UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-Q
| ☒ |
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the quarterly period ended March 31, 2018
OR
| ☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the transition period from to
Commission file number: 001-36829
Rocket Pharmaceuticals, Inc.
(Exact name of registrant as specified in its charter)
|
Delaware
|
04-3475813
|
|
|
(State or other jurisdiction of incorporation or organization)
|
(I.R.S. Employer Identification No.)
|
430 East 29th Street, Suite 1040
New York, NY 10016
(Address of principal executive office) (Zip Code)
Registrant’s telephone number, including area code:
(646) 440-9100
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer
|
☐
|
Accelerated filer ☐
|
|
|
Non-accelerated filer
|
☐ (Do not check if a smaller reporting company)
|
Smaller reporting company
|
☒
|
|
Emerging growth company
|
☒
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of May 10, 2018, there were 39,480,407 shares of common stock, $0.01 par value per share, outstanding.
|
Page
|
||
|
Item 1.
|
3
|
|
|
3
|
||
|
4
|
||
|
5
|
||
|
6
|
||
|
7
|
||
|
8
|
||
|
Item 2.
|
23
|
|
|
Item 3.
|
33
|
|
|
Item 4.
|
33
|
|
|
PART II - OTHER INFORMATION
|
||
|
Item 1.
|
34
|
|
|
Item 1A.
|
34
|
|
|
Item 2.
|
54
|
|
|
Item 3.
|
54
|
|
|
Item 4.
|
54
|
|
|
Item 5.
|
55
|
|
|
Item 6.
|
55
|
|
|
56
|
||
PRESENTATION NOTE: As a result of the Reverse Merger, each outstanding share of Rocket Ltd share capital (including shares of Rocket Ltd share capital to be issued upon exercise of outstanding share options) automatically converted into the right to receive approximately 76.185 shares of Inotek’s common stock, par value $0.01 per share (the “Exchange Ratio”). The historical financial statements, outstanding shares and all other historical share information have been adjusted to reflect the impact of the Exchange Ratio as if the Exchange Ratio had been in effect for all periods presented.
Rocket Pharmaceuticals, Inc.
(in thousands, except share and per share amounts)
|
March 31,
2018
|
December 31,
2017
|
|||||||
|
Assets
|
(unaudited)
|
|||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
171,136
|
$
|
18,142
|
||||
|
Short term investments
|
11,561
|
-
|
||||||
|
Prepaid expenses and other assets
|
2,131
|
813
|
||||||
|
Total current assets
|
184,828
|
18,955
|
||||||
|
Property and equipment, net
|
1,146
|
985
|
||||||
|
Goodwill
|
30,815
|
-
|
||||||
|
Restricted cash
|
208
|
207
|
||||||
|
Deposits
|
168
|
-
|
||||||
|
Total assets
|
$
|
217,165
|
$
|
20,147
|
||||
|
Liabilities and shareholders' equity
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable and accrued expenses
|
$
|
4,762
|
$
|
2,062
|
||||
|
Accrued research and development costs
|
2,575
|
2,459
|
||||||
|
Total current liabilities
|
7,337
|
4,521
|
||||||
|
Convertible notes,net of unamortized discount
|
39,084
|
-
|
||||||
|
Deferred rent and lease liability
|
598
|
107
|
||||||
|
Total liabilities
|
47,019
|
4,628
|
||||||
|
Commitments and contingencies (Note 12)
|
||||||||
|
Shareholders' equity:
|
||||||||
|
Preferred shares, $0.01 par value, authorized 1,000,000 shares
|
||||||||
|
Series A convertible preferred shares; 300,000 shares designated as Series A; 0 and 128,738 shares issued and outstanding at March 31, 2018 and December 31, 2017, respectively
|
-
|
16,060
|
||||||
|
Series B convertible preferred shares; 300,000 shares designated as Series B; 0 and 126,909 shares issued and outstanding at March 31, 2018 and December 31, 2017, respectively
|
-
|
25,406
|
||||||
|
Common stock, $0.01 par value, 120,000,000 shares authorized; 39,403,898 and 6,795,627 shares issued and outstanding at March 31, 2018 and December 31, 2017, respectively
|
394
|
1
|
||||||
|
Additional paid-in capital
|
216,440
|
5,407
|
||||||
|
Accumulated other comprehensive income
|
10
|
-
|
||||||
|
Accumulated deficit
|
(46,698
|
)
|
(31,355
|
)
|
||||
|
Total shareholders' equity
|
170,146
|
15,519
|
||||||
|
Total liabilities and shareholders' equity
|
$
|
217,165
|
$
|
20,147
|
||||
The accompanying notes are an integral part of these consolidated financial statements.
Rocket Pharmaceuticals, Inc.
(in thousands, except share and per share amounts)
(unaudited)
|
Three Months Ended March 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
Revenue
|
$
|
-
|
$
|
-
|
||||
|
Operating expenses:
|
||||||||
|
Research and development
|
5,743
|
2,285
|
||||||
|
General and administrative
|
8,662
|
585
|
||||||
|
Total operating expenses
|
14,405
|
2,870
|
||||||
|
Loss from operations
|
(14,405
|
)
|
(2,870
|
)
|
||||
|
Research and development incentives
|
186
|
192
|
||||||
|
Interest expense
|
(1,427
|
)
|
-
|
|||||
|
Interest income
|
288
|
-
|
||||||
|
Other income
|
15
|
-
|
||||||
|
Net loss
|
$
|
(15,343
|
)
|
$
|
(2,678
|
)
|
||
|
Net loss per share attributable to common shareholders - basic and diluted
|
$
|
(0.42
|
)
|
$
|
(0.39
|
)
|
||
|
Weighted-average common shares outstanding - basic and diluted
|
36,137,120
|
6,795,627
|
||||||
The accompanying notes are an integral part of these consolidated financial statements.
Rocket Pharmaceuticals, Inc.
(in thousands)
(unaudited)
|
Three Months Ended March 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
Net loss
|
$
|
(15,343
|
)
|
$
|
(2,678
|
)
|
||
|
Other comprehensive gain
|
||||||||
|
Net unrealized gain on short term investments
|
10
|
-
|
||||||
|
Total comprehensive loss
|
$
|
(15,333
|
)
|
$
|
(2,678
|
)
|
||
The accompanying notes are an integral part of these consolidated financial statements.
Rocket Pharmaceuticals, Inc.
(in thousands except share amounts)
(unaudited)
|
Series A Convertible
Preferred Shares
|
Series B Convertible
Preferred Shares
|
Common Stock
|
Additional
Paid-In
|
Accumulated
|
Accumulated
Other
Comprehensive
|
Total
Shareholders'
|
||||||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Capital
|
Deficit
|
Income
|
Equity
|
|||||||||||||||||||||||||||||||
|
Balance at December 31, 2017
|
128,738
|
$
|
16,060
|
126,909
|
$
|
25,406
|
6,795,627
|
$
|
68
|
$
|
5,340
|
$
|
(31,355
|
)
|
$
|
-
|
$
|
15,519
|
||||||||||||||||||||||
|
Conversion of convertible preferred shares into common shares
|
(128,738
|
)
|
(16,060
|
)
|
(126,909
|
)
|
(25,406
|
)
|
19,475,788
|
194
|
41,272
|
-
|
-
|
-
|
||||||||||||||||||||||||||
|
Exchange of common shares in connection with the Merger
|
-
|
-
|
-
|
-
|
6,805,608
|
68
|
85,992
|
-
|
-
|
86,060
|
||||||||||||||||||||||||||||||
|
Issuance of common shares, net of issuance costs of $5.3 million
|
-
|
-
|
-
|
-
|
6,325,000
|
63
|
78,455
|
-
|
-
|
78,518
|
||||||||||||||||||||||||||||||
|
Issuance of common shares pursuant to settlement of restricted stock units
|
-
|
-
|
-
|
-
|
1,875
|
1
|
(1
|
)
|
-
|
-
|
-
|
|||||||||||||||||||||||||||||
|
Unrealized gain on short term investments
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
10
|
10
|
||||||||||||||||||||||||||||||
|
Share-based compensation
|
-
|
-
|
-
|
-
|
-
|
-
|
5,382
|
-
|
-
|
5,382
|
||||||||||||||||||||||||||||||
|
Net loss
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
(15,343
|
)
|
-
|
(15,343
|
)
|
||||||||||||||||||||||||||||
|
Balance at March 31, 2018
|
-
|
$
|
-
|
-
|
$
|
-
|
39,403,898
|
$
|
394
|
$
|
216,440
|
$
|
(46,698
|
)
|
$
|
10
|
170,146
|
|||||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
Rocket Pharmaceuticals, Inc.
(In thousands)
(unaudited)
|
Three Months Ended March 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
Operating Activities:
|
||||||||
|
Net loss
|
$
|
(15,343
|
)
|
$
|
(2,678
|
)
|
||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
||||||||
|
Accretion of discount on convertible notes
|
696
|
-
|
||||||
|
Reduction of lease liability
|
(20
|
)
|
-
|
|||||
|
Depreciation expense
|
83
|
28
|
||||||
|
Share-based compensation expense
|
5,382
|
99
|
||||||
|
Amortization of premium on short term investments
|
12
|
-
|
||||||
|
Changes in operating assets and liabilities:
|
||||||||
|
Prepaid expenses
|
(266
|
)
|
(954
|
)
|
||||
|
Accounts payable and accrued expenses
|
(2,261
|
)
|
(282
|
)
|
||||
|
Accrued research and development costs
|
116
|
64
|
||||||
|
Net cash used in operating activities
|
(11,601
|
)
|
(3,723
|
)
|
||||
|
Investing activities:
|
||||||||
|
Cash acquired in connection with the Reverse Merger
|
76,348
|
-
|
||||||
|
Proceeds from maturities of short term investments
|
9,718
|
-
|
||||||
|
Proceeds from sale of property and equipment
|
20
|
-
|
||||||
|
Purchases of property and equipment
|
(8
|
)
|
(131
|
)
|
||||
|
Net cash provided by (used in) investing activities
|
86,078
|
(131
|
)
|
|||||
|
Financing activities:
|
||||||||
|
Proceeds from issuance of common stock, net of issuance costs
|
78,518
|
-
|
||||||
|
Proceeds from issuance of convertible preferred stock, net
|
-
|
21,549
|
||||||
|
Net cash provided by financing activities
|
78,518
|
21,549
|
||||||
|
Net change in cash and restricted cash
|
152,995
|
17,695
|
||||||
|
Cash, cash equivalents and restricted cash at beginning of period
|
18,349
|
9,665
|
||||||
|
Cash, cash equivalents and restricted cash at end of period
|
$
|
171,344
|
$
|
27,360
|
||||
|
Supplemental disclosure of non-cash financing activities:
|
||||||||
|
Conversion of convertible preferred stock into common stock
|
$
|
41,466
|
$
|
-
|
||||
|
Supplemental cash flow information:
|
||||||||
|
Cash paid for interest
|
$
|
1,495
|
$
|
-
|
||||
|
Cash paid for income taxes
|
$
|
-
|
$
|
-
|
||||
The accompanying notes are an integral part of these consolidated financial statements.
ROCKET PHARMACEUTICALS, INC.
(Amounts in thousands, except share and per share data)
(Unaudited)
1. Nature of Business, Merger and Basis of Presentation
Rocket Pharmaceuticals, Inc. (“Rocket” or the “Company”) is a multi-platform biotechnology company focused on the development of first or best-in-class gene therapies for rare and devastating pediatric diseases. Rocket has LVV programs currently undergoing clinical testing for Fanconi Anemia (“FA”), a genetic defect in the bone marrow that reduces production of blood cells or promotes the production of faulty blood cells, and three additional LVV programs targeting other rare genetic diseases. In addition, Rocket has an adeno-associated virus (“AAV”) program, which will permit the commencement of human clinical studies thereafter. Rocket has global commercialization and development rights to all of its product candidates under royalty-bearing license agreements, with the exception of the CRISPR/Cas9 development program (described below) for which Rocket currently only has development rights.
Rocket’s two leading LVV and AAV technology platforms are each being designed in collaboration with leading academic and industry partners. Through its gene therapy platforms, Rocket aims to restore normal cellular function by modifying the defective genes that cause each of the targeted disorders.
Reverse Merger and Exchange Ratio
On January 4, 2018, Rome Merger Sub (“Merger Sub”), a wholly owned subsidiary of Inotek Pharmaceuticals Corporation (“Inotek”), completed its merger with and into Rocket Pharmaceuticals, Ltd. (“Rocket Ltd”), with Rocket Ltd surviving as a wholly owned subsidiary of Inotek. This transaction is referred to as the “Reverse Merger.” The Reverse Merger was effected pursuant to an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”), dated as of September 12, 2017, by and among Inotek, Rocket Ltd and Rome Merger Sub.
As a result of the Reverse Merger, each outstanding share of Rocket Ltd share capital (including shares of Rocket Ltd share capital to be issued upon exercise of outstanding share options) automatically converted into the right to receive approximately 76.185 shares of Inotek’s common stock, par value $0.01 per share (the “Exchange Ratio”). Following the closing of the Reverse Merger, holders of Inotek’s common stock immediately prior to the Reverse Merger owned approximately 18.643% on a fully diluted basis, and holders of Rocket Ltd common stock immediately prior to the Reverse Merger owned approximately 81.357% on a fully diluted basis, of Inotek’s common stock.
The Reverse Merger has been accounted for as a reverse acquisition under the acquisition method of accounting where Rocket Ltd is considered the accounting acquirer and Inotek is the acquired company for financial reporting purposes. Rocket Ltd was determined to be the accounting acquirer based on the terms of the Merger Agreement and other factors, such as relative voting rights and the composition of the combined company’s board of directors and senior management. The pre-acquisition financial statements of Rocket Ltd became the historical financial statements of Rocket following completion of the Reverse Merger. The historical financial statements, outstanding shares and all other historical share information have been adjusted by multiplying the respective share amount by the Exchange Ratio as if the Exchange Ratio had been in effect for all periods presented.
Immediately following the Reverse Merger, the combined company changed its name from “Inotek Pharmaceuticals Corporation” to “Rocket Pharmaceuticals, Inc.” The combined company following the Reverse Merger may be referred to herein as “the combined company,” “Rocket,” or the “Company.”
The Company’s common stock remained listed on the NASDAQ Stock Market, with trading having commenced on a post-split basis (giving effect to the Reverse Stock Split described below) and under the new name as of January 5, 2018. The trading symbol also changed on that date from “ITEK” to “RCKT.”
Unaudited Interim Consolidated Financial Information
The accompanying consolidated balance sheet as of March 31, 2018, the consolidated statements of operations and comprehensive loss and of cash flows for the three months ended March 31, 2018 and 2017, and the consolidated statement of shareholders’ equity for the three months ended as of March 31, 2018 are unaudited. These financial statements should be read in conjunction with the Rocket Ltd 2017 financial statements included in Form 8-K filed on March 7, 2018. The unaudited interim consolidated financial statements have been prepared on the same basis as the audited annual financial statements and, in the opinion of management, reflect all adjustments, which include only normal recurring adjustments, necessary for the fair statement of the Company’s financial position as of March 31, 2018 and the results of its operations and its cash flows for the three months ended March 31, 2018 and 2017. The financial data and other information disclosed in these consolidated notes related to the three months ended March 31, 2018 and 2017 are unaudited. The results for the three months ended March 31, 2018 are not necessarily indicative of results to be expected for the year ending December 31, 2018, any other interim periods or any future year or period.
2. Risks and Liquidity
The Company has not generated any revenue and has incurred losses since inception. Operations of the Company are subject to certain risks and uncertainties, including, among others, uncertainty of drug candidate development, technological uncertainty, uncertainty regarding patents and proprietary rights, having no commercial manufacturing experience, marketing or sales capability or experience, dependency on key personnel, compliance with government regulations and the need to obtain additional financing. Drug candidates currently under development will require significant additional research and development efforts, including extensive preclinical and clinical testing and regulatory approval, prior to commercialization. These efforts require significant amounts of additional capital, adequate personnel infrastructure and extensive compliance-reporting capabilities.
The Company’s drug candidates are in the development stage. There can be no assurance that the Company’s research and development will be successfully completed, that adequate protection for the Company’s intellectual property will be obtained, that any products developed will obtain necessary government approval or that any approved products will be commercially viable. Even if the Company’s product development efforts are successful, it is uncertain when, if ever, the Company will generate significant revenue from product sales. The Company operates in an environment of rapid change in technology and substantial competition from pharmaceutical and biotechnology companies.
The Company’s consolidated financial statements have been prepared on the basis of continuity of operations, realization of assets and the satisfaction of liabilities in the ordinary course of business. The Company has experienced negative cash flows from operations and had an accumulated deficit of $46,698 as of March 31, 2018. As of March 31, 2018, the Company has $182,697 of cash, cash equivalents and short term investments. Rocket expects such resources would be sufficient to fund its operating expenses and capital expenditure requirements into 2020.
In the longer term, the future viability of the Company is dependent on its ability to generate cash from operating activities or to raise additional capital to finance its operations. The Company’s failure to raise capital as and when needed could have a negative impact on its financial condition and ability to pursue its business strategies.
3. Summary of Significant Accounting Policies
Use of Estimates
The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States (“US GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Significant estimates and assumptions reflected in these consolidated financial statements include but are not limited to, goodwill impairment, the accrual of research and development expenses, the valuation of equity transactions and share-based awards. Changes in estimates and assumptions are reflected in reported results in the period in which they become known. Actual results could differ from those estimates.
Cash, Cash Equivalents and Restricted Cash
Cash, cash equivalents and restricted cash consists of bank deposits, certificates of deposit and money market accounts with financial institutions. Cash equivalents are carried at cost which approximates fair value due to their short-term nature and which the Company believes do not have a material exposure to credit risk. The Company considers all highly liquid investments with maturities of three months or less from the date of purchase to be cash equivalents. The Company’s cash and cash equivalent accounts, at times, may exceed federally insured limits. The Company has not experienced any losses in such accounts.
Restricted cash consists of a deposit collateralizing a letter of credit issued by a bank in connection with the Company’s operating lease (See Note 12). As of March 31, 2018 and December 31, 2017, restricted cash was $208 and $207, respectively. Cash, cash equivalents and restricted cash consist of the following:
|
March 31,
2018
|
December 31,
2017
|
|||||||
|
Cash and cash equivalents
|
$
|
171,136
|
$
|
18,142
|
||||
|
Restricted cash
|
208
|
207
|
||||||
|
$
|
171,344
|
$
|
18,349
|
|||||
Short term Investments
Short term investments consist of investments in certificates of deposit and United States Treasury securities. Management determines the appropriate classification of these securities at the time they are acquired and evaluates the appropriateness of such classifications at each balance sheet date. The Company classifies its short-term investments as available-for-sale pursuant to Financial Accounting Standards Board (“FASB”) Accounting Standard Codification (“ASC”) 320, Investments—Debt and Equity Securities. Short-term investments are recorded at fair value, with unrealized gains and losses included as a component of accumulated other comprehensive loss in shareholders’ equity and a component of total comprehensive income in the consolidated statements of comprehensive income, until realized. Realized gains and losses are included in investment income on a specific-identification basis. There were no realized gains or losses on short-term investments for the three months ended March 31, 2018 and 2017, respectively. There was $10 and $0 of net unrealized gains on short term investments for the three months ended March 31, 2018 and 2017, respectively.
The Company reviews short term investments for other-than-temporary impairment whenever the fair value of a short term investment is less than the amortized cost and evidence indicates that a short term investment’s carrying amount is not recoverable within a reasonable period of time. Other-than-temporary impairments of investments are recognized in the consolidated statements of operations if the Company has experienced a credit loss, has the intent to sell the short term investment, or if it is more likely than not that the Company will be required to sell the short term investment before recovery of the amortized cost basis. Evidence considered in this assessment includes reasons for the impairment, compliance with the Company’s investment policy, the severity and the duration of the impairment and changes in value subsequent to the end of the period.
Short term investments at March 31, 2018 consist of the following:
|
Cost
Basis
|
Unrealized
Gains
|
Fair
Value
|
||||||||||
|
Current:
|
||||||||||||
|
Certificates of deposit
|
$
|
1,546
|
$
|
-
|
$
|
1,546
|
||||||
|
United States Treasury securities
|
10,005
|
10
|
10,015
|
|||||||||
|
$
|
11,551
|
$
|
10
|
$
|
11,561
|
|||||||
At March 31, 2018, all short term investments held by the Company had contractual maturities of less than one year. The Company evaluated its securities for other-than-temporary impairment and determined that no such impairment existed at March 31, 2018.
Goodwill
Business combinations are accounted for under the acquisition method (see Note 4). The total cost of an acquisition is allocated to the underlying identifiable net assets, based on their respective estimated fair values as of the acquisition date. Determining the fair value of assets acquired and liabilities assumed requires management’s judgment and often involves the use of significant estimates and assumptions, including assumptions with respect to future cash inflows and outflows, discount rates, asset lives and market multiples, among other items. Assets acquired and liabilities assumed are recorded at their estimated fair values. The excess of the purchase price over the estimated fair values of the net assets acquired is recorded as goodwill.
Goodwill is tested for impairment annually as of December 31, or more frequently when events or changes in circumstances indicate that the asset might be impaired. Examples of such events or circumstances include, but are not limited to, a significant adverse change in legal or business climate, an adverse regulatory action or unanticipated competition.
The Company will assess qualitative factors to determine whether the existence of events or circumstances would indicate that it is more likely than not that the fair value of the reporting unit was less than its carrying amount. If after assessing the totality of events or circumstances, the Company were to determine that it is more likely than not that the fair value of the reporting unit is less than its carrying amount, then the Company would perform a quantitative impairment test.
In the first step, the Company compares the fair value of the reporting unit to its carrying value. If the fair value of the reporting unit exceeds the carrying value of the net assets, goodwill is not impaired, and no further testing is required. If the fair value of the reporting unit is less than the carrying value, the Company measures the amount of impairment loss, if any, as the excess of the carrying value over the fair value of the reporting unit.
The Company has determined there were no indicators of goodwill impairment as of March 31, 2018.
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation. Depreciation expense is recognized using the straight-line method over the useful life of the asset. The estimated useful lives are three to five years. Expenditures for repairs and maintenance of assets are charged to expense as incurred. Upon retirement or sale, the cost and related accumulated depreciation of assets disposed of are removed from the accounts and any resulting gain or loss is included in loss from operations. If the carrying amount of the assets or asset group is not recoverable on an undiscounted cash flow basis, impairment is recognized to the extent that the carrying value exceeds its fair value.
Fair Value Measurements
The Company is required to disclose information on all assets and liabilities reported at fair value that enables an assessment of the inputs used in determining the reported fair values. FASB ASC 820, Fair Value Measurements and Disclosures (“ASC 820”), establishes a hierarchy of inputs used when available. Observable inputs are inputs that market participants would use in pricing the asset or liability based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the inputs that market participants would use in pricing the asset or liability, and are developed based on the best information available in the circumstances. The fair value hierarchy applies only to the valuation inputs used in determining the reported fair value of the investments and is not a measure of the investment credit quality. The three levels of the fair value hierarchy are described below:
Level 1—Valuations based on unadjusted quoted prices in active markets for identical assets or liabilities that the Company has the ability to access at the measurement date.
Level 2—Valuations based on quoted prices for similar assets or liabilities in markets that are not active or for which all significant inputs are observable, either directly or indirectly.
Level 3—Valuations that require inputs that reflect the Company’s own assumptions that are both significant to the fair value measurement and unobservable.
To the extent that valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair value requires more judgment. Accordingly, the degree of judgment exercised by the Company in determining fair value is greatest for instruments categorized in Level 3. A financial instrument’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement. The fair value of the Company’s financial instruments, including cash and cash equivalents, prepaid expenses and other assets, accounts payable and accrued expenses approximate their respective carrying values due to the short-term nature of these instruments. The carrying value of the 2021 Convertible Notes approximates fair value due to the acquisition (see Note 4). The Company’s assets and liabilities measured at fair value on a recurring basis include its short term investments.
