this prospectus and to selling group members at that price less a selling concession of up to $0.672 per share. After the initial public offering the representatives may change the public offering price and selling concession and discount to broker/dealers.
The following table summarizes the compensation we will pay:
| |
Per Share | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Without Over- allotment |
With Over- allotment |
Without Over- allotment |
With Over- allotment |
|||||||||
Underwriting discounts and commissions paid by us |
$ | 1.12 | $ | 1.12 | $ | 3,360,000 | $ | 3,864,000 | |||||
We estimate that our out of pocket expenses for this offering (not including any underwriting discounts and commissions) will be approximately $3.1 million.
We have agreed to reimburse the underwriters for expenses of up to $50,000 related to clearance of this offering with the Financial Industry Regulatory Authority, Inc. or FINRA.
137
The underwriters have informed us that they do not expect sales to accounts over which the underwriters have discretionary authority to exceed 5% of the shares of common stock being offered.
We have agreed that we will not offer, sell, contract to sell, pledge or otherwise dispose of, directly or indirectly, or file with the SEC a registration statement under the Securities Act, relating to any shares of our common stock or securities convertible into or exchangeable or exercisable for any shares of our common stock, or publicly disclose the intention to make any offer, sale, pledge, disposition or filing, without the prior written consent of the representatives for a period of 180 days after the date of this prospectus, or the Lock-Up Period except issuances pursuant to the conversion or exchange of convertible or exchangeable securities outstanding on the date hereof or the exercise of warrants or options outstanding on the date hereof, grants of employee stock options pursuant to our existing plans or issuances pursuant to the exercise of such employee options.
Our officers, directors and substantially all of our existing security holders have agreed that they will not offer, sell, contract to sell, pledge or otherwise dispose of, directly or indirectly, any shares of our common stock or securities convertible into or exchangeable or exercisable for any shares of our common stock, enter into a transaction that would have the same effect, or enter into any swap, hedge or other arrangement that transfers, in whole or in part, any of the economic consequences of ownership of our common stock, whether any of these transactions are to be settled by delivery of our common stock or other securities, in cash or otherwise, or publicly disclose the intention to make any offer, sale, pledge or disposition, or to enter into any transaction, swap, hedge or other arrangement, without, in each case, the prior written consent of the representatives during the Lock-Up Period, subject to limited exceptions.
We have agreed to indemnify the several underwriters against liabilities under the Securities Act, or contribute to payments that the underwriters may be required to make in that respect.
Our common stock has been approved for listing on The NASDAQ Global Market under the symbol "ADMS".
Prior to the offering, there has been no public market for our common stock. The initial public offering price was determined through negotiations between us and the representatives. In determining the initial public offering price, we and the representatives considered a number of factors including:
- •
- the information set forth in this prospectus and otherwise available to the underwriters;
- •
- our prospects and the history and prospects for the industry in which we compete;
- •
- an assessment of our management;
- •
- our prospects for future earnings;
- •
- the recent market prices of, and demand for, publicly-traded common stock of generally comparable companies;
- •
- the general condition of the securities markets at the time of the offering; and
- •
- other factors deemed relevant by the underwriters and us.
Neither we nor the underwriters can assure investors that an active trading market will develop for our common stock, or that shares of our common stock will trade in the public market at or above the initial public offering price.
In connection with the offering the underwriters may engage in stabilizing transactions, over-allotment transactions, syndicate covering transactions, penalty bids and passive market making in accordance with Regulation M under the Exchange Act.
138
- •
- Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a
specified maximum.
- •
- Over-allotment transactions involve sales by the underwriters of shares in excess of the number of shares the underwriters
are obligated to purchase, creating a syndicate short position. The short position may be either a covered short position or a naked short position. In a covered short position, the number of shares
over-allotted by the underwriters is not greater than the number of shares that they may purchase in the over-allotment option. In a naked short position, the number of shares involved is greater than
the number of shares in the over-allotment option. The underwriters may close out any covered short position by either exercising their over-allotment option and/or purchasing shares in the open
market.
- •
- Syndicate covering transactions involve purchases of the common stock in the open market after the distribution has been
completed in order to cover syndicate short positions. In determining the source of shares to close out the short position, the underwriters will consider, among other things, the price of shares
available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. If the underwriters sell more shares than could be covered by
the over-allotment option or a naked short position, the position can only be closed out by buying shares in the open market. A naked short position is more likely to be created if the underwriters
are concerned that there could be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in the offering.
- •
- Penalty bids permit the representatives to reclaim a selling concession from a syndicate member when the common stock
originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions.
- •
- In passive market making, market makers in the common stock who are underwriters or prospective underwriters may, subject to limitations, make bids for or purchases of our common stock until the time, if any, at which a stabilizing bid is made.
These stabilizing transactions, over-allotment transactions, syndicate covering transactions, penalty bids and passive market making may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of the common stock. As a result the price of our common stock may be higher than the price that might otherwise exist in the open market. These transactions may be effected on The NASDAQ Global Market or otherwise and, if commenced, may be discontinued at any time.
A prospectus in electronic format may be made available on the web sites maintained by one or more of the underwriters, or selling group members, if any, participating in this offering and one or more of the underwriters participating in this offering may distribute prospectuses electronically. The representatives may agree to allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the underwriters and selling group members that will make internet distributions on the same basis as other allocations.
Other relationships
Certain of the underwriters and their affiliates have provided in the past to us and our affiliates and may provide from time to time in the future certain commercial banking, financial advisory, investment banking and other services for us and such affiliates in the ordinary course of their business, for which they may receive customary fees and commissions. In addition, from time to time, certain of the underwriters and their affiliates may effect transactions for their own account or the account of customers, and hold on behalf of themselves or their customers, long or short positions in our debt or
139
equity securities or loans, and may do so in the future. The underwriters are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, principal investment, hedging, financing and brokerage activities.
Selling restrictions
Notice to prospective investors in the European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a "Relevant Member State"), each underwriter represents and agrees that with effect from and including the date on which the Prospectus Directive is implemented in that Relevant Member State, it has not made and will not make an offer of shares which are the subject of the offering contemplated by this prospectus to the public in that Relevant Member State other than:
- (a)
- to
any legal entity which is a qualified investor as defined in the Prospectus Directive;
- (b)
- to
fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150, natural or legal persons
(other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject to obtaining the prior consent of the representatives for any such offer;
or
- (c)
- in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of shares shall require us or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Directive.
For the purposes of this provision, the expression an "offer to the public" in relation to any shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the shares to be offered so as to enable an investor to decide to purchase or subscribe the shares, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State, the expression Prospectus Directive means Directive 2003/71/EC (and amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State), and includes any relevant implementing measure in the Relevant Member State and the expression "2010 PD Amending Directive" means Directive 2010/73/EU.
Notice to prospective investors in the United Kingdom
Each of the underwriters severally represents, warrants and agrees as follows:
- (a)
- it
has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in
investment activity (within the meaning of Section 21 of the Financial Services and Markets Act 2000 ("FSMA") received by it in connection with the issue or sale of the shares in circumstances
in which Section 21 of the FSMA does not apply to us; and
- (b)
- it has complied with, and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the shares in, from or otherwise involving the United Kingdom.
Notice to prospective investors in Switzerland
Our shares of common stock may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange, or SIX, or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards
140
for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering or marketing material relating to the shares or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, us or the shares have been or will be filed with or approved by any Swiss regulatory authority. In particular this document will not be filed with, and the offer of shares will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA, and the offer of the shares has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes, or CISA. The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of the shares.
141
Cooley LLP, San Francisco and Palo Alto, California, will pass upon the validity of the shares of common stock offered hereby. The underwriters are being represented by Davis Polk & Wardwell LLP, Menlo Park, California, in connection with the offering.
The consolidated financial statements of Adamas Pharmaceuticals, Inc. as of December 31, 2012 and 2013, and for each of the two years in the period ended December 31, 2013, included in this prospectus have been so included in reliance on the report of PricewaterhouseCoopers LLP, an independent registered public accounting firm, given on the authority of said firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to this offering of our common stock. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement, some items of which are contained in exhibits to the registration statement as permitted by the rules and regulations of the SEC. For further information with respect to us and our common stock, we refer you to the registration statement, including the exhibits and the financial statements and notes filed as a part of the registration statement. Statements contained in this prospectus concerning the contents of any contract or any other document are not necessarily complete. If a contract or document has been filed as an exhibit to the registration statement, please see the copy of the contract or document that has been filed. Each statement in this prospectus relating to a contract or document filed as an exhibit is qualified in all respects by the filed exhibit. A copy of the registration statement and the exhibits filed therewith may be inspected without charge at the public reference room of the SEC, located at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may obtain information on the operation of the public reference rooms by calling the SEC at 1-800-SEC-0330. The SEC also maintains an Internet website that contains reports, proxy statements, and other information about issuers, like us, that file electronically with the SEC. The address of that website is www.sec.gov.
As a result of this offering, we will become subject to the information and reporting requirements of the Exchange Act and, in accordance with this law, will file periodic reports, proxy statements, and other information with the SEC. These periodic reports, proxy statements, and other information will be available for inspection and copying at the SEC's public reference facilities and the website of the SEC referred to above. We also maintain a website at www.adamaspharma.com. After the closing of this offering, you may access our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge at our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not incorporated by reference into this prospectus.
142
ADAMAS PHARMACEUTICALS, INC.
INDEX TO FINANCIAL STATEMENTS
| |
Page | |
|---|---|---|
F-2 |
||
Consolidated Financial Statements |
||
F-3 |
||
Consolidated Statements of Operations and Comprehensive Income |
F-4 |
|
Consolidated Statements of Convertible Preferred Stock and Stockholders' Equity |
F-5 |
|
F-6 |
||
F-7 |
F-1
Report of Independent Registered Public Accounting Firm
To
the Board of Directors and Stockholders
of Adamas Pharmaceuticals, Inc.
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of operations and comprehensive income, of convertible preferred stock and stockholders' equity and cash flows present fairly, in all material respects, the financial position of Adamas Pharmaceuticals, Inc. and its subsidiaries (the "Company") at December 31, 2013 and 2012, and the results of their operations and their cash flows for each of the two years in the period ended December 31, 2013 in conformity with accounting principles generally accepted in the United States of America. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
/s/ PricewaterhouseCoopers LLP
San
Jose, California
March 5, 2014, except for the effects of the stock split described in Note 15, as to which the date is March 24, 2014
F-2
Adamas Pharmaceuticals, Inc.
