Table of Contents
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2013
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number 000-50743
ALNYLAM PHARMACEUTICALS, INC.
(Exact Name of Registrant as Specified in Its Charter)
| Delaware | 77-0602661 | |
| (State or Other Jurisdiction of Incorporation or Organization) |
(I.R.S. Employer Identification No.) |
300 Third Street, Cambridge, MA 02142
(Address of Principal Executive Offices) (Zip Code)
Registrant’s telephone number, including area code: (617) 551-8200
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class |
Name of Each Exchange on Which Registered | |
| Common Stock, $0.01 par value per share |
The Nasdaq Global Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes þ No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes þ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer þ | Accelerated filer ¨ | Non-accelerated filer ¨ | Smaller reporting company ¨ | |||
| (Do not check if a smaller reporting company) | ||||||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No þ
The aggregate market value of the registrant’s common stock, $0.01 par value per share (“Common Stock”), held by non-affiliates of the registrant, based on the last sale price of the Common Stock at the close of business on June 28, 2013, was $1,915,005,225. For the purpose of the foregoing calculation only, all directors and executive officers of the registrant are assumed to be affiliates of the registrant.
At January 31, 2014, the registrant had 63,916,814 shares of Common Stock, $0.01 par value per share, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement for its 2014 annual meeting of stockholders, which the registrant intends to file pursuant to Regulation 14A with the Securities and Exchange Commission not later than 120 days after the registrant’s fiscal year end of December 31, 2013, are incorporated by reference into Part II, Item 5 and Part III of this Form 10-K.
Table of Contents
ANNUAL REPORT ON FORM 10-K
For the Year Ended December 31, 2013
TABLE OF CONTENTS
2
Table of Contents
This annual report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, that involve risks and uncertainties. All statements other than statements relating to historical matters should be considered forward-looking statements. When used in this report, the words “believe,” “expect,” “plan,” “anticipate,” “estimate,” “predict,” “may,” “could,” “should,” “intend,” “will,” “target,” “goal” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these words. Our actual results could differ materially from those discussed in the forward-looking statements as a result of a number of important factors, including the factors discussed in this annual report on Form 10-K, including those discussed in Item 1A of this report under the heading “Risk Factors,” and the risks discussed in our other filings with the Securities and Exchange Commission. Readers are cautioned not to place undue reliance on these forward-looking statements, which reflect management’s analysis, judgment, belief or expectation only as of the date hereof. We explicitly disclaim any obligation to update these forward-looking statements to reflect events or circumstances that arise after the date hereof.
| ITEM 1. | BUSINESS |
Overview
We are a biopharmaceutical company developing novel therapeutics based on RNA interference, or RNAi. RNAi is a naturally occurring biological pathway within cells for selectively silencing and regulating the expression of specific genes. Since many diseases are caused by the inappropriate activity of specific genes, the ability to silence genes selectively through RNAi could provide a new way to treat a wide range of human diseases. We believe that drugs that work through RNAi have the potential to become a broad new class of drugs, like small molecule, protein and antibody drugs. Using our intellectual property and the expertise we have built in RNAi, we are developing a set of biological and chemical methods and know-how that we apply in a systematic way to develop RNAi therapeutics for a variety of diseases.
Our core product strategy, which we refer to as “Alnylam 5x15,” was launched in January 2011 and is focused on the development and commercialization of novel RNAi therapeutics as genetic medicines. Our genetic medicine programs are RNAi therapeutics directed towards genetically defined targets for the treatment of diseases with high unmet medical need. As part of this strategy, our goal is to develop product candidates with the following shared characteristics: a genetically defined target and disease expressed in the liver; the potential to have a major impact in high unmet need patient populations; the ability to leverage our existing RNAi platform with clinically proven delivery to the liver; the opportunity to monitor an early biomarker in Phase 1 clinical trials for human proof of concept; and the existence of clinically relevant endpoints for the filing of a new drug application, or NDA, with a focused patient database and possible accelerated paths for commercialization. Under our core product strategy, we expect to have six to seven genetic medicine product candidates in clinical development, including at least two programs in Phase 3 and five to six programs with human proof of concept, by the end of 2015. We are currently advancing the following core programs in clinical or pre-clinical development: patisiran (the International Nonproprietary Name for ALN-TTR02), an intravenously delivered RNAi therapeutic targeting transthyretin, or TTR, in development for the treatment of TTR-mediated amyloidosis, or ATTR, in patients with familial amyloidotic polyneuropathy, or FAP; ALN-TTRsc, a subcutaneously delivered RNAi therapeutic targeting TTR in development for the treatment of ATTR in patients with TTR cardiac amyloidosis, including familial amyloidotic cardiomyopathy, or FAC, and senile systemic amyloidosis, or SSA; ALN-AT3, an RNAi therapeutic targeting antithrombin, or AT, in development for the treatment of hemophilia and rare bleeding disorders, or RBD; ALN-CC5, an RNAi therapeutic targeting complement component C5 in development for the treatment of complement-mediated diseases; ALN-AS1, an RNAi therapeutic targeting aminolevulinate synthase-1, or ALAS-1, in development for the treatment of hepatic porphyrias, including acute intermittent porphyria, or AIP; ALN-PCSsc, an RNAi therapeutic targeting proprotein convertase subtilisin/kexin type 9, or PCSK9, in development for the treatment of hypercholesterolemia; ALN-AAT, an RNAi therapeutic targeting alpha-1-antitrypsin, or AAT, in development for the treatment of AAT deficiency liver disease; ALN-TMP, an RNAi therapeutic targeting transmembrane
3
Table of Contents
protease, serine 6, or TMPRSS6, in development for the treatment of beta-thalassemia and iron-overload disorders; ALN-ANG, an RNAi therapeutic targeting angioprotein-like 3, or ANGPTL3, in development for the treatment of genetic forms of mixed hyperlipidemia and severe hypertriglyceridemia; and other yet to be disclosed programs. Our strategy is to retain development and commercial rights for our current and future genetic medicine pipeline in North America and Western Europe, while forming alliances with leading, innovative companies for the development and commercialization of these products in the rest of world, or ROW. In early 2014, we formed an alliance with Genzyme Corporation, a Sanofi company, or Genzyme, to develop and commercialize our current “Alnylam 5x15” and future genetic medicine pipeline principally in territories outside of North America and Western Europe, subject to certain broader rights.
We believe that the strength of our intellectual property portfolio relating to the development and commercialization of small interfering RNAs, or siRNAs, as therapeutics provides us a leading position with respect to this therapeutic modality. Our intellectual property portfolio includes ownership of, or exclusive rights to, issued patents and pending patent applications claiming fundamental features of siRNAs and RNAi therapeutics as well as those claiming crucial chemical modifications and promising delivery technologies. We believe that no other company possesses a portfolio of such broad and exclusive rights to the patents and patent applications required for the commercialization of RNAi therapeutics. Given the importance of our intellectual property portfolio to our business operations, we intend to vigorously enforce our rights and defend against challenges that have arisen or may arise in this area.
In addition, our expertise in RNAi therapeutics and broad intellectual property estate have allowed us to form alliances with leading pharmaceutical and life sciences companies, including Isis Pharmaceuticals, Inc., or Isis, Medtronic, Inc., or Medtronic, Novartis Pharma AG, or Novartis, F. Hoffmann-La Roche Ltd, or Roche (which assigned its rights and obligations to Arrowhead Research Corporation, or Arrowhead during 2011), Takeda Pharmaceutical Company Limited, or Takeda, Kyowa Hakko Kirin Co., Ltd., or Kyowa Hakko Kirin, Cubist Pharmaceuticals, Inc., or Cubist, Ascletis BioScience Co., Ltd., or Ascletis, Monsanto Company, or Monsanto, Genzyme and The Medicines Company, or MDCO. We also have established collaborations with and, in some instances, received funding from major medical and disease associations, including CHDI Foundation, Inc., or CHDI. Finally, to further enable the field and monetize our intellectual property rights, we also grant licenses to biotechnology companies for the development and commercialization of RNAi therapeutics for specified targets in which we have no direct strategic interest under our InterfeRx™ program, and to research companies that commercialize RNAi reagents or services under our research product licenses.
We also seek to form or advance new ventures and opportunities in areas outside our primary focus on RNAi therapeutics. In 2007, we and Isis established Regulus Therapeutics Inc., or Regulus, a company focused on the discovery, development and commercialization of microRNA therapeutics. In October 2012, Regulus completed its initial public offering, and currently, we own approximately 15% of Regulus’ outstanding common stock. Through an internal effort we refer to as Alnylam Biotherapeutics, we have evaluated the application of RNAi technology to improve the manufacturing processes for biologics. We have also evaluated the utility of our VaxiRNA™ platform, an RNAi technology developed under our Alnylam Biotherapeutics initiative, for the enhanced production of viruses used in the manufacture of vaccine products.
Recent Developments
Acquisition of Sirna Therapeutics
On January 10, 2014, we entered into a stock purchase agreement with Sirna Therapeutics, Inc., or Sirna, Merck Sharp & Dohme Corp., or Merck, and, for limited purposes, Merck & Co., Inc., pursuant to which we will purchase from Merck all of Merck’s right, title and interest in and to all of the outstanding shares of common stock of Sirna. Sirna possesses intellectual property and RNAi assets including pre-clinical therapeutic candidates, chemistry, and siRNA-conjugate and other delivery technologies that we intend to integrate into our platform for delivery of RNAi therapeutics. We will not acquire any employees, manufacturing or other facilities, developed processes or clinical-stage assets as part of the acquisition of Sirna.
In consideration for the Sirna common stock, we will (i) pay Merck $25.0 million in cash and (ii) issue to Merck 2,520,044 shares of our common stock, having a value of $150.0 million as calculated under the terms of
4
Table of Contents
the stock purchase agreement on the date of execution. Following the closing of this transaction, Merck will beneficially own approximately 4% of our outstanding common stock.
In addition, Merck is eligible to receive the following consideration from us: (i) up to an aggregate of $10.0 million upon the achievement by us or our related parties of specified regulatory milestones for RNAi products covered by Sirna intellectual property, and (ii) up to an aggregate of $105.0 million upon the achievement by us or our related parties of specified development and regulatory ($40.0 million) and commercial ($65.0 million) milestones associated with the clinical development progress of certain pre-clinical candidates discovered by Sirna, together with low single-digit royalties for our products and single-digit royalties for Sirna products, in each case based on annual worldwide net sales, if any, by us and our related parties of any such products.
Under the stock purchase agreement, Merck also agreed not to dispose of (i) any shares of our common stock beneficially owned for a period of six months following the closing date, referred to as the Initial Lock-Up, subject to certain limited exceptions, and (ii) 50% of the shares of our common stock beneficially owned for a period of six additional months following the termination of the Initial Lock-Up, referred to as the Subsequent Lock-Up. During and following the expiration of the Subsequent Lock-Up, Merck will be permitted to sell such shares of our common stock subject to certain limitations, including certain volume and manner of sale restrictions.
The stock purchase agreement contains customary representations, warranties, and covenants of the parties thereto. Subject to customary closing conditions, including the expiration or early termination of the applicable pre-merger waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, the transaction is expected to close during the first quarter of 2014.
Genzyme Collaboration
On January 11, 2014, we entered into a global, strategic collaboration with Genzyme to discover, develop and commercialize RNAi therapeutics as genetic medicines to treat orphan diseases. The 2014 Genzyme collaboration is governed by a master collaboration agreement, including the license terms appended thereto, which will become effective upon closing of the equity transaction, described below. Once effective, the master agreement will supersede and replace the previous collaboration between us and Genzyme entered into in October 2012 to develop and commercialize RNAi therapeutics targeting TTR for the treatment of ATTR, which original Genzyme agreement is described under the heading “Strategic Alliances” in our annual report on Form 10-K for the year ended December 31, 2012.
The 2014 Genzyme collaboration is structured as an exclusive relationship for the worldwide development and commercialization of RNAi therapeutics in the field of genetic medicines, which includes our current and future genetic medicine programs that reach human proof-of-principal study completion, or Human POP, by the end of 2019, subject to extension to the end of 2021 in various circumstances. We will retain product rights in North America and Western Europe, while Genzyme will obtain exclusive rights to develop and commercialize collaboration products in the ROW, referred to as the Genzyme Territory, together with certain broader co-development/co-promote or worldwide rights for certain products. Genzyme’s rights are structured as an opt-in that is triggered upon achievement of Human POP. We will maintain development control for all programs prior to Genzyme’s opt-in and maintain development and commercialization control after Genzyme’s opt-in for all programs in our territory.
Upon the effective date of the 2014 Genzyme collaboration, Genzyme will opt-in to patisiran for the Genzyme Territory, and we will retain full product rights in North America and Western Europe. We and Genzyme have also agreed to expand our current collaboration on ALN-TTRsc, where we and Genzyme will co-develop and co-promote ALN-TTRsc in North America and Western Europe. We will maintain development and commercialization control with ALN-TTRsc and Genzyme will develop and commercialize the product in the Genzyme Territory.
In addition to its regional rights for our current and future genetic medicine programs in the Genzyme Territory, Genzyme will have the right to either (i) co-develop and co-promote ALN-AT3 for the treatment of
5
Table of Contents
hemophilia and other RBDs in our territory, with us maintaining development and commercialization control, or (ii) obtain a global license to ALN-AS1 for the treatment of hepatic porphyrias. Genzyme will exercise this selection right upon Human POP for the ALN-AT3 and ALN-AS1 programs. Finally, Genzyme will have the right for a global license to a single, future genetic medicine program that is not one of our currently defined genetic medicine programs. We will retain global rights to any RNAi therapeutic genetic medicine program that does not reach Human POP by the end of 2019, subject to certain limited exceptions. Under the terms of the master agreement, we will retain full rights to all current and future RNAi therapeutic programs outside of the field of genetic medicines, including the right to form new collaborations.
In consideration for the rights granted to Genzyme under the master agreement and pursuant to the terms of a stock purchase agreement, we agreed to sell to Genzyme 8,766,338 shares of our common stock and Genzyme agreed to pay to us $700.0 million in aggregate cash consideration. Following the closing of the stock purchase, Genzyme will beneficially own approximately 12% of our outstanding common stock. The stock purchase agreement contains customary representations, warranties and covenants of each of the parties thereto. Subject to customary closing conditions, including the expiration or early termination of the applicable pre-merger waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, the stock purchase is expected to close during the first quarter of 2014.
A description of our 2014 Genzyme collaboration and the related stock purchase agreement is included below under the heading “Strategic Alliances.”
RNA Interference
RNAi is a natural biological pathway that occurs within cells and can be harnessed to selectively silence the activity of specific genes. The discovery of RNAi first occurred in plants and worms in 1998, and two of the scientists who made this discovery, Dr. Andrew Fire and Dr. Craig Mello, received the 2006 Nobel Prize for Physiology or Medicine.
Opportunity for Therapeutics Based on RNAi
Beginning in 1999, our scientific founders described and provided evidence that the RNAi mechanism occurs in mammalian cells and that its immediate trigger is a type of molecule known as an siRNA. They showed that laboratory-synthesized siRNAs could be introduced into the cell and suppress production of specific target proteins by cleaving and degrading the messenger RNA, or mRNA, of the specific gene that encodes that specific protein. Because it is possible to design and synthesize siRNAs specific to any gene of interest, the entire human genome is accessible to RNAi, and we therefore believe that RNAi therapeutics have the potential to become a broad new class of drugs.
In May 2001, one of our scientific founders, Dr. Thomas Tuschl, published the first scientific paper demonstrating that siRNAs can be synthesized in the laboratory using chemical or biochemical methods and, when introduced or delivered into mammalian cells, can silence the activity of a specific gene. Since the Tuschl publication and issuance of the seminal Tuschl II patent, which is licensed exclusively to us for therapeutic applications, the use of siRNAs has been broadly adopted by academic and industrial researchers for the fundamental study of the function of genes. This has resulted in a significant number of publications focused on the use of RNAi and has made the Tuschl publication one of the most cited papers in basic biologic research. Reflecting this, siRNAs are a growing segment of the market for research reagents and related products and services.
Beyond its use as a basic research tool, we believe that RNAi can form the basis of a broad new class of drugs for the treatment of genetically defined targets for diseases with high unmet medical need. Drugs based on the RNAi mechanism could offer numerous opportunities and benefits, which may include:
| • | Ability to target proteins that cannot be targeted effectively by existing drug classes. Over the last decade, the understanding of human disease has advanced enormously, and many proteins that play fundamental roles in human disease have been identified. Paradoxically, greater than 80% of these key proteins cannot be targeted effectively with existing drug approaches like small molecules or proteins such as monoclonal antibodies. These so called “undruggable” targets are potentially accessible to siRNAs as they are made by mRNAs that can be targeted with RNAi. Further, certain diseases may be caused by |
6
Table of Contents
| the mutation in one copy of the genetic material, a single allele, in which case a specific siRNA could target the disease-causing mutation leaving the normal allele intact. |
| • | Ability to treat a broad range of diseases. The ability to make siRNAs that target virtually any gene to suppress the production of virtually any protein whose presence or activity causes disease suggests a broad potential for application in a wide range of diseases. |
| • | Inherently potent and natural mechanism of action. We expect the inherent catalytic nature of the RNAi mechanism to allow for a high degree of potency and durability of effect for RNAi-based therapeutics, which we believe distinguishes RNAi from other approaches, like antisense therapeutics, which are not catalytic and therefore require higher levels of drug to achieve mRNA silencing. In addition, since RNAi therapeutics harness a natural mechanism for gene silencing, we believe that this approach will demonstrate improved safety and tolerability as compared with other RNA-targeting approaches. |
| • | Simplified discovery of product candidates. In contrast to the often arduous and slow drug discovery process for proteins and small molecules, the identification of siRNA product candidates has been, and we expect will continue to be, much simpler, quicker and less costly because it involves relatively standard processes that are directed by the known gene target sequences and can be applied in a similar fashion to many successive product candidates. Further, siRNA lead candidates can be designed to be active across a broad range of species, greatly simplifying the translation of animal model data to human disease applications. In addition, certain chemical modifications can be applied to confer “drug-like properties” to siRNAs, making them stable when administered into the bloodstream. Finally, approaches for delivery of RNAi therapeutics have now been engineered to enable a consistent level of target gene silencing in specific organs, such as genes expressed in the liver. There also appears to be a highly correlated level of target knockdown observed in animal studies as compared with results in human clinical trials, ensuring what we believe to be a highly reliable translation of RNAi therapeutics from pre-clinical research into clinical studies. For these reasons, we believe RNAi therapeutics represent a highly modular and reproducible approach for drug discovery and development. |
We have reported on our advances in developing siRNAs as potential drugs in a large number of peer-reviewed publications and many scientific meetings, including publications by Alnylam scientists in the journals Nature, Nature Medicine, Nature Biotechnology, Cell, Proceedings of the National Academy of Sciences, the New England Journal of Medicine and The Lancet.
Our Product Platform
Our product platform provides a capability for a systematic approach to identifying RNAi therapeutic product candidates through sequence selection, potency selection, stabilization by chemical modification, improvement of biodistribution and cellular uptake by various chemical conjugates and formulations. Key to the therapeutic application of siRNAs is the ability to successfully deliver siRNAs to target tissues and achieve cellular uptake of the siRNA into the inside of the cell where the RNAi machinery, called RNA-induced silencing complex, or RISC, is active. We have employed two predominant approaches for delivery of RNAi therapeutics: first, a formulation-based approach with lipid nanoparticles, or LNPs; and, second, a conjugate-based approach involving the modification of siRNAs with small chemical groups, such as triantennary N-acetylgalactosamine, or GalNAc, conjugates. We have demonstrated the ability to achieve delivery of siRNAs to cells in the liver with both intravenous administration using LNPs and subcutaneous administration using GalNAc-siRNA conjugates. Specifically, we have demonstrated RNAi therapeutic activity with these delivery approaches in multiple species, including humans. We have also demonstrated RNAi therapeutic activity towards multiple disease genes expressed in the liver, including results from our Phase 1 clinical trials for ALN-TTR01 and ALN-PCS02, Phase 1 and Phase 2 clinical trials for patisiran, as well as in biopsy results from our Phase 1 clinical trial for ALN-VSP. During 2013, we reported results from our Phase 1 clinical trial of ALN-TTRsc, representing the first human data to be presented for our proprietary GalNAc-siRNA conjugate delivery platform, enabling subcutaneous dosing of RNAi therapeutics with a wide therapeutic index, and demonstrating human translation for our GalNAc platform. In addition, in some tissues, including the respiratory tract and central nervous system, we have employed a direct RNAi delivery approach, which employs the direct or local application of siRNAs, to achieve cellular uptake and gene silencing.
7
Table of Contents
We believe that we have continued to make considerable progress in developing our product platform and to make further advances relating to the delivery of RNAi therapeutics, both internally and together with our collaborators. We believe the acquisition of Sirna, when consummated, will complement and extend our own progress and continued focus on RNAi therapeutics, including with siRNA-conjugate technologies. With the progress we have made to date and expect to make in the future, we believe we are well positioned to pursue multiple therapeutic opportunities.
Our Product Pipeline
Under our core product strategy, we expect to have six to seven genetic medicine product candidates in clinical development, including at least two programs in Phase 3 and five to six programs with human proof of concept, by the end of 2015. We are currently advancing the following core programs in clinical or pre-clinical development: patisiran for the treatment of ATTR in patients with FAP; ALN-TTRsc for the treatment of ATTR in patients with TTR cardiac amyloidosis, including FAC and SSA; ALN-AT3 for the treatment of hemophilia and RBD; ALN-CC5 for the treatment of complement-mediated diseases; ALN-AS1 for the treatment of hepatic porphyrias, including AIP; ALN-PCSsc for the treatment of hypercholesterolemia; ALN-AAT for the treatment of AAT deficiency liver disease; ALN-TMP for the treatment of beta-thalassemia and iron-overload disorders; ALN-ANG for the treatment of genetic forms of mixed hyperlipidemia and severe hypertriglyceridemia; and other yet to be disclosed programs. Our strategy is to retain development and commercial rights for our current and future genetic medicine pipeline in North America and Western Europe, while forming alliances with leading, innovative companies for the development and commercialization of these products in the ROW. In early 2014, we formed an alliance with Genzyme to develop and commercialize our current and future genetic medicine pipeline principally in territories outside of North America and Western Europe, subject to certain broader rights.
While focusing our efforts on our core product strategy, we also intend to continue to advance additional development programs through existing or future alliances. We have two partner-based programs that have entered clinical development, ALN-RSV01 for the treatment of respiratory syncytial virus, or RSV, and ALN-VSP for the treatment of liver cancers, as well as one candidate in pre-clinical development, ALN-HTT for the treatment of Huntington’s disease, or HD.
The following is a summary of our product development programs and our partner-based clinical development programs as of January 31, 2014:
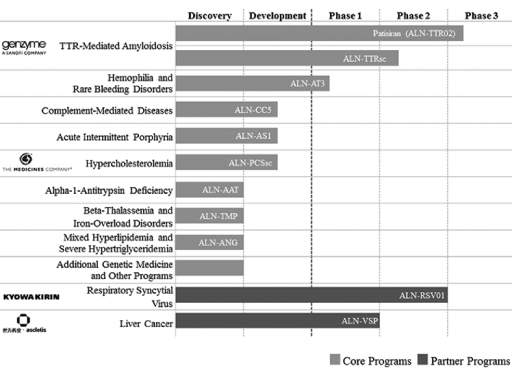
8
Table of Contents
We have spent substantial funds over the past three years to develop our product pipeline and expect to continue to do so in the future. We incurred research and development costs of $113.0 million in 2013, $86.6 million in 2012 and $99.3 million in 2011.
Core Product Development Programs
Our core genetic medicine development programs are described in more detail below.
ALN-TTR — TTR-Mediated Amyloidosis (ATTR)
Our most advanced core product development program, ALN-TTR, targets the TTR gene for the treatment of ATTR. ATTR is an inherited, progressively debilitating and fatal disease caused by a mutation in the TTR gene, of which over 100 mutations have been identified. TTR protein is produced primarily in the liver and is normally a carrier for retinol binding protein. We believe TTR is a suitable target for an RNAi therapeutic formulated to maximize delivery to liver cells, which are the primary source of TTR synthesis. Mutations in TTR result in the accumulation of damaging toxic deposits of the wild-type and mutant protein in several body organs and tissues, including the peripheral nervous system, heart and/or gastrointestinal tract, which leads to FAP and/or FAC. FAP is associated with severe pain and loss of autonomic nervous system function, whereas FAC is associated with heart failure. ALN-TTR targets wild-type and all known mutant forms of TTR, including the V30M mutation, which is the major mutation of ATTR, particularly in FAP, and therefore is a potential therapeutic for the treatment of all forms of ATTR, including FAP and FAC. ATTR represents a major unmet medical need with significant morbidity and mortality as an orphan, or rare, disease. Based on our analysis of the available patient and market data, we estimate that FAP affects approximately 10,000 people worldwide and FAC affects at least 40,000 people worldwide. ATTR patients with FAP have a mean life expectancy of five to 15 years from symptom onset, and the only treatment options for early stage disease are liver transplantation and tafamidis, a small molecule stabilizer of the TTR protein for early-stage FAP patients that has been approved in the European Union, or EU. In addition, a Phase 2/3 clinical trial of diflunisal, a commercially available non-steroidal anti-inflammatory agent, has been conducted in patients with FAP. The mean survival for FAC patients is approximately 2.5 years following diagnosis, and there are no approved therapies. SSA is a non-hereditary form of TTR cardiac amyloidosis caused by idiopathic deposition of wild-type TTR; its prevalence is generally unknown, but is associated with advanced age. Although limited treatment options are available, there remains a significant need for novel therapeutics to treat patients with TTR amyloid polyneuropathy and/or cardiomyopathy.
Patisiran (ALN-TTR02). Patisiran is our most advanced product candidate in clinical development. In July 2012, we reported positive clinical results from our patisiran Phase 1 clinical trial, which was conducted in the United Kingdom as a randomized, single-blind, placebo-controlled, single-ascending dose study, which enrolled 17 healthy volunteer subjects. The primary objective of the study was to evaluate the safety and tolerability of a single dose of patisiran. Secondary objectives of this study included the characterization of pharmacokinetics of patisiran and the assessment of clinical activity as measured by effects on serum TTR levels through at least day 56 following a single dose. Patisiran was found to be generally well tolerated in this Phase 1 clinical trial, consistent with our broader clinical experience with LNP-formulated siRNAs.
In November 2013, we reported the achievement of positive clinical results from our Phase 2 clinical trial of patisiran. The Phase 2 clinical trial with patisiran in ATTR polyneuropathy patients (n=29) was an open-label, multi-center, multi-dose, dose-escalation trial to evaluate the safety and tolerability of two doses of patisiran and to demonstrate clinical activity based on serial measurement of circulating serum levels of wild-type and mutant TTR. Patients received two doses of patisiran in 5 cohorts with doses ranging from 0.01 to 0.30 mg/kg, using either a once-every-four-week or once-every-three-week dosing regimen. The international study included ten sites in Portugal, France, Sweden, Germany, Spain, Brazil and the U.S. The data from 28 patients enrolled and analyzed showed that multiple doses of patisiran resulted in rapid, dose-dependent and durable knockdown of serum TTR levels. As compared with the lowest dose group of 0.01 mg/kg, there was a statistically significant knockdown of serum TTR at doses of 0.15 mg/kg (p<0.05) and 0.30 mg/kg (p<0.001). The study results support further evaluation of patisiran at the 0.30 mg/kg dose administered once every three weeks. With this dose and regimen, mean TTR knockdown at nadir of 83.8% and 86.7% was observed following the first and second doses, respectively, with maximum TTR knockdown of up to 96.0%.
9
Table of Contents
A number of additional analyses were performed in this clinical trial. First, a proprietary mass spectrometry method was used to measure serum levels of wild-type and mutant V30M proteins. These results showed one-to-one correspondence in knockdown of mutant and wild-type TTR (r2=0.95, p<0.001), with essentially superimposable pharmacodynamic effects toward both protein species. Of interest, patients on TTR stabilizer therapy (specifically tafamidis or diflunisal) showed significantly increased baseline levels of serum TTR; regardless, patisiran administration resulted in a similar degree of TTR knockdown in these patients. These results confirm the absence of any interference by TTR stabilizer drugs with the pharmacologic activity of patisiran, and also demonstrate that RNAi-mediated knockdown of TTR is achieved independent of baseline TTR serum levels. Finally, and as expected, serum TTR knockdown was highly correlated with a reduction in circulating levels of retinol binding protein (RBP) (r2=0.89, p<0.001) and vitamin A (r2=0.90, p<0.001).
Multiple doses of patisiran were found to be generally well tolerated in this clinical trial. The majority of the adverse events were mild or moderate. There were no abnormalities seen in liver function tests, renal function or hematologic parameters. There were two serious adverse events, including an episode of self-limiting cellulitis of the arm that occurred as a result of drug extravasation at the infusion site in a patient with poor intravenous access. In addition, an episode of nausea and vomiting occurred in a patient with autonomic involvement due to disease; this patient discontinued the study after one dose. The most common adverse event reported was a mild or moderate infusion-related reaction, or IRR, which occurred in 10.3% (3/29) of patients overall; this adverse event was managed with a prolonged intravenous infusion and was not associated with any patient discontinuations. Notably, no IRRs were reported among 12 patients who received 0.30 mg/kg once every three weeks, nine of whom received their infusion with a proprietary micro-dosing regimen administered over 70 minutes.
During 2013, we also initiated a Phase 2 open-label extension, or OLE, study with patisiran. Eligible patients treated in the Phase 2 clinical trial are being enrolled in the OLE study, where they will receive patisiran at a dose of 0.30 mg/kg every three weeks for up to two years. The primary objective of this study is to evaluate the long-term safety and tolerability of patisiran administration. In addition, the study will measure a number of clinical endpoints every six months. This includes measurement of a modified composite Neuropathy Impairment Score, termed “mNIS+7,” which is an evaluation of muscle weakness, sensory and autonomic function, and nerve conductance across a 304-point scale, where neuropathy progression leads to an increased score over time. A number of additional clinical endpoints will be assessed, including: quality of life; timed ten-meter walk test to evaluate mobility; modified body mass index, or BMI, as a measure of nutritional status; level of disability; and nerve fiber density in skin biopsies. In addition, serum TTR levels will be measured.
In November 2013, we also announced the initiation of our APOLLO Phase 3 clinical trial of patisiran. The APOLLO Phase 3 clinical trial is a randomized, double-blind, placebo-controlled, global study designed to evaluate the efficacy and safety of patisiran in ATTR patients with FAP. The primary endpoint of the study is the difference in the change in mNIS+7 between patisiran and placebo at 18 months. Secondary endpoints include: the Norfolk Quality of Life-Diabetic Neuropathy (QOL-DN) score; NIS-weakness; modified BMI; timed ten-meter walk; and the COMPASS-31 autonomic symptom score. The trial is designed to enroll up to 200 FAP patients with a baseline NIS in the range of ten to 100, which represents patients with Stage 1 or Stage 2 disease. Patients will be randomized two-to-one, patisiran-to-placebo, with patisiran administered at 0.30 mg/kg once every three weeks for 18 months. The study was designed with 90% power to conservatively detect as little as a 37.5% difference in change in mNIS+7 between treatment groups, with a two-sided alpha of 0.05. The placebo mNIS+7 progression rate was derived from an Alnylam analysis of natural history data from 283 FAP patients. Prior to the initiation of the APOLLO study, we obtained protocol assistance for the patisiran Phase 3 clinical trial from the European Medicines Agency, or EMA, and completed our End-of-Phase 2 meeting with the U.S. Food and Drug Administration, or FDA. All patients completing the APOLLO Phase 3 clinical trial will be eligible to enroll in a Phase 3 OLE study.
The Committee for Orphan Medicinal Products, or COMP, of the EMA has designated patisiran as an orphan medicinal product for the treatment of FAP. Orphan Drug Designation, or ODD, by the European Commission, or EC, provides regulatory and financial incentives for companies developing orphan drugs to develop and market therapies that treat a life-threatening or chronically debilitating condition affecting no more than five in 10,000 persons in the EU. In addition to a ten-year period of marketing exclusivity in the EU after
10
Table of Contents
product approval, ODD provides companies with protocol assistance from the EMA during the product development phase, direct access to centralized marketing authorization and reduced regulatory fees. In addition, FDA provided ODD to patisiran as a therapeutic for the treatment of FAP. The FDA’s ODD program provides orphan status to drugs and biologics which are defined as those intended for the safe and effective treatment, diagnosis or prevention of rare diseases/disorders that affect fewer than 200,000 people in the United States.
In November 2013, the FDA granted Fast Track designation to patisiran for the treatment of FAP. According to the FDA, Fast Track is a process designed to facilitate the development and expedite the review of drugs to treat serious conditions and to fill an unmet medical need. The purpose is to get important new drugs to the patient earlier.
ALN-TTRsc. In addition to patisirian, we are also advancing ALN-TTRsc, which utilizes our proprietary GalNAc-siRNA conjugate delivery technology enabling subcutaneous dose administration, with a wide therapeutic index. In September 2013, we reported interim results from our Phase 1 clinical trial of ALN-TTRsc conducted in the United Kingdom. The Phase 1 clinical trial was a randomized, double-blind, placebo-controlled, single- and multi-dose, dose-escalation trial, enrolling up to 40 healthy volunteer subjects. The primary objective of this study was to evaluate the safety and tolerability of single and multiple doses of subcutaneously administered ALN-TTRsc. Secondary objectives included assessment of clinical activity of the ALN-TTRsc as measured by serum TTR levels. In an initial single-ascending dose phase of the study, subjects (n=16) received subcutaneous doses of placebo or ALN-TTRsc from 1.25 to 10.0 mg/kg. In the multiple-ascending dose phase of the study, subjects (n=12) received ten subcutaneous doses of placebo or ALN-TTRsc from 2.5 to 10.0 mg/kg. As reported in September 2013, interim data from the 28 subjects enrolled and analyzed in this study as of that date showed that single- and multi-dose administration of ALN-TTRsc resulted in rapid, dose-dependent, consistent and durable knockdown of serum TTR levels. In the multi-dose cohorts (n=12), there was a statistically significant knockdown of serum TTR at doses of 2.5 mg/kg (p<0.01), 5.0 mg/kg (p<0.001) and 10.0 mg/kg (p<0.001) as compared to placebo. At a dose of 5.0 mg/kg, ALN-TTRsc administration resulted in an up to 93.3% knockdown of serum TTR and a mean TTR knockdown of 87.5% at nadir. At a dose of 10.0 mg/kg, ALN-TTRsc administration led to an up to 94.0% knockdown of serum TTR and a mean TTR knockdown of 92.4% at nadir. Analysis of the TTR knockdown in humans as compared to results obtained in non-human primates, or NHPs, showed a closely correlated, essentially one-to-one relationship on a mg/kg basis (r2=0.83, p<0.001). We believe these results confirm human translation for our GalNAc-siRNA conjugate platform, which is also being employed in the majority of our other pre-clinical and clinical genetic medicine programs.
In this study, as reported in September 2013, single and multiple doses of ALN-TTRsc were found to be generally well tolerated. There were no significant adverse events associated with drug at doses through 10.0 mg/kg. All adverse events were deemed mild or moderate in severity. Injection site reactions were observed in a minority of subjects receiving ALN-TTRsc (24%) or placebo (14%). These were reported as being clinically mild and consisted of transient erythema associated in a minority of cases with edema and/or pain. In all cases, these reactions were self-limiting and resolved within approximately two hours of onset. There were no study discontinuations, flu-like symptoms, or changes in cytokines, C-reactive protein, liver function tests, renal function or hematologic parameters.
In December 2013, we initiated a pilot Phase 2 clinical trial with ALN-TTRsc. The Phase 2 clinical trial is an open-label, multi-dose study of ALN-TTRsc, designed to enroll approximately 15 TTR cardiac amyloidosis patients with FAC or SSA. The primary objective of the study is to evaluate the general tolerability of ALN-TTRsc. Patients will receive five daily doses followed by five weekly doses of five mg/kg, with follow-up through Day 90. Secondary objectives include assessment of clinical activity as measured by knockdown of serum TTR levels and additional tests, such as cardiac imaging (including echocardiography and cardiac MRI), circulating cardiac biomarkers (NT-proBNP and troponins T and I), six-minute walk test, New York Heart Association classification, and measures of heart failure symptoms and quality of life (Kansas City Cardiomyopathy Questionnaire and EQ-5D QOL). Patients completing the Phase 2 clinical trial will be eligible to participate in an OLE study for further assessment of general tolerability and clinical activity with long-term dosing.
In October 2012, we and Genzyme entered into a license and collaboration agreement pursuant to which we granted to Genzyme an exclusive license in Japan and the Asia-Pacific region to develop and commercialize
11
Table of Contents
RNAi therapeutics targeting TTR, including patisirian and ALN-TTRsc, for the treatment of ATTR and other human diseases. In early 2014, this relationship was extended as a significantly broader alliance to advance RNAi therapeutics as genetic medicines. Under the 2014 Genzyme collaboration, we will lead development and commercialization of patisiran in North America and Western Europe while Genzyme will develop and commercialize the product in the Genzyme Territory. In addition, under this new collaboration, we and Genzyme have agreed to co-develop and co-commercialize ALN-TTRsc in North America and Western Europe, with Genzyme developing and commercializing the product in the Genzyme Territory. This broadened relationship on ALN-TTRsc is aimed at expanding and accelerating the product’s potential global value. The 2014 Genzyme collaboration is described below under the heading “Strategic Alliances.”
ALN-AT3 — Hemophilia and Rare Bleeding Disorders (RBD)
ALN-AT3 is an RNAi therapeutic targeting AT, a genetically defined target, for the treatment of hemophilia and RBD. Hemophilias are hereditary disorders caused by genetic deficiencies of various blood clotting factors, resulting in recurrent bleeds into joints, muscles and other major internal organs. Hemophilia A, or HA, is defined by loss-of-function mutations in Factor VIII, and there are greater than 40,000 registered HA patients in the United States and EU. Hemophilia B, or HB, defined by loss-of-function mutations in Factor IX, affects greater than 9,500 registered patients in the United States and EU. RBD are defined by congenital deficiencies of other blood coagulation factors including Factors II, V, VII, X and XI. Based on our analysis of the available patient and market data, we estimate that there are approximately 1,000 RBD patients worldwide with a severe bleeding phenotype. Standard treatment for hemophilia patients involves replacement of the missing clotting factor either as prophylaxis or on-demand therapy. As many as one-third of people with severe HA will develop an antibody to their replacement factor, a very serious complication. These ‘inhibitor’ patients become refractory to standard replacement therapy. There exists a small subset of hemophilia patients who have co-inherited a prothrombotic mutation, such as Factor V Leiden, antithrombin deficiency, protein C deficiency and prothrombin G20210A. People with hemophilia who have co-inherited these prothrombotic mutations are characterized as having milder disease, with a later onset of disease, lower risk of bleeding and reduced requirements for Factor VIII or Factor IX treatment as part of their disease management. There remains a significant need for novel therapeutics to treat people with hemophilia and RBD.
ALN-AT3 is a novel therapeutic approach aimed at re-balancing the coagulation cascade and normalizing hemostasis in severe HA and HB patients, including patients with inhibitors against their replacement factor. The program aims to reproduce the human genetic findings of a milder clinical disease in people with hemophilia who have co-inherited prothrombotic mutations. In January 2014, we initiated a Phase 1 clinical trial with ALN-AT3. The Phase 1 clinical trial is being conducted in the U.K. as a single- and multi-dose, dose-escalation study consisting of two parts. Part A is a randomized, single-blind, placebo-controlled, single-dose, dose-escalation study, enrolling up to 24 healthy volunteer subjects. The primary objective of this part of the study is to evaluate the safety and tolerability of a single low subcutaneous dose of ALN-AT3, with the potential secondarily to show changes in AT plasma levels at sub-pharmacologic doses. Part B of the study will be an open-label, multi-dose, dose-escalation study enrolling up to 18 people with moderate to severe HA or HB. The primary objective of this part of the study is to evaluate the safety and tolerability of multiple doses of subcutaneously administered ALN-AT3 in hemophilia subjects. Secondary objectives include assessment of clinical activity as determined by knockdown of circulating AT levels and increase in thrombin generation at pharmacologic doses of ALN-AT3. Thrombin generation is known to be a biomarker for bleeding frequency and severity in people with hemophilia.
Pre-clinical studies showed that ALN-AT3 achieves rapid, dose-dependent and durable knockdown of AT in rodents and NHPs. In NHPs, weekly subcutaneous doses as low as 0.125 mg/kg led to a 50% knockdown of AT, while weekly doses of 0.50 mg/kg led to approximately 90% knockdown. In addition, ALN-AT3 administration was found to normalize thrombin generation and improve hemostasis in hemophilia mice and fully correct thrombin generation in a NHP hemophilia inhibitor model. Additional pre-clinical results showing that repeat administration of ALN-AT3 was well tolerated in HA mice, with no adverse findings up to dose levels 200 times greater than those required to achieve 50% AT knockdown. Results from these studies also demonstrated that ALN-AT3 administration achieved complete correction in HA mice of the activated Partial Thromboplastin Time (aPTT), an ex vivo measure of blood coagulation that is significantly prolonged in hemophilia.
12
Table of Contents
Under the 2014 Genzyme collaboration, Genzyme will have the right to opt-in to the ALN-AT3 program after completion of Human POP to either co-develop and co-promote ALN-AT3 with us in North America and Western Europe, with Genzyme developing and commercializing in the Genzyme Territory (subject to its development decision regarding ALN-AS1), or to develop and commercialize the product in the Genzyme Territory. In both circumstances, we will lead and control development and commercialization in North America and Western Europe.
ALN-CC5 — Complement-Mediated Diseases
ALN-CC5 is an RNAi therapeutic targeting the C5 component of the complement pathway for the treatment of complement-mediated diseases. The complement system plays a central role in immunity as a protective mechanism for host defense, but its dysregulation results in life-threatening complications in a broad range of human diseases including paroxysmal nocturnal hemoglobinuria, or PNH, atypical hemolytic-uremic syndrome, or aHUS, myasthenia gravis and neuromyelitis optica, amongst many others. Complement component C5, which is predominantly expressed in liver cells, is a genetically and clinically validated target; loss of function human mutations are associated with an attenuated immune response against certain infections. Intravenous anti-C5 monoclonal antibody therapy (eculizumab) has demonstrated clinical activity and tolerability in a number of complement-mediated diseases. It is approved for the treatment PNH and aHUS in the United States, Europe and other countries. A subcutaneously administered RNAi therapeutic that silences C5 represents a novel approach to the treatment of complement-mediated diseases, with a potentially competitive profile compared with intravenously administered anti-C5 monoclonal antibody therapy.
ALN-CC5 utilizes Alnylam’s proprietary GalNAc-siRNA conjugate delivery platform enabling subcutaneous dose administration. In December 2013, we presented pre-clinical data showing robust, dose-dependent and durable knockdown of serum C5 in NHPs. Multiple doses of ALN-CC5 at 2.5 or 5.0 mg/kg led to rapid and dose-dependent knockdown of serum C5 of up to 97.8%, with mean knockdown at nadir of 97.5% (p<0.001) at the top dose. Knockdown of C5 was durable, with greater than 90% knockdown sustained for up to three weeks after the final dose. Further, subcutaneous administration resulted in a consistent greater than 80% knockdown of C5 during the treatment period. The results are consistent with published literature that shows an hepatic origin for the vast majority of circulating C5, and confirms that the serum component of locally produced C5 is minimal at best. In addition, multi-dose administration of ALN-CC5 resulted in robust and durable inhibition of serum hemolytic activity, a measure of complement activity in the blood. At the top dose of 5.0 mg/kg, an up to 94% inhibition of hemolytic activity was observed, with a mean nadir reduction of 92% (p<0.01). Inhibition of hemolytic activity was sustained for at least two weeks after the final dose. Further, inhibition of hemolytic activity was shown to be highly correlated with serum levels of C5 (r2=0.93, p<10-15). Importantly, a greater than 80% inhibition in hemolytic activity has been previously validated in studies of eculizumab in patients with PNH as being associated with clinical benefit. The essentially complete knockdown of C5 was well tolerated in NHPs, as evidenced by no changes in hematology, serum chemistry or coagulation parameters at 24 hours after the last dose. We are continuing to optimize our C5-targeted siRNA lead candidate, and expect to identify our final development candidate for ALN-CC5 in early 2014.
Under the 2014 Genzyme collaboration, Genzyme will have the right to opt-in to the ALN-CC5 program after completion of Human POP to develop and commercialize the product in the Genzyme Territory. We will lead and control development and commercialization in North America and Western Europe.
ALN-AS1 — Hepatic Porphyrias
ALN-AS1 is an RNAi therapeutic targeting ALAS-1 for the treatment of hepatic porphyrias, including AIP. AIP is an ultra-rare autosomal dominant disease caused by loss-of-function mutations in porphobilinogen deaminase, or PBGD, an enzyme in the heme biosynthesis pathway. Exposure of AIP patients to certain drugs, dieting or hormonal changes can trigger strong induction of ALAS-1, another enzyme in the heme biosynthesis pathway, which can lead to accumulation of heme intermediates upstream of PBGD that precipitate attack symptoms. Patients with AIP can suffer acute and/or recurrent life-threatening attacks with severe abdominal pain, peripheral and autonomic neuropathy, and neuropsychiatric manifestations, and possible death if left untreated. Based on our analysis of the available patient and market data, we estimate that approximately 5,000
13
Table of Contents
patients in the United States and Europe suffer acute porphyria attacks annually, and we believe approximately 500 of those patients are afflicted with recurrent debilitating attacks. Treatment options for AIP patients suffering from an acute attack are limited; some patients are treated with intravenous heme analogues that have a slow onset and can result in severe thrombophlebitis and iron overload. Currently, there is no approved prophylactic treatment available to prevent recurrent attacks, which often occur monthly in women associated with menses. There exists a significant need for therapies for AIP patients.
ALN-AS1 is a subcutaneously administered RNAi therepautic targeting ALAS-1. Inhibition of ALAS-1 is known to reduce the accumulation of heme precursors that are believed to cause the clinical manifestations of AIP. ALN-AS1 has the potential to be a therapy for the treatment of hepatic porphyrias, including acute attacks, as well as a potential prophylactic approach for the prevention of recurrent attacks. In October 2013, we announced that we have identified a development candidate for advancement and presented data from pre-clinical models of the human disease showing that multi-dose administration of a GalNAc-siRNA targeting ALAS-1 results in a rapid, dose-dependent and long-lasting knockdown of ALAS-1 mRNA and complete inhibition of the toxic intermediates that mediate the symptoms and pathology of AIP. We have initiated IND-enabling studies, with the goal of filing an IND application or IND equivalent for ALN-AS1.
Under the 2014 Genzyme collaboration, Genzyme will have the right to opt-in to the ALN-AS1 program after completion of Human POP to either obtain a global license to ALN-AS1 for the treatment of hepatic porphyrias (subject to its development decision regarding ALN-AT3), or to develop and commercialize the product in the Genzyme Territory. In the latter case, we will lead and control development and commercialization in North America and Western Europe.
ALN-PCS — Hypercholesterolemia
ALN-PCS is an RNAi therapeutic targeting PCSK9 for the treatment of hypercholesterolemia. PCSK9 is a protein involved in the regulation of low-density lipoprotein, or LDL, receptor levels on hepatocytes and the metabolism of LDL cholesterol, or LDL-C, which is commonly referred to as “bad” cholesterol. PCSK9 is produced by the liver and circulates in the bloodstream. Both intracellular and extracellular PCSK9 reduce the liver’s capacity to absorb LDL-C by decreasing LDL receptor levels. Published studies indicate that, if PCSK9 activity could be reduced, the liver’s uptake of LDL-C should increase and blood cholesterol levels should decrease. In fact, published case reports have shown individuals with loss-of-function genetic mutations in PCSK9 have decreased blood cholesterol levels. In turn, these individuals have been shown to have a dramatically reduced risk of coronary artery disease, or CAD, including myocardial infarction or heart attack. In addition, studies have shown that PCSK9 levels are increased by statin therapy, limiting their effect, suggesting that the introduction of a PCSK9 inhibitor to statin therapy may result in even further reductions in LDL-C levels. Hypercholesterolemia is a condition characterized by very high levels of cholesterol in the blood which is known to increase the risk of coronary artery disease, the leading cause of death in the United States. Some forms of hypercholesterolemia can be treated through dietary restrictions, lifestyle modifications (e.g., exercise and smoking cessation) and medicines such as statins. However, a large proportion of patients with hypercholesterolemia, including genetic familial hypercholesterolemia patients, acute coronary syndrome patients, high-risk patient populations (e.g., patients with CAD, diabetes, symptomatic carotid artery disease) and other patients that are statin intolerant, are not achieving target LDL-C goals with statin therapy. Severe forms of hypercholesterolemia are estimated to affect more than 500,000 patients worldwide, and as a result, there is a significant need for novel therapeutics to treat patients with hypercholesterolemia whose disease is inadequately managed by existing therapies.
In February 2013, we and MDCO entered into a license and collaboration agreement pursuant to which we granted to MDCO an exclusive, worldwide license to develop, manufacture and commercialize RNAi therapeutics targeting PCSK9, including ALN-PCS02 and ALN-PCSsc, for the treatment of hypercholesterolemia and other human diseases. A description of our agreement with MDCO is included below under the heading “Strategic Alliances.”
In April 2012, we reported clinical data from our Phase 1 clinical trial of ALN-PCS02. ALN-PCS02 employs the same LNP formulation used for patisiran. The Phase 1 clinical trial was conducted in the United Kingdom as a randomized, single-blind, placebo-controlled, single-ascending dose study in healthy volunteer
14
Table of Contents
subjects with elevated baseline LDL-C (greater than 116mg/dL). The primary objective of the clinical trial was to evaluate the safety and tolerability of a single dose of ALN-PCS02. Secondary objectives included assessment of pharmacodynamic effects of the drug on plasma PCSK9 protein levels and evaluation of clinical efficacy as measured by LDL-C levels. The clinical trial was performed in the absence of statins or other lipid lowering therapy. A total of 32 subjects were enrolled into six sequential dose cohorts ranging from 0.015 to 0.400 mg/kg in a three-to-one randomization of drug to placebo.
In this clinical trial, as reported in April 2012, administration of ALN-PCS02 resulted in rapid, dose-dependent and durable reductions in LDL-C of up to 50% relative to baseline and placebo, with a statistically significant mean reduction of 41% (p<0.01) at the 0.400 mg/kg dose level. In addition, ALN-PCS02 administration resulted in rapid, dose-dependent and durable knockdown of PCSK9 protein levels in plasma with a maximal 84% reduction relative to baseline and placebo, with a statistically significant mean reduction of 68% in the highest dose group of 0.400 mg/kg (p<0.0001). There was also a dose-dependent increase in the proportion of subjects who achieved “target” levels of LDL-C of less than 100 mg/dL (p<0.05). ALN-PCS02 was found to be generally well tolerated in this study and there were no serious adverse events related to study drug administration. There were no drug-related discontinuations and no liver enzyme elevations. A mild, transient rash, observed in 16 subjects, including four who received placebo, is believed to be related to steroid pre-medication provided to subjects receiving both ALN-PCS02, as well as those receiving placebo. There were no significant changes compared to baseline in levels of high-density lipoprotein, or HDL, also referred to as “good” cholesterol, consistent with the phenotype observed in human PCSK9 loss-of-function mutations.
We are also developing a GalNAc-siRNA conjugate targeting PCSK9 that enables subcutaneous dose administration. In October 2013, we and MDCO announced that we have identified a lead development candidate for the collaboration, ALN-PCSsc, that is a subcutaneously administered RNAi therapeutic. Specifically, pre-clinical studies in NHPs performed in the absence of concomitant statin therapy showed that this new development candidate led to an up to 95% knockdown of plasma PCSK9, with mean PCSK9 knockdown at nadir of 87% (p<0.0001 compared with pre-dose values according to ANOVA models) and an up to 67% lowering of LDL-C, with mean LDL-C lowering at nadir of 62% (p<0.0001 compared with pre-dose values). The level of LDL-C reduction was achieved in the absence of statin co-administration. Knockdown of PCSK9 and lowering of LDL-C was rapid and durable, with effects lasting greater than 50 days after the final dose, supportive of the potential for once-monthly dosing and potentially a highly competitive target product profile. We are advancing ALN-PCSsc to file an IND application or IND equivalent and will lead the program through the completion of Phase 1. MDCO is responsible for leading and funding development from Phase 2 forward and for commercializing the product if development is successful.
Additional Genetic Medicine Programs
In addition to the programs described above, we are also advancing other early stage genetic medicine programs. These programs include: ALN-AAT, an RNAi therapeutic targeting AAT in development for the treatment of AAT deficiency liver disease; ALN-TMP, an RNAi therapeutic targeting TMPRSS6 in development for the treatment of beta-thalassemia and iron-overload disorders; ALN-ANG, an RNAi therapeutic targeting ANGPTL3 in development for the treatment of genetic forms of mixed hyperlipidemia and severe hypertriglyceridemia; and other yet to be disclosed programs. We expect to identify one to two new development candidates by the end of 2014.
Partner-Based Product Development Programs
While focusing our core efforts on advancing our genetic medicine product development programs as described above, we also intend to continue to advance additional product development programs through existing or future alliances, including the clinical and pre-clinical programs described below.
ALN-RSV01 — Respiratory Syncytial Virus (RSV) Infection
ALN-RSV01 is an RNAi therapeutic for the treatment of RSV infection. We have conducted numerous clinical trials of ALN-RSV01, including an international multi-center, randomized, double-blind, placebo-
15
Table of Contents
controlled Phase 2b clinical trial with ALN-RSV01 for the treatment of RSV infection in lung transplant patients. In September 2012, we reported complete results from our Phase 2b clinical trial. During 2012, we met with the FDA and European regulatory authorities regarding the results of the ALN-RSV01 Phase 2b clinical trial and obtained preliminary guidance on the design of a potential Phase 3 clinical trial.
We have a collaboration with Kyowa Hakko Kirin for the development and commercialization of RNAi products for the treatment of RSV in Asia. We also had an agreement with Cubist, pursuant to which Cubist had the right to opt into collaborating with us on ALN-RSV01, subject to certain conditions. In February 2013, Cubist notified us that it would not exercise its opt-in right for ALN-RSV01. In light of this determination, we and Cubist mutually agreed to terminate our agreement. We do not intend to advance the ALN-RSV01 program into a Phase 3 clinical trial unless we are able to identify a partner outside the Kyowa Hakko Kirin territory.
ALN-VSP — Liver Cancer
ALN-VSP is a systemically delivered RNAi therapeutic for the treatment of advanced solid tumors with liver involvement. In January 2013, we published complete study results from our Phase 1 clinical trial for ALN-VSP. The ALN-VSP Phase 1 clinical trial was a multi-center, open-label, dose-escalation study in patients with advanced solid tumors with liver involvement who failed to respond to or had progressed after standard treatment.
In July 2012, we formed a collaboration with Ascletis, a privately held U.S.-China joint venture pharmaceutical company, for the development of ALN-VSP. Under the collaboration agreement, we granted Ascletis exclusive rights to develop and commercialize ALN-VSP in China, including Hong Kong and Macau, and Taiwan. We retain all rights to develop and commercialize ALN-VSP in the rest of the world. We may use the data generated in China by Ascletis under this strategic collaboration for development of ALN-VSP in the rest of the world. We do not intend to advance the ALN-VSP program into a Phase 2 clinical trial unless we are able to identify a partner outside of the Ascletis territory.
Other Partner-Based Programs
In addition to ALN-RSV01 and ALN-VSP, we are also supporting the development of ALN-HTT, a novel drug-device product incorporating an RNAi therapeutic candidate targeting the huntingtin gene, delivered using an implantable infusion device, for the treatment of HD, in collaboration with Medtronic and CHDI. ALN-HTT is currently in pre-clinical development.
Additional Discovery Programs
In addition to our core development efforts on genetic medicine programs and our additional partner-based programs in RSV, liver cancer and HD, we are conducting additional research activities to discover novel RNAi therapeutic product candidates that we can partner with third parties.
Our Collaboration and Licensing Strategy
Our business strategy is to develop and commercialize a pipeline of RNAi therapeutic products directed toward genetically defined targets for the treatment of diseases with high unmet medical need. As part of this strategy, we have entered into, and expect to enter into additional, collaboration and licensing agreements as a means of obtaining resources, capabilities and funding to advance our RNAi therapeutic programs.
Our collaboration strategy is to form alliances that create significant value for ourselves and our collaborators in the advancement of RNAi therapeutics as a new class of innovative medicines. Specifically, our goal is to retain development and commercial rights for our current and future genetic medicine pipeline in North America and Western Europe, while forming alliances with leading, innovative companies for the development and commercialization of these products in the ROW. In early 2014, we formed an alliance with Genzyme to develop and commercialize our current and future genetic medicine pipeline principally in territories outside of North America and Western Europe, subject to certain broader rights. This broad collaboration will replace our 2012 alliance with Genzyme, has been approved by the boards of both companies, and is subject to customary closing conditions and clearances under the Hart-Scott Rodino Antitrust Improvements Act.
16
Table of Contents
With respect to programs that are outside of our core focus on genetic medicines, for example, in the fields of metabolic and infectious disease and in oncology, we intend to seek regional or global alliances. For example, in 2012, we formed a regional alliance with Ascletis for the development and commercialization of ALN-VSP in China and certain other territories. In addition, in early 2013, we established a worldwide alliance with MDCO for the development and commercialization of ALN-PCS. We may also enter into RNAi platform and/or multi-target discovery alliances. For example, we have entered into a broad, non-exclusive platform license agreement with Takeda. In addition, we have formed a platform alliance with Monsanto in the field of agriculture.
We also seek to form or advance new ventures and opportunities in areas outside our primary focus on RNAi therapeutics. In 2007, we and Isis formed Regulus to capitalize on our technology and intellectual property in the field of microRNA therapeutics. Currently, we own approximately 15% of Regulus’ outstanding common stock. Through an internal effort we refer to as Alnylam Biotherapeutics, we have evaluated the application of RNAi technology to improve the manufacturing processes for biologics. We have also evaluated the utility of our VaxiRNA platform, an RNAi technology developed under our Alnylam Biotherapeutics initiative, for the enhanced production of viruses used in the manufacture of vaccine products.
To generate revenues from our intellectual property rights, we also grant licenses to biotechnology companies under our InterfeRx program for the development and commercialization of RNAi therapeutics for specified targets in which we have no direct strategic interest. We also license key aspects of our intellectual property to companies active in the research products and services market, which includes the manufacture and sale of reagents. We expect our InterfeRx and research product licenses to generate modest near-term revenues that we can re-invest in the development of our proprietary RNAi therapeutics pipeline. As of January 31, 2014, we had granted such licenses, on both an exclusive and non-exclusive basis, to approximately 20 companies.
Since delivery of RNAi therapeutics remains an important objective of our research activities, we also look to form collaboration and licensing arrangements with other companies and academic institutions to gain access to delivery technologies. For example, we have entered into agreements with Arrowhead, Tekmira Pharmaceuticals Corporation, or TPC, Protiva Biotherapeutics, Inc., a wholly owned subsidiary of TPC, and together with TPC, referred to as Tekmira, the Massachusetts Institute of Technology, or MIT, The University of British Columbia, or UBC, and Acuitas Therapeutics Inc. (formerly AlCana Technologies, Inc.), or Acuitas, among others, related to various delivery technologies. We have also entered into license agreements with Isis, Max Planck Innovation GmbH (formerly known as Garching Innovation GmbH), or Max Planck Innovation, Tekmira, MIT, Cancer Research Technology Limited, or CRT, and Whitehead Institute for Biomedical Research, or Whitehead, as well as a number of other entities, to obtain rights to intellectual property in the field of RNAi. Finally, we have sought, and may seek in the future, funding for the development of our proprietary RNAi therapeutics pipeline from the government and foundations.
Strategic Alliances
We have formed, and intend to continue to form, strategic alliances to gain access to the financial, technical, clinical and commercial resources necessary to develop and market RNAi therapeutics. We expect these alliances to provide us with financial support in the form of upfront cash payments, license fees, equity investments, research and development funding, milestone payments and/or royalties or profit sharing based on sales of RNAi therapeutics.
Product Alliances.
Genzyme. On January 11, 2014, we entered into a global, strategic collaboration with Genzyme to discover, develop and commercialize RNAi therapeutics as genetic medicines to treat orphan diseases. The 2014 Genzyme collaboration is governed by a master collaboration agreement, including the license terms appended thereto, which will become effective upon closing of the equity transaction, described below. Once effective, the master agreement will supersede and replace the previous collaboration between us and Genzyme entered into in October 2012 to develop and commercialize RNAi therapeutics targeting TTR for the treatment of ATTR, which original Genzyme agreement is described under the heading “Strategic Alliances” in our annual report on Form 10-K for the year ended December 31, 2012.
