Exhibit 15.2


| GSK GSK will unite science, talent and technology to get Ahead of disease Together. We will prioritise innovation in vaccines and specialty medicines, maximising the increasing opportunities to prevent and treat disease. Step change in growth – Expected sales growth of more than 5% and adjusted operating profit growth of more than 10% on a compound basis 2021-26 – R&D focused on the science of the immune system, human genetics and advanced technologies – Positively impacting the health of more than 2.5 billion people over ten years – Leading ESG performance to be maintained  |
Haleon Haleon will be a global leader 100% focused on consumer health. It will have a clear purpose to deliver better everyday health with humanity, and a focused strategy to deliver sustainable above-market growth and attractive returns to shareholders. Strong prospects for growth – Exceptional portfolio of category-leading brands with attractive global footprint and competitive capabilities – Compelling strategy to outperform in a growing, £150 billion plus sector which is more relevant than ever – 4-6% annual organic sales growth in the medium term, sustainable moderate margin expansion and high cash conversion – Attractive growth profile with capacity to invest and deliver shareholder returns  |
| Contents |
||||||||||
| Strategic report |
93 |
168 | ||||||||
01 |
94 |
172 | ||||||||
02 |
95 |
|||||||||
03 |
96 |
|||||||||
05 |
252 | |||||||||
07 |
99 |
| ||||||||
10 |
102 |
Investor information |
||||||||
11 |
103 |
258 | ||||||||
12 |
104 |
263 | ||||||||
13 |
116 |
269 | ||||||||
17 |
117 |
|||||||||
29 |
|
272 | ||||||||
34 |
Remuneration report |
275 | ||||||||
41 |
120 |
288 | ||||||||
44 |
125 |
290 | ||||||||
46 |
143 |
291 | ||||||||
55 |
144 |
291 | ||||||||
| |
|
292 | ||||||||
| Corporate governance |
Financial statements |
294 | ||||||||
| 83 |
296 | |||||||||
| 89 |
154 |
299 | ||||||||
| 92 |
311 | |||||||||

– |
£34.1 billion Group turnover stable at AER, +5% CER |
– |
Pharmaceuticals £17.7 billion +4% AER, +10% CER; new and specialty medicines £10 billion +20% AER, +26% CER |
– |
Vaccines £6.8 billion -3% AER, +2% CER |
– |
COVID-19 solutions sales £1.4 billion |
– |
Consumer Healthcare £9.6 billion -4% AER, stable CER (+4% excluding brands divested/under review) |
– |
Total EPS 87.6p -24% AER, -13% CER |
– |
Adjusted EPS 113.2p -2% AER, +9% CER; contribution to growth from COVID-19 solutions +8% AER, +9% CER |
– |
Total operating profit £6.2 billion -20% AER, -9% CER |
– |
Adjusted operating profit £8.8 billion -1% AER, +9% CER |
– |
Dividend of 80p |
– |
Three major product approvals; 8 phase III starts; 22 vaccines and medicines in pivotal trials |
– |
Strong pipeline of 21 vaccines and 43 medicines, many of which offer potential best or first-in-class opportunities for patients |
– |
20+ deals executed securing access to novel clinical programmes including in immuno-oncology, immuno-neurology and flu, plus technologies that expand our capabilities in human genetics and artificial intelligence/machine learning (AI/ML) |
– |
New GSK investor update in June 2021 set out our new purpose, growth commitments and R&D catalysts. For detail see gsk.com |
– |
Consumer Healthcare capital markets day in February 2022 highlighted our strategic priorities, key growth drivers and detailed financial information. For detail see gsk.com |
– |
1st in the pharmaceutical industry for Dow Jones Sustainability Index |
– |
1st in the Access to Medicine Index |
– |
Gold recognition in S&P’s Sustainability Yearbook |
– |
A- in CDP Climate Change |

 |
Sir Jonathan Symonds |
Chair |

 |
Emma Walmsley |
Chief Executive Officer |
Operating performance – 2021 |
|
|||||||||||
Turnover |
||||||||||||
2021 |
||||||||||||
£m |
Growth £% |
Growth CER% |
||||||||||
| Pharmaceuticals |
17,729 |
4 |
10 |
|||||||||
| Vaccines |
6,778 |
(3 |
) |
2 |
||||||||
| Consumer Healthcare |
9,607 |
(4 |
) |
– |
||||||||
| Group turnover |
34,114 |
– |
5 |
|||||||||
| Financial results |
|
|||||||||||
2021 |
||||||||||||
£m |
£% |
Growth CER% |
||||||||||
| Turnover |
34,114 |
– |
5 |
|||||||||
| Total operating profit |
6,201 |
(20 |
) |
(9 |
) | |||||||
| Total earnings per share |
87.6p |
(24 |
) |
(13 |
) | |||||||
| Adjusted operating profit |
8,806 |
(1 |
) |
9 |
||||||||
| Adjusted earnings per share |
113.2p |
(2 |
) |
9 |
||||||||
| Net cash from operating activities |
7,952 |
(6 |
) |
|||||||||
| Free cash flow |
4,437 |
(18 |
) |
|||||||||

| Adjusting items |
Total results £m |
Intangible asset amortisation £m |
Intangible asset impairment £m |
Major restructuring £m |
Transaction- related £m |
Divestments, significant legal and other items £m |
Separation costs £m |
Adjusted results £m |
||||||||||||||||||||||||
| Turnover |
34,114 |
34,114 |
||||||||||||||||||||||||||||||
| Cost of sales |
(11,603 |
) |
701 |
(33 |
) |
154 |
28 |
27 |
(10,726 |
) | ||||||||||||||||||||||
| Gross profit |
22,511 |
701 |
(33 |
) |
154 |
28 |
27 |
23,388 |
||||||||||||||||||||||||
| Selling, general and administration |
(10,975 |
) |
426 |
25 |
17 |
282 |
(10,225 |
) | ||||||||||||||||||||||||
| Research and development |
(5,278 |
) |
101 |
355 |
46 |
(4,776 |
) | |||||||||||||||||||||||||
| Royalty income |
419 |
419 |
||||||||||||||||||||||||||||||
| Other operating (expense)/income |
(476 |
) |
1,106 |
(662 |
) |
32 |
– |
|||||||||||||||||||||||||
| Operating profit |
6,201 |
802 |
322 |
626 |
1,159 |
(618 |
) |
314 |
8,806 |
|||||||||||||||||||||||
| Net finance costs |
(756 |
) |
2 |
1 |
(753 |
) | ||||||||||||||||||||||||||
| Share of after-tax profits of associates and joint ventures |
33 |
33 |
||||||||||||||||||||||||||||||
| Loss on disposal of interest in associates |
(36 |
) |
36 |
– |
||||||||||||||||||||||||||||
| Profit before taxation |
5,442 |
802 |
322 |
628 |
1,159 |
(581 |
) |
314 |
8,086 |
|||||||||||||||||||||||
| Taxation |
(346 |
) |
(159 |
) |
(81 |
) |
(114 |
) |
(196 |
) |
(470 |
) |
(49 |
) |
(1,415 |
) | ||||||||||||||||
| Tax rate |
6.4 |
% |
17.5 |
% | ||||||||||||||||||||||||||||
| Profit after taxation |
5,096 |
643 |
241 |
514 |
963 |
(1,051 |
) |
265 |
6,671 |
|||||||||||||||||||||||
| Profit attributable to non-controlling interests |
711 |
295 |
1,006 |
|||||||||||||||||||||||||||||
| Profit attributable to shareholders |
4,385 |
643 |
241 |
514 |
668 |
(1,051 |
) |
265 |
5,665 |
|||||||||||||||||||||||
| Earnings per share |
87.6 |
p |
12.9 |
p |
4.8 |
p |
10.3 |
p |
13.3 |
p |
(21.0 |
)p |
5.3 |
p |
113.2 |
p | ||||||||||||||||
2021 |
2020 |
|||||||||||||||||||||||
£m |
% of turnover |
£m |
% of turnover |
£% |
Growth CER% |
|||||||||||||||||||
| Turnover |
34,114 |
100 |
34,099 |
100 |
– |
5 |
||||||||||||||||||
| Cost of sales |
(10,726 |
) |
(31.4 |
) |
(10,191 |
) |
(29.9 |
) |
5 |
8 |
||||||||||||||
| Gross profit |
23,388 |
68.6 |
23,908 |
70.1 |
(2 |
) |
4 |
|||||||||||||||||
| Selling, general and administration |
(10,225 |
) |
(30.0 |
) |
(10,717 |
) |
(31.4 |
) |
(5 |
) |
(1 |
) | ||||||||||||
| Research and development |
(4,776 |
) |
(14.0 |
) |
(4,603 |
) |
(13.5 |
) |
4 |
8 |
||||||||||||||
| Royalty income |
419 |
1.2 |
318 |
0.9 |
32 |
32 |
||||||||||||||||||
| Operating profit |
8,806 |
25.8 |
8,906 |
26.1 |
(1 |
) |
9 |
|||||||||||||||||
| Net finance costs |
(753 |
) |
(844 |
) |
||||||||||||||||||||
| Share of after-tax profits of associates and joint ventures |
33 |
33 |
||||||||||||||||||||||
| Profit before taxation |
8,086 |
8,095 |
– |
11 |
||||||||||||||||||||
| Taxation |
(1,415 |
) |
(1,295 |
) |
||||||||||||||||||||
| Tax rate |
17.5 |
% |
16.0 |
% |
||||||||||||||||||||
| Profit after taxation |
6,671 |
6,800 |
(2 |
) |
9 |
|||||||||||||||||||
| Profit attributable to non-controlling interests |
1,006 |
1,031 |
||||||||||||||||||||||
| Profit attributable to shareholders |
5,665 |
5,769 |
||||||||||||||||||||||
| Earnings per share |
113.2 |
p |
115.9 |
p |
(2 |
) |
9 |
|||||||||||||||||

| Innovation We invest in scientific and technical excellence to develop and launch a pipeline of new products that meet the needs of our patients, payers and consumers. |
|
Performance We deliver growth by investing effectively in our business, developing our people and executing competitively. |
|
Trust We are a responsible company. We commit to use our science and technology to address health needs, make our products affordable and available and be a modern employer. | ||||
| 2021 objectives – Deliver Innovation sales with excellent commercial, R&D and supply chain execution in oncology, HIV and vaccines – Accelerate and strengthen pipeline with robust commercial input, including business development |
2021 objectives – Continue to prioritise spending to deliver growth and return on investment – Continue to deliver two-year programme to prepare GSK for separation into two new leading companies– Build a stronger, more diverse workforce for two new leading companies |
2021 objectives – Continue to deliver on-time, in-full supply of our products– Improve manager capability to motivate, focus, develop and care for people – Continue to deliver progress on Trust commitments | ||||||
| Progress – Received three major approvals in 2021: Apretude Jemperli Xevudy COVID-19 – Strong pipeline of 21 vaccines and 43 medicines, many of which offer potential best or first-in-class – 20+ deals executed securing access to novel clinical programmes including with iTeos in immuno-oncology, Alector in immuno-neurology and Vir Biotechnology in flu, plus technologies that expand our capabilities in human genetics and AI /ML |
Progress – Strong commercial execution across Pharmaceuticals, Vaccines and Consumer Healthcare – Pharmaceuticals £17.7 billion +4% AER, +10% CER with double-digit growth in new and specialty medicines +20% AER, +26% CER – Vaccines £6.8 billion -3% AER, +2% CER– Consumer Healthcare -4% AER, stable CER; -1% AER, +4% CER excluding divestments/brands under review– On track to deliver separation plans in mid-2022 |
Progress – Maintained sector-leading rankings in ESG indices, including the Dow Jones Sustainability Index, Access to Medicine Index and Antimicrobial Resistance Benchmark – Maintained supply and manufacturing without significant disruption throughout the pandemic – Made further progress to deliver on net zero impact on climate, and a net positive impact on nature by 2030 – Rolled out a new training programme to develop our managers to support them to be great managers and lead with care – Continued to prioritise diversity, with good progress made against our gender and ethnicity targets to improve representation in senior roles – WHO recommended wider use of our RTS,S vaccine for children in regions with moderate to high malaria transmission | ||||||
| 2022 priority objectives – Deliver Innovation sales with excellent commercial, R&D and supply chain execution – Further accelerate and strengthen pipeline with dedicated in-house expertise and robust commercial input, including optimised capital allocation and business development |
2022 priority objectives – Deliver more than 5% sales growth and more than 10% adjusted operating profit on a compound basis in the next five years – Continue to prioritise spending to deliver growth and return on investment – Deliver a successful demerger in mid-2022 |
2022 priority objectives – Deliver leading ESG performance and effective risk management with disciplined compliance |
| Culture As we move towards the creation of two new leading companies, we have been embedding a culture where we are all ambitious for patients, accountable for impact, and continue to do the right thing. We track our cultural change with a range of indicators, increasingly embedding assessments in HR processes, and the Board receives regular updates. See pages 99 and 102. Principal risks Our risk management framework is designed to support our long-term priorities. See pages 46 and 112. |
1 |
Innovation sales defined on page 12 |


Innovation |
2021 |
2020 |
2019 |
|||||||||
Innovation sales  |
||||||||||||
Pharmaceuticals and Vaccines – sales of products launched in the last five years |
£6.8bn |
1 |
£4.1bn |
2 |
£3.0bn |
2 | ||||||
Consumer Healthcare – sales from products which are new to a market in the last three years as a % of total sales |
10% |
11% |
12% |
|||||||||
Pipeline value and progress  |
n/r |
n/r |
n/r |
|||||||||
Performance |
2021 |
2020 |
2019 |
|||||||||
Group turnover  |
£34.1bn |
£34.1bn |
£33.8bn |
|||||||||
Profit  |
||||||||||||
Total operating profit – down 20% AER, down 9% CER |
£6.2bn |
£7.8bn |
£7.0bn |
|||||||||
Adjusted operating profit – down 1% AER, up 9% CER |
£8.8bn |
£8.9bn |
£9.0bn |
|||||||||
Total operating margin |
18.2% |
22.8% |
20.6% |
|||||||||
Adjusted operating margin |
25.8% |
26.1% |
26.6% |
|||||||||
Free cash flow  |
£4.4bn |
£5.4bn |
£5.1bn |
|||||||||
Market share |
n/r |
n/r |
n/r |
|||||||||
| Top talent and succession plans for key roles succession plans in place |
n/r |
n/r |
n/r |
|||||||||
Trust |
2021 |
2020 |
2019 |
|||||||||
Employee feedback |
78% |
84% |
78% |
|||||||||
Supply service level on-time, in-full |
n/r |
n/r |
n/r |
|||||||||
| Corporate reputation globally and in top 13 markets |
n/r |
n/r |
n/r |
|||||||||
 |
Linked to Executive LTI awards and annual bonus, see pages 120, 129 and 131 |
1 |
2021 includes products that have benefited from significant lifecycle innovation |
2 |
Comparative information reflects sales of those products that meet the definition for 2020 |
n/r |
Not reported externally due to commercial sensitivities |
1 |
IMF, World Economic Outlook: Recovery During a Pandemic, October 2021 |
2 |
IQVIA, The Global Use of Medicines 2022, January 2022 |
3 |
IQVIA, Global Medicine Spending and Usage Trends Outlook to 2025, April 2021 |

1 |
World Economic Forum, From zero COVID-19 vaccines to 11.2 billion in a year, 4 January 2022 |
2 |
Our World in Data, Coronavirus Vaccinations, as at 19 January 2022 |
3 |
IQVIA, Drug Expenditure Dynamics 1995-2020, October 2021 |
4 |
ABPI, Economic and Societal Impacts of Vaccination, 2020 |
5 |
H.R.5376—Build Back Better Act, 117th Congress, 2021-2022 |
6 |
PharmaExec.com, China 2021: The NRDL Readout, January 2022 |
 For more on pricing see our ESG Performance Report
For more on pricing see our ESG Performance Report 1 |
Digital Health Global Market Report 2021 - COVID 19 Growth and Change, Research and Markets, March 2021 |
2 |
United Nations, World Population Prospects 2019 (Revised), 2019 |
3 |
Brookings, China’s influence on the global middle class, Homi Kharas and Meagan Dooley, October 2020 |

1 |
Lewis S & Maslin M, Five things you need to know about the Glasgow Climate Pact, World Economic Forum, 15 November 2020 |
2 |
Ritchie H and Roser M, CO2 emissions by fuel, Our World in Data, Last accessed 19 January 2022 |
| Pharmaceuticals and Vaccines highlights | ||
| – Strong pipeline of 21 vaccines and 43 medicines, many with the potential to be first or best-in-class – Approval in the US for Apretude – Xevudy COVID-19, approved or authorised for conditional/ temporary use in the US, UK, EU and over 12 other countries |
– Approval for Jemperli – Positive phase III data for daprodustat for patients with anaemia of chronic kidney disease – 20+ deals executed securing access to five novel clinical assets – Approximately 70% of our targets in research are genetically validated, and published scientific research shows that genetically validated targets are at least twice as likely to become medicines | |

– |
Benlysta |
– |
Nucala IL-5 biologic, which is now also approved in the US and Europe for severe eosinophilic asthma, hypereosinophilic syndrome, eosinophilic granulomatosis and polyangitis and chronic rhinosinusitis with nasal polyps. |
– |
Our shingles vaccine, Shingrix |
– |
Expansion of our clinical trial programme for Zejula |
– |
The contribution of Trelegy Ellipta Trelegy |

– |
COVID-19, for which we are working on both treatments and vaccines |
– |
RSV and respiratory conditions, through our efforts to develop RSV vaccines for the populations most at risk, as well as to develop future respiratory medicines |
– |
Hepatitis B, through our antisense oligonucleotide and vaccine technologies in development |
– |
Influenza, for which we are developing vaccines and antibodies |




| Phase III/Registration |
||||
| Bexsero |
Xevudy 1 (sotrovimab/VIR-7831) COVID-19 | |||
| COVID-19 (Medicago)1 vaccine 3 |
Blenrep 1 | |||
| COVID-19 (Sanofi)1 vaccine 3 |
Jemperli 1 (PD-1 antagonist) 1L endometrial cancer2 | |||
| COVID-19 (SK Bioscience)1 vaccine 3 |
letetresgene-autoleucel 1 (NY-ESO-1 2,6 | |||
| MenABCWY (1st gen) vaccine |
Zejula 1 | |||
| Menveo |
4527223 1 (AL001, anti-sortilin) frontotemporal dementia 2,7 | |||
| MMR (US) vaccine |
depemokimab 1 (LA anti-IL5 antagonist) asthma | |||
| Rotarix |
Nucala | |||
| RSV maternal 1, † vaccine |
otilimab 1 (aGM-CSF inhibitor) rheumatoid arthritis | |||
| RSV older adults 1 vaccine |
daprodustat (HIF-PHI) anaemia of chronic kidney disease | |||
| gepotidacin 1 (BTI inhibitor) uUTI and GC |
linerixibat (IBATi) cholestatic pruritus in primary biliary cholangitis | |||
| Phase II |
||||
| Malaria (fractional dose) 1 vaccine |
bepirovirsen 1 (HBV ASO) HBV | |||
| S. aureus 1 vaccine 4 |
3036656 1 (leucyl t-RNA inhibitor) tuberculosis | |||
| Shigella 1 vaccine |
3640254 (maturation inhibitor) HIV | |||
| Therapeutic HBV 1 vaccine 4 |
3810109 1 (broadly neutralising antibody) HIV | |||
| MenABCWY (2nd gen) vaccine 4 |
cobolimab 1 (TIM-3 antagonist) NSCLC | |||
| Varicella new strain vaccine |
||||
| Phase I |
||||
| C. difficile 1 vaccine |
3745417 (STING agonist) cancer | |||
| Klebsiella pneumoniae 1 vaccine |
3845097 1 (NY-ESO-1/TGFbR2 | |||
| SAM (COVID-19 model) vaccine |
3901961 1 (NY-ESO-1/CD8a | |||
| SAM (rabies model) vaccine |
4074386 1 (LAG3 antagonist) cancer | |||
| CMV vaccine |
4362676 1 (Mat2A inhibitor) cancer | |||
| BVL-GSK098 1 (ethionamide booster) tuberculosis |
4428859 1 (EOS - 448, TIGIT antagonist) cancer | |||
| VIR-2482 1 (neutralising monoclonal antibody) influenza 8 |
6097608 (CD96 antagonist) cancer | |||
| 2556286 1 (Mtb inhibitor) tuberculosis |
4527226 1 (AL101, anti-sortilin) neurodegenerative diseases | |||
| 3186899 1 (CRK-12 inhibitor) visceral leishmaniasis5 |
3858279 1 (anti-CCL17) osteoarthritis pain | |||
| 3494245 1 (proteasome inh) visceral leishmaniasis |
3915393 1 (TG2 inhibitor) celiac disease | |||
| 3882347 1 (FimH antagonist) uUTI |
1070806 (anti-IL18) atopic dermatitis | |||
| 3923868 (PI4k b |
3888130 1 (anti-IL7 ) multiple sclerosis | |||
| 4182137 1 (VIR-7832) COVID-19 4 |
4532990 1 (ARO-HSD siRNA) non-alcoholic steatohepatitis | |||
| 3739937 (maturation inhibitor) HIV |
2798745 1 (TRPV4 blocker) diabetic macular edema | |||
| cabotegravir (400 mg/ml formulation) HIV |
3884464 1 heart failure | |||
| 4004280 (capsid protein inhibitor) HIV |
||||
| 1 In-licence or other alliance relationship with third party. |
7 Phase III trial in patients with progranulin gene mutation |
NSCLC: non-small cell lung cancer; uUTI: uncomplicated urinary tract infection; GC: gonorrhea; SS: synovial sarcoma; MRCLS: myxoid/round cell liposarcoma | ||
| 2 Additional indications also under investigation |
8 GSK has exclusive option to co-develop post phase II | |||
| 3 GSK contributing pandemic adjuvant |
† Enrolment and vaccination stopped in February 2022. Further analysis to better understand safety data from these trials is ongoing | |||
| 4 In phase I/II trial | ||||
| 5 Transition activities underway to enable further progression by partner | ||||
| 6 In potentially registrational phase II trial |
Pharmaceuticals highlights |
Vaccines highlights | |
| – Total 2021 turnover £17.7 billion, +4% AER, +10% CER – Sales of new and specialty pharmaceuticals £10 billion +20% AER, +26% CER – Sales of Xevudy – Strong commercial execution of key growth products, including Trelegy Nucala – Better digital capabilities to support more effective engagement with healthcare professionals, higher productivity and a more efficient supply chain  Read more below Read more below |
– Total 2021 turnover £6.8 billion, -3% AER, +2% CER– COVID-19 pandemic sales for Vaccines £447 million including pandemic adjuvant sales of £444 million– Shingles: Shingrix – Meningitis: increased market share in the US for Bexsero Menveo – Maintained market share for key products despite significant disruption from COVID-19 – Excellent supply performance; our Shingrix – Accelerated our digital transformation, helping to drive data-driven decisions in manufacturing and supply  Read more on page 31 Read more on page 31 | |
 See Group financial review on page 62 for more detail
See Group financial review on page 62 for more detail 





| External benchmarking | ||
| We have maintained our acknowledged leadership in ESG, and this continues to be a key driver in our goal to deliver health impact and shareholder returns. Detailed below is how we perform in key ESG ratings that we are frequently asked about by investors. | ||
| – Dow Jones Sustainability Index (DJSI): |
– CDP: A- in Climate Change, B in Water, B in Forests (palm oil and timber) and Supplier Engagement Leader | |
| – S&P Global Sustainability Award: |
– Sustainalytics: | |
| – Access to Medicine Index (ATMI): |
– MSCI: – Vigeo Eiris: | |
| – FTSE4Good: |
||
| Using our science and technology to address health needs |
 |
| Commitment |
Progress in 2021 | |
| New medical innovations Develop differentiated, high-quality and needed medicines, vaccines and consumer healthcare products to improve health |
– 2021 saw three major approvals for medicines, eight phase III starts and have 64 vaccines and medicines in our pipeline. For more details, see the Innovation section on pages 17 to 28. | |
| Global health Improve global health impact through R&D for infectious diseases that affect children and young people in low-income countries, focusing on HIV, malaria and TB |
– Our commitment to improve global health impact through R&D for infectious diseases and access to medicines and vaccines has been recognised in the Access to Medicines Index (ATMI) where we have ranked number one for the last seven years, every year since its inception. – Our RTS,S/AS01e malaria vaccine is the first and only vaccine shown in long-term clinical trials to reduce malaria in children. In 2021, the WHO recommended broader deployment of the vaccine, to reduce illness and deaths in children in sub-Saharan Africa and other regions with moderate to high malaria transmission. This followed new data which showed that the vaccine, in combination with seasonal antimalarials, lowers clinical episodes of malaria, hospital admissions with severe malaria and deaths by around 70% compared to antimalarials alone. In December 2021, Gavi announced its decision to provide funding for the procurement and introduction of the vaccine into routine child immunisation programmes in Gavi eligible countries.– We made good progress in improving availability of age-appropriate HIV treatment options for children around the world. A generic dolutegravir dispersible tablet was made available in key sub-Saharan African countries, less than a year after US FDA approval of this treatment. This work was facilitated by our public-private partnership with the Clinton Health Access Initiative, Unitaid and two generic manufacturers: Mylan (now part of Viatris group) and Macleods.– Shigella is the second biggest cause of morbidity and mortality from diarrhoea worldwide after rotavirus, and no approved vaccine is widely available. In late 2021, the first subjects were vaccinated with our quadrivalent shigella vaccine candidate, in a first-time-in-human, – We have the richest pipeline focused on global health priority diseases in the industry, including ten medicines and vaccines currently in clinical development. – We launched a collaboration with Novartis in 2021, Project Africa Gradient, to support scientific research on the link between genetic diversity and patients’ response to malaria and tuberculosis drugs in three African regions. | |
| Health security Help the world to better prepare for future disease outbreaks with pandemic potential, and tackle antimicrobial resistance |
– We have taken a broad approach to developing COVID-19 solutions. To see how we have applied our science to finding COVID-19 innovations, see page 21.– We were one of five companies to sit on the Pandemic Preparedness Partnership Steering Group, convened by the UK Government in 2021, bringing together industry, international organisations and experts to advise G7 governments on how to speed up the response to a future pandemic. The Trinity Challenge, of which we were a founding member, also announced the winners of its inaugural competition to find innovative ways to better predict and prevent outbreaks of disease, using data and analytics. Winners included the VaccineLedger, which tracks vaccines from manufacture to patient, using blockchain technology. – Our commitment to preventing antimicrobial resistance (AMR) was recognised by the Access to Medicine Foundation’s AMR Benchmark, with GSK an industry leader for the third consecutive time in 2021. The benchmark highlighted in particular the diversity and depth of our R&D pipeline, particularly our AMR-relevant vaccines. | |

 |
For full details of our progress against these commitments, please see our ESG Performance Report | |
| GSK Annual Report 2021 35 |
| Making our products affordable and available |
 |
| Commitment |
Progress in 2021 | |
| Pricing Improve the health of millions of people each year by making our products available at responsible prices that are sustainable for our business |
– In developed markets, pricing of all our new products reflects the value they deliver to patients, healthcare systems and wider society compared to available alternatives, and supports our work to meet future healthcare needs. We offer patient support and, in the US during 2021, provided prescribed vaccines and medicines to more than 87,000 low-income uninsured, underinsured, and Medicare Part D patients through GSK and ViiV Healthcare’s Patient Assistance Programs Foundation.– For pricing in low income countries (LICs) and lower middle income countries (LMICs) we use innovative pricing structures to extend product reach. Our vaccines business has a tiered pricing model based on World Bank gross national income country classifications, and we do not file patents for our medicines or enforce historic patents in low-income countries LICs. | |
| Product reach Use access strategies to reach 800 million underserved people in lower income countries with our products by 2025 |
– Our access strategies continued to reach many more underserved people in lower income countries. We made good progress against our target in 2021, and have now reached over 323 million people with our products using access strategies. These strategies include our advanced market commitments to provide our vaccines to lower income countries through Gavi. Our partnership with Gavi includes supplying Cervarix Synflorix Rotarix | |
| – In 2021, we also made a commitment to supply Rotarix Synflorix | ||
| – ViiV Healthcare has voluntary licensing agreements with generic manufacturers. These have allowed at least 21.3 million people living with HIV across 119 LICs and LMICs access to a generic product containing dolutegravir by the end of 2021. | ||
| – We have donated over ten billion albendazole tablets, including 526.4 million in 2021, to support efforts to end lymphatic filariasis and control intestinal worms in school-age children. | ||
| Healthcare access Partner to improve disease prevention, awareness and access to healthcare services for 12 million people by 2025 |
– We have a number of partnerships with NGOs and multilateral organisations to improve disease prevention, awareness and access to healthcare services. By 2021, these programmes reached 13.9 million people. Over the next year we’re developing an ambitious global health strategy for GSK which will include setting a new target. – Our partnership with Save the Children increased its emergency preparedness and response capability, investing in data analytics and early-action protocols to provide efficient and timely healthcare in crises. Our partnerships with Save the Children, Amref Health Africa and CARE International have trained more than 108,000 front-line health workers since 2011. They reached over 17.3 million people with prevention and treatment for infectious diseases, plus providing maternal/child healthcare, vaccination, hygiene sanitation and nutrition. – ViiV Healthcare’s Positive Action programme aims to explore ways to support people-centred and community-led interventions to help meet the UN targets to end AIDS by 2030. In 2021, the programme reached approximately 274,000 people and funded 66 grants across 28 countries. | |
 |
For full details of our progress against these commitments, please see our ESG Performance Report |
36 |
GSK Annual Report 2021 |
| Being a modern employer |
 | |
| Commitment |
Progress in 2021 | |
| Engaged people Achieve and maintain a competitive employee engagement score by 2022 |
– In early 2022, we launched a new all-company survey focused on purpose, strategy, engagement and culture progress. Engagement remains high at 78% and above the general industry benchmark, settling back to 2019 levels after an extra boost during the early phases of the pandemic. | |
| Inclusion and diversity Accelerate our progress on inclusion and diversity, including aspirational targets for female and ethnically diverse representation in senior roles by the end of 2025, and recognition as a disability confident employer and in LGBT+ indices |
– Our aspiration is that women hold at least 45% of VP and SVP roles by the end of 2025. In 2021, women held 40% of roles at VP and above, up from 38% in 2020. The FTSE Women Leaders ranking showed that we are in the top 10% of FTSE 100 companies based on the proportion of women on our Board and in leadership positions 1 . We also published our fifth annual UK ‘gender pay gap’ report in 2021, which showed that we continue to outperform the national average.– Our aspiration is to have at least 30% ethnically diverse leaders in our roles at VP and above in the US and at least 18% in the UK, by the end of 2025. Our representation as at 31 December 2021 showed that we had 12.9% ethnically diverse leaders in VP and above roles in the UK, up from 11.1% in 2020. In the US, we had 27.1% ethnically diverse leaders in roles at VP and above, up from 23.2% in 2020. This progress is supported by our rigorous focus on equal employment opportunity. We have launched programmes such as Accelerating Difference – Ethnic Diversity, which supports the development of ethnically diverse employees, building on their strengths and addressing development gaps through individual and group coaching. From 2023 we will publish GSK’s ‘ethnicity pay gap’ data for the UK. | |
| – We have developed a three-year plan to increase our disability confidence. As part of this we have rolled out our workplace adjustments programme to our biggest markets, making it available to over 40% of our employee population so far. We also signed up to the International Labour Organization’s Global Business and Disability Network, to promote the inclusion of people with disabilities in workplaces. | ||
| – We continue to be recognised in global LGBT+ indices, including being designated as a Best Place to Work for LGBTQ+ Equality in the Human Rights Campaign Foundation’s 2021 Corporate Equality Index. | ||
| Health, wellbeing and development Be a leading company in how we support employee health, wellbeing and personal development |
– GSK’s Leadership Team has continued to oversee our COVID-19 response, including the health, wellbeing and engagement of our employees in all our locations. We continuously monitor the impact of COVID-19 on our employees and as public health vaccination programmes continue, we’re helping to educate and raise awareness about them. Where there are no public health vaccination programmes available, we have committed to offer vaccinations at minimal cost to our employees and their eligible dependents. | |
| – We continued to make mental health training available for all our employees, and 66% of managers have completed it since it launched in 2019. We make confidential support available through our global Employee Assistance Programme, and we successfully piloted a new wellbeing programme focused on resilience strategies and energy management and will continue to implement a global rollout in 2022. | ||
| – We run health and safety training for our people, which covers how to identify and take measures to reduce workplace risks. In 2021, our reportable injury and illness rate remained at 0.16 per 100,000 hours worked and there were no fatalities. | ||
| – All our employees have access to our internal development portal – the Keep Growing Campus. This offers extensive development courses, videos and articles on a range of topics, including decision making, building change capability, coaching, influencing others and health and wellbeing. In 2021, our people completed 84,493 leadership and business courses. | ||
1 |
Data on employees by gender (including total employees, Board and management) is provided in our non-financial information statement on page 54 |

 |
For full details of our progress against these commitments, please see our ESG Performance Report | |
| GSK Annual Report 2021 37 |
| Being a responsible business |
 |
| Commitment |
Progress in 2021 | |
| Reliable supply Commit to quality, safety and reliable supply of our products for patients and consumers |
– It’s a priority to make sure there is a high-quality and reliable supply of our products for patients and consumers. This has continued to be of high importance throughout the pandemic, which has put increased strain on global supply chains. For more on how we manage continuity of supply, see pages 31 and 33. – Our quality management systems allow for continuous improvement, helping us to keep up high standards for product quality and safety. In 2021, we had 171 external regulatory inspections at our manufacturing sites and local operating companies – many conducted virtually because of the pandemic. We respond to all inspection findings, no matter how minor. We also ran 1,833 quality audits of suppliers, and 312 audits of clinical trials run by, or on behalf of, GSK to assess their quality and safety. Where we find areas to improve, we create improvement plans and track their progress. | |
| Ethics and values Operate an ethical, values- driven culture, in which any issues are responded to swiftly and transparently |
– Everyone at GSK has to complete training on what the company expects from them. In 2021, we renamed this mandatory employee code of conduct training ‘Working at GSK’ and improved the content to focus on risk and compliance, as well as diversity and creating an inclusive workplace. In 2021, 99.4% of employees and 92.9% of contract workers completed this training. – Anyone inside or outside GSK can raise concerns or speak to an independent third party through our Speak Up reporting channels, confidentially or anonymously, without fear of retaliation. We continue to take every concern raised seriously, and review every report to identify whether we need to investigate formally. If investigations show an employee has breached our policies, we take action. | |
| – In 2021, we changed the way we report disciplinary data and expanded the scope to include cases which were initiated in previous years. In 2021, 2,065 employees had concerns raised against them, with an additional 757 employees with concerns raised from prior year’s open cases. We disciplined 1,176 employees (298 of whom initially had concerns raised in previous years), an increase from 2020 primarily driven by late completion of mandatory training. Of these, 265 either left voluntarily or were dismissed, and 923 received a written warning. In other cases, we took action short of a written warning. At the end of 2021, we had 427 cases awaiting investigation or a disciplinary decision. | ||
| – During 2021, we undertook an independent assessment of our approach to managing human rights, to help us better understand how we can continue to improve how we manage our priority human rights areas. The assessment showed that there is good understanding of our human rights impacts and we will be reviewing and addressing the findings in the year to come. | ||
| – How our third parties act can have a direct impact on us meeting our priorities. It is important to manage our relationships with them well, including the way we choose, contract and monitor them. Our Third-Party Oversight (TPO) programme evaluates and mitigates the risks introduced through engaging third-parties to provide goods or services for GSK. We complete assessments for the portion of our third parties that may present greater potential risk, for example, interactions with government officials or annual transfers of value above certain pre-defined limits. In 2021, we ran more than 12,800 assessments of these higher risk third parties across more than 20 risk areas, identifying over 55% as high-risk in one or more areas. Most of these third parties are goods and services providers (70%), contract manufacturers and external suppliers (2%) or distributors and wholesalers (9%). We are evaluating our TPO programme to simplify the upfront assessment and broaden its focus to risk management throughout the third-party relationship, using user feedback and findings from our ongoing monitoring. | ||
 |
For full details of our progress against these commitments, please see our ESG Performance Report |
38 |
GSK Annual Report 2021 |
| Being a responsible business continued | ||
| Commitment |
Progress in 2021 | |
Data and engagement |
– In 2021, we simplified our privacy notices and made them easier to access through a portal on all our websites. Privacy is a key part of the mandatory ‘Working at GSK’ annual training that all our people have to complete. This helps employees to understand that everyone at GSK is responsible for handling personal information in the right way. | |
| – Our patient panels give us insights and advice, as well as building trusting, long-term relationships with patients and carers that help us develop medicines that meet patients’ needs. In 2021, we ran panels in disease areas including cancer, rheumatoid arthritis and hepatitis B. | ||
| – As part of our commitment to data transparency for our clinical studies, we have published 2,776 clinical study reports and 6,239 summaries of results. We have listed 2,550 studies for data sharing via www.vivli.org and www.clinicalstudydatarequest.com. | ||
| – We want our clinical trials to be as representative and accessible as possible, reflecting the patient populations with the disease including age, race, ethnicity, sex and gender. Over the past five years, we have endeavoured to improve patient diversity in our clinical trials by implementing training and support to personnel at investigator sites including awareness training on conducting clinical trials in under served communities. In 2021, we formed a Global Demographics and Diversity team to coordinate our learning about epidemiology, burden of disease and health equity, and how they relate to age, sex, gender, race and ethnicity, so we can apply these lessons when planning our trials. | ||
Environment |
Climate | |
Have a net zero impact on climate and a net positive impact on nature by 2030 |
– To achieve our ambitious net zero goal we have set targets across our value chain carbon footprint. The targets have been accredited by the Science Based Targets Initiative as aligning to a 1.5 o C pathway. | |
| – In 2021, we reduced our operational carbon emissions (scope 1 and 2) by 15% compared to 2020, primarily through increased use of renewable energy 1 . In September 2021, we announced a £50 million investment in UK and US manufacturing sites to secure renewable power generation. This includes new wind turbines and a 20-year power purchase agreement to supply solar electricity for our Irvine facility in Scotland, and solar energy for our Oak Hill facility in New York. | ||
| – In 2020 (our latest available data), emissions from our suppliers, logistics and people using our products (scope 3) reduced by 8% reflecting the evolution of our product portfolio and reductions in business travel and commuting as a result of the pandemic. Our metered dose inhalers for asthma and COPD account for 40% of our carbon footprint so in 2021 we started an R&D programme to find a lower-impact propellant that could reduce emissions from them by about 90%. | ||
Nature | ||
| – Collaboration is an important part of our strategy and during the year we joined nine other global pharmaceutical companies to launch the Energize programme. This is the first collaboration of its kind to use the scale of a single industry’s global supply chain to drive greater use of renewable electricity. We were a Principal Partner of the UN Global Climate Change Conference (COP26) in Glasgow and we championed the need for action on climate and nature to protect health. We also joined the Health Systems Task Force of the Sustainable Markets Initiative to drive collective action in digital healthcare, supply chains and patient care pathways to accelerate the shift to net zero. | ||
| – We make our Climate-Related Financial Disclosure on pages 49 to 52 along with our energy and carbon emissions data. GSK’s carbon reduction pathway to become net zero by 2030 can be found on gsk.com. | ||
1 |
Energy and carbon emissions data is provided in our Climate-related financial disclosure on pages 49 to 52. |

 |
For full details of our progress against these commitments, please see our ESG Performance Report | |
| GSK Annual Report 2021 39 |
| Being a responsible business continued | ||
| Commitment |
Progress in 2021 | |
| Environment continued |
– We are involved in developing standardised guidance on measuring our impact on nature through working with the Science Based Targets for Nature Initiative and the Taskforce on Nature-related Financial Disclosures (TNFD). We will achieve our net nature positive goal by reducing our environmental impacts across water, materials and biodiversity and investing in protecting and restoring nature. | |
| – In 2021, we reduced overall water use in our operations by 16% compared to 2020, and by 21% in sites in high water stress regions. 91% of our sites are now good water stewards, in line with the Alliance for Water Stewardship’s definition. During the year, we joined the Water Resilience Coalition (WRC), partnering to develop our approach to water neutrality in water- stressed regions and to deliver water resilience projects on the ground. Our Cape Town site in South Africa is the first in our network to embark on the journey towards water neutrality, and we are working with the WRC and local partners to address shared water challenges by clearing alien plant species and replanting local flora to create greater resilience in the basin. | ||
| – In 2021, we reduced the waste from our sites by 7% and recovered 43% of these materials through circular routes like reuse or recycling. Consumer Healthcare launched 40 million recycle-ready toothpaste tubes in over 20 markets. | ||
| – In 2021, we piloted our approach to biodiversity at our Stevenage site in the UK, working in partnership with Kew Gardens to deliver a 39% increase of biodiversity at the site. We aim to have measurable and effective biodiversity plans in place across all GSK sites by 2025. | ||
| – In 2021, we joined the public-private Lowering Emissions by Accelerating Forest Finance (LEAF) coalition which contributes high-quality emissions reductions by supporting countries to protect their tropical forests from deforestation. | ||
 |
For full details of our progress against these commitments, please see our ESG Performance Report |
40 |
GSK Annual Report 2021 |
Consumer Healthcare |
| – Consumer Healthcare had 26 first-market launches for new innovations in 2021 |
– Committed to producing one billion recyclable toothpaste tubes by 2025 | |
| – Total 2021 turnover £9.6 billion -1% AER, +4% CER (excluding brands divested/under review) – E-commerce represented 8% of total sales – Delivered 3.7 billion consumer healthcare products |
– Significant investment in on-site solar power towards goal to source 100% of our electricity from renewable sources by 2025– Announced growth ambitions of 4-6% annual organic sales growth in the medium term, sustainable moderate margin expansion and high cash conversion | |
1 |
Therapeutic oral health segment |
2 |
Nicholas Hall’s DB6 Consumer Healthcare (OTC/VMS) Database, 2020 Store and E-commerce sales |

Consumer healthcare |
 |
See Group financial review on page 65 for more detail |
Consumer healthcare |
– |
Go beyond |
– |
Do what matters most |
– |
Keep it human |
– |
Environmenta |
– |
Social |
– |
Governance |

| | ||
| Patients and consumers Insights from patients and consumers enable us to develop products that better meet their needs. How we engage Advisory boards, disease-specific patient panels and Patient Advocacy Leaders Summits to provide patient insights. Engagement and support for patient groups (disclosed on GSK.com), and initiatives that empower patients to get involved in medicine development. Market research including consumer sensory labs. |
What matters to patients and consumers Differentiated product innovation based on patient and consumer needs. Access to a reliable supply of high-quality products. Pricing of healthcare products, particularly out-of-pocket What we’re doing Strengthening our pipeline of innovative products. Maintaining high standards for product quality and safety. Continuing to take a value-based approach to pricing to balance reward for innovation with access and affordability. | |
| | ||
Investors |
What matters to investors | |
| We maintain regular and constructive dialogue with investors to communicate our strategy and performance in order to promote investor confidence and ensure our continued access to capital. How we engage Ongoing communications including the AGM, quarterly results calls, in-person and virtual roadshows and detailed company information online.One-to-one Biennial investors and analysts perception study. |
Sustainable performance for long-term shareholder value. Understanding how our R&D strategy is successfully developing our pipeline. Commitment to strong management of ESG issues. What we’re doing Creating two new leading companies through demerger in 2022. Good financial performance and transparent reporting. Business and R&D updates and events on key pipeline milestones. Driving leading-edge ESG performance and a culture of ambition, accountability and responsibility. | |
| | ||
Healthcare professionals and medical experts |
What matters to HCPs and medical experts | |
| We work with healthcare professionals (HCPs) and medical experts to understand the patients’ journey, partner to resolve unmet medical needs and make sure that our products are used safely and effectively. How we engage Scientific dialogue to increase understanding of disease management and patient experience. Providing high-quality, balanced information about our vaccines and medicines. Collaborating on clinical trials and research. |
Access to product and scientific information. Responsible sales and marketing practices. Safety, efficacy and differentiated innovation. What we’re doing Increasing the use of digital channels to deliver more personalised and effective sharing of information to HCPs. Ensuring we attract and retain the best talent and uphold responsible sales and marketing standards. Using HCP insights on disease management and patient experience to inform the development of our vaccines and medicines. | |
| | ||
| R&D partners and academia |
What matters to R&D partners and academia | |
| We partner with scientific institutions, national health systems, academia and industry partners to help us develop the most effective vaccines and medicines to meet unmet patient needs. How we engage Collaborating with outstanding scientists at academic institutions to accelerate discovery and development of new vaccines and medicines. Licensing advanced technology and potential vaccines and medicines from biotechs. Establishing joint ventures to strengthen innovation and improve efficiency. |
Finding the right partner to identify and accelerate a potential vaccine or medicine to reach the patients that need it. Pushing the science and technology as far as it can go to advance human health. Dissemination and advancement of scientific knowledge. What we’re doing Working with world-leading experts at biotechs, research institutes and universities to improve drug and vaccine discovery to increase the productivity of our R&D pipeline. Collaborating with a broad range of partners to support our R&D focus on the science of the immune system, human genetics and advanced technologies (see pages 17 to 27). Supporting the advancement of scientific knowledge with our long-standing commitment to sharing research see page 39. | |
| | ||
| | ||
Governments and regulators |
What matters to governments and regulators | |
| We work with governments and regulators to advocate for policies that encourage innovation and promote efficient management of healthcare spending. How we engage Meeting with regulatory bodies throughout the development process to ensure high-quality new products. Engaging with government health agencies to demonstrate the value of our products for patients and economies. Working with governments to protect and strengthen the operating environment for life sciences innovation and new medicine and vaccine launches. Participating in international efforts to address global health threats, such as the pandemic. |
Investment in innovation and life sciences. Scientific funding and collaboration. Medicines pricing and reimbursement. Public health threats – COVID-19 and antimicrobial resistance (AMR).Investment in preventive health and strengthening health systems. What we’re doing Engaging in US policy pricing/reimbursement debates and, with phRMA, commenting on legislative proposals for healthcare reform. Partnering across industry and governments to tackle AMR. Engaging with governments, including the US, UK and EU regarding production and procurement of COVID-19 vaccines and treatments. | |
| | ||
NGOs and multilateral organisations |
What matters to NGOs and multilateral organisations | |
| We work with partners to improve access to healthcare services and our products, and to advocate for the policy environment in which we can be successful and deliver on our ambitions for patients. How we engage Working with non-governmental organisations (NGOs) and partners to research and develop products to address global health challenges.Collaborating with NGOs and generic manufacturers to sustainably supply our products to lower income countries. Partnering to strengthen health systems in lower income countries and drive progress on global health priorities. |
Access to vaccines and medicines. UN SDGs and WHO targets for specific disease areas. Universal health coverage and the future of health systems. Financing for global health, including COVID-19 solutions.What we’re doing Focusing on our unique role as a global health partner to develop products where we have scientific expertise. Partnering with organisations that have complementary capabilities and reach to create sustainable models that share risk, including our partnership with Gavi to support access to vaccines in lower income countries. Leveraging our community investment programmes to support our scientific expertise and deliver greater impact for patients. | |
| | ||
Suppliers |
What matters to suppliers | |
| We work with thousands of suppliers, large and small, who provide goods and services that support us in delivering a reliable supply of high-quality, safe products for our patients and consumers. How we engage Regular direct engagement with suppliers to ensure they support GSK’s strategies and targets. Engaging with suppliers through our Third-Party Oversight programme and by conducting in-depth audits.Participating in forums such as the Pharmaceutical Supply Chain Initiative and the Consumer Goods Forum to improve supply chain sustainability. |
Prompt payment to agreed terms. Understanding GSK policies to ensure compliance. Opportunities to innovate and grow the relationship. What we’re doing Engaging with suppliers to develop improvement plans and track progress when we identify areas for improvement. Providing proactive support through our third-party EH&S team in countries where our priority suppliers are located. | |
| | ||
Our people |
What matters to our people | |
| We involve and listen to our people to increase employee engagement, drive business performance and retain talented people. How we engage Regular interactive broadcast events with the GLT and other senior leaders. Facilitating dialogue and collaboration through our internal communications platforms, Works Councils, Employee Forums and Employee Resource Groups. Providing feedback to managers via the global all-company survey and One80 questions. |
Our purpose and being able to see the difference we make. Having a great line manager. Feeling understood and valued. Being part of an inclusive and diverse workplace. What we’re doing Fostering a culture of accountability and ambition, underpinned by integrity and humanity. Launched new leadership programmes to help managers motivate, focus, care for and develop their teams. Campaigns and programmes to support safety, mental wellbeing and enable work-life balance. Driving our diversity and inclusion activities in support of new aspirational targets. | |
| | ||

 COVID-19, see page 54. ARC report, see page 111. Internal control framework, see page 112. |
| 2021 Principal risks summary | ||||
| Risk |
Trend |
Assessment and mitigation activities | ||
Patient safety |
 |
The macro risk level is stable but remains challenging. Public awareness of drug safety has increased following media coverage of the safety and efficacy of COVID-19 vaccines and therapies in 2021. Misinformation and negative characterisations of the industry have fuelled vaccine hesitancy. Highly publicised information security threats and data breaches require us to consider how we securely collect safety information from external sources. | ||
 |
GSK’s risk exposure is stable. Our portfolio is evolving, with a greater focus on advanced therapy medicinal products that may require specialised pharmacovigilance. We need to carefully balance resources to execute routine pharmacovigilance while we manage change initiatives including the separation of the Consumer Healthcare business, the accelerated pace of drug development and the simplification of our safety processes. | |||
Product quality |
 |
The macro risk has increased following COVID-19, with regulators resuming multiple on-site inspections to check that product quality expectations are met. There continues to be a focus on data governance and data integrity requirements, and on evaluation of products for the presence of nitrosamines. | ||
 |
GSK’s risk exposure has increased, as we need to respond to the heightened inspectorate presence. We have launched inspection readiness programmes to ensure full preparedness. We have continued to invest in technology and digital platforms to further strengthen our controls around good data management practices. Governance and control strategies have been deployed for timely nitrosamine evaluations. All these mitigations will require focus and diligence as GSK undergoes significant organisational change. | |||
Financial controls and reporting |
 |
The external environment remains challenging due to political uncertainty, proposed increases in the obligations of directors and auditors, increasing threats of cyber attacks (information security) and fraud, and increasing environmental disclosure requirements. | ||
 |
GSK’s risk exposure has remained stable due to our ongoing focus on the resilience of personnel and the testing of our internal control framework. We implement optimal risk mitigation through transformational programmes, technology, centralised processes, and risk and control assessments, and maintain effective tax and treasury strategies. We continually strengthen our control frameworks and collaborate with external bodies on standard setting. | |||
Anti-bribery and corruption (ABAC) |
 |
The macro risk level for bribery and corruption remained unchanged in 2021. We continued to see the ongoing impact of the pandemic on governments, people and businesses; rigorous anti-bribery and corruption standards aided by improved technology; and continued enforcement with focus on third-party intermediaries. | ||
 |
GSK’s risk exposure is unchanged as we continuously improve our Anti Bribery and Corruption programme to ensure appropriate controls, training, capability building, awareness raising, strong monitoring and use of data analytics. | |||
Commercial practices |
 |
COVID-19 consequences continue to impact the macro level. Competitive pressure has increased in many therapy areas and market segments. Future innovation requires successful launches of key medicines and products. Vaccination rates have been impacted by accessibility and political issues. Governments remain focused on initiatives to drive medicine and vaccine costs down for consumers. | ||
 |
GSK’s risk exposure level remains stable due to our mature and robust control environment. We continue to evolve our commercial practices competitively. We have invested in new technologies that support virtual customer engagement. We maintain proportionate controls, training and monitoring for employees that engage with healthcare organisations and professionals. We train senior business leaders on delivering performance and managing risk. | |||
Non-promotional engagement |
 |
The macro environment for non-promotional activities and scientific engagement with HCPs and patients is stable. It continues to be characterised by complex, dynamic disease areas and treatments with increased patient-centric focus, increasing diversity of engagement platforms, and the continued increase in virtual engagements since the pandemic. | ||
 |
GSK’s risk exposure has remained stable. Our digital practices continued to develop and modernise, and we have applied our internal principles and policies, designed to mitigate risk, to this rapidly evolving environment. We have internal networks to foster collaboration and best practice sharing, as well as the identification of emerging risks associated with non-promotional activities, so we can conduct them in compliance with GSK’s values and policies, local laws and regulations. | |||

| Risk |
Trend |
Assessment and mitigation activities | ||
Privacy |
 |
The macro risk continues to increase, with priority GSK markets such as the UK, EU, US, China and India instituting new privacy laws, and court rulings invalidating established international data transfer mechanisms that international companies had relied on. The increasing trend for data sovereignty initially targeting tech companies could affect healthcare companies in their ability to drive medical innovation and to effectively operate internationally. | ||
 |
GSK’s risk exposure is increasing due to the impact of the unstable privacy regulatory environment preventing us from further standardising our privacy framework globally and due to the scale of the changes necessary to prepare for the creation of two new data-driven companies. | |||
Research practices |
 |
The macro risk level is unchanged. We always need to continually assess how we do R&D in the context of our future ambition, our benchmarks, and the evolving global regulations and quality standards. This is particularly vital when expectations change or there are country-specific requirements (Human Genetic Resources Administration of China, Schrems II). | ||
 |
GSK’s risk exposure is unchanged, as laws and regulations are continually evolving. When regulations change, the accountable R&D function develops an action plan which can include risk and impact assessments to determine how the internal control framework needs to change to meet the new requirements. R&D regularly scans the external environment through membership of professional organisations and consortiums, attendance at industry or agency-sponsored meetings and review of publicly posted regulatory/legal reports. | |||
Environment, health and safety (EHS) |
 |
The macro risk level is unchanged as COVID-19 protocols have been embedded in our ways of working. Site staffing has moved from essential workers only to mostly full staffing. This has meant we have been able to resume more consistent management oversight and on-site global support through senior leaders, subject matter experts and audit teams. | ||
 |
GSK’s risk exposure has levelled out due to consistent work practices related to COVID-19 control measures. However, organisational change continues to be a factor. We have placed continued focus on safety leadership training, embedding our Life Saving Rules, and adhering to our EHS standards. | |||
Environmental sustainability |
 |
The macro risk level continues to increase. Investors, regulators and other stakeholders expect companies to understand and actively reduce the environmental footprint of their operations across their value chain, and to mitigate the impacts climate change could have on their operations and supply chains. | ||
 |
GSK’s risk exposure is unchanged. We set ambitious new environmental sustainability goals at the end of 2020 and have established an enterprise transformation programme addressing climate, water, waste and biodiversity across our operations. We also increased the scope and depth of our Task Force on Climate-related Financial Disclosures (TCFD) analysis, and continued to monitor trends in physical, reputational and regulatory risks from climate change impacts. | |||
Information security |
 |
The macro risk level continues to rise, as large multinationals increase their digital footprints and threats from hackers become more sophisticated. Risks identified as increasing during the pandemic have levelled off but continue to be an ongoing threat. At the same time, governments are tightening the regulatory frameworks, and we can expect enforcement to increase. | ||
 |
GSK’s risk exposure has increased. The targeting of pharmaceutical and vaccine intellectual property, and of third-party service availability, has intensified. In response, our cyber security programme continues to improve our controls to increase our cyber threat intelligence capabilities and protect critical information and systems, including operational technology and networks. | |||
Supply continuity |
 |
The macro risk level remains high due to the ongoing impact of the pandemic on product supply. There is also continuing potential for increasing protectionism, and Brexit uncertainty. Our COVID Issues Management Team is actively managing supply risk and mitigation on an ongoing basis. | ||
 |
GSK’s risk exposure has stabilised. Our Procurement Task Force, a cross-functional group from Procurement and Supply Chain, is accountable for the identification and management of potential bottlenecks in the supply of components. | |||
Transformation and separation |
 |
The macro risk level is unchanged and remains challenging as we set up two new companies in a highly competitive external labour market. | ||
 |
GSK’s risk exposure level remains unchanged. Our transformation and separation projects have progressed as planned throughout 2021, with employee engagement remaining a priority. | |||
– |
The GSK Sustainability Council chaired by Regis Simard which includes leaders from business units and global functions, including manufacturing, R&D, procurement and facilities management, ethics and compliance and finance, who all play a key role in delivering our environmental strategy. The Council is supported by a dedicated Programme Steering Team, which is run by the Global Sustainability Team who also provide specialist expertise and advice to the business. |
– |
The Programme Steering Team who co-ordinate the sustainability programme and associated workstreams and have oversight for monitoring performance and progress of the enablers to deliver the sustainability programme. |
– |
The Capital Allocations Board (CAB) which includes the CFO and Group Financial Controller who review climate-related capital expenditure as part of their annual planning and capital allocation process. |
– |
The Finance Sustainability Network includes leaders from across Finance, Sustainability and Procurement and focuses on key financial enablers to deliver the sustainability programme. |
– |
business-as-usual 3-5°C of warming by 2100. |
– |
low-carbon future: assumes that the global temperature increase by 2100 is limited to well below 2°C by rapid changes in legislation and technology. |
1 |
https://www.gsk.com/media/7180/gsk-carbon-glidepath-010921.pdf 2” |
2 |
Scenarios are based on IPPC Representative Concentration Pathways 2.6, 4.5 and 8.5, the IEA World Energy Outlook 2018 New Policy Scenario, Current Policy Scenario and Sustainable Development Scenario; and data sets from WWF and WRI for water stress and flood risk modelling |

| Physical risk/ description |
Scenario |
Risk management |
Potential profit impact/ timeframe |
Metrics |
Targets | |||||
| Increasing levels of water stress which reduces the availability of water for our operations. GSK uses freshwater as the main source of water to manufacture medicines, vaccines, and consumer health products. If water availability was restricted at a factory then production operations would be interrupted. |
BAU and low carbon |
We have performed water stewardship risk assessments for all our manufacturing sites and we have identified ten sites in our current network that are currently in areas of high-water risk. We are developing plans for these sites to become water neutral by 2030 and will partner with other organisations to address shared water challenges. We are currently piloting this approach in our Cape Town site working with partners including WWF and the Water Resilience Coalition. The TCFD process has helped us develop a watch list of additional sites potentially under long-term threat and we will monitor changes to the risk levels and update our site water risk assessments appropriately. |
Low: <£100m/ Long: 3-10 years |
Sites that have achieved water stewardship* Water use in our operations Sites and supplier sites that have achieved water neutrality |
Achieve good water stewardship at 100% of our sites by 2025 Reduce overall water use in our operations by 20% by 2030 Be water neutral in our own operations and at key suppliers in water stressed regions by 2030 | |||||
| Increasing frequency of extreme weather events causing disruption. Extreme weather events such as flooding, storms etc can result in short-term interruptions to manufacturing and other operations. |
BAU and low carbon |
We have performed risk assessments for our manufacturing and other operations and have business continuity plans in place which are reviewed annually to respond to the impact of extreme weather events including adopting appropriate mitigation plans. The TCFD process has helped us identify a watch list of sites that are in places where the flood risk is expected to increase over time. However, the risk from flooding remains very low. GSK has a well-established loss prevention and risk engineering programme to identify a range of risks that could impact our sites and where flood risks exist, we have taken action to mitigate the risk. |
Low: <£100m/ Long: 3-10 years |
Sites that have business continuity plans |
100% of sites have a response to extreme weather events in their business continuity plans | |||||
| An increased number of very hot days (>35°C) resulting in reduced productivity. Extreme heat could result in heat stress affecting our staff. |
BAU |
GSK has operations in countries that already experience very hot temperatures periodically. We already control the temperature and humidity inside our buildings. As part of our EHS control framework, sites conduct risk assessments on very hot days including adaptations for outside work. |
Low: <£100m/ Long: 3-10 years |
Scope 1, 2 and 3 carbon emissions |
Net zero emissions across all operations by 2030 Net zero emissions across our full value chain by 2030) | |||||
* |
As defined by the Alliance for Water Stewardship |
| Transitional risk/ description |
Scenario |
How the risk is managed |
Potential profit impact/ timeframe |
Metrics |
Targets | |||||
| Regulations governing the use of high global warming potential (GWP) substances are being updated in the UK, EU and US. This could lead to increasing cost and restrictions on the use of the high GWP propellant (HFA134a) in our Metered Dose Inhaler (MDI) products. |
BAU and low carbon |
We have started an R&D programme to find a lower-impact propellant that could reduce emissions from our metered dose inhalers by about 90%. We already have a portfolio of Dry Powder Inhaler products that do not use propellants that are not impacted by this risk. We are monitoring the evolving regulations governing the use of fluorinated gases and will review our assessments in future declarations. |
Medium: £100m to £300m/ Long: 3-10 years |
Scope 3 carbon emissions |
Net zero emissions across all operations by 2030 Net zero emissions across our full value chain by 2030) | |||||
| There is uncertainty over future regulatory policy responses to address climate change that countries around the world will develop including carbon pricing. We anticipate that carbon pricing on operational carbon emissions will come into force in some regions in the medium to long term which could increase our operating costs. |
Low carbon |
We are transitioning to 100% renewable electricity by 2025 and are starting to investigate options for renewable heat technology to reduce our carbon emissions from energy. Our sales fleet aim to transition to electric vehicles by 2030, further reducing our scope 1 carbon emissions. Shadow carbon pricing has been embedded in the capital investment process at $100 per tonne and is driving conversations and decisions around carbon emissions at all levels of the organisation. |
Low: <£100m/ Long: 3-10 years |
Scope 1&2 carbon emissions |
Net zero emissions across all operations by 2030 | |||||
| Opportunities |
Scenario |
How the opportunity is managed |
Potential profit impact/ timeframe |
Metrics |
Targets | |||||
| At COP26 in November 2021, more than 50 countries around the world committed to provide low carbon healthcare systems. This could lead to increasing demand for low carbon vaccines and medicines. |
BAU and low carbon |
We are reducing our own scope 1 & 2 carbon emissions which in turn reduces the scope 3 footprint of our customers and suppliers. We have started a new Eco-design programme to reduce the impacts of all our products and packaging.GSK have certified and published the carbon footprints of our portfolio of respiratory inhalers and have launched our first carbon neutral inhaler in the UK. This enables healthcare providers and patients make informed choices. We have started an R&D programme to find a lower-impact propellant that could reduce emissions from our metered dose inhalers by about 90%. |
Low: <£100m/ Long: 3-10 years |
Scope 1, 2 and 3 carbon emissions Total waste and non-circular waste |
Net zero emissions across our full value chain by 2030 Zero operational waste, including eliminating single-use plastics by 2030 25% environmental impact reduction for our products and packaging by 2030 10% waste reduction from supply chain by 2030 | |||||

Carbon emissions ‘000 tonnes CO 2 |
2021 |
2020 |
2019 |
|||||||
| Scope 1 emissions (from energy) |
393 |
415 |
416 |
|||||||
| Scope 1 emissions (other 3 ) |
288 |
349 |
382 |
|||||||
| Scope 2 emissions (market-based) |
159 |
227 |
518 |
|||||||
| Scope 3 emissions 4 |
Available in 2022 report |
13,427 |
14,260 |
|||||||
| UK Scope 1 & 2 emissions |
130 |
141 |
195 |
|||||||
Energy |
2021 |
2020 |
2019 |
|||||||
| Scope 1 and 2 emissions from energy/sales revenue (tonnes CO 2 e/£m) |
15.1 |
18.8 |
27.7 |
|||||||
| Scope 1 and 2 emissions from energy/FTE (tonnes CO 2 e/FTE) |
6.1 |
6.8 |
9.4 |
|||||||
| Total energy used (GWh) |
3,596 |
3,858 |
4,079 |
|||||||
| UK energy used (GWh) |
850 |
945 |
975 |
|||||||
1 |
Carbon emissions are calculated according to the Greenhouse Gas Protocol: A Corporate Accounting and Reporting Standard (revised edition). GSK uses market-based Scope 2 emissions for reporting purposes and reports Scope 3 emissions across all 15 categories in our ESG Performance Report. We ask external assurance providers, DNV, to provide limited assurance to ISAE 3000 for energy, Scope 1, 2 and selected Scope 3 carbon emission data. Methodologies for reporting and measurements are provided in our ESG Performance Report, on the KPI definitions page |
2 |
GSK asks DNV to provide limited assurance to ISAE 3000 for energy, Scope 1, 2 and selected Scope 3 carbon emissions, water, waste and wastewater data. Methodologies for reporting and measurements are provided in our ESG Performance Report, on the KPI definitions pages |
3 |
“Other” refers to emissions from sales force vehicles, propellant emissions released during manufacture of inhalers, on-site waste, or wastewater treatment and refrigerant gas losses |
4 |
We collect and publish scope 3 data across 15 categories. The most recent scope 3 data available is for 2020 as the process of compiling the 2021 data is not yet complete. We will publish this data once it becomes available and it will be included in the 2022 ESG Performance Report |
Risk management |
||

Description of the business model |
||
The value we create |
01 | |
Social matters |
||
Global health |
35 | |
Health security |
35 | |
Affordability and availability |
36 | |
Employees |
||
Employee engagement |
37 | |
Diversity |
37 | |
Wellbeing and development |
37 | |
Gender pay gap |
37 | |
| Ethics and values |
38 | |
Board diversity |
83 | |
| Human rights |
||
| Ethics and values |
38 | |
Data and engagement |
39 | |
Third parties |
38 | |
| Anti-corruption and bribery |
||
| Ethics and values |
38 | |
Reporting and investigating concerns |
38 | |
Anti-bribery and corruption |
47 | |
| Environmental matters |
||
Carbon, water and waste |
39 | |
Climate-related financial disclosure |
49 | |
Policy, due diligence and outcomes |
||
Summary of our principal risks |
47 | |
Principal risks and uncertainties |
275 | |
Viability statement |
53 | |
Audit & Risk Committee report |
111 | |
Non-financial key performance indicators |
||
Key performance indicators |
12 | |
Our policies |
||
All of our public policies, codes and standards are available on gsk.com |
||
Male |
Female |
Total |
||||||||||
| Board |
8 |
5 |
13 |
|||||||||
| Management* |
10,148 |
9,553 |
19,701 |
|||||||||
| All employees |
47,751 |
42,345 |
90,096 |
|||||||||
* |
Senior managers as defined in the Companies Act 2006 (Strategic Report and Directors’ Report) Regulations 2013 |
| Group financial review |
 | |||||||
| In this section |
||||||||
| 56 |
||||||||
| 60 |
||||||||
| 61 |
||||||||
| 70 |
||||||||
| 73 |
||||||||
| 74 |
||||||||
| 79 |
||||||||
| 80 |
||||||||
– |
amortisation of intangible assets (excluding computer software and capitalised development costs) |
– |
impairment of intangible assets (excluding computer software) and goodwill |
– |
Major restructuring costs, which include impairments of tangible assets and computer software, (under specific Board approved programmes that are structural, of a significant scale and where the costs of individual or related projects exceed £25 million) including integration costs following material acquisitions |
– |
transaction-related accounting or other adjustments related to significant acquisitions |
– |
proceeds and costs of disposals of associates, products and businesses; significant settlement income; significant legal charges (net of insurance recoveries) and expenses on the settlement of litigation and government investigations; other operating income other than royalty income, and other items including the impact of the revaluation of deferred tax assets and liabilities following enactment of the increase in the headline rate of UK corporation tax from 19% to 25% (effective 2023) |
– |
separation costs include costs to establish Consumer Healthcare as an independent business, as well as admission listing and demerger costs |
2021 |
2020 |
2019 |
2018 |
2017 |
||||||||||||||||
£m |
£m |
£m |
£m |
£m |
||||||||||||||||
| Total operating profit |
6,201 |
7,783 |
6,961 |
5,483 |
4,087 |
|||||||||||||||
| Intangible asset amortisation |
802 |
775 |
777 |
580 |
591 |
|||||||||||||||
| Intangible asset impairment |
322 |
263 |
83 |
116 |
688 |
|||||||||||||||
| Major restructuring |
626 |
1,532 |
1,105 |
809 |
1,056 |
|||||||||||||||
| Transaction-related items |
1,159 |
1,308 |
345 |
1,977 |
1,599 |
|||||||||||||||
| Divestments, significant legal and other items |
(618 |
) |
(2,823 |
) |
(299 |
) |
(220 |
) |
(119 |
) | ||||||||||
| Separation costs |
314 |
68 |
– |
– |
– |
|||||||||||||||
| US tax reform |
– |
– |
– |
– |
666 |
|||||||||||||||
| Adjusted operating profit |
8,806 |
8,906 |
8,972 |
8,745 |
8,568 |
|||||||||||||||
2021 |
2020 |
2019 |
2018 |
2017 |
||||||||||||||||
£m |
£m |
£m |
£m |
£m |
||||||||||||||||
| Novartis Consumer Healthcare Joint Venture put option |
– |
– |
– |
658 |
986 |
|||||||||||||||
| Contingent consideration on former Shionogi-ViiV Healthcare JV (including Shionogi preferential dividends) |
1,026 |
1,114 |
31 |
1,188 |
556 |
|||||||||||||||
| ViiV Healthcare put options and Pfizer preferential dividends |
48 |
(52 |
) |
(234 |
) |
(58 |
) |
(126 |
) | |||||||||||
| Contingent consideration on former Novartis Vaccines business |
27 |
172 |
76 |
58 |
101 |
|||||||||||||||
| Release of fair value uplift on acquired Pfizer inventory |
– |
91 |
366 |
– |
– |
|||||||||||||||
| Other adjustments |
58 |
(17 |
) |
106 |
131 |
82 |
||||||||||||||
| Transaction-related items |
1,159 |
1,308 |
345 |
1,977 |
1,599 |
|||||||||||||||