Consolidated Balance Sheets
(in thousands except share and per share data)
| |
|
|
Pro Forma Stockholders' Equity as of December 31, 2013 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
December 31, | |||||||||
| |
2012 | 2013 | ||||||||
| |
|
|
(unaudited) |
|||||||
Assets |
||||||||||
Current assets |
||||||||||
Cash and cash equivalents |
$ | 62,957 | $ | 85,612 | $ | |||||
Accounts receivable, net |
890 | 129 | ||||||||
Prepaid expenses and other current assets |
348 | 267 | ||||||||
| | | | | | | | | | | |
Total current assets |
64,195 | 86,008 | ||||||||
Property and equipment, net |
99 | 199 | ||||||||
Other assets |
9 | 9 | ||||||||
| | | | | | | | | | | |
Total assets |
$ | 64,303 | $ | 86,216 | $ | |||||
| | | | | | | | | | | |
| | | | | | | | | | | |
Liabilities, convertible preferred stock |
||||||||||
Current liabilities |
||||||||||
Accounts payable |
$ | 3,255 | $ | 2,097 | $ | |||||
Accrued liabilities |
1,406 | 2,119 | ||||||||
Convertible notes payable |
4,000 | — | ||||||||
Deferred revenue |
29,611 | — | ||||||||
Interest payable and other current liabilities |
208 | 2 | ||||||||
| | | | | | | | | | | |
Total current liabilities |
38,480 | 4,218 | ||||||||
Warrant liability |
1,706 | 6,232 | — | |||||||
Other non current liabilities |
— | 12 | ||||||||
| | | | | | | | | | | |
Total liabilities |
40,186 | 10,462 | ||||||||
| | | | | | | | | | | |
Commitments and contingencies (Note 7) |
||||||||||
Convertible preferred stock, $0.001 par value—6,700,000 shares authorized in 2012, and 2013 respectively, and 4,719,174 shares issued and outstanding at December 31, 2012 and 2013, respectively and no shares outstanding pro forma (unaudited); liquidation value of $77,433 at December 31, 2012 and 2013, respectively |
19,149 | 19,149 | — | |||||||
| | | | | | | | | | | |
Stockholders' equity |
||||||||||
Common stock, $0.001 par value—100,000,000 shares authorized, 9,499,862 and 9,515,528 shares issued and outstanding at December 31, 2012 and 2013, respectively and 13,422,992 shares outstanding pro forma (unaudited); |
14 | 14 | 14 | |||||||
Additional paid-in capital |
76,447 | 77,163 | 102,544 | |||||||
Accumulated deficit |
(71,493 | ) | (20,572 | ) | (20,572 | ) | ||||
| | | | | | | | | | | |
Total stockholders' equity |
4,968 | 56,605 | 81,986 | |||||||
| | | | | | | | | | | |
Total liabilities, convertible preferred stock and stockholders' equity |
$ | 64,303 | $ | 86,216 | $ | |||||
| | | | | | | | | | | |
| | | | | | | | | | | |
The accompanying notes are an integral part of these financial statements.
F-3
Adamas Pharmaceuticals, Inc.
Consolidated Statements of Operations and Comprehensive Income
(in thousands except per share data)
| |
Years Ended December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Revenue |
$ | 37,471 | $ | 71,095 | |||
| | | | | | | | |
Operating expenses |
|||||||
Research and development |
9,192 | 7,410 | |||||
General and administrative |
8,330 | 6,667 | |||||
| | | | | | | | |
Total operating expenses |
17,522 | 14,077 | |||||
| | | | | | | | |
Income from operations |
19,949 | 57,018 | |||||
Interest and other income (expense), net |
(1,537 | ) | (4,818 | ) | |||
Interest expense |
(376 | ) | (88 | ) | |||
| | | | | | | | |
Income before income taxes |
18,036 | 52,112 | |||||
Income tax expense |
(300 | ) | (1,191 | ) | |||
| | | | | | | | |
Net income |
$ | 17,736 | $ | 50,921 | |||
| | | | | | | | |
| | | | | | | | |
Net income attributable to common stockholders |
|||||||
Basic |
$ | 11,441 | $ | 33,068 | |||
| | | | | | | | |
| | | | | | | | |
Diluted |
$ | 11,596 | $ | 35,353 | |||
| | | | | | | | |
| | | | | | | | |
Net income per share attributable to common stockholders |
|||||||
Basic |
$ | 1.21 | $ | 3.48 | |||
| | | | | | | | |
| | | | | | | | |
Diluted |
$ | 1.17 | $ | 3.00 | |||
| | | | | | | | |
| | | | | | | | |
Weighted average number of shares used in computing net income attributable to common stockholders |
|||||||
Basic |
9,488 | 9,506 | |||||
| | | | | | | | |
| | | | | | | | |
Diluted |
9,924 | 11,806 | |||||
| | | | | | | | |
| | | | | | | | |
Pro forma net income per share attributable to common stockholders (unaudited) |
|||||||
Basic |
$ | 4.13 | |||||
| | | | | | | | |
| | | | | | | | |
Diluted |
$ | 3.51 | |||||
| | | | | | | | |
| | | | | | | | |
Pro forma weighted average number of shares used in computing net income attributable to common stockholders (unaudited) |
|||||||
Basic |
13,414 | ||||||
| | | | | | | | |
| | | | | | | | |
Diluted |
15,790 | ||||||
| | | | | | | | |
| | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Consolidated Statements of Convertible Preferred Stock and Stockholders' Equity
(in thousands except share and per share data)
| |
Convertible Preferred Stock |
|
|
|
|
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Common Stock | |
|
|
||||||||||||||||||
| |
Additional Paid-In Capital |
Accumulated Deficit |
Total Stockholders' Equity |
|||||||||||||||||||
| |
Shares | Amount | Shares | Amount | ||||||||||||||||||
Balances at December 31, 2011 |
3,667,832 | $ | 15,336 | 9,424,862 | $ | 14 | $ | 75,583 | $ | (89,229 | ) | $ | (13,632 | ) | ||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
Issuance of common stock in January 2012 in consideration of service at fair value of $0.99 per share |
— | — | 65,000 | — | 64 | — | 64 | |||||||||||||||
Issuance of common stock in July 2012 from the exercise of options for cash at $0.67 per share |
— | — | 10,000 | — | 1 | — | 1 | |||||||||||||||
Issuance of Series AA convertible preferred stock at $3.81 per share recorded at relative fair value, net of issuance costs of $188 |
1,051,342 | 3,813 | — | — | — | — | — | |||||||||||||||
Vesting of common stock |
— | — | — | — | 2 | — | 2 | |||||||||||||||
Employee stock-based compensation |
— | — | — | — | 221 | — | 221 | |||||||||||||||
Nonemployee stock-based compensation |
— | — | — | — | 576 | — | 576 | |||||||||||||||
Net income |
— | — | — | — | — | 17,736 | 17,736 | |||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2012 |
4,719,174 | 19,149 | 9,499,862 | 14 | 76,447 | (71,493 | ) | 4,968 | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
Issuance of common stock in July 2013 from the exercise of options for cash at $1.37 per share |
— | — | 15,666 | — | 16 | — | 16 | |||||||||||||||
Vesting of common stock |
— | — | — | — | 8 | — | 8 | |||||||||||||||
Modification of common stock purchase warrants |
— | — | — | — | 52 | — | 52 | |||||||||||||||
Employee stock-based compensation |
— | — | — | — | 275 | — | 275 | |||||||||||||||
Nonemployee stock-based compensation |
— | — | — | — | 365 | — | 365 | |||||||||||||||
Net income |
— | — | — | — | — | 50,921 | 50,921 | |||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2013 |
4,719,174 | $ | 19,149 | 9,515,528 | $ | 14 | 77,163 | $ | (20,572 | ) | $ | 56,605 | ||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Adamas Pharmaceuticals, Inc.
Consolidated Statements of Cash Flows
(in thousands)
| |
Years Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Cash flows from operating activities |
|||||||
Net income |
$ | 17,736 | $ | 50,921 | |||
Adjustments to reconcile net income to net cash provided by operating activities |
|||||||
Depreciation and amortization |
41 | 66 | |||||
Stock-based compensation |
797 | 640 | |||||
Change in preferred stock warrant value |
1,330 | 4,526 | |||||
Provision for employee notes receivable |
158 | 1 | |||||
Noncash interest expense |
377 | — | |||||
Issuance of common stock and vesting of restricted common stock for services received |
67 | 52 | |||||
Changes in assets and liabilities |
|||||||
Prepaid expenses and other assets |
(39 | ) | 79 | ||||
Accounts receivable |
(516 | ) | 761 | ||||
Accounts payable |
1,799 | (1,157 | ) | ||||
Accrued liabilities and other liabilities |
600 | 523 | |||||
Deferred revenue |
29,611 | (29,611 | ) | ||||
| | | | | | | | |
Net cash provided by operating activities |
51,961 | 26,801 | |||||
| | | | | | | | |
Cash flows from investing activities |
|||||||
Purchase of property and equipment |
(24 | ) | (167 | ) | |||
| | | | | | | | |
Net cash used in investing activities |
(24 | ) | (167 | ) | |||
| | | | | | | | |
Cash flows from financing activities |
|||||||
Proceeds from issuance of convertible preferred stock, net of issuance costs |
3,948 | — | |||||
Proceeds from issuance of common stock upon exercise of stock options |
7 | 21 | |||||
Proceeds from issuance of convertible promissory notes and related warrants |
3,948 | — | |||||
Principal payments on convertible promissory notes |
— | (4,000 | ) | ||||
| | | | | | | | |
Net cash provided by (used in) financing activities |
7,903 | (3,979 | ) | ||||
| | | | | | | | |
Net increase in cash and cash equivalents |
59,840 | 22,655 | |||||
Cash and cash equivalents at beginning of period |
3,117 | 62,957 | |||||
| | | | | | | | |
Cash and cash equivalents at end of period |
$ | 62,957 | $ | 85,612 | |||
| | | | | | | | |
| | | | | | | | |
Supplemental disclosure |
|||||||
Cash paid for interest |
$ | — | $ | 279 | |||
Cash paid for income taxes |
— | 1,501 | |||||
Supplemental disclosure of noncash items |
|||||||
Issuance of warrants for preferred stock in connection with 2012 notes and preferred stock financing |
269 | 200 | |||||
Accrued deferred offering costs |
— | 85 | |||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
1. The Company
Adamas Pharmaceuticals, Inc. (the "Company") is a specialty pharmaceutical company focused on the development and commercialization of therapeutics targeting chronic disorders of the central nervous systems ("CNS"). The Company achieves this by enhancing the pharmacokinetic profiles of proven drugs to create novel therapeutics for use alone and in fixed-dose combination products. The Company is developing its lead wholly owned product candidate, ADS-5102, for a complication of Parkinson's disease known as levodopa induced dyskinesia ("LID") and as a treatment for chronic behavioral symptoms associated with traumatic brain injury ("TBI"). The Company has successfully completed a Phase 2/3 clinical study in LID and intends to initiate a Phase 3 registration trial in 2014. Its late-stage therapeutics portfolio also includes an NDA-submitted product candidate, MDX-8704, being co-developed with Forest Laboratories, Inc. ("Forest"), and an approved product, Namenda XR, which Forest developed and is marketing in the United States under a license from the Company.
