UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended
OR
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
FOR THE TRANSITION PERIOD FROM TO
Commission File Number
(Exact name of Registrant as specified in its Charter)
|
||
(State or other jurisdiction of incorporation or organization) |
|
(I.R.S. Employer Identification No.) |
|
||
(Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code:
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
|
|
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the Registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit files).
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definition of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
|
☐ |
|
Accelerated filer |
☐ |
|
|
|
|
|
|||
|
☒ |
|
Smaller reporting company |
|
||
|
|
|
|
|
|
|
|
|
|
|
Emerging growth company |
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to § 240.10D-1(b). ☐
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes
i
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the Registrant, based on the closing price of the shares of common stock on The Nasdaq Capital Market on June 30, 2022 (the last business day of the Registrant’s most recently completed second fiscal quarter), was $
The number of shares of Registrant’s Common Stock outstanding as of March 15, 2023 was
ii
Table of Contents
|
|
Page |
PART I |
|
|
Item 1. |
3 |
|
Item 1A. |
24 |
|
Item 1B. |
56 |
|
Item 2. |
56 |
|
Item 3. |
57 |
|
|
|
|
PART II |
|
|
Item 5. |
58 |
|
Item 6. |
58 |
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
59 |
Item 7A. |
68 |
|
Item 8. |
68 |
|
Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
72 |
Item 9A. |
72 |
|
Item 9B. |
73 |
|
Item 9C. |
Disclosure Regarding Foreign Jurisdictions that Prevent Inspections |
73 |
|
|
|
PART III |
|
|
Item 10. |
74 |
|
Item 11. |
82 |
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
95 |
Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
96 |
Item 14. |
98 |
|
|
|
|
PART IV |
|
|
Item 15. |
99 |
|
Item 16. |
103 |
iii
PART I
Unless the context requires otherwise, references to “HTG,” “HTG Molecular Diagnostics,” “we,” “us” and “our” refer to HTG Molecular Diagnostics, Inc.
Forward-Looking Statements
This Annual Report on Form 10-K, including the sections entitled “Business,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” may contain forward-looking statements. We may, in some cases, use words such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “continue,” “seek,” “project,” “should,” “will,” “would” or the negative of those terms, and similar expressions that convey uncertainty of future events or outcomes, to identify these forward-looking statements. Any statements contained herein that are not statements of historical facts may be deemed to be forward-looking statements. Forward-looking statements in this Annual Report include, but are not limited to, statements about:
These forward-looking statements reflect our management’s beliefs and views with respect to future events, are based on estimates and assumptions as of the filing date of this Annual Report and are subject to risks and uncertainties. We discuss many of these risks in greater detail under “Risk Factors.” Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this Annual Report, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Except as required by law, we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise.
1
RISK FACTOR SUMMARY
Below is a summary of the principal factors that make an investment in our common stock speculative or risky. This summary does not address all of the risks that we face. Additional discussion of the risks summarized in this risk factor summary, and other risks that we face, can be found below under the heading “Risk Factors” and should be carefully considered, together with other information in this Annual Report on Form 10-K and our other filings with the SEC before making investment decisions regarding our common stock.
2
Item 1. Business.
Overview
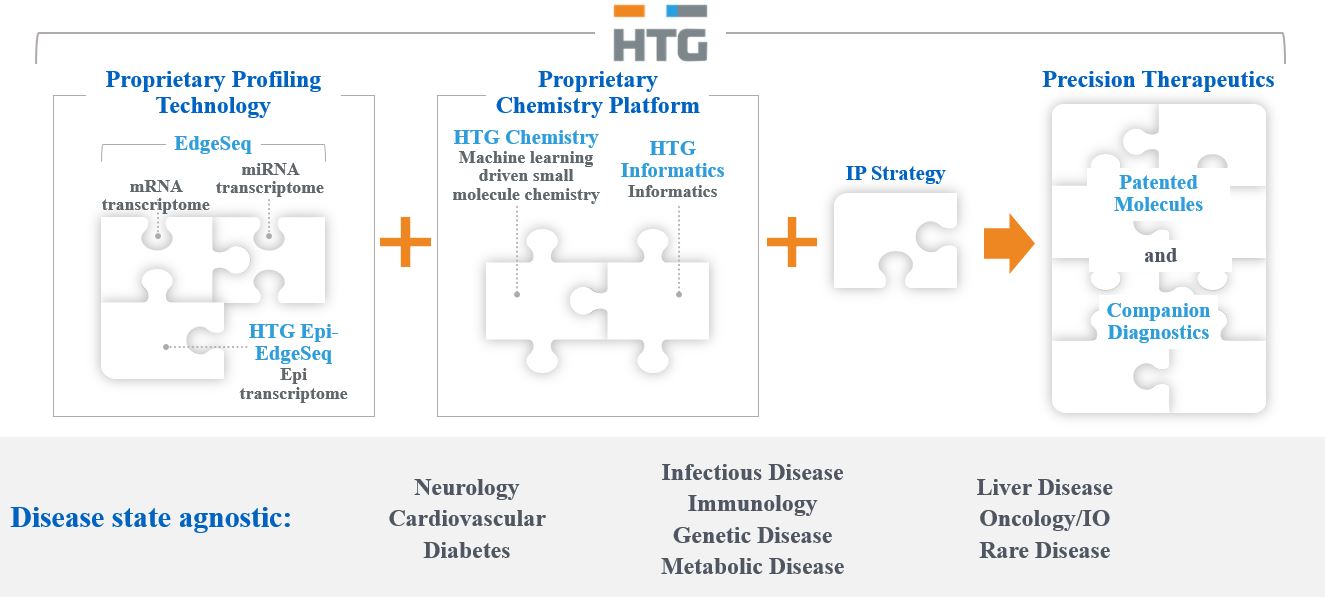
We are focused on advancing precision medicine and drug discovery through our innovative transcriptome-wide profiling and advanced drug discovery platform technologies. Building on more than a decade of pioneering innovation, our proprietary next-generation HTG EdgeSeq technology is the basis for our tech-driven hybrid business model allowing our RNA molecular profiling applications to be more effective, efficient and relevant and also serving as a key component of the engine behind our platform-based drug discovery process. Central to our business strategy is our drug discovery engine, which uses our captive transcriptomic profiling capabilities combined with a proprietary medicinal chemistry machine learning platform to render an artificial intelligence ("AI") -driven drug candidate optimization platform. We are using this platform to innovate drug discovery with the goal of building best-in-class molecules for known pharmacologic targets across multiple disease areas, better, faster and in a more cost-effective manner.
The training data sets for our machine learning platform utilize our own primary data generated specifically for this purpose. This high quality, standardized data provides a clear advantage over other platform approaches which are typically dependent upon publicly available data. The medicinal chemistry portion of our platform allows for rapid design and in silico evaluation of large chemical libraries in order to prioritize and select compounds for synthesis and advancement into early testing. These data are then integrated and processed into an iterative loop using a series of proprietary machine learning algorithms prior to further advancing the molecules to more traditional drug discovery studies. We expect that this will allow for rapid identification, selection and optimization of drug candidates for entrance into development. Further, we believe that our ability to rapidly iterate between primary data and computational analyses gives us valuable information and insights for candidate molecule design and selection.
To date, we have used our transcriptome-informed drug discovery engine to develop an early pipeline of drug candidate molecules for two known pharmacologic targets, both of which can target several potential therapeutic indications, but with a current focus on oncology and neurodegenerative diseases. We believe that our technology provides a differentiated and potentially disruptive approach to drug discovery, that may allow ourselves and our partners to potentially improve upon key attrition factors, namely efficacy and toxicity, early in the discovery process, thereby allowing for better chances for candidate success when entering development.
Our business strategy is to build our drug discovery pipeline in order to out-license certain drug candidates and carry other candidates into preclinical and early development ourselves. In addition, we would expect to retain and potentially capitalize upon clinical diagnostics ("CDx") rights through the clinical development and commercialization of these assets where appropriate.
We also operate a profiling business in life science tools. Our profiling product and service solutions enable targeted RNA profiling using a small amount of biological sample, in liquid or solid forms. Our menu of HTG EdgeSeq assays, including our HTG Transcriptome Panel ("HTP"), which has been designed to measure approximately 20,000 mRNA targets using our HTG EdgeSeq technology, is automated on our HTG EdgeSeq system, which applies NGS tools, enabling the generation of gene expression data in a timely manner utilizing our simplified workflow. We seek to leverage key business drivers in molecular profiling for biomarker analysis and diagnostics, including the acceleration of precision medicine, the migration of molecular testing to NGS-based applications, the movement to smaller and less invasive biopsies, the need for greater diagnostic sensitivity, the need to conform to challenging healthcare economics and the need for automation and an easily deployable workflow, including simplified bioinformatics. These capabilities enable customers to extend the use of limited biological samples for retrospective or prospective analysis, gaining further understanding of the molecular drivers of disease with the goal of developing biomarker-driven targeted therapies.
3
Our existing products include instruments, consumables and software that, as an integrated platform, automate sample processing and can quickly, robustly and simultaneously profile hundreds, thousands or tens of thousands of molecular targets from samples which are a fraction of the size required by many prevailing technologies. Customers can access our technology by purchasing our HTG EdgeSeq system and assays for their internal use or through our Tucson, Arizona-based VERI/O service laboratory, including molecular profiling of cohorts and development of custom research use only ("RUO") panels to support early-stage clinical programs and investigational-use-only assays for clinical trials. However, with the release of our HTP, revenue from our RUO assay design services is expected to be lower than historical levels, as our RUO assay design services revenue is replaced by HTP consumables purchases and sample processing laboratory services using our HTP. Our product and service solutions have enabled us to access a number of early-stage biomarker discovery programs. We believe this approach will enable new opportunities collaborating with biopharmaceutical companies in their future drug development programs.
Our Strategy
Our objective is to establish our transcriptome-informed drug discovery process as the preferred methodology for small molecule drug discovery and our HTG EdgeSeq technology as the standard in profiling and CDx development.
The key components of our strategy are:
4
Our Market Opportunities
Drug Discovery for the Biopharmaceutical Sector
The transitional drug discovery and development process is characterized by substantial financial risks for development programs that often fail to reach patients as marketed products. Historically, it has taken over ten years and average capitalized research and development costs of over $2.0 billion per approved medicine to move a drug discovery project from early discovery to an approved therapeutic. Such productivity outcomes have culminated in an expected industry success rate of 8% to 14% from discovery to commercialization, yielding a rapidly declining internal rate of return for the industry from approximately 10% in 2010 to 2.5% in 2020.
These trends create an environment that is ripe for technological innovation. Traditional drug discovery relies on basic research discoveries from the scientific community for disease-relevant pathways and targets to interrogate. Frequently, drug developers are left to make decisions on potential candidates without fully understanding the incredible complexity of systems biology. Despite decades of accumulated knowledge, the result is that drug discovery has unintentionally become almost artisanal, with little informative biological data available to those in the industry.
In an attempt to address these issues, the biopharmaceutical sector has increasingly relied on the “open science” model whereby companies pursue a diverse set of strategies leveraging internal research and development efforts as well as turning to external research and development, scientific collaborations and in-licensing opportunities to advance new drug discoveries, meet currently unmet needs and help more patients. Fully integrated pharmaceutical companies have evolved to use open science as a ground to supplement their internal pipeline efforts with new drug candidates and/or new emerging technologies. It is not uncommon among the fully integrated companies to have strategic goals where half of the pipeline is from internal efforts whereas the remaining half is populated through assets that are either in-licensed, partnered or acquired through strategic acquisitions. Some fully integrated pharmaceutical companies rely solely on external science and innovation.
We believe that our approach of utilizing our established RNA profiling capabilities, integrated into a drug discovery platform with advanced medicinal chemistry technologies, will result in more well-informed molecule design and selection and potentially provide multiple revenue opportunities. These revenue opportunities may include collaboration or licensing arrangements for any small molecule drug candidates we generate, either at early- or mid-stage development, potentially out-licensing our technology to pharmaceutical companies to enable them to implement our advanced drug discovery approach into their own internal discovery efforts and developing new companion diagnostic assays to support the related clinical development programs for those molecules.
Cancer Molecular Profiling and Genomics in Life Science Research
Molecular profiling is the analysis of biomarkers, including DNA, RNA and protein, in biological samples, such as tissue, cells, blood and other biofluids, to identify gene expression patterns or genomic changes. The HTG EdgeSeq technology coupled with NGS is making it possible to perform these characterizations in unprecedented ways, resulting in a shift from the traditional approach of looking at one target at a time to the simultaneous analysis of potentially tens, hundreds or thousands of gene targets.
Among what we believe are the most promising applications of molecular profiling is the targeted sequencing of RNA from patient samples to identify gene expression patterns or molecular markers of disease that can aid in diagnosis, gauge patient prognosis or predict response to an available therapy. These applications have launched a fundamental shift towards personalized medicine where an individual patient’s molecular profile is used to guide treatment.
The market for RNA-Seq is estimated to be approximately $1.0 billion and growing annually at 10-20%. The gene expression component of that market is estimated to be approximately $820.0 million and growing at the same rate. With these metrics in mind, we expect our target market, NGS-based gene expression profiling, to be between $1.3 billion and $2.0 billion by 2024.
Therapy Driven Diagnostics - Companion Diagnostics
The World Health Organization estimates that cancer will lead to the deaths of approximately 17 million people per year by 2030. As a result, biopharmaceutical companies are aggressively deploying biomarker driven strategies to improve the response rates to drugs, including existing drugs, new drugs and combination therapies. These companies are looking for technology solutions that can more effectively identify the biological root causes of disease and aid the discovery of biomarkers to better develop and target drugs to the correct patients. The companion diagnostic market is currently estimated at $2.6 billion and growing approximately 20% annually. We believe that the acceleration of investment into immunotherapy drugs will also be a catalyst for future companion diagnostics for combination therapies where RNA gene expression classification is expected to be important.
5
When a molecular biomarker panel is used for selection of patients in a Phase 2 or Phase 3 clinical trial to demonstrate safety and efficacy of a new drug, the drug and biomarker test are often submitted to the applicable regulatory agency for approval together. In the United States, upon U.S. Food and Drug Administration (“FDA”) approval or clearance of the CDx test, the patient must be tested with the CDx test prior to being treated with the drug. Companion diagnostic tests have a clear clinical utility that generally supports favorable reimbursement decisions. We believe there are currently approximately 3,100 oncology clinical trials, approximately 24% of which are interrogating RNA. This percentage has more than doubled since 2014, and we believe this percentage will approach 50% by 2025.
Our Technology
HTG has assembled a portfolio of technology platforms to provide highly differentiated capabilities to serve our two business areas. These technologies are as follows and are described in further detail below:
Platform Technology for Drug Discovery

HTG EdgeSeq Profiling Technology
Our HTG EdgeSeq profiling technology measures RNA using DNA nuclease protection probes ("DNA protection probes"). These DNA protection probes include a target-specific region flanked by universal wing sequences and are hybridized in solution to their target RNAs. Target RNA can be both soluble and cross-linked in the biological matrix. Universal DNA wingmen are hybridized to the wings to prevent S1 nuclease digestion. S1 nuclease is added to remove single-stranded nucleic acids, including unhybridized DNA protection probes and RNA. Following S1 nuclease treatment, the only remaining DNA protection probes in the reaction are those hybridized to targeted RNA and wingmen to form a hybridized heteroduplex. This produces an approximately 1:1 ratio of DNA protection probes to the RNA targeted in the sample. DNA protection probes are labeled with sequencing adaptors and molecular barcodes in a PCR reaction. The labeled DNA protection probes are cleaned up, quantified, pooled, and ready for sequencing using standard NGS protocols. Data from the NGS instrument is processed and reported by the parser software provided with the HTG EdgeSeq platform.
6
Key Advantages of our HTG EdgeSeq Profiling Technology
HTG Instrument Platform
Our instrument and assays were developed internally and are manufactured in Tucson, AZ under ISO 13485:2016 certified procedures using our proprietary HTG EdgeSeq chemistry to simplify multiplexed nucleic acid testing in research and clinical laboratories. The entire workflow from sample preparation to a molecular profiling report can be accomplished in as few as 36 hours for 96 samples. With the speed, flexibility, sensitivity, and accuracy of our HTG EdgeSeq platform, combined with the system’s ability to work effectively with small sample volumes, researchers can profile tens, hundreds or thousands of different genes per sample.

7
The HTG EdgeSeq platform consists of a processor (shown above), a host computer and integrated software. The processor is a fully automated instrument that prepares biological samples for quantitation using proprietary, electronically barcoded, single-use consumables. The instrument has barcode scanner units to process the two-dimensional barcodes printed on the consumables loaded into the instrument. The barcoded consumables are single-use to reduce operator errors, eliminate cross-contamination and provide chain of custody traceability for the samples. The robotic liquid handling within the instrument is engineered for reliable performance and low maintenance. The walking path of the robot is programmed to minimize any chance of contamination of the reagents or samples. One host computer supports up to six processors allowing laboratories to easily expand their capacity by adding processors.
Applications of our HTG EdgeSeq technology combine the HTG EdgeSeq platform with a NGS platform to enable the quantitative analysis of hundreds or thousands of RNA targets in a single panel. The sample library is prepared on the processor, then labeled with molecular sequencing adaptors and tags. The labeled samples are cleaned up, quantified, pooled, and sequenced on a NGS platform using standard protocols. Data from the NGS instrument are processed and reported by the parser software included with the system. HTG EdgeSeq panels are currently available to process from one to 96 samples in a single batch.
In addition to direct sales of our systems, we utilize several alternative arrangements to provide customer access to our platform. Our platform can be purchased directly by our customers, who also then purchase HTG EdgeSeq assays and other consumables from us on an as-needed basis. In some instances, we provide our instruments free of charge on a limited basis to facilitate customer evaluation prior to acquisition. We also may choose to install instruments for our customers at no cost, in exchange for an agreement to purchase assays and other consumables from us at a stated price and volume over the term of the agreement or allow customers to rent our instrument for a monthly fee.
Our Drug Discovery Engine
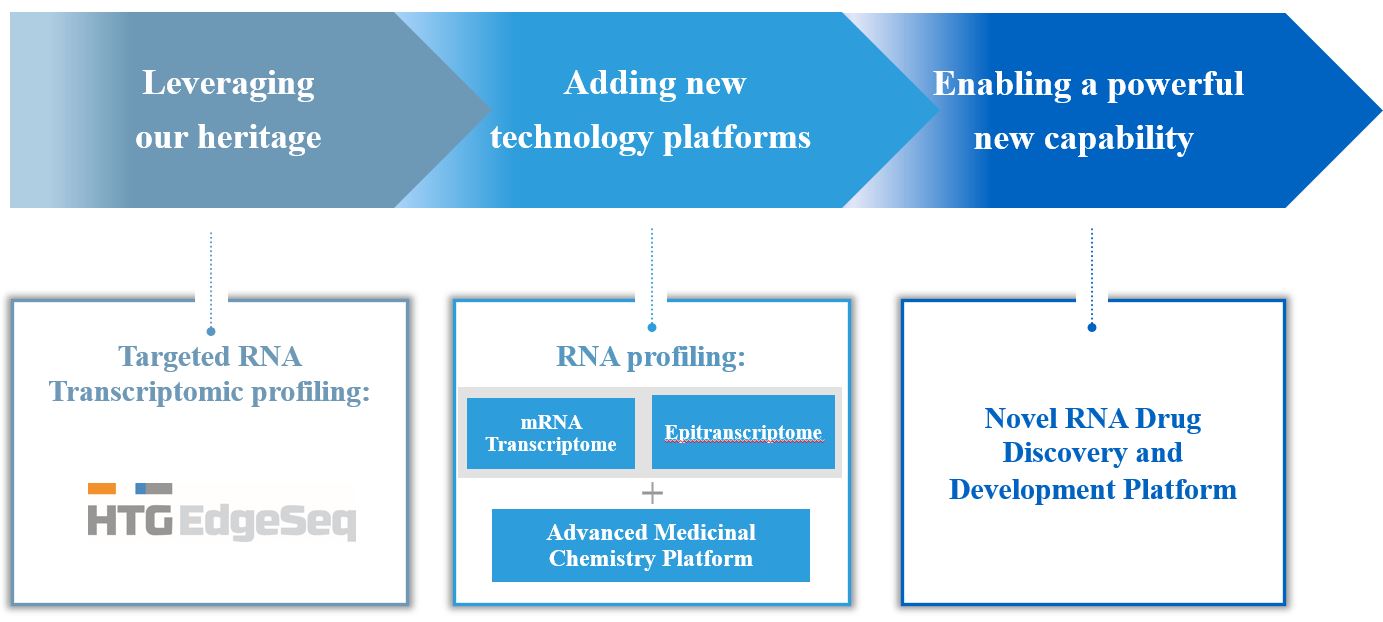
Currently, approximately 90% of drugs fail in clinical development due to insufficient efficacy and/or safety issues and, in many instances, these issues are not revealed until considerable time has passed and tens or even hundreds of millions of dollars have been spent in the discovery and development stages of these programs. Through key learnings from our prior collaborative development services experiences, we have designed a new approach to drug discovery that leverages the benefits of our HTP and epitranscriptomic profiling technologies in RNA profiling, sequencing and other scientific applications, including drug discovery and development. We believe the competitive advantages provided by our technology, compared with other profiling technologies, are the ability to process smaller sample volumes of multiple sample types with faster turnaround times and a simplified workflow.
In June 2021, we announced the formation of HTG Therapeutics, with the addition of several highly experienced drug development professionals to our leadership team. Throughout 2021, we strengthened our HTG EdgeSeq technology platform and added new profiling capabilities, including epitranscriptomic profiling, which currently provides the capability to generate over 40,000 biological data points from each experimental sample. By leveraging these profiling technologies in the drug discovery process, integrated with an advanced AI and machine learning-based medicinal chemistry approach, we have established a novel transcriptome-informed small molecule discovery engine at the core of our HTG Therapeutics business unit which we believe will generate drug candidate molecules that are intrinsically lower risk and will have greater potential for clinical development success when compared to currently existing early-stage drug discovery methods in the biopharmaceutical industry. We further expect that this approach to small molecule discovery can be applied agnostically across therapeutic areas and is scalable and flexible, allowing us to adapt our strategic and therapeutic focus rapidly as new information emerges on the pathogenesis of diseases.
8
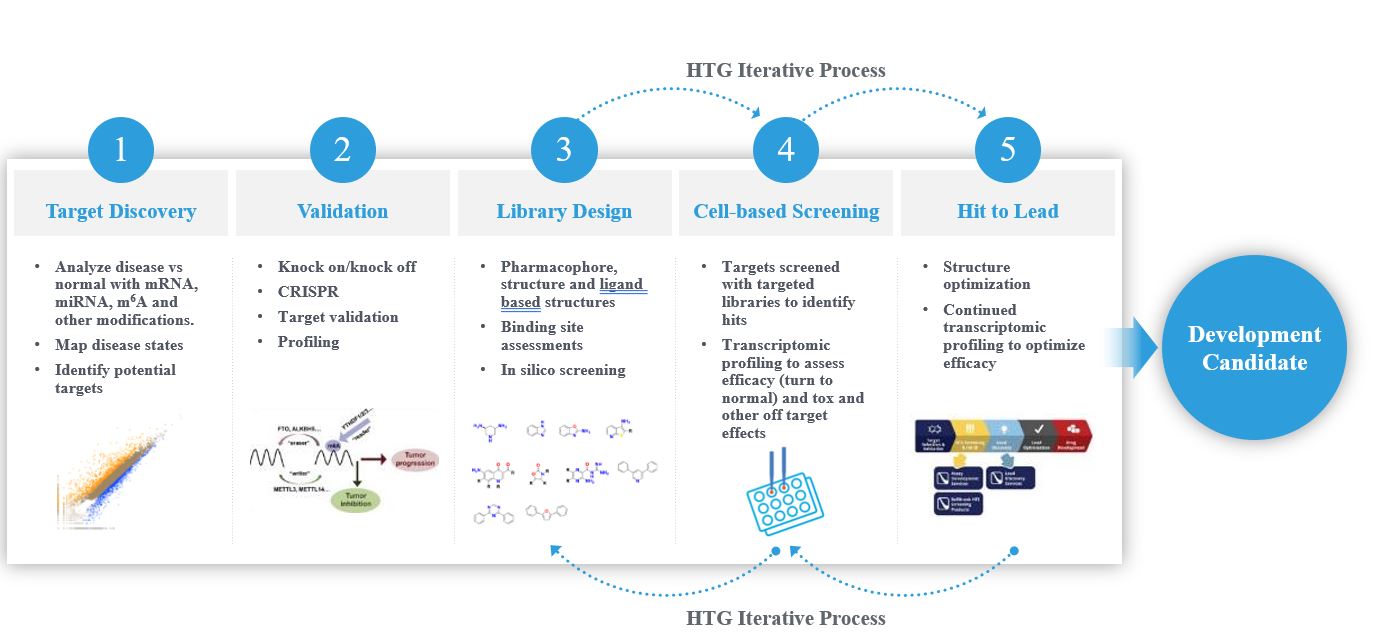
We believe that our approach will potentially provide multiple revenue opportunities, including collaboration or out-licensing arrangements for small molecule drug candidates we generate from as early as lead optimization through early preclinical development, the out-licensing of our technology to pharmaceutical companies to enable them to implement our advanced drug discovery approach into their own internal discovery efforts, and potentially new companion diagnostic opportunities to support the related clinical development programs for molecules that are brought forward through this novel discovery approach.
In the first half of 2022, we released a series of white papers after demonstrating the utility of our proprietary technologies as a key component of our novel transcriptome-informed drug discovery and design approach and applying the approach to our initial therapeutic target. As anticipated, the results of our studies summarized in these white papers supported our approach and its ability to reveal indication-specific effects and potential undesirable effects in our first target through analysis of transcriptomic profiles from compound-treated human cell line test systems.
Throughout the second half of 2022, we continued to work to strengthen our drug discovery core platform technology, including advancing the machine learning component of our platform with the refinement of key proprietary algorithms while continuing to generate our own internal data supporting training sets. In addition, we made capital investments to establish internal cell culture capabilities to support the expansion of our cell-based test system models. Our medicinal chemistry effort has produced a series of chemical libraries for our first target, and our most advanced library for this target has entered preclinical characterization, with a series of data generated including early efficacy in two different disease states.
As a result of the progress made throughout 2022, we filed a patent application in December 2022, which included claims directed toward specific compounds, pharmaceutical compositions and methods of treating or preventing disease by administration of the compounds. Our initial therapeutic pipeline is focused on oncology and degenerative neuroscience, emphasizing pharmacologic targets with understood roles in the progression of diseases in these areas.
The most advanced discovery program in oncology is a small molecule program for treatment of liquid tumors. We expect to continue lead optimization of this program through the end of the first quarter of 2023, with advancement to support entry into preclinical development later in the year. HTG Therapeutics has a second oncology directed small molecule program for the treatment of a solid tumor type that is nearing completion in the hit-to-lead discovery phase, with lead optimization efforts planned through the second quarter of 2023 and subsequent preparation for potential preclinical development expected by the end of 2023. In our neuroscience pipeline, we have completed early discovery stage efforts and chemical library generation for candidate small molecules for application to neurodegenerative conditions which are expected to enter the hit-to-lead phase in the second half of 2023.
9
We expect to initiate several early discovery-stage programs evaluating small molecule candidates against a variety of different cancers, from which we plan to select candidates for additional indications to continually expand our drug discovery pipeline. As additional candidates are identified, we may choose to retain certain candidates internally to be advanced through early development, with the intention to increase the value of these pipeline assets before moving to license or partner for further development. In parallel to these therapy-area specific programs, we continue to enrich the proprietary dataset that supports our transcriptome-informed drug discovery platform and to evolve and refine the complementary AI and machine learning portions of our drug discovery engine throughout these discovery processes. Finally, we would expect to maintain the exclusive rights and the opportunity to solely develop new CDx assays relating to these drug candidates as they move through the increasingly advanced stages of development with our future collaboration partners, further growing our existing gene expression profiling business.
Our Competitive Advantages
We believe that our proprietary technologies provide us with a number of competitive advantages that set us apart from others in our industry and that will continue to drive new customers toward our solutions.
In drug discovery, we intend to use our captive transcriptomic profiling capabilities combined with our captive medicinal chemistry capabilities to create an AI driven drug candidate optimization process. Differentiation begins with our profiling capability. Our HTG EdgeSeq technology uses very little sample, has a high sample pass rate and high sensitivity, uses an automated workflow and produces high quality data in under three days for hundreds of samples at a time. This enables us to use high quality primary transcriptomic data as an input to analyze the biological response of cells to individual molecules in candidate chemical libraries. We believe competing profiling technologies are not as robust, are more variable and take significantly longer to generate data.
10

A further point of differentiation is our chemistry platform. A combination of cheminformatics, molecular docking and machine learning algorithms allow for building and refinement of compound libraries in silico, rapidly generating highly focused compound libraries for specific targets. These small molecule-focused libraries are screened using traditional methods and via molecular profiling, allowing for rapid feedback into the compound design system for optimization of lead compounds. All compound molecules, along with their attendant data, are added to HTG's proprietary compound library.
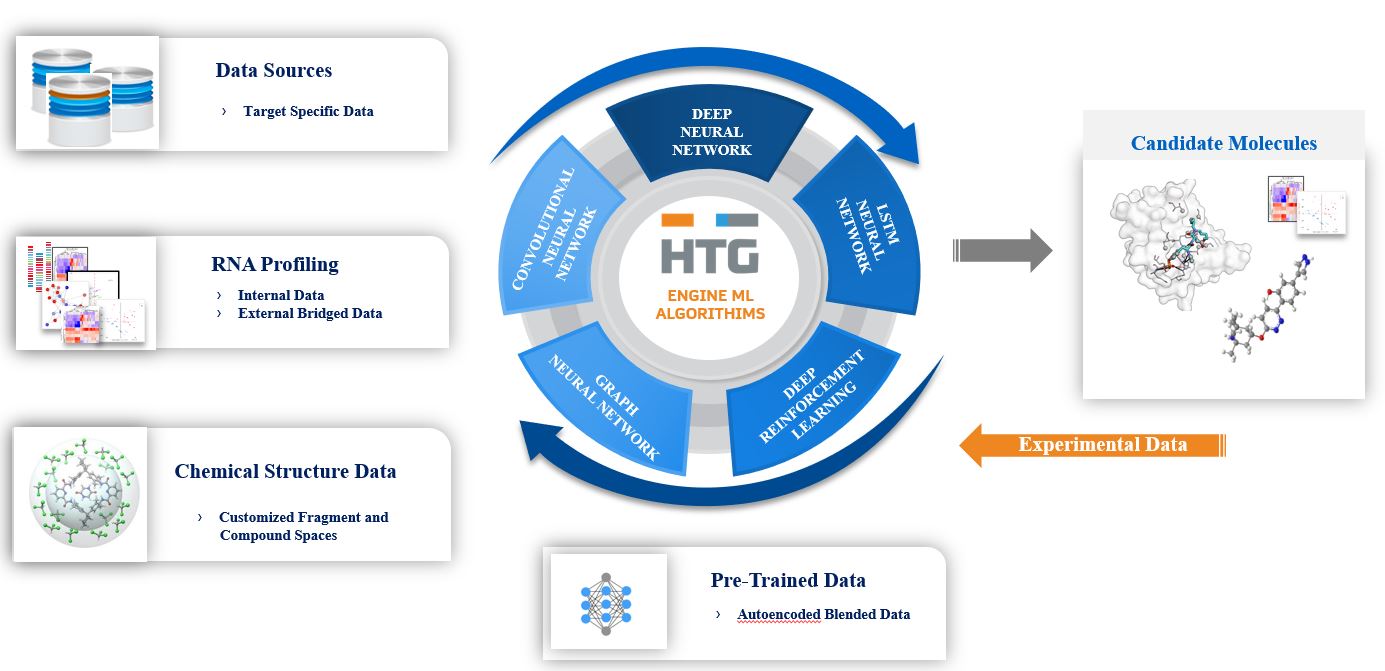
The key element of our drug discovery engine is a machine learning ‘conversation’ between several data sources, including our proprietary transcriptomic data and the chemical structures of the compound used to generate the data. Additional data sources include pre-trained data from multiple databases for RNA and protein biology, pathway analysis, compound properties and compound structures. Data that we are generating are continually being added and experimental data from our compound libraries will be used in an effort to optimize the design and selection of potential drug candidates for considerations based on transcriptomic indicators related to efficacy and safety. Our drug discovery engine is designed and built to be a modular and highly scalable set of machine learning algorithms, with the flexibility to incorporate cutting-edge techniques in this rapidly-changing area.
11
In molecular profiling, our products and services are designed to work with many different biological sample types, can generate robust results from very small samples, and obviate the need for many of the sample-preparation steps associated with traditional molecular techniques. Our platform and assays enable the simultaneous detection and quantitation of tens, hundreds or thousands of molecular targets and are capable of profiling multiple parameters such as RNA expression levels, RNA-expressed gene fusions and RNA modifications in a single testing workflow that can use NGS detection for quantitative measurement.
We believe we are well positioned with the following key product benefits:
Revenue and Commercialization of our Products
Commercialization of our Drug Discovery Assets
We have recently begun partnering conversations regarding our drug discovery assets. These portfolio discussions are being addressed in two ways. First, we will seek to identify biopharmaceutical companies who want to partner with us to further develop individual drug candidates. These partnerships could be molecule, target or indication specific, or a combination of all three. We expect to have drug candidates for the first two indications available for potential partnership opportunities in 2023.
12
Second, we expect to begin partnering conversations with biopharmaceutical companies around the potential licensing of our drug discovery technology. These partnerships could use all of the platform technologies or only certain elements. We expect the supporting data associated with our discovery efforts to serve as tangible and objective proof of our ability to develop differentiated molecules, which would further enable partnership discussions.
Revenue and Commercialization of our Profiling Products
We currently market proprietary molecular profiling panels targeting early and late-stage drug development programs with potential breakthrough therapies. We market these panels to biopharmaceutical companies, with which we may collaborate in biomarker development programs. We believe these programs could facilitate our commercialization of companion diagnostic tests. In addition, our panels are used in pre-clinical and clinical research areas, which we believe will facilitate our commercialization of diagnostic tests, including tumor classifiers and prognostic tests.
Our product and product-related services revenue is generated primarily through the sale of our profiling instruments and consumables and sample processing services to biopharmaceutical companies, academic research centers and molecular testing laboratories.
Customers can purchase our HTG EdgeSeq instrument and related consumables, which consist primarily of our proprietary molecular profiling panels and other assay components. We currently market a number of proprietary profiling panels including, but not limited to, the following panels which profile the full human mRNA and miRNA transcripts, respectively:
Customers can also access our technology through contracted services. Pre-clinical services, including custom assay design and sample processing services provided by our VERI/O laboratory, allow our customers to identify and validate biomarker signatures across their drug portfolios or patient cohorts more efficiently. Our VERI/O laboratory is a high-volume molecular laboratory focused solely on providing high-quality data from our proprietary molecular profiling technology. These services provide our customers expedited access to our technology at a competitive price. For our biopharmaceutical company customers, we offer an end-to-end solution leveraging a single technology from discovery to diagnostics.
Through collaboration with biopharmaceutical company customers, we believe we are uniquely positioned to provide comprehensive services to design, develop and manufacture custom targeted assays with complex molecular diagnostic signatures as investigational use only (“IUO”) assays for use in global prospective or retrospective clinical trials. Our expertise in medical device design control and global regulatory submissions, coupled with our ISO 13485:2016 certified quality system, enable us to support potential CDx programs. Although our initial focus primarily has been in oncology, we offer customers a full solution from biomarker discovery to deployment of CDx assays across numerous disease states. Utilizing NGS as our method of detection provides our customers with the benefits of our highly multiplexed and extraction-free chemistry and the sensitivity and dynamic range of the sequencers, providing a powerful value proposition and complete workflow.
Research and Development
We are committed to the continued evolution of our HTG technology platforms, including our transcriptomic profiling, machine learning chemistry and our AI drug discovery technology. We have assembled an experienced research and development team with the scientific, drug design and development, engineering and software development experience that we believe is necessary to successfully grow our business and allow us to reach our strategic objectives.
13
For the year ended December 31, 2022, the largest portion of our research and development efforts were focused on achievement of our drug discovery milestones. We expect to continue to invest in this area as we expand our pipeline and take candidates into development. Upon commercial release of our HTP in August 2021, primary focus of development efforts related to our profiling business has shifted from development of new products to expansion of sample types and improvement of processes associated with our existing product portfolio. As of December 31, 2022, our research and development team consisted of 15 employees across the disciplines of research and development, platform development and chemistry, therapeutics and bioinformatics, of which 10 held PhDs.
Sales and Marketing
We distribute our instruments and consumables via direct sales in the United States and Europe and through distributors in parts of Europe and other countries.
As of December 31, 2022, our U.S. sales and marketing organization consisted of seven employees including three in direct sales or sales management, two in sales support and two in marketing. In addition to our U.S. sales team, as of December 31, 2022, we had six direct sales and support employees in Europe and distribution agreements in several additional countries. This sales model provides us with direct sales coverage in Austria, Belgium, France, Germany, Luxembourg, the Netherlands, the United Kingdom and Switzerland, with distributors in Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, Hungary, Ireland, Israel, Italy, Kosovo, Leetonia, Lithuania, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain and Sweden.
Our sales and marketing efforts target biopharmaceutical companies, clinical research centers and clinical diagnostic labs focused on sample profiling for translational research, biomarker/companion assay development and lab-developed diagnostic testing. We intend to promote adoption of our HTG EdgeSeq platform, sample profiling panels and future molecular diagnostic assays upon marketing clearance or approval by the FDA, by expanding our U.S. sales force, building a greater direct sales presence in Europe, expanding international distribution and continuing to collaborate with key opinion leaders to validate our platform and to influence utilization of our products.
We expect that our drug discovery assets will be monetized through internal and third-party corporate development resources, and expect initial partnering conversations to be primarily in the United States. We will conduct business development activities internally and with third parties with the objective of partnering our assets as they progress through the preclinical and clinical development pathway.
Manufacturing and Suppliers
We primarily manufacture our products within our facility in Tucson, AZ. External resources are leveraged for their specific expertise in either producing components for our HTG EdgeSeq instrument and raw materials for our consumables in accordance with our designs or based on their catalog products which are utilized as is within our designs. We manufacture HTG EdgeSeq instruments and reagent kits at our Tucson, Arizona facility, which has been certified to ISO 13485:2016 standards. We believe that our existing manufacturing capacity is sufficient to meet our needs for at least the next several years.
We require a wide variety of raw materials, electronic and mechanical components, chemical and biochemical materials and other supplies to manufacture our products. While multiple commercial sources provide the majority of these required components and supplies, we currently rely on a single supplier to manufacture a subcomponent used in our HTG EdgeSeq instrument. As part of our standard supply management process, we continuously monitor material availability, vendor status and supply chain disruptions to identify and mitigate potential risks by expanding material and source alternatives. Although there are a limited number of manufacturers for components of this type, we believe that other suppliers could provide similar products on comparable terms if additional or alternative supply sources should be necessary in the future. In addition, while we attempt to keep our inventory at minimal levels, we closely monitor inventory of this subcomponent and purchase incremental inventory in this area as circumstances warrant to protect our supply chain.
Instruments
We assemble and test our HTG EdgeSeq instruments at our Tucson, Arizona facility. Instrument component vendors are qualified under our quality system and reviewed regularly to ensure that manufacturing standards are met and maintained. We award contracts for estimated annual quantities of components and, considering the replenishment lead times of our vendors, take delivery of batches covering approximately one month of demand at a time.
14
Consumables
We manufacture and test our HTG EdgeSeq consumables at our Tucson, Arizona facility. Raw material vendors are selected using precise standards and are reviewed regularly for compliance with our specific quality requirements. We purchase raw material stock in quantities that often exceed projected annual demand in order to take advantage of bulk pricing discounts and manufacturing efficiencies. We produce batches of finished goods approximating quarterly demand and supervise inventory on a minimum/maximum basis to ensure that we are replenishing our finished goods and raw material ahead of demand.
Competition
We have categorized known competition into:
We believe that the principal competitive factors in all our target markets include:
We believe the automation afforded by our HTG EdgeSeq platform coupled with fast turnaround time, high multiplexing capability, lysis only/no extraction protocol and low sample requirement gives us numerous competitive advantages in our target markets, as discussed in more detail elsewhere in this report.
While we believe that we compete favorably based on the factors described above, many of our competitors are more highly capitalized and/or have been in existence for a longer period, and enjoy several competitive advantages over us, including:
15
The biopharmaceutical sector is populated with companies advancing new or differentiated approaches to discovery and experimental therapeutics by way of different platform technologies or modalities with intent for application to specific disease areas through focus on pharmacologic targets or through phenotypic approaches. Each approach has inherent scientific risks that are intrinsic to the discovery sciences for molecule selection, as these efforts provide the early pipeline assets that progress into the more established and regulated stages of drug development. We believe that our approach, which is grounded in our exceptional RNA profiling capabilities now being applied to experimental systems used in conjunction with an advanced medicinal chemistry technology, can result in more well-informed design and selection of small molecule drug candidates very early on in the drug discovery process, thereby allowing for greater chances for success of these molecules. As such, we believe our approach differentiates us from the competition as the early risk reduction of small molecule design and selection is at the core of our strategy, with the flexibility for application across multiple therapy areas.
Intellectual Property
Our success depends in large part on our ability to develop and maintain intellectual property rights relating to key aspects of the technology employed in our HTG EdgeSeq platform and assays, maintain any strategic licenses to use intellectual property owned by third parties, preserve the confidentiality of our trade secrets and operate without infringing the valid and enforceable patents and other proprietary rights of third parties. We rely upon certain patents, registered and common law trademarks, trade secrets, know-how, invention and patent assignment agreements and continuing technological innovation to develop and maintain our competitive position.
Patents and Patent Applications
As of December 31, 2022, our patent portfolio included seven issued U.S. patents, 58 granted foreign patents (variously in Australia, Canada, China, Japan, France, Germany, Italy, Spain, and United Kingdom), and 22 patent applications pending in the United States and foreign jurisdictions. This portfolio is directed to, inter alia, our nuclease-protection-based technologies, other nucleic-acid detection methods, methods for subtyping diffuse large B-cell lymphoma ("DLBCL"), distinguishing indeterminate nevi from melanoma and methods of therapeutics treatments using novel pharmaceutical compounds. Our patent portfolio will help us maintain an exclusive position in key areas of our business, including in the areas of targeted nuclease-protection based sequencing, and drug discovery applications of our technology. In addition, this portfolio may provide out‑licensing opportunities. There were at least 10 granted patents, including one U.S. patent, directed to our novel HTG EdgeSeq product in the portfolio as of December 31, 2022. Our HTG EdgeSeq patents will begin to expire in 2032. Our patent portfolio includes at least four applications and five patents directed towards our direct-target sequencing HTG EdgeSeq methods, at least three applications and eight patents directed towards our methods of subtyping DLBCL, and at least six applications directed towards our methods of detecting DNA and RNA in the same sample.
Trade Secrets
We also rely on trade secrets, including unpatented know-how, technology and other proprietary information to maintain our competitive position. We seek to protect these trade secrets, in part, by entering into nondisclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, contract manufacturers, consultants, advisors and other third parties. We also enter into invention or patent assignment agreements with our employees and consultants that obligate them to assign to us any inventions developed in the course of their work for us. We cannot provide any assurance, however, that we have entered into such agreements with all relevant parties, or that these parties will abide by the terms of these agreements. Despite measures taken to protect our intellectual property, unauthorized parties might copy or commercially exploit aspects of our technology or obtain and use information that we regard as proprietary.
For additional information relating to the risks associated with our intellectual property position see “Risk Factors – Risks Related to our Intellectual Property.”
Agreements with Third Parties
Asset Purchase Agreement with NuvoGen Research, LLC
We entered into an asset purchase agreement dated January 9, 2001, as amended in November 2003, September 2004, November 2012 and February 2014, with NuvoGen Research, LLC (“NuvoGen”) to acquire certain intellectual property from NuvoGen (“NuvoGen obligation”). The acquired technology generally relates to our former array-based nuclease protection panels. Pursuant to the terms of the agreement, in exchange for the acquired technology, we agreed to pay NuvoGen aggregate cash compensation of $15.0 million. On an annual basis, we are currently obligated to pay the greater of $0.4 million or 6% of our annual revenue, until the total aggregate cash compensation paid to NuvoGen under the agreement totals $15.0 million. Interest on the remaining unpaid obligation has been accrued since January 1, 2019 and compounds annually at a rate of 2.5% per year. Accrued interest on this unpaid obligation is payable on the date that the remaining obligation is paid in full.
16
SVB Term Loan
In June 2020, we entered into a Loan and Security Agreement (the "Loan Agreement") for an asset-secured loan in the principal amount of $10.0 million with Silicon Valley Bank (currently named Silicon Valley Bridge Bank, N.A. following the closure of Silicon Valley Bank on March 10, 2023 by the California Department of Financial Protection and Innovation, which appointed the Federal Deposit Insurance Corporation as receiver) ("SVB"), as lender (the "SVB Term Loan"). The proceeds from the SVB Term Loan were fully funded on the June 25, 2020. Our obligations under the SVB Term Loan are secured by a security interest in substantially all of our assets, excluding intellectual property (which is subject to a negative pledge).
The SVB Term Loan bears interest at a floating rate equal to the greater of 2.5% above the Prime Rate (as defined in the Loan Agreement) and 5.75%. Interest on the SVB Term Loan is due and payable monthly in arrears. The SVB Term Loan originally required interest-only payments through June 30, 2021. As a result of achieving an equity milestone defined in the Loan Agreement during the quarter ended June 30, 2021, the interest only period was extended for six months through December 31, 2021. Following the extended interest-only period, the Loan Agreement required equal monthly payments of principal and interest through the maturity date of December 1, 2023.