2021 |
2020 |
|||||||
£m |
£m |
|||||||
| Contingent consideration at beginning of the year |
5,359 |
5,103 |
||||||
| Remeasurement through income statement |
1,026 |
1,114 |
||||||
| Cash payments: operating cash flows |
(721 |
) |
(751 |
) | ||||
| Cash payments: investing activities |
(105 |
) |
(107 |
) | ||||
| Contingent consideration at end of the year |
5,559 |
5,359 |
||||||
2021 |
2020 |
|||||||
£m |
£m |
|||||||
| Pfizer put option |
1,008 |
960 |
||||||
| Pfizer preferential dividend |
– |
1 |
||||||

£34.1bn |
AER growth 0% |
CER growth 5% |

£6.2bn |
AER growth (20)% |
CER growth (9)% |
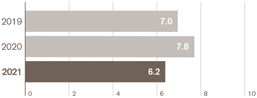
£8.8bn |
AER growth (1)% |
CER growth 9% |
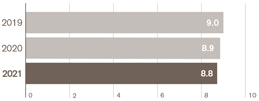
2021 |
2020 |
Growth |
||||||||||||||||||||||
£m |
% of turnover |
£m |
% of turnover |
£% |
CER% |
|||||||||||||||||||
| Turnover |
34,114 |
100 |
34,099 |
100 |
– |
5 |
||||||||||||||||||
| Cost of sales |
(11,603 |
) |
(34.0 |
) |
(11,704 |
) |
(34.3 |
) |
(1 |
) |
2 |
|||||||||||||
| Selling, general and administration |
(10,975 |
) |
(32.1 |
) |
(11,456 |
) |
(33.6 |
) |
(4 |
) |
– |
|||||||||||||
| Research and development |
(5,278 |
) |
(15.5 |
) |
(5,098 |
) |
(15.0 |
) |
4 |
7 |
||||||||||||||
| Royalty income |
419 |
1.2 |
318 |
0.9 |
32 |
32 |
||||||||||||||||||
| Other operating (expenses)/income |
(476 |
) |
(1.4 |
) |
1,624 |
4.8 |
||||||||||||||||||
| Operating profit |
6,201 |
18.2 |
7,783 |
22.8 |
(20 |
) |
(9 |
) | ||||||||||||||||
| Net finance costs |
(756 |
) |
(848 |
) |
||||||||||||||||||||
| Share of after-tax profits of associates and joint ventures |
33 |
33 |
||||||||||||||||||||||
| Loss on disposal of interest in associates |
(36 |
) |
– |
|||||||||||||||||||||
| Profit before taxation |
5,442 |
6,968 |
(22 |
) |
(10 |
) | ||||||||||||||||||
| Taxation |
(346 |
) |
(580 |
) |
||||||||||||||||||||
| Profit after taxation for the year |
5,096 |
6,388 |
(20 |
) |
(9 |
) | ||||||||||||||||||
| Profit attributable to shareholders |
4,385 |
5,749 |
||||||||||||||||||||||
| Earnings per share (p) |
87.6p |
115.5 |
(24 |
) |
(13 |
) | ||||||||||||||||||
| Earnings per ADS (US$) |
2.42 |
2.98 |
||||||||||||||||||||||
2021 |
2020 |
Growth |
||||||||||||||||||||||
£m |
% of turnover |
£m |
% of turnover |
£% |
CER% |
|||||||||||||||||||
| Turnover |
34,114 |
100 |
34,099 |
100 |
– |
5 |
||||||||||||||||||
| Cost of sales |
(10,726 |
) |
(31.4 |
) |
(10,191 |
) |
(29.9 |
) |
5 |
8 |
||||||||||||||
| Selling, general and administration |
(10,225 |
) |
(30.0 |
) |
(10,717 |
) |
(31.4 |
) |
(5 |
) |
(1 |
) | ||||||||||||
| Research and development |
(4,776 |
) |
(14.0 |
) |
(4,603 |
) |
(13.5 |
) |
4 |
8 |
||||||||||||||
| Royalty income |
419 |
1.2 |
318 |
0.9 |
32 |
32 |
||||||||||||||||||
| Adjusted operating profit |
8,806 |
25.8 |
8,906 |
26.1 |
(1 |
) |
9 |
|||||||||||||||||
| Adjusted profit attributable to shareholders |
5,665 |
5,769 |
(2 |
) |
9 |
|||||||||||||||||||
| Adjusted earnings per share (p) |
113.2p |
115.9 |
(2 |
) |
9 |
|||||||||||||||||||

| 2021 £m |
2020 £m |
Growth £% |
Growth CER% |
|||||||||||||
| Pharmaceuticals |
17,729 |
17,056 |
4 |
10 |
||||||||||||
| Vaccines |
6,778 |
6,982 |
(3 |
) |
2 |
|||||||||||
| Consumer Healthcare |
9,607 |
10,033 |
(4 |
) |
– |
|||||||||||
34,114 |
34,071 |
– |
5 |
|||||||||||||
Corporate and other unallocated turnover |
– |
28 |
||||||||||||||
34,114 |
34,099 |
– |
5 |
|||||||||||||
| 2021 £m |
2020 £m |
Growth £% |
Growth CER% |
|||||||||||||
| US |
15,093 |
14,556 |
4 |
10 |
||||||||||||
| Europe |
7,838 |
8,164 |
(4 |
) |
(2 |
) | ||||||||||
| International |
11,183 |
11,379 |
(2 |
) |
4 |
|||||||||||
34,114 |
34,099 |
– |
5 |
|||||||||||||
| £17.7bn 52% of Group turnover |
AER growth 4% |
CER growth 10% |

| 2021 £m |
2020 (revised*) £m |
Growth £% |
Growth CER% |
|||||||||||||
| Respiratory |
2,863 |
2,360 |
21 |
28 |
||||||||||||
| HIV |
4,777 |
4,876 |
(2 |
) |
3 |
|||||||||||
| Immuno-inflammation |
885 |
727 |
22 |
29 |
||||||||||||
| Oncology |
489 |
372 |
31 |
37 |
||||||||||||
| Pandemic |
958 |
– |
– |
– |
||||||||||||
| New and Specialty |
9,972 |
8,335 |
20 |
26 |
||||||||||||
| Established Pharmaceuticals |
7,757 |
8,721 |
(11 |
) |
(6 |
) | ||||||||||
17,729 |
17,056 |
4 |
10 |
|||||||||||||
* |
GSK has reviewed the presentation of its pharmaceuticals products and from 1 January 2021 has moved sales of Arnuity Ellipta, Incruse Ellipta and Relvar/Breo Ellipta |

| £6.8bn 20% of Group turnover |
AER growth (3)% |
CER growth 2% |

2021 £m |
2020 £m |
Growth £% |
Growth CER% |
|||||||||||||
| Meningitis |
961 |
1,029 |
(7 |
) |
(2 |
) | ||||||||||
| Influenza |
679 |
733 |
(7 |
) |
(2 |
) | ||||||||||
| Shingles |
1,721 |
1,989 |
(13 |
) |
(9 |
) | ||||||||||
| Established Vaccines |
2,970 |
3,231 |
(8 |
) |
(4 |
) | ||||||||||
6,331 |
6,982 |
(9 |
) |
(5 |
) | |||||||||||
| Pandemic Vaccines |
447 |
– |
– |
– |
||||||||||||
6,778 |
6,982 |
(3 |
) |
2 |
||||||||||||
| £9.6bn 28% of Group turnover |
AER growth (4)% |
CER growth 0% |

2021 £m |
2020 £m |
Growth £% |
Growth CER% |
|||||||||||||
| Oral health |
2,732 |
2,753 |
(1 |
) |
5 |
|||||||||||
| Pain relief |
2,276 |
2,219 |
3 |
7 |
||||||||||||
| Vitamins, minerals and supplements |
1,512 |
1,506 |
– |
4 |
||||||||||||
| Respiratory health |
1,133 |
1,209 |
(6 |
) |
(1 |
) | ||||||||||
| Digestive health and other |
1,803 |
1,824 |
(1 |
) |
4 |
|||||||||||
9,456 |
9,511 |
(1 |
) |
4 |
||||||||||||
| Brands divested/under review |
151 |
522 |
(71 |
) |
(69 |
) | ||||||||||
9,607 |
10,033 |
(4 |
) |
– |
||||||||||||
2021 £m |
2020 £m |
Growth £% |
Growth CER% |
|||||||||||||
| US |
3,179 |
3,408 |
(7 |
) |
(1 |
) | ||||||||||
| Europe |
2,468 |
2,619 |
(6 |
) |
(3 |
) | ||||||||||
| International |
3,960 |
4,006 |
(1 |
) |
4 |
|||||||||||
9,607 |
10,033 |
(4 |
) |
– |
||||||||||||

| 2021 £m |
2020 £m |
Growth £% |
Growth CER% |
|||||||||||||
| Total cost of sales |
(11,603 |
) |
(11,704 |
) |
(1 |
) |
2 |
|||||||||
| Adjusted cost of sales |
(10,726 |
) |
(10,191 |
) |
5 |
8 |
||||||||||
| 2021 £m |
2020 £m |
Growth £% |
Growth CER% |
|||||||||||||
| Total selling, general and administration |
(10,975 |
) |
(11,456 |
) |
(4 |
) |
– |
|||||||||
| Adjusted selling, general and administration |
(10,225 |
) |
(10,717 |
) |
(5 |
) |
(1 |
) | ||||||||
| 2021 £m |
2020 £m |
Growth £% |
Growth CER% |
|||||||||||||
| Total research and development |
(5,278 |
) |
(5,098 |
) |
4 |
7 |
||||||||||
| Adjusted research and development |
(4,776 |
) |
(4,603 |
) |
4 |
8 |
||||||||||

Finance income |
2021 £m |
2020 £m |
||||||
| Interest and other income |
26 |
39 |
||||||
| Fair value movements |
2 |
5 |
||||||
28 |
44 |
|||||||
| Finance expense |
||||||||
| Interest expense |
(746 |
) |
(822 |
) | ||||
| Unwinding of discounts on provisions |
(2 |
) |
(3 |
) | ||||
| Remeasurements and fair value movements |
– |
(4 |
) | |||||
| Finance expense on lease liabilities |
(31 |
) |
(40 |
) | ||||
| Other finance expense |
(5 |
) |
(23 |
) | ||||
(784 |
) |
(892 |
) | |||||
| 2021 £m |
2020 £m |
|||||||
| UK current year charge |
132 |
30 |
||||||
| Rest of world current year charge |
1,044 |
1,177 |
||||||
| Charge in respect of prior periods |
172 |
66 |
||||||
| Total current taxation |
1,348 |
1,273 |
||||||
| Total deferred taxation |
(1,002 |
) |
(693 |
) | ||||
| Taxation on total profits |
346 |
580 |
||||||

| Adjusted results reconciliation 31 December 2021 |
Total results £m |
Intangible asset amortisation £m |
Intangible asset impairment £m |
Major restructuring £m |
Transaction- related £m |
Divestments, significant legal and other items £m |
Separation costs £m |
Adjusted results £m |
||||||||||||||||||||||||
| Turnover |
34,114 |
34,114 |
||||||||||||||||||||||||||||||
| Cost of sales |
(11,603 |
) |
701 |
(33 |
) |
154 |
28 |
27 |
(10,726 |
) | ||||||||||||||||||||||
| Gross profit |
22,511 |
701 |
(33 |
) |
154 |
28 |
27 |
23,388 |
||||||||||||||||||||||||
| Selling, general and administration |
(10,975 |
) |
426 |
25 |
17 |
282 |
(10,225 |
) | ||||||||||||||||||||||||
| Research and development |
(5,278 |
) |
101 |
355 |
46 |
(4,776 |
) | |||||||||||||||||||||||||
| Royalty income |
419 |
419 |
||||||||||||||||||||||||||||||
| Other operating (expense)/income |
(476 |
) |
1,106 |
(662 |
) |
32 |
– |
|||||||||||||||||||||||||
| Operating profit |
6,201 |
802 |
322 |
626 |
1,159 |
(618 |
) |
314 |
8,806 |
|||||||||||||||||||||||
| Net finance costs |
(756 |
) |
2 |
1 |
(753 |
) | ||||||||||||||||||||||||||
| Loss on disposal of interest in associates |
(36 |
) |
36 |
– |
||||||||||||||||||||||||||||
| Share of after-tax profits of associates and joint ventures |
33 |
33 |
||||||||||||||||||||||||||||||
| Profit before taxation |
5,442 |
802 |
322 |
628 |
1,159 |
(581 |
) |
314 |
8,086 |
|||||||||||||||||||||||
| Taxation |
(346 |
) |
(159 |
) |
(81 |
) |
(114 |
) |
(196 |
) |
(470 |
) |
(49 |
) |
(1,415 |
) | ||||||||||||||||
| Tax rate |
6.4% |
17.5% |
||||||||||||||||||||||||||||||
| Profit after taxation |
5,096 |
643 |
241 |
514 |
963 |
(1,051 |
) |
265 |
6,671 |
|||||||||||||||||||||||
| Profit attributable to non-controlling interests |
711 |
295 |
1,006 |
|||||||||||||||||||||||||||||
| Profit attributable to shareholders |
4,385 |
643 |
241 |
514 |
668 |
(1,051 |
) |
265 |
5,665 |
|||||||||||||||||||||||
| Earnings per share |
87.6p |
12.9p |
4.8p |
10.3p |
13.3p |
(21.0 |
)p |
5.3p |
113.2p |
|||||||||||||||||||||||
| Weighted average number of shares (millions) |
5,003 |
5,003 |
||||||||||||||||||||||||||||||
| Adjusted results reconciliation 31 December 2020 |
Total results £m |
Intangible asset amortisation £m |
Intangible asset impairment £m |
Major restructuring £m |
Transaction- related £m |
Divestments, significant legal and other items £m |
Separation costs £m |
Adjusted results £m |
||||||||||||||||||||||||
| Turnover |
34,099 |
34,099 |
||||||||||||||||||||||||||||||
| Cost of sales |
(11,704 |
) |
699 |
31 |
667 |
116 |
(10,191 |
) | ||||||||||||||||||||||||
| Gross profit |
22,395 |
699 |
31 |
667 |
116 |
23,908 |
||||||||||||||||||||||||||
| Selling, general and administration |
(11,456 |
) |
1 |
18 |
659 |
(23 |
) |
16 |
68 |
(10,717 |
) | |||||||||||||||||||||
| Research and development |
(5,098 |
) |
75 |
214 |
206 |
(4,603 |
) | |||||||||||||||||||||||||
| Royalty income |
318 |
318 |
||||||||||||||||||||||||||||||
| Other operating (expense)/income |
1,624 |
1,215 |
(2,839 |
) |
– |
|||||||||||||||||||||||||||
| Operating profit |
7,783 |
775 |
263 |
1,532 |
1,308 |
(2,823 |
) |
68 |
8,906 |
|||||||||||||||||||||||
| Net finance costs |
(848 |
) |
2 |
2 |
(844 |
) | ||||||||||||||||||||||||||
| Share of after-tax profits of associates and joint ventures |
33 |
33 |
||||||||||||||||||||||||||||||
| Profit before taxation |
6,968 |
775 |
263 |
1,534 |
1,308 |
(2,821 |
) |
68 |
8,095 |
|||||||||||||||||||||||
| Taxation |
(580 |
) |
(150 |
) |
(47 |
) |
(292 |
) |
(229 |
) |
17 |
(14 |
) |
(1,295 |
) | |||||||||||||||||
| Tax rate |
8.3% |
16.0% |
||||||||||||||||||||||||||||||
| Profit after taxation |
6,388 |
625 |
216 |
1,242 |
1,079 |
(2,804 |
) |
54 |
6,800 |
|||||||||||||||||||||||
| Profit attributable to non-controlling interests |
639 |
392 |
1,031 |
|||||||||||||||||||||||||||||
| Profit attributable to shareholders |
5,749 |
625 |
216 |
1,242 |
687 |
(2,804 |
) |
54 |
5,769 |
|||||||||||||||||||||||
| Earnings per share |
115.5p |
12.6p |
4.4p |
25.0p |
13.8p |
(56.5 |
)p |
1.1p |
115.9p |
|||||||||||||||||||||||
| Weighted average number of shares (millions) |
4,976 |
4,976 |
||||||||||||||||||||||||||||||
2021 |
2020 |
|||||||||||||||||||||||
| Cash £m |
Non- cash £m |
Total £m |
Cash £m |
Non- cash £m |
Total £m |
|||||||||||||||||||
| 2018 major restructuring programme (incl. Tesaro) |
18 |
9 |
27 |
105 |
210 |
315 |
||||||||||||||||||
| Consumer Healthcare Joint Venture integration programme |
173 |
11 |
184 |
298 |
28 |
326 |
||||||||||||||||||
| Separation Preparation restructuring programme |
371 |
59 |
430 |
625 |
216 |
841 |
||||||||||||||||||
| Combined restructuring and integration programme |
8 |
(23 |
) |
(15 |
) |
39 |
11 |
50 |
||||||||||||||||
570 |
56 |
626 |
1,067 |
465 |
1,532 |
|||||||||||||||||||
| 2021 £m |
2020 £m |
|||||||
| Pharmaceuticals |
233 |
671 |
||||||
| Vaccines |
(40 |
) |
214 |
|||||
| Consumer Healthcare |
196 |
374 |
||||||
389 |
1,259 |
|||||||
| Corporate and central functions |
237 |
273 |
||||||
| Total Major restructuring charges |
626 |
1,532 |
||||||
| 2021 £m |
2020 £m |
|||||||
| Cost of sales |
154 |
667 |
||||||
| Selling, general and administration |
426 |
659 |
||||||
| Research and development |
46 |
206 |
||||||
| Other operating income/(expense) |
- |
– |
||||||
| Total Major restructuring charges |
626 |
1,532 |
||||||

– |
Drive a common approach to R&D with improved capital allocation |
– |
Align and improve the capabilities and efficiency of global support functions to support new GSK |
– |
Further optimise the supply chain and product portfolio, including the divestment of non-core assets. |
– |
A strategic review of prescription dermatology is underway |
– |
Prepare Consumer Healthcare to operate as a standalone company |
Charge/(credit) |
2021 £m |
2020 £m |
||||||
| Contingent consideration on former Shionogi-ViiV Healthcare Joint Venture (including Shionogi preferential dividends) |
1,026 |
1,114 |
||||||
| ViiV Healthcare put options and Pfizer preferential dividends |
48 |
(52) |
||||||
| Contingent consideration on former Novartis Vaccines business |
27 |
172 |
||||||
| Release of fair value uplift on acquired Pfizer inventory |
– |
91 |
||||||
| Other adjustments |
58 |
(17) |
||||||
| Total transaction-related charges |
1,159 |
1,308 |
||||||
2021 £m |
2020 £m |
|||||||
| Net cash inflow from operating activities |
7,952 |
8,441 |
||||||
| Net cash inflow/(outflow) from investing activities |
(1,777) |
2,161 |
||||||
| Net cash outflow from financing activities |
(7,589) |
(10,132) |
||||||
| Increase in cash and bank overdrafts |
(1,414) |
470 |
||||||
| Cash and bank overdrafts at beginning of year |
5,262 |
4,831 |
||||||
| Increase in cash and bank overdrafts |
(1,414) |
470 |
||||||
| Exchange adjustments |
(29) |
(39) |
||||||
| Cash and bank overdrafts at end of year |
3,819 |
5,262 |
||||||
| Cash and bank overdrafts at end of year comprise: |
||||||||
| Cash and cash equivalents |
4,274 |
6,292 |
||||||
| Overdrafts |
(455) |
(1,030) |
||||||
3,819 |
5,262 |
|||||||
| 2021 £m |
2020 £m |
|||||||
| Free cash inflow |
4,437 |
5,406 |
||||||
| 2021 £m |
2020 £m |
|||||||
| Net cash inflow from operating activities |
7,952 |
8,441 |
||||||
| Purchase of property, plant and equipment |
(1,172) |
(1,226) |
||||||
| Purchase of intangible assets |
(1,759) |
(1,013) |
||||||
| Proceeds from sale of property, plant and equipment |
143 |
68 |
||||||
| Proceeds from disposal of intangible assets |
772 |
1,255 |
||||||
| Interest paid |
(786) |
(864) |
||||||
| Interest received |
27 |
39 |
||||||
| Dividends from associates and joint ventures |
9 |
31 |
||||||
| Contingent consideration paid (reported in investing activities) |
(114) |
(120) |
||||||
| Contribution from non-controlling interests |
7 |
3 |
||||||
| Distributions to non-controlling interests |
(642) |
(1,208) |
||||||
| Free cash flow |
4,437 |
5,406 |
||||||

| 2021 £m |
2020 £m |
|||||||
| Assets |
||||||||
| Non-current assets |
||||||||
| Property, plant and equipment |
9,932 |
10,176 |
||||||
| Right of use assets |
740 |
830 |
||||||
| Goodwill |
10,552 |
10,597 |
||||||
| Other intangible assets |
30,079 |
29,824 |
||||||
| Investments in associates and joint ventures |
88 |
364 |
||||||
| Other investments |
2,126 |
3,060 |
||||||
| Deferred tax assets |
5,218 |
4,287 |
||||||
| Derivative financial instruments |
18 |
5 |
||||||
| Other non-current assets |
1,676 |
1,041 |
||||||
| Total non-current assets |
60,429 |
60,184 |
||||||
| Current assets |
||||||||
| Inventories |
5,783 |
5,996 |
||||||
| Current tax recoverable |
486 |
671 |
||||||
| Trade and other receivables |
7,860 |
6,952 |
||||||
| Derivative financial instruments |
188 |
152 |
||||||
| Liquid investments |
61 |
78 |
||||||
| Cash and cash equivalents |
4,274 |
6,292 |
||||||
| Assets held for sale |
22 |
106 |
||||||
| Total current assets |
18,674 |
20,247 |
||||||
| Total assets |
79,103 |
80,431 |
||||||
| Liabilities |
||||||||
| Current liabilities |
||||||||
| Short-term borrowings |
(3,601) |
(3,725) |
||||||
| Contingent consideration liabilities |
(958) |
(765) |
||||||
| Trade and other payables |
(17,554) |
(15,840) |
||||||
| Derivative financial instruments |
(227) |
(221) |
||||||
| Current tax payable |
(489) |
(545) |
||||||
| Short-term provisions |
(841) |
(1,052) |
||||||
| Total current liabilities |
(23,670) |
(22,148) |
||||||
| Non-current liabilities |
||||||||
| Long-term borrowings |
(20,572) |
(23,425) |
||||||
| Corporation tax payable |
(180) |
(176) |
||||||
| Deferred tax liabilities |
(3,556) |
(3,600) |
||||||
| Pensions and other post-employment benefits |
(3,113) |
(3,650) |
||||||
| Other provisions |
(630) |
(707) |
||||||
| Derivative financial instruments |
(1) |
(10) |
||||||
| Contingent consideration liabilities |
(5,118) |
(5,104) |
||||||
| Other non-current liabilities |
(921) |
(803) |
||||||
| Total non-current liabilities |
(34,091) |
(37,475) |
||||||
| Total liabilities |
(57,761) |
(59,623) |
||||||
| Net assets |
21,342 |
20,808 |
||||||
| Total equity |
21,342 |
20,808 |
||||||


| 2021 £m |
2020 £m |
|||||||
| Cash, cash equivalents and liquid investments |
4,335 |
6,370 |
||||||
| Borrowings – repayable within one year |
(3,601 |
) |
(3,725 |
) | ||||
| Borrowings – repayable after one year |
(20,572 |
) |
(23,425 |
) | ||||
| Net debt |
(19,838 |
) |
(20,780 |
) | ||||
| 2021 £m |
2020 £m |
|||||||
| Bank balances and deposits |
2,825 |
3,000 |
||||||
| US Treasury and Treasury repo only money market funds |
54 |
317 |
||||||
| Liquidity funds |
1,395 |
2,975 |
||||||
| Cash and cash equivalents |
4,274 |
6,292 |
||||||
| Liquid investments – government securities |
61 |
78 |
||||||
4,335 |
6,370 |
|||||||
| 2021 £m |
2020 £m |
|||||||
| Cash and liquid investments |
4,335 |
6,370 |
||||||
| Gross debt – fixed |
(23,167 |
) |
(24,538 |
) | ||||
| – floating |
(1,006 |
) |
(2,612 |
) | ||||
| – non-interest bearing |
– |
– |
||||||
| Net debt |
(19,838 |
) |
(20,780 |
) | ||||
Movements in net debt |
||||||||
| 2021 £m |
2020 £m |
|||||||
| Net debt at beginning of year |
(20,780 |
) |
(25,215 |
) | ||||
| (Decrease)/increase in cash and bank overdrafts |
(1,414 |
) |
470 |
|||||
| (Decrease)/increase in liquid investments |
(18 |
) |
1 |
|||||
| Increase in long-term loans |
– |
(3,298 |
) | |||||
| Net repayment of short-term loans |
1,995 |
7,305 |
||||||
| Repayment of lease liabilities |
215 |
227 |
||||||
| Exchange movements |
314 |
(135 |
) | |||||
| Other movements |
(150 |
) |
(135 |
) | ||||
| Net debt at end of year |
(19,838 |
) |
(20,780 |
) | ||||
| 2021 £m |
2020 £m |
|||||||
| Total equity at beginning of year |
20,808 |
18,357 |
||||||
| Total comprehensive income for the year |
4,759 |
7,358 |
||||||
| Dividends to shareholders |
(3,999 |
) |
(3,977 |
) | ||||
| Ordinary shares issued |
21 |
29 |
||||||
| Changes in non-controlling interests |
– |
(131 |
) | |||||
| Transaction with non-controlling interest |
10 |
– |
||||||
| Share-based incentive plans |
367 |
381 |
||||||
| Tax on share-based incentive plans |
11 |
(4 |
) | |||||
| Contributions from non-controlling interests |
7 |
3 |
||||||
| Distributions to non-controlling interests |
(642 |
) |
(1,208 |
) | ||||
| Total equity at end of year |
21,342 |
20,808 |
||||||

| Total £m |
Under 1 yr £m |
1-3 yrs £m |
3-5 yrs £m |
5 yrs+ £m |
||||||||||||||||
| Loans |
23,296 |
3,399 |
5,624 |
2,800 |
11,473 |
|||||||||||||||
| Interest on loans |
7,603 |
686 |
1,194 |
1,038 |
4,685 |
|||||||||||||||
| Lease obligations |
1,015 |
203 |
305 |
166 |
341 |
|||||||||||||||
| Future finance charges |
153 |
25 |
41 |
30 |
57 |
|||||||||||||||
| Intangible assets |
12,082 |
583 |
1,013 |
1,914 |
8,572 |
|||||||||||||||
| Property, plant & equipment |
616 |
468 |
148 |
– |
– |
|||||||||||||||
| Investments |
146 |
45 |
61 |
40 |
– |
|||||||||||||||
| Purchase commitments |
484 |
360 |
115 |
8 |
1 |
|||||||||||||||
| Pensions |
44 |
44 |
– |
– |
– |
|||||||||||||||
| Total |
45,439 |
5,813 |
8,501 |
5,996 |
25,129 |
|||||||||||||||
| Total £m |
Under 1 yr £m |
1-3 yrs £m |
3-5 yrs £m |
5 yrs+ £m |
||||||||||||||||
| Guarantees |
12 |
9 |
2 |
– |
1 |
|||||||||||||||
| Other contingent liabilities |
114 |
13 |
12 |
31 |
58 |
|||||||||||||||
| Total |
126 |
22 |
14 |
31 |
59 |
|||||||||||||||

– |
Turnover |
– |
Taxation (Note 14) |
– |
Legal and other disputes (Notes 46 and 31) |
– |
Contingent liabilities (Note 34) |
– |
Pensions and other post-employment benefits (Note 30). |
– |
We have arrangements with certain indirect customers whereby the customer is able to buy products from wholesalers at reduced prices. A chargeback represents the difference between the invoice price to the wholesaler and the indirect customer’s contractual discounted price. Accruals for estimating chargebacks are calculated based on the terms of each agreement, historical experience and product growth rates |
– |
Customer rebates are offered to key managed care and Group Purchasing Organisations and other direct and indirect customers. These arrangements require the customer to achieve certain performance targets relating to the value of product purchased, formulary status or pre-determined market shares relative to competitors. The accrual for customer rebates is estimated based on the specific terms in each agreement, historical experience and product growth rates |
– |
The US Medicaid programme is a state-administered programme providing assistance to certain poor and vulnerable patients. In 1990, the Medicaid Drug Rebate Program was established to reduce state and federal expenditure on prescription drugs. In 2010, the Patient Protection and Affordable Care Act became law. We participate by providing rebates to states. Accruals for Medicaid rebates are calculated based on the specific terms of the relevant regulations or the Patient Protection and Affordable Care Act |
– |
Cash discounts are offered to customers to encourage prompt payment. These are accrued for at the time of invoicing and adjusted subsequently to reflect actual experience |
– |
We record an accrual for estimated sales returns by applying historical experience of customer returns to the amounts invoiced, together with market-related information such as stock levels at wholesalers, anticipated price increases and competitor activity. |
2021 |
2020 |
2019 |
||||||||||||||||||||||
£m |
Margin % |
£m |
Margin % |
£m |
Margin % |
|||||||||||||||||||
| Gross turnover |
19,928 |
100 |
20,035 |
100 |
18,471 |
100 |
||||||||||||||||||
| Market-driven segments |
(6,656 |
) |
(33 |
) |
(6,754 |
) |
(34 |
) |
(5,976 |
) |
(32 |
) | ||||||||||||
| Government mandated and state programmes |
(4,553 |
) |
(23 |
) |
(5,205 |
) |
(26 |
) |
(4,264 |
) |
(23 |
) | ||||||||||||
| Cash discounts |
(377 |
) |
(2 |
) |
(388 |
) |
(2 |
) |
(356 |
) |
(2 |
) | ||||||||||||
| Customer returns |
(117 |
) |
(1 |
) |
(117 |
) |
(1 |
) |
(141 |
) |
(1 |
) | ||||||||||||
| Prior year adjustments |
838 |
4 |
402 |
2 |
247 |
1 |
||||||||||||||||||
| Other items |
(621 |
) |
(3 |
) |
(522 |
) |
(2 |
) |
(579 |
) |
(3 |
) | ||||||||||||
| Total deductions |
(11,486 |
) |
(58 |
) |
(12,584 |
) |
(63 |
) |
(11,069 |
) |
(60 |
) | ||||||||||||
| Net turnover |
8,442 |
42 |
7,451 |
37 |
7,402 |
40 |
||||||||||||||||||

| Corporate Governance |
||||||||
| In this section |
||||||||
| 83 |
||||||||
| 89 |
||||||||
| 92 |
||||||||
| 93 |
||||||||
| 94 |
||||||||
| 95 |
||||||||
| 96 |
||||||||
| 99 |
||||||||
| 102 |
||||||||
| 103 |
||||||||
| 104 |
||||||||
| 116 |
||||||||
| 117 |
||||||||
| Board composition |
Board diversity |
International experience |
|
|||||||||||||||||||||||||
| Composition |
Gender |
|
|
|||||||||||||||||||||||||
Executive |
23% |
Male |
62% |
Global |
85 |
% |
||||||||||||||||||||||
Non-Executive |
77% |
Female |
38% |
US |
100 |
% |
||||||||||||||||||||||
| Tenure Non-Executive |
Ethnicity |
Europe EMAP |
77 69 |
% % |
||||||||||||||||||||||||
| Up to 3 years |
40% |
Ethnically diverse |
15% |
|||||||||||||||||||||||||
3-6 years |
20% |
White |
85% |
|||||||||||||||||||||||||
6-9 years |
30% |
|||||||||||||||||||||||||||
9-10 years |
10% |
 |
See more information on page 110 |
|||||||||||||||||||||||||
| , | ||
Sir Jonathan Symonds, CBE |
Skills and experience | |
Non-Executive Chair |
Jon has extensive international financial, life sciences and governance experience. | |
| Age: Nationality: Appointed: |
Jon served as an Independent Non-Executive Director of HSBC Holdings plc from April 2014, and as Deputy Group Chairman from August 2018, until his retirement from the Board in February 2020. He was previously Chairman of HSBC Bank plc, Chief Financial Officer of Novartis AG, Partner and Managing Director of Goldman Sachs, Chief Financial Officer of AstraZeneca plc, and a Partner at KPMG. His governance experience includes roles as Non-Executive Director and Chair of the Audit Committees of Diageo plc and QinetiQ Group plc and Non-Executive Chair of Proteus Digital Health Inc. | |
 |
Jon is a Fellow of the Institute of Chartered Accountants in England and Wales. | |
External appointments | ||
Non-Executive Director, Rubius Therapeutics, Inc; Non-Executive Director, Genomics England Limited having previously served as its Chairman; Member, European Round Table for Industry; Senior Advisor to Chatham House. | ||
| | ||
Dame Emma Walmsley |
Skills and experience | |
| Chief Executive Officer Age: Nationality: Appointed: Chief Executive Officer from 1 April 2017 |
Prior to her appointment as GSK’s CEO, Emma was the CEO of GSK Consumer Healthcare, a Joint Venture between GSK and Novartis, from its creation in March 2015. Emma joined GSK in 2010 from L’Oreal, having worked for 17 years in a variety of roles in Paris, London, New York and Shanghai. Emma was previously a Non-Executive Director of Diageo plc. | |
| Emma holds an MA in Classics and Modern Languages from Oxford University. | ||
External appointments | ||
| Independent director, Microsoft, Inc. | ||
| | ||
Iain Mackay |
Skills and experience | |
| Chief Financial Officer Age: Nationality: Appointed Chief Financial Officer from 1 April 2019 |
Prior to joining GSK, Iain was Group Finance Director at HSBC Holdings plc, a position he held for eight years. A chartered accountant, Iain has lived and worked in Asia, the US and Europe and before HSBC was at General Electric, Schlumberger Dowell and Price Waterhouse. Iain was previously a Trustee of the British Heart Foundation and Chair of its Audit and Risk Committee. | |
| Iain holds an MA in Business Studies and Accounting and holds an Honorary Doctorate from Aberdeen University in Scotland. | ||
Iain is a member of the Institute of Chartered Accountants of Scotland. | ||
| External appointments | ||
| Member, Court of the University of Aberdeen and Chair of its Remuneration Committee; Member, The 100 Group and Chair of its Stakeholder Communications and Reporting Committee. | ||
| | ||
Dr Hal Barron |
Skills and experience | |
| Chief Scientific Officer and President, R&D Age: Nationality: Appointed: Chief Scientific Officer and President, R&D from 1 April 2018 |
Prior to joining GSK, Hal was President, R&D at Calico LLC (California Life Company), an Alphabet-funded company that uses advanced technologies to increase understanding of lifespan biology. Prior to this, Hal was Executive Vice President, Head of Global Product Development, and Chief Medical Officer of Roche, responsible for all the products in the combined portfolio of Roche and Genentech. At Genentech, he was Senior Vice President of Development and Chief Medical Officer. Hal was a Non-Executive Director and Chair of the Science & Technology Committee at Juno Therapeutics, Inc until March 2018, when it was acquired by Celgene Corporation. Hal previously served as a Non-Executive Board Director of GRAIL, Inc and an Advisory Board Member of Verily Life Sciences LLC. | |
As announced on 19 January 2022 Tony Wood will succeed Hal as CSO and Head of R&D with effect from 1 August 2022. From that date, Hal will transition to a non-independent Non-Executive Director with additional responsibilities to support R&D. | ||
External appointments | ||
| Non-Executive Director of Altos Labs Inc; Associate Adjunct Professor, Epidemiology & Biostatistics, University of California, San Francisco. | ||
| | ||
| Key       | ||

| | ||
Charles Bancroft |
Skills and experience | |
Independent Non-Executive Director |
Charlie has a wealth of financial and management experience in global biopharma. Charlie retired from a successful career at Bristol Myers Squibb (BMS) in March 2020 where he held a number of leadership roles in commercial, strategy and finance. Beginning his career at BMS in 1984, he held positions of increasing responsibility within the finance organisation and had commercial operational responsibility for Latin America, Middle East, Africa, Canada, Japan and several Pacific Rim countries. He was appointed Chief Financial Officer in 2010, Chief Financial Officer and Executive Vice President, Global Business Operations in 2016 and Executive Vice President and Head of Integration and Strategy & Business Development in 2019. Charlie successfully steered BMS through a period of strategic transformation, including its recent $74 billion acquisition of Celgene. Charlie also served as a member of the Board of Colgate-Palmolive Company from 2017 until March 2020. | |
| Age: Nationality: Appointed:   | ||
External appointments | ||
Board Member, Kodiak-Sciences Inc; Board Member, BioVector Inc; Advisory Board Member, Drexel University’s LeBow College of Business. | ||
| The Board determined that Charlie has recent and relevant financial experience and agreed that he has the appropriate qualifications and background to be an audit committee financial expert. | ||
| | ||
Manvinder Singh (Vindi) Banga |
Skills and experience | |
Senior Independent Non-Executive Director |
Vindi has many years of commercial experience and a track record of delivering outstanding performance in highly competitive global consumer-focused businesses. | |
| Age: Nationality: Appointed: Senior Independent Non-Executive Director from 5 May 2016   |
Prior to joining GSK, Vindi spent 33 years at Unilever plc, where his last role (amongst several senior positions) was President of the Global Foods, Home and Personal Care businesses, and a member of the Unilever Executive Board. Vindi sat on the Prime Minister of India’s Council of Trade & Industry from 2004 to 2014 and was on the Board of Governors of the Indian Institute of Management (IIM), Ahmedabad. Vindi is also the recipient of the Padma Bhushan, one of India’s highest civilian honours. Vindi has been a Non-Executive Director of the Confederation of British Industry (CBI) and Thomson Reuters Corp, Chairman of the Supervisory Board of Mauser Group, Chairman of Kalle GmbH, Director of High Ridge Brands LLC, Member of the Indo UK CEO Forum, and Senior Independent Director of Marks & Spencer Group plc. | |
| External appointments | ||
Partner, Clayton Dubilier & Rice; Non-Executive Director, The Economist Newspaper Limited; Member, Holdingham International Advisory Board; Board Member, International Chamber of Commerce United Kingdom; Member, Governing Board of the Indian School of Business, Hyderabad; Member, Global Leadership Council of Saïd Business School, Oxford; Chair of the Board of Trustees, Marie Curie; Chairman, UK Government Investments. | ||
| | ||
Dr Anne Beal |
Skills and experience | |
| Independent Non-Executive DirectorAge: Nationality: Appointed:   |
Anne brings extensive healthcare experience to the Board as a physician and entrepreneur combined with a passion for patient advocacy. She is a recognised health policy expert in the development of global and national programmes for improving healthcare access for all patient groups and in ensuring the voice of patients is reflected in research programmes. Prior to her current roles, Anne spent six years at Harvard Medical School and Massachusetts General Hospital, where she was an instructor in paediatrics. She has also held leadership roles at the Commonwealth Fund and the Aetna Foundation. Anne was previously Deputy Executive Director and Chief Engagement Officer for The Patient-Centered Outcomes Research Institute in the U.S. and Chief Patient Officer and Global Head of Patient Solutions at Sanofi. | |
| External appointments | ||
Founder and CEO, AbsoluteJOI Skincare; Board Member, AcademyHealth; Board Member, Prolacta Bioscience. | ||
| | ||
Dame Vivienne Cox |
Skills and experience | |
| Independent Non-Executive Director & Workforce Engagement DirectorAge: Nationality: Appointed:   |
Vivienne has wide experience of business gained in the energy, natural resources and publishing sectors. She also has a deep understanding of regulatory organisations and government. Vivienne worked for BP plc for 28 years, in Britain and Continental Europe, in posts including Executive Vice President and Chief Executive of BP’s gas, power and renewable business and its alternative energy unit. Vivienne was previously a Non-Executive Director of BG Group plc and Rio Tinto plc, the Senior Independent Director of Pearson plc, Chairman of the Supervisory Board of Vallourec and the Lead Independent Director at the UK Government’s Department for International Development. Vivienne was made a Dame Commander of the Order of the British Empire (DBE) in the 2022 UK New Year’s Honours List for services to sustainability, diversity, and inclusion in business. | |
| External appointments | ||
| Chair Designate, Victrex plc; Non-Executive Director, Stena AB; Advisory Board Member, African Leadership Institute; Vice President, Energy Institute; Advisory Board Member, Montrose Associates; Investment Advisor, QantX Ventures; Chair, Rosalind Franklin Institute; Vice Chair, Saïd Business School, Oxford and Member of its Global Leadership Council; Patron, Hospice of St Francis. | ||
| | ||
| Key       | ||
| | ||
| Dr Harry (Hal) C Dietz Independent Non-Executive Director and Scientific & Medical ExpertAge: Nationality: Appointed:  |
Skills and experience | |
Hal brings extensive experience in the field of human genetics which is central to GSK’s approach to R&D. He is a former President of the American Society of Human Genetics and is recognised as the world’s leading authority on a genetic disorder known as Marfan Syndrome. He also brings experience in development of novel therapies, through his role as Founder of and Scientific Adviser to Blade Therapeutics, a biopharmaceutical company focused on disease-modifying treatments for fibrotic and neurodegenerative diseases. In total, Hal has authored 282 original publications in peer-reviewed journals across his career. | ||
| As a physician scientist, he has dedicated his entire career to the care and study of individuals with heritable connective tissue disorders with primary perturbations of extracellular matrix homeostasis and function. His lab has identified the genes for many of these conditions, for which he uses model systems to elucidate disease mechanisms. | ||
| Hal has received multiple prestigious awards including the Curt Stern Award from the American Society of Human Genetics, the Colonel Harland Sanders Lifetime Achievement Award in Medical Genetics, the Taubman Prize for excellence in translational medical science, the Harrington Prize from the American Society for Clinical Investigation and the Harrington Discovery Institute, the Pasarow Award in Cardiovascular Research, the InBev-Baillet Latour Health Prize from the country of Belgium, and the Research Achievement Award from the American Heart Association. | ||
| He is an inductee of the American Society for Clinical Investigation, American Association for the Advancement of Science, Association of American Physicians, National Academy of Medicine, and National Academy of Sciences. | ||
| External appointments | ||
| Victor A. McKusick Professor of Paediatrics, Medicine, and Molecular Biology & Genetics in the Department of Genetic Medicine, The Johns Hopkins University School of Medicine; Investigator, Howard Hughes Medical Institute; Founder and Scientific Advisor, Blade Therapeutics; Consultant and Chair of Scientific Advisory Board, Aytu Biopharma; Independent Chair, GSK’s Human Genetics Scientific Advisory Board. | ||
| | ||
| Lynn Elsenhans Independent Non-Executive DirectorAge: Nationality: Appointed:    |
Skills and experience | |
Lynn has a wealth of experience of running a global business and significant knowledge of the global markets in which GSK operates. | ||
| Lynn served as Chair, President and Chief Executive Officer of Sunoco Inc from 2009 to 2012. Prior to joining Sunoco in 2008 as President and Chief Executive Officer, Lynn worked for Royal Dutch Shell, which she joined in 1980, and where she held a number of senior roles, including Executive Vice President, Global Manufacturing from 2005 to 2008. Lynn was previously a Non-Executive Director of the First Tee of Greater Houston, Flowserve Corporation, the Texas Medical Center, and a Trustee of the United Way of Greater Houston. | ||
| External appointments | ||
| Non-Executive Director and Chair of the Governance and Corporate Responsibility Committee, Baker Hughes Company; Board Director and Chair of the Audit Committee, Saudi Aramco; Advisory Board Member, Johns Hopkins University Whiting School of Engineering; Member, Audit Committee Leadership Network. | ||
| | ||
| Dr Laurie Glimcher Independent Non-Executive Director and Scientific & Medical ExpertAge: Nationality: Appointed:   |
Skills and experience | |
Laurie brings scientific and public health expertise to the Board’s deliberations, and a wealth of global, publicly listed pharmaceutical business experience. | ||
| In addition to a number of senior leadership positions held at both Harvard Medical School and Harvard School of Public Health, Laurie has also served as Stephen and Suzanne Weiss Dean and Professor of Medicine at Weill Cornell Medical College and as an Attending Physician at the New York Presbyterian Hospital/Weill Cornell Medical Center. Laurie stepped down from the Board of Bristol-Myers Squibb (BMS) in 2017 after serving for 20 years on its Board. Laurie was previously a Non-Executive Director of the Waters Corporation and co-founder and Chair of the Scientific Advisory Board of Quentis Therapeutics Inc. | ||
External appointments | ||
Professor of Medicine, Harvard Medical School; CEO, President and an Attending Physician, Dana-Farber Cancer Institute. | ||
| Member, US National Academy of Sciences and the National Academy of Medicine; Member, Scientific Steering Committee of the Parker Institute for Cancer Immunotherapy; Independent Director, Analog Devices Inc; Director and Member of the Executive Committee, Breakthrough Cancer; Member, Scientific Advisory Boards of Repare Therapeutics Inc, Abpro Therapeutics, Kaleido Biosciences Inc, BioCentury Inc and Stand Up 2 Cancer. | ||
| | ||
Judy Lewent joined the Board on 1 April 2011. She retired from the Board on 5 May 2021. | ||
| Key       | ||

| Dr Jesse Goodman Independent Non-Executive Director and Scientific & Medical ExpertAge: Nationality: Appointed:   |
Skills and experience | |
Jesse brings scientific and public health expertise to the Board’s deliberations. He has a wealth of experience spanning science, medicine, vaccines, regulation and public health, and has a proven record in addressing pressing public health needs from both the academic and federal sectors. | ||
| Jesse previously served in senior leadership positions at the US Food and Drug Administration (FDA), including most recently as the FDA’s Chief Scientist and previously as Deputy Commissioner for Science and Public Health and as Director of the Center for Biologics Evaluation and Research (CBER). | ||
| Jesse played a leadership role in developing the FDA’s Regulatory Science and Medical Countermeasures Initiatives and has worked collaboratively with industry, academia, government and global public health and regulatory partners to prepare for and respond to major public health threats, including emerging infectious diseases, disasters and terrorism. He led the FDA’s response to West Nile Virus and to the 2009 H1N1 influenza pandemic and served on the Senior Leadership Team for the 2010 White House Medical Countermeasure Review. Jesse was previously a member of both the Scientific Advisory Committee and the Regulatory and Legal Working Group of the Coalition for Epidemic Preparedness Innovations (CEPI). | ||
| External appointments | ||
| Professor of Medicine and Attending Physician, Infectious Diseases, Georgetown University and directs the Georgetown University Center on Medical Product Access, Safety and Stewardship (COMPASS); Board Member (formerly President), United States Pharmacopeia (USP); Board Member, Scientific Counselors for Infectious Diseases, Centers for Disease Control and Prevention (CDC); Board Member, Intellia Therapeutics Inc; Member, US National Academy of Medicine; Board Member, Adaptive Phage Therapeutics, Inc. | ||
| | ||
| Urs Rohner Independent Non-Executive DirectorAge: Nationality: Appointed:   |
Skills and experience | |
Urs has a broad business, banking and legal background and extensive senior level experience at multinational companies. | ||
| Urs has served as Chairman on a number of Boards, most recently for Credit Suisse Group from 2011 until April 2021. Prior to joining Credit Suisse in 2004, Urs served as Chairman of the Executive Board and CEO of ProSieben and ProSiebenSat.1 Media AG. This followed a number of years in private practice at major law firms in Switzerland and the US, having been admitted to the bars of the canton of Zurich in Switzerland in 1986 and the state of New York in the US in 1990. | ||
| External appointments | ||
Member, International Advisory Board, Investcorp; Chair, Vega Cyber Associates AG. | ||
| | ||






| Skills and experience | ||
| Dr Hal Barron 1 Chief Scientific Officer and President, R&D |
Hal joined GSK and the GSK Leadership Team (GLT) in 2018. See Board biographies on pages 83 to 86. | |
| Roger Connor President, Vaccines and Global Health |
Roger joined the GLT in 2013. He was appointed President of GSK Vaccines in 2018. In addition to leadership of the Vaccines business, he leads GSK’s Global Health organisation since 2021 and is also responsible for GSK’s global procurement organisation. Roger is a member of the Board of Gavi, the Vaccine Alliance, and the Chair of the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA) CEO Vaccines Committee. Previously he was President, Global Manufacturing & Supply and, before that, Vice President, Office of the CEO and Corporate Strategy. Roger joined GSK in 1998 from AstraZeneca. Roger holds a degree in Mechanical and Manufacturing Engineering from Queen’s University, Belfast and a Master’s in Manufacturing Leadership from Cambridge University. He is a Chartered Accountant. | |
| Diana Conrad Chief People Officer |
Diana was appointed Chief People Officer and member of the GLT in April 2019. She was previously Senior Vice President, HR, Pharmaceuticals R&D from 2016 where she played a key strategic role as leader of the R&D people and culture agenda to support its transformation. Diana joined GSK Canada’s HR team in 2000 where she held several roles of increasing responsibility before becoming Senior Vice President, HR for Consumer Healthcare in 2009. Prior to joining GSK, she held HR roles in companies including GE Capital, Gennum Corporation and Zenon Environmental Laboratories. Diana has an Honours Bachelor of Arts from McMaster University in Canada. | |
| James Ford SVP and Group General Counsel, Legal and Compliance |
James joined the GLT in 2018, when he was appointed Senior Vice President and Group General Counsel, Legal and Compliance. He joined GSK in 1995 and has served as General Counsel Consumer Healthcare, General Counsel Global Pharmaceuticals, Vice President of Corporate Legal and was Acting Head of Global Ethics and Compliance. Prior to GSK, James was a solicitor at Clifford Chance and DLA. He holds a law degree from University of East Anglia and a Diploma in Competition Law from King’s College. He is qualified as a solicitor in England and Wales and is an attorney at the New York State Bar. James is based in London but has practised law and lived in the US, Singapore and Hong Kong. James is co-chair of the US-based Civil Justice Reform Group and a director of the European General Counsel Association. | |
| Sally Jackson SVP, Global Communications and CEO Office |
Sally joined the GLT in March 2019 as Senior Vice President, Global Communications and CEO Office. She leads our Communications and Government Affairs function globally and is also the CEO’s Chief of Staff. Prior to this, Sally was Senior Vice President Office of the CEO and CFO and she previously served as Head of Investor Relations. She joined GSK in 2001. Sally holds a degree in Natural Sciences from the University of Cambridge. | |
| Iain Mackay Chief Financial Officer |
Iain joined GSK and the GLT in 2019. See Board biographies on pages 83 to 86. | |
| Brian McNamara CEO, GSK Consumer Healthcare |
Brian is CEO, GSK Consumer Healthcare and CEO designate of the new Consumer Healthcare company, Haleon. He joined GSK in 2015 as Head of Europe and Americas for Consumer Healthcare and has led two successful Joint Ventures, first between GSK and Novartis and, more recently, with Pfizer. Previously, he was head of Novartis’ OTC division. Brian began his career at P&G. Brian is a Board member of the Consumer Goods Forum and a former Chairman and Board member of the Global Self-Care Federation (GSCF). He earned an undergraduate degree in Electrical Engineering from Union College in New York and an MBA in Finance from the University of Cincinnati. | |

| Skills and experience | ||
| Luke Miels Chief Commercial Officer |
Luke joined GSK and the GLT in 2017. As Chief Commercial Officer he is responsible for our commercial portfolio of medicines and vaccines. Luke also co-chairs the Portfolio Investment Board with Hal. | |
He previously worked for AstraZeneca as Executive Vice President of their European business and, prior to that, was Executive Vice President of Global Product and Portfolio Strategy, Global Medical Affairs and Corporate Affairs. Before that, he was head of Asia for Roche, based in Shanghai and then Singapore. Prior to that he held roles of increasing seniority at Roche and Sanofi-Aventis in the US, Europe and Asia. | ||
| Luke holds a Bachelor of Science degree in Biology from Flinders University in Adelaide and a MBA from the Macquarie University, Sydney. | ||
| Shobie Ramakrishnan Chief Digital and Technology Officer |
Shobie joined the GLT in 2021 when she was appointed Chief Digital and Technology Officer. She joined GSK in 2018 and has deep and broad experience in both biotech and hi-tech companies and, most recently, has led Digital and Technology for GSK’s Global Commercial organisation, transforming the company’s capabilities in digital, data and analytics and playing a pivotal role in establishing a more agile commercial operating model. Before joining GSK, Shobie held senior technology leadership roles in organisations including AstraZeneca, Salesforce, Genentech and Roche. She is a board member of Remediant and on the advisory board of Pistoia Alliance. | |
| Shobie holds a Bachelor’s degree in Electronics Engineering from Vellore Institute of Technology, University of Madras, India. | ||
| David Redfern Chief Strategy Officer |
David joined the GLT as Chief Strategy Officer in 2008 and is responsible for corporate development and strategic planning. Previously, he was Senior Vice President, Northern Europe with responsibility for GSK’s pharmaceutical businesses in that region and, before that, he was Senior Vice President for Central and Eastern Europe. He joined GSK in 1994. David was appointed Chairman of the Board of ViiV Healthcare Limited in 2011 and a Non-Executive Director of the Aspen Pharmacare Holdings Limited Board in 2015. | |
| He has a Bachelor of Science degree from Bristol University and is a Chartered Accountant. | ||
| Regis Simard President, Pharmaceuticals Supply Chain |
Regis joined the GLT in 2018, when he became President, Pharmaceuticals Supply Chain. He is responsible for the manufacturing and supply of GSK’s pharmaceutical products. He also leads Quality and Environment, Health, Safety and Sustainability at a corporate level. Regis joined GSK in 2005 as a Site Director in France, rising to become Senior Vice President of Global Pharmaceuticals Manufacturing before his current role. Previously, he held senior positions at Sony, Konica Minolta and Tyco Healthcare. He is a member of the Board of ViiV Healthcare. | |
| He is a mechanical engineer and holds an MBA. | ||
| Phil Thomson President, Global Affairs |
Phil joined the GLT in 2011. He was appointed President, Global Affairs in 2017, and has responsibility for the Group’s strategic approach to stakeholder engagement, reputation and policy development. Previously, Phil was Senior Vice President, Communications and Government Affairs. | |
Phil is Chair of The Whitehall & Industry Group and a Board member of the China–Britain Business Council. | ||
| He earned his degree in English, History and Russian Studies from Durham University. | ||
| Emma Walmsley Chief Executive Officer |
Emma joined GSK in 2010 and the GLT in 2011. See Board biographies on pages 83 to 86. | |
| Deborah Waterhouse CEO, ViiV Healthcare |
Deborah was appointed to the GLT in January 2020. She became Chief Executive Officer of ViiV Healthcare in April 2017. | |
Deborah joined GSK in 1996 and prior to ViiV was the Senior Vice President of Primary Care within GSK’s US business. She has a strong track record of performance in both specialty and primary care. Deborah led the HIV business in the UK before heading the HIV Centre of Excellence for Pharma Europe and held roles as General Manager of Australia and New Zealand and Senior Vice President for Central and Eastern Europe. | ||
Deborah is a Non-Executive Director of Schroders plc and holds a degree in Economic History and English Literature from Liverpool University. | ||
– |
remain objective and act in the best interests of the company and all shareholders |
– |
put sustained value creation at the heart of our agenda |
– |
align the Board agenda with our strategy, performance and pipeline priorities |
– |
ensure management performance and succession is assessed against delivery |
– |
use the IU targets to provide the foundation for enhanced performance management |
– |
ensure that the separation of CH is a value-based process |




Board committee |
Role |
Membership comprises |
Board committee report on page | |||
| Science |
Supports the Board in its understanding of the key strategic themes, upon which the company’s R&D strategy is based, and of any external transactions, by performing in-depth reviews of the underlying scientific assumptions to give the Board technical assurance. It also undertakes more in-depth risk oversight of R&D-related risks |
Dr Jesse Goodman (Chair) Dr Hal Dietz (from January 2022) Dr Laurie Glimcher Charles Bancroft (from May 2021 to February 2022) Judy Lewent (until May 2021) |
105-106 | |||
| Corporate Responsibility |
Considers GSK’s Trust priority and oversight of progress against the associated Trust commitments which reflect the most important issues for responsible and sustainable business growth. It has oversight of the views and interests of our internal and external stakeholders and reviews issues that have the potential for serious impact upon GSK’s business and reputation |
Lynn Elsenhans (Chair) Dr Anne Beal (from May 2021) Dame Vivienne Cox Dr Jesse Goodman |
104-105 | |||
| Transformation & Separation (Devolved into the committee architecture and disbanded in December 2021) |
Advises and assists the Board on the transformation and separation of the company and oversees the associated risks in separating the Group into Biopharma and Consumer Healthcare companies |
Sir Jonathan Symonds (Chair) Charles Bancroft Vindi Banga Dame Vivienne Cox Lynn Elsenhans Urs Rohner Judy Lewent (until May 2021) |
110 | |||
Nominations & Corporate Governance |
Reviews the structure, size and composition of the Board, the appointment of members to Board committees and the appointment of Corporate Officers and makes recommendations to the Board as appropriate. It plans and assesses orderly succession for Executive and Non-Executive directors and reviews management’s Succession Plan to ensure its adequacy |
Sir Jonathan Symonds (Chair) Charles Bancroft (from May 2021) Vindi Banga Lynn Elsenhans Urs Rohner Judy Lewent (until May 2021) |
107-110 | |||
| Is responsible for reporting to the Board, overseeing and monitoring corporate governance arrangements and for making recommendations to the Board to ensure the company’s standards and arrangements are consistent with existing corporate governance standards and emerging best practice. It also reviews the company’s conflicts of interest |
||||||
| Audit & Risk |
Reviews the financial reporting process, the integrity of the company’s financial statements, the external and internal audit process, the system of internal control and the identification and management of risks, and the company’s process for monitoring compliance with laws, regulations and ethical codes of practice |
Charles Bancroft (Chair from March 2021) Vindi Banga Dr Anne Beal (from July 2021) Lynn Elsenhans Dr Laurie Glimcher Judy Lewent (Chair until March 2021 and member until May 2021) |
111-115 | |||
| Initiates audit tenders, the selection and appointment of the external auditor, setting their remuneration and exercising oversight of their work |
||||||
| Remuneration |
Sets the company’s remuneration policy having regard to GSK’s workforce remuneration so that GSK is able to recruit, retain and motivate its executives |
Urs Rohner (Chair) Vindi Banga Dame Vivienne Cox |
119-152 | |||
| The Remuneration policy is regularly reviewed to ensure that it is consistent with the company’s scale and scope of operations, supports the business strategy and growth plans, is aligned to the wider workforce and helps drive the creation of shareholder value |
||||||
| (The Chair and the CEO are responsible for evaluating and making recommendations to the Board on the remuneration of Non-Executive Directors) |
||||||

 |
|
|||||||||||||||||||||||||||||||||
Attendance at scheduled Board and committee meetings during 2021 |
|
|||||||||||||||||||||||||||||||||
| |
Board |
Nominations & Corporate Governance |
Audit & Risk |
Remuneration |
Science |
Corporate Responsibility |
Transformation & Separation |
* |
||||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
| Total number of scheduled meetings |
6 |
6 |
6 |
6 |
3 |
4 |
3 |
|||||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
| Members |
Attended |
Attended |
Attended |
Attended |
Attended |
Attended |
Attended |
|||||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
| Sir Jonathan Symonds |
6 |
6 |
3 |
|||||||||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
| Emma Walmsley |
6 |
|||||||||||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
| Iain Mackay |
6 |
|||||||||||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
| Dr Hal Barron |
6 |
|||||||||||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
| Charles Bancroft |
6 |
3 (3) |
6 |
2 (2) |
3 |
|||||||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
| Vindi Banga |
6 |
6 |
6 |
6 |
3 |
|||||||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
| Dr Anne Beal |
3 (3) |
2 (2) |
3 (3) |
|||||||||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
| Dame Vivienne Cox |
6 |
6 |
4 |
3 |
||||||||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
| Lynn Elsenhans |
6 |
6 |
6 |
4 |
3 |
|||||||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
| Dr Laurie Glimcher |
6 |
6 |
3 |
|||||||||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
| Dr Jesse Goodman |
6 |
3 |
4 |
|||||||||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
| Urs Rohner |
6 |
6 |
6 |
3 |
||||||||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
| Judy Lewent |
3 (3) |
3 (3) |
3 (3) |
3 (3) |
1 (1) |
2 (2) |
||||||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
| Number of ad-hoc meetings |
15 |
7 |
4 |
7 |
6 |
1 |
||||||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
| For Charles Bancroft, Dr Anne Beal and Judy Lewent, the numbers in brackets denote the number of meetings which these individuals were eligible to attend. Dr Beal joined the Board and the Corporate Responsibility Committee on 6 May 2021 and the Audit & Risk Committee on 23 July 2021. Charles Bancroft joined the Science and Nominations & Corporate Governance committees on 6 May 2021. Judy Lewent retired from the Board following the AGM on 5 May 2021. |
|
|||||||||||||||||||||||||||||||||
| In addition to the ad-hoc meetings included in the table above, the Chairs’ Committee, that was established at the end of 2020, met on 15 occasions to consider various items of business during 2021. |
|
|||||||||||||||||||||||||||||||||
| * The Transformation & Separation Committee was devolved into the committee architecture and disbanded in December 2021. |
|
|||||||||||||||||||||||||||||||||
Areas of focus in 2021 | ||
Further strengthening GSK’s fundamentals |
The Board’s oversight of the fundamentals of the commercial execution, cost base, capital allocation, pipeline and culture included: | |
| – receiving regular progress updates and providing input into the company’s Vaccines mRNA strategy plan | ||
| – receiving and discussing commercial strategy performance reports from Pharmaceuticals, Vaccines and ViiV Healthcare businesses | ||
| – reviewing and approving the objectives and ambitions for the company and patients that were announced at the Investor Update in June | ||
| – approving GSK’s progressive new dividend policy | ||
| – approving the Board’s 2021-23 priorities | ||
| – approving business development transactions and strategic partnerships with third parties, including Vir Biotechnology, CureVac, iTeos and Alector | ||
| – receiving updates on R&D strategy, progress and the company’s pipeline | ||
| – receiving quarterly reports from the CEO, CFO and CSO | ||
| – scrutinising the Group’s financial performance | ||
| – setting the company’s new purpose and simplified culture | ||
| – oversight of projects and collaborations with third parties, to develop vaccines and treatments for COVID-19 | ||
| – reviewing the risks and impacts of COVID-19 on the Group’s business and performance | ||
| – approving the terms of the global settlement and licensing agreement with Gilead | ||
Separation of Consumer Healthcare |
The Board’s preparation for the demerger as a value-based process included: | |
| – regularly discussing and scrutinising transformation plans for Consumer Healthcare business | ||
| – receiving and discussing commercial strategy performance reports from Consumer Healthcare business | ||
| – discussing plans for Consumer Healthcare up to and beyond separation as Haleon at the annual Board and GLT strategy day | ||
| – approving the appointment of the Haleon Chair and CEO designates and planning for the Haleon Board composition | ||
| – reviewing and rejecting unsolicited proposals for the Consumer Healthcare business | ||
New GSK |
The Board’s oversight of the creation of GSK as a pure biopharma business and delivering a step change in performance included: | |
| – regularly discussing and scrutinising transformation plans for new GSK | ||
| – discussing plans for the company up to and beyond separation as new GSK a pure biopharma company at the annual Board and GLT strategy day | ||
| – reviewing and approving the objectives and ambitions for the company and patients that were announced at the Investor Update | ||
| – receiving updates on R&D strategy, progress and the company’s pipeline | ||
| – succession planning for the new GSK Board, including approval of the appointments of a new Non-Executive Director and Corporate Responsibility Committee Chair successor and a new Non-Executive Director and designated Scientific & Medical Expert | ||
Maintaining ESG leadership |
The Board’s oversight of Trust and the ESG agenda included: | |
| – approving the Trust section of the Annual Report | ||
| – approving the Task Force on Climate-related Financial Disclosures in the Annual Report | ||
| – delegating specific responsibility to the Corporate Responsibility Committee for oversight of Human Rights in the company’s operations | ||
| Regular governance oversight |
The Board’s focus on a routine programme of good governance activities included: | |
| – reviewing the quarterly financial results, dividend proposal, earnings guidance, investor materials and results announcements and receiving reports from the external auditor | ||
| – approving the Annual Report and Form 20-F | ||
| – setting the annual budget and plan, and the forward-looking three-year forecast | ||
| – conducting an annual review of the Board’s enterprise risk responsibility framework and enterprise-wide risks | ||
| – considering observations and agreeing actions from the Board’s external evaluation | ||
| – reviewing and continuing to evolve the Board’s governance architecture | ||
| – evaluating the CEO’s 2020 performance, and setting her 2021 objectives | ||
| – reviewing the annual talent and succession plan | ||
| – receiving reports from Board committees and the Workforce Engagement Director | ||
| – discussing the employee PULSE survey results | ||
| – receiving reports on corporate governance and regulatory developments and the Company Secretary’s report | ||
| – approving the company’s modern slavery statement and gender pay gap positioning | ||
| – reviewing stakeholder perception research | ||

| Progress area |
Principal decision |
How Board/Committee regarded stakeholder interests |
Stakeholder groups, and other section 172 duties considered | |||
Commercial execution |
China: The Audit & Risk Committee recommended incremental changes to the commercial model in China to the Board for approval Further details are available on page 111 |
The Committee reviewed GSK China’s implementation of the healthcare professionals (HCP) speaker engagements and sales force incentive (SFI) policy changes to date. It noted tangible improvements observed in our people and customer engagement In this context, the Committee considered further incremental changes to our HCP engagement and SFI programme in China. This included a plan for an increase in the number of city-level Healthcare Organisations (HCOs) to increase our reach. Further improvement of HCP coverage across the country enables our innovative Specialty Care products to ultimately reach more patients. To continue to safeguard key stakeholder interests including patients, the Committee reviewed a risk assessment, the training approach and the proposed implementation of controls over the new potential HCOs before recommending this change |
Stakeholders: HCPs and medical experts, employees, investors, governments and regulators, patients and consumers Other s172 duties: Long-term results, our workforce, business relationships and reputation | |||
Cost base |
Transformation programme: The Board concluded its oversight of the savings made from the transformation programme to achieve a cost base competitive with its peers |
The Board agreed to the acceleration of this programme to generate additional savings that could be invested in the R&D pipeline for the potential benefit of patients and to deliver shareholder returns |
Stakeholders: Investors, patients and employees Other s172 duties: Long-term business performance, our workforce and our business relationships | |||
Capital allocation |
Dividend policy change: The Board reviewed and approved the implementation of a new progressive dividend policy for implementation from 2022 Further details are available on page 111 |
The Board, with support from the Audit & Risk Committee, carefully considered this matter before concluding to move to a progressive dividend policy from 2022. In consideration of its duties, the Directors examined the importance of predictable returns, particularly in uncertain times As part of its deliberations, the Board carefully balanced the impact of and trade-offs between reducing the dividend against the importance of setting up new GSK with the right capital structure and the resources to invest, grow and improve shareholder returns over the longer term Ultimately, the Board determined that setting a progressive dividend policy in this way would support the investment needed to deliver growth, unlock further shareholder value and develop an even stronger pipeline of innovative products capable of transforming the lives of our patients |
Stakeholders: Investors, patients and our people Other s172 duties: Our long-term results, workforce and business relationships and reputation and fairness between our shareholders | |||
| Pipeline |
Business development, collaborations and deals: The Science Committee considered the scientific merits of these opportunities prior to the Board’s review and approval Further details are available on page 106 |
The Science Committee and Board reviewed many business development opportunities during the year. Those leading to concluded transactions included: – A collaboration with iTeos Therapeutics to enable next generation immune-oncology combinations – Expansion of the collaboration with Vir Biotechnology to advance new therapeutics for influenza and other respiratory viruses and – Collaboration with Alector to co-develop anti-bodies for neurodegenerative diseasesThese deals were considered in the context of their potential to help GSK deliver transformational medicines to patients |
Stakeholders: Patients, employees and investors Other s172 duties: Our long-term results, workforce and business relationships | |||
Progress area |
Principal decision |
How Board/Committee regarded stakeholder interests |
Stakeholder groups, and other section 172 duties considered | |||
New growth ambitions |
Investor Update: The Board approved the June Investor Update (IU) objectives and ambitions with a focus on GSK’s growth outlook and for maximising shareholder value creation including: – competitive growth and margin outlook – competitive sustainable returns and value creation and – new ambitions for shareholders and society Further details are available on page 111 |
The Board drew on comprehensive investor feedback and other key stakeholder research and outreach to help inform and shape the agreed ambitions shared at the IU event for new GSK, our patients and shareholders, and impacting the health of more than 2.5 billion people over the next ten years. The Audit & Risk Committee also reviewed the proposals The details of how stakeholder interests were then taken into account by the Remuneration Committee when incorporating key IU ambitions into the updated 2022 Remuneration policy for GSK are described in the ‘Remuneration policy review’ principal decision below |
Stakeholders: Patients and consumers, our people and investors, governments and regulators, non-governmental organisations and multilateral organisationsOther s172 duties: Long-term business performance, our workforce, business relationships, the community and our environment, our reputation and fairness between our shareholders | |||
Separation of Consumer Healthcare |
Rejection of unsolicited proposals for CH business: – The Board had ensured that the CH business was well-positioned to sustainably grow ahead of its categories in the years to come and had a highly skilled management team to lead it – It was confident that the CH business could sustainably deliver organic sales growth in the range of 4-6% (CER) over the medium term |
Having completed this foundational work, the Board was well-positioned to consider the unsolicited, conditional and non-binding proposals received to acquire the CH business The proposals were rejected as they were not in the best interests of shareholders since they fundamentally undervalued the business and its future prospects |
Stakeholders: Patients and consumers, our people, investors, governments and regulators, non-governmental organisations and multilateral organisations Other s172 duties: Long-term business performance, our workforce, our business relationships, the community and our environment, our reputation and fairness between our shareholders | |||
| Demerger of CH: TheBoard approved: – the retention of a stake in the Consumer Healthcare company, Haleon, post demerger – Haleon’s opening capital structure and – the separation of CH to create Haleon by mid-2022 Further details are available on page 110 |
The Board, supported by the Transformation & Separation Committee, considered the best way to release maximum shareholder value, and for the two companies be set on firm foundations to be able to most effectively serve their patients and customers respectively This included the most appropriate capital structures required for the two companies to be competitive, how to distribute shares in Haleon to GSK’s shareholders, whether to retain a stake in Haleon, and on which exchanges Haleon should list and why |
Stakeholders: Patients and consumers, our people and investors, governments and regulators, non-governmental organisations and multilateral organisations Other s172 duties Long-term business performance, our workforce, our business relationships, the community and our environment, our reputation, and fairness between our shareholders | ||||
Remuneration |
Remuneration policy review: The Remuneration Committee approved a new 2022 Remuneration policy and measures for the biopharma company, which is subject to a binding shareholder vote at our 2022 Annual General Meeting. It incorporates new long- and short-term incentives including: – Sales and adjusted operating profit growth measures aligned to the IU ambitions and – ESG measures reflecting the company’s work in this regard |
Prior to developing the new 2022 Remuneration policy (the new policy), on behalf of the Remuneration Committee, the Remuneration Committee Chair and the Chair: – considered investor feedback on the key ambitions set out at the IU event and engaged with its major investors, and proxy advisers on the proposed changes – consulted with the Corporate Responsibility Committee on GSK’s ESG commitments and Trust priorities – listened to the views of an ESG expert, outlined in the ‘ESG leadership’ principal decisions below, concerning views of stakeholders on the linkage of ESG to remuneration incentives and – met with the Chief People Officer and the HR leads for each area of the business to hear their views on remuneration arrangements at GSK and wider workforce pay alignment opportunities for new GSK They also consulted with investors and proxy advisers on the new policy proposals. Following engagement, the Committee then carefully considered the feedback before finalising the design of the new policy Further details are available on pages 143 to 152 |
Stakeholders: Our people, investors, patients and consumers, governments and regulators and proxy advisers Other s172 duties: Long-term results, our workforce the community and our environment and our reputation | |||

