Exhibit 99.1

| Company Overview June 2006 |
| The Private Securities Litigation Reform Act of 1995 provides a "safe harbor" for forward-looking statements. All statements made in this presentation that are not statements of historical fact constitute forward-looking statements. The matters referred to in forward-looking statements could be affected by the risks and uncertainties of the Company's business. Such risks inherent to the Company's business will be described in the Company's filings, when they occur, with the Securities and Exchange Commission, as well as in its press releases. The Company's actual results may differ materially from those expressed in or indicated by such forward-looking statements. Safe Harbor |

| Halozyme's core competence is to develop and commercialize biotechnology products both productively and efficiently Early product commercialization and technology partnerships should generate cash that can be reinvested into exciting growth opportunities Broad and deep product pipeline targeting significant unmet needs should provide sustainable long-term value creation Investment Highlights |
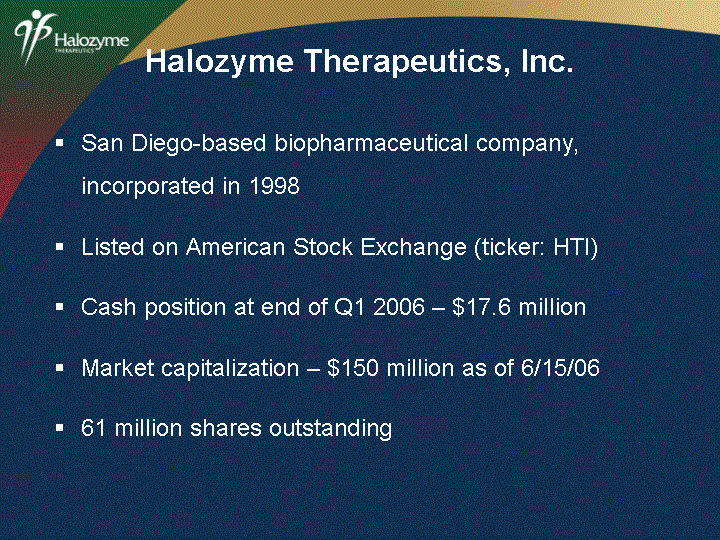
| San Diego-based biopharmaceutical company, incorporated in 1998 Listed on American Stock Exchange (ticker: HTI) Cash position at end of Q1 2006 - $17.6 million Market capitalization - $150 million as of 6/15/06 61 million shares outstanding Halozyme Therapeutics, Inc. |
