As filed with the Securities and Exchange Commission on January 31, 2024
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
☐ REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR 12(g) OF THE SECURITIES EXCHANGE ACT OF 1934
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 for the fiscal year ended December 31, 2023
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
☐ SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Date of event requiring this shell company report……………
For the transition period from _____ to _____
Commission file number 1-15024
(Exact name of Registrant as specified in its charter)
(Translation of Registrant’s name into English)
(Jurisdiction of incorporation or organization)
(Address of principal executive offices)
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
Title of each class
Trading Symbol(s)
Name of each exchange on which registered
American Depositary Shares each representing 1 share
NVS
New York Stock Exchange
Ordinary shares, nominal value CHF 0.49 per share*
NOVN
New York Stock Exchange*
* Not for trading but only in connection with the registration of American Depositary Shares representing such ordinary shares.
Securities registered or to be registered pursuant to Section 12(g) of the Act:
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report:
2 044 033 986 ordinary shares
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
Note—Checking the box above will not relieve any registrant required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 from their obligations under those Sections.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☒ Accelerated filer ☐ Non-accelerated filer ☐ Emerging growth company ☐
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act. ☐
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
U.S. GAAP ☐ International Financial Reporting Standards as issued by the International Accounting Standards Board ☒ Other ☐
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Table of contents
Introduction and use of certain terms
Novartis AG and its consolidated affiliates publish consolidated financial statements expressed in US dollars. Our consolidated financial statements responsive to Item 18 of this Annual Report on Form 20-F (Annual Report) are prepared in accordance with International Financial Reporting Standards Accounting Standards as issued by the International Accounting Standards Board. “Item 5. Operating and Financial Review and Prospects,” together with the sections on products in development and key development projects of our businesses (see “Item 4. Information on the Company—Item 4.B. Business overview”), constitute the Operating and Financial Review (“Lagebericht”), as defined by the Swiss Code of Obligations.
Unless the context requires otherwise, the words “we,” “our,” “us,” “Novartis,” “Group,” “Company,” and similar words or phrases in this Annual Report refer to Novartis AG and its consolidated affiliates. However, each Novartis affiliate is legally separate from all other Novartis affiliate companies and manages its business independently through its respective board of directors or similar supervisory body or other top local management body, if applicable. Each executive identified in this Annual Report reports directly to other executives of the Novartis affiliate company that employs such executive, or to such company’s board of directors.
In this Annual Report, references to “US dollars,” “USD” or “$” are to the lawful currency of the United States of America; references to “CHF” are to Swiss francs; references to “euro” or “EUR” are to the lawful currency of the member states of the European Union in which it is the official currency; references to the “United States” or to “US” are to the United States of America; references to the “European Union” or to “EU” are to the European Union and its 27 member states; references to “Latin America” are to Central and South America, including the Caribbean; references to “Australasia” are to Australia, New Zealand, Melanesia, Micronesia and Polynesia, unless the context otherwise requires; references to the “EC” are to the European Commission; references to “associates” are to employees of our affiliates; references to the “SEC” are to the US Securities and Exchange Commission; references to the “FDA” are to the US Food and Drug Administration; references to the “EMA” are to the European Medicines Agency, an agency of the EU; references to the “CHMP” are to the Committee for Medicinal Products for Human Use of the EMA; references to “ADR” or “ADRs” are to Novartis American Depositary Receipts; references to “ADS” or “ADSs” are to Novartis American Depositary Shares; references to the “NYSE” are to the New York Stock Exchange, and references to “SIX” are to the SIX Swiss Exchange; references to “ECN” are to the Executive Committee of Novartis; references to “Bausch + Lomb” are to Bausch & Lomb Incorporated; references to “GSK” are to GlaxoSmithKline plc; references to “Roche” are to Roche Holding AG; references to “Gyroscope Therapeutics” are to Gyroscope Therapeutics Holdings plc; references to “ADACAP” are to Advanced Accelerator Applications S.A.; references to “Novartis Gene Therapies” are to Novartis Gene Therapies, Inc.; references to “Endocyte” are to Endocyte, Inc.; references to “Chinook” are to Chinook Therapeutics, Inc. and references to “DTx Pharma” are to DTx Pharma, Inc.
All product names appearing in italics are trademarks owned by or licensed to Novartis. Product names identified by a “™” are trademarks that are not owned by or licensed to Novartis and are the property of their respective owners.
Certain documents and information referenced in this Annual Report are available on our website. However, the information contained on our website, or any information that may be accessed by links on our website, is not included as part of, or incorporated by reference into, this Annual Report.
Forward-looking statements
This Annual Report contains certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the United States Private Securities Litigation Reform Act of 1995, as amended. Other written materials filed with or furnished to the SEC by Novartis, as well as other written and oral statements made to the public, may also contain forward-looking statements. Forward-looking statements can be identified by words such as “potential,” “expect,” “will,” “plan,” “pipeline,” “outlook,” “may,” “could,” “would,” “anticipate,” “seek,” “likely,” “ongoing,” “estimate,” “believe,” “target,” “intend,” or similar terms, or by express or implied discussions regarding potential new products, potential new indications for existing products, or regarding potential future revenues from any such products or indications; or regarding the potential outcome, or financial or other impact on Novartis, of any of the transactions described; or regarding the potential impact of share buybacks; or regarding potential future sales or earnings of Novartis or potential shareholder returns; or regarding potential future credit ratings of Novartis; or by discussions of strategy, plans, expectations or intentions. Such forward-looking statements are based on the current beliefs and expectations of management regarding future events, and are subject to significant known and unknown risks and uncertainties. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those set forth in forward-looking statements. You should not place undue reliance on these statements.
In particular, our expectations could be affected by, among other things:
• Uncertainties regarding the success of key products, commercial priorities and strategy
• Uncertainties in the research and development of new healthcare products, including clinical trial results and additional analysis of existing clinical data, and the use of new and disruptive technologies, including artificial intelligence (AI)
• Global trends toward healthcare cost-containment, including new laws and regulations, ongoing government, payer and general public pricing and reimbursement pressures and requirements for increased pricing transparency
• Our ability to realize the strategic benefits, operational efficiencies or opportunities expected from our external business opportunities
• Our ability to realize the intended benefits of our separation of Sandoz into a new publicly traded standalone company
• Our ability to obtain or maintain proprietary intellectual property protection, including the ultimate extent of the impact on Novartis of the loss of patent protection and exclusivity on key products that commenced in prior years and is expected to continue this year
• Our performance on environmental, social and governance matters
• Uncertainties in the development or adoption of potentially transformational digital technologies and business models
• Uncertainties regarding potential significant breaches of information security or disruptions of our information technology systems
• Uncertainties surrounding the implementation of our new IT projects and systems
• Our reliance on outsourcing key business functions to third parties
• Uncertainties regarding actual or potential legal proceedings, including, among others, litigation and other legal disputes with respect to our recent transactions, product liability litigation, litigation and investigations regarding sales and marketing practices, intellectual property disputes and government investigations generally
• Safety, quality, data integrity or manufacturing issues
• Our ability to identify, attract, integrate, develop and retain key personnel and qualified individuals for critical roles
• Regulatory actions or delays or government regulation generally, including potential regulatory actions or delays with respect to the development of the products described in this Annual Report
• Our ability to comply with evolving regulatory requirements and meet societal expectations concerning environmental, social and governance matters
• Our ability to comply with cybersecurity and data privacy laws and regulations, and uncertainties regarding potential significant breaches of data privacy
• Our ability to adapt to major geopolitical and macroeconomic developments, including the effects of and efforts to mitigate pandemic diseases such as COVID-19, and the impact of the war in certain parts of the world
• Uncertainties involved in predicting shareholder returns
• Uncertainties regarding the effects of recent and anticipated future changes in tax laws and their application to us
• Uncertainties regarding future global exchange rates
• Uncertainties regarding our supply chain and future demand for our products
These risks and others are discussed in more detail in this Annual Report, including under “Item 3. Key Information—Item 3.D. Risk factors,” “Item 4. Information on the Company,” and “Item 5. Operating and Financial Review and Prospects.” Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those described in this Annual Report as anticipated, believed, estimated or expected. It is not possible to predict or identify all risk to our business. Consequently, you should not consider the foregoing to be a complete discussion of all potential risks or uncertainties. We provide the information in this Annual Report as of the date of its filing. We do not intend, and do not assume any obligation, to update any information or forward-looking statements set out in this Annual Report as a result of new information, future events or otherwise.
Part I
Item 1. Identity of Directors, Senior Management and Advisers
Not applicable.
Item 2. Offer Statistics and Expected Timetable
Not applicable.
Item 3. Key Information
3.A [Reserved]
3.B Capitalization and indebtedness
Not applicable.
3.C Reasons for the offer and use of proceeds
Not applicable.
3.D Risk factors
Our business faces significant risks and uncertainties. You should carefully consider all of the information set forth in this Annual Report and in other documents we file with or furnish to the SEC, including the following risk factors, before deciding to invest in or to maintain an investment in any Novartis securities. Our business, as well as our reputation, financial condition, results of operations, and share price, could be materially adversely affected by any of these risks, as well as other risks and uncertainties not currently known to us or not currently considered material.
Strategic risks
Key products and commercial priorities
Risk description
Failure to deliver key commercial priorities and successfully launch new products
Context and potential impact
Our ability to maintain and grow our business and to replace revenue and income lost to generic, biosimilar and other competition depends heavily on the commercial success of our new or existing key products. The commercial success of these products could be impacted at any time by a number of factors, including pressure from new or existing competitive products, changes in the prescribing habits of healthcare professionals, slower than expected post-launch adoption, unexpected side effects or safety signals, supply chain issues or other product shortages, pricing pressure, regulatory proceedings, changes in labeling, loss of intellectual property protection, and global pandemics. In addition, our revenue and margins could be significantly impacted by the timing and rate of commercial acceptance of new products.
Healthcare professionals, patients and payers may choose competitor products instead of ours for various reasons, including if they perceive them to be better in terms of efficacy, safety, cost, convenience or other reasons. The commercial success of our key products and launches in the face of increasing competition requires significant attention, management focus and resource allocation. Such competition could significantly affect the revenue from our products and our results of operations. This impact could also be compounded to the extent that such competition results in us making significant additional investments in research and development, marketing or sales.
Furthermore, from time to time, we reassess how our business is organized to ensure we have the optimal structure with which to execute our strategy. An inability to successfully implement new organizational structures and operating models could have a material adverse effect on our results of operations and financial condition.
Research and development
Risk description
Failure to successfully prioritize, integrate and execute our research and development programs for new products or new indications for existing products
Context and potential impact
We engage in extensive and costly research and development activities, both through our own internal resources and through collaborations with third parties, in an effort to identify and develop new products and new indications for existing products that address unmet, ever-changing medical needs, while ensuring commercial viability and success. Our ability to grow our business and our product pipeline; to replace sales lost due to branded competition, entry of generics, or other reasons; and to bring products to market that take
advantage of new and potentially disruptive technologies, including cell, gene and radioligand therapies, depends in significant part on the success of these efforts.
Failure to successfully develop our pipeline products is typically the result of the inherent uncertainty of science, suboptimal internal execution, or both. Key elements of internal execution include our ability to prioritize our investments on our highest potential value assets, optimize the transition of assets from research to development, integrate externally acquired assets in an efficient way, and execute the steps in our drug development process that enable our assets to be approved and reimbursed in a timely manner to positively impact clinical practice. We invest in new businesses, products, services and technologies, including artificial intelligence (AI), to achieve our goals, operate our business and reduce the time, effort and expense associated with identifying, developing and commercializing new products. Our investments in new and disruptive technologies may not ultimately achieve the intended benefits, may not result in an adequate return of capital and, in pursuing new strategies, we may incur unanticipated liabilities. For more information, see also “Item 4. Information on the Company—Item 4.B Business overview—Research and development.”
Our new products must undergo intensive preclinical and clinical testing and are approved by means of a highly complex, lengthy, and expensive approval process that varies substantially from country to country and may have very specific requirements for the recruitment of patients for clinical trials. We face increasing and evolving regulatory approval and reimbursement requirements. Additionally, if we fail to successfully progress late-stage assets and the core elements of drug development for key programs, this could have a negative impact on the development of our product pipeline, and ultimately on the success of our business and our financial results.
Another issue we face is the increasing challenge to adequately recruit a sufficient number of patients in the US for clinical trials due to the cost and effort associated with expanding our operations for the recruitment of patients into such trials. As a result, we may be unable to develop the necessary clinical evidence to support the desired indications and product profile for a particular disease that is needed to drive clinical adoption of our new products, and thereby achieve the full potential of our assets (also known as the “target product profile”). Similarly, the post‑approval regulatory burden has also increased. These requirements make the maintenance of regulatory approvals for our products increasingly expensive, and further heighten the risk of recalls, product withdrawals, changes to product specifications, loss of market share, and loss of revenue and profitability.
The clinical testing, regulatory processes and post-approval activities described above become more difficult during pandemics, such as the COVID-19 pandemic, as well as during periods of geopolitical and economic uncertainty. This is due to challenges related to recruiting, enrolling and treating patients in clinical trials, as well as ensuring the supply of trial materials. For a further description of the research and development of, and approval processes for, our products, see “Research and development” and “Regulation” under “Item 4. Information on the Company—Item 4.B Business overview.”
Furthermore, our research and development activities must be conducted in an ethical and compliant manner. Among other things, we are concerned with patient safety (both pre- and post-product approval), data privacy, current Good Clinical Practices (cGCP) requirements, data integrity, the fair treatment of patients, diversity and inclusion in the recruitment of patients to clinical trials, and animal welfare. If we fail to properly manage such issues, we risk injury to third parties, damage to our reputation, negative financial consequences as a result of potential claims for damages, sanctions and fines, and the potential that investments in research and development activities may not bring the expected benefits to us.
Pricing, reimbursement and access
Risk description
Pricing and reimbursement pressure, including pricing transparency and access to healthcare
Context and potential impact
Our business has continuously experienced significant pressures on the pricing of our products and on our ability to obtain and maintain satisfactory rates of reimbursement for our products by governments, insurers and other payers. These pressures have many sources, including growth of healthcare costs as a percentage of gross domestic product; funding restrictions and policy changes; and public controversies, political debate, investigations and legal proceedings regarding pharmaceutical pricing. Pressures on pricing may negatively impact both our product pricing and the availability of our products.
In addition, we face numerous cost‑containment measures imposed by governments and other payers. These include government‑imposed industrywide price reductions, mandatory pricing systems, reference pricing systems, payers limiting access to treatments based on cost‑benefit analyses, the importation of drugs from lower‑cost countries to higher‑cost countries, the shifting of the payment burden to patients through higher co‑payments and co-pay accumulator programs, the limiting of physicians’ ability to choose among competing medicines, the mandatory substitution of generic drugs for the patented equivalent, pressure on physicians to reduce the prescribing of patented prescription medicines, increasing pressure on intellectual property protections, and growing requirements for increased transparency on pricing. For more information on price controls, see “Item 4. Information on the Company—Item 4.B Business overview—Price controls.”
Recent trends in our external environment may have an impact on the likelihood of these pricing and reimbursement pressures occurring. Slow economic recovery following the COVID-19 pandemic and the onset of war in certain parts of the world (which is contributing to challenges such as high energy costs and inflation) have led to an increased strain on fiscal budgets in many major economies. In addition, legislative developments such as those in the US (e.g., the Inflation Reduction Act) and in Europe (e.g., the EU Joint Health Technology
Assessment and 2023 EU Pharmaceutical Legislation Update) pose potential further pressures on pricing and timelines for reimbursement in these countries. For example, in August 2023, our cardiovascular drug Entresto was selected for the Medicare Drug Price Negotiation Program in the US and additional Novartis products may be selected for price negotiation programs in the future. These external factors may materially affect our ability to achieve value-based prices; to achieve and maintain an acceptable return on our investments in the research and development of our products; and may impact our ability to research and develop new products.
Alliances, acquisitions and divestments
Risk description
Failure to identify, execute or realize the expected benefits from our external business opportunities
Context and potential impact
As part of our strategy, we evaluate external opportunities that could strengthen our portfolio by acquiring and divesting products, entering businesses or entering into strategic alliances and collaborations. For example, in 2023, we closed the acquisitions of Chinook Therapeutics and DTx Pharma. This strategy is partly dependent on our ability to identify strategic external business opportunities, including assessing the value of the early phase companies, and to close transactions with third parties on acceptable terms and timelines.
Once the terms of a strategic transaction have been agreed with a third party, we may not be able to complete the transaction in a timely manner or at all. In addition, we cannot be sure that pre-transaction due diligence will identify all possible issues that might arise during and after the transaction. Our efforts on such transactions can also divert management’s attention from our existing businesses.
After a transaction is closed, efforts to develop and commercialize acquired or licensed products, to integrate the acquired business or to achieve expected synergies may fail or may not fully meet expectations. This may occur due to difficulties in retaining key personnel, customers and suppliers; failure to obtain marketing approval or reimbursement within expected timeframes or at all; differences in corporate culture, standards, controls, processes and policies; or other factors. Transactions can also result in liabilities being incurred that were not known at the time of acquisition, or the creation of tax or accounting issues. Acquired businesses are not always in full compliance with legal, regulatory or Novartis standards, including, for example, Current Good Manufacturing Practices (cGMP) or cGCP standards, which can be costly and time-consuming to remediate. Furthermore, our strategic alliances and collaborations with third parties may not achieve their intended goals and objectives within expected time frames, or at all. For more information about recent business acquisitions, see “Item 18. Financial Statements—Note 2. Significant transactions.”
Similarly, we cannot ensure that we will be able to successfully divest or spin off businesses or other assets that we have identified for this purpose, or that any completed divestment or spin-off will achieve the expected strategic benefits, operational efficiencies or opportunities, or that the divestment or spin-off will ultimately maximize shareholder value.
Intellectual property
Risk description
Expiry, assertion or loss of intellectual property protection
Context and potential impact
Many of our products are protected by intellectual property rights, which may provide us with exclusive rights to market those products for a limited time, and to enable our purpose of reimagining medicine by sustainably financing our research and development. However, the strength and duration of those rights can vary significantly from product to product and from country to country, and they may be successfully challenged by third parties or governmental authorities.
Loss of intellectual property protection and the introduction of generic or biosimilar competition for a patented branded medicine in a country typically result in a significant reduction in net sales and operating income for the branded product. Such competition can occur after successful challenges to intellectual property rights or the regular expiration of the patent term or other intellectual property rights. Such competition can also result from the entry of generic or biosimilar versions of another medicine in the same therapeutic class as one of our drugs or in a competing therapeutic class, from a Declaration of Public Interest or the compulsory licensing of our intellectual property by governmental authorities, or as a result of a general weakening of intellectual property and governing laws in certain countries around the world. In addition, generic or biosimilar manufacturers may sometimes conduct so-called “launches at risk” of products that are still under legal challenge for infringement, or whose patents are still under legal challenge for validity, before final resolution of legal proceedings.
We also rely in all aspects of our businesses on unpatented proprietary technology, know-how, trade secrets and other confidential information, which we seek to protect through various measures, including confidentiality agreements with licensees, employees, third-party collaborators and consultants who may have had access to such information. If these agreements are breached or our other protective measures should fail, then our contractual or other remedies may not be adequate to cover our losses.
We may also be subject to assertions of intellectual property rights against our medicines by third parties. If successful, these actions may involve payment of future royalties or damages, for example for patent infringement, and may also involve injunctive relief requiring the removal of one or more dosage strengths of a product from the market (or removal of a therapeutic indication from the product’s approved labeling) for a period of time or throughout the life of the asserted intellectual property right. Such damages or such an injunction may have a material impact on our operating income and net sales.
In any given year, we may experience a potentially significant impact on our net sales from products that have already lost intellectual property protections, as
well as products that may lose protection during the year. Because we may have substantially reduced marketing and research and development expenses related to products that are in their final years of exclusivity, the initial loss of protection for a product during a given year could also have an impact on our operating income for that year in an amount corresponding to a significant portion of the product’s lost sales. The magnitude of the impact of generic or biosimilar competition on our income could depend on a number of factors. These include, with respect to income in a given year, the time of year at which the generic or biosimilar competitor is launched; the ease or difficulty of manufacturing a competitor product and obtaining regulatory approval to market it; the number of generic or biosimilar competitor products approved, including whether, in the US, a single competitor is granted an exclusive marketing period; whether an authorized generic is launched; the geographies in which generic or biosimilar competitor products are approved, including the strength of the market for generic or biosimilar pharmaceutical products in such geographies, and the comparative profitability of branded pharmaceutical products in such geographies; and our ability to successfully develop and launch new products for patients that may also offset the income lost to generic or biosimilar competition. For more information on the patent and generic competition status of our products, see “Item 4. Information on the Company—Item 4.B Business overview—Intellectual property.”
Sandoz spin-off
Risk description
We may not successfully achieve our goals related to our separation from Sandoz and our failure to do so may have an adverse impact on our business
Context and potential impact
We recently completed the separation of Sandoz, our generics and biosimilars division, into a new Swiss publicly traded independent company, by way of a 100% spin-off. In connection with the Sandoz separation, we entered into a separation and distribution agreement and various other agreements. These agreements govern the separation and distribution and the relationship between Novartis and Sandoz going forward, including with respect to the allocation of assets and liabilities between Novartis and Sandoz. The agreements also provide for the performance of services by each company for the benefit of the other company for a period of time. The terms, scope and/or duration of these agreements could negatively impact our ability to pursue other strategic business interests as we will have to devote resources and capacity to fulfilling our obligations that we may prefer to direct elsewhere. If we or Sandoz are unable to satisfy our respective obligations under these agreements, we could incur losses or experience operational challenges or difficulties. These agreements could also lead to disputes over the performance of obligations under these agreements or the allocation of our respective resources. For example, during the term of these agreements, we may have less flexibility to optimize our biologic manufacturing for our own products (or those of other third parties). In addition, pursuant to these agreements, we will perform technical development services for Sandoz, which may involve certain proprietary know-how. While we intend to retain the personnel involved in our technical research and development and to protect our trade secrets, provision of such services might create the incremental potential for the disclosure or misuse of such proprietary know-how, particularly in connection with technology transfer at the end of such arrangements.
Additionally, we may not realize the anticipated strategic, financial, operational, or other benefits from our separation of Sandoz. We cannot predict with certainty when the benefits expected from the Sandoz spin-off will occur or the extent to which they will be achieved. In addition, we incurred one-time costs and may encounter operational inefficiencies in connection with the Sandoz spin-off that may negate some of the benefits we expect to achieve. If we do not realize these assumed benefits, we could suffer a material adverse effect on our financial condition.
Further, if the spin-off does not generally qualify as a tax-neutral transaction for Swiss and U.S. federal income tax purposes, we, our shareholders, or both, could be subject to significant tax liabilities. The spin-off is intended to qualify for tax-neutral treatment for us and our shareholders for Swiss and U.S. federal income tax purposes. If, however, the spin-off fails to qualify as tax-neutral for Swiss and U.S. federal income tax purposes, we, our shareholders, or both, could recognize taxable gain with respect to the spin-off, resulting in Swiss and U.S. income, withholding and capital gains tax consequences. In particular, if the spin-off does not qualify as tax neutral for Swiss and U.S. federal income tax purposes, our shareholders who received shares of Sandoz in the spin-off as part of the separation would be subject to tax as if they had received a taxable distribution equal to the fair market value of such shares. For additional information about the potential tax consequences of the spin-off, see “Item 10.E Taxation—Tax consequences of the Sandoz spin-off.”
Environmental, social and governance matters
Risk description
Failure to meet rapidly evolving environmental, social and governance expectations
Context and potential impact
Increasingly, in addition to financial results, companies are being judged by their performance on a variety of environmental, social and governance (ESG) matters, which can contribute to the long-term sustainability of a company’s performance. An inability to successfully perform on ESG matters and to meet societal expectations could result in negative impacts on our reputation, recruitment, retention, operations, financial results, and share price.
Topics related to large societal changes such as social inequity, access to medicines and climate change are increasingly important to a wide range of our stakeholders. For example, a variety of organizations measure the performance of companies on ESG topics, and the results of these assessments are widely publicized. In addition, investments in funds that specialize in
companies that perform well in such assessments are increasingly popular, and major institutional investors have publicly emphasized the importance of such ESG measures in making their investment decisions. Our actions related to ESG topics may in the long-term impact our operations and ability to achieve our strategic goals, and ultimately could have a potential negative impact on the value of Novartis.
We actively manage a broad range of ESG matters, taking into consideration their expected impact on the sustainability of our business over time, and the potential impact of our business on society and the environment. We have created a Sustainability & ESG Office, which, in coordination with the ESG Committee of the Executive Committee of Novartis, is tasked with developing our ESG strategy and tracking our performance against our ESG targets. However, considering the fast pace of change of external expectations, and a range of upcoming regulations, there can be no certainty that we will manage such issues successfully, that the ESG standards we currently use to measure our performance against will remain the same, or that we will successfully meet society or investors’ expectations. Failure to meet rapidly evolving regulatory requirements and investor and societal expectations could also result in litigation or regulatory actions, which could have a material adverse impact on our reputation, recruitment, retention, operations, financial results, and share price. Additionally, external partners in our value chain that we do not control may not comply with ESG commitments and goals we set for ourselves, which may have a negative impact on our business.
Operational risks
Cybersecurity and data protection
Risk description
Cybersecurity breaches, data loss and catastrophic loss of IT systems
Context and potential impact
We are heavily dependent on critical, complex and interdependent information technology (IT) systems, including internet‑based systems to support our business processes. We also outsource significant parts of our IT infrastructure to third-party providers, and currently use these providers to perform business-critical IT services for us. We are therefore vulnerable to cybersecurity attacks and incidents on such networks and systems, whether our own or those of the third-party providers that we contract, and we have experienced, and may in the future experience, such cybersecurity threats and attacks. Cybersecurity threats and attacks take many forms, and the size, age and complexity of our IT systems make them potentially vulnerable to external and internal security threats; outages; malicious intrusions and attacks; cybercrimes, including state-sponsored cybercrimes; malware; misplaced data, lost data or data errors; programming or human errors; or other similar events. The risk of such threats and attacks has increased, as virtual and remote working have become more common, and sensitive data is accessed by employees working in less secure, home-based environments. In addition, due to our reliance on third-party providers, we have experienced, and may in the future experience, interruptions, delays or outages in IT service availability due to a variety of factors outside of our control, including technical failures, natural disasters, fraud, or security attacks experienced by or caused by third-party providers. Interruptions in the service provided by these third parties could affect our ability to perform critical tasks.
A significant information security or other event, such as a disruption or loss of availability of one or more of our IT systems, whether managed by us or a third-party service provider, has previously and could in the future negatively impact important business processes, such as the conduct of scientific research and clinical trials, the submission of data and information to health authorities, our manufacturing and supply chain processes, our shipments to customers, our compliance with legal obligations, and communication between employees and with third parties. IT issues have previously led to, and could in the future lead to, the compromise of trade secrets or other intellectual property that could be sold and used by competitors to accelerate the development or manufacturing of competing products; the compromise of personal financial and health information; and the compromise of IT security data such as usernames, passwords and encryption keys, as well as security strategies and information about network infrastructure, which could allow unauthorized parties to gain access to additional systems or data. In addition, malfunctions in software or medical devices that make significant use of IT could lead to a risk of direct harm to patients.
Although we have experienced some of the events described above, to date they have not had a material impact on our operations. Nonetheless, the occurrence of any of the events described above in the future could disrupt our business operations and result in enforcement actions or liability, including potential government fines and penalties, claims for damages, and shareholder litigation or allegations that the public health, or the health of individuals, has been harmed.
Any significant events of this type could require us to expend significant resources beyond those we already invest to remediate any damage, to further modify or enhance our protective measures, and to enable the continuity of our business.
Strategic technology programs implementation
Risk description
Failure to successfully implement our IT strategy may disrupt our core business processes
Context and potential impact
We rely on various IT systems to operate our complex global business and several of our current IT systems are reaching the end of their useful life, which could cause disruptions to our operational stability. As a result, we are implementing several companywide IT programs to replace and consolidate outdated IT systems and to simplify and standardize our processes, systems and tools, and create a unified data marketplace. Implementation and operation of these new systems involves certain risks, including the potential for a failure of the new
systems to operate as expected; a failure to properly integrate new systems with other systems we use; delays in adopting and scaling of new systems; potential loss of data or information; a failure of, or potential issues with, systems related to our payment and procurement processes; compliance issues; and cost overruns and delays. Our inability to timely and successfully implement our IT strategy may prevent us from materializing the expected business benefits and could lead to business disruptions, cost inefficiencies and potential exposure to legal, regulatory and reputational risks as our internal controls could be negatively affected. Any disruptions or malfunctions of new systems could cause critical information to be delayed, lost, defective, corrupted, or rendered inadequate or inaccessible, which could negatively impact our operations, the effectiveness of our internal controls and financial condition.
Talent management
Risk description
Inability to identify, attract, develop and retain qualified talent for critical roles
Context and potential impact
We rely on identifying, attracting, developing and retaining a diverse, highly skilled workforce across our business and functions to achieve our objectives. If we are unable to sustain our supply of key personnel—including senior members of our scientific and management teams, high‑quality researchers and development specialists and skilled employees with key capabilities in key markets—our ability to achieve our major business objectives may be adversely affected. In addition, our brand and reputation could be negatively impacted, and the diversity of our workforce may decline.
The market for skilled talent has become increasingly competitive, and we anticipate this trend will persist in the long term. We face a challenge to attract and retain top talent in several areas, including biology, immunology, chemistry, clinical development, drug manufacturing, data, digital and IT, oncology, and advanced therapy platforms (i.e., gene and cell therapy, radioligand therapy and “xRNA”). In addition, many pharmaceutical and biotechnology companies, universities and research centers, and government entities with significant capital are not only competing with us to attract the same skilled talent but are also aggressively pursuing our experienced talent. Furthermore, if we are unable to retain and engage key talent of companies that we acquire and integrate, we may not be able to realize the full value of these acquisitions.
In recent years, we have adopted new ways of working that include location flexibility and increasingly recruiting from a global pool of talent. However, the success of our business continues to depend on having employees who possess local knowledge of, and experience in, our key markets. The external talent supply is especially limited in many of the geographies that are expected to be sources of growth for us. In the United States, China and several other markets, the geographic mobility of talent is decreasing, as they find ample career opportunities available closer to home.
The risks associated with the challenging talent market will be exacerbated if we are unable to retain and effectively develop employees, and to maintain an internal pipeline with critical skills, experiences, and leadership to deliver our business priorities. As a result, development, engagement, motivation, succession planning and performance rewards for our critical talent are essential to achieve our business priorities.
External partner risk management and human rights
Risk description
Failure to maintain adequate governance and oversight over external partner relationships, and failure of external partners to meet their contractual, regulatory or other obligations
Context and potential impact
We rely on external partners for the performance of certain key business functions and services, including, among others, research and development, manufacturing operations and warehousing and distribution, certain finance functions, sales and marketing activities and data management. Some external partners, particularly those in developing countries, do not have internal compliance systems or resources comparable to ours. As a result, our investment and efforts in relation to external partner management include focusing on risk management and the oversight of such external partners.
Our reliance on external partners poses certain risks, including the misappropriation of our intellectual property, the failure of the external partner to comply with regulatory and quality assurance requirements, the failure of the external partner to comply with environmental, anti-bribery and human rights standards and regulations, unexpected supply disruptions, breach of our agreement by the external partner, and the unexpected termination or nonrenewal of our agreement by the external partner.
In addition, governments require us, and the public expects us, to take responsibility for and report on compliance with various human rights, responsible sourcing and environmental practices, as well as other actions of our external partner contractors around the world.
Ultimately, if external partners fail to meet their obligations to us, we may lose our investment in the relationship with the external partners or fail to receive the expected benefits of our agreements with such external partners. While we aim to identify and assess any risk of harm to society caused by our external partners’ operations, should any of these external partners fail to comply with the law or our standards, or should they otherwise act inappropriately while performing services for us, we could be held responsible for their acts, our reputation may suffer, and penalties could be imposed on us.
Legal, regulatory, ethics and compliance
Risk description
Challenges posed by evolving legal and regulatory requirements, innovative and disruptive technologies, and societal expectations regarding ethical behavior
Context and potential impact
We are subject to an extensive and complex framework of laws and regulations across the jurisdictions in which we operate.
The laws and regulations relevant to the healthcare industry and applicable to us are broad in scope, are subject to change, and have evolving interpretations, which could require us to incur substantial costs associated with compliance or to alter one or more of our business practices. For example, we have been, are currently, and may in the future be, subject to various significant legal proceedings, such as private party litigation, government investigations and law enforcement actions worldwide. These types of matters may take various forms based on evolving government enforcement and private party litigation priorities, and could include, for example, matters pertaining to: pricing; bribery and corruption; trade regulation and embargo legislation; product liability; commercial disputes; employment and wrongful discharge; antitrust and competition; securities; government benefit programs; reimbursement; rebates; healthcare fraud; sales and marketing practices; insider trading; occupational health and safety; environmental regulations; tax; cyber and data security; use of technologies, including AI; data privacy; regulatory interactions; disclosure compliance; and intellectual property. Such matters can involve civil or criminal proceedings and can retroactively challenge practices previously considered to be legal.
There is also a risk that governance of our medical and patient support activities, and of our interactions with governments, public officials/institutions, healthcare professionals, healthcare organizations and patient organizations may be inadequate or fail, or that we may undertake activities based on improper or inadequate scientific justification.
Legal proceedings and investigations are inherently unpredictable, and significant judgments sometimes occur. Consequently, we may in the future incur judgments that could involve large payments, including the potential repayment of amounts allegedly obtained improperly, and other penalties, including treble damages. In addition, such legal proceedings and investigations, even if meritless, may affect our reputation, may create a risk of potential exclusion from government reimbursement programs in the US and other countries, and may lead to civil litigation or criminal exposure. As a result, having considered all relevant factors, we have in the past and may again in the future enter into major settlements of such claims without bringing them to final legal adjudication by courts or other such bodies, despite having potentially significant defenses against them, to limit the risks they pose to our business and reputation. Such settlements may require us to pay significant sums of money and to enter into corporate integrity or similar agreements, which are intended to regulate company behavior for extended periods. From time to time, we may also initiate challenges to laws or regulations that we believe are illegal or unconstitutional. For example, in September 2023, we filed a lawsuit against the US Department of Health and Human Services and the Centers for Medicare and Medicaid Services because we believe the drug price-setting provisions in the Inflation Reduction Act (IRA) are unconstitutional and will have long-lasting negative consequences for patients by limiting access to medicines now and in the future. The result of this and similar litigation we may pursue in the future is inherently uncertain and may negatively impact our business and reputation.
For information on significant legal matters pending against us, see “Item 18. Financial Statements—Note 21. Provisions and other non‑current liabilities” and “Item 18. Financial Statements—Note 29. Commitments and contingent liabilities.”
New requirements may also be imposed on us due to changing government and societal expectations regarding the healthcare industry, and acceptable corporate behavior generally. For example, we are faced with laws and regulations requiring changes in how we do business, including with respect to disclosures concerning our interactions with healthcare professionals, healthcare organizations and patient organizations. These laws and regulations include requirements that we disclose payments or other transfers of value made to healthcare professionals and organizations, as well as information relating to the costs and prices for our products, which represent evolving standards of acceptable corporate behavior. These requirements may cause us to incur significant costs, including substantial time and additional resources, that are necessary to bring our interactions with healthcare professionals and organizations into compliance with these evolving standards.
To support our efforts to comply with the many requirements that impact us, we have a significant global ethics, risk and compliance program in place, and we devote substantial time and resources to efforts to ensure that we conduct business in a lawful manner, and in line with society’s expectations. Despite our efforts, an actual or alleged failure to comply with the law or with heightened public expectations could lead to substantial liabilities that may not be covered by insurance, or to other significant losses.
Manufacturing and product quality
Risk description
Inability to ensure proper controls in product development and product manufacturing, and failure to comply with applicable regulations and standards
Context and potential impact
The development and manufacture of our products is complex and heavily regulated by governmental health authorities around the world. Regardless of whether our products and the related raw materials are developed and manufactured at our own manufacturing sites or by third parties, we must ensure that all development and manufacturing processes comply with regulatory requirements, as well as our own quality standards in order to deliver novel therapies while ensuring patient safety. Failure to comply with regulatory requirements may result in warning letters, suspension of manufacturing, seizure of products, injunctions, product recalls, failure to secure product approvals, debarment or harm to patients or our reputation.
In recent years, global health authorities have substantially intensified their scrutiny of manufacturers’ compliance with regulatory requirements. Any significant
failure by us or our third-party suppliers to comply with regulatory requirements, or with health authorities’ expectations, may create the need to suspend clinical trials, shut down production facilities or production lines, and recall commercial products. A failure to fully comply with regulatory requirements could also lead to a delay in the approval of new products, an inability to ship or import our products, and significant penalties and reputational harm.
In addition, the technically complex manufacturing processes required to manufacture many of our products increase the risk of both production failures and product recalls, and can increase the cost of producing our goods. Some of our products require a supply of highly specialized raw materials, such as cell lines, tissue samples, bacteria, viral strains and radioisotopes. In addition, we manufacture and sell a number of sterile products, biologic products and products that involve advanced therapy platforms, such as gene and cell therapy, radioligand therapy, and “xRNA,” all of which are particularly complex and involve highly specialized manufacturing technologies. For more information, see “Item 4. Information on the Company—Item 4.B. Business overview—Production.” As a result, even slight deviations at any point in their production processes or in the materials used have led to, and may in the future lead to, production failures or recalls.
Supply chain
Risk description
Inability to maintain continuity of product supply
Context and potential impact
Many of our products are produced using technically complex manufacturing processes and require a supply of highly specialized raw materials. For some of our products and raw materials, we may rely on a single source of supply. In addition, we manufacture and sell a number of sterile products, biologic products, and products that involve advanced therapy platforms, such as gene and cell therapy, radioligand therapy, and “xRNA,” all of which are particularly complex and involve highly specialized manufacturing technologies. Due to this complexity, there is a risk of production and supply of critical raw materials failures, which may result in supply interruptions or product recalls due to manufactured products not meeting required specifications.
In addition, due to the inherent complexities of our manufacturing processes and the supply chains for advanced therapy platforms, we are required to plan our production activities and purchase of materials well in advance. If we suffer from third-party raw material shortages, underestimate market demand for a product, or fail to accurately predict when a new product will be approved for sale, then we may not be able to produce sufficient product to meet demand. These issues could be made worse during a pandemic, or geopolitical events, such as wars in certain parts of the world, and could lead to (i) a sudden increase in demand for selected medicinal products, resulting in the short-term unavailability of critical materials; (ii) logistical and supply challenges that may lead to our inability to ship products from one location to another due to restrictions imposed as a result of a pandemic or geopolitical events and any related sanctions, which can also impact transportation and warehousing costs; or (iii) our inability to properly operate a manufacturing site due to restrictions imposed as the result of a pandemic or any issues arising from geopolitical events.
Our or our suppliers’ inability to manage such issues could lead to shutdowns, product shortages, or to us being entirely unable to supply products to patients for an extended period of time. Furthermore, as our products are intended to promote the health of patients, such shortages or shutdowns could endanger our reputation and have led to, and could continue to lead to, significant losses of sales revenue, potential litigation or allegations that the public health, or the health of individuals, has been harmed.
Data privacy
Risk description
Noncompliance with personal data protection laws and regulations
Context and potential impact
We operate in an environment that relies on the collection, processing, analysis and interpretation of large sets of patients and other individuals’ personal information, including via social media and mobile technologies. In addition, the operation of our business requires data to flow across the borders of numerous countries in which there are different, potentially conflicting, and frequently changing, data privacy laws in effect. Examples of such laws include: the EU General Data Protection Regulation (GDPR); the California Consumer Privacy Act; Brazil’s General Personal Data Protection Law; and the Personal Information Protection Law in China. Such laws impose stringent requirements on how we and third parties with whom we contract collect, share, export or otherwise process personal information, and provide for significant penalties for noncompliance. Breaches of our systems or those of our third-party contractors, or other failures to protect the data we collect from misuse or breach by third parties, could expose such personal information to unauthorized persons.
Events involving the substantial loss of personal information, use of personal information without a legal basis, or other privacy violations could give rise to significant liability, reputational harm, damaged relationships with business partners, and potentially substantial monetary penalties and other sanctions under laws enacted or being enacted around the world. Such events could also lead to restrictions on our ability to use personal information and/or transfer personal information across country borders, which could interfere with critical business operations. In addition, there is a trend of increasing divergence of data privacy legal frameworks, not only across these frameworks but also within individual legal frameworks themselves. This divergence may constrain the implementation of global business processes and may lead to different approaches on the use of health data for scientific research, which may have a negative impact on our business and operations.
Falsified medicines
Risk description
Impact of falsified medicines on patient safety, and reputational and financial harm to Novartis and our products
Context and potential impact
We continue to be challenged by the vulnerability of distribution channels to falsified medicines, which include counterfeit, stolen, tampered and illegally diverted medicines, as defined by the World Health Organization.
Falsified medicines pose patient safety risks and can be seriously harmful or life-threatening. Reports of adverse events related to falsified medicines and increased levels of falsified medicines in the healthcare system affect patient confidence in genuine medicines and in healthcare systems in general. These events could also cause us substantial reputational and financial harm, and potentially lead to litigation if the adverse event from the falsified medicine is mistakenly attributed to the genuine one. Stolen or illegally diverted medicines that are not properly stored and later sold through unauthorized channels could adversely impact patient safety, our reputation and our business. Furthermore, there is a direct financial loss when falsified medicines replace sales of genuine medicines, or genuine medicines are recalled following the discovery of falsified products.
Emerging risks
Geopolitical developments
Risk description
Impact of geo- and socio-political threats
Context and potential impact
Geopolitical tensions in various parts of the world worsened in 2023 and could continue to worsen in 2024 and beyond. Direct conflicts, including the ongoing wars in Ukraine and the Middle East, an increasingly challenging economic landscape and social unrest, each have both a direct and indirect impact on the pharmaceutical industry and lead to a degree of uncertainty about the future.
As a result of ongoing geopolitical tensions, certain countries have adopted, and may in the future adopt additional, protectionist measures including the imposition of tariffs. Tariffs that are intended to shield domestic markets from foreign competition and the possibility of additional trade restrictions, such as export controls, could have a material impact on our business. If tariffs or export controls on pharmaceutical products or active pharmaceutical ingredients (APIs) were increased in certain parts of the world, our supply chain and flow of our products could be immediately disrupted. There is also an additional risk that aggressive monetary and fiscal policies by governments and central banks to curb inflation may prompt market-specific recessions and raise the cost-of-living, further putting pressure on pricing and cost containment for the pharmaceutical industry.
Collectively, unstable geo- and socio-political conditions could, among other things, disturb the international flow of goods and increase the costs and difficulties of international transactions. This could potentially impact our ability to develop and supply our products to patients in an undisrupted fashion, and further erode reimbursement mechanisms for our medicines.
Macroeconomic developments
Risk description
Impact of macroeconomic developments
Context and potential impact
Our business may be impacted by deteriorating macroeconomic and financial conditions directly affecting us, our suppliers, payers and consumers. Given that patients, in many countries, directly pay a sizable and increasing portion of their own healthcare costs, there is a risk that consumers may cut back on prescription drugs due to financial constraints.
Negative macroeconomic developments may also adversely affect the ability of payers, as well as our distributors, customers, suppliers, and service providers, to pay for our products, or to buy necessary inventory or raw materials, and to perform their obligations under agreements with us. Weakening growth and rising interest rates may also increase the credit risk of our counterparties. Although we make efforts to monitor the financial condition and liquidity of these third parties, our ability to do so is limited, and some of them may become unable to pay their bills in a timely manner or may even become insolvent. These risks may be elevated with respect to our interactions with fiscally challenged government payers, or with third parties with substantial exposure to such payers.
At the same time, significant changes, and potential future volatility in financial markets, the consumer and business environment, the competitive landscape, and the global political and security landscape make it increasingly difficult for us to predict our revenues and earnings. As a result, any revenue or earnings guidance or outlook that we have given or might give may be overtaken by events or may otherwise prove to be inaccurate. Although we endeavor to give reasonable estimates of future revenues and earnings at the time at which we give such guidance, based on then‑current knowledge and conditions, there is a risk that such guidance or outlook will prove to be incorrect.
Asset price corrections in financial markets may also result in lower returns on our financial investments. In addition, pricing pressures in developed markets resulting from efforts to reduce the cost of healthcare (e.g., the Inflation Reduction Act in the US, which targets drug prices) may have a negative impact on our revenue and our net sales. In addition, inflation may have an impact on our operating costs in the form of higher prices for supplies, energy, raw materials, wages, and capital, which could reduce our net income.
Uncertainties around future central bank and other economic policies in the US and EU, including rising interest rates, as well as high debt levels in some countries could also impact world trade. Sudden increases in economic, currency or financial market volatility in different countries, such as appreciation of the US dollar, have also impacted, and may continue to have an unpredictable impact on our business, or results of operations,
including the conversion of our operating results into our reporting currency, the US dollar, as well as the value of our investments in our pension plans.
For more information about the effect of price controls on our business, see “Item 4. Information on the Company—Item 4.B—Business overview—Price controls.” See also “Item 5. Operating and Financial Review and Prospects—Item 5.B Liquidity and capital resources—Effects of currency fluctuations,” “Item 5. Operating and Financial Review and Prospects—Item 5.B Liquidity and capital resources—Condensed consolidated balance sheets,” “Item 18. Financial Statements—Note 16. Trade receivables” and “Item 18. Financial Statements—Note 30. Financial instruments – additional disclosures.”
Climate change
Risk description
Failure to manage physical and transition risks from climate change
Context and potential impact
We are exposed to a broad range of climate risks such as transition risks (e.g., regulatory frameworks, carbon pricing, and the cost of and access to capital) and physical risks (e.g., heat, water scarcity, rising sea levels, and flooding from severe weather events), which could vary in magnitude and impact across different countries.
Climate change has triggered, and may continue to trigger, the adoption of new regulatory requirements across the globe, as well as rapidly evolving societal expectations. To comply with such legislation and meet such expectations, we may be required to increase our investment in technology to reduce our energy use, water use and greenhouse gas emissions. In addition, legislative and regulatory action, both current and in the future, includes or could include, carbon pricing, climate risk-related disclosures, and changes in zoning or building codes to increase climate resilience. As a result, the combined impact of these transition risks could increase our direct operating costs or be passed on to us through the impact on our supply chain. As a result of these transition risks, we are committed to becoming carbon neutral in our own operations by 2025, and carbon neutral across our value chain by 2030. In addition, we are committed to achieving net zero across our value chain by 2040. Any failure to achieve these commitments in the expected time frame, or at all, could result in negative impacts on our reputation, our operations, and the price of our shares.
Climate change has created, and will continue to create, physical risks to our business. Some of our production facilities that depend on the availability of significant water supplies are located in areas where fresh water is increasingly scarce. Other facilities are located in areas that, due to increasingly violent weather events, rising sea levels, or both, are increasingly at risk of substantial damage. In regions where such a risk is present, this has an impact not only on our own operations but also our distributed supply chain. Such events may result in the loss of life, increased costs, business interruptions, destruction of facilities, and disruption to healthcare systems that patients use to access our medicines.
Tax laws and developments
Risk description
Changes in tax laws or their application
Context and potential impact
Our multinational operations are taxed under the laws of the countries and other jurisdictions in which we operate. Changes in tax laws or in their application could lead to an increased risk of international tax disputes and an increase in our effective tax rate, which could adversely affect our financial results. The integrated nature of our worldwide operations can produce conflicting claims from revenue authorities in different countries as to the profits to be taxed in the individual countries, including potential disputes relating to the prices our subsidiaries charge one another for intercompany transactions, known as transfer pricing. Most of the jurisdictions in which we operate have double tax treaties with other foreign jurisdictions, which provide a framework for mitigating the impact of double taxation on our revenues and capital gains. However, mechanisms developed to resolve such conflicting claims are largely untried and can be expected to be very lengthy. Accruals for tax contingencies are made based on experience, interpretations of tax law, and judgments about potential actions by tax authorities. However, due to the complexity of tax contingencies, the ultimate resolution of any tax matter may result in payments materially different from the amounts accrued.
In 2019, the Organization for Economic Co-operation and Development (OECD) launched a new initiative on behalf of the G20 to minimize profit shifting by working toward a global tax framework that ensures that corporate income taxes are paid where consumption takes place, in addition to introducing a global standard on minimum taxation combined with new tax dispute resolution processes. This project achieved OECD political consensus in October 2021, and the detailed principles are still under discussion by the OECD and political leaders. The OECD expects that the implementation of these new principles will begin globally in 2024. However, some countries already announced postponement to 2025 while others have not taken any implementation steps so far. Once changes to the tax laws in any jurisdiction in which we operate are enacted or substantially enacted, we will be subject to the OECD top-up tax, the aim of which is to bring the total amount of taxes paid on our profit in a given jurisdiction up to a minimum rate of 15%. In June 2023, the Swiss public voted to approve an amendment to the Swiss Constitution that provides the legal basis for the implementation of an OECD compliant minimum tax in Switzerland. In December 2023, the Swiss federal council partially implemented the OECD 15% minimum tax for the financial year 2024 in the form of a qualified domestic top-up tax (QDMTT), which will be assessed on certain qualifying profits earned by companies domiciled in Switzerland. This QDMTT will not be applied to qualifying profits earned by a company’s affiliates domiciled in tax jurisdictions outside of Switzerland. The timing and specific provisions of any further tax regulations remain subject to assessments in political and technical forums at both a federal and cantonal level.
Due to the ongoing discussion in many countries on the implementation and additional guidance from the OECD, the full impact of the OECD minimum tax project on our financial position, income statement and cash flows cannot currently be estimated. On September 12, 2023, the EU Commission published two draft directives relating to international tax. The draft Business in Europe: Framework for Income Taxation (BEFIT) directive provides common rules for determining the corporate tax base for EU-based entities that are part of a group with global consolidated revenues above EUR 750 million. The BEFIT proposal includes provisions for a formula-driven allocation of profits between relevant EU member states which would then be subject to the corporate income tax rate of the respective member state. The draft transfer pricing directive aims to harmonize transfer pricing rules within the EU consistent with the OECD Transfer Pricing Guidelines. It also clarifies processes for relieving double taxation within the EU. Both draft directives require unanimous agreement among EU member states before they can be further implemented. In the US, the IRA was signed into law on August 16, 2022. The IRA creates a 15% corporate alternative minimum tax on the profits of corporations whose average annual adjusted financial statement income exceeds USD 1.0 billion. The IRA also includes a one percent excise tax on certain corporate stock repurchases. Additionally, the IRA also contains provisions that affect tax-exempt entities, including tax credit opportunities to encourage investment in clean energy and expanded incentives for energy-efficient construction by tax-exempt entities.
While we have taken steps to comply with the evolving tax initiatives of the OECD, the US and the EU, and we will continue to do so, significant uncertainties remain as to the outcome of our efforts.
For more information, see “Item 18. Financial Statements—Note 7. Income taxes” and “Item 18. Financial Statements—Note 13. Deferred tax assets and liabilities.”
General risks
Indebtedness
Risk description
Our indebtedness could adversely affect our operations
Context and potential impact
As of December 31, 2023, we had USD 18.4 billion of non‑current financial debt, and USD 6.2 billion of current financial debt. Our current and long‑term debt requires us to dedicate a portion of our cash flow to service interest and principal payments and, if interest rates rise, this amount may increase. As a result, our existing debt may limit our ability to use our cash flow to fund capital expenditures, to engage in transactions, or to meet other capital needs, or otherwise may place us at a competitive disadvantage relative to competitors that have less debt. Our debt could also limit our flexibility to plan for and react to changes in our business or industry, and increase our vulnerability to general adverse economic and industry conditions, including changes in interest rates or a downturn in our business or the economy. We may also have difficulty refinancing our existing debt or incurring new debt on terms that we would consider to be commercially reasonable, if at all.
Goodwill and intangible assets
Risk description
Goodwill and intangible assets resulting in significant impairment charges
Context and potential impact
We carry a significant amount of goodwill and other intangible assets on our consolidated balance sheet, including, in particular, substantial goodwill and other intangible assets obtained through acquisitions, including most recently through our acquisitions of The Medicines Company, Endocyte, Novartis Gene Therapies, ADACAP, and Chinook Therapeutics. As a result, we may incur significant impairment charges in the future if the fair value of the intangible assets and the groupings of cash-generating units containing goodwill would be less than their carrying value on our consolidated balance sheet at any point in time.
We regularly review our intangible and tangible assets for impairment, including identifiable intangible assets and goodwill. Any significant impairment charges could have a material adverse effect on our results of operations and financial condition. In 2023, for example, we recorded intangible asset impairment charges of USD 3.0 billion.
For a detailed discussion about how we determine whether an impairment has occurred, what factors could result in an impairment, and the impact of impairment charges on our results of operations, see Item 18. Financial Statements—Note 1. Accounting policies” and “Item 18. Financial Statements—Note 12. Goodwill and intangible assets.”
Foreign currency exchange rates
Risk description
Negative effect on financial results due to foreign currency exchange rate fluctuations
Context and potential impact
Changes in exchange rates between the US dollar, which is our reporting currency, and other currencies can result in significant increases or decreases in our reported sales, costs and earnings as expressed in US dollars, and in the reported value of our assets, liabilities and cash flows.
In addition to ordinary market risk, there is a risk that countries could take affirmative steps that could significantly impact the value of their currencies. Such steps could include “quantitative easing” measures and potential withdrawals by countries from common currencies. In addition, countries facing local financial difficulties, including countries experiencing high inflation rates, and highly indebted countries facing large capital outflows, may impose controls on the exchange of foreign currency. Currency exchange controls and sanctions could limit our ability to distribute retained earnings from our local affiliates, or to pay intercompany payables due from those countries.
Despite measures undertaken to reduce or hedge against foreign currency exchange risks, as a significant portion of our earnings and expenditures are in currencies other than the US dollar, including expenditures in Swiss francs that are significantly higher than our revenue in Swiss francs, any such exchange rate volatility may negatively and materially impact our results of operations and financial condition, and may impact the reported value of our net sales, earnings, assets and liabilities. In addition, the timing and extent of such volatility can be difficult to predict. Furthermore, depending on the movements of particular foreign exchange rates, we may be materially adversely affected at a time when the same currency movements are benefiting some of our competitors.
For more information on the effects of currency fluctuations on our consolidated financial statements and on how we manage currency risk, see “Item 5. Operating and Financial Review and Prospects—Item 5.B Liquidity and capital resources—Effects of currency fluctuations” and “Item 18. Financial Statements—Note 30. Financial instruments – additional disclosures.”
Key customers
Risk description
Concentration among our key customers
Context and potential impact
A significant portion of our global sales is made to a relatively small number of drug wholesalers, retail chains and other purchasing organizations. For example, our three most important customers globally accounted for approximately 15%, 13% and 8%, respectively, of net sales from continuing operations in 2023. The largest trade receivables outstanding were for these three customers, amounting to 17%, 13% and 8%, respectively, of the trade receivables at December 31, 2023. Historically, there has been a trend of consolidation among our customer base, which may continue in the future. As a result, we are exposed to a concentration of credit risk among our key customers. If one or more of our major customers experienced financial difficulties, the effect on us would be considerable, and could include a substantial loss of sales and an inability to collect amounts owed to us.
Environmental matters
Risk description
Impact of environmental liabilities
Context and potential impact
The environmental laws of various jurisdictions impose actual and potential obligations on us to investigate and remediate contaminated sites, including in connection with activities in the past by businesses that are no longer part of Novartis. In some cases, these remediation efforts may take many years. While we have set aside provisions for known worldwide environmental liabilities that are probable and estimable, there is no guarantee that additional costs will not be incurred beyond the amounts for which we have provided in our consolidated financial statements. If environmental contamination resulting from our facility operations, business activities or products adversely impacts third parties or if we fail to properly manage the safety of our facilities, including the safety of our employees and contractors, and the environmental risks, we may face substantial one-time and recurring costs and other penalties, and be required to increase our provisions for environmental liabilities. Furthermore, our headquarters and a number of our major production and research facilities are located near earthquake fault lines in Basel, Switzerland. Other major facilities are located near major earthquake fault lines in various locations around the world. A major earthquake could result in loss of life, business interruptions and the destruction of our facilities. See also “Item 4. Information on the Company—Item 4.D Property, plants and equipment” and “Item 18. Financial Statements—Note 21. Provisions and other non‑current liabilities.”
Pension plans
Risk description
Inaccuracies in the assumptions and estimates used to calculate our pension plan and other post-employment obligations
Context and potential impact
We sponsor pension and other post-employment benefit plans in various forms that cover a significant portion of our current and former employees. For post-employment plans with defined benefit obligations, we are required to make significant assumptions and estimates about future events in calculating the expense and the present value of the liability related to these plans. These include assumptions about the discount rates we apply to estimate future defined benefit obligations and net periodic pension expense, as well as rates of future pension increases. In addition, our actuarial consultants provide our management with historical statistical information, such as withdrawal and mortality rates in connection with these estimates.
Assumptions and estimates that we use may differ materially from the actual results we experience due to changing market and economic conditions, higher or lower withdrawal rates, and longer or shorter life spans of participants, among other factors. Depending on events, such differences could have a material effect on our total equity, and may require us to make additional contributions to our pension funds.
For more information on obligations under retirement and other post-employment benefit plans and underlying actuarial assumptions, see “Item 18. Financial Statements—Note 26. Post-employment benefits for employees.”
Item 4. Information on the Company
4.A History and development of Novartis
Novartis AG
Novartis AG was incorporated on February 29, 1996, under the laws of Switzerland as a stock corporation (“Aktiengesellschaft”) with an indefinite duration. On December 20, 1996, our predecessor companies, Ciba‑Geigy AG and Sandoz AG, merged into this new entity, creating Novartis. We are domiciled in and governed by the laws of Switzerland. Our registered office is located at the following address:
Novartis AG
Lichtstrasse 35
CH‑4056 Basel, Switzerland
Telephone: +41‑61‑324‑1111
Website: www.novartis.com
Novartis AG, our Swiss holding company, owns, directly or indirectly, all of our significant operating companies. For a list of our significant operating subsidiaries, see “Item 18. Financial Statements—Note 33. Novartis principal subsidiaries and associated companies.”
For a description of important corporate developments since January 1, 2021, see “Item 18. Financial Statements—Note 2. Significant transactions.” For information regarding the Company’s material commitments for capital expenditures, see “Item 5. Operating and Financial Review and Prospects—Material contractual obligations and commitments.”
The SEC maintains an internet site at http://www.sec.gov that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC.
4.B Business overview
Overview
Novartis is an innovative medicines company. Our purpose is to reimagine medicine to improve and extend people’s lives. Our strategy is to focus on high-value, innovative medicines that alleviate society’s greatest disease burdens through technology leadership in R&D and novel access approaches. To support our strategy, we have clear focus areas where we commit most of our time, energy and resources. These core therapeutic areas are cardiovascular, renal and metabolic; immunology; neuroscience; and oncology. For more information about our strategy, see “Item 5. Operating and Financial Review and Prospects—Overview—Our strategy.”
In 2023, Novartis achieved net sales from continuing operations of USD 45.4 billion, and net income from continuing operations amounted to USD 8.6 billion. Headquartered in Basel, Switzerland, we employed 76 057 full‑time equivalent employees as of December 31, 2023. Our products are sold in approximately 130 countries around the world.
Beginning in September 2023, we reorganized our operations into the following five organizational units:
• Biomedical Research is our innovation engine, focused on creating new ways of fighting disease and turning scientific breakthroughs into new medicines with the potential to change lives.
• Development oversees the development of potential new medicines through clinical trials to confirm their safety and efficacy, and steers the way to regulatory approval for use by patients.
• Operations manufactures and delivers our medicines to customers, while also overseeing the global functions of IT, procurement and real estate services.
• The two commercial units, US and International, focus on their respective geographic areas. They work with customers to provide innovative medicines and services that improve treatment options and raise the quality of care for patients.
These organizational units are supported by our global functions in areas such as corporate affairs, ethics, risk and compliance, finance, legal, internal audit, people and organization and strategy and growth. For more information about our Development unit, see “—Research and development—Development program” below. For more information about our Operations unit see “—Item 4.D Property, plants and equipment” and “Item 18. Financial Statements—Note 3. Operating segment and Note 4. Revenues and geographical information.”
In 2023, Novartis completed its transformation into a pure-play innovative medicines business, with the successful spin-off of Sandoz. Effective October 4, 2023, Sandoz was listed on the SIX Swiss Exchange, with a Level 1 ADR program in the United States. To comply with International Financial Reporting Standards (IFRS®) Accounting Standards as a result of the spin-off, Novartis has separated the Company’s reported financial data for the current and prior years into “continuing” and “discontinued” operations. Continuing operations comprises the retained business activities that includes our innovative
medicines business and continued corporate activities. Discontinued operations include the Sandoz generic pharmaceuticals and biosimilars division and certain corporate activities attributable to Sandoz prior to the spin-off up to the distribution date of October 3, 2023, and certain other expenses related to the spin-off. Included in 2023 is also the IFRS Accounting Standards non-cash, non-taxable net gain on distribution of Sandoz Group AG to Novartis AG shareholders. Sandoz operated in the off-patent medicines segment and specialized in the development, manufacturing and marketing of generic pharmaceuticals and biosimilars. The Sandoz business was organized globally into two franchises: Generics and Biosimilars. Except where noted, this Annual Report focuses on continuing operations.
Key marketed products
The following summaries describe certain Novartis key marketed products in certain indications. These products are listed according to year-end net sales within each therapeutic area or reporting category. Some of them have lost patent protection or are otherwise subject to generic competition, while others are subject to patent challenges by potential generic competitors. Please see “—Intellectual property” for general information on intellectual property and regulatory data protection, and for more information on the status of patents and exclusivity for certain key marketed products.
While we typically seek to sell our marketed products throughout the world, not all products and indications are available in every country. The indications described in these summaries may therefore vary by country. In addition, a product may be available under different brand names depending on country and indication.
Cardiovascular, renal and metabolic
• Entresto (sacubitril/valsartan) is an oral, first‑in‑class angiotensin receptor neprilysin inhibitor. Entresto enhances the protective effects of a hormone system called the natriuretic peptide system, and simultaneously suppresses the harmful effects of a hormone system called the renin‑angiotensin‑aldosterone system. It is approved:
• In the US, the EU and other countries to treat adults who have symptomatic heart failure with reduced ejection fraction (HFrEF). HFrEF is a disease in which the heart cannot pump blood efficiently
• In the US and other countries to treat most chronic heart failure patients with preserved ejection fraction (HFpEF). HFpEF is a disease in which the heart’s main pumping chamber (left ventricle) becomes stiff and unable to fill properly with blood
• In the US and other countries to treat children aged 1 year and older who have symptomatic heart failure with systemic left ventricular systolic dysfunction
• In China and Japan to treat patients with essential hypertension (abnormally high blood pressure that is not the result of a medical condition)
• Leqvio (inclisiran) is the first and only approved small-interfering RNA therapy to reduce LDL cholesterol, a risk factor for atherosclerotic cardiovascular disease (ASCVD), which is caused by plaque buildup in the arteries. Leqvio is administered by a healthcare professional twice a year as an injection, following an initial dose and another dose after three months. It is approved:
• In the EU and other countries to treat adults with primary hypercholesterolemia (heterozygous familial and non-familial) or mixed dyslipidemia as an adjunct to diet. Leqvio is used in combination with the maximum tolerated dose of a statin or a statin with other lipid-lowering therapies in patients unable to reach LDL cholesterol goals, or alone or in combination with other lipid-lowering therapies in patients who are statin-intolerant or for whom a statin is contraindicated. Primary hypercholesterolemia and mixed dyslipidemia are disorders characterized by high levels of fats in the blood
• In the US to treat adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (HeFH), as an adjunct to diet and statin therapy to reduce LDL cholesterol. This includes patients who have ASCVD or HeFH, or are at an increased risk of ASCVD, meaning they have not had a cardiovascular event but have other factors that increase their risk. Primary hyperlipidemia, also known as high cholesterol, is characterized by high levels of fats in the blood
Novartis obtained global rights to develop, manufacture and commercialize Leqvio under a license and collaboration agreement with Alnylam Pharmaceuticals, Inc.
Immunology
• Cosentyx (secukinumab) is an injectable, fully-human monoclonal antibody that selectively inhibits interleukin-17A (IL‑17A), a cytokine involved in several immunological diseases. It is approved in the US, the EU and other countries to treat:
• Adults and children aged 6 years and older with moderate‑to‑severe plaque psoriasis (this indication is also approved in China). Psoriasis is a debilitating systemic inflammatory disease that is characterized by the appearance of raised, red patches on the skin
• Adults with active ankylosing spondylitis (AS). AS is a progressive inflammatory disease that is characterized by chronic back pain, is generally visible on X-rays, and can cause structural damage to the bones and joints
• Adults with active non-radiographic axial spondyloarthritis (nr-axSpA). nr-axSpA is a long-term inflammatory disease that is characterized by chronic back pain and is not visible on X-rays
• Adults and children (aged 2 years and older in the US and 6 years and older in the EU) with active psoriatic arthritis (PsA). PsA is a type of progressive inflammatory arthritis that results in swollen and painful joints and tendons, which can cause structural damage to the bones and joints
• Children (aged 4 years and older in the US and 6 years and older in the EU) with enthesitis-related arthritis (ERA) and children (aged 2 years and older in the US and 6 years and older in the EU) with juvenile psoriatic arthritis (JPsA). ERA and JPsA are
subtypes of juvenile idiopathic arthritis. If left untreated, they can lead to high levels of pain and disability
• Adults with moderate to severe hidradenitis suppurativa (HS). HS is a chronic skin disease that causes recurring boil-like lumps that may burst into open wounds and cause irreversible scarring, often in the most intimate parts of the body.
An intravenous formulation of Cosentyx is approved in the US for the treatment of adults with PsA, AS and nr-axSpA.
• Xolair (omalizumab) is an injectable prescription medicine and the only approved antibody designed to target and block immunoglobulin E (IgE). It is approved in the US, the EU and other countries to treat:
• Adults and children aged 6 years and older with moderate‑to‑severe, or severe, persistent allergic asthma
• Adults and children aged 12 years and older with chronic spontaneous urticaria/chronic idiopathic urticaria (hives)
• Adults with nasal polyps or severe chronic rhinosinusitis with nasal polyps (CRSwNP). CRSwNP is a chronic inflammation of the nose and the sinuses with the presence of benign lesions (nasal polyps) on the lining of the nasal sinuses or nasal cavity
Approved indications vary by country. Xolair is provided as lyophilized powder for reconstitution, and as liquid formulation in a pre‑filled syringe. Novartis co-promotes Xolair with Genentech in the US and shares a portion of operating income, but Novartis does not record any US sales. Novartis records all sales of Xolair outside the US. For more information, see “Item 18. Financial Statements—Note 28. Transactions with related parties—Roche Holding AG.”
• Ilaris (canakinumab) is an injectable, selective, high-affinity, fully-human monoclonal antibody that inhibits interleukin-1 beta (IL-1 beta), a key cytokine in the inflammatory pathway. It is approved in the US, the EU and other countries to treat patients with certain debilitating autoinflammatory disorders, including:
• Adults and children with periodic fever syndromes. Periodic fever syndromes are a set of rare disorders characterized by recurrent episodes of illness, with fever as the main symptom
• Patients with Still’s disease, including systemic juvenile idiopathic arthritis and adult-onset Still’s disease. Still’s disease is a disorder that causes fevers, rash and joint pain
• Adults with acute gouty arthritis. Gouty arthritis is a type of arthritis characterized by pain, redness, tenderness and swelling in one or more joints
Approved indications vary by country.
Neuroscience
• Kesimpta (ofatumumab) is an anti-CD20 monoclonal antibody that enables the targeted depletion of B-cells, specifically in lymph nodes. Kesimpta is the only B-cell treatment for relapsing multiple sclerosis that is self-administered once-monthly via the Sensoready autoinjector pen following three weekly starter doses. It is approved:
• In the US to treat adults with relapsing forms of multiple sclerosis, including clinically isolated syndrome, relapsing-remitting multiple sclerosis and active secondary progressive multiple sclerosis. Multiple sclerosis is a disease in which the immune system attacks the protective covering of nerves (known as myelin)
• In the EU to treat adults with relapsing forms of multiple sclerosis with active disease defined by clinical or imaging features (i.e., relapse, disability, or lesions detected by MRI scans)
Approved indications vary across other countries. Ofatumumab was originally developed by Genmab and licensed to GlaxoSmithKline (GSK). Novartis obtained the rights to ofatumumab from GSK across all indications.
• Zolgensma (onasemnogene abeparvovec) is a one-time intravenous gene therapy designed to address the genetic root cause of spinal muscular atrophy (SMA) by replacing the function of the missing or nonworking SMN1 gene. Zolgensma delivers a new working copy of the SMN gene into a patient’s cells. It is approved in the US, the EU and other countries to treat:
• Babies and young children who have SMA with biallelic mutations in the SMN1 gene. SMA is a rare, genetic neuromuscular disease resulting in the progressive and irreversible loss of motor neurons, which causes muscle weakness and atrophy
Approved indications vary by country.
Oncology
• Promacta/Revolade (eltrombopag) is a once‑daily oral thrombopoietin receptor agonist that works by stimulating bone marrow cells to produce platelets. It is approved in the US, the EU and other countries to treat:
• Immune thrombocytopenia (ITP) in patients who have had an insufficient response to or have failed previous therapies. ITP is a bleeding disorder caused by an unusually low number of platelets
• Thrombocytopenia in patients with chronic hepatitis C to allow them to initiate and maintain interferon‑based therapy
• Patients with severe aplastic anemia (SAA). SAA is a condition in which the body does not produce enough blood cells
Promacta/Revolade is marketed under a research, development and license agreement between Novartis and RPI Finance Trust (dba Royalty Pharma), as assignee of Ligand Pharmaceuticals.
• Kisqali (ribociclib) is a selective oral cyclin‑dependent inhibitor of kinases 4 and 6 (CDK4/6) – two enzymes involved in the control of cell cycle progression. Kisqali is approved in the US, the EU and other countries to treat:
• Pre-, peri- and postmenopausal women, and men (US and other countries), with locally advanced or metastatic hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2-)
breast cancer, in combination with an aromatase inhibitor as initial endocrine‑based therapy. HR+/HER2- breast cancer is the most common subtype of breast cancer
• Pre-, peri- (EU) and postmenopausal women, and men (US), with locally advanced or metastatic HR+/HER2- breast cancer, in combination with fulvestrant, as first- or second-line therapy
Kisqali was developed by our Biomedical Research organizational unit (formerly the Novartis Institutes for BioMedical Research) under a research collaboration with Astex Pharmaceuticals.
• Tafinlar + Mekinist (dabrafenib + trametinib) is an oral combination therapy. Tafinlar and Mekinist are kinase inhibitors of the BRAF and MEK1/2 proteins, respectively, approved in combination to treat patients who have certain types of cancer with a change in the BRAF gene (called a BRAF V600 mutation), including:
• Adults in the US, the EU and other countries with unresectable or metastatic melanoma with a BRAF V600 mutation. Melanoma is a form of skin cancer; unresectable melanoma cannot be removed with surgery and metastatic melanoma has spread to other parts of the body. Tafinlar and Mekinist are also approved as single agents for this indication
• Adults in the US, the EU and other countries with stage III melanoma with a BRAF V600 mutation as an adjuvant treatment (following surgery)
• Adults in the US, the EU and other countries with advanced non-small cell lung cancer (NSCLC) with a BRAF V600 mutation. NSCLC is the most common type of lung cancer
• Adults and children aged 1 year and older in the US and 6 years and older in other countries with unresectable or metastatic solid tumors with a BRAF V600E mutation whose cancer has progressed following prior treatment and who have no satisfactory alternative treatment options
Approved indications vary by country. Novartis has worldwide exclusive rights to develop, manufacture and commercialize trametinib granted by Japan Tobacco Inc.
• Tasigna (nilotinib) is a twice-daily oral tyrosine kinase inhibitor that acts by blocking the BCR-ABL protein. It is approved in the US, the EU and other countries to treat:
• Patients with Philadelphia chromosome-positive chronic myeloid leukemia (Ph+ CML) in the chronic and/or accelerated phase who are resistant or intolerant to existing treatment. Ph+ CML is a cancer that starts in the blood-forming cells of bone marrow
• Newly diagnosed adults and children with Ph+ CML in the chronic phase
• Jakavi (ruxolitinib) is an oral inhibitor of the JAK1 and JAK2 tyrosine kinases. It is the first therapy approved in the EU and other countries to treat:
• Adults with myelofibrosis (MF), including primary myelofibrosis, post-polycythemia vera myelofibrosis and post-essential thrombocythemia myelofibrosis. MF is a rare blood cancer characterized by abnormal blood cell production and scarring in the bone marrow, which can lead to an enlarged spleen
• Adults with polycythemia vera (PV) who are resistant or intolerant to a medication called hydroxyurea. PV is a rare blood cancer in which the bone marrow produces too many red blood cells, resulting in serious problems like clots
• Patients aged 12 years and older with acute or chronic graft-versus-host disease (GvHD) and who have had an inadequate response to corticosteroids or other systemic therapies. GvHD occurs in stem-cell transplant patients when donor cells see the recipient’s healthy cells as foreign and attack them
Novartis licensed ruxolitinib from Incyte Corporation for development and commercialization in the indications of oncology, hematology and GvHD outside the US. Incyte Corporation markets ruxolitinib as Jakafi® in the US.
• Pluvicto (lutetium (177Lu) vipivotide tetraxetan) is an intravenous radioligand therapy combining a targeting compound (a ligand) with a therapeutic radionuclide (a radioactive particle, in this case lutetium-177). Pluvicto delivers radiation selectively to PSMA-positive cells and the surrounding cells. It is approved in the US, the EU and other countries to treat:
• Adults with prostate-specific membrane antigen-positive metastatic castration-resistant prostate cancer (PSMA-positive mCRPC), a type of advanced cancer that has spread to other parts of the body (metastatic). These patients have already been treated with other anticancer treatments (androgen receptor pathway inhibition and taxane-based chemotherapy)
• Lutathera (lutetium Lu 177 dotatate/lutetium (177Lu) oxodotreotide) is an intravenous targeted radioligand therapy approved in the US, the EU and other countries to treat:
• Adults with somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs). GEP-NETs are rare tumors found in the digestive tract
Approved indications vary by country.
• Scemblix (asciminib) is an oral kinase inhibitor that works by binding to a part of the BCR-ABL protein called the ABL myristoyl pocket. It is approved:
• In the US, the EU and other countries to treat adults with Philadelphia chromosome-positive chronic myeloid leukemia (Ph+ CML) in the chronic phase who have previously been treated with two or more tyrosine kinase inhibitors (TKIs). Ph+ CML is a cancer that starts in the blood-forming cells of bone marrow
• In the US and other countries to treat adults with Ph+ CML in the chronic phase with the T315I mutation. The T315I mutation causes resistance to most available TKI therapies and, as a result, patients with this mutation would otherwise have limited treatment options
• Fabhalta (iptacopan) is an oral Factor B inhibitor of the alternative complement pathway, a part of the immune system involved in triggering inflammation and fighting infection. It is approved in the US to treat:
• Adults with paroxysmal nocturnal hemoglobinuria (PNH). PNH is a rare chronic blood disorder in which red blood cells are susceptible to premature destruction by the complement system
Established brands
• Lucentis (ranibizumab) is a humanized, high-affinity antibody fragment that binds to vascular endothelial growth factor A (VEGF‑A), a protein that can cause the growth of blood vessels in the eye, potentially leading to vision loss. Lucentis is an anti-VEGF therapy that is injected into the eye. It is approved in the EU and other countries to treat patients with certain eye conditions, including:
• Adults with neovascular (wet) age‑related macular degeneration (AMD). Wet AMD develops when abnormal blood vessels grow under the macula and leak blood and other fluids in the back of the eye, which damages the macula
• Adults with proliferative diabetic retinopathy, moderately severe to severe non-proliferative diabetic retinopathy, and/or visual impairment due to diabetic macular edema. These conditions are complications of diabetes
• Adults with visual impairment due to macular edema secondary to retinal vein occlusion (branch RVO or central RVO). Retinal vein occlusion is a blockage of the branch or central retinal veins, which carry blood away from the retina
Approved indications vary by country. Lucentis is licensed from Genentech, and Novartis holds the rights to commercialize the product outside the US. Genentech holds the rights to commercialize Lucentis in the US. For more information, see “Item 18. Financial Statements—Note 28. Transactions with related parties—Roche Holding AG.”
• Sandostatin SC (octreotide acetate for injection) and Sandostatin LAR (octreotide acetate for injectable suspension) are somatostatin analogs approved in the US, the EU and other countries to treat:
• Adults with acromegaly that is inadequately controlled by surgery or radiotherapy. Acromegaly is a chronic disease caused by the oversecretion of growth hormone
• Patients with certain symptoms associated with carcinoid tumors and other types of functional gastrointestinal and pancreatic neuroendocrine tumors
Sandostatin LAR is also approved in the EU and other countries to treat patients with advanced neuroendocrine tumors of the midgut or of unknown primary tumor origin.
Compounds in development
The following table provides an overview of key projects currently in the Confirmatory Development stage and may also describe certain projects in the Early Development stage. Projects typically enter Confirmatory Development and become the responsibility of our Development organizational unit during Phase II testing. (For more information about our drug development program, see “—Research and development—Development program.”) Projects are listed in alphabetical order by compound code, or by product name where applicable. Projects include those seeking to develop potential uses of new molecular entities as well as potential additional indications or new formulations for already marketed products. The table below, entitled “Projects removed from the development table since 2022,” highlights changes to the table entitled “Selected development projects” from the previous year.
The year that each project entered the current phase of development refers to the year of the first patient’s first visit in the first clinical trial of that phase. For projects in Phase II, the year generally refers to the first patient’s first visit in the first trial in Confirmatory Development. In some cases, the first patient’s first visit in a Phase II trial can occur before the Confirmatory Development stage. Prior to 2020, we reported the current phase based on the year in which the decision to enter the phase was made. To maintain continuity, we have included certain previously disclosed projects, noted below, that have not yet achieved “first patient, first visit” in any Phase I-III study for the reported indication and route of administration. We have disclosed these projects using our previous reporting criteria.
A reference to a project being in registration means that an application has been submitted to a health authority for marketing approval. Compounds and new indications in development are subject to required regulatory approvals and, in certain instances, contractual limitations. These compounds and indications are in various stages of development throughout the world. It may not be possible to obtain regulatory approval for any or all of the new compounds and new indications referred to in this Form 20‑F in any country or in every country. See “—Regulation” for more information on the approval process.
Selected development projects
Compound/
product
| |
Common
name
| |
Mechanism
of action
| |
Potential indication
| |
Category
| |
Formulation/
route of
administration
| | Year project
entered
current
development
phase | |
Planned filing
dates/current
phase
| |
|
AVXS-101
(OAV101) | | onasemno-
gene abepar-
vovec | | Survival motor neuron
(SMN) gene therapy
| | Spinal muscular atrophy
(IT formulation)
| | Neuroscience
| | Intrathecal injection
| | 2021
| | 2025/III
| |
|
Beovu | | brolucizumab | | VEGF inhibitor | | Diabetic retinopathy | | Ophthalmology | | Intravitreal injection | | 2020 | | 2025/III | |
|
CFZ533 | | iscalimab | | CD40 inhibitor | | Sjögren's syndrome | | Immunology | | Subcutaneous injection | | 2019 | | ≥2027/II | |
|
Coartem | | artemether +
lumefantrine
| | PGH-1 (artemisinin
combination therapy)
| | Malaria,
uncomplicated
(<5 kg patients) | | Global Health
| | Oral
| | 2020
| | 2024/III
| |
|
Cosentyx | | secukinumab | | IL‑17A inhibitor | | Giant cell arteritis | | Immunology | | Subcutaneous injection | | 2021 | | 2025/III | |
| | | | | | | | | | | | | | | |
| | | | | | Polymyalgia rheumatica 1 | | Immunology | | Subcutaneous injection | | 2023 | | 2026/III | |
| | | | | | | | | | | | | | | |
| | | | | | Rotator cuff tendinopathy 1 | | Immunology | | Subcutaneous injection | | 2023 | | ≥2027/III | |
|
EXV811 1 | | atrasentan
| | ETA receptor
antagonist
| | IgA nephropathy
| | Cardiovascular,
Renal and
Metabolic | | Oral
| | 2023
| | 2024/III
| |
|
Fabhalta | | iptacopan
| | CFB inhibitor
| | IgA nephropathy
| | Cardiovascular,
Renal and
Metabolic | | Oral
| | 2021
| | 2024/III
| |
| | | | | | | | | | | | | | | |
| |
| |
| | C3 glomerulopathy
| | Cardiovascular,
Renal and
Metabolic | | Oral
| | 2021
| | 2024/III
| |
| | | | | | | | | | | | | | | |
| |
| |
| | IC-MPGN 1
| | Cardiovascular,
Renal and
Metabolic | | Oral
| | 2023
| | ≥2027/III
| |
| | | | | | | | | | | | | | | |
| | | | | | Atypical hemolytic uremic syndrome | | Oncology | | Oral | | 2021 | | ≥2027/III | |
|
FUB523 1 | | zigakibart
| | Anti-APRIL
monoclonal
antibody | | IgA nephropathy
| | Cardiovascular,
Renal and
Metabolic | | Subcutaneous injection
| | 2023
| | ≥2027/III
| |
|
JDQ443 | | opnurasib
| | KRAS inhibitor
| | Non-small cell lung cancer 2
(monotherapy and/or combination therapy) | | Oncology
| | Oral
| | 2022
| | ≥2027/III
| |
|
KAE609 | | cipargamin | | PfATP4 inhibitor | | Malaria, uncomplicated | | Global Health | | Oral | | 2017 | | ≥2027/II | |
| | | | | | | | | | | | | | | |
| | | | | | Malaria, severe | | Global Health | | Oral | | 2022 | | ≥2027/II | |
|
Kisqali | | ribociclib
| | CDK4 inhibitor
| | Hormone receptor-positive
(HR+)/human epidermal growth
factor receptor 2-negative (HER2-)
early breast cancer (adjuvant) | | Oncology
| | Oral
| | 2023
| | US, EU
registration
| |
|
KLU156 3 | | ganaplacide
+
lumefantrine | | Non-artemisinin
plasmodium
falciparum inhibitor | | Malaria, uncomplicated
| | Global Health
| | Oral
| | 2023
| | 2026/II
| |
|
Leqvio | | inclisiran
| | siRNA
(regulation of LDL-C)
| | Secondary prevention of cardiovascular
events in patients with elevated levels
of LDL-C | | Cardiovascular,
Renal and
Metabolic | | Subcutaneous injection
| | 2018
| | ≥2027/III
| |
| | | | | | | | | | | | | | | |
| |
| |
| | Primary prevention cardiovascular 1
risk reduction
| | Cardiovascular,
Renal and
Metabolic | | Subcutaneous injection
| | 2023
| | ≥2027/III
| |
|
LNA043 | | TBD | | ANGPTL3 agonist | | Osteoarthritis 4 | | Immunology | | Intra-articular | | 2021 | | ≥2027/II | |
|
LOU064 | | remibrutinib | | BTK inhibitor | | Chronic spontaneous urticaria | | Immunology | | Oral | | 2021 | | 2024/III | |
| | | | | | | | | | | | | | | |
| | | | | | Chronic inducible urticaria | | Immunology | | Oral | | 2023 | | ≥2027/III | |
| | | | | | | | | | | | | | | |
| | | | | | Multiple sclerosis | | Neuroscience | | Oral | | 2021 | | ≥2027/III | |
|
Lutathera | | lutetium
Lu 177
dotatate/
lutetium
(177Lu)
oxodotreotide | | Radioligand therapy
targeting SSTR
| | Gastroenteropancreatic
neuroendocrine tumors,
1st line in G2/3 tumors
| | Oncology
| | Intravenous infusion
| | 2020
| | 2024/III
| |
|
LXE408 | | TBD | | Proteasome inhibitor | | Visceral leishmaniasis | | Global Health | | Oral | | 2022 | | ≥2027/II | |
|
Pluvicto | | lutetium
Lu 177
vipivotide
tetraxetan/
lutetium
(177Lu)
vipivotide
tetraxetan | | Radioligand therapy
targeting PSMA
| | Metastatic castration-resistant
prostate cancer, pre-taxane
| | Oncology
| | Intravenous infusion
| | 2021
| | 2024/III
| |
| | | | | | | | | | | | | | | |
| |
| |
| | Metastatic hormone-sensitive
prostate cancer | | Oncology
| | Intravenous infusion
| | 2021
| | 2025/III
| |
|
|
1 Project added to selected development projects table in 2023 – entered Confirmatory Development |
2 Previously disclosed as non-small cell lung cancer, 2/3L |
3 Project added to selected development projects table in 2023 (replacing KAF156) – entered Confirmatory Development, FPFV in Phase III expected in early 2024 |
4 Previously disclosed as knee osteoarthritis |
Compound/
product
| |
Common
name
| |
Mechanism
of action
| |
Potential indication
| |
Category
| |
Formulation/
route of
administration
| | Year project
entered
current
development
phase | |
Planned filing
dates/current
phase
| |
|
QGE031 | | ligelizumab | | IgE inhibitor | | Food allergy | | Immunology | | Subcutaneous injection | | 2021 | | ≥2027/III | |
|
Scemblix | | asciminib
| | BCR‑ABL inhibitor
| | Chronic myeloid
leukemia, 1st line | | Oncology
| | Oral
| | 2021
| | 2024/III
| |
|
TQJ230 | | pelacarsen
| | ASO targeting
lipoprotein(a)
| | Secondary prevention of cardiovascular
events in patients with elevated levels
of lipoprotein(a) | | Cardiovascular,
Renal and
Metabolic | | Subcutaneous injection
| | 2019
| | 2025/III
| |
|
VAY736 | | ianalumab | | BAFF-R inhibitor | | Autoimmune hepatitis | | Immunology | | Subcutaneous injection | | 2018 | | ≥2027/II | |
| | | | | | | | | | | | | | | |
| | | | | | Lupus nephritis | | Immunology | | Subcutaneous injection | | 2022 | | ≥2027/III | |
| | | | | | | | | | | | | | | |
| | | | | | Sjögren’s syndrome | | Immunology | | Subcutaneous injection | | 2022 | | 2026/III | |
| | | | | | | | | | | | | | | |
| | | | | | Systemic lupus erythematosus 1 | | Immunology | | Subcutaneous injection | | 2023 | | ≥2027/III | |
| | | | | | | | | | | | | | | |
| |
| |
| | Warm autoimmune hemolytic anemia
(wAIHA) | | Oncology
| | Intravenous infusion
| | 2022
| | 2026/III
| |
| | | | | | | | | | | | | | | |
| | | | | | Immune thrombocytopenia, 1st line 1 | | Oncology | | Intravenous infusion | | 2023 | | 2026/III | |
| | | | | | | | | | | | | | | |
| | | | | | Immune thrombocytopenia, 2nd line 1 | | Oncology | | Intravenous infusion | | 2023 | | 2026/III | |
|
Vijoice | | alpelisib | | PI3K-alpha inhibitor | | Lymphatic malformations | | Oncology | | Oral | | 2023 | | ≥2027/III | |
|
Xolair | | omalizumab | | IgE inhibitor | | Food allergy | | Immunology | | Subcutaneous injection | | 2019 | | US registration | |
|
XXB750 | | TBD
| | NPR1 agonist
| | Hypertension
| | Cardiovascular,
Renal and
Metabolic | | Subcutaneous injection
| | 2022
| | ≥2027/II
| |
|
YTB323 1 | | rapcabtagene
autoleucel | | CD19 CAR-T
| | Severe refractory lupus nephritis/
systemic lupus erythematosus | | Immunology
| | Intravenous infusion
| | 2023
| | ≥2027/II
| |
| | | | | | | | | | | | | | | |
| | | | | | High-risk large B-cell lymphoma, 1st line | | Oncology | | Intravenous infusion | | 2023 | | ≥2027/II | |
|
|
1 Project added to selected development projects table in 2023 – entered Confirmatory Development |
Projects removed from the development table since 2022
Compound/product | | Potential indication | | Change | | Reason | |
|
Cosentyx | | Hidradenitis suppurativa | | Commercialized | | | |
| | | | | | | |
| | Lupus nephritis | | Removed | | Development discontinued | |
| | | | | | | |
| | Psoriatic arthritis (IV formulation) | | Commercialized | | | |
| | | | | | | |
| | Ankylosing spondylitis (IV formulation) | | Commercialized | | | |
|
Fabhalta | | Paroxysmal nocturnal hemoglobinuria | | Commercialized | | | |
|
KAF156 | | Malaria, uncomplicated | | Removed | | Development discontinued 1 | |
|
LOU064 | | Sjögren's syndrome | | Removed | | Development discontinued | |
|
MBG453 | | Myelodysplastic syndrome | | Removed | | Development discontinued | |
| | | | | | | |
| | Unfit acute myeloid leukemia | | Removed | | Development discontinued | |
|
MIJ821 | | Major depressive disorder | | Removed | | Development discontinued | |
|
NIS793 | | Pancreatic cancer, 1st line | | Removed | | Development discontinued | |
|
Piqray | | Ovarian cancer | | Removed | | Development discontinued | |
|
PPY988 | | Geographic atrophy | | Removed | | Development discontinued | |
|
SAF312 | | Chronic ocular surface pain | | Removed | | Development discontinued | |
|
SKO136 | | Coronavirus infection | | Removed | | Development discontinued | |
|
VDT482 | | Esophageal cancer, 2nd line | | Removed | | Development discontinued 2 | |
| | | | | | | |
| | Non‑small cell lung cancer | | Removed | | Development discontinued 2 | |
| | | | | | | |
| | Nasopharyngeal carcinoma, 1st line | | Removed | | Development discontinued 2 | |
| | | | | | | |
| | Gastric cancer, 1st line | | Removed | | Development discontinued 2 | |
| | | | | | | |
| | Esophageal cancer, 1st line | | Removed | | Development discontinued 2 | |
| | | | | | | |
| | Localized esophageal cancer | | Removed | | Development discontinued 2 | |
| | | | | | | |
| | Hepatocellular carcinoma, 1st line | | Removed | | Development discontinued 2 | |
| | | | | | | |
| | Small cell lung cancer, 1st line | | Removed | | Development discontinued 2 | |
| | | | | | | |
| | Urothelial cell carcinoma, 1st line | | Removed | | Development discontinued 2 | |
|
VPM087 | | Colorectal cancer, 1st line | | Removed | | Development discontinued | |
|
|
1 Now in development as KLU156 |
2 Mutual termination of the agreement with BeiGene, Ltd. |
Principal markets
Novartis sells products in approximately 130 countries worldwide. Net sales are primarily concentrated in the US and Europe. The following table sets forth aggregate 2023 net sales by region:
| | 2023 net sales to third parties | |
| | | |
| | | |
| | | |
| | USD millions | | % | |
|
United States | | 17 959 | | 40 | |
|
Europe | | 14 997 | | 33 | |
|
Asia, Africa, Australasia | | 9 308 | | 20 | |
|
Canada and Latin America | | 3 176 | | 7 | |
|
Total | | 45 440 | | 100 | |
|
Of which in established markets 1 | | 33 725 | | 74 | |
|
Of which in emerging growth markets 1 | | 11 715 | | 26 | |
|
|
1 Emerging growth markets comprise all markets other than the established markets of the US, Canada, Western Europe, Japan, Australia and New Zealand. Novartis definition of Western Europe includes Austria, Belgium, Finland, France, Germany, Greece, Iceland, Ireland, Italy, Luxembourg, Malta, The Netherlands, Norway, Portugal, Spain, Sweden, Switzerland, and the United Kingdom. |
Many of our products are used for chronic conditions that require patients to continue dosing of the product over long periods of time, ranging from months to years. However, certain of our marketed products and development projects, such as cell and gene therapies, are administered only once. Net sales of the vast majority of our products are not subject to material changes in seasonal demand.
Production
Our primary goal is to ensure the uninterrupted and timely supply of medicines that meet all product specifications and quality standards, and that are produced in the most cost-effective and sustainable manner. The manufacturing of our products is highly regulated by governmental health authorities around the world, including the US Food and Drug Administration (FDA) and European Medicines Agency (EMA). In addition to regulatory requirements, many of our products involve technically complex manufacturing processes or require highly specialized raw materials.
We are continuing to integrate ADACAP manufacturing sites into our existing manufacturing and supply structure for radioligand therapies. We manufacture our products across the following technologies at facilities worldwide: large molecules, small molecules, cell and gene therapy, xRNA therapy and radioligand therapy (see also “—Item 4.D Property, plants and equipment”). In addition, we generate contract manufacturing sales from biotechnology services that we provide to third parties, which we include under “established brands” in our consolidated financial statements (see “Item 18. Financial Statements—Note 4. Revenues and geographic information”).
In our manufacturing network, we maintain state‑of‑the‑art processes, with quality as a priority, and require our suppliers to adhere to the same high standards we expect from our own people and processes. These processes include chemical and biological syntheses; radioisotope handling; sterile processing, including CAR-T cell processing; gene modification and delivery; and formulation and packaging. We are constantly working to improve our existing manufacturing processes, develop new and innovative technologies, and review and adapt our manufacturing network to meet our needs and those of our patients and customers.
We produce raw materials for manufacturing in‑house or purchase them from third-party suppliers. Where possible, we maintain multiple supply sources so that the business is not dependent on a single or limited number of suppliers. However, our ability to do so may at times be limited by regulatory or other requirements. We monitor market developments that could have an adverse effect on the supply of essential materials. Our suppliers of raw materials are required to comply with applicable regulations and Novartis quality standards.
Because the manufacturing of our products is complex and highly regulated by governmental health authorities, uninterrupted supply cannot be guaranteed. If we or our third-party suppliers fail to comply with applicable regulations, then there could be a product recall or other disruption to our production activities. We have experienced supply interruptions for our products in the past, and there can be no assurance that supply will not be interrupted again in the future. For more information on the risks related to the manufacturing of our products, see “Item 3. Key Information—Item 3.D Risk factors—Manufacturing and product quality—Inability to ensure proper controls in product development and product manufacturing, and failure to comply with applicable regulations and standards.” We have implemented a global manufacturing strategy to maximize business continuity in case of such events.
Marketing and sales
Although specific distribution patterns vary by country, Novartis generally sells its prescription drugs primarily to wholesale and retail drug distributors, hospitals, clinics, government agencies and managed healthcare providers. We reach healthcare professionals and patients in many markets and across our core therapeutic areas through integrated channels including field force
operations, patient support programs and Novartis-owned digital platforms.
We have 19 607 full-time equivalent field force employees, as of December 31, 2023, including supervisors and administrative personnel. These trained representatives present the therapeutic benefits and risks of our products to physicians, pharmacists, hospitals, insurance groups, managed care organizations and other healthcare professionals. In the US, Novartis advertises certain products via digital and traditional media channels, including the internet, television, newspapers and magazines. Novartis also pursues co‑promotion or co‑marketing opportunities as well as licensing and distribution agreements with other companies in various markets.
The marketplace for healthcare is constantly evolving. Customer groups beyond prescribers have increasing influence on treatment decisions and guidelines, while patients continue to become more informed stakeholders in their healthcare decisions and look for solutions to meet their changing needs. Novartis is responding by adapting our business practices to engage appropriately with patients, customer groups and other stakeholders, including by delivering innovative solutions to drive education, access and improved patient care.
The COVID-19 pandemic accelerated additional changes related to marketing and sales techniques in the healthcare industry. For example, many healthcare professionals increased their use of virtual platforms when interacting with pharmaceutical companies, and now prefer to receive information in a more convenient and personalized way. In response, Novartis is combining traditional face-to-face visits with digital and other methods of engaging healthcare professionals to improve the efficiency and effectiveness of every interaction. We are similarly changing our approach to engaging healthcare systems, payers and other healthcare providers.
The growing number of so‑called “specialty” drugs in our portfolio, such as Cosentyx and Kesimpta, has resulted in increased engagement with specialty pharmacies. Because many of these drugs require special handling and administration, we are rolling out an international patient support program across our priority markets that serves as a central resource for onboarding, education and support to help patients navigate their healthcare.
With our radioligand therapy Pluvicto, extra steps must be taken to educate customers, in part because it can only be administered by those who are trained to handle radiopharmaceuticals. We are working to improve the customer experience by connecting patients with healthcare professionals throughout the disease management process, including awareness and engagement, treatment site onboarding, referrals and imaging, preparation and infusion, and treatment follow-up. We have established a dedicated support team, a customer relationship management platform and an order management platform as part of this effort.
In the US, the US Centers for Medicare & Medicaid Services (CMS) is the largest single payer for healthcare services as a result of continuing changes in healthcare economics and an aging population. In addition, both commercial and government-sponsored managed care organizations continue to be among the largest groups of payers for healthcare services in the US. In other countries, national health services are often the only significant payer for healthcare services. In an effort to control prescription drug costs, almost all managed care organizations and national health services use formularies that list specific drugs that may be reimbursed and/or the level of reimbursement for each drug. Managed care organizations and national health services also use cost‑benefit analyses to determine whether or not newly approved drugs will be added to a formulary and/or the level of reimbursement for that drug, and to determine whether or not to continue to reimburse existing drugs. We have dedicated teams that actively seek to optimize patient access, including formulary positions, for our products.
The trend toward consolidation among distributors and retailers of our products continues in the US and internationally, both within and across countries. This has increased our customers’ purchasing leverage and resulted in increased pricing pressure on our products. Moreover, we are exposed to increased concentration of credit risk as a result of the consolidation among our customers.
Drug pricing is an increasingly prominent issue in many countries as healthcare spending continues to rise. This issue has received significant attention in the US, especially with the passage of the Inflation Reduction Act (please see “—Price controls” for more information). At Novartis, we are increasing our efforts to enable patient access through innovative pricing and access initiatives in the US, Europe and other markets. These include contract structures such as pay-over-time and outcome-based agreements.
In 2020, Novartis Gene Therapies established a novel early access program for Zolgensma. It supports early patient access through customizable options including retroactive rebates, deferred payments, installment options, outcome-based rebates, and collaborations with healthcare systems to optimize disease management. We now have more than 40 early access agreements and pay-for-performance agreements (i.e., outcome-based arrangements) in place in various markets around the world. Zolgensma is approved in 51 countries.
Additionally, in 2021 Novartis reached an agreement with the National Health Service (NHS) in England to implement a first-of-its-kind population health management approach designed to provide faster and broader access to Leqvio for certain high-risk patients with atherosclerotic cardiovascular disease. Novartis has engaged in similar collaborations with other countries.
Competition
The global pharmaceutical market is highly competitive. We compete against other major international corporations that have substantial financial and other resources, as well as against smaller companies that operate regionally or nationally. Competition within the industry is intense and extends across a wide range of activities, including pricing, product characteristics, customer service, sales and marketing, and research and development.
Like other companies selling patented pharmaceuticals, Novartis faces challenges from companies selling competing patented products. Generic forms of our products may follow the expiration of intellectual property protection or regulatory exclusivities, and generic companies may also gain entry to the market through successfully challenging our intellectual property rights and exclusivities. We use appropriate, legally permissible measures to defend those rights and exclusivities (see also “—Intellectual property” below). We also may face competition from over-the-counter (OTC) products that do not require a prescription from a physician.
There is ongoing consolidation in the pharmaceutical industry. At the same time, new entrants are looking to use their expertise to establish or expand their presence in healthcare. Technology companies, for instance, are seeking to benefit from the increasing importance of data and data management in our industry, including the use of artificial intelligence.
Research and development
The discovery and development of a new drug usually requires approximately 10 to 15 years from the initial research to bringing a drug to market. This includes approximately six to eight years from Phase I clinical trials to market entry. At each of these steps, there is a substantial risk that a therapeutic candidate will not meet the requirements to progress further. In such an event, we may be required to abandon the development of a potential therapy in which we have made a substantial investment.
We manage our research and development expenditures across our entire portfolio in accordance with our strategic priorities. We make decisions about whether or not to proceed with development projects on a project‑by‑project basis. These decisions are based on the project’s potential to meet a significant unmet medical need or to improve patient outcomes, the strength of the science underlying the project, and the potential of the project (subject to the risks inherent in pharmaceutical development) to generate significant positive financial results for the Company. Once a management decision has been made to proceed with the development of a therapeutic candidate, the level of research and development investment required will be driven by many factors. These include the medical indications for which it is being developed, the number of indications being pursued, whether the therapeutic candidate is of a chemical or biological nature, the stage of development, and the level of evidence necessary to demonstrate clinical efficacy and safety.
Research program
Our research and early development program is conducted by our Biomedical Research organizational unit, which is the innovation engine of Novartis. This unit is responsible for the discovery of new medicines that bring value for patients and the Company. This requires hiring and retaining highly talented employees, focusing on fundamental disease mechanisms that are relevant across different disease areas, continuously improving technologies for drug discovery and potential therapies, working with patients to understand their diseases and the potential benefits of therapies, forming close alliances with clinical and commercial colleagues, and establishing strategic external alliances.
We have 5 511 full‑time-equivalent scientists, physicians and business professionals at Biomedical Research sites in Basel, Switzerland; Cambridge, Massachusetts; East Hanover, New Jersey; San Diego, California; and Emeryville, California. They contribute to research into disease areas such as cardiovascular, renal and metabolic diseases; neuroscience; oncology; immunology; and ophthalmology. Research at the Friedrich Miescher Institute focuses on basic genetic and genomic research, and our Global Health Disease Area (formerly the Novartis Institute for Tropical Diseases) focuses on discovering new medicines to fight tropical diseases, including malaria and cryptosporidiosis. In 2023, we made the decision to discontinue our respiratory research efforts to further focus our resources on priority areas.
All drug candidates go through clinical trials to enable an early assessment of the safety and efficacy of the drug while collecting basic information on how the drug moves through the body and is tolerated, and adhering to the guidance for early clinical testing set forth by health authorities. When assessments are favorable, our Development organizational unit conducts confirmatory trials on the drug candidates to generate data that can be submitted to regulatory authorities to secure approval for patient use.
Development program
Our Development unit oversees and executes drug development activities, working collaboratively with Biomedical Research, our commercial units and other parts of the Company on our overall pipeline strategy. It includes centralized global functions such as Regulatory Affairs and Global Clinical Operations, and global Development Units, and has 12 723 full‑time equivalent employees worldwide.
The traditional model of clinical development consists of three phases:
Phase I: The first clinical trials of a new compound – generally performed in a small number of healthy human volunteers/patients (e.g., in oncology) – to assess the drug’s safety profile, including the safe dosage range. These trials also determine how a drug is absorbed, distributed, metabolized and excreted, and the duration of its action.
Phase II: Studies performed with patients who have the target disease, with the aim of continuing the Phase I safety assessment in a larger group, assessing the efficacy of the drug in the patient population, and determining the appropriate doses for further evaluation.
Phase III: Large‑scale studies with up to several thousand patients, which aim to establish the safety and efficacy of the drug in specific indications for regulatory approval. Phase III trials may also be used to compare a new drug against a current standard of care to evaluate the overall benefit‑risk relationship of the new medicine.
In each of these phases, physicians monitor consenting volunteers or patients closely to assess the safety and efficacy of a potential new drug or indication.
Although we use this traditional model, we have tailored the development process to be simpler, more flexible and more efficient. This design ensures close collaboration across R&D, enabling Development teams to initiate later-stage planning in parallel with early evaluations and research teams to better support later-stage activities.
Our development process consists of two stages: Early Development to build confidence in the overall properties of the compound, followed by Confirmatory Development to confirm the concept in large numbers of patients. Early development consists of both Phase I studies in healthy volunteers as well as Phase Ib and Phase II studies in patients. This work includes a careful review of safety and tolerability, understanding of whether the drug is modulating the target of interest, and understanding of dose response and early evidence of disease efficacy. Biomedical Research conducts these trials and if this evaluation is positive, the drug moves to the Confirmatory Development stage and becomes the responsibility of Development.
Confirmatory Development has elements of traditional Phase II/III testing and includes trials aimed at confirming the safety and efficacy of the drug in the given indication, leading up to submission of a dossier to health authorities for approval. This stage can also include trials that compare the drug to the current standard of care for the disease in order to evaluate the drug’s overall benefit-risk profile. Further, with new treatment approaches such as gene therapy for rare diseases, elements of Early and Confirmatory Development may be combined and suffice for registration under certain conditions such as high unmet medical need and clinical data showing highly favorable benefit-risk profiles. In these cases, additional post-approval studies may be required by the regulatory authorities to continue to gather important data to further support approval.
The vast amount of data that must be collected and evaluated makes clinical testing the most time‑consuming and expensive part of new drug development. The next stage in the drug development process is to seek registration for the new drug. For more information, see “—Regulation.”
The Innovation Management Board (IMB), chaired by our Chief Executive Officer, drives our R&D portfolio strategy. The IMB endorses new early- and late-stage development projects, strategic plans and portfolio-related priorities. It oversees our drug development budget; approves major project phase transitions; and makes key decisions, such as when to submit regulatory applications to health authorities or when to discontinue projects. IMB members include representatives from the Executive Committee of Novartis (ECN) and senior management with expertise in different fields.
To support our R&D strategy, we are investing in artificial intelligence (AI) and other technologies that have the potential to enhance and accelerate the delivery of innovative medicines to patients. We are working with partners on scalable projects in early-stage research and in clinical development to help improve our decision-making and generate actionable insights across our core therapeutic areas—from designing new compounds to predicting drug safety and conducting clinical trials.
Alliances and acquisitions
Novartis enters into business development agreements with other pharmaceutical and biotechnology companies and with academic and other institutions to develop new products and access new markets. We license products that complement our current product line and are appropriate to our business strategy. We focus on strategic alliances and acquisition activities for key disease areas and indications that we expect to be growth drivers in the future. We review products and compounds we are considering licensing, using the same criteria that we use for our own internally discovered drugs.
In July 2023, Novartis acquired DTx Pharma Inc., a US-based, preclinical-stage biotechnology company focused on leveraging its proprietary FALCON platform to develop siRNA therapies for neuroscience indications. Its lead program, DTx-1252, targets the root cause of CMT1A – the overexpression of PMP22, a protein that causes the myelin sheath that supports and insulates nerves in the peripheral nervous system to function abnormally. The transaction also includes two additional preclinical programs for other neuroscience indications.
In August 2023, Novartis acquired Chinook Therapeutics, Inc., a US-based, clinical-stage biopharmaceutical company with two late-stage medicines in development for rare, severe chronic kidney diseases.
For more information about recent business acquisitions, see “Item 18. Financial Statements—Note 2. Significant transactions.”
Regulation
The international pharmaceutical industry is highly regulated. Regulatory authorities around the world administer numerous laws and regulations regarding the testing, approval, manufacturing, importing, labeling and marketing of drugs, and review the safety and efficacy of pharmaceutical products. Extensive controls exist on the non‑clinical and clinical development of pharmaceutical products. These regulatory requirements, and the implementation of them by local health authorities around the globe, are a major factor in determining whether a substance can be developed into a marketable product, and the amount of time and expense associated with that development.
Health authorities, including those in the US and the EU, have high standards of technical evaluation. The introduction of new pharmaceutical products generally entails a lengthy approval process. Products must be authorized or registered prior to marketing, and such authorization or registration must subsequently be maintained. In recent years, the registration process has required increased testing and documentation for the approval of new drugs, with a corresponding increase in the expense of product introduction.
To register a pharmaceutical product, a registration dossier containing evidence establishing the safety, efficacy and quality of the product must be submitted to regulatory authorities. Generally, a therapeutic product must be registered in each country in which it will be sold. In every country, the submission of an application to a
regulatory authority does not guarantee that approval to market the product will be granted. Although the criteria for the registration of therapeutic drugs are similar in most countries, the formal structure of the necessary registration documents and the specific requirements, including risk tolerance, of the local health authorities can vary significantly from country to country. Even if a drug is registered and marketed in one country, the registration authority in another country may request additional information from the pharmaceutical company prior to registration or even reject the product. A drug may be approved for different indications in different countries.
The registration process generally takes between six months and several years, depending on the country, the quality of the data submitted, the efficiency of the registration authority’s procedures, and the nature of the product. Many countries provide for accelerated processing of registration applications for innovative products of particular therapeutic interest. In recent years, the US and the EU have made efforts to harmonize registration requirements in order to achieve shorter development and registration times for medical products. However, the requirement in many countries to negotiate selling prices or reimbursement levels with government regulators and other payers can substantially extend the time until a product may finally be available to patients.
The following provides a summary of the regulatory processes in the principal markets served by our affiliates:
United States
In the US, applications for drug registration are submitted to and reviewed by the FDA. The FDA regulates the testing, manufacturing, labeling and approval for marketing of pharmaceutical products intended for commercialization in the US. The FDA continues to monitor the safety of pharmaceutical products after they have been approved for sale in the US market. The pharmaceutical development and registration process is typically intensive, lengthy and rigorous. When a pharmaceutical company has gathered data that it believes sufficiently demonstrates a drug’s safety, efficacy and quality, the company may file a New Drug Application (NDA) or Biologics License Application (BLA), as applicable, for the compound. The NDA or BLA must contain all the scientific information that has been gathered about the compound. This typically includes information regarding the clinical experiences of patients tested in the drug’s clinical trials. A Supplemental New Drug Application (sNDA) or Supplemental Biologics License Application (sBLA) must be filed for new indications and dosage forms for a previously approved drug.
Once an application is submitted, the FDA assigns reviewers from its staff, including experts in biopharmaceutics, chemistry, clinical microbiology, pharmacology/toxicology, and statistics. After a complete review, these content experts provide written evaluations of the NDA or BLA. These recommendations are consolidated and are used by senior FDA staff in its final evaluation of the NDA or BLA. Based on that final evaluation, the FDA then either approves the NDA or BLA, or provides a “complete response” letter if the NDA or BLA application is not approved. If not approved, the letter will state the specific deficiencies in the NDA or BLA that need to be addressed. The company making the application must then submit an adequate response to the deficiencies in order to restart the review procedure.
Once the FDA has approved an NDA, BLA, sNDA or sBLA, the company can make the new drug available for physicians and other healthcare providers to prescribe. The drug owner must submit periodic reports to the FDA, including any cases of adverse reactions. For some medications, the FDA requires additional post‑approval studies (Phase IV) to evaluate long‑term effects or to gather information on the use of the product under specified conditions.
Throughout the life cycle of a product, the FDA requires compliance with standards relating to good laboratory, clinical and manufacturing practices. The FDA also requires compliance with rules pertaining to the manner in which we may promote our products.
European Union
In the EU, there are three main procedures for application for authorization to market pharmaceutical products in more than one EU member state at the same time: the centralized procedure, the mutual recognition procedure and the decentralized procedure. It is also possible to obtain a national authorization for products intended for commercialization in a single EU member state only. The procedure used for first authorization must continue to be followed for subsequent changes, e.g., to add an indication for a licensed product.
Under the centralized procedure, applications are made to the EMA for an authorization that is valid for the European Union (all member states). The centralized procedure is mandatory for all biotechnology products; new chemical entities in cancer, neurodegenerative disorders, diabetes, AIDS, autoimmune diseases and other immune dysfunctions; advanced therapy medicines, such as gene therapy, somatic cell therapy and tissue-engineered medicines; and orphan medicines (medicines for rare diseases). It is optional for other new chemical entities, innovative medicinal products, and medicines for which authorization would be in the interest of public health. When a pharmaceutical company has gathered data that it believes sufficiently demonstrates a drug’s safety, efficacy and quality, the company may submit an application to the EMA. The EMA then receives and validates the application, and the specialized committee for human medicines, the CHMP, appoints a rapporteur and co‑rapporteur to review it. They use experts from their countries to carry out the assessment but can also draw on expertise from other member states (“multinational teams”). The entire review cycle must be completed within 210 days, although there are “clock stops” to allow the company to respond to questions set forth in the rapporteur and co‑rapporteur’s assessment report and agreed with the CHMP. The first clock stop is at Day 120 and the clock restarts on Day 121, when the company’s complete response is received by the EMA. If there are further aspects of the dossier requiring clarification, the CHMP will issue further questions at Day 180, and may also request an oral explanation, in which case the sponsor must not only respond to the further questions but also appear before the committee to
justify its responses. On Day 210, the CHMP will take a vote to recommend the approval or non‑approval of the application, and their opinion is transferred to the European Commission (EC). The final EC decision under this centralized procedure is a single decision that is applicable to all member states. This decision occurs 60 days, on average, after a positive CHMP recommendation.
Under both the mutual recognition procedure (MRP) and the decentralized procedure (DCP), the assessment is led by one member state, called the reference member state (RMS), which then liaises with other member states, known as the concerned member states. In the MRP, the company first obtains a marketing authorization in the RMS, which is then recognized by the concerned member states in 90 days. In the DCP, the application is done simultaneously in the RMS and all concerned member states. During the DCP, the RMS drafts an assessment report within 120 days. Within an additional 90 days, the concerned member states review the application and can issue objections or requests for additional information. On Day 90, each concerned member state must be assured that the product is safe and effective, and that it will cause no undue risks to the public health. Once an agreement has been reached, each member state grants national marketing authorizations for the product.
After receiving the marketing authorizations, the company must submit periodic safety reports to the relevant health authority (EMA for the centralized procedure, national health authorities for DCP or MRP). In addition, pharmacovigilance measures must be implemented and monitored, including the collection, evaluation and expedited reporting of adverse events, and updates to risk management plans. For some medications, post-approval studies (Phase IV) may be imposed to complement available data with additional data to evaluate long-term effects (called a Post-Approval Safety Study, or PASS) or to gather additional efficacy data (called a Post-Approval Efficacy Study, or PAES).
European marketing authorizations have an initial duration of five years. The holder of the marketing authorization must actively apply for its renewal after this first five-year period. As part of the renewal procedure, the competent authority performs a full benefit‑risk review of the product. Should the authority conclude that the benefit‑risk balance is no longer positive, the marketing authorization can be suspended or revoked. Once renewed, the marketing authorization is valid for an unlimited period, unless it is determined that the product must be further monitored for safety reasons. In this case, the authority may require another renewal at 10 years. If the holder does not apply for renewal, the marketing authorization automatically lapses. Any marketing authorization that is not followed within three years of its granting by the actual placing on the market of the corresponding medicinal product ceases to be valid.
Price controls
In most of the markets where we operate, the prices of pharmaceutical products are subject to both direct and indirect price controls and to drug reimbursement programs with varying price control mechanisms. Due to increasing political pressure and governmental budget constraints, we expect these mechanisms to remain robust – and potentially even be strengthened – and to have a continued negative influence on the prices we are able to charge for our products.
Direct governmental efforts to control prices
United States: The Inflation Reduction Act of 2022 (IRA), signed into law in August 2022, mandates the negotiation of eligible Medicare Part B and Part D drugs; redesigned the Medicare Part D benefit, including a USD 2 000 out-of-pocket cap for Medicare beneficiaries; and imposed penalties for Medicare drugs that increase in price faster than the rate of inflation. Under the IRA, the US government will set Medicare prices for selected products it has defined as single-sourced small-molecule drugs that have been on the market for seven years following FDA approval as well as single-sourced biologics that have been on the market for 11 years after FDA approval.
Medicare drugs with the highest total cost to the US government are being selected for the program as they become eligible. Exemptions include orphan drugs with an indication for one rare disease or condition, drugs with a total cost to the US government of less than USD 200 million, and plasma-derived drugs.
The price set by the government will be publicly available and will become effective for selected drugs nine years after FDA approval for eligible small molecules and 13 years after FDA approval for eligible biologics. It will be implemented as follows:
• Ten eligible Medicare Part D drugs in 2026
• An additional 15 eligible Medicare Part D drugs in 2027
• An additional 15 eligible combined Medicare Part B and Part D drugs in 2028
• An additional 20 eligible combined Medicare Part B and Part D drugs in 2029
• An additional 20 eligible combined Medicare Part B and Part D drugs each year after 2029
On August 29, 2023, the US government released the list of the first 10 drugs to be subject to the program, and Entresto was one of the selected products. Novartis has initiated the process of participating in negotiations because manufacturers that refuse to participate are subject to an excise tax of up to 95% of sales. Novartis has filed a lawsuit against the US Department of Health and Human Services (HHS) and the US Centers for Medicare & Medicaid Services because we believe the IRA’s drug price-setting provisions are unconstitutional and will have long-lasting negative consequences for patients by limiting access to medicines now and in the future (for more information, see “Item 18. Financial Statements—Note 21. Provisions and other non-current liabilities”). Novartis may also be affected by other provisions of the IRA, such as price increase penalties for Medicare Part D drugs starting in 2022 and for Medicare Part B drugs in 2023, and rebates on eligible Medicare Part D sales starting in 2025.
In addition, by December 31, 2023, 23 US states had passed legislation intended to impact pricing or requiring manufacturers to report price increases to states, with eight of these states also allowing for drug affordability (i.e., price control) review boards. The disclosure
requirements vary by state. Many states require multiple types of reporting, including for new drug applications, new drug launches, prior notice of price increases, and quarterly or annual reporting. It is expected that state legislatures will continue to focus on drug pricing in 2024 and that similar bills will be passed in more states.
Europe: In Europe, our operations are subject to significant price and marketing regulations. Many governments are introducing healthcare reforms in a further attempt to curb increasing healthcare costs. In some member states, these include reforms to permit the reimbursed use of off-label medicines, despite the presence of licensed alternatives on the market. In the EU, governments influence the price of pharmaceutical products through their control of national healthcare systems that fund a large part of the cost of such products to patients. The downward pressure on healthcare costs in general in the EU, particularly with regard to prescription drugs, is intense. Increasingly strict analyses are applied when evaluating the entry of new products, and as a result, access to innovative medicines is limited based on strict cost‑benefit assessments. In addition, prices for marketed products are referenced within member states and across international borders, further impacting individual EU member state pricing. Member states also collaborate to enhance pricing transparency and have started conducting joint health technology assessments, joint pricing negotiations and/or joint purchasing. As an additional control for healthcare budgets, some EU countries have passed legislation to impose further mandatory rebates for pharmaceutical products and/or financial claw‑backs on the pharmaceutical industry. The calculation of these rebates and claw‑backs may lack transparency in some cases and can be difficult to predict.
Regulations favoring generics and biosimilars
In response to rising healthcare costs, most governments and private medical care providers have established reimbursement schemes that favor the substitution of more expensive brand‑name pharmaceuticals by generic pharmaceuticals. All US states have generic substitution statutes. These statutes permit or require the dispensing pharmacist to substitute a less expensive generic drug instead of an original drug. Other countries, including many European countries, have similar laws. We expect that the pressure for generic substitution will continue to increase. In addition, the US, the EU and other jurisdictions are increasingly introducing laws and regulations encouraging the development of biosimilar versions of biologic drugs, which can also be expected to have an impact on pricing.
Cross‑border sales
Price controls in one country can have an impact in other countries as a result of cross‑border sales. In the EU, products that we have sold to customers in countries with stringent price controls can be legally resold to customers in other EU countries at a lower price than the price at which the product is otherwise available in the importing country (known as parallel trade). In North America, products that we have sold to customers in Canada – which has relatively stringent price controls – are sometimes resold into the US, again at a lower price than the price at which the product is otherwise sold in the US. Such imports from Canada and other countries into the US are currently illegal in most states. However, six US states (Colorado, Florida, Minnesota, New Hampshire, New Mexico and Vermont) have enacted laws allowing the import of pharmaceutical drugs from select foreign countries. The Secretary of the HHS must certify that each state’s importation plan is safe and cost-effective before it can be implemented.
We expect that pressures on pricing will continue worldwide and will likely increase. Because of these pressures, there can be no certainty that in every instance we will be able to charge prices for a product that, in a particular country or in the aggregate, would enable us to earn an adequate return on our investment in that product.
Intellectual property
Intellectual property (IP) rights are essential to our business as an innovative medicines company since they protect our innovation and investments in research and development, manufacturing and marketing of our products. IP rights include patents, trademarks, copyrights, know‑how, trade secrets and regulatory-based protection.
Patents
Among other things, patents may cover products themselves, including the product’s active ingredient or ingredients and its formulation. Patents may cover processes for manufacturing a product, including processes for manufacturing intermediate substances used in the manufacture of the product. Patents may also cover particular uses of a product, such as its use to treat a particular disease, or its dosage regimen. In addition, patents may cover tests for certain diseases or biomarkers – which can improve patient outcomes when administered with certain drugs – as well as assays, research tools and other techniques used to identify new drugs.
In the US, the EU and other countries, the law recognizes that product development and review by health authorities can take an extended period, and provides an extension of patent term for a period related to the time taken for the conduct of clinical trials and for the health authority’s review. These extensions are termed patent term extensions (PTEs) for the US and supplementary protection certificates (SPCs) for the EU.
United States
• In the US, a patent issued from an application filed today will generally receive a term of 20 years from the earliest application filing date as well as potential patent term adjustments for delays in patent issuance based upon certain delays in prosecution by the United States Patent and Trademark Office (USPTO). A US pharmaceutical patent may also be eligible for a PTE. The PTE may only extend the patent term for a maximum of five years, and may not extend the patent term beyond 14 years from regulatory approval. Only one patent may be extended for a product based on FDA review.
European Union
• Patent applications in Europe may be filed in the European Patent Office (EPO) or in a particular country or countries. The term of a patent granted by the EPO or an EU country office is 20 years from the earliest application filing date. Pharmaceutical patents can be granted a further period of exclusivity under an SPC system. The SPCs may only extend the patent term for a maximum of five years, and may not extend the patent term beyond 15 years from the date of the first EU marketing authorization.
RDP and market exclusivity
In addition to patent protection, various countries provide regulatory-based protection, including regulatory data protection (RDP) and/or other market exclusivities, for a prescribed period of time. RDP is a distinct type of IP right providing exclusivity that precludes a potential competitor from filing a regulatory application that relies on the sponsor’s clinical trial data, or that precludes the regulatory authority from approving the application for a set period of time.
United States
• A new small‑molecule active pharmaceutical ingredient receives five years of RDP, during which time a competitor generally may not obtain final approval of an application to the FDA based on a sponsor’s clinical data.
• A new biologic active pharmaceutical ingredient receives 12 years of regulatory-based market exclusivity, during which time a competitor generally may not market the same or similar drug.
• The FDA may also request that a sponsor conduct pediatric studies and, in exchange, it will grant an additional six‑month period of pediatric market exclusivity if the sponsor makes a timely submission of the reports of the pediatric studies in response to the FDA’s Written Request. The sponsor must also have a patent‑based and/or regulatory‑based exclusivity period for the product to which the pediatric market exclusivity is appended.
• Orphan drug exclusivity (ODE) provides seven years of market exclusivity for drugs designated by the FDA as orphan drugs, meaning drugs that treat rare diseases. During this period, a potential competitor generally may not market the same or similar drug for the same indication even if the competitor’s application does not rely on data from the sponsor.
European Union
• A new pharmaceutical ingredient receives eight years of data protection, during which a competitor cannot rely on the relevant data; a further period of two years of market exclusivity, during which the data can be used to support applications for marketing authorization but a competitive product cannot be launched; and a possible one‑year extension of the market exclusivity period if, during the initial eight‑year data exclusivity period, the sponsor registered a new therapeutic indication with “significant clinical benefit.”
• Orphan drug exclusivity provides for 10 years of market exclusivity, during which time an application for the same or similar medicine for the same indication will not generally be accepted or granted. Under certain circumstances, this exclusivity can be extended with a two‑year pediatric extension.
Third-party patents and challenges to intellectual property
Third parties can challenge our IP, including patents, patent term extensions, RDP and marketing exclusivities (such as pediatric extensions and orphan drug exclusivity), through various proceedings. For example, patents in the US can be challenged in the USPTO through various proceedings, including Inter Partes Review (IPR) and Post-Grant Review (PGR) proceedings. They may also be challenged through patent infringement litigation under the Abbreviated New Drug Application (ANDA) provisions of the Hatch‑Waxman Act or under the Biologics Price Competition and Innovation Act (BPCIA). In the EU, patents may be challenged through oppositions in the EPO, or national patents may be challenged in national courts or national patent offices. The outcomes of such challenges can be difficult to predict.
In addition to directly challenging our IP rights, in some circumstances a competitor may be able to market a generic version of one of our products by, for example, designing around our patents or marketing the generic product for non‑patent-protected indications, or filing a separate New Drug Application (NDA) under the Hatch-Waxman Act (typically referred to as a 505(b)(2) application). Despite RDP, a competitor could opt to incur the costs of conducting its own clinical trials and preparing its own regulatory application, and avoid our RDP altogether. There is a risk that some countries may seek to impose limitations on or seek not to recognize the availability of IP rights for pharmaceutical products, or limit the extent to which such rights may be enforced. Additionally, even though we may own, co‑own or in‑license patents protecting our products, and conduct freedom‑to‑operate analyses, a third party may nevertheless assert that one of our products infringes a third-party patent for which we do not have a license, seeking remedies such as monetary damages or an injunction against our continued marketing of the product.
As a result, there can be no assurance that our IP rights will protect our products or that we will be able to avoid adverse effects from the loss of IP protection or from third-party patents in the future. For more information on the risks related to our IP protection, see “Item 3. Key Information—Item 3.D Risk factors—Intellectual property—Expiry, assertion or loss of intellectual property protection.”
Intellectual property protection for certain key marketed products and compounds in development
We present additional details below regarding certain IP protection in the US and the EU for certain key marketed products. For each, we identify issued, unexpired patents by their general subject matter and, in parentheses, years of expiry, if relevant, in the US and the EU. The identified patents are owned, co‑owned or exclusively in‑licensed by Novartis and relate to at least one dosage strength of the product or to the method of treatment or its use as it is currently approved and marketed or, in the case of a compound in development, as it is currently
submitted to the FDA and/or the EMA for approval. Identification of an EU patent refers to national patents in EU countries and/or to the national patents that have been derived from a patent granted by the EPO. Novartis may own, co-own, control or have rights to additional patents, for example, relating to compound forms, methods of treatment or use, formulations, devices, processes, product-by-process, synthesis, purification and assays. Information on such patents, where available, may be found in publicly accessible databases such as the FDA patent databases, online databases such as Espacenet™ and Pat-INFORMED, and patent office registers.
We identify unexpired RDP periods and, in parentheses, years of expiry if the relevant marketing authorizations have been authorized or granted. We identify certain unexpired patent term extensions and marketing exclusivities and, in parentheses, years of expiry if they are granted; their subject matter scope may be limited and is not specified. Marketing exclusivities and patent term extensions include ODE, pediatric exclusivity (PE), PTE and SPC.
Identification of a patent in the EU refers to national patents in EU countries and/or to the national patents that have been derived from a patent granted by the EPO. In the case of the EU, identification of a patent, supplementary protection certificate, marketing exclusivity or regulatory data protection means grant, authorization and maintenance in at least one EU country. However, it could be pending, not granted, expired or found invalid in others.
We designate certain IP protection as “pending” if such IP protection has been applied for but not granted and includes years of expiration if estimable. Such pending applications ultimately may or may not be granted.
Where relevant, we indicate whether there is current generic or biosimilar competition for one or more product versions in one or more approved indications in either the US or one or more EU countries. We identify certain enforcement actions, or ongoing challenges to the disclosed IP, including IPRs or PGRs if instituted by the USPTO, that have not been finally resolved (including appeals) unless noted. Resolution of challenges to the disclosed IP, which in the EU may involve IP in one or more EU countries, may include settlement agreements under which Novartis permits or does not permit future launch of generic versions of our products before expiration of that IP. We identify certain material terms of such settlement agreements where they could have a material adverse effect on our business. In other cases, such settlement agreements may contain confidentiality obligations restricting what may be disclosed.
In the event that a product listed below does not have identified patents as described above, we provide information only on generic competition.
For additional information regarding commercial arrangements with respect to these products, see “—Key marketed products.”
Cardiovascular renal and metabolic
• Entresto. US: Two patents on combination (2023, 2023), PTE (2025), two PEs (2024, 2025); two patents on complex (2026, 2027), two PEs (2027, 2027); three patents on methods of treatment (2033 (3)); patent on dosage regimen (2036); RDP for labeling changes related to new clinical investigation (2024). EU: Patent on combination (2023), SPC (2028); patent on complex (2026), SPC (2030); patent on formulation (2028); patent on method of use (2034); patent on dosage regimen (2036); RDP (2026). There is no generic competition in the US or the EU. In the US, certain patents, including the combination and complex patents, are being challenged in ANDA proceedings against generic manufacturers. In July 2023, the US District Court for the District of Delaware issued a negative decision regarding the validity of one of the combination patents. Novartis has appealed to reverse the decision. In the EU, certain patents, including the complex patent, are being opposed in the EPO. In some EU countries, the combination patent or its associated SPC is being challenged by generic manufacturers.
• Leqvio. US: Two patents on composition of matter (2027, 2034), PTE pending (2035); two patents on method of treatment and dosing regimen (2027, 2036); RDP (2026). EU: One patent on composition of matter (2033), SPC (2035); RDP (2030). There is no generic competition in the US or the EU.
Immunology
• Cosentyx. US: Five patents on composition of matter (2025 (4), 2026), PTE (2029); patent on psoriatic arthritis use (2031); patent on psoriasis use (2032); two patents on ankylosing spondylitis use (2032, 2033); RDP (2027). EU: Four patents on composition of matter (2025 (4)), SPC (2030), PE (2030); patent on psoriasis use (2031); RDP (2026). There is no generic competition in the US or the EU.
• Xolair. US: Two patents on syringe formulation (2024, 2025). EU: Three patents on syringe formulation (2024, 2024, 2025). There is no generic competition in the US or the EU.
• Ilaris. US: Patent on composition of matter (2024); patent on cryopyrin‑associated periodic syndromes (CAPS) use (2026); patent on familial Mediterranean fever (FMF) use (2026); patent on systemic onset juvenile idiopathic arthritis (SJIA) use (2028); patent on gout use (2028); patent on hyperimmunoglobulin D syndrome, adult-onset Still’s disease (AOSD), and tumor necrosis factor receptor-associated periodic syndrome use (2029); patent on formulation (2029); ODE on AOSD (2027). EU: Patent on composition of matter (2021), SPC (2024), PE (2025); patent on SJIA use (2026); patent on FMF use (2026); patent on CAPS use (2026); two patents on formulation (2029, 2029). There is no generic competition in the US or the EU.
Neuroscience
• Kesimpta. US: Patent on compound (2031); patent on dosing regimen (2037). EU: Patent on use (2023), SPC (2028); patent on formulation (2028), SPC (2033); patent on formulation and use (2028); two patents on dosing regimen (2037, 2037). There is no generic competition in the US or the EU.
• Zolgensma. US: Four patents on composition of matter (2024, 2024, 2026, 2033), PTE pending (2029);
four patents on methods of treatment (2028 (3), 2029); ODE for spinal muscular atrophy (SMA) in patients less than 2 years old with biallelic mutations in the SMN1 gene (2026); RDP (2031). EU: Three patents on composition of matter (2024, 2024, 2028), SPC (2029); two patents on methods of use (2028, 2028), two SPCs (2033, 2033); ODE for SMA in patients with a biallelic mutation in the SMN1 gene and a clinical diagnosis of SMA type 1, or patients with a biallelic mutation in the SMN1 gene and up to three copies of the SMN2 gene (2030); RDP (2030). There is no generic competition in the US or the EU.
Oncology
• Promacta/Revolade. US: Patent on salt form and thrombocytopenia use (2025), PE (2026); five patents on tablet formulations of different dose strengths (2027 (5)), five PEs (2028 (5)); ODE on severe aplastic anemia patients in combination with standard immunosuppressive therapy (2025). EU: Patent on compound (2021), SPC (2025), PE (2025); patent on severe aplastic anemia use (2028). There is no generic competition in the US or the EU. In the US, generic manufacturers have filed ANDAs challenging certain patents other than the compound patent. In the EU, a patent, other than the compound patent, is being opposed in the EPO.
• Kisqali. US: Three patents on compound (2028, 2030, 2031), PTE (2031); three patents on methods of treatment (2029, 2029, 2031); patent on salt form (2031); patent for tablet formulation (2036). EU: Patent on compound (2027); patent on compound (2029), SPC (2032); patent on salt form (2031); patent on methods of use with letrozole (2034); patent on formulation (2036); RDP (2027). There is no generic competition in the US or the EU. In the US, certain patents, including the compound patent, are being challenged in ANDA proceedings against a generic manufacturer. In the EU, a patent, other than the compound patent, is being opposed in the EPO.
• Tafinlar and Mekinist.
Tafinlar. US: Two patents on compound (2030, 2030), two PEs (2030, 2030); patent on method of treatment (2029), PE (2029); patent on pediatric formulation (2038). EU: Patent on compound (2029); RDP (2024). There is no generic competition in the US or the EU. In the EU, patents, other than the compound patent, are being opposed in the EPO.
Mekinist. US: Patent on compound (2025), PTE (2027), PE (2027); patent on method of treatment (2025), PE (2025); four patents on formulation (2032 (4)), four PEs (2032 (4)). EU: Patent on compound (2025), SPC (2029); patent on formulation (2031); RDP (2025). There is no generic competition in the US or the EU. In the US, certain patents, including the compound patent, are being challenged in ANDA proceedings against a generic manufacturer. In the EU, patents other than the compound patent are being opposed in the EPO.
Use of Mekinist with Tafinlar or Tafinlar with Mekinist. US: Patent on combination (2030), PE (2031); four patents on method of use of combination (2025, 2030, 2030, 2033), four PEs (2025, 2031, 2031, 2034); ODE on non‑small cell lung cancer (2024), PE (2024); ODE on adjuvant treatment of melanoma (2025), PE (2025); ODE on anaplastic thyroid cancer (2025), PE (2025); ODE on metastatic solid tumors (2025), PE (2025); ODE on pediatric glioma (2030). EU: Patent on combination (2030); patent on combination for use in lung cancer (2030); patent on adjuvant melanoma use (2033); ODE on pediatric glioma (2035). There is no generic competition in the US or the EU.
• Tasigna. US: Two patents on salt forms (2026, 2028), two PEs (2027, 2029); patent on polymorph compound form (2026), PE (2027); two patents on capsule form (2026, 2027), two PEs (2027, 2028); patent on method of treatment (2032), PE (2032). EU: Patent on salt form (2026); patent on polymorph compound form (2026); three patents on capsule form (2027 (3)); patent on method of treatment (2030). There is no generic competition in the US or the EU. In the US, generic manufacturers have filed ANDAs challenging certain patents other than the compound patent.
• Jakavi. EU: Patent on compound (2026), SPC (2027); two patents on salt form (2028, 2028); patent on compound for polycythemia vera use (2026); patent on use in treatment of graft-versus-host disease (2026); patent on salt form for graft-versus-host disease use (2028). There is no generic competition in the EU.
• Pluvicto. US: Three patents on composition of matter (2028, 2028, 2034); RDP (2027). PTE pending. EU: RDP (2032). There is no generic competition in the US or the EU.
• Lutathera. US: Two patents on formulation (2038, 2038); ODE (2025). EU: RDP (2027); ODE (2027). There is no generic competition in the US or the EU. In the US, certain patents are being challenged in ANDA proceedings against a generic manufacturer.
• Scemblix. US: Patent on compound (2033), PTE pending (2035); patent on polymorph compound form (2040); RDP (2026); ODE (2028). EU: Patent on compound (2033), SPC (2037); RDP (2032); ODE (2032). There is no generic competition in the US or the EU.
• Fabhalta. US: Patent on compound (2034), PTE pending (2037); patent on salt form (2041); RDP (2028); ODE (2030). EU: Patent on compound (2034). There is no generic competition in the US or the EU.
Established brands
• Lucentis. EU: There is generic competition in the EU.
• Sandostatin SC and Sandostatin LAR.
Sandostatin SC: There is generic competition in the US and the EU.
Sandostatin LAR: There is generic competition in most EU countries but no generic competition in the US.
Compounds in development
We provide certain patent information for non‑marketed compounds in development that have been submitted to the FDA and/or the EMA for registration but have not yet been approved by either agency. For these products, Novartis will seek all appropriate RDP, will continue to seek additional intellectual property protection for significant product developments, and will apply for PTEs and SPCs in keeping with the great importance we attach to intellectual property.
4.C Organizational structure
Organizational structure
See “Item 4. Information on the Company—Item 4.A History and development of Novartis” and “Item 4. Information on the Company—Item 4.B Business overview—Overview.”
Significant subsidiaries
See “Item 18. Financial Statements—Note 33. Novartis principal subsidiaries and associated companies.”
4.D Property, plants and equipment
Our principal executive offices are located in Basel, Switzerland. We operate through a number of affiliates that have offices, research and development facilities, and production sites throughout the world.
We generally own our facilities or have entered into long-term lease arrangements for them. Some of our principal facilities are subject to mortgages and other security interests granted to secure certain debts.
Our Operations organizational unit manages the production, supply chains and quality of our products through a network of 33 manufacturing sites, as well as through external suppliers, and warehouse and distribution centers. In addition, our Operations organizational unit also manages non-production real estate owned or leased by Novartis around the world.
The following table sets forth our major headquarters and most significant production, research and development, and administrative facilities. See also “—Item 4.B Business overview—Production” for a discussion of our manufacturing processes.
Major facilities
Location
| | Size of site
(in square meters) | |
Major activity
| |
|
Basel, Switzerland – St. Johann | | 481 448 | | Global Company headquarters; International organizational unit headquarters; research and development; production of drug substances and drug intermediates | |
|
Kundl and Schaftenau, Austria | | 480 000 | | Production of biotechnological products, active drug substances and nucleic acids, drug products and finished products; product development | |
|
East Hanover, New Jersey, US | | 277 015 | | US organizational unit headquarters; research and development | |
|
Cambridge, Massachusetts, US | | 179 939 | | Research and development | |
|
Menges, Slovenia | | 133 763 | | Production of drug substances and drug intermediates; Research and development for Biologics | |
|
Shanghai, China | | 105 614 | | China country headquarters; research and development | |
|
Stein, Switzerland | | 64 700 | | Production of sterile vials, pre‑filled syringes and ampoules; capsules and tablets; active pharmaceutical ingredients; and cell and gene therapies | |
|
Huningue, France | | 35 000 | | Production of drug substances for clinical and commercial supply | |
|
Durham, North Carolina, US | | 15 794 | | Manufacture, package and release commercial Zolgensma product and certain clinical development activities | |
|
Schweizerhalle, Switzerland | | 8 880 | | Manufacture of small-interfering RNA (siRNA) drug substance for Leqvio | |
|
Indianapolis, Indiana, US | | 6 500 | | Manufacture, package and release clinical and commercial Pluvicto product for US and Canada | |
|
Ivrea, Italy | | 2 100 | | Manufacture, package and release clinical and commercial Pluvicto and Lutathera products | |
|
As our product portfolio evolves, the Company's Operations organizational unit is adapting our manufacturing capacity and capabilities to meet our changing needs, shifting from high-volume products toward lower-volume, customized and personalized medicines. As of December 31, 2023, we have closed, exited or sold 13 Novartis manufacturing sites since 2020. We have continued to invest in manufacturing technologies implemented at our
sites, such as our new targeted radioligand therapy production facility in Indianapolis, Indiana. We are leveraging innovation to increase the reliability and productivity of our manufacturing network, including using data and digital technologies. We continue to seek opportunities to manage our production facilities as efficiently as possible, optimize external spend, and simplify and standardize across our manufacturing network to help us increase our cost competitiveness and optimize the value of our products. At the same time, we are working to improve our environmental sustainability, for example by reducing energy, waste disposal and water consumption at our sites by making our manufacturing processes more efficient, introducing new technologies, and switching to clean and renewable energy solutions.
For a description of the impact of environmental matters, see “Item 3. Key Information—Item 3.D Risk factors—Environmental, social and governance matters—Failure to meet rapidly evolving environmental, social and governance expectations,” “Item 3. Key Information—Item 3.D Risk factors—Environmental matters—Impact of environmental liabilities,” and “Item 3. Key Information—Item 3.D Risk factors—Climate change—Failure to manage physical and transition risks from climate change.” See also “Item 18. Financial Statements—Note 21. Provisions and other non‑current liabilities.”
Item 4A. Unresolved Staff Comments
Not applicable.
Item 5. Operating and Financial Review and Prospects
5.A Operating results
This operating and financial review should be read together with our consolidated financial statements in this Annual Report, which have been prepared in accordance with International Financial Reporting Standards (IFRS) Accounting Standards as issued by the International Accounting Standards Board (see “Item 18. Financial Statements”). “Item 5. Operating and Financial Review and Prospects” with the sections on our compounds in development and selected development projects (see “Item 4. Information on the Company—Item 4.B Business overview”) constitute the Operating and Financial Review (Lagebericht), as defined by the Swiss Code of Obligations.
Overview
Novartis is an innovative medicines company. Our purpose is to reimagine medicine to improve and extend people’s lives. In September 2023, we reorganized our operations into five organizational units. These are Biomedical Research, Development, Operations, and two commercial units: US and International. Global functions support these organizational units in the execution of their work. We are engaged in the research, development, manufacturing, distribution, and commercialization and sale of innovative medicines, with a focus on the core therapeutic areas: cardiovascular, renal and metabolic; immunology; neuroscience; and oncology; as well as established brands. For information about this new organizational structure, see “Item 4. Information on the Company—Item 4.B Overview.”
In 2023, Novartis completed its transformation into a pure-play innovative medicines business, with the spin-off of Sandoz. Effective October 4, 2023, Sandoz was listed on the SIX Swiss Exchange, with a Level 1 ADR program in the United States.
To comply with IFRS Accounting Standards, as a result of the spin-off, Novartis has separated the Company’s consolidated financial statements for the current and prior years into “continuing” and “discontinued” operations. For more information, see “Item 18. Financial Statements—Note 1. Accounting policies.”
The disclosures and commentary in this “Item 5. Operating and Financial Review and Prospects” focuses on continuing operations. We also provide information on discontinued operations.
Continuing operations include the retained business activities of Novartis, comprising the innovative medicines business and continued corporate activities.
Discontinued operations include the Sandoz generic pharmaceuticals and biosimilars division and certain corporate activities attributable to Sandoz prior to the spin-off up to the distribution date of October 3, 2023, and certain other expenses related to the spin-off. Included in 2023 is also the IFRS Accounting Standards non-cash, non-taxable net gain on distribution of Sandoz Group AG to Novartis AG shareholders. Sandoz operated in the off-patent medicines segment and specialized in the development, manufacturing, and marketing of generic pharmaceuticals and biosimilars. The Sandoz business was organized globally into two franchises: Generics and Biosimilars.
Significant transactions are discussed in “Item 18. Financial Statements—Note 2. Significant transactions,” and “Item 18. Financial Statements—Note 29. Commitments and contingent liabilities.”
With the spin-off of the Sandoz business, Novartis operates as a single global operating segment, as an innovative medicines company.
Our business environment
Progress in science and technology raises the possibility of new types of medicines and more efficient drug discovery. At the same time, healthcare inequities remain entrenched around the world, while aging populations are putting pressure on healthcare systems in many countries. The major trends currently shaping our business environment include:
• Scientific progress is opening new paths to treat disease. Rapid progress in medical science is creating opportunities for new types of treatments. These advances highlight the importance of investment in R&D, including in next-generation technologies such as radioligand therapies and gene and cell therapies.
• Demand for high-quality healthcare continues to rise. Demand for medicines in areas such as cancer, cardiovascular disease and immunology continues to grow in key markets. The US and EU markets are expanding. China is growing rapidly, while spending in Japan is forecast to remain stable. To meet demand and maintain growth, companies are investing in developing new, innovative treatments.
• Healthcare systems are under strain. In many countries, healthcare systems are under pressure. Healthcare professionals often feel overwhelmed and under-resourced. Staff shortages have occurred in both the US and Europe. This trend began with the COVID-19 pandemic, but there are longer-term factors as well—aging and changes to lifestyle have led to a significant rise in noncommunicable illnesses such as cancer, diabetes and heart disease.
• The policy landscape is changing. New legislation or regulations in the US, EU and China may change how governments pay for medicines. In the US, for example, the IRA will limit price increases in Medicare to inflation and impose price controls on select drugs in the Medicare program beginning in 2026. The EU is revising the legislative framework for medicines, trying
to balance access and affordability, while China has rolled out a volume-based procurement program to reduce prices for eligible medicines. See “Item 3. Key Information—Item 3.D Risk factors—Pricing, reimbursement and access—Pricing and reimbursement pressure, including pricing transparency and access to healthcare,” and “Item 3. Key Information—Item 3.D Risk factors—Macroeconomic developments—Impact of macroeconomic developments.”
• Patients want more say over their treatment. Increasingly, patients want their voice to be heard in the process of developing new medicines, so that the treatments address the outcomes that matter most to them. Patients also want more say over policies that affect their access to medicines and are becoming more important as data owners. As a result, companies are building patient engagement into their approaches—from research and clinical trials to commercialization and access programs.
• Health inequities remain entrenched. Billions of people around the world struggle to get the medicines and healthcare services they need. Many of the issues are in low- and middle-income countries (LMICs), where people face the dual burden of infectious and non-communicable diseases, as well as fragile and under-resourced health systems. Health inequities also affect people in higher-income countries, however, where causes are often linked to structural health system issues as well as demographic, social and economic challenges.
• AI is poised to reshape the industry. Across the biopharmaceutical industry, we are beginning to realize the benefits of new technologies such as AI in automating processes and generating insights that could help us design new compounds, predict drug safety or speed up drug discovery. The extent to which companies can harness this potential will depend on their ability to aggregate and analyze large volumes of anonymized health data. See “Item 3. Key Information—Item 3.D Risk factors—Research and development—Failure to successfully prioritize, integrate and execute our research and development programs for new products or new indications for existing products.”
• Climate change threatens to widen health inequity. Climate change and nature loss continue to have an adverse effect on human health, with people in LMICs disproportionately impacted. The World Health Organization forecasts approximately 250 000 additional deaths per year between 2030 and 2050 due to climate change, mainly from malnutrition, malaria, diarrhea and heat stress. Respiratory illnesses are also on the rise due to air pollution. At the same time, health systems are aiming to build climate resilience—with 29 countries committing to net-zero carbon emissions in their health systems by 2050, according to the WHO. See “Item 3. Key Information—Item 3.D Risk factors—Climate change—Failure to manage physical and transition risks from climate change.”
Our strategy
Our strategy is to deliver high-value medicines that alleviate society’s greatest disease burdens through technology leadership in R&D and novel access approaches. The aim of our strategy is to create long-term value—to contribute to continued advances in human health, to deliver returns to shareholders and to benefit society.
We focus on four core therapeutic areas with strong growth potential and high unmet patient needs: cardiovascular, renal and metabolic; immunology; neuroscience; and oncology. A focused approach enables us to build depth in these areas to find new ways to treat and cure disease, intervene earlier in chronic illnesses and improve quality of life for patients.
We focus on technology platforms where we have the depth and scale to discover, develop and commercialize therapies. These are two established platforms (chemistry and biotherapeutics) plus three newer platforms (xRNA, radioligand therapy and gene and cell therapy) that represent key sources of future growth.
We focus on our priority markets—US, Germany, China and Japan—which together account for most of the expected growth in global healthcare spending over the next five years. In the US, our ambition is to become a top-five player. In Germany we aim to retain our current position as market leader, while in China and Japan we aim to be in third position in each market. Although these are our priority geographies, we maintain a strong presence in other markets worldwide.
To support our focus areas, we have three strategic priorities:
• Deliver high-value medicines. We aim to increase growth, driven by continued strong momentum in our existing portfolio of medicines—including Entresto; Kisqali, Kesimpta, Cosentyx, Scemblix, Pluvicto and Leqvio—and key upcoming launches. Over the longer term, we expect growth will come through delivering high-value medicines that sustain and replace our existing growth drivers.
• Embed operational excellence. In an increasingly competitive environment, we are simplifying processes and reducing costs to become more efficient and effective in our decision-making and to free up resources for investment in new medicines. Our goal is to continue making attractive returns to shareholders while creating value for patients, healthcare systems and society.
• Strengthen the foundations of our business. We continue to invest to strengthen the foundations of our long-term success. We have made progress in strengthening our culture to attract and retain talent, while developing artificial intelligence capabilities in R&D and building stronger trust with stakeholders and society.
Results of operations
Financial year 2023 compared with 2022
Key figures1
(USD millions unless indicated otherwise)
| |
Year ended
Dec 31, 2023
| |
Year ended
Dec 31, 2022
| |
Change
in USD
%
| | Change in
constant
currencies
%2 | |
|
Net sales from continuing operations | | 45 440 | | 42 206 | | 8 | | 10 | |
|
Other revenues | | 1 220 | | 1 255 | | -3 | | -3 | |
|
Cost of goods sold | | -12 472 | | -11 582 | | -8 | | -6 | |
|
Gross profit from continuing operations | | 34 188 | | 31 879 | | 7 | | 11 | |
|
Selling, general and administration | | -12 517 | | -12 193 | | -3 | | -3 | |
|
Research and development | | -11 371 | | -9 172 | | -24 | | -22 | |
|
Other income | | 1 772 | | 696 | | 155 | | 147 | |
|
Other expense | | -2 303 | | -3 264 | | 29 | | 31 | |
|
Operating income from continuing operations | | 9 769 | | 7 946 | | 23 | | 39 | |
|
Return on net sales (%) | | 21.5 | | 18.8 | | | | | |
|
Loss from associated companies | | -13 | | -11 | | -18 | | 1 | |
|
Interest expense | | -855 | | -800 | | -7 | | -11 | |
|
Other financial income and expense | | 222 | | 42 | | nm | | nm | |
|
Income before taxes from continuing operations | | 9 123 | | 7 177 | | 27 | | 45 | |
|
Income taxes | | -551 | | -1 128 | | 51 | | 44 | |
|
Net income from continuing operations | | 8 572 | | 6 049 | | 42 | | 62 | |
|
Net income from discontinued operations before gain on distribution of Sandoz Group AG to Novartis AG shareholders | | 422 | | 906 | | nm | | nm | |
|
Gain on distribution of Sandoz Group AG to Novartis AG shareholders | | 5 860 | | | | nm | | nm | |
|
Net income from discontinued operations | | 6 282 | | 906 | | nm | | nm | |
|
Net income | | 14 854 | | 6 955 | | nm | | nm | |
|
Basic earnings per share from continuing operations (USD) | | 4.13 | | 2.77 | | 49 | | 70 | |
|
Basic earnings per share from discontinued operations (USD) | | 3.02 | | 0.42 | | nm | | nm | |
|
Total basic earnings per share (USD) | | 7.15 | | 3.19 | | nm | | nm | |
|
Net cash flows from operating activities from continuing operations | | 14 220 | | 13 039 | | 9 | | | |
|
Non-IFRS measures 2 | | | | | | | | | |
|
Free cash flow from continuing operations 2, 3 | | 13 160 | | 12 123 | | 9 | | | |
|
|
1 For information on continuing operations and discontinued operations, refer to the Overview section above in this Item 5 and "Item 18. Financial Statements—Note 1. Accounting policies ", "Item 18. Financial Statements—Note 2. Significant transactions—Significant transactions in 2023," and "Item 18. Financial Statements—Note 31. Discontinued operations." |
2 For an explanation of non-IFRS measures and reconciliation tables, see "—Non-IFRS measures as defined by Novartis." |
3 Effective January 1, 2023, Novartis revised its definition of free cash flow, to define free cash flow as net cash flows from operating activities less purchases of property, plant and equipment. To aid in comparability, the prior year free cash flow amounts have been revised to conform with the new free cash flow definition. See "—Non-IFRS measures as defined by Novartis" |
nm = not meaningful |
Company overview
Net sales from continuing operations were USD 45.4 billion, up 8% in USD reported terms and 10% measured in constant currencies (cc) to remove the impact of exchange rate movements. Sales growth was driven by volume growth of 16 percentage points. Generic competition had a negative impact of 4 percentage points and pricing had a negative impact of 2 percentage points. Sales in the US were USD 18.0 billion (+13%) and in the rest of the world USD 27.5 billion (+5%, +8% cc). Net sales growth was mainly driven by continued strong performance from Entresto (USD 6.0 billion, +30%, +31% cc), Kesimpta (USD 2.2 billion, +99%, +99% cc), Kisqali (USD 2.1 billion, +69%, +75% cc), Pluvicto (USD 980 million, +262%, +261% cc), partly offset by generic competition mainly for Gilenya.
In the US (USD 18.0 billion, +13%), sales growth was mainly driven by Entresto, Pluvicto, Kesimpta, Kisqali, Scemblix and Leqvio, partly offset by the impact of generic competition for Gilenya. In Europe (USD 15.0 billion, +4%, +4% cc), sales growth was driven by Kesimpta, Entresto, Kisqali, Cosentyx and Leqvio, partly offset by increased generic competition for Lucentis and Gilenya. Emerging growth markets, which comprise all markets excluding the US, Canada, Western Europe1, Japan, Australia and New Zealand, grew +8% (+17% cc), includes China net sales of USD 3.3 billion (+11%, +17% cc).
Operating income from continuing operations was USD 9.8 billion (+23%, +39% cc), mainly driven by higher net sales, lower restructuring charges, and income from legal matters, partly offset by higher impairments and higher selling, general and administration (SG&A), and research and development (R&D) investments. Operating income margin from continuing operations was 21.5% of net sales, increasing 2.7 percentage points (+5.0 percentage points in cc).
Net income from continuing operations was USD 8.6 billion (+42%, +62% cc), mainly driven by higher operating income and non-recurring favorable tax impacts. Basic earnings per share from continuing operations was USD 4.13 (+49%, +70% cc), growing faster than net income, benefiting from lower weighted average number of shares outstanding.
Net cash flows from operating activities from continuing operations amounted to USD 14.2 billion, compared with USD 13.0 billion in 2022. This increase was mainly driven by higher net income from continuing operations adjusted for non-cash items and other adjustments, including divestment gains, which were partly offset by higher income taxes paid, mainly due to the timing of payments.
Free cash flow from continuing operations amounted to USD 13.2 billion (+9% USD), compared with USD 12.1 billion in 2022, driven by higher net cash flows from operating activities from continuing operations.
We also present our core results2, which exclude the impact of amortization of intangible assets, impairments, business acquisitions, divestments, and other significant items, including restructuring and related items, to help investors understand our underlying performance.
Core operating income from continuing operations was USD 16.4 billion (+11%, +18% cc), mainly driven by higher net sales, partly offset by higher SG&A and R&D investments. Core operating income margin from continuing operations was 36.0% of net sales, increasing 0.9 percentage points (+2.4 percentage points cc).
Core net income from continuing operations was USD 13.4 billion (+13%, +19% cc), mainly due to higher core operating income. Core basic earnings per share from continuing operations was USD 6.47 (+18%, +25% cc), growing faster than core net income from continuing operations, benefiting from lower weighted average number of shares outstanding.
Discontinued operations net sales in 2023 were USD 7.4 billion, compared with USD 9.4 billion in 2022, and operating income amounted to USD 265 million, compared with USD 1.3 billion in 2022. Net income from discontinued operations in 2023 amounted to USD 6.3 billion, compared with USD 906 million in 2022, driven by the IFRS Accounting Standards non-cash, non-taxable, net gain on distribution of Sandoz Group AG to Novartis AG shareholders, which amounted to USD 5.9 billion.
Total Company net income amounted to USD 14.9 billion in 2023, compared with USD 7.0 billion in 2022, and basic earnings per share was USD 7.15, compared with USD 3.19 in the prior year, driven by the IFRS Accounting Standards non-cash, non-taxable, net gain on distribution of Sandoz Group AG to Novartis AG shareholders of USD 5.9 billion. Net cash flows from operating activities for the total Company amounted to USD 14.5 billion, and free cash flow amounted to USD 13.2 billion.
1 Novartis definition of Western Europe includes Austria, Belgium, Finland, France, Germany, Greece, Iceland, Ireland, Italy, Luxembourg, Malta, The Netherlands, Norway, Portugal, Spain, Sweden, Switzerland, and the United Kingdom.
2 For an explanation of non-IFRS measures and reconciliation tables, see “—Non-IFRS measures as defined by Novartis.”
Net sales from continuing operations
The following table provides an overview of net sales from continuing operations by core therapeutic area and established brands:
(USD millions)
| |
Year ended
Dec 31, 2023
| |
Year ended
Dec 31, 20221
| |
Change
in USD
%
| | Change in
constant
currencies
%2 | |
|
Cardiovascular, renal and metabolic | | 6 391 | | 4 756 | | 34 | | 36 | |
|
Immunology | | 7 798 | | 7 287 | | 7 | | 8 | |
|
Neuroscience | | 4 043 | | 3 038 | | 33 | | 34 | |
|
Oncology | | 13 590 | | 11 176 | | 22 | | 23 | |
|
Total promoted brands | | 31 822 | | 26 257 | | 21 | | 23 | |
|
Established brands 3 | | 13 618 | | 15 949 | | -15 | | - 12 | |
|
Total net sales from continuing operations 3 | | 45 440 | | 42 206 | | 8 | | 10 | |
|
|
1 Reclassified to conform with the 2023 organizational structure. |
2 For an explanation of non-IFRS measures and reconciliation tables, see "—Non-IFRS measures as defined by Novartis." |
3 Effective January 1, 2023, the discontinued operations Sandoz business bio-technology manufacturing services to other companies’ activities and the Coartem brand were transferred to the Novartis continuing operations. The financial information of the Novartis continuing operations and discontinued operations were adapted accordingly in 2022, in compliance with IFRS Accounting Standards. See “Item 18. Financial Statements – Note 3. Operating segment.” |
The following table provides the top 20 product net sales from continuing operations in 2023, as well as the change compared with 2022:
| | | | | | US | | Rest of world | | Total | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Brands | | Brand classification by therapeutic area or established brands | | Key indications | | USD m | | % change USD/cc1 | | USD m | | % change USD | | % change cc1 | | USD m | | % change USD | | % change cc1 | |
|
Entresto | | Cardiovascular, renal and metabolic | | Chronic heart failure, hypertension | | 3 067 | | 30 | | 2 968 | | 30 | | 32 | | 6 035 | | 30 | | 31 | |
|
Cosentyx | | Immunology | | Psoriasis (PsO), ankylosing spondylitis (AS), psoriatic arthritis (PsA), non-radiographic axial spondyloarthritis (nr-axSPA), hidradenitis suppurativa (HS) | | 2 636 | | -5 | | 2 344 | | 16 | | 19 | | 4 980 | | 4 | | 5 | |
|
Promacta/Revolade | | Oncology | | Immune thrombocytopenia (ITP), severe aplastic anemia (SAA) | | 1 205 | | 11 | | 1 064 | | 6 | | 8 | | 2 269 | | 9 | | 10 | |
|
Kesimpta | | Neuroscience | | Relapsing-remitting multiple sclerosis (RRMS) | | 1 528 | | 66 | | 643 | | 276 | | 272 | | 2 171 | | 99 | | 99 | |
|
Kisqali | | Oncology | | HR+/HER2- metastatic breast cancer | | 1 032 | | 119 | | 1 048 | | 38 | | 47 | | 2 080 | | 69 | | 75 | |
|
Tafinlar + Mekinist | | Oncology | | BRAF V600+ metastatic adjuvant melanoma, advanced non-small cell lung cancer (NSCLC), tumor agnostic with BRAF mutation indication | | 791 | | 17 | | 1 131 | | 4 | | 8 | | 1 922 | | 9 | | 11 | |
|
Tasigna | | Oncology | | Chronic myeloid leukemia (CML) | | 884 | | 1 | | 964 | | -8 | | -5 | | 1 848 | | -4 | | -3 | |
|
Jakavi | | Oncology | | Myelofibrosis (MF), polycytomia vera (PV), graft-versus-host disease (GvHD) | | | | | | 1 720 | | 10 | | 12 | | 1 720 | | 10 | | 12 | |
|
Lucentis 2 | | Established brands | | Age-related macular degeneration (AMD), diabetic macular edema (DME), retinal vein occlusion (RVO) | | | | | | 1 475 | | -21 | | -20 | | 1 475 | | -21 | | -20 | |
|
Xolair 3 | | Immunology | | Severe allergic asthma (SAA), chronic spontaneous urticaria (CSU), nasal polyps | | | | | | 1 463 | | 7 | | 9 | | 1 463 | | 7 | | 9 | |
|
Ilaris | | Immunology | | Auto-inflammatory (CAPS, TRAPS, HIDS/MKD, FMF, SJIA, AOSD, gout) | | 686 | | 20 | | 669 | | 19 | | 24 | | 1 355 | | 20 | | 22 | |
|
Sandostatin | | Established brands | | Carcinoid tumors, acromegaly | | 829 | | 4 | | 485 | | 11 | | 15 | | 1 314 | | 6 | | 8 | |
|
Zolgensma | | Neuroscience | | Spinal muscular atrophy (SMA) | | 372 | | -14 | | 842 | | -10 | | -7 | | 1 214 | | -11 | | -9 | |
|
Pluvicto | | Oncology | | PSMA-positive mCRPC patients post-ARPI, post-Taxane | | 921 | | 265 | | 59 | | 211 | | 195 | | 980 | | 262 | | 261 | |
|
Gilenya 2 | | Established brands | | Relapsing multiple sclerosis (RMS) | | 359 | | -69 | | 566 | | -34 | | -33 | | 925 | | -54 | | -54 | |
|
Exforge Group | | Established brands | | Hypertension | | 13 | | -7 | | 700 | | -4 | | -1 | | 713 | | -4 | | -1 | |
|
Galvus Group | | Established brands | | Type 2 diabetes | | | | | | 692 | | -19 | | -11 | | 692 | | -19 | | -11 | |
|
Diovan Group | | Established brands | | Hypertension | | 52 | | -5 | | 561 | | -6 | | -1 | | 613 | | -6 | | -1 | |
|
Lutathera | | Oncology | | GEP-NETs gastroenteropancreatic neuroendocrine tumors | | 427 | | 29 | | 178 | | 27 | | 26 | | 605 | | 28 | | 28 | |
|
Gleevec/Glivec | | Established brands | | Chronic myeloid leukemia (CML), gastrointestinal stromal tumors (GIST) | | 150 | | -27 | | 411 | | -24 | | -20 | | 561 | | -25 | | -22 | |
|
Top 20 brands total | | | | | | 14 952 | | 15 | | 19 983 | | 6 | | 9 | | 34 935 | | 10 | | 12 | |
|
Rest of portfolio 4 | | | | | | 3 007 | | 1 | | 7 498 | | 1 | | 5 | | 10 505 | | 1 | | 4 | |
|
Total net sales from continuing operations 4 | | | | | | 17 959 | | 13 | | 27 481 | | 5 | | 8 | | 45 440 | | 8 | | 10 | |
|
|
1 For an explanation of non-IFRS measures and reconciliation tables, see "—Non-IFRS measures as defined by Novartis." |
2 In the first quarter of 2023 Lucentis was reclassified from other promoted brands to established brands and Gilenya was reclassified from neuroscience to established brands. |
3 Net sales reflect Xolair sales for all indications. |
4 Effective January 1, 2023, the discontinued operations Sandoz business bio-technology manufacturing services to other companies’ activities and the Coartem brand were transferred to the Novartis continuing operations. The financial information of the Novartis continuing operations and discontinued operations were adapted accordingly in 2022, in compliance with IFRS Accounting Standards. See “Item 18. Financial Statements – Note 3. Operating segment.” |
|
nm = not meaningful |
For the table providing the top 20 products net sales from continuing operations in 2022 see Results of Operations 2022 compared with 2021, below in this Item 5. For the table providing the net sales from continuing operations by core therapeutic area and established brands for 2023 and 2022, see “Item 18. Financial statements—Note 4. Revenues and geographic information.”
For information about the approved indications for certain products described, see “Item 4. Information on the Company—Item 4.B Business overview— Products.”
Cardiovascular, renal and metabolic
Net sales in the cardiovascular, renal and metabolic therapeutic area were USD 6.4 billion (+34%, +36% cc), sales growth mainly driven by Entresto.
Entresto (USD 6.0 billion, +30%, +31% cc) sustained robust demand-led growth. In the US and Europe, Entresto penetration grew through the continued adoption of guideline-directed medical therapy in heart failure. In China and Japan, Entresto volume growth is fueled by heart failure as well as increased penetration in hypertension. Highlights of the year also included the approval of the pediatric indication and formulation in Europe with a 1-year extension of RDP to November 2026, and the inclusion of Entresto in the 2023 China Hypertension Treatment Guideline as a new drug category and 1st line treatment option. In the US, Novartis is in ANDA litigation with generic manufacturers. Novartis has appealed to reverse the negative US district court decision to uphold the validity of its combination patent covering Entresto and combinations of sacubitril and valsartan, which expires in 2025 (with pediatric exclusivity). No generics have tentative or final approval in the US. Any US commercial launch of a generic Entresto product prior to the final outcome of Novartis combination patent appeal, or ongoing litigations involving other patents, may be at risk of later litigation developments.
Leqvio (USD 0.4 billion, +217%, +217% cc) launch in the US and other markets is ongoing, with focus on patient on-boarding, removing access hurdles and enhancing medical education. In July 2023, FDA expanded the label to include primary hyperlipidemia (patients at increased risk of ASCVD) and removed four adverse reactions from the safety section and Limitations of Use. In Q3 2023, Leqvio was approved in China and in Japan and is now approved in 94 countries. Novartis obtained global rights to develop, manufacture and commercialize Leqvio under a license and collaboration agreement with Alnylam Pharmaceuticals.
Immunology
Net sales in the immunology therapeutic area reached USD 7.8 billion (+7%, +8% cc), sales growth was mainly driven by Ilaris and Cosentyx.
Cosentyx (USD 5.0 billion, +4%, +5% cc) continued demand growth across key regions, partly offset by revenue deduction increases in the US. Ex-US sales grew +19% (cc). Since initial approval in 2015, Cosentyx has shown sustained efficacy and a robust safety profile, treating more than 1 million patients across six systemic inflammatory conditions. Cosentyx demonstrated durable efficacy and symptom improvement at 16 weeks with observed results at 52 weeks in patients with moderate-to-severe hidradenitis suppurativa. In May and October 2023, respectively, the European Commission and FDA approved Cosentyx as the first and only IL-17A inhibitor for hidradenitis suppurativa in adults and the first new biologic therapy for hidradenitis suppurativa in nearly a decade. Cosentyx hidradenitis suppurativa in adults is now approved in more than 60 countries worldwide. In October 2023, FDA has approved Cosentyx intravenous formulation for the treatment of adults with psoriatic arthritis, ankylosing spondylitis, and non-radiographic axial spondyloarthritis.
Xolair (USD 1.5 billion, ex-US +7%, +9% cc) sales grew across all regions. Following EMA positive opinion in February 2023, the Xolair SmPC was updated with long term (48 week) efficacy and safety data on chronic spontaneous urticaria (CSU) allowing continued treatment beyond 24 weeks. In November 2023, Novartis received EU approval for six new Xolair product configurations, including auto injectors and a new 300 mg strength. Novartis co-promotes Xolair with Genentech in the US and shares a portion of revenue as operating income but does not record any US sales.
Ilaris (USD 1.4 billion, +20%, +22% cc) sales grew across all regions. Contributors to growth include strong performance in the Periodic Fever Syndrome (PFS) and Still’s disease indications (SJIA/AOSD) in the US, Europe and Japan, as well as in key markets worldwide.
Neuroscience
Net sales in the neuroscience therapeutic area were USD 4.0 billion (+33%, +34% cc), sales growth was mainly driven by Kesimpta.
Kesimpta (USD 2.2 billion, +99%, +99% cc) sales grew across all regions mainly driven by increased demand and strong access. Kesimpta is a high efficacy B-cell therapy, with a favorable safety and tolerability profile and an at home self-administration for a broad population of RMS patients. Kesimpta is now approved in 87 countries with more than 85,000 patients treated.
Zolgensma (USD 1.2 billion, -11%, -9% cc). Established markets are treating mainly incident patients. Sales declined in the US and Europe. Zolgensma is now approved in 51 countries with more than 3,700 patients treated globally through clinical trials, early access programs and in the commercial setting.
Mayzent (USD 0.4 billion, +10%, +10% cc) sales grew mainly in Europe. Sales continued to grow in patients with multiple sclerosis showing signs of progression despite being on other treatments.
Aimovig (USD 0.3 billion, ex-US, ex-Japan +22%, +21% cc) sales grew mainly in Europe, driven by increased demand in migraine prevention. Novartis commercializes Aimovig ex-US, ex-Japan, while Amgen retains all rights in the US and in Japan.
Oncology
Net sales in the oncology therapeutic area were USD 13.6 billion (+22%, +23% cc), sales growth was mainly driven by Kisqali, Pluvicto, Scemblix and Promacta/Revolade.
Promacta/Revolade (USD 2.3 billion, +9%, +10% cc) sales grew across all regions, driven by increased use in second-line persistent and chronic immune thrombocytopenia and as first-line and/or second-line treatment
for severe aplastic anemia, according to the respective label in the countries.
Kisqali (USD 2.1 billion, +69%, +75% cc) sales grew strongly across all regions, based on increasing recognition of its consistently reported overall survival in HR+/HER2- advanced breast cancer. Updates to the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for breast cancer, released in January 2023, recommend ribociclib (Kisqali) as the only Category 1 Preferred CDK4/6 inhibitor for first-line treatment of patients with HR+/HER2- advanced breast cancer in combination with an aromatase inhibitor (AI). Positive, statistically significant interim and final efficacy results of the iDFS analysis of the early breast cancer pivotal Phase III trial NATALEE were presented at ASCO and SABCS 2023. Additional QOL information presented at ESMO demonstrated that the addition of Ribociclib to endocrine therapy did not compromise the QOL of patients. Submission for approval in early breast cancer was completed in August to EMA and in December to the FDA. Submissions to other regulatory authorities are ongoing.
Tafinlar + Mekinist (USD 1.9 billion, +9%, +11% cc) sales grew mainly in the US and emerging growth markets, driven by demand in BRAF+ adjuvant melanoma and NSCLC indications, while maintaining demand in the highly competitive BRAF+ metastatic melanoma market. In addition, the tumor agnostic indication contributed to growth in the US.
Tasigna (USD 1.8 billion, -4%, -3% cc) sales declined mainly in Europe.
Jakavi (USD 1.7 billion, ex-US +10%, +12% cc) sales grew in emerging growth markets, Europe and Japan, driven by strong demand in both myelofibrosis and polycythemia vera indications. Incyte retains all rights to ruxolitinib (Jakafi®) in the US.
Pluvicto (USD 1.0 billion, +262%, +261% cc) saw continued sales growth in the US. Pluvicto is the first and only radioligand therapy approved by the FDA for the treatment of adult patients with progressive, PSMA-positive metastatic castration-resistant prostate cancer, who have already been treated with other anticancer treatments (ARPI and taxane-based chemotherapy). Data from the Phase III PSMAfore trial was presented at ESMO. Pluvicto met its primary endpoint with a clinically meaningful and statistically significant benefit in radiographic progression-free survival (rPFS) in patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) after treatment with androgen receptor pathway inhibitor (ARPI) therapy, compared with a change in ARPI. In Q2 2023, approval was received for commercial production of Pluvicto for US patients at our radioligand manufacturing facility in Millburn, NJ and the expansion of manufacturing operations for EU commercial supply at our site in Zaragoza, Spain. In January 2024, Novartis received approval from the FDA for the commercial manufacturing of Pluvicto at state-of-the-art radioligand therapy (RLT) manufacturing facility in Indianapolis.
Lutathera (USD 0.6 billion, +28%, +28% cc) sales grew across all regions due to increased demand. Growth in the US was also driven by strong field execution. In Japan, growth was driven by increased demand following the transfer of the marketing authorization (MA) back to Novartis from Fujifilm Toyama Chemical. In Q3 2023, the Phase III NETTER-2 trial with Lutathera met its primary endpoint, showing Lutathera is the first radioligand therapy (RLT) to demonstrate clinically meaningful benefit in a first line setting.
Kymriah (USD 0.5 billion, -5%, -5% cc) sales declined in Europe and the US, partly offset by growth in Japan and follicular lymphoma indication launch across markets.
Piqray / Vijoice (USD 0.5 billion, +35%, +37% cc) sales grew mainly in the US, Europe and emerging growth markets. In addition to PIK3CA-related overgrowth spectrum (PROS), Piqray is the first therapy specifically developed for the approximately 40% of HR+/HER2- advanced breast cancer patients who have a PIK3CA mutation, associated with a worse prognosis.
Scemblix (USD 0.4 billion, +177%, +179% cc) sales grew across all regions, demonstrating the high unmet need for effective and tolerable treatment options for CML patients who have been treated with 2 or more tyrosine kinase inhibitors. Scemblix has now been approved in more than 60 countries for patients with Philadelphia chromosome-positive CML in chronic phase who have been treated with 2 or more TKIs. In January 2024, Novartis announced that the ASC4FIRST trial met both primary endpoints, with clinically meaningful and statistically significant results vs. standard-of-care TKIs in newly diagnosed Ph+CML-CP patients while demonstrating a favorable safety and tolerability profile. Data will be presented at an upcoming medical conference and submitted to regulatory authorities in 2024.
Votrient (USD 0.4 billion, -18%, -17% cc) declined due to increased competition, especially from immuno-oncology agents in metastatic renal cell carcinoma.
Adakveo (USD 0.2 billion, +1%, 0% cc) sales grew (cc) mainly in the US, offset by decline in emerging growth markets and Europe. In August 2023, European Commission endorsed the CHMP’s recommendation to revoke the conditional marketing authorization for Adakveo. Adakveo remains approved for use by the FDA for the reduction in frequency of vaso-occlusive crises (pain crises) in adults and pediatric patients aged 16 years or older with sickle cell disease. Novartis continues to discuss the STAND study results with FDA and other health authorities globally.
Tabrecta (USD 0.2 billion, +16%, +16% cc) sales grew mainly in the US. Tabrecta is the first therapy approved by the FDA to specifically target metastatic NSCLC with a mutation that leads to MET exon 14 skipping (METex14) in line agnostic setting. Novartis obtained global rights to develop, manufacture and commercialize Tabrecta under a license and collaboration agreement with Incyte Corporation.
Established Brands
The established brands had net sales of USD 13.6 billion (-15%, -12% cc).
Lucentis (USD 1.5 billion, ex-US -21%, -20% cc) sales declined in Europe, emerging growth markets and Japan due to competition.
Sandostatin (USD 1.3 million, +6%, +8% cc) sales grew mainly in emerging growth markets, Europe and in the US.
Gilenya (USD 0.9 billion, -54%, -54% cc) sales declined due to generic competition mainly in the US and Europe. Novartis is in litigation against a generic manufacturer on the method of treatment patent in the US, and against generic manufacturers on the dosing regimen patent in Europe.
Exforge Group (USD 0.7 million, -4%, -1% cc) sales declined mainly in Europe, partly offset by growth in emerging growth markets.
Galvus Group (USD 0.7 million, -19%, -11% cc) sales declined mainly in Europe, partly offset by growth in emerging growth markets.
Diovan Group (USD 0.6 million, -6%, -1% cc) sales declined mainly in Europe, Japan and in the US, partly offset by growth in emerging growth markets.
Gleevec/Glivec (USD 0.6 million, -25%, -22% cc) sales declined due to increased generic competition.
Afinitor/Votubia (USD 0.4 million, -20%, -18% cc) sales declined mainly in the US and Europe, driven by generic competition.
Operating income from continuing operations
(USD millions unless indicated otherwise)
| |
Year ended
Dec 31, 2023
| |
Year ended
Dec 31, 2022
| |
Change
in USD
%
| | Change in
constant
currencies
%1 | |
|
Gross profit from continuing operations | | 34 188 | | 31 879 | | 7 | | 11 | |
|
Selling, general and administration | | -12 517 | | -12 193 | | -3 | | -3 | |
|
Research and development | | -11 371 | | -9 172 | | -24 | | -22 | |
|
Other income | | 1 772 | | 696 | | 155 | | 147 | |
|
Other expense | | -2 303 | | -3 264 | | 29 | | 31 | |
|
Operating income from continuing operations | | 9 769 | | 7 946 | | 23 | | 39 | |
|
Return on net sales (%) | | 21.5 | | 18.8 | | | | | |
|
|
1 For an explanation of non-IFRS measures and reconciliation tables, see "—Non-IFRS measures as defined by Novartis." |
Operating income from continuing operations was USD 9.8 billion (+23%, +39% cc), mainly driven by higher net sales, lower restructuring charges, and income from legal matters, partly offset by higher impairments and higher SG&A and R&D investments. Operating income margin from continuing operations was 21.5% of net sales, increasing 2.7 percentage points (+5.0 percentage points in cc). Other revenues as a percentage of net sales from continuing operations decreased by 0.3 percentage points (-0.4 percentage points cc). Cost of goods sold as a percentage of net sales from continuing operations was in line with the prior year (+1.0 percentage points cc). R&D expenses as a percentage of net sales from continuing operations increased by 3.3 percentage points (-2.4 percentage points cc). Selling, general and administration (SG&A) expenses as a percentage of net sales from continuing operations decreased by 1.4 percentage points (+1.7 percentage points cc). Other income and other expense, net as a percentage of net sales from continuing operations, increased the margin by 4.9 percentage points (+5.1 percentage points cc).
Non-IFRS measure Core operating income from continuing operations 1
(USD millions unless indicated otherwise)
| |
Year ended
Dec 31, 2023
| |
Year ended
Dec 31, 2022
| |
Change
in USD
%
| | Change in
constant
currencies
% | |
|
Core gross profit from continuing operations | | 37 959 | | 35 591 | | 7 | | 9 | |
|
Core selling, general and administration | | -12 489 | | -12 143 | | -3 | | -3 | |
|
Core research and development | | -8 600 | | -8 267 | | -4 | | -3 | |
|
Core other income | | 392 | | 291 | | 35 | | 29 | |
|
Core other expense | | -890 | | -678 | | -31 | | -33 | |
|
Core operating income from continuing operations | | 16 372 | | 14 794 | | 11 | | 18 | |
|
Core return on net sales (%) | | 36.0 | | 35.1 | | | | | |
|
|
1 For an explanation of non-IFRS measures and reconciliation tables, see "—Non-IFRS measures as defined by Novartis." |
The adjustments made to operating income from continuing operations to arrive at core operating income from continuing operations amounted to USD 6.6 billion (compared with USD 6.8 billion in the prior year). For more information, see “—Non-IFRS measures as defined by Novartis—2023, 2022 and 2021 reconciliation from IFRS Accounting Standards results to non-IFRS core results.”
Core operating income from continuing operations was USD 16.4 billion (+11%, +18% cc), mainly driven by higher net sales, partly offset by higher SG&A and R&D investments. Core operating income margin from continuing operations was 36.0% of net sales, increasing 0.9 percentage points (+2.4 percentage points cc). Core other revenues as a percentage of sales decreased by 0.2 percentage points (cc). Core cost of goods sold as a percentage of sales increased by 0.1 percentage points (cc). Core R&D expenses as a percentage of net sales decreased by 1.3 percentage points (cc). Core SG&A expenses as a percentage of net sales decreased by 1.6 percentage points (cc). Core other income and expense as a percentage of net sales decreased the margin by 0.2 percentage points (cc).
Research and development
The following table provides an overview of the continuing operations reported research and development expense and the non-IFRS measure core research and development expense1:
(USD millions unless indicated otherwise)
| |
Year ended
Dec 31, 2023
| |
Year ended
Dec 31, 2022
| |
Change
in USD
%
| | Change in
constant
currencies
%1 | |
|
Research and exploratory development | | -3 640 | | -2 938 | | -24 | | -22 | |
|
Confirmatory development | | -7 731 | | -6 234 | | -24 | | -22 | |
|
Total research and development expense | | -11 371 | | -9 172 | | -24 | | -22 | |
|
Research and development as % of net sales from continuing operations | | 25.0 | | 21.7 | | | | | |
|
Non-IFRS measures | | | | | | | | | |
|
Core research and exploratory development1 | | -2 988 | | -2 784 | | -7 | | -5 | |
|
Core confirmatory development1 | | -5 612 | | -5 483 | | -2 | | -1 | |
|
Total core research and development expense | | -8 600 | | -8 267 | | -4 | | -3 | |
|
Core research and development as % of net sales from continuing operations | | 18.9 | | 19.6 | | | | | |
|
|
1 Core research and development expense exclude impairments, amortization and certain other items. For an explanation of non-IFRS measures and reconciliation tables, see "—Non-IFRS measures as defined by Novartis." |
Research and exploratory development expenses increased by 24% (-22% cc) to USD 3.6 billion. Confirmatory development expenses amounted to USD 7.7 billion, increasing by 24% (-22% cc) versus the prior year mainly due to higher impairments from discontinuation of early stage development projects. Research and development as a percentage of net sales from continuing operations increased by 3.3 percentage points to 25.0% of net sales from continuing operations.
Total core research and development expenses amounted to USD 8.6 billion, increasing by 4% (-3% cc) versus the prior year mainly due to higher investments in recently acquired assets. Core research and development as a percentage of net sales from continuing operations decreased by 0.7 percentage points (-1.3 percentage points cc) to 18.9% of net sales from continuing operations.
Non-operating income and expense
The term “non-operating income and expense” includes all income and expense items outside operating income from continuing operations. The following table provides an overview of non-operating income and expense from continuing operations:
(USD millions unless indicated otherwise)
| |
Year ended
Dec 31, 2023
| |
Year ended
Dec 31, 2022
| |
Change
in USD
%
| | Change in
constant
currencies
%1 | |
|
Operating income from continuing operations | | 9 769 | | 7 946 | | 23 | | 39 | |
|
Loss from associated companies | | -13 | | -11 | | -18 | | 1 | |
|
Interest expense | | -855 | | -800 | | -7 | | -11 | |
|
Other financial income and expense | | 222 | | 42 | | nm | | nm | |
|
Income before taxes | | 9 123 | | 7 177 | | 27 | | 45 | |
|
Income taxes | | -551 | | -1 128 | | 51 | | 44 | |
|
Net income from continuing operations | | 8 572 | | 6 049 | | 42 | | 62 | |
|
Net income from discontinued operations before gain on distribution of Sandoz Group AG to Novartis AG shareholders | | 422 | | 906 | | nm | | nm | |
|
Gain on distribution of Sandoz Group AG to Novartis AG shareholders | | 5 860 | | | | nm | | nm | |
|
Net income from discontinued operations | | 6 282 | | 906 | | nm | | nm | |
|
Net income | | 14 854 | | 6 955 | | nm | | nm | |
|
Basic earnings per share from continuing operations (USD) | | 4.13 | | 2.77 | | 49 | | 70 | |
|
Basic earnings per share from discontinued operations (USD) | | 3.02 | | 0.42 | | nm | | nm | |
|
Total basic earnings per share (USD) | | 7.15 | | 3.19 | | nm | | nm | |
|
|
1 For an explanation of non-IFRS measures and reconciliation tables, see "—Non-IFRS measures as defined by Novartis." |
nm = not meaningful |
Interest expense and other financial income and expense
Interest expense amounted to USD 855 million, broadly in line with the prior year.
Other financial income and expense amounted to an income of USD 222 million compared with USD 42 million in the prior year, mainly due to higher interest income partly offset by higher net losses from the impact of IAS 29 “Financial reporting in Hyperinflation Economies.”
Income taxes
The tax rate was 6.0%, compared with 15.7% in the prior year period. The current year tax rate was favorably impacted by the effect of tax benefits from the write-down of investments in subsidiaries, non-taxable net gains on unrealized foreign currency results, recognition of deferred tax assets on prior years tax loss carryforwards, non-taxable income related to legal matters, and other items including impact of tax rate changes. Excluding these impacts, the current year tax rate would have been 15.3% compared with 15.7% in the prior year period. The decrease from the prior year was mainly the result of a change in profit mix.
Net income from continuing operations
Net income from continuing operations was USD 8.6 billion (+42%, +62% cc), mainly due to higher operating income from continuing operations and non-recurring favorable tax impacts.
Earnings per share from continuing operations
Basic earnings per share from continuing operations was USD 4.13 (+49%, +70% cc), growing faster than net income from continuing operations, benefiting from lower weighted average number of shares outstanding.
Non-IFRS measure Core non-operating income and expense1
The following table provides an overview of the non-IFRS measure core non-operating income and expense from continuing operations:
(USD millions unless indicated otherwise)
| |
Year ended
Dec 31, 2023
| |
Year ended
Dec 31, 2022
| |
Change
in USD
%
| | Change in
constant
currencies
% | |
|
Core operating income from continuing operations | | 16 372 | | 14 794 | | 11 | | 18 | |
|
Core loss from associated companies | | -13 | | -11 | | -18 | | 1 | |
|
Core interest expense | | -855 | | -800 | | -7 | | -11 | |
|
Core other financial income and expense | | 430 | | 140 | | nm | | nm | |
|
Core income before taxes from continuing operations | | 15 934 | | 14 123 | | 13 | | 19 | |
|
Core income taxes | | -2 488 | | -2 177 | | -14 | | -21 | |
|
Core net income from continuing operations | | 13 446 | | 11 946 | | 13 | | 19 | |
|
Core basic EPS from continuing operations (USD) | | 6.47 | | 5.48 | | 18 | | 25 | |
|
|
1 For an explanation of non-IFRS measures and reconciliation tables, see "—Non-IFRS measures as defined by Novartis." |
nm = not meaningful |
Core interest expense and other financial income and expense
Core interest expense amounted to USD 855 million, broadly in line with the prior year.
Core other financial income and expense amounted to an income of USD 430 million compared with USD 140 million in the prior year, mainly due to higher interest income.
Core income taxes
The core tax rate (core taxes as a percentage of core income before tax) was 15.6% compared with 15.4% in the prior year period. The increase from the prior year was mainly the result of a change in profit mix.
Core net income from continuing operations
Core net income from continuing operations was USD 13.4 billion (+13%, +19% cc), mainly due to higher core operating income from continuing operations.
Core earnings per share from continuing operations
Core basic earnings per share from continuing operations was USD 6.47 (+18%, +25% cc), growing faster than core net income from continuing operations, benefiting from lower weighted average number of shares outstanding.
Discontinued operations
Discontinued operations include the Sandoz, generic pharmaceuticals and biosimilars division and certain corporate activities attributable to Sandoz prior to the spin-off up to the distribution date of October 3, 2023 and certain other expenses related to the spin-off. Included in 2023 is also the IFRS Accounting Standards non-cash, non-taxable net gain on the distribution of Sandoz Group AG to Novartis AG shareholders of USD 5.9 billion, representing mainly the excess amount of the IFRS Accounting Standards distribution liability, which is the estimated fair value of the Sandoz business distributed to Novartis AG shareholders, over the then carrying value of Sandoz business net assets. There were no operating results for the fourth quarter 2023 following the distribution date. The prior year includes the results for the full year.
Discontinued operations net sales in 2023 were USD 7.4 billion, compared with USD 9.4 billion in 2022 and operating income amounted to USD 265 million compared with USD 1.3 billion in 2022.
Net income from discontinued operations in 2023 amounted to USD 6.3 billion, compared with USD 906 million in 2022, driven by the IFRS Accounting Standards non-cash, non-taxable, net gain on distribution of Sandoz Group AG to Novartis AG shareholders, which amounted to USD 5.9 billion.
For further information, see “Item 18. Financial Statements—Note 1. Accounting policies; Note 2. Significant transactions—Completion of the spin-off of the Sandoz business through a dividend in kind distribution to Novartis AG shareholders and —Note 31. Discontinued operations.”
Total Company
Total Company net income amounted to USD 14.9 billion in 2023, compared with USD 7.0 billion in 2022, and basic earnings per share was USD 7.15, compared with USD 3.19 in the prior year, driven by the IFRS Accounting Standards non-cash, non-taxable, net gain on distribution of Sandoz Group AG to Novartis AG shareholders of USD 5.9 billion. Net cash flows from operating activities for the total Company amounted to USD 14.5 billion, and free cash flow amounted to USD 13.2 billion.
Financial year 2022 compared with 2021
Key figures1
(USD millions unless indicated otherwise)
| |
Year ended
Dec 31, 2022
| |
Year ended
Dec 31, 2021
| |
Change
in USD
%
| | Change in
constant
currencies
%2 | |
|
Net sales from continuing operations | | 42 206 | | 42 781 | | -1 | | 5 | |
|
Other revenues | | 1 255 | | 1 193 | | 5 | | 7 | |
|
Cost of goods sold | | -11 582 | | -11 735 | | 1 | | -5 | |
|
Gross profit from continuing operations | | 31 879 | | 32 239 | | -1 | | 5 | |
|
Selling, general and administration | | -12 193 | | -12 827 | | 5 | | 0 | |
|
Research and development | | -9 172 | | -8 641 | | -6 | | -10 | |
|
Other income | | 696 | | 1 620 | | -57 | | -55 | |
|
Other expense | | -3 264 | | -2 335 | | -40 | | -49 | |
|
Operating income from continuing operations | | 7 946 | | 10 056 | | -21 | | -12 | |
|
Return on net sales (%) | | 18.8 | | 23.5 | | | | | |
|
(Loss)/income from associated companies | | -11 | | 15 337 | | nm | | nm | |
|
Interest expense | | -800 | | -787 | | -2 | | -3 | |
|
Other financial income and expense | | 42 | | -76 | | nm | | nm | |
|
Income before taxes from continuing operations | | 7 177 | | 24 530 | | -71 | | -67 | |
|
Income taxes | | -1 128 | | -1 625 | | 31 | | 21 | |
|
Net income from continuing operations | | 6 049 | | 22 905 | | -74 | | -70 | |
|
Net income from discontinued operations | | 906 | | 1 113 | | -19 | | -11 | |
|
Net income | | 6 955 | | 24 018 | | -71 | | -67 | |
|
Basic earnings per share from continuing operations (USD) | | 2.77 | | 10.22 | | -73 | | -69 | |
|
Basic earnings per share from discontinued operations (USD) | | 0.42 | | 0.49 | | -15 | | -9 | |
|
Total basic earnings per share (USD) | | 3.19 | | 10.71 | | -70 | | -66 | |
|
Net cash flows from operating activities from continuing operations | | 13 039 | | 13 365 | | -2 | | | |
|
Non-IFRS measures 2 | | | | | | | | | |
|
Free cash flow from continuing operations 2, 3 | | 12 123 | | 12 299 | | -1 | | | |
|
|
1 For information on continuing operations and discontinued operations, refer to the Overview section above in this Item 5 and "Item 18. Financial Statements—Note 1. Accounting policies ", "Item 18. Financial Statements—Note 2. Significant transactions—Significant transactions in 2023," and "Item 18. Financial Statements—Note 31. Discontinued operations." |
2 For an explanation of non-IFRS measures and reconciliation tables, see "—Non-IFRS measures as defined by Novartis." |
3 Effective January 1, 2023, Novartis revised its definition of free cash flow, to define free cash flow as net cash flows from operating activities less purchases of property, plant and equipment. To aid in comparability, the prior year free cash flow amounts have been revised to conform with the new free cash flow definition. See "—Non-IFRS measures as defined by Novartis." |
nm = not meaningful |
Company overview
Net sales from continuing operations were USD 42.2 billion in 2022, down 1% in USD reported terms and up 5% measured in constant currencies (cc) to remove the impact of exchange rate movements. Sales growth was driven by volume growth of 13 percentage points, mainly driven by continued strong growth from Entresto, Kesimpta, Kisqali, Pluvicto and Cosentyx. Generic competition had a negative impact of 4 percentage points, mainly due to Gilenya, Afinitor/Votubia, and Gleevec/Glivec. Pricing had a negative impact of 4 percentage points. Net sales from continuing operations in the US were USD 15.9 billion (+7%) and in the rest of the world USD 26.3 billion (-6%, +4% cc).
In emerging growth markets, which comprise all markets excluding the US, Canada, Western Europe1, Japan, Australia and New Zealand, net sales from continuing operations were USD 10.8 billion (+2%, +9% cc), driven by China (USD 2.9 billion) growing +3% (+7% cc).
Operating income from continuing operations was USD 7.9 billion (–21%, –12% cc), mainly due to higher restructuring primarily related to the implementation of the previously announced streamlined organizational model, higher impairments and lower divestment gains. Operating income margin from continuing operations was 18.8% of net sales from continuing operations, decreasing by 4.7 percentage points (-3.8 percentage points cc).
Net income from continuing operations was USD 6.0 billion compared with USD 22.9 billion in the prior year, impacted by Roche income in the prior year. Excluding the impact of Roche income, net income from continuing operations declined 9% (cc). Basic earnings per share from continuing operations were USD 2.77 compared with USD 10.22 in the prior year. Excluding the impact of Roche income, basic earnings per share from continuing operations declined 7% (cc).
Net cash flows from operating activities from continuing operations amounted to USD 13.0 billion, compared with USD 13.4 billion in 2021. This decrease was mainly due to unfavorable changes in working capital and lower dividends from associated companies (2021 included the USD 0.5 billion dividends received from our investment in Roche, which was divested in the fourth quarter of 2021), partly offset by lower income taxes paid, higher interest received and favorable hedging results.
Free cash flow from continuing operations amounted to USD 12.1 billion, broadly in line with USD 12.3 billion in 2021.
We also present our core results2, which exclude the impact of amortization, impairments, disposals, acquisitions, restructurings and other significant items, to help investors understand our underlying performance.
Core operating income from continuing operations was USD 14.8 billion (+2%, +10% cc), benefiting from higher gross margin, partly offset by higher research and development (R&D) investments. Core operating income margin from continuing operations was 35.1% of net sales from continuing operations, increasing by 1.2 percentage points (+1.8 percentage points cc).
Core net income from continuing operations was USD 11.9 billion (–5%, +4% cc) as growth in core operating income was partly offset by the loss of Roche core income. Excluding the impact of Roche core income, core net income grew from continuing operations +13% (cc).
Discontinued operations include the Sandoz generic pharmaceuticals and biosimilars division and certain corporate activities attributable to Sandoz prior to the spin-off up to the distribution date of October 3, 2023, and certain other expenses related to the spin-off. Net sales of discontinued operations were USD 9.4 billion, compared with USD 9.8 billion in 2021, and operating income amounted to USD 1.3 billion, compared with USD 1.6 billion in the prior year. Net income from discontinued operations was USD 0.9 billion compared with USD 1.1 billion in the prior year.
Total Company net income amounted to USD 7.0 billion, and basic earnings per share were USD 3.19, compared with USD 10.71 in the prior year. Net cash flows from operating activities amounted to USD 14.2 billion, and free cash flow amounted to USD 13.0 billion.
1 Novartis definition of Western Europe includes Austria, Belgium, Finland, France, Germany, Greece, Iceland, Ireland, Italy, Luxembourg, Malta, The Netherlands, Norway, Portugal, Spain, Sweden, Switzerland, and the United Kingdom.
2 For an explanation of non-IFRS measures and reconciliation tables, see “—Non-IFRS measures as defined by Novartis.”
Net sales from continuing operations
The following table provides an overview of net sales from continuing operations by core therapeutic area and established brands:
(USD millions)
| |
Year ended
Dec 31, 2022
| |
Year ended
Dec 31, 20211
| |
Change
in USD
%
| | Change in
constant
currencies
%2 | |
|
Cardiovascular, renal and metabolic | | 4 756 | | 3 561 | | 34 | | 40 | |
|
Immunology | | 7 287 | | 7 206 | | 1 | | 7 | |
|
Neuroscience | | 3 038 | | 2 220 | | 37 | | 42 | |
|
Oncology | | 11 176 | | 10 532 | | 6 | | 12 | |
|
Total promoted brands | | 26 257 | | 23 519 | | 12 | | 18 | |
|
Established brands 3 | | 15 949 | | 19 262 | | -17 | | - 11 | |
|
Total net sales from continuing operations 3 | | 42 206 | | 42 781 | | -1 | | 5 | |
|
|
1 Reclassified to conform with the 2023 organizational structure. |
2 For an explanation of non-IFRS measures and reconciliation tables, see "—Non-IFRS measures as defined by Novartis." |
3 Effective January 1, 2023, the discontinued operations Sandoz business bio-technology manufacturing services to other companies’ activities and the Coartem brand were transferred to the Novartis continuing operations. The financial information of the Novartis continuing operations and discontinued operations were adapted accordingly in 2022 and 2021, in compliance with IFRS Accounting Standards. See “Item 18. Financial Statements – Note 3. Operating segment.” |
The following table provides the top 20 product net sales from continuing operations in 2022 as well as the change compared with 2021:
| | | | | | US | | Rest of world | | Total | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Brands | | Brand classification by therapeutic area or established brands | | Key indications | | USD m | | % change USD/cc1 | | USD m | | % change USD | | % change cc1 | | USD m | | % change USD | | % change cc1 | |
|
Cosentyx | | Immunology | | Psoriasis (PsO), ankylosing spondylitis (AS), psoriatic arthritis (PsA), non-radiographic axial spondyloarthritis (nr-axSPA) | | 2 770 | | -4 | | 2 018 | | 10 | | 20 | | 4 788 | | 1 | | 5 | |
|
Entresto | | Cardiovascular, renal and metabolic | | Chronic heart failure, hypertension | | 2 354 | | 38 | | 2 290 | | 25 | | 37 | | 4 644 | | 31 | | 37 | |
|
Promacta/Revolade | | Oncology | | Immune thrombocytopenia (ITP), severe aplastic anemia (SAA) | | 1 083 | | 14 | | 1 005 | | -6 | | 5 | | 2 088 | | 4 | | 9 | |
|
Gilenya 2 | | Established brands | | Relapsing multiple sclerosis (RMS) | | 1 153 | | -19 | | 860 | | -37 | | -29 | | 2 013 | | -28 | | -24 | |
|
Tasigna | | Oncology | | Chronic myeloid leukemia (CML) | | 877 | | -1 | | 1 046 | | -11 | | -2 | | 1 923 | | -7 | | -1 | |
|
Lucentis 2 | | Established brands | | Age-related macular degeneration (AMD), diabetic macular edema (DME), retinal vein occlusion (RVO) | | | | | | 1 874 | | -13 | | -4 | | 1 874 | | -13 | | -4 | |
|
Tafinlar + Mekinist | | Oncology | | BRAF V600+ metastatic adjuvant melanoma, advanced non-small cell lung cancer (NSCLC), tumor agnostic with BRAF mutation indication | | 678 | | 12 | | 1 092 | | 0 | | 10 | | 1 770 | | 5 | | 11 | |
|
Jakavi | | Oncology | | Myelofibrosis (MF), polycytomia vera (PV), graft-versus-host disease (GvHD) | | | | | | 1 561 | | -2 | | 9 | | 1 561 | | -2 | | 9 | |
|
Zolgensma | | Neuroscience | | Spinal muscular atrophy (SMA) | | 434 | | -7 | | 936 | | 6 | | 12 | | 1 370 | | 1 | | 5 | |
|
Xolair 3 | | Immunology | | Severe allergic asthma (SAA), chronic spontaneous urticaria (CSU), nasal polyps | | | | | | 1 365 | | -4 | | 6 | | 1 365 | | -4 | | 6 | |
|
Sandostatin | | Established brands | | Carcinoid tumors, acromegaly | | 800 | | -5 | | 438 | | -23 | | -16 | | 1 238 | | -12 | | -10 | |
|
Kisqali | | Oncology | | HR+/HER2- metastatic breast cancer | | 472 | | 39 | | 759 | | 27 | | 38 | | 1 231 | | 31 | | 38 | |
|
Ilaris | | Immunology | | Auto-inflammatory (CAPS, TRAPS, HIDS/MKD, FMF, SJIA, AOSD, gout) | | 570 | | 14 | | 563 | | 1 | | 16 | | 1 133 | | 7 | | 15 | |
|
Kesimpta | | Neuroscience | | Relapsing-remitting multiple sclerosis (RRMS) | | 921 | | 165 | | 171 | | nm | | nm | | 1 092 | | 194 | | 200 | |
|
Galvus Group | | Established brands | | Type 2 diabetes | | | | | | 859 | | -21 | | -12 | | 859 | | -21 | | -12 | |
|
Gleevec/Glivec | | Established brands | | Chronic myeloid leukemia (CML), gastrointestinal stromal tumors (GIST) | | 205 | | -22 | | 540 | | -29 | | -23 | | 745 | | -27 | | -22 | |
|
Exforge Group | | Established brands | | Hypertension | | 14 | | 0 | | 729 | | -18 | | -12 | | 743 | | -18 | | -12 | |
|
Diovan Group | | Established brands | | Hypertension | | 55 | | 8 | | 597 | | -17 | | -10 | | 652 | | -16 | | -9 | |
|
Kymriah | | Oncology | | r/r pediatric and young adults acute lymphoblastic leukemia (ALL), diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL) | | 196 | | -15 | | 340 | | -5 | | 7 | | 536 | | -9 | | -2 | |
|
Afinitor/Votubia | | Established brands | | Breast cancer/ tuberous sclerosis complex (TSC) | | 171 | | -67 | | 341 | | -18 | | -8 | | 512 | | -45 | | -41 | |
|
Top 20 brands total | | | | | | 12 753 | | 6 | | 19 384 | | -5 | | 5 | | 32 137 | | -1 | | 5 | |
|
Rest of portfolio 4 | | | | | | 3 182 | | 10 | | 6 887 | | -7 | | 1 | | 10 069 | | -3 | | 4 | |
|
Total net sales from continuing operations 4 | | | | | | 15 935 | | 7 | | 26 271 | | -6 | | 4 | | 42 206 | | -1 | | 5 | |
|
|
1 For an explanation of non-IFRS measures and reconciliation tables, see "—Non-IFRS measures as defined by Novartis." |
2 In the first quarter of 2023 Lucentis was reclassified from other promoted brands to established brands and Gilenya was reclassified from neuroscience to established brands. |
3 Net sales from continuing operations reflect Xolair sales for all indications. |
4 Effective January 1, 2023, the discontinued operations Sandoz business bio-technology manufacturing services to other companies’ activities and the Coartem brand were transferred to the Novartis continuing operations. The financial information of the Novartis continuing operations and discontinued operations were adapted accordingly in 2022 and 2021, in compliance with IFRS Accounting Standards. See “Item 18. Financial Statements – Note 3. Operating segment.” |
|
nm = not meaningful |
For the table providing the top 20 product net sales from continuing operations in 2021 and for the table providing the net sales from continuing operations by core therapeutic area and established brands for 2022 and 2021, see “Item 18. Financial statements—Note 4. Revenues and geographic information.”
Cardiovascular, renal and metabolic
Net sales in the cardiovascular, renal and metabolic therapeutic area were USD 4.8 billion (+34%, +40% cc), sales growth mainly driven by Entresto.
Entresto (USD 4.6 billion, +31%, +37% cc) sustained robust demand-led growth, with increased patient share across all geographies. Guidelines position Entresto as the first choice RASi versus ACEi/ARB in patients with HFrEF. Entresto benefits from the adoption of guideline directed medical therapy for these patients in all geographies. In the US, Entresto benefits from being added to guidelines for patients with HFpEF (with LVEF below normal). In China, Entresto has been listed in the National Reimbursement Drug List (NRDL) for both HFrEF and hypertension, effective January 2022. In China and Japan, Entresto volume growth is fueled by increased penetration in hypertension in addition to growth in heart failure. It is estimated that around 10 million patients are on treatment with Entresto.
Leqvio (USD 0.1 billion) launch in the US and other markets is ongoing, with focus on patient on-boarding, removing access hurdles and enhancing medical education. Leqvio is the first and only small interfering RNA (siRNA) therapy to lower low-density lipoprotein cholesterol approved in the US and was launched in January 2022. In the US, Leqvio is covered at or near label for 76% of patients eleven months after launch. Leqvio in the US has been assigned a unique Healthcare Common Procedure Coding System code (J-code) and average sales price. Leqvio is now approved in 70 countries. Novartis obtained global rights to develop, manufacture and commercialize Leqvio under a license and collaboration agreement with Alnylam Pharmaceuticals.
Immunology
Net sales in the immunology therapeutic area reached USD 7.3 billion (+1%, +7% cc), sales growth was mainly driven by Cosentyx and Ilaris.
Cosentyx (USD 4.8 billion, +1%, +5% cc) sales grew in emerging growth markets, Europe and Japan, partly offset by decline in the US due to higher revenue deductions. In China, Cosentyx growth was fueled by increased biologic uptake and inclusion in approximately 1,900 hospital listings. Since initial approval in 2015, Cosentyx has proven its sustained efficacy and consistent safety profile across five systemic inflammatory conditions and has treated more than 960,000 patients worldwide.
Xolair (USD 1.4 billion, -4%, +6% cc) sales grew (cc) in emerging growth markets, Europe and Japan. Novartis co-promotes Xolair with Genentech in the US and shares a portion of revenue as operating income but does not record any US sales.
Ilaris (USD 1.1 billion, +7%, +15% cc) showed continued growth across all geographies. Contributors to growth include the adult-onset Still’s disease indication, together with the other adult rheumatology indications in the US and Europe, as well as strong performance for the Periodic Fevers Syndrome indications in Japan.
Neuroscience
Net sales in the neuroscience therapeutic area were USD 3.0 billion (+37%, +42% cc), sales growth (cc) mainly driven by Kesimpta.
Zolgensma (USD 1.4 billion, +1%, +5% cc) has been approved in 47 countries to date. As this represents most major markets, sales growth is now mainly driven by the Incident patient population where we’ve seen double digit growth in 2022. Access pathways are now in place in 35 countries with negotiations ongoing in additional markets.
Kesimpta (USD 1.1 billion, +194%, +200% cc) showed strong sales growth driven by launch momentum across all geographies. Kesimpta is a targeted B-cell therapy that can deliver powerful and sustained high efficacy, with a favorable safety and tolerability profile and the flexibility of an at home self-administration for a broad population of RMS patients. Kesimpta is now approved in 80 countries with more than 36,000 patients treated.
Mayzent (USD 0.4 billion, +27%, +32% cc) sales grew across all geographies in MS patients showing signs of progression despite being on other treatments. Mayzent is the first and only oral disease-modifying therapy studied and proven to delay disease progression in a broad SPMS patient population.
Aimovig (USD 0.2 billion, +1%, +11% cc) sales grew in Europe and emerging growth markets. Aimovig is reimbursed in 32 markets and has been prescribed to over 759,000 patients worldwide. Earlier this year, Aimovig was submitted for approval in China. In October 2022, Novartis reached an agreement in Germany by which Aimovig is reimbursed as a 1st line prophylactic migraine treatment based on the HER-MES trial.
ONCOLOGY
Net sales in the oncology therapeutic area were USD 11.2 billion (+6%, +12% cc), sales growth was mainly driven by Kisqali, Pluvicto, Promacta/Revolade, Tafinlar + Mekinist.
Promacta/Revolade (USD 2.1 billion, +4%, +9% cc) growth was driven by the US, Europe and emerging growth markets, partly offset by decline in Japan. Sales growth was driven by increased use in second-line persistent and chronic immune thrombocytopenia and as first-line and/or second-line treatment for severe aplastic anemia.
Tasigna (USD 1.9 billion, -7%, -1% cc) sales declined in Europe, Japan and the US, partly offset by growth in emerging growth markets.
Tafinlar + Mekinist (USD 1.8 billion, +5%, +11% cc) sales grew across all geographies, driven by demand in BRAF+ adjuvant melanoma and NSCLC indications, while maintaining demand in the highly competitive BRAF+ metastatic melanoma market. Tafinlar + Mekinist remains the worldwide targeted therapy leader in BRAF+ melanoma. Following FDA approval in late June 2022, Tafinlar + Mekinist is the first and only therapy with a tumor-agnostic indication for adult and pediatric patients with solid tumors that have a BRAF V600E mutation, which drives tumor growth in more than 20 different tumor types.
Jakavi (USD 1.6 billion, -2%, +9% cc) sales grew (cc) in Europe, emerging growth markets, Japan, driven by strong demand in both the myelofibrosis and polycythemia vera indications. In May 2022, EC approved Jakavi for the treatment of patients aged 12 years and older with acute or chronic GvHD who have inadequate response to corticosteroids or other systemic therapies.
Kisqali (USD 1.2 billion, +31%, +38% cc) sales grew strongly across all geographies, based on increasing recognition of its overall survival benefits in HR+/HER2- advanced breast cancer. It is a CDK4/6 inhibitor with proven overall survival benefit across all three Phase III trials of the MONALEESA program regardless of menopausal status, line of therapy, site and number of metastases, endocrine resistance, or endocrine partner.
Kymriah (USD 0.5 billion, -9%, -2% cc) sales declined in the US and Europe due to lower DLBCL demand in both geographies and was partly offset by growth in emerging growth markets and Japan. In May 2022, EC and FDA approved Kymriah for the treatment of adult patients with relapsed or refractory (r/r) follicular lymphoma (FL) after two or more lines of systemic therapy.
Votrient (USD 0.5 billion, -18%, -13% cc) declined due to increased competition, especially from immuno-oncology agents in metastatic renal cell carcinoma.
Lutathera (USD 0.5 billion, -1%, +3% cc) sales grew (cc) in Europe and Japan, partly offset by decline in the US. There are approximately 500 centers actively treating patients globally. In the second quarter of 2022, there was a temporary suspension in manufacturing during the quarter; production and deliveries of patient doses resumed in early June 2022.
Piqray/Vijoice (USD 0.4 billion, +13%, +14% cc) sales grew mainly in the US, benefiting from indication expansion into PIK3CA-related overgrowth spectrum (PROS). Piqray is the first and only therapy specifically developed for the approximately 40% of HR+/HER2- advanced breast cancer patients who have a PIK3CA mutation, which is associated with a worse prognosis.
Pluvicto (USD 0.3 billion) launch is progressing well, with more than 160 active centers ordering. Pluvicto is the first and only radioligand therapy approved by the FDA for the treatment of progressive, PSMA-positive metastatic castration-resistant prostate cancer, who have already been treated with other anticancer treatments (ARPI and taxane-based chemotherapy).
Adakveo (USD 0.2 billion, +18%, +19% cc) continued to grow worldwide, reaching more than 11,800 patients with vaso-occlusive crises caused by sickle cell disease to date.
Scemblix (USD 0.1 billion) continued its strong launch uptake in the US, with launches underway in EU and Japan, demonstrating the high unmet need in CML, particularly patients previously treated with 2 or more tyrosine kinase inhibitors, or with the T315I mutation. In October 2022, US FDA converted the accelerated approval of Scemblix to a full approval, confirming the clinical benefit after longer exposure.
Tabrecta (USD 0.1 billion, +48%, +48% cc) sales grew across all geographies, as the first therapy approved by the FDA to specifically target metastatic NSCLC with a mutation that leads to MET exon 14 skipping (METex14).
Established Brands
The established brands had net sales of USD 15.9 billion (-17%, -11% cc).
Gilenya (USD 2.0 billion, -28%, -24% cc) sales declined mainly in Europe and in the US due to generic pressure.
Lucentis (USD 1.9 billion, -13%, -4% cc) sales declined in Japan and Europe mainly due to competition, which was partly offset by growth in emerging growth markets.
Sandostatin (USD 1.2 billion, –12%, –10% cc) declined across all geographies due to ongoing competitive pressure, including generic competition ex-US.
Galvus Group (USD 0.9 billion, –21%, –12% cc) declined in Japan, Europe and Emerging Growth Markets.
Gleevec/Glivec (USD 0.7 billion, –27%, –22% cc) declined due to increased generic competition.
Exforge Group (USD 0.7 billion, –18%, –12% cc) declined across all geographies.
Diovan Group (USD 0.7 billion, –16%, –9% cc) declined in emerging growth markets, Japan and Europe.
Afinitor/Votubia (USD 0.5 billion, –45%, –41% cc) declined in the US and Europe, driven by generic competition.
Operating income from continuing operations
(USD millions unless indicated otherwise)
| |
Year ended
Dec 31, 2022
| |
Year ended
Dec 31, 2021
| |
Change
in USD
%
| | Change in
constant
currencies
%1 | |
|
Gross profit from continuing operations | | 31 879 | | 32 239 | | -1 | | 5 | |
|
Selling, general and administration | | -12 193 | | -12 827 | | 5 | | 0 | |
|
Research and development | | -9 172 | | -8 641 | | -6 | | -10 | |
|
Other income | | 696 | | 1 620 | | -57 | | -55 | |
|
Other expense | | -3 264 | | -2 335 | | -40 | | -49 | |
|
Operating income from continuing operations | | 7 946 | | 10 056 | | -21 | | -12 | |
|
Return on net sales (%) | | 18.8 | | 23.5 | | | | | |
|
|
1 For an explanation of non-IFRS measures and reconciliation tables, see "—Non-IFRS measures as defined by Novartis." |
Operating income from continuing operations was USD 7.9 billion (–21%, –12% cc), mainly due to higher restructuring primarily related to the implementation of the previously announced streamlined organizational model, higher impairments and lower divestment gains. Operating income margin from continuing operations was 18.8% of net sales from continuing operations, decreasing by 4.7 percentage points (-3.8 percentage points cc). Other revenues as a percentage of net sales from continuing operations increased by 0.2 percentage points (0.0 percentage points cc). Cost of goods sold as a percentage of net sales from continuing operations (0.1 percentage points cc) was in line with the prior year. R&D expenses as a percentage of net sales from continuing operations increased by 1.5 percentage points (1.0 percentage points cc). Selling, general and administration (SG&A) expenses as a percentage of net sales from continuing operations decreased by 1.1 percentage points (1.5 percentage points cc). Other income and other expense, net as a percentage of net sales from continuing operations decreased the margin by 4.5 percentage points (4.4 percentage points cc).
Non-IFRS measure Core operating income from continuing operations 1
(USD millions unless indicated otherwise)
| |
Year ended
Dec 31, 2022
| |
Year ended
Dec 31, 2021
| |
Change
in USD
%
| | Change in
constant
currencies
% | |
|
Core gross profit from continuing operations | | 35 591 | | 36 002 | | -1 | | 5 | |
|
Core selling, general and administration | | -12 143 | | -12 756 | | 5 | | 0 | |
|
Core research and development | | -8 267 | | -8 150 | | -1 | | -5 | |
|
Core other income | | 291 | | 296 | | -2 | | 8 | |
|
Core other expense | | -678 | | -901 | | 25 | | 20 | |
|
Core operating income from continuing operations | | 14 794 | | 14 491 | | 2 | | 10 | |
|
Core return on net sales (%) | | 35.1 | | 33.9 | | | | | |
|
|
1 For an explanation of non-IFRS measures and reconciliation tables, see "—Non-IFRS measures as defined by Novartis." |
The adjustments made to operating income from continuing operations to arrive at core operating income from continuing operations amounted to USD 6.8 billion mainly due to amortization, impairments and restructuring, compared with USD 4.4 billion in the prior year. Core adjustments increased compared with the prior year, mainly due to higher impairments and restructuring. For more information, see “—Non-IFRS measures as defined by Novartis—2023, 2022 and 2021 reconciliation from IFRS Accounting Standards results to non-IFRS core results.”
Core operating income from continuing operations was USD 14.8 billion (+2%, +10% cc), mainly driven by higher gross margin, partly offset by higher R&D investments. Core operating income margin from continuing operations was 35.1% of net sales from continuing operations, increasing 1.2 percentage points (+1.8 percentage points cc). Other revenues as a percentage of net sales from continuing operations decreased by 0.2 percentage points (cc). Core cost of goods sold as a percentage of net sales from continuing operations was in line with the prior year. Core R&D expenses as a percentage of net sales from continuing operations increased by 0.1 percentage points (cc). Core SG&A expenses as a percentage of net sales from continuing operations decreased by 1.6 percentage points (cc). Core other income and expense as a percentage of net sales from continuing operations increased the margin by 0.5 percentage points (cc).
Research and development
The following table provides an overview of the continuing operations reported research and development expense and the non-IFRS measure core research and development expense1:
(USD millions unless indicated otherwise)
| |
Year ended
Dec 31, 2022
| |
Year ended
Dec 31, 2021
| |
Change
in USD
%
| | Change in
constant
currencies
%1 | |
|
Research and exploratory development | | -2 938 | | -3 209 | | 8 | | 6 | |
|
Confirmatory development | | -6 234 | | -5 432 | | -15 | | -20 | |
|
Total research and development expense | | -9 172 | | -8 641 | | -6 | | -10 | |
|
Research and development as % of net sales from continuing operations | | 21.7 | | 20.2 | | | | | |
|
Non-IFRS measures | | | | | | | | | |
|
Core research and exploratory development1 | | -2 784 | | -2 809 | | 1 | | -1 | |
|
Core confirmatory development1 | | -5 483 | | -5 341 | | -3 | | -7 | |
|
Total core research and development expense | | -8 267 | | -8 150 | | -1 | | -5 | |
|
Core research and development as % of net sales from continuing operations | | 19.6 | | 19.1 | | | | | |
|
|
1 Core research and development expense exclude impairments, amortization and certain other items. For an explanation of non-IFRS measures and reconciliation tables, see "—Non-IFRS measures as defined by Novartis." |
Research and exploratory development expense decreased by 8% (+6% cc) to USD 2.9 billion. Confirmatory development expense amounted to USD 6.2 billion, increasing by 15% (-20% cc) versus the prior year mainly due to higher impairment charges and higher investments in development to support acquired assets. Research and development as a percentage of net sales from continuing operations increased by 1.5 percentage points to 21.7% of net sales from continuing operations.
Total core research and development expense as a percentage of net sales from continuing operations increased by 0.5 percentage points (+0.1 percentage points cc) to 19.6% of net sales from continuing operations, mainly driven by higher investments in acquired assets.
Non-operating income and expense
The term “non-operating income and expense” includes all income and expense items outside operating income. The following table provides an overview of non-operating income and expense from continuing operations:
(USD millions unless indicated otherwise)
| |
Year ended
Dec 31, 2022
| |
Year ended
Dec 31, 2021
| |
Change
in USD
%
| | Change in
constant
currencies
%1 | |
|
Operating income from continuing operations | | 7 946 | | 10 056 | | -21 | | -12 | |
|
(Loss)/income from associated companies | | -11 | | 15 337 | | nm | | nm | |
|
Interest expense | | -800 | | -787 | | -2 | | -3 | |
|
Other financial income and expense | | 42 | | -76 | | nm | | nm | |
|
Income before taxes | | 7 177 | | 24 530 | | -71 | | -67 | |
|
Income taxes | | -1 128 | | -1 625 | | 31 | | 21 | |
|
Net income from continuing operations | | 6 049 | | 22 905 | | -74 | | -70 | |
|
Net income from discontinued operations | | 906 | | 1 113 | | -19 | | -11 | |
|
Net income | | 6 955 | | 24 018 | | -71 | | -67 | |
|
Basic earnings per share from continuing operations (USD) | | 2.77 | | 10.22 | | -73 | | -69 | |
|
Basic earnings per share from discontinued operations (USD) | | 0.42 | | 0.49 | | -15 | | -9 | |
|
Total basic earnings per share (USD) | | 3.19 | | 10.71 | | -70 | | -66 | |
|
|
1 For an explanation of non-IFRS measures and reconciliation tables, see "—Non-IFRS measures as defined by Novartis." |
nm = not meaningful |
Income from associated companies
Income from associated companies was a loss of USD 11 million compared with an income of USD 15.3 billion in the prior year. This decrease was due to the divestment of our investment in Roche that closed in the fourth quarter of 2021 where a gain of USD 14.6 billion was recognized.
Interest expense and other financial income and expense
Interest expense amounted to USD 800 million, broadly in line with the prior year.
Other financial income and expense amounted to an income of USD 42 million compared with an expense of USD 76 million in the prior year, as higher interest income was only partly offset by financial expenses and currency losses.
Income taxes
The tax rate was 15.7% compared with 6.6% in the prior year period. In the prior year, the tax rate was impacted by the Roche income from associated companies (including the divestment gain recognized on the sale of our investment in Roche in December 2021), the impact of increases in uncertain tax positions and prior-year items.
For comparability, excluding these impacts, the prior year tax rate would have been 15.4% compared with 15.7% in the current year period. The increase was mainly the result of a change in profit mix.
Net income from continuing operations
Net income from continuing operations was USD 6.0 billion (–74%, –70% cc), impacted by Roche income in the prior year. Excluding the impact of Roche income, net income from continuing operations declined 9% (cc).
Earnings per share from continuing operations
Basic earnings per share from continuing operations were USD 2.77 compared with USD 10.22 in the prior year, mainly due to prior year Roche income. Excluding the impact of Roche income, basic earnings per share from continuing operations declined 7% (cc).
Non-IFRS measure Core non-operating income and expense 1
The following table provides an overview of the non-IFRS measure core non-operating income and expense from continuing operations:
(USD millions unless indicated otherwise)
| |
Year ended
Dec 31, 2022
| |
Year ended
Dec 31, 2021
| |
Change
in USD
%
| | Change in
constant
currencies
% | |
|
Core operating income from continuing operations | | 14 794 | | 14 491 | | 2 | | 10 | |
|
Core (loss)/income from associated companies | | -11 | | 991 | | nm | | nm | |
|
Core interest expense | | -800 | | -787 | | -2 | | -3 | |
|
Core other financial income and expense | | 140 | | -38 | | nm | | nm | |
|
Core income before taxes from continuing operations | | 14 123 | | 14 657 | | -4 | | 5 | |
|
Core income taxes | | -2 177 | | -2 129 | | -2 | | -11 | |
|
Core net income from continuing operations | | 11 946 | | 12 528 | | -5 | | 4 | |
|
Core basic EPS from continuing operations (USD) | | 5.48 | | 5.59 | | -2 | | 6 | |
|
|
1 For an explanation of non-IFRS measures and reconciliation tables, see "—Non-IFRS measures as defined by Novartis." |
nm = not meaningful |
Core income from associated companies
Core income from associated companies was a loss of USD 11 million compared with an income of USD 991 million in the prior year. This decrease was due to the divestment of our investment in Roche that closed in the fourth quarter of 2021.
Core interest expense and other financial income and expense
Core interest expense amounted to USD 800 million, broadly in line with the prior year.
Core other financial income and expense amounted to an income of USD 140 million compared with an expense of USD 38 million in the prior year as higher interest income was only partly offset by currency losses.
Core income taxes
The core tax rate (core taxes as a percentage of core income before tax) was 15.4%, compared with 14.5% in the prior year. For comparability, excluding Roche Income from associated companies (divested in December 2021), the prior year core tax rate would have been 15.4%, in line with 15.4% in the current year.
Core net income from continuing operations
Core net income from continuing operations was USD 11.9 billion (–5%, +4% cc) as growth in core operating income from continuing operations was partly offset by the loss of Roche core income. Excluding the impact of Roche core income, core net income from continuing operations grew 13% (cc).
Core earnings per share from continuing operations
Core basic earnings per share from continuing operations was USD 5.48 (–2%, +6% cc), benefiting from lower weighted average number of shares outstanding. Excluding the impact of Roche core income, core basic earnings per share from continuing operations grew 16% (cc).
Discontinued operations
Discontinued operations net sales were USD 9.4 billion compared with USD 9.8 billion in the prior year. Operating income amounted to USD 1.3 billion, compared with USD 1.6 billion in the prior year.
Net income from discontinued operations amounted to USD 0.9 billion, compared to USD 1.1 billion in the prior year.
Total Company
Total Company net income amounted to USD 7.0 billion in 2022, compared with USD 24.0 billion in the prior year, impacted by Roche income in the prior year (see “Item 18. Financial Statements – Note 2. Significant transactions and Note 5. Associated companies”). Basic earnings per share decreased to USD 3.19 from USD 10.71. Net cash flows from operating activities for the total Company amounted to USD 14.2 billion, and free cash flow amounted to USD 13.0 billion.
Factors affecting comparability of year-on-year results of operations
Significant transactions in 2023, 2022 and 2021
The comparability of the year-on-year results of our operations for the total Company can be significantly affected by acquisitions and divestments. As part of our long-term strategy to focus Novartis as a leading medicines company, we announced and/or completed several acquisitions and divestments during 2023, 2022 and 2021.
A detailed description of significant transactions in 2023, 2022 and 2021, can be found in “Item 18. Financial Statements—Note 2. Significant transactions.”
Internal control over financial reporting
The Company’s management has assessed the effectiveness of internal control over financial reporting. The Company’s independent registered public accounting firm also issued an opinion on the effectiveness of internal control over financial reporting. Both the Company’s management and its independent registered public accounting firm concluded that the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2023. For more information, see “Item 15. Controls and Procedures.”
Approach to risk management
See “Item 6. Directors, Senior Management and Employees—Item 6.C Board practices—Corporate governance—Information and control systems—Risk management” and “Item 18. Financial Statements—Note 30. Financial instruments – additional disclosures.”
Non-IFRS measures as defined by Novartis
Novartis uses certain non-IFRS Accounting Standards metrics when measuring performance, especially when measuring current-year results against prior periods, including core results, constant currencies and free cash flow. These are referred to by Novartis as non-IFRS measures.
Despite the use of these measures by management in setting goals and measuring the Company’s performance, these are non-IFRS measures that have no standardized meaning prescribed by IFRS Accounting Standards. As a result, such measures have limits in their usefulness to investors.
Because of their non-standardized definitions, the non-IFRS measures (unlike IFRS Accounting Standards measures) may not be comparable to the calculation of similar measures of other companies. These non-IFRS measures are presented solely to permit investors to more fully understand how the Company’s management assesses underlying performance. These non-IFRS measures are not, and should not be viewed as, a substitute for IFRS Accounting Standards measures, and should be viewed in conjunction with the consolidated financial statements prepared in accordance with IFRS Accounting Standards.
As an internal measure of Company performance, these non-IFRS measures have limitations, and the Company’s performance management process is not solely restricted to these metrics.
Core results
The Company’s core results – including core operating income, core net income and core earnings per share – exclude fully the amortization and impairment charges of intangible assets, excluding software, net gains and losses on fund investments and equity securities valued at fair value through profit and loss, impact of IAS 29 “Financial reporting in Hyperinflation Economies” to other financial income and expense, and certain acquisition- and divestment-related items. The following items that exceed a threshold of USD 25 million are also excluded: integration- and divestment-related income and expenses; divestment gains and losses; restructuring charges/releases and related items; legal-related items; impairments of property, plant and equipment, software, and financial assets, and income and expense items that management deems exceptional and that are or are expected to accumulate within the year to be over a USD 25 million threshold.
Novartis believes that investor understanding of the Company’s performance is enhanced by disclosing core measures of performance, since core measures exclude items that can vary significantly from year to year, they enable better comparison of business performance across years. For this same reason, Novartis uses these core measures in addition to IFRS Accounting Standards measures and other measures as important factors in assessing the Company’s performance.
The following are examples of how these core measures are used:
• In addition to monthly reports containing financial information prepared under IFRS Accounting Standards, senior management receives a monthly analysis incorporating these non-IFRS core measures.
• Annual budgets are prepared for both IFRS Accounting Standard measures and non-IFRS core measures.
As an internal measure of Company performance, the core results measures have limitations, and the Company’s performance management process is not solely restricted to these metrics. A limitation of the core results measures is that they provide a view of the Company’s operations without including all events during a period, such as the effects of an acquisition, divestment, or amortization/impairments of purchased intangible assets, impairments to property, plant and equipment and restructurings and related items.
Constant currencies
Changes in the relative values of non-US currencies to the US dollar can affect the Company’s financial results and financial position. To provide additional information that may be useful to investors, including changes in sales volume, we present information about our net sales and various values relating to operating and net income that are adjusted for such foreign currency effects.
Constant currency calculations have the goal of eliminating two exchange rate effects so that an estimate can be made of underlying changes in the consolidated income statement excluding the impact of fluctuations in exchanges rates:
• The impact of translating the income statements of consolidated entities from their non-USD functional currencies to USD
• The impact of exchange rate movements on the major transactions of consolidated entities performed in currencies other than their functional currency.
We calculate constant currency measures by translating the current year’s foreign currency values for sales and other income statement items into USD (excluding the IAS 29 “Financial Reporting in Hyperinflationary Economies” adjustments to the local currency income statements of subsidiaries operating in hyperinflationary economies), using the average exchange rates from the prior year and comparing them to the prior year values in USD.
We use these constant currency measures in evaluating the Company’s performance, since they may assist us in evaluating our ongoing performance from year to year. However, in performing our evaluation, we also consider equivalent measures of performance that are not affected by changes in the relative value of currencies.
Growth rate calculation
For ease of understanding, Novartis uses a sign convention for its growth rates such that a reduction in operating expenses or losses compared with the prior year is shown as a positive growth.
Free cash flow
Effective January 1, 2023, Novartis revised its definition of free cash flow, to define free cash flow as net cash flows from operating activities less purchases of property, plant and equipment. This new definition provides a simpler performance measure focusing on core operating activities, and also excludes items that can vary significantly from year to year, thereby enabling better comparison of business performance across years. The prior year free cash flow amounts have been revised to conform with the new free cash flow definition to aid in comparability.
Free cash flow is a non-IFRS measure and is not intended to be a substitute measure for net cash flows from operating activities as determined under IFRS Accounting Standards. Free cash flow is presented as additional information because management believes it is a useful supplemental indicator of the Company’s ability to operate without reliance on additional borrowing or use of existing cash. Free cash flow is a measure of the net cash generated that is available for investment in strategic opportunities, returning to shareholders and for debt repayment. Free cash flow is a non-IFRS measure, which means it should not be interpreted as a measure determined under IFRS Accounting Standards.
Additional information
NET DEBT
Novartis calculates net debt as current financial debts and derivative financial instruments plus non-current financial debt less cash and cash equivalents and marketable securities, commodities, time deposits and derivative financial instruments.
Net debt is presented as additional information because it sets forth how management monitors net debt or liquidity and management believes it is a useful supplemental indicator of the Company’s ability to pay dividends, to meet financial commitments, and to invest in new strategic opportunities, including strengthening its balance sheet.
For the table that shows the Company’s net debt, see “— Item 5.B Liquidity and capital resources — Company liquidity, financial debts and net debt.”
EBITDA
Novartis defines earnings before interest, tax, depreciation and amortization (EBITDA) as operating income, excluding depreciation of property, plant and equipment, depreciation of right-of-use assets, amortization of intangible assets, and net impairments of property, plant and equipment, right-of-use assets and of intangible assets.
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
Operating income from continuing operations | | 9 769 | | 7 946 | | 10 056 | |
|
Depreciation of property, plant and equipment | | 916 | | 967 | | 1 005 | |
|
Depreciation of right-of-use assets | | 259 | | 267 | | 279 | |
|
Amortization of intangible assets | | 3 960 | | 3 760 | | 3 665 | |
|
Impairments of property, plant and equipment, right-of-use assets and intangible assets, net 1 | | 3 142 | | 1 711 | | 648 | |
|
EBITDA from continuing operations | | 18 046 | | 14 651 | | 15 653 | |
|
Operating income from discontinued operations | | 265 | | 1 251 | | 1 633 | |
|
Depreciation of property, plant and equipment | | 144 | | 196 | | 203 | |
|
Depreciation of the right-of-use-assets | | 32 | | 33 | | 39 | |
|
Amortization of intangible assets | | 171 | | 222 | | 238 | |
|
Impairments of property, plant and equipment, right-of-use assets and intangible assets, net 2 | | 56 | | 25 | | 36 | |
|
EBITDA from discontinued operations 3 | | 668 | | 1 727 | | 2 149 | |
|
EBITDA | | 18 714 | | 16 378 | | 17 802 | |
|
|
1 There were no impairments of right-of-use assets in 2021. |
2 There were no impairments of right-of-use assets. |
3 The EBITDA from discontinued operations for 2023 is for the period from January 1, 2023, to the October 3, 2023, Distribution date. |
Enterprise value
Enterprise value represents the total amount that shareholders and debt holders have invested in Novartis, less the Company’s liquidity.
(USD millions) | | Dec 31, 2023 | | Dec 31, 2022 | | Dec 31, 2021 | |
|
Market capitalization | | 206 264 | | 191 530 | | 196 107 | |
|
Non-controlling interests | | 83 | | 81 | | 167 | |
|
Non-current financial debts | | 18 436 | | 20 244 | | 22 902 | |
|
Current financial debts and derivative financial instruments | | 6 175 | | 5 931 | | 6 295 | |
|
Marketable securities, commodities, time deposits and derivative financial instruments | | -1 035 | | -11 413 | | -15 922 | |
|
Cash and cash equivalents | | -13 393 | | -7 517 | | -12 407 | |
|
Enterprise value | | 216 530 | | 198 856 | | 197 142 | |
|
|
Reconciliation from IFRS Accounting Standards results to non-IFRS measure core results
The following tables provide an overview of the reconciliation from IFRS Accounting Standards results to non-IFRS measure core results:
2023, 2022 and 2021 reconciliation from IFRS Accounting Standards results to non-IFRS measure core results – Total Company
(USD millions unless indicated otherwise) | | 2023 | | 2022 | | 2021 | |
|
IFRS Accounting Standards operating income from continuing operations | | 9 769 | | 7 946 | | 10 056 | |
|
Amortization of intangible assets | | 3 730 | | 3 585 | | 3 528 | |
|
Impairments | | | | | | | |
|
Intangible assets | | 3 044 | | 1 293 | | 360 | |
|
Property, plant and equipment related to the company-wide rationalization of manufacturing sites | | 5 | | 286 | | 219 | |
|
Other property, plant and equipment | | 39 | | 85 | | 40 | |
|
Total impairment charges | | 3 088 | | 1 664 | | 619 | |
|
Acquisition or divestment of businesses and related items | | | | | | | |
|
- Income | | -174 | | -4 | | -66 | |
|
- Expense | | 149 | | 8 | | 107 | |
|
Total acquisition or divestment of businesses and related items, net | | -25 | | 4 | | 41 | |
|
Other items | | | | | | | |
|
Divestment gains | | -225 | | -166 | | -724 | |
|
Financial assets - fair value adjustments | | 105 | | 260 | | -38 | |
|
Restructuring and related items | | | | | | | |
|
- Income | | -229 | | -34 | | -38 | |
|
- Expense | | 1 180 | | 1 856 | | 865 | |
|
Legal-related items | | | | | | | |
|
- Income | | -608 | | -51 | | | |
|
- Expense | | 66 | | 364 | | 170 | |
|
Additional income | | -602 | | -698 | | -277 | |
|
Additional expense | | 123 | | 64 | | 289 | |
|
Total other items | | -190 | | 1 595 | | 247 | |
|
Total adjustments | | 6 603 | | 6 848 | | 4 435 | |
|
Core operating income from continuing operations | | 16 372 | | 14 794 | | 14 491 | |
|
as % of net sales | | 36.0% | | 35.1% | | 33.9% | |
|
(Loss)/income from associated companies | | -13 | | -11 | | 15 337 | |
|
Core adjustments to income from associated companies, net of tax | | | | | | -14 346 | |
|
Interest expense | | -855 | | -800 | | -787 | |
|
Other financial income and expense | | 222 | | 42 | | -76 | |
|
Core adjustments to other financial income and expense | | 208 | | 98 | | 38 | |
|
Income taxes, adjusted for above items (core income taxes) | | -2 488 | | -2 177 | | -2 129 | |
|
Core net income from continuing operations | | 13 446 | | 11 946 | | 12 528 | |
|
Core net income from discontinued operations 2 | | 889 | | 1 406 | | 1 566 | |
|
Core net income | | 14 335 | | 13 352 | | 14 094 | |
|
Core net income attributable to shareholders of Novartis AG | | 14 331 | | 13 352 | | 14 097 | |
|
Core basic EPS from continuing operations (USD) 1 | | 6.47 | | 5.48 | | 5.59 | |
|
Core basic EPS from discontinued operations (USD) 1, 2 | | 0.43 | | 0.64 | | 0.70 | |
|
Core basic EPS (USD) 1 | | 6.90 | | 6.12 | | 6.29 | |
|
|
1 Core earnings per share (EPS) is calculated by dividing core net income attributable to shareholders of Novartis AG by the weighted average number of shares used in the basic EPS calculation outstanding in a reporting period. |
2 For details on discontinued operations reconciliation from IFRS Accounting Standards net income to core net income, refer to page 70. |
2023, 2022 and 2021 reconciliation from IFRS Accounting Standards results to non-IFRS measure core results – Total Company
2023 (USD millions unless indicated otherwise)
| | IFRS
Accounting
Standards
results | |
Amortization
of intangible
assets1
| |
Impairments2
| | Acquisition or
divestment of
businesses and
related items3 | |
Other
items4
| |
Core results
| |
|
Gross profit from continuing operations | | 34 188 | | 3 319 | | 310 | | | | 142 | | 37 959 | |
|
Operating income from continuing operations | | 9 769 | | 3 730 | | 3 088 | | -25 | | -190 | | 16 372 | |
|
Income before taxes from continuing operations | | 9 123 | | 3 730 | | 3 088 | | -25 | | 18 | | 15 934 | |
|
Income taxes 5 | | -551 | | | | | | | | | | -2 488 | |
|
Net income from continuing operations | | 8 572 | | | | | | | | | | 13 446 | |
|
Net income from discontinued operations 6 | | 6 282 | | | | | | | | | | 889 | |
|
Net income | | 14 854 | | | | | | | | | | 14 335 | |
|
Basic EPS from continuing operations (USD) 7 | | 4.13 | | | | | | | | | | 6.47 | |
|
Basic EPS from discontinued operations (USD) 7 | | 3.02 | | | | | | | | | | 0.43 | |
|
Basic EPS (USD) 7 | | 7.15 | | | | | | | | | | 6.90 | |
|
|
The following are adjustments to arrive at core gross profit from continuing operations | | | | | | | | | | | | | |
|
Cost of goods sold | | -12 472 | | 3 319 | | 310 | | | | 142 | | -8 701 | |
|
|
The following are adjustments to arrive at core operating income from continuing operations | | | | | | | | | | | | | |
|
Selling, general and administration | | -12 517 | | | | | | | | 28 | | -12 489 | |
|
Research and development | | -11 371 | | 411 | | 2 737 | | 32 | | -409 | | -8 600 | |
|
Other income | | 1 772 | | | | -10 | | -174 | | -1 196 | | 392 | |
|
Other expense | | -2 303 | | | | 51 | | 117 | | 1 245 | | -890 | |
|
|
The following are adjustments to arrive at core income before taxes from continuing operations | | | | | | | | | | | | | |
|
Other financial income and expense | | 222 | | | | | | | | 208 | | 430 | |
|
|
1 Amortization of intangible assets: cost of goods sold includes the amortization of acquired rights to currently marketed products and other production-related intangible assets; research and development includes the amortization of acquired rights to technologies |
2 Impairments: cost of goods sold, research and development, other income and other expense include net impairment charges related to intangible assets; other income and other expense includes also net impairment charges related to property, plant and equipment |
3 Acquisition or divestment of businesses and related items, including restructuring and integration charges: research and development include restructuring and integration cost charges; other income includes a favorable stamp duties tax settlement related to a prior periods acquisition; other income and other expense include also transitional service-fee income and expenses related to the Sandoz distribution, restructuring and integration costs charges and reversals |
4 Other items: cost of goods sold, selling, general and administration, research and development, other income and other expense include restructuring income and charges related to the initiative to implement a new streamlined organizational model, the company-wide rationalization of manufacturing sites and other net restructuring charges and related items; cost of goods sold and research and development also include contingent consideration adjustments; cost of goods sold and selling, general and administration includes also adjustments to provisions; research and development also include a write-off of prepaid expenses for a terminated development project; other income and other expense include fair value adjustments, divestment gains, losses and gains on financial assets, legal related items, adjustments to environmental provisions; other income includes also gains from the divestment of products and curtailment gains; other expenses also includes a fair value adjustment on a contingent receivable and other costs and items; other financial income and expense includes the impact of IAS 29 "Financial reporting in Hyperinflation Economies" for subsidiaries operating in hyperinflation economies and foreign exchange losses |
5 Taxes on the adjustments between IFRS Accounting Standards and core results, for each item included in the adjustment, take into account the tax rate that will finally be applicable to the item based on the jurisdiction where the adjustment will finally have a tax impact. Generally, this results in amortization and impairment of intangible assets and acquisition-related restructuring and integration items having a full tax impact. There is usually a tax impact on other items, although this is not always the case for items arising from legal settlements in certain jurisdictions. Adjustments related to income from associated companies are recorded net of any related tax effect. Due to these factors and the differing effective tax rates in the various jurisdictions, the tax on the total adjustments of USD 6.8 billion to arrive at the core results before tax amounts to USD 1.9 billion and the average tax rate on the adjustments was 28.4%. |
6 For details on discontinued operations reconciliation from IFRS Accounting Standards net ncome to core net income refer to page 70. |
7 Earnings per share (EPS) is calculated on the amount of net income attributable to shareholders of Novartis AG. |
2022 (USD millions unless indicated otherwise)
| | IFRS
Accounting
Standards
results | |
Amortization
of intangible
assets1
| |
Impairments2
| | Acquisition or
divestment of
businesses and
related items3 | |
Other
items4
| |
Core results
| |
|
Gross profit from continuing operations | | 31 879 | | 3 427 | | 314 | | | | -29 | | 35 591 | |
|
Operating income from continuing operations | | 7 946 | | 3 585 | | 1 664 | | 4 | | 1 595 | | 14 794 | |
|
Income before taxes from continuing operations | | 7 177 | | 3 585 | | 1 664 | | 4 | | 1 693 | | 14 123 | |
|
Income taxes from continuing operations 5 | | -1 128 | | | | | | | | | | -2 177 | |
|
Net income from continuing operations | | 6 049 | | | | | | | | | | 11 946 | |
| | | | | | | | | | | | | |
Net income from discontinued operations 6 | | 906 | | | | | | | | | | 1 406 | |
|
Net income | | 6 955 | | | | | | | | | | 13 352 | |
|
Basic EPS from continuing operations (USD) 7 | | 2.77 | | | | | | | | | | 5.48 | |
| | | | | | | | | | | | | |
Basic EPS from discontinued operations (USD) 7 | | 0.42 | | | | | | | | | | 0.64 | |
|
Basic EPS (USD) 7 | | 3.19 | | | | | | | | | | 6.12 | |
|
|
The following are adjustments to arrive at core gross profit from continuing operations | | | | | | | | | | | | | |
|
Other revenues | | 1 255 | | | | | | | | -86 | | 1 169 | |
|
Cost of goods sold | | -11 582 | | 3 427 | | 314 | | | | 57 | | -7 784 | |
|
|
The following are adjustments to arrive at core operating income | | | | | | | | | | | | | |
|
Selling, general and administration | | -12 193 | | | | | | | | 50 | | -12 143 | |
|
Research and development | | -9 172 | | 158 | | 953 | | | | -206 | | -8 267 | |
|
Other income | | 696 | | | | -1 | | -4 | | -400 | | 291 | |
|
Other expense | | -3 264 | | | | 398 | | 8 | | 2 180 | | -678 | |
|
|
The following are adjustments to arrive at core income before taxes from continuing operations | | | | | | | | | | | | | |
|
Other financial income and expense | | 42 | | | | | | | | 98 | | 140 | |
|
|
1 Amortization of intangible assets: cost of goods sold includes the amortization of acquired rights to currently marketed products and other production-related intangible assets; research and development includes the amortization of acquired rights for technologies |
2 Impairments: cost of goods sold, research and development and other expense include impairment charges related to intangible assets; other income and other expense include net impairment charges related to property, plant and equipment |
3 Acquisition or divestment of businesses and related items, including restructuring and integration charges: other income and other expense include transitional service fee income and charges related to divestments; other income also includes adjustments to provisions; other expense includes stamp duties related to an acquisition |
4 Other items: other revenues includes a net income from an outlicensing agreement; cost of goods sold, selling, general and administration, research and development, other income and other expense include restructuring income and charges related to the restructuring initiative to implement a new streamlined organizational model, the company-wide rationalization of manufacturing sites and other net restructuring charges and related items; cost of goods sold, selling, general and administration, research and development and other expense include adjustments to provisions and related items; cost of goods sold and research and development also include contingent consideration adjustments; other income and other expense include fair value adjustments and divestment gains and losses on financial assets and legal-related items; other income also includes gains from the divestment of products and property, curtailment gains and an adjustment to an environmental provision; other expense includes a reversal of an accrual and other costs and items; other financial income and expense includes the impact of IAS 29 "Financial reporting in Hyperinflation Economies" for subsidiaries operating in hyperinflation economies and a revaluation impact of a financial liability incurred through the Alcon distribution |
5 Taxes on the adjustments between IFRS Accounting Standards and core results take into account, for each individual item included in the adjustment, the tax rate that will finally be applicable to the item based on the jurisdiction where the adjustment will finally have a tax impact. Generally, this results in amortization and impairment of intangible assets and acquisition-related restructuring and integration items having a full tax impact. There is usually a tax impact on other items, although this is not always the case for items arising from legal settlements in certain jurisdictions. Adjustments related to income from associated companies are recorded net of any related tax effect. Due to these factors and the differing effective tax rates in the various jurisdictions, the tax on the total adjustments of USD 6.9 billion to arrive at the core results before tax amounts to USD 1.0 billion and the average tax rate on the adjustments was 15.1%. |
6 For details on discontinued operations reconciliation from IFRS Accounting Standards net income to core net income please refer to page 71. |
7 Earnings per share (EPS) is calculated on the amount of net income attributable to shareholders of Novartis AG. |
2021 (USD millions unless indicated otherwise)
| | IFRS
Accounting
Standards
results | |
Amortization
of intangible
assets1
| |
Impairments2
| | Acquisition or
divestment of
businesses and
related items3 | |
Other
items4
| |
Core results
| |
|
Gross profit from continuing operations | | 32 239 | | 3 419 | | | | | | 344 | | 36 002 | |
|
Operating income from continuing operations | | 10 056 | | 3 528 | | 619 | | 41 | | 247 | | 14 491 | |
|
Income before taxes from continuing operations | | 24 530 | | 3 738 | | 619 | | -14 531 | | 301 | | 14 657 | |
|
Income taxes from continuing operations 5 | | -1 625 | | | | | | | | | | -2 129 | |
|
Net income from continuing operations | | 22 905 | | | | | | | | | | 12 528 | |
|
Net income from discontinued operations 6 | | 1 113 | | | | | | | | | | 1 566 | |
|
Net income | | 24 018 | | | | | | | | | | 14 094 | |
|
Basic EPS from continuing operations (USD) 7 | | 10.22 | | | | | | | | | | 5.59 | |
| | | | | | | | | | | | | |
Basic EPS from discontinued operations (USD) 7 | | 0.49 | | | | | | | | | | 0.70 | |
|
Basic EPS (USD) 7 | | 10.71 | | | | | | | | | | 6.29 | |
|
|
The following are adjustments to arrive at core gross profit from continuing operations | | | | | | | | | | | | | |
|
Cost of goods sold | | -11 735 | | 3 419 | | | | | | 344 | | -7 972 | |
|
|
The following are adjustments to arrive at core operating income from continuing operations | | | | | | | | | | | | | |
|
Selling, general and administration | | -12 827 | | | | | | | | 71 | | -12 756 | |
|
Research and development | | -8 641 | | 109 | | 360 | | | | 22 | | -8 150 | |
|
Other income | | 1 620 | | | | -45 | | -66 | | -1 213 | | 296 | |
|
Other expense | | -2 335 | | | | 304 | | 107 | | 1 023 | | -901 | |
|
|
The following are adjustments to arrive at core income before taxes from continuing operations | | | | | | | | | | | | | |
|
Income from associated companies | | 15 337 | | 210 | | | | -14 556 | | | | 991 | |
|
Other financial income and expense | | -76 | | | | | | -16 | | 54 | | -38 | |
|
|
1 Amortization of intangible assets: cost of goods sold includes the amortization of acquired rights to currently marketed products and other production-related intangible assets; research and development includes the amortization of acquired rights for technologies; income from associated companies includes USD 210 million for the Novartis share of the estimated Roche core items |
2 Impairments: cost of goods sold, and research and development include impairment charges related to intangible assets; other income and other expense include reversals of impairment charges and impairment charges related to property, plant and equipment |
3 Acquisition or divestment of businesses and related items, including restructuring and integration charges: other income includes adjustments to portfolio transformation and Alcon spin-off accruals; other income and other expense include transitional service-fee income and expenses related to the Alcon distribution; other expense also includes adjustments to provisions; income from associated companies includes the gain related to the divestment of our investment in Roche; other financial income and expense includes other financial gains related to the divestment of our investment in Roche |
4 Other items: cost of goods sold, research and development, other income and other expense include net restructuring and other charges related to the company-wide rationalization of manufacturing sites; cost of goods sold, selling, general and administration, other income and other expense include other restructuring income and charges and related items; cost of goods sold, research and development, other income and other expense also include adjustments to contingent considerations; selling, general and administration, research and development, other income and other expense include adjustments to provisions; other income and other expense also include gains and losses from the divestment of products and financial assets and fair value adjustments on financial assets, adjustments to environmental provisions and legal-related items; other financial income and expense includes the impact of IAS 29 "Financial reporting in Hyperinflation Economies" for subsidiaries operating in hyperinflation economies and a revaluation impact of a financial liability incurred through the Alcon distribution |
5 Taxes on the adjustments between IFRS Accounting Standards and core results take into account, for each individual item included in the adjustment, the tax rate that will finally be applicable to the item based on the jurisdiction where the adjustment will finally have a tax impact. Generally, this results in amortization and impairment of intangible assets and acquisition-related restructuring and integration items having a full tax impact. There is usually a tax impact on other items, although this is not always the case for items arising from legal settlements in certain jurisdictions. Adjustments related to income from associated companies are recorded net of any related tax effect. Due to these factors and the differing effective tax rates in the various jurisdictions, the tax on the total adjustments of USD 9.9 billion to arrive at the core results before tax amounts to USD 504 million. Excluding the gain on the divestment of our investment in Roche, the tax on the total adjustments of USD 4.5 billion to arrive at the core results before tax amounts to USD 504 million and the average tax rate on the adjustments was 11.3%. |
6 For details on discontinued operations reconciliation from IFRS Accounting Standards net income to core net income please refer to page 72. |
7 Earnings per share (EPS) is calculated on the amount of net income, attributable to shareholders of Novartis AG. |
2023, 2022 and 2021 reconciliation from IFRS Accounting Standards results to non-IFRS measure core results – Discontinued operations
2023 (USD millions unless indicated otherwise)
| | IFRS
Accounting
Standards
results | |
Amortization
of intangible
assets1
| |
Impairments2
| | Acquisition or
divestment of
businesses and
related items | |
Other
items3
| |
Core results
| |
|
Gross profit from discontinued operations | | 3 403 | | 165 | | 34 | | | | 57 | | 3 659 | |
|
Operating income from discontinued operations | | 265 | | 165 | | 43 | | | | 712 | | 1 185 | |
|
Income before taxes from discontinued operations | | 214 | | 165 | | 43 | | | | 718 | | 1 140 | |
|
Income taxes 4 | | 208 | | | | | | | | | | -251 | |
|
Net income from discontinued operations before gain on distribution of Sandoz Group AG to Novartis AG shareholders | | 422 | | | | | | | | | | 889 | |
|
Gain on distribution of Sandoz Group AG to Novartis AG shareholders | | 5 860 | | | | | | -5 860 | | | | | |
|
Net income from discontinued operations | | 6 282 | | | | | | | | | | 889 | |
|
Basic EPS from discontinued operations (USD) 5 | | 3.02 | | | | | | | | | | 0.43 | |
|
|
The following are adjustments to arrive at core gross profit from discontinued operations | | | | | | | | | | | | | |
|
Cost of goods sold | | -4 044 | | 165 | | 34 | | | | 57 | | -3 788 | |
|
|
The following are adjustments to arrive at core operating income from discontinued operations | | | | | | | | | | | | | |
|
Selling, general and administration | | -1 728 | | | | | | | | 25 | | -1 703 | |
|
Research and development | | -671 | | | | 10 | | | | | | -661 | |
|
Other income | | 56 | | | | -1 | | | | -24 | | 31 | |
|
Other expense | | -795 | | | | | | | | 654 | | -141 | |
|
| | | | | | | | | | | | | |
The following are adjustments to arrive at core income before taxes from discontinued operations | | | | | | | | | | | | | |
|
Other financial income and expense | | -20 | | | | | | | | 6 | | -14 | |
|
|
1 Amortization of intangible assets: cost of goods sold includes the amortization of acquired rights to currently marketed products and other production-related intangible assets |
2 Impairments: cost of goods sold and research and development include impairment charges related to intangible assets; other income includes a reversal of impairment charges related to property, plant and equipment |
3 Other items: cost of goods sold, selling, general and administration, other income and other expense include charges related to the Sandoz distribution, the company-wide rationalization of manufacturing sites and other net restructuring charges and related items; cost of goods sold and selling, general and administration also include adjustments to provisions; other expense includes legal-related items; other financial income and expense includes the impact of IAS 29 "Financial reporting in Hyperinflation Economies" for subsidiaries operating in hyperinflation economies |
4 Taxes on the adjustments between IFRS Accounting Standards and core results, for each item included in the adjustment, take into account the tax rate that will finally be applicable to the item based on the jurisdiction where the adjustment will finally have a tax impact. Generally, this results in amortization and impairment of intangible assets and acquisition-related restructuring and integration items having a full tax impact. There is usually a tax impact on other items, although this is not always the case for items arising from legal settlements in certain jurisdictions. Adjustments related to income from associated companies are recorded net of any related tax effect. Due to these factors and the differing effective tax rates in the various jurisdictions, the tax on the total adjustments of USD 926 million to arrive at the core results before tax amounts to USD 459 million and the average tax rate on the adjustments was 49.5%. |
5 Earnings per share (EPS) is calculated on the amount of net income attributable to shareholders of Novartis AG. |
2022 (USD millions unless indicated otherwise)
| | IFRS
Accounting
Standards
results | |
Amortization
of intangible
assets1
| |
Impairments2
| | Acquisition or
divestment of
businesses and
related items | |
Other
items3
| |
Core results
| | |
|
Gross profit from discontinued operations | | 4 463 | | 221 | | 24 | | | | 93 | | 4 801 | | |
|
Operating income from discontinued operations | | 1 251 | | 221 | | 23 | | | | 376 | | 1 871 | | |
|
Income before taxes from discontinued operations | | 1 194 | | 221 | | 23 | | | | 399 | | 1 837 | | |
|
Income taxes from discontinued operations 4 | | -288 | | | | | | | | | | -431 | | |
|
Net income from discontinued operations | | 906 | | | | | | | | | | 1 406 | | |
|
Basic EPS from discontinued operations (USD) 5 | | 0.42 | | | | | | | | | | 0.64 | | |
|
|
The following are adjustments to arrive at core gross profit from discontinued operations | | | | | | | | | | | | | | |
|
Cost of goods sold | | -4 937 | | 221 | | 24 | | | | 93 | | -4 599 | | |
|
|
The following are adjustments to arrive at core operating loss from discontinued operations | | | | | | | | | | | | | | |
|
Selling, general and administration | | -2 060 | | | | | | | | 13 | | -2 047 | | |
|
Research and development | | -824 | | | | 1 | | | | 2 | | -821 | | |
|
Other income | | 109 | | | | -2 | | | | -14 | | 93 | | |
|
Other expense | | -437 | | | | | | | | 282 | | -155 | | |
|
|
The following are adjustments to arrive at core income before taxes from discontinued operations | | | | | | | | | | | | | | |
|
Other financial income and expense | | -22 | | | | | | | | 23 | | 1 | | |
|
|
1 Amortization of intangible assets: cost of goods sold includes the amortization of acquired rights to currently marketed products and other production-related intangible assets |
2 Impairments: cost of goods sold and research and development include impairment charges related to intangible assets; other income includes a reversal of an impairment charge related to property, plant and equipment |
3 Other items: cost of goods sold, selling, general and administration, research and development, other income and other expense include charges related to the Sandoz strategic review, the company-wide rationalization of manufacturing sites and other net restructuring charges and related items; other expense also includes legal-related items; cost of goods sold and selling, general and administration include adjustments to provisions and related items |
4 Taxes on the adjustments between IFRS Accounting Standards and core results take into account, for each individual item included in the adjustment, the tax rate that will finally be applicable to the item based on the jurisdiction where the adjustment will finally have a tax impact. Generally, this results in amortization and impairment of intangible assets and acquisition-related restructuring and integration items having a full tax impact. There is usually a tax impact on other items, although this is not always the case for items arising from legal settlements in certain jurisdictions. Adjustments related to income from associated companies are recorded net of any related tax effect. Due to these factors and the differing effective tax rates in the various jurisdictions, the tax on the total adjustments of USD 643 million to arrive at the core results before tax amounts to USD 143 million and the average tax rate on the adjustments was 22.2%. |
5 Earnings per share (EPS) is calculated on the amount of net income, attributable to shareholders of Novartis AG. |
2021 (USD millions unless indicated otherwise)
| | IFRS
Accounting
Standards
results | |
Amortization
of intangible
assets1
| |
Impairments2
| | Acquisition or
divestment of
businesses and
related items | |
Other
items3
| |
Core results
| | |
|
Gross profit from discontinued operations | | 4 771 | | 236 | | 18 | | | | 70 | | 5 095 | | |
|
Operating income from discontinued operations | | 1 633 | | 236 | | 34 | | | | 194 | | 2 097 | | |
|
Income before taxes from discontinued operations | | 1 607 | | 236 | | 34 | | | | 195 | | 2 072 | | |
|
Income taxes from discontinued operations 4 | | -494 | | | | | | | | | | -506 | | |
|
Net income from discontinued operations | | 1 113 | | | | | | | | | | 1 566 | | |
|
Basic EPS from discontinued operations (USD) 5 | | 0.49 | | | | | | | | | | 0.70 | | |
|
|
The following are adjustments to arrive at core gross profit from discontinued operations | | | | | | | | | | | | | | |
|
Cost of goods sold | | -5 121 | | 236 | | 18 | | | | 70 | | -4 797 | | |
|
|
The following are adjustments to arrive at core operating loss from discontinued operations | | | | | | | | | | | | | | |
|
Research and development | | -899 | | | | 9 | | | | -1 | | -891 | | |
|
Other income | | 232 | | | | -55 | | | | -52 | | 125 | | |
|
Other expense | | -412 | | | | 62 | | | | 177 | | -173 | | |
|
|
The following are adjustments to arrive at core income before taxes from discontinued operations | | | | | | | | | | | | | | |
|
Other financial income and expense | | -4 | | | | | | | | 1 | | -3 | | |
|
|
1 Amortization of intangible assets: cost of goods sold includes the amortization of acquired rights to currently marketed products and other production related intangible assets |
2 Impairments: cost of goods sold and research and development include impairment charges related to intangible assets; other income and other expense include reversals of impairment charges and impairment charges related to property, plant and equipment |
3 Other items: cost of goods sold, other income and other expense include net restructuring charges related to the company-wide rationalization of manufacturing sites and other restructuring income and charges and related items; research and development includes adjustments to provisions; other income includes net gains from the divestment of a product; other income and other expense include legal related items |
4 Taxes on the adjustments between IFRS Accounting Standards and core results take into account, for each individual item included in the adjustment, the tax rate that will finally be applicable to the item based on the jurisdiction where the adjustment will finally have a tax impact. Generally, this results in amortization and impairment of intangible assets and acquisition-related restructuring and integration items having a full tax impact. There is usually a tax impact on other items, although this is not always the case for items arising from legal settlements in certain jurisdictions. Adjustments related to income from associated companies are recorded net of any related tax effect. Due to these factors and the differing effective tax rates in the various jurisdictions, the tax on the total adjustments of USD 465 million to arrive at the core results before tax amounts to USD 12 million and the average tax rate on the adjustments was 2.6%. |
5 Earnings per share (EPS) is calculated on the amount of net income, attributable to shareholders of Novartis AG. |
Reconciliation of 2021 IFRS Accounting Standards results and non-IFRS measures core results and free cash flow to exclude the impacts of the 2021 divestment of our Roche investment
To enhance investor understanding of the Company’s performance in comparison with the prior year, we presented the 2021 IFRS Accounting Standards results and non-IFRS measures core results and free cash flow excluding the impacts related to our Roche investment, due to its divestment in the fourth quarter of 2021.
The following tables provide a reconciliation of our 2021 published IFRS Accounting Standards results and the non-IFRS measures core results and free cash flow to the 2021 results, excluding the impacts related to our Roche investment, due to its divestment. The table also provides a comparison of our 2022 IFRS Accounting Standards results and non-IFRS measures core results and free cash flow from continuing operations as published with the non-IFRS measure 2021 results excluding impacts from the divestment of our Roche investment.
| | 2021 | | 2022 | |
| | | | | |
| | | | | |
| | | | | |
(USD millions unless indicated otherwise)
| |
Results as
published1
| |
Our Roche
investment
impacts
excluding
the divestment
gain
| |
Gain on
divestment
of our
investment
in Roche
| | Results
excluding
impacts
from the
divestment
of our Roche
investment | |
Results as
published
| |
Change
in USD
%
| |
Change in
constant
currencies
%
| |
|
Operating income from continuing operations | | 10 056 | | | | | | 10 056 | | 7 946 | | -21 | | -12 | |
|
Income from associated companies | | 15 337 | | -785 | | -14 556 | | -4 | | -11 | | nm | | nm | |
|
Interest expense | | -787 | | | | | | -787 | | -800 | | -2 | | -3 | |
|
Other financial income and expense | | -76 | | | | -16 | | -92 | | 42 | | nm | | nm | |
|
Income taxes | | -1 625 | | | | | | -1 625 | | -1 128 | | 31 | | | |
|
Net income from continuing operations | | 22 905 | | -785 | | -14 572 | | 7 548 | | 6 049 | | -20 | | -9 | |
|
Basic earnings per share (USD) from continuing operations | | 10.22 | | -0.35 | | -6.50 | | 3.37 | | 2.77 | | -18 | | -7 | |
|
Effective tax rate 2 | | 6.6% | | | | | | 17.7% | | 15.7% | | | | | |
|
| | | | | | | | | | | | | | | |
|
Non-IFRS measures | | | | | | | | | | | | | | | |
Core operating income from continuing operations | | 14 491 | | | | | | 14 491 | | 14 794 | | 2 | | 10 | |
|
Core income from associated companies | | 991 | | -995 | | | | -4 | | -11 | | nm | | nm | |
|
Core interest expense | | -787 | | | | | | -787 | | -800 | | -2 | | -3 | |
|
Core other financial income and expense | | -38 | | | | | | -38 | | 140 | | nm | | nm | |
|
Core income taxes | | -2 129 | | | | | | -2 129 | | -2 177 | | -2 | | -11 | |
|
Core net income from continuing operations | | 12 528 | | -995 | | | | 11 533 | | 11 946 | | 4 | | 13 | |
|
Core basic earnings per share (USD) from continuing operations | | 5.59 | | -0.45 | | | | 5.14 | | 5.48 | | 7 | | 16 | |
|
Core effective tax rate 3 | | 14.5% | | | | | | 15.6% | | 15.4% | | | | | |
|
| | | | | | | | | | | | | | | |
|
Free cash flow from continuing operations 4,5 | | 12 299 | | -522 | | | | 11 777 | | 12 123 | | 3 | | | |
|
|
1 For information on continuing operations and discontinued operations, refer to the Overview section above in this Item 5 and “Item 18. Financial Statements - Note 1. Accounting policies “, “Item 18. Financial Statements - Note 2. Significant transactions - Significant transactions in 2023,” and “Item 18. Financial Statements - Note 31. Discontinued operations.” |
2 Effective tax rate is calculated as Income taxes divided by Income before tax. |
3 Core effective tax rate is calculated as Core income taxes divided by Core income before tax. |
4 Effective January 1, 2023, Novartis revised its definition of free cash flow, to define free cash flow as net cash flows from operating activities less purchases of property, plant and equipment. To aid in comparability, the prior year free cash flow amounts have been revised to conform with the new free cash flow definition. See “-Non-IFRS measures as defined by Novartis." |
5 The free cash flow impact represents the dividend received in Q1 2021 from Roche in relation to the distribution of its 2020 net income. |
| | 2021 | |
| | | |
| | | |
| | | |
(USD millions)
| |
Free cash flow
as published1
| | Dividends
received from
Roche in
relation to
the distribution
of its 2020
net income2 | |
Free cash
flow excluding
dividends
received
from Roche
| |
|
Operating income from continuing operations | | 10 056 | | | | 10 056 | |
|
Adjustments for non-cash items | | 6 419 | | | | 6 419 | |
|
Operating income adjusted for non-cash items from continuing operations | | 16 475 | | | | 16 475 | |
|
Dividends received from associated companies and others | | 523 | | -522 | | 1 | |
|
Interest and other financial payments, net | | -929 | | | | -929 | |
|
Income taxes paid | | -1 856 | | | | -1 856 | |
|
Other operating cash flow items, net | | -848 | | | | -848 | |
|
Net cash flows from operating activities from continuing operations | | 13 365 | | -522 | | 12 843 | |
|
Purchases of property, plant and equipment | | -1 066 | | | | -1 066 | |
|
Free cash flow from continuing operations | | 12 299 | | -522 | | 11 777 | |
|
|
1 Effective January 1, 2023, Novartis revised its definition of free cash flow, to define free cash flow as net cash flows from operating activities less purchases of property, plant and equipment. To aid in comparability, the prior year free cash flow amounts have been revised to conform with the new free cash flow definition. See “-Non-IFRS measures as defined by Novartis." |
2 In 2021, the dividend received from Roche in relation to the distribution of its 2020 net income was received in Q1 2021. |
The following table provides a summary of the percentage point impact from excluding the effect of the divestment of our investment in Roche (in the fourth quarter of 2021) on the USD and constant currencies % change on key Company figures:
| | In USD | | In constant currencies | |
| | | | | |
| | | | | |
| | | | | |
| |
% change
as published
2022
| | % change
excluding
impacts
from the
divestment
of our Roche
investment
2022 | |
Percentage
point
impact
2022
| |
% change
as published
2022
| | % change
excluding
impacts
from the
divestment
of our Roche
investment
2022 | |
Percentage
point
impact
2022
| |
|
Net income from continuing operations | | -74 | | -20 | | -54 | | -70 | | -9 | | -61 | |
|
Basic earnings per share (USD) from continuing operations | | -73 | | -18 | | -55 | | -69 | | -7 | | -62 | |
|
Free cash flow from continuing operations | | -1 | | 3 | | -4 | | | | | | | |
|
Core net income from continuing operations | | -5 | | 4 | | -9 | | 4 | | 13 | | -9 | |
|
Core basic earnings per share (USD) from continuing operations | | -2 | | 7 | | -9 | | 6 | | 16 | | -10 | |
|
5.B Liquidity and capital resources
The following tables summarize the Company’s cash flows and net debt:
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
Net cash flows from operating activities from continuing operations | | 14 220 | | 13 039 | | 13 365 | |
|
Net cash flows from operating activities from discontinued operations | | 238 | | 1 197 | | 1 706 | |
|
Net cash flows from investing activities from continuing operations | | 6 719 | | 1 904 | | 4 897 | |
|
Net cash flows used in investing activities from discontinued operations | | -1 123 | | -436 | | -689 | |
|
Net cash flows used in financing activities from continuing operations | | -17 564 | | -20 681 | | -16 290 | |
|
Net cash flows from financing activities from discontinued operations | | 3 286 | | 119 | | 26 | |
|
Effect of exchange rate changes on cash and cash equivalents | | 100 | | -32 | | -266 | |
|
Net change in cash and cash equivalents | | 5 876 | | -4 890 | | 2 749 | |
|
Change in marketable securities, commodities, time deposits and derivative financial instruments | | -10 378 | | -4 509 | | 14 017 | |
|
Change in current and non-current financial debts and derivative financial instruments | | 1 564 | | 3 022 | | 6 847 | |
|
Change in net debt | | -2 938 | | -6 377 | | 23 613 | |
|
Net debt at January 1 | | -7 245 | | -868 | | -24 481 | |
|
Net debt at December 31 | | -10 183 | | -7 245 | | -868 | |
|
Cash flow
Financial year 2023 compared with 2022
Net cash flows from operating activities from continuing operations amounted to USD 14.2 billion, compared with USD 13.0 billion in 2022. This increase was mainly driven by higher net income from continuing operations adjusted for non-cash items and other adjustments, including divestment gains, which were partly offset by higher income taxes paid, mainly due to the timing of payments.
Net cash flows from operating activities from discontinued operations amounted to USD 0.2 billion, compared with USD 1.2 billion in 2022. This decrease was mainly driven by lower net income from discontinued operations adjusted for non-cash items and other adjustments, including divestment gains and the Distribution (spin-off) of the Sandoz business on October 3, 2023.
Net cash inflows from investing activities from continuing operations amounted to USD 6.7 billion, compared with USD 1.9 billion in 2022.
The current year net cash inflows from investing activities from continuing operations were driven by net proceeds of USD 10.6 billion from the sale of marketable securities, commodities and time deposits; USD 2.0 billion from the sale of intangible assets (including USD 1.75 billion cash proceeds from the divestment of the ‘front of eye’ ophthalmology assets to Bausch + Lomb); USD 0.3 billion from the sale of financial assets; and USD 0.2 billion from the sale of property, plant and equipment (including proceeds from the sale and leaseback of real estate). These cash inflows were partly offset by cash outflows of USD 3.6 billion for acquisitions and divestments of businesses, net (including the acquisition of Chinook Therapeutics, Inc. for USD 3.1 billion, net of cash acquired USD 0.1 billion, and the acquisition of DTx Pharma Inc. for USD 0.5 billion, net of cash acquired USD 0.1 billion); USD 1.7 billion for purchases of intangible assets; USD 1.1 billion for purchases of property, plant and equipment; and USD 0.1 billion for purchases of financial assets.
In 2022, net cash inflows from investing activities from continuing operations of USD 1.9 billion were mainly driven by net proceeds of USD 4.7 billion from the sale of marketable securities, commodities and time deposits; and USD 0.5 billion from the sale of intangible assets, financial assets and property, plant and equipment. These cash inflows were partly offset by cash outflows of USD 1.3 billion for purchases of intangible assets; USD 0.9 billion for purchases of property, plant and equipment; USD 0.1 billion for purchases of financial assets; and USD 0.8 billion for acquisitions and divestments of businesses, net (primarily the acquisition of Gyroscope Therapeutics Holdings plc for USD 0.8 billion).
Net cash outflows used in investing activities from discontinued operations amounted to USD 1.1 billion, compared with USD 0.4 billion in 2022. The current year mainly includes the cash outflow of USD 0.7 billion due to the derecognition of cash and cash equivalents of the Sandoz business following the Distribution (spin-off) on October 3, 2023.
Net cash outflows used in financing activities from continuing operations amounted to USD 17.6 billion, compared with USD 20.7 billion in 2022.
The current year net cash outflows used in financing activities from continuing operations were mainly driven by USD 8.6 billion for net treasury share transactions; USD 7.3 billion for the dividend payment; USD 2.2 billion for the repayment of two EUR denominated bonds (notional amounts of EUR 1.25 billion and of EUR 0.75 billion) at maturity. Payments of lease liabilities amounted to USD 0.3 billion. These cash outflows were partly offset by cash inflows of USD 0.5 billion from the net increase in current financial debts.
In 2022, net cash outflows used in financing activities from continuing operations of USD 20.7 billion were
mainly driven by USD 10.6 billion for net treasury share transactions; USD 7.5 billion for the dividend payment; USD 2.5 billion in aggregate for the repayment of two US dollar bonds; and USD 0.3 billion payments of lease liabilities. These cash outflows were partly offset by cash inflows of USD 0.3 billion from the net increase in current financial debts.
The current year net cash inflows from financing activities from discontinued operations of USD 3.3 billion were mainly driven by USD 3.6 billion cash inflows from bank borrowings (including the USD 3.3 billion Sandoz business borrowings from a group of banks on September 28, 2023) in connection with the Distribution (spin-off) of the Sandoz business to Novartis AG shareholders, partly offset by transaction cost payments of USD 0.2 billion. Net cash inflows from financing activities from discontinued operations in 2022 were USD 119 million.
Financial year 2022 compared with 2021
Net cash flows from operating activities from continuing operations amounted to USD 13.0 billion, compared with USD 13.4 billion in 2021. This decrease was mainly due to unfavorable changes in working capital and lower dividends from associated companies (2021 included the USD 0.5 billion dividends received from our investment in Roche, which was divested in the fourth quarter of 2021), partly offset by lower income taxes paid, higher interest received and favorable hedging results.
Net cash flows from operating activities from discontinued operations amounted to USD 1.2 billion, compared with USD 1.7 billion in 2021. This decrease was mainly driven by lower net income from discontinued operations adjusted for non-cash items and other adjustments, including divestment gains and unfavorable changes in working capital, which were partly offset by lower income taxes paid and lower payments out of provisions.
Net cash inflows from investing activities from continuing operations amounted to USD 1.9 billion, compared with USD 4.9 billion in 2021.
In 2022, net cash inflows from investing activities from continuing operations were mainly driven by net proceeds of USD 4.7 billion from the sale of marketable securities, commodities and time deposits; and USD 0.5 billion from the sale of intangible assets, financial assets and property, plant and equipment. These cash inflows were partly offset by cash outflows of USD 1.3 billion for purchases of intangible assets; USD 0.9 billion for purchases of property, plant and equipment; USD 0.1 billion for purchases of financial assets; and USD 0.8 billion for acquisitions and divestments of businesses, net (primarily the acquisition of Gyroscope Therapeutics Holdings plc for USD 0.8 billion).
In 2021, net cash inflows from investing activities from continuing operations of USD 4.9 billion were mainly driven by proceeds of USD 20.7 billion from the divestment of our investment in Roche; USD 2.3 billion from the sale of marketable securities, commodities and time deposits; and USD 1.3 billion from the sale of intangible assets, financial assets and property, plant and equipment. These cash inflows were partly offset by USD 16.4 billion cash outflows for purchases of marketable securities and time deposits, mainly due to the investment of a portion of the proceeds from the divestment of our investment in Roche; USD 1.5 billion for purchases of intangible assets (including the upfront payment to in-license tislelizumab from an affiliate of BeiGene, Ltd); USD 1.1 billion for purchases of property, plant and equipment; USD 0.2 billion for acquisitions and divestments of businesses, net; and USD 0.2 billion for purchases of financial assets.
Net cash outflows used in investing activities from discontinued operations amounted to USD 0.4 billion, compared with USD 0.7 billion in 2021. The 2021 amount includes the acquisition of GSK’s cephalosporin antibiotics business for USD 351 million.
Net cash outflows used in financing activities from continuing operations amounted to USD 20.7 billion, compared with USD 16.3 billion in 2021.
In 2022, net cash outflows used in financing activities from continuing operations were mainly driven by USD 10.6 billion for net treasury share transactions; USD 7.5 billion for the dividend payment; USD 2.5 billion in aggregate for the repayment of two US dollar bonds; and USD 0.3 billion payments of lease liabilities. These cash outflows were partly offset by cash inflows of USD 0.3 billion from the net increase in current financial debts.
In 2021, net cash outflows used in financing activities from continuing operations of USD 16.3 billion were driven by USD 7.4 billion for the dividend payment; USD 3.0 billion for net treasury share transactions; USD 3.5 billion net decrease in current financial debts; and USD 2.2 billion for the repayment of two EUR denominated bonds (notional amount of EUR 1.25 billion and of EUR 0.6 billion) at maturity. Payments of lease liabilities and other financing cash flows resulted in a net cash outflow of USD 0.2 billion.
Net cash inflows from financing activities from discontinued operations amounted to USD 119 million, compared with USD 26 million in 2021.
Free cash flow
Free cash flow is a non-IFRS measure, see “—Item 5.A Operating results—Non-IFRS measures as defined by Novartis—Free cash flow” for further information.
The following table is a reconciliation of the three major categories of the IFRS Accounting Standards consolidated statements of cash flows to the non-IFRS measure free cash flow:
| | 2023 | | 2022 | | 2021 | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
(USD millions)
| | IFRS
Accounting
Standards
cash flow | |
Adjustments
| |
Free
cash flow
| | IFRS
Accounting
Standards
cash flow | |
Adjustments1
| |
Revised
Free
cash flow1
| | IFRS
Accounting
Standards
cash flow | |
Adjustments1
| |
Revised
Free
cash flow1
| |
|
Net cash flows from operating activities from continuing operations | | 14 220 | | | | 14 220 | | 13 039 | | | | 13 039 | | 13 365 | | | | 13 365 | |
|
Net cash flows from operating activities from discontinued operations | | 238 | | | | 238 | | 1 197 | | | | 1 197 | | 1 706 | | | | 1 706 | |
|
Total net cash flows from operating activities | | 14 458 | | | | 14 458 | | 14 236 | | | | 14 236 | | 15 071 | | | | 15 071 | |
|
| | | | | | | | | | | | | | | | | | | |
|
Net cash flows from/(used in) investing activities from continuing operations | | 6 719 | | -7 779 | | -1 060 | | 1 904 | | -2 820 | | -916 | | 4 897 | | -5 963 | | -1 066 | |
|
Net cash flows used in investing activities from discontinued operations | | -1 123 | | 904 | | -219 | | -436 | | 154 | | -282 | | -689 | | 377 | | -312 | |
|
Total net cash flows from/(used in) investing activities 2 | | 5 596 | | -6 875 | | -1 279 | | 1 468 | | -2 666 | | -1 198 | | 4 208 | | -5 586 | | -1 378 | |
|
| | | | | | | | | | | | | | | | | | | |
|
Net cash flows used in financing activities from continuing operations | | -17 564 | | 17 564 | | 0 | | -20 681 | | 20 681 | | 0 | | -16 290 | | 16 290 | | 0 | |
|
Net cash flows from financing activities from discontinued operations | | 3 286 | | -3 286 | | 0 | | 119 | | -119 | | 0 | | 26 | | -26 | | 0 | |
|
Total net cash flows used in financing activities 3 | | -14 278 | | 14 278 | | 0 | | -20 562 | | 20 562 | | 0 | | -16 264 | | 16 264 | | 0 | |
|
| | | | | | | | | | | | | | | | | | | |
|
Non-IFRS measure free cash flow from continuing operations 1 | | | | | | 13 160 | | | | | | 12 123 | | | | | | 12 299 | |
|
Non-IFRS measure free cash flow from discontinued operations 1 | | | | | | 19 | | | | | | 915 | | | | | | 1 394 | |
|
Total non-IFRS measure free cash flow 1 | | | | | | 13 179 | | | | | | 13 038 | | | | | | 13 693 | |
|
|
1 To aid in comparability, the prior year adjustments and free cash flow amounts have been revised to conform with the new free cash flow definition that was effective as of January 1, 2023. |
2 With the exception of purchases of property, plant and equipment, all net cash flows from/(used in) investing activities from continuing operations and from discontinued operations are excluded from the free cash flow. |
3 Net cash flows (used in)/from financing activities from continuing operations and from discontinued operations are excluded from the free cash flow. |
|
The following table is a summary of the non-IFRS measure free cash flow:
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
Operating income from continuing operations | | 9 769 | | 7 946 | | 10 056 | |
|
Reversal of non-cash items and other adjustments | | | | | | | |
Depreciation, amortization and impairments | | 8 383 | | 6 965 | | 5 559 | |
|
Change in provisions and other non-current liabilities | | 61 | | 1 318 | | 806 | |
|
Other | | 728 | | 451 | | 54 | |
|
Operating income from continuing operations adjusted for non-cash items | | 18 941 | | 16 680 | | 16 475 | |
|
Dividends received from associated companies and others | | 2 | | 1 | | 523 | |
|
Interest received and other financial receipts | | 735 | | 323 | | 11 | |
|
Interest paid and other financial payments | | -768 | | -693 | | -940 | |
|
Income taxes paid | | -2 787 | | -1 702 | | -1 856 | |
|
Payments out of provisions and other net cash movements in non-current liabilities | | -1 534 | | -774 | | -775 | |
|
Change in inventories and trade receivables less trade payables | | -1 571 | | -1 138 | | -565 | |
|
Change in other net current assets and other operating cash flow items | | 1 202 | | 342 | | 492 | |
|
Net cash flows from operating activities from continuing operations | | 14 220 | | 13 039 | | 13 365 | |
|
Purchases of property, plant and equipment | | -1 060 | | -916 | | -1 066 | |
|
Non-IFRS measure free cash flow from continuing operations 1 | | 13 160 | | 12 123 | | 12 299 | |
|
Non-IFRS measure free cash flow from discontinued operations 1, 2 | | 19 | | 915 | | 1 394 | |
|
Total non-IFRS measure free cash flow 1 | | 13 179 | | 13 038 | | 13 693 | |
|
|
1 To aid in comparability, the prior year free cash flow amounts have been revised to conform with the new free cash flow definition that was effective as of January 1, 2023. |
2 In 2023, the free cash flow from discontinued operations was a cash inflow of USD 19 million (2022: USD 915 million, 2021: USD 1 394 million) consisting of USD 238 million (2022: USD 1 197 million, 2021: USD 1 706 million) net cash inflows from operating activities from discontinued operations, less purchases of property, plant and equipment by discontinued operations of USD 219 million (2022: USD 282 million, 2021: USD 312 million). |
| |
Financial year 2023 compared with 2022
Free cash flow from continuing operations amounted to USD 13.2 billion (+9% USD), compared with USD 12.1 billion in 2022, driven by higher net cash flows from operating activities from continuing operations.
For the total Company, free cash flow amounted to USD 13.2 billion, compared with USD 13.0 billion in 2022.
Financial year 2022 compared with 2021
Free cash flow from continuing operations amounted to USD 12.1 billion, broadly in line with USD 12.3 billion in 2021.
For the total Company, free cash flow amounted to USD 13.0 billion, compared with USD 13.7 billion in 2021.
Condensed consolidated balance sheets
(USD millions) | | Dec 31, 2023 | | Dec 31, 2022 | |
|
Assets | | | | | |
|
Property, plant and equipment | | 9 514 | | 10 764 | |
|
Right-of-use assets | | 1 410 | | 1 431 | |
|
Goodwill | | 23 341 | | 29 301 | |
|
Intangible assets other than goodwill | | 26 879 | | 31 644 | |
|
Investments in associated companies | | 205 | | 143 | |
|
Deferred tax assets | | 4 309 | | 3 739 | |
|
Financial assets and other non-current assets | | 3 806 | | 3 521 | |
|
Total non-current assets | | 69 464 | | 80 543 | |
|
Inventories | | 5 913 | | 7 175 | |
|
Trade receivables | | 7 107 | | 8 066 | |
|
Other current assets and income tax receivables | | 3 033 | | 2 739 | |
|
Marketable securities, commodities, time deposits and derivative financial instruments | | 1 035 | | 11 413 | |
|
Cash and cash equivalents | | 13 393 | | 7 517 | |
|
Total current assets | | 30 481 | | 36 910 | |
|
Total assets | | 99 945 | | 117 453 | |
|
Equity and liabilities | | | | | |
|
Total equity | | 46 750 | | 59 423 | |
|
Liabilities | | | | | |
|
Financial debts | | 18 436 | | 20 244 | |
|
Lease liabilities | | 1 598 | | 1 538 | |
|
Deferred tax liabilities | | 2 248 | | 2 686 | |
|
Provisions and other non-current liabilities | | 4 523 | | 4 906 | |
|
Total non-current liabilities | | 26 805 | | 29 374 | |
|
Trade payables | | 4 926 | | 5 146 | |
|
Financial debts and derivative financial instruments | | 6 175 | | 5 931 | |
|
Lease liabilities | | 230 | | 251 | |
|
Provisions and other current liabilities and current income tax liabilities | | 15 059 | | 17 328 | |
|
Total current liabilities | | 26 390 | | 28 656 | |
|
Total liabilities | | 53 195 | | 58 030 | |
|
Total equity and liabilities | | 99 945 | | 117 453 | |
|
|
There has been a significant change to the December 31, 2023 consolidated balance sheet resulting from the presentation of the Sandoz business as discontinued operations. This follows the September 15, 2023 shareholders’ approval to spin off the Sandoz business through a dividend in kind distribution to the Novartis AG share- holders (for further information see Item 18. Financial Statements – Note 1. Accounting policies; and – Note 2. Significant transactions).
The December 31, 2022 consolidated balance sheet includes the assets and liabilities of the Sandoz business. The December 31, 2023 consolidated balance sheet excludes the assets and liabilities of the Sandoz business in the individual lines, due to the derecognition of the Sandoz business at the date of the October 3, 2023 distribution (spin-off).
The consolidated balance sheet discussion and analysis that follows excludes the impacts of the derecognition of the Sandoz business assets and liabilities at the date of the distribution (spin-off). For information on the assets and liabilities of the Sandoz business derecognized at October 3, 2023, the distribution (spin-off) date, see Item 18. Financial Statements – Note 31 – Discontinued operations – Net assets derecognized.”
Assets
Total non-current assets of USD 69.5 billion increased by USD 0.5 billion compared with December 31, 2022, excluding the impact of the derecognition of the Sandoz business non-current assets related to discontinued operations.
Intangible assets other than goodwill decreased by USD 3.3 billion mainly due to amortization and impairments and the divestment of the ‘front of eye’ ophthalmology assets, partially offset by the impact of acquisitions, including Chinook Therapeutics, Inc. and of DTx Pharma Inc., additions, and favorable currency translation adjustments.
Goodwill increased by USD 1.5 billion mainly due to the acquisition of Chinook Therapeutics, Inc and DTx Pharma Inc.
Deferred tax assets increased by USD 1.3 billion mainly due to higher deferred tax assets on intangible assets, inventory and tax loss carryforwards. Property, plant and equipment increased by USD 0.6 billion mainly
as additions and favorable currency translation adjustments exceeded depreciation charge and disposals. Right-of-use assets, investments in associated companies, financial assets, and other non-current assets were broadly in line with December 31, 2022.
Total current assets of USD 30.5 billion decreased by USD 1.7 billion, compared with December 31, 2022, excluding the impact of the derecognition of the Sandoz business non-current assets related to discontinued operations.
Cash and cash equivalents, marketable securities, commodities, time deposits and derivative financial instruments decreased by USD 4.4 billion mainly due to the dividend payment, and net purchases of treasury shares and intangible assets, partially offset by the cash generated through operating activities.
Inventories increased by USD 0.9 billion. Trade receivables increased by USD 1.3 billion, mainly due to the increase in net sales. Other current assets and income tax receivables were broadly in line with December 31, 2022.
We consider our provisions for doubtful trade receivables to be adequate. We particularly monitor the level of trade receivables in countries deemed to have an elevated credit risk. We consider macroeconomic environment, historical experience, country and political risk, in addition to other relevant information when assessing risk. These risk factors are monitored regularly to determine any adjustments in risk classification. The majority of the past due trade receivables from elevated credit risk countries are due from local governments or from government-funded entities. Deteriorating credit and economic conditions as well as other factors in these elevated credit risk countries have resulted in, and may continue to result in, an increase in the average length of time that it takes to collect these trade receivables and may require the Company to re-evaluate the expected credit loss amount of these trade receivables in future periods. As at December 31, 2023, amounts past due for more than one year were not significant in elevated credit risk countries.
For a table showing an overview of the aging analysis of total trade receivables and the total amount of the provision for doubtful trade receivables as at December 31, 2023, and 2022, see “Item 18. Financial Statements—Note 16. Trade receivables.”
There is also a risk that certain countries could devalue their currency. Currency exposures are described in more detail in “—Effects of currency fluctuations.”
Liabilities
Total non-current liabilities of USD 26.8 billion decreased by USD 1.7 billion, compared with December 31, 2022, excluding the impact of the derecognition of the Sandoz business non-current liabilities related to discontinued operations.
Non-current financial debts decreased by USD 1.8 billion mainly due to the reclassification of USD 2.1 billion from non-current to current financial debts of a USD denominated bond with notional amount of USD 2.2 billion maturing in 2024.
Non-current lease liabilities, deferred tax liabilities and provisions and other non-current liabilities were broadly in line with December 31, 2022.
Total current liabilities of USD 26.4 billion increased by USD 1.5 billion, compared with December 31, 2022, excluding the impact of the derecognition of the Sandoz business non-current liabilities related to discontinued operations.
Current financial debts and derivative financial instruments were broadly in line with December 31, 2022, as the repayment of a 0.5% coupon bond with a notional amount of EUR 750 million and a 0.125% coupon bond with a notional amount of EUR 1.25 billion was largely offset by the reclassification of USD 2.1 billion from non-current to current financial debts of a USD denominated bond with notional amount of USD 2.2 billion maturing in 2024.
Provisions and other current liabilities increased by USD 0.6 billion, mainly driven by an increase of the provisions for deductions from revenue. Trade payables increased by USD 0.9 billion. Current income tax liabilities and current lease liabilities were broadly in line with December 31, 2022.
In our key countries, Switzerland and the United States, assessments have been agreed by the tax authorities up to 2019 in Switzerland and up to 2016 in the United States, with the exception of one open United States position related to the 2007 tax filing. Uncertainties also exist on the application of a taxing right based on a German non-resident tax regulation for specific revenues derived from German registered intellectual property rights.
Novartis believes that its total provisions are adequate based upon currently available information. However, given the inherent difficulties in estimating liabilities in this area, Novartis may incur additional costs beyond the amounts provided. Management believes that such additional amounts, if any, would not be material to the Company’s financial condition but could be material to the results of operations or cash flows in a given period.
Equity
The Company’s equity decreased by USD 12.7 billion to USD 46.8 billion, compared with December 31, 2022.
This decrease was mainly due to the dividend in kind to effect the distribution (spin-off) of Sandoz Group AG to the Novartis AG shareholders’ of USD 14.0 billion (for further information see “Item 18. Financial Statements – Note 2. Significant transactions, and Note 31 – Discontinued operations”), the cash-dividend payment of USD 7.3 billion and the purchase of treasury shares of USD 8.5 billion. This was partially offset by the net income of USD 14.9 billion, and equity-based compensation of USD 0.9 billion.
Summary of equity movements attributable to Novartis AG shareholders
| | Number of outstanding shares (in millions) | | Equity attributable to Novartis AG shareholders | |
| | | | | |
| | | | | |
| | | | | |
| |
2023
| |
2022
| | 2023
USD millions | | 2022
USD millions | |
|
Balance at beginning of year | | 2 119.6 | | 2 234.9 | | 59 342 | | 67 655 | |
|
Shares acquired to be canceled | | -87.5 | | -126.2 | | -8 369 | | -10 787 | |
|
Other share purchases | | -1.6 | | -1.4 | | -148 | | -123 | |
|
Exercise of options and employee transactions | | 2.8 | | 1.9 | | 146 | | 88 | |
|
Equity-based compensation | | 10.4 | | 10.4 | | 904 | | 854 | |
|
Shares delivered to Alcon employees as a result of the Alcon spin-off | | | | 0.0 | | | | 5 | |
|
Shares delivered to Sandoz employees as a result of the Sandoz spin-off | | 0.3 | | | | 30 | | | |
|
Taxes on treasury share transactions | | | | | | 14 | | 14 | |
|
Decrease of treasury share repurchase obligation under a share buyback trading plan | | | | | | | | 2 809 | |
|
Transaction costs, net of taxes | | | | | | -214 | | | |
|
Dividends | | | | | | -7 255 | | -7 506 | |
|
Dividend in kind to effect the spin-off of Sandoz | | | | | | -13 962 | | | |
|
Net income of the year attributable to shareholders of Novartis AG | | | | | | 14 850 | | 6 955 | |
|
Other comprehensive income attributable to shareholders of Novartis AG | | | | | | 1 200 | | -839 | |
|
Other movements | | | | | | 129 | | 217 | |
|
Balance at end of year | | 2 044.0 | | 2 119.6 | | 46 667 | | 59 342 | |
|
|
In 2023, Novartis repurchased a total of 87.5 million shares for USD 8.4 billion on the SIX Swiss Exchange second trading line. These repurchases included 52.8 million shares (USD 4.9 billion) under the USD 15 billion share buyback (announced in December 2021 and completed in June 2023) and 23.0 million shares (USD 2.3 billion) under the new up-to USD 15 billion share buyback announced in July 2023. In addition, 11.7 million shares (USD 1.2 billion) were repurchased to mitigate dilution related to participation plans of associates. Furthermore, 1.6 million shares (for an equity value of USD 0.1 billion) were repurchased from associates. In the same period, 13.5 million shares (for an equity value of USD 1.1 billion) were delivered as a result of options exercised and share deliveries related to participation plans of associates. Consequently, the total number of shares outstanding decreased by 75.6 million versus December 31, 2022. These treasury share transactions resulted in an equity decrease of USD 7.4 billion and a net cash outflow of USD 8.6 billion.
In 2022, Novartis repurchased a total of 126.2 million shares for USD 10.8 billion on the SIX Swiss Exchange second trading line, including 115.3 million shares (USD 9.9 billion) under the up-to USD 15 billion share buyback announced in December 2021 and 10.9 million shares (USD 0.9 billion) to mitigate dilution related to participation plans of associates. In addition, 1.4 million shares (USD 0.1 billion) were repurchased from associates. In the same period, 12.3 million shares (for an equity value of USD 0.9 billion) were delivered as a result of option exercises and share deliveries related to participation plans of associates. Consequently, the total number of shares outstanding decreased by 115.3 million versus December 31, 2021. These treasury share transactions resulted in a decrease in equity of USD 10.0 billion and a net cash outflow of USD 10.6 billion.
Treasury shares
As at December 31, 2023, our holding of treasury shares amounted to 233.5 million shares, or approximately 10% of the total number of issued shares. Approximately 93.8 million treasury shares were held in entities that restrict their availability for use.
As at December 31, 2022, our holding of treasury shares amounted to 284.1 million shares, or approximately 12% of the total number of issued shares. Approximately 99.0 million treasury shares were held in entities that restrict their availability for use.
Effects of currency fluctuations
We transact our business in many currencies other than the US dollar, our reporting currency.
The following table provides an overview of net sales from continuing operations and operating expenses based on IFRS Accounting Standards values for 2023, 2022 and 2021, for currencies most important to the Company:
| | 2023 | | 2022 | | 2021 | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
Currency
| |
Net sales
from
continuing
operations
%
| | Operating
expenses
from
continuing
operations
%1 | |
Net sales
from
continuing
operations
%
| | Operating
expenses
from
continuing
operations
%1 | |
Net sales
from
continuing
operations
%
| | Operating
expenses
from
continuing
operations
%1 | |
|
US dollar (USD) | | 42 | | 37 | | 40 | | 38 | | 38 | | 37 | |
|
Euro (EUR) | | 24 | | 20 | | 25 | | 21 | | 27 | | 23 | |
|
Swiss franc (CHF) | | 1 | | 22 | | 2 | | 22 | | 2 | | 20 | |
|
Chinese yuan (CNY) | | 7 | | 4 | | 7 | | 4 | | 7 | | 4 | |
|
Japanese yen (JPY) | | 4 | | 2 | | 4 | | 2 | | 5 | | 3 | |
|
Canadian dollar (CAD) | | 2 | | 1 | | 2 | | 1 | | 2 | | 1 | |
|
British pound (GBP) | | 2 | | 5 | | 2 | | 2 | | 3 | | 2 | |
|
Russian ruble (RUB) | | 1 | | 0 | | 2 | | 1 | | 1 | | 1 | |
|
Brazilian real (BRL) | | 2 | | 1 | | 2 | | 1 | | 1 | | 1 | |
|
Australian dollar (AUD) | | 1 | | 0 | | 1 | | 1 | | 1 | | 1 | |
|
Other currencies | | 14 | | 8 | | 13 | | 7 | | 13 | | 7 | |
|
|
1 Operating expenses include cost of goods sold; selling, general and administration; research and development; other income and other expense. |
We prepare our consolidated financial statements in US dollars. As a result, fluctuations in the exchange rates between the US dollar and other currencies can have a significant effect on both the Company’s results of operations as well as the reported value of our assets, liabilities and cash flows. This in turn may significantly affect reported earnings (both positively and negatively) and the comparability of period-to-period results of operations.
For purposes of our consolidated balance sheets, we translate assets and liabilities denominated in other currencies into US dollars at the prevailing market exchange rates as of the relevant balance sheet date. For purposes of the Company’s consolidated income and cash flow statements, revenue, expense and cash flow items in local currencies are translated into US dollars at average exchange rates prevailing during the relevant period. As a result, even if the amounts or values of these items remain unchanged in the respective local currency, changes in exchange rates have an impact on the amounts or values of these items in our consolidated financial statements.
Because our expenditure in Swiss francs is significantly higher than our revenue in Swiss francs, volatility in the value of the Swiss franc can have a significant impact on the reported value of our earnings, assets and liabilities, and the timing and extent of such volatility can be difficult to predict.
The Company manages its global currency exposure by engaging in hedging transactions where management deems appropriate, after taking into account the natural hedging afforded by our global business activity. In 2023 and 2022, we entered into various contracts that change in value with movements in foreign exchange rates, to preserve the value of assets, commitments and expected transactions. We use forward contracts and foreign currency options to hedge. For more information on how these transactions affect our consolidated financial statements and on how foreign exchange rate exposure is managed, see “Item 18. Financial Statements—Note 1. Accounting policies,” “Item 18. Financial Statements—Note 6. Interest expense and other financial income and expense,” “Item 18. Financial Statements—Note 16. Trade receivables,” “Item 18. Financial Statements—Note 29. Commitments and contingent liabilities” and “Item 18. Financial Statements—Note 30. Financial instruments – additional disclosures.”
The following table sets forth the foreign exchange rates of the US dollar against key currencies used for foreign currency translation when preparing the Company’s consolidated financial statements:
| | Average for year | | Year-end | |
| | | | | |
| | | | | |
| | | | | |
USD per unit | | 2023 | | 2022 | | Change in % | | 2023 | | 2022 | | Change in % | |
|
Australian dollar (AUD) | | 0.665 | | 0.695 | | -4 | | 0.683 | | 0.678 | | 1 | |
|
Brazilian real (BRL) | | 0.200 | | 0.194 | | 3 | | 0.206 | | 0.189 | | 9 | |
|
Canadian dollar (CAD) | | 0.741 | | 0.769 | | -4 | | 0.755 | | 0.738 | | 2 | |
|
Swiss franc (CHF) | | 1.113 | | 1.048 | | 6 | | 1.189 | | 1.081 | | 10 | |
|
Chinese yuan (CNY) | | 0.141 | | 0.149 | | -5 | | 0.141 | | 0.144 | | -2 | |
|
Euro (EUR) | | 1.082 | | 1.054 | | 3 | | 1.107 | | 1.065 | | 4 | |
|
British pound (GBP) | | 1.243 | | 1.237 | | 0 | | 1.275 | | 1.207 | | 6 | |
|
Japanese yen (JPY (100)) | | 0.713 | | 0.766 | | -7 | | 0.707 | | 0.757 | | -7 | |
|
Russian ruble (RUB (100)) | | 1.185 | | 1.481 | | -20 | | 1.111 | | 1.380 | | -19 | |
|
The following table provides a summary of the currency impact on key Company figures due to their conversion into US dollars, the Company’s reporting currency. For additional information on the constant currency calculation (“cc”), see “—Item 5.A Operating results—Non-IFRS measures as defined by Novartis—Constant currencies.”
Currency impact on key figures
| |
Change in
USD %
2023
| | Change in
constant
currencies %
2023 | | Percentage
point currency
impact
2023 | |
Change in
USD %
2022
| | Change in
constant
currencies %
2022 | | Percentage
point currency
impact
2022 | |
|
Net sales from continuing operations | | 8 | | 10 | | -2 | | -1 | | 5 | | -6 | |
|
Operating income from continuing operations | | 23 | | 39 | | -16 | | -21 | | -12 | | -9 | |
|
Net income from continuing operations | | 42 | | 62 | | -20 | | -74 | | -70 | | -4 | |
|
Basic earnings per share (USD) from continuing operations | | 49 | | 70 | | -21 | | -73 | | -69 | | -4 | |
|
Core operating income from continuing operations | | 11 | | 18 | | -7 | | 2 | | 10 | | -8 | |
|
Core net income from continuing operations | | 13 | | 19 | | -6 | | -5 | | 4 | | -9 | |
|
Core basic earnings per share (USD) from continuing operations | | 18 | | 25 | | -7 | | -2 | | 6 | | -8 | |
|
| | | | | | | | | | | | | |
nm = not meaningful |
For additional information on the effects of currency fluctuations, see “Item 18. Financial Statements—Note 30. Financial instruments – additional disclosures.”
Company liquidity, financial debts and net debt
The following table shows Company liquidity, financial debts and net debt:
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
Non-current financial debts | | -18 436 | | -20 244 | | -22 902 | |
|
Current financial debts and derivative financial instruments | | -6 175 | | -5 931 | | -6 295 | |
|
Total financial debts | | -24 611 | | -26 175 | | -29 197 | |
|
Less liquidity | | | | | | | |
|
Cash and cash equivalents | | 13 393 | | 7 517 | | 12 407 | |
|
Marketable securities, commodities, time deposits and derivative financial instruments | | 1 035 | | 11 413 | | 15 922 | |
|
Total liquidity | | 14 428 | | 18 930 | | 28 329 | |
|
Net debt at December 31 1 | | -10 183 | | -7 245 | | -868 | |
|
|
1 For further information about the net debt measure, which is a non-IFRS measure, see “—Item 5.A Operating results—Non-IFRS measures as defined by Novartis—Net debt.” |
Financial year 2023
The Company’s net debt as at December 31, 2023, increased to USD 10.2 billion, compared with USD 7.2 billion as at December 31, 2022.
Total financial debts amounted to USD 24.6 billion as at December 31, 2023, compared with USD 26.2 billion as at December 31, 2022. Non-current financial debts decreased by USD 1.8 billion mainly due to the reclassification of USD 2.1 billion from non-current to current financial debts of a USD denominated bond with notional amount of USD 2.2 billion maturing in 2024.
Current financial debts and derivative financial instruments were broadly in line with December 31, 2022, as the repayment of a 0.5% coupon bond with a notional amount of EUR 750 million and a 0.125% coupon bond with a notional amount of EUR 1.25 billion was largely offset by the reclassification of USD 2.1 billion from non-current to current financial debts of a USD denominated bond with notional amount of USD 2.2 billion maturing in 2024.
Novartis has two US commercial paper programs under which it can issue up to USD 9.0 billion in the aggregate of unsecured commercial paper notes. Novartis also has a Japanese commercial paper program under which it can issue up to JPY 150 billion (approximately USD 1.1 billion) of unsecured commercial paper notes. Commercial paper notes totaling USD 3.3 billion under these three programs were outstanding as per December 31, 2023 (2022: USD 2.8 billion).
Novartis also has a committed credit facility of USD 6.0 billion. This credit facility is provided by a syndicate of banks and is intended to be used as a backstop for the US commercial paper programs. The facility matures in September 2025 and was undrawn as per December 31, 2023, and December 31, 2022.
Total liquidity decreased to USD 14.4 billion compared with USD 18.9 billion as at December 31, 2022.
As of year-end 2023, Moody’s Investors Service rated the Company A1 for long-term maturities and P-1 for short-term maturities and S&P Global Ratings rated the company AA- for long-term maturities and A-1+ for short-term maturities.
Financial year 2022
The Company’s net debt as at December 31, 2022, increased to USD 7.2 billion, compared with USD 0.9 billion as at December 31, 2021.
Total financial debts amounted to USD 26.2 billion as at December 31, 2022, compared with USD 29.2 billion as at December 31, 2021. Non-current financial debts decreased by USD 2.7 billion, mainly due to the reclassification of USD 2.3 billion from non-current to current financial debts of two EUR denominated bonds with notional amounts of EUR 750 million and EUR 1.25 billion maturing in 2023 and favorable foreign currency translation adjustments of USD 0.4 billion.
Current financial debts and derivative financial instruments decreased by USD 0.4 billion, mainly due to the repayment of two US dollar bonds of USD 1.0 billion and USD 1.5 billion, the closure during the third quarter of 2022 of the interest-bearing accounts of employees payable on demand, which amounted to USD 1.8 billion as at December 31, 2021, and favorable currency translation adjustments, partly offset by the reclassification from non-current to current financial debts of USD 2.3 billion and an increase of USD 1.9 billion in commercial paper.
Novartis has two US commercial paper programs under which it can issue up to USD 9.0 billion in the aggregate of unsecured commercial paper notes. Novartis also has a Japanese commercial paper program under which it can issue up to JPY 150 billion (approximately USD 1.1 billion) of unsecured commercial paper notes. Commercial paper notes totaling USD 2.8 billion under these three programs were outstanding as per December 31, 2022 (2021: USD 0.9 billion).
Novartis also has a committed credit facility of USD 6.0 billion, which was extended in 2022. This credit facility is provided by a syndicate of banks and is intended to be used as a backstop for the US commercial paper programs. The extended facility matures in September 2025 and was undrawn as per December 31, 2022, and December 31, 2021.
Total liquidity decreased to USD 18.9 billion compared with USD 28.3 billion as at December 31, 2021.
As of year-end 2022, Moody’s Investors Service rated the Company A1 for long-term maturities and P-1 for short-term maturities and S&P Global Ratings rated the
company AA- for long-term maturities and A-1+ for short-term maturities.
For the tables showing the maturity schedule of our current financial assets, current and non-current financial debts and net debt as at December 31, 2023 and December 31, 2022, see “Item 18. Financial Statements—Note 30. Financial instruments - Additional disclosures—Nature and extent of risks arising from financial instruments—Liquidity risk.”
For a description of risks and restrictions on the ability of subsidiaries to transfer funds to the Company via cash dividends, loan or advances, see “—Liquidity/short-term funding” and “Item 18. Financial Statements—Note 30. Financial instruments - Additional disclosures—Nature and extent of risks arising from financial instruments.”
Information regarding the Company’s material contractual obligations and commitments as of the end of 2023 are provided in “—Material contractual obligations and commitments.”
Liquidity and financial debt by currency
The following table provides a breakdown of liquidity and financial debt by currency as at December 31:
| |
Liquidity
in % 20231
| |
Liquidity
in % 20221
| |
Liquidity
in % 20211
| | Financial
debt in %
20232 | | Financial
debt in %
20222 | | Financial
debt in %
20212 | |
|
USD | | 67 | | 85 | | 92 | | 67 | | 62 | | 57 | |
|
CHF | | 7 | | 4 | | 4 | | 7 | | 6 | | 12 | |
|
EUR | | 22 | | 7 | | 2 | | 23 | | 29 | | 27 | |
|
JPY | | | | | | | | 1 | | 1 | | 1 | |
|
Other | | 4 | | 4 | | 2 | | 2 | | 2 | | 3 | |
|
| | 100 | | 100 | | 100 | | 100 | | 100 | | 100 | |
|
|
1 Liquidity includes cash and cash equivalents and marketable securities, including debt securities, commodities and time deposits. |
2 Financial debt includes non-current and current financial debt. |
Bonds
In August 2023, a 5-year EUR denominated bond of EUR 750 million with a coupon of 0.50% was repaid at maturity.
In September 2023, a 7-year EUR denominated bond of EUR 1.25 billion with a coupon of 0.125% was repaid at maturity.
In April 2022, a 5-year USD denominated bond of USD 1.0 billion with a coupon of 2.40% was repaid, in advance of its maturity date at no additional cost.
In September 2022, a 10-year USD denominated bond of USD 1.5 billion with a coupon of 2.40% was repaid at maturity.
In March 2021, a 4-year EUR denominated bond of EUR 1.25 billion with a coupon of 0.00% was repaid at maturity.
In November 2021, a 7-year EUR denominated bond of EUR 0.6 billion with a coupon of 0.75% was repaid at maturity.
Liquidity/short-term funding
The Company’s liquidity amounted to USD 14.4 billion as at December 31, 2023, compared with USD 18.9 billion as at December 31, 2022. Total non-current and current financial debts, including derivatives, amounted to USD 24.6 billion as at December 31, 2023, compared with USD 26.2 billion as at December 31, 2022.
The debt/equity ratio increased to 0.53:1 as at December 31, 2023, compared with 0.44:1 as at December 31, 2022. The net debt increased to USD 10.2 billion as at December 31, 2023, compared with USD 7.2 billion as at December 31, 2022.
We continuously track our liquidity position and asset/liability profile. This involves modeling cash flow maturity profiles based on both historical experiences and contractual expectations to project our liquidity requirements. We seek to preserve prudent liquidity and funding capabilities. We are confident that we have sufficient liquidity to support our normal business activities for the foreseeable future.
Certain countries have legal or economic restrictions on the ability of subsidiaries to transfer funds to the Company in the form of cash dividends, loans or advances, but these restrictions do not have an impact on the ability of the Company to meet its cash obligations.
We are not aware of any significant demands to change the level of liquidity needed to support our normal business activities. We make use of various borrowing facilities provided by several financial institutions. We also successfully issued various bonds in previous years and raised funds through our commercial paper programs.
The maturity schedule of our net debt can be found in “Item 18. Financial Statements—Note 30. Financial instruments - Additional disclosures—Nature and extent of risks arising from financial instruments—Liquidity risk.”
Material contractual obligations and commitments
The Company’s material contractual obligations and commitments, entered into from time to time, consist of the following:
• Non-current financial debt, including current portion (see “Item 18. Financial Statements—Note 20. Non-current financial debt”). For the table showing the maturity schedule of our current and non-current financial debt, see “Item 18. Financial Statements—Note 30. Financial instruments—additional disclosures—Nature and extent of risks arising from financial instruments— Liquidity risk”;
• Leases on assets used in operations entered into in the ordinary course of business (see “Item 18. Financial Statements— Note 11. Right-of-use assets and lease liabilities”);
• Long-term research and development agreements with various institutions and pharmaceutical companies related to intangible assets. These agreements provide for potential milestone payments by Novartis, which are dependent on successful clinical development, or meeting specified sales targets, or other conditions that are specified in the agreements (see “Item 18. Financial Statements—Note 29. Commitments and contingent liabilities—Research and development commitments”);
• Commitments related to the acquisition of businesses and interests in intellectual property focused on key disease areas and indications that the Company expects to be growth drivers in the future (see “Item 18. Financial Statements—Note 29. Commitments and contingent Liabilities—Other commitments”). In addition, certain business acquisition arrangements include contingent payments, which the shareholders of the acquired company are eligible to receive upon the achievement of specified milestones. For the table showing the maturity schedule of contingent consideration liabilities, see “Item 18. Financial Statements—Note 30. Financial instruments—additional disclosures—Nature and extent of risks arising from financial instruments—Liquidity risk”;
• Independent pension and other post-employment benefit plans (see “Item 18. Financial Statements – Note 26. Post-employment benefits for employees”); and
• Property, plant and equipment purchase commitments in the ordinary course of business (see “Item 18. Financial Statements—Note 10. Property, plant and equipment”).
5.C Research and development, patents and licenses
Our research and development spending from continuing operations totaled USD 11.4 billion, USD 9.2 billion and USD 8.6 billion (non-IFRS measure core research and development from continuing operations USD 8.6 billion, USD 8.3 billion and USD 8.2 billion) for the years 2023, 2022 and 2021, respectively.
Novartis has numerous products in various stages of development. For further information on these policies and these products in development, see “Item 4. Information on the Company—Item 4.B Business overview.”
As described in the risk factors section and elsewhere in this Annual Report, our drug development efforts are subject to the risks and uncertainties inherent in any new drug development program. Due to the risks and uncertainties involved in progressing through preclinical development and clinical trials, and the time and cost involved in obtaining regulatory approvals, among other factors, we cannot reasonably estimate the timing, completion dates and costs, or range of costs, of our drug development programs, or of the development of any particular development compound (see “Item 3. Key Information—Item 3.D Risk factors”). In addition, for a description of the research and development process for the development of new drugs and our other products, and the regulatory process for their approval, see “Item 4. Information on the Company—Item 4.B Business overview.”
5.D Trend information
See “—Item 5.A Operating results”, “—Item 5.B Liquidity and capital resources” and “Item 4. Information on the Company—Item 4.B Business overview” for trend information.
5.E Critical accounting estimates
See “Item 18. Financial Statements—Note 1. Accounting policies” for Critical accounting estimates.
Item 6. Directors, Senior Management and Employees
6.A Directors and senior management
The information set forth under “Item 6. Directors, Senior Management and Employees—Item 6.C Board practices—Corporate governance—Board of Directors” and “Item 6. Directors, Senior Management and Employees—Item 6.C Board practices—Corporate governance—Executive Committee” is incorporated by reference.
6.B Compensation
Dear shareholder,
On behalf of the Compensation Committee, I am pleased to present our 2023 Novartis Compensation Report.
2023 company performance
Novartis delivered a very strong performance in 2023, with a robust strategic (Sandoz spin-off), financial (top-and bottom-line growth) and innovation (large number of positive Phase III readouts) performance. The performance of our product portfolio (including Entresto, Kesimpta, Kisqali and Scemblix) together with the optimization of our commercial and supporting functions contributed to sales growth of 10% (constant currencies ‘cc’) and core operating income growth of 18% (cc), compared with the previous year. Innovation highlights included positive results from several Phase III clinical trials for investigational medicines with significant sales potential, including Pluvicto, remibrutinib and iptacopan, as well as additional approval indications for Entresto (for pediatric heart failure) in the EU and Cosentyx (for hidradenitis suppurativa) in the EU and US. Regulatory submissions were completed for Kisqali (for early breast cancer) in the EU and US. Transactions provided further substance to the pipeline, notably with the acquisition of Chinook Therapeutics to strengthen our renal portfolio. The one-year 2023 TSR performance of 26% was in the top 3 of our global healthcare peers.
On October 4, 2023, we successfully spun off our Sandoz generics and biosimilars business, through a dividend-in-kind distribution to holders of Novartis shares and American Depositary Receipts (ADRs). Each holder received one Sandoz share for every five Novartis shares or one Sandoz ADR for every five Novartis ADRs. While shareholders received the aforementioned dividend in kind, Performance Share Unit (PSU) and Restricted Share Unit (RSU) holders, including active and former members of the Executive Committee of Novartis (ECN), received instead ‘keep whole awards’ with the same vesting and performance conditions as the underlying award. This ensured award holders were treated on an economically consistent basis to Novartis shareholders. For more information, see “—Sandoz equity restoration plan.”
2023 realized compensation
The company’s performance in 2023 resulted in an Annual Incentive payout of 185% for the CEO, and in a Long-Term Performance Plan (LTPP) 2021-2023 payout of 122%. These factors, together with an increase of 16% in the vesting price of the 2021-2023 LTPP (when adjusted for the Sandoz spin-off) resulted in the total realized CEO compensation of CHF 16 248 178 in 2023. These three outcomes contributed to the vast majority of the year-on-year increase in realized pay for the CEO. They also impacted the compensation outcome for the other members of the ECN, whose total aggregated compensation was CHF 47 205 005. For details on the realized compensation, see “—CEO and Executive Committee 2023 realized compensation.”
Changes to the executive compensation system in 2024
As part of our annual review, we identified that our existing CEO compensation practices placed us in the lowest quartile versus global healthcare peers. We engaged extensively with our largest shareholders and proxy advisors to collect feedback about our executive compensation framework, in particular the challenges that European companies face in the competition for talent. Following this engagement and the overall positive nature of the feedback received, the Compensation Committee and the Board of Directors agreed that it is necessary to take a global perspective to attract and retain the best talent at the top of the organization, and that the company could be more competitive in this regard. As a result, and while still mindful of the expectations of European-based investors and proxy advisors, we made the following changes to our compensation system, effective January 1, 2024:
• CEO target compensation: We aspire to continue growing our global business, with a particular focus on the US market. Aligned with this aspiration and our compensation philosophy, the Board of Directors decided to adjust the CEO target pay in a way that preserves alignment with shareholders. Specifically, we increased the LTPP target, which is fully performance driven based on three-year forward-looking targets, from 325% to 400% as a percentage of annual base salary. The additional two-year holding period for the CEO remains unchanged, thereby restricting the equities from sale for five years. The Compensation Committee will continue to set stretch targets, with a robust assessment at the end of the cycle. No changes were made to the CEO base salary (beyond ordinary salary increases received by other Swiss employees) or the Annual Incentive target. This is the first significant increase in CEO target pay since 2019 and places his target compensation just above the lowest quartile of global healthcare peers, based on the last disclosed proxy information.
• Annual Incentive metrics: The Compensation Committee agreed to replace operating income with core operating income in our ECN Annual Incentive. The Compensation Committee agreed that core operating income, which excludes certain one-time or non-recurring items, represents a better measure of the Company’s underlying performance. Additionally, core-adjusted metrics are more commonly used by our global healthcare peers, which would enable easier peer performance comparisons.
• Share ownership and Annual Incentive deferral: The Compensation Committee strongly affirms a commitment to the principle of aligning the interests of executives with shareholders. To that end, we will continue to enforce an obligation that all ECN members become meaningful shareholders with a requirement to hold a multiple of their salary in Novartis shares. Currently, the Annual Incentive has a 50% mandatory deferral into equity, which is blocked for three years. The Compensation Committee decided that this aspect of the Annual Incentive should be more in line with relevant
market practice. For this reason, once an ECN member has met their shareholding requirement, the portion of the Annual Incentive that is mandatorily deferred will be reduced to 30%. To reinforce strong shareholder alignment, the CEO’s shareholding requirement will simultaneously be increased from 5x to 6x annual base salary.
For more information about these changes, see “—2024 Executive Committee compensation system changes and increases.”
2023 compensation report structure and disclosure
The Compensation Committee decided to make the following changes to the 2023 Novartis Compensation Report:
• The Compensation Report has been reorganized to enhance its readability. For this reason, a detailed presentation of executive compensation outcomes can be found in the first part, and an explanation of our underlying compensation philosophy, system and governance in the second part. The Board compensation and governance information is in the final section. We trust that you will find this new structure more accessible.
• In response to shareholder feedback, we provide more disclosure and transparency in the CEO balanced scorecard, with an increased focus on the link between pay and performance.
• Aligned with our evolution from an organization with separate divisions each with a separate leader into a focused medicines company, we disclose target pay for the CEO (as the highest paid), the CFO and the Presidents of our International and US organizations individually, while all other ECN target pay is aggregated, see “—Compensation at grant value for the CEO and Executive Committee”. The Compensation Committee believes that this approach is more commercially appropriate while also maintaining our disclosure at the upper end of Swiss practice. Notwithstanding this change, we continue to disclose the CEO and ECN realized pay, as well as any significant individual pay increases, buyouts and exit packages.
2024 Annual General Meeting (AGM)
On behalf of the Compensation Committee, I would like to thank shareholders for their input and engagement during the consultation process. This has helped us shape the improvements to our compensation system presented here, as well as the changes in the format of the Compensation Report.
At the 2024 AGM, as with prior years, shareholders will be asked to vote on:
• The maximum aggregate amount of compensation for the Board of Directors from the 2024 AGM to the 2025 AGM
• The maximum aggregate amount of compensation for the ECN for the financial year 2025
• This 2023 Compensation Report
We trust that this Report and our 2024 Say-on-Pay brochure provides you with the information required for you to vote in favor of the above. We continue to welcome your feedback, which is invaluable in driving improvements in our compensation system and practices.
Simon Moroney, D.Phil.
Chair of the Compensation Committee
Executive Committee and Board 2023 compensation at a glance
CEO pay for performance
2023 Annual Incentive% of target200%150%100%50%0%MaximumPayout: 185% of targetTarget2021-2023 Long-Term Performance Plan (LTPP) cycle% of target200%150%100%50%0%MaximumTargetPayout: 122% of target3rd party sales CAGR1(102% of target)Core operating income CAGR (158% of target)Innovation (108% of target)Relative TSR (120% of target)
1 CAGR = compound annual growth rate
CEO and Executive Committee total realized compensation
The 2023 total realized compensation for the CEO and Executive Committee members was CHF 63 453 183. For more information, see “—2023 CEO Annual Incentive balanced scorecard”, “ —2021-2023 LTPP cycle performance outcomes” and “ —CEO and Executive Committee 2023 realized compensation.”
| | | | | | | | | | | | | | | |
| | | | 2023 annual base salary | | 2023 pension benefits | | 2023 Annual Incentive | | 2021 - 2023 LTPP cycle | | Other 2023 compensation | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| |
Currency
| |
Cash (amount)
| |
Amount
| |
Cash & Equity
| |
Equity (value
at vesting date)
| |
Amount
| | Total realized
compensation
(incl. share
price movement) | |
|
|
Vasant Narasimhan (CEO) | | CHF | | 1 822 334 | | 170 125 | | 5 075 255 | | 8 921 546 | | 258 918 | | 16 248 178 | |
|
Aggregate realized compensation of the other 11 Executive Committee members, including the member who stepped down during the financial year 2023 | | CHF | | 8 551 936 | | 1 627 708 | | 15 449 571 | | 15 100 093 | | 6 475 697 | | 47 205 005 | |
|
Total | | CHF | | 10 374 269 | | 1 797 833 | | 20 524 826 | | 24 021 639 | | 6 734 615 | | 63 453 183 | |
|
| | | | | | | | | | | | | | | |
Board compensation
The total actual compensation earned by Board members in the 2023 financial year is shown in the table below. For more information, see “—Board member total compensation earned for the financial year 2023.”
CHF
| | 2023
total compensation | |
|
Board Chair | | 3 803 784 | |
|
Other members of the Board | | 4 787 933 | |
|
Total | | 8 591 717 | |
|
|
|
|
CEO compensation and performance
2023 fixed pay and benefits
The CEO 2023 annual base salary was: CHF 1 828 900 (2.2% salary increase effective as of March 1, 2023, in line with ordinary salary increases received by other Swiss employees).
Pension and other benefits
The CEO is a member of the Novartis Swiss pension funds, which provide company contributions on the base salary and Annual Incentive up to the legal cap on the insured salary of CHF 882 000. No supplementary pension plans or savings plans are provided. The CEO’s employer pension contributions represent 9.3% of the base salary.
2023 CEO Annual Incentive balanced scorecard
This section presents the balanced scorecard for the CEO. Financial performance is measured in constant currencies (cc) to reflect operational performance that can be influenced. Performance outcomes for compensation purposes may differ from reported numbers in accordance with our compensation adjustments policy e.g. to reflect any currency impacts.
TargetMeasureWeight (%)TargetPerformanceachievementFinancial performance160Significantly AboveGroup 3rd Party Sales (cc, USD million)244989752 282Significantly aboveGroup Operating Income (cc, USD million)18983310 673Significantly aboveGroup Free Cash Flow as a % of 3rd party sales (cc)1824.6%26.8%Significantly above1Group 3rd Party Sales, Group Operating Income and Group Free Cash Flow as a % of 3rd party sales include the continuing operations’ financial performance for the year ended December 31, 2023 and the Sandoz discontinued operations’ financial performance for the nine months ended September 30, 2023.
Financial targets were re-calibrated following the Sandoz spin-off by excluding Q4 2023 Sandoz targets. This was to ensure targets were as stretched as the original targets after excluding the impact of Sandoz; and that they remain consistent with our financial reporting.
2023 CEO Annual Incentive balanced scorecard (continued)
In addition to the financial targets, the CEO also has five equally weighted strategic objectives across key priority areas, including targets related to environmental, social and governance (ESG) matters. We provide a summary of those targets that most influenced performance and their relevant achievements below. More details can be found in the Novartis in Society Integrated Report 2023.