Progress area |
Principal decision |
How Board/Committee regarded stakeholder interests |
Stakeholder groups, and other section 172 duties considered | |||
ESG leadership |
Leading ESG expert view and insights on GSK: Following a wide-ranging and comprehensive briefing and debate with a recognised ESG expert, the Corporate Responsibility Committee agreed a programme of actions to further improve our ESG communications and IR engagement by: – providing further evidence, metrics and data to investors of how the company’s culture is being transformed – more proactively targeting our long-term investor base and – increasing the availability of our Board committee Chairs to help strengthen understanding of their committees’ approach and work Further details are available on page 105 |
The ESG expert: – provided an overview of ESG investor expectations – described major trends in ESG and the causal drivers – covered GSK and sector specific issues, including culture, net zero and intangibles and – shared developments around ESG links to remuneration The Committee considered the positive and negative historical stakeholder perceptions together with GSK’s focus on purpose, mission and culture The company’s new environmental sustainability goals had been announced the previous year. The company’s approach could be further enhanced by strengthening the alignment to remuneration incentives with delivery of ESG ambitions. The expert’s insights were considered as part of the development by the Remuneration Committee of the ESG remuneration measures explained in the Remuneration Report |
Stakeholders: Investors, patients, employees, governments and regulators, non-governmental organisations and multilateral organisations Other s172 duties: Long-term results, our business relationships, the community and our environment, our reputation and fairness between our shareholders | |||
New GSK |
Board succession planning: The Nominations & Corporate Governance Committee agreed: – a set of key guiding principles for the new GSK Board and – an optimal Board skills matrix This supported the development of a roadmap for future appointments over the medium term to help deliver on our stated ambitions for patients and shareholders Further details are available on page 107 and 108 |
The Committee considered the optimal future composition of the new GSK Board for the future To appropriately reflect stakeholder interests, the Board wished to be constituted so as to: – be diverse in the broadest sense – have appropriate operational depth across the life science value chain and from a general commercial perspective – have experience of major customer markets, and – needed the skills and insights of members who could continue to ensure the company’s leadership position in ESG |
Stakeholders: Patients and consumers, our people and investors Other s172 duties: Our long-term business performance, workforce and business relationships and reputation | |||
Settle significant litigation |
Gilead – Dolutegravir global settlement The Board approved the terms of the global settlement and licensing agreement in which Gilead would: – make an upfront payment of $1.25 billion to ViiV Healthcare and – pay a 3% royalty on all future US sales of Biktarvy and in respect of the bictegravir component of any other bictegravir-containing products sold in the US |
The decision to settle this global litigation was taken after careful consideration in the context of bringing certainty for investors and to support additional investment in the business for the future and thereby benefiting patients and investors Further details are available on page 58 |
Stakeholders: Investors, patients, governments and regulators Other s172 duties: Long-term results, our business relationships and our reputation | |||
– |
Section 172 statement on page 116, and the sections it references in this Annual Report |
– |
principal decisions the Board and its committees made, on pages 96 to 98 |
– |
the CEO’s Board report |
– |
a specific external stakeholders report. This provides strategic insights based on an analysis of key developments, achievements and risks impacting our reputation and the perceptions of external stakeholders |
– |
a monthly investor relations report which summarises investor perceptions |
– |
regular corporate governance and litigation and regulatory updates |
– |
feedback from our global, as well as smaller, more targeted PULSE employee surveys |
– |
the work of our Workforce Engagement Director, Dame Vivienne Cox, who regularly gathers and explains colleagues’ views to the Board, as she outlines below |

| |
||||||||
| Workforce Engagement Director |
||||||||
| This is my third year as Workforce Engagement Director. In this time, I have appreciated the chance to meet with different people across the company and to listen carefully to their views and perspectives. During the year, the engagements I have attended have continued to be virtual; however, I am very pleased that this has not prevented people from being very open and transparent in their discussions with me. |
Other engagement programme highlights I joined a Site Directors’ and Site Quality Leaders’ meeting comprising a group of employees who had recently been appointed to these roles. My meeting with them was part of a longer induction programme they undertake. I took part in the session where they discussed the impact of the new culture on their roles. They stressed the importance of ensuring continuous improvement at their sites. |
|||||||
Purpose, strategy and culture |
In the mid-point of 2021, I met with HR leaders and I was impressed by their energy and commitment, through to and beyond separation. It was clear that their focus on People, Culture, Leadership and Capability would be key to supporting an environment where people can thrive, and additionally how important the new simplified HR systems and operating model would be to ensuring quality support for all our people.The ‘Ahead Together’ session was an ambitious and well- received two-day digital event which brought together 1,500 employees from around the world. The objective was to share thinking and progress on the launch of two new companies and exchange ideas about the opportunities that lie ahead.Finally, I spent time with a group of high potential Commercial employees from the Greater China and Intercontinental region who were completing a virtual development programme. We discussed their key learnings, which were the importance of developing resilience and building trust. After each meeting with an employee group, I share my thoughts and observations with the leaders and the Board on a non-attributable basis. Perhaps the most valuable aspect is that on an ongoing basis, those views and perspectives can be factored into the Board and GLT discussions and decision making.Dame Vivienne Cox Workforce Engagement Director 28 February 2022 |
|||||||
| As I established the programme of visits at the start of 2021, I was conscious that it would be a year of significant change. The transformation programme to restructure the Group in advance of separation was launched in 2020. It has continued throughout 2021 and, with it, there has naturally been some uncertainty for our people. Therefore, I was keen to use my role to understand the impact of these changes on the organisation. Additionally, as the separation has been getting closer, it has raised questions in the minds of our people about the future shape of new GSK and the Consumer Healthcare business as a new listed company. In particular, I wanted to understand how the work done by the Board and the GLT to define a new purpose, strategy and embed a new simplified culture, which is discussed elsewhere in the Annual Report, was being experienced. Probably the most consistent message I have heard this year is the value people attach to working for a company with a strong sense of purpose and a clear strategy. Additionally, the people I have met are supportive of the new culture. They appreciate the simplicity and the clarity that it brings. I have continued, with Jon, our Chair, to engage with our diversity Employee Resource Groups (ERGs), specifically on the impact of the announcement of the company’s public aspirations for improving ethnicity and gender representation in the workforce and leadership positions. Overall, their responses were positive while continuing to encourage the Board and GLT to intensify their efforts to support and promote diverse talent. |
||||||||

– |
are ambitious for patients, by delivering what matters better and faster |
– |
are accountable for impact, by having clear ownership and the support to succeed |
– |
do the right thing, by working with integrity and care and understanding that people count on us |
– |
the rationale for this change |
– |
a review of employee engagement and feedback when trialling this change |
– |
the next steps the Board and GLT needed to take to make this change real for our people |
– |
Go beyond |
– |
Do what matters most |
– |
Keep it human |
– |
Living our values and expectations, which explores our values, expectations and culture and how they apply to our operations and ways of working |
– |
Anti-bribery and corruption |
– |
Inclusion and diversity |
– |
The key challenges and opportunities for GSK over the next five years (eg science, M&A, China, areas of management strength and support) |
– |
The culture of GSK |
– |
Which skills and experience to prioritise in recruiting new Non-Executive Directors to the Board. The imperatives and desirable attributes were considered against the strategic opportunities that lie ahead and |
– |
The workings of Board committees and how they obtained external input |
– |
Corporate Responsibility: |
– |
Audit & Risk: |
– |
Remuneration: |
– |
Nominations & Corporate Governance: |
– |
Science: |
| Progress on 2020 Board evaluation | ||
Areas of focus for 2021 |
Progress/achievements | |
Consideration had and would continue to be given to stop any unnecessary tasks to free more time to focus on the priorities with the pre-condition that creating shareholder value was of prime importance |
Board priorities were agreed and adhered to in structuring Board discussions. Key priorities were the key driver in examining performance and transactions. This would remain a key focus. | |
Consideration would also be given to making the best use of the Board’s time during virtual meetings and incorporating opportunities for ‘unstructured discussions’ where possible |
There was increased use of break-out sessions to focus on and bring different perspectives to particular issues. In October, to facilitate greater in person interaction despite ongoing COVID-19 restrictions, the Board, committee and annual strategy meetings were held at dual sites in the UK and US. The Chair led the meeting in the UK for UK/European-based Directors and the CEO led the meeting for US-based Directors. | |
| In addition, specific time was set aside for GLT members to meet with Board members without a set objective or agenda. These discussions were greatly appreciated by all and a welcome opportunity to connect. | ||
The Science Committee would look to further deepen its understanding of how R&D’s resources were allocated |
The Board and Science Committee meeting agendas were designed to facilitate these deeper dives in line with Board’s agreed key priorities. See page 106 | |
| There was a desire to further enhance root cause analysis that was undertaken when incidents or issues occurred. This was to ensure they could be avoided in the future and as part of the Group’s approach to further improving performance |
This enhanced approach was the foundation of the global safety review. | |

– |
progress on our Trust commitments through regular reports from GLT members and senior managers |
– |
GSK’s approach to managing the risks and opportunities associated with ESG factors that help create value for shareholders and society |
– |
management understanding of key issues and stakeholder perspectives by listening directly to key independent expert voices and |
– |
the principal risks most relevant to its area of expertise and responsibility, namely: product quality, non-promotional engagement, supply continuity, environmental ,sustainability and health and safety |
– |
education and training to further build capabilities; while |
– |
ensuring there is clear accountability from leaders for safety through heightened awareness and application of GSK’s simplified culture to “do the right thing with integrity and care because people count on us” |
– |
Jemperli |
– |
Cabenuva Cabenuva Apretude |

– |
Immunology |
– |
Oncology with a focus on Synthetic Lethality |
– |
Vaccines mRNA Strategy |
Key priorities |
Status | |
Succession planning for the CSO and his R&D Leadership Team |
CSO succession candidate identified and subsequently appointed CSO Designate | |
| Appoint a Chair of the Corporate Responsibility Committee to succeed Lynn Elsenhans |
Dr Anne Beal joined the Board in May 2021 | |
| Appoint a third Scientific & Medical Expert (SME) |
Dr Hal Dietz joined the Board in January 2022 | |
Design target GSK Board composition and recruit high calibre Non-Executives to complete the new GSK biopharma Board |
Target GSK Board composition agreed and search for new Non-Executive Directors for GSK in progress | |
Appoint the Haleon CEO and assemble an appropriately seasoned management team |
Brian McNamara appointed as Haleon CEO Designate in July 2021. Haleon Management Team announced in December 2021 | |
Appoint a Chair for Haleon and support the composition of the Haleon Board |
Sir Dave Lewis appointed Haleon Chair Designate in December 2021. Selection of the remaining Haleon Board in progress | |
– |
An outlook on the future direction of R&D, innovation and the treatment and management of human health |
– |
Experience of people leadership and management at ‘scale’, either in an academic or industry setting |
– |
Interested in, and having a deep understanding of, a breadth of scientific and therapeutic areas, particularly in immunology as well as genomics and genetics. Having perspectives on the ability to harness digital technologies (including Artificial Intelligence) to enhance the research and development of new medicines and |
– |
Able to deliver complex science to a broad audience. Highly collaborative and a willingness to engage proactively on topics beyond their own immediate realm of expertise |

– |
Significant listed Board experience with an understanding of investors, analysts, banks, regulators and governments |
– |
A high degree of financial acumen and successful business track record in creating shareholder value and growing businesses |
– |
A strong emphasis on coaching skills and the ability to create a high-performance environment |
– |
Deep experience of consumer facing businesses, with a high degree of customer-centricity. International experience, preferably in the US and China |
– |
Strong strategic skills and a track record of innovative thinking, coaching and development |
– |
Be well respected and have high credibility with all stakeholders, including investors, capital market participants, regulators and governments |
– |
Have high integrity, strong values and be driven by a strong sense of purpose |
– |
Understand the role of a Chair of a FTSE 100 |
– |
Possession of humility and a subdued ego and a strong emotional commitment and passion for the CH business |
– |
Be committed to diversity in all its forms, resilient and open-minded with strong judgement as well as a natural team builder |
– |
Korn Ferry: general recruitment, executive search and assessment services, coaching and other HR-related services |
– |
Egon Zehnder: executive search, assessment and coaching services to specific senior executives |
– |
Heidrick & Struggles: executive search services |
– |
Spencer Stuart: executive search and assessment services |
Director |
Membership |
Appointment date |
Retirement date | |||
| Charles Bancroft |
Chair of Audit & Risk Committee Chair |
9 March 2021 |
||||
Member of Nominations & Corporate Governance, and Science committees |
6 May 2021 |
8 February 2022 (stepped down from Science Committee after Dr Hal Dietz joined the Committee) | ||||
| Dr Anne Beal |
Member of Corporate Responsibility and Audit & Risk committees |
6 May 2021 23 July 2021 |
||||
| Dr Hal Dietz |
Member of Science Committee |
1 January 2022 |
||||
| Judy Lewent |
Chair of Audit & Risk Committee Chair Member of Audit & Risk, Nominations & Corporate Governance, Remuneration, Science and Transformation & Separation committees |
9 March 2021 5 May 2021 | ||||
| Lynn Elsenhans |
Chair of Corporate Responsibility Committee Member of Audit & Risk, Corporate Responsibility and Nominations & Corporate Governance committees |
4 May 2022 After CH Demerger | ||||
| Dr Anne Beal |
Chair of Corporate Responsibility Committee |
4 May 2022 |
||||
| Shobie Ramakrishnan |
Chief Digital and Technology Officer and member of GLT |
16 December 2021 | ||||

Progress achieved | ||||
Diversity objectives |
Status |
Performance | ||
At least 33% of Board positions held by women |
Exceed objective |
38.4% | ||
At least 33% of GLT positions held by women |
Met objective |
35.7% | ||
At least 33% of combined GLT and direct report positions held by women |
Exceed objective |
42.5% | ||
At least one Board Director is ethnically diverse |
Exceed objective |
Two Directors | ||
| Transformation & Separation Committee report Jonathan Symonds Transformation & Separation Committee I am pleased to present my second and final report as Chair of the Transformation & Separation Committee (the Committee) given that it has now fulfilled its purpose and mandate. The Committee was established in May 2020 charged with two principal functions: – Exercising oversight of the Future Ready transformation programme, – Considering the optimal form of separation. The Committee was pleased that the Future Ready transformation programme was completed to schedule by the end of 2021 and exceeded the cost savings identified to be derived from this programme. During 2021, the Committee undertook a programme of work to understand and consider the key fundamentals of separation. This was not just the technical requirements. It considered how to best unlock, release and maximise long-term shareholder value. This work was supported by guidance and advice from external experts as appropriate. The Committee began by considering how we should separate and the principal value to be achieve from each option available. Discussions then progressed to the capital structures required for the resulting two companies to be competitive as independent entities. Following a decision to demerge the CH business it was important to determine how to distribute shares in Haleon to our shareholders, and on which exchanges Haleon should list and why. The Committee also considered whether to retain a stake in Haleon and how big a stake to retain. This was a very intensive and detailed programme of work as the Committee addressed these major questions and the impact for all our stakeholders. It then reported to the Board accordingly on its conclusions and recommendations. This process is now well into the execution phase. Oversight of the remaining work more appropriately rests with the other specialist Board committees and has been devolved to them as appropriate, or will be reviewed and overseen directly by the Board. Having fulfilled its mandate, it was agreed that the Committee be decommissioned. I would like to thank Board colleagues for their commitment and diligence in supporting the Committee’s work in this respect. Sir Jonathan Symonds Transformation & Separation Committee Chair 28 February 2022 |
– |
further incremental changes to the company’s commercial model in China |
– |
the company’s new growth ambitions before they were shared at the Investor Update in June 2021 and |
– |
the move to a progressive dividend policy from 2022 |

| Significant issues considered by the Committee in relation to the financial statements |
How the issue was addressed by the Committee | |||
Going concern basis for the preparation of the financial statements |
The Committee considered the outcome of management’s half-yearly and year end reviews of current and forecast net debt positions and the various financing facilities and options available to the Group. | |||
| The Committee also considered management’s review of the current and longer-term impacts of the COVID-19 pandemic, at the outbreak of the pandemic and at the year end. Following consideration of these assessments, which included stress testing and viability scenarios, sources of liquidity and funding, forecasts and estimates, the Committee confirmed that the application of the going concern basis for the preparation of the financial statements continued to be appropriate. | ||||
Revenue recognition, including returns and rebates (RAR) accruals |
The Committee reviewed management’s approach to the timing of recognition of revenue and accruals for customer returns and rebates. The US Pharmaceuticals and Vaccines accrual for returns and rebates was £5.0 billion at 31 December 2021 and the Committee reviewed the basis on which the accrual had been made and concurred with management’s judgements on the amounts involved. A fuller description of the process operated in the US Pharmaceuticals and Vaccines business in determining the level of accrual necessary is set out in ‘Critical accounting policies’ on page 80. | |||
Provisions for legal matters, including investigations into the Group’s commercial practices |
The Committee received detailed reports on actual and potential litigation from both internal and external legal counsel, together with a number of detailed updates on investigations into the Group’s commercial practices. Management outlined the levels of provision and corresponding disclosure considered necessary in respect of potential adverse litigation outcomes and also those areas where it was not yet possible to determine if a provision was necessary, or its amount. At 31 December 2021, the provision for legal matters was £0.2 billion, as set out in Note 31 to the financial statements, ‘Other provisions’. | |||
Provisions for uncertain tax positions |
The Committee considered current tax disputes and areas of potential risk and concurred with management’s judgement on the levels of tax contingencies required. At 31 December 2021, a tax payable liability of £0.7 billion, including provisions for uncertain tax positions, was recognised on the Group’s balance sheet. | |||
Impairments of intangible assets |
The Committee reviewed management’s process for reviewing and testing goodwill and other intangible assets for potential impairment. The Committee accepted management’s judgements on the intangible assets that required writing down and the resulting impairment of £455 million in 2021. See Note 20 to the financial statements, ‘Other intangible assets’ for more details. | |||
Valuation of contingent consideration in relation to ViiV Healthcare |
The Committee considered management’s judgement that it was necessary to increase the liability to pay contingent consideration as a result of increases in sales forecasts as well as the unwind of the discount and updated exchange rate assumptions. After cash payments of approximately £0.8 billion in the year, at 31 December 2021, the Group’s Balance sheet included a contingent consideration liability of £5.6 billion in relation to ViiV Healthcare. The settlement with Gilead resulted in a re-measurement of the existing liabilities for the contingent consideration at the year end and is included in the closing balance. | |||
ViiV Healthcare put option |
The Committee reviewed and agreed the accounting for the Pfizer put option and concurred with management’s judgement on the valuation of the put option of £1.0 billion at 31 December 2021. The settlement with Gilead resulted in a re-measurement of the Pfizer put option at the year end and is included in the closing balance. | |||

Auditor’s reappointment | ||
External auditor |
||
| External auditor appointment |
||
| Last tender |
May – December 2016 | |
| Transition year |
2017 | |
| First shareholder approval of current auditor |
May 2018 | |
| First audited Annual Report and 20-F |
Year ending 31 December 2018 | |
| Next audit tender required by regulations |
2026 | |
– |
the overall quality of the audit |
– |
the independence of Deloitte |
– |
whether Deloitte exhibited an appropriate level of challenge and scepticism in its work |
– |
effectiveness of the auditor’s challenge |
– |
integrity of Deloitte |
– |
transparency of its reporting to management and the Committee |
– |
clarity of the auditor’s communication and ways of working |
– |
alignment of the 2021 audit to the Group’s investment in Systems, Applications and Products (SAP) |
– |
quality of the audit team’s leadership |
– |
skills and experience of the audit team |
– |
effectiveness of the auditor and the external audit process and |
– |
auditor’s independence, qualifications, objectivity, expertise and resources |
| Process: |
All non-audit services over £50,000 are put to competitive tender with other financial services providers, in line with the Group’s procurement process, unless the skills and experience of the external auditor make it the only suitable supplier. | |||
| Safeguards: |
Adequate safeguards are established so that the objectivity and independence of the Group audit are not threatened or compromised. | |||
| Fee cap: |
The total fee payable for non-audit services should not exceed 50% of the annual audit fee, except in special circumstances where there would be a clear advantage in the auditor undertaking the additional work. | |||
| Prohibitions: |
GSK’s policy includes a ‘whitelist’ of permitted non-audit services in line with the relevant regulations. Any service not on this list is prohibited. | |||
| Pre-approval: |
All non-audit services require pre-approval as set out in the table below to ensure services approved are consistent with GSK’s non-audit policy for permissible services. This process ensures all services fall within the scope of services permitted and pre-approved by the Committee and does not represent a delegation of authority for pre-approval. | |||
Value |
Pre-approver | |||
More than £50,000 |
Committee Chair and CFO | |||
Between £25,000 and |
Group Financial Controller | |||
£50,000 |
||||
Under £25,000 |
Designate of the Group | |||
Financial Controller | ||||
The fees paid to the company’s auditor and its associates are set out overleaf. Further details are given in Note 8 to the financial statements, ‘Operating profit’ on page 184. | ||||
 During the year, fees for audit related and other assurance services of £4.0 million have increased by £2.4 million compared to 2020. This increase is due to work associated with Deloitte’s reporting accountant role in preparing for the demerger of the Consumer Healthcare business. Including audit fees in respect of the GSK pension schemes of £0.2 million, fees for audit related and other assurance services represent 15.2% of the annual audit service fee (2020: 6.3%). Excluding the demerger work, fees for audit related and other assurance services would have represented 2.2% of the annual audit fee. The Committee considered that hiring Deloitte to undertake the reporting accountant role for the demerger was in the best interests of shareholders because: – Deloitte possessed the type of expertise, experience, size and international scope required to handle a major demerger of this scale and complexity – the company benefited specifically from Deloitte’s in-depth knowledge and understanding of our CH business and their processes and compliance environment and– management time, that would otherwise have been devoted to educating another firm on the company’s business and operations, could instead be spent on delivering the demerger and creation of Haleon. The Committee considered the level of non-audit services incurred as part of its annual review of Deloitte’s independence set out on page 114 and was satisfied that the auditor continued to be independent and exercise objectivity throughout 2021. |
Fair, balanced and understandable assessment The need for an annual report to be fair, balanced and understandable is one of the key compliance requirements for a company’s financial statements. To ensure that GSK’s Annual Report meets this requirement, we have a well-established and documented process governing the coordination and review of Group-wide contributions to the publication. This runs in parallel with the process followed by the external auditor. The Committee received a summary of management’s approach to GSK’s 2021 Annual Report to ensure it met the requirements of the FRC’s. This enabled the Committee, and the Board, to confirm that GSK’s 2021 Annual Report as a whole is fair, balanced and understandable and provides the necessary information for shareholders to assess the company’s position and performance, business model and strategy. Code of Conduct and reporting lines We have a number of well-established policies (including a Code of Conduct), which are available on gsk.com, together with details of our confidential Speak Up lines for reporting and investigating unlawful conduct. Charles Bancroft Audit & Risk Committee Chair 28 February 2022 |

The Board has had regard to the following matters: |
||||
(a) Long-term results |
(b) Our workforce |
(c) Our business relationships | ||
| The likely consequences of any decision in the long-term Strategic report: Our business model (page 1) Chair’s statement (page 3) CEO’s statement (page 5) Key performance indicators (page 12) Risk management (page 46) Viability statement (page 53) Corporate Governance report: Chair’s governance statement (page 89) Board activity (page 95) Board progress in 2021 (page 96) The Board’s approach to continuous engagement (page 99) Board-led purpose and culture (page 102) Audit & Risk Committee report (page 111) |
The interests of the Group’s employees Strategic report: Our business model (page 1) Our culture (page 11) Being a modern employer (page 37) Stakeholder engagement (page 44) Corporate Governance report: Board activity (page 95) Board progress in 2021 (page 96) The Board’s approach to continuous engagement (page 99) Board-led purpose and culture (page 102) Audit & Risk Committee report (page 111) Nominations & Corporate Governance Committee report (page 107) Remuneration report: Remuneration Committee Chair’s statement (page 120) Directors’ pay in a wider setting (page 132) gsk.com: Gender pay gap report |
The importance of developing the Group’s business relationships with suppliers, customers and others Strategic report: Our business model (page 1) Our external environment (page 13) Stakeholder engagement (page 44) Innovation (page 17) Performance (page 29) Reliable supply (page 38) Working with third parties (page 38) Risk management (page 46) Corporate Governance report: Board activity (page 95) Board progress in 2021 (page 96) The Board’s approach to continuous engagement (page 99) Audit & Risk Committee report (page 111) Corporate Responsibility Committee report (page 104) | ||
(d) The community and our environment |
(e) Our reputation |
(f) Fairness between our shareholders | ||
| The impact of the Group’s operations on the community and our environment Strategic report: Trust section including: Environment (page 39) Environment, Health and Safety, and Environmental Sustainability risks (page 48) Climate-related financial disclosure (page 49) Corporate Governance report: Corporate Responsibility Committee report (page 104) gsk.com: ESG Performance Report |
Our desire to maintain our reputation for high standards of business conduct Strategic report: Our culture (page 11) Trust (page 34) Ethics and values (page 38) Human rights (page 38) Reporting and investigating concerns (page 38) Anti-bribery and corruption risk (pages 47 and 279) Non-financial information statement (page 54)Our approach to tax (page 60) Corporate Governance report: Corporate Responsibility Committee report (page 104) gsk.com: Modern slavery statement |
Our aim to act fairly as between members of the Group Corporate Governance report: Chair’s governance statement (page 89) The Board’s approach to continuous engagement (page 99) Transformation & Separation Committee report (page 110) Investor information (page 257) | ||
– |
becomes bankrupt |
– |
ceases to be a Director by virtue of the Companies Act or the Articles |
– |
suffers mental or physical ill health and the Board resolves that he or she shall cease to be a Director |
– |
has missed Directors’ meetings for a continuous period of six months without permission and the Board resolves that he or she shall cease to be a Director |
– |
is prohibited from being a Director by law |
– |
resigns, or offers to resign and the Board accepts that offer |
– |
is required to resign by the Board |

Directors’ report |
||
| Section |
Pages | |
| Corporate governance report |
82 to 118 | |
| Employee engagement |
100 | |
| Directors’ statements of responsibilities |
154 to 155 | |
| Investor information |
257 to 310 | |
Strategic report |
||
Section |
Pages | |
Risk management objectives and policies |
46 to 54 and 275 to 287 | |
Likely future developments of the company |
1 to 81 | |
Research and development activities |
17 to 28 | |
Business relationships |
38 | |
Diversity |
37 | |
Provision of information to and consultations with employees |
11 and 37 | |
Carbon emissions |
39 | |
Section 172 statement |
44 to 45 and 116 | |
Location in Annual Report | ||
Interest capitalised |
Financial statements, Notes 17 and 20 | |
Publication of unaudited financial information |
Group financial review, page 55 | |
Details of any long-term incentive schemes |
Remuneration report | |
Waiver of emoluments by a Director |
Not applicable | |
Waiver of future emoluments by a Director |
Not applicable | |
Non pre-emptive issues of equity for cash |
Not applicable | |
Non pre-emptive issues of equity for cash by any unlisted major subsidiary undertaking |
Not applicable | |
Parent company participation in a placing by a listed subsidiary |
Not applicable | |
Provision of services by a controlling shareholder |
Not applicable | |
Shareholder waiver of dividends |
Financial statements, Notes 16 and 44 | |
Shareholder waiver of future dividends |
Financial statements, Notes 16 and 44 | |
Agreements with controlling shareholders |
Not applicable | |
– |
has been drawn up and presented in accordance with and in reliance upon English company law and the liabilities of the Directors in connection with that Report shall be subject to the limitations and restrictions provided by such law. |
– |
was approved by the Board of Directors on 28 February 2022 and signed on its behalf by: |
| Remuneration |
||||||||
| In this section |
||||||||
| 120 |
||||||||
| 125 |
||||||||
| 143 |
||||||||
| 144 |
||||||||

– |
Bonus |
– |
Vesting of LTI awards pre-agreed measures for this award were: R&D new product performance; adjusted free cash flow; and relative TSR, each of which was equally weighted. Performance was measured over the three years to 31 December 2021. 74% of the R&D new product measure vested. This reflected delivery in strengthening the pipeline and the successful commercialisation of newly launched products. The continued strong focus on cash management and generation resulted in full delivery of the adjusted free cash flow measure. Disappointingly, the company’s relative TSR performance over the last three years has again resulted in this part of the award lapsing in full. The vested shares will be deferred for two years. See page 130. |
– |
Base salary |
– |
raise the target performance level to align to delivery at or above the IU ambitions; |
– |
reduce the reward previously available for lower than “on target” performance; |
– |
change the financial bonus measure from adjusted group PBIT to sales growth and adjusted operating profit growth in line with the key IU ambitions; |
– |
strengthen and focus strategic and operational measures for the Executive Directors to a few stretch and personal objectives aligned to quantifiable IU ambitions, reflecting personal areas of accountability. These would also reinforce our culture and Trust priority; and |
– |
given how fundamental ESG is to our DNA and success, it is important to recognise this through a specific performance condition to incentivise incremental year on year improvements against our public ambitions. |

– |
Annual Bonus measures will be: |
– |
LTI measures will be: |
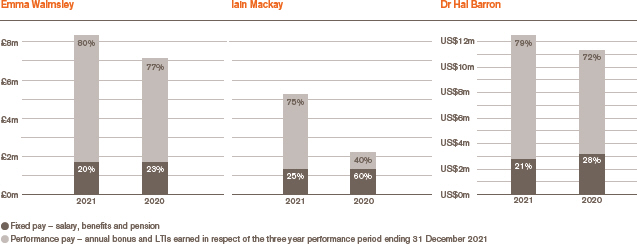

To align the interests of Executive Directors with those of shareholders, they are required to build and maintain significant holdings of shares in GSK over time. Executive Directors are required to continue to satisfy these Share Ownership Requirements (SOR) by holding 100% of their SOR for the first 12 months after leaving GSK and not less than 50% of their SOR for months 13-24 after leaving GSK. |
| Executive Directors and GLT |
SOR % of salary |
|||
| CEO |
650 |
|||
| Other Executive Directors |
300 |
|||
| Other GSK Leadership Team members |
200 |
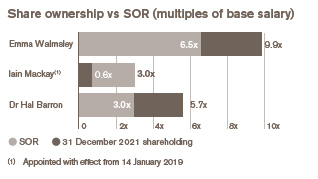

Key change: stronger link between short and long-term performance | ||
| – Annual Bonus and LTI performance measures are directly aligned to the Investor Update (IU) ambitions – The measures are complementary by design to ensure in-year performance delivers long-term sustained results– Annual Bonus and LTI performance calibration has been toughened meaning reduced reward for below target performance and maximum reward only for exceptional performance – Maximum annual bonus opportunity increased to 300% of salary (from 200% of salary) to enable recognition of exceptional outperformance when achieved |
– Target payout under the annual bonus of 100% of salary will align with our IU ambitions (ie. no increase for delivering our core ambitions) – Any reward for the incremental exceptional performance opportunity to be delivered fully in shares deferred for three years so as to align to shareholder experience, and – Annual Bonus and LTI measures and their alignment with the IU ambitions will be cascaded down to the GLT and wider organisation | |
How our incentive measures align to our strategy | ||
Performance measures |
Alignment to strategy |
 Weighting |
 Weighting | |||||||||||||
Relative total shareholder return |
 |
Alignment with shareholders as participants are only rewarded for strong shareholder returns |
– |
30% | ||||||||||||
| Total sales growth |
 |
 |
Top line growth to deliver against our IU ambition of more than 5% sales growth |
30% |
20% | |||||||||||
Adjusted operating profit growth |
 |
 |
Bottom line growth to deliver against our IU ambition of more than 10% profit growth |
30% |
20% | |||||||||||
| Pipeline |
 |
Increases the emphasis on Innovation and rewards the acceleration and strengthening of our pipeline |
– |
20% | ||||||||||||
| ESG ambitions |
 |
 |
Focus on our key ESG ambitions, including our Human Capital Management: I&D priorities and Nature Net Positive and Climate Net Zero 2030 ambitions |
10% |
10% | |||||||||||
Strategic and operational |
 |
Focus on key areas of individual accountability to underpin delivery of our strategy and public ambitions |
30% |
– | ||||||||||||
Key |
 |
Annual bonus |
 |
Long-term incentives | ||||||||||
2022 Executive Director Remuneration | ||||||||||||||
| |
Emma Walmsley |
Iain Mackay | ||||
| Fixed remuneration |
Salary |
£1,259,855 |
£915,335 | |||
Pension (% of salary) |
Will reduce to align with wider workforce by 1 January 2023 |
|||||
| Annual bonus (% of salary) |
Maximum opportunity: for three years |
|||||
Incremental Exceptional Performance: paid in shares all deferred for three years |
||||||
| LTI (% of salary) |
575% |
400% | ||||
Share ownership requirement (% of salary) |
650% |
300% | ||||
| Dr Hal Barron will transition to a Non-Executive Director with effect from 1 August 2022. | ||||||


Emma Walmsley |
Iain Mackay |
Dr Hal Barron(2) |
||||||||||||||||||||||||||||||||
2021 £000 |
2020 £000 |
2021 £000 |
2020 £000 |
2021 $000 |
2020 $000 |
|||||||||||||||||||||||||||||
| Fixed pay |
||||||||||||||||||||||||||||||||||
| Salary |
1,223 |
1,199 |
889 |
871 |
1,883 |
1,786 |
||||||||||||||||||||||||||||
| Benefits |
134 |
141 |
242 |
155 |
145 |
58 |
||||||||||||||||||||||||||||
| Pension |
245 |
245 |
178 |
175 |
651 |
1,247 |
||||||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Total fixed pay |
1,602 |
1,585 |
1,309 |
1,201 |
2,679 |
3,091 |
||||||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Pay for performance |
||||||||||||||||||||||||||||||||||
| Annual bonus (1) |
2,275 |
1,169 |
1,573 |
810 |
3,483 |
1,741 |
||||||||||||||||||||||||||||
| Vesting of LTI awards: |
||||||||||||||||||||||||||||||||||
| PSP (3) |
4,326 |
4,277 |
2,408 |
– |
6,371 |
6,387 |
||||||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Total pay for performance (4) |
6,601 |
5,446 |
3,981 |
810 |
9,854 |
8,128 |
||||||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Total remuneration |
£ |
8,203 |
£ |
7,031 |
£ |
5,290 |
£ |
2,011 |
$ |
12,533 |
$ |
11,219 |
||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
(1) |
Details of the mandatory bonus deferrals in 2021 and 2022 under the Deferred Annual Bonus Plan (DABP) are set out on page 140. |
(2) |
From 1 August 2021 Dr Barron’s base salary increased by 8% to reflect the creation of the One R&D organisation. This has brought scientists and governance across Pharmaceuticals and Vaccines together to focus on and invest in what matters most across the Group. |
(3) |
The 2019 PSP was valued based on the closing share price on 16 February 2022 of £15.76 and the closing ADS price of $43.39. Of the vested amounts for the Executive Directors, the amount attributable to share price appreciation over the performance period was for the CEO £149,246, the CFO £83,092 and the CSO $411,869. The Committee did not exercise any discretion in relation to the vesting of the awards or share price changes. |
(4) |
The Committee may in specific circumstances, and in line with stated principles, apply clawback/malus, as it determines appropriate. Following due consideration by the Committee, there has been no recovery of sums paid (clawback) or reduction of outstanding awards or vesting levels (malus) applied during 2021 in respect of any of the current Executive Directors. |


| % change and 2021 effective date |
Base salary |
|||||||||||
2021 |
2020 |
|||||||||||
| Emma Walmsley |
£1,223,160 |
£1,199,176 |
||||||||||
| Iain Mackay |
2% from 1 January |
£888,675 |
£871,250 |
|||||||||
| Dr Hal Barron |
$1,821,781 |
$1,786,060 |
||||||||||
| Dr Hal Barron (1) |
8% from 1 August |
$1,967,523 |
– |
|||||||||
| UK & US employees |
2% from 1 April |
– |
– |
|||||||||
(1) |
Base salary increased by 8% from 1 August 2021 to reflect the creation of the One R&D organisation. This has brought scientists and governance across Pharmaceuticals and Vaccines together to focus on and invest in what matters most across the Group. |
– |
Employee benefits |
– |
Business related services |
– |
Business travel: includes travel costs for the Executive Director and as appropriate for their spouse/partner associated with accompanying the Executive Director on GSK business which are deemed to be taxable benefits for the Executive Director. |
– |
Accommodation whilst on business travel. |
– |
Other benefits. |
| 2021 Benefits £000 |
2020 Benefits £000 |
|||||
| Emma Walmsley |
||||||
| Benefits available to employees |
71 |
62 |
||||
| Business related services (1) |
||||||
| Business travel |
22 |
36 |
||||
| Other benefits |
41 |
43 |
||||
| Total benefits |
134 |
141 |
||||
| Iain Mackay |
||||||
| Benefits available to employees |
131 |
149 |
||||
| Business related services (1) |
||||||
| Business travel |
9 |
5 |
||||
| Other benefits (2) |
102 |
1 |
||||
| Total benefits |
242 |
155 |
||||
| Dr Hal Barron |
$000 |
$ |
000 |
|||
| Benefits available to employees |
83 |
58 |
||||
| Business related services (1) |
||||||
| Business travel (3) |
63 |
– |
||||
| Accommodation whilst on business travel (4) |
(2) |
– |
||||
| Other benefits |
1 |
– |
||||
| Total benefits |
145 |
58 |
||||
(1) |
Business related services which tax regulations deem to be a taxable benefit in the UK and/or the US. |
(2) |
Iain Mackay’s Other benefits have increased year on year. This is mainly due to membership of a global business organisation which supports his work as CFO and is not recognised by UK HM Revenue & Customs so is therefore deemed to be a taxable benefit. This was not incurred in 2020. |
(3) |
Increased travel costs compared with 2020 following changes to COVID-19 restrictions. |
(4) |
One-off refund of accommodation costs relating to prior year. |
Executive Director |
Member since |
Pension arrangements in 2021 | ||
Emma Walmsley |
2010 |
Pension contributions of 20% of base salary and matching contributions on the first £13,333 of salary, with a cash | ||
Iain Mackay |
2019 |
supplement of 20% of base salary in lieu of pension on salary in excess of £13,333 (1) (2) . | ||
Dr Hal Barron |
2018 |
The CSO is a member of the 401(k) plan open to all US employees and the Executive Supplemental Savings Plan (ESSP), a savings scheme open to US executives to accrue benefits above the 401(k) plan limits. He receives 38% of base salary, less a contribution to the 401(k) and ESSP equivalent to 5% of total base salary and bonus (net of the bonus deferred under the DABP). In addition, in line with the wider US workforce, from 1 January 2021, a combined contribution rate under the 401(k) and ESSP plans of 11% (7% core contribution plus a match of up to 4%) of total base salary and bonus (net of the bonus deferred under the DABP). | ||
(1) |
As a member of the defined contribution plan, Emma Walmsley and Iain Mackay are eligible to receive a matching award of up to 5% on the first £13,333 of their salaries in accordance with the terms of the plan. |
(2) |
Emma Walmsley and Iain Mackay receive cash payments in lieu of pension of 20% of base salary in excess of £13,333, in line with GSK’s defined contribution pension plan rates. |
| Emma Walmsley |
Iain Mackay |
Dr Hal Barron |
||||||||||||||||||||||||||
Pension remuneration values |
2021 £000 |
2020 £000 |
2021 £000 |
2020 £000 |
2021 $000 |
2020 $000 |
||||||||||||||||||||||
| UK defined contribution |
3 |
5 |
3 |
5 |
– |
– |
||||||||||||||||||||||
| US defined benefit |
– |
– |
– |
– |
350 |
1,059 |
||||||||||||||||||||||
| Employer cash contributions |
242 |
240 |
175 |
170 |
301 |
188 |
||||||||||||||||||||||
| Total pension remuneration value |
245 |
245 |
178 |
175 |
651 |
1,247 |
||||||||||||||||||||||
Accrued pension |
Pension remuneration value for 2021 $000 |
|||||||||||||
Dr Hal Barron pension values |
31 December 2021 $000 |
31 December 2020 $000 |
||||||||||||
| US – Funded |
2 |
2 |
(6 |
) | ||||||||||
| US – Unfunded |
187 |
158 |
356 |
|||||||||||
| Total |
189 |
160 |
350 |
|||||||||||


Weighting |
2021 Adjusted Group PBIT performance |
|||||||||||||||
Performance measure |
Executive Directors |
2021 target |
Outcome |
Positioning against target |
||||||||||||
| Adjusted Group PBIT |
70 |
% |
£ |
8,254m |
£ |
8,562m |
104 |
% | ||||||||
| Individual objectives |
30 |
% |
||||||||||||||
2021 bonus opportunity |
2021 bonus outcome | |||||||||||||
Bonus |
Target (% of salary) |
Maximum (% of salary) |
2021 Base salary |
Financial performance (% of salary) |
Individual objectives (% of salary) |
Total 2021 bonus (% of salary) |
Total 2021 bonus 000 | |||||||
| Emma Walmsley |
£1,223,160 |
60 |
186 |
£2,275 | ||||||||||
| Iain Mackay |
100 |
200 |
£888,675 |
126 |
51 |
177 |
£1,573 | |||||||
| Dr Hal Barron |
$1,967,523 |
51 |
177 |
$3,483 | ||||||||||
Financial performance |
– |
Overall an encouraging performance exceeding updated guidance despite the uncertainties of the COVID-19 pandemic. |
– |
Delivered full-year reported Group sales of £34 billion (stable AER, +5% CER) with strong commercial execution driving CER growth across Pharmaceuticals, Vaccines and Consumer Healthcare (excluding brands divested/under review) including COVID-19 solutions sales of £1.4 billion. |
– |
Adjusted Group PBIT of £8,839 million above target driven by higher sales and effective cost control. Outcome adjusted to exclude the commercial benefit from COVID-19 solutions. |
– |
Adjusted EPS of 113.2p (-2% AER, +9% CER), ahead of guidance including COVID-19 solutions, delivery driven by higher sales and effective cost control. |
| Individual objectives |
Achievements | |
Emma Walmsley |
||
The Committee determined that the CEO clearly exceeded or met her individual objectives. 2021 was a highly successful year of focus and acceleration against GSK’s long-term IPT priorities, and the company exceeded its financial targets. GSK is on track for separation to unlock the potential of two new growth companies in a landmark year for the company in 2022: | ||
| Strengthen pipeline and build GSK’s reputation for Innovation |
– Continued progress in strengthening and advancing Pharmaceuticals and Vaccines pipeline, with 43 potential new medicines and 21 vaccine candidates in development – COVID-19 solutions focussed on prevention and treatment, including Xevudy (sotrovimab) launched for treatment, with positive data against Omicron | |
| Drive growth and return on investment |
– Delivered EPS ahead of initial and updated guidance, with sales growth driven by commercial execution excellence. Pharmaceuticals sales £17.7 billion, Vaccines £6.8 billion and Consumer Healthcare £9.6 billion | |
| Demonstrate continued commercial execution excellence |
– Transformed Specialty Medicine commercial capabilities and effectiveness across key markets – Exceptional supply chain reliability through continued COVID-19 disruption, and continued network strengthening and simplification | |
| Deliver separation programme milestones |
– All demerger milestones on track. – New ambitions set out for new GSK to deliver a step change in growth and performance, and health impact at scale | |
| Demonstrate strong Environmental, Social and Governance (ESG) credentials and build trust in future delivery |
– Sustained leading ESG performance, with delivery against all Global Health, Environment and Inclusion and Diversity commitments. Maintained sector-leading rankings in key ESG indices, as well as progress to deliver on climate and nature commitments | |
| Demonstrate strong culture and leadership |
– Culture and talent to deliver success for both new companies, and strong progress to build a stronger, more diverse workforce (40% senior female representation; on track for 2025 gender and race & ethnicity aspirations) – Continued development and succession planning for leadership team roles, with internal candidates appointed Chief Scientific Officer Designate and Chief Digital and Technology Officer | |
Iain Mackay |
||
The Committee determined that the CFO successfully met his individual objectives: | ||
| Demonstrate financial leadership |
– Strong financial leadership, with key role in delivery of Investor Update setting out competitive growth profile for new GSK – Delivered full year reported Group sales of £34.1 billion (stable at AER, +5% CER) | |
| Demonstrate financial oversight and cost discipline |
– Adjusted EPS of 113.2p (-2% AER, +9% CER) ahead of updated guidance, delivery supported by cost discipline and initial savings from scale transformation programme | |
| Deliver separation programme milestones |
– Separation preparations on track, including corporate finance and capital market readiness | |
| Demonstrate strong culture and leadership |
– Strong oversight across Finance and Tech during transformation, including appointment of new Head of Investor Relations and Chief Digital and Technology Officer | |
Dr Hal Barron |
||
The Committee determined that the CSO successfully met his individual objectives: | ||
| Strengthen pipeline and build GSK’s reputation for Innovation |
– Continued R&D momentum both in R&D delivery and strengthening of pipeline, with pipeline progress targets exceeded. 12 approvals, 8 Phase III starts and 6 Phase II starts. 43 potential new medicines and 21 vaccine candidates in development. Business development to augment the pipeline, including: Vir, iTeos, Alector and Halozyme | |
| Drive growth and return on investment |
– Continued progress to improve R&D productivity and success rates, including achieving US FDA emergency use authorisation for Xevudy in 13 months from deal signing with Vir in pre-clinical phase. This medicine has proven effective against multiple COVID-19 variants, including Omicron– Creation of One R&D organisation, bringing scientists and governance across Pharmaceuticals and Vaccines together to focus on and invest in what matters most | |
| Demonstrate strong culture and leadership |
– Continuing focus on top talent in key roles in R&D (80%, with 31% of new talent in key roles external hires). Robust succession planning, including appointment of new Global Head of Vaccines R&D and Global Head of Oncology Development | |

| Performance measures and relative weighting |
Outcome and vesting level |
|||||||||||||||||||||||||||
Performance targets |
Outcome |
% of maximum |
% of award |
|||||||||||||||||||||||||
R&D new product performance (1/3rd) |
R&D new product sales performance measures aggregate three-year sales for new products launched in the three-year performance period and the preceding two years, ie 2017-21. |
|
£ |
11.12bn |
74 |
24.66 |
||||||||||||||||||||||
Target |
% vesting |
|||||||||||||||||||||||||||
Maximum |
£12.25bn |
100% |
||||||||||||||||||||||||||
£11.14bn |
75% |
|||||||||||||||||||||||||||
£10.58bn |
50% |
|||||||||||||||||||||||||||
Threshold |
£10.02bn |
25% |
||||||||||||||||||||||||||
Adjusted free cash flow performance (1/3rd) |
In line with the company’s agreed principles, the AFCF figures included adjustments for a number of material distorting items, including legal settlements, exchange rate movements and special pension contributions. |
|
£ |
14.53bn |
100 |
33.33 |
||||||||||||||||||||||
Original target |
Revised target (1) |
% vesting |
||||||||||||||||||||||||||
Maximum |
£13.91bn |
£13.20bn |
100% |
|||||||||||||||||||||||||
£13.31bn |
£12.63bn |
75% |
||||||||||||||||||||||||||
£12.10bn |
£11.48bn |
50% |
||||||||||||||||||||||||||
Threshold |
£11.74bn |
£11.14bn |
25% |
|||||||||||||||||||||||||
| (1) The revised target has been further adjusted since the 2020 Annual Report as noted above. |
|
|||||||||||||||||||||||||||
| Relative TSR performance (1/3rd) |
TSR ranking within comparator group (2) |
% vesting |
Ranked 10th |
0 |
0 |
|||||||||||||||||||||||
Maximum |
1st, 2nd, 3rd |
|
100% |
|||||||||||||||||||||||||
4th |
|
70% |
||||||||||||||||||||||||||
5th |
|
40% |
||||||||||||||||||||||||||
Threshold (3) |
Median |
|
25% |
|||||||||||||||||||||||||
6th to 10th |
|
0% |
||||||||||||||||||||||||||
| (2) TSR comparator group: AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GSK, Johnson & Johnson, Merck & Co, Novartis, Pfizer, Roche Holdings and Sanofi. |
|
|||||||||||||||||||||||||||
| (3) The vesting schedule is based on delivering 25% vesting for median performance. In a comparator group of ten companies, median falls between two companies. |
|
|||||||||||||||||||||||||||
| Total vesting in respect of 2019 awards |
58 |
% |
57.99 |
% | ||||||||||||||||||||||||


Vesting% |
||||||||||||||||||||||||
Year of grant |
Relative TSR |
Adjusted free cash flow |
R&D new product |
Business diversification |
Lapsed % |
Total vested % |
||||||||||||||||||
| 2011 |
0 |
13 |
16 |
11 |
60 |
40 |
||||||||||||||||||
| 2012 |
0 |
0 |
7 |
7 |
86 |
14 |
||||||||||||||||||
| 2013 |
0 |
0 |
21 |
17 |
62 |
38 |
||||||||||||||||||
| 2014 |
0 |
0 |
33 |
67 |
33 |
|||||||||||||||||||
| 2015 |
15 |
21 |
33 |
31 |
69 |
|||||||||||||||||||
| 2016 |
0 |
26 |
33 |
41 |
59 |
|||||||||||||||||||
| 2017 |
0 |
33 |
33 |
33 |
67 |
|||||||||||||||||||
| 2018 |
0 |
33 |
33 |
33 |
67 |
|||||||||||||||||||
| 2019 |
0 |
33 |
25 |
42 |
58 |
|||||||||||||||||||
2021 DABP awards |
2021 PSP awards |
|||||||||||||||||||||||
2020 % of total bonus deferred |
Number of shares |
Face value of award (1) |
Award level as % of base salary |
Number of shares |
Face value of award (2)(3) |
|||||||||||||||||||
| Emma Walmsley |
45,779 shares |
£ |
0.585m |
575 |
% |
550,757 shares |
£ |
7.0m |
||||||||||||||||
| Iain Mackay |
50 |
% |
31,725 shares |
£ |
0.405m |
400 |
% |
278,363 shares |
£ |
3.6m |
||||||||||||||
| Dr Hal Barron |
24,355 ADS |
$ |
0.871m |
500 |
% |
254,794 ADS |
$ |
9.1m |
||||||||||||||||
(1) |
The face values of the DABP awards have been calculated based on a share price of £12.77 and an ADS price of $35.75, being the closing prices on 9 February 2021 (the day before grant). These are nil-cost options for the UK Executive Directors and restricted shares for the US Executive Director. No performance conditions are attached to the DABP awards, as they reflect the mandatory 3 year deferrals in respect of the 2020 annual bonus earned. |
(2) |
The face values of the PSP awards have been calculated based on a share price of £12.77, and an ADS price of $35.75, being the closing prices on 9 February 2021 (the day before grant). These are conditional shares, based on the performance measures outlined above. |
(3) |
The performance period for the 2021 PSP awards is from 1 January 2021 to 31 December 2023. Awards vest at 25% of maximum for threshold performance. |

| In setting executive pay it is important that the Committee and I do so with a good understanding of our wider workforce pay. To that end on an annual basis I meet with our Human Resources Business Leaders of Global Support Functions, Pharmaceuticals, ViiV Healthcare, Vaccines and Consumer Healthcare to understand perspectives on pay and GSK’s remuneration package for the wider workforce. This year was the third such annual meeting I have held. I was pleased to discuss progress on the Group’s human capital management and I&D agenda to attract and retain diverse talent which lies at the heart of the company’s fundamental commitment to the equity of its employment and reward practices. At the meeting, we covered the current Reward environment for employees across the enterprise and notable global competitive challenges facing the company; namely: – Competitive pressures for in-high demand skills in our businesses and the actions taken to attract and retain key talent in these areas– Handling different pay levels across the Group and in different geographies. This included where the company was experiencing particular pay challenges currently or were anticipated to experience in the future and the mitigatory steps that were being taken to address these – Preparation of a competitive Reward strategy and programmes for the Consumer company for implementation after the demerger – Progress against the company’s publicly disclosed gender and ethnically diverse leader aspirations. We discussed the country-based reviews and the clear guidance, tools and support provided to markets to ensure pay equity Finally, Dame Vivienne Cox, our Workforce Engagement Director and member of the Committee, ensures that employee views and perspectives on pay and reward are reflected in the Committee’s discussions. Urs Rohner Remuneration Committee Chair |
Element |
Wider workforce pay |
Comparison with Executive Director and GLT pay | ||
Salary |
– The market competitiveness of salaries across the company is assessed at a local market level. The competitiveness of roles, which is measured against the external market and internal peers, is kept under regular review |
– For our Executive Directors and for the GLT, ordinarily following a performance review, increases in base salaries are in line with the average of the wider employee population unless there is a change in scope of the individual’s role, responsibilities or experience | ||
Pensions and benefits |
– The company seeks to provide an appropriate pensions and benefits package that is aligned to competitive market practices in those countries in which the company operates and our employees are based |
– Our Executive Directors and the GLT are eligible to receive benefits broadly in line with the policy for our other employees, which may vary by location – Pension arrangements are structured in accordance with where our Executive Director or GLT member is expected to retire. Current and future UK and US Executive Directors will have their pension arrangements aligned to the wider UK and US workforce by 1 January 2023 | ||
Annual bonus |
– With the exception of our sales force, who participate in separate arrangements, our wider workforce participates in a plan based on performance against four business and financial measures (three measures for Consumer Healthcare). This is structured to reflect the priorities of the specific business area – This plan is designed to reward our employees’ collective contribution to business achievement. Separate mechanisms are in place to recognise outstanding individual performance or to address under-performance |
– Our Executive Directors and the GLT participate in a plan based on an assessment of a combination of stretching financial / business and personal objectives – Our Executive Directors are required to defer 50% – and the GLT 25% – of any bonus earned into shares or ADSs as appropriate for three years – Clawback and/or malus provisions apply | ||
LTI plans |
– Our employees at Senior Vice President (SVP) and Vice President (VP) level participate in the same PSP as our Executive Directors and the GLT with the same performance targets and periods – Clawback and/or malus provisions apply – Our SVP and VP employees, together with Directors and Managers below the GLT, receive annual Share Value Plan awards of restricted shares |
– Our Executive Directors and the GLT are granted annual PSP awards with the same performance targets and periods – Our Executive Directors are required to hold vested awards for an additional two-year period– Clawback and/or malus provisions apply – Our Executive Directors and the GLT do not receive Share Value Plan awards following appointment |
Financial year |
Methodology |
(Lower Quartile) P25 |
(Median) P50 |
(Upper Quartile) P75 |
||||||||||
| 2021 |
154:1 |
108:1 |
67:1 |
|||||||||||
| 2020 |
Option A |
130:1 |
96:1 |
62:1 |
||||||||||
| 2019 |
160:1 |
119:1 |
73:1 |
|||||||||||
2021 |
2020 |
2019 |
2021 |
2020 |
2019 |
2021 |
2020 |
2019 | ||||||||||
£ |
P25 |
P50 |
P75 | |||||||||||||||
| Salary |
37,251 |
36,924 |
34,510 |
51,492 |
50,000 |
47,029 |
72,997 |
70,203 |
66,561 | |||||||||
| Total pay and benefits |
53,151 |
54,133 |
50,467 |
76,234 |
73,340 |
68,200 |
122,852 |
113,830 |
110,638 | |||||||||
Financial Year |
Methodology |
P25 |
P50 |
P75 | ||||
| 2021 |
73:1 |
51:1 |
34:1 | |||||
| 2020 |
Option A* |
51:1 |
38:1 |
26:1 | ||||
| 2019 |
65:1 |
48:1 |
32:1 | |||||
* |
Total remuneration less vesting of long-term incentive awards. |
Emma Walmsley |
£000 |
|||||||||||||||||||
2021 |
2020 |
2019 |
2018 |
2017 |
||||||||||||||||
| Total remuneration |
8,203 |
7,031 |
8,094 |
5,887 |
4,883 (1) |
|||||||||||||||
| Annual bonus award (2) (% of maximum) |
93% |
49% |
79% |
93% |
77% |
|||||||||||||||
| Vesting of LTI awards (% of maximum) |
58% |
67% |
67% |
59% |
69% |
|||||||||||||||
Sir Andrew Witty |
£000 |
|||||||||||||||||||
2017 |
2016 |
2015 |
2014 |
2013 |
||||||||||||||||
| Total remuneration |
715 (2) |
6,830 |
6,661 |
3,902 |
7,207 |
|||||||||||||||
| Annual bonus award (2) (% of maximum) |
0% (2) |
97% 100% |
42% |
88% |
||||||||||||||||
| Vesting of LTI awards (% of maximum) |
0% (3) |
33% |
38% |
14% |
31% |
|||||||||||||||
(1) |
Emma Walmsley’s total remuneration includes her pay for the period 1 January to 31 March 2017, before she became CEO. |
(2) |
Sir Andrew Witty received a pro-rata payment for 2017 in lieu of a variable bonus opportunity, in accordance with the 2014 Remuneration policy. |
(3) |
PSP and DABP awards for Sir Andrew Witty granted in 2015 did not vest until April 2018, in accordance with the terms of the Executive financial recoupment policy. |

2021 percentage change |
2020 percentage change |
|||||||||||||||||||||||
Salary/fee |
Benefits |
Bonus |
Salary/fee |
Benefits |
Bonus |
|||||||||||||||||||
% |
% |
% |
% |
% |
% |
|||||||||||||||||||
| UK Employees (1) |
2.0 |
0.0 |
4.85 |
2.5 |
0.0 |
1.1 |
||||||||||||||||||
| Executive Directors (2,3) |
||||||||||||||||||||||||
| Emma Walmsley |
2.0 |
(5.0 |
) |
94.6 |
8.0 |
(26.6 |
) |
(33.4 |
) | |||||||||||||||
| Iain Mackay |
2.0 |
56.1 |
94.2 |
5.6 |
11.5 |
(31.6 |
) | |||||||||||||||||
| Dr Hal Barron |
5.4 |
150.0 |
100.1 |
2.5 |
(91.2 |
) |
(34.9 |
) | ||||||||||||||||
| Non-Executive Directors(2,4) |
||||||||||||||||||||||||
| Sir Jonathan Symonds |
– |
50.0 |
– |
201.7 |
0.0 |
– |
||||||||||||||||||
| Charles Bancroft (5) |
156.1 |
– |
– |
– |
– |
– |
||||||||||||||||||
| Vindi Banga |
(4.6 |
) |
(50.0 |
) |
– |
23.6 |
(50.0 |
) |
– |
|||||||||||||||
| Dr Anne Beal (5) |
– |
– |
– |
– |
– |
– |
||||||||||||||||||
| Dame Vivienne Cox |
(5.6 |
) |
(50.0 |
) |
– |
55.4 |
(75.0 |
) |
– |
|||||||||||||||
| Lynn Elsenhans |
(7.3 |
) |
(75.0 |
) |
– |
(12.3 |
) |
(73.3 |
) |
– |
||||||||||||||
| Dr Laurie Glimcher |
(8.3 |
) |
(61.8 |
) |
– |
(18.2 |
) |
(55.3 |
) |
– |
||||||||||||||
| Dr Jesse Goodman |
(5.6 |
) |
– |
– |
(12.5 |
) |
(65.2 |
) |
– |
|||||||||||||||
| Urs Rohner |
(5.6 |
) |
175.0 |
– |
16.3 |
(69.2 |
) |
– |
||||||||||||||||
| Judy Lewent (6) |
(73.8 |
) |
(25.0 |
) |
– |
(17.6 |
) |
(85.4 |
) |
– |
||||||||||||||
(1) |
The UK employee population was considered to be the most relevant comparison as it most closely reflects the economic environment encountered by the majority of the Executive Directors. |
(2) |
Percentage changes have been calculated based on the 2021 Total remuneration table on page 125 for Executive Directors and the 2021 Total fees table on page 139 for Non-Executive Directors. |
(3) |
Further information on salary and benefits for Executive Directors can be found on page 126. Further information on annual bonus for Executive Directors can be found on page 128. |
(4) |
Fees of Non-Executive Directors include fees received as cash and in the form of shares or ADS under the terms of the Non-Executive Directors’ share allocation plan. |
(5) |
Charles Bancroft and Dr Anne Beal were appointed to the Board on 1 May 2020 and 6 May 2021 respectively. |
(6) |
Judy Lewent retired from the Board on 5 May 2021. |
Change |
2021 |
2020 |
||||||||||
% |
£m |
£m |
||||||||||
| Total employee pay |
(12.2 |
) |
9,003 |
10,249 |
||||||||
| Dividends paid in the year |
0.6 |
3,999 |
3,977 |
|||||||||

European cross-industry comparator group | ||||||
| Emma Walmsley |
Roche Holding AG |
Linde |
Deutsche Telekom | |||
| Iain Mackay |
Novartis |
Sanofi |
Kering | |||
LVMH |
AstraZeneca |
Heineken | ||||
Anheuser-Busch Inbev |
Diageo |
BASF | ||||
Unilever |
Siemens |
Vinci | ||||
SAP |
Christian Dior |
Adidas | ||||
L’Oreal |
Inditex |
Bayer | ||||
Novo Nordisk A/S |
BAT |
Safran | ||||
Airbus |
Volkswagen |
Reckitt Benckiser | ||||
Global pharmaceutical comparator group | ||||||
| Dr Hal Barron |
France |
US |
||||
Sanofi |
AbbVie (1) |
|||||
Switzerland |
Amgen (1) |
|||||
Novartis |
Bristol-Myers Squibb |
|||||
Roche Holdings |
Eli Lilly |
|||||
Johnson & Johnson |
||||||
UK |
Merck & Co |
|||||
AstraZeneca |
Pfizer |
|||||
(1) |
AbbVie and Amgen are included for remuneration benchmarking, but are not included in the relative TSR comparator group. |


* |
This index comprises AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck & Co, Novartis, Pfizer, Roche Holdings and Sanofi. |

Base salary |
2022 |
% change |
||||||
| Wider workforce (1) |
– |
|||||||
| Emma Walmsley |
£ |
1,259,855 |
||||||
| Iain Mackay |
£915,335 |
3.0 |
||||||
| Dr Hal Barron (2) |
$ |
2,026,549 |
||||||
(1) |
Based on the average increase budget for employees below the level of GLT in the UK and US. |
(2) |
Dr Barron will transition to a Non-Executive Director with effect from 1 August 2022. |
2022 Pension contribution | ||
| Emma Walmsley Iain Mackay |
20% of base salary and matching contributions of 5% on the first £13,333 of salary in accordance with the terms of the plan open to all employees, and 20% of base salary in lieu of pension on salary in excess of £13,333 | |
Dr Hal Barron (1) |
38% of base salary, less a contribution to the 401(k) and ESSP equivalent to 5% of total base salary and bonus (net of the bonus deferred under the DABP). In addition, in line with the wider US workforce, from 1 January 2021, a combined contribution rate under the 401(k) and ESSP plans of 11% (7% core contribution plus a match of up to 4%) of total base salary and bonus (net of the bonus deferred under the DABP). | |
(1) |
Dr Barron will transition to a Non-Executive Director with effect from 1 August 2022. |
| Bonus opportunity % of salary |
||||||||||||
Target |
Maximum |
Exceptional performance (1) |
||||||||||
| Emma Walmsley |
||||||||||||
| Iain Mackay |
100 |
200 |
300 |
|||||||||
| Dr Hal Barron |
||||||||||||
(1) |
Exceptional performance: up to an additional 100% of salary fully paid in shares deferred for three years. |
Weighting of performance measures % |
||||||||||||||||
Total sales growth |
Adjusted operating profit growth |
Strategic and operational measures |
ESG: Inclusion & Diversity |
|||||||||||||
| Emma Walmsley |
||||||||||||||||
| Iain Mackay |
30 |
30 |
30 |
10 |
||||||||||||
| Dr Hal Barron |
||||||||||||||||
| Total bonus deferred into shares % |
DABP awards |
|||||||||||
Shares |
ADS |
|||||||||||
| Emma Walmsley |
72,399 |
|||||||||||
| Iain Mackay |
50 |
50,056 |
||||||||||
| Dr Hal Barron |
40,617 |
|||||||||||
% of salary |
Shares |
|||||||
| Emma Walmsley |
575 |
461,059 |
||||||
| Iain Mackay |
400 |
233,028 |
||||||
LTI measure |
Measure |
Weighting |
||||||
| Innovation |
Pipeline progress |
20% |
||||||
| Performance |
Relative TSR |
30% |
||||||
Total Sales Growth |
20% |
|||||||
Adjusted Operating Profit Growth |
20% |
|||||||
| Trust |
ESG: Environment (1) |
10% |
||||||
(1) |
A composite scorecard incorporating Scope 1 & 2 Targets for which assessment of performance against this metric will be determined in line with the World Resources Institute/World Business Council for Sustainable Development GHG Protocol methodology for accounting and reporting of our emissions footprint. |
Performance level |
Points |
Payout |
||||||
| Below Threshold |
<11 |
Nil |
||||||
| Threshold |
11 |
25% |
||||||
13 |
50% |
|||||||
15 |
75% |
|||||||
| Maximum Major Regulatory Approvals |
17 |
100% |
||||||
Performance level |
Points |
Payout |
||||||
| Below Threshold |
<16 |
Nil |
||||||
| Threshold |
16 |
25% |
||||||
18 |
50% |
|||||||
20 |
75% |
|||||||
| Maximum |
22 |
100% |
||||||
Value of holdings as % of salary |
||||||||||||
SOR % of salary |
27 February 2022 |
31 December 2021 |
||||||||||
| Emma Walmsley |
650 |
1,292 |
985 |
|||||||||
| Iain Mackay |
300 |
261 |
64 |
|||||||||
| Dr Hal Barron |
300 |
799 |
566 |
|||||||||