Deferred Rent and Lease Liability
The Company recognizes rent expense on a straight-line basis, after considering the effect of rent escalation provisions resulting in a level rent expense recognized over the lease term. For the lease liability, the Company reduces the rent expense on a straight-line basis over the remaining life of the lease.
Research and Development
Research and development costs, which include salaries and staff costs, license costs, regulatory and scientific consulting fees, as well as contract research, and share-based compensation expense, are accounted for in accordance with ASC Topic 730, Research and Development, (“ASC 730”).
The Company does not currently have any commercial biopharmaceutical products, and does not expect to have any for several years, if at all. Accordingly, research and development costs are expensed as incurred. While certain of the Company’s research and development costs may have future benefits, the policy of expensing all research and development expenditures is predicated on the fact that the Company has no history of successful commercialization of product candidates to base any estimate of the number of future periods that would be benefited.
Foreign Currency Transactions
Certain transactions during the three months ended March 31, 2018 and 2017 are denominated in Euros. Gains and losses on foreign currency transactions are not significant for the three months ended March 31, 2018 and 2017.
Share-Based Compensation
The Company measures the cost of employee services received in exchange for an award of equity instruments based on the fair value of the award on the grant date. That cost is recognized on a straight-line basis over the period during which the employee is required to provide service in exchange for the award. The fair value of options on the date of grant is calculated using the Black-Scholes option pricing model based on key assumptions such as stock price, expected volatility and expected term. The Company’s estimates of these assumptions are primarily based on the trading price of Company’s stock, historical data, peer company data and judgment regarding future trends and factors. The fair value of restricted stock awards is based on the intrinsic value of such awards on the date of grant. Compensation cost for stock purchase rights under the employee stock purchase plan is measured and recognized on the date the Company becomes obligated to issue shares of common stock and is based on the difference between the fair value of the Company’s common stock and the purchase price on such date.
The Company classifies share-based compensation expense in its statement of operations in the same manner in which the award recipient’s payroll costs are classified or in which the award recipients’ service payments are classified.
The Company recognizes compensation expense for only the portion of awards that are expected to vest. Forfeitures are recorded as they occur.
Prior to the Reverse Merger, the Company was a private company and lacked company-specific historical and implied volatility information. Therefore, it estimated its expected share volatility based on the historical volatility of a publicly traded set of peer companies. The expected term of the Company’s share options was determined utilizing the “simplified” method for awards that qualify as “plain vanilla” options. The expected term of share options granted to non-employees was equal to the contractual term of the option award. The risk-free interest rate was determined by reference to the U.S. Treasury yield curve in effect at the time of grant of the award for time periods approximately equal to the expected term of the award. Expected dividend yield was based on the fact that the Company had never paid cash dividends and did not expect to pay any cash dividends in the foreseeable future.
The Company measured the compensation expense of share options and other share-based awards granted to employees and directors using the grant date fair value of the award and recognized compensation expense as determined by the Black-Scholes Option pricing model on a straight-line basis over their requisite service period, which was generally the vesting period of the respective award.
The Company initially measured the compensation expense of share-based awards granted to consultants using the grant date fair value of the award. Compensation expense was recognized over the period during which services were rendered by such consultants. At the end of each financial reporting period prior to completion of services being rendered, the compensation expense was remeasured using the then current fair value of the share-based award, based on updated assumption inputs in the Black-Scholes option pricing model.
NYC Biotechnology Tax Credit Program
New York City allows investors and owners of emerging technology companies focused on biotechnology to claim a tax credit against the General Corporation Tax and Unincorporated Business Tax for amounts paid or incurred for certain facilities, operations, and employee training in New York City. The credit is recognized as research and development incentives when approved by New York City of the eligibility for the credit and the credit amount. During the three months ended March 31, 2018 and 2017, the Company recorded research and development incentive income and a related receivable of $186 and $192, respectively, related to this credit.
Income Taxes
The Company accounts for income taxes under the asset and liability method. The Company recognizes deferred tax assets and liabilities for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases, as well as for operating loss and tax credit carry-forwards. The Company measures deferred tax assets and liabilities using enacted tax rates expected to apply to taxable income in the years in which the Company expects to recover or settle those temporary differences. The Company recognizes the effect of a change in tax rates on deferred tax assets and liabilities in the results of operations in the period that includes the enactment date. The Company reduces the measurement of a deferred tax asset, if necessary, by a valuation allowance if it is more likely than not that the Company will not realize some or all of the deferred tax asset.
The Company accounts for uncertainty in income taxes recognized in the financial statements by applying a two-step process to determine the amount of tax benefit to be recognized. First, the tax position must be evaluated to determine the likelihood that it will be sustained upon external examination by the taxing authorities. If the tax position is deemed more-likely-than-not to be sustained, the tax position is then assessed to determine the amount of benefit to recognize in the financial statements. The amount of the benefit that may be recognized is the largest amount that has a greater than 50% likelihood of being realized upon ultimate settlement. The provision for income taxes includes the effects of any resulting tax reserves, or unrecognized tax benefits, that are considered appropriate as well as the related net interest and penalties.
On December 22, 2017, the Tax Cut and Jobs Act (the “Act”), was signed into law by the President of the United States. The Act includes a number of provisions, including the lowering of the U.S. corporate tax rate from 34 percent to 21 percent, effective January 1, 2018 and the establishment of a territorial-style system for taxing foreign-source income of domestic multinational corporations. The Company is in the process of quantifying the tax impacts of The Act, but at this time does not believe the provisions will have a material impact on the Company’s financial reporting. The Company will continue to monitor and quantify the impact of the Act and will record any adjustments in accordance with the guidance in Staff Accounting Bulletin No. 118.
Net Loss Per Share
The Company follows the two-class method when computing net income (loss) per share as the Company has issued shares that meet the definition of participating securities. The two-class method determines net income (loss) per share for each class of common shares and participating securities according to dividends declared or accumulated and participation rights in undistributed earnings. The two-class method requires income available to common shareholders for the period to be allocated between common shares and participating securities based upon their respective rights to receive dividends as if all income for the period had been distributed.
Basic net income (loss) per share attributable to common shareholders is computed by dividing the net income (loss) attributable to common shareholders by the weighted average number of common shares outstanding for the period. Diluted net income (loss) attributable to common shareholders is computed by adjusting net income (loss) attributable to common shareholders to reallocate undistributed earnings based on the potential impact of dilutive securities. Diluted net income (loss) per share attributable to common shareholders is computed by dividing the diluted net income (loss) attributable to common shareholders by the weighted average number of common shares outstanding for the period, including potential dilutive common shares. For purposes of this calculation, outstanding options and convertible preferred shares are considered potential dilutive common shares.
The Company’s convertible preferred shares contractually entitled the holders of such shares to participate in dividends but contractually did not require the holders of such shares to participate in losses of the Company. Accordingly, in periods in which the Company reports a net loss attributable to common shareholders, diluted net loss per share attributable to common shareholders is the same as basic net loss per share attributable to common shareholders, since dilutive common shares are not assumed to have been issued if their effect is anti-dilutive. In connection with the Reverse Merger, all of the convertible preferred shares were converted into common stock. This conversion was in accordance with the original terms.
Segment Reporting
Operating segments are defined as components of an enterprise about which separate discrete information is available for evaluation by the chief operating decision maker, or decision-making group, in deciding how to allocate resources and in assessing performance. The Company views its operations and manages its business in one operating segment.
Comprehensive loss
Comprehensive loss is defined as the change in equity of a business enterprise during a period from transactions, and other events and circumstances from non-owner sources, and currently consists of net loss and changes in unrealized gains and losses on short-term investments as of March 31, 2018 and December 31, 2017.
Recent Accounting Pronouncements
In January 2016, the FASB issued Accounting Standards Update (“ASU”) ASU 2016-01, "Financial Instruments—Overall (Topic 825-10): "Recognition and Measurement of Financial Assets and Financial Liabilities." ASU 2016-01 amends the guidance on the classification and measurement of financial instruments. Some of the amendments in ASU 2016-01 include the following: 1) requires equity investments (except those accounted for under the equity method of accounting or those that result in consolidation of the investee) to be measured at fair value with changes in fair value recognized in net income; 2) simplifies the impairment assessment of equity investments without readily determinable fair values by requiring a qualitative assessment to identify impairment; 3) requires public business entities to use the exit price notion when measuring the fair value of financial instruments for disclosure purposes; and 4) requires an entity to present separately in other comprehensive income the portion of the total change in the fair value of a liability resulting from a change in the instrument-specific credit risk when the entity has elected to measure the liability at fair value; among others. For public business entities, the amendments of ASU 2016-01 are effective for fiscal years beginning after December 15, 2017, including interim periods within those fiscal years. The Company adopted ASU 2016-01 effective January 1, 2018 and it did not have a material impact on the Company’s consolidated financial statements.
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842), which supersedes the current leasing guidance and upon adoption, will require lessees to recognize right-of-use assets and lease liabilities on the balance sheet for all leases with terms longer than 12 months. The new standard is effective for the Company for the annual period beginning after December 15, 2018, and can be early adopted by applying a modified retrospective approach for leases existing at, and entered into after, the beginning of the earliest comparable period presented in the financial statements. The Company is currently evaluating the impact of this accounting standard on the Company’s consolidated financial statements.
In May 2017, the FASB issued ASU 2017-09, Compensation-Stock Compensation (Topic 718): Scope of Modification Accounting, to provide guidance about which changes to the terms or conditions of a share-based payment award require an entity to apply modification accounting in Topic 718. ASU 2017-09 was effective for the annual periods beginning after December 15, 2017. The Company adopted this standard on January 1, 2018. The adoption of ASU 2017-09 did not have a material impact on the Company’s consolidated financial position or results of operations.
In January 2017, the FASB issued ASU 2017-01, “Business Combinations (Topic 805): Clarifying the Definition of a Business (“ASU 2017-01)”. ASU 2017-01 provides that when substantially all of the fair value of the assets acquired is concentrated in a single identifiable asset or group of similar identifiable assets, the set is not a business. ASU 2017-01 is effective to annual period beginning after December 31, 2018 and interim period within annual periods beginning after December 31, 2019. Adoption of ASU 2017-09 may impact the Company’s accounting for future acquisitions.
In January 2017, the FASB issued ASU No. 2017-04, “Intangibles- Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment”, an amendment to simply the subsequent quantitative measurement of goodwill by eliminating step two from the goodwill impairment test. As amended, an entity will recognize an impairment charge for the amount by which the carrying amount of a reporting unit exceeds its fair value, not to exceed the total amount of goodwill allocated to the reporting unit. An entity still has the option to perform the qualitative test for a reporting unit to determine if the quantitative impairment test is necessary. This amendment is effective for annual or interim goodwill impairment tests in fiscal years beginning after December 31, 2021. Entities should apply for the amendment prospectively. Early adoption is permitted. The Company early adopted this guidance as of January 1, 2018 and will apply it when performing its annual goodwill impairment test.
4. Acquisition Accounting
The identifiable assets and liabilities of Inotek are allocated in the Company’s consolidated financial statements at their provisional fair values at the acquisition date, January 4, 2018. Goodwill, is calculated as the excess value of consideration paid over the fair value of assets acquired and liabilities assumed.
The acquisition-date fair value of the consideration transferred is as follows:
|
Number of shares of the combined company owned by Inotek shareholders
|
6,805,608
|
|||
|
Number of shares issuable in connection with fully vested RSUs of Inotek immediately prior to the Reverse Merger
|
271,718
|
|||
|
Inotek common stock on the acquisition date
|
7,077,326
|
|||
|
Price per share of Inotek common stock on acquisition date
|
$
|
12.16
|
||
|
Total purchase price
|
$
|
86,060
|
The following table summarizes the provisional fair value purchase price allocation of the assets acquired and liabilities assumed at the date of acquisition which is subject to adjustment as the Company finalizes it valuation:
|
Cash and cash equivalents
|
$
|
76,348
|
||
|
Short term investments
|
21,292
|
|||
|
Prepaid expense and other assets
|
1,041
|
|||
|
Property and equipment
|
256
|
|||
|
Deposits
|
168
|
|||
|
Goodwill
|
30,815
|
|||
|
Accounts payable and accrued expenses
|
(4,961
|
)
|
||
|
Convertible notes
|
(38,388
|
)
|
||
|
Unfavorable lease liability
|
(511
|
)
|
||
|
Net assets acquired
|
$
|
86,060
|
The goodwill of $30,815 represents the premium over the purchase price. Goodwill is mainly attributable to the value of cash and cash equivalents and short term investments acquired as of the acquisition date and access to capital markets. The allocation of the purchase price with the assistance of a third party valuation, is based on certain management assumptions. The Company incurred acquisition costs of $132 for the three months ended March 31, 2018.
The following supplemental unaudited pro forma information presents the Company’s financial results as if the acquisition of Inotek had occurred on January 1, 2017:
|
Three Months Ended March 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
Revenue
|
$
|
-
|
$
|
-
|
||||
|
Net loss
|
(15,998
|
)
|
(15,670
|
)
|
||||
The above unaudited pro forma information was determined based on the historical US GAAP results of the Company and Inotek. The unaudited pro forma consolidated results are not necessarily indicative of what the Company’s consolidated results of operations actually would have been if the acquisition was completed on January 1, 2017. The unaudited pro forma consolidated net loss includes pro forma adjustments primarily relating to the following non-recurring items directly attributable to the business combination:
| (1) |
Elimination of $4,512 of transaction costs for both the Company and Inotek from the three months ended March 31, 2018;
|
| (2) |
Elimination of $3,459 of stock-based compensation expense related to the acceleration of vesting and modification of certain previously unvested Inotek awards in connection with the Reverse Merger from the three months ended March 31, 2018;
|
| (3) |
Elimination of $1,622 of expense related to severance and stay bonuses from the three months ended March 31, 2018;
|
| (4) |
To adjust interest expense incurred in connection with the 2021 Convertible Notes assumed in connection with the Reverse Merger based on the fair value of the 2021 Convertible Notes on the date of the Reverse Merger, as if it occurred on January 1, 2017;
|
| (5) |
To adjust depreciation expense associated with property and equipment acquired in connection with the Reverse Merger based on the fair value of the property and equipment on the date of the Reverse Merger, as if it occurred on January 1, 2017; and
|
| (6) |
To adjust expense associated with operating lease obligations assumed in connection with the Merger based on the fair value of the leases on the date of the Merger, as if it occurred on January 1, 2017.
|
5. Fair Value of Financial Instruments
Items measured at fair value on a recurring basis are short-term investments. The following table sets forth the Company’s financial instruments that were measured at fair value on a recurring basis by level within the fair value hierarchy:
|
Fair Value Measurements as of
March 31, 2018 Using:
|
||||||||||||||||
|
Level 1
|
Level 2
|
Level 3
|
Total
|
|||||||||||||
|
Assets:
|
||||||||||||||||
|
Money market mutual funds (included in cash and cash equivalents)
|
$
|
79,910
|
$
|
-
|
$
|
-
|
$
|
79,910
|
||||||||
|
Certificates of deposit
|
$
|
1,546
|
$
|
-
|
$
|
-
|
$
|
1,546
|
||||||||
|
United States Treasury securities
|
10,015
|
-
|
-
|
10,015
|
||||||||||||
|
Short-term investments
|
11,561
|
-
|
-
|
11,561
|
||||||||||||
|
$
|
91,471
|
$
|
-
|
$
|
-
|
$
|
91,471
|
|||||||||
The Company classifies its money market mutual funds as Level 1 assets under the fair value hierarchy, as these assets have been valued using quoted market prices in active markets without any valuation adjustment.
6. Property and Equipment
The Company’s property and equipment consisted of the following:
|
March 31,
2018
|
December 31,
2017
|
|||||||
|
Laboratory equipment
|
$
|
1,042
|
$
|
1,042
|
||||
|
Computer equipment
|
106
|
98
|
||||||
|
Furniture and fixtures
|
351
|
115
|
||||||
|
1,499
|
1,255
|
|||||||
|
Less: Accumulated depreciation
|
(353
|
)
|
(270
|
)
|
||||
|
$
|
1,146
|
$
|
985
|
|||||
During the three months ended March 31, 2018 and 2017, the Company recognized $83 and $28 of depreciation expense, respectively.
7. Accounts Payable and Accrued Expenses
At March 31, 2018 and December 31, 2017, the Company’s accounts payable and accrued expenses consisted of the following:
|
March 31,
2018
|
December 31,
2017
|
|||||||
|
Bonus
|
$ |
956
|
$ |
703
|
||||
|
Research and development
|
956
|
814
|
||||||
|
Severance and benefits
|
891
|
88
|
||||||
|
Professional fees
|
609
|
382
|
||||||
|
Government payable
|
513
|
—
|
||||||
|
Accrued interest
|
493
|
—
|
||||||
|
Other
|
149
|
25
|
||||||
|
Compensation and benefits
|
|
128
|
|
50
|
||||
|
Accounts payable
|
67
|
—
|
||||||
|
$
|
4,762
|
$
|
2,062
|
|||||
8. Debt
On January 4, 2018, in connection with the Reverse Merger, Inotek’s obligations under its outstanding convertible notes, with an aggregate principal value of $52,000, were assumed by the Company (the “2021 Convertible Notes”). The 2021 Convertible Notes were issued in 2016 and mature on August 1, 2021 (“Maturity Date”). The 2021 Convertible Notes are unsecured, accrue interest at a rate of 5.75% per annum and interest is payable semi-annually on February 1 and August 1 of each year.
Each holder of a 2021 Convertible Note (the “Holder”) has the option until the close of business on the second business day immediately preceding the Maturity Date to convert all, or any portion, of the 2021 Convertible Notes held by it at a conversion rate of 31.1876 shares of the Company’s common stock per $1 principal amount of 2021 Convertible Notes (the “Conversion Rate”). The Conversion Rate is subject to adjustment from time to time upon the occurrence of certain events, including the issuance of stock dividends and payment of cash dividends.
In addition, in certain circumstances, the Conversion Rate will be increased in respect of a Holder’s conversion of 2021 Convertible Notes in connection with the occurrence of one or more corporate events specified in the indenture (as supplemented, the “Indenture”) governing the 2021 Convertible Notes (each such specified corporate event, a “Make-Whole Fundamental Change”) that occurs prior to the Maturity Date (a “Make-Whole Fundamental Change Conversion”) or in respect of a Holder’s voluntary conversion of 2021 Convertible Notes other than in connection with a Make-Whole Fundamental Change (a “Voluntary Conversion”). In connection with a Make-Whole Fundamental Change Conversion or a Voluntary Conversion, the Company will increase the Conversion Rate for the 2021 Convertible Notes surrendered for conversion by a number of additional shares of the Company’s common stock set forth in the Additional Shares Make-Whole Table in the Indenture, based on the applicable Stock Price (as defined in the Indenture) and Effective Date (as defined in the Indenture) for such conversion. The additional shares potentially issuable in connection with a Make-Whole Fundamental Change Conversion or a Voluntary Conversion range from 0 to 6.2375 per $1 principal amount of 2021 Convertible Notes, subject to adjustment. If the Stock Price applicable to any conversion is greater than $160.00 per share, the Conversion Rate will not be increased. If the Stock Price applicable to any conversion is less than $26.72 per share, the Conversion Rate in connection with a Make-Whole Fundamental Change Conversion will not be increased but it will be increased by 6.2375 shares in connection with a Voluntary Conversion. Upon conversion, Holders of the 2021 Convertible Notes will receive shares of the Company’s common stock and cash in lieu of fractional shares.
Upon the occurrence of a Fundamental Change, the occurrence of certain change of control transactions or delisting events (as defined in the Indenture), each Holder may require the Company to repurchase for cash all or any portion of the 2021 Convertible Notes held by such Holder at a repurchase price equal to 100% of the principal amount thereof, plus accrued and unpaid interest thereon.
The Company, at its option, may redeem for cash all or any portion of the 2021 Convertible Notes if the last reported sale price of a share of the Company’s common stock is equal to or greater than 200% of the conversion price for the 2021 Convertible Notes then in effect for at least 20 trading days (whether or not consecutive) during any 30 consecutive trading day period (including the last trading day of such period) ending within the five trading days immediately preceding the date on which the Company provides notice of redemption, at a redemption price equal to 100% of the principal amount of the 2021 Convertible Notes to be redeemed, plus accrued and unpaid interest to, but excluding, the redemption date.
If an Event of Default (as defined in the Indenture), other than certain events of bankruptcy, insolvency or reorganization involving the Company, occurs and is continuing, the trustee under the Indenture (the “Trustee”) or the Holders of at least 25% in principal amount of the outstanding 2021 Convertible Notes may declare 100% of the principal of and accrued and unpaid interest, if any, on all of the 2021 Convertible Notes to be due and payable immediately. Upon the occurrence of an Event of Default relating to bankruptcy, insolvency or reorganization involving the Company, 100% of the principal of and accrued and unpaid interest, if any, on all of the 2021 Convertible Notes would become due and payable automatically.
Notwithstanding the foregoing, the Indenture provides that, to the extent the Company elects, the sole remedy for an Event of Default relating to certain failures by the Company to comply with certain reporting covenants in the Indenture, will (i) for the first 90 days after the occurrence of such an Event of Default, consist exclusively of the right to receive additional interest on the 2021 Convertible Notes at a rate equal to 0.25% per annum of the principal amount of the 2021 Convertible Notes outstanding for each day during such 90-day period on which such an Event of Default is continuing and (ii) for the period from, and including, the 91st day after the occurrence of such an Event of Default to, and including, the 180th day after the occurrence of such an Event of Default, consist exclusively of the right to receive additional interest on the 2021 Convertible Notes at a rate equal to 0.50% per annum of the principal amount of the 2021 Convertible Notes outstanding for each day during such additional 90-day period on which such an Event of Default is continuing (such additional interest, “Additional Interest”). After 180 days, if such Event of Default is not cured or waived, the 2021 Convertible Notes would be subject to acceleration in accordance with the Indenture.
The 2021 Convertible Notes are considered a hybrid financial instrument consisting of a fixed interest rate “host” and various embedded features that required evaluation as potential embedded derivatives under FASB ASC 815, Derivatives and Hedging (“ASC 815”). Based on the nature of the host instrument and the embedded features, management concluded that none of the conversion, put and redemption features required bifurcation and separate accounting from the host instrument. The Company determined that the Additional Interest was an embedded derivative that contains non-credit related events of default. As a result, the Additional Interest feature required bifurcation and separate accounting under ASC 815. Based on the amount of Additional Interest that would be owed and the likelihood of occurrence, Rocket estimated the fair value of the Additional Interest feature to be insignificant upon issuance and as of March 31, 2018 and December 31, 2017.
The Company recorded the 2021 Convertible Notes at their provisional fair value of $38,388 on January 4, 2018, the date of the acquisition. The difference between the provisional fair value of the 2021 Convertible Notes and the principal value represents a discount on the notes that is being amortized to interest expense over the remaining term using the effective interest method. As of March 31, 2018, the stated interest rate was 5.75%, and the effective interest rate was 15.3%.
The table below summarizes the carrying value of the 2021 Convertible Notes as of March 31, 2018:
|
Principal amount
|
$
|
52,000
|
||
|
Discount
|
(12,916
|
)
|
||
|
Carrying value as of March 31, 2018
|
$
|
39,084
|
Accretion of the discount was $696 for the three months ended March 31, 2018.
9. Shareholders’ Equity
Preferred Shares
On January 4, 2018, immediately prior to and in connection with the closing of the Reverse Merger, and in accordance with the original terms of the convertible preferred shares, all of the outstanding convertible preferred shares of Rocket Ltd were converted into an aggregate of 19,475,788 shares of common stock.