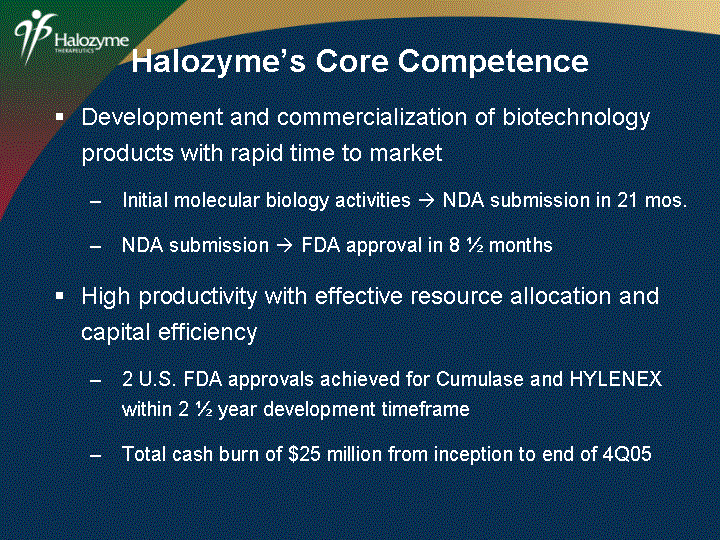
| Development and commercialization of biotechnology products with rapid time to market Initial molecular biology activities ? NDA submission in 21 mos. NDA submission ? FDA approval in 8 1/2 months High productivity with effective resource allocation and capital efficiency 2 U.S. FDA approvals achieved for Cumulase and HYLENEX within 2 1/2 year development timeframe Total cash burn of $25 million from inception to end of 4Q05 Halozyme's Core Competence |
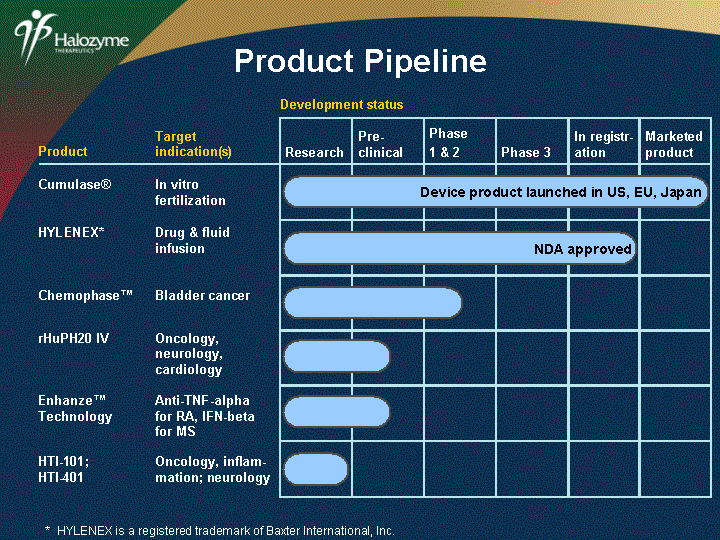
| Product Target indication(s) HYLENEX* Drug & fluid infusion Chemophase(tm) Bladder cancer rHuPH20 IV Oncology, neurology, cardiology Enhanze(tm) Technology Anti-TNF-alpha for RA, IFN-beta for MS HTI-101; HTI-401 Oncology, inflam- mation; neurology Research Pre- clinical Phase 1 & 2 Phase 3 In registr- ation Marketed product Development status NDA approved Product Pipeline Cumulase(r) In vitro fertilization Device product launched in US, EU, Japan * HYLENEX is a registered trademark of Baxter International, Inc. |