Total votes cast (billion) |
Total votes for (%) |
Total votes against (%) |
Votes withheld (million) |
|||||||||||||
| Remuneration report |
||||||||||||||||
| 2021 AGM |
3.5 |
93.1 |
6.9 |
15.4 |
||||||||||||
| Remuneration policy |
||||||||||||||||
| 2020 AGM |
2.7 |
88.2 |
11.8 |
620.1 |
||||||||||||
Date of contract |
Effective date |
Expiry date | ||||
| Emma Walmsley |
29.03.17 |
01.04.17 |
30.06.34 | |||
| Iain Mackay |
18.09.18 |
14.01.19 |
n/a | |||
| Dr Hal Barron (1) |
16.12.17 |
01.01.18 |
31.12.24 |
(1) |
Dr Barron will transition to a Non-Executive Director (with a letter of appointment) with effect from 1 August 2022. |
– |
Proposed 2022 Remuneration policy |
– |
Remuneration impact of major Group restructuring and CH demerger |
– |
Engagement with shareholders and consideration of feedback |
– |
Review of remuneration environment and wider employee trends |
– |
Executive Director and GLT benchmarking, competitiveness and GSK comparator groups |
– |
GLT and Company Secretary salary review and recommendations for 2021 |
– |
Executive Director salary review and recommendations for 2022 |
– |
LTI performance outcomes and vesting of LTI awards for GLT and below |
– |
Confirmation of LTI grants for GLT and below |
– |
Remuneration considerations and committee programme for 2021 |
– |
Review of Terms of Reference |
– |
Committee evaluation annual review |
– |
2020 Remuneration report |
– |
Confirmation of 2021 Group Budget for remuneration purposes |
– |
AGM and Remuneration report feedback, the external remuneration environment and performance target disclosure for incentive plans |
– |
2021 Remuneration report disclosures, including CEO pay ratio |
– |
Annual governance meeting key Committee messages |
– |
Committee Chair consultation with employee representatives on setting pay and wider workforce pay practices |
Per annum | ||
Standard annual fee |
£95,000 | |
Supplemental fees |
||
Chair of the Audit & Risk Committee |
£80,000 | |
Senior Independent Director |
£50,000 | |
Scientific & Medical Experts |
£30,000 | |
| Chairs of the Remuneration, Corporate Responsibility and Science Committees Workforce Engagement Director |
£40,000 | |
Non-Executive Director undertaking intercontinental travel to meetings |
£7,500 per meeting | |
| Non-Executive Directors’emoluments (000) (audited) |
2021 |
2020 |
||||||||||||||||||||||||||||||
| Fixed fees |
Fixed fees |
|||||||||||||||||||||||||||||||
Cash |
Shares/ADS |
Benefits |
Total pay |
Cash |
Shares/ADS |
Benefits |
Total pay |
|||||||||||||||||||||||||
| Sir Jonathan Symonds |
£525 |
£175 |
£3 |
£703 |
£525 |
£175 |
£2 |
£702 |
||||||||||||||||||||||||
| Vindi Banga |
£109 |
£36 |
£1 |
£146 |
£114 |
£38 |
£2 |
£154 |
||||||||||||||||||||||||
| Charles Bancroft |
– |
$210 |
$5 |
$215 |
– |
$82 |
– |
$82 |
||||||||||||||||||||||||
| Dr Anne Beal |
$62 |
$21 |
– |
$83 |
– |
– |
– |
– |
||||||||||||||||||||||||
| Dame Vivienne Cox |
£101 |
£34 |
£1 |
£136 |
£107 |
£36 |
£2 |
£145 |
||||||||||||||||||||||||
| Lynn Elsenhans |
$134 |
$45 |
$5 |
$184 |
$93 |
$100 |
$20 |
$213 |
||||||||||||||||||||||||
| Dr Laurie Glimcher |
– |
$165 |
$13 |
$178 |
– |
$180 |
$34 |
$214 |
||||||||||||||||||||||||
| Dr Jesse Goodman |
$164 |
$55 |
$23 |
$242 |
$174 |
$58 |
$23 |
$255 |
||||||||||||||||||||||||
| Urs Rohner |
£101 |
£34 |
£11 |
£146 |
£107 |
£36 |
£4 |
£147 |
||||||||||||||||||||||||
| Judy Lewent (1) |
$48 |
$16 |
$9 |
$73 |
$183 |
$61 |
$12 |
$256 |
||||||||||||||||||||||||
(1) |
Retired from the Board on 5 May 2021. |

As at 31 December 2021 |
||||||||||||||||||||||||
| |
Unvested share plan interests |
|||||||||||||||||||||||
Total directors’ interests as at |
Beneficial interests |
Not subject to performance |
Subject to performance |
|||||||||||||||||||||
27 February 2022 (1) |
31 December 2021 (1) |
Shares/ADS (2) |
Shares/ADS (3,7) |
Options (4,7) |
Shares/ADS (5) |
|||||||||||||||||||
| |
|
|
|
|
|
|
||||||||||||||||||
Shares |
||||||||||||||||||||||||
| Emma Walmsley |
1,521,133 |
1,195,364 |
364,520 |
654,043 |
176,801 |
1,495,049 |
||||||||||||||||||
| |
|
|
|
|
|
|
||||||||||||||||||
| Iain Mackay |
275,681 |
71,972 |
– |
– |
71,972 |
779,782 |
||||||||||||||||||
| |
|
|
|
|
|
|
||||||||||||||||||
ADS |
||||||||||||||||||||||||
| Dr Hal Barron |
519,723 |
424,186 |
224,353 |
199,833 |
– |
740,680 |
||||||||||||||||||
| |
|
|
|
|
|
|
||||||||||||||||||
1) |
Total directors’ interests |
2) |
Beneficial interests |
3) |
Unvested shares/ADS not subject to performance two-year holding period for Emma Walmsley and Dr Barron. Unvested ADS not subject to performance for Dr Barron also represent bonus deferrals (as described in note 7 below). |
4) |
Unvested options not subject to performance nil-cost options (as described in note 7 below). |
5) |
Unvested shares/ADS subject to performance |
6) |
Vested but unexercised options: |
7) |
DABP: |
Deferred Annual Bonus Plan (Bonus deferrals) |
27 February 2022 |
31 December 2021 |
1 January 2021 |
|||||||||
| Shares |
||||||||||||
| Emma Walmsley |
178,962 |
176,801 |
189,554 |
|||||||||
| Iain Mackay |
122,866 |
71,972 |
36,655 |
|||||||||
| ADS |
||||||||||||
| Dr Hal Barron |
100,301 |
101,801 |
72,192 |
|||||||||
DABP |
Date of grant |
Number of shares under option |
Date of exercise |
Grant price |
Market price at exercise |
Gain on exercise (000) |
||||||||||||||||||
| Emma Walmsley |
||||||||||||||||||||||||
| Deferral award |
01.03.18 |
68,716 |
01.03.21 |
£ |
0.00 |
£ |
12.11 |
£ |
832 |
|||||||||||||||
| Share allocation plan for Non-Executive Directors |
||||||||||||||||||||||||||||||||
Total directors’ interests as at (1) |
Number of shares/ADS |
|||||||||||||||||||||||||||||||
27 February 2022 |
31 December 2021 |
Beneficial interests at 31 December 2021 (2) |
Dividends reinvested after year end |
31 December 2021 |
Elected & allocated during the year (3) |
1 January 2021 |
||||||||||||||||||||||||||
| Shares |
||||||||||||||||||||||||||||||||
| Sir Jonathan Symonds |
64,467 |
63,474 |
35,757 |
993 |
27,717 |
15,865 |
11,851 |
|||||||||||||||||||||||||
| Vindi Banga |
106,013 |
104,473 |
71,800 |
1,541 |
32,673 |
4,780 |
27,893 |
|||||||||||||||||||||||||
| Dame Vivienne Cox |
10,997 |
10,548 |
– |
449 |
10,548 |
3,345 |
7,203 |
|||||||||||||||||||||||||
| Urs Rohner |
17,168 |
16,427 |
– |
741 |
16,427 |
3,673 |
12,754 |
|||||||||||||||||||||||||
| ADS |
||||||||||||||||||||||||||||||||
| Charles Bancroft |
7,665 |
7,466 |
– |
199 |
7,466 |
6,099 |
1,367 |
|||||||||||||||||||||||||
| Dr Anne Beal |
509 |
504 |
◾ |
5 |
504 |
504 |
– |
|||||||||||||||||||||||||
| Dr Hal Dietz |
– |
– |
– |
– |
– |
– |
– |
|||||||||||||||||||||||||
| Lynn Elsenhans |
47,168 |
44,984 |
1,000 |
2,184 |
43,984 |
3,849 |
40,135 |
|||||||||||||||||||||||||
| Dr Laurie Glimcher |
23,664 |
22,653 |
– |
1,011 |
22,653 |
6,039 |
16,614 |
|||||||||||||||||||||||||
| Dr Jesse Goodman |
10,695 |
10,223 |
– |
472 |
10,223 |
2,136 |
8,086 |
|||||||||||||||||||||||||
| Judy Lewent (4) |
– |
– |
– |
– |
– |
1,928 |
18,892 |
|||||||||||||||||||||||||
1) |
Total directors’ interests Non-Executive Directors’ share allocation plan. Dividends received on shares/ADS under the plan during the year and in January 2022 were converted into shares/ADS as at 13 January 2022. |
2) |
Beneficial interests Non-Executive Directors and their PCAs. |
3) |
Shares/ADS allocated during the year Non-Executive Directors’ share allocation plan cover five quarters of allocations for the period from October 2020 to December 2021 due to a change in the timing of allocations during 2021. Shares/ADS allocated also includes dividends reinvested during the year. |
4) |
Judy Lewent retired from the Board on 5 May 2021, at which time her holding of 20,820 ADS under the Non-Executive Directors’ share allocation plan was released to her under the terms of the plan. The holding was subject to UK income tax. |
Remuneration for 2021 |
£ |
|||
| Total compensation paid |
29,205,417 |
|||
| Aggregate increase in accrued pension benefits (net of inflation) |
39,483 |
|||
Aggregate payments to defined contribution schemes |
1,421,723 |
|||
| |
Awards |
|
Dividend reinvestment awards |
|||||||||||||||
Awarded during 2021 |
Shares |
ADS |
Shares |
ADS |
||||||||||||||
| Performance Share Plan |
2,305,483 |
471,211 |
351,369 |
83,884 |
||||||||||||||
| Deferred Investment Awards (1,2) |
274,510 |
– |
18,759 |
– |
||||||||||||||
| Share Value Plan (2) |
16,380 |
– |
– |
– |
||||||||||||||
1) |
Notional shares and ADS. |
2) |
Executive Directors are not eligible to receive Deferred Investment Awards or participate in the Share Value Plan. |

Interests at 27 February 2022 |
Shares |
ADS |
||||||
| Owned |
2,482,185 |
526,342 |
||||||
| Unexercised options |
3,440 |
– |
||||||
| Deferred Annual Bonus Plan |
588,815 |
121,198 |
||||||
| Performance Share Plan |
7,245,586 |
959,612 |
||||||
| Deferred Investment Awards (1,2) |
348,947 |
8,563 |
||||||
| Share Value Plan (2) |
32,760 |
11,480 |
||||||
(1) |
Notional shares. |
(2) |
Executive Directors are not eligible to receive Deferred Investment Awards or participate in the Share Value Plan. |
Date of vesting |
Number of shares vested | |||
| 2019 DABP |
14 February 2022 |
51,712 |
– |
Align with the company’s business priorities, culture, wider workforce pay policies and emerging best practice |
– |
Support the bold performance ambitions announced to investors in June 2021 and company’s key ESG commitments |
– |
Create long-term shareholder value, and |
– |
Drive the success of the company for the benefit of shareholders, patients, our people and other key stakeholders |
Remuneration element |
Proposed changes to policy |
Rationale for the change | ||
Pension |
– The description of the policy has been updated to reflect that the pension arrangements of any current UK and US Executive Directors will be aligned to the new Executive Directors’ arrangements from 1 January 2023 – The US contribution rates have been updated |
– This reflects the commitment given in the 2020 Remuneration Report that the pension arrangements of US Executive Directors would also be aligned to those of the new Executive Directors from January 2023 – The US references have been updated to reflect the latest contribution rates for the US wider workforce which came into effect in January 2021 | ||
Annual bonus |
– The maximum bonus opportunity for Executive Directors will be 300% of salary. For target performance, the bonus payout will be 100% of salary – For bonus up to an equivalent of 200% of salary, Executive Directors are required to defer 50% of any bonus earned into shares, or ADS as appropriate, for three years. Any portion of the bonus earned in excess of 200% of salary must be deferred 100% on the same basis |
– The additional opportunity of 100% is being introduced in the annual bonus to appropriately focus and reward executives to deliver and exceed our public ambitions and to secure strong performance for all our stakeholders – The additional opportunity would be deferred in full to ensure alignment with shareholders’ interests | ||
Non-Executive Directors’ fees |
– Authority is sought for a Non-Executive Director who is a member of the Science Committee to be remunerated up to £200,000 per annum for undertaking additional responsibilities on behalf of GSK and to support R&D– The current requirement for Non-Executive Directors and the Chair to invest 25% of their net basic fees in shares or ADS of the company is retained, but the company may choose to replace this for the Chair or one or more Non-Executive Directors with a minimum share or ADS ownership requirement of at least one times their gross annual standard fee until their retirement from the Board. Shares or ADS previously acquired through investment of fees would continue to be held under those arrangements and would be delivered or released following retirement from the Board. Such shares or ADS would count towards any expected minimum ownership requirement |
– To appropriately remunerate Non-Executive Directors for their work– If the company chooses to replace the current investment requirement, the minimum ownership requirement would continue to maintain a meaningful and prudent level of investment to align Non- Executive Directors’ interests with shareholders – The ability to replace the current investment requirement would facilitate greater flexibility in operation of these arrangements | ||

| Salary No change |
To provide a core reward for the role. Set at a level appropriate to secure and retain high calibre individuals needed to deliver the Group’s strategic priorities. |
| Benefits No change |
Levels are set to recruit and retain high calibre individuals to execute the business strategy. |
Pension |
Pension arrangements provide a competitive level of retirement income. | |
Change |
– |
20% of base salary contribution to defined contribution plan and further 5% in matched contributions subject to any relevant cap and in line with implementation principles for other members of the plan; and |
– |
20% of base salary as a cash payment in lieu of pension contribution for the portion above the relevant cap; |
– |
20% of base salary as a cash payment in lieu of pension contribution. |
– |
7% of base salary contribution to defined contribution plan and further 3% in matched contributions subject to any relevant cap and in line with implementation principles for other members of the plan; and |
– |
7% of base salary as a cash payment in lieu of pension contribution for the portion above the relevant cap; |
or |
– |
7% of base salary as a cash payment in lieu of pension contribution. |
– |
Supplemental Cash Balance pension plan, providing annual contribution of 38% of base salary, less 5% of total base salary and bonus (net of the bonus deferred under the DABP)(3). |
– |
GSK 401(k) plan(1) and the ESSP(1) with core contributions of 7% of salary and bonus(2) and matched contributions of 4% of salary and bonus(2). |
– |
GSK 401(k) plan(1) and the ESSP(1) with core contributions of 7% of salary and bonus(2) and matched contributions of 4% of salary and bonus(2). |
– |
Eligible for appropriate equivalent arrangement not in excess of the US/UK arrangements. |
(1) |
In the event of any change to the plans operated in the US, a similar treatment would be provided under any successor arrangements introduced within the market |
(2) |
Less bonus deferred under the DABP |
(3) |
The 5% offset is equal to the contribution to the 401(k) and ESSP which was moved from the pension plans, in line with the wider US workforce, from 1 January 2021 |
| Annual bonus Change |
To incentivise and recognise execution of the business strategy on an annual basis. Rewards the achievement of stretching annual financial, strategic and operational measures. |

| Performance Share Plan (PSP) No change |
To incentivise and recognise delivery of the longer term business priorities, financial growth and increases in shareholder value compared to other pharmaceutical companies. In addition, to provide alignment with shareholder interests, a retention element, to encourage long-term shareholding and discourage excessive risk taking. |
% of salary |
||||
| CEO |
600 |
|||
| CFO |
400 |
|||
| Other Executive Directors |
500 |
|||
| Share Ownership Requirements No change |
||||
| To align the interests of Executive Directors with those of shareholders, they are required to build and maintain significant holdings of shares in GSK over time. The requirements for each Executive Director are as follows: |
As a minimum, Executive Directors are required to maintain 100% of their share ownership requirements to the end of the first year following retirement from the company and 50% to the end of the second year. | |||
% salary |
||||
| CEO |
650 |
|||
| Other Executive Directors |
300 |
|||
| Clawback and malus |
No change |
| Approach to recruitment remuneration |
No change |

Loss of office payment policy |
No change | |
| Element of Remuneration |
Loss of office payment policy | |
| Termination payment |
Termination by notice: (pro-rated where part of the notice period is worked). No termination payment is made in respect of any part of a notice period that extends beyond the contract expiry date. | |
| A bonus element is not normally included in the termination payment. However, the terms of the contracts seek to balance commercial imperatives and best practice. | ||
| Redundancy: | ||
| Retirement, death and ill-health, injury or disability: | ||
| LTI awards |
PSP awards are governed by the plan rules as approved by shareholders. | |
| The following provisions will normally apply: | ||
| Termination by notice: | ||
| Redundancy, retirement, death, ill-health, injury, disability or any other reason:pro-rated for time. | ||
| In the event of a change of control, PSP awards will vest, taking into account performance to date and normally taking into account the proportion of the performance period that has elapsed. Alternatively, the awards may be exchanged for new awards. | ||
| Annual bonus |
Termination by notice by individual: | |
| Termination by notice by the company, redundancy, retirement, death, ill-health, injury or disability:pro-rated on-target bonus (if employed on 31 December, bonus payable based on actual results). | ||
| Mandatorily deferred bonus under the DABP |
DABP deferred bonus awards in respect of mandatorily deferred bonus amounts are governed by the plan rules as approved by shareholders. The following provisions will normally apply: Termination for gross misconduct: Any other reason: In the event of a change of control, awards will vest or may be exchanged for new awards. | |
| Pensions |
Pension scheme contributions by the individual and the company, and any pension scheme benefit accruals, generally cease at the termination date in accordance with pension scheme rules. Access to pension scheme benefits is governed by the pension scheme rules and country legislation. | |
| Benefits |
Generally, benefits will continue to apply until the termination date. The Committee may make payments in connection with an existing legal obligation or in respect of any claim related to the cessation of employment. This may include fees for outplacement assistance, legal and/or professional advice. | |
| Termination by notice by the company and retirement (US executives): | ||
– |
Salary and benefits (including pension) are tailored to the local market. |
– |
The annual bonus plan applies to the wider employee population and is based on business performance. |
– |
A combination of performance-related and restricted share plans apply to the wider employee population. |
– |
All-employee share plans are available to employees in the UK, including the HM Revenue & Customs approved UK Share Save and Share Reward Plans. |

– |
2022 base salary has been used. |
– |
2021 benefits figures have been used, ie. based on actual amounts received in 2021. |
– |
Pensions for Emma Walmsley and Iain Mackay are based upon their 2022 salaries. |
– |
The amounts shown under value of PSP awards are based upon the relevant multiples for 2022. They do not include amounts in respect of dividends reinvested and do not factor in changes in share price over the vesting period (except as described below). |

Non-Executive Directors’ fees |
Change | |
| Element |
Purpose and link to strategy |
Operation | ||
Chair’s fees |
To provide an inclusive flat rate fee that is competitive with those paid by other companies of equivalent size and complexity subject to the limits contained in GSK’s Articles of Association. |
There is no formal maximum. However, fees are reviewed annually and set by reference to a review of the Chair’s performance and independently sourced market data. The Committee is responsible for evaluating and making recommendations to the Board on the fees payable to the Chair. The Chair does not participate in discussions in respect of their fees. | ||
Basic fees |
As above |
There is no formal maximum. As with the Chair, fees are reviewed annually and set by reference to independently sourced data. The Chair and CEO are responsible for evaluating and making recommendations to the Board on the fees payable to the company’s Non-Executive Directors. | ||
Fee payment |
Alignment with shareholders |
Fees are paid in cash. Non-Executive Directors (including the Chair) are required to invest at least 25% of their total net fees in shares or ADS of the company, but the company may choose to replace this with an ownership requirement to hold shares or ADS with an aggregate value at or above one times their gross annual standard fee until their retirement from the Board. If the current investment requirement is replaced with this ownership requirement, shares or ADS previously acquired through investment of fees would continue to be held under those arrangements and would be delivered or released following retirement from the Board. Such shares or ADS would count towards any minimum ownership requirement. | ||
Supplemental fees |
To compensate Non-Executive Directors (other than the Chair) for taking on additional Board responsibilities or undertaking intercontinental travel. |
Additional fees for the Senior Independent Director, Committee Chairs, Science and Medical Experts, the Workforce Engagement Director role and intercontinental travel. The company has the authority to pay an additional fee, up to the equivalent of the Committee Chair supplement to a Non-Executive Director, should the company require significant additional time commitment in exceptional or unforeseen circumstances. The company has the authority to pay an additional fee of up to £200,000 to Non-Executive Directors (excluding the Chair) who are members of the Science Committee for undertaking additional responsibilities on behalf of GSK and to support R&D. | ||
Benefits |
To facilitate execution of responsibilities and duties required by the role. |
Travel and subsistence costs for Non-Executive Directors are incurred in the normal course of business in relation to meetings on Board and Committee matters and other GSK-hosted events. For overseas-based Non-Executive Directors, this includes travel to meetings in the UK. In the event it is necessary for business purposes, whilst not normal practice, Non-Executive Directors may be accompanied by their spouse or partner to these meetings or events. The costs associated with the above are all met by the company and, in some instances, they are deemed to be taxable and therefore treated as benefits for the Non-Executive Director. |
Approach to recruitment remuneration |
No change | |
| The following policy and principles apply to the roles of Chair and Non-Executive Director. It seeks to ensure alignment withshareholders through the requirement to invest in company shares and ADS. Chair Fees will be set at a level that is competitive with those paid by other companies of equivalent size and complexity. Fees will be paid partly in shares. |
Non-Executive DirectorsFee levels for new Non-Executive Directors will be set on the same basis as for existing Non-Executive Directors of the company, subject to local laws and regulations.In the event of a Non-Executive Director with a different role and responsibilities being appointed, fee levels will be benchmarked and set by reference to comparable roles in companies of equivalent size and complexity. |
Loss of office |
No change | |

| Financial statements |
||||||||
| In this section |
||||||||
| 154 |
||||||||
| 168 |
||||||||
| 172 |
||||||||
| 252 |
||||||||

– |
select suitable accounting policies and then apply them consistently; |
– |
make judgements and accounting estimates that are reasonable and prudent; |
– |
state that the Group financial statements comply with IFRS, as issued by the IASB and in conformity with the requirements of the Companies Act 2006; |
– |
state with regard to the parent company financial statements that applicable UK Accounting Standards have been followed, subject to any material departures disclosed and explained in the parent company financial statements; and |
– |
prepare the financial statements on a going concern basis unless it is inappropriate to presume that the Group and the parent company will continue in business. |
– |
the Group financial statements, which have been prepared in accordance with IFRS, as issued by the IASB and in conformity with the requirements of Companies Act 2006, give a true and fair view of the assets, liabilities, financial position and profit of the Group; and |
– |
the Strategic report and risk sections of the Annual Report, which represent the management report, include a fair review of the development and performance of the business and the position of the company and the Group taken as a whole, together with a description of the principal risks and uncertainties that it faces. |
– |
so far as he or she is aware, there is no relevant audit information of which the company’s auditor is unaware; and |
– |
he or she has taken all the steps that he or she ought to have taken as a Director to make himself or herself aware of any relevant audit information and to establish that the company’s auditor is aware of that information. |







Notes |
2021 £m |
2020 £m |
2019 £m |
|||||||||||||
| Turnover |
6 |
|||||||||||||||
| Cost of sales |
( |
) |
( |
) |
( |
) | ||||||||||
| Gross profit |
||||||||||||||||
| Selling, general and administration |
( |
) |
( |
) |
( |
) | ||||||||||
| Research and development |
( |
) |
( |
) |
( |
) | ||||||||||
| Royalty income |
||||||||||||||||
| Other operating (expense)/income |
7 |
( |
) |
|||||||||||||
| Operating profit |
8 |
|||||||||||||||
| Finance income |
11 |
|||||||||||||||
| Finance expense |
12 |
( |
) |
( |
) |
( |
) | |||||||||
| Share of after tax profits of associates and joint ventures |
13 |
|||||||||||||||
| Loss on disposal of interest in associates |
( |
) |
||||||||||||||
| Profit before taxation |
||||||||||||||||
| Taxation |
14 |
( |
) |
( |
) |
( |
) | |||||||||
| Profit after taxation for the year |
||||||||||||||||
| Profit attributable to non-controlling interests |
||||||||||||||||
| Profit attributable to shareholders |
||||||||||||||||
| Basic earnings per share (pence) |
15 |
p |
p |
p |
||||||||||||
| Diluted earnings per share (pence) |
15 |
p |
p |
p |
||||||||||||
Notes |
2021 £m |
2020 £m |
2019 £m |
|||||||||||||
| Profit for the year |
||||||||||||||||
| Other comprehensive income/(expense) for the year |
||||||||||||||||
| Items that may be subsequently reclassified to income statement: |
||||||||||||||||
| Exchange movements on overseas net assets and net investment hedges |
37 |
( |
) |
( |
) |
( |
) | |||||||||
| Reclassification of exchange movements on liquidation or disposal of overseas subsidiaries and associates |
37 |
( |
) |
( |
) | |||||||||||
| Fair value movements on cash flow hedges |
( |
) |
( |
) | ||||||||||||
| Tax on fair value movements on cash flow hedges |
( |
) |
( |
) |
||||||||||||
| Reclassification of cash flow hedges to income statement |
||||||||||||||||
( |
) |
( |
) |
( |
) | |||||||||||
| Items that will not be reclassified to income statement: |
||||||||||||||||
| Exchange movements on overseas net assets of non-controlling interests |
37 |
( |
) |
( |
) |
( |
) | |||||||||
| Fair value movements on equity investments |
( |
) |
||||||||||||||
| Tax on fair value movements on equity investments |
( |
) |
( |
) | ||||||||||||
| Remeasurement gains/(losses) on defined benefit plans |
( |
) |
( |
) | ||||||||||||
| Tax on remeasurement of defined benefit plans |
( |
) |
||||||||||||||
( |
) |
( |
) | |||||||||||||
| Other comprehensive (expense)/income for the year |
37 |
( |
) |
( |
) | |||||||||||
| Total comprehensive income for the year |
||||||||||||||||
| Total comprehensive income for the year attributable to: |
||||||||||||||||
| Shareholders |
||||||||||||||||
| Non-controlling interests |
||||||||||||||||
| Total comprehensive income for the year |
||||||||||||||||
Notes |
2021 £m |
2020 £m |
||||||||||
| Non-current assets |
||||||||||||
| Property, plant and equipment |
17 |
|||||||||||
| Right of use assets |
18 |
|||||||||||
| Goodwill |
19 |
|||||||||||
| Other intangible assets |
20 |
|||||||||||
| Investments in associates and joint ventures |
21 |
|||||||||||
| Other investments |
22 |
|||||||||||
| Deferred tax assets |
14 |
|||||||||||
| Derivative financial instruments |
43 |
|||||||||||
| Other non-current assets |
23 |
|||||||||||
| Total non-current assets |
||||||||||||
| Current assets |
||||||||||||
| Inventories |
24 |
|||||||||||
| Current tax recoverable |
14 |
|||||||||||
| Trade and other receivables |
25 |
|||||||||||
| Derivative financial instruments |
43 |
|||||||||||
| Liquid investments |
29 |
|||||||||||
| Cash and cash equivalents |
26 |
|||||||||||
| Assets held for sale |
27 |
|||||||||||
| Total current assets |
||||||||||||
| Total assets |
||||||||||||
| Current liabilities |
||||||||||||
| Short-term borrowings |
29 |
( |
) |
( |
) | |||||||
| Contingent consideration liabilities |
32 |
( |
) |
( |
) | |||||||
| Trade and other payables |
28 |
( |
) |
( |
) | |||||||
| Derivative financial instruments |
43 |
( |
) |
( |
) | |||||||
| Current tax payable |
14 |
( |
) |
( |
) | |||||||
| Short-term provisions |
31 |
( |
) |
( |
) | |||||||
| Total current liabilities |
( |
) |
( |
) | ||||||||
| Non-current liabilities |
||||||||||||
| Long-term borrowings |
29 |
( |
) |
( |
) | |||||||
| Corporation tax payable |
14 |
( |
) |
( |
) | |||||||
| Deferred tax liabilities |
14 |
( |
) |
( |
) | |||||||
| Pensions and other post-employment benefits |
30 |
( |
) |
( |
) | |||||||
| Other provisions |
31 |
( |
) |
( |
) | |||||||
| Derivative financial instruments |
43 |
( |
) |
( |
) | |||||||
| Contingent consideration liabilities |
32 |
( |
) |
( |
) | |||||||
| Other non-current liabilities |
33 |
( |
) |
( |
) | |||||||
| Total non-current liabilities |
( |
) |
( |
) | ||||||||
| Total liabilities |
( |
) |
( |
) | ||||||||
| Net assets |
||||||||||||
| Equity |
||||||||||||
| Share capital |
36 |
|||||||||||
| Share premium account |
36 |
|||||||||||
| Retained earnings |
37 |
|||||||||||
| Other reserves |
37 |
|||||||||||
| Shareholders’ equity |
||||||||||||
| Non-controlling interests |
||||||||||||
| Total equity |
||||||||||||

Shareholders’ equity |
||||||||||||||||||||||||||||
| Share capital £m |
Share premium £m |
Retained earnings £m |
Other reserves* £m |
Total £m |
Non-controlling interests £m |
Total equity £m |
||||||||||||||||||||||
| At 31 December 2018, as revised |
( |
) |
( |
) |
||||||||||||||||||||||||
| Implementation of IFRS 16 |
– |
– |
( |
) |
– |
( |
) |
– |
( |
) | ||||||||||||||||||
| At 31 December 2018, as adjusted |
( |
) |
( |
) |
||||||||||||||||||||||||
| Profit for the year |
– |
– |
– |
|||||||||||||||||||||||||
| Other comprehensive (expense)/income for the year |
– |
– |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||||||||
| Total comprehensive income for the year |
– |
– |
||||||||||||||||||||||||||
| Distributions to non-controlling interests |
– |
– |
– |
– |
– |
( |
) |
( |
) | |||||||||||||||||||
| Changes in non-controlling interests |
– |
– |
– |
– |
– |
( |
) |
( |
) | |||||||||||||||||||
| Dividends to shareholders |
– |
– |
( |
) |
– |
( |
) |
– |
( |
) | ||||||||||||||||||
| Recognition of interest in Consumer Healthcare JV |
– |
– |
– |
|||||||||||||||||||||||||
| Realised losses on disposal of equity investments |
– |
– |
( |
) |
– |
– |
– |
|||||||||||||||||||||
| Shares issued |
– |
– |
– |
|||||||||||||||||||||||||
| Shares acquired by ESOP Trusts |
– |
( |
) |
– |
– |
– |
||||||||||||||||||||||
| Write-down of shares held by ESOP Trusts |
– |
– |
( |
) |
– |
– |
– |
|||||||||||||||||||||
| Share-based incentive plans |
– |
– |
– |
– |
||||||||||||||||||||||||
| Tax on share-based incentive plans |
– |
– |
– |
– |
||||||||||||||||||||||||
| At 31 December 2019 |
||||||||||||||||||||||||||||
| Profit for the year |
– |
– |
– |
|||||||||||||||||||||||||
| Other comprehensive (expense)/income for the year |
– |
– |
( |
) |
( |
) |
||||||||||||||||||||||
| Total comprehensive income for the year |
– |
– |
||||||||||||||||||||||||||
| Distributions to non-controlling interests |
– |
– |
– |
– |
– |
( |
) |
( |
) | |||||||||||||||||||
| Contributions from non-controlling interests |
– |
– |
– |
– |
– |
|||||||||||||||||||||||
| Changes in non-controlling interests |
– |
– |
– |
– |
– |
( |
) |
( |
) | |||||||||||||||||||
| Dividends to shareholders |
– |
– |
( |
) |
– |
( |
) |
– |
( |
) | ||||||||||||||||||
| Shares issued |
– |
– |
– |
– |
||||||||||||||||||||||||
| Realised profits on disposal of equity investments |
– |
– |
( |
) |
– |
– |
– |
|||||||||||||||||||||
| Share of associates and joint ventures realised profits on disposal of equity investments |
– |
– |
( |
) |
– |
– |
– |
|||||||||||||||||||||
| Shares acquired by ESOP Trusts |
– |
( |
) |
– |
– |
– |
||||||||||||||||||||||
| Write-down of shares held by ESOP Trusts |
– |
– |
( |
) |
– |
– |
– |
|||||||||||||||||||||
| Share-based incentive plans |
– |
– |
– |
– |
||||||||||||||||||||||||
| Tax on share-based incentive plans |
– |
– |
( |
) |
– |
( |
) |
– |
( |
) | ||||||||||||||||||
| At 31 December 2020 |
||||||||||||||||||||||||||||
| Profit for the year |
– |
– |
– |
|||||||||||||||||||||||||
| Other comprehensive (expense)/income for the year |
– |
– |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||||||||
| Total comprehensive income for the year |
– |
– |
( |
) |
||||||||||||||||||||||||
| Distributions to non-controlling interests |
( |
) |
( |
) | ||||||||||||||||||||||||
| Contributions from non-controlling interests |
||||||||||||||||||||||||||||
| Dividends to shareholders |
( |
) |
( |
) |
( |
) | ||||||||||||||||||||||
| Realised profits on disposal of equity investments |
( |
) |
||||||||||||||||||||||||||
| Share of associates and joint ventures realised profits on disposal of equity investments |
( |
) |
||||||||||||||||||||||||||
| Shares issued |
||||||||||||||||||||||||||||
| Write-down of shares held by ESOP Trusts |
( |
) |
||||||||||||||||||||||||||
| Share-based incentive plans |
||||||||||||||||||||||||||||
| Transactions with non-controlling interests |
– |
– |
– |
– |
– |
|||||||||||||||||||||||
| Tax on share-based incentive plans |
||||||||||||||||||||||||||||
| At 31 December 2021 |
||||||||||||||||||||||||||||
* |
an analysis of Other reserves is presented as part of Note 37, ‘Movements in equity’. |
Notes |
2021 £m |
2020 £m |
2019 £m |
|||||||||||||
| Cash flow from operating activities |
||||||||||||||||
| Profit after taxation for the year |
||||||||||||||||
| Adjustments reconciling profit after tax to operating cash flows |
41 |
|||||||||||||||
| Cash generated from operations |
||||||||||||||||
| Taxation paid |
( |
) |
( |
) |
( |
) | ||||||||||
| Net cash inflow from operating activities |
||||||||||||||||
| Cash flow from investing activities |
||||||||||||||||
| Purchase of property, plant and equipment |
( |
) |
( |
) |
( |
) | ||||||||||
| Proceeds from sale of property, plant and equipment |
||||||||||||||||
| Purchase of intangible assets |
( |
) |
( |
) |
( |
) | ||||||||||
| Proceeds from sale of intangible assets |
||||||||||||||||
| Purchase of equity investments |
( |
) |
( |
) |
( |
) | ||||||||||
| Proceeds from sale of equity investments |
||||||||||||||||
| Contingent consideration paid |
( |
) |
( |
) |
( |
) | ||||||||||
| Purchase of businesses, net of cash acquired |
40 |
– |
( |
) | ||||||||||||
| Disposal of businesses, net of cash disposed |
40 |
( |
) |
|||||||||||||
| Investments in associates and joint ventures |
40 |
( |
) |
( |
) |
( |
) | |||||||||
| (Increase)/decrease in liquid investments |
( |
) |
||||||||||||||
| Interest received |
||||||||||||||||
| Proceeds from disposal of associates and joint ventures |
– |
– |
||||||||||||||
| Dividends from associates, joint ventures and equity investments |
||||||||||||||||
| Net cash inflow/(outflow) from investing activities |
( |
) |
( |
) | ||||||||||||
| Cash flow from financing activities |
||||||||||||||||
| Issue of share capital |
36 |
|||||||||||||||
| Purchase of non-controlling interests |
( |
) | ||||||||||||||
| Increase in long-term loans |
||||||||||||||||
| Repayment of short-term Notes |
( |
) |
( |
) |
( |
) | ||||||||||
| (Repayment of)/increase in other short-term loans |
( |
) |
||||||||||||||
| Repayment of lease liabilities |
( |
) |
( |
) |
( |
) | ||||||||||
| Interest paid |
( |
) |
( |
) |
( |
) | ||||||||||
| Dividends paid to shareholders |
( |
) |
( |
) |
( |
) | ||||||||||
| Distributions to non-controlling interests |
( |
) |
( |
) |
( |
) | ||||||||||
| Contributions from non-controlling interests |
||||||||||||||||
| Other financing cash flows |
( |
) | ||||||||||||||
| Net cash outflow from financing activities |
( |
) |
( |
) |
( |
) | ||||||||||
| (Decrease)/increase in cash and bank overdrafts |
42 |
( |
) |
|||||||||||||
| Cash and bank overdrafts at beginning of year |
||||||||||||||||
| Exchange adjustments |
( |
) |
( |
) |
( |
) | ||||||||||
| (Decrease)/increase in cash and bank overdrafts |
( |
) |
||||||||||||||
| Cash and bank overdrafts at end of year |
||||||||||||||||
| Cash and bank overdrafts at end of year comprise: |
||||||||||||||||
| Cash and cash equivalents |
||||||||||||||||
| Cash and cash equivalents reported in assets held for sale |
||||||||||||||||
| Overdrafts |
( |
) |
( |
) |
( |
) | ||||||||||

– |
Consolidated income statement |
– |
Consolidated statement of comprehensive income |
– |
Consolidated balance sheet |
– |
Consolidated statement of changes in equity |
– |
Consolidated cash flow statement |
– |
Notes to the financial statements. |
– |
the assets and liabilities, and the results and cash flows, of the company and its subsidiaries, including ESOP Trusts |
– |
the Group’s share of the results and net assets of associates and joint ventures |
– |
the Group’s share of assets, liabilities, revenue and expenses of joint operations. |

Freehold buildings |
||
Leasehold land and buildings |
||
Plant and machinery |
||
Equipment and vehicles |
||



2021 |
2020 |
2019 |
||||||||||
Average rates: |
||||||||||||
US$/£ |
||||||||||||
Euro/£ |
||||||||||||
Yen/£ |
||||||||||||
2021 |
2020 |
2019 |
||||||||||
Period end rates: |
||||||||||||
US$/£ |
||||||||||||
Euro/£ |
||||||||||||
Yen/£ |
||||||||||||
Turnover by segment |
2021 £m |
2020 £m |
2019 £m |
|||||||||
Pharmaceuticals |
||||||||||||
Vaccines |
||||||||||||
Consumer Healthcare |
||||||||||||
Segment turnover |
||||||||||||
Corporate and other unallocated turnover |
– |
|||||||||||
Pharmaceuticals turnover by therapeutic area |
2021 £m |
2020 (revised) £m |
2019 (revised) £m |
|||||||||
Respiratory |
||||||||||||
HIV |
||||||||||||
Immuno-inflammation |
||||||||||||
Oncology |
||||||||||||
Pandemic |
– |
– |
||||||||||
New and Specialty |
||||||||||||
Established Pharmaceuticals |
||||||||||||
Vaccines turnover by category |
2021 £m |
2020 £m |
2019 £m |
|||||||||
Meningitis |
||||||||||||
Influenza |
||||||||||||
Shingles |
||||||||||||
Established Vaccines |
||||||||||||
Pandemic Vaccines |
– |
– |
||||||||||
| Consumer Healthcare turnover by category |
2021 £m |
2020 £m |
2019 £m |
|||||||||
| Oral health |
||||||||||||
| Pain relief |
||||||||||||
| Vitamins, minerals and supplements |
||||||||||||
| Respiratory health |
||||||||||||
| Digestive health and other |
||||||||||||
| Brands divested/under review |
||||||||||||
| Segment profit |
2021 £m |
2020 £m |
2019 £m |
|||||||||
| Pharmaceuticals |
||||||||||||
| Pharmaceuticals R&D |
( |
) |
( |
) |
( |
) | ||||||
| Pharmaceuticals, including R&D |
||||||||||||
| Vaccines |
||||||||||||
| Consumer Healthcare |
||||||||||||
| Segment profit |
||||||||||||
| Corporate and other unallocated costs |
( |
) |
( |
) |
( |
) | ||||||
| Other reconciling items between segment profit and operating profit |
( |
) |
( |
) |
( |
) | ||||||
| Operating profit |
||||||||||||
| Finance income |
||||||||||||
| Finance costs |
( |
) |
( |
) |
( |
) | ||||||
| Loss on disposal of interest in associates |
( |
) |
– |
– |
||||||||
| Share of after-tax profits of associates and joint ventures |
||||||||||||
| Profit before taxation |
||||||||||||
| Taxation |
( |
) |
( |
) |
( |
) | ||||||
| Profit after taxation for the year |
||||||||||||
| Depreciation and amortisation by segment |
2021 £m |
2020 £m |
2019 £m |
|||||||||
| Pharmaceuticals |
||||||||||||
| Pharmaceuticals R&D |
||||||||||||
| Pharmaceuticals, including R&D |
||||||||||||
| Vaccines |
||||||||||||
| Consumer Healthcare |
||||||||||||
| Segment depreciation and amortisation |
||||||||||||
| Corporate and other unallocated depreciation and amortisation |
||||||||||||
| Other reconciling items between segment depreciation and amortisation and total depreciation and amortisation |
||||||||||||
| Total depreciation and amortisation |
||||||||||||

| PP&E, intangible asset and goodwill impairment by segment |
2021 £m |
2020 £m |
2019 £m |
|||||||||
| Pharmaceuticals |
||||||||||||
| Pharmaceuticals R&D |
||||||||||||
| Pharmaceuticals, including R&D |
||||||||||||
| Vaccines |
||||||||||||
| Consumer Healthcare |
– |
|||||||||||
| Segment impairment |
||||||||||||
| Corporate and other unallocated impairment |
||||||||||||
| Other reconciling items between segment impairment and total impairment |
||||||||||||
| Total impairment |
||||||||||||
| PP&E and intangible asset impairment reversals by segment |
||||||||||||
| Pharmaceuticals |
( |
) |
( |
) |
( |
) | ||||||
| Pharmaceuticals R&D |
( |
) |
( |
) |
– |
|||||||
| Pharmaceuticals, including R&D |
( |
) |
( |
) |
( |
) | ||||||
| Vaccines |
( |
) |
( |
) |
( |
) | ||||||
| Consumer Healthcare |
– |
– |
– |
|||||||||
| Segment impairment reversals |
( |
) |
( |
) |
( |
) | ||||||
| Corporate and other unallocated impairment reversals |
– |
( |
) |
( |
) | |||||||
| Other reconciling items between segment impairment reversals and total impairment reversals |
( |
) |
( |
) |
( |
) | ||||||
| Total impairment reversals |
( |
) |
( |
) |
( |
) | ||||||
| Net operating assets by segment |
2021 £m |
2020 £m |
||||||||||
| Pharmaceuticals |
( |
) |
||||||||||
| Pharmaceuticals R&D |
||||||||||||
| Pharmaceuticals, including R&D |
||||||||||||
| Vaccines |
||||||||||||
| Consumer Healthcare |
||||||||||||
| Segment net operating assets |
||||||||||||
| Corporate and other unallocated net operating assets |
||||||||||||
| Net operating assets |
||||||||||||
| Net debt |
( |
) |
( |
) |
||||||||
| Investments in associates and joint ventures |
||||||||||||
| Derivative financial instruments |
( |
) |
( |
) |
||||||||
| Current and deferred taxation |
||||||||||||
| Assets held for sale (excluding cash and cash equivalents) |
||||||||||||
| Net assets |
||||||||||||
| Turnover by location of customer |
2021 £m |
2020 £m |
2019 £m |
|||||||||
| UK |
||||||||||||
| US |
||||||||||||
| Rest of World |
||||||||||||
| External turnover |
||||||||||||
| Non-current assets by location of subsidiary |
2021 £m |
2020 (revised) £m |
||||||||||
| UK |
||||||||||||
| US |
||||||||||||
| Belgium |
||||||||||||
| Switzerland |
||||||||||||
| Rest of World |
||||||||||||
| Non-current assets |
||||||||||||
2021 £m |
2020 £m |
2019 £m |
||||||||||
| Fair value remeasurements of equity investments |
( |
) |
( |
) | ||||||||
| Disposal of businesses and assets |
||||||||||||
| Fair value remeasurements on contingent consideration recognised in business combinations |
( |
) |
( |
) |
( |
) | ||||||
| Remeasurement of ViiV Healthcare put option liabilities and preferential dividends |
( |
) |
||||||||||
| Fair value adjustments on derivative financial instruments |
( |
) | ||||||||||
| Other incom e |
||||||||||||
( |
) |
|||||||||||

| The following items have been included in operating profit: |
2021 £m |
2020 £m |
2019 £m |
|||||||||
| Employee costs (Note 9) |
||||||||||||
| Advertising |
||||||||||||
| Distribution costs |
||||||||||||
| Depreciation of property, plant and equipment |
||||||||||||
| Impairment of property, plant and equipment, net of reversals |
||||||||||||
| Depreciation of right of use assets |
||||||||||||
| Impairment of right of use assets |
||||||||||||
| Amortisation of intangible assets |
||||||||||||
| Impairment of intangible assets, net of reversals |
||||||||||||
| Impairment of property, plant and equipment held for sale, net of reversals |
||||||||||||
| Impairment of intangible assets held for sale, net of reversals |
||||||||||||
| Impairment of goodwill allocated to a disposal group, net of reversals |
||||||||||||
| Net foreign exchange (gains)/losses |
( |
) | ( |
) | ||||||||
| Inventories: |
||||||||||||
| Cost of inventories included in cost of sales |
||||||||||||
| Write-down of inventories |
||||||||||||
| Reversal of prior year write-down of inventories |
( |
) |
( |
) |
( |
) | ||||||
| Short-term lease charge |
||||||||||||
| Low-value lease charge |
||||||||||||
| Variable lease payments |
||||||||||||
| Fees payable to the company’s auditor and its associates in relation to the Group (see below) |
||||||||||||
| Fees payable to the company’s auditor and its associates: |
2021 £m |
2020 £m |
2019 £m |
|||||||||
| Audit of parent company and consolidated financial statements including attestation under s.404 of Sarbanes-Oxley Act 2002 |
||||||||||||
| Audit of the company’s subsidiaries |
||||||||||||
| Total audit services |
||||||||||||
| Audit related and other assurance services |
||||||||||||
| All other services |
||||||||||||
| Total audit-related and non-audit services |
||||||||||||
2021 £m |
2020 £m |
2019 £m |
||||||||||
| Audit |
||||||||||||
| Other services |
||||||||||||
| 2021 £m |
2020 £m |
2019 £m | ||||
| Wages and salaries |
||||||
| Social security costs |
||||||
| Pension and other post-employment costs, including augmentations (Note 30) |
||||||
| Cost of share-based incentive plans |
||||||
| Severance and other costs from integration and restructuring activities |
||||||
| 2021 £m |
2020 £m |
2019 £m | ||||
| Share Value Plan |
||||||
| Performance Share Plan |
||||||
| Share option plans |
||||||
| Cash settled and other plans |
||||||
| 2021 Number |
2020 Number |
2019 Number | ||||
| Manufacturing |
||||||
| Selling, general and administration |
||||||
| Research and development |
||||||
| 2021 £m |
2020 £m |
2019 £m | ||||
| Wages and salaries |
||||||
| Social security costs |
||||||
| Pension and other post-employment costs |
||||||
| Cost of share-based incentive plans |
||||||

– |
Restructuring costs to prepare for separation of GSK into two companies |
– |
Restructuring following the integration of the Pfizer consumer healthcare business into GSK Consumer Healthcare |
– |
Continued implementation of the restructuring programme that started in July 2018, to simplify the operating models and improve resource allocation of the Pharmaceutical and Consumer Healthcare supply chains |
– |
Continued transformation of central functions, including GSK technology platforms and interfaces, to deliver greater digital synergies, simplification of applications and staff reductions. |
| 2021 £m |
2020 £m |
2019 £m |
||||||||||
| Increase in provision for Major restructuring programmes (see Note 31) |
||||||||||||
| Amount of provision reversed unused (see Note 31) |
( |
) |
( |
) |
( |
) | ||||||
| Impairment losses recognised |
||||||||||||
| Other non-cash charges |
||||||||||||
| Other cash costs |
||||||||||||
2021 |
||||||||||
| Cash £m |
Non-cash £m |
Total £m |
||||||||
| Separation Preparation programme |
||||||||||
| Consumer Healthcare Joint Venture integration programme |
||||||||||
| 2018 Major restructuring programme (including Tesaro) |
||||||||||
| Combined restructuring and integration programme |
( |
) |
( |
) | ||||||
2020 |
||||||||||
| Cash £m |
Non-cash £m |
Total £m |
||||||||
| Separation Preparation programme |
||||||||||
| Consumer Healthcare Joint Venture integration programme |
||||||||||
| 2018 Major restructuring programme (including Tesaro) |
||||||||||
| Combined restructuring and integration programme |
||||||||||
| 2021 £m |
2020 £m |
2019 £m | ||||
| Cost of sales |
||||||
| Selling, general and administration |
||||||
| Research and development |
||||||
| Other operating expense |
||||||
| 2021 £m |
2020 £m |
2019 £m | ||||
| Finance income arising from: |
||||||
| Financial assets measured at amortised cost |
||||||
| Financial assets measured at fair value through profit or loss |
||||||
| Net gains arising from the forward element of forward contracts in net investment hedge relationships |
||||||
| Other finance income |
||||||
| 2021 £m |
2020 £m |
2019 £m |
||||||
| Finance expense arising on: |
||||||||
| Financial liabilities at amortised cost |
( |
( |
( |
) | ||||
| Derivatives at fair value through profit or loss |
( |
( |
) | |||||
| Net losses arising from: |
||||||||
| Financial instruments mandatorily measured at fair value through profit or loss |
( |
( |
) | |||||
| Retranslation of loans |
( |
|||||||
| Reclassification of hedges from other comprehensive income |
( |
( |
( |
) | ||||
| Unwinding of discounts on provisions |
( |
( |
( |
) | ||||
| Finance expense arising on lease liabilities |
( |
( |
( |
) | ||||
| Other finance expense |
( |
( |
( |
) | ||||
( |
( |
( |
) | |||||

| 2021 £m |
2020 £m |
2019 £m |
||||||||||
| Share of after-tax profits of associates |
||||||||||||
| Share of after-tax losses of joint ventures |
( |
) |
( |
) | ||||||||
| 2021 £m |
2020 £m |
2019 £m |
||||||||||
| Turnover |
||||||||||||
| Profit after taxation |
||||||||||||
| Total comprehensive income |
||||||||||||
| 2021 £m |
2020 £m |
2019 £m |
||||||||||
| Share of turnover |
||||||||||||
| Share of after-tax losses |
( |
) |
( |
) | ||||||||
| Share of other comprehensive income |
||||||||||||
| Share of total comprehensive income/(expense) |
( |
) | ||||||||||
Taxation charge based on profits for the year |
2021 £m |
2020 £m |
2019 £m |
|||||||||
| UK current year charge |
||||||||||||
| Rest of World current year charge |
||||||||||||
| Charge/(credit) in respect of prior periods |
( |
) | ||||||||||
| Current taxation |
||||||||||||
| Deferred taxation |
( |
) |
( |
) |
( |
) | ||||||
Reconciliation of taxation on Group profits |
2021 £m |
2021 % |
2020 £m |
2020 % |
2019 £m |
2019 % |
||||||||||||||||||
| Profit before tax |
||||||||||||||||||||||||
| UK statutory rate of taxation |
||||||||||||||||||||||||
| Differences in overseas taxation rates |
||||||||||||||||||||||||
| Benefit of intellectual property incentives |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
| R&D credits |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
| Fair value remeasurement of non-taxable put options |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||||||
| Tax losses where no benefit is recognised |
||||||||||||||||||||||||
| Permanent differences on disposals, acquisitions and transfers |
( |
) |
( |
) |
( |
) |
( |
) |
||||||||||||||||
| Other permanent differences |
||||||||||||||||||||||||
| Reassessments of prior year estimates |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
| Changes in tax rates |
( |
) |
( |
) |
( |
) |
( |
) |
||||||||||||||||
| Tax charge/tax rate |
||||||||||||||||||||||||

Tax on items charged to equity and statement of comprehensive income |
2021 £m |
2020 £m |
2019 £m |
|||||||||
| Current taxation |
||||||||||||
| Share-based payments |
– |
( |
) |
|||||||||
| Defined benefit plans |
– |
( |
) |
|||||||||
| Fair value movements on cash flow hedges |
– |
|||||||||||
| Fair value movements on equity investments |
– |
|||||||||||
| Deferred taxation |
||||||||||||
| Share-based payments |
( |
) |
||||||||||
| Defined benefit plans |
( |
) |
||||||||||
| Fair value movements on cash flow hedges |
||||||||||||
| Fair value movements on equity investments |
( |
) |
( |
) | ||||||||
| Total credit to equity and statement of comprehensive income |
||||||||||||
| Accelerated capital allowances £m |
Intangible assets £m |
Contingent consideration £m |
Intra-Group profit £m |
Pensions & other post employment benefits £m |
Tax losses £m |
Share option and award schemes £m |
Other net temporary differences £m |
Total £m |
||||||||||||||||||||||||||||
| At 1 January 2020 |
( |
) |
( |
) |
||||||||||||||||||||||||||||||||
| Exchange adjustments |
( |
) |
– |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | |||||||||||||||||||||||
| Credit/(charge) to income statement |
( |
) |
( |
) |
( |
) |
( |
) |
||||||||||||||||||||||||||||
| Credit/(charge) to statement of comprehensive income |
– |
– |
– |
– |
– |
( |
) |
( |
) |
( |
) | |||||||||||||||||||||||||
| Acquisitions / Disposals |
( |
) |
– |
– |
( |
) | ||||||||||||||||||||||||||||||
| R&D credits utilisation |
– |
– |
– |
– |
– |
– |
– |
( |
) |
( |
) | |||||||||||||||||||||||||
| At 31 December 2020 |
( |
) |
( |
) |
||||||||||||||||||||||||||||||||
| Exchange adjustments |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||||||||||||||||||
| Credit/(charge) to income statement |
( |
) |
||||||||||||||||||||||||||||||||||
| Credit/(charge) to statement of comprehensive income |
( |
) |
( |
) | ||||||||||||||||||||||||||||||||
| Acquisitions / Disposals |
( |
) |
( |
) | ||||||||||||||||||||||||||||||||
| R&D credits utilisation |
||||||||||||||||||||||||||||||||||||
| At 31 December 2021 |
( |
) |
( |
) |
||||||||||||||||||||||||||||||||
| 2021 £m |
2020 £m |
|||||||
| Deferred tax assets |
||||||||
| Deferred tax liabilities |
( |
) |
( |
) | ||||
2021 |
2020 |
|||||||||||||||
Unrecognised tax losses |
Tax losses £m |
Unrecognised deferred tax asset £m |
Tax losses £m |
Unrecognised deferred tax asset £m |
||||||||||||
| Trading losses expiring: |
||||||||||||||||
| Within 10 years |
||||||||||||||||
| More than 10 years |
||||||||||||||||
| Available indefinitely |
||||||||||||||||
| At 31 December |
||||||||||||||||
| Capital losses expiring: |
||||||||||||||||
| Available indefinitely |
||||||||||||||||
| At 31 December |
||||||||||||||||

2021 pence |
2020 pence |
2019 pence |
||||||||||
| Basic earnings per share |
.6 |
.5 |
9 |
|||||||||
| Diluted earnings per share |
6 |
.1 |
6 |
|||||||||
Weighted average number of shares in issue |
2021 millions |
2020 millions |
2019 millions |
|||||||||
| Basic |
||||||||||||
| Dilution for share options and awards |
||||||||||||
| Diluted |
||||||||||||
2021 |
2020 |
2019 |
||||||||||||||||||||||||||||
Paid/payable |
Dividend per share (pence) |
Total dividend £m |
Paid |
Dividend per share (pence) |
Total dividend £m |
Paid |
Dividend per share (pence) |
Total dividend £m |
||||||||||||||||||||||
| First interim |
||||||||||||||||||||||||||||||
| Second interim |
||||||||||||||||||||||||||||||
| Third interim |
||||||||||||||||||||||||||||||
| Fourth interim |
* |
|||||||||||||||||||||||||||||
| Total |
||||||||||||||||||||||||||||||
* |
The estimate for the fourth interim dividend for 2020 disclosed in the 2020 annual report and accounts was £ |
| 2021 £m |
2020 £m |
2019 £m |
||||||||||
| Dividends to shareholders |
||||||||||||
| Land and buildings £m |
Plant, equipment and vehicles £m |
Assets in construction £m |
Total £m |
|||||||||||||
| Cost at 1 January 2020 |
||||||||||||||||
| Exchange adjustments |
||||||||||||||||
| Additions through business combinations |
– |
– |
||||||||||||||
| Other additions |
||||||||||||||||
| Capitalised borrowing costs |
– |
– |
||||||||||||||
| Disposals and write-offs |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Reclassifications |
( |
) |
( |
) | ||||||||||||
| Transfer to assets held for sale |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Cost at 31 December 2020 |
||||||||||||||||
| Exchange adjustments |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Other additions |
||||||||||||||||
| Capitalised borrowing costs |
– |
– |
||||||||||||||
| Disposals and write-offs |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Reclassifications |
( |
) |
( |
) | ||||||||||||
| Transfer to assets held for sale |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Cost at 31 December 2021 |
||||||||||||||||
| Depreciation at 1 January 2020 |
( |
) |
( |
) |
– |
( |
) | |||||||||
| Exchange adjustments |
( |
) |
( |
) |
– |
( |
) | |||||||||
| Charge for the year |
( |
) |
( |
) |
– |
( |
) | |||||||||
| Disposals and write-offs |
– |
|||||||||||||||
| Transfer to assets held for sale |
– |
|||||||||||||||
| Depreciation at 31 December 2020 |
( |
) |
( |
) |
– |
( |
) | |||||||||
| Exchange adjustments |
– |
|||||||||||||||
| Charge for the year |
( |
) |
( |
) |
– |
( |
) | |||||||||
| Disposals and write-offs |
– |
|||||||||||||||
| Transfer to assets held for sale |
– |
|||||||||||||||
| Depreciation at 31 December 2021 |
( |
) |
( |
) |
– |
( |
) | |||||||||
| Impairment at 1 January 2020 |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Exchange adjustments |
( |
) |
– |
( |
) | |||||||||||
| Disposals and write-offs |
||||||||||||||||
| Impairment losses |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Reversal of impairments |
||||||||||||||||
| Transfer to assets held for sale |
||||||||||||||||
| Impairment at 31 December 2020 |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Exchange adjustments |
||||||||||||||||
| Disposals and write-offs |
||||||||||||||||
| Impairment losses |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Reversal of impairments |
– |
|||||||||||||||
| Impairment at 31 December 2021 |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Total depreciation and impairment at 31 December 2020 |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Total depreciation and impairment at 31 December 2021 |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Net book value at 1 January 2020 |
||||||||||||||||
| Net book value at 31 December 2020 |
||||||||||||||||
| Net book value at 31 December 2021 |
( |
) | ||||||||||||||

| Land and buildings £m |
Plant and equipment £m |
Vehicles £m |
Total £m |
|||||||||||||
| Net book value at 1 January 2020 |
||||||||||||||||
| Exchange adjustments |
( |
) |
( |
) | ||||||||||||
| Additions |
||||||||||||||||
| Depreciation |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Disposals |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Impairments |
( |
) |
– |
– |
( |
) | ||||||||||
| Reclassifications |
( |
) |
– |
– |
( |
) | ||||||||||
| Net book value at 31 December 2020 |
||||||||||||||||
| Exchange adjustments |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Additions |
||||||||||||||||
| Depreciation |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Disposals |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Impairments |
( |
) |
– |
– |
( |
) | ||||||||||
| Net book value at 31 December 2021 |
||||||||||||||||
| 2021 £m |
2020 £m |
|||||||
| Cost at 1 January |
||||||||
| Exchange adjustments |
( |
) |
( |
) | ||||
| Additions through business combinations (Note 40) |
– |
|||||||
| Other movements |
– |
|||||||
| Transfer to assets held for sale |
– |
( |
) | |||||
| Cost at 31 December |
||||||||
| Net book value at 1 January |
||||||||
| Net book value at 31 December |
||||||||
| 2021 £m |
2020 £m | |||
| Pharmaceuticals |
||||
| Vaccines |
||||
| Consumer Healthcare |
||||
| Net book value at 31 December |
||||
| Valuation basis |
||||||
| Key assumptions |
Sales growth rates Profit margins Terminal growth rate Discount rate Taxation rate |
|||||
| Determination of assumptions |
Growth rates are internal forecasts based on both internal and external market information. Margins reflect past experience, adjusted for expected changes. Terminal growth rates based on management’s estimate of future long-term average growth rates. Discount rates based on Group WACC, adjusted where appropriate. Taxation rates based on appropriate rates for each jurisdiction. | |||||
| Period of specific projected cash flows |
||||||
| Terminal growth rate and discount rate |
Terminal growth rate |
Discount rate | ||||
Pharmaceuticals |
||||||
Vaccines |
||||||
Consumer Healthcare |
||||||

| Computer software £m |
Licences, patents, amortised brands etc. £m |
Indefinite life brands £m |
Total £m |
|||||||||||||
| Cost at 1 January 2020 |
||||||||||||||||
| Exchange adjustments |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Capitalised development costs |
– |
– |
||||||||||||||
| Additions through business combinations |
– |
– |
||||||||||||||
| Other additions |
– |
|||||||||||||||
| Disposals and asset write-offs |
( |
) |
( |
) |
– |
( |
) | |||||||||
| Transfer to assets held for sale |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Reclassifications |
( |
) |
||||||||||||||
| Cost at 31 December 2020 |
||||||||||||||||
| Exchange adjustments |
( |
) |
( |
) |
( |
) | ||||||||||
| Capitalised development costs |
– |
– |
||||||||||||||
| Other additions |
– |
|||||||||||||||
| Disposals and asset write-offs |
( |
) |
( |
) |
– |
( |
) | |||||||||
| Transfer to assets held for sale |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Reclassifications |
( |
) |
||||||||||||||
| Cost at 31 December 2021 |
||||||||||||||||
| Amortisation at 1 January 2020 |
( |
) |
( |
) |
– |
( |
) | |||||||||
| Exchange adjustments |
( |
) |
– |
|||||||||||||
| Charge for the year |
( |
) |
( |
) |
– |
( |
) | |||||||||
| Disposals and asset write-offs |
– |
|||||||||||||||
| Transfer to assets held for sale |
– |
|||||||||||||||
| Amortisation at 31 December 2020 |
( |
) |
( |
) |
– |
( |
) | |||||||||
| Exchange adjustments |
– |
|||||||||||||||
| Charge for the year |
( |
) |
( |
) |
– |
( |
) | |||||||||
| Disposals and asset write-offs |
– |
|||||||||||||||
| Transfer to assets held for sale |
– |
– |
||||||||||||||
| Amortisation at 31 December 2021 |
( |
) |
( |
) |
– |
( |
) | |||||||||
| Impairment at 1 January 2020 |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Exchange adjustments |
– |
|||||||||||||||
| Impairment losses |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Reversal of impairments |
– |
– |
||||||||||||||
| Disposals and asset write-offs |
– |
– |
||||||||||||||
| Transfer to assets held for sale |
– |
– |
||||||||||||||
| Reclassification |
– |
( |
) |
– |
||||||||||||
| Impairment at 31 December 2020 |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Exchange adjustments |
– |
– |
||||||||||||||
| Impairment losses |
( |
) |
( |
) |
– |
( |
) | |||||||||
| Reversal of impairments |
– |
|||||||||||||||
| Disposals and asset write-offs |
– |
|||||||||||||||
| Impairment at 31 December 2021 |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Total amortisation and impairment at 31 December 2020 |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Total amortisation and impairment at 31 December 2021 |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
| Net book value at 1 January 2020 |
||||||||||||||||
| Net book value at 31 December 2020 |
||||||||||||||||
| Net book value at 31 December 2021 |
||||||||||||||||
Amortisation |
Net impairment losses |
|||||||||||||||
| 2021 £m |
2020 £m |
2021 £m |
2020 £m |
|||||||||||||
| Cost of sales |
( |
) |
||||||||||||||
| Selling, general and administration |
||||||||||||||||
| Research and development |
||||||||||||||||
| 2021 £m |
2020 £m |
|||||||
| Tesaro Assets |
||||||||
| Meningitis portfolio |
||||||||
| Dolutegravir |
||||||||
| Benlysta |
||||||||
| Alector Assets |
– |
|||||||
| iTeos Assets |
– |
|||||||
| Lamisil |
||||||||
| Merck Assets |
– |
|||||||
| Vir Assets |
||||||||
| BMS Assets |
||||||||
| Fluarix/FluLaval |
||||||||
| Okairos |
||||||||
| CureVac Assets |
||||||||
| Stiefel trade name |
||||||||
| Others |
||||||||
| 2021 £m |
2020 £m |
|||||||
| Advil |
||||||||
| Voltaren |
||||||||
| Centrum |
||||||||
| Caltrate |
||||||||
| Otrivin |
||||||||
| Preparation H |
||||||||
| Robitussin |
||||||||
| Nexium |
||||||||
| Fenistil |
||||||||
| Chapstick |
||||||||
| Emergen-C |
||||||||
| Theraflu |
||||||||
| Panadol |
||||||||
| Sensodyne |
||||||||
| Others |
||||||||

Joint ventures £m |
Associates £m |
2021 Total £m |
Joint ventures £m |
Associates £m |
2020 Total £m |
|||||||||||||||||||
| At 1 January |
||||||||||||||||||||||||
| Exchange adjustments |
– |
( |
) |
( |
) |
– |
( |
) |
( |
) | ||||||||||||||
| Additions |
– |
– |
||||||||||||||||||||||
| Disposals |
– |
( |
) |
( |
) |
– |
– |
– |
||||||||||||||||
| Distributions received |
– |
( |
) |
( |
) |
– |
( |
) |
( |
) | ||||||||||||||
| Net fair value movements through Other comprehensive income |
– |
– |
||||||||||||||||||||||
| Impairment of interest in associates |
– |
( |
) |
( |
) |
– |
– |
– |
||||||||||||||||
| Profit/(loss) after tax recognised in the consolidated income statement |
( |
) |
– |
|||||||||||||||||||||
| At 31 December |
||||||||||||||||||||||||
At 31 December 2020 £m |
||||
Non-current assets |
||||
Current assets |
||||
Current liabilities |
( |
) | ||
Non-current liabilities |
( |
) | ||
Net assets |
||||
The carrying value of the Group’s investment in Innoviva in 2020 is analysed as follows: |
||||
2020 |
||||
£m |
||||
Interest in net assets of associate |
||||
Goodwill |
||||
Fair value and other adjustments |
||||
Carrying value at 31 December |
||||
The investment in Innoviva had a market value of £ |
||||
Investments designated as measured at FVTOCI £m |
Investments measured at FVTPL £m |
2021 £m |
Investments designated as measured at FVTOCI £m |
Investments measured at FVTPL £m |
2020 £m |
|||||||||||||||||||
At 1 January |
||||||||||||||||||||||||
Additions |
||||||||||||||||||||||||
Net fair value movements through Other comprehensive income |
( |
) |
– |
( |
) |
– |
||||||||||||||||||
Net fair value movements through profit or loss |
– |
– |
( |
) |
( |
) | ||||||||||||||||||
Disposals and settlements |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
At 31 December |
||||||||||||||||||||||||

2021 £m |
2020 £m |
|||||||
Amounts receivable under insurance contracts |
||||||||
Pension schemes in surplus |
||||||||
Other receivables |
||||||||
2021 £m |
2020 £m |
|||||||
Raw materials and consumables |
||||||||
Work in progress |
||||||||
Finished goods |
||||||||
2021 £m |
2020 £m |
|||||||
Trade receivables, net of loss allowance |
||||||||
Accrued income |
||||||||
Prepayments |
||||||||
Interest receivable |
||||||||
Employee loans and advances |
||||||||
Other receivables |
||||||||
Loss allowance - trade receivables |
2021 £m |
2020 £m |
||||||
At 1 January |
||||||||
Exchange adjustments |
( |
) |
( |
) | ||||
Charge for the year |
||||||||
Subsequent recoveries of amounts provided for |
( |
) |
( |
) | ||||
Utilised |
( |
) |
( |
) | ||||
At 31 December |
||||||||
2021 £m |
2020 £m |
|||||||
Cash at bank and in hand |
||||||||
Short-term deposits |
||||||||
2021 £m |
2020 £m |
|||||||
Property, plant and equipment |
||||||||
Other intangibles |
– |
|||||||
Inventory |
– |
|||||||