Exchange Ratio
On January 4, 2018, in connection with the Reverse Merger, Rocket’s historical share and per share information has been adjusted in the consolidated financial statements presented to give effect to the Exchange Ratio.
Common Shares
At the time of the Reverse Merger, Rocket Ltd’s outstanding shares of common stock were 26,281,396 which includes 19,475,788 issued upon the conversion of Rocket Ltd’s convertible preferred stock.
On January 24, 2018, the Company entered into an underwriting agreement (the “Underwriting Agreement”) with Cowen and Company, LLC and Evercore Group L.L.C., as representatives (the “Representatives”) of the several underwriters (collectively with the Representatives, the “Underwriters”), pursuant to which the Company sold 6,325,000 shares of common stock (the “Shares”), which includes 825,000 shares that were sold pursuant to an option granted to the Underwriters (the “Offering”). The Shares were sold in the Offering at a public offering price of $13.25 per share in which the Company received gross proceeds of $83,806 net of $5,288 of offering costs, commission and legal and other expenses for net proceeds from the Offering of $78,518, after deducting the underwriting discounts and commissions and legal and accounting costs.
10. Share-Based Awards
2015 Share Option Plan
The Rocket Ltd 2015 Share Option Plan provides for the Company to grant incentive stock options or nonqualified stock options for the purchase of common shares to employees, members of the board of directors and consultants. The 2015 Share Option Plan is administered by an administrative committee appointed by the board of directors or, in the absence of such appointment, the entire board of directors. The exercise prices, vesting and other restrictions are determined at the discretion of the board of directors, or their committee if so delegated, except that the exercise price per share of share options may not be less than 100% of the fair market value of the share of common shares on the date of grant (or 110% of the fair market value in the case of an employee who owns shares representing more than 10% of the voting power of all classes of shares for the Company) and the term of share options may not be greater than ten years (or five years in the case of an employee who owns shares representing more than 10% of the voting power of all classes of shares for the Company). The Company generally grants share-based awards with service conditions only (“service-based” awards).
As required by the 2015 Share Option Plan, the exercise price for share options granted was not to be less than the fair value of common shares as determined by the Company as of the date of grant. The Company valued its common shares by taking into consideration its most recently available valuation of common shares performed by management and the board of directors as well as additional factors which may have changed since the date of the most recent contemporaneous valuation through the date of grant.
The total number of shares that may be issued under the 2015 Share Option Plan was 9,904,050 shares; however, the 2,944,702 shares that remained available under the 2015 Share Option Plan were added to the share reserve of the 2014 Plan in connection with the Reverse Merger.
By virtue of the terms of the Merger Agreement, each stock option outstanding under the Rocket Ltd 2015 Share Option Plan immediately prior to the consummation of the Reverse Merger was automatically converted into a stock option exercisable for a number of shares of the Company’s common stock calculated based on the exchange ratio and the exercise price per share of such outstanding stock option.
Pursuant to the Merger Agreement, the Company sponsors Inotek’s equity compensation plans: the Amended and Restated 2014 Stock Option and Incentive Plan (the “2014 Plan”), the 2004 Stock Option and Incentive Plan (the “2004 Plan”), and the 2014 Employee Stock Purchase Plan (“ESPP”) and assumed all stock options and restricted stock units (“RSUs”) outstanding under each of the plans immediately prior to the effective time of the Reverse Merger.
Amended and Restated 2014 Stock Option and Incentive Plan
In August 2014, Inotek’s board of directors adopted the 2014 Plan for the issuance of incentive and non-qualified stock options, restricted stock, and other equity awards, all for common stock, as determined by the board of directors, to employees, officers, directors, consultants, and advisors of Inotek and its subsidiaries. Pursuant to the provisions of the 2014 Plan and approval by the board of directors, on January 1, 2018 an additional 272,227 shares were added to the 2014 Plan representing 4% of total common shares issued and outstanding at December 31, 2017. The 2014 Plan expires in August 2024.
2004 Stock Option and Incentive Plan
In July 2004, Inotek’s board of directors adopted the 2004 Plan for the issuance of incentive stock options, restricted stock, and other equity awards, all for common stock, as determined by the board of directors to employees, officers, directors, consultants, and advisors of Inotek and its subsidiaries. Only stock options were granted under the 2004 Plan. The 2004 Plan expired in February 2014 but remains effective for all outstanding options. All of the stock options granted under the 2004 Plan were fully vested at the time of the Reverse Merger.
Vesting of all unvested Inotek option awards issued and outstanding was accelerated at the effective time of the Reverse Merger, and all such option awards issued and outstanding at the time of the Reverse Merger, aggregating to 523,520, remained issued and outstanding. For accounting purposes, since the acceleration of vesting was negotiated in contemplation of the Reverse Merger, the remaining unrecognized compensation expense associated with the original grant date fair value of the options of $2,997 was recognized in the Company’s consolidated statement of operations for the three months ended March 31, 2018. In addition, the exercise period for all Inotek options outstanding at the effective time of the Reverse Merger was extended beyond the respective periods provided in the original awards. The Company recorded $462 in connection with the extension of the exercise periods in the consolidated statement of operations for the three months ended March 31, 2018 equal to the difference in the fair value of the options immediately prior to and immediately following the modification of the exercise period.
Share Option Valuation
The weighted average assumptions that the Company used in the Black-Scholes pricing model to determine the fair value of the share options granted to employees and directors were as follows:
|
Three Months Ended March 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
Risk-free interest rate
|
2.57
|
%
|
2.03
|
%
|
||||
|
Expected term (in years)
|
5.76
|
5.86
|
||||||
|
Expected volatility
|
88.60
|
%
|
94.30
|
%
|
||||
|
Expected dividend yield
|
0.00
|
%
|
0.00
|
%
|
||||
|
Exercise price
|
$
|
17.52
|
$
|
1.21
|
||||
|
Fair value of common stock
|
$
|
17.52
|
$
|
1.21
|
||||
The weighted average assumptions that the Company used in the Black-Scholes pricing model to determine the fair value of the share options granted to non-employees were as follows:
|
Three Months Ended
March 31, 2018
|
||||
|
Risk-free interest rate
|
2.74
|
%
|
||
|
Expected term (in years)
|
10.0
|
|||
|
Expected volatility
|
83.79
|
%
|
||
|
Expected dividend yield
|
0.00
|
%
|
||
|
Exercise price
|
$
|
18.75
|
||
|
Fair value of common stock
|
$
|
18.75
|
||
The Company recognizes compensation expense for only the portion of awards that are expected to vest.
A summary of activity under the Company’s equity plans is as follows:
|
Number of
Shares
|
Weighted
Average
Exercise
Price
|
Weighted
Average
Contractual
Term (Years)
|
Aggregate
Intrinsic
Value
|
|||||||||||||
|
Outstanding as of December 31,2017 *
|
6,959,347
|
$
|
1.06
|
8.17
|
$
|
27,175
|
||||||||||
|
Assumed as part of merger with Inotek
|
523,520
|
2.31
|
7.53
|
|||||||||||||
|
Granted
|
1,190,378
|
17.53
|
9.95
|
|||||||||||||
|
Forfeited
|
(38,156
|
)
|
1.37
|
|||||||||||||
|
Outstanding as of March 31,2018 (unaudited)
|
8,635,089
|
$
|
3.58
|
8.18
|
$
|
130,982
|
||||||||||
|
Options vested and exercisable as of March 31, 2018 (unaudited)
|
6,357,746
|
$
|
1.16
|
7.10
|
$
|
111,852
|
||||||||||
* Effected by Exchange Ratio
Restricted Stock Units
All unvested Inotek’s RSU awards issued and outstanding were accelerated at the effective time of the Reverse Merger. For accounting purposes, since the acceleration of vesting upon change of control was included in the original terms of the award, the remaining unrecognized compensation expense associated with the original grant date fair value of the RSU awards was recognized as a pre-merger expense of Inotek.
The following table summarizes the RSU activity for the three months ended March 31, 2018 under the 2014 Stock Option and Incentive Plan:
|
Number of Shares
|
||||
|
Outstanding as of December 31, 2017
|
-
|
|||
|
Assumed as part of merger with Inotek
|
271,719
|
|||
|
Settled
|
(1,875
|
)
|
||
|
Outstanding as of March 31, 2018
|
269,844
|
|||
As of March 31, 2018, due to lockup agreements signed in conjunction with the Reverse Merger, 269,844 RSU’s remain unsettled.
The aggregate intrinsic value of stock options is calculated as the difference between the exercise price of the stock options and the fair value of the Company’s common stock for those stock options that had exercise prices lower than the fair value of the Company’s common stock.
The weighted average grant-date fair value per share of stock options granted during the three months ended March 31, 2018 and 2017 was $12.84 and $1.21, respectively.
The total fair value of options vested during the year ended March 31, 2018 and the three months ended March 31, 2018 and 2017 was $24,724 and $1,583, respectively.
Employee Stock Purchase Plan
In November 2014, Inotek’s board of directors adopted and the stockholders approved the ESPP. The ESPP provides that the number of shares reserved and available for issuance under the ESPP shall be cumulatively increased each January 1, beginning on January 1, 2016, by the lesser of (i) 150,000 shares of common stock or (ii) the number of shares necessary to set the number of shares of common stock under the ESPP at 1% percent of the outstanding number of shares as of January 1 of the applicable year. However, the board of directors reserves the right to determine that there will be no increase for any year or that any increase will be for a lesser number of shares. On January 1, 2018, 6,562 shares were added to the ESPP. As of March 31, 2018, there were 68,256 shares available for issuance under the ESPP. During the three months ended March 31, 2018, 0 shares of common stock were purchased pursuant to the ESPP. The Company recorded $0 of stock-based compensation expense pursuant to the ESPP during the three months ended March 31, 2018.
Share-Based Compensation
Share-based compensation expense is reflected in the consolidated statements of operations as follows:
|
Three Months Ended March 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
Research and development
|
$
|
2,207
|
$
|
61
|
||||
|
General and administrative
|
3,175
|
38
|
||||||
|
Total share based compensation expense
|
$
|
5,382
|
$
|
99
|
||||
As of March 31, 2018, the Company had an aggregate of $25,836 of unrecognized share-based compensation cost, which is expected to be recognized over the weighted average period of 2.45 years.
11. Net Loss Per Share
Basic and diluted net loss per share attributable to common shareholders was calculated as follows:
|
Three Months Ended March 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
Numerator:
|
||||||||
|
Net loss attributable to common shareholders
|
$
|
(15,343
|
)
|
$
|
(2,678
|
)
|
||
|
Denominator:
|
||||||||
|
Weighted-average common shares outstanding - basic and diluted
|
36,137,120
|
6,795,627
|
||||||
|
Net loss per share attributable to common shareholders - basic and diluted
|
$
|
(0.42
|
)
|
$
|
(0.39
|
)
|
||
The Company excluded the following potential common shares, presented based on amounts outstanding at each period end, from the computation of diluted net loss per share attributable to common shareholders for the periods indicated because including them would have had an anti-dilutive effect:
|
Three Months Ended March 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
Shares issuable upon conversion of the 2021 Convertible Notes
|
1,620,948
|
-
|
||||||
|
Warrants exercisable for common shares
|
14,102
|
-
|
||||||
|
Options to purchase common shares
|
8,635,089
|
6,207,401
|
||||||
|
Redeemable Series A convertible preferred shares (as converted to common shares)
|
-
|
9,807,564
|
||||||
|
Redeemable Series A convertible preferred shares (as converted to common shares)
|
-
|
9,668,224
|
||||||
|
10,270,139
|
25,683,189
|
|||||||
12. Commitments and Contingencies
Operating Leases
On March 31, 2016, the Company entered into a lease agreement for its office space in New York, New York whose term ends on July 31, 2021. In connection with the lease agreement, the Company established an irrevocable standby letter of credit (“LOC”) with a bank. The LOC serves as the Company’s security deposit on the lease, in which the landlord is the beneficiary. The LOC expires and is automatically renewed April 8 of each succeeding calendar year up to October 29, 2020, unless written notice is provided no later than 90 days before the then existing expiration date. The Company has a certificate of deposit with a bank as collateral for the stand by letter of credit which is classified as restricted cash in the consolidated balance sheets. The restricted cash balance at March 31, 2018 is $208. Rent expense was $140 and $139 for the three months ended March 31, 2018 and 2017, respectively.
In January 2018, in connection with the Reverse Merger, the Company assumed an operating lease of Inotek for its former headquarters in Lexington, Massachusetts, whose term ends in February 2023. Rent expense was $83 during the three months ended March 31, 2018 in connection with the assumed lease.
As of March 31, 2018, the remaining aggregate annual commitments pursuant to the leases, as amended, are as follows:
|
2018 (remaining nine months)
|
$
|
641
|
||
|
2019
|
874
|
|||
|
2020
|
897
|
|||
|
2021
|
718
|
|||
|
2022
|
445
|
|||
|
Thereafter
|
74
|
|||
|
Total
|
$
|
3,649
|
Securities Litigation
On January 6, 2017, a purported stockholder of Inotek filed a putative class action in the U.S. District Court for the District of Massachusetts, captioned Whitehead v. Inotek Pharmaceuticals Corporation, et al., No. 1:17-cv-10025. An amended complaint was filed on July 10, 2017, and a second amended complaint was filed on September 5, 2017. The second amended complaint alleges violations of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 and SEC Rule 10b-5 against the Company, David Southwell, and Rudolf Baumgartner based on allegedly false and misleading statements and omissions regarding Inotek’s phase 2 and phase 3 clinical trials of trabodenoson. The lawsuit seeks, among other things, unspecified compensatory damages for purchasers of Inotek’s common stock between July 23, 2015 and July 10, 2017, as well as interest and attorneys’ fees and costs. The defendants filed a motion to dismiss the second amended complaint on October 6, 2017, the plaintiffs opposed the motion on December 5, 2017, and the defendants filed a reply on January 16, 2018. The Company continues to vigorously defend itself against this claim.
From time to time, the Company may be subject to other various legal proceedings and claims that arise in the ordinary course of its business activities. Although the results of litigation and claims cannot be predicted with certainty, the Company does not believe it is party to any other claim or litigation the outcome of which, if determined adversely to the Company, would individually or in the aggregate be reasonably expected to have a material adverse effect on its business. Regardless of the outcome, litigation can have an adverse impact on the Company because of defense and settlement costs, diversion of management resources and other factors.
Indemnification Arrangements
Pursuant to its bylaws and as permitted under Delaware law, the Company has indemnification obligations to directors, officers, employees or agents of the Company or anyone serving in these capacities. The maximum potential amount of future payments the Company could be required to pay is unlimited. The Company has insurance that reduces its monetary exposure and would enable it to recover a portion of any future amounts paid. As a result, the Company believes that the estimated fair value of these indemnification commitments is minimal.
Throughout the normal course of business, the Company has agreements with vendors that provide goods and services required by the Company to run its business. In some instances, vendor agreements include language that requires the Company to indemnify the vendor from certain damages caused by the Company’s use of the vendor’s goods and/or services. The Company has insurance that would allow it to recover a portion of any future amounts that could arise from these indemnifications. As a result, the Company believes that the estimated fair value of these indemnification commitments is minimal.
13. Agreements Related to Intellectual Property
The Company has entered into various license and research and collaboration arrangements. The transactions principally resulted in the acquisition of rights to intellectual property which is in the preclinical phase and have not been tested for safety or feasibility. In all cases, the Company did not acquire tangible assets, processes, protocols or operating systems. The Company expenses the acquired intellectual property rights as of the acquisition date on the basis that the cost of intangible assets purchased from others for use in research and development activities, has no alternative future uses.
License 161101 and SRA 161101
On April 20, 2018, the Company entered into Amendment No. 1 to the clinical trial agreement with the Fred Hutchinson Research Cancer Center (“Hutch”) for the clinical trial entitled: Gene Therapy for Patients with Fanconi Anemia Complementation Group A. The Company agreed to pay $108 for additional budgeted amounts for the period from March 2018 through August 2018.
LAD-I (leukocyte adhesion deficiency-I) Agreement with CIEMAT
On March 1, 2018, the Company entered into Amendment No. 1 to the Master Research Agreement (“MRA”) whereby the Company and Centro de Investigaciones Energéticas, Medioambientales y Tecnológicas (“CIEMAT”) agreed to a modification of the original commitment from Rocket down to approximately $444.
14. Related Party Transactions
During March 2018, the Company entered into a consulting agreement with a member of the Board of Directors for strategic and corporate consulting services to be provided to the Company. The Company accrued $48 for the three months ended March 31, 2018 relating to services provided under this consulting agreement which was paid in April 2018.
During January 2018, the Company sold certain furniture and fixtures from the Inotek former corporate headquarter to a director of the Company for $20.
15. 401(k) Savings Plan
The Company has a defined contribution savings plan (the “Plan”) under Section 401(k) of the Internal Revenue Code of 1986 (the “Code”). This Plan covers substantially all employees who meet minimum age and service requirements and allows participants to defer a portion of their annual compensation on a pre-tax basis. Company contributions to the Plan may be made at the discretion of the Company’s board of directors. The Company has elected to match 4% of employee contributions to the Plan, subject to certain limitations. The Company’s matching contribution for the three months ended March 31, 2018 and 2017, was $30 and $27, respectively.
16. Subsequent Events
In April 2018, the Company entered into a consulting agreement with a different member of the Board of Directors for business development consulting services. Payments for the services under the agreement are $28 per quarter, and the Company may terminate the agreement with 14 days’ notice.
The information set forth below should be read in conjunction with the consolidated financial statements and the notes thereto included elsewhere in this Quarterly Report on Form 10-Q as well as the audited financial statements and the notes thereto contained in our current report on Form 8-K and Form 10-K filed with the Securities and Exchange Commission (the “SEC”) on March 7, 2018. Unless stated otherwise, references in this Quarterly Report on Form 10-Q to “us,” “we,” “our,” or our “Company” and similar terms refer to Rocket Pharmaceuticals, Inc. References to “Inotek” refer to the company prior to the Reverse Merger (as defined below).
Recent Developments
Rocket Pharmaceuticals, Inc. (“Rocket” or the “Company”) is a multi-platform biotechnology company focused on the development of first or best-in-class gene therapies for rare and devastating pediatric diseases. Rocket has LVV programs currently undergoing clinical testing for Fanconi Anemia (“FA”), a genetic defect in the bone marrow that reduces production of blood cells or promotes the production of faulty blood cells, and three additional LVV programs targeting other rare genetic diseases. In addition, Rocket has an adeno-associated virus (“AAV”) program, which will permit the commencement of human clinical studies thereafter. Rocket has global commercialization and development rights to all of its product candidates under royalty-bearing license agreements, with the exception of the CRISPR/Cas9 development program (described below) for which Rocket currently only has development rights.
Rocket’s two leading LVV and AAV technology platforms are each being designed in collaboration with leading academic and industry partners. Through its gene therapy platforms, Rocket aims to restore normal cellular function by modifying the defective genes that cause each of the targeted disorders.
Reverse Merger and Exchange Ratio
On January 4, 2018, Rome Merger Sub (“Merger Sub”), a wholly owned subsidiary of Inotek Pharmaceuticals Corporation (“Inotek”), completed its merger with and into Rocket Pharmaceuticals, Ltd. (“Rocket Ltd”), with Rocket Ltd surviving as a wholly owned subsidiary of Inotek. This transaction is referred to as the “Reverse Merger.” The Reverse Merger was effected pursuant to an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”), dated as of September 12, 2017, by and among Inotek, Rocket Ltd and Rome Merger Sub.
As a result of the Reverse Merger, each outstanding share of Rocket Ltd share capital (including shares of Rocket Ltd share capital to be issued upon exercise of outstanding share options) automatically converted into the right to receive approximately 76.185 shares of Inotek’s common stock, par value $0.01 per share (the “Exchange Ratio”). Following the closing of the Reverse Merger, holders of Inotek’s common stock immediately prior to the Reverse Merger owned approximately 18.643% on a fully diluted basis, and holders of Rocket Ltd common stock immediately prior to the Reverse Merger owned approximately 81.357% on a fully diluted basis, of Rocket Ltd’s common stock.
The Reverse Merger has been accounted for as a reverse acquisition under the acquisition method of accounting where Rocket Ltd is considered the accounting acquirer and Inotek is the acquired company for financial reporting purposes. Rocket Ltd was determined to be the accounting acquirer based on the terms of the Merger Agreement and other factors, such as relative voting rights and the composition of the combined company’s board of directors and senior management. The pre-acquisition financial statements of Rocket Ltd became the historical financial statements of Rocket following completion of the Reverse Merger. The historical financial statements, outstanding shares and all other historical share information have been adjusted by multiplying the respective share amount by the Exchange Ratio as if the Exchange Ratio had been in effect for all periods presented.
Immediately following the Reverse Merger, the combined company changed its name from “Inotek Pharmaceuticals Corporation” to “Rocket Pharmaceuticals, Inc.” The combined company following the Reverse Merger may be referred to herein as “the combined company,” “Rocket,” or “the Company.”
The Company’s common stock remained listed on the NASDAQ Stock Market, with trading having commenced on a post-split basis and under the new name as of January 5, 2018. The trading symbol also changed on that date from “ITEK” to “RCKT.”
On January 24, 2018, the Company entered into an underwriting agreement (the “Underwriting Agreement”) with Cowen and Company, LLC and Evercore Group L.L.C., as representatives (the “Representatives”) of the several underwriters (collectively with the Representatives, the “Underwriters”), pursuant to which the Company sold 6,325,000 shares of common stock (the “Shares”), which includes 825,000 shares that were sold pursuant to an option granted to the Underwriters (the “Offering”). The Shares were sold in the Offering at a public offering price of $13.25 per share in which the Company received gross proceeds of $83,806 net of $5,288 of offering costs, commission and legal and other expenses for net proceeds from the Offering of $78,518, after deducting the underwriting discounts and commissions and legal and accounting costs.
Gene Therapy Overview
Genes are composed of sequences of deoxyribonucleic acid (“DNA”), which code for proteins that perform a broad range of physiologic functions in all living organisms. Although genes are passed on from generation to generation, genetic changes, also known as mutations, can occur in this process. These changes can result in the lack of production of proteins or the production of altered proteins with reduced or abnormal function, which can in turn result in disease.
Gene therapy is a therapeutic approach in which an isolated gene sequence or segment of DNA is administered to a patient, most commonly for the purpose of treating a genetic disease that is caused by genetic mutations. Currently available therapies for many genetic diseases focus on administration of large proteins or enzymes and typically address only the symptoms of the disease. Gene therapy aims to address the disease-causing effects of absent or dysfunctional genes by delivering functional copies of the gene sequence directly into the patient’s cells, offering the potential for curing the genetic disease, rather than simply addressing symptoms.
Rocket is using modified non-pathogenic viruses for the development of its gene therapy treatments. Viruses are particularly well suited as delivery vehicles, because they are adept at penetrating cells and delivering genetic material inside a cell. In creating Rocket’s viral delivery vehicles, the viral (pathogenic) genes are removed and are replaced with a functional form of the missing or mutant gene that is the cause of the patient’s genetic disease. The functional form of a missing or mutant gene is called a therapeutic gene, or the “transgene.” The process of inserting the transgene is called “transduction.” Once a virus is modified by replacement of the viral genes with a transgene, the modified virus is called a “viral vector.” The viral vector delivers the transgene into the targeted tissue or organ (such as the cells inside a patient’s bone marrow). Rocket has two types of viral vectors in development, lentiviral vector (“LVV”) and adeno-associated viral vector (“AAV”). Rocket believes that its LVV and AAV-based programs have the potential to offer a significant therapeutic benefit to patients that is durable (long-lasting).
