Exhibit 99.1
Transforming Oncology With Precision Medicine Solutions Company Overview January 2017

Forward-looking statements Statements in this presentation about the Company's expectations, applications of its technology, markets, launch of tests and other statements that are not historical facts constitute “forward-looking statements” for purposes of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and are based on management's current beliefs, assumptions, estimates and projections. Actual results may differ materially from those projected in the forward-looking statements for various reasons, including, without limitation, risks associated with product and test development, test transfer to contracting labs, government regulation, market acceptance, limited commercial experience, dependence on key personnel, obtaining financing and other factors discussed in the Company's periodic reports filed with the Securities and Exchange Commission, and the Company anticipates that subsequent events and developments will cause its views to change. While the Company may elect to update these forward-looking statements in the future, it specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date of this presentation.

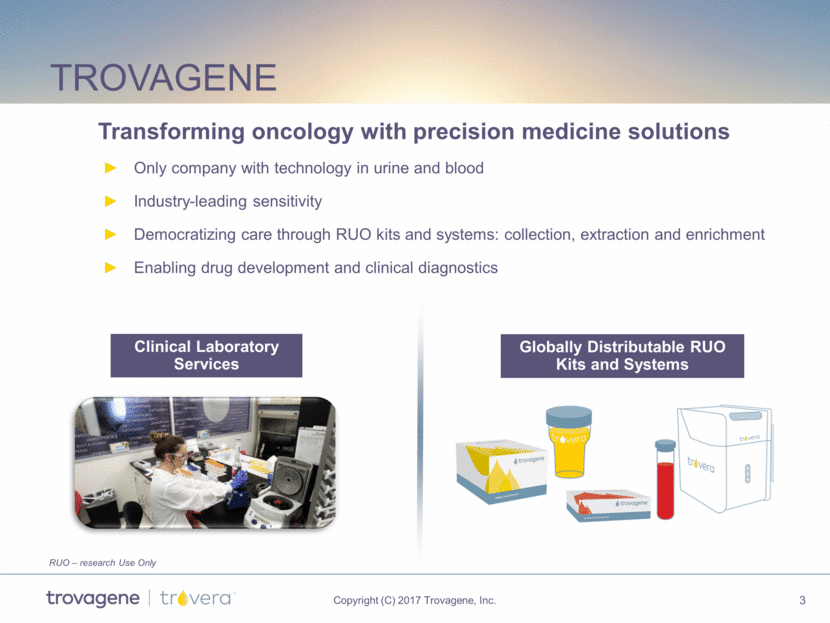
Trovagene Only company with technology in urine and blood Industry-leading sensitivity Democratizing care through RUO kits and systems: collection, extraction and enrichment Enabling drug development and clinical diagnostics Clinical Laboratory Services Globally Distributable RUO Kits and Systems Transforming oncology with precision medicine solutions RUO – research Use Only
Current status First clinically actionable multigene pan-cancer panel Industry-leading sensitivity across 7 oncogenes >200 SNVs, insertions and deletions Analytical validation completed NEXTcollect urine collection and preservation kit Design finalized Mold and process at third party manufacturing for production Development of final kits in process Global distribution of Trovera™ CLIA tests 6 agreements signed with major ex-US distribution partners Option to provide RUO kits and systems upon availability

Blood Urine Noninvasive tumor profiling Circulating Tumor DNA (ctDNA) in Urine and Blood Trovagene is the only company that can harness ctDNA in urine and blood Bardelli and Diaz. JCO. 2014 1Tumor growth causes an increase in tumor cell turnover Tumor cells Turnover of tumor cells: apoptosis or necrosis – tumor DNA (ctDNA) is released1 ctDNA enters blood stream, then excreted in urine via kidneys

