
| ☐ | Preliminary Proxy Statement | |
Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) | ||
| ☒ | Definitive Proxy Statement | |
| ☐ | Definitive Additional Materials | |
| ☐ | Soliciting Material under §240.14a-12 | |
| Payment of Filing Fee (Check the appropriate box): | ||
| ☒ | No fee required. | |
| ☐ | Fee paid previously with preliminary materials. | |
| ☐ | Fee computed on table in exhibit required by Item 25(b) per Exchange Act Rules 14a6(i)(1) and 0-11 | |


May 1, 2023
DEAR STOCKHOLDERS:
I am pleased to report on our achievements since the start of the 2022 fiscal year and to invite you to attend the 2023 Annual Meeting of Stockholders of Madrigal Pharmaceuticals, Inc. (the “Annual Meeting”) on Thursday, June 15, 2023 at 9:00 a.m. Eastern Time.
Highlights
We delivered on key objectives during the 2022 fiscal year, including:
| • | announcing successful topline results from our 969-patient MAESTRO-NAFLD-1 safety study in January of 2022; |
| • | securing a $250 million credit facility with Hercules Capital and affiliates in May of 2022; |
| • | initiating in August 2022 a 700-patient Phase 3 MAESTRO-NASH outcomes study for well-compensated NASH cirrhosis patients; |
| • | announcing in December 2022 achievement of both primary endpoints (with both oral doses) from our pivotal Phase 3 MAESTRO-NASH trial; |
| • | raising $260 million in equity securities proceeds in December of 2022; and |
| • | delivering a 422% return over the last twelve months. |
We plan to build on this success by filing a new drug application in the second quarter of 2023 for resmetirom and expanding personnel and resources in preparation for a planned launch of resmetirom in the first quarter of 2024.
Annual Meeting Details
Madrigal again has decided to employ a virtual format for our Annual Meeting, which is designed to provide a consistent and convenient remote access and attendance experience for all stockholders, regardless of location. You may attend the Annual Meeting virtually via the Internet at www.proxydocs.com/MDGL. In order to attend you must register in advance at www.proxydocs.com/MDGL prior to the deadline of June 8, 2023 at 5:00 p.m. Eastern Time. Upon completing your registration, you will receive further instructions via email, including your unique links that will permit you to submit questions in advance of the Annual Meeting and allow you access to the meeting. Please be sure to follow instructions found on your Proxy Card and/or Voting Authorization Form and subsequent instructions that will be delivered to you via email.
The Notice of the 2023 Annual Meeting of Stockholders and the proxy statement contain details of the business to be conducted at the Annual Meeting, including the nominees for election as directors. Only stockholders of record at the close of business on April 27, 2023 will be entitled to notice of, and to vote at, the Annual Meeting.
Your vote is important. Regardless of whether you plan to participate in the Annual Meeting, we hope that you will vote as soon as possible. You may vote by proxy over the Internet (as described below), by telephone, or by mail (if you received paper copies of the proxy materials) by following the instructions on the proxy card or voting instruction card. Voting over the Internet or by telephone, written proxy or voting instruction card will ensure your representation at the Annual Meeting.
Thank you for your ongoing support of Madrigal.
Sincerely,

Paul A. Friedman
Chairman and Chief Executive Officer

NOTICE OF 2023 ANNUAL MEETING OF STOCKHOLDERS
TO BE HELD JUNE 15, 2023
To the Stockholders of Madrigal Pharmaceuticals, Inc.:
NOTICE IS HEREBY GIVEN that the 2023 Annual Meeting of Stockholders of Madrigal Pharmaceuticals, Inc., a Delaware corporation, will be held on Thursday, June 15, 2023, at 9:00 a.m. Eastern Time for the following purposes:
| 1. | to elect three Class I director nominees named in the accompanying proxy statement to serve on the Board of Directors for three-year terms expiring at the Annual Meeting of Stockholders to be held in 2026 or until their successors are duly elected and qualified; |
| 2. | to ratify the appointment of PricewaterhouseCoopers LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2023; |
| 3. | to approve, on an advisory basis, the compensation of our named executive officers as disclosed in the accompanying proxy statement, pursuant to the compensation disclosure rules of the Securities and Exchange Commission; |
| 4. | to approve an amendment to the Company’s Restated Certificate of Incorporation to reflect new Delaware law provisions regarding officer exculpation; and |
| 5. | to transact such other business that is properly presented at the meeting and any adjournments or postponements thereof. |
The 2023 Annual Meeting of Stockholders will be a completely virtual meeting. There will be no physical meeting location. The meeting will only be conducted via live webcast. To participate in the 2023 Annual Meeting virtually via the Internet, please visit www.proxydocs.com/MDGL. Through this webcast, stockholders and proxyholders will be deemed to be present in person for purposes of conducting a vote at such meeting. In order to attend this webcast, you must register in advance at www.proxydocs.com/MDGL prior to the deadline of June 8, 2023 at 5:00 p.m. Eastern Time, as more fully described in the accompanying proxy statement.
In accordance with rules established by the Securities and Exchange Commission, we are providing you access to our proxy materials over the Internet. Accordingly, we plan to mail a Notice of Internet Availability of Proxy Materials (the “Notice”), to our stockholders on or about May 3, 2023. The Notice will describe how to access and review our proxy materials, including our proxy statement and Annual Report on Form 10-K for the fiscal year ended December 31, 2022, as amended by our Annual Report on Form 10-K/A for the fiscal year ended December 31, 2022 filed on March 3, 2023. The Notice, as well as our proxy card, will also describe how you may submit your proxy electronically. If you received just a Notice by mail and would like to receive a printed copy of our proxy materials, you should follow the instructions for requesting such materials included in the Notice.
Only stockholders of record at the close of business on April 27, 2023, are entitled to notice of and to vote at the Annual Meeting. A list of stockholders entitled to vote at the Annual Meeting will be available for inspection at our executive offices for the ten days prior to the Annual Meeting. All stockholders are cordially invited to attend the Annual Meeting via live webcast. Whether or not you plan to attend the virtual Annual Meeting, please vote as soon as possible.
| BY ORDER OF THE BOARD OF DIRECTORS |

|
| Brian J. Lynch |
| Senior Vice President and General Counsel |
| West Conshohocken, Pennsylvania |
| May 1, 2023 |
YOUR VOTE IS IMPORTANT. PLEASE CAST YOUR VOTE PROMPTLY.
PROXY STATEMENT TABLE OF CONTENTS
| Page | ||||
| i | ||||
| QUESTIONS AND ANSWERS ABOUT THESE PROXY MATERIALS AND VOTING |
1 | |||
| SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT |
7 | |||
| 10 | ||||
|
|
10 |
| ||
|
|
15 |
| ||
|
|
15 |
| ||
|
|
19 |
| ||
|
|
19 |
| ||
|
|
20 |
| ||
|
|
21 |
| ||
| 23 | ||||
|
|
23 |
| ||
|
|
26 |
| ||
|
|
27 |
| ||
|
|
29 |
| ||
|
|
32 |
| ||
|
|
33 |
| ||
|
|
33 |
| ||
| 34 | ||||
|
|
34 |
| ||
|
|
35 |
| ||
|
|
36 |
| ||
|
|
37 |
| ||
|
|
37 |
| ||
|
|
38 |
| ||
| Employment Retention, Severance and Change in Control Arrangements |
|
41 |
| |
| Potential Qualifying Separation and Change of Control Payments |
|
44 |
| |
|
|
45 |
| ||
|
|
46 |
| ||
| Report of the Compensation Committee of the Board of Directors |
|
47 |
| |
|
|
47 |
| ||
|
|
47 |
| ||
|
|
48 |
| ||
|
|
51 |
| ||
|
|
2023 PROXY STATEMENT |
TABLE OF CONTENTS
| Page | ||||
| PROPOSAL NO. 2: RATIFICATION OF APPOINTMENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM |
|
52 |
| |
|
|
55 |
| ||
| PROPOSAL NO. 4: AMENDMENT OF RESTATED CERTIFICATE OF INCORPORATION |
|
56 |
| |
|
|
58 |
| ||
|
|
58 |
| ||
|
|
58 |
| ||
|
|
58 |
| ||
|
|
59 |
| ||
|
|
60 |
| ||
Important Notice Regarding the Availability of Proxy Materials
For the Stockholder Meeting to be Held on June 15, 2023
at 9:00 a.m. Eastern Time
The Proxy Statement, Proxy Card and Annual Report on Form 10-K for the fiscal year ended December 31, 2022, as amended by the Annual Report on Form 10-K/A for the fiscal year ended December 31, 2022 are available at:
www.proxydocs.com/MDGL
YOUR VOTE IS VERY IMPORTANT
You are cordially invited to attend the Annual Meeting, which will be a virtual meeting and therefore will not be held at a physical location. To participate in the 2023 Annual Meeting, virtually via the Internet, please visit www.proxydocs.com/MDGL. In order to attend, you must register in advance at www.proxydocs.com/MDGL prior to the deadline of June 8, 2023 at 5:00 p.m. Eastern Time. Whether or not you expect to attend the Annual Meeting virtually, please vote over the telephone or the Internet, or, if you receive a paper proxy card by mail, by completing, dating, signing and returning the proxy mailed to you, as promptly as possible in order to ensure your representation at the Annual Meeting.

|
|
PROXY STATEMENT SUMMARY
This summary highlights information contained elsewhere in this proxy statement and does not contain all of the information that you should consider. You should read the entire proxy statement carefully before voting.
| Date and Time Thursday, June 15, 2023 9:00 a.m. Eastern Time |
Record Date April 27, 2023 | |||
| Voting Matters |
Page(s) | Board’s Recommendation | ||
| Proposal 1 Election of Directors |
51 | FOR each | ||
| Proposal 2 Ratification of Appointment of Independent Registered Public Accounting Firm for 2023 |
52 | FOR | ||
| Proposal 3 Non-binding Advisory Vote to Approve the Compensation of our Named Executive Officers (Say-on-Pay) |
55 | FOR | ||
| Proposal 4 Amendment of Restated Certificate of Incorporation to provide for officer exculpation permitted by Delaware law |
56 | FOR | ||
| Recent Business Highlights | ||
| • Successful topline results for MAESTRO-NAFLD-1 Safety Study (January 2022)
• $250 million credit facility established (May 2022)
• Initiated Phase 3 Outcomes Study for well-compensated cirrhotic NASH patients (August 2022)
• Achieved both primary endpoints (at both doses) for pivotal Phase 3 MAESTRO-NASH trial (December 2022)
• Raised $260 million in equity proceeds (December 2022)
• Completed enrollment for Phase 3 MAESTRO-NASH
• Received Breakthrough Therapy designation from FDA for Resmetirom (April 2023) | ||

| * | LTM before Proxy Statement filing — April 26, 2022 to April 26, 2023 |
|
|
2023 PROXY STATEMENT | i |
PROXY STATEMENT SUMMARY
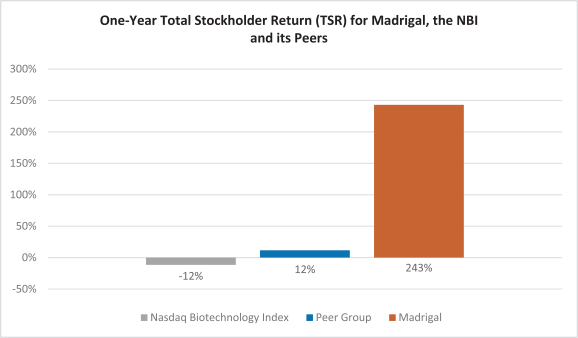
Delivering Robust Total Stockholder Return
The charts below highlight the performance of Madrigal’s (“MDGL”) total stockholder return (“TSR”) on the Nasdaq Stock Market LLC compared to: (1) the TSR of our compensation peer group’s 75th percentile (identified on page 29); and (2) the Nasdaq Biotechnology Index (“NBI”), for applicable periods ending December 31, 2022. One-Year TSR measures performance from January 1, 2022 through December 31, 2022. Since Inception of Public Trading TSR measures performance from July 22, 2016, the first date of trading of our common stock on the Nasdaq after our merger with Synta Pharmaceuticals Corp., through December 31, 2022.

| ii |

|
|
PROXY STATEMENT SUMMARY
Proposal No.1, Election of Class I Directors; Board Composition (Page 10; 52)
Our Board of Directors has eight members divided into three classes, six of whom are independent. Three Class I Directors, Paul A. Friedman, M.D., Kenneth Bate and James Daly are standing for re-election at the 2023 Annual Meeting of Stockholders.
Our Board of Directors is responsible for overseeing the affairs and performance of Madrigal and acting in the best interests of stockholders. The Board selects senior management and has oversight responsibility over senior management and the conduct of Madrigal’s business operations. The experience of our Board members is summarized below and described in greater detail beginning on page 10.
We are committed to a Board composition that reflects an optimal mix of skills and experience necessary to carry out critical oversight of operations, clinical and commercial objectives and overall strategy. We believe our current Board membership effectively oversees our business strategy and operations, contributes to the long-term, forward-looking strategy and performance objectives of the Company and complements senior management’s objectives and efforts to create and sustain value associated with the long-term interests of the Company’s stockholders.
Board Skills Matrix
Our Board possesses a broad range of qualifications and skills that facilitate strong oversight of the Company’s management and strategy. The following table provides a summary of our directors’ key skills and core competencies*:
| Paul A. Friedman, M.D. |
Kenneth M. Bate |
Fred B. Craves, M.D. |
James M. Daly |
Keith R. Gollust |
Richard S. Levy, M.D. |
David Milligan, Ph.D. |
Rebecca Taub, M.D. | |||||||||
| Biopharma/healthcare leadership |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ | ||||||||
| Drug discovery and clinical development strategy | execution |
✓ |
✓ |
✓ |
✓ |
✓ | |||||||||||
| Commercialization | sales marketing |
✓ |
✓ |
✓ |
✓ |
✓ |
|||||||||||
| Government |legal | FDA regulatory experience |
✓ |
✓ |
✓ | |||||||||||||
| Public company management | oversight | governance expertise |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ | ||||||||
| Business and corporate development |
✓ |
✓ |
✓ |
✓ |
✓ |
|||||||||||
| Global competition and positioning |
✓ |
✓ |
✓ |
✓ |
✓ | |||||||||||
| Corporate accounting and finance |
✓ |
✓ |
✓ |
✓ |
| * | The absence of a check mark does not necessarily indicate that the director lacks such a qualification or skill. |
|
|
2023 PROXY STATEMENT | iii |
PROXY STATEMENT SUMMARY
Board Diversity Matrix
The following table illustrates the composition of our directors by self-identified diversity statistics. Each of the categories listed in the below table has the meaning used in Nasdaq Listing Rule 5605(f).
| Board Diversity Matrix | ||||||||
| Part I: Gender Identity |
Female | Male | Non-Binary | Did Not Disclose Gender | ||||
| Directors |
1 | 6 | – | 1 | ||||
| Part II: Demographic Background |
||||||||
| African American or Black |
– | – | – | – | ||||
| Alaskan Native or Native American |
– | – | – | – | ||||
| Asian |
– | – | – | – | ||||
| Hispanic or Latinx |
– | – | – | – | ||||
| Native Hawaiian or Pacific Islander |
– | – | – | – | ||||
| White |
1 | 6 | – | – | ||||
| Two or More Races or Ethnicities |
– | – | – | – | ||||
| LGBTQ+ |
– | |||||||
| Did Not Disclose Demographic Background |
2 | |||||||
| Total Number of Directors |
8 | |||||||
The information set forth in the table above did not change from our 2022 director diversity disclosure published on our website under Nasdaq rules.
Madrigal’s Officer Diversity Representation
The charts below illustrate the diversity statistics of our named executive officers and our employees with a title of Vice President or above.


| * | Represents diversity in accordance with the categories set forth in Nasdaq Listing Rule 5605(f). |
| iv |

|
|
PROXY STATEMENT SUMMARY
Proposal No. 2, Ratification of Auditors (Page 53)
As in previous annual meetings, we are requesting stockholders to ratify our selection of auditors for the current fiscal year.
Proposal No. 3, Say-On-Pay Advisory Vote (Page 56) and Management Compensation (Page 23)
In 2022, stockholders continued their historically strong support for our executive compensation programs with 99% of the votes cast voting in favor of approving the say-on-pay proposal. The charts below demonstrate our say-on-pay support for the last three years. As required by SEC rules, we are seeking a stockholder advisory vote on compensation, or “say-on-pay.”

Proposal No. 4, Amendment to Restated Certificate of Incorporation to Add Officer Exculpation Under Delaware Law (Page 57).
Based on recent changes to Delaware law, we are seeking stockholder support for officer exculpation, similar to long-standing exculpation for directors.
|
|
2023 PROXY STATEMENT | v |

PROXY STATEMENT
We prepared this proxy statement under the direction of our Board of Directors to solicit your proxy for use at our 2023 Annual Meeting of Stockholders to be held via internet webcast on Thursday, June 15, 2023, at 9:00 a.m. Eastern Time. As used in this proxy statement, the terms “Madrigal,” “Public Madrigal,” the “Company,” “we,” “our” and “us” refer to Madrigal Pharmaceuticals, Inc., a Delaware corporation that has been publicly traded since July 22, 2016.
QUESTIONS AND ANSWERS ABOUT THESE PROXY MATERIALS AND VOTING
How do I attend the Annual Meeting?
The 2023 Annual Meeting will be a completely virtual meeting. There will be no physical meeting location. The meeting will only be conducted via live webcast.
To participate in the 2023 Annual Meeting virtually, via the Internet, please visit www.proxydocs.com/MDGL. In order to attend, you must register in advance at www.proxydocs.com/MDGL prior to the deadline of June 8, 2023 at 5:00 p.m. Eastern Time. Upon completing your registration, you will receive further instructions via email, including your unique links that will allow you access to the meeting and will permit you to submit questions. You will not be able to attend the 2023 Annual Meeting in person.
Why did I receive a notice regarding the availability of proxy materials on the Internet?
Pursuant to rules adopted by the Securities and Exchange Commission (the “SEC”), we have elected to provide access to our proxy materials over the Internet. Accordingly, we have sent you a Notice of Internet Availability of Proxy Materials (the “Notice”), because the Board of Directors is soliciting your proxy to vote at the Annual Meeting, including at any adjournments or postponements of the Annual Meeting. All stockholders will have the ability to access the proxy materials on the website referred to in the Notice or request to receive a printed set of the proxy materials. Instructions on how to access the proxy materials over the Internet or to request a printed copy may be found in the Notice.
We intend to mail the Notice on or about May 3, 2023 to all stockholders of record entitled to vote at the Annual Meeting.
Who can vote at the Annual Meeting?
Only stockholders of record at the close of business on April 27, 2023, will be entitled to vote at the Annual Meeting. On April 1, 2023, there were 18,283,074 shares of common stock outstanding and entitled to vote.
Stockholder of Record: Shares Registered in Your Name
If on April 27, 2023, your shares were registered directly in your name with our transfer agent, then you are a stockholder of record. As a stockholder of record, you may vote directly through any of the following means: (1) electronically over the Internet, (2) by telephone, or (3) by completing and returning a printed proxy card that you may request or that we may elect to deliver at a later time, to ensure your vote is counted.
|
|
2023 PROXY STATEMENT | 1 |
QUESTIONS AND ANSWERS ABOUT THESE PROXY MATERIALS AND VOTING
Beneficial Owner: Shares Registered in the Name of a Broker or Bank
If on April 27, 2023, your shares were held, not in your name, but rather in an account at a brokerage firm, bank or other similar organization, then you are the beneficial owner of shares held in “street name” and the Notice is being forwarded to you by that organization. The organization holding your account is considered to be the stockholder of record for purposes of voting at the Annual Meeting. As a beneficial owner, you have the right to direct your broker, bank or other agent regarding how to vote the shares in your account. You are also invited to attend the Annual Meeting, on a virtual basis.
What am I voting on?
There are four matters scheduled for a vote:
| • | election of three Class I directors (Proposal No. 1); |
| • | ratification of the appointment of PricewaterhouseCoopers LLP as our independent registered public accounting firm for our fiscal year ending December 31, 2023 (Proposal No. 2); and |
| • | approval, on an advisory basis, of the compensation of our named executive officers, as disclosed in this proxy statement pursuant to the compensation disclosure rules of the SEC (Proposal No. 3). |
| • | approval of an amendment to our Restated Certificate Incorporation to reflect new Delaware law provisions regarding officer exculpation (Proposal No. 4). |
What if another matter is properly brought before the meeting?
As of the date of this proxy statement, the Board of Directors knows of no other matters that will be presented for consideration at the Annual Meeting. If any other matters are properly brought before the Annual Meeting, it is the intention of the persons acting as proxies to vote on those matters in accordance with their best judgment.
How do I vote?
For Proposal No. 1 to be voted on at the Annual Meeting, you may vote “For” or “Withhold” for each nominee for director. For each of Proposal Nos. 2, 3 and 4 to be voted on at the Annual Meeting, you may vote “For” or “Against” or abstain from voting. See the table below for more details.
Stockholder of Record: Shares Registered in Your Name
If you are a stockholder of record, you may vote by proxy over the telephone, vote by proxy through the Internet or vote by proxy using a proxy card that you may request. Whether or not you plan to participate in the virtual Annual Meeting webcast, we urge you to vote in advance by proxy by one of the foregoing means to ensure your vote is counted.
| • | To vote through the Internet, go to www.proxypush.com/MDGL to complete an electronic proxy card. You will be asked to provide the company number and control number from the Notice. You must cast your Internet vote by 11:59 p.m. Eastern Time on June 14, 2023. |
| • | To vote using the proxy card, simply complete, sign and date the proxy card that may be delivered and return it promptly in the envelope provided. If you return your signed proxy card to us before the Annual Meeting, we will vote your shares as you direct. |
| • | To vote over the telephone, dial toll-free 1-866-249-5094 using a touch-tone phone and follow the recorded instructions. You will be asked to provide the company number and control number from the Notice. You must cast your telephone vote by 11:59 p.m. Eastern Time on June 14, 2023. |
| 2 |

|
|
QUESTIONS AND ANSWERS ABOUT THESE PROXY MATERIALS AND VOTING
Beneficial Owner: Shares Registered in the Name of Broker or Bank
If you are a beneficial owner of shares registered in the name of your broker, bank or other agent, you should have received a Notice containing voting instructions from that organization rather than from us. Simply follow the voting instructions in the Notice to ensure that your vote is counted. Alternatively, you may vote by telephone or over the Internet as instructed by your broker or bank. Follow the instructions from your broker, bank or other agent included with these proxy materials, or contact that organization for assistance on how to vote.
How many votes do I have?
On each matter to be voted upon, you have one vote for each share of common stock you owned as of April 27, 2023.
If I am a stockholder of record and I do not vote, or if I return a proxy card or otherwise vote without giving specific voting instructions, what happens?
If you are a stockholder of record and do not vote through the Internet, by telephone, or by completing a proxy card that may be delivered to you, your shares will not be voted.
If you return a signed and dated proxy card or otherwise vote without marking voting selections, your shares will be voted “For” each of the proposals, including for each nominee for director. If any other matter is properly presented at the meeting, your proxyholder (one of the individuals named on your proxy card) will vote your shares in his or her discretion.
If I am a beneficial owner of shares held in street name and I do not provide my broker or bank with voting instructions, what happens?
If you are a beneficial owner of shares held in street name and you do not instruct your broker, bank or other agent how to vote your shares, your broker, bank or other agent may still be able to vote your shares in its discretion. In this regard, under the rules of the New York Stock Exchange, or the NYSE, brokers, banks and other securities intermediaries that are subject to NYSE rules may use their discretion to vote your “uninstructed” shares with respect to matters considered to be “routine” under NYSE rules, but not with respect to “non-routine” matters. In this regard, we believe that Proposals Nos. 1, 3 and 4 are considered to be “non-routine” under NYSE rules meaning that your broker may not vote your shares on those proposals in the absence of your voting instructions. However, Proposal No. 2 is considered a “routine” matter under stock exchange rules, meaning that if you do not return voting instructions to your broker by its deadline, your shares may be voted by your broker in its discretion on Proposal No. 2.
If you are a beneficial owner of shares held in street name, to ensure your shares are voted in the way you would prefer, you must provide voting instructions to your broker, bank or other agent by the deadline provided in the materials you receive from your broker, bank or other agent.
Who is paying for this proxy solicitation?
We will pay for the entire cost of soliciting proxies. In addition to these proxy materials, our directors and employees may also solicit proxies in person, by telephone, or by other means of communication. Directors and employees will not be paid any additional compensation for soliciting proxies. We may also reimburse brokerage firms, banks and other agents for the cost of forwarding proxy materials to beneficial owners.
What does it mean if I receive more than one Notice?
If you receive more than one Notice, your shares may be registered in more than one name or in different accounts. Please follow the voting instructions on the Notices to ensure that all of your shares are voted.
|
|
2023 PROXY STATEMENT | 3 |
QUESTIONS AND ANSWERS ABOUT THESE PROXY MATERIALS AND VOTING
Can I change my vote after submitting my proxy?
Stockholder of Record: Shares Registered in Your Name
Yes. You can revoke your proxy before the final vote at the Annual Meeting. If you are the record holder of your shares, you may revoke your proxy in any one of the following ways:
| • | You may grant a subsequent proxy by telephone or through the Internet. |
| • | You may submit another properly completed proxy card with a later date. |
| • | You may send a timely written notice that you are revoking your proxy to our Secretary at Four Tower Bridge, 200 Barr Harbor Drive, Suite 200, West Conshohocken, Pennsylvania 19428. Such notice will be considered timely if it is received at the indicated address by the close of business on Wednesday, June 14, 2023. |
Your most current proxy card or most current vote via telephone or Internet proxy will be the vote that is counted.
Beneficial Owner: Shares Registered in the Name of Broker or Bank
If your shares are held by your broker, bank or other agent, you should follow the instructions provided by your broker, bank or other agent.
How are votes counted?
Votes will be counted by the inspector of election appointed for the meeting, who will separately count votes in accordance with the requirements summarized in the table below.
What are “broker non-votes?”
When a beneficial owner of shares held in street name does not give voting instructions to his or her broker, bank or other securities intermediary holding his or her shares as to how to vote on matters deemed to be “non-routine” under NYSE rules (as described above), the broker, bank or other such agent cannot vote the shares. These un-voted shares are counted as “broker non-votes.”
As a reminder, if you are a beneficial owner of shares held in street name, to ensure your shares are voted in the way you would prefer, you must provide voting instructions to your broker, bank or other agent by the deadline provided in the materials you receive from your broker, bank or other agent.
What Vote is Required to Approve Each Proposal and How Are Votes Counted?
| Proposal No. 1: Election of Directors | The three nominees for director who receive the most votes (also known as a “plurality” of the votes cast) will be elected. You may vote either FOR any one or more of the nominees, or WITHHOLD your vote from any one or more of the nominees. WITHHOLD votes will not be included in the vote tally for the election of the directors. Brokerage firms do not have authority to vote customers’ unvoted shares held by the firms in street name for the election of directors. As a result, any shares not voted by a customer will be treated as a broker non-vote. Such broker non-votes will have no effect on the results of this vote.
| |
| Proposal No. 2: Ratify Appointment of Independent Registered Public Accounting Firm | The vote of a majority of the shares of our common stock cast affirmatively at the Annual Meeting is required to ratify the appointment of our independent registered public accounting firm. Abstentions are not considered votes cast on Proposal No. 2 and will have no effect on the results of this vote. Brokerage firms
|
| 4 |