TargetMeasureWeight (%) / PerformanceachievementStrategic objectives40 AboveAdvance our new focused strategy•Sandoz spin-off successfully completed as planned on October 4, 2023 with positive value creation•More than 15 business development and M&A deals signed, strengthening the pipeline across key therapeutic areas and technology platforms AboveMaintain growth momentum and ensure successful launches•Strong sales performance for growth drivers: Entresto, Cosentyx, Kesimpta, Kisqali and Zolgensma were 105% of 2023 target in cc•Recent launches: Leqvioand Pluvicto, delivered 105% of 2023 target in cc•Fabhalta (iptacopan) launch preparations on track, after US approval was secured for paroxysmal nocturnal hemoglobinuria (PNH) in DecemberSignificantly aboveDeliver pipeline and drive R&D productivity•22 regulatory approvals achieved in US, EU, China and Japan, including: Cosentyx for hidradenitis suppurativa (HS) in US and EU; intravenous (IV) formulation of Cosentyx in US; Leqvio for hypercholesterolemia in Japan and China•18 submissions filed in the US, EU, China and Japan, including for Fabhalta (iptacopan) for PNH in EU and Japan and Kisqali(for early breast cancer) in EU and US•Nine positive phase III readouts/presentations including for Kisqali, Pluvicto, iptacopan, Lutathera, remibrutinib and atrasentan•Strong progress was made in the early-stage pipeline, with seven new molecular entity first patient first visits (NME FPFVs) and six pivotal trial-enabling first patient first visits (PTE-FPFVs), including two NMEs •23 entries were made into the pre-clinical portfolioAboveExecute on operational excellence & productivity•Core Margin exceeded target 37.9% in cc (vs. 2023 target 36.2%), when including policy adjustments and considering 3rd party sales •Organizational transformation initiated in 2022 was largely completed and is on track to deliver over USD 1.5bn in savings• Technology transformation programs for our new ERP (Enterprise Resource Planning) system and master data management are on track•Increased weekly production of Pluvictofollowing US and EU production approval in Milburn and Zaragoza sites. Expanded capacity of sites in Slovenia and Italy to support Kisqalidemand•99.1% regulatory inspections of clinical and manufacturing operations acceptableSignificantly aboveStrengthen foundations (ESG/ Human Capital) •Targets for our sustainability-linked bond were exceeded: 1.6m patients reached in LMICs with strategic innovative therapies (vs. 2023 target 1.1m); 28.7m patients reached with flagship programs (vs. 2023 target 22.6m)•Invested USD 98.4m in R&D for neglected tropical diseases and malaria•100% new launches with a global access strategy (vs. 2023 target 100%)•Reduction of greenhouse gas emissions by 63%, water consumption by 50%, and waste sent for disposal by 66% (all in our own operations; vs 2016 baseline)•Employee engagement score was 75 (vs. industry benchmark of 74), an increase of 2 percentage points over prior year •Employee share purchase plan on track: launched in North America, 27 countries in Europe, and 11 countries in Asia and the Middle East. A rollout to employees in other countries is scheduled in the near future•EPIC commitments achieved: 48% female representation in management (vs. 2023 aspiration of 48%52%); 98% of total headcount with pay transparency to external and/or internal benchmarks, where available (100% when considering exclusions mainly due to contractual or legal constraints and the ongoing integration of acquired businesses); pay gap at -0.9%, compared with external median +19%; 100% of recruitment no longer using historical salary data•100% of eligible suppliers risk-assessed using our External Partner Risk Management frameworkAboveTotal100Overall assessment and payout for CEOSignificantly AboveNovartis delivered a very strong performance in 2023, surpassing its targets on almost all measures, a significant improvement versus 2022. Financial results exceeded target due to a strong performance on sales and significant savings from our organizational restructuring. Our TSR performance for the year 2023 was in the top quartile of the industry. Strategic objectives were also well executed: the spin-off of Sandoz was completed in Q4 2023 and our organic pipeline made good progress with several positive clinical trial results and important regulatory filings. We maintained strong positions in our priority ESG ratings, underscoring our progress in delivering on our ESG commitments. In view of these achievements, the Board of Directors decided on an Annual Incentive payout of 185% (within the range of 0200%), leading to a total amount ofCHF 5 075 255.
2021-2023 LTPP cycle performance outcomes
The charts below illustrate the performance of the 2021-2023 LTPP cycle against target. These targets were introduced in early 2021 during the Covid pandemic and were set at ambitious levels relative to prevailing market and internal expectations at that time. The Board reviewed these targets again at the end of the cycle and decided they are still appropriate.
As with the Annual Incentive, the financial LTPP targets were recalibrated to take the Sandoz spin-off into account. Given that these metrics measure the compound annual growth rate, Sandoz targets were removed for the financial year 2023. For the relative TSR measure, the dividend in kind distribution was treated as a one-time dividend that is not reinvested. A similar approach was taken for the targets in other ongoing cycles.

