e10vk
UNITED STATES SECURITIES AND
EXCHANGE COMMISSION
Washington, D.C.
20549
Form 10-K
| |
|
|
|
þ
|
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
|
|
|
|
For the fiscal year ended
December 31, 2008
|
|
OR
|
|
o
|
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
|
|
|
|
For the transition period
from to
|
Commission File Number
000-50743
ALNYLAM PHARMACEUTICALS,
INC.
(Exact Name of Registrant as
Specified in Its Charter)
| |
|
|
|
Delaware
|
|
77-0602661
|
|
(State or Other Jurisdiction
of
|
|
(I.R.S. Employer
|
|
Incorporation or
Organization)
|
|
Identification
No.)
|
300 Third Street, Cambridge, MA 02142
(Address of Principal Executive
Offices) (Zip Code)
Registrant’s telephone number, including area code:
(617) 551-8200
Securities registered pursuant to Section 12(b) of the
Act:
| |
|
|
|
Title of Each Class
|
|
Name of Each Exchange on Which Registered
|
|
Common Stock, $0.01 par value per share
|
|
The Nasdaq Global Market
|
Securities registered pursuant to Section 12(g) of the
Act: None
Indicate by check mark if the registrant is a well-known
seasoned issuer, as defined in Rule 405 of the Securities
Act. Yes þ No o
Indicate by check mark if the registrant is not required to file
reports pursuant to Section 13 or Section 15(d) of the
Act. Yes o No þ
Indicate by check mark whether the registrant (1) has filed
all reports required to be filed by Section 13 or 15(d) of
the Securities Exchange Act of 1934 during the preceding
12 months (or for such shorter period that the registrant
was required to file such reports), and (2) has been
subject to such filing requirements for the past
90 days. Yes þ No o
Indicate by check mark if disclosure of delinquent filers
pursuant to Item 405 of
Regulation S-K
is not contained herein, and will not be contained, to the best
of the registrant’s knowledge, in definitive proxy or
information statements incorporated by reference in
Part III of this
Form 10-K
or any amendment to this
Form 10-K. þ
Indicate by check mark whether the registrant is a large
accelerated filer, an accelerated filer, a non-accelerated
filer, or a smaller reporting company. See the definitions of
“large accelerated filer,” “accelerated
filer” and “smaller reporting company” in Rule
12b-2 of the
Exchange Act. (Check one):
| |
|
|
|
|
|
|
|
Large accelerated
filer þ
|
|
Accelerated
filer o
|
|
Non-accelerated
filer o
(do not check if a smaller reporting company)
|
|
Smaller reporting
company o
|
Indicate by check mark whether the registrant is a shell company
(as defined in
Rule 12b-2
of the Exchange
Act). Yes o No þ
The aggregate market value of the voting Common Stock held by
non-affiliates of the registrant, based on the last sale price
of the registrant’s Common Stock at the close of business
on June 30, 2008, was $1,082,053,308.
As of January 31, 2009, the registrant had
41,424,759 shares of Common Stock, $0.01 par value per
share, outstanding.
DOCUMENTS
INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement for
its 2009 annual meeting of stockholders, to be filed pursuant to
Regulation 14A with the Securities and Exchange Commission
not later than 120 days after the registrant’s fiscal
year end of December 31, 2008, are incorporated by
reference into Part III of this
Form 10-K.
ALNYLAM
PHARMACEUTICALS, INC.
ANNUAL REPORT ON
FORM 10-K
For the Year Ended December 31, 2008
TABLE OF
CONTENTS
1
This annual report on
Form 10-K
contains forward-looking statements within the meaning of
Section 27A of the Securities Act of 1933, as amended, and
Section 21E of the Securities Exchange Act of 1934, as
amended, that involve risks and uncertainties. All statements
other than statements relating to historical matters should be
considered forward-looking statements. When used in this report
the words “believe,” “expect,”
“anticipate,” “will,” “plan,”
“target,” “goal” and similar expressions are
intended to identify forward-looking statements, although not
all forward-looking statements contain these words. Our actual
results could differ materially from those discussed in the
forward-looking statements as a result of a number of important
factors, including the factors discussed in this section and
elsewhere in this annual report on
Form 10-K,
including those discussed in Item 1A of this report under
the heading “Risk Factors,” and the risks discussed in
our other filings with the Securities and Exchange Commission.
Readers are cautioned not to place undue reliance on these
forward-looking statements, which reflect management’s
analysis, judgment, belief or expectation only as of the date
hereof. We explicitly disclaim any obligation to update these
forward-looking statements to reflect events or circumstances
that arise after the date hereof.
PART I
Overview
We are a biopharmaceutical company developing novel therapeutics
based on RNA interference, or RNAi. RNAi is a naturally
occurring biological pathway within cells for selectively
silencing and regulating the expression of specific genes. Since
many diseases are caused by the inappropriate activity of
specific genes, the ability to silence genes selectively through
RNAi could provide a new way to treat a wide range of human
diseases. We believe that drugs that work through RNAi have the
potential to become a broad new class of drugs, like small
molecule, protein and antibody drugs. Using our intellectual
property and the expertise we have built in RNAi, we are
developing a set of biological and chemical methods and know-how
that we apply in a systematic way to develop RNAi therapeutics
for a variety of diseases.
We are applying our technological expertise to build a pipeline
of RNAi therapeutics to address significant medical needs, many
of which cannot effectively be addressed with small molecules or
antibodies, the current major classes of drugs. Our lead RNAi
therapeutic program, ALN-RSV01, is in Phase II clinical
trials for the treatment of human respiratory syncytial virus,
or RSV, infection, which is reported to be the leading cause of
hospitalization in infants in the United States and also occurs
in the elderly and in immune compromised adults. In February
2008, we reported positive results from our Phase II
experimental RSV infection clinical trial, referred to as the
GEMINI study. In April 2008, we initiated a second Phase II
human clinical trial, which is currently ongoing, to assess the
safety and tolerability of aerosolized ALN-RSV01 versus placebo
in adult lung transplant patients naturally infected with RSV.
We recently formed collaborations with Cubist Pharmaceuticals,
Inc., or Cubist, and Kyowa Hakko Kirin Co., Ltd., or Kyowa
Hakko, for the development and commercialization of products for
RSV. We will jointly develop and commercialize products for RSV
in partnership with Cubist in North America, Cubist has
responsibility for developing and commercializing these products
in the rest of the world outside of Asia, and Kyowa Hakko has
the responsibility for developing and commercializing these
products in Asia.
In December 2008, we submitted an investigational new drug
application, or IND, to the United States Food and Drug
Administration, or FDA, for ALN-VSP, our first systemically
delivered RNAi therapeutic candidate. We are developing ALN-VSP
for the treatment of liver cancers, including hepatocellular
carcinoma and other solid tumors with liver involvement. We
received clearance from the FDA in January 2009 to proceed with
a Phase I study. We expect to initiate this study during the
first half of 2009. The Phase I study will be a multi-center,
open label, dose escalation trial to evaluate the safety,
tolerability, pharmacokinetics and pharmacodynamics of
intravenous ALN-VSP in patients with advanced solid tumors with
liver involvement.
We are also working on a number of programs in pre-clinical
development, including ALN-PCS, ALN-TTR and ALN-HTT, focused on
the treatment of hypercholesterolemia, transthyretin, or TTR,
amyloidosis, and Huntington’s disease, respectively. We
have additional discovery programs for RNAi therapeutics for the
treatment of a broad range of diseases.
2
In addition, we are working internally and with third-party
collaborators to develop capabilities to deliver our RNAi
therapeutics directly to specific sites of disease, such as the
delivery of ALN-RSV01 to the lungs, which we refer to as Direct
RNAi. We are also working to extend our capabilities to advance
the development of RNAi therapeutics that are administered by
intravenous, subcutaneous or intramuscular approaches, which we
refer to as Systemic RNAi. We have numerous RNAi therapeutic
delivery collaborations and intend to continue to collaborate
with government, academic and corporate third parties to
evaluate different delivery options, including with respect to
Direct RNAi and Systemic RNAi.
We rely on the strength of our intellectual property portfolio
relating to the development and commercialization of small
interfering RNAs, or siRNAs, as therapeutics. This includes
ownership of, or exclusive rights to, issued patents and pending
patent applications claiming fundamental features of siRNAs and
RNAi therapeutics as well as those claiming crucial chemical
modifications and promising delivery technologies. We believe
that no other company possesses a portfolio of such broad and
exclusive rights to the patents and patent applications required
for the commercialization of RNAi therapeutics. Given the
importance of our intellectual property portfolio to our
business operations, we intend to vigorously enforce our rights
and defend against any challenges that have arisen or may arise
in this area.
In addition, our expertise in RNAi therapeutics and broad
intellectual property estate have allowed us to form alliances
with leading companies, including Isis Pharmaceuticals, Inc., or
Isis, Medtronic, Inc., or Medtronic, Novartis Pharma AG, or
Novartis, Biogen Idec Inc., or Biogen Idec, F.
Hoffmann-La Roche Ltd, or Roche, Takeda Pharmaceutical
Company Limited, or Takeda, Kyowa Hakko and Cubist. We have also
entered into contracts with government agencies, including the
National Institute of Allergy and Infectious Diseases, or NIAID,
a component of the National Institutes of Health, or NIH. We
have established collaborations with and, in some instances,
received funding from major medical and disease associations.
Finally, to further enable the field and monetize our
intellectual property rights, we also grant licenses to
biotechnology companies for the development and
commercialization of RNAi therapeutics for specified targets in
which we have no direct strategic interest under our
InterfeRxtm
program and to research companies that commercialize RNAi
reagents or services under our research product licenses.
We also seek opportunities to form new ventures in areas outside
our core strategic interest. For example, in 2007, we and Isis
established Regulus Therapeutics LLC, or Regulus Therapeutics, a
company focused on the discovery, development and
commercialization of microRNA-based therapeutics. Because
microRNAs are believed to regulate whole networks of genes that
can be involved in discrete disease processes, microRNA-based
therapeutics represent a possible new approach to target the
pathways of human disease. Given the broad applications for RNAi
technology, we believe additional opportunities exist for new
ventures to be formed.
Below is a list of some of our key developments in 2008 and
early 2009.
2008 and
Early 2009 Key Developments
Product
Pipeline and Scientific Developments
|
|
|
| |
•
|
We achieved human proof of concept with an RNAi therapeutic,
ALN-RSV01, in our GEMINI study, which we believe is a first for
RNAi technology and for the industry.
|
| |
| |
•
|
We expanded the development of ALN-RSV01 with the initiation of
a Phase II clinical trial to assess the safety and
tolerability of aerosolized ALN-RSV01 versus placebo in adult
lung transplant patients naturally infected with RSV.
|
| |
| |
•
|
After filing an IND in December 2008, we received clearance from
the FDA to initiate clinical studies with ALN-VSP, an RNAi
therapeutic candidate that we are developing for the treatment
of liver cancers. We expect to initiate the Phase I trial in the
first half of 2009.
|
| |
| |
•
|
We initiated new development programs in 2008 including ALN-HTT,
an RNAi therapeutic candidate targeting the huntingtin gene for
the treatment of Huntington’s disease, and ALN-TTR, an RNAi
therapeutic candidate targeting the TTR gene for the treatment
of TTR amyloidosis. These two programs, along with ALN-PCS for
the treatment of hypercholesterolemia, represent potential IND
candidates.
|
3
|
|
|
| |
•
|
We entered into new agreements with Tekmira Pharmaceuticals
Corporation, or Tekmira, related to its business combination
with Protiva Biotherapeutics Inc., or Protiva, augmenting our
platform for the systemic delivery of RNAi therapeutics.
|
| |
| |
•
|
During 2008, our scientists demonstrated continued scientific
leadership in RNAi research with the publication of 14 papers in
some of the world’s top journals, including Nature,
Nature Medicine, Nature Biotechnology and PNAS, as
well as the presentation of peer-reviewed research at key
scientific meetings.
|
Business
Execution
|
|
|
| |
•
|
We formed a strategic worldwide collaboration with Takeda,
providing Takeda with non-exclusive access to and enablement
with our RNAi therapeutics platform technology and intellectual
property in two therapeutic fields.
|
| |
| |
•
|
We formed strategic collaborations with Kyowa Hakko in Asia and
Cubist in the rest of the world outside of Asia for the
development and commercialization of our ALN-RSV program.
|
| |
| |
•
|
In 2008, Novartis elected to extend its RNAi therapeutics
collaboration for at least one additional year, through October
2009, resulting in continued research and development funding to
us.
|
| |
| |
•
|
Regulus Therapeutics, of which we own 49%, and GlaxoSmithKline
formed a strategic alliance to discover, develop and market
novel microRNA-based therapeutics to treat inflammatory diseases.
|
Intellectual
Property
|
|
|
| |
•
|
Fundamental patents from our exclusively held Tuschl II
patent series were granted by the European Patent Office, or
EPO, and the Japanese Patent Office, extending the geographic
scope of the patents and adding to the previous issuances of
the Tuschl II patent series in the United States.
|
| |
| |
•
|
We strengthened our intellectual property estate through the
acquisition of the patent assets of Nucleonics, Inc., covering
broad structural features of RNAi therapeutics.
|
| |
| |
•
|
Our partner Isis obtained additional issued claims for the
Crooke patent, which is licensed exclusively to us in the field
of RNAi therapeutics.
|
| |
| |
•
|
With respect to our Kreutzer-Limmer patent series, the EPO
granted EU 1550719 in Europe in January 2009; the EPO ruled in
favor of the opposing parties in an opposition proceeding
relating to EP 1214945, which initial ruling we intend to
appeal; and the EPO rendered a final ruling overturning EP
1144623.
|
| |
| |
•
|
In February 2009, we received the first patent grant for the
Kay & McCaffrey patent.
|
| |
| |
•
|
The Glover patent, which is exclusively licensed to us from
Cancer Research Technology Limited, was overturned by the EPO.
We intend to appeal this initial ruling.
|
Organizational
Developments
|
|
|
| |
•
|
In 2008, in addition to other promotions and appointments, we
hired Jack Schmidt, M.D., as our Chief Scientific Officer.
|
| |
| |
•
|
We elected Edward Scolnick, M.D., to our Board of Directors.
|
| |
| |
•
|
Regulus Therapeutics elected Stelios Papadopoulos, Ph.D.,
to its Board of Directors and made several key appointments to
its management team.
|
RNA
Interference
RNAi is a natural biological pathway that occurs within cells
and can be harnessed to selectively silence the activity of
specific genes. The discovery of RNAi first occurred in plants
and worms in 1998, and two of the
4
scientists who made this discovery, Dr. Andrew Fire and
Dr. Craig Mello, received the 2006 Nobel Prize for
Physiology or Medicine.
Opportunity
for Therapeutics Based on RNAi
Beginning in 1999, our scientific founders described and
provided evidence that the RNAi mechanism occurs in mammalian
cells and that its immediate trigger is a type of molecule known
as a small interfering RNA, or siRNA. They showed that
laboratory-synthesized siRNAs could be introduced into the cell
and suppress production of specific target proteins by cleaving
and degrading the messenger RNA, or mRNA, of the specific gene
that encodes that specific protein. Because it is possible to
design and synthesize siRNAs specific to any gene of interest,
the entire human genome is accessible to RNAi, and we therefore
believe that RNAi therapeutics have the potential to become a
broad new class of drugs.
In May 2001, one of our scientific founders, Thomas
Tuschl, Ph.D., published the first scientific paper
demonstrating that siRNAs can be synthesized in the laboratory
using chemical or biochemical methods and when introduced, or
delivered, into mammalian cells can silence the activity of a
specific gene. Since the Tuschl publication, the use of siRNAs
has been broadly adopted by academic and industrial researchers
for the fundamental study of the function of genes, has resulted
in a significant number of publications focused on the use of
RNAi and has made the 2001 paper one of the most cited papers in
basic biologic research. Reflecting this, siRNAs are a growing
segment of the market for research reagents and related products
and services.
Beyond its use as a basic research tool, we believe that RNAi
can form the basis of a completely new class of drug for the
treatment of disease. Drugs based on the RNAi mechanism could
offer numerous opportunities and benefits, which may include:
|
|
|
| |
•
|
Ability to target proteins that cannot be targeted
effectively by existing drug classes. Over the
last decade, the understanding of human disease has advanced
enormously and many proteins have been identified that play
fundamental roles in human disease. Paradoxically, many of these
key proteins (greater than 80%) cannot be targeted effectively
with existing drug approaches like small molecules or proteins
such as monoclonal antibodies. These so called
“undruggable” targets are potentially accessible to
siRNAs as they are made by mRNAs that can be targeted with RNAi.
|
| |
| |
•
|
Ability to treat a broad range of
diseases. The ability to make siRNAs that target
virtually any gene to suppress the production of virtually any
protein whose presence or activity causes disease suggests a
broad potential for application in a wide range of diseases.
|
| |
| |
•
|
Inherently potent mechanism of action. We
expect the inherent catalytic nature of the RNAi mechanism to
allow for a high degree of potency and durability of effect for
RNAi-based therapeutics, which distinguishes RNAi from other
approaches.
|
| |
| |
•
|
Simplified discovery of drug candidates. In
contrast to the often arduous and slow drug discovery process
for proteins and small molecules, the identification of siRNA
drug candidates has been, and we expect will continue to be,
much simpler, quicker and less costly because it involves
relatively standard processes that are directed by the known
gene target sequences and can be applied in a similar fashion to
many successive product candidates.
|
We have reported on our advances in developing siRNAs as
potential drugs in a large number of peer-reviewed publications
and meetings, including publications by Alnylam scientists in
the journals Nature, Cell, Nature Medicine, Nature
Biotechnology and PNAS.
Our
Product Platform
Our product platform provides a capability for a systematic
approach to identifying RNAi drug candidates through sequence
selection, potency selection, stabilization by chemical
modification, improvement of biodistribution and cellular
uptake by various chemical conjugates and formulations. Key to
the therapeutic application of siRNAs is the ability to
successfully deliver siRNAs to target tissues and achieve
cellular uptake of the siRNA into the inside of the cell where
the RNAi machinery, called RNA induced silencing complex, or
RISC, is
5
active. In some tissues, including the lung and central nervous
system, the direct application of formulated siRNAs, which we
refer to as the Direct RNAi approach, achieves cellular uptake
and gene knockdown. For other tissues, such as the liver,
Systemic RNAi delivery has been employed, where tissue access
comes via intravenous or subcutaneous injection of the siRNA
into the bloodstream and where cellular uptake can be achieved
by the conjugation of the siRNA with other molecules, such as
small chemical groups, or by formulation with other
biomaterials, such as lipid nanoparticles. Delivery is a key
focus for our internal research team and is also the focus of
numerous current government, academic and corporate
collaborations. We have demonstrated RNAi therapeutic activity
towards multiple genes, in multiple organs and in multiple
species, including humans, as demonstrated by our results in the
GEMINI trial for ALN-RSV01. We believe that we have made
considerable progress in developing our product platform, as
documented in many recent publications. With the progress we
have made to date and expect to make in the future, we believe
we will be well positioned to pursue multiple therapeutic
opportunities.
Our progress has enabled us to advance a number of development
programs for RNAi therapeutics that are administered directly to
diseased tissues, including ALN-RSV01 and ALN-HTT. Our progress
in achieving delivery of RNAi therapeutics through Systemic RNAi
has been recently demonstrated by the advancement our first
systemically delivered RNAi therapeutic candidate ALN-VSP, for
the treatment of liver cancers, to the clinic. We received
clearance from the FDA in January 2009 to proceed with a Phase I
study and expect to initiate this study during the first half of
2009. In addition we have published pre-clinical results from
development programs for other systemically delivered RNAi
therapeutic candidates, including ALN-PCS and ALN-TTR. We
recognize, however, that challenges remain with respect to the
development of RNAi-based therapeutics, including achieving
effective delivery of siRNAs to target cells and tissues, and we
therefore regard further development of our product platform as
an ongoing priority.
Our
Product Pipeline
The following is a summary of our development programs as of
January 31, 2009:
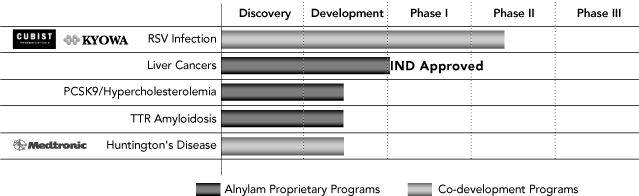
Our most advanced program is focused on RSV, a virus that
infects the respiratory tract. In January 2008, we completed our
GEMINI study, a Phase II human clinical trial of ALN-RSV01,
an RNAi therapeutic candidate for the treatment of RSV
infection, designed to evaluate the safety, tolerability and
anti-viral activity of ALN-RSV01 in adult subjects
experimentally infected with RSV. In April 2008, we initiated a
second Phase II human clinical trial, which is currently
ongoing, to assess the safety and tolerability of aerosolized
ALN-RSV01 versus placebo in adult lung transplant patients
naturally infected with RSV. We recently formed collaborations
with Cubist and Kyowa Hakko for the development and
commercialization of products for RSV. We will jointly develop
and commercialize products for RSV in partnership with Cubist in
North America, Cubist has responsibility for developing and
commercializing these products in the rest of the world outside
of Asia, and Kyowa Hakko has the responsibility for developing
and commercializing these products in Asia.
In December 2008, we submitted an IND to the FDA for ALN-VSP,
our first systemically delivered RNAi therapeutic candidate for
the treatment of liver cancers, including hepatocellular
carcinoma and other solid tumors with liver involvement. We
received clearance from the FDA in January 2009 to proceed with
a Phase I study. We
6
expect to initiate this study during the first half of 2009. The
Phase I study will be a multi-center, open label, dose
escalation trial to evaluate the safety, tolerability,
pharmacokinetics and pharmacodynamics of intravenous ALN-VSP in
patients with advanced solid tumors with liver involvement.
Our ALN-PCS program is focused on the treatment of
hypercholesterolemia with an RNAi therapeutic candidate that
targets a gene called proprotein convertase subtilisin/kexin
type 9, or PCSK9. Other development programs include ALN-TTR, an
RNAi therapeutic candidate targeting the TTR gene for the
treatment of TTR amyloidosis, which we advanced to a development
program during 2008, and ALN-HTT, an RNAi therapeutic candidate
for the treatment of Huntington’s disease, which we are
developing jointly with Medtronic.
We have spent substantial funds over the past three years to
develop our product pipeline and expect to continue to do so in
the future. We incurred research and development costs of
$96.9 million in 2008, $120.7 million in 2007 and
$49.3 million in 2006. Research and development costs in
2007 included $27.5 million in license fees paid to certain
entities, primarily Isis, in connection with our alliance with
Roche.
Development
Programs
Respiratory
Syncytial Virus Infection
Market Opportunity. RSV is a highly contagious
virus that causes infections in both the upper and lower
respiratory tract. RSV infects nearly every child by the age of
two years and is responsible for a significant percentage of
hospitalizations of infants, children with lung or congenital
heart disease, the elderly and adults with immune-compromised
systems. RSV infection typically results in cold-like symptoms,
but can lead to more serious respiratory illnesses such as
croup, pneumonia and bronchiolitis, and in extreme cases, severe
illness and death. According to NIH, up to 125,000 children are
hospitalized each year due to RSV infection. A study published
in the New England Journal of Medicine estimates that
over 170,000 elderly adults are hospitalized with RSV each year.
As a result, there is a significant need for novel therapeutics
to treat patients infected with RSV.
Current Treatments. The only product currently
approved for the treatment of RSV infection is Ribavirin, which
is marketed as
Virazole®
by Valeant Pharmaceuticals International, or Valeant. However,
this product is approved only for treatment of hospitalized
infants and young children with severe lower respiratory tract
infections due to RSV. Moreover, administration of the drug is
complicated and requires elaborate environmental reclamation
devices because of potential harmful effects on healthcare
personnel exposed to the drug.
Two other products, a monoclonal antibody known as
Synagis®
and an immune globulin called
Respigam®,
have been approved for the prevention of severe lower
respiratory tract disease caused by RSV in infants at high risk
of such disease. Neither of these products is approved for
treatment of an existing RSV infection.
Alnylam Program. In June 2007, we initiated
the GEMINI study, a double-blind, placebo-controlled, randomized
Phase II trial designed to evaluate the safety,
tolerability and anti-viral activity of ALN-RSV01 in adult
subjects experimentally infected with RSV. In total, 88 subjects
were randomized 1:1 to receive either ALN-RSV01 or placebo
treatment prior to and after experimental infection with a wild
type clinical strain of RSV. In February 2008, we reported
positive results from the GEMINI study. ALN-RSV01 was found to
be safe and well tolerated and demonstrated statistically
significant anti-viral activity, including an approximate 40%
reduction in viral infection rate and 95% increase in
infection-free patients (p<0.01), as compared to placebo.
In April 2008, we initiated a second Phase II human
clinical trial, which is currently ongoing in multiple sites
across both hemispheres. This is a double-blind, randomized
Phase II clinical trial to assess the safety and antiviral
activity of aerosolized ALN-RSV01 versus placebo in adult lung
transplant patients naturally infected with RSV.
Prior to the GEMINI study, ALN-RSV01 was shown in pre-clinical
testing to be effective in both preventing and treating RSV
infection in mice when administered intranasally. ALN-RSV01 also
showed no significant toxicities in animal toxicology studies
performed to enable the filing of an IND. We submitted an IND
for ALN-RSV01 to the FDA in November 2005, and have completed a
number of Phase I clinical trials in both the United States and
Europe. In these Phase I trials, ALN-RSV01 was found to be
generally safe and well tolerated when administered by single or
repeat administration at doses up to 150 milligrams intranasally
or at doses up to 0.6 milligrams per kilogram by nebulizer. The
results of our completed ALN-RSV01 clinical trials have been
presented
7
at medical conferences. We also have an active program to
identify second-generation RNAi-based RSV inhibitors, and have
identified several potent and specific candidates in
pre-clinical studies. We recently formed collaborations with
Cubist and Kyowa Hakko for the development and commercialization
of products for RSV. We will jointly develop and commercialize
products for RSV in partnership with Cubist in North America,
Cubist has responsibility for developing and commercializing
these products in the rest of the world outside of Asia, and
Kyowa Hakko has the responsibility for developing and
commercializing these products in Asia.
Liver
Cancer
Market Opportunity. An estimated 600,000
patients worldwide are diagnosed with primary liver cancer each
year. Hepatocellular carcinoma, or HCC, is the most common form
of liver cancer and is responsible for about 90% of primary
malignant liver tumors in adults. HCC is the sixth most common
cancer in the world and the third leading cause of
cancer-related deaths globally. In addition to primary liver
cancer patients, in whom the disease starts in the liver,
another 500,000 patients are identified each year with
secondary liver cancer, whereby the primary tumor of another
tissue, such as colorectal, stomach, pancreatic, breast, lung or
skin, has metastasized to the liver.
Current Treatments. The treatment options for
liver cancer are dependent on the stage of disease, site of
tumor and condition of the patient, but can include surgical
resection, radiation, chemotherapy, chemoembolism, liver
transplantation and various combinations of these approaches. In
November 2007, the FDA approved Sorafenib, also called
Nexavar®,
for the treatment of un-resectable liver cancer. Even with
relatively early diagnosis and resection, the prognosis remains
very poor for liver cancer patients, who are often diagnosed
late in their clinical course of disease. For primary liver
cancer, with early diagnosis and a resectable tumor, the
five-year disease free survival rate has been reported at
approximately 20%. However, this applies only to about 15% of
primary liver cancer patients. For most primary liver cancer
patients, the disease is fatal within three to six months. The
prognosis for secondary liver cancer is generally also very
poor, due often to the late stage of the disease at the time of
diagnosis and metastatic nature of the neoplasm. For example, in
the absence of treatment, the prognosis for patients with
hepatic colorectal metastases is extremely poor, with five-year
survival rates of 3% or less. Among patients that can be treated
with complete resection of hepatic colorectal metastases, only
30% to 40% will survive for five years following resection.
Alnylam Program. In December 2008, we
submitted an IND to the FDA for ALN-VSP, our first systemically
delivered RNAi therapeutic candidate for the treatment of liver
cancers, including hepatocellular carcinoma and other solid
tumors with liver involvement. We received clearance from the
FDA in January 2009 to proceed with a Phase I study. We expect
to initiate this study during the first half of 2009. ALN-VSP
contains two siRNAs formulated in a lipid nanoparticle developed
by Tekmira. ALN-VSP is designed to target two genes critical in
the growth and development of cancer: kinesin spindle protein,
or KSP, and vascular endothelial growth factor, or VEGF,
required for tumor growth. KSP is a key component of the
cellular machinery that mediates chromosome separation during
cell division, which is critical for tumor proliferation. As
such, it represents a potent target as an anti-tumor mechanism.
VEGF is a potent angiogenic factor that drives the development
of blood vessels that are critical to ensuring adequate blood
supply to the growing tumor.
Pre-clinical data in mouse tumor model studies have demonstrated
efficacy of ALN-VSP, including suppression of these targeted
genes, demonstration of an RNAi mechanism of action, tumor
reduction and extension of survival. We believe our strategy of
using an RNAi therapeutic targeting two well-validated genes
critical for tumor proliferation and survival has the potential
of achieving meaningful clinical benefit for patients with liver
cancer. In addition, we believe that this is the first dual
targeting RNAi therapeutic program to advance to clinical
development. This is an important milestone, as we view the
ability to design and formulate multiple siRNAs against more
than one target as a potentially attractive feature of our RNAi
therapeutics platform, particularly in the setting of oncology
drug development.
The Phase I study will be a multi-center, open label, dose
escalation trial to evaluate the safety, tolerability,
pharmacokinetics and pharmacodynamics of intravenous ALN-VSP in
patients with advanced solid tumors with liver involvement.
8
Hypercholesterolemia
Market Opportunity. Coronary artery disease,
or CAD, is the leading cause of mortality in the
United States, responsible for 40% of all deaths annually.
Hypercholesterolemia, defined as a high level of LDL
cholesterol, or LDL-c, in the blood, is one of the major risk
factors for CAD. Although current therapies are effective in
many patients, studies have shown that as many as 45% of these
patients do not achieve adequate control of their high
cholesterol level with existing treatments, which include drugs
known as statins. Currently in the United States, there are
almost 500,000 patients with high cholesterol levels not
controlled by the use of existing lipid lowering therapies.
These patients are said to have refractory or poorly controlled
hypercholesterolemia and constitute a potential target
population for our product candidate.
Current Treatments. The current standard of
care for patients with hypercholesterolemia includes the use of
several agents. The first treatment often prescribed is a drug
from the statin family. Commonly prescribed statins include
Lipitor®
(atorvastatin),
Zocor®
(simvastatin),
Crestor®
(rosuvastatin) and
Pravachol®
(pravastatin). A different type of drug, such as
Zetia®
(ezetimibe) and
Vytorin®
(ezetimibe/simvastatin), which reduces dietary cholesterol
uptake from the gut, may also be used either on its own or in
combination with a statin. Despite these therapies, there are
many patients who have refractory or poorly controlled
hypercholesterolemia and require more intensive treatment. In
addition, some patients do not tolerate current treatments and
at least 5% of those treated with a statin have to stop because
of side-effects. In patients with very high uncontrolled
cholesterol levels, a procedure called lipid apheresis is used,
which effectively removes cholesterol from the blood using a
machine specifically designed for this process. However, this
procedure is inconvenient and uncomfortable, requiring regular
weekly visits to a doctor’s office.
Alnylam Program. PCSK9 is a widely
acknowledged target for the treatment of hypercholesterolemia by
lowering of LDL-c levels. PCSK9 is a protein that is produced by
the liver but circulates in the bloodstream. The liver
determines cholesterol levels, in part by taking up or absorbing
LDL-c from the bloodstream. PCSK9 reduces the liver’s
capacity to absorb LDL-c. Recent evidence indicates that, if
PCSK9 activity could be reduced, the liver should increase its
uptake of LDL-c and blood cholesterol levels should decrease. In
fact, some individuals have been shown to have a genetic
mutation in PCSK9 that lowers its activity and results in
increased liver LDL-c uptake and lowered blood cholesterol
levels. In turn, these individuals have been shown to have a
dramatically reduced risk of CAD, including myocardial
infarction or heart attack. In addition, a recent study has
shown that PCSK9 levels are increased by statin therapy while
LDL-c levels are decreased, suggesting that the introduction of
a PCSK9 inhibitor to statin therapy may result in even further
reductions in LDL-c levels.
In July 2006, in collaboration with The University of Texas
Southwestern Medical Center, or UTSW, we began our ALN-PCS
program focused on the development of an RNAi therapeutic
candidate directed at PCSK9. As part of the UTSW collaboration,
we and UTSW are testing RNAi therapeutic candidates targeting
PCSK9 in certain UTSW animal models. Non-human primate data for
our ALN-PCS program has demonstrated efficient silencing of
PCSK9 and rapid and durable reductions in LDL blood cholesterol
levels by approximately 50%.
TTR
Amyloidosis
Market Opportunity. TTR amyloidosis is a
hereditary, systemic disease caused by a mutation in the TTR
gene. The resulting abnormal protein is deposited as
TTR-containing amyloid fibrils in extrahepatic tissues including
peripheral nerves, which is referred to as familial amylodotic
polyneuropathy, or FAP, and the heart, referred to as familial
amyloidotic cardiomyopathy, FAC. The condition is associated
with neuropathy, or severe pain and loss of autonomic nervous
function,
and/or heart
failure. Typical onset for TTR amyloidosis is between the third
and fifth decades of life and the disease may be fatal within
five to 15 years of onset. In its severest form,
TTR-mediated FAP and FAC present a tremendous unmet medical need
with significant morbidity and mortality. As an orphan, or rare,
disease, TTR-mediated FAP is estimated to affect approximately
5,000 to 10,000 people worldwide. There are also other
forms of TTR amyloidosis that are more common and also
associated with significant morbidity.
Current Treatments. There are no existing
disease-modifying treatments to address TTR amyloidosis.
Currently, liver transplantation is the only available treatment
for TTR-mediated FAP. However, only a subset of FAP patients
qualify for this costly and invasive procedure and, even
following liver transplantation, the disease
9
continues to progress for many of these patients, presumably due
to normal TTR being deposited into preexisting fibrils.
Moreover, there is a shortage of donors to provide healthy
livers for transplantation into eligible patients.
Alnylam Program. ALN-TTR is an RNAi
therapeutic candidate targeting the TTR gene for the treatment
of TTR amyloidosis. TTR is a carrier for thyroid hormone and
retinol binding protein and is produced almost exclusively in
the liver. Thus, we believe TTR is a suitable target for an RNAi
therapeutic formulated to maximize delivery to liver cells. Our
scientists recently presented data from our ALN-TTR program
which demonstrated that highly potent RNAi therapeutic
candidates targeting TTR significantly reduced the levels of
target mRNA in the liver and TTR protein in circulation. Using
our proprietary lipid nanoparticle formulation, studies were
performed in a transgenic mouse model where human TTR, or hTTR,
with a certain mutation known as V30M is
over-expressed.
Studies were also performed in non-human primates using the
lipid nanoparticle formulation from Tekmira. Specifically, data
from these studies showed that administration of ALN-TTR
resulted in
dose-dependent
silencing of TTR in vitro with the absence of any
measurable immune stimulatory effects; reduced TTR plasma levels
and liver TTR mRNA by greater than 70% in the V30M-hTTR
transgenic mouse model; and reduced liver TTR mRNA levels by
approximately 80% in non-human primates.
Our findings demonstrate the potential therapeutic benefit of an
RNAi therapeutic targeting TTR for the treatment of TTR
amyloidosis, including FAP. Moreover, siRNA treatment may be
superior to liver transplantation based on the ability to
simultaneously reduce the expression of mutant, as well as
wild-type, TTR. ALN-TTR is also one example of a number of
orphan-like indications where there is a very high unmet need
and the potential for early biomarker data in clinical studies
that enables rapid proof-of-concept and a clear opportunity for
a large therapeutic impact for patients.
Huntington’s
Disease
Market Opportunity. Huntington’s disease,
or HD, is a fatal, inherited and progressive brain disease that
results in uncontrolled movements, loss of intellectual
faculties, emotional disturbance and premature death. HD
patients typically first start to develop the disease in their
third or fourth decade of life and have an average survival of
only 10 to 20 years after initial diagnosis. The disease is
associated with the production of an altered form of a protein
known as huntingtin, the presence of which is believed to
trigger the death of important cells in the brain. This
autosomal dominant, neurodegenerative disease afflicts
approximately 30,000 patients in the United States. An
estimated 150,000 additional people in the United States carry
the mutant huntingtin gene and, therefore, have an approximate
50% risk of developing the disease in their lifetimes.
Current Treatments. The current treatment of
this severe disease is supportive care and symptomatic therapy,
with no drugs or therapies available that have been shown to
slow the underlying disease progress and the inexorable erosion
of the patient’s nerve cell functionality.
Alnylam Program. In collaboration with
Medtronic, we are seeking to develop a novel drug-device product
incorporating an RNAi therapeutic candidate targeting the
huntingtin gene that will protect these cells by suppressing
huntingtin mRNA and the disease causing protein. Alnylam
scientists and collaborators have published and presented in
vivo data demonstrating efficacy for an siRNA targeting the
huntingtin gene in a mouse model of the disease. The program,
ALN-HTT, is part of a
50-50
co-development/profit share relationship with Medtronic for the
United States market. Outside the United States, Medtronic will
be solely responsible for the development and commercialization
of the drug-device.
Discovery
Programs
In addition to our development efforts on RSV, liver cancer,
hypercholesterolemia, TTR amyloidosis and Huntington’s
disease, we are conducting research activities to discover RNAi
therapeutics to treat various diseases. The diseases for which
we have discovery programs include: viral hemorrhagic fever,
including the Ebola virus, which can cause severe, often fatal
infection and poses a potential biological safety risk and
bioterrorism threat; progressive multifocal leukoencephalopathy,
or PML, which is a disease of the central nervous system caused
by viral infection in immune compromised patients; and
Parkinson’s disease, a progressive brain disease which is
characterized by uncontrollable tremor, and in some cases, may
result in dementia. We are also pursuing many other undisclosed
internal programs.
10
In addition to these programs, as part of our collaborations
with Novartis and Takeda, we have research activities to
discover RNAi therapeutics directed to a number of undisclosed
targets. Our alliance with Roche also contemplates such research
activities.
Our
Collaboration and Licensing Strategy
Our business strategy is to develop and commercialize a pipeline
of RNAi therapeutic products. As part of this strategy, we have
entered into, and expect to enter into additional, collaboration
and licensing agreements as a means of obtaining resources,
capabilities and funding to advance our RNAi therapeutic
programs.
Our collaboration strategy is to form (1) non-exclusive
platform alliances where our collaborators obtain access to our
capabilities and intellectual property to develop their own RNAi
therapeutic products; and
(2) 50-50
co-development
and/or
ex-U.S. market geographic partnerships on specific RNAi
therapeutic programs. We have entered into broad, non-exclusive
platform license agreements with Roche and Takeda, under which
we will also collaborate with each of Roche and Takeda on RNAi
drug discovery for one or more disease targets. We are pursuing
50-50
co-development programs with Cubist and Medtronic for the
development and commercialization of ALN-RSV and ALN-HTT,
respectively. In addition, we have entered into a product
alliance with Kyowa Hakko for the development and
commercialization of ALN-RSV in territories not covered by the
Cubist agreement, which includes Japan and other markets in
Asia. We also have discovery and development alliances with
Isis, Novartis and Biogen Idec.
We also seek opportunities to form new ventures in areas outside
our core strategic interest. For example, we formed Regulus
Therapeutics, together with Isis, to capitalize on our
technology and intellectual property in the field of
microRNA-based therapeutics. Given the broad applications for
RNAi technology, we believe additional opportunities exist for
new ventures to be formed.
To generate revenues from our intellectual property rights, we
also grant licenses to biotechnology companies under our
InterfeRx program for the development and commercialization of
RNAi therapeutics for specified targets in which we have no
direct strategic interest. We also license key aspects of our
intellectual property to companies active in the research
products and services market, which includes the manufacture and
sale of reagents. Our InterfeRx and research product licenses
aim to generate modest near-term revenues that we can re-invest
in the development of our proprietary RNAi therapeutics
pipeline. As of January 31, 2009, we had granted such
licenses, on an exclusive or nonexclusive basis, to
approximately 20 companies.
Since delivery of RNAi therapeutics remains a major objective of
our research activities, we also look to enter into
collaboration and licensing agreements with other companies and
academic institutions to gain access to delivery technologies.
For example, we have entered into agreements with Tekmira and
MIT, among others, to focus on various delivery strategies. We
have also entered into license agreements with Isis, Max Planck
Innovation, as well as a number of other entities, to obtain
rights to important intellectual property in the field of RNAi.
Finally, we seek funding for the development of our proprietary
RNAi therapeutics pipeline from foundations and government
sources. In 2006, NIAID awarded us a contract to advance the
development of a broad spectrum RNAi anti-viral therapeutic
against hemorrhagic fever virus, including the Ebola virus. In
2007, the Defense Threat Reduction Agency, or DTRA, an agency of
the United States Department of Defense, awarded us a contract
to advance the development of a broad spectrum RNAi anti-viral
therapeutic for hemorrhagic fever virus, which ended in February
2009. In addition, we have obtained funding for pre-clinical
discovery programs from organizations such as The Michael J. Fox
Foundation.
Strategic
Alliances
We have formed, and intend to continue to form, strategic
alliances to gain access to the financial, technical, clinical
and commercial resources necessary to develop and market RNAi
therapeutics. We expect these alliances to provide us with
financial support in the form of upfront cash payments, license
fees, equity investments, research and development funding,
milestone payments
and/or
royalties or profit sharing based on sales of RNAi therapeutics.
11
Platform
Alliances.
Roche. In July 2007, we and, for limited
purposes, Alnylam Europe AG, or Alnylam Europe, entered into a
license and collaboration agreement with Roche. Under the
license and collaboration agreement, which became effective in
August 2007, we granted Roche a non-exclusive license to our
intellectual property to develop and commercialize therapeutic
products that function through RNAi, subject to our existing
contractual obligations to third parties. The license is
initially limited to the therapeutic areas of oncology,
respiratory diseases, metabolic diseases and certain liver
diseases, and may be expanded to include up to 18 additional
therapeutic areas, comprising all other fields of human disease,
as identified and agreed upon by the parties, upon payment to us
by Roche of an additional $50.0 million for each additional
therapeutic area, if any.
In consideration for the rights granted to Roche under the
license and collaboration agreement, Roche paid us
$273.5 million in upfront cash payments. In addition, in
exchange for our contributions under the collaboration
agreement, for each RNAi therapeutic product successfully
developed by Roche, its affiliates or sublicensees under the
collaboration agreement, if any, we are entitled to receive
milestone payments upon achievement of specified development and
sales events, totaling up to an aggregate of $100.0 million
per therapeutic target, together with royalty payments based on
worldwide annual net sales, if any. Due to the uncertainty of
pharmaceutical development and the high historical failure
rates generally associated with drug development, we may not
receive any milestone or royalty payments from Roche.
Under the license and collaboration agreement, we and Roche also
agreed to collaborate on the discovery of RNAi therapeutic
products directed to one or more disease targets, subject to our
contractual obligations to third parties. The discovery
collaboration between Roche and us will be governed by a joint
steering committee for a period of five years that is comprised
of an equal number of representatives from each party. In
exchange for our contributions to the collaboration, Roche will
be required to make additional milestone and royalty payments to
us.
The term of the license and collaboration agreement generally
ends upon the later of ten years from the first commercial sale
of a licensed product and the expiration of the last-to-expire
patent covering a licensed product. We estimate that our
fundamental RNAi patents covered under the license and
collaboration agreement will expire both in and outside the
United States generally between 2016 and 2025, subject to any
potential patent term extensions
and/or
supplemental protection certificates extending such term
extensions in countries where such extensions may become
available. After the first anniversary of the effective date,
Roche may terminate the license and collaboration agreement, on
a licensed
product-by-licensed
product, licensed
patent-by-licensed
patent, and
country-by-country
basis, upon
180-days’
prior written notice to us, but is required to continue to make
milestone and royalty payments to us if any royalties were
payable on net sales of a terminated licensed product during the
previous 12 months. The license and collaboration agreement
may also be terminated by either party in the event the other
party fails to cure a material breach under the license and
collaboration agreement.
In connection with the execution of the license and
collaboration agreement, we executed a common stock purchase
agreement with Roche Finance Ltd, or Roche Finance, an affiliate
of Roche. Under the terms of the common stock purchase
agreement, in August 2007, Roche Finance purchased
1,975,000 shares of our common stock at $21.50 per share,
for an aggregate purchase price of $42.5 million. Under the
terms of the common stock purchase agreement, in the event we
propose to sell or issue any of our equity securities, subject
to specified exceptions, we agreed to grant to Roche Finance the
right to acquire additional securities, such that Roche Finance
would be able to maintain its ownership percentage in us. Roche
Finance agreed that until August 9, 2010, neither it nor
any specified affiliates will acquire any of our securities or
assets (other than an acquisition resulting in such entities
beneficially owning less than 5% of our total outstanding voting
securities), participate in any tender or exchange offer, merger
or other business combination involving us or seek to control
our management, board of directors or policies, subject to
specified exceptions. Roche Finance also agreed that neither it
nor any specified affiliates will sell or transfer any of our
equity securities during the period prior to August 9, 2009
and will limit the volume of such sales or transfers in a single
day during the following one-year period, in each case, for so
long as Roche Finance and such affiliates beneficially own more
than 2.5% of the total outstanding shares of our common stock.
In connection with the execution of the license and
collaboration agreement and the common stock purchase agreement,
we also executed a stock purchase agreement with Alnylam Europe
and Roche Beteiligungs GmbH, or
12
Roche Germany, an affiliate of Roche. Under the terms of the
Alnylam Europe stock purchase agreement, we created a new,
wholly-owned German limited liability company, Roche Kulmbach,
into which substantially all of the non-intellectual property
assets of Alnylam Europe were transferred, and Roche Germany
purchased from us all of the issued and outstanding shares of
Roche Kulmbach for an aggregate purchase price of
$15.0 million. The Alnylam Europe stock purchase agreement
included transition services that were performed by Roche
Kulmbach employees at various levels through August 2008. We
reimbursed Roche for these services at an
agreed-upon
rate.
In connection with the license and collaboration agreement and
the common stock purchase agreement, we paid $27.5 million
in license fees to our licensors, primarily Isis, in accordance
with the applicable license agreements with those parties.
Takeda. In May 2008, we entered into a license
and collaboration agreement with Takeda to pursue the
development and commercialization of RNAi therapeutics. Under
the Takeda agreement, we granted to Takeda a non-exclusive,
worldwide, royalty-bearing license to our intellectual property
to develop, manufacture, use and commercialize RNAi
therapeutics, subject to our existing contractual obligations to
third parties. The license initially is limited to the fields of
oncology and metabolic disease and may be expanded at
Takeda’s option to include other therapeutic areas, subject
to specified conditions. Under the Takeda agreement, Takeda will
be our exclusive platform partner in the Asian territory, as
defined in the agreement, for a period of five years.
In consideration for the rights granted to Takeda under the
Takeda agreement, Takeda agreed to pay us $150.0 million in
upfront and near-term technology transfer payments. In addition,
we have the option, exercisable until the start of
Phase III development, to opt-in under a
50-50 profit
sharing agreement to the development and commercialization in
the United States of up to four Takeda licensed products, and
would be entitled to opt-in rights for two additional products
for each additional field expansion, if any, elected by Takeda
under the Takeda agreement. In June 2008, Takeda paid us an
upfront payment of $100.0 million. Takeda is also required
to make the additional $50.0 million in payments to us upon
achievement of specified technology transfer milestones,
$20.0 million of which was achieved in September 2008 and
paid in October 2008, $20.0 million of which is required to
be paid within 12 to 24 months of execution of the
agreement and $10.0 million of which is required to be paid
within 24 to 36 months of execution of the agreement. If
Takeda elects to expand its license to additional therapeutic
areas, Takeda will be required to pay us $50.0 million for
each of up to approximately 20 total additional fields selected,
comprising all other fields of human disease, as identified and
agreed upon by the parties. In addition, for each RNAi
therapeutic product successfully developed by Takeda, its
affiliates and sublicensees, if any, we are entitled to receive
specified development and commercialization milestones, totaling
up to $171.0 million per product, together with royalty
payments based on worldwide annual net sales, if any. Due to the
uncertainty of pharmaceutical development and the high
historical failure rates generally associated with drug
development, we may not receive any milestone or royalty
payments from Takeda.
Pursuant to the Takeda agreement, we and Takeda have also agreed
to collaborate on the research of RNAi therapeutics directed to
one or two disease targets agreed to by the parties, subject to
our existing contractual obligations with third parties. Takeda
also has the option, subject to certain conditions, to
collaborate with us on the research and development of RNAi drug
delivery technology for targets agreed to by the parties. In
addition, Takeda has a right of first negotiation for the
development and commercialization of our RNAi therapeutic
products in the Asian territory, excluding our ALN-RSV01
program. In addition to our
50-50 profit
sharing option, we have a similar right of first negotiation to
participate with Takeda in the development and commercialization
in the United States of licensed products. The
collaboration is governed by a joint technology transfer
committee, or JTTC, a joint research collaboration committee, or
JRCC, and a joint delivery collaboration committee, or JDCC,
each of which is comprised of an equal number of representatives
from each party.
The term of the Takeda agreement generally ends upon the later
of (i) the expiration of our last-to-expire patent covering
a licensed product and (ii) the last-to-expire term of a
profit sharing agreement in the event we elect to enter into
such an agreement. We estimate that our fundamental RNAi patents
covered under the Takeda agreement will expire both in and
outside the United States generally between 2016 and 2025,
subject to any potential patent term extensions
and/or
supplemental protection certificates extending such term
extensions in countries where such extensions may become
available. The Takeda agreement may be terminated by either
party in the event the other party fails to cure a material
breach under the agreement. In addition, after the first
anniversary of the effective date
13
of the Takeda agreement, Takeda may terminate the agreement on a
licensed
product-by-licensed
product or
country-by-country
basis upon
180-days’
prior written notice to us, provided, however, that Takeda is
required to continue to make royalty payments to us for the
duration of the royalty term with respect to a licensed product.
In connection with the Takeda agreement, we paid
$5.0 million of license fees to our licensors, primarily
Isis, in accordance with the applicable license agreements with
those parties.
Discovery
and Development Alliances.
Isis. In March 2004, we entered into a
collaboration and license agreement with Isis, a leading
developer of antisense oligonucleotide drugs that target RNA.
The Isis agreement significantly enhanced our intellectual
property position with respect to RNA-based therapeutic products
and our ability to develop siRNAs for RNAi therapeutic products,
and provided us with the opportunity to defer investment in
manufacturing technology. Isis granted us licenses to its
current and future patents and patent applications relating to
chemistry and to RNA-targeting mechanisms for the research,
development and commercialization of siRNA products. We have the
right to use Isis technologies in our development programs or in
collaborations, and Isis has agreed not to grant licenses under
these patents to any other organization for any siRNA products
designed to work through an RNAi mechanism, except in the
context of a collaboration in which Isis plays an active role.
The licenses that we have granted to Isis are non-exclusive
licenses to our current and future patents and patent
applications relating to RNA-targeting mechanisms and to
chemistry for research use. We have also granted Isis the
non-exclusive right to develop and commercialize siRNA products
against a limited number of targets. In addition, we have
granted Isis non-exclusive rights to our patents and patent
applications for research, development and commercialization of
antisense RNA products.
Under the terms of the Isis agreement, we paid Isis an upfront
license fee of $5.0 million. We also agreed to pay Isis
milestone payments, totaling up to approximately
$3.4 million, upon the occurrence of specified development
and regulatory events, and royalties on sales, if any, for each
product that we or a collaborator develops using Isis
intellectual property. In addition, we agreed to pay to Isis a
percentage of specified fees from strategic collaborations we
may enter into that include access to the Isis intellectual
property.
In conjunction with the agreement, Isis made a
$10.0 million equity investment in us. In addition, Isis
has agreed to pay us, per therapeutic target, a license fee of
$0.5 million, and milestone payments totaling approximately
$3.4 million, payable upon the occurrence of specified
development and regulatory events, and royalties on sales, if
any, for each product developed by Isis or a collaborator that
utilizes our intellectual property. Isis has the right to elect
up to ten non-exclusive target licenses under the agreement and
has the right to purchase one additional non-exclusive target
per year during the term of the collaboration.
The Isis agreement also gives us an option to use Isis’
manufacturing services for RNA-based therapeutic products. In
addition, under the Isis agreement, we have the exclusive right
to grant sub-licenses for Isis technology to third parties with
whom we are not collaborating. We may include these sub-licenses
in our non-exclusive platform licenses and our InterfeRx
licenses. If a license includes rights to Isis’
intellectual property, we will share revenues from that license
equally with Isis.
The term of the Isis agreement generally ends upon the
expiration of the last-to-expire patent licensed thereunder,
whether such patent is a patent licensed by us to Isis, or vice
versa. As the license will include additional patents, if any,
filed to cover future inventions, if any, the date of expiration
cannot be calculated at this time.
Novartis. We have formed two alliances with
Novartis. We refer to the first of these, which was initiated in
September 2005, as the broad Novartis alliance, and to the
second, which was initiated in February 2006, as the Novartis
flu alliance.
In connection with the broad Novartis alliance, we entered into
a series of transactions with Novartis beginning in September
2005. At that time, we and Novartis executed a stock purchase
agreement and an investor rights agreement. When the
transactions contemplated by the stock purchase agreement closed
in October 2005, the investor rights agreement became effective
and we and Novartis executed a research collaboration and
license agreement. The collaboration and license agreement had
an initial term of three years, with an option for two
additional one-year extensions at the election of Novartis. In
July 2008, Novartis elected to extend the initial term of
14
the agreement for an additional year, through October 2009.
Novartis retains the right to extend the term for a second
additional year, which right must be exercised no later than
July 2009.
Under the terms of the collaboration and license agreement, the
parties will work together on selected targets, as defined in
the collaboration and license agreement, to discover and develop
therapeutics based on RNAi. In consideration for rights granted
to Novartis under the collaboration and license agreement,
Novartis made an upfront payment of $10.0 million to us in
October 2005, partly to reimburse prior costs incurred by us to
develop in vivo RNAi technology. The collaboration and
license agreement also includes terms under which Novartis will
provide us with research funding and development and sales
milestone payments as well as royalties on annual net sales of
products resulting from the collaboration, if any. The amount of
research funding provided by Novartis under the collaboration
and license agreement during the research term is dependent upon
the number of active programs on which we are collaborating with
them at any given time and the number of our employees that are
working on those programs, in respect of which Novartis
reimburses us at an agreed upon rate. Under the terms of the
collaboration and license agreement, Novartis has the right to
select up to 30 exclusive targets to include in the
collaboration, which number may be increased to 40 under certain
circumstances and upon additional payments. For RNAi therapeutic
products successfully developed under the agreement, if any, we
would be entitled to receive milestone payments upon achievement
of certain specified development and annual net sales events, up
to an aggregate of $75.0 million per therapeutic product.
Due to the uncertainty of pharmaceutical development and the
high historical failure rates generally associated with drug
development, we may not receive any milestone or royalty
payments from Novartis.
Under the terms of the collaboration and license agreement, we
retain the right to discover, develop, commercialize and
manufacture compounds that function through the mechanism of
RNAi, or products that contain such compounds as an active
ingredient, with respect to targets not selected by Novartis for
inclusion in the collaboration, provided that Novartis has a
right of first offer with respect to an exclusive license for
additional targets before we partner any of those additional
targets with third parties.
The collaboration and license agreement also provides Novartis
with a non-exclusive option to integrate into its operations our
intellectual property relating to RNAi technology, excluding any
technology related to delivery of nucleic acid based molecules.
Novartis may exercise this integration option at any point
during the research term, as defined in the collaboration and
license agreement. The research term expires in October 2009 and
may be extended until October 2010 at Novartis’ election.
In connection with the exercise of the integration option,
Novartis would be required to make additional payments to us
totaling $100.0 million, payable in full at the time of
exercise, which payments would include an option exercise fee, a
milestone based on the overall success of the collaboration and
pre-paid milestones and royalties that could become due as a
result of future development of products using our technology.
In addition, under this license grant, Novartis may be required
to make milestone and royalty payments to us in connection with
the successful development and commercialization of RNAi
therapeutic products, if any. The license grant under the
integration option, if exercised by Novartis, would be
structured similarly to our non-exclusive platform licenses with
Roche and Takeda.
Novartis may terminate the collaboration and license agreement
in the event that we materially breach our obligations. We may
terminate the agreement with respect to particular programs,
products
and/or
countries in the event of specified material breaches by
Novartis of its obligations, or in its entirety under specified
circumstances for multiple such breaches.
Under the terms of the stock purchase agreement, in October
2005, Novartis purchased 5,267,865 shares of our common
stock at a purchase price of $11.11 per share for an aggregate
purchase price of $58.5 million, which, after such
issuance, represented 19.9% of our outstanding common stock as
of the date of issuance. In addition, under the investor rights
agreement, we granted Novartis rights to acquire additional
equity securities in the event that we propose to sell or issue
any equity securities, subject to specified exceptions, as
described in the investor rights agreement, such that Novartis
would be able to maintain its then-current ownership percentage
in our outstanding common stock. Pursuant to terms of the
investor rights agreement, in May 2008, Novartis purchased
213,888 shares of our common stock at a purchase price of
$25.29 per share resulting in a payment to us of
$5.4 million. At December 31, 2008, Novartis owned
13.2% of our outstanding common stock.