Prepayments of the remaining SVB Term Loan, in whole or in part, will be subject to early termination fees of 1% and we will be required to pay a final fee premium equal to 8% of the principal amount of the SVB Term Loan upon termination of the Loan Agreement.
The Loan Agreement contains customary affirmative covenants and customary negative covenants limiting our ability and the ability of our subsidiaries, if any, to, among other things, dispose of assets, undergo a change in control, merge or consolidate, make acquisitions, incur debt, incur liens, pay dividends, repurchase stock and make investments, in each case subject to certain exceptions.
In July 2022, the Company entered into an amendment to the SVB Term Loan (the "Term Loan Amendment"). Under the Term Loan Amendment, SVB agreed to remove a financial covenant under the Loan Agreement that had required the Company to maintain a minimum unrestricted cash balance. In exchange for this accommodation, the Company prepaid $2.5 million of outstanding principal under the SVB Term Loan (the "Prepayment"). SVB waived the prepayment fee that otherwise would have applied to the Prepayment. The remaining outstanding principal amount due under the Term Loan will continue to be paid in equal monthly payments of principal and interest through the maturity date of December 31, 2023.
Third-Party Coverage and Reimbursement
Clinical laboratories acquire our instrumentation through a capital purchase, capital lease or reagent purchasing agreement. These laboratories offer their customers a menu of testing services using laboratory-developed tests ("LDTs"), which they may develop using consumables they purchase from us. Our customers generate revenue for these testing services by collecting payments from third-party payors, including public and private payors, as well as patient co-payments. In the United States, claims for Medicare coverage are processed by private Medicare Administrative Contractors (“MACs”) such as Novitas and Cahaba on behalf of the Centers for Medicare & Medicaid Services (“CMS”), and coverage for specific test codes are specified in Local Coverage Determinations (“LCDs”) issued by individual MACs or National Coverage Determinations (“NCDs”) which apply to all MACs. Private payors issue their own coverage determinations that are largely reflective of the CMS LCDs and NCDs. HTG closely monitors trends in coverage through interactions with customers, industry associations such as the College of American Pathologists (“CAP”) and the Association for Molecular Pathology (“AMP”) and industry consultants; these trends are key considerations in our product development plans. In Europe, coverage for molecular diagnostic testing is varied. Countries with statutory health insurance (e.g., Germany, France, the Netherlands) tend to be more progressive in technology adoption with favorable reimbursement for molecular diagnostic testing. In countries such as the United Kingdom with tax-based insurance, adoption and reimbursement for molecular diagnostic testing is not uniform and is influenced by local budgets. Failure by our U.S. and ex-U.S. customers who use our tests to obtain coverage and sufficient reimbursement from healthcare payors or adverse changes in government and private third-party payors’ policies could have a material adverse effect on our business, financial condition, results of operations and future growth prospects.
17
Government Regulation – Medical Device Regulations
United States
Our products and operations are subject to extensive and rigorous regulation by the FDA and other federal, state, local and foreign authorities. Currently we are limited to marketing our products in the United States for research use only, which means that we cannot make any diagnostic or clinical claims. However, we intend to seek regulatory clearances or approvals in the United States and other jurisdictions to market certain assays for diagnostic purposes. The companion diagnostic tests under development by HTG are classified as “medical devices” under the United States Food, Drug and Cosmetic Act (“FDCA”). The FDA regulates, among other things, the research, development, testing, manufacturing, approval, labeling, storage, recordkeeping, advertising, promotion and marketing, distribution, post approval monitoring and reporting and import and export of medical devices in the United States to assure the safety and effectiveness of such products for their intended use.
Unless an exemption applies, each new or significantly modified medical device we seek to commercially distribute in the United States will require either a premarket notification to the FDA requesting permission for commercial distribution under Section 510(k) of the FDCA, also referred to as a 510(k) clearance, or approval from the FDA of a premarket approval (“PMA”) application. Both the 510(k) clearance and PMA submission can be expensive, and lengthy, and require payment of significant user fees, unless an exemption is available. We believe that our companion diagnostic tests under development would be eligible for the less burdensome 510(k) regulatory pathway.
Device Classification
Under the FDCA, medical devices are classified into one of three classes – Class I, Class II or Class III – depending on the degree of risk associated with each medical device and the extent of control needed to provide reasonable assurances with respect to safety and effectiveness.
Class I devices are those for which safety and effectiveness can be reasonably assured by adherence to a set of regulations, referred to as General Controls, which require compliance with the applicable portions of the FDA’s Quality System Regulation (“QSR”) facility registration and product listing, reporting of adverse events and malfunctions, and appropriate, truthful and non-misleading labeling and promotional materials. Most Class I products are exempt from the premarket notification requirements.
Class II devices are those that are subject to the General Controls, as well as Special Controls, which can include performance standards, guidelines and post market surveillance. Most Class II devices are subject to premarket review and clearance by the FDA. Premarket review and clearance by the FDA for Class II devices is accomplished through the 510(k) premarket notification process. Under the 510(k) process, the manufacturer must submit to the FDA a premarket notification, demonstrating that the device is “substantially equivalent,” to either:
To be “substantially equivalent,” the proposed device must have the same intended use as the predicate device, and either have the same technological characteristics as the predicate device or have different technological characteristics and not raise different questions of safety or effectiveness than the predicate device. Clinical data are sometimes required to support substantial equivalence.
After a 510(k) notice is submitted, the FDA determines whether to accept it for substantive review. If it lacks necessary information for substantive review, the FDA will refuse to accept the 510(k) notification. If it is accepted for filing, the FDA begins a substantive review. By statute, the FDA is required to complete its review of a 510(k) notification within 90 days of receiving the 510(k) notification. As a practical matter, clearance often takes longer, and clearance is never assured. Although many 510(k) premarket notifications are cleared without clinical data, the FDA may require further information, including clinical data, to make a determination regarding substantial equivalence, which may significantly prolong the review process. If the FDA agrees that the device is substantially equivalent, it will grant clearance to commercially market the device.
18
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a new or major change in its intended use, will require a new 510(k) clearance or, depending on the modification, could require a PMA application. The FDA requires each manufacturer to make this determination initially, but the FDA can review any such decision and can disagree with a manufacturer’s determination. If the FDA disagrees with a manufacturer’s determination regarding whether a new premarket submission is required for the modification of an existing device, the FDA can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or approval of a PMA application is obtained. If the FDA requires us to seek 510(k) clearance or approval of a PMA application for any modifications to a previously cleared product, we may be required to cease marketing or recall the modified device until we obtain this clearance or approval. In addition, in these circumstances, we may be subject to significant regulatory fines or penalties for failure to submit the requisite PMA application(s). In addition, the FDA is currently evaluating the 510(k) process and may make substantial changes to industry requirements.
The PMA Process
If the FDA determines that the device is not “substantially equivalent” to a predicate device, or if the device is classified into Class III, the device sponsor must then fulfill the much more rigorous premarketing requirements of the PMA process, or seek reclassification of the device through the de novo process. A manufacturer can also submit a petition for direct de novo review if the manufacturer is unable to identify an appropriate predicate device and the new device or new use of the device presents a moderate or low risk.
A PMA application typically includes, but is not limited to, extensive technical information regarding device design and development, pre-clinical and clinical study data, manufacturing information, labeling and financial disclosure information for the clinical investigators in the device studies.
Post-Approval Requirements
After the FDA permits a device to enter commercial distribution, numerous regulatory requirements apply. These include, but are not limited to:
Our facilities, records and manufacturing processes are subject to periodic unscheduled inspections by the FDA. Failure to comply with the applicable United States medical device regulatory requirements could result in, among other things, warning letters, untitled letters, fines, injunctions, consent decrees, civil penalties, unanticipated expenditures, repairs, replacements, refunds, recalls or seizures of products, operating restrictions, total or partial suspension of production, the FDA’s refusal to issue certificates to foreign governments needed to export products for sale in other countries, the FDA’s refusal to grant future premarket clearances or approvals, withdrawals or suspensions of current product clearances or approvals and criminal prosecution.
19
Research Use Only
An RUO product is one that is not intended for clinical diagnostic use and must be labeled “For Research Use Only”. Not for use in diagnostic procedures.” Products that are intended for research use only and are properly labeled as RUO are exempt from compliance with the FDA requirements discussed above, including the approval or clearance and most QSR requirements. A product labeled RUO but intended to be used diagnostically may be viewed by the FDA as adulterated and misbranded under the FDC Act and is subject to FDA enforcement activities. The FDA may consider the totality of the circumstances surrounding distribution and use of an RUO product, including how the product is marketed, when determining its intended use. In November 2013 the FDA issued a guidance document entitled “Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only” (the “RUO Guidance”) which highlights the FDA’s interpretation that distribution of RUO products with any labeling, advertising or promotion that suggests that clinical laboratories can validate the test through their own procedures and subsequently offer it for clinical diagnostic use as a laboratory developed test is in conflict with RUO status. The RUO Guidance further articulates the FDA’s position that any assistance offered in performing clinical validation or verification, or similar specialized technical support, to clinical laboratories, conflicts with RUO status.
European Union
The European Union (“EU”) has also adopted requirements that affect our products. These requirements include establishing standards that address creating a certified quality system as well as several directives that address specific product areas. The most significant of these currently effective directives is the In Vitro Diagnostic Medical Device Directive (“IVDD”) which includes:
On May 26, 2017, the EU released a new regulatory framework, the In Vitro Diagnostic Medical Device Regulation (“IVDR”) which is expected to replace IVDD. Our CE/IVD marked products continued to meet the requirements of IVDD for commercialization in the EU until the requirements of IVDR took effect on May 26, 2022. At this time we do not anticipate moving to the requirements of IVDR for our existing CE/IVD marked products. As such, these products are no longer available other than for research use only.
Other International
Several other countries, including Australia, Canada, China and Japan, have adopted or are in the process of adopting standards for medical devices sold in those countries. Many of these standards are loosely patterned after those adopted by the EU, but with elements unique to each country. Although there is a trend towards harmonization of quality system standards, regulations in each country may vary substantially, which can affect timelines of introduction. We routinely monitor these developments and address compliance with the various country requirements as new standards are adopted.
Government Regulation – Fraud and Abuse and Other Healthcare Regulation
We may be subject to various federal and state healthcare laws, including, but not limited to, anti-kickback, false claims, data privacy and security, and transparency laws. Penalties for violations of these healthcare laws include, but are not limited to, significant criminal, civil and administrative penalties, damages, fines, disgorgement, imprisonment, possible exclusion from Medicare, Medicaid and other federal healthcare programs, additional reporting requirements and/or oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws and the curtailment or restructuring of operations. These laws include the following:
20
21
Healthcare Reform
There have been and we anticipate that there will be healthcare reform measures that may be adopted in the future that may result in more rigorous coverage criteria and additional downward pressure on the reimbursement for healthcare products and services. For example, the ACA, which substantially changed healthcare financing and delivery by both governmental and private insurers, remains subject to challenge. On June 17, 2021, the U.S. Supreme Court dismissed a challenge on procedural grounds that argued the ACA is unconstitutional in its entirety because the “individual mandate” was repealed by Congress. Further, on August 16, 2022, President Biden signed the Inflation Reduction Act of 2022, or IRA, into law, which, among other things, extends enhanced subsidies for individuals purchasing health insurance coverage in ACA marketplaces through plan year 2025. The IRA also eliminates the “donut hole” under the Medicare Part D program beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost and creating a new manufacturer discount program. The IRA also includes measures designed to lower the cost of certain pharmaceutical products under the Medicare program. It is possible that the ACA will be subject to judicial or Congressional challenges in the future. Congress and the Biden administration are considering various health reform measures. Moreover, payment methodologies may be subject to changes in healthcare legislation and regulatory initiatives.
The Foreign Corrupt Practices Act
The Foreign Corrupt Practices Act (“FCPA”) prohibits any U.S. individual or business from paying, offering, or authorizing payment or offering of anything of value, directly or indirectly, to any foreign official, political party or candidate for the purpose of influencing any act or decision of the foreign entity to assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the United States to comply with accounting provisions requiring the company to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations.
Human Capital
Our ability to identify and recruit strong candidates into our company and to retain and develop current talent within our organization is a critical factor in our continued growth and performance improvement. We continue to initiate programs to promote our organizational culture and to identify the best possible new talent as the organization grows and new positions are made available. We believe our culture and commitment to our employees result in the attraction and retention of qualified talent, while providing significant value to our company and its stockholders. As of December 31, 2022, we had 53 full-time and one part-time employee, of which 11 are employed in administration, 13 in manufacturing and operations, 15 in research and development, two in regulatory and quality affairs, and 13 in direct sales and marketing. Of these employees, six were located in Europe and all others were located in the United States. We believe that our success will depend, in part, on our ability to attract and retain qualified personnel. We have never experienced a work stoppage due to labor difficulties and believe that our relations with our employees are good. None of our U.S. employees are represented by labor unions. Collective bargaining is established by law in France. We and our French employees have agreed to the terms of the applicable collective bargaining agreements.
Corporate Information
We were originally incorporated in Arizona in October 1997 as “High Throughput Genomics, Inc.” In December 2000, we reincorporated in Delaware as “HTG, Inc.” and in March 2011 we changed our name to “HTG Molecular Diagnostics, Inc.” Our principal executive offices are located at 3430 E. Global Loop, Tucson, AZ 85706, and our telephone number is (877) 289-2615. Our corporate website address is www.htgmolecular.com. Information contained on or accessible through our website is not a part of this report, and the inclusion of our website address in this report is an inactive textual reference only.
This report contains references to our trademarks, including VERI/O and HTG EdgeSeq, and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this report, including logos, artwork and other visual displays, may appear without the ® or TM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
We are also a “smaller reporting company” as defined in the Securities Exchange Act of 1934 (the “Exchange Act”) and have elected to take advantage of certain of the scaled disclosures available to smaller reporting companies.
22
Where You Can Find Additional Information
We make available free of charge through our investor relations website, www.htgmolecular.com, our annual reports, quarterly reports, current reports, proxy statements and all amendments to those reports as soon as reasonably practicable after such material is electronically filed or furnished with the SEC. These reports may also be obtained without charge by contacting Investor Relations, HTG Molecular Diagnostics, Inc., 3430 E. Global Loop, Tucson, Arizona 85706, e-mail: info@htgmolecular.com. Our Internet website and the information contained therein or incorporated therein are not intended to be incorporated into this Annual Report on Form 10-K. In addition, the SEC maintains an Internet site that contains reports, proxy and information statements and other information regarding reports that we file or furnish electronically with them at www.sec.gov.
23
Item 1A. Risk Factors.
RISK FACTORS
An investment in shares of our common stock involves a high degree of risk. You should carefully consider the following risk factors, as well as the other information in this report, and in our other public filings, before deciding to purchase, hold or sell shares of our common stock. The occurrence of any of the following risks could have a material adverse effect on our business, financial condition, results of operations and future growth prospects or cause our actual results to differ materially from those contained in forward-looking statements we have made in this report and those we may make from time to time. In these circumstances, the market price of our common stock could decline, and you may lose all or part of your investment. You should consider all of the risk factors described when evaluating our business.
Risks Related to our Business and Strategy
There is substantial doubt about our ability to continue as a going concern. We will need to raise additional capital to fund our operations in the future. If we are unsuccessful in attracting new capital, we may be forced to delay, reduce or eliminate at least some of our product development programs or business development plans, may not be able to continue operations or may be forced to sell assets to do so. Alternatively, capital may not be available to us on favorable terms, or if at all. If available, financing terms may lead to significant dilution of our stockholders’ equity.
We are not profitable and have had negative cash flow from operations since our inception. To fund our operations and develop and commercialize our products, we have relied primarily on equity and debt financings and revenue generated from the sale of our HTG EdgeSeq products and related services. We currently expect that our existing resources will only be sufficient to fund our planned operations and expenditures until at least July 2023. In addition, potentially changing circumstances may also result in the depletion of our capital resources more rapidly than we currently anticipate. These circumstances raise substantial doubt about our ability to continue as a going concern.
Our efforts to use our transcriptome-based drug discovery engine to deliver new drug candidates in areas of significant unmet medical need are broad, expensive to achieve and will require substantial additional capital in the future. Our existing programs span early and late-stage discovery, with some approaching entrance into preclinical development. We expect our expenses to increase in connection with our ongoing activities as we continue research and development and add to our pipeline that we believe will be an accelerating number of additional programs. Preclinical testing is expensive and can take many years, which may make equity and debt financing more difficult to obtain.
We will need to obtain additional funds to finance our operations. Additional capital may not be available at such times or in amounts needed by us. Historically we have financed our business in part by access to the capital markets. However, the current volatility in the equity markets creates additional challenges to raising a sufficient amount of capital through an equity financing in the near term. Even if capital is available, it might be available only on unfavorable terms. Any additional equity or convertible debt financing into which we enter could be dilutive to our existing stockholders. Any future debt financing into which we enter may impose covenants upon us that restrict our operations, including limitations on our ability to incur liens or additional debt, pay dividends, repurchase our stock, make certain investments and engage in certain merger, consolidation or asset sale transactions. Any debt financing or additional equity that we raise may contain terms that are not favorable to us or our stockholders. If we raise additional funds through strategic collaborations, partnerships or licensing arrangements with third parties, we may need to relinquish rights to our technologies, our products or our drug candidates or grant licenses on terms that are not favorable to us. If access to sufficient capital is not available as and when needed, our business will be materially impaired, and we may be required to cease operations, curtail one or more product development or commercialization programs, or significantly reduce expenses, sell assets, seek a merger or joint venture partner, file for protection from creditors or liquidate all of our assets. Any of these factors could harm our operating results.
We have incurred losses since our inception and expect to incur losses for the foreseeable future. We cannot be certain that we will achieve or sustain profitability.
We have incurred losses since our inception and expect to incur losses in the future. We incurred net losses of $21.6 million and $17.1 million for the years ended December 31, 2022 and 2021, respectively. As of December 31, 2022, we had an accumulated deficit of $229.9 million. We expect that our losses will continue for the foreseeable future as we will be required to invest significant additional funds to support product development, including the commercialization of our HTG EdgeSeq platform and proprietary consumables and advancement of our HTG Therapeutics business unit. Our ability to achieve or, if achieved, sustain profitability is based on numerous factors, many of which are beyond our control, including the market acceptance of our products and services, competitive product development and our market penetration and margins. We may never be able to generate sufficient revenue to achieve or, if achieved, sustain profitability.
24
Payments under the instruments governing our indebtedness may reduce our working capital. In addition, a default under our SVB Term Loan could cause a material adverse effect on our financial position.
Pursuant to the terms of the NuvoGen obligation, we have paid NuvoGen $11.1 million, and are required to annually pay NuvoGen the greater of $400,000 or 6% of our yearly revenue until the total aggregate cash compensation paid to NuvoGen under the agreement equals $15.0 million. Payments to NuvoGen will result in a reduction in our working capital as we continue to make payments on this obligation.
The SVB Term Loan requires us, and any debt arrangements we may enter into in the future may require us, to comply with various covenants that limit our ability to, among other things:
These restrictions could inhibit our ability to pursue our business strategies. If we default under our obligations under the SVB Term Loan, including as a result of a “material adverse change,” the lender could proceed against the collateral granted to them to secure our indebtedness or declare all obligations under the SVB Term Loan to be due and payable. The definition of “material adverse change” is broad and includes a material impairment in the value of the collateral securing the SVB Term Loan, a material adverse change in our business, operations, or condition (financial or otherwise), and a material impairment of the prospect of repayment of any portion of the SVB Term Loan. Moreover, the determination by the lender as to whether a “material adverse change” has occurred is not within our control. This risk may be exacerbated by the recent closure of SVB. On March 10, 2023, the FDIC took control and was appointed receiver of SVB. The SVB Term Loan remains intact and we will continue to make required payments through the end of the current year, at which time the SVB Term Loan will be repaid in full. However, it is unclear how the current managers of SVB will view the SVB Term Loan from a risk standpoint and what actions they may elect to take under the SVB Term Loan to protect the financial interests of SVB.
In certain circumstances, procedures by the lender could result in a loss by us of all of our equipment and inventory, which are included in the collateral granted to the lender. Our intellectual property is not included in the collateral granted to the lender but is subject to a negative pledge. In addition, upon any distribution of assets pursuant to any liquidation, insolvency, dissolution, reorganization or similar proceeding, the holders of secured indebtedness will be entitled to receive payment in full from the proceeds of the collateral securing our secured indebtedness before the holders of other indebtedness or our common stock will be entitled to receive any distribution with respect thereto.
Unstable market and economic conditions may have serious adverse consequences on our business, financial condition and share price.
The global economy, including credit and financial markets particularly in the emerging biotech sector, has experienced extreme volatility and disruptions, including severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, record inflation and uncertainty about economic stability. For example, the COVID-19 pandemic resulted in widespread unemployment, economic slowdown and extreme volatility in the capital markets. Similarly, the current Russia-Ukraine conflict has resulted and may result in the future in volatility in the global capital markets and has disrupted the global supply chain and energy markets. Any such volatility and disruptions may have adverse consequences on us or the third parties on whom we rely. If the equity and credit markets deteriorate, including as a result of bank failures, political unrest or war, it may make any necessary debt or equity financing more difficult to obtain in a timely manner or on favorable terms, more costly or more dilutive.
25
Our HTG Therapeutics business strategy is unique, may not lead to successful drug products for various reasons, may require significant investments in working capital and may not generate any revenue.
In July 2021, we formed a new drug discovery business unit, HTG Therapeutics. This business unit uses our HTP and our epitranscriptomic profiling technologies to more thoroughly understand at the cellular level how cells respond to pharmacologic perturbation. By leveraging these profiling technologies earlier in the drug discovery process, our objective is for HTG Therapeutics to generate lead compounds faster, and with potentially more favorable efficacy and toxicity profiles, with the ultimate goal of generating interest from pharmaceutical companies that results in research or licensing collaborations for, or acquisitions of, these compounds. While we have hired experienced employees and added drug development depth to our Board of Directors, as a company we have no prior experience with drug discovery and development and may not be successful in this endeavor. If studying the transcriptomic profile does not prove to be a more insightful approach to understand diseases and the effects of molecules or does not lead to the biological insights for selection and design of drug candidates that we anticipate, our drug discovery platform may not be as useful or may not lead to as successful drug products, or we may have to move to a new business strategy, any of which could have an adverse effect on our reputation and results of operations.
Moreover, drug discovery and development is expensive and will require investments in working capital by us that may be significant. Our current drug candidates are discovery stage and are approaching entrance to preclinical development. Before we or a partner can bring any drug candidate to market, we must, among other things, complete preclinical studies, have the candidate manufactured to appropriate specifications, conduct extensive clinical trials to demonstrate safety and efficacy in humans, prepare regulatory registration packages and ultimately obtain marketing approval from global regulatory authorities, which, based on our early stage, we have not yet demonstrated our ability to do. Even if we are successful in partnering for one or more early-stage drug discovery programs with a pharmaceutical company, we will need to expend potentially significant capital resources on these programs prior to any such partnering, and potentially after, and there can be no assurance that we will generate meaningful revenue from these programs. Preclinical and clinical development are expensive, difficult to design and implement, can take many years to complete and are uncertain as to outcome. A failure of a clinical trial can occur at any stage of testing. The outcome of preclinical development testing and early clinical trials may not be predictive of success in later clinical trials, and interim results of a clinical trial do not necessarily predict final results.
We have a limited history of operations for our preclinical-stage platform-based drug discovery business and no products approved by regulators for commercial sale, which may make it difficult to evaluate our current and future business prospects.
Since the creation of our drug discovery business in July 2021, we have focused substantial efforts and financial resources on building our drug discovery platform and developing our initial target compounds. All of our compounds are in the discovery stages and/or approaching preclinical development. We may never establish an out-licensing or collaboration arrangement for any compounds we develop, or if we do, the terms may not be favorable to us. Until we successfully partner, out-license develop and/or commercialize drug candidates for these targets, which may never occur, we expect to finance our operations through a combination of equity offerings, debt financings and strategic collaborations or similar arrangements. Biopharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk. For these and other reasons discussed elsewhere in this Risk Factors section, it may be difficult to evaluate our current business and our future prospects.
As part of our current business model, we intend to seek to enter into strategic collaborations and licensing arrangements with third parties.
We have relied, and expect to continue to rely, on strategic development collaborations and licensing agreements with third parties to develop or in-license technologies based on which products or services we may develop or offer.
We have entered into agreements with third parties to facilitate or enable our development of assays, and ultimately diagnostic tests, to aid in the diagnosis of oncology diseases, such as breast cancer and melanoma, and other diseases. We intend to enter into additional similar agreements with life sciences companies, biopharmaceutical companies and other researchers for future diagnostic products.
In addition, we intend to seek strategic collaborations, partnerships and licensing arrangements with pharmaceutical and biotechnology companies related to preclinical or clinical development or commercialization to fund expenses associated with development, registration and commercialization of our potential drug candidates. In the near term, the value of our company will depend in part on the number and quality of the collaborations and similar arrangements that we create. Whether we reach a definitive agreement for a collaboration will depend, among other things, on our assessment of the collaborator's resources and expertise, the terms and conditions of the proposed collaboration and the potential collaborator's evaluation of our technologies and capabilities.
26
We cannot guarantee that we will enter into any additional agreements or collaborations. For example, our life sciences research or biopharmaceutical customers are not obligated to collaborate with us or license technology to us, and they may choose to develop diagnostic products themselves or collaborate with our competitors. Establishing strategic collaborations and licensing arrangements is difficult and time-consuming. Discussions may not lead to development collaborations or licenses on favorable terms, or at all. Potential collaborators or licensors may elect not to work with us based upon their assessment of our financial, regulatory or intellectual property position. To the extent that we enter new collaborative development or licensing agreements, they may never result in the successful development or commercialization of future drug candidates or other products or the generation of future sales revenue for a variety of reasons. The success of these arrangements will depend heavily on the efforts and activities of our collaborators. We cannot control the amount and timing of our collaborators’ resources that will be devoted to performing their responsibilities under our agreements with them. Moreover, to the extent we agree to work exclusively with a party in a given area, our opportunities to collaborate with others would be limited. Disputes with our collaborators could also impair our reputation or result in development delays, could consume time and divert management resources away from operations, impact our ability to enter into future collaboration agreements, decrease future revenue and or result litigation expenses or payments to settle disputes.
If we are unable to successfully commercialize our products, our business may be adversely affected.
Our sales of life science research products, profiling and diagnostic products, and potential future products will depend in large part on our ability to successfully increase the scope of our marketing efforts and establish and maintain a sales force commensurate with our then applicable markets. We currently market our products through our own sales force in the United States and Europe and have distributors in parts of Europe, though we may choose to expand our marketing and sales efforts into other parts of the world. However, we may not be able to market and sell our products effectively. If we do not build and maintain an efficient and effective sales force and distributor relationships targeting new markets, our business and operating results will be adversely affected. Further, if our products fail to achieve and sustain sufficient market acceptance, or we are not able to continue to expand our service relationships with third parties, we may not generate the expected revenues and our prospects could be harmed.
If our HTG EdgeSeq platform and proprietary profiling panels fail to achieve and sustain sufficient market acceptance, or we are not able to continue to expand our service relationships with biopharmaceutical customers, either directly or through a partner, we will not generate expected revenue, and our prospects may be harmed. If the utility of our HTG EdgeSeq platform, proprietary profiling panels, services and solutions in development is not supported by studies published in peer-reviewed medical publications, the rate of adoption of our current and future products and the rate of reimbursement of our future products by third-party payors may be negatively affected. We may provide our HTG EdgeSeq instrument and profiling panels free of charge or through other arrangements to customers or key opinion leaders through evaluation agreements or reagent rental programs, and these programs may not be successful in generating recurring revenue from sales of our systems and proprietary panels.
In addition, a component of our strategy is to develop diagnostic tools in conjunction with biopharmaceutical companies’ drug development programs, to help assess the proper course of treatment for specific diseases. Even if we are successful in developing those diagnostic tools and receive regulatory approval, we still may not be successful in marketing those diagnostic tests. Furthermore, the decision to advance an underlying drug candidate through clinical trials and ultimately to commercialization is at the discretion of biopharmaceutical companies with which we collaborate. Our biopharmaceutical partners may take certain actions that could negatively impact the utility and marketability of our diagnostic tests. For example, our biopharmaceutical partners could:
To the extent that we develop diagnostic assays for a biopharmaceutical company in collaboration with a collaboration partner, we may not have responsibility for some or all aspects of developing, marketing or commercializing any resulting diagnostic tests. In addition to this biopharmaceutical partner risk, a collaboration partner may take certain actions that could negatively impact the development, utility and marketability of the applicable diagnostic tests. For example, a collaboration partner could fail to satisfy or fall behind in its obligations to us or to the biopharmaceutical company for which we develop a companion diagnostic test, which may delay development, regulatory approvals, market development and/or commercialization of the applicable companion diagnostic test.
27
COVID-19 has adversely affected our business and a resurgence of COVID-19 or another health epidemic or pandemic may have an adverse impact on our business in the future.
Our business, including our workforce, supply chain and customer base, has been adversely affected by COVID‑19 in the past and a resurgence of COVID-19 or another health epidemic or pandemic may adversely affect us in the future.
COVID-19 had a negative impact on our product and product-related services revenue in 2020, 2021 and 2022. While we saw some recovery in customers returning to work in 2022, the period of reduced revenue continued in 2022 as many customers did not return to historical operating levels, did not allow visitors on site at their facilities for some portion of the year or have not resumed previously planned studies. The extent of this impact has varied from customer to customer depending upon how they have been directly or indirectly impacted by local stay-at-home orders and other social distancing measures, priorities for the customers when the immediate impacts of the pandemic had passed, and the workforce and supplier impacts that each customer experienced during the pandemic.
It is also possible that COVID-19 or another epidemic or pandemic will impact our workforce, supply chains or distribution networks or otherwise impact our ability to conduct sample processing services in our laboratory or to travel to customer facilities for commercial or support functions in the future. Governmental mandates may require forced shutdowns of our facilities for extended or indefinite periods. A public health crisis could also substantially interfere with general commercial activity related to our supply chain and customer base, which could have a material adverse effect on our financial condition, results of operations, business or prospects. Restrictions resulting from a public health crisis may disrupt our supply chains or distribution networks or limit our ability to obtain sufficient materials for our consumables or instruments and may disrupt our ability to process customer samples or, to the extent we enter into collaborative services agreements with biopharmaceutical customers, perform collaborative development services. Further, to the extent our customers’ businesses are adversely affected by the pandemic, they might delay or reduce purchases from us or development projects with us, which could adversely affect our results of operations. The effects of ongoing or future health epidemics on our business remain uncertain and subject to change. While we do not know the full extent of potential delays or impacts on the global economy, these effects could have a material adverse impact on our operations, financial position and liquidity.
Our business operations might be disrupted or adversely affected by catastrophic events.
We manufacture our HTG EdgeSeq instrument and consumable products and perform our RUO profiling and custom RUO assay design services in our Tucson, Arizona facilities. In addition, our Tucson facilities are the center for order processing, receipt of critical components of our HTG EdgeSeq instrument and shipping products to customers. We do not have redundant facilities. Damage or the inability to utilize our Tucson facilities and the equipment we use to perform research, development or services and manufacture our products could be costly, and we would require substantial lead-time to repair or replace this facility and equipment. The Tucson facilities may be harmed or rendered inoperable by natural or man-made disasters, including flooding, wind damage, power spikes and power outages, which may render it difficult or impossible for us to perform these critical functions for some period of time. The inability to manufacture consumables or instruments, process customer samples, perform development services or ship products to customers for even a short period of time may result in the loss of customers or harm our reputation, and we may be unable to regain those customers in the future. Although we possess insurance for damage to our property and the disruption of our business, this insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all. In addition, natural disasters or other catastrophic events in various parts of the world, including interruptions in the supply of natural resources, political and governmental changes, disruption in transportation networks or delivery services, severe weather conditions, wildfires and other fires, explosions, actions of animal rights activists, terrorist attacks, earthquakes, wars, conflicts (including the current Russia-Ukraine conflict), and public health issues could disrupt our operations or those of our collaborators, contractors and vendors or contribute to unfavorable economic or other conditions that could adversely impact us.
Our financial results may vary significantly from quarter to quarter or may fall below the expectations of investors or securities analysts, each of which may adversely affect our stock price.
Investors should consider our business and prospects considering the risks and difficulties we expect to encounter in the new, uncertain and rapidly evolving markets in which we compete. Because these markets are new and evolving, predicting their future growth and size is difficult. We expect that our visibility into future sales of our products, including volumes, prices and product mix between instruments, consumables and services, will continue to be limited and could result in unexpected fluctuations in our quarterly and annual operating results.
28
Numerous other factors, many of which are outside our control, may cause or contribute to significant fluctuations in our quarterly and annual operating results. For example, two customers accounted for 16% and 14% of our revenue for the year ended December 31, 2022. The three largest customers accounted for 37%, 24%, and 13% of our accounts receivable balance as of December 31, 2022. If orders from our top customers are discontinued and we are unable to establish new projects or continue to expand our customer base, our revenue in future periods may materially decrease. In addition, we experienced a significant slowing of product and product-related services revenue generation beginning in March 2020 as a result of COVID-19. This period of reduced revenue continued through the remainder of 2020, 2021 and into 2022 due to disruptions to our customers’ businesses as a result of the pandemic. The extent of this impact on our ongoing business is likely to vary from customer to customer depending upon how they are directly or indirectly impacted by local stay-at-home orders and other social distancing measures, priorities for the customers when the immediate impacts of the pandemic have passed, and the workforce and supplier impacts that each customer has experienced during the pandemic. Fluctuations in our operating results may make financial planning and forecasting difficult. In addition, these fluctuations may result in unanticipated decreases in our available cash, which could negatively affect our business and prospects. Factors that may contribute to fluctuations in our operating results include many of the risks described under the caption “Risk Factors – Risks Related to Our Business and Strategy” of this report. In addition, one or more of such factors may cause our revenue or operating expenses in one period to be disproportionately higher or lower relative to the others. Our products involve a significant capital commitment from our customers or may depend on customer studies that have variable or indefinite timelines and accordingly, involve a lengthy sales cycle. We may expend significant effort in attempting to make a particular sale, which may be deferred by the customer or never occur. Accordingly, comparing our operating results on a period-to-period basis may not be meaningful, and investors should not rely on our past results as an indication of our future performance. If such fluctuations occur or if our operating results deviate from our expectations or the expectations of investors or securities analysts, our stock price may be adversely affected.
Our sales cycle is lengthy and variable, which makes it difficult for us to forecast revenue and other operating results.
Our sales process involves numerous interactions with multiple individuals within any given organization, and often includes in-depth analysis by potential customers of our products (where in some instances we will provide a demonstration unit for their use and evaluation), performance of proof-of-principle studies, preparation of extensive documentation and a lengthy review process. As a result of these factors, the capital investment required in purchasing our instrument and the budget cycles of our customers, the time from initial contact with a customer to our receipt of a purchase order can vary significantly and be up to 12 months or longer. Given the length and uncertainty of our sales cycle, we have in the past experienced, and likely will in the future experience, fluctuations in our product and product-related services revenue on a period-to-period basis. In addition, any failure to meet customer expectations could result in customers choosing to retain their existing systems or service providers or to purchase systems or services other than ours. To the extent we enter into collaborative services agreements with biopharmaceutical customers, the revenue that we expect to earn from our collaborative development services are also subject to an extended, variable timeline based on each project agreement, which will likely result in fluctuations in our collaborative development services revenue on a period-to-period basis as well.
We may not be able to develop new products or enhance the capabilities of our systems to keep pace with rapidly changing technology and customer requirements, which could have a material adverse effect on our business and operating results.
Our success depends on our ability to develop new products and applications for our technology in existing and new markets, while improving the performance and cost-effectiveness of our systems. If we do not successfully manage the development and launch of new products, our products or our financial results could be adversely affected. Developing new products and applications may require large investments in working capital and/or the development of new methods or technologies.
Although we believe that our HTG Transcriptome Panel will be a foundational product for RUO profiling, future companion diagnostics and potential proprietary diagnostic products, and will allow us to further expand our product offerings outside of oncology and autoimmune, we have only recently initiated commercial sales of this panel and it may not have the commercial success that we anticipate or hope for, and we may not be able to continue to expand our product offerings.
In July 2021, we formed a new drug discovery business unit, HTG Therapeutics, which uses our HTP and epitranscriptomic profiling technologies in RNA profiling, and we expect that, by leveraging these profiling technologies earlier in the drug discovery process, HTG Therapeutics will generate lead compounds faster, and with potentially more favorable efficacy and toxicity profiles. However, there can be no assurance that HTG Therapeutics will be able to accomplish these goals or will otherwise be successful. In addition, we have built a machine learning-based chemical library design platform, which is expected to better predict the binding properties of a drug candidate to its target. If we are unsuccessful at developing this full machine learning-based chemical library design platform, or it, HTG Therapeutics or our HTP do not provide the benefits that we anticipate, our future revenue opportunities will be limited.
29
New technologies, techniques or products could emerge that might offer better combinations of price and performance than our current or future products and systems. Existing or future markets for our products, including gene expression analysis, liquid-based specimen analysis (e.g., plasma, blood and urine) and single-cell analysis, as well as potential markets for our diagnostic product candidates, are characterized by rapid technological change and innovation. It is critical to our success that we anticipate changes in technology and customer requirements and successfully introduce new, enhanced and competitive technologies to meet our customers’ and prospective customers’ needs on a timely and cost-effective basis. At the same time, however, we must carefully manage the introduction of new products. If customers believe that such products will offer enhanced features or be sold for a more attractive price, they may delay purchases until such products are available. We may also have excess or obsolete inventory of older products as we transition to new products and our experience in managing product transitions is very limited. If we do not successfully innovate and introduce new technology into our product lines or effectively manage the transitions to new product offerings, our revenue and results of operations will be adversely impacted.
Competitors may respond more quickly and effectively than we do to new or changing opportunities, technologies, standards or customer requirements. We anticipate that we will face increased competition in the future as existing companies and competitors develop new or improved products and as new companies enter the market with new technologies.
If we do not successfully manage the development and launch of new products, our financial results could be adversely affected.
We face risks associated with launching new products and with undertaking to comply with regulatory requirements for certain types of our products. If we encounter development or manufacturing challenges, adjust our product development priorities, or discover deficiencies during our product development cycle, the product launch date(s) may be delayed, or certain product development projects may be terminated. The expenses or losses associated with unsuccessful product development or launch activities or lack of market acceptance of our new products could adversely affect our business or financial condition.
We may not be successful in expanding our customer base and introducing new applications for our profiling business.
Our current customer base is primarily composed of biopharmaceutical companies, academic institutions and molecular labs that perform analyses using or directly or indirectly obtain services based on our HTG EdgeSeq platform and consumables for research use only, which means that the products or data from services may not be used for clinical diagnostic purposes. The success of our profiling business will depend, in part, upon our ability to increase our market penetration among our customer bases, to continue to introduce new applications for our existing technology and to expand existing customer adoption of our RUO applications (whether product or service). To achieve these goals, we will need to enter into service arrangements with biopharmaceutical company customers, expand and adjust our sales strategy, continue to train our sales force and support publication of scientific publications that reflect the application of our technology in various areas. Additionally, we must demonstrate to laboratory directors, physicians and third-party payors that our products are effective in obtaining relevant information, and that our HTG EdgeSeq platform and related panels can enable an equivalent or superior approach than other available technology. Furthermore, we expect that a combination of increasing the installed base of our HTG EdgeSeq instruments and entering into additional service agreements with biopharmaceutical customers will drive increased demand for our relatively high margin panels. If we are not able to successfully increase our installed base and biopharmaceutical customer relationships, then sales of our products and services, and our margins for these revenue items may not meet expectations. Attracting new customers and introducing new applications for our products and services requires time and expense. Any failure to expand adoption of our technology would adversely affect our ability to improve our operating results.
Our HTG EdgeSeq product portfolio requires the use of NGS instrumentation and reagents and could be adversely affected by actions of third-party NGS product manufacturers over whom we have no control.
We depend at least in part on the availability of NGS instrumentation and reagents, and the ability of our HTG EdgeSeq products to operate seamlessly with NGS instrumentation. Any significant interruption or delay in the ability of our HTG EdgeSeq products to operate on or with NGS instrumentation could reduce demand for our products and result in a loss of customers.
Our reputation, and our ability to continue to establish or develop our technology for clinical applications of next-generation sequencers, are dependent upon the availability of NGS instrumentation and the reliable performance of our products with NGS instrumentation. We are not able to control the providers of NGS instrumentation, which increases our vulnerability to interoperability problems with the products that they provide. For example, providers of NGS instruments may discontinue existing products, or introduce new NGS instrumentation products with little or no notice to us.
30
If we do not achieve, sustain or successfully manage our anticipated growth, our business and growth prospects will be harmed.
Our current personnel, systems and facilities may not be adequate to support our business plan and future growth. Our need to effectively manage our operations, growth and various projects requires that we, among other things:
Moreover, growth will place significant strains on our management and our operational and financial systems and processes. For example, expanded market penetration of our HTG EdgeSeq platform and related proprietary panels, and future development and approval of diagnostic products, are key elements of our growth strategy that will require us to hire and retain additional sales and marketing, regulatory, manufacturing and quality assurance personnel. If we do not successfully forecast the timing and cost of the development of new panels and diagnostic products, the regulatory clearance or approval for product marketing of any future diagnostic products or the demand and commercialization costs of such products, or manage our anticipated expenses accordingly, our operating results will be harmed.
We expect to generate a portion of our revenue internationally and are subject to various risks relating to our international activities, which could adversely affect our operating results.
For the year ended December 31, 2022, approximately 35% of our revenue was generated from sales originated by customers located outside of the United States, respectively, compared with 31% for the year ended December 31, 2021. We expect that a percentage of our future revenue will continue to come from international sources, and we expect to expand our overseas operations and develop opportunities in additional areas. Engaging in international business involves a number of difficulties and risks, including:
31
As we expand internationally our results of operations and cash flows will become increasingly subject to fluctuations due to changes in foreign currency exchange rates. Historically, most of our revenue has been denominated in U.S. dollars, although we have sold our products and services in local currency outside of the United States, principally the Euro. Our expenses are generally denominated in the currencies in which our operations are located, which is primarily in the United States. As our operations in countries outside of the United States grows, our results of operations and cash flows will increasingly be subject to fluctuations due to changes in foreign currency exchange rates, which could negatively impact our results of operations in the future. For example, if the value of the U.S. dollar increases relative to foreign currencies, in the absence of an offsetting change in local currency prices, our revenue could be adversely affected as we convert revenue from local currencies to U.S. dollars.
If we dedicate significant resources to our international operations and are unable to manage these risks effectively, our business, operating results and prospects will suffer. Moreover, we cannot be certain that the investment and additional resources required in establishing operations in other countries will produce desired levels of revenue or profitability.
In addition, any failure to comply with applicable legal and regulatory obligations could negatively impact us in a variety of ways that include, but are not limited to, significant criminal, civil and administrative penalties, including imprisonment of individuals, fines and penalties, denial of export privileges, seizure of products and restrictions on certain business activities.
If the utility of our HTG EdgeSeq platform, proprietary profiling panels, services and solutions in development is not supported by studies published in peer-reviewed medical publications, the rate of adoption of our current and future products and the rate of reimbursement of our future products by third-party payors may be negatively affected.
We anticipate that we will need to maintain a continuing presence in peer-reviewed publications to promote adoption of our products by biopharmaceutical companies, academic institutions and molecular labs and to promote favorable coverage and reimbursement decisions. We believe that peer-reviewed journal articles that provide evidence of the utility of our current and future products or the technology underlying the HTG EdgeSeq platform, consumables and services are important to our commercial success. It is critical to the success of our sales efforts that we educate a sufficient number of clinicians and administrators about our HTG EdgeSeq technology, including our epitranscriptomic profiling technology, our current panels and services and our future solutions, and demonstrate the research and clinical benefits of these solutions. Our customers may not adopt our current and future solutions, and third-party payors may not cover or adequately reimburse our future products, unless they determine, based on published peer-reviewed journal articles and the experience of other researchers and clinicians, that our products provide accurate, reliable, useful and cost-effective information. Peer-reviewed publications regarding our products and solutions may be limited by many factors, including delays in the completion of, poor design of, or lack of compelling data from studies that would be the subject of the article. If our current and future product and product-related service solutions or the technology underlying such products and services do not receive sufficient favorable exposure in peer-reviewed publications, the rate of research and clinical adoption and positive coverage and reimbursement decisions could be negatively affected.
We may provide our HTG EdgeSeq instrument and profiling panels free of charge or through other arrangements to customers or key opinion leaders through evaluation agreements or reagent rental programs, and these programs may not be successful in generating recurring revenue from sales of our systems and proprietary panels.
We sell our HTG EdgeSeq instrument and profiling panels under different arrangements to expand our installed base and facilitate the adoption of our platform.
In some instances, we provide equipment free of charge under evaluation agreements for a limited period of time to permit the user to evaluate the system for their purposes in anticipation of a decision to purchase the system. We retain title to the equipment under such arrangements unless the evaluator purchases the equipment, and in most cases, require evaluation customers to purchase a minimum quantity of consumables during the evaluation period.
When we place a system under a reagent rental agreement, we install equipment in the customer’s facility without a fee and the customer agrees to purchase consumable products at a stated price over the term of the agreement. While some of these agreements did not historically contain a minimum purchase requirement, we have included a minimum purchase requirement in all current reagent rental agreements and will continue to do so in the future. We retain title to the equipment and such title is transferred to the customer at no additional charge at the end of the initial arrangement. The cost of the instrument under the agreement is expected to be recovered in the fees charged for consumables, to the extent sold, over the term of the agreement.
Other arrangements might include a research agreement whereby an academic collaborator agrees to provide biological samples in exchange for the use of an HTG EdgeSeq instrument at no cost in furtherance of the collaborator’s professional goals and/or the educational or research objectives of an applicable institution.
