UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
For the quarterly period ended
OR
From the transition period from to .
Commission File Number
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction of | (IRS Employer | |
| incorporation) | Identification No.) |
(Address of principal executive offices)
(Zip Code)
Registrant’s telephone number, including area
code:
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on with registered |
Indicate by check mark whether the registrant (1) has filed all
reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or
for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements
for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically
every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during
the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act:
| Large accelerated filer | Accelerated filer ☐ | |
| Smaller reporting company | ||
| Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as
defined in Rule 12b-2 of the Exchange Act). Yes ☐ No
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Section 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court. Yes ☒ No ☐
As of May 3, 2023, there were
Unless the context indicates otherwise, the terms “Humanigen,” “we,” “us” and “our” refer to Humanigen, Inc., and its consolidated subsidiaries. This report also may include trademarks, service marks and trade names owned by us or other companies. All trademarks, service marks and trade names included in this report are the property of their respective owners.
| 2 |
TABLE OF CONTENTS
HUMANIGEN, INC.
FORM 10-Q
| 3 |
PART I. FINANCIAL INFORMATION
| Item 1. | Financial Statements |
Humanigen, Inc.
Condensed Consolidated Balance Sheets
(in thousands, except share data)
(Unaudited)
| March 31, 2023 | December 31, 2022 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | $ | ||||||
| Prepaid expenses and other current assets | ||||||||
| Total current assets | ||||||||
| Other assets | ||||||||
| Total assets | $ | $ | ||||||
| Liabilities and stockholders’ deficit | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | $ | ||||||
| Accrued expenses | ||||||||
| Deferred revenue | ||||||||
| Total current liabilities | ||||||||
| Non-current liabilities: | ||||||||
| Deferred revenue | ||||||||
| Total liabilities | ||||||||
| Stockholders’ deficit: | ||||||||
| Common stock, $ | ||||||||
March 31, 2023 and December 31, 2022; outstanding at March 31, 2023 and December 31, 2022 | ||||||||
| Additional paid-in capital | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Total stockholders’ deficit | ( | ) | ( | ) | ||||
| Total liabilities and stockholders’ deficit | $ | $ | ||||||
See accompanying notes.
| 4 |
Humanigen, Inc.
Condensed Consolidated Statements of Operations
(in thousands, except share and per share data)
(Unaudited)
| Three Months Ended March 31, | ||||||||
| 2023 | 2022 | |||||||
| Revenue: | ||||||||
| License revenue | $ | $ | ||||||
| Total revenue | ||||||||
| Operating expenses: | ||||||||
| Research and development | ||||||||
| General and administrative | ||||||||
| Total operating expenses | ||||||||
| Loss from operations | ( | ) | ( | ) | ||||
| Other income (expense): | ||||||||
| Interest expense | ( | ) | ( | ) | ||||
| Other income (expense), net | ( | ) | ||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| $ | ( | ) | $ | ( | ) | |||
See accompanying notes.
| 5 |
Humanigen, Inc.
Condensed Consolidated Statements of Cash Flows
(in thousands)
(Unaudited)
| Three Months Ended March 31, | ||||||||
| 2023 | 2022 | |||||||
| Operating activities: | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Stock based compensation expense | ||||||||
| Non-cash interest expense related to debt financing | ||||||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses and other assets | ( | ) | ( | ) | ||||
| Accounts payable | ( | ) | ||||||
| Accrued expenses | ( | ) | ( | ) | ||||
| Deferred revenue | ( | ) | ( | ) | ||||
| Net cash used in operating activities | ( | ) | ( | ) | ||||
| Financing activities: | ||||||||
| Net proceeds from issuance of common stock | ||||||||
| Net cash provided by financing activities | ||||||||
| Net decrease in cash and cash equivalents | ( | ) | ( | ) | ||||
| Cash and cash equivalents, beginning of period | ||||||||
| Cash and cash equivalents, end of period | $ | $ | ||||||
| Supplemental cash flow disclosure: | ||||||||
| Cash paid for interest | $ | $ | ||||||
See accompanying notes.
| 6 |
Humanigen, Inc.
Condensed Consolidated Statements of Stockholders’ Deficit
(in thousands, except share data)
(Unaudited)
| Three Months Ended March 31, 2023 | ||||||||||||||||||||
| Additional | Total | |||||||||||||||||||
| Common Stock | Paid-In | Accumulated | Stockholders’ | |||||||||||||||||
| Shares | Amount | Capital | Deficit | Deficit | ||||||||||||||||
| Balances at January 1, 2023 | $ | $ | $ | ( | ) | $ | ( | ) | ||||||||||||
| Stock-based compensation expense | - | |||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||
| Balances at March 31, 2023 | $ | $ | $ | ( | ) | $ | ( | ) | ||||||||||||
| Three Months Ended March 31, 2022 | ||||||||||||||||||||
| Additional | Total | |||||||||||||||||||
| Common Stock | Paid-In | Accumulated | Stockholders’ | |||||||||||||||||
| Shares | Amount | Capital | Deficit | Deficit | ||||||||||||||||
| Balances at January 1, 2022 | $ | $ | $ | ( | ) | $ | ( | ) | ||||||||||||
| Issuance of common stock, net of expenses | ||||||||||||||||||||
| Stock-based compensation expense | - | |||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||
| Balances at March 31, 2022 | $ | $ | $ | ( | ) | $ | ( | ) | ||||||||||||
See accompanying notes.
| 7 |
Humanigen, Inc.
Notes to Condensed Consolidated Financial Statements
(Unaudited)
1. Nature of Operations
Description of the Business
The Company is a clinical stage biopharmaceutical company, developing its portfolio of proprietary Humaneered® anti-inflammatory immunology and immuno-oncology monoclonal antibodies. The Company’s proprietary, patented Humaneered technology platform is a method for converting existing antibodies (typically murine) into engineered, high-affinity human antibodies designed for therapeutic use, particularly with acute and chronic conditions. Humanigen has developed or in-licensed targets or research antibodies, typically from academic institutions, and then applied its Humaneered technology to optimize them. The Company’s lead product candidate, lenzilumab, or LENZ®, and its other product candidate, ifabotuzumab (“iFab”), are Humaneered monoclonal antibodies. The Company’s Humaneered antibodies are closer to human antibodies than chimeric or conventionally humanized antibodies and have a high affinity for their target. In addition, the Company believes its Humaneered antibodies offer further important advantages, such as high potency, a slow off-rate and a lower likelihood to induce an inappropriate immune response or infusion related reactions.