15
Under the terms of the investor rights agreement, we also
granted Novartis demand and piggyback registration rights under
the Securities Act of 1933 for the shares of our common stock
held by Novartis. Novartis agreed, until the later of
(1) October 12, 2008 and (2) the date of
termination or expiration of the selection term, as defined in
the collaboration and license agreement, not to acquire any of
our securities, other than an acquisition resulting in Novartis
and its affiliates beneficially owning less than 20% of our
total outstanding voting securities, participate in any tender
or exchange offer, merger or other business combination
involving us or seek to control or influence our management,
board of directors or policies, subject to specified exceptions
described in the investor rights agreement.
In February 2006, we entered into the Novartis flu alliance.
Under the terms of the Novartis flu alliance, we and Novartis
have joint responsibility for the development of RNAi
therapeutics for pandemic flu. This program was stopped and
currently there are no specific resource commitments for this
program.
Biogen Idec. In September 2006, we entered
into a collaboration and license agreement with Biogen Idec. The
collaboration is focused on the discovery and development of
therapeutics based on RNAi for the potential treatment of PML.
Under the terms of the Biogen Idec agreement, we granted Biogen
Idec an exclusive license to distribute, market and sell certain
RNAi therapeutics to treat PML and Biogen Idec has agreed to
fund all related research and development activities. We
received an upfront $5.0 million payment from Biogen Idec.
In addition, upon the successful development and utilization of
a product resulting from the collaboration, if any, Biogen Idec
would be required to pay us milestone payments, totaling
$51.0 million, and royalty payments on sales, if any. Due
to the uncertainty of pharmaceutical development and the high
historical failure rates generally associated with drug
development, we may not receive any milestone or royalty
payments from Biogen Idec. The pace and scope of future
development of this program is the responsibility of Biogen
Idec. We expect to expend limited resources on this program in
2009.
Unless earlier terminated, the Biogen Idec agreement will remain
in effect until the expiration of all payment obligations under
the agreement. Either we or Biogen Idec may terminate the
agreement in the event that the other party breaches its
obligations thereunder. Biogen Idec may also terminate the
agreement, on a
country-by-country
basis, without cause upon 90 days prior written notice.
Product
Alliances.
Medtronic. In July 2007, we entered into an
amended and restated collaboration agreement with Medtronic to
pursue the development of therapeutic products for the treatment
of neurodegenerative disorders. The amended and restated
collaboration agreement supersedes the collaboration agreement
entered into by the parties in February 2005, and continues the
existing collaboration between the parties focusing on the
delivery of RNAi therapeutics to specific areas of the brain
using implantable infusion systems.
Under the terms of the amended and restated collaboration
agreement, we and Medtronic are continuing our existing
development program focused on developing a combination
drug-device product for the treatment of Huntington’s
disease. In addition, we and Medtronic may jointly agree to
collaborate on additional product development programs for the
treatment of other neurodegenerative diseases, which can be
addressed by the delivery of siRNAs discovered and developed
using our RNAi therapeutics platform to the human nervous system
through implantable infusion devices developed by Medtronic. We
will be responsible for supplying the siRNA component and
Medtronic will be responsible for supplying the device component
of any product resulting from the collaboration.
With respect to the initial product development program focused
on Huntington’s disease, each party is funding 50% of the
development efforts for the United States while Medtronic is
responsible for funding development efforts outside the United
States. Medtronic will commercialize any resulting products and
pay royalties to us based on net sales of any such products, if
any, which royalties in the United States are designed to
approximate 50% of the profit associated with the sale of such
product and which royalties in Europe are similar to more
traditional pharmaceutical royalties, in that they are intended
to reflect each party’s contribution.
Each party has the right to opt out of its obligation to fund
the program under the agreement at certain stages, and the
agreement provides for revised economics based on the timing of
any such opt out. Other than pursuant to
16
the initial product development program, and subject to
specified exceptions, neither party may research, develop,
manufacture or commercialize products that use implanted
infusion devices for the direct delivery of siRNAs to the human
nervous system to treat Huntington’s disease during the
term of such program.
The amended and restated collaboration agreement expires, on a
product-by-product
and
country-by-country
basis, upon expiration of the royalty term for the applicable
product. The royalty term is the longer of a specified number of
years from the first commercial sale of the applicable product
and the expiration of the last-to-expire of specified patent
rights. Royalties are paid at a lower level during any part of a
royalty term in which specified patent coverage does not exist.
Either party may terminate the amended and restated
collaboration agreement on 60 days’ prior written
notice if the other party materially breaches the agreement in
specified ways and fails to cure the breach within the
60-day
notice period. Either party may also terminate the agreement in
the event that specified pre-clinical testing does not yield
results meeting specified success criteria.
Kyowa Hakko. In June 2008, we entered into a
license and collaboration agreement with Kyowa Hakko. Under the
Kyowa Hakko agreement, we granted Kyowa Hakko an exclusive
license to our intellectual property in Japan and other markets
in Asia for the development and commercialization of ALN-RSV01
for the treatment of RSV infection. The Kyowa Hakko agreement
also covers additional RSV-specific RNAi therapeutic compounds
that comprise the ALN-RSV program. We retain all development and
commercialization rights worldwide excluding the licensed
territory.
Under the terms of the Kyowa Hakko agreement, in June 2008,
Kyowa Hakko paid us an upfront cash payment of
$15.0 million. In addition, Kyowa Hakko is required to make
payments to us upon achievement of specified development and
sales milestones totaling up to $78.0 million, and royalty
payments based on annual net sales, if any, of ALN-RSV01 by
Kyowa Hakko, its affiliates and sublicensees in the licensed
territory. Due to the uncertainty of pharmaceutical development
and the high historical failure rates generally associated with
drug development, we may not receive any milestone or royalty
payments from Kyowa Hakko.
Our collaboration with Kyowa Hakko is governed by a joint
steering committee that is comprised of an equal number of
representatives from each party. Under the agreement, Kyowa
Hakko is establishing a development plan for ALN-RSV01 relating
to the development activities to be undertaken in the licensed
territory, with the initial focus on Japan. Kyowa Hakko is
responsible, at its expense, for all development activities
under the development plan that are reasonably necessary for the
regulatory approval and commercialization of ALN-RSV01 in Japan
and the rest of the licensed territory. We will be responsible
for supply of the product to Kyowa Hakko under a supply
agreement unless Kyowa Hakko elects, prior to the first
commercial sale of the product in the licensed territory, to
manufacture the product itself or arrange for a third party to
manufacture the product.
The term of the Kyowa Hakko agreement generally ends on a
country-by-country
basis upon the later of (1) the expiration of our
last-to-expire patent covering a licensed product and
(2) the tenth anniversary of the first commercial sale in
the country of sale. We estimate that our principal patents
covered under the Kyowa Hakko agreement will expire both in and
outside the United States generally between 2016 and 2025. These
patent rights are subject to any potential patent term
extensions
and/or
supplemental protection certificates extending such term
extensions in countries where such extensions may become
available. Additional patent filings relating to the
collaboration may be made in the future. The Kyowa Hakko
agreement may be terminated by either party in the event the
other party fails to cure a material breach under the agreement.
In addition, Kyowa Hakko may terminate the agreement without
cause upon
180-days’
prior written notice to us, subject to certain conditions.
Cubist. In January 2009, we entered into a
license and collaboration agreement with Cubist to develop and
commercialize therapeutic products based on certain of our RNAi
technology for the treatment of RSV, including ALN-RSV01, which
is currently in Phase II clinical development for the
treatment of RSV infection in adult lung transplant patients, as
well as several other second-generation RNAi-based RSV
inhibitors, which currently are in pre-clinical studies.
Under the terms of the Cubist agreement, we and Cubist will
share responsibility for developing licensed products in North
America and will each bear one-half of the related development
costs. Our collaboration with Cubist for the development of
licensed products in North America will be governed by a joint
steering committee comprised of an equal number of
representatives from each party. Cubist will have the sole right
to commercialize
17
licensed products in North America with costs associated with
such activities and any resulting profits or losses to be split
equally between us and Cubist. Throughout the rest of the world,
referred to as the Royalty Territory, excluding Asia, where we
have previously partnered our ALN-RSV program with Kyowa Hakko,
Cubist will have an exclusive, royalty-bearing license to
develop and commercialize licensed products.
In consideration for the rights granted to Cubist under the
agreement, in January 2009, Cubist paid us an upfront cash
payment of $20.0 million. Cubist also has an obligation
under the agreement to pay us milestone payments, totaling up to
an aggregate of $82.5 million, upon the achievement of
specified development and sales events in the Royalty Territory.
In addition, if licensed products are successfully developed,
Cubist will be required to pay us double digit royalties on net
sales of licensed products in the Royalty Territory, if any,
subject to offsets under certain circumstances. Upon achievement
of certain development milestones, we will have the right to
convert the North American co-development and profit sharing
arrangement into a royalty-bearing license and, in addition to
royalties on net sales in North America, will be entitled to
receive additional milestone payments totaling up to an
aggregate of $130.0 million upon achievement of specified
development and sales events in North America, subject to
the timing of the conversion by us and the regulatory status of
a licensed product at the time of conversion. If we make the
conversion to a royalty-bearing license with respect to North
America, then North America becomes part of the Royalty
Territory. Due to the uncertainty of pharmaceutical development
and the high historical failure rates generally associated with
drug development, we may not receive any milestone or royalty
payments from Cubist.
Unless terminated earlier in accordance with the agreement, the
agreement expires on a
country-by-country
and licensed
product-by-licensed
product basis, (a) with respect to the Royalty Territory,
upon the latest to occur of (1) the expiration of the
last-to-expire Alnylam patent covering a licensed product,
(2) the expiration of the Regulatory-Based Exclusivity
Period (as defined in the Cubist agreement) and (3) ten
years from first commercial sale in such country of such
licensed product by Cubist or its affiliates or sublicensees,
and (b) with respect to North America, if we have not
converted North America into the Royalty Territory, upon the
termination of the agreement by Cubist upon specified prior
written notice. We estimate that our fundamental RNAi patents
covered under the Cubist agreement will expire both in and
outside of the United States generally between 2016 and 2025.
Allowed claims covering ALN-RSV01 in the United States would
expire in 2026. These patent rights are subject to any potential
patent term extensions
and/or
supplemental protection certificates extending such term
extensions in countries where such extensions may become
available. In addition, more patent filings relating to the
collaboration may be made in the future. Cubist has the right to
terminate the agreement at any time (1) upon three
months’ prior written notice if such notice is given prior
to the acceptance for filing of the first application for
regulatory approval of a licensed product or (2) upon nine
months prior written notice if such notice is given after the
acceptance for filing of the first application for regulatory
approval. Either party may terminate the agreement in the event
the other party fails to cure a material breach or upon
patent-related challenges by the other party.
During the term of the Cubist agreement, neither party nor its
affiliates may develop, manufacture or commercialize anywhere in
the world, outside of Asia, a therapeutic or prophylactic
product that specifically targets RSV, except for licensed
products developed, manufactured or commercialized pursuant to
the agreement.
microRNAi-based
Therapeutics
Regulus Therapeutics. In September 2007, we
and Isis established Regulus Therapeutics, a company focused on
the discovery, development and commercialization of
microRNA-based therapeutics. Regulus Therapeutics combines our
and Isis’ technologies, know-how and intellectual property
relating to microRNA-based therapeutics. In addition, we believe
that Regulus Therapeutics has assembled a strong leadership
team, as well as leading authorities in the field of microRNA
research to lead this new venture. In December 2007, Kleanthis
G. Xanthopoulos, Ph.D. was appointed as President and Chief
Executive Officer of Regulus Therapeutics.
Regulus Therapeutics is exploring therapeutic opportunities that
arise from alterations in microRNA expression. Since microRNAs
are believed to regulate the expression of broad networks of
genes and biological pathways, microRNA-based therapeutics
define a new and potentially high-impact strategy to target
multiple points on disease pathways. Conventional messenger RNAs
are genetically encoded and in turn instruct the creation of
proteins through the process of translation. However, these
small microRNAs do not instruct creation of
18
proteins but instead regulate the expression of other genes.
There are approximately 700 microRNAs that have been identified
in the human genome, and these are believed to regulate the
expression of up to 30% of all human genes. Since microRNAs may
act as master regulators for physiological pathways or genetic
networks to achieve integrated biological functions, affecting
the expression of multiple genes in the pathway of disease,
microRNAs potentially represent an exciting new platform for
drug discovery and development.
To date, microRNAs have been implicated in several disease areas
such as cancer, viral infection, inflammatory diseases and
metabolic disorders. Regulus Therapeutics’ most advanced
program, which is in pre-clinical research, is a microRNA-based
therapeutic candidate that targets miR-122, an endogenous host
gene required for viral infection by the hepatitis C virus,
or HCV. HCV infection is a significant disease worldwide, for
which emerging therapies target viral genes and, therefore, are
prone to viral resistance. Regulus Therapeutics is also pursuing
a program that targets miR-21. Pre-clinical studies by Regulus
Therapeutics and collaborators have shown that
miR-21 is
implicated in several therapeutic areas, including heart failure
and fibrosis. In addition to these programs, Regulus
Therapeutics is also actively exploring additional areas for
development of microRNA-based therapeutics, including cancer,
other viral diseases, metabolic disorders and inflammatory
diseases.
In April 2008, Regulus Therapeutics entered into a worldwide
strategic alliance with GlaxoSmithKline, or GSK, to discover,
develop and market novel microRNA-targeted therapeutics to treat
inflammatory diseases such as rheumatoid arthritis and
inflammatory bowel disease. In connection with this alliance,
Regulus Therapeutics received $20.0 million in upfront
payments from GSK, including a $15.0 million option fee and
a loan of $5.0 million evidenced by a promissory note
(guaranteed by Isis and us) that will convert into Regulus
Therapeutics common stock under certain specified circumstances.
Regulus Therapeutics could be eligible to receive development,
regulatory and sales milestone payments for each of the four
microRNA-targeted therapeutics discovered and developed as part
of the alliance, and would also receive royalty payments on
worldwide sales of products resulting from the alliance, if any.
Regulus Therapeutics, which was initially established as a
limited liability company, converted to a C corporation as
of January 2, 2009 and changed its name to Regulus
Therapeutics Inc. We and Isis continue to own 49% and 51%,
respectively, of Regulus Therapeutics and Regulus Therapeutics
continues to operate as an independent company with a separate
board of directors, scientific advisory board and management
team. In September 2007, we, Isis and Regulus Therapeutics
also entered into a license and collaboration agreement to
pursue the discovery, development and commercialization of
therapeutic products directed to microRNAs. Under the terms of
the license and collaboration agreement, we and Isis assigned to
Regulus Therapeutics specified patents and contracts covering
microRNA-specific technology. In addition, each of us granted to
Regulus Therapeutics an exclusive, worldwide license under our
rights to other microRNA-related patents and know-how to develop
and commercialize therapeutic products containing compounds that
are designed to interfere with or inhibit a particular microRNA,
subject to our and Isis’ existing contractual obligations
to third parties. Regulus Therapeutics also has the right to
request a license from us and Isis to develop and commercialize
therapeutic products directed to other microRNA compounds, which
license is subject to our and Isis’ approval and to each
such party’s existing contractual obligations to third
parties. Regulus Therapeutics granted to us and Isis an
exclusive license to technology developed or acquired by Regulus
Therapeutics for use solely within our respective fields (as
defined in the license and collaboration agreement), but
specifically excluding the right to develop, manufacture or
commercialize the therapeutic products for which we and Isis
granted rights to Regulus Therapeutics. In addition, we made an
initial cash contribution to Regulus Therapeutics of
$10.0 million, resulting in us and Isis making initial
capital contributions to Regulus Therapeutics of approximately
equal aggregate value.
After a sufficient portfolio of data is obtained with respect to
each microRNA drug candidate developed by Regulus Therapeutics,
Regulus Therapeutics may elect to continue to pursue the
development and commercialization of products directed to such
microRNA compound and related microRNA compounds, in which event
Regulus Therapeutics would be obligated to pay us and Isis a
royalty on net sales of any such resulting products. If Regulus
Therapeutics decides not to continue to pursue the development
and commercialization of products directed to particular
microRNA compounds, either we or Isis may pursue development and
commercialization of such Regulus Therapeutics products.
Development and commercialization of such products by either
party would be subject to the payment to Regulus Therapeutics of
a specified upfront fee,
19
milestone payments upon achievement of specified regulatory
events, royalties on net sales and a portion of income received
from sublicensing rights.
In addition, we are also a party to a services agreement with
Isis and Regulus Therapeutics. Under the terms of the services
agreement, we and Isis agreed to provide to Regulus Therapeutics
certain research and development and general and administrative
services, as set forth in an operating plan mutually agreed upon
by us and Isis. Pursuant to this agreement, we and Isis are paid
by Regulus Therapeutics for these services.
Other
RNAi Areas of Opportunity
We continue to seek opportunities to form new ventures in areas
outside our core strategic interest. For example, during 2008,
we further expanded our technology platform with the acquisition
of RNA activation, or RNAa, technology. As part of our overall
strategy to be the leader in the field of RNA therapeutics,
including RNAi and microRNA-based therapeutics, we consolidated
key intellectual property in the emerging biological field of
RNAa. RNAa technology has the potential to activate gene
expression, which may have multiple potential therapeutic
applications, including the treatment of certain genetic
diseases and cancer. We have entered into exclusive license
agreements with UTSW, the University of California
San Francisco and the Salk Institute for Biological
Studies. RNAa technology represents a potential new product
platform in our efforts to advance innovative medicines to
patients.
We are also evaluating various opportunities in the areas of
stem cell research, genomics, bioprocessing and biologics,
vaccines, and other non-coding RNAs. Given the broad
applications for RNAi technology, we believe additional
opportunities exist for new ventures to be formed.
Licenses
To further enable the field and monetize our intellectual
property rights, we have established our InterfeRx program and
our research reagents and services licensing program.
InterfeRx Program. Our InterfeRx program
consists of the licensing of our intellectual property to others
for the development and commercialization of RNAi therapeutic
products relating to specific targets outside our direct
strategic focus. We expect to receive license fees, annual
maintenance fees, milestone payments and royalties on sales of
any resulting RNAi therapeutic products. Generally, we do not
expect to collaborate with our InterfeRx licensees in the
development of RNAi therapeutic products, but may do so in
certain circumstances. To date, we have granted InterfeRx
licenses to a number of companies, including GeneCare Research
Institute Co., Ltd., or GeneCare, Quark Biotech, Inc., or Quark,
Calando Pharmaceuticals, Inc., or Calando, and Tekmira. In
general, these licenses allow the licensees to discover, develop
and commercialize RNAi therapeutics for a limited number of
targets in return for upfront, milestone, license maintenance
and/or
royalty payments to us. In some cases, we also retained a right
to negotiate the ability to co-promote
and/or
co-commercialize the licensed product, and in one case, we
included the rights to discover, develop and commercialize RNAi
therapeutics utilizing expressed RNAi (i.e., RNAi mediated by
siRNAs generated from DNA constructs introduced into cells). In
addition, Benitec Ltd., or Benitec, has an option to take an
InterfeRx license, subject to certain conditions. We have
granted InterfeRx licenses relating to fewer than 15 gene
targets in total, under both exclusive and non-exclusive
licenses.
Research Reagents and Services. We have
granted approximately 15 licenses to our intellectual property
for the development and commercialization of research reagents
and services, and intend to enter into additional licenses on an
ongoing basis. Our target licensees are vendors that provide
siRNAs and related products and services for use in biological
research. We offer these licenses in return for an initial
license fee, annual renewal fees and royalties from sales of
siRNA research reagents and services. No single research reagent
or research services license is material to our business.
Delivery
Initiatives
We are working to extend our capabilities in developing
technology to achieve effective and safe delivery of RNAi
therapeutics to a broad spectrum of organ and tissue types. In
connection with these efforts, we have entered
20
into a number of agreements to evaluate and gain access to
certain delivery technologies. In some instances, we are also
providing funding to support the advancement of these delivery
technologies.
For example, in May of 2007, we entered into an agreement with
MIT, Center for Cancer Research under which we are sponsoring an
exclusive five-year research program focused on the delivery of
RNAi therapeutics. In addition, during 2007, we obtained an
exclusive worldwide license to the liposomal delivery
formulation technology of Tekmira for the discovery,
development and commercialization of lipid-based nanoparticle
formulations for the delivery of RNAi therapeutics. In May 2008,
Tekmira acquired Protiva. In connection with this acquisition,
we entered into new agreements with Tekmira and Protiva, which
provide us access to key existing and future technology and
intellectual property for the systemic delivery of RNAi
therapeutics with liposomal delivery technologies. Under the new
agreements with Tekmira and Protiva, we continue to have
exclusive rights to the Semple (U.S. Patent
No. 6,858,225) and Wheeler (U.S. Patent Nos. 5,976,567
and 6,815,432) patents for RNAi, which we believe are critical
for the use of cationic liposomal delivery technology.
As noted above, we are developing ALN-VSP, a systemically
delivered RNAi therapeutic candidate, for the treatment of liver
cancers, including hepatocellular carcinoma and other solid
tumors with liver involvement.
ALN-VSP
comprises two siRNAs formulated using Tekmira’s stable
nucleic acid-lipid particles, or SNALP, technology. We also have
rights to use SNALP technology in the advancement of our other
systemically delivered RNAi therapeutic programs, including
ALN-PCS for the treatment of hypercholesterolemia and ALN-TTR
for the treatment of TTR amyloidosis.
We have other RNAi therapeutic delivery collaborations and
intend to continue to collaborate with government, academic and
corporate third parties to evaluate different delivery
technologies, including with respect to Direct RNAi and Systemic
RNAi.
Government
Funding
NIH. In September 2006, NIAID awarded us a
contract for up to $23.0 million over four years to advance
the development of a broad spectrum RNAi anti-viral therapeutic
for hemorrhagic fever virus, including the Ebola virus. The
Ebola virus can cause a severe, often fatal infection, and poses
a potential biological safety risk and bioterrorism threat. Of
the $23.0 million in funding, the government initially
committed to pay us up to $14.2 million over the first two
years of the contract. In June 2008, as a result of the progress
of the program, the government awarded us an additional
$7.5 million, to be paid through September 2009 for the
third year of the contract, together with any remaining funds
carried over from the funding allocated for the first two years
of the contract.
Department of Defense. In August 2007, DTRA
awarded us a contract to advance the development of a broad
spectrum RNAi anti-viral therapeutic for hemorrhagic fever
virus. The government initially committed to pay us up to
$10.9 million through February 2009, which included a
six-month extension granted by DTRA in July 2008. Following a
program review in early 2009, we and DTRA have determined to end
this program and accordingly, the remaining funds will not be
accessed.
Patents
and Proprietary Rights
We have devoted considerable effort and resources to establish
what we believe to be a strong intellectual property position
relevant to RNAi therapeutic products and delivery technologies.
In this regard, we have amassed a portfolio of patents, patent
applications and other intellectual property covering:
|
|
|
| |
•
|
fundamental aspects of the structure and uses of siRNAs,
including their use as therapeutics, and RNAi-related mechanisms;
|
| |
| |
•
|
chemical modifications to siRNAs that improve their suitability
for therapeutic uses;
|
| |
| |
•
|
siRNAs directed to specific targets as treatments for particular
diseases;
|
| |
| |
•
|
delivery technologies, such as in the field of cationic
liposomes; and
|
| |
| |
•
|
all aspects of our specific development candidates.
|
21
We believe that no other company possesses a portfolio of such
broad and exclusive rights to the patents and patent
applications required for the commercialization of RNAi
therapeutics. Our intellectual property estate for RNAi
therapeutics includes over 1,800 active cases and over 700
granted or issued patents, of which over 300 are issued or
granted in the United States, the European Union, or EU, and
Japan. Given the importance of our intellectual property
portfolio to our business operations, we intend to vigorously
enforce our rights and defend against any challenges that have
arisen or may arise in this area.
Intellectual
Property Related to Fundamental Aspects and Uses of siRNA and
RNAi-related Mechanisms
In this category, we include United States and foreign patents
and patent applications that claim key aspects of siRNA
architecture and RNAi-related mechanisms. Specifically included
are patents and patent applications covering targeted cleavage
of mRNA directed by RNA-like oligonucleotides, double-stranded
RNAs of particular lengths and particular structural features,
such as blunt
and/or
overhanging ends. Our strategy has been to secure exclusive
rights where possible and appropriate to key patents and patent
applications that we believe cover fundamental aspects of RNAi.
The following table lists patents
and/or
patent applications to which we have secured rights that we
regard as being fundamental for the use of siRNAs as
therapeutics.
| |
|
|
|
|
|
|
|
|
|
|
|
|
Patent
|
|
|
|
First
|
|
|
|
|
|
|
|
|
|
Licensor/Owner
|
|
Subject Matter
|
|
Priority Date
|
|
Inventors
|
|
Status
|
|
Expiration Date*
|
|
Alnylam Rights
|
|
|
|
Isis
|
|
Inactivation of
target mRNA
|
|
6/6/1996 and 6/6/1997
|
|
S. Crooke
|
|
U.S. 5,898,031, U.S. 6,107,094 & U.S. 7,432,250
EP 0928290
Additional applications pending in the U.S. and several foreign jurisdictions
|
|
06/06/2016
06/06/2017
|
|
Exclusive rights for therapeutic
purposes related
to siRNAs**
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carnegie
Institution of
Washington
|
|
Double-stranded
RNAs to induce
RNAi
|
|
12/23/1997
|
|
A. Fire,
C. Mello
|
|
U.S. 6,506,559
Additional applications pending in the U.S. and several foreign jurisdictions
|
|
12/18/2018
|
|
Non-exclusive rights for therapeutic purposes
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Medical
College of
Georgia
Research
Institute, Inc.
|
|
Methods for
inhibiting gene
expression using double-stranded
RNA
|
|
1/28/1999
|
|
Y. Li,
M. Farrell,
M. Kirby
|
|
AU 776150 (Australia)
Additional applications pending in the U.S., Europe and Canada
|
|
1/28/2020
|
|
Exclusive rights
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Alnylam
|
|
Small double-
stranded RNAs
as therapeutic
products
|
|
1/30/1999
|
|
R. Kreutzer,
S. Limmer
|
|
EP 1214945 (revoked/to be appealed) & EP 1550719, CA 2359180 (Canada), AU 778474 (Australia), ZA 2001/5909 (South Africa), DE 20023125 U1, DE 10066235 & DE 10080167 (Germany)
Additional applications pending in the U.S. and several foreign jurisdictions
|
|
01/29/2020
|
|
Owned
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Alnylam
|
|
Composition and methods for
inhibiting a target nucleic acid
with double-
stranded RNA
|
|
4/21/1999
|
|
C. Pachuk,
C. Satishchandran
|
|
AU 781598 (Australia)
Additional applications pending in the U.S. and several foreign jurisdictions
|
|
4/19/2020
|
|
Owned
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cancer
Research
Technology
Limited
|
|
RNAi uses in
mammalian
oocytes,
preimplantation
embryos and
somatic cells
|
|
11/19/1999
|
|
M. Zernicka-Goetz,
M.J. Evans,
D.M. Glover
|
|
EP 1230375 (revoked/to be appealed), SG 89569 (Singapore), AU 774285 (Australia)
Additional applications pending in the U.S. and several foreign jurisdictions
|
|
11/17/2020
|
|
Exclusive rights for therapeutic purposes
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Massachusetts
Institute of
Technology,
Whitehead
Institute, Max
Planck
Gesellschaft***
|
|
Mediation of RNAi by
small RNAs
21-23 base
pairs long
|
|
3/30/2000
|
|
D.P. Bartel,
P.A. Sharp,
T. Tuschl,
P.D. Zamore
|
|
AU 2001249622 (Australia)
Additional applications pending in the U.S. and several foreign jurisdictions
|
|
03/30/2020
|
|
Non-exclusive rights for therapeutic purposes***
|
22
| |
|
|
|
|
|
|
|
|
|
|
|
|
Patent
|
|
|
|
First
|
|
|
|
|
|
|
|
|
|
Licensor/Owner
|
|
Subject Matter
|
|
Priority Date
|
|
Inventors
|
|
Status
|
|
Expiration Date*
|
|
Alnylam Rights
|
|
|
Max Planck
Gesellschaft
|
|
Synthetic and
chemically
modified
siRNAs as
therapeutic
products
|
|
12/01/2000, 04/24/2004 and 04/27/2004
|
|
T. Tuschl,
S. Elbashir,
W. Lendeckel
|
|
U.S. 7,056,704 & U.S. 7,078,196, EP 1407044, AU 2002235744 (Australia), ZA 2003/3929 (South Africa), SG 96891 (Singapore), NZ 52588 (New Zealand), JP 4 095 895 (Japan), RU 2322500 (Russia)
Additional applications pending in the U.S. and several foreign jurisdictions
|
|
11/29/2021
|
|
Exclusive
rights for therapeutic purposes
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Alnylam
|
|
Methods for
inhibiting a target nucleic acid via the introduction
of a vector encoding a
double-stranded
RNA
|
|
1/31/2001
|
|
T. Giordano,
C. Pachuk,
C. Satishchandran
|
|
AU 785395 (Australia)
Additional applications pending in the U.S., Australia and Canada
|
|
1/31/2021
|
|
Owned
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cold Spring
Harbor
Laboratory
|
|
RNAi uses in
mammalian cells
|
|
3/16/2001
|
|
D. Beach,
G. Hannon
|
|
Pending in the U.S. and several foreign jurisdictions
|
|
|
|
Non-exclusive rights for therapeutic purposes
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stanford
University
|
|
RNAi uses in vivo in mammalian liver
|
|
7/23/2001
|
|
M.A. Kay,
A.P. McCaffrey
|
|
AU 2002326410 (Australia)
Additional applications pending in the U.S. and several foreign jurisdictions
|
|
7/23/2021
|
|
Exclusive rights
for therapeutic purposes
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* |
|
For applications filed after June 7, 1995, the patent term
generally is 20 years from the earliest application filing
date. However, under the Drug Price Competition and Patent Term
Extension Act of 1984, known as the Hatch-Waxman Act, we may be
able to apply for patent term extensions for our U.S. patents.
We cannot predict whether or not any patent term extensions will
be granted or the length of any patent term extension that might
be granted. |
| |
|
** |
|
We hold co-exclusive therapeutic rights with Isis. However, Isis
has agreed not to license such rights to any third party, except
in the context of a collaboration in which Isis plays an active
role. |
| |
|
*** |
|
We hold exclusive rights to the interest owned by three
co-owners. A separate entity, the University of Massachusetts,
has licensed its purported interest separately to third parties. |
We believe that we have a strong portfolio of broad rights to
fundamental RNAi patents and patent applications. Many of these
rights are exclusive, which we believe prevents potential
competitors from entering the field of RNAi without taking a
license from us. In securing these rights, we have focused on
obtaining the strongest rights for those intellectual property
assets we believe will be most important in providing
competitive advantage with respect to RNAi therapeutic products.
We believe that the Crooke patent series, issued in several
countries around the world, covers the use of all modified
oligonucleotides to achieve enzyme-mediated cleavage of a target
mRNA and, as such, has broad issued claims that cover RNAi. We
have obtained rights to the Crooke patents through a license
agreement with Isis. Under the terms of our license agreement,
Isis agreed not to grant licenses under these patents to any
other organization for oligonucleotide products designed to work
through an RNAi mechanism, except in the context of a
collaboration in which Isis plays an active role.
Through our acquisition of Ribopharma AG, now known as Alnylam
Europe, we own the entire
Kreutzer-Limmer
patent portfolio, which includes pending applications in the
United States and many countries worldwide. The first patent to
issue in the Kreutzer-Limmer series (EP 1144623) was
granted in Europe in 2002, and specifically covered the use of
small double-stranded RNAs, or dsRNAs, as therapeutics. This
patent was recently revoked in appeal. The second European
Kreutzer-Limmer patent (EP 1214945) to issue in the series
was granted in Europe in 2005. This patent covers dsRNA
structures of 15 to 49 successive nucleotide pairs in length. In
January 2009, the EPO ruled in favor of the opposing parties in
an opposition proceeding related to the second Kreutzer-Limmer
patent. We intend to appeal this ruling. In December 2008, the
EPO granted a third patent in the Kreutzer-Limmer series (EP
1550719). This patent covers therapeutic dsRNAs which are 15 to
21
23
consecutive nucleotide pairs in length. We have also received
grants for patents in the Kreutzer-Limmer series in several
other countries, as reflected in the table above. The outcome of
the EP 1144623 appeal and the EP 1214945 opposition will only
affect the granted or pending claims of other members of the
Kreutzer-Limmer patent series to the extent the same issue
arises in the formal examination or post-grant review
proceedings of the other members of the series. We do not expect
this to occur, but in the event it does, the ruling in the
former proceeding would be controlling.
The Glover patent series has resulted in several patent grants,
including in Europe (EP 1230375). The European Glover patent was
revoked in June 2008 during opposition proceedings and our
appeal of this decision is pending. Broad claims from this
patent cover dsRNAs of any length or structure as mediators of
RNAi in mammalian systems. We have an exclusive license to the
Glover patent for therapeutic uses from Cancer Research
Technology Limited.
The Tuschl patent applications filed by Max Planck Gesellschaft
zur Förderung der Wissenschaften e.V, on the invention by
Dr. Tuschl and his colleagues, which we call the
Tuschl II patent series, cover what we believe are key
structural features of siRNAs. Specifically, the Tuschl II
patents and patent applications include claims directed to
synthetic siRNAs and the use of chemical modifications to
stabilize siRNAs. In June 2006, the United States Patent and
Trademark Office, or USPTO, issued U.S. Patent
No. 7,056,704 and in July 2006 the USPTO issued
U.S. Patent No. 7,078,196, each covering methods of
making dsRNAs having a 3’ overhang structure. In September
2007, the EPO granted broad claims for the Tuschl II patent
in Europe (EP 1407044). Five parties have filed Notices of
Opposition in the EPO against EP 1407044. The Japanese Patent
Office has granted the Tuschl II patent in Japan (JP 4 095
895). We have also received grants for patents in the
Tuschl II series in several other countries, as reflected
in the table above. We have obtained an exclusive license to
claims in the Tuschl II patent series uniquely covering the
use of RNAi for therapeutic purposes.
The Fire and Mello patent owned by the Carnegie Institution
covers the use of dsRNAs to induce RNAi. The Carnegie
Institution has made this patent broadly available for licensing
and we, like many companies, have taken a non-exclusive license
to the patent for therapeutic purposes. We believe, however,
that the claims of the Fire and Mello patent do not cover the
structural features of dsRNAs that are important for the
biological activity of siRNAs in mammalian cells. We believe
that these specific features are the subjects of the Crooke,
Kreutzer-Limmer, Glover and Tuschl II patents and patent
applications for which we have secured exclusive rights.
The other pending patent applications listed in the table above
either provide further coverage for structural features of
siRNAs or relate to the use of siRNAs in mammalian cells. For
some of these, we have exclusive rights, and for others, we have
non-exclusive rights. In addition, in December 2008, we acquired
the intellectual property assets of Nucleonics, Inc., a
privately held biotechnology company. This acquisition included
over 100 active patent filings, including 15 patents that have
been granted worldwide, of which five have been granted in the
United States and Europe. With this acquisition, we obtained
patents and patent applications with early priority dates,
notably the “Li & Kirby,” “Pachuk
I” and “Giordano” patent families, that cover
broad structural features of RNAi therapeutics, thus extending
the breadth of our fundamental intellectual property.
Intellectual
Property Related to Chemical Modifications
Our collaboration and license agreement with Isis provides us
with rights to practice the inventions covered by over 200
issued patents worldwide, as well as rights based on future
chemistry patent applications through March 2009. These
patents will expire both in and outside the United States
generally between 2009 and 2029, subject to any potential patent
term extensions
and/or
supplemental protection certificates extending such term
extensions in countries where such extensions may become
available. These inventions cover chemical modifications we may
wish to incorporate into our RNAi therapeutic products. Under
the terms of our license agreement, Isis agreed not to grant
licenses under these patents to any other organization for dsRNA
products designed to work through an RNAi mechanism, except in
the context of a collaboration in which Isis plays an active
role.
In addition to licensing these intellectual property rights from
Isis, we are also working to develop our own proprietary
chemical modifications that may be incorporated into siRNAs to
endow them with drug-like properties. We have filed a large
number of patent applications relating to these novel and
proprietary chemical modifications.
24
With the combination of the technology we have licensed from
Isis, U.S. Patent No. 7,078,196, a patent in the
Tuschl II patent series, and our own patent application
filings, we possess issued claims that cover methods of making
siRNAs that incorporate any of various chemical modifications,
including the use of phosphorothioates,
2’-O-methyl,
and/or
2’-fluoro modifications. These modifications are believed
to be important for achieving “drug-like” properties
for RNAi therapeutics. We hold exclusive worldwide rights to
these claims for RNAi therapeutics.
Intellectual
Property Related to siRNAs Directed to Specific
Targets
We have filed a number of patent applications claiming specific
siRNAs directed to various gene targets that correlate to
specific diseases. While there may be a significant number of
competing applications filed by other organizations claiming
siRNAs to treat the same gene target, we were among the first
companies to focus and file on RNAi therapeutics, and thus, we
believe that a number of our patent applications may predate
competing applications that others may have filed. Reflecting
this, in August 2005, the EPO granted a broad patent, which we
call the Kreutzer-Limmer II patent, with 103 allowed claims
on therapeutic compositions, methods and uses comprising siRNAs
that are complementary to mRNA sequences in over 125 disease
target genes. The Kreutzer-Limmer II patent will expire on
January 9, 2022, subject to any potential patent term
extensions
and/or
supplemental protection certificates extending such term
extensions in countries where such extensions may become
available. Some of these claimed gene targets are being pursued
by our development and pre-clinical programs, such as those
expressed by viral pathogens including RSV and influenza virus.
In addition, the claimed targets include oncogenes, cytokines,
cell adhesion receptors, angiogenesis targets, apoptosis and
cell cycle targets, and additional viral disease targets, such
as hepatitis C virus and HIV. The Kreutzer-Limmer II
patent series is pending in the United States and many foreign
countries. Moreover, a patent in the Tuschl II patent
series, U.S. Patent No. 7,078,196, claims methods of
preparing siRNAs that mediate cleavage of an mRNA in mammalian
cells and, therefore, covers methods of making siRNAs directed
toward any and all target genes. We hold exclusive worldwide
rights to these claims for RNAi therapeutics.
With respect to specific siRNAs, we believe that patent coverage
will result from demonstrating that particular compositions
exert suitable biological and therapeutic effects. Accordingly,
we are focused on achieving such demonstrations for siRNAs in
key therapeutic programs.
Intellectual
Property Related to the Delivery of siRNAs to
Cells
We are pursuing internal research and collaborative approaches
regarding the delivery of siRNAs to mammalian cells. These
approaches include exploring technology that may allow delivery
of siRNAs to cells through the use of cationic lipids,
cholesterol and carbohydrate conjugation, peptide and
antibody-based targeting, and polymer conjugations. Our
collaborative efforts include working with academic and
corporate third parties to examine specific embodiments of these
various approaches to delivery of siRNAs to appropriate cell
tissue, and in-licensing of the most promising technology. For
example, we have obtained an exclusive license from the
University of British Columbia and Tekmira in the field of RNAi
therapeutics to intellectual property covering cationic
liposomes and their use to deliver nucleic acid to cells. The
issued United States patents and foreign counterparts, including
the Semple (U.S. Patent No 6,858,225) and Wheeler
(U.S. Patent Nos. 5,976,567 and 6,815,432) patents, cover
compositions, methods of making and methods of using cationic
liposomes to deliver agents, such as nucleic acid molecules, to
cells. These patents will expire both in and outside the United
States on October 30, 2017, January 6, 2015 and
June 7, 2015, respectively, subject to any potential patent
term extensions
and/or
supplemental protection certificates extending such term
extensions in countries where such extensions may become
available.
Intellectual
Property Related to Our Development Candidates
As our development pipeline matures, we have made and will
continue to make patent filings that claim all aspects of our
development candidates, including dose, method of administration
and manufacture.
As the field of RNAi therapeutics is maturing, patent
applications are being fully processed by national patent
offices around the world. There is uncertainty about which
patents will issue, and, if they do, as to when, to whom, and
with what claims. It is likely that there will be significant
litigation and other proceedings, such as interference,
25
reexamination and opposition proceedings, in various patent
offices relating to patent rights in the RNAi field. For
example, as noted above, various third parties have initiated
oppositions to patents in our Kreutzer-Limmer and Tuschl II
series in the EPO, as well as in other jurisdictions. We expect
that additional oppositions will be filed in the EPO and
elsewhere, and other challenges will be raised relating to other
patents and patent applications in our portfolio. In many cases,
the possibility of appeal exists for either us or our opponents,
and it may be years before final, unappealable rulings are made
with respect to these patents in certain jurisdictions. Given
the importance of our intellectual property portfolio to our
business operations, we intend to vigorously enforce our rights
and defend against any challenges that have arisen or may arise
in this area.
Competition
The pharmaceutical marketplace is extremely competitive, with
hundreds of companies competing to discover, develop and market
new drugs. We face a broad spectrum of current and potential
competitors, ranging from very large, global pharmaceutical
companies with significant resources, to other biotechnology
companies with resources and expertise comparable to our own and
to smaller biotechnology companies with fewer resources and
expertise than we have. We believe that for most or all of our
drug development programs, there will be one or more competing
programs under development at other companies. In many cases,
the companies with competing programs will have access to
greater resources and expertise than we do and may be more
advanced in those programs.
The competition we face can be grouped into three broad
categories:
|
|
|
| |
•
|
other companies working to develop RNAi therapeutic products;
|
| |
| |
•
|
companies developing technology known as antisense, which, like
RNAi, attempts to silence the activity of specific genes by
targeting the mRNAs copied from them; and
|
| |
| |
•
|
marketed products and development programs for therapeutics that
treat the same diseases for which we may also be developing
treatments.
|
We are aware of several other companies that are working to
develop RNAi therapeutic products. Some of these companies are
seeking, as we are, to develop chemically synthesized siRNAs as
drugs. Others are following a gene therapy approach, with the
goal of treating patients not with synthetic siRNAs but with
synthetic, exogenously-introduced genes designed to produce
siRNA-like molecules within cells.
Companies working on chemically synthesized siRNAs include
Merck, Roche, Takeda, Opko Corporation, MDRNA, Inc., Calando,
Quark, Silence Therapeutics plc, RXi Pharmaceuticals
Corporation, Tekmira, Intradigm, Inc. and Dicerna
Pharmaceuticals, Inc. Many of these companies have licensed our
intellectual property.
Companies working on gene therapy approaches to RNAi
therapeutics include Benitec, Cequent, Inc. and Targeted
Genetics Corporation.
Companies working on microRNA-based therapeutics include Rosetta
Genomics, Santaris Pharma A/S, Miragen Therapeutics Inc. and
Asuragen, Inc.
Antisense technology uses short, single-stranded, DNA-like
molecules to block mRNAs encoding specific proteins. An
antisense oligonucleotide, or ASO, contains a sequence of bases
complementary to a sequence within its target mRNA, enabling it
to attach to the mRNA by base-pairing. The attachment of the ASO
may lead to breakdown of the mRNA, or may physically block the
mRNA from associating with the protein synthesis machinery of
the cell. In either case, production of the protein encoded by
the mRNA may be reduced. Typically, the backbone of an ASO, the
linkages that hold its constituent bases together, will carry a
number of chemical modifications that do not exist in naturally
occurring DNA. These modifications are intended to improve the
stability and pharmaceutical properties of the ASO.
While we believe that RNAi drugs may potentially have
significant advantages over ASOs, including greater potency and
specificity, others are developing ASO drugs that are currently
at a more advanced stage of development than RNAi drugs. For
example, Isis has developed an ASO drug,
Vitravene®,
which is currently on the market, and has several ASO drug
candidates in clinical trials. In addition, a number of other
companies have
26
product candidates in various stages of pre-clinical and
clinical development. Included in these companies are Genta
Incorporated and AVI BioPharma, Inc. Because of their later
stage of development, ASOs, rather than siRNAs, may become the
preferred technology for drugs that target mRNAs in order to
turn off the activity of specific genes.
The competitive landscape continues to expand and we expect that
additional companies will initiate programs focused on the
development of RNAi therapeutic products using the approaches
described above as well as potentially new approaches that may
result in the more rapid development of RNAi therapeutics or
more effective technologies for RNAi drug development or
delivery.
Competing
Drugs for RSV
The only product currently approved for the treatment of RSV
infection is Ribavirin, which is marketed as Virazole by
Valeant. This is approved only for treatment of hospitalized
infants and young children with severe lower respiratory tract
infections due to RSV. However, Ribavirin has been reported to
have limited efficacy and limited anti-viral activity against
RSV. Moreover, administration of the drug is complicated and
requires elaborate environmental reclamation devices because of
potential harmful effects on health care personnel exposed to
the drug. According to published reports by Valeant, sales of
Virazole were $14.3 million in 2007. Other current RSV
therapies consist of primarily treating the symptoms or
preventing the viral infection by using the prophylactic drug
Synagis (palivizumab), which is marketed by MedImmune, Inc.
Synagis is a neutralizing monoclonal antibody that prevents the
virus from infecting the cell by blocking the RSV F protein.
Synagis is injected intramuscularly once a month during the RSV
season to prevent infection. According to published reports by
MedImmune and AstraZeneca PLC, which acquired MedImmune during
2007, worldwide Synagis sales were greater than
$1.2 billion in 2008. MedImmune is also developing
motavizumab (formerly known as
Numax®),
a humanized monoclonal antibody, which is being evaluated for
its potential to prevent serious lower respiratory tract disease
caused by RSV in pediatric patients at high risk of contracting
RSV disease. MedImmune submitted a biologic license application
for motavizumab to the FDA in early 2008. MedImmune has also
recently initiated a Phase I/IIa clinical trial of a live,
attenuated intranasal vaccine in development to help prevent
severe RSV infections. In addition, Novartis has a small
molecule drug, RSV604, licensed from Arrow Therapeutics Ltd,
which is in Phase II clinical trials. RSV604 is an oral
drug that targets the viral N protein.
Competing
Drugs for Liver Cancer
There are a variety of surgical procedures, chemotherapeutics,
radiation and other approaches that are used in the management
of both primary and secondary liver cancer. However, for the
majority of patients the prognosis remains poor with fatal
outcomes within several months of diagnosis. In November 2007,
the FDA approved Sorafenib, also called
Nexavar®,
for the treatment of un-resectable liver cancer. Nexavar is the
product of Onyx Pharmaceuticals, Inc., developed in
collaboration with Bayer Pharmaceuticals Corporation. Worldwide
sales of Nexavar were $677.8 million in 2008.
There are also a large number of drugs in various stages of
clinical development as cancer therapeutics, although the
efficacy and safety of these newer drugs are difficult to
ascertain at this point of development.
Competing
Drugs for Hypercholesterolemia
The current standard of care for patients with
hypercholesterolemia includes the use of several agents. Front
line therapy consists of HMG CoA reductase inhibitors, commonly
known as statins, which block production of cholesterol by the
liver and increase clearance of LDL-c from the bloodstream.
These include Lipitor, Zocor, Crestor and Pravachol. A different
class of compounds, which includes Zetia and Vytorin, function
by blocking cholesterol uptake from the diet and are utilized on
their own or in combination with statins. Each of these
competing drugs had sales of greater than $1.0 billion
during 2007, according to published reports. With regard to
future therapies, mipomersen, formerly ISIS 301012, is a
lipid-lowering drug targeting apolipoprotein B-100 being
developed by Isis in collaboration with Genzyme Corporation.
Currently in Phase III development, mipomersen has been
shown in Phase II trials to reduce cholesterol and other
atherogenic lipids more than 40% beyond reductions achieved with
current standard lipid-lowering drugs. A weekly injectable
therapeutic, mipomersen is being
27
developed primarily for patients at significant cardiovascular
risk who are unable to achieve target cholesterol levels with
statins alone or who are intolerant of statins.
Competing
Drugs for TTR Amyloidosis
Currently, liver transplantation is the only available treatment
option for TTR-mediated FAP. However, only a subset of FAP
patients qualify for this costly and invasive procedure and,
even following liver transplantation, the disease continues to
progress for many patients, presumably due to normal TTR being
deposited into preexisting fibrils. Moreover, there is a
shortage of donors to provide healthy livers for transplantation
into eligible patients. There are no existing disease-modifying
treatments to address TTR amyloidosis.
There are a few drugs in clinical development for the treatment
of TTR amyloidosis. FoldRx Pharmaceuticals, Inc. is currently
conducting a Phase II/III trial of Fx-1006A for FAP and a
Phase II trial of Fx-1006A for FAC.
Fx-1006A is
a small-molecule compound that is intended to stabilize
wild-type and variant TTR, prevent misfolding and inhibit the
formation of TTR amyloid fibrils. In a Phase I study in healthy
volunteers,
Fx-1006A was
found to be safe and well-tolerated, and demonstrated TTR
stabilization in the plasma of participants. Researchers at
Boston University, in collaboration with the National Institute
of Neurological Disorders and Stroke, are currently conducting a
Phase II/III study of diflunisal for the treatment of
TTR-mediated FAP. Diflunisal is a commercially available
non-steroidal anti-inflammatory agent that has been found to
stabilize TTR in vitro.
Competing
Drugs for Huntington’s Disease
While certain drugs are currently used to treat some of the
symptoms of HD, no drug has been approved in the United States
for the treatment of the underlying disease. Current
pharmacological therapy for HD is limited to the management or
alleviation of neurobehavioral or movement abnormalities
associated with the disease. No disease modifying, disease
slowing or neuroprotective agent is currently approved or used
to treat HD, although there are several drugs in development.
Avicena Group Inc.’s HD-02, an ultra-pure creatine, is a
candidate for prophylactic use for Huntington’s disease
which has shown potential neuroprotective properties in HD
patients in Phase II trials. Medivation Inc.’s
Dimebontm
is an orally- available small molecule that is believed to block
the mitochondrial permeability transition pore, or MPTP, the
glutamate N-methyl
D-asparate,
or NMDA, receptor and cholinesterase activity. The drug is
currently being investigated as a treatment for HD and results
from a Phase II trial completed in early 2008 showed
significantly improved cognitive function in patients with
mild-to-moderate HD over placebo.
Other
Competition
Finally, for many of the diseases that are the subject of our
RNAi therapeutics discovery programs, there are already drugs on
the market or in development. However, notwithstanding the
availability of these drugs or drug candidates, we believe there
currently exists sufficient unmet medical need to warrant the
advancement of RNAi therapeutic programs.
Regulatory
Matters
The research, testing, manufacture and marketing of drug
products and their delivery systems are extensively regulated in
the United States and the rest of the world. In the United
States, drugs are subject to rigorous regulation by the FDA. The
Federal Food, Drug, and Cosmetic Act and other federal and state
statutes and regulations govern, among other things, the
research, development, testing, manufacture, storage, record
keeping, packaging, labeling, promotion and advertising,
marketing and distribution of pharmaceutical products. Failure
to comply with the applicable regulatory requirements may
subject a company to a variety of administrative or
judicially-imposed sanctions and the inability to obtain or
maintain required approvals to test or market drug products.
These sanctions could include warning letters, product recalls,
product seizures, total or partial suspension of production or
distribution, clinical holds, injunctions, fines, civil
penalties or criminal prosecution.
28
The steps ordinarily required before a new pharmaceutical
product may be marketed in the United States include
non-clinical laboratory tests, animal tests and formulation
studies, the submission to the FDA of an IND, which must become
effective prior to commencement of clinical testing, adequate
and well-controlled clinical trials to establish that the drug
product is safe and effective for the indication for which FDA
approval is sought, submission to the FDA of a new drug
application, or NDA, satisfactory completion of an FDA
inspection of the manufacturing facility or facilities at which
the product is produced to assess compliance with current good
manufacturing practice, or cGMP, requirements and FDA review and
approval of the NDA. Satisfaction of FDA pre-market approval
requirements typically takes several years, but may vary
substantially depending upon the complexity of the product and
the nature of the disease. Government regulation may delay or
prevent marketing of potential products for a considerable
period of time and impose costly procedures on a company’s
activities. Success in early stage clinical trials does not
necessarily assure success in later stage clinical trials. Data
obtained from clinical activities, including the data derived
from our clinical trials such as the GEMINI study, is not always
conclusive and may be subject to alternative interpretations
that could delay, limit or even prevent regulatory approval.
Even if a product receives regulatory approval, later discovery
of previously unknown problems with a product, including new
safety risks, may result in restrictions on the product or even
complete withdrawal of the product from the market.
Non-clinical tests include laboratory evaluation of product
chemistry and formulation, as well as animal testing to assess
the potential safety and efficacy of the product. The conduct of
the non-clinical tests and formulation of compounds for testing
must comply with federal regulations and requirements. The
results of non-clinical testing are submitted to the FDA as part
of an IND, together with manufacturing information, analytical
and stability data, a proposed clinical trial protocol and other
information.
A 30-day
waiting period after the filing of an IND application is
required prior to such application becoming effective and the
commencement of clinical testing in humans. If the FDA has not
commented on, or questioned, the application during this
30-day
waiting period, clinical trials may begin. If the FDA has
comments or questions, these must be resolved to the
satisfaction of the FDA prior to commencement of clinical
trials. The IND approval process can result in substantial delay
and expense. We, an institutional review board, or IRB, or the
FDA may, at any time, suspend, terminate or impose a clinical
hold on ongoing clinical trials. If the FDA imposes a clinical
hold, clinical trials cannot commence or recommence without FDA
authorization and then only under terms authorized by the FDA.
Clinical trials involve the administration of the
investigational new drug to healthy volunteers or patients under
the supervision of a qualified investigator. Clinical trials
must be conducted in compliance with federal regulations and
requirements, under protocols detailing, among other things, the
objectives of the trial and the safety and effectiveness
criteria to be evaluated. Each protocol involving testing on
human subjects in the United States, or in foreign countries if
such tests are intended to support approval in the United
States, must be submitted to the FDA as part of the IND
application. The study protocol and informed consent information
for patients in clinical trials must be submitted to IRBs for
approval prior to initiation of the trial.
Clinical trials to support NDAs for marketing approval are
typically conducted in three sequential phases, which may
overlap or be combined. In Phase I, the initial
introduction of the drug into healthy human subjects or
patients, the drug is tested to primarily assess safety,
tolerability, pharmacokinetics, pharmacological actions and
metabolism associated with increasing doses. Phase II
usually involves trials in a limited patient population, to
assess the optimum dosage, identify possible adverse effects and
safety risks and provide preliminary support for the efficacy of
the drug in the indication being studied.
If a compound demonstrates evidence of effectiveness and an
acceptable safety profile in Phase II trials,
Phase III trials are undertaken to further evaluate
clinical efficacy and to further test for safety in an expanded
patient population, typically at geographically dispersed
clinical trial sites. Phase I, Phase II or
Phase III testing of any product candidates may not be
completed successfully within any specified time period, if at
all. After successful completion of the required clinical
testing, generally an NDA is prepared and submitted to the FDA.
We believe that any RNAi product candidate we develop, whether
for RSV, liver cancer, hypercholesterolemia, TTR amyloidosis, HD
or the various indications targeted in our preclinical discovery
programs, will be regulated as a new drug by the FDA. FDA
approval of an NDA is required before marketing of the product
may begin in the
29
United States. The NDA must include the results of extensive
clinical and other testing, as described above, and a
compilation of data relating to the product’s pharmacology,
chemistry, manufacture and controls. In addition, an NDA for a
new active ingredient, new indication, new dosage form, new
dosing regimen, or new route of administration must contain data
assessing the safety and efficacy for the claimed indication in
all relevant pediatric subpopulations, and support dosing and
administration for each pediatric subpopulation for which the
drug is shown to be safe and effective. In some circumstances,
the FDA may grant deferrals for the submission of some or all
pediatric data, or full or partial waivers. The cost of
preparing and submitting an NDA is substantial. Under federal
law, NDAs are subject to substantial application user fees and
the sponsor of an approved NDA is also subject to annual product
and establishment user fees.
The FDA has 60 days from its receipt of an NDA to determine
whether the application will be accepted for filing based on the
agency’s threshold determination that the NDA is
sufficiently complete to permit substantive review. Once the
submission is accepted for filing, the FDA begins an in-depth
review of the NDA. The review process is often significantly
extended by FDA requests for additional information or
clarification regarding information already provided in the
submission. The FDA may also refer applications for novel drug
products or drug products that present difficult questions of
safety or efficacy to an advisory committee, typically a panel
that includes clinicians and other experts, for review,
evaluation and a recommendation as to whether the application
should be approved. The FDA is not bound by the recommendation
of an advisory committee. The FDA normally also will conduct a
pre-approval inspection to ensure the manufacturing facility,
methods and controls are adequate to preserve the drug’s
identity, strength, quality, purity and stability, and are in
compliance with regulations governing cGMPs.
If the FDA evaluation of the NDA and the inspection of
manufacturing facilities are favorable, the FDA may issue an
approval letter, which authorizes commercial marketing of the
drug with specific prescribing information for a specific
indication. As a condition of NDA approval, the FDA may require
post approval testing, including Phase IV trials, and
surveillance to monitor the drug’s safety or efficacy and
may impose other conditions, including labeling restrictions,
which can materially impact the potential market and
profitability of the drug. Once granted, product approvals may
be withdrawn if compliance with regulatory standards is not
maintained or problems are identified following initial
marketing.
Under FDA regulations in effect prior to August 11, 2008,
if the NDA did not warrant approval the FDA would instead issue
either an approvable letter or a not-approvable letter.
Approvable letters generally contained a statement of specific
conditions that must be met in order to secure final approval of
the NDA. If and when those conditions were met to the FDA’s
satisfaction, the FDA would typically issue an approval letter.
If the FDA’s evaluation of the NDA or inspection of the
manufacturing facilities was not favorable, the FDA may refuse
to approve the NDA or, prior to August 11, 2008, could
issue a not-approvable letter. Not-approvable letters outlined
the deficiencies in the submission and often required additional
testing or information in order for the FDA to reconsider the
application. Even with submission of this additional
information, the FDA ultimately could decide that the
application does not satisfy the regulatory criteria for
approval.