Issues with tissue Addressed with liquid biopsy insufficient DNA tumor heterogeneity inconclusive results inadvisable due to tumor location and/or patient health status results can take up to 30 days significant cost over course of treatment Inherent limitations with tissue biopsies Meric-Bernstrom, JCO, 2015. Gerlinger, NEJM, 2012 Lokhandwala T, Chicago Multidisciplinary Symposium in Thoracic Oncology, 2014

liquid biopsy kit opportunity Broad and established base of next generation sequencers (NGS) globally at molecular laboratories - Illumina, ThermoFisher, Bio-Rad, others Strong desire by clinical research pathologists to utilize liquid biopsy testing at their institutions2 30%1 of clinical molecular labs already perform NGS based tests Trovagene’s multigene panels Broad, highly sensitive, clinically actionable Capable of monitoring for emergence of resistance mutations 1McEvoy and Farmer 2015 Research report > $5 billion global market opportunity1 2Oncology, neo- and pre-natal testing, inherited diseases and genetics. Defined as all collection, reagents, markers and systems prior to NGS: Applications of next-generation sequencing technologies in functional genomics - Genomics 92 (2008) 255–264

Intellectual Property supporting liquid biopsy franchise 51-110 base pair TrNA Family Urine Collection and Concentration Family Patent Term 2035 US app filed 2034 US app filed 2029 US & EU 2018 US & EU Small Footprint Family Monitoring Disease Family 20-50 Base Pair TrNA Family Anion Exchange Purification Family Viral and Pathogen TrNA Families Patent Families Transrenal Nucleic Acid (tRNA) Patent Family 2026 US, EU, JP, China, Australia, Canada, India 2034 US app filed 2034 US app filed 2027 US, EU, Canada 90 issued and 73 pending patents

Technology enablement Urine Collection Kit NEXTcollect: Proprietary DNA preservation DNA Extraction Kit Reagents for extraction, isolation and purification Mutation-Enrichment Technology Multigene panel kit targeting oncogenes of interest System for separation and identification NEXTcollect Urine Collection Kit Multigene Panel Kit DNA Extraction Kit Mutation-Enrichment System

NEXTcollect Final mold fabricated and with manufacturer and distributor Completion steps: Preservative sealing process underway Packaging and kitting Scaled for global distribution Multiple use applications Wide-mouth opening Proprietary preservative Graduation (0 – 250 mL) Concealed piercing tips 1 patent pending Centrifuge Adaptable Urine DNA Collection and Preservation kit1

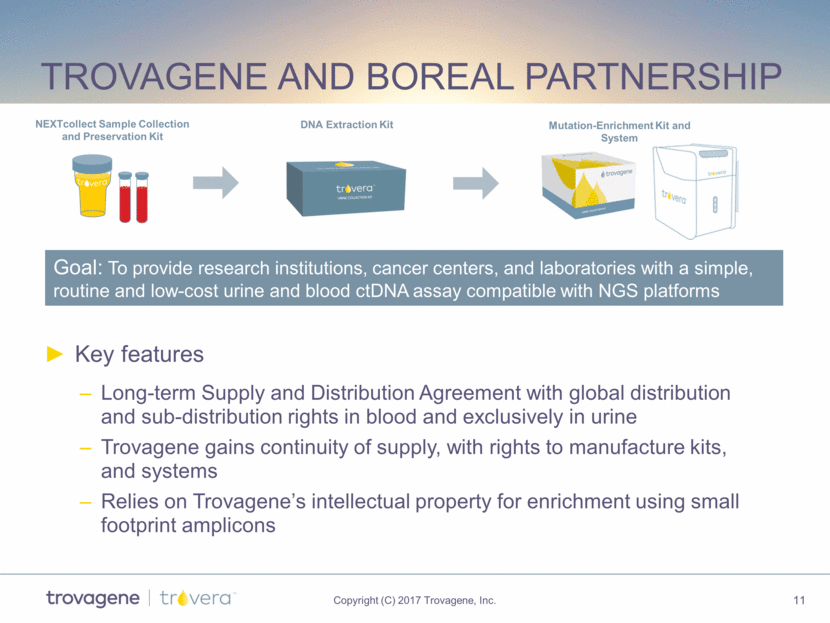
Trovagene and Boreal Partnership Key features Long-term Supply and Distribution Agreement with global distribution and sub-distribution rights in blood and exclusively in urine Trovagene gains continuity of supply, with rights to manufacture kits, and systems Relies on Trovagene’s intellectual property for enrichment using small footprint amplicons Goal: To provide research institutions, cancer centers, and laboratories with a simple, routine and low-cost urine and blood ctDNA assay compatible with NGS platforms NEXTcollect Sample Collection and Preservation Kit DNA Extraction Kit Mutation-Enrichment Kit and System
High sensitivity and quantitation in a multigene panel Trovera™ Multigene Panel Applications Single gene markers Multigene markers (2-10) Assays EGFR, KRAS, BRAF Panel 1: 7 oncogenes across solid tumors Panel 2: 3 resistance oncogenes (lung cancer) Lower Limit of Detection 0.006% 0.003% Technology wild-type blocking alleles to enrich for mutant identification and amplification electrophoretic separation to remove wild-type alleles prior to identification and amplification