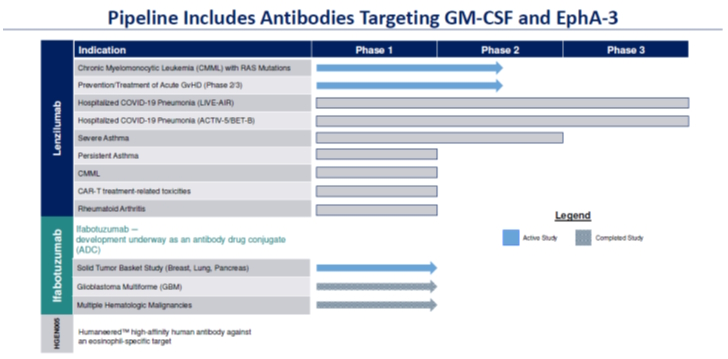
The Company is developing lenzilumab in chronic myelomonocytic leukemia (“CMML”), a rare blood cancer, for which the Precision Approach to Chronic Myelomonocytic Leukemia (“PREACH-M”) study is underway, and is continuing its plans for the Risk Adapted Therapy in Acute GvHD (“RATinG”) study in acute graft versus host disease (“aGvHD”) that occurs in patients undergoing bone marrow transplant, as these studies are majority funded by its partners. In April 2023, the Company announced that as of December 31, 2022, eleven subjects had been dosed with lenzilumab and with current standard of care, azacytidine, in the PREACH-M study. Six subjects were evaluable based on at least three months of follow-up, including those with high risk CMML, and all demonstrated clinical benefit. In addition, LENZ appeared to be well-tolerated. The Company anticipates the first patient dosing in the RATinG study to occur in the second quarter of 2023. A leading network of centers, The Mayo Clinics, is currently progressing with an investigator-initiated trial (“IIT”) of lenzilumab in combination with CAR-T therapies. With the exception of the one lenzilumab batch in process, the Company has discontinued the manufacturing of lenzilumab and is consolidating the remaining inventory of lenzilumab bulk drug substance and drug product in a central location for potential future use. The Company is also developing iFab, an EpAh-3 targeted monoclonal antibody, currently in Phase 1 development, as part of an antibody drug conjugate (“ADC”), for certain solid tumors.
See Management’s Discussion and Analysis of Financial Condition and Results of Operations included in Item 7 of the Company’s 2022 Annual Report on Form 10-K for additional information regarding the business.
Liquidity and Going Concern
The Condensed Consolidated Financial Statements for the three months ended March 31, 2023 were prepared on the basis of a going concern, which contemplates that the Company will be able to realize assets and discharge liabilities in the normal course of business. However, the Company has incurred net losses since its inception, and has negative operating cash flows and its total liabilities exceed total assets. These conditions raise substantial doubt about the Company’s ability to continue as a going concern.
As of March 31, 2023, the Company had cash and
cash equivalents of $
As previously reported, the Company has executed a non-binding letter of intent and is engaged in exclusive negotiations relating to a proposed business combination with a privately held biopharmaceutical company, which contemplates a tax-free stock-for-stock merger. The Company is seeking external financing in connection with the potential business combination. There can be no assurance that the potential business combination or financing will be consummated on favorable terms or at all.
| 8 |
The Company also may seek to raise additional capital through public or private equity offerings, including under the Controlled Equity OfferingSM Sales Agreement (the “Sales Agreement”) with Cantor Fitzgerald & Co. (“Cantor”), grant financing, convertible and other debt financings, collaborations, strategic alliances, or licensing arrangements involving LENZ and iFab. Additional funds may not be available when the Company needs them on terms that are acceptable to the Company, or at all. If adequate funds are not available, the Company may be required to delay or reduce the scope of or eliminate one or more of its research or development programs and may not be able to continue as a going concern. In addition, if the Company raises additional funds through collaborations, strategic alliances, or licensing arrangements with third parties, the Company may have to relinquish rights to its technologies, future revenue streams or product candidates or to grant licenses on terms that may not be favorable to the Company. While management believes its realignment plans and its plans to raise additional funds will alleviate the conditions that raise substantial doubt about the Company’s ability to continue as a going concern, these plans are not entirely within the Company’s control and cannot be assessed as being probable of occurring.
Basis of Presentation
The accompanying interim unaudited Condensed Consolidated Financial Statements have been prepared in accordance with US generally accepted accounting principles (“US GAAP”) for interim financial information and on a basis consistent with the annual consolidated financial statements and include all adjustments necessary for the presentation of the Company’s condensed consolidated financial position, results of operations and cash flows for the periods presented.
The Condensed Consolidated Financial Statements include the accounts of the Company and its wholly-owned subsidiaries. These financial statements have been prepared on a basis that assumes that the Company will continue as a going concern, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The December 31, 2022 Condensed Consolidated Balance Sheet was derived from the audited financial statements but does not include all disclosures required by US GAAP. These interim financial results are not necessarily indicative of the results to be expected for the year ending December 31, 2023, or for any other future annual or interim period. The accompanying unaudited Condensed Consolidated Financial Statements should be read in conjunction with the audited consolidated financial statements and the related notes thereto included in the Company’s 2022 Annual Report on Form 10-K.
The preparation of financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the amounts and disclosures reported in the Condensed Consolidated Financial Statements and accompanying notes. Actual results could differ materially from those estimates. The Company believes judgment is involved in accounting for the determination of revenue recognition, fair value-based measurement of stock-based compensation and accruals. The Company evaluates its estimates and assumptions as facts and circumstances dictate. As future events and their effects cannot be determined with precision, actual results could differ from these estimates and assumptions, and those differences could be material to the Condensed Consolidated Financial Statements.
2. Summary of Significant Accounting Policies
The Company’s significant accounting policies are detailed in its Annual Report on Form 10-K for the year ended December 31, 2022. There have been no significant changes to the Company’s significant accounting policies during the three months ended March 31, 2023, from those previously disclosed in its 2022 Annual Report on Form 10-K.
3. Potentially Dilutive Securities
The Company’s potentially dilutive securities, which include stock options and warrants and shares of common stock issuable upon conversion of convertible debt, have been excluded from the computation of diluted net loss per common share as the effect of including those securities would be to reduce the net loss per common share and be antidilutive. Therefore, the denominator used to calculate both basic and diluted net loss per common share is the same in each period presented.