Under new regulations, effective August 11, 2008, the FDA
has discontinued use of approvable and not-approvable letters
when taking action on marketing applications. Instead, the FDA
now issues a “complete response” letter to notify a
sponsor that the review period for an application is complete
and that the application is not ready for approval. A complete
response letter describes the deficiencies that the FDA has
identified in an application and, when possible, recommends
actions that the applicant might take to place the application
in condition for approval. Such actions may include, among other
things, conducting additional safety or efficacy studies after
which the sponsor may resubmit the application for further
review. The new regulations also clarify the anticipated
additional review time for the FDA to respond to resubmissions,
which for significant resubmissions may be six months or longer.
While we believe that any RNAi therapeutic we develop will be
regulated as a new drug under the Federal Food, Drug, and
Cosmetic Act, the FDA could decide to regulate certain RNAi
therapeutic products as biologics under the Public Health
Service Act. Biologics must have a biologics license
application, or BLA, approved prior to commercialization. Like
NDAs, BLAs are subject to user fees. To obtain BLA approval, an
applicant must provide non-clinical and clinical evidence and
other information to demonstrate that the biologic product is
safe, pure and
30
potent, and like NDAs, must complete clinical trials that are
typically conducted in three sequential phases
(Phase I, II and III). Additionally, the applicant
must demonstrate that the facilities in which the product is
manufactured, processed, packaged or held meet standards,
including cGMPs and any additional standards in the license
designed to ensure its continued safety, purity and potency.
Biologics establishments are subject to pre-approval
inspections. The review process for BLAs is also time consuming
and uncertain, and BLA approval may be conditioned on
post-approval testing and surveillance. Once granted, BLA
approvals may be suspended or revoked under certain
circumstances, such as if the product fails to conform to the
standards established in the license.
Once an NDA or BLA is approved, a product will be subject to
certain post-approval requirements, including requirements for
adverse event reporting, submission of periodic reports,
recordkeeping, product sampling and distribution. Additionally,
the FDA also strictly regulates the promotional claims that may
be made about prescription drug products and biologics. In
particular, a drug or biologic may not be promoted for uses that
are not approved by the FDA as reflected in the product’s
approved labeling. In addition, the FDA requires substantiation
of any claims of superiority of one product over another,
including that such claims be proven by adequate and
well-controlled head-to-head clinical trials. To the extent that
market acceptance of our products may depend on their
superiority over existing therapies, any restriction on our
ability to advertise or otherwise promote claims of superiority,
or requirements to conduct additional expensive clinical trials
to provide proof of such claims, could negatively affect the
sales of our products or our costs. We must also notify the FDA
of any change in an approved product beyond variations already
allowed in the approval. Certain changes to the product, its
labeling or its manufacturing require prior FDA approval and may
require the conduct of further clinical investigations to
support the change. Such approvals may be expensive and
time-consuming and, if not approved, the FDA will not allow the
product to be marketed as modified.
Some of our drug candidates may need to be administered using
specialized drug delivery systems. We may rely on drug delivery
systems that are already approved to deliver drugs like ours to
similar physiological sites or, in some instances, we may need
to modify the design or labeling of the legally available device
for delivery of our product candidate. In such an event, the FDA
may regulate the product as a combination product or require
additional approvals or clearances for the modified device. In
addition, to the extent the delivery device is owned by another
company, we would need that company’s cooperation to
implement the necessary changes to the device and to obtain any
additional approvals or clearances. Obtaining such additional
approvals or clearances, and cooperation of other companies,
when necessary, could significantly delay, and increase the cost
of obtaining marketing approval, which could reduce the
commercial viability of a drug candidate. To the extent that we
rely on previously unapproved drug delivery systems, we may be
subject to additional testing and approval requirements from the
FDA above and beyond those described above.
Once an NDA or BLA is approved, the product covered thereby
becomes a listed drug that can, in turn, be cited by potential
competitors in support of approval of an abbreviated new drug
application, or ANDA, upon expiration of relevant patents, if
any. An approved ANDA provides for marketing of a drug product
that has the same active ingredients in the same strength,
dosage form and route of administration as the listed drug and
has been shown through bioequivalence testing to be
therapeutically equivalent to the listed drug. There is no
requirement, other than the requirement for bioequivalence
testing, for an ANDA applicant to conduct or submit results of
non-clinical or clinical tests to prove the safety or
effectiveness of its drug product. Drugs approved in this way
are commonly referred to as generic equivalents to the listed
drug, are listed as such by the FDA and can often be substituted
by pharmacists under prescriptions written for the original
listed drug.
Federal law provides for a period of three years of exclusivity
following approval of a listed drug that contains previously
approved active ingredients but is approved in a new dosage,
dosage form, route of administration or combination, or for a
new use, if the FDA deems that the sponsor was required to
provide support from new clinical trials to obtain such
marketing approval. During such three-year exclusivity period,
the FDA cannot grant approval of an ANDA to commercially
distribute a generic version of the drug based on that listed
drug. However, the FDA can approve generic or other versions of
that listed drug, such as a drug that is the same in every way
but its indication for use, and thus the value of such
exclusivity may be undermined. Federal law also provides a
period of up to five years exclusively following approval of a
drug containing no previously approved active ingredients,
during which ANDAs
31
for generic versions of those drugs cannot be submitted unless
the submission accompanies a challenge to a listed patent, in
which case the submission may be made four years following the
original product approval.
Additionally, in the event that the sponsor of the listed drug
has properly informed the FDA of patents covering its listed
drug, applicants submitting an ANDA referencing that drug are
required to make one of four patent certifications, including
certifying that it believes one or more listed patents are
invalid or not infringed. If an applicant certifies invalidity
or non-infringement, it is required to provide notice of its
filing to the NDA sponsor and the patent holder. If the patent
holder then initiates a suit for patent infringement against the
ANDA sponsor within 45 days of receipt of the notice, the
FDA cannot grant effective approval of the ANDA until either
30 months have passed or there has been a court decision
holding that the patents in question are invalid, unenforceable
or not infringed. If the patent holder does not initiate a suit
for patent infringement within the 45 days, the ANDA may be
approved immediately upon successful completion of FDA review,
unless blocked by a regulatory exclusivity period. If the ANDA
applicant certifies that it does not intend to market its
generic product before some or all listed patents on the listed
drug expire, then the FDA cannot grant effective approval of the
ANDA until those patents expire. The first of the ANDA
applicants submitting substantially complete applications
certifying that one or more listed patents for a particular
product are invalid or not infringed may qualify for an
exclusivity period of 180 days running from when the
generic product is first marketed, during which subsequently
submitted ANDAs cannot be granted effective approval. The
180-day
generic exclusivity can be forfeited in various ways, including
if the first applicant does not market its product within
specified statutory timelines. If more than one applicant files
a substantially complete ANDA on the same day, each such first
applicant will be entitled to share the
180-day
exclusivity period, but there will only be one such period,
beginning on the date of first marketing by any of the first
applicants.
From time to time, legislation is drafted and introduced in
Congress that could significantly change the statutory
provisions governing the approval, manufacturing and marketing
of drug products. For example, the Food and Drug Administration
Amendments Act of 2007, or FDAAA, granted significant new powers
to the FDA, many of which are aimed at improving the safety of
drug products before and after approval. In particular, the
FDAAA authorizes the FDA to, among other things, require
post-approval studies and clinical trials, mandate changes to
drug labeling to reflect new safety information, and require
risk evaluation and mitigation strategies, or REMS, for certain
drugs, including certain currently approved drugs. In addition,
it significantly expands the federal government’s clinical
trial registry and results databank and creates new restrictions
on the advertising and promotion of drug products. Under the
FDAAA, companies that violate these and other provisions of the
law are subject to substantial civil monetary penalties.
In addition, FDA regulations and guidance are often revised or
reinterpreted by the agency in ways that may significantly
affect our business and development of our product candidates
and any products that we may commercialize. It is impossible to
predict whether additional legislative changes will be enacted,
or FDA regulations, guidance or interpretations changed, or what
the impact of any such changes may be.
Foreign
Regulation of New Drug Compounds
Approval of a drug or biologic product by comparable regulatory
authorities will be necessary in all or most foreign countries
prior to the commencement of marketing of the product in those
countries, whether or not FDA approval has been obtained. The
approval procedure varies among countries and can involve
requirements for additional testing. The time required may
differ from that required for FDA approval. Although there are
some procedures for unified filings in the European Union, in
general, each country has its own procedures and requirements,
many of which are time consuming and expensive. Thus, there can
be substantial delays in obtaining required approvals from
foreign regulatory authorities after the relevant applications
are filed.
In Europe, marketing authorizations may be submitted under a
centralized or decentralized procedure. The centralized
procedure is mandatory for the approval of biotechnology and
many pharmaceutical products and provides for the grant of a
single marketing authorization that is valid in all European
Union member states. The decentralized procedure is a mutual
recognition procedure that is available at the request of the
applicant for medicinal products that are not subject to the
centralized procedure. We will strive to choose the appropriate
route of European regulatory filing to accomplish the most rapid
regulatory approvals. However, our chosen regulatory strategy
may not secure regulatory approvals on a timely basis or at all.
32
Hazardous
Materials
Our research and development processes involve the controlled
use of hazardous materials, chemicals and radioactive materials
and produce waste products. We are subject to federal, state and
local laws and regulations governing the use, manufacture,
storage, handling and disposal of hazardous materials and waste
products. We do not expect the cost of complying with these laws
and regulations to be material.
Manufacturing
We have no commercial manufacturing capabilities. We may
manufacture material for use in IND-enabling toxicology studies
in animals at our own facility, but we do not anticipate
manufacturing the substantial portion of material for human
clinical use ourselves. We have contracted with several
third-party contract manufacturing organizations for the supply
of certain amounts of material to meet our testing needs for
pre-clinical toxicology and clinical testing. Commercial
quantities of any drugs that we may seek to develop will have to
be manufactured in facilities, and by processes, that comply
with FDA regulations and other federal, state and local
regulations. We plan to rely on third parties to manufacture
commercial quantities of any product that we successfully
develop. Under our agreement with Isis, at our request, we may
negotiate a manufacturing services agreement with Isis for
double-stranded RNA products designated to work through an RNAi
mechanism.
33
Scientific
Advisors
We seek advice from our scientific advisory board, which
consists of a number of leading scientists and physicians, on
scientific and medical matters. Our scientific advisory board
meets regularly to assess:
|
|
|
| |
•
|
our research and development programs;
|
| |
| |
•
|
the design and implementation of our clinical programs;
|
| |
| |
•
|
our patent and publication strategies;
|
| |
| |
•
|
new technologies relevant to our research and development
programs; and
|
| |
| |
•
|
specific scientific and technical issues relevant to our
business.
|
The current members of our scientific advisory board are:
| |
|
|
|
Name
|
|
Position/Institutional Affiliation
|
|
|
|
David P. Bartel, Ph.D.
|
|
Member/Whitehead Institute for Biomedical Research;
Professor/ Massachusetts Institute of Technology
|
|
Fritz Eckstein, Ph.D.
|
|
Professor/Max Planck Institute
|
|
Edward E. Harlow, Ph.D.
|
|
Chair/Harvard Medical School
|
|
Robert S. Langer, Ph.D.
|
|
Institute Professor/Massachusetts Institute of Technology
|
|
Judy Lieberman, M.D., Ph.D.
|
|
Senior Investigator/Immune Disease Institute — Harvard
Medical School; Professor/Harvard Medical School
|
|
Stephen N. Oesterle, M.D.*
|
|
Senior Vice President for Medicine and Technology/Medtronic, Inc.
|
|
Paul R. Schimmel, Ph.D.
|
|
Ernest and Jean Hahn Professor/Skaggs Institute for Chemical
Biology
|
|
Phillip A. Sharp, Ph.D.
|
|
Institute Professor/The Koch Institute for Integrative Cancer
Research, Massachusetts Institute of Technology
|
|
Markus Stoffel, M.D., Ph.D.
|
|
Professor/Institute of Molecular Systems Biology at the ETH
Zurich
|
|
Thomas H. Tuschl, Ph.D.
|
|
Associate Professor/Rockefeller University
|
|
Phillip D. Zamore, Ph.D.
|
|
Gretchen Stone Cook Professor/University of Massachusetts
Medical School
|
|
|
|
|
* |
|
Dr. Oesterle participates as an observer on our scientific
advisory board. |
Employees
As of January 31, 2009, we had 170 employees, 137 of
whom were engaged in research and development. None of our
employees is represented by a labor union or covered by a
collective bargaining agreement, nor have we experienced work
stoppages. We believe that relations with our employees are good.
Financial
Information About Geographic Areas
See Note 2 to our Consolidated Financial Statements,
entitled “Segment Information,” for financial
information about geographic areas. The Notes to our
consolidated financial statements are contained herein in
Item 8.
Corporate
Information
The company comprises four entities, Alnylam Pharmaceuticals,
Inc. and three wholly owned subsidiaries (Alnylam U.S., Inc.,
Alnylam Europe AG and Alnylam Securities Corporation). Alnylam
Pharmaceuticals, Inc. is a Delaware corporation that was formed
in May 2003. Alnylam U.S., Inc. is also a Delaware corporation
that was formed in June 2002. Alnylam Securities Corporation is
a Massachusetts corporation that was formed in December 2006.
Alnylam Europe AG, which was incorporated in Germany in June
2000 under the name Ribopharma AG, was acquired by Alnylam
Pharmaceuticals, Inc. in July 2003. Our principal executive
office is located at 300 Third Street, Cambridge, Massachusetts
02142, and our telephone number is
(617) 551-8200.
34
Investor
Information
We maintain an internet website at
http://www.alnylam.com. The information on our website is
not incorporated by reference into this annual report on
Form 10-K
and should not be considered to be a part of this annual report
on
Form 10-K.
Our website address is included in this annual report on
Form 10-K
as an inactive technical reference only. Our reports filed or
furnished pursuant to Section 13(a) or 15(d) of the
Securities Exchange Act of 1934, including our annual reports on
Form 10-K,
our quarterly reports on
Form 10-Q
and our current reports on
Form 8-K,
and amendments to those reports, are accessible through our
website, free of charge, as soon as reasonably practicable after
these reports are filed electronically with, or otherwise
furnished to, the Securities and Exchange Commission, or SEC. We
also make available on our website the charters of our audit
committee, compensation committee and nominating and corporate
governance committee, our corporate governance guidelines and
our code of business conduct and ethics. In addition, we intend
to disclose on our web site any amendments to, or waivers from,
our code of business conduct and ethics that are required to be
disclosed pursuant to the SEC rules.
You may read and copy any materials we file with the SEC at the
SEC’s Public Reference Room at 100 F Street, NE,
Washington, DC 20549. You may obtain information on the
operation of the Public Reference Room by calling the SEC at
1-800-SEC-0330.
The SEC also maintains an Internet website that contains
reports, proxy and information statements, and other information
regarding Alnylam and other issuers that file electronically
with the SEC. The SEC’s Internet website address is
http://www.sec.gov.
Executive
Officers of the Registrant
| |
|
|
|
|
|
|
|
Name
|
|
Age
|
|
Position
|
|
|
|
John M. Maraganore, Ph.D.
|
|
|
46
|
|
|
Chief Executive Officer and Director
|
|
Barry E. Greene
|
|
|
45
|
|
|
President and Chief Operating Officer
|
|
John (Jack) A. Schmidt, Jr., M.D.
|
|
|
58
|
|
|
Senior Vice President and Chief Scientific Officer
|
|
Patricia L. Allen
|
|
|
47
|
|
|
Vice President of Finance and Treasurer
|
John M. Maraganore, Ph.D. has served as our Chief
Executive Officer and as a member of our board of directors
since December 2002. Dr. Maraganore also served as our
President from December 2002 to December 2007. From April 2000
to December 2002, Dr. Maraganore served as Senior Vice
President, Strategic Product Development at Millennium
Pharmaceuticals, Inc., a biopharmaceutical company.
Dr. Maraganore serves as a member of the board of directors
of the Biotechnology Industry Organization.
Barry E. Greene has served as our President and Chief
Operating Officer since December 2007, as our Chief Operating
Officer since he joined us in October 2003, and from February
2004 through December 2005, as our Treasurer. From February 2001
to September 2003, Mr. Greene served as General Manager of
Oncology at Millennium Pharmaceuticals, Inc., a
biopharmaceutical company. Mr. Greene serves as a member of
the board of directors of Acorda Therapeutics, Inc., a
biotechnology company.
John (Jack) A. Schmidt, Jr., M.D. has served as
our Senior Vice President and Chief Scientific Officer since he
joined us in September 2008. From August 2004 to September 2008,
Dr. Schmidt served as Vice President and Associate
Director, Discovery Research and a U.S. member of the
Sanofi-aventis Global Discovery Leadership Team where he was
also responsible for initiatives in biotherapeutics, external
innovation, and discovery initiatives in China. From 2000 to
2004, he served as Vice President and Head of the Respiratory
and Rheumatoid Arthritis Disease Group at Aventis.
Patricia L. Allen has served as our Vice President of
Finance since she joined us in May 2004, and as our Treasurer
since January 2006. From March 1992 to May 2004, Ms. Allen
held various positions at Alkermes, Inc., a biopharmaceutical
company, most recently as Director of Finance. Ms. Allen is
a certified public accountant.
35
Our business is subject to numerous risks. We caution you
that the following important factors, among others, could cause
our actual results to differ materially from those expressed in
forward-looking statements made by us or on our behalf in
filings with the SEC, press releases, communications with
investors and oral statements. Any or all of our forward-looking
statements in this annual report on
Form 10-K
and in any other public statements we make may turn out to be
wrong. They can be affected by inaccurate assumptions we might
make or by known or unknown risks and uncertainties. Many
factors mentioned in the discussion below will be important in
determining future results. Consequently, no forward-looking
statement can be guaranteed. Actual future results may vary
materially from those anticipated in forward-looking statements.
We explicitly disclaim any obligation to update any
forward-looking
statements to reflect events or circumstances that arise after
the date hereof. You are advised, however, to consult any
further disclosure we make in our reports filed with the SEC.
Risks
Related to Our Business
Risks
Related to Being an Early Stage Company
Because
we have a short operating history, there is a limited amount of
information about us upon which you can evaluate our business
and prospects.
Our operations began in 2002 and we have only a limited
operating history upon which you can evaluate our business and
prospects. In addition, as an early-stage company, we have
limited experience and have not yet demonstrated an ability to
successfully overcome many of the risks and uncertainties
frequently encountered by companies in new and rapidly evolving
fields, particularly in the biopharmaceutical area. For example,
to execute our business plan, we will need to successfully:
|
|
|
| |
•
|
execute product development activities using unproven
technologies related to both RNAi and to the delivery of siRNAs
to the relevant cell tissue;
|
| |
| |
•
|
build and maintain a strong intellectual property portfolio;
|
| |
| |
•
|
gain acceptance for the development of our product candidates
and any products we commercialize;
|
| |
| |
•
|
develop and maintain successful strategic alliances; and
|
| |
| |
•
|
manage our spending as costs and expenses increase due to
clinical trials, regulatory approvals and commercialization.
|
If we are unsuccessful in accomplishing these objectives, we may
not be able to develop product candidates, commercialize
products, raise capital, expand our business or continue our
operations.
The
approach we are taking to discover and develop novel RNAi
therapeutics is unproven and may never lead to marketable
products.
We have concentrated our efforts and therapeutic product
research on RNAi technology, and our future success depends on
the successful development of this technology and products based
on it. Neither we nor any other company has received regulatory
approval to market therapeutics utilizing siRNAs, the class of
molecule we are trying to develop into drugs. The scientific
discoveries that form the basis for our efforts to discover and
develop new drugs are relatively new. The scientific evidence to
support the feasibility of developing drugs based on these
discoveries is both preliminary and limited. Skepticism as to
the feasibility of developing RNAi therapeutics has been
expressed in scientific literature. For example, there are
potential challenges to achieving safe RNAi therapeutics based
on the so-called off-target effects and activation of the
interferon response.
Relatively few drug candidates based on these discoveries have
ever been tested in animals or humans. siRNAs may not naturally
possess the inherent properties typically required of drugs,
such as the ability to be stable in the body long enough to
reach the tissues in which their effects are required, nor the
ability to enter cells within these tissues in order to exert
their effects. We currently have only limited data, and no
conclusive evidence, to suggest that we can introduce these
drug-like properties into siRNAs. We may spend large amounts of
money trying to
36
introduce these properties, and may never succeed in doing so.
In addition, these compounds may not demonstrate in patients the
chemical and pharmacological properties ascribed to them in
laboratory studies, and they may interact with human biological
systems in unforeseen, ineffective or harmful ways. As a result,
we may never succeed in developing a marketable product. If we
do not successfully develop and commercialize drugs based upon
our technological approach, we may not become profitable and the
value of our common stock will decline.
Further, our focus solely on RNAi technology for developing
drugs, as opposed to multiple, more proven technologies for drug
development, increases the risks associated with the ownership
of our common stock. If we are not successful in developing a
product candidate using RNAi technology, we may be required to
change the scope and direction of our product development
activities. In that case, we may not be able to identify and
implement successfully an alternative product development
strategy.
Risks
Related to Our Financial Results and Need for
Financing
We
have a history of losses and may never be
profitable.
We have experienced significant operating losses since our
inception. As of December 31, 2008, we had an accumulated
deficit of $252.2 million. To date, we have not developed
any products nor generated any revenues from the sale of
products. Further, we do not expect to generate any such
revenues in the foreseeable future. We expect to continue to
incur annual net operating losses over the next several years
and will require substantial resources over the next several
years as we expand our efforts to discover, develop and
commercialize RNAi therapeutics. We anticipate that the majority
of any revenue we generate over the next several years will be
from alliances with pharmaceutical companies or funding from
contracts with the government, but cannot be certain that we
will be able to secure or maintain these alliances or contracts,
or meet the obligations or achieve any milestones that we may be
required to meet or achieve to receive payments.
To become and remain consistently profitable, we must succeed in
discovering, developing and commercializing novel drugs with
significant market potential. This will require us to be
successful in a range of challenging activities, including
pre-clinical testing and clinical trial stages of development,
obtaining regulatory approval for these novel drugs and
manufacturing, marketing and selling them. We may never succeed
in these activities, and may never generate revenues that are
significant enough to achieve profitability. Even if we do
achieve profitability, we may not be able to sustain or increase
profitability on a quarterly or annual basis. If we cannot
become and remain consistently profitable, the market price of
our common stock could decline. In addition, we may be unable to
raise capital, expand our business, diversify our product
offerings or continue our operations.
We
will require substantial additional funds to complete our
research and development activities and if additional funds are
not available, we may need to critically limit, significantly
scale back or cease our operations.
We have used substantial funds to develop our RNAi technologies
and will require substantial funds to conduct further research
and development, including pre-clinical testing and clinical
trials of any product candidates, and to manufacture and market
any products that are approved for commercial sale. Because the
successful development of our products is uncertain, we are
unable to estimate the actual funds we will require to develop
and commercialize them.
Our future capital requirements and the period for which we
expect our existing resources to support our operations may vary
from what we expect. We have based our expectations on a number
of factors, many of which are difficult to predict or are
outside of our control, including:
|
|
|
| |
•
|
our progress in demonstrating that siRNAs can be active as drugs;
|
| |
| |
•
|
our ability to develop relatively standard procedures for
selecting and modifying siRNA drug candidates;
|
| |
| |
•
|
progress in our research and development programs, as well as
the magnitude of these programs;
|
| |
| |
•
|
the timing, receipt and amount of milestone and other payments,
if any, from present and future collaborators, if any;
|
37
|
|
|
| |
•
|
the timing, receipt and amount of funding under current and
future government contracts, if any;
|
| |
| |
•
|
our ability to maintain and establish additional collaborative
arrangements;
|
| |
| |
•
|
the resources, time and costs required to initiate and complete
our pre-clinical and clinical trials, obtain regulatory
approvals, protect our intellectual property and obtain and
maintain licenses to third-party intellectual property;
|
| |
| |
•
|
the cost of preparing, filing, prosecuting, maintaining and
enforcing patent claims;
|
| |
| |
•
|
progress in the research and development programs of Regulus
Therapeutics; and
|
| |
| |
•
|
the timing, receipt and amount of sales and royalties, if any,
from our potential products.
|
If our estimates and predictions relating to these factors are
incorrect, we may need to modify our operating plan.
We will be required to seek additional funding in the future and
intend to do so through either collaborative arrangements,
public or private equity offerings or debt financings, or a
combination of one or more of these funding sources. Additional
funds may not be available to us on acceptable terms or at all.
In addition, the terms of any financing may adversely affect the
holdings or the rights of our stockholders. For example, if we
raise additional funds by issuing equity securities, further
dilution to our stockholders will result. In addition, our
investor rights agreement with Novartis provides Novartis with
the right generally to maintain its ownership percentage in us
and our common stock purchase agreement with Roche contains a
similar provision. In May 2008, Novartis purchased
213,888 shares of our common stock at a purchase price of
$25.29 per share, which resulted in a 0.5% increase in
Novartis’ ownership position. At December 31, 2008,
Novartis’ ownership position was 13.2%. While the exercise
of these rights by Novartis provided us with $5.4 million
in cash, and the exercise in the future by Novartis or Roche may
provide us with additional funding under some circumstances,
this exercise and any future exercise of these rights by
Novartis or Roche will also cause further dilution to our
stockholders. Debt financing, if available, may involve
restrictive covenants that could limit our flexibility in
conducting future business activities. If we are unable to
obtain funding on a timely basis, we may be required to
significantly curtail one or more of our research or development
programs. We also could be required to seek funds through
arrangements with collaborators or others that may require us to
relinquish rights to some of our technologies, product
candidates or products that we would otherwise pursue on our own.
If the
estimates we make, or the assumptions on which we rely, in
preparing our consolidated financial statements prove
inaccurate, our actual results may vary from those reflected in
our projections and accruals.
Our consolidated financial statements have been prepared in
accordance with accounting principles generally accepted in the
United States. The preparation of these consolidated financial
statements requires us to make estimates and judgments that
affect the reported amounts of our assets, liabilities, revenues
and expenses, the amounts of charges accrued by us and related
disclosure of contingent assets and liabilities. We base our
estimates on historical experience and on various other
assumptions that we believe to be reasonable under the
circumstances. We cannot assure you, however, that our
estimates, or the assumptions underlying them, will be correct.
The
investment of our cash balance and our investments in marketable
debt securities are subject to risks which may cause losses and
affect the liquidity of these investments.
At December 31, 2008, we had $512.7 million in cash,
cash equivalents and marketable securities. We historically have
invested these amounts in corporate bonds, commercial paper,
securities issued by the U.S. government, certificates of
deposit and money market funds meeting the criteria of our
investment policy, which is focused on the preservation of our
capital. These investments are subject to general credit,
liquidity, market and interest rate risks, which may be affected
by U.S. sub-prime mortgage defaults that have affected
various sectors of the financial markets and caused credit and
liquidity issues. We may realize losses in the fair value of
these investments or a complete loss of these investments, which
would have a negative effect on our consolidated financial
statements. In addition, should our investments cease paying or
reduce the amount of interest paid to us,
38
our interest income would suffer. For example, due to recent
market conditions, interest rates have fallen, and accordingly,
despite a higher average cash balance in 2008, our interest
income decreased to $14.4 million in 2008 from
$15.4 million in 2007. These market risks associated with
our investment portfolio may have a negative adverse effect on
our results of operations, liquidity and financial condition.
Risks
Related to Our Dependence on Third Parties
Our
collaboration with Novartis is important to our business. If
this collaboration is unsuccessful, Novartis terminates this
collaboration or this collaboration results in competition
between us and Novartis for the development of drugs targeting
the same diseases, our business could be adversely
affected.
In October 2005, we entered into a collaboration agreement with
Novartis. Under this agreement, Novartis can select up to 30
exclusive targets to include in the collaboration, which number
may be increased to 40 under certain circumstances and upon
additional payments. Novartis pays the costs to develop these
drug candidates and will commercialize and market any products
derived from this collaboration. For RNAi therapeutic products
successfully developed under the agreement, if any, we would be
entitled to receive milestone payments upon achievement of
certain specified development and annual net sales events, up to
an aggregate of $75.0 million per therapeutic product, as
well as royalties on the annual net sales, if any. The Novartis
agreement has an initial term of three years, with the option
for two additional one-year extensions at the election of
Novartis. In July 2008, Novartis elected to extend the initial
term for an additional year, through October 2009. Novartis
retains the right to extend the term for a second additional
year, which right must be exercised no later than July 2009.
Novartis may elect to terminate this collaboration in the event
of a material uncured breach by us. We expect that a substantial
amount of funding will come from this collaboration. If this
collaboration is unsuccessful, or if it is terminated, our
business could be adversely affected.
This agreement also provides Novartis with a non-exclusive
option to integrate into its operations our intellectual
property relating to RNAi technology, excluding any technology
related to delivery of nucleic acid based molecules. Novartis
may exercise this integration option at any point during the
research term, as defined in the collaboration and license
agreement. The research term expires in October 2009 and may be
extended until October 2010 at Novartis’ election. The
license grant under the integration option, if exercised, would
be structured similarly to our non-exclusive platform licenses
with Roche and Takeda. If Novartis elects to exercise this
option, Novartis could become a competitor of ours in the
development of RNAi-based drugs targeting the same diseases.
Novartis has significantly greater financial resources and far
more experience than we do in developing and marketing drugs,
which could put us at a competitive disadvantage if we were to
compete with Novartis in the development of RNAi-based drugs
targeting the same disease. Accordingly, the exercise by
Novartis of this option could adversely affect our business.
Our agreement with Novartis allows us to continue to develop
products on an exclusive basis on our own with respect to
targets not selected by Novartis for inclusion in the
collaboration. We may need to form additional alliances to
develop products. However, our agreement with Novartis provides
Novartis with a right of first offer, for a defined term, in the
event that we propose to enter into an agreement with a third
party with respect to such targets. This right of first offer
may make it difficult for us to form future alliances around
specific targets with other parties.
Our
license and collaboration agreements with Roche and Takeda are
important to our business. If Roche and/or Takeda do not
successfully develop drugs pursuant to these agreements or these
agreements result in competition between us and Roche and/or
Takeda for the development of drugs targeting the same diseases,
our business could be adversely affected.
In July 2007, we entered into a license and collaboration
agreement with Roche. Under the license and collaboration
agreement we granted Roche a non-exclusive license to our
intellectual property to develop and commercialize therapeutic
products that function through RNAi, subject to our existing
contractual obligations to third parties. The license is limited
to the therapeutic areas of oncology, respiratory diseases,
metabolic diseases and certain liver diseases and may be
expanded to include up to 18 additional therapeutic areas,
comprising all other fields of human disease, as identified and
agreed upon by the parties, upon payment to us by Roche of an
additional
39
$50.0 million for each additional therapeutic area, if any.
In addition, in exchange for our contributions under the
collaboration agreement, for each RNAi therapeutic product
successfully developed by Roche, its affiliates, or sublicensees
under the collaboration agreement, if any, we are entitled to
receive milestone payments upon achievement of specified
development and sales events, totaling up to an aggregate of
$100.0 million per therapeutic target, together with
royalty payments based on worldwide annual net sales, if any. In
May 2008, we entered into a similar license and collaboration
agreement with Takeda, which is limited to the therapeutic areas
of oncology and metabolic diseases, and which may be expanded to
include up to 20 additional therapeutic areas, comprising all
other fields of human disease, as identified and agreed upon by
the parties, upon payment to us by Takeda of an additional
$50.0 million for each additional therapeutic area, if any.
For each RNAi therapeutic product successfully developed by
Takeda, its affiliates and sublicensees, if any, we are entitled
to receive specified development and commercialization
milestones, totaling up to $171.0 million per product,
together with royalty payments based on worldwide annual net
sales, if any. In addition, we have agreed that for a period of
five years, we will not grant any other party rights to develop
RNAi therapeutics in the Asian territory.
If Roche or Takeda fails to successfully develop products using
our technology, we may not receive any milestone or royalty
payments under these agreements. In addition, even if Takeda is
not successful in its efforts, for a period of five years we
will be limited in our ability to form alliances with other
parties in the Asia territory. We also have the option under the
Takeda agreement, exercisable until the start of Phase III
development, to opt-in under a
50-50 profit
sharing agreement to the development and commercialization in
the United States of up to four Takeda licensed products, and
would be entitled to opt-in rights for two additional products
for each additional field expansion, if any, elected by Takeda
under the collaboration agreement. If Takeda fails to
successfully develop products, we may not realize any economic
benefit from these opt-in rights.
Finally, either Roche or Takeda could become a competitor of
ours in the development of RNAi-based drugs targeting the same
diseases. Each of these companies has significantly greater
financial resources than we do and has far more experience in
developing and marketing drugs, which could put us at a
competitive disadvantage if we were to compete with either Roche
or Takeda in the development of RNAi-based drugs targeting the
same disease.
We may
not be able to execute our business strategy if we are unable to
enter into alliances with other companies that can provide
business and scientific capabilities and funds for the
development and commercialization of our drug candidates. If we
are unsuccessful in forming or maintaining these alliances on
favorable terms, our business may not succeed.
We do not have any capability for sales, marketing or
distribution and have limited capabilities for drug development.
In addition, we believe that other companies are expending
substantial resources in developing safe and effective means of
delivering siRNAs to relevant cell and tissue types.
Accordingly, we have entered into alliances with other companies
and collaborators that we believe can provide such capabilities,
and we intend to enter into additional alliances in the future.
For example, we intend to enter into (1) non-exclusive
platform alliances which will enable our collaborators to
develop RNAi therapeutics and will bring in additional funding
with which we can develop our RNAi therapeutics, and
(2) alliances to jointly develop specific drug candidates
and to jointly commercialize RNAi therapeutics, if they are
approved,
and/or
ex-U.S. market geographic partnerships on specific RNAi
therapeutic programs. In such alliances, we may expect our
collaborators to provide substantial capabilities in delivery of
RNAi therapeutics to the relevant cell or tissue type, clinical
development, regulatory affairs,
and/or
marketing, sales and distribution. For example, under our
collaboration with Medtronic, we are jointly developing ALN-HTT,
an RNAi therapeutic for Huntington’s disease, which would
be delivered using an implanted infusion device developed by
Medtronic. The success of this collaboration will depend, in
part, on Medtronic’s expertise in the area of delivery by
infusion device. In other alliances, we may expect our
collaborators to develop, market and sell certain of our product
candidates. We may have limited or no control over the sales,
marketing and distribution activities of these third parties.
Our future revenues may depend heavily on the success of the
efforts of these third parties. For example, we will jointly
develop and commercialize products for RSV in partnership with
Cubist in North America. We will rely entirely on Cubist for the
development and commercialization of products for RSV in the
rest of the world outside of Asia, where we will rely on Kyowa
Hakko for development and commercialization of products for RSV.
If Cubist and Kyowa Hakko are not successful in their
commercialization efforts, our future revenues for RSV may be
adversely affected.
40
We may not be successful in entering into such alliances on
favorable terms due to various factors, including Novartis’
right of first offer on our drug targets. Even if we do succeed
in securing such alliances, we may not be able to maintain them
if, for example, development or approval of a drug candidate is
delayed or sales of an approved drug are disappointing.
Furthermore, any delay in entering into collaboration agreements
could delay the development and commercialization of our drug
candidates and reduce their competitiveness even if they reach
the market. Any such delay related to our collaborations could
adversely affect our business.
For certain drug candidates that we may develop, we have formed
collaborations to fund all or part of the costs of drug
development and commercialization, such as our collaborations
with Novartis, as well as collaborations with Cubist, Medtronic
and NIAID. We may not, however, be able to enter into additional
collaborations, and the terms of any collaboration agreement we
do secure may not be favorable to us. If we are not successful
in our efforts to enter into future collaboration arrangements
with respect to a particular drug candidate, we may not have
sufficient funds to develop that or any other drug candidate
internally, or to bring any drug candidates to market. If we do
not have sufficient funds to develop and bring our drug
candidates to market, we will not be able to generate sales
revenues from these drug candidates, and this will substantially
harm our business.
If any
collaborator terminates or fails to perform its obligations
under agreements with us, the development and commercialization
of our drug candidates could be delayed or
terminated.
Our dependence on collaborators for capabilities and funding
means that our business could be adversely affected if any
collaborator terminates its collaboration agreement with us or
fails to perform its obligations under that agreement. Our
current or future collaborations, if any, may not be
scientifically or commercially successful. Disputes may arise in
the future with respect to the ownership of rights to technology
or products developed with collaborators, which could have an
adverse effect on our ability to develop and commercialize any
affected product candidate.
Our current collaborations allow, and we expect that any future
collaborations will allow, either party to terminate the
collaboration for a material breach by the other party. Our
agreement with Kyowa Hakko for the development and
commercialization of ALN-RSV01 for the treatment of RSV
infection in Japan and other major markets in Asia may be
terminated by Kyowa Hakko without cause upon
180-days’
prior written notice to us, subject to certain conditions, and
our agreement with Cubist relating to the development and
commercialization of RSV therapeutics in territories outside of
Asia may be terminated by Cubist at any time upon as little as
three months prior written notice, if such notice is given prior
to the acceptance for filing of the first application for
regulatory approval of a licensed product. If we were to lose a
commercialization collaborator, we would have to attract a new
collaborator or develop internal sales, distribution and
marketing capabilities, which would require us to invest
significant amounts of financial and management resources.
In addition, if a collaborator terminates its collaboration with
us, for breach or otherwise, it would be difficult for us to
attract new collaborators and could adversely affect how we are
perceived in the business and financial communities. A
collaborator, or in the event of a change in control of a
collaborator, the successor entity, could determine that it is
in its financial interest to:
|
|
|
| |
•
|
pursue alternative technologies or develop alternative products,
either on its own or jointly with others, that may be
competitive with the products on which it is collaborating with
us or which could affect its commitment to the collaboration
with us;
|
| |
| |
•
|
pursue higher-priority programs or change the focus of its
development programs, which could affect the collaborator’s
commitment to us; or
|
| |
| |
•
|
if it has marketing rights, choose to devote fewer resources to
the marketing of our product candidates, if any are approved for
marketing, than it does for product candidates developed without
us.
|
If any of these occur, the development and commercialization of
one or more drug candidates could be delayed, curtailed or
terminated because we may not have sufficient financial
resources or capabilities to continue such development and
commercialization on our own.
41
We
depend on government contracts to partially fund our research
and development efforts and may enter into additional government
contracts in the future. If current or future government
funding, if any, is reduced or delayed, our drug development
efforts for such funded programs may be negatively
affected.
In September 2006, NIAID awarded us a contract for up to
$23.0 million over four years to advance the development of
a broad spectrum RNAi anti-viral therapeutic for hemorrhagic
fever virus, including the Ebola virus. Of the
$23.0 million in funding, the government initially
committed to pay us up to $14.2 million over the first two
years of the contract. In June 2008, as a result of the progress
of the program, the government awarded us an additional
$7.5 million, to be paid through September 2009 for the
third year of the contract, together with any remaining funds
carried over from the funding allocated for the first two years
of the contract. We cannot be certain that the government will
appropriate the funds necessary for this contract in future
budgets. In addition, the government can terminate the agreement
in specified circumstances. If we do not receive the
$23.0 million we expect to receive under this contract, we
may not be able to develop therapeutics to treat Ebola.
For example, in August 2007, DTRA awarded us a contract to
advance the development of a broad spectrum RNAi anti-viral
therapeutic for hemorrhagic fever virus infection. When granted,
this federal contract provided for potential funding of up to
$38.6 million through February 2011. Of this amount, the
government initially committed to pay us up to
$10.9 million through February 2009, which term includes a
six-month extension granted by DTRA in July 2008. However,
following a program review in early 2009, we and DTRA have
determined to end this program and accordingly, the remaining
funds will not be accessed.
Regulus
Therapeutics is important to our business. If Regulus
Therapeutics does not successfully develop drugs pursuant to
this license and collaboration agreement or Regulus Therapeutics
is sold to Isis or a third-party, our business could be
adversely affected.
In September 2007, we and Isis formed Regulus Therapeutics, of
which we own 49%, to discover, develop and commercialize
microRNA-based therapeutics. Regulus Therapeutics intends to
address therapeutic opportunities that arise from abnormal
expression or mutations in microRNAs. Generally, we do not have
rights to pursue microRNA-based therapeutics independently of
Regulus Therapeutics. If Regulus Therapeutics is unable to
discover, develop and commercialize microRNA-based therapeutics,
our business could be adversely affected.
In addition, subject to certain conditions, we and Isis each
have the right to initiate a buy-out of Regulus
Therapeutics’ assets, including Regulus Therapeutics’
intellectual property and rights to licensed intellectual
property. Following the initiation of such a buy-out, we and
Isis will mutually determine whether to sell Regulus
Therapeutics to us, Isis or a third party. We may not have
sufficient funds to buy out Isis’ interest in Regulus
Therapeutics and we may not be able to obtain the financing to
do so. In addition, Isis may not be willing to sell their
interest in Regulus Therapeutics. If Regulus Therapeutics is
sold to Isis or a third party, we may lose our rights to
participate in the development and commercialization of
microRNA-based therapeutics. If we and Isis are unable to
negotiate a sale of Regulus Therapeutics, Regulus Therapeutics
will distribute and assign its rights, interests and assets to
us and Isis in accordance with our percentage interests, except
for Regulus Therapeutics’ intellectual property and license
rights, to which each of us and Isis will receive co-exclusive
rights, subject to certain specified exceptions. In this event,
we could face competition from Isis in the development of
microRNA-based therapeutics.
We
have very limited manufacturing experience or resources and we
must incur significant costs to develop this expertise or rely
on third parties to manufacture our products.
We have very limited manufacturing experience. Our internal
manufacturing capabilities are limited to
small-scale
production of non-good manufacturing practice material for use
in in vitro and in vivo experiments. Our
products utilize specialized formulations, such as liposomes,
whose
scale-up and
manufacturing could be very difficult. We also have very limited
experience in such
scale-up and
manufacturing, requiring us to depend on third parties, who
might not be able to deliver in a timely manner, or at all. In
order to develop products, apply for regulatory approvals and
commercialize our products, we will need to develop, contract
for, or otherwise arrange for the necessary manufacturing
capabilities. We may manufacture clinical trial materials
ourselves or we may rely on others to manufacture the materials
we will require for any clinical trials that we initiate. Only a
limited number of manufacturers supply synthetic siRNAs. We
currently rely on several contract manufacturers for our supply
of
42
synthetic siRNAs. There are risks inherent in pharmaceutical
manufacturing that could affect the ability of our contract
manufacturers to meet our delivery time requirements or provide
adequate amounts of material to meet our needs. Included in
these risks are synthesis and purification failures and
contamination during the manufacturing process, which could
result in unusable product and cause delays in our development
process, as well as additional expense to us. To fulfill our
siRNA requirements, we may also need to secure alternative
suppliers of synthetic siRNAs. In addition to the manufacture of
the synthetic siRNAs, we may have additional manufacturing
requirements related to the technology required to deliver the
siRNA to the relevant cell or tissue type. In some cases, the
delivery technology we utilize is highly specialized or
proprietary, and for technical and legal reasons, we may have
access to only one or a limited number of potential
manufacturers for such delivery technology. Failure by these
manufacturers to properly formulate our siRNAs for delivery
could also result in unusable product and cause delays in our
discovery and development process, as well as additional expense
to us.
The manufacturing process for any products that we may develop
is subject to the FDA and foreign regulatory authority approval
process and we will need to contract with manufacturers who can
meet all applicable FDA and foreign regulatory authority
requirements on an ongoing basis. In addition, if we receive the
necessary regulatory approval for any product candidate, we also
expect to rely on third parties, including our commercial
collaborators, to produce materials required for commercial
supply. We may experience difficulty in obtaining adequate
manufacturing capacity for our needs. If we are unable to obtain
or maintain contract manufacturing for these product candidates,
or to do so on commercially reasonable terms, we may not be able
to successfully develop and commercialize our products.
To the extent that we enter into manufacturing arrangements with
third parties, we will depend on these third parties to perform
their obligations in a timely manner and consistent with
regulatory requirements, including those related to quality
control and quality assurance. The failure of a third-party
manufacturer to perform its obligations as expected could
adversely affect our business in a number of ways, including:
|
|
|
| |
•
|
we may not be able to initiate or continue clinical trials of
products that are under development;
|
| |
| |
•
|
we may be delayed in submitting regulatory applications, or
receiving regulatory approvals, for our products candidates;
|
| |
| |
•
|
we may lose the cooperation of our collaborators;
|
| |
| |
•
|
we may be required to cease distribution or recall some or all
batches of our products; and
|
| |
| |
•
|
ultimately, we may not be able to meet commercial demands for
our products.
|
If a third-party manufacturer with whom we contract fails to
perform its obligations, we may be forced to manufacture the
materials ourselves, for which we may not have the capabilities
or resources, or enter into an agreement with a different
third-party manufacturer, which we may not be able to do with
reasonable terms, if at all. In some cases, the technical skills
required to manufacture our product may be unique to the
original manufacturer and we may have difficulty transferring
such skills to a
back-up nor
alternate supplier, or we may be unable to transfer such skills
at all. In addition, if we are required to change manufacturers
for any reason, we will be required to verify that the new
manufacturer maintains facilities and procedures that comply
with quality standards and with all applicable regulations and
guidelines. The delays associated with the verification of a new
manufacturer could negatively affect our ability to develop
product candidates in a timely manner or within budget.
Furthermore, a manufacturer may possess technology related to
the manufacture of our product candidate that such manufacturer
owns independently. This would increase our reliance on such
manufacturer or require us to obtain a license from such
manufacturer in order to have another third party manufacture
our products.
We
have no sales, marketing or distribution experience and would
have to invest significant financial and management resources to
establish these capabilities.
We have no sales, marketing or distribution experience. We
currently expect to rely heavily on third parties to launch and
market certain of our product candidates, if approved. However,
if we elect to develop internal sales, distribution and
marketing capabilities, we will need to invest significant
financial and management resources. For
43
products where we decide to perform sales, marketing and
distribution functions ourselves, we could face a number of
additional risks, including:
|
|
|
| |
•
|
we may not be able to attract and build a significant marketing
or sales force;
|
| |
| |
•
|
the cost of establishing a marketing or sales force may not be
justifiable in light of the revenues generated by any particular
product; and
|
| |
| |
•
|
our direct sales and marketing efforts may not be successful.
|
If we are unable to develop our own sales, marketing and
distribution capabilities, we will not be able to successfully
commercialize our products without reliance on third parties.
The
current credit and financial market conditions may exacerbate
certain risks affecting our business.
Due to the recent tightening of global credit, there may be a
disruption or delay in the performance of our
third-party
contractors, suppliers or collaborators. We rely on third
parties for several important aspects of our business, including
significant portions of our manufacturing needs, development of
product candidates and conduct of clinical trials. If such third
parties are unable to satisfy their commitments to us, our
business could be adversely affected.
Risks
Related to Managing Our Operations
If we
are unable to attract and retain qualified key management and
scientists, staff consultants and advisors, our ability to
implement our business plan may be adversely
affected.
We are highly dependent upon our senior management and
scientific staff. The loss of the service of any of the members
of our senior management, including Dr. John Maraganore,
our Chief Executive Officer, may significantly delay or prevent
the achievement of product development and other business
objectives. Our employment agreements with our key personnel are
terminable without notice. We do not carry key man life
insurance on any of our employees.
Although we have generally been successful in our recruiting
efforts, as well as our retention of employees, we face intense
competition for qualified individuals from numerous
pharmaceutical and biotechnology companies, universities,
governmental entities and other research institutions, many of
which have substantially greater resources with which to reward
qualified individuals than we do. We may be unable to attract
and retain suitably qualified individuals, and our failure to do
so could have an adverse effect on our ability to implement our
business plan.
We may
have difficulty managing our growth and expanding our operations
successfully as we seek to evolve from a company primarily
involved in discovery and pre-clinical testing into one that
develops and commercializes drugs.
Since we commenced operations in 2002, we have grown
substantially. As of December 31, 2008, we had
approximately 164 employees in our facility in Cambridge,
Massachusetts. Our rapid and substantial growth may place a
strain on our administrative and operational infrastructure. If
drug candidates we develop enter and advance through clinical
trials, we will need to expand our development, regulatory,
manufacturing, marketing and sales capabilities or contract with
other organizations to provide these capabilities for us. As our
operations expand, we expect that we will need to manage
additional relationships with various collaborators, suppliers
and other organizations. Our ability to manage our operations
and growth will require us to continue to improve our
operational, financial and management controls, reporting
systems and procedures. We may not be able to implement
improvements to our management information and control systems
in an efficient or timely manner and may discover deficiencies
in existing systems and controls.
44
Risks
Related to Our Industry
Risks
Related to Development, Clinical Testing and Regulatory Approval
of Our Drug Candidates
Any
drug candidates we develop may fail in development or be delayed
to a point where they do not become commercially
viable.
Pre-clinical testing and clinical trials of new drug candidates
are lengthy and expensive and the historical failure rate for
drug candidates is high. We are developing our most advanced
product candidate, ALN-RSV01, for the treatment of RSV
infection. In January 2008, we completed our GEMINI study, a
Phase II trial designed to evaluate the safety,
tolerability and anti-viral activity of ALN-RSV01 in adult
subjects experimentally infected with RSV. We commenced a second
Phase II trial in April 2008 to assess the safety and
tolerability of ALN-RSV01 in adult lung transplant patients
naturally infected with RSV and we intend to continue the
clinical development of ALN-RSV01. In addition, in December
2008, we submitted an IND to the FDA for ALN-VSP, our first
systemically delivered RNAi therapeutic. We are developing
ALN-VSP for the treatment of certain liver cancers. We received
clearance from the FDA in January 2009 to proceed with a Phase I
study. However, we may not be able to further advance these or
any other product candidate through clinical trials. If we
successfully enter into clinical studies, the results from
pre-clinical testing or early clinical trials of a drug
candidate may not predict the results that will be obtained in
subsequent human clinical trials. For example, ALN-VSP and our
other systemically delivered therapeutic candidates employ novel
delivery formulations that have yet to be evaluated in human
studies and have yet to be proven safe and effective in clinical
trials. We, the FDA or other applicable regulatory authorities,
or an institutional review board, or IRB, may suspend clinical
trials of a drug candidate at any time for various reasons,
including if we or they believe the subjects or patients
participating in such trials are being exposed to unacceptable
health risks. Among other reasons, adverse side effects of a
drug candidate on subjects or patients in a clinical trial could
result in the FDA or foreign regulatory authorities suspending
or terminating the trial and refusing to approve a particular
drug candidate for any or all indications of use.
Clinical trials of a new drug candidate require the enrollment
of a sufficient number of patients, including patients who are
suffering from the disease the drug candidate is intended to
treat and who meet other eligibility criteria. Rates of patient
enrollment are affected by many factors, including the size of
the patient population, the age and condition of the patients,
the nature of the protocol, the proximity of patients to
clinical sites, the availability of effective treatments for the
relevant disease, the seasonality of infections and the
eligibility criteria for the clinical trial. Delays in patient
enrollment can result in increased costs and longer development
times.
Clinical trials also require the review and oversight of IRBs,
which approve and continually review clinical investigations and
protect the rights and welfare of human subjects. Inability to
obtain or delay in obtaining IRB approval can prevent or delay
the initiation and completion of clinical trials, and the FDA
may decide not to consider any data or information derived from
a clinical investigation not subject to initial and continuing
IRB review and approval in support of a marketing application.
Our drug candidates that we develop may encounter problems
during clinical trials that will cause us, an IRB or regulatory
authorities to delay, suspend or terminate these trials, or that
will delay the analysis of data from these trials. If we
experience any such problems, we may not have the financial
resources to continue development of the drug candidate that is
affected, or development of any of our other drug candidates. We
may also lose, or be unable to enter into, collaborative
arrangements for the affected drug candidate and for other drug
candidates we are developing.
Delays in clinical trials could reduce the commercial viability
of our drug candidates. Any of the following could, among other
things, delay our clinical trials:
|
|
|
| |
•
|
delays in filing initial drug applications;
|
| |
| |
•
|
conditions imposed on us by the FDA or comparable foreign
authorities regarding the scope or design of our clinical trials;
|
| |
| |
•
|
problems in engaging IRBs to oversee trials or problems in
obtaining or maintaining IRB approval of trials;
|
| |
| |
•
|
delays in enrolling patients and volunteers into clinical trials;
|
45
|
|
|
| |
•
|
high drop-out rates for patients and volunteers in clinical
trials;
|
| |
| |
•
|
negative or inconclusive results from our clinical trials or the
clinical trials of others for drug candidates similar to ours;
|
| |
| |
•
|
inadequate supply or quality of drug candidate materials or
other materials necessary for the conduct of our clinical trials;
|
| |
| |
•
|
serious and unexpected drug-related side effects experienced by
participants in our clinical trials or by individuals using
drugs similar to our product candidates; or
|
| |
| |
•
|
unfavorable FDA or other regulatory agency inspection and review
of a clinical trial site or records of any clinical or
pre-clinical investigation.
|
Even if we successfully complete clinical trials of our drug
candidates, any given drug candidate may not prove to be an
effective treatment for the diseases for which it was being
tested.
The
FDA approval process may be delayed for any drugs we develop
that require the use of specialized drug delivery
devices.
Some drug candidates that we develop may need to be administered
using specialized drug delivery devices that deliver RNAi
therapeutics directly to diseased parts of the body. For
example, we believe that product candidates we develop for
Parkinson’s disease, HD or other central nervous system
diseases may need to be administered using such a device. For
neurodegenerative diseases, we have entered into a collaboration
agreement with Medtronic to pursue potential development of
drug-device combinations incorporating RNAi therapeutics. We may
not achieve successful development results under this
collaboration and may need to seek other collaboration partners
to develop alternative drug delivery systems, or utilize
existing drug delivery systems, for the direct delivery of RNAi
therapeutics for these diseases. While we expect to rely on drug
delivery systems that have been approved by the FDA or other
regulatory agencies to deliver drugs like ours to similar
physiological sites, we, or our collaborator, may need to modify
the design or labeling of such delivery device for some products
we may develop. In such an event, the FDA may regulate the
product as a combination product or require additional approvals
or clearances for the modified delivery device. Further, to the
extent the specialized delivery device is owned by another
company, we would need that company’s cooperation to
implement the necessary changes to the device, or its labeling,
and to obtain any additional approvals or clearances. In cases
where we do not have an ongoing collaboration with the company
that makes the device, obtaining such additional approvals or
clearances and the cooperation of such other company could
significantly delay and increase the cost of obtaining marketing
approval, which could reduce the commercial viability of our
drug candidate. In summary, we may be unable to find, or
experience delays in finding, suitable drug delivery systems to
administer RNAi therapeutics directly to diseased parts of the
body, which could negatively affect our ability to successfully
commercialize these RNAi therapeutics.
We may
be unable to obtain United States or foreign regulatory approval
and, as a result, be unable to commercialize our drug
candidates.
Our drug candidates are subject to extensive governmental
regulations relating to, among other things, research, testing,
development, manufacturing, safety, efficacy, recordkeeping,
labeling, marketing and distribution of drugs. Rigorous
pre-clinical testing and clinical trials and an extensive
regulatory approval process are required to be successfully
completed in the United States and in many foreign jurisdictions
before a new drug can be marketed. Satisfaction of these and
other regulatory requirements is costly, time consuming,
uncertain and subject to unanticipated delays. It is possible
that none of the drug candidates we may develop will obtain the
appropriate regulatory approvals necessary for us or our
collaborators to begin selling them.
We have very limited experience in conducting and managing the
clinical trials necessary to obtain regulatory approvals,
including approval by the FDA. The time required to obtain FDA
and other approvals is unpredictable but typically takes many
years following the commencement of clinical trials, depending
upon the complexity of the drug candidate. Any analysis we
perform of data from pre-clinical and clinical activities is
subject to confirmation and interpretation by regulatory
authorities, which could delay, limit or prevent regulatory
approval. We may also encounter unexpected delays or increased
costs due to new government regulations, for example, from
future legislation or
46
administrative action, or from changes in FDA policy during the
period of product development, clinical trials and FDA
regulatory review. For example, the Food and Drug Administration
Amendments Act of 2007, or FDAAA, may make it more difficult and
costly for us to obtain regulatory approval of our product
candidates and to produce, market and distribute products after
approval. The FDAAA granted a variety of new powers to the FDA,
many of which are aimed at improving the safety of drug products
before and after approval. In particular, it authorizes the FDA
to, among other things, require post-approval studies and
clinical trials, mandate changes to drug labeling to reflect new
safety information, and require risk evaluation and mitigation
strategies, or REMS, for certain drugs, including certain
currently approved drugs. In addition, it significantly expanded
the federal government’s clinical trial registry and
results databank and creates new restrictions on the advertising
and promotion of drug products. Under the FDAAA, companies that
violate the new law are subject to substantial civil monetary
penalties.
Because the drugs we are intending to develop may represent a
new class of drug, the FDA has not yet established any
definitive policies, practices or guidelines in relation to
these drugs. While the product candidates that we are currently
developing are regulated as a new drug under the Federal Food,
Drug, and Cosmetic Act, the FDA could decide to regulate them or
other products we may develop as biologics under the Public
Health Service Act. The lack of policies, practices or
guidelines may hinder or slow review by the FDA of any
regulatory filings that we may submit. Moreover, the FDA may
respond to these submissions by defining requirements we may not
have anticipated. Such responses could lead to significant
delays in the clinical development of our product candidates. In
addition, because there may be approved treatments for some of
the diseases for which we may seek approval, in order to receive
regulatory approval, we will need to demonstrate through
clinical trials that the product candidates we develop to treat
these diseases, if any, are not only safe and effective, but
safer or more effective than existing products. Furthermore, in
recent years, there has been increased public and political
pressure on the FDA with respect to the approval process for new
drugs, and the number of approvals to market new drugs has
declined.