| Labeled indications* "For use as an adjunct to increase the absorption and dispersion of other injected drugs; for hypodermoclysis; as an adjunct in subcutaneous urography for improving the resorption of radiopaque agents." * DESI review findings published in the Federal Register (September 23, 1970; 35(185):14800-1) Product Highlights Approved by FDA in December 2005 HYLENEX sales and marketing partnership with Baxter Healthcare Targeted launch planned for 1H06 Estimated $200 - 300 million U.S. market opportunity for drug and fluid infusion Post-marketing clinical trials ongoing HYLENEX: For Drug and Fluid Infusion |
| "It's tough to deal with kids and their parents if you have to stick them several times to start the IV..." "After we have tried and failed, we give up and call an outside specialized company... that's about half the time and it adds significant cost" Intravenous Access: When Things Go Right... |

| Bruising Infiltration Infection Phlebitis Extravasation Extravasation + 10 days Intravenous Access: When Things Go Wrong... |
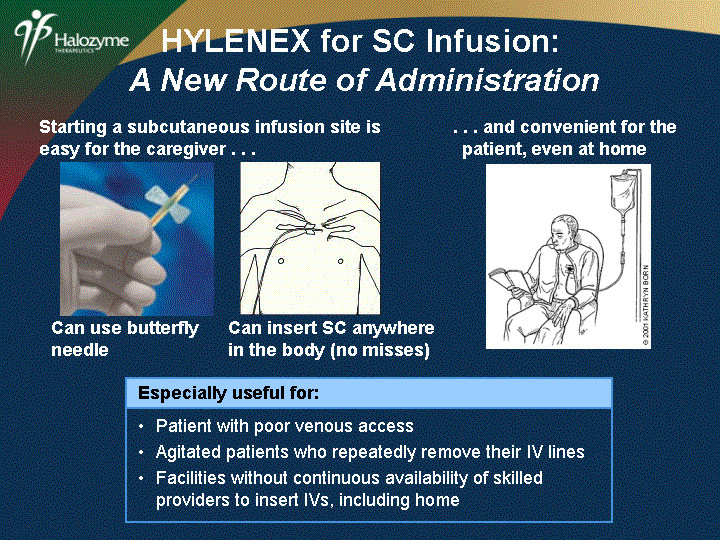
| Starting a subcutaneous infusion site is easy for the caregiver . . . . . . and convenient for the patient, even at home Can use butterfly needle Can insert SC anywhere in the body (no misses) Especially useful for: Patient with poor venous access Agitated patients who repeatedly remove their IV lines Facilities without continuous availability of skilled providers to insert IVs, including home HYLENEX for SC Infusion: A New Route of Administration |