The Company was incorporated in the State of Delaware on November 15, 2000. The Company's headquarters and operations are located in Emeryville, California. The Company has two subsidiaries: Adamas Pharmaceuticals Asia Pte Limited (inactive) and Adamas India Pharmaceuticals Private Limited, which ceased operations in August 2013.
2. Summary of Significant Accounting Policies
Basis of Presentation and Use of Estimates
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States ("U.S. GAAP"). The preparation of the accompanying consolidated financial statements in accordance with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities as of the date of the consolidated financial statements, and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates.
To date, nearly all of the Company's resources have been dedicated to the research and development of its products, and the Company has not generated any commercial revenue from the sale of its products. The Company does not anticipate the generation of any commercial product revenue until it receives the necessary regulatory approvals to launch one of its products.
Based upon the current status of, and plans for, its product development, the Company believes that the existing cash and cash equivalents will be adequate to satisfy the Company's capital needs through at least the next twelve months. However, the process of developing and commercializing products requires significant research and development, preclinical testing and clinical trials, manufacturing arrangements as well as regulatory approvals. These activities, together with the Company's general and administrative expenses, are expected to result in significant operating losses until the commercialization of the Company's products or partner collaborations generate sufficient revenue to cover expenses. While the Company had net income during 2012 and 2013, it has not generated any commercial revenue from sales of its products. To achieve sustained profitability, the Company, alone or with others, must successfully develop its product candidates, obtain required regulatory approvals and successfully manufacture and market its products.
F-7
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
2. Summary of Significant Accounting Policies (Continued)
Unaudited Pro Forma Balance Sheet Information
The unaudited pro forma stockholders' equity information in the accompanying consolidated balance sheet reflects that there are 13,422,992 shares of common stock outstanding and assumes: (i) the automatic conversion of all outstanding shares of convertible preferred stock into shares of common stock, (ii) the issuance of common stock upon conversion of the Series AA preferred stock issued upon net exercise of warrants, (iii) the related reclassification of the convertible preferred warrant liability to additional paid-in-capital. The unaudited pro forma stockholders' equity does not assume any proceeds from the proposed initial public offering.
Stock Splits
In June 2011, the Board of Directors of the Company approved a reverse stock split of the Company's common stock. As a result, common stock, stock options and warrants to purchase common stock were adjusted in the ratio of 1:3, effective June 30, 2011. All common shares and per share amounts presented in these consolidated financial statements for all periods have been retroactively adjusted to reflect this reverse stock split (see Note 10). The Company completed a 2-for-1 forward stock split on March 24, 2014 (see Note 15). No fractional shares were issued.
Consolidation
The consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries. Intercompany balances and transactions have been eliminated in consolidation.
Segments
The Company operates in one segment. Management uses one measurement of profitability and does not segregate its business for internal reporting. All long-lived assets are maintained in the United States.
Cash and Cash Equivalents
The Company considers all highly liquid investments purchased with an original maturity of three months or less to be cash equivalents.
Revenue Recognition
The Company recognizes revenue when all four of the following criteria have been met: (i) persuasive evidence of an arrangement exists, (ii) delivery has occurred or services have been rendered, (iii) the fee is fixed or determinable and (iv) collectability is reasonably assured. Revenue under license and collaboration arrangements is recognized based on the performance requirements of the contract. Determinations of whether persuasive evidence of an arrangement exists and whether delivery has occurred or services have been rendered are based on management's judgments regarding the fixed nature of the fees charged for deliverables and the collectability of those fees. Should changes in conditions cause management to determine that these criteria are not met for any new or modified transactions, revenue recognized could be adversely affected.
The Company generates revenue from collaboration and license agreements for the development and commercialization of products. Collaboration and license agreements may include non-refundable upfront license fees, partial or complete reimbursement of research and development costs, contingent
F-8
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
2. Summary of Significant Accounting Policies (Continued)
consideration payments based on the achievement of defined collaboration objectives and royalties on sales of commercialized products. The Company's performance obligations under the collaborations may include the license or transfer of intellectual property rights, obligations to provide research and development services and related materials and obligations to participate on certain development and/or commercialization committees with the collaborators.
On January 1, 2011, the Company adopted an accounting standards update that amends the guidance on accounting for new arrangements, or those materially modified, with multiple deliverables. This guidance eliminates the requirement for objective and reliable evidence of fair value of the undelivered items in order to consider a deliverable a separate unit of accounting. It also changes the allocation method such that the relative-selling-price method must be used to allocate arrangement consideration to the units of accounting in an arrangement. This guidance establishes the following estimation hierarchy that must be used in estimating selling price under the relative-selling-price method: (i) vendor-specific objective evidence of fair value of the deliverable, if it exists, (ii) third-party evidence of selling price, if vendor-specific objective evidence is not available or (iii) vendor's best estimate of selling price, if neither vendor-specific nor third-party evidence is available.
On January 1, 2011, the Company adopted an accounting standards update that provides guidance on revenue recognition using the milestone method. Payments that are contingent upon achievement of a substantive milestone are recognized in their entirety in the period in which the milestone is achieved. Milestones are defined as events that can only be achieved based on the Company's performance and there is substantive uncertainty about whether the event will be achieved at the inception of the arrangement. Events that are contingent only on the passage of time or only on counterparty performance are not considered milestones subject to this guidance. Further, the amounts received must relate solely to prior performance, be reasonable relative to all of the deliverables and payment terms within the agreement and commensurate with the Company's performance to achieve the milestone after commencement of the agreement.
Amounts related to research and development funding are recognized as the related services or activities are performed, in accordance with the contract terms. Payments may be made to or by the Company based on the number of full-time equivalent researchers assigned to the collaboration project and the related research and development expenses incurred.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to credit risk consist principally of cash, cash equivalents and accounts receivable. Substantially all the Company's cash and cash equivalents are held at one financial institution that management believes is of high credit quality. Such deposits generally exceed federally insured limits.
Risk and Uncertainties
The Company's future results of operations involve a number of risks and uncertainties. Factors that could affect the Company's future operating results and cause actual results to vary materially from expectations include, but are not limited to, rapid technological change, uncertainty of results of clinical trials and reaching milestones, uncertainty of market acceptance of the Company's products, competition from substitute products and larger companies, protection of proprietary technology, strategic relationships and dependence on key individuals.
F-9
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
2. Summary of Significant Accounting Policies (Continued)
Products developed by the Company require approvals from the U.S. Food and Drug Administration ("FDA") or other international regulatory agencies prior to commercial sales. There can be no assurance that the products will receive the necessary approvals. If the Company was denied approval, approval was delayed or the Company was unable to maintain approval, it could have a materially adverse impact on the Company.
The Company has expended and will continue to expend substantial funds to complete the research, development and clinical testing of product candidates. The Company also will be required to expend additional funds to establish commercial-scale manufacturing arrangements and to provide for the marketing and distribution of products that receive regulatory approval. The Company may require additional funds to commercialize its products. The Company is unable to entirely fund these efforts with its current financial resources. Additional funds may not be available on acceptable terms, if at all. If adequate funds are unavailable on a timely basis from operations or additional sources of financing, the Company may have to delay, reduce the scope of or eliminate one or more of its research or development programs which would materially and adversely affect its business, financial condition and operations.
Property and Equipment
Property and equipment are stated at cost. Depreciation is computed using the straight-line method over the estimated useful lives of the assets, generally between three and ten years. Leasehold improvements are amortized on a straight-line basis over the lesser of their useful life or the term of the lease. Maintenance and repairs are charged to expense as incurred, and improvements and betterments are capitalized. When assets are retired or otherwise disposed of, the cost and accumulated depreciation are removed from the consolidated balance sheet and any resulting gain or loss is reflected in operations in the period realized. Costs of maintenance and repairs are charged to expense as incurred, and improvements and betterments are capitalized.
Accounting for Long-Lived Assets
The Company reviews property and equipment for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability is measured by the comparison of the carrying amount to the future net cash flows that the assets are expected to generate. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the projected discounted future net cash flows arising from the asset. There have been no such impairments of long-lived assets as of December 31, 2013.
Preclinical and Clinical Trial Accruals
The Company's clinical trial accruals are based on estimates of patient enrollment and related costs at clinical investigator sites as well as estimates for the services received and efforts expended pursuant to contracts with multiple research institutions and clinical research organizations ("CROs") that conduct and manage clinical trials on the Company's behalf.
The Company estimates preclinical and clinical trial expenses based on the services performed pursuant to contracts with research institutions and clinical research organizations that conduct and manage preclinical studies and clinical trials on its behalf. In accruing service fees, the Company
F-10
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
2. Summary of Significant Accounting Policies (Continued)
estimates the time period over which services will be performed and the level of patient enrollment and activity expended in each period. If the actual timing of the performance of services or the level of effort varies from the estimate, the Company will adjust the accrual accordingly. Payments made to third parties under these arrangements in advance of the receipt of the related services are recorded as prepaid expenses until the services are rendered. The Company has had no significant adjustments to accrued preclinical and clinical trial expenses through December 31, 2013.
Convertible Preferred Stock
The Company has classified the convertible preferred stock as temporary equity in the balance sheets due to certain change in control events that are outside the Company's control, including liquidation, sale or transfer of the Company, as holders of the convertible preferred stock can cause redemption of the shares.