|
|
QUESTIONS AND ANSWERS ABOUT THESE PROXY MATERIALS AND VOTING
| have authority to vote customers’ unvoted shares held by the firms in street name on Proposal No. 2. If a broker does not exercise this authority, such broker non-votes will have no effect on the results of this vote. We are not required to obtain the approval of our stockholders to select our independent registered public accounting firm. However, if our stockholders do not ratify the appointment of PricewaterhouseCoopers LLP as our independent registered public accounting firm for the 2023 fiscal year, our Audit Committee will reconsider its selection.
| ||
| Proposal No. 3: Approve an Advisory Vote on the Compensation of our Named Executive Officers | The vote of a majority of the shares of our common stock cast affirmatively at the Annual Meeting is required to approve on an advisory basis the compensation of our named executive officers, as described in this proxy statement. Abstentions are not considered votes cast and will have no effect on the results of this vote. Brokerage firms do not have authority to vote customers’ unvoted shares held by the firms in street name on this proposal. As a result, any shares not voted by a customer will be treated as a broker non-vote. Such broker non-votes will have no effect on the results of this vote. Although the advisory vote is non-binding, the Compensation Committee and the Board of Directors will review the voting results and take them into consideration when making future decisions regarding executive compensation.
| |
| Proposal No. 4: Approve an Amendment to our Restated Certificate of Incorporation to Reflect new Delaware Law Provisions Regarding Officer Exculpation | The vote of a majority of the outstanding shares of our common stock entitled to vote thereon is required to approve the proposal to amend our Restated Certificate of Incorporation to reflect new Delaware law provisions regarding officer exculpation, as described in this proxy statement. Abstentions have the same effect as a vote cast against the proposal because abstentions are treated as shares entitled to vote. Brokerage firms do not have authority to vote customers’ unvoted shares held by the firms in street name on this proposal. As a result, any shares not voted by a customer will be treated as a broker non-vote. Broker non-votes will have the same effect as a vote against the proposal.
|
|
|
2023 PROXY STATEMENT | 5 |
QUESTIONS AND ANSWERS ABOUT THESE PROXY MATERIALS AND VOTING
The following table summarizes the applicable vote required for approval of each item of business to be transacted at the Annual Meeting, assuming there is a quorum. In addition, the table shows the effect on the outcome of the vote of: (i) abstentions (or, in the case of Proposal No. 1, WITHHOLD votes); (ii) “broker non-votes” or shares held by brokers when a beneficial owner of shares held in “street name” does not provide voting instructions for non-routine matters and, as a result, the broker/nominee is prohibited from voting those shares); and (iii) signed but unmarked proxy cards.
| Proposal |
Vote Required for Approval | Effect of Abstentions/ Withhold Votes (1) |
Uninstructed Shares/ Non-Votes (1) |
Signed but Unmarked Proxy Cards (2) | ||||
|
1. Election of Directors |
Plurality of Votes Cast |
No effect |
Not voted / no effect |
Voted “For” each nominee | ||||
|
2. Ratification of selection of independent registered public accounting firm |
The vote of a majority of the shares of our common stock cast affirmatively or negatively |
No effect |
Not applicable— discretionary vote by broker |
Voted “For” | ||||
|
3. Advisory Vote on Compensation of Our Named Executive Officers |
The vote of a majority of the shares of our common stock cast affirmatively or negatively |
No effect |
Not voted / no effect |
Voted “For” | ||||
|
4. Amendment of Restated Certificate of Incorporation |
The vote of a majority of the outstanding shares of our common stock entitled to vote thereon |
Same as a vote “Against” |
Same as a vote “Against” |
Voted “For” |
| (1) | Abstentions, withheld votes and broker non-votes are included for purposes of determining whether a quorum is present. |
| (2) | If you sign and return your proxy card properly, but do not provide instructions on your proxy card as to how to vote your shares, your shares will be voted as shown in this column, and in accordance with the judgment of the individuals named as proxies on the proxy card as to any other matter properly brought before the Annual Meeting. |
Under stock exchange rules, without voting instructions from beneficial owners, brokers will have discretion to vote on the ratification of the appointment of the independent registered public accounting firm but not on the other proposals.
Is Voting Confidential?
We will keep all the proxies and voting tabulations private. We only let our Inspector of Elections examine these documents. Management will not know how you voted on a specific proposal unless it is necessary to meet legal requirements. We will, however, forward to management any written comments you make, on the proxy card or elsewhere.
Where Can I Find the Voting Results of the Annual Meeting?
The preliminary voting results will be announced at the Annual Meeting, and we will publish preliminary, or final results if available, in a Current Report on Form 8-K within four business days of the Annual Meeting. If final results are unavailable at the time, we file the Form 8-K, then we will file an amended report on Form 8-K to disclose the final voting results within four business days after the final voting results are known.
What Constitutes a Quorum for the Meeting?
The presence, in person or by proxy, of the holders of a majority of the voting power of all outstanding shares of our common stock entitled to vote at the meeting is necessary to constitute a quorum at the meeting. Votes of stockholders of record who are deemed present at the meeting in person or by proxy, abstentions, and broker non-votes are counted for purposes of determining whether a quorum exists.
| 6 |

|
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
Except as otherwise noted, the following table sets forth certain information regarding the beneficial ownership of our common stock as of March 31, 2023 by:
| • | each of our executive officers listed in this proxy statement; |
| • | each of our directors and director nominees; |
| • | all of our current directors and executive officers as a group; and |
| • | each stockholder known by us to own beneficially more than 5% of our common stock. |
Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to the securities. Shares of common stock that may be acquired by an individual or group within 60 days of March 31, 2023 pursuant to the exercise of options or warrants, are deemed to be outstanding for the purpose of computing the percentage ownership of such individual or group, but are not deemed to be outstanding for the purpose of computing the percentage ownership of any other person shown in the table. Percentage of ownership is based on 18,283,074 shares of common stock outstanding on March 31, 2023.
Except as indicated in footnotes to this table, we believe that the stockholders named in this table have sole voting and investment power with respect to all shares of common stock shown to be beneficially owned by them, based on information disclosed in SEC filings by stockholders or provided to us by such stockholders. Unless otherwise indicated, the address for each director and executive officer listed is: c/o Madrigal Pharmaceuticals, Inc., Four Tower Bridge, 200 Barr Harbor Drive, Suite 200, West Conshohocken, Pennsylvania 19428.
| Beneficial Owner |
Number of Shares Beneficially Owned |
Percentage of Common Stock Beneficially Owned |
||||||
| Directors and Named Executive Officers |
||||||||
| Paul A. Friedman, M.D. (1) |
|
2,139,997 |
|
|
11.16 |
% | ||
| Rebecca Taub, M.D. (2) |
|
2,139,997 |
|
|
11.16 |
% | ||
| Brian J. Lynch (3) |
|
91,343 |
|
|
* |
| ||
| Alex Howarth (4) |
|
48,438 |
|
|
* |
| ||
| Remy Sukhija (5) |
|
41,063 |
|
|
* |
| ||
| Kenneth M. Bate (6) |
|
68,489 |
|
|
* |
| ||
| Fred B. Craves, Ph.D. (7) |
|
2,049,629 |
|
|
11.17 |
% | ||
| James M. Daly (8) |
|
38,489 |
|
|
* |
| ||
| Keith R. Gollust (9) |
|
128,941 |
|
|
* |
| ||
| Richard S. Levy, M.D. (10) |
|
55,100 |
|
|
* |
| ||
| David Milligan, Ph.D. (11) |
|
38,489 |
|
|
* |
| ||
| All current executive officers and directors as a group (11 persons) (12) |
|
4,709,119 |
|
|
23.92 |
% | ||
| Five Percent Stockholders |
||||||||
| Entities Affiliated with Avoro Capital Advisors LLC (13) |
|
1,660,000 |
|
|
9.08 |
% | ||
| Entities Affiliated with Baker Bros. Advisors LP (14) |
|
1,545,113 |
|
|
8.45 |
% | ||
| Entities Affiliated with Bay City Capital LLC (15) |
|
1,511,782 |
|
|
8.27 |
% | ||
| Entities Affiliated with The Vanguard Group (16) |
|
1,266,375 |
|
|
6.93 |
% | ||
| Entities Affiliated with State Street Corporation (17) |
|
1,260,993 |
|
|
6.9 |
% | ||
| Entities Affiliated with BlackRock, Inc. (18) |
|
986,572 |
|
|
5.4 |
% | ||
| * | Represents beneficial ownership of less than 1% of the shares of common stock. |
|
|
2023 PROXY STATEMENT | 7 |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
| (1) | Includes: 540,231shares of common stock issuable upon the exercise of Dr. Friedman’s options that are exercisable within sixty days of March 31, 2023; 655,540 shares of common stock owned and held by SQN, LLC, or SQN; shares held of record or in street name by Dr. Friedman; 8,000 shares held in a grantor retained annuity trust (the “GRAT”); and the deemed beneficial ownership of shares not reflected in the foregoing clauses that are beneficially owned by his spouse, Dr. Taub. See footnote 2 below for Dr. Taub’s beneficial ownership information, but note that the shares described therein should not be double-counted with Dr. Freidman’s shares described herein. Dr. Friedman is a managing member of SQN and may be deemed to share voting and investment power over our common stock that is owned and held by SQN. Dr. Friedman disclaims beneficial ownership of our common stock held by SQN and the GRAT and shares of our common stock beneficially owned by Dr. Taub, except to the extent of his pecuniary interest therein. |
| (2) | Includes: 348,147 shares of common stock issuable upon the exercise of Dr. Taub’s options that are exercisable within sixty days of March 31, 2023; 655,540 shares of common stock held by SQN; shares held of record or in street name by Dr. Taub; 8,000 shares held in the GRAT; and the deemed beneficial ownership of shares not reflected in the foregoing clauses that are beneficially owned by her spouse, Dr. Friedman. See footnote 1 above for Dr. Freidman’s beneficial ownership information, but note that the shares described therein should not be double-counted with Dr. Taub’s shares described herein. Dr. Taub is a managing member of SQN and may be deemed to share voting and investment power over our common stock that is owned and held by SQN. The 655,540 shares held by SQN and the 8,000 shares held by the GRAT are the same shares of our common stock as are listed in footnote 1. Dr. Taub disclaims beneficial ownership of our common stock held by SQN and the GRAT and shares of our common stock beneficially owned by Dr. Friedman, except to the extent of her pecuniary interest therein. |
| (3) | Includes 90,813 shares of common stock issuable upon the exercise of options exercisable within sixty days of March 31, 2023. |
| (4) | Consists of 48,438 shares of common stock issuable upon the exercise of options exercisable within sixty days of March 31, 2023. |
| (5) | Consists of 41,063 shares of common stock issuable upon the exercise of options exercisable within sixty days of March 31, 2023. |
| (6) | Consists of 68,489 shares of common stock issuable upon the exercise of options exercisable within sixty days of March 31, 2023. |
| (7) | Number of shares listed in table and in this footnote as beneficially owned is based solely on disclosures made in a Form 5 filed with the SEC on February 2, 2022. Includes 361,358 shares of common stock held directly, 1,511,782 shares of common stock held by investment entities affiliated with Bay City Capital LLC, or BCC, as set forth in footnote 15 below, 90,000 shares of common stock held in a GRAT, 18,000 shares of common stock held by the Craves Family Foundation and 68,489 shares of common stock issuable upon the exercise of options exercisable within sixty days of March 31, 2023 issued to Dr. Craves, a director of the Issuer, in connection with his service as a director of the Issuer. Dr. Craves is a party to an agreement whereby he must transfer such stock options to BCC upon receipt. Dr. Craves is a managing director of BCC and thus may be deemed to share voting and investment power and therefore beneficially own shares held by the entities listed in footnote 15. Dr. Craves disclaims beneficial ownership of the shares beneficially owned by the entities listed in footnote 15 except to the extent of his pecuniary interest therein. |
| (8) | Consists of 38,489 shares of common stock issuable upon the exercise of options exercisable within sixty days of March 31, 2023. |
| (9) | Includes 68,489 shares of common stock issuable upon the exercise of options exercisable within sixty days of March 31, 2023, 43,975 shares of common stock owned of record by Wyandanch Partners, L.P., 11,477 shares held directly and 5,000 shares of common stock owned of record by Keith R. Gollust Roth IRA. Mr. Gollust is the president and sole stockholder of Gollust Management, Inc., which is the general partner of Wyandanch Partners, L.P. |
| (10) | Includes 46,000 shares of common stock issuable upon the exercise of options exercisable within sixty days of March 31, 2023. |
| (11) | Consists of 38,489 shares of common stock issuable upon the exercise of options exercisable within sixty days of March 31, 2023. |
| (12) | Percentage calculation includes common stock issuable upon the exercise of options as set forth in footnotes 1 through 11. Common stock beneficially owned by Drs. Friedman and Taub and referenced in footnotes 1 and 2 above have not been double-counted in the number of shares beneficially owned on this line. |
| (13) | Number of shares listed in table as beneficially owned is based solely upon information disclosed via Amendment No. 1 to Schedule 13G filed with the SEC on February 14, 2023. The address for the entities listed above is c/o Avoro Capital Advisors LLC, 110 Greene Street, Suite 800, New York, NY 10012. |
| (14) | Number of shares listed in table and in this footnote as beneficially owned is based solely on information disclosed via Amendment No. 2 to Schedule 13G filed with the SEC on February 14, 2023 (the “Baker Bros. 13G”). The number of shares in the table reflects common stock of Madrigal directly held by certain affiliated funds. The shares described in the table do not include 2,369,797 shares of our common stock that may be acquired upon conversion of Madrigal convertible preferred stock, common stock equivalents with no voting rights, which is convertible into shares of common stock on a 1-for-1 basis only to the extent that after giving effect to such conversion the holders thereof (and their affiliates and any persons who are members of a Section 13(d) group with the holders or their affiliates) would beneficially own (in the aggregate, for purposes of Rule 13d-3 under the Securities Exchange Act of 1934) no more than 4.99% of the outstanding Madrigal common stock (the “Beneficial Ownership Limitation”). Such 2,369,797 shares of Madrigal common stock underlying Madrigal convertible preferred stock, disregarding the Beneficial Ownership Limitation and assuming the full conversion of all currently outstanding preferred shares owned by entities affiliated with Baker Bros. Advisors LP, plus such 1,545,113 additional shares of common stock directly owned by entities affiliated with Baker Bros. Advisors LP collectively would represent 18.96% of our common stock on an as converted, fully-converted basis. The address for the entities listed above and additional reporting persons described in the Baker Bros. 13G is c/o Baker Bros. Advisors LP, 860 Washington Street, 3rd Floor, New York, NY 10014. |
| 8 |

|
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
| (15) | Number of shares listed in table and in this footnote as beneficially owned is based solely on disclosures made in Amendment No. 5 to Schedule 13D filed with the SEC on December 7, 2021. Of the 1,511,782 shares listed above, 1,510,521 shares of common stock are held of record by Bay City Capital Fund IV, L.P., or Fund IV, and 1,261 shares of common stock are held of record by BCC. Bay City Capital Management IV LLC, or BCC Management IV, is the general partner of Fund IV. BCC Management IV holds no shares of common stock directly, but is deemed to have beneficial ownership of common stock owned by Fund IV due to its role as general partner of such funds. Investment and voting decisions by BCC Management IV are exercised by BCC as manager. Due to its role as manager of BCC Management IV, BCC is deemed to have beneficial ownership of common stock deemed to be beneficially owned by BCC Management IV. Dr. Craves is a managing director of BCC and thus may be deemed to share investment and voting power and therefore beneficially own shares held by these entities. Dr. Craves disclaims beneficial ownership of the shares beneficially owned by these entities except to the extent of his pecuniary interest therein. The address for BCC and the entities affiliated with BCC is 1000 4th Street, Suite 500, San Rafael, California 94901. |
| (16) | Number of shares listed in table as beneficially owned is based solely upon information disclosed via Amendment No. 3 to Schedule 13G filed with the SEC February 9, 2023. The address for the entities listed above is 100 Vanguard Blvd., Malvern, PA 19355. |
| (17) | Number of shares listed in table as beneficially owned is based solely upon information disclosed via Schedule 13G filed with the SEC February 2, 2023. The address for the entities listed above is State Street Financial Center, 1 Lincoln Street, Boston, MA 02111. |
| (18) | Number of shares listed in table as beneficially owned is based solely upon information disclosed via Amendment No. 2 to Schedule 13G filed with the SEC on February 1, 2023. The address for the entities listed above is c/o BlackRock, Inc., 55 East 52nd Street New York, NY 10055. |
|
|
2023 PROXY STATEMENT | 9 |
MANAGEMENT AND CORPORATE GOVERNANCE
The Board of Directors
Our restated certificate of incorporation and restated bylaws provide that our business is to be managed by or under the direction of our Board of Directors. Our Board of Directors is divided into three classes for purposes of election. One class is elected at each Annual Meeting of Stockholders to serve for a three-year term. Our Board of Directors, which consists of eight members, is classified into three classes as follows:
| • | the Class I directors are Paul A. Friedman, M.D., Kenneth M. Bate and James M. Daly, and their terms are scheduled to expire at this year’s Annual Meeting; |
| • | the Class II directors are Rebecca Taub, M.D. and Fred B. Craves, Ph.D., and their terms will expire at the Annual Meeting of Stockholders to be held in 2024; and |
| • | the Class III directors are Keith R. Gollust, David Milligan, Ph.D. and Richard S. Levy, M.D., and their terms will expire at the Annual Meeting of Stockholders to be held in 2025. |
On March 9, 2023, our Nominating and Governance Committee nominated, and our Board thereafter approved on the same date, Paul A. Friedman, M.D., Kenneth M. Bate and James M. Daly for re-election at the Annual Meeting for a term of three years to serve until the 2026 Annual Meeting of Stockholders, and until their respective successors have been elected and qualified.
Set forth below are the names of the persons nominated as directors and directors, their ages, their offices in the Company, if any, their principal occupations or employment for at least the past five years, the length of their tenure as directors and the names of other public companies in which such persons hold or have held directorships during the past five years. Except as otherwise specified, such information is reflected as of March 31, 2023. As used in certain of the following biographical descriptions, the term “Private Madrigal” refers to Madrigal Pharmaceuticals, Inc., a privately-held Delaware corporation focused on developing innovative therapeutic candidates for the treatment of cardiovascular, metabolic and liver diseases prior to the consummation of the Merger; the term “Merger” means the July 22, 2016 business combination transaction and name change which involved the merger of Private Madrigal and a subsidiary of Synta and resulted in the establishment of Public Madrigal; and the term “Synta” refers to Synta Pharmaceuticals Corp., a publicly traded Delaware corporation prior to the consummation of the Merger. Additionally, information is set forth below about the specific experience, qualifications, attributes or skills relevant to service on our Board of Directors.
| Name |
Age |
Position | ||
| Kenneth M. Bate (1)(2) |
72 |
Class I Director | ||
| Fred B. Craves, Ph.D. (2)(3) |
77 |
Lead Director and Class II Director | ||
| James M. Daly (1)(3) |
61 |
Class I Director | ||
| Paul A. Friedman, M.D. |
80 |
Chairman of the Board of Directors, Chief Executive Officer and Class I Director | ||
| Keith R. Gollust (1)(3) |
77 |
Class III Director | ||
| Richard S. Levy, M.D. (2)(3) |
65 |
Class III Director | ||
| David Milligan, Ph.D. (1)(2) |
82 |
Class III Director | ||
| Rebecca Taub, M.D. |
71 |
President, Research & Development, Chief Medical Officer and Class II Director |
| (1) | Member of our Audit Committee. |
| (2) | Member of our Compensation Committee. |
| (3) | Member of our Nominating and Governance Committee. |
| 10 |