32
Any of the foregoing arrangements could result in lost revenue and profit and potentially harm our long-term goal of achieving profitable operations. In addition, we require customers who receive systems that we continue to own to carry insurance sufficient to protect us against any equipment losses, we cannot guarantee that they will maintain such coverage, which may expose us to a loss of the value of the equipment in the event of any loss or damage.
There are instances where we provide our systems to key opinion leaders free of charge, to gather data and publish the results of their research to assist our marketing efforts. We have no control over some of the work being performed by these key opinion leaders, or whether the results will be satisfactory. It is possible that the key opinion leader may generate data that is unsatisfactory and could potentially harm our marketing efforts. In addition, customers may from time to time create negative publicity about their experience with our systems, which could harm our reputation and negatively affect market perception and adoption of our platform.
Placing our HTG EdgeSeq instruments under evaluation agreements, under reagent rental agreements or with our key opinion leaders without receiving payment for the instruments could require substantial additional working capital to provide additional units for sale to our customers.
We face risks related to handling of hazardous materials and other regulations governing environmental safety.
Our operations are subject to complex and stringent environmental, health, safety and other governmental laws and regulations that both public officials and private individuals may seek to enforce. Our activities that are subject to these regulations include, among other things, our use of hazardous materials and the generation, transportation and storage of waste. We could discover that we or an acquired business is not in material compliance with these regulations. Existing laws and regulations may also be revised or reinterpreted, or new laws and regulations may become applicable to us, whether retroactively or prospectively, that may have a negative effect on our business and results of operations. It is also impossible to eliminate completely the risk of accidental environmental contamination or injury to individuals. In such an event, we could be liable for any damages that result, and any liability could exceed our resources or any applicable insurance coverage we may have, which events could adversely affect our business.
The life sciences research and diagnostic markets are highly competitive. We face competition from enhanced or alternative technologies and products, which could render our products and/or technologies obsolete. If we fail to compete effectively, our business and operating results will suffer.
We face significant competition in the drug discovery, life sciences research and diagnostics markets.
The biopharmaceutical sector is populated with companies seeking to advance new and differentiated approaches to discovery and experimental therapeutics by way of different platform technologies or modalities with the intent for application to specific disease areas through focus on pharmacologic targets or through phenotypic approaches. In drug discovery, companies such as Accent Therapeutics, Arrakis Therapeutics, Storm Therapeutics and other discovery-stage biotechnology companies are focused in similar therapeutic areas.
We currently compete with both established and early-stage life sciences research companies that design, manufacture and market instruments and consumables for gene expression analysis, liquid-based specimen analysis (e.g., plasma, blood and urine), single-cell analysis, PCR, digital PCR, other nucleic acid detection and additional applications. These companies use well-established laboratory techniques such as microarrays or qPCR as well as newer technologies such as next-generation sequencing. We believe our principal competitors in the life sciences research market are Abbott Molecular, Affymetrix, Inc., Agilent Technologies, Inc., BioRad Laboratories, Invitae, Fluidigm Corporation, Illumina, Inc., Luminex Corporation, NanoString Technologies, Inc., Personal Genome Diagnostics (acquired by Labcorp), entities owned and controlled by QIAGEN N.V., Roche Diagnostics, a division of the Roche Group of companies, and Thermo Fisher Scientific, Inc. In addition, there are several other market entrants in the process of developing novel technologies for the life sciences market. One or more of our competitors could develop a product that is superior to a product we offer or intend to offer, or our technology and products may be rendered obsolete or uneconomical by advances in existing technologies.
Within the diagnostic market, there are competitors that manufacture systems for sales to hospitals and laboratories and other competitors that offer tests conducted through CLIA certified laboratories. We will also compete with commercial diagnostics companies. Most of our current competitors are either publicly traded, or are divisions of publicly traded companies, and enjoy a number of competitive advantages over us, including:
33
We believe that the principal competitive factors in all of our target markets include:
We believe that additional competitive factors specific to the diagnostics market include:
Our products may not compete favorably, and we may not be successful in the face of increasing competition from new products and technologies introduced by our existing competitors or new companies entering our markets. In addition, our competitors may have or may develop products or technologies that currently or in the future will enable them to produce competitive products with greater capabilities or at lower costs than ours. Any failure to compete effectively could materially and adversely affect our business, financial condition and operating results.
Our revenue currently depends in part on research and development spending by academic and governmental research institutions and biopharmaceutical companies, a reduction in which could limit demand for our products and adversely affect our business and operating results.
Our revenue is currently derived from sales of our HTG EdgeSeq instrument and related proprietary panels, the design of custom RUO assays and sample processing for research applications to biopharmaceutical companies, academic institutions and molecular labs, predominantly in the United States and Europe. The demand for our products and services will depend in part upon the research and development budgets of these customers, which are impacted by factors beyond our control, such as:
We believe that any uncertainty regarding the availability of research funding may adversely affect our operating results and may adversely affect sales to customers or potential customers that rely on government funding. In addition, academic, governmental and other research institutions that fund research and development activities may be subject to stringent budgetary constraints that could result in spending reductions, reduced allocations or budget cutbacks, which could jeopardize the ability of these customers to purchase our products or services. Our operating results may fluctuate substantially due to reductions and delays in research and development expenditures by these customers. Any decrease in our customers’ budgets or expenditures, or in the size, scope or frequency of capital or operating expenditures, could materially and adversely affect our business, operating results and financial condition.
34
Our research and development efforts will be hindered if we are not able to contract with third parties for access to archival patient samples.
Our future development of products for clinical indications will require access to archival patient samples for which data relevant to the clinical indication of interest is known. We rely on our ability to secure access to these archived patient samples, including FFPE tissue, plasma, serum, whole blood preserved in PAXgene, or various cytology preparations, together with the information pertaining to the clinical outcomes of the patients from which the samples were taken. Owners or custodians of relevant samples may be difficult to identify and/or identified samples may be of poor quality or limited in number or amount. Additionally, others compete with us for access to these samples for both research and commercial purposes. Even when an appropriate cohort of samples is identified, the process of negotiating access to these samples can be lengthy because it typically involves numerous parties and approval levels to resolve complex issues such as usage rights, institutional review board approval, privacy rights, publication rights, and intellectual property ownership. In addition, in some instances the cost to acquire samples can be prohibitively expensive. If we are not able to negotiate access to archived patient samples on a timely basis and on acceptable terms, or at all, or if our competitors or others secure access to these samples before us, our ability to research, develop and commercialize future products will be limited or delayed.
We are dependent on third-party suppliers for certain subcomponents of our products, including a single supplier for one subcomponent of our HTG EdgeSeq instruments.
We rely on third-party suppliers to supply certain subcomponents used in our HTG EdgeSeq instruments and consumables, including a single supplier, In Position Technologies, to produce a certain subcomponent used in our HTG EdgeSeq instruments. While we periodically forecast our needs for these subcomponents, our contracts with these suppliers do not commit them to carry inventory or make available any particular quantities, and the suppliers may give other customers’ needs higher priority than ours and we may not be able to obtain adequate supplies in a timely manner or on commercially reasonable terms. If we were to lose any of these suppliers, we may not be able to identify or enter into agreements with alternative suppliers on a timely basis on acceptable terms, or at all. In addition, we have in the past experienced supply issues, as well as quality control problems such as shipment errors, with certain of our suppliers, and may experience problems in the future. If we should encounter delays or difficulties in securing the quality and quantity of subcomponents we require for our products, our supply chain would be interrupted or our products may not perform as expected, which would adversely affect our sales. A loss or performance failure of any of these suppliers could significantly delay the delivery or impact the performance of our products, which in turn would materially affect our ability to generate revenue. If any of these events occur, our business and operating results could be materially harmed.
We rely on distributors for sales of our products in several markets outside of the United States.
We have established exclusive and non-exclusive distribution agreements for our HTG EdgeSeq platform and related profiling panels within parts of Europe and the Middle East. We intend to continue to grow our business internationally, and to do so, in addition to expanding our own direct sales and support team, we plan to attract additional distributors and sales partners to maximize the commercial opportunity for our products. We cannot guarantee that we will be successful in attracting desirable distribution and sales partners or that we will be able to enter into such arrangements on favorable terms. Distributors and sales partners may not commit the necessary resources to market and sell our products to the level of our expectations or may favor marketing the products of our competitors. If current or future distributors or sales partners do not perform adequately, or we are unable to enter into effective arrangements with distributors or sales partners in particular geographic areas, we may not realize long-term international revenue growth.
Limitations in the use of our products could harm our reputation or decrease market acceptance of our products; undetected errors or defects in our products could harm our reputation, decrease market acceptance of our products or expose us to product liability claims.
Our products are subject to the limitations set forth in the product labeling, which may not satisfy the needs of all customers. For example, in the past we have introduced new panels that initially were intended to be used with specific sample types. Because our customers desire that our panels be broadly applicable to many biological sample types, these initial limitations could harm our reputation or decrease market acceptance of our products. If that occurs, we may incur significant costs, the attention of our key personnel could be diverted, or other significant customer relations problems may arise, which could harm our business and operating results.
35
Similarly, our products may contain undetected errors or defects when first introduced or as new versions are released. Since our current customers use our products for research and, if cleared or approved for diagnostic applications, disruptions or other performance problems with our products may damage our customers’ businesses and could harm our reputation. If that occurs, we may incur significant costs, the attention of our key personnel could be diverted, or other significant customer relations problems may arise. We may also be subject to warranty and liability claims for damages related to errors or defects in our products. A material liability claim or other occurrence that harms our reputation or decreases market acceptance of our products could harm our business and operating results.
The sale and use of products or services based on our technologies, or activities related to our research and clinical studies, could lead to the filing of product liability claims if someone were to allege that one of our products contained a design or manufacturing defect which resulted in the failure to adequately perform the analysis for which it was designed. A product liability claim could result in substantial damages and be costly and time consuming to defend, either of which could materially harm our business or financial condition. We cannot assure investors that our product liability insurance could adequately protect our assets from the financial impact of defending a product liability claim. Any product liability claim brought against us, with or without merit, could increase our product liability insurance rates or prevent us from securing insurance coverage in the future.
Uncertainties in the interpretation and application of existing, new and proposed tax laws and regulations could materially affect our tax obligations and effective tax rate.
The tax regimes to which we are subject or under which we operate are unsettled and may be subject to significant change. The issuance of additional guidance related to existing or future tax laws, or changes to tax laws or regulations proposed or implemented by the current or a future U.S. presidential administration, Congress, or taxing authorities in other jurisdictions, including jurisdictions outside of the United States, could materially affect our tax obligations and effective tax rate. To the extent that such changes have a negative impact on us, including as a result of related uncertainty, these changes may adversely impact our business, financial condition, results of operations, and cash flows.
The amount of taxes we pay in different jurisdictions depends on the application of the tax laws of various jurisdictions, including the United States, to our international business activities, tax rates, new or revised tax laws, or interpretations of tax laws and policies, and our ability to operate our business in a manner consistent with our corporate structure and intercompany arrangements. The taxing authorities of the jurisdictions in which we operate may challenge our methodologies for pricing intercompany transactions pursuant to our intercompany arrangements or disagree with our determinations as to the income and expenses attributable to specific jurisdictions. If such a challenge or disagreement were to occur, and our position was not sustained, we could be required to pay additional taxes, interest, and penalties, which could result in one-time tax charges, higher effective tax rates, reduced cash flows, and lower overall profitability of our operations. Our financial statements could fail to reflect adequate reserves to cover such a contingency. Similarly, a taxing authority could assert that we are subject to tax in a jurisdiction where we believe we have not established a taxable connection, often referred to as a “permanent establishment” under international tax treaties, and such an assertion, if successful, could increase our expected tax liability in one or more jurisdictions.
Effective January 1, 2022, legislation enacted in 2017, informally titled the Tax Cuts and Jobs Act (the “Tax Act”) eliminated the option to deduct research and development expenses for tax purposes in the year incurred and requires taxpayers to capitalize and subsequently amortize such expenses over five years for research activities conducted in the United States and over 15 years for research activities conducted outside the United States. Although there have been legislative proposals to repeal or defer the capitalization requirement to later years, there can be no assurance that the provision will be repealed or otherwise modified. Future guidance from the Internal Revenue Service and other tax authorities with respect to such legislation may affect us, and certain aspects of such legislation could be repealed or modified in future legislation.
36
Our ability to use net operating loss carryforwards and certain other tax attributes may be limited.
As of December 31, 2022, we had federal net operating loss carryforwards (“NOLs”) to offset future taxable income of $203.0 million, of which $121.6 million will begin to expire after 2023 if not utilized, while the remainder can be carried forward indefinitely. A lack of future taxable income would adversely affect our ability to utilize these NOLs. Under current law, our federal NOLs incurred in tax years beginning after December 31, 2017 may be carried forward indefinitely but the deductibility of these federal NOLs is limited to 80% of taxable income. In addition, under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, and corresponding provisions of state law, a corporation that undergoes an “ownership change” (generally defined as a greater than 50% change, by value, in its equity ownership over a three-year period) is subject to limitations on its ability to utilize its pre-ownership change NOL carryforwards and certain other pre-ownership change tax attributes to offset post-ownership change income or taxes. We believe we may have already experienced one or more ownership changes and may in the future experience one or more additional ownership changes, and thus, our ability to utilize pre-ownership change NOL carryforwards and other pre-ownership change tax attributes to offset post-ownership change income or taxes may be limited. Such limitations may cause a portion of our NOL and credit carryforwards to expire before we are able to utilize them. In addition, at the state level, there may be periods during which the use of NOL carryforwards is suspended or otherwise limited, which could accelerate or permanently increase state taxes owed. We have recorded a full valuation allowance related to our NOLs and other deferred tax assets due to the uncertainty of the ultimate realization of the future benefits of those assets.
Acquisitions or joint ventures could disrupt our business, cause dilution to our stockholders and otherwise harm our business.
We may acquire other businesses, products or technologies as well as pursue strategic alliances, partnerships, joint ventures, technology licenses or investments in complementary businesses. We have limited experience with respect to business, product or technology acquisitions or the formation of collaborations, strategic alliances and joint ventures or investing in complementary businesses. Any of these transactions could be material to our financial condition and operating results and expose us to many risks, including:
Foreign acquisitions involve unique risks in addition to those mentioned above, including those related to integration of operations across different cultures and languages, currency risks and the particular economic, political and regulatory risks associated with specific countries. Also, the anticipated benefit of any acquisition may not materialize. Future acquisitions or dispositions could result in potentially dilutive issuances of our equity securities, the incurrence of debt, contingent liabilities or amortization expenses or write-offs of goodwill, any of which could harm our financial condition. We cannot predict the number, timing or size of future joint ventures or acquisitions, or the effect that any such transactions might have on our operating results.
37
If any members of our management team were to leave us or we are unable to recruit, train and retain key personnel, we may not achieve our goals.
Our future success depends on our ability to recruit, train, retain and motivate key personnel, including our senior management, research and development, manufacturing, service and sales and marketing personnel. If we were to lose one or more of our key employees, we may experience difficulties in competing effectively, developing our technologies and implementing our business strategies. Competition for qualified personnel is intense, and we may not be able to attract talent. Our growth depends, in part, on attracting, retaining and motivating highly trained sales personnel with the necessary scientific background and ability to understand our systems at a technical level to effectively identify and sell to potential new customers, including new biopharmaceutical company customers. In particular, our HTG Therapeutics business division requires us to continue to establish and maintain scientific expertise in drug discovery and early development and may require in the future additional support in the areas of expertise that contribute to our transcriptome-informed drug discovery and design platforms and well as to our therapeutics pipeline. In addition, the commercialization of our HTG EdgeSeq platform and related panels requires us to continue to establish and maintain sales and support teams to optimize the markets for research tools and, where approved, diagnostic assays, and to fully optimize a broad array of diagnostic market opportunities as we receive approval for any future diagnostic products. We do not maintain fixed term employment contracts or key man life insurance relating to any of our employees. Because of the complex and technical nature of our products and the dynamic market in which we compete, any failure to retain our management team or to attract, train, retain and motivate other qualified personnel could materially harm our operating results and growth prospects.
Our operating results may be harmed if we are required to collect sales, services or other related taxes for our products and services in jurisdictions where we have not historically done so.
We do not believe that we are required to collect sales, use, services or other similar taxes from our customers in certain jurisdictions. However, one or more countries or states may seek to impose sales, use, services, or other tax collection obligations on us, including for past sales. A successful assertion by one or more jurisdictions that we should collect sales or other taxes on the sale of our products and services could result in substantial tax liabilities for past sales and decrease our ability to compete for future sales. Each country and each state has different rules and regulations governing sales and use taxes and these rules and regulations are subject to varying interpretations that may change over time. We review these rules and regulations periodically and, when we believe sales and use taxes apply in a particular jurisdiction, voluntarily engage tax authorities in order to determine how to comply with their rules and regulations. However, we cannot guarantee that we will not be subject to sales and use taxes or related penalties for past sales in jurisdictions where we presently believe sales and use taxes are not due.
Providers of goods or services are typically held responsible by taxing authorities for the collection and payment of any applicable sales and similar taxes. If one or more taxing authorities determines that taxes should have, but have not, been paid with respect to our products and services, we may be liable for past taxes in addition to being required to collect sales or similar taxes in respect of our products and services going forward. Liability for past taxes may also include substantial interest and penalty charges. Our customer contracts provide that our customers must pay all applicable sales and similar taxes. Nevertheless, customers may be reluctant to pay back taxes and may refuse responsibility for interest or penalties associated with those taxes or we may determine that it would not be feasible to seek reimbursement. If we are required to collect and pay back taxes and the associated interest and penalties and if our customers do not reimburse us for all or a portion of these amounts, we will have incurred unplanned expenses that may be substantial. Moreover, imposition of such taxes on our products and services going forward will effectively increase the cost of such products and services to our customers.
Many states are also pursuing legislative expansion of the scope of goods and services that are subject to sales and similar taxes as well as the circumstances in which a vendor of goods and services must collect such taxes. Following the U.S. Supreme Court decision in South Dakota v. Wayfair, Inc., states are now free to levy taxes on sales of goods and services based on an “economic nexus,” regardless of whether the seller has a physical presence in the state. Furthermore, legislative proposals have been introduced in Congress that would provide states with additional authority to impose such taxes. Accordingly, it is possible that either federal or state legislative changes may require us to collect additional sales and similar taxes from our customers in the future.
Our insurance policies are expensive and protect us only from some business risks, which may leave us exposed to significant uninsured liabilities.
We do not carry insurance for all categories of risk that our business may encounter. Some of the policies we currently maintain include general liability, foreign liability, employee benefits liability, property, automobile, umbrella, workers’ compensation, crime (including cybercrime), fiduciary, products liability, pollution, errors and omissions and directors and officers insurance. We do not know, however, if we will be able to maintain existing insurance with adequate levels of coverage. Any significant uninsured liability may require us to pay substantial amounts, which would adversely affect our cash position and results of operations.
38
Performance issues, service interruptions or price increases by our shipping carriers could adversely affect our business and harm our reputation and ability to provide our services on a timely basis.
Expedited, reliable shipping is essential to our operations. We rely heavily on providers of transport services for reliable and secure point-to-point transport of our HTG EdgeSeq instrument and consumables to our customers and, as applicable, customers’ samples to our laboratory, and for enhanced tracking of these shipments. Should a carrier encounter delivery performance issues such as loss, damage or destruction of any instrumentation, consumables or samples, it would be costly to replace such instrumentation or consumables in a timely manner and may be difficult to replace customers’ samples lost or damaged in shipping, and such occurrences may damage our reputation and lead to decreased demand for our products and increased cost and expense to our business. In addition, any significant increase in shipping rates could adversely affect our operating margins and results of operations. Similarly, strikes, severe weather, natural disasters or other service interruptions affecting delivery services we use would adversely affect our ability to process orders for our products or receive recipient samples on a timely basis.
If our information technology systems or those of third parties upon which we rely are or were compromised, we could experience adverse consequences resulting from such consequences including but not limited to damage to our reputation and/or subject us to costs, fines, penalties, lawsuits, business interruption or otherwise adversely affect our business.
Our business requires collecting, receiving, processing, generating, transferring, disposing of, transmitting, sharing, manipulating, analyzing, disclosing, storing, making accessible and otherwise using (collectively, processing) large amounts of proprietary, confidential and sensitive data, including personal data about our employees and others, information we collect from samples we process, intellectual property, trade secrets, and proprietary business information owned or controlled by ourselves or other third parties (collectively, sensitive data).
The confidentiality, availability, integrity and protection of our data and information technology systems is critical to our business and relevant stakeholders have a high expectation that we will adequately protect sensitive data. Our business therefore depends on the continuous, effective, reliable and secure operation of our data and information technology systems.
To the extent that our information technology systems malfunction or access to our data is interrupted or otherwise compromised, our business could suffer. If we or the third parties upon which we rely have experienced or in the future experience any security incident(s) or other interruption(s) that result in any data loss, deletion or destruction, unauthorized, unlawful or accidental access to, loss of, acquisition of or disclosure of, alteration, encryption or exposure of sensitive data, or compromise related to the security, confidentiality, integrity or availability of our (or their) information technology systems or data, it may result in material adverse impacts.
We and third parties upon which we rely are vulnerable to cyberattacks, malicious internet-based activity and online and offline fraud and other similar activities, such as social-engineering attacks, credential harvesting, supply-chain attacks, software bugs, malicious code (such as viruses and worms), employee theft or misuse, denial-of-service attacks (such as credential stuffing), ransomware attacks, phishing attacks, viruses, malware installation, server malfunction, software or hardware failures, telecommunications failures, physical or software break-ins, loss of data and other computer assets, adware or other similar issues. Such threats are prevalent and continue to increase and come from a variety of sources, including traditional computer “hackers”, threat actors, “hacktivists”, organized criminal threat actors, personnel (such as through theft or misuse), sophisticated nation states, and nation-state-supported actors. Some actors now engage and are expected to continue to engage in cyber-attacks, including without limitation nation-state actors for geopolitical reasons and in conjunction with military conflicts and defense activities. During times of war and other major conflicts, we and the third parties upon which we rely may be vulnerable to a heightened risk of these attacks, including retaliatory cyber-attacks, that could materially disrupt our systems and operations, supply chain, and ability to produce, sell and distribute our services. For example, some third parties upon which we rely to support our business are located in unstable regions and regions experiencing (or expected to experience) geopolitical or other conflicts, including Ukraine which was attacked by Russia in February 2022 through various means, including cyberattacks.
In particular, severe ransomware attacks, including those from organized criminal threat actors, nation-states and nation-state supported actors, are becoming increasingly prevalent and severe and can lead to significant interruptions, delays, or outages in our operations, loss of data (including sensitive data) and income, significant extra expenses to restore data or systems, reputational loss and the diversion of funds. To alleviate the financial, operational and reputational impact of a ransomware attack, it may be preferable to make extortion payments, but we may be unwilling or unable to do so (including, for example, if applicable laws or regulations prohibit such payments).
39
Additionally, as remote work has become more common, there is an increased risk to our information technology systems and data, as more of our employees utilize network connections, computers and devices outside our premises or network, including working at home, while in transit and in public locations. Moreover, future or past business transactions (such as acquisitions or integrations) could expose us to additional cybersecurity risks and vulnerabilities, as our systems could be negatively affected by vulnerabilities present in acquired or integrated entities’ systems and technologies. Furthermore, we may discover security issues that were not found during due diligence of such acquired or integrated entities, and it may be difficult to integrate companies into our information technology environment and security program.
In addition, we rely on enterprise software systems and third-party service providers and sub-processors to operate and manage our business, and to process sensitive data in a variety of contexts, including, without limitation, cloud-based infrastructure, data center facilities, encryption and authentication technology, employee email, content delivery to customers, and other functions. Our ability to monitor these third parties’ information security practices is limited, and these third parties may not have adequate information security measures in place. If our third-party service providers experience a security incident or other interruption, we could experience adverse consequences. While we may be entitled to damages if our third-party service providers fail to satisfy their privacy or security-related obligations to us, any award may be insufficient to cover our damages, or we may be unable to recover such award. Additionally, supply chain attacks have increased in frequency and severity, and we cannot guarantee that third parties and infrastructure in our supply chain have not been compromised or that they do not contain exploitable defects or bugs that could result in a breach of or disruption to our systems and networks or the systems and networks of third parties that support us and our services.
While we have implemented security measures designed to protect against security incidents, and take steps to detect and remediate vulnerabilities, we may not be able to detect and remediate all vulnerabilities because the threats and techniques used to exploit the vulnerability change frequently and are often sophisticated in nature. Therefore, such vulnerabilities could be exploited but may not be detected until after a security incident has occurred. Further, we may experience delays in developing and deploying remedial measures designed to address any such identified vulnerabilities. We could be required to expend significant resources, fundamentally change our business activities and practices or modify our services, software, operations or information technology in an effort to protect against security incidents and to mitigate, detect and remediate actual and potential vulnerabilities and security incidents. There can be no assurances that our security measures or those of the third parties upon which we rely will be effective in protecting against all security incidents and the material adverse impacts that may arise from such incidents.
Despite the security controls we have in place, security incidents are very difficult to avoid. We have experienced specific instances of cyber events, including attempted compromises, in the past, and there could be unauthorized access, acquisition, disclosure and use of non-public information (including personal data) in the future. The techniques used to attack information technology systems are sophisticated and change. As a result, we or the third parties upon which we rely may not be able to address these techniques proactively or implement adequate preventative measures. If our data or information technology systems (or those of third parties upon which we rely) are compromised, we could be subject to restrictions on processing sensitive data (including personal data), negative publicity, monetary fund diversions, interruptions in our operations (including availability of data), financial loss, reputational damage, fines, penalties, damages, litigation (including class claims) and enforcement actions (for example, investigations, audits and inspections), and we could lose trade secrets, the occurrence of which could harm our business. In addition, a security incident may require notification to governmental agencies, supervisory bodies, credit reporting agencies, the media or individuals pursuant to various obligations. Such disclosures are costly, and the disclosures or the failure to comply with such requirements, could lead to material adverse impacts, including without limitation, negative publicity, a loss of customer confidence in our services or security measures or breach of contract claims.
There can be no assurance that the limitations of liability in our contracts would be enforceable or adequate or would otherwise protect us from liabilities or damages if we fail to comply with obligations related to security incidents. We cannot be sure that our insurance coverage will be adequate or sufficient to protect us from or adequately mitigate liabilities or damages with respect to claims, costs, expenses, litigation, fines, penalties, business loss, data loss, regulatory actions or material adverse impacts arising out of our privacy and security practices, processing or security incidents we may experience, or that such coverage will continue to be available on commercially reasonable terms or at all. The successful assertion of one or more large claims against us that exceeds our available insurance coverage, or results in changes to our insurance policies (including premium increases or the imposition of large deductible or co-insurance requirements) could have an adverse effect on our business. In addition, we cannot be sure that our existing insurance coverage and coverage for errors and omissions will continue to be available on acceptable terms or that our insurers will not deny coverage as to any future claim.
40
We are subject to stringent and evolving U.S. and foreign laws, regulations, rules, contractual obligations, policies, self-regulatory schemes, government regulation, and other obligations or standards related to data privacy and security. The actual or perceived failure by us, our customers, partners or vendors to comply with such obligations could lead to regulatory investigations or actions; litigation; fines and penalties; disruptions of our business operations; reputational harm; loss of revenue or profits; and other adverse business consequences.
In the ordinary course of business, we process sensitive data. We are subject to numerous federal, state, local and foreign laws and regulations, as well as regulatory guidance, industry standards, and other obligations relating to data privacy and security, governing the processing of personal data, such as information that we collect about employees and patients in the United States and abroad. Our data processing activities may subject us to numerous data privacy and security obligations, such as various laws, regulations, guidance, industry standards, external and internal privacy and security policies, contractual requirements, and other obligations relating to data privacy and security.
In the United States, federal, state, and local governments have enacted numerous data privacy and security laws, including data breach notification laws, personal data privacy laws, consumer protection laws (e.g., Section 5 of the Federal Trade Commission Act), and other similar laws (e.g., wiretapping laws). For example, HIPAA, as amended by HITECH, imposes specific requirements relating to the privacy, security, and transmission of individually identifiable health information. Additionally, the California Consumer Privacy Act, as amended by the California Privacy Rights Act (“CPRA”) (collectively, “CCPA”), applies to personal data of California-resident consumers, business representatives, and employees, and establishes a privacy framework for covered businesses. The CCPA provides for administrative fines of up to $7,500 per violation and allows private rights of action for certain data breaches. Although the CCPA exempts some data processed in the context of clinical trials, as we expand our operations, the CCPA may increase our compliance costs and potential liability. Additionally, the CPRA expanded the CCPA’s requirements, including by expanding consumers’ rights with respect to certain sensitive personal data and creating a new state agency to implement and enforce the law. We may be subject to additional U.S. privacy regulations in the future, including the Virginia Consumer Data Protection Act, and the Colorado Privacy Act, both of which either took or take effect in 2023. While these states, like the CCPA, also exempt some data processed in the context of clinical trials, these developments further complicate compliance efforts, and increase legal risk and compliance costs for us and the third parties upon which we rely.
Our operations abroad may also be subject to increased scrutiny or attention from data protection authorities. Many countries in the regions in which we operate or may operate have established or are in the process of establishing privacy and data security legal frameworks with which we and the third parties upon which we rely must comply. For example, the EU has adopted the General Data Protection Regulation (EU) 2016/679 (“EU GDPR”), which went into effect in May 2018, and the United Kingdom has adopted the United Kingdom’s GDPR (“UK GDPR”). These regulations introduce strict requirements for processing the personal data of individuals in the EU and UK. The EU and UK GDPR have and will continue to increase compliance burdens on us, including by mandating potentially burdensome documentation requirements and granting certain rights to individuals to control how we collect, use, disclose, retain and process information about them. Processing sensitive personal data, such as health information, may impose heightened compliance burdens under the EU and UK GDPR and is a topic of active interest among foreign regulators. In addition, the EU and UK GDPR provide for more robust regulatory enforcement and fines of up to €20 million under the EU GDPR (or £17.5 million under the UK GDPR) or 4% of the annual global revenue of the noncompliant company, whichever is greater. Under the EU GDPR, companies may face private litigation related to processing of personal data brought by classes of data subjects or consumer protection organizations authorized at law to represent their interests. As we expand into other foreign countries and jurisdictions, we may be subject to additional laws and regulations that may affect how we conduct business.
Certain data protection laws, including the EU GDPR, restrict the transfer of personal data from Europe, including the European Economic Area (“EEA”), UK and Switzerland, and other jurisdictions to the United States or other countries unless the parties to the transfer have implemented specific safeguards to protect the transferred personal data. In particular, the EEA and UK have significantly restricted the transfer of personal data to the United States and other countries whose privacy laws it believes are inadequate. Other jurisdictions may adopt similarly stringent interpretations of their data localization and cross-border data transfer laws. Although there are currently various mechanisms that may be used to transfer personal data from the EEA and UK to the United States in compliance with law, such as the EEA and UK’s standard contractual clauses, these mechanisms are subject to legal challenges, and there is no assurance that we can satisfy or rely on these measures to lawfully transfer personal data to the United States. As such, if there is no lawful manner for us to transfer personal data from the EEA, the UK or other jurisdictions to the United States, or if the requirements for a legally-compliant transfer are too onerous, we could face significant adverse consequences, including the interruption or degradation of our operations, the need to relocate part of or all of our business or data processing activities to other jurisdictions at significant expense, increased exposure to regulatory actions, substantial fines and penalties and injunctions against our processing or transferring of personal data necessary to operate our business. Companies that transfer personal data out of the EEA and UK to other jurisdictions, particularly to the United States, are subject to increased scrutiny from regulators, individual litigants, and activist groups. Some European regulators have ordered certain companies to suspend or permanently cease certain transfers out of Europe for allegedly violating the EU GDPR’s cross-border data transfer limitations.
41
In addition to data privacy and security laws, we may be contractually subject to industry standards adopted by industry groups and may become subject to such obligations in the future. We are also bound by contractual obligations related to data privacy and security, and our efforts to comply with such obligations may not be successful. We publish privacy policies, marketing materials and other statements regarding data privacy and security. If these policies, materials or statements are found to be deficient, lacking in transparency, deceptive, unfair, or misrepresentative of our practices, we may be subject to investigation, enforcement actions by regulators or other adverse consequences.
The global data protection landscape is rapidly evolving, and implementation standards and enforcement practices are likely to remain uncertain for the foreseeable future. Furthermore, the obligations are not consistent and may conflict with each other. This evolution may create uncertainty in our business, affect our or the third parties upon which we rely ability to operate in certain jurisdictions or to process personal data, necessitate the acceptance of more onerous obligations in our contracts, or result in liability or impose additional costs on us. The cost of compliance with these obligations is high and is likely to increase in the future. Compliance with these obligations is a rigorous and time-intensive process, and we may be required to put in place additional mechanisms, potentially at significant expense, to ensure compliance with such obligations.
Although we endeavor to comply with our obligations, we may at times fail to do so or may be perceived to have failed to do so. Any failure or perceived failure by us or the third parties upon which we rely to comply with data privacy and security obligations could result in negative publicity, disruptions or interruptions in our operations, fines, penalties (including changes to our data processing practices), lawsuits, liability, an inability to process personal data, diversion of management time and effort and proceedings against us by governmental entities or others, any of which could interrupt or stop our business operations (including our clinical trials) and adversely affect our business, financial condition, results of operations and growth prospects. In many jurisdictions, enforcement actions and consequences for noncompliance are rising.
Risks Related to Government Regulation and Diagnostic Product Reimbursement
Changes in laws or regulations may have a material adverse effect on our business, cash flow, financial conditions or results of operations.
New laws, statutes, rules, regulations or ordinances could be enacted at any time which could adversely affect our business operations and financial performance. Further, existing laws, statutes, rules, regulations or ordinances could be interpreted, changed, modified or applied in ways that are detrimental to us or our customers. For example, if regulatory limitations are placed on our products or if tax laws are changed or reinterpreted, our business and growth could be harmed.
New income, sales, use or other tax laws, statutes, rules, regulations or ordinances could be enacted at any time, which could adversely affect our business operations and financial performance. Further, existing tax laws, statutes, rules, regulations or ordinances could be interpreted, changed, modified or applied adversely to us. For example, the Biden administration and Congress have proposed various U.S. federal tax law changes, which if enacted could have a material impact on our business, cash flow, financial condition or results of operations. In addition, it is uncertain if and to what extent various states will conform to federal tax laws. Future tax reform legislation could have a material impact on the value of our deferred tax assets, could result in significant one-time charges, and could increase our future U.S. tax expense.
We do not believe that we are required to collect sales, use, services or other similar taxes from our customers in certain jurisdictions. However, one or more countries or states may seek to impose sales, use, services, or other tax collection obligations on us, including for past sales. A successful assertion by one or more jurisdictions that we should collect sales or other taxes on the sale of our products and services could result in substantial tax liabilities for past sales and decrease our ability to compete for future sales. Each country and each state has different rules and regulations governing sales and use taxes and these rules and regulations are subject to varying interpretations that may change over time. We review these rules and regulations periodically and, when we believe sales and use taxes apply in a particular jurisdiction, voluntarily engage tax authorities in order to determine how to comply with their rules and regulations. However, we cannot guarantee that we will not be subject to sales and use taxes or related penalties for past sales in jurisdictions where we presently believe sales and use taxes are not due.
42
Providers of goods or services are typically held responsible by taxing authorities for the collection and payment of any applicable sales and similar taxes. If one or more taxing authorities determines that taxes should have, but have not, been paid with respect to our products and services, we may be liable for past taxes in addition to being required to collect sales or similar taxes in respect of our products and services going forward. Liability for past taxes may also include substantial interest and penalty charges. Our customer contracts provide that our customers must pay all applicable sales and similar taxes. Nevertheless, customers may be reluctant to pay back taxes and may refuse responsibility for interest or penalties associated with those taxes or we may determine that it would not be feasible to seek reimbursement. If we are required to collect and pay back taxes and the associated interest and penalties and if our customers do not reimburse us for all or a portion of these amounts, we will have incurred unplanned expenses that may be substantial. Moreover, imposition of such taxes on our products and services going forward will effectively increase the cost of such products and services to our customers.
Many states are also pursuing legislative expansion of the scope of goods and services that are subject to sales and similar taxes as well as the circumstances in which a vendor of goods and services must collect such taxes. Following the U.S. Supreme Court decision in South Dakota v. Wayfair, Inc., states are now free to levy taxes on sales of goods and services based on an “economic nexus,” regardless of whether the seller has a physical presence in the state. Furthermore, legislative proposals have been introduced in Congress that would provide states with additional authority to impose such taxes. Accordingly, it is possible that either federal or state legislative changes may require us to collect additional sales and similar taxes from our customers in the future.
Our research use only products for the life sciences market could become subject to regulation as medical devices by the FDA or other regulatory agencies in the future, which could increase our costs and delay our commercialization efforts, thereby materially and adversely affecting our life sciences business and results of operations.
In the United States, our products are currently labeled and sold for research use only, and not for the diagnosis or treatment of disease, and are sold to a variety of parties, including biopharmaceutical companies, academic institutions and molecular labs. Because such products are not intended for use in clinical practice in diagnostics, and the products cannot include clinical or diagnostic claims, they are exempt from many regulatory requirements otherwise applicable to medical devices. In particular, while the FDA regulations require that RUO products be labeled, “For Research Use Only. Not for use in diagnostic procedures,” the regulations do not otherwise subject such products to the FDA’s pre- and post-market controls for medical devices.
A significant change in the laws governing RUO products or how they are enforced may require us to change our business model in order to maintain compliance. For instance, in November 2013 the FDA issued a guidance document entitled “Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only” (the “RUO Guidance”) which highlights the FDA’s interpretation that distribution of RUO products with any labeling, advertising or promotion that suggests that clinical laboratories can validate the test through their own procedures and subsequently offer it for clinical diagnostic use as a laboratory developed test is in conflict with RUO status. The RUO Guidance further articulates the FDA’s position that any assistance offered in performing clinical validation or verification, or similar specialized technical support, to clinical laboratories, conflicts with RUO status. If we engage in any activities that the FDA deems to be in conflict with the RUO status held by the products that we sell, we may be subject to immediate, severe and broad FDA enforcement action that would adversely affect our ability to continue operations. Accordingly, if the FDA finds that we are distributing our RUO products in a manner that is inconsistent with its regulations or guidance, we may be forced to stop distribution of our RUO tests until we are in compliance, which would reduce our revenue, increase our costs and adversely affect our business, prospects, results of operations and financial condition. In addition, the FDA’s proposed implementation for a new framework for the regulation of LDTs may negatively impact the LDT market and thereby reduce demand for RUO products.
If the FDA requires marketing authorization of our RUO products in the future, there can be no assurance that the FDA will ultimately grant any clearance or approval requested by us in a timely manner, or at all.
43
We expect to rely on third parties to conduct any future studies of our diagnostic products that may be required by the FDA or other regulatory authorities, and those third parties may not perform satisfactorily.
We do not have the ability to independently conduct the clinical studies or other studies that may be required to obtain FDA and other regulatory clearance or approval for our diagnostic products, including the HTG EdgeSeq instrument and related proprietary panels. Accordingly, we expect to rely on third parties, such as medical institutions, contract research organizations and clinical investigators, and providers of NGS instrumentation, to conduct such studies and/or to provide information necessary for our submissions to regulatory authorities. Our reliance on these third parties for clinical development activities or information will reduce our control over these activities. These third parties may not complete activities on schedule or conduct studies in accordance with regulatory requirements or our study design. Similarly, providers of NGS instrumentation may not place the same importance on our regulatory submissions as we do. Our reliance on third parties that we do not control will not relieve us of any applicable requirement to prepare, and ensure compliance with, the various procedures required under good clinical practices, or the submission of all information required in connection with requested regulatory approvals. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines if the third parties need to be replaced or if the quality or accuracy of the data they obtain is compromised due to their failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our studies may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for our diagnostic products.
If Medicare and other third-party payors in the United States and foreign countries do not approve coverage and adequate reimbursement for our future clinical diagnostic tests enabled by our technology, the commercial success of our diagnostic products would be compromised.
We plan to develop, obtain regulatory approval for and sell clinical diagnostics products for a number of different indications. Successful commercialization of our clinical diagnostic products depends, in large part, on the availability of coverage and adequate reimbursement for testing services using our diagnostic products from third-party payors, including government insurance plans, managed care organizations and private insurance plans. There is significant uncertainty surrounding third-party coverage and reimbursement for the use of tests that incorporate new technology, such as the HTG EdgeSeq platform and related applications and assays. Reimbursement rates have the potential to fluctuate depending on the region in which the testing is provided, the type of facility or treatment center at which the testing is done, and the third-party payor responsible for payment. If our customers are unable to obtain positive coverage decisions from third-party payors approving reimbursement for our tests at adequate levels, the commercial success of our products would be compromised, and our revenue would be significantly limited. Even if we do obtain favorable reimbursement for our tests, third-party payors may withdraw their coverage policies, review and adjust the rate of reimbursement, require co-payments from patients or stop paying for our tests, which would reduce revenue for testing services based on our technology and demand for our diagnostic products.
The American Medical Association Current Procedural Terminology (“CPT”) Editorial Panel created CPT codes that could be used by our customers to report testing for certain large-scale multianalyte genomic sequencing procedures (“GSPs”), including our diagnostic products, if approved. Effective January 1, 2015, these codes allow for uniform reporting of broad genomic testing panels using technology similar to ours. While these codes standardize reporting for these tests, coverage and payment rates for GSPs remain uncertain and we cannot guarantee that coverage and reimbursement for these tests will be provided in the amounts we expect, or at all. We cannot assure that CMS and other third-party payors will establish reimbursement rates sufficient to cover the costs incurred by our customers in using our clinical diagnostic products, if approved.
Even if we are able to establish coverage and reimbursement codes for our clinical diagnostic products in development, we will continue to be subject to significant pricing pressure, which could harm our business, results of operations, financial condition and prospects.
Third-party payors, including managed care organizations as well as government payors such as Medicare and Medicaid, have increased their efforts to control the cost, utilization and delivery of healthcare services, which may include decreased coverage or reduced reimbursement. From time to time, Congress has considered and implemented changes to the Medicare fee schedules in conjunction with budgetary legislation, and pricing and payment terms, including the possible requirement of a patient co-payment for Medicare beneficiaries for laboratory tests covered by Medicare, and are subject to change at any time. Reductions in the reimbursement rate of third-party payors have occurred and may occur in the future. Reductions in the prices at which testing services based on our technology are reimbursed in the future could result in pricing pressures and have a negative impact on our revenue. In many countries outside of the United States, various coverage, pricing and reimbursement approvals are required. We expect that it will take several years to establish broad coverage and reimbursement for testing services based on our products with payors in countries outside of the United States, and our efforts may not be successful.
44
We may be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws and other federal and state healthcare laws applicable to our business and marketing practices. If we are unable to comply, or have not complied, with such laws, we could face substantial penalties.
Our operations may be, and may continue to be, directly, or indirectly through our customers, subject to various federal and state fraud and abuse laws, including, without limitation, the federal and state anti-kickback statutes, false claims statutes, civil monetary penalties laws, patient data privacy and security laws, physician transparency laws and marketing compliance laws. These laws may impact, among other things, our proposed sales and marketing and education programs.
The laws that may affect our ability to operate include, but are not limited to:
45
Promotional activities for FDA-regulated products have been the subject of significant enforcement actions brought under healthcare reimbursement laws, fraud and abuse laws, and consumer protection statutes, among other theories. Advertising and promotion of medical devices are also regulated by the Federal Trade Commission and by state regulatory and enforcement authorities. In addition, under the Federal Lanham Act and similar state laws, competitors and others can initiate litigation relating to advertising claims.
In addition, the approval and commercialization of any of our product candidates outside the United States will also likely subject us to foreign equivalents of the healthcare laws mentioned above, among other foreign laws.
Because of the breadth of these laws and the narrowness of the statutory exceptions and regulatory safe harbors available under such laws, it is possible that some of our business activities, including our relationships with physicians and other health care providers, and our evaluation, reagent rental and collaborative development agreements with customers, and sales and marketing efforts could be subject to challenge under one or more of such laws.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to significant penalties, including administrative, civil and criminal penalties, damages, fines, imprisonment, disgorgement, exclusion from participation in federal healthcare programs, such as Medicare and Medicaid, contractual damages, reputational harm, additional reporting requirements and/or oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
46
Our employees, independent contractors, principal investigators, consultants, commercial partners and vendors may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements.
We are exposed to the risk of fraud or other misconduct by our employees, independent contractors, principal investigators, consultants, commercial partners and vendors. Misconduct by these parties could include intentional, reckless or negligent failures to, among other things: (i) comply with the regulations of the FDA, CMS, the Department of Health and Human Services Office of Inspector General (“OIG”) and other similar foreign regulatory bodies; (ii) provide true, complete and accurate information to the FDA and other similar regulatory bodies; (iii) comply with manufacturing standards we have established; (iv) comply with healthcare fraud and abuse laws and regulations in the United States and similar foreign fraudulent misconduct laws; or (v) report financial information or data accurately, or disclose unauthorized activities to us. These laws may impact, among other things, our activities with collaborators and key opinion leaders, as well as our sales, marketing and education programs. In particular, the promotion, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Such misconduct could also involve the improper use of information obtained in the course of clinical studies, which could result in regulatory sanctions and cause serious harm to our reputation. We currently have a code of conduct applicable to all of our employees, but it is not always possible to identify and deter employee misconduct, and our code of conduct and the other precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses, or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant civil, criminal and administrative penalties, damages, monetary fines, disgorgement, imprisonment, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, additional reporting requirements and/or oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, and curtailment of our operations. Any of these actions or investigations could result in substantial costs to us, including legal fees, and divert the attention of management from operating our business.
Healthcare policy changes, including recently enacted legislation reforming the United States healthcare system, may have a material adverse effect on our financial condition and results of operations.