3rd pARTY sales CAGR(25% weighting)Vesting range 0–200% of target8%6%4%2%0%Maximum: 8.8% (CAGR)Actual: 5.9% (CAGR)Target: 5.8% (CAGR)3rd party sales CAGR payout102% of targetNotes: A minimum achievement of 3.2% CAGR was required to receive a payout under this performance measureNovartis achieved 3rd party sales CAGR of 5.9% (in constant currencies – cc) against the 5.8% target set at the beginning of the performance cycle, in large part due to Entresto, Kesimpta and Promacta, partly offset by Beovu, Kymriah and Cosentyx.Following the application of the payout curve, the 3rd party sales CAGR (cc) achievement generates a payout factor of 102% for this metric.Core operating income (COI) CAGR(25% weighting)Vesting range 0–200% of target14%10.5%7%3.5%0%Maximum: 14.4% (CAGR)Actual: 12.3% (CAGR)Target: 8.4% (CAGR)COI CAGR payout158% of targetNotes: A minimum achievement of 2.8% CAGR was required to receive a payout under this performance measureNovartis achieved a COI CAGR of 12.3% (cc) against the 8.4% target set at the beginning of the performance cycle as a result of significant productivity savings generated from the creation of our new organizational structure. Following the application of the payout curve, the COI CAGR (cc) achievement generates a payout factor of 158% for this metric.Relative Total Shareholder Return (TSR)(25% weighting)Novartis position Payout rangein the peer group(% of target)Position 1 – 2Position 3 – 5Position 6 – 8Position 9 – 15170% – 200% 130% – 160%80% – 120%0%Actual ranking 6th = 120% of targetTSR for the 2021-2023 cycle was 31.2%. As a result, Novartis ranked No. 6 out of 15 healthcare companies (including Novartis) resulting in a payout of 120% for this metric.Innovation(25% weighting)The following outcomes were considered in our 2021-2023 LTPP cycle innovation performance:•Cosentyx approved in the EU for childhood arthritic conditions; approved in the EU and US for HS; IV formulation approved in US •Entresto approved in the EU for pediatric heart failure; extending regulatory data protection to November 2026•Kymriah approved in the EU and US for adults with relapsed or refractory follicular lymphoma•Pluvicto approved in the US for the treatment of progressive PSMA positive mCRPC•Kisqali submissions in EU and US (for early breast cancer)•Iptacopan submission in EU and approval in US for patients with PNH•Submissions for Pluvicto treating metastatic castration-resistant prostate cancer, ligelizumab in chronic spontaneous urticaria and canakinumabas adjuvant treatment in non-small cell lung cancer were delayed or not submitted•In Biomedical Research, 19 pivotal trial enabling First Patient First Visits were achieved. Two Oncology targeted therapies and two radio-ligand NMEs were progressed to clinical investigationBased on input from the Science & Technology Committee (STC), the Board of Directors approved a payout of 108% for this metric.2021-2023 LTPP CYCLE PayoutOverall, the Board of Directors approved a 2021-2023 LTPP cycle payout at 122% of target, within the range of 0–200%. This resulted in an LTPP payout of CHF 8 921 546 for the CEO, including dividend equivalents of CHF 759 557 and Keep Whole Awards of CHF 523 862. The Committee did not exercise any discretion in relation to the vesting or share price changes following the spin-off.3rd party sales CAGR102% x 25%+COI CAGR158% x 25%+Innovation108% x 25%+Relative TSR120% x 25%Final vesting122% of target
Historic CEO incentive payouts since appointment
The table below presents the CEO Annual Incentive and LTI payouts over the last six years since his appointment, out of a maximum of 200%. The average Annual Incentive and LTI payouts over this period were 132% and 111%, respectively. The high variability of the incentive payouts demonstrates a strong link between pay and performance, and provides evidence of stretch in the targets. For instance, the three lowest LTI payouts correspond to the cycles for which relative TSR performance was below the median of the peer group, and the payout was therefore 0% under this metric.
Year ending | | 20181 | | 20191 | | 20201 | | 2021 | | 2022 | | 2023 | |
|
STI payout | | 145% | | 160% | | 100% | | 100% | | 100% | | 185% | |
|
LTI payout | | 99% | | 157% | | 126% | | 107% | | 57% | | 122% | |
|
|
1 For these cycles, two LTI plans existed: LTPP and LTRPP. Payouts represent the average CEO weighted payout |
Interim update regarding ongoing LTPP cycles
How performance is tracking against target for our ongoing LTPP performance cycles is reported below.
2022-2024 LTPP cycle
After the first two years of the three-year LTPP cycle, 3rd party sales CAGR and core operating income CAGR are tracking ahead of target. Innovation is on track. At the end of 2023, the relative TSR for Novartis was above median among our global healthcare peer group.
Performance measures | | Tracking | |
|
3rd party sales CAGR (25%) | |  | |
|
Core operating income CAGR (25%) | |  | |
|
Innovation (25%) | |  | |
|
Relative TSR (25%) | |  |
|
2023-2025 LTPP cycle
After the first year of the three-year LTPP cycle, 3rd party sales CAGR and core operating income CAGR are ahead of target and innovation is tracking on target. At the end of 2023, the relative TSR for Novartis was in the top three of our global healthcare peer group.
Performance measures | | Tracking | |
|
3rd party sales CAGR (25%) | |  | |
|
Core operating income CAGR (25%) | |  | |
|
Innovation (25%) | |  | |
|
Relative TSR (25%) | |  | |
|