The gene therapies can be delivered either (1) ex vivo (outside the body), in which case the patient’s cells are extracted and the vector is delivered to these cells in a controlled, safe laboratory setting, with the modified cells then being reinserted into the patient, or (2) - in vivo - (inside the body), in which the vector is injected directly into the patient, either intravenously (IV) or directly into a specific tissue at a targeted site, with the aim of the vector delivering the transgene to the targeted cells.
Rocket believes that scientific advances, clinical progress, and the greater regulatory acceptance of gene therapy have created a promising environment to advance gene therapy products as these products are being designed to restore cell function and improve clinical outcomes, which in many cases include prevention of death at an early age. The recent approval by the U.S. Food and Drug Administration (“FDA”) of Novartis’s treatment for pediatric acute lymphoblastic leukemia, Gilead Science’s treatment for relapsed or refractory large B-Cell lymphoma, and Spark Therapeutic’ s treatment for biallelic RPE65 mutation-associated retinal dystrophy, indicate that there is a regulatory pathway forward for cell and gene therapy products.
Pipeline Overview
LVV Programs. Rocket’s LVV-based programs utilize third-generation, self-inactivating lentiviral vectors to target selected rare diseases. Currently, Rocket is developing LVV programs to treat FA, LAD-I, Pyruvate Kinase Deficiency (“PKD”), and Infantile Malignant Osteopetrosis (“IMO”). Brief descriptions of these conditions and the Rocket programs for each is set forth below.
Fanconi Anemia (FA)
Rocket’s LVV-based programs utilize third-generation, self-inactivating lentiviral vectors to correct defects in patients’ hematopoietic stem cells (“HSCs”), which are the cells found in bone marrow that are capable of generating blood cells over a patient’s lifetime. Defects in the genetic coding of hematopoietic stem cells can result in severe, and potentially life-threatening anemia, which is when a patient’s blood lacks enough properly functioning red blood cells to carry oxygen throughout the body. Stem cell defects can also result in severe and potentially life-threatening decreases in white blood cells resulting in susceptibility to infections, and in platelets responsible for blood clotting, which may result in severe and potentially life-threatening bleeding episodes. Patients with FA have a genetic defect that prevents the normal repair of genes and chromosomes within blood cells in the bone marrow, which frequently results in the development of AML (acute myeloid leukemia, a type of blood cancer), as well as bone marrow failure and congenital defects. The average lifespan of an FA patient is estimated to be 30 to 40 years. The prevalence of FA in the US/EU is estimated to be about 2,000.
Rocket currently has the following two LVV-based programs targeting FA:
| • |
RP-L101: RP-L101 is a program that Rocket in-licensed from Fred Hutchinson Cancer Center in Seattle, Washington (“Hutch”). RP-L101 is currently being studied in a Phase 1 clinical trial that is treating FA patients at Hutch under an IND sponsored by Hutch. Rocket is entitled to the data from this clinical study and has the commercial rights to the drug being studied under this IND.
|
| • |
RP-L102: RP-L102 is a program that Rocket in-licensed from CIEMAT (Centro de Investigaciones Energéticas, Medioambientales y Tecnológicas), which is a leading research institute in Madrid, Spain. RP-L102 is currently being studied in a Phase 1/2 clinical trial treating FA patients with a modified process under an Investigational Medicinal Product Dossier (“IMPD”) sponsored by CIEMAT. Rocket is entitled to the data from this clinical study and has the commercial rights to the drug being studied under this IMPD.
|
Leukocyte Adhesion Deficiency-I (“LAD-I”)
LAD-I is a genetic disorder that causes the immune system to malfunction, resulting in a form of immunodeficiency. Immunodeficiencies are conditions in which the immune system is unable to protect the body effectively from foreign invaders such as viruses, bacteria, and fungi. Starting from birth, people with LAD-I frequently develop serious bacterial and fungal infections. Life expectancy in individuals with LAD-I is often severely shortened. Due to repeat infections, affected individuals may not survive past infancy.
Rocket currently has one LVV-based program targeting LAD-I, RP-L201. RP-L201 is a preclinical program that Rocket in-licensed from CIEMAT. This program is currently being developed through an ongoing collaboration with CIEMAT.
Pyruvate Kinase Deficiency (“PKD”)
PKD is an inherited lack of the enzyme “pyruvate kinase,” which is used by red blood cells. Without this enzyme, red blood cells break down too easily, resulting in a low level of these cells, which in turn causes a form of anemia that can range in severity from mild (asymptomatic) to severe (resulting in childhood mortality or the requirement for frequent, lifelong blood transfusions). The pediatric population is the most commonly and severely affected subgroup of patients with PKD, and pediatric patients often undergo splenectomy (removal of the spleen) and experience jaundice and chronic iron overload.
Rocket currently has one LVV-based program targeting PKD, RP-L301. RP-L301 is a preclinical program that Rocket in-licensed from CIEMAT. This program is currently being developed through an ongoing collaboration with CIEMAT.
Infantile Malignant Osteopetrosis (“IMO”)
IMO is a genetic disorder characterized by increased bone density and bone mass secondary to impaired bone resorption. Osteopetrosis is a disorder of bone development in which the bones become thickened. Normally, small areas of bone are constantly being broken down by special cells called osteoclasts, then made again by cells called osteoblasts. In osteopetrosis, the cells that break down bone (osteoclasts) do not work properly, which leads to the bones becoming thicker and not as healthy. Untreated, IMO patients may suffer from a compression of the bone-marrow space, which results in bone marrow failure, anemia and increased infection risk due to the lack of production of white blood cells. Untreated IMO patients may also suffer from a compression of cranial nerves, which transmit signals between vital organs and the brain, resulting in blindness, hearing loss and other neurologic deficits.
Rocket currently has one LVV-based program targeting IMO, RP-L401. RP-L401 is a preclinical program that Rocket in-licensed from Lund University, Sweden.
AAV-based Program
Rocket’s AAV-based program for an undisclosed rare disease involves the direct injection of the viral vector into the patient, rather than modifying the patient’s cells ex-vivo. In Rocket’s preclinical studies of its AAV-based program to date, this method of therapy has displayed substantial tropism, which is the ability to hone in on the organs most afflicted by the underlying disorder, with the aim of modifying cellular function to enable the production of sufficient quantities of a missing protein to restore proper function to the afflicted cells.
Rocket is currently developing RP-A501, which is an AAV-based program for an undisclosed rare disease. This program is currently in preclinical development, with IND-enabling studies ongoing.
CRISPR/Cas9-based program
In addition to its LVV and AAV programs, Rocket also has a program evaluating CRISPR/Cas9-based gene editing for FA. This program is currently in the discovery phase. CRISPR/Cas9-based gene editing is a different method of correcting the defective genes in a patient, where the editing is very specific and targeted to a particular gene sequence. “CRISPR/Cas9” stands for Clustered, Regularly Interspaced Short Palindromic Repeats (“CRISPR”) Associated protein-9. The CRISPR/Cas9 technology can be used to make “cuts” in DNA at specific sites of targeted genes, making it potentially more precise in delivering gene therapies than traditional vector-based delivery approaches. CRISPR/Cas9 can also be adapted to regulate the activity of an existing gene without modifying the actual DNA sequence, which is referred to as gene regulation.
The chart below shows the current phases of development of Rocket’s programs and product candidates:
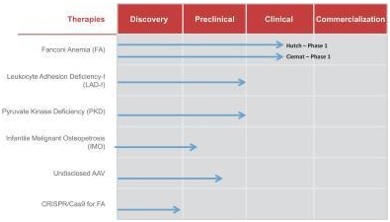
Strategy
Rocket seeks to bring hope and relief to patients with devastating, undertreated, rare pediatric diseases through the development and commercialization of potentially curative first-in-class gene therapies. To achieve these objectives, Rocket intends to develop into a fully integrated biotechnology company. In the near- and medium-term, Rocket intends to develop its first-in-class product candidates, which are targeting devastating diseases with substantial unmet need. In the medium- and long-term, Rocket expects to develop proprietary in-house analytics and manufacturing capabilities, commence registration trials for its currently planned programs and submit its first biologics license applications (“BLAs”), and establish its gene therapy platform and expand its pipeline to target additional indications that Rocket believes to be potentially compatible with its gene therapy technologies. In addition, during that time, Rocket believes that its currently planned programs will become eligible for priority review vouchers from the FDA that provide for expedited review. Rocket has assembled a leadership and research team with expertise in cell and gene therapy, rare disease drug development and commercialization.
Rocket believes that its competitive advantage lies in its disease-based selection approach, a rigorous process with defined criteria to identify target diseases. Rocket believes that this approach to asset development differentiates it as a gene therapy company and potentially provides Rocket with a first-mover advantage.
Overview
Since our inception, we have devoted substantially all of our resources to organizing and staffing the company, business planning, raising capital, acquiring or discovering product candidates and securing related intellectual property rights, conducting discovery, research and development activities for the programs and planning for potential commercialization. We do not have any products approved for sale and have not generated any revenue from product sales. From inception through March 31, 2018, Rocket raised net cash proceeds of approximately $120.7 million from private investors through both equity and convertible debt financing to fund operating activities. In addition, in conjunction with the closing of the Reverse Merger, Rocket received additional proceeds of $76.3 million.
Since inception, we have incurred significant operating losses. Our ability to generate product revenue sufficient to achieve profitability will depend heavily on the successful development and eventual commercialization of one or more of the current or future product candidates and programs. Rocket Ltd had net losses of $19.6 million for the year ended December 31, 2017 and Rocket’s net loss is $15.3 million for the three months ended March 31, 2018. As of March 31, 2018, we had an accumulated deficit of $46.7 million. We expect to continue to incur significant expenses and higher operating losses for the foreseeable future as we advance our current product candidates from discovery through preclinical development and clinical trials and seek regulatory approval of our product candidates. In addition, if we obtain marketing approval for any of their product candidates, we expect to incur significant commercialization expenses related to product manufacturing, marketing, sales and distribution. Furthermore, we expect to incur additional costs as a public company. Accordingly, we will need additional financing to support continuing operations and potential acquisitions of licensing or other rights for product candidates.
Until such a time as we can generate significant revenue from product sales, if ever, we will seek to fund our operations through public or private equity or debt financings or other sources, which may include collaborations with third parties and government programs or grants. Adequate additional financing may not be available to us on acceptable terms, or at all. We can make no assurances that we will be able to raise the cash needed to fund our operations and, if we fail to raise capital when needed, we may have to significantly delay, scale back or discontinue the development and commercialization of one or more product candidates or delay pursuit of potential in-licenses or acquisitions.
Because of the numerous risks and uncertainties associated with product development, we are unable to predict the timing or amount of increased expenses or when or if we will be able to achieve or maintain profitability. Even if we are able to generate product sales, we may not become profitable. If we fail to become profitable or are unable to sustain profitability on a continuing basis, then we may be unable to continue our operations at planned levels and be forced to reduce or terminate our operations.
Financial Operations Overview
Revenue
To date, we have not generated any revenue from any sources, including from product sales, and we do not expect to generate any revenue from the sale of products in the near future. If our development efforts for product candidates are successful and result in regulatory approval or license agreements with third parties, we may generate revenue in the future from product sales.
Operating Expenses
Research and Development Expenses
Our research and development program expenses consist primarily of external costs incurred for the development of our product candidates. These expenses include:
| • |
expenses incurred under agreements with research institutions that conduct research and development activities including, process development, preclinical, and clinical activities on Rocket’s behalf;
|
| • |
costs related to process development, production of preclinical and clinical materials, including fees paid to contract manufacturers and manufacturing input costs for use in internal manufacturing processes;
|
| • |
consultants supporting process development and regulatory activities; and
|
| • |
costs related to in-licensing of rights to develop and commercialize our product candidate portfolio.
|
We recognize external development costs based on contractual payment schedules aligned with program activities, invoices for work incurred, and milestones which correspond with costs incurred by the third parties. Nonrefundable advance payments for goods or services to be received in the future for use in research and development activities are recorded as prepaid expenses.
Our direct research and development expenses are tracked on a program-by-program basis for product candidates and consist primarily of external costs, such as research collaborations and third party manufacturing agreements associated with our preclinical research, process development, manufacturing, and clinical development activities. Our direct research and development expenses by program also include fees incurred under license agreements. Our personnel, non-program and unallocated program expenses include costs associated with activities performed by our internal research and development organization and generally benefit multiple programs. These costs are not separately allocated by product candidate and consist primarily of:
| • |
salaries and personnel-related costs, including benefits, travel and share-based compensation, for our scientific personnel performing research and development activities;
|
| • |
facilities and other expenses, which include expenses for rent and maintenance of facilities, and depreciation expense; and
|
| • |
laboratory supplies and equipment used for internal research and development activities.
|
Our research and development activities are central to our business model. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development. As a result, we expect that research and development expenses will increase substantially over the next several years as the Company increases personnel costs, including share-based compensation, supports ongoing clinical studies, seeks to achieve proof-of-concept in one or more product candidates, advances preclinical programs to clinical programs, and prepares regulatory filings for product candidates.
We cannot determine with certainty the duration and costs to complete current or future clinical studies of product candidates or if, when, or to what extent we will generate revenues from the commercialization and sale of any of its product candidates that obtain regulatory approval. We may never succeed in achieving regulatory approval for any of our product candidates. The duration, costs, and timing of clinical studies and development of product candidates will depend on a variety of factors, including:
| • |
the scope, rate of progress, and expense of ongoing as well as any clinical studies and other research and development activities that we undertake;
|
| • |
future clinical study results;
|
| • |
uncertainties in clinical study enrollment rates;
|
| • |
changing standards for regulatory approval; and
|
| • |
the timing and receipt of any regulatory approvals.
|
We expect research and development expenses to increase for the foreseeable future as we continue to invest in research and development activities related to developing product candidates, including investments in manufacturing, as our programs advance into later stages of development and as it conducts additional clinical trials. The process of conducting the necessary clinical research to obtain regulatory approval is costly and time-consuming, and the successful development of product candidates is highly uncertain. As a result, we are unable to determine the duration and completion costs of research and development projects or when and to what extent we will generate revenue from the commercialization and sale of any of our product candidates.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related costs for personnel, including share-based compensation and travel expenses for our employees in executive, operational, finance, legal, business development, and human resource functions. Other general and administrative expenses include facility-related costs, professional fees for accounting, tax and legal and consulting services.
We expect general and administrative expenses to increase for the foreseeable future due to anticipated increases in headcount to support the continued advancement of our product candidates. We also anticipate that we will incur increased accounting, audit, legal, regulatory, compliance and director and officer insurance costs as well as investor and public relations expenses associated with being a public company.
Research and development incentives
New York City allows investors and owners of emerging technology companies focused on biotechnology to claim a tax credit against the General Corporation Tax and Unincorporated Business Tax for amounts paid or incurred for certain facilities, operations, and employee training in New York City. The credit is recognized as research and development incentives when approved by New York City of the eligibility for the credit and the credit amount.
Interest Expense
Interest expense relates to our 2021 Convertible Notes, which are due in August 2021.
Interest Income
Interest income mainly relates to interest earned from cash, cash equivalents and short term investments.
Critical Accounting Policies and Significant Judgments and Estimates
Our consolidated financial statements are prepared in accordance with generally accepted accounting principles in the United States. The preparation of our financial statements and related disclosures requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, costs and expenses, and the disclosure of contingent assets and liabilities in our financial statements. We base our estimates on historical experience, known trends and events and various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. We evaluate estimates and assumptions on an ongoing basis. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are described in more detail in Note 3 to our financial statements appearing elsewhere in this Quarterly Report on Form 10-Q, we believe that the following accounting policies are those most critical to the judgments and estimates used in the preparation of our consolidated financial statements.
Accrued Research and Development Expenses
We estimate our accrued research and development expenses as of the date of each of our balance sheets. We recognize external development costs based on contractual payment schedules aligned with program activities, invoices for work performed, and milestones which correspond with costs incurred by the third parties. This process involves reviewing contracts and purchase orders with service providers, identifying services that have been performed on our behalf, confirming the level of service performed are aligned with the contract, expected remaining period of performance and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of actual cost. Expenses that are paid in advance of performance are deferred as a prepaid expense and expensed as the services are provided.
Examples of estimated accrued research and development expenses include fees paid to:
| • |
research organizations for collaborations for preclinical development, process development and clinical studies;
|
| • |
contract manufacturing organizations and other vendors related to process development and manufacturing of materials for use in preclinical development and clinical studies; and
|
| • |
service providers for professional service fees such as consulting and other research and development related services.
|
Our understanding of the status and timing of services performed relative to the actual status and timing may vary and may result in our reporting changes in estimates in any particular period. To date, there have been no material differences from our estimates to the amounts actually incurred.
Share-based compensation
We measure the cost of employee services received in exchange for an award of equity instruments based on the grant date fair value of the award. That cost is recognized on a straight-line basis over the period during which the employee is required to provide service in exchange for the award. The fair value of options on the date of grant is calculated using the Black-Scholes option pricing model based on key assumptions such as stock price, expected volatility and expected term. The fair value of restricted stock awards is based on the intrinsic value of such awards on the date of grant. Compensation cost for stock purchase rights under our employee stock purchase plan is measured and recognized on the date that we become obligated to issue shares of our common stock and is based on the difference between the fair value of our common stock and the purchase price on such date. Our estimates of these assumptions are primarily based on third-party valuations, historical data, peer company data and judgment regarding future trends and factors.
Prior to the listing of our common stock on the NASDAQ capital market, our board of directors historically determined, as of the date of each option grant, with input from our management, the assistant of a third-party valuation specialist and the guidance outlined in the American Institute of Certified Public Accountants’ Accounting and Valuation Guide, Valuation of Privately-Held-Company Equity Securities Issued as Compensation, estimate the fair value of our common stock on the date of grant based on a number of objectives and subjective factors
Since the Reverse Merger and the listing of our common stock on the NASDAQ Capital Market, we have relied on the market price of our common stock to determine its fair value on the date of grant for purposes of determining our stock-based compensation expense.
The assumptions underlying these valuations represent the best estimates of our management, which involve inherent uncertainties and the application of our judgment. As a result, if factors or expected outcomes change and we use significantly different assumptions or estimates, the resulting share-based compensation expense could be materially different.
Goodwill
Goodwill represents the difference between the consideration transferred and the fair value of the net assets acquired and liabilities assumed under the acquisition method of accounting. Goodwill is evaluated for impairment within our single reporting unit on an annual basis, during the fourth quarter, or more frequently if an event occurs or circumstances change that would more likely than not reduce the fair value of our reporting unit below our carrying amount. When performing the impairment assessment, the accounting standard for testing goodwill for impairment permits us to first assess the qualitative factors to determine whether the existence of events and circumstances indicates that it is more likely than not that the goodwill is impaired. If we believe, as a result of the qualitative assessment, that it is more likely than not that the fair value of goodwill is impaired, we must perform the quantitative goodwill impairment test.
Results of Operations
Comparison of the Three Months Ended March 31, 2018 and 2017
The following table summarizes the results of operations for the three months ended March 31, 2018 and 2017:
|
Three Months Ended
March 31,
|
||||||||||||
|
2018
|
2017
|
Change
|
||||||||||
|
Operating expenses:
|
||||||||||||
|
Research and development
|
$
|
5,743
|
$
|
2,285
|
$
|
3,458
|
||||||
|
General and administrative
|
8,662
|
585
|
8,077
|
|||||||||
|
Total operating expenses
|
14,405
|
2,870
|
11,535
|
|||||||||
|
Loss from operations
|
(14,405
|
)
|
(2,870
|
)
|
(11,535
|
)
|
||||||
|
Research and development incentives
|
186
|
192
|
(6
|
)
|
||||||||
|
Interest expense
|
(1,427
|
)
|
-
|
(1,427
|
)
|
|||||||
|
Interest income
|
288
|
-
|
288
|
|||||||||
|
Other income
|
15
|
-
|
15
|
|||||||||
|
Total other income (expense), net
|
(938
|
)
|
192
|
(1,130
|
)
|
|||||||
|
Net loss
|
$
|
(15,343
|
)
|
$
|
(2,678
|
)
|
$
|
12,665
|
||||
Research and Development Expenses
Research and development expenses (“R&D”) increased $3.5 million to $5.7 million for the three months ended March 31, 2018 compared to the three months ended March 31, 2017. The increases were primarily a result of increases in manufacturing and process development expenses of $2.4 million and $1.4 million increase in share-based compensation expense for the three months ended March 31, 2018 as compared to the three months ended March 31, 2017.
General and Administrative Expenses
General and administrative expense (“G&A”) increased $8.1 million to $8.7 million for the three months ended March 31, 2018 compared to the three months ended March 31, 2017. The increase in G&A for the three months ended March 31, 2018 was primarily due to merger-related expenses due to the Reverse Merger of $5.3 million which were incurred for the three months ended March 31, 2018, including $3.4 million share-based compensation expenses and post-merger transition expenses including payroll and severance payments for remaining Inotek employees retained for the post-merger transition. The remaining increase of $2.8 million in G&A for the three months ended March 31, 2018 as compared to the three months ended March 31, 2017 was primarily due to an increase in personnel costs due to headcount additions as of March 31, 2018 as compared to March 31, 2017 and an increase in legal costs of $0.4 million in connection with supporting the growth in our business and becoming a public company. In addition, there was a $0.7 million increase in rent and office related expense primarily due to an increase in insurance expenses to support the Company’s transition to a public company, and an increase of $0.5 million increase in G&A share-based compensation expense. We expect an increase in general administrative expense in future periods, as we operate as a public company.
Other Income (Expense)
Other expense increased $1.1 million for the three months ended March 31, 2018 compared to the three months ended March 31, 2017 primarily due to increase in interest expense of $1.4 million, offset by an increase in interest income of $0.3 million. The increase in interest expense is due to the assumption by the Company of the 2021 Convertible Notes in connection with the Reverse Merger. The increase in interest income is due to interest on the Company’s short term investments in connection with the Reverse Merger.
Liquidity, Capital Resources and Plan of Operations
Since inception, we have not generated any revenue from any sources, including from product sales, and have incurred significant operating losses and negative cash flows from our operations. We have funded operations to date primarily with proceeds from the sale of preferred shares, common stock and the issuance of convertible notes. We received $78.5 million from the sale of our common shares in January 2018.
On January 24, 2018, the Company entered into an underwriting agreement (the “Underwriting Agreement”) with Cowen and Company, LLC and Evercore Group L.L.C., as representatives (the “Representatives”) of the several underwriters (collectively with the Representatives, the “Underwriters”), pursuant to which the Company sold 6,325,000 shares of common stock (the “Shares”), which includes 825,000 shares that were sold pursuant to an option granted to the Underwriters (the “Offering”). The Shares were sold in the Offering at a public offering price of $13.25 per share in which the Company received gross proceeds of $83,806 million, net of $5,288 million of offering costs, commission and legal and other expenses for net proceeds of $78,518 million.
As of March 31, 2018, we had cash, cash equivalents and short term investments of $182.7 million. Based upon current operating plans, we expect that our existing cash will be sufficient to fund operations into 2020.
Cash Flows
The following table summarizes our cash flows for each of the periods presented:
|
Three Months Ended
March 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
(in thousands)
|
||||||||
|
Net cash used in operating activities
|
$
|
(11,601
|
)
|
$
|
(3,723
|
)
|
||
|
Net cash provided by (used in) investing activities
|
86,078
|
(131
|
)
|
|||||
|
Net cash provided by financing activities
|
78,518
|
21,549
|
||||||
|
Net increase in cash, cash equivalents and restricted cash
|
$
|
152,995
|
$
|
17,695
|
||||
Operating Activities
During the three months ended March 31, 2018, operating activities used $11.6 million of cash, primarily resulting from our net loss of $15.3 million and net changes in our operating assets and liabilities of $2.4 million, partially offset by net non-cash charges of $6.2 million, including share-based compensation expense of $5.4 million. Changes in our operating assets and liabilities for the three months ended March 31, 2018 consisted primarily of an increase in prepaid expenses of $0.3 million and a decrease in accounts payable and accrued expenses of $2.3 million.