TROVAGENE CAPTURES BROADEST RANGE OF ctDNA FRAGMENTS Size of assay footprint determines sensitivity Trovagene markers can detect entire spectrum of ctDNA fragments Underhill, et al. (2016). Fragment Length of Circulating Tumor DNA. PLoS Genetics Traditional Methods Trovagene Reference Sequence 250 bp ctDNA Fragments 150 bp ctDNA Fragments 50 bp ctDNA Fragments

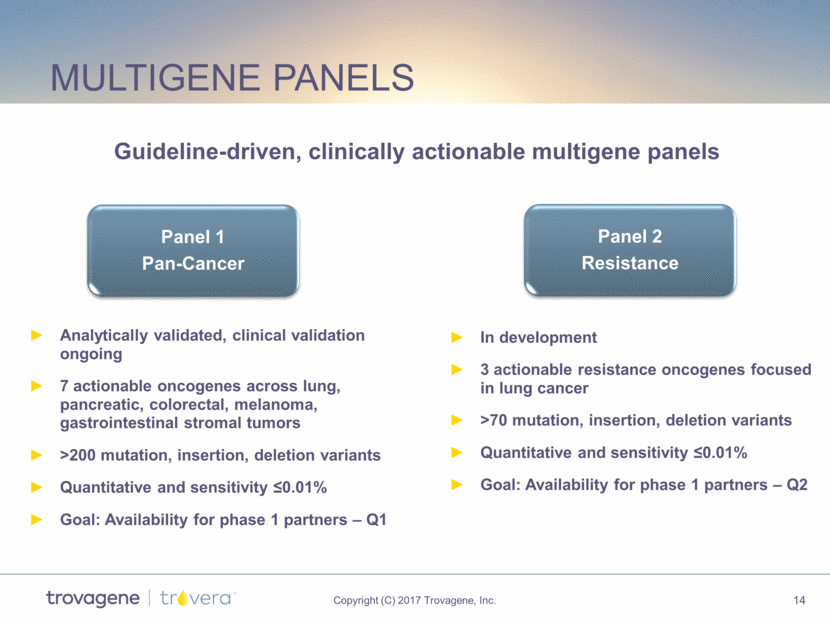
Multigene panels Analytically validated, clinical validation ongoing 7 actionable oncogenes across lung, pancreatic, colorectal, melanoma, gastrointestinal stromal tumors >200 mutation, insertion, deletion variants Quantitative and sensitivity 0.01% Goal: Availability for phase 1 partners – Q1 In development 3 actionable resistance oncogenes focused in lung cancer >70 mutation, insertion, deletion variants Quantitative and sensitivity 0.01% Goal: Availability for phase 1 partners – Q2 Guideline-driven, clinically actionable multigene panels Panel 1 Pan-Cancer Panel 2 Resistance
Pan-cancer panel vs droplet digital PCR Accuracy and quantitation1 Pan-cancer panel is the first multigene panel to show accuracy and quantitation equivalent to single assay ddPCR Highly sensitive assessment of multiple clinically actionable biomarkers in a single test 1Data from analytical validation represents selected mutations: KRAS G12D, KRAS G12V, KRAS Q61H, EGFR T790M, EGFR L858R, EGFR exon19 del Equivalent Quantitative Accuracy Equivalent Linearity for Quantitation