On or ahead of target
CEO and Executive Committee
2023 compensation of joining and departing Executive Committee members
Appointment of President, International
Patrick Horber
Patrick Horber joined the Executive Committee on December 1, 2023 as President, International. In line with the buyout policy of Novartis (see “—CEO and Executive Committee: appointments”), to replace entitlements forfeited from his previous employer as a result of joining Novartis, he was granted buyout awards of CHF 1 058 274 in cash to be paid out in March 2024 as well as CHF 3 084 694 in target PSUs and CHF 2 292 624 in RSUs, both of which will vest between 2024 and 2026.
Departure of President, Innovative Medicines International & Chief Commercial Officer
Marie-France Tschudin
Marie-France Tschudin stepped down from the Executive Committee on September 15, 2023 and started her notice period on October 1, 2023. In determining her termination arrangement, the Compensation Committee ensured that contractual entitlements were respected, and all payments are in line with our plan rules and the Swiss Code of Obligations.
Per policy (see “—CEO and Executive Committee: termination arrangements”), during her 12-month notice period, Marie-France is entitled to her base salary, pension, Annual Incentive and other benefits. No severance payments were made. Outstanding equity grants will vest in line with the respective plan rules and are subject to malus and clawback, including requirements defined by the U.S. Securities and Exchange Commission, as well as non-compete restrictions. No new LTPP grants will be made during the notice period.
CEO and Executive Committee 2023 realized compensation
To aid shareholders’ understanding of the link between pay and performance, the Compensation Report discloses the realized compensation for the CEO on an individual basis, and for the other ECN members on an aggregated basis. Disclosing realized compensation means that the Annual Incentive and the LTPP are disclosed at the end of their respective performance cycles, reflecting actual payouts based on performance.
The total actual payout may vary year on year depending on multiple factors, including the composition of the Executive Committee and the tenure of its members (as new members may not have equity vestings), compensation increases, payout of variable compensation based on actual performance, share price fluctuations, and dividend equivalents.
The table below shows compensation for all ECN members for the 2023 financial year, including base salary, pension, other benefits, 2023 Annual Incentive, 2021-23 LTPP cycle payout, and any buyouts paid or vesting within the year. Base salary increases were in line with the average of other Novartis employees, with the exception of three individuals as disclosed in Item 6B of the 2022 Annual Report. The portion of the Annual Incentive paid in shares for the year 2023 is disclosed using the underlying value of Novartis shares at the date of grant. The realized values of any other equity awards (including dividend equivalents) are calculated using the share price on the date of vesting. The table also includes the total 2022 realized compensation for all Executive Committee members for comparative purposes.
To determine the appropriateness of 2023 CEO and executive compensation payouts under the Annual Incentive and LTPP, the Board of Directors and the Compensation Committee reviewed management’s performance against targets set at the beginning of the cycles as described in “—2023 CEO Annual Incentive balanced scorecard” and “—2021-2023 LTPP cycle performance outcomes.”
The incentive performance outcomes, combined with base salary and other benefits, pension, keep whole awards and dividend equivalents, resulted in 2023 total realized compensation for the CEO of CHF 16 248 178.
Realized compensation for the CEO and Executive Committee (2023 compared with 2022)
| | 2023 | | 2022 | |
| | | | | |
| | | | | |
| | | | | |
In CHF (gross) 1 | | CEO | | Other ECN2 | | Total | | CEO | | Other ECN3 | | Total | |
|
Annual Base Salary | | 1 822 334 | | 8 551 936 | | 10 374 269 | | 1 786 500 | | 9 122 792 | | 10 909 292 | |
|
Annual Incentive (performance achieved) | | 5 075 255 | | 15 449 571 | | 20 524 826 | | 2 684 321 | | 10 130 159 | | 12 814 480 | |
|
Thereof cash | | 2 537 599 | | 6 149 179 | | 8 686 778 | | 1 342 125 | | 4 211 841 | | 5 553 966 | |
|
Thereof equity | | 2 537 656 | | 9 300 392 | | 11 838 048 4 | | 1 342 196 | | 5 918 318 | | 7 260 514 5 | |
|
LTPP | | 8 921 546 | | 15 100 093 | | 24 021 639 6 | | 3 307 422 | | 10 025 047 | | 13 332 469 7 | |
|
Other payments 8 | | 258 918 | | 6 475 697 | | 6 734 615 9 | | 499 445 | | 9 716 294 | | 10 215 739 | |
|
Pension benefits 10 | | 170 125 | | 1 627 708 | | 1 797 833 11 | | 174 488 | | 1 978 304 | | 2 152 792 12 | |
|
Total | | 16 248 178 | | 47 205 005 13 | | 63 453 183 | | 8 452 176 | | 40 972 595 14 | | 49 424 771 | |
|
|
1 All compensation amounts are stated gross, before the deduction of social security contributions and income tax paid by the Executive Committee members. Amounts for Executive Committee members paid in USD were converted at a rate of USD 1.00 = CHF 0.8986, which is the same average exchange rate used in the Company's 2023 consolidated financial statements (a similar rule applies to payments made in other currencies during the year). |
2 Aggregate realized compensation of the other 11 Executive Committee members, including Marie-France Tschudin who stepped down during the financial year 2023. |
3 Aggregate realized compensation of the other 15 Executive Committee members, including the members who stepped down during the financial year 2022. For more information, see item 6B of the 2022 Annual Report. |
4 The portion of the Annual Incentive delivered in equity is rounded up to the nearest share, based on the closing share price on the grant date (January 24, 2024) of CHF 93.53 per Novartis share and USD 107.55 per ADR. |
5 The portion of the Annual Incentive delivered in equity is rounded up to the nearest share, based on the closing share price on the grant date (January 25, 2023) of CHF 85.30 per Novartis share and USD 92.81 per ADR. |
6 The amount represents the underlying share value of the 264 799 realized LTPP PSUs to the CEO and other Executive Committee members for the 2021-2023 LTPP cycle, including dividend equivalents for the three-year cycle of value CHF 759 557 for the CEO and CHF 1 228 736 for the other Executive Committee members, including the one who stepped down during the financial year 2023. The taxable value is determined using the closing share price, on the day the payout factor is approved by the Board of Directors (i.e., January 24, 2024), of CHF 93.53 per Novartis share and USD 107.55 per ADR. Includes vested keep-whole shares received in connection to the Sandoz spin-off. Robert Kowalski was promoted to the Executive Committee during the course of the 2021 performance period, and Victor Bulto during the course of the 2022 performance period. As such, the information disclosed reflects their pro-rata 2021-2023 LTPP payouts attributable to the period in which they were members of the Executive Committee. Shreeram Aradhye rejoined Novartis, and Patrick Horber, Aharon Gal and Fiona Marshall joined Novartis after the 2021-2023 LTPP awards were made, and therefore did not receive an LTPP award for the 2021-2023 LTPP cycle. |
7 Based on the closing share price of January 25, 2023 of CHF 85.30 per Novartis share and USD 92.81 per ADR for all members. |
8 Includes any other perquisites, benefits in kind, and international assignment benefits as per the global mobility policy (e.g., housing, international health insurance, children's school fees, tax equalization). The compensation and benefits elements related to the period after the step-down dates are also reported under ‘other payments’. |
9 Includes 2 042 realized PSUs, which vested on March 31, 2023 and 6 212 realized RSUs, which vested on May 1, 2023 and a cash buyout of CHF 469 071 (for a total value of CHF 1 553 135), to Fiona Marshall in lieu of the Annual Incentive and LTI that she forfeited when leaving her previous employer. |
10 Includes social security contributions to the extent that they result in a pension entitlement. Includes also contributions to company provided pension plans. |
11 This amount is out of total social security employer contributions of CHF 1 933 476 and pension employer contributions of CHF 1 852 898 paid in 2023 for all Executive Committee members. |
12 This amount is out of total social security employer contributions of CHF 3 266 972 and pension employer contributions of CHF 2 608 462 paid in 2022 for all Executive Committee members. |
13 Includes CHF 5 975 824 for the member who stepped down during 2023. |
14 Includes CHF 20 967 229 for members who stepped down during 2022. |
|
The 2023 total realized compensation for the CEO increased compared with the prior year, driven by (i) the higher performance payouts of the 2023 Annual Incentive (185% compared with 100% in 2022) and the 2021-2023 LTPP (122% compared with 57% payout for cycle 2020-2022), which will be blocked until January 2026, and (ii) the vesting share price of the 2021-2023 LTPP, which was 16% higher than the prior cycle (when adjusted for the Sandoz spin-off). For more information on the performance outcomes, see “—2023 Annual Incentive CEO balanced scorecard” and “—2021-2023 LTPP cycle performance outcomes.” The performance outcomes of each measure compared with 2022 are provided below:
Annual Incentive | | 2023 | | 2022 | |
|
3rd party sales | | Significantly above | | Met | |
|
Operating income | | Significantly above | | Met | |
|
Free cash flow/3rd party sales | | Significantly above | | Below | |
|
Share of peers | | - 1 | | Met | |
|
Strategic objectives | | Above | | Met | |
|
Overall assessment | | Significantly above | | Met | |
|
CEO payout | | 185% | | 100% | |
|
|
1 Share of peers was removed from the ECN Annual Incentive from performance year 2023, as disclosed in the 2022 Compensation Report |
LTPP cycle ending | | 2023 | | 2022 | |
|
3rd party sales CAGR | | 102% | | 43% | |
|
Core operating income CAGR | | 158% | | 93% | |
|
Innovation | | 108% | | 92% | |
|
Relative TSR | | 120% | | 0% | |
|
Payout (weighted) | | 122% | | 57% | |
|
The strong performance outcomes in 2023 were driven by several factors including:
• Sales of Entresto, Kesimpta, Promacta, Lucentis and Ilaris
• Savings generated from the new, more efficient organizational structure of Novartis
• Strategic milestones achieved, including clinically meaningful Phase III data for multiple assets with blockbuster potential, patient reach in low-and middle-income countries, achievements against our EPIC pledge commitment and the launch of our all-employee share purchase plan
• Significant improvement in the rTSR; 6th position for the 2021-2023 cycle (versus 12th position for the cycle ending in 2022), driven by a strong one-year 2023 TSR performance of 26%
Sandoz equity restoration plan
Novartis shareholders received a dividend in kind in Sandoz shares at the spin-off date. PSUs and RSUs held by Novartis employees are not entitled to dividends. To ensure equal treatment of PSU and RSU holders relative to Novartis shareholders, Novartis granted keep whole awards to its employees, including the CEO and members and former members of the Executive Committee. This was done in accordance with the Sandoz spin-off equity restoration plan as described in the 2022 Compensation Report:
• The keep whole awards had a value similar to that of the dividend in kind that the beneficiary would have received had they been holding Novartis shares
• The keep whole awards were granted in the same equity instrument (i.e., PSUs or RSUs) with the same vesting terms and performance conditions (if applicable) as the underlying award
The total value of keep whole awards granted to the active members of the Executive Committee was CHF 4 704 902. This is equivalent to the estimated reduction in the value of the Novartis share price due to the dividend in kind distribution, and as such is not considered additional compensation. The amounts realized from the vesting of keep whole awards will however be reported in the ECN realized pay for each respective year.
CEO and Executive Committee 2023 compensation at grant
In accordance with the Swiss Code of Obligations, Novartis discloses total compensation at grant value for the CEO and Executive Committee.
The CEO 2023 compensation at grant increase was mainly driven by the stronger performance on the 2023 Annual Incentive compared with 2022, as detailed in “—CEO and Executive Committee 2023 realized compensation.” 2023 compensation at grant for all ECN members remained broadly the same compared with 2022 (CHF 68 365 598 versus CHF 70 819 358), as the higher performance payouts were offset by a reduction in the number of members reported. In 2022, five members stepped down, including the Sandoz CEO, who was not replaced, compared with one in 2023.
The table below discloses the following information about compensation for the CEO and Executive Committee:
• 2023 base salary
• Actual cash portion and portion deferred in equity of the 2023 Annual Incentive
• 2023-2025 LTPP cycle awards, which are reported at target grant date value, based on the assumption that the awards will vest at 100% achievement, excluding any share price movement and dividend equivalents that may be accrued over the performance cycle. The future payout will be determined only after the performance cycle concludes in three years (i.e., at the end of 2025), with a payout range of 0% to 200% of the target value
• Other payments for 2023, which include other benefits, either paid in cash or granted in equity during the year
• 2023 pension benefits
• Total 2023 and total 2022 compensation at grant, for comparative purposes
The highest-paid individual in 2023 was Vasant Narasimhan, CEO of Novartis.
Compensation at grant value for the CEO and Executive Committee (2023 compared with 2022)
|
|
In CHF (gross)1
| |
Annual
Base Salary
| | 2023 Annual
Incentive
(performance
achieved) | | 2023-2025
LTPP cycle
PSUs
(target amount)2 | |
Other
payments3
| |
Pension
benefits4
| |
Total 20235
| |
Total 20226
| |
|
Vasant Narasimhan | | 1 822 334 | | 5 075 255 | | 5 943 960 | | 258 918 | | 170 125 | | 13 270 592 | | 10 960 639 | |
|
Victor Bulto | | 859 085 | | 1 642 520 | | 2 161 211 | | 359 772 | | 137 180 | | 5 159 769 | | 2 948 867 | |
|
Patrick Horber (from December 1, 2023) 7 | | 83 333 | | 132 560 | | - | | 6 455 092 | | 14 007 | | 6 684 992 | | - | |
|
Harry Kirsch | | 1 103 917 | | 2 315 616 | | 2 880 581 | | 47 744 | | 180 055 | | 6 527 912 | | 5 426 373 | |
|
Other ECN members | | 5 789 756 | | 10 093 882 | | 12 714 201 | | 1 348 250 | | 1 178 184 | | 31 124 272 | | 30 516 250 | |
|
Subtotal | | 9 658 425 | | 19 259 833 | | 23 699 952 | | 8 469 775 | | 1 679 551 | | 62 767 536 | | 49 852 130 | |
|
|
Members who stepped down | | 715 845 | | 1 264 993 | | 2 767 559 | | 731 384 | | 118 282 | | 5 598 062 | | 20 967 229 8 | |
|
Subtotal | | 715 845 | | 1 264 993 | | 2 767 559 | | 731 384 | | 118 282 | | 5 598 062 | | 20 967 229 | |
|
Total | | 10 374 269 | | 20 524 826 9 | | 26 467 511 | | 9 201 159 | | 1 797 833 10 | | 68 365 598 | | 70 819 358 | |
|
|
1 All compensation amounts are stated gross, before the deduction of social security contributions and income tax paid by the Executive Committee members. Amounts for Executive Committee members paid in USD were converted at a rate of USD 1.00 = CHF 0.8986, which is the same average exchange rate used in the Company's 2023 consolidated financial statements (a similar rule applies to payments made in other currencies during the year). |
2 The amounts represent the underlying share value of the target number of PSUs granted to Executive Committee members for the 3-year performance cycle, based on the closing share price on the grant date (January 24, 2024) of CHF 93.53 per Novartis share and USD 107.55 per ADR for all members. |
3 Includes any other perquisites, benefits in kind, buyouts and international assignment benefits as per the global mobility policy (e.g., housing, international health insurance, children's school fees, tax equalization). The compensation and benefits elements related to the period after the step-down dates are also reported under ‘other payments’. |
4 Includes social security contributions to the extent that they result in a pension entitlement. Includes also contributions to company provided pension plans. |
5 Aggregate compensation at grant for the 12 Executive Committee members, including Marie-France Tschudin who stepped down during the financial year 2023. |
6 Aggregate compensation at grant for the 16 Executive Committee members, including the members who stepped down during the financial year 2022. For more information, see item 6B of the 2022 Annual Report. |
7 In line with the Company’s buyout policy (see “—CEO and Executive Committee: appointments”), Patrick Horber received buyout awards of CHF 1 058 274 in cash to be paid out in March 2024 as well as CHF 3 084 694 in PSUs, and CHF 2 292 624 in RSUs, both of which will vest between 2024 and 2026, in lieu of the Annual Incentive and LTI that he forfeited when leaving his previous employer. |
8 Includes five members (James Bradner, Richard Saynor, John Tsai, Susanne Schaffert and Robert Weltevreden) who stepped down during the financial year 2022. |
9 The portion of the Annual Incentive delivered in equity is rounded up to the nearest share, based on the closing share price on the grant date (January 24, 2024) of CHF 93.53 per Novartis share and USD 107.55 per ADR. |
10 This amount is out of total social security employer contributions of CHF 1 933 476 and pension employer contributions of CHF 1 852 898 paid in 2023 for all Executive Committee members. |
|
|
Number of equity instruments granted to the CEO and Executive Committee (2023 compared with 2022)
| | Variable compensation1 | |
| | | |
| | | |
| | | |
| | 2023 Annual Incentive
(performance achieved)
Equity
(number)2 | |
2023-2025 LTPP
PSUs
(target number)3
| |
Other
Equity/PSUs
(number)
| |
Total
2023
| |
Total
2022
| |
|
|
Vasant Narasimhan | | 27 132 | | 69 683 | | - | | 96 815 | | 90 145 | |
|
Victor Bulto | | 8 498 | | 25 914 | | - | | 34 412 | | 14 016 | |
|
Patrick Horber (from December 1, 2023) 5 | | 709 | | - | | 62 981 | | 63 690 | | - | |
|
Harry Kirsch | | 24 758 | | 33 770 | | - | | 58 528 | | 43 749 | |
|
Other ECN members | | 58 099 | | 149 713 | | - | | 207 812 | | 218 563 | |
|
Subtotal | | 119 196 | | 279 080 | | 62 981 | | 461 257 | | 366 473 | |
|
|
Members who stepped down 4 | | 6 763 | | 32 445 | | - | | 39 208 | | 178 653 | |
|
Subtotal | | 6 763 | | 32 445 | | - | | 39 208 | | 178 653 | |
|
Total | | 125 959 | | 311 525 | | 62 981 | | 500 465 | | 545 126 | |
|
|
1 The values of these awards are reported in the table "—Compensation at grant value for the CEO and Executive Committee." |
2 Vested shares, restricted shares and/or RSUs granted under the Annual Incentive for the 2023 performance period. |
3 Target number of PSUs granted under the LTPP for the 2023-2025 performance cycle. |
4 Marie-France Tschudin stepped down from the Executive Committee on September 15, 2023, and will end her contractual notice period on September 30, 2024. The LTPP grant for the 2023-2025 performance cycle, included in the table above, will vest at the end of the performance cycle on a pro-rata basis subject to the plan rules. |
5 In line with the Company’s buyout policy (see “—CEO and Executive Committee: appointments”), Patrick Horber received buyout awards of 36 129 PSUs, and 26 852 RSUs, both of which will vest between 2024 and 2026, in lieu of the Annual Incentive and LTI that he forfeited when leaving his previous employer. |
Additional disclosures and other statutory information
Fixed and variable compensation
The following table summarizes the annual base salary and variable compensation at grant for the financial year 2023 for the CEO and Executive Committee.
| | Annual
Base Salary1 | | Variable
Compensation2 | |
|
Vasant Narasimhan | | 13.9% | | 86.1% | |
|
Victor Bulto | | 17.1% | | 82.9% | |
|
Patrick Horber (from December 1, 2023) | | 1.2% | | 98.8% | |
|
Harry Kirsch | | 17.4% | | 82.6% | |
|
Other ECN members 3, 4 | | 19.3% | | 80.7% | |
|
Total | | 15.8% | | 84.2% | |
|
| | | | | |
|
1 Pro-rated for ECN time. |
2 See the table “–Compensation at grant value for the CEO and Executive Committee” with regard to the disclosure principles of variable compensation. |
3 For the other seven active members at December 31, 2023. |
4 Excludes the member who stepped down during the financial year 2023. |
Other payments to Executive Committee members
During 2023, no other payments or waivers of claims other than those set out in the tables (including the footnotes) contained in this Compensation Report were made to Executive Committee members or to “persons closely linked” to them.
Payments to former Executive Committee members
Under the contracts of Executive Committee members and in line with the Company’s LTI plan rules, payments were made to 12 former members. Of this, CHF 8 725 507 relates to the vesting of LTI awards. In addition, contractual amounts totaling CHF 5 028 812 were made (comprising the base salary, the Annual Incentive and other benefits), and tax equalization on variable compensation granted during international assignments/commuter arrangements amounted to a total of CHF 221 718. The highest paid former Executive Committee member was John Tsai, who received CHF 3 537 225 (comprising the base salary, the Annual Incentive, realized LTI and other benefits). No other payments (or waivers of claims) were made to former Executive Committee members or to “persons closely linked” to them during 2023.
Persons closely linked
“Persons closely linked”, a definition used throughout the Annual Report, are (i) their spouse or equivalent, (ii) their children (under 18 years of age), (iii) any legal entities that they own or otherwise control, and (iv) any legal or natural person who is acting as their fiduciary.
Malus and clawback
Consistent with our “—CEO and Executive Committee compensation philosophy and system,” in 2023 there was no legal or factual basis on which to exercise malus or clawback for current or former Executive Committee members.
Award and delivery of equity to Novartis employees
During 2023, 11.8 million unvested restricted shares (or ADRs), RSUs and target PSUs were granted, and 10.7 million Novartis vested shares (or ADRs) were delivered to Novartis employees under various equity-based participation plans. Current unvested equity instruments (restricted shares, RSUs and target PSUs) held by employees represent 0.92% of issued shares. Novartis delivers treasury shares to employees to fulfill these obligations and aims to offset the dilutive impact from its equity-based participation plans.
Note 28 to the Company’s audited consolidated financial statements
The total expense for the year for compensation awarded to Executive Committee, using IFRS Accounting Standards measurement rules, is presented in Note 28 to the Company’s audited consolidated financial statements.
Shares, ADRs and other equity rights owned by Executive Committee members as at December 31, 20231
The following table shows, in alphabetical order after the CEO, the total number of shares, ADRs and other equity rights owned by the CEO and the other Executive Committee members and “persons closely linked” to them as at December 31, 2023. At this date, no members of the Executive Committee, either individually or together with “persons closely linked” to them, owned 1% or more of the outstanding shares or ADRs of Novartis. As at December 31, 2023, all members who had served at least five years on the Executive Committee had met or exceeded their personal Novartis share ownership requirements.
| |
Vested shares
and ADRs1
| |
Unvested shares
and other equity rights2
| | Equity ownership level
as a multiple of
annual base salary3 | |
Unvested target PSUs
(e.g., LTPP)4
| | Total as at
December 31,
2023 | | Total as at
December 31,
2022 |
|
Vasant Narasimhan | | 250 240 | | 77 324 | | 15x | | 169 770 | | 497 334 | | 406 502 |
|
Shreeram Aradhye | | 0 | | 17 523 | | 1x | | 19 573 | | 37 096 | | 14 394 |
|
Victor Bulto | | 1 780 | | 32 000 | | 3x | | 28 310 | | 62 090 | | 36 386 |
|
Aharon Gal | | 47 660 | | 39 241 | | 9x | | 6 862 | | 93 763 | | 62 960 |
|
Karen Hale | | 5 481 | | 18 527 | | 2x | | 48 182 | | 72 190 | | 28 568 |
|
Patrick Horber | | 0 | | 27 561 | | 2x | | 22 083 | | 49 644 | | 0 |
|
Harry Kirsch | | 342 730 | | 35 944 | | 29x | | 82 290 | | 460 964 | | 399 948 |
|
Robert Kowalski | | 0 | | 21 450 | | 2x | | 26 085 | | 47 535 | | 32 495 |
|
Steffen Lang | | 124 349 | | 28 821 | | 14x | | 49 080 | | 202 250 | | 174 237 |
|
Fiona Marshall | | 5 010 | | 44 643 | | 4x | | 16 864 | | 66 517 | | 34 980 |
|
Klaus Moosmayer | | 21 559 | | 15 396 | | 4x | | 31 187 | | 68 142 | | 47 421 |
|
Subtotal | | 798 809 | | 358 430 | | | | 500 286 | | 1 657 525 | | 1 237 891 |
|
| | | | | | | | | | | | |
Members who stepped down | | 36 174 | | 33 493 | | | | 81 135 | | 150 802 | | 667 092 |
|
Subtotal | | 36 174 | | 33 493 | | | | 81 135 | | 150 802 | | 667 092 |
|
Total | | 834 983 | | 391 923 | | | | 581 421 | | 1 808 327 | | 1 904 983 |
|
|
1 Includes holdings of persons closely linked to Executive Committee members (see definition "—Persons closely linked"). |
2 Includes unvested shares and ADRs as well as other equity rights applicable for the determination of equity amounts for the share ownership requirements, as per the definition "—CEO and Executive Committee: share ownership requirements." Also includes unvested keep-whole awards received in connection to the Sandoz spin-off. |
3 The multiple is calculated based on the full-year annual base salary and the closing share price as at the end of the 2023 financial year. The share price and ADR price on the final trading day of 2023 was CHF 84.87 and USD 100.97, respectively. |
4 The target number of PSUs is disclosed pro-rata to December 31, 2023, unless the award qualified for full vesting under the relevant plan rules. Also includes unvested keep-whole awards received in connection to the Sandoz spin-off. |
Executive Committee compensation approved by shareholders
The total compensation dispensed by the Company in 2023 is within the Say-on-Pay budget approved by the shareholders at the 2022 AGM, including active Executive Committee members and those who stepped down in the course of 2023.
CEO and Executive Committee compensation philosophy and system
Compensation philosophy
Our compensation philosophy aims to ensure that we attract and retain outstanding Executive Committee members and reward them according to their success in implementing the Company strategy, as well as their contribution to the Company performance and long-term value creation. The main elements of our compensation philosophy are set out in the table below.