Convertible Preferred Stock Warrants
The Company accounts for its convertible preferred stock warrants as a liability based upon the characteristics and provisions of each instrument. Convertible preferred stock warrants classified as a liability are recorded on the Company's balance sheet at their fair value on the date of issuance and are revalued on each subsequent balance sheet, with fair value changes recognized as increases or reductions in the statements of operations. The Company adjusts the liability for changes in fair value of these warrants until the earlier of: (i) exercise of warrants, (ii) expiration of warrants, (iii) a change of control of the Company or (iv) the closing of the Company's initial public offering ("IPO"). At that time, the convertible preferred stock warrant liability will be adjusted to fair value in the consolidated statements of operations and comprehensive income (loss) with the final fair value reclassified to additional paid-in capital.
Research and Development
Research and development costs are charged to operations as incurred. Research and development costs include, but are not limited to, payroll and personnel expenses, laboratory supplies, consulting costs, and allocated overhead, including rent, equipment depreciation, and utilities.
Fair Value of Financial Instruments
The carrying value of the Company's cash and cash equivalents, accounts receivable, prepaid expenses and other current assets, other assets, accounts payable, accrued liabilities, and convertible notes payable approximate fair value due to the short-term nature of these items. Based on the borrowing rates currently available to the Company for debt with similar terms and consideration of default and credit risk, the carrying value of the convertible notes payable approximates their fair value. The convertible preferred stock warrant liability is carried at fair value (see Note 8).
Fair value is defined as the exchange price that would be received for an asset or an exit price paid to transfer a liability in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs.
F-11
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
2. Summary of Significant Accounting Policies (Continued)
The fair value hierarchy has three levels that prioritize the inputs used in fair value measurements:
| Level 1 | Unadjusted quoted prices in active markets for identical assets or liabilities; | |
Level 2 |
Inputs other than quoted prices included within Level 1 that are observable, unadjusted quoted prices in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the related assets or liabilities; and |
|
Level 3 |
Unobservable inputs that are supported by little or no market activity for the related assets or liabilities. |
The categorization of a financial instrument within the valuation hierarchy is based upon the lowest level of input that is significant to the fair value measurement.
The Company's financial instruments consist of Level 1 assets and Level 3 liabilities. Level 1 securities include highly liquid money market funds. Level 3 liabilities that are measured at fair value on a recurring basis consist of the convertible preferred stock warrant liability.
Income Taxes
The Company accounts for income taxes under the liability method. Under this method, deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized.
The Company assesses all material positions taken in any income tax return, including all significant uncertain positions, in all tax years that are still subject to assessment or challenge by relevant taxing authorities. Assessing an uncertain tax position begins with the initial determination of the position's sustainability and is measured at the largest amount of benefit that is greater than fifty percent likely of being realized upon ultimate settlement. As of each balance sheet date, unresolved uncertain tax positions must be reassessed, and the Company will determine whether (i) the factors underlying the sustainability assertion have changed and (ii) the amount of the recognized tax benefit is still appropriate. The recognition and measurement of tax benefits requires significant judgment. Judgments concerning the recognition and measurement of a tax benefit might change as new information becomes available.
Foreign Currency Translation
For non U.S. operations, the U.S. dollar is the functional currency. Monetary assets and liabilities of the foreign subsidiary are translated into U.S. dollars at current exchange rates. Nonmonetary assets such as property and equipment are translated at historical rates. Income and expense items are translated at average rates of exchange prevailing during the period of the related transactions, except that depreciation charged to operations is translated at historical rates.
Stock-Based Compensation
The Company accounts for stock-based employee compensation arrangements in accordance with provisions of ASC 718, "Compensation—Stock Compensation." ASC 718 requires the recognition of
F-12
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
2. Summary of Significant Accounting Policies (Continued)
compensation expense, using a fair-value based method, for costs related to all share-based payments including stock options. ASC 718 requires companies to estimate the fair value of share-based payment awards on the date of grant using an option-pricing model.
The Company accounts for equity instruments issued to nonemployees in accordance with the provisions of ASC 505-50, "Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services." Equity instruments issued to nonemployees are recorded at their fair value on the measurement date and are subject to periodic adjustment as the underlying equity instruments vest.
Comprehensive Income (Loss)
Comprehensive income (loss) is defined as a change in equity of a business enterprise during a period, resulting from transactions from non-owner sources. There have been no items qualifying as comprehensive income (loss) and, therefore, for all periods presented, the Company's comprehensive income (loss) was the same as its reported net income (loss).
Deferred Offering Costs
Deferred offering costs, which primarily consist of direct incremental legal and accounting fees relating to the initial public offering ("IPO"), are capitalized. The deferred offering costs will be offset against IPO proceeds upon the consummation of the offering. In the event the offering is terminated, deferred offering costs will be expensed.
Net Income (Loss) Per Share Attributable to Common Stockholders
The Company calculates its basic and diluted net income (loss) per share attributable to common stockholders in conformity with the two-class method required for companies with participating securities. Under the two-class method, the Company determines whether it has net income attributable to common stockholders, which includes the results of operations less current period convertible preferred stock non-cumulative dividends. If it is determined that the Company does have net income attributable to common stockholders during a period, the related undistributed earnings are then allocated between common stock and the convertible preferred stock based on the weighted average number of shares outstanding during the period to determine the numerator for the basic net income per share attributable to common stockholders. In computing diluted net income attributable to common stockholders, undistributed earnings are re-allocated to reflect the potential impact of dilutive securities to determine the numerator for the diluted net income per share attributable to common stockholders. The Company's basic net income (loss) per share attributable to common stockholders is calculated by dividing the net income (loss) by the weighted average number of shares of common stock outstanding for the period. The diluted net income (loss) per share attributable to common stockholders is computed by giving effect to all potential dilutive common stock equivalents outstanding for the period. For purposes of this calculation, options to purchase common stock and common stock warrants are considered common stock equivalents.
Recent Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the FASB or other standard setting bodies and adopted by us as of the specified effective date. Unless otherwise discussed, the
F-13
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
2. Summary of Significant Accounting Policies (Continued)
impact of recently issued standards that are not yet effective will not have a material impact on the Company's financial position or results of operations upon adoption.
In February 2013, the FASB issued guidance which addresses the presentation of amounts reclassified from accumulated other comprehensive income. This guidance does not change current financial reporting requirements, instead an entity is required to cross-reference to other required disclosures that provide additional detail about amounts reclassified out of accumulated other comprehensive income. In addition, the guidance requires an entity to present significant amounts reclassified out of accumulated other comprehensive income by line item of net income if the amount reclassified is required to be reclassified to net income in its entirety in the same reporting period. Adoption of this standard is required for periods beginning after December 15, 2012 for public companies. The amended guidance became effective for the Company in the first quarter of fiscal year 2013. The adoption of this guidance did not have a material impact on the Company's consolidated financial statements.
3. Balance Sheet Components
Prepaid expenses and other current assets (in thousands)
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Prepaid expenses |
$ | 200 | $ | 237 | |||
Prepaid clinical trial |
16 | 10 | |||||
Other current assets |
132 | 20 | |||||
| | | | | | | | |
|
$ | 348 | $ | 267 | |||
| | | | | | | | |
| | | | | | | | |
Property and equipment, net (in thousands)
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Computer equipment and software |
$ | 206 | $ | 330 | |||
Office equipment |
84 | 60 | |||||
Furniture and fixtures |
137 | 121 | |||||
Leasehold improvements |
6 | 25 | |||||
| | | | | | | | |
|
433 | 536 | |||||
Less: Accumulated depreciation and amortization |
(334 | ) | (337 | ) | |||
| | | | | | | | |
|
$ | 99 | $ | 199 | |||
| | | | | | | | |
| | | | | | | | |
F-14
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
3. Balance Sheet Components (Continued)
Accrued liabilities (in thousands)
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Accrued vacation |
$ | 277 | $ | 317 | |||
Accrued salaries and bonus |
— | 1,349 | |||||
Preclinical and clinical trial accruals |
535 | 104 | |||||
Consulting expenses |
73 | 180 | |||||
Income and other taxes |
362 | 101 | |||||
Other |
159 | 68 | |||||
| | | | | | | | |
|
$ | 1,406 | $ | 2,119 | |||
| | | | | | | | |
| | | | | | | | |
4. Collaboration and License Agreements
In November 2012, the Company entered into a license agreement with a wholly owned subsidiary of Forest, which granted Forest an exclusive license with right to sublicense certain of the Company's intellectual property rights in the United States in connection with the development and commercialization of MDX-8704 and marketing of Forest's approved product Namenda XR for the treatment of moderate to severe dementia related to Alzheimer's disease. Pursuant to the agreement, Forest made an upfront payment of $65.0 million. The Company was eligible to receive additional cash payments totaling up to $95.0 million upon achievement by Forest of certain development and regulatory milestones in addition to tiered royalty payments based on future net sales of the product upon commercialization.
The Company identified the following two non-contingent performance deliverables under the license agreement: (i) transfer of intellectual property rights, inclusive of the related technology know-how conveyance ("license and know-how" or "license") and (ii) the obligation to participate on the Joint Development Committee ("JDC"). The Company concluded that the license and the know-how together represent a single deliverable, and therefore the two together have been accounted for as a single unit of accounting. There was no separate consideration identified in the agreement for the deliverables and there was no right of return under the agreement. The Company considered the provisions of the multiple-element arrangement guidance in determining whether the deliverables outlined above have standalone value. The transfer of license and know-how has standalone value separate from the JDC, as the agreement allows Forest to sublicense its rights to the acquired license to a third party. Further, the Company believes that Forest has research and development expertise with compounds similar to those licensed under the agreement and has the ability to engage other third parties to develop these compounds allowing Forest to realize the value of the license and know-how without receiving the JDC participation.
The Company developed its best estimates of selling prices ("BESP") for each deliverable in order to allocate the non-contingent arrangement consideration to the two units of accounting. Based on BESP analysis, value assigned to the JDC was a negligible amount. Accordingly, the entire upfront license fee of $65.0 million was allocated to the transfer of license and technical know-how. Revenue recognition commenced upon delivery of the license and was recognized on a straight-line basis through the period of the transfer of the know-how. Forest was able to derive value from the license as the know-how was transferred. A straight-line pattern of revenue recognition is only acceptable when a more precise pattern cannot be discerned. The way in which the transfer of know-how occured did not
F-15
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
4. Collaboration and License Agreements (Continued)
give rise to a more precise pattern of recognition and the Company therefore recognized revenue on a straight-line basis over the period of the transfer of the know-how (November 2012 to February 2013).