Multigene panel sensitivity comparable or better than ddPCR Demonstrates high analytical sensitivity Test Technology Sensitivity / LOD Trovagene PanCancer PCR + SCODAphoresis + NGS >200 mutations in 7 genes 0.003% (average in 300ng of input DNA) Bio-Rad QX 200 PrimePCR KRAS G12V Mutation Assay Detection Kit Droplet digital PCR (ddPCR) single mutation assays, only 0.025% in 130ng of input DNA1 Roche Cobas® EGFR Mutation Test V2 Real-time PCR 42 EGFR mutations (exons 18-21) 0.4 – 2% in 10 – 20ng of input DNA2 Guardant Health Guardant360 73 gene panel NGS 0.25% in 80% of plasma samples3 0.1% in 28% of plasma samples3 1Olmedillas López S et al, KRAS G12V Mutation Detection by Droplet Digital PCR in Circulating Cell-Free DNA of Colorectal Cancer Patients. Int J Mol Sci. 2016 Apr 1;17(4):484. 2cobas® EGFR Mutation Test v2 (US-IVD), PMA P150047: FDA Summary of Safety and Effectiveness Data (http://www.accessdata.fda.gov/cdrh_docs/pdf15/P150047b.pdf) 3Lanman et al. Analytical and Clinical Validation of a Digital Sequencing Panel for Quantitative, Highly Accurate Evaluation of Cell-Free Circulating Tumor DNA . PLoS One. 2015 Oct 16;10(10):e0140712.

Kit1 Commercialization Demonstrate Clinical Evidence Validation sites (target up to 6) Discussions underway with top research cancer centers Transition to centers of excellence for ctDNA analysis Joint publication of results Q1 and Q2 Phase 1 Phase 2 Commercial Execution Broad commercial distribution of kits and systems from distribution center Cancer Centers, Academic, Pharma, Commercial Reference Labs, Integrated Delivery Networks Global distribution Q2 and Q3 1Kits for research use only (RUO)

Signed ex-US Commercial Partners Six commercial partners recently signed1 with others in progress France, Italy, Benelux, Spain, Portugal, Israel and select eastern Europe Partners to offer Trovera™ CLIA tests to customers in their regions Urine (and blood) specimen sent to Trovagene CLIA laboratory Trovagene reports to partner who reports to prescribing physician Trovagene bills at flat test fee to partner Partner collects from prescribing physician/patient Gateway to technology adoption – supply partners with kits and systems 1 Instituto Diagnostico Varelli; NM Genomics; Progenetics; Amplitech: Diagnostica Longwood; Sorgente Genetica

Clinical evidence Ultra-sensitive mutant allele enrichment – industry leading assay performance Measure tumor burden changes over time Provide highly accurate information to increase confidence in treatment decisions Identify a greater number of patients who may benefit from targeted therapies
High T790M sensitivity in NSCLC Tissue, plasma, and urine testing identify unique subsets of T790M+ patients Urine and plasma testing combined identify more T790M+ patients than tissue alone Urine (Trovera) Tissue (therascreen) Plasma (Trovera) 16 4 5 21 5 100 19 170 T790M-positive cases 174 matched tissue, plasma and urine specimens % T790M positive Positive by any one specimen type 97.7 Positive by plasma and urine combined 94.8 Negative/invalid by all three specimens 2.3 Positive by tissue 83.3 Positive by plasma 81.6 Positive by urine 79.9 IASLC 17th World Conference on Lung Cancer: A highly sensitive next-generation sequencing platform for detection of NSCLC EGFR T790M mutation in urine and plasma - H. Wakelee 95% detection in prospective clinical cohort