|
|
MANAGEMENT AND CORPORATE GOVERNANCE
In addition to the information presented below regarding each of our director’s specific experience, qualifications, attributes and skills that led our Board to the conclusion that he or she should serve as a director, we also believe that all of our directors have a reputation for integrity, honesty and adherence to high ethical standards. They each have demonstrated business acumen and an ability to exercise sound judgment, as well as a commitment of service to our company and our Board of Directors.
| Kenneth M. Bate | ||
|
Age: 72
Director Since: July 2016 |
Biographical Information Mr. Bate currently works as an independent consultant. Previously, Mr. Bate was the President and Chief Executive Officer of Archemix Corp., a privately-held biopharmaceutical company, a position he held from April 2009 through December 2011. From 2006 to April 2009, he served in various positions at NitroMed, Inc., a publicly-held pharmaceutical company, most recently as President and Chief Executive Officer. From 2002 to 2005, Mr. Bate served as Head of Commercial Operations and Chief Financial Officer at Millennium Pharmaceuticals, Inc., a biopharmaceutical company. Prior to joining Millennium Pharmaceuticals, Mr. Bate co-founded JSB Partners, LLC, a banking and advisory services firm for biopharmaceutical and life sciences companies. From 1990 to 1996, Mr. Bate was employed with Biogen, Inc., a publicly-held biotechnology company, first as its Chief Financial Officer and then as head of the commercial organization responsible for launching its multiple sclerosis business. Mr. Bate currently serves as chairman of the board of directors of Astria Therapeutics, Inc., a publicly-held biopharmaceutical company. Mr. Bate previously served as chairman of the board of directors of Cubist Pharmaceuticals, Inc., AVEO Pharmaceuticals Inc. and Genocea Biosciences, Inc., as well as a director of BioMarin Pharmaceuticals, Inc. and Vanda Pharmaceuticals Inc., each publicly-held biopharmaceutical companies. Mr. Bate holds a B.A. in Chemistry from Williams College and an M.B.A. from The Wharton School of the University of Pennsylvania.
| |
| Qualifications We believe that Mr. Bate’s qualifications to serve on our board of directors include his operating, finance, commercial, transactional and senior management experience in the industry, such as his experience as chief executive officer of Archemix and NitroMed, as head of commercial operations and chief financial officer at Millennium Pharmaceuticals, and as chief financial officer and vice president of sales and marketing at Biogen, as well as his experience serving on the board of directors of other public companies in the life sciences industry. | ||
| Fred B. Craves, Ph.D. | ||
|
Age: 77
Director Since: July 2016 |
Biographical Information Dr. Craves co-founded and served as Chairman of the Board of Private Madrigal, a privately-held biopharmaceutical company, from its inception in September 2011 through the Merger involving Synta, in July of 2016. Dr. Craves is a Managing Director and co-founder of Bay City Capital. In the course of his career, Dr. Craves has founded and managed several biotechnology companies. Dr. Craves previously served on the boards of directors of several privately-held and publicly-held companies. Dr. Craves currently serves as a member of the board of directors of Synchronicity Pharma, Inc. and IMIDomics, Inc., each a privately-held life science company. During the past five years, Dr. Craves served as a member of the board of directors of Dermira, Inc., KBP Pharmaceuticals, Inc. and Twist Bioscience, Inc., each a publicly-held life science company. Dr. Craves earned a B.S. degree in biology from Georgetown University, an M.S. in biochemical pharmacology from Wayne State University and a Ph.D. in pharmacology and experimental toxicology from the University of California, San Francisco. | |
| Qualifications We believe that Dr. Craves is qualified to serve on our board of directors due to his extensive experience with founding, managing and serving on the boards of directors of life sciences companies, both public and private, and his extensive knowledge of the life sciences industry. | ||
|
|
2023 PROXY STATEMENT | 11 |
MANAGEMENT AND CORPORATE GOVERNANCE
| James M. Daly
| ||
|
Age: 61
Director Since: June 2019 |
Biographical Information On June 27, 2019, our Board of Directors appointed James M. Daly as a member of our Board of Directors. Mr. Daly has over 30 years of experience leading U.S. and global businesses in the biopharmaceutical industry and also currently serves as a Director of Acadia Pharmaceuticals, argenx SE, Bellicum Pharmaceuticals, and Halozyme Therapeutics*. He previously served on the board of directors of Chimerix, Inc. Most recently, Mr. Daly served as Executive Vice President and Chief Commercial Officer at Incyte Corporation, a publicly-held biopharmaceutical company, or Incyte, from 2012 to 2015. Previously, Mr. Daly worked for Amgen, Inc. and held various leadership positions over a 10-year period, including his last role as Senior Vice President, North America Commercial Operations, Global Marketing and Commercial Development. Earlier in his career, he spent over 16 years with Glaxo Wellcome/GlaxoSmithKline (GSK), where he held roles of increasing responsibility, including his last role as Senior Vice President, General Manager of the Respiratory and Anti-Infective Business Unit. He earned a B.S. in Pharmacy and an M.B.A. from the University at Buffalo, The State University of New York.
*Mr. Daly will resign from the Halozyme Therapeutics board effective December of 2023.
| |
| Qualifications We believe that Mr. Daly is qualified to serve as a member of our board of directors due to his extensive experience as a pharmaceutical executive heading up major commercialization programs and given his extensive experience as a director with public biopharmaceutical companies. | ||
| Paul A. Friedman, M.D.
| ||
| Age: 80
Director Since: July 2016 |
Biographical Information Dr. Friedman has served as our Chairman and Chief Executive Officer since July 22, 2016. Dr. Friedman also currently serves on the Board of Prelude Therapeutics, a publicly-held biopharmaceutical company, where he serves as non-executive Chairman. Dr. Friedman served as the Chief Executive Officer of Incyte from November 2001 until his retirement in January 2014. Dr. Friedman served from 1994 to 1998, as President of Research & Development for the DuPont-Merck Pharmaceutical Company and from 1998 to 2001 as President of DuPont Pharmaceuticals Research Laboratories, a wholly-owned subsidiary of the DuPont Company. From 1991 to 1994, he served as Senior Vice President at Merck Research Laboratories. Prior to his tenures at Merck and DuPont, Dr. Friedman was an Associate Professor of Medicine and Pharmacology at Harvard Medical School. Dr. Friedman is a diplomat of the American Board of Internal Medicine and a member of the American Society of Clinical Investigation. He also previously served on the board of directors of the following publicly-held pharmaceutical companies in the last five years: Incyte (from November 2001 through May of 2021); Alexion Pharmaceuticals (through its acquisition by AstraZeneca in 2021); Cerulean Pharma Inc. (now Daré Bioscience, Inc.) (through January of 2017); and Verastem, Inc. (through April of 2017). Dr. Friedman received his A.B. in Biology from Princeton University and his M.D. from Harvard Medical School. Dr. Friedman and Dr. Taub, our Chief Medical Officer, President, Research & Development, and a director, are married to each other.
| |
| Qualifications We believe that Dr. Friedman is qualified to serve as Chief Executive Officer and as Chairman of our board of directors due to his: extensive experience in the biopharmaceutical industry; deep experience in research and both early and late stage clinical development; extensive experience building and leading R&D organizations, expanding company pipelines of assets, and overseeing the commercial development of innovative therapeutic products across a range of areas; and valuable perspectives to the Board as Madrigal’s Chief Executive Officer. | ||
| 12 |

|
|
MANAGEMENT AND CORPORATE GOVERNANCE
| Keith R. Gollust
| ||
| Age: 77
Director Since: July 2016 |
Biographical Information Mr. Gollust has served on our Board since July of 2016, on the board of directors of Synta from July 2002 through the Merger in July 2016 and as Chairman of the Synta board of directors from September 2002 through the Merger in July 2016. Mr. Gollust is a private investor and President of Gollust Management, Inc., the general partner of Wyandanch Partners, an investment partnership. In the past, Mr. Gollust has served as a director of numerous public and private companies. Mr. Gollust received a B.A. from Princeton University and an M.S.I.A. from Carnegie Mellon University.
| |
| Qualifications We believe that Mr. Gollust is qualified to serve as a member of our board of directors due to his experience as managing general partner of various investment partnerships which have given him the responsibility for investing over $1 billion as a fiduciary. | ||
| Richard S. Levy, M.D.
| ||
| Age: 65
Director Since: August 2016 |
Biographical Information Dr. Levy has served on Madrigal’s board of directors since August of 2016. Dr. Levy also serves on the board of directors of ProTara Therapeutics, Inc., Kodiak Sciences Inc. and Kiniksa Pharmaceuticals, Ltd., each a publicly-held pharmaceutical company. Dr. Levy previously served on the board of Aquinox Pharmaceuticals, Inc. and Constellation Pharmaceuticals. Previously, from December 2016 to May 2019, Dr. Levy was a part-time Senior Advisor for Baker Bros. Advisors, L.P., a firm that primarily manages long-term investment funds focused on publicly traded life sciences companies. Dr. Levy served as Executive Vice President and Chief Drug Development Officer at Incyte from January 2009 until his retirement in April 2016, and as Senior Vice President of Drug Development at Incyte from August 2003 to January 2009. Prior to joining Incyte, Dr. Levy served as Vice President, Biologic Therapies, at Celgene Corporation, a publicly-held biopharmaceutical company, from 2002 to 2003. From 1997 to 2002, Dr. Levy served in various executive positions with DuPont Pharmaceuticals Company, first as Vice President, Regulatory Affairs and Pharmacovigilence, and thereafter as Vice President, Medical and Commercial Strategy. Dr. Levy served at Novartis, and its predecessor company Sandoz, from 1991 to 1997 in positions of increasing responsibility in clinical research and regulatory affairs. Dr. Levy has more than 30 years’ experience in the pharmaceutical and biotechnology industries through his prior positions at Incyte, Celgene, DuPont Pharmaceuticals and Novartis, has extensive clinical research, regulatory and product development skills and has worked in multiple therapeutic areas. Prior to joining the pharmaceutical industry, Dr. Levy served as an Assistant Professor of Medicine at the UCLA School of Medicine. Dr. Levy is Board Certified in Internal Medicine and Gastroenterology and received his A.B. in Biology from Brown University, his M.D. from the University of Pennsylvania School of Medicine, and completed his training in Internal Medicine at the Hospital of the University of Pennsylvania and a fellowship in Gastroenterology and Hepatology at UCLA.
| |
| Qualifications We believe that Dr. Levy is qualified to serve on our board of directors due to his extensive and diverse experience in the pharmaceutical and biotechnology industries. | ||
|
|
2023 PROXY STATEMENT | 13 |
MANAGEMENT AND CORPORATE GOVERNANCE
| David Milligan, Ph.D.
| ||
| Age: 82
Director Since: July 2016 |
Biographical Information Dr. Milligan currently works as an independent consultant. Previously, Dr. Milligan was a partner at Bay City Capital LLC, a life sciences investment firm, a position he held from 1997 through 2013. From 1979 to 1996, Dr. Milligan served in a variety of roles retiring as Senior Vice President and Chief Scientific Officer at Abbott Laboratories, a publicly-held healthcare products company. Dr. Milligan currently serves as Chairman of Air Answers, previously Inspirotech, Director of Minute Molecular Diagnostics and Chairman of Ekatra. From 2016 to 2018 he was a board member for WebLoq Inc, a privately-held information technology security company that is no longer in business. Starting in 1995, Dr. Milligan served as a director of Caliper Life Sciences, a publicly-held pharmaceutical and biotechnology company that was acquired by PerkinElmer Company in 2011, as well as ICOS, a publicly-held pharmaceutical company where he was a board member and later lead director before it was acquired by Eli Lilly in 2008. Dr. Milligan also served as Chairman and a director at Vicuron Pharmaceuticals, Inc., a privately-held biopharmaceutical company from 1997 to 2005 when it was acquired by Pfizer. He was also a director of Reliant Pharmaceuticals, Inc., a privately-held pharmaceutical company from 1999 until acquired by GlaxoSmithKline, in 2007. In addition, he was a director of Pathway Diagnostics Corporation, a privately-held diagnostics company acquired by Quest Diagnostics, Inc., and a director of Maxia Pharmaceuticals, Inc., a privately-held pharmaceutical company acquired by Incyte, from 1999 to 2003. Dr. Milligan received an A.B. in Chemistry from Princeton University and an M.S. and Ph.D. in Organic Chemistry from the University of Illinois.
| |
| Qualifications We believe that Dr. Milligan’s qualifications to serve on our board of directors include his operating, finance, commercial, transactional and senior management experience in the life sciences industry, as well as his experience serving on the boards of directors of publicly- and privately-held companies in the life sciences industry. | ||
| Rebecca Taub, M.D.
| ||
| Age: 71
Director Since: July 2016 |
Biographical Information Dr. Taub has been a member of our Board of Directors since July 2016 and has served as our President, Research & Development since June 2019 and our Chief Medical Officer since July 2016. She also served as Executive Vice President, Research & Development, from July 2016 through June 2019. Dr. Taub also currently serves on the board of directors of BriaCell Therapeutics Corp., a publicly-held immuno-oncology biotechnology company. Dr. Taub served on the board of directors of Private Madrigal and as Chief Executive Officer of Private Madrigal from its inception in September 2011 through the Merger in July 2016. Prior to joining Private Madrigal, Dr. Taub served as Senior Vice President, Research and Development of VIA Pharmaceuticals from 2008 to 2011 and as Vice President, Research, Metabolic Diseases at Hoffmann-La Roche from 2004 to 2008. In those positions, Dr. Taub oversaw clinical development and drug discovery programs in cardiovascular and metabolic diseases including the conduct of a series of Phase I and II proof of concept clinical trials. Dr. Taub led drug discovery including target identification, lead optimization and advancement of preclinical candidates into clinical development. From 2000 through 2003, Dr. Taub worked at Bristol-Myers Squibb Co. and DuPont Pharmaceutical Company, in a variety of positions, including Executive Director of CNS and metabolic diseases research. Before becoming a pharmaceutical executive, Dr. Taub was a tenured Professor of Genetics and Medicine at the University of Pennsylvania, and remains an adjunct professor. Dr. Taub is the author of more than 120 research articles. Before joining the faculty of the University of Pennsylvania, Dr. Taub served as an Assistant Professor at the Joslin Diabetes Center of Harvard Medical School, Harvard University and an associate investigator with the Howard Hughes Medical Institute. Dr. Taub received her M.D. from Yale University School of Medicine and B.A. from Yale College. Dr. Taub and Dr. Friedman, our Chief Executive Officer and Chairman of the Board of Directors, are married to each other.
| |
| Qualifications We believe that Dr. Taub is qualified to serve on our board based on her experience as our Chief Medical Officer and President, Research and Development, and due to her extensive experience as a pharmaceutical executive heading up major development programs in non-alcoholic steatohepatitis, or NASH. | ||
| 14 |

|
|
MANAGEMENT AND CORPORATE GOVERNANCE
Director Independence
Our Board of Directors has reviewed the materiality of any relationship that each of our directors has with our company, either directly or indirectly. Based on this review, our Board of Directors has determined that each of its current and nominated directors is independent under applicable listing standards of the Nasdaq Stock Market LLC, or Nasdaq, other than Dr. Friedman, who is our Chief Executive Officer, and Dr. Taub, who is our Chief Medical Officer and President, Research & Development. Six of our eight Board members therefore satisfy such independence requirements.
Committees of the Board of Directors and Meetings
Meeting Attendance
Our Board of Directors held ten meetings during 2022. Our Board of Directors has an Audit Committee, a Compensation Committee, and a Nominating and Governance Committee. Each director attended or participated in at least 75% (or more) of the aggregate of (i) the total number of meetings of the Board of Directors and (ii) the total number of meetings held by all committees of the Board of Directors on which he or she served during 2022. The Board of Directors has adopted a policy under which each member of the Board of Directors is encouraged, but not required, to attend each Annual Meeting of Stockholders.
Audit Committee
Our Audit Committee is composed of Messrs. Bate (Chairman), Daly and Gollust and Dr. Milligan. Our Audit Committee held four meetings during 2022. Our Board of Directors has determined that each member of the Audit Committee is independent under SEC rules and the applicable listing standards of Nasdaq, as such rules and standards apply specifically to members of audit committees. Our Board of Directors has determined that Mr. Bate is an “audit committee financial expert,” as the SEC has defined that term, and has the requisite financial sophistication in accordance with applicable Nasdaq listing standards. Please also see the report of the Audit Committee set forth elsewhere in this proxy statement. Our Audit Committee’s role and responsibilities are set forth in the Audit Committee’s written charter and include the authority to:
| • | approve and retain the independent auditors to conduct the annual audit of our consolidated financial statements; |
| • | review the proposed scope and results of the audit; |
| • | review and pre-approve the independent auditor’s audit and non-audit services rendered; |
| • | approve the audit fees to be paid; |
| • | review accounting and financial controls with the independent auditors and our financial and accounting staff; |
| • | review and approve transactions between us and our directors, officers and affiliates; |
| • | review the effectiveness of the Company’s information technology security and controls; |
| • | recognize and prevent prohibited non-audit services; |
| • | establish procedures for complaints received by us regarding accounting matters; |
| • | oversee internal audit functions, if any; |
| • | review, with counsel, any legal or regulatory matter that could have a significant impact on the Corporation’s financial statements; and |
| • | prepare the report of the Audit Committee that the rules of the SEC require to be included in our Annual Meeting proxy statement. |
A copy of the Audit Committee’s written charter is publicly available through the “Investors—Corporate Governance” section of our website at www.madrigalpharma.com.
|
|
2023 PROXY STATEMENT | 15 |
MANAGEMENT AND CORPORATE GOVERNANCE
Compensation Committee
Our Compensation Committee is composed of Mr. Bate and Drs. Craves, Levy (Chairman) and Milligan. Our Compensation Committee held five meetings during 2022. Our Board of Directors has determined that each member of the Compensation Committee is independent under SEC rules and the applicable listing standards of Nasdaq. Our Compensation Committee’s role and responsibilities are set forth in the Compensation Committee’s written charter and include the authority to:
| • | review and establish the compensation arrangements for management, including the compensation for our Chief Executive Officer; |
| • | establish and review general compensation policies with the objective to attract and retain superior talent, to reward individual performance and to achieve our financial goals; |
| • | administer our stock incentive plan; |
| • | review the Compensation Discussion and Analysis, or CD&A, discuss the CD&A with management and, based on such review and discussions, recommend to our Board of Directors that the CD&A be included in our Annual Report on Form 10-K, Annual Meeting proxy statement, or any other applicable filing as required by the SEC; and |
| • | prepare the report of the Compensation Committee that is required to be included in our Annual Meeting proxy statement. |
The Compensation Committee is charged with establishing a compensation policy for our executives and directors that is designed to attract and retain the best possible executive talent, to motivate them to achieve corporate objectives, and reward them for superior performance. Our Compensation Committee is also responsible for establishing and administering our executive compensation policies and equity compensation plans. The Compensation Committee meets at least twice per year and more often as necessary to review and make decisions with regard to executive compensation matters. As part of its review of executive compensation matters, the Compensation Committee may delegate any of the powers given to it to a subcommittee of the committee consisting of one or more members of the Compensation Committee or certain members of executive management, with regard to non-executive equity awards and new hire awards.
The Compensation Committee has the authority to directly retain the services of independent consultants and other experts to assist in fulfilling its responsibilities. In October 2016, the Compensation Committee engaged Compensia, Inc., or Compensia, as its independent compensation consultant, continuing through to the present. Compensia was engaged to review all aspects of our executive compensation programs. As described in the CD&A, Compensia assists the Compensation Committee in defining the appropriate peer companies for executive compensation and practices and in benchmarking our executive compensation program against the peer group. The Compensation Committee also uses information obtained from Compensia, as well as survey data, for evaluating our executive compensation practices, measuring the competitiveness of our practices, and reviewing and implementing our cash bonus policy, equity awards, and base salary benchmarks across all levels of the Company. The Compensation Committee has assessed the independence of Compensia pursuant to SEC rules and the corporate governance rules of Nasdaq and concluded that no conflict of interest exists that would prevent Compensia from independently representing the Compensation Committee. In compliance with SEC rules and the corporate governance rules of Nasdaq, Compensia provided the Compensation Committee with a letter addressing each of the six independence factors. Their responses affirm the independence of Compensia and the partners, consultants, and employees who service the Compensation Committee on executive compensation matters and governance issues.
Please also see the CD&A and the report of the Compensation Committee set forth elsewhere in this proxy statement.
| 16 |

|
|
MANAGEMENT AND CORPORATE GOVERNANCE
A copy of the Compensation Committee’s written charter is publicly available through the “Investors—Corporate Governance” section of our website at www.madrigalpharma.com.
Nominating and Governance Committee
Our Nominating and Governance Committee is composed of Drs. Craves and Levy and Messrs. Daly and Gollust (Chairman). Our Nominating and Governance Committee held one meeting during 2022 and also covered nomination and governance topics with the full Board in the context of regularly scheduled meetings of the Board of Directors throughout the fiscal year. Our Board of Directors has determined that each member of the Nominating and Governance Committee is independent under SEC rules and the applicable listing standards of Nasdaq. Our Nominating and Governance Committee’s role and responsibilities are set forth in the Nominating and Governance Committee’s written charter and include the authority to:
| • | identify and nominate members of the Board of Directors; |
| • | develop and recommend to the Board of Directors a set of corporate governance principles applicable to our company; and |
| • | oversee the evaluation of the Board of Directors and management pursuant to annual and ongoing evaluation of Board and Committee structure, performance and open-access. |
In future annual meetings, if a stockholder wishes to propose to nominate a director for election, in order to be properly considered by our Nominating and Governance Committee, such proposal must follow the procedures described below as well as under our bylaws, as summarized under “Stockholder Proposals at Future Annual Meetings” at the end of this proxy statement (our “Bylaw Provisions Related to Stockholder Nominations of Director Candidates”).
The Nominating and Governance Committee may consider candidates recommended by stockholders as well as from other sources such as other directors or officers, third-party search firms or other appropriate sources. For all potential candidates, our Nominating and Governance Committee may consider all factors it deems relevant, such as a candidate’s: personal integrity and sound judgment, business and professional skills and experience, independence, knowledge of the industry in which we operate, ability to bring skill sets necessary to contribute to the long-term forward-looking strategy and performance objectives of the Company, possible conflicts of interest, diversity, capacity to provide needed depth or complementary skills considered relevant for Board service, capabilities to fill a present need on the Board of Directors, and ability to contribute to, create and sustain value associate with the long-term interests of the Company’s stockholders. Director candidates nominated by stockholders for election at an Annual Meeting must be made in accordance with our Bylaw Provisions Related to Stockholder Nominations of Director Candidates, and are subject to the following requirements and/or considerations. Our Nominating and Governance Committee will consider a recommended nominee from any stockholder or group of affiliated stockholders, and such recommending stockholder or group must have held at least 5% of our common stock for at least one year as of the date the recommendation was made.
Additionally, stockholder recommendations for proposed director nominees must be in writing to the Nominating and Governance Committee, care of our Secretary at Four Tower Bridge, 200 Barr Harbor Drive, Suite 200, West Conshohocken, Pennsylvania 19428. The recommendation must be accompanied by the following information concerning the recommending stockholder:
| • | name, address and telephone number of the recommending stockholder; |
| • | the number of shares of our common stock owned by the recommending stockholder and the time period for which such shares have been held; |
| • | if the recommending stockholder is not a stockholder of record, a statement from the record holder verifying the holdings of the recommending stockholder and a statement from the recommending stockholder of the length of |
|
|
2023 PROXY STATEMENT | 17 |
MANAGEMENT AND CORPORATE GOVERNANCE
| time such shares have been held (alternatively the recommending stockholder may furnish a current Schedule 13D, Schedule 13G, Form 3, Form 4 or Form 5 filed with the SEC, together with a statement of the length of time that the shares have been held); and |
| • | a statement from the recommending stockholder as to a good faith intention to continue to hold such shares through the date of the next Annual Meeting. |
The recommendation must also be accompanied by the following information concerning the proposed director nominee:
| • | the information required by Items 401, 403 and 404 of Regulation S-K under the Securities Act of 1933, as amended (the “Securities Act”); |
| • | a description of all relationships between the proposed nominee and the recommending stockholder, including any agreements or understandings regarding the nomination; |
| • | a description of all relationships between the proposed nominee and any of our competitors, customers, suppliers, labor unions or other persons with special interests regarding our company; and |
| • | the contact information of the proposed nominee. |
The recommending stockholder must also furnish a statement supporting a view that the proposed nominee possesses the minimum qualifications as set forth below for director nominees and describing the contributions that the proposed nominee would be expected to make to the Board of Directors and to the governance of our company and must state whether, in its view, the proposed nominee, if elected, would represent all stockholders and not serve for the purpose of advancing or favoring any particular stockholder or other constituency of our company. The recommendation must also be accompanied by the written consent of the proposed nominee (1) to be considered by the Nominating and Governance Committee and interviewed if the committee chooses to do so in its discretion, and (2) if nominated and elected, to serve as a director.
For all potential candidates, the Nominating and Governance Committee may consider all factors it deems relevant, including the following threshold criteria:
| • | candidates should possess the highest personal and professional standards of integrity and ethical values; |
| • | candidates must be committed to promoting and enhancing the long-term value of our company for our stockholders; |
| • | candidates must be able to represent fairly and equally all stockholders without favoring or advancing any particular stockholder or other constituency of our company; |
| • | candidates must have demonstrated achievement in one or more fields of business, professional, governmental, community, scientific or educational endeavor, and possess mature and objective business judgment and expertise; |
| • | candidates are expected to have sound judgment, derived from management or policy making experience that demonstrates an ability to function effectively in an oversight role; and |
| • | candidates must have, and be prepared to devote, adequate time to the Board of Directors and its committees. |
Our Nominating and Governance Committee considers diversity of experience as one of the factors it considers in conducting its assessment of director nominees, along with such other factors as it deems appropriate given the then current needs of the Board and the Company, to maintain a balance of knowledge, experience and capability. Consistent with its charter, the Nominating and Governance Committee will strive where appropriate to achieve a diverse balance of backgrounds, perspectives, experience, age, gender, ethnicity and country of citizenship on the Board and its committees.
| 18 |