In November and December 2013, the Company received a total of $40.0 million in milestone payments under its license agreement with Forest. The milestone payments were for the successful completion of studies that support the planned New Drug Application filing with the FDA for MDX-8704 by Forest. These amounts have been recorded as revenue in the consolidated statement of operations and comprehensive income during 2013.
5. Borrowings
2012 Notes and Preferred stock
In March 2012, the Company issued convertible promissory notes ("2012 Notes") pursuant to a convertible notes, Series AA preferred stock and warrants agreement ("2012 Notes and Preferred Stock Agreement") with various investors, raising proceeds of $9.3 million in multiple drawdowns with each drawdown to be divided in equal parts between convertible note and Series AA convertible preferred stock.
The 2012 Notes accrue at an interest rate of 10.0% per annum (upon an event of default, all unpaid principal and accrued interest will accrue at the default rate of 20%) and had a maturity date at the earliest of:
- i.
- March
2013,
- ii.
- upon
an event of default or
- iii.
- pursuant
to prepayment:
- a.
- at
the Company's election at its sole discretion following a subsequent sale of the Company's equity securities led by an investor not currently affiliated
with the Company and from which the Company receives $15.0 million or such lower amount approved by a majority of the holders of the 2012 notes (a "Subsequent Financing") in which the financing
is priced by a strategic investor as determined in good faith by the Company's board of directors (a "Strategic Financing") or
- b.
- automatically upon a "Corporate Transaction," defined as a liquidation event, a deemed liquidation event, a firm commitment underwritten IPO, or a Strategic Financing in which $50.0 million or more in cash payments are made to the Company.
The principal and accrued interest on the 2012 Notes are convertible into "Conversion Shares" (defined as common stock, preferred stock or any securities conferring the right to purchase, convertible into, or exchangeable for the Company's common stock or preferred stock) upon (a) a Subsequent Financing or (b) a Strategic Financing at the sole discretion of the Company. The number of conversion shares shall be equal to the quotient obtained by dividing the outstanding principal and unpaid accrued interest on date of conversion by conversion price (defined as 80% of price paid per share by investors in subsequent financing).
In connection with the execution of the 2012 Note and Preferred Stock Agreement, the investors deposited $9.3 million into an escrow account in March and May 2012. The Company drew down $8.0 million in six closings between March and November 2012. In November 2012, the Company determined that no further drawdowns were needed and the remaining $1.3 million (plus interest accrued within the escrow account) were released from the escrow account to the investors.
F-16
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
5. Borrowings (Continued)
The Company also issued warrants to purchase equity securities upon each drawdown (see Note 6). The warrant liability has been recorded at its estimated full fair value. The remaining proceeds have been allocated between Series AA preferred stock and 2012 Notes based on their relative fair values.
In connection with the 2012 Notes, the Company determined that the subsequent financing and strategic financing put options were embedded derivatives but had negligible value. Further, the cash settled put option upon an event of default or Corporate Transaction was not considered to be an embedded derivative as the debt did not result in a substantial premium or discount in the recorded basis of debt. The Company repaid all principal and accrued interest on these notes during 2013.
6. Warrants to Purchase Common or Preferred Stock
Common stock warrants
In June 2011, the Company recapitalized its outstanding preferred stock and warrants to purchase preferred stock as part of Series AA financing. Pursuant to the recapitalization agreement, the Company raised $7.0 million in gross proceeds by issuing 1,814,010 shares of Series AA preferred stock at $3.81 per share (incurred $176,650 as stock issuance costs) and each then outstanding Series A, B, C, C-1, and D preferred stock converted into common stock. Upon conversion, there was a reverse stock split in the ratio of 1:3.
In conjunction with the convertible promissory notes issued in November 2002 and September 2003, the Company issued warrants to purchase 22,220 shares and 88,884 shares, respectively, of the Company's Series A convertible preferred stock. The warrants expire ten years from issuance. The relative fair value of these warrants was determined to be approximately $49,188 and $205,001, respectively, and was amortized to interest expense over the term of each loan. These preferred stock warrants converted to warrants to purchase common stock as part of the Company's recapitalization transaction during 2011. The warrants to purchase 22,220 shares of common stock expired in November 2012 while warrants to purchase 88,884 shares remain outstanding as of December 31, 2012 and December 31, 2013.
The warrants to purchase 88,884 shares of common stock that were scheduled to expire in September 2013 were modified to extend their term by one additional year. As such, the Company has recorded an expense of $51,636. These warrants were outstanding as of December 31, 2013.
In conjunction with the convertible promissory notes issued in November 2004, the Company issued warrants to purchase 114,448 shares of common stock at $0.53 per share. The warrants expire on November 26, 2014. The relative fair value of these warrants was determined to be $53,496 and is being amortized to interest expense over the term of the convertible note. To induce payment of the note in cash rather than the holder exercising their conversion option, the warrant holder relinquished the right to purchase 28,616 shares of the Company's common stock provided for under the original warrant. Upon modification of the warrants, the Company recorded a gain of $29,149 during the year ended December 31, 2006 which was equal to the fair market value of the relinquished share purchase right. The gain was recorded in interest and other income and as a reduction in additional paid in capital. As of December 31, 2012, the warrant holder had the right to purchase 85,832 shares of the Company's common stock. The warrants were outstanding as of December 31, 2012 and December 31, 2013.
F-17
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
6. Warrants to Purchase Common or Preferred Stock (Continued)
In 2006, the Company issued a warrant to purchase 13,332 shares of common stock at an exercise price of $1.88 per share to a consultant in consideration for the provision of such third-party services. The common stock warrant was exercisable for a period of 10 years. The Company recorded $12,837 in additional paid in capital at the time of issuance. The warrant was outstanding as of December 31, 2012 and December 31, 2013.
In connection with the first two draw-downs pertaining to the 2006 Term Loan, made in 2006 for a total of $1.5 million, the Company issued warrants to purchase a total of 13,586 shares of the Company's Series B preferred stock. Using the Black-Scholes model with a volatility of 84%, term of ten years and a risk-free interest rate of 4.72%, the fair value of the warrants was determined to be $121,336 and was recorded as warrant liability and discount against the borrowings and is being amortized to interest expense over the term of the loan. These preferred stock warrants converted to warrants to purchase common stock as part of the Company's recapitalization transaction during 2011. The common stock warrants were outstanding as of December 31, 2012 and December 31, 2013.
In connection with the third draw-down pertaining to the 2006 Term Loan, made in 2007 for $500,000, the Company issued a warrant to purchase 4,528 shares of the Company's Series B preferred stock. Using the Black-Scholes model with a volatility of 73%, term of ten years and a risk free interest rate of 4.72%, the fair value of the warrant was determined to be $47,278 and was recorded as warrant liability and discount against the borrowings and is being amortized to interest expense over the term of the loan. These preferred stock warrants converted to warrants to purchase common stock as part of the Company's recapitalization transaction during 2011. The common stock warrants were outstanding as of December 31, 2012 and December 31, 2013.
In connection with the first draw-down of $500,000 in June 2008 pertaining to the 2008 Term Loan, the Company issued warrants to purchase a total of 3,914 shares of the Company's Series D preferred stock. Using the Black-Scholes model with a volatility of 82%, term of ten years and a risk-free interest rate of 3.99%, the fair value of the warrants was determined to be $46,266 and was recorded as warrant liability and discount against the borrowings and is being amortized to interest expense over the term of the loan. These preferred stock warrants converted to warrants to purchase common stock as part of the Company's recapitalization transaction during 2011. The common stock warrants were outstanding as of December 31, 2012 and December 31, 2013.
In connection with the second draw-down of $1.5 million in December 2008 pertaining to the 2008 Term Loan, the Company issued warrants to purchase a total of 3,202 shares of the Company's Series D preferred stock. Using the Black-Scholes model with a volatility of 85%, term of ten years and a risk-free interest rate of 2.25%, the fair value of the warrants was determined to be $20,239 and was recorded as warrant liability and discount against the borrowings and is being amortized to interest expense over the term of the loan. These preferred stock warrants converted to warrants to purchase common stock as part of the Company's recapitalization transaction during 2011. The common stock warrants were outstanding as of December 31, 2012 and December 31, 2013.
Convertible preferred stock warrants
In connection with the 2011 Notes and warrant purchase agreement, the Company issued warrants to purchase 55,848 shares of Series AA preferred stock. Using the Black-Scholes model with a volatility of 90%, expected term of 3 years and risk-free interest rate of 0.82%, the fair value of the warrant
F-18
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
6. Warrants to Purchase Common or Preferred Stock (Continued)
liability was determined to be $12,504 and was recorded as debt discount and amortized in 2011. The warrants were outstanding as of December 31, 2012 and December 31, 2013.
In connection with the issuance of Series AA preferred stock in June 2011, the Company issued warrants to purchase 462,762 shares of Series AA preferred stock. Using the Black-Scholes model with a volatility of 90%, expected term of 3 years and a risk-free interest rate of 0.82%, the fair value of the warrant liability was determined to be $64,787 and was recorded as a reduction against the value of Series AA preferred stock. The warrants were outstanding as of December 31, 2012 and December 31, 2013.
In conjunction with the issuance of the 2012 Notes, the Company issued warrants to purchase equity securities (the "2012 Warrants"). The 2012 Warrants become exercisable on the date the 2012 Notes are converted into the Company's equity securities (or upon cash settlement of the strategic financing put option) and expire on March 22, 2019, or if there is a Corporate Transaction prior to the date the 2012 Notes are converted, the 2012 Warrants will be automatically net exercised immediately prior to the closing of a Corporate Transaction.
The number and class of shares into which the 2012 Warrants are exercisable are determined as follows:
- •
- Number of shares
- •
- If the 2012 Notes convert into shares of the Company's equity securities through the financing put options, then the 2012
Warrants are exercisable into a number of shares equal to: (1) 10% of the principal amount of the 2012 Notes issued to the warrant holder divided by (2) the "Conversion Price," which is
the greater of (a) $3.81 and (b) 80% of the price paid by subsequent investors.
- •
- Upon a Corporate Transaction or in the event the Company elects to settle the strategic financing put option in cash, then
the 2012 warrants are exercisable into a number of AA Preferred equal to: (1) 10% of the principal of the 2012 Notes issued to the warrant holder divided by (2) $3.81.