17
Table of Contents
The 2014 Genzyme collaboration is structured as an exclusive relationship for the worldwide development and commercialization of RNAi therapeutics in the field of genetic medicines, which includes our current and future genetic medicine programs that reach Human POP by the end of 2019, subject to extension to the end of 2021 in various circumstances. We will retain product rights in North America and Western Europe, while Genzyme will obtain exclusive rights to develop and commercialize collaboration products in the Genzyme Territory, together with certain broader co-development/co-promote or worldwide rights for certain products. Genzyme’s rights are structured as an opt-in that is triggered upon achievement of Human POP. We will maintain development control for all programs prior to Genzyme’s opt-in and maintain development and commercialization control after Genzyme’s opt-in for all programs in our territory.
Upon the effective date of the 2014 Genzyme collaboration, Genzyme will opt-in to patisiran for the Genzyme Territory, and we will retain full product rights in North America and Western Europe. We and Genzyme have also agreed to expand our current collaboration on ALN-TTRsc, where we and Genzyme will co-develop and co-promote ALN-TTRsc in North America and Western Europe. We will maintain development and commercialization control with ALN-TTRsc and Genzyme will develop and commercialize the product in the Genzyme Territory.
In addition to its regional rights for our current and future genetic medicine programs in the Genzyme Territory, Genzyme will have the right to either (i) co-develop and co-promote ALN-AT3 for the treatment of hemophilia and other RBDs in our territory, with us maintaining development and commercialization control, or (ii) obtain a global license to ALN-AS1 for the treatment of hepatic porphyrias. Genzyme will exercise this selection right upon Human POP for the ALN-AT3 and ALN-AS1 programs. Finally, Genzyme will have the right for a global license to a single, future genetic medicine program that is not one of our currently defined genetic medicine programs. We will retain global rights to any RNAi therapeutic genetic medicine program that does not reach Human POP by the end of 2019, subject to certain limited exceptions. Under the terms of the master agreement, we will retain full rights to all current and future RNAi therapeutic programs outside of the field of genetic medicines, including the right to form new collaborations.
In consideration for the rights granted to Genzyme under the master agreement, Genzyme is required to make payments to us for each collaboration product upon the achievement of specified development, regulatory and commercial milestones for each (i) regional (e.g., patisiran) and co-developed/co-promoted (e.g., ALN-TTRsc) collaboration product totaling up to $75.0 million and (ii) global collaboration product up to $200.0 million, and to pay tiered double-digit royalties up to twenty percent for each regional and global collaboration product based on annual net sales, if any, of each collaboration product by Genzyme, its affiliates and sublicensees. In the case of co-developed/co-promoted collaboration products, the parties will share profits equally and we will book product sales in North America and Western Europe. Due to the uncertainty of pharmaceutical development and the high historical failure rates generally associated with drug development, we may not receive any milestone or royalty payments from Genzyme under the 2014 Genzyme collaboration.
Under the master agreement, the parties will collaborate in the development of option products, with us leading development for all programs prior to Genzyme’s opt-in and also leading development and commercialization for all programs in our territory after Genzyme’s opt-in. Development costs for collaboration products once Genzyme exercises an option (or as of the effective date for patisiran and ALN-TTRsc) will be shared between Genzyme and us as follows, subject to the provisions of the relevant license terms: (a) for all regional collaboration products, Genzyme shall be responsible for twenty percent of the global development costs, (b) for all co-develop/co-promote collaboration products, Genzyme shall be responsible for fifty percent of the global development costs, and (c) for all global collaboration products, Genzyme shall be responsible for one hundred percent of global development costs. If Genzyme does not exercise its option to license rights to a particular program, we will retain the exclusive right to develop and commercialize such program throughout the world, including the right to sublicense to third parties.
The 2014 Genzyme collaboration will be governed by an alliance joint steering committee that will be comprised of an equal number of representatives from each party. There will also be additional committees to manage various aspects of each regional, co-developed/co-promoted and global program. We and Genzyme
18
Table of Contents
intend to enter into supply agreements to provide for supply of collaboration products to Genzyme for clinical manage various aspects of each regional, co-developed/co-promoted and global program. We and Genzyme intend to enter into supply agreements to provide for supply of collaboration products to Genzyme for clinical studies, and, at Genzyme’s request, commercial sales. Genzyme also has certain rights to manufacture collaboration products. Additionally, Genzyme has certain limited opt-out rights, as specified in the master agreement, upon which products revert fully back to us with no further obligations to Genzyme.
In addition, under the master agreement, we and Genzyme have agreed to enter into exclusive discussions and negotiations regarding a potential collaboration around the delivery of siRNAs to the central nervous system, or CNS, with the objective of enabling the discovery of siRNAs for the treatment of CNS disorders.
The master agreement (including the license terms appended thereto) contains certain termination provisions, including for material breach by the other party. Unless terminated earlier pursuant to its terms, the master agreement will terminate upon the last to expire of any of the option periods under the master agreement or the license terms appended thereto.
In consideration for the rights granted to Genzyme under the master agreement and pursuant to the terms of a stock purchase agreement, we agreed to sell to Genzyme 8,766,338 shares of our common stock and Genzyme agreed to pay to us $700.0 million in aggregate cash consideration. Following the closing of the stock purchase, Genzyme will beneficially own approximately 12% of our outstanding common stock. The stock purchase agreement contains customary representations, warranties and covenants of each of the parties thereto. Subject to customary closing conditions, including the expiration or early termination of the applicable pre-merger waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, the stock purchase is expected to close during the first quarter of 2014.
As a condition to the closing of the stock purchase, Genzyme will enter into an investor agreement with us. Under the investor agreement, until the earlier of the fifth anniversary of the expiration or earlier termination of the 2014 Genzyme collaboration and the date on which Genzyme and its affiliates cease to beneficially own at least 5% of our outstanding common stock, Genzyme and its affiliates will be bound by certain “standstill” provisions. The standstill provisions include agreements not to acquire more than 30% of our outstanding common stock, call stockholder meetings, nominate directors other than those approved by our board of directors, subject to certain limited exceptions, or propose or support a proposal to acquire us.
Further, Genzyme will agree to vote, and cause its affiliates to vote, all shares of our voting securities they are entitled to vote, up to a maximum of 20% of our outstanding common stock, in a manner either as recommended by our board of directors or proportionally with the votes cast by our other stockholders, except with respect to certain change of control transactions or our liquidation or dissolution. Until Genzyme owns less than 7.5% of our outstanding common stock, subject to Genzyme’s limited right to maintain its ownership percentage as described below, if we issue common stock or securities convertible into or exercisable for common stock to a third party that holds at least 30% of our outstanding common stock or, in connection with a collaboration or license transaction, to a third party that will initially hold at least the percentage of our outstanding shares of common stock represented by the shares purchased by Genzyme at the closing of the stock purchase, we will offer Genzyme an opportunity to amend the standstill and voting provisions in the investor agreement to be consistent with the terms provided to such third party.
Under the investor agreement, Genzyme will agree not to dispose of any shares of common stock beneficially owned by it immediately after the closing of the stock purchase until the earlier of (i) December 31, 2019 (subject to extension by up to two years if Genzyme’s option to select additional compounds under the master agreement is extended beyond December 31, 2019) and (ii) six months after the expiration or earlier valid termination of the collaboration, in each case subject to earlier termination in the event certain clinical activities under the collaboration fail to occur. Following the expiration of this lock-up period, Genzyme will be permitted to sell such shares of common stock subject to certain limitations, including volume and manner of sale restrictions. Notwithstanding the foregoing, following the two-year anniversary of the closing of the stock purchase, in the event that the market price per share of our common stock is at least 100% higher than the market price per share of our common stock at closing of the stock purchase (in each case based upon a ten-day
19
Table of Contents
trailing average), Genzyme may sell up to 25% of its initial shares, subject to certain restrictions on post-lock-up period dispositions as described above.
Under the investor agreement, following the lock-up period, Genzyme will have three demand rights to require us to conduct a registered underwritten public offering with respect to the shares of common stock beneficially owned by Genzyme immediately after the closing of the stock purchase. In addition, following the lock-up period, subject to certain conditions, Genzyme will be entitled to participate in registered underwritten public offerings by us if other selling stockholders are included in the registration.
The investor agreement provides that, until Genzyme owns less than 7.5% of our outstanding common stock, subject to Genzyme’s limited right to maintain its ownership percentage as described herein, in connection with new issuances of common stock, subject to certain exceptions, Genzyme will be entitled to a right of first offer to participate proportionally to maintain its then-current ownership percentage of our common stock. If Genzyme is not entitled to a right of first offer with respect to a new issuance, Genzyme will have the opportunity, on a post-transaction basis, to purchase additional shares sufficient to maintain its pre-transaction ownership percentage of our common stock (subject to the same 7.5% ownership threshold).
In addition, in the event Genzyme and its affiliates acquire at least 20% or more of our outstanding common stock, Genzyme will be entitled to appoint one individual to our board of directors. Genzyme will also be entitled to certain information rights, including with respect to financial information in the event Genzyme or its affiliates require such information for its own financial reporting purposes. The rights and restrictions under the investor agreement are subject to termination upon the occurrence of certain events.
The Medicines Company. On February 4, 2013, we and MDCO entered into a license and collaboration agreement pursuant to which we granted to MDCO an exclusive, worldwide license to develop, manufacture and commercialize RNAi therapeutics targeting PCSK9, including ALN-PCS02 and ALN-PCSsc, for the treatment of hypercholesterolemia and other human diseases. In consideration for the rights granted to MDCO under the MDCO agreement, MDCO paid us an upfront cash payment of $25.0 million. In addition, MDCO is required to make payments to us upon the achievement of specified clinical development, regulatory approval and commercialization milestones totaling up to $180.0 million. In addition, we will be entitled to royalties ranging from the low- to high-teens, based on annual worldwide net sales, if any, of licensed products by MDCO, its affiliates and sublicensees, subject to reduction under specified circumstances. Due to the uncertainty of pharmaceutical development and the high historical failure rates generally associated with drug development, we may not receive any milestone or royalty payments from MDCO.
Under the MDCO agreement, we and MDCO will collaborate in the further development of licensed products. We will retain responsibility for the development of licensed products until Phase 1 completion, as defined in the MDCO agreement, at our cost, up to an agreed upon initial development cost cap. MDCO will assume all other responsibility for the development and commercialization of licensed products, at its sole cost. Initially the collaboration included the development of both ALN-PCS02 and ALN-PSCsc in parallel. In October 2013, the parties announced the selection of ALN-PCSsc for ongoing development, in accordance with the terms of the MDCO agreement. The collaboration between us and MDCO is governed by a joint steering committee comprised of an equal number of representatives from each party.
We are solely responsible for obtaining supply of finished product reasonably required for the conduct of our obligations under the initial development plan through Phase 1 completion, and supplying MDCO with finished product reasonably required for the first Phase 2 clinical trial of a licensed product conducted by MDCO, at our expense, provided such costs do not exceed the development costs cap, subject to certain exceptions. After such time, MDCO will have the sole right and responsibility to manufacture and supply licensed product for development and commercialization under the MDCO development plan, subject to the terms of the MDCO agreement. We and MDCO intend to enter into a supply and technical transfer agreement to provide for supply of licensed products to MDCO within a specified time following the effective date of the MDCO agreement.
Unless terminated earlier in accordance with the terms of the agreement, the MDCO agreement expires on a licensed product-by-licensed product and country-by-country basis upon expiration of the last royalty term for
20
Table of Contents
any licensed product in any country, where a royalty term is defined as the latest to occur of (1) the expiration of the last valid claim of patent rights covering a licensed product, (2) the expiration of the Regulatory Exclusivity, as defined in the MDCO agreement, and (3) the twelfth anniversary of the first commercial sale of the licensed product in such country. We estimate that our fundamental RNAi patents covering licensed products under the MDCO agreement will expire both in and outside of the United States generally between 2015 and 2023. We also estimate that our ALN-PCS product-specific patents covering licensed products under the MDCO agreement in the United States and elsewhere will expire at the end of 2033. These patent rights are subject to potential patent term extensions and/or supplemental protection certificates extending such terms in countries where such extensions may become available. In addition, more patent filings relating to the collaboration may be made in the future.
Either party may terminate the MDCO agreement in the event the other party fails to cure a material breach or upon patent-related challenges by the other party. We may terminate the agreement in the event that a lead licensed product has not been designated by the joint steering committee within a designated time period. In addition, MDCO has the right to terminate the agreement without cause at any time upon four months’ prior written notice.
During the term of the MDCO agreement, neither party will, alone or with an affiliate or third party, research, develop or commercialize, or grant a license to any third party to research, develop or commercialize, in any country, any product directed to the PCSK9 gene, other than a licensed product, without the prior written agreement of the other party, subject to the terms of the MDCO agreement.
Kyowa Hakko Kirin. In June 2008, we entered into a license and collaboration agreement with Kyowa Hakko Kirin, under which we granted Kyowa Hakko Kirin an exclusive license to our intellectual property in Japan and other markets in Asia for the development and commercialization of an RNAi therapeutic for the treatment of RSV infection. The Kyowa Hakko Kirin agreement covers ALN-RSV01, as well as additional RSV-specific RNAi therapeutic compounds that comprise the ALN-RSV program. We retain all development and commercialization rights worldwide outside of the licensed territory.
Under the terms of the Kyowa Hakko Kirin agreement, in June 2008, Kyowa Hakko Kirin paid us an upfront cash payment of $15.0 million. In addition, Kyowa Hakko Kirin is required to make payments to us upon achievement of specified development and sales milestones totaling up to $78.0 million, and royalty payments based on annual net sales, if any, of RNAi therapeutics for the treatment of RSV by Kyowa Hakko Kirin, its affiliates and sublicensees in the licensed territory. Due to the uncertainty of pharmaceutical development and the high historical failure rates generally associated with drug development, we may not receive any milestone or royalty payments from Kyowa Hakko Kirin.
Under the agreement, Kyowa Hakko Kirin is responsible, at its expense, for all development activities under the development plan that are reasonably necessary for the regulatory approval and commercialization of an RNAi therapeutic for the treatment of RSV in Japan and the rest of the licensed territory. We are responsible for supply of the product to Kyowa Hakko Kirin under a supply agreement unless Kyowa Hakko Kirin elects, prior to the first commercial sale of the product in the licensed territory, to manufacture the product itself or arrange for a third party to manufacture the product.
The term of the Kyowa Hakko Kirin agreement generally ends on a country-by-country basis upon the later of (1) the expiration of our last-to-expire patent covering a licensed product and (2) the tenth anniversary of the first commercial sale in the country of sale. We estimate that our principal patents covered under the Kyowa Hakko Kirin agreement will expire both in and outside the United States generally between 2016 and 2025. These patent rights are subject to any potential patent term extensions and/or supplemental protection certificates extending such term extensions in countries where such extensions may become available. Additional patent filings relating to the collaboration may be made in the future. The Kyowa Hakko Kirin agreement may be terminated by either party in the event the other party fails to cure a material breach under the agreement. In addition, Kyowa Hakko Kirin may terminate the agreement without cause upon 180 days’ prior written notice to us, subject to certain conditions.
Cubist. In January 2009, we entered into a license and collaboration agreement with Cubist to develop and commercialize therapeutic products based on certain of our RNAi technology for the treatment of RSV.
21
Table of Contents
Licensed products initially included ALN-RSV01, as well as several other second-generation RNAi-based RSV inhibitors. In November 2009, we and Cubist entered into an amendment to our license and collaboration agreement, which provided that we and Cubist would focus our collaboration and joint development efforts on ALN-RSV02, a second-generation compound, intended for use in pediatric patients. In December 2010, we and Cubist jointly made a portfolio decision to put the development of ALN-RSV02 on hold. Pursuant to the terms of the amendment, we continued to develop ALN-RSV01 for adult transplant patients at our sole discretion and expense and Cubist had the right to opt into collaborating with us on ALN-RSV01, subject to specified conditions. In February 2013, Cubist notified us that it would not exercise its opt-in right for ALN-RSV01. In light of this determination, we and Cubist mutually agreed to terminate the license and collaboration agreement. As of the termination date, the parties have no further rights and obligations under the license and collaboration agreement, notwithstanding anything to the contrary in the agreement. We do not intend to advance the ALN-RSV01 program into a Phase 3 clinical trial unless we are able to identify a partner outside the Kyowa Hakko Kirin territory.
Platform Alliances.
Monsanto. In August 2012, we and Monsanto entered into a license and collaboration agreement, pursuant to which we granted to Monsanto a worldwide, exclusive, royalty bearing right and license, including the right to grant sublicenses, to our RNAi platform technology and intellectual property controlled by us as of the date of the Monsanto agreement or during the 30 months thereafter, in the field of agriculture. The Monsanto agreement also includes the transfer of technology from us to Monsanto and a collaborative research project. Under the Monsanto agreement, Monsanto will be our exclusive collaborator in the agriculture field for a ten-year period.
In consideration for the rights granted to Monsanto under the Monsanto agreement, Monsanto paid us $29.2 million in upfront cash payments. Monsanto is also required to make near-term milestone payments to us upon the achievement of specified technology transfer and patent-related milestones. We are also entitled to receive additional funding for collaborative research efforts. In the aggregate, we can earn up to $5.0 million in potential future milestone payments and research funding under the Monsanto alliance. In December 2012, we received $1.5 million of the $5.0 million in potential milestone payments from Monsanto based upon the achievement of a specified patent-related event. In August 2013, we received an additional milestone payment of $2.5 million based upon the completion of technology transfer activities. We could potentially earn the next and final milestone payment of $1.0 million under the Monsanto agreement in connection with the collaborative research efforts contemplated under the Monsanto agreement. In addition, Monsanto is required to pay to us a percentage of specified fees from certain sublicense agreements Monsanto may enter into that include access to our intellectual property, as well as low single-digit royalty payments on worldwide, net sales by Monsanto, its affiliates and sublicensees of certain licensed products, as defined in the Monsanto agreement, if any. Due to the uncertainty of the application of RNAi technology in the field of agriculture, we may not receive any additional milestone payments or any royalty payments from Monsanto.
The term of the Monsanto agreement generally ends upon the expiration of the last-to-expire patent licensed under the agreement. We estimate that our fundamental RNAi patents licensed under the Monsanto agreement will expire both in and outside the United States generally between 2016 and 2025, subject to any potential patent term extensions and/or supplemental protection certificates extending such term extensions in countries where such extensions may become available. Monsanto may terminate the Monsanto agreement in its entirety upon 30-days’ prior written notice to us, provided, however, that Monsanto is required to continue to make royalty payments to us if any royalties were payable on net sales of a licensed product during the previous 24 months. The Monsanto agreement may also be terminated by either party in the event the other party fails to cure a material breach under the Monsanto agreement.
Under the terms of the Monsanto agreement, in the event that during the exclusivity period we cease to own or otherwise exclusively control certain licensed patent rights in the agriculture field, for any reason other than Monsanto’s breach of the Monsanto agreement or its negligence or willful misconduct, resulting in the loss of exclusivity with respect to Monsanto’s rights to such patent rights, and such loss of exclusivity has a material adverse effect on the licensed products, then we would be required to pay Monsanto up to $5.0 million as
22
Table of Contents
liquidated damages, and Monsanto’s royalty obligations to us under the Monsanto agreement would be reduced or, under certain circumstances, terminated. We have the right to cure any such loss of patent rights under the Monsanto agreement.
Takeda. In May 2008, we entered into a license and collaboration agreement with Takeda to pursue the development and commercialization of RNAi therapeutics. Under the Takeda agreement, we granted to Takeda a non-exclusive, worldwide, royalty-bearing license to our intellectual property, including delivery-related intellectual property, controlled by us as of the date of the Takeda agreement or during the five years thereafter, to develop, manufacture, use and commercialize RNAi therapeutics, subject to our existing contractual obligations to third parties. The license initially is limited to the fields of oncology and metabolic disease and may be expanded at Takeda’s option to include other therapeutic areas, subject to specified conditions.
In consideration for the rights granted to Takeda under the Takeda agreement, Takeda agreed to pay us $150.0 million in upfront and near-term technology transfer payments. In addition, we have the option, exercisable until the start of Phase 3 development, to opt-in under a 50-50 profit sharing agreement to the development and commercialization in the United States of up to four Takeda licensed products, and would be entitled to opt-in rights for two additional products for each additional field expansion, if any, elected by Takeda under the Takeda agreement. In June 2008, Takeda paid us an upfront payment of $100.0 million and agreed to pay us an additional $50.0 million upon achievement of specified technology transfer milestones, which payment we have received. If Takeda elects to expand its license to additional therapeutic areas, Takeda will be required to pay us $50.0 million for each additional field selected, if any. In addition, for each RNAi therapeutic product developed by Takeda, its affiliates and sublicensees, we are entitled to receive specified development, regulatory and commercialization milestone payments, totaling up to $171.0 million per product, together with a double-digit percentage royalty payment based on worldwide annual net sales, if any. The potential future milestone payments per product include up to $26.0 million for the achievement of specified development milestones, up to $40.0 million for the achievement of specified regulatory milestones and up to $105.0 million for the achievement of specified commercialization milestones. Due to the uncertainty of pharmaceutical development and the high historical failure rates generally associated with drug development, we may not receive any additional milestone payments or any royalty payments from Takeda.
The collaboration is governed by a joint technology transfer committee, a joint research collaboration committee and a joint delivery collaboration committee, each of which is comprised of an equal number of representatives from each party. The term of the Takeda agreement generally ends upon the later of (i) the expiration of our last-to-expire patent covering a licensed product and (ii) the last-to-expire term of a profit sharing agreement in the event we elect to enter into such an agreement. We estimate that our fundamental RNAi patents covered under the Takeda agreement will expire both in and outside the United States generally between 2016 and 2025, subject to any potential patent term extensions and/or supplemental protection certificates extending such term extensions in countries where such extensions may become available. The Takeda agreement may be terminated by either party in the event the other party fails to cure a material breach under the agreement. In addition, Takeda may terminate the agreement on a licensed product-by-licensed product or country-by-country basis upon 180-days’ prior written notice to us, provided, however, that Takeda is required to continue to make royalty payments to us for the duration of the royalty term with respect to a licensed product.
Roche/Arrowhead. In July 2007, we and, for limited purposes, Alnylam Europe AG, or Alnylam Europe, entered into a license and collaboration agreement with Roche. Under the license and collaboration agreement, which became effective in August 2007, we granted Roche a non-exclusive license to our intellectual property, including delivery-related intellectual property existing as of the date of the license and collaboration agreement, to develop and commercialize therapeutic products that function through RNAi, subject to our existing contractual obligations to third parties. In November 2010, Roche announced the discontinuation of certain activities in research and early development, including its RNAi research efforts. In October 2011, Arrowhead announced its acquisition of RNA therapeutics assets from Roche, including the license and collaboration agreement. As a result of this acquisition, Arrowhead owns all of the rights and obligations of Roche under the license and collaboration agreement. The license is initially limited to four therapeutic areas, and may be
23
Table of Contents
expanded to include additional therapeutic areas upon payment to us by Arrowhead of an additional $50.0 million for each additional therapeutic area, if any.
In consideration for the rights we granted under the license and collaboration agreement, Roche paid us $273.5 million in upfront cash payments. In addition, in exchange for our contributions under the collaboration agreement, for each RNAi therapeutic product developed by Arrowhead, its affiliates or sublicensees under the collaboration agreement, we are entitled to receive milestone payments upon achievement of specified development, regulatory and commercialization events, totaling up to an aggregate of $100.0 million per therapeutic target, together with a single-digit percentage royalty payment based on worldwide annual net sales, if any. The potential future milestone payments for each therapeutic target include up to $17.5 million for the achievement of specified development milestones, up to $62.5 million for the achievement of specified regulatory milestones and up to $20.0 million for the achievement of specified commercialization milestones. Due to the uncertainty of pharmaceutical development and the high historical failure rates generally associated with drug development, we may not receive any milestone or royalty payments from Arrowhead.
The term of the license and collaboration agreement generally ends upon the later of ten years from the first commercial sale of a licensed product and the expiration of the last-to-expire patent covering a licensed product. We estimate that our fundamental RNAi patents covered under the license and collaboration agreement will expire both in and outside the United States generally between 2016 and 2025, subject to any potential patent term extensions and/or supplemental protection certificates extending such term extensions in countries where such extensions may become available. Arrowhead may terminate the license and collaboration agreement, on a licensed product-by-licensed product, licensed patent-by-licensed patent, and country-by-country basis, upon 180-days’ prior written notice to us, but is required to continue to make milestone and royalty payments to us if any royalties were payable on net sales of a terminated licensed product during the previous 12 months. The license and collaboration agreement may also be terminated by either party in the event the other party fails to cure a material breach under the license and collaboration agreement.
Discovery and Development Alliances.
Isis. In April 2009, we and Isis amended and restated our existing strategic collaboration and license agreement, originally entered into in March 2004, to extend the broad cross-licensing arrangement regarding double-stranded RNAi that was established in 2004, pursuant to which Isis granted us licenses to its current and future patents and patent applications relating to chemistry and to RNA-targeting mechanisms for the research, development or commercialization of double-stranded RNA, or dsRNA, products. We have the right to use Isis technologies in our development programs or in collaborations and Isis agreed not to grant licenses under these patents to any other organization for the discovery, development and commercialization of dsRNA products designed to work through an RNAi mechanism, except in the context of a collaboration in which Isis plays an active role. We granted Isis non-exclusive licenses to our current and future patents and patent applications relating to RNA-targeting mechanisms and to chemistry for research use. We also granted Isis the non-exclusive right to develop and commercialize dsRNA products developed using RNAi technology against a limited number of targets. In addition, we granted Isis non-exclusive rights to research, develop and commercialize single-stranded RNA products. In August 2012, we and Isis amended the amended and restated Isis agreement to provide for the discovery, development and commercialization of dsRNA products by us or our sublicensees in the field of agriculture.
We agreed to pay Isis milestone payments, totaling up to approximately $3.4 million, upon the occurrence of specified development and regulatory events, and low single-digit royalties on sales, if any, for each product that we or a collaborator develop using Isis intellectual property. In addition, we agreed to pay to Isis a percentage of specified fees from strategic collaborations we may enter into that include access to Isis’ intellectual property. Isis agreed to pay us, per therapeutic target, a license fee of $0.5 million, milestone payments totaling approximately $3.4 million, payable upon the occurrence of specified development and regulatory events, and low single-digit royalties on sales, if any, for each product developed by Isis or a collaborator that utilizes our intellectual property. Isis has the right to elect up to ten non-exclusive target licenses under the agreement and has the right to purchase one additional non-exclusive target per year during the term of the collaboration. Due to the uncertainty of
24
Table of Contents
pharmaceutical development and the high historical failure rates generally associated with drug development, we may not receive any milestone or royalty payments from Isis.
As part of the amended and restated Isis agreement, we and Isis established a collaborative effort focused on single-stranded RNAi, or ssRNAi, technology, and we obtained from Isis a co-exclusive, worldwide license to research, develop and commercialize ssRNAi products. In November 2010, we exercised our right to terminate the ssRNAi collaborative effort, and all licenses to ssRNAi products granted by Isis to us, and any obligation thereunder requiring us to provide further research funding or pay additional license fees, milestone payments, royalties or sublicense payments to Isis for such ssRNAi products, also terminated. The termination of this collaborative effort did not affect the remainder of the amended and restated Isis agreement, including our licenses to Isis’ current and future patents and patent applications relating to dsRNAs, which remains in effect.
The term of the Isis agreement generally ends upon the expiration of the last-to-expire patent licensed thereunder, whether such patent is a patent licensed by us to Isis, or vice versa. As the license will include additional patents, if any, filed to cover future inventions, if any, the date of expiration cannot be determined at this time.
Novartis. In the second half of 2005, we entered into a series of transactions with Novartis, which included a stock purchase agreement, an investor rights agreement and a research collaboration and license agreement. In October 2010, the research program under the collaboration and license agreement was substantially completed in accordance with the terms of such agreement, subject to certain surviving rights and obligations of the parties.
In consideration for the rights granted to Novartis under the collaboration and license agreement, Novartis made an upfront payment of $10.0 million to us in October 2005, partly to reimburse prior costs incurred by us to develop in vivo RNAi technology. We also received research funding and development milestone payments from Novartis.
In September 2010, Novartis exercised its right under the collaboration and license agreement to select 31 designated gene targets, for which Novartis has exclusive rights to discover, develop and commercialize RNAi therapeutic products using our intellectual property and technology, including delivery-related intellectual property and related technology. Novartis’ right of first offer with respect to an exclusive license for additional targets has terminated. Under the terms of the collaboration and license agreement, for any RNAi therapeutic products Novartis develops against its selected targets, we are entitled to receive milestone payments upon achievement of certain specified development and annual net sales events, up to an aggregate of $75.0 million per therapeutic product, as well as royalties on annual net sales of any such product. Due to the uncertainty of pharmaceutical development and the high historical failure rates generally associated with drug development, we may not receive any milestone or royalty payments from Novartis.
Intellectual Property Licenses
In December 2002, we entered into a co-exclusive license with Max Planck Innovation for the worldwide rights to use and sublicense certain patented technology to develop and commercialize therapeutic products and related applications. We also obtained the rights to use, without the right to sublicense, the technology for all diagnostic uses other than for the purposes of therapeutic monitoring. We were also given the right to acquire the remaining 50% exclusive rights, which right we exercised upon our acquisition of Ribopharma AG in July 2003. In June 2005, we entered into an amendment to our agreement with Max Planck Innovation that secured our exclusivity to use and sublicense certain patented technology to develop and commercialize therapeutic products and related applications.
We are not obligated to pay any development or sales milestone payments to Max Planck Innovation, however, we will be required to pay Max Planck Innovation future single-digit royalties on net sales of all therapeutic and prophylactic products developed with the technology, if any.
Our agreements with Max Planck Innovation generally remain in effect until the expiration of the last-to-expire patent licensed thereunder. We estimate that the principal issued patents covered under the Max Planck Innovation agreements will expire both in and outside the United States during 2021, subject to any potential patent term extensions, restoration and/or supplemental protection certificates extending such term extensions in
25
Table of Contents
countries where such extensions may become available. We may terminate the agreements without cause with six months’ prior notice to Max Planck Innovation, and Max Planck Innovation may terminate the agreements in the event that we materially breach our obligations thereunder. Max Planck Innovation also has the right to terminate the agreements in the event that we, independently or through a third party, attack the validity of any of the licensed patents.
Delivery-Related Licenses and Collaborations
We have worked internally and with third-party collaborators with the goal of developing new technologies to achieve effective and safe delivery of RNAi therapeutics to a broad spectrum of organ and tissue types. In connection with these efforts, we have entered into a number of agreements to evaluate and gain access to certain delivery technologies. In some instances, we are providing or have previously provided funding to support the advancement of these delivery technologies. During 2013, we continued to make advances relating to the delivery of RNAi therapeutics, both internally and together with our collaborators.
Arrowhead. In January 2012, we and Arrowhead entered into collaboration and joint licensing agreements, pursuant to which we received a license from Arrowhead to utilize their dynamic polyconjugate, or DPC, delivery technology for an RNAi therapeutic product. Arrowhead is eligible to receive from us milestone payments and royalties, if any, on sales of product resulting from the license. In addition, we granted Arrowhead a license under our intellectual property that enables the discovery, development and commercialization of an RNAi therapeutic targeting the hepatitis B virus, or HBV, and utilizing DPC delivery technology. We are eligible to receive from Arrowhead milestone payments and royalties, if any, on sales of any product resulting from the license.
MIT. In November 2011, we extended for an additional three years, through May 2015, the term of our agreement with the David H. Koch Institute for Integrative Cancer Research at MIT, under which we are sponsoring an exclusive research program focused on the discovery of new materials and formulations for the delivery of RNAi therapeutics. We and MIT have published data describing advancements in the discovery and development of LNPs based on novel “lipidoid” formulations for the systemic delivery of RNAi therapeutics. Lipidoids are lipid-like materials discovered for the delivery of RNAi therapeutics, and were originally described by us and our collaborators at MIT. Lipidoid formulations represent one of several approaches we are pursuing for systemic delivery of RNAi therapeutics under our research agreement with MIT.
Tekmira. In November 2012, we, TPC and Protiva agreed to restructure our existing contractual relationship. In connection with this restructuring, the parties entered into a cross-license agreement, and agreed to terminate certain prior agreements, including: the May 2008 amended and restated license and collaboration between us and TPC, the May 2008 amended and restated cross-license agreement between us and Protiva, and the January 2009 manufacturing agreement between us and TPC.
Under the 2012 cross-license agreement, the parties consolidated certain intellectual property related to LNP technology for the systemic delivery of RNAi therapeutics. Specifically, certain patents and patent applications, including the MC3 lipid family, were assigned by us to TPC. We retain rights to use this intellectual property for the research, development and commercialization of RNAi therapeutic products, including the rights to sublicense this intellectual property on a product-by-product basis. Tekmira has also granted us a worldwide license to its LNP technology for the research, development and commercialization of LNP-based RNAi therapeutics, which license shall be exclusive for up to eight targets designated by us, and otherwise shall be non-exclusive. We have the right to sublicense on a product-by-product basis.
In addition, we elected to buy out our manufacturing obligations to TPC with respect to our LNP-based pipeline programs. Pursuant to the terms of the 2012 cross-license agreement, we made a one-time payment of $30.0 million to TPC for the termination of, and our release from, all of our obligations under the manufacturing agreement, including without limitation the obligations to obtain materials and/or services from TPC. We also have the right to manufacture and have manufactured our LNP-based RNAi therapeutics, which right may be sublicensed to our collaborators.
26
Table of Contents
Further, as part of this restructuring, we elected to buy-down certain future potential milestone and royalty payments due to Tekmira for certain of our RNAi therapeutics, formulated using LNP technology. Specifically, pursuant to the cross-license agreement, we made a one-time payment of $35.0 million to TPC, which amount constituted payment for the termination of the 2008 license agreements with TPC and Protiva and the parties’ rights and obligations thereunder, as well as the buy-down of certain milestone payments and the significant reduction of royalty rates for ALN-VSP, ALN-PCS02 and patisirian. As a result of this buy-down, future royalty rates for these products are now further reduced from the low single-digit royalties due on other products, as described below. Under the 2012 cross-license agreement, we are obligated to pay TPC an aggregate of $10.0 million in contingent milestone payments related to advancement of ALN-VSP and ALN-TTR, which represent the only potential milestones due to Tekmira for ALN-VSP, ALN-PCS02 and patisiran, LNP-based RNAi therapeutics. In December 2013, we paid to TPC $5.0 million in connection with the initiation of our Phase 3 clinical trial for patisiran, fulfilling one of these milestone obligations. With respect to the second $5.0 million milestone, in August 2013, we initiated binding arbitration proceedings to resolve a disagreement with TPC regarding the achievement by TPC of this milestone under our cross-license agreement relating to the manufacture of ALN-VSP clinical trial material for use in China.
Consistent with the prior license agreements, under the 2012 cross-license agreement, we are obligated to pay TPC up to an aggregate of $16.0 million in milestone payments for any future RNAi therapeutic formulated using Tekmira LNP technology, excluding ALN-VSP, ALN-PCS02 and patisiran, together with low single-digit royalty payments on annual product sales, if any.
Under the 2012 cross-license Agreement, Tekmira maintains the three exclusive and five non-exclusive licenses previously granted by us under the prior license agreements to research, develop and commercialize RNAi therapeutics directed to up to eight gene targets. In addition, we granted Tekmira a non-exclusive license for two additional gene targets, on the same terms and conditions as the prior non-exclusive licenses. Tekmira also acquired from Acuitas its existing options for three additional non-exclusive licenses, which were included under the 2012 cross-license agreement. Tekmira may sublicense these rights on a product-by-product basis. We waived our right under the prior license agreements to opt-in to the Tekmira research program directed to polo-like kinase 1, or PLK1. Under the 2012 cross-license agreement, we are eligible to receive from Tekmira up to an aggregate of $8.5 million in milestone payments for RNAi therapeutics directed to nine of the targets for which we have granted licenses to Tekmira, together with single-digit royalties on annual product sales, if any, of RNAi therapeutic products directed to all thirteen of the targets for which we have granted licenses to Tekmira. Due to the uncertainty of pharmaceutical development and the high historical failure rates generally associated with drug development, we may not receive any milestone or royalty payments from Tekmira.
The term of the 2012 cross-license agreement generally ends upon the expiration of the last-to-expire royalty term. Royalties are payable on a product-by-product and country-by-country basis commencing on the first commercial sale of a product in a country and continuing during any period in which (a) in the case of us, a valid claim within the Tekmira Royalty-Bearing Patents (as defined in the 2012 cross-license agreement) covers our applicable product in such country of sale, or (b) in the case of Tekmira products, a valid claim within our patents covers the applicable Tekmira product in such country of sale. We estimate that our fundamental RNAi patents covered under the 2012 cross-license agreement will expire both in and outside the United States generally between 2019 and 2021, and that the Tekmira LNP patents covered under the 2012 cross-license agreement will expire both in and outside the United States generally between 2020 and 2030, subject in each case to any potential patent term extensions and/or supplemental protection certificates extending such term extensions in countries where such extensions may become available. Either party may terminate a license it granted to the other in the event that the other party fails to cure a material breach of its obligations relating to that license. Furthermore, either party may terminate the 2012 cross-license agreement in the event the other party fails to cure a material breach of an obligation under the agreement. In addition, either party may terminate the 2012 cross-license agreement upon patent-related challenges by the other party.
UBC and Acuitas. In July 2009, we entered into a research agreement with UBC and Acuitas that was focused on the discovery of novel lipids, such as the MC3 lipid, employed in second-generation LNP formulations for the systemic delivery of RNAi therapeutics. Pursuant to the terms of the research agreement, we funded collaborative
27
Table of Contents
research through July 2012, which was conducted by our scientists, together with scientists at UBC and Acuitas. Under the research agreement, UBC and Acuitas are eligible to receive up to an aggregate of $1.3 million in milestone payments from us for each licensed product (as defined in the research agreement) directed to a particular target (as defined in the research agreement), together with single-digit royalty payments on annual product sales, if any.
Concurrent with the execution of the research agreement, we also entered into a supplemental agreement with TPC, Protiva, UBC and Acuitas, which contains additional terms regarding the intellectual property rights arising out of the research agreement. In connection with 2012 cross-license agreement with Tekmira described above, we and Tekmira agreed to supersede the rights and obligations under the supplemental agreement as between ourselves, with the rights and obligations set forth in the 2012 cross-license agreement.
microRNA Therapeutics
Regulus. In September 2007, we and Isis established Regulus, a company focused on the discovery, development and commercialization of microRNA therapeutics. Regulus leverages our and Isis’ technologies, know-how and intellectual property relating to microRNA therapeutics.
Regulus, which initially was established as a limited liability company, converted to a C corporation as of January 2, 2009 and changed its name to Regulus Therapeutics Inc. In consideration for our and Isis’ initial interests in Regulus, we and Isis each granted Regulus exclusive licenses to our intellectual property for certain microRNA therapeutics as well as certain patents in the microRNA field. Regulus operates as an independent company with a separate board of directors, scientific advisory board and management team, some of whom have options to purchase common stock of Regulus. In October 2012, Regulus completed an underwritten initial public offering, raising $45.0 million in net proceeds, including proceeds from the exercise by the underwriters of the over-allotment option. In July 2013, Regulus completed an additional underwritten public offering, raising an additional $45.8 million in net proceeds, including proceeds from the exercise of the over-allotment option. Currently, we own approximately 15% of Regulus’ outstanding common stock.
Regulus is exploring therapeutic opportunities that arise from microRNA dysregulation. Since microRNAs are believed to regulate broad networks of genes and biological pathways, microRNA therapeutics define a new and potentially high-impact strategy to target multiple nodes on disease pathways. microRNAs are small non-coding RNAs that regulate the expression of other genes. More than 500 microRNAs have been identified to date in humans, each of which is believed to interact with a specific set of genes that control key aspects of cell biology. Since microRNAs may act as master regulators of the genome and are often found to be dysregulated in disease, microRNAs potentially represent an exciting new platform for drug discovery and development. Regulus is advancing microRNA therapeutics in several areas including oncology, fibrosis, hepatitis C virus, or HCV, and metabolic diseases.
Regulus has entered into a number of strategic alliances with leading pharmaceutical companies, including GlaxoSmithKline, or GSK, Sanofi, Biogen Idec Inc., or Biogen Idec, and AstraZeneca. Each of Alnylam and Isis is entitled to receive specified sublicense income in connection with certain collaborative agreements entered into by Regulus, as well as royalties on net sales, if any, of certain products developed by Regulus or its collaborators, in each case subject to the terms and conditions of the license and collaboration agreement among Regulus, Isis and Alnylam.
Licenses
To further enable the field and monetize our intellectual property rights, we have established our InterfeRx program and our research reagents and services licensing program.
InterfeRx Program. Our InterfeRx program consists of the licensing of our intellectual property to others for the development and commercialization of RNAi therapeutic products relating to specific targets outside our direct strategic focus. We expect to receive license fees, annual maintenance fees, milestone payments and royalties on sales of any resulting RNAi therapeutic products. Generally, we do not expect to collaborate with our InterfeRx licensees in the development of RNAi therapeutic products, but may do so in certain circumstances. To date, we have granted InterfeRx licenses to several companies, including Quark Pharmaceuticals, Inc., or Quark,
28
Table of Contents
Arrowhead and its subsidiary Calando Pharmaceuticals, Inc., or Calando, and Tekmira. In general, these licenses allow the licensees to discover, develop and commercialize RNAi therapeutics for a limited number of targets in return for upfront, milestone, license maintenance and/or royalty payments to us. In some cases, we also retained a right to negotiate the ability to co-promote and/or co-commercialize the licensed product, and in one case, we included the rights to discover, develop and commercialize RNAi therapeutics utilizing expressed RNAi (i.e., RNAi mediated by siRNAs generated from DNA constructs introduced into cells). In addition, Sylentis, S.A.U., or Sylentis, and Benitec Ltd., or Benitec, each have an option to take an InterfeRx license, subject to certain conditions. We have granted InterfeRx licenses or options relating to approximately 23 gene targets and, as of January 31, 2014, 11 of these targets have been selected by InterfeRx partners.
Research Reagents and Services. We have granted approximately 14 licenses to our intellectual property for the development and commercialization of research reagents and services, and intend to enter into additional licenses on an ongoing basis. Our target licensees are vendors that provide siRNAs and related products and services for use in biological research. We offer these licenses in return for an initial license fee, annual renewal fees and royalties from sales of siRNA research reagents and services. No single research reagent or research services license is material to our business.
Patents and Proprietary Rights
We have devoted considerable effort and resources to establish what we believe to be a strong intellectual property position relevant to RNAi therapeutic products and delivery technologies. In this regard, we have amassed a portfolio of patents, patent applications and other intellectual property covering:
| • | fundamental aspects of the structure and uses of siRNAs, including their use as therapeutics, and RNAi-related mechanisms; |
| • | chemical modifications to siRNAs that improve their suitability for therapeutic and other uses; |
| • | siRNAs directed to specific targets as treatments for particular diseases; |
| • | delivery technologies, such as in the fields of carbohydrate conjugates and cationic liposomes; and |
| • | all aspects of our specific development candidates. |
We believe that no other company possesses a portfolio of such broad and exclusive rights to the patents and patent applications required for the commercialization of RNAi therapeutics. Our intellectual property estate for RNAi therapeutics includes over 1,800 active cases and over 700 granted or issued patents, of which over 300 are issued or granted in the United States, the EU, including by the European Patent Office, or EPO, and Japan. Given the importance of our intellectual property portfolio to our business operations, we intend to vigorously enforce our rights and defend against challenges that have arisen or may arise in this area.
Intellectual Property Related to Fundamental Aspects and Uses of siRNA and RNAi-related Mechanisms
In this category, we include United States and foreign patents and patent applications that claim key aspects of siRNA architecture and RNAi-related mechanisms. Specifically included are patents and patent applications covering targeted cleavage of mRNA directed by RNA-like oligonucleotides and dsRNAs of particular lengths and particular structural features, such as blunt and/or overhanging ends. Our strategy has been to secure exclusive rights where possible and appropriate to key patents and patent applications that we believe cover fundamental aspects of RNAi. The following table lists patents and/or patent applications to which we have secured rights that we regard as being fundamental for the use of siRNAs as therapeutics.
29
Table of Contents
| Patent Licensor/Owner |
Subject Matter |
First Priority Date |
Inventors |
Status |
Expiration Date* |
Alnylam Rights | ||||||
| Isis | Inactivation of target mRNA | 6/6/1996 (EP) and 6/6/1997 (U.S.) | S. Crooke | U.S. 5,898,031, U.S. 6,107,094, U.S. 7,432,250 & U.S. 7,695,902 EP 0928290
Additional applications pending in the U.S. and several foreign jurisdictions |
6/6/2016
6/6/2017 |
Exclusive rights for therapeutic purposes related to siRNAs** | ||||||
| Carnegie Institution of Washington |
Double-stranded RNAs to induce RNAi | 12/23/1997 | A. Fire, C. Mello |
U.S. 6,506,559, U.S. 7,560,438 & U.S. 7,538,095
Additional applications pending in the U.S. and several foreign jurisdictions |
12/18/2018 | Non-exclusive rights for therapeutic purposes | ||||||
| Medical College of Georgia Research Institute, Inc. |
Methods for inhibiting gene expression using double-stranded RNA | 1/28/1999 | Y. Li, M. Farrell, M. Kirby |
AU 776150 (Australia)
Additional applications pending in the U.S., Europe and Canada |
1/28/2020 | Exclusive rights | ||||||
| Alnylam | Small double-stranded RNAs as therapeutic products | 1/30/1999 | R. Kreutzer, S. Limmer | EP 1214945 (revoked/under appeal), EP 1550719 (revoked/under appeal), CA 2359180 (Canada), AU 778474 (Australia), ZA 2001/5909 (South Africa), DE 20023125 U1, DE 10066235 & DE 10080167 (Germany)
Additional applications pending in the U.S. and several foreign jurisdictions |
1/29/2020 | Owned | ||||||
| Alnylam | Medicament for inhibiting the expression of a target gene and medicament for treating a tumor disease | 1/9/2001 | R. Kreutzer, S. Limmer, H-P.Vornlocher, P. Hadwiger, A. Geick, M. Ocker, C. Herold, D. Schuppan |
EP 1349927 (opposed and maintained in amended form) | 1/9/2022 | Owned | ||||||
| Alnylam | Method for inhibiting the expression of a target gene | 1/9/2001 | R. Kreutzer, S. Limmer, P. Hadwiger |
EP 1352061 (opposed, maintained and under appeal) | 1/9/2022 | Owned | ||||||
| Alnylam | Composition and methods for inhibiting a target nucleic acid with double-stranded RNA | 4/21/1999 | C. Pachuk, C. Satishchandran | AU 781598 (Australia)
Additional applications pending in the U.S. and several foreign jurisdictions |
4/19/2020 | Owned | ||||||
| Cancer Research Technology Limited |
RNAi uses in mammalian oocytes, preimplantation embryos and somatic cells | 11/19/1999 | M. Zernicka- Goetz, F. Wianny, M.J. Evans, D.M. Glover |
EP 1230375 (revoked/under appeal), SG 89569 (Singapore), AU 774285 (Australia)
Additional applications pending in the U.S. and several foreign jurisdictions |
11/17/2020 | Exclusive rights for therapeutic purposes | ||||||
| Massachusetts Institute of Technology, Whitehead Institute for Biomedical Research, Max Planck Gesellschaft, University of Massachusetts *** |
Mediation of RNAi by small RNAs 21-23 base pairs long | 3/30/2000 | D.P. Bartel, P.A. Sharp, T. Tuschl, P.D. Zamore | U.S. 8,420,391 & U.S. 8,395,628
EP 2361981 (opposed and maintained in amended form/under appeal), AU 2001249622 (Australia), NZ 522045 (New Zealand), KR 08724437 & KR 10-0909681 (Korea)
Additional applications pending in the U.S. and several foreign jurisdictions |
3/30/2021 | Non-exclusive rights for therapeutic purposes*** | ||||||
30
Table of Contents
| Patent Licensor/Owner |
Subject Matter |
First Priority Date |
Inventors |
Status |
Expiration Date* |
Alnylam Rights | ||||||
| Massachusetts Institute of Technology, Whitehead Institute, University of Massachusetts, Max Planck Gesellschaft (U.S.)****
Max Planck Gesellschaft (ex-U.S.), European Molecular Biology Laboratory (ex-U.S.)***** |
Synthetic and chemically modified siRNAs as therapeutic products | 12/1/2000 (EP), 4/24/2004 and 4/27/2004 | T. Tuschl, S. Elbashir, W. Lendeckel, M. Wilm#, R. Lührmann#
#EMBL inventors |
U.S. 7,056,704, U.S. 7,078,196, U.S. 8,329,463, U.S. 8,372,968, U.S. 8,362,231 & U.S. 8,445,237 EP 1407044 (opposed and maintained in amended form/under appeal), EP 1873259, EP 2348134 & EP 2351852, AU 2002235744 (Australia), ZA 2003/3929 (South Africa), SG 96891 (Singapore), NZ 52588 (New Zealand), JP 4 095 895 (opposed and maintained in amended form/under appeal), JP 4 494 392 (Japan), RU 2322500 (Russia), CN 1568373 (China)
Additional applications pending in the U.S. and several foreign jurisdictions |
11/29/2021 | Exclusive rights for therapeutic purposes**** | ||||||
| Alnylam | Methods for inhibiting a target nucleic acid via the introduction of a vector encoding a double-stranded RNA | 1/31/2001 | T. Giordano, C. Pachuk, C. Satishchandran | AU 785395 (Australia)
Additional applications pending in the U.S., Australia and Canada |
1/31/2021 | Owned | ||||||
| Stanford University | RNAi uses in vivo in mammalian liver | 7/23/2001 | M.A. Kay, A.P. McCaffrey | AU 2002326410 (Australia)
Additional applications pending in the U.S. and several foreign jurisdictions |
7/23/2021 | Exclusive rights for therapeutic purposes | ||||||
| Alnylam | Claims directed to carbohydrate conjugates linked to siRNA | 4/17/2003 | M. Manoharan | U.S. 7,723,509, U.S. 7,745,608, U.S. 7,851,615, U.S. 8,017,762, U.S. 8,507,661, U.S. 8,344,125 & U.S. 8,426,377 | 9/21/2024 | Owned | ||||||
| Alnylam | Claims directed to GalNAc-conjugated siRNA | 12/13/2007 | M. Manoharan | U.S. 8,106,022 & U.S. 8,450,467 | 12/12/2029; 12/12/2029 |
Owned | ||||||
| * | For applications filed after June 7, 1995, the patent term generally is 20 years from the earliest application filing date. However, under the Drug Price Competition and Patent Term Extension Act of 1984, known as the Hatch-Waxman Act, we may be able to apply for patent term extensions for our U.S. patents. We cannot predict whether or not any patent term extensions will be granted or the length of any patent term extension that might be granted. |
| ** | We hold co-exclusive therapeutic rights with Isis. However, Isis has agreed not to license such rights to any third party, except in the context of a collaboration in which Isis plays an active role. |
| *** | We hold exclusive rights to the interest owned by three co-owners. The University of Massachusetts, or UMass, has licensed its interest separately to third parties. |
| **** | We hold exclusive rights to the interest owned by all co-owners in the U.S., subject to the right of UMass to sublicense the U.S. Tuschl II patent family to Merck & Co., Inc. |
| ***** | European Molecular Biology Laboratory, or EMBL, has a limited ownership interest in certain ex-US cases in this family with no rights to control or otherwise affect patent prosecution. |
We believe that we have a strong portfolio of broad rights to fundamental RNAi patents and patent applications. Many of these rights are exclusive, which we believe prevents potential competitors from commercializing products in the field of RNAi without taking a license from us. In securing these rights, we have focused on obtaining the strongest rights for those intellectual property assets we believe will be most important in providing competitive advantage with respect to RNAi therapeutic products.
We believe that the Crooke patent series, issued in several countries around the world, covers the use of modified oligonucleotides to achieve enzyme-mediated cleavage of a target mRNA. We have obtained rights to the Crooke patents for use with dsRNA products, through a license agreement with Isis. Under the terms of our amended and restated Isis agreement, Isis agreed not to grant licenses under these patents to any other organization for dsRNA products designed to work through an RNAi mechanism, except in the context of a collaboration in which Isis plays an active role.
31
Table of Contents
Through our acquisition of Ribopharma AG, now known as Alnylam Europe, we own the entire Kreutzer-Limmer patent portfolio, which includes pending applications in the United States and many countries worldwide. The first patent to issue in the Kreutzer-Limmer series (EP 1144623) was granted in Europe in 2002, and specifically covered the use of small dsRNAs as therapeutics. This patent was revoked on appeal. The second European Kreutzer-Limmer patent (EP 1214945) to issue in the series was granted in Europe in 2005. This patent covers methods using, medicaments comprising and uses of dsRNA structures of 15 to 49 successive nucleotide pairs in length. In January 2009, the Opposition Division of the EPO ruled in favor of the opposing parties in an opposition proceeding related to the second Kreutzer-Limmer patent. We appealed this decision, and in May 2010, the Board of Appeals of the EPO ruled in our favor, rejecting the Opposition Division’s ruling that the second Kreutzer-Limmer patent was invalid. The patent was sent back to the Opposition Division to address the remaining grounds asserted by the opponents. In July 2013, the Opposition Division revoked the patent and this decision is currently under appeal. In December 2008, the EPO granted a third patent in the Kreutzer-Limmer series (EP 1550719). This patent covers therapeutic dsRNAs which are 15 to 21 consecutive nucleotide pairs in length. The third Kreutzer-Limmer patent was opposed and revoked in July 2012. We have appealed this decision. We have also received grants for patents in the Kreutzer-Limmer series in several other countries, as reflected in the table above. The decision with respect to EP 1144623 will only affect the granted or pending claims of other members of the Kreutzer-Limmer patent series to the extent the same issue arises in the formal examination or post-grant review proceedings of the other members of the series. In the event this happens, we believe that the ruling in the EP 1144623 proceeding would be controlling. In March 2010, the EPO issued a further patent designating Kreutzer and Limmer (among others) as inventors (EP 1349927). This patent covers methods and medicaments having dsRNAs that are less than 25 nucleotides in length having a 3’ nucleotide overhang on the antisense strand which inhibit anti-apoptotic genes in tumor cells. This fourth Kreutzer-Limmer patent had also been opposed and in December 2012, the EPO limited the claims to dsRNAs specific for members of the BCL-2 gene family, a family of anti-apoptotic genes implicated in cancer.
The Glover patent series has resulted in several patent grants, including in Europe (EP 1230375). The European Glover patent was revoked in July 2008 during opposition proceedings and our appeal of this decision is pending. Broad claims from this patent cover dsRNAs of any length or structure as mediators of RNAi in mammalian systems. We have an exclusive license to the Glover patent for therapeutic uses from CRT.
The Tuschl patent applications filed by Whitehead, MIT, UMass and Max Planck Gesellschaft zur Foerderung der Wissenschaften e.V. on the invention by Dr. Tuschl and his colleagues, which we call the Tuschl I patent series, cover compositions and methods important for RNAi discovery. Two patents in this family have been granted in the United States, U.S. Patent No. 8,420,391 with claims directed to a method of producing knock-down cells with dsRNAs of between 21 to 23 nucleotides, and U.S. Patent No. 8,394,628 with claims directed to a method of mediating RNAi in vitro with dsRNAs of between 21 to 23 nucleotides. The EPO granted patent EP 1309726, which has been opposed and consists of 19 claims broadly covering in vitro RNAi methods, including methods of reducing the expression of a gene, including those of mammalian or viral origin, with dsRNAs between 21 and 23 nucleotides in length. In addition, the patent also includes claims covering methods of examining the function of a gene, as well as the use of both unmodified and chemically modified dsRNAs. This patent was opposed and claims in the main request were maintained. This ruling is under appeal. A second patent was granted by the EPO, EP 2361981, and includes claims directed to dsRNAs of 21 to 23 nucleotides for use in treating a disease. The Tuschl I series has also been granted in New Zealand (NZ 522045) and Korea (KR 08724437 and 10-0909681). We are the exclusive licensee of the ownership interests of the Max Planck Gesellschaft zur Foerderung der Wissenschaften e.V., MIT and Whitehead in the Tuschl I patent series for RNAi therapeutics.
The Tuschl patent applications filed by Max Planck Gesellschaft zur Foerderung der Wissenschaften e.V. on the invention by Dr. Tuschl and his colleagues, which we call the Tuschl II patent series, cover what we believe are key structural features of siRNAs. Specifically, the Tuschl II patents and patent applications include claims directed to synthetic siRNAs and the use of chemical modifications to stabilize siRNAs. In June 2006, the United States Patent and Trademark Office, or USPTO, issued U.S. Patent No. 7,056,704 and in July 2006, the USPTO issued U.S. Patent No. 7,078,196, each covering methods of making dsRNAs having a 3’ overhang structure. In
32
Table of Contents
December 2012, the USPTO granted U.S. Patent No. 8,329,463 with claims directed to in vitro methods of use of dsRNA of 19 to 23 nucleotides with a 3’overhang of from one to three nucleotides. In January 2013, the USPTO granted U.S. Patent No. 8,362,231 with claims directed to dsRNA compositions consisting of 19 to 23 nucleotides with a 3’ overhang of from 1 to 3 nucleotides that contains at least one nucleotide analogue, and in February, 2013, the USPTO granted U.S. Patent No. 8,372,968 with claims directed to dsRNA compositions consisting of 19 to 25 nucleotides with a 3’ overhang of from one to five nucleotides. An additional patent was granted in March 2013 by the USPTO, U.S. Patent No. 8,445,237, with claims directed to a method of preparing dsRNAs of between 19 to 25 nucleotides with a 3’ overhang of between one and five nucleotides. In September 2007, the EPO granted broad claims for the Tuschl II patent in Europe (EP 1407044). Five parties filed Notices of Opposition in the EPO against EP 1407044. In December 2010, the Opposition Division of the EPO ruled in our favor maintaining the patent in amended form. All of the opponents have appealed the decision of the Opposition Division. The Japanese Patent Office has granted the Tuschl II patent in Japan (JP 4 095 895 and JP 4 494 392) and the Chinese Patent Office has granted the Tuschl II patent in China (CN 1568373). JP 4 095 895 was the subject of an Invalidation Trial which was requested by a Japanese company. The Japanese court ruled that claims directed to dsRNA of 19 to 23 nucleotides with a 3’ overhang of from one to three nucleotides be maintained with the additional feature that the 3’ overhang is stabilized against degradation. We have appealed the inclusion of this additional feature. The Japanese court granted our appeal and restored the original claims as granted. We have also received grants for patents in the Tuschl II series in several other countries, as reflected in the table above. We have obtained an exclusive license to claims in the Tuschl II patent series uniquely covering the use of RNAi for therapeutic purposes.
Collectively, the Tuschl I and II patent families cover a wide range of dsRNA molecules including those unmodified and those comprising chemical modifications. Examples of those chemical modifications encompassed by the Tuschl claims include those modifications made in the ribose ring, e.g., at the 2’ position such as 2’-OMe, 2’-F or modifications such as those found in locked and unlocked (acyclic) nucleotides.
The Fire and Mello patent owned by the Carnegie Institution covers the use of dsRNAs to induce RNAi. The Carnegie Institution has made this patent broadly available for licensing and we, like many companies, have taken a non-exclusive license to the patent for therapeutic purposes. We believe, however, that the claims of the Fire and Mello patent do not cover the structural features of dsRNAs that are important for the biological activity of siRNAs in mammalian cells. We believe that these specific features are the subjects of the Crooke, Kreutzer-Limmer, Glover and Tuschl II patents and patent applications for which we have secured exclusive rights.
The other pending patent applications listed in the table above either provide further coverage for structural features of siRNAs or relate to the use of siRNAs in mammalian cells. For some of these, we have exclusive rights, and for others, we have non-exclusive rights. In addition, in December 2008, we acquired the intellectual property assets of Nucleonics, Inc., a privately held biotechnology company. This acquisition included over 100 active patent filings, including 15 patents that have been granted worldwide, of which five have been granted in the United States and Europe. With this acquisition, we obtained patents and patent applications with early priority dates, notably the “Li & Kirby,” “Pachuk I” and “Giordano” patent families, that cover broad structural features of RNAi therapeutics, thus extending the breadth of our fundamental intellectual property.
Intellectual Property Related to Chemical Modifications
Our amended and restated collaboration and license agreement with Isis provides us with rights to practice the inventions covered by over 200 issued patents worldwide, as well as rights based on future chemistry patent applications through April 2014 for use with dsRNA products. These patents will expire both in and outside the United States generally between 2011 and 2029, subject to any potential patent term extensions and/or supplemental protection certificates extending such term extensions in countries where such extensions may become available. These inventions cover chemical modifications we may wish to incorporate into dsRNA therapeutic products designed to work through an RNAi mechanism. Under the terms of our amended and restated license agreement, Isis agreed not to grant licenses under these patents to any other organization for dsRNA products designed to work through an RNAi mechanism, except in the context of a collaboration in which Isis plays an active role.
33
Table of Contents
In addition to licensing these intellectual property rights from Isis, we are also working to develop our own proprietary chemical modifications that may be incorporated into siRNAs to endow them with drug-like properties. We have filed a large number of patent applications relating to these novel and proprietary chemical modifications.