|
|
MANAGEMENT AND CORPORATE GOVERNANCE
In addition, the Nominating and Governance Committee will also take into account the extent to which the candidate would fill a present need on the Board of Directors, including the extent to which a candidate meets the independence and experience standards promulgated by the SEC and by Nasdaq.
A copy of the Nominating and Governance Committee’s written charter is publicly available through the “Investors—Corporate Governance” section of our website at www.madrigalpharma.com.
Compensation Committee Interlocks and Insider Participation
Our Compensation Committee is composed of Mr. Bate and Drs. Craves, Levy and Milligan. No member of our Compensation Committee has at any time been an employee of our company. None of our executive officers serve as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving as a member of our Board of Directors or Compensation Committee.
Board Leadership Structure
Currently, our Chief Executive Officer, Dr. Friedman, serves as Chairman of our Board of Directors. Our Board of Directors believes that Dr. Friedman is the director best situated to identify strategic opportunities for our company and to focus the activities of our Board of Directors due to his full-time commitment to our business operations. Our Board of Directors also believes that Dr. Friedman’s dual roles as Chairman of the Board and Chief Executive Officer promotes effective execution of our business strategy and facilitates information flow between management and our Board of Directors. Our Board of Directors has appointed Fred Craves as lead independent director to enhance the independence of our Board leadership function.
Our Board of Directors has determined that maintaining the independence of a majority of our directors helps maintain the Board’s independent oversight of management and ensures that the appropriate level of independence is applied to all Board decisions. Moreover, in addition to feedback provided during the course of Board meetings, the independent directors conduct regular executive sessions without the presence of Dr. Friedman, Dr. Taub or any other members of management. Our Audit, Compensation, and Nominating and Governance Committees, each consisting of independent directors, oversee critical matters such as our: (i) accounting policies, financial reporting processes, internal control assessment over financial reporting (Audit Committee); (ii) executive compensation program (Compensation Committee); and (iii) selection and evaluation of our directors and director nominees (Nominating and Governance Committee).
Our Board of Directors’ Role in Risk Oversight
Our Board of Directors has overall responsibility for risk oversight, including, as part of regular Board and committee meetings, general oversight of our executive officers’ management of risks relevant to us. A fundamental part of risk oversight is not only understanding the material risks our company faces and the steps management is taking to manage those risks, but also understanding what level of risk is appropriate for us. The involvement of our Board of Directors in reviewing our business strategy is an integral aspect of its assessment of our management’s tolerance for risk and also its determination of what constitutes an appropriate level of risk for us.
While it has overall responsibility for risk oversight, our Board of Directors has delegated oversight responsibility related to certain risks to committees of the Board. Our Audit Committee is responsible for reviewing our policies with respect to risk assessment and risk management, as well as coordinating our internal control over financial reporting, disclosure controls and procedures and code of conduct controls. Our Audit Committee receives periodic reports from officers responsible for oversight of particular risks within our company at regularly scheduled meetings and other reports as requested by our Audit Committee from time to time. The Nominating and Governance Committee reviews our risks associated with governance matters and non-compensation related human resources matters. In addition, our Compensation Committee has authority to oversee risks as to our compensation programs and discusses with management its annual assessment of our employee compensation policies and programs.
|
|
2023 PROXY STATEMENT | 19 |
MANAGEMENT AND CORPORATE GOVERNANCE
In addition, our Audit Committee has primary responsibility for overseeing risks associated with our information systems and technology, including cybersecurity. Our Audit Committee receives periodic reports from our information security officer and finance team regarding our information systems and technology and associated policies, processes and practices for managing and mitigating cybersecurity and technology-related risks. We also invest in data protection and information technology.
Our Board of Directors satisfies its overall responsibility through full reports by each committee chair regarding the committee’s considerations and actions, as well as through regular reports directly from relevant officers within our company. Our Board of Directors believes that full and open communication between management and the board is essential for effective risk management and oversight.
Stockholder Communications to the Board
Generally, stockholders who have questions or concerns should contact our Investor Relations department at ir@madrigalpharma.com. However, any stockholders who wish to address questions regarding our business directly with the Board of Directors, or any individual director, must prepare the communication in written form and mail or hand deliver the same to the following address:
ATTN: SECURITY HOLDER COMMUNICATION
Board of Directors
Madrigal Pharmaceuticals, Inc.
Four Tower Bridge
200 Barr Harbor Drive, Suite 200
West Conshohocken, PA 19428
Such communications should not exceed 500 words in length and must be accompanied by the following information:
| • | a statement of the type and amount of the securities of our company that the person holds; |
| • | any special interest, meaning an interest not in the capacity as a stockholder of our company, that the person has in the subject matter of the communication; and |
| • | the address, telephone number and e-mail address, if any, of the person submitting the communication. |
The following types of communications are not appropriate for delivery to directors under these procedures:
| • | communications regarding individual grievances or other interests that are personal to the party submitting the communication and could not reasonably be construed to be of concern to security holders or other constituencies of our company (such as employees, members of the communities in which we operate our businesses, customers and suppliers) generally; |
| • | communications that advocate engaging in illegal activities; |
| • | communications that, under community standards, contain offensive, scurrilous or abusive content; and |
| • | communications that have no rational relevance to the business or operations of our company. |
Communications will be distributed to the Board of Directors, or to any individual director or directors as appropriate, depending on the facts and circumstances outlined in the communications. Items that are unrelated to the duties and responsibilities of the Board of Directors may be excluded, such as:
| • | junk mail and mass mailings; |
| • | résumés and other forms of job inquiries; |
| 20 |

|
|
MANAGEMENT AND CORPORATE GOVERNANCE
| • | surveys; and |
| • | solicitations or advertisements. |
In addition, any material that is unduly hostile, threatening, or illegal in nature may be excluded, provided that any communication that is filtered out will be made available to any outside director upon request.
Information About Our Executive Officers
The following table sets forth certain information regarding our executive officers as of March 31, 2023:
| Name |
Age |
Position | ||
| Paul A. Friedman, M.D. |
80 |
Chairman of the Board and Chief Executive Officer | ||
| Rebecca Taub, M.D. |
71 |
President, Research & Development, and Chief Medical Officer | ||
| Brian J. Lynch |
61 |
Senior Vice President and General Counsel | ||
| Remy Sukhija |
51 |
Senior Vice President, Chief Commercial Officer | ||
| Alex Howarth |
54 |
Senior Vice President and Chief Financial Officer | ||
| Robert Waltermire, Ph.D. |
59 |
Chief Pharmaceutical Development Officer |
Paul A. Friedman, M.D., Dr. Friedman’s biographical information is set forth above under “Management and Corporate Governance—The Board of Directors.”
Rebecca Taub, M.D., Dr. Taub’s biographical information is set forth above under “Management and Corporate Governance—The Board of Directors.”
Brian J. Lynch has been at Madrigal since June of 2018. For over 29 years before joining Madrigal, Mr. Lynch engaged in private law practice where he focused on value-enhancing M&A, equity and debt capital markets and collaboration transactions and a broad range of legal representations for life sciences companies, including Madrigal. During his private practice career, he was a partner at the leading international law firms headquartered in Washington, DC (Hogan Lovells), Philadelphia (Morgan Lewis) and Silicon Valley (Cooley). Mr. Lynch has a Juris Doctor degree and a Bachelor’s degree in Accounting. He worked within the United States Department of Treasury and the Securities and Exchange Commission’s Division of Corporation Finance before entering private practice.
Remy Sukhija, who joined Madrigal as Senior Vice President, Chief Commercial Officer, in April of 2020, has over 25 years of commercial experience with large pharmaceutical and biopharmaceutical companies. Before joining Madrigal, from January of 2017 to March of 2020, Mr. Sukhija served as Senior Vice President, US Commercial, for Otsuka Pharmaceutical, and also as CEO of Otsuka Digital Health, Inc. from June of 2018 to June of 2019. From May of 2015 to December of 2016, Remy was Chief Operating Officer for Merganser Biotech, Inc. From April 2010 to April 2015, Mr. Sukhija served as Vice President and Business Unit Head for Biogen. While at Biogen, Remy was responsible for building a commercial organization in support of the launch of two rare disease therapies (ALPROLIX and ELOCTATE), and he was a member of the U.S. leadership team responsible for the launch of Tecfidera® and Plegridy® for multiple sclerosis. Earlier in his career, he held positions of increasing responsibility for Pfizer and GlaxoSmithKline. Mr. Sukhija earned his degree in business from The University of Akron.
Alex Howarth joined Madrigal in May of 2021, bringing more than 30 years of experience in healthcare finance, corporate development and operational roles. Prior to joining Madrigal, he worked at Akcea Therapeutics, Inc. where he held the role of Chief Operating Officer from December of 2019 through the acquisition (in October of 2020) by Ionis Pharmaceuticals, Inc. until December 2020. Before Akcea, Mr. Howarth was the President (from January to December of 2019) and Chief Financial Officer (from September of 2015 to January of 2019) at Lycera Corp. He has
|
|
2023 PROXY STATEMENT | 21 |
MANAGEMENT AND CORPORATE GOVERNANCE
also held roles as Chief Financial Officer and Chief Business Officer at moksha8 Pharmaceuticals, Inc., from April 2007 to September of 2015, and prior to this served as Chief Business Officer at Vitae Pharmaceuticals, Inc. and Head of Venture Partnerships at GlaxoSmithKline plc.
Robert Waltermire, Ph.D., joined Madrigal in August of 2021, bringing proven expertise in all aspects of chemistry, manufacturing and control (CMC), as well as experience in manufacturing new commercial products. Prior to joining Madrigal, Dr. Waltermire served as Senior Vice President, CMC at VenatoRx from November 2020 to July 2021 and as Senior Vice President, Product Development, of Palatin Technologies from February 2020 to November 2020. From 1988 to 2020, Dr. Waltermire held positions of increasing responsibility at Bristol-Myers Squibb (including Vice President Chemical and Synthetic Development from 2016 to January 2020) and Dupont Pharmaceutical Company.
| 22 |

|
|
COMPENSATION DISCUSSION AND ANALYSIS
Table of Contents
| Page |
||||
|
|
23 |
| ||
|
|
23 |
| ||
|
|
26 |
| ||
|
|
27 |
| ||
|
|
29 |
| ||
|
|
32 |
| ||
|
|
33 |
| ||
|
|
33 |
| ||
This Compensation Discussion and Analysis, or CD&A, explains the policies and objectives underlying our executive compensation program as it relates to the following “named executive officers” whose compensation information is presented in the tables following this discussion under “Executive Officer and Director Compensation,” in accordance with SEC rules.
| Name | Position | |
| Paul A. Friedman, M.D. |
Chief Executive Officer | |
| Rebecca Taub, M.D. |
President, Research & Development, and Chief Medical Officer | |
| Brian J. Lynch |
Senior Vice President and General Counsel | |
| Remy Sukhija |
Senior Vice President, Chief Commercial Officer | |
| Alex Howarth |
Senior Vice President and Chief Financial Officer |
Madrigal’s Focus and Achievements
Our Focus. Madrigal is a clinical-stage biopharmaceutical company pursuing novel therapeutics for nonalcoholic steatohepatitis, or NASH. Our lead product candidate, resmetirom, is a proprietary, liver-directed, selective thyroid hormone receptor-ß, or THR-ß, agonist being developed as a once-daily oral pill for the treatment of NASH.
Our Patient Market Opportunity. NASH is a serious inflammatory form of nonalcoholic fatty liver disease, or NAFLD. NAFLD has become the most common liver disease in the United States and other developed countries and is characterized by an accumulation of fat in the liver with no other apparent causes. NASH can progress to cirrhosis or liver failure, require liver transplantation and can also result in liver cancer. Progression of NASH to end stage liver disease will soon surpass all other causes of liver failure requiring liver transplantation. Importantly, beyond these critical conditions, NASH and NAFLD patients additionally suffer heightened cardiovascular risk and, in fact, die more frequently from cardiovascular events than from liver disease. NASH and NAFLD have grown as a consequence of rising worldwide obesity-related disorders. In the United States, NAFLD is estimated to affect approximately 25% of the population, and approximately 25% of those will progress from NAFLD to NASH. Current estimates place NASH prevalence at approximately 22 million people in the United States by 2024, with similar prevalence in Europe and Asia. The prevalence of NASH is also increasing in developing regions due to the adoption of a more sedentary lifestyle and a diet consisting of processed foods with high fat and fructose content.
|
|
2023 PROXY STATEMENT | 23 |
COMPENSATION DISCUSSION AND ANALYSIS
Our Completed Studies. For NASH, we enrolled 125 patients in a Phase 2 clinical trial with resmetirom. We achieved the 12-week primary endpoint for this Phase 2 clinical trial and reported the results in December 2017, and we reported positive topline 36-week results at the conclusion of the Phase 2 clinical trial in May 2018. We also completed a 36-week, open-label extension study in 31 participating NASH patients from our Phase 2 clinical trial, which included 14 patients who received placebo in the main study.
On December 18, 2019 we announced we had opened for enrollment MAESTRO-NAFLD-1, a 52-week, non-invasive, multi-center, double-blind, placebo-controlled Phase 3 clinical study of patients with biopsy-confirmed or presumed NASH recruited from sites in the U.S. Key endpoints are safety, including safety biomarkers. Secondary endpoints include LDL cholesterol, lipid biomarkers, MRI-PDFF, NASH and fibrosis biomarkers. Except for serial liver biopsies, the study protocol is similar to the MAESTRO-NASH study (discussed below under “—Our Ongoing and Planned Studies”), with resmetirom doses of 80 mg or 100 mg or placebo. Enrollment objectives for this study were exceeded, with approximately 1,300 patients enrolled overall. The MAESTRO-NAFLD-1 study will help support the adequacy of the safety database at the time of NDA submission for Subpart H approval for treatment of patients with NASH with fibrosis. In November of 2021, we reported data from the open label non-cirrhotic arm of MAESTRO-NAFLD-1, and in January 2022, we announced that we had achieved primary and secondary endpoints for the double-blind portion of MAESTRO-NAFLD-1, as summarized in “– Key Developments” below.
Our Ongoing and Planned Studies. On March 28, 2019, we announced that we had initiated MAESTRO-NASH, a Phase 3 trial in NASH with its once daily, oral thyroid hormone receptor beta selective agonist, resmetirom. This double-blind, placebo-controlled study is being conducted at more than 220 sites in the United States and the rest of the world. MAESTRO-NASH is a multicenter, randomized, double-blind, placebo-controlled Phase 3 study of resmetirom in patients with liver biopsy-confirmed NASH and was initiated in March 2019. The subpart H portion of the study enrolled more than 1,000 patients with biopsy-proven NASH (at least half with F3 (advanced) fibrosis, the remainder F2 or F1B (moderate fibrosis) with a few earlier F1 patients), randomized 1:1:1 to receive once-daily resmetirom 80 mg, resmetirom 100 mg, or placebo. After 52 weeks of treatment, a second liver biopsy is performed. The dual primary surrogate endpoints on biopsy are NASH resolution with ≥2-point reduction in NAS (NAFLD Activity Score), and with no worsening of fibrosis OR a 1-point decrease in fibrosis with no worsening of NAS. Achievement of either primary endpoint is considered a successful trial outcome. A key secondary endpoint is lowering of LDL-C. All patients enrolled in the MAESTRO-NASH study continue on therapy after the initial 52-week treatment period for up to 54 months to accrue and measure hepatic clinical outcome events including progression to cirrhosis on biopsy (52 weeks and 54 months) and hepatic decompensation events, as well as all-cause mortality. In December 2022, we reported topline results from the subpart H portion of the study: resmetirom achieved both primary endpoints with both daily oral doses, 80 mg and 100 mg, relative to placebo, as summarized in “- Key Developments” below.
On July 13, 2021 we announced first patient dosed in a planned 52-week open label active treatment extension study of MAESTRO-NAFLD-1, named MAESTRO-NAFLD-Open Label Extension (OLE). The OLE study allows patients who complete MAESTRO-NAFLD-1 to consent to 52 weeks of active treatment with resmetirom, making this treatment available to both patients who were assigned to placebo in MAESTRO-NAFLD-1 and patients who were on resmetirom in MAESTRO-NAFLD-1.
In August 2022, we initiated MAESTRO-NASH-OUTCOMES, a randomized double-blind placebo-controlled study in approximately 700 patients with early NASH cirrhosis to allow for noninvasive monitoring of progression to liver decompensation events. A positive outcome is expected to support the full approval of resmetirom for noncirrhotic NASH, potentially accelerating the timeline to full approval. In addition, this study has the potential to support an additional indication for resmetirom in patients with well-compensated NASH cirrhosis.
| 24 |

|
|
COMPENSATION DISCUSSION AND ANALYSIS
The following chart summarizes the status of our product candidate development programs for resmetirom as of March 31 2023:

Key Developments
MAESTRO-NASH TRIAL. In December 2022, we announced topline results from the pivotal Phase 3 MAESTRO-NASH biopsy study of resmetirom. We reported that resmetirom achieved both primary endpoints with both daily oral doses, 80 mg and 100 mg, relative to placebo.
Patients meeting eligibility requirements for MAESTRO-NASH were randomized 1:1:1 to receive resmetirom 80 mg, resmetirom 100 mg, or placebo taken orally once daily. Baseline characteristics in the 966 randomized patients in the primary analysis NASH population (ITT, safety population) were balanced across treatment arms and include age 57 (10) (mean (SD)), female 56%, white 89%, Hispanic 21%, BMI 36 (7) kg/m2, type 2 diabetes 67%, hypertension 78%, dyslipidemia 71%, hypothyroidism 13%, FibroScan, kilopascals (kPa) 13 (7), CAP 348 (38), MRI-PDFF 18% (7), FIB-4 1.4 (0.7), ALT 55 (32) IU, AST 41 (23) IU, LDL 99 (40) mg/dL, triglycerides 188 (132) mg/dL, hemoglobin A1C 6.6 (1) %, ELF 9.8 (0.9). Medications included 49% on statins, 14% on GLP-1 agonists, 14% on SGLT2 inhibitors. Baseline liver biopsy fibrosis scores included F3 (~60%), F2 (~35%), F1B (~5%) (primary analysis population) with 84% with NAS ≥5 based on independent primary glass slide reads of the entire study by two central pathologists.
A second biopsy was conducted after 52 weeks of treatment for assessment of the dual primary endpoints. The primary efficacy analysis assessed histological response at 52 weeks in 955 patients with biopsy-confirmed NASH with fibrosis (modified intent-to-treat (mITT) population) that excluded 11 ITT patients who had their Week 52 biopsy after Week 60 due to COVID-related reasons per regulatory guidelines. Patients without a second biopsy due to early study discontinuation or missing liver biopsy (~17% across treatment arms) were included and considered as non-responders in the primary efficacy analyses (mITT). The compliance to treatment was high and minimally impacted by COVID-19 pandemic restrictions.
Dual Primary Endpoints (52 Weeks) and Key Secondary Endpoint (24 weeks)
| Primary Endpoint |
Resmetirom 80 mg (n=316) |
p-value | Resmetirom 100 mg (n=321) |
p-value | Placebo (n=318) |
|||||||||||||||
| NASH resolution (ballooning o, inflammation 0,1) with ≥2-point reduction in NAS and no worsening of fibrosis |
26 | % | <0.0001 | 30 | % | <0.0001 | 10 | % | ||||||||||||
| ≥-stage improvement in fibrosis with no worsening of NAS |
24 | % | 0.0002 | 26 | % | <0.0001 | 14 | % | ||||||||||||
| Key Secondary Endpoint |
||||||||||||||||||||
| LDL-C lowering (24 weeks) |
-12 | % | <0.0001 | -16 | % | <0.0001 | 1 | % | ||||||||||||
|
|
2023 PROXY STATEMENT | 25 |
COMPENSATION DISCUSSION AND ANALYSIS
All biopsies were read independently by two central pathologists. Each pathologist’s scores showed a similar statistically significant magnitude of response at both doses for both liver biopsy endpoints. Biopsy endpoints were achieved independent of baseline fibrosis stage or diabetes status, including similar statistical significance and magnitude of effect at both doses in subgroups of F2, F3, and F2/F3 patients. Other secondary liver biopsy endpoints that were achieved at both doses include ≥2 point reduction in NAS with no worsening of fibrosis, ≥2 point reduction in NAS with ≥1-stage improvement in fibrosis, NASH resolution (with ≥2 point reduction in NAS) with ≥1-stage improvement in fibrosis, and a 2-stage reduction in fibrosis without worsening of NAS.
Multiple secondary endpoints were achieved, including statistically significant reduction from baseline in liver enzymes (ALT, AST and GGT). Reductions in atherogenic lipids and lipoproteins, fibrosis biomarkers and imaging tests (MRI-PDFF, CAP and liver stiffness measures) were observed in resmetirom treatment arms as compared with placebo. MAESTRO-NASH included many biomarker and imaging assessments that may be used in real world clinical practice to identify appropriate patients for treatment and monitor response to resmetirom, if approved.
MAESTRO-NAFLD-1. In January 2022, we announced topline results from the Phase 3 MAESTRO-NAFLD-1 safety study of resmetirom. We reported that resmetirom demonstrated statistical significance for primary and key secondary endpoints from the double-blind placebo-controlled 969-patient portion of the study. These endpoints indicated that resmetirom (1) was well-tolerated at 80 and 100 mg in patients treated for 52 weeks, (2) provided significant and clinically relevant reductions in liver fat as measured by MRI-PDFF and (3) significantly reduced atherogenic lipids, including LDLc, apolipoprotein B and triglycerides.
A total of 972 patients were randomized in the double-blind arms of the MAESTRO-NAFLD-1 study: 969 patients were included in the safety population and 943 patients in a modified intent-to-treat population for evaluation of key secondary and other endpoints. Important inclusion criteria included the presence of three risk factors of metabolic syndrome, a level of liver fibrosis (measured by FibroScan) consistent with a range of stages of liver fibrosis, and >=8% liver fat (measured by MRI-PDFF).
MASESTRO-NASH OUTCOMES. In August 2022, we announced initiation of MAESTRO-NASH OUTCOMES, a Phase 3, double-blind, randomized, placebo-controlled study that will noninvasively measure progression to liver decompensation events in approximately 700 patients with compensated NASH cirrhosis.
The primary endpoint of MAESTRO-NASH OUTCOMES is the incidence of composite liver-related outcome events, including all-cause mortality, liver transplant, hepatic decompensation (ascites, hepatic encephalopathy, gastroesophageal variceal hemorrhage), and confirmed increase of Model for End-Stage Liver Disease (MELD) score from <12 to ≥15 due to progression of NASH cirrhosis. Key inclusion criteria are well-compensated NASH cirrhosis (Child-Pugh A) and presence of three metabolic risk factors (metabolic syndrome). Patients will be randomized 3:1 in a blinded manner to receive 80 mg resmetirom or matching placebo, given orally once daily. The study duration is expected to be two to three years for accrual of the required number of composite clinical outcome events.
A positive outcome is expected to support the full approval of resmetirom for noncirrhotic NASH, potentially accelerating the timeline to full approval. In addition, this study has the potential to support an additional indication for resmetirom in patients with well-compensated NASH cirrhosis.
2022 Executive Compensation Highlights
As discussed in this CD&A, the Compensation Committee strives to create a positive relationship between our compensation program and our operational performance and stockholder return. The Compensation Committee established our 2022 compensation program and the associated corporate goals in accordance with this philosophy based on our business plans and objectives. Our compensation practices keep the best interests of our stockholders in mind. Below is a summary of the best practices that we have implemented.
| 26 |