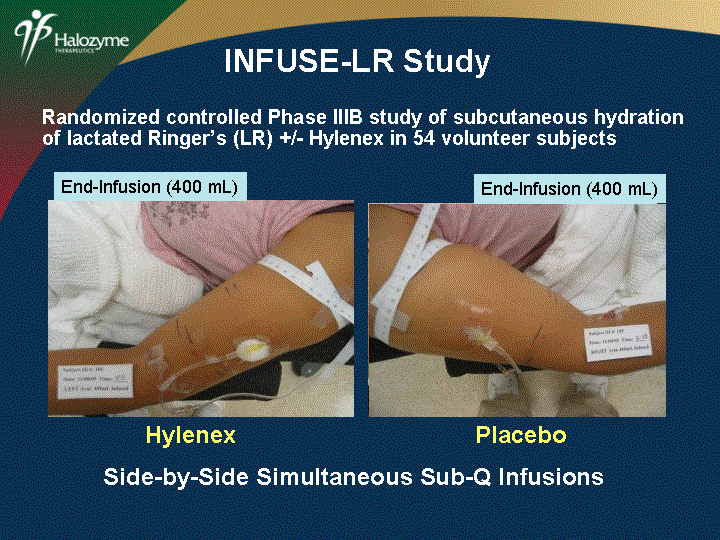
| Baseline Baseline Mid-Infusion (200 cc) Mid-Infusion (200 cc) INFUSE-LR Study End-Infusion (400 mL) End-Infusion (400 mL) Side-by-Side Simultaneous Sub-Q Infusions Randomized controlled Phase IIIB study of subcutaneous hydration of lactated Ringer's (LR) +/- Hylenex in 54 volunteer subjects Hylenex Placebo |
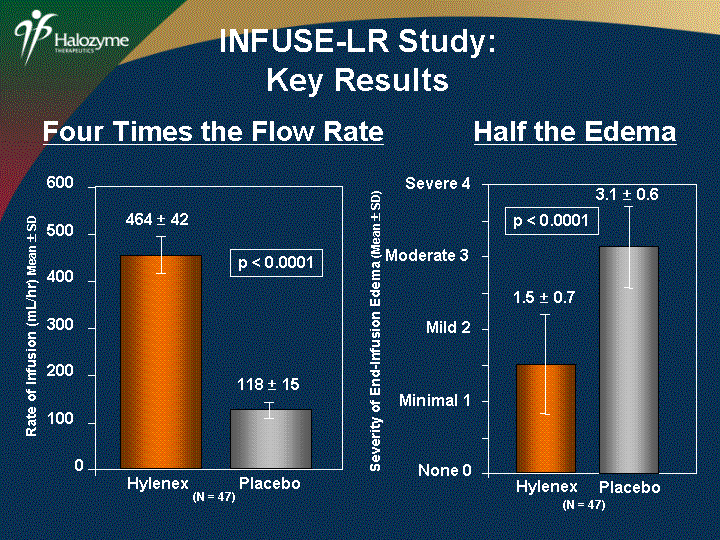
| Four Times the Flow Rate Half the Edema Severity of End-Infusion Edema (Mean ? SD) 3.1 ? 0.6 None 0 Minimal 1 Mild 2 Moderate 3 Severe 4 1.5 ? 0.7 (N = 47) Hylenex Placebo p < 0.0001 Rate of Infusion (mL/hr) Mean ? SD 0 100 200 300 400 500 600 p < 0.0001 464 ? 42 Hylenex (N = 47) Placebo 118 ? 15 INFUSE-LR Study: Key Results |

| Baxter to market, distribute, and sell HYLENEX in US and Puerto Rico Baxter and Halozyme are equal partners in commercialization of HYLENEX Initial target is drug & fluid infusion indication Partners may mutually agree to pursue other dosage forms and indications Baxter has exercised its option to market, sell, and distribute HYLENEX in the European Union HYLENEX: Sales & Marketing Deal with Baxter |
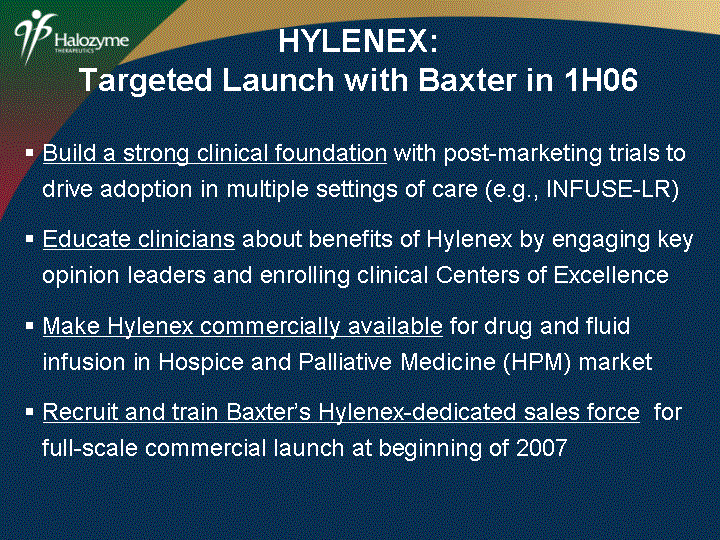