Pay for performance•Variable compensation is tied directly to the achievement of strategic Company targetsShareholderalignment•Our incentives are significantly weighted toward long-term equity-based plans•Measures under the Long-Term Incentive plans are calibrated to promote the creation of shareholder value•Executive Committee members are expected to build and maintain substantial shareholdingsBalanced rewards•Balanced set of measures to create sustainable value•Mix of targets based on financial metrics, strategic objectives, and performance versus our competitorsBusiness ethics•The Novartis Values and Behaviors are an integral part of our compensation system•They underpin the assessment of overall performance for the Annual IncentiveCompetitive compensation•Total compensation must be sufficient to attract and retain key global talent•Overarching emphasis on pay for performance
Alignment with Company strategy
Our strategy is to focus on high-value, innovative medicines that alleviate society’s greatest disease burdens through technology leadership in R&D and novel access approaches.
We made some strategic updates to our compensation framework to ensure it remains aligned to our Company strategy and compensation philosophy, while being market competitive. Details of these changes are provided in “—2024 Executive Committee compensation system changes and increases.”
Approach to market benchmarking
Significant competition continues to exist for top executive talent with deep expertise and the requisite competencies and proven performance within the pharmaceutical and biotechnology industries. For this reason, external peer compensation data is one of a number of key reference points considered by the Board of Directors and the Compensation Committee when making decisions on executive pay, so as to help ensure that the compensation system and levels at Novartis remain competitive. Novartis is committed to confirming benchmarking practices, including the healthcare peer group, to shareholders on an annual basis.
The Compensation Committee believes in a rigorous approach to peer group construction and maintenance. Furthermore, it believes that using a consistent set of global peers that is similar in size and scope of the operations of Novartis enables shareholders to evaluate the compensation year on year and make pay-for-performance comparisons.
Although Novartis is headquartered in Switzerland, more than a third of its sales come from the US market, and the US therefore represents a significant talent pool for the recruitment of executives by the Company. The Compensation Committee uses a pay comparator group of global healthcare companies to ensure that Novartis is able to attract and retain key talent globally. To ensure European and local practices are fully taken into account, the Compensation Committee also uses a cross-industry peer group of Europe-headquartered multinational companies of a similar size and scope.
global healthcare peer companies
AbbVie
Biogen
GlaxoSmithKline
Novo Nordisk
Roche
Amgen
Bristol-Myers Squibb
Gilead Sciences
Merck & Co.
Sanofi
AstraZeneca
Eli Lilly & Co.
Johnson & Johnson
Pfizer
European peer companies
Anheuser-Busch InBev
AstraZeneca
Bayer
BMW
GlaxoSmithKline
L’Oréal
Merck KGaA
Nestlé
Novo Nordisk
Reckitt Benckiser
Roche
Siemens
Sanofi
Unilever
Components of CEO and Executive Committee compensation
The compensation of the CEO and Executive Committee is comprised of fixed pay, including an annual base salary, pension and other benefits, in addition to a variable annual incentive and long-term incentive, which are entirely performance based.
• The annual base salary is based on the individual’s role, skills and experience. It is reviewed on an annual basis based on an external benchmark for the role, the performance of the individual, business performance and the external environment, salary increases across the Company and market movements.
Pension and other benefits
• Pension and other benefits are provided to the ECN members on the same terms as to all other employees based on local country practices and regulations. No supplementary pension plans or savings plans are provided.
• Pension and other benefits do not constitute a significant proportion of total compensation.
• Globally the Company operates both defined benefit and defined contribution pension plans (see also Note 26 to the Company’s consolidated financial statements).
• Novartis may provide other benefits according to local market practice. These include the provision of a company car, tax and financial planning, and insurance benefits.