On April 1, 2014, the Protecting Access to Medicare Act of 2014 (“PAMA”) was signed into law, which, among other things, significantly altered the current payment methodology under the Medicare Clinical Laboratory Fee Schedule (“CLFS”). Effective January 1, 2018, the CLFS is based on weighted median private payor rates as required by PAMA. Under the law, starting January 1, 2016 and every three years thereafter (or annually in the case of advanced diagnostic lab tests), applicable clinical laboratories must report laboratory test payment data for each Medicare-covered clinical diagnostic lab test that it furnishes. The reported data must include the payment rate (reflecting all discounts, rebates, coupons and other price concessions) and the volume of each test that was paid by each private payor (including health insurance issuers, group health plans, Medicare Advantage plans and Medicaid managed care organizations). Reporting of payment data under PAMA for clinical diagnostic laboratory tests has been delayed on numerous occasions. Based on current law, between January 1, 2023 and March 31, 2023, applicable laboratories will be required to report on data collected during January 1, 2019 and June 30, 2019. This data will be utilized to determine 2024 to 2026 clinical diagnostic laboratory test rates. The payment rate applies to laboratory tests furnished by a hospital laboratory if the test is separately paid under the hospital outpatient prospective payment system. In addition, CMS updated the statutory phase-in provisions such that the rates for clinical diagnostic laboratory tests in 2020 could not be reduced by more than 10% of the rates for 2019. Pursuant to the Coronavirus Aid, Relief, and Economic Security Act (the “CARES Act”), as updated by the Consolidated Appropriations Act, 2023, the statutory phase-in of the payment reductions has been extended through 2026, with a 0% reduction cap for 2021-2023, and a 15% reduction cap for 2024 through 2026. It is still too early to predict the full impact on reimbursement for our products in development.
Also, under PAMA, CMS is required to adopt temporary billing codes to identify new tests and new advanced diagnostic laboratory tests that have been cleared or approved by the FDA. For an existing test that is cleared or approved by the FDA and for which Medicare payment is made as of April 1, 2014, CMS is required to assign a unique billing code if one has not already been assigned by the agency. In addition to assigning the code, CMS was required to publicly report payment for the tests. We cannot determine at this time the full impact of the law, including its implementing regulations, on our business, financial condition and results of operations.
The Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010 (collectively, the “ACA”), made changes that significantly impacted the biopharmaceutical and medical device industries and clinical laboratories. For example, the ACA imposes a multifactor productivity adjustment to the reimbursement rate paid under Medicare for certain clinical diagnostic laboratory tests, which may reduce payment rates. These or any future proposed or mandated reductions in payments may apply to some or all of the clinical laboratory tests that our diagnostics customers use our technology to deliver to Medicare beneficiaries and may reduce demand for our diagnostic products.
47
Other significant measures contained in the ACA include, for example, coordination and promotion of research on comparative clinical effectiveness of different technologies and procedures, initiatives to revise Medicare payment methodologies, such as bundling of payments across the continuum of care by providers and physicians, and initiatives to promote quality indicators in payment methodologies. The ACA also includes significant new fraud and abuse measures, including required disclosures of financial arrangements with physician customers, lower thresholds for violations and increasing potential penalties for such violations. However, the future of the ACA is uncertain. There have been executive, judicial and Congressional challenges to certain aspects of the ACA. On June 17, 2021, the U.S. Supreme Court dismissed a challenge on procedural grounds that argued the ACA is unconstitutional in its entirety because the “individual mandate” was repealed by Congress. Further, prior to the U.S. Supreme Court ruling, on January 28, 2021, President Biden issued an executive order that initiated a special enrollment period for purposes of obtaining health insurance coverage through the ACA marketplace. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA. On August 16, 2022, President Biden signed the Inflation Reduction Act of 2022 (“IRA”) into law, which, among other things, extends enhanced subsidies for individuals purchasing health insurance coverage in ACA marketplaces through plan year 2025. The IRA also eliminates the “donut hole” under the Medicare Part D program beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost and creating a new manufacturer discount program. The IRA also includes measures designed to lower the cost of certain pharmaceutical products under the Medicare program. It is currently unclear how the IRA will be implemented but is likely to have a significant impact on the pharmaceutical industry. It is possible that the ACA will be subject to judicial or Congressional challenges in the future. It is unclear how any additional healthcare reform measures of the Biden administration will impact the ACA and our business.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. On August 2, 2011, then-President Obama signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes reductions to Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013, and, following the passage of other legislative amendments, will stay in effect until 2031 unless additional Congressional action is taken. Under current legislation, the actual reduction in Medicare payments will vary from 1% in 2022 to up to 4% in the final fiscal year of this sequester. Further, Congress and the Biden administration are considering additional health reform measures. On January 2, 2013, then-President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, further reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
Various healthcare reform proposals have also emerged from federal and state governments. Changes in healthcare law or policy, such as the creation of broad test utilization limits for diagnostic products in general or requirements that Medicare patients pay for portions of clinical laboratory tests or services received, could substantially impact the sales of our tests, increase costs and divert management’s attention from our business. In addition, sales of our tests outside of the United States will subject us to foreign regulatory requirements, which may also change over time.
We cannot predict whether future healthcare initiatives will be implemented at the federal or state level or in countries outside of the United States in which we may do business, or the effect any future legislation or regulation will have on us. The full impact of the ACA, as well as other laws and reform measures that may be proposed and adopted in the future, remains uncertain, but may continue the downward pressure on medical device pricing, especially under the Medicare program, and may also increase our regulatory burdens and operating costs, which could have a material adverse effect on our business operations.
Changes in funding for the FDA, the SEC and other government agencies could hinder their ability to hire and retain key leadership and other personnel, prevent new products and services from being developed or commercialized in a timely manner or otherwise prevent those agencies from performing normal functions on which the operation of our business may rely, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept payment of user fees, and statutory, regulatory, and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of the SEC and other government agencies on which our operations may rely, including those that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
48
Disruptions at the FDA and other agencies may also slow the time necessary for new drugs or diagnostic products to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, over the last several years, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA and the SEC, have had to furlough critical FDA, SEC and other government employees and stop critical activities. If a prolonged government shutdown occurs, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Further, future government shutdowns could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations.
Risks Related to Intellectual Property
If we are unable to protect our intellectual property effectively, our business will be harmed.
We rely on patent protection as well as trademark, copyright, trade secret and other intellectual property rights protection and contractual restrictions to protect our proprietary technologies, all of which provide limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. Our U.S. and foreign patent and patent application portfolio relates to our nuclease-protection-based technologies as well as to lung cancer and melanoma and DLBCL biomarker panels discovered using our nuclease-protection-based technology. We have exclusive or non-exclusive licenses to multiple U.S. and foreign patents and patent applications covering technologies that we may elect to utilize in developing diagnostic tests for use on our HTG EdgeSeq platform. Those licensed patents and patent applications cover technologies related to the diagnosis of breast cancer and melanoma.
If we fail to protect our intellectual property, third parties may be able to compete more effectively against us and we may incur substantial litigation costs in our attempts to recover or restrict use of our intellectual property.
We cannot assure investors that any of our currently pending or future patent applications will result in issued patents, and we cannot predict how long it will take for such patents to be issued. Further, we cannot assure investors that other parties will not challenge any patents issued to us or that courts or regulatory agencies will hold our patents to be valid or enforceable. We cannot guarantee investors that we will be successful in defending challenges made against our patents. Any successful third-party challenge to our patents could result in the unenforceability or invalidity of such patents.
The patent positions of life sciences companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in such companies’ patents has emerged to date in the United States. Furthermore, in the biotechnology field, courts frequently render opinions that may adversely affect the patentability of certain inventions or discoveries, including opinions that may adversely affect the patentability of methods for analyzing or comparing nucleic acids molecules, such as RNA or DNA.
The patent positions of companies engaged in development and commercialization of molecular diagnostic tests are particularly uncertain. Various courts, including the U.S. Supreme Court, have recently rendered decisions that impact the scope of patentability of certain inventions or discoveries relating to molecular diagnostics. Specifically, these decisions stand for the proposition that patent claims that recite laws of nature (for example, the relationships between gene expression levels and the likelihood of risk of recurrence of cancer) are not themselves patentable unless those patent claims have sufficient additional features that provide practical assurance that the processes are genuine inventive applications of those laws rather than patent drafting efforts designed to monopolize the law of nature itself. What constitutes a “sufficient” additional feature is uncertain. Accordingly, this evolving case law in the United States may adversely impact our ability to obtain new patents and may facilitate third-party challenges to our existing owned and licensed patents.
The laws of some non-U.S. countries do not protect intellectual property rights to the same extent as the laws of the United States, and many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biotechnology, which could make it difficult for us to stop the infringement of our patents. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
Changes in either the patent laws or in interpretations of patent laws in the United States or other countries may diminish the value of our intellectual property. We cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents. For example:
49
In addition to pursuing patents on our technology, we take steps to protect our intellectual property and proprietary technology by entering into confidentiality agreements and intellectual property assignment agreements with our employees, consultants, corporate partners and, when needed, our advisors. Such agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements, and we may not be able to prevent such unauthorized disclosure. Monitoring unauthorized disclosure is difficult, and we do not know whether the steps we have taken to prevent such disclosure are, or will be, adequate. If we were to enforce a claim that a third-party had illegally obtained and was using our trade secrets, it would be expensive and time consuming, and the outcome would be unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets.
In addition, competitors could purchase our products and attempt to replicate some or all of the competitive advantages we derive from our development efforts, willfully infringe our intellectual property rights, design around our protected technology or develop their own competitive technologies that fall outside of our intellectual property rights. If our intellectual property is not adequately protected so as to protect our market against competitors’ products and methods, our competitive position could be adversely affected, as could our business.
We have not yet registered certain of our trademarks, including “HTG Edge,” “HTG EdgeSeq,” “VERI/O,” “qNPA,” and “HTG Transcriptome Panel” in all of our potential markets. If we apply to register these trademarks, our applications may not be allowed for registration, and our registered trademarks may not be maintained or enforced. In addition, opposition or cancellation proceedings may be filed against our trademark applications and registrations, and our trademarks may not survive such proceedings. If we do not secure registrations for our trademarks, we may encounter more difficulty in enforcing them against third parties than we otherwise would.
To the extent our intellectual property, including licensed intellectual property, offers inadequate protection, or is found to be invalid or unenforceable, we would be exposed to a greater risk of direct competition. If our intellectual property does not provide adequate protection against our competitors’ products, our competitive position could be adversely affected, as could our business. Both the patent application process and the process of managing patent disputes can be time consuming and expensive.
We may be involved in lawsuits to protect or enforce our patent or other proprietary rights, to determine the scope, coverage and validity of others’ patent or other proprietary rights, or to defend against third-party claims of intellectual property infringement, any of which could be time-intensive and costly and may adversely impact our business or stock price.
We may from time to time receive notices of claims of infringement and misappropriation or misuse of other parties’ proprietary rights, including with respect to third-party trade secrets, infringement by us of third-party patents and trademarks or other rights, or challenges to the validity or enforceability of our patents, trademarks or other rights. Some of these claims may lead to litigation. We cannot assure investors that such actions will not be asserted or prosecuted against us or that we will prevail in any or all such actions.
50
As we move into new markets and applications for our products, incumbent participants in such markets may assert their patents and other proprietary rights against us as a means of slowing our entry into such markets or as a means to extract substantial license and royalty payments from us. Our competitors and others may now and in the future have significantly larger and more mature patent portfolios than we currently have. In addition, future litigation may involve patent holding companies or other adverse patent owners who have no relevant product revenue and against whom our own patents may provide little or no deterrence or protection. Therefore, our commercial success may depend in part on our non-infringement of the patents or proprietary rights of third parties. Numerous significant intellectual property issues have been litigated, and will likely continue to be litigated, between existing and new participants in our existing and targeted markets and competitors may assert that our products infringe their intellectual property rights as part of a business strategy to impede our successful entry into those markets. We have not conducted comprehensive freedom-to-operate searches to determine whether the commercialization of our products or other business activities would infringe patents issued to third parties. Third parties may assert that we are employing their proprietary technology without authorization. In addition, our competitors and others may have patents or may in the future obtain patents and claim that use of our products infringes these patents. We could incur substantial costs and divert the attention of our management and technical personnel in defending against any of these claims. Parties making claims against us may be able to obtain injunctive or other relief, which could block our ability to develop, commercialize and sell products, and could result in the award of substantial damages against us. In the event of a successful claim of infringement against us, we may be required to pay damages and obtain one or more licenses from third parties or be prohibited from selling certain products. We may not be able to obtain these licenses at a reasonable cost, if at all. We could therefore incur substantial costs related to royalty payments for licenses obtained from third parties, which could negatively affect our margins. In addition, we could encounter delays in product introductions while we attempt to develop alternative methods or products to avoid infringing third-party patents or proprietary rights. Defense of any lawsuit or failure to obtain any of these licenses on favorable terms could prevent us from commercializing products, and the prohibition of sale of any of our products could materially affect our ability to grow and gain market acceptance for our products.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, during the course of this kind of litigation, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock.
In addition, our agreements with some of our suppliers, distributors, customers and other entities with whom we do business require us to defend or indemnify these parties to the extent they become involved in infringement claims against us, including the claims described above. We could also voluntarily agree to defend or indemnify third parties in instances where we are not obligated to do so if we determine it would be important to our business relationships. If we are required or agree to defend or indemnify any of these third parties in connection with any infringement claims, we could incur significant costs and expenses that could adversely affect our business, operating results, or financial condition.
We may need to depend on certain technologies that are licensed to us. We do not control these technologies and any loss of our rights to them could prevent us from selling some of our products.
We have entered into several license agreements with third parties for certain licensed technologies that are, or may become relevant to the products we market, or plan to market. In addition, we may in the future elect to license third-party intellectual property to further our business objectives and/or as needed for freedom to operate for our products. We do not and will not own the patents, patent applications or other intellectual property rights that are a subject of these licenses. Our rights to use these technologies and employ the inventions claimed in the licensed patents, patent applications and other intellectual property rights are or will be subject to the continuation of and compliance with the terms of those licenses.
We might not be able to obtain licenses to technology or other intellectual property rights that we require. Even if such licenses are obtainable, they may not be available at a reasonable cost or multiple licenses may be needed for the same product (e.g., stacked royalties). We could therefore incur substantial costs related to royalty payments for licenses obtained from third parties, which could negatively affect our margins. Further, we could encounter delays in product introductions, or interruptions in product sales, as we develop alternative methods or products.
In some cases, we do not or may not control the prosecution, maintenance, or filing of the patents or patent applications to which we hold licenses, or the enforcement of these patents against third parties. As a result, we cannot be certain that drafting or prosecution of the licensed patents and patent applications by the licensors have been or will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents and other intellectual property rights.
51
Certain of the U.S. patent rights we own, have licensed or may license relate to technology that was developed with U.S. government grants, in which case the U.S. government has certain rights in those inventions, including, among others, march-in license rights. In addition, federal regulations impose certain domestic manufacturing requirements with respect to any products within the scope of those U.S. patent claims.
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of our employees’ former employers.
Many of our employees were previously employed at other medical diagnostic companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights. A loss of key research personnel work product could hamper or prevent our ability to commercialize certain potential products, which could severely harm our business. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Our products contain third-party open-source software components, and failure to comply with the terms of the underlying open-source software licenses could restrict our ability to sell our products.
Our products contain software tools licensed by third-party authors under “open-source” licenses. Use and distribution of open-source software may entail greater risks than use of third-party commercial software, as open-source licensors generally do not provide warranties or other contractual protections regarding infringement claims or the quality of the code. Some open-source licenses contain requirements that we make available source code for modifications or derivative works we create based upon the type of open-source software we use. If we combine our proprietary software with open-source software in a certain manner, we could, under certain open-source licenses, be required to release the source code of our proprietary software to the public. This would allow our competitors to create similar products with less development effort and time and ultimately could result in a loss of product sales.
Although we monitor our use of open-source software to avoid subjecting our products to conditions we do not intend, the terms of many open-source licenses have not been interpreted by U.S. courts, and there is a risk that these licenses could be construed in a way that could impose unanticipated conditions or restrictions on our ability to commercialize our products. Moreover, we cannot assure investors that our processes for controlling our use of open-source software in our products will be effective. If we are held to have breached the terms of an open-source software license, we could be required to seek licenses from third parties to continue offering our products on terms that are not economically feasible, to re-engineer our products, to discontinue the sale of our products if re-engineering could not be accomplished on a timely basis, or to make generally available, in source code form, our proprietary code, any of which could adversely affect our business, operating results, and financial condition.
We use third-party software that may be difficult to replace or cause errors or failures of our products that could lead to lost customers or harm to our reputation.
We use software licensed from third parties in our products. In the future, this software may not be available to us on commercially reasonable terms, or at all. Any loss of the right to use any of this software could result in delays in the production of our products until equivalent technology is either developed by us, or, if available, is identified, obtained and integrated, which could harm our business. In addition, any errors or defects in third-party software, or other third-party software failures could result in errors, defects or cause our products to fail, which could harm our business and be costly to correct. Many of these providers attempt to impose limitations on their liability for such errors, defects or failures, and if enforceable, we may have additional liability to our customers or third-party providers that could harm our reputation and increase our operating costs.
We will need to maintain our relationships with third-party software providers and to obtain software from such providers that do not contain any errors or defects. Any failure to do so could adversely impact our ability to deliver reliable products to our customers and could harm our results of operations.
52
Risks Related to Being a Public Company
Complying with the laws and regulations affecting public companies increases our costs and the demands on management and could harm our operating results.
As a public company, we will continue to incur significant legal, accounting and other expenses. The Exchange Act requires, among other things, that we file annual, quarterly and current reports with respect to our business and operating results. In addition, the Sarbanes-Oxley Act and rules subsequently implemented by the SEC and Nasdaq, impose numerous requirements on public companies, including corporate governance requirements. Our management and other personnel will need to continue to devote a substantial amount of time to compliance with these laws and regulations. These requirements have resulted and will continue to result in significant legal, accounting, and financial compliance costs and have made and will continue to make some activities more time consuming and costly.
As a “non-accelerated filer” we have availed ourselves of the exemption from the requirement that our independent registered public accounting firm attest to the effectiveness of our internal control over financial reporting under Section 404. When our independent registered public accounting firm is required to undertake an assessment of our internal control over financial reporting, the cost of our compliance with Section 404 will correspondingly increase. Our compliance with applicable provisions of Section 404 will require that we incur substantial accounting expense and expend significant management time on compliance-related issues as we implement additional corporate governance practices and comply with reporting requirements. Moreover, if we are not able to comply with the requirements of Section 404 applicable to us in a timely manner, or if we or our independent registered public accounting firm identifies deficiencies in our internal control over financial reporting that are deemed to be material weaknesses, the market price of our stock could decline and we could be subject to sanctions or investigations by the SEC or other regulatory authorities, which would require additional financial and management resources.
Furthermore, investor perceptions of our company may suffer if deficiencies are found, and this could cause a decline in the market price of our stock. Irrespective of compliance with Section 404, any failure of our internal control over financial reporting could have a material adverse effect on our stated operating results and harm our reputation. If we are unable to implement these requirements effectively or efficiently, it could harm our operations, financial reporting, or financial results and could result in an adverse opinion on our internal controls from our independent registered public accounting firm.
We are a “smaller reporting company” and a “non-accelerated filer” and any decision on our part to comply only with certain reduced reporting and disclosure requirements applicable to smaller reporting companies or non-accelerated filers could make our common stock less attractive to investors.
We are a “smaller reporting company” and a “non-accelerated filer” as defined in the Exchange Act, and for as long as we continue to be a “smaller reporting company” or a “non-accelerated filer,” we may choose to take advantage of exemptions from various reporting requirements applicable to other public companies but not to “smaller reporting companies” or “non-accelerated filers,” including, but not limited to, not being required to have our independent registered public accounting firm audit our internal control over financial reporting under Section 404 (for so long as we are a “non-accelerated filer”) and reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements (for so long as we are a “smaller reporting company”). We cannot predict if investors will find our common stock less attractive if we choose to rely on these exemptions. If some investors find our common stock less attractive as a result of any choices to reduce future disclosure, there may be a less active trading market for our common stock and our stock price may be more volatile.
Risks Related to Our Common Stock
We expect that our stock price will fluctuate significantly.
The trading price of our common stock may be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. These factors include:
53
The stock market in general, and market prices for the securities of health technology companies like ours in particular, have from time-to-time experienced volatility that often has been unrelated to the operating performance of the underlying companies. COVID-19, for example, has resulted in significant volatility in the stock market over the last several months. These broad market and industry fluctuations may adversely affect the market price of our common stock, regardless of our operating performance. In several recent situations where the market price of a stock has been volatile, holders of that stock have instituted securities class action litigation against the company that issued the stock. If any of our stockholders were to bring a lawsuit against us, the defense and disposition of the lawsuit could be costly and divert the time and attention of our management and harm our operating results.
In addition, to date our common stock has generally been sporadically and thinly traded. As a consequence, the trading of relatively small quantities of our shares may disproportionately influence the price of our common stock in either direction. The price for our common stock could decline precipitously if even a moderate amount of our common stock is sold on the market without commensurate demand.
If we are unable to continue to satisfy the applicable continued listing requirements of Nasdaq, our common stock could be delisted.
Our common stock is currently listed on The Nasdaq Capital Market under the symbol “HTGM.” In order to maintain this listing, we must continue to satisfy minimum financial and other continued listing requirements and standards. There can be no assurance that we will be able to continue to comply with the applicable listing standards. If we were not able to comply with applicable listing standards, our shares of common stock would be subject to delisting. The delisting of our common stock from trading on Nasdaq may have a material adverse effect on the market for, and liquidity and price of, our common stock and impair our ability to raise capital. Delisting from Nasdaq could also have other negative results, including, without limitation, the potential loss of confidence by customers and employees, the loss of institutional investor interest and fewer business development opportunities. In the event that our common stock is delisted from Nasdaq and is not eligible for quotation or listing on another market or exchange, trading of our common stock could be conducted only in the over-the-counter market or on an electronic bulletin board established for unlisted securities such as the Pink Sheets or the OTC Bulletin Board. In such event, it could become more difficult to dispose of, or obtain accurate price quotations for, our common stock, and there would likely also be a reduction in our coverage by securities analysts and the news media, which could cause the price of our common stock to decline further.
Future sales and issuances of our common stock or rights to purchase common stock, including pursuant to our equity incentive plans, could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
We expect that significant additional capital will be needed in the future to continue our planned operations. We may sell common stock, convertible securities or other equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell common stock, convertible securities or other equity securities in more than one transaction, investors may be materially diluted by these and subsequent sales. New investors could also gain rights superior to our existing stockholders.
54
Pursuant to our 2020 Equity Incentive Plan (“2020 Plan”), we are authorized to grant stock options and other equity-based awards to our employees, directors and consultants. Pursuant to our 2021 Inducement Plan (“Inducement Plan”), we are authorized to grant up to 300,000 shares to new employees as inducements material to such new employees entering into employment with us. The number of shares which may be granted under the Inducement Plan may be increased in the future by our board of directors without stockholder approval. In addition, our amended and restated 2014 Employee Stock Purchase Plan (“ESPP”) authorizes us to offer, sell and issue shares to our employees. Increases in the number of shares available for future grant or purchase may result in additional dilution, which could cause our stock price to decline.
We do not intend to pay dividends on our common stock in the foreseeable future.
We have never declared or paid any cash dividend on our common stock. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. In addition, our ability to pay cash dividends is currently prohibited by the terms of our debt facility, and any future debt financing arrangement may contain terms prohibiting or limiting the amount of dividends that may be declared or paid on our common stock. Any return to stockholders will therefore be limited to the appreciation of their stock.
Provisions in our amended and restated certificate of incorporation and bylaws, as well as provisions of Delaware law, could make it more difficult for a third-party to acquire us or increase the cost of acquiring us, even if doing so would benefit our stockholders or remove our current management.
Some provisions of our charter documents and Delaware law may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our stockholders and may prevent attempts by our stockholders to replace or remove our current management. These provisions include:
These provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our Board of Directors, which is responsible for appointing the members of our management. In addition, we are subject to Section 203 of the Delaware General Corporation Law, which generally prohibits a Delaware corporation from engaging in any of a broad range of business combinations with an interested stockholder for a period of three years following the date on which the stockholder became an interested stockholder, unless such transactions are approved by our Board of Directors. This provision could have the effect of delaying or preventing a change of control, whether or not it is desired by or beneficial to our stockholders. Further, other provisions of Delaware law may also discourage, delay or prevent someone from acquiring us or merging with us.
Our amended and restated certificate of incorporation and amended and restated bylaws provide that the Court of Chancery of the State of Delaware is the exclusive forum for certain disputes between us and our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers, or employees.
Our amended and restated certificate of incorporation and our amended and restated bylaws provide that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall, to the fullest extent permitted by law, be the sole and exclusive forum for (1) any derivative action or proceeding brought on our behalf; (2) any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers or other employees to us or our stockholders; (3) any action asserting a claim against us or any of our directors or officers or other employees arising pursuant to any provision of the Delaware General Corporation Law, our amended and restated certificate or our amended and restated bylaws; and/or (4) any action asserting a claim against us or any of our directors or officers or other employees governed by the internal affairs doctrine. The foregoing provisions do not apply to actions brought to enforce a duty or liability created by the Securities Act or the Exchange Act, or any other claim for which the federal courts have exclusive jurisdiction.
55
These exclusive forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers, or other employees, which may discourage lawsuits against us and our directors, officers and other employees. If a court were to find the exclusive forum provision in our governing documents to be inapplicable or unenforceable in an action, we may incur further significant additional costs associated with resolving the dispute in other jurisdictions, all of which could seriously harm our business.
General Risk Factors
Sales of a substantial number of shares of our common stock in the public market could cause our stock price to fall.
Sales of a substantial number of shares of our common stock in the public market or the perception that these sales might occur, could depress the market price of our common stock and could impair our ability to raise capital through the sale of additional equity securities. We are unable to predict the effect that sales may have on the prevailing market price of our common stock.
If we fail to maintain proper and effective internal controls, our ability to produce accurate consolidated financial statements on a timely basis could be impaired.
We are subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act and the rules and regulations of The Nasdaq Stock Market (“Nasdaq”). The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements in accordance with accounting principles generally accepted in the United States of America (“GAAP”). We have performed system and process evaluation and testing of our internal control over financial reporting to allow management to report annually on the effectiveness of our internal control over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act. This has required and will require that we incur substantial professional fees and internal costs to augment our accounting and finance functions and that we expend significant management efforts as we continue to make this assessment and ensure maintenance of proper internal controls on an ongoing basis.
If we are not able to comply with the requirements of Section 404 of the Sarbanes-Oxley Act in a timely manner, or if we fail to establish and maintain proper and effective internal control over financial reporting, we may not be able to produce timely and accurate consolidated financial statements, and our ability to accurately report our financial results could be adversely affected. If that were to happen, the market price of our stock could decline, and we could be subject to sanctions or investigations by Nasdaq, the SEC or other regulatory authorities.
In addition, for so long as we remain a non-accelerated filer, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal control over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act. An independent assessment of the effectiveness of our internal controls could detect problems that our management’s assessment might not. Undetected material weaknesses in our internal controls could lead to financial statement restatements and require us to incur the expense of remediation.
If securities or industry analysts do not publish research reports about our business, or if they issue an adverse opinion about our business, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about us or our business. If one or more of the analysts who cover us issues an adverse opinion about our company, our stock price would likely decline. If one or more of these analysts ceases coverage of us or fails to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
Item 1B. Unresolved Staff Comments.
None.
Item 2. Properties.
Our corporate facilities are comprised of 37,100 square feet of administrative, laboratory and manufacturing spaces located in Tucson, Arizona. We occupy these facilities pursuant to two separate leases. Following its amendment in January 2019, which amended the lease to add approximately 7,000 square feet of additional administrative, manufacturing and laboratory space effective August 2019, the first lease concerns 24,500 square feet housing our administrative, manufacturing, and lab services facilities. The second lease concerns 12,600 square feet of space used for our research and development facilities.
56
We first amended these leases in August 2015 to, among other things, align and extend the lease terms to expire in January 2021. Upon amendment of the first lease in 2019, the lease for the additional space was aligned to this January 2021 expiration. In December 2020, the leases were again amended to extend their terms for one additional year, through January 2022 and again in September 2021 to extend the terms of the leases for three years, through January 31, 2025. The lease extension allows for an additional extension of two years upon the same terms and conditions of the existing amended lease agreements, except that lease rates would be adjusted to rates applicable to like-kind buildings within the market at the time we elect to exercise the extension option, but in no event to less than the last applicable rental rate. Base rent payable is currently approximately $24,000 per month and $16,000 per month, respectively, under the first and second leases, in each case for the remaining terms of the respective leases.
We believe that our existing facilities are adequate to meet our business requirements for the reasonably foreseeable future and that additional space will be available on commercially reasonable terms, if required.
Item 3. Legal Proceedings.
We are not engaged in any material legal proceedings. However, in the normal course of business, we may from time to time be named as a party to legal claims, actions and complaints, including matters involving employment, intellectual property others. Although we anticipate that we will continue to incur legal fees in the coming periods to defend our intellectual property rights, we do not believe that there are any claims or actions pending against us currently, the ultimate disposition of which could have a material adverse effect on our consolidated results of operation, financial condition or cash flows.
Item 4. Mine Safety Disclosures.
Not applicable.
57
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Our common stock is traded on The Nasdaq Capital Market under the symbol “HTGM.” Trading of our common stock on The Nasdaq Stock Market commenced on May 6, 2015 in connection with our initial public offering.
On March 15, 2023, the last reported sale price of our common stock was $3.10 per share.
Holders
As of March 15, 2023, there were approximately 57 registered holders of our common stock. The actual number of stockholders is greater than this number of record holders and includes stockholders who are beneficial owners but whose shares are held in street name by brokers and other nominees.
Dividends
We have never declared or paid any cash dividends on our common stock. We anticipate that we will retain all available funds and any future earnings, if any, for use in the operation of our business and do not anticipate paying cash dividends in the foreseeable future. In addition, the Loan Agreement materially restricts, and future debt instruments we issue may materially restrict, our ability to pay dividends on our common stock. Payment of future cash dividends, if any, will be at the discretion of the board of directors after considering various factors, including our financial condition, operating results, current and anticipated cash needs, the requirements of current or then-existing debt instruments and other factors the board of directors deems relevant.
Item 6. Reserved.
58
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
You should read the following discussion and analysis together with our consolidated financial statements and related notes included elsewhere in this Annual Report. The following discussion contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those expressed or implied in any forward-looking statements due to various factors, including those set forth under the caption “Item 1A. Risk Factors.” All forward-looking statements included in this Annual Report are based on information available to us as of the time we file this Annual Report and, except as required by law, we undertake no obligation to update publicly or revise any forward-looking statements. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this Annual Report, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain.
In December 2022, we completed a reverse stock split of our outstanding shares of common stock pursuant to which every 12 shares of issued and outstanding common stock were exchanged for one share of common stock. All share and per share amounts within this Annual Report have been adjusted to reflect the reverse stock split for all periods and dates presented.
Overview
We are focused on advancing precision medicine and drug discovery through our innovative transcriptome-wide profiling and advanced drug discovery platform technologies. Building on more than a decade of pioneering innovation, our proprietary next-generation HTG EdgeSeq technology is the basis for our tech-driven hybrid business model allowing our RNA molecular profiling applications to be more effective, efficient and relevant and also serving as a key component of the engine behind our platform-based drug discovery process. Central to our business strategy is our drug discovery engine, which uses our captive transcriptomic profiling capabilities combined with a proprietary medicinal chemistry machine learning platform to render an AI-driven drug candidate optimization platform. We are using this platform to innovate drug discovery with the goal of building best-in-class molecules for known pharmacologic targets across multiple disease areas, better, faster and in a more cost-effective manner.
The training data sets for our machine learning platform utilize our own primary data generated specifically for this purpose. This high quality, standardized data provides a clear advantage over other platform approaches which are typically dependent upon publicly available data. The medicinal chemistry portion of our platform allows for rapid design and in silico evaluation of large chemical libraries in order to prioritize and select compounds for synthesis and advancement into early testing. These data are then integrated and processed into an iterative loop using a series of proprietary machine learning algorithms prior to further advancing the molecules to more traditional drug discovery studies. We expect that this will allow for rapid identification, selection and optimization of drug candidates for entrance into development. Further, we believe that our ability to rapidly iterate between primary data and computational analyses gives us valuable information and insights for candidate molecule design and selection.
To date, we have used our transcriptome-informed drug discovery engine to develop an early pipeline of drug candidate molecules for two known pharmacologic targets, both of which can target several potential therapeutic indications, but with a current focus on oncology and neurodegenerative diseases. We believe that our technology provides a differentiated and potentially disruptive approach to drug discovery, that may allow ourselves and our partners to potentially improve upon key attrition factors, namely efficacy and toxicity, early in the discovery process, thereby allowing for better chances for candidate success when entering development.
Our business strategy is to build our drug discovery pipeline in order to out-license certain drug candidates and carry other candidates into preclinical and early development ourselves. In addition, we would expect to retain and potentially capitalize upon CDx rights through the clinical development and commercialization of these assets where appropriate.
We also operate a profiling business in life science tools. Our profiling product and service solutions enable targeted RNA profiling using a small amount of biological sample, in liquid or solid forms. Our menu of HTG EdgeSeq assays, including our HTP, which has been designed to measure approximately 20,000 mRNA targets using our HTG EdgeSeq technology, is automated on our HTG EdgeSeq system, which applies NGS tools, enabling the generation of gene expression data in a timely manner utilizing our simplified workflow. We seek to leverage key business drivers in molecular profiling for biomarker analysis and diagnostics, including the acceleration of precision medicine, the migration of molecular testing to NGS-based applications, the movement to smaller and less invasive biopsies, the need for greater diagnostic sensitivity, the need to conform to challenging healthcare economics and the need for automation and an easily deployable workflow, including simplified bioinformatics. These capabilities enable customers to extend the use of limited biological samples for retrospective or prospective analysis, gaining further understanding of the molecular drivers of disease with the goal of developing biomarker-driven targeted therapies.
59
Our existing products include instruments, consumables and software that, as an integrated platform, automate sample processing and can quickly, robustly and simultaneously profile hundreds, thousands or tens of thousands of molecular targets from samples which are a fraction of the size required by many prevailing technologies. Customers can access our technology by purchasing our HTG EdgeSeq system and assays for their internal use or through our Tucson, Arizona-based VERI/O service laboratory, including molecular profiling of cohorts and development of custom RUO panels to support early-stage clinical programs and investigational-use-only assays for clinical trials. However, with the release of our HTP, revenue from our RUO assay design services is expected to be lower than historical levels, as our RUO assay design services revenue is replaced by HTP consumables purchases and sample processing laboratory services using our HTP. Our product and service solutions have enabled us to access a number of early-stage biomarker discovery programs. We believe this approach will enable new opportunities collaborating with biopharmaceutical companies in their future drug development programs.
Our Drug Discovery Approach
In June 2021, we announced the formation of HTG Therapeutics, with the addition of several highly experienced drug development professionals to our leadership team. Throughout 2021, we strengthened our HTG EdgeSeq technology platform and added new profiling capabilities, including epitranscriptomic profiling, which currently provides the capability to generate over 40,000 biological data points from each experimental sample. By leveraging these profiling technologies in the drug discovery process, integrated with an advanced AI and machine learning-based medicinal chemistry approach, we have established a novel transcriptome-informed small molecule discovery engine at the core of our HTG Therapeutics business unit which we believe will generate drug candidate molecules that are intrinsically lower risk and will have greater potential for clinical development success when compared to currently existing early-stage drug discovery methods in the biopharmaceutical industry. We further expect that this approach to small molecule discovery can be applied agnostically across therapeutic areas and is scalable and flexible, allowing us to adapt our strategic and therapeutic focus rapidly as new information emerges on the pathogenesis of diseases.
We believe that our approach will potentially provide multiple revenue opportunities, including collaboration or out-licensing arrangements for small molecule drug candidates we generate from as early as lead optimization through early preclinical development, the out-licensing of our technology to pharmaceutical companies to enable them to implement our advanced drug discovery approach into their own internal discovery efforts, and potentially new companion diagnostic opportunities to support the related clinical development programs for molecules that are brought forward through this novel discovery approach.
In the first half of 2022, we released a series of white papers after demonstrating the utility of our proprietary technologies as a key component of our novel transcriptome-informed drug discovery and design approach and applying the approach to our initial therapeutic target. As anticipated, the results of our studies summarized in these white papers supported our approach and its ability to reveal indication-specific effects and potential undesirable effects in our first target through analysis of transcriptomic profiles from compound-treated human cell line test systems.
Throughout the second half of 2022, we continued to work to strengthen our drug discovery core platform technology, including advancing the machine learning component of our platform with the refinement of key proprietary algorithms while continuing to generate our own internal data supporting training sets. In addition, we made capital investments to establish internal cell culture capabilities to support the expansion of our cell-based test system models. Our medicinal chemistry effort has produced a series of chemical libraries for our first target, and our most advanced library for this target has entered preclinical characterization, with a series of data generated including early efficacy in two different disease states.
As a result of the progress made throughout 2022, we filed a patent application in December 2022, which included claims directed toward specific compounds, pharmaceutical compositions and methods of treating or preventing disease by administration of the compounds. Our initial therapeutic pipeline is focused on oncology and degenerative neuroscience, emphasizing pharmacologic targets with understood roles in the progression of diseases in these areas.
The most advanced discovery program in oncology is a small molecule program for treatment of liquid tumors. We expect to continue lead optimization of this program through the end of the first quarter of 2023, with advancement to support entry into preclinical development later in the year. HTG Therapeutics has a second oncology directed small molecule program for the treatment of a solid tumor type that is nearing completion in the hit-to-lead discovery phase, with lead optimization efforts planned through the second quarter of 2023 and subsequent preparation for potential preclinical development expected by the end of 2023. In our neuroscience pipeline, we have completed early discovery stage efforts and chemical library generation for candidate small molecules for application to neurodegenerative conditions which are expected to enter the hit-to-lead phase in the second half of 2023.
60
We expect to initiate several early discovery-stage programs evaluating small molecule candidates against a variety of different cancers, from which we plan to select candidates for additional indications to continually expand our drug discovery pipeline. As additional candidates are identified, we may choose to retain certain candidates internally to be advanced through early development, with the intention to increase the value of these pipeline assets before moving to license or partner for further development. In parallel to these therapy-area specific programs, we continue to enrich the proprietary dataset that supports our transcriptome-informed drug discovery platform and to evolve and refine the complementary AI and machine learning portions of our drug discovery engine throughout these discovery processes. Finally, we would expect to maintain the exclusive rights and the opportunity to solely develop new CDx assays relating to these drug candidates as they move through the increasingly advanced stages of development with our future collaboration partners, further growing our existing gene expression profiling business.
Revenue and Commercialization of our Profiling Products
We believe the future financial performance of our profiling business will continue to be driven by adoption and utilization of our HTG EdgeSeq instruments and consumables, and an overall increase in the number and type of customers using our technology. As such, we believe the primary measures of adoption for our profiling technology are the number of total active customers, the number of active programs in our biopharmaceutical company customer pipeline, the number of instruments actively producing revenue in our installed base and revenue growth relating to new and existing customers. Total active customers and active installed base reflect customers and instruments that have generated revenue for the Company within the last 12 months. To be included in our active programs metric, a program needs to be associated with a pharma sponsored clinical trial, be traceable to a program on clinicaltrials.gov and have generated revenue for the Company within the last 12 months. As of December 31, 2022, we had 78 active customers, 73 active programs and 42 instruments actively producing revenue in our installed base, compared with 82 active customers, 62 active programs and 51 instruments actively producing revenue in our installed base as of December 31, 2021.
Our profiling business continues to experience the ripple effects of the COVID-19 pandemic and has not yet recovered to pre-pandemic revenue levels, as seen in a year over year decrease in two out of three of the metrics discussed above, active customer base and active instruments. This trend reflects the continued challenge of the significant reduction of and delay in clinical trial activities during the pandemic generating lower quantities of retrospective samples for testing, budget reductions, labor shortages and supply chain issues being faced by a number of our customers. In response to these trends, our focus has shifted to the quality and sustainability of future revenue, including higher revenue per sample, larger cohorts and minimum batch sizes for service in our VERI/O laboratory. Given the length of our profiling business sales cycle and ongoing concerns regarding the economy throughout our industry, we expect to continue to see fluctuations in our profiling revenue on a period-to-period basis despite our ongoing focus on continuing to identify new opportunities to effectively commercialize our profiling technology and seek expanded commercial partnering channels. As a result of these profiling revenue trends, we have taken actions to reduce operating expenses and minimize the impact of reduced revenue on our operating loss and cash utilization, including a significant reduction in force late in the second quarter of 2022. We will continue to make appropriate operating adjustments in support of what we believe will be a quickly evolving, best-in-class drug discovery company and our existing gene expression profiling business.
2022 Equity Financings
In March 2022, we entered into a Securities Purchase Agreement (the “March 2022 Securities Purchase Agreement”) with a single investor pursuant to which we agreed to issue to the investor 270,415 units at a price of $27.744 per unit (less $0.012 for each pre-funded warrant purchased in lieu of a share of common stock) for net proceeds, after deducting the placement agent fees and other fees and expenses, of approximately $7.0 million. Each unit consisted of one share of common stock (or one pre-funded warrant in lieu thereof), a common warrant to purchase one share of common stock with a term of 24 months from the issuance date, and a common warrant to purchase one share of common stock with a term of 66 months from the issuance date. Each of the common warrants became exercisable commencing on September 21, 2022 and has an exercise price of $24.744 per share. Each pre-funded warrant had an exercise price of $0.012 per share. May 2022, the 200,911 pre-funded warrants were exercised for proceeds of $2,411.
In December 2022, in connection with a best-efforts public offering, we entered into a Securities Purchase Agreement (the "December 2022 Securities Purchase Agreement") with a certain institutional investor, pursuant to which we issued and sold to the investor 1,290,322 units at a combined public offering price of $7.75 per share (less $0.001 for each pre-funded warrant purchased in lieu of a share of common stock) for net proceeds, after deducting the placement agent fees and expenses and other fees and expenses, of approximately $8.7 million. Each unit consisted of one share of common stock (or one pre-funded warrant in lieu thereof), a common warrant to purchase one share of common stock with a term of 24 months from the issuance date, and a common warrant to purchase one share of common stock with a term of 60 months from the issuance date. Each of these common warrants has an exercise price of $7.50 per share. In December 2022, the 1,188,322 pre-funded warrants were exercised for proceeds of $1,188.
61
Financial Operations Overview and Consolidated Results of Operations
Comparison of the Years Ended December 31, 2022 and 2021
|
|
Years Ended December 31, |
|
|
Change |
|
||||||||||
|
|
2022 |
|
|
2021 |
|
|
$ |
|
|
% |
|
||||
Product and product-related services revenue |
|
$ |
6,366,220 |
|
|
$ |
8,906,828 |
|
|
$ |
(2,540,608 |
) |
|
|
(29 |
%) |
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Cost of product and product-related services revenue |
|
|
4,572,134 |
|
|
|
4,094,980 |
|
|
|
477,154 |
|
|
|
12 |
% |
Selling, general and administrative |
|
|
15,841,790 |
|
|
|
16,546,740 |
|
|
|
(704,950 |
) |
|
|
(4 |
%) |
Research and development |
|
|
6,781,892 |
|
|
|
6,088,934 |
|
|
|
692,958 |
|
|
|
11 |
% |
Total operating expenses |
|
|
27,195,816 |
|
|
|
26,730,654 |
|
|
|
465,162 |
|
|
|
2 |
% |
Operating loss |
|
|
(20,829,596 |
) |
|
|
(17,823,826 |
) |
|
|
(3,005,770 |
) |
|
|
17 |
% |
Gain on forgiveness of PPP Loan |
|
|
— |
|
|
|
1,735,792 |
|
|
|
(1,735,792 |
) |
|
|
(100 |
%) |
Other income (expense), net |
|
|
(754,043 |
) |
|
|
(1,034,661 |
) |
|
|
280,618 |
|
|
|
(27 |
%) |
Net loss before income taxes |
|
$ |
(21,583,639 |
) |
|
$ |
(17,122,695 |
) |
|
$ |
(4,460,944 |
) |
|
|
26 |
% |
Product and product-related services revenue
Our product and product-related services revenue is generated primarily through the sale of our profiling instruments and consumables and sample processing services performed on behalf of pharmaceutical companies, academic research centers and molecular testing laboratories.
RUO profiling is currently made available to our customers through product and service offerings. Customers can purchase our HTG EdgeSeq instrument and related consumables, which consist primarily of our proprietary molecular profiling panels and other assay components, for use in their own facilities. They can also access our technology through contracted RUO profiling services using our HTG EdgeSeq instruments and RUO consumables to process their samples in our VERI/O laboratory and through the development of custom RUO panels which are expected to generate future sample processing or RUO consumables revenue.