|
|
COMPENSATION DISCUSSION AND ANALYSIS
| • | In order to assure that the compensation programs for our executive officers remain competitive, we benchmark peer companies’ programs in order to establish our objectives and create rewards for the realization of our long-term strategic goals. We also evaluate survey data to supplement these analyses for our executive officers and to support cash compensation and equity award values by grade level for employees below our executive officers. The Compensation Committee works with a compensation consultant, Compensia, to obtain the advice and market data needed to structure compensation programs consistent with these goals, and it supplements such benchmarking as appropriate with market survey data. |
| • | The Compensation Committee approved an average increase in base salaries for executive officers in 2022 to remain competitive with peers and after considering benchmarking of executive salaries to peer companies of 5%. |
| • | The Compensation Committee continued the Company’s cash bonus program in 2022 to provide our executive officers with a direct financial incentive in the form of a cash bonus award tied to our achievement of pre-established objectives, including clinical, research and development, commercial development, medical affairs, CMC, operations, business development, finance, investor relations and communications objectives for the 2022 year. The performance goals had to be attained at a minimum threshold of 75% in order for a bonus to be paid. |
| • | We do not offer a defined benefit pension plan, deferred compensation plan or supplemental executive retirement plan. Instead, we rely on equity compensation (in the form of stock options in 2022) in order to attract and retain key employees, align the interests of our executive officers with those of our stockholders and to provide our executive officers and other employees with the opportunity to accumulate retirement income. |
| • | The Compensation Committee annually assesses whether our compensation programs have potential risks that are reasonably likely to have a material adverse effect on the Company. |
| • | We do not offer any significant perquisites or personal benefits to our executive officers. |
Overview of Our Executive Compensation Program
Corporate Governance and the Role of the Compensation Committee
The Compensation Committee assists the Board of Directors in fulfilling its fiduciary responsibilities with respect to the oversight of our compensation plans, policies and programs, especially those regarding executive compensation and employee benefits. The Compensation Committee’s responsibilities include reviewing and establishing the compensation arrangements for management, including the compensation for our Chief Executive Officer, and establishing and reviewing general compensation policies with the objective to attract and retain industry-leading talent, to reward individual performance and to achieve our financial goals.
Objectives of the Company’s Compensation Program
We are focused on developing and commercializing innovative therapeutic candidates for the treatment of cardiovascular, metabolic and liver diseases. To achieve this objective, we have recruited executives with significant industry or scientific experience, including in the areas of commercialization, development and research. The biotechnology industry is very competitive and our success depends upon our ability to attract and retain qualified executives through competitive compensation packages. The Compensation Committee administers the compensation programs for our executive officers with this competitive environment in mind. The Compensation Committee believes our compensation program must balance long-term incentives that create rewards for the realization of our long-term strategic objectives and near-term compensation that rewards our executives for the achievement of annual goals. We believe this approach motivates the attainment of our long-term objectives and aligns the interests of our executives with those of our stockholders. At the same time, our compensation programs are designed to serve as an important retention tool.
|
|
2023 PROXY STATEMENT | 27 |
COMPENSATION DISCUSSION AND ANALYSIS
To this end, the primary objectives of our compensation program are to:
| • | Enable us to attract and retain highly qualified executives with extensive industry, business or scientific experience by providing a competitive compensation package that includes long-term incentives that provide significant retentive value; |
| • | Reward our executives for our success in achieving significant operational goals; and |
| • | Align the interests of our executives with those of our stockholders. |
Executive Compensation Determination Procedures and Policies
The Compensation Committee reviews executive compensation annually. As part of this process, our Chief Executive Officer makes recommendations to the Compensation Committee with respect to the compensation levels for individual executives other than himself. The Compensation Committee reviews this information and adjusts or approves the recommendations as appropriate. In making its determination for each named executive officer, the Compensation Committee considers our performance against established performance objectives and market data regarding executive compensation at comparable companies. In the case of our Chief Executive Officer, the Compensation Committee evaluates his performance against our established performance objectives and market data regarding executive compensation at comparable companies.
In October 2016, the Compensation Committee initially retained the services of Compensia to review all aspects of our executive compensation. Since then we have continued to work with Compensia, which assists the Compensation Committee in defining the appropriate peer companies for executive compensation and practices and in benchmarking our executive compensation program against the peer group. The Compensation Committee uses the information obtained from Compensia primarily for evaluating our executive compensation practices, including measuring the competitiveness of our practices. The Compensation Committee also uses information obtained from Compensia to review our cash bonus policy, equity awards, and base salary benchmarks across all levels of the Company. The Compensation Committee determined that Compensia was independent pursuant to SEC rules and the corporate governance rules of Nasdaq and concluded that no conflict of interest exists that would prevent Compensia from independently representing the Compensation Committee.
Comparative Analysis
For purposes of measuring the competitive positioning of our compensation packages, peer companies are generally selected by the Compensation Committee with input from Compensia, primarily using the following criteria: publicly-held pre-commercial or early-commercial U.S. biotechnology companies; and companies that fall within a specific market capitalization range relative to our market capitalization at the time of the peer evaluation. Because the biotechnology industry is a dynamic industry, the comparator group used by the Compensation Committee to measure the competitive positioning of our compensation packages is periodically updated to ensure that companies continue to meet the established criteria. During the 2022 year, adjustments to peer group composition were made for a number of factors, including adjustments associated with market capitalization alignment between Madrigal and the peer group of companies (i.e., the removal of Akero Therapeutics, CytoDyn, Inovio, Karyopharm, Phathom, Provention Bio and Rhythm Pharmaceuticals from the peer group), phase of development alignment between Madrigal and the peer group of companies, and the occurrence of acquisitions of peer companies (i.e., removal of Dicerna and Epizyme from the peer group), each of which occurred subsequent to the previous annual determination of our peer group. We also added new peer companies which met the foregoing criteria, as noted below.
| 28 |

|
|
COMPENSATION DISCUSSION AND ANALYSIS
The list of selected comparable companies for purposes of measuring the competitive positioning of the base salary, cash incentive bonus and equity compensation elements of the compensation packages was adjusted during 2022 to add new companies, with the full list is as follows:
| Agios Pharmaceuticals | Concept Therapeutics* | Intra-Cellular Therapies | ||
| Amicus Therapeutics* | Cytokinetics* | IVERIC bio* | ||
| Arrowhead Therapeutics* | Denali Therapeutics* | Reata Pharmaceuticals | ||
| Axsome Therapeutics | FibroGen | REGENXBIO | ||
| Blueprint Medicines | Global Blood Therapeutics | Travere Therapeutics | ||
| BridgeBio Pharma* | Harmony Biosciences* | |||
| Cara Therapeutics | Insmed | |||
| * | Reflects new additions to peer group during 2022. |
Direct Compensation Components
The components of our direct compensation package are as follows:
| Element
|
Fixed or Variable
|
Compensation Objective
| ||
| Base Salary |
Fixed | To attract and retain executives by offering fixed compensation that is competitive with market opportunities and that recognizes each executive’s position, role, responsibility and experience. | ||
| Annual Cash Incentive Bonus |
Variable | To motivate and reward the achievement of annual individual performance objectives and corporate goals. | ||
| Equity Awards |
Variable | To align our executives’ interests with the interests of stockholders and to promote the long-term retention of our executives and all employees through equity-based compensation in the form of stock options and/or restricted stock or RSUs. | ||
Base Salary
Our Compensation Committee reviews base salaries for our named executive officers on an annual basis as part of our compensation program and, in general, may adjust base salaries for a variety of reasons, such as: an executive officer’s success in meeting or exceeding individual performance objectives established by our Compensation Committee; the collective achievement of significant corporate goals, as established by our Compensation Committee; or other factors warranted throughout the year, including for changes in the scope or breadth of an executive officer’s role or responsibilities. Our Compensation Committee will also evaluate an executive officer’s base salary together with other components of the executive officer’s compensation to ensure that the executive officer’s total compensation is in line with our overall compensation philosophy.
|
|
2023 PROXY STATEMENT | 29 |
COMPENSATION DISCUSSION AND ANALYSIS
Our Compensation Committee will also realign base salaries with market levels for the same positions if our Compensation Committee, with input from Compensia, identifies significant disparities in peer pay or market changes in its data analysis, or if a change is needed to align our pay practices so we can attract and retain top talent necessary to advance our strategic and operational goals. In light of market rate adjustments and comparisons of Madrigal and peer executive salaries, the Compensation Committee made 5% annualized increases in executive salaries for 2022, as detailed below.
These salary adjustments were approved after considering prior year salary data and consultation with Compensia, which reported that such salary amounts aligned with the following benchmarked percentiles of our peer companies, as follows: Dr. Friedman, CEO (35th percentile); Mr. Lynch, General Counsel (55th percentile); Mr. Sukhija, CCO (55th percentile); and Mr. Howarth, CFO (55th percentile). There was no direct comparable peer group benchmark for Dr. Taub’s position of President, R&D, but there was a CMO peer group relative to her additional title as CMO and a peer grouping for second highest paid executive. As benchmarked to the second highest paid executive, Dr. Taub’s salary corresponded to approximately the 70th percentile of this peer group. As a result, in 2022, the Compensation Committee established the annual salary of each of our named executive officers as set forth in the table:
| Name |
2022 Base Salary ($) |
2021 Base Salary ($) |
Percent Increase |
|||||||||
| Paul A. Friedman, M.D. |
663,000 | (1) | 631,700 | 5 | % | |||||||
| Rebecca Taub, M.D. |
539,000 | (1) | 513,800 | 5 | % | |||||||
| Brian J. Lynch |
471,000 | (1) | 448,900 | 5 | % | |||||||
| Remy Sukhija |
487,000 | (1) | 463,500 | 5 | % | |||||||
| Alex Howarth |
469,000 | (1) | 450,000 | 4 | % | |||||||
| (1) | Effective as of March 1, 2022. The actual salary amount paid in 2022, as disclosed in the Summary Compensation Table, utilized 2021 salary levels through March 1, 2022 and the salary level disclosed above for 2022, as prorated from March 1, 2022. |
Annual Cash Incentive Bonus
In addition to base salaries, our named executive officers are eligible to receive annual cash incentive bonuses. The annual cash incentive bonus each named executive officer is eligible to receive is based on the individual’s target bonus, as a percentage of base salary, and an assessment of individual performance and achievement of pre-established objectives, including clinical, research and development, commercial development, medical affairs, CMC, operations, business development, finance, investor relations and communications for the 2022 year. The Compensation Committee determined the size of potential cash bonuses by reference to target bonus amounts, based on market data from the peer group. The specified percentages are intended to make our total cash compensation competitive when compared to the peer companies. It is also designed to allocate a significant portion of each executive’s cash compensation opportunity to be contingent on goal achievement and create a significant performance-based component of each executive’s total compensation. Our annual cash incentive bonuses are disclosed in the Summary Compensation Table in this proxy statement under the column “Non-Equity Incentive Plan Compensation.”
For 2022, the target cash bonus percentage for each of our named executive officers remained in line with 2021 levels as follows: Dr. Friedman, at 50% of base salary; Dr. Taub at 50% of base salary; and Messrs. Lynch, Sukhija, and Howarth at 40% of base salary.
For 2022, the Compensation Committee assigned points to each bonus objective based on its determination, with input from our senior management team, of the value of the objective in light of our overall corporate goals and relevant objective achievement for 2022. The Compensation Committee also assigned bonus points for certain
| 30 |

|
|
COMPENSATION DISCUSSION AND ANALYSIS
ancillary performance objectives within each primary group of performance objectives. If we achieved a performance objective at the target level, 100% of the available points for such objective would be assigned. If we achieved a performance objective above the target level, up to 150% of the available points for such objective would be assigned. If we achieved a performance objective at less than 75% of the target level, zero points for such objective would be assigned.
The performance objectives and associated allocation goal percentage at target for 2022 may be summarized as follows:
| 2022 Corporate Performance Objectives |
Percentage of Overall Objectives at Target | |
| Research and Development Objectives |
45% | |
| Medical Affairs Objectives |
10% | |
| CMC and Operations Objectives |
15% | |
| Commercial Objectives |
10% | |
| Finance, Business Development, Investor Relations and Communications Objectives |
20% | |
| Total
|
100%
| |
In the first quarter of 2023, the Compensation Committee, with input from our senior management team, assessed our achievement of the performance objectives set forth above. Based on its assessment, the Compensation Committee determined that we outperformed and achieved bonus point performance in a number of categories established previously by the Compensation Committee (consisting of 131% of previously established objectives plus a 19% positive adjustment based on exceptional overall performance and TSR performance in 2022), such that overall performance was achieved at 150% of the target level (out of a maximum 150% objective opportunity). As a result, in the first quarter of 2023, the Compensation Committee awarded the following cash bonuses in respect of such 2022 performance to our named executives as of December 31, 2022, based upon 150% of his or her stated target bonus (please see right column below for amount at 150% of target) relative to the target dollar value at 100% (see left column for amount at 100% of target).
| Name | Target Award for 2022 ($) |
Actual Award Paid for 2022 ($) |
||||||
| Paul A. Friedman, M.D. |
331,500 | 497,464 | ||||||
| Rebecca Taub, M.D. |
269,500 | 404,618 | ||||||
| Brian J. Lynch |
188,400 | 282,807 | ||||||
| Remy Sukhija |
194,800 | 292,005 | ||||||
| Alex Howarth |
187,600 | 281,250 | ||||||
Long-Term Incentives
We believe that long-term incentives in the form of equity-based awards are critical to meeting the following objectives:
| • | focus all employees, including our named executive officers, on our long-term performance by aligning their interests with those of our stockholders; |
| • | retain our key employees and executives and maintain management continuity through longer-term vesting of our equity-based awards; and |
|
|
2023 PROXY STATEMENT | 31 |
COMPENSATION DISCUSSION AND ANALYSIS
| • | promote an ownership culture through participation in equity-based compensation programs. |
Our 2015 Stock Plan allows the grant of stock options, restricted stock, RSUs and other equity-based awards to employees, consultants and directors. We typically make an initial equity award of stock options to new employees, including our executive officers, and annual equity grants as part of our overall compensation program. Our Compensation Committee authorized Dr. Friedman to make new hire stock option grants to our employees, except for our executive officers, within certain parameters, beyond which approval of our Compensation Committee is required. Dr. Friedman may award new hire stock option grants as of the employee’s initial commencement of employment with an exercise price equal to the closing price of our common stock on the date of grant, in accordance with our 2015 Stock Plan. The Compensation Committee also has delegated to senior management authority, within certain bounds, to award equity awards to employees who are not executive officers.
Annual grants of equity awards to our executive officers are approved by our Compensation Committee. The timing of annual equity grants has been generally consistent, with a first quarter grant each year at a regularly scheduled meeting of our Compensation Committee. Such awards have not been coordinated with the public release of material non-public information. In February of 2022, at a regularly scheduled meeting, the Compensation Committee made an annual grant of stock options concerning 70,000 shares to Dr. Friedman, 61,500 shares to Dr. Taub, and 45,000 shares to Messrs. Lynch, Howarth and Sukhija. Each of the foregoing options vests as follows: 25% on the first anniversary of the grant date, and 6.25% each quarter thereafter until fully-vested. The exercise price of these stock options is determined by the Compensation Committee based on the closing price of the Company’s common stock on the Nasdaq on the grant date. Mr. Lynch also received an option grant for 25,000 shares in August 2022 in consideration of long-term performance and internal equity considerations.
Other Benefits
We maintain broad-based benefits that are provided to all employees, including health insurance, life and disability insurance, dental insurance, and a 401(k) plan with a matching company contribution. Our named executive officers participate in the benefits programs generally on the same basis as all employees, except that Drs. Friedman and Taub have elected to forego group life and disability insurance and 401(k) plan participation, and Dr. Friedman has elected to forego health and dental insurance plan participation.
Agreements
We have entered into agreements with each of our named executive officers, which agreements provide financial protection against the potential loss of employment in designated circumstances, and which the Compensation Committee believes will allow the executives to focus attention on the best interests of the stockholders, without undue concern as to the executive’s own financial situation. None of these agreements contains a tax gross-up provision. A summary of the material terms of these agreements may be found in this proxy statement under the section entitled “Executive Officer and Director Compensation; Employment Retention, Severance and Change in Control Arrangements” and “-; Potential Qualifying Separation and Change of Control Payments.”
Executive Compensation and Risk-Taking
We have sought to align the equity and cash components of our executive compensation program with industry peers in order to offer compensation packages that enable us to attract and retain talented executive officers. The Compensation Committee continues to evaluate the relative importance of equity and cash components of total compensation. We do not believe that our executive compensation program encourages excessive risk-taking by our executive officers. For example, long-term equity awards tied to the value of our common stock represent a significant component of an executive officer’s total direct compensation, as evidenced by the compensation breakdown contained in the Summary Compensation Table that follows. Those awards promote a long-term
| 32 |

|
|
COMPENSATION DISCUSSION AND ANALYSIS
commonality of interest between our executive officers and our stockholders in sustaining and increasing stockholder value. Because the equity awards are typically made on an annual basis to our executive officers, vested and unvested awards that are outstanding can decrease in value or have no value in the event of stock price declines, whether due to market factors or whether our business is not managed to achieve its long-term goals. Thus, our executive compensation program is not heavily weighted toward short-term incentives, and we have taken what we believe are reasonable steps to protect against the potential of disproportionately large short-term incentives that might encourage excessive risk taking.
Clawback Policy
Pursuant to the requirements of the Sarbanes-Oxley Act of 2002, if we are required to prepare an accounting restatement due to material noncompliance, as a result of misconduct, with any financial reporting requirement under the securities laws, then any incentive- or equity-based compensation received by our Chief Executive Officer and Senior Vice President and Chief Financial Officer during the 12-month period following the filing that included the financials that were restated is subject to potential forfeiture. In addition, the Company is monitoring ongoing rulemaking efforts and intends to update its executive compensation clawback requirements as necessary to comply with the SEC’s and Nasdaq’s evolving requirements for executive compensation recovery.
Advisory Vote on Executive Compensation
At our 2022 Annual Meeting of Stockholders, we conducted an advisory vote on executive compensation. Approximately 99.0% of the votes cast on this advisory vote proposal were in favor of our named executive officer compensation as disclosed in last year’s proxy statement. The Board of Directors and Compensation Committee reviews the advisory vote results in the context of our overall compensation philosophy and programs and other relevant competitive, incentive and market developments affecting executive officer compensation, in order to assess the need for changes to our executive compensation programs and policies.
|
|
2023 PROXY STATEMENT | 33 |
EXECUTIVE OFFICER AND DIRECTOR COMPENSATION
Summary Compensation Table
The following table shows for the fiscal years ended December 31, 2022, 2021, and 2020, the compensation awarded to or earned by our named executive officers.
| Name and Principal Position | Year | Salary ($) |
Option Awards ($)(1) |
Non-Equity Incentive Plan Compensation ($) |
Bonus ($) | All Other Compensation ($)(2) |
Total ($) |
|||||||||||||||||||||
| Paul A. Friedman, M.D. Chairman and Chief Executive Officer |
|
2022 |
|
|
658,021 |
|
|
4,239,900 |
|
|
497,464 |
|
|
— |
|
|
— |
|
|
5,395,385 |
| |||||||
|
|
2021 |
|
|
627,650 |
|
|
4,267,500 |
|
|
423,239 |
|
|
— |
|
|
— |
|
|
5,318,389 |
| ||||||||
|
|
2020 |
|
|
603,500 |
|
|
3,468,500 |
|
|
463,446 |
|
|
— |
|
|
— |
|
|
4,535,446 |
| ||||||||
|
Rebecca Taub, M.D. President, Research & Development and Chief Medical Officer |
|
2022 |
|
|
535,208 |
|
|
3,725,055 |
|
|
404,618 |
|
|
— |
|
|
— |
|
|
4,664,881 |
| |||||||
|
|
2021 |
|
|
510,500 |
|
|
3,755,400 |
|
|
344,246 |
|
|
— |
|
|
— |
|
|
4,610,146 |
| ||||||||
|
|
2020 |
|
|
490,833 |
|
|
3,052,280 |
|
|
339,230 |
|
|
— |
|
|
— |
|
|
3,882,343 |
| ||||||||
|
Brian J. Lynch Senior Vice President and General Counsel |
|
2022 |
|
|
467,604 |
|
|
3,994,400 |
|
|
282,807 |
|
|
— |
|
|
13,338 |
|
|
4,758,149 |
| |||||||
|
|
2021 |
|
|
445,333 |
|
|
2,731,200 |
|
|
240,610 |
|
|
— |
|
|
13,338 |
|
|
3,430,481 |
| ||||||||
|
|
2020 |
|
|
425,417 |
|
|
3,749,840 |
|
|
260,946 |
|
|
— |
|
|
11,690 |
|
|
4,447,893 |
| ||||||||
|
Remy Sukhija Senior Vice President, Chief Commercial Officer |
|
2022 |
|
|
482,813 |
|
|
2,725,650 |
|
|
292,005 |
|
|
— |
|
|
10,381 |
|
|
3,510,849 |
| |||||||
|
|
2021 |
|
|
461,250 |
|
|
2,048,400 |
|
|
248,436 |
|
|
— |
|
|
10,381 |
|
|
2,768,467 |
| ||||||||
|
|
2020 |
|
|
337,500 |
|
|
2,724,000 |
|
|
274,680 |
|
|
100,000 |
|
|
2,722 |
|
|
3,438,902 |
| ||||||||
|
Alex Howarth Senior Vice President and Chief Financial Officer |
|
2022 |
|
|
465,625 |
|
|
2,725,650 |
|
|
281,250 |
|
|
— |
|
|
10,381 |
|
|
3,482,906 |
| |||||||
|
|
2021 |
|
|
281,250 |
|
|
6,526,500 |
|
|
241,200 |
|
|
75,000 |
|
|
7,854 |
|
|
7,131,804 |
| ||||||||
| (1) | These amounts represent the aggregate grant date fair value of each option award, calculated in accordance with Financial Accounting Standards Board Accounting Standards Codification, or FASB ASC, Topic 718, Compensation—Stock Compensation. See our discussion of “Stock-Based Compensation” under Notes 2 and 8 to our audited consolidated financial statements included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2022 filed on February 23, 2023, as amended by our Annual Report on Form 10-K/A for the fiscal year ended December 31, 2022 filed on March 3, 2023 (the “Annual Report”); these Notes include a discussion of all assumptions made by us in determining the grant date fair values of our equity awards. See also our discussion of stock-based compensation under Item 7 “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Estimates” of the Annual Report. |
| (2) | All Other Compensation includes life and disability insurance premiums paid, and matching contributions under our 401(k) plan for each applicable executive officer listed above, with no such individual amount exceeding $10,000 for any executive officer. |
| 34 |

|
|
EXECUTIVE OFFICER AND DIRECTOR COMPENSATION
Grants of Plan-Based Awards
The following table shows information regarding grants of plan-based awards during the fiscal year end December 31, 2022 to our named executive officers.
| Name and principal position |
Grant date | Estimated future payouts under non- ($)(1) |
All other stock awards: Number of shares of stock or units (#) |
All other stock and option awards: Number of securities underlying options (#) |
Exercise or base price of stock and option awards ($/share) (2) |
Grant date fair value of stock and option award (3) |
||||||||||||||||||||||||
| Threshold | Target | Maximum | ||||||||||||||||||||||||||||
|
Paul A. Friedman, M.D. Chairman and Chief Executive Officer |
February 22, 2022 |
|
248,625 |
|
|
331,500 |
|
|
497,464 |
|
|
-0- |
|
|
70,000 |
|
|
87.09 |
|
|
4,239,900 |
| ||||||||
|
Rebecca Taub, M.D. Chief Medical Officer, President, R&D |
February 22, 2022 |
|
202,125 |
|
|
269,500 |
|
|
404,618 |
|
|
-0- |
|
|
61,500 |
|
|
87.09 |
|
|
3,725,055 |
| ||||||||
| Brian J. Lynch Senior Vice President, and General Counsel |
February 22, 2022 |
|
141,300 |
|
|
188,400 |
|
|
282,807 |
|
|
-0- |
|
|
45,000 |
|
|
87.09 |
|
|
2,731,200 |
| ||||||||
| August 10, 2022 |
|
— |
|
|
— |
|
|
— |
|
|
-0- |
|
|
25,000 |
|
|
73.75 |
|
|
1,623,200 |
| |||||||||
| Remy Sukhija Senior Vice President, and Chief Commercial Officer |
February 22, 2022 |
|
146,100 |
|
|
194,800 |
|
|
292,005 |
|
|
-0- |
|
|
45,000 |
|
|
87.09 |
|
|
2,725,650 |
| ||||||||
| Alex Howarth Senior Vice President and Chief Financial Officer |
February 22, 2022 |
|
140,700 |
|
|
187,600 |
|
|
281,250 |
|
|
-0- |
|
|
45,000 |
|
|
87.09 |
|
|
2,731,200 |
| ||||||||
| (1) | The material terms of the 2022 non-equity incentive awards are described above in the Compensation Discussion and Analysis in the section entitled “Annual Cash Incentive Bonus.” The threshold value above represents performance at 75% of target without achievement of bonus points or outperformance objectives. The target value above represents performance at 100% of target without achievement of bonus points or outperformance objectives. If bonus points or outperformance objectives are earned, the threshold and target amounts could increase above target. The maximum value above represents performance at 150% of target, including maximum achievement of bonus points and outperformance objectives. |
| (2) | The exercise price of these stock options is determined by the Company based on the closing price of its common stock on the Nasdaq Stock Market on the grant date. |
| (3) | Each amount represents the grant date fair value of the executive officer’s stock options, calculated in accordance with FASB ASC Topic 718, using a Black-Scholes valuation model for stock options. See Note 1 under “—Summary Compensation Table” for a discussion of the assumptions made by the Company in determining the grant date fair value of our option awards for the fiscal year ended December 31, 2022. Options were awarded with time-based vesting criteria established by the Compensation Committee and described in Compensation Discussion and Analysis above. |
|
|
2023 PROXY STATEMENT | 35 |
EXECUTIVE OFFICER AND DIRECTOR COMPENSATION
Outstanding Equity Awards at Fiscal Year End
The following table shows stock options held and vesting status for each of our named executive officers as of December 31, 2022.
| Option Awards | ||||||||||||||||||||||||
| Name | Date of Grant |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Option Exercise Price ($) |
Option Expiration Date |
|||||||||||||||||||
| Paul A. Friedman, M.D. Chief Executive Officer |
|
2/22/2022 |
|
|
— |
|
|
70,000 |
(1) |
|
87.09 |
|
|
2/22/2032 |
|
|||||||||
|
|
3/1/2021 |
|
|
21,875 |
|
|
28,125 |
(1) |
|
117.55 |
|
|
3/1/2031 |
|
||||||||||
|
|
3/5/2020 |
|
|
34,375 |
|
|
15,625 |
(1) |
|
91.79 |
|
|
3/5/2030 |
|
||||||||||
|
|
3/7/2019 |
|
|
46,875 |
|
|
3,125 |
(1) |
|
127.96 |
|
|
3/7/2029 |
|
||||||||||
|
|
3/1/2018 |
|
|
50,000 |
|
|
— |
|
|
124.45 |
|
|
3/1/2028 |
|
||||||||||
|
|
3/2/2017 |
|
|
49,600 |
|
|
— |
|
|
15.80 |
|
|
3/2/2027 |
|
||||||||||
|
|
7/22/2016 |
|
|
306,256 |
|
|
— |
|
|
9.45 |
|
|
7/22/2026 |
|
||||||||||
| Rebecca Taub, M.D. President, Research & Development, Chief Medical Officer |
|
2/22/2022 |
|
|
— |
|
|
61,500 |
(1) |
|
87.09 |
|
|
2/22/2032 |
|
|||||||||
|
|
3/1/2021 |
|
|
19,250 |
|
|
24,750 |
(1) |
|
117.55 |
|
|
3/1/2031 |
|
||||||||||
|
|
3/5/2020 |
|
|
30,250 |
|
|
13,750 |
(1) |
|
91.79 |
|
|
3/5/2030 |
|
||||||||||
|
|
7/10/2019 |
|
|
6,500 |
|
|
1,500 |
(1) |
|
101.30 |
|
|
7/10/2029 |
|
||||||||||
|
|
3/7/2019 |
|
|
35,625 |
|
|
2,375 |
(1) |
|
127.96 |
|
|
3/7/2029 |
|
||||||||||
|
|
3/1/2018 |
|
|
38,000 |
|
|
— |
|
|
124.45 |
|
|
3/1/2028 |
|
||||||||||
|
|
3/2/2017 |
|
|
37,300 |
|
|
— |
|
|
15.80 |
|
|
3/2/2027 |
|
||||||||||
|
|
7/22/2016 |
|
|
153,128 |
|
|
— |
|
|
9.45 |
|
|
7/22/2026 |
|
||||||||||
| Brian J. Lynch Senior Vice President and General Counsel |
|
8/10/2022 |
|
|
— |
|
|
25,000 |
(1) |
|
73.75 |
|
|
8/10/2023 |
|
|||||||||
|
|
2/22/2022 |
|
|
— |
|
|
45,000 |
(1) |
|
87.09 |
|
|
2/22/2032 |
|
||||||||||
|
|
3/1/2021 |
|
|
14,000 |
|
|
18,000 |
(1) |
|
117.55 |
|
|
3/1/2031 |
|
||||||||||
|
|
8/6/2020 |
|
|
11,250 |
|
|
8,750 |
(1) |
|
103.10 |
|
|
8/6/2030 |
|
||||||||||
|
|
3/5/2020 |
|
|
15,000 |
(2) |
|
10,000 |
(1) |
|
91.79 |
|
|
3/5/2030 |
|
||||||||||
|
|
2/19/2019 |
|
|
17,813 |
|
|
1,187 |
(1) |
|
134.25 |
|
|
2/19/2029 |
|
||||||||||
|
|
2/5/2019 |
|
|
15,000 |
|
|
1,000 |
(1) |
|
114.27 |
|
|
2/5/2029 |
|
||||||||||
|
|
1/14/2019 |
|
|
3,000 |
|
|
— |
|
|
114.55 |
|
|
1/14/2029 |
|
||||||||||
|
|
7/1/2018 |
|
|
5,000 |
|
|
— |
|
|
279.69 |
|
|
7/1/2028 |
|
||||||||||
| Remy Sukhija Senior Vice President and Chief Commercial Officer |
|
2/22/2022 |
|
|
— |
|
|
45,000 |
(1) |
|
87.09 |
|
|
2/22/2032 |
|
|||||||||
|
|
3/1/2021 |
|
|
10,500 |
|
|
13,500 |
(1) |
|
117.55 |
|
|
3/1/2031 |
|
||||||||||
|
|
4/1/2020 |
|
|
7,500 |
(3) |
|
22,500 |
(1) |
|
60.26 |
|
|
4/1/2030 |
|
||||||||||
| Alex Howarth Senior Vice President and Chief Financial Officer |
|
2/22/2022 |
|
|
— |
|
|
45,000 |
|
|
87.09 |
|
|
2/22/2032 |
|
|||||||||
|
|
5/17/2021 |
|
|
24,375 |
|
|
40,625 |
(1) |
|
134.00 |
|
|
5/17/2031 |
|
||||||||||
|
|
11/1/2021 |
|
|
1,250 |
|
|
3,750 |
(1) |
|
81.95 |
|
|
11/1/2031 |
|
||||||||||
| (1) | These stock options vest as to 25% of the underlying shares on the first anniversary of the grant date and, thereafter, as to 6.25% of the underlying shares on the last day of each successive three-month period. |
| (2) | Represents the remaining unexercised options following an exercise of 7,000 options on December 23, 2022. |
| (3) | Represents the remaining unexercised options following an exercise of 30,000 options on December 23, 2022. |
| 36 |