The following outstanding potentially dilutive securities have been excluded from the computations of diluted net loss per common share:
| As of March 31, | ||||||||
| 2023 | 2022 | |||||||
| Options to purchase common stock | ||||||||
| Warrants to purchase common stock | ||||||||
| Convertible debt | ||||||||
| 9 |
4. License Revenue
On November 3, 2020, the Company entered into a License Agreement (the “South Korea Agreement”) with KPM Tech Co., Ltd. (“KPM”) and its affiliate, Telcon RF Pharmaceutical, Inc. (together with KPM, the “Licensee”). Pursuant to the South Korea Agreement, among other things, the Company granted the Licensee a license under certain patents and other intellectual property to develop and commercialize lenzilumab for treatment of COVID-19 pneumonia, in South Korea and the Philippines (the “Territory”), subject to certain reservations and limitations. The Licensee will be responsible for gaining regulatory approval for, and subsequent commercialization of, lenzilumab in the Territory.
As consideration for the license, the Licensee
agreed to pay the Company (i) an up-front license fee of $
Since the provision of the license and the cooperation
and assistance to be provided by the Company to the Licensee with regulatory authorities in the Territory and the Company’s obligation
to serve on a joint steering committee (the “Services”) are considered a single performance obligation, the $
Licensee’s purchases of lenzilumab for development purposes or for commercial requirements, represent options under the agreement and revenues will therefore be recognized when control of the product is transferred to Licensee.
Contract Liabilities
A contract liability of $
The following table presents changes in the Company’s contract liability for the three months ended March 31, 2023 (in thousands):
| Balance at January 1, 2023 | $ | |||
| Deductions for performance obligations satisfied: | ||||
| In current period | ( | ) | ||
| Balance at March 31, 2023 | $ |
5. Long-Term Debt
Secured Term Loan Facility
On March 10, 2021, the Company executed the Loan
and Security Agreement with Hercules Capital as agent for its affiliates serving as lenders thereunder (the “Term Loan”) which
provided a loan in the aggregate principal amount of up to $
| 10 |
In July 2022, the Company prepaid $
6. Commitments and Contingencies
Manufacturing Agreements
As of March 31, 2023, the Company estimates that
its commitments remaining to be incurred under its contract manufacturing organization (“CMO”) agreements are approximately
$
7. Stockholders’ Equity
Controlled Equity Offering
On December 31, 2020, the Company entered into
a Sales Agreement with Cantor, under which the Company could issue and sell, from time-to-time, shares of the Company’s common stock,
having an aggregate gross sales price of up to $
8. Stock-Based Compensation
A summary of stock option activity for the three months ended March 31, 2023 under all the Company’s options plans is as follows:
| Options | Weighted Average Exercise Price | |||||||
| Outstanding at January 1, 2023 | $ | |||||||
| Granted | $ | |||||||
| Exercised | $ | |||||||
| Cancelled (expired) | ( | ) | $ | |||||
| Outstanding at March 31, 2023 | $ | |||||||
The Company recorded stock-based compensation expense in the Condensed Consolidated Statements of Operations as follows (in thousands):
| Three Months Ended March 31, | ||||||||
| 2023 | 2022 | |||||||
| General and administrative | $ | $ | ||||||
| Research and development | ||||||||
| Total stock-based compensation | $ | $ | ||||||
At March 31, 2023, the Company had $
9. Litigation
Eversana Arbitration
On May 19, 2022, Eversana Life Science Services, LLC (“Eversana”)
filed a Demand for Arbitration claiming approximately $
| 11 |
Avid Settlement
On February 21, 2023, the Company and Avid Bioservices, Inc. (“Avid”) entered into a Settlement Agreement (the “Avid Settlement Agreement”) providing for a conditional resolution of certain previously reported disputes between the Company and Avid arising pursuant to the commercial agreements between the two parties (collectively, the “Lenzilumab Disputes”).
Pursuant to the Settlement Agreement, the Company
made a one-time payment of $
Thermo Litigation
On October 24, 2022, one of the Company’s former CMOs, Thermo
Fisher Scientific, Inc. (“Thermo”) filed a lawsuit against the Company in Delaware Superior Court (Patheon Biologics, Inc.
v. Humanigen, Inc., Case No. N22C-10-185 MMJ) for $
Securities Class Action Litigation
On August 26, 2022, a putative securities class action complaint captioned Pieroni v. Humanigen Inc., et al., Case No. 22-cv-05258, was filed in the United States District Court for the District of New Jersey against the Company, its Chief Executive Officer, Dr. Cameron Durrant, and its former Chief Financial Officer, Timothy Morris. On October 17, 2022, a second putative securities class action complaint captioned Greenbaum v. Humanigen Inc., et al., Case No. 22-cv-06118, was filed in the United States District Court for the District of New Jersey against the Company, Dr. Durrant and the Company’s Chief Scientific Officer, Dale Chappell. The complaints assert claims and seek damages for alleged violations of sections 10(b) and 20(a) of the Securities Exchange Act of 1934 and Rule 10b-5 promulgated thereunder. The two actions have been consolidated into a single action captioned In re Humanigen, Inc. Securities Litigation, Case No. 2:22-cv-05258 and co-lead plaintiffs and co-lead law firms have been appointed. The Company believes that the allegations in the putative complaints are without merit and will vigorously defend against them.
Shareholder Derivative Litigation
On January 17, 2023, a derivative lawsuit captioned Chul Yang derivatively on behalf of Humanigen, Inc. v. Durrant, et al., Case No. 2:23-cv-00235, was filed in the United States District Court for the District of New Jersey against the company’s Chief Executive Officer, Dr. Cameron Durrant, its former Chief Financial Officer, Timothy Morris, and each of its Directors. The complaint asserts claims and seeks damages against all of the defendants for alleged violations of section 14(a) of the Securities Exchange Act of 1934 and Rule 14a-9 promulgated thereunder, breach of fiduciary duty, unjust enrichment, abuse of control, gross mismanagement, and waste of corporate assets, and against Dr. Durrant and Mr. Morris for alleged violations of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 and Rule 10b-5 promulgated thereunder. This matter has been stayed pending initial rulings in the consolidated securities class action matter. The Company believes that the allegations in the putative derivative action are without merit and will vigorously defend against them.