Product and product-related services revenue, which includes revenue generated through the sale of our HTG EdgeSeq instruments and consumables and from services performed for customers using our proprietary RUO technology, decreased by 29% to $6.4 million for the year ended December 31, 2022 compared with $8.9 million for the year ended December 31, 2021, and was comprised of the following:
|
|
Years Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Product revenue: |
|
|
|
|
|
|
||
Instrument |
|
$ |
609,627 |
|
|
$ |
1,385,665 |
|
Consumables |
|
|
3,140,420 |
|
|
|
3,786,923 |
|
Total product revenue |
|
|
3,750,047 |
|
|
|
5,172,588 |
|
Product-related services revenue: |
|
|
|
|
|
|
||
Custom RUO assay design |
|
|
20,000 |
|
|
|
48,350 |
|
RUO sample processing |
|
|
2,596,173 |
|
|
|
3,685,890 |
|
Total product-related services revenue |
|
|
2,616,173 |
|
|
|
3,734,240 |
|
Total product and product-related services revenue |
|
$ |
6,366,220 |
|
|
$ |
8,906,828 |
|
Product revenue, which includes gene expression profiling revenue generated through the sale of our HTG EdgeSeq instruments and consumables, decreased by 28% to $3.8 million for the year ended December 31, 2022, compared with $5.2 million for the year ended December 31, 2021. The decrease in new instrument placements when compared with the prior year is consistent with the decrease in new customers added in 2022 compared with 2021. As we have worked to right size our business to profiling revenue trends experienced since the beginning of the COVID-19 pandemic in March 2020, our commercial team has prioritized its efforts on expanding business with existing customers and seeking out new customers with larger studies and those with expectations of more extensive future profiling needs. This resulted in the need to place fewer new instruments in 2022, as many of our customers purchased instruments in prior years or have opted to use our laboratory or a certified reference laboratory who previously purchased our instruments to run their samples. Consumables revenue reflected the slower than anticipated recovery of our business to pre-COVID-19 levels. Consumables revenue generated from the sale of our HTP, commercially launched in August 2021, was $1.8 million and $1.3 million for the years ended December 31, 2022 and 2021, respectively. HTP consumables revenue represented 47% of our product revenue for the year ended December 31, 2022, compared with 25% of our product revenue for the year ended December 31, 2021 reflecting expanding adoption of that product by new and existing customers since its launch.
62
Product-related services revenue, consisting of RUO sample processing using our HTG EdgeSeq instruments and consumables in our VERI/O laboratory and custom RUO assay design, decreased by 30% to $2.6 million for the year ended December 31, 2022, compared with $3.7 million for the year ended December 31, 2021. RUO sample processing revenue decreased primarily due to the timing of several biopharma programs pending decisions from data generated in previous studies using our technology, continued delays in our ability to obtain customer samples for planned sample processing programs, and our customers' reprioritizing their programs due to continuing impacts of COVID-19 on their operations. Revenue generated from sample processing services using our HTP was $0.9 million and $0.1 million for the years ended December 31, 2022 and 2021, respectively, and represented 34% and 3% of our product-related services revenue for the years ended December 31, 2022 and 2021, respectively.
Cost of product and product-related services revenue
Cost of product and product-related services revenue includes both product-related and services-related costs. Product-related costs include the aggregate costs incurred in manufacturing, delivering, installing and servicing instruments and consumables. The components of our product-related costs of revenue include consumables and lab supplies, subcomponent and servicing costs, manufacturing costs incurred internally (which include direct labor costs), and equipment and infrastructure expenses associated with the manufacturing and distribution of our products. Due to the fixed nature of certain of these expenses, such as overhead, equipment and infrastructure, associated with our regulated industry and our expectations for further growth in customer demand, we expect our cost of product and product-related services revenue as a percentage to decrease over time as our product and product-related services revenue increases, further absorbing these fixed costs.
Cost of product and product-related services revenue increased by 12% to $4.6 million for the year ended December 31, 2022 compared with $4.1 million for the year ended December 31, 2021. This increase primarily reflects an increase in excess inventory allowance of $1.1 million in the fourth quarter of 2022, reflecting our estimation of inventory in excess of our projections of future demand for certain of our products and $0.5 million of Employee Retention Credit ("ERC") benefits that served to partially offset compensation expense in 2021 but did not recur in 2022. This increase was partially offset by a decrease in direct and indirect costs incurred consistent with lower year over year product and product-related services revenue.
Selling, general and administrative expenses
Selling, general and administrative expenses consist primarily of personnel costs for our sales and marketing, regulatory, legal, executive management and finance functions. The expenses also include third-party professional and consulting fees incurred by these functions, promotional expenses and facility and overhead costs relating to our administrative offices. Selling, general and administrative expenses decreased by 4% to $15.8 million for the year ended December 31, 2022 compared with $16.5 million for the year ended December 31, 2021. This decrease primarily reflects a decrease in legal fees incurred to protect our intellectual property and decreased compensation and stock-based compensation expenses following a reduction in force completed in the second quarter of 2022. This decrease was partially offset by $0.8 million of ERC benefits that served to offset a portion of compensation expense in 2021 but did not recur in 2022.
Research and development expenses
Research and development expenses increased by 11% to $6.8 million for the year ended December 31, 2022, compared with $6.1 million for the year ended December 31, 2021. This increase in research and development expense for the year ended December 31, 2022 compared with the same period in 2021 reflects $0.4 million of ERC benefits that served to offset a portion of compensation expense in 2021, and an increase in research and development spending associated with efforts to build and strengthen our drug discovery engine in 2021. This increase was partially offset by a decrease in profiling product development as we shifted focus to our therapeutics efforts and to maintenance and marketing of our existing product portfolio following commercial release of our HTP in August 2021.
Gain on forgiveness of PPP Loan
In May 2021, upon receipt of the notification that the PPP Loan and related accrued interest had been forgiven by the U.S. Small Business Administration and that the note associated with the PPP Loan had been cancelled, we reversed the liabilities related to the PPP Loan and recorded a gain on forgiveness of PPP Loan of approximately $1.7 million.
Other income (expense)
As of both December 31, 2022 and 2021, we had outstanding obligations due to NuvoGen under an asset purchase agreement and to SVB under the SVB Term Loan. Interest expense related to these obligations and to the discount, deferred financing fee and final fee premium amortization of amounts associated with these obligations was $0.9 million and $1.1 for the years ended December 31, 2022 and 2021, respectively. This decrease in interest expense was primarily the result of a $2.5 million payment made to SVB as part of the Term Loan Amendment which, in addition to regularly scheduled SVB Term Loan and NuvoGen obligation payments, resulted in a decreased balance on which interest is being accrued and/or paid.
63
Cash Flows for the Years Ended December 31, 2022 and 2021
The following table summarizes the primary sources and uses of cash for each of the periods presented:
|
|
Years Ended December 31, |
|
|
Change |
|
||||||||||
|
|
2022 |
|
|
2021 |
|
|
$ |
|
|
% |
|
||||
Net cash provided by (used in): |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Operating activities |
|
$ |
(18,407,791 |
) |
|
$ |
(16,508,554 |
) |
|
$ |
(1,899,237 |
) |
|
|
12 |
% |
Investing activities |
|
|
12,420,233 |
|
|
|
(6,664,744 |
) |
|
|
19,084,977 |
|
|
|
(286 |
%) |
Financing activities |
|
|
8,604,820 |
|
|
|
10,391,622 |
|
|
|
(1,786,802 |
) |
|
|
(17 |
%) |
Effect of exchange rate on cash |
|
|
(6,355 |
) |
|
|
(16,186 |
) |
|
|
9,831 |
|
|
|
(61 |
%) |
Increase (decrease) in cash and cash equivalents |
|
$ |
2,610,907 |
|
|
$ |
(12,797,862 |
) |
|
$ |
15,408,769 |
|
|
|
(120 |
%) |
Operating Activities
Net cash used in operating activities for the year ended December 31, 2022 increased by 12% to $18.4 million compared with $16.5 million for the year ended December 31, 2021. This increase for the year ended December 31, 2022 reflected (i) the net loss of $21.6 million and (ii) net non-cash items of $3.3 million consisting primarily of provision for excess inventory of $1.2 million, stock-based compensation expense of $0.8 million, depreciation and amortization expense of $0.6 million, amortization of loan discount and issuance costs of $0.4 million, non-cash operating lease expense of $0.4 million and gain on abandonment and disposal of assets of $0.1 million; and (iii) a net cash outflow from changes in balances of operating assets and liabilities of $0.1 million.
Net cash used in operating activities for the year ended December 31, 2021 was $16.5 million and reflected (i) the net loss of $17.1 million and (ii) net non-cash items of $1.6 million consisting primarily of the gain on the forgiveness of our PPP loan of $1.7 million, stock-based compensation expense of $1.3 million, depreciation and amortization expense of $0.7 million, amortization of loan discount and issuance costs of $0.5 million, non-cash operating lease expense of $0.5 million and loss on abandonment and disposal of assets of $0.2 million; and (iii) a net cash outflow from changes in balances of operating assets and liabilities of $1.0 million.
Investing Activities
Net cash provided by investing activities for the year ended December 31, 2022 increased by 286% to $12.4 million compared with net cash used in investing activities of $6.7 million for the year ended December 31, 2021. Net cash provided by investing activities for the year ended December 31, 2022 consisted primarily of the maturity of $20.0 million of the available-for-sale securities and the proceeds from the sale of property and equipment of $0.1 million, partially offset by the purchases of available-for-sale securities of $7.6 million.
Net cash used in investing activities for the year ended December 31, 2021 was $6.7 million and consisted primarily of purchases of available-for-sale securities of $18.6 million and purchases of laboratory equipment and other fixed assets during the year of $0.6 million, partially offset by the maturity of $12.6 million of the available-for-sale securities.
Financing Activities
Net cash provided by financing activities for the year ended December 31, 2022 decreased by 17% to $8.6 million compared with $10.4 million for the year ended December 31, 2021. This activity for the year ended December 31, 2022 consisted primarily of $9.1 million of proceeds net of commissions and issuance costs from the December 2022 Securities Purchase Agreement (see Note 14 of the accompanying consolidated financial statements), $7.0 million in net proceeds from the March 2022 Securities Purchase Agreement (see Note 14 to the accompanying consolidated financial statements) and $0.8 million in proceeds from our 2022 Insurance Note, partially offset by $6.8 million of payments on our SVB Term Loan, $0.5 million of payments made on our outstanding NuvoGen obligation, and $1.0 million of payments made on our 2021 and 2022 Insurance Notes.
Net cash provided by financing activities for the year ended December 31, 2021 was $10.4 million and consisted primarily of $10.7 million in net proceeds from sales of our common stock in an “at the market offering” and $0.9 million in proceeds from our stock purchase agreement (the “LP Purchase Agreement”) with Lincoln Park Capital Fund, LLC (“Lincoln Park”), $0.1 million in proceeds from shares purchased under stock purchase plans, partially offset by $0.5 million of payments made on our outstanding NuvoGen obligation, and $0.7 million of payments made on our 2020 and 2021 Insurance Notes.
64
Liquidity and Capital Resources
Since our inception, our operations have primarily been financed through the issuance of our common stock, preferred stock, the incurrence of debt and cash received from product sales, services revenue and other income. As of December 31, 2022, we had $12.2 million in cash and cash equivalents, current liabilities of $8.3 million and $4.1 million of long-term liabilities primarily relating to our NuvoGen obligation and operating leases.
In June 2020, we entered into the SVB Term Loan with SVB. The proceeds from the SVB Term Loan, together with cash on hand, were used to repay in full all outstanding amounts and fees due under our prior MidCap Credit Facility and a subordinated convertible note that has since been repaid. Our SVB Term Loan bears interest at a floating rate equal to the greater of 2.50% above the Prime Rate (as defined in the Loan Agreement) and 5.75%. In July 2022, we entered into the Term Loan Amendment with SVB. Under the Term Loan Amendment, SVB agreed to remove the financial covenant under the Loan Agreement. In exchange for this accommodation, we prepaid $2.5 million of outstanding principal under the SVB Term Loan. The remaining outstanding principal amount due under the SVB Term Loan will continue to be paid in equal monthly payments of principal and interest through the maturity date of December 1, 2023.
In March 2022, we entered into a Securities Purchase Agreement with a single investor pursuant to which we issued and sold to the investor 270,415 units at a price of $27.744 per unit (less $0.012 for each pre-funded warrant purchased in lieu of a share of common stock) for net proceeds, after deducting the placement agent fees and other fees and expenses, of approximately $7.0 million. Each unit consisted of one share of common stock (or one pre-funded warrant in lieu thereof), a common warrant to purchase one share of our common stock with a term of 24 months from the issuance date, and a common warrant to purchase one share of our common stock with a term of 66 months from the issuance date. Each of these common warrants became exercisable commencing on September 21, 2022 and has an exercise price of $24.744 per share. Each pre-funded warrant had an exercise price of $0.012 per share and had no expiration date. In May 2022, all of the 200,911 pre-funded warrants were exercised for proceeds of $2,411.
In December 2022, in connection with a best-efforts public offering, we entered into a Securities Purchase Agreement (the "December 2022 Securities Purchase Agreement") with a certain institutional investor, pursuant to which we issued and sold to the investor 1,290,322 units at a combined public offering price of $7.75 per share (less $0.001 for each pre-funded warrant purchased in lieu of a share of common stock) for net proceeds, after deducting the Placement Agent fees and expenses and other estimated fees and expenses of approximately $8.7 million. Each unit consisted of one share of common stock (or one pre-funded warrant in lieu thereof), a common warrant to purchase one share of common stock with a term of 24 months from the issuance date, and a common warrant to purchase one share of common stock with a term of 60 months from the issuance date. Each of these common warrants has an exercise price of $7.50 per share. In December 2022, all of the 1,188,322 pre-funded warrants were exercised for proceeds of $1,188.
The current volatility in the equity markets may create additional challenges to raising a sufficient amount of capital through an equity financing in the near term. If sufficient additional capital is not available as and when needed, we may have to delay, scale back or discontinue one or more product development programs, curtail our commercial activities, significantly reduce expenses, sell assets (potentially at a discount to their fair value or carrying value), enter into relationships with third parties to develop or commercialize products or technologies that we otherwise would have sought to develop or commercialize independently, pursue a sale of the Company at a price that may result in a significant loss on investment for our stockholders, file for bankruptcy or seek other protection from creditors, or liquidate all assets. In addition, if we default under any of the terms of the Loan Agreement, including as a result of a material adverse change, Silicon Valley Bridge Bank could accelerate the payment of the SVB Term Loan and ultimately foreclose on our assets.
Contractual Obligations, Commitments and Material Cash Requirements
We have had recurring operating losses and negative cash flows from operations since our inception and have an accumulated deficit of $229.9 million as of December 31, 2022. As of December 31, 2022, we had cash and cash equivalents of $12.2 million and had current liabilities of $8.3 million. As of December 31, 2022, we also had approximately $4.1 million of long-term liabilities outstanding, relating to our NuvoGen obligation, and our financing and operating leases.
We currently expect that our existing resources will only be sufficient to fund our planned operations and expenditures until at least July 2023. In addition, potentially changing circumstances, including those related to a resurgence of COVID-19, inflation and high interest rates, may also result in the depletion of our capital resources more rapidly than we currently anticipate. These circumstances raise substantial doubt about our ability to continue as a going concern.
65
Our primary capital needs, including contractual obligations and commitments, which are subject to change, include:
Until our revenue reaches a level sufficient to support self-sustaining cash flows, if ever, we expect to finance our cash needs through public or private equity offerings, debt financings, or other capital sources which may include strategic collaborations, licensing arrangements or other arrangements with third parties. The current volatility in the equity markets may create additional challenges to raising a sufficient amount of capital through an equity financing in the near term. Future funding requirements will depend on a number of factors, including our ability to generate significant revenue, our ability to repay our debt obligations as they become due, the cost and timing of establishing additional sales, marketing and distribution capabilities, the ongoing cost of research and development activities, the cost and timing of regulatory clearances and approvals, the effect of competing technology and market developments, the nature and timing of companion diagnostic development collaborations we may establish and the extent to which we acquire or invest in businesses, products and technologies.
Additional capital may not be available at such times or in amounts needed by us. Even if sufficient capital is available to us, it might be available only on unfavorable terms. If we are unable to raise additional capital in the future when required and in sufficient amounts or on terms acceptable to us, we may have to delay, scale back or discontinue one or more product development programs, curtail our commercialization activities, significantly reduce expenses, sell assets (potentially at a discount to their fair value or carrying value), enter into relationships with third parties to develop or commercialize products or technologies that we otherwise would have sought to develop or commercialize independently, cease operations altogether, pursue an acquisition of our company at a price that may result in a significant loss on investment to our stockholders, file for bankruptcy, seek other protection from creditors, or liquidate all of our assets. In addition, if we default under our SVB Term Loan agreement, including as a result of a “material adverse change,” our lender could foreclose on our assets. The definition of “material adverse change” is broad and includes a material impairment in the value of the collateral securing the SVB Term Loan, a material adverse change in our business, operations, or condition (financial or otherwise), and a material impairment of the prospect of repayment of any portion of the SVB Term Loan. As the remaining payments under the SVB Term Loan are due within twelve months of December 31, 2022, the impact of a material adverse change would be to accelerate the payment of this short-term debt further.
Recent Accounting Pronouncements
For a summary of recent accounting pronouncements applicable to our consolidated financial statements, see “Note 2. Basis of Presentation and Summary of Significant Accounting Policies” in Part II, Item 8, Notes to Consolidated Financial Statements.
Critical Accounting Policies and Significant Judgments and Critical Accounting Estimates
Management’s discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance GAAP. The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Critical accounting policies and estimates are those that we consider most important to the portrayal of our financial condition and results of operations because they require our most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain. Our critical accounting policies and estimates include those related to revenue recognition, fair value measurements and inventory valuation. Actual results could materially differ from these estimates and such differences could affect the results of operations in future periods.
66
Revenue from Contracts with Customers
Revenue from contracts with customers is recognized when, or as, we satisfy our performance obligations by delivering the promised goods or service deliverables to our customers. A good or service deliverable is transferred to a customer when, or as, the customer obtains control of that good or service deliverable. A performance obligation may be satisfied over time or at a point in time. Revenue from a performance obligation satisfied over time is recognized by measuring our progress in satisfying the performance obligation in a manner that depicts the transfer of the goods or services to the customer. Revenue from a performance obligation satisfied at a point in time is recognized at the point in time that we determine the customer obtains control over the promised good or service deliverable. The amount of revenue recognized reflects the consideration we expect to be entitled to in exchange for those promised goods or services (i.e., the “transaction price”). In determining the transaction price, we consider multiple factors, including the effects of variable consideration. Variable consideration is included in the transaction price only to the extent it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainties with respect to the amount are resolved. In determining when to include variable consideration in the transaction price, we consider the range of possible outcomes, the predictive value of our past experiences, the time period of when uncertainties expect to be resolved and the amount of consideration that is susceptible to factors outside of our influence, such as the judgment and actions of third parties.
For contracts where the period between when we transfer a promised good or service to the customer and when the customer pays is one year or less, we have elected the practical expedient to not adjust the promised amount of consideration for the effects of a significant financing component.
We have made a policy election to exclude from the measurement of the transaction price all taxes assessed by a government authority that are both imposed on and concurrent with a specific revenue producing transaction and collected from a customer. Such taxes may include but are not limited to sales, use, value added and certain excise taxes.
Product and Product-related Services Revenue
Sale of instruments and consumables
The delivery of each instrument and related installation and calibration are considered to be a single performance obligation, as the HTG EdgeSeq instrument must be professionally installed and calibrated prior to use. Instrument product revenue is generally recognized upon installation and calibration of the instrument by field service engineers, which represents the point at which the customer has the ability to use the instrument and has accepted the asset. Installation generally occurs within one month of instrument shipment.
The delivery of each consumable is a separate performance obligation. Consumables revenue is recognized upon transfer of control, which represents the point when the customer has legal title and the significant risks of ownership of the asset. Our standard terms and conditions provide that no right of return exists for instruments and consumables, unless replacement is necessary due to delivery of defective or damaged product. Customer payment terms vary but are typically between 30 and 90 days of revenue being earned from shipment or delivery, as applicable.
Shipping and handling fees charged to customers for instruments shipped are included in the consolidated statements of operations as part of product and product-related services revenue. Shipping and handling costs for products shipped to customers are included in the consolidated statements of operations as part of cost of product and product-related services revenue.
For sales of consumables in the United States, standard delivery terms are FOB shipping point, unless otherwise specified in the customer contract, reflecting transfer of control to the customer upon shipment. Standard delivery terms for sales to customers outside of the United States are FOB delivery point, unless otherwise specified in the customer contract. We have elected the practical expedient to account for shipping and handling as activities to fulfill the promise to transfer the consumables.
We provide instruments to certain customers under reagent rental agreements. Under these agreements, an instrument is installed in the customer’s facility without a fee and the customer agrees to purchase consumable products at a stated price over the term of the agreement; in some instances, the agreements do not contain a minimum purchase requirement. Terms range from several months to multiple years and may automatically renew in several month or multiple year increments unless either party notifies the other in advance that the agreement will not renew. We measure progress toward complete satisfaction of this performance obligation to provide the instrument and deliver the consumables using an output method based on the number of consumables delivered in relation to the total consumables to be provided under the reagent rental agreement. This is considered to be representative of the delivery of outputs under the arrangement and the best measure of progress because the customer benefits from the instrument only in conjunction with the consumables. We expect to recover the cost of the instrument under the agreement through the fees charged for consumables, to the extent sold, over the term of the agreement.
67
In reagent rental agreements, we retain title to the instrument and title is transferred to the customer at no additional charge at the conclusion of the initial arrangement. The cost of the instrument is amortized on a straight-line basis over the term of the arrangement, unless there is no minimum consumable product purchase, in which case the instrument would be expensed as cost of product and product-related services revenue upon installation. Cost to maintain the instrument while we hold title is charged to selling, general and administrative expense as incurred.
Service revenue
Sample Processing Services
We also provide sample preparation and processing services and molecular profiling of retrospective cohorts for our customers through our VERI/O laboratory, whereby the customer provides samples to be processed using HTG EdgeSeq technology specified in the order. Customers are charged a per sample fee for sample processing services which is recognized as revenue upon delivery of a data file to the customer showing the results of testing and completing delivery of the agreed upon service. This is when the customer can use and benefit from the results of testing and we have the present right to payment.
Fair Value Measurements
We establish the fair value of all of our financial assets and liabilities, which are recognized and disclosed at fair value in the consolidated financial statements, using the price that would be received to sell an asset or paid to transfer a financial liability in an orderly transaction between market participants at the measurement date. A fair value hierarchy is used to measure fair value. The three levels of the fair value hierarchy are as follows:
Level 1 – Quoted prices in active markets for identical assets and liabilities.
Level 2 – Pricing inputs are based on quoted prices for similar instruments in active markets, quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations in which all significant inputs and significant value drivers are observable in active markets.
Level 3 – Valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable and include situations where there is little, if any, market activity for the investment.
Our portfolio of securities comprises high credit quality corporate debt securities classified as available-for-sale securities.
Inventory Valuation
Inventory consists of raw materials and finished goods which are stated at the lower of cost (first-in, first-out) or net realizable value. We assess the valuation of our inventory on a periodic basis and make adjustments to the value for estimated obsolescence, inventory in excess of reasonably expected near term sales or unmarketable inventory, in an amount equal to the difference between the cost of inventory and the estimated market value, based upon assumption about future demand and market conditions. Such estimates are difficult to make under most economic conditions. Our excess inventory review process includes analysis of sales forecasts and expected customer demand, careful management of product utilization and future purchasing and coordinating with manufacturing to maximize recovery of excess inventory. If actual market conditions are less favorable than those projected, additional inventory write-downs may be required. Inventory impairment charges establish a new cost basis for inventory and charges are not reversed subsequently to income, even if circumstances later suggest that increased carrying amounts are recoverable. If actual market conditions are more favorable than anticipated, inventory previously written down may be sold to customers, resulting in lower cost of sales and lower operating loss than expected in that period.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
We are a “smaller reporting company” as defined by Rule 12b-2 of the Exchange Act and are not required to provide the information otherwise required under this item.
Item 8. Financial Statements and Supplementary Data.
68
INDEX TO FINANCIAL STATEMENTS
Report of Independent Registered Public Accounting Firm (BDO USA, LLP; Los Angeles, California USA; PCAOB ID#: |
F-2 |
|
|
Consolidated Balance Sheets as of December 31, 2022 and 2021 |
F-4 |
|
|
Consolidated Statements of Operations for the Years ended December 31, 2022 and 2021 |
F-5 |
|
|
Consolidated Statements of Comprehensive Loss for the Years ended December 31, 2022 and 2021 |
F-6 |
|
|
F-7 |
|
|
|
Consolidated Statements of Cash Flows for the Years ended December 31, 2022 and 2021 |
F-8 |
|
|
F-9 |
F-1
Report of Independent Registered Public Accounting Firm
Stockholders and Board of Directors
HTG Molecular Diagnostics, Inc.
Tucson, Arizona
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of HTG Molecular Diagnostics, Inc. (the “Company”) as of December 31, 2022 and 2021, the related consolidated statements of operations, comprehensive loss, changes in stockholders’ equity, and cash flows for the years then ended, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2022 and 2021, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Going Concern Uncertainty
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has suffered recurring losses from operations and negative operating cash flows that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of the critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which it relates.
Reserve for Excess and Obsolete Inventory
As more fully described in Notes 2 and 3 to the consolidated financial statements, the Company’s consolidated inventory balance was approximately $1.3 million at December 31, 2022. Inventory, consisting of raw materials, work in process and finished goods, is stated at the lower of cost (first-in, first-out) or net realizable value. The Company reserves its inventory for estimated obsolescence or inventory in excess of expected sales or unmarketable inventory, based upon assumptions about future demand and market conditions.
F-2
We identified the valuation of inventories with respect to excess and obsolete inventory as a critical audit matter. Specifically, the determination of the reserve for excess and obsolete inventory requires management to make judgments and assumptions about the future usage and sales of inventory. Auditing these elements involved especially challenging auditor judgment due to the nature and extent of audit effort required to address this matter.
The primary procedures we performed to address this critical audit matter included:
/s/
We have served as the Company's auditor since 2014.
March 30, 2023
F-3
HTG Molecular Diagnostics, Inc.
Consolidated Balance Sheets
|
|
December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Assets |
|
|
|
|
|
|
||
Current assets: |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
|
|
$ |
|
||
Investments available-for-sale, at fair value |
|
|
|
|
|
|
||
Accounts receivable, net of allowance of $ |
|
|
|
|
|
|
||
Inventory, net |
|
|
|
|
|
|
||
Prepaid expenses and other |
|
|
|
|
|
|
||
Total current assets |
|
|
|
|
|
|
||
Operating lease right-of-use assets |
|
|
|
|
|
|
||
Property and equipment, net |
|
|
|
|
|
|
||
Other non-current assets |
|
|
|
|
|
|
||
Total assets |
|
$ |
|
|
$ |
|
||
Liabilities and stockholders’ equity |
|
|
|
|
|
|
||
Current liabilities: |
|
|
|
|
|
|
||
Accounts payable |
|
$ |
|
|
$ |
|
||
Accrued liabilities |
|
|
|
|
|
|
||
Current portion of long-term debt, net of discount and debt issuance costs |
|
|
|
|
|
|
||
NuvoGen obligation - current |
|
|
|
|
|
|
||
Operating lease liabilities - current |
|
|
|
|
|
|
||
Other current liabilities |
|
|
|
|
|
|
||
Total current liabilities |
|
|
|
|
|
|
||
NuvoGen obligation - non-current, net of discount |
|
|
|
|
|
|
||
Long-term debt, net of current portion, discount and debt issuance costs |
|
|
— |
|
|
|
|
|
Operating lease liabilities - non-current, net of discount |
|
|
|
|
|
|
||
Other non-current liabilities |
|
|
|
|
|
|
||
Total liabilities |
|
|
|
|
|
|
||
|
|
|
|
|
|
|||
Stockholders’ equity: |
|
|
|
|
|
|
||
Series A convertible preferred stock, $ |
|
|
— |
|
|
|
|
|
Common stock, $ |
|
|
|
|
|
|
||
Additional paid-in-capital |
|
|
|
|
|
|
||
Accumulated other comprehensive income |
|
|
|
|
|
|
||
Accumulated deficit |
|
|
( |
) |
|
|
( |
) |
Total stockholders’ equity |
|
|
|
|
|
|
||
Total liabilities and stockholders' equity |
|
$ |
|
|
$ |
|
||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
HTG Molecular Diagnostics, Inc.
Consolidated Statements of Operations
|
|
Years Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Product and product-related services revenue |
|
$ |
|
|
$ |
|
||
|
|
|
|
|
|
|
||
Operating expenses: |
|
|
|
|
|
|
||
Cost of product and product-related services revenue |
|
|
|
|
|
|
||
Selling, general and administrative |
|
|
|
|
|
|
||
Research and development |
|
|
|
|
|
|
||
Total operating expenses |
|
|
|
|
|
|
||
Operating loss |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
||
Other income (expense): |
|
|
|
|
|
|
||
Interest expense |
|
|
( |
) |
|
|
( |
) |
Interest income |
|
|
|
|
|
|
||
Other income |
|
|
|
|
|
|
||
Gain on forgiveness of PPP Loan |
|
|
|
|
|
|
||
Total other income (expense) |
|
|
( |
) |
|
|
|
|
Net loss before income taxes |
|
|
( |
) |
|
|
( |
) |
Provision for income taxes |
|
|
( |
) |
|
|
( |
) |
Net loss |
|
$ |
( |
) |
|
$ |
( |
) |
Net loss per share, basic and diluted |
|
$ |
( |
) |
|
$ |
( |
) |
Shares used in computing net loss per share, basic and diluted |
|
|
|
|
|
|
||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
HTG Molecular Diagnostics, Inc.
Consolidated Statements of Comprehensive Loss
|
|
Years Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Net loss |
|
$ |
( |
) |
|
$ |
( |
) |
Other comprehensive loss, net of tax effect: |
|
|
|
|
|
|
||
Foreign currency translation adjustment |
|
|
|
|
|
( |
) |
|
Comprehensive loss |
|
$ |
( |
) |
|
$ |
( |
) |
The accompanying notes are an integral part of these consolidated financial statements.
F-6
HTG Molecular Diagnostics, Inc.
Consolidated Statements of Changes in Stockholders’ Equity
|
|
Series A Convertible |
|
|
Common Stock |
|
|
Additional |
|
|
Accumulated |
|
|
Accumulated |
|
|
Total |
|
||||||||||||||
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Income (Loss) |
|
|
Deficit |
|
|
Equity |
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
Balance at January 1, 2021 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|||||||
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Release of restricted stock awards |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||||
Net share settlement of restricted stock awards |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
Employee stock purchase plan expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Stock issued under stock purchase plans |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||||
Issuance of common stock from ATM Offering, net of commissions of approximately $ |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||||
Issuance of common stock in connection with LP Purchase Agreement |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||||
Exercise of September 2019 Securities Purchase Agreement pre-funded warrants |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
||
Exercise of stock options |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|||
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
Foreign currency translation adjustment |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
Balance at December 31, 2021 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|||||||
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Release of restricted stock awards |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
||
Net share settlement of restricted stock awards |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
Employee stock purchase plan expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Stock issued under stock purchase plans |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||||
Conversion of Series A convertible preferred stock for common stock |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|||
Issuance of common stock and pre-funded warrants from March 2022 Securities Purchase Agreement, net of issuance costs of approximately $ |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||||
Issuance of common stock and pre-funded warrants from December 2022 Securities Purchase Agreement, net of commissions and issuance costs of approximately $ |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||||
Exercise of March 2022 Securities Purchase Agreement pre-funded warrants |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||||
Exercise of December 2022 Securities Purchase Agreement pre-funded warrants |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|||
Cash in lieu of fractional shares related to reverse stock split |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
Foreign currency translation adjustment |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|||
Balance at December 31, 2022 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
HTG Molecular Diagnostics, Inc.
Consolidated Statements of Cash Flows
|
|
Years Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Operating activities |
|
|
|
|
|
|
||
Net loss |
|
$ |
( |
) |
|
$ |
( |
) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
||
Depreciation and amortization |
|
|
|
|
|
|
||
Accretion of interest on NuvoGen obligation |
|
|
( |
) |
|
|
( |
) |
Provision for excess inventory |
|
|
|
|
|
|
||
Amortization of SVB Term Loan discount and issuance costs |
|
|
|
|
|
|
||
Stock-based compensation expense |
|
|
|
|
|
|
||
Employee stock purchase plan expense |
|
|
|
|
|
|
||
Bad debt expense |
|
|
|
|
|
|
||
Non-cash operating lease expense |
|
|
|
|
|
|
||
Accrued interest on available-for-sale securities investments |
|
|
|
|
|
( |
) |
|
Gain on forgiveness of PPP Loan |
|
|
|
|
|
( |
) |
|
(Gain) loss on abandonment and disposal of assets, net |
|
|
( |
) |
|
|
|
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
||
Accounts receivable |
|
|
|
|
|
( |
) |
|
Inventory |
|
|
|
|
|
( |
) |
|
Prepaid expenses and other |
|
|
|
|
|
|
||
Accounts payable |
|
|
( |
) |
|
|
|
|
Accrued liabilities |
|
|
( |
) |
|
|
|
|
Contract liabilities |
|
|
|
|
|
( |
) |
|
Operating lease liabilities |
|
|
( |
) |
|
|
( |
) |
Net cash used in operating activities |
|
|
( |
) |
|
|
( |
) |
Investing activities |
|
|
|
|
|
|
||
Purchase of property and equipment |
|
|
( |
) |
|
|
( |
) |
Proceeds from the sale of property and equipment |
|
|
|
|
|
|
||
Maturities of available-for-sale securities |
|
|
|
|
|
|
||
Purchase of available-for-sale securities |
|
|
( |
) |
|
|
( |
) |
Net cash (used in) provided by investing activities |
|
|
|
|
|
( |
) |
|
Financing activities |
|
|
|
|
|
|
||
Proceeds from ATM Offering, net of commissions of approximately $ |
|
|
|
|
|
|
||
Proceeds from March 2022 Securities Purchase Agreement, net of issuance costs of approximately $ |
|
|
|
|
|
|
||
Proceeds from LP Purchase Agreement |
|
|
|
|
|
|
||
Payments on NuvoGen obligation |
|
|
( |
) |
|
|
( |
) |
Payments on SVB Term Loan |
|
|
( |
) |
|
|
|
|
Payments on SVB Term Loan Amendment issuance costs |
|
|
( |
) |
|
|
|
|
Payments on deferred offering costs |
|
|
( |
) |
|
|
|
|
Payments on financing leases |
|
|
( |
) |
|
|
( |
) |
Proceeds from exercise of stock options |
|
|
|
|
|
|
||
Taxes paid for net share settlement of restricted stock awards |
|
|
( |
) |
|
|
( |
) |
Proceeds from shares purchased under stock purchase plans |
|
|
|
|
|
|
||
Proceeds from exercise of March 2022 Securities Purchase Agreement pre-funded warrants |
|
|
|
|
|
|
||
Proceeds from December 2022 Securities Purchase Agreement, net of commissions and issuance costs of approximately $ |
|
|
|
|
|
|
||
Proceeds from exercise of December 2022 Securities Purchase Agreement pre-funded warrants |
|
|
|
|
|
|
||
Cash in lieu of fractional shares related to reverse stock split |
|
|
( |
) |
|
|
|
|
Proceeds from insurance note |
|
|
|
|
|
|
||
Payments on insurance notes |
|
|
( |
) |
|
|
( |
) |
Net cash provided by financing activities |
|
|
|
|
|
|
||
Effect of exchange rates on cash |
|
|
( |
) |
|
|
( |
) |
Increase (decrease) in cash and cash equivalents |
|
|
|
|
|
( |
) |
|
Cash and cash equivalents at beginning of year |
|
|
|
|
|
|
||
Cash and cash equivalents at end of year |
|
$ |
|
|
$ |
|
||
Supplemental disclosure of noncash investing and financing activities |
|
|
|
|
|
|
||
Fixed asset purchases payable at year end |
|
$ |
|
|
$ |
|
||
Issuance costs payable and accrued at year end |
|
|
|
|
|
|
||
Issuance of common stock upon conversion of Series A convertible preferred stock |
|
|
|
|
|
— |
|
|
Issuance of common stock from cashless exercise of pre-funded warrants |
|
|
|
|
|
|
||
2021 Insurance Note issued for insurance premiums |
|
|
|
|
|
|
||
Gain on forgiveness of PPP Loan |
|
|
|
|
|
|
||
Operating lease right-of-use assets obtained in exchange for operating lease liabilities |
|
|
|
|
|
|
||
Carrying value of demonstration units transferred from property and equipment to inventory |
|
|
|
|
|
|
||
Disposal of fully depreciated assets |
|
|
|
|
|
|
||
Reclassification of instrument from inventory to property and equipment |
|
|
|
|
|
|
||
Supplemental cash flow information |
|
|
|
|
|
|
||
Cash paid for interest |
|
$ |
|
|
$ |
|
||
Cash paid for taxes |
|
|
|
|
|
|
||
The accompanying notes are an integral part of these consolidated financial statements.
F-8
HTG Molecular Diagnostics, Inc.
Notes to Consolidated Financial Statements
Note 1. Description of Business and Basis of Presentation
HTG Molecular Diagnostics, Inc. (the “Company”) is a life science company whose mission is to advance precision medicine through its innovative transcriptome-wide profiling technology and advanced medicinal chemistry technology. The Company derives revenue primarily from sales of its HTG EdgeSeq system and integrated next-generation sequencing-based (“NGS-based”) HTG EdgeSeq research use only (“RUO”) assays and from sample processing services performed in its VERI/O laboratory.
The Company operates in
Basis of Presentation
The consolidated financial statements and accompanying notes were prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”).
Principles of Consolidation
The Company formed a French subsidiary, HTG Molecular Diagnostics France SARL, in November 2018. The consolidated financial statements include the accounts of the Company and this wholly owned subsidiary after elimination of intercompany transactions and balances as of December 31, 2022 and 2021.
Going Concern and Liquidity
Management has assessed the Company’s ability to continue as a going concern within one year of issuance of these consolidated financial statements. The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates the realization of the assets and satisfaction of liabilities in the normal course of business. However, the Company has had recurring operating losses and negative operating cash flows since its inception and has an accumulated deficit of approximately $
F-9
The Company will need to raise additional capital to fund its operations and service its long-term debt obligations until its revenue reaches a level sufficient to provide for self-sustaining cash flows. There can be no assurance that additional capital will be available on acceptable terms, or at all, or that the Company’s revenue will reach a level sufficient to provide for self-sustaining cash flows. If the Company is not able to generate additional capital, the Company may have to delay, scale back or discontinue one or more of its therapeutics development programs, curtail its commercial activities, significantly reduce expenses, sell assets (potentially at a discount to their fair value or carrying value), enter into relationships with third parties to develop or commercialize products or technologies that the Company otherwise would have sought to develop or commercialize independently, cease operations altogether, pursue a sale of the Company at a price that may result in a significant loss on investment for its stockholders, file for bankruptcy or seek other protection from creditors. In addition, if the Company defaults under any of the provisions of the Loan and Security Agreement for the SVB Term Loan (the "Loan Agreement"), SVB could charge an interest rate of
COVID-19 Pandemic and Relief
The Company experienced a significant slowing of product and product-related services revenue generation beginning in March 2020 as a result of COVID-19. The extent of this impact has varied from customer to customer depending upon how they have been directly or indirectly impacted by local stay-at-home orders and other social distancing measures, how they have prioritized studies and previously planned trials as the immediate impacts of the pandemic have passed, and how significantly their workforces and supplier networks have been impacted by the pandemic. The Company has not experienced delays in development even with its efforts to prioritize the safety of its employees during the pandemic. In addition, the impact of COVID-19 on the Company’s ability to source raw materials and other supplies has not been significant to date. However, a change in or loss of suppliers or other supply chain or distribution network partners due to the ongoing impacts of the pandemic or a resurgence of COVID-19 on the global economy could adversely affect the Company’s business and the business of its vendors, partners and customers, and could result in future reductions in sales and operating results.
While there remains uncertainty as to the future impact of COVID-19, the Company has considered the known impacts on its business as of the date these consolidated financial statements were issued and has reflected any known or expected impacts in its consolidated financial statements, including consideration of potential impairment risks to its long-lived assets, potential accounts receivable collection risks and potential impacts to its overall liquidity position.
As a result of various government programs enacted to address the ongoing impacts of COVID-19, the Company was able to qualify for and receive Employee Retention Credits (“ERC”) during the year ended December 31, 2021. ERC benefits of approximately $
In April 2020, the Company received proceeds from a loan pursuant to the Paycheck Protection Program (“PPP”) of the CARES Act (the “PPP Loan”) in the amount of approximately $
Laws and regulations concerning government programs, including the ERC and PPP Loan, are complex and subject to varying interpretations. Claims made under these programs may also be subject to retroactive audit and review. While the Company does not believe there is a basis for estimation of an audit or recapture risk at this time, there can be no assurance that regulatory authorities will not challenge the Company’s claim to the ERC or PPP Loan in a future period.
F-10
Note 2. Summary of Significant Accounting Policies
Use of Estimates
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosures of contingent assets and liabilities at the date of the consolidated financial statements, and the reported amounts of revenues and expenses during the reporting period. The Company’s estimates include revenue recognition, stock-based compensation expense, bonus and warranty accrual, income tax valuation allowances and reserves, recovery of long‑lived assets, lease liability, inventory valuation, allowance for doubtful accounts and available-for-sale securities. Actual results could materially differ from those estimates.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less to be cash equivalents. Cash and cash equivalents consist of cash on deposit with financial institutions, money market instruments and high credit quality corporate debt securities purchased with a term of three months or less.
Accounts Receivable
Accounts receivable represent valid claims against debtors. Management reviews accounts receivable regularly to determine, using the specific identification method, if any receivable amounts will potentially be uncollectible and to estimate the amount of allowance for doubtful accounts necessary to reduce accounts receivable to its estimated net realizable value.
Investments in Available-for-Sale Securities
The Company classifies its debt securities, which are reported at estimated fair value with unrealized gains and losses included in accumulated other comprehensive income, net of tax, as available-for-sale securities. Investments in securities with maturities of
The Company recognizes other-than-temporary impairment (“OTTI”) of a debt security for which there has been a decline in fair value below amortized cost if (i) management intends to sell the security, (ii) it is more likely than not that the Company will be required to sell the security before recovery of its amortized cost basis, or (iii) the Company does not expect to recover the entire amortized cost basis of the security. The amount by which amortized cost exceeds the fair value of a debt security that is considered to have OTTI is separated into a component representing the credit loss, which is recognized in earnings, and a component related to all other factors, which is recognized in other comprehensive loss. The measurement of the credit loss component is equal to the difference between the debt security’s amortized cost basis and the present value of its expected future cash flows discounted at the security’s effective yield. If the Company intends to sell the security, or if it is more likely than not it will be required to sell the security before recovery, an OTTI write-down is recognized in earnings equal to the entire difference between the amortized cost basis and fair value of the security. As the Company did
F-11
Fair Value of Financial Instruments
Fair value measurements are based on the premise that fair value is an exit price representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions, the following three-tier fair value hierarchy has been used in determining the inputs used in measuring fair value:
Level 1 |
|
– |
|
Quoted prices in active markets for identical assets or liabilities on the reporting date. |
|
|
|
|
|
Level 2 |
|
– |
|
Pricing inputs are based on quoted prices for similar instruments in active markets, quoted prices for identical or similar instruments in markets that are not active and model-based valuation techniques for which all significant assumptions are observable in the market or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
|
|
|
|
|
Level 3 |
|
– |
|
Pricing inputs are generally unobservable and include situations where there is little, if any, market activity for the investment. The inputs into the determination of fair value require management’s judgment or estimation of assumptions that market participants would use in pricing the assets or liabilities. The fair values are therefore determined using factors that involve considerable judgment and interpretations, including but not limited to private and public comparables, third-party appraisals, discounted cash flow models, and fund manager estimates. |
The carrying value of financial instruments classified as current assets and current liabilities approximate fair value due to their liquidity and short-term nature. Investments that are classified as available-for-sale are recorded at fair value, which is determined using quoted market prices, broker or dealer quotations or alternative pricing sources with reasonable levels of price transparency. The carrying value of the SVB Term Loan (see Note 8) is estimated to approximate its fair value as the interest rate approximates the market rate for debt with similar terms and risk characteristics.
The NuvoGen obligation relates to an asset purchase transaction with a then-common stockholder of the Company (see Note 10). As of December 31, 2022, the estimated aggregate fair value of the NuvoGen obligation is approximately $
Inventory
Inventory is stated at the lower of cost or net realizable value. The Company determines the cost of inventory using the first-in, first out method. The Company estimates the recoverability of inventory by reference to internal estimates of future demands and product life cycles, including expiration. The Company periodically analyzes its inventory levels to identify inventory that may expire prior to expected sale or has a cost basis in excess of its estimated realizable value and records a charge to expense for such inventory as appropriate. The Company classifies inventory as long-term when it expects to utilize the inventory beyond its normal operating cycle.
The Company charges cost of sales for inventory provisions to write-down our inventory to the lower of cost or net realizable value or for obsolete or excess inventory. Most of its inventory provisions relate to excess quantities of products, based on our inventory levels and future product purchase commitments compared to assumptions about future demand and market conditions. Once inventory has been written-off or written-down, it creates a new cost basis for the inventory that is not subsequently written-up, any increase in demand forecasted or inventory value of such inventory is not realized until such inventory is sold.
Equipment that is under evaluation for purchase remains in inventory as the Company maintains title to the equipment throughout the evaluation period. The period of time customers use to evaluate the Company’s equipment generally ranges from
F-12
Property and Equipment
Property and equipment are stated at historical cost and depreciated over their useful lives, which range from to
Costs incurred in the development and installation of software for internal use and in the development of the Company’s website are expensed or capitalized, depending on whether they are incurred in the preliminary project stage (expensed), application development stage (capitalized), or post-implementation stage (expensed). Amounts capitalized following project completion are amortized on a straight-line basis over the useful life of the developed asset, which is generally
Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset group to the estimated undiscounted future cash flows expected to be generated by the asset group. If the carrying amount of an asset group exceeds its estimated future cash flow, an impairment charge is recognized in the amount by which the carrying amount of the asset group exceeds the fair value of the asset group. Although the Company has accumulated losses since inception, the Company believes the future cash flows will be sufficient to exceed the carrying value of the Company’s long-lived assets. There were
Leases
Arrangements meeting the definition of a lease are classified as operating or financing leases and are recorded on the consolidated balance sheets as both a right-of-use asset and a lease liability for each type of lease, calculated by discounting fixed lease payments over the lease term at the rate implicit in the lease or the Company’s incremental borrowing rate. Lease liabilities are increased by interest and reduced by payments each period, and the right-of-use asset is amortized over the lease term. For operating leases, interest on the lease liability and the amortization of the right-of-use asset result in straight-line rent expense over the lease term. For financing leases, interest on the lease liability and the amortization of the right-of-use asset results in front-loaded expense over the lease term. Variable lease expenses are recorded to rent expense as incurred.