| Build a strong clinical foundation with post-marketing trials to drive adoption in multiple settings of care (e.g., INFUSE-LR) Educate clinicians about benefits of Hylenex by engaging key opinion leaders and enrolling clinical Centers of Excellence Make Hylenex commercially available for drug and fluid infusion in Hospice and Palliative Medicine (HPM) market Recruit and train Baxter's Hylenex-dedicated sales force for full-scale commercial launch at beginning of 2007 HYLENEX: Targeted Launch with Baxter in 1H06 |
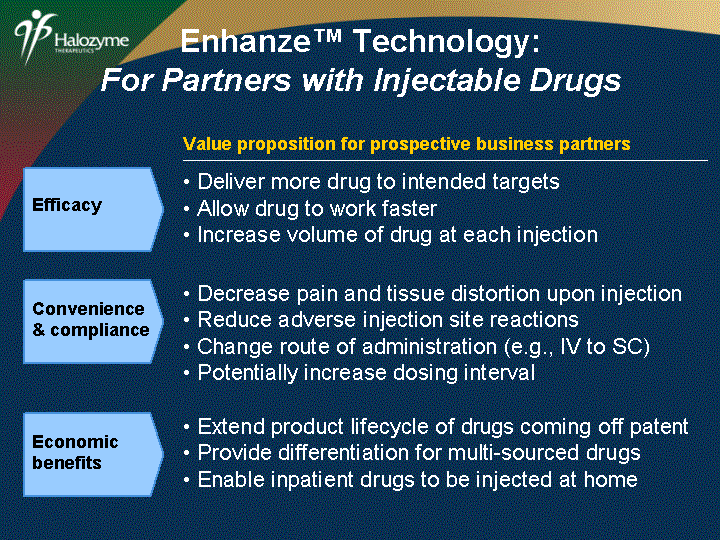
| Decrease pain and tissue distortion upon injection Reduce adverse injection site reactions Change route of administration (e.g., IV to SC) Potentially increase dosing interval Convenience & compliance Extend product lifecycle of drugs coming off patent Provide differentiation for multi-sourced drugs Enable inpatient drugs to be injected at home Economic benefits Value proposition for prospective business partners Deliver more drug to intended targets Allow drug to work faster Increase volume of drug at each injection Efficacy Enhanze(tm) Technology: For Partners with Injectable Drugs |
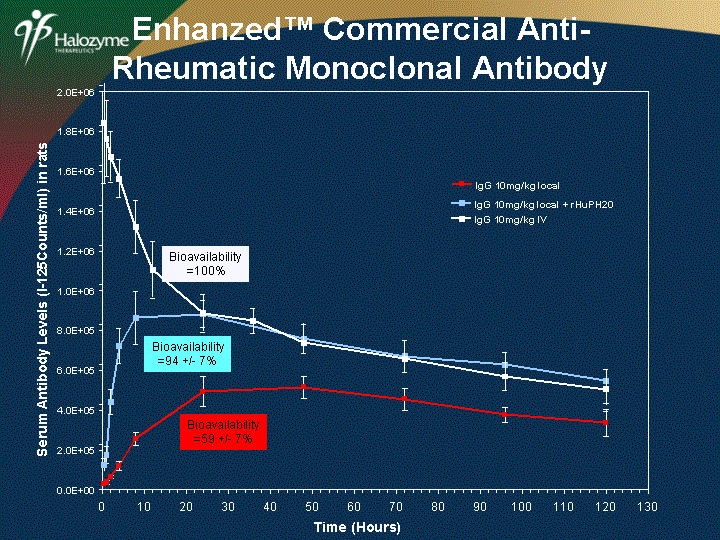
| 0.0E+00 2.0E+05 4.0E+05 6.0E+05 8.0E+05 1.0E+06 1.2E+06 1.4E+06 1.6E+06 1.8E+06 2.0E+06 0 10 20 30 40 50 60 70 80 90 100 110 120 130 Time (Hours) Serum Antibody Levels (I-125Counts/ml) in rats IgG 10mg/kg local Bioavailability =59 +/- 7% Enhanzed(tm) Commercial Anti- Rheumatic Monoclonal Antibody IgG 10mg/kg local + rHuPH20 Bioavailability =94 +/- 7% Bioavailability =100% IgG 10mg/kg IV |