| 12 |
| Item 2. | Management’s Discussion and Analysis of Financial Condition and Results of Operations. |
You should read the following discussion and analysis together with our financial statements and the notes to those statements included elsewhere in this Quarterly Report on Form 10-Q and our Annual Report on Form 10-K for the fiscal year ended December 31, 2022 (the “2022 Annual Report”). This Quarterly Report on Form 10-Q contains statements that discuss future events or expectations, projections of results of operations or financial condition, trends in our business, business prospects and strategies and other “forward-looking” information. In some cases, you can identify “forward-looking statements” by words like “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “intends,” “potential” or “continue” or the negative of those words and other comparable words. These forward-looking statements are not guarantees of future performance or development and involve known and unknown risks, uncertainties and other factors that are in some cases beyond our control. These statements may relate to, among other things, our expectations regarding the scope, progress, timing, expansion, and costs of researching, developing and commercializing our product candidates; our expectations relating to regulatory pathways to marketing authorization and the opportunity to benefit from various regulatory incentives; expectations for our financial results, revenue, operating expenses and other financial measures in future periods; the adequacy of our sources of liquidity to satisfy our working capital needs, capital expenditures, and other liquidity requirements; and our exploration of strategic alternatives. Among the factors that could cause actual results to differ materially are the factors discussed under “Risk Factors” in “Part I, Item 1A - Risk Factors” in the 2022 Annual Report, and the additional or modified risk factors disclosed in this Quarterly Report on Form 10-Q and each subsequently filed Quarterly Report on Form 10-Q. Some additional factors that could cause actual results to differ include:
| ● | our ability to attain the significant amount of additional financing we need to continue as a going concern on favorable terms or at all, and the availability of future financing; |
| ● | our ability to complete the proposed business combination and related financing transactions described in the 2022 Annual Report under “Item 1. Business – Overview”, or to identify and execute upon another strategic transaction or alternative to maximize value for our stakeholders; |
| ● | our ability to demonstrate by August 21, 2023, and thereafter maintain compliance with, the listing requirements of the Nasdaq Capital Market; |
| ● | the outcome of pending, threatened or future litigation or arbitration; |
| ● | our ability to resolve pending or threatened litigation regarding payment disputes with certain Contract Manufacturing Organizations (“CMOs”) and other parties, and our ability to defer payments, negotiate lower amounts or successfully pursue other courses of action for certain amounts accrued at March 31, 2023; |
| ● | our ability to successfully execute the strategic realignment of our pipeline and resources; |
| ● | the timing of the initiation, enrollment and completion and results of ongoing or planned clinical trials; |
| ● | our ability to research, develop and commercialize our product candidates, including our ability to do so after our competitors have developed and commercialized competing products or alternative therapies; |
| ● | the ability of partners to initiate and conduct the PREACH-M and RATinG studies (as described below) of lenzilumab in chronic myelomonocytic leukemia (“CMML”) and in patients with acute Graft versus Host Disease (“aGvHD”), respectively, as currently planned; |
| ● | our ability to assess and support further clinical assessment of lenzilumab as a companion therapy with commercially available chimeric antigen receptor T-cell (“CAR-T”) therapies in non-Hodgkin lymphoma through an investigator-initiated trial (“IIT”); |
| ● | increasing levels of market acceptance of CAR-T therapies and stem cell transplants and the development of a market for lenzilumab in these therapies; |
| ● | our ability to maintain licenses with third parties; |
| ● | our ability to attain market exclusivity and/or to obtain, maintain, protect and enforce our intellectual property and to operate our business without infringing, misappropriating or otherwise violating, the intellectual property rights of others; |
| ● | our ability to achieve collaborations, strategic alliances, or licensing arrangements for lenzilumab; |
| ● | acquisitions or in-licensing or out-licensing transactions that we may pursue may fail to perform as expected; |
| ● | changes in the regulatory landscape that may prevent us from pursuing or realizing any of the expected benefits from the various regulatory incentives, or the imposition of regulations that affect our products; and |
| ● | the accuracy of our estimates regarding expenses, future revenues, capital requirements and needs for additional financing. |
These are only some of the factors that may affect the forward-looking statements contained in this Form 10-Q. For a discussion identifying additional important factors that could cause actual results to vary materially from those anticipated in the forward-looking statements, see “Risk Factors” in Item 1A of Part II below and in Part I, Item 1A of the 2022 Annual Report. You should review these risk factors, together, for a more complete understanding of the risks associated with an investment in our securities. However, we operate in a competitive and rapidly changing environment and new risks and uncertainties emerge, are identified or become apparent from time-to-time. It is not possible for us to predict all risks and uncertainties that could have an impact on the forward-looking statements contained in this Form 10-Q. You should be aware that the forward-looking statements contained in this Form 10-Q are based on our current views and assumptions. We undertake no obligation to revise or update any forward-looking statements made in this Form 10-Q to reflect events or circumstances after the date hereof or to reflect new information or the occurrence of unanticipated events, except as required by law.
| 13 |
Overview
We are a clinical stage biopharmaceutical company, developing our portfolio of proprietary Humaneered® anti-inflammatory immunology and immuno-oncology monoclonal antibodies. Our proprietary, patented Humaneered technology platform is a method for converting existing antibodies (typically murine) into engineered, high-affinity human antibodies designed for therapeutic use, particularly with acute and chronic conditions. We have developed or in-licensed targets or research antibodies, typically from academic institutions, and then applied our Humaneered technology to optimize them. Our lead product candidate, lenzilumab, and our other product candidate, ifabotuzumab (“iFab”), are Humaneered monoclonal antibodies. Our Humaneered antibodies are closer to human antibodies than chimeric or conventionally humanized antibodies and have a high affinity for their target. In addition, we believe our Humaneered antibodies offer further important advantages, such as high potency, a slow off-rate and a lower likelihood to induce an inappropriate immune response or infusion related reaction.
We are focusing our efforts on the development of our lead product candidate, lenzilumab. Lenzilumab is a monoclonal antibody that has been demonstrated to neutralize human granulocyte-macrophage colony-stimulating factor (“GM-CSF”), a cytokine that we believe leads to the overproduction of monocytes which are responsible for CMML, a rare blood cancer and is of critical importance in aGvHD associated with bone marrow transplants.
We are currently developing lenzilumab in CMML for which the Precision Approach to Chronic Myelomonocytic Leukemia (“PREACH-M”) study is underway, and we are continuing plans for the Risk Adapted Therapy in Acute GvHD (“RATinG”) study in aGvHD, as these studies are majority funded by our partners.