During the three months ended March 31, 2017, operating activities used $3.7 million of cash, primarily resulting from our net loss of $2.7 million and net cash used by changes in our operating assets and liabilities of $1.2 million, partially offset by non-cash charges including share-based compensation expense of $0.1 million. Net cash used by changes in our operating assets and liabilities for the three months ended March 31, 2017 consisted primarily of a $1.0 million increase in prepaid expenses primarily due to preclinical expenses.
Investing Activities
During the three months ended March 31, 2018, net cash provided by investing activities was $86.1 million, consisting primarily of $76.3 million of cash acquired in connection with the Reverse Merger and $9.7 million from the maturities of short-term investments.
During the three months ended March 31, 2017, we used $0.1 million of cash in investing activities, consisting of purchases of property and equipment.
Financing Activities
During the three months ended March 31, 2018, net cash provided by financing activities was $78.5 million, consisting entirely of proceeds from the issuance of common stock.
During the three months ended March 31, 2017, net cash provided by financing activities was $21.6 million, consisting entirely of proceeds from the issuance of convertible preferred stock.
Funding Requirements
We expect expenses to increase substantially in connection with our ongoing activities, particularly as we advance our preclinical activities, initiate additional clinical trials and manufacturing of our product candidates. In addition, we expect to incur additional costs associated with operating as a public company. Our expenses will also increase as we:
|
•
|
leverage our programs to advance other product candidates into preclinical and clinical development;
|
| • |
seek regulatory agreements to initiate clinical trials in the EU, US and ROW;
|
| • |
establish a sales, marketing, medical affairs and distribution infrastructure to commercialize any product candidates for which Rocket may obtain marketing approval and intend to commercialize on its own or jointly;
|
| • |
hire additional preclinical, clinical, regulatory, quality and scientific personnel;
|
| • |
expand our operational, financial and management systems and increase personnel, including personnel to support our clinical development, manufacturing and commercialization efforts and our operations as a public company;
|
| • |
maintain, expand and protect our intellectual property portfolio; and
|
| • |
acquire or in-license other product candidates and technologies.
|
As of March 31, 2018, we had cash, cash equivalents and short term investments of $182.7 million. We believe that our existing cash will be sufficient to fund operations into May 2020. Because of the numerous risks and uncertainties associated with research, development and commercialization of pharmaceutical product candidates, we are unable to estimate the exact amount of working capital requirements. Our future funding requirements will depend on, and could increase significantly as a result of, many factors, including:
| • |
the scope, progress, results and costs of researching and developing our product candidates, and conducting preclinical studies and clinical trials;
|
| • |
the costs, timing and outcome of regulatory review of our product candidates;
|
| • |
the costs of future activities, including product sales, medical affairs, marketing, manufacturing and distribution, for any of our product candidates for which we receive marketing approval;
|
| • |
the costs of manufacturing commercial-grade product to support commercial launch;
|
| • |
the ability to receive additional non-dilutive funding, including grants from organizations and foundations;
|
| • |
the revenue, if any, received from commercial sale of its products, should any of its product candidates receive marketing approval;
|
| • |
the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property-related claims;
|
| • |
our ability to establish and maintain collaborations on favorable terms, if at all;
|
| • |
the extent to which we acquire or in-license other product candidates and technologies; and
|
| • |
the timing, receipt and amount of sales of, or milestone payments related to our royalties on, current or future product candidates, if any.
|
Until such time, if ever, as we can generate substantial product revenue, we expect to finance our cash needs through a combination of public or private equity offerings, debt financings, collaborations, strategic partnerships or marketing, distribution or licensing arrangements with third parties. To the extent that we raise additional capital through the sale of equity or convertible debt securities, our ownership interest may be materially diluted, and the terms of such securities could include liquidation or other preferences that adversely affect the rights of our common stockholders. Debt financing and preferred equity financing, if available, may involve agreements that include restrictive covenants that limit our ability to take specified actions, such as incurring additional debt, making capital expenditures or declaring dividends. In addition, additional debt financing would result in increased fixed payment obligations.
If we raise funds through governmental funding, collaborations, strategic partnerships or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, reduce or eliminate our product development or future commercialization efforts or grant rights to develop and market product candidates that it would otherwise prefer to develop and market themselves.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the Securities and Exchange Commission.
JOBS Act
Under Section 107(b) of the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), an “emerging growth company” can delay the adoption of new or revised accounting standards until such time as those standards would apply to private companies. We have irrevocably elected not to avail ourselves of this exemption and, as a result, we will adopt new or revised accounting standards at the same time as other public companies that are not emerging growth companies. There are other exemptions and reduced reporting requirements provided by the JOBS Act that we are currently evaluating. For example, as an emerging growth company, we are exempt from Sections 14A(a) and (b) of the Securities Exchange Act of 1934 (the “Exchange Act”), which would otherwise require us to (i) submit certain executive compensation matters to stockholder advisory votes, such as “say-on-pay,” “say-on-frequency” and “golden parachutes” and (ii) disclose certain executive compensation related items such as the correlation between executive compensation and performance and comparisons of our Chief Executive Officer’s compensation to our median employee compensation. We also intend to rely on an exemption from the rule requiring us to provide an auditor’s attestation report on our internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act and the rule requiring us to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board (“PCAOB”) regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements as the auditor discussion and analysis. We will continue to remain an “emerging growth company” until the earliest of the following: December 31, 2020; the last day of the fiscal year in which our total annual gross revenue is equal to or more than $1.07 billion; the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; or the date on which we are deemed to be a large accelerated filer under the rules of the SEC.
Recently Issued Accounting Pronouncements
A description of recently issued accounting pronouncements that may potentially impact our financial position and results of operations is disclosed in Note 3 of our “Consolidated Unaudited Financial Statements,” in this Quarterly Report on Form 10-Q.
We are exposed to market risks in the ordinary course of our business. These market risks are principally limited to interest rate fluctuations. We had cash and cash equivalents of $171.1 million at March 31, 2018, consisting primarily of funds in money market accounts. We also had $11.6 million in short-term investments consisting of certificates of deposit and United States Treasury securities. The primary objective of our investment activities is to preserve principal and liquidity while maximizing income without significantly increasing risk. We do not enter into investments for trading or speculative purposes. Due to the short-term nature of our investment portfolio, we do not believe an immediate 1.0% increase in interest rates would have a material effect on the fair market value of our portfolio, and accordingly we do not expect a sudden change in market interest rates to affect materially our operating results or cash flows.
Our 2021 Convertible Notes bear interest at a fixed rate and therefore a change in interest rates would not impact the amount of interest we would have to pay on this indebtedness.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and our principal financial officer, evaluated, as of the end of the period covered by this Quarterly Report on Form 10-Q, the effectiveness of our disclosure controls and procedures. Based on that evaluation of our disclosure controls and procedures as of March 31, 2018, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures as of such date are effective at the reasonable assurance level. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act are recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports we file or submit under the Exchange Act is accumulated and communicated to our management, including our principal executive officer and principal financial and accounting officer, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and our management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Inherent Limitations of Internal Controls
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during the quarter ended March 31, 2018, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
PART II – OTHER INFORMATION
On January 6, 2017, a purported stockholder of Inotek filed a putative class action in the U.S. District Court for the District of Massachusetts, captioned Whitehead v. Inotek Pharmaceuticals Corporation, et al., No. 1:17-cv-10025. An amended complaint was filed on July 10, 2017, and a second amended complaint was filed on September 5, 2017. The second amended complaint alleges violations of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 and SEC Rule 10b-5 against the Company, David Southwell, and Rudolf Baumgartner based on allegedly false and misleading statements and omissions regarding Inotek’s phase 2 and phase 3 clinical trials of trabodenoson. The lawsuit seeks, among other things, unspecified compensatory damages for purchasers of Inotek’s common stock between July 23, 2015 and July 10, 2017, as well as interest and attorneys’ fees and costs. The defendants filed a motion to dismiss the second amended complaint on October 6, 2017, the plaintiffs opposed the motion on December 5, 2017, and the defendants filed a reply on January 16, 2018. The Company continues to vigorously defend itself against this claim.
From time to time, we may be subject to other various legal proceedings and claims that arise in the ordinary course of our business activities. Although the results of litigation and claims cannot be predicted with certainty, we do not believe we are party to any other claim or litigation the outcome of which, if determined adversely to us, would individually or in the aggregate be reasonably expected to have a material adverse effect on our business. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
We operate in an industry that involves numerous risks and uncertainties. You should carefully consider the following information about these risks, together with the other information appearing elsewhere in this Quarterly Report on Form 10-Q, including our financial statements and related notes hereto. The occurrence of any of the following risks could have a material adverse effect on our business, financial condition, results of operations and future growth prospects. The risks and uncertainties described below may change over time and other risks and uncertainties, including those that we do not currently consider material, may impair our business. In these circumstances, the market price of our common stock could decline. The following Risk Factors were consistent with those previously disclosed in the 2017 Form 10-K
Risks Related to Rocket’s Financial Position
Rocket has a history of operating losses, and Rocket may not achieve or sustain profitability. Rocket anticipates that it will continue to incur losses for the foreseeable future. If Rocket fails to obtain additional funding to conduct its planned research and development effort, Rocket could be forced to delay, reduce or eliminate its product development programs or commercial development efforts.
Rocket is an early-stage gene therapy company with a limited operating history on which to base your investment decision. Gene therapy product development is a highly speculative undertaking and involves a substantial degree of risk. Rocket’s operations to date have been limited primarily to organizing and staffing its company, business planning, raising capital, acquiring and developing product and technology rights and conducting preclinical research and development activities for its product candidates. Rocket has never generated any revenue from product sales. Rocket has not obtained regulatory approvals for any of its product candidates, and has funded its operations to date through proceeds from sales of its preferred stock, common stock and the issuance of convertible notes.
Rocket has incurred net losses since its inception. Rocket has incurred net losses of $15.3 million for the three months ended March 31, 2018, and Rocket Ltd. incurred losses of $19.6 million and $7.6 million for the years ended December 31, 2017 and 2016, respectively. As of March 31, 2018 and December 31, 2017, Rocket had an accumulated deficit of $46.7 million and Rocket Ltd had an accumulated deficit of $31.4 million, respectively. Substantially all of its operating losses have resulted from costs incurred in connection with its research and development programs and from general and administrative costs associated with its operations. Rocket expects to continue to incur significant expenses and operating losses over the next several years and for the foreseeable future, as Rocket intends to continue to conduct research and development, clinical testing, regulatory compliance activities, manufacturing activities, and, if any of its product candidates is approved, sales and marketing activities that, together with anticipated general and administrative expenses, will likely result in Rocket incurring significant losses for the foreseeable future. Rocket’s prior losses, combined with expected future losses, have had and will continue to have an adverse effect on Rocket’s stockholders’ deficit and working capital.
Rocket may need to raise additional funding, which may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force Rocket to delay, limit or terminate certain of its licensing activities, product development efforts or other operations.
Rocket expects to require substantial future capital in order to seek to broaden licensing of its gene therapy platforms, complete preclinical and clinical development for its current product candidates and other future product candidates, if any, and potentially commercialize these product candidates. Rocket expects its spending levels to increase in connection with its preclinical and clinical trials. In addition, if Rocket obtains marketing approval for any of its product candidates, Rocket expects to incur significant expenses related to product sales, medical affairs, marketing, manufacturing and distribution. Furthermore, Rocket expects to incur additional costs associated with operating as a public company. Accordingly, Rocket will need to obtain substantial additional funding in connection with its continuing operations. If Rocket is unable to raise capital when needed or on acceptable terms, Rocket could be forced to delay, reduce or eliminate certain of its licensing activities, its research and development programs or other operations.
Rocket’s operations have consumed significant amounts of cash since inception. As of March 31, 2018 and December 31, 2017, Rocket’s cash, cash equivalents and short term investments was $182.7 million and $18.1 million, respectively. Rocket’s future capital requirements will depend on many factors, including:
| • |
the timing of enrollment, commencement, completion and results of Rocket’s clinical trials, including Rocket’s current clinical trials for Fanconi Anemia;
|
| • |
the results of Rocket’s preclinical studies for Rocket’s current product candidates and any subsequent clinical trials;
|
| • |
the scope, progress, results and costs of drug discovery, laboratory testing, preclinical development and clinical trials, if any, for Rocket’s internal product candidates;
|
| • |
the costs associated with building out additional laboratory and manufacturing capacity, if any;
|
| • |
the costs, timing and outcome of regulatory review of Rocket’s product candidates;
|
| • |
the costs of future activities, including product sales, medical affairs, marketing, manufacturing and distribution, for any of Rocket’s product candidates for which Rocket receives marketing approval;
|
| • |
the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing its intellectual property rights and defending any intellectual property-related claims;
|
| • |
Rocket’s current licensing agreements or collaborations remaining in effect;
|
| • |
Rocket’s ability to establish and maintain additional licensing agreements or collaborations on favorable terms, if at all;
|
| • |
the extent to which Rocket acquires or in-licenses other product candidates and technologies; and
|
| • |
the costs associated with being a public company.
|
Many of these factors are outside of Rocket’s control. Identifying potential product candidates and conducting preclinical testing and clinical trials is a time-consuming, expensive and uncertain process that takes years to complete, and Rocket may never generate the necessary data or results required to obtain regulatory and marketing approval and achieve product sales. In addition, Rocket’s product candidates, if approved, may not achieve commercial success. Accordingly, Rocket will need to continue to rely on additional financing to achieve its business objectives.
To the extent that additional capital is raised through the sale of equity or equity-linked securities, the issuance of those securities could result in substantial dilution for Rocket’s current shareholders and the terms may include liquidation or other preferences that adversely affect the rights of Rocket’s current shareholders. Adequate additional financing may not be available to Rocket on acceptable terms, or at all. Rocket also could be required to seek funds through arrangements with partners or otherwise that may require Rocket to relinquish rights to its intellectual property, its product candidates or otherwise agree to terms unfavorable to Rocket.
Rocket’s limited operating history may make it difficult for Rocket to evaluate the success of its business to date and to assess Rocket’s future viability.
Rocket’s operations to date have predominantly focused on organizing and staffing its company, business planning, raising capital, acquiring its technology, administering and expanding its gene therapy platforms, identifying potential product candidates, undertaking research, preclinical studies and clinical trials of its product candidates and establishing licensing arrangements and collaborations. Rocket has not yet completed clinical trials of its product candidates, obtained marketing approvals, manufactured a commercial-scale product or conducted sales and marketing activities necessary for successful commercialization. Consequently, any predictions made about Rocket’s future success or viability may not be as accurate as they could be if Rocket had a longer operating history.
In addition, as a new business, Rocket may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors. Rocket expects to eventually transition from a company with a licensing and research focus to a company that is also capable of supporting clinical development activities and Rocket may need to transition to supporting commercial activities in the future. Rocket cannot guarantee that it will be successful in these transitions.
Rocket’s ability to use its net operating loss carryforwards and certain other tax attributes may be limited.
Under Section 382 of the Internal Revenue Code of 1986, as amended, if a corporation undergoes an “ownership change,” generally defined as a greater than 50% change (by value) in its equity ownership over a three-year period, the corporation’s ability to use its pre-change net operating loss (“NOL”) carryforwards and other pre-change tax attributes to offset its post-change income may be limited. Rocket may experience ownership changes in the future. As a result, if Rocket earns net taxable income, Rocket’s ability to use its pre-change net NOL carryforwards to offset U.S. federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to Rocket. Furthermore, Rocket’s ability to use NOL carryforwards to offset U.S. federal taxable income in the future may be further limited by certain provisions set forth in The Tax Cuts and Jobs Act, which could potentially result in increased future tax liability to Rocket. In addition, at the state level, there may be periods during which the use of NOL carryforwards is suspended or otherwise limited, which could accelerate or permanently increase state taxes owed. As of December 31, 2017, Rocket had net operating losses of approximately $24.8 million for New York City tax purposes. As of December 31, 2017, Rocket had no unrecognized tax benefits or liabilities for uncertain tax positions. Rocket files income tax returns in the United States and New York State and New York City, but for the year ended December 31, 2017, did not report any income effectively connected with a U.S. trade or business.
As of December 31, 2017, Inotek had federal NOL carryforwards for income tax purposes of $127.1 million that will expire at various dates through 2037 and state NOL carryforwards of $83.4 million that will expire at various dates through 2037, available to reduce future federal and state income taxes, if any. As of December 31, 2017, Inotek had federal research and development tax credits of $5.2 million and state research and development tax credits of $0.8 million. The pre-change NOL carryforwards, although subject to an annual limitation, as well as any post-change NOL carryforwards, can be utilized in future years, provided that sufficient income is generated and no future ownership changes occur that may limit Inotek’s NOL carryforwards. Additionally, the Reverse Merger on January 4, 2018 is expected to significantly limit utilization of Inotek’s NOL carryforwards as the Reverse Merger was co considered to be an ownership change, under Section 382 of the Code, though the actual amount of the NOL limitation has not yet been determined.
Rocket has never generated any revenue from product sales and may never be profitable.
Rocket’s ability to generate revenue and achieve profitability depends on Rocket’s ability, alone or with strategic collaboration partners, to successfully complete the development of, and obtain the regulatory, pricing and reimbursement approvals necessary to commercialize its product candidates. Rocket does not anticipate generating revenues from product sales for the foreseeable future, if ever. Rocket’s ability to generate future revenues from product sales depends heavily on its success in:
| • |
completing research and preclinical and clinical development of Rocket’s product candidates;
|
| • |
seeking and obtaining regulatory and marketing approvals for product candidates for which Rocket completes clinical studies;
|
| • |
developing a sustainable, commercial-scale, reproducible, and transferable manufacturing process for Rocket’s vectors and product candidates;
|
|
•
|
establishing and maintaining supply and manufacturing relationships with third parties that can provide adequate (in amount and quality) products and services to support clinical development and the market demand for Rocket’s product candidates, if approved;
|
| • |
launching and commercializing product candidates for which Rocket obtains regulatory and marketing approval, either by collaborating with a partner or, if launched independently, by establishing a sales force, marketing and distribution infrastructure;
|
| • |
obtaining sufficient pricing and reimbursement for Rocket’s product candidates from private and governmental payors;
|
| • |
obtaining market acceptance of Rocket’s product candidates and gene therapy as a viable treatment option;
|
| • |
addressing any competing technological and market developments;
|
| • |
identifying and validating new gene therapy product candidates;
|
| • |
negotiating favorable terms in any collaboration, licensing or other arrangements into which Rocket may enter; and
|
| • |
maintaining, protecting and expanding Rocket’s portfolio of intellectual property rights, including patents, trade secrets and know-how.
|
Even if one or more of the product candidates that Rocket will develop is approved for commercial sale, Rocket anticipates incurring significant costs associated with commercializing any approved product candidate. Rocket’s expenses could increase beyond expectations if Rocket is required by the FDA, the EMA, or other regulatory agencies, domestic or foreign, to perform clinical and other studies in addition to those that Rocket currently anticipates. Even if Rocket is able to generate revenues from the sale of any approved products, Rocket may not become profitable and may need to obtain additional funding to continue operations.
Risks Related to Product Regulatory Matters
Rocket’s gene therapy product candidates are based on novel technology, which makes it difficult to predict the time and cost of product candidate development and subsequently obtaining regulatory approval. Currently, only a few gene therapy products have been approved in the United States and the European Union.
Rocket has concentrated its research and development efforts to date on a gene therapy platform, and Rocket’s future success depends on the successful development of viable gene therapy product candidates. Rocket cannot guarantee that it will not experience problems or delays in developing current or future product candidates or that such problems or delays will not cause unanticipated costs, or that any such development problems or delays can be resolved. Rocket may also experience unanticipated problems or delays in developing Rocket’s manufacturing capacity or transferring Rocket’s manufacturing process to commercial partners, which may prevent Rocket from completing its clinical studies or commercializing its products on a timely or profitable basis, if at all.
In addition, the clinical study requirements of the FDA, the European Medicines Agency, (“EMA”), and other regulatory agencies and the criteria these regulators use to determine the safety and efficacy of a product candidate vary substantially according to the type, complexity, novelty and intended use and market of the potential products. The regulatory approval process for novel product candidates such as Rocket’s can be more expensive and take longer than for other, better known or more extensively studied pharmaceutical or other product candidates. Currently, only a few gene therapy products have received marketing authorization in the U.S. or the European Union, including Novartis’ Kymriah, Kite Pharma’s Yescarta, and Spark Therapeutics’ Luxturna. It is therefore difficult to determine how long it will take or how much it will cost to obtain regulatory approvals for Rocket’s product candidates in the United States, the European Union or other jurisdictions. Approvals by the EMA may not be indicative of what the FDA may require for approval. Delay or failure to obtain, or unexpected costs in obtaining, the regulatory approvals necessary to bring a potential product to market could decrease Rocket’s ability to generate sufficient product revenue and Rocket’s business, financial condition, results of operations and prospects could be materially harmed.
Regulatory requirements governing gene therapy products have evolved and may continue to change in the future. For example, FDA’s Center for Biologics Evaluation and Research (“CBER”) may require Rocket to perform additional nonclinical studies or clinical trials that may increase Rocket’s development costs, lead to changes in regulatory positions and interpretations, delay or prevent approval and commercialization of Rocket’s gene therapy product candidates or lead to significant post-approval limitations or restrictions.
In addition, EMA’s Committee for Advanced Therapies (“CAT”) and other regulatory review committees and advisory groups and any new guidelines they promulgate may lengthen the regulatory review process, require us to perform additional studies, increase our development costs, lead to changes in regulatory positions and interpretations, delay or prevent approval and commercialization of our product candidates or lead to significant post-approval limitations or restrictions. As we advance our product candidates, we will be required to consult with these regulatory and advisory groups, and comply with applicable guidelines. If we fail to do so, we may be required to delay or discontinue development of certain of our product candidates. These additional processes may result in a review and approval process that is longer than we otherwise would have expected. Delay or failure to obtain, or unexpected costs in obtaining, the regulatory approval necessary to bring a potential product to market could decrease our ability to generate product revenue, and our business, financial condition, results of operations and prospects would be materially harmed.
Rocket may encounter substantial delays in commencement, enrollment or completion of Rocket’s clinical trials or may fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory authorities, which could prevent Rocket from commercializing its current and future product candidates on a timely basis, if at all.
Before obtaining marketing approval from regulatory authorities for the sale of Rocket’s current and future product candidates, Rocket must conduct extensive clinical trials to demonstrate the safety and efficacy of Rocket’s product candidates. Clinical trials are expensive, time-consuming, and outcomes are uncertain.
To date, Rocket’s experience with clinical trials has been limited. Rocket’s only clinical programs to date have been performed under a physician-sponsored investigational new drug application, or IND, held by the Fred Hutchinson Cancer Research Center in Seattle, Washington, or Hutch, and under an IMPD, in Spain sponsored by CIEMAT. The clinical trials performed by these sponsors are for a lentiviral treatment for Fanconi Anemia, a rare mutation of the FANC-A gene, which are still ongoing. Rocket intends to assume responsibility for or obtain the authority to reference the clinical trials performed under one or both of the IND and IMPD held by its collaborators, but has not completed any clinical trials to date. Rocket cannot guarantee that any clinical trials will be conducted as planned or completed on schedule, if at all. A clinical trial failure can occur at any stage of testing.