Any delay or failure in obtaining required approvals could have
a material adverse effect on our ability to generate revenues
from the particular drug candidate. Furthermore, any regulatory
approval to market a product may be subject to limitations on
the indicated uses for which we may market the product. These
limitations may limit the size of the market for the product and
affect reimbursement by third-party payors.
We are also subject to numerous foreign regulatory requirements
governing, among other things, the conduct of clinical trials,
manufacturing and marketing authorization, pricing and
third-party reimbursement. The foreign regulatory approval
process includes all of the risks associated with FDA approval
described above as well as risks attributable to the
satisfaction of local regulations in foreign jurisdictions.
Approval by the FDA does not assure approval by regulatory
authorities outside the United States and vice versa.
If our
pre-clinical testing does not produce successful results or our
clinical trials do not demonstrate safety and efficacy in
humans, we will not be able to commercialize our drug
candidates.
Before obtaining regulatory approval for the sale of our drug
candidates, we must conduct, at our own expense, extensive
pre-clinical tests and clinical trials to demonstrate the safety
and efficacy in humans of our drug candidates. Pre-clinical and
clinical testing is expensive, difficult to design and
implement, can take many years to complete and is uncertain as
to outcome. Success in pre-clinical testing and early clinical
trials does not ensure that later clinical trials will be
successful, and interim results of a clinical trial do not
necessarily predict final results.
A failure of one of more of our clinical trials can occur at any
stage of testing. We may experience numerous unforeseen events
during, or as a result of, pre-clinical testing and the clinical
trial process that could delay or prevent our ability to receive
regulatory approval or commercialize our drug candidates,
including:
|
|
|
| |
•
|
regulators or IRBs may not authorize us to commence or continue
a clinical trial or conduct a clinical trial at a prospective
trial site;
|
| |
| |
•
|
our pre-clinical tests or clinical trials may produce negative
or inconclusive results, and we may decide, or regulators may
require us, to conduct additional pre-clinical testing or
clinical trials, or we may abandon projects that we expect to be
promising;
|
| |
| |
•
|
enrollment in our clinical trials may be slower than we
anticipate or participants may drop out of our clinical trials
at a higher rate than we anticipate, resulting in significant
delays;
|
47
|
|
|
| |
•
|
our third party contractors may fail to comply with regulatory
requirements or meet their contractual obligations to us in a
timely manner;
|
| |
| |
•
|
we might have to suspend or terminate our clinical trials if the
participants are being exposed to unacceptable health risks;
|
| |
| |
•
|
IRBs or regulators, including the FDA, may require that we hold,
suspend or terminate clinical research for various reasons,
including noncompliance with regulatory requirements;
|
| |
| |
•
|
the cost of our clinical trials may be greater than we
anticipate;
|
| |
| |
•
|
the supply or quality of our drug candidates or other materials
necessary to conduct our clinical trials may be insufficient or
inadequate;
|
| |
| |
•
|
effects of our drug candidates may not be the desired effects or
may include undesirable side effects or the drug candidates may
have other unexpected characteristics; and
|
| |
| |
•
|
effects of our drug candidates may not be clear, or we may
disagree with regulatory authorities, including the FDA, about
how to interpret the data generated in our clinical trials.
|
Even
if we obtain regulatory approvals, our marketed drugs will be
subject to ongoing regulatory review. If we fail to comply with
continuing United States and foreign regulations, we could lose
our approvals to market drugs and our business would be
seriously harmed.
Following any initial regulatory approval of any drugs we may
develop, we will also be subject to continuing regulatory
review, including the review of adverse drug experiences and
clinical results that are reported after our drug products are
made commercially available. This would include results from any
post-marketing tests or vigilance required as a condition of
approval. The manufacturer and manufacturing facilities we use
to make any of our drug candidates will also be subject to
periodic review and inspection by the FDA. The discovery of any
new or previously unknown problems with the product,
manufacturer or facility may result in restrictions on the drug
or manufacturer or facility, including withdrawal of the drug
from the market. We do not have, and currently do not intend to
develop, the ability to manufacture material for our clinical
trials or on a commercial scale. We may manufacture clinical
trial materials or we may contract a third party to manufacture
these materials for us. Reliance on third-party manufacturers
entails risks to which we would not be subject if we
manufactured products ourselves, including reliance on the
third-party manufacturer for regulatory compliance. Our product
promotion and advertising is also subject to regulatory
requirements and continuing regulatory review.
If we fail to comply with applicable continuing regulatory
requirements, we may be subject to fines, suspension or
withdrawal of regulatory approval, product recalls and seizures,
operating restrictions and criminal prosecution.
Even
if we receive regulatory approval to market our product
candidates, the market may not be receptive to our product
candidates upon their commercial introduction, which will
prevent us from becoming profitable.
The product candidates that we are developing are based upon new
technologies or therapeutic approaches. Key participants in
pharmaceutical marketplaces, such as physicians, third-party
payors and consumers, may not accept a product intended to
improve therapeutic results based on RNAi technology. As a
result, it may be more difficult for us to convince the medical
community and third-party payors to accept and use our product,
or to provide favorable reimbursement.
Other factors that we believe will materially affect market
acceptance of our product candidates include:
|
|
|
| |
•
|
the timing of our receipt of any marketing approvals, the terms
of any approvals and the countries in which approvals are
obtained;
|
| |
| |
•
|
the safety, efficacy and ease of administration of our product
candidates;
|
| |
| |
•
|
the willingness of patients to accept potentially new routes of
administration;
|
48
|
|
|
| |
•
|
the success of our physician education programs;
|
| |
| |
•
|
the availability of government and third-party payor
reimbursement;
|
| |
| |
•
|
the pricing of our products, particularly as compared to
alternative treatments; and
|
| |
| |
•
|
availability of alternative effective treatments for the
diseases that product candidates we develop are intended to
treat and the relative risks, benefits and costs of the
treatments.
|
Even
if we develop a RNAi therapeutic product for the prevention or
treatment of infection by hemorrhagic fever viruses such as
Ebola, governments may not elect to purchase such a product,
which could adversely affect our business.
We expect that governments will be the only purchasers of any
products we may develop for the prevention or treatment of
hemorrhagic fever viruses such as Ebola. In the future, we may
also initiate additional programs for the development of product
candidates for which governments may be the only or primary
purchasers. However, governments will not be required to
purchase any such products from us and may elect not to do so,
which could adversely affect our business. For example, although
the focus of our Ebola program is to develop RNAi therapeutic
targeting gene sequences that are highly conserved across known
Ebola viruses, if the sequence of any Ebola virus that emerges
is not sufficiently similar to those we are targeting, any
product candidate that we develop may not be effective against
that virus. Accordingly, while we expect that any RNAi
therapeutic we develop for the treatment of Ebola could be
stockpiled by governments as part of their biodefense
preparations, they may not elect to purchase such product, or if
they purchase our products, they may not do so at prices and
volume levels that are profitable for us.
If we
or our collaborators, manufacturers or service providers fail to
comply with regulatory laws and regulations, we or they could be
subject to enforcement actions, which could affect our ability
to market and sell our products and may harm our
reputation.
If we or our collaborators, manufacturers or service providers
fail to comply with applicable federal, state or foreign laws or
regulations, we could be subject to enforcement actions, which
could affect our ability to develop, market and sell our
products successfully and could harm our reputation and lead to
reduced acceptance of our products by the market. These
enforcement actions include:
|
|
|
| |
•
|
warning letters;
|
| |
| |
•
|
product recalls or public notification or medical product safety
alerts to healthcare professionals;
|
| |
| |
•
|
restrictions on, or prohibitions against, marketing our products;
|
| |
| |
•
|
restrictions on importation or exportation of our products;
|
| |
| |
•
|
suspension of review or refusal to approve pending applications;
|
| |
| |
•
|
exclusion from participation in government-funded healthcare
programs;
|
| |
| |
•
|
exclusion from eligibility for the award of government contracts
for our products;
|
| |
| |
•
|
suspension or withdrawal of product approvals;
|
| |
| |
•
|
product seizures;
|
| |
| |
•
|
injunctions; and
|
| |
| |
•
|
civil and criminal penalties and fines.
|
Any
drugs we develop may become subject to unfavorable pricing
regulations, third-party reimbursement practices or healthcare
reform initiatives, thereby harming our business.
The regulations that govern marketing approvals, pricing and
reimbursement for new drugs vary widely from country to country.
Some countries require approval of the sale price of a drug
before it can be marketed. In many countries, the pricing review
period begins after marketing or product licensing approval is
granted. In some foreign
49
markets, prescription pharmaceutical pricing remains subject to
continuing governmental control even after initial approval is
granted. Although we intend to monitor these regulations, our
programs are currently in the early stages of development and we
will not be able to assess the impact of price regulations for a
number of years. As a result, we might obtain regulatory
approval for a product in a particular country, but then be
subject to price regulations that delay our commercial launch of
the product and negatively impact the revenues we are able to
generate from the sale of the product in that country.
Our ability to commercialize any products successfully also will
depend in part on the extent to which reimbursement for these
products and related treatments will be available from
government health administration authorities, private health
insurers and other organizations. Even if we succeed in bringing
one or more products to the market, these products may not be
considered cost-effective, and the amount reimbursed for any
products may be insufficient to allow us to sell our products on
a competitive basis. Because our programs are in the early
stages of development, we are unable at this time to determine
their cost effectiveness or the likely level or method of
reimbursement. Increasingly, the third-party payors who
reimburse patients, such as government and private insurance
plans, are requiring that drug companies provide them with
predetermined discounts from list prices, and are seeking to
reduce the prices charged for pharmaceutical products. If the
price we are able to charge for any products we develop is
inadequate in light of our development and other costs, our
profitability could be adversely affected.
We currently expect that any drugs we develop may need to be
administered under the supervision of a physician. Under
currently applicable United States law, drugs that are not
usually self-administered may be eligible for coverage by the
Medicare program if:
|
|
|
| |
•
|
they are incident to a physician’s services;
|
| |
| |
•
|
they are “reasonable and necessary” for the diagnosis
or treatment of the illness or injury for which they are
administered according to accepted standard of medical practice;
|
| |
| |
•
|
they are not excluded as immunizations; and
|
| |
| |
•
|
they have been approved by the FDA.
|
There may be significant delays in obtaining coverage for
newly-approved drugs, and coverage may be more limited than the
purposes for which the drug is approved by the FDA. Moreover,
eligibility for coverage does not imply that any drug will be
reimbursed in all cases or at a rate that covers our costs,
including research, development, manufacture, sale and
distribution. Interim payments for new drugs, if applicable, may
also not be sufficient to cover our costs and may not be made
permanent. Reimbursement may be based on payments allowed for
lower-cost drugs that are already reimbursed, may be
incorporated into existing payments for other services and may
reflect budgetary constraints or imperfections in Medicare data.
Net prices for drugs may be reduced by mandatory discounts or
rebates required by government health care programs or private
payors and by any future relaxation of laws that presently
restrict imports of drugs from countries where they may be sold
at lower prices than in the United States. Third party payors
often rely upon Medicare coverage policy and payment limitations
in setting their own reimbursement rates. Our inability to
promptly obtain coverage and profitable reimbursement rates from
both government-funded and private payors for new drugs that we
develop could have a material adverse effect on our operating
results, our ability to raise capital needed to commercialize
products, and our overall financial condition.
We believe that the efforts of governments and third-party
payors to contain or reduce the cost of healthcare will continue
to affect the business and financial condition of pharmaceutical
and biopharmaceutical companies. A number of legislative and
regulatory proposals to change the healthcare system in the
United States and other major healthcare markets have been
proposed in recent years. These proposals have included
prescription drug benefit legislation that was enacted and took
effect in January 2006 and healthcare reform legislation
recently enacted by certain states. Further federal and state
legislative and regulatory developments are possible and we
expect ongoing initiatives in the United States to increase
pressure on drug pricing. Such reforms could have an adverse
effect on anticipated revenues from drug candidates that we may
successfully develop.
50
Another development that may affect the pricing of drugs is
Congressional action regarding drug reimportation into the
United States. Recent proposed legislation has been introduced
in Congress that, if enacted, would permit more widespread
re-importation of drugs from foreign countries into the United
States. This could include
reh-importation
from foreign countries where the drugs are sold at lower prices
than in the United States. Such legislation, or similar
regulatory changes, could lead to a decrease in the price we
receive for any approved products, which, in turn, could impair
our ability to generate revenue. Alternatively, in response to
legislation such as this, we might elect not to seek approval
for or market our products in foreign jurisdictions in order to
minimize the risk of re-importation, which could also reduce the
revenue we generate from our product sales.
There
is a substantial risk of product liability claims in our
business. If we are unable to obtain sufficient insurance, a
product liability claim against us could adversely affect our
business.
Our business exposes us to significant potential product
liability risks that are inherent in the development,
manufacturing and marketing of human therapeutic products.
Product liability claims could delay or prevent completion of
our clinical development programs. If we succeed in marketing
products, such claims could result in an FDA investigation of
the safety and effectiveness of our products, our manufacturing
processes and facilities or our marketing programs, and
potentially a recall of our products or more serious enforcement
action, limitations on the indications for which they may be
used, or suspension or withdrawal of approvals. We currently
have product liability insurance that we believe is appropriate
for our stage of development and may need to obtain higher
levels prior to marketing any of our drug candidates. Any
insurance we have or may obtain may not provide sufficient
coverage against potential liabilities. Furthermore, clinical
trial and product liability insurance is becoming increasingly
expensive. As a result, we may be unable to obtain sufficient
insurance at a reasonable cost to protect us against losses
caused by product liability claims that could have a material
adverse effect on our business.
If we
do not comply with laws regulating the protection of the
environment and health and human safety, our business could be
adversely affected.
Our research and development involves the use of hazardous
materials, chemicals and various radioactive compounds. We
maintain quantities of various flammable and toxic chemicals in
our facilities in Cambridge that are required for our research
and development activities. We are subject to federal, state and
local laws and regulations governing the use, manufacture,
storage, handling and disposal of these hazardous materials. We
believe our procedures for storing, handling and disposing these
materials in our Cambridge facility comply with the relevant
guidelines of the City of Cambridge and the Commonwealth of
Massachusetts. Although we believe that our safety procedures
for handling and disposing of these materials comply with the
standards mandated by applicable regulations, the risk of
accidental contamination or injury from these materials cannot
be eliminated. If an accident occurs, we could be held liable
for resulting damages, which could be substantial. We are also
subject to numerous environmental, health and workplace safety
laws and regulations, including those governing laboratory
procedures, exposure to blood-borne pathogens and the handling
of biohazardous materials.
Although we maintain workers’ compensation insurance to
cover us for costs and expenses we may incur due to injuries to
our employees resulting from the use of these materials, this
insurance may not provide adequate coverage against potential
liabilities. We do not maintain insurance for environmental
liability or toxic tort claims that may be asserted against us
in connection with our storage or disposal of biological,
hazardous or radioactive materials. Additional federal, state
and local laws and regulations affecting our operations may be
adopted in the future. We may incur substantial costs to comply
with, and substantial fines or penalties if we violate, any of
these laws or regulations.
Risks
Related to Patents, Licenses and Trade Secrets
If we
are not able to obtain and enforce patent protection for our
discoveries, our ability to develop and commercialize our
product candidates will be harmed.
Our success depends, in part, on our ability to protect
proprietary methods and technologies that we develop under the
patent and other intellectual property laws of the United States
and other countries, so that we can prevent others from
unlawfully using our inventions and proprietary information.
However, we may not hold proprietary
51
rights to some patents required for us to commercialize our
proposed products. Because certain U.S. patent applications
are confidential until the patents issue, such as applications
filed prior to November 29, 2000, or applications filed
after such date which will not be filed in foreign countries,
third parties may have filed patent applications for technology
covered by our pending patent applications without our being
aware of those applications, and our patent applications may not
have priority over those applications. For this and other
reasons, we may be unable to secure desired patent rights,
thereby losing desired exclusivity. Further, we may be required
to obtain licenses under third-party patents to market our
proposed products or conduct our research and development or
other activities. If licenses are not available to us on
acceptable terms, we will not be able to market the affected
products or conduct the desired activities.
Our strategy depends on our ability to rapidly identify and seek
patent protection for our discoveries. In addition, we may rely
on third-party collaborators to file patent applications
relating to proprietary technology that we develop jointly
during certain collaborations. The process of obtaining patent
protection is expensive and time-consuming. If our present or
future collaborators fail to file and prosecute all necessary
and desirable patent applications at a reasonable cost and in a
timely manner, our business will be adversely affected. Despite
our efforts and the efforts of our collaborators to protect our
proprietary rights, unauthorized parties may be able to obtain
and use information that we regard as proprietary. While issued
patents are presumed valid, this does not guarantee that the
patent will survive a validity challenge or be held enforceable.
Any patents we have obtained, or obtain in the future, may be
challenged, invalidated, adjudged unenforceable or circumvented
by parties attempting to design around our intellectual
property. Moreover, third parties or the USPTO may commence
interference proceedings involving our patents or patent
applications. Any challenge to, finding of unenforceability or
invalidation or circumvention of, our patents or patent
applications would be costly, would require significant time and
attention of our management and could have a material adverse
effect on our business.
Our pending patent applications may not result in issued
patents. The patent position of pharmaceutical or biotechnology
companies, including ours, is generally uncertain and involves
complex legal and factual considerations. The standards that the
USPTO and its foreign counterparts use to grant patents are not
always applied predictably or uniformly and can change. Adding
to the uncertainty of our current intellectual property
portfolio and our ability to secure and enforce future patent
rights are the outcome of a legal dispute surrounding the
implementation of certain continuation and claims rules
promulgated by the USPTO, which were scheduled to take effect
November 1, 2007, but which are now enjoined and on appeal,
and the outcome of Congressional efforts to reform the Patent
Act of 1952. There is also no uniform, worldwide policy
regarding the subject matter and scope of claims granted or
allowable in pharmaceutical or biotechnology patents.
Accordingly, we do not know the degree of future protection for
our proprietary rights or the breadth of claims that will be
allowed in any patents issued to us or to others.
We also rely to a certain extent on trade secrets, know-how and
technology, which are not protected by patents, to maintain our
competitive position. If any trade secret, know-how or other
technology not protected by a patent were to be disclosed to or
independently developed by a competitor, our business and
financial condition could be materially adversely affected.
We
license patent rights from third party owners. If such owners do
not properly maintain or enforce the patents underlying such
licenses, our competitive position and business prospects will
be harmed.
We are a party to a number of licenses that give us rights to
third party intellectual property that is necessary or useful
for our business. In particular, we have obtained licenses from,
among others, Isis, MIT, the Whitehead Institute for Biomedical
Research, Max Planck Innovation, Stanford University, Tekmira
and UTSW. We also intend to enter into additional licenses to
third party intellectual property in the future.
Our success will depend in part on the ability of our licensors
to obtain, maintain and enforce patent protection for our
licensed intellectual property, in particular, those patents to
which we have secured exclusive rights. Our licensors may not
successfully prosecute the patent applications to which we are
licensed. Even if patents issue in respect of these patent
applications, our licensors may fail to maintain these patents,
may determine not to pursue litigation against other companies
that are infringing these patents, or may pursue such litigation
less aggressively than we would. Without protection for the
intellectual property we license, other companies might be able
to offer
52
substantially identical products for sale, which could adversely
affect our competitive business position and harm our business
prospects.
Other
companies or organizations may challenge our patent rights or
may assert patent rights that prevent us from developing and
commercializing our products.
RNA interference is a relatively new scientific field, the
commercial exploitation of which has resulted in many different
patents and patent applications from organizations and
individuals seeking to obtain patent protection in the field. We
have obtained grants and issuances of RNAi patents and have
licensed many of these patents from third parties on an
exclusive basis. The issued patents and pending patent
applications in the United States and in key markets around the
world that we own or license claim many different methods,
compositions and processes relating to the discovery,
development, manufacture and commercialization of RNAi
therapeutics. Specifically, we have a portfolio of patents,
patent applications and other intellectual property covering:
fundamental aspects of the structure and uses of siRNAs,
including their manufacture and use as therapeutics, and
RNAi-related mechanisms; chemical modifications to siRNAs that
improve their suitability for therapeutic uses; siRNAs directed
to specific targets as treatments for particular diseases; and
delivery technologies, such as in the field of cationic
liposomes.
As the field of RNAi therapeutics is maturing, patent
applications are being fully processed by national patent
offices around the world. There is uncertainty about which
patents will issue, and, if they do, as to when, to whom, and
with what claims. It is likely that there will be significant
litigation and other proceedings, such as interference,
reexamination and opposition proceedings, in various patent
offices relating to patent rights in the RNAi field. For
example, various third parties have initiated oppositions to
patents in our Kreutzer-Limmer and Tuschl II series in the
EPO and in other jurisdictions. We expect that additional
oppositions will be filed in the EPO and elsewhere, and other
challenges will be raised relating to other patents and patent
applications in our portfolio. In many cases, the possibility of
appeal exists for either us or our opponents, and it may be
years before final, unappealable rulings are made with respect
to these patents in certain jurisdictions. The timing and
outcome of these and other proceedings is uncertain and may
adversely affect our business if we are not successful in
defending the patentability and scope of our pending and issued
patent claims. In addition, third parties may attempt to
invalidate our intellectual property rights. Even if our rights
are not directly challenged, disputes could lead to the
weakening of our intellectual property rights. Our defense
against any attempt by third parties to circumvent or invalidate
our intellectual property rights could be costly to us, could
require significant time and attention of our management and
could have a material adverse effect on our business and our
ability to successfully compete in the field of RNAi.
There are many issued and pending patents that claim aspects of
oligonucleotide chemistry that we may need to apply to our siRNA
drug candidates. There are also many issued patents that claim
targeting genes or portions of genes that may be relevant for
siRNA drugs we wish to develop. Thus, it is possible that one or
more organizations will hold patent rights to which we will need
a license. If those organizations refuse to grant us a license
to such patent rights on reasonable terms, we may not be able to
market products or perform research and development or other
activities covered by these patents.
If we
become involved in patent litigation or other proceedings
related to a determination of rights, we could incur substantial
costs and expenses, substantial liability for damages or be
required to stop our product development and commercialization
efforts.
Third parties may sue us for infringing their patent rights.
Likewise, we may need to resort to litigation to enforce a
patent issued or licensed to us or to determine the scope and
validity of proprietary rights of others. In addition, a third
party may claim that we have improperly obtained or used its
confidential or proprietary information. Furthermore, in
connection with a license agreement, we have agreed to indemnify
the licensor for costs incurred in connection with litigation
relating to intellectual property rights. The cost to us of any
litigation or other proceeding relating to intellectual property
rights, even if resolved in our favor, could be substantial, and
the litigation would divert our management’s efforts. Some
of our competitors may be able to sustain the costs of complex
patent litigation more effectively than we can because they have
substantially greater resources. Uncertainties resulting from
the initiation and continuation of any litigation could limit
our ability to continue our operations.
53
If any parties successfully claim that our creation or use of
proprietary technologies infringes upon their intellectual
property rights, we might be forced to pay damages, potentially
including treble damages, if we are found to have willfully
infringed on such parties’ patent rights. In addition to
any damages we might have to pay, a court could require us to
stop the infringing activity or obtain a license. Any license
required under any patent may not be made available on
commercially acceptable terms, if at all. In addition, such
licenses are likely to be non-exclusive and, therefore, our
competitors may have access to the same technology licensed to
us. If we fail to obtain a required license and are unable to
design around a patent, we may be unable to effectively market
some of our technology and products, which could limit our
ability to generate revenues or achieve profitability and
possibly prevent us from generating revenue sufficient to
sustain our operations. Moreover, we expect that a number of our
collaborations will provide that royalties payable to us for
licenses to our intellectual property may be offset by amounts
paid by our collaborators to third parties who have competing or
superior intellectual property positions in the relevant fields,
which could result in significant reductions in our revenues
from products developed through collaborations.
If we
fail to comply with our obligations under any licenses or
related agreements, we could lose license rights that are
necessary for developing and protecting our RNAi technology and
any related product candidates that we develop, or we could lose
certain exclusive rights to grant sublicenses.
Our current licenses impose, and any future licenses we enter
into are likely to impose, various development,
commercialization, funding, royalty, diligence, sublicensing,
insurance and other obligations on us. If we breach any of these
obligations, the licensor may have the right to terminate the
license or render the license non-exclusive, which could result
in us being unable to develop, manufacture and sell products
that are covered by the licensed technology or enable a
competitor to gain access to the licensed technology. In
addition, while we cannot currently determine the amount of the
royalty obligations we will be required to pay on sales of
future products, if any, the amounts may be significant. The
amount of our future royalty obligations will depend on the
technology and intellectual property we use in products that we
successfully develop and commercialize, if any. Therefore, even
if we successfully develop and commercialize products, we may be
unable to achieve or maintain profitability.
Confidentiality
agreements with employees and others may not adequately prevent
disclosure of trade secrets and other proprietary
information.
In order to protect our proprietary technology and processes, we
rely in part on confidentiality agreements with our
collaborators, employees, consultants, outside scientific
collaborators and sponsored researchers and other advisors.
These agreements may not effectively prevent disclosure of
confidential information and may not provide an adequate remedy
in the event of unauthorized disclosure of confidential
information. In addition, others may independently discover
trade secrets and proprietary information, and in such cases we
could not assert any trade secret rights against such party.
Costly and time-consuming litigation could be necessary to
enforce and determine the scope of our proprietary rights, and
failure to obtain or maintain trade secret protection could
adversely affect our competitive business position.
Risks
Related to Competition
The
pharmaceutical market is intensely competitive. If we are unable
to compete effectively with existing drugs, new treatment
methods and new technologies, we may be unable to commercialize
successfully any drugs that we develop.
The pharmaceutical market is intensely competitive and rapidly
changing. Many large pharmaceutical and biotechnology companies,
academic institutions, governmental agencies and other public
and private research organizations are pursuing the development
of novel drugs for the same diseases that we are targeting or
expect to target. Many of our competitors have:
|
|
|
| |
•
|
much greater financial, technical and human resources than we
have at every stage of the discovery, development, manufacture
and commercialization of products;
|
| |
| |
•
|
more extensive experience in pre-clinical testing, conducting
clinical trials, obtaining regulatory approvals, and in
manufacturing, marketing and selling pharmaceutical products;
|
54
|
|
|
| |
•
|
product candidates that are based on previously tested or
accepted technologies;
|
| |
| |
•
|
products that have been approved or are in late stages of
development; and
|
| |
| |
•
|
collaborative arrangements in our target markets with leading
companies and research institutions.
|
We will face intense competition from drugs that have already
been approved and accepted by the medical community for the
treatment of the conditions for which we may develop drugs. We
also expect to face competition from new drugs that enter the
market. We believe a significant number of drugs are currently
under development, and may become commercially available in the
future, for the treatment of conditions for which we may try to
develop drugs. For instance, we are currently evaluating RNAi
therapeutics for RSV, liver cancer, hypercholesterolemia, TTR
amyloidosis and HD, and have a number of additional discovery
programs targeting other diseases. Virazole and Synagis are
currently marketed for the treatment of certain RSV patients,
and numerous drugs are currently marketed or used for the
treatment of liver cancer, hypercholesterolemia and HD as well.
These drugs, or other of our competitors’ products, may be
more effective, safer, less expensive or marketed and sold more
effectively, than any products we develop.
If we successfully develop drug candidates, and obtain approval
for them, we will face competition based on many different
factors, including:
|
|
|
| |
•
|
the safety and effectiveness of our products;
|
| |
| |
•
|
the ease with which our products can be administered and the
extent to which patients accept relatively new routes of
administration;
|
| |
| |
•
|
the timing and scope of regulatory approvals for these products;
|
| |
| |
•
|
the availability and cost of manufacturing, marketing and sales
capabilities;
|
| |
| |
•
|
price;
|
| |
| |
•
|
reimbursement coverage; and
|
| |
| |
•
|
patent position.
|
Our competitors may develop or commercialize products with
significant advantages over any products we develop based on any
of the factors listed above or on other factors. Our competitors
may therefore be more successful in commercializing their
products than we are, which could adversely affect our
competitive position and business. Competitive products may make
any products we develop obsolete or noncompetitive before we can
recover the expenses of developing and commercializing our drug
candidates. Furthermore, we also face competition from existing
and new treatment methods that reduce or eliminate the need for
drugs, such as the use of advanced medical devices. The
development of new medical devices or other treatment methods
for the diseases we are targeting could make our drug candidates
noncompetitive, obsolete or uneconomical.
We
face competition from other companies that are working to
develop novel drugs using technology similar to ours. If these
companies develop drugs more rapidly than we do or their
technologies, including delivery technologies, are more
effective, our ability to successfully commercialize drugs will
be adversely affected.
In addition to the competition we face from competing drugs in
general, we also face competition from other companies working
to develop novel drugs using technology that competes more
directly with our own. We are aware of several companies that
are working in the field of RNAi. In addition, we granted
licenses or options for licenses to Isis, GeneCare, Benitec,
Calando, Tekmira, Quark and others under which these companies
may independently develop RNAi therapeutics against a limited
number of targets. Any of these companies may develop its RNAi
technology more rapidly and more effectively than us. Merck was
one of our collaborators and a licensee under our intellectual
property for specified disease targets until September 2007, at
which time we and Merck agreed to terminate our collaboration.
As a result of its acquisition of Sirna Therapeutics Inc. in
December 2006, and in light of the mutual termination of our
collaboration, Merck, which has substantially more resources and
experience in developing drugs than we do, may become a direct
competitor.
55
In addition, as a result of agreements that we have entered
into, Roche and Takeda have obtained, and Novartis has the right
to obtain, broad, non-exclusive licenses to certain aspects of
our technology that give them the right to compete with us in
certain circumstances.
We also compete with companies working to develop
antisense-based drugs. Like RNAi product candidates, antisense
drugs target messenger RNAs, or mRNAs, in order to suppress the
activity of specific genes. Isis is currently marketing an
antisense drug and has several antisense drug candidates in
clinical trials. The development of antisense drugs is more
advanced than that of RNAi therapeutics, and antisense
technology may become the preferred technology for drugs that
target mRNAs to silence specific genes.
In addition to competition with respect to RNAi and with respect
to specific products, we face substantial competition to
discover and develop safe and effective means to deliver siRNAs
to the relevant cell and tissue types. Safe and effective means
to deliver siRNAs to the relevant cell and tissue types may be
developed by our competitors, and our ability to successfully
commercialize a competitive product would be adversely affected.
In addition, substantial resources are being expended by third
parties in the effort to discover and develop a safe and
effective means of delivering siRNAs into the relevant cell and
tissue types, both in academic laboratories and in the corporate
sector. Some of our competitors have substantially greater
resources than we do, and if our competitors are able to
negotiate exclusive access to those delivery solutions developed
by third parties, we may be unable to successfully commercialize
our product candidates.
Risks
Related to Our Common Stock
If our
stock price fluctuates, purchasers of our common stock could
incur substantial losses.
The market price of our common stock may fluctuate significantly
in response to factors that are beyond our control. The stock
market in general has recently experienced extreme price and
volume fluctuations. The market prices of securities of
pharmaceutical and biotechnology companies have been extremely
volatile, and have experienced fluctuations that often have been
unrelated or disproportionate to the operating performance of
these companies. These broad market fluctuations could result in
extreme fluctuations in the price of our common stock, which
could cause purchasers of our common stock to incur substantial
losses.
We may
incur significant costs from class action litigation due to our
expected stock volatility.
Our stock price may fluctuate for many reasons, including as a
result of public announcements regarding the progress of our
development efforts, the addition or departure of our key
personnel, variations in our quarterly operating results and
changes in market valuations of pharmaceutical and biotechnology
companies. Recently, when the market price of a stock has been
volatile as our stock price may be, holders of that stock have
occasionally brought securities class action litigation against
the company that issued the stock. If any of our stockholders
were to bring a lawsuit of this type against us, even if the
lawsuit is without merit, we could incur substantial costs
defending the lawsuit. The lawsuit could also divert the time
and attention of our management.
Novartis’
ownership of our common stock could delay or prevent a change in
corporate control or cause a decline in our common stock should
Novartis decide to sell all or a portion of its
shares.
As of December 31, 2008, Novartis holds 13.2% of our
outstanding common stock and has the right to maintain its
ownership percentage through the expiration or termination of
our broad alliance. This concentration of ownership may harm the
market price of our common stock by:
|
|
|
| |
•
|
delaying, deferring or preventing a change in control of our
company;
|
| |
| |
•
|
impeding a merger, consolidation, takeover or other business
combination involving our company; or
|
| |
| |
•
|
discouraging a potential acquirer from making a tender offer or
otherwise attempting to obtain control of our company.
|
In addition, if Novartis decides to sell all or a portion of its
shares in a rapid or disorderly manner, our stock price could be
negatively impacted.
56
Anti-takeover
provisions in our charter documents and under Delaware law and
our stockholder rights plan could make an acquisition of us,
which may be beneficial to our stockholders, more difficult and
may prevent attempts by our stockholders to replace or remove
our current management.
Provisions in our certificate of incorporation and our bylaws
may delay or prevent an acquisition of us or a change in our
management. In addition, these provisions may frustrate or
prevent any attempts by our stockholders to replace or remove
our current management by making it more difficult for
stockholders to replace members of our board of directors.
Because our board of directors is responsible for appointing the
members of our management team, these provisions could in turn
affect any attempt by our stockholders to replace current
members of our management team. These provisions include:
|
|
|
| |
•
|
a classified board of directors;
|
| |
| |
•
|
a prohibition on actions by our stockholders by written consent;
|
| |
| |
•
|
limitations on the removal of directors; and
|
| |
| |
•
|
advance notice requirements for election to our board of
directors and for proposing matters that can be acted upon at
stockholder meetings.
|
In addition our board of directors has adopted a stockholder
rights plan, the provisions of which could make it difficult for
a potential acquirer of Alnylam to consummate an acquisition
transaction.
Moreover, because we are incorporated in Delaware, we are
governed by the provisions of Section 203 of the Delaware
General Corporation Law, which prohibits a person who owns in
excess of 15% of our outstanding voting stock from merging or
combining with us for a period of three years after the date of
the transaction in which the person acquired in excess of 15% of
our outstanding voting stock, unless the merger or combination
is approved in a prescribed manner. These provisions would apply
even if the proposed merger or acquisition could be considered
beneficial by some stockholders.
|
|
|
ITEM 1B.
|
UNRESOLVED
STAFF COMMENTS.
|
Not applicable.
Our operations are based primarily in Cambridge, Massachusetts.
As of January 31, 2009, we lease approximately
84,000 square feet of office and laboratory space in
Cambridge, Massachusetts. The two leases for this property
expire in September 2011. We believe that the total space
available to us under our current leases will meet our needs for
the foreseeable future and that additional space would be
available to us on commercially reasonable terms if required.
|
|
|
ITEM 3.
|
LEGAL
PROCEEDINGS
|
We are currently not a party to any material legal proceedings.
|
|
|
ITEM 4.
|
SUBMISSION
OF MATTERS TO A VOTE OF SECURITY HOLDERS
|
No matters were submitted to a vote of our security holders
during the fourth quarter of the fiscal year ended
December 31, 2008.
57
PART II
|
|
|
ITEM 5.
|
MARKET
FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS
AND ISSUER PURCHASES OF EQUITY SECURITIES
|
Market
Information
Our common stock began trading on The NASDAQ Global Market on
May 28, 2004 under the symbol “ALNY”. Prior to
that time, there was no established public trading market for
our common stock. The following table sets forth the high and
low sale prices per share for our common stock on The NASDAQ
Global Market for the periods indicated:
| |
|
|
|
|
|
|
|
Year Ended December 31, 2007:
|
|
High
|
|
|
Low
|
|
|
|
First Quarter
|
|
$
|
22.94
|
|
|
$16.66
|
|
Second Quarter
|
|
$
|
20.68
|
|
|
$15.06
|
|
Third Quarter
|
|
$
|
34.85
|
|
|
$14.87
|
|
Fourth Quarter
|
|
$
|
37.35
|
|
|
$26.84
|
| |
|
|
|
|
|
|
|
|
|
Year Ended December 31, 2008:
|
|
High
|
|
|
Low
|
|
|
|
|
First Quarter
|
|
$
|
35.19
|
|
|
$
|
22.25
|
|
|
Second Quarter
|
|
$
|
30.74
|
|
|
$
|
22.55
|
|
|
Third Quarter
|
|
$
|
36.37
|
|
|
$
|
25.07
|
|
|
Fourth Quarter
|
|
$
|
28.95
|
|
|
$
|
16.37
|
|
Holders
of record
As of January 31, 2009, there were approximately 97 holders
of record of our common stock. Because many of our shares are
held by brokers and other institutions on behalf of
stockholders, we are unable to estimate the total number of
individual stockholders represented by these record holders.
Dividends
We have never paid or declared any cash dividends on our common
stock. We currently intend to retain any earnings for future
growth and, therefore, do not expect to pay cash dividends in
the foreseeable future.
Securities
Authorized for Issuance Under Equity Compensation
Plans
Information relating to our equity compensation plans will be
included in our proxy statement in connection with our 2009
Annual Meeting of Stockholders, under the caption “Equity
Compensation Plan Information.” That portion of our proxy
statement is incorporated herein by reference.
58
Stock
Performance Graph
The following performance graph and related information shall
not be deemed “soliciting material” or to be
“filed” with the SEC, nor shall such information be
incorporated by reference into any future filing under the
Securities Act of 1933 or Securities Exchange Act of 1934, each
as amended, except to the extent that we specifically
incorporate it by reference into such filing.
The comparative stock performance graph below compares the
cumulative total stockholder return (assuming reinvestment of
dividends, if any) from investing $100 on May 28, 2004, the
date on which our common stock was first publicly traded, to the
close of the last trading day of 2008, in each of (i) our
common stock, (ii) the NASDAQ Stock Market (U.S.) Index and
(iii) the NASDAQ Pharmaceutical Index.
Comparison
of Cumulative Total Return
Among Alnylam Pharmaceuticals, Inc.,
NASDAQ Stock Market (U.S.) Index and NASDAQ Pharmaceuticals
Index
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5/28/2004
|
|
|
12/31/2004
|
|
|
12/30/2005
|
|
|
12/29/2006
|
|
|
12/31/2007
|
|
|
12/31/2008
|
|
Alnylam Pharmaceuticals, Inc.
|
|
|
$
|
100.00
|
|
|
|
$
|
124.29
|
|
|
|
$
|
222.30
|
|
|
|
$
|
356.07
|
|
|
|
$
|
483.86
|
|
|
|
$
|
411.48
|
|
|
Nasdaq Stock Market (U.S.) Index
|
|
|
$
|
100.00
|
|
|
|
$
|
109.70
|
|
|
|
$
|
112.03
|
|
|
|
$
|
123.08
|
|
|
|
$
|
133.48
|
|
|
|
$
|
64.38
|
|
|
Nasdaq Pharmaceutical Index
|
|
|
$
|
100.00
|
|
|
|
$
|
103.32
|
|
|
|
$
|
113.77
|
|
|
|
$
|
111.36
|
|
|
|
$
|
117.11
|
|
|
|
$
|
108.98
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
59
|
|
|
ITEM 6.
|
SELECTED
CONSOLIDATED FINANCIAL DATA
|
The following selected consolidated financial data for each of
the five years in the period ended December 31, 2008 are
derived from our audited consolidated financial statements. The
selected consolidated financial data set forth below should be
read in conjunction with “Management’s Discussion and
Analysis of Financial Condition and Results of Operations”
and the financial statements, and the related Notes, included
elsewhere in this annual report on
Form 10-K.
Historical results are not necessarily indicative of future
results.
Selected
Consolidated Financial Data
(In thousands, except per share data)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
2005
|
|
|
2004
|
|
|
|
|
Statement of Operations Data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net revenues
|
|
$
|
96,163
|
|
|
$
|
50,897
|
|
|
$
|
26,930
|
|
|
$
|
5,716
|
|
|
$
|
4,278
|
|
|
Operating expenses(1)
|
|
|
123,998
|
|
|
|
144,074
|
|
|
|
66,431
|
|
|
|
49,188
|
|
|
|
36,542
|
|
|
Loss from operations
|
|
|
(27,835
|
)
|
|
|
(93,177
|
)
|
|
|
(39,501
|
)
|
|
|
(43,472
|
)
|
|
|
(32,264
|
)
|
|
Net loss
|
|
|
(26,249
|
)
|
|
|
(85,466
|
)
|
|
|
(34,608
|
)
|
|
|
(42,914
|
)
|
|
|
(32,654
|
)
|
|
Net loss attributable to common stockholders
|
|
$
|
(26,249
|
)
|
|
$
|
(85,466
|
)
|
|
$
|
(34,608
|
)
|
|
$
|
(42,914
|
)
|
|
$
|
(35,367
|
)
|
|
Net loss per common share — basic and diluted
|
|
$
|
(0.64
|
)
|
|
$
|
(2.21
|
)
|
|
$
|
(1.09
|
)
|
|
$
|
(1.96
|
)
|
|
$
|
(2.98
|
)
|
|
Weighted average common shares outstanding — basic and
diluted
|
|
|
41,077
|
|
|
|
38,657
|
|
|
|
31,890
|
|
|
|
21,949
|
|
|
|
11,886
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) Non-cash stock-based compensation included in operating
expenses
|
|
$
|
16,382
|
|
|
$
|
14,472
|
|
|
$
|
8,304
|
|
|
$
|
4,597
|
|
|
$
|
4,106
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
2005
|
|
|
2004
|
|
|
|
|
Balance Sheet Data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash, cash equivalents and marketable securities
|
|
$
|
512,709
|
|
|
$
|
455,602
|
|
|
$
|
217,260
|
|
|
$
|
80,002
|
|
|
$
|
46,046
|
|
|
Working capital
|
|
|
342,797
|
|
|
|
314,427
|
|
|
|
199,859
|
|
|
|
63,930
|
|
|
|
41,606
|
|
|
Total assets
|
|
|
554,676
|
|
|
|
493,791
|
|
|
|
240,006
|
|
|
|
98,348
|
|
|
|
66,107
|
|
|
Notes payable
|
|
|
—
|
|
|
|
6,758
|
|
|
|
9,136
|
|
|
|
7,395
|
|
|
|
7,201
|
|
|
Total stockholders’ equity
|
|
|
202,125
|
|
|
|
199,168
|
|
|
|
201,174
|
|
|
|
61,779
|
|
|
|
46,142
|
|
60
|
|
|
ITEM 7.
|
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF
OPERATIONS
|
Overview
We are a biopharmaceutical company developing novel therapeutics
based on RNA interference, or RNAi. RNAi is a naturally
occurring biological pathway within cells for selectively
silencing and regulating the expression of specific genes. Since
many diseases are caused by the inappropriate activity of
specific genes, the ability to silence genes selectively through
RNAi could provide a new way to treat a wide range of human
diseases. We believe that drugs that work through RNAi have the
potential to become a broad new class of drugs, like small
molecule, protein and antibody drugs. Using our intellectual
property and the expertise we have built in RNAi, we are
developing a set of biological and chemical methods and know-how
that we apply in a systematic way to develop RNAi therapeutics
for a variety of diseases.
We are applying our technological expertise to build a pipeline
of RNAi therapeutics to address significant medical needs, many
of which cannot effectively be addressed with small molecules or
antibodies, the current major classes of drugs. Our lead RNAi
therapeutic program, ALN-RSV01, is in Phase II clinical
trials for the treatment of human respiratory syncytial virus,
or RSV, infection, which is reported to be the leading cause of
hospitalization in infants in the United States and also occurs
in the elderly and in immune compromised adults. In February
2008, we reported positive results from our Phase II
experimental RSV infection clinical trial, referred to as the
GEMINI study. In April 2008, we initiated a second Phase II
human clinical trial, which is currently ongoing, to assess the
safety and tolerability of aerosolized ALN-RSV01 versus placebo
in adult lung transplant patients naturally infected with RSV.
We recently formed collaborations with Cubist Pharmaceuticals,
Inc., or Cubist, and Kyowa Hakko Kirin Co., Ltd., or Kyowa
Hakko, for the development and commercialization of products for
RSV. We will jointly develop and commercialize products for RSV
in partnership with Cubist in North America, Cubist has
responsibility for developing and commercializing these products
in the rest of the world outside of Asia, and Kyowa Hakko has
the responsibility for developing and commercializing these
products in Asia.
In December 2008, we submitted an investigational new drug
application, or IND, to the United States Food and Drug
Administration, or FDA, for ALN-VSP, our first systemically
delivered RNAi therapeutic candidate. We are developing ALN-VSP
for the treatment of liver cancers, including hepatocellular
carcinoma and other solid tumors with liver involvement. We
received clearance from the FDA in January 2009 to proceed with
a Phase I study. We expect to initiate this study during the
first half of 2009. The Phase I study will be a multi-center,
open label, dose escalation trial to evaluate the safety,
tolerability, pharmacokinetics and pharmacodynamics of
intravenous ALN-VSP in patients with advanced solid tumors with
liver involvement.
We are also working on a number of programs in pre-clinical
development, including:
|
|
|
| |
•
|
ALN-PCS, an RNAi therapeutic candidate targeting a gene called
proprotein convertase subtilisin/kexin type 9, or PCSK9, for the
treatment of hypercholesterolemia;
|
| |
| |
•
|
ALN-TTR, an RNAi therapeutic candidate targeting the
transthyretin, or TTR, gene, for the treatment of TTR
amyloidosis; and
|
| |
| |
•
|
ALN-HTT, an RNAi therapeutic candidate targeting the huntingtin
gene, for the treatment of Huntington’s disease, which we
are developing in collaboration with Medtronic, Inc., or
Medtronic.
|
We have additional discovery programs for RNAi therapeutics for
the treatment of a broad range of diseases.
In addition, we are working internally and with third-party
collaborators to develop capabilities to deliver our RNAi
therapeutics directly to specific sites of disease, such as the
delivery of ALN-RSV01 to the lungs, which we refer to as Direct
RNAi. We are also working to extend our capabilities to advance
the development of RNAi therapeutics that are administered by
intravenous, subcutaneous or intramuscular approaches, which we
refer to as Systemic RNAi. We have numerous RNAi therapeutic
delivery collaborations and intend to continue to collaborate
with government, academic and corporate third parties to
evaluate different delivery options, including with respect to
Direct RNAi and Systemic RNAi.
61
We rely on the strength of our intellectual property portfolio
relating to the development and commercialization of small
interfering RNAs, or siRNAs, as therapeutics. This includes
ownership of, or exclusive rights to, issued patents and pending
patent applications claiming fundamental features of siRNAs and
RNAi therapeutics as well as those claiming crucial chemical
modifications and promising delivery technologies. We believe
that no other company possesses a portfolio of such broad and
exclusive rights to the patents and patent applications required
for the commercialization of RNAi therapeutics. Given the
importance of our intellectual property portfolio to our
business operations, we intend to vigorously enforce our rights
and defend against any challenges that have arisen or may arise
in this area.
In addition, our expertise in RNAi therapeutics and broad
intellectual property estate have allowed us to form alliances
with leading companies, including Isis Pharmaceuticals, Inc., or
Isis, Medtronic, Novartis Pharma AG, or Novartis, Biogen Idec
Inc., or Biogen Idec, F. Hoffmann-La Roche Ltd, or Roche,
Takeda Pharmaceutical Company Limited, or Takeda, Kyowa Hakko
and Cubist. We have also entered into contracts with government
agencies, including the National Institute of Allergy and
Infectious Diseases, or NIAID, a component of the National
Institutes of Health, or NIH. We have established collaborations
with and, in some instances, received funding from major medical
and disease associations. Finally, to further enable the field
and monetize our intellectual property rights, we also grant
licenses to biotechnology companies for the development and
commercialization of RNAi therapeutics for specified targets in
which we have no direct strategic interest under our
InterfeRxtm
program and to research companies that commercialize RNAi
reagents or services under our research product licenses.
We also seek opportunities to form new ventures in areas outside
our core strategic interest. For example, in 2007, we and Isis
established Regulus Therapeutics LLC, or Regulus Therapeutics, a
company focused on the discovery, development and
commercialization of microRNA-based therapeutics. Because
microRNAs are believed to regulate whole networks of genes that
can be involved in discrete disease processes, microRNA-based
therapeutics represent a possible new approach to target the
pathways of human disease. Given the broad applications for RNAi
technology, we believe additional opportunities exist for new
ventures to be formed.
Alnylam commenced operations in June 2002. We have focused our
efforts since inception primarily on business planning, research
and development, acquiring, filing and expanding intellectual
property rights, recruiting management and technical staff, and
raising capital. Since our inception, we have generated
significant losses. As of December 31, 2008, we had an
accumulated deficit of $252.2 million. Through
December 31, 2008, we have funded our operations primarily
through the net proceeds from the sale of equity securities and
payments we have received under strategic alliances. Through
December 31, 2008, a substantial portion of our total net
revenues have been collaboration revenues derived from our
strategic alliances with Roche, Takeda and Novartis, and from
the United States government in connection with our development
of treatments for hemorrhagic fever viruses, including Ebola. We
expect our revenues to continue to be derived primarily from new
and existing strategic alliances, government and foundation
funding and license fee revenues.
We currently have programs focused in a number of therapeutic
areas. However, we are unable to predict when, if ever, we will
successfully develop or be able to commence sales of any
product. We have never achieved profitability on an annual basis
and we expect to incur additional losses over the next several
years. We expect our net losses to continue due primarily to
research and development activities relating to our drug
development programs, collaborations and other general corporate
activities. We anticipate that our operating results will
fluctuate for the foreseeable future. Therefore,
period-to-period comparisons should not be relied upon as
predictive of the results in future periods. Our sources of
potential funding for the next several years are expected to be
derived primarily from payments under new and existing strategic
alliances, which may include license and other fees, funded
research and development payments and milestone payments,
government and foundation funding and proceeds from the sale of
equity.
Research
and Development
Since our inception, we have focused on drug discovery and
development programs. Research and development expenses
represent a substantial percentage of our total operating
expenses. Our most advanced program is focused on the treatment
of RSV infection and is in Phase II clinical studies. In
January 2009, we received clearance from the FDA to proceed with
a Phase I study of ALN-VSP for the treatment of liver cancers
and
62
potentially other solid tumors. Our other development programs
are focused on the treatment of hypercholesterolemia, TTR
amyloidosis and Huntington’s disease. We also have
discovery programs to develop RNAi therapeutics for the
treatment of a broad range of diseases, such as viral
hemorrhagic fever, including the Ebola virus, progressive
multifocal leukoencephalopathy, or PML, Parkinson’s disease
and many other undisclosed programs, as well as several other
diseases that are the subject of our strategic alliances. In
addition, we are working internally and with third-party
collaborators to develop capabilities to deliver our RNAi
therapeutics both directly to the specific sites of disease and
systemically, and we intend to continue to collaborate with
government, academic and corporate third parties to evaluate
different delivery options.
There is a risk that any drug discovery or development program
may not produce revenue for a variety of reasons, including the
possibility that we will not be able to adequately demonstrate
the safety and efficacy of the product candidate. Moreover,
there are uncertainties specific to any new field of drug
discovery, including RNAi. The successful development of any
product candidate we develop is highly uncertain. Due to the
numerous risks associated with developing drugs, we cannot
reasonably estimate or know the nature, timing and estimated
costs of the efforts necessary to complete the development of,
or the period, if any, in which material net cash inflows will
commence from, any potential product candidate. These risks
include the uncertainty of:
|
|
|
| |
•
|
our ability to progress product candidates into pre-clinical and
clinical trials;
|
| |
| |
•
|
the scope, rate and progress of our pre-clinical trials and
other research and development activities, including those
related to developing safe and effective ways of delivering
siRNAs into cells and tissues;
|
| |
| |
•
|
the scope, rate of progress and cost of any clinical trials we
commence;
|
| |
| |
•
|
clinical trial results;
|
| |
| |
•
|
the cost of filing, prosecuting, defending and enforcing any
patent claims and other intellectual property rights;
|
| |
| |
•
|
the terms, timing and success of any collaborative, licensing
and other arrangements that we may establish;
|
| |
| |
•
|
the cost, timing and success of regulatory filings and approvals;
|
| |
| |
•
|
the cost and timing of establishing sufficient sales, marketing
and distribution capabilities;
|
| |
| |
•
|
the cost and timing of establishing sufficient clinical and
commercial supplies of any products that we may develop; and
|
| |
| |
•
|
the effect of competing technological and market developments.
|
Any failure to complete any stage of the development of any
potential products in a timely manner could have a material
adverse effect on our operations, financial position and
liquidity. A discussion of some of the risks and uncertainties
associated with completing our projects on schedule, or at all,
and the potential consequences of failing to do so, are set
forth in Item 1A above under the heading “Risk
Factors.”
Strategic
Alliances
A significant component of our business plan is to enter into
strategic alliances and collaborations with pharmaceutical and
biotechnology companies, academic institutions, research
foundations and others, as appropriate, to gain access to
funding, capabilities, technical resources and intellectual
property to further our development efforts and to generate
revenues. Our collaboration strategy is to form
(1) non-exclusive platform alliances where our
collaborators obtain access to our capabilities and intellectual
property to develop their own RNAi therapeutic products; and
(2) 50-50
co-development
and/or
ex-U.S. market geographic partnerships on specific RNAi
therapeutic programs. We have entered into broad, non-exclusive
platform license agreements with Roche and Takeda, under which
we will also collaborate with each of Roche and Takeda on RNAi
drug discovery for one or more disease targets. We are pursuing
50-50
co-development programs with Cubist and Medtronic for the
development and commercialization of ALN-RSV and ALN-HTT,
respectively. In addition, we have entered into a product
alliance with Kyowa Hakko for the development and
commercialization of ALN-RSV in territories not
63
covered by the Cubist agreement, which include Japan and other
markets in Asia. We also have discovery and development
alliances with Isis, Novartis and Biogen Idec.
We also seek opportunities to form new ventures in areas outside
our core strategic interest. For example, we formed Regulus
Therapeutics, together with Isis, to capitalize on our
technology and intellectual property in the field of
microRNA-based therapeutics. Given the broad applications for
RNAi technology, we believe additional opportunities exist for
new ventures to be formed.
To generate revenues from our intellectual property rights, we
also grant licenses to biotechnology companies under our
InterfeRx program for the development and commercialization of
RNAi therapeutics for specified targets in which we have no
direct strategic interest. We also license key aspects of our
intellectual property to companies active in the research
products and services market, which includes the manufacture and
sale of reagents. Our InterfeRx and research product licenses
aim to generate modest near-term revenues that we can re-invest
in the development of our proprietary RNAi therapeutics
pipeline. As of January 31, 2009, we had granted such
licenses, on both an exclusive and nonexclusive basis, to
approximately 20 companies.
Since delivery of RNAi therapeutics remains a major objective of
our research activities, we also look to form collaboration and
licensing agreements with other companies and academic
institutions to gain access to delivery technologies. For
example, we have formed agreements with Tekmira Pharmaceuticals
Corporation, or Tekmira, and Massachusetts Institute of
Technology, or MIT, among others, to focus on various delivery
strategies. We have also entered into license agreements with
Isis, Max Planck Innovation, Tekmira and MIT, as well as a
number of other entities, to obtain rights to important
intellectual property in the field of RNAi.
Finally, we seek funding for the development of our proprietary
RNAi therapeutics pipeline from foundations and government
sources. In 2006, NIAID awarded us a contract to advance the
development of a broad spectrum RNAi anti-viral therapeutic
against hemorrhagic fever virus, including the Ebola virus. In
2007, the Defense Threat Reduction Agency, or DTRA, an agency of
the United States Department of Defense, awarded us a contract
to advance the development of a broad spectrum RNAi anti-viral
therapeutic for hemorrhagic fever virus, which contract ended in
February 2009. In addition, we have obtained funding for
pre-clinical discovery programs from organizations such as The
Michael J. Fox Foundation.
In September 2007, we terminated our amended and restated
research collaboration and license agreement with
Merck & Co., Inc., or Merck. Pursuant to the
termination agreement, all license grants of intellectual
property to develop, manufacture
and/or
commercialize RNAi therapeutic products under the amended and
restated research collaboration and license agreement ceased as
of the date of the termination agreement, subject to certain
specified exceptions. The termination agreement further provides
that, subject to certain conditions, we and Merck will each
retain sole ownership and rights in our own intellectual
property.
Platform
Alliances.
Roche. In July 2007, we and, for limited
purposes, Alnylam Europe AG, or Alnylam Europe, entered into a
license and collaboration agreement with Roche. Under the
license and collaboration agreement, which became effective in
August 2007, we granted Roche a non-exclusive license to our
intellectual property to develop and commercialize therapeutic
products that function through RNAi, subject to our existing
contractual obligations to third parties. The license is
initially limited to the therapeutic areas of oncology,
respiratory diseases, metabolic diseases and certain liver
diseases, and may be expanded to include up to 18 additional
therapeutic areas, comprising all other fields of human disease,
as identified and agreed upon by the parties, upon payment to us
by Roche of an additional $50.0 million for each additional
therapeutic area, if any.
In consideration for the rights granted to Roche under the
license and collaboration agreement, Roche paid us
$273.5 million in upfront cash payments. In addition, in
exchange for our contributions under the collaboration
agreement, for each RNAi therapeutic product successfully
developed by Roche, its affiliates or sublicensees under the
collaboration agreement, if any, we are entitled to receive
milestone payments upon achievement of specified development and
sales events, totaling up to an aggregate of $100.0 million
per therapeutic target, together with royalty payments based on
worldwide annual net sales, if any. Due to the uncertainty of
pharmaceutical
64
development and the high historical failure rates generally
associated with drug development, we may not receive any
milestone or royalty payments from Roche.