- •
- Class of shares
- •
- If the conversion price is equal to $3.81, the 2012 Warrants become exercisable into AA Preferred.
- •
- If the conversion price is greater than $3.81, the 2012 Warrants convert into the class of equity securities issued through the exercise of the financing put options.
In order to determine a fair value for the 2012 Warrants upon issuance of the 2012 Notes, the Company evaluated multiple potential outcomes using the option pricing model value depending on the scenario while applying estimated probabilities to each scenario value. These scenarios included potential subsequent financing, strategic financing and corporate transaction at different times during 2012. Accordingly, the Company determined the fair value of the warrants to be $268,763, which was recorded as a convertible preferred stock warrant liability and a debt discount. Upon repayment of the 2012 Notes in 2013, the warrants became exercisable to purchase 104,050 shares of Series AA convertible preferred stock at $3.81 per share.
F-19
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
6. Warrants to Purchase Common or Preferred Stock (Continued)
The Company remeasures its preferred stock warrants at each reporting period and records the change in fair value in the consolidated statement of operations and comprehensive income. The Company remeasured their preferred warrants at December 31, 2012 and 2013 and recorded a change in fair value of $1.3 million and $4.5 million, respectively, in the consolidated statement of operations and comprehensive income under interest and other income (expense), net. The warrants were outstanding as of December 31, 2012 and 2013.
The following table summarizes the outstanding warrants as of:
| |
Number of shares outstanding |
||||||
|---|---|---|---|---|---|---|---|
| |
December 31, | ||||||
| |
2012 | 2013 | |||||
Series AA convertible preferred stock warrants issued in 2011 |
518,610 | 518,610 | |||||
Series AA convertible preferred stock warrants issued in 2012 |
* | 104,050 | |||||
Common stock warrants |
213,278 | 213,278 | |||||
- *
- not determinable as the number of shares is dependant upon the conversion or repayment of the 2012 Notes.
7. Commitments and Contingencies
The Company leases various facilities under operating leases with various expiration dates. In 2013, the Company amended its facility lease agreement which expires on February 28, 2016. Rent expense for the years ended December 31, 2012 and 2013 was $122,148 and $213,960, respectively.
As of December 31, 2013, future minimum lease payments under non-cancelable facility operating leases were as follows:
2014 |
$ |
227,769 |
||
2015 |
234,603 | |||
2016 |
39,291 | |||
| | | | | |
Total |
$ | 501,663 | ||
| | | | | |
| | | | | |
Contingencies
In the normal course of business, the Company enters into contracts and agreements that contain a variety of representations and warranties and provide for general indemnifications. The Company's exposure under these agreements is unknown because it involves claims that may be made against the Company in the future, but have not yet been made. The Company accrues a liability for such matters when it is probable that future expenditures will be made and such expenditures can be reasonably estimated.
Indemnification
In accordance with the Company's amended and restated certificate of incorporation and amended and restated bylaws, the Company has indemnification obligations to its officers and directors for
F-20
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
7. Commitments and Contingencies (Continued)
certain events or occurrences, subject to certain limits, while they are serving in such capacity. There have been no claims to date and the Company has a director and officer insurance policy that may enable it to recover a portion of any amounts paid for future claims.
Litigation
The Company is not a party to any material litigation and does not have contingent reserves established for any litigation liabilities.
8. Fair Value Measurements
The following table sets forth the Company's financial instruments that were measured at fair value on a recurring basis at December 31, 2012 by level within the fair value hierarchy (in thousands):
| |
Fair Value Measurements at December 31, 2012 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Total | Level 1 | Level 2 | Level 3 | |||||||||
Assets |
|||||||||||||
Money market fund |
$ | 61,703 | $ | 61,703 | $ | — | $ | — | |||||
Liabilities |
|||||||||||||
Preferred stock warrant liability |
$ | 1,706 | $ | — | $ | — | $ | 1,706 | |||||
The following table sets forth the Company's financial instruments that were measured at fair value on a recurring basis at December 31, 2013 by level within the fair value hierarchy (in thousands):
| |
Fair Value Measurements at December 31, 2013 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Total | Level 1 | Level 2 | Level 3 | |||||||||
Assets |
|||||||||||||
Money market fund |
$ | 83,700 | $ | 83,700 | $ | — | $ | — | |||||
Liabilities |
|||||||||||||
Preferred stock warrant liability |
$ | 6,232 | $ | — | $ | — | $ | 6,232 | |||||
Upon issuance of the convertible preferred stock warrants, the Company estimates the fair value of the liability and subsequent remeasurement using the option pricing model at each reporting date, using the following inputs: the risk-free interest rates; the expected dividend rates; the remaining expected life of the warrants; and the expected volatility of the price of the underlying stock. The estimates are based, in part, on subjective assumptions and could differ materially in the future.
F-21
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
8. Fair Value Measurements (Continued)
The following table includes a roll forward of the financial instruments classified within Level 3 of the fair value hierarchy (in thousands):
| |
Amounts | |||
|---|---|---|---|---|
Fair Value Using Level 3 Inputs |
||||
Balance at December 31, 2011 |
$ |
107 |
||
Issuance of preferred stock warrant liability |
269 | |||
Change in fair value recorded in interest and other income/expense |
1,330 | |||
| | | | | |
Balance at December 31, 2012 |
1,706 | |||
Change in fair value recorded in interest and other income/expense |
4,526 | |||
| | | | | |
Balance at December 31, 2013 |
$ | 6,232 | ||
| | | | | |
| | | | | |
9. Convertible Preferred Stock
The Company's amended and restated certificate of incorporation authorizes 6,700,000 shares of convertible preferred stock, 5,000,000 of which are designated as Series AA and 1,700,000 of which are designated as Series AA-1.
At December 31, 2012 and 2013, the convertible preferred stock consists of the following (in thousands except share and per share data):
| |
Shares | |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Per Share Liquidation Preference |
Carrying Value |
|||||||||||
| Series |
Authorized | Outstanding | |||||||||||
Series AA |
5,000,000 | 3,431,620 | $ | 3.81 | $ | 6,521 | |||||||
Series AA-1 |
1,700,000 | 1,287,554 | $ | 50.00 | 12,628 | ||||||||
| | | | | | | | | | | | | | |
Total |
6,700,000 | 4,719,174 | $ | 19,149 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Amended and Restated Certificate of Incorporation
In June 2011, in connection with the recapitalization transaction and Series AA preferred stock financing, the Company amended and restated its certificate of incorporation. The significant rights and preferences of preferred stock are as follows:
Dividends
The preferred stockholders are entitled to receive, when and as declared by the board of directors, out of funds legally available, cash dividends at the rate of 8% of the original issue price per annum on each outstanding share of Series AA and AA-1 preferred stock. Such dividends are payable when, as and if declared by the board of directors and are noncumulative. No dividends have been declared to date.
F-22
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
9. Convertible Preferred Stock (Continued)
Liquidation
Upon liquidation, dissolution or winding up of the Company and including a merger, acquisition or sale of assets, before distribution or payment is made to the holders of common stock, the holders of Series AA and Series AA-1 preferred stock are entitled to a liquidation preference of an amount per share equal to the original issue price for the preferred stock (as adjusted for any stock dividends, stock splits or recapitalization and similar events), plus all declared and unpaid dividends thereon to the date fixed for such distribution. After the payment of the full liquidation preference, all remaining assets available for distribution, if any, shall be distributed among the holders of common stock. If available assets are insufficient to pay the full liquidation preference, the available assets will be distributed pro rata to the holders of Series AA and Series AA-1 preferred stock based on their liquidation preferences. The holders of Series AA-1 preferred stock will not participate in cash distributions upon liquidation if sufficient liquidation proceeds exist to fully satisfy their $50.00 per share liquidation preference. In such a situation, the holders of Series AA-1 preferred stock would instead participate through common shares held, which would entitle the holder to participate in the residual assets on a pro-rata basis.
Conversion
At the option of the holder thereof, each share of the Series AA preferred stock is convertible, at any time or from time to time, into fully paid and nonassessable shares of common stock as is determined by dividing the applicable original issue price for the Series AA preferred stock by the applicable conversion price for such series.
Automatic Conversion
Each share of the Series AA preferred stock shall automatically be converted into shares of common stock immediately upon the earlier of (i) at anytime upon the affirmative election of the holders of a majority of the then-outstanding shares of the preferred stock or (ii) immediately upon the closing of a firmly underwritten public offering, the public offering price which results in proceeds to the Company of at least $40.0 million, net of underwriting discounts and commissions. Upon the closing of a firmly underwritten public offering the Series AA-1 preferred stock shall automatically be converted into one one-thousandth of a share of common stock.
Voting
Each holder of Series AA preferred stock is entitled to the number of votes equal to the number of shares of common stock into which such holder's shares of preferred stock could be converted as of the record date. The Series AA-1 preferred stock is non-voting stock except as required by law.
10. Shareholders' Equity
Common Stock
The amended and restated certificate of incorporation authorizes the Company to issue 100 million shares of common stock. Common stockholders are entitled to dividends as and when declared by the board of directors, subject to the rights of holders of all classes of stock outstanding having priority
F-23
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
10. Shareholders' Equity (Continued)
rights as to dividends. There have been no dividends declared to date. Each share of common stock is entitled to one vote.
The Company has classified all early exercised options as employee deposits (a liability) as these options are not considered to be substantive exercises until vested. At December 31, 2012 and 2013, 5,000 and zero shares of common stock respectively from early exercised options were unvested.
Shares reserved for Future Issuance
Shares of Company's common stock reserved for future issuance are as follows:
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Conversion of convertible preferred stock |
3,432,908 | 3,432,908 | |||||
Common stock options outstanding |
3,155,182 | 3,567,858 | |||||
Common stock options available for grant |
199,554 | 1,771,212 | |||||
Warrants to purchase common stock |
213,290 | 213,290 | |||||
Warrants to purchase convertible preferred stock |
518,610 | 622,660 | |||||
| | | | | | | | |
Total |
7,519,544 | 9,607,928 | |||||
| | | | | | | | |
| | | | | | | | |
The above table does not include the Series AA preferred stock warrants issued in connection with the 2012 Notes, as they are dependent on the repayment or conversion of the 2012 Notes.
Restricted Stock
The Company has entered into Restricted Stock Agreements with founders, employees or consultants. Pursuant to these agreements, the Company has the right but not the obligation, to repurchase the unvested shares of common stock upon termination of employment or consulting arrangement at the original purchase price per share. The repurchase rights with respect to these shares lapse over a term of up to four years. At December 31, 2012 and 2013, 5,000 and zero shares of common stock, respectively, were subject to repurchase by the Company.