PLAN OVERVIEWTarget Annual Incentive On-target opportunities•CEO: 150% of annual base salary.•Other Executive Committee members: 80% to 120% of annual base salary.Performance measures•An Annual Incentive balanced scorecard containing:•Financial performance measures (60% weighting) related to the Company •Strategic objectives (40% weighting)•The balanced scorecard targets and achievements of the CEO are detailed in “2023 CEO Annual Incentive balanced scorecard.”•The balanced scorecards for individual Executive Committee members include the same company financial targets (60% weighting) as well as individual qualitative and quantitative targets (40% weighting).•Values and Behaviors are a key component of the Annual Incentive and are embedded in our culture. As such, members of the Executive Committee are expected to demonstrate these to the highest standards.Target setting•Financial targets are set at the beginning of each financial year and align with the strategic plan proposed by management to the Board of Directors for approval.•The strategic objectives are aligned with the most important priorities in any performance year.Payout ranges•The payout schedule for the Annual Incentive incorporates performance against financial and strategic objectives. The payout range is 0% to 200% of on-target opportunity based on performance, as shown below:PERFORMANCEPAYOUT (% of on-target)Outstanding170% 200%Exceeds expectations130% 160%Meets expectations80% 120%Partially meets expectations40% 70%Below expectations0%Payout formulaPayout vehicle•At the end of the performance period, 50% is paid in cash, and the remaining 50% is delivered in Novartis restricted shares or RSUs, deferred for three years.•Executives may choose to receive all or part of the cash portion of their Annual Incentive in Novartis shares or American Depositary Receipts (ADRs; US only) that will not be subject to forfeiture conditions. In the US, awards may also be delivered in cash under the US deferred compensation plan.Dividend rights, voting rights and settlement•Novartis restricted shares and ADRs carry voting rights and dividends during the vesting period. RSUs are of equivalent value but do not carry voting rights and dividends during the vesting period.•Following the vesting period, settlement of RSUs is made in unrestricted Novartis shares or ADRs. Annual base salaryxTargetincentive % =Target Annual IncentiveAnnual base salaryxTarget incentive %xPayout factor (% of target: 0%200%)=Realized Annual Incentive

PLAN OVERVIEWAward vehiclePerformance share units (PSUs) are granted at the beginning of the three-year performance cycle and vest at the end of the cycle to the extent that performance conditions have been met. At the time of vesting, they are converted into Novartis shares.PSUs carry dividend equivalents that are paid in shares at the end of the cycle.Grant formula At the start of the performance cycle, PSUs are granted under the LTPP, as follows:Target opportunity •CEO: 325% of annual base salary•Other Executive Committee members: between 180% and 260% of annual base salaryPerformance measures•3rd party sales CAGR (25%)•Core operating income CAGR (25%)•Innovation (25%)•Relative TSR (25%)Target settingFinancial targets: Targets for 3rd party sales CAGR and core operating income CAGR are set based on the strategic plan of the Company. Innovation: Development targets are based on targeted filings communicated at the start of each performance cycle, weighted 70%. The Science & Technology Committee (STC) determines the most important Biomedical Research milestones, weighted 30%. Payout rangeFinancial targets: When assessing performance, achievements for threshold, target and maximum payout are defined for each metric, and a payout curve is applied to determine the corresponding payout between 0-200% against target.Innovation: At the end of the cycle, the Compensation Committee determines the payout factor in the range of 0150% based on the performance assessment made by the STC. A payout between 150-200% of target is only delivered for truly exceptional performance.Relative TSR: Performance on TSR is assessed relative to our global healthcare peer group, as outlined below. A three-month averaging method is used for both the start and the end of the performance cycle. Companies are then ranked in order of highest to lowest TSR in USD. No payout for below median TSR applies.The Compensation Committee may use its discretion on each metric, including deciding on the payout within the ranges where appropriate. In doing so, it takes into consideration factors such as the underlying assumptions of the targets set at the beginning of the cycle, overall economic conditions, currency fluctuations and other unforeseeable situations.Payout formulaStep 1Annual base salaryxTarget incentive %=Grant valueStep 2Grant value/Share price=Target number of PSUsGlobal healthcare peer groupAbbVieBiogenGlaxoSmithKlineNovo NordiskRocheAmgen Bristol-Myers SquibbGilead SciencesMerck & Co.SanofiAstraZenecaEli Lilly & Co Johnson & JohnsonPfizerNovartis position Payout rangein the peer group(% of target)Position 1 2Position 3 5Position 6 8Position 9 15170% 200% 130% 160%80% 120%0%Target number of PSUsxPayout factor+Dividend equivalents=Realized PSUs
CEO and Executive Committee: share ownership requirements
CEO and Executive Committee members are required to own at least a minimum multiple of their annual base salary in Novartis equity as set out in the table below. The Compensation Committee reviews compliance with the share ownership guideline on an annual basis.
FunctionOwnership levelAdditional holding requirementsTime for achieving levelEquity included in determinationCEOCFOOther EC members5 x annual base salary3 x annual base salaryEquity vesting under the LTPP for a minimum of two years after the vesting dateNone5 years within hire or promotion. In the event of a substantial rise or drop in the share price, the Board of Directors may, at its discretion, amend the time period accordingly.•Vested and unvested Novartis shares or ADRs, and RSUs acquired under Novartis compensation plans (unvested PSUs excluded)•Other shares and vested options of Novartis shares or ADRs that are owned directly or indirectly by “Persons closely linked” to an Executive Committee member
CEO and Executive Committee: appointments

Element of compensationPolicyLevelThe overall package should be market-competitive to enable the recruitment of global executive talent with deep expertise and competencies.Annual base salaryThe Compensation Committee may appoint individuals who are new to a role on an annual base salary that is below the market level, with a view to increase this toward market level over a period of three to four years as an individual develops in the role.This prudent approach ensures pay levels are merit-based, with increases dependent on strong performance and proven ability in the role over a sustained period.If the scope of an existing Executive Committee member’s role changes significantly during the year, the Compensation Committee may make adjustments to the individual’s base salary (and/or incentives) in consideration of the benchmark of the new role and the Executive Committee appointments compensation policy.IncentivesThe compensation package will normally include the key compensation elements and incentive opportunities in line with those offered to current Executive Committee members.In exceptional circumstances, higher incentive opportunities than those offered to current Executive Committee members may be provided at the Compensation Committee’s discretion.Performance measures may include business-specific measures tailored to the specific role.Pension and other benefitsNewly appointed Executive Committee members are eligible for the local country pension plan and other benefits in line with the wider employee group.BuyoutsThe Compensation Committee seeks to balance the need to offer competitive compensation opportunities to acquire the talent required by the business with the principle of maintaining a strong focus on pay for performance.As such, when an individual forfeits variable compensation as a result of an appointment at Novartis, the Compensation Committee may offer replacement awards to compensate the commercial equivalent value or fair value of payments and awards forfeited by the individual, in such form as the Compensation Committee considers appropriate, taking into account relevant factors.Relevant factors include the expected value of the forfeited award, the replacement vehicle (i.e., cash, restricted share units, restricted shares or performance share units), whether the award is contingent on meeting performance conditions or not, the timing of forfeiture (i.e., Novartis mirrors the blocking or vesting period of the forfeited award) and the leaver conditions, in case the recruited individual leaves Novartis prior to the end of the blocking or vesting period.International mobility If individuals are required to relocate or be assigned away from their home location to take up their position, relocation support may be provided in line with our global mobility policies (e.g., relocation support, tax equalization). This includes ongoing US state income tax liabilities on behalf of US citizens locally employed outside the US who have US workdays and therefore, US state taxable compensation that generates a US state tax liability.
CEO and Executive Committee: termination arrangements

ElementsRetirement, termination by the Company for reasons other than performance or conduct, and change of controlVoluntary resignationTermination by the Company for misconduct or poor performanceDeath or long-term disabilityAnnual Incentive for period between start of notice and termination datePro-rata Annual Incentive is paid to reflect the portion of the year the individual was employed.Annual Incentive is fully forfeited.Pro-rata Annual Incentive is paid to reflect the portion of the year the individual was employed.Unvested equity: mandatory deferral of Annual Incentive into restricted shares/ restricted share units (RSUs)Awards are released on the original blocking end date. Is subject to forfeiture in the event that a leaver joins a competitor company before the original vesting date.Unvested restricted shares and restricted share units (RSUs) are forfeited.Accelerated vesting is applied.Unvested equity: voluntary deferral of Annual Incentive into restricted shares/RSUs/American Depository Receipts (ADRs) (ADRs applicable for US employees only)Awards are not subject to forfeiture during the deferral period. Unvested equity: mandatory Long-Term Incentive performance share units (PSUs)Awards vest on the regular vesting date, subject to performance, on a pro-rata basis for time spent with the Company during the performance cycle. Is subject to forfeiture in the event that a leaver joins a competitor company before the vesting date.All of the award is forfeited.Accelerated vesting at target is applied.Unvested equity: Buyouts or previous equity grants in restricted shares/ restricted share units (RSUs)Accelerated vesting is applied to equity pro-rated until last date of employmentAll of the award is forfeited.Accelerated vesting is applied
Further details are provided in in our “—Risk Management principles.”
Malus and clawback policy
Any incentive compensation paid to Executive Committee members is subject to malus and clawback rules. This means that the Board of Directors for the CEO, and the Compensation Committee for the other Executive Committee members, may decide – subject to applicable law – to retain any unpaid or unvested incentive compensation (malus), or to recover incentive compensation that has been paid or vested in the past (clawback). This applies in cases where the payout has resulted from a violation of laws or conflicts with internal management standards, including Company and accounting policies.
This principle applies to both the short-term Annual Incentive and all long-term incentive plans.
In October 2023, the Compensation Committee adopted a no-fault compensation clawback policy “for the recovery of erroneously awarded compensation” to all members of the Executive Committee and certain executive officers, in the event that the Company is required to prepare an accounting restatement, in full compliance with the U.S. Securities and Exchange Commission (SEC) Rule.
CEO and Executive Committee performance management
To foster a high-performance culture, the Company applies a performance management process based on quantitative and qualitative criteria. The CEO and the other Executive Committee members are subject to a formal three-step process, which consists of objective setting, performance evaluation and compensation determination. This process is explained in the chart below.
Performance targets are generally set before the start of the relevant performance cycle. A rigorous framework is in place for establishing targets to ensure they are suitably robust, challenging and align with the strategic priorities of the Company.
The key factors taken into account when setting targets include:
• Internal and external market expectations
• The strategic priorities of Novartis
• Regulatory factors (e.g., new launches, patent expiries)
• Investment in capital expenditure
• Novartis Values and Behaviors
The targets are challenged at multiple stages before they are ultimately approved by the Board of Directors. In line with good governance practices, the Compensation Committee works to set targets that are ambitious and challenging but do not encourage undue risk-taking.
Following the end of the performance cycle, the Board of Directors and the Compensation Committee consider performance against the targets originally set. The CEO and Executive Committee members are not present while the Board of Directors and the Compensation Committee discuss their individual performance evaluations and determine their individual compensation. Prior to determining the final outcome, related factors such as performance relative to peers, wider market conditions, general industry trends and best practice are used to inform the overall performance assessment.
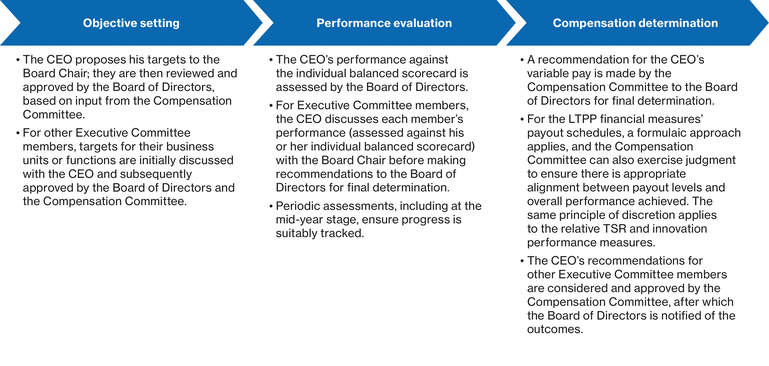
Objective setting•The CEO proposes his targets to the Board Chair; they are then reviewed and approved by the Board of Directors, based on input from the Compensation Committee.•For other Executive Committee members, targets for their business units or functions are initially discussed with the CEO and subsequently approved by the Board of Directors and the Compensation Committee.Performance evaluation•The CEO’s performance against the individual balanced scorecard is assessed by the Board of Directors.•For Executive Committee members, the CEO discusses each member’s performance (assessed against his or her individual balanced scorecard) with the Board Chair before making recommendations to the Board of Directors for final determination.•Periodic assessments, including at the mid-year stage, ensure progress is suitably tracked.Compensation determination •A recommendation for the CEO’s variable pay is made by the Compensation Committee to the Board of Directors for final determination.•For the LTPP financial measures’ payout schedules, a formulaic approach applies, and the Compensation Committee can also exercise judgment to ensure there is appropriate alignment between payout levels and overall performance achieved. The same principle of discretion applies to the relative TSR and innovation performance measures.•The CEO’s recommendations for other Executive Committee members are considered and approved by the Compensation Committee, after which the Board of Directors is notified of the outcomes.
2024 Executive Committee compensation system changes and increases
Novartis is today a pure-play innovative medicines company with a focused strategy. The Board of Directors and the Compensation Committee therefore decided to make certain changes to the compensation system, in particular to reflect the ambition of Novartis to build its US business organically and become a top player in the US.
In this context, the following changes align with our compensation philosophy, and will enable Novartis to compete for talent globally (see “—Compensation philosophy”):
Annual Incentive metrics
As of the 2024 performance year, core operating income (which includes adjustments for certain one-time/non-recurring items such as restructuring and M&A write-downs) replaces operating income in the financial objectives of the Annual Incentive. The weighting remains at 30% of the financial objectives. The Compensation Committee decided to make this change as core operating income:
• Is consistent with how investors and analysts measure underlying performance
• Encourages executives to make bold investments with high return potential
• Aligns with our global healthcare peers and the broader market, enabling more effective performance comparisons
A reconciliation between our core operating income and operating income will continue to be provided in “—Item 5. Operating and Financial Review and Prospects —Non-IFRS measures as defined by Novartis —Reconciliation from IFRS Accounting Standards results to non-IFRS measures core results.”
To maintain strong alignment between performance and pay outcomes, the Board of Directors will retain its discretion to consider any adjustment to the Annual Incentive payout of the CEO and other Executive Committee members. Any such decisions will be disclosed in the Compensation Report.
Share ownership and Annual Incentive deferral
The CEO shareholding requirement increased from 5x to 6x the CEO’s annual base salary. Increasing the CEO shareholding requirement better aligns with shareholder interests and brings Novartis in line with its global healthcare peers.
The Compensation Committee also decided to amend the portion of the Annual Incentive that is mandatorily deferred into equity to make the compensation system more competitive, particularly in markets where bonus deferrals are uncommon (such as Switzerland and US, where much of our executive talent is sourced). As of the 2024 performance year, the portion of the Annual Incentive that is mandatorily deferred into equity is reduced from 50% to 30% for all Executive Committee members, provided that their shareholding requirement is met. Compliance with their shareholding requirement must then be maintained.
CEO target compensation
Since 2019, the Board of Directors has made no material increases to the CEO compensation. As a result, the CEO’s target pay has fallen below the 25th percentile of global healthcare peers.
Given the competitive landscape of the industry, the Board of Directors decided to increase the CEO LTPP target from 325% to 400% of his annual base salary as from the cycle 2024-26. This represents a 15.8% increase in total target compensation and moves him out of the bottom quartile to just above the 25th percentile of global healthcare peers, as shown in the figure below. The maximum payout for the LTPP remains at 200% of target, and there will be no change to the metrics. For this review, we used the same global healthcare companies specified in “—Approach to market benchmarking.”
Last disclosed global healthcare peer CEO target compensation (CHF millions)2023 Novartis CEO target2024 Novartis CEO target 510152025Bottom quartile to medianMedian to upper quartile
In making its decision, the Board of Directors was mindful of investor perspectives toward executive compensation of European companies. It therefore chose to make an increase to the LTPP target only (rather than to the base salary or Annual Incentive), which is fully performance-based (i.e. no use of restricted shares or stock options as used by many peers), to align with the Company’s long term strategy. Targets will continue to be stretched as demonstrated in “—Historic CEO incentive payouts since appointment.”
The Board of Directors considered it appropriate to act now so as to:
• Align with the Company’s ambition to become a top player in the US. This requires a leader with significant knowledge and experience of the US market, and executive compensation in US peer companies is more competitive than in Europe
• Proactively avoid a further decline of the pay positioning of our CEO, while providing a fair compensation system that rewards performance
• Demonstrate its commitment to establishing a competitive system that promotes the retention and attraction of executive talent capable of delivering value to shareholders
Executive Committee compensation increases
Each year, we collaborate with our independent external advisors to benchmark the compensation levels of the Executive Committee members and assess the competitiveness of their total target compensation. 2024 compensation increases have been made in line with demonstrated performance and ability in role as outlined in “—CEO and Executive Committee: appointments.” Accordingly, the following Executive members will receive the following increases, effective 2024:
Shreeram Aradhye, President, Development and Chief Medical Officer
Dr. Aradhye, appointed in May 2022, led an operational improvement of our Development function, resulting in significant approvals and submissions for new medicines across US, EU, China and Japan, as well as promising results for many ongoing Phase III programs. Dr. Aradhye will receive a 4.7% increase in annual base salary and a 20% increase in LTPP target, as a percentage of annual base salary.
Victor Bulto, President, US
Mr. Bulto, appointed in April 2022, delivered a strong performance on almost all brands including Pluvicto, Kisqali, Kesimpta and Scemblix and ensured proactive launch-readiness for Cosentyx HS and Cosentyx IV. Mr. Bulto will receive a 4% increase in annual base salary and a 20% increase in Annual Incentive target, as a percentage of annual base salary.
Aharon (Ronny) Gal, Chief Strategy & Growth Officer
Mr. Gal, appointed in July 2022, made a significant impact in his first full year with the Company, integrating the new Strategy and Growth team across the key decision-making bodies. Under his leadership, several business development deals were completed in the year, including Xiidra, and Chinook. Mr. Gal will receive a 4% increase in annual base salary and a 20% increase in LTPP target, as a percentage of annual base salary.
Karen Hale, Chief Legal Officer
Ms. Hale, appointed in May 2021, successfully managed several large-scale transactions, such as the Sandoz spin-off, as well as successfully handling a number of important legal matters, a civil investigative demand involving Entresto, the Exforge antitrust litigation, a shareholder derivative lawsuit, and critical intellectual property matters. She also played a critical role in managing the obligations under the US Department of Justice’s deferred prosecution agreement and SEC Order and led both to a timely and satisfactory conclusion. Ms. Hale will receive a 2.8% increase in annual base salary and a 20% increase in LTPP target, as a percentage of annual base salary.
Rob Kowalski, Chief People & Organization Officer
Mr. Kowalski, appointed in September 2021, provided critical support to the transformation of the company, including outstanding progress of the restructuring of the organization, successful completion of consultations with works councils and developing the ECN into a high-performing team. Mr. Kowalski will receive a 3.8% increase in annual base salary and a 10% increase in LTPP target, as a percentage of annual base salary.
All other Executive Committee members will receive ordinary base salary increases received by other employees in Switzerland or the US, effective March 1, 2024. Their Annual Incentive and LTPP targets remain unchanged.
Pay practice for other employees
The Board of Directors is equally committed to ensuring fair and competitive compensation practices across the entire organization in 2024. Recent such examples include an approved global budget of USD 420 million for salary adjustments during the year 2024, achieving our EPIC commitments, further closing our global pay gap, and launching the second wave of our all-employee share purchase plan which is now available to 64% of our global population. More details can be found in the Novartis in Society Integrated Report 2023.
Board compensation
Board member total compensation earned for the financial year 2023 (compared with 2022)
| | Positions as per 31 December | | | | Share-Based compensation | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | Board membership | | Audit and Compliance Committee | | Compensation Committee | | Governance, Sustainability and Nomination Committee | | Science & Technology Committee | | Risk Committee | | Cash (CHF) (A) | | Shares (number)1 | | Shares (CHF) (B) | | Social Security (CHF) (C) | | Total 2023 (CHF) (A)+(B)+(C)2 | | Total 2022 (CHF) | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
|
Joerg Reinhardt 3 | | Board Chair | | | | | | | | Chair | | | | 1 900 000 | | 22 606 | | 1 900 000 | | 3 784 | | 3 803 784 | | 3 803 670 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Simon Moroney | | Vice-Chair | | | | Chair | | | | • | | | | 230 000 | | 2 736 | | 230 000 | | - | | 460 000 | | 456 228 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Patrice Bula | | Lead Independent Director | | | | • | | Chair | | | | | | 205 000 | | 2 438 | | 205 000 | | 3 784 | | 413 784 | | 398 670 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Nancy C. Andrews | | • | | | | | | | | • | | • | | 180 000 | | 2 141 | | 180 000 | | - | | 360 000 | | 360 000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Ton Buechner | | • | | • | | | | | | | | Chair | | 210 000 | | 2 497 | | 210 000 | | 4 675 | | 424 675 | | 424 560 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Elizabeth Doherty | | • | | Chair | | | | | | | | • | | 225 000 | | 2 676 | | 225 000 | | - | | 450 000 | | 450 000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Bridgette Heller | | • | | • | | • | | • | | | | | | 215 000 | | 2 557 | | 215 000 | | - | | 430 000 | | 423 334 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Daniel Hochstrasser | | • | | • | | | | • | | | | | | 185 833 | | 2 085 | | 185 833 | | 4 675 | | 376 341 | | 237 894 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Frans van Houten | | • | | • | | | | | | • | | | | 162 500 | | 3 565 | | 227 500 | | 4 675 | | 394 675 | | 390 000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Ana de Pro Gonzalo | | • | | • | | | | | | | | • | | 195 000 | | 2 319 | | 195 000 | | - | | 390 000 | | 329 560 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Charles L. Sawyers | | • | | | | | | • | | • | | | | 180 000 | | 2 141 | | 180 000 | | - | | 360 000 | | 360 000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
William T. Winters | | • | | | | • | | • | | | | | | - | | 4 283 | | 360 000 | | - | | 360 000 | | 360 000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
John D. Young 4 | | • 4 | | | | | | | | • 4 | | • 4 | | 150 000 | | 991 | | 150 000 | | 4 675 | | 304 675 | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Subtotal | | | | | | | | | | | | | | 4 038 333 | | 53 035 | | 4 463 333 | | 26 267 | | 8 527 933 | | 7 993 916 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Board members who stepped down 5 | | | | | | | | | | | | | | 30 000 | | 1 150 | | 30 000 | | 3 784 | | 63 784 | | 512 339 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Subtotal | | | | | | | | | | | | | | 30 000 | | 1 150 | | 30 000 | | 3 784 | | 63 784 | | 512 339 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Total | | | | | | | | | | | | | | 4 068 333 | | 54 185 | | 4 493 333 | | 30 051 | | 8 591 717 | | 8 506 255 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
|
1 The amounts shown represent the gross number of shares delivered to each Board member in 2023 for the respective Board member’s service period. The number of shares reported in this column represent: (i) the second and final equity installment delivered in February 2023 for their service from the 2022 AGM to the 2023 AGM; and (ii) the first of two equity installments delivered in August 2023 for their service from the 2023 AGM to the 2024 AGM. The second and final equity installment for their service from the 2023 AGM to the 2024 AGM will take place in February 2024. |
2 All amounts are before the deduction of social security contributions and income tax paid by the Board members. |
3 No additional committee fees for chairing the Science & Technology Committee were delivered to Joerg Reinhardt. |
4 From March 7, 2023. |
5 Includes the compensation earned by Andreas von Planta, who stepped down at the 2023 AGM, as well as Ann Fudge and Enrico Vanni, who stepped down at the 2022 AGM. |
|
Compensation approved and dispensed
In CHF | | | | Board of Directors | |
|
Compensation earned during the financial year 2023 | | A | | 8 591 717 | |
|
Compensation earned for the period January 1 to February 28, 2023 (2 months) | | B | | 1 417 153 | |
|
Compensation to be earned for the period from January 1 to February 29, 2024 (2 months) | | C | | 1 435 008 | |
|
Total compensation earned for the period from the 2023 AGM to the 2024 AGM | | A-B+C | | 8 609 573 | |
|
Amount approved by shareholders at the 2023 AGM | | | | 8 750 000 | |
|
Compensation dispensed by the Company within the approved amount | | | | Yes | |
|
|
|
Shares, ADRs and share options owned by Board members
The total number of vested Novartis shares and ADRs owned by members of the Board of Directors and “persons closely linked” to them as at December 31, 2023, is shown in the table below. As at this date, no members of the Board, either individually or together with “persons closely linked” to them, owned 1% or more of the outstanding shares (or ADRs) of Novartis. As at the same date, no members of the Board of Directors held any share options to purchase Novartis shares.
| | Number of shares
at December 31, 20231,2 | |
|
Joerg Reinhardt | | 655 336 | |
|
Simon Moroney | | 5 992 | |
|
Patrice Bula | | 11 240 | |
|
Nancy C. Andrews | | 10 536 | |
|
Ton Buechner | | 22 958 | |
|
Elizabeth Doherty | | 14 843 | |
|
Bridgette Heller | | 6 214 | |
|
Daniel Hochstrasser | | 2 824 | |
|
Frans van Houten | | 17 115 | |
|
Ana de Pro Gonzalo | | 2 422 | |
|
Charles L. Sawyers | | 17 493 | |
|
William T. Winters | | 30 777 | |
|
John D. Young | | 682 | |
|
Subtotal | | 798 432 | |
|
| | | |
Board members who stepped down at the 2023 AGM |
Andreas von Planta | | 169 867 | |
|
Subtotal | | 169 867 | |
|
Total | | 968 299 | |
|
|
1 Includes holdings of persons closely linked to Board members (see definition "—persons closely linked"). |
2 Each share provides entitlement to one vote. |
Additional disclosures and other statutory information
Other payments to Board members
During 2023, no payments (or waivers of claims) other than those set out in the Board member compensation table titled “—Board member total compensation earned for the financial year 2023” (including in the table footnotes) were made to current members of the Board or to “persons closely linked” to them.
Payments to former Board members
During 2023, no payments (or waivers of claims) were made to former Board members or to “persons closely linked” to them.
Note 28 to the Group’s audited consolidated financial statements
The total expense for the year for compensation awarded to Board members, using IFRS Accounting Standards measurement rules, is presented in Note 28 to the Group’s audited consolidated financial statements.
Board compensation philosophy and fee structure
Philosophy and benchmarking
Aligned with market practice in Switzerland, the Board of Directors sets compensation for its members at a level that allows for the attraction of high-caliber individuals, including both Swiss and international members, who have global experience.
Given their focus on corporate strategy, supervision and governance, Board members do not receive variable compensation. Each year at the AGM, shareholders are requested to approve, in a binding vote, the total compensation of the Board of Directors until the following AGM.
The Board of Directors sets the level of compensation for its Chair and the other members to be in line with relevant benchmark companies, including other large Switzerland-based multinational companies. Following the acquisition of Credit Suisse by UBS in 2023, the Board of Directors revised its peer benchmarking group to a larger set of companies comprising ABB, Holcim, Nestle, Richemont, Roche, SwissRe, UBS and Zurich Insurance. This peer group was chosen for Board compensation due to the comparability of Swiss legal requirements, including broad personal and individual liabilities under Swiss law (and criminal liability under Swiss rules regarding board and executive committee compensation related to the Swiss Code of Obligations), and under US law, where applicable (due to the Company’s secondary listing on the New York Stock Exchange). Each year, the Board of Directors reviews the compensation of its members, including the Board Chair, based on a proposal by the Compensation Committee and advice from its independent advisor, including relevant benchmarking information. To ensure independence of decision-making, the peer group used for the Board of Directors is different to that used for the Executive Committee.
The Board Chair’s contract and the Board of Directors compensation policy do not provide for any termination-related payments.
Share ownership requirements for Board members
To ensure their interests are aligned with those of shareholders the Board Chair is required to own a minimum of 30 000 Novartis shares, and other members of the Board of Directors are required to own at least 5 000 Novartis shares within five years of having joined the Board of Directors.
Board members are prohibited from hedging or pledging their ownership positions in Novartis shares that are part of their guideline share ownership requirement and are required to hold these shares for 12 months after having retired from the Board of Directors. As at December 31, 2023, all current and former members of the Board of Directors who were required to meet the minimum share ownership requirements did so.
Board fee structure
The annual fee rates for Board membership and additional functions are included in the table below. These were approved by the Board of Directors and remain unchanged from the prior term. Aggregate Board compensation is aligned with other large Swiss companies.
| | | |
CHF 000s
| | 2023-2024 AGM
annual fee | |
|
Board Chair | | 3 800 | |
|
Board membership | | 280 | |
|
Vice-Chair | | 50 | |
|
Lead Independent Director | | 20 | |
|
Chair of the Audit and Compliance Committee | | 130 | |
|
Chair of the Compensation Committee | | 90 | |
|
Chair of the following committees:
• Governance, Nomination and
Corporate Responsibilities Committee
• Science & Technology Committee
• Risk Committee | |
70
| |
|
Membership of the Audit
and Compliance Committee | |
70
| |
|
Membership of the following committees:
• Compensation Committee
• Governance, Nomination and
Corporate Responsibilities Committee
• Science & Technology Committee
• Risk Committee | |
40
| |
|
In addition, the following policies apply regarding Board compensation:
• 50% of compensation is delivered in cash, paid on a quarterly basis in arrears. Board members may choose to receive more of their compensation in shares instead of cash
• At least 50% of compensation is delivered in shares in two installments: one six months after the AGM; and one 12 months after the AGM
Board members bear the full cost of their employee social security contributions, if any, and do not receive share options or pension benefits.
For 2023, the Board Chair voluntarily waived the increase in compensation to which he is contractually entitled.
Compensation governance
Legal framework
The Swiss Code of Obligations and the corporate governance guidelines of the SIX Swiss Exchange require listed companies to disclose certain information about the compensation of board and executive committee members, their equity participation, and loans made to them. This Annual Report fulfills that requirement in addition to being in line with the principles of the Swiss Code of Best Practice for Corporate Governance of the Swiss Business Federation (economiesuisse). For more information, see “—Corporate Governance” in Section 6C of this Report.
Compensation decision-making authorities
Authority for decisions related to compensation is governed by the Articles of Incorporation, Board Regulations and the Compensation Committee Charter, which are all published on the Company website: www.novartis.com/investors/company-overview/corporate-governance. The Compensation Committee serves as the supervisory and governing body for compensation policies and plans within Novartis, and has overall responsibility for determining, reviewing and proposing compensation policies and plans for approval by the Board of Directors in line with the Compensation Committee Charter. The discussions and conclusions of each committee meeting are delivered to the full Board of Directors. A summary of the compensation decision-making authorities is set out below.
Approval process for key compensation decisions
|
CEO
| Compensation
Committee | Board
Chair | Board of
Directors |
AGM
|
|
|
Executive Compensation | | | | | | |
CEO | | | | | | |
Performance target setting and assessment | | |  |  | | |
|
Individual compensation | |  | |  | | |
|
Other EC members | | | | | | |
Performance target setting and assessment |  | |  | | | |
|
Individual compensation |  |  | | | | |
|
All Executive Committee | | | | | | |
Maximum aggregate amount of fixed and variable long-term compensation | |  | |  | Binding Vote | |
|
Board Compensation | | | | | | |
Board of Directors | | | | | | |
Fee structure for individual roles on the Board of Directors | |  | |  | | |
|
Maximum aggregate amount of compensation for the next term of office | |  | |  | Binding Vote | |
|
Other | | | | | | |
Board members, Executive Committee and other employees | | | | | | |
Compensation report | |  | |  | Consultative Vote | |
|
Compensation policy and principles | |  | |  | | |
|
Variable short-term and long-term compensation payout factors for the Group | |  | |  | | |
|
Committee member independence
The Compensation Committee is composed exclusively of members of the Board of Directors who meet the independence criteria set forth in the Board Regulations. From the 2023 AGM, the Compensation Committee consisted of the following four members: Simon Moroney (as Chair), Patrice Bula, Bridgette Heller, and William Winters.
Role of the Compensation Committee’s independent advisor
The independent external compensation advisor supports the Compensation Committee in determining the design and implementation of compensation and benefits.
In 2023, the Compensation Committee retained Mitul Shah of Deloitte LLP, who was appointed in July 2022, as its independent compensation advisor. The independent advisor from Deloitte LLP and his respective team that advised and supported the Compensation Committee are not responsible or rewarded for work on senior compensation beyond support provided to the Compensation Committee and the People & Organization function.
Meetings held in 2023 and self-evaluation
In 2023, the Compensation Committee held six formal meetings. For the approval of the Board of Directors, in line with prior years, it collaborated with the Science & Technology Committee to review and endorse the innovation targets and achievements of the Annual Incentive and LTPP. The Compensation Committee will conduct a self-evaluation in 2024.
Risk management principles
The Compensation Committee, with support from its independent advisor, reviews market trends in compensation, and changes in corporate governance rules and best practices. Together with the Risk Committee, it also reviews the Novartis compensation systems to ensure that they do not encourage inappropriate or excessive risk-taking, and instead encourage behaviors that support sustainable value creation. A summary of the risk management principles is outlined below.
RISK MANAGEMENT PRINCIPLES
• Rigorous performance management process, with approval of targets and evaluation of performance of the CEO by the Board of Directors
• Balanced mix of short-term and long-term variable compensation elements
• Novartis Values and Behaviors are a key component of the Annual Incentive and are embedded in our culture
• Clawback and malus principles apply to all elements of the variable compensation
• Performance-vesting Long-Term Incentives only, with three-year cycles
• All variable compensation is capped at 200% of target
• Contractual notice period of 12 months
• Post-contractual non-compete period is limited to a maximum of 12 months from the end of employment. Resulting compensation, if applicable, will not exceed the average annual compensation (annual base salary plus Annual Incentive) of the previous three financial years
• Good and bad leaver provisions apply to variable compensation of leavers
• No severance payments or change-of-control clauses
• Share ownership requirements; no hedging or pledging of Novartis share ownership
• No loans granted to current or former members of the Executive Committee and the Board of Directors or to “Persons closely linked” to them
Mandates outside the Novartis Group
According to article 34 of the Articles of Incorporation (https://www.novartis.com/investors/company-overview/corporate-governance), limitations apply to mandates outside the Novartis Group for Board members and Executive Committee members (see “-Item 6.C Board Practices-Board of Directors-Mandates outside the Novartis Group” and “-Item 6.C Board Practices-Executive Committee-Mandates outside the Novartis Group”). The following external mandates are subject to these limitations and are therefore presented in the Compensation Report.
Board Members
Joerg Reinhardt
Swiss Re AG, Switzerland