With the combination of the technology we have licensed from Isis, U.S. Patent No. 7,078,196, a patent in the Tuschl II patent series, and our own patent application filings, we possess issued claims that cover methods of making siRNAs that incorporate any of various chemical modifications, including the use of phosphorothioates, 2’-O-methyl and/or 2’-fluoro modifications and modifications such as those found in locked and unlocked (acyclic) nucleotides. These modifications are believed to be important for achieving “drug-like” properties for RNAi therapeutics. We hold exclusive worldwide rights to these claims for RNAi therapeutics.
Intellectual Property Related to the Delivery of siRNAs to Cells
We are pursuing internal research and collaborative approaches regarding the delivery of siRNAs to mammalian cells. These approaches include exploring technology that may allow delivery of siRNAs to cells through the use of cholesterol and carbohydrate conjugation, cationic lipids, peptide and antibody-based targeting, and polymer conjugations. Our collaborative efforts include working with academic and corporate third parties to examine specific embodiments of these various approaches to delivery of siRNAs to appropriate cell tissue, and in-licensing of the most promising technology. For example, we have obtained a license from UBC and Tekmira in the field of RNAi therapeutics to intellectual property covering cationic liposomes and their use to deliver nucleic acid to cells. The issued United States patents and foreign counterparts, including the Semple (U.S. Patent No 6,858,225) and Wheeler (U.S. Patent Nos. 5,976,567 and 6,815,432) patents, cover compositions, methods of making and methods of using cationic liposomes to deliver agents, such as nucleic acid molecules, to cells. These patents will expire both in and outside the United States on October 30, 2017, January 6, 2015 and June 7, 2015, respectively, subject to any potential patent term extensions and/or supplemental protection certificates extending such term extensions in countries where such extensions may become available.
In addition, in April 2012, the USPTO granted U.S. Patent No. 8,158,601, which includes 30 claims covering composition of matter and formulations of the MC3 lipid, as well as methods of using these compositions and formulations. The patent lists inventors who are, or were, our employees as well as employees of Acuitas. MC3 is being utilized in our patisiran and ALN-PCS02 development programs and may potentially be utilized in other development programs. We assigned this patent, amongst other patents and patent applications relating to lipids and LNP technology, to Tekmira in connection with our November 2012 restructuring and cross-license agreement. We retain rights to use this intellectual property for the research, development and commercialization of RNAi therapeutic products, including the rights to sublicense this intellectual property on a product-by-product basis. A description of our 2012 restructuring and cross-license agreement with Tekmira is set forth above under “Strategic Alliances—Delivery-Related Licenses and Collaborations – Tekmira.”
Intellectual Property Related to siRNAs Directed to Specific Targets
We have filed a number of patent applications claiming specific siRNAs directed to various gene targets that correlate to specific diseases. While there may be a significant number of competing applications filed by other organizations claiming siRNAs to treat the same gene target, we were among the first companies to focus and file on RNAi therapeutics, and thus, we believe that a number of our patent applications may predate competing applications that others may have filed. Reflecting this, in August 2005, the EPO granted a broad patent, which we call the Kreutzer-Limmer II patent, with 103 allowed claims on therapeutic compositions, methods and uses comprising siRNAs that are complementary to mRNA sequences in over 125 disease target genes. In July 2009, the EPO ruled in our favor in an opposition proceeding related to the Kreutzer-Limmer II patent. The decision has been appealed by the opponents. The Kreutzer-Limmer II patent will expire on January 9, 2022, subject to any potential patent term extensions and/or supplemental protection certificates extending such term extensions in countries where such extensions may become available. Some of these claimed gene targets are being pursued
34
Table of Contents
by our development and pre-clinical programs, such as those expressed by viral pathogens including RSV and influenza virus. In addition, the claimed targets include oncogenes, cytokines, cell adhesion receptors, angiogenesis targets, apoptosis and cell cycle targets, and additional viral disease targets, such as hepatitis C virus and HIV. The Kreutzer-Limmer II patent series is pending in the United States and many foreign countries. Moreover, a patent in the Tuschl II patent series, U.S. Patent No. 7,078,196, claims methods of preparing siRNAs that mediate cleavage of an mRNA in mammalian cells and, therefore, covers methods of making siRNAs directed toward any and all target genes. We hold exclusive worldwide rights to these claims for RNAi therapeutics.
In addition, during 2011, the USPTO declared an interference between our issued patent covering ALN-VSP, our RNAi therapeutic undergoing clinical testing for the treatment of liver cancers, and a pending third-party application assigned to Protiva. In connection with the settlement of all outstanding litigation with Tekmira in November of 2012, the interference was settled and the Protiva application was assigned to us. A description of our settlement with Tekmira is set forth in Note 6 to our consolidated financial statements included Part II, Item 8, “Financial Statements and Supplementary Data,” of this annual report on Form 10-K. With respect to specific siRNAs, we believe that patent coverage will result from demonstrating that particular compositions exert suitable biological and therapeutic effects. Accordingly, we are focused on achieving such demonstrations for siRNAs in key therapeutic programs.
Intellectual Property Related to Our Development Candidates
As our development pipeline matures, we have made and plan to continue to make patent filings that claim all aspects of our development candidates, including dose, method of administration and manufacture.
Intellectual Property Challenges
As the field of RNAi therapeutics is maturing, patent applications are being fully processed by national patent offices around the world. There is uncertainty about which patents will issue, and, if they do, as to when, to whom and with what claims. It is likely that there will be significant litigation and other proceedings, such as interference, reexamination and opposition proceedings, in various patent offices relating to patent rights in the RNAi field. For example, as noted above, various third parties have initiated oppositions to patents in our Kreutzer-Limmer and Tuschl II series in the EPO, as well as in other jurisdictions. We expect that additional oppositions will be filed in the EPO and elsewhere, and other challenges will be raised relating to other patents and patent applications in our portfolio. In many cases, the possibility of appeal exists for either us or our opponents, and it may be years before final, unappealable rulings are made with respect to these patents in certain jurisdictions. Given the importance of our intellectual property portfolio to our business operations, we intend to vigorously enforce our rights and defend against challenges that have arisen or may arise in this area. A description of ongoing legal matters relating to certain aspects of our intellectual property portfolio is set forth in Part I, Item 3, “Legal Proceedings,” of this annual report on Form 10-K.
Competition
The pharmaceutical marketplace is extremely competitive, with hundreds of companies competing to discover, develop and market new drugs. We face a broad spectrum of current and potential competitors, ranging from very large, global pharmaceutical companies with significant resources, to other biotechnology companies with resources and expertise comparable to our own and to smaller biotechnology companies with fewer resources and expertise than we have. We believe that for most or all of our drug development programs, there will be one or more competing programs under development at other companies. In many cases, the companies with competing programs will have access to greater resources and expertise than we do and may be more advanced in those programs.
The competition we face can be grouped into three broad categories:
| • | other companies working to develop RNAi and microRNA therapeutic products; |
| • | companies developing technology known as antisense, which, like RNAi, attempts to silence the activity of specific genes by targeting the mRNAs copied from them; and |
35
Table of Contents
| • | marketed products and development programs for therapeutics that treat the same diseases for which we may also be developing treatments. |
We are aware of several other companies that are working to develop RNAi therapeutic products. Some of these companies are seeking, as we are, to develop chemically synthesized siRNAs as drugs. Others are following a gene therapy approach, with the goal of treating patients not with synthetic siRNAs but with synthetic, exogenously-introduced genes designed to produce siRNA-like molecules within cells.
Companies working on chemically synthesized siRNAs include Novartis, Takeda, Kyowa Hakko Kirin, Marina Biotech, Inc., Arrowhead and its subsidiary, Calando, Quark, Silence Therapeutics plc, Tekmira, Sylentis and Dicerna Pharmaceuticals, Inc. Many of these companies have licensed our intellectual property. Benitec is working on gene therapy approaches to RNAi therapeutics.
Companies working on microRNA therapeutics include Regulus, Rosetta Genomics, Santaris Pharma A/S, or Santaris, miRagen Therapeutics, Inc., Mirna Therapeutics, Inc. and Asuragen, Inc.
Antisense technology uses short, single-stranded, DNA-like molecules to block mRNAs encoding specific proteins. While we believe that RNAi drugs may potentially have significant advantages over antisense oligonucleotides, or ASOs, including greater potency and specificity, others are developing ASO drugs that are currently at a more advanced stage of development than RNAi drugs. For example, Isis has developed an ASO drug, Vitravene®, which was approved by the FDA in 1998, but is no longer available in the U.S. Isis also has several ASO product candidates in clinical development, including mipomersen, which is a lipid-lowering drug being developed by Isis in collaboration with Genzyme. In December 2012, the Committee for Medicinal Products for Human Use, or CHMP, recommended against approval of the drug in Europe for familial hypercholesterolemia. In January 2013, the FDA approved mipomersen for the treatment of patients with homozygous familial hypercholesterolemia, or HoFH. In addition, a number of other companies have product candidates in various stages of pre-clinical and clinical development, including Santaris and Sarepta Therapeutics, Inc. Because of their later stage of development, ASOs, rather than siRNAs, may become the preferred technology for drugs that target mRNAs in order to turn off the activity of specific genes.
The competitive landscape continues to expand and we expect that additional companies will initiate programs focused on the development of RNAi therapeutic products using the approaches described above as well as potentially new approaches that may result in the more rapid development of RNAi therapeutics or more effective technologies for RNAi drug development or delivery.
Competing Drugs for Our RNAi Therapeutics in Clinical Development
TTR-Mediated Amyloidosis (ATTR). Until recently, organ transplantation was the only treatment option for patients with ATTR. Liver or combined heart/liver transplantations were the only available treatment options for FAP. Only a subset of FAP patients with early stage disease qualify for this costly and invasive procedure and, even following liver transplantation, the disease continues to progress for many patients, presumably due to normal TTR being deposited into preexisting fibrils. Moreover, there is a shortage of donors to provide healthy livers for transplantation. In November 2011, Pfizer received marketing approval from the EC for tafamidis for the treatment of transthyretin amyloidosis in adult patients with stage 1 symptomatic polyneuropathy to delay peripheral neurologic impairment. Tafamidis has ODD in the EU for the treatment of FAP and cardiomyopathy associated with ATTR. Tafamidis is intended to stabilize wild-type and variant TTR, to prevent dissociation of the TTR protein and thereby inhibit the formation of TTR oligomers and amyloid fibrils.
The only currently available treatments for FAC are aimed at relief of symptoms, such as diuretics, or water pills, to treat the swelling of the ankles, one of the symptoms of FAC. For patients with advanced cardiomyopathy (FAC and SSA) heart transplant is a therapeutic option. Again, the scarcity of organs for transplantation, and the mortality, morbidity and cost associated with this procedure render it a realistic option for only a very small number of patients. In December 2013, Pfizer initiated a Phase 3 clinical trial of tafamidis in patients with TTR amyloidosis cardiomyopathy. The study is expected to enroll up to 400 patients and study two dose levels, with a 30-month treatment duration for each patient.
36
Table of Contents
There are a few drugs in clinical development for the treatment of ATTR. Researchers at Boston University, in collaboration with the National Institute of Neurological Disorders and Stroke, conducted a Phase 2/3 clinical trial for diflunisal for the treatment of FAP. Diflunisal is a commercially available non-steroidal anti-inflammatory agent that has been found to stabilize TTR in vitro. The results of this clinical trial were recently published, concluding that the use of diflunisal compared with placebo for two years reduced the rate of progression of neurological impairment and preserved quality of life. As published, the discontinuation rate was high in this clinical trial in both treatment arms (approximately 50% overall) and the majority of patients continued to deteriorate, including patients on diflunisal. Furthermore, the safety profile of this drug and its known adverse effects, particularly on the kidney and heart, could likely limit the potential use of it in this disease.
In addition, Isis, together with its partner GSK, is developing ISIS-TTRRx, an ASO designed to treat patients with FAP. Isis has completed a Phase 1 clinical trial evaluating the safety and activity of six subcutaneous doses of ISIS-TTRRx over four weeks in healthy volunteers. Isis reported that in this clinical trial, ISIS-TTRRx produced significant reductions of approximately 80% in TTR protein at the highest dose studied, and reported that ISIS-TTRRx was generally well tolerated with no significant adverse events, although Isis has also reported the occurrence of injection site reactions and flu-like symptoms for ISIS-TTRRx. Isis has announced that ISIS-TTRRx was granted fast track designation by the FDA for the treatment of patients with FAP, and that it initiated a Phase 2/3 clinical trial in early 2013. The trial is expected to enroll up to 195 patients and the treatment duration is 15 months for each patient.
Hemophilia. The global market for treatments of hemophila and bleeding disorders is valued at approximately $9.0 billion. Products on the market include: two Factor VIII replacements marketed by Baxter Healthcare Corporation, or Baxter; a Factor VIII replacement marketed by Bayer Healthcare; a Factor VIII replacement and a Factor IX replacement marketed by Pfizer (Wyeth); a Factor VIIa replacement marketed by Novo Nordisk; two plasma proteins marketed by Baxter; as well as concentrated products available for fibrinogen, FII, FX and FXI deficiencies.
In addition, there are a number of products in development. Long-acting factor therapies will be on the market in the near future, offering weekly intravenous dosing. There are other bypass products that may offer alternatives to patients with inhibitors. None of the mid- or late-stage programs are administered subcutaneously. Biogen Idec has submitted and received acceptances of a biologics license application, or BLA, for long-lasting recombinant Factor VIII (rFVIIIFc) and long-lasting recombinant Factor IX (rFIXFc). FDA decisions on both products are expected in 2014. Novo Nordisk is developing long-acting recombinant derivatives: N8-GP (NN7088), a Factor VIII analog for the treatment of HA, and N9-GP (NN7999), a Factor IX analog for the treatment of HB. Both programs are in Phase 3 clinical trials. Novo Nordisk is also developing Anti-TFPI (NN7415), a monoclonal antibody against a tissue factor pathway inhibitor, for the treatment of HA and HB. It is intended to offer a potential prophylactic treatment of hemophilia with subcutaneous administration. No clinical trials are ongoing. During 2013, Cangene Corporation acquired a recombinant Factor IX candidate, (IB1001) for HB, from Inspiration Biopharmaceuticals, Inc. In addition, Baxter acquired worldwide rights from Inspiration to the HA candidate (OBI-1), a recombinant porcine Factor VIII. Pfizer is developing PF-05280602, a recombinant Factor VIIa variant (813d). It is in a Phase 1 clinical trial in adults with HA or HB. Chugai Pharmaceutical Co. is developing a bispecific antibody that mimics the function of factor VIII by simultaneously binding factor IXa and factor X to promote factor X activation and consequent activation of the blood coagulation cascade. Chugai initiated a Phase 1 clinical trial in healthy adults and HA patients (both with inhibitors and without inhibitors) in Japan in August 2012.
There is a particular segment of hemophilia patients who develop neutralizing antibodies to the factors; these are called patients with inhibitors. They cannot be given replacement factors since the inhibitor, an antibody, will lead to immediate clearance of any Factor VIII or Factor IX that is administered. These patients therefore cannot get any prophylaxis treatment. Generally speaking, they monitor for signs of a bleed and then are given recombinant Factor VIIa, or FEIBA. FEIBA is an anti-inhibitor coagulant complex from Baxter. It is approved in both the United States and the EU for the treatment of acute bleeds and in the EU for prophylactic treatment as well. A BLA is pending in the United States for the prophylaxis indication as well. There are a number of other approaches in development targeting tissue factor pathway inhibitor, another naturally occurring anticoagulant protein.
37
Table of Contents
Hypercholesterolemia. The current standard of care for patients with hypercholesterolemia includes the use of several agents. Front line therapy consists of HMG CoA reductase inhibitors, commonly known as statins, which block production of cholesterol by the liver and increase clearance of LDL-C from the bloodstream. These include atorvastatin, simvastatin, rosuvastatin and pravastatin. A different class of compounds, which includes ezetimibe and ezetimibe/simvastatin, function by blocking cholesterol uptake from the diet and are utilized on their own or in combination with statins. Aegerion Pharmaceuticals, Inc. is developing lomitapide, an microsomal triglyceride protein, or MTP, inhibitor for the treatment of dyslipidemia. In December 2012, the FDA approved lomitapide for use in patients with HoFH and in July 2013 lomitapide was approved for HoFH in the EU.
In addition, mipomersen is a lipid-lowering drug targeting apolipoprotein B-100 being developed by Isis in collaboration with Genzyme. Isis and Genzyme have evaluated mipomersen in four positive Phase 3 clinical trials in which its primary endpoints were met. In all four Phase 3 clinical trials, treatment with mipomersen lowered LDL-C and had a beneficial impact on other atherogenic lipids. A weekly injectable therapeutic, mipomersen is being developed primarily for patients at significant cardiovascular risk who are unable to achieve target cholesterol lowering levels with statins alone or who are intolerant of statins. In July 2011, Genzyme submitted a marketing authorization application for mipomersen in Europe. In December 2012, the CHMP recommended against approval of the drug in Europe for familial hypercholesterolemia. In January 2013, the FDA approved mipomersen for the treatment of patients with HoFH.
With regard to future therapies in clinical development, several anti-PCSK9 antibodies have advanced into clinical development, including REGN727/SAR236553, which is being developed by Regeneron Pharmaceuticals, Inc., or Regeneron, in collaboration with Sanofi. Data reported from one REGN727/SAR236553 Phase 2 clinical trial in patients with severe hypercholesterolemia have demonstrated mean reductions in LDL-C from baseline ranging from approximately 30% to greater than 65% depending on the dosing regimen of REGN727/SAR236553 compared to a mean reduction of 10% with placebo (p<0.05 for all dose groups). Regeneron announced the launch of ODYSSEY OUTCOMES, an 18,000 patient Phase 3 clinical trial designed to test the efficacy and safety of REGN727 added to maximal doses of statins in reducing cardiovascular morbidity and mortality in patients with recent acute coronary syndrome, a population at high risk of cardiovascular events despite best contemporary therapy. Amgen Inc., Eli Lilly and Company and Pfizer also have anti-PCSK9 antibodies in clinical development and we are aware of several additional similar compounds in advanced pre-clinical development.
Other Competition
Finally, for many of the diseases that are the subject of our RNAi therapeutics pre-clinical development and discovery programs, there are already drugs on the market or in development. For example, with respect to ALN-CC5, an intravenous anti-C5 monoclonal antibody therapy (eculizumab) has demonstrated clinical activity and tolerability in a number of complement-mediated diseases. It is approved for the treatment PNH and aHUS in the United States, Europe and other countries. However, notwithstanding the availability of existing drugs or drug candidates, we believe there currently exists sufficient unmet medical need to warrant the advancement of RNAi therapeutic programs.
Regulatory Matters
The research, testing, manufacture and marketing of drug products and their delivery systems are extensively regulated in the United States and the rest of the world. In the United States, drugs are subject to rigorous regulation by the FDA. The Federal Food, Drug, and Cosmetic Act, or FDCA, and other federal and state statutes and regulations govern, among other things, the research, development, testing, approval, manufacture, storage, record keeping, reporting, packaging, labeling, promotion and advertising, marketing and distribution of pharmaceutical products. Failure to comply with the applicable regulatory requirements may subject a company to a variety of administrative or judicially-imposed sanctions and the inability to obtain or maintain required approvals to test or market drug products. These sanctions could include, among other things, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, clinical holds, injunctions, fines, civil penalties or criminal prosecution.
38
Table of Contents
The steps ordinarily required before a new pharmaceutical product may be marketed in the United States include nonclinical laboratory tests, animal tests and formulation studies, the submission to the FDA of an IND, which must become effective prior to commencement of clinical testing, approval by an independent review board, or IRB, at each clinical site before each trial may be initiated, completion of adequate and well-controlled clinical trials to establish that the drug product is safe and effective for the indication for which FDA approval is sought, submission to the FDA of an NDA, review and recommendation by an advisory committee of independent experts (particularly for new chemical entities), satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with current good manufacturing practice, or cGMP, requirements, satisfactory completion of an FDA inspection of the major investigational sites to ensure data integrity and assess compliance with good clinical practice requirements, or GCPs, and FDA review and approval of the NDA. Satisfaction of FDA pre-market approval requirements typically takes several years, but may vary substantially depending upon the complexity of the product and the nature of the disease. Government regulation may delay or prevent marketing of potential products for a considerable period of time and impose costly procedures on a company’s activities. Success in early stage clinical trials does not necessarily assure success in later stage clinical trials. Data obtained from clinical activities, including the data derived from our clinical trials for ALN-TTR01, patisiran, ALN-TTRsc, ALN-PCS02 and ALN-AT3 is not always conclusive and may be subject to alternative interpretations that could delay, limit or even prevent regulatory approval. Even if a product receives regulatory approval, later discovery of previously unknown problems with a product, including new safety risks, may result in restrictions on the product or even complete withdrawal of the product from the market.
Nonclinical tests include laboratory evaluation of product chemistry and formulation, as well as animal testing to assess the potential safety and efficacy of the product. The conduct of the non-clinical tests and formulation of compounds for testing must comply with federal regulations and requirements. The results of nonclinical testing are submitted to the FDA as part of an IND, together with manufacturing information, analytical and stability data, a proposed clinical trial protocol and other information.
A 30-day waiting period after the filing of an IND is required prior to such application becoming effective and the commencement of clinical testing in humans. If the FDA has not commented on, or questioned, the application during this 30-day waiting period, clinical trials may begin. If the FDA has comments or questions, these must be resolved to the satisfaction of the FDA prior to commencement of clinical trials. The IND review process can result in substantial delay and expense. We, an IRB, or the FDA may, at any time, suspend, terminate or impose a clinical hold on ongoing clinical trials. If the FDA imposes a clinical hold, clinical trials cannot commence or recommence without FDA authorization and then only under terms authorized by the FDA.
Clinical trials involve the administration of an investigational new drug to healthy volunteers or patients under the supervision of a qualified investigator. Clinical trials must be conducted in compliance with federal regulations and requirements, including GCPs, which are ethical and scientific quality standards and FDA requirements for conducting, recording and reporting clinical trials to assure the rights, safety and well-being of trial participants are protected and include the requirement that all research subjects provide their informed consent for their participation in any clinical study. Clinical studies are conducted under protocols detailing, among other things, the objectives of the trial and the safety and effectiveness criteria to be evaluated. Each protocol involving testing on human subjects in the United States must be submitted to the FDA as part of the IND. In addition, an IRB at each institution participating in the clinical trial must review and approve the plan for any clinical trial before it commences at that institution, and the IRB must conduct continuing review. The IRB must review and approve, among other things, the study protocol and informed consent information to be provided to study subjects. An IRB must operate in compliance with FDA regulations.
Clinical trials to support NDAs for marketing approval are typically conducted in three sequential phases, which may overlap or be combined. In Phase 1, the initial introduction of the drug into healthy human subjects or patients, the drug is tested to primarily assess safety, tolerability, pharmacokinetics, pharmacological actions and metabolism associated with increasing doses. Phase 2 usually involves trials in a limited patient population, to assess the optimum dosage, identify possible adverse effects and safety risks, and provide preliminary support for the efficacy of the drug in the indication being studied.
39
Table of Contents
If a compound demonstrates evidence of effectiveness and an acceptable safety profile in Phase 2 clinical trials, Phase 3 clinical trials may be undertaken to further evaluate clinical efficacy and to further test for safety in an expanded patient population, typically at geographically dispersed clinical trial sites, to establish the overall benefit-risk relationship of the drug and to provide adequate information for the labeling of the drug. Phase 1, Phase 2 or Phase 3 testing of any drug candidates may not be completed successfully within any specified time period, if at all. The FDA closely monitors the progress of each of the three phases of clinical trials that are conducted in the United States. The FDA may, at its discretion, reevaluate, alter, suspend or terminate the testing based upon the data accumulated to that point and the FDA’s assessment of the risk/benefit ratio to the subject. The FDA, an IRB, or a clinical trial sponsor may suspend or terminate clinical trials at any time for various reasons, including a finding that the subjects or patients are being exposed to an unacceptable health risk. The FDA can also request that additional clinical trials be conducted as a condition to product approval. Finally, sponsors are required to publicly disseminate information about ongoing and completed clinical trials on a government website administered by the National Institutes of Health, or NIH, and are subject to civil monetary penalties and other civil and criminal sanctions for failing to meet these obligations. After successful completion of the required clinical testing, generally an NDA is prepared and submitted to the FDA.
We believe that any RNAi product candidate we develop, whether for the treatment of ATTR, hemophilia and RBD, complement mediated diseases, AIP, hypercholesterolemia or the various indications targeted in our development or pre-clinical discovery programs, will be regulated as a new drug by the FDA. FDA approval of an NDA is required before marketing of a new drug may begin in the United States. The NDA must include the results of extensive pre-clinical, clinical and other testing, as described above, a compilation of data relating to the product’s pharmacology, chemistry, manufacture and controls, proposed labeling and other information. In addition, an NDA for a new active ingredient, new indication, new dosage form, new dosing regimen, or new route of administration must contain data assessing the safety and efficacy for the claimed indication in all relevant pediatric subpopulations, and support dosing and administration for each pediatric subpopulation for which the drug is shown to be safe and effective. In some circumstances, the FDA may grant deferrals for the submission of some or all pediatric data, or full or partial waivers. The cost of preparing and submitting an NDA is substantial. Under federal law, NDAs are subject to substantial application user fees and the sponsor of an approved NDA is also subject to annual product and establishment user fees.
The FDA conducts a preliminary review of all NDAs within the first 60 days after submission before accepting them for filing to determine whether they are sufficiently complete to permit substantive review. The FDA may request additional information rather than accept an NDA for filing. If the submission is accepted for filing, the FDA begins an in-depth review of the NDA. The FDA has agreed to specified performance goals regarding the timing of its review of NDAs, although the FDA does not always meet these goals. The review process is often significantly extended by FDA requests for additional information or clarification regarding information already provided in the submission. The FDA may also refer applications for novel drug products or drug products that present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. The FDA normally conducts a pre-approval inspection to ensure the manufacturing facility, methods and controls are adequate to preserve the drug’s identity, strength, quality, purity and stability, and are in compliance with regulations governing cGMPs. In addition, the FDA often will conduct a bioresearch monitoring inspection of the clinical trial sites involved in conducting pivotal studies to ensure data integrity and compliance with applicable GCP requirements.
If the FDA evaluation of the NDA and the inspections of manufacturing facilities and clinical trial sites are favorable, the FDA may issue an approval letter, which authorizes commercial marketing of the drug with specific prescribing information for a specific indication. As a condition of NDA approval, the FDA may require post-approval testing, sometimes referred to as Phase 4 trials, and surveillance to monitor the drug’s safety or efficacy and may impose other conditions, including labeling restrictions, which can materially impact the potential market and profitability of the drug. In addition, the FDA may impose distribution and use restrictions and other limitations on labeling and communication activities with respect to an approved drug product through
40
Table of Contents
a Risk Evaluation and Mitigation Strategy, or REMS, plan. Once granted, product approvals may be further limited or withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
Once an NDA is approved, a product will be subject to certain post-approval requirements, including requirements for adverse event reporting, submission of periodic reports, recordkeeping, product sampling and distribution. Additionally, the FDA also strictly regulates the promotional claims that may be made about prescription drug products and biologics. In particular, the FDA generally prohibits pharmaceutical companies from promoting their drugs or biologics for uses that are not approved by the FDA as reflected in the product’s approved labeling. In addition, the FDA requires substantiation of any safety or effectiveness claims, including claims that one product is superior in terms of safety or effectiveness to another. Superiority claims generally must be supported by two adequate and well-controlled head-to-head clinical trials. To the extent that market acceptance of our products may depend on their superiority over existing therapies, any restriction on our ability to advertise or otherwise promote claims of superiority, or requirements to conduct additional expensive clinical trials to provide proof of such claims, could negatively affect the sales of our products or our costs. We must also notify the FDA of any change in an approved product beyond variations already allowed in the approval. Certain changes to the product, its labeling or its manufacturing require prior FDA approval and may require the conduct of further clinical investigations to support the change, which may require the payment of additional, substantial user fees. Such approvals may be expensive and time-consuming and, if not approved, the FDA will not allow the product to be marketed as modified.
If the FDA’s evaluation of the NDA submission or manufacturing facilities is not favorable, the FDA may refuse to approve the NDA or issue a complete response letter. The complete response letter describes the deficiencies that the FDA has identified in an application and, when possible, recommends actions that the applicant might take to place the application in condition for approval. Such actions may include, among other things, conducting additional safety or efficacy studies after which the sponsor may resubmit the application for further review. Even with the completion of this additional testing or the submission of additional requested information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval. With limited exceptions, the FDA may withhold approval of an NDA regardless of prior advice it may have provided or commitments it may have made to the sponsor.
Some of our product candidates may need to be administered using specialized drug delivery systems. We may rely on drug delivery systems that are already approved to deliver drugs like ours to similar physiological sites or, in some instances, we may need to modify the design or labeling of the legally available device for delivery of our product candidate. In such an event, the FDA may regulate the product as a combination product or require additional approvals or clearances for the modified device. In addition, to the extent the delivery device is owned by another company, we would need that company’s cooperation to implement the necessary changes to the device and to obtain any additional approvals or clearances. Obtaining such additional approvals or clearances, and cooperation of other companies, when necessary, could significantly delay, and increase the cost of obtaining marketing approval, which could reduce the commercial viability of a product candidate. To the extent that we rely on previously unapproved drug delivery systems, we may be subject to additional testing and approval requirements from the FDA above and beyond those described above.
Once an NDA is approved, the product covered thereby becomes a listed drug that can, in turn, be relied upon by potential competitors in support of approval of an abbreviated new drug application, or ANDA, or 505(b)(2) application upon expiration of certain patent and non-patent exclusivity periods, if any. An approved ANDA generally provides for marketing of a drug product that has the same active ingredients in the same strength, dosage form and route of administration as the listed drug and has been shown through appropriate testing (unless waived) to be bioequivalent to the listed drug. There is no requirement, other than the requirement for bioequivalence testing (which may be waived by the FDA), for an ANDA applicant to conduct or submit results of nonclinical or clinical tests to prove the safety or effectiveness of its drug product. Drugs approved in this way are commonly referred to as generic equivalents to the listed drug, are listed as such by the FDA and can often be substituted by pharmacists under prescriptions written for the original listed drug. A 505(b)(2) application is a type of NDA that relies, in part, upon data the applicant does not own and to which it does not have a right of reference. Such applications typically are submitted for changes to previously approved drug products.
41
Table of Contents
Federal law provides for a period of three years of exclusivity following approval of a listed drug that contains a previously approved active ingredient but is approved in, among other things, a new dosage, dosage form, route of administration or combination, or for a new use, if the FDA determines that new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are essential to the approval of the application. This three-year exclusivity covers only the conditions of use associated with the new clinical investigations and, as a general matter, does not prohibit the FDA from approving ANDAs or 505(b)(2) applications for generic versions of the original, unmodified drug product. Federal law also provides a period of up to five years exclusivity following approval of a drug containing no previously approved active moiety, which is the molecule or ion responsible for the action of the drug substance, during which ANDAs and 505(b)(2) applications referencing the protected listed drug cannot be submitted unless the submission accompanies a challenge to a listed patent, in which case the submission may be made four years following the original product approval. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA; however, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the pre-clinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
Additionally, in the event that the sponsor of the listed drug has properly informed the FDA of patents covering its listed drug, applicants submitting an ANDA or 505(b)(2) application referencing the listed drug are required to make one of four patent certifications for each listed patent, except for patents covering methods of use for which the ANDA or 505(b)(2) applicant is not seeking approval. If an applicant certifies its belief that one or more listed patents are invalid, unenforceable, or not infringed (and thereby indicates it is seeking approval prior to patent expiration), it is required to provide notice of its filing to the NDA sponsor and the patent holder within certain time limits. If the patent holder then initiates a suit for patent infringement against the ANDA or 505(b)(2) applicant within 45 days of receipt of the notice, the FDA cannot grant effective approval of the ANDA or 505(b)(2) application until either 30 months have passed or there has been a court decision or settlement order holding or stating that the patents in question are invalid, unenforceable or not infringed. If the patent holder does not initiate a suit for patent infringement within the 45 days, the ANDA or 505(b)(2) application may be approved immediately upon successful completion of FDA review, unless blocked by another listed patent or regulatory exclusivity period. If the ANDA or 505(b)(2) applicant certifies that it does not intend to market its generic product before some or all listed patents on the listed drug expire, then the FDA cannot grant effective approval of the ANDA or 505(b)(2) application until those patents expire. The first of the ANDA applicants submitting substantially complete applications certifying that one or more listed patents for a particular product are invalid, unenforceable, or not infringed may qualify for an exclusivity period of 180 days running from when the generic product is first marketed, during which subsequently submitted ANDAs containing similar certifications cannot be granted effective approval. The 180-day generic exclusivity can be forfeited in various ways, including if the first applicant does not market its product within specified statutory timelines. If more than one applicant files a substantially complete ANDA on the same day, each such first applicant will be entitled to share the 180-day exclusivity period, but there will only be one such period, beginning on the date of first marketing by any of the first applicants.
The Patient Protection and Affordable Care Act, or Affordable Care Act, signed into law on March 23, 2010, includes a subtitle called the Biologics Price Competition and Innovation Act of 2009, or BPCI Act, which created an abbreviated approval pathway for biological products shown to be similar to, or interchangeable with, an FDA-licensed reference biological product. This amendment to the PHSA attempts to minimize duplicative testing. Biosimilarity, which requires that there be no clinically meaningful differences between the biological product and the reference product in terms of safety, purity, and potency, can be shown through analytical studies, animal studies, and a clinical study or studies. Interchangeability requires that a product is biosimilar to the reference product and the product must demonstrate that it can be expected to produce the same clinical results as the reference product and, for products administered multiple times, the biologic and the reference biologic may be switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biologic. However, complexities associated with the larger, and often more complex, structure of biological products, as well as the process by which such products are manufactured, pose significant hurdles to implementation that are still being worked out by the FDA.
42
Table of Contents
A reference biologic is granted twelve years of exclusivity from the time of first licensure of the reference product. The first biologic product submitted under the abbreviated approval pathway that is determined to be interchangeable with the reference product has exclusivity against other biologics submitting under the abbreviated approval pathway for the lesser of (i) one year after the first commercial marketing, (ii) 18 months after approval if there is no legal challenge, (iii) 18 months after the resolution in the applicant’s favor of a lawsuit challenging the biologics’ patents if an application has been submitted, or (iv) 42 months after the application has been approved if a lawsuit is ongoing within the 42-month period.
Under the Orphan Drug Act, the FDA may grant ODD to a drug intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making available in the United States a drug for this type of disease or condition will be recovered from sales in the United States for that drug. Orphan drug designation must be requested before submitting an NDA. After the FDA grants ODD, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA.
If a product that has ODD subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications, including a full NDA, to market the same drug for the same indication, except in very limited circumstances, for seven years. For purposes of small molecule drugs, the FDA defines “same drug” as a drug that contains the same active moiety and is intended for the same use as the previously approved orphan drug. For purposes of large molecule drugs, the FDA defines “same drug” as a drug that contains the same principal molecular structural features, but not necessarily all of the same structural features, and is intended for the same use as the drug in question. Notwithstanding the above definitions, a drug that is clinically superior to an orphan drug will not be considered the “same drug” and thus will not be blocked by orphan drug exclusivity.
A designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. In addition, orphan drug exclusive marketing rights in the United States may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the drug to meet the needs of patients with the rare disease or condition.
The Orphan Products Grants Program in FDA’s Office of Orphan Products Development, or OOPD, with an annual budget of approximately $14.0 million, supports clinical development of products including drugs, biologics, medical devices and medical foods for use in rare diseases and conditions where no current therapy exists or where the proposed product will be superior to the existing therapy. This program provides grants for clinical studies on safety and/or effectiveness that will either result in, or substantially contribute to, market approval of these products. In addition, OOPD will administer a new grant program, the Pediatric Device Consortia Grant Program, resulting from the 2007 FDAAA legislation, with an annual budget of $2.0 million to support nonprofit consortia to facilitate pediatric medical device development. The future availability of such grants is subject to uncertainties regarding continued federal funding.
From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the approval, manufacturing and marketing of drug products. In addition, FDA regulations and guidance are often revised or reinterpreted by the agency or reviewing courts in ways that may significantly affect our business and development of our product candidates and any products that we may commercialize. It is impossible to predict whether additional legislative changes will be enacted, or FDA regulations, guidance or interpretations changed, or what the impact of any such changes may be. Federal budget uncertainties or spending reductions may reduce the capabilities of the FDA, extend the duration of required regulatory reviews, and reduce the availability of clinical research grants.
Recently, the Food and Drug Administration Safety and Innovation Act, or FDASIA, which was signed into law on July 9, 2012, amended the FDCA. FDASIA requires that a sponsor who is planning to submit a marketing application for a drug or biological product that includes a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration submit an initial Pediatric Study Plan, or PSP,
43
Table of Contents
within sixty days of an end-of-phase 2 meeting or as may be agreed between the sponsor and FDA. The initial PSP must include an outline of the pediatric study or studies that the sponsor plans to conduct, including study objectives and design, age groups, relevant endpoints and statistical approach, or a justification for not including such detailed information, and any request for a deferral of pediatric assessments or a full or partial waiver of the requirement to provide data from pediatric studies along with supporting information. FDA and the sponsor must reach agreement on the PSP. A sponsor can submit amendments to an agreed-upon initial PSP at any time if changes to the pediatric plan need to be considered based on data collected from nonclinical studies, early phase clinical trials, and/or other clinical development programs.
The FDA has a Fast Track program that is intended to expedite or facilitate the process for reviewing new drugs and biological products that meet certain criteria. Specifically, new drugs and biological products are eligible for Fast Track designation if they are intended to treat a serious or life-threatening condition and demonstrate the potential to address unmet medical needs for the condition. Fast Track designation applies to the combination of the product and the specific indication for which it is being studied. The sponsor of a new drug or biological may request the FDA to designate the drug or biologic as a Fast Track product at any time during the clinical development of the product. Unique to a Fast Track product, the FDA may consider for review sections of the marketing application on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the application, the FDA agrees to accept sections of the application and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the application.
Any product submitted to the FDA for marketing, including under a Fast Track program, may be eligible for other types of FDA programs intended to expedite development and review, such as priority review and accelerated approval. Any product is eligible for priority review if it has the potential to provide safe and effective therapy where no satisfactory alternative therapy exists or a significant improvement in the treatment, diagnosis or prevention of a disease compared to marketed products. The FDA will attempt to direct additional resources to the evaluation of an application for a new drug or biological product designated for priority review in an effort to facilitate the review. Additionally, a product may be eligible for accelerated approval. Drug or biological products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may receive accelerated approval, which means that they may be approved on the basis of adequate and well-controlled clinical studies establishing that the product has an effect on a surrogate endpoint that is reasonably likely to predict a clinical benefit, or on the basis of an effect on a clinical endpoint other than survival or irreversible morbidity. As a condition of approval, the FDA may require that a sponsor of a drug or biological product receiving accelerated approval perform adequate and well-controlled post-marketing clinical studies. In addition, the FDA currently requires as a condition for accelerated approval pre-approval of promotional materials, which could adversely impact the timing of the commercial launch of the product. Fast Track designation, priority review and accelerated approval do not change the standards for approval but may expedite the development or approval process.
The Food and Drug Administration Safety and Innovation Act of 2012 also amended the FDCA to require FDA to expedite the development and review of a breakthrough therapy. A drug or biological product can be designated as a breakthrough therapy if it is intended to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that it may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. A sponsor may request that a drug or biological product be designated as a breakthrough therapy at any time during the clinical development of the product. If so designated, FDA shall act to expedite the development and review of the product’s marketing application, including by meeting with the sponsor throughout the product’s development, providing timely advice to the sponsor to ensure that the development program to gather nonclinical and clinical data is as efficient as practicable, involving senior managers and experienced review staff in a cross-disciplinary review, assigning a cross-disciplinary project lead for the FDA review team to facilitate an efficient review of the development program and to serve as a scientific liaison between the review team and the sponsor, and taking steps to ensure that the design of the clinical trials is as efficient as practicable.
44
Table of Contents
Foreign Regulation of New Drug Compounds
In addition to regulations in the United States, we are subject to a variety of regulations in other jurisdictions governing, among other things, clinical trials and any commercial sales and distribution of our products.
Whether or not we obtain FDA approval for a product, we must obtain the requisite approvals from regulatory authorities in all or most foreign countries prior to the commencement of clinical trials or marketing of the product in those countries. Certain countries outside of the United States have a similar process that requires the submission of a clinical trial application, or CTA, much like the IND prior to the commencement of human clinical trials. In Europe, for example, a CTA must be submitted to each country’s national health authority and an independent ethics committee, much like the FDA and IRB, respectively. Once the CTA is approved in accordance with a country’s requirements, clinical trial development may proceed. Similarly, all clinical trials in Australia require review and approval of clinical trial proposals by an ethics committee, which provides a combined ethical and scientific review process.
The requirements and process governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country. In all cases, the clinical trials must be conducted in accordance with GCP, which have their origin in the World Medical Association’s Declaration of Helsinki, the applicable regulatory requirements, and guidelines developed by the International Conference on Harmonization, or ICH, for GCP practices in clinical trials.
The approval procedure also varies among countries and can involve requirements for additional testing. The time required may differ from that required for FDA approval and may be longer than that required to obtain FDA approval. Although there are some procedures for unified filings in the EU, in general, each country has its own procedures and requirements, many of which are time consuming and expensive. Thus, there can be substantial delays in obtaining required approvals from foreign regulatory authorities after the relevant applications are filed.
In Europe, marketing authorizations may be submitted under a centralized or decentralized procedure. The centralized procedure is mandatory for the approval of biotechnology and many pharmaceutical products and provides for the grant of a single marketing authorization that is valid in all EU member states. The decentralized procedure is a mutual recognition procedure that is available at the request of the applicant for medicinal products that are not subject to the centralized procedure. We intend to strive to choose the appropriate route of European regulatory filing to accomplish the most rapid regulatory approvals. However, our chosen regulatory strategy may not secure regulatory approvals on a timely basis or at all.
If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Pharmaceutical Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any drug products for which we obtain regulatory approval. In the United States and markets in other countries, sales of any products for which we may receive regulatory approval for commercial sale will depend in part on the availability of reimbursement from third-party payors. Third-party payors include government health administrative authorities, managed care providers, private health insurers and other organizations. The process for determining whether a payor will provide coverage for a drug product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the drug product. Third-party payors may limit coverage to specific drug products on an approved list, or formulary, which might not include all of the FDA-approved drugs for a particular indication. Third-party payors may provide coverage, but place stringent limitations on such coverage, such as requiring alternative treatments to be tried first. These third-party payors are increasingly challenging the price and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. In addition, significant uncertainty exists as to the reimbursement status of newly approved healthcare product candidates. We may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of our products,
45
Table of Contents
in addition to incurring the costs required to obtain FDA approvals. Our product candidates may not be considered medically reasonable or necessary or cost-effective. Even if a drug product is covered, a payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
Federal, state and local governments in the United States continue to consider legislation to limit the growth of healthcare costs, including the cost of prescription drugs. Future legislation could limit payments for pharmaceuticals such as the drug candidates that we are developing.
Different pricing and reimbursement schemes exist in other countries. In the EU, governments influence the price of pharmaceutical products through their pricing and reimbursement rules and control of national health care systems that fund a large part of the cost of those products to consumers. Some jurisdictions operate systems under which products may be marketed only after a reimbursement price has been agreed. To obtain reimbursement or pricing approval, some of these countries may require the completion of clinical trials that compare the cost-effectiveness of a particular product candidate to currently available therapies. Other member states allow companies to set their own prices for medicines, but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products. In addition, in some countries, cross-border imports from low-priced markets exert competitive pressure that may reduce pricing within a country.
The marketability of any products for which we receive regulatory approval for commercial sale may suffer if the government and third-party payors fail to provide adequate coverage and reimbursement. In addition, the emphasis on managed care in the United States has increased and we expect will continue to exert downward pressure on pharmaceutical pricing. Coverage policies, third-party reimbursement rates and pharmaceutical pricing regulations may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
In March 2010, the Patient Protection and Affordable Health Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, collectively, PPACA, was enacted, which includes measures that have or will significantly change the way health care is financed by both governmental and private insurers. Among the provisions of PPACA of greatest importance to the pharmaceutical industry are the following:
| • | The Medicaid Drug Rebate Program requires pharmaceutical manufacturers to enter into and have in effect a national rebate agreement with the Secretary of the Department of Health and Human Services a condition for states to receive federal matching funds for the manufacturer’s outpatient drugs furnished to Medicaid patients. Effective in 2010, PPACA made several changes to the Medicaid Drug Rebate Program, including increasing pharmaceutical manufacturers’ rebate liability by raising the minimum basic Medicaid rebate on most branded prescription drugs and biologic agents from 15.1% of average manufacturer price, or AMP, to 23.1% of AMP and adding a new rebate calculation for “line extensions” (i.e., new formulations, such as extended release formulations) of solid oral dosage forms of branded products, as well as potentially impacting their rebate liability by modifying the statutory definition of AMP. PPACA also expanded the universe of Medicaid utilization subject to drug rebates by requiring pharmaceutical manufacturers to pay rebates on Medicaid managed care utilization as of 2010 and by expanding the population potentially eligible for Medicaid drug benefits, to be phased-in by 2014. The Centers for Medicare and Medicaid Services, or CMS, have proposed to expand Medicaid rebate liability to the territories of the United States as well. In addition, PPACA provides for the public availability of retail survey prices and certain weighted average AMPs under the Medicaid program. The implementation of this requirement by the CMS may also provide for the public availability of pharmacy acquisition of cost data, which could negatively impact our sales. |
| • | In order for a pharmaceutical product to receive federal reimbursement under the Medicare Part B and Medicaid programs or to be sold directly to U.S. government agencies, the manufacturer must extend |
46
Table of Contents
| discounts to entities eligible to participate in the 340B drug pricing program. The required 340B discount on a given product is calculated based on the AMP and Medicaid rebate amounts reported by the manufacturer. Effective in 2010, PPACA expanded the types of entities eligible to receive discounted 340B pricing, although, under the current state of the law, with the exception of children’s hospitals, these newly eligible entities will not be eligible to receive discounted 340B pricing on orphan drugs when used for the orphan indication. In addition, as 340B drug pricing is determined based on AMP and Medicaid rebate data, the revisions to the Medicaid rebate formula and AMP definition described above could cause the required 340B discount to increase. |
| • | Effective in 2011, PPACA imposed a requirement on manufacturers of branded drugs and biologic agents to provide a 50% discount off the negotiated price of branded drugs dispensed to Medicare Part D patients in the coverage gap (i.e., “donut hole”). |
| • | Effective in 2011, PPACA imposed an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs, although this fee would not apply to sales of certain products approved exclusively for orphan indications. |
| • | Effective in 2012, PPACA required pharmaceutical manufacturers to track certain financial arrangements with physicians and teaching hospitals, including any “transfer of value” made or distributed to such entities, as well as any investment interests held by physicians and their immediate family members. Manufacturers were required to begin tracking this information in 2013 and to report this information to CMS beginning in 2014. |
| • | As of 2010, a new Patient-Centered Outcomes Research Institute was established pursuant to PPACA to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research. The research conducted by the Patient-Centered Outcomes Research Institute may affect the market for certain pharmaceutical products. |
| • | PPACA created the Independent Payment Advisory Board which, beginning in 2014, will have authority to recommend certain changes to the Medicare program to reduce expenditures by the program that could result in reduced payments for prescription drugs. Under certain circumstances, these recommendations will become law unless Congress enacts legislation that will achieve the same or greater Medicare cost savings. |
| • | PPACA established the Center for Medicare and Medicaid Innovation within CMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending. Funding has been allocated to support the mission of the Center for Medicare and Medicaid Innovation from 2011 to 2019. |
Hazardous Materials
Our research and development processes involve the controlled use of hazardous materials, chemicals and radioactive materials and produce waste products. We are subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of hazardous materials and waste products. We do not expect the cost of complying with these laws and regulations to be material.
Manufacturing
To date, we have manufactured only limited supplies of drug substance for use in IND-enabling toxicology studies in animals at our own facility and have contracted with several third-party contract manufacturing organizations for the supply of drug substance and finished product to meet our testing needs for pre-clinical toxicology and clinical testing. We expect to continue to rely on third-party contract manufacturing organizations for the supply of drug substance and certain drug product, including siRNAs and siRNA conjugates, for our product candidates for the foreseeable future. In November 2012, we elected to buy out our manufacturing obligations to TPC with respect to our LNP-based pipeline programs. During 2012, we established a
47
Table of Contents
manufacturing facility and have developed cGMP capabilities and processes for the manufacture of patisiran formulated bulk drug product for late-stage clinical trials and early commercial use. During 2013, we manufactured our first cGMP batches of patisiran for use in our Phase 2 OLE and Phase 3 clinical trials. We expect to manufacture late-stage clinical and early stage commercial supply for patisiran formulated bulk drug product in our facility. In the future, we may also develop our own capabilities to manufacture drug substance, including siRNAs and siRNA conjugates for human clinical use. Commercial quantities of any drugs that we may seek to develop will have to be manufactured in facilities, and by processes, that comply with FDA regulations and other federal, state and local regulations, as well as comparable foreign regulations.
We believe we have sufficient manufacturing capacity through our third-party contract manufacturers and our internal cGMP manufacturing facility to meet our current research and clinical needs. We believe that the supply capacity we have established externally, together with the internal capacity we developed to support clinical trials, will be sufficient to meet our anticipated needs. We also believe that with reasonably anticipated benefits from increases in scale and improvements in chemistry, we will be able to manufacture our product candidates at commercially competitive prices.
Scientific Advisors
We seek advice from our scientific advisory board, which consists of a number of leading scientists and physicians, on scientific and medical matters. Our scientific advisory board meets regularly to assess:
| • | our research and development programs; |
| • | the design and implementation of our clinical programs; |
| • | our patent and publication strategies; |
| • | new technologies relevant to our research and development programs; and |
| • | specific scientific and technical issues relevant to our business. |
The current members of our scientific advisory board are:
| Name |
Position/Institutional Affiliation | |
| Dennis A. Ausiello, M.D. |
Jackson Professor of Clinical Medicine/Harvard Medical School; Chief of Medicine/Massachusetts General Hospital | |
| David P. Bartel, Ph.D. |
Member/Whitehead Institute for Biomedical Research; Professor/Massachusetts Institute of Technology; Investigator/Howard Hughes Medical Institute | |
| Katherine A. High, M.D. |
Professor of Pediatrics/Perelman School of Medicine, University of Pennsylvania | |
| Robert S. Langer, Ph.D. |
Institute Professor/Massachusetts Institute of Technology | |
| Judy Lieberman, M.D., Ph.D. |
Senior Investigator/Immune Disease Institute — Harvard Medical School; Professor/Harvard Medical School | |
| Paul R. Schimmel, Ph.D. |
Ernest and Jean Hahn Professor/Skaggs Institute for Chemical Biology, The Scripps Research Institute | |
| Phillip A. Sharp, Ph.D. | Institute Professor/The Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology | |
| Daniel J. Rader, M.D. |
Professor of Medicine and Chief, Division of Translational Medicine and Human Genetics/Perelman School of Medicine, University of Pennsylvania | |
| Markus Stoffel, M.D., Ph.D. |
Professor/Institute of Molecular Systems Biology, Swiss Federal Institute of Technology (ETH) Zurich | |
| Thomas H. Tuschl, Ph.D. |
Professor/Rockefeller University; Investigator/Howard Hughes Medical Institute | |
| Phillip D. Zamore, Ph.D. |
Gretchen Stone Cook Professor/University of Massachusetts Medical School; Co-Director/RNAi Therapeutics Institute, University of Massachusetts Medical School; Investigator/Howard Hughes Medical Institute |
48
Table of Contents
Employees
At January 31, 2014, we had 165 employees, 139 of whom were engaged in research and development. None of our employees are represented by a labor union or covered by a collective bargaining agreement, nor have we experienced work stoppages. We believe that relations with our employees are good.
Financial Information About Geographic Areas
See the section entitled “Segment Information” appearing in Note 2 to our consolidated financial statements for financial information about geographic areas. The Notes to our consolidated financial statements are contained in Part II, Item 8, “Financial Statements and Supplementary Data,” of this annual report on Form 10-K.
Corporate Information
The company comprises six entities, Alnylam Pharmaceuticals, Inc. and five wholly owned subsidiaries (Alnylam U.S., Inc., Alnylam Europe AG, Alnylam (Bermuda) Ltd., Alnylam UK Limited and Alnylam Securities Corporation). Alnylam Pharmaceuticals, Inc. is a Delaware corporation that was formed in May 2003. Alnylam U.S., Inc. is also a Delaware corporation that was formed in June 2002. Alnylam Securities Corporation is a Massachusetts corporation that was formed in December 2006. Alnylam Europe AG, which was incorporated in Germany in June 2000 under the name Ribopharma AG, was acquired by Alnylam Pharmaceuticals, Inc. in July 2003. Alnylam (Bermuda) Ltd., an entity organized under the laws of Bermuda, was formed on August 7, 2013. Alnylam UK Limited, an entity organized under the laws of the United Kingdom, was formed on January 31, 2014. Our principal executive office is located at 300 Third Street, Cambridge, Massachusetts 02142, and our telephone number is (617) 551-8200.
Investor Information
We maintain an internet website at http://www.alnylam.com. The information on our website is not incorporated by reference into this annual report on Form 10-K and should not be considered to be a part of this annual report on Form 10-K. Our website address is included in this annual report on Form 10-K as an inactive technical reference only. Our reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, including our annual reports on Form 10-K, our quarterly reports on Form 10-Q and our current reports on Form 8-K, and amendments to those reports, are accessible through our website, free of charge, as soon as reasonably practicable after these reports are filed electronically with, or otherwise furnished to, the SEC. We also make available on our website the charters of our audit committee, compensation committee, nominating and corporate governance committee, and science and technology committee, as well as our corporate governance guidelines and our code of business conduct and ethics. In addition, we intend to disclose on our web site any amendments to, or waivers from, our code of business conduct and ethics that are required to be disclosed pursuant to the SEC rules.
You may read and copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC also maintains an Internet website that contains reports, proxy and information statements, and other information regarding Alnylam and other issuers that file electronically with the SEC. The SEC’s Internet website address is http://www.sec.gov.
Executive Officers of the Registrant
Set forth below is information about our executive officers, as of December 31, 2013.
| Name |
Age | Position | ||||
| John M. Maraganore, Ph.D |
51 | Chief Executive Officer and Director | ||||
| Barry E. Greene |
50 | President and Chief Operating Officer | ||||
| Akshay K. Vaishnaw, M.D., Ph.D. | 51 | Executive Vice President and Chief Medical Officer | ||||
| Laurence E. Reid, Ph.D. |
50 | Senior Vice President and Chief Business Officer | ||||
| Michael P. Mason |
39 | Vice President of Finance and Treasurer | ||||
49
Table of Contents
John M. Maraganore, Ph.D. has served as our Chief Executive Officer and as a member of our board of directors since December 2002. Dr. Maraganore also served as our President from December 2002 to December 2007. From April 2000 to December 2002, Dr. Maraganore served as Senior Vice President, Strategic Product Development at Millennium Pharmaceuticals, Inc., a biopharmaceutical company. Dr. Maraganore serves as a member of the board of directors of Agios Pharmaceuticals, Inc., a biotechnology company, bluebird bio, Inc., a biotechnology company, Regulus Therapeutics Inc., a biotechnology company, and the Biotechnology Industry Organization.
Barry E. Greene has served as our President and Chief Operating Officer since December 2007, as our Chief Operating Officer since he joined us in October 2003, and from February 2004 through December 2005, as our Treasurer. From February 2001 to September 2003, Mr. Greene served as General Manager of Oncology at Millennium Pharmaceuticals, Inc., a biopharmaceutical company. Mr. Greene serves as a member of the board of directors of Acorda Therapeutics, Inc., a biotechnology company and Karyopharm Therapeutics Inc., a clinical-stage pharmaceutical company.
Akshay K. Vaishnaw, M.D., Ph.D. has served as our Executive Vice President and Chief Medical Officer since June 2012 and prior to that as our Senior Vice President and Chief Medical Officer from June 2011 to June 2012. He served as our Senior Vice President, Clinical Research from December 2008 to June 2011, and prior to that served as our Vice President, Clinical Research from the time he joined us in January 2006. From December 1998 through December 2005, Dr. Vaishnaw held various positions at Biogen Idec Inc. (formerly Biogen, Inc.), a biopharmaceutical company. Dr. Vaishnaw is a Member of the Royal College of Physicians, United Kingdom.
Laurence E. Reid, Ph.D. has served as our Senior Vice President and Chief Business Officer since he joined us in June 2010. From January 2006 through May 2010, Dr. Reid served as the Chief Business Officer at Ensemble Therapeutics, a biotechnology company. Prior to joining Ensemble Therapeutics, Dr. Reid worked as a founder of two start-up companies in the fields of stem cell therapeutics and inflammation. Dr. Reid previously spent ten years at Millennium Pharmaceuticals, Inc., a biopharmaceutical company, from 1993 through 2003, where he served in a range of general management and business development positions.
Michael P. Mason has served as our Vice President of Finance and Treasurer since February 2011. From December 2005 to February 2011, Mr. Mason served as our Corporate Controller, and from August 2009 to February 2011, as our Senior Director of Finance. From June 2006 to July 2009, Mr. Mason served as our Director of Finance. From May 2000 through November 2005, Mr. Mason served in several finance and commercial roles at Praecis Pharmaceuticals Incorporated, a public biotechnology company, most recently as Corporate Controller. Prior to Praecis, Mr. Mason worked in the audit practice at KPMG LLP, a national audit, tax and advisory services firm. Mr. Mason has an MBA and is a certified public accountant.
50
Table of Contents
| ITEM 1A. | RISK FACTORS |
Our business is subject to numerous risks. We caution you that the following important factors, among others, could cause our actual results to differ materially from those expressed in forward-looking statements made by us or on our behalf in filings with the SEC, press releases, communications with investors and oral statements. All statements other than statements relating to historical matters should be considered forward-looking statements. When used in this report, the words “believe,” “expect,” “plan,” “anticipate,” “estimate,” “predict,” “may” “could” “should,” “intend,” “will,” “target,” “goal” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these words. Any or all of our forward-looking statements in this annual report on Form 10-K and in any other public statements we make may turn out to be wrong. They can be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties. Many factors mentioned in the discussion below will be important in determining future results. Consequently, no forward-looking statement can be guaranteed. Actual future results may vary materially from those anticipated in forward-looking statements. We explicitly disclaim any obligation to update any forward-looking statements to reflect events or circumstances that arise after the date hereof. You are advised, however, to consult any further disclosure we make in our reports filed with the SEC.
Risks Related to Our Business
Risks Related to Being a Clinical Stage Company
Because we are in clinical development, there is limited information about our ability to successfully overcome many of the risks and uncertainties encountered by companies in the biopharmaceutical industry.
As a company in clinical development, we have limited experience and have not yet demonstrated an ability to successfully overcome many of the risks and uncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in the biopharmaceutical area. For example, to execute our business plan, we will need to successfully:
| • | execute product development activities using unproven technologies related to both RNAi and to the delivery of siRNAs to the relevant tissues and cells; |
| • | build and maintain a strong intellectual property portfolio; |
| • | gain regulatory acceptance for the development of our product candidates and market success for any products we commercialize; |
| • | develop and maintain successful strategic alliances; and |
| • | manage our spending as costs and expenses increase due to clinical trials, regulatory approvals and commercialization. |
If we are unsuccessful in accomplishing these objectives, we may not be able to develop product candidates, commercialize products, raise capital, expand our business or continue our operations.
The approach we are taking to discover and develop novel RNAi therapeutics is unproven and may never lead to marketable products.
We have concentrated our efforts and therapeutic product research on RNAi technology, and our future success depends on the successful development of this technology and products based on it. Neither we nor any other company has received regulatory approval to market therapeutics utilizing siRNAs, the class of molecule we are trying to develop into drugs. The scientific discoveries that form the basis for our efforts to discover and develop new drugs are relatively new. The scientific evidence to support the feasibility of developing drugs based on these discoveries is both preliminary and limited. Skepticism as to the feasibility of developing RNAi therapeutics has been expressed in scientific literature. For example, there are potential challenges to achieving safe RNAi therapeutics based on the so-called off-target effects and activation of the interferon response. In addition, decisions by other companies with respect to their RNAi development efforts may increase skepticism
in the marketplace regarding the potential for RNAi therapeutics.
51
Table of Contents
Relatively few product candidates based on these discoveries have ever been tested in animals or humans. siRNAs may not naturally possess the inherent properties typically required of drugs, such as the ability to be stable in the body long enough to reach the tissues in which their effects are required, nor the ability to enter cells within these tissues in order to exert their effects. We currently have only limited data, and no conclusive evidence, to suggest that we can introduce these drug-like properties into siRNAs. We may spend large amounts of money trying to introduce these properties, and may never succeed in doing so. In addition, these compounds may not demonstrate in patients the chemical and pharmacological properties ascribed to them in laboratory studies, and they may interact with human biological systems in unforeseen, ineffective or harmful ways. As a result, we may never succeed in developing a marketable product, we may not become profitable and the value of our common stock will decline.