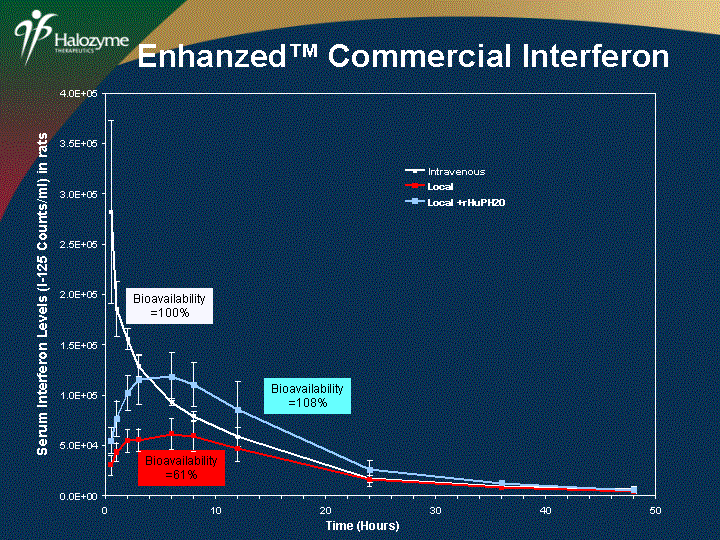
| 0.0E+00 5.0E+04 1.0E+05 1.5E+05 2.0E+05 2.5E+05 3.0E+05 3.5E+05 4.0E+05 0 10 20 30 40 50 Time (Hours) Local Serum Interferon Levels (I-125 Counts/ml) in rats Bioavailability =61% Local +rHuPH20 Intravenous Local Local +rHuPH20 Enhanzed(tm) Commercial Interferon |
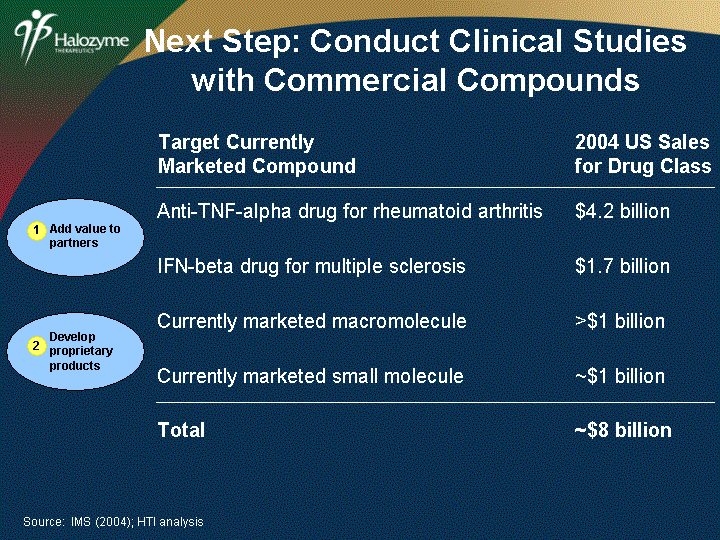
| 2004 US Sales for Drug Class Anti-TNF-alpha drug for rheumatoid arthritis $4.2 billion IFN-beta drug for multiple sclerosis $1.7 billion Currently marketed macromolecule >$1 billion Currently marketed small molecule ~$1 billion Total ~$8 billion Source: IMS (2004); HTI analysis Target Currently Marketed Compound Next Step: Conduct Clinical Studies with Commercial Compounds Add value to partners 1 Develop proprietary products 2 |
| Chemophase: For Superficial Bladder Cancer |
| Chemophase: For Superficial Bladder Cancer |
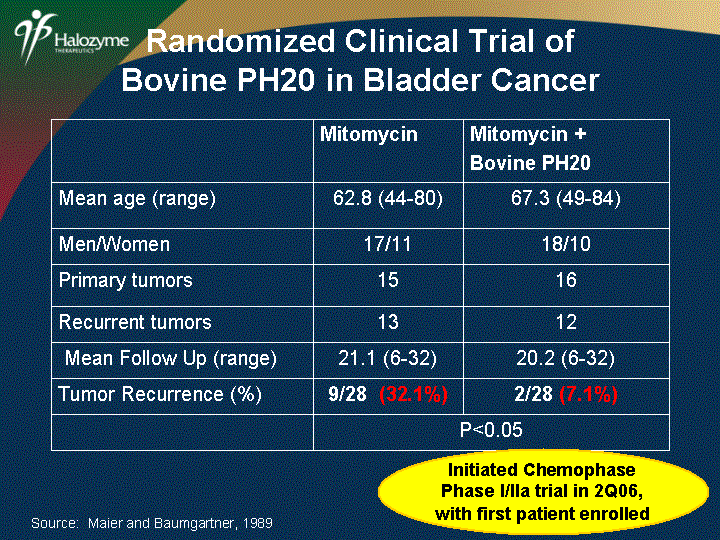
| Mitomycin Mitomycin + Bovine PH20 Mean age (range) 62.8 (44-80) 67.3 (49-84) Men/Women 17/11 18/10 Primary tumors 15 16 Recurrent tumors 13 12 Mean Follow Up (range) 21.1 (6-32) 20.2 (6-32) Tumor Recurrence (%) 9/28 (32.1%) 2/28 (7.1%) P<0.05 P<0.05 Source: Maier and Baumgartner, 1989 Randomized Clinical Trial of Bovine PH20 in Bladder Cancer Initiated Chemophase Phase I/IIa trial in 2Q06, with first patient enrolled |