In calculating the right-of-use asset and lease liability, the Company elects to combine lease and non-lease components for all classes of assets currently under lease, including facilities and computer equipment. The Company excludes short-term leases having initial terms of 12 months or less as an accounting policy election and recognizes rent expense on short-term leases on a straight-line basis over the lease term for these leases.
Debt Issuance Costs and Debt Discounts
Costs incurred to issue non-revolving debt instruments are recognized as a reduction to the related debt balance in the consolidated balance sheets and amortized to interest expense over the contractual term of the related debt using the effective interest method. Costs incurred to issue the Loan Agreement with SVB were deferred as an asset in the consolidated balance sheets and are being amortized on a straight-line basis to interest expense over the term of the loan (see Note 8).
Contract Liabilities
Contract liabilities represent cash receipts for products or services to be delivered in future periods. When products or services are delivered to customers, contract liabilities are recognized as earned. Up-front fees received for custom RUO assay design are recognized over time based on the costs incurred to date compared with total expected costs as design or development procedures are completed and outputs are produced.
F-13
Revenue Recognition
Revenue from contracts with customers is recognized when, or as, the Company satisfies its performance obligations by delivering the promised goods or service deliverables to the customers. A good or service deliverable is transferred to a customer when, or as, the customer obtains control of that good or service deliverable. A performance obligation may be satisfied over time or at a point in time. Revenue from a performance obligation satisfied over time is recognized by measuring the Company’s progress in satisfying the performance obligation in a manner that depicts the transfer of the goods or services to the customer. Revenue from a performance obligation satisfied at a point in time is recognized at the point in time that the Company determines the customer obtains control over the promised good or service deliverable. The amount of revenue recognized reflects the consideration the Company expects to be entitled to in exchange for those promised goods or services (i.e., the “transaction price”). In determining the transaction price, the Company considers multiple factors, including the effects of variable consideration. Variable consideration is included in the transaction price only to the extent it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainties with respect to the amount are resolved. In determining when to include variable consideration in the transaction price, the Company considers the range of possible outcomes, the predictive value of its past experiences, the time period of when uncertainties expect to be resolved and the amount of consideration that is susceptible to factors outside of the Company’s influence, such as the judgment and actions of third parties.
For contracts where the period between when the Company transfers a promised good or service to the customer and when the customer pays is one year or less, the Company has elected the practical expedient to not adjust the promised amount of consideration for the effects of a significant financing component.
As the Company’s agreements for product and product-related services revenue have an expected duration of one year or less, the Company has elected the practical expedient to not disclose information about its remaining performance obligations.
The Company has also made a policy election to exclude from the measurement of the transaction price all taxes assessed by a government authority that are both imposed on and concurrent with a specific revenue producing transaction and collected by the Company from a customer. Such taxes may include but are not limited to sales, use, value added and certain excise taxes.
See Note 9 for additional discussion of the Company’s revenue recognition policies.
Product Warranty
The Company generally provides a one-year warranty on its HTG EdgeSeq platform covering the performance of system hardware and software in conformance with customer specifications under normal use and protecting against defects in materials and workmanship. The Company may, at its option, replace, repair or exchange products covered under valid warranty claims. A provision for estimated warranty costs is recognized at the time of sale, through cost of product and product-related services revenue, based upon recent historical experience and other relevant information as it becomes available. Customers have the option to purchase an extended warranty after the one-year warranty period expires. The Company continuously assesses the adequacy of its product warranty accrual by reviewing actual claims and adjusts the provision as needed. Warranty accrual is included in accrued liabilities and other non-current liabilities in the consolidated balance sheets.
Research and Development Expenses
Research and development expenses represent costs incurred internally for and externally in support of research and development activities. These costs include those generated through research and development efforts for the improvement and expansion of the Company’s proprietary profiling technology and product offerings, and payroll, related expenses, consulting expenses, laboratory supplies, facilities and equipment costs incurred to complete development milestones associated with the Company's transcriptome-informed drug discovery business, HTG Therapeutics.
Stock-based Compensation
The Company incurs stock-based compensation expense relating to grants of restricted stock units (“RSUs”) and stock options to employees, consultants and non-employee directors under its equity incentive plans, and stock purchase rights granted under its employee stock purchase plans. The Company recognizes expense for stock-based awards based on the fair value of awards on the date of grant. The fair value of RSUs is based on the quoted market price of the Company’s common stock on the date of grant. The fair value of stock purchase rights and stock options granted pursuant to the Company’s equity incentive plans is estimated on the date of grant using the Black-Scholes option pricing model. The determination of the fair value utilizing the Black-Scholes option pricing model is affected by the fair value of the Company’s stock price and several assumptions, including volatility, expected term, risk-free interest rate, and dividend yield. Generally, these assumptions are based on historical information and judgment is required to determine if historical trends may be indicators of future outcomes. The Company accounts for forfeitures as they occur.
F-14
Income Taxes
The Company accounts for income taxes under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to the differences between the financial statement carrying amounts and tax base of assets and liabilities using enacted tax rates and laws that will be in effect when the differences are expected to reverse. A valuation allowance is established against net deferred tax assets for the uncertainty it presents of our ability to use the net deferred tax assets, in this case, primarily carryforwards of net operating tax losses and research and development tax credits. In assessing the realizability of net deferred tax assets the Company has assessed the likelihood that net deferred tax assets will be recovered from future taxable income, and to the extent that it is “more likely than not” that the assets will not be recovered or there is an insufficient history of operating profits, a valuation allowance is established. The Company records the valuation allowance in the period it determines that it is more likely than not that net deferred tax assets will not be realized. For the years ended December 31, 2022 and 2021, the Company has provided a full valuation allowance for all net deferred tax assets due to their current realization being considered remote in the near term. Uncertain tax positions taken or expected to be taken in a tax return are accounted for using the more likely than not threshold for financial statement recognition and measurement. Therefore, for income tax positions where it is not more likely than not that a tax benefit will be sustained in a court of last resort, the Company does not recognize a tax benefit in its financial statements.
Foreign Currency Translation and Foreign Currency Transactions
The Company has assets and liabilities, including accounts receivable and accounts payable, which are denominated in currencies other than its functional currency. These assets and liabilities are subject to re-measurement, the impact of which is recorded in selling, general and administrative expense within the consolidated statements of operations.
Adjustments resulting from translating foreign functional currency financial statements of the Company’s wholly owned subsidiary into U.S. Dollars are included in the foreign currency translation adjustment, a component of accumulated other comprehensive income in the consolidated statements of changes in stockholders' equity.
Comprehensive Loss
Comprehensive loss includes certain changes in equity that are excluded from net loss. Specifically, unrealized gains and losses on short-term available-for-sale investments and adjustments resulting from translating foreign functional currency financial statements into U.S. Dollars are included in comprehensive loss.
Concentration Risks
Financial instruments that potentially subject the Company to credit risk consist principally of cash and cash equivalents and accounts receivable. The Company maintains the majority of its cash balances in the form of cash deposits in checking and money market accounts in amounts in excess of federally insured limits. In accordance with the Loan Agreement, as of December 31, 2022, the Company's cash balances were held in operating accounts at and in a custodial account at U.S. Bank subject to a control agreement with Silicon Valley Bank. On March 12, 2023, the U.S. Treasury, Federal Reserve and FDIC announced that SVB depositors will have access to all of their money beginning on March 13, 2023. Management believes that the credit risk with regard to these deposits is not significant based on the quality of the financial institution or, with respect to deposits with SVB, as a result of the guarantee provided by the U.S. Treasury, Federal Reserve and FDIC.
The Company sells its instruments, consumables, sample processing services, custom RUO assay design and collaborative development services primarily to biopharmaceutical companies, academic institutions and molecular labs. The Company routinely assesses the financial strength of its customers and credit losses have been minimal to date.
The Company’s top
F-15
The Company is also subject to supply chain risks related to the reliance on a single supplier to manufacture a subcomponent used in its HTG EdgeSeq instruments. Although there are a limited number of manufacturers for components of this type, the Company believes that other suppliers could provide similar products on comparable terms. However, a change in or loss of this supplier could significantly delay the delivery of products, which in turn would materially affect the Company’s ability to generate revenue.
Recent Accounting Pronouncements
The following are new FASB Accounting Standard Updates ("ASU") that had not been adopted by the Company as of December 31, 2022. The Company's management does not believe that any other recently issued, but not yet effective, accounting standards updates, if currently adopted, would have a material effect on the accompanying consolidated financial statements.
In June 2022, the FASB issued ASU 2022-03, Fair Value Measurement of Equity Securities Subject to Contractual Sale Restrictions ("ASU 2022-03"), which amends ASC 820 to clarify that a contractual sales restriction is not considered in measuring an equity security at fair value and to introduce new disclosure requirements for equity securities subject to contractual sale restrictions that are measured at fair value. ASU 2022-03 applies to both holders and issuers of equity and equity-linked securities measured at fair value. The amendments in this ASU are effective for the Company in fiscal years and interim periods within those fiscal years, beginning after December 15, 2023. Early adoption is permitted for both interim and annual financial statements that have not yet been issued or made available for issuance. The Company is still evaluating the impact of this pronouncement on the financial statements.
In August 2020, the FASB issued ASU No. 2020-06, Debt – Debt with Conversion and other Options (Subtopic 470-20) and Derivatives and Hedging – Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (“ASU 2020-06”), which simplifies accounting for convertible instruments by removing major separation models required under current GAAP. ASU 2020-06 removes certain settlement conditions that are required for equity contracts to qualify for the derivative scope exception and also simplifies the diluted earnings per share calculation in certain areas. The standard is effective for the Company effective for fiscal years beginning after December 15, 2023. Early adoption is permitted, and adoption must be as of the beginning of the Company’s annual fiscal year. The Company is currently evaluating the impact of this standard on its consolidated financial statements and related disclosures.
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments - Credit Losses, which was subsequently amended by ASU 2018-19, ASU 2019-10 and ASU 2020-02, and requires the measurement of expected credit losses for financial instruments carried at amortized cost held at the reporting date based on historical experience, current conditions and reasonable forecasts. The updated guidance also amends the current other-than-temporary impairment model for available-for-sale debt securities by requiring the recognition of impairments relating to credit losses through an allowance account and limits the amount of credit loss to the difference between a security’s amortized cost basis and its fair value. In addition, the length of time a security has been in an unrealized loss position will no longer impact the determination of whether a credit loss exists. The main objective of this ASU is to provide financial statement users with more decision-useful information about the expected credit losses on financial instruments and other commitments to extend credit held by a reporting entity at each reporting date. With the issuance of ASU 2019-10 in November 2019, the standard will be effective for the Company for fiscal years and interim periods within those fiscal years beginning after December 15, 2022. The Company's adoption of this standard on January 1, 2023 is not expected to have a material impact on its consolidated financial statements or related footnote disclosures, given the high credit quality of the obligors to its available-for-sale debt securities and its history of minimal bad debt expense relating to trade accounts receivable.
Note 3. Inventory
Inventory - current, net of allowance, consisted of the following as of the dates indicated:
|
|
December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Raw materials |
|
$ |
|
|
$ |
|
||
Work in process |
|
|
|
|
|
|
||
Finished goods |
|
|
|
|
|
|
||
Total gross inventory - current |
|
|
|
|
|
|
||
Less general inventory allowance |
|
|
( |
) |
|
|
( |
) |
|
|
$ |
|
|
$ |
|
||
F-16
Inventory - non-current, net of excess inventory allowance, included in other non-current assets on the consolidated balance sheets, consisted of the following as of the dates indicated:
|
|
December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Raw materials - non-current, net |
|
$ |
|
|
$ |
|
||
Work in process - non-current, net |
|
|
|
|
|
|
||
Finished goods - non-current |
|
|
|
|
|
|
||
|
|
$ |
|
|
$ |
|
||
For the year ended December 31, 2022, the Company recorded adjustments to its specific inventory reserve of $
For the year ended December 31, 2021, the Company recorded adjustments to the general inventory allowance of approximately $
Note 4. Fair Value
Financial assets and liabilities measured at fair value are classified in their entirety in the fair value hierarchy, based on the lowest level input significant to the fair value measurement.
|
|
December 31, 2022 |
|
|||||||||||||
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
Asset included in: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Cash and cash equivalents |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Money market securities |
|
$ |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
|
||
Total |
|
$ |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
December 31, 2021 |
|
|||||||||||||
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
Asset included in: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Cash and cash equivalents |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Money market securities |
|
$ |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
|
||
Investments available-for-sale at fair value |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Corporate debt securities |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
||
Total |
|
$ |
|
|
$ |
|
|
$ |
— |
|
|
$ |
|
|||
There were no other financial instruments subject to fair value measurement on a recurring basis. Transfers to and from Levels 1, 2 and 3 are recognized at the end of the reporting period. There were
Level 1 instruments include investments in money market securities. These instruments are valued using quoted market prices for identical unrestricted instruments in active markets. The Company defines active markets for debt instruments based on both the average daily trading volume and the number of days with trading activity. Level 2 instruments as of December 31, 2021 included corporate debt securities, including commercial paper and corporate bonds. Valuations of Level 2 instruments can be verified to quoted prices, recent trading activity for identical or similar instruments, broker or dealer quotations or alternative pricing sources with reasonable levels of price transparency. Consideration is given to the nature of the quotations (e.g. indicative or firm) and the relationship of recent market activity to the prices provided from alternative pricing sources.
F-17
Fair values of these assets are based on prices provided by independent market participants that are based on observable inputs using market-based valuation techniques. These valuation models and analytical tools use market pricing or similar instruments that are both objective and publicly available, including matrix pricing or reported trades, benchmark yields, broker/dealer quotes, issuer spreads, two-sided markets, benchmark securities, bids and/or offers. The Company did not adjust any of the valuations received from these third parties with respect to any of its Level 1 or 2 securities for either of the years ended December 31, 2022 or 2021 and did not have any Level 3 financial assets or liabilities during either of these periods.
Note 5. Available-for-Sale Securities
The Company did
|
December 31, 2021 |
|
|||||||||||||
|
|
|
|
Gross |
|
|
Gross |
|
|
Fair Value |
|
||||
|
Amortized |
|
|
Unrealized |
|
|
Unrealized |
|
|
(Net Carrying |
|
||||
|
Cost |
|
|
Gains |
|
|
Losses |
|
|
Amount) |
|
||||
Corporate debt securities |
$ |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
|
||
Total available-for-sale securities |
$ |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
|
||
There were
Note 6. Property and Equipment
Property and equipment, net consisted of the following as of the dates indicated:
|
|
December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Furniture & fixtures |
|
$ |
|
|
$ |
|
||
Leasehold improvements |
|
|
|
|
|
|
||
Equipment used in manufacturing |
|
|
|
|
|
|
||
Equipment used in research & development |
|
|
|
|
|
|
||
Equipment used in the field |
|
|
|
|
|
|
||
Software |
|
|
|
|
|
|
||
Property and equipment |
|
|
|
|
|
|
||
Less: accumulated depreciation and amortization |
|
|
( |
) |
|
|
( |
) |
|
|
$ |
|
|
$ |
|
||
Depreciation and leasehold improvement amortization expense was approximately $
Note 7. Accrued Liabilities
Accrued liabilities consisted of the following as of the dates indicated:
|
|
December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Accrued employee bonuses |
|
$ |
|
|
$ |
|
||
Payroll and employee benefit accruals |
|
|
|
|
|
|
||
Accrued professional fees |
|
|
|
|
|
|
||
Other accrued liabilities |
|
|
|
|
|
|
||
|
|
$ |
|
|
$ |
|
||
F-18
Note 8. Debt Obligations
Current portion of long-term debt consisted of the following as of the dates indicated:
|
|
December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
SVB Term Loan, net of discount and debt issuance costs |
|
$ |
|
|
$ |
|
||
2021 Insurance Note |
|
|
|
|
|
|
||
|
|
$ |
|
|
$ |
|
||
Long-term debt, net of current portion, discount and debt issuance costs, consisted of the following as of the dates indicated:
|
|
December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
SVB Term Loan, net of discount and debt issuance costs |
|
$ |
|
|
$ |
|
||
SVB Term Loan
On June 24, 2020 (the “Closing Date”), the Company entered into the SVB Term Loan with SVB, which provided a secured term loan in the principal amount of $
The SVB Term Loan bears interest at a floating rate equal to the greater of
Prepayments of the SVB Term Loan, in whole or in part, are subject to early termination fees in an amount equal to
In July 2022, the Company and SVB entered into an amendment to the SVB Term Loan (the "Term Loan Amendment"). Under the Term Loan Amendment, the Company and SVB agreed to remove the financial covenant under the Loan Agreement that had required the Company to maintain unrestricted cash, including short term investments available-for-sale, of not less than the greater of (i) $
On March 10, 2023, the FDIC took control and was appointed receiver of SVB. The SVB Term Loan remains intact and the Company will continue to make required payments through the end of the current year, at which time the SVB Term Loan will be repaid in full.
The Company’s obligations under the Loan Agreement are secured by a security interest in substantially all of its assets, excluding intellectual property (which is subject to a negative pledge), and the Company’s future subsidiaries, if any, may be required to become co-borrowers or guarantors under the Loan Agreement. If we default under our obligations under the SVB Term Loan, including as a result of a material adverse change, as defined in the SVB Term Loan, the lender could proceed against the collateral granted to them to secure our indebtedness or declare all obligations under the SVB Term Loan to be due and payable. The determination as to whether a material adverse change has occurred is not within the Company's control and it is unclear how the current managers of Silicon Valley Bridge Bank will view the SVB Term Loan from a risk standpoint and what actions they may elect to take under the SVB Term Loan to protect the financial interests of the lender.
F-19
The remaining principal repayments due under the SVB Term Loan as of December 31, 2022 are as follows:
2023 |
|
$ |
|
|
Less discount and deferred financing costs |
|
|
( |
) |
Plus final fee premium |
|
|
|
|
Total SVB Term Loan, net |
|
$ |
|
The Company included $
Insurance Note
In May 2021, the Company entered into a new commercial financing agreement to extend the payment period related to its directors and officers insurance policy (the “2021 Insurance Note”). The 2021 Insurance Note required a down payment to be made upon signing the agreement equal to approximately $
In May 2022, the Company entered into a new commercial financing agreement to extend the payment period related to its directors and officers insurance policy (the "2022 Insurance Note"). The 2022 Insurance Note required a down payment to be made upon signing the agreement equal to approximately $
Note 9. Revenue from Contracts with Customers
Product and Product-related Services Revenue
The Company had product and product-related services revenue consisting of revenue from the sale of instruments and consumables and the use of the HTG EdgeSeq proprietary technology to process samples and design custom RUO assays for the years ended December 31, 2022 and 2021 as follows:
|
|
Years Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Product revenue: |
|
|
|
|
|
|
||
Instrument |
|
$ |
|
|
$ |
|
||
Consumables |
|
|
|
|
|
|
||
Total product revenue |
|
|
|
|
|
|
||
Product-related services revenue: |
|
|
|
|
|
|
||
Custom RUO assay design |
|
|
|
|
|
|
||
RUO sample processing |
|
|
|
|
|
|
||
Total product-related services revenue |
|
|
|
|
|
|
||
Total product and product-related services revenue |
|
$ |
|
|
$ |
|
||
Revenue by primary geographic market for the years ended December 31, 2022 and 2021 was as follows:
|
|
December 31, 2022 |
|
|||||||||
|
|
United States |
|
|
Europe |
|
|
Other |
|
|||
Product revenue |
|
|
|
|
|
|
|
|
|
|||
Product-related services revenue |
|
|
|
|
|
|
|
|
|
|||
Total product and product-related services revenue |
|
$ |
|
|
$ |
|
|
$ |
|
|||
F-20
|
|
December 31, 2021 |
|
|||||||||
|
|
United States |
|
|
Europe |
|
|
Other |
|
|||
Product revenue |
|
|
|
|
|
|
|
|
|
|||
Product-related services revenue |
|
|
|
|
|
|
|
|
|
|||
Total product and product-related services revenue |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Sale of instruments and consumables
The delivery of each instrument and the related installation and calibration are considered to be a single performance obligation, as the HTG EdgeSeq instrument must be professionally installed and calibrated prior to use. Instrument product revenue is generally recognized upon installation and calibration of the instrument by field service engineers, which represents the point at which the customer has the ability to use the instrument and has accepted the asset. Installation generally occurs within one month of instrument shipment.
The delivery of each consumable is a separate performance obligation. Consumables revenue is recognized upon transfer of control, which represents the point when the customer has legal title and the significant risks of ownership of the asset. The Company’s standard terms and conditions provide that no right of return exists for instruments and consumables, unless replacement is necessary due to delivery of defective or damaged product. Customer payment terms vary but are typically between
Shipping and handling fees charged to customers for instruments shipped are included in the consolidated statements of operations as part of product and product-related services revenue. Shipping and handling costs for products shipped to customers are included in the consolidated statements of operations as part of cost of product and product-related services revenue. We have elected the practical expedient to account for shipping and handling as activities to fulfill the promise to transfer the consumables.
The Company provides instruments to certain customers under reagent rental agreements. Under these agreements, the Company installs an instrument in the customer’s facility without a fee and the customer agrees to purchase consumable products at a stated price over the term of the agreement; in some instances, the agreements do not contain a minimum purchase requirement. Terms range from several months to multiple years and may automatically renew in several month or multiple year increments unless either party notifies the other in advance that the agreement will not renew. The Company measures progress toward complete satisfaction of this performance obligation to provide the instrument and deliver the consumables using an output method based on the number of consumables delivered in relation to the total consumables to be provided under the reagent rental agreement. This is considered to be representative of the delivery of outputs under the arrangement and the best measure of progress because the customer benefits from the instrument only in conjunction with the consumables. The Company expects to recover the cost of the instrument under the agreement through the fees charged for consumables, to the extent sold, over the term of the agreement.
RUO Sample Processing
The Company also provides sample preparation and processing services and molecular profiling of retrospective cohorts for its customers through its VERI/O laboratory, whereby the customer provides samples to be processed using HTG EdgeSeq technology specified in the order. Customers are charged a per sample fee for sample processing services which is recognized as revenue upon delivery of a data file to the customer showing the results of testing and completing delivery of the agreed upon service. This is when the customer can use and benefit from the results of testing and the Company has the present right to payment.
Custom RUO Assay Design
The Company enters into custom RUO assay design agreements that may generate up-front fees and subsequent payments that may be earned upon completion of design process phases. The Company measures progress toward complete satisfaction of its performance obligation to perform custom RUO assay design procedures using an output method based on the costs incurred to date compared with total expected costs, as this is representative of the delivery of outputs under the arrangements and the best measure of progress. However, because in most instances the assay development fees are contingent upon completion of each phase of the design project and the decision of the customer to proceed to the next phase, the amount to be included in the transaction price and recognized as revenue is limited to that which the customer is contractually obligated to pay upon completion of that phase, which is when it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur. Changes in estimates of total expected costs are accounted for prospectively as a change in estimate. From period to period, custom RUO design services revenue can fluctuate substantially based on the completion of design-related phases.
The Company did
F-21
Contract Liabilities
The Company may receive up-front payments from customers for custom RUO assay design and sample processing services. In addition, payments for instrument extended warranty contracts are required to be made in advance. The Company recognizes such up-front payments as contract liabilities. The contract liabilities are subsequently reduced as revenue is recognized. Contract liabilities of approximately $
Changes in the Company’s contract liabilities were as follows as of the dates indicated:
|
|
Product |
|
|
Sample |
|
|
Total Contract |
|
|||
Balance at January 1, 2022 |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Deferral of revenue |
|
|
|
|
|
|
|
|
|
|||
Recognition of deferred revenue |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Balance at December 31, 2022 |
|
$ |
|
|
$ |
|
|
$ |
|
|||
|
|
Product |
|
|
Sample |
|
|
Total Contract |
|
|||
Balance at January 1, 2021 |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Deferral of revenue |
|
|
|
|
|
|
|
|
|
|||
Recognition of deferred revenue |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Balance at December 31, 2021 |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Note 10. Other Agreements
NuvoGen Obligation
The Company entered into an asset purchase agreement in 2001, as amended, with NuvoGen Research, LLC (“NuvoGen”) to acquire certain intellectual property from NuvoGen. The Company accounted for the transaction as an asset acquisition. However, as the intellectual property was determined to not have an alternative future use, the upfront consideration was expensed. In exchange for the intellectual property, the Company agreed to pay total aggregate cash compensation to NuvoGen under the agreement of $
Pursuant to the latest amendment to the agreement, the Company is obligated to pay the greater of $
Minimum payments to be made in 2023 include $
2023 |
|
$ |
|
|
2024 |
|
|
|
|
2025 |
|
|
|
|
2026 |
|
|
|
|
2027 |
|
|
|
|
2028 and beyond |
|
|
|
|
Total NuvoGen obligation payments |
|
|
|
|
Plus interest accretion |
|
|
|
|
Total NuvoGen obligation, net |
|
$ |
|
F-22
The Company recorded the obligation at the estimated present value of the future payments using a discount rate of
Note 11. Leases
Operating Leases
The Company leases office space under agreements classified as operating leases.
In the fourth quarter of 2022, the Company recorded an increase to its operating lease liability as the result of leasehold improvements financed by the landlord, to be repaid in equal installments over the remaining term of the lease.
In the first quarter of 2021, the Company closed its development laboratory in San Carlos, California and, as a result, $
Variable expenses generally represent the Company’s share of the landlord’s operating expenses and are recorded when incurred. Incremental borrowing rates used to discount future lease payments in calculating lease liabilities were estimated by reference to the rates for similar length secured lines of credit to the Company’s lease agreements provided by the Company’s lenders at the time that the lease liabilities were recorded, as these rates represented the cost of borrowing for secured loans of similar duration. The Company does not have any operating lease arrangements where it acts as a lessor.
The components of lease cost for operating leases were as follows:
|
|
Years Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Operating leases |
|
|
|
|
|
|
||
Operating lease cost |
|
$ |
|
|
$ |
|
||
Variable lease cost |
|
|
|
|
|
|
||
Total rent expense |
|
$ |
|
|
$ |
|
||
The table below summarizes other information related to the Company’s operating leases:
|
|
Years Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Cash paid for amounts included in measurement of operating lease liabilities |
|
$ |
|
|
$ |
|
||
Establishment of operating lease liabilities arising from obtaining right-of-use- assets |
|
|
|
|
|
|
||
Weighted-average remaining lease term – operating leases |
|
|
|
|
|
|
||
Weighted-average discount rate – operating leases |
|
|
% |
|
|
% |
||
F-23
Remaining maturities of the Company’s operating leases, included in operating lease liabilities – current and operating lease liabilities - non-current, net of discount, in the consolidated balance sheets as of December 31, 2022, are as follows:
2023 |
|
|
|
$ |
|
|
2024 |
|
|
|
|
|
|
2025 |
|
|
|
|
|
|
Total |
|
|
|
|
|
|
Less present value discount |
|
|
|
|
( |
) |
Total operating lease liabilities |
|
|
|
|
|
|
Less operating lease liabilities - current |
|
|
|
|
( |
) |
Operating lease liabilities - non-current |
|
|
|
$ |
|
Financing Leases
The Company has a small number of computer and copier equipment leases that are classified as financing leases. Incremental borrowing rates used to discount future lease payments in calculating lease liabilities were estimated by reference to information received by the Company from bankers regarding estimated current borrowing rates for collateralized loans with similar amount and duration as the leases. The Company did not have any material financing leases as of either the year ended December 31, 2022 or 2021.
Note 12. Net Loss Per Share
Basic loss per common share is computed by dividing the net loss allocable to common stockholders by the weighted-average number of shares of common stock or common stock equivalents outstanding. Diluted loss per common share is computed similar to basic loss per common share except that it reflects the potential dilution that could occur if dilutive securities or other obligations to issue common stock were exercised or converted into common stock.
The following table provides a reconciliation of the numerator and denominator used in computing basic and diluted net loss per share for the periods presented:
|
|
Years Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Numerator: |
|
|
|
|
|
|
||
Net loss |
|
$ |
( |
) |
|
$ |
( |
) |
Denominator: |
|
|
|
|
|
|
||
Weighted-average shares outstanding-basic and diluted * |
|
|
|
|
|
|
||
Net loss per share, basic and diluted |
|
$ |
( |
) |
|
$ |
( |
) |
*Reflects the retrospective adjustment related to the reverse stock split completed on December 20, 2022.
The following common stock equivalents were excluded from the computation of diluted net loss per share for the periods presented because their effect would have been anti-dilutive:
|
|
Years Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Options to purchase common stock |
|
|
|
|
|
|
||
Series A Preferred |
|
|
— |
|
|
|
|
|
Common stock warrants |
|
|
|
|
|
|
||
Unvested restricted stock units |
|
|
|
|
|
|
||
Note 13. Warrants
In connection with certain of its redeemable convertible preferred stock issuances, debt agreements, convertible debt and other financing arrangements, the Company has issued warrants for shares of its common stock and various issues of its redeemable convertible preferred stock which have since been converted to common stock warrants.
F-24
In connection with the March 2022 Securities Purchase Agreement (see Note 14), the Company issued and sold pre-funded warrants exercisable for an aggregate of
In connection with the December 2022 Securities Purchase Agreement (see Note 14), the Company issued and sold pre-funded warrants exercisable for an aggregate of
Also in connection with the December 2022 Securities Purchase Agreement, the Company issued warrants to purchase up to an aggregate of
The following table shows the common stock warrants outstanding as of December 31, 2022:
Warrant Issuance Date |
|
Shares of |
|
|
Exercise |
|
|
Expiration Date |
||
|
|
|
|
$ |
|
|
||||
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
||||
Note 14. Stockholders’ Equity
Reverse Stock Split
Equity Offerings
September 2019 Securities Purchase Agreement
In September 2019, concurrently with the closing of an underwritten public offering, the Company entered into a Securities Purchase Agreement (the "September 2019 Securities Purchase Agreement") with certain institutional accredited investors (the "Purchasers"), pursuant to which the Company sold the Purchasers, in a private placement transaction, warrants to purchase up to an aggregate of
F-25
ATM Offering
In November 2019, the Company entered into a Controlled Equity Offering Sales Agreement with Cantor Fitzgerald & Co. ("Cantor") as sales agent, pursuant to which the Company was able to offer and sell, from time to time, through Cantor, shares of its common stock, par value $
During the year ended December 31, 2021, the Company sold
LP Purchase Agreement
In March 2020, the Company entered into a purchase agreement ("LP Purchase Agreement") with Lincoln Park Capital Fund, LLC ("Lincoln Park"), pursuant to which, upon the terms and subject to the conditions and limitations set forth therein, the Company had the right to sell to Lincoln Park up to $
Exchange and Private Placement
In February 2020, the Company entered into an Exchange and Purchase Agreement with certain accredited investors pursuant to which the Company agreed to (i) issue the investors an aggregate of
In March 2022, the remaining
March 2022 Securities Purchase Agreement
In March 2022, the Company entered into a Securities Purchase Agreement (the “March 2022 Securities Purchase Agreement”) with a single investor pursuant to which it agreed to issue to the investor
The common warrants issued in this transaction may not be exercised if the aggregate number of shares of common stock beneficially owned by the holder thereof would exceed
Cantor served as the placement agent in connection with the March 2022 Securities Purchase Agreement. The Company paid Cantor a fee of approximately $
December 2022 Securities Purchase Agreement
In December 2022, in connection with a best-efforts public offering, the Company entered into a Securities Purchase Agreement (the "December 2022 Securities Purchase Agreement") with a certain institutional investor, pursuant to which the Company sold the investor
F-26
The common warrants issued in this transaction may not be exercised if the aggregate number of shares of common stock beneficially owned by the holder thereof would exceed
Each pre-funded warrant had an exercise price of $
H.C. Wainwright & Co., LLC (the "Placement Agent") served as the exclusive placement agent in connection with the December 2022 Securities Purchase Agreement. The Company paid the Placement Agent a cash fee of
Common Stock
Pursuant to its amended and restated certificate of incorporation, the Company is authorized to issue
Preferred Stock
Pursuant to its amended and restated certificate of incorporation, the Company has been authorized to issue
Series A Preferred Stock
In October 2022, the Company entered into a Purchase Agreement (the "Purchase Agreement" with Ann Hanham, Ph.D., the Chair of the Company's Board of Directors (the "Purchaser"), pursuant to which the Company agreed to issue and sell one share of the Company's newly designated Series A Preferred Stock, par value $
Stock-based Compensation
The Company incurs stock-based compensation expense relating to the grants of RSUs and stock options to employees, non-employee directors and consultants under its equity incentive plans and through stock purchase rights granted under the ESPP.
Equity Incentive Plans
In August 2020, the Company’s stockholders, upon the recommendation of the Company’s Board of Directors, approved the 2020 Equity Incentive Plan (the “2020 Plan”) as a successor to and continuation of the previous 2001 Stock Option Plan, 2011 Equity Incentive Plan and 2014 Equity Incentive Plan (the "2014 Plan"). Upon approval of the 2020 Plan,
F-27
There were
In July 2021, the Company’s Board of Directors adopted the Company’s 2021 Inducement Plan (the “2021 Inducement Plan”), pursuant to which
The Company’s Board of Directors determines the grant date for all awards granted under the equity plans. The exercise price of stock options granted is generally equal to the closing price of the Company’s common stock on the date of grant. All stock options granted have a
Amounts recognized in the consolidated statements of operations with respect to the Company’s equity incentive plans were as follows:
|
|
Years Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Selling, general and administrative |
|
$ |
|
|
$ |
|
||
Research and development |
|
|
|
|
|
|
||
Cost of product and product-related services revenue |
|
|
|
|
|
|
||
|
|
$ |
|
|
$ |
|
||
The following table summarizes stock option activity (including Inducement Award activity) during the two-year period ended December 31, 2022:
|
|
Number of |
|
|
Weighted- |
|
|
Weighted- |
|
|
Aggregate |
|
||||
Balance at January 1, 2021 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
Granted |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Exercised |
|
|
( |
) |
|
|
|
|
|
|
|
$ |
|
|||
Forfeited |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|||
Expired/Cancelled |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|||
Balance at December 31, 2021 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
Granted |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Exercised |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
||
Forfeited |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|||
Expired/Cancelled |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|||
Balance at December 31, 2022 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
Exercisable at December 31, 2021 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
Exercisable at December 31, 2022 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
The weighted-average fair value of stock options granted was $
F-28
The fair value of each stock option granted has been determined using the Black-Scholes option pricing model.
|
|
2022 |
|
2021 |
Fair value of common stock on grant date |
|
$ |
|
$ |
Risk-free interest rate |
|
|
||
Expected volatility |
|
|
||
Expected term |
|
|
||
Expected dividend yield |
|
|
In preparing its Black-Scholes option-pricing model fair value calculations, the Company does not estimate a forfeiture rate to calculate stock-based compensation. The Company uses judgment in evaluating the expected volatility and expected terms utilized for the Company’s stock-based compensation calculations on a prospective basis.
The following table summarizes RSU activity (including Inducement Award activity) during the two-year period ended December 31, 2022:
|
|
Number of |
|
|
Weighted- |
|
||
Balance at January 1, 2021 |
|
|
|
|
$ |
|
||
Granted |
|
|
|
|
|
|
||
Released |
|
|
( |
) |
|
|
|
|
Forfeited |
|
|
( |
) |
|
|
|
|
Balance at December 31, 2021 |
|
|
|
|
$ |
|
||
Granted |
|
|
|
|
|
|
||
Released |
|
|
( |
) |
|
|
|
|
Forfeited |
|
|
( |
) |
|
|
|
|
Balance at December 31, 2022 |
|
|
|
|
$ |
|
||
Vested and unissued at December 31, 2022 |
|
|
|
|
$ |
|
||
The weighted-average fair value of RSUs granted was $
RSU activity includes
F-29
2014 Employee Stock Purchase Plan
In April 2015, the Company’s stockholders approved the 2014 Employee Stock Purchase Plan (the “2014 ESPP"), which became effective in May 2015.
In August 2021, the Company’s stockholders, upon the recommendation of the Company’s Board of Directors, approved the Amended and Restated 2014 Employee Stock Purchase Plan (the “Amended 2014 ESPP”). Upon approval of the Amended 2014 ESPP,
Amounts recognized in the consolidated statements of operations with respect to the Amended 2014 ESPP were as follows:
|
|
Years Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Selling, general and administrative |
|
$ |
|
|
$ |
|
||
Research and development |
|
|
|
|
|
|
||
Cost of product and product-related services revenue |
|
|
|
|
|
|
||
|
|
$ |
|
|
$ |
|
||
During the year ended December 31, 2022, employees purchased the following shares at the end of each of the six-month purchase periods:
|
|
June 2022 |
|
|
December 2022 |
|
||||||||||
|
|
Number of |
|
|
Price |
|
|
Number of |
|
|
Price |
|
||||
Total number of shares purchased |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
During the year ended December 31, 2021, employees entering the plan at various times throughout the offering period purchased the following shares at the end of each of the six-month purchase periods:
|
|
June 2021 |
|
|
December 2021 |
|
||||||||||
|
|
Number of |
|
|
Price |
|
|
Number of |
|
|
Price |
|
||||
Total number of shares purchased |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
As of December 31, 2022, approximately
The Company recognizes employee stock purchase plan expense based on the fair value of stock purchase rights, estimated for each six-month purchase period using the Black-Scholes option pricing model. The model requires the Company to make subjective assumptions, including expected stock price volatility, risk free rate of return and estimated life. The fair value of equity-based awards is amortized straight-line over the vesting period of the award.
The material factors incorporated in the Black-Scholes model in estimating the fair value of employee stock purchase plan stock purchase rights for the periods presented were as follows:
|
|
2022 |
|
2021 |
Fair value of common stock |
|
$ |
|
$ |
Risk-free interest rate |
|
|
F-30
Expected volatility |
|
|
||
Expected term |
|
|
||
Expected dividend yield |
|
|
Note 15. Commitments and Contingencies
Legal Matters
The Company’s industry is characterized by frequent claims and litigation, including claims regarding intellectual property and product liability. As a result, the Company may be subject to various legal proceedings from time to time. The results of any current or future litigation cannot be predicted with certainty, and regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources, and other factors. Any current litigation is considered immaterial and counter claims have been assessed as remote.
Employee Agreements
The Company has entered into Severance and Change in Control Plan agreements with certain named executive officers and various other members of management, which provide salary continuation payments, bonuses and, in certain instances, the acceleration of the vesting of certain equity awards to individuals in the event that the individual is terminated other than for cause, as defined in the applicable agreement.
Indemnification Agreements
In the course of operating its business, the Company has entered into, and continues to enter into, separate indemnification agreements with the Company’s directors and executive officers, in addition to the indemnification provided for in the Company’s amended and restated bylaws. These agreements may require the Company to indemnify its directors and executive officers for certain expenses incurred in any action or proceeding arising out of their services as one of the Company’s directors or executive officers.
Product Warranty
The following is a summary of the Company’s general product warranty liability. Product warranty liabilities of approximately $
|
|
December 31, |
|
|||
|
|
2022 |
|
|
2021 |
|
F-31
Beginning balance |
|
$ |
|
|
$ |
|
||
Cost of warranty claims |
|
|
( |
) |
|
|
( |
) |
Increase in warranty reserve |
|
|
|
|
|
|
||
Ending balance |
|
$ |
|
|
$ |
|
Defined Contribution Plan
In January 2003, the Company established a defined contribution plan (“401(k) Plan”) under section 401(k) of the Internal Revenue Code of 1986, as amended. All employees who are over the age of 21 and who are expected to work at least 1,000 hours in a calendar year are eligible for participation in the 401(k) Plan upon commencement of employment with the Company. The Company may make discretionary contributions to the 401(k) Plan but has
Note 16. Income Taxes
The Company provides for income taxes based upon management’s estimate of taxable income or loss for each respective period. The Company recognizes an asset or liability for the deferred tax consequences of temporary differences between the tax bases of assets and liabilities and their reported amounts in the financial statements. These temporary differences would result in deductible or taxable amounts in future years, when the reported amounts of the assets are recovered or liabilities are settled, respectively.
In each period since inception, the Company has recorded a valuation allowance for the full amount of its net deferred tax assets, as the realization of the net deferred tax assets is uncertain. As a result, the Company has not recorded any federal or state income tax benefit in the accompanying consolidated statements of operations; however, income tax expense has been recorded for state minimum and foreign income taxes.
The Company periodically reviews its filing positions for all open tax years in all U.S. federal, state and international jurisdictions where the Company is or might be required to file tax returns or other required reports. The Company applies a two-step approach to recognizing and measuring uncertain tax positions. The Company evaluates the tax position for recognition by determining if the weight of available evidence indicates that it is “more likely than not” that the position will be sustained on audit, including resolution of related appeals or litigation process, if any. The term “more likely than not” means a likelihood of more than 50 percent. If the tax position is not more likely than not to be sustained in a court of last resort, the Company may not recognize any of the potential tax benefit associated with the position. The Company recognizes a benefit for a tax position that meets the more likely than not criterion at the largest amount of tax benefit that is greater than 50 percent likely of being realized upon its effective resolution. Unrecognized tax benefits involve management’s judgment regarding the likelihood of the benefit being sustained. The final resolution of uncertain tax positions could result in adjustments to recorded amounts and may affect the Company’s results of operations, financial position and cash flows. As discussed below, the Company has estimated $3.4 million and $3.2 million of uncertain tax positions as of December 31, 2022 and 2021, respectively, related to certain tax credit carryforwards.
The Company’s policy is to recognize interest and/or penalties related to income tax matters in income tax expense. The Company had no accrual for interest or penalties at December 31, 2022 or 2021, and has not recognized interest or penalties during the years ended December 31, 2022 and 2021, since there was no reduction in income taxes paid due to uncertain tax positions. Management of the Company believes no significant change to the amount of unrecognized tax benefits will occur within the next 12 months.
The following table summarizes loss before income taxes:
|
|
Years Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
U.S. pre-tax loss |
|
$ |
( |
) |
|
$ |
( |
) |
Foreign pre-tax gain (loss) |
|
|
|
|
|
|
||
Loss before income taxes |
|
$ |
( |
) |
|
$ |
( |
) |
The components of income tax expense are as follows:
|
|
Years Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Current: |
|
|
|
|
|
|
||
Federal |
|
$ |
— |
|
|
$ |
— |
|
State |
|
|
|
|
|
|
||
Foreign |
|
|
|
|
|
|
||
F-32
Total current income tax expense |
|
$ |
|
|
$ |
|
||
|
|
|
|
|
|
|
||
Deferred: |
|
|
|
|
|
|
||
Federal |
|
$ |
— |
|
|
$ |
— |
|
State |
|
|
— |
|
|
|
— |
|
Foreign |
|
|
— |
|
|
|
— |
|
Total deferred income tax expense |
|
$ |
— |
|
|
$ |
— |
|
Total income tax expense |
|
$ |
|
|
$ |
|
||
The Company’s actual income tax expense for the years ended December 31, 2022 and 2021 differ from the expected amount computed by applying the statutory federal income tax rate to loss before income taxes as follows:
|
|
Years Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Computed tax (benefit) at |
|
$ |
( |
) |
|
$ |
( |
) |
State taxes, net of federal benefit |
|
|
( |
) |
|
|
( |
) |
Stock-based compensation |
|
|
|
|
|
|
||
Foreign tax rate differential |
|
|
|
|
|
( |
) |
|
Return to provision |
|
|
|
|
|
|
||
Nontaxable loan forgiveness |
|
|
|
|
|
( |
) |
|
Other |
|
|
|
|
|
|
||
Research and development tax credit - state |
|
|
( |
) |
|
|
( |
) |
Research and development tax credit - federal |
|
|
( |
) |
|
|
( |
) |
Uncertain tax position adjustment for prior periods |
|
|
( |
) |
|
|
( |
) |
Increase in valuation allowance |
|
|
|
|
|
|
||
|
|
$ |
|
|
$ |
|
||
Deferred tax assets and liabilities comprise the following:
|
|
Years Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Deferred tax assets: |
|
|
|
|
|
|
||
Net operating loss carryforwards |
|
$ |
|
|
$ |
|
||
Research and development credits |
|
|
|
|
|
|
||
R&D expenditures capitalization |
|
|
|
|
|
|
||
Deferred revenue |
|
|
|
|
|
|
||
Inventory reserve |
|
|
|
|
|
|
||
Fixed assets and intangibles |
|
|
|
|
|
|
||
Accrued NuvoGen liability |
|
|
|
|
|
|
||
Lease liability |
|
|
|
|
|
|
||
Other |
|
|
|
|
|
|
||
Gross deferred tax assets |
|
|
|
|
|
|
||
Valuation allowance |
|
|
( |
) |
|
|
( |
) |
Deferred tax assets, net |
|
|
|
|
|
|
||
Deferred tax liabilities: |
|
|
|
|
|
|
||
Right of use asset |
|
|
|
|
|
|
||
Total deferred tax liabilities |
|
|
|
|
|
|
||
Net deferred tax assets (liabilities) |
|
$ |
|
|
$ |
|
||
As of December 31, 2022, the Company has estimated federal and state net operating loss (“NOL”) carryforwards of approximately $
F-33
For financial reporting purposes, valuation allowances of $
Pursuant to Sections 382 and 383 of the IRC, annual use of the Company’s NOLs and research and development credit carryforwards may be limited if there is a cumulative change in ownership of greater than
A reconciliation of the Company’s gross unrecognized tax benefits is as follows:
|
|
Years Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Balance at beginning of year |
|
$ |
|
|
$ |
|
||
Increases to prior positions |
|
|
— |
|
|
|
— |
|
Decreases to prior positions |
|
|
( |
) |
|
|
( |
) |
Increases for current year positions |
|
|
|
|
|
|
||
Balance at end of year |
|
$ |
|
|
$ |
|
||
As of December 31, 2022, the Company had $
On August 16, 2022, the President signed into law the Inflation Reduction Act of 2022 which contained provisions effective January 1, 2023, including a 15% corporate minimum tax and a 1% excise tax on stock buybacks, both of which we expect to be immaterial to our financial results, financial position and cash flows.