• Member of the Board
Nancy C. Andrews
Charles River Laboratories International, Inc., US

• Member of the Board
• Chair of the Science and Technology Committee
Maze Therapeutics, Inc., US
• Member of the Board
Ton Buechner
Burckhardt Compression AG, Switzerland

• Board Chair
• Chair of the Strategy and Sustainability Committee
Swiss Prime Site AG, Switzerland

• Board Chair
• Chair of the Sustainability Board
Tonality Holding AG, Switzerland (private holding)*
• Director
Bandinnera GmbH, Switzerland (private holding)*
• Manager
Great Apes Aviation GmbH, Switzerland (private holding)*
• Manager
Patrice Bula
Schindler AG, Switzerland

• Member and Vice Chair of the Board
Froneri Lux Topco Sarl, Luxembourg
• Board Chair
New Tiger LLC, US
• Member of the Board
• Chair of the ESG Committee
Elizabeth (Liz) Doherty
Corbion NV, Netherlands

• Member of the Board
• Chair of the Audit Committee
Royal Philips NV, Netherlands

• Member of the Supervisory Board
• Chair of the Audit Committee
Bridgette Heller
Aramark, US

• Member of the Board
DexCom, Inc., US

• Member of the Board
Integral Ad Science Inc., US

• Member of the Board
Newman’s Own Inc., US
• Member of the Board
Daniel Hochstrasser
Daniel Hochstrasser AG, Switzerland
• Board Chair
• CEO
Frans van Houten
Absci Corporation, US

• Member of the Board
Castor EDC, NL
• Board Chair
Synthesis Health Inc. US
• Member of the Board
FvH Capital BV, NL (private family holding)
• Director
Simon Moroney
Biotalys NV, Belgium

• Board Chair
• Chair of the Remuneration and Nomination Committee
Ana de Pro Gonzalo
Mobico Group PLC, UK

• Member of the Board
STMicroelectronics NV, Switzerland

• Member of the Supervisory Board
• Chair of the Audit Committee
Charles Sawyers
–
William Winters
Standard Chartered Bank plc., UK

• Member of the Board
• CEO
John Young
Arvinas Inc, US

• Member of the Board
Johnson Controls International plc., Ireland

• Member of the Board
Imbria Pharmaceuticals Inc., US
• Member of the Board
Executive Committee members
Steffen Lang
Bachem Holding AG, Switzerland

• Board member
Other Executive Committee members
–

in listed companies
* under common ownership
(This page has been left blank intentionally.)
(This page has been left blank intentionally.)
6.C Board practices
Corporate governance
Framework
Novartis is committed to effective corporate governance, and our corporate governance framework is intended to support sustainable financial performance and long-term value creation for our shareholders, patients, employees and other stakeholders based on our Values and Behaviors.
Novartis AG is subject to and compliant with the laws and regulations of Switzerland (in particular, Swiss company and securities law, SIX Swiss Exchange rules and the Swiss Code of Best Practice for Corporate Governance) and the securities laws of the United States, including New York Stock Exchange (NYSE) rules, applicable to foreign private issuers of securities.
The Novartis corporate governance principles are described in key governance documents, in particular in our Articles of Incorporation and the Regulations of the Board, the Board Committees and the Executive Committee (“Board Regulations”) (www.novartis.com/investors/company-overview/corporate-governance).
The Governance, Sustainability and Nomination Committee (GSNC) regularly reviews both the corporate governance principles and the key governance documents against evolving best practice standards and new developments in line with our commitment to maintaining the highest standards.
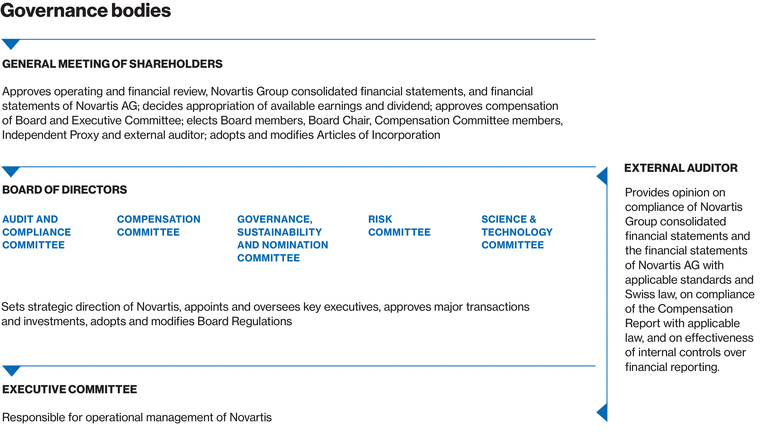
Our leadership structureGovernance bodies General Meeting of ShareholdersApproves operating and financial review, Novartis Group consolidated financial statements, and financial statements of Novartis AG; decides appropriation of available earnings and dividend; approves compensation of Board and Executive Committee; elects Board members, Board Chair, Compensation Committee members, Independent Proxy and external auditor; adopts and modifies Articles of IncorporationExternal auditorProvides opinion on compliance of Novartis Group consolidated financial statements and the financial statements of NovartisAG with applicable standards and Swiss law, on compliance of the Compensation Report with applicable law, and on effectiveness of internal controls over financial reporting.Board of DirectorsAudit and Compliance CommitteeCompensation CommitteeGovernance, Sustainability and Nomination CommitteeRISK CommitteeSCIENCE &TECHNOLOGYCommitteeSets strategic direction of Novartis, appoints and oversees key executives, approves major transactions andinvestments, adopts and modifies Board RegulationsExecutive CommitteeResponsible for operational management of Novartis
Group structure and shareholders
Group structure
Novartis AG and Group companies
Novartis AG, the Group’s holding company, is a corporation organized under Swiss law with issued registered shares and registered office at Lichtstrasse 35, CH-4056 Basel, Switzerland.
The principal subsidiaries and associated companies of the Novartis Group are shown in “Item 18. Financial Statements—Note 33. Novartis principal subsidiaries and associated companies.”
Organizational structure
Novartis is an innovative medicines company. Following the spin-off of Sandoz, it no longer has divisions. Its five organizational units represent parts of the Company along the research and development/production/commercialization continuum. These are Biomedical Research, Development, Operations and the two commercial units – US and International – which are, focused on their respective geographic areas.
Shareholdings
Majority holdings in publicly traded Group companies
The Novartis Group owns 70.68% of Novartis India Ltd., with its registered office in Mumbai, India, and a listing on the BSE (formerly known as the Bombay Stock Exchange) (ISIN INE234A01025, symbol: HCBA). The total market value of the 29.32% free float of Novartis India Ltd. was USD 66.8 million on December 31, 2023, using the quoted market share price at year-end. Applying this share price to all the shares of the Company, the market capitalization of the whole company was USD 227.8 million, and that of the shares owned by Novartis was USD 161.0 million.
Shareholders
Significant shareholders
According to the Share Register, as of December 31, 2023, the following registered shareholders, including nominees and the American Depositary Share (ADS) depositary, held more than 2% of the total share capital, with the right to vote all their shares based on exemptions granted by the Board (see “—Item 6.C Board practices—Shareholder participation—Voting rights, restrictions and representation—Registration restrictions”):*
| | % holding of
share capital
Dec 31, 2023 | |
|
Shareholders registered for their own account: | | | |
Emasan AG, Basel 1 | | 3.9 | |
|
UBS Fund Management (Switzerland) AG, Basel | | 2.7 | |
|
Credit Suisse Funds AG, Zurich | | 2.2 | |
|
|
1 According to a disclosure notification filed with Novartis AG and the SIX Swiss Exchange, the beneficial owner of the shares registered for Emasan AG is Sandoz - Fondation de Famille, Liechtenstein. |
| | % holding of
share capital
Dec 31, 2023 | |
|
Shareholders registered as nominees: | | | |
Nortrust Nominees Ltd., London | | 3.6 | |
|
The Bank of New York Mellon, New York | | 2.9 | |
Through The Bank of New York Mellon, Everett | | 1.5 | |
Through The Bank of New York Mellon, New York | | 1.0 | |
Through The Bank of New York Mellon, SA/NV, Brussels | | 0.4 | |
|
Chase Nominees Ltd., London 1 | | | |
|
Shareholder acting as American Depositary Share (ADS) depositary: | | | |
JPMorgan Chase Bank, N.A., New York | | 8.3 | |
|
|
1 Chase Nominees, Ltd. (Chase) has informed us that as of December 2023, it will no longer register any shareholding positions on its own behalf. Shares held by customers of Chase will be registered for such customer’s own account. |
According to a disclosure notification filed with Novartis AG, Norges Bank (Central Bank of Norway), Oslo, held 2.4% of the share capital but was not registered in the Share Register as of December 31, 2023.
According to a disclosure notification filed with Novartis AG and the SIX Swiss Exchange, BlackRock, Inc., New York, held between 5% and 10% but was registered with less than 2% of the share capital as of December 31, 2023.
Disclosure notifications pertaining to shareholdings filed with Novartis AG and the SIX Swiss Exchange are published on the latter’s electronic publication platform:
www.ser-ag.com/en/resources/notifications-market-participants/significant-shareholders.html.
* Excluding 10.2% of the share capital held as treasury shares by Novartis AG or its fully owned subsidiaries (including Swiss foundations controlled by Novartis AG)
Duty to make an offer
According to the Swiss Federal Act on Financial Infrastructures, anyone who – directly, indirectly or acting in concert with third parties – acquires equity securities exceeding 33 1/3% of the voting rights of a company (whether or not such rights are exercisable) is required to make an offer to acquire all listed equity securities of that company. A company may raise this threshold up to 49% of the voting rights (“opting up”) or may, under certain circumstances, waive the threshold (“opting out”). Novartis AG has not adopted any such measures.
Cross shareholdings
Novartis AG has no cross shareholdings in excess of 5% of capital, or voting rights with any other company.
Overview on shareholder structure
The following tables relate only to registered shareholders and cannot be assumed to represent the entire investor base because nominees and JPMorgan Chase Bank, N.A., as ADS depositary, are registered as shareholders for a large number of beneficial owners.
As of December 31, 2023, Novartis AG had approximately 183 000 registered shareholders.
Number of registered shareholders/shares
As of December 31, 20231
| | Number of
registered
shareholders | |
% of
share capital
| |
|
1–100 | | 34 384 | | 0.09 | |
|
101–1 000 | | 108 966 | | 1.92 | |
|
1 001–10 000 | | 36 299 | | 4.38 | |
|
10 001–100 000 | | 3 071 | | 3.46 | |
|
100 001–1 000 000 | | 458 | | 6.01 | |
|
1 000 001–5 000 000 | | 61 | | 5.46 | |
|
5 000 001 or more 2 | | 26 | | 35.45 | |
|
Total registered shareholders/shares | | 183 265 | | 56.77 | |
|
Unregistered shares | | | | 43.23 | |
|
Total | | | | 100.00 | |
|
|
1 At the record date of the 2023 Annual General Meeting of Shareholders (AGM), unregistered shares amounted to 16.3%. |
2 Including significant registered shareholders as listed above |
Registered shareholders by type
As of December 31, 2023 | | Shareholders in % | | Shares in % | |
|
Individual shareholders | | 96.79 | | 19.98 | |
|
Legal entities 1 | | 3.17 | | 41.74 | |
|
Nominees, fiduciaries and ADS depositary | | 0.04 | | 38.28 | |
|
Total | | 100.00 | | 100.00 | |
|
|
1 Excluding 10.2% of the share capital held as treasury shares by Novartis AG or its fully owned subsidiaries (including Swiss foundations controlled by Novartis AG) |
Registered shareholders by country1
As of December 31, 2023 | | Shareholders in % | | Shares in % | |
|
Belgium | | 0.11 | | 1.01 | |
|
Canada | | 0.04 | | 0.68 | |
|
France | | 1.99 | | 0.48 | |
|
Germany | | 5.82 | | 2.15 | |
|
Ireland | | 0.48 | | 0.55 | |
|
Japan | | 0.18 | | 0.52 | |
|
Luxembourg | | 0.06 | | 0.96 | |
|
Switzerland 2 | | 86.84 | | 54.61 | |
|
United Kingdom | | 0.67 | | 11.99 | |
|
United States | | 0.24 | | 24.68 | |
|
Other countries | | 3.57 | | 2.37 | |
|
Total | | 100.00 | | 100.00 | |
|
|
1 Registered shares held by nominees are shown in the country where the company/affiliate entered in the Share Register as shareholder has its registered seat. |
2 Excluding 10.2% of the share capital held as treasury shares by Novartis AG or its fully owned subsidiaries (including Swiss foundations controlled by Novartis AG) |
Capital structure
Share capital
As of December 31, 2023, the share capital amounted to CHF 1 115 964 098.48 fully paid-in and divided into 2 277 477 752 registered shares with a nominal value of CHF 0.49 each.
Shares are listed on the SIX Swiss Exchange (ISIN CH0012005267, symbol: NOVN) and on the New York Stock Exchange (NYSE) in the form of American Depositary Receipts (ADRs) representing American Depositary Shares (ADSs) (ISIN US66987V1098, symbol: NVS).
No conditional capital exists as of December 31, 2023 nor has a capital band been introduced in the Company’s Articles of Incorporation.
Shares, participation certificates, non-voting equity securities, profit-sharing certificates
Shares are issued as uncertificated securities (in the sense of the Swiss Code of Obligations) and as book entry securities (in terms of the Swiss Act on Intermediated Securities). All shares have equal voting rights and carry equal entitlements to dividends. No participation certificates, non-voting equity securities (Genussscheine) or profit-sharing certificates have been issued.
Convertible securities and options
Novartis AG has not issued convertible or exchangeable bonds, warrants, options or other securities granting rights to shares, other than certain instruments granted under or in connection with equity-based participation plans of employees.
Limitation on transferability
No transferability restrictions are imposed on shares (for registration restrictions, see “—Item 6.C Board practices—Shareholder participation—Voting rights, restrictions and representation—Registration restrictions”). The registration of shareholders in the Share Register or in the ADR register kept by JPMorgan Chase Bank, N.A., does not affect the tradability of shares or ADRs.
Changes to share capital
AGM
| |
Shareholder decision
| |
Shares canceled
| | Average repurchase
share price (CHF)1 | |
|
2021 | | • Capital reduction by CHF 16.32 million (from CHF 1 233 530 460.00 to CHF 1 217 210 460.00)
• Authorization of the Board to repurchase shares up to a maximum of CHF 10 billion between the 2021 AGM and the 2024 AGM | | 32 640 000 | | 80.57 | |
|
2022 | | • Capital reduction by CHF 15.35 million (from CHF 1 217 210 460.00 to CHF 1 201 860 626.00)
• Authorization of the Board to repurchase shares up to a maximum of CHF 10 billion between the 2022 AGM and the 2025 AGM2 | | 30 699 668 | | 81.82 | |
|
2023 | | • Capital reduction by CHF 63.12 million (from CHF 1 201 860 626.00 to CHF 1 138 738 876.00)
• Authorization of the Board to repurchase shares up to a maximum of CHF 10 billion between the 2023 AGM and the 2026 AGM3 | | 126 243 500 | | 81.56 | |
|
| | | | | | | |
| | | | | | | |
EGM | | Shareholder decision | | | | | |
|
2023 | | • Capital reduction by CHF 22.77 million (from CHF 1 138 738 876.00 to CHF 1 115 964 098.48) by reducing the par value of each share from CHF 0.50 to CHF 0.49 | | | | | |
|
| | | | | | | |
| | | | | | | |
AGM
| |
Proposal to the shareholders
| |
Shares to be canceled
| | Average repurchase
share price (CHF)1 | |
|
2024 | | • Capital reduction by CHF 42.90 million (from CHF 1 115 964 098.48 to CHF 1 073 065 943.53) | | 87 547 255 | | 86.36 | |
|
|
1 All shares were repurchased on the SIX Swiss Exchange second trading line. |
2 In addition to the remaining authorization from the 2021 AGM |
3 In addition to the remaining authorization from the 2022 AGM |
Key Novartis share data
| | 2023 | | 2022 | | 2021 | |
|
Issued shares | | 2 277 477 752 | | 2 403 721 252 | | 2 434 420 920 | |
|
Treasury shares 1 | | 233 443 766 | | 284 112 195 | | 199 480 972 | |
|
Outstanding shares at December 31 | | 2 044 033 986 | | 2 119 609 057 | | 2 234 939 948 | |
|
Weighted average number of shares outstanding | | 2 076 794 140 | | 2 181 180 341 | | 2 242 601 173 | |
|
|
1 Approximately 94 million treasury shares (2022: 99 million 2021: 102 million) are held in Novartis entities that restrict their availability for use. |
Per-share information1
| | 2023 | | 2022 | | 2021 | |
|
Basic earnings per share from continuing operations (USD) | | 4.13 | | 2.77 | | 10.21 | |
|
Diluted earnings per share from continuing operations (USD) | | 4.10 | | 2.76 | | 10.14 | |
|
Net cash flows from operating activities from continuing operations (USD) | | 6.85 | | 5.98 | | 5.96 | |
|
Year-end equity for Novartis AG shareholders (USD) | | 22.83 | | 28.00 | | 30.31 | |
|
Dividend (CHF) 2 | | 3.30 | | 3.20 | | 3.10 | |
|
Dividend (USD) 3 | | 3.92 | | 3.51 | | 3.33 | |
|
|
1 Calculated on the weighted average number of shares outstanding, except year-end equity |
2 2023: proposal to shareholders for approval at the AGM on March 5, 2024. |
3 Translated into US dollars at the December 31, 2023, rate of USD 1.189 to the Swiss franc. This translation is an example only, and should not be construed as a representation that the Swiss franc amount represents, or has been or could be converted into US dollars at that or any other rate. 2022 and 2021, dividends are translated into US dollars at the Bloomberg Market System Rate on the payment date. |
Key ratios – December 31
| | 2023 | | 2022 | | 2021 | |
|
Price/earnings ratio 1 | | 14.1 | | 28.3 | | 8.2 | |
|
Dividend yield (%) 1 | | 3.9 | | 3.8 | | 3.9 | |
|
|
1 Based on the Novartis share price at December 31 of each year |
Key data on ADRs issued in the US
| | 2023 | | 2022 | | 2021 | |
|
Year-end ADR price (USD) | | 100.97 | | 90.72 | | 87.47 | |
|
High 1 | | 105.13 | | 93.75 | | 98.47 | |
|
Low 1 | | 80.03 | | 74.61 | | 79.70 | |
|
Number of ADRs outstanding 2 | | 189 633 312 | | 225 435 680 | | 269 891 321 | |
|
|
|
1 Based on daily closing prices |
2 The depositary, JPMorgan Chase Bank, N.A., holds one Novartis AG share for every ADR issued. |
Share price (CHF)
| | 2023 | | 2022 | | 2021 | |
|
Year-end share price | | 84.87 | | 83.59 | | 80.28 | |
|
High 1 | | 93.87 | | 87.82 | | 86.75 | |
|
Low 1 | | 74.62 | | 73.98 | | 73.44 | |
|
Year-end market capitalization (USD billions) 2 | | 206.3 | | 191.5 | | 196.1 | |
|
Year-end market capitalization (CHF billions) 2 | | 173.5 | | 177.2 | | 179.4 | |
|
|
|
1 Based on daily closing prices |
2 Market capitalization is calculated based on the number of shares outstanding (excluding treasury shares). Market capitalization in USD is based on the market capitalization in CHF converted at the year-end CHF/USD exchange rate. |
Shareholder participation
Shareholder engagement
Shareholder engagement is fundamental to our commitment to governance and transparency, and the feedback we receive during these engagements helps us create long-term and sustainable value.
We concentrate our outreach efforts on our largest 100 shareholders – portfolio managers, buy-side professionals, stewardship teams and ESG analysts – who represent 60% of our ownership. While the Board Chair, CEO and CFO, together with Investor Relations, are accountable for ensuring effective shareholder engagement, other senior managers from within and outside the Executive Committee also participate in the meetings. We conduct regular outreach to investors throughout the year.
Types of engagements (select examples):
• AGM and quarterly results teleconferences (TCs)
• Bank conferences and management roadshows
• “Meet Novartis Management” and “R&D day” capital markets event
• Governance roadshow and TCs
• Board Chair’s meetings with Swiss, US and UK investors
• Annual ESG investor event, captioned “Impact and Health Equity”
• Sandoz spin-off EGM
Topics discussed with shareholders during 2023:
Growth:
• Replacement power
• Growth drivers (including Cosentyx, Entresto, Kisqali, Kesimpta and Pluvicto)
• Policy and pricing environment
• Life cycle management
INNOVATION:
• Progress and milestones
• Data of pipeline projects
• Return on R&D investments
PRODUCTIVITY:
• Progress on financial, strategic and operational performance
• Long-term sustainability of financial performance
• Capital allocation strategy
• New organization model
• Sandoz spin-off
BUILDING TRUST WITH SOCIETY AND CULTURE:
• Board accountability on ESG, and integration of ESG and compensation
• Strong governance, enhanced process and focus on material ESG factors, leading to improved rating agency scores
• Patient access to innovative medicines
• Learning from Novartis Access programs implemented over the decades, including integrated sustainable business models and access principles to help address access and health inequity
• ESG targets: full carbon neutrality, patient access targets for strategic innovative therapies, and global health flagship programs
• Progress on culture and other human capital metrics
Compensation AND Governance:
• Diversity of the Board, the Executive Committee, and the Company
• Board renewal, succession planning and evaluation
• The linking of the compensation system to key strategic priorities
• Risk oversight
• Stakeholder expectations from the Board on ESG matters
Voting rights, restrictions and representation
Registration
Shareholders have the right to vote and to execute all other rights as granted under Swiss law and the Articles of Incorporation (see, in particular, articles 17 and 18 of the Articles of Incorporation).
Each share registered with the right to vote by the third business day before the General Meeting entitles the holder to one vote at General Meetings. Article 5, paragraph 2 of the Articles of Incorporation provides that to be registered with voting rights, shareholders must declare that they acquired the shares in their own name and for their own account. According to article 5, paragraph 3 of the Articles of Incorporation, the Board may register nominees with the right to vote. The Share Register is a non-public register subject to statutory confidentiality and data privacy.
The Articles of Incorporation are available at www.novartis.com/investors/company-overview/corporate-governance.
Registration restrictions
Article 5, paragraph 2 of the Articles of Incorporation provides that no shareholder shall be registered with the right to vote for more than 2% of the share capital. Given that shareholder representation at General Meetings has traditionally been comparatively low in Switzerland, Novartis AG considers registration restrictions necessary to prevent a minority shareholder from dominating a General Meeting. The Board may, upon request, grant an exemption. Considerations include if the shareholder supports our goal of creating sustainable value and has a long-term investment horizon. Exemptions are in force for the registered shareholders listed in “—Item 6.C Board practices—Group structure and shareholders—Shareholders—Significant shareholders.” Exemptions also apply to the Novartis Foundation for Employee Participation, Basel, which as of December 31, 2023, was registered in the Share Register with less than 2% of the share capital, and to Norges Bank (Central Bank of Norway), Oslo, which as of December 31, 2023, was not registered but held 2.4% according to a disclosure notification filed with Novartis AG. No further exemptions were requested in 2023. The same restrictions indirectly apply to ADR holders.
Article 5, paragraph 3 of the Articles of Incorporation provides that no nominee shall be registered with the right to vote for more than 0.5% of the registered share capital. The Board may, upon request, grant an exemption from this restriction if the nominee discloses the names, addresses and number of shares of the persons for whose account it holds 0.5% or more of the registered share capital. Exemptions are in force for the nominees listed in “—Item 6.C Board practices—Group structure and shareholders—Shareholders—Significant shareholders,” and for the nominee Citibank, London, which in 2015 requested an exemption, but as of December 31, 2023, was not registered in the Share Register. The same restrictions indirectly apply to ADR holders.
According to article 5, paragraph 4 of the Articles of Incorporation, shareholders, ADR holders, or nominees who are linked to each other or who act in concert to circumvent
registration restrictions are treated as one person or nominee for the purposes of the restrictions on registration.
The registration restrictions may be changed by resolution of the General Meeting, with approval of at least two-thirds of the votes represented at the meeting.
The Articles of Incorporation are available at www.novartis.com/investors/company-overview/corporate-governance.
Attendance, Representation and WEB PORTAL
Registered shareholders will receive personal invitations to the General Meetings along with a registration/proxy form as well as a personal access code and a QR code to log in to our web portal. By returning the registration/proxy form or using the web portal, shareholders can order an admission ticket for the General Meeting or appoint a representative of their choice by means of a written proxy or the Independent Proxy to vote their shares on their behalf.
If the Independent Proxy is appointed, shareholders can also give voting instructions on agenda items or on alternative or additional motions related to the agenda items either (i) following the recommendations of the Board for such alternative or additional motions; or (ii) opposing such alternative or additional motions. They can also abstain from voting.
Shareholders choosing not to receive the comprehensive invitation materials will be informed of upcoming General Meetings through a letter containing the login credentials to access the web portal as well as a reference to www.novartis.com/investors/shareholder-information/general-meetings, where all relevant information is available.
ADR holders
ADR holders have the rights enumerated in the deposit agreement (such as the right to give voting instructions and to receive dividends). The ADS depositary of Novartis AG – JPMorgan Chase Bank, N.A., New York – holds the shares underlying the ADRs and is registered as a shareholder in the Share Register. An ADR is not a share, and an ADR holder is not a Novartis AG shareholder. Each ADR represents one share. ADR holders exercise their voting rights by instructing the depositary to exercise their voting rights. The ADS depositary exercises the voting rights for registered shares underlying ADRs for which no voting instructions have been given by providing a discretionary proxy to an uninstructed independent designee. Such designee has to be a shareholder.
Annual General Meeting (AGM)
Convening
The AGM must be held within six months of the end of our financial year (December 31), and normally takes place in late February or early March. According to article 12a of the Articles of Incorporation (www.novartis.com/investors/company-overview/corporate-governance), the Board may foresee that shareholders who cannot be present at the venue of the AGM may exercise their rights through electronic means. The Board may at any time until June 30, 20281 also order that the AGM be held electronically without a venue. Extraordinary General Meetings may be requested by the Board, the external auditor, or shareholders representing at least 5% of the share capital.
Agenda
Shareholders representing shares with an aggregate nominal value of at least CHF 1 million may request that an item be included in a General Meeting agenda. Such requests must be made in writing at least 45 days before the meeting, specifying the requested item and proposal. If an explanatory statement is to be included in the notice of meeting, it must be submitted within the same period, and formulated in a short, clear and concise manner.
Powers
According to article 17 of the Articles of Incorporation (www.novartis.com/investors/company-overview/corporate-governance), the following powers are vested exclusively in the General Meeting:
• Adoption and amendment of the Articles of Incorporation
• Election and removal of the Board Chair, the Board and Compensation Committee members, the Independent Proxy and the external auditor
• Approval of the management report, the consolidated financial statements and the report on non-financial matters
• Approval of the financial statements of Novartis AG, and the decision on the appropriation of available earnings shown on the balance sheet, in particular with regard to dividends (including any repayment of the statutory capital reserves and the approval of interim dividends and the interim financial statements required for such purpose)
• Approval of the maximum aggregate compensation of the Board (from an AGM until the next AGM) and of the Executive Committee (for the financial year following the AGM). If the maximum aggregate amount of compensation already approved by the AGM is not sufficient to cover the compensation of newly appointed or promoted Executive Committee members, Novartis may use up to 40% of the amount last approved for the newly appointed or promoted Executive Committee members
• Discharge of Board and Executive Committee members
• Delisting of the shares of Novartis AG
• Decision on other matters that are reserved by law or by the Articles of Incorporation (e.g., advisory vote on the Compensation Report) to the General Meeting
1 In accordance with the statement by the Board issued on February 10, 2023, Novartis commits to submitting the corresponding authorization again to a shareholder vote at the 2025 Annual General Meeting, regardless of the time limitation stipulated in the Articles of Incorporation.
Statutory Quorums
The General Meeting passes resolutions and elections with the absolute majority of the votes represented at the meeting. However, under article 18 of the Articles of Incorporation (www.novartis.com/investors/company-overview/corporate-governance), the approval of two-thirds of the votes represented at the meeting is required for:
• An alteration of the purpose of Novartis AG
• The consolidation of shares, unless the approval of all affected shareholders is required
• An increase of the share capital out of equity, by contribution in kind, for the purpose of an acquisition of property or the grant of special rights
• An increase of the share capital out of equity, by contributions in kind by way of set-off against a receivable and the grant of special rights
• A restriction or cancellation of rights of options to subscribe
• The introduction of a conditional capital or a capital band
• An implementation of restrictions on the transfer of registered shares, and the removal of such restrictions
• The creation of shares with increased voting powers
• The change of the currency of the share capital
• The introduction of the deciding vote for the presiding officer at the General Meeting of Shareholders
• A provision in the Articles of Incorporation allowing to hold the General Meeting of Shareholders abroad
• The delisting of the shares of Novartis AG
• A change of the registered office of Novartis AG
• The introduction of an arbitration clause in the Articles of Incorporation
• The merger, split or transformation of Novartis AG under the Merger Act (subject to mandatory provisions)
• The dissolution of Novartis AG
Board of Directors
Composition (as per December 31, 2023)BOARD Chair: J. ReinhardtVice-Chair:S. Moroneylead independent Director: P. BulaN. AndrewsT. BuechnerE. DohertyB. HellerD. HochstrasserF. van HoutenA. de Pro GonzaloC. SawyersW. WintersJ. YoungAudit and Compliance CommitteeE. Doherty (Chair)T. BuechnerB. HellerD. Hochstrasser F. van HoutenA. de Pro GonzaloCompensation CommitteeS. Moroney (Chair)P. BulaB. HellerW. WintersGovernance, Sustainability and Nomination CommitteeP. Bula (Chair)B. HellerD. HochstrasserC. SawyersW. WintersRisk CommitteeT. Buechner (Chair)N. AndrewsE. DohertyA. de Pro Gonzalo J. Young Science & Technology CommitteeJ. Reinhardt (Chair)N. AndrewsF. van HoutenS. MoroneyC. SawyersJ. Young
Changes to the Board of Directors
John Young was elected as a new Board member at the 2023 AGM. Andreas von Planta, who had been a Board member since 2006, did not stand for re-election at the 2023 AGM. The biography of Mr. von Planta can be found in the 2022 Annual Report (page 136), which is available at www.novartis.com/news/media-library/novartis-annual-report-2022.
Election and term of office
Board members (including the Board Chair) and Compensation Committee members are elected individually by shareholders at the General Meeting for a one-year term of office. The term of office expires at the end of the next AGM.
According to article 20, paragraph 3 of the Articles of Incorporation, a member shall not serve on the Board for more than 12 years. Under special circumstances and if deemed to be in the best interest of the Company, the Board may recommend exceptions to the shareholders (www.novartis.com/investors/company-overview/corporate-governance).
The term limit supports our commitment to renew the Board on an ongoing basis and follows international best practice. We believe age is still a relevant factor in Board composition, and the GSNC will consider this and other factors – including gender, nationality and ethnicity – when evaluating candidates and exploring ways to increase Board diversity.
Succession planning
The GSNC prepares and reviews succession plans for the Board on an annual basis. These plans are discussed by the Board in private meetings. A search for a new Board member is launched – normally with the support of a professional executive search company – with individual selection criteria defined based on the evolving needs of the Company and a continuing focus on diversity, skills and experience. The set of competencies (further explained in “—Item 6.C Board practices—Board of Directors—Board skills”) and a balance between continuity of experience and fresh perspectives are also important criteria for the GSNC when evaluating new candidates. Candidates are interviewed by the Board Chair, members of the GSNC, other Board members, and members of the Executive Committee. The GSNC then makes a recommendation to the full Board, and the Board ultimately decides who should be proposed for election at the upcoming AGM.
Independence
All Board members – including the Board Chair – are non-executive and independent, pursuant to applicable corporate governance rules and Novartis independence criteria, which are outlined in Appendix II to the Board Regulations (www.novartis.com/investors/company-overview/corporate-governance). In particular, no Board member is or was a member of the management of Novartis AG or of any other Novartis Group company in the last three financial years up to December 31, 2023, or has or had, a significant business relationship with Novartis AG or with any other Novartis Group company. No separate meetings of independent Board members were held in 2023.
The independence of Board members is assessed annually. Each Board member completes an independence questionnaire that is reviewed by the GSNC. The GSNC then submits a proposal to the full Board, and the Board determines the independence status of each Board member.
Diversity
Novartis is dedicated to fostering an inclusive board where individuals from all genders and ethnic backgrounds can thrive and contribute their unique insights. We pledge to continuously advance our efforts to promote gender parity in the composition of our Board of Directors within a range of +/- 10 %. A diverse Board ensures that the appropriate balance of skills, expertise, experience and cultural background is represented to discharge its responsibilities and to support long-term value creation for shareholders, patients, employees and other stakeholders. Diversity remains a critical focus area for the Board, and the GSNC continuously examines opportunities to further increase the Board’s diversity when identifying new Board member candidates. We firmly believe that by valuing and respecting these differences, we can drive innovation and make more informed decisions and better serve our stakeholders.

Diversity profileNationality1pAmerican27%pSwiss23%pBritish11.5%pDutch11.5%pGerman11.5%pSpanish7.5%pIrish4%pNew Zealander4%GenderpMale69%pFemale31%Agep556023%p616554%p>6523%Tenurep<3y23%p36y31%p79y23%p>9y23%1Please note that six Board members have dual nationalities. Each of these nationalities is counted as a half in the above chart.
Board skills
Upon proposal by the GSNC, the Board has determined a diverse set of competencies for its members that aligns with our status as a listed company, as well as our business portfolio, geographic reach and culture. Within this set of competencies, the Board members were asked to identify their most relevant skills based on their educational background, professional experience and personal achievements.
The GSNC assesses the set of competencies as well as the individual skills annually to ensure that an appropriate balance of skills, expertise, experience and diversity is represented on the Board.
To learn more about our Board members and their individual skills, see “—Item 6.C Board practices—Board of Directors—Members of the Board of Directors.”
Board skill distributionMedicine/healthcare/R&D54%7/13Environmental, social 61%8/13and governance (ESG)Data/digital38%5/13Leadership/management 85%11/13Finance/accounting61%8/13Law/regulatory/risk management77%10/13
Members of the Board of Directors
Joerg Reinhardt, Ph.D.
Chair since 2013 | Nationality: German | Year of birth: 1956
Joerg Reinhardt is a healthcare industry veteran whose career spans nearly 40 years. After receiving his doctorate in pharmaceutical sciences, Mr. Reinhardt joined Sandoz Pharma Ltd., a predecessor to Novartis, in 1982. He held a number of senior leadership positions at Novartis, including Chief Operating Officer and Head of the Vaccines and Diagnostics Division. Additionally, he led Bayer HealthCare AG as chair of the board of management and the executive committee from 2010 to 2013.
Professional experience
• Chair of the board of management and the executive committee, Bayer HealthCare AG, Germany (2010–2013)
• Chief Operating Officer, Novartis AG, Switzerland (2008–2010)
• Head of the Vaccines and Diagnostics Division, Novartis AG, Switzerland (2006–2008)
• Various managerial positions at Sandoz Pharma Ltd. and Novartis AG, Switzerland (1982–2006)
Mandates
• Senate member, Helmholtz Association of German Research Centers, Germany
• Chair of the board of trustees, Institute of Molecular and Clinical Ophthalmology Basel (IOB), Switzerland
• Chair of the board of trustees, Novartis Foundation, Switzerland
• Board member, Swiss Re AG, Switzerland
Education
• Doctorate in pharmaceutical sciences, Saarland University, Germany
Key skills
Medicine/healthcare/R&D

Environmental, social and governance (ESG)

Leadership/management

Law/regulatory/risk management
Simon Moroney, D.Phil.
Board member since 2020 | Vice-Chair since March 4, 2022 | Nationality: German/New Zealander |
Year of birth: 1959
As co-founder and CEO of MorphoSys AG, Simon Moroney played a central role in establishing the company as a force in the field of therapeutic antibodies, with one of the broadest pipelines of drug candidates in the industry. Mr. Moroney holds both a doctorate and a Master’s degree in chemistry.
Professional experience
• Co-founder and CEO, MorphoSys AG, Germany (1992–2019)
• Research associate, Department of Pharmacology, University of Cambridge, UK (1991–1992)
• Assistant professor, Department of Chemistry, University of British Columbia, Canada (1989–1990)
Mandates
• Chair of the board of directors and the remuneration and nomination committee, Biotalys NV, Belgium
Education
• Doctorate in chemistry, University of Oxford, UK
• Master’s degree in chemistry, University of Waikato, New Zealand
Key skills
Medicine/healthcare/R&D

Environmental, social and governance (ESG)

Leadership/management

Law/regulatory/risk management
Nancy C. Andrews, M.D., Ph.D.
Board member since 2015 | Nationality: American/Swiss | Year of birth: 1958
Nancy C. Andrews has extensive experience as a physician, scientist, professor and senior administrator at leading academic institutions and hospitals. Her distinguished career spans more than 30 years, with leadership roles at both Harvard Medical School and the Duke University School of Medicine. Since 2023, Dr. Andrews is professor in residence of pediatrics at Harvard Medical School and is credited with conducting research that led to advances in understanding iron biology and iron diseases.
Professional experience
• Professor in residence of pediatrics, Harvard Medical School, US (2023-present)
• Executive vice president and chief scientific officer, Boston Children’s Hospital, US (2021–present)
• Dean emerita, Duke University School of Medicine, and vice chancellor emerita for academic affairs, Duke University, US (2017–present)
• Dean, Duke University School of Medicine, and vice chancellor for academic affairs, Duke University, US (2007–2017)
• Professor of pediatrics, pharmacology and cancer biology, Duke University, US (2007–2021)
• Dean for basic sciences and graduate studies, Harvard Medical School, US (2003–2007)
• Director, Harvard/MIT M.D.-Ph.D. Program, US (1999–2003)
• Biomedical research investigator, Howard Hughes Medical Institute, US (1993–2006)
Mandates
• Board member, Maze Therapeutics Inc., US
• Board member and chair of the science and technology committee, Charles River Laboratories International Inc., US
• Home secretary (since July 2023) and council member, National Academy of Sciences, US
• Former council member (2013–2019) and member, National Academy of Medicine, US
• Fellow (since April 2007) and former chair (2017 – 2023), American Academy of Arts and Sciences, US
• Member of the executive committee of the corporation, Massachusetts Institute of Technology, US (2019-2022)
• Member of the scientific management review board, National Institutes of Health, US (2014–2019)
• Board member and former chair, Burroughs Wellcome Fund, US (2011–2019)
Education
• Doctor of medicine, Harvard Medical School, US
• Doctorate in biology, Massachusetts Institute of Technology, US
• Master’s and bachelor’s degrees in molecular biophysics and biochemistry, Yale University, US
Key skills
Medicine/healthcare/R&D

Leadership/management
Ton Buechner
Board member since 2016 | Nationality: Dutch/Swiss | Year of birth: 1965
Ton Buechner is an engineer by training who started his career in the oil and gas construction industry. Before becoming the CEO of Sulzer AG, he held several divisional leadership roles at the company and worked in markets including Asia. Mr. Buechner most recently served as CEO and chair of the executive board of AkzoNobel NV, where he introduced industry-leading ESG policies.
Professional experience
• CEO and chair of the executive board, AkzoNobel NV, Netherlands (2012–2017)
• CEO, Sulzer AG, Switzerland (2007–2011)
• President, Sulzer Pumps, Switzerland (2003–2006)
• President, Sulzer Turbomachinery Services, Switzerland (2000–2002)
• Various managerial positions at Sulzer AG, China and Switzerland (1994–2000)
Mandates
• Chair of the board of directors and the sustainability committee, Swiss Prime Site AG, Switzerland
• Chair of the board of directors and the strategy and sustainability committee, Burckhardt Compression AG, Switzerland
• Member of advisory committee to the Ministry of Economic Affairs and Climate Policy (“Adviescommissie Maatwerkafspraken Verduurzaming Industrie”), Netherlands
• Member of the presidential and shareholder committees, Voith GmbH & Co. KGaA, Germany (2014–2020)
• Member of the supervisory board, Voith GmbH & Co. KGaA, Germany (2014–2018)
Education
• Master of business administration, IMD business school, Switzerland
• Master’s degree in civil engineering, Delft University of Technology, Netherlands
Key skills

Environmental, social and governance (ESG)

Leadership/management

Finance/accounting

Law/regulatory/risk management
Patrice Bula
Board member since 2019 | Lead Independent Director since March 4, 2022 | Nationality: Swiss | Year of birth: 1956
Patrice Bula has 40 years of global management experience and is a leader in the consumer goods industry across established and emerging markets. He has served in various senior roles at Nestlé SA, including as general manager of its businesses in China, Germany and South Africa. Most recently, he successfully led the Nestlé Group’s brand strategies, digital marketing transformation and Nespresso business.
Professional experience
• Executive vice president and head of strategic business units, marketing, sales and Nespresso, Nestlé SA, Switzerland (2011–2021)
• Market head of the Greater China region, Nestlé SA, Switzerland (2007–2011)
• Market head of Germany, Nestlé SA, Switzerland (2003–2007)
• Head of the confectionery and biscuits strategic business unit, Nestlé SA, Switzerland (2000–2003)
• Various managerial positions at Nestlé SA, Switzerland (1980–2000)
Mandates
• Chair, Froneri Lux Topco Sarl, Luxembourg
• Board member and vice chair, Schindler AG, Switzerland
• Board member and chair of the ESG committee, New Tiger LLC, US
• Co-chair (2020–2021) and board member (2015–2021), Cereal Partners Worldwide SA, Switzerland (Nestlé representative)
• Board member, Froneri Lux Topco Sarl, Luxembourg (Nestlé representative) (2016–2020)
• Board member, Bobst Group SA, Switzerland (2017–2019)
• Chair, Blue Bottle Coffee Inc., US (Nestlé representative) (2017–2019)
• Chair, Nestlé Nespresso SA, Switzerland (Nestlé representative) (2011–2019)
• Board member, Hsu Fu Chi Food Companies, China (Nestlé representative) (2011–2019)
Education
• Program for executive development, IMD business school, Switzerland
• Master’s degree in economic sciences, HEC Lausanne, Switzerland
Key skills

Environmental, social and governance (ESG)

Data/digital

Leadership/management

Finance/accounting
Elizabeth (Liz) Doherty
Board member since 2016 | Nationality: British/Irish | Year of birth: 1957 | Audit Committee Financial Expert
Elizabeth (Liz) Doherty is an expert in finance and accounting who has broad operational experience in international consumer and retail businesses. She began her career in internal audit at Unilever PLC and has held senior finance and accounting roles there and at other companies including Tesco PLC and Reckitt Benckiser Group PLC.
Professional experience
• CFO (interim), Cognita Schools Ltd., UK (2014–2015)
• CFO and board member, Reckitt Benckiser Group PLC, UK (2011–2013)
• CFO (interim), City Inn, UK (2010)
• CFO, Brambles Ltd., Australia (2007–2009)
• Group international finance director, Tesco PLC, UK (2001–2007)
• Various managerial positions at Unilever PLC, UK (1981–2001)
Mandates
• Board member and chair of the audit committee, Corbion NV, Netherlands
• Member of the supervisory board and chair of the audit committee, Royal Philips NV, Netherlands
• Advisor, Affinity Petcare SA and GB Foods SA, Spain (2017–2023)
• Board member, Dunelm Group PLC, UK (2013–2019)
• Board member, HM Courts & Tribunals Service, UK (2015–2019)
• Board member, Ministry of Justice, UK (2015–2019)
• Board member, Delhaize Group, Belgium (2013–2016)
• Board member, Nokia Corp., Finland (2013–2016)
Education
• Fellow, Chartered Institute of Management Accountants, UK
• Bachelor’s degree in liberal studies in science (physics), University of Manchester, UK
Key skills

Leadership/management

Finance/accounting

Law/regulatory/risk management
Bridgette Heller
Board member since 2020 | Nationality: American | Year of birth: 1961
Bridgette Heller has proven experience in the standalone divisions of companies such as Johnson & Johnson, Merck & Co. Inc. and Danone SA, and has served on the audit committees of ADT Corp. and Tech Data Corp. During her career, she has overseen the performance of CFOs and made decisions on strategic R&D priorities. Ms. Heller is an advocate for diversity, equity and inclusion, and traveled globally to reinforce Danone’s commitment to infant and maternal health, inclusive diversity, an equitable workforce for women, and sustainable communities. She is co-founder and CEO of the Shirley Proctor Puller Foundation, an education and youth empowerment nonprofit, and devotes much of her time to strengthening education and sustainability in an underserved community in the US.
Professional experience
• Co-founder and CEO, Shirley Proctor Puller Foundation, US (2019–present)
• EVP and president of specialized nutrition, Danone SA, Netherlands (2017–2019)
• EVP of early life nutrition, Danone SA, Netherlands (2016–2019)
• EVP and president of consumer care, Merck & Co. Inc., US (2010–2015)
• Global president of the baby global business unit, Johnson & Johnson, US (2007–2009)
• President of the US baby, kids and wound care business and of global innovation development, Johnson & Johnson, US (2005–2007)
• Managing partner, Heller Associates: Ideas for Growth Inc., US (2004–2005)
• CEO, Chung’s Gourmet Foods, US (2003–2004)
• Various managerial positions at Kraft Foods Inc., US (1985–2003)
Mandates
• Board member, Integral Ad Science Inc., US
• Board member, Aramark, US
• Board member, Dexcom Inc., US
• Board member, Newman’s Own Inc., US
• Member of the board of trustees, Northwestern University, US
• Member of the advisory board, Kellogg School of Management at Northwestern University, US
• Board member, Shirley Proctor Puller Foundation, US
• Board member, Newman’s Own Foundation, US
• Board member, Tech Data Corp., US (2016–2020)
• Board member, ADT Corp., US (2012–2016)
• Board member, Girls Inc., US (2002–2014)
Education
• Master’s degree in marketing and management policy, Kellogg School of Management at Northwestern University, US
• Bachelor’s degree in economics and computer studies, Northwestern University, US
Key skills
Medicine/healthcare/R&D

Environmental, social and governance (ESG)

Data/digital

Leadership/management

Finance/accounting

Law/regulatory/risk management
Daniel Hochstrasser
Board member since March 4, 2022 | Nationality: Swiss | Year of birth: 1960
Daniel Hochstrasser is an independent dispute resolution specialist practicing in Zurich, Switzerland. Until the end of 2022, he has been leading Bär & Karrer’s arbitration practice for 15 years. He frequently represented parties in complex disputes arising from matters such as M&A transactions, industrial and infrastructure projects, and license, distribution and development agreements, particularly in the pharmaceutical industry. In addition, he led the firm as senior partner from 2011 until 2021. He has published extensively on arbitration and litigation, and lectures at the University of Zurich and the University of St. Gallen in Switzerland.
Professional experience
• Attorney-at-law, Daniel Hochstrasser AG, Switzerland (since January 2023)
• Attorney-at-law and partner, Bär & Karrer AG, Switzerland (1993–December 2022)
• Senior partner and chair of the board of directors, Bär & Karrer AG, Switzerland (2011-2021)
• Lawyer, District Court of Affoltern, Court of Appeals/Court of Cassation of Zurich, Switzerland (1987–1992)
• In-house lawyer, Staubli SA, France (1986–1987)
Mandates
• Chair of the board of directors, Daniel Hochstrasser AG, Switzerland
• Member (2015–2021) and vice president (since 2021), ICC Court of Arbitration, France
• Member of the Ethics Court, Zurich Bar Association, Switzerland (since 2004)
• Board member, Finland Arbitration Institute, Finland (since 2020)
• Chair of the board of directors, Bär & Karrer AG, Switzerland (2011-2021)
• Member of the Court, Swiss Arbitration Chambers, Switzerland (2004–2014)
Education
• Master of laws (LL.M.), Cornell Law School, US
• Bar examination, Switzerland
• Licentiatus iuris, University of Zurich, Switzerland
Key skills

Law/regulatory/risk management
Frans van Houten
Board member since 2017 | Nationality: Dutch | Year of birth: 1960
Frans van Houten is passionate about purpose-driven innovation, entrepreneurship and business transformation to drive customer value and competitiveness. Under his leadership as CEO of Royal Philips, the company transformed into a leading health technology solutions company, leveraging data and informatics to improve healthcare provider results, and became a forerunner across ESG dimensions, having become carbon neutral in its operations since 2020 and recycling over 90% of its waste. Mr. van Houten was an initiator of the World Economic Forum Compact for Responsive and Responsible Leadership as well as founder and co-chair of the Platform to Accelerate the Circular Economy.
Professional experience
• Advisor, Royal Philips NV, Netherlands (October 2022–April 2023)
• CEO and chair of the executive committee and the board of management, Royal Philips NV, Netherlands (2011–October 2022)
• Interim management, ING Group NV, Netherlands (2009–2010)
• CEO and chair of the management board, NXP Semiconductors NV (formerly Philips Semiconductors NV), Netherlands (2004–2009)
• Various managerial positions at Royal Philips Electronics NV, Netherlands (1986–2004)
Mandates
• Board member, Absci Corporation, US
• Board member, Synthesis Health Inc. US
• Chair, Castor EDC, Netherlands
• Member of the steering committee, European Round Table for Industry (ERT), Belgium (2014-November 2022)
• Vice chair and member of the supervisory board, Philips Lighting, Netherlands (2016–2017)
Education
• Master’s degree in economics and business management, Erasmus University Rotterdam, Netherlands
• Bachelor’s degree in economics, Erasmus University Rotterdam, Netherlands
Key skills
Medicine/healthcare/R&D

Environmental, social and governance (ESG)

Data/digital

Leadership/management

Finance/accounting

Law/regulatory/risk management
Ana de Pro Gonzalo
Board member since March 4, 2022 | Nationality: Spanish | Year of birth: 1967 | Audit Committee Financial Expert
Since starting her career at Arthur Andersen, Ana de Pro Gonzalo has worked across a variety of industries, ranging from construction and real estate to engineering and telecommunications. With deep expertise in finance, capital markets and technology, she has held executive positions at several multinational companies. Most recently, she spent 10 years as chief financial officer of Amadeus IT Group, a leading software provider for the global travel and tourism industry.
Professional experience
• Chief financial officer, Amadeus IT Group SA, Spain (2010–2020)
• Corporate general manager, Sacyr Vallehermoso SA, Spain (2002–2010)
• Deputy general manager and finance director, Metrovacesa SA, Spain (1994–2002)
• Senior auditor, Arthur Andersen SA, Spain (1990–1994)
Mandates
• Member of the supervisory board and chair of the Audit Committee, STMicroelectronics NV, Netherlands
• Board member, Mobico Group PLC, UK
• Board member, Indra Sistemas SA, Spain (2020-2022)
• Board member, Merlin Properties Socimi SA, Spain (2015–2017)
Education
• General management program (PDG), IESE Business School, Spain
• Bachelor’s degree in business studies, Complutense University of Madrid, Spain
Key skills

Environmental, social and governance (ESG)

Data/digital

Leadership/management

Finance/accounting

Law/regulatory/risk management
Charles L. Sawyers, M.D.
Board member since 2013 | Nationality: American | Year of birth: 1959
Charles L. Sawyers is a highly accomplished expert and leader in cancer research. As a physician and prominent scientist, he has a deep understanding of the benefits of drugs for patients and society at large, and the importance of access to medicines. Dr. Sawyers co-developed the Novartis cancer drug Gleevec/Glivec and has received numerous honors and awards, including the Lasker-DeBakey Clinical Medical Research Award.
Professional experience
• Chair of the human oncology and pathogenesis program, Memorial Sloan Kettering Cancer Center, US (2006–present)
• Professor of medicine (2008–present), and professor of cell and developmental biology (2011–present), Weill Cornell Graduate School of Medical Sciences, US
• Investigator, Howard Hughes Medical Institute, US (2002–2006 and 2008–present)
• Associate chief, division of hematology-oncology, University of California, Los Angeles, US (1996–2006)
Mandates
• Member, National Academy of Medicine, US
• Member, National Academy of Sciences, US
• Investigator, Howard Hughes Medical Institute, US
• Member, National Cancer Advisory Board, US (2012–2020)
• President, American Association for Cancer Research, US (2013–2014)
Education
• Doctor of medicine, Johns Hopkins University School of Medicine, US
• Bachelor’s degree, Princeton University, US
Key skills
Medicine/healthcare/R&D
William T. Winters
Board member since 2013 | Nationality: British/American | Year of birth: 1961
William T. Winters has extensive leadership experience in the financial sector. He began his career at JPMorgan Chase & Co. in 1983 and has held management roles across several market areas and in corporate finance. Mr. Winters founded Renshaw Bay LLP, an alternative asset management firm, and now serves as CEO of Standard Chartered PLC, where he is leading a digital transformation of the global bank.
Professional experience
• CEO, Standard Chartered PLC, UK (2015–present)
• Chair and CEO, Renshaw Bay LLP, UK (2011–2015)
• Co-CEO of the investment bank, JPMorgan Chase & Co., UK (2004–2010)
• Various managerial positions at JPMorgan Chase & Co., UK and US (1983–2004)
Mandates
• Board member, Standard Chartered Bank PLC, UK
• Commissioner, Independent Commission on Banking, UK (2010–2011)
Education
• Master of business administration, Wharton School of the University of Pennsylvania, US
• Bachelor’s degree in international relations, Colgate University, US
Key skills

Environmental, social and governance (ESG)

Data/digital

Leadership/management

Finance/accounting

Law/regulatory/risk management
John D. Young
Board member since March 7, 2023 | Nationality: British/American | Year of birth: 1964
A scientist by training, John D. Young has over 35 years of experience in the healthcare industry and will bring a wealth of experience in leadership, strategy, business development and commercialization of innovative medicines to the Novartis Board of Directors. He joined Pfizer in 1987 as a sales representative and held positions of increasing seniority across the company, including as a member of Pfizer’s executive leadership team from 2012. As Pfizer’s group president and chief business officer from 2019 until 2022, John also played an integral role in the development and delivery of the Pfizer-BioNTech COVID-19 vaccine.
Professional experience
• Senior advisor to the CEO, Pfizer, US (January-June 2022)
• Group president and chief business officer, Pfizer, US (2019-2022)
• Group president, innovative health business, Pfizer, US (2018)
• Group president, essential health business, Pfizer, US (2014-2017)
• President and general manager, global primary care business unit, Pfizer, US (2012-2013)
• Regional president, primary care business unit for Europe and Canada, Pfizer, UK (2009-2012)
• Various managerial positions, Pfizer, UK and Australia (1987–2008)
Mandates
• Board member, Johnson Controls International, Ireland
• Board member, Arvinas Inc, US
• Board member, Imbria Pharmaceuticals, US
• Board member, Haleon, UK (2022-February 2023)
• Board member, GSK Consumer Health Joint Venture, UK (2019-2022)
• Board member, Biotechnology Innovation Organization (BIO), US (2018-2021)
• US bio-pharmaceutical representative, UK Government Life Sciences Council, UK (2007-2021)
• Board member, National Committee for US China Relations, US (2014-2017)
• Board member, European Federation of Pharmaceutical Industries and Associations (EFPIA), Belgium (2012-2017)
Education
• Master of business administration, University of Strathclyde, UK
• Bachelor’s degree in biological sciences, University of Glasgow, UK
Key skills
Medicine/healthcare/R&D

Leadership/management

Finance/accounting

Law/regulatory/risk management
Corporate Secretary
Charlotte Pamer-Wieser, Ph.D.
Self-assessment
The Board and its committees conduct a self-assessment once a year, covering topics including Board composition, purpose, scope and responsibilities; succession planning; Board processes and governance; interaction between the Board and the Executive Committee; Board meetings and pre-reading material; team effectiveness; and Board Chair and peer evaluation. Every third year, this process is conducted by an independent external consultant. The 2023 review is currently being undertaken by the consulting firm Egon Zehnder. Its results will be discussed during the second quarter of 2024.