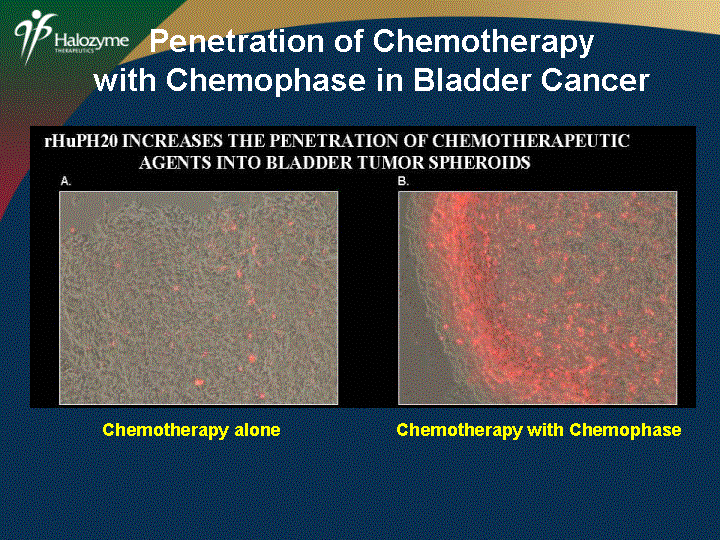
| Penetration of Chemotherapy with Chemophase in Bladder Cancer Chemotherapy alone Chemotherapy with Chemophase |
| 63,210 new cases of urinary bladder cancer in the US1,2 499,199 prevalent cases of urinary bladder cancer3,4 At least 6 treatment cycles of mitomycin and Chemophase Source: 1 American Cancer Society, 2005; 2 ~70% of new cases are "superficial" disease (AUA Bladder Cancer Guidelines Panel, 1999); 3 NCI SEER Cancer Statistics Review, 2002; 4 ~30% recurrence rate of treated patients within 12 months (Southwest Oncology Group Study, 1995) Chemophase, if adopted as first line therapy with mitomycin, would target ~100,000 potential patients and ~600,000 doses/year, or a potential market size of about $600 - 700 million Chemophase Market Opportunity in Superficial Bladder Cancer |
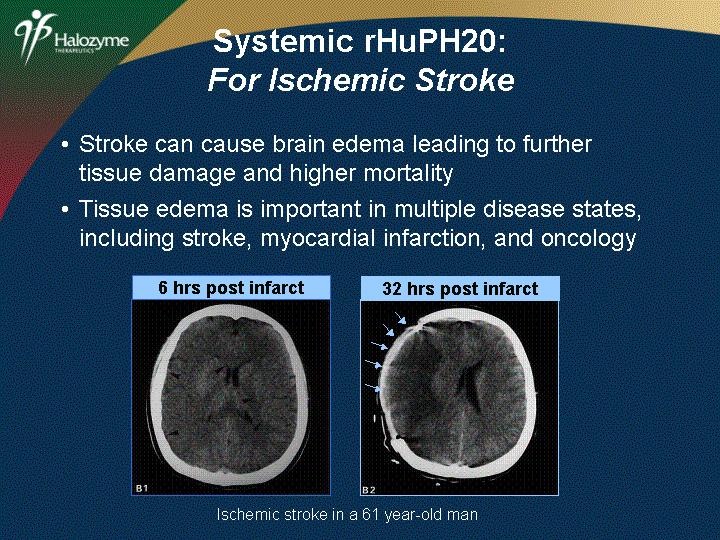
| Stroke can cause brain edema leading to further tissue damage and higher mortality Tissue edema is important in multiple disease states, including stroke, myocardial infarction, and oncology 6 hrs post infarct Ischemic stroke in a 61 year-old man Systemic rHuPH20: For Ischemic Stroke |
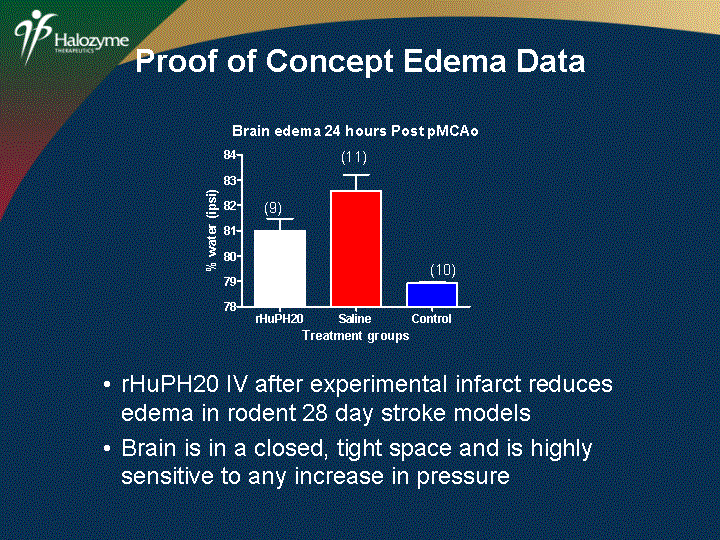