Further, our focus solely on RNAi technology for developing drugs, as opposed to multiple, more proven technologies for drug development, increases the risks associated with the ownership of our common stock. If we are not successful in developing a product candidate using RNAi technology, we may be required to change the scope and direction of our product development activities. In that case, we may not be able to identify and implement successfully an alternative product development strategy.
Risks Related to Our Financial Results and Need for Financing
We have a history of losses and may never become and remain consistently profitable.
We have experienced significant operating losses since our inception. At December 31, 2013, we had an accumulated deficit of $596.2 million. To date, we have not developed any products nor generated any revenues from the sale of products. Further, we do not expect to generate any product revenues in the foreseeable future. We expect to continue to incur annual net operating losses over the next several years and will require substantial resources over the next several years as we expand our efforts to discover, develop and commercialize RNAi therapeutics. We anticipate that the majority of any revenues we generate over the next several years will be from alliances with pharmaceutical and biotechnology companies, but cannot be certain that we will be able to secure or maintain these alliances, or meet the obligations or achieve any milestones that we may be required to meet or achieve to receive payments. We anticipate that revenues derived from such sources will not be sufficient to make us consistently profitable.
We believe that to become and remain consistently profitable, we must succeed in discovering, developing and commercializing novel drugs with significant market potential. This will require us to be successful in a range of challenging activities, including pre-clinical testing and clinical trial stages of development, obtaining regulatory approval for these novel drugs and manufacturing, marketing and selling them. We may never succeed in these activities, and may never generate revenues that are significant enough to achieve profitability. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. If we cannot become and remain consistently profitable, the market price of our common stock could decline. In addition, we may be unable to raise capital, expand our business, develop additional product candidates or continue our operations.
We will require substantial additional funds to complete our research and development activities and if additional funds are not available, we may need to critically limit, significantly scale back or cease our operations.
We have used substantial funds to develop our RNAi technologies and will require substantial funds to conduct further research and development, including pre-clinical testing and clinical trials of our product candidates, and to manufacture and market any products that are approved for commercial sale. Because we cannot be certain of the length of time or activities associated with successful development of our product candidates, we are unable to estimate the actual funds we will require to develop and commercialize them.
Our future capital requirements and the period for which we expect our existing resources to support our operations may vary from what we expect. We have based our expectations on a number of factors, many of which are difficult to predict or are outside of our control, including:
| • | our progress in demonstrating that siRNAs can be active as drugs; |
52
Table of Contents
| • | our ability to develop relatively standard procedures for selecting and modifying siRNA product candidates; |
| • | progress in our research and development programs, as well as the magnitude of these programs; |
| • | the timing, receipt and amount of milestone and other payments, if any, from present and future collaborators, if any; |
| • | our ability to maintain and establish additional collaborative arrangements and/or new business initiatives; |
| • | the resources, time and costs required to initiate and complete our pre-clinical and clinical trials, obtain regulatory approvals, and obtain and maintain licenses to third-party intellectual property; |
| • | our ability to manufacture, or contract with third-parties for the manufacture of, our product candidates for clinical testing and commercial sale; |
| • | the resources, time and cost required for the preparation, filing, prosecution, maintenance and enforcement of patent claims; |
| • | the costs associated with legal activities, including litigation, arising in the course of our business activities and our ability to prevail in any such legal disputes; |
| • | progress in the research and development programs of Regulus; and |
| • | the timing, receipt and amount of sales and royalties, if any, from our potential products. |
If our estimates and predictions relating to these factors are incorrect, we may need to modify our operating plan.
Even if our estimates are correct, we will be required to seek additional funding in the future and intend to do so through either collaborative arrangements, public or private equity offerings or debt financings, or a combination of one or more of these funding sources. Additional funds may not be available to us on acceptable terms or at all.
In addition, the terms of any financing may adversely affect the holdings or the rights of our stockholders. For example, if we raise additional funds by issuing equity securities, under our shelf registration statement or otherwise, further dilution to our stockholders will result. In addition, as a condition to providing additional funds to us, future investors may demand, and may be granted, rights superior to those of existing stockholders. Debt financing, if available, may involve restrictive covenants that could limit our flexibility in conducting future business activities and, in the event of insolvency, would be paid before holders of equity securities received any distribution of corporate assets.
If we are unable to obtain funding on a timely basis, we may be required to significantly delay or curtail one or more of our research or development programs or undergo future reductions in our workforce or other corporate restructuring activities. We also could be required to seek funds through arrangements with collaborators or others that may require us to relinquish rights to some of our technologies, product candidates or products that we would otherwise pursue on our own.
If the estimates we make, or the assumptions on which we rely, in preparing our consolidated financial statements prove inaccurate, our actual results may vary from those reflected in our projections and accruals.
Our consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America, or GAAP. The preparation of these consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of our assets, liabilities, revenues and expenses, the amounts of charges accrued by us and related disclosure of contingent assets and liabilities. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. We cannot assure you, however, that our estimates, or the assumptions underlying them, will be correct.
53
Table of Contents
The investment of our cash, cash equivalents and fixed income marketable securities is subject to risks which may cause losses and affect the liquidity of these investments.
At December 31, 2013, we had $350.5 million in cash, cash equivalents and fixed income marketable securities. We historically have invested these amounts in corporate bonds, commercial paper, securities issued by the U.S. government, certificates of deposit and money market funds meeting the criteria of our investment policy, which is focused on the preservation of our capital. These investments are subject to general credit, liquidity, market and interest rate risks. We may realize losses in the fair value of these investments or a complete loss of these investments, which would have a negative effect on our consolidated financial statements. In addition, should our investments cease paying or reduce the amount of interest paid to us, our interest income would suffer. The market risks associated with our investment portfolio may have an adverse effect on our results of operations, liquidity and financial condition.
Risks Related to Our Dependence on Third Parties
Our license and collaboration agreements with pharmaceutical companies are important to our business. If these pharmaceutical companies do not successfully develop drugs pursuant to these agreements or we develop drugs targeting the same diseases as our non-exclusive licensees, our business could be adversely affected.
In July 2007, we entered into a license and collaboration agreement with Roche. Under the license and collaboration agreement we granted Roche a non-exclusive license to our intellectual property to develop and commercialize therapeutic products that function through RNAi, subject to our existing contractual obligations to third parties. In November 2010, Roche announced the discontinuation of certain activities in research and early development, including their RNAi research efforts. In October 2011, Arrowhead announced its acquisition of RNA therapeutics assets from Roche, including our license and collaboration agreement with Roche. As a result of the assignment, Arrowhead now has all of the rights and obligations of Roche under that agreement. The license is limited to four therapeutic areas and may be expanded to include additional therapeutic areas, upon payment to us by Arrowhead of an additional $50.0 million for each additional therapeutic area, if any. In addition, for each RNAi therapeutic product developed by Arrowhead, its affiliates, or sublicensees under the collaboration agreement, we are entitled to receive milestone payments upon achievement of specified development and sales events, totaling up to an aggregate of $100.0 million per therapeutic target, together with royalty payments based on worldwide annual net sales, if any. Our receipt of milestone payments under this agreement is dependent upon Arrowhead’s ability to successfully develop and commercialize RNAi therapeutic products.
In May 2008, we entered into a similar license and collaboration agreement with Takeda, which is limited to two therapeutic areas, and which may be expanded to include additional therapeutic areas, upon payment to us by Takeda of an additional $50.0 million for each additional therapeutic area, if any. For each RNAi therapeutic product developed by Takeda, its affiliates and sublicensees, we are entitled to receive specified development and commercialization milestone payments, totaling up to $171.0 million per product, together with royalty payments based on worldwide annual net sales, if any.
In September 2010, Novartis exercised its right under our collaboration and license agreement to select 31 designated gene targets, for which Novartis has exclusive rights to discover, develop and commercialize RNAi therapeutic products using our intellectual property and technology. Under the terms of the collaboration and license agreement, for any RNAi therapeutic products Novartis develops against these targets, we are entitled to receive milestone payments upon achievement of certain specified development and annual net sales events, up to an aggregate of $75.0 million per therapeutic product, as well as royalties on annual net sales of any such product, if any.
If Takeda, Novartis or Arrowhead fails to successfully develop products using our technology, we may not receive any milestone or royalty payments under our agreements with them. Finally, Takeda could become a competitor of ours in the development of RNAi-based drugs targeting the same diseases that we choose to target.
54
Table of Contents
Takeda has significantly greater financial resources than we do and far more experience in developing and marketing drugs, which could put us at a competitive disadvantage if we were to compete with them in the development of RNAi-based drugs targeting the same disease.
We may not be able to execute our business strategy if we are unable to enter into alliances with other companies that can provide business and scientific capabilities and funds for the development and commercialization of our product candidates. If we are unsuccessful in forming or maintaining these alliances on terms favorable to us, our business may not succeed.
We do not have any capability for sales, marketing or distribution and have limited capabilities for drug development. Accordingly, we have entered into alliances with other companies and collaborators that we believe can provide such capabilities, and we intend to enter into additional such alliances in the future. Our collaboration strategy is to form alliances that create significant value for ourselves and our collaborators in the advancement of RNAi therapeutics as a new class of innovative medicines. Specifically, our goal is to retain development and commercial rights for our current and future genetic medicine pipeline in North America and Western Europe, while forming alliances with leading, innovative companies for the development and commercialization of these products in the ROW. In early 2014, we formed an alliance with Genzyme to develop and commercialize our current and future genetic medicine pipeline principally in territories outside of North America and Western Europe, subject to certain broader rights. This broad collaboration will replace our 2012 alliance with Genzyme, has been approved by the boards of both companies, and is subject to customary closing conditions and clearances under the Hart-Scott Rodino Antitrust Improvements Act. With respect to programs that are outside of our core focus on genetic medicines (for example, in the fields of metabolic and infectious disease and in oncology), we intend to seek regional or global alliances. For example, in February 2013, we entered into a global alliance with MDCO to advance our ALN-PCS program.
In such alliances, we expect our current, and may expect our future, collaborators to provide substantial capabilities in clinical development, regulatory affairs, and/or marketing, sales and distribution. Under certain of our alliances, we also may expect our collaborators to develop, market and/or sell certain of our product candidates. We may have limited or no control over the development, sales, marketing and distribution activities of these third parties. Our future revenues may depend heavily on the success of the efforts of these third parties. For example, we will rely entirely on (i) Genzyme for the development and commercialization of ALN-TTR, ALN-TTRsc and potentially other of our genetic medicine programs in territories outside of North America and Western Europe under the 2014 Genzyme Collaboration, and (ii) MDCO for later stage development and commercialization of ALN-PCSsc worldwide. If Genzyme and/or MDCO are not successful in their commercialization efforts, our future revenues from RNAi therapeutics for these indications may be adversely affected.
We may not be successful in entering into such alliances on terms favorable to us due to various factors, including our ability to successfully demonstrate proof of concept for our technology in humans, our ability to demonstrate the safety and efficacy of our specific drug candidates, our ability to manufacture or have third parties manufacture RNAi therapeutics, the strength of our intellectual property and/or concerns around challenges to our intellectual property. Even if we do succeed in securing any such alliances, we may not be able to maintain them if, for example, development or approval of a product candidate is delayed, challenges are raised as to the validity or scope of our intellectual property or sales of an approved drug are lower than we expected. In the case of the Monsanto agreement, if we cease to own or otherwise exclusively control certain licensed patent rights in the agriculture field, resulting in the loss of exclusivity with respect to Monsanto’s rights to such patent rights, and such loss of exclusivity has a material adverse effect on the licensed products (as defined in the agreement), we would be required to pay Monsanto up to $5.0 million in liquidated damages, and Monsanto’s royalty obligations to us would be reduced or, under certain circumstances, terminated.
Furthermore, any delay in entering into collaboration agreements would likely either delay the development and commercialization of certain of our product candidates and reduce their competitiveness even if they reach the market, or prevent the development of certain product candidates. Any such delay related to our collaborations could adversely affect our business.
55
Table of Contents
For certain product candidates that we may develop, we have formed collaborations to fund all or part of the costs of drug development and commercialization, such as our collaboration with MDCO. We may not, however, be able to enter into additional collaborations for certain other programs, and the terms of any collaboration agreement we do secure may not be favorable to us. If we are not successful in our efforts to enter into future collaboration arrangements with respect to one or more of these product candidates, we may not have sufficient funds to develop that or any other product candidate internally, or to bring any product candidates to market. If we do not have sufficient funds to develop and bring our product candidates to market, we will not be able to generate sales revenues from these product candidates, and this will substantially harm our business.
If any collaborator terminates or fails to perform its obligations under agreements with us, the development and commercialization of our product candidates could be delayed or terminated.
Our dependence on collaborators for capabilities and funding means that our business could be adversely affected if any collaborator terminates its collaboration agreement with us or fails to perform its obligations under that agreement. Our current or future collaborations, if any, may not be scientifically or commercially successful. Disputes may arise in the future with respect to the ownership of rights to technology or products developed with collaborators, which could have an adverse effect on our ability to develop and commercialize any affected product candidate.
Our current collaborations allow, and we expect that any future collaborations will allow, either party to terminate the collaboration for a material breach by the other party. For example, our agreement with MDCO relating to the development and commercialization of ALN-PCS worldwide may be terminated by MDCO at any time upon four months’ prior written notice. If we were to lose a commercialization collaborator, we would have to attract a new collaborator or develop internal sales, distribution and marketing capabilities, which would require us to invest significant amounts of financial and management resources.
In addition, if we have a dispute with a collaborator over the ownership of technology or other matters, or if a collaborator terminates its collaboration with us, for breach or otherwise, or determines not to pursue the research and development of RNAi therapeutics, it could delay our development of product candidates, result in the need for additional company resources to develop product candidates, make it more difficult for us to attract new collaborators and could adversely affect how we are perceived in the business and financial communities.
For example, in March 2011, Tekmira filed a civil complaint against us claiming, among other things. misappropriation of its confidential and proprietary information and trade secrets. As a result of the litigation, which was settled in November 2012, we were required to expend resources and management attention that would otherwise have been engaged in other activities. In addition, in August 2013, we initiated binding arbitration proceedings to resolve a disagreement with TPC regarding the achievement by TPC of a $5.0 million milestone payment under our cross-license agreement relating to the manufacture of ALN-VSP clinical trial material for use in China. Moreover, a collaborator, or in the event of a change in control of a collaborator or the assignment of a collaboration agreement to a third-party, the successor entity or assignee, could determine that it is in its interests to:
| • | pursue alternative technologies or develop alternative products, either on its own or jointly with others, that may be competitive with the products on which it is collaborating with us or which could affect its commitment to the collaboration with us; |
| • | pursue higher-priority programs or change the focus of its development programs, which could affect the collaborator’s commitment to us; or |
| • | if it has marketing rights, choose to devote fewer resources to the marketing of our product candidates, if any are approved for marketing, than it does for product candidates developed without us. |
If any of these occur, the development and commercialization of one or more product candidates could be delayed, curtailed or terminated because we may not have sufficient financial resources or capabilities to continue such development and commercialization on our own.
56
Table of Contents
We rely on third parties to conduct our clinical trials, and if they fail to fulfill their obligations, our development plans may be adversely affected.
We rely on independent clinical investigators, contract research organizations and other third-party service providers to assist us in managing, monitoring and otherwise carrying out our clinical trials. We have contracted, and we plan to continue to contract with certain third-parties to provide certain services, including site selection, enrollment, monitoring and data management services. Although we depend heavily on these parties, we do not control them and therefore, we cannot be assured that these third-parties will adequately perform all of their contractual obligations to us. If our third-party service providers cannot adequately and timely fulfill their obligations to us, or if the quality and accuracy of our clinical trial data is compromised due to failure by such third-party to adhere to our protocols or regulatory requirements or if such third-parties otherwise fail to meet deadlines, our development plans may be delayed or terminated.
We have very limited manufacturing experience or resources and we must incur significant costs to develop this expertise and/or rely on third parties to manufacture our products.
We have very limited manufacturing experience. Some of our product candidates utilize specialized formulations, such as LNP-based formulations, whose scale-up and manufacturing could be very difficult. We also have very limited experience in such scale-up and manufacturing, requiring us to depend on a limited number of third parties, who might not be able to deliver in a timely manner, or at all. In order to develop products, apply for regulatory approvals and commercialize our products, we will need to develop, contract for, or otherwise arrange for the necessary manufacturing capabilities. Our internal manufacturing capabilities have been limited to small-scale production of material for use in in vitro and in vivo experiments that is not required to be produced under current good manufacturing practice, or cGMP, standards. During 2012, we developed cGMP capabilities and processes for the manufacture of patisiran for late-stage clinical trial use and early commercial supply.
We may manufacture clinical trial materials ourselves or we may rely on others to manufacture the materials we will require for any clinical trials that we initiate. There are a limited number of manufacturers that supply synthetic siRNAs. We currently rely on several contract manufacturers for our supply of synthetic siRNAs. There are risks inherent in pharmaceutical manufacturing that could affect the ability of our contract manufacturers to meet our delivery time requirements or provide adequate amounts of material to meet our needs. Included in these risks are synthesis and purification failures and contamination during the manufacturing process, which could result in unusable product and cause delays in our development process, as well as additional expense to us. To fulfill our siRNA requirements, we may also need to secure alternative suppliers of synthetic siRNAs and such alternative suppliers may not be available, or we may be unable to enter into agreements with them on reasonable terms and in a timely manner.
In addition to the manufacture of the synthetic siRNAs, we may have additional manufacturing requirements related to the technology required to deliver the siRNA to the relevant cell or tissue type, such as LNPs or conjugates. In some cases, the delivery technology we utilize is highly specialized or proprietary, and for technical and legal reasons, we may have access to only one or a limited number of potential manufacturers for such delivery technology. Failure by manufacturers to properly formulate our siRNAs for delivery could result in unusable product. Furthermore, a breach by such manufacturers of their contractual obligations or a dispute with such manufacturers would cause delays in our discovery and development efforts, as well as additional expense to us. Given the limited number of suppliers for our delivery technology and other materials, we have developed cGMP capabilities and processes for the manufacture of patisiran formulated bulk drug product for late-stage clinical use and early commercial supply, and in the future, we may also develop our own capabilities to manufacture drug substance, including siRNAs and siRNA conjugates for human clinical use. In developing these manufacturing capabilities by building our own manufacturing facility, we have incurred substantial expenditures. Also, we have had to, and will likely need to continue to, hire and train qualified employees to staff our new facility. We do not currently have a second source of supply for patisiran formulated bulk drug product. If we are unable to manufacture sufficient quantatites of material or if we encounter problems with our facility in
57
Table of Contents
the future, we may also need to secure alternative suppliers of for patisiran formulated bulk drug product and such alternative suppliers may not be available, or we may be unable to enter into agreements with them on reasonable terms and in a timely manner.
The manufacturing process for any products that we may develop is subject to FDA and foreign regulatory authority approval process and we will need to meet, and will need to contract with manufacturers who can meet, all applicable FDA and foreign regulatory authority requirements on an ongoing basis. In addition, if we receive the necessary regulatory approval for any product candidate, we also expect to rely on third parties, including our commercial collaborators, to produce materials required for commercial supply. We may experience difficulty in obtaining adequate manufacturing capacity for our needs. If we are unable to obtain or maintain contract manufacturing for these product candidates, or to do so on commercially reasonable terms, we may not be able to successfully develop and commercialize our products.
To the extent that we have existing, or enter into future, manufacturing arrangements with third parties, we depend, and will depend in the future, on these third parties to perform their obligations in a timely manner and consistent with contractual and regulatory requirements, including those related to quality control and quality assurance. The failure of a third-party manufacturer to perform its obligations as expected, or, to the extent we manufacture all or a portion of our product candidates ourselves, our failure to execute on our manufacturing requirements could adversely affect our business in a number of ways, including:
| • | we or our current or future collaborators may not be able to initiate or continue clinical trials of products that are under development; |
| • | we or our current or future collaborators may be delayed in submitting regulatory applications, or receiving regulatory approvals, for our product candidates; |
| • | we may lose the cooperation of our collaborators; |
| • | our facilities and products could be the subject of inspections by regulatory authorities; |
| • | we may be required to cease distribution or recall some or all batches of our products; and |
| • | ultimately, we may not be able to meet commercial demands for our products. |
If any third-party manufacturer with whom we contract fails to perform its obligations, we may be forced to manufacture the materials ourselves, for which we may not have the capabilities or resources, or enter into an agreement with a different third-party manufacturer, which we may not be able to do on reasonable terms, if at all. In some cases, the technical skills required to manufacture our products or product candidates may be unique or proprietary to the original manufacturer and we may have difficulty, or there may be contractual restrictions prohibiting us from, transferring such skills to a back-up or alternate supplier, or we may be unable to transfer such skills at all. In addition, if we are required to change manufacturers for any reason, we will be required to verify that the new manufacturer maintains facilities and procedures that comply with quality standards and with all applicable regulations and guidelines. We will also need to verify, such as through a manufacturing comparability study, that any new manufacturing process will produce our product according to the specifications approved by FDA. The delays associated with the verification of a new manufacturer could negatively affect our ability to develop product candidates in a timely manner or within budget. Furthermore, a manufacturer may possess technology related to the manufacture of our product candidate that such manufacturer owns independently. This would increase our reliance on such manufacturer or require us to obtain a license from such manufacturer in order to have another third-party manufacture our products or product candidates.
We have no sales, marketing or distribution experience and would have to invest significant financial and management resources to establish these capabilities.
We have no sales, marketing or distribution experience. We currently expect to rely heavily on third parties to launch and market certain of our product candidates in certain geographies, if approved. However, we intend to commercialize the majority of our genetic medicine programs on our own in North America and Western Europe, and accordingly, we will need to develop internal sales, distribution and marketing capabilities as part of
58
Table of Contents
our core product strategy, which will require significant financial and management resources. For our genetic medicine programs where we will perform sales, marketing and distribution functions ourselves, we could face a number of additional risks, including:
| • | we may not be able to attract and build a significant marketing or sales force; |
| • | the cost of establishing a marketing or sales force may not be justifiable in light of the revenues generated by any particular product; and |
| • | our direct sales and marketing efforts may not be successful. |
If we are unable to develop our own sales, marketing and distribution capabilities, we will not be able to successfully commercialize our genetic medicine programs without reliance on third parties.
The current credit and financial market conditions may exacerbate certain risks affecting our business.
Due to the tightening of global credit, there may be a disruption or delay in the performance of our third-party contractors, suppliers or collaborators. We rely on third parties for several important aspects of our business, including significant portions of our manufacturing needs, development of product candidates and conduct of clinical trials. If such third parties are unable to satisfy their commitments to us, our business could be adversely affected.
Risks Related to Managing Our Operations
If we are unable to attract and retain qualified key management and scientists, staff, consultants and advisors, our ability to implement our business plan may be adversely affected.
We are highly dependent upon our senior management and scientific staff. The loss of the service of any of the members of our senior management, including Dr. John Maraganore, our Chief Executive Officer, may significantly delay or prevent the achievement of product development and other business objectives. Our employment agreements with our key personnel are terminable without notice. We do not carry key man life insurance on any of our employees.
We face intense competition for qualified individuals from numerous pharmaceutical and biotechnology companies, universities, governmental entities and other research institutions, many of which have substantially greater resources with which to reward qualified individuals than we do. We may be unable to attract and retain suitably qualified individuals, and our failure to do so could have an adverse effect on our ability to implement our future business plan.
We may have difficulty expanding our operations successfully as we evolve from a company primarily involved in discovery and pre-clinical testing into one that develops and commercializes drugs.
We expect that as we increase the number of product candidates we are developing we will also need to expand our operations. This expected growth may place a strain on our administrative and operational infrastructure. As product candidates we develop enter and advance through clinical trials, we will need to expand our development, regulatory, manufacturing, marketing and sales capabilities or contract with other organizations to provide these capabilities for us. As our operations expand due to our development progress, we expect that we will need to manage additional relationships with various collaborators, suppliers and other organizations. Our ability to manage our operations and future growth will require us to continue to improve our operational, financial and management controls, reporting systems and procedures. We may not be able to implement improvements to our management information and control systems in an efficient or timely manner and may discover deficiencies in existing systems and controls.
Our business and operations could suffer in the event of system failures.
Despite the implementation of security measures, our internal computer systems and those of our contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war, and telecommunication and electrical failures. Such events could cause interruption of
59
Table of Contents
our operations. For example, the loss of pre-clinical trial data or data from completed or ongoing clinical trials for our product candidates could result in delays in our regulatory filings and development efforts and significantly increase our costs. To the extent that any disruption or security breach were to result in a loss of or damage to our data, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the development of our product candidates could be delayed.
Risks Related to Our Industry
Risks Related to Development, Clinical Testing and Regulatory Approval of Our Product Candidates
Any product candidates we develop may fail in development or be delayed to a point where they do not become commercially viable.
Before obtaining regulatory approval for the commercial distribution of our product candidates, we must conduct, at our own expense, extensive pre-clinical tests and clinical trials to demonstrate the safety and efficacy in humans of our product candidates. Pre-clinical and clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome and the historical failure rate for product candidates is high. We currently have several programs in clinical development, including patisiran in a Phase 3 clinical trial, ALN-TTRsc in a Phase 2 clinical trial and ALN-AT3 in a Phase 1 clinical trial. We have also conducted Phase 1 clinical trials for ALN-PCS02 and ALN-VSP, and Phase 1 and 2 clinical trials for ALN-RSV. However, we may not be able to further advance these or any other product candidate through clinical trials.
If we enter into clinical trials, the results from pre-clinical testing or early clinical trials of a product candidate may not predict the results that will be obtained in subsequent human clinical trials of that product candidate or any other product candidate. For example, patisiran and ALN-TTRsc employ novel delivery formulations that have yet to be extensively evaluated in human clinical trials and proven safe and effective. We, the FDA or other applicable regulatory authorities, or an institutional review board, or IRB, or similar foreign review board or committee, may suspend clinical trials of a product candidate at any time for various reasons, including if we or they believe the subjects or patients participating in such trials are being exposed to unacceptable health risks. Among other reasons, adverse side effects of a product candidate on subjects or patients in a clinical trial could result in the FDA or foreign regulatory authorities suspending or terminating the trial and refusing to approve a particular product candidate for any or all indications of use.
Clinical trials of a new product candidate require the enrollment of a sufficient number of patients, including patients who are suffering from the disease the product candidate is intended to treat and who meet other eligibility criteria. Rates of patient enrollment are affected by many factors, including the size of the patient population, the age and condition of the patients, the stage and severity of disease, the nature of the protocol, the proximity of patients to clinical sites, the availability of effective treatments for the relevant disease and the eligibility criteria for the clinical trial. For example, we may experience difficulty enrolling our clinical trials, including, but not limited to, our clinical trials for patisiran, due to the small population of ATTR patients suffering from FAP and the availability of existing approved treatments, as well as other investigational treatments in development. Although our RNAi therapeutics have been generally well tolerated in our clinical trials to date, in our ALN-VSP clinical trial, one patient with advanced pancreatic neuroendocrine cancer with extensive involvement of the liver developed hepatic failure five days following the second dose of ALN-VSP and subsequently died; this was deemed possibly related to the study drug. In addition, in our ALN-VSP and ALN-TTR01 Phase 1 clinical trials, we have reported an incidence of acute infusion reactions occurring in 15-20% of patients. These were graded as mild or moderate in severity and readily responded to slowing of the infusion rate; all patients completed dosing without further incident. The frequency of acute infusion reactions in our ALN-PCS02 and patisiran Phase 1 clinical trials has been less than three percent. In our ALN-PCS02 Phase 1 clinical trial, we reported the occurrence of a mild, transient rash that was observed in sixteen subjects, including four who received placebo; the incidence of this finding was the same in both placebo and drug treatment arms. In our ALN-TTRsc clinical trial, we observed injection site reactions in a minority of subjects receiving ALN-TTRsc or placebo. These were reported as being clinically mild and consisted of transient erythema associated in
60
Table of Contents
a minority of cases with edema and/or pain. In all cases, these reactions were self-limiting and resolved within approximately two hours of onset. Delays or difficulties in patient enrollment or difficulties retaining trial participants, including as a result of the availability of existing or other investigational treatments or the occurrence of adverse events, can result in increased costs, longer development times or termination of a clinical trial.
Clinical trials also require the review, oversight and approval of IRBs, which continually review clinical investigations and protect the rights and welfare of human subjects. Inability to obtain or delay in obtaining IRB approval can prevent or delay the initiation and completion of clinical trials, and the FDA or foreign regulatory authorities may decide not to consider any data or information derived from a clinical investigation not subject to initial and continuing IRB review and approval in support of a marketing application.
Our product candidates that we develop may encounter problems during clinical trials that will cause us, an IRB or regulatory authorities to delay, suspend or terminate these trials, or that will delay or confound the analysis of data from these trials. If we experience any such problems, we may not have the financial resources to continue development of the product candidate that is affected, or development of any of our other product candidates. We may also lose, or be unable to enter into, collaborative arrangements for the affected product candidate and for other product candidates we are developing.
A failure of one or more of our clinical trials can occur at any stage of testing. We may experience numerous unforeseen events during, or as a result of, pre-clinical testing and the clinical trial process that could delay or prevent regulatory approval or our ability to commercialize our product candidates, including:
| • | our pre-clinical tests or clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional pre-clinical testing or clinical trials, or we may abandon projects that we expect to be promising; |
| • | delays in filing INDs or comparable foreign applications or delays or failure in obtaining the necessary approvals from regulators or IRBs in order to commence a clinical trial at a prospective trial site, or their suspension or termination of a clinical trial once commenced; |
| • | conditions imposed on us by the FDA or comparable foreign authorities regarding the scope or design of our clinical trials; |
| • | problems in engaging IRBs to oversee clinical trials or problems in obtaining or maintaining IRB approval of trials; |
| • | delays in enrolling patients and volunteers into clinical trials, and variability in the number and types of patients and volunteers available for clinical trials; |
| • | high drop-out rates for patients and volunteers in clinical trials; |
| • | negative or inconclusive results from our clinical trials or the clinical trials of others for product candidates similar to ours; |
| • | inadequate supply or quality of product candidate materials or other materials necessary for the conduct of our clinical trials; |
| • | greater than anticipated clinical trial costs; |
| • | serious and unexpected drug-related side effects experienced by participants in our clinical trials or by individuals using drugs similar to our product candidates; |
| • | poor effectiveness of our product candidates during clinical trials; |
| • | unfavorable FDA or other regulatory agency inspection and review of a clinical trial site or records of any clinical or pre-clinical investigation; |
| • | failure of our third-party contractors or investigators to comply with regulatory requirements or otherwise meet their contractual obligations in a timely manner, or at all; |
61
Table of Contents
| • | governmental or regulatory delays and changes in regulatory requirements, policy and guidelines, including the imposition of additional regulatory oversight around clinical testing generally or with respect to our technology in particular; or |
| • | varying interpretations of data by the FDA and similar foreign regulatory agencies. |
Even if we successfully complete clinical trials of our product candidates, any given product candidate may not prove to be a safe and effective treatment for the diseases for which it was being tested.
We may be unable to obtain United States or foreign regulatory approval and, as a result, unable to commercialize our product candidates.
Our product candidates are subject to extensive governmental regulations relating to, among other things, research, testing, development, manufacturing, safety, efficacy, approval, recordkeeping, reporting, labeling, storage, packaging, advertising and promotion, pricing, marketing and distribution of drugs. Rigorous pre-clinical testing and clinical trials and an extensive regulatory approval process are required to be successfully completed in the United States and in many foreign jurisdictions before a new drug can be marketed. Satisfaction of these and other regulatory requirements is costly, time consuming, uncertain and subject to unanticipated delays. It is possible that none of the product candidates we may develop will obtain the regulatory approvals necessary for us or our collaborators to begin selling them.
We have limited experience in conducting and managing the clinical trials necessary to obtain regulatory approvals, including approval by the FDA. The time required to obtain FDA and other approvals is unpredictable but typically takes many years following the commencement of clinical trials, depending upon the type, complexity and novelty of the product candidate. The standards that the FDA and its foreign counterparts use when regulating us are not always applied predictably or uniformly and can change. Any analysis we perform of data from pre-clinical and clinical activities is subject to confirmation and interpretation by regulatory authorities, which could delay, limit or prevent regulatory approval. We may also encounter unexpected delays or increased costs due to new government regulations, for example, from future legislation or administrative action, or from changes in FDA policy during the period of product development, clinical trials and FDA regulatory review. It is impossible to predict whether legislative changes will be enacted, or whether FDA or foreign regulations, guidance or interpretations will be changed, or what the impact of such changes, if any, may be.
Because the drugs we are developing may represent a new class of drug, the FDA and its foreign counterparts have not yet established any definitive policies, practices or guidelines in relation to these drugs. While we believe the product candidates that we are currently developing are regulated as new drugs under the Federal Food, Drug, and Cosmetic Act, the FDA could decide to regulate them or other products we may develop as biologics under the Public Health Service Act. The lack of policies, practices or guidelines may hinder or slow review by the FDA of any regulatory filings that we may submit. Moreover, the FDA may respond to these submissions by defining requirements we may not have anticipated. Such responses could lead to significant delays in the clinical development of our product candidates. In addition, because there may be approved treatments for some of the diseases for which we may seek approval, in order to receive regulatory approval, we may need to demonstrate through clinical trials that the product candidates we develop to treat these diseases, if any, are not only safe and effective, but safer or more effective than existing products. Furthermore, in recent years, there has been increased public and political pressure on the FDA with respect to the approval process for new drugs, and the FDA’s standards, especially regarding drug safety, appear to have become more stringent.
Any delay or failure in obtaining required approvals could have a material adverse effect on our ability to generate revenues from the particular product candidate for which we are seeking approval. Furthermore, any regulatory approval to market a product may be subject to limitations on the approved uses for which we may market the product or the labeling or other restrictions. In addition, the FDA has the authority to require a REMS plan as part of an NDA, or after approval, which may impose further requirements or restrictions on the distribution or use of an approved drug or biologic, such as limiting prescribing to certain physicians or medical centers that have undergone specialized training, limiting treatment to patients who meet certain safe-use criteria and requiring treated patients to enroll in a registry. These limitations and restrictions may limit the size of the market for the product and affect reimbursement by third-party payors.
62
Table of Contents
We are also subject to numerous foreign regulatory requirements governing, among other things, the conduct of clinical trials, manufacturing and marketing authorization, pricing and third-party reimbursement. The foreign regulatory approval process varies among countries and includes all of the risks associated with FDA approval described above as well as risks attributable to the satisfaction of local regulations in foreign jurisdictions. Approval by the FDA does not ensure approval by regulatory authorities outside the United States and vice versa.
Even if we obtain regulatory approvals, our marketed drugs will be subject to ongoing regulatory review. If we fail to comply with continuing U.S. and foreign requirements, our approvals could be limited or withdrawn, we could be subject to other penalties, and our business would be seriously harmed.
Following any initial regulatory approval of any drugs we may develop, we will also be subject to continuing regulatory review, including the review of adverse drug experiences and clinical results that are reported after our drug products are made commercially available. This would include results from any post-marketing tests or surveillance to monitor the safety and efficacy of the drug product required as a condition of approval or agreed to by us. Any regulatory approvals that we receive for our product candidates may also be subject to limitations on the approved uses for which the product may be marketed. Other ongoing regulatory requirements include, among other things, submissions of safety and other post-marketing information and reports, registration and listing, as well as continued compliance with cGMP requirements and GCP requirements for any clinical trials that we conduct post-approval. In addition, we are conducting, and intend to continue to conduct, clinical trials for our product candidates, and we intend to seek approval to market our product candidates, in jurisdictions outside of the United States, and therefore will be subject to, and must comply with, regulatory requirements in those jurisdictions.
The FDA has significant post-market authority, including, for example, the authority to require labeling changes based on new safety information and to require post-market studies or clinical trials to evaluate serious safety risks related to the use of a drug and to require withdrawal of the product from the market. The FDA also has the authority to require a REMS plan after approval, which may impose further requirements or restrictions on the distribution or use of an approved drug.
The manufacturer and manufacturing facilities we use to make any of our product candidates, including our Cambridge facility, will also be subject to periodic review and inspection by the FDA and other regulatory agencies. To date, our manufacturing facility has not been subject to an inspection by any regulatory authority. The discovery of any new or previously unknown problems with us or our third-party manufacturers, or our or their manufacturing processes or facilities, may result in restrictions on the drug or manufacturer or facility, including withdrawal of the drug from the market. We have recently developed cGMP capabilities and processes for the manufacture of patisiran for Phase 3 clinical and early commercial use. We may not have the ability to manufacture material for a broader commercial scale in the future. We may manufacture clinical trial materials or we may contract a third party to manufacture these materials for us. Reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured products ourselves, including reliance on the third-party manufacturer for regulatory compliance. We will not have control over our third-party manufacturers’ compliance with applicable rules and regulations. Our product promotion and advertising is also subject to regulatory requirements and continuing regulatory review.
If we or our collaborators, manufacturers or service providers fail to comply with applicable continuing regulatory requirements in the United States or foreign jurisdictions in which we may seek to market our products, we or they may be subject to, among other things, fines, warning letters, holds on clinical trials, refusal by the FDA to approve pending applications or supplements to approved applications, suspension or withdrawal of regulatory approval, product recalls and seizures, refusal to permit the import or export of products, operating restrictions, injunction, civil penalties and criminal prosecution.
63
Table of Contents
Even if we receive regulatory approval to market our product candidates, the market may not be receptive to our product candidates upon their commercial introduction, which will prevent us from becoming profitable.
The product candidates that we are developing are based upon new technologies or therapeutic approaches. Key participants in pharmaceutical marketplaces, such as physicians, third-party payors and consumers, may not accept a product intended to improve therapeutic results based on RNAi technology. As a result, it may be more difficult for us to convince the medical community and third-party payors to accept and use our product, or to provide favorable reimbursement.
Other factors that we believe will materially affect market acceptance of our product candidates include:
| • | the timing of our receipt of any marketing approvals, the terms of any approvals and the countries in which approvals are obtained; |
| • | the safety and efficacy of our product candidates, as demonstrated in clinical trials; |
| • | relative convenience and ease of administration of our product candidates; |
| • | the willingness of patients to accept potentially new routes of administration; |
| • | the success of our physician education programs; |
| • | the availability of adequate government and third-party payor reimbursement; |
| • | the pricing of our products, particularly as compared to alternative treatments; and |
| • | availability of alternative effective treatments for the diseases that product candidates we develop are intended to treat and the relative risks, benefits and costs of the treatments. |
In addition, our estimates regarding the potential market size may be materially different from what we currently expect at the time we commence commercialization, which could result in significant changes in our business plan and may have a material adverse effect on our results of operations and financial condition.
If we or our collaborators, manufacturers or service providers fail to comply with healthcare laws and regulations, we or they could be subject to enforcement actions, which could affect our ability to develop, market and sell our products and may harm our reputation.
As a manufacturer of pharmaceuticals, we are subject to federal, state, and foreign healthcare laws and regulations pertaining to fraud and abuse and patients’ rights. These laws and regulations include:
| • | the U.S. federal healthcare program anti-kickback law, which prohibits, among other things, persons from soliciting, receiving or providing remuneration, directly or indirectly, to induce either the referral of an individual for a healthcare item or service, or the purchasing or ordering of an item or service, for which payment may be made under a federal healthcare program such as Medicare or Medicaid; |
| • | the U.S. federal false claims law, which prohibits, among other things, individuals or entities from knowingly presenting or causing to be presented, claims for payment by government funded programs such as Medicare or Medicaid that are false or fraudulent, and which may apply to us by virtue of statements and representations made to customers or third parties; |
| • | the U.S. federal Health Insurance Portability and Accountability Act, or HIPAA, and Health Information Technology for Economic and Clinical Health, or HITECH, Act, which impose requirements relating to the privacy, security, and transmission of individually identifiable health information; and require notification to affected individuals and regulatory authorities of certain breaches of security of individually identifiable health information; |
| • | the federal Open Payments requirements under the National Physician Payment Transparency Program require that manufacturers of pharmaceutical and biological drugs covered by Medicare, Medicaid, and Children’s Health Insurance Programs report all consulting fees, travel reimbursements, research grants, and other payments or gifts with values over $10 made to physicians and teaching hospitals; |
64
Table of Contents
| • | pharmaceutical manufacturers are required to track certain financial arrangements with physicians and teaching hospitals, including any “transfer of value” made or distributed to such entities, as well as any investment interests held by physicians and their immediate family members. Manufacturers were required by PPACA to begin tracking this information in 2013 and to report this information to CMS beginning in 2014; and |
| • | state laws comparable to each of the above federal laws, such as, for example, anti-kickback and false claims laws applicable to commercial insurers and other non-federal payors, requirements for mandatory corporate regulatory compliance programs, and laws relating to patient data privacy and security. |
If our operations are found to be in violation of any such requirements, we may be subject to penalties, including civil or criminal penalties, monetary damages, the curtailment or restructuring of our operations, loss of eligibility to obtain approvals from the FDA, or exclusion from participation in government contracting, healthcare reimbursement or other government programs, including Medicare and Medicaid, any of which could adversely our financial results. Although effective compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, these risks cannot be entirely eliminated. Any action against us for an alleged or suspected violation could cause us to incur significant legal expenses and could divert our management’s attention from the operation of our business, even if our defense is successful. In addition, achieving and sustaining compliance with applicable laws and regulations may be costly to us in terms of money, time and resources.
If we or our collaborators, manufacturers or service providers fail to comply with applicable federal, state or foreign laws or regulations, we could be subject to enforcement actions, which could affect our ability to develop, market and sell our products successfully and could harm our reputation and lead to reduced acceptance of our products by the market. These enforcement actions include, among others:
| • | adverse regulatory inspection findings; |
| • | warning letters; |
| • | voluntary or mandatory product recalls or public notification or medical product safety alerts to healthcare professionals; |
| • | restrictions on, or prohibitions against, marketing our products; |
| • | restrictions on, or prohibitions against, importation or exportation of our products; |
| • | suspension of review or refusal to approve pending applications or supplements to approved applications; |
| • | exclusion from participation in government-funded healthcare programs; |
| • | exclusion from eligibility for the award of government contracts for our products; |
| • | suspension or withdrawal of product approvals; |
| • | product seizures; |
| • | injunctions; and |
| • | civil and criminal penalties and fines. |
Moreover, federal, state or foreign laws or regulations are subject to change, and while we, our collaborators, manufacturers and/or service providers currently may be compliant, that could change due to changes in interpretation, prevailing industry standards or the legal structure.
Any drugs we develop may become subject to unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives, thereby harming our business.
The regulations that govern marketing approvals, pricing and reimbursement for new drugs vary widely from country to country. Some countries require approval of the sale price of a drug before it can be marketed. In
65
Table of Contents
many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. Although we intend to monitor these regulations, our programs are currently in the early stages of development and we will not be able to assess the impact of price regulations for a number of years. As a result, we might obtain regulatory approval for a product in a particular country, but then be subject to price regulations that delay our commercial launch of the product and negatively impact the revenues we are able to generate from the sale of the product in that country.
Our ability to commercialize any products successfully also will depend in part on the extent to which reimbursement for these products and related treatments will be available from government health administration authorities, private health insurers and other organizations. Even if we succeed in bringing one or more products to the market, these products may not be considered cost-effective, and the amount reimbursed for any products may be insufficient to allow us to sell our products on a competitive basis. Because our programs are in the early stages of development, we are unable at this time to determine their cost effectiveness or the likely level or method of reimbursement. Increasingly, the third-party payors who reimburse patients or healthcare providers, such as government and private insurance plans, are requiring that drug companies provide them with predetermined discounts from list prices, and are seeking to reduce the prices charged or the amounts reimbursed for pharmaceutical products. If the price we are able to charge for any products we develop, or the reimbursement provided for such products, is inadequate in light of our development and other costs, our return on investment could be adversely affected.
We currently expect that any drugs we develop may need to be administered under the supervision of a physician on an outpatient basis. Under currently applicable U.S. law, certain drugs that are not usually self-administered (including injectable drugs) may be eligible for coverage under the Medicare Part B program if:
| • | they are incident to a physician’s services; |
| • | they are reasonable and necessary for the diagnosis or treatment of the illness or injury for which they are administered according to accepted standards of medical practice; and |
| • | they have been approved by the FDA and meet other requirements of the statute. |
There may be significant delays in obtaining coverage for newly-approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA. Moreover, eligibility for coverage does not imply that any drug will be reimbursed in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Interim payments for new drugs, if applicable, may also not be sufficient to cover our costs and may not be made permanent. Reimbursement may be based on payments allowed for lower-cost drugs that are already reimbursed, may be incorporated into existing payments for other services and may reflect budgetary constraints or imperfections in Medicare data. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. Third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement rates. Our inability to promptly obtain coverage and adequate reimbursement rates from both government-funded and private payors for new drugs that we develop and for which we obtain regulatory approval could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products, and our overall financial condition.
We believe that the efforts of governments and third-party payors to contain or reduce the cost of healthcare and legislative and regulatory proposals to broaden the availability of healthcare will continue to affect the business and financial condition of pharmaceutical and biopharmaceutical companies. A number of legislative and regulatory changes in the healthcare system in the United States and other major healthcare markets have been proposed in recent years, and such efforts have expanded substantially in recent years. These developments have included prescription drug benefit legislation that was enacted and took effect in January 2006, healthcare reform legislation enacted by certain states, and major healthcare reform legislation that was passed by Congress and enacted into law in the United States in 2010. These developments could, directly or indirectly, affect our ability to sell our products, if approved, at a favorable price.
66
Table of Contents
In particular, in March 2010, the PPACA was signed into law. This new legislation changes the current system of healthcare insurance and benefits intended to broaden coverage and control costs. The new law also contains provisions that will affect companies in the pharmaceutical industry and other healthcare related industries by imposing additional costs and changes to business practices. Provisions affecting pharmaceutical companies include the following:
| • | Mandatory rebates for drugs sold into the Medicaid program have been increased, and the rebate requirement has been extended to drugs used in risk-based Medicaid managed care plans. |
| • | The 340B Drug Pricing Program under the Public Health Services Act has been extended to require mandatory discounts for drug products sold to certain critical access hospitals, cancer hospitals and other covered entities. |
| • | Pharmaceutical companies are required to offer discounts on brand-name drugs to patients who fall within the Medicare Part D coverage gap, commonly referred to as the “Donut Hole.” |
| • | Pharmaceutical companies are required to pay an annual non-tax deductible fee to the federal government based on each company’s market share of prior year total sales of branded products to certain federal healthcare programs, such as Medicare, Medicaid, Department of Veterans Affairs and Department of Defense. Since we expect our branded pharmaceutical sales to constitute a small portion of the total federal health program pharmaceutical market, we do not expect this annual assessment to have a material impact on our financial condition. |
| • | The new law provides that approval of an application for a follow-on biologic product may not become effective until 12 years after the date on which the reference innovator biologic product was first licensed by the FDA, with a possible six-month extension for pediatric products. After this exclusivity ends, it will be easier for generic manufacturers to enter the market, which is likely to reduce the pricing for such products and could affect our profitability. |
The full effects of the U.S. healthcare reform legislation cannot be known until the new law is fully implemented through regulations or guidance issued by the Centers for Medicare & Medicaid Services and other federal and state healthcare agencies. The financial impact of the U.S. healthcare reform legislation over the next few years will depend on a number of factors, including but not limited, to the policies reflected in implementing regulations and guidance, and changes in sales volumes for products affected by the new system of rebates, discounts and fees. The new legislation may also have a positive impact on our future net sales, if any, by increasing the aggregate number of persons with healthcare coverage in the United States, but such increases are unlikely to be realized until approximately 2014 at the earliest.
Moreover, we cannot predict what healthcare reform initiatives may be adopted in the future. Further federal and state legislative and regulatory developments are likely, and we expect ongoing initiatives in the United States to increase pressure on drug pricing. Such reforms could have an adverse effect on anticipated revenues from product candidates that we may successfully develop and for which we may obtain regulatory approval and may affect our overall financial condition and ability to develop drug candidates.
Our ability to obtain services, reimbursement or funding from the federal government may be impacted by possible reductions in federal spending.
Our ability to obtain services, reimbursement or funding from the federal government may be impacted by possible reductions in federal spending. Under the Budget Control Act of 2011, the failure of Congress to enact deficit reduction measures of at least $1.2 trillion for the years 2013 through 2021 triggered automatic cuts to most federal programs. These cuts included aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, starting in 2013. Under the American Taxpayer Relief Act of 2012, which was enacted on January 1, 2013, the imposition of these automatic cuts was delayed until March 1, 2013. As required by law, President Obama issued a sequestration order on March 1, 2013. Certain of these automatic cuts have been implemented resulting in reductions in Medicare payments to physicians, hospitals, and other healthcare providers, among other things. The full impact on our business of these automatic cuts is uncertain.
67
Table of Contents
If other federal spending is reduced, any budgetary shortfalls may also impact the ability of relevant agencies, such as the FDA or NIH to continue to function. Amounts allocated to federal grants and contracts may be reduced or eliminated. These reductions may also impact the ability of relevant agencies to timely review and approve drug research and development, manufacturing, and marketing activities, which may delay our ability to develop, market and sell any products we may develop.
There is a substantial risk of product liability claims in our business. If we are unable to obtain sufficient insurance, a product liability claim against us could adversely affect our business.
Our business exposes us to significant potential product liability risks that are inherent in the development, testing, manufacturing and marketing of human therapeutic products. Product liability claims could delay or prevent completion of our clinical development programs. If we succeed in marketing products, such claims could result in an FDA investigation of the safety and effectiveness of our products, our manufacturing processes and facilities or our marketing programs, and potentially a recall of our products or more serious enforcement action, limitations on the approved indications for which they may be used, or suspension or withdrawal of approvals. Regardless of the merits or eventual outcome, liability claims may also result in decreased demand for our products, injury to our reputation, costs to defend the related litigation, a diversion of management’s time and our resources, substantial monetary awards to trial participants or patients and a decline in our stock price. We currently have product liability insurance that we believe is appropriate for our stage of development and may need to obtain higher levels prior to marketing any of our product candidates. Any insurance we have or may obtain may not provide sufficient coverage against potential liabilities. Furthermore, clinical trial and product liability insurance is becoming increasingly expensive. As a result, we may be unable to obtain sufficient insurance at a reasonable cost to protect us against losses caused by product liability claims that could have a material adverse effect on our business.
If we do not comply with laws regulating the protection of the environment and health and human safety, our business could be adversely affected.
Our research, development and manufacturing involves the use of hazardous materials, chemicals and various radioactive compounds. We maintain quantities of various flammable and toxic chemicals in our facilities in Cambridge that are required for our research, development and manufacturing activities. We are subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of these hazardous materials. We believe our procedures for storing, handling and disposing these materials in our Cambridge facilities comply with the relevant guidelines of the City of Cambridge, the Commonwealth of Massachusetts and the Occupational Safety and Health Administration of the U.S. Department of Labor. Although we believe that our safety procedures for handling and disposing of these materials comply with the standards mandated by applicable regulations, the risk of accidental contamination or injury from these materials cannot be eliminated. If an accident occurs, we could be held liable for resulting damages, which could be substantial. We are also subject to numerous environmental, health and workplace safety laws and regulations, including those governing laboratory procedures, exposure to blood-borne pathogens and the handling of biohazardous materials.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of these materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials. Additional federal, state and local laws and regulations affecting our operations may be adopted in the future. We may incur substantial costs to comply with, and substantial fines or penalties if we violate, any of these laws or regulations.
Risks Related to Patents, Licenses and Trade Secrets
If we are not able to obtain and enforce patent protection for our discoveries, our ability to develop and commercialize our product candidates will be harmed.
Our success depends, in part, on our ability to protect proprietary methods and technologies that we develop under the patent and other intellectual property laws of the United States and other countries, so that we can
68
Table of Contents
prevent others from unlawfully using our inventions and proprietary information. However, we may not hold proprietary rights to some patents required for us to commercialize our proposed products. Because certain U.S. patent applications are confidential until the patents issue, such as applications filed prior to November 29, 2000, or applications filed after such date which will not be filed in foreign countries, third parties may have filed patent applications for technology covered by our pending patent applications without our being aware of those applications, and our patent applications may not have priority over those applications. For this and other reasons, we may be unable to secure desired patent rights, thereby losing desired exclusivity. Further, we may be required to obtain licenses under third-party patents to market our proposed products or conduct our research and development or other activities. If licenses are not available to us on acceptable terms, we will not be able to market the affected products or conduct the desired activities.
Our strategy depends on our ability to rapidly identify and seek patent protection for our discoveries. In addition, we may rely on third-party collaborators to file patent applications relating to proprietary technology that we develop jointly during certain collaborations. The process of obtaining patent protection is expensive and time-consuming. If our present or future collaborators fail to file and prosecute all necessary and desirable patent applications at a reasonable cost and in a timely manner, our business will be adversely affected. Despite our efforts and the efforts of our collaborators to protect our proprietary rights, unauthorized parties may be able to obtain and use information that we regard as proprietary. While issued patents are presumed valid, this does not guarantee that the patent will survive a validity challenge or be held enforceable. Any patents we have obtained, or obtain in the future, may be challenged, invalidated, adjudged unenforceable or circumvented by parties attempting to design around our intellectual property. Moreover, third parties or the USPTO may commence interference proceedings involving our patents or patent applications. Any challenge to, finding of unenforceability or invalidation or circumvention of, our patents or patent applications, would be costly, would require significant time and attention of our management and could have a material adverse effect on our business.
Our pending patent applications may not result in issued patents. The patent position of pharmaceutical or biotechnology companies, including ours, is generally uncertain and involves complex legal and factual considerations. The standards that the USPTO and its foreign counterparts use to grant patents are not always applied predictably or uniformly and can change. Similarly, the ultimate degree of protection that will be afforded to biotechnology inventions, including ours, in the United States and foreign countries, remains uncertain and is dependent upon the scope of the protection decided upon by patent offices, courts and lawmakers. Moreover, there are periodic discussions in the Congress of the United States and in international jurisdictions about modifying various aspects of patent law. For example, the America Invents Act includes a number of changes to the patent laws of the United States. If any of the enacted changes do not provide adequate protection for discoveries, including our ability to pursue infringers of our patents for substantial damages, our business could be adversely affected. One major provision of the America Invents Act, which took effect in March 2013, changed United States patent practice from a first-to-invent to a first-to-file system. If we fail to file an invention before a competitor files on the same invention, we no longer have the ability to provide proof that we were in possession of the invention prior to the competitor’s filing date, and thus would not be able to obtain patent protection for our invention. There is also no uniform, worldwide policy regarding the subject matter and scope of claims granted or allowable in pharmaceutical or biotechnology patents.
Accordingly, we do not know the degree of future protection for our proprietary rights or the breadth of claims that will be allowed in any patents issued to us or to others. We also rely to a certain extent on trade secrets, know-how and technology, which are not protected by patents, to maintain our competitive position. If any trade secret, know-how or other technology not protected by a patent were to be disclosed to or independently developed by a competitor, our business and financial condition could be materially adversely affected.
We license patent rights from third-party owners. If such owners do not properly or successfully obtain, maintain or enforce the patents underlying such licenses, our competitive position and business prospects will be harmed.
We are a party to a number of licenses that give us rights to third-party intellectual property that is necessary or useful for our business. In particular, we have obtained licenses from, among others, CRT, Isis, MIT,
69
Table of Contents
Whitehead, Max Planck Innovation, Tekmira and Arrowhead. We also intend to enter into additional licenses to third-party intellectual property in the future.
Our success will depend in part on the ability of our licensors to obtain, maintain and enforce patent protection for our licensed intellectual property, in particular, those patents to which we have secured exclusive rights. Our licensors may not successfully prosecute the patent applications to which we are licensed. Even if patents issue in respect of these patent applications, our licensors may fail to maintain these patents, may determine not to pursue litigation against other companies that are infringing these patents, or may pursue such litigation less aggressively than we would. Without protection for the intellectual property we license, other companies might be able to offer substantially identical products for sale, which could adversely affect our competitive business position and harm our business prospects. In addition, we sublicense our rights under various third-party licenses to our collaborators. Any impairment of these sublicensed rights could result in reduced revenues under our collaboration agreements or result in termination of an agreement by one or more of our collaborators.
Other companies or organizations may challenge our patent rights or may assert patent rights that prevent us from developing and commercializing our products.
RNAi is a relatively new scientific field, the commercial exploitation of which has resulted in many different patents and patent applications from organizations and individuals seeking to obtain patent protection in the field. We have obtained grants and issuances of RNAi patents and have licensed many of these patents from third parties on an exclusive basis. The issued patents and pending patent applications in the United States and in key markets around the world that we own or license claim many different methods, compositions and processes relating to the discovery, development, manufacture and commercialization of RNAi therapeutics.
Specifically, we have a portfolio of patents, patent applications and other intellectual property covering: fundamental aspects of the structure and uses of siRNAs, including their manufacture and use as therapeutics, and RNAi-related mechanisms; chemical modifications to siRNAs that improve their suitability for therapeutic uses; siRNAs directed to specific targets as treatments for particular diseases; and delivery technologies, such as in the field of cationic liposomes.
As the field of RNAi therapeutics is maturing, patent applications are being fully processed by national patent offices around the world. There is uncertainty about which patents will issue, and, if they do, as to when, to whom, and with what claims. It is likely that there will be significant litigation and other proceedings, such as interference, reexamination and opposition proceedings, in various patent offices relating to patent rights in the RNAi field. For example, various third parties have initiated oppositions to patents in our Kreutzer-Limmer and Tuschl II series in the EPO and in other jurisdictions. We expect that additional oppositions will be filed in the EPO and elsewhere, and other challenges will be raised relating to other patents and patent applications in our portfolio. In many cases, the possibility of appeal exists for either us or our opponents, and it may be years before final, unappealable rulings are made with respect to these patents in certain jurisdictions. The timing and outcome of these and other proceedings is uncertain and may adversely affect our business if we are not successful in defending the patentability and scope of our pending and issued patent claims. In addition, third parties may attempt to invalidate our intellectual property rights. Even if our rights are not directly challenged, disputes could lead to the weakening of our intellectual property rights. Our defense against any attempt by third parties to circumvent or invalidate our intellectual property rights could be costly to us, could require significant time and attention of our management and could have a material adverse effect on our business and our ability to successfully compete in the field of RNAi.
There are many issued and pending patents that claim aspects of oligonucleotide chemistry and modifications that we may need to apply to our siRNA therapeutic candidates. There are also many issued patents that claim targeting genes or portions of genes that may be relevant for siRNA drugs we wish to develop. Thus, it is possible that one or more organizations will hold patent rights to which we will need a license. If those organizations refuse to grant us a license to such patent rights on reasonable terms, we may not be able to market products or perform research and development or other activities covered by these patents.
70
Table of Contents
If we become involved in patent litigation or other proceedings related to a determination of rights, we could incur substantial costs and expenses, substantial liability for damages or be required to stop our product development and commercialization efforts.
Third parties may sue us for infringing their patent rights. Likewise, we may need to resort to litigation to enforce a patent issued or licensed to us or to determine the scope and validity of proprietary rights of others. In addition, a third party may claim that we have improperly obtained or used its confidential or proprietary information. For example, in March 2011, Tekmira filed a civil complaint against us alleging, among other things, misappropriation of the plaintiffs’ confidential and proprietary information and trade secrets. In November 2012, we settled this litigation and restructured our contractual relationship with Tekmira. In connection with this restructuring, we incurred a $65.0 million charge to operating expenses during the quarter ended December 31, 2012. In addition, during the pendency of the litigation, we incurred significant costs, and the defense of this litigation diverted the attention of our management and other resources that would otherwise have been engaged in other activities.
Furthermore, third parties may challenge the inventorship of our patents or licensed patents. For example, in March 2011, the University of Utah, or Utah, filed a complaint in the United States District Court for the District of Massachusetts against us, Max Planck Gesellschaft Zur Foerderung Der Wissenschaften e.V. and Max Planck Innovation, together, Max Planck, Whitehead, MIT and UMass, claiming that a professor of Utah is the sole inventor, or in the alternative, a joint inventor of certain of our in-licensed patents. The original complaint was not served on any of the parties and, in July 2011, Utah filed an amended complaint containing substantially the same claims as the original complaint against us, Max Planck, Whitehead, MIT and UMass. The amended complaint alleges the defendants have incorrectly determined inventorship of some of our in-licensed patents and further claims unjust enrichment, unfair competition, false advertising and seeks correction of inventorship, injunctive relief and unspecified damages. In October 2011, we, Max Planck, Whitehead, MIT and UMass filed a motion to dismiss and UMass filed a motion to dismiss on separate grounds, which we, Max Planck, Whitehead and MIT have joined. In December 2011, Utah filed a second amended complaint dropping UMass as a defendant and adding as defendants several UMass officials. In June 2012, the Court denied both motions to dismiss. We, Max Planck, Whitehead, MIT and UMass filed an appeal of the Court’s ruling on the motion to dismiss for lack of jurisdiction and a motion requesting that the Court stay the case pending the outcome of the appeal. In July 2012, the Court stayed discovery in the case pending the outcome of the defendants’ appeal. Oral arguments in the appeal were heard in early March 2013 in the United States Court of Appeals for the Federal Circuit, or CAFC. In August 2013, the CAFC affirmed the lower Court’s ruling, in a split decision. We believe the majority made an error in law when affirming the lower Court’s decision, and in September 2013, we filed a petition with the CAFC for rehearing or rehearing en banc. In October 2013, the CAFC invited Utah to file an answer to the petition. In November 2013, the CAFC denied our petition for rehearing or rehearing en banc and remanded the case back to the lower Court. In February 2014, we filed a petition for writ of certiorari from the Supreme Court and a motion to stay the lower Court’ proceedings pending a decision from the Supreme Court on our petition. We are awaiting the Courts’ decisions. We intend to vigorously defend ourselves in this matter, however, litigation is subject to inherent uncertainty and a court could ultimately rule against us.