Identifying and qualifying patients to participate in clinical trials of Rocket’s product candidates is critical to Rocket’s success. Rocket may not be able to identify, recruit and enroll a sufficient number of patients, or those with required or desired characteristics, to complete clinical trials in a timely manner. Patient enrollment and trial completion is affected by numerous factors including:
| • |
severity of the disease under investigation;
|
| • |
design of the study protocol;
|
| • |
size of the patient population;
|
| • |
eligibility criteria for the study in question;
|
| • |
perceived risks and benefits of the product candidate under study, including as a result of adverse effects observed in similar or competing therapies;
|
| • |
proximity and availability of clinical study sites for prospective patients;
|
| • |
availability of competing therapies and clinical studies;
|
| • |
efforts to facilitate timely enrollment in clinical studies;
|
| • |
patient referral practices of physicians; and
|
| • |
ability to monitor patients adequately during and after treatment.
|
In particular, each of the conditions for which Rocket plans to evaluate its current product candidates are rare genetic diseases with limited patient pools from which to draw for clinical studies. Additionally, the process of finding and diagnosing patients may prove costly. Finally, the treatment process requires that the cells be obtained from patients and then shipped to a transduction facility within the required timelines, and this may introduce unacceptable shipping-related delays to the process.
In addition, to the extent Rocket seeks to obtain regulatory approval for its product candidates in foreign countries, Rocket’s ability to successfully initiate, enroll and complete a clinical study in any foreign country is subject to numerous risks unique to conducting business in foreign countries, including:
| • |
difficulty in establishing or managing relationships with clinical research organizations (“CROs”), and physicians;
|
| • |
different standards for the conduct of clinical trials;
|
| • |
absence in some countries of established groups with sufficient regulatory expertise for review of AAV gene therapy protocols;
|
| • |
Rocket’s inability to locate qualified local partners or collaborators for such clinical trials; and
|
| • |
the potential burden of complying with a variety of foreign laws, medical standards and regulatory requirements, including the regulation of pharmaceutical and biotechnology products and treatment.
|
If Rocket has difficulty enrolling a sufficient number of patients to conduct its clinical trials as planned, Rocket may need to delay, limit or terminate planned clinical trials, the occurrence of any of which would harm our business, financial condition, results of operations and prospects. Moreover, Rocket intends to rely on the nonclinical studies and clinical trials performed by Hutch and CIEMAT, and the FDA or the regulatory authority in any other country in which we decide to perform clinical trials or seek approval may not accept that results of the Hutch and CIEMAT studies and trials. Any inability to successfully complete preclinical studies and clinical trials could result in additional costs to Rocket or impair Rocket’s ability to generate revenues from product sales, regulatory and commercialization milestones and royalties.
Rocket has not completed any clinical studies of its current product candidates. Initial results in Rocket’s ongoing clinical studies may not be indicative of results obtained when these studies are completed. Furthermore, success in early clinical studies may not be indicative of results obtained in later studies.
Rocket’s Fanconi Anemia gene therapy treatments are currently in clinical trials being conducted by Rocket’s partners, Hutch and CIEMAT. Several of Rocket’s other gene therapy programs are in the preclinical stages. Study designs and results from previous or ongoing studies and clinical trials are not necessarily predictive of future study or clinical trial results, and initial or interim results may not continue or be confirmed upon completion of the study or trial. Positive data may not continue or occur for subjects in Rocket’s clinical studies or for any future subjects in Rocket’s ongoing or future clinical studies and may not be repeated or observed in ongoing or future studies involving Rocket’s product candidates. Furthermore, Rocket’s product candidates may also fail to show the desired safety and efficacy in later stages of clinical development despite having successfully advanced through initial clinical studies. Rocket cannot guarantee that any of these studies will ultimately be successful or that preclinical or early stage clinical studies will support further clinical advancement or regulatory approval of Rocket’s product candidates.
Data obtained from preclinical and clinical activities are subject to varying interpretations, which may delay, limit or prevent regulatory approval. In addition, regulatory delays or rejections may be encountered as a result of many factors, including changes in regulatory policy during the period of product development.
Even if Rocket successfully completes the necessary preclinical studies and clinical trials, Rocket cannot predict when, or if, Rocket will obtain regulatory approval to commercialize a product candidate and the approval may be for a more narrow indication than Rocket seeks.
Rocket cannot commercialize a product candidate until the appropriate regulatory authorities have reviewed and approved the product candidate. Rocket has not received approval from regulatory authorities in any jurisdiction to market any of its product candidates. Even if Rocket’s product candidates meet their safety and efficacy endpoints in clinical trials, the regulatory authorities may not complete their review processes in a timely manner, issue a complete response letter, or ultimately, Rocket may not be able to obtain regulatory approval. In addition, Rocket may experience delays or rejections if an FDA Advisory Committee recommends disapproval or restrictions on use. In addition, Rocket may experience delays or rejections based upon additional government regulation from future legislation or administrative actions, or changes in regulatory authority policy during the period of product development, clinical trials and the review process. Regulatory authorities have substantial discretion in the approval process and may refuse to accept any application or may decide that Rocket’s data are insufficient for approval and require additional preclinical, clinical or other studies. In addition, varying interpretations of data obtained from preclinical and clinical testing could delay, limit or prevent the receipt of marketing approval for a product candidate.
Regulatory authorities also may approve a product candidate for more limited indications than requested or they may impose significant limitations in the form of narrow indications, warnings or Risk Evaluation and Mitigation Strategies (“REMS”). These regulatory authorities may require precautions or contra-indications with respect to conditions of use or they may grant approval subject to the performance of costly post-marketing clinical trials. In addition, regulatory authorities may not approve the labeling claims that are necessary or desirable for the successful commercialization of Rocket’s product candidates. Any of the foregoing scenarios could materially harm the commercial prospects for Rocket’s product candidates and materially harm its business, financial condition, results of operations and prospects.
Even if Rocket obtains regulatory approval for a product candidate, its products will remain subject to regulatory scrutiny.
Even if Rocket obtains regulatory approval in a jurisdiction, the applicable regulatory authority may still impose significant restrictions on the indicated uses or marketing of Rocket’s product candidates or impose ongoing requirements for potentially costly post-approval studies, post-market surveillance or patient or drug restrictions. Additionally, the holder of an approved Biologics License Application, or BLA, is obligated to monitor and report adverse events and any failure of a product to meet the specifications in the BLA. The holder of an approved BLA must also submit new or supplemental applications and obtain FDA approval for certain changes to the approved product, product labeling or manufacturing process. FDA guidance advises that patients treated with some types of gene therapy undergo follow-up observations for potential adverse events for as long as 15 years. Advertising and promotional materials must comply with FDA rules and are subject to FDA review, in addition to other potentially applicable federal and state laws.
In addition, product manufacturers and their facilities are subject to payment of user fees and continual review and periodic inspections by the FDA and other regulatory authorities for compliance with good manufacturing practices (“GMP”), current good tissue practice (“cGMP”), and adherence to commitments made in the BLA. If Rocket or a regulatory agency discovers previously unknown problems with a product such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory agency may impose restrictions relative to that product or the manufacturing facility, including requiring recall or withdrawal of the product from the market or suspension of manufacturing.
If Rocket fails to comply with applicable regulatory requirements following approval of any of its product candidates, a regulatory agency may take a variety of actions, including:
| • |
issue a warning letter asserting that Rocket is in violation of the law;
|
| • |
seek an injunction or impose civil or criminal penalties or monetary fines;
|
| • |
suspend or withdraw regulatory approval;
|
| • |
suspend any ongoing clinical studies;
|
| • |
refuse to approve a pending marketing application, such as a BLA or supplements to a BLA submitted by Rocket;
|
|
•
|
seize products; or
|
| • |
refuse to allow Rocket to enter into supply contracts, including government contracts.
|
Any government investigation of alleged violations of law could require Rocket to expend significant time and resources in response and could generate negative publicity. The occurrence of any event or penalty described above may inhibit Rocket’s ability to commercialize its product candidates and generate revenues and could harm its business, financial condition, results of operations and prospects.
In addition, the FDA’s policies, and those of comparable foreign regulatory authorities, may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of Rocket’s product candidates. Rocket cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative actions, either in the U.S. or abroad. If Rocket is slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if Rocket is not able to maintain regulatory compliance, Rocket may lose any marketing approval which Rocket may have obtained and Rocket may not achieve or sustain profitability, which would materially harm Rocket’s business, financial condition, results of operations and prospects.
Rocket may never obtain FDA approval for any of its product candidates in the United States, and even if Rocket does, Rocket may never obtain approval for or commercialize any of its product candidates in any other jurisdiction, which would limit Rocket’s ability to realize its full market potential.
In order to eventually market any of Rocket’s product candidates in any particular foreign jurisdiction, Rocket must establish and comply with numerous and varying regulatory requirements regarding safety and efficacy on a jurisdiction-by-jurisdiction basis. Approval by the FDA in the United States, if obtained, does not ensure approval by regulatory authorities in other countries or jurisdictions. In addition, preclinical studies and clinical trials conducted in one country may not be accepted by regulatory authorities in other countries, and regulatory approval in one country does not guarantee regulatory approval in any other country. Approval processes vary among countries and can involve additional product testing and validation and additional administrative review periods. Seeking foreign regulatory approval could result in difficulties and costs for Rocket and require additional preclinical studies or clinical trials which could be costly and time-consuming. Regulatory requirements can vary widely from country to country and could delay or prevent the introduction of Rocket’s products in those countries. The foreign regulatory approval process involves similar risks to those associated with FDA approval. Rocket does not have any product candidates approved for sale in any jurisdiction, including international markets, nor has Rocket attempted to obtain such approval. If Rocket fails to comply with regulatory requirements in international markets or to obtain and maintain required approvals, or if regulatory approvals in international markets are delayed, Rocket’s target market will be reduced and Rocket’s ability to realize the full market potential of its products will be unrealized.
Rocket’s product candidates may cause undesirable and unforeseen side effects or be perceived by the public as unsafe, which could delay or prevent their advancement into clinical trials or regulatory approval, limit the commercial potential or result in significant negative consequences.
Gene therapy is still a relatively new approach to disease treatment and adverse side effects could develop with Rocket’s product candidates. There also is the potential risk of delayed adverse events following exposure to gene therapy products due to persistent biologic activity of the genetic material or other components of products used to carry the genetic material.
Possible adverse side effects that could occur with treatment with gene therapy products include an immunologic reaction soon after administration which could substantially limit the effectiveness and durability of the treatment. If certain side effects are observed in testing of Rocket’s potential product candidates, Rocket may decide or be required to halt or delay further clinical development of its product candidates.
In addition to side effects caused by the product candidate, the administration process or related procedures associated with a given product candidate also can cause adverse side effects. If any such adverse events occur, Rocket’s clinical trials could be suspended or terminated. Under certain circumstances, the FDA, the European Commission, the EMA or other regulatory authorities could order Rocket to cease further development of, or deny approval of, Rocket’s product candidates for any or all targeted indications. Moreover, if Rocket elects, or is required, to not initiate or to delay, suspend or terminate any future clinical trial of any of its product candidates, the commercial prospects of such product candidates may be harmed and Rocket’s ability to generate product revenues from any of these product candidates may be delayed or eliminated. Any of these occurrences may harm Rocket’s ability to develop other product candidates, and may harm Rocket’s business, financial condition and prospects significantly.
Furthermore, if undesirable side effects caused by Rocket’s product candidate are identified following regulatory approval of a product candidate, several potentially significant negative consequences could result, including:
| • |
regulatory authorities may suspend or withdraw approvals of such product candidate;
|
| • |
regulatory authorities may require additional warnings on the label;
|
| • |
Rocket may be required to change the way a product candidate is administered or conduct additional clinical trials; and
|
| • |
Rocket’s reputation may suffer.
|
Any of these occurrences may harm Rocket’s business, financial condition and prospects significantly.
Rocket may be unable to obtain orphan drug designation or exclusivity for some product candidates. If Rocket’s competitors are able to obtain orphan drug exclusivity for products that constitute the same drug and treat the same indications as its product candidates, Rocket may not be able to have competing products approved by the applicable regulatory authority for a significant period of time.
Regulatory authorities in some jurisdictions, including the U.S. and the European Union, may designate drugs for relatively small patient populations as orphan drugs. Under the Orphan Drug Act of 1983, the FDA may designate a product candidate as an orphan drug if it is intended to treat a rare disease or condition, which is generally defined as having a patient population of fewer than 200,000 individuals in the U.S., or a patient population greater than 200,000 in the U.S. where there is no reasonable expectation that the cost of developing the drug will be recovered from sales in the U.S. In the European Union, following the opinion of the EMA’s Committee for Orphan Medicinal Products, the European Commission grants orphan drug designation to promote the development of products that are intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition affecting not more than five in 10,000 persons in the European Union. Additionally, orphan designation is granted for products intended for the diagnosis, prevention or treatment of a life-threatening, seriously debilitating or serious and chronic condition and when, without incentives, it is unlikely that sales of the drug in the European Union would be sufficient to justify the necessary investment in developing the drug or biologic product.
Generally, if a product candidate with an orphan drug designation receives the first marketing approval for the indication for which it has such designation, the product is entitled to a period of marketing exclusivity, which precludes the FDA or the European Commission from approving another marketing application for a product that constitutes the same drug treating the same indication for that marketing exclusivity period, except in limited circumstances. If another sponsor receives such approval before Rocket does (regardless of Rocket’s orphan drug designation), Rocket will be precluded from receiving marketing approval for Rocket’s product for the applicable exclusivity period. The applicable period is seven years in the U.S. and 10 years in the European Union. The exclusivity period in the U.S. can be extended by six months if the BLA sponsor submits pediatric data that fairly respond to a written request from the FDA for such data. The exclusivity period in the European Union can be reduced to six years if a product no longer meets the criteria for orphan drug designation or if the product is sufficiently profitable so that market exclusivity is no longer justified. Orphan drug exclusivity may be revoked if any regulatory agency determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the product to meet the needs of patients with the rare disease or condition.
Even if Rocket requests orphan drug designation for any of its product candidates, Rocket cannot guarantee that the FDA or the European Commission will grant any of its product candidates such designation. Additionally, the designation of any of Rocket’s product candidates as an orphan product does not guarantee that any regulatory agency will accelerate regulatory review of, or ultimately approve, that product candidate, nor does it limit the ability of any regulatory agency to grant orphan drug designation to product candidates of other companies that treat the same indications as Rocket’s product candidates prior to Rocket’s product candidates receiving exclusive marketing approval.
Even if Rocket obtains orphan drug exclusivity for a product candidate, that exclusivity may not effectively protect the product candidate from competition because different drugs can be approved for the same condition. In the U.S., even after an orphan drug is approved, the FDA may subsequently approve another drug for the same condition if the FDA concludes that the latter drug is not the same drug or is clinically superior in that it is shown to be safer, more effective or makes a major contribution to patient care. In the European Union, marketing authorization may be granted to a similar medicinal product for the same orphan indication if:
| • |
the second applicant can establish in its application that its medicinal product, although similar to the orphan medicinal product already authorized, is safer, more effective or otherwise clinically superior;
|
| • |
the holder of the marketing authorization for the original orphan medicinal product consents to a second orphan medicinal product application; or
|
| • |
the holder of the marketing authorization for the original orphan medicinal product cannot supply sufficient quantities of orphan medicinal product.
|
Risks Related to Manufacturing, Development and Commercialization of Rocket’s Product Candidates
Products intended for use in gene therapies are novel, complex and difficult to manufacture. Rocket could experience production problems that result in delays in its development or commercialization programs, limit the supply of its products or otherwise harm its business.
Rocket currently has development, manufacturing and testing agreements with third parties to manufacture supplies of its product candidates. Several factors could cause production interruptions, including equipment malfunctions, facility contamination, raw material shortages or contamination, natural disasters, disruption in utility services, human error or disruptions in the operations of suppliers.
Rocket’s product candidates require processing steps that are more complex than those required for small molecule pharmaceuticals.
Rocket may encounter problems contracting with, hiring and retaining the experienced scientific, quality control and manufacturing personnel needed to operate Rocket’s manufacturing process which could result in delays in Rocket’s production or difficulties in maintaining compliance with applicable regulatory requirements.
Any problems in Rocket’s manufacturing process or the facilities with which Rocket contracts could make Rocket a less attractive collaborator for potential partners, including larger pharmaceutical companies and academic research institutions, which could limit Rocket’s access to attractive development programs. Problems in third-party manufacturing processes or facilities also could restrict Rocket’s ability to meet market demand for Rocket’s products. Additionally, should Rocket manufacturing agreements with third parties be terminated for any reason, there may be a limited number of manufacturers who would be suitable replacements and it could take a significant amount of time to transition the manufacturing to a replacement.
Rocket may not successfully commercialize Rocket’s drug candidates.
Rocket’s gene therapy product candidates are subject to the risks of failure inherent in the development of pharmaceutical products based on new technologies, and Rocket’s failure to develop safe, commercially viable products would severely limit Rocket’s ability to become profitable or to achieve significant revenues. Rocket may be unable to successfully commercialize Rocket’s product candidates because of several reasons, including:
| • |
some or all of Rocket’s product candidates may be found to be unsafe or ineffective or otherwise fail to meet applicable regulatory standards or receive necessary regulatory clearances;
|
| • |
Rocket’s product candidates, if safe and effective, may nonetheless not be able to be developed into commercially viable products;
|
| • |
it may be difficult to manufacture or market its product candidates on a scale that is necessary to ultimately deliver its products to end-users;
|
| • |
proprietary rights of third parties may preclude Rocket from marketing its product candidates; and
|
| • |
third parties may market superior or equivalent drugs which could adversely affect the commercial viability and success of Rocket’s product candidates.
|
Rocket’s ability to successfully develop and commercialize its product candidates will substantially depend upon the availability of reimbursement funds for the costs of the resulting drugs and related treatments.
Market acceptance and sales of Rocket’s product candidates may depend on coverage and reimbursement policies and health care reform measures. Decisions about formulary coverage as well as levels at which government authorities and third-party payors, such as private health insurers and health maintenance organizations, reimburse patients for the price they pay for Rocket’s products as well as levels at which these payors pay directly for Rocket’s products, where applicable, could affect whether Rocket is able to successfully commercialize these products. Rocket cannot guarantee that reimbursement will be available for any of its product candidates. Nor can Rocket guarantee that coverage or reimbursement amounts will not reduce the demand for, or the price of, its product candidates. Rocket has not commenced efforts to have its product candidates reimbursed by government or third-party payors. If coverage and reimbursement are not available or are available only at limited levels, Rocket may not be able to successfully commercialize its products. In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or the PPACA, was signed into law, and in recent years, numerous proposals to change the health care system in the U.S. have been made. These reform proposals include measures that would limit or prohibit payments for certain medical treatments or subject the pricing of drugs to government control. In addition, in many foreign countries, particularly the countries of the European Union, the pricing of prescription drugs is subject to government control. If Rocket’s products are or become subject to government regulation that limits or prohibits payment for Rocket’s products, or that subjects the price of Rocket’s products to governmental control, Rocket may not be able to generate revenue, attain profitability or commercialize its products.
In addition, third-party payors are increasingly limiting both coverage and the level of reimbursement of new drugs. They may also impose strict prior authorization requirements and/or refuse to provide any coverage of uses of approved products for medical indications other than those for which the FDA has granted market approvals. As a result, significant uncertainty exists as to whether and how much third-party payors will reimburse patients for their use of newly-approved drugs. If Rocket is unable to obtain adequate levels of reimbursement for its product candidates, Rocket’s ability to successfully market and sell its product candidates will be harmed. The manner and level at which reimbursement is provided for services related to Rocket’s product candidates (e.g., for administration of Rocket’s product to patients) is also important to successful commercialization of its product candidates. Inadequate reimbursement for such services may lead to physician resistance and limit Rocket’s ability to market or sell its products.
Rocket faces intense competition and rapid technological change and the possibility that its competitors may develop therapies that are more advanced or effective than Rocket’s, which may adversely affect Rocket’s financial condition and its ability to successfully commercialize its product candidates.
Rocket is engaged in gene therapy for severe genetic and rare diseases, which is a competitive and rapidly changing field. Although Rocket is not currently aware of any gene therapy competitors addressing any of the same indications as those in Rocket’s pipeline, Rocket may have competitors both in the United States and internationally, including major multinational pharmaceutical companies, biotechnology companies and universities and other research institutions.
Rocket’s potential competitors may have substantially greater financial, technical and other resources, such as larger research and development staff, manufacturing capabilities, experienced marketing and manufacturing organizations. These competitors may succeed in developing, acquiring or licensing on an exclusive basis, products that are more effective or less costly than any product candidate that Rocket may develop, or achieve earlier patent protection, regulatory approval, product commercialization and market penetration than Rocket. Additionally, technologies developed by Rocket’s competitors may render its potential product candidates uneconomical or obsolete, and Rocket may not be successful in marketing Rocket’s product candidates against those of Rocket’s competitors.
In addition, as a result of the expiration or successful challenge of Rocket’s patent rights, Rocket could face increased litigation with respect to the validity and/or scope of patents relating to Rocket’s competitors’ products. The availability of Rocket’s competitors’ products could limit the demand, and the price Rocket is able to charge, for any products that Rocket may develop and commercialize, thereby causing harm to Rocket’s business, financial condition, results of operations and prospects.
Rocket may not be successful in its efforts to build a pipeline of additional product candidates.
Rocket’s business model is centered on applying its expertise in rare genetic diseases by establishing focused selection criteria to develop and advance a portfolio of gene therapy product candidates through development into commercialization. Rocket may not be able to continue to identify and develop new product candidates in addition to the pipeline of product candidates that its research and development efforts to date have resulted in. Even if Rocket is successful in continuing to build Rocket’s pipeline, the potential product candidates that Rocket identifies may not be suitable for clinical development. If Rocket does not successfully develop and commercialize product candidates based upon its approach, Rocket will not be able to obtain product revenue in future periods, which would likely result in significant harm to Rocket’s financial position and results of operations.
The success of Rocket’s research and development activities, upon which Rocket primarily focuses, is uncertain.
Rocket’s primary focus is on its research and development activities and the clinical testing and commercialization of its product candidates. Research and development was Rocket’s most significant operating expense for the year ended December 31, 2017. Research and development activities, by their nature, preclude definitive statements as to the time required and costs involved in reaching certain objectives. Actual research and development costs, therefore, could significantly exceed budgeted amounts and estimated time frames may require significant extension. Cost overruns, unanticipated regulatory delays or demands, unexpected adverse side effects or insufficient therapeutic efficacy will prevent or substantially slow Rocket’s research and development effort and Rocket’s business could ultimately suffer. Rocket anticipates that it will remain principally engaged in research and development activities for an indeterminate, but substantial, period of time.
Risks Related to Third Parties
Rocket relies on third parties to conduct its preclinical studies and clinical trials and perform other tasks for Rocket. If these third parties do not successfully carry out their contractual duties, meet expected deadlines, or comply with regulatory requirements, Rocket may not be able to obtain regulatory approval for or commercialize Rocket’s product candidates and Rocket’s business, financial condition and results of operations could be substantially harmed.
Rocket has relied upon and plans to continue to rely upon third parties, including contract research organizations, which we refer to as CROs, medical institutions, and contract laboratories to monitor and manage data for Rocket’s ongoing preclinical and clinical programs. Nevertheless, Rocket maintains responsibility for ensuring that each of Rocket’s clinical trials and preclinical studies is conducted in accordance with the applicable protocol, legal, regulatory, and scientific standards and Rocket’s reliance on these third parties does not relieve Rocket of its regulatory responsibilities. Rocket and its vendors are required to comply with current requirements on GMP, good clinical practice, or GCP, and good laboratory practice, or GLP, which are a collection of laws and regulations enforced by the FDA, EMA or comparable foreign authorities for all of Rocket’s drug candidates in clinical development.