Under the license and collaboration agreement, we and Roche also
agreed to collaborate on the discovery of RNAi therapeutic
products directed to one or more disease targets, subject to our
contractual obligations to third parties. The discovery
collaboration between Roche and us will be governed by a joint
steering committee for a period of five years that is comprised
of an equal number of representatives from each party. In
exchange for our contributions to the collaboration, Roche will
be required to make additional milestone and royalty payments to
us.
In connection with the execution of the license and
collaboration agreement, we executed a common stock purchase
agreement with Roche Finance Ltd, or Roche Finance, an affiliate
of Roche. Under the terms of the common stock purchase
agreement, in August 2007, Roche Finance purchased
1,975,000 shares of our common stock at $21.50 per share,
for an aggregate purchase price of $42.5 million.
In connection with the execution of the license and
collaboration agreement and the common stock purchase agreement,
we also executed a stock purchase agreement with Alnylam Europe
and Roche Beteiligungs GmbH, or Roche Germany, an affiliate of
Roche. Under the terms of the Alnylam Europe stock purchase
agreement, we created a new, wholly-owned German limited
liability company, Roche Kulmbach, into which substantially all
of the non-intellectual property assets of Alnylam Europe were
transferred, and Roche Germany purchased from us all of the
issued and outstanding shares of Roche Kulmbach for an aggregate
purchase price of $15.0 million. The Alnylam Europe stock
purchase agreement included transition services that were
performed by Roche Kulmbach employees at various levels through
August 2008. We reimbursed Roche for these services at an
agreed-upon
rate.
In connection with the license and collaboration agreement and
the common stock purchase agreement, we paid $27.5 million
in license fees to our licensors, primarily Isis, in accordance
with the applicable license agreements with those parties.
Takeda. In May 2008, we entered into a license
and collaboration agreement with Takeda to pursue the
development and commercialization of RNAi therapeutics. Under
the Takeda agreement, we granted to Takeda a non-exclusive,
worldwide, royalty-bearing license to our intellectual property
to develop, manufacture, use and commercialize RNAi
therapeutics, subject to our existing contractual obligations to
third parties. The license initially is limited to the fields of
oncology and metabolic disease and may be expanded at
Takeda’s option to include other therapeutic areas, subject
to specified conditions. Under the Takeda agreement, Takeda will
be our exclusive platform partner in the Asian territory, as
defined in the agreement, for a period of five years.
In consideration for the rights granted to Takeda under the
Takeda agreement, Takeda agreed to pay us $150.0 million in
upfront and near-term technology transfer payments. In addition,
we have the option, exercisable until the start of
Phase III development, to opt-in under a
50-50 profit
sharing agreement to the development and commercialization in
the United States of up to four Takeda licensed products, and
would be entitled to opt-in rights for two additional products
for each additional field expansion, if any, elected by Takeda
under the Takeda agreement. In June 2008, Takeda paid us an
upfront payment of $100.0 million. Takeda is also required
to make the additional $50.0 million in payments to us upon
achievement of specified technology transfer milestones,
$20.0 million of which was achieved in September 2008 and
paid in October 2008, $20.0 million of which is required to
be paid within 12 to 24 months of execution of the
agreement and $10.0 million of which is required to be paid
within 24 to 36 months of execution of the agreement. If
Takeda elects to expand its license to additional therapeutic
areas, Takeda will be required to pay us $50.0 million for
each of up to approximately 20 total additional fields selected,
comprising all other fields of human disease, as identified and
agreed upon by the parties. In addition, for each RNAi
therapeutic product successfully developed by Takeda, its
affiliates and sublicensees, if any, we are entitled to receive
specified development and commercialization milestones, totaling
up to $171.0 million per product, together with royalty
payments based on worldwide annual net sales, if any. Due to the
uncertainty of pharmaceutical development and the high
historical failure rates generally associated with drug
development, we may not receive any milestone or royalty
payments from Takeda.
Pursuant to the Takeda agreement, we and Takeda have also agreed
to collaborate on the research of RNAi therapeutics directed to
one or two disease targets agreed to by the parties, subject to
our existing contractual obligations with third parties. Takeda
also has the option, subject to certain conditions, to
collaborate with us on the
65
research and development of RNAi drug delivery technology for
targets agreed to by the parties. In addition, Takeda has a
right of first negotiation for the development and
commercialization of our RNAi therapeutic products in the Asian
territory, excluding our ALN-RSV01 program. In addition to our
50-50 profit
sharing option, we have a similar right of first negotiation to
participate with Takeda in the development and commercialization
in the United States of licensed products. The collaboration is
governed by a joint technology transfer committee, or JTTC, a
joint research collaboration committee, or JRCC, and a joint
delivery collaboration committee, or JDCC, each of which is
comprised of an equal number of representatives from each party.
In connection with the Takeda agreement, we paid
$5.0 million of license fees to our licensors, primarily
Isis, in accordance with the applicable license agreements with
those parties.
Discovery
and Development Alliances.
Isis. In March 2004, we entered into a
collaboration and license agreement with Isis, a leading
developer of antisense oligonucleotide drugs that target RNA.
The Isis agreement significantly enhanced our intellectual
property position with respect to RNA-based therapeutic products
and our ability to develop siRNAs for RNAi therapeutic products,
and provided us with the opportunity to defer investment in
manufacturing technology. Isis granted us licenses to its
current and future patents and patent applications relating to
chemistry and to RNA-targeting mechanisms for the research,
development and commercialization of siRNA products. We have the
right to use Isis technologies in our development programs or in
collaborations, and Isis has agreed not to grant licenses under
these patents to any other organization for any siRNA products
designed to work through an RNAi mechanism, except in the
context of a collaboration in which Isis plays an active role.
The licenses that we have granted to Isis are non-exclusive
licenses to our current and future patents and patent
applications relating to RNA-targeting mechanisms and to
chemistry for research use. We have also granted Isis the
non-exclusive right to develop and commercialize siRNA products
against a limited number of targets. In addition, we have
granted Isis non-exclusive rights to our patents and patent
applications for research, development and commercialization of
antisense RNA products.
Under the terms of the Isis agreement, we paid Isis an upfront
license fee of $5.0 million. We also agreed to pay Isis
milestone payments, totaling up to approximately
$3.4 million, upon the occurrence of specified development
and regulatory events, and royalties on sales, if any, for each
product that we or a collaborator develops using Isis
intellectual property. In addition, we agreed to pay to Isis a
percentage of specified fees from strategic collaborations we
may enter into that include access to the Isis intellectual
property.
In conjunction with the agreement, Isis made a
$10.0 million equity investment in us. In addition, Isis
has agreed to pay us, per therapeutic target, a license fee of
$0.5 million, and milestone payments totaling approximately
$3.4 million, payable upon the occurrence of specified
development and regulatory events, and royalties on sales, if
any, for each product developed by Isis or a collaborator that
utilizes our intellectual property. Isis has the right to elect
up to ten non-exclusive target licenses under the agreement and
has the right to purchase one additional non-exclusive target
per year during the term of the collaboration.
The Isis agreement also gives us an option to use Isis’
manufacturing services for RNA-based therapeutic products. In
addition, under the Isis agreement, we have the exclusive right
to grant sub-licenses for Isis technology to third parties with
whom we are not collaborating. We may include these sub-licenses
in our non-exclusive platform licenses and our InterfeRx
licenses. If a license includes rights to Isis’
intellectual property, we will share revenues from that license
equally with Isis.
Novartis. We have formed two alliances with
Novartis. We refer to the first of these, which was initiated in
September 2005, as the broad Novartis alliance, and to the
second, which was initiated in February 2006, as the Novartis
flu alliance.
In connection with the broad Novartis alliance, we entered into
a series of transactions with Novartis beginning in September
2005. At that time, we and Novartis executed a stock purchase
agreement and an investor rights agreement. When the
transactions contemplated by the stock purchase agreement closed
in October 2005, the investor rights agreement became effective
and we and Novartis executed a research collaboration and
license agreement. The collaboration and license agreement had
an initial term of three years, with an option for two
66
additional one-year extensions at the election of Novartis. In
July 2008, Novartis elected to extend the initial term for an
additional year, through October 2009. Novartis retains the
right to extend the term for a second additional year, which
right must be exercised no later than July 2009.
Under the terms of the collaboration and license agreement, the
parties will work together on selected targets, as defined in
the collaboration and license agreement, to discover and develop
therapeutics based on RNAi. In consideration for rights granted
to Novartis under the collaboration and license agreement,
Novartis made an upfront payment of $10.0 million to us in
October 2005, partly to reimburse prior costs incurred by us to
develop in vivo RNAi technology. The collaboration and
license agreement also includes terms under which Novartis will
provide us with research funding and development and sales
milestone payments as well as royalties on annual net sales of
products resulting from the collaboration, if any. The amount of
research funding provided by Novartis under the collaboration
and license agreement during the research term is dependent upon
the number of active programs on which we are collaborating with
them at any given time and the number of our employees that are
working on those programs, in respect of which Novartis
reimburses us at an agreed upon rate. Under the terms of the
collaboration and license agreement, Novartis has the right to
select up to 30 exclusive targets to include in the
collaboration, which number may be increased to 40 under certain
circumstances and upon additional payments. For RNAi therapeutic
products successfully developed under the agreement, if any, we
would be entitled to receive milestone payments upon achievement
of certain specified development and annual net sales events, up
to an aggregate of $75.0 million per therapeutic product.
Due to the uncertainty of pharmaceutical development and the
high historical failure rates generally associated with drug
development, we may not receive any milestone or royalty
payments from Novartis.
The collaboration and license agreement also provides Novartis
with a non-exclusive option to integrate into its operations our
intellectual property relating to RNAi technology, excluding any
technology related to delivery of nucleic acid based molecules.
Novartis may exercise this integration option at any point
during the research term, as defined in the collaboration and
license agreement. The research term expires in October 2009 and
may be extended until October 2010 at Novartis’ election.
In connection with the exercise of the integration option,
Novartis would be required to make additional payments to us
totaling $100.0 million, payable in full at the time of
exercise, which payments would include an option exercise fee, a
milestone based on the overall success of the collaboration and
pre-paid milestones and royalties that could become due as a
result of future development of products using our technology.
In addition, under this license grant, Novartis may be required
to make milestone and royalty payments to us in connection with
the successful development and commercialization of RNAi
therapeutic products, if any. The license grant under the
integration option, if exercised by Novartis, would be
structured similarly to our non-exclusive platform licenses with
Roche and Takeda.
Under the terms of the stock purchase agreement, in October
2005, Novartis purchased 5,267,865 shares of our common
stock at a purchase price of $11.11 per share for an aggregate
purchase price of $58.5 million, which, after such
issuance, represented 19.9% of our outstanding common stock as
of the date of issuance. In addition, under the investor rights
agreement, we granted Novartis rights to acquire additional
equity securities in the event that we propose to sell or issue
any equity securities, subject to specified exceptions, as
described in the investor rights agreement, such that Novartis
would be able to maintain its then-current ownership percentage
in our outstanding common stock. Pursuant to terms of the
investor rights agreement, in May 2008, Novartis purchased
213,888 shares of our common stock at a purchase price of
$25.29 per share resulting in a payment to us of
$5.4 million. At December 31, 2008, Novartis owned
13.2% of our outstanding common stock.
In February 2006, we entered into the Novartis flu alliance.
Under the terms of the Novartis flu alliance, we and Novartis
have joint responsibility for the development of RNAi
therapeutics for pandemic flu. This program was stopped and
currently there are no specific resource commitments for this
program.
Biogen Idec. In September 2006, we entered
into a collaboration and license agreement with Biogen Idec. The
collaboration is focused on the discovery and development of
therapeutics based on RNAi for the potential treatment of PML.
Under the terms of the Biogen Idec agreement, we granted Biogen
Idec an exclusive license to distribute, market and sell certain
RNAi therapeutics to treat PML and Biogen Idec has agreed to
fund all related research and development activities. We
received an upfront $5.0 million payment from Biogen Idec.
In addition, upon the successful development and utilization of
a product resulting from the collaboration, if any, Biogen Idec
67
would be required to pay us milestone payments, totaling
$51.0 million, and royalty payments on sales, if any. Due
to the uncertainty of pharmaceutical development and the high
historical failure rates generally associated with drug
development, we may not receive any milestone or royalty
payments from Biogen Idec. The pace and scope of future
development of this program is the responsibility of Biogen
Idec. We expect to expend limited resources on this program in
2009.
Product
Alliances.
Medtronic. In July 2007, we entered into an
amended and restated collaboration agreement with Medtronic to
pursue the development of therapeutic products for the treatment
of neurodegenerative disorders. The amended and restated
collaboration agreement supersedes the collaboration agreement
entered into by the parties in February 2005, and continues the
existing collaboration between the parties focusing on the
delivery of RNAi therapeutics to specific areas of the brain
using implantable infusion systems.
Under the terms of the amended and restated collaboration
agreement, we and Medtronic are continuing our existing
development program focused on developing a combination
drug-device product for the treatment of Huntington’s
disease. In addition, we and Medtronic may jointly agree to
collaborate on additional product development programs for the
treatment of other neurodegenerative diseases, which can be
addressed by the delivery of siRNAs discovered and developed
using our RNAi therapeutics platform to the human nervous system
through implantable infusion devices developed by Medtronic. We
will be responsible for supplying the siRNA component and
Medtronic will be responsible for supplying the device component
of any product resulting from the collaboration.
With respect to the initial product development program focused
on Huntington’s disease, each party is funding 50% of the
development efforts for the United States while Medtronic is
responsible for funding development efforts outside the United
States. Medtronic will commercialize any resulting products and
pay royalties to us based on net sales of any such products, if
any, which royalties in the United States are designed to
approximate 50% of the profit associated with the sale of such
product and which royalties in Europe are similar to more
traditional pharmaceutical royalties, in that they are intended
to reflect each party’s contribution.
Each party has the right to opt out of its obligation to fund
the program under the agreement at certain stages, and the
agreement provides for revised economics based on the timing of
any such opt out. Other than pursuant to the initial product
development program, and subject to specified exceptions,
neither party may research, develop, manufacture or
commercialize products that use implanted infusion devices for
the direct delivery of siRNAs to the human nervous system to
treat Huntington’s disease during the term of such program.
Kyowa Hakko. In June 2008, we entered into a
license and collaboration agreement with Kyowa Hakko. Under the
Kyowa Hakko agreement, we granted Kyowa Hakko an exclusive
license to our intellectual property in Japan and other markets
in Asia for the development and commercialization of ALN-RSV01
for the treatment of RSV infection. The Kyowa Hakko agreement
also covers additional RSV-specific RNAi therapeutic compounds
that comprise the ALN-RSV program. We retain all development and
commercialization rights worldwide excluding the licensed
territory.
Under the terms of the Kyowa Hakko agreement, in June 2008,
Kyowa Hakko paid us an upfront cash payment of
$15.0 million. In addition, Kyowa Hakko is required to make
payments to us upon achievement of specified development and
sales milestones totaling up to $78.0 million, and royalty
payments based on annual net sales, if any, of ALN-RSV01 by
Kyowa Hakko, its affiliates and sublicensees in the licensed
territory. Due to the uncertainty of pharmaceutical development
and the high historical failure rates generally associated with
drug development, we may not receive any milestone or royalty
payments from Kyowa Hakko.
Our collaboration with Kyowa Hakko is governed by a joint
steering committee that is comprised of an equal number of
representatives from each party. Under the agreement, Kyowa
Hakko is establishing a development plan for ALN-RSV01 relating
to the development activities to be undertaken in the licensed
territory, with the initial focus on Japan. Kyowa Hakko is
responsible, at its expense, for all development activities
under the development plan that are reasonably necessary for the
regulatory approval and commercialization of ALN-RSV01 in Japan
and the rest of the licensed territory. We will be responsible
for supply of the product to Kyowa Hakko under a supply
68
agreement unless Kyowa Hakko elects, prior to the first
commercial sale of the product in the licensed territory, to
manufacture the product itself or arrange for a third party to
manufacture the product.
Cubist. In January 2009, we entered into a
license and collaboration agreement with Cubist to develop and
commercialize therapeutic products based on certain of our RNAi
technology for the treatment of RSV, including ALN-RSV01, which
is currently in Phase II clinical development for the
treatment of RSV infection in adult lung transplant patients, as
well as several other second-generation RNAi-based RSV
inhibitors, which currently are in pre-clinical studies.
Under the terms of the Cubist agreement, we and Cubist will
share responsibility for developing licensed products in North
America and will each bear one-half of the related development
costs. Our collaboration with Cubist for the development of
licensed products in North America will be governed by a joint
steering committee comprised of an equal number of
representatives from each party. Cubist will have the sole right
to commercialize licensed products in North America with costs
associated with such activities and any resulting profits or
losses to be split equally between us and Cubist. Throughout the
rest of the world, referred to as the Royalty Territory,
excluding Asia, where we have previously partnered our ALN-RSV
program with Kyowa Hakko, Cubist will have an exclusive,
royalty-bearing license to develop and commercialize licensed
products.
In consideration for the rights granted to Cubist under the
agreement, in January 2009, Cubist paid us an upfront cash
payment of $20.0 million. Cubist also has an obligation
under the agreement to pay us milestone payments, totaling up to
an aggregate of $82.5 million, upon the achievement of
specified development and sales events in the Royalty Territory.
In addition, if licensed products are successfully developed,
Cubist will be required to pay us double digit royalties on net
sales of licensed products in the Royalty Territory, if any,
subject to offsets under certain circumstances. Upon achievement
of certain development milestones, we will have the right to
convert the North American co-development and profit sharing
arrangement into a royalty-bearing license and, in addition to
royalties on net sales in North America, will be entitled to
receive additional milestone payments totaling up to an
aggregate of $130.0 million upon achievement of specified
development and sales events in North America, subject to the
timing of the conversion by us and the regulatory status of a
licensed product at the time of conversion. If we make the
conversion to a royalty-bearing license with respect to North
America, then North America becomes part of the Royalty
Territory. Due to the uncertainty of pharmaceutical development
and the high historical failure rates generally associated with
drug development, we may not receive any milestone or royalty
payments from Cubist.
Delivery
Initiatives
We are working to extend our capabilities in developing
technology to achieve effective and safe delivery of RNAi
therapeutics to a broad spectrum of organ and tissue types. In
connection with these efforts, we have entered into a number of
agreements to evaluate and gain access to certain delivery
technologies. In some instances, we are also providing funding
to support the advancement of these delivery technologies.
For example, in May of 2007, we entered into an agreement with
the MIT, Center for Cancer Research under which we are
sponsoring an exclusive five-year research program focused on
the delivery of RNAi therapeutics. In addition, during 2007, we
obtained an exclusive worldwide license to the liposomal
delivery formulation technology of Tekmira for the discovery,
development and commercialization of lipid-based nanoparticle
formulations for the delivery of RNAi therapeutics. In May 2008,
Tekmira acquired Protiva Biotherapeutics Inc., or Protiva. In
connection with this acquisition, we entered into new agreements
with Tekmira and Protiva, which provide us access to key
existing and future technology and intellectual property for the
systemic delivery of RNAi therapeutics with liposomal delivery
technologies. Under the new agreements with Tekmira and Protiva,
we continue to have exclusive rights to the Semple
(U.S. Patent No. 6,858,225) and Wheeler
(U.S. Patent Nos. 5,976,567 and 6,815,432) patents for
RNAi, which we believe are critical for the use of cationic
liposomal delivery technology.
As noted above, we are developing ALN-VSP, a systemically
delivered RNAi therapeutic candidate, for the treatment of liver
cancers, including hepatocellular carcinoma and other solid
tumors with liver involvement. ALN-VSP comprises two siRNAs
formulated using Tekmira’s stable nucleic acid-lipid
particles, or SNALP, technology. We also have rights to use
SNALP technology in the advancement of our other systemically
delivered RNAi
69
therapeutic programs, including ALN-PCS for the treatment of
hypercholesterolemia and ALN-TTR for the treatment of TTR
amyloidosis.
In connection with Tekmira’s acquisition of Protiva, in May
2008 we made an equity investment of $5.0 million in
Tekmira, purchasing 2,083,333 shares of Tekmira common
stock at a price of $2.40 per share, which represented a premium
of $1.00 per share, or an aggregate of $2.1 million. This
premium was calculated on the difference between the purchase
price and the closing price of Tekmira’s common stock on
the effective date of the acquisition. We allocated this
$2.1 million premium to the expansion of our access to key
technology and intellectual property rights and, accordingly,
recorded a charge to research and development expense during the
year ended December 31, 2008. During 2008, we recorded an
impairment charge of $1.6 million related to our investment
in Tekmira, as the decrease in the fair value of this investment
was deemed to be other than temporary. In connection with these
transactions, we and Tekmira cancelled our $5.0 million
capital equipment loan to Tekmira, which was never drawn down by
Tekmira.
We have other RNAi therapeutic delivery collaborations and
intend to continue to collaborate with government, academic and
corporate third parties to evaluate different delivery
technologies, including with respect to Direct RNAi and Systemic
RNAi.
Government
Funding
NIH. In September 2006, NIAID awarded us a
contract for up to $23.0 million over four years to advance
the development of a broad spectrum RNAi anti-viral therapeutic
for hemorrhagic fever virus, including the Ebola virus. The
Ebola virus can cause a severe, often fatal infection, and poses
a potential biological safety risk and bioterrorism threat. Of
the $23.0 million in funding, the government initially
committed to pay us up to $14.2 million over the first two
years of the contract. In June 2008, as a result of the progress
of the program, the government awarded us an additional
$7.5 million, to be paid through September 2009 for the
third year of the contract, together with any remaining funds
carried over from the funding allocated for the first two years
of the contract.
Department of Defense. In August 2007, DTRA
awarded us a contract to advance the development of a broad
spectrum RNAi anti-viral therapeutic for hemorrhagic fever
virus. The government initially committed to pay us up to
$10.9 million through February 2009, which included a
six-month extension granted by DTRA in July 2008. Following a
program review in early 2009, we and DTRA have determined to end
this program and accordingly, the remaining funds of up to
$27.7 million will not be accessed.
microRNAi-based
Therapeutics
Regulus Therapeutics. In September 2007, we
and Isis established Regulus Therapeutics, a company focused on
the discovery, development and commercialization of
microRNA-based therapeutics. Regulus Therapeutics combines our
and Isis’ technologies, know-how and intellectual property
relating to microRNA-based therapeutics. Since microRNAs are
believed to regulate the expression of broad networks of genes
and biological pathways, microRNA-based therapeutics define a
new and potentially high-impact strategy to target multiple
points on disease pathways. Regulus Therapeutics, which had
initially been established as a limited liability company,
converted to a C corporation as of January 2, 2009 and
changed its name to Regulus Therapeutics Inc.. We and Isis
continue to own 49% and 51%, respectively, of Regulus
Therapeutics. Regulus Therapeutics continues to operate as an
independent company with a separate board of directors,
scientific advisory board and management team.
Regulus Therapeutics’ most advanced program, which is in
pre-clinical research, is a microRNA-based therapeutic candidate
that targets miR-122, an endogenous host gene required for viral
infection by the hepatitis C virus, or HCV. HCV infection
is a significant disease worldwide, for which emerging therapies
target viral genes and, therefore, are prone to viral
resistance. Regulus Therapeutics is also pursuing a program that
targets miR-21. Pre-clinical studies by Regulus Therapeutics and
collaborators have shown that miR-21 is implicated in several
therapeutic areas, including heart failure and fibrosis. In
addition to these programs, Regulus Therapeutics is also
actively exploring additional areas for development of
microRNA-based therapeutics, including cancer, other viral
diseases, metabolic disorders and inflammatory diseases.
70
In April 2008, Regulus Therapeutics entered into a worldwide
strategic alliance with GlaxoSmithKline, or GSK, to discover,
develop and market novel microRNA-targeted therapeutics to treat
inflammatory diseases such as rheumatoid arthritis and
inflammatory bowel disease. In connection with this alliance,
Regulus Therapeutics received $20.0 million in upfront
payments from GSK, including a $15.0 million option fee and
a loan of $5.0 million evidenced by a promissory note
(guaranteed by Isis and us) that will convert into Regulus
Therapeutics common stock under certain specified circumstances.
Regulus Therapeutics could be eligible to receive development,
regulatory and sales milestone payments for each of the four
microRNA-targeted therapeutics discovered and developed as part
of the alliance, and would also receive royalty payments on
worldwide sales of products resulting from the alliance, if any.
Critical
Accounting Policies and Estimates
Our discussion and analysis of our financial condition and
results of operations is based on our consolidated financial
statements, which have been prepared in accordance with
accounting principles generally accepted in the United States.
The preparation of our consolidated financial statements
requires us to make estimates and judgments that affect the
reported amounts of assets, liabilities, revenues and expenses
and disclosure of contingent liabilities in our consolidated
financial statements. Actual results may differ from these
estimates under different assumptions or conditions and could
have a material impact on our reported results. While our
significant accounting policies are more fully described in the
Notes to our consolidated financial statements included
elsewhere in this annual report on
Form 10-K,
we believe the following accounting policies to be the most
critical in understanding the judgments and estimates we use in
preparing our consolidated financial statements:
Revenue
Recognition
Our business strategy includes entering into collaborative
license and development agreements with biotechnology and
pharmaceutical companies for the development and
commercialization of our product candidates. The terms of the
agreements typically include non-refundable license fees,
funding of research and development, payments based upon
achievement of clinical and pre-clinical development milestones
and royalties on product sales. We follow the provisions of the
Securities and Exchange Commission’s Staff Accounting
Bulletin No. 104, “Revenue Recognition,”
Emerging Issues Task Force, or EITF, Issue
No. 00-21,
“Accounting for Revenue Arrangements with Multiple
Deliverables,” or
EITF 00-21,
EITF Issue
No. 99-19,
“Reporting Revenue Gross as a Principal Versus Net as an
Agent,” and EITF Issue
No. 01-9,
“Accounting for Consideration Given by a Vendor to a
Customer (Including a Reseller of the Vendor’s
Products),” or
EITF 01-9.
Non-refundable license fees are recognized as revenue upon
delivery of the license only if we have a contractual right to
receive such payment, the contract price is fixed or
determinable, the collection of the resulting receivable is
reasonably assured and we have no further performance
obligations under the license agreement. Multiple element
arrangements, such as license and development arrangements, are
analyzed to determine whether the deliverables, which often
include a license and performance obligations such as research
and steering committee services, can be separated or whether
they must be accounted for as a single unit of accounting in
accordance with
EITF 00-21.
We recognize upfront license payments as revenue upon delivery
of the license only if the license has stand-alone value and the
fair value of the undelivered performance obligations, typically
including research
and/or
steering committee services, can be determined. If the fair
value of the undelivered performance obligations can be
determined, such obligations would then be accounted for
separately as performed. If the license is considered to either
not have stand-alone value or have stand-alone value but the
fair value of any of the undelivered performance obligations
cannot be determined, the arrangement would then be accounted
for as a single unit of accounting and the license payments and
payments for performance obligations are recognized as revenue
over the estimated period of when the performance obligations
are performed.
Whenever we determine that an arrangement should be accounted
for as a single unit of accounting, we must determine the period
over which the performance obligations will be performed and
revenue will be recognized. Revenue will be recognized using
either a proportional performance or straight-line method. We
recognize revenue using the proportional performance method when
we can reasonably estimate the level of effort required to
complete our performance obligations under an arrangement and
such performance obligations are provided on a best-efforts
basis. Direct labor hours or full-time equivalents are typically
used as the measure of performance.
71
Revenue recognized under the proportional performance method
would be determined by multiplying the total payments under the
contract, excluding royalties and payments contingent upon
achievement of substantive milestones, by the ratio of level of
effort incurred to date to estimated total level of effort
required to complete our performance obligations under the
arrangement. Revenue is limited to the lesser of the cumulative
amount of payments received or the cumulative amount of revenue
earned, as determined using the proportional performance method,
as of the period ending date.
If we cannot reasonably estimate the level of effort required to
complete our performance obligations under an arrangement, then
revenue under the arrangement will be recognized as revenue on a
straight-line basis over the period we expect to complete our
performance obligations. Revenue is limited to the lesser of the
cumulative amount of payments received or the cumulative amount
of revenue earned, as determined using the straight-line method,
as of the period ending date.
Significant management judgment is required in determining the
level of effort required under an arrangement and the period
over which we are expected to complete our performance
obligations under an arrangement. Steering committee services
that are not inconsequential or perfunctory and that are
determined to be performance obligations are combined with other
research services or performance obligations required under an
arrangement, if any, in determining the level of effort required
in an arrangement and the period over which we expect to
complete our aggregate performance obligations.
Many of our collaboration agreements entitle us to additional
payments upon the achievement of performance-based milestones.
If the achievement of a milestone is considered probable at the
inception of the collaboration, the related milestone payment is
included with other collaboration consideration, such as
up-front fees and research funding, in our revenue model.
Milestones that involve substantial effort on our part and the
achievement of which are not considered probable at the
inception of the collaboration are considered “substantive
milestones.” Substantive milestones are included in our
revenue model when achievement of the milestone is considered
probable. As future substantive milestones are achieved, a
portion of the milestone payment, equal to the percentage of the
performance period completed when the milestone is achieved,
multiplied by the amount of the milestone payment, will be
recognized as revenue upon achievement of such milestone. The
remaining portion of the milestone will be recognized over the
remaining performance period using the proportional performance
or
straight-line
method. Milestones that are tied to regulatory approval are not
considered probable of being achieved until such approval is
received. Milestones tied to counter-party performance are not
included in our revenue model until the performance conditions
are met.
For revenue generating arrangements where we, as a vendor,
provide consideration to a licensor or collaborator, as a
customer, we apply the provisions of
EITF 01-9.
EITF 01-9
addresses the accounting for revenue arrangements where both the
vendor and the customer make cash payments to each other for
services
and/or
products. A payment to a customer is presumed to be a reduction
of the selling price unless we receive an identifiable benefit
for the payment and we can reasonably estimate the fair value of
the benefit received. Payments to a customer that are deemed a
reduction of selling price are recorded first as a reduction of
revenue, to the extent of both cumulative revenue recorded to
date and probable future revenues, which include any unamortized
deferred revenue balances, under all arrangements with such
customer, and then as an expense. Payments that are not deemed
to be a reduction of selling price would be recorded as an
expense.
Amounts received prior to satisfying the above revenue
recognition criteria are recorded as deferred revenue in the
accompanying consolidated balance sheets. Amounts not expected
to be recognized within the next twelve months are classified as
long-term deferred revenue. As of December 31, 2008, we had
short-term and long-term deferred revenue of $79.9 million
and $250.1 million, respectively, related to our
collaborations.
Although we follow detailed guidelines in measuring revenue,
certain judgments affect the application of our revenue policy.
For example, in connection with our existing collaboration
agreements, we have recorded on our balance sheet short-term and
long-term deferred revenue based on our best estimate of when
such revenue will be recognized. Short-term deferred revenue
consists of amounts that are expected to be recognized as
revenue in the next twelve months. Amounts that we expect will
not be recognized prior to the next twelve months are classified
as long-term deferred revenue. However, this estimate is based
on our current operating plan and, if our operating plan should
change in the future, we may recognize a different amount of
deferred revenue over the next twelve-month period.
72
The estimate of deferred revenue also reflects management’s
estimate of the periods of our involvement in certain of our
collaborations. Our performance obligations under these
collaborations consist of participation on steering committees
and the performance of other research and development services.
In certain instances, the timing of satisfying these obligations
can be difficult to estimate. Accordingly, our estimates may
change in the future. Such changes to estimates would result in
a change in revenue recognition amounts. If these estimates and
judgments change over the course of these agreements, it may
affect the timing and amount of revenue that we recognize and
record in future periods.
Novartis. In consideration for rights granted
to Novartis under the collaboration and license agreement,
Novartis made an up-front payment of $10.0 million to us in
October 2005 to partly reimburse costs previously incurred by us
to develop in vivo RNAi technology. The collaboration and
license agreement includes terms under which Novartis will
provide us with research funding. In addition, for RNAi
therapeutic products successfully developed under the agreement,
if any, we would be entitled to receive milestone payments upon
achievement of certain specified development and annual net
sales events, up to an aggregate of $75.0 million per
therapeutic product. We initially recorded as deferred revenue
the non-refundable $10.0 million up-front payment and
$6.4 million premium that represents the difference between
the purchase price and the closing price of our common stock on
the date of the stock purchase from Novartis. In addition to
these payments, research funding and certain milestone payments,
the receipt of which is considered probable, are being amortized
into revenue using the proportional performance method over the
estimated duration of the Novartis agreement, or ten years. We
only include milestone payments that we believe are probable of
being received. Under this method, we estimate the level of
effort to be expended over the term of the agreement and
recognize revenue based on the lesser of the amount calculated
based on the proportional performance of total expected revenue
or the amount of non-refundable payments earned. As future
substantive milestones are achieved, and to the extent they are
within the period of performance, milestone payments will be
recognized as revenue on a proportional performance basis over
the contract’s entire performance period, starting with the
contract’s commencement. A portion of the milestone
payment, equal to the percentage of total performance completed
when the milestone is achieved, multiplied by the milestone
payment, will be recognized as revenue upon achievement of the
milestone. The remaining portion of the milestone will be
recognized over the remaining performance period under the
proportional performance method.
We believe our estimated period of performance under the
Novartis agreement includes the three-year term of the
agreement, two one-year extensions at the election of Novartis
and limited support as part of a technology transfer until the
fifth anniversary of the termination of the agreement. In July
2008, Novartis elected to extend the initial term of the
agreement for an additional year, through October 2009. We
continue to use an expected term of ten years in our
proportional performance model. We reevaluate the expected term
when new information is known that could affect our estimate. In
the event our period of performance is different than we
estimated, revenue recognition will be adjusted on a prospective
basis.
Roche. We recorded $278.2 million as
deferred revenue in connection with the Roche alliance. This
amount represents the aggregate proceeds received from Roche of
$331.0 million, net of the amount allocated for financial
statement purposes to the common stock issuance of
$51.3 million and the net book value of Alnylam Europe of
$1.5 million. In exchange for our contributions under the
collaboration agreement, for each RNAi therapeutic product
successfully developed by Roche, its affiliates or sublicensees
under the collaboration agreement, if any, we are entitled to
receive milestone payments upon achievement of specified
development and sales events, totaling up to an aggregate of
$100.0 million per therapeutic target, together with
royalty payments based on worldwide annual net sales, if any. In
addition, we have agreed with Roche to collaborate on the
discovery of RNAi therapeutic products directed to one or more
disease targets, referred to as the Discovery Collaboration,
subject to our existing contractual obligations to third
parties. The collaboration between Roche and us will be governed
by a joint steering committee for a period of five years that is
comprised of an equal number of representatives from each party.
Our performance obligations under the license and collaboration
agreement, including participation in the steering committee and
research conducted as part of the Discovery Collaboration, are
expected to cease five years from the effective date of the
license and collaboration agreement.
The proceeds allocated to the common stock issuance of
$51.3 million were based on the fair value on the date the
shares were issued. We have concluded that the license issued to
Roche, the Alnylam Europe assets and employees, the steering
committee services and the services we will be required to
perform under the Discovery
73
Collaboration should be treated as a single unit of accounting.
Accordingly, the remaining consideration received has been
recorded as deferred revenue and will be amortized into revenue
over the five-year period during which we are required to
provide services under the license and collaboration agreement.
We are recognizing revenue on a straight-line basis over five
years because we are unable to reasonably estimate the total
level of effort required under the license and collaboration
agreement. When, and if, we are able to make a reasonable
estimate of our remaining efforts under the collaboration, we
will modify the method of recognition and utilize the
proportional performance method. As future substantive
milestones are achieved, a portion of the milestone payment,
equal to the percentage of the performance period completed when
the milestone is achieved, multiplied by the amount of the
milestone payment, will be recognized as revenue upon
achievement of such milestone. The remaining portion of the
milestone will be recognized over the remaining performance
period on a straight-line basis.
Takeda. In consideration for the rights
granted to Takeda under the Takeda agreement, Takeda agreed to
pay us $150.0 million in upfront and near-term technology
transfer payments. In June 2008, Takeda paid us an upfront
payment of $100.0 million. Takeda is also required to make
an additional $50.0 million in payments to us upon
achievement of specified technology transfer milestones,
$20.0 million of which was paid in October 2008,
$20.0 million of which is required to be paid within 12 to
24 months of execution of the agreement and
$10.0 million of which is required to be paid within 24 to
36 months of execution of the agreement. If Takeda elects
to expand its license to additional therapeutic areas, Takeda
will be required to pay us $50.0 million for each of up to
approximately 20 total additional fields selected, comprising
all other fields of human disease, as identified and agreed upon
by the parties. In addition, for each RNAi therapeutic product
successfully developed by Takeda, its affiliates and
sublicensees, if any, we are entitled to receive specified
development and commercialization milestones, totaling up to
$171.0 million per product, together with royalty payments
based on worldwide annual net sales, if any.
Pursuant to the Takeda agreement, we and Takeda have also agreed
to collaborate on the research of RNAi therapeutics directed to
one or two disease targets agreed to by the parties, subject to
our existing contractual obligations with third parties. Takeda
also has the option, subject to certain conditions, to
collaborate with us on the research and development of RNAi drug
delivery technology for targets agreed to by the parties. In
addition, Takeda has a right of first negotiation for the
development and commercialization of our RNAi therapeutic
products in the Asian territory, excluding our ALN-RSV01
program. In addition to our
50-50 profit
sharing option, we have a similar right of first negotiation to
participate with Takeda in the development and commercialization
in the United States of licensed products. The collaboration is
governed by a joint technology transfer committee, or JTTC, a
joint research collaboration committee, or JRCC, and a joint
delivery collaboration committee, or JDCC, each of which is
comprised of an equal number of representatives from each party.
We have determined that the deliverables under the Takeda
agreement include the license, the joint committees (the JTTC,
JRCC and JDCC), the technology transfer activities and the
services that we will be obligated to perform under the research
collaboration with Takeda. We have determined that, pursuant to
EITF 00-21,
the license and undelivered services (i.e., the joint committees
and the research collaboration) are not separable and,
accordingly, the license and services are being treated as a
single unit of accounting. Under the Takeda agreement, the last
elements to be delivered are the JDCC and JTTC services, each of
which has a life of no more than seven years. We are initially
recognizing the upfront payment of $100.0 million, the
first technology transfer milestone of $20.0 million and
the $30.0 million of remaining technology transfer
milestones, the receipt of which we believe is probable, on a
straight-line basis over seven years because we are unable to
reasonably estimate the level of effort to fulfill these
obligations, primarily because the effort required under the
research collaboration is largely unknown. As future substantive
milestones are achieved, a portion of the milestone payment,
equal to the percentage of the performance period completed when
the milestone is achieved, multiplied by the amount of the
milestone payment, will be recognized as revenue upon
achievement of such milestone. The remaining portion of the
milestone will be recognized over the remaining performance
period on a straight-line basis. We will continue to reassess
whether we can reasonably estimate the level of effort required
to fulfill our obligations under the Takeda agreement. When, and
if, we can make a reasonable estimate of our remaining efforts
under the collaboration, we would modify our method of
recognition and utilize a proportional performance method.
Kyowa Hakko. Under the terms of the Kyowa
Hakko agreement, in June 2008, Kyowa Hakko paid us an upfront
cash payment of $15.0 million. In addition, Kyowa Hakko is
required to make payments to us upon achievement of specified
development and sales milestones totaling up to
$78.0 million, and royalty payments
74
based on annual net sales, if any, of ALN-RSV01 by Kyowa Hakko,
its affiliates and sublicenses in the licensed territory.
Our collaboration with Kyowa Hakko is governed by a joint
steering committee that is comprised of an equal number of
representatives from each party. Kyowa Hakko is responsible, at
its expense, for all development activities under the
development plan that are reasonably necessary for the
regulatory approval and commercialization of ALN-RSV01 in Japan
and the rest of the licensed territory. We will be responsible
for supply of the product to Kyowa Hakko under a supply
agreement unless Kyowa Hakko elects, prior to the first
commercial sale of the product in the licensed territory, to
manufacture the product itself or arrange for a third party to
manufacture the product.
We have determined that the deliverables under the Kyowa Hakko
agreement include the license, the joint steering committee, the
manufacturing services and any additional RSV-specific RNAi
therapeutic compounds that comprise the ALN-RSV program. We have
determined that pursuant to
EITF 00-21,
the individual deliverables are not separable and, accordingly,
must be accounted for as a single unit of accounting. We are
currently unable to reasonably estimate our period of
performance under the Kyowa Hakko agreement, as we are unable to
estimate the timeline of our deliverables related to the
fixed-price option granted to Kyowa Hakko for any additional
compounds. We are deferring all revenue under the Kyowa Hakko
agreement until we are able to reasonably estimate our period of
performance. We will continue to reassess whether we can
reasonably estimate the period of performance to fulfill our
obligations under the Kyowa Hakko agreement.
Government Contracts. Revenue under government
cost reimbursement contracts is recognized as we perform the
underlying research and development activities.
Accounting
for Income Taxes
Effective January 1, 2007, we adopted the provisions of
Financial Accounting Standards Board, or FASB, Interpretation
No. 48, “Accounting for Uncertainty in Income
Taxes — an interpretation of FASB Statement 109,”
or FIN 48, which clarifies the accounting for income tax
positions by prescribing a minimum recognition threshold that a
tax position is required to meet before being recognized in the
financial statements. FIN 48 also provides guidance on the
derecognition of previously recognized deferred tax items,
measurement, classification, interest and penalties, accounting
in interim periods, disclosure and transition. Under
FIN 48, we recognize the tax benefit from an uncertain tax
position only if it is more likely than not that the tax
position will be sustained upon examination by the taxing
authorities, based on the technical merits of the tax position.
The tax benefits recognized in our financial statements from
such a position are measured based on the largest benefit that
has a greater than 50% likelihood of being realized upon
ultimate resolution.
We operate in the United States and Germany where our income tax
returns are subject to audit and adjustment by local tax
authorities. The nature of the uncertain tax positions is often
very complex and subject to change and the amounts at issue can
be substantial. We develop our cumulative probability assessment
of the measurement of uncertain tax positions using internal
experience, judgment and assistance from professional advisors.
Estimates are refined as additional information becomes known.
Any outcome upon settlement that differs from our current
estimate may result in additional tax expense in future periods.
We recognize income taxes when transactions are recorded in our
consolidated statements of operations, with deferred taxes
provided for items that are recognized in different periods for
financial statement and tax reporting purposes. We record a
valuation allowance to reduce the deferred tax assets to the
amount that is more likely than not to be realized. In addition,
we estimate our exposures relating to uncertain tax positions
and establish reserves for such exposures when they become
probable and reasonably estimable.
For the years ended December 31, 2008 and 2007, we recorded
a provision for income taxes of $0.7 million and
$5.2 million, respectively. We provide income tax expense
for federal alternative minimum tax, state and foreign taxes. We
generated U.S. taxable income during 2008 due to the
recognition of certain proceeds received from the Roche
alliance. Our 2008 U.S. taxable income will be offset by
net operating loss carry forwards and other deferred tax
attributes. However, we anticipate being subject to federal
alternative minimum tax and state income taxes.
75
At December 31, 2008, we had a valuation allowance against
our net deferred tax assets to the extent it is more likely than
not that the assets will not be realized. At December 31,
2008, we had utilized all federal and Massachusetts state net
operating loss carryforwards and all federal and a significant
portion of Massachusetts state research and development credits
and all foreign tax credits. At December 31, 2008, we had
$0.5 million of state research and development credits
remaining and $8.6 million of California net operating
losses. We have placed a valuation allowance against the state
net operating loss and state credit deferred tax assets as it is
unlikely that we will realize these assets. Ownership changes,
as defined in the Internal Revenue Code, including those
resulting from the issuance of common stock in connection with
our public offerings, may limit the amount of net operating loss
and tax credit carryforwards that can be utilized to offset
future taxable income or tax liability. The amount of the
limitation is determined in accordance with Section 382 of
the Internal Revenue Code. We have determined that there is no
limitation on the utilization of net operating loss and tax
credit carryforwards in accordance with Section 382 of the
Internal Revenue Code in 2008.
Accounting
for Stock-Based Compensation
Effective January 1, 2006, we adopted the fair value
recognition provisions of FASB Statement of Financial Accounting
Standards, or SFAS, No. 123R, “Share-Based Payment, an
amendment of FASB Statements Nos. 123 and 95,” or
SFAS 123R, using the modified prospective transition
method. All stock-based awards granted to non-employees are
accounted for at their fair value in accordance with
SFAS No. 123, “Accounting for Stock Based
Compensation” and EITF Issue
No. 96-18,
“Accounting for Equity Instruments that are Issued to Other
Than Employees for Acquiring, or in Conjunction with Selling,
Goods or Services,” under which compensation expense is
generally recognized over the vesting period of the award.
Determining the amount of stock-based compensation to be
recorded requires us to develop estimates of fair values of
stock options as of the grant date. We calculate the grant date
fair values using the Black-Scholes valuation model. Our
expected stock price volatility assumption is based on a
combination of the historical and implied volatility of our
publicly traded stock. For stock option grants issued during the
year ended December 31, 2008, we used a weighted-average
expected stock-price volatility assumption of 66%. Our expected
life assumption is based on the equal weighting of our
historical data and the historical data of our pharmaceutical
and biotechnology peers. Our weighted average expected term was
6.1 years for the year ended December 31, 2008. We
utilize a dividend yield of zero based on the fact that we have
never paid cash dividends and have no present intention to pay
cash dividends. The risk-free interest rate used for each grant
is based on the U.S. Treasury yield curve in effect at the
time of grant for instruments with a similar expected life.
As of December 31, 2008, the estimated fair value of
unvested employee awards was $50.0 million, net of
estimated forfeitures. This amount will be recognized over the
weighted average remaining vesting period of approximately
1.6 years for these awards. Stock-based employee
compensation expense was $16.4 million for the year ended
December 31, 2008. However, the total amount of stock-based
compensation expense recognized in any future period cannot be
predicted at this time because it will depend on levels of
stock-based payments granted in the future as well as the
portion of the awards that actually vest. SFAS 123R
requires forfeitures to be estimated at the time of grant and
revised, if necessary, in subsequent periods if actual
forfeitures differ from those estimates. The term
“forfeitures” is distinct from
“cancellations” or “expirations” and
represents only the unvested portion of the surrendered option.
We currently expect, based on an analysis of our historical
forfeitures, that approximately 86% of our options will actually
vest, and therefore have applied an annual forfeiture rate of
3.7% to all unvested options as of December 31, 2008.
Ultimately, the actual expense recognized over the vesting
period will only be for those shares that vest.
Accounting
for Joint Venture
We account for our interest in Regulus Therapeutics using the
equity method of accounting. We have concluded that Regulus
Therapeutics qualifies as a variable interest entity under FASB
Interpretation No. 46R, “Consolidation of Variable
Interest Entities — an interpretation of Accounting
Research Bulletin No. 51,” or FIN 46R. The
limited liability company agreement contains transfer
restrictions on each of Isis’ and our interests and, as a
result, we and Isis are considered related parties under
paragraph 16(d)(1) of FIN 46R. Because we and Isis are
related parties and collectively own 100% of Regulus
Therapeutics, the determination of which entity would be
considered the primary beneficiary is based on which entity is
most closely associated with Regulus Therapeutics.
76
Following the guidance in paragraph 17 of FIN 46R, we
have concluded that Isis is the primary beneficiary and,
accordingly, we have not consolidated Regulus Therapeutics and
account for our investment under the equity method of accounting.
Results
of Operations
The following data summarizes the results of our operations for
the periods indicated, in thousands:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
|
|
Net revenues
|
|
$
|
96,163
|
|
|
$
|
50,897
|
|
|
$
|
26,930
|
|
|
Operating expenses
|
|
|
123,998
|
|
|
|
144,074
|
|
|
|
66,431
|
|
|
Loss from operations
|
|
|
(27,835
|
)
|
|
|
(93,177
|
)
|
|
|
(39,501
|
)
|
|
Net loss
|
|
$
|
(26,249
|
)
|
|
$
|
(85,466
|
)
|
|
$
|
(34,608
|
)
|
Discussion
of Results of Operations for 2008 and 2007
The following table summarizes our total consolidated net
revenues from research collaborators, for the periods indicated,
in thousands:
| |
|
|
|
|
|
|
|
|
|
|
|
Year Ended
|
|
|
|
|
December 31,
|
|
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Roche
|
|
$
|
54,427
|
|
|
$
|
17,571
|
|
|
Government contract
|
|
|
14,172
|
|
|
|
9,800
|
|
|
Takeda
|
|
|
12,794
|
|
|
|
—
|
|
|
Novartis
|
|
|
11,635
|
|
|
|
14,670
|
|
|
InterfeRx program, research reagent license and other
|
|
|
2,207
|
|
|
|
1,526
|
|
|
Other research collaborator
|
|
|
928
|
|
|
|
7,330
|
|
|
|
|
|
|
|
|
|
|
|
|
Total net revenues from research collaborators
|
|
$
|
96,163
|
|
|
$
|
50,897
|
|
|
|
|
|
|
|
|
|
|
|
Revenues increased significantly for the year ended
December 31, 2008 as compared to the year ended
December 31, 2007 primarily as a result of our August 2007
alliance with Roche, as well as our May 2008 alliance with
Takeda. Under the Roche alliance, $278.2 million is being
recognized as revenue on a straight-line basis over five years,
which equates to approximately $14.0 million per quarter.
In connection with the Roche alliance, Roche Kulmbach employees
performed certain transition services for us at various levels
through August 2008. We reimbursed Roche for these services at
an
agreed-upon
rate. We recorded as contra revenue (a reduction of revenues)
$1.0 million and $4.2 million for these services
during the years ended December 31, 2008 and 2007,
respectively. Under the Takeda alliance, the $150.0 million
in upfront and technology transfer milestone payments made or
due to us are being recognized as revenue on a straight-line
basis over seven years, which equates to approximately
$5.0 million per quarter.
For the year ended December 31, 2008 as compared to the
year ended December 31, 2007, government contract revenues
increased primarily as a result of our collaboration with DTRA,
which began in the third quarter of 2007. Following a program
review in early 2009, we and DTRA have determined to end this
program after February 2009 and accordingly, no additional funds
will be accessed.
The increase in InterfeRx program, research reagent licenses and
other revenues for the year ended December 31, 2008
compared to the prior year was due to milestone payments from
certain InterfeRx licensees.
The decrease in Novartis revenues during the year ended
December 31, 2008 as compared to the year ended
December 31, 2007 was due in part to the wind down of the
Novartis flu alliance.
Other research collaborator revenues decreased in the year ended
December 31, 2008 as compared to the year ended
December 31, 2007 due primarily to our termination of the
Merck collaboration agreement in September 2007. We were
recognizing the remaining deferred revenue under the Merck
agreement on a straight-line basis over the
77
remaining period of expected performance of four years. As a
result of the termination, we recognized an aggregate of
$3.5 million during the third quarter of 2007, which
represented all of the remaining deferred revenue under the
Merck agreement. In addition, during 2008, we reduced the number
of resources allocated to, and received lower external expense
reimbursement under, our collaboration with Biogen Idec. The
pace and scope of future development under this collaboration is
the responsibility of Biogen Idec. We expect to expend limited
resources on this program during 2009.
Total deferred revenue of $330.0 million at
December 31, 2008 consists of payments received from
collaborators, primarily Roche, Takeda and Kyowa Hakko, that we
have yet to recognize pursuant to our revenue recognition
policies.
For the foreseeable future, we expect our revenues to continue
to be derived primarily from our alliances with Roche, Takeda
and Novartis, as well as other strategic alliances,
collaborations, government contracts and licensing activities.
Operating
Expenses
The following tables summarize our operating expenses for the
periods indicated, in thousands and as a percentage of total
operating expenses, together with the changes, in thousands and
percentages:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
% of
|
|
|
|
|
|
% of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
|
|
|
Total
|
|
|
Increase
|
|
|
|
|
|
|
|
Operating
|
|
|
|
|
|
Operating
|
|
|
(Decrease)
|
|
|
|
|
2008
|
|
|
Expenses
|
|
|
2007
|
|
|
Expenses
|
|
|
$
|
|
|
%
|
|
|
|
|
Research and development
|
|
$
|
96,883
|
|
|
|
78
|
%
|
|
$
|
120,686
|
|
|
|
84
|
%
|
|
$
|
(23,803
|
)
|
|
|
(20
|
)%
|
|
General and administrative
|
|
|
27,115
|
|
|
|
22
|
%
|
|
|
23,388
|
|
|
|
16
|
%
|
|
|
3,727
|
|
|
|
16
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
$
|
123,998
|
|
|
|
100
|
%
|
|
$
|
144,074
|
|
|
|
100
|
%
|
|
$
|
(20,076
|
)
|
|
|
(14
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development. The following table
summarizes the components of our research and development
expenses for the periods indicated, in thousands and as a
percentage of total research and development expenses, together
with the changes, in thousands and percentages:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
% of
|
|
|
|
|
|
% of
|
|
|
Increase
|
|
|
|
|
|
|
|
Expense
|
|
|
|
|
|
Expense
|
|
|
(Decrease)
|
|
|
|
|
2008
|
|
|
Category
|
|
|
2007
|
|
|
Category
|
|
|
$
|
|
|
%
|
|
|
|
|
Research and development
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
External services
|
|
$
|
23,732
|
|
|
|
24
|
%
|
|
$
|
18,417
|
|
|
|
15
|
%
|
|
$
|
5,315
|
|
|
|
29
|
%
|
|
Compensation and related
|
|
|
17,299
|
|
|
|
18
|
%
|
|
|
13,201
|
|
|
|
11
|
%
|
|
|
4,098
|
|
|
|
31
|
%
|
|
Clinical trial and manufacturing
|
|
|
13,342
|
|
|
|
14
|
%
|
|
|
20,662
|
|
|
|
17
|
%
|
|
|
(7,320
|
)
|
|
|
(35
|
)%
|
|
License fees
|
|
|
12,554
|
|
|
|
13
|
%
|
|
|
42,207
|
|
|
|
35
|
%
|
|
|
(29,653
|
)
|
|
|
(70
|
)%
|
|
Facilities-related
|
|
|
10,181
|
|
|
|
11
|
%
|
|
|
8,511
|
|
|
|
7
|
%
|
|
|
1,670
|
|
|
|
20
|
%
|
|
Non-cash stock-based compensation
|
|
|
9,575
|
|
|
|
10
|
%
|
|
|
9,363
|
|
|
|
8
|
%
|
|
|
212
|
|
|
|
2
|
%
|
|
Lab supplies and materials
|
|
|
7,838
|
|
|
|
8
|
%
|
|
|
6,154
|
|
|
|
5
|
%
|
|
|
1,684
|
|
|
|
27
|
%
|
|
Other
|
|
|
2,362
|
|
|
|
2
|
%
|
|
|
2,171
|
|
|
|
2
|
%
|
|
|
191
|
|
|
|
9
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total research and development expenses
|
|
$
|
96,883
|
|
|
|
100
|
%
|
|
$
|
120,686
|
|
|
|
100
|
%
|
|
$
|
(23,803
|
)
|
|
|
(20
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development expenses decreased during the year
ended December 31, 2008 as compared to the year ended
December 31, 2007 due primarily to higher license fees
during the prior period consisting of $27.5 million in
payments to certain entities, primarily Isis, as a result of our
alliance with Roche, a non-cash license fee of $7.9 million
and a cash license fee of $0.4 million related to the
issuance of our stock to Tekmira during 2007 in connection with
our original license agreement with Tekmira, and
$6.0 million in payments for drug delivery-related
activities. Partially offsetting this decrease was
$5.0 million in payments made in 2008 to certain entities,
primarily Isis, as a result of the Takeda alliance, as well as a
charge of $2.1 million in connection with our new Tekmira
license agreement and $3.2 million associated with various
intellectual property assets.
78
Clinical trial and manufacturing expenses decreased during the
year ended December 31, 2008 as compared to the year ended
December 31, 2007 as a result of higher clinical trial and
manufacturing expenses in the prior period in support of our
clinical program for RSV, for which we began Phase II
trials in June 2007.
Partially offsetting these decreases, external services expenses
increased during the year ended December 31, 2008 as
compared to the year ended December 31, 2007 as a result of
higher expenses related to our government programs, our RSV
program and our pre-clinical programs for the treatment of liver
cancer and Huntington’s disease, as well as higher expenses
associated with our drug delivery-related collaborations. In
addition, compensation and related, lab supplies and materials,
and facilities-related expenses increased during the year ended
December 31, 2008 as compared to the prior year due to
additional research and development headcount to support our
alliances and expanding product pipeline.
We expect to continue to devote a substantial portion of our
resources to research and development expenses and we expect
that research and development expenses will remain consistent or
increase slightly in 2009 as we continue development of our and
our collaborators’ product candidates and focus on drug
delivery-related technologies.
We do not track actual costs for most of our research and
development programs or our personnel and personnel-related
costs on a
project-by-project
basis because all of our programs are in the early stages of
development. In addition, a significant portion of our research
and development costs are not tracked by project as they benefit
multiple projects or our technology platform. However, our
collaboration agreements contain cost-sharing arrangements
whereby certain costs incurred under the project are reimbursed.
Costs reimbursed under the agreements typically include certain
direct external costs and a negotiated full-time equivalent
labor rate for the actual time worked on the project. In
addition, we are reimbursed under our government contracts for
certain allowable costs including direct internal and external
costs. As a result, although a significant portion of our
research and development expenses are not tracked on a
project-by-project
basis, we do track direct external costs attributable to, and
the actual time our employees worked on, our collaborations and
government contracts.