|
|
EXECUTIVE OFFICER AND DIRECTOR COMPENSATION
Option Exercises and Stock Vested
The following table shows the number of shares acquired upon exercise of stock options held by each of our named executive officers during the fiscal year ended December 31, 2022.
| Name | Option awards number of shares |
Option awards value realized |
||||||||||
| Paul A. Friedman, M.D. Chief Executive Officer |
|
— |
|
|
— |
|
||||||
| Rebecca Taub, M.D. Chief Medical Officer and President, Research & Development |
|
— |
|
|
— |
|
||||||
| Brian J. Lynch Senior Vice President and General Counsel |
|
7,000 |
|
|
1,272,390 |
|
||||||
| Remy Sukhija Senior Vice President and Chief Commercial Officer |
|
30,000 |
|
|
6,399,000 |
|
||||||
| Alex Howarth Senior Vice President and Chief Financial Officer |
|
— |
|
|
— |
|
||||||
| (1) | Value realized on exercise calculated based on the difference between the closing price of our common stock on the date of exercise and the option exercise price, multiplied by the number of shares exercised. |
Pay Ratio
The following is a reasonable estimate, prepared under applicable SEC rules, of the ratio of the annual total compensation of our Chief Executive Officer to the median of the annual total compensation of our other employees. We calculated this ratio after examining the total compensation amounts for all of our employees for the 2022 fiscal year, which included the following elements: base salary (annualized where applicable for those who commenced employment during 2022); bonus payments made in respect of the 2022 fiscal year (also annualized where applicable); the grant date value of equity awards made in 2022; and 401(k) plan matching contributions. We calculated the median total compensation of this employee population (consisting of 91 employees, 5 of whom were employed outside the U.S.) and selected the median-compensated employee from within that group as our median employee. This median employee’s annual total compensation for 2022 was $441,396 calculated in the same manner as our Chief Executive Officer’s total compensation for 2021 as disclosed in the Summary Compensation Table. The Chief Executive Officer’s total compensation for 2022 was $5,395,385, as disclosed in the Summary Compensation Table. Therefore, our estimate of the ratio of Chief Executive Officer pay to median employee pay is 12:1. Given the different methodologies that various public companies will use to determine an estimate of their pay ratio, the ratio reported above should not be used as a direct basis for comparison between companies.
|
|
2023 PROXY STATEMENT | 37 |
Year |
Summary Compensation Table Total Pay for CEO (1)(2) |
CAP to CEO (3) |
Average Summary Compensation Table Total Pay for Other NEOs (1)(2) |
Average CAP to Other NEOs (3) |
Value of Initial Fixed $100 Investment Based on: |
GAAP Net Income (Loss) (5) |
||||||||||||||||||||||
Company TSR (4) |
Peer Group TSR (4) |
|||||||||||||||||||||||||||
| 2022 | $ | $ | $ | $ | $ | $ | $ | ( |
) | |||||||||||||||||||
| 2021 | ( |
) | ||||||||||||||||||||||||||
| 2020 | ( |
) | ||||||||||||||||||||||||||
| (1) | For each year shown, the CEO was |
| (2) | The values reflected in this column reflect the “Total” compensation set forth in the Summary Compensation Table (“SCT”) on p. 34. See the footnotes to the SCT for further detail regarding the amounts in this column. |
| (3) | CAP is defined by the SEC and is computed in accordance with SEC rules by subtracting the amounts in the “Stock Awards” (as applicable) and “Option Awards” column of the SCT for each year from the “Total” column of the SCT and then: (i) adding the fair value as of the end of the reported year of all awards granted during the reporting year that are outstanding and unvested as of the end of the reporting year; (ii) adding the amount equal t o the change a s of the end of the reporting year (from the end of the prior year) in fair value (whether positive or negative) of any awards granted in any prior year that are outstanding and unvested as of the end of the reporting year; (iii) adding, for awards that are granted and vest in the reporting year, the fair value as of the vesting date; (iv) adding the amount equal to the change as of the vesting date (from the end of the prior fiscal year) in fair value (whether positive or negative) of any awards granted in any prior year for which all applicable vesting conditions were satisfied at the end of or during the reporting year; and (v) subtracting, for any awards granted in any prior year that are forfeited during the reporting year, the amount equal to the fair value at the end of the prior year. Valuation assumptions used to calculate fair values did not materially differ from those used to calculate fair values at the time of grant as reflected in the SCT. The following tables reflect the adjustments made to SCT total compensation to compute CAP for our CEO and average CAP for our other NEOs. |
SCT Total Comp |
Minus SCT Equity Awards |
Plus Year-End Fair Valueof Equity Awards Granted During Year That Remained Unvested as of Last Day of Year |
Plus Change in Fair Value from Last Day of Prior Year to Last Day of Year of Unvested Equity Awards |
Plus Change in Fair Value from Last Day of Prior Year to Vesting Date of Unvested Equity Awards that Vested During Year |
Equals CAP |
|||||||||||||||||||
| 2022 | $ | $ | ( |
) | $ | $ | $ | $ | ||||||||||||||||
| 2021 | ( |
) | ( |
) | ( |
) | ||||||||||||||||||
| 2020 | ( |
) | ||||||||||||||||||||||
38 |
 |
SCT Total Comp |
Minus SCT Equity Awards |
Plus Year-End FairValue of Equity Awards Granted During Year That Remained Unvested as of Last Day of Year |
Plus Change in Fair Value from Last Day of Prior Year to Last Day of Year of Unvested Equity Awards |
Plus Change in Fair Value from Last Day of Prior Year to Vesting Date of Unvested Equity Awards that Vested During Year |
Equals CAP |
|||||||||||||||||||
2022 |
$ | $ | ( |
) | $ | $ | $ | $ | ||||||||||||||||
2021 |
( |
) | ( |
) | ( |
) | ||||||||||||||||||
2020 |
( |
) | ||||||||||||||||||||||
| (4) | two-years ended December 31, 2021 and the three years ended December 31, 2022, assuming a $100 investment at the closing price on December 31, 2019 and the reinvestment of all dividends. |
| (5) | Amounts in thousands. |

| 2023 PROXY STATEMENT |
39 |

40 |
 |
EMPLOYMENT RETENTION, SEVERANCE AND CHANGE IN CONTROL ARRANGEMENTS
Paul A. Friedman, M.D.
On April 13, 2016, Private Madrigal entered into an agreement (the “Friedman Letter Agreement”), with Dr. Friedman, for the position of Chairman and Chief Executive Officer of the Company following the completion of the Merger. We assumed the Friedman Letter Agreement upon completion of the Merger. Under the terms of the Friedman Letter Agreement, Dr. Friedman is entitled to an annual base salary of $400,000 (subject to adjustment by the Compensation Committee) and an annual target bonus of 50% of his base salary (subject to adjustment by the Compensation Committee). In addition, upon the consummation of the Merger, Dr. Friedman received equity awards, including stock options to purchase 306,256 shares of our common stock and a restricted stock award representing 153,128 shares of our common stock.
The foregoing stock options vested as to 25% of the shares on the grant date and the remainder vested in equal annual installments on the first, second and third anniversaries of the grant date. The repurchase right relating to the foregoing restricted stock award lapsed as to 25% of the shares on the grant date and the repurchase right on the remaining shares lapsed in equal annual installments on the first, second and third anniversaries of the grant date.
Dr. Friedman is also entitled to severance benefits if we terminate his employment without “Cause” (as defined) or if Dr. Friedman voluntarily resigns for “Good Reason” (as defined), which we refer to herein collectively as a “Qualifying Separation,” consisting of:
| • | a severance payment equal to 12 months of Dr. Friedman’s then-current base salary and target bonus, payable (i) in a lump sum if the Qualifying Separation occurs following a change of control of the Company, or (ii) in 12 equal monthly payments if the Qualifying Separation does not occur following a change of control of the Company; |
| • | full vesting of restricted stock and stock options held by Dr. Friedman if a Qualifying Separation occurs following a change of control of the Company; and 12-months’ vesting of restricted stock and stock options held by Dr. Friedman upon a Qualifying Separation, if such Qualifying Separation does not occur following a change of control of the Company; and |
| • | reimbursement of and continuation of medical benefits for 12 months following a Qualifying Separation. |
Dr. Friedman has also entered into a customary indemnification agreement with us with respect to his service as an officer and director of the Company.
Rebecca Taub, M.D.
On April 13, 2016, Private Madrigal entered into an agreement, (the “Taub Letter Agreement”), with Rebecca Taub, M.D. for the position of Chief Medical Officer and Executive Vice President, Research & Development, of the Company following the completion of the Merger. We assumed the Taub Letter Agreement upon completion of the Merger. Under the terms of the Taub Letter Agreement, Dr. Taub is entitled to an annual base salary of $370,000 (subject to adjustment by the Compensation Committee) and an annual target bonus of 40% of her base salary (subject to adjustment by the Compensation Committee, which has since been adjusted to 50%). In addition, upon the consummation of the Merger, Dr. Taub received equity awards, including stock options to purchase 153,128 shares of our common stock and a restricted stock award representing 30,626 shares of our common stock.
The foregoing stock options vested as to 25% of the shares on the grant date and the remainder vested in equal annual installments on the first, second and third anniversaries of the grant date. The repurchase right relating to the
|
|
2023 PROXY STATEMENT | 41 |
EMPLOYMENT RETENTION, SEVERANCE AND CHANGE IN CONTROL ARRANGEMENTS
foregoing restricted stock award lapsed as to 25% of the shares on the grant date and the repurchase right on the remaining shares lapsed in equal annual installments on the first, second and third anniversaries of the grant date.
Dr. Taub is also entitled to severance benefits upon a Qualifying Separation consisting of:
| • | a severance payment equal to 12 months of Dr. Taub’s then-current base salary and target bonus, payable (i) in a lump sum if the Qualifying Separation occurs following a change of control of the Company, or (ii) in 12 equal monthly payments if the Qualifying Separation does not occur following a change of control of the Company; |
| • | full vesting of restricted stock and stock options held by Dr. Taub, in particular if a Qualifying Separation occurs following a change of control of the Company; and 12-months’ vesting of restricted stock and stock options held by Dr. Taub upon a Qualifying Separation, if such Qualifying Separation does not occur following a change of control of the Company; and |
| • | reimbursement of and continuation of medical benefits for 12 months following a Qualifying Separation. |
Dr. Taub has also entered into a customary indemnification agreement with us with respect to her service as an officer and director of the Company.
Brian J. Lynch
Pursuant to a letter agreement dated February 19, 2019, with Mr. Lynch, Mr. Lynch is entitled to receive an annual base salary of $415,000 (subject to adjustment by the Compensation Committee). Under our bonus policy, Mr. Lynch is eligible to receive an annual performance-based bonus with a target at 40% of his base salary (subject to adjustment by the Compensation Committee). In addition, in connection the signing of the letter agreement, Mr. Lynch was granted stock options to purchase 16,000 and 19,000 shares of our common stock at exercise prices of $114.27 and $134.25, per share respectively. The foregoing stock options vest as to 25% of the shares on the first anniversary of the grant date as to 6.25% of the shares on each quarterly anniversary after the first anniversary of the grant date.
Pursuant to the terms of a severance and change of control agreement entered into with Mr. Lynch, he is also entitled to severance benefits upon a Qualifying Separation consisting of:
| • | a severance payment equal to 12 months of Mr. Lynch’s then-current base salary and target bonus, payable (i) in a lump sum if such Qualifying Separation occurs following a change of control of the Company, or (ii) in 12 equal monthly payments if such Qualifying Separation does not occur following a change of control of the Company; |
| • | (i) full vesting of restricted stock, if any, and stock options held by Mr. Lynch upon a Qualifying Separation, if such Qualifying Separation occurs following a change in control of the Company, or (ii) 12-months’ vesting of restricted stock and stock options held by Mr. Lynch upon a Qualifying Separation, if such Qualifying Separation does not occur following a change of control of the Company; and |
| • | reimbursement of and continuation of medical benefits for 12 months following a Qualifying Separation. |
Mr. Lynch has also entered into a customary indemnification agreement with us with respect to his service as an officer of the Company.
Remy Sukhija
Pursuant to a letter agreement dated April 1, 2020, with Mr. Sukhija, Mr. Sukhija is entitled to receive an annual base salary of $450,000 (subject to adjustment by the Compensation Committee). Under our bonus policy, Mr. Sukhija is eligible to receive an annual performance-based bonus with a target at 40% of his base salary (subject to adjustment by the Compensation Committee). In addition, in connection with the signing of the letter
| 42 |

|
|
EMPLOYMENT RETENTION, SEVERANCE AND CHANGE IN CONTROL ARRANGEMENTS
agreement, Mr. Sukhija (1) became eligible to receive a signing bonus of $100,000 (which was subject to repayment equal to $50,000 in the event his employment with the Company cease before his first anniversary with the Company) and (2) was granted stock options to purchase 60,000 shares of our common stock at an exercise price of $60.26 per share. The foregoing stock options vest as to 25% of the shares on the first anniversary of the grant date and as to 6.25% of the shares on each quarterly anniversary after the first anniversary of the grant date.
Pursuant to the terms of a severance and change of control agreement entered into with Mr. Sukhija, he is also entitled to severance benefits upon a Qualifying Separation consisting of:
| • | a severance payment equal to 12 months of Mr. Sukhija’s then-current base salary and target bonus, payable (i) in a lump sum if such Qualifying Separation occurs following a change of control of the Company, or (ii) in 12 equal monthly payments if such Qualifying Separation does not occur following a change of control of the Company; |
| • | (i) full vesting of restricted stock, if any, and stock options held by Mr. Sukhija upon a Qualifying Separation, if such Qualifying Separation occurs following a change in control of the Company, or (ii) 12-months’ vesting of restricted stock and stock options held by Mr. Sukhija upon a Qualifying Separation, if such Qualifying Separation does not occur following a change of control of the Company; and |
| • | reimbursement of and continuation of medical benefits for 12 months following a Qualifying Separation. |
Mr. Sukhija has also entered into a customary indemnification agreement with us with respect to his service as an officer of the Company.
Alex Howarth
Pursuant to a letter agreement dated April 18, 2021, with Mr. Howarth, Mr. Howarth is entitled to receive an annual base salary of $450,000 (subject to adjustment by the Compensation Committee). Under our bonus policy, Mr. Howarth is eligible to receive an annual performance-based bonus with a target at 40% of his base salary (subject to adjustment by the Compensation Committee). In addition, in connection therewith, Mr. Howarth was granted stock options to purchase 65,000 and 5,000 shares of our common stock on May 17, 2021 and November 1, 2021, respectively, at exercise prices of $134.00 and $81.95 per share, respectively. The foregoing stock options vest as to 25% of the shares on the first anniversary of the grant date as to 6.25% of the shares on each quarterly anniversary after the first anniversary of the grant date. In addition, in connection with the signing of the letter agreement, Mr. Howarth became eligible to receive a signing bonus of $75,000 (which is subject to repayment equal to $37,500 in the event his employment with the Company ceases before his first anniversary with the Company, subject to certain exceptions).
Pursuant to the terms of a severance and change of control agreement entered into with Mr. Howarth, he is also entitled to severance benefits upon a Qualifying Separation consisting of:
| • | a severance payment equal to 12 months of Mr. Howarth’s then-current base salary and target bonus, payable (i) in a lump sum if such Qualifying Separation occurs following a change of control of the Company, or (ii) in 12 equal monthly payments if such Qualifying Separation does not occur following a change of control of the Company; |
| • | (i) full vesting of restricted stock, if any, and stock options held by Mr. Howarth upon a Qualifying Separation, if such Qualifying Separation occurs following a change in control of the Company, or (ii) 12-months’ vesting of restricted stock and stock options held by Mr. Howarth upon a Qualifying Separation, if such Qualifying Separation does not occur following a change of control of the Company; and |
| • | reimbursement of and continuation of medical benefits for 12 months following a Qualifying Separation. |
Mr. Howarth has also entered into a customary indemnification agreement with us with respect to his service as an officer of the Company.
|
|
2023 PROXY STATEMENT | 43 |
EMPLOYMENT RETENTION, SEVERANCE AND CHANGE IN CONTROL ARRANGEMENTS
Potential Qualifying Separation and Change of Control Payments
Potential Qualifying Separation without change of control payments and Qualifying Separation with change of control payments pursuant to existing agreements, assuming the termination event occurred on December 31, 2022, are set forth in the table below using our common stock price of $290.25, the closing price of our common stock on the Nasdaq Stock Market on December 30, 2022. This presentation is required by SEC disclosure rules. In addition to the amounts shown in the table below, each executive would receive payments for base salary and vacation time accrued through the date of termination and payment for any reimbursable business expenses incurred.
| Triggering event | ||||||||||
| Name | Benefit | Qualifying Separation Only |
Qualifying Separation within 12 months after a Change of Control |
|||||||
| Paul A. Friedman, M.D. Chairman and Chief Executive Officer |
Salary-based Severance | $663,000 | (1) | $ | 663,000 | (2) | ||||
| Bonus-based Severance | 331,500 | (1) | 331,500 | (2) | ||||||
| Continuation of Benefits | — | — | ||||||||
| Market Value of Vesting | 11,368,431 | (3) | 22,686,481 | (3) | ||||||
| Rebecca Taub, M.D. Chief Medical Officer, President, R & D, |
Salary-based Severance | $539,000 | (1) | $ | 539,000 | (2) | ||||
| Bonus-based Severance | 269,500 | (1) | 269,500 | (2) | ||||||
| Continuation of Benefits | 10,700 | (4) | 10,700 | (4) | ||||||
| Market Value of Vesting | 10,217,847 | (3) | 20,166,354 | (3) | ||||||
| Brian J. Lynch Senior Vice President and General Counsel |
Salary-based Severance | $471,000 | (1) | $ | 471,000 | (2) | ||||
| Bonus-based Severance | 188,400 | (1) | 188,400 | (2) | ||||||
| Continuation of Benefits | 25,100 | (4) | 25,100 | (4) | ||||||
| Market Value of Vesting | 9,957,511 | (3) | 21,646,615 | (3) | ||||||
| Remy Sukhija Senior Vice President and Chief Commercial Officer |
Salary-based Severance | $487,000 | (1) | $ | $487,000 | (2) | ||||
| Bonus-based Severance | 194,800 | (1) | 194,800 | (2) | ||||||
| Continuation of Benefits | 31,300 | (4) | 31,300 | (4) | ||||||
| Market Value of Vesting | 8,485,864 | (3) | $ | 16,648,425 | (3) | |||||
| Alex Howarth Senior Vice President and Chief Financial Officer |
Salary-based Severance | $469,000 | (1) | $ | 469,000 | (2) | ||||
| Bonus-based Severance | 187,600 | (1) | 187,600 | (2) | ||||||
| Continuation of Benefits | 31,300 | (4) | 31,300 | (4) | ||||||
| Market Value of Vesting | 6,799,252 | (3) | 16,270,981 | (3) | ||||||
| (1) | Upon such Qualifying Separation, each named executive officer is entitled to severance paid over time as described in “Employment Retention, Severance and Change in Control Arrangements” outside a change of control. |
| (2) | Upon such Qualifying Separation, each named executive officer is entitled to severance paid in a lump sum as described in “Employment Retention, Severance and Change in Control Arrangements” in connection with a change of control. |
| (3) | Represents value associated with certain acceleration and cancellation of unvested stock option awards, as described in “Employment Retention, Severance and Change in Control Arrangements,” assuming cancellation occurred at December 31, 2022 and involved a payment equal to the difference between the closing price on December 30, 2022 ($290.25) and the exercise price of such unvested in-the-money stock options. |
| (4) | Upon Qualifying Separation, such named executive officer is eligible for the Company to continue to pay the employer-paid portion of health benefits as described in “Employment Retention, Severance and Change in Control Arrangement.” |
| 44 |