Regulatory authorities enforce these regulations through periodic inspections of preclinical study and clinical trial sponsors, principal investigators, preclinical study and clinical trial sites, and other contractors. If Rocket or any of its vendors fails to comply with applicable regulations, the data generated in Rocket’s preclinical studies and clinical trials may be deemed unreliable and the FDA, EMA or comparable foreign authorities may require Rocket to perform additional preclinical studies and clinical trials before approving Rocket’s marketing applications. Rocket cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of Rocket’s clinical trials comply with GCP regulations. In addition, Rocket’s clinical trials must be conducted with products produced consistent with GMP regulations. Rocket’s failure to comply with these regulations may require Rocket to repeat clinical trials, which would delay the development and regulatory approval processes.
If any of Rocket’s relationships with these third parties, medical institutions, clinical investigators or contract laboratories terminate, Rocket may not be able to enter into arrangements with alternative CROs on commercially reasonable terms, or at all. In addition, Rocket’s CROs are not its employees, and except for remedies available to Rocket under its agreements with such CROs, Rocket cannot control whether or not they devote sufficient time and resources to Rocket’s ongoing preclinical and clinical programs.
If Rocket’s CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to Rocket’s protocols, regulatory requirements, or for other reasons, Rocket’s clinical trials may be extended, delayed or terminated and Rocket may not be able to obtain regulatory approval for or successfully commercialize its product candidates. CROs may also generate higher costs than anticipated. As a result, Rocket’s business, financial condition and results of operations and the commercial prospects for Rocket’s product candidates could be materially and adversely affected, Rocket’s costs could increase, and its ability to generate revenue could be delayed.
Switching or adding additional CROs, medical institutions, clinical investigators or contract laboratories involves additional cost and requires management time and focus. In addition, there is a natural transition period when a new CRO commences work replacing a previous CRO. As a result, delays occur, which can materially impact Rocket’s ability to meet its desired clinical development timelines. Though Rocket carefully manages its relationships with its CROs, Rocket cannot guarantee that Rocket will not encounter similar challenges or delays in the future or that these delays or challenges will not have a material adverse effect on its business, financial condition or results of operations.
Rocket expects to rely on third parties to conduct some or all aspects of its drug product manufacturing, research and preclinical and clinical testing, and these third parties may not perform satisfactorily.
Rocket does not expect to independently conduct all aspects of its gene therapy production, product manufacturing, research and preclinical and clinical testing. Rocket currently relies, and expects to continue to rely, on third parties with respect to these items. In some cases, these third parties are academic, research or similar institutions that may not apply the same quality control protocols utilized in certain commercial settings.
Rocket’s reliance on these third parties for research and development activities will reduce Rocket’s control over these activities but will not relieve Rocket of its responsibility to ensure compliance with all required regulations and study protocols. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct Rocket’s studies in accordance with regulatory requirements or Rocket’s stated study plans and protocols, Rocket will not be able to complete, or may be delayed in completing, the preclinical and clinical studies required to support future product submissions and approval of its product candidates.
Generally, these third parties may terminate their engagements with Rocket at will upon notice. If Rocket needs to enter into alternative arrangements, it could delay Rocket’s product development activities.
Reliance on third-party manufacturers entails risks to which Rocket would not be subject if Rocket manufactured the product candidates itself, including:
| • |
the inability to negotiate manufacturing agreements with third parties under commercially reasonable terms;
|
| • |
reduced control as a result of using third-party manufacturers for all aspects of manufacturing activities;
|
| • |
the risk that these activities are not conducted in accordance with Rocket’s study plans and protocols;
|
| • |
termination or nonrenewal of manufacturing agreements with third parties in a manner or at a time that is costly or damaging to Rocket; and
|
| • |
disruptions to the operations of its third-party manufacturers or suppliers caused by conditions unrelated to its business or operations, including the bankruptcy of the manufacturer or supplier.
|
Any of these events could lead to clinical study delays or failure to obtain regulatory approval, or impact Rocket’s ability to successfully commercialize future products. Some of these events could be the basis for FDA action, including an injunction, recall, seizure or total or partial suspension of production.
Rocket may not be successful in finding strategic collaborators for continuing development of certain of its product candidates or successfully commercializing its product candidates.
Rocket may seek to establish strategic partnerships for developing and/or commercializing certain of Rocket’s product candidates due to relatively high capital costs required to develop the product candidates, manufacturing constraints or other reasons. Rocket may not be successful in its efforts to establish such strategic partnerships or other alternative arrangements for its product candidates for several reasons, including because its research and development pipeline may be insufficient, Rocket’s product candidates may be deemed to be at too early of a stage of development for collaborative effort or third parties may not view Rocket’s product candidates as having the requisite potential to demonstrate efficacy or market opportunity. In addition, Rocket may be restricted under existing agreements from entering into future agreements with potential collaborators.
If Rocket is unable to reach agreements with suitable licensees or collaborators on a timely basis, on acceptable terms or at all, Rocket may have to curtail the development of a product candidate, reduce or delay its development program, delay its potential commercialization, reduce the scope of any sales or marketing activities or increase Rocket’s expenditures and undertake development or commercialization activities at its own expense. If Rocket elects to independently fund development or commercialization activities, Rocket may need to obtain additional expertise and additional capital, which may not be available on acceptable terms or at all. If Rocket fails to enter into collaboration arrangements and does not have sufficient funds or expertise to undertake necessary development and commercialization activities, Rocket may not be able to further develop its product candidates and Rocket’s business, financial condition, results of operations and prospects may be materially harmed.
The commercial success of any of Rocket’s product candidates will depend upon its degree of market acceptance by physicians, patients, third-party payors and others in the medical community.
Ethical, social, legal and other concerns about gene therapy could result in additional regulations restricting or prohibiting Rocket’s products. Even with the requisite approvals from the FDA in the United States, the EMA in the European Union and other regulatory authorities internationally, the commercial success of Rocket’s product candidates will depend, in part, on the acceptance of physicians, patients and health care payors of gene therapy products in general, and Rocket’s product candidates in particular, as medically beneficial, cost-effective and safe. Any product that Rocket commercializes may not gain acceptance by physicians, patients, health care payors and others in the medical community. If these products do not achieve an adequate level of acceptance, Rocket may not generate significant product revenue and may not become profitable. The degree of market acceptance of gene therapy products and, in particular, Rocket’s product candidates, if approved for commercial sale, will depend on several factors, including:
| • |
the efficacy and safety of such product candidates as demonstrated in preclinical studies and clinical trials;
|
| • |
the potential and perceived advantages of product candidates over alternative treatments;
|
| • |
the cost of Rocket’s treatment relative to alternative treatments;
|
| • |
the clinical indications for which the product candidate is approved by the FDA or the European Commission;
|
| • |
patient awareness of, and willingness to seek, gene therapy;
|
| • |
the willingness of physicians to prescribe new therapies;
|
| • |
the willingness of physicians to undergo specialized training with respect to administration of Rocket’s product candidates;
|
| • |
the willingness of the target patient population to try new therapies;
|
| • |
the prevalence and severity of any side effects;
|
| • |
product labeling or product insert requirements of the FDA, EMA or other regulatory authorities, including any limitations or warnings contained in a product’s approved labeling;
|
| • |
relative convenience and ease of administration;
|
| • |
the strength of marketing and distribution support;
|
| • |
the timing of market introduction of competitive products;
|
| • |
publicity concerning Rocket’s products or competing products and treatments; and
|
| • |
sufficient third-party payor coverage and reimbursement.
|
Even if a potential product displays a favorable efficacy and safety profile in preclinical studies and clinical trials, market acceptance of the product will not be fully known until after it is approved and launched. The failure of any of Rocket’s product candidates to achieve market acceptance could materially harm Rocket’s business, financial condition, results of operations and prospects.
RTW Investments, LP, Rocket’s principal stockholder, may have the ability to significantly influence all matters submitted to stockholders for approval.
RTW Investments, LP (“RTW”), in the aggregate, beneficially owns approximately 39.15% of Rocket’s outstanding shares of common stock. This concentration of voting power gives RTW the power to significantly influence all matters submitted to our stockholders for approval, as well as our management and affairs. For example, RTW could significantly influence the election of directors and approval of any merger, consolidation or sale of all or substantially all of our assets.
Risks Related to Personnel and Other Risks Related to Rocket’s Business
Rocket’s business could suffer if it loses the services of, or fails to attract, key personnel.
Rocket is highly dependent upon the efforts of the company’s senior management, including Rocket’s Chief Executive Officer, Gaurav Shah, MD; Rocket’s Chief Medical Officer and Head of Clinical Development, Jonathan Schwartz, MD; and Rocket’s Chief Operating Officer and Head of Development, Kinnari Patel. The loss of the services of these individuals and other members of Rocket’s senior management could delay or prevent the achievement of research, development, marketing, or product commercialization objectives. Rocket’s employment arrangements with the key personnel are “at-will.” Rocket does not maintain any “key-man” insurance policies on any of the key employees nor does Rocket intend to obtain such insurance. In addition, due to the specialized scientific nature of Rocket’s business, Rocket is highly dependent upon its ability to attract and retain qualified scientific and technical personnel and consultants. In view of the stage of Rocket’s organizational development and research and development programs, Rocket has restricted its hiring to research scientists, consultants and a small administrative staff and has made only limited investments in manufacturing, production, sales or regulatory compliance resources. There is intense competition among major pharmaceutical and chemical companies, specialized biotechnology firms and universities and other research institutions for qualified personnel in the areas of Rocket’s operations, however, and Rocket may be unsuccessful in attracting and retaining these personnel.
Rocket may need to expand its organization and may experience difficulties in managing this growth, which could disrupt its operations.
As of March 1, 2018, Rocket had 20 full-time employees. As Rocket’s business activities expand, Rocket may expand its full-time employee base and hire more consultants and contractors. Rocket’s management may need to divert a disproportionate amount of its attention away from day-to-day activities and devote a substantial amount of time to managing these growth activities. Rocket may not be able to effectively manage the expansion of its operations, which may result in weaknesses in Rocket’s infrastructure, operational setbacks, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Rocket’s expected growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of additional product candidates. If Rocket’s management is unable to effectively manage Rocket’s growth, Rocket’s expenses may increase more than expected, Rocket’s ability to generate and/or grow revenues could be reduced and Rocket may not be able to implement its business strategy.
Rocket’s employees, principal investigators, consultants and commercial partners may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements and insider trading.
Rocket is exposed to the risk of fraud or other misconduct by its employees, consultants and commercial partners. Misconduct by these parties could include intentional failures to comply with the regulations of the FDA and non-U.S. regulators, provide accurate information to the FDA and non-U.S. regulators, comply with healthcare fraud and abuse laws and regulations in the United States and abroad, report financial information or data accurately or disclose unauthorized activities to Rocket. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Such misconduct could also involve the improper use of information obtained in the course of clinical studies, which could result in regulatory sanctions and cause serious harm to Rocket’s reputation or could cause regulatory agencies not to approve Rocket’s product candidates. Rocket has a code of business ethics and conduct applicable to all employees, but it is not always possible to identify and deter employee or third-party misconduct, and the precautions Rocket takes to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting Rocket from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against Rocket, and Rocket is not successful in defending the company or asserting its rights, those actions could have a significant impact on Rocket’s business, including the imposition of significant fines or other sanctions.
Rocket’s internal computer systems, or those of its third-party collaborators or other contractors, may fail or suffer security breaches, which could result in a material disruption of Rocket’s development programs.
Rocket’s internal computer systems and those of its current and any future collaborators and other consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. While Rocket has not experienced any such material system failure, accident or security breach to date, if such an event were to occur and cause interruptions in Rocket’s operations, it could result in a material disruption of Rocket’s development programs and its business operations, whether due to a loss of its trade secrets or other proprietary information or other similar disruptions. For example, the loss of clinical trial data from completed or future clinical trials could result in delays in Rocket’s regulatory approval efforts and significantly increase Rocket’s costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of, or damage to, Rocket’s data or applications, or inappropriate disclosure of confidential or proprietary information, Rocket could incur liability, its competitive position could be harmed and the further development and commercialization of Rocket’s product candidates could be delayed.
Rocket may be subject to claims that its employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties or that Rocket’s employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Rocket employs individuals who were previously employed at universities or other biotechnology or pharmaceutical companies, including its competitors or potential competitors. Although Rocket endeavors to ensure that its employees, consultants and independent contractors do not use the proprietary information or know-how of others in their work for Rocket, Rocket may be subject to claims that Rocket or its employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of any of Rocket’s employee’s former employer or other third parties. Litigation may be necessary to defend against these claims. If Rocket fails in defending any such claims, in addition to paying monetary damages, Rocket may lose valuable intellectual property rights or personnel, which could adversely impact Rocket’s business. Even if Rocket is successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Given Rocket’s commercial relationships outside of the United States, in particular in the European Union, a variety of risks associated with international operations could harm its business.
Rocket engages in various commercial relationships outside the United States and Rocket may commercialize its product candidates outside of the United States. In many foreign countries, it is common for others to engage in business practices that are prohibited by U.S. laws and regulations applicable to Rocket, including the Foreign Corrupt Practices Act. Although Rocket may implement policies and procedures specifically designed to comply with these laws and policies, there can be no assurance that Rocket’s employees, contractors and agents will comply with these laws and policies. If Rocket is unable to successfully manage the challenges of international expansion and operations, Rocket’s business and operating results could be harmed.
Rocket may be, and expect that it will be to the extent Rocket commercializes its product candidates outside the United States, subject to various risks associated with operating internationally, including:
| • |
different regulatory requirements for approval of drugs and biologics in foreign countries;
|
| • |
reduced protection for intellectual property rights;
|
| • |
unexpected changes in tariffs, trade barriers and regulatory requirements;
|
| • |
economic weakness, including inflation, or political instability in particular foreign economies and markets;
|
| • |
compliance with tax, employment, immigration and labor laws for employees living or traveling abroad;
|
| • |
foreign currency fluctuations, which could result in increased operating expenses and reduced revenues, and other obligations incident to doing business in another country;
|
| • |
workforce uncertainty in countries where labor unrest is more common than in the United States;
|
| • |
shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad;
|
| • |
business interruptions resulting from geopolitical actions, including war and terrorism or natural disasters including earthquakes, typhoons, floods and fires, or from economic or political instability; and
|
| • |
greater difficulty with enforcing Rocket’s contracts in jurisdictions outside of the United States.
|
These and related risks could materially harm Rocket’s business, financial condition, results of operations and prospects.
Risks Related to Rocket’s Intellectual Property
Rocket’s rights to intellectual property for the development and commercialization of its product candidates are subject to the terms and conditions of licenses granted to Rocket by others.
Rocket is heavily reliant upon licenses to certain patent rights and proprietary technology from third parties that are important or necessary to the development of its technology and products, including technology related to Rocket’s manufacturing process and Rocket’s gene therapy product candidates. These and other licenses may not provide exclusive rights to use such intellectual property and technology in all relevant fields of use and in all territories in which Rocket may wish to license its platform or develop or commercialize its technology and products in the future. As a result, Rocket may not be able to prevent competitors from developing and commercializing competitive products in territories not included in all of its licenses.
Licenses to additional third-party technology that may be required for Rocket’s licensing or development programs may not be available in the future or may not be available on commercially reasonable terms, or at all, which could materially harm Rocket’s business and financial condition.
In some circumstances, Rocket may not have the right to control the preparation, filing and prosecution of patent applications, or to maintain or enforce the patents, covering technology that Rocket licenses from third parties. If Rocket’s licensors fail to maintain such patents, or lose rights to those patents or patent applications, the rights Rocket has licensed may be reduced or eliminated and Rocket’s right to develop and commercialize any of its products that are the subject of such licensed rights could be impacted. In addition to the foregoing, the risks associated with patent rights that Rocket licenses from third parties will also apply to patent rights Rocket may own in the future.
Furthermore, the research resulting in certain of Rocket’s licensed patent rights and technology was funded by the U.S. government. As a result, the government may have certain rights, or march-in rights, to such patent rights and technology. When new technologies are developed with government funding, the government generally obtains certain rights in any resulting patents, including a non-exclusive license authorizing the government to use the invention for non-commercial purposes. These rights may permit the government to disclose Rocket’s confidential information to third parties and to exercise march-in rights to use or allow third parties to use Rocket’s licensed technology. The government can exercise its march-in rights if it determines that action is necessary because Rocket fails to achieve practical application of the government-funded technology, because action is necessary to alleviate health or safety needs, to meet requirements of federal regulations or to give preference to U.S. industry. In addition, Rocket’s rights in such inventions may be subject to certain requirements to manufacture products embodying such inventions in the U.S. Any exercise by the government of such rights could harm Rocket’s competitive position, business, financial condition, results of operations and prospects.
If Rocket is unable to obtain and maintain patent protection for is products and related technology, or if the scope of the patent protection obtained is not sufficiently broad, Rocket’s competitors could develop and commercialize products and technology similar or identical to Rocket’s, and Rocket’s ability to successfully commercialize its products may be harmed.
Rocket’s success depends, in large part, on its ability to obtain and maintain patent protection in the U.S. and other countries with respect to its product candidates and its manufacturing technology. Rocket’s licensors have sought and Rocket may intend to seek, to protect their respective proprietary position by filing patent applications in the U.S. and abroad related to many of their novel technologies and product candidates that are important to Rocket’s business.
The patent prosecution process is expensive, time-consuming and complex, and Rocket may not be able to file, prosecute, maintain, enforce or license all necessary or desirable patent applications at a reasonable cost or in a timely manner. In addition, certain patents in the field of gene therapy that may have otherwise potentially provided patent protection for certain of Rocket’s product candidates have expired or will soon expire. In some cases, the work of certain academic researchers in the gene therapy field has entered the public domain, which Rocket believes precludes its ability to obtain patent protection for certain inventions relating to such work. It is also possible that Rocket will fail to identify patentable aspects of its research and development output before it is too late to obtain patent protection.
Rocket is party to intellectual property license agreements with several entities, each of which is important to its business, and Rocket expects to enter into additional license agreements in the future. Rocket’s patent portfolio consists solely of patent applications in-licensed pursuant to those license agreements, and those agreements impose, and Rocket expects that future license agreements will impose, various diligence, development and commercialization timelines, milestone obligations, payments and other obligations on Rocket. If Rocket or its licensees fail to comply with Rocket’s obligations under these agreements, or Rocket is subject to a bankruptcy, the licensor may have the right to terminate the license, in which event Rocket could lose certain rights provided by the licenses, including that Rocket may not be able to market products covered by the license. In addition, the patent rights that we have in-licensed from Hutch relate only to Hutch’s “Prodigy” platform, a portable platform for hematopoietic stem/progenitor cell gene therapy, and not to RP-L101, Rocket’s LVV-based program targeting FA that is in-licensed from Hutch.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has, in recent years, been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of Rocket’s patent rights are highly uncertain. Pending and future patent applications may not result in patents being issued which protect Rocket’s technology or product candidates or which effectively prevent others from commercializing competitive technologies and product candidates. Changes in either the patent laws or interpretation of the patent laws in the U.S. and other countries may diminish the value of Rocket’s patent rights or narrow the scope of Rocket’s patent protection.
While we believe our intellectual property allows us to pursue our current development programs, several companies and academic institutions are pursuing alternate approaches to gene therapy and have built intellectual property around these approaches and methods. For example, Institute Pasteur controls a patent family related to vector elements for lentiviral-based gene therapy. These patents relate to an element that improves nuclear localization. While these patents expire from 2019 to 2023, if our products were to launch before these dates, we may need to secure a license. In addition, Rocket may not be aware of all third-party intellectual property rights potentially relating to its technology and product candidates. Publications of discoveries in the scientific literature often lag the actual discoveries, and patent applications in the U.S. and other jurisdictions are typically not published until 18 months after filing or, in some cases, not at all. Therefore, Rocket cannot be certain that Rocket was the first to make the inventions claimed in any owned or any licensed patents or pending patent applications, or that Rocket was the first to file for patent protection of such inventions.
Even if the patent applications Rocket licenses or may own in the future do issue as patents, they may not issue in a form that will provide Rocket with any meaningful protection, prevent competitors or other third parties from competing with Rocket or otherwise provide Rocket with any competitive advantage. Rocket’s competitors or other third parties may avail themselves of safe harbor under the Drug Price Competition and Patent Term Restoration Act of 1984 (Hatch-Waxman Amendments) to conduct research and clinical trials and may be able to circumvent Rocket’s patent rights by developing similar or alternative technologies or products in a non-infringing manner.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and Rocket’s patent rights may be challenged in the courts or patent offices in the U.S. and abroad. Such challenges may result in loss of exclusivity or in patent claims being narrowed, invalidated or held unenforceable, which could limit Rocket’s ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of its technology and product candidates. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, Rocket’s intellectual property may not provide sufficient rights to exclude others from commercializing products similar or identical to Rocket’s.
If Rocket breaches its license agreements, it could have a material adverse effect on Rocket’s commercialization efforts for its product candidates.
If Rocket breaches any of the agreements under which Rocket licenses intellectual property relating to the use, development and commercialization rights to its product candidates or technology from third parties, Rocket could lose license rights that are important to its business. Licensing of intellectual property is of critical importance to Rocket’s business and involves complex legal, business and scientific issues. Disputes may arise between Rocket and its licensors regarding intellectual property subject to a license agreement, including:
| • |
the scope of rights granted under the license agreement;
|
| • |
whether and the extent to which Rocket technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement;
|
| • |
Rocket’s right to sublicense patent and other intellectual property rights to third parties under collaborative development relationships;
|
| • |
Rocket’s diligence obligations with respect to the use of the licensed technology in relation to its development and commercialization of its product candidates, and what activities satisfy those diligence obligations;
|
| • |
the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by Rocket’s licensors and Rocket and its partners; and
|
| • |
whether and the extent to which inventors are able to contest to the assignment of their rights to Rocket’s licensors.
|
If disputes over intellectual property that Rocket has in-licensed prevent or impair Rocket’s ability to maintain its current licensing arrangements on acceptable terms, Rocket may be unable to successfully develop and commercialize the affected product candidates. In addition, if disputes arise as to ownership of licensed intellectual property, Rocket’s ability to pursue or enforce the licensed patent rights may be jeopardized. If Rocket or its licensors fail to adequately protect this intellectual property, Rocket’s ability to commercialize its products could suffer.
Rocket may incur substantial costs as a result of litigation or other proceedings relating to patent and other intellectual property rights and Rocket may be unable to protect its rights to, or use, its technology.
If Rocket chooses to engage in legal action to prevent a third-party from using the inventions claimed in its patents or patents which Rocket licenses, that third-party has the right to ask the court to rule that these patents are invalid and/or should not be enforced against that third-party. These lawsuits are expensive and would consume time and other resources even if Rocket were successful in stopping the infringement of these patents. In addition, there is a risk that the court will decide that these patents are not valid and that Rocket does not have the right to stop the other party from using the inventions. There is also the risk that, even if the validity of these patents is upheld, the court will refuse to stop the other party on the ground that such other party’s activities do not infringe Rocket’s rights to these patents.
Furthermore, a third-party may claim that Rocket is using inventions covered by the third-party’s patent rights and may go to court to stop Rocket from engaging in its normal operations and activities, including making or selling its product candidates. These lawsuits are costly and could affect Rocket’s results of operations and divert the attention of managerial and technical personnel. There is a risk that a court would decide that Rocket is infringing the third-party’s patents and would order Rocket to stop the activities covered by the patents. In addition, there is a risk that a court will order Rocket to pay the other party damages for having violated the other party’s patents. The biotechnology industry has produced a proliferation of patents, and it is not always clear to industry participants which patents cover various types of products or methods of use. The coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform. If Rocket is sued for patent infringement, Rocket would need to demonstrate that its products or methods of use either do not infringe the patent claims of the relevant patent and/or that the patent claims are invalid. Proving invalidity, in particular, is difficult since it requires a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents. Rocket’s competitors have filed, and may in the future file, patent applications covering technology similar to Rocket’s. Any such patent application may have priority over Rocket’s in-licensed patent applications and could further require Rocket to obtain rights to issued patents covering such technologies. If another party has filed a U.S. patent application on inventions similar to Rocket’s, Rocket may have to participate in an interference proceeding declared by the U.S. Patent and Trademark Office, to determine priority of invention in the U.S. The costs of these proceedings could be substantial, and it is possible that such efforts would be unsuccessful, resulting in a loss of Rocket’s United States patent position with respect to such inventions.