In addition, in connection with certain license and collaboration agreements, we have agreed to indemnify certain third parties for certain costs incurred in connection with litigation relating to intellectual property rights or the subject matter of the agreements. The cost to us of any litigation or other proceeding relating to intellectual property rights, even if resolved in our favor, could be substantial, and litigation would divert our management’s efforts. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Uncertainties resulting from the initiation and continuation of any litigation could delay our research and development efforts and limit our ability to continue our operations.
If any parties successfully claim that our creation or use of proprietary technologies infringes upon or otherwise violates their intellectual property rights, we might be forced to pay damages, potentially including treble damages, if we are found to have willfully infringed on such parties’ patent rights. In addition to any damages we might have to pay, a court could require us to stop the infringing activity or obtain a license. Any
71
Table of Contents
license required under any patent may not be made available on commercially acceptable terms, if at all. In addition, such licenses are likely to be non-exclusive and, therefore, our competitors may have access to the same technology licensed to us. If we fail to obtain a required license and are unable to design around a patent, we may be unable to effectively market some of our technology and products, which could limit our ability to generate revenues or achieve profitability and possibly prevent us from generating revenue sufficient to sustain our operations. Moreover, we expect that a number of our collaborations will provide that royalties payable to us for licenses to our intellectual property may be offset by amounts paid by our collaborators to third parties who have competing or superior intellectual property positions in the relevant fields, which could result in significant reductions in our revenues from products developed through collaborations.
If we fail to comply with our obligations under any licenses or related agreements, we may be required to pay damages and could lose license or other rights that are necessary for developing and protecting our RNAi technology and any related product candidates that we develop, or we could lose certain rights to grant sublicenses.
Our current licenses impose, and any future licenses we enter into are likely to impose, various development, commercialization, funding, milestone, royalty, diligence, sublicensing, insurance, patent prosecution and enforcement, and other obligations on us. If we breach any of these obligations, or use the intellectual property licensed to us in an unauthorized manner, we may be required to pay damages and the licensor may have the right to terminate the license or render the license non-exclusive, which could result in us being unable to develop, manufacture and sell products that are covered by the licensed technology or enable a competitor to gain access to the licensed technology. For example, TPC has notified us that it believes it has achieved a $5.0 million milestone payment under our cross-license agreement relating to the manufacture of ALN-VSP clinical trial material for use in China. We have notified TPC that we do not believe that the milestone has been achieved under the terms of the cross-license agreement. In August 2013, we initiated binding arbitration proceedings seeking a declaratory judgment that TPC has not yet met the conditions of the milestone and is not entitled to payment at this time. If it is determined through arbitration that TPC has met the requirements of the milestone, we will have to pay TPC the milestone.
Moreover, our licensors may own or control intellectual property that has not been licensed to us and, as a result, we may be subject to claims, regardless of their merit, that we are infringing or otherwise violating the licensor’s rights. In addition, while we cannot currently determine the amount of the royalty obligations we will be required to pay on sales of future products, if any, the amounts may be significant. The amount of our future royalty obligations will depend on the technology and intellectual property we use in products that we successfully develop and commercialize, if any. Therefore, even if we successfully develop and commercialize products, we may be unable to achieve or maintain profitability.
Confidentiality agreements with employees and others may not adequately prevent disclosure of trade secrets and other proprietary information.
In order to protect our proprietary technology and processes, we rely in part on confidentiality agreements with our collaborators, employees, consultants, outside scientific collaborators and sponsored researchers, and other advisors. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover trade secrets and proprietary information, and in such cases we could not assert any trade secret rights against such party. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
72
Table of Contents
Risks Related to Competition
The pharmaceutical market is intensely competitive. If we are unable to compete effectively with existing drugs, new treatment methods and new technologies, we may be unable to commercialize successfully any drugs that we develop.
The pharmaceutical market is intensely competitive and rapidly changing. Many large pharmaceutical and biotechnology companies, academic institutions, governmental agencies and other public and private research organizations are pursuing the development of novel drugs for the same diseases that we are targeting or expect to target. Many of our competitors have:
| • | much greater financial, technical and human resources than we have at every stage of the discovery, development, manufacture and commercialization of products; |
| • | more extensive experience in pre-clinical testing, conducting clinical trials, obtaining regulatory approvals, and in manufacturing, marketing and selling pharmaceutical products; |
| • | product candidates that are based on previously tested or accepted technologies; |
| • | products that have been approved or are in late stages of development; and |
| • | collaborative arrangements in our target markets with leading companies and research institutions. |
We will face intense competition from drugs that have already been approved and accepted by the medical community for the treatment of the conditions for which we may develop drugs. We also expect to face competition from new drugs that enter the market. We believe a significant number of drugs are currently under development, and may become commercially available in the future, for the treatment of conditions for which we may try to develop drugs. These drugs may be more effective, safer, less expensive, or marketed and sold more effectively, than any products we develop. For example, we are developing patisiran for the treatment of ATTR patients suffering from FAP. We are aware of other approved products used to treat this disease, as well as product candidates in various stages of clinical development. Patisiran may not compete favorably with these products and product candidates, and even if approved, it may not achieve commercial success.
If we successfully develop product candidates, and obtain approval for them, we will face competition based on many different factors, including:
| • | the safety and effectiveness of our products; |
| • | the ease with which our products can be administered and the extent to which patients accept relatively new routes of administration; |
| • | the timing and scope of regulatory approvals for these products; |
| • | the availability and cost of manufacturing, marketing and sales capabilities; |
| • | price; |
| • | reimbursement coverage; and |
| • | patent position. |
Our competitors may develop or commercialize products with significant advantages over any products we develop based on any of the factors listed above or on other factors. Our competitors may therefore be more successful in commercializing their products than we are, which could adversely affect our competitive position and business. Competitive products may make any products we develop obsolete or noncompetitive before we can recover the expenses of developing and commercializing our product candidates. Such competitors could also recruit our employees, which could negatively impact our level of expertise and the ability to execute on our business plan. Furthermore, we also face competition from existing and new treatment methods that reduce or eliminate the need for drugs, such as the use of advanced medical devices. The development of new medical devices or other treatment methods for the diseases we are targeting could make our product candidates noncompetitive, obsolete or uneconomical.
73
Table of Contents
We face competition from other companies that are working to develop novel drugs and technology platforms using technology similar to ours. If these companies develop drugs more rapidly than we do or their technologies, including delivery technologies, are more effective, our ability to successfully commercialize drugs may be adversely affected.
In addition to the competition we face from competing drugs in general, we also face competition from other companies working to develop novel drugs using technology that competes more directly with our own. We are aware of multiple companies that are working in the field of RNAi. In addition, we granted licenses or options for licenses to Isis, GeneCare Research Institute Co., Ltd., Benitec Ltd., Arrowhead and its subsidiary, Calando Pharmaceuticals, Inc., Tekmira, Quark Pharmaceuticals, Inc., Sylentis S.A.U. and others under which these companies may independently develop RNAi therapeutics against a limited number of targets. Any of these companies may develop its RNAi technology more rapidly and more effectively than us.
In addition, as a result of agreements that we have entered into, Arrowhead, as the assignee of Roche, and Takeda have obtained non-exclusive licenses, and Novartis has obtained specific exclusive licenses for 31 gene targets, to certain aspects of our technology that give them the right to compete with us in certain circumstances We also compete with companies working to develop antisense-based drugs. Like RNAi therapeutics, antisense drugs target mRNAs, in order to suppress the activity of specific genes. Isis is currently marketing an antisense drug and has several antisense product candidates in clinical trials, including one for the treatment of ATTR. The development of antisense drugs is more advanced than that of RNAi therapeutics, and antisense technology may become the preferred technology for drugs that target mRNAs to silence specific genes.
In addition to competition with respect to RNAi and with respect to specific products, we face substantial competition to discover and develop safe and effective means to deliver siRNAs to the relevant cell and tissue types. Safe and effective means to deliver siRNAs to the relevant cell and tissue types may be developed by our competitors, and our ability to successfully commercialize a competitive product would be adversely affected. In addition, substantial resources are being expended by third parties in the effort to discover and develop a safe and effective means of delivering siRNAs into the relevant cell and tissue types, both in academic laboratories and in the corporate sector. Some of our competitors have substantially greater resources than we do, and if our competitors are able to negotiate exclusive access to those delivery solutions developed by third parties, we may be unable to successfully commercialize our product candidates.
Risks Related to Our Common Stock
If our stock price fluctuates, purchasers of our common stock could incur substantial losses.
The market price of our common stock has fluctuated and may continue to fluctuate significantly in response to factors that are beyond our control. The stock market in general has recently experienced extreme price and volume fluctuations. The market prices of securities of pharmaceutical and biotechnology companies have been extremely volatile, and have experienced fluctuations that often have been unrelated or disproportionate to the operating performance of these companies. These broad market fluctuations could result in extreme fluctuations in the price of our common stock, which could cause purchasers of our common stock to incur substantial losses.
We may incur significant costs from class action litigation due to stock volatility.
Our stock price may fluctuate for many reasons, including as a result of public announcements regarding the progress of our development efforts or the development efforts of our collaborators and/or competitors, the addition or departure of our key personnel, variations in our quarterly operating results and changes in market valuations of pharmaceutical and biotechnology companies. When the market price of a stock has been volatile as our stock price may be, holders of that stock have occasionally brought securities class action litigation against the company that issued the stock. If any of our stockholders were to bring a lawsuit of this type against us, even if the lawsuit is without merit, we could incur substantial costs defending the lawsuit. The lawsuit could also divert the time and attention of our management.
74
Table of Contents
Sales of additional shares of our common stock, including by us or our directors and officers, could cause the price of our common stock to decline.
Sales of substantial amounts of our common stock in the public market, or the availability of such shares for sale, by us or our officers and directors, or others, including the issuance of common stock upon exercise of outstanding options or restricted stock, could adversely affect the price of our common stock.
Genzyme’s ownership of our common stock following the closing of the 2014 Genzyme collaboration could delay or prevent a change in corporate control.
As of closing of the stock purchase in connection with the 2014 Genzyme collaboration, Genzyme will hold approximately 12% of our outstanding common stock and will have the right to increase its ownership up to 30%, as well as the right to maintain its ownership percentage through the term of our collaboration, subject to certain limitations. This concentration of ownership may harm the market price of our common stock by:
| • | delaying, deferring or preventing a change in control of our company; |
| • | impeding a merger, consolidation, takeover or other business combination involving our company; or |
| • | discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of our company. |
Anti-takeover provisions in our charter documents and under Delaware law and our stockholder rights plan could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our certificate of incorporation and our bylaws may delay or prevent an acquisition of us or a change in our management. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors. Because our board of directors is responsible for appointing the members of our management team, these provisions could in turn affect any attempt by our stockholders to replace current members of our management team. These provisions include:
| • | a classified board of directors; |
| • | a prohibition on actions by our stockholders by written consent; |
| • | limitations on the removal of directors; and |
| • | advance notice requirements for election to our board of directors and for proposing matters that can be acted upon at stockholder meetings. |
In addition, our board of directors has adopted a stockholder rights plan, the provisions of which could make it difficult for a potential acquirer of Alnylam to consummate an acquisition transaction. Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner. These provisions would apply even if the proposed merger or acquisition could be considered beneficial by some stockholders.
75
Table of Contents
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
Not applicable.
| ITEM 2. | PROPERTIES |
Our operations are based primarily in Cambridge, Massachusetts. As of January 31, 2014, we leased approximately 129,000 square feet of office and laboratory space in Cambridge, Massachusetts for our corporate headquarters and primary research facility, of which approximately 18,000 square feet is under sublease to a third party through September 2016. The lease for this property expires in September 2016, and we have the option to extend the lease for two successive five-year periods. In February 2012, we executed a lease for approximately 15,000 square feet of additional office and laboratory space in Cambridge, Massachusetts for our cGMP manufacturing facility. The lease for this property expires in August 2017, and we have the option to extend this lease for two successive five-year periods.
We believe that the total space available to us under our current leases will meet our needs for the foreseeable future and that additional space would be available to us on commercially reasonable terms if required.
| ITEM 3. | LEGAL PROCEEDINGS |
University of Utah Litigation
On March 22, 2011, Utah filed a civil complaint in the United States District Court for the District of Massachusetts against us, Max Planck, Whitehead, MIT and UMass, claiming a professor at Utah is the sole inventor or, in the alternative, a joint inventor, of the Tuschl patents. Utah did not serve the original complaint on us or the other defendants. On July 6, 2011, Utah filed an amended complaint alleging substantially the same claims against us, Max Planck, Whitehead, MIT and UMass. The amended complaint was served on us on July 14, 2011. Utah is seeking changes to the inventorship of the Tuschl patents, unspecified damages and other relief. On October 31, 2011, we, Max Planck, Whitehead, MIT and UMass filed a motion to dismiss. Also on October 31, 2011, UMass filed a motion to dismiss on separate grounds, which we, Max Planck, Whitehead and MIT joined. On December 31, 2011, Utah filed a second amended complaint dropping UMass as a defendant and adding as defendants several UMass officials. In June 2012, the Court denied both motions to dismiss. We, Max Planck, Whitehead, MIT and UMass filed an appeal of the Court’s ruling on the motion to dismiss for lack of jurisdiction and a motion requesting that the Court stay the case pending the outcome of the appeal. In July 2012, the Court stayed discovery in the case pending the outcome of the defendants’ appeal. Oral arguments in the appeal were heard in early March 2013 in the United States Court of Appeals for the Federal Circuit, or CAFC. In August 2013, the CAFC affirmed the lower Court’s ruling, in a split decision. We believe the majority made an error in law when affirming the lower Court’s decision, and in September 2013, we filed a petition with the CAFC for rehearing or rehearing en banc. In October 2013, the CAFC invited Utah to file an answer to the petition. In November 2013, the CAFC denied our petition for rehearing or rehearing en banc and remanded the case back to the lower Court. In February 2014, we filed a petition for writ of certiorari from the Supreme Court and a motion to stay the lower Court’ proceedings pending a decision from the Supreme Court on our petition. We are awaiting the Courts’ decisions.
Although we believe we have meritorious defenses and intend to vigorously defend ourselves in this matter, litigation is subject to inherent uncertainty and a court could ultimately rule against us. In addition, the defense of litigation and related matters are costly and may divert the attention of our management and other resources that would otherwise be engaged in other activities. We have not recorded an estimate of the possible loss associated with this legal proceeding due to the uncertainties related to both the likelihood and the amount of any possible loss or range of loss.
| ITEM 4. | MINE SAFETY DISCLOSURES |
Not applicable.
76
Table of Contents
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Our common stock began trading on The NASDAQ Global Select Market on May 28, 2004 under the symbol “ALNY.” Prior to that time, there was no established public trading market for our common stock. The following table sets forth the high and low sale prices per share for our common stock on The NASDAQ Global Select Market for the periods indicated:
| Year Ended December 31, 2012: |
High | Low | ||||||
| First Quarter |
$ | 13.75 | $ | 8.33 | ||||
| Second Quarter |
$ | 12.05 | $ | 9.51 | ||||
| Third Quarter |
$ | 21.38 | $ | 11.64 | ||||
| Fourth Quarter |
$ | 19.48 | $ | 14.88 | ||||
| Year Ended December 31, 2013: |
High | Low | ||||||
| First Quarter |
$ | 25.80 | $ | 18.61 | ||||
| Second Quarter |
$ | 31.79 | $ | 21.60 | ||||
| Third Quarter |
$ | 65.83 | $ | 31.75 | ||||
| Fourth Quarter |
$ | 67.97 | $ | 48.16 | ||||
Holders of record
At January 31, 2014, there were 36 holders of record of our common stock. Because many of our shares are held by brokers and other institutions on behalf of stockholders, we are unable to estimate the total number of beneficial holders represented by these record holders.
Dividends
We have never paid or declared any cash dividends on our common stock. We currently intend to retain any earnings for future growth and, therefore, do not expect to pay cash dividends in the foreseeable future.
Securities Authorized for Issuance Under Equity Compensation Plans
We intend to file with the SEC a definitive Proxy Statement, which we refer to herein as the Proxy Statement, not later than 120 days after the close of the fiscal year ended December 31, 2013. The information required by this item relating to our equity compensation plans is incorporated herein by reference to the information contained under the section captioned “Equity Compensation Plan Information” of the Proxy Statement.
77
Table of Contents
Stock Performance Graph
The following performance graph and related information shall not be deemed “soliciting material” or to be “filed” with the SEC, nor shall such information be incorporated by reference into any future filing under the Securities Act of 1933 or Securities Exchange Act of 1934, each as amended, except to the extent that we specifically incorporate it by reference into such filing.
The comparative stock performance graph below compares the five-year cumulative total stockholder return (assuming reinvestment of dividends, if any) from investing $100 on December 31, 2008, to the close of the last trading day of 2013, in each of (i) our common stock, (ii) the NASDAQ Stock Market (U.S.) Index and (iii) the NASDAQ Pharmaceutical Index. The stock price performance reflected in the graph below is not necessarily indicative of future price performance.
Comparison of Five-Year Cumulative Total Return
Among Alnylam Pharmaceuticals, Inc.,
NASDAQ Stock Market (U.S.) Index and NASDAQ Pharmaceutical Index
| 12/31/2008 | 12/31/2009 | 12/31/2010 | 12/30/2011 | 12/31/2012 | 12/31/2013 | |||||||||||||||||||
| Alnylam Pharmaceuticals, Inc. |
$ | 100.00 | $ | 71.25 | $ | 39.87 | $ | 32.96 | $ | 73.80 | $ | 260.01 | ||||||||||||
| NASDAQ Stock Market (U.S.) Index |
$ | 100.00 | $ | 143.74 | $ | 170.17 | $ | 171.08 | $ | 202.40 | $ | 281.91 | ||||||||||||
| NASDAQ Pharmaceutical Index |
$ | 100.00 | $ | 112.36 | $ | 121.80 | $ | 130.38 | $ | 173.46 | $ | 285.96 | ||||||||||||
78
Table of Contents
| ITEM 6. | SELECTED CONSOLIDATED FINANCIAL DATA |
The following selected consolidated financial data for each of the five years in the period ended December 31, 2013 are derived from our audited consolidated financial statements. The selected consolidated financial data set forth below should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the financial statements, and the related Notes, included elsewhere in this annual report on Form 10-K. Historical results are not necessarily indicative of future results.
Selected Consolidated Financial Data
(In thousands, except per share data)
| Year Ended December 31, | ||||||||||||||||||||
| 2013 | 2012 | 2011 | 2010 | 2009 | ||||||||||||||||
| Statements of Comprehensive Loss Data: |
||||||||||||||||||||
| Net revenues from collaborators |
$ | 47,167 | $ | 66,725 | $ | 82,757 | $ | 100,041 | $ | 100,533 | ||||||||||
| Operating expenses(1) |
140,109 | 196,181 | 137,575 | 144,111 | 148,644 | |||||||||||||||
| Loss from operations |
(92,942 | ) | (129,456 | ) | (54,818 | ) | (44,070 | ) | (48,111 | ) | ||||||||||
| Net loss |
(89,225 | ) | (106,014 | ) | (57,649 | ) | (43,515 | ) | (47,590 | ) | ||||||||||
| Net loss per common share — basic and diluted |
$ | (1.45 | ) | $ | (2.11 | ) | $ | (1.36 | ) | $ | (1.04 | ) | $ | (1.14 | ) | |||||
| Weighted average common shares outstanding — basic and diluted |
61,551 | 50,286 | 42,410 | 42,040 | 41,633 | |||||||||||||||
|
(1) Non-cash stock-based compensation expenses included in operating expenses |
$ | 20,703 | $ | 12,360 | $ | 16,676 | $ | 19,118 | $ | 19,727 | ||||||||||
| December 31, | ||||||||||||||||||||
| 2013 | 2012 | 2011 | 2010 | 2009 | ||||||||||||||||
| Balance Sheet Data: |
||||||||||||||||||||
| Cash, cash equivalents and marketable securities |
$ | 350,472 | $ | 226,228 | $ | 260,809 | $ | 349,904 | $ | 435,316 | ||||||||||
| Working capital |
200,164 | 77,212 | 71,038 | 152,093 | 182,801 | |||||||||||||||
| Total assets |
420,530 | 287,520 | 281,917 | 393,265 | 481,385 | |||||||||||||||
| Total stockholders’ equity |
270,347 | 134,053 | 117,997 | 158,233 | 177,965 | |||||||||||||||
79
Table of Contents
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Overview
We are a biopharmaceutical company developing novel therapeutics based on RNAi. RNAi is a naturally occurring biological pathway within cells for selectively silencing and regulating the expression of specific genes. Since many diseases are caused by the inappropriate activity of specific genes, the ability to silence genes selectively through RNAi could provide a new way to treat a wide range of human diseases. We believe that drugs that work through RNAi have the potential to become a broad new class of drugs, like small molecule, protein and antibody drugs. Using our intellectual property and the expertise we have built in RNAi, we are developing a set of biological and chemical methods and know-how that we apply in a systematic way to develop RNAi therapeutics for a variety of diseases.
Our core product strategy was launched in January 2011 and is focused on the development and commercialization of novel RNAi therapeutics as genetic medicines. Under our core product strategy, we expect to have six to seven genetic medicine product candidates in clinical development, including at least two programs in Phase 3 and five to six programs with human proof of concept, by the end of 2015. We are currently advancing the following core programs in clinical or pre-clinical development: patisiran, for the treatment of ATTR in patients with FAP; ALN-TTRsc, for the treatment of ATTR in patients with TTR cardiac amyloidosis, including FAC and SSA; ALN-AT3, for the treatment of hemophilia and RBD; ALN-CC5, for the treatment of complement-mediated diseases; ALN-AS1, for the treatment of hepatic porphyrias, including AIP; ALN-PCSsc, for the treatment of hypercholesterolemia; ALN-AAT, for the treatment of AAT deficiency liver disease; ALN-TMP, for the treatment of beta-thalassemia and iron-overload disorders; ALN-ANG, for the treatment of genetic forms of mixed hyperlipidemia and severe hypertriglyceridemia; and other yet to be disclosed programs. Our strategy is to retain development and commercial rights for our current and future genetic medicine pipeline in North America and Western Europe, while forming alliances with leading, innovative companies for the development and commercialization of these products in the ROW. In early 2014, we formed an alliance with Genzyme to develop and commercialize our current and future genetic medicine pipeline principally in territories outside of North America and Western Europe, subject to certain broader rights.
In January 2014, we entered into a stock purchase agreement with Sirna, Merck and, for limited purposes, Merck & Co., Inc., pursuant to which we will purchase from Merck all of Merck’s right, title and interest in and to all of the outstanding shares of common stock of Sirna. Sirna possesses intellectual property and RNAi assets including pre-clinical therapeutic candidates, chemistry, and siRNA-conjugate and other delivery technologies that we intend to integrate into our platform for delivery of RNAi therapeutics. We will not acquire any employees, manufacturing or other facilities, developed processes or clinical-stage assets as part of the acquisition of Sirna. In consideration for the Sirna common stock, we will (i) pay Merck $25.0 million in cash and (ii) issue to Merck 2,520,044 shares of our common stock, having a value of $150.0 million as calculated under the terms of the stock purchase agreement on the date of execution. Following the closing of this transaction, Merck will beneficially own approximately 4% of our outstanding common stock.
In addition, Merck is eligible to receive the following consideration from us: (i) up to an aggregate of $10.0 million upon the achievement by us or our related parties of specified regulatory milestones for RNAi products covered by Sirna intellectual property, and (ii) up to an aggregate of $105.0 million upon the achievement by us or our related parties of specified development and regulatory ($40.0 million) and commercial ($65.0 million) milestones associated with the clinical development progress of certain pre-clinical candidates discovered by Sirna, together with low single-digit royalties for our products and single-digit royalties for Sirna products, in each case based on annual worldwide net sales, if any, by us and our related parties of any such products. The stock purchase agreement contains customary representations, warranties, and covenants of the parties thereto. Subject to customary closing conditions, including the expiration or early termination of the applicable pre-merger waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, the transaction is expected to close during the first quarter of 2014.
80
Table of Contents
In addition, in January 2014, we entered into a global, strategic collaboration with Genzyme to discover, develop and commercialize RNAi therapeutics as genetic medicines to treat orphan diseases. The 2014 Genzyme collaboration is governed by a master collaboration agreement, including the license terms appended thereto, which will become effective upon closing of the equity transaction, described below. Once effective, the master agreement will supersede and replace the 2012 Genzyme agreement, which original Genzyme agreement is described under the heading “Strategic Alliances” in our annual report on Form 10-K for the year ended December 31, 2012.
The 2014 Genzyme collaboration is structured as an exclusive relationship for the worldwide development and commercialization of RNAi therapeutics in the field of genetic medicines, which includes our current and future genetic medicine programs that reach Human POP by the end of 2019, subject to extension to the end of 2021 in various circumstances. We will retain product rights in North America and Western Europe, while Genzyme will obtain exclusive rights to develop and commercialize collaboration products in the Genzyme Territory, together with certain broader co-development/co-promote or worldwide rights for certain products. Genzyme’s rights are structured as an opt-in that is triggered upon achievement of Human POP. We will maintain development control for all programs prior to Genzyme’s opt-in and maintain development and commercialization control after Genzyme’s opt-in for all programs in our territory.
Upon the effective date of the 2014 Genzyme collaboration, Genzyme will opt-in to patisiran for the Genzyme Territory, and we will retain full product rights in North America and Western Europe. We and Genzyme have also agreed to expand our current collaboration on ALN-TTRsc, where we and Genzyme will co-develop and co-promote ALN-TTRsc in North America and Western Europe. We will maintain development and commercialization control with ALN-TTRsc and Genzyme will develop and commercialize the product in the Genzyme Territory.
In addition to its regional rights for our current and future genetic medicine programs in the Genzyme Territory, Genzyme will have the right to either (i) co-develop and co-promote ALN-AT3 for the treatment of hemophilia and other RBDs in our territory, with us maintaining development and commercialization control, or (ii) obtain a global license to ALN-AS1 for the treatment of hepatic porphyrias. Genzyme will exercise this selection right upon Human POP for the ALN-AT3 and ALN-AS1 programs. Finally, Genzyme will have the right for a global license to a single, future genetic medicine program that is not one of our currently defined genetic medicine programs. We will retain global rights to any RNAi therapeutic genetic medicine program that does not reach Human POP by the end of 2019, subject to certain limited exceptions. Under the terms of the master agreement, we will retain full rights to all current and future RNAi therapeutic programs outside of the field of genetic medicines, including the right to form new collaborations.
In consideration for the rights granted to Genzyme under the master agreement and pursuant to the terms of a stock purchase agreement, we agreed to sell to Genzyme 8,766,338 shares of our common stock and Genzyme agreed to pay to us $700.0 million in aggregate cash consideration. Following the closing of the stock purchase, Genzyme will beneficially own approximately 12% of our outstanding common stock. Subject to customary closing conditions, including the expiration or early termination of the applicable pre-merger waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, the stock purchase is expected to close during the first quarter of 2014.
A description of our 2014 Genzyme collaboration and the related stock purchase agreement is included in Part I, Item 1, “Strategic Alliances—Product Alliances – Genzyme.”
We have incurred significant losses since we commenced operations in 2002 and expect such losses to continue for the foreseeable future. At December 31, 2013, we had an accumulated deficit of $596.2 million. Historically, we have generated losses principally from costs associated with research and development activities, acquiring, filing and expanding intellectual property rights and general administrative costs. As a result of planned expenditures for research and development activities relating to our drug development programs, including the development and optimization of drug delivery technologies and clinical trial costs, extension of the capabilities of our technology platform, including through business initiatives, continued management and
81
Table of Contents
growth of our patent portfolio, collaborations and general corporate activities, we expect to incur additional operating losses for the foreseeable future. We anticipate that our operating results will fluctuate for the foreseeable future. Therefore, period-to-period comparisons should not be relied upon as predictive of the results in future periods.
Although we currently have programs focused on a number of therapeutic areas, we are unable to predict when, if ever, we will successfully develop or be able to commence sales of any product. To date, a substantial portion of our total net revenues has been derived from collaboration revenues from strategic alliances with Roche/Arrowhead, Takeda, Cubist and Novartis, and from the United States government in connection with our development of treatments for hemorrhagic fever viruses, including Ebola. We expect our sources of potential funding for the next several years to be derived primarily from new and existing strategic alliances, which may include license and other fees, funded research and development and milestone payments, and proceeds from the sale of equity or debt.
In February 2012, we sold an aggregate of 8,625,000 shares of our common stock through an underwritten public offering at a price to the public of $10.75 per share. As a result of this offering, we received aggregate net proceeds of approximately $86.8 million, after deducting underwriting discounts and commissions and other estimated offering expenses of approximately $5.9 million. In January 2013, we sold an aggregate of 9,200,000 shares of our common stock through an underwritten public offering at a price to the public of $20.13 per share. As a result of this offering, we received aggregate net proceeds of approximately $173.6 million, after deducting underwriting discounts and commissions and other estimated offering expenses of approximately $11.6 million.
Research and Development
Since our inception, we have focused on drug discovery and development programs. Research and development expenses represent a substantial percentage of our total operating expenses. Under our core product strategy, we expect to have six to seven genetic medicine product candidates in clinical development, including at least two programs in Phase 3 and five to six programs with human proof of concept, by the end of 2015. While focusing our efforts on our core product strategy, we also intend to continue to advance additional partner-based development programs through existing or future alliances. In addition, we continue to work internally and with third-party collaborators to develop and optimize new technologies to deliver our RNAi therapeutics both directly to specific sites of disease, and systemically by intravenous or subcutaneous administration.
There is a risk that any drug discovery or development program may not produce revenue for a variety of reasons, including the possibility that we will not be able to adequately demonstrate the safety and efficacy of the product candidate. Moreover, there are uncertainties specific to any new field of drug discovery, including RNAi. The successful development of any product candidate we develop is highly uncertain. Due to the numerous risks associated with developing drugs, we cannot reasonably estimate or know the nature, timing and estimated costs of the efforts necessary to complete the development of, or the period, if any, in which material net cash inflows will commence from, any potential product candidate. These risks include the uncertainty of:
| • | our ability to discover new product candidates; |
| • | our ability to progress product candidates into pre-clinical and clinical trials; |
| • | the scope, rate of progress and cost of our pre-clinical trials and other research and development activities, including those related to developing safe and effective ways of delivering siRNAs into cells and tissues; |
| • | the scope, rate of progress and cost of any clinical trials we commence; |
| • | clinical trial results; |
| • | the cost of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; |
| • | the terms, timing and success of any collaboration, licensing and other arrangements that we may establish; |
82
Table of Contents
| • | the cost, timing and success of regulatory filings and approvals or potential changes in regulations that govern our industry or the way in which they are interpreted or enforced; |
| • | the cost and timing of establishing sufficient sales, marketing and distribution capabilities; |
| • | the cost and timing of establishing sufficient clinical and commercial supplies for any product candidates and products that we may develop; |
| • | limits on our ability to research, develop, or manufacture our product candidates as a result of contractual obligations to third parties or intellectual property held by third parties; |
| • | the costs associated with legal activities, including litigation, arising in the course of our business activities and our ability to prevail in any such legal disputes; and |
| • | the effect of competing technological and market developments. |
Any failure to complete any stage of the development of any potential products in a timely manner could have a material adverse effect on our operations, financial position and liquidity. A discussion of some of the risks and uncertainties associated with completing our projects on schedule, or at all, and the potential consequences of failing to do so, are set forth in Part I, Item 1A of this annual report on Form 10-K under the heading “Risk Factors.”
Strategic Alliances
A significant component of our business plan is to enter into strategic alliances and collaborations with leading pharmaceutical and life sciences companies, academic institutions, research foundations and others, as appropriate, to gain access to funding, capabilities, technical resources and intellectual property to further our development efforts and to generate revenues. We also seek to form or advance new ventures and opportunities in areas outside our primary focus on RNAi therapeutics.
To generate revenues from our intellectual property rights, we also grant licenses to biotechnology companies under our InterfeRx program for the development and commercialization of RNAi therapeutics for specified targets in which we have no direct strategic interest. We also license key aspects of our intellectual property to companies active in the research products and services market, which includes the manufacture and sale of reagents. We expect our InterfeRx and research product licenses to generate modest near-term revenues that we can re-invest in the development of our proprietary RNAi therapeutics pipeline. At January 31, 2014, we had granted such licenses, on both an exclusive and non-exclusive basis, to approximately 20 companies.
Since delivery of RNAi therapeutics remains an important objective of our research activities, we also look to form collaboration and licensing arrangements with other companies and academic institutions to gain access to delivery technologies. For example, we have entered into agreements with Arrowhead, Tekmira, MIT, UBC and Acuitas, among others, to focus on various delivery strategies. We have also entered into license agreements with Isis, Max Planck Innovation, Tekmira, MIT, CRT and Whitehead, as well as a number of other entities, to obtain rights to intellectual property in the field of RNAi.
Finally, we have sought, and may seek in the future, funding for the development of our proprietary RNAi therapeutics pipeline from the government and foundations.
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with GAAP. The preparation of our consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses and disclosure of contingent liabilities in our consolidated financial statements. Actual results may differ from these estimates under different assumptions or conditions and could have a material impact on our reported results. While our significant accounting policies are more fully described in the Notes to our consolidated financial statements included elsewhere in this annual report on Form 10-K, we believe
83
Table of Contents
the following accounting policies to be the most critical in understanding the judgments and estimates we use in preparing our consolidated financial statements:
Revenue Recognition
Our business strategy includes entering into collaborative license and development agreements with with leading pharmaceutical and life sciences companies for the development and commercialization of our product candidates. We have entered into collaboration agreements with Novartis, Biogen Idec, Roche/Arrowhead, Takeda, Kyowa Hakko Kirin, Cubist, Ascletis, Monsanto, Genzyme and MDCO. The terms of the agreements typically include non-refundable license fees, funding of research and development, payments based upon achievement of clinical and pre-clinical development milestones, regulatory milestones, manufacturing services, sales milestones and royalties on product sales.
In January 2011, we adopted new authoritative guidance on revenue recognition for multiple element arrangements. The guidance, which applies to multiple element arrangements entered into or materially modified on or after January 1, 2011, amends the criteria for separating and allocating consideration in a multiple element arrangement by modifying the fair value requirements for revenue recognition and eliminating the use of the residual method. The fair value of deliverables under the arrangement may be derived using a “best estimate of selling price” if vendor specific objective evidence and third-party evidence is not available. Deliverables under the arrangement will be separate units of accounting provided that (i) a delivered item has value to the customer on a standalone basis and (ii) if the arrangement includes a general right of return relative to the delivered item, delivery or performance of the undelivered item is considered probable and substantially in the control of the vendor. The new guidance did not change the criteria for standalone value. As a biotechnology entity with unique and specialized delivered and undelivered performance obligations, we have been unable to demonstrate standalone value in our multiple element arrangements. For example, we applied the new rules to collaborations executed with Monsanto and Genzyme during 2012, but we were unable to demonstrate standalone value. In addition, we have not materially modified any of our multiple element arrangements. As such, we will continue to account for our other license and collaboration agreements under previously issued revenue recognition guidance for multiple element arrangements, as described below.
Non-refundable license fees are recognized as revenue upon delivery of the license only if we have a contractual right to receive such payment, the contract price is fixed or determinable, the collection of the resulting receivable is reasonably assured and we have no further performance obligations under the license agreement. Multiple element arrangements, such as license and development arrangements, are analyzed to determine whether the deliverables, which often include a license and performance obligations such as research and steering committee services, can be separated or whether they must be accounted for as a single unit of accounting. We recognize upfront license payments as revenue upon delivery of the license only if the license has standalone value and the fair value of the undelivered performance obligations, typically including research and/or steering committee services, can be determined. If the fair value of the undelivered performance obligations can be determined, such obligations would then be accounted for separately as performed. If the license is considered to either not have standalone value or have standalone value but the fair value of any of the undelivered performance obligations cannot be determined, the arrangement would then be accounted for as a single unit of accounting and the license payments and payments for performance obligations are recognized as revenue over the estimated period of when the performance obligations are performed.
Whenever we determine that an arrangement should be accounted for as a single unit of accounting, we must determine the period over which the performance obligations will be performed and revenue will be recognized. We recognize revenue using either a proportional performance or straight-line method. We recognize revenue using the proportional performance method when we can reasonably estimate the level of effort required to complete our performance obligations under an arrangement and such performance obligations are provided on a best-efforts basis. Direct labor hours or full-time equivalents are typically used as the measure of performance. The amount of revenue recognized under the proportional performance method is determined by multiplying the total payments under the contract, excluding royalties and payments contingent upon achievement of milestones, by the ratio of level of effort incurred to date to estimated total level of effort
84
Table of Contents
required to complete our performance obligations under the arrangement. Revenue is limited to the lesser of the cumulative amount of payments received or the cumulative amount of revenue earned, as determined using the proportional performance method, as of the period ending date.
If we cannot reasonably estimate the level of effort required to complete our performance obligations under an arrangement, we recognize revenue under the arrangement on a straight-line basis over the period we expect to complete our performance obligations. Revenue is limited to the lesser of the cumulative amount of payments received or the cumulative amount of revenue earned, as determined using the straight-line method, as of the period ending date.
Significant management judgment is required in determining the level of effort required under an arrangement and the period over which we are expected to complete our performance obligations under an arrangement. Steering committee services that are not inconsequential or perfunctory and that are determined to be performance obligations are combined with other research services or performance obligations required under an arrangement, if any, in determining the level of effort required in an arrangement and the period over which we expect to complete our aggregate performance obligations.
Many of our collaboration agreements entitle us to additional payments upon the achievement of performance-based milestones. These milestones are generally categorized into three types; development milestones which are generally based on the advancement of our pipeline and initiation of clinical trials, regulatory milestones which are generally based on the submission, filing or approval of regulatory applications such as an NDA in the United States, and commercialization milestones which are generally based on meeting specific thresholds of sales in certain geographic areas. If the achievement of a milestone is considered probable at the inception of the collaboration, the related milestone payment is included with other collaboration consideration, such as upfront fees and research funding, in our revenue model. Milestones that are tied to regulatory approval are not considered probable of being achieved until such approval is received. Milestones tied to counter-party performance are not included in our revenue model until the performance conditions are met.
We perform an assessment to determine whether a substantive milestone exists at the inception of our collaborative arrangements. In evaluating if a milestone is substantive, we consider whether uncertainty exists as to the achievement of the milestone event at the inception of the arrangement, the achievement of the milestone involves substantive effort and can only be achieved based in whole or part on the performance or the occurrence of a specific outcome resulting from our performance, the amount of the milestone payment appears reasonable either in relation to the effort expected to be expended or to the projected enhancement of the value of the delivered items, there is any future performance required to earn the milestone, and the consideration is reasonable relative to all deliverables and payment terms in the arrangement. When a substantive milestone is achieved, the accounting rules permit us to recognize revenue related to the milestone payment in its entirety.
To date, we have not recorded any substantive milestones under our collaborations because we have not identified any milestones that meet the required criteria listed above. We have deferred recognition of payments for achievement of non-substantive milestones and recognized revenue over the estimated period of performance applicable to each collaborative arrangement. As these milestones are achieved, we will recognize as revenue a portion of the milestone payment, which is equal to the percentage of the performance period completed, when the milestone is achieved, multiplied by the amount of the milestone payment, upon achievement of such milestone. We will recognize the remaining portion of the milestone payment over the remaining performance period under the proportional performance method or on a straight-line basis.
For revenue generating arrangements where we, as a vendor, provide consideration to a licensor or collaborator, as a customer, we apply the accounting standard that governs such transactions. This standard addresses the accounting for revenue arrangements where both the vendor and the customer make cash payments to each other for services and/or products. A payment to a customer is presumed to be a reduction of the selling price unless we receive an identifiable benefit for the payment and we can reasonably estimate the fair value of the benefit received. Payments to a customer that are deemed a reduction of selling price are recorded first as a reduction of revenue, to the extent of both cumulative revenue recorded to date and probable future revenues,
85
Table of Contents
which include any unamortized deferred revenue balances, under all arrangements with such customer, and then as an expense. Payments that are not deemed to be a reduction of selling price are recorded as an expense.
We evaluate our collaborative agreements for proper classification in our consolidated statements of comprehensive loss based on the nature of the underlying activity. Transactions between collaborators recorded in our consolidated statements of comprehensive loss are recorded on either a gross or net basis, depending on the characteristics of the collaborative relationship. We generally reflect amounts due under our collaborative agreements related to cost-sharing of development activities as a reduction of research and development expense.
Amounts received prior to satisfying the above revenue recognition criteria are recorded as deferred revenue in the accompanying consolidated balance sheets. Although we follow detailed guidelines in measuring revenue, certain judgments affect the application of our revenue policy. For example, in connection with our existing collaboration agreements, we have recorded on our balance sheet short-term and long-term deferred revenue based on our best estimate of when such revenue will be recognized. Short-term deferred revenue consists of amounts that are expected to be recognized as revenue in the next 12 months. Amounts that we expect will not be recognized prior to the next 12 months are classified as long-term deferred revenue. However, this estimate is based on our current operating plan and, if our operating plan should change in the future, we may recognize a different amount of deferred revenue over the next 12-month period.
The estimate of deferred revenue also reflects management’s estimate of the periods of our involvement in certain of our collaborations. Our performance obligations under these collaborations consist of participation on steering committees and the performance of other research and development services. In certain instances, the timing of satisfying these obligations can be difficult to estimate. Accordingly, our estimates may change in the future. Such changes to estimates would result in a change in revenue recognition amounts. If these estimates and judgments change over the course of these agreements, it may affect the timing and amount of revenue that we recognize and record in future periods. At December 31, 2013, we had short-term and long-term deferred revenue of $32.7 million and $93.4 million, respectively, related to our collaborations.
Genzyme. In January 2014, we entered into a global, strategic collaboration with Genzyme to discover, develop and commercialize RNAi therapeutics as genetic medicines to treat orphan diseases. The 2014 Genzyme collaboration is governed by a master collaboration agreement, including the license terms appended thereto, which will become effective upon closing of the equity transaction. A description of our 2014 Genzyme collaboration and the related stock purchase agreement is included in Part I, Item 1, “Strategic Alliances—Product Alliances – Genzyme.” Once effective, the master agreement will supersede and replace the 2012 Genzyme agreement. In consideration for the rights granted to Genzyme under the 2012 Genzyme agreement, Genzyme paid us an upfront cash payment of $22.5 million. We were also entitled to receive certain milestone payments under the 2012 Genzyme agreement. In the fourth quarter of 2013, we earned a milestone of $7.0 million based upon the completion of a successful patisiran Phase 2 clinical trial and a milestone of $4.0 million based upon the initiation of the Phase 3 clinical trial for patisiran. For purposes of potential future revenue recognition, we do not believe these milestones or any future milestones are substantive. Due to the uncertainty of pharmaceutical development and the high historical failure rates generally associated with drug development, we may not receive any further milestones or any royalty payments from Genzyme under the 2014 Genzyme collaboration.
Under the original 2012 Genzyme agreement, the parties agreed to collaborate in the development of licensed products, with Genzyme assuming primary responsibility for the development and commercialization of licensed products in the Genzyme territory and us retaining primary responsibility for the development and commercialization of licensed products in the rest of the world. The collaboration between Genzyme and us was governed by a joint steering committee comprised of an equal number of representatives from each party. Genzyme was responsible, at its expense, for all development activities under the development plan reasonably necessary for the regulatory approval and commercialization of an RNAi therapeutic for the treatment of ATTR in the Genzyme territory.
We determined that the deliverables under the original 2012 Genzyme agreement included the license, the joint steering committee and any additional TTR-specific RNAi therapeutic compounds that comprised the ALN-TTR program. We also determined that, pursuant to the accounting guidance governing revenue recognition on
86
Table of Contents
multiple element arrangements, the license and undelivered joint steering committee and any additional TTR-specific RNAi therapeutic compounds did not have standalone value due to the specialized nature of the services to be provided by us. In addition, while Genzyme had the ability to grant sublicenses, it could not sublicense all or substantially all of its rights under the 2012 Genzyme agreement. The uniqueness of our services and the limited sublicense right are indicators that standalone value is not present in the arrangement. Therefore the deliverables are not separable and, accordingly, the license and undelivered services were treated as a single unit of accounting. We were unable to reasonably estimate our period of performance under the 2012 Genzyme agreement, as we were unable to estimate the timeline of our deliverables related to the option granted to Genzyme for any additional compounds. Through December 31, 2013, we deferred all revenue under the 2012 Genzyme agreement. At December 31, 2013, deferred revenue under the 2012 Genzyme agreement was $33.5 million. We expect that the $33.5 million received under the 2012 Genzyme agreement, which is currently included in deferred revenue, will be accounted for in conjunction with the 2014 Genzyme collaboration.
The Medicines Company. In February 2013, we and MDCO entered into a license and collaboration agreement pursuant to which we granted to MDCO an exclusive, worldwide license to develop, manufacture and commercialize RNAi therapeutics targeting PCSK9, including ALN-PCS Licensed Products. ALN-PCS02 is an intravenously administered RNAi therapeutic for we completed a Phase 1 clinical trial, and ALN-PCSsc is a subcutaneously administered RNAi therapeutic currently in pre-clinical development.
In consideration for the rights granted to MDCO under the MDCO agreement, MDCO paid us an upfront cash payment of $25.0 million. Upon achievement of certain milestones, we will be entitled to receive milestone payments, up to an aggregate of $180.0 million, including up to $30.0 million in specified development milestones, $50.0 million in specified regulatory milestones and $100.0 million in specified commercialization milestones. In addition, we will be entitled to royalties ranging from the low- to high- teens based on annual worldwide net sales, if any, of ALN-PCS Licensed Products by MDCO, its affiliates and sublicensees, subject to reduction under specified circumstances. We could potentially earn the next development milestone payment of $10.0 million under the MDCO agreement based upon the initiation of an ALN-PCSsc Phase 1 clinical trial. For purposes of potential future revenue recognition, we do not believe this milestone or any future milestones are substantive. Due to the uncertainty of pharmaceutical development and the high historical failure rates generally associated with drug development, we may not receive any milestone or royalty payments from MDCO.
Under the MDCO agreement, the parties are collaborating in the further development of ALN-PCS Licensed Products. we retain responsibility for the development of ALN-PCS Licensed Products until Phase 1 Completion (as defined in the MDCO agreement) at our cost, up to an agreed upon initial development cost cap. MDCO will assume all other responsibility for the development and commercialization of ALN-PCS Licensed Products, at its sole cost. Initially the collaboration included the development of both ALN-PCS02 and ALN-PCSsc in parallel. In October 2013, the parties announced the selection of ALN-PCSsc for ongoing development, in accordance with the terms of the MDCO agreement. The collaboration between us and MDCO is governed by a joint steering committee that is comprised of an equal number of representatives from each party. We are solely responsible for obtaining supply of finished product reasonably required for the conduct of its obligations through Phase 1 Completion, and supplying MDCO with finished product reasonably required for the first Phase 2 clinical trial of an ALN-PCS Licensed Product conducted by MDCO, at our expense, provided such costs do not exceed the development costs cap, subject to certain exceptions. After such time, MDCO will have the sole right and responsibility to manufacture and supply ALN-PCS Licensed Product for development and commercialization under the MDCO development plan, subject to the terms of the MDCO agreement.
We have determined that the significant deliverables under the MDCO agreement include the license, the joint steering committee, technology transfer obligations, development activities through Phase 1 Completion and supply of product for a Phase 2 clinical trial. We also determined that, pursuant to the accounting guidance governing revenue recognition on multiple element arrangements, the license and collective undelivered activities and services do not have standalone value due to the specialized nature of the activities and services to be provided by us. In addition, while MDCO has the ability to grant sublicenses, it must receive our prior written consent to sublicense all or substantially all of its rights. The uniqueness of our services and the limited sublicense right are indicators that standalone value is not present in the arrangement. Therefore the deliverables
87
Table of Contents
are not separable and, accordingly, the license and undelivered services are being treated as a single unit of accounting. When multiple deliverables are accounted for as a single unit of accounting, we base our revenue recognition pattern on the final deliverable. Under the MDCO agreement, all deliverables are expected to be completed within five years. We are recognizing revenue under the MDCO agreement on a straight-line basis over five years. We are not utilizing a proportional performance model since we are unable to reasonably estimate the level of effort to fulfill these obligations, primarily because the effort required under the development activities is largely unknown.
The initial upfront payment of $25.0 million from MDCO was initially recorded as deferred revenue. As future milestones are achieved, if any, we will recognize as revenue a portion of the milestone payment equal to the percentage of the performance period completed when the milestone is achieved, multiplied by the amount of the milestone payment. At December 31, 2013, deferred revenue under the MDCO agreement was $20.5 million.
Monsanto. In consideration for the rights granted to Monsanto under the Monsanto agreement, Monsanto paid us $29.2 million in upfront cash payments. Monsanto is also required to make near-term milestone payments to us upon the achievement of specified technology transfer and patent-related milestones. We are also entitled to receive additional funding for collaborative research efforts. In the aggregate, we can earn up to $5.0 million in potential future milestone payments and research funding under the Monsanto alliance. In December 2012, we received $1.5 million of the $5.0 million in potential milestone payments from Monsanto based upon the achievement of a specified patent-related event. In August 2013, we received an additional milestone payment of $2.5 million based upon the completion of technology transfer activities. In addition, Monsanto is required to pay to us a percentage of specified fees from certain sublicense agreements Monsanto may enter into that include access to our intellectual property, as well as low single-digit royalty payments on worldwide, net sales by Monsanto, its affiliates and sublicensees of certain licensed products, as defined in the Monsanto agreement, if any. We could potentially earn the next and final milestone payment of $1.0 million under the Monsanto agreement in connection with the collaborative research efforts contemplated under the Monsanto agreement. For purposes of potential future revenue recognition, we do not believe this milestone or any future milestones are substantive. Due to the uncertainty of the application of RNAi technology in the field of agriculture, we may not receive any additional milestone payments or any royalty payments from Monsanto.
Under the terms of the Monsanto agreement, in the event that during the exclusivity period Monsanto loses certain patent rights, and such loss has a material adverse effect on the licensed products, then we would be required to pay Monsanto up to $5.0 million as liquidated damages, and Monsanto’s royalty obligations to us under the Monsanto agreement would be reduced or, under certain circumstances, terminated. We have the right to cure any such loss of patent rights under the Monsanto agreement.
We have determined that the significant deliverables under the Monsanto agreement include the license, the technology transfer activities and the services that we will be obligated to perform under the Monsanto discovery collaboration. We have also determined that, pursuant to the accounting guidance governing revenue recognition on multiple element arrangements, the license and undelivered technical transfer activities and Monsanto discovery collaboration services do not have standalone value due to the specialized nature of the services to be provided by us. In addition, while Monsanto has the ability to grant sublicenses, it cannot grant access to certain of our proprietary technology. The uniqueness of our services and the limited sublicense right are indicators that standalone value is not present in the arrangement. Therefore the deliverables are not separable and, accordingly, the license and undelivered technical transfer activities and Monsanto discovery collaboration services are being treated as a single unit of accounting. When multiple deliverables are accounted for as a single unit of accounting, we base our revenue recognition model on the final deliverable. Under the Monsanto agreement, the last deliverable to be completed is the Monsanto discovery collaboration, which must be completed within five years. We are recognizing revenue under the Monsanto agreement on a straight-line basis over five years. We are not utilizing a proportional performance model since we are unable to reasonably estimate the level of effort to fulfill these obligations, primarily because the effort required under the Monsanto discovery collaboration is largely unknown. At December 31, 2013, deferred revenue under the Monsanto agreement was $25.6 million.
Kyowa Hakko Kirin. Under the terms of the Kyowa Hakko Kirin agreement, in June 2008, Kyowa Hakko Kirin paid us an upfront cash payment of $15.0 million. In addition, Kyowa Hakko Kirin is required to
88
Table of Contents
make payments to us upon achievement of specified development and sales milestones totaling up to $78.0 million, and double-digit royalty payments based on annual net sales, if any, of RNAi therapeutics for the treatment of RSV by Kyowa Hakko Kirin, its affiliates and sublicenses in the licensed territory. Due to the uncertainty of pharmaceutical development and the high historical failure rates generally associated with drug development, we may not receive any milestone or royalty payments from Kyowa Hakko Kirin.
Our collaboration with Kyowa Hakko Kirin is governed by a joint steering committee that is comprised of an equal number of representatives from each party. We are responsible for supply of the product to Kyowa Hakko Kirin under a supply agreement unless Kyowa Hakko Kirin elects, prior to the first commercial sale of the product in the licensed territory, to manufacture the product itself or arrange for a third party to manufacture the product.
We have determined that the deliverables under the Kyowa Hakko Kirin agreement include the license, the joint steering committee, the manufacturing services and any additional RSV-specific RNAi therapeutic compounds that comprise the ALN-RSV program. We have determined that, pursuant to the accounting guidance governing revenue recognition on multiple element arrangements, the individual deliverables are not separable and, accordingly, must be accounted for as a single unit of accounting. We are currently unable to reasonably estimate our period of performance under the Kyowa Hakko Kirin agreement, as we are unable to estimate the timeline of our deliverables related to the fixed-price option granted to Kyowa Hakko Kirin for any additional compounds. We are deferring all revenue under the Kyowa Hakko Kirin agreement until we are able to reasonably estimate our period of performance. We will continue to reassess whether we can reasonably estimate the period of performance to fulfill our obligations under the Kyowa Hakko Kirin agreement. At December 31, 2013, deferred revenue under the Kyowa Hakko Kirin agreement was $15.5 million.
Cubist. In January 2009, we entered into a license and collaboration agreement with Cubist to develop and commercialize therapeutic products based on certain of our RNAi technology for the treatment of RSV infection. Licensed products initially included ALN-RSV01, as well as several other second-generation RNAi-based RSV inhibitors. In November 2009, we and Cubist entered into an amendment to our license and collaboration agreement, which provided that we and Cubist would focus our collaboration and joint development efforts on ALN-RSV02, a second-generation compound, intended for use in pediatric patients. In December 2010, we and Cubist jointly made a portfolio decision to put the development of ALN-RSV02 on hold. Pursuant to the terms of the amendment, we continued to develop ALN-RSV01 for adult transplant patients at our sole discretion and expense and Cubist had the right to opt into collaborating with us on ALN-RSV01, subject to specified conditions. In February 2013, Cubist notified us that it would not exercise its opt-in right for ALN-RSV01. In light of this determination, we and Cubist mutually agreed to terminate the license and collaboration agreement. As of the termination date, the parties have no further rights and obligations under the license and collaboration agreement, notwithstanding anything to the contrary in the agreement, as amended.
In consideration for the rights granted to Cubist under the Cubist Agreement, in January 2009, Cubist paid us an upfront cash payment of $20.0 million. Under the terms of the Cubist Agreement, we and Cubist shared responsibility for developing licensed products in North America and each was responsible for one-half of the related development costs, subject to the terms of the Amendment. Our collaboration with Cubist for the development of licensed products in North America was governed by a joint steering committee comprised of an equal number of representatives from each party. We determined that the deliverables under the Cubist agreement included the licenses, technology transfer related to the ALN-RSV program, the joint steering committee and the development and manufacturing services that we were obligated to perform during the development period. We also determined that, pursuant to the accounting guidance governing revenue recognition on multiple element arrangements, the licenses and undelivered services were not separable and, accordingly, the licenses and services were treated as a single unit of accounting. Under the Cubist agreement, the last element expected to be delivered was the development and manufacturing services, which had an expected life of approximately eight years. We were recognizing the upfront payment of $20.0 million on a straight-line basis over approximately eight years because we were unable to reasonably estimate the level of effort to fulfill our performance obligations, and therefore, could not utilize a proportional performance model. As a result of the termination of the Cubist agreement in February 2013 and the end of our performance obligations thereunder, we recognized the remaining deferred revenue of $9.7 million during 2013.
89
Table of Contents
Takeda. In consideration for the rights granted to Takeda under the Takeda agreement, Takeda paid us an upfront payment of $100.0 million in June 2008 and agreed to pay us an additional $50.0 million upon achievement of specified technology transfer milestones. Of this $50.0 million, $20.0 million was paid in October 2008, $20.0 million was paid in March 2010 and $10.0 million was paid in March 2011. If Takeda elects to expand its license to additional therapeutic areas, Takeda will be required to pay us $50.0 million for each additional field selected, if any. In addition, for each RNAi therapeutic product developed by Takeda, its affiliates and sublicensees, we are entitled to receive specified development, regulatory and commercialization milestone payments, totaling up to $171.0 million per product, together with up to a double-digit percentage royalty payment based on worldwide annual net sales, if any. The potential future milestone payments per product include up to $26.0 million for the achievement of specified development milestones, up to $40.0 million for the achievement of specified regulatory milestones and up to $105.0 million for the achievement of specified commercialization milestones. We could potentially earn the next milestone payment of $2.0 million under the Takeda agreement based upon the achievement of a specified pre-clinical event by Takeda for an RNAi therapeutic product. For purposes of potential future revenue recognition, we do not believe this milestone or any future milestones are substantive. Due to the uncertainty of pharmaceutical development and the high historical failure rates generally associated with drug development, we may not receive any additional milestone payments or any royalty payments from Takeda.
Pursuant to the Takeda agreement, we and Takeda have also agreed to collaborate on the research of RNAi therapeutics directed to one or two disease targets agreed to by the parties, subject to our existing contractual obligations with third parties. The collaboration is governed by a JTTC, a JRCC and a JDCC, each of which is comprised of an equal number of representatives from each party.
We have determined that the deliverables under the Takeda agreement include the license, the joint committees (the JTTC, JRCC and JDCC), the technology transfer activities and the services that we will be obligated to perform under the research collaboration with Takeda. We also have determined that, pursuant to the accounting guidance governing revenue recognition on multiple element arrangements, the license and undelivered services (i.e., the joint committees and the research collaboration) are not separable and, accordingly, the license and services are being treated as a single unit of accounting. Under the Takeda agreement, the last elements to be delivered are the JDCC and JTTC services, each of which has a life of no more than seven years. We are recognizing the upfront payment of $100.0 million and the technology transfer milestones of $50.0 million, the receipt of which we believed was probable at the commencement of the collaboration, on a straight-line basis over seven years because we are unable to reasonably estimate the level of effort to fulfill these obligations, primarily because the effort required under the research collaboration is largely unknown, and therefore, cannot utilize a proportional performance model. As future milestones are achieved, we will recognize as revenue a portion of the milestone payment equal to the percentage of the performance period completed when the milestone is achieved, multiplied by the amount of the milestone payment. At December 31, 2013, deferred revenue under the Takeda agreement was $30.8 million.
Roche/Arrowhead. We received aggregate proceeds from Roche of $331.0 million in August 2007, of which $278.2 million was recorded as deferred revenue in connection with this alliance. We and Roche established a discovery collaboration in October 2009, pursuant to the terms of the Roche license and collaboration agreement and subject to our existing contractual obligations to third parties. In November 2010, Roche announced the discontinuation of certain activities in research and early development, including its RNAi research efforts. In October 2011, Arrowhead announced its acquisition of RNA therapeutics assets from Roche, including the license and collaboration agreement. As a result of this acquisition, Arrowhead owns all of the rights and obligations of Roche under that agreement. The license is initially limited to four therapeutic areas, and may be expanded to include additional therapeutic areas upon payment to us by Arrowhead of an additional $50.0 million for each additional therapeutic area, if any. In exchange for our contributions under the collaboration agreement, for each RNAi therapeutic product developed by Arrowhead, its affiliates or sublicensees under the collaboration agreement, we are entitled to receive milestone payments upon achievement of specified development, regulatory and commercialization events, totaling up to an aggregate of $100.0 million per therapeutic target, together with a single-digit percentage royalty payment based on worldwide annual net
90
Table of Contents
sales, if any. The potential future milestone payments for each therapeutic target include up to $17.5 million for the achievement of specified development milestones, up to $62.5 million for the achievement of specified regulatory milestones and up to $20.0 million for the achievement of specified commercialization milestones. We could potentially earn the next development milestone payment of $1.0 million under the license and collaboration agreement based upon the initiation of the first Phase 1 clinical trial by Arrowhead for an RNAi therapeutic product covered under the license and collaboration agreement. For purposes of potential future revenue recognition, we do not believe this milestone or any future milestones are substantive. Due to the uncertainty of pharmaceutical development and the high historical failure rates generally associated with drug development, we may not receive any milestone or royalty payments from Arrowhead.