28. Trade and other payables |
||||||||
2021 £m |
2020 £m |
|||||||
Trade payables |
||||||||
Wages and salaries |
||||||||
Social security |
||||||||
ViiV Healthcare put option |
||||||||
Other payables |
||||||||
Deferred income |
||||||||
Customer return and rebate accruals |
||||||||
Other accruals |
||||||||
Increase/(decrease) in financial liability and loss/(gain) in Income statement |
2021 £m |
2020 £m |
||||||
10% increase in sales forecasts* |
||||||||
10% decrease in sales forecasts* |
( |
) |
( |
) | ||||
1% (100 basis points) increase in discount rate |
( |
) |
( |
) | ||||
1% (100 basis points) decrease in discount rate |
||||||||
10 cent appreciation of US Dollar |
||||||||
10 cent depreciation of US Dollar |
( |
) |
( |
) | ||||
10 cent appreciation of Euro |
||||||||
10 cent depreciation of Euro |
( |
) |
( |
) | ||||
Listing exchange |
2021 £m |
2020 £m |
||||||||
| Current assets: |
||||||||||
| Liquid investments |
||||||||||
| Cash and cash equivalents |
||||||||||
| Short-term borrowings: |
||||||||||
| Commercial paper |
( |
) |
( |
) | ||||||
| Bank loans, overdrafts and other |
( |
) |
( |
) | ||||||
| LIBOR +0.35% US$ US Medium Term Note 2021 |
New York Stock Exchange |
– |
( |
) | ||||||
| EURIBOR +60% € Euro Medium Term Note 2021 |
London Stock Exchange |
– |
( |
) | ||||||
| 0.000% € Euro Medium Term Note 2021 |
London Stock Exchange |
– |
( |
) | ||||||
| 2.850% US$ US Medium Term Note 2022 |
New York Stock Exchange |
( |
) |
– |
||||||
| 2.875% US$ US Medium Term Note 2022 |
New York Stock Exchange |
( |
) |
– |
||||||
| Lease liabilities |
( |
) |
( |
) | ||||||
( |
) |
( |
) | |||||||
| Long-term borrowings: |
||||||||||
| 2.850% US$ US Medium Term Note 2022 |
New York Stock Exchange |
– |
( |
) | ||||||
| 2.875% US$ US Medium Term Note 2022 |
New York Stock Exchange |
– |
( |
) | ||||||
| 2.800% US$ US Medium Term Note 2023 |
New York Stock Exchange |
( |
) |
( |
) | |||||
| 0.125% € Euro Medium Term Note 2023 |
London Stock Exchange |
( |
) |
( |
) | |||||
| Exchangeable US$ US Medium Term Note 2023 |
New York Stock Exchange |
( |
) |
( |
) | |||||
| 3.375% US$ US Medium Term Note 2023 |
New York Stock Exchange |
( |
) |
( |
) | |||||
| 0.000% € Euro Medium Term Note 2023 |
London Stock Exchange |
( |
) |
( |
) | |||||
| 0.534% US$ US Medium Term Note 2023 |
New York Stock Exchange |
( |
) |
( |
) | |||||
| 3.000% US$ US Medium Term Note 2024 |
New York Stock Exchange |
( |
) |
( |
) | |||||
| 1.375% € Euro Medium Term Note 2024 |
London Stock Exchange |
( |
) |
( |
) | |||||
| 4.000% € Euro Medium Term Note 2025 |
London Stock Exchange |
( |
) |
( |
) | |||||
| 3.625% US$ US Medium Term Note 2025 |
New York Stock Exchange |
( |
) |
( |
) | |||||
| 1.000% € Euro Medium Term Note 2026 |
London Stock Exchange |
( |
) |
( |
) | |||||
| 1.250% € Euro Medium Term Note 2026 |
London Stock Exchange |
( |
) |
( |
) | |||||
| 3.375% £ Euro Medium Term Note 2027 |
London Stock Exchange |
( |
) |
( |
) | |||||
| 3.875% US$ US Medium Term Note 2028 |
New York Stock Exchange |
( |
) |
( |
) | |||||
| 1.250% £ Euro Medium Term Note 2028 |
London Stock Exchange |
( |
) |
( |
) | |||||
| 3.375% US$ US Medium Term Note 2029 |
New York Stock Exchange |
( |
) |
( |
) | |||||
| 1.375% € Euro Medium Term Note 2029 |
London Stock Exchange |
( |
) |
( |
) | |||||
| 1.750% € Euro Medium Term Note 2030 |
London Stock Exchange |
( |
) |
( |
) | |||||
| 5.250% £ Euro Medium Term Note 2033 |
London Stock Exchange |
( |
) |
( |
) | |||||
| 5.375% US$ US Medium Term Note 2034 |
London Stock Exchange |
( |
) |
( |
) | |||||
| 1.625% £ Euro Medium Term Note 2035 |
London Stock Exchange |
( |
) |
( |
) | |||||
| 6.375% US$ US Medium Term Note 2038 |
New York Stock Exchange |
( |
) |
( |
) | |||||
| 6.375% £ Euro Medium Term Note 2039 |
London Stock Exchange |
( |
) |
( |
) | |||||
| 5.250% £ Euro Medium Term Note 2042 |
London Stock Exchange |
( |
) |
( |
) | |||||
| 4.200% US$ US Medium Term Note 2043 |
New York Stock Exchange |
( |
) |
( |
) | |||||
| 4.250% £ Euro Medium Term Note 2045 |
London Stock Exchange |
( |
) |
( |
) | |||||
| Other long-term borrowings |
( |
) |
( |
) | ||||||
| Lease liabilities |
( |
) |
( |
) | ||||||
( |
) |
( |
) | |||||||
| Net debt |
( |
) |
( |
) | ||||||

2021 |
2020 |
|||||||
£m |
£m |
|||||||
| Rental payments due within one year |
||||||||
| Rental payments due between one and two years |
||||||||
| Rental payments due between two and three years |
||||||||
| Rental payments due between three and four years |
||||||||
| Rental payments due between four and five years |
||||||||
| Rental payments due after five years |
||||||||
| Total lease liabilities |
||||||||
2021 |
2020 |
2019 |
||||||||||
Pension and other post-employment costs |
£m |
£m |
£m |
|||||||||
| UK pension schemes |
||||||||||||
| US pension schemes |
||||||||||||
| Other overseas pension schemes |
||||||||||||
| Unfunded post-retirement healthcare schemes |
||||||||||||
| Analysed as: |
||||||||||||
| Funded defined benefit/hybrid pension schemes |
||||||||||||
| Unfunded defined benefit pension schemes |
||||||||||||
| Unfunded post-retirement healthcare schemes |
||||||||||||
| Defined benefit schemes |
||||||||||||
| Defined contribution pension schemes |
||||||||||||
2021 |
2020 |
2019 |
||||||||||
£m |
£m |
£m |
||||||||||
| Cost of sales |
||||||||||||
| Selling, general and administration |
||||||||||||
| Research and development |
||||||||||||

UK |
US |
|||||||||||||||
Male Years |
Female Years |
Male Years |
Female Years |
|||||||||||||
| Current |
||||||||||||||||
| Projected for 2041 |
||||||||||||||||
UK |
US |
Rest of World |
||||||||||||||||||||||||||||||||||||||||||||||
| 2021 % pa |
2020 % pa |
2019 % pa |
2021 % pa |
2020 % pa |
2019 % pa |
2021 % pa |
2020 % pa |
2019 % pa |
||||||||||||||||||||||||||||||||||||||||
| Rate of increase of future earnings |
n/a |
n/a |
||||||||||||||||||||||||||||||||||||||||||||||
| Discount rate |
||||||||||||||||||||||||||||||||||||||||||||||||
| Expected pension increases |
n/a |
n/a |
n/a |
|||||||||||||||||||||||||||||||||||||||||||||
| Cash balance credit/conversion rate |
n/a |
n/a |
n/a |
|||||||||||||||||||||||||||||||||||||||||||||
| Inflation rate |
||||||||||||||||||||||||||||||||||||||||||||||||
Post-retirement |
||||||||||||||||||||
Pensions |
benefits |
|||||||||||||||||||
| 2021 |
UK £m |
US £m |
Rest of World £m |
Group £m |
Group £m |
|||||||||||||||
| Amounts charged to operating profit |
||||||||||||||||||||
| Current service cost |
||||||||||||||||||||
| Past service cost |
( |
) |
||||||||||||||||||
| Net interest cost |
||||||||||||||||||||
| Gains from settlements |
( |
) |
( |
) |
||||||||||||||||
| Expenses |
||||||||||||||||||||
| Remeasurement gains/(losses) recorded in the statement of comprehensive income |
||||||||||||||||||||
Post-retirement |
||||||||||||||||||||
Pensions |
benefits |
|||||||||||||||||||
| 2020 |
UK £m |
US £m |
Rest of World £m |
Group £m |
Group £m |
|||||||||||||||
| Amounts charged to operating profit |
||||||||||||||||||||
| Current service cost |
||||||||||||||||||||
| Past service cost/(credit) |
( |
) |
( |
) | ||||||||||||||||
| Net interest (income)/cost |
||||||||||||||||||||
| Gains from settlements |
( |
) |
( |
) |
( |
) | ||||||||||||||
| Expenses |
||||||||||||||||||||
| Remeasurement gains/(losses) recorded in the statement of comprehensive income |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
Post-retirement |
||||||||||||||||||||
Pensions |
benefits |
|||||||||||||||||||
| 2019 |
UK £m |
US £m |
Rest of World £m |
Group £m |
Group £m |
|||||||||||||||
| Amounts charged to operating profit |
||||||||||||||||||||
| Current service cost |
||||||||||||||||||||
| Past service cost/(credit) |
( |
) |
( |
) |
||||||||||||||||
| Net interest (income)/cost |
( |
) |
||||||||||||||||||
| Gains from settlements |
( |
) |
( |
) |
||||||||||||||||
| Expenses |
||||||||||||||||||||
| Remeasurement losses recorded in the statement of comprehensive income |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||

| 2021 £m |
2020 £m |
2019 £m |
||||||||||
| Recognised in Other non-current assets: |
||||||||||||
| Pension schemes in surplus |
||||||||||||
| Recognised in Assets held for sale: |
||||||||||||
| Post-retirement benefits |
( |
) | ||||||||||
| Recognised in Pensions and other post-employment benefits: |
||||||||||||
| Pension schemes in deficit |
( |
) |
( |
) |
( |
) | ||||||
| Post-retirement benefits |
( |
) |
( |
) |
( |
) | ||||||
( |
) |
( |
) |
( |
) | |||||||
At 31 December 2021 |
UK £m |
US £m |
Rest of World £m |
Group £m |
||||||||||||||
| Equities: |
– listed |
|||||||||||||||||
– unlisted |
||||||||||||||||||
| Multi-asset funds |
||||||||||||||||||
| Property: |
– listed |
|||||||||||||||||
– unlisted |
||||||||||||||||||
| Corporate bonds: |
– listed |
|||||||||||||||||
– unlisted |
||||||||||||||||||
| Government bonds: |
– listed |
|||||||||||||||||
| Insurance contracts |
||||||||||||||||||
| Other (liabilities)/assets |
( |
) |
||||||||||||||||
| Fair value of assets |
||||||||||||||||||
| Asset ceiling restrictions |
( |
) |
( |
) | ||||||||||||||
| Present value of scheme obligations |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Net surplus/(obligation) |
( |
) |
( |
) |
( |
) | ||||||||||||
| Included in Other non-current assets |
||||||||||||||||||
| Included in Pensions and other post-employment benefits |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
( |
) |
( |
) |
( |
) | |||||||||||||
| Actual return on plan assets |
||||||||||||||||||
At 31 December 2020 |
UK £m |
US £m |
Rest of World £m |
Group £m |
||||||||||||||
| Equities: |
– listed |
|||||||||||||||||
– unlisted |
||||||||||||||||||
| Multi-asset funds |
||||||||||||||||||
| Property: |
– listed |
|||||||||||||||||
– unlisted |
||||||||||||||||||
| Corporate bonds: |
– listed |
|||||||||||||||||
– unlisted |
||||||||||||||||||
| Government bonds: |
– listed |
|||||||||||||||||
| Insurance contracts |
||||||||||||||||||
| Other (liabilities)/assets |
( |
) |
||||||||||||||||
| Fair value of assets |
||||||||||||||||||
| Present value of scheme obligations |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Net surplus/(obligation) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Included in Other non-current assets |
||||||||||||||||||
| Included in Pensions and other post-employment benefits |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
( |
) |
( |
) |
( |
) |
( |
) | |||||||||||
| Actual return on plan assets |
||||||||||||||||||
At 31 December 2019 |
UK £m |
US £m |
Rest of World £m |
Group £m |
||||||||||||||
| Equities: |
– listed |
|||||||||||||||||
– unlisted |
||||||||||||||||||
| Multi-asset funds |
||||||||||||||||||
| Property: |
– listed |
|||||||||||||||||
– unlisted |
||||||||||||||||||
| Corporate bonds: |
– listed |
|||||||||||||||||
– unlisted |
||||||||||||||||||
| Government bonds: |
– listed |
|||||||||||||||||
| Insurance contracts |
||||||||||||||||||
| Other (liabilities)/assets |
( |
) |
||||||||||||||||
| Fair value of assets |
||||||||||||||||||
| Present value of scheme obligations |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Net surplus/(obligation) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Included in Other non-current assets |
||||||||||||||||||
| Included in Pensions and other post-employment benefits |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
( |
) |
( |
) |
( |
) |
( |
) | |||||||||||
| Actual return on plan assets |
||||||||||||||||||

Pensions |
Post-retirement benefits |
|||||||||||||||||||
Movements in fair values of assets |
UK £m |
US £m |
Rest of World £m |
Group £m |
Group £m |
|||||||||||||||
| Assets at 1 January 2019 |
– |
|||||||||||||||||||
| Exchange adjustments |
– |
( |
) |
( |
) |
( |
) |
– |
||||||||||||
| Additions through business combinations |
– |
– |
– |
|||||||||||||||||
| Interest income |
– |
|||||||||||||||||||
| Expenses |
( |
) |
( |
) |
– |
( |
) |
– |
||||||||||||
| Settlements and curtailments |
– |
– |
– |
|||||||||||||||||
| Remeasurement |
– |
|||||||||||||||||||
| Employer contributions |
||||||||||||||||||||
| Scheme participants’ contributions |
– |
|||||||||||||||||||
| Benefits paid |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Assets at 31 December 2019 |
– |
|||||||||||||||||||
| Exchange adjustments |
– |
( |
) |
– |
||||||||||||||||
| Interest income |
– |
|||||||||||||||||||
| Expenses |
( |
) |
( |
) |
– |
( |
) |
– |
||||||||||||
| Settlements and curtailments |
– |
– |
( |
) |
( |
) |
– |
|||||||||||||
| Remeasurement |
– |
|||||||||||||||||||
| Employer contributions |
||||||||||||||||||||
| Scheme participants’ contributions |
– |
|||||||||||||||||||
| Benefits paid |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Assets at 31 December 2020 |
– |
|||||||||||||||||||
| Exchange adjustments |
– |
( |
) |
( |
) |
– |
||||||||||||||
| Interest income |
– |
|||||||||||||||||||
| Expenses |
( |
) |
( |
) |
– |
( |
) |
– |
||||||||||||
| Settlements and curtailments |
– |
– |
( |
) |
( |
) |
– |
|||||||||||||
| Remeasurement |
– |
|||||||||||||||||||
| Employer contributions |
||||||||||||||||||||
| Scheme participants’ contributions |
– |
|||||||||||||||||||
| Benefits paid |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Assets at 31 December 2021 |
– |
|||||||||||||||||||
| Pensions |
Post-retirement benefits |
|||||||||||||||||||
Movements in defined benefit obligations |
UK £m |
US £m |
Rest of World £m |
Group £m |
Group £m |
|||||||||||||||
| Obligations at 1 January 2019 |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Exchange adjustments |
– |
|||||||||||||||||||
| Additions through business combinations |
– |
– |
( |
) |
( |
) |
( |
) | ||||||||||||
| Service cost |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Past service cost |
( |
) |
( |
) |
||||||||||||||||
| Interest cost |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Settlements and curtailments |
– |
– |
– |
|||||||||||||||||
| Remeasurement |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Scheme participants’ contributions |
( |
) |
– |
( |
) |
( |
) |
( |
) | |||||||||||
| Benefits paid |
||||||||||||||||||||
| Obligations at 31 December 2019 |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Exchange adjustments |
– |
( |
) |
( |
) |
|||||||||||||||
| Disposals |
– |
– |
– |
– |
||||||||||||||||
| Service cost |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Past service cost |
( |
) |
( |
) |
( |
) |
||||||||||||||
| Interest cost |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Settlements and curtailments |
– |
– |
||||||||||||||||||
| Remeasurement |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Scheme participants’ contributions |
( |
) |
– |
( |
) |
( |
) |
( |
) | |||||||||||
| Benefits paid |
||||||||||||||||||||
| Obligations at 31 December 2020 |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Exchange adjustments |
( |
) |
||||||||||||||||||
| Service cost |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Past service cost |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
| Interest cost |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Settlements and curtailments |
||||||||||||||||||||
| Remeasurement |
||||||||||||||||||||
| Scheme participants’ contributions |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
| Benefits paid |
||||||||||||||||||||
| Obligations at 31 December 2021 |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
The defined benefit pension obligation is analysed as follows : |
| |||||||||||||||||||
| 2021 £m |
2020 £m |
2019 £m |
||||||||||||||||||
| Funded |
( |
) |
( |
) |
( |
) | ||||||||||||||
| Unfunded |
( |
) |
( |
) |
( |
) | ||||||||||||||
( |
) |
( |
) |
( |
) | |||||||||||||||

| 2021 £m |
2020 £m |
2019 £m |
||||||||||
| At 1 January |
( |
) |
( |
) |
( |
) | ||||||
| Exchange adjustments |
( |
) |
||||||||||
| Additions through business combinations |
– |
– |
( |
) | ||||||||
| Service cost |
( |
) |
( |
) |
( |
) | ||||||
| Past service cost |
( |
) |
( |
) |
( |
) | ||||||
| Interest cost |
( |
) |
( |
) |
( |
) | ||||||
| Settlements and curtailments |
||||||||||||
| Remeasurements: |
||||||||||||
| Return on plan assets, excluding amounts included in interest |
||||||||||||
| (Loss)/gain from change in demographic assumptions |
( |
) |
||||||||||
| Gain/(loss) from change in financial assumptions |
( |
) |
( |
) | ||||||||
| Experience (loss)/gain |
( |
) |
( |
) | ||||||||
| Employer contributions |
||||||||||||
| Expenses |
( |
) |
( |
) |
( |
) | ||||||
| At 31 December |
( |
) |
( |
) |
( |
) | ||||||
The remeasurements included within post-retirement benefits are detailed below : |
| |||||||||||
| 2021 £m |
2020 £m |
2019 £m |
||||||||||
| Gain from change in demographic assumptions |
– |
|||||||||||
| Gain/(loss) from change in financial assumptions |
( |
) |
( |
) | ||||||||
| Experience gains |
||||||||||||
( |
) |
( |
) | |||||||||
The defined benefit pension obligation analysed by membership category is as follows: |
| |||||||||||
| 2021 £m |
2020 £m |
2019 £m |
||||||||||
| Active |
||||||||||||
| Retired |
||||||||||||
| Deferred |
||||||||||||
The post-retirement benefit obligation analysed by membership category is as follows: |
| |||||||||||
| 2021 £m |
2020 £m |
2019 £m |
||||||||||
| Active |
||||||||||||
| Retired |
||||||||||||
| Deferred |
– |
|||||||||||
The weighted average duration of the defined benefit obligation is as follows: |
| |||||||||||
| 2021 years |
2020 years |
2019 years |
||||||||||
| Pension benefits |
||||||||||||
| Post-retirement benefits |
||||||||||||
0.25% increase £m |
0.25% decrease £m |
|||||||
| Discount rate |
||||||||
| (Decrease)/increase in annual pension cost |
( |
) |
||||||
| Increase/(decrease) in annual post-retirement benefits cost |
( |
) | ||||||
| (Decrease)/increase in pension obligation |
( |
) |
||||||
| (Decrease)/increase in post-retirement benefits obligation |
( |
) |
||||||
0.5% increase £m |
0.5% decrease £m |
|||||||
| (Decrease)/increase in annual pension cost |
( |
) |
||||||
| Increase/(decrease) in annual post-retirement benefits cost |
( |
) | ||||||
| (Decrease)/increase in pension obligation |
( |
) |
||||||
| (Decrease)/increase in post-retirement benefits obligation |
( |
) |
||||||
0.25% increase £m |
0.25% decrease £m |
|||||||
| Inflation rate |
||||||||
| Increase/(decrease) in annual pension cost |
( |
) | ||||||
| Increase/(decrease) in pension obligation |
( |
) | ||||||
1 year increase £m |
||||||||
| Life expectancy |
||||||||
| Increase in annual pension cost |
||||||||
| Increase in annual post-retirement benefits cost |
||||||||
| Increase in pension obligation |
||||||||
| Increase in post-retirement benefits obligation |
||||||||
| 1% increase £m |
||||||||
| Rate of future healthcare inflation |
||||||||
| Increase in annual post-retirement benefits cost |
||||||||
| Increase in post-retirement benefits obligation |
||||||||

Legal and other disputes £m |
Major restructuring programmes £m |
Employee related provisions £m |
Other provisions £m |
Total £m |
||||||||||||||||
| At 1 January 2021 |
||||||||||||||||||||
| Exchange adjustments |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
| Charge for the year |
||||||||||||||||||||
| Reversed unused |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Unwinding of discount |
||||||||||||||||||||
| Utilised |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Reclassifications and other movements |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
| Transfer to Pension obligations |
( |
) |
( |
) | ||||||||||||||||
| At 31 December 2021 |
||||||||||||||||||||
| To be settled within one year |
||||||||||||||||||||
| To be settled after one year |
||||||||||||||||||||
| At 31 December 2021 |
||||||||||||||||||||
Shionogi- ViiV Healthcare £m |
Novartis Vaccines £m |
Other £m |
Total £m |
|||||||||||||
At 1 January 2019 |
||||||||||||||||
Remeasurement through income statement |
( |
) |
||||||||||||||
Cash payments: operating cash flows |
( |
) |
( |
) |
( |
) | ||||||||||
Cash payments: investing activities |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
Other movements |
||||||||||||||||
At 31 December 2019 |
||||||||||||||||
Remeasurement through income statement |
||||||||||||||||
Cash payments: operating cash flows |
( |
) |
( |
) |
( |
) | ||||||||||
Cash payments: investing activities |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
At 31 December 2020 |
||||||||||||||||
Remeasurement through income statement |
||||||||||||||||
Cash payments: operating cash flows |
( |
) |
( |
) |
( |
) | ||||||||||
Cash payments: investing activities |
( |
) |
( |
) |
( |
) | ||||||||||
At 31 December 2021 |
||||||||||||||||
2021 |
2020 |
|||||||||||||||
Increase/(decrease) in financial liability and loss/(gain) in Income statement |
Shionogi- ViiV Healthcare £m |
Novartis Vaccines £m |
Shionogi- ViiV Healthcare £m |
Novartis Vaccines £m |
||||||||||||
10% increase in sales forecasts* |
||||||||||||||||
10% decrease in sales forecasts* |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
1% increase in discount rate |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
1% decrease in discount rate |
||||||||||||||||
10 cent appreciation of US Dollar |
||||||||||||||||
10 cent depreciation of US Dollar |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
10 cent appreciation of Euro |
||||||||||||||||
10 cent depreciation of Euro |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||
* |
The sales forecast is for ViiV Healthcare sales only in respect of the Shionogi-ViiV Healthcare contingent consideration. |

2021 £m |
2020 £m |
|||||||
Accruals |
||||||||
Deferred income |
||||||||
Other payables |
||||||||
Contractual obligations and commitments |
2021 £m |
2020 £m |
||||||
Contracted for but not provided in the financial statements: |
||||||||
Intangible assets |
||||||||
Property, plant and equipment |
||||||||
Investments |
||||||||
Purchase commitments |
||||||||
Pensions |
||||||||
Interest on loans |
||||||||
Future finance charges on leases |
||||||||
Ordinary Shares of 25p each |
Share premium |
|||||||||||
Number |
£m |
£m |
||||||||||
| Share capital issued and fully paid |
||||||||||||
| At 1 January 2019 |
||||||||||||
| Issued under employee share schemes |
||||||||||||
| Ordinary shares acquired by ESOP Trusts |
– |
– |
||||||||||
| At 31 December 2019 |
||||||||||||
| Issued under employee share schemes |
– |
|||||||||||
| Ordinary shares acquired by ESOP Trusts |
– |
– |
||||||||||
| At 31 December 2020 |
||||||||||||
| Issued under employee share schemes |
||||||||||||
| Ordinary shares acquired by ESOP Trusts |
||||||||||||
| At 31 December 2021 |
||||||||||||
| 31 December 2021 000 |
31 December 2020 000 |
|||||||||||
| Number of shares issuable under employee share schemes |
||||||||||||
| Number of unissued shares not under option |
||||||||||||
Net translation exchange included in: |
||||||||||||||||||
Retained earnings £m |
Fair value reserve £m |
Non- controlling interests £m |
Total translation exchange £m |
|||||||||||||||
| At 1 January 2019 |
( |
) |
||||||||||||||||
| Exchange movements on overseas net assets and net investment hedges |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Reclassification of exchange movements on liquidation or disposal of overseas subsidiaries |
( |
) |
– |
– |
( |
) | ||||||||||||
| At 31 December 2019 |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Exchange movements on overseas net assets and net investment hedges |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Reclassification of exchange movements on liquidation or disposal of overseas subsidiaries |
– |
– |
||||||||||||||||
| At 31 December 2020 |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||
| Exchange movements on overseas net assets and net investment hedges |
( |
) |
– |
( |
) |
( |
) | |||||||||||
| Reclassification of exchange movements on liquidation or disposal of overseas subsidiaries and associates |
( |
) |
– |
– |
( |
) | ||||||||||||
| At 31 December 2021 |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||

2021 |
Retained earnings £m |
Other reserves £m |
Non- controlling interests £m |
Total £m |
||||||||||||
| Items that may be subsequently reclassified to income statement: |
||||||||||||||||
| Exchange movements on overseas net assets and net investment hedges |
( |
) |
– |
– |
( |
) | ||||||||||
| Reclassification of exchange movements on liquidation or disposal of overseas subsidiaries and associates |
( |
) |
– |
– |
( |
) | ||||||||||
| Fair value movements on cash flow hedges |
– |
– |
||||||||||||||
| Reclassification of cash flow hedges to income and expense |
– |
– |
||||||||||||||
| Tax on fair value movements on cash flow hedges |
– |
( |
) |
– |
( |
) | ||||||||||
| Items that will not be reclassified to income statement: |
||||||||||||||||
| Exchange movements on overseas net assets of non-controlling interests |
– |
( |
) |
( |
) | |||||||||||
| Fair value movements on equity investments |
– |
( |
) |
– |
( |
) | ||||||||||
| Tax on fair value movements on equity investments |
– |
– |
||||||||||||||
| Remeasurement losses on defined benefit plans |
– |
– |
||||||||||||||
| Tax on remeasurement losses in defined benefit plans |
( |
) |
– |
– |
( |
) | ||||||||||
| Other comprehensive (expense)/income for the year |
( |
) |
( |
) |
( |
) | ||||||||||
2020 |
Retained earnings £m |
Other reserves £m |
Non- controlling interests £m |
Total £m |
||||||||||||
| Items that may be subsequently reclassified to income statement: |
||||||||||||||||
| Exchange movements on overseas net assets and net investment hedges |
( |
) |
( |
) |
– |
( |
) | |||||||||
| Reclassification of exchange movements on liquidation or disposal of overseas subsidiaries |
– |
– |
||||||||||||||
| Fair value movements on cash flow hedges |
– |
( |
) |
– |
( |
) | ||||||||||
| Reclassification of cash flow hedges to income and expense |
– |
– |
||||||||||||||
| Tax on fair value movements on cash flow hedges |
– |
( |
) |
– |
( |
) | ||||||||||
| Items that will not be reclassified to income statement: |
||||||||||||||||
| Exchange movements on overseas net assets of non-controlling interests |
– |
– |
( |
) |
( |
) | ||||||||||
| Fair value movements on equity investments |
– |
– |
||||||||||||||
| Tax on fair value movements on equity investments |
– |
( |
) |
– |
( |
) | ||||||||||
| Remeasurement losses on defined benefit plans |
( |
) |
– |
– |
( |
) | ||||||||||
| Tax on remeasurement losses in defined benefit plans |
– |
– |
||||||||||||||
| Other comprehensive (expense)/income for the year |
( |
) |
( |
) |
||||||||||||
2019 |
Retained earnings £m |
Other reserves £m |
Non- controlling interests £m |
Total £m |
||||||||||||
| Items that may be subsequently reclassified to income statement: |
||||||||||||||||
| Exchange movements on overseas net assets and net investment hedges |
( |
) |
( |
) |
– |
( |
) | |||||||||
| Reclassification of exchange movements on liquidation or disposal of overseas subsidiaries |
( |
) |
– |
– |
( |
) | ||||||||||
| Fair value movements on cash flow hedges |
– |
( |
) |
– |
( |
) | ||||||||||
| Reclassification of cash flow hedges to income and expense |
– |
– |
||||||||||||||
| Tax on fair value movements on cash flow hedges |
– |
– |
||||||||||||||
| Items that will not be reclassified to income statement: |
||||||||||||||||
| Exchange movements on overseas net assets of non-controlling interests |
– |
– |
( |
) |
( |
) | ||||||||||
| Fair value movements on equity investments |
– |
– |
||||||||||||||
| Tax on fair value movements on equity investments |
– |
( |
) |
– |
( |
) | ||||||||||
| Remeasurement gains on defined benefit plans |
( |
) |
– |
– |
( |
) | ||||||||||
| Tax on remeasurement gains in defined benefit plans |
– |
– |
||||||||||||||
| Other comprehensive (expense)/income for the year |
( |
) |
( |
) |
( |
) | ||||||||||
| ESOP Trust shares £m |
Fair value reserve £m |
Cash flow hedge reserve £m |
Other reserves £m |
Total £m |
||||||||||||||||
| At 1 January 2019 |
( |
) |
( |
) |
||||||||||||||||
| Exchange adjustments |
– |
– |
– |
|||||||||||||||||
| Transferred to Retained earnings in the year on disposal of equity investments |
– |
– |
– |
|||||||||||||||||
| Net fair value movement in the year |
– |
( |
) |
– |
||||||||||||||||
| Ordinary shares acquired by ESOP Trusts |
( |
) |
– |
– |
– |
( |
) | |||||||||||||
| Write-down of shares held by ESOP Trusts |
– |
– |
– |
|||||||||||||||||
| At 31 December 2019 |
( |
) |
( |
) |
||||||||||||||||
| Exchange adjustments |
– |
– |
– |
|||||||||||||||||
| Transferred to Retained earnings in the year on disposal of equity investments |
– |
( |
) |
– |
– |
( |
) | |||||||||||||
| Net fair value movement in the year |
– |
– |
||||||||||||||||||
| Ordinary shares acquired by ESOP Trusts |
( |
) |
– |
– |
– |
( |
) | |||||||||||||
| Write-down of shares held by ESOP Trusts |
– |
– |
– |
|||||||||||||||||
| At 31 December 2020 |
( |
) |
( |
) |
||||||||||||||||
| Exchange adjustments |
( |
) |
– |
– |
– |
( |
) | |||||||||||||
| Transferred to Retained earnings in the year on disposal of equity investments |
– |
( |
) |
– |
– |
( |
) | |||||||||||||
| Net fair value movement in the year |
– |
( |
) |
– |
( |
) | ||||||||||||||
| Transferred to income an d expense in the year on impairments of equity investments |
– |
– |
– |
|||||||||||||||||
| At 31 December 2021 |
( |
) |
( |
) |
||||||||||||||||
| 2021 £m |
2020 £m |
2019 £m |
||||||||||
| Turnover |
||||||||||||
| Profit after taxation |
||||||||||||
| Other comprehensive income/(expense) |
( |
) |
( |
) | ||||||||
| Total comprehensive income |
||||||||||||
| 2021 £m |
2020 £m |
|
||||||||||
| Non-current assets |
||||||||||||
| Current assets |
||||||||||||
| Total assets |
||||||||||||
| Current liabilities |
( |
) |
( |
) |
||||||||
| Non-current liabilities |
( |
) |
( |
) |
||||||||
| Total liabilities |
( |
) |
( |
) |
||||||||
| Net liabilities |
( |
) |
( |
) |
||||||||
| 2021 £m |
2020 £m |
2019 £m |
||||||||||
| Net cash inflow from operating activities |
||||||||||||
| Net cash outflow from investing activities |
( |
) |
( |
) |
( |
) | ||||||
| Net cash outflow from financing activities |
( |
) |
( |
) |
( |
) | ||||||
| (Decrease)/increase in cash and bank overdrafts in the year |
( |
) |
||||||||||

| 2021 £m |
2020 £m |
2019 £m |
||||||||||
| Share of profit for the year attributable to non-controlling interest |
||||||||||||
| Dividends paid to non-controlling interest |
||||||||||||
| Non-controlling interest in the Consolidated balance sheet |
( |
) |
( |
) |
( |
) | ||||||
| 2021 £m |
2020 £m |
2019 £m |
||||||||||
| Turnover |
||||||||||||
| Profit after taxation |
||||||||||||
| Other comprehensive expenses |
( |
) |
( |
) |
( |
) | ||||||
| Total comprehensive income/(expenses) |
( |
) | ||||||||||
| 2021 £m |
2020 £m |
|||||||||||
| Non-current assets |
||||||||||||
| Current assets |
||||||||||||
| Total assets |
||||||||||||
| Current liabilities |
( |
) |
( |
) |
||||||||
| Non-current liabilities |
( |
) |
( |
) |
||||||||
| Total liabilities |
( |
) |
( |
) |
||||||||
| Net assets |
||||||||||||
| 2021 £m |
2020 £m |
2019 £m |
||||||||||
| Net cash inflow from operating activities |
||||||||||||
| Net cash inflow/(outflow) from investing activities |
( |
) |
( |
) | ||||||||
| Net cash outflow from financing activities |
( |
) |
( |
) |
( |
) | ||||||
| Increase in cash and bank overdraft in the year/period |
– |
|||||||||||
| 2021 £m |
2020 £m |
2019 £m |
||||||||||
| Share of profit for the year/period attributable to non-controlling interest |
||||||||||||
| Dividends paid to non-controlling interest |
– |
|||||||||||
| Non-controlling interest in the Consolidated balance sheet |
||||||||||||
| Total £m |
||||
| Consideration: |
||||
| Cash consideration including currency forwards, purchase adjustments and deferred consideration |
||||
| Total |
||||
| Net assets sold: |
||||
| Property, plant and equipment |
||||
| Cash and cash equivalents |
||||
| Other net assets |
||||
| Total |
||||
| Costs: |
||||
| Deal costs |
( |
) | ||
| Reclassification of exchange from other comprehensive income |
||||
| Gain on disposals in 2021 |
||||
| Business disposals £m |
Associates and joint ventures disposals £m |
|||||||
| Cash consideration received |
||||||||
| Net deferred consideration paid |
( |
) |
– |
|||||
| Transaction costs |
( |
) |
– |
|||||
| Cash and cash equivalents (divested)/acquired |
( |
) |
– |
|||||
| Cash (outflow)/inflow |
( |
) |
||||||

| Total £m |
||||
| Net assets acquired: |
||||
| Intangible assets |
||||
| Property, plant and equipment |
||||
| Inventory |
||||
| Trade and other receivables |
||||
| Cash and cash equivalents |
||||
| Trade and other payables |
( |
) | ||
| Non-controlling interest |
( |
) | ||
| Goodwill |
||||
| Non-cash consideration (settlement of a promissory note) |
||||
| Total consideration |
||||
| Horlicks divestment £m |
Other £m |
Total £m |
||||||||||
| Consideration: |
||||||||||||
| Cash consideration receivable including currency forwards and purchase adjustments |
||||||||||||
| Equity investment in Hindustan Unilever Limited |
– |
|||||||||||
| Total |
||||||||||||
| Net assets disposed: |
||||||||||||
| Goodwill |
||||||||||||
| Intangible assets |
||||||||||||
| Property, plant and equipment |
||||||||||||
| Inventory |
– |
|||||||||||
| Cash and cash equivalents |
||||||||||||
| Other net (liabilities)/assets |
( |
) |
( |
) | ||||||||
| Total |
||||||||||||
| Costs: |
||||||||||||
| Transaction costs |
||||||||||||
| Derivative |
– |
|||||||||||
| Reclassification of exchange from other comprehensive income |
– |
|||||||||||
| Total |
||||||||||||
| Gain on disposals |
||||||||||||
Business acquisitions £m |
Business disposals £m |
Associates and joint ventures investments £m |
||||||||||
| Cash consideration received/(paid) |
– |
( |
) | |||||||||
| Net deferred consideration |
– |
( |
) |
– |
||||||||
| Transaction costs |
( |
) |
( |
) |
– |
|||||||
| Cash and cash equivalents acquired/(divested) |
( |
) |
– |
|||||||||
| Cash inflow/(outflow) |
( |
) | ||||||||||

Pfizer consumer healthcare business £m |
Tesaro £m |
Other £m |
||||||||||
| Net assets acquired: |
||||||||||||
| Intangible assets |
– |
|||||||||||
| Property, plant and equipment |
– |
|||||||||||
| Right of use assets |
– |
|||||||||||
| Inventory |
– |
|||||||||||
| Trade and other receivables |
||||||||||||
| Other assets including cash and cash equivalents |
||||||||||||
| Trade and other payables |
( |
) |
( |
) |
( |
) | ||||||
| Net deferred tax liabilities |
( |
) |
( |
) |
– |
|||||||
| Other liabilities |
( |
) |
( |
) |
– |
|||||||
| Term loan |
– |
( |
) |
– |
||||||||
| Non-controlling interest |
( |
) |
– |
– |
||||||||
| Goodwill |
– |
|||||||||||
| Total |
||||||||||||
| Consideration settled by shares in GSK Consumer Healthcare Joint Venture |
– |
– |
||||||||||
| Cash consideration paid |
– |
|||||||||||
| Fair value of investment in joint venture converted into subsidiary |
– |
– |
||||||||||
| Total consideration |
||||||||||||
£m |
Total £m |
|||||||
| Cash consideration receivable net of subsidy payable |
||||||||
| Net assets disposed: |
||||||||
| Goodwill |
( |
) |
||||||
| Intangible assets |
( |
) |
||||||
| Property, plant and equipment |
( |
) |
||||||
| Inventory |
( |
) |
||||||
| Cash and cash equivalents |
( |
) |
||||||
| Other net assets |
( |
) |
||||||
( |
) | |||||||
| Transaction costs |
( |
) | ||||||
| Reclassification of exchange from other comprehensive income |
||||||||
| Non-controlling interest divested |
||||||||
| Transaction signed but not yet completed – gain on embedded derivative |
||||||||
| Transaction signed but not yet completed – transaction costs |
( |
) | ||||||
| Total profit on disposal |
||||||||
Business acquisitions £m |
Business disposals £m |
Associates and joint venture investments £m |
||||||||||
| Cash consideration (paid)/received |
( |
) |
( |
) | ||||||||
| Net deferred consideration received |
– |
– |
||||||||||
| Transaction costs |
( |
) |
( |
) |
– |
|||||||
| Cash and cash equivalents acquired/divested |
( |
) |
– |
|||||||||
| Cash (outflow)/inflow |
( |
) |
( |
) | ||||||||
2021 £m |
2020 £m |
2019 £m |
||||||||||
| Profit after tax |
||||||||||||
| Tax on profits |
||||||||||||
| Share of after-tax profits of associates and joint ventures |
( |
) |
( |
) |
( |
) | ||||||
| Finance expense net of finance income |
||||||||||||
| Depreciation |
||||||||||||
| Amortisation of intangible assets |
||||||||||||
| Impairment and assets written off |
||||||||||||
| Profit on sale of businesses |
( |
) |
( |
) |
( |
) | ||||||
| Profit on sale of intangible assets |
( |
) |
( |
) |
( |
) | ||||||
| Loss on sale of investments in associates |
– |
– |
||||||||||
| Profit on sale of equity investments |
( |
) |
( |
) |
( |
) | ||||||
| Business acquisition costs |
– |
|||||||||||
| Changes in working capital: |
||||||||||||
| Decrease in inventories |
||||||||||||
| Increase in trade receivables |
( |
) |
( |
) |
( |
) | ||||||
| Increase in trade payables |
||||||||||||
| (Increase) in other receivables |
( |
) |
( |
) |
( |
) | ||||||
| Contingent consideration paid (see Note 32) |
( |
) |
( |
) |
( |
) | ||||||
| Other non-cash increase in contingent consideration liabilities |
||||||||||||
| Increase in other payables |
||||||||||||
| Increase/(decrease) in pension and other provisions |
( |
) |
( |
) | ||||||||
| Share-based incentive plans |
||||||||||||
| Fair value adjustments |
( |
) |
||||||||||
| Other |
( |
) |
( |
) |
( |
) | ||||||
| Cash generated from operations |
||||||||||||

2021 £m |
2020 £m |
2019 £m |
||||||||||
| Net debt, as previously reported |
( |
) |
( |
) |
( |
) | ||||||
| Implementation of IFRS 16 |
– |
– |
( |
) | ||||||||
| Net debt at beginning of year, as adjusted |
( |
) |
( |
) |
( |
) | ||||||
| Increase in cash and bank overdrafts |
( |
) |
||||||||||
| Increase/(decrease) in liquid investments |
( |
) |
( |
) | ||||||||
| Increase in long-term loans |
– |
( |
) |
( |
) | |||||||
| Repayment of short-term Notes |
||||||||||||
| Repayment of/(increase in) other short-term loans |
( |
) |
( |
) | ||||||||
| Repayment of lease liabilities |
||||||||||||
| Debt of subsidiary undertakings acquired |
– |
– |
( |
) | ||||||||
| Exchange adjustments |
( |
) |
||||||||||
| Other non-cash movements |
( |
) |
( |
) |
( |
) | ||||||
| Movement in net debt |
( |
) | ||||||||||
| Net debt at end of year |
( |
) |
( |
) |
( |
) | ||||||
Analysis of changes in net debt |
At 1 January 2021 £m |
Exchange £m |
Other £m |
Interest expense £m |
Change in fair value £m |
Reclass- ifications £m |
Cash flow £m |
At 31 December 2021 £m |
||||||||||||||||||||||||
| Liquid investments |
– |
– |
– |
– |
( |
) |
||||||||||||||||||||||||||
| Cash and cash equivalents |
( |
) |
( |
) |
– |
– |
– |
( |
) |
|||||||||||||||||||||||
| Overdrafts |
( |
) |
– |
– |
– |
– |
– |
( |
) | |||||||||||||||||||||||
( |
) |
( |
) |
– |
– |
– |
( |
) |
||||||||||||||||||||||||
| Debt due within one year: |
||||||||||||||||||||||||||||||||
| Commercial paper |
( |
) |
– |
– |
– |
– |
( |
) |
( |
) | ||||||||||||||||||||||
| European/US MTN & Bank facilities |
( |
) |
– |
– |
– |
( |
) |
( |
) | |||||||||||||||||||||||
| Lease liabilities |
( |
) |
– |
– |
( |
) |
( |
) | ||||||||||||||||||||||||
| Other |
( |
) |
( |
) |
– |
– |
– |
( |
) |
( |
) | |||||||||||||||||||||
( |
) |
– |
– |
( |
) |
( |
) | |||||||||||||||||||||||||
| Debt due after one year: |
||||||||||||||||||||||||||||||||
| European/US MTN & Bank facilities |
( |
) |
– |
( |
) |
– |
– |
( |
) | |||||||||||||||||||||||
| Lease liabilities |
( |
) |
( |
) |
– |
– |
– |
( |
) | |||||||||||||||||||||||
( |
) |
( |
) |
( |
) |
– |
– |
( |
) | |||||||||||||||||||||||
| Net debt |
( |
) |
( |
) |
( |
) |
– |
– |
( |
) | ||||||||||||||||||||||
| Interest payable |
( |
) |
– |
( |
) |
( |
) |
– |
– |
( |
) | |||||||||||||||||||||
| Derivative financial instruments |
( |
) |
– |
– |
– |
– |
( |
) |
( |
) | ||||||||||||||||||||||
| Total liabilities from financing activities* |
( |
) |
( |
) |
( |
) |
– |
( |
) | |||||||||||||||||||||||
* |
Excluding cash and cash equivalents, overdrafts and liquid investments. |
Analysis of changes in net debt |
At 1 January 2020 £m |
Exchange £m |
Other £m |
Interest expense £m |
Change in fair value £m |
Reclass- ifications £m |
Cash flow £m |
At 31 December 2020 £m |
||||||||||||||||||||||||
Liquid investments |
– |
– |
– |
– |
– |
( |
) |
|||||||||||||||||||||||||
Cash and cash equivalents |
( |
) |
– |
– |
– |
– |
||||||||||||||||||||||||||
Cash and cash equivalents - AHFS |
– |
– |
– |
– |
– |
( |
) |
– |
||||||||||||||||||||||||
Overdrafts |
( |
) |
– |
– |
– |
– |
( |
) |
( |
) | ||||||||||||||||||||||
( |
) |
– |
– |
– |
– |
|||||||||||||||||||||||||||
Debt due within one year: |
||||||||||||||||||||||||||||||||
Commercial paper |
( |
) |
( |
) |
– |
– |
– |
– |
( |
) | ||||||||||||||||||||||
European/US MTN and Bank facilities |
( |
) |
– |
– |
– |
( |
) |
( |
) | |||||||||||||||||||||||
Lease liabilities |
( |
) |
( |
) |
– |
– |
( |
) |
( |
) | ||||||||||||||||||||||
Other |
( |
) |
( |
) |
– |
– |
– |
( |
) |
( |
) | |||||||||||||||||||||
( |
) |
( |
) |
– |
– |
( |
) |
( |
) | |||||||||||||||||||||||
Debt due after one year: |
||||||||||||||||||||||||||||||||
European/US MTN & Bank facilities |
( |
) |
( |
) |
( |
) |
( |
) |
– |
( |
) |
( |
) | |||||||||||||||||||
Lease liabilities |
( |
) |
( |
) |
– |
– |
– |
( |
) | |||||||||||||||||||||||
( |
) |
( |
) |
( |
) |
( |
) |
– |
( |
) |
( |
) | ||||||||||||||||||||
Net debt |
( |
) |
( |
) |
( |
) |
( |
) |
– |
– |
( |
) | ||||||||||||||||||||
Interest payable |
( |
) |
– |
( |
) |
– |
– |
( |
) | |||||||||||||||||||||||
Derivative financial instruments |
– |
– |
– |
( |
) |
– |
( |
) |
( |
) | ||||||||||||||||||||||
Total liabilities from financing activities* |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
– |
( |
) | |||||||||||||||||||
* |
Excluding cash and cash equivalents, overdrafts and liquid investments. |


2021 |
AAA/Aaa £m |
AA/Aa £m |
A/A £m |
BBB/Baa £m |
BB+/Ba1 and below /unrated £m |
Total £m |
||||||||||||||||||
Bank balances and deposits |
– |
|||||||||||||||||||||||
US Treasury and Treasury repo only money market funds |
– |
– |
– |
– |
||||||||||||||||||||
Liquidity funds |
– |
– |
– |
– |
||||||||||||||||||||
Government securities |
– |
– |
– |
|||||||||||||||||||||
3rd party financial derivatives |
– |
– |
– |
– |
||||||||||||||||||||
Total |
||||||||||||||||||||||||
2020 |
AAA/Aaa £m |
AA/Aa £m |
A/A £m |
BBB/Baa £m |
BB+/Ba1 and below /unrated £m |
Total £m |
||||||||||||||||||
Bank balances and deposits |
– |
|||||||||||||||||||||||
US Treasury and Treasury repo only money market funds |
– |
– |
– |
– |
||||||||||||||||||||
Liquidity funds |
– |
– |
– |
– |
||||||||||||||||||||
Government securities |
– |
– |
– |
|||||||||||||||||||||
3rd party financial derivatives |
– |
– |
– |
|||||||||||||||||||||
Total |
||||||||||||||||||||||||
– |
Other investments – equity investments traded in an active market determined by reference to the relevant stock exchange quoted bid price; other equity investments determined by reference to the current market value of similar instruments, recent financing rounds or the discounted cash flows of the underlying net assets |
– |
Trade receivables carried at fair value – based on invoiced amount |
– |
Interest rate swaps, foreign exchange forward contracts, swaps and options – based on the present value of contractual cash flows or option valuation models using market sourced data (exchange rates or interest rates) at the balance sheet date |
– |
Cash and cash equivalents carried at fair value – based on net asset value of the funds |
– |
Contingent consideration for business acquisitions and divestments – based on present values of expected future cash flows. |
– |
Receivables and payables, including put options, carried at amortised cost – approximates to the carrying amount |
– |
Liquid investments – approximates to the carrying amount |
– |
Cash and cash equivalents carried at amortised cost – approximates to the carrying amount |
– |
Long-term loans – based on quoted market prices (a level 1 fair value measurement) in the case of European and US Medium Term Notes; approximates to the carrying amount in the case of other fixed rate borrowings and floating rate bank loans |
– |
Short-term loans, overdrafts and commercial paper – approximates to the carrying amount because of the short maturity of these instruments. |

2021 |
2020 |
|||||||||||||||||||
Notes |
Carrying value £m |
Fair value £m |
Carrying value £m |
Fair value £m |
||||||||||||||||
Financial assets measured at amortised cost: |
||||||||||||||||||||
Other non-current assets |
b |
|||||||||||||||||||
Trade and other receivables |
b |
|||||||||||||||||||
Liquid investments |
||||||||||||||||||||
Cash and cash equivalents |
||||||||||||||||||||
Financial assets measured at fair value through other comprehensive income (FVTOCI): |
||||||||||||||||||||
Other investments designated at FVTOCI |
a |
|||||||||||||||||||
Trade and other receivables |
a,b |
|||||||||||||||||||
Financial assets mandatorily measured at fair value through profit or loss (FVTPL): |
||||||||||||||||||||
Other investments |
a |
|||||||||||||||||||
Other non-current assets |
a,b |
|||||||||||||||||||
Trade and other receivables |
a,b |
|||||||||||||||||||
Held for trading derivatives that are not in a designated and effective hedging relationship |
a,d,e |
|||||||||||||||||||
Cash and cash equivalents |
a |
|||||||||||||||||||
Derivatives designated and effective as hedging instruments (fair value movements through Other comprehensive income) |
a,d,e |
|||||||||||||||||||
Total financial assets |
||||||||||||||||||||
Financial liabilities measured at amortised cost: |
||||||||||||||||||||
Borrowings excluding obligations under lease liabilities: |
||||||||||||||||||||
– bonds in a designated hedging relationship |
d |
( |
) |
( |
) |
( |
) |
( |
) | |||||||||||
– other bonds |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
– bank loans and overdrafts |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
– commercial paper |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
– other borrowings |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
Total borrowings excluding lease liabilities |
f |
( |
) |
( |
) |
( |
) |
( |
) | |||||||||||
Trade and other payables |
c |
( |
) |
( |
) |
( |
) |
( |
) | |||||||||||
Other provisions |
c |
( |
) |
( |
) |
( |
) |
( |
) | |||||||||||
Other non-current liabilities |
c |
( |
) |
( |
) |
( |
) |
( |
) | |||||||||||
Financial liabilities mandatorily measured at fair value through profit or loss (FVTPL): |
||||||||||||||||||||
Contingent consideration liabilities |
a,c |
( |
) |
( |
) |
( |
) |
( |
) | |||||||||||
Held for trading derivatives that are not in a designated and effective hedging relationship |
a,d,e |
( |
) |
( |
) |
( |
) |
( |
) | |||||||||||
Derivatives designated and effective as hedging instruments (fair value movements through Other comprehensive income) |
a,d,e |
( |
) |
( |
) |
( |
) |
( |
) | |||||||||||
Total financial liabilities excluding lease liabilities |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
Net financial assets and financial liabilities excluding lease liabilities |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
At 31 December 2021 |
Level 1 £m |
Level 2 £m |
Level 3 £m |
Total £m |
||||||||||||
Financial assets at fair value |
||||||||||||||||
Financial assets measured at fair value through other comprehensive income (FVTOCI): |
||||||||||||||||
Other investments designated at FVTOCI |
||||||||||||||||
Trade and other receivables |
||||||||||||||||
Financial assets mandatorily measured at fair value through profit or loss (FVTPL): |
||||||||||||||||
Other investments |
||||||||||||||||
Other non-current assets |
||||||||||||||||
Trade and other receivables |
||||||||||||||||
Held for trading derivatives that are not in a designated and effective hedging relationship |
||||||||||||||||
Cash and cash equivalents |
||||||||||||||||
Derivatives designated and effective as hedging instruments (fair value movements through OCI) |
||||||||||||||||
Financial liabilities at fair value |
||||||||||||||||
Financial liabilities mandatorily measured at fair value through profit or loss (FVTPL): |
||||||||||||||||
Contingent consideration liabilities |
( |
) |
( |
) | ||||||||||||
Held for trading derivatives that are not in a designated and effective hedging relationship |
( |
) |
( |
) | ||||||||||||
Derivatives designated and effective as hedging instruments (fair value movements through OCI) |
( |
) |
( |
) | ||||||||||||
( |
) |
( |
) |
( |
) | |||||||||||
At 31 December 2020 |
Level 1 £m |
Level 2 £m |
Level 3 £m |
Total £m |
||||||||||||
Financial assets at fair value |
||||||||||||||||
Financial assets measured at fair value through other comprehensive income (FVTOCI): |
||||||||||||||||
Other investments designated at FVTOCI |
||||||||||||||||
Trade and other receivables |
||||||||||||||||
Financial assets mandatorily measured at fair value through profit or loss (FVTPL): |
||||||||||||||||
Other investments |
||||||||||||||||
Other non-current assets |
||||||||||||||||
Trade and other receivables |
||||||||||||||||
Held for trading derivatives that are not in a designated and effective hedging relationship |
||||||||||||||||
Cash and cash equivalents |
||||||||||||||||
Derivatives designated and effective as hedging instruments (fair value movements through OCI) |
||||||||||||||||
Financial liabilities at fair value |
||||||||||||||||
Financial liabilities mandatorily measured at fair value through profit or loss (FVTPL): |
||||||||||||||||
Contingent consideration liabilities |
( |
) |
( |
) | ||||||||||||
Held for trading derivatives that are not in a designated and effective hedging relationship |
( |
) |
( |
) |
( |
) | ||||||||||
Derivatives designated and effective as hedging instruments (fair value movements through OCI) |
( |
) |
( |
) | ||||||||||||
( |
) |
( |
) |
( |
) | |||||||||||

2021 £m |
2020 £m |
|||||||
At 1 January |
( |
) |
( |
) | ||||
Net losses recognised in the income statement |
( |
) |
( |
) | ||||
Net gains recognised in other comprehensive income |
||||||||
Settlement of contingent consideration liabilities |
||||||||
Additions |
||||||||
Disposals and settlements |
( |
) |
( |
) | ||||
Transfers from Level 3 |
( |
) |
( |
) | ||||
At 31 December |
( |
) |
( |
) | ||||
2021 |
2020 |
|||||||||||||||||||||||||||||||||||||||||||||||
At FVTPL £m |
At FVTOCI £m |
Amortised cost £m |
Financial instruments £m |
Non- financial instruments £m |
Total £m |
At FVTPL £m |
At FVTOCI £m |
Amortised cost £m |
Financial instruments £m |
Non- financial instruments £m |
Total £m |
|||||||||||||||||||||||||||||||||||||
Trade and other receivables (Note 25) |
||||||||||||||||||||||||||||||||||||||||||||||||
Other non-current assets(Note 23 ) |
– |
|||||||||||||||||||||||||||||||||||||||||||||||
2021 |
2020 |
|||||||||||||||||||||||||||||||||||||||
At FVTPL £m |
Amortised cost £m |
Financial instruments £m |
Non- financial instruments £m |
Total £m |
At FVTPL £m |
Amortised cost £m |
Financial instruments £m |
Non- financial instruments £m |
Total £m |
|||||||||||||||||||||||||||||||
Trade and other payables (Note 28) |
( |
) |
( |
) |
( |
) |
( |
) |
– |
( |
) |
( |
) |
( |
) |
( |
) | |||||||||||||||||||||||
Other provisions (Note 31) |
( |
) |
( |
) |
( |
) |
( |
) |
– |
( |
) |
( |
) |
( |
) |
( |
) | |||||||||||||||||||||||
Contingent consideration liabilities (Note 32) |
( |
) |
( |
) |
( |
) |
( |
) |
– |
( |
) |
– |
( |
) | ||||||||||||||||||||||||||
Other non-current liabilities (Note 33) |
( |
) |
( |
) |
( |
) |
( |
) |
– |
( |
) |
( |
) |
( |
) |
( |
) | |||||||||||||||||||||||
( |
) |
( |
) |
( |
) | ( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | |||||||||||||||||||||
2021 Fair value |
2020 Fair value |
|||||||||||||||
Assets £m |
Liabilities £m |
Assets £m |
Liabilities £m |
|||||||||||||
Non-current |
||||||||||||||||
Cash flow hedges – Interest rate swap contracts (principal amount – £ |
( |
) |
– |
– |
||||||||||||
Current |
||||||||||||||||
Cash flow hedges – Interest rate swap contracts (principal amount – £ 2020 – £ |
– |
( |
) | |||||||||||||
Net investment hedges – Cross currency swaps (principal amount – £ |
– |
( |
) | |||||||||||||
Cash flow hedges – Foreign exchange contracts (principal amount – £ |
( |
) |
– |
– |
||||||||||||
Net investment hedges – Foreign exchange contracts (principal amount – £ |
( |
) |
( |
) | ||||||||||||
Derivatives designated and effective as hedging instruments |
( |
) |
( |
) | ||||||||||||
Non-current |
||||||||||||||||
Embedded and other derivatives |
( |
) | ||||||||||||||
Current |
||||||||||||||||
Foreign exchange contracts (principal amount – £ |
( |
) |
( |
) | ||||||||||||
Embedded and other derivatives |
( |
) |
– |
|||||||||||||
Derivatives classified as held for trading |
( |
) |
( |
) | ||||||||||||
Total derivative instruments |
( |
) |
( |
) | ||||||||||||
2021 |
||||||||||||||||||||
Hedging instruments |
Average exchange rate |
Foreign currency |
Notional value £m |
Carrying value £m |
Periodic change in value for calculating hedge ineffectiveness £m |
|||||||||||||||
Cash flow hedges |
||||||||||||||||||||
Foreign exchange contracts |
||||||||||||||||||||
Buy foreign currency: |
||||||||||||||||||||
Less than 3 months |
( |
) |
– |
|||||||||||||||||
3 to 6 months |
( |
) |
( |
) | ||||||||||||||||
Over 6 months |
– |
– |
||||||||||||||||||
( |
) | ( |
) | |||||||||||||||||
2021 |
||||||||||||||||||||
Hedging instruments |
Average exchange rate |
Foreign currency |
Notional value £m |
Carrying value £m |
Periodic change in value for calculating hedge ineffectiveness £m |
|||||||||||||||
Net investment hedges |
||||||||||||||||||||
Foreign exchange contracts |
||||||||||||||||||||
Sell foreign currency: |
||||||||||||||||||||
Less than 3 months |
||||||||||||||||||||
– |
– |
|||||||||||||||||||
– |
||||||||||||||||||||
Borrowings |
||||||||||||||||||||
Less than 3 months |
( |
) |
||||||||||||||||||
Over 6 months |
( |
) |
||||||||||||||||||
( |
) |
|||||||||||||||||||
2021 |
||||||||
Hedged items |
Periodic change in value for calculating hedge ineffectiveness £m |
Cumulative balance in cash flow hedge reserve/foreign currency translation reserve for continuing hedges £m |
||||||
Cash flow hedges |
||||||||
Variability in cash flows from foreign exchange exposure arising on Euro denominated coupon payments relating to debt issued |
( |
) | ||||||
Net investment hedges |
||||||||
Net investment in foreign operations |
( |
) |
( |
) | ||||
2020 |
||||||||||||||||||||
Hedging instruments |
Average exchange rate |
Foreign currency |
Notional value £m |
Carrying value £m |
Periodic change in value for calculating hedge ineffectiveness £m |
|||||||||||||||
Cash flow hedges |
||||||||||||||||||||
Foreign exchange contracts |
||||||||||||||||||||
Buy foreign currency: |
||||||||||||||||||||
3 to 6 months |
– |
|||||||||||||||||||
– |
||||||||||||||||||||
2020 |
||||||||||||||||||||
Hedging instruments |
Average exchange rate |
Foreign currency |
Notional value £m |
Carrying value £m |
Periodic change in value for calculating hedge ineffectiveness £m |
|||||||||||||||
Net investment hedges |
||||||||||||||||||||
Foreign exchange contracts |
||||||||||||||||||||
Sell foreign currency: |
||||||||||||||||||||
Less than 3 months |
( |
) | ||||||||||||||||||
Less than 3 months |
||||||||||||||||||||
Less than 3 months |
( |
) | ||||||||||||||||||
Borrowings (including cross currency interest rate swaps): |
||||||||||||||||||||
3 to 6 months |
( |
) |
( |
) | ||||||||||||||||
Over 6 months |
( |
) |
( |
) | ||||||||||||||||
( |
) |
( |
) | |||||||||||||||||
2020 |
||||||||
Hedged items |
Periodic change in value for calculating hedge ineffectiveness £m |
Cumulative balance in cash flow hedge reserve/foreign currency translation reserve for continuing hedges £m |
||||||
Cash flow hedges |
||||||||
Variability in cash flows from a highly probable forecast transaction |
– |
– |
||||||
Variability in cash flows from foreign exchange exposure arising on Euro denominated coupon payments relating to debt issued |
– |
– |
||||||
Net investment hedges |
||||||||
Net investment in foreign operations |
( |
) | ||||||
2021 |
||||||||||||||||||||||||
Amount reclassified to profit or loss |
||||||||||||||||||||||||
Hedging gains/(losses) recognised in reserves £m |
Amount of hedge ineffectiveness gains/(losses) recognised in profit or loss £m |
Line item in profit or loss in which hedge ineffectiveness is included |
Hedged future cash flows no longer expected to occur £m |
As hedged item affects profit or loss £m |
Line item in which reclassification adjustment is included |
|||||||||||||||||||
Cash flow hedges |
||||||||||||||||||||||||
Variability in cash flows from a highly probable forecast transaction |
Other operating income/ (expense) |
( |
) |
Other operating income/ (expense) |
||||||||||||||||||||
Variability in cash flows from foreign exchange exposure arising on Euro denominated coupon payments relating to debt issued |
( |
) |
Finance income/ (expense) |
Finance income/ (expense) |
||||||||||||||||||||
Net investment hedges |
||||||||||||||||||||||||
Net investment in foreign operations |
Finance income/ (expense) |
( |
) |
Finance income/ (expense) |
||||||||||||||||||||
2020 |
||||||||||||||||||||||||
Amount reclassified to profit or loss |
||||||||||||||||||||||||
Hedging gains/(losses) recognised in reserves £m |
Amount of hedge ineffectiveness gains/(losses) recognised in profit or loss £m |
Line item in profit or loss in which hedge ineffectiveness is included |
Hedged future cash flows no longer expected to occur £m |
As hedged item affects profit or loss £m |
Line item in which reclassification adjustment is included |
|||||||||||||||||||
Cash flow hedges |
||||||||||||||||||||||||
Variability in cash flows from a highly probable forecast transaction |
( |
) |
Other operating income/ (expense) |
– |
Other operating income/ (expense) |
|||||||||||||||||||
Variability in cash flows from foreign exchange exposure arising on Euro denominated coupon payments relating to debt issued |
– |
– |
Finance income/ (expense) |
– |
– |
Finance income/ (expense) |
||||||||||||||||||
Net investment hedges |
||||||||||||||||||||||||
Net investment in foreign operations |
( |
) |
– |
Finance income/ (expense) |
– |
– |
Finance income/ (expense) |
|||||||||||||||||
2021 |
||||||||||||||||
Hedging instruments |
Average contracted fixed rate % |
Notional principal value £m |
Change in fair value for recognising hedge ineffectiveness £m |
Fair value assets/ (liabilities) £m |
||||||||||||
5-10 years |
||||||||||||||||
10-30 years |
||||||||||||||||
More than 30 years |
||||||||||||||||
2021 |
||||||||
Hedged items |
Change in value used for calculating hedge ineffectiveness £m |
Balance in cash flow hedge reserve for continuing hedges after tax £m |
||||||
Pre-hedging of long-term interest rate |
( |
( |
||||||
2020 |
||||||||||||||||
Hedging instruments |
Average contracted fixed rate % |
Notional principal value £m |
Change in fair value for recognising hedge ineffectiveness £m |
Fair value assets/ (liabilities) £m |
||||||||||||
Less than 1 year |
( |
|||||||||||||||
1 to 2 years |
||||||||||||||||
2020 |
||||||||
Hedged items |
Change in value used for calculating hedge ineffectiveness £m |
Balance in cash flow hedge reserve for continuing hedges after tax £m |
||||||
Variable rate borrowings |
( |
|||||||

2021 |
||||||||||||||||||||||||
Amount reclassified to profit or loss |
||||||||||||||||||||||||
Hedging gains/(losses) recognised in reserves |
Amount of hedge ineffectiveness recognised in profit or loss |
Line item in profit or loss in which hedge ineffectiveness is included |
Hedged future cash flows no longer expected to occur |
As hedged item affects profit or loss |
Line item in which reclassification adjustment is included |
|||||||||||||||||||
£m |
£m |
£m |
£m |
|||||||||||||||||||||
Cash flow hedges |
||||||||||||||||||||||||
Variability in cash flows |
( |
) |
e income/ (expense |
) |
e income/ (expense |
) | ||||||||||||||||||
Pre-hedging of long-term interest rates: |
||||||||||||||||||||||||
Matured in the past |
e |
e |
||||||||||||||||||||||
5-10 years |
income/ |
income/ |
||||||||||||||||||||||
10-30 years |
(expense |
) |
(expense |
) | ||||||||||||||||||||
>30 years |
||||||||||||||||||||||||
2020 |
||||||||||||||||||||||||
Amount reclassified to profit or loss |
||||||||||||||||||||||||
Hedging gains/(losses) recognised in reserves |
Amount of hedge ineffectiveness recognised in profit or loss |
Line item in profit or loss in which hedge ineffectiveness is included |
Hedged future cash flows no longer expected to occur |
As hedged item affects profit or loss |
Line item in which reclassification adjustment is included |
|||||||||||||||||||
£m |
£m |
£m |
£m |
|||||||||||||||||||||
Cash flow hedges |
||||||||||||||||||||||||
Variability in cash flows |
e income/ (expense) |
e income/ (expense) |
||||||||||||||||||||||
Pre-hedging of long-term interest rates |
( |
) |
e |
e |
||||||||||||||||||||
income/ (expense |
) |
income/ (expense) |
||||||||||||||||||||||
At 31 December 2021 |
Gross financial assets/ (liabilities) £m |
Financial (liabilities)/ assets offset £m |
Net financial assets/ (liabilities) £m |
Related amounts not offset £m |
Net amount £m |
|||||||||||||||
Financial assets |
||||||||||||||||||||
Trade and other receivables |
( |
) |
( |
) |
||||||||||||||||
Derivative financial instruments |
( |
) |
||||||||||||||||||
Financial liabilities |
||||||||||||||||||||
Trade and other payables |
( |
) |
( |
) |
( |
) | ||||||||||||||
Derivative financial instruments |
( |
) |
( |
) |
( |
) | ||||||||||||||
At 31 December 2020 |
Gross financial assets/ (liabilities) £m |
Financial (liabilities)/ assets offset £m |
Net financial assets/ (liabilities) £m |
Related amounts not offset £m |
Net balance £m |
|||||||||||||||
Financial assets |
||||||||||||||||||||
Trade and other receivables |
( |
) |
( |
) |
||||||||||||||||
Derivative financial instruments |
( |
) |
||||||||||||||||||
Financial liabilities |
||||||||||||||||||||
Trade and other payables |
( |
) |
( |
) |
( |
) | ||||||||||||||
Derivative financial instruments |
( |
) |
( |
) |
( |
) | ||||||||||||||
2021 |
2020 |
|||||||
Total debt £m |
Total £m |
|||||||
Floating and fixed rate debt less than one year |
( |
) |
( |
) | ||||
Between one and two years |
( |
) |
( |
) | ||||
Between two and three years |
( |
) |
( |
) | ||||
Between three and four years |
( |
) |
( |
) | ||||
Between four and five years |
( |
) |
( |
) | ||||
Between five and ten years |
( |
) |
( |
) | ||||
Greater than ten years |
( |
) |
( |
) | ||||
Total |
( |
) |
( |
) | ||||
Original issuance profile: |
||||||||
Fixed rate interest |
( |
) |
( |
) | ||||
Floating rate interest |
( |
) |
( |
) | ||||
( |
) |
( |
) | |||||