|
|
DIRECTOR COMPENSATION
Non-Employee Director Compensation Program
A non-employee director is a director who is not employed by us and who does not receive compensation from us through any business relationship with us that is inconsistent with applicable Nasdaq or SEC independence rules. Our Board of Directors approves the form and amount of non-employee director compensation. Our Compensation Committee makes recommendations on the form and amount of non-employee director compensation. In July 2016, we adopted a non-employee director compensation program that became effective upon its adoption, and which has been amended from time to time, as described below.
Policy for Non-Employee Director Equity Awards. Under our policy for equity grants to our non-employee directors included in the Amended 2015 Stock Plan, as of, or as soon as practicable following, the regularly scheduled annual equity award grant date for the Company’s executive officers, an annual equity grant is made to each non-employee director serving on the Board of Directors with a grant-date fair value equal to the 50th percentile value of the director annual equity awards for the Company’s peer group, as benchmarked with the advice of the Company’s compensation consultant in the ordinary course. In the case of annual stock option awards, the amount of shares underlying the stock options is determined as of the date of grant by applying the applicable stock option value equal to the 50th percentile value of the director annual equity awards for the Company’s peer group to the Company’s then-applicable Black-Scholes inputs and formula under Financial Accounting Standards Board Accounting Standards Codification, Topic 718, Compensation—Stock Compensation. Such annual grant vests in full on the first anniversary of such date of grant, subject to the non-employee director’s continued service on such date. In accordance with this policy, in June of 2022, our non-employee directors received an annual grant of stock options for 9,470 shares of common stock at an exercise price of $65.06 per share.
In addition, pursuant to the Amended 2015 Stock Plan (as amended by the 2021 Amendment), one-time equity grants will be made to each new non-employee director upon his/her first election to the Board of Directors, with a value equivalent to two times (2x) the 50th percentile value of the Company’s peer group director annual equity awards as benchmarked with the advice of the Company’s compensation consultant. Such initial grant would vest as to 50% of the underlying shares on the first anniversary of the grant date and as to an additional 12.5% of the underlying shares on the last day of each successive quarterly period thereafter for four successive quarterly periods, subject to the non-employee director’s continued service on such dates.
Annual Cash Compensation
In addition to stock options, each non-employee director was eligible during 2022 to receive for his or her service on our Board of Directors or committees thereof annual cash retainers (payable quarterly, in arrears), as follows:
| Position |
Retainer ($) |
|||
| Board Member |
|
42,500 |
| |
| Lead Independent Director |
|
25,000 |
| |
| Audit Committee Chair |
|
20,000 |
| |
| Compensation Committee Chair |
|
15,000 |
| |
| Nominating and Governance Committee Chair |
|
10,000 |
| |
| Audit Committee Member |
|
10,000 |
| |
| Compensation Committee Member |
|
7,500 |
| |
| Nominating and Governance Committee Member |
|
5,000 |
| |
|
|
2023 PROXY STATEMENT | 45 |
DIRECTOR COMPENSATION
Expenses
Finally, we reimburse our non-employee directors for their reasonable out-of-pocket expenses incurred in attending meetings of our Board of Directors and committee meetings.
Director Compensation Tables
The following tables set forth information about the compensation paid to the non-employee members of our Board of Directors who served as a director during the year ended December 31, 2022. Other than as set forth in the tables and described more fully below, during the year ended December 31, 2022, we did not pay any fees to, make any equity awards to or pay any other compensation to the non-employee members of our Board of Directors. The stock options granted in 2022 and disclosed in the table below were granted under our Amended 2015 Stock Plan. Neither Dr. Friedman, our Chief Executive Officer and Chairman of our Board of Directors, nor Dr. Taub, our President, Research & Development, and Chief Medical Officer, and a director, received any compensation from us in 2022 for service as a director and, therefore, they are not included in the table below.
| Name | Fees Earned or Paid in Cash ($) (1) |
Option Awards ($) (2)(3) |
All Other Compensation ($) |
Total ($) | ||||||||||||||||
| Kenneth M. Bate |
|
68,125 |
|
|
180,309 |
|
|
0 |
|
|
248,434 |
|
||||||||
| Fred B. Craves, Ph.D. |
|
55,000 |
|
|
180,309 |
|
|
0 |
|
|
235,309 |
|
||||||||
| James M. Daly |
|
55,000 |
|
|
180,309 |
|
|
0 |
|
|
235,309 |
|
||||||||
| Keith R. Gollust |
|
60,625 |
|
|
180,309 |
|
|
0 |
|
|
240,934 |
|
||||||||
| Richard S. Levy, M.D. |
|
60,625 |
|
|
180,309 |
|
|
0 |
|
|
240,934 |
|
||||||||
| David Milligan, Ph.D. |
|
56,250 |
|
|
180,309 |
|
|
0 |
|
|
236,559 |
|
||||||||
| (1) | Consists of the annual retainer fee for service as a member of the Board of Directors or any Board committee. For further information concerning such fees, see the section above entitled “—Annual Cash Compensation.” The amount under “All Other Compensation” reflects fees for consultations concerning drug development and regulatory matters. |
| (2) | Mr. Bate, Dr. Craves, Mr. Daly, Mr. Gollust, Dr. Levy, and Dr. Milligan each received an option to purchase 9,470 shares of common stock with an exercise price per share of $65.06 at our 2022 Annual Meeting of Stockholders. The amounts in this column represent the aggregate grant date fair value of the option awards granted to applicable director in our fiscal year 2022 computed in accordance with FASB ASC Topic 718. See our discussion of “Stock-Based Compensation” under Notes 2 and 8 to our audited consolidated financial statements included in the Annual Report for a discussion of all assumptions made by us in determining the grant date fair values of our equity awards. See also our discussion of stock-based compensation under Item 7 “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Estimates” of the Annual Report. |
| (3) | The following table shows the total number of outstanding and vested stock options as of December 31, 2022, the last day of our fiscal year, that have been issued as director compensation to our non-employee directors. |
| Name
|
# of Stock Options Outstanding
|
# of Stock Options Vested
|
||||||||||
| Kenneth M. Bate |
|
77,959 |
|
|
68,489 |
|
||||||
| Fred B. Craves, Ph.D. |
|
77,959 |
|
|
68,489 |
|
||||||
| James M. Daly |
|
47,959 |
|
|
38,489 |
|
||||||
| Keith R. Gollust |
|
77,959 |
|
|
68,489 |
|
||||||
| Richard S. Levy, M.D. |
|
77,959 |
|
|
68,489 |
|
||||||
| David Milligan, Ph.D. |
|
47,959 |
|
|
38,489 |
|
||||||
| 46 |

|
|
DIRECTOR COMPENSATION
Report of the Compensation Committee of the Board of Directors
The Compensation Committee of our Board of Directors has reviewed and discussed the Compensation Discussion and Analysis required by Item 402(b) of Regulation S-K, which appears elsewhere in this proxy statement, with our management. Based on this review and discussion, the Compensation Committee has recommended to the Board of Directors that the Compensation Discussion and Analysis be included in our proxy statement and incorporated by reference in our Annual Report on Form 10-K for the fiscal year ended December 31, 2022.
MEMBERS OF THE COMPENSATION COMMITTEE:
Richard S. Levy, M.D. (Chairman)
Kenneth M. Bate
Fred B. Craves, Ph.D.
David Milligan, Ph.D.
EQUITY COMPENSATION PLAN INFORMATION
The following table provides certain aggregate information with respect to all of our equity compensation plans in effect as of December 31, 2022:
| Plan Category |
(a) Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights |
(b) Weighted Average Exercise Price of Outstanding Options, Warrants and Rights |
(c) Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding securities reflected in column (a)) |
|||||||||||||||||
| Equity Compensation Plans Approved by Security Holders (1) |
2,857,054 | $81.78 | 890,029 | |||||||||||||||||
| Total |
2,857,054 | $81.78 | 890,029 | |||||||||||||||||
| (1) | These shares relate to those authorized under our Amended 2015 Stock Plan, as amended at the 2021 Annual Meeting of Stockholders. |
DELINQUENT SECTION 16(a) REPORTS
The members of our Board of Directors, our executive officers, and persons who beneficially own more than ten percent of our outstanding common stock are subject to the reporting requirements of Section 16 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), which require them to file reports with respect to their beneficial ownership of our common stock and their transactions in our common stock. Based upon (i) the copies of Section 16 reports which we received from such persons for their 2022 year transactions in our common stock and their common stock holdings, and (ii) written representation that no other reports were required, we believe that all reporting requirements under Section 16 for such year were met in a timely manner by our directors, executive officers and greater than ten percent beneficial owners.
|
|
2023 PROXY STATEMENT | 47 |
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Other than compensation arrangements for our named executive officers and directors, and except as disclosed below, there have been no transaction or series of similar transactions, since January 1, 2022, to which we were a party or will be a party, in which:
| • | the amounts involved exceeded or will exceed the lesser of $120,000 or one percent of the average of our total assets at year-end; and |
| • | any of our directors, executive officers or holders of more than 5% of our capital stock, or any member of the immediate family of the foregoing persons, had or will have a direct or indirect material interest. |
December 21, 2022 Securities Purchase Agreement
On December 21, 2022, the Company entered into a Securities Purchase Agreement (the “Securities Purchase Agreement”) with funds affiliated with Baker Bros. Advisors LP (the “Baker Bros. Investors”) and Avoro Life Sciences Fund LLC (the “Avoro Investors,” and together with the Baker Bros. Investors, the “Investors”). Each of the Investors are beneficial owners of more than 5% of our capital stock, or affiliates thereof. Pursuant to the Securities Purchase Agreement, we issued and sold to Baker Bros. Investors 400,000 shares of the Company’s new Series B Convertible Preferred Stock (“Series B Preferred Stock”) at a price of $225.00 per share, for aggregate consideration of $90 million. We issued and sold to the Avoro Investors 44,444 shares of common stock at a price of $225.00 per share, for aggregate cash consideration of approximately $10.0 million.
Each share of the Series B Preferred Stock is convertible into shares of the Company’s common stock at any time at the holder’s option at a one-to-one ratio, subject to adjustment as provided in the Certificate of Designation of Preferences, Rights and Limitations of Series B Preferred Stock. A holder of Series B Preferred Stock, however, will be prohibited from converting shares of the Series B Preferred Stock into shares of our common stock if, as a result of such conversion, the holder, together with its affiliates, would own more than 4.99% of the shares of our common stock or any other class of any equity security of ours (other than an exempted security) that is registered pursuant to Section 12 of the Exchange Act, which may be increased or decreased up to 19.99% at the holder’s election on 61 days’ notice delivered to the Company.
Upon our liquidation, dissolution or winding-up, whether voluntary or involuntary, after the satisfaction in full of our debts and the payment of any liquidation preference owed to the holders of shares of our capital stock ranking prior to the Series B Preferred Stock upon liquidation, the holders of the Series B Preferred Stock shall participate pari passu with the holders of our common stock and the holders of the Series A Convertible Preferred Stock (on an as-if-converted-to-common-stock basis) in our net assets. Shares of the Series B Preferred Stock will generally
| 48 |

|
|
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
have no voting rights, except as required by law. Shares of the Series B Preferred Stock will be entitled to receive dividends pari passu with the shares of the Series A Convertible Preferred Stock, and before shares of any other class or series of capital stock of the Company (other than dividends in the form of the Common Stock) equal to the dividend payable on each share of our common stock, on an as-converted basis.
Amendment to June 20, 2017 Securities Purchase Agreement
On December 22, 2022, the Company entered into an amendment (the “Amendment”) to that certain Securities Purchase Agreement, dated June 20, 2017, with the Baker Bros. Investors. Pursuant to the Amendment, the Baker Bros. Investors’ right to nominate one director to the Company’s board of directors was amended such that in order to exercise the right, among other conditions, the Baker Bros. Investors must hold both (i) at least 50% of the Company’s Series A Convertible Preferred Stock (including conversion shares) that they originally acquired and (ii) at least 9.5% of the outstanding shares of the Company’s Common Stock. In addition to these amended terms, other conditions exist with regard to the Baker Bros. Investors’. director nomination right, as previously disclosed in SEC filings.
Indemnification Agreements
We have entered into indemnification agreements with each of our directors and executive officers and certain other key employees. The indemnification agreements provide that we will indemnify each of our directors, executive officers and such other key employees against any and all expenses incurred by that director, executive officer, or other key employee because of his or her status as one of our directors, executive officers, or other key employees, to the fullest extent permitted by Delaware law, our restated certificate of incorporation and our amended and restated bylaws. In addition, the indemnification agreements provide that, to the fullest extent permitted by Delaware law, we will advance all expenses incurred by our directors, executive officers and other key employees in connection with a legal proceeding.
Policy for Approval of Related Person Transactions
Pursuant to the written charter of our Audit Committee, the Audit Committee is responsible for reviewing and approving, prior to our entry into any such transaction, all transactions in which we are a participant and in which any of the following persons has or will have a direct or indirect material interest:
| • | our executive officers; |
| • | our directors; |
| • | the beneficial owners of more than 5% of our securities; |
| • | the immediate family members of any of the foregoing persons; and |
| • | any other persons whom the Board of Directors determines may be considered related persons. |
For purposes of these procedures, “immediate family members” means any child, stepchild, parent, stepparent, spouse, sibling, mother-in-law, father-in-law, son-in-law, daughter-in-law, brother-in-law, or sister-in-law, and any person (other than a tenant or employee) sharing the household with the executive officer, director or 5% beneficial owner.
In reviewing and approving such transactions, the Audit Committee shall obtain, or shall direct our management to obtain on its behalf, all information that the committee believes to be relevant and important to a review of the transaction prior to its approval. Following receipt of the necessary information, a discussion shall be held of the relevant factors if deemed to be necessary by the committee prior to approval. If a discussion is not deemed to be necessary, approval may be given by written consent of the committee. This approval authority may also be delegated to the chairman of the Audit Committee in some circumstances. No related person transaction shall be entered into prior to the completion of these procedures.
|
|
2023 PROXY STATEMENT | 49 |
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The Audit Committee or its chairman, as the case may be, shall approve only those related person transactions that are determined to be in, or not inconsistent with, the best interests of the Company and our stockholders, taking into account all available facts and circumstances as the committee or the chairman determines in good faith to be necessary. These facts and circumstances will typically include, but not be limited to, the benefits of the transaction to our company; the impact on a director’s independence in the event that the related person is a director, an immediate family member of a director or an entity in which a director is a partner, stockholder or executive officer; the availability of other sources for comparable products or services; the terms of the transaction; and the terms of comparable transactions that would be available to unrelated third parties or to employees generally. No member of the Audit Committee shall participate in any review, consideration or approval of any related person transaction with respect to which the member or any of his or her immediate family members is the related person.
| 50 |

|
|
PROPOSAL NO. 1: ELECTION OF DIRECTORS
Summary
Our Board of Directors currently consists of eight members, classified into three classes as follows: Paul A. Friedman, M.D., Kenneth M. Bate and James M. Daly are the Class I directors with a term ending at this year’s Annual Meeting; Rebecca Taub, M.D. and Fred B. Craves, Ph.D. are the Class II directors with a term ending at the 2024 Annual Meeting of Stockholders; and Keith R. Gollust, David Milligan, Ph.D. and Richard S. Levy, M.D. are the Class III directors with a term ending at the 2025 Annual Meeting of Stockholders. At each Annual Meeting of Stockholders, directors are elected for a full term of three years to succeed those directors whose terms are expiring.
On March 9,2023, our Nominating and Governance Committee nominated, and our Board thereafter approved, Paul A. Friedman, M.D., Kenneth M. Bate and James M. Daly as Class I director nominees for election at the Annual Meeting for a term of three years to serve until the 2026 Annual Meeting of Stockholders, and until their respective successors have been elected and qualified, or until their earlier death, resignation, retirement or removal. Unless authority to vote for any of these nominees is withheld, the shares represented by a validly executed proxy will be voted FOR the election of Paul A. Friedman, M.D., Kenneth M. Bate and James M. Daly as directors. In the event that either nominee should become unable or unwilling to serve, the shares represented by a validly executed proxy will be voted for the election of such other person as the Board of Directors may recommend in his or her place, as the case may be, unless the Board of Directors chooses to reduce the number of directors serving on the Board of Directors. We have no reason to believe that any nominee will be unable or unwilling to serve as a director.
Vote Required
Directors are elected by a plurality of the affirmative votes cast by those shares deemed present in person or represented by proxy and entitled to vote at the Annual Meeting. The three nominees for director receiving the highest number of affirmative votes will be elected.
Recommendation of the Board of Directors
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT OUR STOCKHOLDERS VOTE “FOR” THE ELECTION OF PAUL A. FRIEDMAN, M.D., KENNETH M. BATE AND JAMES M. DALY AS DIRECTORS, AND PROXIES SOLICITED BY THE BOARD WILL BE VOTED IN ACCORDANCE WITH THE BOARD’S RECOMMENDATION UNLESS A STOCKHOLDER HAS INDICATED OTHERWISE ON THE PROXY.
|
|
2023 PROXY STATEMENT | 51 |
PROPOSAL NO. 2: RATIFICATION OF APPOINTMENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Summary
The Audit Committee has appointed PricewaterhouseCoopers LLP, independent registered public accounting firm, to audit our financial statements for the fiscal year ending December 31, 2023. PricewaterhouseCoopers LLP audited our financial statements for the fiscal year ended December 31, 2022. The Board of Directors proposes that our stockholders ratify this appointment. In the event our stockholders do not ratify the appointment of PricewaterhouseCoopers LLP as our independent registered public accounting firm, the Audit Committee will reconsider its appointment. We do not expect a representative of PricewaterhouseCoopers LLP to be present at the Annual Meeting or to otherwise be available to make a statement or respond to questions.
Accounting Fees and Services
The following table summarizes the aggregate fees for professional services rendered to us by PricewaterhouseCoopers LLP for the years ended December 31, 2022 and December 31, 2021. The Audit Committee pre-approved all services fees described below.
| 2022 ($) | 2021 ($) | |||||||||||
| Audit fees |
726,500 | 631,000 | ||||||||||
| Audit-related fees |
— | — | ||||||||||
| Tax fees |
45,000 | 34,000 | (1) | |||||||||
| All other fees |
3,000 | 3,000 | ||||||||||
| Total |
774,500 | 668,000 | (1) | |||||||||
| (1) | Reflects additional $6,000 in tax fees related to 2021 relative to 2021 Proxy Statement disclosure. |
Audit Fees
Audit services consist of fees and expenses for the audit of our annual financial statements included in our Forms 10-K, and the related audit of internal control over financial reporting included in our Annual Report on Form 10-K, review of interim financial statements included in our Forms 10-Q, consultations regarding accounting and auditing matters, and fees in connection with our underwritten public offering of shares of common stock.
Tax Fees
Tax fees consist of fees billed for professional services for tax compliance, tax planning, and tax advice.
Policy on Audit Committee Pre-Approval of Audit and Permissible Non-audit Services of Independent Auditors.
Consistent with policies of the SEC regarding auditor independence, the Audit Committee has responsibility for appointing, setting compensation and overseeing the work of our independent registered public accounting firm. In recognition of this responsibility, the Audit Committee has established a policy to pre-approve all audit and permissible non-audit services provided by our independent registered public accounting firm.
Prior to engagement of an independent registered public accounting firm for the next year’s audit, management will submit an aggregate of services expected to be rendered during that year for each of four categories of services to the Audit Committee for approval.
1. Audit services include audit work performed in the preparation of financial statements, as well as work that generally only an independent registered public accounting firm can reasonably be expected to provide, including comfort letters, statutory audits, and attest services and consultation regarding financial accounting and/or reporting standards.
| 52 |

|
|
PROPOSAL NO. 2: RATIFICATION OF APPOINTMENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
2. Audit-Related services are for assurance and related services that are traditionally performed by an independent registered public accounting firm, including due diligence services, employee benefit plan audits, and special procedures required to meet certain regulatory requirements.
3. Tax services include all services performed by an independent registered public accounting firm’s tax personnel except those services specifically related to the audit of the financial statements, and includes fees in the areas of tax compliance, tax planning, and tax advice.
4. Other Fees are those associated with services not captured in the other categories.
Prior to engagement, the Audit Committee pre-approves these services by category of service. The fees are budgeted and the Audit Committee requires our independent registered public accounting firm and management to report actual fees versus the budget periodically throughout the year by category of service. During the year, circumstances may arise when it may become necessary to engage our independent registered public accounting firm for additional services not contemplated in the original pre-approval. In those instances, the Audit Committee requires specific pre-approval before engaging our independent registered public accounting firm.
The Audit Committee may delegate pre-approval authority to one or more of its members. The member to whom such authority is delegated must report, for informational purposes only, any pre-approval decisions to the Audit Committee at its next scheduled meeting.
Report of the Audit Committee of the Board of Directors
The information contained in this “Report of the Audit Committee of the Board of Directors” shall not be: deemed to be “soliciting material” or to be “filed” with the SEC, nor shall such information be incorporated by reference into any of our filings with the SEC, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing; or subject to the liabilities of Section 18 of the Exchange Act.
The Audit Committee, which consists entirely of directors who meet the independence and experience requirements of The Nasdaq Stock Market, has furnished the following report:
The Audit Committee assists the Board of Directors in overseeing and monitoring the integrity of our financial reporting process, compliance with legal and regulatory requirements and the quality of internal and external audit processes. The Audit Committee’s role and responsibilities are set forth in a charter adopted by the Board of Directors, which is publicly available through the “Investors—Corporate Governance” section of our website at www.madrigalpharma.com. The Audit Committee reviews and reassesses its charter annually and recommends any changes to the Board of Directors for approval. The Audit Committee is responsible for overseeing our overall financial reporting process, and for the appointment, compensation, retention, and oversight of the work of our independent registered public accounting firm. In fulfilling its responsibilities for the financial statements for the fiscal year ended December 31, 2022, the Audit Committee took the following actions:
| • | Reviewed and discussed the audited financial statements for the fiscal year ended December 31, 2022 with management and PricewaterhouseCoopers LLP, the independent registered public accounting firm that audited our financial statements for the fiscal year ended December 31, 2022, and the critical audit matter addressed during the audit; |
| • | Discussed with PricewaterhouseCoopers LLP the matters required to be discussed by the applicable requirements of the Public Company Accounting Oversight Board, or PCAOB; and |
| • | Received written disclosures and the letter from PricewaterhouseCoopers LLP regarding its independence as required by the applicable requirements of the PCAOB regarding PricewaterhouseCoopers LLP’s communications with the Audit Committee and the Audit Committee further discussed with PricewaterhouseCoopers LLP their |
|
|
2023 PROXY STATEMENT | 53 |
PROPOSAL NO. 2: RATIFICATION OF APPOINTMENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
| independence. The Audit Committee also considered the status of taxation matters and other areas of oversight relating to the financial reporting and audit process that the committee determined appropriate. |
Based on the Audit Committee’s review of the audited financial statements and discussions with management and PricewaterhouseCoopers LLP, the Audit Committee recommended to the Board that the audited financial statements be included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, as amended, in our filing with the SEC.
| MEMBERS OF THE AUDIT COMMITTEE: |
| Kenneth M. Bate (Chairman) James M. Daly Keith R. Gollust David Milligan, Ph.D. |
Vote Required
The majority of the shares of our common stock cast affirmatively at the Annual Meeting on this proposal is required to ratify the appointment of PricewaterhouseCoopers LLP as our independent registered public accounting firm.
If our stockholders ratify the appointment of PricewaterhouseCoopers LLP as our independent registered public accounting firm, the Audit Committee may still, in its discretion, decide to appoint a different independent registered public accounting firm at any time during the year ending December 31, 2023, if it concludes that such a change would be in the best interests of the Company and our stockholders. If our stockholders fail to ratify the appointment, the Audit Committee will reconsider, but not necessarily rescind, the appointment.
Recommendation of the Board of Directors
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT OUR STOCKHOLDERS VOTE “FOR” RATIFICATION OF THE APPOINTMENT OF PRICEWATERHOUSECOOPERS LLP AS OUR INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM, AND PROXIES SOLICITED BY THE BOARD WILL BE VOTED IN ACCORDANCE WITH THE BOARD’S RECOMMENDATION UNLESS A STOCKHOLDER INDICATES OTHERWISE ON THE PROXY.
| 54 |