In April 2023, we announced that as of December 31, 2022, eleven subjects had been dosed with lenzilumab and with current standard of care, azacytidine, in the PREACH-M study. Six subjects were evaluable based on at least three months of follow-up, including those with high risk CMML, and all demonstrated clinical benefit. In addition, lenzilumab appeared to be well-tolerated. We anticipate the first patient dosing in the RATinG study to occur in the second quarter of 2023.
A leading network of centers, The Mayo Clinics, is currently progressing with an IIT of lenzilumab in combination with CAR-T therapies. We are also developing iFab, an EpAh-3 targeted monoclonal antibody, currently in Phase 1 development, as part of an antibody drug conjugate (“ADC”), for certain solid tumors.
Recent Developments
Our current capital resources are not sufficient to fund our operations for the remainder of 2023. Accordingly, as previously disclosed, we have been pursuing strategic alternatives and seeking to raise additional capital and settle or otherwise resolve payment disputes and other ongoing arbitration and litigation.
As previously reported, during 2022 we engaged SC&H Capital, an affiliate of SC&H Group, (“SC&H”) to advise us on exploration of strategic options. SC&H is an investment banking and advisory firm providing merger and acquisition (M&A), financial restructuring and related business advisory solutions. SC&H has acted as our advisor as we explore strategic options to maximize value around lenzilumab and ifabotuzumab. We also have considered and pursued a full range of options to raise additional capital and to address, satisfy, defer or restructure our accounts payable and accrued liabilities to manufacturing and other parties.
We have executed a non-binding letter of intent and are engaged in exclusive negotiations relating to a proposed business combination with a privately held biopharmaceutical company (the “Partner Company”). The proposed terms for the business combination contemplate a tax-free stock-for-stock merger, as a result of which we would issue shares of our capital stock to stockholders of the Partner Company which are expected to represent roughly two times the number of our currently outstanding shares of common stock.
We cannot assure you that we and the Partner Company will enter into a definitive agreement for the proposed transaction, and the final form and terms of any such transaction may be materially different from the terms described above. Our ability to enter into a definitive agreement is subject to conditions, including that we have received binding commitments for investment of additional capital that will be necessary to fund the operations of the combined company going forward and enable the combined company to maintain a listing of its common stock on the Nasdaq Capital Market or another national securities exchange, as well as customary matters such as approval of the terms of the definitive agreement by the Partner Company’s board of directors and stockholders. Certain of these conditions will be out of our control. Accordingly, we cannot provide any assurance that we will effect the proposed business combination or related financing transactions. If we are unable to complete the proposed transactions or identify and complete another strategic or financing transaction in the first half of 2023, we may elect or be required to pursue a reorganization or seek other protection under the federal bankruptcy code. See Part I, Item 1A, “Risk Factors” to the 2022 Annual Report.
| 14 |
Our Pipeline
Our product candidates are in the clinical stage of development and require substantial time, resources, research and development, and regulatory approval prior to commercialization. Our pipeline is depicted below:
Nasdaq Listing Deficiencies
As previously reported, we have received two notices from The Nasdaq Stock Market, LLC (“Nasdaq”) regarding our failures to satisfy the $1 minimum bid price and $35 million total market value of listed securities standards for continued listing. On April 18, 2023, we were notified that we had been granted an extension until August 21, 2023 to demonstrate compliance with all applicable requirements for listing of our common stock on Nasdaq by the Nasdaq Hearings Panel (the “Panel”), subject to our compliance with the Panel’s requirements for periodic updates relating to the status of our progress against achievement of the compliance plan presented at the April 6, 2023 hearing with the Panel. There can be no assurance that we will be successful in our execution of the compliance plan or otherwise regain compliance with the applicable Nasdaq listing requirements within the extended compliance period. In addition, our common stock may be subject to immediate delisting from the Nasdaq Capital Market if our common stock has a closing bid price of $0.10 or less for any ten consecutive trading days. See Part I, Item 1A, “Risk Factors” to the 2022 Annual Report.
Critical Accounting Policies and Use of Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our Condensed Consolidated Financial Statements, which have been prepared in accordance with accounting principles generally accepted in the US, or GAAP. The preparation of our financial statements in conformity with GAAP requires our management to make estimates and assumptions that affect the amounts and disclosures reported in the financial statements and accompanying notes. Actual results could differ materially from those estimates. Our management believes judgment is involved in determining revenue recognition, the fair value-based measurement of stock-based compensation, and accruals. Our management evaluates estimates and assumptions as facts and circumstances dictate. As future events and their effects cannot be determined with precision, actual results could differ from these estimates and assumptions, and those differences could be material to the Condensed Consolidated Financial Statements. If our assumptions change, we may need to revise our estimates, or take other corrective actions, either of which may also have a material adverse effect on our statements of operations, liquidity and financial condition.
| 15 |
There were no significant and material changes in our critical accounting policies and use of estimates during the three months ended March 31, 2023, as compared to those disclosed in “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Use of Estimates” in the 2022 Annual Report.
Results of Operations
At March 31, 2023, we had an accumulated deficit of $686.0 million, primarily as a result of research and development and general and administrative expenses. Since inception, we have recognized a nominal amount of revenue from payments for license or collaboration fees. While we may in the future generate additional revenue from a variety of sources, including license fees, milestone payments, and research and development payments in connection with strategic partnerships, our product candidates may never be successfully developed or commercialized and we may therefore never realize revenue from any product sales. Accordingly, we expect to continue to incur substantial losses from operations for the foreseeable future, and there can be no assurance that we will ever generate significant revenue or profits. Our ability to continue as a going concern depends on our ability to attain a significant amount of additional financing, as more fully described under “—Liquidity and Capital Resources” below and in “Risk Factors” in the 2022 Annual Report.