2021 |
2020 |
|||||||
Income statement impact of non-functional currency foreign exchange exposures |
Increase/(decrease) in income £m |
Increase/(decrease) in income £m |
||||||
10 cent appreciation of the US Dollar |
||||||||
10 cent appreciation of the Euro |
( |
) |
( |
) | ||||
10 yen appreciation of the Yen |
( |
) | ||||||
2021 |
2020 |
|||||||
Income statement impact of non-functional currency foreign exchange exposures |
Increase/(decrease) in income £m |
Increase/(decrease) in income £m |
||||||
10 cent depreciation of the US Dollar |
( |
) |
( |
) | ||||
10 cent depreciation of the Euro |
||||||||
10 yen depreciation of the Yen |
||||||||
2021 |
2020 |
|||||||
Equity impact of non-functional currency foreign exchange exposures |
Increase/(decrease) in equity £m |
Increase/(decrease) in equity £m |
||||||
10 cent appreciation of the Euro |
( |
) |
( |
) | ||||
2021 |
2020 |
|||||||
Equity impact of non-functional currency foreign exchange exposures |
Increase/(decrease) in equity £m |
Increase/(decrease) in equity £m |
||||||
10 cent depreciation of the Euro |
||||||||
2021 |
2020 |
|||||||
Impact of foreign exchange movements on net debt |
(Increase)/decrease in net debt £m |
(Increase)/decrease in net debt £m |
||||||
10 cent appreciation of the US Dollar |
( |
) |
( |
) | ||||
10 cent appreciation of the Euro |
||||||||
10 yen appreciation of the Yen |
||||||||
2021 |
2020 |
|||||||
Impact of foreign exchange movements on net debt |
(Increase)/decrease in net debt £m |
(Increase)/decrease in net debt £m |
||||||
10 cent depreciation of the US Dollar |
||||||||
10 cent depreciation of the Euro |
( |
) |
( |
) | ||||
10 yen depreciation of the Yen |
( |
) |
( |
) | ||||
2021 |
2020 |
|||||||
Income statement impact of interest rate movements |
Increase/(decrease) in income £m |
Increase/(decrease) in income £m |
||||||
1% (100 basis points) increase in Sterling interest rates |
( |
) |
||||||
1% (100 basis points) increase in US Dollar interest rates |
||||||||
1% (100 basis points) increase in Euro interest rates |
( |
) | ||||||

At 31 December 2021 |
Debt £m |
Interest on debt £m |
Lease liabilities £m |
Finance charge on lease liabilities £m |
Trade payables and other liabilities not in net debt £m |
Total £m |
||||||||||||||||||
Due in less than one year |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
Between one and two years |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
Between two and three years |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
Between three and four years |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
Between four and five years |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
Between five and ten years |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
Greater than ten years |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
Gross contractual cash flows |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
At 31 December 2020 |
Debt £m |
Interest on debt £m |
Lease liabilities £m |
Finance charge on lease liabilities £m |
Trade payables and other liabilities not in net debt £m |
Total £m |
||||||||||||||||||
Due in less than one year |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
Between one and two years |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
Between two and three years |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
Between three and four years |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
Between four and five years |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
Between five and ten years |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
Greater than ten years |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
Gross contractual cash flows |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||
2021 |
2020 |
|||||||||||||||||||||||||||||||
Gross cash inflows |
Gross cash ouflows |
Gross cash inflows |
Gross cash outflows |
|||||||||||||||||||||||||||||
Forward starting interest rate swaps £m |
Foreign exchange forward contracts and swaps £m |
Forward starting interest rate swaps £m |
Foreign exchange forward contracts and swaps £m |
Cross currency interest rate swaps £m |
Foreign exchange forward contracts and swaps £m |
Cross currency interest rate swaps £m |
Foreign exchange forward contracts and swaps £m |
|||||||||||||||||||||||||
Less than one year |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||||||||||||||
Between one and two years |
( |
) |
||||||||||||||||||||||||||||||
Between two and three years |
– |
( |
) |
– |
– |
– |
– |
– |
||||||||||||||||||||||||
Between three and four years |
– |
( |
) |
– |
– |
– |
– |
– |
||||||||||||||||||||||||
Between four and five years |
– |
( |
) |
– |
– |
– |
– |
– |
||||||||||||||||||||||||
Greater than five years |
– |
( |
) |
– |
– |
– |
– |
– |
||||||||||||||||||||||||
Gross contractual cash flows |
( |
) |
( |
) |
( |
) |
( |
) | ||||||||||||||||||||||||
Number of shares and ADS issuable |
Shares Number (000) |
Weighted fair value |
ADS Number (000) |
Weighted fair value |
||||||||||||
| At 1 January 2019 |
||||||||||||||||
| Awards granted |
£ |
$ |
||||||||||||||
| Awards exercised |
( |
) |
( |
) |
||||||||||||
| Awards cancelled |
( |
) |
( |
) |
||||||||||||
| At 31 December 2019 |
||||||||||||||||
| Awards granted |
£ |
$ |
||||||||||||||
| Awards exercised |
( |
) |
( |
) |
||||||||||||
| Awards cancelled |
( |
) |
( |
) |
||||||||||||
| At 31 December 2020 |
||||||||||||||||
| Awards granted |
£ |
$ |
||||||||||||||
| Awards exercised |
( |
) |
( |
) |
||||||||||||
| Awards cancelled |
( |
) |
( |
) |
||||||||||||
| At 31 December 2021 |
||||||||||||||||

2021 Grant |
2020 Grant |
2019 Grant |
||||||||||
| Risk-free interest rate |
( |
|||||||||||
| Dividend yield |
||||||||||||
| Volatility |
||||||||||||
| Expected life |
||||||||||||
| Savings-related options grant price (including 20% discount) |
£ |
£ |
£ |
|||||||||
Options outstanding |
Savings-related share option schemes |
|||||||||
Number 000 |
Weighted exercise price |
|||||||||
| At 31 December 2021 |
£ |
|||||||||
| Range of exercise prices on options outstanding at year end |
£ |
– £ |
||||||||
| Weighted average market price on exercise during year |
£ |
|||||||||
| Weighted average remaining contractual life |
||||||||||
Shares held for share award schemes |
2021 |
2020 |
||||||
| Number of shares (000) |
||||||||
£m |
£m |
|||||||
| Nominal value |
||||||||
| Carrying value |
||||||||
| Market value |
||||||||
Shares held for share option schemes |
2021 |
2020 |
||||||
| Number of shares (000) |
||||||||
£m |
£m |
|||||||
| Nominal value |
||||||||
| Carrying value |
||||||||
| Market value |
||||||||
% |
||||
Europe |
% |
|||
GlaxoSmithKline Consumer Healthcare S.r. l |
||||
% |
||||
Others |
% |
|||
De Sante Aux Consommateurs SRI |
||||
* |
Directly held wholly-owned subsidiary of GlaxoSmithKline plc. |



Notes |
2021 £m |
2021 £m |
2020 £m |
2020 £m |
||||||||||||||||
| Fixed assets – investments |
E |
54,995 |
54,992 |
|||||||||||||||||
| Current assets: |
||||||||||||||||||||
| Trade and other receivables |
F |
2,720 |
1,689 |
|||||||||||||||||
| Cash at bank |
17 |
14 |
||||||||||||||||||
| Total current assets |
2,737 |
1,703 |
||||||||||||||||||
| Trade and other payables |
G |
(598 |
) |
(531 |
) | |||||||||||||||
| Total current liabilities |
(598 |
) |
(531 |
) | ||||||||||||||||
| Net current assets |
2,139 |
1,172 |
||||||||||||||||||
| Total assets less current liabilities |
57,134 |
56,164 |
||||||||||||||||||
| Provisions for liabilities |
H |
(12 |
) |
(7 |
) | |||||||||||||||
| Other non-current liabilities |
I |
(458 |
) |
(457 |
) | |||||||||||||||
| Net assets |
56,664 |
55,700 |
||||||||||||||||||
| Capital and reserves |
||||||||||||||||||||
| Share capital |
J |
1,347 |
1,346 |
|||||||||||||||||
| Share premium account |
J |
3,301 |
3,281 |
|||||||||||||||||
| Other reserves |
K |
1,420 |
1,420 |
|||||||||||||||||
| Retained earnings: |
||||||||||||||||||||
| At 1 January |
49,653 |
49,206 |
||||||||||||||||||
| Profit/(loss) for the year |
4,942 |
3,893 |
||||||||||||||||||
| Other changes in retained earnings |
(3,999 |
) |
(3,446 |
) |
||||||||||||||||
K |
50,596 |
49,653 |
||||||||||||||||||
| Equity shareholders’ funds |
56,664 |
55,700 |
||||||||||||||||||
| Share capital £m |
Share premium account £m |
Other reserves £m |
Retained earnings £m |
Total equity £m |
||||||||||||||||
| At 1 January 2020 |
1,346 |
3,174 |
1,420 |
49,206 |
55,146 |
|||||||||||||||
| Profit and Total comprehensive income attributable to shareholders |
– |
– |
– |
3,893 |
3,893 |
|||||||||||||||
| Dividends to shareholders |
– |
– |
– |
(3,977 |
) |
(3,977 |
) | |||||||||||||
| Shares issued under employee share schemes |
– |
29 |
– |
– |
29 |
|||||||||||||||
| Treasury shares transferred to the ESOP Trusts |
– |
78 |
– |
531 |
609 |
|||||||||||||||
| At 31 December 2020 |
1,346 |
3,281 |
1,420 |
49,653 |
55,700 |
|||||||||||||||
| Profit and Total comprehensive income attributable to shareholders |
– |
– |
– |
4,942 |
4,942 |
|||||||||||||||
| Dividends to shareholders |
– |
– |
– |
(3,999 |
) |
(3,999 |
) | |||||||||||||
| Shares issued under employee share schemes |
1 |
20 |
– |
– |
21 |
|||||||||||||||
| At 31 December 2021 |
1,347 |
3,301 |
1,420 |
50,596 |
56,664 |
|||||||||||||||
- |
Paragraphs 45(b) and 46 to 52 of IFRS 2, ‘Share-based payment’ |
- |
IFRS 7, ‘Financial Instruments – Disclosures’ |
- |
Paragraphs 91-99 of IFRS 13, ‘Fair value measurement’ |
- |
Paragraph 38 of IAS 1, ‘Presentation of financial statements’ comparative information requirements in respect of paragraph 79(a) (iv) of IAS 1 |
- |
Paragraphs 10(d), 10(f), 16, 38(A), 38 (B to D), 40 (A to D), 111 and 134 to 136 of IAS 1, ‘Presentation of financial statements’ |
- |
IAS 7, ‘Statement of cash flows’ |
- |
Paragraph 30 and 31 of IAS 8, ‘Accounting policies, changes in accounting estimates and errors’ |
- |
Paragraph 17 of IAS 24, ‘Related party disclosures’ and the further requirement in IAS 24 to disclose related party transactions entered into between two or more members of a Group. |

| 2021 £m |
2020 £m |
|||||||
| Shares in GlaxoSmithKline Services Unlimited |
637 |
637 |
||||||
| Shares in GlaxoSmithKline Holdings (One) Limited |
18 |
18 |
||||||
| Shares in GlaxoSmithKline Holdings Limited |
17,888 |
17,888 |
||||||
| Shares in GlaxoSmithKline Consumer Healthcare Holdings Limited |
34,800 |
34,800 |
||||||
| Shares in GlaxoSmithKline Mercury Limited |
33 |
33 |
||||||
53,376 |
53,376 |
|||||||
| Capital contribution relating to share-based payments |
1,139 |
1,139 |
||||||
| Contribution relating to contingent consideration |
480 |
477 |
||||||
54,995 |
54,992 |
|||||||
| 2021 £m |
2020 £m |
|||||||
| Amounts due within one year: |
||||||||
| UK Corporation tax recoverable |
9 |
10 |
||||||
| Amounts owed by Group undertakings |
2,319 |
1,231 |
||||||
2,328 |
1,241 |
|||||||
| Amounts due after more than one year: |
||||||||
| Amounts owed by Group undertakings |
392 |
448 |
||||||
2,720 |
1,689 |
|||||||
| 2021 £m |
2020 £m |
|||||||
| Amounts due within one year: |
||||||||
| Other creditors |
457 |
511 |
||||||
| Contingent consideration payable |
22 |
20 |
||||||
| Amounts owed to Group undertakings |
119 |
– |
||||||
598 |
531 |
|||||||
| 2021 £m |
2020 £m |
|||||||
| At 1 January |
7 |
4 |
||||||
| Charge for the year |
24 |
15 |
||||||
| Utilised |
(19 |
) |
(12 |
) | ||||
| At 31 December |
12 |
7 |
||||||
| 2021 £m |
2020 £m |
|||||||
| Contingent consideration payable |
458 |
457 |
||||||

| Ordinary Shares of 25p each |
Share premium account |
|||||||||||
Number |
£m |
£m |
||||||||||
| Share capital issued and fully paid |
||||||||||||
| At 1 January 2020 |
5,383,102,231 |
1,346 |
3,174 |
|||||||||
| Issued under employee share schemes |
2,087,386 |
– |
29 |
|||||||||
| Ordinary shares acquired by ESOP trusts |
– |
– |
78 |
|||||||||
| At 31 December 2020 |
5,385,189,617 |
1,346 |
3,281 |
|||||||||
| Issued under employee share schemes |
1,825,442 |
1 |
20 |
|||||||||
| At 31 December 2021 |
5,387,015,059 |
1,347 |
3,301 |
|||||||||
| 31 December 2021 000 |
31 December 2020 000 |
|||||||||||
| Number of shares issuable under employee share schemes |
75,210 |
48,205 |
||||||||||
| Number of unissued shares not under option |
4,537,775 |
4,566,605 |
||||||||||
| Investor information |
||||||||
| In this section |
||||||||
| 258 |
||||||||
| 260 |
||||||||
| 262 |
||||||||
| 263 |
||||||||
| 269 |
||||||||
| 272 |
||||||||
| 275 |
||||||||
| 288 |
||||||||
| 290 |
||||||||
| 291 |
||||||||
| 291 |
||||||||
| 292 |
||||||||
| 294 |
||||||||
| 296 |
||||||||
| 299 |
||||||||
| |
311 |
|||||||

12 months 2021 |
Q4 2021 |
|||||||||||||||||||||||||||
Reported |
Reported |
|||||||||||||||||||||||||||
£m |
£% |
CER% |
£m |
£% |
CER% |
|||||||||||||||||||||||
| Turnover |
||||||||||||||||||||||||||||
| Pharmaceuticals |
17,729 |
4 |
10 |
5,221 |
20 |
25 |
||||||||||||||||||||||
| Vaccines |
6,778 |
(3 |
) |
2 |
1,809 |
(10 |
) |
(7 |
) | |||||||||||||||||||
| Consumer Healthcare |
9,607 |
(4 |
) |
– |
2,497 |
6 |
10 |
|||||||||||||||||||||
| Total turnover |
34,114 |
– |
5 |
9,527 |
9 |
13 |
||||||||||||||||||||||
| Cost of sales |
(11,603 |
) |
(1 |
) |
2 |
(3,680 |
) |
16 |
19 |
|||||||||||||||||||
| Selling, general and administration |
(10,975 |
) |
(4 |
) |
– |
(3,260 |
) |
3 |
6 |
|||||||||||||||||||
| Research and development |
(5,278 |
) |
4 |
7 |
(1,448 |
) |
(2 |
) |
1 |
|||||||||||||||||||
| Royalty income |
419 |
32 |
32 |
135 |
48 |
46 |
||||||||||||||||||||||
| Other operating income/(expense) |
(476 |
) |
(379 |
) |
||||||||||||||||||||||||
| Operating profit |
6,201 |
(20 |
) |
(9 |
) |
895 |
(16 |
) |
1 |
|||||||||||||||||||
| Net finance costs |
(756 |
) |
(187 |
) |
||||||||||||||||||||||||
| Loss on disposal of interest in associates |
(36 |
) |
– |
|||||||||||||||||||||||||
| Share of after-tax profits/(losses) of associates and joint ventures |
33 |
(2 |
) |
|||||||||||||||||||||||||
| Profit before taxation |
5,442 |
(22 |
) |
(10 |
) |
706 |
(14 |
) |
8 |
|||||||||||||||||||
| Taxation |
(346 |
) |
224 |
|||||||||||||||||||||||||
| Tax rate % |
6.4 |
% |
(31.7 |
)% |
||||||||||||||||||||||||
| Profit after taxation for the period |
5,096 |
(20 |
) |
(9 |
) |
930 |
11 |
30 |
||||||||||||||||||||
| Profit attributable to non-controlling interests |
711 |
181 |
||||||||||||||||||||||||||
| Profit attributable to shareholders |
4,385 |
749 |
||||||||||||||||||||||||||
| Basic earnings per share (pence) |
87.6p |
(24 |
) |
(13 |
) |
15.0p |
10 |
31 |
||||||||||||||||||||
| Diluted earnings per share (pence) |
86.6p |
14.7p |
||||||||||||||||||||||||||
Income statement – Adjusted |
||||||||||||||||||||||||||||
| Total turnover |
34,114 |
– |
5 |
9,527 |
9 |
13 |
||||||||||||||||||||||
| Cost of sales |
(10,726 |
) |
5 |
8 |
(3,496 |
) |
25 |
28 |
||||||||||||||||||||
| Selling, general and administration |
(10,225 |
) |
(5 |
) |
(1 |
) |
(2,908 |
) |
(1 |
) |
2 |
|||||||||||||||||
| Research and development |
(4,776 |
) |
4 |
8 |
(1,365 |
) |
5 |
7 |
||||||||||||||||||||
| Royalty income |
419 |
32 |
32 |
135 |
48 |
46 |
||||||||||||||||||||||
| Operating profit |
8,806 |
(1 |
) |
9 |
1,893 |
4 |
15 |
|||||||||||||||||||||
| Net finance costs |
(753 |
) |
(186 |
) |
||||||||||||||||||||||||
| Share of after-tax profits/(losses) of associates and joint ventures |
33 |
(2 |
) |
|||||||||||||||||||||||||
| Profit before taxation |
8,086 |
– |
11 |
1,705 |
8 |
20 |
||||||||||||||||||||||
| Taxation |
(1,415 |
) |
(177 |
) |
||||||||||||||||||||||||
| Tax rate % |
17.5 |
% |
10.4 |
% |
||||||||||||||||||||||||
| Profit after taxation for the period |
6,671 |
(2 |
) |
9 |
1,528 |
13 |
25 |
|||||||||||||||||||||
| Profit attributable to non-controlling interests |
1,006 |
248 |
||||||||||||||||||||||||||
| Profit attributable to shareholders |
5,665 |
1,280 |
||||||||||||||||||||||||||
| Adjusted earnings per share (pence) |
113.2p |
(2 |
) |
9 |
25.6p |
9 |
22 |
|||||||||||||||||||||
 |
The calculation of Adjusted results is described on page 56. |
Q3 2021 |
Q2 2021 |
Q1 2021 | ||||||||||||||||||||||||||||||||||||||||
Reported |
Reported |
Reported | ||||||||||||||||||||||||||||||||||||||||
£m |
£% |
CER% |
£ m |
£% |
CER% |
£ m |
£% |
CER% | ||||||||||||||||||||||||||||||||||
4,397 |
5 |
10 |
4,229 |
3 |
12 |
3,882 |
(12 |
) |
(8) | |||||||||||||||||||||||||||||||||
2,174 |
7 |
13 |
1,571 |
39 |
49 |
1,224 |
(32 |
) |
(30) | |||||||||||||||||||||||||||||||||
2,506 |
3 |
8 |
2,292 |
(4 |
) |
3 |
2,312 |
(19 |
) |
(16) | ||||||||||||||||||||||||||||||||
9,077 |
5 |
10 |
8,092 |
6 |
15 |
7,418 |
(18 |
) |
(15) | |||||||||||||||||||||||||||||||||
(2,889 |
) |
- |
3 |
(2,554 |
) |
4 |
9 |
(2,480 |
) |
(22 |
) |
(21) | ||||||||||||||||||||||||||||||
(2,646 |
) |
(1 |
) |
4 |
(2,642 |
) |
(2 |
) |
3 |
(2,427 |
) |
(17 |
) |
(15) | ||||||||||||||||||||||||||||
(1,490 |
) |
31 |
34 |
(1,222 |
) |
(6 |
) |
– |
(1,118 |
) |
(6 |
) |
(3) | |||||||||||||||||||||||||||||
116 |
36 |
40 |
77 |
3 |
– |
91 |
36 |
39 | ||||||||||||||||||||||||||||||||||
(230 |
) |
(76 |
) |
209 |
||||||||||||||||||||||||||||||||||||||
1,938 |
4 |
15 |
1,675 |
(41 |
) |
(30) |
1,693 |
(16 |
) |
(8) | ||||||||||||||||||||||||||||||||
(193 |
) |
(185 |
) |
(191 |
) |
|||||||||||||||||||||||||||||||||||||
– |
(36 |
) |
– |
|||||||||||||||||||||||||||||||||||||||
3 |
16 |
16 |
||||||||||||||||||||||||||||||||||||||||
1,748 |
5 |
16 |
1,470 |
(44 |
) |
(32) |
1,518 |
(17 |
) |
(9) | ||||||||||||||||||||||||||||||||
(380 |
) |
68 |
(258 |
) |
||||||||||||||||||||||||||||||||||||||
21.7 |
% |
(4.6 |
)% |
17.0 |
% |
|||||||||||||||||||||||||||||||||||||
1,368 |
(4 |
) |
6 |
1,538 |
(37 |
) |
(26) |
1,260 |
(25 |
) |
(17) | |||||||||||||||||||||||||||||||
200 |
143 |
187 |
||||||||||||||||||||||||||||||||||||||||
1,168 |
1,395 |
1,073 |
||||||||||||||||||||||||||||||||||||||||
23.3 |
p |
(7 |
) |
3 |
27.9 |
p |
(39 |
) |
(28) |
21.5 |
p |
(32 |
) |
(25) | ||||||||||||||||||||||||||||
23.1 |
p |
27.6 |
p |
21.3 |
p |
|||||||||||||||||||||||||||||||||||||
9,077 |
5 |
10 |
8,092 |
6 |
15 |
7,418 |
(18 |
) |
(15) | |||||||||||||||||||||||||||||||||
(2,646 |
) |
4 |
7 |
(2,348 |
) |
4 |
9 |
(2,236 |
) |
(14 |
) |
(13) | ||||||||||||||||||||||||||||||
(2,504 |
) |
1 |
7 |
(2,498 |
) |
(1 |
) |
5 |
(2,315 |
) |
(17 |
) |
(15) | |||||||||||||||||||||||||||||
(1,169 |
) |
11 |
15 |
(1,165 |
) |
(1 |
) |
6 |
(1,077 |
) |
(1 |
) |
3 | |||||||||||||||||||||||||||||
116 |
36 |
40 |
77 |
3 |
– |
91 |
36 |
39 | ||||||||||||||||||||||||||||||||||
2,874 |
8 |
16 |
2,158 |
23 |
43 |
1,881 |
(30 |
) |
(23) | |||||||||||||||||||||||||||||||||
(192 |
) |
(185 |
) |
(190 |
) |
|||||||||||||||||||||||||||||||||||||
3 |
16 |
16 |
||||||||||||||||||||||||||||||||||||||||
2,685 |
8 |
16 |
1,989 |
29 |
50 |
1,707 |
(32 |
) |
(25) | |||||||||||||||||||||||||||||||||
(554 |
) |
(366 |
) |
(318 |
) |
|||||||||||||||||||||||||||||||||||||
20.6 |
% |
18.4 |
% |
18.6 |
% |
|||||||||||||||||||||||||||||||||||||
2,131 |
3 |
11 |
1,623 |
32 |
54 |
1,389 |
(36 |
) |
(29) | |||||||||||||||||||||||||||||||||
296 |
216 |
246 |
||||||||||||||||||||||||||||||||||||||||
1,835 |
1,407 |
1,143 |
||||||||||||||||||||||||||||||||||||||||
36.6 |
p |
3 |
10 |
28.1 |
p |
46 |
71 |
22.9 |
p |
(39 |
) |
(33) | ||||||||||||||||||||||||||||||

Total |
US |
Europe |
International |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2021 |
2020 |
Growth |
2021 |
Growth |
2021 |
Growth |
2021 |
Growth |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Therapeutic area/major products |
£m |
£m |
£% |
CER% |
£m |
£% |
CER% |
£m |
£% |
CER% |
£m |
£% |
CER% |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Respiratory |
2,863 |
2,360 |
|
21 |
|
28 |
1,822 |
23 |
30 |
606 |
11 |
13 |
435 |
33 |
42 |
|||||||||||||||||||||||||||||||||||||||||||||||||
| Anoro Ellipta |
504 |
547 |
(8 |
) |
(3 |
) |
278 |
(15 |
) |
(9 |
) |
149 |
5 |
8 |
77 |
(1 |
) |
3 |
||||||||||||||||||||||||||||||||||||||||||||||
| Trelegy Ellipta |
1,217 |
819 |
49 |
57 |
854 |
52 |
62 |
200 |
19 |
21 |
163 |
81 |
92 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Nucala |
1,142 |
994 |
15 |
22 |
690 |
15 |
23 |
257 |
8 |
11 |
195 |
23 |
34 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| HIV |
4,777 |
4,876 |
(2 |
) |
3 |
2,898 |
(4 |
) |
3 |
1,194 |
(2 |
) |
1 |
685 |
4 |
11 |
||||||||||||||||||||||||||||||||||||||||||||||||
| Dolutegravir products |
4,567 |
4,702 |
(3 |
) |
2 |
2,774 |
(6 |
) |
– |
1,151 |
(1 |
) |
1 |
642 |
7 |
14 |
||||||||||||||||||||||||||||||||||||||||||||||||
| Tivicay |
1,381 |
1,527 |
(10 |
) |
(4 |
) |
763 |
(12 |
) |
(7 |
) |
286 |
(22 |
) |
(20 |
) |
332 |
15 |
24 |
|||||||||||||||||||||||||||||||||||||||||||||
| Triumeq |
1,882 |
2,306 |
(18 |
) |
(14 |
) |
1,190 |
(18 |
) |
(13 |
) |
452 |
(20 |
) |
(18 |
) |
240 |
(15 |
) |
(12 |
) | |||||||||||||||||||||||||||||||||||||||||||
| Juluca |
517 |
495 |
4 |
10 |
393 |
2 |
8 |
111 |
14 |
18 |
13 |
18 |
27 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Dovato |
787 |
374 |
>100 |
>100 |
428 |
87 |
99 |
302 |
>100 |
>100 |
57 |
>100 |
>100 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Rukobia |
45 |
11 |
>100 |
>100 |
43 |
>100 |
>100 |
2 |
>100 |
>100 |
– |
– |
– |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Cabenuva |
38 |
– |
>100 |
>100 |
32 |
– |
– |
5 |
– |
– |
1 |
>100 |
(>100 |
) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Other |
127 |
163 |
(22 |
) |
(18 |
) |
49 |
(8 |
) |
(4 |
) |
36 |
(28 |
) |
(26 |
) |
42 |
(30 |
) |
(23 |
) | |||||||||||||||||||||||||||||||||||||||||||
| Immuno-inflammation |
885 |
727 |
22 |
29 |
727 |
19 |
26 |
68 |
21 |
25 |
90 |
53 |
63 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Benlysta |
874 |
719 |
22 |
29 |
727 |
19 |
26 |
68 |
21 |
25 |
79 |
55 |
67 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Oncology |
489 |
372 |
31 |
37 |
274 |
19 |
26 |
195 |
43 |
46 |
20 |
>100 |
>100 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Zejula |
395 |
339 |
17 |
22 |
212 |
3 |
10 |
163 |
27 |
30 |
20 |
>100 |
>100 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Blenrep |
89 |
33 |
>100 |
>100 |
61 |
>100 |
>100 |
28 |
>100 |
>100 |
– |
– |
– |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Jemperli |
5 |
– |
>100 |
>100 |
2 |
– |
– |
3 |
>100 |
>100 |
– |
– |
– |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Pandemic |
958 |
– |
– |
– |
602 |
– |
– |
69 |
– |
– |
287 |
– |
– |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Xevudy |
958 |
– |
– |
– |
602 |
– |
– |
69 |
– |
– |
287 |
– |
– |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| New and Specialty |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pharmaceuticals |
9,972 |
8,335 |
20 |
26 |
6,323 |
19 |
26 |
2,132 |
9 |
12 |
1,517 |
45 |
54 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Established pharmaceuticals |
7,757 |
8,721 |
(11 |
) |
(6 |
) |
2,119 |
– |
6 |
1,802 |
(16 |
) |
(14 |
) |
3,836 |
(14 |
) |
(8 |
) | |||||||||||||||||||||||||||||||||||||||||||||
| Established Respiratory |
4,327 |
4,640 |
(7 |
) |
(2 |
) |
1,788 |
7 |
13 |
995 |
(12 |
) |
(10 |
) |
1,544 |
(16 |
) |
(10 |
) | |||||||||||||||||||||||||||||||||||||||||||||
| Arnuity Ellipta |
47 |
45 |
4 |
11 |
40 |
8 |
16 |
– |
– |
– |
7 |
(12 |
) |
(13 |
) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Avamys/Veramyst |
298 |
297 |
– |
7 |
– |
– |
– |
65 |
(2 |
) |
2 |
233 |
1 |
8 |
||||||||||||||||||||||||||||||||||||||||||||||||||
| Flixotide/Flovent |
444 |
419 |
6 |
12 |
275 |
50 |
60 |
69 |
(14 |
) |
(11 |
) |
100 |
(36 |
) |
(32 |
) | |||||||||||||||||||||||||||||||||||||||||||||||
| Incruse Ellipta |
205 |
220 |
(7 |
) |
(3 |
) |
109 |
(7 |
) |
(2 |
) |
70 |
(5 |
) |
(3 |
) |
26 |
(10 |
) |
(7 |
) | |||||||||||||||||||||||||||||||||||||||||||
| Relvar/Breo Ellipta |
1,121 |
1,124 |
– |
5 |
488 |
3 |
9 |
334 |
4 |
6 |
299 |
(9 |
) |
(2 |
) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Seretide/Advair |
1,357 |
1,535 |
(12 |
) |
(7 |
) |
486 |
12 |
19 |
322 |
(28 |
) |
(27 |
) |
549 |
(16 |
) |
(11 |
) | |||||||||||||||||||||||||||||||||||||||||||||
| Ventolin |
718 |
785 |
(9 |
) |
(4 |
) |
390 |
(9 |
) |
(3 |
) |
108 |
(7 |
) |
(5 |
) |
220 |
(8 |
) |
(3 |
) | |||||||||||||||||||||||||||||||||||||||||||
| Other Respiratory |
137 |
215 |
(36 |
) |
(31 |
) |
– |
– |
– |
27 |
– |
– |
110 |
(41 |
) |
(36 |
) | |||||||||||||||||||||||||||||||||||||||||||||||
| Dermatology |
399 |
425 |
(6 |
) |
(1 |
) |
(1 |
) |
>(100 |
) |
>(100 |
) |
131 |
(6 |
) |
(4 |
) |
269 |
(5 |
) |
2 |
|||||||||||||||||||||||||||||||||||||||||||
| Augmentin |
426 |
490 |
(13 |
) |
(7 |
) |
– |
– |
– |
124 |
(14 |
) |
(12 |
) |
302 |
(12 |
) |
(4 |
) | |||||||||||||||||||||||||||||||||||||||||||||
| Avodart |
332 |
466 |
(29 |
) |
(25 |
) |
1 |
(80 |
) |
(80 |
) |
118 |
(25 |
) |
(23 |
) |
213 |
(30 |
) |
(25 |
) | |||||||||||||||||||||||||||||||||||||||||||
| Imigran/Imitrex |
105 |
118 |
(11 |
) |
(8 |
) |
29 |
(31 |
) |
(31 |
) |
51 |
– |
2 |
25 |
– |
8 |
|||||||||||||||||||||||||||||||||||||||||||||||
| Lamictal |
478 |
537 |
(11 |
) |
(6 |
) |
232 |
(14 |
) |
(9 |
) |
112 |
(7 |
) |
(5 |
) |
134 |
(9 |
) |
(3 |
) | |||||||||||||||||||||||||||||||||||||||||||
| Seroxat/Paxil |
128 |
146 |
(12 |
) |
(6 |
) |
– |
– |
– |
35 |
(5 |
) |
(5 |
) |
93 |
(15 |
) |
(6 |
) | |||||||||||||||||||||||||||||||||||||||||||||
| Valtrex |
92 |
103 |
(11 |
) |
(5 |
) |
11 |
(27 |
) |
(20 |
) |
33 |
3 |
3 |
48 |
(14 |
) |
(5 |
) | |||||||||||||||||||||||||||||||||||||||||||||
| Other |
1,470 |
1,796 |
(18 |
) |
(13 |
) |
59 |
(46 |
) |
(40 |
) |
203 |
(39 |
) |
(37 |
) |
1,208 |
(11 |
) |
(5 |
) | |||||||||||||||||||||||||||||||||||||||||||
| Pharmaceuticals |
17,729 |
17,056 |
4 |
10 |
8,442 |
13 |
21 |
3,934 |
(4 |
) |
(2 |
) |
5,353 |
(3 |
) |
4 |
||||||||||||||||||||||||||||||||||||||||||||||||
Total |
US |
Europe |
International |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
2020 |
2019 |
Growth |
2020 |
Growth |
2020 |
Growth |
2020 |
Growth |
||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic area/major products |
£m |
£m |
£% |
CER% |
£m |
£% |
CER% |
£m |
£% |
CER% |
£m |
£% |
CER% |
|||||||||||||||||||||||||||||||||||||||||||||
| Respiratory |
2,360 |
1,800 |
31 |
32 |
1,486 |
28 |
30 |
548 |
28 |
27 |
326 |
53 |
56 |
|||||||||||||||||||||||||||||||||||||||||||||
| Anoro Ellipta |
547 |
514 |
6 |
8 |
327 |
1 |
2 |
142 |
18 |
17 |
78 |
11 |
17 |
|||||||||||||||||||||||||||||||||||||||||||||
| Trelegy Ellipta |
819 |
518 |
58 |
59 |
561 |
47 |
48 |
168 |
65 |
65 |
90 |
>100 |
>100 |
|||||||||||||||||||||||||||||||||||||||||||||
| Nucala |
994 |
768 |
29 |
30 |
598 |
32 |
33 |
238 |
16 |
15 |
158 |
45 |
46 |
|||||||||||||||||||||||||||||||||||||||||||||
| HIV |
4,876 |
4,854 |
– |
1 |
3,005 |
– |
1 |
1,213 |
5 |
4 |
658 |
(5 |
) |
(1 |
) | |||||||||||||||||||||||||||||||||||||||||||
| Dolutegravir products |
4,702 |
4,633 |
1 |
2 |
2,941 |
– |
1 |
1,163 |
7 |
6 |
598 |
(2 |
) |
3 |
||||||||||||||||||||||||||||||||||||||||||||
| Tivicay |
1,527 |
1,662 |
(8 |
) |
(7 |
) |
871 |
(11 |
) |
(10 |
) |
368 |
(7 |
) |
(8 |
) |
288 |
(1 |
) |
5 |
||||||||||||||||||||||||||||||||||||||
| Triumeq |
2,306 |
2,549 |
(10 |
) |
(9 |
) |
1,454 |
(10 |
) |
(9 |
) |
568 |
(9 |
) |
(10 |
) |
284 |
(9 |
) |
(6 |
) | |||||||||||||||||||||||||||||||||||||
| Juluca |
495 |
366 |
35 |
36 |
387 |
28 |
29 |
97 |
73 |
71 |
11 |
57 |
71 |
|||||||||||||||||||||||||||||||||||||||||||||
| Dovato |
374 |
56 |
>100 |
>100 |
229 |
>100 |
>100 |
130 |
>100 |
>100 |
15 |
>100 |
>100 |
|||||||||||||||||||||||||||||||||||||||||||||
| Rukobia |
11 |
– |
– |
– |
11 |
– |
>100 |
– |
– |
– |
– |
– |
– |
|||||||||||||||||||||||||||||||||||||||||||||
| Cabenuva |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|||||||||||||||||||||||||||||||||||||||||||||
| Other |
163 |
221 |
(26 |
) |
(25 |
) |
53 |
(20 |
) |
(18 |
) |
50 |
(29 |
) |
(27 |
) |
60 |
(29 |
) |
(28 |
) | |||||||||||||||||||||||||||||||||||||
| Immuno-inflammation |
727 |
613 |
19 |
20 |
612 |
14 |
16 |
56 |
22 |
20 |
59 |
84 |
91 |
|||||||||||||||||||||||||||||||||||||||||||||
| Benlysta |
719 |
613 |
17 |
19 |
612 |
14 |
16 |
56 |
22 |
20 |
51 |
59 |
66 |
|||||||||||||||||||||||||||||||||||||||||||||
| Oncology |
372 |
230 |
62 |
62 |
231 |
72 |
74 |
136 |
42 |
40 |
5 |
– |
– |
|||||||||||||||||||||||||||||||||||||||||||||
| Zejula |
339 |
229 |
48 |
48 |
206 |
54 |
55 |
128 |
35 |
33 |
5 |
– |
– |
|||||||||||||||||||||||||||||||||||||||||||||
| Blenrep |
33 |
– |
– |
– |
25 |
– |
– |
8 |
– |
– |
– |
– |
– |
|||||||||||||||||||||||||||||||||||||||||||||
| New and Specialty |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pharmaceuticals |
8,335 |
7,497 |
11 |
12 |
5,334 |
10 |
12 |
1,953 |
13 |
12 |
1,048 |
12 |
16 |
|||||||||||||||||||||||||||||||||||||||||||||
| Established pharmaceuticals |
8,721 |
10,057 |
(13 |
) |
(12 |
) |
2,117 |
(18 |
) |
(17 |
) |
2,151 |
(10 |
) |
(11 |
) |
4,453 |
(12 |
) |
(9 |
) | |||||||||||||||||||||||||||||||||||||
| Established Respiratory |
4,640 |
5,181 |
(10 |
) |
(9 |
) |
1,676 |
(16 |
) |
(15 |
) |
1,134 |
(2 |
) |
(3 |
) |
1,830 |
(9 |
) |
(6 |
) | |||||||||||||||||||||||||||||||||||||
| Arnuity Ellipta |
45 |
48 |
(6 |
) |
(6 |
) |
37 |
(10 |
) |
(7 |
) |
– |
– |
– |
8 |
14 |
– |
|||||||||||||||||||||||||||||||||||||||||
| Avamys/Veramyst |
297 |
324 |
(8 |
) |
(6 |
) |
– |
– |
– |
66 |
(4 |
) |
(4 |
) |
231 |
(10 |
) |
(7 |
) | |||||||||||||||||||||||||||||||||||||||
| Flixotide/Flovent |
419 |
629 |
(33 |
) |
(32 |
) |
183 |
(50 |
) |
(50 |
) |
80 |
(9 |
) |
(10 |
) |
156 |
(10 |
) |
(5 |
) | |||||||||||||||||||||||||||||||||||||
| Incruse Ellipta |
220 |
262 |
(16 |
) |
(15 |
) |
117 |
(27 |
) |
(27 |
) |
74 |
1 |
1 |
29 |
4 |
7 |
|||||||||||||||||||||||||||||||||||||||||
| Relvar/Breo Ellipta |
1,124 |
971 |
16 |
17 |
474 |
24 |
25 |
322 |
14 |
13 |
328 |
6 |
9 |
|||||||||||||||||||||||||||||||||||||||||||||
| Seretide/Advair |
1,535 |
1,730 |
(11 |
) |
(10 |
) |
434 |
(14 |
) |
(13 |
) |
449 |
(11 |
) |
(11 |
) |
652 |
(10 |
) |
(7 |
) | |||||||||||||||||||||||||||||||||||||
| Ventolin |
785 |
938 |
(16 |
) |
(14 |
) |
430 |
(21 |
) |
(20 |
) |
116 |
(3 |
) |
(4 |
) |
239 |
(12 |
) |
(7 |
) | |||||||||||||||||||||||||||||||||||||
| Other Respiratory |
215 |
279 |
(23 |
) |
(23 |
) |
1 |
>100 |
>100 |
27 |
(4 |
) |
– |
187 |
(25 |
) |
(26 |
) | ||||||||||||||||||||||||||||||||||||||||
| Dermatology |
425 |
445 |
(4 |
) |
(1 |
) |
1 |
(67 |
) |
(67 |
) |
140 |
(12 |
) |
(13 |
) |
284 |
– |
6 |
|||||||||||||||||||||||||||||||||||||||
| Augmentin |
490 |
602 |
(19 |
) |
(15 |
) |
– |
– |
– |
145 |
(16 |
) |
(16 |
) |
345 |
(20 |
) |
(15 |
) | |||||||||||||||||||||||||||||||||||||||
| Avodart |
466 |
574 |
(19 |
) |
(17 |
) |
5 |
25 |
25 |
158 |
(24 |
) |
(25 |
) |
303 |
(16 |
) |
(13 |
) | |||||||||||||||||||||||||||||||||||||||
| Imigran/Imitrex |
118 |
138 |
(14 |
) |
(14 |
) |
42 |
(29 |
) |
(29 |
) |
51 |
(2 |
) |
(4 |
) |
25 |
(7 |
) |
(4 |
) | |||||||||||||||||||||||||||||||||||||
| Lamictal |
537 |
566 |
(5 |
) |
(4 |
) |
269 |
(5 |
) |
(5 |
) |
120 |
7 |
6 |
148 |
(13 |
) |
(9 |
) | |||||||||||||||||||||||||||||||||||||||
| Seroxat/Paxil |
146 |
160 |
(9 |
) |
(6 |
) |
– |
– |
– |
37 |
– |
(3 |
) |
109 |
(11 |
) |
(7 |
) | ||||||||||||||||||||||||||||||||||||||||
| Valtrex |
103 |
107 |
(4 |
) |
(2 |
) |
15 |
7 |
7 |
32 |
3 |
– |
56 |
(10 |
) |
(5 |
) | |||||||||||||||||||||||||||||||||||||||||
| Other |
1,796 |
2,284 |
(21 |
) |
(20 |
) |
109 |
(48 |
) |
(47 |
) |
334 |
(28 |
) |
(28 |
) |
1,353 |
(16 |
) |
(14 |
) | |||||||||||||||||||||||||||||||||||||
| Pharmaceuticals |
17,056 |
17,554 |
(3 |
) |
(1 |
) |
7,451 |
1 |
2 |
4,104 |
(1 |
) |
(1 |
) |
5,501 |
(9 |
) |
(5 |
) | |||||||||||||||||||||||||||||||||||||||

Total |
US |
Europe |
International |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2021 |
2020 |
Growth |
2021 |
Growth |
2021 |
Growth |
2021 |
Growth |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Major products |
£m |
£m |
£% |
CER% |
£m |
£% |
CER% |
£m |
£% |
CER% |
£m |
£% |
CER% |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Meningitis |
961 |
1,029 |
(7 |
) |
(2 |
) |
453 |
5 |
11 |
354 |
(1 |
) |
2 |
154 |
(36 |
) |
(30 |
) | ||||||||||||||||||||||||||||||||||||||||||||||
| Bexsero |
650 |
650 |
– |
5 |
253 |
(3 |
) |
3 |
328 |
1 |
4 |
69 |
5 |
20 |
||||||||||||||||||||||||||||||||||||||||||||||||||
| Menveo |
272 |
265 |
3 |
9 |
200 |
16 |
23 |
21 |
(19 |
) |
(15 |
) |
51 |
(23 |
) |
(18 |
) | |||||||||||||||||||||||||||||||||||||||||||||||
| Other |
39 |
114 |
(66 |
) |
(65 |
) |
– |
– |
– |
5 |
(17 |
) |
(17 |
) |
34 |
(69 |
) |
(68 |
) | |||||||||||||||||||||||||||||||||||||||||||||
| Influenza |
679 |
733 |
(7 |
) |
(2 |
) |
456 |
(15 |
) |
(9 |
) |
101 |
3 |
6 |
122 |
22 |
28 |
|||||||||||||||||||||||||||||||||||||||||||||||
| Fluarix, FluLaval |
679 |
733 |
(7 |
) |
(2 |
) |
456 |
(15 |
) |
(9 |
) |
101 |
3 |
6 |
122 |
22 |
28 |
|||||||||||||||||||||||||||||||||||||||||||||||
| Shingles |
1,721 |
1,989 |
(13 |
) |
(9 |
) |
1,344 |
(20 |
) |
(15 |
) |
281 |
51 |
54 |
96 |
(25 |
) |
(23 |
) | |||||||||||||||||||||||||||||||||||||||||||||
| Shingrix |
1,721 |
1,989 |
(13 |
) |
(9 |
) |
1,344 |
(20 |
) |
(15 |
) |
281 |
51 |
54 |
96 |
(25 |
) |
(23 |
) | |||||||||||||||||||||||||||||||||||||||||||||
| Established vaccines |
2,970 |
3,231 |
(8 |
) |
(4 |
) |
977 |
(7 |
) |
(1 |
) |
700 |
(13 |
) |
(10 |
) |
1,293 |
(6 |
) |
(3 |
) | |||||||||||||||||||||||||||||||||||||||||||
| Infanrix, Pediarix |
543 |
629 |
(14 |
) |
(9 |
) |
303 |
(3 |
) |
4 |
116 |
(33 |
) |
(32 |
) |
124 |
(14 |
) |
(10 |
) | ||||||||||||||||||||||||||||||||||||||||||||
| Boostrix |
521 |
476 |
9 |
14 |
270 |
5 |
12 |
140 |
– |
2 |
111 |
41 |
44 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Hepatitis |
460 |
576 |
(20 |
) |
(16 |
) |
269 |
(19 |
) |
(14 |
) |
109 |
(22 |
) |
(21 |
) |
82 |
(20 |
) |
(17 |
) | |||||||||||||||||||||||||||||||||||||||||||
| Rotarix |
541 |
559 |
(3 |
) |
1 |
111 |
(10 |
) |
(4 |
) |
118 |
(1 |
) |
2 |
312 |
(2 |
) |
3 |
||||||||||||||||||||||||||||||||||||||||||||||
| Synflorix |
357 |
402 |
(11 |
) |
(8 |
) |
– |
– |
– |
45 |
(15 |
) |
(13 |
) |
312 |
(11 |
) |
(7 |
) | |||||||||||||||||||||||||||||||||||||||||||||
| Priorix, Priorix Tetra, Varilrix |
260 |
261 |
– |
4 |
– |
– |
– |
125 |
(1 |
) |
2 |
135 |
– |
5 |
||||||||||||||||||||||||||||||||||||||||||||||||||
| Cervarix |
138 |
139 |
(1 |
) |
– |
– |
– |
– |
25 |
(17 |
) |
(17 |
) |
113 |
4 |
5 |
||||||||||||||||||||||||||||||||||||||||||||||||
| Other |
150 |
189 |
(21 |
) |
(19 |
) |
24 |
(20 |
) |
(13 |
) |
22 |
16 |
26 |
104 |
(26 |
) |
(26 |
) | |||||||||||||||||||||||||||||||||||||||||||||
| Vaccines excluding pandemic |
6,331 |
6,982 |
(9 |
) |
(5 |
) |
3,230 |
(13 |
) |
(7 |
) |
1,436 |
– |
2 |
1,665 |
(10 |
) |
(6 |
) | |||||||||||||||||||||||||||||||||||||||||||||
| Pandemic vaccines |
447 |
– |
– |
– |
242 |
– |
– |
– |
– |
– |
205 |
– |
– |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Pandemic adjuvant |
444 |
– |
– |
– |
242 |
– |
– |
– |
– |
– |
202 |
– |
– |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Total vaccines |
6,778 |
6,982 |
(3 |
) |
2 |
3,472 |
(6 |
) |
– |
1,436 |
– |
2 |
1,870 |
1 |
5 |
|||||||||||||||||||||||||||||||||||||||||||||||||
| £% represents growth at actual exchange rates. CER% represents growth at constant exchange rates. |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vaccines turnover 2020 |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Total |
US |
Europe |
International |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2020 |
2019 |
Growth |
2020 |
Growth |
2020 |
Growth |
2020 |
Growth |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Major products |
£m |
£m |
£% |
CER% |
£m |
£% |
CER% |
£m |
£% |
CER% |
£m |
£% |
CER% |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Meningitis |
1,029 |
1,018 |
1 |
3 |
433 |
1 |
2 |
356 |
4 |
3 |
240 |
(2 |
) |
4 |
||||||||||||||||||||||||||||||||||||||||||||||||||
| Bexsero |
650 |
679 |
(4 |
) |
(2 |
) |
260 |
– |
1 |
324 |
2 |
1 |
66 |
(34 |
) |
(20 |
) | |||||||||||||||||||||||||||||||||||||||||||||||
| Menveo |
265 |
267 |
(1 |
) |
1 |
173 |
2 |
3 |
26 |
44 |
39 |
66 |
(16 |
) |
(13 |
) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Other |
114 |
72 |
58 |
57 |
– |
– |
– |
6 |
– |
– |
108 |
64 |
62 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Influenza |
733 |
541 |
35 |
37 |
535 |
30 |
31 |
98 |
75 |
73 |
100 |
37 |
42 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Fluarix, FluLaval |
733 |
541 |
35 |
37 |
535 |
30 |
31 |
98 |
75 |
73 |
100 |
37 |
42 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Shingles |
1,989 |
1,810 |
10 |
11 |
1,675 |
– |
1 |
186 |
>100 |
>100 |
128 |
47 |
49 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Shingrix |
1,989 |
1,810 |
10 |
11 |
1,675 |
– |
1 |
186 |
>100 |
>100 |
128 |
47 |
49 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Established vaccines |
3,231 |
3,788 |
(15 |
) |
(14 |
) |
1,054 |
(24 |
) |
(24 |
) |
801 |
(23 |
) |
(23 |
) |
1,376 |
1 |
3 |
|||||||||||||||||||||||||||||||||||||||||||||
| Infanrix, Pediarix |
629 |
733 |
(14 |
) |
(13 |
) |
311 |
(14 |
) |
(13 |
) |
174 |
(18 |
) |
(19 |
) |
144 |
(10 |
) |
(6 |
) | |||||||||||||||||||||||||||||||||||||||||||
| Boostrix |
476 |
584 |
(18 |
) |
(18 |
) |
257 |
(14 |
) |
(13 |
) |
140 |
(10 |
) |
(11 |
) |
79 |
(39 |
) |
(36 |
) | |||||||||||||||||||||||||||||||||||||||||||
| Hepatitis |
576 |
874 |
(34 |
) |
(33 |
) |
333 |
(37 |
) |
(36 |
) |
140 |
(39 |
) |
(39 |
) |
103 |
(10 |
) |
(6 |
) | |||||||||||||||||||||||||||||||||||||||||||
| Rotarix |
559 |
558 |
– |
1 |
123 |
(12 |
) |
(11 |
) |
119 |
6 |
6 |
317 |
4 |
5 |
|||||||||||||||||||||||||||||||||||||||||||||||||
| Synflorix |
402 |
468 |
(14 |
) |
(14 |
) |
– |
– |
– |
53 |
(2 |
) |
(2 |
) |
349 |
(16 |
) |
(15 |
) | |||||||||||||||||||||||||||||||||||||||||||||
| Priorix, Priorix Tetra, Varilrix |
261 |
232 |
13 |
14 |
– |
– |
– |
126 |
26 |
25 |
135 |
2 |
5 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Cervarix |
139 |
50 |
>100 |
>100 |
– |
– |
– |
30 |
43 |
43 |
109 |
>100 |
>100 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Other |
189 |
289 |
(35 |
) |
(35 |
) |
30 |
(55 |
) |
(56 |
) |
19 |
(87 |
) |
(87 |
) |
140 |
87 |
85 |
|||||||||||||||||||||||||||||||||||||||||||||
| Total vaccines |
6,982 |
7,157 |
(2 |
) |
(1 |
) |
3,697 |
(5 |
) |
(4 |
) |
1,441 |
(3 |
) |
(4 |
) |
1,844 |
5 |
7 |
|||||||||||||||||||||||||||||||||||||||||||||
£% represents growth at actual exchange rates. CER% represents growth at constant exchange rates. |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Group turnover by geographic region |
2021 £m |
2020 £m |
2019 £m |
2018 £m |
2017 £m |
|||||||||||||||
| US |
15,093 |
14,556 |
13,890 |
11,982 |
11,263 |
|||||||||||||||
| Europe |
7,838 |
8,164 |
8,069 |
7,973 |
7,943 |
|||||||||||||||
| International |
11,183 |
11,379 |
11,795 |
10,866 |
10,980 |
|||||||||||||||
34,114 |
34,099 |
33,754 |
30,821 |
30,186 |
||||||||||||||||
Group turnover by segment |
2021 £m |
2020 £m |
2019 £m |
2018 £m |
2017 £m |
|||||||||||||||
| Pharmaceuticals |
17,729 |
17,056 |
17,554 |
17,269 |
17,276 |
|||||||||||||||
| Vaccines |
6,778 |
6,982 |
7,157 |
5,894 |
5,160 |
|||||||||||||||
| Consumer Healthcare |
9,607 |
10,033 |
8,995 |
7,658 |
7,750 |
|||||||||||||||
| Segment turnover |
34,114 |
34,071 |
33,706 |
30,821 |
30,186 |
|||||||||||||||
| Corporate and other unallocated turnover |
– |
28 |
48 |
– |
– |
|||||||||||||||
34,114 |
34,099 |
33,754 |
30,821 |
30,186 |
||||||||||||||||
Pharmaceuticals turnover |
2021 £m |
2020 (revised) £m |
2019 (revised) £m |
2018 (revised) £m |
2017 (revised) £m |
|||||||||||||||
| Respiratory |
2,863 |
2,360 |
1,800 |
1,195 |
688 |
|||||||||||||||
| HIV |
4,777 |
4,876 |
4,854 |
4,722 |
4,350 |
|||||||||||||||
| Immuno-inflammation |
885 |
727 |
613 |
472 |
377 |
|||||||||||||||
| Oncology |
489 |
372 |
230 |
– |
– |
|||||||||||||||
| Pandemic |
958 |
– |
– |
– |
– |
|||||||||||||||
| New and Specialty |
9,972 |
8,335 |
7,497 |
6,389 |
5,415 |
|||||||||||||||
| Established Pharmaceuticals |
7,757 |
8,721 |
10,057 |
10,880 |
11,861 |
|||||||||||||||
17,729 |
17,056 |
17,554 |
17,269 |
17,276 |
||||||||||||||||
Vaccines turnover |
2021 £m |
2020 £m |
2019 £m |
2018 £m |
2017 £m |
|||||||||||||||
| Meningitis |
961 |
1,029 |
1,018 |
881 |
890 |
|||||||||||||||
| Influenza |
679 |
733 |
541 |
523 |
488 |
|||||||||||||||
| Shingles |
1,721 |
1,989 |
1,810 |
784 |
22 |
|||||||||||||||
| Established Vaccines |
2,970 |
3,231 |
3,788 |
3,706 |
3,760 |
|||||||||||||||
6,331 |
6,982 |
7,157 |
5,894 |
5,160 |
||||||||||||||||
| Pandemic Vaccines |
447 |
– |
– |
– |
– |
|||||||||||||||
6,778 |
6,982 |
7,157 |
5,894 |
5,160 |
||||||||||||||||
Consumer Healthcare turnover |
2021 £m |
2020 £m |
2019 £m |
2018 £m |
2017 £m |
|||||||||||||||
| Oral health |
2,732 |
2,753 |
2,673 |
2,496 |
2,466 |
|||||||||||||||
| Pain relief |
2,276 |
2,219 |
1,781 |
1,440 |
1,465 |
|||||||||||||||
| Vitamins, minerals and supplements |
1,512 |
1,506 |
611 |
103 |
105 |
|||||||||||||||
| Respiratory health |
1,133 |
1,209 |
1,186 |
1,085 |
1,057 |
|||||||||||||||
| Digestive health and other |
1,803 |
1,824 |
1,646 |
1,435 |
1,447 |
|||||||||||||||
| Sub-total |
9,456 |
9,511 |
7,897 |
6,559 |
6,540 |
|||||||||||||||
| Brands divested/under review |
151 |
522 |
1,098 |
1,099 |
1,210 |
|||||||||||||||
9,607 |
10,033 |
8,995 |
7,658 |
7,750 |
||||||||||||||||

Financial results – Total |
2021 £m |
2020 £m |
2019 £m |
2018 £m |
2017 £m |
|||||||||||||||
| Turnover |
34,114 |
34,099 |
33,754 |
30,821 |
30,186 |
|||||||||||||||
| Operating profit |
6,201 |
7,783 |
6,961 |
5,483 |
4,087 |
|||||||||||||||
| Profit before taxation |
5,442 |
6,968 |
6,221 |
4,800 |
3,525 |
|||||||||||||||
| Profit after taxation |
5,096 |
6,388 |
5,268 |
4,046 |
2,169 |
|||||||||||||||
pence |
pence |
pence |
pence |
pence |
||||||||||||||||
| Basic earnings per share |
87.6 |
115.5 |
93.9 |
73.7 |
31.4 |
|||||||||||||||
| Diluted earnings per share |
86.6 |
114.1 |
92.6 |
72.9 |
31.0 |
|||||||||||||||
2021 millions |
2020 millions |
2019 millions |
2018 millions |
2017 millions |
||||||||||||||||
| Weighted average number of shares in issue: |
||||||||||||||||||||
| Basic |
5,003 |
4,976 |
4,947 |
4,914 |
4,886 |
|||||||||||||||
| Diluted |
5,065 |
5,038 |
5,016 |
4,971 |
4,941 |
|||||||||||||||
Financial results – Adjusted |
2021 £m |
2020 £m |
2019 £m |
2018 £m |
2017 £m |
|||||||||||||||
| Turnover |
34,114 |
34,099 |
33,754 |
30,821 |
30,186 |
|||||||||||||||
| Operating profit |
8,806 |
8,906 |
8,972 |
8,745 |
8,568 |
|||||||||||||||
| Profit before taxation |
8,086 |
8,095 |
8,236 |
8,078 |
7,924 |
|||||||||||||||
| Profit after taxation |
6,671 |
6,800 |
6,918 |
6,543 |
6,257 |
|||||||||||||||
pence |
pence |
pence |
pence |
pence |
||||||||||||||||
| Adjusted earnings per share |
113.2 |
115.9 |
123.9 |
119.4 |
111.8 |
|||||||||||||||
% |
% |
% |
% |
% |
||||||||||||||||
| Return on capital employed |
25.8 |
35.6 |
56.5 |
134.0 |
83.4 |
|||||||||||||||
Balance sheet |
2021 £m |
2020 £m |
2019 £m |
2018 £m |
2017 £m |
|||||||||||||||
| Non-current assets |
60,429 |
60,184 |
60,201 |
41,139 |
40,474 |
|||||||||||||||
| Current assets |
18,674 |
20,247 |
19,491 |
16,927 |
15,907 |
|||||||||||||||
| Total assets |
79,103 |
80,431 |
79,692 |
58,066 |
56,381 |
|||||||||||||||
| Current liabilities |
(23,670 |
) |
(22,148 |
) |
(24,050 |
) |
(22,491 |
) |
(26,569 |
) | ||||||||||
| Non-current liabilities |
(34,091 |
) |
(37,475 |
) |
(37,285 |
) |
(31,903 |
) |
(26,323 |
) | ||||||||||
| Total liabilities |
(57,761 |
) |
(59,623 |
) |
(61,335 |
) |
(54,394 |
) |
(52,892 |
) | ||||||||||
| Net assets |
21,342 |
20,808 |
18,357 |
3,672 |
3,489 |
|||||||||||||||
| Shareholders’ equity |
15,055 |
14,587 |
11,405 |
3,781 |
(68 |
) | ||||||||||||||
| Non-controlling interests |
6,287 |
6,221 |
6,952 |
(109 |
) |
3,557 |
||||||||||||||
| Total equity |
21,342 |
20,808 |
18,357 |
3,672 |
3,489 |
|||||||||||||||
| Number of employees |
||||||||||||||||||||
2021 |
2020 |
2019 |
2018 |
2017 |
||||||||||||||||
| US |
14,289 |
15,706 |
16,676 |
13,804 |
14,526 |
|||||||||||||||
| Europe |
38,809 |
40,711 |
40,524 |
41,943 |
43,002 |
|||||||||||||||
| International |
36,998 |
37,649 |
42,237 |
39,743 |
40,934 |
|||||||||||||||
90,096 |
94,066 |
99,437 |
95,490 |
98,462 |
||||||||||||||||
| Manufacturing |
32,141 |
33,848 |
36,925 |
36,527 |
38,245 |
|||||||||||||||
| Selling |
34,846 |
36,391 |
39,184 |
36,351 |
37,374 |
|||||||||||||||
| Administration |
11,014 |
11,730 |
11,249 |
10,768 |
11,307 |
|||||||||||||||
| Research and development |
12,095 |
12,097 |
12,079 |
11,844 |
11,536 |
|||||||||||||||
90,096 |
94,066 |
99,437 |
95,490 |
98,462 |
||||||||||||||||
2021 |
2020 |
2019 |
2018 |
2017 | ||||||||
| Average |
1.38 |
1.29 |
1.28 |
1.34 |
1.29 | |||||||
| 2022 Feb |
2022 Jan |
2021 Dec |
2021 Nov |
2021 Oct |
2021 Sep | |||||||
| High |
1.36 |
1.37 |
1.35 |
1.37 |
1.38 |
1.39 | ||||||
| Low |
1.33 |
1.34 |
1.32 |
1.32 |
1.35 |
1.34 | ||||||