11. Stock Option Plans
In October 2002, the Company established its 2002 Employee, Director and Consultant Stock Plan (the "2002 Plan") which provides for the granting of stock options to employees and consultants of the Company and issuance of restricted shares of common stock. Options granted under the 2002 Plan could be either incentive stock options ("ISOs") or nonqualified stock options ("NSOs"). ISOs could be granted only to Company employees. NSOs could be granted to Company employees and consultants.
In December 2007, the Company established its 2007 Stock Plan. No further grants will be made under the 2002 Plan. The 2007 Stock Plan provides both for the direct award or sale of shares and for the grant of options to purchase shares. Options granted under the 2007 Stock Plan could either be ISOs or NSOs. ISOs could be granted only to Company employees. NSOs could be granted to Company employees and consultants.
F-24
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
11. Stock Option Plans (Continued)
Options granted under the 2007 Stock Plan may have terms of up to ten years. All options issued to date have had a ten year life. The exercise price of an ISO shall not be less than 100% of the estimated fair value of the shares on the date of grant, as determined by the board of directors. The exercise price of an ISO and NSO granted to a 10% shareholder shall not be less than 110% of the estimated fair value of the shares on the date of grant, respectively, as determined by the board of directors. The exercise price of a NSO shall not be less than the par value per share of common stock. The options granted generally vest over five years and vest at a rate of 20% upon the first anniversary of the issuance date and 1/48th per month thereafter.
Under the terms of the 2002 Plan and 2007 Stock Plan, all options are fully exercisable on the grant date, subject to the Company's repurchase right, which under the 2002 Plan is at the original exercise price and under the 2007 Stock Plan is at the lower of original exercise price or fair value. The repurchase rights lapse over the options' vesting period of generally five years.
Activity under the Company's stock option plans is set forth below:
| |
|
Outstanding Options | |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Shares Available for Grant |
Number of Shares |
Weighted Average Exercise Price |
Aggregate Intrinsic Value (thousands) |
|||||||||
Balances, December 31, 2011 |
675,432 | 2,806,568 | $ | 1.18 | |||||||||
Options granted |
(545,000 | ) | 545,000 | 0.67 | |||||||||
Options exercised |
— | (10,000 | ) | 0.67 | |||||||||
Options cancelled |
186,386 | (186,386 | ) | 1.06 | |||||||||
Options retired |
(117,264 | ) | — | — | |||||||||
| | | | | | | | | | | | | | |
Balances, December 31, 2012 |
199,554 | 3,155,182 | $ | 1.10 | $ | 599 | |||||||
Additional shares reserved |
2,000,000 | — | — | ||||||||||
Options granted |
(616,000 | ) | 616,000 | 3.08 | |||||||||
Options exercised |
— | (15,666 | ) | 1.37 | |||||||||
Options cancelled |
187,658 | (187,658 | ) | 1.00 | |||||||||
| | | | | | | | | | | | | | |
Balances, December 31, 2013 |
1,771,212 | 3,567,858 | $ | 1.45 | $ | 3,701 | |||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
The aggregate intrinsic value of options exercised was $8,100 and $28,762 for the years ended December 31, 2012 and 2013, respectively.
The following table summarizes information about stock options as of December 31, 2012:
| |
Options Outstanding and Exercisable | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
Exercise Price
|
Number Outstanding |
Weighted Average Remaining Contractual Life (in Years) |
Weighted Average Exercise Price |
|||||||
$0.08-0.23 |
3,332 | 1.00 | $ | 0.08 | ||||||
$0.53-0.67 |
2,112,848 | 8.58 | 0.66 | |||||||
$1.74-2.25 |
1,039,002 | 5.16 | 2.01 | |||||||
| | | | | | | | | | | |
|
3,155,182 | 7.46 | $ | 1.10 | ||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
F-25
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
11. Stock Option Plans (Continued)
The following table summarizes information about stock options as of December 31, 2013:
| |
Options Outstanding and Exercisable | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
Exercise Price
|
Number Outstanding |
Weighted Average Remaining Contractual Life (in Years) |
Weighted Average Exercise Price |
|||||||
$0.08-0.23 |
3,332 | 0.01 | $ | 0.08 | ||||||
$0.53-0.67 |
1,961,930 | 7.55 | 0.66 | |||||||
$1.69-3.31 |
1,602,596 | 6.31 | 2.42 | |||||||
| | | | | | | | | | | |
|
3,567,858 | 6.99 | $ | 1.45 | ||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
Stock-Based Compensation
During the years ended December 31, 2012 and 2013, the Company granted stock options to employees to purchase 440,000 and 522,000 shares of common stock respectively with a weighted-average grant date fair value of $1.09 per share and $8.73 per share, respectively. As of December 31, 2013, there was total unrecognized compensation cost of $5.0 million. This cost is expected to be recognized over a period of 4.55 years. The total fair value of employee stock options vested for the years ended December 31, 2012 and 2013 was $183,324, and $240,650, respectively.
Stock-based compensation expense related to employee options for the years ended December 31, 2012 and 2013 was $220,732, and $275,073, respectively.
The Company estimated the fair value of stock options using the Black-Scholes option pricing model. The fair value of employee stock options is being amortized on a straight-line basis over the requisite service period of the awards.
The fair value of employee stock options was estimated using the following assumptions:
| |
Year Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Expected volatility |
91-92% | 89-100% | |||||
Risk-free interest rate |
1.15%-1.41% | 1.45%-2.48% | |||||
Dividend yield |
— | — | |||||
Expected term (in years) |
7.00 | 7.25 | |||||
Determining Fair Value of Stock Options
The fair value of each grant of stock options was determined by the Company using the methods and assumptions discussed below. Each of these inputs is subjective and generally requires significant judgment to determine.
Weighted-Average Expected Term: The expected term of options granted is determined using the average period the stock options are expected to remain outstanding and is based on the options vesting term, contractual terms and historical exercise and vesting information used to develop
F-26
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
11. Stock Option Plans (Continued)
reasonable expectations about future exercise patterns and post-vesting employment termination behavior.
Volatility: The expected stock price volatility assumption was determined by examining the historical volatilities of a group of industry peers, as the Company did not have any trading history for the Company's common stock. The Company will continue to analyze the historical stock price volatility and expected term assumptions as more historical data for the Company's common stock becomes available.
Risk-Free Interest Rate: The risk-free rate is based on U.S. Treasury zero-coupon issues with remaining terms similar to the expected term on the options.
Dividend Yield: The expected dividend assumption was based on the Company's history and expectation of dividend payouts.
Forfeitures: Forfeitures were estimated based on historical experience.
Fair Value of Common Stock: The fair value of the shares of common stock underlying the stock options has historically been the responsibility of and determined by the Company's board of directors. Because there has been no public market for the Company's common stock, the board of directors determined fair value of common stock at the time of grant of the option by considering a number of objective and subjective factors including independent third-party valuations of the Company's common stock, sales of convertible preferred stock to unrelated third parties, operating and financial performance, the lack of liquidity of capital stock and general and industry specific economic outlook, amongst other factors. The fair value of the underlying common stock will be determined by the Company's board of directors until such time as the Company's common stock is listed on an established exchange, after which it will be determined based on the closing price on that exchange.
Nonemployee Stock-Based Compensation
During the years ended December 31, 2012 and 2013, the Company granted options to purchase 105,000 and 94,000 shares of common stock to consultants, respectively. These options are granted in exchange for consulting services to be rendered and vest over the term of the consulting agreement.
The Company has estimated fair value of common stock options granted to nonemployees using the Black-Scholes option pricing model with the following assumptions:
| |
Year Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Expected volatility |
89%-92% | 88%-98% | |||||
Risk-free interest rate |
0.87%-1.93% | 1.02%-2.72% | |||||
Dividend yield |
— | — | |||||
Contractual life (in years) |
6.25-10.00 | 5.25-10.00 | |||||
Compensation expense related to nonemployee options for the years ended December 31, 2012 and 2013 was $575,460 and $364,490, respectively.
F-27
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
11. Stock Option Plans (Continued)
Total Stock-Based Compensation
Total stock-based compensation expense was allocated as follows (in thousands):
| |
Year Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Research and development |
$ | 269 | $ | 304 | |||
General and administrative |
528 | 336 | |||||
| | | | | | | | |
|
$ | 797 | $ | 640 | |||
| | | | | | | | |
| | | | | | | | |
12. Related Party Transactions
In July 2008, the Company loaned an officer, who is no longer associated with the Company, $200,000 by issuing a full recourse promissory note bearing interest at 3.45% per annum. The note is due at the earliest of (i) July 2012, (ii) the Company's initial filing for an IPO, or (iii) the termination of employment of the officer. The Company recorded accrued interest income of $20,634 as of December 31, 2013. Subsequent amendments were made to the terms of the note to (a) reduce the interest rate to 0.55% effective April 1, 2011, and (b) extension the maturity date to July 1, 2014. A reserve in the amount of $220,634 for the outstanding principal and accrued interest has been reflected in the consolidated financial statements as of December 31, 2013.
The officer notes are included in prepaid and other current assets on the consolidated balance sheets.