General and administrative. The following
table summarizes the components of our general and
administrative expenses for the periods indicated, in thousands
and as a percentage of total general and administrative
expenses, together with the changes, in thousands and
percentages:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
% of
|
|
|
|
|
|
% of
|
|
|
Increase
|
|
|
|
|
|
|
|
Expense
|
|
|
|
|
|
Expense
|
|
|
(Decrease)
|
|
|
|
|
2008
|
|
|
Category
|
|
|
2007
|
|
|
Category
|
|
|
$
|
|
|
%
|
|
|
|
|
General and administrative
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consulting and professional services
|
|
$
|
9,281
|
|
|
|
34
|
%
|
|
$
|
8,547
|
|
|
|
36
|
%
|
|
$
|
734
|
|
|
|
9
|
%
|
|
Non-cash stock-based compensation
|
|
|
6,807
|
|
|
|
25
|
%
|
|
|
5,109
|
|
|
|
22
|
%
|
|
|
1,698
|
|
|
|
33
|
%
|
|
Compensation and related
|
|
|
5,757
|
|
|
|
21
|
%
|
|
|
4,647
|
|
|
|
20
|
%
|
|
|
1,110
|
|
|
|
24
|
%
|
|
Facilities-related
|
|
|
2,401
|
|
|
|
9
|
%
|
|
|
2,486
|
|
|
|
11
|
%
|
|
|
(85
|
)
|
|
|
(3
|
)%
|
|
Insurance
|
|
|
682
|
|
|
|
3
|
%
|
|
|
654
|
|
|
|
3
|
%
|
|
|
28
|
|
|
|
4
|
%
|
|
Other
|
|
|
2,187
|
|
|
|
8
|
%
|
|
|
1,945
|
|
|
|
8
|
%
|
|
|
242
|
|
|
|
12
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total general and administrative expenses
|
|
$
|
27,115
|
|
|
|
100
|
%
|
|
$
|
23,388
|
|
|
|
100
|
%
|
|
$
|
3,727
|
|
|
|
16
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The increase in general and administrative expenses during the
year ended December 31, 2008 as compared to the prior year
was due primarily to an increase in general and administrative
headcount during 2008 to support our growth and higher non-cash
stock-based compensation. We expect that general and
administrative expenses will remain consistent or increase
slightly in 2009.
Other
income (expense)
Equity in loss of joint venture was $9.3 million and
$1.1 million during the years ended December 31, 2008
and 2007, respectively, and in each year related to our share of
the net losses incurred by Regulus Therapeutics, which was
formed in September 2007. The increase was a result of Regulus
Therapeutics ramping up its operations
79
throughout 2008. Separate financial information for Regulus
Therapeutics is included in Exhibit 99.1 to this annual
report on
Form 10-K.
Interest income was $14.4 million in 2008 as compared to
$15.4 million in 2007. The decrease was due to our lower
average interest rates during the year ended December 31,
2008, partially offset by higher average cash, cash equivalent
and marketable securities balances.
Interest expense was $0.9 million in 2008 as compared to
$1.1 million in 2007. Interest expense in each period was
related to borrowings under our lines of credit with Oxford
Finance Corporation, or Oxford, and Lighthouse Capital
Partners V, L.P., or Lighthouse, used to finance capital
equipment purchases. In December 2008, we defeased the aggregate
outstanding balance under the Oxford and Lighthouse credit lines
and expect to have no interest expense in 2009.
Included in other expense during the year ended
December 31, 2008 was an impairment charge of
$1.6 million related to our May 2008 investment in Tekmira,
as the decrease in the fair value of this investment was deemed
to be other than temporary.
Our provision for income taxes was $0.7 million for year
ended December 31, 2008 primarily as a result of our 2007
alliance with Roche. Income tax expense was $5.2 million
for the prior year primarily as a result of the sale of our
German operations to Roche in August 2007 for $15.0 million.
Discussion
of Results of Operations for 2007 and 2006
The following table summarizes our total consolidated net
revenues from research collaborators, for the periods indicated,
in thousands:
| |
|
|
|
|
|
|
|
|
|
|
|
Year Ended
|
|
|
|
|
December 31,
|
|
|
|
|
2007
|
|
|
2006
|
|
|
|
|
Roche
|
|
$
|
17,571
|
|
|
$
|
—
|
|
|
Novartis
|
|
|
14,670
|
|
|
|
21,775
|
|
|
Government contract
|
|
|
9,800
|
|
|
|
786
|
|
|
Other research collaborator
|
|
|
7,330
|
|
|
|
2,508
|
|
|
InterfeRx program, research reagent license and other
|
|
|
1,526
|
|
|
|
1,861
|
|
|
|
|
|
|
|
|
|
|
|
|
Total net revenues from research collaborators
|
|
$
|
50,897
|
|
|
$
|
26,930
|
|
|
|
|
|
|
|
|
|
|
|
Revenues increased significantly for the year ended
December 31, 2007 as compared to the year ended
December 31, 2006 primarily as a result of our August 2007
alliance with Roche. Under the Roche alliance,
$278.2 million is being recognized as revenue on a
straight-line basis over five years. In connection with the
Roche alliance, Roche Kulmbach employees performed certain
transition services for us at various levels through August
2008. We reimbursed Roche for these services at an
agreed-upon
rate. We recorded $4.2 million of these services as contra
revenue (a reduction of revenues) during 2007.
The decrease in Novartis revenues during the year ended
December 31, 2007 compared to the year ended
December 31, 2006 was due to a decrease in the number of
resources allocated to the broad Novartis alliance. The decrease
in Novartis revenues was also due to a decrease in the number of
resources allocated to, as well as lower external expense
reimbursement under, our Novartis flu alliance, as a result of
the shift in focus during 2007 on additional pre-clinical
research prior to advancing the pandemic flu program into
development. During 2008, the pandemic flu program was stopped
and currently there are no specific resource commitments for
this program.
For the year ended December 31, 2007, government contract
revenues increased as a result of our collaboration with NIAID,
which began in the fourth quarter of 2006, and our collaboration
with DTRA, which began in the third quarter of 2007.
Other research collaborator revenues consisted primarily of
research funding and amortization of upfront payments or license
fees from Biogen Idec and Merck. The increase in other research
collaborator revenues in 2007 was primarily the result of our
collaboration with Biogen Idec, which began in the fourth
quarter of 2006 and the
80
termination of our Merck collaboration agreement in September
2007. We were recognizing the remaining deferred revenue under
the Merck agreement on a straight-line basis over the remaining
period of expected performance of four years. As a result of the
termination, we recognized an aggregate of $3.5 million
during the third quarter of 2007, which represented all of the
remaining deferred revenue under the Merck agreement.
The decrease in InterfeRx program, research reagent license and
other revenues for the year ended December 31, 2007
compared to the prior year was due to upfront payments pursuant
to license agreements entered into under our InterfeRx program
in the prior year.
Total deferred revenues of $263.3 million at
December 31, 2007 consists of payments received from
collaborators, primarily Roche, that we have yet to recognize
pursuant to our revenue recognition policies.
Operating
Expenses
The following tables summarize our operating expenses for the
periods indicated, in thousands and as a percentage of total
operating expenses, together with the changes, in thousands and
percentages:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
% of
|
|
|
|
|
|
% of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
|
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating
|
|
|
|
|
|
Operating
|
|
|
Increase
|
|
|
|
|
2007
|
|
|
Expenses
|
|
|
2006
|
|
|
Expenses
|
|
|
$
|
|
|
%
|
|
|
|
|
Research and development
|
|
$
|
120,686
|
|
|
|
84
|
%
|
|
$
|
49,260
|
|
|
|
74
|
%
|
|
$
|
71,426
|
|
|
|
145
|
%
|
|
General and administrative
|
|
|
23,388
|
|
|
|
16
|
%
|
|
|
17,171
|
|
|
|
26
|
%
|
|
|
6,217
|
|
|
|
36
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
$
|
144,074
|
|
|
|
100
|
%
|
|
$
|
66,431
|
|
|
|
100
|
%
|
|
$
|
77,643
|
|
|
|
117
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Certain reclassifications have been made to prior years’
financial statements to conform to the 2007 presentation.
Research and development. The following table
summarizes the components of our research and development
expenses for the periods indicated, in thousands and as a
percentage of total research and development expenses, together
with the changes, in thousands and percentages:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
% of
|
|
|
|
|
|
% of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expense
|
|
|
|
|
|
Expense
|
|
|
Increase
|
|
|
|
|
2007
|
|
|
Category
|
|
|
2006
|
|
|
Category
|
|
|
$
|
|
|
%
|
|
|
|
|
Research and development
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
License fees
|
|
$
|
42,207
|
|
|
|
35
|
%
|
|
$
|
4,040
|
|
|
|
8
|
%
|
|
$
|
38,167
|
|
|
|
945
|
%
|
|
Clinical trial and manufacturing
|
|
|
20,662
|
|
|
|
17
|
%
|
|
|
10,019
|
|
|
|
20
|
%
|
|
|
10,643
|
|
|
|
106
|
%
|
|
External services
|
|
|
18,417
|
|
|
|
15
|
%
|
|
|
6,001
|
|
|
|
12
|
%
|
|
|
12,416
|
|
|
|
207
|
%
|
|
Compensation and related
|
|
|
13,201
|
|
|
|
11
|
%
|
|
|
10,666
|
|
|
|
22
|
%
|
|
|
2,535
|
|
|
|
24
|
%
|
|
Non-cash stock-based compensation
|
|
|
9,363
|
|
|
|
8
|
%
|
|
|
5,006
|
|
|
|
10
|
%
|
|
|
4,357
|
|
|
|
87
|
%
|
|
Facilities-related
|
|
|
8,511
|
|
|
|
7
|
%
|
|
|
6,315
|
|
|
|
13
|
%
|
|
|
2,196
|
|
|
|
35
|
%
|
|
Lab supplies and materials
|
|
|
6,154
|
|
|
|
5
|
%
|
|
|
5,462
|
|
|
|
11
|
%
|
|
|
692
|
|
|
|
13
|
%
|
|
Other
|
|
|
2,171
|
|
|
|
2
|
%
|
|
|
1,751
|
|
|
|
4
|
%
|
|
|
420
|
|
|
|
24
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total research and development expenses
|
|
$
|
120,686
|
|
|
|
100
|
%
|
|
$
|
49,260
|
|
|
|
100
|
%
|
|
$
|
71,426
|
|
|
|
145
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
During the year ended December 31, 2007, our research and
development expenses increased significantly compared to the
year ended December 31, 2006 primarily as a result of an
increase in license fees consisting of $27.5 million in
payments to certain entities, primarily Isis, in connection with
the Roche alliance and $14.7 million in charges for
licenses for certain delivery technologies. The increase was
also due to an increase in clinical trial and manufacturing
expenses in support of our clinical program for RSV, for which
we began Phase II trials in June 2007, as well as higher
manufacturing expenses related to our pre-clinical development
programs. The increase in external services was due to higher
expenses related to our pre-clinical programs for the treatment
of hypercholesterolemia, liver cancer and Ebola, as well as
higher expenses associated with our delivery-related
81
collaborations. The increase in compensation and related
expenses and facilities-related expenses was due to additional
research and development headcount over the past year to support
our alliances and expanding product pipeline. The increase in
non-cash stock-based compensation was due primarily to one-time
charges of $2.9 million from restricted stock grants and
stock option modifications in August 2007 relating to the
transfer of our former German employees to Roche Kulmbach as
part of our alliance with Roche.
General and administrative. The following
table summarizes the components of our general and
administrative expenses for the periods indicated, in thousands
and as a percentage of total general and administrative
expenses, together with the changes, in thousands and
percentages:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
% of
|
|
|
|
|
|
% of
|
|
|
Increase
|
|
|
|
|
|
|
|
Expense
|
|
|
|
|
|
Expense
|
|
|
(Decrease)
|
|
|
|
|
2007
|
|
|
Category
|
|
|
2006
|
|
|
Category
|
|
|
$
|
|
|
%
|
|
|
|
|
General and administrative
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consulting and professional services
|
|
$
|
8,547
|
|
|
|
36
|
%
|
|
$
|
5,162
|
|
|
|
30
|
%
|
|
$
|
3,385
|
|
|
|
66
|
%
|
|
Non-cash stock-based compensation
|
|
|
5,109
|
|
|
|
22
|
%
|
|
|
3,298
|
|
|
|
19
|
%
|
|
|
1,811
|
|
|
|
55
|
%
|
|
Compensation and related
|
|
|
4,647
|
|
|
|
20
|
%
|
|
|
3,546
|
|
|
|
21
|
%
|
|
|
1,101
|
|
|
|
31
|
%
|
|
Facilities-related
|
|
|
2,486
|
|
|
|
11
|
%
|
|
|
2,736
|
|
|
|
16
|
%
|
|
|
(250
|
)
|
|
|
(9
|
)%
|
|
Insurance
|
|
|
654
|
|
|
|
3
|
%
|
|
|
652
|
|
|
|
4
|
%
|
|
|
2
|
|
|
|
0
|
%
|
|
Other
|
|
|
1,945
|
|
|
|
8
|
%
|
|
|
1,777
|
|
|
|
10
|
%
|
|
|
168
|
|
|
|
9
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total general and administrative expenses
|
|
$
|
23,388
|
|
|
|
100
|
%
|
|
$
|
17,171
|
|
|
|
100
|
%
|
|
$
|
6,217
|
|
|
|
36
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The increase in general and administrative expenses in 2007
compared to the prior year was due primarily to professional
service fees, which were the result of increased business
development activities, including work related to our alliance
with Roche and with Regulus Therapeutics, the company we formed
with Isis to pursue microRNA-based therapeutics. The increase in
compensation and related expenses was due primarily to an
increase in headcount to support the overall corporate growth of
the company. The increase in non-cash stock-based compensation
was due primarily to one-time charges of $0.9 million from
restricted stock grants and stock option modifications in August
2007 relating to the transfer of our former German employees to
Roche Kulmbach as part of our alliance with Roche.
Other
income (expense)
Interest income was $15.4 million in 2007 compared to
$6.2 million in 2006. The increase was due to our higher
average cash, cash equivalent and marketable securities
balances, primarily from the $331.0 million in proceeds we
received in August 2007 from our alliance with Roche.
Interest expense was $1.1 million in 2007 and
$1.0 million in 2006. Interest expense in each year related
to borrowings under our lines of credit used to finance capital
equipment purchases.
Our provision for income taxes was $5.2 million in 2007
primarily as a result of the sale of our German operations to
Roche in August 2007 for $15.0 million.
82
Liquidity
and Capital Resources
The following table summarizes our cash flow activities for the
periods indicated, in thousands:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
|
|
Net loss
|
|
$
|
(26,249
|
)
|
|
|
$(85,466
|
)
|
|
$
|
(34,608
|
)
|
|
Adjustments to reconcile net loss to net cash used in operating
activities
|
|
|
27,840
|
|
|
|
29,834
|
|
|
|
12,633
|
|
|
Changes in operating assets and liabilities
|
|
|
63,900
|
|
|
|
252,151
|
|
|
|
(2,655
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) operating activities
|
|
|
65,491
|
|
|
|
196,519
|
|
|
|
(24,630
|
)
|
|
Net cash provided by (used in) investing activities
|
|
|
17,936
|
|
|
|
(277,425
|
)
|
|
|
(30,046
|
)
|
|
Net cash provided by financing activities
|
|
|
3,155
|
|
|
|
58,635
|
|
|
|
166,631
|
|
|
Effect of exchange rate on cash
|
|
|
53
|
|
|
|
(527
|
)
|
|
|
243
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase (decrease) in cash and cash equivalents
|
|
|
86,635
|
|
|
|
(22,798
|
)
|
|
|
112,198
|
|
|
Cash and cash equivalents, beginning of period
|
|
|
105,157
|
|
|
|
127,955
|
|
|
|
15,757
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, end of period
|
|
$
|
191,792
|
|
|
|
$105,157
|
|
|
$
|
127,955
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Since we commenced operations in 2002, we have generated
significant losses. As of December 31, 2008, we had an
accumulated deficit of $252.2 million. As of
December 31, 2008, we had cash, cash equivalents and
marketable securities of $512.7 million, compared to cash,
cash equivalents and marketable securities of
$455.6 million as of December 31, 2007. The increase
in our cash, cash equivalents and marketable securities at
December 31, 2008 was due primarily to our receipt of
$120.0 million of upfront cash and technology transfer
milestone payments under our Takeda alliance, partially offset
by the funding of our operating expenses. We invest primarily in
cash equivalents, U.S. government obligations, high-grade
corporate notes and commercial paper. Our investment objectives
are, primarily, to assure liquidity and preservation of capital
and, secondarily, to obtain investment income. All of our
investments in debt securities are recorded at fair value and
are available-for-sale. Fair value is determined based on quoted
market prices and models using observable data inputs. We have
not recorded any impairment charges to our fixed income
marketable securities as of December 31, 2008.
Operating
activities
We have required significant amounts of cash to fund our
operating activities as a result of net losses since our
inception. The decrease in net cash provided by operating
activities for the year ended December 31, 2008 compared to
the year ended December 31, 2007 was due primarily to the
proceeds received from our August 2007 Roche alliance, partially
offset by the proceeds received from our May 2008 alliance with
Takeda. Offsetting the proceeds from the Takeda alliance, the
main components of our use of cash in operating activities for
the year ended December 31, 2008 consisted of the net loss
and changes in our working capital. Cash used in operating
activities is adjusted for non-cash items to reconcile net loss
to net cash provided by or used in operating activities. These
non-cash adjustments consist primarily of stock-based
compensation, equity in loss of joint venture and depreciation
and amortization. We had an increase in deferred revenue of
$66.7 million for the year ended December 31, 2008 due
primarily to the proceeds received from our Takeda and Kyowa
Hakko alliances.
We expect that we will require significant amounts of cash to
fund our operating activities for the foreseeable future as we
continue to develop and advance our research and development
initiatives. The actual amount of overall expenditures will
depend on numerous factors, including the timing of expenses,
the timing and terms of collaboration agreements or other
strategic transactions, if any, and the timing and progress of
our research and development efforts.
Investing
activities
For the year ended December 31, 2008, net cash provided by
investing activities resulted primarily from net sales and
maturities of marketable securities of $28.8 million. Also
included in our investing activities for the year ended
December 31, 2008 were purchases of property and equipment
of $10.8 million related to the expansion of our Cambridge
facility. For the year ended December 31, 2007, net cash
used in investing activities of $277.4 million resulted
primarily from purchases of marketable securities of
$544.4 million due primarily to
83
the investments made with the proceeds from the Roche alliance,
partially offset by sales and maturities of marketable
securities of $283.3 million. Also included in our
investing activities for the year ended December 31, 2007
was a $10.0 million cash contribution made to Regulus
Therapeutics in connection with its formation, as well as
purchases of property and equipment of $7.8 million related
to the expansion of our Cambridge facility.
Financing
activities
For the year ended December 31, 2008, net cash provided by
financing activities was $3.2 million as compared to
$58.6 million provided by financing activities for the year
ended December 31, 2007. For the year ended
December 31, 2008, net cash provided by financing
activities was due primarily to proceeds of $5.4 million
from our issuance of shares to Novartis in May 2008. Net cash
provided by financing activities for the year ended
December 31, 2007 consisted primarily of proceeds of
$42.5 million from our sale of 1,975,000 shares of our
common stock to Roche in connection with the establishment of
the Roche alliance.
In March 2006, we entered into an agreement with Oxford to
establish an equipment line of credit for up to
$7.0 million to help support capital expansion of our
facility in Cambridge, Massachusetts and capital equipment
purchases. During 2006, we borrowed an aggregate of
$4.2 million from Oxford pursuant to the agreement. In May
2007, we borrowed an aggregate of $1.0 million from Oxford
pursuant to the agreement. In March 2004, we entered into an
equipment line of credit with Lighthouse to finance leasehold
improvements and equipment purchases of up to
$10.0 million. On the maturity of each equipment advance
under the Lighthouse line of credit, we were required to pay, in
addition to the principal and interest due, an additional amount
of 11.5% of the original principal. This amount was being
accrued over the applicable borrowing period as additional
interest expense. In December 2008, we defeased the aggregate
outstanding balance under the Oxford and Lighthouse credit lines.
During the current downturn in global financial markets, some
companies have experienced difficulties accessing their cash
equivalents, investment securities and raising capital
generally, which have had a material adverse impact on their
liquidity. In addition, the current economic downturn has
severely diminished the availability of capital and may limit
our ability to access these markets to obtain financing in the
future. Based on our current operating plan, we believe that our
existing cash, cash equivalents and fixed income marketable
securities, for which we have not recognized any impairment
charges, together with the cash we expect to generate under our
current alliances, including our Novartis, Roche and Takeda
alliances, will be sufficient to fund our planned operations for
at least the next several years, during which time we expect to
further the development of our product candidates, conduct
clinical trials, extend the capabilities of our technology
platform and continue to prosecute patent applications and
otherwise build and maintain our patent portfolio. However, we
may require significant additional funds earlier than we
currently expect in order to develop, commence clinical trials
for and commercialize any product candidates.
In the longer term, we may seek additional funding through
additional collaborative arrangements and public or private
financings. Additional funding may not be available to us on
acceptable terms or at all. In addition, the terms of any
financing may adversely affect the holdings or the rights of our
stockholders. For example, if we raise additional funds by
issuing equity securities, further dilution to our existing
stockholders may result. If we are unable to obtain funding on a
timely basis, we may be required to significantly curtail one or
more of our research or development programs. We also could be
required to seek funds through arrangements with collaborators
or others that may require us to relinquish rights to some of
our technologies or product candidates that we would otherwise
pursue.
Even if we are able to raise additional funds in a timely
manner, our future capital requirements may vary from what we
expect and will depend on many factors, including:
|
|
|
| |
•
|
our progress in demonstrating that siRNAs can be active as drugs;
|
| |
| |
•
|
our ability to develop relatively standard procedures for
selecting and modifying siRNA drug candidates;
|
| |
| |
•
|
progress in our research and development programs, as well as
the magnitude of these programs;
|
| |
| |
•
|
the timing, receipt and amount of milestone and other payments,
if any, from present and future collaborators, if any;
|
| |
| |
•
|
the timing, receipt and amount of funding under current and
future government contracts, if any;
|
| |
| |
•
|
our ability to maintain and establish additional collaborative
arrangements;
|
84
|
|
|
| |
•
|
the resources, time and costs required to successfully initiate
and complete our pre-clinical and clinical trials, obtain
regulatory approvals, protect our intellectual property and
obtain and maintain licenses to third-party intellectual
property;
|
| |
| |
•
|
the cost of preparing, filing, prosecuting, maintaining and
enforcing patent claims;
|
| |
| |
•
|
progress in the research and development programs of Regulus
Therapeutics; and
|
| |
| |
•
|
the timing, receipt and amount of sales and royalties, if any,
from our potential products.
|
Off-Balance
Sheet Arrangements
In connection with a certain license agreement, we are required
to indemnify the licensor for certain damages arising in
connection with the intellectual property rights licensed under
the agreement. In addition, we are a party to a number of
agreements entered into in the ordinary course of business,
which contain typical provisions, which obligate us to indemnify
the other parties to such agreements upon the occurrence of
certain events. These indemnification obligations are considered
off-balance sheet arrangements in accordance with FASB
Interpretation No. 45, “Guarantor’s Accounting
and Disclosure Requirements for Guarantees, Including Indirect
Guarantees of Indebtedness of Others.” To date, we have not
encountered material costs as a result of such obligations and
have not accrued any liabilities related to such obligations and
have not accrued any liabilities related to such obligations in
our financial statements. See Note 7 to our consolidated
financial statements included in this annual report on
Form 10-K
for further discussion of these indemnification agreements.
Contractual
Obligations
In the table below, we set forth our enforceable and legally
binding obligations and future commitments as of
December 31, 2008, as well as obligations related to
contracts that we are likely to continue, regardless of the fact
that they were cancelable as of December 31, 2008. Some of
the figures that we include in this table are based on
management’s estimate and assumptions about these
obligations, including their duration, the possibility of
renewal, anticipated actions by third parties, and other
factors. Because these estimates and assumptions are necessarily
subjective, the obligations we will actually pay in future
periods may vary from those reflected in the table.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Payments Due by Period
|
|
|
|
|
|
|
|
2010 and
|
|
|
2012 and
|
|
|
|
|
|
|
|
|
Contractual Obligations
|
|
2009
|
|
|
2011
|
|
|
2013
|
|
|
After 2013
|
|
|
Total
|
|
|
|
|
Operating lease obligations(1)
|
|
$
|
3,296
|
|
|
$
|
5,768
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
9,064
|
|
|
Purchase commitments(2)
|
|
|
8,721
|
|
|
|
523
|
|
|
|
—
|
|
|
|
—
|
|
|
|
9,244
|
|
|
Technology-related commitments(3)
|
|
|
3,612
|
|
|
|
5,091
|
|
|
|
1,922
|
|
|
|
5,700
|
|
|
|
16,325
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total contractual cash obligations
|
|
$
|
15,629
|
|
|
$
|
11,382
|
|
|
$
|
1,922
|
|
|
$
|
5,700
|
|
|
$
|
34,633
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
Relates to our Cambridge, Massachusetts non-cancelable operating
lease agreements. |
| |
|
(2) |
|
Includes commitments related to non-cancelable purchase orders,
clinical and pre-clinical agreements and other significant
purchase commitments for good or services. Amounts have not been
adjusted to reflect our January 2009 agreement with Cubist,
under which Cubist will bear one-half of the development costs
related to our ALN-RSV program. |
| |
|
(3) |
|
Relates to our fixed payment obligations under license
agreements, as well as other payments related to technology
research and development. Does not include license fees due to
certain of our licensors in accordance with the applicable
license agreements with those parties as a result of our January
2009 agreement with Cubist. |
We in-license technology from a number of sources. Pursuant to
these in-license agreements, we will be required to make
additional payments if and when we achieve specified development
and regulatory milestones. To the extent we are unable to
reasonably predict the likelihood, timing or amount of such
payments, we have excluded them from the table above.
85
Recent
Accounting Pronouncements
Effective January 1, 2008, we implemented
SFAS No. 157, “Fair Value Measurements,” or
SFAS 157. SFAS 157 clarifies the principle that fair
value should be based on the assumptions market participants
would use when pricing an asset or liability and establishes a
fair value hierarchy that prioritizes the information used to
develop those assumptions. Under the standard, fair value
measurements would be separately disclosed by level within the
fair value hierarchy. Our partial adoption of SFAS 157 has
had no impact on our operating results or financial position. We
are evaluating the impact, if any, full adoption of
SFAS 157 will have on our nonfinancial assets and
liabilities within the scope of SFAS 157.
In December 2007, the FASB reached a consensus on EITF Issue
No. 07-1,
“Accounting for Collaborative Arrangements,” or
EITF 07-1.
EITF 07-1
requires collaborators to present the results of activities for
which they act as the principal on a gross basis and report any
payments received from (made to) other collaborators based on
other applicable GAAP or, in the absence of other applicable
GAAP, based on analogy to authoritative accounting literature or
a reasonable, rational and consistently applied accounting
policy election. Further,
EITF 07-1
clarifies that the determination of whether transactions within
a collaborative arrangement are part of a vendor-customer (or
analogous) relationship subject to
EITF 01-9.
EITF 07-1
became effective on January 1, 2009. We are evaluating the
potential impact of
EITF 07-1
on our consolidated financial statements.
In December 2007, the FASB issued SFAS No. 141R,
“Business Combinations,” or SFAS 141R.
SFAS 141R establishes principles and requirements for how
an acquirer recognizes and measures in its financial statements
the identifiable assets acquired, the liabilities assumed, any
noncontrolling interest in the acquiree and the goodwill
acquired. SFAS 141R also establishes disclosure
requirements to enable the evaluation of the nature and
financial effects of the business combination. SFAS 141R is
effective for fiscal year 2009 and is not expected to have a
material impact on our consolidated financial statements.
In December 2007, the FASB issued SFAS No. 160,
“Noncontrolling Interests in Consolidated Financial
Statements, an amendment of ARB No. 51,” or
SFAS 160. SFAS 160 changes the accounting for and
reporting of noncontrolling or minority interests (now called
noncontrolling interests) in consolidated financial statements.
SFAS 160 become effective on January 1, 2009. When
implemented, prior periods will be recast for the changes
required by SFAS 160. We do not anticipate the adoption of
SFAS 160 will have a material impact on our consolidated
financial statements.
In May 2008, the FASB issued SFAS No. 162, “The
Hierarchy of Generally Accepted Accounting Principles,” or
SFAS 162. SFAS 162 identifies the sources of
accounting principles and the framework for selecting the
principles used (order of authority) in the preparation of
financial statements that are presented in conformity with
generally accepted accounting standards in the United States.
The adoption of SFAS 162 did not have a material impact on
our consolidated financial statements.
|
|
|
ITEM 7A.
|
QUANTITATIVE
AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
As part of our investment portfolio, we own financial
instruments that are sensitive to market risks. The investment
portfolio is used to preserve our capital until it is required
to fund operations, including our research and development
activities. Our marketable securities consist of
U.S. government obligations, high-grade corporate notes and
commercial paper. All of our investments in debt securities are
classified as “available-for-sale” and are recorded at
fair value. Our available-for-sale investments in debt
securities are sensitive to changes in interest rates and
changes in the credit ratings of the issuers. Interest rate
changes would result in a change in the net fair value of these
financial instruments due to the difference between the market
interest rate and the market interest rate at the date of
purchase of the financial instrument. A 10% decrease in market
interest rates at December 31, 2008 would impact the net
fair value of such interest-sensitive financial instruments by
$1.8 million. A downgrade in the credit rating of an issuer
of a debt security or further deterioration of the credit
markets could result in a decline in the fair value of the debt
instruments. Our investment guidelines prohibit investment in
auction rate securities and we do not believe we have any direct
exposure to losses relating from mortgage-based securities or
derivatives related thereto such as credit-default swaps. We
have not recorded any impairment charges to our fixed income
marketable securities as of December 31, 2008.
86
|
|
|
ITEM 8.
|
FINANCIAL
STATEMENTS AND SUPPLEMENTARY DATA
|
INDEX TO
CONSOLIDATED FINANCIAL STATEMENTS
| |
|
|
|
|
|
|
|
Page
|
|
|
|
|
|
|
88
|
|
|
|
|
|
89
|
|
|
|
|
|
90
|
|
|
|
|
|
91
|
|
|
|
|
|
92
|
|
|
|
|
|
93
|
|
|
|
|
|
94
|
|
87
Management’s
Annual Report on Internal Control Over Financial
Reporting
The management of the Company is responsible for establishing
and maintaining adequate internal control over financial
reporting. Internal control over financial reporting is defined
in
Rule 13a-15(f)
or 15d-15(f)
promulgated under the Securities Exchange Act of 1934 as a
process designed by, or under the supervision of, the
company’s principal executive and principal financial
officers and effected by the company’s board of directors,
management and other personnel, to provide reasonable assurance
regarding the reliability of financial reporting and the
preparation of financial statements for external purposes in
accordance with generally accepted accounting principles and
includes those policies and procedures that:
|
|
|
| |
•
|
Pertain to the maintenance of records that in reasonable detail
accurately and fairly reflect the transactions and dispositions
of the assets of the Company;
|
| |
| |
•
|
Provide reasonable assurance that transactions are recorded as
necessary to permit preparation of financial statements in
accordance with generally accepted accounting principles, and
that receipts and expenditures of the Company are being made
only in accordance with authorizations of management and
directors of the Company; and
|
| |
| |
•
|
Provide reasonable assurance regarding prevention or timely
detection of unauthorized acquisition, use or disposition of the
Company’s assets that could have a material effect on the
financial statements.
|
Because of its inherent limitations, internal control over
financial reporting may not prevent or detect misstatements.
Projections of any evaluation of effectiveness to future periods
are subject to the risk that controls may become inadequate
because of changes in conditions, or that the degree of
compliance with the policies or procedures may deteriorate.
The Company’s management assessed the effectiveness of the
Company’s internal control over financial reporting as of
December 31, 2008. In making this assessment, the
Company’s management used the criteria set forth by the
Committee of Sponsoring Organizations of the Treadway Commission
(COSO) in Internal Control-Integrated Framework.
Based on our assessment, management concluded that, as of
December 31, 2008, the Company’s internal control over
financial reporting is effective based on those criteria.
The effectiveness of the Company’s internal control over
financial reporting as of December 31, 2008 has been
audited by PricewaterhouseCoopers LLP, an independent registered
public accounting firm, as stated in their report. This report
appears on page 89.
88
Report of
Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Alnylam
Pharmaceuticals, Inc.:
In our opinion, based on our audits and the report of other
auditors, the accompanying consolidated balance sheets and the
related consolidated statements of operations and comprehensive
loss, stockholders’ equity and cash flows present fairly,
in all material respects, the financial position of Alnylam
Pharmaceuticals, Inc. and its subsidiaries at December 31,
2008 and 2007, and the results of their operations and their
cash flows for each of the three years in the period ended
December 31, 2008 in conformity with accounting principles
generally accepted in the United States of America. Also in our
opinion, the Company maintained, in all material respects,
effective internal control over financial reporting as of
December 31, 2008, based on criteria established in
Internal Control — Integrated Framework issued
by the Committee of Sponsoring Organizations of the Treadway
Commission (COSO). The Company’s management is responsible
for these financial statements, for maintaining effective
internal control over financial reporting and for its assessment
of the effectiveness of internal control over financial
reporting, included in Management’s Annual Report on
Internal Control over Financial Reporting. Our responsibility is
to express opinions on these financial statements and on the
Company’s internal control over financial reporting based
on our integrated audits. We did not audit the financial
statements of Regulus Therapeutics LLC, an approximate
49 percent-owned equity investment, which were audited by
other auditors whose report thereon has been furnished to us.
Our opinion expressed herein, insofar as it relates to the
Company’s net investment in (approximately
$1.6 million and $9.1 million at December 31,
2008 and 2007, respectively) and equity in the net loss
(approximately $9.3 million and $1.1 million for the
year ended December 31, 2008 and for the period from
September 6, 2007 (inception) to December 31, 2007,
respectively) of Regulus Therapeutics LLC, is based solely on
the report of the other auditors. We conducted our audits in
accordance with the standards of the Public Company Accounting
Oversight Board (United States). Those standards require that we
plan and perform the audits to obtain reasonable assurance about
whether the financial statements are free of material
misstatement and whether effective internal control over
financial reporting was maintained in all material respects. Our
audits of the financial statements included examining, on a test
basis, evidence supporting the amounts and disclosures in the
financial statements, assessing the accounting principles used
and significant estimates made by management, and evaluating the
overall financial statement presentation. Our audit of internal
control over financial reporting included obtaining an
understanding of internal control over financial reporting,
assessing the risk that a material weakness exists, and testing
and evaluating the design and operating effectiveness of
internal control based on the assessed risk. Our audits also
included performing such other procedures as we considered
necessary in the circumstances. We believe that our audits and
the report of other auditors provide a reasonable basis for our
opinions.
A company’s internal control over financial reporting is a
process designed to provide reasonable assurance regarding the
reliability of financial reporting and the preparation of
financial statements for external purposes in accordance with
generally accepted accounting principles. A company’s
internal control over financial reporting includes those
policies and procedures that (i) pertain to the maintenance
of records that, in reasonable detail, accurately and fairly
reflect the transactions and dispositions of the assets of the
company; (ii) provide reasonable assurance that
transactions are recorded as necessary to permit preparation of
financial statements in accordance with generally accepted
accounting principles, and that receipts and expenditures of the
company are being made only in accordance with authorizations of
management and directors of the company; and (iii) provide
reasonable assurance regarding prevention or timely detection of
unauthorized acquisition, use, or disposition of the
company’s assets that could have a material effect on the
financial statements.
Because of its inherent limitations, internal control over
financial reporting may not prevent or detect misstatements.
Also, projections of any evaluation of effectiveness to future
periods are subject to the risk that controls may become
inadequate because of changes in conditions, or that the degree
of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
Boston, Massachusetts
March 2, 2009
89
ALNYLAM
PHARMACEUTICALS, INC.
CONSOLIDATED
BALANCE SHEETS
(In thousands, except share and per share amounts)
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2008
|
|
|
2007
|
|
|
|
|
ASSETS
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
191,792
|
|
|
$
|
105,157
|
|
|
Marketable securities
|
|
|
238,596
|
|
|
|
284,791
|
|
|
Collaboration receivables
|
|
|
4,188
|
|
|
|
5,031
|
|
|
Prepaid expenses and other current assets
|
|
|
4,674
|
|
|
|
2,926
|
|
|
Restricted cash
|
|
|
2,999
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
442,249
|
|
|
|
397,905
|
|
|
Marketable securities
|
|
|
82,321
|
|
|
|
65,654
|
|
|
Property and equipment, net
|
|
|
19,194
|
|
|
|
13,810
|
|
|
Deferred tax assets
|
|
|
5,382
|
|
|
|
—
|
|
|
Investment in joint venture (Regulus Therapeutics LLC)
|
|
|
1,583
|
|
|
|
9,129
|
|
|
Intangible assets, net
|
|
|
795
|
|
|
|
968
|
|
|
Restricted cash, net of current portion
|
|
|
3,152
|
|
|
|
6,152
|
|
|
Other assets
|
|
|
—
|
|
|
|
173
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
554,676
|
|
|
$
|
493,791
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
2,588
|
|
|
$
|
3,826
|
|
|
Accrued expenses
|
|
|
9,328
|
|
|
|
11,724
|
|
|
Income taxes payable
|
|
|
6,111
|
|
|
|
3,497
|
|
|
Current portion of notes payable
|
|
|
—
|
|
|
|
3,795
|
|
|
Deferred rent
|
|
|
1,561
|
|
|
|
1,387
|
|
|
Deferred revenue
|
|
|
79,864
|
|
|
|
59,249
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
99,452
|
|
|
|
83,478
|
|
|
Deferred rent, net of current portion
|
|
|
2,732
|
|
|
|
3,813
|
|
|
Deferred revenue, net of current portion
|
|
|
250,121
|
|
|
|
204,067
|
|
|
Notes payable, net of current portion
|
|
|
—
|
|
|
|
2,963
|
|
|
Other long-term liabilities
|
|
|
246
|
|
|
|
302
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
352,551
|
|
|
|
294,623
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies (Notes 7 and 12)
|
|
|
—
|
|
|
|
—
|
|
|
Stockholders’ equity:
|
|
|
|
|
|
|
|
|
|
Preferred stock, $0.01 par value, 5,000,000 shares
authorized and no shares issued and outstanding at
December 31, 2008 and 2007
|
|
|
—
|
|
|
|
—
|
|
|
Common stock, $0.01 par value, 125,000,000 shares
authorized; 41,413,828 shares issued and outstanding at
December 31, 2008; 40,772,967 shares issued and
outstanding at December 31, 2007
|
|
|
414
|
|
|
|
408
|
|
|
Additional paid-in capital
|
|
|
452,767
|
|
|
|
424,453
|
|
|
Accumulated other comprehensive income
|
|
|
1,186
|
|
|
|
300
|
|
|
Accumulated deficit
|
|
|
(252,242
|
)
|
|
|
(225,993
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
202,125
|
|
|
|
199,168
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity
|
|
$
|
554,676
|
|
|
$
|
493,791
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these
consolidated financial statements.
90
ALNYLAM
PHARMACEUTICALS, INC.
CONSOLIDATED
STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(In thousands, except per share amounts)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
|
|
Net revenues from research collaborators
|
|
$
|
96,163
|
|
|
$
|
50,897
|
|
|
$
|
26,930
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development(1)
|
|
|
96,883
|
|
|
|
120,686
|
|
|
|
49,260
|
|
|
General and administrative(1)
|
|
|
27,115
|
|
|
|
23,388
|
|
|
|
17,171
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
|
123,998
|
|
|
|
144,074
|
|
|
|
66,431
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(27,835
|
)
|
|
|
(93,177
|
)
|
|
|
(39,501
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity in loss of joint venture (Regulus Therapeutics LLC)
|
|
|
(9,290
|
)
|
|
|
(1,075
|
)
|
|
|
—
|
|
|
Interest income
|
|
|
14,414
|
|
|
|
15,393
|
|
|
|
6,177
|
|
|
Interest expense
|
|
|
(872
|
)
|
|
|
(1,083
|
)
|
|
|
(1,029
|
)
|
|
Other expense
|
|
|
(1,947
|
)
|
|
|
(279
|
)
|
|
|
(255
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total other income (expense)
|
|
|
2,305
|
|
|
|
12,956
|
|
|
|
4,893
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss before income taxes
|
|
|
(25,530
|
)
|
|
|
(80,221
|
)
|
|
|
(34,608
|
)
|
|
Provision for income taxes
|
|
|
(719
|
)
|
|
|
(5,245
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(26,249
|
)
|
|
$
|
(85,466
|
)
|
|
$
|
(34,608
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per common share — basic and diluted
|
|
$
|
(0.64
|
)
|
|
$
|
(2.21
|
)
|
|
$
|
(1.09
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares used to compute basic and diluted
net loss per common share
|
|
|
41,077
|
|
|
|
38,657
|
|
|
|
31,890
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive loss:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(26,249
|
)
|
|
$
|
(85,466
|
)
|
|
$
|
(34,608
|
)
|
|
Foreign currency translation
|
|
|
53
|
|
|
|
(598
|
)
|
|
|
665
|
|
|
Unrealized gain on marketable securities
|
|
|
833
|
|
|
|
258
|
|
|
|
117
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive loss
|
|
$
|
(25,363
|
)
|
|
$
|
(85,806
|
)
|
|
$
|
(33,826
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
Non-cash stock-based compensation expenses included in operating
expenses are as follows: |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
$
|
9,575
|
|
|
$
|
9,363
|
|
|
$
|
5,006
|
|
|
General and administrative
|
|
|
6,807
|
|
|
|
5,109
|
|
|
|
3,298
|
|
The accompanying notes are an integral part of these
consolidated financial statements.
91
ALNYLAM
PHARMACEUTICALS, INC.
CONSOLIDATED
STATEMENTS OF STOCKHOLDERS’ EQUITY
(In thousands, except share amounts)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional
|
|
|
Deferred
|
|
|
Other
|
|
|
|
|
|
Total
|
|
|
|
|
Common Stock
|
|
|
Paid-in
|
|
|
Stock-based
|
|
|
Comprehensive
|
|
|
Accumulated
|
|
|
Stockholders’
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
Capital
|
|
|
Compensation
|
|
|
(Loss) Income
|
|
|
Deficit
|
|
|
Equity
|
|
|
|
|
Balance at December 31, 2005
|
|
|
26,638,255
|
|
|
$
|
267
|
|
|
$
|
170,033
|
|
|
$
|
(2,460
|
)
|
|
$
|
(142
|
)
|
|
$
|
(105,919
|
)
|
|
$
|
61,779
|
|
|
Exercise of common stock options and warrants
|
|
|
539,425
|
|
|
|
5
|
|
|
|
998
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,003
|
|
|
Issuance of common stock
|
|
|
56,990
|
|
|
|
1
|
|
|
|
591
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
592
|
|
|
Deferred compensation related to stock options and restricted
stock
|
|
|
—
|
|
|
|
—
|
|
|
|
1,614
|
|
|
|
(467
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
1,147
|
|
|
Amortization of deferred compensation expense related to stock
options and restricted stock
|
|
|
—
|
|
|
|
—
|
|
|
|
4,318
|
|
|
|
2,838
|
|
|
|
—
|
|
|
|
—
|
|
|
|
7,156
|
|
|
Issuance of common stock upon public offerings, net of offering
costs of $6,586
|
|
|
9,815,961
|
|
|
|
98
|
|
|
|
163,225
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
163,323
|
|
|
Foreign currency translation
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
665
|
|
|
|
—
|
|
|
|
665
|
|
|
Unrealized gain on marketable securities
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
117
|
|
|
|
—
|
|
|
|
117
|
|
|
Net loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(34,608
|
)
|
|
|
(34,608
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2006
|
|
|
37,050,631
|
|
|
|
371
|
|
|
|
340,779
|
|
|
|
(89
|
)
|
|
|
640
|
|
|
|
(140,527
|
)
|
|
|
201,174
|
|
|
Exercise of common stock options
|
|
|
1,247,808
|
|
|
|
12
|
|
|
|
9,232
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
9,244
|
|
|
Issuance of common stock
|
|
|
2,474,528
|
|
|
|
25
|
|
|
|
59,874
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
59,899
|
|
|
Stock-based compensation expense
|
|
|
—
|
|
|
|
—
|
|
|
|
14,364
|
|
|
|
89
|
|
|
|
—
|
|
|
|
—
|
|
|
|
14,453
|
|
|
Foreign currency translation
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(598
|
)
|
|
|
—
|
|
|
|
(598
|
)
|
|
Joint venture stock compensation (Regulus Therapeutics LLC)
|
|
|
—
|
|
|
|
—
|
|
|
|
204
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
204
|
|
|
Unrealized gain on marketable securities
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
258
|
|
|
|
—
|
|
|
|
258
|
|
|
Net loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(85,466
|
)
|
|
|
(85,466
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2007
|
|
|
40,772,967
|
|
|
|
408
|
|
|
|
424,453
|
|
|
|
—
|
|
|
|
300
|
|
|
|
(225,993
|
)
|
|
|
199,168
|
|
|
Exercise of common stock options
|
|
|
377,228
|
|
|
|
4
|
|
|
|
3,782
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
3,786
|
|
|
Issuance of common stock
|
|
|
263,633
|
|
|
|
2
|
|
|
|
6,507
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
6,509
|
|
|
Stock-based compensation expense
|
|
|
—
|
|
|
|
—
|
|
|
|
16,381
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
16,381
|
|
|
Foreign currency translation
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
53
|
|
|
|
—
|
|
|
|
53
|
|
|
Joint venture stock compensation (Regulus Therapeutics LLC)
|
|
|
—
|
|
|
|
—
|
|
|
|
1,644
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,644
|
|
|
Unrealized gain on marketable securities
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
833
|
|
|
|
—
|
|
|
|
833
|
|
|
Net loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(26,249
|
)
|
|
|
(26,249
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2008
|
|
|
41,413,828
|
|
|
$
|
414
|
|
|
$
|
452,767
|
|
|
$
|
—
|
|
|
$
|
1,186
|
|
|
$
|
(252,242
|
)
|
|
$
|
202,125
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these
consolidated financial statements.
92
ALNYLAM
PHARMACEUTICALS, INC.
CONSOLIDATED
STATEMENTS OF CASH FLOWS
(In thousands)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
|
|
Cash flows from operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(26,249
|
)
|
|
$
|
(85,466
|
)
|
|
$
|
(34,608
|
)
|
|
Adjustments to reconcile net loss to net cash provided by (used
in) operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
5,726
|
|
|
|
4,082
|
|
|
|
4,070
|
|
|
Deferred income tax (benefit) provision
|
|
|
(5,501
|
)
|
|
|
1,889
|
|
|
|
—
|
|
|
Non-cash stock-based compensation
|
|
|
18,026
|
|
|
|
14,676
|
|
|
|
8,304
|
|
|
Non-cash license expense
|
|
|
—
|
|
|
|
7,909
|
|
|
|
130
|
|
|
Charge for 401(k) company stock match
|
|
|
382
|
|
|
|
407
|
|
|
|
129
|
|
|
Equity in loss of joint venture (Regulus Therapeutics LLC)
|
|
|
7,646
|
|
|
|
871
|
|
|
|
—
|
|
|
Impairment on equity investment
|
|
|
1,561
|
|
|
|
—
|
|
|
|
—
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from landlord tenant improvements
|
|
|
581
|
|
|
|
2,621
|
|
|
|
1,106
|
|
|
Collaboration receivables
|
|
|
843
|
|
|
|
(1,194
|
)
|
|
|
(3,220
|
)
|
|
Prepaid expenses and other assets
|
|
|
(1,748
|
)
|
|
|
(4,348
|
)
|
|
|
(336
|
)
|
|
Accounts payable
|
|
|
(1,238
|
)
|
|
|
(264
|
)
|
|
|
2,088
|
|
|
Income taxes payable
|
|
|
2,614
|
|
|
|
3,497
|
|
|
|
—
|
|
|
Accrued expenses and other
|
|
|
(3,821
|
)
|
|
|
6,843
|
|
|
|
611
|
|
|
Deferred revenue
|
|
|
66,669
|
|
|
|
244,996
|
|
|
|
(2,904
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) operating activities
|
|
|
65,491
|
|
|
|
196,519
|
|
|
|
(24,630
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from investing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of property and equipment
|
|
|
(10,764
|
)
|
|
|
(7,788
|
)
|
|
|
(4,986
|
)
|
|
Disposals of property and equipment
|
|
|
—
|
|
|
|
2,342
|
|
|
|
—
|
|
|
Increase in restricted cash
|
|
|
—
|
|
|
|
(839
|
)
|
|
|
—
|
|
|
Purchases of marketable securities
|
|
|
(482,244
|
)
|
|
|
(544,394
|
)
|
|
|
(172,303
|
)
|
|
Sales and maturities of marketable securities
|
|
|
511,044
|
|
|
|
283,254
|
|
|
|
147,243
|
|
|
Investment in joint venture (Regulus Therapeutics LLC)
|
|
|
(100
|
)
|
|
|
(10,000
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) investing activities
|
|
|
17,936
|
|
|
|
(277,425
|
)
|
|
|
(30,046
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of common stock, net of issuance costs
|
|
|
4,505
|
|
|
|
61,011
|
|
|
|
164,890
|
|
|
Proceeds from issuance of shares to Novartis
|
|
|
5,408
|
|
|
|
—
|
|
|
|
—
|
|
|
Proceeds from notes payable
|
|
|
—
|
|
|
|
957
|
|
|
|
4,000
|
|
|
Repayments of notes payable
|
|
|
(6,758
|
)
|
|
|
(3,333
|
)
|
|
|
(2,259
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by financing activities
|
|
|
3,155
|
|
|
|
58,635
|
|
|
|
166,631
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Effect of exchange rate on cash
|
|
|
53
|
|
|
|
(527
|
)
|
|
|
243
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase (decrease) in cash and cash equivalents
|
|
|
86,635
|
|
|
|
(22,798
|
)
|
|
|
112,198
|
|
|
Cash and cash equivalents, beginning of period
|
|
|
105,157
|
|
|
|
127,955
|
|
|
|
15,757
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, end of period
|
|
$
|
191,792
|
|
|
$
|
105,157
|
|
|
$
|
127,955
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosure of cash flows
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash paid for interest
|
|
$
|
1,499
|
|
|
$
|
890
|
|
|
$
|
726
|
|
|
Cash paid for income taxes
|
|
$
|
2,671
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
Supplemental disclosure of non-cash financing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued in connection with license agreements
|
|
$
|
—
|
|
|
$
|
7,909
|
|
|
$
|
130
|
|
The accompanying notes are an integral part of these
consolidated financial statements.
93
ALNYLAM
PHARMACEUTICALS, INC.
Alnylam Pharmaceuticals, Inc. (the “Company” or
“Alnylam”) commenced operations on June 14, 2002
as a biopharmaceutical company seeking to develop and
commercialize novel therapeutics based on RNA interference
(“RNAi”). Alnylam is focused on discovering,
developing and commercializing RNAi therapeutics by establishing
strategic alliances with leading pharmaceutical and
biotechnology companies, establishing and maintaining a strong
intellectual property position in the RNAi field, generating
revenues through licensing agreements and ultimately developing
and commercializing RNAi therapeutics for its own account. The
Company has devoted substantially all of its efforts to business
planning, research and development, acquiring, filing and
expanding intellectual property rights, recruiting management
and technical staff, and raising capital.
|
|
|
2.
|
SUMMARY
OF SIGNIFICANT ACCOUNTING POLICIES
|
Basis
of Presentation and Principles of Consolidation
The Company comprises four entities, Alnylam Pharmaceuticals,
Inc. (the parent company) and three wholly-owned subsidiaries
(Alnylam U.S., Inc., Alnylam Europe AG (“Alnylam
Europe”) and Alnylam Securities Corporation). Alnylam
Pharmaceuticals, Inc. is a Delaware corporation that was formed
on May 8, 2003. Alnylam U.S., Inc. is also a Delaware
corporation that was formed on June 14, 2002. Alnylam
Securities Corporation is a Massachusetts corporation that was
formed on December 19, 2006. Alnylam Europe was
incorporated in Germany in June 2000 under the name Ribopharma
AG. The Company acquired Alnylam Europe in July 2003.
The accompanying consolidated financial statements reflect the
operations of the Company and its wholly-owned subsidiaries. All
significant intercompany accounts and transactions have been
eliminated. The Company uses the equity method of accounting to
account for its investment in Regulus Therapeutics LLC
(“Regulus Therapeutics”).
Reclassifications
Certain reclassifications have been made to prior years’
financial statements to conform to the 2008 presentation.
Use of
Estimates
The preparation of financial statements in conformity with
accounting principles generally accepted in the United States of
America requires management to make estimates and assumptions
that affect the reported amounts of assets and liabilities and
the disclosure of contingent assets and liabilities at the date
of the consolidated financial statements and the reported
amounts of revenues and expenses during the reporting period.
Actual results could differ from those estimates.
Concentrations
of Credit Risk and Significant Customers
Financial instruments which potentially expose the Company to
concentrations of credit risk consist primarily of cash, cash
equivalents and marketable securities. As of December 31,
2008 and 2007, substantially all of the Company’s cash,
cash equivalents and marketable securities were invested in
money market mutual funds, commercial paper, corporate notes and
government securities through highly rated financial
institutions.
To date, the Company’s revenues from collaborations have
been generated from primarily F. Hoffmann-La Roche Ltd and
certain of its affiliates (collectively, “Roche”),
Novartis Pharma AG and one of its affiliates (collectively,
“Novartis”) and Takeda Pharmaceutical Company Limited
(“Takeda”). Novartis owned approximately 13.2% of the
Company’s outstanding common stock as of December 31,
2008. In 2008, the Company had revenue from Roche, Takeda and
Novartis, which accounted for 57%, 13% and 12%, respectively, of
94
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
the Company’s total revenue. In 2007, the Company had
revenue from Roche, Novartis and the National Institute of
Allergy and Infectious Diseases (“NIAID”), a component
of the National Institutes of Health (“NIH”), which
accounted for 35%, 29% and 15%, respectively, of the
Company’s total revenue. In 2006, the Company had revenue
from Novartis, which accounted for 81% of the Company’s
total revenue. Receivables from Novartis, NIAID and the Defense
Threat Reduction Agency (“DTRA”), an agency of the
United States Department of Defense, represented approximately
55%, 22% and 10%, respectively, of the Company’s
collaboration receivables balance at December 31, 2008.
Receivables from Novartis, NIAID and DTRA represented
approximately 43%, 36% and 15%, respectively, of the
Company’s collaboration receivables balance at
December 31, 2007.
Fair
Value Measurements
Effective January 1, 2008, the Company implemented
Statement of Financial Accounting Standards (“SFAS”)
No. 157, “Fair Value Measurements”
(“SFAS 157”), which addresses how companies
should measure fair value when they are required to do so for
recognition or disclosure purposes. The standard provides a
common definition of fair value and is intended to make the
measurement of fair value more consistent and comparable as well
as to improve disclosures about those measures. This standard
formalizes the measurement principles to be utilized in
determining fair value for purposes such as derivative valuation
and impairment analysis. The partial adoption of SFAS 157
by the Company has had no impact on the Company’s operating
results or financial position. The Company is evaluating the
impact, if any, full adoption of this standard will have on its
non-financial assets and liabilities within the scope of
SFAS 157. For recognition purposes, on a recurring basis,
the Company is required to measure certain cash equivalents and
available-for-sale investments at fair value. Changes in the
fair value of these investments historically have been
insignificant.
The following table presents information about the
Company’s assets that are measured at fair value on a
recurring basis as of December 31, 2008, and indicates the
fair value hierarchy of the valuation techniques the Company
utilized to determine such fair value. In general, fair values
determined by Level 1 inputs utilize quoted prices
(unadjusted) in active markets for identical assets or
liabilities. Fair values determined by Level 2 inputs
utilize data points that are observable, such as quoted prices
(adjusted), interest rates and yield curves. Fair values
determined by Level 3 inputs utilize unobservable data
points for the asset or liability, and include situations where
there is little, if any, market activity for the asset or
liability. Financial assets and liabilities measured at fair
value on a recurring basis are summarized as follows, in
thousands:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quoted
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Prices in
|
|
|
Significant
|
|
|
Significant
|
|
|
|
|
As of
|
|
|
Active
|
|
|
Observable
|
|
|
Unobservable
|
|
|
|
|
December 31,
|
|
|
Markets
|
|
|
Inputs
|
|
|
Inputs
|
|
|
Description
|
|
2008
|
|
|
(Level 1)
|
|
|
(Level 2)
|
|
|
(Level 3)
|
|
|
|
|
Cash equivalents
|
|
$
|
187,057
|
|
|
$
|
167,293
|
|
|
$
|
19,764
|
|
|
$
|
—
|
|
|
Marketable securities (fixed income)
|
|
|
320,269
|
|
|
|
—
|
|
|
|
320,269
|
|
|
|
—
|
|
|
Marketable securities (equity holdings)
|
|
|
648
|
|
|
|
—
|
|
|
|
648
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
507,974
|
|
|
$
|
167,293
|
|
|
$
|
340,681
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The carrying amounts reflected in the Company’s
consolidated balance sheets for cash, collaboration receivables,
other current assets, accounts payable and accrued expenses
approximate fair value due to their short-term maturities.
The Company adopted the provisions of SFAS No. 159,
“The Fair Value Option for Financial Assets and
Financial Liabilities” (“SFAS 159”),
effective January 1, 2008. SFAS 159 allows an entity
the irrevocable option to elect fair value for the initial and
subsequent measurement for certain financial assets and
liabilities on a
contract-by-contract
basis. The Company has not elected the fair value option for any
of its financial assets or liabilities in the year ended
December 31, 2008.