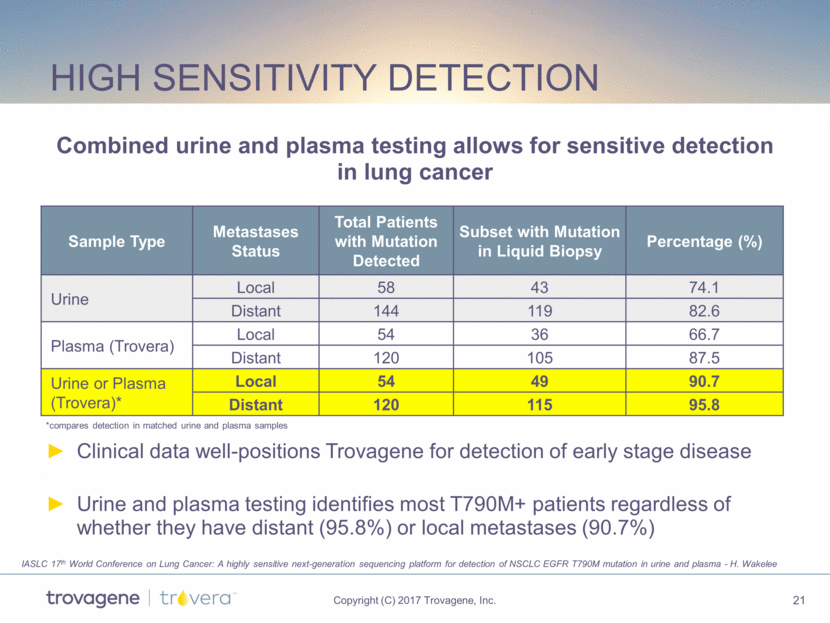
High sensitivity detection Combined urine and plasma testing allows for sensitive detection in lung cancer Sample Type Metastases Status Total Patients with Mutation Detected Subset with Mutation in Liquid Biopsy Percentage (%) Urine Local 58 43 74.1 Distant 144 119 82.6 Plasma (Trovera) Local 54 36 66.7 Distant 120 105 87.5 Urine or Plasma (Trovera)* Local 54 49 90.7 Distant 120 115 95.8 *compares detection in matched urine and plasma samples Clinical data well-positions Trovagene for detection of early stage disease Urine and plasma testing identifies most T790M+ patients regardless of whether they have distant (95.8%) or local metastases (90.7%) IASLC 17th World Conference on Lung Cancer: A highly sensitive next-generation sequencing platform for detection of NSCLC EGFR T790M mutation in urine and plasma - H. Wakelee
monitoring of cancer in urine CONCORDANT WITH RADIOGRAPHIC RESPONSE Multiple peer-reviewed studies demonstrate that Trovagene’s ctDNA tests can be effectively used to monitor therapeutic response, regardless of cancer type or drug class NSCLC 3rd Generation TKI/EGFR T790M1 Histiocytic Tumor/BRAF Inhibitor2 Colorectal Neuroendocrine/BRAF Inhibitor3 1Husain, Kurzrock et al, World Lung Conference, 2015 2Hyman et al., Cancer Discovery 2015 3Klempner et al., Cancer Discovery 2016