|
|
PROPOSAL NO. 3: ADVISORY VOTE ON EXECUTIVE COMPENSATION
General
Pursuant to Section 14A(a)(1) of the Exchange Act, we are providing our stockholders with an opportunity to approve, on a non-binding advisory basis, the compensation of our named executive officers as disclosed in this proxy statement in accordance with the compensation disclosure rules of the SEC (commonly referred to as a “say-on-pay” vote).
Prior to casting your vote on this proposal, you are encouraged to read the proxy statement sections entitled “Compensation Discussion and Analysis” and “Executive Officer and Director Compensation” for a detailed discussion of our policies and practices relating to the compensation of our named executive officers.
Our Compensation Committee believes that the objectives of our executive compensation program, as it relates to our named executive officers, are appropriate for a company of our size and stage of development and that our compensation policies and practices help meet those objectives. In addition, our Compensation Committee believes that our executive compensation program, as it relates to our named executive officers, achieves an appropriate balance between fixed compensation and variable incentive compensation, pays for performance and promotes an alignment between the interests of our named executive officers and our stockholders. Accordingly, we are asking our stockholders to approve the compensation of our named executive officers. This advisory vote is not intended to be limited or specific to any particular element of compensation, but rather to cover the overall compensation of our named executive officers and the compensation policies and practices described in this proxy statement as it relates to our named executive officers.
Resolution
Our Board of Directors is asking our stockholders to indicate their support for the compensation of our named executive officers, as described in this proxy statement, by casting a non-binding advisory vote “FOR” the following resolution:
“RESOLVED, that the compensation paid to the named executive officers of Madrigal Pharmaceuticals, Inc., as disclosed pursuant to the compensation disclosure rules of the Securities and Exchange Commission, including the Compensation Discussion and Analysis, the compensation tables and the related material disclosed in this proxy statement, is hereby APPROVED.”
Vote Required
The foregoing resolution will be deemed approved on an advisory basis by the majority of the shares of our common stock cast affirmatively at the Annual Meeting on this proposal. Because this proposal is advisory, the results of the vote will not be binding on us, our Board of Directors or our Compensation Committee.
Recommendation of the Board of Directors
OUR BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT OUR STOCKHOLDERS VOTE “FOR” THE APPROVAL ON AN ADVISORY BASIS OF THE COMPENSATION OF OUR NAMED EXECUTIVE OFFICERS, AND PROXIES SOLICITED BY THE BOARD WILL BE VOTED IN ACCORDANCE WITH THE BOARD’S RECOMMENDATION UNLESS A STOCKHOLDER INDICATES OTHERWISE ON THE PROXY.
|
|
2023 PROXY STATEMENT | 55 |
PROPOSAL NO. 4: APPROVAL OF AMENDMENT TO RESTATED CERTFICATE OF INCORPORATION TO UPDATE THE EXCULPATION PROVISION TO ALIGN WITH DELAWARE LAW
We are asking our stockholders to approve an amendment to our Restated Certificate of Incorporation, as amended (our “Certificate of Incorporation”), to provide exculpation from liability for certain officers of the Company from certain claims of breach of the fiduciary duty of care, similar to protections currently available to directors of the Company.
On August 1, 2022, the state of Delaware, which is our state of incorporation, amended Section 102(b)(7) of the Delaware General Corporation Law (the “DGCL”) to allow Delaware corporations to exculpate (i.e., to limit or eliminate the monetary liability of) certain officers from certain claims of breaches of the fiduciary duty of care, affording them certain protections previously available only to directors. Consistent with prior law before this change, the Ninth Article of our Certificate of Incorporation provides exculpation for directors, but not senior officers. The Company is proposing to amend our Certificate of Incorporation to provide exculpation for senior officers, in addition to directors, consistent with, and to the extent permitted by, Delaware law.
The Board has approved a Certificate of Amendment to our Certificate of Incorporation (the “Amendment”), subject to approval from our stockholders at the Annual Meeting. The Amendment would add a new Article Twelfth to the Certificate of Incorporation as follows:
“TWELFTH: To the fullest extent that the Delaware General Corporation Law, as the same exists or may hereafter be amended, permits the limitation or elimination of the liability of officers, no officer of the Corporation shall be personally liable to the Corporation or its stockholders for any monetary damages for breach of fiduciary duty as an officer. No amendment to, or modification or repeal of this Article TWELFTH, nor the adoption of any provision of this Restated Certificate of Incorporation inconsistent with this Article TWELFTH, shall adversely affect any right or protection of an officer existing hereunder with respect to any act or omission occurring prior to such amendment, modification, repeal or adoption of an inconsistent provision. If the Delaware General Corporation Law is amended to authorize corporate action further eliminating or limiting the personal liability of officers, then the liability of an officer of the Corporation shall be eliminated or limited to the fullest extent permitted by the Delaware General Corporation Law, as so amended.”
The full text of the amendment is attached to this proxy statement as Appendix A.
Background
Section 102(b)(7) of the DGCL previously permitted Delaware corporations to exculpate directors, but not officers. In August of 2022, the Delaware legislature amended Section 102(b)(7) to permit Delaware corporations to exculpate certain senior officers, in addition to directors. However, for both directors and officers, the amended Section 102(b)(7) does not permit exculpation with respect to breaches of the duty of loyalty; acts or omissions not in good faith or those that involve intentional misconduct or a knowing violation of law; or any transactions in which a director or officer derived an improper personal benefit. Further, specifically for senior officers, the amended Section 102(b)(7) only permits exculpation for direct claims brought by stockholders (as opposed to derivative claims made by stockholders on behalf of the corporation, with respect to which exculpation of officers is not permitted).
We are proposing to add a new Twelfth Article to our Certificate of Incorporation to be consistent with the recently amended Section 102(b)(7) of the DGCL. If the Amendment is approved by our stockholders, certain senior officers will be exculpated from monetary liability from certain breaches of fiduciary duty, solely to the extent permitted by Section 102(b)(7) of the DGCL.
| 56 |

|
|
PROPOSAL NO. 4: APPROVAL OF AMENDMENT TO RESTATED CERTFICATE OF INCORPORATION TO UPDATE THE EXCULPATION PROVISION TO ALIGN WITH DELAWARE LAW
Reasons for the Amendment
The Board believes that providing exculpation to senior officers (to the extent permitted by Delaware law) is critical to attract and retain executive talent who can deliver performance for our stockholders, particularly as we approach FDA approval and commercialization of resmetirom and we expand headcount significantly, including senior officials. Exculpation has been available to directors of Delaware corporations since 1987, and we expect our peers to exculpate their senior officers now that Delaware law permits this. If our peers (including current peers and larger biopharmaceutical companies with whom we expect to compete) adopt such exculpation provisions and we do not, our ability to attract and retain highly qualified officer candidates in the competitive marketplace may be adversely impacted. Officer candidates for biopharmaceutical companies often weigh the benefits of serving as an officer with potential exposure to liability, costs of defense and other risks of proceedings, given the current litigious environment and the complex regulatory environment in which we operate.
We believe that adopting an exculpation provision that aligns with current Delaware law could prevent expensive and protracted litigation that distracts our directors and senior officers from important commercial development, ongoing clinical trials, and operational and strategic matters. Delaware corporations that fail to adopt officer exculpation provisions may experience a disproportionate amount of nuisance litigation and disproportionately increased costs in the form of increased director and officer liability insurance premiums. Going forward, we believe that our senior officers, in addition to our directors, will be called upon to make critical development, regulatory, operational, financing and strategic decisions in response to time-sensitive challenges and opportunities, including in light of competitive and regulatory challenges as well as changes in economic conditions and financial markets. Given the current litigious environment of our country, these decisions can create the potential for substantial risk of claims, actions, suits or proceedings seeking to impose liability on the basis of hindsight. An exculpation provision that is fully consistent with current Delaware law would empower both directors and senior officers to exercise their business judgment in furtherance of stockholder interests. Consistent with longstanding laws, our directors or senior officers would not be protected after this Amendment from liability for breaches of the duty of loyalty, acts or omissions not in good faith or those that involve intentional misconduct or a knowing violation of law, or any transactions in which a director or officer derived an improper personal benefit.
For these reasons, the Board believes this proposal to approve the Amendment, which would update the exculpation provision to align with Delaware law, is in the best interests of the Company and our stockholders. If stockholders approve this Proposal No. 4, the Company’s Amended Certificate of Incorporation will become effective upon its filing with the Delaware Secretary of State, which we anticipate doing as soon as practicable following stockholder approval. In accordance with the DGCL, however, the Board may abandon the Amendment without further action by the stockholders at any time prior to the effectiveness of the filing of the Amendment with the Delaware Secretary of State, notwithstanding stockholder approval.
Vote Required
The vote of a majority of the outstanding shares of our common stock entitled to vote thereon is required to approve the Amendment. Abstentions have the same effect as a vote cast against the proposal because abstentions are treated as shares entitled to vote. Brokerage firms do not have authority to vote customers’ unvoted shares held by the firms in street name on this proposal. As a result, any shares not voted by a customer will be treated as a broker non-vote. Broker non-votes will have the same effect as a vote against the proposal.
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT OUR STOCKHOLDERS VOTE “FOR” THE APPROVAL OF THE AMENDMENT TO THE COMPANY’S RESTATED CERTIFICATE OF INCORPORATION TO REFLECT NEW DELAWARE LAW PROVISIONS REGARDING OFFICER EXCULPATION, AND PROXIES SOLICITED BY THE BOARD WILL BE VOTED IN ACCORDANCE WITH THE BOARD’S RECOMMENDATION UNLESS A STOCKHOLDER HAS INDICATED OTHERWISE ON THE PROXY.
|
|
2023 PROXY STATEMENT | 57 |
CODE OF CONDUCT AND ETHICS
We have adopted a code of conduct and ethics that applies to all of our directors and employees, including our chief executive officer and chief financial and accounting officer. The text of the code of conduct and ethics is posted on the “Investors—Corporate Governance” section of our website at www.madrigalpharma.com. Disclosure regarding any amendments to, or waivers from, provisions of the code of conduct and ethics that apply to our directors or principal executive and financial officers will be included in a Current Report on Form 8-K within four business days following the date of the amendment or waiver, unless website posting or the issuance of a press release of such amendments or waivers is then permitted by the rules of Nasdaq.
HEDGING AND PLEDGING POLICY
Pursuant to our Insider Trading Policy, we prohibit employees, officers and directors from pledging any Company securities as collateral for a loan, or from holding any Company securities in a margin account. This policy also prohibits employees, officers and directors from entering into hedging transactions involving Company securities, including purchasing financial instruments such as options, prepaid variable forwards, equity swaps, collars, exchange funds and similar transactions. Hedging transactions means any transaction that hedges or offsets, or is designed to hedge or offset, any decrease in the market value of Company securities.
OTHER MATTERS
The Board of Directors knows of no other business which will be presented at the Annual Meeting. If any other business is properly brought before the Annual Meeting, proxies in the enclosed form will be voted in accordance with the judgment of the persons named therein.
STOCKHOLDER PROPOSALS AT FUTURE ANNUAL MEETINGS
To be considered for inclusion in the proxy statement relating to our 2024 Annual Meeting of Stockholders, we must receive stockholder proposals subject to Rule 14a-8 under the Exchange Act no later than January 3, 2024. Such proposals must comply with SEC regulations regarding the inclusion of stockholder proposals in Company-sponsored proxy materials. A stockholder’s nomination of persons for election to the Board of Directors (a “Director Nomination Proposal”) or for the proposal of business to be considered by the stockholders (a “Business Proposal”) may be made at an annual meeting of stockholders if certain requirements are met including the following procedural requirements: (1) the stockholder must have given timely notice thereof in writing to the Secretary of the Corporation (as described in greater detail below), (2) such other business must otherwise be a proper matter for stockholder action under the Delaware General Corporation Law, and (3) if the stockholder intends to deliver a proxy statement and form of proxy to stockholders of, in the case of a proposal, at least the percentage of the Corporation’s voting shares required under applicable law to carry the proposal or, in the case of a nomination or nominations, a sufficient number of holders of the Corporation’s voting shares to elect such nominee or nominees, the stockholder shall have satisfied applicable proxy statement and form of proxy notice and delivery requirements sufficient to carry the applicable Director Nomination Proposal or Business Proposal under applicable law or
| 58 |

|
|
STOCKHOLDER PROPOSALS AT FUTURE ANNUAL MEETINGS
satisfied solicitation requirements, in each case, as set forth in our Bylaws. To be timely, a stockholder’s notice concerning the foregoing matters pertaining to an annual meeting shall be delivered to the Secretary at the principal executive offices of the Corporation not less than forty-five (45) or more than seventy-five (75) days prior to the first anniversary of the date on which the Corporation first mailed its proxy materials for the preceding year’s annual meeting; provided, however, that in the event that the date of the annual meeting is more than thirty (30) days before or more than thirty (30) days after the anniversary date of the preceding year’s annual meeting, notice by the stockholder to be timely must be so delivered not earlier than the close of business on the ninetieth (90th) day prior to such annual meeting and not later than the close of business on the later of the sixtieth (60th) day prior to such annual meeting or the close of business on the tenth (10th) day following the day on which public announcement of the date of such meeting is first made by the Corporation (collectively, the “Meeting Matter Notice Requirement”). The submission window to meet the Meeting Matter Notice Requirement for the 2024 Annual Meeting of Stockholders runs from February 17, 2024 to March 19, 2024. In addition, such stockholder’s notice for an annual meeting shall set forth: (a) as to each person whom the stockholder proposes in a Director Nomination Proposal all information relating to such person that is required to be disclosed in solicitations of proxies for election of directors, or is otherwise required, in each case, pursuant to Regulation 14A under the Exchange Act (including such person’s written consent to being named in the proxy statement as a nominee and to serving as a director if elected); (b) as to any Business Proposal a brief description of the business desired to be brought before the meeting, the reasons for conducting such business at the meeting and any material interest in such business of such stockholder and the beneficial owner, if any, on whose behalf the proposal is made; and (c) as to the stockholder giving the notice and the beneficial owner, if any, on whose behalf the nomination or proposal is made (i) the name and address of such stockholder, as they appear on the Corporation’s books, and of such beneficial owner, (ii) the class and number of shares of the Corporation that are owned beneficially and held of record by such stockholder and such beneficial owner, and (iii) whether either such stockholder or beneficial owner intends to deliver a proxy statement and form of proxy to holders at least the percentage of the Corporation’s voting shares required under applicable law to carry the proposal or, in the case of a nomination or nominations, a sufficient number of holders of the Corporation’s voting shares to elect such nominee or nominees as described above. The foregoing Bylaw provisions related to a Director Nomination Proposal are referenced to elsewhere in this proxy statement as Bylaw Provisions Related to Stockholder Nominations of Director Candidates and such nominations are subject to additional requirements as set forth in this proxy statement. See “Management and Corporate Governance — Committees of the Board of Directors and Meetings; Nominating and Governance Committee.”
In addition to satisfying the foregoing requirements under our Bylaws (including our advance notice requirements), to comply with the universal proxy rules under the Exchange Act, stockholders who intend to solicit proxies in support of director nominees other than the Company’s nominees must provide notice that sets forth the other information required by Rule 14a-19 under the Exchange Act no later than April 16, 2024.
Proposals that are not a proper subject for consideration or that are not received in a timely manner will not be voted on at the 2024 Annual Meeting. If a proposal is proper and received on time, the proxies that management solicits for the meeting may still exercise discretionary voting authority on the proposal under circumstances consistent with the proxy rules of the SEC. All stockholder proposals should be marked for the attention of Corporate Secretary, Madrigal Pharmaceuticals, Inc., Four Tower Bridge, 200 Barr Harbor Drive, Suite 200, West Conshohocken, Pennsylvania 19428.
HOUSEHOLDING OF PROXY MATERIALS
The SEC has adopted rules that permit companies and intermediaries to satisfy the delivery requirements for Notices of Internet Availability of Proxy Materials or other Annual Meeting materials with respect to two or more stockholders sharing the same address by delivering a single Notice of Internet Availability of Proxy Materials or
|
|
2023 PROXY STATEMENT | 59 |
HOUSEHOLDING OF PROXY MATERIALS
other Annual Meeting materials addressed to those stockholders. This process, which is commonly referred to as “householding,” potentially means extra convenience for stockholders and cost savings for companies.
This year, a number of brokers with account holders who are our stockholders will be “householding” our proxy materials. A single Notice of Internet Availability of Proxy Materials will be delivered to multiple stockholders sharing an address unless contrary instructions have been received from the affected stockholders. Once you have received notice from your broker that they will be “householding” communications to your address, “householding” will continue until you are notified otherwise or until you revoke your consent. If, at any time, you no longer wish to participate in “householding” and would prefer to receive a separate Notice of Internet Availability of Proxy Materials, please notify your broker or us. We will promptly deliver, upon written request to the address above, a separate copy of the Notice of Internet Availability of Proxy Materials or the full set of proxy materials, as applicable, to a stockholder at a shared address to which a single copy of the documents was delivered. Direct your written request to Madrigal Pharmaceuticals, Inc., Corporate Secretary, Four Tower Bridge, 200 Barr Harbor Drive, Suite 200, West Conshohocken, Pennsylvania 19428. Stockholders who currently receive multiple copies of the Notices of Internet Availability of Proxy Materials at their addresses and would like to request “householding” of their communications should contact their brokers or us at the above address.
Our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, as amended by our Annual Report on Form 10-K/A for the fiscal year ended December 31, 2022 filed on March 3, 2023 is available without charge upon written request to: Madrigal Pharmaceuticals, Inc., Corporate Secretary, Four Tower Bridge, 200 Barr Harbor Drive, Suite 200, West Conshohocken, Pennsylvania 19428. Our Annual Report on Form 10-K, as amended by our Annual Report on Form 10-K/A, is not incorporated into this Proxy Statement and is not considered proxy soliciting material.
DISCLOSURE REGARDING FORWARD-LOOKING STATEMENTS
This proxy statement contains “forward-looking statements” made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, that are based on Madrigal’s beliefs and assumptions and on information currently available to us, but are subject to factors beyond our control. Forward-looking statements reflect management’s current knowledge, assumptions, judgment and expectations regarding future performance or events. Forward-looking statements include: all statements that are not historical facts; statements referenced by forward-looking statement identifiers, including the examples in the paragraph below; resmetirom’s potential to be a cost-effective specialty therapy for NASH patients with significant liver fibrosis; and statements or references concerning—the potential efficacy and safety of resmetirom for noncirrhotic NASH patients and cirrhotic NASH patients, possible or assumed future results of operations and expenses, business strategies and plans (including ex-US. Launch/partnering plans), research and development activities, and the timing and results associated with the future development of resmetirom, the timing and completion of projected future clinical milestone events, including enrollment, additional studies, top-line data and open label projections, plans, objectives, timing and support for making for making a Subpart H (Accelerated Approval of New Drugs for Serious or Life-Threatening Illnesses) submission to FDA, projections or objectives for obtaining accelerated or full approval for resmetirom, Madrigal’s primary and key secondary study endpoints for resmetirom and the potential for achieving such endpoints and projections, the potential to support an additional indication for resmetirom in patients with well-compensated NASH cirrhosis, optimal dosing levels for resmetirom and projections regarding potential NASH or NAFLD and potential patient benefits with resmetirom, including future NASH resolution, safety, fibrosis treatment, cardiovascular effects, lipid treatment, and/or biomarker effects with resmetirom.
Forward-looking statements can be identified by terms such as “accelerate,” “achieve,” “allow,” “anticipates,” “appear,” “be,” “believes,” “can,” “continue,” “could,” “demonstrates,” ”design,” “estimates,” “expectation,” “expects,” “forecasts,” “future,” “goal,” “help,” “hopeful,” “inform,” inform,” “intended,” “intends,” “may,” “might,” “on track,” “planned,” “planning,” “plans,” “positions,” “potential,” “powers,” “predicts,” ”predictive,” “projects,” “seeks,” “should,” “will,” “will achieve,” “will be,” “would” or similar expressions and the negatives of those terms.
| 60 |

|
|
DISCLOSURE REGARDING FORWARD-LOOKING STATEMENTS
Forward-looking statements are subject to a number of risks and uncertainties including, but not limited to: the assumptions underlying the forward-looking statements; risks of obtaining and maintaining regulatory approvals, including, but not limited to, potential regulatory delays or rejections; risks associated with meeting the objectives of Madrigal’s clinical studies, including, but not limited to Madrigal’s ability to achieve enrollment objectives concerning patient numbers (including an adequate safety database), outcomes objectives and/or timing objectives for Madrigal’s studies; any delays or failures in enrollment, and the occurrence of adverse safety events; risks related to the effects of resmetirom’s mechanism of action; the achievement of enrollment objectives concerning patient number, safety database and/or timing for Madrigal’s studies; enrollment and trial conclusion uncertainties, generally and in relation to COVID-19 related measures and individual precautionary measures that may be implemented or continued for an uncertain period of time; market demand for and acceptance of our products; the potential inability to raise sufficient capital to fund ongoing operations as currently planned or to obtain financings on terms similar to those arranged in the past; the ability to service indebtedness and otherwise comply with debt covenants; outcomes or trends from competitive studies; future topline data timing or results; the risks of achieving potential benefits in studies that includes substantially more patients, and patients with different disease states, than prior studies; the timing and outcomes of clinical studies of resmetirom; and the uncertainties inherent in clinical testing. Undue reliance should not be placed on forward-looking statements, which speak only as of the date they are made. Madrigal undertakes no obligation to update any forward-looking statements to reflect new information, events or circumstances after the date they are made, or to reflect the occurrence of unanticipated events. Please refer to Madrigal’s submissions filed with the U.S. Securities and Exchange Commission, or SEC, for more detailed information regarding these risks and uncertainties and other factors that may cause actual results to differ materially from those expressed or implied. Madrigal specifically discusses these risks and uncertainties in greater detail in the section appearing in Part I, Item 1A of its Annual Report on Form 10-K for the year ended December 31, 2022, filed with the SEC on February 23, 2023, as amended by its Annual Report on Form 10-K/A filed on March 3, 2023, and as updated from time to time by Madrigal’s other filings with the SEC.
Moreover, we operate in an evolving environment. New risks and uncertainties emerge from time to time and it is not possible for our management to predict all risks and uncertainties, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual future results to be materially different from those expressed or implied by any forward-looking statements. Except as required by applicable law or the rules of the Nasdaq, we assume no obligation to update any forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future. We qualify all of our forward-looking statements by these cautionary statements.
MADRIGAL PHARMACEUTICALS, INC.
FOUR TOWER BRIDGE
200 BARR HARBOR DRIVE, SUITE 200
WEST CONSHOHOCKEN, PENNSYLVANIA 19428
(267) 824-2827
West Conshohocken, Pennsylvania
May 1, 2023
|
|
2023 PROXY STATEMENT | 61 |
APPENDIX A
CERTIFICATE OF AMENDMENT OF
RESTATED CERTIFICATE OF INCORPORATION
MADRIGAL PHARMACEUTICALS, INC.
(Pursuant to Section 242 of the
General Corporation Law of the State of Delaware)
Madrigal Pharmaceuticals, Inc. (the “Corporation”), a corporation organized and existing under the laws of the State of Delaware, does hereby certify:
1. This Certificate of Amendment (the “Certificate of Amendment”) amends the provisions of the Restated Certificate of Incorporation of the Corporation (the “Restated Certificate of Incorporation”) filed with the Secretary of State of the State of Delaware on February 9, 2007, as amended.
2. The Restated Certificate of Incorporation is hereby amended by adding Article TWELFTH to read in its entirety as follows:
“TWELFTH: To the fullest extent that the Delaware General Corporation Law, as the same exists or may hereafter be amended, permits the limitation or elimination of the liability of officers, no officer of the Corporation shall be personally liable to the Corporation or its stockholders for any monetary damages for breach of fiduciary duty as an officer. No amendment to, or modification or repeal of this Article TWELFTH, nor the adoption of any provision of this Restated Certificate of Incorporation inconsistent with this Article TWELFTH, shall adversely affect any right or protection of an officer existing hereunder with respect to any act or omission occurring prior to such amendment, modification, repeal or adoption of an inconsistent provision. If the Delaware General Corporation Law is amended to authorize corporate action further eliminating or limiting the personal liability of officers, then the liability of an officer of the Corporation shall be eliminated or limited to the fullest extent permitted by the Delaware General Corporation Law, as so amended.”
3. This Certificate of Amendment was duly adopted by the Board of Directors of the Corporation and by the stockholders of the Corporation in accordance with Section 242 of the General Corporation Law of the State of Delaware.
EXECUTED, this [ ] day of [ ], 2023.
| Madrigal Pharmaceuticals, Inc. | ||||||
| By: |
|
|||||
|
|
2023 PROXY STATEMENT | A-1 |


Madrigal Pharmaceuticals, Inc. Annual Meeting of Stockholders Please make your marks like this: X THE BOARD OF DIRECTORS RECOMMENDS A VOTE: FOR PROPOSALS 1, 2, 3 AND 4 BOARD OF DIRECTORS PROPOSAL YOUR VOTE RECOMMENDS 1. Re-election of Class I Directors FOR WITHHOLD 1.01 Paul A. Friedman, M.D. FOR P2 P2 1.02 Kenneth M. Bate FOR P3 P3 1.03 James M. Daly FOR P4 P4 FOR AGAINST ABSTAIN 2. To ratify the appointment of PricewaterhouseCoopers LLP as the Company’s independent FOR registered public accounting firm for the fiscal year ending December 31, 2023. P5 P5 P5 3. Advisory vote to approve executive compensation. FOR P6 P6 P6 4. Amendment of Restated Certificate of Incorporation to reflect new Delaware law provisions FOR regarding officer exculpation. P7 P7 P7 To adjournments transact such or postponements other business that thereof is properly and use presented the discretion at the to meeting vote on and such any other matters as may be properly brought beforethe 2023 Annual Stockholder Meeting. Authorized Signatures - Must be completed for your instructions to be executed. Please sign exactly as your name(s) appears on your account. If held in joint tenancy, all persons should sign. Trustees, administrators, etc., should include title and authority. Corporations should provide full name of corporation and title of authorized officer signing the Proxy/Vote Form. Signature (and Title if applicable) Date Signature (if held jointly) Date