The Company files income tax returns in the United States, Arizona, California, Texas, various other state jurisdictions, and France, with varying statutes of limitations. As of December 31, 2022, the earliest year subject to examination is 2019 for U.S. federal tax purposes. The earliest year subject to examination is 2018 for the state jurisdictions, and 2019 for France. However, the Company’s federal and state NOLs and tax credit carryforwards for periods ending December 31, 2003 and thereafter remain subject to examination by the United States and certain states.
F-34
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures.
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our periodic and current reports that we file with the SEC is recorded, processed, summarized and reported with the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
In designing and evaluating the disclosure controls and procedures, management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable and not absolute assurance of achieving the desired control objectives. In reaching a reasonable level of assurance, management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures. In addition, the design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions; over time, control may become inadequate because of changes in conditions, or the degree of compliance with policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
As of December 31, 2022, we carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and our Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended. Based on that evaluation, our Chief Executive Officer and our Chief Financial Officer have concluded that, as of December 31, 2022, our disclosure controls and procedures were effective at the reasonable assurance level.
Management’s Report on Internal Control over Financial Reporting.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f). Internal control over financial reporting is a process designed under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America. The effectiveness of any system of internal control over financial reporting, including ours, is subject to inherent limitations, including the exercise of judgment in designing, implementing, operating and evaluating the controls and procedures, and the inability to eliminate misconduct completely. Accordingly, any system of internal control over financial reporting, including ours, no matter how well designed and operated, can only provide reasonable, not absolute assurances. In addition, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
We conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework). Based on our evaluation of the framework in Internal Control – Integrated Framework, our management concluded that our internal control over financial reporting was effective at the reasonable assurance level as of December 31, 2022.
Changes in Internal Control Over Financial Reporting
An evaluation was performed under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of any change in our internal control over financial reporting that occurred during our last fiscal quarter and that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting. That evaluation did not identify any change in our internal control over financial reporting that occurred during the quarter ended December 31, 2022 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
72
Item 9B. Other Information.
None.
Item 9C. Disclosure Regarding Foreign Jurisdictions That Prevent Inspections.
Not applicable.
73
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
Executive Officers and Directors
The following table sets forth certain information regarding our current executive officers and directors:
Name |
|
Age |
|
|
Position(s) |
|
Executive Officers |
|
|
|
|
|
|
John L. Lubniewski |
|
|
59 |
|
|
President, Chief Executive Officer and Director |
Shaun D. McMeans |
|
|
61 |
|
|
Senior Vice President, Chief Financial Officer, Treasurer and Secretary |
Byron T. Lawson |
|
|
48 |
|
|
Senior Vice President, Chief Commercial Officer |
Stephen A. Barat |
|
|
53 |
|
|
Senior Vice President, Therapeutics |
|
|
|
|
|
|
|
Non-Employee Directors |
|
|
|
|
|
|
Ann F. Hanham, Ph.D. (3) |
|
|
70 |
|
|
Chair of Board of Directors |
Thomas W. Dubensky Jr.(2)(4) |
|
|
65 |
|
|
Director |
Michelle R. Griffin (1)(2) |
|
|
57 |
|
|
Director |
Donnie M. Hardison (2) |
|
|
72 |
|
|
Director |
Christopher P. Kiritsy (1) |
|
|
58 |
|
|
Director |
Lee R. McCracken (1)(2)(3) |
|
|
65 |
|
|
Director |
Executive Officers
John L. Lubniewski. Mr. Lubniewski has served as our President and Chief Executive Officer and as a member of our Board of Directors since April 2019 and previously served as our President and Chief Operating Officer since April 2018. Prior to this, he served as our Senior Vice President and Chief Business Officer since April 2011. Mr. Lubniewski joined us from Ventana Medical Systems, Inc. (“Ventana”), a medical diagnostics company and member of the Roche Group, and the global headquarters of Roche Tissue Diagnostics (“RTD”) where he served in leadership roles for nine years both before and after the acquisition of Ventana by Roche Holdings, Inc. (“Roche”) in March 2008. From August 2010 to April 2011, Mr. Lubniewski was Senior Vice President and Lifecycle Leader, Advanced Staining Platforms at Ventana. From January 2008 to August 2010, Mr. Lubniewski served as Senior Vice President and Lifecycle Leader, Clinical Assays at RTD, with responsibility for three lifecycle teams, technical marketing and medical marketing and global accountability for all RTD clinical assay products. Prior to the Roche acquisition of Ventana, Mr. Lubniewski served at Ventana as Senior Vice President, Advanced Staining Business Unit, Vice President Worldwide Marketing and Translational Diagnostic Business Unit, and General Manager, Research Products. In these roles, Mr. Lubniewski was responsible for a variety of assay and platform development and commercialization efforts. Prior to Ventana, Mr. Lubniewski worked for over ten years in a variety of divisional, sector and corporate leadership roles at Corning, Incorporated, a multinational technology company that specializes in specialty glass, ceramics and related materials and technologies including advanced optics, primarily for industrial and scientific applications. Mr. Lubniewski earned a B.S. in Chemical Engineering from Clarkson University. Our Board of Directors believes that Mr. Lubniewski’s extensive executive management experience in commercialization, marketing, strategic planning and management of operations, as well as his service as our Chief Executive Officer, qualify him to serve on our Board of Directors.
74
Shaun D. McMeans. Mr. McMeans has served as our Chief Financial Officer since February 2012. He previously was our Vice President of Finance & Administration from February 2012 until February 2018. Prior to joining us, Mr. McMeans was Vice President – Finance of Securaplane Technologies, Inc., a product supply company and division of Meggitt PLC, an aerospace, defense and energy conglomerate, from May 2011 to February 2012. Mr. McMeans was a financial consultant from February 2008 to April 2011, working both in an individual capacity and as a partner for Tatum LLC, a consulting company. Prior to February 2008, Mr. McMeans was Chief Financial Officer for The Long Companies, a full service residential and commercial real estate division of Berkshire Hathaway, Inc. Mr. McMeans also worked for over five years at LXU Healthcare, Inc., a manufacturer and distributor of specialty surgical equipment, as Controller and then Chief Financial and Operating Officer. In his early career, Mr. McMeans worked in roles of increasing responsibility, including Director of Finance, for Burnham Holdings, Inc., formerly Burnham Corporation, a manufacturer and distributor of residential and commercial hydronic heating equipment. Mr. McMeans received his B.S. in Accounting from Pennsylvania State University.
Byron T. Lawson. Mr. Lawson has served as our Senior Vice President and Chief Commercial Officer since January 2020 and previously served as our Senior Vice President, Pharma Business Unit since January 2018. Prior to this, he served as our Vice President, Commercial Operations since April 2016 and Senior Director, Commercial Options since October 2012. Mr. Lawson joined us from Ventana, where he worked for nearly 15 years and served in a variety of roles with increasing responsibility in the North American commercial organization. He also served in the United States Air Force for nearly 10 years between Active and Reserve Duty as a certified Histology Technician.
Stephen A. Barat, Ph.D. Dr. Barat has served as our Senior Vice President of Therapeutics since joining our company in October 2021. Our Board of Directors designated him an executive officer in February 2023. Prior to joining us, Dr. Barat was the U.S.-head of the non-clinical safety leaders at Janssen Pharmaceutical Companies of Johnson and Johnson ("Janssen"), the world's largest healthcare company, from March 2020 to October 2021. Prior to Janssen, from January 2017 to March 2020, he was vice president of research and early development at Scynexis, a biotechnology company focused on discovering and developing novel anti-fungal agents. Prior to January 2017, Dr. Barat served in a variety of leadership positions through a series of mergers and acquisitions with Forest Laboratories/Actavis/Allergan, having joined from Merck (Schering-Plough). Professionally, Dr. Barat is an internationally recognized speaker having delivered numerous talks on subjects related to global drug development, having served as a course creator and continuing education faculty member and having been active with industry expert working groups including U.S. Pharmacopeia and Product Quality Research Institute. A pharmacologist by training, Dr. Barat received his B.S. in Clinical Laboratory Sciences (Toxicology) from Rutgers University and his Ph.D. degree in Biomedical Sciences (Pharmacology and Toxicology) from New Jersey Medical School.
Non-Employee Directors
Ann F. Hanham Ph.D. Dr. Hanham has served on our Board of Directors since August 2016 and has served as chair of our Board of Directors since January 2021. Prior to this, Dr. Hanham served as Lead Independent Director from April 2019 to December 2020 and as chair of our Board of Directors from March 2017 to March 2019. Since March 2017, Dr. Hanham has provided independent management consulting as a sole proprietor. Previously, she was the founding and managing partner of BAR Capital LLC, an investment company, a position she has held from December 2013 to March 2017. From February 2000 to November 2013, Dr. Hanham was the Managing Director and General Partner of Burrill and Company, a life science investment company. Prior to that, Dr. Hanham held positions of increasing responsibility in product development, medical affairs, and clinical and regulatory affairs at various companies, including InterMune Inc., Otsuka America Pharmaceuticals, Inc. (“Otsuka”), Celtrix Pharmaceuticals, Inc. (“Celtrix”), and Becton Dickinson and Company (“BD”). InterMune, Inc., Otsuka and Celtrix are, or prior to respective acquisitions, were clinical-stage biopharmaceutical companies, and BD is a life sciences discovery and diagnostics company. Dr. Hanham also currently serves on the board of directors of SCYNEXIS (Nasdaq: SCYX). Dr. Hanham received her B.Sc. degree from the University of Toronto, Canada; her M.Sc. degree, in biology, from Simon Fraser University, Canada; and her Ph.D. degree, in biology, from the University of British Columbia, Canada. Our Board of Directors believes that Dr. Hanham’s extensive industry and executive experience, and her experience serving on the board of directors of other public companies qualifies her to serve on our Board of Directors.
75
Thomas W. Dubensky Jr., Ph.D. Dr. Dubensky joined our Board of Directors in August 2022. Dr. Dubensky was the Chief Executive Officer and a director of Tempest Therapeutics, Inc. (“Legacy Tempest”) since 2017 until its merger with Millendo Therapeutics, Inc. in June 2021, which combined company is now called Tempest Therapeutics, Inc. (Nasdaq: TPST) ("Tempest"), and has served as the President and a member of the Board of Tempest since June 2021. Prior to Legacy Tempest, Dr. Dubensky was the Chief Scientific Officer of Aduro Biotech, where he led the development of STRING agonists. Additionally, Dr. Dubensky served in executive and principal roles in leading discovery biology, development and clinical translation of multiple first-in-class agents in cancer immunotherapy and infectious disease indications at several biotech companies, including Viagene Biotech, Inc., Chiron Corporation, Onyx Pharmaceuticals, Inc., Cerus Corporation and Immune Design, Inc. Dr. Dubensky has served on the scientific advisory boards of Turnstone Biologics, Oncorus, Inc., Remedy Plan, Inc., Vaccitech PLC-ADR and Tyligand Bioscience. Dr. Dubensky has an extensive publication and patent record. Dr. Dubensky received his B.A. in Bacteriology and Immunology from the University of California, Berkeley, his Ph.D. from the University of Colorado Health Sciences Center, conducted his post-doctoral studies at Harvard Medical School in the Department of Pathology, and received executive training at the University of California, San Diego, in the Executive Program for Scientists and Engineers. Our Board of Directors believes that Dr. Dubensky's extensive industry and executive experience, especially in the areas of drug discovery and development, qualifies him to serve on our Board of Directors.
Michelle R. Griffin. Ms. Griffin has served on our Board of Directors since August 2018. Ms. Griffin currently serves as a member of the board of directors and chair of the compensation committee for Acer Therapeutics, Inc (Nasdaq: ACER), Adaptive Biotechnologies Corp (Nasdaq: ADPT) and Chinook Therapeutics, Inc. (Nasdaq KDNY). She has also served on the board of directors and as audit committee chair for PhaseRx, Inc. (Nasdaq:PZRX) from 2016 to 2018, OncoGenex Pharmaceuticals Inc. (Nasdaq: OGXI) from 2008 to 2011, and Sonus Pharmaceuticals, Inc. (Nasdaq: SNUS) from 2004 to 2008. Ms. Griffin served as Executive Vice President, Operations, and Chief Financial Officer at OncoGenex from 2011 to 2013; served as acting Chief Executive, Senior Vice President and Chief Operating Officer at Trubion Pharmaceuticals, Inc. (Nasdaq: TRBN) from 2009 until its acquisition in 2010 and as its Chief Financial Officer from 2006 to 2009; and served as Senior Vice President and Chief Financial Officer of Dendreon Corp. (Nasdaq: DNRN) from 2005 to 2006. Ms. Griffin began her career in the biopharmaceuticals industry in 1994 at Corixa Corp. (Nasdaq: CRXA) and served as its Chief Financial Officer from its IPO in 1997 until 2005 when Corixa was acquired by GlaxoSmithKline plc. She received a post‐graduate certificate in accounting and an MBA from Seattle University, a B.S. in statistics and marketing from George Mason University and has passed the certified public accountant exam. Our Board of Directors believes that Ms. Griffin’s financial and accounting expertise and extensive executive experience qualifies her to serve on our Board of Directors.
Donnie M. Hardison. Mr. Hardison has served on our Board of Directors since May 2016. Since February 2021, he has been working as an independent consultant. He was most recently the President and Chief Executive Officer, and served on the board of directors, of Biotheranostics, Inc., a molecular diagnostic company focused on oncology, from February 2017 until it was acquired by Hologic, Inc. in February 2021. From April 2010 to March 2016, Mr. Hardison was the President and Chief Executive Officer of Good Start Genetics, a molecular genetic testing and information company. For more than 20 years prior to that, Mr. Hardison held various executive and senior management positions at companies including Laboratory Corporation of America (“LabCorp”) a clinical laboratory company, Exact Sciences Corporation, a molecular diagnostics company, OnTarget, Inc., a sales and marketing consulting company, Quest Diagnostics Inc., a clinical laboratory company, SmithKline Beecham Corporation, a pharmaceutical company, and others. He currently serves on the board of directors of Cytek Biosciences (Nasdaq: CTKB), MDxHealth (Nasdaq: MDXH) and BioPorto, Inc. (BIOPOR.CO) and as an independent director or advisor for several private companies, including Stemina Biomarker Discovery, Inc., YourBio Health, Breath BioMedical and IQuity, Inc. He also previously served on the board of directors of Exact Sciences Corporation (Nasdaq: EXAS) from May 2000, through its initial public offering in February 2001, until August 2007. Mr. Hardison received his Bachelor of Arts degree, in political science, from the University of North Carolina, Chapel Hill. Our Board of Directors believes that Mr. Hardison’s broad private and public company background, his extensive executive and industry experience, his experience with newly emerging and well-established companies, and his extensive commercial and operational experience qualify him to serve on our Board of Directors.
Christopher P. Kiritsy. Mr. Kiritsy has served on our Board of Directors since January 2022. Mr. Kiritsy currently serves as audit and compensation committee chairs and on the board of directors of Pieris Pharmaceuticals, Inc. (Nasdaq: PIRS). Since 2018, Mr. Kiritsy has been the managing member of Precision Kapital, LLC, a private investment and advisory firm that he founded. Prior to forming Precision Kapital, Mr. Kiritsy co-founded Arisaph Pharmaceuticals, Inc. (“Arisaph”) and served as Arisaph’s President and Chief Executive Officer until its exit in 2018. At Arisaph, Mr. Kiritsy evolved the drug discovery organization from an academic orientation to a clinical development enterprise, taking several cardiometabolic products into clinical development. Prior to Arisaph, Mr. Kiritsy served as Executive Vice President, Corporate Development and Chief Financial Officer of Kos Pharmaceuticals, Inc. (Nasdaq: KOSP). Mr. Kiritsy is a seasoned entrepreneur, who possesses more than 25 years of business and technical experience in the biopharmaceutical industry. He received his A.B. in Biology from Bowdoin College and an MBA from Boston University. Our Board of Directors believes that Mr. Kiritsy’s extensive industry and executive experience, and his experience serving on the board of directors of another public company qualify him to serve on our Board of Directors.
76
Lee R. McCracken. Mr. McCracken has served on our Board of Directors since October 2015. Since 2022, Mr. McCracken has served as the Chief Executive Officer of NephroSant, a diagnostics company developing innovative tests focused on early detection of kidney disease and injury. From May 2021 to October 2022, Mr. McCracken was an Entrepreneur in Residence at Thorne HealthTech, a leader in the development of innovative solutions for personalized approaches to health and wellbeing, following its acquisition of Drawbridge Health, where he served as Chief Executive Officer from June 2018 to April 2021 and became the Chair of the board of directors in May 2021. From May 2016 to May 2017 and from April 2013 to March 2014, Mr. McCracken was a strategic and restructuring consultant in the regenerative medicine and diagnostics industries through his firm, McCracken Consulting. In addition, he served as Chief Executive Officer of Gensignia Life Sciences, Inc., a molecular diagnostics company, from April 2014 through May 2016. Mr. McCracken previously held executive positions or roles with significant responsibility at several biotechnology and therapeutics companies, including Pathwork Diagnostics, Inc., Prometheus Laboratories Inc., GenStar Therapeutics Corporation, CombiChem Inc., and Allergan Inc., as well as at the investment companies, 3i Capital and Union Venture. Mr. McCracken received his M.B.A. from the Anderson School of Management at the University of California, Los Angeles, his Master of Computer Science (MCS) from the University of Dayton, and his B.S. in Commerce from Santa Clara University. Our Board of Directors believes Mr. McCracken’s extensive executive and industry experience and his broad knowledge of molecular diagnostics qualify him to serve on our Board of Directors.
Board Composition
Our business and affairs are organized under the direction of our Board of Directors, which currently consists of six members. The primary responsibilities of our Board of Directors are to provide oversight, strategic guidance, counseling and direction to our management. Our Board of Directors meets on a regular basis and additionally as required.
Our Board of Directors has determined that all of our directors other than Mr. Lubniewski are independent directors, as defined by Rule 5605(a)(2) of the Nasdaq Listing Rules. The Nasdaq independence definition includes a series of objective tests, including that the director is not, and has not been for at least three years, one of our employees and that neither the director nor any of his family members has engaged in various types of business dealings with us. In addition, as required by Nasdaq rules, our Board of Directors has made a subjective determination as to each independent director that no relationships exist, which, in the opinion of our Board of Directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. In making these determinations, our Board of Directors reviewed and discussed information provided by the directors and us regarding each director’s business and personal activities and relationships as they may relate to us and our management. There are no family relationships among any of our directors or executive officers.
In accordance with the terms of our amended and restated certificate of incorporation, our Board of Directors is divided into three classes, as follows:
At each annual meeting of stockholders, the successors to directors whose terms then expire will serve until the third annual meeting following their election and until their successors are duly elected and qualified. The authorized number of directors may be changed only by resolution of our Board of Directors. Any additional directorships resulting from an increase in the number of directors will be distributed between the three classes so that, as nearly as possible, each class will consist of one-third of the directors. This classification of the board of directors may have the effect of delaying or preventing changes in our control or management. Our directors may be removed for cause by the affirmative vote of the holders of at least 66 2/3% of our voting stock.
Board Leadership Structure
As a general policy, our Board of Directors believes that separation of the positions of Chair and Chief Executive Officer reinforces the independence of the Board from management, creates an environment that encourages objective oversight of management’s performance and enhances the effectiveness of the Board as a whole.
77
Dr. Hanham serves as Chair of our Board of Directors and Mr. Lubniewski serves as our Chief Executive Officer. Dr. Hanham presides over Board of Directors meetings, sets meeting agendas, ensures the duties, responsibilities and roles of members of our Board of Directors are clearly understood, ensures that our Board of Directors receives appropriate and timely information, material and reports from management regarding our business, provides input to the Board regarding candidates for nomination or appointment to the Board and Board committees, and performs such additional duties as set forth in our bylaws and as our Board of Directors may otherwise determine and delegate.
We also have a separate chair for each committee of our Board of Directors. The chair of each committee is expected to report at least annually to our Board of Directors on the activities of their respective committee in fulfilling their responsibilities as detailed in their respective charters or specify any shortcomings should that be the case.
Role of the Board in Risk Oversight
One of the key functions of our Board of Directors is informed oversight of our risk management process. The Board does not have a standing risk management committee, but rather administers this oversight function directly through our Board of Directors as a whole, as well as through various standing Board committees that address risks inherent in their respective areas of oversight. The risk oversight process includes receiving regular reports from Board committees and members of senior management to enable our Board of Directors to understand our risk identification, risk management and risk mitigation strategies with respect to areas of potential material risk, including operations, finance, legal, regulatory, strategic and reputational risk. In particular, our Board of Directors is responsible for monitoring and assessing strategic risk exposure and our Audit Committee has the responsibility to consider and discuss our major financial risk exposures and the steps our management has taken to monitor and control these exposures, including guidelines and policies to govern the process by which risk assessment and management is undertaken. The Audit Committee also monitors compliance with legal and regulatory requirements. Oversight by the Audit Committee includes direct communication with our external auditors. Our Nominating and Governance Committee monitors the effectiveness of our corporate governance practices, including whether they are successful in preventing illegal or improper liability-creating conduct. Our Compensation Committee assesses and monitors whether any of our compensation policies and programs has the potential to encourage excessive risk-taking.
Board Committees
Our Board of Directors has established an audit committee, a compensation committee and a nominating and governance committee.
Audit Committee
Our Audit Committee consists of Ms. Griffin, Mr. Kiritsy and Mr. McCracken. Mr. Kiritsy serves as the chair of our Audit Committee. Our Board of Directors has determined that each of the members of our Audit Committee satisfies the Nasdaq Stock Market and SEC independence requirements. The functions of this committee include, among other things:
78
Our Board of Directors has determined that each member of the audit committee meets the requirements for financial literacy under the applicable rules and regulations of the SEC and the Nasdaq Stock Market. It has also determined that Mr. Kiritsy qualifies as an audit committee financial expert within the meaning of SEC regulations and meets the financial sophistication requirements of the Nasdaq Listing Rules. In making this determination, our Board of Directors has considered Mr. Kiritsy’s formal education and experience in financial and executive roles. Both our independent registered public accounting firm and management periodically meet privately with our Audit Committee. The audit committee operates under a written charter that satisfies the applicable standards of the SEC and the Nasdaq Stock Market.
Compensation Committee
Our Compensation Committee consists of Dr. Dubensky, Ms. Griffin, Mr. Hardison, and Mr. McCracken. Mr. Hardison serves as the chair of our Compensation Committee. Our Board of Directors has determined that each of the members of our Compensation Committee is a non-employee director, as defined in Rule 16b-3 promulgated under the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and satisfies the Nasdaq Stock Market independence requirements. None of these individuals has ever been an executive officer or employee of ours. The functions of this committee include, among other things:
79
The compensation committee operates under a written charter that satisfies the applicable standards of the SEC and the Nasdaq Stock Market.
In 2022, our Compensation Committee retained Radford, an Aon Hewitt company and a provider of compensation market intelligence to the technology and life sciences industries, to provide a report summarizing relevant benchmark data relating to industry-appropriate peers and make recommendations regarding base salary, target total cash (base salary plus target cash incentives) and the amounts and terms of long-term equity incentive awards for our executives as well as to benchmark and make recommendations regarding the initial and annual cash retainer amounts for directors and chairpersons of our Board of Directors and the various committees and the amounts and terms of initial and annual long-term equity incentive awards for directors. No work performed by Radford during fiscal year 2022 raised a conflict of interest.
None of our executive officers currently serves, or has served during the last completed fiscal year, on the compensation committee or board of directors of any other entity that has one or more executive officers serving as a member of our Board of Directors or Compensation Committee.
Nominating and Governance Committee
Our Nominating and Governance Committee consists of Dr. Hanham, and Mr. McCracken. Dr. Hanham serves as the chair of our Nominating and Governance Committee. Our Board of Directors has determined that each of the members of this committee satisfies the Nasdaq Stock Market independence requirements. The functions of this committee include, among other things:
80
The nominating and governance committee operates under a written charter, which the nominating and governance committee reviews and evaluates at least annually.
The nominating and governance committee will consider qualified director candidates recommended by stockholders in compliance with our procedures and subject to applicable inquiries. The nominating and governance committee’s evaluation of candidates recommended by stockholders does not differ materially from its evaluation of candidates recommended from other sources. Any stockholder may recommend nominees for director by writing to Dr. Ann F. Hanham, Ph.D., Chair of the Nominating and Governance Committee of the Board of Directors, HTG Molecular Diagnostics, Inc., 3430 E. Global Loop, Tucson, Arizona 85706, giving the name and address of the stockholder on whose behalf the submission is made, the number of Company shares that are owned beneficially by such stockholder as of the date of the submission, the full name of the proposed candidate, a description of the proposed candidate’s business experience for at least the previous five years, complete biographical information for the proposed candidate and a description of the proposed candidate’s qualifications as a director. All of these communications will be reviewed by our Nominating and Governance Committee, for further review and consideration in accordance with this policy.
Limitation on Liability and Indemnification of Directors and Officers
Our amended and restated certificate of incorporation and bylaws limits our directors’ and officers’ liability to the fullest extent permitted under Delaware corporate law. Delaware corporate law provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except for liability:
If the Delaware General Corporation Law is amended to authorize corporate action further eliminating or limiting the personal liability of directors or officers, then the liability of our directors or officers shall be eliminated or limited to the fullest extent permitted by the Delaware General Corporation Law, as so amended.
Delaware law and our amended and restated bylaws provide that we will, in certain situations, indemnify any person made or threatened to be made a party to a proceeding by reason of that person’s former or present official capacity with us against judgments, penalties, fines, settlements and reasonable expenses. Any person is also entitled, subject to certain limitations, to payment or reimbursement of reasonable expenses (including attorneys’ fees and disbursements) in advance of the final disposition of the proceeding.
In addition, we have entered, and intend to continue to enter, into separate indemnification agreements with our directors and executive officers. These agreements, among other things, require us to indemnify our directors and executive officers for certain expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by a director or executive officer in any action or proceeding arising out of their services as one of our directors or executive officers or as a director or executive officer of any other company or enterprise to which the person provides services at our request.
We believe that these provisions in our amended and restated certificate of incorporation and amended bylaws and these indemnification agreements are necessary to attract and retain qualified persons as directors and officers. We also maintain a directors’ and officers’ insurance policy pursuant to which our directors and officers are insured against liability for actions taken in their capacities as directors and officers.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or control persons, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
81
Stockholder Communications with the Board of Directors
We have adopted a formal process by which stockholders may communicate with the Board or any of its directors. Stockholders who wish to communicate with the Board may do so by sending written communications addressed to: Attn: Corporate Secretary, 3430 E. Global Loop, Tucson, Arizona, 85706. These communications will be reviewed by the Secretary, who will determine whether the communication is appropriate for presentation to the Board or the relevant director. The purpose of this screening is to allow the Board to avoid having to consider irrelevant or inappropriate communications (such as advertisements, solicitations and hostile communications).
Code of Ethics
We have adopted a Code of Business Conduct and Ethics that applies to all officers, directors and employees. The Code of Business Conduct and Ethics is available on our website at www.htgmolecular.com. If we make any substantive amendments to the Code of Business Conduct and Ethics or grants any waiver from a provision of the Code to any executive officer or director, we will promptly disclose the nature of the amendment or waiver on our website.
Item 11. Executive Compensation.
Our named executive officers for the year ended December 31, 2022, which consist of our principal executive officer and our two other most highly compensated executive officers as of December 31, 2022, are as follows:
Summary Compensation Table
Name and principal position |
|
Year |
|
Salary |
|
|
Option |
|
|
Non-equity |
|
|
|
All other |
|
|
Total |
|
||||||
John L. Lubniewski |
|
2022 |
|
|
538,000 |
|
|
|
92,700 |
|
|
|
282,450 |
|
|
(4 |
) |
|
741 |
|
|
|
913,891 |
|
President and Chief Executive Officer |
|
2021 |
|
|
460,000 |
|
|
|
— |
|
|
|
276,000 |
|
|
|
|
741 |
|
|
|
736,741 |
|
|
Shaun D. McMeans |
|
2022 |
|
|
385,000 |
|
|
|
46,350 |
|
|
|
148,225 |
|
|
(4 |
) |
|
741 |
|
|
|
580,316 |
|
Senior Vice President and Chief Financial Officer |
|
2021 |
|
|
370,000 |
|
|
|
— |
|
|
|
139,860 |
|
|
|
|
741 |
|
|
|
510,601 |
|
|
Byron T. Lawson |
|
2022 |
|
|
345,000 |
|
|
|
15,444 |
|
|
|
60,375 |
|
|
|
|
741 |
|
|
|
421,560 |
|
|
Chief Commercial Officer |
|
2021 |
|
|
333,000 |
|
|
|
— |
|
|
|
106,560 |
|
|
|
|
741 |
|
|
|
440,301 |
|
|
82
Annual Base Salary
The base salary of our named executive officers is generally set forth in each officer’s employment letter agreement with us and periodically reviewed and adjusted by our Board of Directors, based on the recommendation of our Compensation Committee and following analyses conducted by independent third-party consultants. The 2022 base salaries for Mr. Lubniewski, Mr. McMeans and Mr. Lawson were $538,000, $385,000 and $345,000, respectively.
Annual Performance-Based Bonus Opportunity
In addition to base salaries, our named executive officers are eligible to receive annual performance-based bonuses, which are designed to provide appropriate incentives to our executives to achieve defined annual corporate goals and to reward our executives for individual achievement towards these goals. As with annual base salary, the target annual performance-based bonus percentage for each of our named executive officers is determined by our Board of Directors, based on the recommendation of our Compensation Committee and input from independent third-party consultants. The annual performance-based bonus each named executive officer is awarded is generally based on the extent to which we achieve the corporate goals that our Board of Directors establishes each year. At the end of the year, our Board of Directors reviews our performance against each corporate goal and approves the extent to which we achieved each of our corporate goals.
Our Board of Directors will generally consider each named executive officer’s individual contributions towards reaching our annual corporate goals but does not typically establish specific individual goals for our named executive officers. There is no minimum bonus percentage or amount established for the named executive officers and, thus, the bonus amounts vary from year to year based on corporate and individual performance. For 2022, Mr. Lubniewski was eligible to receive a target bonus of up to 75% of his base salary pursuant to the terms of his employment letter agreement described below. For 2022, Mr. McMeans was eligible to receive a target bonus of up to 55% of his base salary pursuant to the terms of his employment letter agreement described below. For 2022, Mr. Lawson was eligible to receive a target bonus of up to 50% of his base salary pursuant to the terms of his employment letter agreement described below.
The corporate goals established by our Board of Directors for 2022 were based upon financial and strategic goals. Specific goals included direct revenue and cash, customer metrics and strategic metrics related to technology development and Therapeutics. The financial and strategic goals were weighted at 20%, 15% and 65%, respectively, towards overall corporate goal achievement. There was no minimum percentage of corporate goals that was required to be achieved to earn a bonus. No specific individual goals were established for any of our named executive officers for 2022.
In February 2023, our Board of Directors determined that the 2022 corporate goals related to direct revenue and cash, customer metrics and strategic metrics related to technology development and Therapeutics had been achieved at an aggregate level of 70%, to be allocated based on individual performance objectives. As a result, our Board of Directors awarded bonuses of $282,450, $148,225 and $60,375 to Mr. Lubniewski, Mr. McMeans and Mr. Lawson, respectively, representing the performance-adjusted percentage of each executive’s target bonus for the period.
Equity-Based Incentive Awards
Our equity-based incentive awards are designed to align our interests with those of our employees and consultants, including our named executive officers. Our Board of Directors or any authorized committee thereof is responsible for approving equity grants, which include to date, stock options and RSUs. Vesting of the stock option and RSU awards is tied to continuous service with us and serves as an additional retention measure. Our executives generally are awarded an initial stock option grant upon commencement of employment. Additional equity awards may occur periodically to specifically incentivize executives to achieve certain corporate goals or to reward executives for exceptional performance. As of December 31, 2022, our named executive officers have been granted both stock option awards and RSUs.
Prior to the initial public offering, we granted all equity awards pursuant to the 2011 Plan and the 2001 Plan. All equity awards granted since our initial public offering have been granted pursuant to the 2014 Plan, the 2020 Plan and the 2021 Inducement Plan, the terms of which are described below under “—Equity Benefit Plans.” All stock options are granted with a per share exercise price equal to no less than the fair market value of a share of our common stock on the date of the grant and a term of up to 10 years from the date of grant.
83
Generally, our stock option and RSU awards vest over a one to four-year period subject to the holder’s continuous service to us. Should the Board of Directors deem it appropriate, stock option awards may be granted with an early exercise feature which would allow the holder to exercise and receive unvested shares of our stock, so that the holder may have a greater opportunity for gains on the shares to be taxed at long-term capital gains rates rather than ordinary income rates. From time to time as our Board of Directors considers appropriate, we may grant stock options or RSUs that vest upon achievement of performance goals.
In 2022, Mr. Lubniewski, Mr. McMeans and Mr. Lawson received grants of options to purchase 10,000, 5,000, and 1,666 shares, respectively, of our common stock, vesting in equal quarterly installments over a two-year period, subject to each individual’s continued service with us through each vesting date.
401(k) Plan
We maintain a tax-qualified retirement plan that provides eligible employees with an opportunity to save for retirement on a tax advantaged basis. All participants’ interests in their deferrals are 100% vested when contributed. We made no matching contributions into the 401(k) plan for either of the years ended December 31, 2022 or 2021. Pre-tax contributions are allocated to each participant’s individual account and are then invested in selected investment alternatives according to the participants’ directions. The 401(k) plan is intended to qualify under Sections 401(a) and 501(a) of the Code. As a tax-qualified retirement plan, contributions to the 401(k) plan and earnings on those contributions are not taxable to the employees until distributed from the 401(k) plan, and all contributions are deductible by us when made.
Agreements with Named Executive Officers
We have entered into letter agreements with each of our named executive officers. The letter agreements generally provide for at-will employment and set forth the named executive officer’s initial base salary, eligibility for employee benefits, in some cases, and severance benefits upon a qualifying termination of employment. In addition, each of our named executive officers has executed a form of our standard confidential information and invention assignment agreement. The key terms of the letter agreements with our named executive officers are described below. Any potential payments and benefits due upon a qualifying termination of employment or a change in control are further described below under “– Potential Payments and Benefits upon Termination or Change in Control.”
Employment Letter Agreement with Mr. Lubniewski. We entered into an amended and restated letter agreement with Mr. Lubniewski in March 2019 that replaced his previous December 2014 letter agreement. The agreement sets forth certain agreed upon terms and conditions of employment. Mr. Lubniewski was initially entitled to receive an annual base salary of $460,000 (which has been increased, most recently in January 2022 to $538,000), an annual target performance bonus of up to 75% of his base salary as determined by the Board of Directors following analysis conducted by independent third-party consultants, and certain severance benefits, which were superseded and replaced by the terms of our Severance Plan, as further described below under “—Potential Payments and Benefits upon Termination or Change of Control.” Mr. Lubniewski’s base salary and target bonus percentage are subject to modification from time to time in the discretion of our Board of Directors or any authorized committee thereof.
Employment Letter Agreement with Mr. McMeans. We entered into an amended and restated letter agreement with Mr. McMeans in July 2019 that replaced his previous December 2014 letter agreement. The agreement sets forth certain agreed upon terms and conditions of employment. Mr. McMeans was initially entitled to an annual base salary of $370,000 (which has been increased, most recently in January 2022 to $385,000), an annual target performance bonus of up to 55% of his base salary as determined by the board of directors, and certain severance benefits, which were superseded and replaced by the terms of our Severance Plan, as further described below under “—Potential Payments and Benefits upon Termination or Change of Control.” Mr. McMeans’ base salary and target bonus percentage are subject to modification from time to time in the discretion of our Board of Directors or any authorized committee thereof.
Employment Letter Agreement with Mr. Lawson. We entered into an amended and restated letter agreement with Mr. Lawson in July 2019 that replaced his previous letter agreement that became effective in June 2017. The agreement sets forth certain agreed upon terms and conditions of employment. Mr. Lawson was initially entitled to receive an annual base salary of $333,000 (which has been increased, most recently in January 2022 to $345,000), an annual target performance bonus of up to 50% of his base salary as determined by our Board of Directors, and certain severance benefits, which were superseded and replaced by the terms of our Severance Plan, as further described below under “—Potential Payments and Benefits upon Termination or Change of Control.” Mr. Lawson's base salary and target bonus percentage are subject to modification from time to time in the discretion of our Board of Directors or any authorized committee thereof.
84
Potential Payments and Benefits upon Termination or Change of Control
In October 2020, our Compensation Committee adopted our Severance and Change in Control Plan, or the Severance Plan, which provides for severance and/or change in control benefits to our named executive officers upon (i) a “change in control termination” or (ii) a “regular termination” (each as described below). Upon a change in control termination, each of our named executive officers is entitled to receive continued payment of his base salary for a specified period of time (18 months for Mr. Lubniewski, 15 months for Mr. McMeans and 12 months for Mr. Lawson), payment of COBRA premiums for a period of time (up to 18 months for Mr. Lubniewski, 15 months for Mr. McMeans and 12 months for Mr. Lawson) and accelerated vesting of outstanding time-vesting equity awards. Upon a regular termination, each of our named executive officers is entitled to receive continued payment of his base salary for a specified period of time (12 months for Mr. Lubniewski, 12 months for Mr. McMeans and 9 months for Mr. Lawson) and payment of COBRA premiums for a period of time (up to 12 months for Mr. Lubniewski, 12 months for Mr. McMeans and 9 months for Mr. Lawson). All severance benefits under the Severance Plan are subject to the executive’s execution of an effective release of claims against the Company. The Severance Plan superseded and replaced any change in control or severance benefit plans previously provided to our named executive officers, including any such benefits in their amended and restated letter agreements with us.
For purposes of the Severance Plan, a “regular termination” is an involuntary termination (i.e., a termination other than for cause (and not as a result of death or disability) or a resignation for good reason, as defined in the Severance Plan) that does not occur during the period of time beginning three months prior to, and ending 12 months following, a “change in control” (as defined in the 2020 Plan), or the “change in control period.” A “change in control termination” is a regular termination that occurs during the change in control period.
For purposes of the Severance Plan, “cause” generally means the occurrence of any of the following events, conditions or actions with respect to the executive: (1) conviction of any felony or crime involving fraud or dishonesty; (2) participation in any material fraud, material act of dishonesty or other material act of misconduct against us; (3) willful and habitual neglect of the executive’s duties after written notice and opportunity to cure; (4) material violation of any fiduciary duty or duty of loyalty owed to us; (5) breach of any material term of any material contract with us which has a material adverse effect on us; (6) knowing violation of any material company policy which has a material adverse effect on us; or (7) knowing violation of state or federal law in connection with the performance of the executive’s job which has a material adverse effect on us.
For purposes of the Severance Plan, “good reason” generally means the following undertaken by us with respect to the executive without the executive’s prior written consent: (1) a material reduction in base salary; (2) a material reduction in the executive’s authority, duties or responsibilities; (3) a material reduction in the authority, duties or responsibilities of the supervisor to whom the executive is required to report (which, with respect to Mr. Lubniewski, includes a change requiring him to report to a corporate officer or employee rather than directly to the Board of Directors); (4) a material breach by the Company of any provision of the Severance Plan or any other material agreement between the executive and the Company concerning the terms and conditions of the executive’s employment; or (5) a relocation of the executive’s principal place of employment to a place that increases the executive’s one-way commute by more than 50 miles.
Each of our named executive officers holds stock options under our equity incentive plans that were granted subject to our form of stock option agreements. A description of the termination and change of control provisions in such equity incentive plans and stock options granted thereunder is provided below under “– Equity Benefit Plans” and the specific vesting terms of each named executive officer’s stock options are described below under “– Outstanding Equity Awards at Fiscal Year-End.”
85
Outstanding Equity Awards at Fiscal Year-End
The following table presents information concerning equity awards held by our named executive officers as of December 31, 2022.
|
|
|
|
|
Option Awards |
|
Stock Awards |
|
|||||||||||||||||
Name |
|
|
Grant Date/Vesting Commencement Date |
|
Number of Securities Underlying Unexercised Options (#) Exercisable |
|
Number of Securities Underlying Unexercised Options (#) Unexercisable |
|
|
|
Option Exercise Price ($) |
|
|
Option Expiration Date |
|
Number of Shares or Units of Stock That Have Not Vested (#) |
|
|
Market Value of Shares or Units of Stock That Have Not Vested ($) |
|
|||||
John L. Lubniewski |
|
|
2/01/2013 |
|
|
19 |
|
|
— |
|
|
|
|
387.00 |
|
|
2/01/2023 |
|
|
— |
|
|
|
— |
|
|
|
|
8/06/2013 |
|
|
72 |
|
|
— |
|
|
|
|
387.00 |
|
|
8/06/2023 |
|
|
— |
|
|
|
— |
|
|
|
|
3/20/2014 |
|
|
165 |
|
|
— |
|
|
|
|
387.00 |
|
|
3/20/2024 |
|
|
— |
|
|
|
— |
|
|
|
|
12/29/2014 |
|
|
20 |
|
|
— |
|
|
|
|
2,320.20 |
|
|
12/29/2024 |
|
|
— |
|
|
|
— |
|
|
|
|
2/16/2016 |
|
|
194 |
|
|
— |
|
|
|
|
424.80 |
|
|
2/16/2026 |
|
|
— |
|
|
|
— |
|
|
|
|
2/13/2017 |
|
|
111 |
|
|
— |
|
|
|
|
345.60 |
|
|
2/13/2027 |
|
|
— |
|
|
|
— |
|
|
|
|
8/16/2018 |
|
|
555 |
|
|
— |
|
|
|
|
612.00 |
|
|
8/16/2028 |
|
|
— |
|
|
|
— |
|
|
|
|
5/23/2019 |
|
|
1,666 |
|
|
— |
|
|
|
|
399.60 |
|
|
5/23/2029 |
|
|
— |
|
|
|
— |
|
|
|
|
8/15/2019 |
|
|
625 |
|
|
— |
|
|
|
|
171.00 |
|
|
8/15/2029 |
|
|
— |
|
|
|
— |
|
|
|
|
1/23/2020 |
|
|
2,986 |
|
|
— |
|
|
|
|
118.80 |
|
|
1/23/2030 |
|
|
— |
|
|
|
— |
|
|
|
(1) |
8/20/2020 |
|
|
4,028 |
|
|
2,638 |
|
|
|
|
86.40 |
|
|
8/20/2030 |
|
|
— |
|
|
|
— |
|
|
|
(2) |
8/18/2022 |
|
|
2,500 |
|
|
7,500 |
|
|
|
|
11.28 |
|
|
8/18/2032 |
|
|
— |
|
|
|
— |
|
Shaun D. McMeans |
|
|
2/01/2013 |
|
|
10 |
|
|
— |
|
|
|
|
387.00 |
|
|
2/01/2023 |
|
|
— |
|
|
|
— |
|
|
|
|
8/06/2013 |
|
|
72 |
|
|
— |
|
|
|
|
387.00 |
|
|
8/06/2023 |
|
|
— |
|
|
|
— |
|
|
|
|
3/20/2014 |
|
|
168 |
|
|
— |
|
|
|
|
387.00 |
|
|
3/20/2024 |
|
|
— |
|
|
|
— |
|
|
|
|
12/29/2014 |
|
|
12 |
|
|
— |
|
|
|
|
2,320.20 |
|
|
12/29/2024 |
|
|
— |
|
|
|
— |
|
|
|
|
2/16/2016 |
|
|
111 |
|
|
— |
|
|
|
|
424.80 |
|
|
2/16/2026 |
|
|
— |
|
|
|
— |
|
|
|
|
2/13/2017 |
|
|
102 |
|
|
— |
|
|
|
|
345.60 |
|
|
2/13/2027 |
|
|
— |
|
|
|
— |
|
|
|
|
8/16/2018 |
|
|
555 |
|
|
— |
|
|
|
|
612.00 |
|
|
8/16/2028 |
|
|
— |
|
|
|
— |
|
|
|
|
8/15/2019 |
|
|
263 |
|
|
— |
|
|
|
|
171.00 |
|
|
8/15/2029 |
|
|
— |
|
|
|
— |
|
|
|
|
1/23/2020 |
|
|
1,319 |
|
|
— |
|
|
|
|
118.80 |
|
|
1/23/2030 |
|
|
— |
|
|
|
— |
|
|
|
(1) |
8/20/2020 |
|
|
1,678 |
|
|
1,099 |
|
|
|
|
86.40 |
|
|
8/20/2030 |
|
|
— |
|
|
|
— |
|
|
|
(2) |
8/18/2022 |
|
|
1,250 |
|
|
3,750 |
|
|
|
|
11.28 |
|
|
8/18/2032 |
|
|
— |
|
|
|
— |
|
Byron T. Lawson |
|
|
2/1/2013 |
|
|
1 |
|
|
— |
|
|
|
|
387.00 |
|
|
2/1/2023 |
|
|
— |
|
|
|
— |
|
|
|
|
8/6/2013 |
|
|
10 |
|
|
— |
|
|
|
|
387.00 |
|
|
8/6/2023 |
|
|
— |
|
|
|
— |
|
|
|
|
3/20/2014 |
|
|
29 |
|
|
— |
|
|
|
|
387.00 |
|
|
3/20/2024 |
|
|
— |
|
|
|
— |
|
|
|
|
12/29/2014 |
|
|
10 |
|
|
— |
|
|
|
|
2,320.20 |
|
|
12/29/2024 |
|
|
— |
|
|
|
— |
|
|
|
|
11/25/2015 |
|
|
55 |
|
|
— |
|
|
|
|
914.40 |
|
|
11/25/2025 |
|
|
— |
|
|
|
— |
|
|
|
|
5/25/2016 |
|
|
27 |
|
|
— |
|
|
|
|
509.40 |
|
|
5/25/2026 |
|
|
— |
|
|
|
— |
|
|
|
|
1/31/2017 |
|
|
55 |
|
|
— |
|
|
|
|
315.00 |
|
|
1/31/2027 |
|
|
— |
|
|
|
— |
|
|
|
|
5/31/2017 |
|
|
27 |
|
|
— |
|
|
|
|
622.80 |
|
|
5/31/2027 |
|
|
— |
|
|
|
— |
|
|
|
|
7/25/2017 |
|
|
41 |
|
|
— |
|
|
|
|
430.20 |
|
|
7/25/2027 |
|
|
— |
|
|
|
— |
|
|
|
|
8/16/2018 |
|
|
166 |
|
|
— |
|
|
|
|
612.00 |
|
|
8/16/2028 |
|
|
— |
|
|
|
— |
|
|
|
|
8/6/2019 |
|
|
361 |
|
|
— |
|
|
|
|
248.40 |
|
|
8/6/2029 |
|
|
— |
|
|
|
— |
|
|
|
|
9/12/2019 |
|
|
194 |
|
|
— |
|
|
|
|
144.00 |
|
|
9/12/2029 |
|
|
— |
|
|
|
— |
|
|
|
|
1/7/2020 |
|
|
388 |
|
|
— |
|
|
|
|
135.00 |
|
|
1/7/2030 |
|
|
— |
|
|
|
— |
|
|
|
(1) |
8/20/2020 |
|
|
907 |
|
|
593 |
|
|
|
|
86.40 |
|
|
8/20/2030 |
|
|
— |
|
|
|
— |
|
|
|
(2) |
8/18/2022 |
|
|
416 |
|
|
1,250 |
|
|
|
|
11.28 |
|
|
8/18/2032 |
|
|
— |
|
|
|
— |
|
86
Equity Benefit Plans
2021 Inducement Plan
In July 2021, the Company’s Board of Directors adopted the Company’s 2021 Inducement Plan (the “2021 Inducement Plan”), pursuant to which 25,000 shares were initially authorized and reserved for issuance exclusively for the grant of awards to individuals who were not previously employees or non-employee directors of the Company, as inducement material to the individuals’ entering into employment with the Company (“Inducement Awards”). There were 13,961 shares of the Company’s stock available for issuance under the 2021 Inducement Plan as of December 31, 2022, in addition to shares that may become available from time to time as shares of the Company’s common stock subject to outstanding awards granted under the 2021 Inducement Plan are forfeited back to or repurchased by the Company because of the failure to meet a contingency or condition required for the vesting of such shares.