Adjusted results reconciliation 31 December 2021 |
Total results £m |
Intangible asset amortisation £m |
Intangible asset impairment £m |
Major restructuring £m |
Transaction- related £m |
Divestments, significant legal and other items £m |
Separation costs £m |
Adjusted results £m |
||||||||||||||||||||||||
| Turnover |
34,114 |
34,114 |
||||||||||||||||||||||||||||||
| Cost of sales |
(11,603 |
) |
701 |
(33 |
) |
154 |
28 |
27 |
(10,726 |
) | ||||||||||||||||||||||
| Gross profit |
22,511 |
701 |
(33 |
) |
154 |
28 |
27 |
23,388 |
||||||||||||||||||||||||
| Selling, general and administration |
(10,975 |
) |
426 |
25 |
17 |
282 |
(10,225 |
) | ||||||||||||||||||||||||
| Research and development |
(5,278 |
) |
101 |
355 |
46 |
(4,776 |
) | |||||||||||||||||||||||||
| Royalty income |
419 |
419 |
||||||||||||||||||||||||||||||
| Other operating (expense)/income |
(476 |
) |
1,106 |
(662 |
) |
32 |
– |
|||||||||||||||||||||||||
| Operating profit |
6,201 |
802 |
322 |
626 |
1,159 |
(618 |
) |
314 |
8,806 |
|||||||||||||||||||||||
| Net finance costs |
(756 |
) |
2 |
1 |
(753 |
) | ||||||||||||||||||||||||||
| Loss on disposal of interest in associates |
(36 |
) |
36 |
– |
||||||||||||||||||||||||||||
| Share of after-tax profits of associates and joint ventures |
33 |
33 |
||||||||||||||||||||||||||||||
| Profit before taxation |
5,442 |
802 |
322 |
628 |
1,159 |
(581 |
) |
314 |
8,086 |
|||||||||||||||||||||||
| Taxation |
(346 |
) |
(159 |
) |
(81 |
) |
(114 |
) |
(196 |
) |
(470 |
) |
(49 |
) |
(1,415 |
) | ||||||||||||||||
| Tax rate |
6.4% |
17.5% |
||||||||||||||||||||||||||||||
| Profit after taxation |
5,096 |
643 |
241 |
514 |
963 |
(1,051 |
) |
265 |
6,671 |
|||||||||||||||||||||||
| Profit attributable to non-controlling interests |
711 |
295 |
1,006 |
|||||||||||||||||||||||||||||
| Profit attributable to shareholders |
4,385 |
643 |
241 |
514 |
668 |
(1,051 |
) |
265 |
5,665 |
|||||||||||||||||||||||
| Earnings per share |
87.6p |
12.9p |
4.8p |
10.3p |
13.3p |
(21.0)p |
5.3p |
113.2p |
||||||||||||||||||||||||
| Weighted average number of shares (millions) |
5,003 |
5,003 |
||||||||||||||||||||||||||||||
Adjusted results reconciliation 31 December 2020 |
Total results £m |
Intangible asset amortisation £m |
Intangible asset impairment £m |
Major restructuring £m |
Transaction- related £m |
Divestments, significant legal and other items £m |
Separation costs £m |
Adjusted results £m |
||||||||||||||||||||||||
| Turnover |
34,099 |
34,099 |
||||||||||||||||||||||||||||||
| Cost of sales |
(11,704 |
) |
699 |
31 |
667 |
116 |
(10,191 |
) | ||||||||||||||||||||||||
| Gross profit |
22,395 |
699 |
31 |
667 |
116 |
23,908 |
||||||||||||||||||||||||||
| Selling, general and administration |
(11,456 |
) |
1 |
18 |
659 |
(23 |
) |
16 |
68 |
(10,717 |
) | |||||||||||||||||||||
| Research and development |
(5,098 |
) |
75 |
214 |
206 |
(4,603 |
) | |||||||||||||||||||||||||
| Royalty income |
318 |
318 |
||||||||||||||||||||||||||||||
| Other operating (expense)/income |
1,624 |
1,215 |
(2,839 |
) |
– |
|||||||||||||||||||||||||||
| Operating profit |
7,783 |
775 |
263 |
1,532 |
1,308 |
(2,823 |
) |
68 |
8,906 |
|||||||||||||||||||||||
| Net finance costs |
(848 |
) |
2 |
2 |
(844 |
) | ||||||||||||||||||||||||||
| Share of after-tax profits of associates and joint ventures |
33 |
33 |
||||||||||||||||||||||||||||||
| Profit before taxation |
6,968 |
775 |
263 |
1,534 |
1,308 |
(2,821 |
) |
68 |
8,095 |
|||||||||||||||||||||||
| Taxation |
(580 |
) |
(150 |
) |
(47 |
) |
(292 |
) |
(229 |
) |
17 |
(14 |
) |
(1,295 |
) | |||||||||||||||||
| Tax rate |
8.3% |
16.0% |
||||||||||||||||||||||||||||||
| Profit after taxation |
6,388 |
625 |
216 |
1,242 |
1,079 |
(2,804 |
) |
54 |
6,800 |
|||||||||||||||||||||||
| Profit attributable to non-controlling interests |
639 |
392 |
1,031 |
|||||||||||||||||||||||||||||
| Profit attributable to shareholders |
5,749 |
625 |
216 |
1,242 |
687 |
(2,804 |
) |
54 |
5,769 |
|||||||||||||||||||||||
| Earnings per share |
115.5p |
12.6p |
4.4p |
25.0p |
13.8p |
(56.5)p |
1.1p |
115.9p |
||||||||||||||||||||||||
| Weighted average number of shares (millions) |
4,976 |
4,976 |
||||||||||||||||||||||||||||||
| Adjusted results reconciliation 31 December 2019 |
Total results £m |
Intangible asset amortisation £m |
Intangible asset impairment £m |
Major restructuring £m |
Transaction- related £m |
Divestments, significant legal and other items £m |
Adjusted results £m |
|||||||||||||||||||||
| Turnover |
33,754 |
33,754 |
||||||||||||||||||||||||||
| Cost of sales |
(11,863 |
) |
713 |
30 |
658 |
383 |
(10,079 |
) | ||||||||||||||||||||
| Gross profit |
21,891 |
713 |
30 |
658 |
383 |
23,675 |
||||||||||||||||||||||
| Selling, general and administration |
(11,402 |
) |
4 |
332 |
104 |
247 |
(10,715 |
) | ||||||||||||||||||||
| Research and development |
(4,568 |
) |
64 |
49 |
114 |
2 |
(4,339 |
) | ||||||||||||||||||||
| Royalty income |
351 |
351 |
||||||||||||||||||||||||||
| Other operating (expense)/income |
689 |
1 |
(142 |
) |
(548 |
) |
– |
|||||||||||||||||||||
| Operating profit |
6,961 |
777 |
83 |
1,105 |
345 |
(299 |
) |
8,972 |
||||||||||||||||||||
| Net finance costs |
(814 |
) |
5 |
(1 |
) |
(810 |
) | |||||||||||||||||||||
| Share of after-tax profits of associates and joint ventures |
74 |
74 |
||||||||||||||||||||||||||
| Profit before taxation |
6,221 |
777 |
83 |
1,110 |
345 |
(300 |
) |
8,236 |
||||||||||||||||||||
| Taxation |
(953 |
) |
(156 |
) |
(17 |
) |
(208 |
) |
(124 |
) |
140 |
(1,318 |
) | |||||||||||||||
| Tax rate |
15.3% |
16.0% |
||||||||||||||||||||||||||
| Profit after taxation |
5,268 |
621 |
66 |
902 |
221 |
(160 |
) |
6,918 |
||||||||||||||||||||
| Profit attributable to non-controlling interests |
623 |
164 |
787 |
|||||||||||||||||||||||||
| Profit attributable to shareholders |
4,645 |
621 |
66 |
902 |
57 |
(160 |
) |
6,131 |
||||||||||||||||||||
| Earnings per share |
93.9p |
12.6p |
1.3p |
18.2p |
1.2p |
(3.3)p |
123.9p |
|||||||||||||||||||||
| Weighted average number of shares (millions) |
4,947 |
4,947 |
||||||||||||||||||||||||||
| Adjusted results reconciliation 31 December 2018 |
Total results £m |
Intangible asset amortisation £m |
Intangible asset impairment £m |
Major restructuring £m |
Transaction- related £m |
Divestments, significant legal and other items £m |
Adjusted results £m |
|||||||||||||||||||||
| Turnover |
30,821 |
30,821 |
||||||||||||||||||||||||||
| Cost of sales |
(10,241 |
) |
536 |
69 |
443 |
15 |
(9,178 |
) | ||||||||||||||||||||
| Gross profit |
20,580 |
536 |
69 |
443 |
15 |
21,643 |
||||||||||||||||||||||
| Selling, general and administration |
(9,915 |
) |
2 |
315 |
98 |
38 |
(9,462 |
) | ||||||||||||||||||||
| Research and development |
(3,893 |
) |
44 |
45 |
49 |
20 |
(3,735 |
) | ||||||||||||||||||||
| Royalty income |
299 |
299 |
||||||||||||||||||||||||||
| Other operating (expense)/income |
(1,588 |
) |
2 |
1,864 |
(278 |
) |
– |
|||||||||||||||||||||
| Operating profit |
5,483 |
580 |
116 |
809 |
1,977 |
(220 |
) |
8,745 |
||||||||||||||||||||
| Net finance costs |
(717 |
) |
4 |
(3 |
) |
18 |
(698 |
) | ||||||||||||||||||||
| Profit on disposal of associates |
3 |
(3 |
) |
– |
||||||||||||||||||||||||
| Share of after-tax profits of associates and joint ventures |
31 |
31 |
||||||||||||||||||||||||||
| Profit before taxation |
4,800 |
580 |
116 |
813 |
1,974 |
(205 |
) |
8,078 |
||||||||||||||||||||
| Taxation |
(754 |
) |
(109 |
) |
(19 |
) |
(170 |
) |
(239 |
) |
(244 |
) |
(1,535 |
) | ||||||||||||||
| Tax rate |
15.7% |
19.0% |
||||||||||||||||||||||||||
| Profit after taxation |
4,046 |
471 |
97 |
643 |
1,735 |
(449 |
) |
6,543 |
||||||||||||||||||||
| Profit attributable to non-controlling interests |
423 |
251 |
674 |
|||||||||||||||||||||||||
| Profit attributable to shareholders |
3,623 |
471 |
97 |
643 |
1,484 |
(449 |
) |
5,869 |
||||||||||||||||||||
| Earnings per share |
73.7p |
9.6p |
2.0p |
13.1p |
30.2p |
(9.2)p |
119.4p |
|||||||||||||||||||||
| Weighted average number of shares (millions) |
4,914 |
4,914 |
||||||||||||||||||||||||||

| Adjusted results reconciliation 31 December 2017 |
Total results £m |
Intangible asset amortisation £m |
Intangible asset impairment £m |
Major restructuring £m |
Transaction- related £m |
Divestments, significant legal and other items £m |
US tax reform £m |
Adjusted results £m |
||||||||||||||||||||||||
| Turnover |
30,186 |
30,186 |
||||||||||||||||||||||||||||||
| Cost of sales |
(10,342 |
) |
546 |
400 |
545 |
80 |
(8,771 |
) | ||||||||||||||||||||||||
| Gross profit |
19,844 |
546 |
400 |
545 |
80 |
21,415 |
||||||||||||||||||||||||||
| Selling, general and administration |
(9,672 |
) |
248 |
83 |
(9,341 |
) | ||||||||||||||||||||||||||
| Research and development |
(4,476 |
) |
45 |
288 |
263 |
18 |
(3,862 |
) | ||||||||||||||||||||||||
| Royalty income |
356 |
356 |
||||||||||||||||||||||||||||||
| Other operating (expense)/income |
(1,965 |
) |
1,519 |
(220 |
) |
666 |
– |
|||||||||||||||||||||||||
| Operating profit |
4,087 |
591 |
688 |
1,056 |
1,599 |
(119 |
) |
666 |
8,568 |
|||||||||||||||||||||||
| Net finance costs |
(669 |
) |
4 |
8 |
(657 |
) | ||||||||||||||||||||||||||
| Profit on disposal of associates |
94 |
(94 |
) |
– |
||||||||||||||||||||||||||||
| Share of after-tax profits of associates and joint ventures |
13 |
13 |
||||||||||||||||||||||||||||||
| Profit before taxation |
3,525 |
591 |
688 |
1,060 |
1,599 |
(205 |
) |
666 |
7,924 |
|||||||||||||||||||||||
| Taxation |
(1,356 |
) |
(134 |
) |
(176 |
) |
(209 |
) |
(619 |
) |
(251 |
) |
1,078 |
(1,667 |
) | |||||||||||||||||
| Tax rate |
38.5% |
21.0% |
||||||||||||||||||||||||||||||
| Profit after taxation |
2,169 |
457 |
512 |
851 |
980 |
(456 |
) |
1,744 |
6,257 |
|||||||||||||||||||||||
| Profit attributable to non-controlling interests |
637 |
42 |
114 |
793 |
||||||||||||||||||||||||||||
| Profit attributable to shareholders |
1,532 |
457 |
512 |
851 |
938 |
(456 |
) |
1,630 |
5,464 |
|||||||||||||||||||||||
| Earnings per share |
31.4p |
9.4p |
10.5p |
17.4p |
19.2p |
(9.4)p |
33.3p |
111.8p |
||||||||||||||||||||||||
| Weighted average number of shares (millions) |
4,886 |
4,886 |
||||||||||||||||||||||||||||||
| Key |
† In-license or other alliance relationship with third party^ ViiV Healthcare, a global specialist HIV company with GSK, Pfizer, Inc. and Shionogi Limited as shareholders, is responsible for developing and delivering HIV medicines. BLA Biological Licence Application MAA Marketing Authorisation Application (Europe) NDA New Drug Application (US) A Approved S Submitted |
EUA Emergency Use Authorisation Phase I Evaluation of clinical pharmacology, usually conducted in volunteers Phase II Determination of dose and initial evaluation of efficacy, conducted in a small number of patients Phase III Large comparative study (compound versus placebo and/or established treatment) in patients to establish clinical benefit and safety | ||||
| Achieved regulatory review milestones | ||||||||||
Compound |
Mechanism of Action/Vaccine Type |
Indication |
Phase |
MAA |
NDA/BLA | |||||
Oncology |
||||||||||
Jemperli † |
Anti-Programmed Cell Death protein 1 receptor (PD-1) antibody |
2L dMMR/MSI-H endometrial cancer2L dMMR solid tumours 1L endometrial cancer 1L endometrial cancer combination with niraparib Non-small cell lung cancer1 |
Approved Approved III III II |
A: Jun21 |
A: Apr21 A: Aug21 | |||||
Zejula † |
Poly (ADP-ribose) polymerase (PARP) 1/2 inhibitor |
1L maintenance ovarian cancer combination with dostarlimab |
III |
|||||||
1L maintenance non small cell lung cancer (NSCLC) combination with pembrolizumab |
III |
|||||||||
Pre-metastatic, select biomarker population Breast Cancer |
III |
|||||||||
Blenrep † |
ADC targeting B-cell maturation antigen |
3L multiple myeloma |
III |
|||||||
2L+ multiple myeloma combination with Pomalyst and dexamethasone |
III |
|||||||||
2L+ multiple myeloma combination with Velcade and dexamethasone |
III |
|||||||||
Multiple myeloma in combination with anti-cancer treatments (platform study) |
II |
|||||||||
1L multiple myeloma combination with Velcade, Revlimid and dexamethasone |
I |
|||||||||
letetresgene- autoleucel (3377794) † |
Engineered TCR T-cells targeting NY-ESO-1 |
2L+ synovial sarcoma and myxoid/round cell liposarcoma 2L+ non-small cell lung cancer |
II (pivotal) II |
|||||||
cobolimab (4069889) † |
Anti-T-cell domain-3 (TIM-3) antibody |
Non-small cell lung cancer combination with Jemperli (dostarlimab) and docetaxel |
II |
|||||||
4074386 † |
Anti-lymphocyte activation gene-3 (LAG-3) antibody |
Cancer |
I |
|||||||
3745417 |
STING cytosolic DNA pathway agonist |
Cancer |
I |
|||||||
6097608 |
CD96 antagonist |
Cancer |
I |
|||||||
3901961 † |
Engineered TCR T-cells, co-expressing the CD8a cell surface receptor, targeting NY-ESO-1 |
Cancer |
I |
|||||||
3845097 † |
Engineered TCR T-cells, co-expressing the dnTGF-ßRII cell surface receptor, targeting NY-ESO-1 |
Cancer |
I |
|||||||
4362676 † |
Methionine adenosyltransferase 2A (MAT2A) inhibitor |
Cancer |
I |
|||||||
4428859 (EOS-448) † |
TIGIT antagonist |
Cancer |
I |
|||||||
1 |
non-registrational |
2 |
transition activities underway to enable further progression by partner |
3 |
GSK has exclusive option to co-develop post Ph2 |
4 |
Ph3 trial in patients with progranulin gene mutation |
5 |
GSK is contributing pandemic adjuvant to COVID-19 vaccines collaborations |
6 |
Submitted in Canada |
7 |
Enrolment and vaccination stopped in February 2022. Further analysis to better understand safety data from these trials is ongoing |

| Achieved regulatory review milestones | ||||||||||
Compound |
Mechanism of Action/Vaccine Type |
Indication |
Phase |
MAA |
NDA/BLA | |||||
HIV^ | ||||||||||
Apretude |
HIV integrase strand transfer inhibitor (long-acting) |
HIV pre-exposure prophylaxisHIV (400 mg/ml formulation) |
Approved I |
A: Dec21 | ||||||
3640254 |
HIV maturation inhibitor |
HIV infection |
II |
|||||||
| 3810109 † |
HIV broadly neutralising antibody |
HIV infection |
II |
|||||||
3739937 |
HIV maturation inhibitor |
HIV infection |
I |
|||||||
4004280 |
HIV capsid protein inhibitor |
HIV infection |
I |
|||||||
Infectious Diseases | ||||||||||
| Xevudy (sotrovimab) † |
Anti-spike protein antibody |
COVID-19 |
Approved |
A:Dec21 |
EUA: May21 | |||||
| gepotidacin † |
Triazaacenaphthylene bacterial type II topoisomerase inhibitor |
Uncomplicated urinary tract infection (uUTI) and gonorrhea (GC) |
III |
|||||||
| 3036656 † |
Leucyl t-RNA synthetase inhibitor |
Tuberculosis |
II |
|||||||
| bepirovirsen † |
HBV antisense |
Hepatitis B |
II |
|||||||
| 3882347 † |
FimH antagonist |
Uncomplicated urinary tract infection (uUTI) |
I |
|||||||
3186899 † 2 |
CRK-12 inhibitor |
Visceral leishmaniasis |
I |
|||||||
| 3494245 † |
Proteasome inhibitor |
Visceral leishmaniasis |
I |
|||||||
| 2556286 † |
Mtb cholesterol dependent inhibitor |
Tuberculosis |
I |
|||||||
| BVL-GSK098 † |
Ethionamide booster |
Tuberculosis |
I |
|||||||
| 4182137 (VIR-7832) † |
Anti-spike protein Antibody |
COVID-19 |
I |
|||||||
| VIR-2482 † 3 |
Neutralizing monoclonal antibody |
Influenza |
I |
|||||||
3923868 |
PI4K beta inhibitor |
Viral COPD exacerbations |
I |
|||||||
Priorix |
Live attenuated |
Measles, mumps, rubella prophylaxis (US) |
Registration |
S: Jun21 | ||||||
Menveo |
Conjugated-liquid formulation |
Meningococcal A,C,W and Y disease prophylaxis in adolescents |
Registration |
S: Sep21 | ||||||
Rotarix |
Live attenuated, PCV (Porcine circovirus) free |
Rotavirus prophylaxis (US) |
Registration |
S Dec21 | ||||||
Bexsero |
Recombinant protein |
Meningococcal B disease prophylaxis in infants (US) |
III |
|||||||
Men ABCWY vaccine |
Recombinant protein – conjugated |
Meningococcal A,B,C,W and Y disease prophylaxis in adolescents |
III |
|||||||
RSV vaccine |
Recombinant protein |
Respiratory syncytial virus prophylaxis in pregnant woman population to prevent respiratory syncitial virus lower respiratory tract illness in infants during first Months of life by transfer of maternal antibodies † 7 |
III |
|||||||
Recombinant protein – adjuvanted |
Respiratory syncytial virus prophylaxis in older adult population † |
III |
||||||||
COVID-19 plant-derived virus-like particles vaccine (Medicago) † 5 |
Recombinant protein-adjuvanted vaccine |
COVID-19 |
Registration 6 |
|||||||
COVID-19 vaccine (Sanofi) † 5 |
Recombinant protein-adjuvanted vaccine |
COVID-19 |
III |
|||||||
COVID-19 vaccine(SK Bioscience) † 5 |
Recombinant protein nanoparticle- adjuvanted vaccine |
COVID-19 |
III |
|||||||
SAM vaccine (COVID-19 model) |
Self-Amplifying mRNA vaccine |
COVID-19 |
I |
|||||||
1 |
non-registrational |
2 |
transition activities underway to enable further progression by partner |
3 |
GSK has exclusive option to co-develop post Ph2 |
4 |
Ph3 trial in patients with progranulin gene mutation |
5 |
GSK is contributing pandemic adjuvant to COVID-19 vaccines collaborations |
6 |
Submitted in Canada |
7 |
Enrolment and vaccination stopped in February 2022. Further analysis to better understand safety data from these trials is ongoing |
| Achieved regulatory review milestones | ||||||||||
Compound |
Mechanism of Action/Vaccine Type |
Indication |
Phase |
MAA |
NDA/BLA | |||||
Infectious Diseases | ||||||||||
| Malaria next generation vaccine † (fractional dose) |
Recombinant protein – adjuvanted vaccine |
Malaria prophylaxis (Plasmodium falciparum) |
II |
|||||||
Shigella vaccine † |
Bioconjugated (tetravalent) vaccine |
Shigella diarrhea prophylaxis |
II |
|||||||
Therapeutic HBV vaccine † |
Prime-boost with viral vector vaccines co- or sequentially administrated with adjuvanted recombinant proteins |
Treatment of chronic Hepatitis B infections – aims at functional cure by controlling and resolving the infection and reducing the need for further treatment |
II |
|||||||
S. aureus vaccine † |
Recombinant protein – bioconjugated – adjuvanted vaccine |
Active immunization for the prevention of primary and recurrent Soft-Skin-Tissue Infections caused by S. aureus |
II |
|||||||
Men ABCWY vaccine (2nd Gen) |
Recombinant protein – conjugated vaccine |
Meningococcal A, B, C, W,Y disease prophylaxis in adolescents and infants |
II |
|||||||
Varicella New Strain |
Live attenuated vaccine |
Active immunization for the prevention of varicella in individuals from 12 months of age and older |
II |
|||||||
| C. difficile vaccine † |
Recombinant protein – adjuvanted vaccine |
Active immunization for the prevention of the primary C. Diff diseases and for prevention of recurrences |
I |
|||||||
SAM vaccine (Rabies model) |
Self-Amplifying mRNA |
Rabies prophylaxis |
I |
|||||||
Klebsiella pneumoniae |
Recombinant protein – bioconjugated – adjuvanted vaccine |
Klebsiella pneumoniae prophylaxis |
I |
|||||||
CMV |
Recombinant subunit – adjuvanted vaccine |
Cytomegalovirus (CMV) infection prophylaxis in females 16-49 years of age |
I |
|||||||
| Immunology and Respiratory | ||||||||||
Nucala |
Interleukin 5 (IL5) antagonist |
Hypereosinophilic syndrome Nasal polyposis EGPA COPD |
Approved Approved Approved III |
A: Nov21 A: Nov21 A: Nov21 |
A: Jul21 | |||||
otilimab † |
Granulocyte macrophage colony-stimulating factor inhibitor |
Rheumatoid arthritis |
III |
|||||||
| depemokimab † |
Interleukin 5 (IL5) antagonist (long-acting) |
Asthma |
III |
|||||||
| 4527223 (AL001) † |
Anti-Sortilin monoclonal antibody |
Frontotemporal dementia (FTD) 4 Amyotrophic Lateral Sclerosis (ALS) |
III II |
|||||||
| 3858279 † |
Anti-CCL17 antibody |
Osteoarthritis pain |
I |
|||||||
| 3915393 † |
Transglutaminase 2 (TG2) inhibitor |
Celiac disease |
I |
|||||||
| 4527226 (AL101) † |
Anti-sortilin monoclonal antibody |
Neurodegenerative disease |
I |
|||||||
1070806 |
Anti-IL18 antibody |
Atopic dermatitis |
I |
|||||||
| 3888130 † |
Anti-IL7 antibody |
Multiple sclerosis (MS) |
I |
|||||||
| 4532990 † |
HSD17B13 silencer |
Non-alcoholic steatohepatitis (NASH) |
I |
|||||||
| Opportunity Driven | ||||||||||
daprodustat |
HIF Prolyl hydroxylase inhibitor |
Anaemia of chronic kidney disease |
III (RoW) |
|||||||
linerixibat |
Ileal bile acid transporter (IBAT) inhibitor |
Cholestatic pruritus in PBC (primary biliary cholangitis) |
III |
|||||||
| 2798745 † |
TRPV4 channel blocker |
Diabetic macular edema (DME) |
I |
|||||||
| 3884464 † |
Novel mechanism |
Heart failure |
I |
|||||||
1 |
non-registrational |
2 |
transition activities underway to enable further progression by partner |
3 |
GSK has exclusive option to co-develop post Ph2 |
4 |
Ph3 trial in patients with progranulin gene mutation |
5 |
GSK is contributing pandemic adjuvant to COVID-19 vaccines collaborations |
6 |
Submitted in Canada |
7 |
Enrolment and vaccination stopped in February 2022. Further analysis to better understand safety data from these trials is ongoing |

Major |
Patent expiry dates 1 | |||||||||
Products |
Compounds |
Indication(s) |
competitor brands |
US |
EU | |||||
Respiratory |
||||||||||
Anoro Ellipta |
umeclidinium bromide/ vilanterol trifenatate |
COPD |
Stiolto Respimat, Utibron/Ultibro Breezhaler, Duaklir Genuair Bevespi Aerosphere, Brimica Genuair |
2027 (NCE) 2027-2030 (device) |
2029 (NCE) 2022-2026 (device) | |||||
Arnuity Ellipta |
fluticasone furoate |
asthma |
Beclazone, Pulmicort, Budesonide Gx, Asmanex, Alvesco |
2021 (NCE) 2027-2030 (device) |
2023 (NCE) 2022-2026 (device) | |||||
Avamys/Veramyst |
fluticasone furoate |
rhinitis |
Dymista, Xhance, Nasonex, Fluticasone Gx |
expired |
2023 | |||||
Flixotide/Flovent |
fluticasone propionate |
asthma/COPD |
Beclazone, Pulmicort, Budesonide Gx, Asmanex, Alvesco |
expired ( Diskus 2023-2026 (HFA-device) |
expired ( Diskus device (HFA-device) | |||||
Incruse Ellipta |
umeclidinium bromide |
COPD |
Spiriva Handihaler/ Respimat, Yupelri, Braltus, Seebri Breezhaler, Bretaris Genuair |
2027 (NCE) 2027-2030 (device) |
2029 (NCE) 2022-2026 (device) | |||||
Nucala |
mepolizumab |
severe eosinophilic asthma, EGPA hypereosinophilic syndrome, chronic rhinosinusitis with nasal polyps |
Xolair, Cinqair, Fasenra, Dupixent |
expired 2 |
expired 2 | |||||
Relvar/Breo Ellipta |
fluticasone furoate/vilanterol trifenatate |
asthma/COPD |
Symbicort, Foster, Budesonide/Formetrol Gx Sirdupla, Dulera |
2025 (NCE) 2027-2030 (device) |
2027 (NCE) 2022-2026 (device) | |||||
Seretide/Advair |
salmeterol xinafoate/fluticasone propionate |
asthma/COPD |
Symbicort, Foster, Budesonide/Formetrol Gx Sirdupla, Dulera |
expired ( Diskus device 2023-2026 (HFA-device) |
expired ( Diskus device (HFA-device) | |||||
Trelegy Ellipta |
fluticasone furoate/vilanterol trifenatate umeclidinium bromide |
COPD |
Trimbow, Breztri Aerosphere, Trixeo Aerosphere, Enerzair Breezhaler |
2027 (NCE) 2027-2030 (device) |
2029 (NCE) 2022-2026 (device) | |||||
Ventolin HFA |
albuterol sulphate |
asthma/COPD |
generic companies |
2023-2026 (HFA-device) |
expired (HFA-device) | |||||
Xevudy |
sotrovimab |
Early treatment of COVID-19 |
REGEN-COV, bamlanivimab/etesevimab, Evusheld |
2041 (NBE) |
NA | |||||
| Anti-virals |
||||||||||
Valtrex |
valaciclovir |
genital herpes, coldsores, shingles |
Prevymis, Valacyclovir Gx, Valcyte |
expired |
expired | |||||
| Central nervous system |
||||||||||
Lamictal |
lamotrigine |
epilepsy, bipolar disorder |
Vimpat, Trokendi XR, Inovelon |
expired |
expired | |||||
Imigran/Imitrex |
sumatriptan |
migraine |
Zomig, Maxalt, Relpax |
expired |
expired | |||||
Seroxat/Paxil |
paroxetine |
depression, various anxiety disorders |
Trintellix, Aplenzin Viibryd, Zoloft |
expired |
expired | |||||
| Cardiovascular and urogenital |
||||||||||
Avodart |
dutasteride |
benign prostatic hyperplasia |
Harnal, Vesomni, Urorec |
expired |
expired | |||||
| Anti-bacterials |
||||||||||
Augmentin |
amoxicillin/clavulanate potassium |
common bacterial infections |
generic products |
NA |
expired | |||||
1 |
Includes Supplementary Protection Certificates which were granted in multiple countries in EU (including the UK) and patent term extensions granted in the US. |
2 |
Data exclusivity expires 2026 (EU) and 2027 (US). |
Major |
Patent expiry dates 1 | |||||||||
Products |
Compounds |
Indication(s) |
competitor brands |
US |
EU | |||||
Oncology |
||||||||||
Zejula |
niraparib |
ovarian cancer |
Lynparza, Rubraca |
2031 (NCE) |
2028 (NCE) | |||||
Blenrep |
belantamab mafodotin |
relapsed/refractory multiple myeloma |
Sarclisa, Xpovio |
2032 |
2032 | |||||
Jemperli |
dostarlimab |
dMMR recurrent or advanced endometrial cancer, solid tumours |
Keytruda |
2034 (NBE) |
2034 (NBE) | |||||
Immuno-inflammation |
||||||||||
| Benlysta, Benlysta (SC and IV) |
belimumab |
systemic lupus erythematosus, lupus nephritis |
Lupkynis, Saphnelo |
2025 |
2026 | |||||
HIV |
||||||||||
Apretude |
Cabotegravir |
HIV prevention |
Descovy, Truvada |
2026 (NCE) |
2026 (NCE) | |||||
Cabenuva/Vocabria + Rekambys |
Cabotegravir, rilpivirine |
HIV/AIDS |
Descovy, Genvoya, Odefsey, Biktarvy |
2026 (NCE) |
2026 (NCE) | |||||
Rukobia |
Fostemsavir |
HIV/AIDS |
Trogarzo |
2025 (NCE) |
2025 (NCE) | |||||
Dovato |
Dolutegravir, lamivudine |
HIV/AIDS |
Descovy, Genvoya, Odefsey, Biktarvy |
2027 (NCE) |
2029 (NCE) | |||||
Juluca |
Dolutegravir, rilpivirine |
HIV/AIDS |
Descovy, Genvoya, Odefsey, Biktarvy |
2027 (NCE) |
2029 (NCE) | |||||
Triumeq |
Dolutegravir, lamivudine and abacavir |
HIV/AIDS |
Descovy, Genvoya, Odefsey, Biktarvy |
2027 (NCE) |
2029 (NCE) | |||||
Tivicay |
Dolutegravir |
HIV/AIDS |
Isentress, Prezista Symtuza, Reyataz, Biktarvy |
2027 (NCE) |
2029 (NCE) | |||||
| Vaccine products, competition and intellectual property | ||||||||||
Major |
Patent expiry dates 2 | |||||||||
Products |
Compounds |
Indication(s) |
competitor brands |
US |
EU | |||||
Bexsero |
meningococcal group-B vaccine |
Meningitis group B prevention |
Trumenba |
2027 |
2028 | |||||
Boostrix |
diphtheria, tetanus, acellular pertussis |
diphtheria, tetanus, acellular Pertussis booster vaccination |
Adacel |
expired |
expired | |||||
Infanrix Hexa/Pediarix |
diphtheria, tetanus, pertussis, polio, hepatitis B, Haemophilus influenzae type B (EU) |
Prophylaxis against diphtheria, tetanus, pertussis, polio, hepatitis B, Haemophilus influenzae type B (EU) |
Pentacel, Pediacel, Pentaxim, Pentavac, Hexaxim, Hexyon Vaxelis |
expired |
expired | |||||
Cervarix |
HPV 16 & 18 virus like particles (VLPs), AS04 adjuvant (MPL + aluminium hydroxide) |
human papilloma virus type 16 and 18 |
Gardasil (Silgard) |
2028 |
2022 | |||||
Fluarix Tetra |
split inactivated influenza antigens (2 virus subtypes A and 2 subtype B) |
seasonal influenza prophylaxis |
Intenza, Flumist QIV, Vaxigrip QIV, Fluzone QIV, Fluzone High Dose |
2022 |
2022 | |||||
FluLaval |
split inactivated influenza antigens (2 virus subtypes A and 2 subtype B) |
seasonal influenza prophylaxis |
Vaxigrip, Mutagrip, Fluzone, Influvac, Aggripal, Fluad, Intenza, Flumist |
2022 |
2022 | |||||
Menveo |
meningococcal group A, C, W- 135 and Y conjugate vaccine |
Meningitis group A, C, W-135 and Y prophylaxis |
Nimenrix, Menactra |
2025 |
2025 | |||||
Priorix, Priorix Tetra a,b Varilrix b |
live attenuated measles, mumps, rubella and varicella vaccine |
measles, mumps, rubella and chickenpox prophylaxis |
MMR II (M-M-RVaxPro) |
expired |
expired | |||||
Rotarix |
Human rotavirus RIX4414 strain |
Rotavirus prophylaxis |
Rotateq |
2022 |
2026 | |||||
Synflorix |
conjugated pneumococcal polysaccharide |
Prophylaxis against invasive disease, pneumonia, acute otitis media |
Prevenar (Prevnar) |
NA |
2026 | |||||
Shingrix |
zoster vaccine recombinant, adjuvanted |
herpes zoster (shingles) |
Zostavax |
2029 |
2031 | |||||
1 |
See Note 46 to the financial statements, ‘Legal proceedings’. |
2 |
Includes Supplementary Protection Certificates which were granted in multiple countries in EU (including the UK), and patent term extensions granted in the US. |
a |
Related compounds/indications are measles, mumps and rubella vaccine/prophylaxisb. |
b |
Related compound is varicella vaccine. |

Brand |
Products |
Application |
Markets |
Competition | ||||
Oral health |
||||||||
Sensodyne, Pronamel |
toothpastes, toothbrushes, mouth rinse |
relief of dentinal hypersensitivity. Pronamel |
global |
Colgate Sensitive Pro-Relief, Colgate-PalmoliveElmex, Colgate-Palmolive Oral B, Procter & Gamble | ||||
parodontax/ Corsodyl |
toothpaste, daily/medicated mouthwash, gel and spray |
helps stop and prevent bleeding gums, treats and prevents gingivitis |
global |
Colgate Total Gum Health, Colgate-Palmolive Oral B Gum & Enamel Repair, Crest Gum Detoxify, Procter & Gamble | ||||
Polident, Poligrip, Corega |
denture adhesive, denture cleanser, wipes |
improve retention and comfort of dentures, cleans dentures |
global |
Fixodent and Kukident, Procter & Gamble, Steradent, Reckitt Benckiser | ||||
Aquafresh |
toothpastes, toothbrushes mouthwashes |
aids prevention of dental cavities, maintains healthy teeth, gums and fresh breath |
global |
Colgate, Colgate-Palmolive Crest, Procter & Gamble Oral-B, Procter & Gamble | ||||
Pain relief |
||||||||
Panadol |
tablets, caplets, infant syrup |
paracetamol-based treatment for headache, joint pain, fever, cold symptoms |
global (except US) |
Aspirin, Bayer Tylenol, Johnson & Johnson Nurofen, Reckitt Benckiser | ||||
Voltaren |
topical gel, diclofenac tablets and patches |
non-steroidal, diclofenac based anti-inflammatory |
global |
Salonpas, Hisamitsu Aspirin, Bayer Tylenol, Johnson & Johnson Nurofen, Reckitt Benckiser Icy Hot, Sanofi | ||||
Advil non-respiratory range |
tablets, caplets, gel caplets, liquid filled suspension, drops (children’s) |
ibuprofen based treatment for headache, toothache, backache, menstrual cramps, muscular pains, minor pain of arthritis |
US, Canada, Brazil, Colombia, Mexico |
Tylenol, Tylenol PM, Tylenol Children’s Motrin, Motrin Children’s, Johnson & Johnson Aleve, Aleve PM, Bayer | ||||
Vitamins, minerals and supplements |
||||||||
Centrum |
tablets, gummies, capsules, chewables |
vitamin supplement |
global |
Nutralite, Infinitus Cheong-Kwan-Jung, By-Health, Nature Made, Herbalife, Swisse | ||||
Caltrate |
tablets, gummies, soft chews |
calcium supplement |
global |
Citracal, Bayer, OS-Cal, Nature Made and private label | ||||
Emergen-C |
powder, gummies |
immune support dietary supplement |
US, Canada |
Airborne, Reckitt Benckiser Zicam, Church & Dwight Nature made, Pharmavite Sambucol, Healthcare Brands International Ester-C, American Health | ||||
Respiratory health |
||||||||
Otrivin |
nasal spray |
nasal decongestant |
Germany, Netherlands, Norway, Russia, Sweden |
Afrin, Bayer, Nasivin, Proctor & Gamble, Tyzine, Johnson & Johnson | ||||
Theraflu |
hot liquids, tablets, syrups |
cold and flu relief |
Russia, Poland, US |
Tylenol Cold & Flu, Johnson & Johnson Mucinex, Reckitt Benckiser Lemsip, Reckitt Benckiser | ||||
Advil Respiratory Advil |
tablets |
allergy relief and cold & flu relief |
Tylenol Cold & Flu, Johnson & Johnson, Lemsip, Mucinex, Reckit Benckiser | |||||
Flixonase/Flonase Piriton |
nasal spray, tablets |
allergy relief |
US, China, UK, Ireland |
Claritin, Bayer, Allegra, Sanofi Zyrtec, Johnson & Johnson | ||||
Robitussin |
syrup, tablets |
cough/cold |
US, Canada, Singapore, Philippines, Australia |
Mucinex, Reckitt Benckiser Dimetapp, Foundation Consumer Healthcare | ||||
Digestive health and other |
||||||||
Nexium 24HR |
capsules, clear minis, tablets |
treatment of frequent heartburn (two or more days a week) in adults (18 years and older) |
US, Canada, Australia |
Prilosec, Prevacid | ||||
Zovirax Abreva |
topical cream and non-medicated patch |
lip care to treat and prevent the onset of cold sores |
global |
Compeed, Johnson & Johnson Carmex, Carma Labs Blistex, Blistex Incorporated retail own label | ||||
ChapStick |
lip balm |
protect, moisturise, prevent and soothe chapped lips |
global |
Blistex, Burt’s Bees, Carmex, Carma Labs, EOS, Nivea, Beiersdorf, Vaseline, Unilever | ||||
ENO |
effervescent |
immediate relief antacid |
global (except US) |
Estomazil, Hypermarca, Gelusil | ||||
Tums |
chewable tablets |
immediate relief antacid |
US |
Alka-Seltzer, Bayer Gaviscon, Reckitt Benckiser Rolaids, Sanofi | ||||
Nicorette , NicoDerm, Nicotinell |
lozenges, gum and trans-dermal patches |
treatment of nicotine withdrawal as an aid to smoking reduction and cessation |
global |
Nicorette, Johnson & Johnson NiQuitin, Perrigo | ||||

– |
Appropriate controls and governance of quality in product development; |
– |
Compliance with good manufacturing practice or good distribution practice regulations in commercial or clinical trials manufacture and distribution activities; |
– |
Compliance with the terms of GSK product licences and supporting regulatory activities. |

| Mitigating activities Financial results are reviewed and approved by regional management, before being reviewed by GSK’s Group Financial Controller and Chief Financial Officer (CFO). This allows our Financial Controller and CFO to assess the evolution of the business over time, and to evaluate its performance to plan. Significant judgements are reviewed and confirmed by senior management. We integrate technical or organisational transformation, newly acquired activities and external risks, such as the COVID-19 pandemic, into our risk assessments, and apply appropriate controls and reviews.We maintain a control environment designed to identify material errors in financial reporting and disclosure. The design and operating effectiveness of key financial reporting controls are regularly reviewed by management and tested by external third parties. A minimum standard control set is in place for all finance locations, irrespective of size, which is reviewed by management and monitored independently. This gives us assurance that controls over key financial reporting and disclosure processes are operating effectively. Our Global Finance Risk Management and Controls (FRMC) group provides extra support during significant transformations, such as system deployment or management/structural reorganisations. We add operational resources and adapt programme timelines to ensure processes and controls are maintained during significant changes. The Disclosure Committee, reporting to the Board, reviews GSK’s quarterly results and annual report. Throughout the year, in consultation with its legal advisors, the Disclosure Committee also determines whether it is necessary to disclose publicly information about the Group through stock exchange announcements. We keep up to date with the latest developments in financial reporting requirements by working with our external auditor and legal advisors. The Treasury Management Group (TMG) meets regularly to ensure that liquidity, interest rate, counterparty, foreign currency transaction and foreign currency translation risks are all managed in line with the prudent approach detailed in the risk strategies and policies adopted by our Board. |
Counterparty exposure is subject to defined limits approved by the Board for both credit rating and individual counterparties. The Middle Office within Treasury monitor the management of counterparty risk in line with agreed policy with oversight from a corporate compliance officer, operating independently of Treasury. Further details on mitigation of Treasury risks can be found on pages 228 to 244. We manage tax risk through robust internal policies, processes, training, and compliance programmes. We maintain open and constructive relationships with tax authorities worldwide. We monitor government debate on tax policy in our key jurisdictions, so that we can understand any potential future changes in tax law and share an informed point of view. Where relevant, we provide pragmatic and constructive business input to tax policy makers, either directly or through industry trade bodies. This includes advocating reform to support economic growth and job creation, as well as the needs of our patients and other key stakeholders. We submit significant tax decisions to our Tax Governance Board, which meets quarterly comprised of senior GSK Finance colleagues. Our tax affairs are managed on a global basis by a team of tax professionals, led by the Global Head of Tax, who work closely with the business on a day-to-day We submit tax returns according to statutory time limits and engage proactively with tax authorities to ensure our tax affairs are current, entering into continuous audit programmes and advance pricing agreements where appropriate. These arrangements provide long-term certainty for both tax authorities and GSK over the tax treatment of our business, based on full disclosure of all relevant facts. We seek to resolve any differences of interpretation in tax legislation with tax authorities in a cooperative manner. In exceptional cases, we may have to resolve disputes through formal proceedings. |


– |
operating and improving the centralised global privacy control framework; |
– |
continuously assessing and providing relevant and proportionate controls and aid to non-deployed markets; |
– |
monitoring new, or changing, laws and adapting the privacy framework; accordingly, and |
– |
deploying a comprehensive training programme to drive greater awareness and accountability for managing personal information across the entire organisation. |

– |
execution of hazardous activities; |
– |
GSK’s physical assets and infrastructure; |
– |
handling and processing of hazardous chemicals and biological agents; |
– |
control of releases of substances harmful to the environment in both the short and long-term; |
– |
Physical climate and environmental risks; |
– |
Current and future regulatory requirements for environmental policies and taxes; |
– |
Delivery and performance of management environmental objectives; |

– |
Modernising cyber operations with consistent evaluation of our security solutions and deployment of best of class cyber security technology to ensure the timely detection and response to information security incidents, with particular focus on ransomware preparation and awareness. |
– |
Modernising cyber security within manufacturing and R&D sites to address the age, complexity, and global footprint of those environments. |
– |
Optimising security architecture to mitigate the risk of data loss intentionally or unintentionally, implementing a cloud security strategy and ensuring new solution development includes security by design. We are also continuing to remediate and improve the control environment for privileged or elevated user rights across our systems. |
– |
Transferring third party risk management to a managed service partner. This organisation will process our critical and sensitive information and supports the solution that will enable us to move all third parties that access our IT resources remotely via a more secure environment. |
– |
Enabling business performance in high risk markets by assessing data and information originating in, and flowing to, international markets where local laws and norms represent a heightened risk to the confidentiality, integrity, and availability of our operational systems. |
– |
Product shortages and product recalls |
– |
Regulatory intervention |
– |
Reputational harm |
– |
Lost sales revenue |

31 December 2021 |
27 February 2022 |
|||||||||||||||
| No. of voting rights |
Percentage of total voting rights (1) |
No. of voting rights |
Percentage of total voting rights (1) |
|||||||||||||
| BlackRock, Inc |
332,238,289 |
(2) |
6.40 |
% |
332,238,289 |
6.40% |
||||||||||
| Dodge & Cox |
253,464,108 |
(3) |
5.04 |
% |
253,464,108 |
5.04% |
||||||||||
(1) |
Percentage of total voting rights at the date of notification to the company. |
(2) |
Comprising an indirect interest in 329,124,508 Ordinary Shares and a holding of 3,113,781 Qualifying Financial Instruments (Contract for Difference). |
(3) |
Comprising an indirect interest in 99,377,874 Ordinary Shares and 154,086,234 American Depositary Shares. |
| Share price |
2021 £ |
2020 £ |
2019 £ |
|||||||||
| At 1 January |
13.42 |
17.79 |
14.91 |
|||||||||
| At 31 December |
16.07 |
13.42 |
17.79 |
|||||||||
| Increase/(decrease) |
20% |
(24.6)% |
19.3% |
|||||||||
| High during the year |
16.19 |
18.46 |
18.19 |
|||||||||
| Low during the year |
11.91 |
12.92 |
14.36 |
|||||||||

Ordinary Shares |
ADS |
|||||||||||||||
UK£ per share |
US$ per share |
|||||||||||||||
High |
Low |
High |
Low |
|||||||||||||
| February 2022* |
16.50 |
15.05 |
45.70 |
41.19 |
||||||||||||
| January 2022 |
17.08 |
15.89 |
46.82 |
43.37 |
||||||||||||
| December 2021 |
16.19 |
15.34 |
44.44 |
41.25 |
||||||||||||
| November 2021 |
15.93 |
15.11 |
45.53 |
41.02 |
||||||||||||
| October 2021 |
15.09 |
13.80 |
42.33 |
38.13 |
||||||||||||
| September 2021 |
14.88 |
13.83 |
41.61 |
38.05 |
||||||||||||
| Quarter ended 31 December 2021 |
16.19 |
13.80 |
44.44 |
38.13 |
||||||||||||
| Quarter ended 30 September 2021 |
15.26 |
13.83 |
42.33 |
38.05 |
||||||||||||
| Quarter ended 30 June 2021 |
14.36 |
12.78 |
40.66 |
35.82 |
||||||||||||
| Quarter ended 31 March 2021 |
14.14 |
11.91 |
39.24 |
33.61 |
||||||||||||
| Quarter ended 31 December 2020 |
14.68 |
12.92 |
39.17 |
33.42 |
||||||||||||
| Quarter ended 30 September 2020 |
16.60 |
14.35 |
42.16 |
37.38 |
||||||||||||
| Quarter ended 30 June 2020 |
17.42 |
14.89 |
42.74 |
37.14 |
||||||||||||
| Quarter ended 31 March 2020 |
18.46 |
13.75 |
47.89 |
31.85 |
||||||||||||
| Year ended 31 December 2020 |
14.68 |
12.92 |
39.17 |
33.42 |
||||||||||||
| Year ended 31 December 2019 |
18.19 |
14.36 |
47.32 |
37.83 |
||||||||||||
| Year ended 31 December 2018 |
16.22 |
12.43 |
41.94 |
35.49 |
||||||||||||
| Year ended 31 December 2017 |
17.22 |
12.76 |
44.37 |
34.66 |
||||||||||||

| Number of accounts |
% of total accounts |
% of total shares |
Number of shares |
|||||||||||||
| Holding of shares |
||||||||||||||||
| Up to 1,000 |
69,334 |
71.06 |
0.44 |
23,444,870 |
||||||||||||
| 1,001 to 5,000 |
21,872 |
22.41 |
0.88 |
47,264,152 |
||||||||||||
| 5,001 to 100,000 |
5,220 |
5.35 |
1.50 |
80,804,464 |
||||||||||||
| 100,001 to 1,000,000 |
776 |
0.80 |
5.04 |
271,429,821 |
||||||||||||
| Over 1,000,000 |
368 |
0.38 |
92.14 |
4,964,071,752 |
||||||||||||
97,570 |
100.00 |
100.00 |
5,387,015,059 |
|||||||||||||
| Held by |
||||||||||||||||
| Institutional and Corporate holders |
2,723 |
2.79 |
61.96 |
3,337,598,976 |
||||||||||||
| Individuals and other corporate bodies |
94,845 |
97.21 |
13.55 |
729,773,041 |
||||||||||||
| Guaranty Nominees Limited |
1 |
0.00 |
17.90 |
964,437,092 |
||||||||||||
| Held as Treasury shares by GlaxoSmithKline |
1 |
0.00 |
6.59 |
355,205,950 |
||||||||||||
Year |
pence |
US$ |
||||||
| 2021 |
80 |
–* |
||||||
| 2020 |
80 |
2.09 |
||||||
| 2019 |
80 |
1.98 |
||||||
| 2018 |
80 |
2.08 |
||||||
| 2017 |
80 |
2.16 |
||||||
* |
The Q4 2021 ordinary dividend receivable by ADS holders will be calculated based on the exchange rate on 7 April 2022. An annual fee of $0.03 per ADS (or $0.0075 per ADS per quarter) will be charged by the Depository. The cumulative dividend receivable by ADS holders for Q1, Q2 and Q3 2021 was $1.56. |
Quarter |
Ex-dividend date |
Record date |
Payment date |
|||||||||
| Q4 2021 |
24 February 2022 |
25 February 2022 |
7 April 2022 |
|||||||||
| Q1 2022 |
19 May 2022 |
20 May 2022 |
7 July 2022 |
|||||||||
| Q2 2022 |
18 August 2022 |
19 August 2022 |
6 October 2022 |
|||||||||
| Q3 2022 |
17 November 2022 |
18 November 2022 |
12 January 2023 |
|||||||||
| Q4 2022 |
23 February 2023 |
24 February 2023 |
13 April 2023 |
|||||||||
Event |
Date | |
| Quarter 1 Results announcement |
27 April 2022 | |
| Annual General Meeting |
4 May 2022 | |
| Quarter 2 Results announcement |
27 July 2022 | |
| Quarter 3 Results announcement |
2 November 2022 | |
| Preliminary/Quarter 4 Results announcement |
1 February 2023 | |
| Annual Report publication |
February/March 2023 | |
| Annual Report distribution |
March 2023 | |

| Taxation of dividends The gross amount of dividends received is treated as foreign source dividend income for US tax purposes. It is not eligible for the dividend received deduction allowed to US corporations. Dividends on ADS are payable in US dollars; dividends on Ordinary Shares are payable in Sterling. Dividends paid in Sterling will be included in income in the US dollar amount calculated by reference to the exchange rate on the day the dividends are received by the holder. Subject to certain exceptions for short-term or hedged positions, an individual eligible US holder will be subject to US taxation at a maximum federal rate of 23.8% plus applicable state and local tax in respect of qualified dividends. A qualified dividend as defined by the US Internal Revenue Service (IRS) is a dividend that meets the following criteria: 1. Must be issued by a US corporation, a corporation incorporated in a US possession, or a corporation that is eligible for the benefits of a comprehensive income tax treaty deemed satisfactory, as published by the IRS. 2. The dividends are not of a type listed by the IRS as dividends that do not qualify. 3. The required dividend holding period has been met. The shares must have been owned by you for more than 60 days of the ‘holding period’ – which is defined as the 121-day period that begins 60 days before the ex-dividend date, or the day in which the stock trades without the dividend priced in. For example, if a stock’s ex-dividend date is 1 October, the shares must be held for more than 60 days in the period between 2 August and 30 November of that year in order to count as a qualified dividend.Dividends that are not qualified are subject to taxation at the US federal graduated tax rates, at a maximum rate of 40.8%. Some types of dividends are automatically excluded from being qualified dividends, even if they meet the other requirements. These include (but are not limited to): 1. Capital gains distributions 2. Dividends on bank deposits 3. Dividends held by a corporation in an Employee Stock Ownership Plan (ESOP) 4. Dividends paid by tax-exempt corporations.US state and local tax rates on qualified and non-qualified dividends may vary and would be assessed in addition to the federal tax rates communicated above. |
Taxation of capital gains Generally, US holders will not be subject to UK capital gains tax, but will be subject to US tax on capital gains realised on the sale or other disposal of shares or ADS. Such gains will be long-term capital gains (subject to reduced rates of taxation for individual holders) if the shares or ADS were held for more than one year, from the date the shares were vested/released. Short-term capital gains can be subject to taxation of rates of up to 40.8%, whereas long-term capital gains may be subject to rates of up to 23.8%. State and local tax rates on capital gains may also apply. Information reporting and backup withholding Dividends and payments of the proceeds on a sale of shares or ADS, paid within the US or through certain US-related financial intermediaries, are subject to information reporting and may be subject to backup withholding unless the US holder is a corporation or other exempt recipient or provides a taxpayer identification number and certifies that no loss of exemption has occurred. Non-US holders generally are not subject to information reporting or backup withholding, but may be required to provide a certification of their non-US status in connection with payments received. Any amounts withheld will be allowed as a refund or credit against a holder’s US federal income tax liability provided the required information is furnished to the IRS.Estate and gift taxes Under the Estate and Gift Tax Convention, a US shareholder is not generally subject to UK inheritance tax. However, a US holder may be subject to US federal estate and gift tax. Stamp duty UK stamp duty and/or SDRT will, subject to certain exemptions, be payable on any transfer of shares to the ADS custodian or depository at a rate of 1.5% of the amount of any consideration provided (if transferred on sale), or their value (if transferred for no consideration). However, no stamp duty or SDRT should be payable on the transfer of, or agreement to transfer, an ADS. |

| Service |
What it offers |
How to participate | ||
Dividend Reinvestment Plan (DRIP) |
As an alternative to receiving cash dividends you may choose to reinvest your dividends to buy more GSK shares. |
A DRIP election form can be downloaded from www.shareview.co.uk or requested by contacting Equiniti. | ||
Dividend payment direct to your bank account (Bank Mandate) |
All dividends are paid directly into your bank or building society account. To receive your cash dividends, you must provide Equiniti with your bank or building society account details. This is a quick and secure method of payment. |
A dividend bank mandate form can be downloaded from www.shareview.co.uk or requested by contacting Equiniti. | ||
Dividend payment direct to bank account for overseas shareholders |
Equiniti can convert your dividend into your local currency and send it direct to your local bank account. This service is available in over 100 countries worldwide. |
For more details on this service and the costs involved please contact Equiniti. | ||
Electronic communications |
Shareholders may elect to receive electronic notifications of company communications including our Annual Report, dividend payments, dividend confirmations and the availability of online voting for all general meetings. Each time GSK publishes shareholder documents you will receive an email containing a link to the document or relevant website. |
Please register at www.shareview.co.uk. | ||
Shareview portfolio service |
This enables you to create a free online portfolio to view your share balance and movements, update your address and dividend payment instructions and register your votes for our general meetings. |
Please register at www.shareview.co.uk. | ||
Deduplication of publications or mailings |
If you receive duplicate copies of mailings, you may have more than one account. Please contact Equiniti and they will arrange for your accounts to be merged into one for your convenience and to avoid waste and unnecessary costs. |
Please contact Equiniti. | ||
Share dealing service † (please note that market trading hours are from 8.00am to 4.30pm UK time, Monday to Friday (excluding public holidays in England and Wales)) |
Shareholders may trade shares, either held in certificated form or in our Corporate Sponsored Nominee, online, by telephone or via postal dealing service provided by Equiniti Financial Services Limited. |
For online transactions, please log on to: www.shareview.co.uk/dealing. For telephone transactions, please call: 0345 603 7037 (in the UK) or +44 (0)121 415 7560 (outside the UK). Lines are open from 8.00am to 4.30pm UK time, Monday to Friday (excluding UK public holidays). For postal transactions, please call: 0371 384 2991* to request a dealing form. | ||
Corporate Sponsored Nominee Account |
This is a convenient way to manage your shares without requiring a share certificate. The service provides a facility for you to hold your shares in a nominee account sponsored by the company. You will continue to receive dividend payments and can attend and vote at the company’s general meetings. Shareholders’ names do not appear on the publicly available share register and the service is free to join. |
An application form can be requested from www.shareview.co.uk or by contacting Equiniti. | ||
Individual Savings Accounts (ISAs) † |
The company has arranged for Equiniti Financial Services Limited to provide a GSK Corporate ISA to hold GSK shares. |
Details are available from www.shareview.co.uk or can be requested by telephoning Equiniti, on 0345 0700 720. Lines are open 8.00am to 4.30pm for dealing, and until 5.30pm for enquiries Monday to Friday (excluding public holidays in England and Wales). | ||
* |
Lines are open from 8.30am to 5.30pm, Monday to Friday (excluding public holidays in England and Wales). |
† |
The provision of share dealing details is not intended to be an invitation or inducement to engage in an investment activity. Advice on share dealing should be obtained from a stockbroker or independent financial adviser. |
† |
The provision of share dealing details is not intended to be an invitation or inducement to engage in an investment activity. Advice on share dealing should be obtained from a stockbroker or independent financial adviser. |
Contacts |
* |
Lines are open from 8.00am to 6.00pm, UK time, Monday to Friday, except UK public holidays, and 9.00am to 1.00pm on Saturdays. |

– |
they have each reviewed the annual report on Form 20-F; |
– |
based on their knowledge, the annual report on Form 20-F contains no material misstatements or omissions; |
– |
based on their knowledge, the financial statements and other financial information fairly present, in all material respects, the financial condition, results of operations and cash flows as of the dates, and for the periods, presented in the annual report on Form 20-F; |
– |
they are responsible for establishing and maintaining disclosure controls and procedures that ensure that material information is made known to them, and have evaluated the effectiveness of these controls and procedures as at the year end, the results of such evaluation being contained in the annual report on Form 20-F; |
– |
they are responsible for establishing and maintaining internal control over financial reporting that provides reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; and |
– |
they have disclosed in the annual report on Form 20-F any changes in internal controls over financial reporting during the period covered by the annual report on Form 20-F that have materially affected, or are reasonably likely to affect materially, the company’s internal control over financial reporting, and they have disclosed, based on their most recent evaluation of internal control over financial reporting, to the external auditor and the ARC, all significant deficiencies and material weaknesses in the design or operation of internal controls over financial reporting which are reasonably likely to affect adversely the company’s ability to record, process, summarise and report financial information, and any fraud (regardless of materiality) involving persons that have a significant role in the company’s internal control over financial reporting. |
– |
management is responsible for establishing and maintaining adequate internal control over financial reporting for the Group. Internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with IFRS; |
– |
management conducted an evaluation of the effectiveness of internal control over financial reporting based on the framework, Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organisations of the Treadway Commission (COSO); |
– |
there have been no changes in the Group’s internal control over financial reporting during 2021 that have materially affected, or are reasonably likely to materially affect, the Group’s internal control over financial reporting; |
– |
management has assessed the effectiveness of internal control over financial reporting as at 31 December 2021 and its conclusion will be filed as part of the Group’s Form 20-F; and |
– |
Deloitte LLP, which has audited the consolidated financial statements of the Group for the year ended 31 December 2021, has also assessed the effectiveness of the Group’s internal control over financial reporting under Auditing Standard 2201 of the Public Company Accounting Oversight Board (United States). Their audit report will be filed with the Group’s Form 20-F. |

Name |
Security |
Registered address | ||
Wholly owned subsidiaries |
||||
1506369 Alberta ULC |
Common |
3500 855-2nd Street SW, Calgary AB T2P 4J8, Canada | ||
Action Potential Venture Capital Limited |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
Adechsa GmbH (ii) |
Ordinary |
c/o PRV Provides Treuhandgesellschaft AG, Dorfstrasse 38, 6341, Baar, Switzerland | ||
Allen & Hanburys Limited (ii) |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
Allen & Hanburys Pharmaceutical Nigeria Limited |
Ordinary |
24 Abimbola Way, Ilasamaja, Isolo, Lagos, Nigeria | ||
Allen Farmaceutica, S.A. |
Ordinary |
Severo Ochoa, 2, Parque Tecnológico de Madrid, Tres Cantos, 28760, Madrid, Spain | ||
Allen Pharmazeutika Gesellschaft m.b.H. |
Ordinary |
Wagenseilgasse 3, Euro Plaza, Gebäude 5i, 4.Stock, 1120, Vienna, Austria | ||
Beecham Group p.l.c |
5p Shares ‘B’; 20p Shares ‘A’ |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
Beecham Pharmaceuticals (Pte) Limited |
Ordinary |
38 Quality Road, Jurong Industrial Estate, Jurong, 618809, Singapore | ||
Beecham Portuguesa-Produtos Farmacêuticos e Químicos, Lda |
Ordinary Quota |
Rua Dr Antonio Loureiro Borges No 3, Arquiparque, Miraflores, 1495-131, Alges, Portugal | ||
Beecham S.A. (ii) |
Ordinary |
avenue Fleming 20, 1300 Wavre, Belgium | ||
Biovesta Ilaçlari Ltd. Sti. (ii) |
Nominative |
Büyükdere Caddesi No. 173, 1.Levent Plaza B Blok, 1.Levent, Istanbul, 34394, Turkey | ||
Cascan GmbH & Co. KG |
Partnership Capital |
Prinzregentenplatz 9, D-81675, Munich, Germany | ||
Castleton Investment Ltd (In Liquidation) |
Ordinary |
C/O DTOS, 19 Cybercity, 10th Floor Standard Chartered Tower, Ebene, Mauritius | ||
Cellzome GmbH |
Ordinary |
Meyerhofstrasse 1, 69117, Heidelberg, Germany | ||
Cellzome Limited (in liquidation since year end) |
Ordinary |
55 Baker Street, London, W1U 7EU, United Kingdom | ||
Charles Midgley Limited (in liquidation since year end) |
7% Cumulative Preference; Ordinary |
55 Baker Street, London, W1U 7EU, United Kingdom | ||
Clarges Pharmaceuticals Limited (in liquidation since year end) |
Ordinary; Preference (99.97%) |
55 Baker Street, London, W1U 7EU, United Kingdom | ||
Clarges Pharmaceutical Trustees Limited (ii) (iv) |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
Colleen Corporation |
Common |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | ||
Corixa Corporation |
Common |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | ||
Dealcyber Limited |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
Desarrollo Energía Solar Alternativa S.L. |
Ordinary |
Severo Ochoa, 2, Parque Tecnológico de Madrid, Tres Cantos, 28760, Madrid, Spain | ||
Duncan Flockhart Australia Pty Limited (ii) (iv) |
Ordinary |
1061 Mountain Highway, Boronia Victoria VIC 3155, Australia | ||
Duncan Pharmaceuticals Philippines Inc. |
Common |
23rd Floor, The Finance Centre, 26th Street Corner 9th Avenue, Bonifacio Global City, Taguig City, 1634, Philippines | ||
Etex Farmaceutica Ltda |
Social Capital |
Avenue Andres Bello 2687, Piso 19, Las Condes, Santiago, C.P. 7550611, Chile | ||
Genelabs Technologies, Inc. |
Common |
Corporation Service Company, 2710 Gateway Oaks Drive, Suite 150N, Sacramento CA 95833, United States | ||
Glaxo Group Limited |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
Glaxo Kabushiki Kaisha (ii) |
Ordinary |
1-8-1 Minato-ku, Tokyo, Japan | ||
Glaxo Laboratories (Nigeria) Limited (ii) |
Ordinary |
82 Marine Road, Apapa, Lagos, Nigeria | ||
Glaxo Laboratories Limited (In Liquidation) |
Ordinary |
55 Baker Street, London, W1U 7EU, United Kingdom | ||
Glaxo New Zealand Pension Plan Trustee Limited |
Ordinary |
Level 2 E.2, Generator at GridAKL, 12 Madden Street, Wynyard Quarter, Auckland, 1010, New Zealand | ||
Glaxo Operations UK Limited |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
Glaxo Properties BV |
Ordinary |
Van Asch van Wijckstraat 55h, 3811 LP, Amersfoort, Netherlands | ||
Glaxo Trustees Limited (ii) (in liquidation) |
Ordinary |
55 Baker Street, London, W1U 7EU, United Kingdom | ||
Glaxo Verwaltungs GmbH |
Ordinary |
Prinzregentenplatz 9, D-81675, Munich, Germany | ||
Glaxo Wellcome Australia Pty Ltd (ii) (iv) |
Ordinary |
1061 Mountain Highway, Boronia Victoria VIC 3155, Australia | ||
Glaxo Wellcome Farmacêutica, Limitada |
Ordinary Quota |
Rua Dr Antonio Loureiro Borges No 3, Arquiparque, Miraflores, 1495-131, Alges, Portugal | ||
Glaxo Wellcome International B.V. (ii) (iii) |
Ordinary |
Huis ter Heideweg 62, 3705 LZ, Zeist, Netherlands | ||
Glaxo Wellcome Manufacturing Pte Ltd |
Ordinary |
1 Pioneer Sector 1, Jurong Industrial Estate, Jurong, 628413, Singapore | ||
Glaxo Wellcome Production |
Ordinary |
23 rue François Jacob, 92500, Rueil-Malmaison, France | ||
Glaxo Wellcome Vidhyasom Limited (ii) |
Ordinary |
12th Floor Wave Place, 55 Wireless Road, Lumpini, Pathumwan, Bangkok, 10330, Thailand | ||

Name |
Security |
Registered address | ||
Wholly owned subsidiaries |
||||
Glaxo Wellcome, S.A. |
Ordinary |
Poligono Industrial Allendeduero, Avenida de Extremadura, 3, Aranda de Duero, 09400, Burgos, Spain | ||
Glaxo, S.A. |
Ordinary |
Severo Ochoa, 2, Parque Tecnologico de Madrid, Tres Cantos, 28760, Madrid, Spain | ||
Glaxo-Allenburys (Nigeria) Limited (ii) |
Ordinary |
41 Creek Road, Apapa, Lagos, PMB 1401, Nigeria | ||
Glaxochem Pte Ltd (iii) |
Ordinary |
23 Rochester Park, 139234, Singapore | ||
GlaxoSmithKline - Produtos Farmacêuticos, Limitada |
Ordinary Quota |
Rua Dr Antonio Loureiro Borges No 3, Arquiparque, Miraflores, 1495-131, Alges, Portugal | ||
GlaxoSmithKline (Cambodia) Co., Ltd. (In Liquidation) |
Ordinary |
5th Floor DKSH Building, No.797 Preah Monivong Boulevard (Co, Sangkat Phsar Deum Thakov, Khan Chamkarmon, Phnom Penh, Cambodia | ||
GlaxoSmithKline (China) Investment Co Ltd |
Ordinary |
Room 901, 902, 903, 905, 908, 909 and 910, Unit 901, Floor 9, No. 56 Mid 4th East Ring Road, Chaoyang District, Beijing, China | ||
GlaxoSmithKline (China) R&D Company Limited |
Equity |
F1-3, No.18 Building, 999 Huanke Road, Pilot Free Trade Zone, Shanghai, 201210, China | ||
GlaxoSmithKline (Cyprus) Limited |
Ordinary |
Arch. Makariou III, 2-4, Capital Center, 9th Floor, Nicosia, P.C. 1065, Cyprus | ||
GlaxoSmithKline (GSK) S.R.L. |
Ordinary |
1-5 Costache Negri Street, Opera Center One, 5th and 6th floors, Zone 1, District 5, Bucharest, Romania | ||
GlaxoSmithKline (Ireland) Limited |
Ordinary |
12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland | ||
GlaxoSmithKline (Israel) Ltd |
Ordinary |
25 Basel Street, PO Box 10283, Petach-Tikva, 49002, Israel | ||
GlaxoSmithKline (Malta) Limited |
Ordinary |
1, First Floor, De La Cruz Avenue, Qormi, QRM2458, Malta | ||
GlaxoSmithKline (Private) Limited (ii) |
Ordinary |
Unit 3, 20 Anthony Road, Msasa, Harare, Zimbabwe | ||
GlaxoSmithKline (Thailand) Limited |
Ordinary |
12th Floor Wave Place, 55 Wireless Road, Lumpini, Pathumwan, Bangkok, 10330, Thailand | ||
GlaxoSmithKline AB |
Ordinary |
Hemvarnsg. 9, 171 54, Solna, Sweden | ||
GlaxoSmithKline AG |
Ordinary |
Talstrasse 3-5, 3053 Muenchenbuchsee, Switzerland | ||
GlaxoSmithKline Angola Unipessoal Limitada (iv) |
Quota |
Luanda, Bairro Petrangol, Estrada de Cacuaco n ° 288, Angola | ||
GlaxoSmithKline Argentina S.A. |
Ordinary |
Tucumán 1, piso 4, Buenos Aires, C1049AAA, Argentina | ||
GlaxoSmithKline AS |
Ordinary |
Drammensveien 288, Oslo, NO-0283, Norway | ||
GlaxoSmithKline Asia Private Limited |
Equity |
Patiala Road, Nabha 147201, Dist Patiala, Punjab, India | ||
GlaxoSmithKline Australia Pty Ltd |
Ordinary |
1061 Mountain Highway, Boronia Victoria VIC 3155, Australia | ||
GlaxoSmithKline B.V. |
Ordinary |
Van Asch van, Wijckstraat 55h, 3811 LP Amersfoort, The Netherlands, Netherlands | ||
GlaxoSmithKline Beteiligungs GmbH |
Ordinary |
Prinzregentenplatz 9, 81675, Munchen, Germany | ||
GlaxoSmithKline Biologicals (Shanghai) Ltd. |
Ordinary |
277 Niudun Road, Pilot Free Trade Zone, Shanhai, China | ||
GlaxoSmithKline Biologicals Kft. |
Ordinary |
2100 Gödöllõ, Homoki Nagy István utca 1, Hungary | ||
GlaxoSmithKline Biologicals S.A.S. |
Ordinary |
637 Rue des Aulnois, Saint-Amand Les Eaux, 59230, France | ||
GlaxoSmithKline Biologicals SA |
Ordinary: Preference |
Rue de l’Institut 89 B-1330 Rixensart, Belgium | ||
GlaxoSmithKline Brasil Limitada |
Quotas |
Estrada dos Banderiantes, 8464, Rio de Janeiro, 22783-110, Brazil | ||
GlaxoSmithKline Capital Inc. |
Common |
Wilmington Trust SP Services Inc., 1105 North Market Street, Suite 1300, Wilmington DE 19801, United States | ||
GlaxoSmithKline Capital plc |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GlaxoSmithKline Caribbean Limited |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GlaxoSmithKline Chile Farmaceutica Limitada |
Social Capital |
Avenue Andres Bello No. 2687, Piso 19, Las Condes, Santiago, C.P. 7550611, Chile | ||
GlaxoSmithKline Colombia S.A. |
Ordinary |
Avenida El Dorado, #69B-45/Piso 9, Bogotá, Colombia | ||
GlaxoSmithKline Consumer Healthcare Holdings Limited (i) |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GlaxoSmithKline Consumer Holding B.V. (ii) |
Ordinary |
Van Asch van Wijckstraat 55h, 3811 LP, Amersfoort, Netherlands | ||
GlaxoSmithKline d.o.o. |
Quotas |
Zmja od Bosne broj 7-7a, Sarajevo, 71000, Bosnia and Herzegovina | ||
GlaxoSmithKline d.o.o. |
Equity Capital |
Ulica Damira Tomljanovica Gavrana 15, Zagreb, Croatia | ||
GlaxoSmithKline doo Beograd |
Ordinary |
Omladinskih brigada 88, New Belgrade, City of Belgrade, 11070, Serbia | ||
GlaxoSmithKline Ecuador S.A. |
Ordinary |
Av 10 De Agosto N36-239, y Naciones Unidas, Edificio Electroectuatoriana, 2do piso, Quito, Ecuador | ||
GlaxoSmithKline Eesti OU |
Ordinary |
Lõõtsa 8a, Tallinn, 11415, Estonia | ||
GlaxoSmithKline El Salvador S.A. de C.V. |
Ordinary |
Municipio de San Salvador, Departamento de San Salvador, El Salvador | ||
GlaxoSmithKline EOOD |
Ordinary |
115 G Tsarigradsko Shose Blvd., floor 9, Mladost Region, Sofia, 1784, Bulgaria | ||
GlaxoSmithKline Export Limited |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GlaxoSmithKline Export Panama S.A. |
Ordinary |
Panama City, Republic of Panama, Panama | ||
GlaxoSmithKline Far East B.V. |
Ordinary |
Van Asch van Wijckstraat 55h, 3811 LP, Amersfoort, Netherlands | ||
GlaxoSmithKline Finance plc |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GlaxoSmithKline GmbH & Co. KG |
Partnership Capital |
Prinzregentenplatz 9, 81675, Munchen, Germany | ||
GlaxoSmithKline Guatemala S.A. |
Ordinary |
3ra. Av. 13-78 Zona 10, Torre Citibank, Nivel 8, Guatemala City, Guatemala | ||
| Name |
Security |
Registered address | ||
Wholly owned subsidiaries |
||||
GlaxoSmithKline Holding AS |
Ordinary |
Drammensveien 288, Oslo, NO-0283, Norway | ||
GlaxoSmithKline Holdings (Americas) Inc. |
Common |
Wilmington Trust SP Services Inc., 1105 North Market Street, Suite 1300, Wilmington DE 19801, United States | ||
GlaxoSmithKline Holdings (One) Limited (i) |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GlaxoSmithKline Holdings Limited (i) |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GlaxoSmithKline Holdings Pty Ltd |
Ordinary |
1061 Mountain Highway, Boronia Victoria VIC 3155, Australia | ||
GlaxoSmithKline Honduras S.A. |
Ordinary |
Tegucigalpa, MDC, Honduras | ||
GlaxoSmithKline IHC Limited |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GlaxoSmithKline Ilaclari Sanayi ve Ticaret A.S. |
Nominative |
Büyükdere Caddesi No. 173, 1.Levent Plaza B Blok, 1.Levent, Istanbul, 34394, Turkey | ||
GlaxoSmithKline Inc. |
Class A Common; Class C Preference |
7333 Mississauga Road North, Mississauga Ontario L5N 6L4, Canada | ||
GlaxoSmithKline Insurance Ltd |
Ordinary |
19 Par-La-Ville | ||
GlaxoSmithKline Intellectual Property (No.2) Limited |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GlaxoSmithKline Intellectual Property Development Limited |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GlaxoSmithKline Intellectual Property Holdings Limited |
A Ordinary; B Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GlaxoSmithKline Intellectual Property Limited |
Deferred; Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GlaxoSmithKline Intellectual Property Management Limited |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GlaxoSmithKline Investigación y Desarrollo, S.L. |
Ordinary |
Severo Ochoa 2 Parque Tecnológico de Madrid, Tres Cantos, 28760, Madrid, Spain | ||
GlaxoSmithKline Investment Holdings Limited (In Liquidation) |
Ordinary |
55 Baker Street, London, W1U 7EU, United Kingdom | ||
GlaxoSmithKline Investment Services Limited (In Liquidation) |
Ordinary |
55 Baker Street, London, W1U 7EU, United Kingdom | ||
GlaxoSmithKline Investments Pty Ltd |
Ordinary |
1061 Mountain Highway, Boronia Victoria VIC 3155, Australia | ||
GlaxoSmithKline K.K. |
Ordinary |
1-8-1 Minato-ku, Tokyo, Japan | ||
GlaxoSmithKline Korea Limited |
Ordinary |
9F LS Yongsan Tower, 92 Hangang-daero, Yongsan-gu, Seoul, 04386, Korea, Republic of | ||
GlaxoSmithKline Latin America, S.A. |
Ordinary |
Panama City, Republic of Panama, Panama | ||
GlaxoSmithKline Latvia SIA |
Ordinary |
Duntes iela 3, Riga, Latvia | ||
GlaxoSmithKline Lietuva UAB |
Ordinary |
Ukmerges st. 120, Vilnius, LT-08105, Lithuania | ||
GlaxoSmithKline Limited |
Ordinary |
23/F., Tower 6, The Gateway, 9 Canton Road, Tsimshatsui, Kowloon, Hong Kong | ||
GlaxoSmithKline LLC |
LLC Interests |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | ||
GlaxoSmithKline Manufacturing SpA |
Ordinary |
Viale dell’Agricoltura 7, 37135, Verona, Italy | ||
GlaxoSmithKline Maroc S.A. |
Ordinary |
42-44 Angle Bd, Rachidi et Abou Hamed El Glaza, Casablanca, Morocco | ||
GlaxoSmithKline Medical and Healthcare Products Limited |
Ordinary |
H-1124, Csorsz utca 43, Budapest, Hungary | ||
GlaxoSmithKline Mercury Limited (i) |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GlaxoSmithKline Mexico S.A. de C.V. |
Ordinary A: Ordinary B |
Calzada, Mexico-Xochimilco 4900, Colonia San Lorenzo, Huipulco, Delegacion Tlalpan, 14370, Mexico | ||
GlaxoSmithKline NZ Limited |
Ordinary |
Level 2 E.2, Generator @GridAKL, 12 Madden Street, Wynyard Quarter, Auckland, 1010, New Zealand | ||
GlaxoSmithKline Oy |
Ordinary |
Piispansilta 9A, P.O. Box 24, Espoo, FIN-02230, Finland | ||
GlaxoSmithKline Peru S.A. |
Ordinary |
Av. Javier Prado Oeste, 995, San Isidro, LIMA 27, Peru | ||
GlaxoSmithKline Pharma A/S |
Ordinary |
Nykaer 68, DK-2605, Brondby, Denmark | ||
GlaxoSmithKline Pharma GmbH |
Ordinary |
Wagenseilgasse 3, Euro Plaza, Gebäude 5i, 4.Stock, Wien, 1120 | ||
GlaxoSmithKline Pharmaceutical Kenya Limited |
Ordinary |
Likoni Road, Nairobi, 78392 - 00507, Kenya | ||
GlaxoSmithKline Pharmaceutical Nigeria Limited |
Ordinary |
1 Industrial Avenue, Ilupeju, Ikeja, Lagos, PM B 21218, Nigeria | ||
GlaxoSmithKline Pharmaceutical Sdn Bhd |
Ordinary |
Level 6, Quill 9, 112 Jalan Prof. Khoo Kay Kim, Petaling Jaya, 46300 Selangor, Malaysia | ||
GlaxoSmithKline Pharmaceuticals (Pvt) Ltd |
Ordinary |
121 Galle Road, Kaldemulla, Moratuwa, Sri Lanka | ||
GlaxoSmithKline Pharmaceuticals Costa Rica S.A. |
Ordinary |
Autopista Florencia del Castillo, kilómetro siete, Oficentro TerraCampus, edificio uno, cuarto piso, San Diego, Cartago, 30302, Costa Rica | ||
GlaxoSmithKline Pharmaceuticals S.A. |
Ordinary A; Ordinary B; Ordinary C; Ordinary D |
Ul. Grunwaldzka 189, 60-322, Poznan, Poland | ||
GlaxoSmithKline Pharmaceuticals S.A. |
Ordinary |
Site Apollo, Avenue Pascal 2-4-6, | ||
GlaxoSmithKline Pharmaceuticals Ukraine LLC |
Chartered Capital |
Pavla Tychyny avenue, 1-V, Kiev, 02152, Ukraine | ||
GlaxoSmithKline Philippines Inc |
Ordinary |
23rd Floor, The Finance Centre, 26th Street Corner 9th Avenue, Bonifacio Global City, Taguig City, 1634, Philippines | ||
GlaxoSmithKline Pte Ltd |
Ordinary |
23 Rochester Park, 139234, Singapore | ||
GlaxoSmithKline Puerto Rico, Inc. |
Common |
The Prentice-Hall Corporation System, Puerto Rico, Inc, c/o Fast Solutions, LLC, 252 Ponce de Leon Avenue, Floor 20, San Juan, 00918, Puerto Rico | ||