| rHuPH20 IV after experimental infarct reduces edema in rodent 28 day stroke models Brain is in a closed, tight space and is highly sensitive to any increase in pressure Proof of Concept Edema Data |
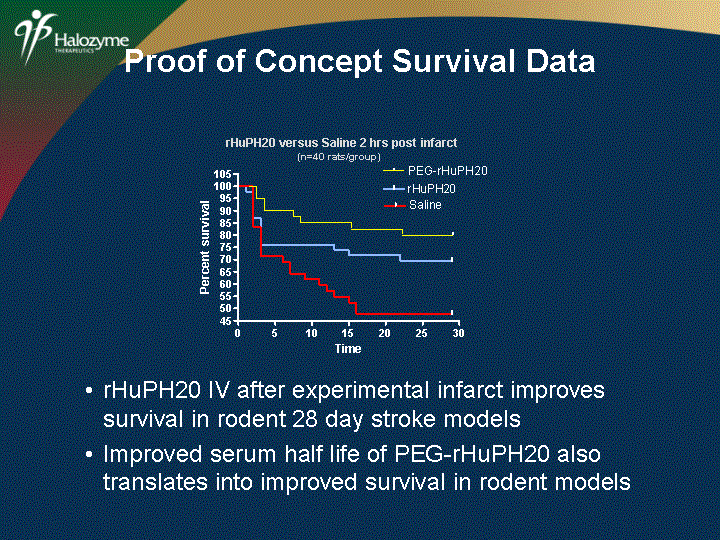
| PEG-rHuPH20 rHuPH20 versus Saline 2 hrs post infarct (n=40 rats/group) rHuPH20 IV after experimental infarct improves survival in rodent 28 day stroke models Improved serum half life of PEG-rHuPH20 also translates into improved survival in rodent models Proof of Concept Survival Data |

| 2005: Completed on time Cumulase 510(k) cleared and product launched, 1H05 Chemophase IND filed, 2Q05; initiated Phase I trial, 4Q05 HYLENEX NDA approved, 4Q05 2005 - 2006 Milestones* * Forward-looking statement 2006: Upcoming Complete INFUSE-LR trial for HYLENEX, 1H06 Complete enrollment of Chemophase Ph I and initiate Ph I/IIa, 1H06 Begin targeted launch of HYLENEX, 1H06 Complete enrollment of INFUSE-Morphine trial for HYLENEX, 4Q06 Initiate clinical studies for Enhanzed(tm) commercial targets, 4Q06 |

| Productive and efficient development and commercialization of biotechnology products with track record of success with FDA Near-term value from commercialization of approved products and prospective deal flow from technology partnerships Long-term value from broad and deep product pipeline targeting significant unmet needs Investment Highlights |