Stock Awards. The 2021 Inducement Plan provides for the grant of nonstatutory stock options (“NSOs”), stock appreciation rights, restricted stock awards, restricted stock unit awards, performance awards and other awards.
Share Reserve. Subject to adjustment for certain changes in our capitalization, the aggregate number of shares of our common stock that may be issued under the 2021 Inducement Plan will not exceed 25,000 shares.
If any shares of our common stock issued pursuant to an award granted under the 2021 Inducement Plan are forfeited back to or redeemed or repurchased by us because of the failure to meet a contingency or condition required for the vesting of such shares, then such shares will become available again for issuance under the 2021 Inducement Plan.
The following shares of our common stock will not become available again for issuance under the 2021 Inducement Plan: (i) any shares repurchased by us on the open market with the proceeds of the exercise or strike price of an award granted under the 2021 Inducement Plan or a Prior Plan Award; and (ii) in the event that a stock appreciation right granted under the 2021 Inducement Plan or a stock appreciation right that is a Prior Plan Award is settled in shares, the gross number of shares subject to such award.
Eligibility. Awards may only be granted to persons who are Eligible Employees described in Section 1(a) of the Plan, where the Award is an inducement material to the individual’s entering into employment with the Company or an Affiliate within the meaning of Rule 5635(c)(4) of the Nasdaq Marketplace Rules or is otherwise permitted pursuant to Rule 5635(c) of the Nasdaq Marketplace Rules.
Administration. The 2021 Inducement Plan will be administered by our Board of Directors, which may in turn delegate some or all of the administration of the 2021 Inducement Plan to a committee or committees composed of members of the board of directors. Our Board of Directors has delegated concurrent authority to administer the 2021 Inducement Plan to our Compensation Committee, but may, at any time, revest in itself some or all of the power delegated to our Compensation Committee. We refer to the plan administrator as the “Plan Administrator” herein.
2020 Equity Incentive Plan
In August 2020, the Company’s stockholders, upon the recommendation of the Company’s Board of Directors, approved the 2020 Equity Incentive Plan (the “2020 Plan”) as a successor to and continuation of the 2014 Plan. As of December 31, 2022, option awards covering an aggregate of 54,042 shares of our common stock under the 2020 Plan were outstanding.
Stock Awards. The 2020 Plan provides for the grant of incentive stock options (“ISOs”), nonstatutory stock options (“NSOs”), stock appreciation rights, restricted stock awards, restricted stock unit awards, performance awards and other awards.
Share Reserve. Subject to adjustment for certain changes in our capitalization, the aggregate number of shares of our common stock that may be issued under the 2020 Plan will not exceed 62,057 shares, which number is the sum of (i) the number of shares remaining available for the grant of new awards under the 2014 Plan (excluding shares available for the grant of inducement awards under the 2014 Plan’s inducement share pool) as of immediately prior to the effective date of the 2020 Plan; (ii) 56,344 new shares; and (iii) the number of the Prior Plan Returning Shares (as defined below), if any, as such shares become available from time to time.
87
The “Prior Plan Returning Shares” are shares of our common stock subject to outstanding awards granted under the 2014 Plan (excluding shares available for the granting of inducement awards under the 2014 Plan), the 2011 Plan or 2001 Plan (together, the “Prior Plans,” and each such award, a “Prior Plan Award”) that, following the effective date of the 2020 Plan: (i) are not issued because such award or any portion thereof expires or otherwise terminates without all of the shares covered by such award having been issued; (ii) are not issued because such award or any portion thereof is settled in cash; or (iii) are forfeited back to or repurchased by us because of the failure to meet a contingency or condition required for the vesting of such shares. The number of shares of our common stock available for issuance under the 2020 Plan will be reduced or increased by (i) one share for each share of common stock issued pursuant to an Appreciation Award (as defined in the 2020 Plan), and (ii) 1.5 shares for each share of common stock issued pursuant to a Full Value Award (as defined in the 2020 Plan). The following actions will not result in an issuance of shares of our common stock under the 2020 Plan and accordingly will not reduce the number of shares of our common stock available for issuance under the 2020 Plan: (i) the expiration or termination of any portion of an award granted under the 2020 Plan without the shares covered by such portion of the award having been issued; or (ii) the settlement of any portion of an award granted under the 2020 Plan in cash.
If any shares of our common stock issued pursuant to an award granted under the 2020 Plan are forfeited back to or redeemed or repurchased by us because of the failure to meet a contingency or condition required for the vesting of such shares, then such shares will become available again for issuance under the 2020 Plan.
The following shares of our common stock will not become available again for issuance under the 2020 Plan: (i) any shares that are reacquired or withheld (or not issued) by us to satisfy the exercise or strike price of an award granted under the 2020 Plan or a Prior Plan Award (including any shares subject to such award that are not delivered because such award is exercised through a reduction of shares subject to such award); (ii) any shares that are reacquired or withheld (or not issued) by us to satisfy a tax withholding obligation in connection with an award granted under the 2020 Plan or a Prior Plan Award; (iii) any shares repurchased by us on the open market with the proceeds of the exercise or strike price of an award granted under the 2020 Plan or a Prior Plan Award; and (iv) in the event that a stock appreciation right granted under the 2020 Plan or a stock appreciation right that is a Prior Plan Award is settled in shares, the gross number of shares subject to such award.
Eligibility. All of our (including our affiliates’) employees, non-employee directors and consultants are eligible to participate in the 2020 Plan and may receive all types of awards other than incentive stock options. Incentive stock options may be granted under the 2020 Plan only to our (including our affiliates’) employees.
Administration. The 2020 Plan will be administered by our Board of Directors, which may in turn delegate some or all of the administration of the 2020 Plan to a committee or committees composed of members of the board of directors. Our Board of Directors has delegated concurrent authority to administer the 2020 Plan to our Compensation Committee, but may, at any time, revest in itself some or all of the power delegated to our Compensation Committee. We refer to the plan administrator as the “Plan Administrator” herein.
Subject to the terms of the 2020 Plan, the Plan Administrator may determine the recipients, the types of awards to be granted, the number of shares of our common stock subject to or the cash value of awards, and the terms and conditions of awards granted under the 2020 Plan, including the period of their exercisability and vesting. The Plan Administrator has the authority to provide for accelerated exercisability and vesting of awards. Subject to the limitations set forth below, the Plan Administrator also determines the fair market value applicable to an award and the exercise or strike price of stock options and stock appreciation rights granted under the 2020 Plan.
In addition, the Plan Administrator may delegate to one or more executive officers the authority to designate employees who are not executive officers to be recipients of certain awards and the number of shares of our common stock subject to such awards. Under any such delegation, the Plan Administrator will specify the total number of shares of our common stock that may be subject to the awards granted by such executive officer. The executive officer may not grant an award to himself or herself.
Repricing; Cancellation and Re-Grant of Stock Options or Stock Appreciation Rights. Under the 2020 Plan, unless our stockholders have approved such an action within 12 months prior to such an event, the Plan Administrator does not have the authority to reprice any outstanding stock option or stock appreciation right by (1) reducing the exercise or strike price of the stock option or stock appreciation right, or (2) canceling any outstanding stock option or stock appreciation right that has an exercise or strike price greater than the then-current fair market value of our common stock in exchange for cash or other awards.
88
Limit on Non-Employee Director Compensation. Pursuant to the 2020 Plan, the aggregate value of all compensation granted or paid, as applicable, by the Company to any individual for service as a non-employee director with respect to any period commencing on the date of the annual meeting of stockholders for a particular year and ending on the day immediately prior to the date of the annual meeting of stockholders for the next subsequent year (the “Annual Period”), including awards granted and cash fees paid by the Company to such non-employee director, will not exceed (i) $400,000 in total value or (ii) in the event such non-employee director is first appointed or elected to the Board of Directors during such Annual Period, $600,000 in total value, in each case calculating the value of any equity awards based on the grant date fair value of such equity awards for financial reporting purposes.
Dividends and Dividend Equivalents. The 2020 Plan provides that dividends or dividend equivalents may be paid or credited with respect to any shares of our common stock subject to an award other than an option or stock appreciation right, as determined by the Plan Administrator and contained in the applicable award agreement; provided, however, that (i) no dividends or dividend equivalents may be paid with respect to any such shares before the date such shares have vested, (ii) any dividends or dividend equivalents that are credited with respect to any such shares will be subject to all of the terms and conditions applicable to such shares under the terms of the applicable award agreement (including any vesting conditions), and (iii) any dividends or dividend equivalents that are credited with respect to any such shares will be forfeited to us on the date such shares are forfeited to or repurchased by us due to a failure to vest.
Stock Options. Stock options may be granted under the 2020 Plan pursuant to stock option agreements. The 2020 Plan permits the grant of stock options that are intended to qualify as ISOs and NSOs.
The exercise price of a stock option granted under the 2020 Plan may not be less than 100% of the fair market value of the common stock subject to the stock option on the date of grant and, in some cases (see “-Limitations on Incentive Stock Options” below), may not be less than 110% of such fair market value.
The term of stock options granted under the 2020 Plan may not exceed ten years from the date of grant and, in some cases (see “-Limitations on Incentive Stock Options” below), may not exceed five years from the date of grant. Except as otherwise provided in a participant’s stock option agreement or other written agreement with us or one of our affiliates, if a participant’s service relationship with us or any of our affiliates (“continuous service” as defined in the 2020 Plan) terminates (other than for cause (as defined in the 2020 Plan) or the participant’s death or disability (as defined in the 2020 Plan)), the participant may exercise any vested stock options for up to three months following the participant’s termination of continuous service. Except as otherwise provided in a participant’s stock option agreement or other written agreement with us or one of our affiliates, if a participant’s continuous service terminates due to the participant’s disability, the participant may exercise any vested stock options for up to 12 months following the participant’s termination due to the participant’s disability. Except as otherwise provided in a participant’s stock option agreement or other written agreement with us or one of our affiliates, if a participant’s continuous service terminates due to the participant’s death (or the participant dies within a specified period following termination of continuous service), the participant’s beneficiary may exercise any vested stock options for up to 18 months following the participant’s death. Except as explicitly provided otherwise in a participant’s stock option agreement or other written agreement with us or one of our affiliates, if a participant’s continuous service is terminated for cause, all stock options held by the participant will terminate upon the participant’s termination of continuous service and the participant will be prohibited from exercising any stock option from and after such termination date. Except as otherwise provided in a participant’s stock option agreement or other written agreement with us or one of our affiliates, the term of a stock option may be extended if a participant’s continuous service terminates for any reason other than for cause and, at any time during the applicable post-termination exercise period, the exercise of the stock option would be prohibited by applicable laws or the sale of any common stock received upon such exercise would violate our insider trading policy. In no event, however, may a stock option be exercised after its original expiration date.
Acceptable forms of consideration for the purchase of our common stock pursuant to the exercise of a stock option under the 2020 Plan will be determined by the Plan Administrator and may include payment: (i) by cash, check, bank draft or money order payable to us; (ii) pursuant to a program developed under Regulation T as promulgated by the Federal Reserve Board; (iii) by delivery to us of shares of our common stock (either by actual delivery or attestation); (iv) by a net exercise arrangement (for NSOs only); or (v) in other legal consideration approved by the Plan Administrator.
Stock options granted under the 2020 Plan may become exercisable in cumulative increments, or “vest,” as determined by the Plan Administrator at the rate specified in the stock option agreement. Shares covered by different stock options granted under the 2020 Plan may be subject to different vesting schedules as the Plan Administrator may determine.
89
The Plan Administrator may impose limitations on the transferability of stock options granted under the 2020 Plan in its discretion. Generally, a participant may not transfer a stock option granted under the 2020 Plan other than by will or the laws of descent and distribution or, subject to approval by the Plan Administrator, pursuant to a domestic relations order. However, the Plan Administrator may permit transfer of a stock option in a manner that is not prohibited by applicable tax and securities laws. Options may not be transferred to a third-party financial institution for value.
Limitations on Incentive Stock Options. In accordance with current federal tax laws, the aggregate fair market value, determined at the time of grant, of shares of our common stock with respect to ISOs that are exercisable for the first time by a participant during any calendar year under all of our stock plans may not exceed $100,000. The stock options or portions of stock options that exceed this limit or otherwise fail to qualify as ISOs are treated as NSOs. No ISO may be granted to any person who, at the time of grant, owns or is deemed to own stock possessing more than 10% of our total combined voting power unless the following conditions are satisfied:
Subject to adjustment for certain changes in our capitalization, the aggregate maximum number of shares of our common stock that may be issued pursuant to the exercise of ISOs under the 2020 Plan is 124,591 shares.
Stock Appreciation Rights. Stock appreciation rights may be granted under the 2020 Plan pursuant to stock appreciation right agreements. Each stock appreciation right is denominated in common stock share equivalents. The strike price of each stock appreciation right will be determined by the Plan Administrator but will in no event be less than 100% of the fair market value of the common stock subject to the stock appreciation right on the date of grant. The term of stock appreciation rights granted under the 2020 Plan may not exceed ten years from the date of grant. The Plan Administrator may also impose restrictions or conditions upon the vesting of stock appreciation rights that it deems appropriate. The appreciation distribution payable upon exercise of a stock appreciation right may be paid in shares of our common stock, in cash, in a combination of cash and stock, or in any other form of consideration determined by the Plan Administrator and set forth in the stock appreciation right agreement. Stock appreciation rights will be subject to the same conditions upon termination of continuous service and restrictions on transfer as stock options under the 2020 Plan.
Restricted Stock Awards. Restricted stock awards may be granted under the 2020 Plan pursuant to restricted stock award agreements. A restricted stock award may be granted in consideration for cash, check, bank draft or money order payable to us, the participant’s services performed for us, or any other form of legal consideration acceptable to the Plan Administrator. Shares of our common stock acquired under a restricted stock award may be subject to forfeiture to or repurchase by us in accordance with a vesting schedule to be determined by the Plan Administrator. Rights to acquire shares of our common stock under a restricted stock award may be transferred only upon such terms and conditions as are set forth in the restricted stock award agreement. Upon a participant’s termination of continuous service for any reason, any shares subject to restricted stock awards held by the participant that have not vested as of such termination date may be forfeited to or repurchased by us.
Restricted Stock Unit Awards. Restricted stock unit awards may be granted under the 2020 Plan pursuant to restricted stock unit award agreements. Payment of any purchase price may be made in any form of legal consideration acceptable to the Plan Administrator. A restricted stock unit award may be settled by the delivery of shares of our common stock, in cash, in a combination of cash and stock, or in any other form of consideration determined by the Plan Administrator and set forth in the restricted stock unit award agreement. Restricted stock unit awards may be subject to vesting in accordance with a vesting schedule to be determined by the Plan Administrator. Except as otherwise provided in a participant’s restricted stock unit award agreement or other written agreement with us, restricted stock units that have not vested will be forfeited upon the participant’s termination of continuous service for any reason.
Performance Awards. The 2020 Plan allows us to grant performance awards. A performance award is an award that may vest or may be exercised, or that may become earned and paid, contingent upon the attainment of certain performance goals during a performance period. A performance award may require the completion of a specified period of continuous service. The length of any performance period, the performance goals to be achieved during the performance period, and the measure of whether and to what degree such performance goals have been attained will be determined by the Plan Administrator in its discretion. In addition, to the extent permitted by applicable law and the applicable award agreement, the Plan Administrator may determine that cash may be used in payment of performance awards.
Performance goals under the 2020 Plan will be established by the board of directors for a performance period. The performance criteria used to establish such goals may be based on any measure of performance selected by the board of directors.
90
Performance goals may be based on a Company-wide basis, with respect to one or more business units, divisions, affiliates or business segments, and in either absolute terms or relative to the performance of one or more comparable companies or the performance of one or more relevant indices. Unless specified otherwise by the Plan Administrator (i) in the award agreement at the time the award is granted or (ii) in such other document setting forth the performance goals at the time the performance goals are established, the Plan Administrator will appropriately make adjustments in the method of calculating the attainment of the performance goals for a performance period as follows: (1) to exclude restructuring and/or other nonrecurring charges; (2) to exclude exchange rate effects; (3) to exclude the effects of changes to generally accepted accounting principles; (4) to exclude the effects of any statutory adjustments to corporate tax rates; (5) to exclude the effects of items that are “unusual” in nature or occur “infrequently” as determined under generally accepted accounting principles; (6) to exclude the dilutive effects of acquisitions or joint ventures; (7) to assume that any business divested by the Company achieved performance objectives at targeted levels during the balance of a performance period following such divestiture; (8) to exclude the effect of any change in the outstanding shares of common stock of the Company by reason of any stock dividend or split, stock repurchase, reorganization, recapitalization, merger, consolidation, spin-off, combination or exchange of shares or other similar corporate change, or any distributions to common stockholders other than regular cash dividends; (9) to exclude the effects of stock based compensation and the award of bonuses under the Company’s bonus plans; (10) to exclude costs incurred in connection with potential acquisitions or divestitures that are required to be expensed under generally accepted accounting principles; (11) to exclude the goodwill and intangible asset impairment charges that are required to be recorded under generally accepted accounting principles; (12) to exclude the effect of any other unusual, non-recurring gain or loss or other extraordinary item; and (13) to exclude the effects of the timing of acceptance for review and/or approval of submissions to the U.S. Food and Drug Administration or any other regulatory body.
In addition, the Plan Administrator retains the discretion to define the manner of calculating the performance criteria it selects to use for a performance period and to reduce, increase or eliminate the compensation or economic benefit due upon the attainment of any performance goal.
Other Awards. Other forms of awards valued in whole or in part by reference to, or otherwise based on, our common stock, may be granted either alone or in addition to other awards under the 2020 Plan; provided that any such award will be treated as a Full Value Award. Subject to the terms of the 2020 Plan, the Plan Administrator will have sole and complete authority to determine the persons to whom and the time or times at which such other awards will be granted, the number of shares of our common stock to be granted and all other terms and conditions of such other awards.
Clawback Policy. Awards granted under the 2020 Plan will be subject to recoupment in accordance with any clawback policy that we are required to adopt pursuant to the listing standards of any national securities exchange or association on which our securities are listed or as is otherwise required by the Dodd-Frank Wall Street Reform and Consumer Protection Act or other applicable law, and any other clawback policy that the Company adopts. In addition, the board of directors may impose such other clawback, recovery or recoupment provisions in an award agreement as the board of directors determines necessary or appropriate, including but not limited to a reacquisition right in respect of previously acquired shares of common stock or other cash or property upon the occurrence of cause.
Changes to Capital Structure. In the event of certain capitalization adjustments, the Plan Administrator will appropriately and proportionately adjust: (i) the class(es) and maximum number of shares of our common stock subject to the 2020 Plan; (ii) the class(es) and maximum number of shares of our common stock that may be issued pursuant to the exercise of ISOs; and (iii) the class(es) and number of shares of our common stock and the exercise, strike or purchase price per share of our common stock subject to outstanding awards.
Corporate Transaction and Change in Control. The following applies to each outstanding award under the 2020 Plan in the event of a corporate transaction (as defined in the 2020 Plan and described below) or a change in control (as defined in the 2020 Plan and described below), unless provided otherwise in the applicable award agreement or in any other written agreement between a participant and the Company or an affiliate. The term “Transaction” will mean such corporate transaction or change in control.
91
In the event of a Transaction, any awards outstanding under the 2020 Plan may be assumed, continued or substituted for by any surviving or acquiring corporation (or its parent company) (such entity, the “acquiring entity”), and any reacquisition or repurchase rights held by us with respect to the award may be assigned to the acquiring entity. If the acquiring entity does not assume, continue or substitute for such awards, then with respect to any such awards that are held by participants whose continuous service has not terminated prior to the effective time of the Transaction (such participants, the “current participants”), the vesting (and exercisability, if applicable) of such awards will be accelerated in full to a date prior to the effective time of the Transaction (contingent upon the effectiveness of the Transaction), and such awards will terminate if not exercised (if applicable) at or prior to the effective time of the Transaction, and any reacquisition or repurchase rights held by us with respect to such awards will lapse (contingent upon the effectiveness of the Transaction). With respect to the vesting of performance awards that will accelerate upon the occurrence of a Transaction, unless otherwise provided in the relevant award agreement, the vesting of such performance awards will accelerate at 100% of the target level upon the occurrence of the Transaction. If the acquiring entity does not assume, continue or substitute for such awards, then any such awards that are held by persons other than current participants will terminate if not exercised (if applicable) at or prior to the effective time of the Transaction, except that any reacquisition or repurchase rights held by us with respect to such awards will not terminate and may continue to be exercised notwithstanding the Transaction.
In the event an award will terminate if not exercised at or prior to the effective time of a Transaction, the Plan Administrator may provide that the holder of such award may not exercise such award but instead will receive a payment equal in value to the excess, if any, of (i) the value of the property the participant would have received upon the exercise of the award, over (ii) any exercise price payable by such holder in connection with such exercise.
Under the 2020 Plan, a “corporate transaction” generally means the consummation of any one or more of the following events: (1) a sale or other disposition of all or substantially all of our assets; (2) a sale or other disposition of at least 50% of our outstanding securities; (3) a merger, consolidation or similar transaction where we do not survive the transaction; or (4) a merger, consolidation or similar transaction where we do survive the transaction but the shares of our common stock outstanding immediately before such transaction are converted or exchanged into other property by virtue of the transaction.
Under the 2020 Plan, a “change in control” generally means the occurrence of any one or more of the following events: (1) the acquisition by any person, entity or group of our securities representing more than 50% of the combined voting power of our then outstanding securities, other than by virtue of a merger, consolidation, or similar transaction; (2) a consummated merger, consolidation or similar transaction in which our stockholders immediately before such transaction do not own, directly or indirectly, more than 50% of the combined voting power of the surviving entity (or the parent of the surviving entity) in substantially the same proportions as their ownership immediately prior to such transaction; or (3) a consummated sale, lease, exclusive license or other disposition of all or substantially all of our assets, other than to an entity, more than 50% of the combined voting power of which is owned by our stockholders in substantially the same proportions as their ownership of our outstanding voting securities immediately prior to such transaction.
Plan Amendments and Termination. The Plan Administrator will have the authority to amend or terminate the 2020 Plan at any time. However, except as otherwise provided in the 2020 Plan, no amendment or termination of the 2020 Plan may materially impair a participant’s rights under his or her outstanding awards without the participant’s consent. We will obtain stockholder approval of any amendment to the 2020 Plan as required by applicable law and listing requirements.
2014 Equity Incentive Plan
Our Board of Directors adopted the 2014 Plan in December 2014 and our stockholders approved the 2014 Plan in April 2015. The 2014 Plan became effective on May 5, 2015 in connection with our initial public offering. In August 2020, upon the effective date of the 2020 Plan, the 2014 Plan ceased to be available for new grants of equity awards, and any shares remaining available for issuance under the 2014 Plan (excluding shares available for the granting of inducement awards under the 2014 Plan’s inducement share pool) became available for issuance under the 2020 Plan.
In May 2019, 1,111 shares were reserved for issuance under the 2014 Plan pursuant to an amendment approved by our Board of Directors pursuant to Rule 5635(c)(4) of the Nasdaq Listing Rules, to be used exclusively for the grant of awards to individuals who were not previously employees or non-employee directors of the Company, as inducement material to the individuals’ entering into employment (“Inducement Awards”).
As of December 31, 2022, option awards covering an aggregate of 12,672 shares of our common stock have been granted under the 2014 Plan and were outstanding.
92
2011 Equity Incentive Plan
General. Our Board of Directors and our stockholders approved our 2011 Plan in March 2011. The 2011 Plan was subsequently amended by our Board of Directors and our stockholders, most recently in February 2014. The 2011 Plan is the successor to and continuation of our 2001 Plan. As of December 31, 2022, option awards under the 2011 Plan covering an aggregate of 637 shares of our common stock were outstanding. No additional awards will be granted under the 2011 Plan and all outstanding awards granted under the 2011 Plan that are repurchased, forfeited, expire or are cancelled will become available for grant under the 2020 Plan in accordance with its terms.
2014 Employee Stock Purchase Plan
General. Our Board of Directors adopted the 2014 ESPP in December 2014 and our stockholders approved the 2014 ESPP in April 2015. The 2014 ESPP became effective on May 5, 2015 in connection with our initial public offering. The purpose of our employee stock purchase plans is to retain the services of new employees and secure the services of new and existing employees while providing incentives for such individuals to exert maximum efforts toward our success and that of our affiliates. The employee stock purchase plans are intended to qualify as an “employee stock purchase plan” within the meaning of Section 423 of the Code. Our Board of Directors has delegated its authority to administer the ESPP to our compensation committee.
The 2014 ESPP initially authorized the issuance of 615 shares of our common stock pursuant to purchase rights granted to our employees or to employees of any of our designated affiliates. The number of shares of our common stock reserved for issuance automatically increases on January 1 of each calendar year, from January 1, 2016 through January 1, 2024 by the least of (1) 1% of the total number of shares of our common stock outstanding on December 31 of the preceding calendar year, (2) 1,083 shares, or (3) a number determined by our Board of Directors that is less than (1) and (2).
In August 2021, the Company’s stockholders, upon the recommendation of the Company’s Board of Directors, approved the Amended and Restated 2014 Employee Stock Purchase Plan (the “Amended 2014 ESPP”). Upon approval of the Amended 2014 ESPP, 41,666 shares of the Company’s common stock were reserved for issuance under the Amended 2014 ESPP in addition to 2,023 shares of the Company’s common stock reserved for issuance under the original 2014 Employee Stock Purchase Plan. The Amended 2014 ESPP does not contain an evergreen provision.
Offerings and Purchases. The Amended 2014 ESPP is implemented through a series of offerings of purchase rights to eligible employees. Under the Amended 2014 ESPP, we may specify offerings with durations of not more than 27 months and may specify shorter purchase periods within each offering. Each offering will have one or more purchase dates on which shares of our common stock will be purchased for employees participating in the offering. Generally, all regular employees, including executive officers, subject to certain restrictions, employed by us or by any of our designated affiliates, may participate in the Amended 2014 ESPP and may contribute, normally through payroll deductions, up to 15% of their earnings for the purchase of our common stock under the Amended 2014 ESPP. Unless otherwise determined by our Board of Directors, common stock will be purchased for accounts of employees participating in the Amended 2014 ESPP at a price per share equal to the lower of (1) 85% of the fair market value of a share of our common stock on the first date of an offering or (2) 85% of the fair market value of a share of our common stock on the date of purchase.
Changes to Capital Structure. In the event that there occurs a change in our capital structure through such actions as a stock split, merger, consolidation, reorganization, recapitalization, reincorporation, stock dividend, dividend in property other than cash, large nonrecurring cash dividend, liquidating dividend, combination of shares, exchange of shares, change in corporate structure or similar transaction, the board of directors will make appropriate adjustments to (1) the number of shares reserved under the Amended 2014 ESPP, (2) the maximum number of shares by which the share reserve may increase automatically each year and (3) the number of shares and purchase price of all outstanding purchase rights.
Corporate Transactions. In the event of certain significant corporate transactions, including the consummation of: (1) a sale of all our assets, (2) the sale or disposition of 90% of our outstanding securities, (3) a merger or consolidation where we do not survive the transaction and (4) a merger or consolidation where we do survive the transaction but the shares of our common stock outstanding immediately prior to such transaction are converted or exchanged into other property by virtue of the transaction, any then-outstanding rights to purchase our stock under the Amended 2014 ESPP may be assumed, continued or substituted for by any surviving or acquiring entity (or its parent company). If the surviving or acquiring entity (or its parent company) elects not to assume, continue or substitute for such purchase rights, then the participants’ accumulated payroll contributions will be used to purchase shares of our common stock within ten business days prior to such corporate transaction, and such purchase rights will terminate immediately.
Plan Amendments, Termination. Our Board of Directors has the authority to amend or terminate our Amended 2014 ESPP, provided that except in certain circumstances any such amendment or termination may not materially impair any outstanding purchase rights without the holder’s consent. We will obtain stockholder approval of any amendment to our Amended 2014 ESPP as required by applicable law or listing requirements.
93
Director Compensation
The following table sets forth in summary form information concerning the compensation that we paid or awarded during the year ended December 31, 2022 to each of our non-employee directors:
Name |
|
Fees Earned or Paid in Cash ($) |
|
|
Option Awards ($) (1) |
|
|
Total ($) |
|
|||
Ann F. Hanham |
|
|
75,000 |
|
|
|
5,048 |
|
|
|
80,048 |
|
Thomas W. Dubensky Jr. (2) |
|
|
4,830 |
|
|
|
6,174 |
|
|
|
11,004 |
|
Michelle R. Griffin |
|
|
52,229 |
|
|
|
2,524 |
|
|
|
54,753 |
|
Donnie M. Hardison |
|
|
47,000 |
|
|
|
2,524 |
|
|
|
49,524 |
|
Christopher P. Kiritsy (3) |
|
|
32,473 |
|
|
|
21,359 |
|
|
|
53,832 |
|
Lee R. McCracken |
|
|
46,883 |
|
|
|
2,524 |
|
|
|
49,407 |
|
Harry A. George (4) |
|
|
37,580 |
|
|
|
— |
|
|
|
37,580 |
|
James T. LaFrance (4) |
|
|
42,002 |
|
|
|
— |
|
|
|
42,002 |
|
We have reimbursed and will continue to reimburse all of our non-employee directors for their travel, lodging and other reasonable expenses incurred in attending meetings of our Board of Directors and committees of our Board of Directors, and will pay for the travel, lodging and other reasonable expenses incurred by our employee directors to attend meetings of our Board of Directors and, as applicable, committees of our Board of Directors.
Pursuant to our non-employee director compensation policy, non-employee director compensation for service on our Board of Directors was as follows as of January 1, 2022:
Each of the option grants described above will vest and become exercisable subject to the director’s continuous service with us through each applicable vesting date, provided that each option will vest in full upon a change of control, as defined under the 2020 Plan. The stock options will be granted under the 2020 Plan, the terms of which are described in more detail above under “– Equity Benefit Plans – 2020 Equity Incentive Plan.”
94
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Securities Authorized for Issuance under Equity Compensation Plans
The following table provides certain information with respect to all of the Company’s equity compensation plans in effect as of December 31, 2022.
Equity Compensation Plan Information
Plan Category |
|
Number of |
|
|
Weighted-average exercise price of |
|
|
Number of |
|
|||
Equity compensation plans approved by security |
|
|
|
|
|
|
|
|
|
|||
2011 Equity Incentive Plan |
|
|
637 |
|
|
|
596.40 |
|
|
|
— |
|
2014 Equity Incentive Plan (1) |
|
|
12,672 |
|
|
|
293.71 |
|
|
|
— |
|
2014 Employee Stock Purchase Plan (2) |
|
|
— |
|
|
N/A |
|
|
|
35,120 |
|
|
2020 Equity Incentive Plan |
|
|
54,042 |
|
|
|
35.65 |
|
|
|
16,668 |
|
Equity compensation plans not approved by security holders (3) (4) |
|
|
9,997 |
|
|
|
47.90 |
|
|
|
13,961 |
|
Total |
|
|
77,348 |
|
|
|
|
|
|
51,788 |
|
|
Principal Stockholders
The following table sets forth certain information regarding the ownership of the Company’s common stock as of March 1, 2023 by: (i) each director; (ii) each of our executive officers named in the Summary Compensation Table above; and (iii) all executive officers and directors of the Company as a group. The Company was not aware of any beneficial owners of more than five percent of its common stock as of this date.
95
The table is based upon information supplied by officers, directors and principal stockholders, Schedules 13G filed with the SEC and other sources believed to be reliable by us. Unless otherwise indicated in the footnotes to this table and subject to community property laws where applicable, the Company believes that each of the stockholders named in this table has sole voting and investment power with respect to the shares indicated as beneficially owned. Applicable percentages are based on 2,214,155 shares outstanding on March 1, 2023, adjusted as required by rules promulgated by the SEC. Unless otherwise indicated, the address for each person or entity listed in the table is c/o HTG Molecular Diagnostics, Inc., 3430 E. Global Loop, Tucson, Arizona 85706.
|
|
Common Stock Beneficially Owned |
|
|||||
|
|
Shares |
|
|
Percentage |
|
||
Directors and named executive officers |
|
|
|
|
|
|
||
John L. Lubniewski (1) |
|
|
15,904 |
|
|
* |
|
|
Shaun D. McMeans (2) |
|
|
7,266 |
|
|
* |
|
|
Byron T. Lawson (3) |
|
|
3,521 |
|
|
* |
|
|
Ann Hanham (4) |
|
|
904 |
|
|
* |
|
|
Michelle R. Griffin (5) |
|
|
845 |
|
|
* |
|
|
Donnie M. Hardison (6) |
|
|
966 |
|
|
* |
|
|
Lee McCracken (7) |
|
|
923 |
|
|
* |
|
|
Christopher P. Kiritsy (8) |
|
|
296 |
|
|
* |
|
|
All current executive officers and directors as a |
|
|
33,912 |
|
|
|
1.5 |
% |
* Represents beneficial ownership of less than one percent.
We have adopted a written related-person transactions policy that sets forth our policies and procedures regarding the identification, review, consideration and oversight of “related-person transactions.” For purposes of our policy only, a “related-person transaction” is a transaction, arrangement or relationship (or any series of similar transactions, arrangements or relationships) in which we and any “related person” are participants involving an amount that exceeds the lesser of $120,000 or one percent of the average of the Company’s total assets at year-end for the last two completed fiscal years.
Transactions involving compensation for services provided to us as an employee, consultant or director are not considered related-person transactions under this policy. A “related person” is any executive officer, director or a holder of more than five percent of our common stock, including any of their immediate family members and any entity owned or controlled by such persons.
96
Under the policy, where a transaction has been identified as a related-person transaction, management must present information regarding the proposed related-person transaction to our Audit Committee (or, where review by our Audit Committee would be inappropriate, to another independent body of our Board of Directors) for review. The presentation must include a description of, among other things, the material facts, the direct and indirect interests of the related persons, the benefits of the transaction to us and whether any alternative transactions are available. To identify related-person transactions in advance, we rely on information supplied by our executive officers, directors and certain significant stockholders. In considering related-person transactions, our Audit Committee or other independent body of our Board of Directors takes into account the relevant available facts and circumstances including, but not limited to:
In the event a director has an interest in the proposed transaction, the director must recuse himself or herself from the deliberations and approval.
The following sections summarize transactions since January 1, 2021 to which we have been a party, in which the amount involved in the transaction exceeded the lesser of $120,000 or one percent of the average of the Company’s total assets at year-end for the last two completed fiscal years, and in which any of our directors, executive officers or, to our knowledge, beneficial owners of more than 5% of our capital stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest, other than equity and other compensation, termination, change in control and other arrangements, which are described under “Executive Compensation” and “Director Compensation.”
Indemnification Agreements
We have entered into, and intend to continue to enter into, separate indemnification agreements with our directors and executive officers, in addition to the indemnification provided for in our amended and restated bylaws. These agreements, among other things, require us to indemnify our directors and executive officers for certain expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by a director or executive officer in any action or proceeding arising out of their services as one of our directors or executive officers or any other company or enterprise to which the person provides services at our request. We believe that these bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors and officers.
The limitation of liability and indemnification provisions in our amended and restated certificate of incorporation and amended and restated bylaws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might benefit us and our stockholders. A stockholder’s investment may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions.
Director Independence
Our Board of Directors has determined that all of our directors other than Mr. Lubniewski are independent directors, as defined by Rule 5605(a)(2) of the Nasdaq Listing Rules. The Nasdaq independence definition includes a series of objective tests, including that the director is not, and has not been for at least three years, one of our employees and that neither the director nor any of his family members has engaged in various types of business dealings with us. In addition, as required by Nasdaq rules, our Board of Directors has made a subjective determination as to each independent director that no relationships exist, which, in the opinion of our Board of Directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. In making these determinations, our Board of Directors reviewed and discussed information provided by the directors and us with regard to each director’s business and personal activities and relationships as they may relate to us and our management.
97
Item 14. Principal Accounting Fees and Services.
The following table summarizes the fees of BDO USA, LLP, our independent registered public accounting firm, for 2022 and 2021.
|
|
December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Fee Category |
|
|
|
|
|
|
||
Audit fees (1) |
|
$ |
746,500 |
|
|
$ |
409,889 |
|
Audit-related fees |
|
|
— |
|
|
|
— |
|
Tax fees |
|
|
— |
|
|
|
— |
|
All other fees |
|
|
— |
|
|
|
— |
|
Total fees |
|
$ |
746,500 |
|
|
$ |
409,889 |
|
Pre-Approval Policies and Procedures
Pursuant to its charter, the audit committee must review and approve, in advance, the scope and plans for the audits and the audit fees and approve in advance (or, where permitted under the rules and regulations of the SEC, subsequently) all non-audit services to be performed by the independent auditor that are not otherwise prohibited by law and any associated fees. The audit committee may delegate to one or more members of the committee the authority to pre-approve audit and permissible non-audit services, as long as this pre-approval is presented to the full committee at scheduled meetings. All fees described above were pre-approved by the audit committee.
98
PART IV
Item 15. Exhibits, Financial Statement Schedules.
(a)(1) Consolidated Financial Statements - The consolidated financial statements filed as part of this Annual Report on Form 10-K are listed on the Index to Financial Statements in Item 8.
(a)(2) Financial Statement Schedules
All schedules are omitted because they are not applicable or the required information is shown in the consolidated financial statements or the notes thereto.
(a)(3) Exhibits
The exhibits required by Item 601 of Regulation S-K are listed in paragraph (b) below.
(b) Exhibits.
The exhibits listed on the Exhibit Index immediately preceding the signature page to this Annual Report on Form 10-K are filed herewith or are incorporated by reference to exhibits previously filed with the SEC.
99
Exhibit Index
Exhibit Number |
|
Description |
|
|
|
2.1 |
|
|
|
|
|
3.1 |
|
|
|
|
|
3.2 |
|
|
|
|
|
3.3 |
|
|
|
|
|
3.4 |
|
|
|
|
|
3.5 |
|
|
|
|
|
4.1 |
|
|
|
|
|
4.2 |
|
|
|
|
|
4.3 |
|
|
|
|
|
4.4 |
|
|
|
|
|
4.5 |
|
|
|
|
|
4.6 |
|
|
|
|
|
4.7 |
|
|
|
|
|
4.8 |
|
|
|
|
|
4.9 |
|
|
|
|
|
4.10 |
|
|
|
|
|
4.11 |
|
|
|
|
|
4.12 |
|
|
|
|
|
100
4.13 |
|
|
|
|
|
4.14 |
|
|
|
|
|
4.15 |
|
|
|
|
|
4.16 |
|
|
|
|
|
10.1+ |
|
|
|
|
|
10.2+ |
|
|
|
|
|
10.3+ |
|
|
|
|
|
10.4+ |
|
|
|
|
|
10.5+ |
|
|
|
|
|
10.6+ |
|
|
|
|
|
10.7+ |
|
|
|
|
|
10.8+ |
|
|
|
|
|
10.9+ |
|
HTG Molecular Diagnostics, Inc. Amended and Restated 2014 Employee Stock Purchase Plan. |
|
|
|
10.10 |
|
|
|
|
|
10.11 |
|
|
|
|
|
10.12 |
|
|
|
|
|
10.13 |
|
|
|
|
|
10.14+ |
|
HTG Molecular Diagnostics, Inc. Amended and Restated Non-Employee Director Compensation Policy. |
|
|
|
101
10.15+ |
|
|
|
|
|
10.16+ |
|
|
|
|
|
10.17+ |
|
|
|
|
|
10.18+ |
|
|
|
|
|
10.19 |
|
|
|
|
|
10.20 |
|
|
|
|
|
10.21 |
|
|
|
|
|
10.22 |
|
|
|
|
|
10.23 |
|
Third Amendment, dated September 27, 2021, to Standard Commercial-Industrial Multi Tenant Triple Net Lease, dated July 11, 2008, between the Company and Pegasus Properties L.P. (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K (File No. 0001-37369), filed with the SEC on September 29, 2021). |
|
|
|
10.24 |
|
|
|
|
|
10.25 |
|
|
|
|
|
10.26 |
|
|
|
|
|
10.27 |
|
|
|
|
|
10.28 |
|
|
|
|
|
23.1 |
|
|
|
|
|
24.1 |
|
Power of Attorney. Reference is made to the signature page hereto. |
|
|
|
31.1 |
|
|
|
|
|
31.2 |
|
|
|
|
|
102
32.1 |
|
|
|
|
|
32.2 |
|
|
|
|
|
101.INS |
|
Inline XBRL Instance Document |
|
|
|
101.SCH |
|
Inline XBRL Taxonomy Extension Schema Document |
|
|
|
101.CAL |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
101.DEF |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
101.LAB |
|
Inline XBRL Taxonomy Extension Label Linkbase Document |
|
|
|
101.PRE |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
|
|
|
104 |
|
Cover Page Interactive Data File (formatted as inline XBRL and contained in Exhibit 101) |
|
|
|
|
|
|
+ Indicates management contract or compensatory plan.
Item 16. Form 10-K Summary.
None.
103
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
HTG Molecular Diagnostics, Inc. |
|
|
|
|
|
Date: March 30, 2023 |
|
By: |
/s/ John L. Lubniewski |
|
|
|
John L. Lubniewski |
|
|
|
Chief Executive Officer (Principal Executive Officer) |
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints John L. Lubniewski and Shaun D. McMeans, jointly and severally, his or her attorneys-in-fact, each with the power of substitution, for him or her in any and all capacities, to sign any amendments to this report, and to file the same, with exhibits thereto and other documents in connection therewith with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys-in-fact, or his or her substitute or substitutes may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant in the capacities and on the dates indicated:
Signature |
|
Title |
|
Date |
|
|
|
||
|
|
|
||
/s/ John L. Lubniewski |
|
President and Chief Executive Officer and Director |
|
March 30, 2023 |
John L. Lubniewski |
|
(Principal Executive Officer) |
|
|
|
|
|
|
|
/s/ Shaun D. McMeans |
|
Senior Vice President and Chief Financial Officer |
|
March 30, 2023 |
Shaun D. McMeans |
|
(Principal Financial Officer) |
|
|
|
|
|
|
|
/s/ Laura L. Godlewski |
|
Senior Vice President of Finance and Administration |
|
March 30, 2023 |
Laura L. Godlewski |
|
(Principal Accounting Officer) |
|
|
|
|
|
|
|
/s/ Ann F. Hanham |
|
Chair of Board of Directors |
|
March 30, 2023 |
Ann F. Hanham |
|
|
|
|
|
|
|
|
|
/s/ Thomas W. Dubensky, Jr. |
|
Director |
|
March 30, 2023 |
Thomas W. Dubensky, Jr. |
|
|
|
|
|
|
|
|
|
/s/ Michelle R. Griffin |
|
Director |
|
March 30, 2023 |
Michelle R. Griffin |
|
|
|
|
|
|
|
|
|
/s/ Donnie M. Hardison |
|
Director |
|
March 30, 2023 |
Donnie M. Hardison |
|
|
|
|
|
|
|
|
|
/s/ Christopher P. Kiritsy |
|
Director |
|
March 30, 2023 |
Christopher P. Kiritsy |
|
|
|
|
|
|
|
|
|
/s/ Lee R. McCracken |
|
Director |
|
March 30, 2023 |
Lee R. McCracken |
|
|
|
|
|
|
|
|
|
104