We determined that the deliverables under these agreements included the license, the Alnylam Europe assets and employees, the steering committees (joint steering committee and future technology committee) and the services under the discovery collaboration. We also determined that, pursuant to the accounting guidance governing revenue recognition on multiple element arrangements, the license and assets of Alnylam Europe were not separable from the undelivered services (i.e., the steering committees and discovery collaboration) and, accordingly, the license and the services were treated as a single unit of accounting. When multiple deliverables are accounted for as a single unit of accounting, we base our revenue recognition pattern on the final deliverable. Under the Arrowhead alliance, the steering committee services and the discovery collaboration services were the final deliverables and all such services ended, contractually, in August 2012, five years from the effective date of the license and collaboration agreement. We recognized revenue related to these agreements on a straight-line basis over five years because we could not reasonably estimate the total level of effort required to complete our service obligations under the license and collaboration agreement, and therefore, could not utilize a proportional performance model. At December 31, 2013, there was no remaining deferred revenue under the license and collaboration agreement as we recognized all remaining Roche/Arrowhead revenue during the quarter ended September 30, 2012. We will recognize future milestones under the license and collaboration agreement, if any, when such milestones are achieved.
Accounting for Income Taxes
We recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained upon examination by the taxing authorities, based on the technical merits of the tax position. The tax benefits recognized in our financial statements from such a position are measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate resolution. Our policy is to accrue interest and penalties related to unrecognized tax positions in income tax expense. As of December 31, 2013, we have not recorded significant interest and penalty expense related to uncertain tax positions.
We operate in the United States, as well as in several countries outside of the United States, where our income tax returns are subject to audit and adjustment by local tax authorities. The nature of the uncertain tax positions is often very complex and subject to change, and the amounts at issue can be substantial. We develop our cumulative probability assessment of the measurement of uncertain tax positions using internal experience, judgment and assistance from professional advisors. We refine estimates as we become aware of additional information. Any outcome upon settlement that differs from our current estimate may result in additional tax expense in future periods. At December 31, 2013, we had no unrecognized tax benefits that, if recognized, would favorably impact our effective income tax rate in future periods.
We recognize income taxes when transactions are recorded in our consolidated statements of comprehensive loss, with deferred taxes provided for items that are recognized in different periods for financial statement and tax reporting purposes. We record a valuation allowance to reduce the deferred tax assets to the amount that is more likely than not to be realized. In addition, we estimate our exposures relating to uncertain tax positions and establish reserves for such exposures when they become probable and reasonably estimable.
For the years ended December 31, 2013 and 2012, we recorded a benefit from income taxes of $2.7 million and $10.6 million, respectively. For the year ended December 31, 2011, we recorded a provision for income taxes of zero. The benefit of $2.7 million and $10.6 million for the years ended December 31, 2013
91
Table of Contents
and 2012, respectively, was due to the recognition of corresponding income tax expense, recorded in other comprehensive income, associated with the increase in the value of our investment in Regulus that we carried at fair market value during the same period. At December 31, 2013, we had a valuation allowance against our net deferred tax assets to the extent it is more likely than not that the assets will not be realized. At December 31, 2013, we had federal and state net operating loss carryforwards of $372.4 million and $441.6 million, respectively, to reduce future taxable income that will expire at various dates through 2033. At December 31, 2013, we had federal and state research and development credit carryforwards of $20.8 million and $6.8 million, respectively, available to reduce future tax liabilities that expire at various dates through 2033. At December 31, 2013, we had foreign tax credit carryforwards of $3.2 million available to reduce future tax liabilities that expire in 2017. At December 31, 2013, we had alternative minimum tax credits of $0.8 million available to reduce future regular tax liabilities to the extent such regular tax less other non-refundable credits exceeds the tentative minimum tax. We have a valuation allowance against the net operating loss and credit deferred tax assets as it is unlikely that we will realize these assets. Ownership changes, as defined in the Internal Revenue Code, including those resulting from the issuance of common stock in connection with our public offerings, may limit the amount of net operating loss and tax credit carryforwards that can be utilized to offset future taxable income or tax liability. The amount of the limitation is determined in accordance with Section 382 of the Internal Revenue Code. We have determined that based on our value, in the event there was an annual limitation under Section 382, all net operating loss and tax credit carryforwards would still be available to offset taxable income.
Accounting for Stock-Based Compensation
We have stock incentive plans and an employee stock purchase plan under which we grant equity instruments. We account for all stock-based awards granted to employees at their fair value and generally recognize compensation expense over the vesting period of the award. Determining the amount of stock-based compensation to be recorded requires us to develop estimates of fair values of stock options as of the grant date. We calculate the grant date fair values using the Black-Scholes valuation model. Our expected stock price volatility assumption is based on a combination of the historical and implied volatility of our publicly traded stock.
For stock option awards granted during the year ended December 31, 2013, we used a weighted-average expected stock-price volatility assumption of 55%. Our expected life assumption is based on our historical data. Our weighted average expected term was 5.6 years for the year ended December 31, 2013. We utilize a dividend yield of zero based on the fact that we have never paid cash dividends and currently have no intention to pay cash dividends. The risk-free interest rate used for each grant is based on the U.S. Treasury yield curve in effect at the time of grant for instruments with a similar expected life.
For stock-based awards granted to non-employees, we generally recognize compensation expense over the vesting period of the award, which is generally the period during which services are rendered by such non-employees. At the end of each financial reporting period prior to vesting, we re-measure the value of these stock-based awards (as calculated using the Black-Scholes option-pricing model) using the then current fair value of our common stock. Stock options granted to non-employees, other than members of our board of directors and scientific advisory board members, generally vest over the service period.
The fair value of restricted stock awards granted to employees is based upon the quoted closing market price per share on the date of grant, adjusted for assumed forfeitures. Expense is recognized over the vesting period, commencing when we determine that it is probable that the awards will vest.
For performance-based stock awards, the value of the awards is measured when we determine the achievement of such performance conditions is deemed probable. This determination requires significant judgment by management. Expense is recognized over the vesting period, commencing when we determine that it is probable that the awards will vest.
At December 31, 2013, the estimated fair value of unvested employee stock options and restricted stock awards was $32.1 million, net of estimated forfeitures. We will recognize this amount over the weighted average remaining vesting period of approximately three years for these awards. Stock-based employee compensation expense was $17.5 million for the year ended December 31, 2013. However, we cannot currently predict the total
92
Table of Contents
amount of stock-based compensation expense to be recognized in any future period because such amounts will depend on levels of stock-based payments granted in the future as well as the portion of the awards that actually vest. The stock compensation accounting standard requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The term “forfeitures” is distinct from “cancellations” or “expirations” and represents only the unvested portion of the surrendered stock option. We have applied an annual forfeiture rate of 8.2% to all unvested employee stock options and restricted stock at December 31, 2013 based on an analysis of its historical forfeitures. Ultimately, the actual expense recognized over the vesting period will only be for those shares that vest.
Estimated Liability for Development Costs
We record accrued liabilities related to expenses for which service providers have not yet billed us with respect to products or services that we have received, specifically related to ongoing pre-clinical studies and clinical trials. These costs primarily relate to third-party clinical management costs, laboratory and analysis costs, toxicology studies and investigator fees. We have multiple product candidates in concurrent pre-clinical studies and clinical trials at multiple clinical sites throughout the world. In order to ensure that we have adequately provided for ongoing pre-clinical and clinical development costs during the period in which we incur such costs, we maintain an accrual to cover these expenses. We update our estimate for this accrual on at least a quarterly basis. The assessment of these costs is a subjective process that requires judgment. Upon settlement, these costs may differ materially from the amounts accrued in our consolidated financial statements. Our historical accrual estimates have not been materially different from our actual costs.
Results of Operations
The following data summarizes the results of our operations for the periods indicated, in thousands:
| Year Ended December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| Net revenues from collaborators |
$ | 47,167 | $ | 66,725 | $ | 82,757 | ||||||
| Operating expenses |
140,109 | 196,181 | 137,575 | |||||||||
| Loss from operations |
(92,942 | ) | (129,456 | ) | (54,818 | ) | ||||||
| Net loss |
$ | (89,225 | ) | $ | (106,014 | ) | $ | (57,649 | ) | |||
The increase in operating expenses for the year ended December 31, 2012 resulted from a $65.0 million charge to operating expenses in connection with the restructuring of our license agreement with Tekmira in November 2012, which is described below under the heading “Restructuring of Tekmira licensing agreement.”
We expect to record a significant charge to operating expenses in connection with our acquisition of Sirna, which we expect to close in the first quarter of 2014. The expected total amount of this expense will be based on a cash payment of $25.0 million and a charge for the value of the 2,520,044 shares of common stock we issue to Merck, multiplied by the closing price of our common stock at the closing of the transaction.
Discussion of Results of Operations for 2013 and 2012
Net revenues from collaborators
We generate revenues through research and development collaborations. The following table summarizes our total consolidated net revenues from collaborators, for the periods indicated, in thousands:
| Year Ended December 31, |
||||||||
| 2013 | 2012 | |||||||
| Takeda |
$ | 21,973 | $ | 21,973 | ||||
| Cubist |
9,721 | 2,777 | ||||||
| Monsanto |
5,640 | 1,954 | ||||||
| MDCO |
4,604 | — | ||||||
| Roche/Arrowhead |
— | 37,318 | ||||||
| Other |
5,229 | 2,703 | ||||||
|
|
|
|
|
|||||
| Total net revenues from collaborators |
$ | 47,167 | $ | 66,725 | ||||
|
|
|
|
|
|||||
93
Table of Contents
Net revenues from collaborators decreased for the year ended December 31, 2013 as compared to the year ended December 31, 2012 due primarily to the completion of our remaining performance obligations under the Roche/Arrowhead alliance in August 2012. This decrease was partially offset by recognition of the remaining deferred revenue under the Cubist agreement of $9.7 million due to the termination of the Cubist agreement in February 2013 and the end of our performance obligations thereunder. In addition, the decrease was partially offset by revenues recorded under our agreements with Monsanto and MDCO.
We expect net revenues from collaborators to decrease during 2014 on a comparative basis due primarily to the termination of the Cubist agreement in February 2013 and the end of our performance obligations thereunder.
We also had $126.1 million of deferred revenue at December 31, 2013, which consists of payments we have received from collaborators, primarily Takeda, Kyowa Hakko Kirin, Monsanto, MDCO and Genzyme, but have not yet recognized pursuant to our revenue recognition policies.
For the foreseeable future, we expect our revenues to continue to be derived primarily from our alliances with Takeda, Monsanto, MDCO, Genzyme and other strategic alliances, as well as new collaborations and licensing activities.
Operating Expenses
The following table summarizes our operating expenses for the periods indicated, in thousands and as a percentage of total operating expenses, together with the changes, in thousands and percentages:
| 2013 | % of Total Operating Expenses |
2012 | % of Total Operating Expenses |
Increase (Decrease) |
||||||||||||||||||||
| $ | % | |||||||||||||||||||||||
| Research and development |
$ | 112,957 | 81 | % | $ | 86,569 | 44 | % | $ | 26,388 | 30 | % | ||||||||||||
| General and administrative |
27,152 | 19 | % | 44,612 | 23 | % | (17,460 | ) | (39 | )% | ||||||||||||||
| Restructuring of Tekmira license agreement |
— | — | 65,000 | 33 | % | (65,000 | ) | (100 | )% | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total operating expenses |
$ | 140,109 | 100 | % | $ | 196,181 | 100 | % | $ | (56,072 | ) | (29 | )% | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
Research and development. The following table summarizes the components of our research and development expenses for the periods indicated, in thousands and as a percentage of total research and development expenses, together with the changes, in thousands and percentages:
| 2013 | % of Expense Category |
2012 | % of Expense Category |
Increase (Decrease) |
||||||||||||||||||||
| $ | % | |||||||||||||||||||||||
| Research and development |
||||||||||||||||||||||||
| Clinical trial and manufacturing |
$ | 27,415 | 24 | % | $ | 15,930 | 18 | % | $ | 11,485 | 72 | % | ||||||||||||
| Compensation and related |
25,518 | 23 | % | 20,438 | 24 | % | 5,080 | 25 | % | |||||||||||||||
| External services |
15,320 | 14 | % | 13,743 | 16 | % | 1,577 | 11 | % | |||||||||||||||
| Non-cash stock-based compensation |
14,369 | 13 | % | 8,041 | 9 | % | 6,328 | 79 | % | |||||||||||||||
| Facilities-related |
14,299 | 13 | % | 12,647 | 15 | % | 1,652 | 13 | % | |||||||||||||||
| License fees |
7,968 | 7 | % | 5,693 | 7 | % | 2,275 | 40 | % | |||||||||||||||
| Lab supplies and materials |
4,983 | 4 | % | 4,422 | 5 | % | 561 | 13 | % | |||||||||||||||
| Restructuring |
— | — | 2,817 | 3 | % | (2,817 | ) | (100 | )% | |||||||||||||||
| Other |
3,085 | 2 | % | 2,838 | 3 | % | 247 | 9 | % | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total research and development expenses |
$ | 112,957 | 100 | % | $ | 86,569 | 100 | % | $ | 26,388 | 30 | % | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
94
Table of Contents
Research and development expenses increased during the year ended December 31, 2013 as compared to the year ended December 31, 2012 due primarily to higher clinical trial and manufacturing costs related to our patisiran, ALN-TTRsc and ALN-AT3 programs. In addition, stock-based compensation increased during the year ended December 31, 2013 as compared to the year ended December 31, 2012 due primarily to an increase in the Black-Scholes value and vesting of stock options granted in 2013. Compensation and related expenses also increased due primarily to the increase in workforce during the year. License fees increased for the year ended December 31, 2013 as compared to the year ended December 31, 2012 due primarily to payments to certain entities for the advancement of our patisiran program into a Phase 3 clinical trial during the fourth quarter, including a $5.0 million milestone paid to TPC. Partially offsetting these increases was a one-time charge related to our 2012 restructuring, including employee severance, benefits and other related costs.
We expect to continue to devote a substantial portion of our resources to research and development expenses as we continue development of our and our collaborators’ product candidates and focus on continuing to develop and optimize drug delivery-related technologies. We expect that research and development expenses will increase significantly in 2014 as we continue to develop our pipeline and advance our product candidates into clinical trials.
A significant portion of our research and development costs are not tracked by project as they benefit multiple projects or our technology platform and because our most-advanced programs are not yet in late-stage clinical development. However, our collaboration agreements contain cost-sharing arrangements pursuant to which certain costs incurred under the project are reimbursed. Costs reimbursed under the agreements typically include certain direct external costs and a negotiated full-time equivalent labor rate for the actual time worked on the project. In addition, we have been reimbursed under government contracts for certain allowable costs including direct internal and external costs. As a result, although a significant portion of our research and development expenses are not tracked on a project-by-project basis, we do track direct external costs attributable to, and the actual time our employees worked on, our collaborations and government contracts.
General and administrative. The following table summarizes the components of our general and administrative expenses for the periods indicated, in thousands and as a percentage of total general and administrative expenses, together with the changes, in thousands and percentages:
| 2013 | % of Expense Category |
2012 | % of Expense Category |
Increase (Decrease) |
||||||||||||||||||||
| $ | % | |||||||||||||||||||||||
| General and administrative |
||||||||||||||||||||||||
| Consulting and professional services |
$ | 9,706 | 36 | % | $ | 28,949 | 65 | % | $ | (19,243 | ) | (66 | )% | |||||||||||
| Compensation and related |
7,102 | 26 | % | 6,384 | 14 | % | 718 | 11 | % | |||||||||||||||
| Non-cash stock-based compensation |
6,334 | 23 | % | 4,319 | 10 | % | 2,015 | 47 | % | |||||||||||||||
| Facilities-related |
1,450 | 5 | % | 1,648 | 4 | % | (198 | ) | (12 | )% | ||||||||||||||
| Restructuring |
— | — | 890 | 2 | % | (890 | ) | (100 | )% | |||||||||||||||
| Other |
2,560 | 10 | % | 2,422 | 5 | % | 138 | 6 | % | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total general and administrative expenses |
$ | 27,152 | 100 | % | $ | 44,612 | 100 | % | $ | (17,460 | ) | (39 | )% | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
The decrease in general and administrative expenses during the year ended December 31, 2013 as compared to the year ended December 31, 2012 was due primarily to a decrease in consulting and professional services expenses related to business activities, primarily legal activities related to litigation with Tekmira that was resolved in November 2012. Also included in the year ended December 31, 2012 was a one-time charge related to our January 2012 strategic corporate restructuring, including employee severance, benefits and other related costs.
We expect that general and administrative expenses will increase slightly in 2014 as we continue to grow our operations.
95
Table of Contents
Other income (expense)
We incurred zero in equity in loss of joint venture (Regulus Therapeutics Inc.) for the year ended December 31, 2013, as compared to $4.5 million for the year ended December 31, 2012 related to our share of the net losses incurred by Regulus. In October 2012, Regulus completed an initial public offering, resulting in our ownership percentage decreasing from approximately 44% to 17% of Regulus’ outstanding common stock. As of December 31, 2013, our current ownership percentage had decreased to approximately 15%.
Interest income was $1.1 million in 2013 as compared to $1.0 million in 2012. The increase in 2013 was due primarily to higher average cash, cash equivalent and fixed income marketable securities balances.
Our benefit from income taxes was $2.7 million in 2013 as compared to $10.6 million for 2012. The change was due to our recognition of the corresponding income tax benefit associated with the increase in the value of our investment in Regulus that we carried at fair market value during the same respective period.
Discussion of Results of Operations for 2012 and 2011
Net revenues from collaborators
We generate revenues through research and development collaborations. The following table summarizes our total consolidated net revenues from collaborators, for the periods indicated, in thousands:
| Year Ended December 31, |
||||||||
| 2012 | 2011 | |||||||
| Roche/Arrowhead |
$ | 37,318 | $ | 55,978 | ||||
| Takeda |
21,973 | 22,248 | ||||||
| Monsanto |
1,954 | — | ||||||
| Other |
5,480 | 4,531 | ||||||
|
|
|
|
|
|||||
| Total net revenues from collaborators |
$ | 66,725 | $ | 82,757 | ||||
|
|
|
|
|
|||||
Net revenues from collaborators declined for the year ended December 31, 2012 as compared to the year ended December 31, 2011 due primarily to the completion of our remaining performance obligations under the Roche/Arrowhead alliance in August 2012.
We had $132.3 million of deferred revenue at December 31, 2012, which consisted of payments we had received from collaborators, primarily Takeda, Kyowa Hakko Kirin, Cubist, Monsanto and Genzyme, but have not yet recognized pursuant to our revenue recognition policies.
Operating Expenses
The following table summarizes our operating expenses for the periods indicated, in thousands and as a percentage of total operating expenses, together with the changes, in thousands and percentages:
| 2012 | % of Total Operating Expenses |
2011 | % of Total Operating Expenses |
Increase (Decrease) |
||||||||||||||||||||
| $ | % | |||||||||||||||||||||||
| Research and development |
$ | 86,569 | 44 | % | $ | 99,295 | 72 | % | $ | (12,726 | ) | (13 | )% | |||||||||||
| General and administrative |
44,612 | 23 | % | 38,280 | 28 | % | 6,332 | 17 | % | |||||||||||||||
| Restructuring of Tekmira license agreement |
65,000 | 33 | % | — | — | 65,000 | 100 | % | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total operating expenses |
$ | 196,181 | 100 | % | $ | 137,575 | 100 | % | $ | 58,606 | 43 | % | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
96
Table of Contents
Research and development. The following table summarizes the components of our research and development expenses for the periods indicated, in thousands and as a percentage of total research and development expenses, together with the changes, in thousands and percentages:
| 2012 | % of Expense Category |
2011 | % of Expense Category |
Increase (Decrease) |
||||||||||||||||||||
| $ | % | |||||||||||||||||||||||
| Research and development |
||||||||||||||||||||||||
| Compensation and related |
$ | 20,438 | 24 | % | $ | 23,743 | 24 | % | $ | (3,305 | ) | (14 | )% | |||||||||||
| Clinical trial and manufacturing |
15,930 | 18 | % | 25,258 | 26 | % | (9,328 | ) | (37 | )% | ||||||||||||||
| External services |
13,743 | 16 | % | 15,653 | 16 | % | (1,910 | ) | (12 | )% | ||||||||||||||
| Facilities-related |
12,647 | 15 | % | 12,751 | 13 | % | (104 | ) | (1 | )% | ||||||||||||||
| Non-cash stock-based compensation |
8,041 | 9 | % | 10,921 | 11 | % | (2,880 | ) | (26 | )% | ||||||||||||||
| License fees |
5,693 | 7 | % | 1,381 | 1 | % | 4,312 | 312 | % | |||||||||||||||
| Lab supplies and materials |
4,422 | 5 | % | 6,283 | 6 | % | (1,861 | ) | (30 | )% | ||||||||||||||
| Restructuring |
2,817 | 3 | % | — | — | 2,817 | 100 | % | ||||||||||||||||
| Other |
2,838 | 3 | % | 3,305 | 3 | % | (467 | ) | (14 | )% | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total research and development expenses |
$ | 86,569 | 100 | % | $ | 99,295 | 100 | % | $ | (12,726 | ) | (13 | )% | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
Research and development expenses decreased during the year ended December 31, 2012 as compared to the year ended December 31, 2011 due primarily to lower clinical trial and manufacturing expenses related to our ALN-RSV, ALN-PCS and ALN-VSP programs and decreases in compensation-related expenses. Partially offsetting these decreases were additional expenses related to the advancement of our ALN-TTR program, as well as license fees due to certain entities, primarily fees due to Isis as a result of the Monsanto and Genzyme alliances. Also included in the year ended December 31, 2012 was a one-time charge related to our January 2012 strategic corporate restructuring, including employee severance, benefits and other related costs.
General and administrative. The following table summarizes the components of our general and administrative expenses for the periods indicated, in thousands and as a percentage of total general and administrative expenses, together with the changes, in thousands and percentages:
| 2012 | % of Expense Category |
2011 | % of Expense Category |
Increase (Decrease) |
||||||||||||||||||||
| $ | % | |||||||||||||||||||||||
| General and administrative |
||||||||||||||||||||||||
| Consulting and professional services |
$ | 28,949 | 65 | % | $ | 21,032 | 55 | % | $ | 7,917 | 38 | % | ||||||||||||
| Compensation and related |
6,384 | 14 | % | 7,074 | 18 | % | (690 | ) | (10 | )% | ||||||||||||||
| Non-cash stock-based compensation |
4,319 | 10 | % | 5,755 | 15 | % | (1,436 | ) | (25 | )% | ||||||||||||||
| Facilities-related |
1,648 | 4 | % | 2,254 | 6 | % | (606 | ) | (27 | )% | ||||||||||||||
| Restructuring |
890 | 2 | % | — | — | 890 | 100 | % | ||||||||||||||||
| Other |
2,422 | 5 | % | 2,165 | 6 | % | 257 | 12 | % | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total general and administrative expenses |
$ | 44,612 | 100 | % | $ | 38,280 | 100 | % | $ | 6,332 | 17 | % | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
The increase in general and administrative expenses during the year ended December 31, 2012 as compared to the year ended December 31, 2011 was due primarily to higher consulting and professional services expenses related to business activities, primarily legal activities, a description of which is set forth in Note 6 to our consolidated financial statements included in Part II, Item 8, “Financial Statements and Supplementary Data,” of this annual report on Form 10-K. Also included in the year ended December 31, 2012 was a one-time charge related to our January 2012 strategic corporate restructuring, including employee severance, benefits and other related costs. These increases were partially offset by a decrease in all compensation expenses and facilities-related expenses due primarily to the reduction in workforce in connection with the January 2012 strategic corporate restructuring.
97
Table of Contents
Restructuring of Tekmira license agreement. For the year ended December 31, 2012, we incurred a $65.0 million charge to operating expenses in connection with the restructuring of our license agreement with Tekmira in November 2012. A description of our 2012 cross-license agreement with Tekmira and the terms of the restructuring is set forth in Part I, Item 1, “Strategic Alliances—Delivery-Related Licenses and Collaborations – Tekmira” of this annual report on Form 10-K.
Other income (expense)
We incurred $4.5 million equity in loss of joint venture (Regulus Therapeutics Inc.) for the year ended December 31, 2012 as compared to $3.5 million for the year ended December 31, 2011 related to our share of the net losses incurred by Regulus.
Interest income was $1.0 million in 2012 as compared to $1.2 million in 2011. The decrease in 2012 was due primarily to lower average interest rates as well as lower average cash, cash equivalent and fixed income marketable securities balances.
Other income was $16.4 million in 2012 due primarily to a gain recorded in connection with the issuance of common stock by Regulus. In October 2012, Regulus completed an initial public offering, resulting in our ownership percentage decreasing from approximately 44% to 17% of Regulus’ outstanding common stock. As a result of this issuance of stock by Regulus, we recognized a gain of $16.1 million. Other expense in 2011 was $0.5 million and was due primarily to an impairment charge related to our former investment in Tekmira equity securities, as the decrease in the fair value of this investment was deemed to be other than temporary.
Our benefit from income taxes was $10.6 million in 2012 as compared to zero for 2011. The increase in 2012 was due to our recognition of corresponding income tax benefit associated with the increase in the value of our investment in Regulus that we carried at fair market value during the same respective period.
Liquidity and Capital Resources
The following table summarizes our cash flow activities for the periods indicated, in thousands:
| Year Ended December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| Net loss |
$ | (89,225 | ) | $ | (106,014 | ) | $ | (57,649 | ) | |||
| Adjustments to reconcile net loss to net cash (used in) provided by operating activities |
28,686 | (630 | ) | 26,509 | ||||||||
| Changes in operating assets and liabilities |
(8,118 | ) | (8,965 | ) | (55,928 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(68,657 | ) | (115,609 | ) | (87,068 | ) | ||||||
| Net cash (used in) provided by investing activities |
(130,505 | ) | 3,374 | 81,959 | ||||||||
| Net cash provided by financing activities |
200,926 | 93,412 | 738 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net increase (decrease) in cash and cash equivalents |
1,764 | (18,823 | ) | (4,371 | ) | |||||||
| Cash and cash equivalents, beginning of period |
51,405 | 70,228 | 74,599 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of period |
$ | 53,169 | $ | 51,405 | $ | 70,228 | ||||||
|
|
|
|
|
|
|
|||||||
Since we commenced operations in 2002, we have generated significant losses. At December 31, 2013, we had an accumulated deficit of $596.2 million. At December 31, 2013, we had cash, cash equivalents and fixed income marketable securities of $350.5 million, excluding our investment in equity securities of Regulus, compared to cash, cash equivalents and fixed income marketable securities of $226.2 million at December 31, 2012. Included in our December 31, 2012 cash, cash equivalents and fixed income marketable securities are the proceeds from our sale, in February 2012, of an aggregate of 8,625,000 shares of our common stock through an underwritten public offering at a price to the public of $10.75 per share. As a result of this offering, we received aggregate net proceeds of approximately $86.8 million, after deducting underwriting discounts and commissions and other estimated offering
98
Table of Contents
expenses of approximately $5.9 million. In January 2013, we sold an aggregate of 9,200,000 shares of our common stock through an underwritten public offering at a price to the public of $20.13 per share. As a result of this offering, we received aggregate net proceeds of approximately $173.6 million, after deducting underwriting discounts and commissions and other estimated offering expenses of approximately $11.6 million. We invest primarily in cash equivalents, U.S. government obligations, high-grade corporate notes and commercial paper. Our investment objectives are, primarily, to assure liquidity and preservation of capital and, secondarily, to obtain investment income. All of our investments in debt securities are recorded at fair value and are available-for-sale. Fair value is determined based on quoted market prices and models using observable data inputs. We have not recorded any impairment charges to our fixed income marketable securities at December 31, 2013.
Operating activities
We have required significant amounts of cash to fund our operating activities as a result of net losses since our inception. The decrease in net cash used in operating activities for the year ended December 31, 2013 compared to the year ended December 31, 2012 was due primarily to our net loss and other changes in our working capital and a decrease in deferred revenue of $6.2 million. The increase in net cash used in operating activities for the year ended December 31, 2012 compared to the year ended December 31, 2011 was due primarily to our net loss and other changes in our working capital, adjustments for noncash gains and benefits of $26.9 million, and a decrease in deferred revenue of $8.6 million. The increase in net cash used in operating activities for the year ended December 31, 2011 compared to the year ended December 31, 2010 was due primarily to our net loss and other changes in our working capital, as well as a decrease in deferred revenue of $70.3 million. Cash used in operating activities is adjusted for non-cash items to reconcile net loss to net cash provided by or used in operating activities. These non-cash adjustments consist primarily of stock-based compensation, equity in loss of joint venture (Regulus Therapeutics Inc.), tax benefit and depreciation and amortization.
We expect that we will require significant amounts of cash to fund our operating activities for the foreseeable future as we continue to develop and advance our research and development initiatives. The actual amount of overall expenditures will depend on numerous factors, including the timing of expenses, the timing and terms of collaboration agreements or other strategic transactions, if any, and the timing and progress of our research and development efforts.
Investing activities
For the year ended December 31, 2013, net cash used by investing activities of $130.5 million resulted primarily from net purchases of fixed income marketable securities of $126.5 million and purchases of property and equipment of $4.0 million. For the year ended December 31, 2012, net cash provided by investing activities of $3.4 million resulted primarily from sales and maturities of fixed income marketable securities of $11.9 million offset by purchases of property and equipment of $8.3 million primarily in connection with the build-out of our cGMP manufacturing facility. For the year ended December 31, 2011, net cash provided by investing activities of $82.0 million resulted primarily from net sales and maturities of fixed income marketable securities of $83.3 million, offset by purchases of property and equipment of $1.3 million.
Financing activities
For the year ended December 31, 2013, net cash provided by financing activities of $200.9 million was due primarily to proceeds of $173.6 million received from our January 2013 underwritten public offering, as well as proceeds of $28.7 million from the issuance of common stock in connection with stock option exercises and other types of equity. For the year ended December 31, 2012, net cash of $93.4 million provided by financing activities was due primarily to proceeds of $86.8 million received from our February 2012 underwritten public offering, as well proceeds of $7.0 million from the issuance of common stock in connection with stock option exercises and other types of equity. For the year ended December 31, 2011, net cash provided by financing activities of $0.7 million was due to proceeds from the issuance of common stock in connection with stock option exercises and other types of equity.
99
Table of Contents
Operating Capital Requirements
We do not know when, if ever, we will successfully develop or be able to commence sales of any product. Therefore, we anticipate that we will continue to generate significant losses for the foreseeable future as a result of planned expenditures for research and development activities relating to our drug development programs, including the development and optimization of drug delivery technologies and clinical trial costs, extension of the capabilities of our technology platform, including through business initiatives, continued management and growth of our patent portfolio, collaborations and general corporate activities. Based on our current operating plan, we believe that our existing cash, cash equivalents and fixed income marketable securities, together with the cash we expect to generate under our current alliances and upon the closing of the 2014 Genzyme collaboration and stock purchase, will be sufficient to fund our planned operations through the launch of our first commercial product. For reasons discussed below, we may require significant additional funds earlier than we currently expect in order to develop, conduct clinical trials for and commercialize any product candidates.
In the future, we may seek additional funding through additional collaborative arrangements and public or private financings. In December 2012, we filed an automatically effective shelf registration statement with the SEC for an indeterminate number of shares. Additional funding may not be available to us on acceptable terms or at all. In addition, the terms of any additional financing may further adversely affect the holdings or the rights of our stockholders. For example, if we raise additional funds by issuing equity securities, further dilution to our existing stockholders may result. In addition, as a condition to providing additional funds to us, future investors may demand, and may be granted, rights superior to those of existing stockholders. If we are unable to obtain funding on a timely basis, we may be required to significantly delay or curtail one or more of our research or development programs. We also could be required to seek funds through arrangements with collaborators or others that may require us to relinquish rights to some of our technologies or product candidates that we would otherwise pursue.
Even if we are able to raise additional funds in a timely manner, our future capital requirements may vary from what we expect and will depend on many factors, including:
| • | our progress in demonstrating that siRNAs can be active as drugs; |
| • | our ability to develop relatively standard procedures for selecting and modifying siRNA product candidates; |
| • | progress in our research and development programs, as well as the magnitude of these programs; |
| • | the timing, receipt and amount of milestone and other payments, if any, from present and future collaborators, if any; |
| • | our ability to maintain and establish additional collaborative arrangements and/or new business initiatives; |
| • | the resources, time and costs required to successfully initiate and complete our pre-clinical and clinical trials, obtain regulatory approvals, and obtain and maintain licenses to third-party intellectual property; |
| • | our ability to manufacture, or contract with third-parties for the manufacture of, our product candidates for clinical testing and commercial sale; |
| • | the resources, time and cost required for the preparation, filing, prosecution, maintenance and enforcement of patent claims; |
| • | the costs associated with legal activities, including litigation, arising in the course of our business activities and our ability to prevail in any such legal disputes; |
| • | progress in the research and development programs of Regulus; and |
| • | the timing, receipt and amount of sales and royalties, if any, from our potential products. |
100
Table of Contents
Off-Balance Sheet Arrangements
In connection with our license agreements with Max Planck relating to the Tuschl I and II patent applications, we are required to indemnify Max Planck for certain damages arising in connection with the intellectual property rights licensed under the agreements. Under this indemnification agreement with Max Planck, we are responsible for paying the costs of any litigation relating to the license agreements or the underlying intellectual property rights. In connection with our research agreement with Acuitas, we have agreed to indemnify Acuitas for certain legal costs, subject to certain exceptions and limitations. Amounts paid under the Acuitas indemnification agreement in connection with our previous litigation with Tekmira were charged to general and administrative expense. In addition, we are a party to a number of agreements entered into in the ordinary course of business, which contain typical provisions that obligate us to indemnify the other parties to such agreements upon the occurrence of certain events. These indemnification obligations are considered off-balance sheet arrangements in accordance with GAAP. To date, other than certain costs associated with the certain previously settled litigation related to the Tuschl patents and our disputes with Tekmira, we have not encountered material costs as a result of such obligations and have not accrued any liabilities related to such obligations in our consolidated financial statements. See Note 6 to our consolidated financial statements included in Part II, Item 8, “Financial Statements and Supplementary Data,” of this annual report on Form 10-K for further discussion of these indemnification agreements and guarantee obligations.
Contractual Obligations
In the table below, we set forth our enforceable and legally binding obligations and future commitments at December 31, 2013, as well as obligations related to contracts that we are likely to continue, regardless of the fact that they were cancelable at December 31, 2013. Some of the figures that we include in this table are based on management’s estimates and assumptions about these obligations, including their duration, the possibility of renewal, anticipated actions by third parties and other factors. Because these estimates and assumptions are necessarily subjective, the obligations we will actually pay in future periods may vary from those reflected in the table.
| Payments Due by Period | ||||||||||||||||||||
| Contractual Obligations |
2014 | 2015 and 2016 |
2017 and 2018 |
After 2018 |
Total | |||||||||||||||
| Operating lease obligations(1) |
$ | 6,303 | $ | 11,742 | $ | 364 | $ | — | $ | 18,409 | ||||||||||
| Purchase commitments(2) |
$ | 33,590 | $ | 29,097 | $ | — | $ | — | $ | 62,687 | ||||||||||
| Technology license commitments(3) |
$ | 7,533 | $ | 1,343 | $ | 1,398 | $ | 7,196 | $ | 17,470 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total contractual cash obligations |
$ | 47,426 | $ | 42,182 | $ | 1,762 | $ | 7,196 | $ | 98,566 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | Relates to our Cambridge, Massachusetts non-cancelable operating lease agreements. |
| (2) | Includes commitments related to purchase orders, clinical and pre-clinical agreements, and other purchase commitments for goods or services. |
| (3) | Relates to our fixed payment obligations under license agreements, as well as other payments related to technology research and development. |
We in-license technology from a number of sources. Pursuant to these in-license agreements, we will be required to make additional payments if and when we achieve specified development, regulatory and commercialization milestones. To the extent we are unable to reasonably predict the likelihood, timing or amount of such payments, we have excluded them from the table above.
Recent Accounting Pronouncements
See Note 2 to our consolidated financial statements included in Part II, Item 8, “Financial Statements and Supplementary Data,” of this annual report on Form 10-K for a description of recent accounting pronouncements applicable to our business.
101
Table of Contents
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
As part of our investment portfolio, we own financial instruments that are sensitive to market risks. The investment portfolio is used to preserve our capital until it is required to fund operations, including our research and development activities. Our fixed income marketable securities consist of U.S. government obligations, high-grade corporate notes and commercial paper. All of our investments in debt securities are classified as available-for-sale and are recorded at fair value. Our available-for-sale investments in debt securities are sensitive to changes in interest rates and changes in the credit ratings of the issuers. Interest rate changes would result in a change in the net fair value of these financial instruments due to the difference between the market interest rate and the market interest rate at the date of purchase of the financial instrument. If market interest rates were to increase immediately and uniformly by 50 basis points, or one-half of a percentage point, from levels at December 31, 2013, the net fair value of our interest-sensitive financial instruments would have resulted in a hypothetical decline of $1.2 million. A downgrade in the credit rating of an issuer of a debt security or further deterioration of the credit markets could result in a decline in the fair value of the debt instruments. Our investment guidelines prohibit investment in auction rate securities and we do not believe we have any direct exposure to losses relating from mortgage-based securities or derivatives related thereto such as credit-default swaps. We did not record any impairment charges to our fixed income marketable securities during the year ended December 31, 2013.
102
Table of Contents
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
103
Table of Contents
Management’s Annual Report on Internal Control Over Financial Reporting
The management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) or 15d-15(f) promulgated under the Securities Exchange Act of 1934 as a process designed by, or under the supervision of, the company’s principal executive and principal financial officers and effected by the company’s board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
| • | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the Company; |
| • | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and |
| • | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The Company’s management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2013. In making this assessment, the Company’s management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework (1992).
Based on its assessment, management concluded that, as of December 31, 2013, the Company’s internal control over financial reporting is effective based on those criteria.
The effectiveness of the Company’s internal control over financial reporting as of December 31, 2013 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report. This report appears on page 105.
104
Table of Contents
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Alnylam Pharmaceuticals, Inc.
In our opinion, based on our audits and the report of other auditors with respect to 2011, the accompanying consolidated balance sheets and the related consolidated statements of comprehensive loss, stockholders’ equity and cash flows present fairly, in all material respects, the financial position of Alnylam Pharmaceuticals, Inc. and its subsidiaries at December 31, 2013 and 2012, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2013 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2013, based on criteria established in Internal Control - Integrated Framework (1992) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for these financial statements, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Annual Report on Internal Control over Financial Reporting. Our responsibility is to express opinions on these financial statements and on the Company’s internal control over financial reporting based on our integrated audits. We did not audit the 2011 financial statements of Regulus Therapeutics Inc., an approximate 45 percent-owned equity investment, insofar as it relates to the Company’s equity in the net loss of Regulus Therapeutics, Inc. for the year ended December 31, 2011 of approximately $3.5 million. The 2011 financial statements of Regulus Therapeutics, Inc. were audited by other auditors whose report thereon has been furnished to us, and our opinion on the results of operations and cash flows for the year ended December 31, 2011 expressed herein, insofar as it relates to the amounts included for Regulus Therapeutics, Inc., is based solely on the report of the other auditors. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits and the report of other auditors provide a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
Boston, Massachusetts
February 20, 2014
105
Table of Contents
CONSOLIDATED BALANCE SHEETS
(In thousands, except share and per share amounts)
| December 31, | ||||||||
| 2013 | 2012 | |||||||
| ASSETS | ||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 53,169 | $ | 51,405 | ||||
| Marketable securities |
192,701 | 71,407 | ||||||
| Billed and unbilled collaboration receivables |
4,248 | 104 | ||||||
| Prepaid expenses and other current assets |
3,910 | 2,641 | ||||||
|
|
|
|
|
|||||
| Total current assets |
254,028 | 125,557 | ||||||
| Marketable securities |
104,602 | 103,416 | ||||||
| Investment in equity securities of Regulus Therapeutics Inc. |
45,452 | 38,748 | ||||||
| Property and equipment, net |
16,448 | 19,799 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 420,530 | $ | 287,520 | ||||
|
|
|
|
|
|||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 5,896 | $ | 4,420 | ||||
| Accrued expenses |
14,160 | 11,558 | ||||||
| Deferred rent |
1,112 | 950 | ||||||
| Deferred revenue |
32,696 | 31,417 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
53,864 | 48,345 | ||||||
| Deferred rent, net of current portion |
2,925 | 4,248 | ||||||
| Deferred revenue, net of current portion |
93,394 | 100,874 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
150,183 | 153,467 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies (Note 6) |
||||||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $0.01 par value per share, 5,000,000 shares authorized and no shares issued and outstanding at December 31, 2013 and 2012 |
— | — | ||||||
| Common stock, $0.01 par value per share, 125,000,000 shares authorized; 63,741,573 shares issued and outstanding at December 31, 2013; 52,489,936 shares issued and outstanding at December 31, 2012 |
637 | 525 | ||||||
| Additional paid-in capital |
846,228 | 624,876 | ||||||
| Accumulated other comprehensive income |
19,717 | 15,662 | ||||||
| Accumulated deficit |
(596,235 | ) | (507,010 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
270,347 | 134,053 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 420,530 | $ | 287,520 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these consolidated financial statements.
106
Table of Contents
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(In thousands, except per share amounts)
| Year Ended December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| Net revenues from collaborators |
$ | 47,167 | $ | 66,725 | $ | 82,757 | ||||||
|
|
|
|
|
|
|
|||||||
| Operating expenses: |
||||||||||||
| Research and development(1) |
112,957 | 86,569 | 99,295 | |||||||||
| General and administrative(1) |
27,152 | 44,612 | 38,280 | |||||||||
| Restructuring of Tekmira license agreement |
— | 65,000 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
140,109 | 196,181 | 137,575 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(92,942 | ) | (129,456 | ) | (54,818 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Other income (expense): |
||||||||||||
| Equity in loss of joint venture (Regulus Therapeutics Inc.) |
— | (4,522 | ) | (3,505 | ) | |||||||
| Gain on issuance of stock by Regulus Therapeutics Inc. |
— | 16,084 | — | |||||||||
| Interest income |
1,069 | 977 | 1,205 | |||||||||
| Other (expense) income |
(47 | ) | 331 | (531 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Total other income (expense) |
1,022 | 12,870 | (2,831 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Loss before income taxes |
(91,920 | ) | (116,586 | ) | (57,649 | ) | ||||||
| Benefit from income taxes |
2,695 | 10,572 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (89,225 | ) | $ | (106,014 | ) | $ | (57,649 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net loss per common share — basic and diluted |
$ | (1.45 | ) | $ | (2.11 | ) | $ | (1.36 | ) | |||
|
|
|
|
|
|
|
|||||||
| Weighted average common shares used to compute basic and diluted net loss per common share |
61,551 | 50,286 | 42,410 | |||||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss: |
||||||||||||
| Net loss |
$ | (89,225 | ) | $ | (106,014 | ) | $ | (57,649 | ) | |||
| Unrealized gain (loss) on marketable securities, net of tax |
4,055 | 15,827 | (879 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss |
$ | (85,170 | ) | $ | (90,187 | ) | $ | (58,528 | ) | |||
|
|
|
|
|
|
|
|||||||
| (1) | Non-cash stock-based compensation expenses included in operating expenses are as follows: |
| Research and development |
$ | 14,369 | $ | 8,041 | $ | 10,921 | ||||||
| General and administrative |
6,334 | 4,319 | 5,755 |
The accompanying notes are an integral part of these consolidated financial statements.
107
Table of Contents
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(In thousands, except share amounts)
| Common Stock | Additional Paid-in Capital |
Accumulated Other Comprehensive Income (Loss) |
Accumulated Deficit |
Total Stockholders’ Equity |
||||||||||||||||||||
| Shares | Amount | |||||||||||||||||||||||
| Balance at December 31, 2010 |
42,343,423 | $ | 423 | $ | 500,443 | $ | 714 | $ | (343,347 | ) | $ | 158,233 | ||||||||||||
| Exercise of common stock options |
16,800 | — | 103 | — | — | 103 | ||||||||||||||||||
| Issuance of common stock under other types of equity plans |
124,815 | 2 | 1,021 | — | — | 1,023 | ||||||||||||||||||
| Issuance of restricted stock |
236,904 | 2 | (2 | ) | — | — | — | |||||||||||||||||
| Stock-based compensation expense |
— | — | 16,676 | — | — | 16,676 | ||||||||||||||||||
| Joint venture stock-based compensation (Regulus Therapeutics Inc.) |
— | — | 370 | — | — | 370 | ||||||||||||||||||
| Tax benefit from stock-based compensation |
— | — | 120 | — | — | 120 | ||||||||||||||||||
| Other comprehensive loss |
— | — | — | (879 | ) | — | (879 | ) | ||||||||||||||||
| Net loss |
— | — | — | — | (57,649 | ) | (57,649 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at December 31, 2011 |
42,721,942 | 427 | 518,731 | (165 | ) | (400,996 | ) | 117,997 | ||||||||||||||||
| Exercise of common stock options |
661,909 | 7 | 6,395 | — | — | 6,402 | ||||||||||||||||||
| Issuance of common stock under other types of equity plans |
97,459 | 1 | 878 | — | — | 879 | ||||||||||||||||||
| Issuance of restricted stock, net of cancellations and tax withholdings |
383,626 | 4 | (523 | ) | — | — | (519 | ) | ||||||||||||||||
| Issuance of common stock, net of offering costs |
8,625,000 | 86 | 86,714 | — | — | 86,800 | ||||||||||||||||||
| Stock-based compensation expense |
— | — | 12,360 | — | — | 12,360 | ||||||||||||||||||
| Joint venture stock-based compensation (Regulus Therapeutics Inc.) |
— | — | 321 | — | — | 321 | ||||||||||||||||||
| Other comprehensive income |
— | — | — | 15,827 | — | 15,827 | ||||||||||||||||||
| Net loss |
— | — | — | — | (106,014 | ) | (106,014 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at December 31, 2012 |
52,489,936 | 525 | 624,876 | 15,662 | (507,010 | ) | 134,053 | |||||||||||||||||
| Exercise of common stock options |
2,031,916 | 20 | 27,974 | — | — | 27,994 | ||||||||||||||||||
| Issuance of common stock under other types of equity plans |
60,180 | 1 | 1,183 | — | — | 1,184 | ||||||||||||||||||
| Tax withholdings and cancellations of restricted stock, net of issuances of new awards |
(40,459 | ) | (1 | ) | (1,988 | ) | — | — | (1,989 | ) | ||||||||||||||
| Issuance of common stock, net of offering costs |
9,200,000 | 92 | 173,480 | — | — | 173,572 | ||||||||||||||||||
| Stock-based compensation expense |
— | — | 20,703 | — | — | 20,703 | ||||||||||||||||||
| Other comprehensive income |
— | — | — | 4,055 | — | 4,055 | ||||||||||||||||||
| Net loss |
— | — | — | — | (89,225 | ) | (89,225 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at December 31, 2013 |
63,741,573 | $ | 637 | $ | 846,228 | $ | 19,717 | $ | (596,235 | ) | $ | 270,347 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
108
Table of Contents
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| Year Ended December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| Cash flows from operating activities: |
||||||||||||
| Net loss |
$ | (89,225 | ) | $ | (106,014 | ) | $ | (57,649 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Depreciation and amortization |
10,229 | 9,035 | 5,125 | |||||||||
| Non-cash stock-based compensation |
20,703 | 12,360 | 16,676 | |||||||||
| Charge for 401(k) company stock match |
449 | 364 | 488 | |||||||||
| Equity in loss of joint venture (Regulus Therapeutics Inc.) |
— | 4,522 | 3,505 | |||||||||
| Tax benefit from stock-based compensation |
— | — | 120 | |||||||||
| Other than temporary impairment on equity securities |
— | — | 595 | |||||||||
| Realized gain on sale of marketable securities |
— | (255 | ) | — | ||||||||
| Gain on issuance of stock by joint venture |
— | (16,084 | ) | — | ||||||||
| Benefit from intraperiod tax allocation on marketable securities |
(2,695 | ) | (10,572 | ) | — | |||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Proceeds from landlord tenant improvements |
204 | 1,780 | — | |||||||||
| Billed and unbilled collaboration receivables |
(4,144 | ) | 1,364 | 1,982 | ||||||||
| Income taxes receivable |
— | — | 10,669 | |||||||||
| Prepaid expenses and other assets |
(1,356 | ) | 1,780 | 2,731 | ||||||||
| Accounts payable |
1,832 | (1,736 | ) | (3,512 | ) | |||||||
| Accrued expenses and other |
1,547 | (3,591 | ) | 2,457 | ||||||||
| Deferred revenue |
(6,201 | ) | (8,562 | ) | (70,255 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(68,657 | ) | (115,609 | ) | (87,068 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from investing activities: |
||||||||||||
| Purchases of property and equipment |
(4,006 | ) | (8,348 | ) | (1,291 | ) | ||||||
| Increase in restricted cash |
— | (162 | ) | — | ||||||||
| Purchases of marketable securities |
(364,305 | ) | (277,129 | ) | (293,115 | ) | ||||||
| Sales and maturities of marketable securities |
237,806 | 289,013 | 376,365 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash (used in) provided by investing activities |
(130,505 | ) | 3,374 | 81,959 | ||||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from financing activities: |
||||||||||||
| Proceeds from exercise of stock options and other types of equity |
28,743 | 6,971 | 738 | |||||||||
| Proceeds from issuance of common stock, net of offering costs |
173,572 | 86,800 | — | |||||||||
| Payments for repurchase of common stock for employee tax withholding |
(1,389 | ) | (359 | ) | — | |||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
200,926 | 93,412 | 738 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net increase (decrease) in cash and cash equivalents |
1,764 | (18,823 | ) | (4,371 | ) | |||||||
| Cash and cash equivalents, beginning of period |
51,405 | 70,228 | 74,599 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of period |
$ | 53,169 | $ | 51,405 | $ | 70,228 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of cash flows: |
||||||||||||
| Cash (paid for income taxes) proceeds from income tax refunds |
$ | (11 | ) | $ | (17 | ) | $ | 10,657 | ||||
| Supplemental disclosure of noncash investing activities: |
||||||||||||
| Fixed asset expenditures included in accounts payable and accrued expenses |
$ | 161 | $ | 1,441 | $ | — | ||||||
| Repurchase of common stock for employee tax withholding in accrued expenses |
$ | 600 | $ | — | $ | — | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
109
Table of Contents
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 1. | NATURE OF BUSINESS |
Alnylam Pharmaceuticals, Inc. (the “Company” or “Alnylam”) commenced operations on June 14, 2002 as a biopharmaceutical company seeking to develop and commercialize novel therapeutics based on RNA interference (“RNAi”). Alnylam is focused on discovering, developing and commercializing RNAi therapeutics by establishing strategic alliances with leading pharmaceutical and life sciences companies, establishing and maintaining a strong intellectual property position in the RNAi field, generating revenues through licensing agreements, and ultimately developing and commercializing RNAi therapeutics for its own account. The Company has devoted substantially all of its efforts to business planning, research and development, acquiring, filing and expanding intellectual property rights, recruiting management and technical staff, and raising capital.
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Basis of Presentation and Principles of Consolidation
The accompanying consolidated financial statements reflect the operations of the Company and its wholly-owned subsidiaries. All intercompany accounts and transactions have been eliminated.
Reclassifications
Certain reclassifications have been made to prior years’ consolidated financial statements to conform to the 2013 presentation.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America (“GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Concentrations of Credit Risk and Significant Customers
Financial instruments that potentially expose the Company to concentrations of credit risk consist primarily of cash, cash equivalents and fixed income marketable securities. At December 31, 2013 and 2012, substantially all of the Company’s cash, cash equivalents and fixed income marketable securities were invested in money market mutual funds, commercial paper, corporate notes and U.S. government securities through highly rated financial institutions. Investments are restricted, in accordance with the Company’s investment policy, to a concentration limit per issuer.
In recent periods, the Company’s revenues from collaborations have been generated from primarily F. Hoffmann-La Roche Ltd and certain of its affiliates (collectively, “Roche”) (which assigned its rights and obligations to Arrowhead Research Corporation (“Arrowhead”) in 2011), Takeda Pharmaceutical Company Limited (“Takeda”), Cubist Pharmaceuticals, Inc. (“Cubist”), Monsanto Company (“Monsanto”) and The Medicines Company (“MDCO”). For the year ended December 31, 2013, the composition of the Company’s billed and unbilled collaboration receivables was primarily composed of an amount due from Genzyme Corporation (“Genzyme”) based upon the initiation of a Phase 3 clinical trial. For the year ended December 31, 2012, the composition of the Company’s billed and unbilled collaboration receivables was entirely composed of amounts due from the U.S. Government for the wind-down of completed government contracts.
110
Table of Contents
The following table summarizes customers that represent greater than 10% of the Company’s net revenues from collaborators, for the periods indicated:
| Year Ended December 31, |
||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| Takeda |
47 | % | 33 | % | 27 | % | ||||||
| Cubist |
21 | % | * | % | * | % | ||||||
| Monsanto |
12 | % | * | % | * | % | ||||||
| Roche/Arrowhead |
* | % | 56 | % | 68 | % | ||||||
The following table summarizes customers with amounts due that represent greater than 10% of the Company’s billed and unbilled collaboration receivables balance, at the periods indicated:
| At December 31, | ||||||||
| 2013 | 2012 | |||||||
| Genzyme |
94 | % | * | % | ||||
| U.S. Government |
* | % | 100 | % | ||||
| * | Represents 10% or less |
Fair Value Measurements
The fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The following tables present information about the Company’s assets that are measured at fair value on a recurring basis at December 31, 2013 and 2012, and indicate the fair value hierarchy of the valuation techniques the Company utilized to determine such fair value. In general, fair values determined by Level 1 inputs utilize quoted prices (unadjusted) in active markets for identical assets or liabilities. Fair values determined by Level 2 inputs utilize data points that are observable, such as quoted prices (adjusted), interest rates and yield curves. Fair values determined by Level 3 inputs utilize unobservable data points for the asset or liability, and include situations where there is little, if any, market activity for the asset or liability. The fair value hierarchy level is determined by the lowest level of significant input. Financial assets and liabilities measured at fair value on a recurring basis are summarized as follows, in thousands:
| Description |
At December 31, 2013 |
Quoted Prices in Active Markets (Level 1) |
Significant Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
||||||||||||
| Cash equivalents |
$ | 32,571 | $ | 32,571 | $ | — | $ | — | ||||||||
| Marketable securities (fixed income): |
||||||||||||||||
| Corporate notes |
228,462 | — | 228,462 | — | ||||||||||||
| U.S. Government obligations |
56,863 | — | 56,863 | — | ||||||||||||
| Commercial paper |
11,978 | — | 11,978 | — | ||||||||||||
| Marketable securities (Regulus equity holdings) |
45,452 | 45,452 | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 375,326 | $ | 78,023 | $ | 297,303 | $ | — | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
111
Table of Contents
| Description |
At December 31, 2012 |
Quoted Prices in Active Markets (Level 1) |
Significant Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
||||||||||||
| Cash equivalents |
$ | 50,213 | $ | 50,213 | $ | — | $ | — | ||||||||
| Marketable securities (fixed income): |
||||||||||||||||
| Corporate notes |
91,523 | — | 91,523 | — | ||||||||||||
| U.S. Government obligations |
60,661 | — | 60,661 | — | ||||||||||||
| Commercial paper |
22,639 | — | 22,639 | — | ||||||||||||
| Marketable securities (Regulus equity holdings) |
38,748 | — | 38,748 | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 263,784 | $ | 50,213 | $ | 213,571 | $ | — | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
For the year ended December 31, 2013, the Company transferred $45.5 million of marketable securities (equity holdings in Regulus Therapeutics Inc. (“Regulus”)) from Level 2 to Level 1 due to the increased volume in average daily trading on the NASDAQ stock market for these instruments. For the year ended December 31, 2012, there were no transfers between Level 1 and Level 2 financial assets. The carrying amounts reflected in the Company’s consolidated balance sheets for cash, billed and unbilled collaboration receivables, other current assets, accounts payable and accrued expenses approximate fair value due to their short-term maturities.
Investments in Marketable Securities
The Company invests its excess cash balances in short-term and long-term marketable debt and equity securities. The Company classifies its investments in marketable debt securities as either held-to-maturity or available-for-sale based on facts and circumstances present at the time it purchased the securities. At each balance sheet date presented, the Company classified all of its investments in debt and equity securities as available-for-sale. The Company reports available-for-sale investments at fair value at each balance sheet date and includes any unrealized holding gains and losses (the adjustment to fair value) in accumulated other comprehensive income (loss), a component of stockholders’ equity. Realized gains and losses are determined using the specific identification method and are included in other income. If any adjustment to fair value reflects a decline in the value of the investment, the Company considers all available evidence to evaluate the extent to which the decline is “other than temporary” and, if so, marks the investment to market through a charge to its consolidated statements of comprehensive loss. The Company did not record any impairment charges related to its fixed income marketable securities during the years ended December 31, 2013, 2012 or 2011. During 2011, the Company recorded an impairment charge of $0.6 million related to its former investment in equity securities of Tekmira Pharmaceuticals Corporation (“TPC”), as the decrease in the fair value of this investment was deemed to be other than temporary. The Company’s marketable securities are classified as cash equivalents if the original maturity, from the date of purchase, is 90 days or less, and as marketable securities if the original maturity, from the date of purchase, is in excess of 90 days. The Company’s cash equivalents are composed of money market funds.
112
Table of Contents
In the fourth quarter of 2012, the Company began accounting for its investment in Regulus as an available-for-sale marketable security. Intraperiod tax allocation rules require the Company to allocate its provision for income taxes between continuing operations and other categories of earnings, such as other comprehensive income. In periods in which the Company has a year-to-date pre-tax loss from continuing operations and pre-tax income in other categories of earnings, such as other comprehensive income, the Company must allocate the tax provision to the other categories of earnings. The Company then records a related tax benefit in continuing operations. The following tables summarize the fair value, accumulated other comprehensive income and intraperiod tax allocation regarding the Company’s investment in Regulus available-for-sale marketable securities at December 31, 2013 and 2012, and for the activity recorded in the year, in thousands:
| Description |
At December 31, 2012 |
During Twelve Months Ended December 31, 2013 |
Balance at December 31, 2013 |
|||||||||
| Carrying value |
$ | 12,449 | $ | — | $ | 12,449 | ||||||
| Accumulated other comprehensive income, before tax |
26,299 | 6,704 | 33,003 | |||||||||
|
|
|
|
|
|
|
|||||||
| Investment in equity securities of Regulus Therapeutics Inc., as reported |
$ | 38,748 | $ | 6,704 | $ | 45,452 | ||||||
|
|
|
|
|
|
|
|||||||
| Accumulated other comprehensive income, before tax |
$ | 26,299 | $ | 6,704 | $ | 33,003 | ||||||
| Intraperiod tax allocation recorded as a benefit from income taxes |
(10,572 | ) | (2,695 | ) | (13,267 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Accumulated other comprehensive income, net of tax |
$ | 15,727 | $ | 4,009 | $ | 19,736 | ||||||
|
|
|
|
|
|
|
|||||||
The Company obtains fair value measurement data for its marketable securities from independent pricing services. The Company performs validation procedures to ensure the reasonableness of this data. This includes meeting with the independent pricing services to understand the methods and data sources used. Additionally, the Company performs its own review of prices received from the independent pricing services by comparing these prices to other sources and confirming those securities are trading in active markets.