Number of Days from Start of Chemotherapy ctDNA KRAS associated with pancreatic cancer patient outcome Monitoring with ctDNA KRAS Associated with Patient Response Baseline ctDNA KRAS Levels Associated with Overall Survival Chen, et al. Manuscript Under Review. 2017 Baseline quantitation of ctDNA KRAS informs clinical outcome in patients with stage III/IV pancreatic cancer Longitudinal monitoring of dynamic changes in ctDNA KRAS mutation load correlates with clinical response by CT scan Overall Survival (Probability) Days Stage Baseline ctDNA KRAS Number of patients Median Overall Survival (in Days) III Low 26 326 - High 6 - IV Low 55 217 High 84 139 CT Scan – Stable Disease CT Scan – Progressive Disease Quantitative ctDNA KRAS level CT Scans

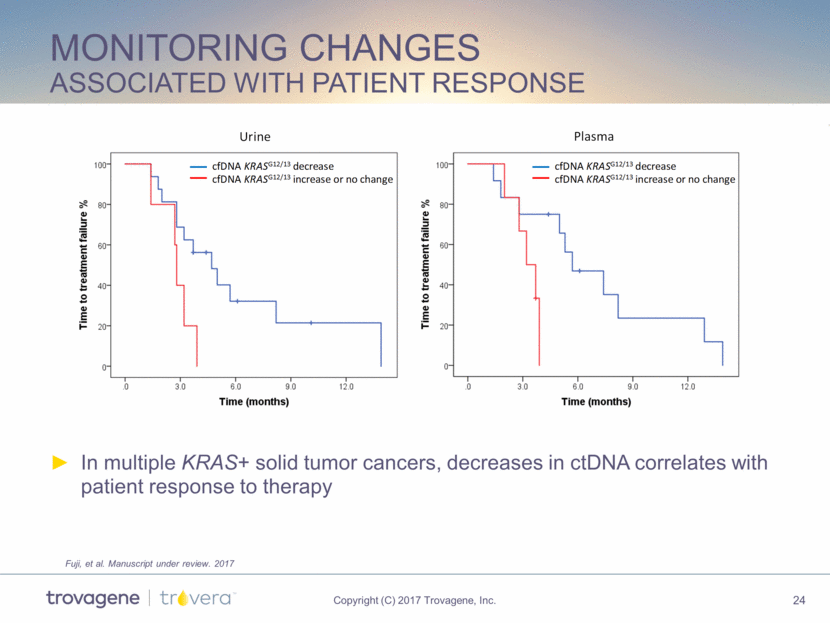
monitoring changes associated with patient response In multiple KRAS+ solid tumor cancers, decreases in ctDNA correlates with patient response to therapy Fuji, et al. Manuscript under review. 2017 Plasma cfDNA KRAS G12/13 decrease cfDNA KRAS G12/13 increase or no change Urine cfDNA KRAS G12/13 decrease cfDNA KRAS G12/13 increase or no change
Clinical utility of ctDNA in urine and blood Publication Highlights Use of urinary ctDNA is a clinically viable biopsy type for mutation detection and monitoring therapeutic response across multiple cancer types Trovagene technology can be used in urine and blood: Industry-leading clinical sensitivity (95%) Demonstrated clinical utility in non-small cell lung cancer, pancreatic cancer, colorectal cancer, melanoma and histiocytic disorders Noninvasive urine liquid biopsy enables repeat and frequent testing to assess changes in tumor mutation burden 6 publications 6 manuscripts under review

precision medicine solutions Trovagene only ctDNA platform in urine & blood Industry-leading sensitivities and quantification Clinically actionable markers and panels Both predictive and early response biomarkers Predictive: identification Early response: monitoring sustainable results Benefit to drug development Smaller, less costly clinical studies Increase probability of clinical success Working with Pharma on drug development and licensing Enabling drug development / measuring drug response

summary Only company with technology in urine and blood Industry-leading sensitivity Democratizing cancer care through RUO kits and systems: collection/preservation, extraction and enrichment NEXTcollect urine collection and preservation kit Multigene pan-cancer panel Multigene resistance panel Enabling drug development and clinical diagnostics

Thank you for more information, please email ir@trovagene.com