95
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
Investments
in Marketable Securities
The Company invests its excess cash balances in short-term and
long-term marketable debt and equity securities. The Company
accounts for its investments in debt and equity securities under
SFAS No. 115, “Accounting for Certain
Investments in Debt and Equity Securities.” The Company
classifies its investments in marketable debt securities as
either held-to-maturity or available-for-sale based on facts and
circumstances present at the time it purchased the securities.
As of each balance sheet date presented, the Company classified
all of its investments in debt securities as available-for-sale.
The Company reports available-for-sale investments at fair value
as of each balance sheet date and includes any unrealized
holding gains and losses (the adjustment to fair value) in
stockholders’ equity. Realized gains and losses are
determined using the specific identification method and are
included in investment income. If any adjustment to fair value
reflects a decline in the value of the investment, the Company
considers all available evidence to evaluate the extent to which
the decline is “other than temporary” and, if so,
marks the investment to market through a charge to its
consolidated statement of operations. The Company did not record
any impairment charges related to its fixed income marketable
securities during the years ended December 31, 2008, 2007
or 2006. During 2008, the Company recorded an impairment charge
of $1.6 million related to its equity investment in Tekmira
Pharmaceuticals Corporation (“Tekmira”), as the
decrease in the fair value of this investment was deemed to be
other than temporary. The Company’s marketable securities
are classified as cash equivalents if the original maturity,
from the date of purchase, is 90 days or less, and as
marketable securities if the original maturity, from the date of
purchase, is in excess of 90 days.
The following tables summarize the Company’s marketable
securities at December 31, 2008 and 2007, in thousands:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2008
|
|
|
|
|
|
|
|
Gross
|
|
|
Gross
|
|
|
|
|
|
|
|
Amortized
|
|
|
Unrealized
|
|
|
Unrealized
|
|
|
|
|
|
|
|
Cost
|
|
|
Gains
|
|
|
Losses
|
|
|
Fair Value
|
|
|
|
|
Commercial paper (Due within 1 year)
|
|
$
|
56,014
|
|
|
$
|
119
|
|
|
$
|
—
|
|
|
$
|
56,133
|
|
|
Corporate notes (Due within 1 year)
|
|
|
37,504
|
|
|
|
262
|
|
|
|
(102
|
)
|
|
|
37,664
|
|
|
Corporate notes (Due after 1 year through 2 years)
|
|
|
30,497
|
|
|
|
81
|
|
|
|
(106
|
)
|
|
|
30,472
|
|
|
U.S. Government obligations (Due within 1 year)
|
|
|
143,872
|
|
|
|
927
|
|
|
|
—
|
|
|
|
144,799
|
|
|
U.S. Government obligations (Due after 1 year through
2 years)
|
|
|
50,649
|
|
|
|
552
|
|
|
|
—
|
|
|
|
51,201
|
|
|
Equity securities
|
|
|
1,345
|
|
|
|
—
|
|
|
|
(697
|
)
|
|
|
648
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
319,881
|
|
|
$
|
1,941
|
|
|
$
|
(905
|
)
|
|
$
|
320,917
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2007
|
|
|
|
|
|
|
|
Gross
|
|
|
Gross
|
|
|
|
|
|
|
|
Amortized
|
|
|
Unrealized
|
|
|
Unrealized
|
|
|
|
|
|
|
|
Cost
|
|
|
Gains
|
|
|
Losses
|
|
|
Fair Value
|
|
|
|
|
Commercial paper (Due within 1 year)
|
|
$
|
210,213
|
|
|
$
|
177
|
|
|
$
|
(1
|
)
|
|
$
|
210,389
|
|
|
Municipal notes (Due within 1 year)
|
|
|
61,605
|
|
|
|
—
|
|
|
|
—
|
|
|
|
61,605
|
|
|
Municipal notes (Due after 1 year through 2 years)
|
|
|
7,340
|
|
|
|
12
|
|
|
|
—
|
|
|
|
7,352
|
|
|
Corporate notes (Due within 1 year)
|
|
|
6,452
|
|
|
|
—
|
|
|
|
(12
|
)
|
|
|
6,440
|
|
|
Corporate notes (Due after 1 year through 2 years)
|
|
|
37,345
|
|
|
|
108
|
|
|
|
(41
|
)
|
|
|
37,412
|
|
|
U.S. Government obligations (Due after 1 year through
2 years)
|
|
|
27,193
|
|
|
|
54
|
|
|
|
—
|
|
|
|
27,247
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
350,148
|
|
|
$
|
351
|
|
|
$
|
(54
|
)
|
|
$
|
350,445
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
96
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
Revenue
Recognition
The Company has entered into collaboration agreements with
biotechnology and pharmaceutical companies, including Roche,
Takeda, Novartis, Kyowa Hakko Kirin Co., Ltd. (“Kyowa
Hakko”), Biogen Idec Inc. (“Biogen Idec”) and
Merck & Co., Inc. (“Merck”). The terms of
the Company’s collaboration agreements typically include
non-refundable license fees, funding of research and
development, payments based upon achievement of clinical and
pre-clinical development milestones and royalties on product
sales. The Company follows the provisions of the Securities and
Exchange Commission’s (“SEC”) Staff Accounting
Bulletin (“SAB”) No. 104, “Revenue
Recognition in Financial Statements,” Emerging Issues
Task Force (“EITF”) Issue
No. 00-21,
“Revenue Arrangements with Multiple Deliverables”
(“EITF 00-21”),
EITF Issue
No. 99-19,
“Reporting Revenue Gross as a Principal Versus Net as an
Agent,” and EITF Issue
No. 01-9,
“Accounting for Consideration Given by a Vendor to a
Customer (Including a Reseller of the Vendor’s
Products)”
(“EITF 01-9”).
Non-refundable license fees are recognized as revenue upon
delivery of the license only if the Company has a contractual
right to receive such payment, the contract price is fixed or
determinable, the collection of the resulting receivable is
reasonably assured and the Company has no further performance
obligations under the license agreement. Multiple element
arrangements, such as license and development arrangements, are
analyzed to determine whether the deliverables, which often
include a license and performance obligations such as research
and steering committee services, can be separated or whether
they must be accounted for as a single unit of accounting in
accordance with
EITF 00-21.
The Company recognizes up-front license payments as revenue upon
delivery of the license only if the license has stand-alone
value and the fair value of the undelivered performance
obligations, typically including research
and/or
steering committee services, can be determined. If the fair
value of the undelivered performance obligations can be
determined, such obligations are accounted for separately as
such obligations are fulfilled. If the license is considered to
either not have stand-alone value or have stand-alone value but
the fair value of any of the undelivered performance obligations
cannot be determined, the arrangement would then be accounted
for as a single unit of accounting and the license payments and
payments for performance obligations are recognized as revenue
over the estimated period of when the performance obligations
are performed.
Whenever the Company determines that an arrangement should be
accounted for as a single unit of accounting, the Company
determines the period over which the performance obligations
will be performed and revenue will be recognized. Revenue will
be recognized using either a proportional performance or
straight-line method. The Company recognizes revenue using the
proportional performance method when the level of effort
required to complete its performance obligations under an
arrangement can be reasonably estimated and such performance
obligations are provided on a best-efforts basis. Direct labor
hours or full-time equivalents are typically used as the measure
of performance. Revenue recognized under the proportional
performance method would be determined by multiplying the total
payments under the contract, excluding royalties and payments
contingent upon achievement of substantive milestones, by the
ratio of level of effort incurred to date to estimated total
level of effort required to complete the Company’s
performance obligations under the arrangement. Revenue is
limited to the lesser of the cumulative amount of payments
received or the cumulative amount of revenue earned, as
determined using the proportional performance method, as of the
period ending date.
If the level of effort to complete its performance obligations
under an arrangement cannot be reasonably estimated, then
revenue under the arrangement would be recognized as revenue on
a straight-line basis over the period the Company is expected to
complete its performance obligations. Revenue is limited to the
lesser of the cumulative amount of payments received or the
cumulative amount of revenue earned, as determined using the
straight-line method, as of the period ending date.
Many of the Company’s collaboration agreements entitle it
to additional payments upon the achievement of performance-based
milestones. If the achievement of a milestone is considered
probable at the inception of the collaboration, the related
milestone payment is included with other collaboration
consideration, such as up-front fees and research funding, in
the Company’s revenue model. Milestones that involve
substantial effort on the Company’s part and the
achievement of which are not considered probable at the
inception of the collaboration are
97
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
considered “substantive milestones.” Substantive
milestones are included in the Company’s revenue model when
achievement of the milestone is considered probable. As future
substantive milestones are achieved, a portion of the milestone
payment, equal to the percentage of the performance period
completed when the milestone is achieved, multiplied by the
amount of the milestone payment, will be recognized as revenue
upon achievement of such milestone. The remaining portion of the
milestone will be recognized over the remaining performance
period using the proportional performance or straight-line
method. Milestones that are tied to regulatory approval are not
considered probable of being achieved until such approval is
received. Milestones tied to counter-party performance are not
included in the Company’s revenue model until the
performance conditions are met.
Steering committee services that are not inconsequential or
perfunctory and that are determined to be performance
obligations are combined with other research services or
performance obligations required under an arrangement, if any,
in determining the level of effort required in an arrangement
and the period over which the Company expects to complete its
aggregate performance obligations.
For revenue generating arrangements where the Company, as a
vendor, provides consideration to a licensor or collaborator, as
a customer, the Company applies the provisions of
EITF 01-9.
EITF 01-9
addresses the accounting for revenue arrangements where both the
vendor and the customer make cash payments to each other for
services
and/or
products. A payment to a customer is presumed to be a reduction
of the selling price unless the Company receives an identifiable
benefit for the payment and it can reasonably estimate the fair
value of the benefit received. Payments to a customer that are
deemed a reduction of selling price are recorded first as a
reduction of revenue, to the extent of both cumulative revenue
recorded to date and probable future revenues, which include any
unamortized deferred revenue balances, under all arrangements
with such customer, and then as an expense. Payments that are
not deemed to be a reduction of selling price would be recorded
as an expense.
Amounts received prior to satisfying the above revenue
recognition criteria are recorded as deferred revenue in the
accompanying consolidated balance sheets. Amounts not expected
to be recognized within the next 12 months are classified
as long-term deferred revenue. As of December 31, 2008, the
Company had short-term and long-term deferred revenue of
$79.9 million and $250.1 million, respectively,
related to its collaborations.
Income
Taxes
The Company uses the asset and liability method of accounting
for income taxes. Under the asset and liability method, deferred
tax assets and liabilities reflect the impact of temporary
differences between amounts of assets and liabilities for
financial reporting purposes and such amounts as measured under
enacted tax laws. A valuation allowance is required to offset
any net deferred tax assets if, based upon the available
evidence, it is more likely than not that some or all of the
deferred tax asset will not be realized.
Research
and Development Costs
Research and development costs are expensed as incurred.
Included in research and development costs are wages, benefits
and other operating costs, facilities, supplies, external
services, clinical trial and manufacturing costs and overhead
directly related to the Company’s research and development
department as well as costs to acquire technology licenses.
The Company has entered into several license agreements for
rights to utilize certain technologies. The terms of the
licenses may provide for upfront payments, annual maintenance
payments, milestone payments based upon certain specified events
being achieved and royalties on product sales. Costs to acquire
and maintain licensed technology that has not reached
technological feasibility and does not have alternative future
use are charged to research and development expense as incurred.
During the years ended December 31, 2008, 2007 and 2006,
the Company charged to research and development expense
$12.6 million, $42.2 million and $4.0 million,
respectively, of costs associated with license fees. License
fees for 2007 were primarily the result of $27.5 million in
payments to
98
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
certain entities, primarily Isis Pharmaceuticals, Inc.
(“Isis”), in connection with the Roche alliance and
$14.7 million in charges for licenses for certain delivery
technologies.
Accounting
for Stock-Based Compensation
Effective January 1, 2006, the Company adopted the fair
value recognition provisions of SFAS No. 123R,
“Share-Based Payment, an amendment of FASB Statements
Nos. 123 and 95” (“SFAS 123R”), using
the modified-prospective-transition method. The Company has
stock option plans and an employee stock purchase plan under
which it grants equity instruments that are required to be
evaluated under SFAS 123R. For stock options granted to
non-employees, the Company recognizes compensation expense in
accordance with the requirements of SFAS No. 123,
“Accounting for Stock-Based Compensation”
(“SFAS 123”) and EITF Issue
No. 96-18,
“Accounting for Equity Instruments that are Issued to
Other Than Employees for Acquiring, or in Conjunction with
Selling, Goods or Services” under which compensation
expense is generally recognized over the vesting period of the
award, which is generally the period during which services are
rendered by such non-employees. At the end of each financial
reporting period prior to vesting, the value of these options
(as calculated using the Black-Scholes option-pricing model) is
re-measured using the then-current fair value of the
Company’s common stock. Stock options granted by the
Company to non-employees, other than members of the
Company’s Board of Directors, generally vest over a
four-year service period. The Company accounts for non-employee
grants as an expense over the vesting period of the underlying
stock options using the method prescribed by Financial
Accounting Standards Board (“FASB”) Interpretation
No. 28, “Accounting for Stock Appreciation Rights
and Other Variable Stock Option or Award Plans — an
interpretation of APB Opinions No. 15 and 25”
(“FIN 28”).
Foreign
Currency
The Company’s foreign subsidiary, Alnylam Europe (a
German-based company), has designated its local currency, the
Euro, as its functional currency. Financial statements of this
foreign subsidiary are translated to United States dollars for
consolidation purposes using current rates of exchange for
assets and liabilities; equity is translated using historical
exchange rates; and revenue and expense amounts are translated
using the average exchange rate for the period. Net unrealized
gains and losses resulting from foreign currency translation are
included in other comprehensive income (loss) which is a
separate component of stockholders’ equity. Net realized
gains and losses from foreign currency transactions are included
in the consolidated statements of operations. The Company
recognized a loss of $0.4 million during 2008, a gain of
$0.1 million during 2007 and a loss of $0.3 million
during 2006 from foreign currency transactions.
Comprehensive
Loss
Comprehensive loss is comprised of net loss and certain changes
in stockholders’ equity that are excluded from net loss.
The Company includes foreign currency translation adjustments in
other comprehensive loss for Alnylam Europe as the functional
currency is not the United States dollar. The Company also
includes unrealized gains and losses on certain marketable
securities in other comprehensive loss.
Net
Loss Per Common Share
The Company accounts for and discloses net loss per common share
in accordance with SFAS No. 128, “Earnings per
Share.” Basic net loss per common share is computed by
dividing net loss attributable to common stockholders by the
weighted average number of common shares outstanding. Diluted
net loss per common share is computed by dividing net loss
attributable to common stockholders by the weighted average
number of common shares and dilutive potential common share
equivalents then outstanding. Potential common shares consist of
shares issuable upon the exercise of stock options (using the
treasury stock method), and unvested restricted stock awards.
Because the inclusion of potential common shares would be
anti-dilutive for all periods presented, diluted net loss per
common share is the same as basic net loss per common share.
99
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
The following table sets forth for the periods presented the
potential common shares (prior to consideration of the treasury
stock method) excluded from the calculation of net loss per
common share because their inclusion would be anti-dilutive, in
thousands:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
|
|
Options to purchase common stock
|
|
|
7,037
|
|
|
|
5,304
|
|
|
|
4,650
|
|
|
Unvested restricted common stock
|
|
|
29
|
|
|
|
57
|
|
|
|
—
|
|
|
Options that were exercised before vesting
|
|
|
—
|
|
|
|
11
|
|
|
|
40
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7,066
|
|
|
|
5,372
|
|
|
|
4,690
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Segment
Information
The Company operates in a single reporting segment, the
discovery, development and commercialization of RNAi
therapeutics. The majority of the Company’s net revenues
from research collaborators was derived in the United States.
Recent
Accounting Pronouncements
Effective January 1, 2008, the Company implemented
SFAS 157. SFAS 157 clarifies the principle that fair
value should be based on the assumptions market participants
would use when pricing an asset or liability and establishes a
fair value hierarchy that prioritizes the information used to
develop those assumptions. Under the standard, fair value
measurements would be separately disclosed by level within the
fair value hierarchy. The partial adoption of SFAS 157 by
the Company has had no impact on the Company’s operating
results or financial position. The Company is evaluating the
impact, if any, full adoption of SFAS 157 will have on its
nonfinancial assets and liabilities within the scope of
SFAS 157.
In December 2007, the FASB reached a consensus on EITF Issue
No. 07-1,
“Accounting for Collaborative Arrangements”
(“EITF 07-1”).
EITF 07-1
requires collaborators to present the results of activities for
which they act as the principal on a gross basis and report any
payments received from (made to) other collaborators based on
other applicable GAAP or, in the absence of other applicable
GAAP, based on analogy to authoritative accounting literature or
a reasonable, rational and consistently applied accounting
policy election. Further,
EITF 07-1
clarifies that the determination of whether transactions within
a collaborative arrangement are part of a vendor-customer (or
analogous) relationship subject to
EITF 01-9.
EITF 07-1
became effective on January 1, 2009. The Company is
evaluating the potential impact of
EITF 07-1
on its consolidated financial statements.
In December 2007, the FASB issued SFAS No. 141R,
“Business Combinations”
(“SFAS 141R”). SFAS 141R establishes
principles and requirements for how an acquirer recognizes and
measures in its financial statements the identifiable assets
acquired, the liabilities assumed, any noncontrolling interest
in the acquiree and the goodwill acquired. SFAS 141R also
establishes disclosure requirements to enable the evaluation of
the nature and financial effects of the business combination.
SFAS 141R is effective for fiscal year 2009 and is not
expected to have a material impact on the Company’s
consolidated financial statements.
In December 2007, the FASB issued SFAS No. 160,
“Noncontrolling Interests in Consolidated Financial
Statements, an amendment of ARB No. 51”
(“SFAS 160”). SFAS 160 changes the
accounting for and reporting of noncontrolling or minority
interests (now called noncontrolling interests) in consolidated
financial statements. SFAS 160 became effective on
January 1, 2009. When implemented, prior periods will be
recast for the changes required by SFAS 160. The Company
does not anticipate the adoption of SFAS 160 will have a
material impact on its consolidated financial statements.
In May 2008, the FASB issued SFAS No. 162,
“The Hierarchy of Generally Accepted Accounting
Principles” (“SFAS 162”). SFAS 162
identifies the sources of accounting principles and the
framework for selecting the
100
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
principles used (order of authority) in the preparation of
financial statements that are presented in conformity with
generally accepted accounting standards in the United States.
The adoption of SFAS 162 did not have a material impact on
the Company’s consolidated financial statements.
|
|
|
3.
|
SIGNIFICANT
AGREEMENTS
|
Platform
Alliances
Roche
Alliance
In July 2007, the Company and, for limited purposes, Alnylam
Europe, entered into a License and Collaboration Agreement (the
“LCA”) with Roche. Under the LCA, which became
effective in August 2007, the Company granted Roche a
non-exclusive license to the Company’s intellectual
property to develop and commercialize therapeutic products that
function through RNAi, subject to the Company’s existing
contractual obligations to third parties. The license is
initially limited to the therapeutic areas of oncology,
respiratory diseases, metabolic diseases and certain liver
diseases, and may be expanded to include up to 18 additional
therapeutic areas, comprising all other fields of human disease,
as identified and agreed upon by the parties, upon payment to
the Company by Roche of an additional $50.0 million for
each additional therapeutic area, if any.
In consideration for the rights granted to Roche under the LCA,
Roche paid the Company $273.5 million in upfront cash
payments. In addition, in exchange for the Company’s
contributions under the LCA, for each RNAi therapeutic product
successfully developed by Roche, its affiliates or sublicensees
under the LCA, if any, the Company is entitled to receive
milestone payments upon achievement of specified development and
sales events, totaling up to an aggregate of $100.0 million
per therapeutic target, together with royalty payments based on
worldwide annual net sales, if any.
Under the LCA, the Company and Roche also agreed to collaborate
on the discovery of RNAi therapeutic products directed to one or
more disease targets (“Discovery Collaboration”),
subject to the Company’s existing contractual obligations
to third parties. The collaboration between Roche and the
Company will be governed by a joint steering committee for a
period of five years that is comprised of an equal number of
representatives from each party. In exchange for the
Company’s contributions to the collaboration, Roche will be
required to make additional milestone and royalty payments to
the Company.
The term of the LCA generally ends upon the later of ten years
from the first commercial sale of a licensed product and the
expiration of the last-to-expire patent covering a licensed
product. After the first anniversary of the effective date,
Roche may terminate the LCA, on a licensed
product-by-licensed
product, licensed
patent-by-licensed
patent, and
country-by-country
basis, upon
180-days’
prior written notice, but is required to continue to make
milestone and royalty payments to the Company if any royalties
were payable on net sales of a terminated licensed product
during the previous 12 months. The LCA may also be
terminated by either party in the event the other party fails to
cure a material breach under the LCA.
In July 2007, the Company executed a Common Stock Purchase
Agreement (the “Common Stock Purchase Agreement”) with
Roche Finance Ltd, an affiliate of Roche (“Roche
Finance”). Under the terms of the Common Stock Purchase
Agreement, on August 9, 2007, Roche Finance purchased
1,975,000 shares of the Company’s common stock at
$21.50 per share, for an aggregate purchase price of
$42.5 million. The Company recorded this issuance using the
closing price of the Company’s common stock on
August 9, 2007, the date the shares were issued to Roche.
Based on the closing price of $25.98, the fair value of the
shares issued was $51.3 million, which was
$8.8 million in excess of the proceeds received from Roche
for the issuance of the Company’s common stock. As a
result, the Company allocated $8.8 million of the up-front
payment from the LCA to the common stock issuance.
Under the terms of the Common Stock Purchase Agreement, in the
event the Company proposes to sell or issue any of its equity
securities, subject to specified exceptions, it has agreed to
grant to Roche Finance the right to
101
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
acquire, at fair value, additional securities, such that Roche
Finance would be able to maintain its ownership percentage in
the Company.
In connection with the execution of the LCA and the Common Stock
Purchase Agreement, the Company also executed a Share Purchase
Agreement (the “Alnylam Europe Purchase Agreement”)
with Alnylam Europe and Roche Beteiligungs GmbH, an affiliate of
Roche (“Roche Germany”). Under the terms of the
Alnylam Europe Purchase Agreement, which became effective in
August 2007, the Company created a new, wholly-owned German
limited liability company (“Roche Kulmbach”) into
which substantially all of the non-intellectual property assets
of Alnylam Europe were transferred, and Roche Germany purchased
from the Company all of the issued and outstanding shares of
Roche Kulmbach for an aggregate purchase price of
$15.0 million. The Alnylam Europe Purchase Agreement also
included transition services that were performed by Roche
Kulmbach employees at various levels through August 2008. The
Company reimbursed Roche for these services at an
agreed-upon
rate. The Company recorded as contra revenue (a reduction of
revenues) $1.0 million and $4.2 million for these
services for the years ended December 31, 2008 and 2007,
respectively.
In addition, in connection with the closing of the Alnylam
Europe Purchase Agreement, the Company granted restricted stock
of the Company to certain employees of Roche Kulmbach. In
connection with the closing, the Company also accelerated the
unvested portion of the outstanding stock options of certain
Alnylam Europe employees. The Company recorded $3.8 million
of stock-based compensation expense during 2007 related to the
restricted share grants and the stock option modifications.
In summary, the Company received upfront payments totaling
$331.0 million under the Roche alliance, which include an
upfront payment under the LCA of $273.5 million,
$42.5 million under the Common Stock Purchase Agreement and
$15.0 million for the Roche Kulmbach shares under the
Alnylam Europe Purchase Agreement.
The Company recorded $278.2 million as deferred revenue in
connection with the Roche alliance. This amount represents the
aggregate proceeds received from Roche of $331.0 million,
net of the amount allocated to the common stock issuance of
$51.3 million, and the net book value of Alnylam Europe of
$1.5 million.
The Company has determined that the deliverables under the Roche
alliance include the license, the Alnylam Europe assets and
employees, the steering committees (Joint Steering Committee and
Future Technology Committee) and the services that Alnylam will
be obligated to perform under the Discovery Collaboration. The
Company has concluded that, pursuant to paragraph 9 of
EITF 00-21,
the license and assets of Alnylam Europe are not separable from
the undelivered services (i.e., the steering committees and
Discovery Collaboration) and, accordingly the license and the
services are being treated as a single unit of accounting. When
multiple deliverables are accounted for as a single unit of
accounting, the Company bases its revenue recognition pattern on
the final deliverable. Under the Roche alliance, the steering
committee services and the Discovery Collaboration services are
the final deliverables and all such services will end,
contractually, five years from the effective date of the LCA.
The Company is recognizing the Roche-related revenue on a
straight-line basis over five years because the Company cannot
reasonably estimate the total level of effort required to
complete its service obligations under the LCA. The Company will
continue to reassess whether it can reasonably estimate the
level of effort required to fulfill its obligations under the
Roche alliance. In particular, when the Discovery Collaboration
commences, the Company may be able to make such an estimate.
When, and if, the Company can make a reasonable estimate of its
remaining efforts under the collaboration, the Company would
modify its method of recognition and utilize a proportional
performance method. As future substantive milestones are
achieved, a portion of the milestone payment, equal to the
percentage of the performance period completed when the
milestone is achieved, multiplied by the amount of the milestone
payment, will be recognized as revenue upon achievement of such
milestone. The remaining portion of the milestone will be
recognized over the remaining performance period on a
straight-line basis.
In connection with the LCA and the Common Stock Purchase
Agreement, the Company paid $27.5 million of license fees
to the Company’s licensors, primarily Isis, during 2007, in
accordance with the applicable license agreements with those
parties. These fees were charged to research and development
expense.
102
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
Takeda
Alliance
In May 2008, the Company entered into a license and
collaboration agreement (the “Takeda Collaboration
Agreement”) with Takeda to pursue the development and
commercialization of RNAi therapeutics. Under the Takeda
Collaboration Agreement, the Company granted Takeda a
non-exclusive, worldwide, royalty-bearing license to the
Company’s intellectual property to develop, manufacture,
use and commercialize RNAi therapeutics, subject to the
Company’s existing contractual obligations to third
parties. The license initially is limited to the fields of
oncology and metabolic disease and may be expanded at
Takeda’s option to include other therapeutic areas, subject
to specified conditions. Under the Takeda Collaboration
Agreement, Takeda will be the Company’s exclusive platform
partner in the Asian territory, as defined in the Takeda
Collaboration Agreement, for a period of five years.
In consideration for the rights granted to Takeda under the
Takeda Collaboration Agreement, Takeda agreed to pay the Company
$150.0 million in upfront and near-term technology transfer
payments. In addition, the Company has the option, exercisable
until the start of Phase III development, to opt-in under a
50-50 profit
sharing agreement to the development and commercialization in
the United States of up to four Takeda licensed products, and
would be entitled to opt-in rights for two additional products
for each additional field expansion, if any, elected by Takeda
under the Takeda Collaboration Agreement. In June 2008, Takeda
paid the Company an upfront payment of $100.0 million.
Takeda is also required to make the additional
$50.0 million in payments to the Company upon achievement
of specified technology transfer milestones, $20.0 million
of which was achieved in September 2008 and paid in October
2008, $20.0 million of which is required to be paid within
12 to 24 months of execution of the Takeda Collaboration
Agreement and $10.0 million of which is required to be paid
within 24 to 36 months of execution of the Takeda
Collaboration Agreement (collectively, the “Technology
Transfer Milestones”). If Takeda elects to expand its
license to additional therapeutic areas, Takeda will be required
to pay the Company $50.0 million for each of up to
approximately 20 total additional fields selected, comprising
all other fields of human disease, as identified and agreed upon
by the parties. In addition, for each RNAi therapeutic product
developed by Takeda, its affiliates and sublicensees, if any,
the Company is entitled to receive specified development and
commercialization milestones, totaling up to $171.0 million
per product, together with royalty payments based on worldwide
annual net sales, if any.
Pursuant to the Takeda Collaboration Agreement, the Company and
Takeda have also agreed to collaborate on the research of RNAi
therapeutics directed to one or two disease targets agreed to by
the parties (the “Research Collaboration”), subject to
the Company’s existing contractual obligations with third
parties. Takeda also has the option, subject to certain
conditions, to collaborate with the Company on the research and
development of RNAi drug delivery technology for targets agreed
to by the parties. In addition, Takeda has a right of first
negotiation for the development and commercialization of the
Company’s RNAi therapeutic products in the Asian territory,
excluding the Company’s ALN-RSV01 program. In addition to
the 50-50
profit sharing option, the Company has a similar right of first
negotiation to participate with Takeda in the development and
commercialization in the United States of licensed products. The
collaboration between the Company and Takeda is governed by a
joint technology transfer committee (the “JTTC”), a
joint research collaboration committee (the “JRCC”)
and a joint delivery collaboration committee (the
“JDCC”), each of which is comprised of an equal number
of representatives from each party.
The term of the Takeda Collaboration Agreement generally ends
upon the later of (1) the expiration of the Company’s
last-to-expire patent covering a licensed product and
(2) the last-to-expire term of a profit sharing agreement
in the event the Company elects to enter into such an agreement.
The Takeda Collaboration Agreement may be terminated by either
party in the event the other party fails to cure a material
breach under the agreement. In addition, after the first
anniversary of the effective date of the Takeda Collaboration
Agreement, Takeda may terminate the agreement on a licensed
product-by-licensed
product or
country-by-country
basis upon
180-days’
prior written notice to the Company, provided, however, that
Takeda is required to continue to make royalty payments to the
Company for the duration of the royalty term with respect to a
licensed product.
103
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
The Company has determined that the deliverables under the
Takeda agreement include the license, the joint committees (the
JTTC, JRCC and JDCC), the technology transfer activities and the
services that the Company will be obligated to perform under the
Research Collaboration. The Company has determined that,
pursuant to
EITF 00-21,
the license and undelivered services (i.e., the joint committees
and the Research Collaboration) are not separable and,
accordingly, the license and services are being treated as a
single unit of accounting.
When multiple deliverables are accounted for as a single unit of
accounting, the Company bases its revenue recognition pattern on
the final deliverable. Under the Takeda Collaboration Agreement,
the last elements to be delivered are the JDCC and JTTC
services, each of which has a life of no more than seven years.
The Company is recognizing the upfront payment of
$100.0 million, the first Technology Transfer Milestone of
$20.0 million and the $30.0 million of remaining
Technology Transfer Milestones, the receipt of which the Company
believes is probable, on a straight-line basis over seven years
because the Company is unable to reasonably estimate the level
of effort to fulfill these obligations, primarily because the
effort required under the Research Collaboration is largely
unknown. As future substantive milestones are achieved, a
portion of the milestone payment, equal to the percentage of the
performance period completed when the milestone is achieved,
multiplied by the amount of the milestone payment, will be
recognized as revenue upon achievement of such milestone. The
remaining portion of the milestone will be recognized over the
remaining performance period on a straight-line basis. The
Company will continue to reassess whether it can reasonably
estimate the level of effort required to fulfill its obligations
under the Takeda Collaboration Agreement. When, and if, the
Company can make a reasonable estimate of its remaining efforts
under the collaboration, the Company would modify its method of
recognition and utilize a proportional performance method.
In connection with the Takeda Collaboration Agreement, the
Company paid $5.0 million of license fees to the
Company’s licensors, primarily Isis, during 2008, in
accordance with the applicable license agreements with those
parties. These fees were charged to research and development
expense.
Discovery
and Development Alliances
Isis
Collaboration and License Agreement
In March 2004, the Company entered into a collaboration and
license agreement with Isis. Isis granted the Company licenses
to its current and future patents and patent applications
relating to chemistry and to RNA-targeting mechanisms for the
research, development and commercialization of double-stranded
RNA products. The Company has the right to use Isis technologies
in its development programs or in collaborations and Isis has
agreed not to grant licenses under these patents to any other
organization for the discovery, development and
commercialization of double-stranded RNA products designed to
work through an RNAi mechanism, except in the context of a
collaboration in which Isis plays an active role. The Company
granted Isis non-exclusive licenses to its current and future
patents and patent applications relating to RNA-targeting
mechanisms and to chemistry for research use. The Company also
granted Isis the non-exclusive right to develop and
commercialize double-stranded RNA products developed using RNAi
technology against a limited number of targets. In addition, the
Company granted Isis non-exclusive rights to research, develop
and commercialize single-stranded RNA products.
Under the terms of the Isis agreement, the Company paid Isis an
upfront license fee of $5.0 million. The Company also
agreed to pay Isis milestone payments, totaling up to
approximately $3.4 million, upon the occurrence of
specified development and regulatory events, and royalties on
sales, if any, for each product that the Company or a
collaborator develops using Isis intellectual property. In
addition, the Company agreed to pay to Isis a percentage of
specified fees from strategic collaborations the Company may
enter into that include access to the Isis intellectual property.
In conjunction with the agreement, Isis made a
$10.0 million equity investment in the Company. In
addition, Isis has agreed to pay the Company, per therapeutic
target, a license fee of $0.5 million, and milestone
payments totaling approximately $3.4 million, payable upon
the occurrence of specified development and regulatory events,
104
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
and royalties on sales, if any, for each product developed by
Isis or a collaborator that utilizes the Company’s
intellectual property. Isis has the right to elect up to ten
non-exclusive target licenses under the agreement and has the
right to purchase one additional non-exclusive target per year
during the term of the collaboration.
The Isis agreement also gives the Company an option to use
Isis’ manufacturing services for RNA-based therapeutic
products. In addition, under the Isis agreement, the Company has
the exclusive right to grant sub-licenses for Isis technology to
third parties with whom the Company is not collaborating. The
Company may include these sub-licenses in its non-exclusive
platform licenses and its InterfeRx licenses. If a license
includes rights to Isis’ intellectual property, the Company
will share revenues from that license equally with Isis.
The term of the Isis agreement generally ends upon the
expiration of the last-to-expire patent licensed thereunder,
whether such patent is a patent licensed by the Company to Isis,
or vice versa. As the license will include additional patents,
if any, filed to cover future inventions, if any, the date of
expiration cannot be calculated at this time.
In May 2008, as a result of certain payments received by the
Company in connection with the Takeda alliance, the Company paid
$4.6 million to Isis. In August 2007, as a result of
certain payments received by the Company in connection with the
Roche alliance, the Company paid $26.5 million to Isis.
These license fees were charged to research and development
expenses in the respective periods.
Novartis
Broad Alliance
Beginning in September 2005, the Company entered into a series
of transactions with Novartis. In September 2005, the Company
and Novartis executed a stock purchase agreement (the
“Stock Purchase Agreement”) and an investor rights
agreement (the “Investor Rights Agreement”). In
October 2005, in connection with the closing of the transactions
contemplated by the Stock Purchase Agreement, the Investor
Rights Agreement became effective and the Company and Novartis
executed a research collaboration and license agreement (the
“Collaboration and License Agreement”) (collectively
the “Novartis Agreements”). The Collaboration and
License Agreement had an initial term of three years, with an
option for two additional one-year extensions at the election of
Novartis. In July 2008, Novartis elected to extend the
initial term for an additional year, through October 2009.
Novartis retains the right to extend the term for a second
additional year, which right must be exercised no later than
July 2009. Novartis may terminate the Collaboration and License
Agreement in the event that the Company materially breaches its
obligations. The Company may terminate the agreement with
respect to particular programs, products and or countries in the
event of certain material breaches of obligations by Novartis,
or in its entirety under certain circumstances for multiple such
breaches.
Under the terms of the Stock Purchase Agreement, in October
2005, Novartis purchased 5,267,865 shares of the
Company’s common stock at a purchase price of $11.11 per
share for an aggregate purchase price of $58.5 million,
which, after such issuance, represented 19.9% of the
Company’s outstanding common stock as of the date of
issuance. In addition, under the Investor Rights Agreement, the
Company granted Novartis rights to acquire additional equity
securities in the event that the Company proposes to sell or
issue any equity securities, subject to specified exceptions, as
described in the Investor Rights Agreement, such that Novartis
would be able to maintain its then-current ownership percentage
in the Company’s outstanding common stock. Pursuant to
terms of the Investor Rights Agreement, in May 2008, Novartis
purchased 213,888 shares of the Company’s common stock
at a purchase price of $25.29 per share resulting in a payment
to the Company of $5.4 million. At December 31, 2008,
Novartis owned 13.2% of the Company’s outstanding common
stock.
Under the terms of the Collaboration and License Agreement, the
parties will work together on a defined number of selected
targets, as defined in the Collaboration and License Agreement,
to discover and develop therapeutics based on RNAi. In
consideration for the rights granted to Novartis under the
Collaboration and License Agreement, Novartis made upfront
payments totaling $10.0 million to the Company in October
2005, partly to reimburse prior costs incurred by the Company to
develop in vivo RNAi technology. The Collaboration and
License Agreement also includes terms under which Novartis will
provide the Company with research funding and
105
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
milestone payments as well as royalties on annual net sales of
products resulting from the Collaboration and License Agreement,
if any. The amount of research funding provided by Novartis
under the Collaboration and License Agreement during the
research term is dependent upon the number of active programs on
which the Company is collaborating with Novartis at any given
time and the number of Company employees that are working on
those programs, in respect of which Novartis reimburses the
Company at an agreed upon rate. Under the terms of the
Collaboration and License Agreement, Novartis has the right to
select up to 30 exclusive targets to include in the
collaboration, which number may be increased to 40 under certain
circumstances and upon additional payments. For RNAi therapeutic
products successfully developed under the Collaboration and
License Agreement, if any, the Company would be entitled to
receive milestone payments upon achievement of certain specified
development and annual net sales events, up to an aggregate of
$75.0 million per therapeutic product.
Under the terms of the Collaboration and License Agreement, the
Company retains the right to discover, develop, commercialize
and manufacture compounds that function through the mechanism of
RNAi, or products that contain such compounds as an active
ingredient, with respect to targets not selected by Novartis for
inclusion in the collaboration, provided that Novartis has a
right of first offer with respect to an exclusive license for
additional targets before the Company partners any of those
additional targets with third parties.
Novartis may exercise this Integration Option at any point
during the research term, as defined in the Collaboration and
License Agreement. The research term expires in October 2009 and
may be extended until October 2010 at Novartis’ election.
In connection with the exercise of the Integration Option,
Novartis would be required to make additional payments to the
Company totaling $100.0 million, payable in full at the
time of exercise, which payments would include an option
exercise fee, a milestone based on the overall success of the
collaboration and pre-paid milestones and royalties that could
become due as a result of future development of products using
the Company’s technology. In addition, under this license
grant, Novartis may be required to make milestone and royalty
payments to the Company in connection with the successful
development and commercialization of RNAi therapeutic products,
if any. The license grant under the integration option, if
exercised by Novartis, would be structured similarly to the
Company’s non-exclusive platform licenses with Roche and
Takeda.
The Company initially deferred the non-refundable
$10.0 million upfront payment and the $6.4 million
premium received that represents the difference between the
purchase price and the closing price of the common stock of the
Company on the date of the stock purchase from Novartis. These
payments, in addition to research funding and certain milestone
payments, the receipt of which is considered probable, are being
amortized into revenue using the proportional performance method
over the estimated duration of the Novartis agreement or ten
years. Under this model, the Company estimates the level of
effort to be expended over the term of the agreement and
recognizes revenue based on the lesser of the amount calculated
based on proportional performance of total expected revenue or
the amount of non-refundable payments earned.
As future substantive milestones are achieved, and to the extent
they are within the period of performance, milestone payments
will be recognized as revenue on a proportional performance
basis over the contract’s entire performance period,
starting with the contract’s commencement. A portion of the
milestone payment, equal to the percentage of total performance
completed when the milestone is achieved, multiplied by the
milestone payment, will be recognized as revenue upon
achievement of the milestone. The remaining portion of the
milestone will be recognized over the remaining performance
period under the proportional performance method.
The Company believes the estimated period of performance under
the Novartis agreement includes the three-year term of the
agreement, two one-year extensions at the election of Novartis
and limited support as part of a technology transfer until the
fifth anniversary of the termination of the agreement. In July
2008, Novartis elected to extend the initial term for an
additional year, through October 2009. The Company continues to
use an expected term of ten years in its proportional
performance model. The Company reevaluates the expected term
when new information is known that could affect the
Company’s estimate. In the event the Company’s period
of performance is different than estimated, revenue recognition
will be adjusted on a prospective basis.
106
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
Novartis
Pandemic Flu Alliance
In February 2006, the Company entered into an alliance with
Novartis for the development of RNAi therapeutics for pandemic
flu (“Novartis Flu Agreement”). Under the terms of the
Novartis Flu Agreement, the Company and Novartis have joint
responsibility for development of RNAi therapeutics for pandemic
flu. This program was stopped and currently there are no
specific resource commitments for this program.
Biogen
Idec Collaboration Agreement
In September 2006, the Company entered into a Collaboration and
License Agreement (the “Biogen Idec Collaboration
Agreement”) with Biogen Idec focused on the discovery and
development of therapeutics based on RNAi for the potential
treatment of progressive multifocal leukoencephalopathy
(“PML”). Under the terms of the Biogen Idec
Collaboration Agreement, the Company granted Biogen Idec an
exclusive license to distribute, market and sell certain RNAi
therapeutics to treat PML and Biogen Idec has agreed to fund all
related research and development activities. The Company
received an upfront $5.0 million payment from Biogen Idec.
In addition, upon the successful development and utilization of
a product resulting from the collaboration, if any, Biogen Idec
would be required to pay the Company milestone payments,
totaling $51.0 million, and royalty payments on sales, if
any. The Company is recognizing revenue under the Biogen Idec
collaboration on a straight-line basis over five years because
the Company cannot reasonably estimate the total level of effort
required to fulfill its obligations under this collaboration.
The pace and scope of future development of this program is the
responsibility of Biogen Idec.
Unless earlier terminated, the Biogen Idec agreement will remain
in effect until the expiration of all payment obligations under
the agreement. Either the Company or Biogen Idec may terminate
the agreement in the event that the other party breaches its
obligations thereunder. Biogen Idec may also terminate the
agreement, on a
country-by-country
basis, without cause upon 90 days prior written notice.
Merck
Agreement
In July 2006, the Company executed an Amended and Restated
Research Collaboration and License Agreement (the “Amended
License Agreement”) with Merck. In September 2007, the
Company and Merck terminated the Amended License Agreement (the
“Termination Agreement”). Pursuant to the Termination
Agreement, all license grants of intellectual property to
develop, manufacture
and/or
commercialize RNAi therapeutic products under the Amended
License Agreement ceased as of the date of the Termination
Agreement, subject to certain specified exceptions. The
Termination Agreement further provides that, subject to certain
conditions, the Company and Merck will each retain sole
ownership and rights in their own intellectual property. The
Company has no remaining deliverables under the Amended License
Agreement. The Company was recognizing the remaining deferred
revenue of $3.5 million under the Amended License
Agreement, related to upfront cash payments and additional
license fee payments received from Merck, on a straight-line
basis over the remaining period of expected performance of four
years. As a result of the Termination Agreement, the Company
recognized this remaining deferred revenue of $3.5 million
during 2007.
Product
Alliances
Kyowa
Hakko Alliance
In June 2008, the Company entered into a License and
Collaboration Agreement (the “Kyowa Hakko Agreement”)
with Kyowa Hakko. Under the Kyowa Hakko Agreement, the Company
granted Kyowa Hakko an exclusive license to its intellectual
property in Japan and other markets in Asia (the “Licensed
Territory”) for the development and commercialization of
ALN-RSV01, an RNAi therapeutic for the treatment of respiratory
syncytial virus (“RSV”) infection, for which the
Company is currently conducting Phase II clinical trials.
The Kyowa Hakko Agreement also covers additional RSV-specific
RNAi therapeutic compounds that comprise the
107
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
ALN-RSV program (“Additional Compounds”). The Company
retains all development and commercialization rights worldwide
excluding the Licensed Territory.
Under the terms of the Kyowa Hakko Agreement, in June 2008,
Kyowa Hakko paid the Company an upfront cash payment of
$15.0 million. In addition, Kyowa Hakko is required to make
payments to the Company upon achievement of specified
development and sales milestones totaling up to
$78.0 million, and royalty payments based on annual net
sales, if any, of ALN-RSV01 by Kyowa Hakko, its affiliates and
sublicensees in the licensed territory.
The collaboration between Kyowa Hakko and the Company is
governed by a joint steering committee that is comprised of an
equal number of representatives from each party. Under the
agreement, Kyowa Hakko is establishing a development plan for
ALN-RSV01 relating to the development activities to be
undertaken in the Licensed Territory, with the initial focus on
Japan. Kyowa Hakko is responsible, at its expense, for all
development activities under the development plan that are
reasonably necessary for the regulatory approval and
commercialization of ALN-RSV01 in Japan and the rest of the
Licensed Territory. The Company will be responsible for supply
of the product to Kyowa Hakko under a supply agreement unless
Kyowa Hakko elects, prior to the first commercial sale of the
product in the Licensed Territory, to manufacture the product
itself or arrange for a third party to manufacture the product.
The term of the Kyowa Hakko agreement generally ends on a
country-by-country
basis upon the later of (1) the expiration of the
Company’s last-to-expire patent covering a licensed product
and (2) the tenth anniversary of the first commercial sale
in the country of sale. Additional patent filings relating to
the collaboration may be made in the future. The Kyowa Hakko
agreement may be terminated by either party in the event the
other party fails to cure a material breach under the agreement.
In addition, Kyowa Hakko may terminate the agreement without
cause upon
180-days’
prior written notice to the Company, subject to certain
conditions.
The Company has determined that the deliverables under the Kyowa
Hakko Agreement include the license, the joint steering
committee, the manufacturing services and any Additional
Compounds. The Company has determined that, pursuant to
EITF 00-21,
the individual deliverables are not separable and, accordingly,
must be accounted for as a single unit of accounting.
When multiple deliverables are accounted for as a single unit of
accounting, the Company bases its revenue recognition pattern on
the final deliverable. The Company is currently unable to
reasonably estimate its period of performance under the Kyowa
Hakko Agreement, as it is unable to estimate the timeline of its
deliverables related to the fixed-price option granted to Kyowa
Hakko for any Additional Compounds. The Company is deferring all
revenue under the Kyowa Hakko Agreement until it is able to
reasonably estimate its period of performance. The Company will
continue to reassess whether it can reasonably estimate the
period of performance to fulfill its obligations under the Kyowa
Hakko Agreement.
Government
Funding
NIH
Contract
In September 2006, NIAID awarded the Company a contract for up
to $23.0 million over four years to advance the development
of a broad spectrum RNAi anti-viral therapeutic for hemorrhagic
fever virus, including the Ebola virus. Of the
$23.0 million in funding, the government initially
committed to pay the Company up to $14.2 million over the
first two years of the contract. In June 2008, as a result of
the progress of the program, the government awarded the Company
an additional $7.5 million, to be paid through September
2009 for the third year of the contract, together with any
remaining funds carried over from the funding allocated for the
first two years of the contract. The Company recognizes revenue
under government cost reimbursement contracts as it performs the
underlying research and development activities.
108
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
Department
of Defense Contract
In August 2007, DTRA awarded the Company a contract to advance
the development of a broad spectrum RNAi anti-viral therapeutic
for hemorrhagic fever virus. The government initially committed
to pay the Company up to $10.9 million through February
2009, which included a six-month extension granted by DTRA in
July 2008. Following a program review in early 2009, the Company
and DTRA have determined to end this program and accordingly,
the remaining funds of up to $27.7 million will not be
accessed. The Company recognizes revenue under government cost
reimbursement contracts as it performs the underlying research
and development activities.
Delivery
Technology
The Company is working to extend its capabilities in developing
technology to achieve efficacious and safe delivery of RNAi
therapeutics to a broad spectrum of organ and tissue types. In
connection with these efforts, the Company has entered into a
number of agreements to evaluate and gain access to certain
delivery technologies. In some instances, the Company is also
providing funding to support the advancement of these delivery
technologies.
In January 2007, the Company obtained an exclusive worldwide
license to the liposomal delivery formulation technology of
Tekmira for the discovery, development and commercialization of
lipid-based nanoparticle formulations for the delivery of RNAi
therapeutics. In connection with its original agreement with
Tekmira, the Company issued to Tekmira 361,990 shares of
common stock. These shares had a value of $7.9 million at
the time of issuance, which amount was expensed during the first
quarter of 2007. In May 2008, Tekmira acquired Protiva
Biotherapeutics Inc. (“Protiva”). In connection with
this acquisition, the Company entered into new agreements with
Tekmira and Protiva which provide the Company with access to key
existing and future technology and intellectual property for the
systemic delivery of RNAi therapeutics with liposomal delivery
technologies. In addition, the Company made an equity investment
of $5.0 million in Tekmira, purchasing
2,083,333 shares of Tekmira common stock at a price of
$2.40 per share, which represented a premium of $1.00 per share,
or an aggregate of $2.1 million. This premium was
calculated as the difference between the purchase price and the
closing price of Tekmira’s common stock on the effective
date of the acquisition. The Company allocated this
$2.1 million premium to the expansion of the Company’s
access to key technology and intellectual property rights and,
accordingly, recorded a charge to research and development
expense during the second quarter of 2008. The Company recorded
this investment as an available-for-sale security in marketable
securities on its consolidated balance sheets. During the year
ended December 31, 2008, the Company recorded an impairment
charge of $1.6 million related to its investment in
Tekmira, as the decrease in the fair value of this investment
was deemed to be other than temporary. In connection with these
transactions, the Company and Tekmira cancelled the
Company’s $5.0 million capital equipment loan to
Tekmira, which was never drawn down by Tekmira.
Intangible assets at December 31, 2008 and 2007 are as
follows, in thousands:
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Core Technology
|
|
$
|
2,410
|
|
|
$
|
2,410
|
|
|
Less — accumulated amortization:
|
|
|
(1,615
|
)
|
|
|
(1,442
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
795
|
|
|
$
|
968
|
|
|
|
|
|
|
|
|
|
|
|
During the years ended December 31, 2008, 2007 and 2006,
the Company recorded $0.2 million, $0.3 million and
$0.4 million, respectively, of amortization expense related
to its core technology and workforce intangibles, of which the
entire amount is included in research and development expenses.
Workforce intangibles were fully amortized during 2007. Core
technology is being amortized over its estimated useful life of
ten years through 2013.
109
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
During 2007, the Company reduced its intangible assets by
$0.6 million as a result of the realization of
pre-acquisition deferred tax assets associated with net
operating loss carryforwards.
|
|
|
5.
|
PROPERTY
AND EQUIPMENT
|
Property and equipment consist of the following at
December 31, 2008 and 2007, in thousands:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
Useful Life
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Laboratory equipment
|
|
|
5 years
|
|
|
$
|
12,617
|
|
|
$
|
7,963
|
|
|
Computer equipment and software
|
|
|
3 years
|
|
|
|
2,653
|
|
|
|
2,080
|
|
|
Furniture and fixtures
|
|
|
5 years
|
|
|
|
1,532
|
|
|
|
1,271
|
|
|
Leasehold improvements
|
|
|
|
*
|
|
|
18,126
|
|
|
|
9,172
|
|
|
Construction in progress
|
|
|
—
|
|
|
|
23
|
|
|
|
3,702
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
34,951
|
|
|
|
24,188
|
|
|
Less: accumulated depreciation
|
|
|
|
|
|
|
(15,757
|
)
|
|
|
(10,378
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
19,194
|
|
|
$
|
13,810
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* |
|
shorter of asset life or lease term |
Depreciation expense was $5.4 million, $3.8 million
and $3.4 million for the years ended December 31,
2008, 2007 and 2006, respectively.
Equipment
Lines of Credit
In March 2006, the Company entered into an agreement with Oxford
Finance Corporation (“Oxford”) to establish an
equipment line of credit for up to $7.0 million to help
support capital expansion of the Company’s facility in
Cambridge, Massachusetts and capital equipment purchases. The
agreement allowed the Company to draw down amounts under the
line of credit through December 31, 2007 upon adherence to
certain conditions. During 2006 and 2007, the Company borrowed
an aggregate of $5.2 million from Oxford pursuant to the
agreement at fixed rates ranging from 10.0% to 10.4%. In
December 2008, the Company defeased the outstanding balance of
$1.7 million under the Oxford line of credit.
In March 2004, the Company entered into an agreement with
Lighthouse Capital Partners V, L.P.
(“Lighthouse”) to establish an equipment line of
credit for $10.0 million. In June 2005, the parties amended
the agreement to allow the Company the ability to draw down
amounts under the line of credit through December 31, 2005
upon adherence to certain conditions. Interest on the
outstanding principal balance accrued at fixed rates of 9.25% to
10.25%. On the maturity of each equipment advance under the line
of credit, the Company was required to pay, in addition to the
principal and interest due, an additional amount of 11.5% of the
original principal. This amount was accrued over the applicable
borrowing period as additional interest expense. In December
2008, the Company defeased the outstanding balance of
$2.2 million under the Lighthouse line of credit.
At December 31, 2008, the Company had no outstanding debt.
|
|
|
7.
|
COMMITMENTS
AND CONTINGENCIES
|
Indemnifications
Licensor indemnification — In connection with a
certain license agreement, the Company is required to indemnify
the licensor for certain damages arising in connection with the
intellectual property rights licensed under
110
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
the agreement. The Company believes that the probability of
receiving a claim is remote and, as such, no amounts have been
accrued related to this indemnification at December 31,
2008 and 2007.
The Company is also a party to a number of agreements entered
into in the ordinary course of business, which contain typical
provisions, which obligate the Company to indemnify the other
parties to such agreements upon the occurrence of certain
events. Such indemnification obligations are usually in effect
from the date of execution of the applicable agreement for a
period equal to the applicable statute of limitations. The
aggregate maximum potential future liability of the Company
under such indemnification provisions is uncertain. Since its
inception, the Company has not incurred any expenses as a result
of such indemnification provisions. Accordingly, the Company has
determined that the estimated aggregate fair value of its
potential liabilities under such indemnification provisions is
minimal and has not recorded any liability related to such
indemnification provisions at December 31, 2008 and 2007.
Technology
License Commitments
The Company has licensed from third parties the rights to use
certain technologies in its research process as well as in any
products the Company may develop including these licensed
technologies. In accordance with the related license agreements,
the Company is required to make certain fixed payments to the
licensor or a designee of the licensor over various agreement
terms. Many of these agreement terms are consistent with the
remaining lives of the underlying intellectual property that the
Company has licensed. At December 31, 2008, the Company was
committed to make the following fixed license payments under
existing license agreements, in thousands:
| |
|
|
|
|
|
Year Ending December 31,
|
|
|
|
|
|
|
2009
|
|
$
|
3,612
|
|
|
2010
|
|
|
2,552
|
|
|
2011
|
|
|
2,539
|
|
|
2012
|
|
|
1,507
|
|
|
2013
|
|
|
415
|
|
|
Thereafter
|
|
|
5,700
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
16,325
|
|
|
|
|
|
|
|
Operating
Leases
The Company leases office and laboratory space in Cambridge,
Massachusetts for its corporate headquarters under
non-cancelable operating lease agreements. The Company also had
a lease in Kulmbach, Germany through August 2007. Total rent
expense, including operating expenses, under these operating
leases was $4.6 million, $4.7 million and
$2.6 million, for the years ended December 31, 2008,
2007 and 2006, respectively.
In 2003, the Company entered into an operating lease to rent
laboratory and office space in Cambridge, Massachusetts through
September 2011. In March 2006, the Company amended its lease
agreement to rent additional space in this same facility. The
Company has the option to extend the lease for two successive
five-year extensions.
Pursuant to the terms of the lease agreement, the Company
secured a $2.3 million letter of credit as security for its
leased facility. The underlying cash securing this letter of
credit has been classified as long-term restricted cash in the
accompanying consolidated balance sheets.
In October 2007, the Company subleased from Archemix Corp.
(“Archemix”) 22,456 rentable square feet of
office and laboratory space in the same location as the
Company’s corporate headquarters (the
“Sublease”). The initial term of the Sublease will
expire in September 2011, and the Company holds an option to
extend the lease for an additional
48-month
period, subject to certain termination rights granted to each of
the Company and Archemix.
111
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
In addition and in connection with the execution of the
Sublease, the Company issued a letter of credit in favor of
Archemix in the amount of $0.8 million. The underlying cash
securing this letter of credit has been classified as long-term
restricted cash in the accompanying consolidated balance sheets.
The Company received $7.3 million in leasehold improvement
incentives from its landlords in connection with its leases.
These leasehold improvement incentives are being accounted for
as a reduction in rent expense ratably over the lease term. The
balance from these leasehold improvement incentives is included
in current portion of deferred rent and deferred rent, net of
current portion in the balance sheets at December 31, 2008
and 2007.
In connection with the Roche alliance in August 2007, Roche
purchased the assets of Alnylam Europe, which included the lease
for the facility in Kulmbach, Germany.
Future minimum lease payments under these non-cancelable leases
are approximately as follows, in thousands:
| |
|
|
|
|
|
Year Ending December 31,
|
|
|
|
|
|
|
2009
|
|
$
|
3,296
|
|
|
2010
|
|
|
3,296
|
|
|
2011
|
|
|
2,472
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
9,064
|
|
|
|
|
|
|
|
Legal
Proceedings
The Company may periodically become subject to legal proceedings
and claims arising in connection with on-going business
activities, including being subject to claims or disputes
related to patents that have been issued or are pending in the
field of research the Company is focused on. The Company does
not believe that there were any material claims against the
Company at December 31, 2008.
Preferred
Stock
The Company has authorized up to 5,000,000 shares of
preferred stock, $0.01 par value per share, for issuance.
The preferred stock will have such rights, preferences,
privileges and restrictions, including voting rights, dividend
rights, conversion rights, redemption privileges and liquidation
preferences, as shall be determined by the Company’s Board
of Directors upon its issuance. At December 31, 2008 and
2007, there were no shares of preferred stock outstanding.