13. Income Taxes
Income before provision for income tax is summarized as follows (in thousands):
| |
Year Ended December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
United States |
$ | 17,989 | $ | 52,095 | |||
International |
47 | 18 | |||||
| | | | | | | | |
Total |
$ | 18,036 | $ | 52,113 | |||
| | | | | | | | |
| | | | | | | | |
F-28
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
13. Income Taxes (Continued)
The income tax provision is summarized as follows (in thousands):
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Current: |
|||||||
Federal |
$ | 297 | $ | 1,190 | |||
State |
6 | 1 | |||||
Foreign |
(2 | ) | (1 | ) | |||
| | | | | | | | |
|
301 | 1,190 | |||||
| | | | | | | | |
Deferred: |
|||||||
Federal |
— | — | |||||
State |
— | — | |||||
Foreign |
(1 | ) | 1 | ||||
| | | | | | | | |
|
(1 | ) | 1 | ||||
| | | | | | | | |
Provision for income tax |
$ | 300 | $ | 1,191 | |||
| | | | | | | | |
| | | | | | | | |
The provision for income taxes differs from the amount computed by applying the federal income tax rate of 35% to pretax income from operations as a result of the following:
| |
2012 | 2013 | |||||
|---|---|---|---|---|---|---|---|
Statutory federal income tax rate |
$ | 6,313 | $ | 18,239 | |||
AMT taxes |
263 | — | |||||
State income taxes, net of federal tax benefits |
4 | 1 | |||||
Warrants |
466 | 1,584 | |||||
Foreign rate differential |
(20 | ) | (6 | ) | |||
Tax credits |
(263 | ) | (119 | ) | |||
Change in statutory rates |
(804 | ) | (61 | ) | |||
Other |
176 | (59 | ) | ||||
Change in valuation allowance |
(5,835 | ) | (18,388 | ) | |||
| | | | | | | | |
Income tax provision |
$ | 300 | $ | 1,191 | |||
| | | | | | | | |
| | | | | | | | |
F-29
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
13. Income Taxes (Continued)
Significant components of the Company's deferred tax assets are as follows (in thousands):
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Net operating loss carryforwards |
$ | 23,537 | $ | 5,459 | |||
Research and development tax credits |
1,760 | 1,187 | |||||
Accruals and reserves |
124 | 103 | |||||
Stock compensation |
506 | 660 | |||||
Depreciation and amortization |
1,752 | 1,804 | |||||
| | | | | | | | |
Total deferred tax assets |
27,679 | 9,213 | |||||
Less: Valuation allowance |
(27,679 | ) | (9,213 | ) | |||
| | | | | | | | |
Net deferred tax assets |
$ | — | $ | — | |||
| | | | | | | | |
| | | | | | | | |
The deferred income tax assets have been fully offset by a valuation allowance, as realization is dependent on future earnings, if any, the timing and amount of which are uncertain. The net valuation allowance decreased by $5.8 million for the year ended December 31, 2012 and decreased by $18.5 million during the year ended December 31, 2013.
The Company's accounting for deferred taxes involves the evaluation of a number of factors concerning the realizability of its net deferred tax assets. The Company primarily considered such factors as its history of operating losses, the nature of the Company's deferred tax assets, and the timing, likelihood and amount, if any, of future taxable income during the periods in which those temporary differences and carryforwards become deductible. At present, the Company does not believe that it is more likely than not that the deferred tax assets will be realized; accordingly, a full valuation allowance has been established and no deferred tax asset is shown in the accompanying balance sheets.
As of December 31, 2013 the Company had federal net operating loss carryforwards of approximately $3.5 million available to reduce future taxable income. The Company also had state net operating loss carryforwards of approximately $73.4 million. The federal net operating loss carryforward begins expiring in 2022, and the state net operating loss carryforward began expiring in 2012.
The Company also had federal research and development tax credit carryforwards of approximately $1.4 million. If not utilized, the carryforwards will begin expiring in 2030. The Company has state research and development credit carryforwards or approximately $2.2 million which do not expire.
Under federal and similar state tax statutes, changes in our ownership, including ownership changes resulting from the offering contemplated by this prospectus, may limit our ability to use our available net operating loss and tax credit carryforwards. The annual limitation, as a result of a change of control, may result in the expiration of net operating losses and credits before utilization.
We have determined that an ownership change occurred on June 25, 2008 and our annual limitation is $2.0 million. We do not currently have any prior ownership changes that will have a material impact on our ability to utilize our existing Federal net operating losses and credit.
F-30
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
13. Income Taxes (Continued)
Our ability to use our remaining net operating losses carryforwards may be further limited if we experience a Section 382 ownership change in connection with this offering or as a result of future changes in our stock ownership.
Uncertain Tax Positions
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows (in thousands):
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Balance at the beginning of the year |
$ | 1,676 | $ | 1,880 | |||
Additions based on prior period tax positions |
— | 184 | |||||
Reductions based on prior period tax positions |
— | — | |||||
Additions based on current period tax positions |
204 | 206 | |||||
| | | | | | | | |
Balance at the end of the year |
$ | 1,880 | $ | 2,270 | |||
| | | | | | | | |
| | | | | | | | |
The Company's policy is to account for interest and penalties as income tax expense. As of December 31, 2013, the Company had no interest related to unrecognized tax benefits. No amounts of penalties related to unrecognized tax were recognized in the provision for income taxes.
The Company files income tax returns in the U.S. federal jurisdiction, California and India. The Company is subject to U.S. federal income tax examination for the calendar years ending 2001 through 2013. Additionally, the Company is subject to state income tax examinations for the 2001 through 2013 calendar years. The U.S. federal and U.S. state taxing authorities may choose to audit tax returns for tax years beyond the statute of limitation period due to significant tax attribute carryforwards from prior years, making adjustments only to carryforward attributes. The Company is subject to audit by the Indian tax authorities from 2009 onward. The Company is not currently under audit in any major tax jurisdiction.
F-31
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
14. Net Income per Share
A reconciliation of the numerator and denominator used in the calculation of the basic and diluted net income per share is as follows (in thousands except per share data):
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Historical net income per share |
|||||||
Numerator: |
|||||||
Net income |
$ | 17,736 | $ | 50,921 | |||
Noncumulative dividend on preferred stock |
(1,268 | ) | (1,436 | ) | |||
Undistributed earnings allocated to preferred stock holders |
(5,027 | ) | (16,417 | ) | |||
| | | | | | | | |
Basic net income attributable to common stockholders |
11,441 | 33,068 | |||||
Adjustment to net income for dilutive securities |
155 | 2,285 | |||||
| | | | | | | | |
Diluted net income attributable to common stockholders |
$ | 11,596 | $ | 35,353 | |||
| | | | | | | | |
| | | | | | | | |
Denominator: |
|||||||
Basic common shares outstanding: |
|||||||
Basic common shares outstanding: weighted average common shares outstanding |
9,490 | 9,508 | |||||
Less: weighted average unvested common shares subject to repurchase |
(2 | ) | (2 | ) | |||
| | | | | | | | |
Weighted average number of common shares used in calculating net income per share—basic |
9,488 | 9,506 | |||||
| | | | | | | | |
| | | | | | | | |
Dilutive securities: |
|||||||
Common stock options |
436 | 2,204 | |||||
Warrants to purchase common stock |
— | 96 | |||||
| | | | | | | | |
Weighted average number of common shares used in calculating net income per share—diluted |
9,924 | 11,806 | |||||
| | | | | | | | |
| | | | | | | | |
Net income per share to attributable to common stockholders |
|||||||
Basic |
$ | 1.21 | $ | 3.48 | |||
| | | | | | | | |
| | | | | | | | |
Diluted |
$ | 1.17 | $ | 3.00 | |||
| | | | | | | | |
| | | | | | | | |
F-32
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
14. Net Income per Share (Continued)
The following outstanding shares of potentially dilutive securities were excluded from the computation of diluted net income per share of common stock for the periods presented, because including them would have been anti-dilutive:
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Convertible preferred stock |
9,499,862 | 4,719,174 | |||||
Options to purchase common stock |
— | — | |||||
Warrants to purchase convertible preferred stock |
622,660 | 622,660 | |||||
Warrants to purchase common stock |
213,278 | — | |||||
| | | | | | | | |
Total |
10,335,800 | 5,341,834 | |||||
| | | | | | | | |
| | | | | | | | |
Unaudited pro forma basic and diluted income per share attributable to common stockholders is computed as follows (in thousands, except per share data):
| |
December 31, 2013 |
|||
|---|---|---|---|---|
| |
(unaudited) |
|||
Pro forma income per share—basic and diluted |
||||
Numerator: |
||||
Net income—basic and diluted |
$ | 33,069 | ||
Noncumulative dividend on preferred stock |
1,436 | |||
Undistributed earnings allocated to preferred stock holders |
16,416 | |||
Change in fair value of preferred stock warrants |
4,526 | |||
| | | | | |
Pro forma net income attributable to common stockholders |
$ | 55,447 | ||
| | | | | |
| | | | | |
Denominator: |
||||
Weighted-average shares used to compute basic and diluted net income per share |
9,506 | |||
Adjustments to reflect the assumed conversion of convertible preferred stock |
3,433 | |||
Adjustment to reflect net exercise of preferred stock warrants |
475 | |||
| | | | | |
Pro forma weighted average number of shares outstanding—basic net income per share |
13,414 | |||
| | | | | |
| | | | | |
Dilutive securities |
||||
Common stock options |
2,203 | |||
Warrants to purchase common stock |
173 | |||
| | | | | |
Pro forma weighted average number of shares outstanding—diluted net income per share |
15,790 | |||
| | | | | |
| | | | | |
Net income per share attributable to common stockholders |
||||
Pro forma net income per share—basic |
$ | 4.13 | ||
| | | | | |
| | | | | |
Pro forma net income per share—diluted |
$ | 3.51 | ||
| | | | | |
| | | | | |
15. Subsequent Events
In February 2014, the Company's board of directors adopted, subject to the approval of the Company's stockholders, the 2014 Equity Incentive Plan (the "2014 Plan"), which will become effective
F-33
Adamas Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
15. Subsequent Events (Continued)
on the completion of this offering and will serve as a successor to the 2007 Plan. 1,380,728 shares of the Company's common stock will be available for issuance under the 2014 Plan, plus an additional number of shares that will be added to the 2014 Plan as of the effective time equal to the sum of (i) all shares that, as of the effective time, were reserved for issuance pursuant to the 2007 Plan, plus (ii) all shares that are subject to outstanding options under the 2007 Plan and the 2002 Plan as of the effective time that thereafter expire, terminate, or otherwise are forfeited or reacquired.
In February 2014, the Company's board of directors adopted, subject to the approval of the Company's stockholders, the 2014 Employee Stock Purchase Plan (the "ESPP"). 262,762 shares of the Company's common stock will be available for future grant or issuance under the ESPP, which will become effective on the completion of this offering.
In late March 2014, the Company's board of directors and stockholders approved amending and restating the Company's amended and restated certificate of incorporation to effect a 2-for-1 forward split of the Company's common stock and preferred stock. The amended and restated certificate of incorporation giving effect to the stock split was filed on March 24, 2014. All information related to common stock, preferred stock, stock options and earnings per share, as well as all references to common stock or preferred stock warrants as converted into common stock, have been adjusted to reflect the 2-for-1 forward stock split.
The Company applies ASC 855 for the accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued. For the financial statements as of December 31, 2012 and 2013, the Company has evaluated subsequent events through March 5, 2014, the date these financial statements were issued.
F-34