The following tables summarize the Company’s marketable securities, other than its holdings in Regulus noted above, at December 31, 2013 and 2012, in thousands:
| December 31, 2013 | ||||||||||||||||
| Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Value | |||||||||||||
| Commercial paper (Due within 1 year) |
$ | 11,983 | $ | — | $ | (5 | ) | $ | 11,978 | |||||||
| Corporate notes (Due within 1 year) |
154,175 | 33 | (46 | ) | 154,162 | |||||||||||
| Corporate notes (Due after 1 year through 2 years) |
74,312 | 23 | (35 | ) | 74,300 | |||||||||||
| U.S. Government obligations (Due within 1 year) |
26,553 | 8 | — | 26,561 | ||||||||||||
| U.S. Government obligations (Due after 1 year through 2 years) |
30,300 | 2 | — | 30,302 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 297,323 | $ | 66 | $ | (86 | ) | $ | 297,303 | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
| December 31, 2012 | ||||||||||||||||
| Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Value | |||||||||||||
| Commercial paper (Due within 1 year) |
$ | 22,650 | $ | 1 | $ | (12 | ) | $ | 22,639 | |||||||
| Corporate notes (Due within 1 year) |
41,249 | 23 | (4 | ) | 41,268 | |||||||||||
| Corporate notes (Due after 1 year through 2 years) |
50,322 | 5 | (72 | ) | 50,255 | |||||||||||
| U.S. Government obligations (Due within 1 year) |
7,500 | — | — | 7,500 | ||||||||||||
| U.S. Government obligations (Due after 1 year through 2 years) |
53,168 | 2 | (9 | ) | 53,161 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 174,889 | $ | 31 | $ | (97 | ) | $ | 174,823 | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
113
Table of Contents
Estimated Liability for Development Costs
The Company records accrued liabilities related to expenses for which service providers have not yet billed the Company with respect to products or services that the Company has received, specifically related to ongoing pre-clinical studies and clinical trials. These costs primarily relate to third-party clinical management costs, laboratory and analysis costs, toxicology studies and investigator fees. The Company has multiple product candidates in concurrent pre-clinical studies and clinical trials at multiple clinical sites throughout the world. In order to ensure that the Company has adequately provided for ongoing pre-clinical and clinical development costs during the period in which the Company incurs such costs, the Company maintains an accrual to cover these expenses. The Company updates the estimate for this accrual on at least a quarterly basis. The assessment of these costs is a subjective process that requires judgment. Upon settlement, these costs may differ materially from the amounts accrued in the Company’s consolidated financial statements. The Company’s historical accrual estimates have not been materially different from the Company’s actual costs.
Revenue Recognition
The Company has entered into collaboration agreements with leading pharmaceutical and life sciences companies, including Novartis Pharma AG and one of its affiliates (together, “Novartis”), Roche, Takeda, Kyowa Hakko Kirin Co., Ltd. (“Kyowa Hakko Kirin”), Cubist, Monsanto, Genzyme and MDCO. The terms of the Company’s collaboration agreements typically include non-refundable license fees, funding of research and development, payments based upon achievement of clinical and pre-clinical development milestones, regulatory milestones, manufacturing services, sales milestones and royalties on product sales.
In January 2011, the Company adopted new authoritative guidance on revenue recognition for multiple element arrangements. The guidance, which applied to multiple element arrangements entered into or materially modified on or after January 1, 2011, amended the criteria for separating and allocating consideration in a multiple element arrangement by modifying the fair value requirements for revenue recognition and eliminating the use of the residual method. The fair value of deliverables under the arrangement may be derived using a “best estimate of selling price” if vendor specific objective evidence and third-party evidence is not available. Deliverables under the arrangement could be considered separate units of accounting provided that (i) a delivered item has value to the customer on a standalone basis and (ii) if the arrangement includes a general right of return relative to the delivered item, delivery or performance of the undelivered item is considered probable and substantially in the control of the vendor. The new guidance did not change the criteria for standalone value. As a biotechnology entity with unique and specialized delivered and undelivered performance obligations, the Company has been unable to demonstrate standalone value in its multiple element arrangements. For example, the Company applied the new rules to collaborations executed with Monsanto and Genzyme during 2012, but it was unable to demonstrate standalone value. In addition, the Company has not materially modified any of its multiple element arrangements. As such, the Company has continued to account for license and collaboration agreements under previously issued revenue recognition guidance for multiple element arrangements, as described below.
Non-refundable license fees are recognized as revenue upon delivery of the license only if the Company has a contractual right to receive such payment, the contract price is fixed or determinable, the collection of the resulting receivable is reasonably assured and the Company has no further performance obligations under the license agreement. Multiple element arrangements, such as license and development arrangements, are analyzed to determine whether the deliverables, which often include a license and performance obligations such as research and steering committee services, can be separated or whether they must be accounted for as a single unit of accounting. The Company recognizes upfront license payments as revenue upon delivery of the license only if the license has standalone value and the fair value of the undelivered performance obligations, typically including research and/or steering committee services, can be determined. If the fair value of the undelivered performance obligations can be determined, such obligations are accounted for separately as such obligations are fulfilled. If the license is considered to either not have standalone value or have standalone value but the fair value of any of the undelivered performance obligations cannot be determined, the arrangement would then be accounted for as a single unit of accounting and the license payments and payments for performance obligations are recognized as revenue over the estimated period of when the performance obligations are performed.
114
Table of Contents
Whenever the Company determines that an arrangement should be accounted for as a single unit of accounting, the Company determines the period over which the performance obligations will be performed and revenue will be recognized. Revenue is recognized using either a proportional performance or straight-line method. The Company recognizes revenue using the proportional performance method when the level of effort required to complete its performance obligations under an arrangement can be reasonably estimated and such performance obligations are provided on a best-efforts basis. Direct labor hours or full-time equivalents are typically used as the measure of performance. The amount of revenue recognized under the proportional performance method is determined by multiplying the total payments under the contract, excluding royalties and payments contingent upon achievement of milestones, by the ratio of level of effort incurred to date to estimated total level of effort required to complete the Company’s performance obligations under the arrangement. Revenue is limited to the lesser of the cumulative amount of payments received or the cumulative amount of revenue earned, as determined using the proportional performance method, as of the period ending date.
If the Company cannot reasonably estimate the level of effort to complete its performance obligations under an arrangement, the Company recognizes revenue under the arrangement on a straight-line basis over the period the Company is expected to complete its performance obligations. Revenue is limited to the lesser of the cumulative amount of payments received or the cumulative amount of revenue earned, as determined using the straight-line method, as of the period ending date.
Significant management judgment is required in determining the level of effort required under an arrangement and the period over which the Company is expected to complete its performance obligations under an arrangement. Steering committee services that are not inconsequential or perfunctory and that are determined to be performance obligations are combined with other research services or performance obligations required under an arrangement, if any, in determining the level of effort required in an arrangement and the period over which the Company expects to complete its aggregate performance obligations.
Many of the Company’s collaboration agreements entitle it to additional payments upon the achievement of performance-based milestones. These milestones are generally categorized into three types; development milestones which are generally based on the advancement of the Company’s pipeline and initiation of clinical trials, regulatory milestones which are generally based on the submission, filing or approval of regulatory applications such as a new drug application in the United States, and commercialization milestones which are generally based on meeting specific thresholds of sales in certain geographic areas. If the achievement of a milestone is considered probable at the inception of the collaboration, the related milestone payment is included with other collaboration consideration, such as upfront fees and research funding, in the Company’s revenue model. Milestones that are tied to regulatory approval are not considered probable of being achieved until such approval is received. Milestones tied to counter-party performance are not included in the Company’s revenue model until the performance conditions are met.
The Company performs an assessment to determine whether a substantive milestone exists at the inception of its collaborative arrangements. In evaluating if a milestone is substantive, the Company considers whether uncertainty exists as to the achievement of the milestone event at the inception of the arrangement, the achievement of the milestone involves substantive effort and can only be achieved based in whole or part on the performance or the occurrence of a specific outcome resulting from the Company’s performance, the amount of the milestone payment appears reasonable either in relation to the effort expected to be expended or to the projected enhancement of the value of the delivered items, there is any future performance required to earn the milestone, and the consideration is reasonable relative to all deliverables and payment terms in the arrangement. When a substantive milestone is achieved, the accounting rules permit the Company to recognize revenue related to the milestone payment in its entirety.
To date, the Company has not recorded any substantive milestones under its collaborations because the Company has not identified any milestones that meet the required criteria listed above. The Company has deferred recognition of payments for achievement of non-substantive milestones and recognized revenue over the estimated period of performance applicable to each collaborative arrangement. As these milestones are achieved, the Company will recognize as revenue a portion of the milestone payment, which is equal to the percentage of the performance period completed when the milestone is achieved, multiplied by the amount of the milestone
115
Table of Contents
payment, upon achievement of such milestone. The Company will recognize the remaining portion of the milestone payment over the remaining performance period under the proportional performance method or on a straight-line basis.
For revenue generating arrangements where the Company, as a vendor, provides consideration to a licensor or collaborator, as a customer, the Company applies the accounting standard that governs such transactions. This standard addresses the accounting for revenue arrangements where both the vendor and the customer make cash payments to each other for services and/or products. A payment to a customer is presumed to be a reduction of the selling price unless the Company receives an identifiable benefit for the payment and it can reasonably estimate the fair value of the benefit received. Payments to a customer that are deemed a reduction of selling price are recorded first as a reduction of revenue, to the extent of both cumulative revenue recorded to date and probable future revenues, which include any unamortized deferred revenue balances, under all arrangements with such customer, and then as an expense. Payments that are not deemed to be a reduction of selling price are recorded as an expense.
The Company evaluates its collaborative agreements for proper classification in its consolidated statements of comprehensive loss based on the nature of the underlying activity. Transactions between collaborators recorded in the Company’s consolidated statements of comprehensive loss are recorded on either a gross or net basis, depending on the characteristics of the collaborative relationship. The Company generally reflects amounts due under its collaborative agreements related to cost-sharing of development activities as a reduction of research and development expense.
Amounts received prior to satisfying the above revenue recognition criteria are recorded as deferred revenue in the accompanying consolidated balance sheets. Although the Company follows detailed guidelines in measuring revenue, certain judgments affect the application of its revenue policy. For example, in connection with the Company’s existing collaboration agreements, the Company has recorded on its balance sheet short-term and long-term deferred revenue based on its best estimate of when such revenue will be recognized. Short-term deferred revenue consists of amounts that are expected to be recognized as revenue in the next 12 months. Amounts that the Company expects will not be recognized within the next 12 months are classified as long-term deferred revenue. However, this estimate is based on the Company’s current operating plan and, if its operating plan should change in the future, the Company may recognize a different amount of deferred revenue over the next 12-month period.
The estimate of deferred revenue also reflects management’s estimate of the periods of the Company’s involvement in certain of its collaborations. The Company’s performance obligations under these collaborations consist of participation on steering committees and the performance of other research and development services. In certain instances, the timing of satisfying these obligations can be difficult to estimate. Accordingly, the Company’s estimates may change in the future. Such changes to estimates would result in a change in revenue recognition amounts. If these estimates and judgments change over the course of these agreements, it may affect the timing and amount of revenue that the Company recognizes and records in future periods. At December 31, 2013, the Company had short-term and long-term deferred revenue of $32.7 million and $93.4 million, respectively, related to its collaborations.
Income Taxes
The Company uses the asset and liability method of accounting for income taxes. Under the asset and liability method, deferred tax assets and liabilities reflect the impact of temporary differences between amounts of assets and liabilities for financial reporting purposes and such amounts as measured under enacted tax laws. A valuation allowance is required to offset any net deferred tax assets if, based upon the available evidence, it is more likely than not that some or all of the deferred tax asset will not be realized.
The Company accounts for uncertain tax positions using a “more-likely-than-not” threshold for recognizing and resolving uncertain tax positions. The evaluation of uncertain tax positions is based on factors that include, but are not limited to, changes in tax law, the measurement of tax positions taken or expected to be taken in tax returns, the effective settlement of matters subject to audit, new audit activity and changes in facts or
116
Table of Contents
circumstances related to a tax position. The Company’s policy is to accrue interest and penalties related to unrecognized tax positions in income tax expense. As of December 31, 2013, the Company has not recorded significant interest and penalty expense related to uncertain tax positions.
Research and Development Costs
The Company expenses research and development costs as incurred. Included in research and development costs are wages, benefits and other operating costs, facilities, supplies, external services, clinical trial and manufacturing costs, and overhead directly related to the Company’s research and development operations, as well as costs to acquire technology licenses.
The Company has entered into several license agreements for rights to utilize certain technologies. The terms of the licenses may provide for upfront payments, annual maintenance payments, milestone payments based upon certain specified events being achieved and royalties on product sales. The Company charges costs to acquire and maintain licensed technology that has not reached technological feasibility and does not have alternative future use to research and development expense as incurred. During the years ended December 31, 2013, 2012 and 2011, the Company charged to research and development expense costs associated with license fees of $8.0 million, $5.7 million and $1.4 million, respectively.
Accounting for Stock-Based Compensation
The Company has stock incentive plans and an employee stock purchase plan under which it grants equity instruments. The Company accounts for all stock-based awards granted to employees at their fair value and generally recognizes compensation expense over the vesting period of the award. Determining the amount of stock-based compensation to be recorded requires the Company to develop estimates of fair values of stock options as of the grant date. The Company calculates the grant date fair values using the Black-Scholes valuation model. The Company’s expected stock price volatility assumption is based on a combination of the historical and implied volatility of the Company’s publicly traded stock.
For stock-based awards granted to non-employees, the Company generally recognizes compensation expense over the vesting period of the award, which is generally the period during which services are rendered by such non-employees. At the end of each financial reporting period prior to vesting, the Company re-measures the value of these stock-based awards (as calculated using the Black-Scholes option-pricing model) using the then-current fair value of the Company’s common stock. Stock options granted by the Company to non-employees, other than members of the Company’s Board of Directors and Scientific Advisory Board members, generally vest over the service period.
The fair value of restricted stock awards granted to employees is based upon the quoted closing market price per share on the date of grant, adjusted for assumed forfeitures. Expense is recognized over the vesting period, commencing when the Company determines that it is probable that the awards will vest.
For performance-based stock awards, expense is first recorded when the Company determines that the achievement of such performance conditions is deemed probable. This determination requires significant judgment by management. At the probable date, the Company records a cumulative expense catch-up, with remaining expense amortized over the remaining service period.
Comprehensive Loss
Comprehensive loss is comprised of net loss and certain changes in stockholders’ equity that are excluded from net loss. The Company includes foreign currency translation adjustments in other comprehensive loss for Alnylam Europe AG (“Alnylam Europe”) as the functional currency is not the United States dollar. The Company includes unrealized gains and losses on certain marketable securities in other comprehensive loss.
Net Loss Per Common Share
The Company computes basic net loss per common share by dividing net loss by the weighted average number of common shares outstanding. The Company computes diluted net loss per common share by dividing
117
Table of Contents
net loss by the weighted average number of common shares and dilutive potential common share equivalents then outstanding. Potential common shares consist of shares issuable upon the exercise of stock options (using the treasury stock method), and unvested restricted stock awards. Because the inclusion of potential common shares would be anti-dilutive for all periods presented, diluted net loss per common share is the same as basic net loss per common share.
The following table sets forth for the periods presented the potential common shares (prior to consideration of the treasury stock method) excluded from the calculation of net loss per common share because their inclusion would be anti-dilutive, in thousands:
| December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| Options to purchase common stock |
8,713 | 8,932 | 9,779 | |||||||||
| Unvested restricted common stock |
500 | 604 | 312 | |||||||||
|
|
|
|
|
|
|
|||||||
| 9,213 | 9,536 | 10,091 | ||||||||||
|
|
|
|
|
|
|
|||||||
Segment Information
The Company operates in a single reporting segment, the discovery, development and commercialization of RNAi therapeutics.
Subsequent Events
The Company evaluated all events or transactions that occurred after December 31, 2013 up through the date these consolidated financial statements were issued. During this period, the Company did not have any material recognized subsequent events. However, the Company did have the following nonrecognized subsequent events, which are more fully described in Note 12, “Subsequent Events”:
| • | In January 2014, the Company entered into a stock purchase agreement with Sirna Therapeutics, Inc. (“Sirna”), Merck Sharp & Dohme Corp. (“Merck”), and, for limited purposes, Merck & Co., Inc., pursuant to which the Company will purchase from Merck all of Merck’s right, title and interest in and to all of the outstanding shares of common stock, par value $0.01 per share, of Sirna (the “Sirna Common Stock”). See Note 12. |
| • | In January 2014, the Company and Genzyme entered into a new global strategic collaboration agreement to develop and commercialize the Company’s current and future genetic medicine pipeline principally in territories outside of North America and Western Europe, subject to certain broader rights. See Note 12. |
Recent Accounting Pronouncements
In February 2013, the Financial Accounting Standards Board (“FASB”) issued amendments to the accounting guidance for presentation of comprehensive income to improve the reporting of reclassifications out of accumulated other comprehensive income. The amendments did not change the current requirements for reporting net income or other comprehensive income, but require an entity to provide information about the amounts reclassified out of accumulated other comprehensive income by component. In addition, an entity is now required to present, either on the face of the statement where the net income is presented or in the notes, significant amounts reclassified out of accumulated other comprehensive income by the respective line items of net income but only if the amount reclassified is required under GAAP to be reclassified to net income in its entirety in the same reporting period. For other amounts that are not required under GAAP to be reclassified in their entirety to net income, an entity is required to cross-reference to other disclosures required under GAAP that provide additional detail about these amounts. For public companies, these amendments were effective prospectively for reporting periods beginning after December 15, 2012. The Company adopted this guidance on January 1, 2013. Other than a change in presentation, the adoption of this guidance did not have a material impact on the Company’s consolidated financial statements.
118
Table of Contents
In July 2013, the FASB issued new accounting guidance specific to income taxes. The new guidance requires an entity to present an unrecognized tax benefit and a net operating loss carryforward, a similar tax loss, or a tax credit carryforward on a net basis as part of a deferred tax asset, unless the unrecognized tax benefit is not available to reduce the deferred tax asset component or would not be utilized for that purpose, then a liability would be recognized. The updated accounting guidance is effective for fiscal years beginning after December 15, 2013. The Company does not expect the adoption of this guidance to have a material impact on the Company’s consolidated financial statements.
| 3. | SIGNIFICANT AGREEMENTS |
The following table summarizes the Company’s total consolidated net revenues from collaborators, for the periods indicated, in thousands:
| Year Ended December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| Takeda |
$ | 21,973 | $ | 21,973 | $ | 22,248 | ||||||
| Cubist |
9,721 | 2,777 | 2,467 | |||||||||
| Monsanto |
5,640 | 1,954 | — | |||||||||
| MDCO |
4,604 | — | — | |||||||||
| Roche/Arrowhead |
— | 37,318 | 55,978 | |||||||||
| Other |
5,229 | 2,703 | 2,064 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total net revenues from collaborators |
$ | 47,167 | $ | 66,725 | $ | 82,757 | ||||||
|
|
|
|
|
|
|
|||||||
Product Alliances
Genzyme Alliance
In January 2014, the Company entered into a global, strategic collaboration with Genzyme to discover, develop and commercialize RNAi therapeutics as genetic medicines to treat orphan diseases (the “2014 Genzyme Collaboration”). The 2014 Genzyme Collaboration is governed by a Master Collaboration Agreement, dated January 11, 2014, by and between the Company and Genzyme (including the License Terms appended thereto, the “Master Agreement”), which will become effective upon closing of the equity transaction, described below under Note 12, “Subsequent Events.” Once effective, the Master Agreement will supersede and replace the previous collaboration between the Company and Genzyme entered into in October 2012 to develop and commercialize RNAi therapeutics targeting targeting transthyretin (“TTR”) for the treatment of transthyretin-mediated amyloidosis (“ATTR”) (the “2012 Genzyme Agreement”).
In consideration for the rights granted to Genzyme under the 2012 Genzyme Agreement, Genzyme paid the Company an upfront cash payment of $22.5 million. The Company was also entitled to receive certain milestone payments under the 2012 Genzyme Agreement. In the fourth quarter of 2013, the Company earned a milestone of $7.0 million based upon the completion of a successful patisiran Phase 2 clinical trial and a milestone of $4.0 million based upon the initiation of the Phase 3 clinical trial for patisiran.
Under the 2012 Genzyme Agreement, the parties agreed to collaborate in the development of licensed products, with Genzyme assuming primary responsibility for the development and commercialization of licensed products in the Genzyme territory and the Company retaining primary responsibility for the development and commercialization of licensed products in the rest of the world. The collaboration between Genzyme and the Company was governed by a joint steering committee comprised of an equal number of representatives from each party. Genzyme was responsible, at its expense, for all development activities under the development plan reasonably necessary for the regulatory approval and commercialization of an RNAi therapeutic for the treatment of ATTR in the Genzyme territory.
The Company determined that the deliverables under the 2012 Genzyme Agreement included the license, the joint steering committee and any additional TTR-specific RNAi therapeutic compounds that comprised the
119
Table of Contents
ALN-TTR program. The Company also determined that, pursuant to the accounting guidance governing revenue recognition on multiple element arrangements, the license and undelivered joint steering committee and any additional TTR-specific RNAi therapeutic compounds did not have standalone value due to the specialized nature of the services to be provided by the Company. In addition, while Genzyme had the ability to grant sublicenses, it could not sublicense all or substantially all of its rights under the 2012 Genzyme Agreement. The uniqueness of the Company’s services and the limited sublicense right were indicators that standalone value was not present in the arrangement. Therefore the deliverables were not separable and, accordingly, the license and undelivered services were treated as a single unit of accounting. Because the Company was unable to reasonably estimate our period of performance under the Genzyme agreement, as the Company was unable to estimate the timeline of deliverables related to the option granted to Genzyme for any additional compounds, the Company deferred all revenue under the 2012 Genzyme Agreement until the Company was able to reasonably estimate its period of performance. The Company was unable to reasonably estimate the period of performance under the 2012 Genzyme Agreement, as it was unable to estimate the timeline of its deliverables related to the deliverable for any additional TTR-specific RNAi therapeutic compounds. Through Decembe 31, 2013, the Company has deferred all revenue under the 2012 Genzyme Agreement. At December 31, 2013, deferred revenue under the 2012 Genzyme Agreement was $33.5 million. The Company expects that the $33.5 million received under the 2012 Genzyme Agreement, which is currently included in deferred revenue, will be accounted for in conjunction with the 2014 Genzyme Collaboration.
The Medicines Company Alliance
In February 2013, the Company and MDCO entered into a license and collaboration agreement (the “MDCO Agreement”) pursuant to which the Company granted to MDCO an exclusive, worldwide license to develop, manufacture and commercialize RNAi therapeutics targeting proprotein convertase subtilisin/kexin type 9 (“PCSK9”), including ALN-PCS02 and ALN-PCSsc, for the treatment of hypercholesterolemia and other human diseases (collectively, “ALN-PCS Licensed Products”). ALN-PCS02 is an intravenously administered RNAi therapeutic for which the Company completed a Phase 1 clinical trial, and ALN-PCSsc is a subcutaneously administered RNAi therapeutic currently in pre-clinical development. In consideration for the rights granted to MDCO under the MDCO Agreement, MDCO paid the Company an upfront cash payment of $25.0 million. Upon achievement of certain milestones, the Company will be entitled to receive milestone payments, up to an aggregate of $180.0 million, including up to $30.0 million in specified development milestones, $50.0 million in specified regulatory milestones and $100.0 million in specified commercialization milestones. In addition, the Company will be entitled to royalties ranging from the low- to high- teens based on annual worldwide net sales, if any, of ALN-PCS Licensed Products by MDCO, its affiliates and sublicensees, subject to reduction under specified circumstances. The Company could potentially earn the next development milestone payment of $10.0 million under the MDCO Agreement based upon the initiation of an ALN-PCSsc Phase 1 clinical trial. For purposes of potential future revenue recognition, the Company does not believe this milestone or any future milestones are substantive. Due to the uncertainty of pharmaceutical development and the high historical failure rates generally associated with drug development, the Company may not receive any milestone or royalty payments from MDCO.
Under the MDCO Agreement, the parties will collaborate in the further development of ALN-PCS Licensed Products. The Company will retain responsibility for the development of ALN-PCS Licensed Products until Phase 1 Completion (as defined in the MDCO Agreement) at its cost, up to an agreed upon initial development cost cap. MDCO will assume all other responsibility for the development and commercialization of ALN-PCS Licensed Products, at its sole cost. Initially the collaboration included the development of both ALN-PCS02 and ALN-PCSsc in parallel. In October 2013, the parties announced the selection of ALN-PCSsc for ongoing development, in accordance with the terms of the MDCO Agreement. The collaboration between MDCO and the Company will be governed by a joint steering committee that will be comprised of an equal number of representatives from each party. The Company will be solely responsible for obtaining supply of finished product
120
Table of Contents
reasonably required for the conduct of its obligations through Phase 1 Completion, and supplying MDCO with finished product reasonably required for the first Phase 2 clinical trial of an ALN-PCS Licensed Product conducted by MDCO, at the Company’s expense, provided such costs do not exceed the development costs cap, subject to certain exceptions. After such time, MDCO will have the sole right and responsibility to manufacture and supply ALN-PCS Licensed Product for development and commercialization under the MDCO development plan, subject to the terms of the MDCO Agreement. The Company also has obligations under the MDCO Agreement to transfer certain technology to MDCO.
Unless terminated earlier in accordance with the terms of the agreement, the MDCO Agreement expires on a licensed product-by-licensed product and country-by-country basis upon expiration of the last royalty term for any licensed product in any country, where a royalty term is defined as the latest to occur of (1) the expiration of the last valid claim of patent rights covering a licensed product, (2) the expiration of the Regulatory Exclusivity, as defined in the MDCO Agreement, and (3) the twelfth anniversary of the first commercial sale of the licensed product in such country. The Company estimates that its fundamental RNAi patents covering licensed products under the MDCO Agreement will expire both in and outside of the United States generally between 2015 and 2023. The Company also estimates that its ALN-PCS product-specific patents covering licensed products under the MDCO Agreement in the United States and elsewhere will expire at the end of 2033. These patent rights are subject to potential patent term extensions and/or supplemental protection certificates extending such terms in countries where such extensions may become available. In addition, more patent filings relating to the collaboration may be made in the future.
Either party may terminate the MDCO Agreement in the event the other party fails to cure a material breach or upon patent-related challenges by the other party. The Company may terminate the agreement in the event that a lead licensed product has not been designated by the joint steering committee within a designated time period. In addition, MDCO has the right to terminate the agreement without cause at any time upon four months’ prior written notice.
The Company has determined that the significant deliverables under the MDCO Agreement include the license, the joint steering committee, technology transfer obligations, development activities through Phase 1 Completion and supply of product for a Phase 2 clinical trial. The Company also determined that, pursuant to the accounting guidance governing revenue recognition on multiple element arrangements, the license and collective undelivered activities and services do not have standalone value due to the specialized nature of the activities and services to be provided by the Company. In addition, while MDCO has the ability to grant sublicenses, it must receive the Company’s prior written consent to sublicense all or substantially all of its rights. The uniqueness of the Company’s services and the limited sublicense right are indicators that standalone value is not present in the arrangement. Therefore the deliverables are not separable and, accordingly, the license and undelivered services are being treated as a single unit of accounting. When multiple deliverables are accounted for as a single unit of accounting, the Company bases its revenue recognition pattern on the final deliverable. Under the MDCO Agreement, all deliverables are expected to be completed within five years. The Company is recognizing revenue under the MDCO Agreement on a straight-line basis over five years. The Company is not utilizing a proportional performance model since it is unable to reasonably estimate the level of effort to fulfill these obligations, primarily because the effort required under the development activities is largely unknown.
The Company received the upfront payment of $25.0 million from MDCO in February 2013, which was initially recorded as deferred revenue. As future milestones are achieved, if any, the Company will recognize as revenue a portion of the milestone payment equal to the percentage of the performance period completed when the milestone is achieved, multiplied by the amount of the milestone payment. At December 31, 2013, deferred revenue under the MDCO Agreement was $20.5 million.
Cubist Alliance
In January 2009, the Company entered into a license and collaboration agreement with Cubist (the “Cubist Agreement”) to develop and commercialize therapeutic products based on certain of the Company’s RNAi technology for the treatment of RSV infection. Licensed products initially included ALN-RSV01, as well as
121
Table of Contents
several other second-generation RNAi-based RSV inhibitors. In consideration for the rights granted to Cubist under the Cubist Agreement, in January 2009, Cubist paid the Company an upfront cash payment of $20.0 million. Under the terms of the Cubist Agreement, the Company and Cubist shared responsibility for developing licensed products in North America and each was responsible for one-half of the related development costs, subject to certain exceptions. In February 2013, Cubist notified the Company that it would not exercise its opt-in right for ALN-RSV01. In light of this determination, the Company and Cubist mutually agreed to terminate the license and collaboration agreement. As of the termination date, the parties have no further rights and obligations under the Cubist Agreement, as amended, notwithstanding anything to the contrary in the Cubist Agreement, as amended.
Under the Cubist Agreement, the last element to be delivered was the development and manufacturing services, which had an expected life of approximately eight years. The Company was recognizing the upfront payment of $20.0 million on a straight-line basis over approximately eight years because the Company was unable to reasonably estimate the level of effort to fulfill its performance obligations, and therefore, could not utilize a proportional performance model. As a result of the termination of the Cubist Agreement in February 2013 and the end of the Company’s performance obligations thereunder, the Company recognized the remaining deferred revenue of $9.7 million during 2013.
Platform Alliances
Monsanto Alliance
In August 2012, the Company and Monsanto entered into a license and collaboration agreement (the “Monsanto Agreement”), pursuant to which the Company granted to Monsanto a worldwide, exclusive, royalty bearing right and license, including the right to grant sublicenses, to the Company’s RNAi platform technology and intellectual property controlled by the Company as of the date of the Monsanto Agreement or during the 30 months thereafter, in the field of agriculture. The Monsanto Agreement also includes the transfer of technology from the Company to Monsanto and a collaborative research project (the “Monsanto Discovery Collaboration”). Under the Monsanto Agreement, Monsanto will be the Company’s exclusive collaborator in the agriculture field for a ten-year period (the “Exclusivity Period”).
In consideration for the rights granted to Monsanto under the Monsanto Agreement, Monsanto paid the Company $29.2 million in upfront cash payments. Monsanto is also required to make near-term milestone payments to the Company upon the achievement of specified technology transfer and patent-related milestones. The Company is also entitled to receive additional funding for collaborative research efforts. In the aggregate, the Company can earn up to $5.0 million in potential future milestone payments and research funding under the Monsanto Agreement. In addition, Monsanto is required to pay to the Company a percentage of specified fees from certain sublicense agreements Monsanto may enter into that include access to the Company’s intellectual property, as well as low single-digit royalty payments on worldwide, net sales by Monsanto, its affiliates and sublicensees of certain Licensed Products (as defined in the Monsanto Agreement), if any. In December 2012, the Company received a milestone payment of $1.5 million of the $5.0 million in potential milestone payments under the Monsanto Agreement based upon the achievement of a specified patent-related event. In August 2013, the Company received an additional milestone payment of $2.5 million based upon the completion of technology transfer activities. The Company could potentially earn the next and final milestone payment of $1.0 million under the Monsanto Agreement in connection with the Monsanto Discovery Collaboration. For purposes of potential future revenue recognition, the Company does not believe this milestone or any future milestones are substantive. Due to the uncertainty of the application of RNAi technology in the field of agriculture, the Company may not receive any additional milestone payments or any royalty payments from Monsanto.
The term of the Monsanto Agreement generally ends upon the expiration of the last-to-expire patent licensed under the agreement. The Company estimates that its fundamental RNAi patents licensed under the Monsanto Agreement will expire both in and outside the United States generally between 2016 and 2025, subject to any potential patent term extensions and/or supplemental protection certificates extending such term extensions in countries where such extensions may become available. Monsanto may terminate the Monsanto
122
Table of Contents
Agreement in its entirety upon 30-days’ prior written notice to the Company, provided, however, that Monsanto is required to continue to make royalty payments to the Company if any royalties were payable on net sales of a Licensed Product during the previous 24 months. The Monsanto Agreement may also be terminated by either party in the event the other party fails to cure a material breach under the agreement.
The Company determined that the significant deliverables under the Monsanto Agreement include the license, the technology transfer activities and the services that the Company will be obligated to perform under the Monsanto Discovery Collaboration. The Company also determined that, pursuant to the accounting guidance governing revenue recognition on multiple element arrangements, the license and undelivered technical transfer activities and Monsanto Discovery Collaboration services do not have standalone value due to the specialized nature of the services to be provided by the Company. In addition, while Monsanto has the ability to grant sublicenses, it cannot grant access to certain of the Company’s proprietary technology. The uniqueness of the Company’s services and the limited sublicense right are indicators that standalone value is not present in the arrangement. Therefore the deliverables are not separable and, accordingly, the license and undelivered technical transfer activities and Monsanto Discovery Collaboration services are being treated as a single unit of accounting. When multiple deliverables are accounted for as a single unit of accounting, the Company bases its revenue recognition model on the final deliverable. Under the Monsanto Agreement, the last deliverable to be completed is the Monsanto Discovery Collaboration, which must be completed within five years. The Company is recognizing revenue under the Monsanto Agreement on a straight-line basis over five years. The Company is not utilizing a proportional performance model since it is unable to reasonably estimate the level of effort to fulfill these obligations, primarily because the effort required under the Monsanto Discovery Collaboration is largely unknown.
The Company received a payment of $29.2 million from Monsanto in August 2012, which was initially recorded as deferred revenue. Under the terms of the Monsanto Agreement, in the event that during the Exclusivity Period Monsanto loses certain patent rights, and such loss has a material adverse effect on the Licensed Products, then the Company would be required to pay Monsanto up to $5.0 million as liquidated damages, and Monsanto’s royalty obligations to the Company under the Monsanto Agreement would be reduced or, under certain circumstances, terminated. The Company has the right to cure any such loss of patent rights under the Monsanto Agreement. The Company has determined that this amount is not fixed and determinable and therefore, the Company has excluded this amount from its revenue model and is deferring the recognition of $5.0 million of revenue. The Company will continue to reassess when this amount can be considered fixed and determinable. If the achievement of a milestone is considered probable at the inception of the collaboration, the Company’s policy is to include the related payment in its revenue model. The Company concluded that the receipt of the technology transfer payment of $2.5 million was probable, and therefore included this amount in the Company’s revenue model. As future milestones are achieved, if any, the Company will recognize as revenue a portion of the milestone payment equal to the percentage of the performance period completed when the milestone is achieved, multiplied by the amount of the milestone payment. At December 31, 2013, deferred revenue under the Monsanto Agreement was $25.6 million.
Takeda Alliance
In May 2008, the Company entered into a license and collaboration agreement (the “Takeda Agreement”) with Takeda to pursue the development and commercialization of RNAi therapeutics. Under the Takeda Agreement, the Company granted to Takeda a non-exclusive, worldwide, royalty-bearing license to the Company’s intellectual property, including delivery-related intellectual property, controlled by the Company as of the date of the agreement or during the five years thereafter, to develop, manufacture, use and commercialize RNAi therapeutics, subject to the Company’s existing contractual obligations to third parties. The license initially is limited to the fields of oncology and metabolic disease and may be expanded at Takeda’s option to include other therapeutic areas, subject to specified conditions.
In consideration for the rights granted to Takeda under the Takeda Agreement, Takeda agreed to pay the Company $150.0 million in upfront and near-term technology transfer payments. In addition, the Company has the option, exercisable until the start of Phase 3 development, to opt-in under a 50-50 profit sharing agreement to the development and commercialization in the United States of up to four Takeda licensed products, and would
123
Table of Contents
be entitled to opt-in rights for two additional products for each additional field expansion, if any, elected by Takeda under the Takeda Agreement. In June 2008, Takeda paid the Company an upfront payment of $100.0 million and agreed to pay to the Company an additional $50.0 million upon achievement of specified technology transfer milestones. Of this $50.0 million, $20.0 million was paid to the Company in October 2008, $20.0 million was paid to the Company in March 2010, and $10.0 million was paid to the Company in March 2011 (collectively, the “Technology Transfer Milestones”). If Takeda elects to expand its license to additional therapeutic areas, Takeda will be required to pay the Company $50.0 million for each additional field selected, if any. In addition, for each RNAi therapeutic product developed by Takeda, its affiliates and sublicensees, the Company is entitled to receive specified development, regulatory and commercialization milestone payments, totaling up to $171.0 million per product, together with up to a double-digit percentage royalty payment based on worldwide annual net sales, if any. The potential future milestone payments per product include up to $26.0 million for the achievement of specified development milestones, up to $40.0 million for the achievement of specified regulatory milestones and up to $105.0 million for the achievement of specified commercialization milestones. The Company could potentially earn the next milestone payment of $2.0 million under the Takeda Agreement based upon the achievement of a specified pre-clinical event by Takeda for an RNAi therapeutic product. For purposes of potential future revenue recognition, the Company does not believe this milestone or any future milestones are substantive. Due to the uncertainty of pharmaceutical development and the high historical failure rates generally associated with drug development, the Company may not receive any additional milestone payments or any royalty payments from Takeda.
Pursuant to the Takeda Agreement, the Company and Takeda are also collaborating on the research of RNAi therapeutics directed to one or two disease targets agreed to by the parties (the “Research Collaboration”), subject to the Company’s existing contractual obligations with third parties. Takeda also has the option, subject to certain conditions, to collaborate with the Company on the research and development of RNAi drug delivery technology for targets agreed to by the parties. In addition, the Company has a right of first negotiation to participate with Takeda in the development and commercialization of licensed products in the United States. The collaboration is governed by a joint technology transfer committee (the “JTTC”), a joint research collaboration committee (the “JRCC”) and a joint delivery collaboration committee (the “JDCC”), each of which is comprised of an equal number of representatives from each party.
The term of the Takeda Agreement generally ends upon the later of (1) the expiration of the Company’s last-to-expire patent covering a licensed product and (2) the last-to-expire term of a profit sharing agreement in the event the Company elects to enter into such an agreement. The Takeda Agreement may be terminated by either party in the event the other party fails to cure a material breach under the agreement. In addition, Takeda may terminate the agreement on a licensed product-by-licensed product or country-by-country basis upon 180-days’ prior written notice to the Company, provided, however, that Takeda is required to continue to make royalty payments to the Company for the duration of the royalty term with respect to a licensed product.
The Company has determined that the deliverables under the Takeda Agreement include the license, the joint committees (the JTTC, JRCC and JDCC), the technology transfer activities and the services that the Company will be obligated to perform under the Research Collaboration. The Company also determined that, pursuant to the accounting guidance governing revenue recognition on multiple element arrangements, the license and undelivered services (i.e., the joint committees and the Research Collaboration) are not separable and, accordingly, the license and services are being treated as a single unit of accounting. When multiple deliverables are accounted for as a single unit of accounting, the Company bases its revenue recognition pattern on the final deliverable. Under the Takeda Agreement, the last elements to be delivered are the JDCC and JTTC services, each of which has a life of no more than seven years.
The Company is recognizing the upfront payment of $100.0 million and the Technology Transfer Milestones of $50.0 million, the receipt of which the Company believed was probable at the commencement of the collaboration, on a straight-line basis over seven years because the Company is unable to reasonably estimate the level of effort to fulfill these obligations, primarily because the effort required under the Research Collaboration is largely unknown, and therefore, cannot utilize a proportional performance model. As future milestones are achieved, if any, the Company will recognize as revenue a portion of the milestone payment equal to the percentage
124
Table of Contents
of the performance period completed when the milestone is achieved, multiplied by the amount of the milestone payment. At December 31, 2013, deferred revenue under the Takeda Agreement was $30.8 million.
Roche/Arrowhead Alliance
In July 2007, the Company and, for limited purposes, Alnylam Europe, entered into a license and collaboration agreement (the “LCA”) with Roche. Under the LCA, which became effective in August 2007, the Company granted Roche a non-exclusive license to the Company’s intellectual property, including delivery-related intellectual property existing as of the date of the LCA, to develop and commercialize therapeutic products that function through RNAi, subject to the Company’s existing contractual obligations to third parties. In November 2010, Roche announced the discontinuation of certain activities in research and early development, including its RNAi research efforts. In October 2011, Arrowhead announced its acquisition of RNA therapeutics assets from Roche, including the LCA. As a result of this acquisition, Arrowhead owns all of the rights and obligations of Roche under the LCA. The license is initially limited to four therapeutic areas, and may be expanded to include additional therapeutic areas upon payment to the Company by Arrowhead of an additional $50.0 million for each additional therapeutic area, if any.
In consideration for the rights the Company granted under the LCA, Roche paid the Company $273.5 million in upfront cash payments. In addition, in exchange for the Company’s contributions under the LCA, for each RNAi therapeutic product developed by Arrowhead, its affiliates or sublicensees under the LCA, the Company is entitled to receive milestone payments upon achievement of specified development, regulatory and commercialization events, totaling up to an aggregate of $100.0 million per therapeutic target, together with a single-digit percentage royalty payment based on worldwide annual net sales, if any. The potential future milestone payments for each therapeutic target include up to $17.5 million for the achievement of specified development milestones, up to $62.5 million for the achievement of specified regulatory milestones and up to $20.0 million for the achievement of specified commercialization milestones. The Company could potentially earn the next development milestone payment of $1.0 million under the LCA based upon the initiation of the first Phase 1 clinical trial by Arrowhead for an RNAi therapeutic product. For purposes of potential future revenue recognition, the Company does not believe this milestone or any future milestones are substantive. Due to the uncertainty of pharmaceutical development and the high historical failure rates generally associated with drug development, the Company may not receive any milestone or royalty payments from Arrowhead. Under the LCA, the Company and Roche also established a discovery collaboration in October 2009 (“Discovery Collaboration”), subject to the Company’s existing contractual obligations to third parties.
The term of the LCA generally ends upon the later of ten years from the first commercial sale of a licensed product and the expiration of the last-to-expire patent covering a licensed product. Arrowhead may terminate the LCA, on a licensed product-by-licensed product, licensed patent-by-licensed patent, and country-by-country basis, upon 180-days’ prior written notice, but is required to continue to make milestone and royalty payments to the Company if any royalties were payable on net sales of a terminated licensed product during the previous 12 months. The LCA may also be terminated by either party in the event the other party fails to cure a material breach under the LCA.
In July 2007, the Company executed a common stock purchase agreement (the “Common Stock Purchase Agreement”) with Roche Finance Ltd, an affiliate of Roche. In connection with the execution of the LCA and the Common Stock Purchase Agreement, the Company also executed a share purchase agreement (the “Alnylam Europe Purchase Agreement”) with Alnylam Europe and Roche Beteiligungs GmbH, an affiliate of Roche (“Roche Germany”). Under the terms of the Alnylam Europe Purchase Agreement, the Company sold substantially all of the non-intellectual property assets of Alnylam Europe to Roche Germany for an aggregate purchase price of $15.0 million.
In summary, the Company received upfront payments totaling $331.0 million under the Roche alliance, which included an upfront payment under the LCA of $273.5 million, $42.5 million under the Common Stock Purchase Agreement and $15.0 million under the Alnylam Europe Purchase Agreement. The Company initially recorded $278.2 million of these proceeds as deferred revenue in connection with this alliance.
125
Table of Contents
The Company determined that the deliverables under these agreements included the license, the Alnylam Europe assets and employees, the steering committees (joint steering committee and future technology committee) and the services under the Discovery Collaboration. The Company also determined that, pursuant to the accounting guidance governing revenue recognition on multiple element arrangements, the license and assets of Alnylam Europe are not separable from the undelivered services (i.e., the steering committees and Discovery Collaboration) and, accordingly the license and the services were treated as a single unit of accounting. When multiple deliverables are accounted for as a single unit of accounting, the Company bases its revenue recognition pattern on the final deliverable. Under the Arrowhead alliance, the steering committee services and the Discovery Collaboration services are the final deliverables and all such services ended, contractually, in August 2012, five years from the effective date of the LCA.
The Company recognized the revenue related to these agreements on a straight-line basis over five years because the Company could not reasonably estimate the total level of effort required to complete its service obligations under the LCA, and therefore, could not utilize a proportional performance model. At December 31, 2013, there was no remaining deferred revenue under the LCA as the Company recognized all remaining Roche/Arrowhead revenue during the quarter ended September 30, 2012. The Company will recognize future milestones under the LCA, if any, when such milestones are achieved.
Discovery and Development Alliances
Isis Collaboration and License Agreement
In April 2009, the Company and Isis amended and restated their existing strategic collaboration and license agreement (as amended and restated, the “Amended and Restated Isis Agreement”), originally entered into in March 2004, to extend the broad cross-licensing arrangement regarding double-stranded RNAi that was established in 2004, pursuant to which Isis granted the Company licenses to its current and future patents and patent applications relating to chemistry and to RNA-targeting mechanisms for the research, development and commercialization of double-stranded RNA (“dsRNA”) products. The Company has the right to use Isis technologies in its development programs or in collaborations and Isis agreed not to grant licenses under these patents to any other organization for the discovery, development and commercialization of dsRNA products designed to work through an RNAi mechanism, except in the context of a collaboration in which Isis plays an active role. The Company granted Isis non-exclusive licenses to its current and future patents and patent applications relating to RNA-targeting mechanisms and to chemistry for research use. The Company also granted Isis the non-exclusive right to develop and commercialize dsRNA products developed using RNAi technology against a limited number of targets. In addition, the Company granted Isis non-exclusive rights to research, develop and commercialize single-stranded RNA products. In August 2012, the Company and Isis amended the Amended and Restated Isis Agreement to provide for the discovery, development and commercialization of dsRNA products by the Company or its sublicensees in the field of agriculture.
In 2004, under the terms of the original Isis agreement, the Company paid Isis an upfront license fee of $5.0 million. The Company also agreed to pay Isis milestone payments, totaling up to approximately $3.4 million, upon the occurrence of specified development and regulatory events, and low single-digit royalties on sales, if any, for each product that the Company or a collaborator develops using Isis intellectual property. In addition, the Company agreed to pay to Isis a percentage of specified fees from strategic collaborations the Company may enter into that include access to Isis’ intellectual property.
Isis agreed to pay the Company, per therapeutic target, a license fee of $0.5 million, milestone payments totaling approximately $3.4 million, payable upon the occurrence of specified development and regulatory events, and low single-digit royalties on sales, if any, for each product developed by Isis or a collaborator that utilizes the Company’s intellectual property. Isis has the right to elect up to ten non-exclusive target licenses under the agreement and has the right to purchase one additional non-exclusive target per year during the term of the collaboration.
126
Table of Contents
The term of the Amended and Restated Isis Agreement generally ends upon the expiration of the last-to-expire patent licensed thereunder, whether such patent is a patent licensed by the Company to Isis, or vice versa. As the license will include additional patents, if any, filed to cover future inventions, if any, the date of expiration cannot be determined at this time.
During the years ended December 31, 2013, 2012 and 2011, as a result of certain payments received by the Company in connection with the MDCO, Monsanto and Genzyme alliances, the Company paid $0.7 million, $2.5 million and zero to Isis, respectively. These license fees were charged to research and development expense.
| 4. | PROPERTY AND EQUIPMENT, NET |
Property and equipment consist of the following at December 31, 2013 and 2012, in thousands:
| December 31, | ||||||||||||
| Useful Life | 2013 | 2012 | ||||||||||
| Laboratory equipment |
5 years | $ | 22,729 | $ | 21,201 | |||||||
| Computer equipment and software |
3 years | 4,261 | 4,203 | |||||||||
| Furniture and fixtures |
5 years | 1,793 | 1,793 | |||||||||
| Leasehold improvements |
* | 28,953 | 19,862 | |||||||||
| Construction in progress |
— | 28 | 8,209 | |||||||||
|
|
|
|
|
|||||||||
| 57,764 | 55,268 | |||||||||||
| Less: accumulated depreciation |
(41,316 | ) | (35,469 | ) | ||||||||
|
|
|
|
|
|||||||||
| $ | 16,448 | $ | 19,799 | |||||||||
|
|
|
|
|
|||||||||
| * | Shorter of asset life or lease term |
During the years ended December 31, 2013, 2012 and 2011, the Company recorded $6.1 million, $4.6 million and $5.0 million, respectively, of depreciation expense related to its property and equipment.
| 5. | 2012 RESTRUCTURING |
In January 2012, the Company’s Board of Directors approved, and the Company implemented, a strategic corporate restructuring pursuant to which the Company reduced its overall workforce by approximately 33%, to approximately 115 employees. During the three months ended March 31, 2012, the Company substantially completed the implementation of the strategic corporate restructuring and recorded $3.9 million of restructuring-related costs in operating expenses, including employee severance, benefits and related costs. The Company paid substantially all of these restructuring costs during 2012. The Company did not incur any additional significant costs associated with this restructuring and does not expect to incur any additional significant costs in the future.
The following table summarizes the components of the Company’s restructuring expenses recorded in operating expenses and in current liabilities, in thousands:
| Original Charges and Amounts Accrued |
(Reversals) or Adjustments to Charges |
Amounts Paid Through December 31, 2013 |
Amounts Accrued at December 31, 2013 |
|||||||||||||
| Employee severance, benefits and related costs |
$ | 3,909 | $ | (179 | ) | $ | 3,730 | $ | — | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
127
Table of Contents
| 6. | COMMITMENTS AND CONTINGENCIES |
Purchase Commitments
The Company has future purchase commitments totaling $62.7 million at December 31, 2013, of which $33.6 million is expected to be incurred in 2014 and $29.1 million is expected to be incurred past 2014. These commitments are related to purchase orders, clinical and pre-clinical agreements, and other purchase commitments for goods or services that the Company is likely to continue, regardless of the fact that they were cancelable at December 31, 2013.
Technology License Commitments
The Company has licensed from third parties the rights to use certain technologies in its research processes as well as in any products the Company may develop including these licensed technologies. In accordance with the related license agreements, the Company is required to make certain fixed payments to the licensor or a designee of the licensor over various agreement terms. Many of these agreement terms are consistent with the remaining lives of the underlying intellectual property that the Company has licensed. At December 31, 2013, the Company was committed to make the following fixed, estimated and cancelable payments under existing license agreements, in thousands:
| Year Ending December 31, |
||||
| 2014 |
$ | 7,533 | ||
| 2015 |
694 | |||
| 2016 |
649 | |||
| 2017 |
669 | |||
| 2018 |
729 | |||
| Thereafter |
7,196 | |||
|
|
|
|||
| Total |
$ | 17,470 | ||
|
|
|
|||
Operating Leases
The Company leases office and laboratory space located at 300 Third Street, Cambridge, Massachusetts (the “Premises”) for its corporate headquarters and primary research facility under a non-cancelable operating lease agreement (the “Third Street Lease”) with ARE-MA Region No. 28 LLC (the “Landlord”). Under the Third Street Lease, the Company leases a total of approximately 129,000 square feet of office and laboratory space at the Premises. The term of the Third Street Lease expires in September 2016. The Company has the option to extend the Third Street Lease for two successive five-year extensions.
The Company separately agreed, with the Landlord’s consent, to sublease a portion of the Premises consisting of 34,014 square feet (the “Subleased Premises) beginning on September 1, 2010 pursuant to a sublease agreement between the Company and sanofi-aventis U.S. Inc. (“sanofi”) dated August 3, 2010 (the “Sublease”). In November 2011, the Company and sanofi entered into a first amendment to the Sublease, pursuant to which the Company agreed, with the Landlord’s consent, to extend the Sublease of the Subleased Premises through September 30, 2016 (the Sublease, as so amended by the first amendment, the “Amended Sublease”). Pursuant to the terms of the Amended Sublease, sanofi had an option to terminate the Amended Sublease as of December 31, 2013, with advance notice and payment of a termination fee to the Company. In connection with the execution of the Amended Sublease, the Company and the Landlord entered into an amendment to the Third Street Lease (the Third Street Lease, as so amended, the “Amended Third Street Lease”) to, among other things, change the allocation as between the Company and the Landlord of Excess Income (as defined in the Amended Third Street Lease) received by the Company in connection with any assignment or subletting of any or all of the Premises (including the Subleased Premises). In March 2013, sanofi notified the Company that it was exercising its right to terminate Amended Sublease as of December 31, 2013. As a result of this early termination, sanofi paid the Company a termination fee. The Company does not believe that the
128
Table of Contents
termination of the sanofi sublease was material to its business. In December 2013, the Company agreed, with the Landlord’s consent, to sublease a portion of the Premises consisting of approximately 18,000 square feet through September 30, 2016 pursuant to a sublease agreement between the Company and a third party.
On February 10, 2012, the Company entered into a non-cancelable real property lease agreement (“the BMR Lease”) with BMR-Fresh Pond Research Park LLC (“BMR”) for the Company’s manufacturing facility. Under the BMR Lease, the Company leases approximately 15,000 square feet of office and laboratory space located at 665 Concord Avenue, Cambridge, Massachusetts. The term of the BMR Lease expires August 31, 2017. The Company has the option to extend the BMR Lease for two successive five-year extensions.
From 2004 through 2008, the Company received $7.3 million in leasehold improvement incentives from the Landlord in connection with the Third Street Lease. In addition, the Company received $0.2 million and $1.8 million in leasehold improvement incentives from BMR during the years ended December 31, 2013 and 2012, respectively. These leasehold improvement incentives are being accounted for as a reduction in rent expense ratably over the Amended Third Street Lease and BMR Lease terms. The balance from these leasehold improvement incentives is included in current portion of deferred rent and deferred rent, net of current portion in the consolidated balance sheets at December 31, 2013 and 2012.
Total rent expense, including operating expenses, under the Company’s real property leases was $4.9 million, $6.4 million and $6.5 million for the years ended December 31, 2013, 2012 and 2011, respectively.
Future minimum payments under the Company’s non-cancelable leases are approximately as follows, in thousands:
| Year Ending December 31, |
||||
| 2014 |
$ | 6,303 | ||
| 2015 |
6,550 | |||
| 2016 |
5,192 | |||
| 2017 |
364 | |||
| 2018 |
— | |||
|
|
|
|||
| Total |
$ | 18,409 | ||
|
|
|
|||
Litigation
University of Utah Litigation
On March 22, 2011, The University of Utah (“Utah”) filed a civil complaint in the United States District Court for the District of Massachusetts against the Company, Max Planck Gesellschaft Zur Foerderung Der Wissenschaften e.V. and Max Planck Innovation GmbH (together, “Max Planck”), the Whitehead Institute for Biomedical Research (“Whitehead”), the Massachusetts Institute of Technology (“MIT”) and the University of Massachusetts (“UMass”), claiming a professor at Utah is the sole inventor or, in the alternative, a joint inventor, of the Tuschl patents. Utah did not serve the original complaint on the Company or the other defendants. On July 6, 2011, Utah filed an amended complaint alleging substantially the same claims against the Company, Max Planck, Whitehead, MIT and UMass. The amended complaint was served on the Company on July 14, 2011. Utah is seeking changes to the inventorship of the Tuschl patents, unspecified damages and other relief. On October 31, 2011, the Company, Max Planck, Whitehead, MIT and UMass filed a motion to dismiss. Also on October 31, 2011, UMass filed a motion to dismiss on separate grounds, which the Company, Max Planck, Whitehead and MIT have joined. On December 31, 2011, Utah filed a second amended complaint dropping UMass as a defendant and adding as defendants several UMass officials. In June 2012, the Court denied both motions to dismiss. The Company, Max Planck, Whitehead, MIT and UMass filed an appeal of the Court’s ruling on the motion to dismiss for lack of jurisdiction and a motion requesting that the Court stay the case pending the outcome of the appeal. In July 2012, the Court stayed discovery in the case pending the outcome of the defendants’ appeal. Oral arguments in the appeal were heard in early March 2013 in the United States Court of
129
Table of Contents
Appeals for the Federal Circuit (the “CAFC”). In August 2013, the CAFC affirmed the lower Court’s ruling, in a split decision. The Company believes the majority made an error in law when affirming the lower Court’s decision, and in September 2013, the Company filed a petition with the CAFC for rehearing or rehearing en banc. In October 2013, the CAFC invited Utah to file an answer to the petition. In November 2013, the CAFC denied the Company’s petition for rehearing or rehearing en banc and remanded the case back to the lower Court. In February 2014, the Company filed a petition for writ of certiorari from the Supreme Court and a motion to stay the lower Court proceedings pending a decision from the Supreme Court on the Company’s petition. The Company is awaiting the Courts’ decisions.
Although the Company believes it has meritorious defenses and intends to vigorously defend itself in this matter, litigation is subject to inherent uncertainty and a court could ultimately rule against the Company. In addition, the defense of litigation and related matters are costly and may divert the attention of the Company’s management and other resources that would otherwise be engaged in other activities. The Company has not recorded an estimate of the possible loss associated with this legal proceeding due to the uncertainties related to both the likelihood and the amount of any possible loss or range of loss.
The Company’s accounting policy for accrual of legal costs is to recognize such expenses as incurred.
Tekmira Settlement Agreement
On November 12, 2012, the Company, TPC, Protiva Biotherapeutics, Inc., a wholly owned subsidiary of TPC (“Protiva,” and together with TPC, “Tekmira”) and Acuitas Therapeutics Inc. (formerly AlCana Technologies, Inc.) (“Acuitas”) entered into a settlement agreement and general release resolving all ongoing litigation, as well as a patent interference proceeding between the Company and Protiva. The terms of the settlement agreement include mutual releases and dismissal with prejudice of all claims and counterclaims in the following litigation between the parties: (i) Tekmira Pharmaceuticals Corp., et al. v. Alnylam Pharmaceuticals, Inc., et al., Civ. A. No. 11-1010-BLS2, pending in the Business Litigation Section of the Massachusetts Superior Court for Suffolk County; (ii) Tekmira Pharmaceuticals Corp. v. Michael Hope, et al., No. S117660, pending in the Supreme Court of British Columbia, Canada; (iii) Alnylam Pharmaceuticals, Inc., et al. v. Tekmira Pharmaceuticals Corp., Civ. A. No. 1:12-CV-10087, pending in the United States District Court for the District of Massachusetts; and (iv) Alnylam Pharmaceuticals, Inc., et al. v. Tekmira Pharmaceuticals Corp., Court File No. T-1783-12, pending in the Federal Court of Canada. In addition, as part of the settlement agreement, the parties agreed to a covenant not to sue one another in the future on matters released under the settlement agreement, as well as substantial liquidated damages to be paid by any party that breaches such covenant. The parties have also agreed to resolve any future disputes that may arise over the next three years through binding arbitration.
Pursuant to the settlement agreement, the Company and Tekmira also agreed to resolve the interference proceeding declared by the United States Board of Patent Appeals and Interferences between the Company and Protiva, captioned Protiva Biotherapeutics, Inc. v. Alnylam Pharmaceuticals, Inc., Patent Interference No. 105792.
Contemporaneously with the execution of the settlement agreement, the Company and Tekmira restructured their contractual relationship and entered into a cross-license agreement that supersedes the prior license and manufacturing agreements among the Company, TPC and Protiva. In connection with this restructuring, the Company incurred a $65.0 million charge to operating expenses for the year ended December 31, 2012. Specifically, the Company made a one-time payment of $30.0 million to Tekmira for the termination of, and its release from, all of its obligations under the manufacturing agreement with TPC, including without limitation the obligations to obtain materials and/or services from TPC. Further, the Company elected to buy-down certain future potential milestone and royalty payments due to Tekmira for certain of the Company’s RNAi therapeutics, formulated using LNP technology. Specifically, pursuant to the cross-license agreement, the Company made a one-time payment of $35.0 million to Tekmira, which amount constituted payment for the termination of the 2008 license agreements with TPC and Protiva and the parties’ rights and obligations thereunder, as well as the buy-down of certain milestone payments and the significant reduction of royalty rates for ALN-VSP, ALN-PCS02 and ALN-TTR02 (“patisiran”). In addition, under the 2012 cross-license agreement, the Company was
130
Table of Contents
obligated to pay TPC an aggregate of $10.0 million in contingent milestone payments related to advancement of ALN-VSP and patisiran, which now represent the only potential milestones due to Tekmira for ALN-VSP, ALN-PCS02 and patisiran lipid nanoparticle (“LNP”)-based RNAi therapeutics. In the fourth quarter of 2013, the Company paid TPC $5.0 million in connection with the initiation of the patisiran Phase 3 clinical trial. With respect to the second $5.0 million milestone, in August 2013, the Company initiated binding arbitration proceedings to resolve a disagreement with TPC regarding the achievement by TPC of this milestone under the parties cross-license agreement relating to the manufacture of ALN-VSP clinical trial material for use in China. The Company’s policy is to expense these potential milestones when incurred and record them as research and development expense. A description of the Company’s cross-license agreement with Tekmira is included in Part I, Item 1, “ —Delivery-Related Licenses and Collaborations—Tekmira,” of this annual report on Form 10-K.
Indemnifications
Licensor indemnification — In connection with the Company’s license agreements with Max Planck relating to the Tuschl I and Tuschl II patent applications, the Company is required to indemnify Max Planck for certain damages arising in connection with the intellectual property rights licensed under the agreements. Under the Max Planck indemnification agreement, the Company is responsible for paying the costs of any litigation relating to the license agreements or the underlying intellectual property rights, including the costs associated with certain litigation regarding the Tuschl patents, which was settled during 2011. In connection with the Company’s research agreement with Acuitas, the Company agreed to indemnify Acuitas for certain legal costs, subject to certain exceptions and limitations, associated with the Tekmira litigation described above. These indemnification costs were charged to general and administrative expense. The Company is also a party to a number of agreements entered into in the ordinary course of business, which contain typical provisions that obligate the Company to indemnify the other parties to such agreements upon the occurrence of certain events. Such indemnification obligations are usually in effect from the date of execution of the applicable agreement for a period equal to the applicable statute of limitations.
The maximum potential future liability of the Company under any such indemnification provisions is uncertain. However, to date, other than certain costs associated with certain previously settled litigation related to the Tuschl patents and the Tekmira litigation described above, the Company has not incurred material costs to defend lawsuits or settle claims related to these indemnification provisions. The Company has determined that the estimated aggregate fair value of its potential liabilities under all such indemnification provisions is minimal and has not recorded any liability related to such indemnification provisions at December 31, 2013 or 2012.
| 7. | STOCKHOLDERS’ EQUITY |
Preferred Stock
The Company has authorized up to 5,000,000 shares of preferred stock, $0.01 par value per share, for issuance. The preferred stock will have such rights, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences, as shall be determined by the Company’s Board of Directors upon its issuance. At December 31, 2013 and 2012, there were no shares of preferred stock outstanding.