Comparison of Three Months Ended March 31, 2023 and 2022
The following table summarizes the results of our operations for the periods indicated (amounts in thousands, except percentages):
| Three Months Ended March 31, | Increase/ (Decrease) | |||||||||||||||
| (in thousands) | 2023 | 2022 | Amount | % | ||||||||||||
| Revenue: | ||||||||||||||||
| License revenue | $ | 221 | $ | 1,036 | $ | (815 | ) | (79 | ) | |||||||
| Total revenue | 221 | 1,036 | (815 | ) | ||||||||||||
| Operating expenses: | ||||||||||||||||
| Research and development | 737 | 17,220 | (16,483 | ) | (96 | ) | ||||||||||
| General and administrative | 3,719 | 4,345 | (626 | ) | (14 | ) | ||||||||||
| Total operating expenses | 4,456 | 21,565 | (17,109 | ) | (79 | ) | ||||||||||
| Loss from operations | (4,235 | ) | (20,529 | ) | (16,294 | ) | (79 | ) | ||||||||
| Other income (expense): | ||||||||||||||||
| Interest expense | (18 | ) | (734 | ) | (716 | ) | (98 | ) | ||||||||
| Other income (expense), net | 71 | (15 | ) | (86 | ) | (573 | ) | |||||||||
| Net loss | $ | (4,182 | ) | $ | (21,278 | ) | $ | (17,096 | ) | (80 | ) | |||||
Revenue
Revenue in the three months ended March 31, 2023 and 2022, represents license revenue under the license agreement (the “South Korea Agreement”) with KPM Tech Co., Ltd. (“KPM”) and its affiliate, Telcon RF Pharmaceutical, Inc. (together with KPM, the “Licensee”) described in more detail in Note 4 to the Condensed Consolidated Financial Statements included in this Quarterly Report on Form 10-Q. License revenue was $0.2 million for the three months ended March 31, 2023, as compared to $1.0 million for the three months ended March 31, 2022. The decrease in revenue for the three months ended March 31, 2023, compared to March 31, 2022, is due to a change in the length of the performance period in the third quarter of 2022, as described in more detail in Note 4 to the Condensed Consolidated Financial Statements included in this Quarterly Report on Form 10-Q.
Research and Development Expenses
Conducting research and development is central to our business model. We expense both internal and external research and development costs as incurred. We track external research and development costs incurred by project for each of our clinical programs. Our external research and development costs consist primarily of:
| 16 |
| ● | expenses incurred under agreements with contract research organizations, investigative sites, and consultants that conduct our clinical trials and our pre-clinical activities; |
| ● | the cost of acquiring and manufacturing clinical trial, pre-commercial and other materials, the cost to transfer the manufacturing process for bulk drug substance and fill/finish production, development of and periodic performance of a variety of tests and assays for stability, release, comparability and product characterization, costs associated with quality management, the preparation of documents and information necessary to file with regulatory authorities; and |
| ● | other costs associated with development activities, including additional studies. |
Other research and development costs consist primarily of internal research and development costs such as salaries and related fringe benefit costs for our employees, stock-based compensation charges, and travel costs not allocated to one of our clinical programs. Internal research and development costs generally benefit multiple projects and are not separately tracked per project.
The following table shows our total research and development expenses for the three months ended March 31, 2023 and 2022:
| Three Months Ended March 31, | ||||||||
| (in thousands) | 2023 | 2022 | ||||||
| External Costs | ||||||||
| Lenzilumab | $ | 489 | $ | 16,448 | ||||
| Ifabotuzumab | 26 | 158 | ||||||
| Internal costs | 222 | 614 | ||||||
| Total research and development | $ | 737 | $ | 17,220 | ||||
Research and development expenses decreased by $16.5 million from $17.2 million for the three months ended March 31, 2022 to $0.7 million for the three months ended March 31, 2023. The decrease is primarily due to a $13.9 million decrease in lenzilumab manufacturing costs and a $2.6 million decrease in clinical trial expenses as the LIVE-AIR study has been completed.
We expect our development costs will decrease in 2023 as compared to 2022. In connection with our realignment to deemphasize the deployment of certain resources for the development of lenzilumab for COVID-19, with the exception of one lenzilumab batch in process, we have discontinued the manufacturing of lenzilumab and are consolidating the remaining inventory of lenzilumab bulk drug substance and drug product in a central location for potential future use. We believe we have sufficient drug product for our currently planned clinical trials.
General and Administrative Expenses
General and administrative expenses consist principally of personnel-related costs (including stock-based compensation), professional fees for legal and patent expenses, insurance, consulting, audit, investor relations costs, and other general operating expenses not otherwise included in research and development.
General and administrative expenses decreased by $0.6 million from $4.3 million for the three months ended March 31, 2022 to $3.7 million for the three months ended March 31, 2023. The decrease for the three months ended March 31, 2023, is primarily due to a reduction in headcount in 2023 as compared to 2022. We expect our overall general and administrative costs to continue to decrease as compared to 2022.
Interest Expense
Interest expense for the three months ended March 31, 2022 related to the Loan and Security Agreement with Hercules Capital as agent for its affiliates serving as lenders thereunder (the “Term Loan”). See Note 5 to the Condensed Consolidated Financial Statements of this Quarterly Report on Form 10-Q for additional information on the Term Loan.
Liquidity and Capital Resources
Since our inception, we have financed our operations primarily through proceeds from the public offerings of our common stock, private placements of our common and preferred stock, debt financings, interest income earned on cash, and cash equivalents, and marketable securities, and borrowings against lines of credit, and with the proceeds under the South Korea Agreement. At March 31, 2023, we had cash and cash equivalents of $3.1 million. In the first quarter of 2022, we sold an aggregate of 5,926,748 shares of our common stock under the Sales Agreement for net proceeds of $18.4 million. No shares were sold under the Sales Agreement in the first quarter of 2023.
| 17 |
Primary Sources of and Uses of Cash
The following table sets forth the primary sources and uses of cash and cash equivalents for each of the periods presented below:
| Three Months Ended March 31, | ||||||||
| (In thousands) | 2023 | 2022 | ||||||
| Net cash (used in) provided by: | ||||||||
| Operating activities | $ | (7,059 | ) | $ | (19,442 | ) | ||
| Financing activities | - | 18,374 | ||||||
| Net decrease in cash and cash equivalents | $ | (7,059 | ) | $ | (1,068 | ) | ||
Net cash used in operating activities was $7.1 million and $19.4 million for the three months ended March 31, 2023 and 2022, respectively. Cash used in operating activities of $7.1 million for the three months ended March 31, 2023, primarily related to our net loss of $4.2 million, adjusted for non-cash items, such as $1.2 million in stock-based compensation, and a net change in operating assets and liabilities of $4.1 million.