Name |
Security |
Registered address | ||
Wholly owned subsidiaries |
||||
GlaxoSmithKline Republica Dominicana S.A. |
Ordinary |
Blue Mall Tower, Floor 23 Ave., Winston Churchill 95, Santo Domingo, Dominican Republic | ||
GlaxoSmithKline Research & Development Limited |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GlaxoSmithKline S.A. |
Ordinary |
Severo Ochoa, 2, Parque Tecnológico de Madrid, Tres Cantos, 28760, Madrid, Spain | ||
GlaxoSmithKline S.p.A. |
Ordinary |
Viale dell’Agricoltura 7, 37135, Verona, Italy | ||
GlaxoSmithKline s.r.o. |
Ordinary |
Hvezdova 1734/2c, Prague, 4 140 00, Czech Republic | ||
GlaxoSmithKline Services GmbH & Co. KG |
Partnership Capital |
Prinzregentenplatz 9, 81675, Munchen, Germany | ||
GlaxoSmithKline Services Unlimited (i) |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GlaxoSmithKline Single Member A.E.B.E. |
Ordinary |
266 Kifissias Avenue, Halandri, Athens, 152 32, Greece | ||
GlaxoSmithKline SL LLC |
LLC Interests |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | ||
GlaxoSmithKline SL LP (ii) (viii) |
Partnership |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GlaxoSmithKline Slovakia s.r.o. |
Ordinary |
Galvaniho 7/A, Bratislava, 821 04, Slovakia | ||
GlaxoSmithKline South Africa (Pty) Limited |
Ordinary |
Flushing Meadows Building, The Campus, 57 Sloane Street, Bryanston 2021, South Africa | ||
GlaxoSmithKline Trading Services Limited (iii) |
Ordinary |
12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland | ||
GlaxoSmithKline Tunisia S.A.R.L. |
Ordinary |
Immeuble REGUS, Lot B17, Centre Urbain Nord, Tunis, Tunisia | ||
GlaxoSmithKline UK Limited |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GlaxoSmithKline Uruguay S.A. |
Registered Provisory Stock |
Salto 1105, CP 11.200 Montevideo, Uruguay | ||
GlaxoSmithKline US Trading Limited |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GlaxoSmithKline Venezuela C.A. |
Ordinary |
Urbanizacion La Trinidad, Calle luis De Camoems, Edif No 115-117 Apatado Posta, Caracas, 1010, Venezuela, Bolivarian Republic of | ||
GlaxoSmithKline Vietnam Limited Liability Company (ii) (iv) |
Equity Capital |
The Metropolitan, 235 Dong Khoi Street, District 1, 7th Floor Unit 701, Ho Chi Minh City, Vietnam | ||
GlycoVaxyn AG (iv) |
Common; Preferred A; Preferred B; Preferred C |
Grabenstrasse 3, 8952 Schlieren, Switzerland | ||
Groupe GlaxoSmithKline |
Ordinary |
23 rue François Jacob, 92500, Rueil-Malmaison, France | ||
GSK Australia NVD Pty Ltd (ii) (iv) |
Ordinary |
1061 Mountain Highway, Boronia Victoria VIC 3155, Australia | ||
GSK Bangladesh Private Limited |
Ordinary |
Sweden Tower, 1, Harinnachala, Konabari, Gazipur, Bangladesh | ||
GSK Biopharma Argentina S.A. |
Nominative Non Endorseable Ordinary |
Tucumán 1, piso 4, Buenos Aires, C1049AAA, Argentina | ||
GSK Business Service Centre Sdn Bhd |
Ordinary |
Level 6, Quill 9, 112 Jalan Prof. Khoo Kay Kim, Petaling Jaya,, 46300 Selangor, Malaysia | ||
GSK Capital B.V. (iii) (v) |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GSK Capital K.K. |
Ordinary |
1-8-1 Minato-ku, Tokyo, Japan | ||
GSK Commercial Sp. z o.o. |
Ordinary |
ul. Rzymowskiego 53, 02-697, Warsaw, Poland | ||
GSK d.o.o., Ljubljana |
Ordinary |
Ameriška ulica 8,, Ljubljana, 1000, Slovenia | ||
GSK Enterprise Management Co, Ltd |
Ordinary |
Floor 4, 18 Lane 999 Huanke Road, No. 1358 Zhongke Road, Shanghai, China | ||
GSK Equity Investments, Limited |
Units |
Corporation Service Company, 2595 Interstate Drive, Suite 103, Harrisburg PA 17110, United States | ||
GSK Finance (No 2) Limited |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GSK Finance (No.3) plc |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GSK India Global Services Private Limited |
Equity |
Level 1, 2 & 3 Luxor North Tower, Bagmane Capital Business Park Outer Ring Road, Bangalore, Karnataka, 560037, India | ||
GSK International Holding and Finance BV |
Ordinary |
Van Asch van Wijckstraat 55h, 3811 LP, Amersfoort, Netherlands | ||
GSK Kazakhstan LLP |
Participation Interest |
273, Furmanov Street, Almaty, Medeu District, 050059, Kazakhstan | ||
GSK Limited |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
GSK Pharma Vietnam Company Limited |
Chartered Capital |
Unit 702/703 7th Floor, The Metropolitan Tower, 235 Dong Khoi Street, Ben Nghe Ward, District 1, Ho Chi Minh, Vietnam | ||
GSK Pharmaceutical Trading S.A. (ii) (iv) |
Ordinary |
Bucharest, 1-5 Costache Negri Street, Opera Center One, 5th floor, discussions room 01, District 5, Romania | ||
GSK PSC Poland sp. z o.o. |
Equal and indivisible shares |
ul. Grunwaldzka 189, Poznań, 60-322, Pol | ||
GSK Services Sp z o.o. |
Ordinary |
Ul. Grunwaldzka 189, 60-322, Poznan, Poland | ||
GSK Vaccines BV |
Ordinary |
Hullenbergweg 85, 1101 CL, Amsterdam, Netherlands | ||
GSK Vaccines GmbH |
Ordinary |
Emil-von-Behring-Str.76, | ||
GSK Vaccines Institute for Global Health S.r.l. |
Quotas |
Via Fiorentina 1, 53100, Siena, Italy | ||
GSK Vaccines S.r.l. |
Quotas |
Via Fiorentina 1, 53100, Siena, Italy | ||
GSK Vaccines Vertriebs GmbH (ii) |
Ordinary |
Rudolf-Diesel-Ring 27, 83607, Holzkirchen, Germany | ||
HGS France S.a.r.l. (ii) (iv) |
Ordinary |
52-54, Rue de la Belle Feuille, Boulogne-Billancourt, 92100, France | ||
Human Genome Sciences, Inc. |
Common |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | ||
ID Biomedical Corporation of Quebec |
Common |
2323, boul. Du Parc Technologique, Québec Québec G1P 4R8, Canada | ||
Name |
Security |
Registered address | ||
Wholly owned subsidiaries |
||||
Instituto Luso Farmaco, Limitada (ii) |
Quotas |
Rua Dr Antonio Loureiro Borges No 3, Arquiparque, Miraflores, 1495-131, Algés, Portugal | ||
InterPharma Dienstleistungen GmbH (ii) |
Quotas |
Wagenseilgasse 3, Euro Plaza, Gebäude I, 4. Stock, A-1120, Vienna, Austria | ||
J&J Technologies, LC |
LLC Interests |
Corporation Service Company, 100 Shockoe Slip, 2nd Floor, Richmond VA 23219,, United States | ||
JSC GlaxoSmithKline Trading |
Ordinary |
Leningradskiy Prospect 37A, Building 4, Floor 3, Premises XV, Room 1, 125167, Moscow, Russian Federation | ||
Laboratoire GlaxoSmithKline |
Ordinary |
23 rue François Jacob, 92500, Rueil-Malmaison, France | ||
Laboratoire Pharmaceutique Algérien LPA Production SPA |
Ordinary |
Zone Industrielle Est, Boudouaou, Boumerdes, Algeria | ||
Laboratoire Pharmaceutique Algérien SPA |
Ordinary |
Zone Industrielle Est, Boudouaou, Boumerdes, Algeria | ||
Laboratoires Paucourt (ii) |
Ordinary |
23 rue François Jacob, 92500, Rueil-Malmaison, France | ||
Laboratoires Saint-Germain (ii) |
Ordinary |
23 rue François Jacob, 92500, Rueil-Malmaison, France | ||
Laboratorios Dermatologicos Darier, S.A de C.V. |
Ordinary A; Ordinary B |
Calzada Mexico Xochimilco, 4900 San Lorenzo Huipulco, District Federal Mexico, 14370, Mexico | ||
Laboratórios Farmaceuticos Stiefel (Portugal) LTDA (ii) |
Ordinary |
Rua Dr Antonio Loureiro Borges No 3, Arquiparque, Miraflores, 1495-131, Algés, Portugal | ||
Laboratorios Stiefel de Venezuela SA |
Ordinary |
Calle Luis de Camoens, Edificio GlaxoSmithKline, No. 115-117, Urb. La Trinidad, Caracas, Venezuela, Bolivarian Republic of | ||
Laboratorios Stiefel Ltda. |
Ordinary |
Rua Professor Joao Cavalheiro Salem, no.1077, Bairro de Bonsucesso, Municipality of Guarulhos, Sao Paulo, CEP 07243-580, Brazil | ||
Laboratorios Wellcome De Portugal Limitada (ii) |
Quota |
Rua Dr Antonio Loureiro Borges No 3, Arquiparque, Miraflores, 1495-131, Alges, Portugal | ||
Mixis Genetics Limited (In Liquidation) |
Ordinary |
BDO LLP, 5 Temple Square Temple Street, Liverpool, L2 5RH | ||
Montrose Pharma Company Limited (ii) (iv) |
Ordinary Quota |
H-1124, Csorsz utca 43, Budapest, Hungary | ||
Penn Labs Inc. (ii) |
Common |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | ||
Setfirst Limited |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
Sitari Pharma, Inc. |
Common Stock |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | ||
Smith Kline & French Portuguesa-Produtos Farmaceuticos, LDA (ii) |
Ordinary |
Rua Dr Antonio Loureiro Borges No 3, Arquiparque, Miraflores, 1495-131, Alges, Portugal | ||
SmithKline Beecham (Bangladesh) Private Limited (ii) |
Ordinary |
House-2/A, Road-138,Gulshan-1, | ||
SmithKline Beecham (Cork) Limited |
Ordinary |
12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland | ||
SmithKline Beecham (Manufacturing) Limited (In Liquidation) |
Ordinary |
BDO, Beax Lane House, Mercer Street, Lower, D02 DH60, Dublin, D02 DH60, Ireland | ||
SmithKline Beecham (SWG) Limited (In Liquidation) |
Ordinary |
BDO LLP, 5 Temple Square Temple Street, Liverpool, L2 5RH | ||
SmithKline Beecham Egypt L.L.C. |
Quotas |
Amoun Street, El Salam City, Cairo, Egypt | ||
SmithKline Beecham Farma, S.A. |
Ordinary |
Severo Ochoa, 2, Parque Tecnologico de Madrid, Tres Cantos, 28760, Madrid, Spain | ||
SmithKline Beecham Limited |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
SmithKline Beecham Pension Plan Trustee Limited (ii) |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
SmithKline Beecham Pension Trustees Limited (In Liquidation) |
Ordinary |
55 Baker Street, London, W1U 7EU, United Kingdom | ||
SmithKline Beecham Pharma GmbH & Co KG |
Partnership Capital |
Prinzregentenplatz 9, 81675, Munchen, Germany | ||
SmithKline Beecham Pharma Verwaltungs GmbH |
Ordinary |
Prinzregentenplatz 9, 81675, Munchen, Germany | ||
SmithKline Beecham Pharmaceuticals (Pty) Limited (ii) (iv) |
Ordinary |
Flushing Meadows Building, The Campus, 57 Sloane Street, Bryanston 2021, South Africa | ||
SmithKline Beecham Pharmaceuticals Co. |
Common |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | ||
SmithKline Beecham Port Louis Limited (In Liquidation) |
Ordinary |
C/o CIM Corporate Services Ltd, Les Cascades Building, Edith Cavell Street, Port Louis, Mauritius | ||
SmithKline Beecham Senior Executive Pension Plan Trustee Limited (ii) |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||
Stiefel Dominicana, S.R.L. (ii) (iv) |
Ordinary |
Ave. Lope de Vega #29, Torre NovoCentro, Local 406, Santo Domingo, Dominican Republic | ||
Stiefel Farma, S.A. |
Ordinary |
Severo Ochoa, 2, Parque Tecnologico de Madrid, Tres Cantos, 28760, Madrid, Spain | ||
Stiefel GmbH & Co. KG |
Partnership Capital |
Prinzregentenplatz 9, 81675, Munchen, Germany | ||
Stiefel India Private Limited |
Equity |
1, Battery House, Bhulabhai Desai Raod, Mumbai, Maharashtra, 400026, India | ||
Stiefel Laboratories (Maidenhead) Ltd (In Liquidation) |
Ordinary |
BDO LLP, 5 Temple Square Temple Street, Liverpool, L2 5RH | ||
Stiefel Laboratories Legacy (Ireland) Limited |
Ordinary |
Unit 2 Building 2500, Avenue 2000 Cork Airport Business Park, Cork, Ireland | ||
Stiefel Laboratories Limited (in liquidation since year end) |
Ordinary |
55 Baker Street, London, W1U 7EU, United Kingdom | ||
Stiefel Laboratories Pte Limited |
Ordinary |
1 Pioneer Sector, 628413, Singapore | ||

Name |
Security |
Registered address | ||||
Wholly owned subsidiaries |
||||||
Stiefel Laboratories, Inc. |
Common |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | ||||
Stiefel Maroc SARL (ii) (iv) |
Ordinary |
275 Boulevard Zerktouni, Casablanca, Morocco | ||||
Stiefel Research (Australia) Holdings Pty Ltd |
Ordinary |
1061 Mountain Highway, Boronia Victoria VIC 3155, Australia | ||||
Stiefel Research Australia Pty Ltd |
Ordinary |
1061 Mountain Highway, Boronia Victoria VIC 3155, Australia | ||||
Stiefel West Coast LLC |
LLC Interests |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | ||||
Strebor Inc. |
Common |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | ||||
Tesaro Bio GmbH (In Liquidation) |
Ordinary |
Poststrasse 6, 6300 Zug, Switzerland | ||||
Tesaro Bio Netherlands B.V |
Ordinary |
Joop Geesinkweg 901, 1114 AB, Amsterdam-Duivendrecht, Netherlands | ||||
Tesaro Bio Sweden AB |
Common |
c/o BDO Malardalen AB, Skatt Box 24193, 104 51, Stockholm, Sweden | ||||
Tesaro Development, Ltd. |
Ordinary |
Clarendon House, 2 Church Street, Hamilton HM11, Bermuda | ||||
Tesaro, Inc. |
Common |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | ||||
The Sydney Ross Co. (ii) |
Ordinary |
Corporation Service Company, Princeton South Corporate Center, Suite 160, 100 Charles Ewing Blvd, Ewing NJ 08628, United States | ||||
UCB Pharma Asia Pacific Sdn Bhd (ii) |
Ordinary |
12th Floor, Menara Symphony, No. 5, Jalan Prof. Khoo Kay Kim,, Seksyen 13, 46200 Petaling Jaya, Malaysia | ||||
Wellcome Consumer Healthcare Limited (ii) |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||||
| Wellcome Consumer Products Limited (in liquidation since year end) |
Ordinary |
BDO LLP, 5 Temple Square Temple Street, Liverpool, L2 5RH | ||||
Wellcome Developments Pty Ltd (ii) (iv) |
Ordinary |
1061 Mountain Highway, Boronia Victoria VIC 3155, Australia | ||||
Wellcome Limited |
Ordinary |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | ||||
Wellcome Operations Pty Ltd (ii) (iv) |
Ordinary |
1061 Mountain Highway, Boronia Victoria VIC 3155, Australia | ||||
Name |
Security |
Effective % Ownership |
Registered address | |||
Subsidiaries where the effective interest is less than 100% | ||||||
Alacer Corp. |
Common |
68 |
Corporate Service Company d/b/a CSC - Lawyers Incorporating , Service, 2710 Gateway Oaks Drive, Suite 150N , Sacramento, California 95833-3505, United States | |||
Amoun Pharmaceutical Industries Co. S.A.E. |
New Monetary Shares (99.5%) |
90.7 |
El Salam City 11491, PO Box 3001, Cairo, Egypt | |||
Beecham Enterprises Inc. (ii) |
Common |
59.8 |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | |||
Biddle Sawyer Limited |
Equity |
68 |
252 Dr Annie Besant Road, Mumbai, 400030, India | |||
Block Drug Company, Inc. |
Common |
68 |
Corporation Service Company, Princeton South Corporate Center, Suite 160, 100 Charles Ewing Blvd, Ewing NJ 08628, United States | |||
Block Drug Corporation (ii) |
Common |
68 |
Corporation Service Company, Princeton South Corporate Center, Suite 160, 100 Charles Ewing Blvd, Ewing NJ 08628, United States | |||
British Pharma Group Limited (i) |
Capital (50%) |
50 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
Consumer Healthcare Holdings Limited |
Ordinary |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
Consumer Healthcare Intermediate Holdings Limited |
Ordinary |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
Duncan Consumer Healthcare Philippines Inc |
Common |
68 |
23rd Floor, The Finance Centre, 26th Street Corner 9th Avenue, Bonifacio Global City, Taguig City, 1634, Philippines | |||
Ex-Lax, Inc. |
Common |
68 |
The Prentice Hall Corporation System, Puerto Rico, Inc., c/o, Citi Tower, 252 Ponce de Leon Avenue, Floor 20, San Juan, 00918, Puerto Rico | |||
Ferrosan ApS |
A Shares; B Shares |
68 |
Delta Park 37, 2665, Vallensbæk Strand, Denmark | |||
Ferrosan International ApS |
Ordinary |
68 |
Delta Park 37, 2665, Vallensbæk Strand, Denmark | |||
Ferrosan S.R.L. |
Registered Capital |
68 |
178/C Calea Turzii, Cluj-Napoca, Cluj County, Romania | |||
Galvani Bioelectronics Inc. |
Common |
55 |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | |||
Galvani Bioelectronics Limited |
A Ordinary; B Ordinary (0%) |
55 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
Glaxo Saudi Arabia Limited |
Ordinary |
75 |
PO Box 22617, Area No 56 to 73, Warehouse City, First Stage Al Khomrah, Jeddah 21416, Saudi Arabia | |||
Glaxo Wellcome Ceylon Limited |
Ordinary; Ordinary B |
67.8 |
121 Galle Road, Kaldemulla, Moratuwa, Sri Lanka | |||
GlaxoSmithKline (Suzhou) Trading Co., Ltd |
Registered Capital |
68 |
No.699 Gangpu Road, Wusongjiang Science and Technology Industrial Park, Wuzhong Economic & Technical Development Zone, Suzhou, China | |||
GlaxoSmithKline (Tianjin) Co. Ltd |
Ordinary |
90 |
No. 65, the Fifth Avenue, Tai Feng Industrial Park, Tianjin Economic and Technolog, Tianjin, 300457, China | |||
GlaxoSmithKline Algérie S.P.A. |
Ordinary |
99.99 |
Zone Industrielle Est, Boudouaou, Wilaya de Boumerdes, Algeria | |||
GlaxoSmithKline Brasil Produtos para Consumo e Saude Ltda |
Quotas |
68 |
Av das Americas, 3500, 4th floor, rooms 407-420, , Rio de Janeiro, RJ, 22621-000, Brazil | |||
Name |
Security |
Effective % Ownership |
Registered address | |||
Subsidiaries where the effective interest is less than 100% | ||||||
GlaxoSmithKline Consumer Healthcare (China) Co. Ltd |
Ordinary |
68 |
Room 506, No. 1 Shen’gang Boulevard, Lin-gang Special Area of China Pilot Free Trade Z, Shanghai, Shanghai, 200000, China | |||
GlaxoSmithKline Consumer Healthcare (Hong Kong) Limited |
Ordinary |
68 |
23/F., Tower 6, The Gateway, 9 Canton Road, Tsimshatsui, Kowloon, Hong Kong | |||
GlaxoSmithKline Consumer Healthcare (Ireland) Limited |
Ordinary |
68 |
12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland | |||
GlaxoSmithKline Consumer Healthcare (Overseas) Limited |
Ordinary |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
GlaxoSmithKline Consumer Healthcare (Thailand) Limited |
Ordinary |
68 |
13th Floor, Unit 13.05 and 13.06 Wave Place, 55 Wireless Road, Lumpini, Pathumwan, Bangkok, 10330, Thailand | |||
GlaxoSmithKline Consumer Healthcare (UK) IP Limited (iv) |
Ordinary |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
GlaxoSmithKline Consumer Healthcare (UK) Trading Limited |
Ordinary |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
GlaxoSmithKline Consumer Healthcare (US) IP LLC |
LLC Interests |
68 |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | |||
GlaxoSmithKline Consumer Healthcare AB |
Ordinary |
68 |
Hemvärnsgatan 9, P.O. Box 516, 169 29, Solna, Sweden | |||
GlaxoSmithKline Consumer Healthcare Aps |
Ordinary |
68 |
Delta Park 37, 2665, Vallensbæk Strand, Denmark | |||
GlaxoSmithKline Consumer Healthcare Australia Pty Ltd |
Ordinary |
68 |
82 Hughes Avenue, Ermington New South Wales NSW 2115, Australia | |||
GlaxoSmithKline Consumer Healthcare B.V. |
Ordinary |
68 |
Van Asch van Wijckstraat 55G, 3811 LP, Amersfoort, Netherlands | |||
GlaxoSmithKline Consumer Healthcare Colombia SAS |
Ordinary |
68 |
Carrera 7 No. 113 - 43 Piso 4, Colombia | |||
GlaxoSmithKline Consumer Healthcare Czech Republic s.r.o. |
Ordinary |
68 |
Hvezdova 1734/2c, Prague, 4 140 00, Czech Republic | |||
GlaxoSmithKline Consumer Healthcare Finance Limited |
Ordinary |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
GlaxoSmithKline Consumer Healthcare Finance No.2 Limited |
Ordinary |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
GlaxoSmithKline Consumer Healthcare Finland Oy |
Ordinary |
68 |
Piispansilta 9A, Fin-02230, Espoo, Finland | |||
GlaxoSmithKline Consumer Healthcare GmbH |
Ordinary |
68 |
Wagenseilgasse 3, Euro Plaza, Gebäude I, 4. Stock, A-1120, Vienna, Austria | |||
GlaxoSmithKline Consumer Healthcare GmbH & Co. KG |
Partnership Capital |
68 |
Barthstr. 4, 80339, München, Germany | |||
GlaxoSmithKline Consumer Healthcare Hellas Single Member Societe Anonyme |
Ordinary |
68 |
274 Kifissias Avenue Halandri, Athens, 152 32, Greece | |||
GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited |
A; B (0%); Preference |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC |
LLC Interests |
68 |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | |||
GlaxoSmithKline Consumer Healthcare Investments (Ireland) (No 3) Limited (iii) (In Liquidation) |
Ordinary |
68 |
Knockbrack, Dungarvan, Co Waterford, X35 RY76, Ireland | |||
GlaxoSmithKline Consumer Healthcare Investments (Ireland) (No.2) Unlimited Company (iii) (In Liquidation) |
Ordinary |
68 |
Knockbrack, Dungarvan, Co Waterford, X35 RY76, Ireland | |||
GlaxoSmithKline Consumer Healthcare Japan K.K. |
Ordinary |
68 |
1-8-1 Minato-ku, Tokyo, Japan | |||
GlaxoSmithKline Consumer Healthcare Korea Co., Ltd. |
Ordinary |
68 |
9F LS Yongsan Tower, 92 Hangang-daero, Yongsan-gu, Seoul, 04386, Korea, Republic of | |||
GlaxoSmithKline Consumer Healthcare L.L.C. |
LLC Interests |
68 |
Corporation Service Company, 2595 Interstate Drive Suite 103, Harrisburg PA 17110, United States | |||
GlaxoSmithKline Consumer Healthcare Mexico, S. De R.L. de C.V. |
Ordinary |
68 |
Boulevard Adolfo Ruiz Cortines No. 3720, Torre 3 Piso 11, Colonia Jardines del Pedregal, Alcaldía Alvaro Obregón, Ciudad de México , C.P. 01900, Mexico | |||
GlaxoSmithKline Consumer Healthcare New Zealand ULC |
Ordinary |
68 |
Level 2 E.2 12 Madden Street, Auckland Central, Auckland, 1010, New Zealand | |||
GlaxoSmithKline Consumer Healthcare Norway AS |
Ordinary |
68 |
Drammensveien 288, Lysaker, 1326, Norway | |||
GlaxoSmithKline Consumer Healthcare Pakistan Limited |
Ordinary (85.8%) |
58.3 |
The Sykes Building, 35 Dockyard Road, West Wharf, Karachi, 74000, Pakistan | |||
GlaxoSmithKline Consumer Healthcare Philippines Inc |
Common |
68 |
23rd Floor, The Finance Centre, 26th Street Corner 9th Avenue, Bonifacio Global City, Taguig City, 1634, Philippines | |||
GlaxoSmithKline Consumer Healthcare Pte. Ltd. |
Ordinary |
68 |
23 Rochester Park, 139234, Singapore | |||
GlaxoSmithKline Consumer Healthcare S.A. |
Ordinary |
68 |
Site Apollo, Avenue Pascal 2-4-6, | |||
GlaxoSmithKline Consumer Healthcare S.A. |
Ordinary |
68 |
Severo Ochoa, 2, Parque Tecnológico de Madrid, Tres Cantos, 28760, Madrid, Spain | |||
GlaxoSmithKline Consumer Healthcare S.r.l |
Ordinary |
68 |
Via Zambeletti snc, Baranzate, 20021, Milan, Italy | |||
GlaxoSmithKline Consumer Healthcare Saudi Limited |
Ordinary |
68 |
603 Salamah Tower, 6th Floor, Madinah Road, Al-Salamah District, Jeddah 21425, Saudi Arabia | |||
GlaxoSmithKline Consumer Healthcare Sdn. Bhd. |
Ordinary |
68 |
Lot 89, Jalan Enggang,, Ampang / Hulu Kelang Industrial Estate, Selangor Darul Ehsan, 68000 Ampang, Malaysia | |||
GlaxoSmithKline Consumer Healthcare Slovakia s. r. o. |
Ownership Interest |
68 |
Galvaniho 7/A, Bratislava, 821 04, Slovakia | |||
GlaxoSmithKline Consumer Healthcare South Africa (Pty) Ltd |
Ordinary |
68 |
Flushing Meadows Building, The Campus, 57 Sloane Street, Bryanston 2021, South Africa | |||
GlaxoSmithKline Consumer Healthcare Sp.z.o.o. |
Ordinary |
68 |
Ul. Grunwaldzka 189, 60-322, Poznan, Poland | |||
GlaxoSmithKline Consumer Healthcare SRL |
Ordinary |
68 |
1-5 Costache Negri Street, Opera Center One, 6th floor (Zone 2), District 5, Bucharest, Romania | |||

Name |
Security |
Effective % Ownership |
Registered address | |||
Subsidiaries where the effective interest is less than 100% | ||||||
GlaxoSmithKline Consumer Healthcare ULC / GlaxoSmithKline Soins De Sante Aux Consommateurs SRI |
A Class Preference; Common |
68 |
595 Burrard Street, Suite 2600 Three Bentall Centre, P.O. Box 49314 Vancouver BC V7X 1L3, Canada | |||
| GlaxoSmithKline Consumer Healthcare Vietnam Company Limited (ii) |
Charter Capital |
68 |
Floor 16, Metropolitan, 235 Dong Khoi, Ben Nghe Ward, District 1, Ho Chi Minh City, Vietnam | |||
GlaxoSmithKline Consumer Healthcare, L.P. |
Partnership Capital |
59.8 |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | |||
GlaxoSmithKline Consumer Healthcare, Produtos para a Saude e Higiene, Lda |
Ordinary Quota |
68 |
Rua Dr Antonio Loureiro Borges No 3, Arquiparque, Miraflores, 1495-131, Algés, Portugal | |||
GlaxoSmithKline Consumer Nigeria plc (vi) |
Ordinary |
46.4 |
1 Industrial Avenue, Ilupeju, Ikeja, Lagos, PM B 21218, Nigeria | |||
GlaxoSmithKline Consumer Private Limited |
Equity |
68 |
Patiala Road, Nabha 147201, Dist Patiala, Punjab, India | |||
GlaxoSmithKline Consumer Trading Services Limited |
Ordinary |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
GlaxoSmithKline Costa Rica S.A. |
Ordinary |
68 |
San José 300 Este de la Rotonda Betania, Carretera a Sabanilla, Costa Rica | |||
GlaxoSmithKline Dungarvan Limited |
Ordinary |
68 |
Knockbrack, Dungarvan, Co Waterford, X35 RY76, Ireland | |||
GlaxoSmithKline Healthcare AO |
Ordinary |
68 |
premises III, Room 9, floor 6, Presnenskaya nab. 10, 123112, Moscow, Russian Federation | |||
GlaxoSmithKline Healthcare GmbH |
Ordinary |
68 |
Barthstr. 4, 80339, München, Germany | |||
GlaxoSmithKline Healthcare Ukraine O.O.O. |
Ownership Interest |
68 |
Pavla Tychyny avenue, 1-V, Kiev, 02152, Ukraine | |||
GlaxoSmithKline Limited |
Cumulative Redeemable Preference; Ordinary |
68 |
Likoni Road, PO Box 78392, Nairobi, Kenya | |||
GlaxoSmithKline Pakistan Limited |
Ordinary |
82.6 |
The Sykes Building, 35 Dockyard Road, West Wharf, Karachi, 74000, Pakistan | |||
GlaxoSmithKline Panama S.A. |
Non-qualified preference shares;Ordinary |
68 |
Urbanizacion Industrial Juan D, Calles A Y B, Republic of Panama, Panama | |||
GlaxoSmithKline Paraguay S.A. |
Ordinary |
68 |
Oficial Gilberto Aranda 333, Planta Alta casi Salvador del Mundo, Asunción, Paraguay | |||
GlaxoSmithKline Pharmaceuticals Limited |
Equity |
75 |
252 Dr Annie Besant Road, Mumbai, 400030, India | |||
GlaxoSmithKline S.A.E. |
Ordinary |
91.2 |
Boomerang Office Building - Land No. 46, Zone (J) - 1st District, Town Center - 5th Tagammoe, New Cairo City, Egypt | |||
GlaxoSmithKline Santé Grand Public |
Ordinary |
68 |
23 rue François Jacob, 92500, Rueil-Malmaison, France | |||
GlaxoSmithKline Technology (Taizhou) Co., Ltd |
Ordinary |
68 |
Room 708 in Building D, Phase II of New Drug Innovation Base, Taizhou, Jiangsu Province, 225300, China | |||
GlaxoSmithKline Tuketici Sagligi Anonim Sirketi |
Nominative |
68 |
Büyükdere Caddesi No. 173, 1.Levent Plaza B Blok, 1.Levent, Istanbul, 34394, Turkey | |||
GlaxoSmithKline-Consumer Kft. |
Membership |
68 |
H-1124, Csorsz utca 43, Budapest, Hungary | |||
GSK Canada Holding Company Limited |
Ordinary |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
GSK CH Caricam Sociedad De Responsabilidad Limitada (ii) |
Participation |
68 |
Urbanizacion Industrial Juan D, Calles A Y B, Republic of Panama, Panama | |||
GSK CH Kazakhstan LLP |
Charter Capital |
68 |
32 A Manasa Str., Bostandyk District, Almaty, 050008, Kazakhstan | |||
| GSK Consumer Health, Inc. |
Common |
68 |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | |||
GSK Consumer Healthcare Capital NL B.V. (iii) (v) |
Shares |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
GSK Consumer Healthcare Capital US LLC |
LLC Interests |
68 |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | |||
GSK Consumer Healthcare Chile SpA |
CLP Interests |
68 |
Av. Andrés Bello N°2687, 25th floor, Las Condes, Chile | |||
GSK Consumer Healthcare Egypt Limited |
Ordinary |
68 |
North 90th street, Boomerang Building, 5th District, Cairo, Egypt | |||
GSK Consumer Healthcare Egypt LLC |
Quotas |
68 |
North 90th street, Boomerang Building, 5th District, Cairo, Egypt | |||
GSK Consumer Healthcare Export Limited |
Ordinary |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
GSK Consumer Healthcare Holdings (No.1) Limited |
Non-voting preference shares;Ordinary |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
GSK Consumer Healthcare Holdings (No.3) Limited |
Non-voting preference shares;Ordinary |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
GSK Consumer Healthcare Holdings (No.5) Limited |
Ordinary |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
GSK Consumer Healthcare Holdings (No.6) Limited |
Ordinary |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
GSK Consumer Healthcare Holdings (No.7) Limited |
Ordinary |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
GSK Consumer Healthcare Holdings (US) Inc. |
Common; Preference Stock |
68 |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | |||
Name |
Security |
Effective % Ownership |
Registered address | |||
Subsidiaries where the effective interest is less than 100% | ||||||
GSK Consumer Healthcare Holdings No. 2 LLC (iii) |
Unit |
68 |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | |||
GSK Consumer Healthcare Insurance Limited |
Ordinary |
68 |
Dorey Court, Admiral Park, St Peter Port, GY1 4AT, Guernsey | |||
GSK Consumer Healthcare Israel Ltd (iv) |
Ordinary |
68 |
25 Basel Street, Petech Tikva 49510, Israel | |||
GSK Consumer Healthcare Levice s.r.o. |
Ordinary |
68 |
Priemyselny Park Gena, Ul. E. Sachsa 4-6, 934 01, Levice, Slovakia | |||
GSK Consumer Healthcare Peru S.R.L |
Ordinary |
68 |
Av Jorge Basadre 349, piso 5, San Isidro, Lima, 05W-109, Peru | |||
GSK Consumer Healthcare SARL |
Ordinary |
68 |
Route de I’Etraz, 1197 Prangins, Switzerland | |||
GSK Consumer Healthcare Schweiz AG |
Ordinary |
68 |
Suurstoffi 14, 6343, Rotkreuz, Switzerland | |||
GSK Consumer Healthcare Services, Inc. |
Common |
68 |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | |||
GSK Consumer Healthcare Singapore Pte. Ltd. |
Ordinary |
68 |
23 Rochester Park, 139234, Singapore | |||
GSK Consumer Healthcare Trinidad and Tobago Limited |
Ordinary: Preference |
68 |
5th Floor Algico Plaza, 91-93 St. Vincent Street, Port of Spain, Trinidad and Tobago | |||
GSK-Gebro Consumer Healthcare GmbH |
Ordinary (60%) |
40.8 |
Bahnhofbichl 13, 6391 Fieberbrunn, Kitzbühel, Austria | |||
Iodosan S.p.A. |
Ordinary |
68 |
Via Zambeletti snc,, Baranzate,, 20021, Milan, Italy | |||
Kuhs GmbH |
Ordinary |
68 |
Barthstr. 4, 80339, München, Germany | |||
Laboratorios ViiV Healthcare, S.L. |
Ordinary |
78.3 |
Severo Ochoa, 2, Parque Tecnológico de Madrid, Tres Cantos, 28760, Madrid, Spain | |||
Modern Pharma Trading Company L.L.C. |
Quotas |
98.2 |
Amoun Street, PO Box 3001, El Salam City, Cairo, 11491, Egypt | |||
N.C.H. – Nutrition Consumer Health Ltd (ii) |
Ordinary |
68 |
14 Hamephalsim St, Petach Tikva, Israel | |||
P.T. SmithKline Beecham Pharmaceuticals |
Ordinary A; Ordinary B (0%) |
99 |
Jl. Pulobuaran Raya, Kav. III DD/2,3,4, Kawasan Industri Pulogadung, Jakarta, 13930, Indonesia | |||
P.T. Sterling Products Indonesia |
A Shares; B Shares |
68 |
Graha Paramita Building, 5th F, Jalan Denpasar Raya Blok D-2, Jakarta, 12940, Indonesia | |||
Panadol GmbH |
Ordinary |
68 |
Barthstr. 4, 80339, München, Germany | |||
PF Consumer Healthcare 1 LLC |
Membership Interest |
68 |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | |||
PF Consumer Healthcare B.V. |
Class A; Class B |
68 |
Van Asch van Wijckstraat 55G, 3811 LP Amersfoort, Netherlands | |||
PF Consumer Healthcare Brazil Importadora e Distribuidora de Medicamentos Ltda |
Quota |
68 |
Barueri, at Avenida Ceci, No.1900, Block III, Part 67, Tambore District, São Paulo, 06460, Brazil | |||
PF Consumer Healthcare Canada ULC/PF Soins De Sante SRI |
Common; Preferred |
68 |
595 Burrard Street, Suite 2600 Three Bentall Centre, P.O. Box 49314 Vancouver BC V7X 1L3, Canada | |||
PF Consumer Healthcare Holding B.V. |
Ordinary |
68 |
Van Asch van Wijckstraat 55G, 3811 LP Amersfoort, Netherlands | |||
PF Consumer Healthcare UK Limited (In Liquidation) |
Ordinary |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
PF Consumer Ireland Company Limited (In Liquidation) |
Ordinary |
68 |
BDO, Beax Lane House, Mercer Street, Lower, D02 DH60, Dublin, D02 DH60, Ireland | |||
PF Consumer Taiwan LLC |
Interests |
68 |
The Corporation Trust Company, Corporation Trust Center, 1209 Orange Street, Wilmington DE 19801, United States | |||
Pfizer Biotech Corporation |
Ordinary (55%) |
37.4 |
24F, No. 66, Sec 1, Zhong Xiao W. Rd, Taipei 100, Taiwan | |||
Pfizer Consumer Healthcare AB |
Ordinary |
68 |
Vetenskapsvagen 10, SE-191 90, Sollentuna, Sweden | |||
Pfizer Consumer Healthcare GmbH |
Ordinary |
68 |
Linkstrasse 10, 10785, Berlin, Germany | |||
Pfizer Consumer Manufacturing Italy S.r.l. |
Quota (no stock) |
68 |
90, Via Nettunese, 04011, Aprilia (Prov. di Latin), Italy | |||
Pfizer Laboratories PFE (Pty) Ltd. |
Common |
68 |
Flushing Meadows Building, The Campus, 57 Sloane Street, Bryanston 2021, South Africa | |||
Pfizer PFE Colombia S.A.S |
Common |
68 |
Carrera 7 No. 113 - 43 Piso 4, Colombia | |||
PHIVCO-1 LLC |
LLC Interests |
78.3 |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | |||
PHIVCO-2 LLC |
LLC Interests |
78.3 |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | |||
PRISM PCH Limited |
Non-Voting Shares; Voting Shares; |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
PT Glaxo Wellcome Indonesia |
Class A; Class B (0%) |
95 |
JL. Pulobuaran Raya Kav.III/DD 2,3,4 KWS. Industri, Pulogadung, Jatinegara, Cakung, Jakarta Timur, Indonesia | |||
PT GSK Consumer Healthcare Indonesia |
Ordinary |
68 |
Graha Paramita Building, 5th F, Jalan Denpasar Raya Blok D-2,, Kuningan, JAKARTA SELATAN, 12940, Indonesia | |||
PT. Bina Dentalindo (In Liquidation) |
Ordinary |
68 |
Gedung Graha Ganesha Lantai 3, Jl Raya Bekasi Km 17, No5, Jakarta Timur 13930, Indonesia | |||
Shionogi-ViiV Healthcare LLC (ii) |
Common Interests |
78.3 |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | |||
Sino-American Tianjin Smith Kline & French Laboratories Ltd |
Ordinary (55%) |
37.4 |
Cheng Lin Zhuang Industrial Zone, Dong Li District, Tianjin, 300163, China | |||
SmithKline Beecham (Private) Limited |
Ordinary (99.6%) |
67.8 |
World Trade Center, Level 34, West Tower, Echelon Square, Colombo 1, Sri Lanka | |||

Name |
Security |
Effective % Ownership |
Registered address | |||
Subsidiaries where the effective interest is less than 100% | ||||||
SmithKline Beecham Research Limited |
Ordinary |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
SmithKline Beecham S.A. |
Ordinary |
68 |
Ctra de Ajalvir Km 2.500, Alcala de Henares, 28806, Madrid, Spain | |||
SmithKline Beecham-Biomed O.O.O. |
Participation Interest |
97 |
Leningradskiy Prospect 37A, Building 4, Floor 2, Premises XIV, Room 42, 125167, Moscow, Russian Federation | |||
Stafford-Miller (Ireland) Limited |
Ordinary |
68 |
Clocherane, Youghal Road, Dungarvan, Co. Waterford, Ireland | |||
Stafford-Miller Limited (In Liquidation) |
Ordinary; Non-Cumulative Non Redeemable Preference |
68 |
Clocherane, Youghal Road, Dungarvan, Co. Waterford, Dungarvan, Waterford, Ireland | |||
Sterling Drug (Malaya) Sdn Berhad |
Ordinary |
68 |
Lot 89, Jalan Enggang, Ampang/Hulu Kelang Industrial Estate, Selangor Darul Ehsan, 68000 Ampang, Malaysia | |||
Sterling Products International, Incorporated (ii) |
Common |
68 |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | |||
Stiefel Consumer Healthcare (UK) Limited |
Ordinary |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
Stiefel Egypt LLC (ii) |
Quotas |
99 |
Amoun Street, PO Box 3001, El Salam City, Cairo, 11491, Egypt | |||
Stiefel Laboratories (Ireland) Limited (In Liquidation) |
Ordinary |
68 |
BDO, Beax Lane House, Mercer Street, Lower, D02 DH60, Dublin, D02 DH60, Ireland | |||
Treerly Health Co., Ltd |
Capital Contribution |
68 |
Unit 01A, Room 3901, No 16. East Zhujiang Road, Tianhe District, Guangzhou City, the PRC, China | |||
ViiV Healthcare (South Africa) (Proprietary) Limited (ii); (iv) |
Ordinary |
78.3 |
Flushing Meadows Building, The Campus, 57 Sloane Street, Bryanston 2021, South Africa | |||
ViiV HealthCare BV |
Ordinary |
78.3 |
Van Asch van, Wijckstraat 55h, 3811 LP Amersfoort, The Netherlands, Netherlands | |||
ViiV Healthcare Company |
Common |
78.3 |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | |||
ViiV Healthcare Finance 1 Limited (In Liquidation) |
Ordinary |
78.3 |
55 Baker Street, London, W1U 7EU, United Kingdom | |||
ViiV Healthcare Finance 2 Limited |
Ordinary |
78.3 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
ViiV Healthcare Finance Limited |
Ordinary; Redeemable Preference |
78.3 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
ViiV Healthcare GmbH |
Ordinary |
78.3 |
Prinzregentenplatz 9, 81675, Munchen, Germany | |||
ViiV Healthcare GmbH |
Ordinary |
78.3 |
Talstrasse 3-5, 3053 Muenchenbuchsee, Switzerland | |||
ViiV Healthcare Hong Kong Limited (ii) |
Ordinary |
78.3 |
23/F Tower 6, The Gateway, 9 Canton Road, Harbour City, Tsimshatsui, Kowloon, Hong Kong | |||
ViiV Healthcare K.K. |
Ordinary |
78.3 |
1-8-1 Minato-ku, Tokyo, Japan | |||
ViiV Healthcare Limited |
Class A; Class B (0%); Class C (0%); Class D1 (0%); Class D2 (0%); Deferred; Class E 5% Cumulative Preference (0%) |
78.3 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
ViiV Healthcare Pty Ltd |
Ordinary |
78.3 |
1061 Mountain Highway, Boronia Victoria VIC 3155, Australia | |||
ViiV Healthcare Puerto Rico, LLC |
LLC Interests |
78.3 |
Centro International de Mercadeo, 90 carr. 165 Torre 2, Suite 800, Guaynabo, 00968, Puerto Rico | |||
ViiV Healthcare S.r.l. |
Quota |
78.3 |
Viale dell’Agricoltura 7, 37135, Verona, Italy | |||
ViiV Healthcare SAS |
Ordinary |
78.3 |
23 rue François Jacob, 92500, Rueil-Malmaison, France | |||
ViiV Healthcare sprl |
Ordinary |
78.3 |
Site Apollo, Avenue Pascal 2-4-6, | |||
ViiV Healthcare Trading LLC (ii) |
Participation Interest |
78.3 |
Leningradskiy Prospect 37A, Building 4, Floor 2, Premises XIV, Room 28, 125167, Moscow, Russian Federation | |||
ViiV Healthcare Trading Services UK Limited |
Ordinary |
78.3 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
ViiV Healthcare UK (No.3) Limited |
Ordinary |
78.3 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
ViiV Healthcare UK (No.4) Limited |
Ordinary |
78.3 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
ViiV Healthcare UK (No.5) Limited |
Ordinary |
78.3 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
ViiV Healthcare UK (No.6) Limited |
Ordinary |
78.3 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
ViiV Healthcare UK (No.7) Limited |
Ordinary |
78.3 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
ViiV Healthcare UK Limited |
Ordinary |
78.3 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England | |||
ViiV Healthcare ULC |
Common |
78.3 |
3500 855-2nd Street SW, Calgary AB T2P 4J8, Canada | |||
ViiV Healthcare Venture LLC |
LLC Interests |
78.3 |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | |||
ViiVHIV Healthcare Unipessoal Lda |
Quota |
78.3 |
Rua Dr Antonio Loureiro Borges No 3, Arquiparque, Miraflores, 1495-131, Algés, Portugal | |||
Vog AU PTY LTD (ii) |
Ordinary; Redeemable Preference |
68 |
82 Hughes Avenue, Ermington New South Wales NSW 2115, Australia | |||
Winster Pharmaceuticals Limited (ii) |
Ordinary |
46.4 |
2A Association Avenue, Ilupeju Industrial Estate, Lagos, PO Box 3199, Nigeria | |||
Wyeth Pharmaceutical Co. Ltd |
Registered Capital |
68 |
4 Baodai West Road, Suzhou, Jiangsu Province, 215128, China | |||
Wyeth Pharmaceuticals Company (vii) |
Partnership |
68 |
No registered address required, Puerto Rico general partnership, Contact entity contact for any questions, Puerto Rico | |||
Name |
Security |
Effective % Ownership |
Registered address | |||
Associates |
||||||
GlaxoSmithKline Landholding Company, Inc |
Common (40%) |
39.9 |
23rd Floor, The Finance Centre, 26th Street Corner 9th Avenue, Bonifacio Global City, Taguig City, 1634, Philippines | |||
Index Ventures Life VI (Jersey) LP |
Partnership Interest (25%) |
25 |
44 Esplanade, St Helier, Jersey, JE4 9WG, Channel Islands | |||
Kurma Biofund II FCPR |
Partnership Interest (32.1%) |
32.1 |
24 rue Royale, 75008, Paris, France | |||
Longwood Fund I, LP |
Partnership Interest (35%) |
35 |
The Prudential Tower, Suite 1555, 800 Boylston Street, Boston, MA 02199 | |||
Medicxi Ventures I LP |
Partnership Interest (26.2%) |
26.2 |
44 Esplanade, St Helier, Jersey, JE4 9WG, Channel Islands | |||
Joint Ventures |
||||||
Chiron Panacea Vaccines Private Limited |
Equity Shares (50%) |
50 |
708/718, 7th Floor, A Wing, Sagar Tech Plaza, Saki Naka, Andheri East, Mumbai, Maharashtra, 400072, India | |||
Qualivax Pte. Limited |
Ordinary (50%) |
50 |
80 Robinson Road, #02-00, 068898, Singapore | |||
Qura Therapeutics, LLC |
Units (39.2%) |
39.2 |
Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States | |||
Other significant holdings |
||||||
Axon Therapies, Inc |
Common (5%); Series A Preference (15%) |
20 |
315 west 36th street, New York 10018, Delaware, USA | |||
Global Farm S.A. |
A Shares (0%) B Shares (0%) C Shares (100%) D Shares (0%) E Shares (0%) F Shares (0%) |
16.7 |
Cazadores de Coquimbo 2841 piso 3, Munro, Argentina | |||
Longwood Fund II, LP |
Partnership Interest (20%) |
20 |
The Prudential Tower, Suite 1555, 800 Boylston Street, Boston, MA 02199 | |||
Sanderling Ventures VII, L.P. A63 |
Partnership Interest (25.3%) |
25.3 |
400 S. El Camino Real, Suite 1200, San Mateo, CA 94402 | |||
SR One Capital Fund I-B, LP |
Partnership Interest (44%) |
44 |
Corporation service company, 251 Little Falls Drive, City of Wilmington, County of New Castle, Delaware 19808 | |||
Name |
Security |
Effective % Ownership |
Registered address |
Company Number | ||||
UK registered subsidiaries exempted from audit | ||||||||
Burroughs Wellcome International Limited |
Ordinary |
100 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
00543757 | ||||
Domantis Limited |
Ordinary |
100 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
03907643 | ||||
Edinburgh Pharmaceutical Industries Limited (ii) |
Ordinary; Preference |
100 |
Shewalton Road, Irvine, Ayrshire, KA11 5AP, United Kingdom |
SC005534 | ||||
Eskaylab Limited |
Ordinary |
100 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
00099025 | ||||
Glaxo Wellcome UK Limited |
Ordinary |
100 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
00480080 | ||||
Glaxochem (UK) Unlimited |
Ordinary; Ordinary B; Ordinary C |
100 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
04299472 | ||||
GlaxoSmithKline Consumer Healthcare (UK) (No.1) Limited |
Ordinary |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
00753340 | ||||
GlaxoSmithKline Consumer Healthcare Sri Lanka Holdings Limited |
Ordinary |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
9400298 | ||||
GlaxoSmithKline Intellectual Property (No.3) Limited |
Ordinary |
100 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
11480952 | ||||
GlaxoSmithKline Intellectual Property (No.4) Limited |
Ordinary |
100 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
11721880 | ||||
GlaxoSmithKline Intellectual Property (No.5) Limited |
Ordinary |
100 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
11959399 | ||||
GlaxoSmithKline International Limited |
Ordinary |
100 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
02298366 | ||||
GSK Consumer Healthcare Capital UK PLC |
Ordinary |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
13481162 | ||||
GSK Consumer Healthcare Holdings (No.4) Limited |
Ordinary |
100 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
13401336 | ||||
GSK Consumer Healthcare Holdings (No.8) Limited |
Ordinary |
100 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
13434151 | ||||
GSK New Zealand Holding Company Limited |
Ordinary |
68 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
12342879 | ||||
Montrose Fine Chemical Company Ltd |
Ordinary |
100 |
Shewalton Road, Irvine, Ayrshire, KA11 5AP, United Kingdom |
SC190635 | ||||
PHIVCO UK II Limited |
Ordinary |
78.3 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
06944229 | ||||
PHIVCO UK Limited |
Ordinary |
78.3 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
06944223 | ||||
Smith Kline & French Laboratories Limited (iv) |
Ordinary |
100 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
00052207 | ||||
SmithKline Beecham (Export) Limited |
Ordinary |
100 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
02860752 | ||||
SmithKline Beecham (H) Limited |
Non-cumulative Non-redeemable; Ordinary |
100 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
03296131 | ||||

Name |
Security |
Effective % Ownership |
Registered address |
Company Number | ||||
UK registered subsidiaries exempted from audit |
||||||||
SmithKline Beecham (Investments) Limited |
Ordinary |
100 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
00302065 | ||||
SmithKline Beecham Legacy H Limited |
Ordinary |
100 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
00210281 | ||||
SmithKline Beecham Marketing and Technical Services Limited |
Ordinary |
100 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
00494385 | ||||
SmithKline Beecham Nominees Limited |
Ordinary |
100 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
00503868 | ||||
SmithKline Beecham Overseas Limited |
Ordinary |
100 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
02552828 | ||||
Stiefel Laboratories (U.K.) Ltd |
Ordinary |
100 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
00831160 | ||||
Tesaro UK Limited |
Ordinary |
100 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
07890847 | ||||
The Wellcome Foundation Limited |
Ordinary |
100 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
00194814 | ||||
ViiV Healthcare Overseas Limited |
Ordinary |
78.3 |
980 Great West Road, Brentford, Middlesex, TW8 9GS, England |
07027385 | ||||
(i) |
Directly owned by GlaxoSmithKline plc. |
(ii) |
Dormant entity. |
(iii) |
Tax resident in the UK. |
(iv) |
Entity expected to be disposed of or removed. |
(v) |
Incorporated in the Netherlands |
(vi) |
Consolidated as a subsidiary in accordance with section 1162 (4)(a) of the Companies Act 2006 on the grounds of dominant influence. |
(vii) |
Principal business address in Puerto Rico. |
(viii) |
Exempt from the provisions of Regulations 4-6 of the Partnership (Accounts) Regulation 2008, in accordance with the exemptions noted in Regulation 7 of that Regulation. |
| Terms used in the Annual Report |
US equivalent or brief description | |
| Accelerated capital allowances |
Tax allowance in excess of depreciation arising from the purchase of fixed assets that delay the charging and payment of tax. The equivalent of tax depreciation. | |
| American Depositary Receipt (ADR) |
Receipt evidencing title to an ADS. Each GSK ADR represents two Ordinary Shares. | |
| American Depositary Shares (ADS) |
Listed on the New York Stock Exchange; represents two Ordinary Shares. | |
| Basic earnings per share |
Basic income per share. | |
| Called up share capital |
Ordinary Shares, issued and fully paid. | |
| CER growth |
Growth at constant exchange rates. | |
| The company |
GlaxoSmithKline plc. | |
| Currency swap |
An exchange of two currencies, coupled with a subsequent re-exchange of those currencies, at agreed exchange rates and dates. | |
| Defined benefit plan |
Pension plan with specific employee benefits, often called ‘final salary scheme’. | |
| Defined contribution plan |
Pension plan with specific contributions and a level of pension dependent upon the growth of the pension fund. | |
| Derivative financial instrument |
A financial instrument that derives its value from the price or rate of some underlying item. | |
| Diluted earnings per share |
Diluted income per share. | |
| Employee Share Ownership Plan Trusts |
Trusts established by the Group to satisfy share-based employee incentive plans. | |
| Equity Shareholders’ funds |
Shareholders’ equity. | |
| Finance lease |
Capital lease. | |
| Freehold |
Ownership with absolute rights in perpetuity. | |
| The Group |
GlaxoSmithKline plc and its subsidiary undertakings. | |
| GSK |
GlaxoSmithKline plc and its subsidiary undertakings. | |
| Hedging |
The reduction of risk, normally in relation to foreign currency or interest rate movements, by making off-setting commitments. | |
| Intangible fixed assets |
Assets without physical substance, such as computer software, brands, licences, patents, know-how and marketing rights purchased from outside parties. | |
| Ordinary Share |
A fully paid up ordinary share in the capital of the company. | |
| Profit |
Income. | |
| Profit attributable to shareholders |
Net income. | |
| Share capital |
Ordinary Shares, capital stock or common stock issued and fully paid. | |
| Share option |
Stock option. | |
| Share premium account |
Additional paid-up capital or paid-in surplus (not distributable). | |
| Shares in issue |
The number of shares outstanding. | |
| Subsidiary |
An entity in which GSK exercises control. | |
| Treasury share |
Treasury stock. | |
| Turnover |
Revenue. | |
| UK Corporate Governance Code |
As required by the UK Listing Authority, the company has disclosed in the Annual Report how it has applied the best practice corporate governance provisions of the Financial Reporting Council’s UK Corporate Governance Code. | |

Page |
Page | |||||||
2021 Remuneration policy summary |
Investor relations |
295 | ||||||
Accounting principles and policies |
171 |
Key accounting judgements and estimates |
252 | |||||
Acquisitions and disposals |
220 |
Key performance indicators |
12 | |||||
| Adjustments reconciling profit after tax to operating cash flows |
224 |
Legal proceedings |
247 | |||||
Affordability and availability |
36 |
Major restructuring costs |
185 | |||||
Annual General Meeting 2022 |
291 |
Modern employer |
37 | |||||
Approach to tax |
60 |
Movements in equity |
216 | |||||
Assets held for sale |
200 |
Net debt |
202 | |||||
Associates and joint ventures |
187 |
New accounting requirements |
178 | |||||
Audit & Risk Committee Report |
110 |
Nominations Committee Report |
||||||
Being a responsible business |
38 |
Non-controlling interests |
09,68,218 | |||||
Business model |
01 |
Non-controlling interests in ViiV Healthcare |
57 | |||||
Cash and cash equivalents |
200 |
Non-Executive Directors’ fees |
138,150 | |||||
Cash generation and conversion |
73 |
Non-financial information statement |
54 | |||||
CEO’s statement |
05 |
Notes to the financial statements |
171 | |||||
Chairman’s statement |
03 |
Operating profit |
07,09,67 | |||||
Chairman’s Governance statement |
89 |
Other intangible assets |
74,174,190 | |||||
Chairman’s Remuneration annual statement |
119 |
Other investments |
75,198 | |||||
Climate-related financial disclosure |
49 |
Other non-current assets |
199 | |||||
Commitments |
215 |
Other non-current liabilities |
75,215 | |||||
Consolidated balance sheet |
168 |
Other operating income/(expense) |
67,177 | |||||
Consolidated cash flow statement |
170 |
Other provisions |
213 | |||||
Consolidated income statement |
167 |
Our culture |
11,110 | |||||
Consolidated statement of changes in equity |
169 |
Our long-term priorities |
12 | |||||
Consolidated statement of comprehensive income |
167 |
Pensions and other post-employment benefits |
204 | |||||
Consumer Healthcare |
41 |
Performance |
29 | |||||
Consumer Healthcare products and competition |
273 |
Pharmaceuticals |
17,29 | |||||
Contingent consideration liabilities |
214 |
Pharmaceutical products, competition and intellectual property |
271 | |||||
Contingent liabilities |
215 |
Pipeline |
268 | |||||
Corporate Executive Team |
Post balance sheet events |
250 | ||||||
Corporate governance |
82 |
Presentation of the financial statements |
171 | |||||
Corporate Responsibility Committee Report |
104 |
Principal Group companies |
246 | |||||
Critical accounting policies |
80 |
Principal risks and uncertainties |
274 | |||||
Data and engagement |
39 |
Property, plant and equipment |
192 | |||||
Directors and senior management |
140 |
Quarterly trend |
257 | |||||
Directors’ interests in shares |
139 |
Reconciliation of net cash flow to movement in net debt |
225 | |||||
Directors’ report |
116 |
Registrar |
294 | |||||
Directors’ statement of responsibilities |
153 |
Related party transactions |
220 | |||||
Dividends |
191,290 |
Reliable supply |
38 | |||||
Donations to political organisations and political expenditure |
298 |
Remuneration governance |
137 | |||||
Earnings per share |
191 |
Remuneration report |
124 | |||||
Employee costs |
184 |
Reporting framework |
56 | |||||
Employee share schemes |
244 |
Responsible business |
16 | |||||
Environment |
39 |
Right of use assets |
193 | |||||
Ethics and values |
38 |
Risk management |
46 | |||||
Exchange rates |
179 |
Science and technology |
35 | |||||
Finance expense |
186 |
Science Committee report |
105 | |||||
Finance income |
186 |
Section 172 statement |
115 | |||||
Financial calendar 2022 |
291 |
Share capital and control |
288 | |||||
Financial instruments and related disclosures |
227 |
Share capital and share premium account |
216 | |||||
Financial performance |
7,61 |
Shareholder information |
288 | |||||
Financial position and resources |
74 |
Shareholder services and contacts |
294 | |||||
| Financial statements of GlaxoSmithKline plc, prepared under UK GAAP |
251 |
Stakeholder engagement |
44 | |||||
Five year record |
262 |
Task Force on Climate-related Financial Disclosures |
49 | |||||
Glossary of terms |
311 |
Taxation |
188 | |||||
Goodwill |
194 |
Tax information for shareholders |
292 | |||||
Group companies |
299 |
The Board |
83 | |||||
Group financial review |
55 |
Trade and other payables |
201 | |||||
GSK Leadership Team |
87 |
Trade and other receivables |
199 | |||||
Industry trends |
Transformation & Separation Committee report |
110 | ||||||
Innovation |
17 |
Treasury policies |
79 | |||||
Inventories |
199 |
Trust |
34 | |||||
Investments in associates and joint ventures |
197 |
Turnover and segment information |
179 | |||||
US law and regulation |
296 | |||||||
Vaccines |
17,31 | |||||||
Vaccine products, competition and intellectual property |
272 | |||||||
Viability statement |
53 | |||||||
| About GSK |
||
| GlaxoSmithKline plc was incorporated as an English public limited company on 6 December 1999. We were formed by a merger between Glaxo Wellcome plc and SmithKline Beecham plc. GSK acquired these two English companies on 27 December 2000 as part of the merger arrangements. Our shares are listed on the London Stock Exchange and the New York Stock Exchange.  www.gsk.com |
Brand names Brand names appearing in italics throughout this report are trade marks either owned by and/or licensed to GSK or associated companies. All other trade marks are the property of their respective owners. Acknowledgements Printing Printed sustainably in the UK by Pureprint, a CarbonNeutral ® company with FSC® chain of custody and an ISO 14001 certified environmental management system recycling over 99% of all dry waste.Paper Printed on Innovation Premium, an FSC certified paper. The pulps used are Totally Chlorine Free and the manufacturing mill has ISO 14001 environmental management certification. The mill’s energy is produced from 100% biomass fuels sourced from local forestry and no fossil fuels are used. The carbon emissions have been measured and offset using the World Land Trust’s Carbon Balanced scheme. Download PDFs:   20-F |
| Cautionary statement regarding forward-looking statements The Group’s reports filed with or furnished to the US Securities and Exchange Commission (SEC), including this document, and any other written information released, or oral statements made, to the public in the future by or on behalf of the Group, may contain forward-looking statements. Forward-looking statements give the Group’s current expectations or forecasts of future events. An investor can identify these statements by the fact that they do not relate strictly to historical or current facts. They use words such as ‘anticipate’, ‘estimate’, ‘expect’, ‘intend’, ‘will’, ‘project’, ‘plan’, ‘believe’, ‘target’ and other words and terms of similar meaning in connection with any discussion of future operating or financial performance. In particular, these include statements relating to future actions, prospective products or product approvals, future performance or results of current and anticipated products, sales efforts, expenses, the outcome of contingencies such as legal proceedings, dividend payments and financial results. Other than in accordance with its legal or regulatory obligations (including under the Market Abuse Regulations, the UK Listing Rules and the Disclosure and Transparency Rules of the Financial Conduct Authority), the Group undertakes no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise. The reader should, however, consult any additional disclosures that the Group may make in any documents which it publishes and/or files with the SEC. All readers, wherever located, should take note of these disclosures. Accordingly, no assurance can be given that any particular expectation will be met and investors are cautioned not to place undue reliance on the forward-looking statements. Forward-looking statements are subject to assumptions, inherent risks and uncertainties, many of which relate to factors that are beyond the Group’s control or precise estimate. The Group cautions investors that a number of important factors, including those in this document, could cause actual results to differ materially from those expressed or implied in any forward-looking statement. Such factors include, but are not limited to, those discussed under ‘Principal risks and uncertainties’ on pages 275 to 287 of this Annual Report and any impacts of the COVID-19 pandemic. Any forward-looking statements made by or on behalf of the Group speak only as of the date they are made and are based upon the knowledge and information available to the Directors on the date of this Annual Report. |
A number of non-IFRS measures are used to report the performance of our business. These measures are defined on pages 56 and 59 and a reconciliation of Adjusted results to Total results is set out on page 70. The information in this document does not constitute an offer to sell or an invitation to buy shares in GlaxoSmithKline plc or an invitation or inducement to engage in any other investment activities. Past performance cannot be relied upon as a guide to future performance. Nothing in this Annual Report should be construed as a profit forecast. Assumptions related to 2022 guidance In outlining the guidance for 2022, the Group has made certain assumptions about the healthcare sector, the different markets in which the Group operates and the delivery of revenues and financial benefits from its current portfolio, pipeline and restructuring programmes. The Group also assumes that the demerger of our Consumer Healthcare business will be delivered in mid-2022 and this guidance relates only to new GSK. The Group has made planning assumptions for 2022 that healthcare systems will approach normality as the year progresses, and we expect sales of Specialty Medicines to grow approximately 10% at CER and sales of General Medicines to show a slight decrease, primarily reflecting increased genericisation of established Respiratory products. Vaccines sales are expected to grow at a low teens percentage at CER for the year as a whole. However, governments’ prioritisation of COVID-19 vaccination programmes and ongoing measures to contain the pandemic are expected to result in some continued disruption to adult immunisations, with the impact weighted to the first half. For Shingrix These planning assumptions as well as operating profit guidance and dividend expectations assume no material interruptions to supply of the Group’s products, no material mergers, acquisitions or disposals, no material litigation or investigation costs for the company (save for those that are already recognised or for which provisions have been made) and no change in the Group’s shareholdings in ViiV Healthcare. The assumptions also assume no material changes in the healthcare environment or unexpected significant changes in pricing as a result of government or competitor action. The 2022 guidance factors in all divestments and product exits announced to date. |
The Group’s guidance assumes successful delivery of the Group’s integration and restructuring plans. It also assumes that the separation programme to deliver the demerger of the Consumer Healthcare business is delivered successfully. Material costs for investment in new product launches and R&D have been factored into the expectations given. Given the potential development options in the Group’s pipeline, the outlook may be affected by additional data-driven R&D investment decisions. The guidance is given on a constant currency basis. 2021-2026 outlooks, 2031 sales ambition and 2021-2023 dividend expectations should be read together with the section “Basis of preparation, assumptions and cautionary statements” on pages 5-7 of our stock-exchange announcement relating to an update to investors dated 23 June 2021. All outlook and ambition statements are given on a constant currency basis and use 2021 actual exchange rates as a base. Notice regarding limitations on Director Liability under English Law Under the UK Companies Act 2006, a safe harbour limits the liability of Directors in respect of statements in and omissions from the Directors’ Report (for which see page 117), the Strategic report and the Remuneration report. Under English law the Directors would be liable to the company, but not to any third party, if one or more of these reports contained errors as a result of recklessness or knowing misstatement or dishonest concealment of a material fact, but would otherwise not be liable. Pages 82 to 118, 154 to 155, and 275 to 310 inclusive comprise the Directors’ Report, pages 1 to 81 inclusive comprise the Strategic report and pages 119 to 152 inclusive comprise the Remuneration report, each of which have been drawn up and presented in accordance with and in reliance upon English company law and the liabilities of the Directors in connection with these reports shall be subject to the limitations and restrictions provided by such law. Website GSK’s website www.gsk.com gives additional information on the Group. Notwithstanding the references we make in this Annual Report to GSK’s website, none of the information made available on the website constitutes part of this Annual Report or shall be deemed to be incorporated by reference herein. |