Stockholder Rights Agreement
On July 13, 2005, the Board of Directors of the Company declared a dividend of one right (collectively, the “Rights”) to buy one one-thousandth of a share of newly designated Series A Junior Participating Preferred Stock (“Series A Junior Preferred Stock”) for each outstanding share of the Company’s common stock to stockholders of record at the close of business on July 26, 2005. Initially, the Rights are not exercisable and will be attached to all certificates representing outstanding shares of common stock. The Rights will expire at the close of business on July 13, 2015 unless earlier redeemed or exchanged. Until a Right is exercised, the holder thereof will have no rights as a stockholder of the Company, including the right to vote or to receive dividends. Subject to the terms
131
Table of Contents
and conditions of the rights agreement (the “Rights Agreement”), the Rights will become exercisable upon the earlier of (1) ten business days following the later of (a) the first date of a public announcement that a person or group (an “Acquiring Person”) acquires, or obtained the right to acquire, beneficial ownership of 20% or more of the outstanding shares of common stock of the Company or (b) the first date on which an executive officer of the Company has actual knowledge that an Acquiring Person has become such or (2) ten business days following the commencement of a tender offer or exchange offer that would result in a person or group beneficially owning more than 20% of the outstanding shares of common stock of the Company. Each Right entitles the holder to purchase one one-thousandth of a share of Series A Junior Preferred Stock at an initial purchase price of $80.00 in cash, subject to adjustment. In the event that any person or group becomes an Acquiring Person, unless the event causing the 20% threshold to be crossed is a Permitted Offer (as defined in the Rights Agreement), each Right not owned by the Acquiring Person will entitle its holder to receive, upon exercise, that number of shares of common stock of the Company (or in certain circumstances, cash, property or other securities of the Company) which equals the exercise price of the Right divided by 50% of the current market price (as defined in the Rights Agreement) per share of such common stock at the date of the occurrence of the event. In the event that, at any time after any person or group becomes an Acquiring Person, (i) the Company is consolidated with, or merged with and into, another entity and the Company is not the surviving entity of such consolidation or merger (other than a consolidation or merger which follows a Permitted Offer) or if the Company is the surviving entity, but shares of its outstanding common stock are changed or exchanged for stock or securities (of any other person) or cash or any other property, or (ii) more than 50% of the Company’s assets or earning power is sold or transferred, each holder of a Right (except Rights which previously have been voided as set forth in the Rights Agreement) shall thereafter have the right to receive, upon exercise, that number of shares of common stock of the acquiring company which equals the exercise price of the Right divided by 50% of the current market price of such common stock at the date of the occurrence of the event.
Public Offerings
In February 2012, the Company sold an aggregate of 8,625,000 shares of its common stock through an underwritten public offering at a price to the public of $10.75 per share. As a result of this offering, the Company received aggregate net proceeds of approximately $86.8 million, after deducting underwriting discounts and commissions and other estimated offering expenses of approximately $5.9 million.
In January 2013, the Company sold an aggregate of 9,200,000 shares of its common stock through an underwritten public offering at a price to the public of $20.13 per share. As a result of this offering, the Company received aggregate net proceeds of approximately $173.6 million, after deducting underwriting discounts and commissions and other estimated offering expenses of approximately $11.6 million.
| 8. | STOCK INCENTIVE PLANS |
Stock Plans
In June 2009, the Company’s stockholders approved an amendment and restatement of the Company’s 2004 Stock Incentive Plan (the “Amended and Restated 2004 Plan”), which replaced the Company’s 2004 Stock Incentive Plan, as amended (the “2004 Plan”). At December 31, 2013, the Amended and Restated 2004 Plan provided for the granting of stock options to purchase up to 12,366,485 shares of common stock. Prior to the adoption of the Amended and Restated 2004 Plan, the Company was authorized to grant both stock options and restricted stock awards under the 2004 Plan. As of the effective date of the Amended and Restated 2004 Plan, the Company may only grant stock options under the Amended and Restated 2004 Plan, provided that the terms and conditions of any restricted stock awards outstanding under the 2004 Plan will continue to be governed by the Amended and Restated 2004 Plan.
In June 2009, the Company’s stockholders also approved the Company’s 2009 Stock Incentive Plan (the “2009 Plan”). The 2009 Plan provides for the granting of stock options, restricted stock awards and units, stock appreciation rights and other stock-based awards. In June 2013, the Company’s stockholders approved an amendment to the 2009 Plan, which increased the number of shares of common stock authorized for issuance from 2,200,000 to 5,900,000. The 2009 Plan has a fungible share pool. Any award that is not a full value award is counted against the authorized share limits specified in the 2009 Plan as one share for each share of common
132
Table of Contents
stock subject to the award, and all full value awards, defined in the 2009 Plan as restricted stock awards or other stock-based awards, are counted as one and a half shares for each one share of common stock subject to such full value award. In addition, the 2009 Plan includes a non-employee director stock option program under which each eligible non-employee director is entitled to (1) a grant of an option to purchase 30,000 shares of common stock upon his or her initial appointment to the Board of Directors, or such other amount as the Board of Directors deems appropriate, and (2) a subsequent annual grant of an option to purchase 15,000 shares of common stock based on continued service, made on the date of each annual meeting of stockholders, provided the non-employee director has served as a director for at least six months and is serving as a director immediately prior to and following such annual meeting. The chairman of the audit committee will receive an additional annual grant of an option to purchase 10,000 shares of common stock based on continued service and the chairman of the science and technology committee will receive an additional annual grant of an option to purchase 15,000 shares of common stock based on continued service. Stock options granted by the Company to non-employee directors upon their appointment to the Board of Directors vest as to one-third of such shares on each of the first, second and third anniversaries of the date of grant, and those granted at each year’s annual meeting at which they serve as a director vest in full on the first anniversary of the date of grant.
At December 31, 2013, an aggregate of 11,369,175 shares of common stock were reserved for issuance under the Company’s stock plans, including outstanding stock options to purchase 8,713,395 shares of common stock, 2,426,811 shares of common stock available for additional equity awards and 228,969 shares available for future grant under the Company’s 2004 Employee Stock Purchase Plan (the “2004 Purchase Plan”). Each option shall expire within ten years of issuance. Stock options granted by the Company to employees generally vest as to 25% of the shares on the first anniversary of the grant date and 6.25% of the shares at the end of each successive three-month period until fully vested.
Stock-Based Compensation
The Company recorded $14.1 million, $9.0 million and $14.8 million of stock-based compensation expense for the years ended December 31, 2013, 2012 and 2011, respectively, related to employee stock options and the 2004 Purchase Plan.
The Company accounts for non-employee grants as an expense over the vesting period of the underlying stock options. At the end of each financial reporting period prior to vesting, the Company re-measures the value of these stock options (as calculated using the Black-Scholes option-pricing model) using the then-current fair value of the Company’s common stock. The Company recognized $3.2 million, $1.0 million and $0.4 million of non-employee stock-based compensation expense for the years ended December 31, 2013, 2012 and 2011, respectively.
In October 2010, the Company granted 113,370 shares of restricted stock of the Company to certain employees. These restricted stock awards were valued at $1.4 million on the grant date. These restricted stock awards vested ratably over an approximate three-year period. In May 2011, the Company granted an aggregate of 229,806 shares of performance-based restricted stock awards to its employees, excluding the Company’s leadership team. These restricted stock awards were valued at $2.3 million on the grant date and had a contractual term of five years. The vesting of these awards was predicated on the Company’s achievement of certain clinical development goals. These awards have vested in full due to the achievement of these goals in 2011 and 2013. In January 2012, as part of its post-restructuring retention program, the Company granted an aggregate of 508,928 shares of restricted stock to its retained employees, excluding the Company’s chief executive officer and president and chief operating officer. These restricted stock awards were valued at $5.3 million on the grant date and vest in full on the second anniversary of the grant date. The Company recognized an aggregate of $3.4 million, $2.4 million and $1.5 million of stock-based compensation expense related to all of these restricted stock awards for the years ended December 31, 2013, 2012 and 2011, respectively.
133
Table of Contents
Valuation Assumptions for Stock Options
The fair value of stock options at date of grant, based on the following assumptions, was estimated using the Black-Scholes option-pricing model. The Company’s expected stock-price volatility assumption is based on the historical volatility of the Company’s publicly traded stock. The expected life assumption is based on the Company’s historical data. The dividend yield assumption is based on the fact that the Company has never paid cash dividends and has no present intention to pay cash dividends. The risk-free interest rate used for each grant is equal to the zero coupon rate for instruments with a similar expected life. The Company has applied an annual forfeiture rate of 8.2% to all unvested employee stock options and restricted stock at December 31, 2013 based on an analysis of its historical forfeitures. The Company will record additional expense if the actual forfeitures are lower than estimated and will record a recovery of prior expense if the actual forfeitures are higher than estimated.
| 2013 | 2012 | 2011 | ||||||||||
| Risk-free interest rate |
1.0-2.0 | % | 0.8-1.0 | % | 1.2-2.6 | % | ||||||
| Expected dividend yield |
— | — | — | |||||||||
| Expected option life |
5.6 years | 5.6-5.9 years | 5.8-5.9 years | |||||||||
| Expected volatility |
55-56 | % | 57 | % | 55-57 | % | ||||||
At December 31, 2013, there was $31.8 million of unearned compensation expense remaining related to unvested employee stock options to be recognized as expense over a weighted-average period of approximately three years.
Stock Option Activity
The following table summarizes the activity of the Company’s stock option plans:
| Number of Options |
Weighted Average Exercise Price |
Weighted Average Remaining Contractual Term (in years) |
Aggregate Intrinsic Value (in thousands) |
|||||||||||||
| Outstanding, December 31, 2012 |
8,931,583 | $ | 15.71 | |||||||||||||
| Granted |
1,849,104 | $ | 54.23 | |||||||||||||
| Exercised |
(2,031,916 | ) | $ | 13.96 | ||||||||||||
| Cancelled |
(35,376 | ) | $ | 21.56 | ||||||||||||
|
|
|
|
|
|||||||||||||
| Outstanding, December 31, 2013 |
8,713,395 | $ | 24.26 | 6.5 | $ | 348,864 | ||||||||||
|
|
|
|
|
|||||||||||||
| Exercisable at December 31, 2013 |
5,324,421 | $ | 18.21 | 5.0 | $ | 245,378 | ||||||||||
| Vested or expected to vest at December 31, 2013 |
7,650,775 | $ | 20.06 | 6.1 | $ | 338,514 | ||||||||||
The weighted average fair value of stock options granted was $26.33, $8.68 and $7.68 per share for the years ended December 31, 2013, 2012 and 2011, respectively. The intrinsic value of stock options exercised was $57.9 million, $4.8 million and $40,000 for the years ended December 31, 2013, 2012 and 2011, respectively.
134
Table of Contents
Restricted Stock Awards
The following table summarizes the activity of the Company’s restricted stock awards:
| Number of Awards |
Weighted Average Grant Date Fair Value |
|||||||
| Unvested at December 31, 2012 |
603,760 | $ | 9.37 | |||||
| Granted |
1,966 | $ | 61.03 | |||||
| Vested |
(105,093 | ) | $ | 10.58 | ||||
| Forfeited |
(1,121 | ) | $ | 10.23 | ||||
|
|
|
|||||||
| Unvested at December 31, 2013 |
499,512 | $ | 10.69 | |||||
|
|
|
|||||||
The total grant date fair value of restricted stock awards that vested during the years ended December 31, 2013, 2012 and 2011 was $1.1 million, $1.5 million and $0.5 million, respectively. At December 31, 2013, there remained $0.3 million of unearned compensation expense related to unvested restricted stock awards to be recognized as expense over a weighted-average period of approximately one month.
Employee Stock Purchase Plan
In 2004, the Company adopted the 2004 Purchase Plan with 315,789 shares authorized for issuance. In June 2010, the Company’s stockholders approved an amendment to the 2004 Purchase Plan, which increased the shares authorized for issuance from 315,789 shares to 715,789 shares. Under the 2004 Purchase Plan, each offering period is six months, at the end of which employees may purchase shares of common stock through payroll deductions made over the term of the offering. The per-share purchase price at the end of each offering period is equal to the lesser of 85% of the closing price of the common stock at the beginning or end of the offering period. The Company issued 45,141, 73,590 and 79,038 shares during the years ended December 31, 2013, 2012 and 2011, respectively, and at December 31, 2013, 228,969 shares were available for issuance under the 2004 Purchase Plan.
The weighted average fair value of stock purchase rights granted as part of the 2004 Purchase Plan was $11.49, $2.82 and $3.46 per share for the years ended December 31, 2013, 2012 and 2011, respectively. The fair value was estimated using the Black-Scholes option-pricing model. The Company used a weighted-average stock-price volatility of 55%, expected option life assumption of six months and a risk-free interest rate of 0.1%. The Company recorded $0.3 million, $0.2 million and $0.3 million of stock-based compensation expense for the years ended December 31, 2013, 2012 and 2011, respectively, related to the 2004 Purchase Plan.
| 9. | INCOME TAXES |
The domestic and foreign components of loss before income taxes are as follows, in thousands:
| 2013 | 2012 | 2011 | ||||||||||
| Domestic |
$ | (81,109 | ) | $ | (116,400 | ) | $ | (57,481 | ) | |||
| Foreign |
(10,811 | ) | (186 | ) | (168 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Loss before income taxes |
$ | (91,920 | ) | $ | (116,586 | ) | $ | (57,649 | ) | |||
|
|
|
|
|
|
|
|||||||
135
Table of Contents
Deferred income taxes reflect the tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting and income tax purposes. The Company establishes a valuation allowance when uncertainty exists as to whether all or a portion of the net deferred tax assets will be realized. Components of the net deferred tax (liability) asset at December 31, 2013 and 2012 are as follows, in thousands:
| 2013 | 2012 | |||||||
| Deferred tax assets: |
||||||||
| Net operating loss carryforwards |
$ | 124,797 | $ | 102,819 | ||||
| Research and development credits |
23,902 | 17,443 | ||||||
| AMT credits |
788 | 788 | ||||||
| Foreign tax credits |
3,196 | 3,196 | ||||||
| Capitalized research and development and start-up costs |
16,214 | 16,102 | ||||||
| Deferred revenue |
37,273 | 30,776 | ||||||
| Deferred compensation |
21,706 | 24,804 | ||||||
| Intangible assets |
2,210 | 2,540 | ||||||
| Partnership interest |
689 | 7,118 | ||||||
| Other |
4,885 | 4,173 | ||||||
|
|
|
|
|
|||||
| Total deferred tax assets |
235,660 | 209,759 | ||||||
| Deferred tax liabilities: |
||||||||
| Intangible assets |
(7 | ) | (38 | ) | ||||
| Unrealized gain on available-for-sale securities |
(13,267 | ) | (10,572 | ) | ||||
| Gain on issuance of stock by Regulus |
— | (6,466 | ) | |||||
| Deferred tax asset valuation allowance |
(222,393 | ) | (192,721 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax liability |
$ | (7 | ) | $ | (38 | ) | ||
|
|
|
|
|
|||||
The benefit from income taxes for the years ended December 31, 2013, 2012 and 2011 are as follows, in thousands:
| 2013 | 2012 | 2011 | ||||||||||
| U.S.: |
||||||||||||
| Current |
$ | 34 | $ | 52 | $ | 52 | ||||||
| Deferred |
(2,695 | ) | (10,572 | ) | — | |||||||
|
|
|
|
|
|
|
|||||||
| Total U.S. |
(2,661 | ) | (10,520 | ) | 52 | |||||||
|
|
|
|
|
|
|
|||||||
| Foreign: |
||||||||||||
| Current |
— | — | — | |||||||||
| Deferred |
(34 | ) | (52 | ) | (52 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total Foreign |
(34 | ) | (52 | ) | (52 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Benefit from income taxes |
$ | (2,695 | ) | $ | (10,572 | ) | $ | — | ||||
|
|
|
|
|
|
|
|||||||
136
Table of Contents
The Company’s effective income tax rate differs from the statutory federal income tax rate as follows for the years ended December 31, 2013, 2012 and 2011:
| 2013 | 2012 | 2011 | ||||||||||
| At U.S. federal statutory rate |
35.0 | % | 35.0 | % | 35.0 | % | ||||||
| State taxes, net of federal effect |
4.3 | 4.9 | 4.6 | |||||||||
| Stock compensation |
0.8 | (0.7 | ) | (3.3 | ) | |||||||
| Tax credits |
8.5 | 4.2 | 3.7 | |||||||||
| Orphan drug credit addback |
(2.7 | ) | — | — | ||||||||
| Other permanent items |
(0.3 | ) | (1.6 | ) | (0.2 | ) | ||||||
| Foreign rate differential |
(4.1 | ) | (0.1 | ) | (0.1 | ) | ||||||
| Valuation allowance |
(38.5 | ) | (32.6 | ) | (39.7 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Effective income tax rate |
3.0 | % | 9.1 | % | — | % | ||||||
|
|
|
|
|
|
|
|||||||
The Company has evaluated the positive and negative evidence bearing upon the realizability of its deferred tax assets. The Company has concluded, in accordance with the applicable accounting standards, that it is more likely than not that the Company may not realize the benefit of all of its deferred tax assets. Accordingly, the Company has recorded a valuation allowance against the deferred tax assets that management believes will not be realized. The Company reevaluates the positive and negative evidence on a quarterly basis. The valuation allowance increased by $29.7 million, $38.3 million and $23.0 million for the years ended December 31, 2013, 2012 and 2011 respectively, due primarily to additional operating losses and deferred revenue. Increases to the valuation allowance were partially offset by decreases related to the Company’s partnership interest in Regulus.
For the years ended December 31, 2013 and 2012, the Company recorded a tax benefit of $2.7 million and $10.6 million, respectively. For the year ended December 31, 2011, the Company recorded a provision for income taxes of zero. For the years ended December 31, 2013 and 2012, the Company recorded unrealized gains on its investments in available-for-sale securities in other comprehensive income. The benefit of $2.7 million and $10.6 million for the years ended December 31, 2013 and 2012, respectively, is due to the recognition of corresponding income tax expense associated with the increase in the value of the Company’s investment in Regulus that the Company carried at fair market value during the same period. The corresponding income tax expense has been recorded in other comprehensive income. Intraperiod tax allocation rules require the Company to allocate its provision for income taxes between continuing operations and other categories of earnings, such as other comprehensive income. In periods in which the Company has a year-to-date pre-tax loss from continuing operations and pre-tax income in other categories of earnings, such as other comprehensive income, the Company must allocate the tax provision to the other categories of earnings. The Company then records a related tax benefit in continuing operations.
The deferred tax assets above exclude $26.6 million of net operating losses and $0.5 million of federal and state research and development credits related to tax deductions from the exercise of stock options subsequent to the adoption of the 2006 accounting standard on stock-based compensation. This amount represents an excess tax benefit and has not been included in the gross deferred tax assets.
At December 31, 2013, the Company had federal and state net operating loss carryforwards of $372.4 million and $441.6 million, respectively, to reduce future taxable income that will expire at various dates through 2033. At December 31, 2013, federal and state research and development credit carryforwards were $20.8 million and $6.8 million, respectively, available to reduce future tax liabilities that expire at various dates through 2033. At December 31, 2013, foreign tax credit carryforwards were $3.2 million available to reduce future tax liabilities that expire in 2017. At December 31, 2013, alternative minimum tax credits of $0.8 million are available to reduce future regular tax liabilities to the extent such regular tax less other non-refundable credits exceeds the tentative minimum tax. Ownership changes, as defined in the Internal Revenue Code, including those resulting from the issuance of common stock in connection with the Company’s public offerings, may limit the
137
Table of Contents
amount of net operating loss that can be utilized to offset future taxable income or tax liability. The Company has determined that based on the value of the Company, in the event there was an annual limitation under Section 382, all net operating loss and tax credit carryforwards would still be available to offset taxable income.
At December 31, 2013, the Company had no unrecognized tax benefits that, if recognized, would favorably impact the Company’s effective income tax rate in future periods. A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows, in thousands:
| Balance at December 31, 2011 |
$ | 128 | ||
| Subtractions for tax positions related to the prior years |
(128 | ) | ||
|
|
|
|||
| Balance at December 31, 2012 |
— | |||
| Subtractions for tax positions related to the prior years |
— | |||
|
|
|
|||
| Balance at December 31, 2013 |
$ | — | ||
|
|
|
The tax years 2010 through 2013 remain open to examination by major taxing jurisdictions to which the Company is subject, which are primarily in the United States, as carryforward attributes generated in years past may still be adjusted upon examination by the Internal Revenue Service or state tax authorities if they have or will be used in a future period. In July 2011, the Internal Revenue Service completed its audits of the Company’s 2008 and 2009 tax years. The Company did not record any tax expense related to these audits. The Company has not recorded any interest and penalties on any unrecognized tax benefits since its inception.
| 10. | REGULUS |
In September 2007, the Company and Isis established Regulus, a company focused on the discovery, development and commercialization of microRNA therapeutics, a potential new class of drugs to treat the pathways of human disease. Regulus, which initially was established as a limited liability company, converted to a C corporation in January 2009 and changed its name to Regulus Therapeutics Inc. Regulus operates as an independent company with a separate board of directors, scientific advisory board and management team.
In consideration for the Company’s and Isis’ initial interests in Regulus, each party granted Regulus exclusive licenses to its intellectual property for certain microRNA therapeutic applications as well as certain patents in the microRNA field. In addition, the Company made an initial cash contribution to Regulus of $10.0 million, resulting in the Company and Isis making approximately equal aggregate initial capital contributions to Regulus. In March 2009, the Company and Isis each purchased $10.0 million of Series A preferred stock of Regulus.
From the formation of Regulus in September 2007 to October 2012, the Company accounted for its interest in Regulus using the equity method of accounting. The Company reviewed the consolidation guidance that defines a variable interest entity (“VIE”), and concluded that Regulus qualified as a VIE during such time period. The Company did not consolidate Regulus as the Company lacked the power to direct the activities that could significantly impact the economic success of this entity.
138
Table of Contents
Summary results of Regulus’ statements of comprehensive loss for the nine months ended September 30, 2012 and the year ended December 31, 2011 are presented in the tables below, in thousands:
| Nine months
ended September 30, |
Year ended December 31, |
|||||||
| 2012 | 2011 | |||||||
| Statements of Comprehensive Loss Data: |
||||||||
| Net revenues |
$ | 9,462 | $ | 13,789 | ||||
| Operating expenses |
17,733 | 20,926 | ||||||
|
|
|
|
|
|||||
| Loss from operations |
(8,271 | ) | (7,137 | ) | ||||
| Other (expense) income |
(2,289 | ) | (259 | ) | ||||
| Income tax benefit (expense) |
28 | (206 | ) | |||||
|
|
|
|
|
|||||
| Net loss |
$ | (10,532 | ) | $ | (7,602 | ) | ||
|
|
|
|
|
|||||
Under the equity method of accounting, the Company was required to recognize losses up to the amount of Regulus’ debt, which was guaranteed by the Company. This resulted in a negative carrying amount. In October 2012, Regulus completed an initial public offering, resulting in the Company’s ownership percentage decreasing from approximately 44% to 17% of Regulus’ outstanding common stock. Upon the completion of the Regulus’ initial public offering, the Company’s debt guarantee was terminated.
Based upon the Company’s ownership percentage of 17% after the initial public offering, as well as a review of qualitative factors, the Company did not believe that it had the ability to exercise significant influence over the operating decisions and financial policies of Regulus and therefore discontinued the equity method of accounting for Regulus at September 30, 2012. The Company determined that the period between September 30, 2012 and the date on which Regulus’ closed its initial public offering was immaterial for additional equity method accounting. Accordingly, beginning October 10, 2012, the Company accounted for its investment in Regulus as an available-for-sale marketable security due to its readily determinable fair value. At December 31, 2012, the fair value of the Regulus equity securities was $38.7 million. As a result of the issuance of additional common stock by Regulus, the Company recognized a gain of $16.1 million. This amount was recorded as other income in the Company’s consolidated statements of comprehensive loss for the year ended December 31, 2012. The Company’s carrying amount in Regulus increased to $12.4 million following the initial public offering, which became the initial basis of its investment in Regulus under the accounting standard for marketable securities. In addition, the Company recorded $15.7 million as an unrealized gain in other comprehensive income, net of an intraperiod tax benefit of $10.6 million. The Company has historically classified the equity method investment amount in the financial statement caption “Investment in joint venture (Regulus Therapeutics Inc.)” on the Company’s consolidated balance sheets. For the purposes of the 2012 balance sheet, this amount is zero and the Company has reclassified the 2011 balance of $0.6 million to the financial statement caption “Other assets.”
| 11. | QUARTERLY FINANCIAL DATA (UNAUDITED) |
The following information has been derived from unaudited consolidated financial statements that, in the opinion of management, include all recurring adjustments necessary for a fair statement of such information.
| Three Months Ended | ||||||||||||||||
| March 31, 2013 |
June 30, 2013 |
September 30, 2013 |
December 31, 2013 |
|||||||||||||
| (In thousands, except per share data) | ||||||||||||||||
| Revenues |
$ | 18,642 | $ | 8,687 | $ | 8,991 | $ | 10,847 | ||||||||
| Operating expenses |
28,446 | 29,999 | 41,225 | 40,439 | ||||||||||||
| Net loss |
(9,013 | ) | (18,169 | ) | (29,686 | ) | (32,357 | ) | ||||||||
| Net loss per common share — basic and diluted |
$ | (0.15 | ) | $ | (0.29 | ) | $ | (0.48 | ) | $ | (0.51 | ) | ||||
| Weighted average common shares — basic and diluted |
59,173 | 61,661 | 62,416 | 62,909 | ||||||||||||
139
Table of Contents
| Three Months Ended | ||||||||||||||||
| March 31, 2012 |
June 30, 2012 |
September 30, 2012 |
December 31, 2012 |
|||||||||||||
| (In thousands, except per share data) | ||||||||||||||||
| Revenues |
$ | 20,587 | $ | 20,884 | $ | 16,759 | $ | 8,495 | ||||||||
| Operating expenses |
31,480 | 32,951 | 34,906 | 96,844 | ||||||||||||
| Net loss |
(11,368 | ) | (12,956 | ) | (19,502 | ) | (62,188 | ) | ||||||||
| Net loss per common share — basic and diluted |
$ | (0.25 | ) | $ | (0.25 | ) | $ | (0.38 | ) | $ | (1.20 | ) | ||||
| Weighted average common shares — basic and diluted |
46,210 | 51,280 | 51,542 | 51,821 | ||||||||||||
The increase in operating expenses for the three months ended December 31, 2012 resulted from a $65.0 million charge to operating expenses in connection with the restructuring of the Company’s license agreement with Tekmira in November 2012. This increase in operating expenses was offset by a gain in other income of $16.1 million and a tax benefit of $10.6 million recorded as part of the Company’s accounting for the Regulus initial public offering.
| 12. | SUBSEQUENT EVENTS |
Acquisition of Sirna Therapeutics
On January 10, 2014, the Company entered into a Stock Purchase Agreement (the “Sirna Agreement”) with Sirna, Merck and, for limited purposes, Merck & Co., Inc., pursuant to which the Company will purchase from Merck all of Merck’s right, title and interest in and to all of the Sirna Common Stock. Sirna possesses intellectual property and RNAi assets including pre-clinical therapeutic candidates, chemistry, and siRNA-conjugate and other delivery technologies that the Company intends to integrate into its platform for delivery of RNAi therapeutics. The Company will not acquire any employees, manufacturing or other facilities, developed processes or clinical-stage assets as part of the acquisition of Sirna.
In consideration for the Sirna Common Stock, the Company will (i) pay Merck $25.0 million in cash and (ii) issue to Merck 2,520,044 shares of the Company’s common stock, par value $0.01 per share (“Company Common Stock”), having a value of $150.0 million as calculated under the terms of the Sirna Agreement on the date of execution. In addition, Merck is eligible to receive the following consideration from the Company: (i) up to an aggregate of $10.0 million upon the achievement by the Company or its Related Parties (as defined in the Agreement) of specified regulatory milestones for RNAi products covered by Sirna intellectual property (“Company Products”), and (ii) up to an aggregate of $105.0 million upon the achievement by the Company or its Related Parties of specified development and regulatory ($40.0 million) and commercial ($65.0 million) milestones associated with the clinical development progress of certain pre-clinical candidates discovered by Sirna (“Sirna Products”), together with low single-digit royalties for Company Products and single-digit royalties for Sirna Products, in each case based on annual worldwide net sales, if any, by the Company and its Related Parties of any such products.
Under the Sirna Agreement, Merck also agreed not to dispose of (i) any shares of Company Common Stock beneficially owned for a period of six months following the closing date (the “Initial Lock-Up”), subject to certain limited exceptions, and (ii) 50% of the shares of Company Common Stock beneficially owned for a period of six additional months following the termination of the Initial Lock-Up (the “Subsequent Lock-Up”). During and following the expiration of the Subsequent Lock-Up, Merck will be permitted to sell such shares of Company Common Stock subject to certain limitations, including certain volume and manner of sale restrictions.
The Sirna Agreement contains customary representations, warranties, and covenants of the parties thereto. Subject to customary closing conditions, including the expiration or early termination of the applicable pre-merger waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, the transaction is expected to close during the first quarter of 2014.
2014 Genzyme Collaboration
On January 11, 2014, the Company entered into the 2014 Genzyme Collaboration. The 2014 Genzyme Collaboration is governed by a Master Collaboration Agreement, dated January 11, 2014, by and between the
140
Table of Contents
Company and Genzyme (including the License Terms appended thereto, the “Master Agreement”), which will become effective upon closing of the Equity Transaction (as defined below) (the “Effective Date”) and will supersede and replace the 2012 Genzyme Agreement.
The 2014 Genzyme Collaboration is structured as an exclusive relationship for the worldwide development and commercialization of RNAi therapeutics in the field of genetic medicines, which includes the Company’s current and future genetic medicine programs that reach Human Proof-of-Principal (POP) Study Completion (as defined in the Master Agreement) (“Human POP”) by the end of 2019 (collectively, “Genzyme Collaboration Products”), subject to extension to the end of 2021 in various circumstances. The Company will retain product rights in North America and Western Europe, while Genzyme will obtain exclusive rights to develop and commercialize Genzyme Collaboration Products in the rest of the world (the “Genzyme Territory”), together with certain broader co-development/co-promote or worldwide rights for certain products. Genzyme’s rights are structured as an opt-in that is triggered upon achievement of Human POP. The Company maintains development control for all programs prior to Genzyme’s opt-in and maintains development and commercialization control after Genzyme’s opt-in for all programs in its territory.
Upon the Effective Date, Genzyme will opt-in to patisiran, an RNAi therapeutic currently in a Phase 3 clinical trial for the treatment of ATTR patients with Familial Amyloidotic Polyneuropathy, for the Genzyme Territory, and the Company will retain full product rights in North America and Western Europe. The Company and Genzyme have also agreed to expand their current collaboration on ALN-TTRsc, an RNAi therapeutic currently in a Phase 2 clinical trial for the treatment of ATTR patients with TTR amyloid cardiomyopathy, where the parties will co-develop and co-promote ALN-TTRsc in North America and Western Europe. The Company will maintain development and commercialization control with ALN-TTRsc and Genzyme will develop and commercialize the product in the Genzyme Territory.
In addition to its regional rights for the Company’s current and future genetic medicine programs in the Genzyme Territory, Genzyme will have the right to either (i) co-develop and co-promote ALN-AT3 for the treatment of hemophilia and other rare bleeding disorders in the Company’s territory, with the Company maintaining development and commercialization control, or (ii) obtain a global license to ALN-AS1 for the treatment of hepatic porphyrias. Genzyme will exercise this selection right upon Human POP for the ALN-AT3 and ALN-AS1 programs. Finally, Genzyme will have the right for a global license to a single, future genetic medicine program that is not one of the currently defined genetic medicine programs. The Company will retain global rights to any RNAi therapeutic genetic medicine program that does not reach Human POP by the end of 2019, subject to certain limited exceptions. Under the terms of the Master Agreement, the Company retains full rights to all current and future RNAi therapeutic programs outside of the field of genetic medicines, including the right to form new collaborations.
In consideration for the rights granted to Genzyme under the Master Agreement, Genzyme will be required to make payments to the Company for each Genzyme Collaboration Product upon the achievement of specified development, regulatory and commercial milestones for each (i) regional (e.g., patisiran) and co-developed/co-promoted (e.g., ALN-TTRsc) Genzyme Collaboration Product totaling up to $75.0 million and (ii) global Genzyme Collaboration Product up to $200.0 million, and to pay tiered double-digit royalties up to twenty percent for each regional and global Genzyme Collaboration Product based on annual net sales, if any, of each Genzyme Collaboration Product by Genzyme, its affiliates and sublicensees. The Company could potentially earn the next development milestone payment of $25.0 million under the 2014 Genzyme Collaboration based upon the initiation of the first global Phase 3 clinical trial. In the case of co-developed/co-promoted Genzyme Collaboration Products, the parties will share profits equally and the Company will book product sales in North America and Western Europe.
Under the Master Agreement, the parties will collaborate in the development of option products, with the Company leading development for all programs prior to Genzyme’s opt-in and also leading development and commercialization for all programs in the Company’s territory after Genzyme’s opt-in. Development costs for Genzyme Collaboration Products once Genzyme exercises an option (or as of the Effective Date for patisiran and ALN-TTRsc) will be shared between Genzyme and the Company as follows, subject to the provisions of the
141
Table of Contents
relevant License Terms: (a) for all regional Genzyme Collaboration Products, Genzyme shall be responsible for twenty percent of the global development costs, (b) for all co-develop/co-promote Genzyme Collaboration Products, Genzyme shall be responsible for fifty percent of the global development costs, and (c) for all global Genzyme Collaboration Products, Genzyme shall be responsible for one hundred percent of global development costs. If Genzyme does not exercise its option to license rights to a particular program, the Company will retain the exclusive right to develop and commercialize such program throughout the world, including the right to sublicense to third parties.
The 2014 Genzyme Collaboration will be governed by an alliance joint steering committee that will be comprised of an equal number of representatives from each party. There will also be additional committees to manage various aspects of each regional, co-developed/co-promoted and global program. The Company and Genzyme intend to enter into supply agreements to provide for supply of Genzyme Collaboration Products to Genzyme for clinical studies, and, at Genzyme’s request, commercial sales. Genzyme also has certain rights to manufacture Genzyme Collaboration Products. Additionally, Genzyme has certain limited opt-out rights, as specified in the Master Agreement, upon which products revert fully back to the Company with no further obligations to Genzyme.
In addition, under the Master Agreement, the Company and Genzyme have agreed to enter into exclusive discussions and negotiations regarding a potential collaboration around the delivery of small interfering RNAs (“siRNAs”) to the central nervous system (“CNS”) with the objective of enabling the discovery of siRNAs for the treatment of CNS disorders.
The Master Agreement (including the License Terms appended thereto) contains certain termination provisions, including for material breach by the other party. Unless terminated earlier pursuant to its terms, the Master Agreement will terminate upon the last to expire of any of the option periods under the Master Agreement or the License Terms appended thereto.
In consideration for the rights granted to Genzyme under the Master Agreement and pursuant to the terms of a Stock Purchase Agreement, dated January 11, 2014, by and between Genzyme and the Company, the Company has agreed to sell to Genzyme 8,766,338 shares of its common stock, par value $0.01 per share (the “Common Stock”), and Genzyme agreed to pay $700.0 million in aggregate cash consideration to the Company (the “Equity Transaction”). This sale does not involve a public offering and is therefore exempt from registration under Section 4(a)(2) of the Securities Act of 1933, as amended. Subject to customary closing conditions, including the expiration or early termination of the applicable pre-merger waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, the Equity Transaction is expected to close during the first quarter of 2014. Following the closing of the Equity Transaction, Genzyme will beneficially own approximately 12% of the Company’s outstanding common stock.
As a condition to the closing of the Equity Transaction, Genzyme will enter into an investor agreement with the Company (the “Investor Agreement”). Under the Investor Agreement, until the earlier of the fifth anniversary of the expiration or earlier termination of the Collaboration and the date on which Genzyme and its affiliates cease to beneficially own at least 5% of the Company’s outstanding common stock, Genzyme and its affiliates will be bound by certain “standstill” provisions. The standstill provisions include agreements not to acquire more than 30% of the Company’s outstanding common stock, call stockholder meetings, nominate directors other than those approved by the Company’s Board of Directors, subject to certain limited exceptions, or propose or support a proposal to acquire the Company.
Further, Genzyme will agree to vote, and cause its affiliates to vote, all shares of the Company’s voting securities they are entitled to vote, up to a maximum of 20% of the Company’s outstanding common stock, in a manner either as recommended by the Company’s Board of Directors or proportionally with the votes cast by other stockholders of the Company, except with respect to certain change of control transactions or liquidation or dissolution of the Company. Until Genzyme owns less than 7.5% of the Company’s outstanding common stock, subject to Genzyme’s limited right to maintain its ownership percentage as described below, if the Company issues common stock or securities convertible into or exercisable for common stock to a third party that holds at least 30% of the Company’s outstanding common stock or, in connection with a collaboration or license
142
Table of Contents
transaction, to a third party that will initially hold at least the percentage of the Company’s outstanding common stock represented by the shares purchased by Genzyme at the closing of the Equity Transaction, the Company will offer Genzyme an opportunity to amend the standstill and voting provisions in the Investor Agreement to be consistent with the terms provided to such third party.
143
Table of Contents
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| ITEM 9A. | CONTROLS AND PROCEDURES |
Our management, with the participation of our chief executive officer and vice president of finance and treasurer, evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2013. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of December 31, 2013, the Company’s chief executive officer and vice president of finance and treasurer concluded that, as of such date, our disclosure controls and procedures were effective at the reasonable assurance level.
Management’s report on our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) and the independent registered public accounting firm’s report on the effectiveness of our internal control over financial reporting are included in Item 8 of this annual report on Form 10-K and are incorporated herein by reference.
No change in our internal control over financial reporting occurred during the fiscal quarter ended December 31, 2013 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
| ITEM 9B. | OTHER INFORMATION |
None.
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information required by this item is incorporated herein by reference to the information contained under the sections captioned “Proposal One — Election of Class I Directors,” “Section 16(a) Beneficial Ownership Reporting Compliance” and “Corporate Governance” of the Proxy Statement. The information required by this item relating to executive officers is included in Part I, Item 1, “— Business-Executive Officers of the Registrant,” of this annual report on Form 10-K.
| ITEM 11. | EXECUTIVE COMPENSATION |
The information required by this item is incorporated herein by reference to the information contained under the sections captioned “Information about Executive Officer and Director Compensation,” “Compensation Committee Interlocks and Insider Participation,” “Employment Arrangements” and “Compensation Committee Report” of the Proxy Statement.
144
Table of Contents
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by this item is incorporated herein by reference to the information contained under the sections captioned “Security Ownership of Certain Beneficial Owners and Management,” “Information about Executive Officer and Director Compensation” and “Securities Authorized for Issuance Under Equity Compensation Plans” of the Proxy Statement.
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information required by this item is incorporated herein by reference to the information contained under the sections captioned “Corporate Governance,” “Employment Arrangements” and “Certain Relationships and Related Transactions” of the Proxy Statement.
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The information required by this item is incorporated herein by reference to the information contained under the sections captioned “Corporate Governance,” “Principal Accountant Fees and Services” and “Pre-Approval Policies and Procedures” of the Proxy Statement.
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
(a) (1) Financial Statements
The following consolidated financial statements are filed as part of this report under “Item 8 — Financial Statements and Supplementary Data”:
(a) (2) List of Schedules
All schedules to the consolidated financial statements are omitted as the required information is either inapplicable or presented in the consolidated financial statements.
(a) (3) List of Exhibits
The exhibits which are filed with this report or which are incorporated herein by reference are set forth in the Exhibit Index hereto.
145
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized, on February 20, 2014.
| ALNYLAM PHARMACEUTICALS, INC. | ||
| By: |
/s/ John M. Maraganore, Ph.D. | |
| John M. Maraganore, Ph.D. | ||
| Chief Executive Officer | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, the Report has been signed below by the following persons on behalf of the Registrant and in the capacities indicated as of February 20, 2014.
| Name |
Title | |
| /s/ John M. Maraganore, Ph.D. John M. Maraganore, Ph.D. |
Director and Chief Executive Officer (Principal Executive Officer) | |
| /s/ Michael P. Mason Michael P. Mason |
Vice President of Finance and Treasurer (Principal Financial and Accounting Officer) | |
| /s/ Dennis A. Ausiello, M.D. Dennis A. Ausiello, M.D. |
Director | |
| /s/ John K. Clarke John K. Clarke |
Director | |
| /s/ Victor J. Dzau, M.D. Victor J. Dzau, M.D. |
Director | |
| /s/ Marsha H. Fanucci Marsha H. Fanucci |
Director | |
| /s/ Steven M. Paul, M.D. Steven M. Paul, M.D. |
Director | |
| /s/ Paul R. Schimmel, Ph.D. Paul R. Schimmel, Ph.D. |
Director | |
| /s/ Phillip A. Sharp, Ph.D. Phillip A. Sharp, Ph.D. |
Director | |
| /s/ Kevin P. Starr Kevin P. Starr |
Director | |
146
Table of Contents
EXHIBIT INDEX
| Exhibit No. |
Exhibit | |
| 3.1 | Restated Certificate of Incorporation of the Registrant (filed as Exhibit 3.1 to the Registrant’s Quarterly Report on Form 10-Q filed on August 11, 2005 (File No. 000-50743) for the quarterly period ended June 30, 2005 and incorporated herein by reference) | |
| 3.2 | Amended and Restated Bylaws of the Registrant (filed as Exhibit 3.4 to the Registrant’s Registration Statement on Form S-1 (File No. 333-113162) and incorporated herein by reference) | |
| 4.1 | Specimen certificate evidencing shares of common stock (filed as Exhibit 4.1 to the Registrant’s Registration Statement on Form S-1 (File No. 333-113162) and incorporated herein by reference) | |
| 4.2 | Rights Agreement dated as of July 13, 2005 between the Registrant and EquiServe Trust Company, N.A., as Rights Agent, which includes as Exhibit A the Form of Certificate of Designations of Series A Junior Participating Preferred Stock, as Exhibit B the Form of Rights Certificate and as Exhibit C the Summary of Rights to Purchase Preferred Stock (filed as Exhibit 4.1 to the Registrant’s Current Report on Form 8-K filed on July 14, 2005 (File No. 000-50743) and incorporated herein by reference) | |
| 10.1* | 2002 Employee, Director and Consultant Stock Plan, as amended, together with forms of Incentive Stock Option Agreement, Non-qualified Stock Option Agreement and Restricted Stock Agreement (filed as Exhibit 10.1 to the Registrant’s Registration Statement on Form S-1 (File No. 333-113162) and incorporated herein by reference) | |
| 10.2* | 2003 Employee, Director and Consultant Stock Plan, as amended, together with forms of Incentive Stock Option Agreement, Non-qualified Stock Option Agreement and Restricted Stock Agreement (filed as Exhibit 10.2 to the Registrant’s Registration Statement on Form S-1 (File No. 333-113162) and incorporated herein by reference) | |
| 10.3* | Amended and Restated 2004 Stock Incentive Plan (filed as Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q filed on August 7, 2009 (File No. 000-50743) for the quarterly period ended June 30, 2009 and incorporated herein by reference) | |
| 10.4* | Forms of Incentive Stock Option Agreement and Nonstatutory Stock Option Agreement under 2004 Stock Incentive Plan, as amended (filed as Exhibit 10.3 to the Registrant’s Quarterly Report on Form 10-Q filed on August 11, 2005 (File No. 000-50743) for the quarterly period ended June 30, 2005 and incorporated herein by reference) | |
| 10.5* | Form of Nonstatutory Stock Option Agreement under 2004 Stock Incentive Plan granted to John M. Maraganore, Ph.D., on December 21, 2004 (filed as Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed on December 28, 2004 (File No. 000-50743) and incorporated herein by reference) | |
| 10.6* | 2009 Stock Incentive Plan, as amended (filed as Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q filed on August 9, 2013 (File No. 000-50743) for the quarterly period ended June 30, 2013 and incorporated herein by reference) | |
| 10.7* | Forms of Incentive Stock Option Agreement and Nonstatutory Stock Option Agreement under 2009 Stock Incentive Plan (filed as Exhibit 10.9 to the Registrant’s Annual Report on Form 10-K filed on February 26, 2010 (File No. 000-50743) for the year ended December 31, 2009 and incorporated herein by reference) | |
| 10.8* | Form of Restricted Stock Agreement under 2009 Stock Incentive Plan (filed as Exhibit 10.10 to the Registrant’s Annual Report on Form 10-K filed on February 18, 2011 (File No. 000-50743) for the year ended December 31, 2010 and incorporated herein by reference) | |
| 10.9* | 2004 Employee Stock Purchase Plan, as amended (filed as Appendix A to the Registrant’s Definitive Proxy Statement on Schedule 14A filed on April 20, 2010 (File No. 000-50743) and incorporated herein by reference) |
147
Table of Contents
| Exhibit No. |
Exhibit | |
| 10.10 | Stock Purchase Agreement, dated as of September 6, 2005, by and between the Registrant and Novartis Pharma AG (filed as Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed on September 12, 2005 (File No. 000-50743) and incorporated herein by reference) | |
| 10.11 | Investor Rights Agreement, dated as of September 6, 2005, by and between the Registrant. and Novartis Pharma AG (filed as Exhibit 10.2 to the Registrant’s Current Report on Form 8-K filed on September 12, 2005 (File No. 000-50743) and incorporated herein by reference) | |
| 10.12 | Letter Agreement dated as of September 20, 2012 by and between the Registrant and Novartis Pharma AG (filed as Exhibit 10.3 to the Registrant’s Quarterly Report on Form 10-Q filed on November 5, 2012 (File No. 000-50743) for the quarterly period ended September 30, 2012 and incorporated herein by reference) | |
| 10.13* | Letter Agreement between the Registrant and John M. Maraganore, Ph.D. dated October 30, 2002 (filed as Exhibit 10.7 to the Registrant’s Registration Statement on Form S-1 (File No. 333-113162) and incorporated herein by reference) | |
| 10.14* | Letter Agreement between the Registrant and Barry E. Greene dated September 29, 2003 (filed as Exhibit 10.10 to the Registrant’s Registration Statement on Form S-1 (File No. 333-113162) and incorporated herein by reference) | |
| 10.15* | Consulting Agreement dated as of March 1, 2006 by and between the Registrant and Phillip A. Sharp, Ph.D., as amended (filed as Exhibit 10.16 to the Registrant’s Annual Report on Form 10-K filed on February 19, 2013 (File No. 000-50743) for the year ended December 31, 2012 and incorporated herein by reference) | |
| 10.16* | Consulting Agreement dated as of April 20, 2012 by and between the Registrant and Dennis A. Ausiello, M.D. (filed as Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed on April 23, 2012 (File No. 000-50743) and incorporated herein by reference) | |
| 10.17 | Lease, dated as of September 26, 2003 by and between the Registrant and Three Hundred Third Street LLC (filed as Exhibit 10.15 to the Registrant’s Registration Statement on Form S-1 (File No. 333-113162) and incorporated herein by reference) | |
| 10.18 | First Amendment to Lease, dated March 16, 2006, by and between the Registrant and ARE-MA Region No. 28, LLC (filed as Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed on March 17, 2006 (File No. 000-50743) and incorporated herein by reference) | |
| 10.19 | Second Amendment to Lease, dated June 26, 2009, by and between the Registrant and ARE-MA Region No. 28, LLC (filed as Exhibit 10.4 to the Registrant’s Quarterly Report on Form 10-Q filed on August 7, 2009 (File No. 000-50743) for the quarterly period ended June 30, 2009 and incorporated herein by reference) | |
| 10.20 | Third Amendment to Lease, dated May 11, 2010, by and between the Registrant and ARE-MA Region No. 28, LLC (filed as Exhibit 10.2 to the Registrant’s Quarterly Report on Form 10-Q filed on August 5, 2010 (File No. 000-50743) for the quarterly period ended June 30, 2010 and incorporated herein by reference) | |
| 10.21 | Fourth Amendment to Lease, dated November 4, 2011, by and between the Registrant and ARE-MA Region No. 28, LLC (filed as Exhibit 10.19 to the Registrant’s Annual Report on Form 10-K filed on February 13, 2012 (File No. 000-50743) for the year ended December 31, 2011 and incorporated herein by reference) | |
| 10.22† | Lease entered into as of February 10, 2012 by and between BMR-Fresh Pond Research Park LLC and the Registrant (filed as Exhibit 10.2 to the Registrant’s Quarterly Report on Form 10-Q filed on May 3, 2012 (File No. 000-50743) for the quarterly period ended March 31, 2012 and incorporated herein by reference) |
148
Table of Contents
| Exhibit No. |
Exhibit | |
| 10.23† | Co-exclusive License Agreement between Garching Innovation GmbH (now known as Max Planck Innovation GmbH) and Alnylam U.S., Inc. dated December 20, 2002, as amended by Amendment dated July 8, 2003 together with Indemnification Agreement by and between Garching Innovation GmbH (now known as Max Planck Innovation GmbH) and Alnylam Pharmaceuticals, Inc. effective April 1, 2004 (filed as Exhibit 10.19 to the Registrant’s Registration Statement on Form S-1 (File No. 333-113162) and incorporated herein by reference) | |
| 10.24† | Co-exclusive License Agreement between Garching Innovation GmbH (now known as Max Planck Innovation GmbH) and Alnylam Europe, AG dated July 30, 2003 (filed as Exhibit 10.20 to the Registrant’s Registration Statement on Form S-1 (File No. 333-113162) and incorporated herein by reference) | |
| 10.25† | Agreement between the Registrant, Garching Innovation GmbH (now known as Max Planck Innovation GmbH), Alnylam U.S., Inc. and Alnylam Europe AG dated June 14, 2005 (filed as Exhibit 10.8 to the Registrant’s Quarterly Report on Form 10-Q filed on August 11, 2005 (File No. 000-50743) for the quarterly period ended June 30, 2005 and incorporated herein by reference) | |
| 10.26† | Confidential Settlement Agreement and Mutual Release entered into as of March 14, 2011 by and between Max-Planck-Gesellschaft zur Förderung der Wissenschaften e. V., Max-Planck-Innovation GmbH and the Registrant, on the one hand, and Whitehead Institute for Biomedical Research, Massachusetts Institute of Technology, and the University of Massachusetts, on the other hand (filed as Exhibit 10.2 to the Registrant’s Quarterly Report on Form 10-Q filed on May 5, 2011 (File No. 000-50743) for the quarterly period ended March 31, 2011 and incorporated herein by reference) | |
| 10.27† | Exclusive License Agreement for Tuschl II United States Patents and Patent Applications dated as of March 14, 2011, by and between the Registrant and University of Massachusetts (filed as Exhibit 10.3 to the Registrant’s Quarterly Report on Form 10-Q filed on May 5, 2011 (File No. 000-50743) for the quarterly period ended March 31, 2011 and incorporated herein by reference) | |
| 10.28 | Amendment to Co-Exclusive License Agreement dated as of March 14, 2011, by and between the Registrant, on the one hand, and Whitehead Institute for Biomedical Research, Massachusetts Institute of Technology and Max-Planck-Innovation GmbH (filed as Exhibit 10.4 to the Registrant’s Quarterly Report on Form 10-Q filed on May 5, 2011 (File No. 000-50743) for the quarterly period ended March 31, 2011 and incorporated herein by reference) | |
| 10.29† | Research Collaboration and License Agreement effective as of October 12, 2005 by and between the Registrant and Novartis Institutes for BioMedical Research, Inc. (filed as Exhibit 10.23 to the Registrant’s Annual Report on Form 10-K filed on March 2, 2009 (File No. 000-50743) for the quarterly and annual period ended December 31, 2008 and incorporated herein by reference) | |
| 10.30† | License and Collaboration Agreement, entered into as of July 8, 2007, by and among F. Hoffmann-La Roche, Ltd, Hoffmann-La Roche Inc. (which assigned its rights and obligations to Arrowhead Research Corporation), the Registrant and, for limited purposes, Alnylam Europe AG (filed as Exhibit 10.26 to the Registrant’s Annual Report on Form 10-K filed on March 2, 2009 (File No. 000-50743) for the quarterly and annual period ended December 31, 2008 and incorporated herein by reference) | |
| 10.31† | Share Purchase Agreement, dated as of July 8, 2007, among Alnylam Europe AG, the Registrant and Roche Pharmaceuticals GmbH (filed as Exhibit 10.3 to the Registrant’s Quarterly Report on Form 10-Q filed on November 8, 2007 (File No. 000-50743) for the quarterly period ended September 30, 2007 and incorporated herein by reference) | |
| 10.32† | Termination Agreement, dated as of September 18, 2007, by and between Merck & Co., Inc. and the Registrant (filed as Exhibit 10.7 to the Registrant’s Quarterly Report on Form 10-Q filed on November 8, 2007 (File No. 000-50743) for the quarterly period ended September 30, 2007 and incorporated herein by reference) |
149
Table of Contents
| Exhibit No. |
Exhibit | |
| 10.33† | License and Collaboration Agreement entered into as of May 27, 2008 by and among Takeda Pharmaceutical Company Limited and the Registrant (filed as Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q filed on August 8, 2008 (File No. 000-50743) for the quarterly period ended June 30, 2008 and incorporated herein by reference) | |
| 10.34† | Amended and Restated License and Collaboration Agreement, entered into as of January 1, 2009, by and among the Registrant, Isis Pharmaceuticals, Inc. and Regulus Therapeutics Inc. (filed as Exhibit 10.3 to the Registrant’s Quarterly Report on Form 10-Q filed on May 8, 2009 (File No. 000-50743) for the quarterly period ended March 31, 2009 and incorporated herein by reference) | |
| 10.35† | Founding Investor Rights Agreement entered into as of January 1, 2009, by and among Regulus Therapeutics Inc., Isis Pharmaceuticals, Inc. and the Registrant (filed as Exhibit 10.4 to the Registrant’s Quarterly Report on Form 10-Q filed on May 8, 2009 (File No. 000-50743) for the quarterly period ended March 31, 2009 and incorporated herein by reference) | |
| 10.36† | Amended and Restated Strategic Collaboration and License Agreement effective as of April 28, 2009 between Isis Pharmaceuticals, Inc. and the Registrant (filed as Exhibit 10.3 to the Registrant’s Quarterly Report on Form 10-Q filed on August 7, 2009 (File No. 000-50743) for the quarterly period ended June 30, 2009 and incorporated herein by reference) | |
| 10.37† | Letter Agreement Amendment dated August 27, 2012, amending the Amended and Restated Strategic Collaboration and License Agreement effective as of April 28, 2009 between Isis Pharmaceuticals, Inc. and the Registrant (filed as Exhibit 10.2 to the Registrant’s Quarterly Report on Form 10-Q filed on November 5, 2012 (File No. 000-50743) for the quarterly period ended September 30, 2012 and incorporated herein by reference) | |
| 10.38† | Sublicense Agreement dated effective January 8, 2007 among the Registrant and INEX Pharmaceuticals Corporation (now Tekmira Pharmaceuticals Corporation, as successor in interest) (filed as Exhibit 10.38 to the Registrant’s Annual Report on Form 10-K filed on February 18, 2011 (File No. 000-50743) for the year ended December 31, 2010 and incorporated herein by reference) | |
| 10.39† | License and Collaboration Agreement effective as of June 19, 2008 by and between the Registrant and Kyowa Hakko Kirin Co., Ltd. (formerly Kyowa Hakko Kogyo Co., Ltd.), as amended as of February 1, 2010 and June 3, 2010 (filed as Exhibit 10.42 to the Registrant’s Annual Report on Form 10-K filed on February 18, 2011 (File No. 000-50743) for the year ended December 31, 2010 and incorporated herein by reference) | |
| 10.40† | Sponsored Research Agreement dated as of July 27, 2009 by and among the Registrant, The University of British Columbia and Acuitas Therapeutics Inc. (formerly AlCana Technologies, Inc.) (filed as Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed on June 29, 2011 (File No. 000-50743) and incorporated herein by reference) | |
| 10.41† | Supplemental Agreement effective July 27, 2009 by and among the Registrant, Tekmira Pharmaceuticals Corporation, Protiva Biotherapeutics Inc., The University of British Columbia and Acuitas Therapeutics Inc. (formerly AlCana Technologies, Inc.) (filed as Exhibit 10.2 to the Registrant’s Current Report on Form 8-K filed on June 29, 2011 (File No. 000-50743) and incorporated herein by reference) | |
| 10.42† | Amendment No. 1, dated as of July 27, 2011, to the Sponsored Research Agreement dated as of July 27, 2009 by and among the Registrant, The University of British Columbia and Acuitas Therapeutics Inc. (formerly AlCana Technologies, Inc.) (filed as Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q filed on November 3, 2011 (File No. 000-50743) for the quarterly period ended September 30, 2011 and incorporated herein by reference) | |
| 10.43† | License and Collaboration Agreement dated as of August 27, 2012 by and among Monsanto Company and the Registrant (filed as Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q filed on November 5, 2012 (File No. 000-50743) for the quarterly period ended September 30, 2012 and incorporated herein by reference) |
150
Table of Contents
| Exhibit No. |
Exhibit | |
| 10.44† | License and Collaboration Agreement dated as of October 18, 2012 by and between the Registrant and Genzyme Corporation, as amended (filed as Exhibit 10.49 to the Registrant’s Annual Report on Form 10-K filed on February 19, 2013 (File No. 000-50743) for the year ended December 31, 2012 and incorporated herein by reference) | |
| 10.45† | Cross-License Agreement dated as of November 12, 2012 by and among the Registrant, Tekmira Pharmaceuticals Corporation and Protiva Biotherapeutics Inc. (filed as Exhibit 10.50 to the Registrant’s Annual Report on Form 10-K filed on February 19, 2013 (File No. 000-50743) for the year ended December 31, 2012 and incorporated herein by reference) | |
| 10.46† | Settlement Agreement and General Release entered into as of November 12, 2012 by and among Tekmira Pharmaceuticals Corporation, Protiva Biotherapeutics Inc., the Registrant and Acuitas Therapeutics Inc. (formerly AlCana Technologies, Inc.) (filed as Exhibit 10.51 to the Registrant’s Annual Report on Form 10-K filed on February 19, 2013 (File No. 000-50743) for the year ended December 31, 2012 and incorporated herein by reference) | |
| 10.47 | Letter Agreement dated as of February 6, 2013 by and between Cubist Pharmaceuticals, Inc. and the Registrant (filed as Exhibit 10.52 to the Registrant’s Annual Report on Form 10-K filed on February 19, 2013 (File No. 000-50743) for the year ended December 31, 2012 and incorporated herein by reference) | |
| 10.48† | License and Collaboration Agreement dated as of February 3, 2013 by and among The Medicines Company and the Registrant (filed as Exhibit 10.2 to the Registrant’s Amendment No. 1 to its Quarterly Report on Form 10-Q/A filed on July 26, 2013 (File No. 000-50743) for the quarterly period ended March 31, 2013 and incorporated herein by reference) | |
| 10.49†# | Master Services Agreement dated as of January 15, 2010 between Medpace, Inc. and the Registrant, as amended, together with Task Order No. 10 thereto, dated July 2, 2013 | |
| 12# | Computation of Consolidated Ratios of Earnings/Deficiencies to Fixed Charges | |
| 21.1# | Subsidiaries of the Registrant | |
| 23.1# | Consent of PricewaterhouseCoopers LLP, an Independent Registered Public Accounting Firm | |
| 23.2# | Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm of Regulus Therapeutics Inc. | |
| 31.1# | Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002, Rule 13(a)- 14(a)/15d-14(a), by Principal Executive Officer | |
| 31.2# | Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002, Rule 13(a)- 14(a)/15d-14(a), by Principal Financial Officer | |
| 32.1# | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, by Principal Executive Officer | |
| 32.2# | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, by Principal Financial Officer | |
| 99.1# | Report of Ernst & Young LLP, Independent Registered Public Accounting Firm of Regulus Therapeutics Inc. | |
| 101# | The following materials from Registrant’s Annual Report on Form 10-K for the year ended December 31, 2013, formatted in XBRL (Extensible Business Reporting Language): (i) the Consolidated Balance Sheets, (ii) the Consolidated Statements of Comprehensive Loss, (iii) the Consolidated Statements of Stockholders’ Equity, (iv) the Consolidated Statements of Cash Flows, and (v) Notes to Consolidated Financial Statements. |
| * | Management contracts or compensatory plans or arrangements required to be filed as an exhibit hereto pursuant to Item 15(a) of Form 10-K. |
| † | Indicates confidential treatment requested as to certain portions, which portions were omitted and filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Request. |
| # | Filed herewith. |
151