Cash used in operating activities of $19.4 million for the three months ended March 31, 2022, primarily related to our net loss of $21.3 million, adjusted for non-cash items, such as $1.5 million in stock-based compensation, and a net change in operating assets and liabilities of $0.1 million.
There were no financing activities for the three months ended March 31, 2023. Net cash provided by financing activities was $18.4 million for the three months ended March 31, 2022 and consists of net proceeds received from the issuance of common stock in connection with the Sales Agreement with Cantor.
Liquidity and Manufacturing Commitments
As of March 31, 2023, we had cash and cash equivalents of $3.1 million; combined accounts payable and accrued expenses of $52.4 million, certain of which were in dispute; and manufacturing commitments of $1.8 million for the remainder of 2023 with no significant commitments, thereafter, as further described below (see “– Contracts”). We intend to seek to defer these disputed payment obligations, negotiate lower amounts or seek other courses of action, which may include legal recourse for the amounts in question. Our capital resources are not sufficient to fund our operations for the remainder of 2023.
Our ability to enter into a definitive agreement with the Partner Company for the strategic transaction described above is subject to numerous conditions, including (among others) that we have received binding commitments for investment of additional capital that will be necessary to enable us to fund the operations of the combined company going forward and enable the combined company to maintain a listing of its common stock on the Nasdaq Capital Market or another national securities exchange. We cannot provide any assurance that we will be able to raise sufficient funds to permit us to effect the proposed business combination. If we are unable to complete the proposed transactions or identify and complete another strategic or financing transaction in the first half of 2023, we may elect or be required to pursue a reorganization or seek other protection under the federal bankruptcy code. If the proposed business combination with the Partner Company and related financing is completed, we would expect to issue a significant number of shares of common stock and/or convertible equity securities to the stockholders of the Partner Company as discussed above and to new investors in the financing, each of which would have a significant dilutive effect on our existing stockholders.
See Part I, Item 1A, “Risk Factors” in the 2022 Annual Report for further discussion of the risks surrounding the proposed transaction and our company.
Contracts
Eversana Agreement
On January 10, 2021, we announced that we had entered into a master services agreement (the “Eversana Agreement”) with Eversana Life Science Services, LLC (“Eversana”) pursuant to which Eversana will provide us with services in connection with the potential launch of lenzilumab.
| 18 |
On September 21, 2021, we notified Eversana that due to the Emergency Use Authorization status in the US, we were terminating the initial statement of work related to commercialization support of lenzilumab for the treatment of COVID-19 in the United States. Eversana disputed the termination notice and requested payment of approximately $4.5 million it asserted we owed for services rendered from April 1, 2021 to September 30, 2021. We have agreed in principle on a conditional resolution of the matters being disputed with Eversana and are negotiating a formal settlement agreement. See Note 9 to the Condensed Consolidated Financial Statements of this Quarterly Report on Form 10-Q for additional information.
Manufacturing Agreements
We entered into agreements with several CMOs to manufacture bulk drug substance (“BDS”) and fill/finish/drug product (“DP”) for our lenzilumab clinical trial activities. We also entered into agreements for packaging of the drug. These agreements provided for upfront amounts prior to commencement of manufacturing and progress payments through the course of the manufacturing process and payments for technology transfer. Certain of these CMOs were unsuccessful in their efforts to manufacture some batches of lenzilumab to our specifications for various reasons. We have amended, and in some cases canceled, certain of these agreements. In addition, we have sought to mitigate our financial commitments by ceasing additional manufacturing of lenzilumab in connection with our realignment plan and, more recently, we have settled our disputes with two of our CMOs. See Note 11 to the Consolidated Financial Statements in the 2022 Annual Report for more information on these settlement agreements.
We believe we have sufficient supply to conduct our contemplated clinical development efforts. We have discontinued the manufacturing of lenzilumab, with the exception of one batch in process at one of our CMOs, Catalent Pharma Solutions, LLC (“Catalent”). If we are unable to obtain regulatory approval for lenzilumab prior to the expiration of the shelf life at that time, the remaining inventory will not be available for commercial use.
There is significant drug product that was in production at one of our other CMOs, Thermo Fisher Scientific, Inc. (“Thermo”), for which material has not yet been released by us because the batches produced are out of specification. Nonetheless, Thermo has notified us that they have stopped production and have recently filed a lawsuit against us in Delaware Superior Court for $25.9 million. We have filed a countersuit against Thermo for breach of contract seeking more than $37.5 million. We have agreed in principle on a conditional resolution of the matters being disputed with Thermo and are negotiating a formal settlement agreement. See Note 9 to the Condensed Consolidated Financial Statements of this Quarterly Report on Form 10-Q for additional information.
Please see our Form 10-K for the year ended December 31, 2022, Part I, Item 1A - Risk Factors—“Risks Related to our Financial Condition, Need for Additional Capital and Ability to Continue as a Going Concern— Currently pending, threatened or future litigation, arbitration, governmental proceedings or inquiries could result in material adverse consequences, including judgments or settlements, and adversely affect our ability to continue as a going concern.”
License Agreements
We are obligated to make future payments to third parties under in-license agreements, including sublicense fees, royalties, and payments that become due and payable on the achievement of certain development and commercialization milestones.
We record upfront and milestone payments made to third parties under licensing arrangements as an expense. Upfront payments are recorded when incurred and milestone payments are recorded when the specific milestone has been achieved.
Outlicensing Agreements
The South Korea Agreement
On November 3, 2020, we entered into a License Agreement (the “South Korea Agreement”) with KPM and Telcon (together, the “Licensee”). Pursuant to the South Korea Agreement, among other things, we granted the Licensee a license under certain patents and other intellectual property to develop and commercialize our lead product candidate, lenzilumab (the “Product”), for treatment of COVID-19 pneumonia, in South Korea and the Philippines (the “Territory”), subject to certain reservations and limitations. The Licensee will be responsible for gaining regulatory approval for, and subsequent commercialization of, lenzilumab in those territories.