Some of Rocket’s competitors may be able to sustain the costs of complex patent litigation more effectively than Rocket can because they have substantially greater resources. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on Rocket’s ability to raise the funds necessary to continue its operations.
If Rocket is unable to protect the confidentiality of its trade secrets, its business and competitive position may be harmed.
In addition to the protection afforded by patents, Rocket relies upon unpatented trade secret protection, unpatented know-how and continuing technological innovation to develop and maintain its competitive position. Rocket seeks to protect its proprietary technology and processes, in part, by entering into confidentiality agreements with its contractors, collaborators, employees and consultants. Nonetheless, Rocket may not be able to prevent the unauthorized disclosure or use of its technical know-how or other trade secrets by the parties to these agreements, however, despite the existence generally of confidentiality agreements and other contractual restrictions. Monitoring unauthorized uses and disclosures is difficult and Rocket does not know whether the steps Rocket has taken to protect its proprietary technologies will be effective. If any of the contractors, collaborators, employees and consultants who are parties to these agreements breaches or violates the terms of any of these agreements, Rocket may not have adequate remedies for any such breach or violation. As a result, Rocket could lose its trade secrets. Enforcing a claim that a third-party illegally obtained and is using its trade secrets, like patent litigation, is expensive and time consuming and the outcome is unpredictable. In addition, courts outside the United States are sometimes less willing or unwilling to protect trade secrets.
Rocket’s trade secrets could otherwise become known or be independently discovered by Rocket’s competitors. Competitors could purchase Rocket’s product candidates and attempt to replicate some or all of the competitive advantages Rocket derives from its development efforts, willfully infringe Rocket’s intellectual property rights, design around Rocket’s protected technology or develop their own competitive technologies that fall outside of Rocket’s intellectual property rights. If any of Rocket’s trade secrets were to be lawfully obtained or independently developed by a competitor, Rocket would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with Rocket. If Rocket’s trade secrets are not adequately protected or sufficient to provide an advantage over Rocket’s competitors, Rocket’s competitive position could be adversely affected, as could Rocket’s business. Additionally, if the steps taken to maintain Rocket’s trade secrets are deemed inadequate, Rocket may have insufficient recourse against third parties for misappropriating Rocket’s trade secrets.
If Rocket is unable to obtain or protect intellectual property rights related to its product candidates, Rocket may not be able to compete effectively in its markets.
Rocket relies upon a combination of patents, trade secret protection and confidentiality agreements to protect the intellectual property related to its product candidates. The strength of patents in the biotechnology and pharmaceutical field involves complex legal and scientific questions and can be uncertain. The patent applications that Rocket owns or in-licenses may fail to result in issued patents with claims that cover its product candidates in the United States or in other foreign countries. There is no assurance that all of the potentially relevant prior art relating to patents and patent applications owned or in-licensed by Rocket has been found, which can invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue and even if such patents cover Rocket’s product candidates, third parties may challenge their validity, enforceability or scope, which may result in such patents being narrowed or invalidated. Furthermore, even if they are unchallenged, patents and patent applications owned or in-licensed by Rocket may not adequately protect Rocket’s intellectual property, provide exclusivity for Rocket’s product candidates or prevent others from designing around Rocket’s claims. Any of these outcomes could impair Rocket’s ability to prevent competition from third parties, which may have an adverse impact on Rocket’s business.
If the patent applications Rocket holds or has in-licensed with respect to its programs or product candidates fail to issue, if their breadth or strength of protection is threatened, or if they fail to provide meaningful exclusivity for Rocket’s product candidates, it could dissuade companies from collaborating with it to develop product candidates, and threaten Rocket’s ability to commercialize, future products. In addition to Rocket’s existing patent application filings, Rocket expects to continue to file additional patent applications covering Rocket’s product candidates. Further, Rocket intends to pursue additional activities to protect the patents, trade secrets and other intellectual property covering its product candidates. Rocket cannot offer any assurances about which, if any, patents will issue, the breadth of any such patent or whether any issued patents will be found invalid and unenforceable or will be threatened by third parties. Any successful opposition to these patents or any other patents owned by or licensed to us could deprive Rocket of rights necessary for the successful commercialization of any product candidates that Rocket may develop. Further, if Rocket or the relevant licensor encounters delays in regulatory approvals, the period of time during which Rocket could market a product candidate under patent protection could be reduced. Since patent applications in the United States and most other countries are confidential for a period of time after filing, and some remain so until issued, Rocket cannot be certain that Rocket or the relevant licensor was the first to file any patent application related to a product candidate. Furthermore, if third parties have filed such patent applications, an interference proceeding in the United States can be initiated by a third-party to determine who was the first to invent any of the subject matter covered by the patent claims of Rocket’s applications. In addition, patents have a limited lifespan. In the United States, the natural expiration of a patent is generally 20 years after it is filed. Various extensions may be available however the life of a patent, and the protection it affords, is limited. Even if patents covering Rocket’s product candidates are obtained, once the patent life has expired for a product, Rocket may be open to competition from generic medications.
In addition to the protection afforded by patents, Rocket relies on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or that Rocket elects not to patent, processes for which patents are difficult to enforce and any other elements of Rocket’s product candidate discovery and development processes that involve proprietary know-how, information or technology that is not covered by patents. However, trade secrets can be difficult to protect. Rocket seeks to protect its proprietary technology and processes, in part, by entering into confidentiality agreements with its employees, consultants, scientific advisors and contractors. Rocket also seeks to preserve the integrity and confidentiality of its data and trade secrets by maintaining physical security of its premises and physical and electronic security of its information technology systems. While Rocket has confidence in these individuals, organizations and systems, agreements or security measures may be breached, and Rocket may not have adequate remedies for any breach. In addition, Rocket’s trade secrets may otherwise become known or be independently discovered by competitors.
Although Rocket expects all of its employees and consultants to assign their inventions to Rocket, and all of Rocket’s employees, consultants, advisors and any third parties who have access to its proprietary know-how, information or technology to enter into confidentiality agreements, Rocket cannot provide any assurances that all such agreements have been duly executed or that its trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to its trade secrets or independently develop substantially equivalent information and techniques. Misappropriation or unauthorized disclosure of Rocket’s trade secrets could impair its competitive position and may have a material adverse effect on its business. Additionally, if the steps taken to maintain Rocket’s trade secrets are deemed inadequate, Rocket may have insufficient recourse against third parties for misappropriating its trade secret. In addition, others may independently discover Rocket’s trade secrets and proprietary information. For example, the FDA, as part of its Transparency Initiative, is currently considering whether to make additional information publicly available on a routine basis, including information that Rocket may consider to be trade secrets or other proprietary information, and it is not clear at the present time how the FDA’s disclosure policies may change in the future, if at all.
Further, the laws of some foreign countries do not protect proprietary rights to the same extent or in the same manner as the laws of the United States. As a result, Rocket may encounter significant problems in protecting and defending its intellectual property, both in the United States and abroad. If Rocket is unable to prevent material disclosure of the non-patented intellectual property related to its technologies to third parties, and there is no guarantee that Rocket will have any such enforceable trade secret protection, it may not be able to establish or maintain a competitive advantage in its market, which could materially adversely affect its business, results of operations and financial condition.
Third-party claims of intellectual property infringement may prevent or delay Rocket’s development and commercialization efforts.
Rocket’s commercial success depends in part on its avoiding infringement of the patents and proprietary rights of third parties. There is a substantial amount of litigation, both within and outside the United States, involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries, including patent infringement lawsuits, interferences, oppositions, ex parte reexaminations, post-grant review, and inter partes review proceedings before the U.S. Patent and Trademark Office, or U.S. PTO, and corresponding foreign patent offices. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which Rocket is pursuing development candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that Rocket’s product candidates may be subject to claims of infringement of the patent rights of third parties.
Third parties may assert that Rocket is employing their proprietary technology without authorization. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of Rocket’s product candidates. Because patent applications can take many years to issue, there may be currently pending patent applications which may later result in issued patents that Rocket’s product candidates may infringe. In addition, third parties may obtain patents in the future and claim that use of Rocket’s technologies infringes upon these patents. If any third-party patents were held by a court of competent jurisdiction to cover the manufacturing process of any of Rocket’s product candidates, any molecules formed during the manufacturing process or any final product itself, the holders of any such patents may be able to block Rocket’s ability to commercialize such product candidate unless Rocket obtained a license under the applicable patents, or until such patents expire. Similarly, if any third-party patents were held by a court of competent jurisdiction to cover aspects of Rocket’s formulations, processes for manufacture or methods of use, including combination therapy, the holders of any such patents may be able to block Rocket’s ability to develop and commercialize the applicable product candidate unless Rocket obtained a license or until such patent expires. In either case, such a license may not be available on commercially reasonable terms or at all.
Parties making claims against Rocket may obtain injunctive or other equitable relief, which could effectively block Rocket’s ability to further develop and commercialize one or more of its product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from Rocket’s business. In the event of a successful claim of infringement against Rocket, Rocket may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, pay royalties, redesign Rocket’s infringing products or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure.
Rocket may not be successful in obtaining or maintaining necessary rights to gene therapy product components and processes for its development pipeline through acquisitions and in-licenses.
Presently Rocket has rights to the intellectual property, through licenses from third parties and under patents that Rocket owns, to develop its gene therapy product candidates. Because Rocket’s programs may involve additional product candidates that may require the use of proprietary rights held by third parties, the growth of Rocket’s business will likely depend in part on its ability to acquire, in-license or use these proprietary rights. In addition, Rocket’s product candidates may require specific formulations to work effectively and efficiently and these rights may be held by others. Rocket may be unable to acquire or in-license any compositions, methods of use, processes or other third-party intellectual property rights from third parties that Rocket identifies. The licensing and acquisition of third-party intellectual property rights is a competitive area, and a number of more established companies are also pursuing strategies to license or acquire third-party intellectual property rights that Rocket may consider attractive. These established companies may have a competitive advantage over Rocket due to their size, cash resources and greater clinical development and commercialization capabilities.
For example, Rocket sometimes collaborates with U.S. and foreign academic institutions to accelerate its preclinical research or development under written agreements with these institutions. Typically, these institutions provide Rocket with an option to negotiate a license to any of the institution’s rights in technology resulting from the collaboration. Regardless of such right of first negotiation for intellectual property, Rocket may be unable to negotiate a license within the specified time frame or under terms that are acceptable to it. If Rocket is unable to do so, the institution may offer the intellectual property rights to other parties, potentially blocking Rocket’s ability to pursue its program.
In addition, companies that perceive Rocket to be a competitor may be unwilling to assign or license rights to it. Rocket also may be unable to license or acquire third-party intellectual property rights on terms that would allow it to make an appropriate return on its investment. If Rocket is unable to successfully obtain rights to required third-party intellectual property rights, Rocket’s business, financial condition and prospects for growth could suffer.
If Rocket fails to comply with its obligations in the agreements under which Rocket licenses intellectual property rights from third parties or otherwise experiences disruptions to Rocket’s business relationships with its licensors, Rocket could lose license rights that are important to its business.
Rocket is a party to a number of intellectual property license agreements that are important to its business and expect to enter into additional license agreements in the future. Rocket’s existing license agreements impose, and Rocket expects that future license agreements will impose, various diligence, milestone payment, royalty and other obligations on Rocket. If Rocket fails to comply with its obligations under these agreements, or Rocket is subject to a bankruptcy, the licensor may have the right to terminate the license, in which event Rocket would not be able to market products covered by the license.
Rocket may need to obtain licenses from third parties to advance its research or allow commercialization of its product candidates, and it has done so from time to time. Rocket may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. In that event, Rocket may be required to expend significant time and resources to develop or license replacement technology. If Rocket is unable to do so, it may be unable to develop or commercialize the affected product candidates, which could harm its business significantly. Rocket cannot provide any assurances that third-party patents do not exist which might be enforced against its current product candidates or future products, resulting in either an injunction prohibiting its sales, or, with respect to its sales, an obligation on Rocket’s part to pay royalties and/or other forms of compensation to third parties.
In many cases, patent prosecution of Rocket’s licensed technology is controlled solely by the licensor. If Rocket’s licensors fail to obtain and maintain patent or other protection for the proprietary intellectual property Rocket licenses from them, Rocket could lose its rights to the intellectual property or its exclusivity with respect to those rights, and its competitors could market competing products using the intellectual property. In certain cases, Rocket controls the prosecution of patents resulting from licensed technology. In the event Rocket breaches any of its obligations related to such prosecution, Rocket may incur significant liability to its licensing partners. Licensing of intellectual property is of critical importance to Rocket’s business and involves complex legal, business and scientific issues and is complicated by the rapid pace of scientific discovery in Rocket’s industry. Disputes may arise regarding intellectual property subject to a licensing agreement, including:
| • |
the scope of rights granted under the license agreement and other interpretation-related issues;
|
| • |
the extent to which Rocket’s technology and processes infringe intellectual property of the licensor that is not subject to the licensing agreement;
|
| • |
the sublicensing of patent and other rights under Rocket’s collaborative development relationships;
|
| • |
Rocket’s diligence obligations under the license agreement and what activities satisfy those diligence obligations;
|
| • |
the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by Rocket’s licensors and Rocket and Rocket’s partners; and
|
| • |
the priority of invention of patented technology.
|
If disputes over intellectual property that Rocket has licensed prevent or impair Rocket’s ability to maintain its current licensing arrangements on acceptable terms, Rocket may be unable to successfully develop and commercialize the affected product candidates.
Rocket may be involved in lawsuits to protect or enforce its patents or the patents of its licensors, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe Rocket’s patents or the patents of Rocket’s licensors. To counter infringement or unauthorized use, Rocket may be required to file infringement claims, which can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that a patent of Rocket’s or Rocket’s licensors is not valid, is unenforceable and/or is not infringed, or may refuse to stop the other party from using the technology at issue on the grounds that Rocket’s patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put one or more of Rocket’s patents at risk of being invalidated or interpreted narrowly and could put Rocket’s patent applications at risk of not issuing.
Interference proceedings provoked by third parties or brought by Rocket may be necessary to determine the priority of inventions with respect to Rocket’s patents or patent applications or those of Rocket’s licensors. An unfavorable outcome could require Rocket to cease using the related technology or to attempt to license rights to it from the prevailing party. Rocket’s business could be harmed if the prevailing party does not offer it a license on commercially reasonable terms. Rocket’s defense of litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract Rocket’s management and other employees. Rocket may not be able to prevent, alone or with its licensors, misappropriation of its intellectual property rights, particularly in countries where the laws may not protect those rights as fully as in the United States.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of Rocket’s confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of Rocket’s common stock.
Patent reform legislation could increase the uncertainties and costs surrounding the prosecution of Rocket’s patent applications and the enforcement or defense of Rocket’s issued patents.
On September 16, 2011, the Leahy-Smith America Invents Act, (the “Leahy-Smith Act”), was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law, including provisions that affect the way patent applications will be prosecuted and may also affect patent litigation. The U.S. PTO is currently developing regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the “first to file” provisions, which were enacted on March 16, 2013. However, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of Rocket’s business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of Rocket’s patent applications and the enforcement or defense of Rocket’s issued patents, all of which could have a material adverse effect on Rocket’s business and financial condition.
Rocket may be subject to claims challenging the inventorship or ownership of its patents and other intellectual property.
Rocket may also be subject to claims that former employees, collaborators or other third parties have an ownership interest in its patents or other intellectual property. Rocket has had in the past, and it may also have in the future, ownership disputes arising, for example, from conflicting obligations of consultants or others who are involved in developing Rocket’s product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship or ownership. If Rocket fails in defending any such claims, in addition to paying monetary damages, it may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on Rocket’s business. Even if Rocket is successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Obtaining and maintaining Rocket’s patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and Rocket’s patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and/or applications will be due to be paid to the U.S. PTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents and/or applications. Rocket and, to its knowledge, its licensors have systems in place to remind them to pay these fees, and Rocket and, to its knowledge, its licensors employ outside firms and rely on their respective outside counsel to pay these fees due to non-U.S. patent agencies. The U.S. PTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. Rocket and, to its knowledge, its licensors employ reputable law firms and other professionals to help them comply, and in many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. However, there are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, Rocket’s competitors might be able to enter the market and this circumstance would have a material adverse effect on Rocket’s business.
Issued patents covering Rocket’s product candidates could be found invalid or unenforceable if challenged in court.
If Rocket or one of Rocket’s licensing partners initiated legal proceedings against a third-party to enforce a patent covering one of Rocket’s product candidates, the defendant could counterclaim that the patent covering Rocket’s product candidate is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including patent eligible subject matter, lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the U.S. PTO, or made a misleading statement, during prosecution. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re-examination, post-grant review, and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). Such proceedings could result in revocation or amendment to Rocket’s or its licensing partners’ patents in such a way that they no longer cover Rocket’s product candidates. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, Rocket cannot be certain that there is no invalidating prior art, of which Rocket and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, Rocket would lose at least part, and perhaps all, of the patent protection on its product candidates. Such a loss of patent protection would have a material adverse impact on Rocket’s business.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing Rocket’s ability to protect its products.
As is the case with other biotechnology companies, Rocket’s success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biotechnology industry involve both technological and legal complexity, and therefore obtaining and enforcing biotechnology patents is costly, time-consuming and inherently uncertain. In addition, the United States has recently enacted and is currently implementing wide-ranging patent reform legislation. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to Rocket’s ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the U.S. PTO, the laws and regulations governing patents could change in unpredictable ways that would weaken Rocket’s ability to obtain new patents or to enforce its existing patents and patents that it might obtain in the future.
Rocket may not be able to protect its intellectual property rights throughout the world.
Filing, prosecuting and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and Rocket’s intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, Rocket may not be able to prevent third parties from practicing its inventions in all countries outside the United States, or from selling or importing products made using Rocket’s inventions in and into the United States or other jurisdictions. Competitors may use Rocket’s technologies in jurisdictions where it has not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where Rocket has patent protection, but enforcement is not as strong as that in the United States. These products may compete with Rocket’s products and its patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for Rocket to stop the infringement of its patents or marketing of competing products in violation of its proprietary rights generally. Proceedings to enforce Rocket’s patent rights in foreign jurisdictions could result in substantial costs and divert its efforts and attention from other aspects of its business, could put its patents at risk of being invalidated or interpreted narrowly and its patent applications at risk of not issuing and could provoke third parties to assert claims against it. Rocket may not prevail in any lawsuits that it initiates and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, Rocket’s efforts to enforce its intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that Rocket develops or licenses.
None.
None.
Not applicable.
None.
|
Number
|
Description of Exhibit
|
|
|
|
||
|
2.1
|
Agreement and Plan of Merger and Reorganization, dated as of September 12, 2017, by and among Inotek Pharmaceuticals Corporation, Rocket Pharmaceuticals, Ltd. and Rome Merger Sub (1)
|
|
|
3.1
|
Seventh Amended and Restated Certificate of Incorporation of Rocket Pharmaceuticals, Inc., effective as of February 23, 2015 (2)
|
|
|
3.2
|
Certificate of Amendment (Reverse Stock Split) to the Seventh Amended and Restated Certificate of Incorporation of the Registrant, effective as of January 4, 2018 (3)
|
|
|
3.3
|
Certificate of Amendment (Name Change) to the Seventh Amended and Restated Certificate of Incorporation of the Registrant, effective January 4, 2018 (3)
|
|
|
3.4
|
Amended and Restated By-Laws of Rocket Pharmaceuticals, Inc., effective as of March 29, 2018 (4)
|
|
|
4.1
|
Form of Common Stock Certificate of Rocket Pharmaceuticals, Inc. (3)
|
|
|
4.2
|
Base Indenture, dated as of August 5, 2016, by and between Inotek Pharmaceuticals Corporation and Wilmington Trust, National Association (5)
|
|
|
4.3
|
First Supplemental Indenture, dated as of August 5, 2016, by and between Inotek Pharmaceuticals Corporation and Wilmington Trust, National Association (5)
|
|
|
4.4
|
Form of 5.75% Convertible Senior Note due 2021 (5)
|
|
|
10.1
|
2004 Stock Option and Incentive Plan (6)
|
|
|
10.2
|
Amended and Restated 2014 Stock Option and Incentive Plan and forms of agreements thereunder (7)
|
|
|
10.3
|
|
Rocket Pharmaceuticals, Ltd. 2015 Share Option Plan (7)
|
|
10.4
|
|
Letter Agreement, dated as of January 4, 2018, by and between Inotek Pharmaceuticals Corporation and Dale Ritter (7)
|
|
10.5
|
|
Rocket Pharmaceuticals, Inc. Amended and Restated 2014 Employee Stock Purchase Plan (7)
|
|
10.6
|
|
Form of Indemnification Agreement, to be entered into between the Registrant and its directors (3)
|
|
10.7
|
|
Form of Indemnification Agreement, to be entered into between the Registrant and its officers (3)
|
|
|
Certification of Principal Executive Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
|
|
|
Certification of Principal Financial Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
|
|
|
Certification of Principal Executive Officer and Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
|
|
|
|
|
|
101.INS
|
|
XBRL Instance Document.
|
|
101.SCH
|
|
XBRL Taxonomy Extension Schema Document.
|
|
101.CAL
|
|
XBRL Taxonomy Extension Calculation Document.
|
|
101.DEF
|
|
XBRL Taxonomy Extension Definition Linkbase Document.
|
|
101.LAB
|
|
XBRL Taxonomy Extension Labels Linkbase Document.
|
|
101.PRE
|
|
XBRL Taxonomy Extension Presentation Link Document.
|
|
*
|
Filed herewith.
|
| (1) |
Filed as an Exhibit to the Company’s current report on Form 8-K (001-36829), filed with the SEC on September 13, 2017, and incorporated herein by reference.
|
| (2) |
Filed as an Exhibit to the Company’s annual report on Form 10-K (001-36829), filed with the SEC on March 31, 2015, and incorporated herein by reference.
|
| (3) |
Filed as an Exhibit to the Company’s current report on Form 8-K (001-36829), filed with the SEC on January 5, 2018, and incorporated herein by reference.
|
| (4) |
Filed as an Exhibit to the Company’s registration statement on Form 8-K, (001-36829), filed with the SEC on April 4, 2018, and incorporated herein by reference.
|
| (5) |
Filed as an Exhibit to the Company’s current report on Form 8-K (001-36829), filed with the SEC on August 5, 2016, and incorporated herein by reference.
|
| (6) |
Filed as an Exhibit to the Company’s registration statement on Form S-1 (333-199859), filed with the SEC on November 5, 2014, as amended, and incorporated herein by reference.
|
| (7) |
Filed as an Exhibit to the Company’s annual report on Form 10-K (001-36829), filed with the SEC on March 7, 2018, and incorporated herein by reference.
|
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
ROCKET PHARMACEUTICALS, INC.
|
||
|
|
|
|
|
May 11, 2018
|
By:
|
/s/ Gaurav Shah, MD
|
|
|
Gaurav Shah, MD
|
|
|
|
|
President, Chief Executive Officer and Director
|
|
(Principal Executive Officer)
|
||
|
|
|
|
|
May 11, 2018
|
By:
|
/s/ John Militello
|
|
|
|
John Militello
|
|
|
|
Controller
|
|
(Principal Financial and Accounting Officer)
|
||
56