Stockholder
Rights Agreement
On July 13, 2005, the Board of Directors of the Company
declared a dividend of one right (collectively, the
“Rights”) to buy one one-thousandth of a share of
newly designated Series A Junior Participating Preferred
Stock (“Series A Junior Preferred Stock”) for
each outstanding share of the Company’s common stock to
stockholders of record at the close of business on July 26,
2005. Initially, the Rights are not exercisable and will be
attached to all certificates representing outstanding shares of
common stock, and no separate Rights Certificates will be
distributed. The Rights will expire at the close of business on
July 13, 2015 unless earlier redeemed or exchanged. Until a
right is exercised, the holder thereof, as such, will have no
rights as a stockholder of the Company, including the right to
vote or to receive dividends. The rights are not immediately
exercisable. Subject to the terms and conditions of the Rights
Agreement entered into by the Company with Computershare
(formerly EquiServe Trust Company, N.A.), as Rights Agent
(the “Rights Agreement”), the Rights will become
exercisable upon the earlier of (1) 10 business days
following the later of (a) the first date of a public
announcement that a person or group (an “Acquiring
Person”) acquires, or obtained the right to acquire,
beneficial ownership of 20 percent or
112
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
more of the outstanding shares of common stock of the Company or
(b) the first date on which an executive officer of the
Company has actual knowledge that an Acquiring Person has become
such or (2) 10 business days following the commencement of
a tender offer or exchange offer that would result in a person
or group beneficially owning more than 20 percent of the
outstanding shares of common stock of the Company. Each right
entitles the holder to purchase one one-thousandth of a share of
Series A Junior Preferred Stock at an initial purchase
price of $80.00 in cash, subject to adjustment. In the event
that any person or group becomes an Acquiring Person, unless the
event causing the 20% threshold to be crossed is a Permitted
Offer (as defined in the Rights Agreement), each Right not owned
by the Acquiring Person will entitle its holder to receive, upon
exercise, that number of shares of common stock of the Company
(or in certain circumstances, cash, property or other securities
of the Company) which equals the exercise price of the Right
divided by 50% of the current market price (as defined in the
Rights Agreement) per share of such common stock at the date of
the occurrence of the event. In the event that, at any time
after any person or group becomes an Acquiring Person,
(i) the Company is consolidated with, or merged with and
into, another entity and the Company is not the surviving entity
of such consolidation or merger (other than a consolidation or
merger which follows a Permitted Offer) or if the Company is the
surviving entity, but shares of its outstanding common stock are
changed or exchanged for stock or securities (of any other
person) or cash or any other property, or (ii) more than
50% of the Company’s assets or earning power is sold or
transferred, each holder of a Right (except Rights which
previously have been voided as set forth in the Rights
Agreement) shall thereafter have the right to receive, upon
exercise, that number of shares of common stock of the acquiring
company which equals the exercise price of the Right divided by
50% of the current market price of such common stock at the date
of the occurrence of the event.
Public
Offerings of Common Stock
In January 2006, the Company completed a public offering of its
common stock. The public offering consisted of the sale and
issuance of 5,115,961 shares of the Company’s common
stock. The price to the public was $13.00 per share, and
proceeds to the Company from the offering, net of expenses, were
approximately $62.2 million. The shares of common stock
were registered pursuant to registration statements filed with
the SEC in 2006 and 2005.
In December 2006, the Company completed a public offering of its
common stock. The public offering consisted of the sale and
issuance of 4,700,000 shares of the Company’s common
stock. The price to the public was $22.00 per share, and
proceeds to the Company from the offering, net of expenses, were
approximately $101.1 million. The shares of common stock
were registered pursuant to a registration statement filed with
the SEC in November 2006.
Stock
Plans
As of December 31, 2008, the Company’s 2004 Stock
Incentive Plan, as amended (the “2004 Plan”), provides
for the granting of restricted stock awards and stock options to
purchase up to 10,295,794 shares of common stock. The 2004
Plan provides for an annual increase in the number of shares
available for issuance under the plan equal to the lesser of
2,631,578 shares of common stock, 5% of the Company’s
outstanding shares or an amount determined by the Board of
Directors. In addition, the 2004 Plan includes a non-employee
director stock option program under which each eligible
non-employee director is entitled to (1) a grant of an
option to purchase 30,000 shares of common stock upon his
or her initial appointment to the Board of Directors, or such
other amount as the Board of Directors deems appropriate, and
(2) a subsequent annual grant of an option to purchase
15,000 shares of common stock based on continued service,
made on the date of each annual meeting of stockholders,
provided the non-employee director has served as a director for
at least six months and is serving as a director immediately
prior to and following such annual meeting. The chairman of the
audit committee will receive an additional annual grant of an
option to purchase 10,000 shares of common stock based on
continued service. Stock options granted by the Company to
non-employee directors (i) upon their appointment to the
Board of Directors vest as to one-third of such
113
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
shares on each of the first, second and third anniversaries of
the date of grant and (ii) at each year’s annual
meeting at which they serve as a director vest in full on the
first anniversary of the date of grant.
At December 31, 2008, an aggregate of 7,560,298 shares
of common stock were reserved for issuance under the
Company’s stock plans, including outstanding options to
purchase 7,037,214 shares of common stock and
523,084 shares were available for future grant under the
2004 Plan. Each option shall expire within 10 years of
issuance. Stock options granted by the Company generally vest as
to 25% of the shares on the first anniversary of the grant date
and 6.25% of the shares at the end of each successive
three-month period until fully vested.
Stock-Based
Compensation
Effective January 1, 2006, the Company adopted the fair
value recognition provisions of SFAS 123R using the
modified-prospective-transition method. Under the provisions of
SFAS 123R, the Company recorded $14.3 million,
$11.9 million and $6.1 million of stock-based
compensation for the years ended December 31, 2008, 2007
and 2006, respectively, related to employee stock options and
the employee stock purchase plan.
The Company accounts for non-employee grants as an expense over
the vesting period of the underlying stock options using the
method prescribed by FIN 28. At the end of each financial
reporting period prior to vesting, the value of these options
(as calculated using the Black-Scholes option-pricing model) is
re-measured using the then-current fair value of the
Company’s common stock. The Company recognized
$2.1 million, $2.6 million and $2.2 million of
non-employee stock-based compensation expense for the years
ended December 31, 2008, 2007 and 2006, respectively.
The Company granted the members of the Regulus
Therapeutics’ scientific advisory board and board of
directors options to purchase 30,000 and 68,500 shares of
common stock during 2008 and 2007, respectively. In addition,
the Company granted options to purchase 60,000 shares of
common stock to two officers of Regulus Therapeutics during 2008
and 60,000 shares of common stock to the chief executive
officer of Regulus Therapeutics in 2007. In addition to the
total stock-based compensation expense stated above, the Company
recorded $1.6 million and $0.2 million of stock-based
compensation expense related to these option grants in equity in
loss of joint venture in its consolidated statements of
operations for the years ended December 31, 2008 and 2007,
respectively, using the method prescribed by FIN 28.
In connection with the closing of the sale of Alnylam Europe to
Roche, the Company granted 95,109 shares of restricted
stock of the Company to certain employees of Roche Kulmbach. In
connection with the closing, the Company also accelerated
177,233 of unvested outstanding stock options of certain Alnylam
Europe employees. The Company recorded $3.8 million of
stock-based compensation expense during 2007 related to the
restricted stock grants and the stock option modifications.
Total compensation cost for all stock-based payment arrangements
for the years ended December 31, 2008, 2007 and 2006 was
$18.0 million, $14.7 million and $8.3 million,
respectively. No amounts relating to the stock-based
compensation have been capitalized.
Valuation
Assumptions for Stock Plans and Employee Stock Purchase
Plan
The fair value of stock options at date of grant, based on the
following assumptions, was estimated using the Black-Scholes
option-pricing model. The Company’s expected stock-price
volatility assumption for 2008 and the three months ended
December 31, 2007 is based on a combination of implied
volatilities of its publicly traded stock option prices as well
as the historical volatility of the Company’s publicly
traded stock. During the nine months ended September 30,
2007 and during 2006, the Company’s expected stock-price
volatility assumption was based on a combination of implied
volatilities of similar entities whose share or option prices
are publicly available as well as the historical volatility of
the Company’s publicly traded stock. The expected life
assumption for 2008 and for the three months ended
December 31, 2007 is based on the equal weighting of the
Company’s historical data and the historical data of the
Company’s pharmaceutical and biotechnology peers. During
the nine months ended
114
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
September 30, 2007 and during 2006, the expected life
assumption is based on the simplified method provided for under
SAB No. 107 (“SAB 107”), which averages
the contractual term of the Company’s options
(ten years) with the ordinary vesting term
(2.2 years). The dividend yield assumption is based on the
fact that the Company has never paid cash dividends and has no
present intention to pay cash dividends. The risk-free interest
rate used for each grant is equal to the zero coupon rate in
effect at the time of grant for instruments with a similar
expected life. The Company currently expects, based on an
analysis of its historical forfeitures, that approximately 86%
of its options will actually vest, and therefore have applied an
annual forfeiture rate of 3.7% to all unvested options as of
December 31, 2008. The Company will record additional
expense if the actual forfeitures are lower than estimated and
will record a recovery of prior expense if the actual
forfeitures are higher than estimated.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
|
|
Risk-free interest rate
|
|
|
1.5-3.5
|
%
|
|
|
4.4-4.7
|
%
|
|
|
4.70
|
%
|
|
Expected dividend yield
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Expected option life
|
|
|
5.7-6.3 years
|
|
|
|
6.0-6.1 years
|
|
|
|
6.1 years
|
|
|
Expected volatility
|
|
|
66-67
|
%
|
|
|
64-67
|
%
|
|
|
67
|
%
|
At December 31, 2008, there remained $50.0 million of
unearned compensation expense related to unvested employee stock
options to be recognized as expense over a weighted-average
period of approximately 1.6 years.
Stock
Option Activity
The following table summarizes the activity of the
Company’s stock option plans:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
Average
|
|
|
|
|
Number of
|
|
|
Exercise
|
|
|
|
|
Options
|
|
|
Price
|
|
|
|
|
Outstanding, December 31, 2007
|
|
|
5,304,021
|
|
|
$
|
17.18
|
|
|
Granted
|
|
|
2,155,056
|
|
|
$
|
24.87
|
|
|
Exercised
|
|
|
(377,228
|
)
|
|
$
|
10.04
|
|
|
Cancelled
|
|
|
(44,635
|
)
|
|
$
|
25.19
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding, December 31, 2008
|
|
|
7,037,214
|
|
|
$
|
19.87
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable at December 31, 2006
|
|
|
1,953,502
|
|
|
$
|
4.44
|
|
|
Exercisable at December 31, 2007
|
|
|
1,913,468
|
|
|
$
|
7.49
|
|
|
Exercisable at December 31, 2008
|
|
|
2,903,479
|
|
|
$
|
12.87
|
|
The weighted average remaining contractual life for options
outstanding and exercisable at December 31, 2008 was
8.1 years and 6.8 years, respectively.
The aggregate intrinsic value of outstanding options at
December 31, 2008 was $48.3 million, of which
$37.2 million related to exercisable options. The intrinsic
value of options exercised was $8.2 million,
$24.6 million and $7.6 million for the years ended
December 31, 2008, 2007 and 2006, respectively. The
weighted average fair value of stock options granted as part of
the 2004 Plan was $15.02, $16.58 and $12.95 per share for the
years ended December 31, 2008, 2007 and 2006, respectively.
The aggregate intrinsic value of options expected to vest at
December 31, 2008 was $10.6 million. The weighted
average fair value of stock options expected to vest as part of
the 2004 Plan was $12.10 per share as of December 31, 2008.
The weighted average remaining contractual life for options
expected to vest at December 31, 2008 was 9.0 years
and the weighted average exercise price for these options was
$24.76 per share.
115
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
Restricted
Stock Awards
The following table summarizes the activity of the
Company’s restricted stock awards:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
Average
|
|
|
|
|
Number of
|
|
|
Grant Date
|
|
|
|
|
Awards
|
|
|
Fair Value
|
|
|
|
|
Unvested at December 31, 2007
|
|
|
57,049
|
|
|
$
|
25.98
|
|
|
Granted
|
|
|
—
|
|
|
$
|
—
|
|
|
Vested
|
|
|
(28,531
|
)
|
|
$
|
25.98
|
|
|
Forfeited
|
|
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
Unvested at December 31, 2008
|
|
|
28,518
|
|
|
$
|
25.98
|
|
|
|
|
|
|
|
|
|
|
|
The total fair value of restricted stock awards that vested
during the year ended December 31, 2008 and 2007 was
$0.7 million and $1.0 million, respectively. The
weighted average remaining contractual life for restricted stock
awards at December 31, 2008 was 1.0 years.
Employee
Stock Purchase Plan
In 2004, the Company adopted the 2004 Employee Stock Purchase
Plan (the “2004 Purchase Plan”) with
315,789 shares authorized for issuance. Under the 2004
Purchase Plan as adopted, the Company made one offering each
year, at the end of which employees could purchase shares of
common stock through payroll deductions made over the term of
the offering. Initially, the annual offering period began on the
1st day of November each year and ended on the
31st day of October the following year. In June 2007, the
Compensation Committee of the Board of Directors amended the
offering period of the 2004 Purchase Plan to provide that each
offering period will be for a period of six months, beginning
with the offering period commencing on November 1, 2007.
The per-share purchase price at the end of the offering is equal
to the lesser of 85% of the closing price of the common stock at
the beginning or end of the offering period. The Company issued
35,065, 29,723 and 40,530 shares during 2008, 2007 and
2006, respectively, and as of December 31, 2008,
158,679 shares were available for issuance under the 2004
Purchase Plan.
The weighted average fair value of stock purchase rights granted
as part of the 2004 Purchase Plan was $9.51, $10.61 and $8.16
for the years ended December 31, 2008, 2007 and 2006,
respectively. The fair value was estimated using the
Black-Scholes option-pricing model. The Company used a
weighted-average stock-price volatility of 66%, expected option
life assumption of one year and risk-free interest rate of 2.9%.
The Company recorded $0.3 million, $0.2 million and
$0.2 million of stock-based compensation for the years
ended December 31, 2008, 2007 and 2006, respectively,
related to the 2004 Purchase Plan.
116
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
Deferred income taxes reflect the tax effects of temporary
differences between the carrying amounts of assets and
liabilities for financial reporting and income tax purposes. A
valuation allowance is established when uncertainty exists as to
whether all or a portion of the net deferred tax assets will be
realized. Components of the net deferred tax asset (liability)
as of December 31, 2008, 2007 and 2006 are as follows, in
thousands:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
|
|
Deferred tax assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net operating loss carryforwards
|
|
$
|
298
|
|
|
$
|
49,910
|
|
|
$
|
21,656
|
|
|
Research and development credits
|
|
|
496
|
|
|
|
3,582
|
|
|
|
3,456
|
|
|
Foreign tax credits
|
|
|
—
|
|
|
|
3,072
|
|
|
|
—
|
|
|
Capitalized research and development and
start-up
costs
|
|
|
9,558
|
|
|
|
12,750
|
|
|
|
13,674
|
|
|
Deferred revenue
|
|
|
74,423
|
|
|
|
2,745
|
|
|
|
7,211
|
|
|
Deferred compensation
|
|
|
7,532
|
|
|
|
3,237
|
|
|
|
1,731
|
|
|
Intangible assets
|
|
|
5,422
|
|
|
|
1,540
|
|
|
|
3,100
|
|
|
Other
|
|
|
2,686
|
|
|
|
3,674
|
|
|
|
1,706
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total deferred tax assets
|
|
|
100,415
|
|
|
|
80,510
|
|
|
|
52,534
|
|
|
Deferred tax liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Intangible assets
|
|
|
(246
|
)
|
|
|
(365
|
)
|
|
|
(1,004
|
)
|
|
Deferred tax asset valuation allowance
|
|
|
(95,033
|
)
|
|
|
(80,510
|
)
|
|
|
(50,863
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net deferred tax asset (liability)
|
|
$
|
5,136
|
|
|
$
|
(365
|
)
|
|
$
|
667
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The provision for income taxes for the years ended
December 31, 2008 and 2007 was as follows, in thousands:
| |
|
|
|
|
|
|
|
|
|
|
|
2008
|
|
|
2007
|
|
|
|
|
U.S.:
|
|
|
|
|
|
|
|
|
|
Current
|
|
$
|
5,978
|
|
|
$
|
—
|
|
|
Deferred
|
|
|
(5,382
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
Total U.S.
|
|
|
596
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign:
|
|
|
|
|
|
|
|
|
|
Current
|
|
|
242
|
|
|
|
3,356
|
|
|
Deferred
|
|
|
(119
|
)
|
|
|
1,889
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Foreign
|
|
|
123
|
|
|
|
5,245
|
|
|
|
|
|
|
|
|
|
|
|
|
Provision for income taxes
|
|
$
|
719
|
|
|
$
|
5,245
|
|
|
|
|
|
|
|
|
|
|
|
117
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
The Company’s effective income tax rate differs from the
statutory federal income tax rate as follows for the years ended
December 31, 2008, 2007 and 2006:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
|
|
At U.S. federal statutory rate
|
|
|
35.0
|
%
|
|
|
34.0
|
%
|
|
|
34.0
|
%
|
|
State taxes, net of federal effect
|
|
|
(0.5
|
)
|
|
|
5.8
|
|
|
|
5.3
|
|
|
Foreign tax credit
|
|
|
—
|
|
|
|
3.8
|
|
|
|
—
|
|
|
Foreign dividends
|
|
|
—
|
|
|
|
(6.6
|
)
|
|
|
—
|
|
|
Stock compensation
|
|
|
(6.0
|
)
|
|
|
(2.4
|
)
|
|
|
—
|
|
|
Other
|
|
|
(0.3
|
)
|
|
|
—
|
|
|
|
—
|
|
|
Other permanent items
|
|
|
(0.3
|
)
|
|
|
1.6
|
|
|
|
(5.4
|
)
|
|
Deemed gain on Roche Germany transaction
|
|
|
—
|
|
|
|
(6.3
|
)
|
|
|
—
|
|
|
Research credits
|
|
|
—
|
|
|
|
0.4
|
|
|
|
4.2
|
|
|
Valuation allowance
|
|
|
(30.7
|
)
|
|
|
(36.7
|
)
|
|
|
(38.0
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Effective income tax rate
|
|
|
(2.8
|
)%
|
|
|
(6.4
|
)%
|
|
|
0.1
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As required by SFAS No. 109, “Accounting for
Income Taxes,” management of the Company has evaluated
the positive and negative evidence bearing upon the
realizability of its deferred tax assets. Management has
concluded, in accordance with the applicable accounting
standards, that it is more likely than not that the Company may
not realize the benefit of all its deferred tax assets.
Accordingly, a valuation allowance has been recorded against the
deferred tax assets that management believes will not be
realized. Management reevaluates the positive and negative
evidence on a quarterly basis. The valuation allowance increased
by $14.5 million, $29.6 million and $13.2 million
for the years ended December 31, 2008, 2007 and 2006,
respectively, due primarily to net operating loss carryforwards
and deferred revenue.
During 2008, the Company utilized certain tax attributes,
including net operating loss and tax credit carryforwards as a
result of the recognition of revenue for certain proceeds
received from the Roche alliance. However, the Company also
generated a deferred tax asset related to the recognition of
this revenue for tax purposes. As a result, the Company has
recorded a net deferred tax asset to the extent it is more
likely than not that the asset will be realized. The remaining
deferred tax assets are subject to a valuation allowance as it
is more likely than not that those assets will not be realized.
The deferred tax assets above exclude $9.4 million of
deductions related to the exercise of stock options subsequent
to the adoption of SFAS 123R. This amount represents an
excess tax benefit as defined under SFAS 123R and has not
been included in the gross deferred tax assets.
At December 31, 2008, the state net operating loss
carryforward was $8.6 million and the state research and
development tax credit carryforward was $0.5 million. These
attributes are available to reduce future tax liabilities and
expire at various dates through 2023. Ownership changes, as
defined in the Internal Revenue Code, including those resulting
from the issuance of common stock in connection with the
Company’s public offerings, may limit the amount of net
operating loss and tax credit carryforwards that can be utilized
to offset future taxable income or tax liability. The Company
has determined that there is no limitation on the utilization of
net operating loss and tax credit carryforwards in accordance
with Section 382 of the Internal Revenue Code in 2008.
On January 1, 2007, the Company adopted the provisions of
FASB Interpretation No. 48, “Accounting for
Uncertainty in Income Taxes — an interpretation of
FASB Statement 109” (“FIN 48”), which
was issued in July 2006. The implementation of FIN 48 did
not result in any adjustment to the Company’s beginning tax
positions. The Company continues to recognize fully its tax
benefits which are offset by a valuation allowance to the extent
that it is more likely than not that the deferred tax assets
will not be realized. At December 31, 2008, the Company had
an unrecognized tax benefit of $0.2 million.
118
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
The tax years 2002 through 2006 remain open to examination by
major taxing jurisdictions to which the Company is subject,
which are primarily in the United States, as carryforward
attributes generated in years past may still be adjusted upon
examination by the Internal Revenue Service or state tax
authorities if they have or will be used in a future period. The
Company is currently not under examination by the Internal
Revenue Service or any other jurisdictions for any tax years.
The Company recognizes both accrued interest and penalties
related to unrecognized benefits in income tax expense. The
Company has not recorded any interest and penalties on any
unrecognized tax benefits since its inception.
The Company sponsors a savings plan for its employees in the
United States, who meet certain eligibility requirements, which
is designed to be a qualified plan under section 401(k) of
the Internal Revenue Code (the “401(k) Plan”).
Participants may contribute up to 60% of their annual base
salary to the 401(k) Plan, subject to certain limitations.
Beginning in April 2006, the Company began matching in its
common stock up to 3% of a participant’s base salary.
Employer common stock matches vest anywhere from immediately to
two years, depending on years of service with the Company.
Employees have the ability to transfer funds from the Company
stock fund to other plan funds as they choose, subject to
blackout periods. The Company issued 14,679 and
12,706 shares of common stock during the years ended
December 31, 2008 and 2007, respectively, in connection
with matching contributions under the 401(k) Plan.
In September 2007, the Company and Isis established Regulus
Therapeutics, a company focused on the discovery, development
and commercialization of microRNA-based therapeutics, a
potential new class of drugs to treat the pathways of human
disease. The Company and Isis own 49% and 51%, respectively, of
Regulus Therapeutics.
Regulus Therapeutics is operated as an independent company and
governed by a managing board comprised of an equal number of
directors appointed by each of the Company and Isis. In
consideration for the Company’s and Isis’ initial
interests in Regulus Therapeutics, each party granted Regulus
Therapeutics exclusive licenses to its intellectual property for
certain microRNA-based therapeutic applications as well as
certain patents in the microRNA field. In addition, the Company
made an initial cash contribution to Regulus Therapeutics of
$10.0 million, resulting in the Company and Isis making
approximately equal aggregate initial capital contributions to
Regulus Therapeutics.
In addition, the Company, Isis and Regulus Therapeutics entered
into a license and collaboration agreement (the “Regulus
Therapeutics Collaboration Agreement”) to pursue the
discovery, development and commercialization of therapeutic
products directed to microRNAs. Under the terms of the Regulus
Therapeutics Collaboration Agreement, the Company and Isis each
assigned to Regulus Therapeutics specified patents and contracts
covering microRNA-specific technology. In addition, each of the
Company and Isis granted to Regulus Therapeutics an exclusive,
worldwide license under its rights to other microRNA-related
patents and know-how to develop and commercialize therapeutic
products containing compounds that are designed to interfere
with or inhibit a particular microRNA, subject to the
Company’s and Isis’ existing contractual obligations
to third parties. Regulus Therapeutics was also granted the
right to request a license from the Company and Isis to develop
and commercialize therapeutic products directed to other
microRNA compounds, which license is subject to the
Company’s and Isis’ approval and to each such
party’s existing contractual obligations to third parties.
Regulus Therapeutics also granted to the Company and Isis an
exclusive license to technology developed or acquired by Regulus
Therapeutics for use solely within the Company’s and
Isis’ respective fields (as defined in the Regulus
Therapeutics Collaboration Agreement), but specifically
excluding the right to develop, manufacture or commercialize the
therapeutic products for which the Company and Isis granted
rights to Regulus Therapeutics.
The Regulus Therapeutics Collaboration Agreement ends if, prior
to first commercial sale of any product, all development
activities cease under the collaboration. The Regulus
Therapeutics Collaboration Agreement
119
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
otherwise expires, on a
product-by-product
and
country-by-country
basis, upon the later of expiration of marketing exclusivity for
such product or a specified number of years from first
commercial sale. If Regulus Therapeutics, the Company or Isis
commits an uncured material breach of the Regulus Therapeutics
Collaboration Agreement, the Regulus Therapeutics Collaboration
Agreement may be terminated with respect to the breaching party
or a buy-out may be initiated, depending on the nature of the
breach.
In September 2007, the Company also executed a Services
Agreement (the “Services Agreement”) with Isis and
Regulus Therapeutics. Under the terms of the Services Agreement,
the Company and Isis provide to Regulus Therapeutics, for the
benefit of Regulus Therapeutics, certain research and
development and general and administrative services for which
they are paid by Regulus Therapeutics.
In April 2008, Regulus Therapeutics entered into a worldwide
strategic alliance with GlaxoSmithKline (“GSK”) to
discover, develop and commercialize up to four novel
microRNA-targeted therapeutics to treat inflammatory diseases
such as rheumatoid arthritis and inflammatory bowel disease. In
connection with this alliance, Regulus Therapeutics received
$20.0 million in upfront payments from GSK, including a
$15.0 million option fee and a loan of $5.0 million
evidenced by a promissory note (guaranteed by Isis and the
Company) that will convert into Regulus Therapeutics common
stock under certain specified circumstances. Regulus
Therapeutics could be eligible to receive development,
regulatory and sales milestone payments for each of the
microRNA-targeted therapeutics discovered and developed as part
of the alliance. Regulus Therapeutics would also receive royalty
payments on worldwide sales of products resulting from the
alliance.
The Company has concluded that Regulus Therapeutics qualifies as
a variable interest entity under FASB Interpretation
No. 46R, “Consolidation of Variable Interest
Entities — an interpretation of Accounting Research
Bulletin No. 51” (“FIN 46R”).
The LLC Agreement contains transfer restrictions on each of
Isis’ and the Company’s LLC interests and, as a
result, Isis and the Company are considered related parties
under paragraph 16(d)(1) of FIN 46R. The Company has
assessed which entity would be considered the primary
beneficiary under FIN 46R and has concluded that Isis is
the primary beneficiary and, accordingly, the Company has not
consolidated Regulus Therapeutics.
The Company accounts for its investment in Regulus Therapeutics
using the equity method of accounting. The Company is
recognizing the first $10.0 million of losses of Regulus
Therapeutics as equity in loss of joint venture (Regulus
Therapeutics LLC) in its consolidated statements of
operations because the Company is responsible for funding those
losses through its initial $10.0 million cash contribution.
Thereafter, the Company will recognize 49% of the income and
losses of Regulus Therapeutics. Under the equity method, the
reimbursement of expenses to the Company is recorded as a
reduction to research and development expenses. At
December 31, 2008, the Company’s investment in the
joint venture was $1.6 million, which is recorded as an
investment in joint venture (Regulus Therapeutics LLC) in
the consolidated balance sheets under the equity method. Summary
results of Regulus Therapeutics’ operations for the years
ended December 31, 2008 and 2007 and balance sheets as of
December 31, 2008 and 2007 are presented in the table
below, in thousands:
| |
|
|
|
|
|
|
|
|
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Statement of Operations Data:
|
|
|
|
|
|
|
|
|
|
Net revenues
|
|
$
|
2,111
|
|
|
$
|
120
|
|
|
Operating expenses(1)
|
|
|
12,029
|
|
|
|
1,541
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(9,918
|
)
|
|
|
(1,421
|
)
|
|
Other income
|
|
|
256
|
|
|
|
138
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(9,662
|
)
|
|
$
|
(1,283
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) Non-cash stock-based compensation included in operating
expenses
|
|
$
|
2,017
|
|
|
$
|
412
|
|
120
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Balance Sheet Data:
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$
|
22,411
|
|
|
$
|
10,138
|
|
|
Working capital
|
|
|
16,467
|
|
|
|
9,118
|
|
|
Total assets
|
|
|
23,678
|
|
|
|
10,446
|
|
|
Notes payable
|
|
|
5,179
|
|
|
|
—
|
|
|
Total stockholders’ equity
|
|
|
1,745
|
|
|
|
9,290
|
|
Separate financial information for Regulus Therapeutics is
included in Exhibit 99.1 to this annual report on
Form 10-K.
Regulus Therapeutics, which was initially established as a
limited liability company, converted to a C corporation as of
January 2, 2009 and changed its name to Regulus
Therapeutics Inc. In connection with such conversion, the
Company, Isis and Regulus Therapeutics terminated the limited
liability company agreement which had previously been in effect.
|
|
|
13.
|
QUARTERLY
FINANCIAL DATA (UNAUDITED)
|
The following information has been derived from unaudited
consolidated financial statements that, in the opinion of
management, include all recurring adjustments necessary for a
fair presentation of such information.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
|
|
|
|
|
March 31,
|
|
|
June 30,
|
|
|
September 30,
|
|
|
December 31,
|
|
|
|
|
2008
|
|
|
2008
|
|
|
2008
|
|
|
2008
|
|
|
|
|
(In thousands, except per share data)
|
|
|
|
|
Revenues
|
|
$
|
22,192
|
|
|
$
|
23,833
|
|
|
$
|
25,734
|
|
|
$
|
24,404
|
|
|
Operating expenses
|
|
|
26,149
|
|
|
|
36,664
|
|
|
|
28,968
|
|
|
|
32,217
|
|
|
Net loss
|
|
|
(1,239
|
)
|
|
|
(12,760
|
)
|
|
|
(2,858
|
)
|
|
|
(9,392
|
)
|
|
Net loss per common share — basic and diluted
|
|
$
|
(0.03
|
)
|
|
$
|
(0.31
|
)
|
|
$
|
(0.07
|
)
|
|
$
|
(0.23
|
)
|
|
Weighted average common shares — basic and diluted
|
|
|
40,736
|
|
|
|
40,908
|
|
|
|
41,197
|
|
|
|
41,375
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
|
|
|
|
|
March 31,
|
|
|
June 30,
|
|
|
September 30,
|
|
|
December 31,
|
|
|
|
|
2007
|
|
|
2007
|
|
|
2007
|
|
|
2007
|
|
|
|
|
(In thousands, except per share data)
|
|
|
|
|
Revenues
|
|
$
|
7,217
|
|
|
$
|
9,133
|
|
|
$
|
16,315
|
|
|
$
|
18,232
|
|
|
Operating expenses
|
|
|
31,211
|
|
|
|
24,086
|
|
|
|
67,653
|
|
|
|
21,124
|
|
|
Net (loss) income
|
|
|
(21,645
|
)
|
|
|
(12,691
|
)
|
|
|
(52,792
|
)
|
|
|
1,662
|
|
|
Net (loss) income per common share — basic and diluted
|
|
$
|
(0.58
|
)
|
|
$
|
(0.34
|
)
|
|
$
|
(1.35
|
)
|
|
$
|
0.04
|
|
|
Weighted average common shares — basic
|
|
|
37,376
|
|
|
|
37,534
|
|
|
|
39,025
|
|
|
|
40,710
|
|
|
Weighted average common shares — diluted
|
|
|
37,376
|
|
|
|
37,534
|
|
|
|
39,025
|
|
|
|
42,763
|
|
For the three months ended December 31, 2008, the Company
discovered that it had understated equity in loss of joint
venture by $0.6 million related to non-cash stock-based
compensation. To correct its equity in loss of joint venture,
the Company recorded a charge of $0.6 million in the three
months ended December 31, 2008.
On January 9, 2009, the Company entered into a license and
collaboration agreement (the “Cubist Agreement”) with
Cubist Pharmaceuticals, Inc. (“Cubist”) to develop and
commercialize therapeutic products
121
ALNYLAM
PHARMACEUTICALS, INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(“Licensed Products”) based on certain of the
Company’s RNAi technology for the treatment of RSV.
Licensed Products include ALN-RSV01, which is currently in
Phase II clinical development for the treatment of RSV
infection in adult lung transplant patients, as well as several
other second-generation RNAi-based RSV inhibitors, which
currently are in pre-clinical studies.
Under the terms of the Cubist Agreement, the Company and Cubist
will share responsibility for developing Licensed Products in
North America and will each bear one-half of the related
development costs. The Company’s collaboration with Cubist
for the development of Licensed Products in North America will
be governed by a joint steering committee comprised of an equal
number of representatives from each party. Cubist will have the
sole right to commercialize Licensed Products in North America
with costs associated with such activities and any resulting
profits or losses to be split equally between the Company and
Cubist. Throughout the rest of the world (the “Royalty
Territory”), excluding Asia, where the Company has
previously partnered its ALN-RSV program with Kyowa Hakko,
Cubist will have an exclusive, royalty-bearing license to
develop and commercialize Licensed Products.
In consideration for the rights granted to Cubist under the
Cubist Agreement, Cubist made a $20.0 million upfront cash
payment to the Company. Cubist also has an obligation under the
Cubist Agreement to pay the Company milestone payments, totaling
up to an aggregate of $82.5 million, upon the achievement
of specified development and sales events in the Royalty
Territory. In addition, if Licensed Products are successfully
developed, Cubist will be required to pay to the company double
digit royalties on net sales of Licensed Products in the Royalty
Territory, if any, subject to offsets under certain
circumstances. Upon achievement of certain development
milestones, the Company will have the right to convert the North
American co-development and profit sharing arrangement into a
royalty-bearing license and, in addition to royalties on net
sales in North America, will be entitled to receive additional
milestone payments totaling up to an aggregate of
$130.0 million upon achievement of specified development
and sales events in North America, subject to the timing of the
conversion by the Company and the regulatory status of a
Licensed Product at the time of conversion. If the Company makes
the conversion to a royalty-bearing license with respect to
North America, then North America becomes part of the Royalty
Territory.
Unless terminated earlier in accordance with the Cubist
Agreement, the agreement expires on a
country-by-country
and Licensed
Product-by-Licensed
Product basis, (a) with respect to the Royalty Territory,
upon the latest to occur of: (1) the expiration of the
last-to-expire Company patent covering a Licensed Product,
(2) the expiration of the Regulatory-Based Exclusivity
Period (as defined in the Cubist Agreement) and (3) ten
years from first commercial sale in such country of such License
Product by Cubist or its affiliates or sublicensees, and
(b) with respect to North America, if the Company has not
converted North America into the Royalty Territory, upon the
termination of the Cubist Agreement by Cubist upon specified
prior written notice. The Company estimates that its fundamental
RNAi patents covered under the Agreement will expire both in and
outside of the United States generally between 2016 and 2025.
Allowed claims covering ALN-RSV01 in the United States would
expire in 2026. These patent rights are subject to any potential
patent term extensions
and/or
supplemental protection certificates extending such term
extensions in countries where such extensions may become
available. In addition, more patent filings relating to the
collaboration may be made in the future. Cubist has the right to
terminate the Cubist Agreement at any time (1) upon three
months’ prior written notice if such notice is given prior
to the acceptance for filing of the first application for
regulatory approval of a Licensed Product or (2) upon nine
months’ prior written notice if such notice is given after
the acceptance for filing of the first application for
regulatory approval. Either party may terminate the Cubist
Agreement in the event the other party fails to cure a material
breach or upon patent-related challenges by the other party.
During the term of the Cubist Agreement, neither party nor its
affiliates may develop, manufacture or commercialize anywhere in
the world, outside of Asia, a therapeutic or prophylactic
product that specifically targets RSV, except for Licensed
Products developed, manufactured or commercialized pursuant to
the Cubist Agreement.
122
|
|
|
ITEM 9.
|
CHANGES
IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND
FINANCIAL DISCLOSURE
|
None.
|
|
|
ITEM 9A.
|
CONTROLS
AND PROCEDURES
|
Our management, with the participation of our chief executive
officer and vice president of finance and treasurer, evaluated
the effectiveness of our disclosure controls and procedures as
of December 31, 2008. The term “disclosure controls
and procedures,” as defined in
Rules 13a-15(e)
and
15d-15(e)
under the Exchange Act, means controls and other procedures of a
company that are designed to ensure that information required to
be disclosed by a company in the reports that it files or
submits under the Exchange Act is recorded, processed,
summarized and reported, within the time periods specified in
the SEC’s rules and forms. Disclosure controls and
procedures include, without limitation, controls and procedures
designed to ensure that information required to be disclosed by
a company in the reports that it files or submits under the
Exchange Act is accumulated and communicated to the
company’s management, including its principal executive and
principal financial officers, as appropriate to allow timely
decisions regarding required disclosure. Management recognizes
that any controls and procedures, no matter how well designed
and operated, can provide only reasonable assurance of achieving
their objectives and management necessarily applies its judgment
in evaluating the cost-benefit relationship of possible controls
and procedures. Based on the evaluation of our disclosure
controls and procedures as of December 31, 2008, the
Company’s chief executive officer and vice president of
finance and treasurer concluded that, as of such date, our
disclosure controls and procedures were effective at the
reasonable assurance level.
Management’s report on our internal control over financial
reporting (as defined in
Rules 13a-15(f)
and
15d-15(f)
under the Exchange Act) and the independent registered public
accounting firm’s report on the effectiveness of our
internal control over financial reporting are included in
Item 8 of this annual report on
Form 10-K
and are incorporated herein by reference.
No change in our internal control over financial reporting
occurred during the fiscal quarter ended December 31, 2008
that has materially affected, or is reasonably likely to
materially affect, our internal control over financial reporting.
|
|
|
ITEM 9B.
|
OTHER
INFORMATION
|
None.
PART III
|
|
|
ITEM 10.
|
DIRECTORS,
EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
We will file with the SEC a definitive Proxy Statement, which we
refer to herein as the Proxy Statement, not later than
120 days after the close of the fiscal year ended
December 31, 2008. The information required by this item is
incorporated herein by reference to the information contained
under the sections captioned “Proposal One —
Election of Class I Directors,”
“Section 16(a) Beneficial Ownership Reporting
Compliance” and “Corporate Governance” of the
Proxy Statement. The information required by this item relating
to executive officers is included in “Part I,
Item 1 — Business- Executive Officers of the
Registrant” of this annual report on
Form 10-K.
|
|
|
ITEM 11.
|
EXECUTIVE
COMPENSATION
|
The information required by this item is incorporated herein by
reference to the information contained under the sections
captioned “Information about Executive Officer and Director
Compensation,” “Compensation Committee Interlocks and
Insider Participation”, “Employment Arrangements”
and “Compensation Committee Report” of the Proxy
Statement.
123
|
|
|
ITEM 12.
|
SECURITY
OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND
RELATED STOCKHOLDER MATTERS
|
The information required by this item is incorporated herein by
reference to the information contained under the sections
captioned “Security Ownership of Certain Beneficial Owners
and Management” “Information about Executive Officer
and Director Compensation” and “Securities Authorized
for Issuance Under Equity Compensation Plans” of the Proxy
Statement.
|
|
|
ITEM 13.
|
CERTAIN
RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR
INDEPENDENCE
|
The information required by this item is incorporated herein by
reference to the information contained under the sections
captioned “Corporate Governance,” “Employment
Arrangements” and “Certain Relationships and Related
Transactions” of the Proxy Statement.
|
|
|
ITEM 14.
|
PRINCIPAL
ACCOUNTING FEES AND SERVICES
|
The information required by this item is incorporated herein by
reference to the information contained under the sections
captioned “Corporate Governance,” “Principal
Accountant Fees and Services” and “Pre-Approval
Policies and Procedures” of the Proxy Statement.
PART IV
|
|
|
ITEM 15.
|
EXHIBITS AND
FINANCIAL STATEMENT SCHEDULES
|
(a) (1) Financial Statements
The following consolidated financial statements are filed as
part of this report under “Item 8 —
Financial Statements and Supplementary Data”:
| |
|
|
|
|
|
|
|
Page
|
|
|
|
Management’s Annual Report on Internal Control Over
Financial Reporting
|
|
|
88
|
|
|
Report of Independent Registered Public Accounting Firm
|
|
|
89
|
|
|
Consolidated Balance Sheets as of December 31, 2008 and 2007
|
|
|
90
|
|
|
Consolidated Statements of Operations and Comprehensive Loss for
the Years Ended December 31, 2008, 2007 and 2006
|
|
|
91
|
|
|
Consolidated Statements of Stockholders’ Equity for the
Years Ended December 31, 2006, 2007 and 2008
|
|
|
92
|
|
|
Consolidated Statements of Cash Flows for the Years Ended
December 31, 2008, 2007 and 2006
|
|
|
93
|
|
|
Notes to Consolidated Financial Statements
|
|
|
94
|
|
(a) (2) List of Schedules
All schedules to the consolidated financial statements are
omitted as the required information is either inapplicable or
presented in the consolidated financial statements.
(a) (3) List of Exhibits
The exhibits which are filed with this report or which are
incorporated herein by reference are set forth in the
Exhibit Index hereto.
124
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the
Securities Exchange Act of 1934, the Registrant has duly caused
this Report to be signed on its behalf by the undersigned,
thereunto duly authorized, on March 2, 2009.
ALNYLAM PHARMACEUTICALS, INC.
|
|
|
| |
By:
|
/s/ John
M. Maraganore, Ph.D.
|
John M. Maraganore, Ph.D.
Chief Executive Officer
Pursuant to the requirements of the Securities Exchange Act of
1934, the Report has been signed below by the following persons
on behalf of the Registrant and in the capacities indicated as
of March 2, 2009.
| |
|
|
|
|
|
Name
|
|
Title
|
|
|
|
|
|
|
|
/s/ John
M. Maraganore, Ph.D.
John
M. Maraganore, Ph.D.
|
|
Director and Chief Executive Officer
(Principal Executive Officer)
|
|
|
|
|
|
/s/ Patricia
L. Allen
Patricia
L. Allen
|
|
Vice President of Finance and Treasurer
(Principal Financial and Accounting Officer)
|
|
|
|
|
|
/s/ John
K. Clarke
John
K. Clarke
|
|
Director
|
|
|
|
|
|
/s/ Victor
J. Dzau, M.D.
Victor
J. Dzau, M.D.
|
|
Director
|
|
|
|
|
|
/s/ Vicki
L. Sato, Ph.D.
Vicki
L. Sato, Ph.D.
|
|
Director
|
|
|
|
|
|
/s/ Paul
R. Schimmel, Ph.D.
Paul
R. Schimmel, Ph.D.
|
|
Director
|
|
|
|
|
|
/s/ Edward
M. Scolnick, M.D.
Edward
M. Scolnick, M.D.
|
|
Director
|
|
|
|
|
|
/s/ Phillip
A. Sharp, Ph.D.
Phillip
A. Sharp, Ph.D.
|
|
Director
|
|
|
|
|
|
/s/ Kevin
P. Starr
Kevin
P. Starr
|
|
Director
|
|
|
|
|
|
/s/ James
L. Vincent
James
L. Vincent
|
|
Director
|
125
EXHIBIT INDEX
| |
|
|
|
|
|
Exhibit No.
|
|
Exhibit
|
|
|
|
|
3
|
.1
|
|
Restated Certificate of Incorporation of the Registrant (filed
as Exhibit 3.1 to the Registrant’s Quarterly Report on
Form 10-Q
filed on August 11, 2005 (File
No. 000-50743)
for the quarterly period ended June 30, 2005 and
incorporated herein by reference)
|
|
|
3
|
.2
|
|
Amended and Restated Bylaws of the Registrant (filed as
Exhibit 3.4 to the Registrant’s Registration Statement
on
Form S-1
(File
No. 333-113162)
and incorporated herein by reference)
|
|
|
4
|
.1
|
|
Specimen certificate evidencing shares of common stock (filed as
Exhibit 4.1 to the Registrant’s Registration Statement
on
Form S-1
(File
No. 333-113162)
and incorporated herein by reference)
|
|
|
4
|
.2
|
|
Rights Agreement dated as of July 13, 2005 between the
Registrant and EquiServe Trust Company, N.A., as Rights
Agent, which includes as Exhibit A the Form of Certificate
of Designations of Series A Junior Participating Preferred
Stock, as Exhibit B the Form of Rights Certificate and as
Exhibit C the Summary of Rights to Purchase Preferred Stock
(filed as Exhibit 4.1 to the Registrant’s Current
Report on
Form 8-K
filed on July 14, 2005 (File
No. 000-50743)
and incorporated herein by reference)
|
|
|
10
|
.1*
|
|
2002 Employee, Director and Consultant Stock Plan, as amended,
together with forms of Incentive Stock Option Agreement,
Non-qualified Stock Option Agreement and Restricted Stock
Agreement (filed as Exhibit 10.1 to the Registrant’s
Registration Statement on
Form S-1
(File
No. 333-113162)
and incorporated herein by reference)
|
|
|
10
|
.2*
|
|
2003 Employee, Director and Consultant Stock Plan, as amended,
together with forms of Incentive Stock Option Agreement,
Non-qualified Stock Option Agreement and Restricted Stock
Agreement (filed as Exhibit 10.2 to the Registrant’s
Registration Statement on
Form S-1
(File
No. 333-113162)
and incorporated herein by reference)
|
|
|
10
|
.3*
|
|
2004 Stock Incentive Plan, as amended (filed as
Exhibit 10.3 to the Registrant’s Annual Report on
Form 10-K
filed on March 7, 2008 (File
No. 000-50743)
for the annual period ended December 31, 2007 and
incorporated herein by reference)
|
|
|
10
|
.4*
|
|
Forms of Incentive Stock Option Agreement and Nonstatutory Stock
Option Agreement under 2004 Stock Incentive Plan, as amended
(filed as Exhibit 10.3 to the Registrant’s Quarterly
Report on
Form 10-Q
filed on August 11, 2005 (File
No. 000-50743)
for the quarterly period ended June 30, 2005 and
incorporated herein by reference)
|
|
|
10
|
.5*
|
|
Form of Nonstatutory Stock Option Agreement under 2004 Stock
Incentive Plan granted to John M. Maraganore, Ph.D., on
December 21, 2004 (filed as Exhibit 10.1 to the
Registrant’s Current Report on
Form 8-K
filed on December 28, 2004 (File
No. 000-50743)
and incorporated herein by reference)
|
|
|
10
|
.6*
|
|
Form of Nonstatutory Stock Option Agreement under 2004 Stock
Incentive Plan granted to James L. Vincent on July 12, 2005
(filed as Exhibit 10.1 to the Registrant’s Current
Report on
Form 8-K
filed on July 13, 2005 (File
No. 000-50743)
and incorporated herein by reference)
|
|
|
10
|
.7*
|
|
Form of Restricted Stock Agreement under 2004 Stock Incentive
Plan issued to James L. Vincent on July 12, 2005 (filed as
Exhibit 10.2 to the Registrant’s Current Report on
Form 8-K
filed on July 13, 2005 (File
No. 000-50743)
and incorporated herein by reference)
|
|
|
10
|
.8*
|
|
2004 Employee Stock Purchase Plan, as amended (filed as
Exhibit 10.8 to the Registrant’s Annual Report on
Form 10-K
filed on March 7, 2008 (File
No. 000-50743)
for the annual period ended December 31, 2007 and
incorporated herein by reference)
|
|
|
10
|
.9
|
|
Investor Rights Agreement entered into as of March 11, 2004
by and between the Registrant and Isis Pharmaceuticals, Inc.
(filed as Exhibit 10.25 to the Registrant’s
Registration Statement on
Form S-1
(File
No. 333-113162)
and incorporated herein by reference)
|
|
|
10
|
.10
|
|
Stock Purchase Agreement, dated as of September 6, 2005, by
and between the Registrant and Novartis Pharma AG (filed as
Exhibit 10.1 to the Registrant’s Current Report on
Form 8-K
filed on September 12, 2005 (File
No. 000-50743)
and incorporated herein by reference)
|
|
|
10
|
.11
|
|
Investor Rights Agreement, dated as of September 6, 2005,
by and between the Registrant. and Novartis Pharma AG (filed as
Exhibit 10.2 to the Registrant’s Current Report on
Form 8-K
filed on September 12, 2005 (File
No. 000-50743)
and incorporated herein by reference)
|
126
| |
|
|
|
|
|
Exhibit No.
|
|
Exhibit
|
|
|
|
|
10
|
.12*
|
|
Letter Agreement between the Registrant and John M.
Maraganore, Ph.D. dated October 30, 2002 (filed as
Exhibit 10.7 to the Registrant’s Registration
Statement on
Form S-1
(File
No. 333-113162)
and incorporated herein by reference)
|
|
|
10
|
.13*
|
|
Letter Agreement between the Registrant and Barry E. Greene
dated September 29, 2003 (filed as Exhibit 10.10 to
the Registrant’s Registration Statement on
Form S-1
(File
No. 333-113162)
and incorporated herein by reference)
|
|
|
10
|
.14
|
|
Lease, dated as of September 26, 2003 by and between the
Registrant and Three Hundred Third Street LLC (filed as
Exhibit 10.15 to the Registrant’s Registration
Statement on
Form S-1
(File No. 333-113162)
and incorporated herein by reference)
|
|
|
10
|
.15†
|
|
License Agreement between Cancer Research Technology Limited and
Alnylam U.S., Inc. dated July 18, 2003 (filed as
Exhibit 10.16 to the Registrant’s Registration
Statement on
Form S-1
(File No. 333-113162)
and incorporated herein by reference)
|
|
|
10
|
.16†
|
|
License Agreement between the Carnegie Institution of Washington
and Alnylam Europe, AG, effective March 1, 2002, as amended
by letter agreements dated September 2, 2002 and
October 28, 2003 (filed as Exhibit 10.17 to the
Registrant’s Registration Statement on
Form S-1
(File
No. 333-113162)
and incorporated herein by reference)
|
|
|
10
|
.17†
|
|
License Agreement by and between the Cold Spring Harbor
Laboratory and Alnylam U.S., Inc. dated December 30, 2003
(filed as Exhibit 10.18 to the Registrant’s
Registration Statement on
Form S-1
(File No. 333-113162)
and incorporated herein by reference)
|
|
|
10
|
.18†
|
|
Co-exclusive License Agreement between Garching Innovation GmbH
(now known as Max Planck Innovation GmbH) and Alnylam U.S., Inc.
dated December 20, 2002, as amended by Amendment dated
July 8, 2003 together with Indemnification Agreement by and
between Garching Innovation GmbH (now known as Max Planck
Innovation GmbH) and Alnylam Pharmaceuticals, Inc. effective
April 1, 2004 (filed as Exhibit 10.19 to the
Registrant’s Registration Statement on
Form S-1
(File No. 333-113162)
and incorporated herein by reference)
|
|
|
10
|
.19†
|
|
Co-exclusive License Agreement between Garching Innovation GmbH
(now known as Max Planck Innovation GmbH) and Alnylam Europe, AG
dated July 30, 2003 (filed as Exhibit 10.20 to the
Registrant’s Registration Statement on
Form S-1
(File
No. 333-113162)
and incorporated herein by reference)
|
|
|
10
|
.20
|
|
Agreement between the Registrant, Garching Innovation GmbH (now
known as Max Planck Innovation GmbH), Alnylam U.S., Inc. and
Alnylam Europe AG dated June 14, 2005 (filed as
Exhibit 10.8 to the Registrant’s Quarterly Report on
Form 10-Q
filed on August 11, 2005
(File No. 000-50743)
for the quarterly period ended June 30, 2005 and
incorporated herein by reference)
|
|
|
10
|
.21†
|
|
Agreement between The Board of Trustees of the Leland Stanford
Junior University and Alnylam U.S., Inc. effective as of
September 17, 2003 (filed as Exhibit 10.21 to the
Registrant’s Registration Statement on
Form S-1
(File
No. 333-113162)
and incorporated herein by reference)
|
|
|
10
|
.22†
|
|
Strategic Collaboration and License Agreement effective as of
March 11, 2004 between Isis Pharmaceuticals, Inc. and the
Registrant (filed as Exhibit 10.24 to the Registrant’s
Registration Statement on
Form S-1
(File
No. 333-113162)
and incorporated herein by reference)
|
|
|
10
|
.23†#
|
|
Research Collaboration and License Agreement effective as of
October 12, 2005 by and between the Registrant and Novartis
Institutes for BioMedical Research, Inc. (previously filed as
Exhibit 10.1 to the Registrant’s Current Report on
Form 8-K
filed on October 12, 2005 (File
No. 000-50743))
|
|
|
10
|
.24
|
|
First Amendment to Lease, dated March 16, 2006, by and
between the Registrant and ARE-MA Region No. 28, LLC (filed
as Exhibit 10.1 to the Registrant’s Current Report on
Form 8-K
filed on March 17, 2006 (File
No. 000-50743)
and incorporated herein by reference)
|
|
|
10
|
.25†
|
|
Collaboration and License Agreement dated September 20,
2006, by and between the Registrant and Biogen Idec Inc. (filed
as Exhibit 10.1 to the Registrant’s Quarterly Report
on
Form 10-Q
filed on November 9, 2006 (File
No. 000-50743)
for the quarterly period ended September 30, 2006 and
incorporated herein by reference)
|
127
| |
|
|
|
|
|
Exhibit No.
|
|
Exhibit
|
|
|
|
|
10
|
.26†#
|
|
License and Collaboration Agreement, entered into as of
July 8, 2007, by and among F. Hoffmann-La Roche, Ltd,
Hoffman-La Roche Inc., the Registrant and, for limited
purposes, Alnylam Europe AG (previously filed as
Exhibit 10.1 to the Registrant’s Quarterly Report on
Form 10-Q
filed on November 8, 2007 (File
No. 000-50743)
for the quarterly period ended September 30, 2007)
|
|
|
10
|
.27
|
|
Common Stock Purchase Agreement dated as of July 8, 2007
between the Registrant and Roche Finance Ltd (filed as
Exhibit 10.2 to the Registrant’s Quarterly Report on
Form 10-Q
filed on November 8, 2007 (File
No. 000-50743)
for the quarterly period ended September 30, 2007 and
incorporated herein by reference)
|
|
|
10
|
.28†
|
|
Share Purchase Agreement, dated as of July 8, 2007, among
Alnylam Europe AG, the Registrant and Roche Pharmaceuticals GmbH
(filed as Exhibit 10.3 to the Registrant’s Quarterly
Report on
Form 10-Q
filed on November 8, 2007 (File
No. 000-50743)
for the quarterly period ended September 30, 2007 and
incorporated herein by reference)
|
|
|
10
|
.29†
|
|
Amended and Restated Collaboration Agreement, entered into as of
July 27, 2007, by and between the Registrant and Medtronic,
Inc. (filed as Exhibit 10.4 to the Registrant’s
Quarterly Report on
Form 10-Q
filed on November 8, 2007 (File
No. 000-50743)
for the quarterly period ended September 30, 2007 and
incorporated herein by reference)
|
|
|
10
|
.30†
|
|
License and Collaboration Agreement, entered into as of
September 6, 2007, by and among the Registrant, Isis
Pharmaceuticals, Inc. and Regulus Therapeutics LLC (filed as
Exhibit 10.5 to the Registrant’s Quarterly Report on
Form 10-Q
filed on November 8, 2007 (File
No. 000-50743)
for the quarterly period ended September 30, 2007 and
incorporated herein by reference)
|
|
|
10
|
.31†
|
|
Termination Agreement, dated as of September 18, 2007, by
and between Merck & Co., Inc. and the Registrant
(filed as Exhibit 10.7 to the Registrant’s Quarterly
Report on
Form 10-Q
filed on November 8, 2007 (File
No. 000-50743)
for the quarterly period ended September 30, 2007 and
incorporated herein by reference)
|
|
|
10
|
.32†
|
|
License and Collaboration Agreement entered into as of
May 27, 2008 by and among Takeda Pharmaceutical Company
Limited and the Registrant (filed as Exhibit 10.1 to the
Registrant’s Quarterly Report on
Form 10-Q
filed on August 8, 2008 (File
No. 000-50743)
for the quarterly period ended June 30, 2008 and
incorporated herein by reference)
|
|
|
21
|
.1#
|
|
Subsidiaries of the Registrant
|
|
|
23
|
.1#
|
|
Consent of PricewaterhouseCoopers LLP, Independent Registered
Public Accounting Firm
|
|
|
23
|
.2#
|
|
Consent of Ernst & Young LLP, Independent Auditors of
Regulus Therapeutics LLC
|
|
|
31
|
.1#
|
|
Certification pursuant to Section 302 of the Sarbanes-Oxley
Act of 2002,
Rule 13(a)-
14(a)/15d-14(a), by Chief Executive Officer
|
|
|
31
|
.2#
|
|
Certification pursuant to Section 302 of the Sarbanes-Oxley
Act of 2002,
Rule 13(a)-
14(a)/15d-14(a), by Vice President of Finance and Treasurer
|
|
|
32
|
.1#
|
|
Certification pursuant to 18 U.S.C. Section 1350, as
adopted pursuant to Section 906 of the Sarbanes-Oxley Act
of 2002, by Chief Executive Officer
|
|
|
32
|
.2#
|
|
Certification pursuant to 18 U.S.C. Section 1350, as
adopted pursuant to Section 906 of the Sarbanes-Oxley Act
of 2002, by Vice President of Finance and Treasurer
|
|
|
99
|
.1#
|
|
Regulus Therapeutics LLC Financial Statements
|
|
|
|
|
* |
|
Management contracts or compensatory plans or arrangements
required to be filed as an exhibit hereto pursuant to
Item 15(a) of
Form 10-K. |
| |
|
† |
|
Indicates confidential treatment requested as to certain
portions, which portions were omitted and filed separately with
the Securities and Exchange Commission pursuant to a
Confidential Treatment Request. |
| |
|
# |
|
Filed herewith. The Registrant is refiling Exhibits 10.23
and 10.26, which include portions previously omitted pursuant to
Confidential Treatment Requests. |
128