As consideration for the license, the Licensee has agreed to pay us (i) an up-front license fee of $6.0 million (or $4.5 million net of withholding taxes and other fees and royalties), payable promptly following the execution of the License Agreement, which was received in the fourth quarter of 2020, (ii) up to an aggregate of $14.0 million in two payments based on our achievement of two specified milestones in the US, of which the first milestone was met in the first quarter of 2021 and $6.0 million (or $4.5 million net of withholding taxes and other fees and royalties) was received in the second quarter of 2021, and (iii) subsequent to the receipt by the Licensee of the requisite regulatory approvals, double-digit royalties on the net sales of lenzilumab in South Korea and the Philippines. The Licensee has agreed to certain development and commercial performance obligations. It is expected that we will supply lenzilumab to the Licensee for a minimum of 7.5 years at a cost-plus basis from an existing or future manufacturer. The Licensee has agreed to certain minimum purchases of lenzilumab on an annual basis.
| 19 |
Indemnification
In the normal course of business, we enter into contracts and agreements that contain a variety of representations and warranties and provide for general indemnifications. Our exposure under these agreements is unknown because it involves claims that may be made against us in the future but have not yet been made. To date, we have not paid any claims or been required to defend any action related to our indemnification obligations. However, we may record charges in the future as a result of these indemnification obligations.
| Item 3. | Quantitative and Qualitative Disclosures About Market Risk |
We are a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and are not required to provide the information specified under this item.
| Item 4. | Controls and Procedures |
We maintain disclosure controls and procedures (as defined in Exchange Act Rule 13a–15(e) and 15d-15(e)) that are designed to ensure that information required to be disclosed in our reports under the Securities Exchange Act of 1934, as amended, or the Exchange Act, and the rules and regulations thereunder, is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and that such information is accumulated and communicated to our management, including our Chief Executive Officer, who is also acting as our Chief Financial Officer, to allow for timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Evaluation of disclosure controls and procedures. As required by Rule 13a-15(b) under the Exchange Act, we carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer, who is also acting as our Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures as of the end of the period covered by this Quarterly Report on Form 10-Q. Based on the foregoing, our Chief Executive Officer, who is also acting as our Chief Financial Officer concluded that our disclosure controls and procedures were effective at the reasonable assurance level.
Changes in internal control over financial reporting. There have been no changes in our internal control over financial reporting during our most recent fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| 20 |
PART II. OTHER INFORMATION
| Item 1. | Legal Proceedings. |
Please see Note 9 to the Condensed Consolidated Financial Statements included in Item 1 of this Quarterly Report on Form 10-Q for a summary of legal proceedings and developments during the quarter ended March 31, 2023.
| Item 1A. | Risk Factors. |
Our operations and financial results are subject to various risks and uncertainties, including those described in Part I, Item
1A, "Risk Factors" in the 2022 Annual Report, which could adversely affect our business, financial condition, results of operations, cash flows, and the trading price of our common stock. Other than the updated risk factors below, there have been no material changes in our risk factors as previously disclosed in Part I, Item 1A of the 2022 Annual Report.
Risks Related to Our Continued Listing on the Nasdaq Capital Market
The suspension and delisting of our common stock on the Nasdaq Capital Market has been stayed through the extended compliance period of August 21, 2023 granted by the Nasdaq Hearings Panel. There can be no assurance that we will be able to demonstrate compliance with the requirements for listing on the Nasdaq Capital Market by August 21, 2023. If we do not demonstrate compliance with the listing requirements by August 21, 2023, our common stock will be subject to delisting from the Nasdaq Capital Market.
On August 24, 2022, Nasdaq notified us that, for 30 consecutive business days, the bid price for our common stock had closed below the minimum $1.00 per share requirement for continued inclusion on the Nasdaq Capital Market pursuant to Nasdaq Listing Rule 5550(a)(2) (the “Bid Price Rule”). In accordance with Nasdaq Listing Rule 5810(c)(3)(A) we were provided an initial period of 180 calendar days, or until February 20, 2023, to regain compliance with the Bid Price Rule. We did not regain compliance by February 20, 2023. On February 21, 2023, we received a letter from the Staff of Nasdaq notifying us that we had not regained compliance with the minimum bid price requirement as of February 20, 2023 and that we were not eligible for a second 180-day extension period, and that accordingly our common stock would be delisted unless we were to appeal successfully to a Nasdaq Hearings Panel. The Nasdaq Staff’s letter specifically noted that the Company does not comply with the stockholders’ equity initial listing requirement for the Nasdaq Capital Market. The total market value of the Company’s listed securities also remains below the $35 million requirement for continued listing on the Nasdaq Capital Market pursuant to Nasdaq Listing Rule 5550(b)(2) (the “MVLS Rule”).
We appealed the delisting determination to a Nasdaq Hearings Panel, which such hearing was held on April 6, 2023. On April 18, 2023, the Nasdaq Hearings Panel granted us an extension until August 21, 2023, to execute our compliance plan and to demonstrate compliance with all applicable criteria for listing on The Nasdaq Capital Market, subject to our compliance with the Nasdaq Hearings Panel’s requirements for periodic updates relating to the status of the Company’s progress against achievement of the compliance plan presented at the April 6, 2023 hearing with the Nasdaq Hearings Panel.
If we fail to regain, by August 21, 2023, and thereafter maintain compliance with the requirements for listing on the Nasdaq Capital Market, our common stock will be subject to suspension from trading and delisting, which would adversely affect the liquidity of our common stock and our ability to raise additional capital.
Delisting from Nasdaq could adversely affect our ability to consummate a strategic transaction, including the proposed business combination with the Partner Company, and raise additional financing through the public or private sale of equity securities and would significantly affect the ability of investors to trade our securities and negatively affect the value and liquidity of our common stock.
| Item 2. | Unregistered Sales of Equity Securities and Use of Proceeds |
None.
| Item 3. | Defaults Upon Senior Securities |
None.
| Item 4. | Mine Safety Disclosures |
Not applicable.
| 21 |
| Item 5. | Other Information |
None.
| Item 6. | Exhibits. |
| 101.INS |
XBRL Instance Document. The instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. | |
| 101.SCH | XBRL Taxonomy Extension Schema | |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document | |
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document | |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document | |
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). |
***The certifications attached as Exhibits 32.1 and 32.2 that accompanies this Quarterly Report on Form 10-Q are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of Registrant under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Quarterly Report on Form 10-Q, irrespective of any general incorporation language contained in such filing.
| 22 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| HUMANIGEN, INC. | |||
| Date: May 15, 2023 | By: | /s/ Cameron Durrant | |
| Cameron Durrant | |||
| Chief Executive Officer | |||
| (Principal Executive Officer) | |||
| Date: May 15, 2023 | By: | /s/ Cameron Durrant | |
| Cameron Durrant | |||
| Acting Chief Financial Officer | |||
| (Principal Accounting and Financial Officer) | |||
23