Questionnaire and Interviews•Each Board member fills out a questionnaire prepared by Egon Zehnder.•Individual interviews are scheduled, during which Board members have the opportunity to share their perspectives on their strengths and areas for development, best practices, and interaction between Board members, and between the Board and the Executive Committee.•The Executive Committee members are also interviewed individually to capture their perspectives on the Board’s functioning and its interactions with the Executive Committee, including focusing on ways to improve efficiency.Review•The preliminary results of the questionnaires and interviews are then presented to the Board Chair. This qualitative review covers topics including Board composition; purpose, scope and responsibilities; processes and governance of the Board and its committees; meetings and pre-reading material; team effectiveness; and leadership and culture.•Thereafter, Egon Zehnder also undertakes a qualitative review with the full Board, sharing its key observations and recommendations, and holds additional individual feedback sessions with each Board member.Outcome•The results of the in-depth 2023 assessment will be discussed during a Board Meeting in the first half of 2024.•Conclusions and results will be shared in the Annual Report 2024.
Trainings
The Board receives regular briefings and trainings on ethics, risks and compliance, ESG and other relevant topics. In 2023, each Board member completed trainings on the following:
• ESG, tailored for Board members
• Doing business ethically
• ‘Fit to Commit’, which focused on anti-bribery, insider trading and procurement
• Information Management
Our Chief Legal Officer also provides regular updates to the Board members on developments related to insider trading laws and regulations and briefs the members of the Board and the Executive Committee on an annual basis on their respective duties. In addition, the Company offers a broad range of external trainings to its Board members.
Role of the Board and its committees
The Board is responsible for the overall direction and oversight of management, and holds the ultimate decision-making authority, with the exception of decisions reserved for shareholders. Board members are expected to commit the time and effort required to fulfil all their Board and committee responsibilities.
The Board has delegated certain duties and responsibilities to its five committees led by a Board-elected committee chair, as set out in the Board Regulations (www.novartis.com/investors/company-overview/corporate-governance). In some cases, these responsibilities are of an advisory or preparatory nature. In other cases, the committee has decision-making power that is subject to final Board approval, or the responsibilities have been fully delegated to the committee. All committees have the authority to retain external consultants.
Any Board member may request a Board or committee meeting and the inclusion of an agenda item. Before meetings, Board members receive materials to help them prepare for the discussions and to inform decision-making.
Attendance at Board and Board Committee Meetings in 2023
Name
| |
Position
| |
Board
| |
Audit and
Compliance
Committee
| |
Compensation
Committee
| | Governance,
Sustainability
and Nomination
Committee | |
Risk
Committee
| |
Science &
Technology
Committee
| |
|
J. Reinhardt | | Board Chair | | 9/9 | | | | | | | | | | 3/3 | |
|
S. Moroney | | Vice-Chair | | 9/9 | | | | 6/6 | | | | | | 3/3 | |
|
P. Bula | | Lead Independent Director | | 9/9 | | | | 6/6 | | 4/4 | | | | | |
|
N. Andrews | | Member | | 9/9 | | | | | | | | 4/4 | | 3/3 | |
|
T. Buechner | | Member | | 9/9 | | 8/8 | | | | | | 4/4 | | | |
|
E. Doherty | | Member | | 9/9 | | 8/8 | | | | | | 4/4 | | | |
|
B. Heller | | Member | | 9/9 | | 8/8 | | 6/6 | | 4/4 | | | | | |
|
F. van Houten | | Member | | 9/9 | | 8/8 | | | | | | | | 3/3 | |
|
D. Hochstrasser | | Member | | 9/9 | | 8/8 | | | | 4/4 | | | | | |
|
A. de Pro Gonzalo | | Member | | 9/9 | | 8/8 | | | | | | 4/4 | | | |
|
C. Sawyers | | Member | | 9/9 | | | | | | 4/4 | | | | 3/3 | |
|
W. Winters | | Member | | 9/9 | | | | 6/6 | | 4/4 | | | | | |
|
J. Young 1 | | Member | | 7/7 | | | | | | | | 4/4 | | 3/3 | |
|
|
1 Mr. Young was elected at the 2023 AGM. |
|
Further details can be found on pages 137 – 142. |
Board of Directors
Primary responsibilities
• Strategy: decides on the ultimate direction of the Company’s business (including portfolio, markets, acquisitions and divestments), considering also key ESG aspects
• Structure and organization: determines major changes in the Group’s structure and organization
• Culture: oversees the strategy and implementation of the corporate culture
• Ethics and compliance: oversees the Group’s ethics and compliance framework, including the approval of fundamental corporate policies such as the Novartis Code of Ethics
• Risk management: oversees the Group’s risk management system, the most significant risks, and how these risks are managed
• Finance: determines the Group’s accounting system, financial controls and financial planning; and reviews and approves the Annual Report (including the Compensation Report)
• Non-financial reporting: reviews and approves the Group’s annual reporting on non-financial matters
• People and organization: nominates or appoints, removes, and determines responsibilities of key executives, and succession planning
• Oversaw the Company’s strategy to become an innovative medicines company with leading technology in key therapeutic and geographic areas
• Reviewed the set-up and functioning of the Executive Committee in the context of the Company’s organizational structure
• Reviewed in depth the US market and our priorities to accelerate growth and become a top player in the market, including a briefing on our markets
• Discussed updates from all organizational units
• Reviewed and discussed strategic considerations around mergers and acquisitions (including the acquisition of Chinook Therapeutics), and the Company’s larger strategic moves to drive sustainable growth
• Assessed and decided on the structure of Sandoz following the spin-off, including the designated Sandoz board (and its Committees) and the designated leadership team
• Conducted the Sandoz separation through a 100% spin-off, including securing shareholder approval at the extraordinary General Meeting held on September 15, 2023
• Discussed the Company’s ESG strategy, plans and developments, and attended an ESG session jointly organized by the Audit and Compliance Committee and the Governance, Sustainability and Nomination Committee.
• Discussed the upcoming non-financial disclosure regulations and Novartis non-financial reporting governance
• Discussed longer-term Board succession planning and required profiles
• Discussed and reviewed the annual Board self-evaluation
Meetings
Number of meetings held
9
Approximate average duration (hours)
6:12
The Board met nine times in 2023. This included regular meetings in January, April, June, August, October and December, and additional special meetings to deal with ad hoc matters. Board committees typically meet the day before the meetings of the full Board. The Board held virtual, hybrid and physical meetings, with participants joining in person whenever possible.
J. Reinhardt (Board Chair)
9
S. Moroney (Vice-Chair)
9
P. Bula (Lead Independent Director)
9
Documents
• Articles of Incorporation of Novartis AG
• Board Regulations
www.novartis.com/investors/company-overview/corporate-governance
1 Mr. Young was elected at the 2023 AGM and has attended all Board meetings in 2023 following his election.
Audit and Compliance Committee
Primary responsibilities
• Supervises the external auditor, and selects and nominates the external auditor for election by the shareholders (FD)**
• Oversees Internal Audit (FD)**
• Oversees accounting policies, financial controls, and compliance with accounting and internal control standards (FD)**
• Approves financial statements for the first three quarters of each calendar year and the corresponding financial results releases (FD)**, and reviews the annual financial statements and the corresponding financial results releases (FBA)***
• Reviews the non-financial data contained in the Group’s annual reporting (FBA)***
• Oversees compliance with laws, regulations and internal policies related to its subject matter expertise (FD)**
• Reviews updates with regards to Quality Assurance and patient safety twice a year and Health Safety & Environment once a year (FD)**
• Reviews updates from the SpeakUp Office twice a year (FD)**
• Reviews the Group’s tax policy every two years (FD)**
• Reviews updates in closed sessions with the Chief Financial Officer, Chief Audit Officer, and external auditor (FD)**
• Reviewed the timelines, milestones and accounting treatment of the Sandoz spin-off
• Reviewed the non-financial reporting
• Reviewed the accounting and financial reporting, focusing on those areas involving significant risk or judgment
• Reviewed and discussed the Company’s approach to non-financial reporting and assurance, including audit scope, processes and the required involvement of the Audit and Compliance Committee (ACC) and the full Board
• In a joint session with the GSNC received an overview of the current and emerging ESG reporting standards and regulations, in addition to the ESG reporting roadmap of Novartis
• Monitored progress on the delivery of the transformation for growth targets
• Received an update on the Novartis fraud risk management framework, including the assessment against the Committee of Sponsoring Organizations of the Treadway Commission (COSO) principles
• Liaised with the Risk Committee to ensure adequate oversight of the Company’s key transformation projects (Enterprise Data Governance and Management and Lean Digital Core (LDC) program)
• Reviewed how Novartis is approaching integrated assurance
• Reviewed the Novartis tax policy
• Evaluated the performance of the external auditor of Novartis, KPMG, during 2023
• Received reports and updates from Internal Audit; Quality; Ethics, Risk & Compliance; the SpeakUp Office; Health, Safety & Environment; and Legal, and discussed progress on identifying and remedying the root causes of any associated issues or problems
Meetings
Number of meetings held
8
Approximate average duration (hours)
2:30
E. Doherty (Chair, Audit Committee Financial Expert)
8
A. de Pro Gonzalo (Audit Committee Financial Expert)
8
Documents
• Board Committees Charter, Appendix I to the Board Regulations
www.novartis.com/investors/company-overview/corporate-governance
* A/P = advisory or preparatory task
** FD = fully delegated task
*** FBA = task subject to final Board approval
Compensation Committee
Primary responsibilities
• Designs, reviews and recommends to the Board the compensation policies and programs (FBA)***
• Advises the Board on the compensation of Board members and the CEO (A/P)*
• Decides on the compensation of Executive Committee members (FD)**
• Prepares the Compensation Report and the Say-on-Pay brochure, and submits them to the Board for approval (FBA)***
• Made decisions relating to Executive Committee and wider employee compensation during the year
• Reviewed the Sandoz Swiss listing prospectus, Say-on-Pay and incentive plan rules including restoration principles for shareholders and equity holders
• Determined the critical performance measures (including financial, strategic, operational, innovation and ESG) to be considered in the Executive incentive plan targets
• Assessed the achievement of incentive plan targets for the Executive Committee members
• Reviewed shareholder and proxy advisor feedback related to Novartis compensation practices and disclosures, in addition to those of peer companies
• Reviewed format of disclosures in the Novartis Compensation Report
• Proposed appropriate peer companies for comparisons of board and executive committee compensation, and assessed the Company’s level of compensation against the peer group
• Reviewed incentive plan rules to secure pay-for-performance alignment while preserving market competitiveness
• Reflected on the effectiveness of the Company’s compensation programs in view of becoming a pure-play innovative medicines company
Meetings
Number of meetings held
6
Approximate average duration (hours)
1:55
Documents
• Board Committees Charter, Appendix I to the Board Regulations
www.novartis.com/investors/company-overview/corporate-governance
* A/P = advisory or preparatory task
** FD = fully delegated task
*** FBA = task subject to final Board approval
Governance, Sustainability and Nomination Committee
Primary responsibilities
• Oversees the Company’s strategy, governance and progress on sustainability, including access to medicine and healthcare, global health, environmental sustainability, human capital management and other material ESG aspects (FBA)***
• Recommends corporate governance best practices to the Board (FBA)***
• Reviews the Articles of Incorporation and Board Regulations on a periodic basis (FD)**
• Reviews the composition and size of the Board and its committees as well as the skills matrix on a regular basis (FBA)***
• Identifies new Board member candidates and recommends to the Board whether existing Board members should stand for re-election (FBA)***
• Prepares and reviews succession plans for the Board Chair, the Vice-Chair, the Lead Independent Director, Board members, committee members and chairs, and the CEO (FBA)***
• Reviews the independence of each Board member on an annual basis (FBA)***
• Reviews directorships and agreements of Board members for conflicts of interest, and deals with conflicts of interest (FBA)***
• Discussed the composition of, and the succession for, the Novartis Board and its committees on a regular basis
• Discussed benchmarking data concerning the board size, composition, diversity, and committee structure of peer companies
• Discussed the candidates for the Sandoz board and the Sandoz board committee structure
• Discussed the new Swiss legal requirements on non-financial reporting and the corresponding shareholder vote on the 2023 report on non-financial matters at the 2024 AGM
• Reviewed an update on ESG Strategy with a focus on trends regarding ESG disclosure regulations, access to medicines and environmental sustainability
• Regularly reviewed updates on the ESG Scorecard to track progress against the sustainability targets for Innovation & Access, Human Capital Management, Environmental Sustainability and Ethical Standards; reviewed the 2024 ESG targets
• Received an update on access to medicines, including the implementation of the Novartis access principles
• Received an update on human capital management focused on leadership development, our company culture, the care agenda for associates, and hybrid working
• Received an update on environmental sustainability, which covered performance against the targets of Novartis for climate, water, and waste, the impact of the Sandoz separation, the approach to reducing scope 3 emissions (including supplier engagement), and the Novartis carbon reduction glidepath to Net Zero in 2024.
• Reviewed the company’s performance to date and upcoming regulations and future Novartis targets on gender balance, equal pay, and pay transparency
• In a joint education session with the ACC received an overview of the current and emerging ESG reporting standards and regulations, in addition to the ESG reporting roadmap of Novartis
• Evaluated the results of the 2023 AGM as well as investor and analyst feedback from ESG and Governance roadshows held during 2023
• Reviewed and updated the Board Skills Matrix
Meetings
Number of meetings held
4
Approximate average duration (hours)
2:03
Documents
• Board Committees Charter, Appendix I to the Board Regulations
www.novartis.com/investors/company-overview/corporate-governance
* A/P = advisory or preparatory task
** FD = fully delegated task
*** FBA = task subject to final Board approval
Risk Committee
Primary responsibilities
• Oversees the risk management system and processes (FBA)***
• Reviews, together with management, the prioritization and handling of risks, the risk portfolio, and actions implemented by management (FBA)***
• Performs deep dives into key risk areas and fosters a culture of smart risk-taking (FBA)***
• Reviews updates on cyber security on an annual basis (FD)**
• Reviews regular updates from designated risk owners as well as the Chief Ethics, Risk & Compliance Officer and/or the Head of Risk & Resilience (FD)**
• Received updates on Enterprise Risk Management mitigation measures and results
• Received an update on European Union regulatory measures and its associated risks and opportunities
• Reviewed and discussed the current risks associated with key product launches in China and the US
• Evaluated the risks associated with current geopolitical developments
• Discussed the key risks associated with data science and artificial intelligence
• Received an update on the Company’s supply network
• Received an update on the main risks in our Research and Development organizational units
• Reviewed the Source-to-Pay risks and mitigations
• Received a deep-dive update on cyber security, including on data loss protection
• Discussed enterprise data management and Lean Digital Core/ERP design and implementation
Meetings
Number of meetings held
4
Approximate average duration (hours)
1:52
Documents
• Board Committees Charter, Appendix I to the Board Regulations
www.novartis.com/investors/company-overview/corporate-governance
* A/P = advisory or preparatory task
** FD = fully delegated task
*** FBA = task subject to final Board approval
1 Mr. Young became a member of the Risk Committee after the 2023 AGM and has attended all Risk Committee meetings in 2023 following his election.
Science & Technology Committee
Primary responsibilities
• Monitors emerging scientific, data-related, technological and research trends and issues, and brings recommendations to the Board (FBA)***
• Informs the Board on a periodic basis about critical developments for the success of the portfolio and for scientific, technological and research activities as well as benchmarking (A/P)*
• Assists the Board with setting the Company’s strategy for science, data, technology and research (A/P)*
• Assists the Board with oversight and evaluation of the performance of the Company’s scientific, technological and R&D activities (FBA)***
• Reviews performance and proposed targets in the area of science, technology and research (FD)**
• Reviews other matters in relation to science, data, technology and research that the committee may, at its own discretion, deem desirable in connection with its responsibilities (A/P)*
• Reviewed the strategy of our Biomedical Research organizational unit with its new leadership
• Reviewed the strategy of the Novartis Venture Fund, and provided input on its future direction
• Provided input on the Novartis plan for future equity investing
• Reviewed R&D performance metrics, including benchmarking, and the Biomedical Research and Development organizational units’ plans to enhance performance
• Provided guidance to Merger & Acquisition (M&A) and Business Development & Licensing (BD&L) teams on scientific aspects of key deals
• Discussed the strategy of our Development organizational unit with its new leadership
Meetings
Number of meetings held
3
Approximate average duration (hours)
3:35
Documents
• Board Committees Charter, Appendix I to the Board Regulations
www.novartis.com/investors/company-overview/corporate-governance
* A/P = advisory or preparatory task
** FD = fully delegated task
*** FBA = task subject to final Board approval
1 Mr. Young became a member of the Science and Technology Committee after the 2023 AGM and has attended all Science and Technology Committee meetings in 2023 following his election.
Board Chair
The Board Chair leads the Board to represent the interests of all stakeholders and ensures an appropriate balance of power between the Board and the Executive Committee. In this role, the Board Chair:
• Provides leadership to the Board
• Supports and mentors the CEO
• Ensures that the Board and its committees work effectively
• Sets the agenda, style and tone of Board discussions, promoting constructive dialogue and effective decision-making
• Ensures onboarding programs for new Board members, and continuous education for and specialization of all Board members
• Ensures the Board’s annual performance evaluation
• Promotes effective relationships and communication between Board and Executive Committee members
• Ensures effective communication with the Company’s shareholders, other stakeholders and the public
Vice-Chair and Lead Independent Director
Vice-Chair
The Vice-Chair has the following responsibilities:
• Leads the Board in the event that, and for as long as, the Board Chair is incapacitated
• Leads the yearly session of the Board members to evaluate the performance of the Board Chair, during which the Board Chair is not present
The Vice-Chair also provides advice and support to the Board Chair.
Lead Independent Director
To support adequate control mechanisms, the Board Regulations outline the role of the Lead Independent Director. The Lead Independent Director has the following responsibilities:
• Chairs the sessions of the independent Board members
• Leads the independent Board members in the event of a crisis or matter requiring their separate consideration or decision
The roles of the Vice-Chair and the Lead Independent Director can be held by two Board members or by one Board member (combined role).
The Board appointed Simon Moroney as Vice-Chair and Patrice Bula as Lead Independent Director, both roles effective as of March 4, 2022.
Honorary Chairman
Alex Krauer and Daniel Vasella were appointed Honorary Chairmen in recognition of their significant achievements on behalf of Novartis. In December 2021, Mr. Krauer passed away at the age of 90.
Mr. Vasella is not provided with Board documents and does not attend Board meetings.
Mandates outside the Novartis Group
According to article 34, paragraph 1 of the Articles of Incorporation (www.novartis.com/investors/company- overview/corporate-governance), the following limitations on mandates apply:
| | Maximum number
of mandates | |
|
Mandates | | 10 | |
|
Other listed companies 1 | | 4 | |
|
|
1 Holding a chair position of the board of directors in other listed companies counts as two mandates. |
According to article 34, paragraph 3 of the Articles of Incorporation (www.novartis.com/investors/company- overview/corporate-governance), the following mandates are not subject to the above-mentioned limitations:
| | Maximum number
of mandates | |
|
Mandates in companies that are controlled by Novartis AG | | No limit | |
|
Mandates held at the request of Novartis AG or companies controlled by it | | 5 | |
|
”Mandates” shall mean any membership in the board of directors, in the executive board or in the advisory board, or a comparable function under foreign law, in a company with an economic purpose. Mandates in different legal entities that are under joint control are deemed to be one mandate.
For a full list of all external mandates subject to the above-mentioned limitations, please refer to the Compensation Report (see “—Item 6.B Compensation—”Mandates outside the Novartis Group”).
Executive Committee
Composition (as per December 31, 2023)Vasant (Vas) NarasimhanChief Executive OfficerShreeram AradhyePresident, Development& Chief Medical OfficerVictor BultoPresident, USAharon (Ronny) GalChief Strategy & Growth OfficerKaren L. HaleChief Legal OfficerPatrick HorberPresident,InternationalHarry KirschChief Financial OfficerRobert (Rob) KowalskiChief People & Organization OfficerSteffen LangPresident, OperationsFiona H. MarshallPresident, BiomedicalResearchKlaus MoosmayerChief Ethics, Risk & Compliance Officer
Changes to the Executive Committee
Marie-France Tschudin, President of the Innovative Medicines International unit and Chief Commercial Officer since 2022, stepped down from her role effective September 15, 2023. The unit was renamed ‘International’ and was led by Mukul Mehta, its chief financial officer, on an ad interim basis from September 16, 2023 to November 30, 2023. Mukul was not a member of the Executive Committee during this period. Patrick Horber, M.D. became President, International, and a member of the Executive Committee effective December 1, 2023. The biography of Marie-France Tschudin can be found in the 2022 Annual Report (page 151), available at www.novartis.com/news/media-library/novartis-annual-report-2022.
Role of the Executive Committee
The Board has appointed the Executive Committee members and delegated the overall responsibility for and oversight of the operational management of Novartis to them, including:
• Recruiting, appointing and promoting senior management
• Ensuring the efficient operation of the Group and the achievement of optimal results
• Promoting an active internal and external communications policy
• Developing policies and strategic plans for Board approval, and implementing those approved
• Submitting the following to the Board for approval: investments, divestments, transactions, contracts and litigations with a value exceeding USD 500 million, and capital market and other important financing transactions, as well as all other matters of fundamental significance to the Novartis Group
• Preparing and submitting quarterly and annual reports to the Board and its committees
• Informing the Board of all matters of fundamental significance to the businesses
• Dealing with any other matters delegated by the Board
There are no contracts between Novartis and third parties whereby Novartis would delegate any business management tasks to such third parties.
CEO
With the support of the Executive Committee, the CEO is responsible for the operational management of Novartis. This includes effectively implementing the Company strategy, delivering financial results, and shaping a corporate culture of empowerment and responsibility to help drive innovation, performance and reputation.
In addition to other Board-assigned duties, the CEO leads the Executive Committee, and is responsible for building and maintaining an effective executive team. With the support of the Executive Committee, the CEO is responsible for:
• Ensuring Novartis has the capabilities to achieve its long-term strategic objectives
• Developing robust management succession and development plans for presentation to the Board
• Promoting effective communication with shareholders and other stakeholders
• Ensuring Novartis conducts its business in a legal and ethical manner
• Developing an effective risk control framework for all business activities
• Ensuring the flow of information to the Board is accurate, timely and clear
Diversity
The composition of the Executive Committee of Novartis as of December 31, 2023, in terms of nationality, gender, age and length of tenure, is shown in the following charts:
Diversity profileNationality1pAmerican41%pGerman18%pSwiss18%pBritish9%pSpanish9%pIsraeli5%GenderpMale82%pFemale18%Agep<450%p455018%p>5082%Tenurep<2y45.5%p24y18%p>4y36.5%1Please note that three Executive Committee members have dual nationalities. Each of these nationalities is counted as a half in the above chart.
Mandates outside the Novartis Group
According to article 34, paragraph 2 of the Articles of Incorporation (www.novartis.com/investors/company-overview/corporate-governance), the following limitations on mandates apply:
| | Maximum number
of mandates | |
|
Mandates | | 6 | |
|
Other listed companies 1 | | 2 | |
|
|
1 Holding a chair position of the board of directors in other listed companies is not allowed. |
According to article 34, paragraph 3 of the Articles of Incorporation (www.novartis.com/investors/company-overview/corporate-governance), the following mandates are not subject to the above-mentioned limitations:
| | Maximum number
of mandates | |
|
Mandates in companies that are controlled by Novartis AG | | No limit | |
|
Mandates held at the request of Novartis AG or companies controlled by it | | 5 | |
|
”Mandates” shall mean any membership in the board of directors, in the executive board or in the advisory board, or a comparable function under foreign law, in a company with an economic purpose. Mandates in different legal entities which are under joint control are deemed one mandate.
For a full list of all external mandates subject to the above-mentioned limitations, please refer to the Compensation Report (see “—Item 6.B Compensation—”Mandates outside the Novartis Group”).
Members of the Executive Committee
Vasant (Vas) Narasimhan, M.D.
Chief Executive Officer of Novartis since 2018 | Nationality: American | Year of birth: 1976
Professional experience
• Global Head of Drug Development and Chief Medical Officer, Novartis AG, Switzerland (2016–2018)
• Global Head of Development, Novartis Pharmaceuticals, Switzerland (2014–2016)
• Global Head of Biopharmaceuticals and Oncology Injectables, Sandoz International, Germany (2014)
• Global Head of Development, Novartis Vaccines, US (2012–2014)
• North America Region Head, Novartis Vaccines, and US Country President, Novartis Vaccines and Diagnostics, US (2008–2012)
• Joined Novartis in 2005
Mandates
• Member, National Academy of Medicine, US
• Committee member, Biopharmaceutical CEOs Roundtable (BCR), International Federation of Pharmaceutical Manufacturers & Associations (IFPMA), Switzerland
• Board member and treasurer, Pharmaceutical Research and Manufacturers of America (PhRMA), US
Education
• Doctor of medicine, Harvard Medical School, US
• Master’s degree in public policy, John F. Kennedy School of Government, Harvard University, US
• Bachelor’s degree in biological sciences, University of Chicago, US
Shreeram Aradhye, M.D.
President, Development & Chief Medical Officer since May 16, 2022 | Nationality: American | Year of birth: 1962
Professional experience
• Executive vice president & chief medical officer, Dicerna Pharmaceuticals, US (2020–March 2022)
• Executive vice president & chief development officer, Axcella Health, US (2019–2020)
• Global Head, Medical Affairs and Chief Medical Officer, Pharmaceuticals, Novartis, US & Switzerland (2017–2019)
• Global Head, Development Franchise, Neuroscience, and US Head, Development, Novartis, US & Switzerland (2013–2017)
• Executive Global Program Head, Multiple Sclerosis, Novartis, Switzerland (2012–2013)
• Head, Global Development India, Novartis, India (2011–2012)
• Head, Global Clinical Development & Medical Affairs, Biosimilars, Sandoz, Germany (2009–2011)
• Joined Novartis in 1999 holding positions of increasing responsibility
Education
• Chief resident and teaching fellow in internal medicine, Newton Wellesley Hospital, US
• Resident in internal medicine, Newton Wellesley Hospital, US
• Fellow in nephrology, St Luke’s Roosevelt Medical Center, US
• Resident in internal medicine (M.D.), All India Institute of Medical Sciences, India
• Bachelor of medicine and bachelor of surgery, All India Institute of Medical Sciences, India
Victor Bulto
President, US since April 4, 2022 | Member of the Executive Committee as of May 1, 2022 | Nationality: Spanish |
Year of birth: 1978
Professional experience
• President, Novartis Pharmaceuticals Corporation, US (2019–April 2022)
• Vice President & Head US Immunology & Dermatology Franchise, US (2017–2019)
• Vice President & Head US Alcon Pharmaceuticals, US (2016–2017)
• Head Neuroscience Franchise, Region Europe, Novartis, Switzerland (2013–2016)
• Business Franchise Head Neuroscience, Novartis, Spain (2012–2013)
• Business Franchise Head Neuroscience/MS, Respiratory, Osteoarticular, Spain, Novartis (2010–2012)
• Marketing Head Respiratory, Osteoarticular, Novartis, Spain (2009–2010)
Mandates
• Board member, Biotechnology Innovation Organization (BIO), US
Education
• Master of business administration, ESADE Business School, Spain
• Master’s degree in health economics and pharmacoeconomics, Pompeu Fabra University Spain
• Master’s degree in chemical engineering, Ramon Llull University, Spain
• Bachelor’s of science degree in chemistry, Ramon Llull University, Spain
Aharon (Ronny) Gal, Ph.D.
Chief Strategy & Growth Officer since July 18, 2022 | Nationality: Israeli/American | Year of birth: 1966
Professional experience
• Senior analyst, US biopharmaceutical, Sanford Bernstein, US (2020–June 2022)
• Senior analyst, US specialty pharmaceuticals and Biotech, Sanford Bernstein, US (2016–2020)
• Senior analyst, US specialty pharmaceuticals and EU mid-cap pharmaceuticals, Sanford Bernstein, US, UK (2013–2016)
• Senior analyst, US specialty pharmaceuticals, Sanford Bernstein, US (2004–2013)
• Vice president, Canon US Life Sciences, US (2003–2004)
• Consultant, team leader, manager, The Boston Consulting Group, Inc., US, Singapore, China (1996–2002)
Education
• Doctorate in biochemistry, Massachusetts Institute of Technology, US
• Bachelor’s degree in chemistry, Emory University, US
Karen L. Hale
Chief Legal Officer of Novartis since May 15, 2021 | Nationality: American | Year of birth: 1968
Professional experience
• Vice president, deputy general counsel, AbbVie Inc., US (2019–2021)
• Vice president, chief ethics and compliance officer, AbbVie Inc., US (2013–2019)
• Vice president, litigation and legal specialty operations, AbbVie Inc., US (2013)
• Divisional vice president, commercial litigation, Abbott Laboratories, US (2006–2012)
• Began practicing law in 1994 and joined Abbott in 1997
Education
• Bar memberships: Illinois and Virginia, US
• Juris doctor, William & Mary Law School, US
• Bachelor’s degree in economics, Duke University, US
Patrick Horber
President, International since December 1, 2023 | Nationality: Swiss | Year of birth: 1970
Professional experience
• Senior vice president, AbbVie, president Immunology, AbbVie, US (July 2023–September 2023)
• President, US commercial operations, Immunology, AbbVie, US (2020–June 2023)
• Vice president and head of global marketing and commercial operations, AbbVie, US (2019–2020)
• Vice president and managing director, AbbVie, Germany (2015–2019)
• Managing director, AbbVie, Switzerland (2013–2015)
• Managing director, Abbott, Switzerland (2012–2012)
• Leadership roles at headquarters and country operations, Roche (2005–2012)
Mandates
• Member of the board and chair of the strategy and politics committee, Verband Forschender Arzneimittelhersteller, Germany (2016–2019)
• Chair of the executive committee and member of the presidents bureau, Interpharma, the association of Switzerland’s research-based pharmaceutical industry (2015–2015)
• Member of the executive committee and the board, Interpharma (2013–2015)
Education
• Doctor of medicine (M.D.), University of Zurich, Switzerland
Harry Kirsch
Chief Financial Officer of Novartis since 2013 | Nationality: German/Swiss | Year of birth: 1965
Professional experience
• Chief Financial Officer of the Pharmaceuticals Division, Novartis Pharmaceuticals, Switzerland (2010–2013)
• Chief Financial Officer of Pharma Europe, Novartis Pharmaceuticals, Switzerland (2008–2010)
• Head of Business Planning & Analysis for the Pharmaceuticals Division, Novartis Pharmaceuticals, Switzerland (2005–2008)
• Joined Novartis in 2003 as Head Finance Global Primary Care, and over the years held positions of increasing responsibility within Finance
Mandates
• Represented Novartis on the board of GlaxoSmithKline Consumer Healthcare Holdings Ltd. (2015–2018)
Education
• Diploma degree in industrial engineering and economics, University of Karlsruhe, Germany
Robert (Rob) Kowalski
Chief People & Organization Officer of Novartis since September 1, 2021 | Nationality: American | Year of birth: 1968
Professional experience
• Executive Vice President and Global Head of Regulatory Affairs (2018–2021), and US Head of Global Drug Development (2009–2015 and 2017–2021), Novartis Pharmaceuticals Corporation, US
• Ad interim President, Novartis Corporation, US (2021)
• Ad interim Head of Global Drug Development and Chief Medical Officer, Novartis AG, Switzerland (2018)
• Senior Vice President and Head of Regulatory Affairs, Novartis Pharmaceuticals Corporation, US (2009–2015 and 2017–2018)
• Senior Vice President and Head of Regulatory Affairs, Novartis Pharma AG, Switzerland (2015–2017)
• Global Head of Country Medical Development, Novartis Pharmaceuticals Corporation, US (2010–2011)
• Previously held regulatory leadership roles at Schering-Plough Corporation (now Merck) and Pharmacia Corporation (now Pfizer)
Mandates
• Member of the advisory board, Industry Pharmacists Organization, US
Education
• Doctor of pharmacy, University of Wisconsin-Madison, US
• Bachelor’s degree in pharmaceutical sciences, University of Wisconsin-Madison, US
Steffen Lang, Ph.D.
President, Operations since April 4, 2022 | Nationality: German/Swiss | Year of birth: 1967
Professional experience
• Global Head of Novartis Technical Operations (NTO), Switzerland (2017–April 2022)
• Global Head of Biologics Technical Development and Manufacturing, Novartis Technical Operations, Switzerland (2015–2017)
• Global Head of Technical Research and Development, Novartis Pharmaceuticals, Switzerland (2009–2015)
• Joined Novartis in 1994 as Head of Laboratory in Research, and over the years held positions of increasing responsibility within Pharmaceuticals Development
Mandates
• Board member, Bachem Holding AG, Switzerland
Education
• Doctorate in pharmaceutical technology, Swiss Federal Institute of Technology, Switzerland
• Master’s degree in pharmaceutical sciences, University of Heidelberg, Germany
Fiona H. Marshall, Ph.D.
President, Biomedical Research since November 1, 2022 | Nationality: British | Year of birth: 1964
Professional experience
• Senior vice president, head of discovery, preclinical and translational medicine, Merck & Co., US, (2021–September 2022)
• Vice president, global head of neuroscience, Merck & Co., US (2019–2021)
• Vice president, head of UK discovery research, Merck & Co., UK (2018–2019)
• Executive vice president and chief scientific officer, Sosei Heptares, UK (2015–2018)
• Chief scientific officer and founder, Heptares Therapeutics, UK (2006–2018)
Mandates
• Member of the Scientific Advisory Board, SciLifeLab, Sweden
• Fellow, Royal Society, UK Academy of Medical Sciences, and Royal Society of Biology, all UK
Education
• Doctorate in neuroscience, University of Cambridge, UK
• Bachelor’s degree in biochemistry, University of Bath, UK
Klaus Moosmayer, Ph.D.
Chief Ethics, Risk & Compliance Officer of Novartis since 2018 | Nationality: German | Year of birth: 1968
Professional experience
• Chief compliance officer, Siemens AG, Germany (2014–2018)
• Chief counsel compliance, Siemens AG, Germany (2009–2013)
• Compliance operating officer, Siemens AG, Germany (2007–2009)
Mandates
• Board member, SwissHoldings, the Swiss federation representing Swiss-based multinational companies, Switzerland
• Member of the executive board, Business at OECD (BIAC), Paris
• Co-founder and honorary board member, European Chief Compliance and Integrity Officers’ Forum
• Co-chair, B20 Integrity & Compliance Task Force under the G20 presidencies of Indonesia (2022), Italy (2021), Saudi Arabia (2020), Argentina (2018), and Chair of the Task Force under the G20 presidency of Germany (2017)
• Chair of the Anti-Corruption Committee of the Business and Industry Advisory Committee (BIAC), Organization for Economic Co-operation and Development (OECD), Paris (2013–2020)
Education
• First and second state examination in law, Germany
• Doctor of jurisprudence, University of Freiburg, Germany
Information and control systems
The Board’s information and control systems vis-à-vis management include a steady flow of information from senior management; monthly financial reports; a comprehensive and integrated risk management framework; and the independent evaluation of our risk management and internal control framework by the Internal Audit function (see “Item 15. Controls and Procedures”).
Information from senior management
The Board ensures that it receives sufficient information from the Executive Committee through:
• Monthly CEO reporting (encompassing progress against company targets, including financial results) and frequent communications from the CEO on current developments
• Executive Committee meeting minutes
• Regular meetings and teleconferences by the Board and/or Board committees with the CEO and/or other members of the Executive Committee (e.g., the CFO, the Chief Legal Officer, the Chief Ethics, Risk & Compliance Officer), and regular meetings and teleconferences with senior management (e.g., the Chief Audit Officer)
• Information from Executive Committee members or other Novartis employees, and visits to Novartis sites
To obtain an outside view, the Board and/or Board committees occasionally invite external advisors (e.g., the independent advisor of the Compensation Committee, the external auditor) to attend a meeting and/or share their observations about a specific topic.
Monthly financial reports
Novartis produces comprehensive, consolidated (unaudited) financial statements on a monthly basis for the Company. These are typically available within 10 days after the end of the month, and include the following:
• Consolidated income statement of the month and year to date, in accordance with IFRS Accounting Standards, as well as adjustments to arrive at non-IFRS measures core results, as defined by Novartis (see “Item 5. Operating and Financial Review and Prospects—Item 5.A Operating results—Non-IFRS measures as defined by Novartis”). The IFRS Accounting Standards and non-IFRS measures core figures are compared with the prior-year period and targets in both USD and on a constant currency basis.
• Supplementary data on a monthly and year-to-date basis, such as free cash flow and earnings per share on a USD basis
Management information related to the consolidated income statements and free cash flow is made available to Board members through the monthly CEO Report, which includes an analysis of key deviations from the prior year or target.
Prior to the release of each quarter’s results, the Board receives the actual consolidated financial statement information and an outlook of the full-year results in accordance with IFRS Accounting Standards and non-IFRS measures core results (as defined by Novartis), together with related commentary.
Annually, during the third quarter, the Board approves the Company’s strategic plan for the next three years. In the fourth quarter of the year, the Board approves the operating targets for the following year as well as the financial targets for the following three-year period, including a projected consolidated income statement in USD prepared in accordance with IFRS Accounting Standards and non-IFRS measures as defined by Novartis (core results).
The Board does not have direct access to the Novartis financial and management reporting systems but can, at any time, request more detailed information.
Risk management
Overview
At Novartis, our continued success depends on our ability to manage risk. Our Board has ultimate oversight of the Enterprise Risk Management (ERM) system and regularly reviews the most significant risks and how these risks are managed. As explained further below, the Board is supported by its committees. Furthermore, our Internal Audit function provides an independent evaluation of risk management (see “—Item 6.C Board practices—Information and control systems—Internal Audit”).
Risk committee
• Oversees the risk management system and processes
• Reviews, together with management, the prioritization and handling of risks, the risk portfolio, and actions implemented by management
• Performs deep dives into key risk areas and fosters a culture of smart risk-taking
• Receives updates on cyber security on an annual basis
• Receives regular updates from designated risk owners as well as the Chief Ethics, Risk & Compliance Officer and/or the Head of Risk & Resilience
Audit and compliance committee
• Ensures that Internal Audit plans are aligned with key risks, and that the function provides independent assurance and insights around these risks
• Works closely with the Risk Committee to minimize gaps in risk coverage
• Receives a semiannual presentation from the Chief Ethics, Risk & Compliance Officer
• Receives a quarterly presentation from the Chief Audit Officer on progress achieved in implementing the risk-based audit plan, and key insights about audit and advisory activities
• Pays particular attention to financial risk
• Has closed sessions with the Chief Audit Officer and, upon request, with the Chief Ethics, Risk & Compliance Officer
Compensation committee
• Works closely with the Risk Committee to ensure that the compensation system does not lead to excessive risk-taking (see “—Item 6.B Compensation—Compensation governance—Risk management principles”)
Executive committee of NOVARTIS
• Regularly assesses risks and fosters a culture of risk awareness, in line with the Novartis Values and Behaviors and the Novartis Code of Ethics
Ethics, risk & compliance
• Governs the Novartis Code of Ethics
• Provides an integrated ERM framework (which is further described in the following section)
• Governs the global compliance program within Novartis
• Administers the Enterprise Policy Management and global Internal Controls framework
SENIOR LEADERS OF ORGANIZATIONAL UNITS AND GLOBAL FUNCTIONS, AT ALL LEVELS
• Provide appropriate risk management within their area of responsibility
• Establish adequate risk prevention and mitigation strategies when risk exposure is identified, including tracking progress and providing resources for possible actions
• Assess emerging risks, trends and overall exposure as part of the ERM process
Enterprise Risk Management framework
The Ethics, Risk & Compliance (ERC) function provides an integrated ERM framework to obtain a holistic view of Company risks and drive a culture of smart risk-taking. Under the leadership of the Chief Ethics, Risk & Compliance Officer, the Risk & Resilience team is responsible for the overall ERM process. This process covers, but is not limited to, risks associated with:
• The research, development, manufacturing, marketing and sales of products
• Finance, taxes, intellectual property, compliance with law and regulations, security, product safety, technology, human resources, and health, safety and environmental protection
• Business objectives and strategies, including mergers and acquisitions
• External factors such as the social, political and economic environment
The ERM process continued to evolve in 2023. The Risk & Resilience team conducted risk workshops and collaborated with all risk assurance and monitoring functions to identify key risks across the Company. Each Novartis unit organized a focused risk workshop including leadership team members. In parallel, risk workshops were held in top countries by revenue and in certain focus markets. Once key risks were identified, mitigation action plans were created to address them in an effective way. The findings from these workshops were consolidated into the Novartis Risk Compass, which enables senior management, the Executive Committee and the Board to focus discussions on key risks and more closely align our corporate strategy with our risk exposure and ways of working.
In 2023, we further matured our ERM framework within the Novartis Risk & Resilience organization and developed additional risk management trainings and our risk intelligence forum, an event that brought together internal and external speakers to address emerging trends and threats. We also integrated a critical scope of activities (Health, Safety and Environment environmental remediation) into the Risk & Resilience department. Furthermore, the Enterprise Policy & Internal Control team is progressing as planned to create a holistic framework by linking process governance with policies and controls and the Central Monitoring Coordination team is expanding its scope to ensure a harmonized and coordinated monitoring process across the Company.
SpeakUp Office
Our SpeakUp Office provides a safe place for employees to report potential misconduct, including the option to do so anonymously.
Global Security
Global Security proactively collects and shares threat intelligence to protect Novartis from situations that may compromise the safety of people, products and assets, and/or the reputation of our organization. Global Security protects patients from counterfeit products and, as part of the SpeakUp process, performs fair and timely investigations into high-risk cases of alleged internal misconduct. It also provides personal security advice and support for Novartis executives and other employees with the utmost discretion.
Internal Audit
The purpose of Internal Audit is to assist the Board and the Executive Committee in discharging their governance responsibilities by providing independent assurance and advice on the effectiveness, efficiency and adequacy of processes and controls that support Novartis in achieving its objectives, managing its major risks, and ensuring compliance with applicable policies, laws and regulations.
The Chief Audit Officer reports administratively to the CEO, and functionally to the chair of the Audit and Compliance Committee (ACC). The Chief Audit Officer meets with the ACC at least once a quarter and confirms the organizational independence of the Internal Audit function to the ACC on an annual basis.
In 2023, our Internal Audit function executed a risk-based audit plan and reported the results to the audited units, the Executive Committee and the ACC. Audit findings and action plans are stored and monitored in a single location to enable efficient and effective follow-up. The following outlines the number of audits and advisories performed in 2023, and key methodology steps when managing the Internal Audit cycle.
2023 Internal Audit Activitiesaudits 28advisories18Internal Audit cycle methodology includes:Planning: Monitoring and information gathering via continuous risk assessment based on data analytics, business interviews and quarterly calibration of the audit plan. The audit plan is approved by the ACC biannually. Execution and Reporting: 46 engagements delivered in 2023, all linked to group risks, emerging topics and company-wide initiatives.Follow Up: Management is responsible for resolving issues, supported by Internal Audit to ensure timely closure of high-risk observations.
Internal Audit performed 77% of planned activities (equating to 46 of 60 engagements) in 2023. Due to methodology redesign and tool development, the internal audit function’s engagement volume was impacted as associates were actively involved in those projects.
Auditors
Duration of the mandate and terms of office
On behalf of the Board, the ACC selects and nominates an independent auditor for election at the AGM. KPMG commenced its auditing mandate for Novartis in 2022. Richard Broadbelt, Auditor in charge, and Heidi Broom-Hirst, Global Audit Partner, began serving in their roles in 2022 and 2023, respectively. The ACC together with KPMG will ensure that these partners are rotated at least every five years.
Auditing fees and additional fees
The ACC monitors and preapproves the fees paid to the external auditor for all audit and non-audit services. It has developed and approved a policy with clear guidelines on the engagement of the independent auditor firm. This policy is designed to help ensure that the independence of the external auditor is maintained. It limits the scope of services that the external auditor may provide to the Company, stipulating certain permissible types of audit-related and non-audit services, including tax services and other services that have been preapproved by the ACC. The ACC preapproves all other services on a case-by-case basis.
The external auditor is required to report periodically to the ACC about the scope of the services it has provided to the Company and the fees for the services it has performed to date. KPMG fees for professional services related to the 12-month periods ended December 31, 2023, and December 31, 2022, are as follows:
| | 2023
USD million | | 2022
USD million | |
|
Audit services | | 26.8 | | 22.5 | |
|
Audit-related services | | 2.5 | | 0.7 | |
|
Tax services | | 0.3 | | 1.2 | |
|
Other services | | 0.0 | | 0.0 | |
|
Total | | 29.6 | | 24.4 | |
|
|
|
Audit services include work performed to issue opinions on consolidated financial statements and parent company financial statements of Novartis AG, to issue opinions related to the effectiveness of the Company’s internal control over financial reporting, and to issue reports on local statutory financial statements. Also included are audit services that can generally only be provided by the statutory auditor, such as the audits of the Compensation Report, special purpose financial statement in connection with divestment transactions, information systems and the related control environment; and reviews of quarterly financial results.
Audit-related services include other assurance services provided by the independent auditor but not restricted to those that can only be provided by the statutory auditor. They include services such as: the limited assurance over the Novartis in Society Integrated Report, audits of pension and other employee benefit plans; audits in connection with non-recurring transactions; contract audits of third-party arrangements; corporate responsibility assurance; and other audit-related services.
Tax services include tax compliance, assistance with historical tax matters, and other tax-related services.
Other services in 2023 included procedures related to company specific training on emerging topics, benchmarking studies, and license fees for use of accounting and other reporting guidance databases.
Information to the Board and the ACC
The ACC, acting on behalf of the Board, is responsible for overseeing the activities of the external auditor. In 2023, this committee held seven meetings. KPMG was invited to all of these meetings to attend the discussions on auditing matters and any other matters relevant to its audit.
The ACC recommended to the Board to approve the audited consolidated financial statements and the separate parent company financial statements of Novartis AG for the year ended December 31, 2023. The Board proposed the acceptance of these financial statements for approval by the shareholders at the next AGM.
The ACC regularly evaluates the performance of the external auditor and, based on this, once a year determines whether the external auditor should be proposed to the shareholders for re-election. To assess the performance of the external auditor, the ACC requests input from management and holds private meetings with the CFO and the Chief Audit Officer and, if necessary, obtains an independent external assessment. Criteria applied for the performance assessment of the external auditor include an evaluation of: its technical and operational competence; its independence and objectivity; the sufficiency of the resources it has employed; its focus on areas of significant risk to Novartis; its willingness to probe and challenge; its ability to provide effective, practical recommendations; and the openness and effectiveness of its communications and coordination with the ACC, the Internal Audit function and management.
Once a year, the Auditor in charge and the Global Audit Partner report to the Board on the external auditor’s activities during the current year, and on the audit plan for the coming year.
On an annual basis, the external auditor provides the ACC with written disclosures required by the US Public Company Accounting Oversight Board, and the committee and the external auditor discuss the external auditor’s independence from Novartis.
Information policy
Novartis is committed to open and transparent communication with shareholders, investors, financial analysts, customers, suppliers and other stakeholders. Novartis disseminates information about material developments in its businesses in a broad and timely manner that complies with the rules of the SIX Swiss Exchange and the NYSE.
Communications
Novartis publishes this Annual Report to provide information on the Group’s results and operations. Novartis discloses financial results in accordance with IFRS Accounting Standards on a quarterly basis, and issues press releases from time to time regarding business developments.
Novartis publishes press releases related to financial results and material events to the US Securities and Exchange Commission (SEC) via Form 6-K. An archive containing annual reports, US SEC Form 20-F, quarterly results releases and all related materials – including presentations and conference call webcasts – is available at www.novartis.com/investors.
Novartis also publishes the Novartis in Society Integrated Report, available at www.novartis.com/reportinghub, which provides an overview of our business, strategy and performance, and describes how we create value for stakeholders and society. The Novartis in Society Integrated Report is prepared in accordance with Art. 964b of the Swiss Code of Obligations, and in alignment with recommendations and standards issued by the Integrated Reporting Framework, the Sustainability Accounting Standards Board (SASB), the Global Reporting Initiative (GRI), and the Task Force on Climate-related Financial Disclosures (TCFD).
The information on Board and Executive Committee compensation is outlined in the Compensation Report (see “—Item 6.B Compensation” in general, and for certain compensation information with respect to our Board that is responsive to Item 6.C.2 of Form 20-F, see “—Item 6.B Compensation—2022 Board compensation—Philosophy and benchmarking”). Please also refer to articles 29-35 of the Articles of Incorporation (www.novartis.com/investors/company-overview/corporate-governance). No change-of-control or ‘golden parachute’ clauses benefit Board members, Executive Committee members, or other members of senior management. Employment contracts with Executive Committee members are either for a fixed term not exceeding one year or for an indefinite period with a notice period not exceeding 12 months, and do not contain commissions for the acquisition or transfer of enterprises or severance payments. No loans or credits are granted to Board and Executive Committee members.
Information contained in reports and releases issued by Novartis is only correct and accurate at the time of release. Novartis does not update past releases to reflect subsequent events, and advises against relying on them for current information.
Investor Relations
Investor Relations manages the Company’s interactions with the international financial community. Several events are held each year to provide institutional investors and analysts with various opportunities to learn more about Novartis.
Investor Relations is based at the Company’s headquarters in Basel. Part of the team is located in the US to coordinate interaction with US investors. More information is available at www.novartis.com/investors.
Website information
Topic | | Information | |
|
Share capital | | Articles of Incorporation of Novartis AG
https://www.novartis.com/sites/novartiscom/files/statuten-en.pdf
Novartis key share data
www.novartis.com/investors/share-data-analysis | |
|
Shareholder rights | | Articles of Incorporation of Novartis AG
www.novartis.com/investors/company-overview/corporate-governance | |
|
Annual General Meeting of Shareholders | | Annual General Meeting of Shareholders
www.novartis.com/investors/shareholder-information/annual-general-meeting | |
|
Board Regulations | | Board Regulations
www.novartis.com/investors/company-overview/corporate-governance | |
|
Novartis code for senior financial officers | | Novartis Code of Ethical Conduct for CEO, ECN and Senior Financial
Officers of Novartis
www.novartis.com/investors/company-overview/corporate-governance | |
|
Novartis in Society Integrated Report | | Novartis in Society Integrated Report
www.novartis.com/reportinghub | |
|
Novartis Annual Report and Form 20-F | | Novartis Annual Report and Form 20-F
www.novartis.com/reportinghub | |
|
Novartis Financial Data | | Novartis Financial Data
www.novartis.com/investors/financial-data | |
|
Press releases | | Press releases
www.novartis.com/news/news-archive?type=media_release
Email service
www.novartis.com/news/stay-up-to-date | |
|
Additional information (including event calendar, registered
office, contact and email addresses, phone numbers, etc.) | | Novartis Investor Relations
www.novartis.com/investors | |
|
|
The information on our website is not, and shall not be deemed to be, a part of this Annual Report or incorporated herein. |
Quiet periods
According to our Global Insider Policy, employees who have access to material non-public information on a regular basis are designated as Continuing Insiders and are banned from trading in Novartis securities during quiet periods. Limited exemptions for the expiry of options or warrants within a quiet period apply. Our quarterly quiet periods commence on the first trading day of each calendar quarter and end at the beginning of the first trading day after the subsequent release of the quarterly and/or annual results.
In 2023, the following quiet periods applied:
• January 1, 2023, until (and including) February 1, 2023
• April 1, 2023, until (and including) April 25, 2023
• July 1, 2023, until (and including) July 18, 2023
• October 1, 2023, until (and including) October 24, 2023
6.D Employees
The table below sets forth the breakdown of the total year-end number of our full-time equivalent employees by main category of activity and geographic area for the past three years.
For the year ended
December 31, 2023
(full-time equivalents) | |
Marketing and
sales
| |
Research and
development
| |
Production and
supply
| |
Operations1
| |
General and
administration
| |
Total
| |
|
USA | | 5 219 | | 5 194 | | 1 310 | | 659 | | 464 | | 12 846 | |
|
Canada and Latin America | | 1 732 | | 461 | | 327 | | 1 019 | | 182 | | 3 721 | |
|
Europe | | 8 426 | | 8 519 | | 11 811 | | 4 035 | | 1 668 | | 34 459 | |
|
Asia/Africa/Australasia | | 12 347 | | 4 061 | | 2 718 | | 5 182 | | 723 | | 25 031 | |
|
Total | | 27 724 | | 18 235 | | 16 166 | | 10 895 | | 3 037 | | 76 057 | |
|
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
For the year ended
December 31, 2022
(full-time equivalents) | |
Marketing and
sales
| |
Research and
development
| |
Production and
supply
| |
Operations1
| |
General and
administration
| |
Total
| |
|
USA | | 6 003 | | 5 358 | | 1 740 | | 825 | | 599 | | 14 525 | |
|
Canada and Latin America | | 2 678 | | 514 | | 809 | | 1 071 | | 270 | | 5 342 | |
|
Europe | | 14 078 | | 10 483 | | 18 781 | | 5 028 | | 2 483 | | 50 853 | |
|
Asia/Africa/Australasia | | 15 856 | | 4 841 | | 3 841 | | 5 513 | | 932 | | 30 983 | |
|
Total | | 38 615 | | 21 196 | | 25 171 | | 12 437 | | 4 284 | | 101 703 | |
|
Thereof continuing operations 2 | | 30 420 | | 18 681 | | 14 826 | | 12 437 | | 3 313 | | 79 677 | |
|
Thereof discontinued operations 2 | | 8 195 | | 2 515 | | 10 345 | | | | 971 | | 22 026 | |
|
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
For the year ended
December 31, 2021
(full-time equivalents) | |
Marketing and
sales
| |
Research and
development
| |
Production and
supply
| |
Operations1
| |
General and
administration
| |
Total
| |
|
USA | | 6 074 | | 5 324 | | 1 938 | | 879 | | 654 | | 14 869 | |
|
Canada and Latin America | | 3 116 | | 510 | | 1 426 | | 1 116 | | 370 | | 6 538 | |
|
Europe | | 15 163 | | 10 307 | | 17 630 | | 5 108 | | 2 613 | | 50 821 | |
|
Asia/Africa/Australasia | | 16 927 | | 4 812 | | 3 570 | | 5 696 | | 1 090 | | 32 095 | |
|
Total | | 41 280 | | 20 953 | | 24 564 | | 12 799 | | 4 727 | | 104 323 | |
|
Thereof continuing operations 2 | | 32 973 | | 18 425 | | 14 165 | | 12 799 | | 3 765 | | 82 127 | |
|
Thereof discontinued operations 2 | | 8 307 | | 2 528 | | 10 399 | | | | 962 | | 22 196 | |
|
|
1 relates to full time equivalent employees (FTEs) from our Operations unit, excluding the Operations units’ production and supply FTEs |
2 Continuing operations include the retained business activities of Novartis, comprising the innovative medicines business and continued corporate activities. Discontinued operations include the Sandoz generic pharmaceuticals and biosimilars division and certain corporate activities attributable to Sandoz prior to the spin-off up to the distribution date of October 3, 2023. |
As of December 31, 2023, the total number of our full-time equivalent employees decreased by 25 646 compared with December 31, 2022, mainly driven by the Sandoz spin-off at the October 3, 2023, distribution date, and the initiative announced in April 2022 to implement a new, streamlined organizational model.
A significant number of our employees are represented by unions or works councils. We have not experienced any material work stoppages in recent years, and we consider our employee relations to be good.
6.E Share ownership
The information set forth under “Item 6. Directors, Senior Management and Employees—Item 6.B Compensation—CEO and Executive Committee—Additional disclosures and other statutory information—Shares, ADRs and other equity rights owned by Executive Committee members as at December 31, 2023” and under “Item 6. Directors, Senior Management and Employees—Item 6.B Compensation—Board compensation—Shares, ADRs and share options owned by Board members” is incorporated by reference. For more information on our equity-based participation plans, see the information set forth under “Item 18. Financial Statements—Note 27. Equity‑based participation plans for employees,” which is incorporated by reference.
6.F Erroneously awarded compensation
Not applicable.
Item 7. Major Shareholders and Related Party Transactions
7.A Major shareholders
Novartis shares are widely held. As of December 31, 2023, Novartis had approximately 183 000 shareholders listed in the Share Register of Novartis, representing approximately 56.8% of issued shares. Based on the Novartis Share Register and excluding treasury shares, approximately 54.6% of the shares registered by name were held in Switzerland, and approximately 24.7% were held in the US. Approximately 20.0% of the shares registered in the Share Register were held by individual investors, while approximately 41.7% were held by legal entities, excluding 10.2% of our share capital held as treasury shares by Novartis AG or its fully owned subsidiaries (including Swiss foundations controlled by Novartis AG), and 38.3% were held by nominees, fiduciaries and the ADS depositary. Due to a change in Swiss corporate law, as of January 1, 2023, Novartis ordinary shares held by Swiss foundations controlled by Novartis AG (Foundation Shares) no longer carry the right to vote. As a result, these Foundation Shares are excluded from the calculation of the shares registered in the Share Register in the same way, as described above, that our treasury shares are excluded.
Based on the Share Register, we believe that we are not directly or indirectly owned or controlled by another corporation or government, or by any other natural or legal persons. There are no arrangements that may result in a change of control.
The tables below set forth information with respect to our major shareholders according to the Share Register as of December 31, 2023, excluding 10.2% of our share capital held as treasury shares by Novartis AG or its fully owned subsidiaries (including Swiss foundations controlled by Novartis AG). The following registered shareholders (including nominees and the ADS depositary) held more than 2% of the total share capital of Novartis with the right to vote all their Novartis shares based on an exemption granted by the Board of Directors:
| | | | % of respective share capital beneficially owned as of: | |
| | | | | |
| | | | | |
| | | | | |
| | Ordinary shares
beneficially owned as of
Dec 31, 2023 | |
Dec 31, 2023
| |
Dec 31, 2022
| |
Dec 31, 2021
| |
|
Shareholders registered for their own account: | | | | | | | | | |
Emasan AG, Basel, Switzerland 1 | | 89 135 960 | | 3.9 | | 3.7 | | 3.7 | |
|
UBS Fund Management (Switzerland) AG, Basel, Switzerland | | 60 962 889 | | 2.7 | | 2.3 | | 2.3 | |
|
Credit Suisse Funds AG, Zurich, Switzerland | | 50 178 623 | | 2.2 | | 2.1 | | 2.1 | |
|
|
1 According to a disclosure notification filed with Novartis AG and the SIX Swiss Exchange, the beneficial owner of the shares registered for Emasan AG is Sandoz - Fondation de Famille, Liechtenstein. |
| | | | % of respective share capital held as of: | |
| | | | | |
| | | | | |
| | | | | |
| | Ordinary shares
held as of
Dec 31, 2023 | |
Dec 31, 2023
| |
Dec 31, 2022
| |
Dec 31, 2021
| |
|
Shareholders registered as nominees: | | | | | | | | | |
Nortrust Nominees Ltd., London, England | | 82 324 675 | | 3.6 | | 3.8 | | 4.2 | |
|
The Bank of New York Mellon, New York, NY | | 66 451 778 | | 2.9 | | 2.9 | | 3.0 | |
Through The Bank of New York Mellon, Everett, MA | | 34 094 134 | | 1.5 | | 1.6 | | 1.6 | |
Through The Bank of New York Mellon, New York, NY | | 22 963 391 | | 1.0 | | 0.9 | | 1.1 | |
Through The Bank of New York Mellon, SA/NV, Brussels, Belgium | | 9 394 253 | | 0.4 | | 0.4 | | 0.3 | |
|
Chase Nominees Ltd., London, England | | — 1 | | — | | 8.4 | | 8.8 | |
|
Shareholder acting as American Depositary Share (ADS) depositary: | | | | | | | | | |
JPMorgan Chase Bank, N.A., New York, NY | | 190 038 312 | | 8.3 | | 9.4 | | 11.1 | |
|
|
1 Chase Nominees Ltd. (Chase) has informed us that as of December 2023, it will no longer register any shareholding positions on its own behalf. Shares held by customers of Chase will be registered for such customer’s own account. |
According to a disclosure notification filed with Novartis AG, Norges Bank (Central Bank of Norway), Oslo, Norway, held 2.4% of the share capital of Novartis AG, or 55 319 441 shares, as of December 31, 2023, but was not registered in the Share Register as of December 31, 2023. Provided that these shares are registered in the
Share Register on the record date of the Annual General Meeting, Norges Bank will have full voting rights for all of these shares.
According to a disclosure notification filed with Novartis AG and the SIX Swiss Exchange, BlackRock, Inc., New York, NY, held between 5% and 10%, but was registered with less than 2% of the share capital of Novartis AG in the Share Register as of December 31, 2023.
As of December 31, 2023, no other shareholder was registered as owner of more than 2% of the registered share capital.
The Articles of Incorporation provide that no shareholder shall be registered with the right to vote shares comprising more than 2% of the registered share capital. The Board of Directors may, upon request, grant an exemption from this restriction. Considerations include whether the shareholder supports the Novartis goal of creating sustainable value and has a long-term investment horizon. Exemptions are in force for the registered major shareholders as described above. Novartis has not entered into any agreement with any shareholder regarding the voting or holding of Novartis shares.
7.B Related party transactions
The information set forth under “Item 18. Financial Statements—Note 28. Transactions with related parties” is incorporated by reference.
7.C Interests of experts and counsel
Not applicable.
Item 8. Financial Information
8.A Consolidated statements and other financial information
See “Item 18. Financial Statements.”
Dividend policy
Subject to the dividend policy described below, our Board of Directors expects to recommend the payment of a dividend in respect of each financial year. If approved by our shareholders at the relevant annual shareholders’ meeting, the dividends will be payable shortly following such approval. Any shareholder who purchases our shares before the ex‑dividend date and holds the shares until that date shall be deemed to be entitled to receive the dividends approved at that meeting. Dividends are reflected in our financial statements in the year in which they are approved by our shareholders.
Our dividend policy is to pay a growing annual dividend in Swiss francs per share. This policy is subject to our financial conditions and outlook at the time, the results of our operations, and other factors.
The Board will propose a dividend of CHF 3.30 per share to the shareholders for approval at the Annual General Meeting to be held on March 5, 2024. Because we pay dividends in Swiss francs, exchange rate fluctuations will affect the US dollar amounts received by holders of ADRs. For the amount of dividends we paid in the past three years, see “Item 18. Financial Statements—Note 19—Equity.”
Disclosure pursuant to Section 219 of the Iran Threat Reduction and Syria Human Rights Act (ITRA)
At Novartis, our purpose is to reimagine medicine to improve and extend people’s lives, regardless of where they live. This includes the compliant sale of medicines and other healthcare products worldwide. To help us fulfill this mission, we have for many years maintained two representative offices located in Iran.
As of October 18, 2010, a non‑US Novartis affiliate entered into a non‑binding Memorandum of Understanding (MoU) with the Ministry of Health and Medical Education of the Islamic Republic of Iran. Pursuant to the MoU, the Iranian Ministry of Health acknowledges certain benefits that may apply to sales of certain of our medicines by third‑party distributors in Iran. These include fast‑track registration, market exclusivity, end‑user subsidies, and exemptions from customs tariffs. Novartis receives no payments from the Iranian Ministry of Health under the MoU, and the MoU creates no obligations on the part of either Novartis or the Iranian Ministry of Health.
From time to time, including in 2023, certain Novartis non‑US affiliates made payments to government entities in Iran related to patents, trademarks, exit fees and other transactions ordinarily incident to travel by doctors and other medical professionals resident in Iran to attend conferences or other events outside Iran.
From time to time, including in 2023, certain Novartis non‑US affiliates enter into agreements with hospitals, research institutes, medical associations and universities in Iran to provide grants and sponsor congresses, seminars and symposia, and with doctors and other healthcare professionals for consulting services, including participation in advisory boards and investigator services for observational (non‑interventional) studies. Some hospitals and research institutes are owned or controlled by the government of Iran, and some doctors and healthcare professionals are employed by hospitals that may be public or government‑owned.
Because we have operations in Iran, including employees, we obtain services and have other dealings incidental to our activities in that country, including paying taxes and salaries either directly or indirectly through a service provider, and obtaining office rentals, insurance, electricity, water and telecommunications services, office and similar supplies, and customs‑related services from Iranian companies that may be owned or controlled by the government of Iran. In addition, from time to time, representatives of our non-US affiliates participate in meetings with Iranian officials to discuss issues relevant to our business and the pharmaceutical industry.
Certain Novartis non-US affiliates maintain local accounts at banks that are, as of November 5, 2018, on the Specially Designated Nationals and Blocked Persons List (SDN List). These non-US affiliates make local transactions for employee payroll and local vendor payment purposes. These transactions are conducted for the purpose of facilitating the provision of medicine to Iran, in line with the humanitarian exceptions contained in Section 11 of Executive Order 13902 and other applicable sanctions legal authorities. No transactions are made with an Iranian financial institution designated on the SDN List in connection with Iran’s support for international terrorism or proliferation of weapons of mass destruction.
8.B Significant changes
None.
Item 9. The Offer and Listing
9.A Offer and listing details
Our ordinary shares are listed in Switzerland on the SIX Swiss Exchange under the symbol “NOVN.” Our ADSs, each representing one ordinary share, are traded on the New York Stock Exchange under the symbol “NVS.”
9.B Plan of distribution
Not applicable.
9.C Markets
See “—Item 9.A Offer and listing details.”
9.D Selling shareholders
Not applicable.
9.E Dilution
Not applicable.
9.F Expenses of the issue
Not applicable.
Item 10. Additional Information
10.A Share capital
Not applicable.
10.B Memorandum and articles of association
The following is a non-exhaustive summary of certain provisions of our Articles of Incorporation (“Articles”); our Regulations of the Board, the Board Committees and the Executive Committee (“Board Regulations”); and Swiss law, particularly the Swiss Code of Obligations (“Swiss CO”), and is qualified in its entirety by reference to the Articles and the Board Regulations, which are an exhibit to this Form 20‑F, and to Swiss law.
10.B.1 Company purpose
Novartis AG is registered in the commercial register of the canton of Basel‑Stadt, Switzerland, under number CHE‑103.867.266. Our business purpose, as stated in Article 2 of the Articles, is to hold interests in enterprises in the area of healthcare or nutrition. We may also hold interests in enterprises in the areas of biology, chemistry, physics, information technology or related areas. We may acquire, mortgage, liquidate or sell real estate and intellectual property rights in Switzerland or abroad. In pursuing our business purpose, we strive to create sustainable value.
10.B.2 Directors
According to our Articles, the Board of Directors (“Board”) consists of a minimum of eight and a maximum of 16 members. The members of the Board (including the Board Chair) are elected individually by the General Meeting of Shareholders (“General Meeting”) for a one-year term of office lasting until the completion of the next Annual General Meeting of Shareholders (“AGM”).
(a) A Board resolution requires the affirmative majority of the votes cast. According to our Board Regulations, a member of our Board (“Director”) may not participate in decisions and resolutions on matters that affect, or reasonably might affect, the Director’s interests or the interests of a person close to the Director (but the Director may participate in the discussion).
(b) Compensation of the Directors is subject to the approval of the aggregate amounts of such compensation by a shareholders’ resolution under the Ordinance against Excessive Compensation in Public Companies of the Swiss Federal Council.
(c) The Articles prohibit the granting of loans or credits to Directors.
(d) The Articles provide that a Director shall not serve on the Board for more than 12 years. The Board may, under certain circumstances and if deemed in the best interests of the Company, recommend exceptions to this rule to the General Meeting.
(e) Our Directors are not required to be shareholders at the time of the election by the General Meeting. However, according to our share ownership guidelines, to ensure their interests are aligned with those of our shareholders, the Board Chair is required to own a minimum of 30 000 Novartis AG shares, and other Directors are required to own at least 5 000 Novartis AG shares within five years of having joined the Board.
10.B.3 Shareholder rights
Because Novartis AG has only one class of registered shares, the following information applies to all shareholders.
(a) Under the Swiss CO, we may only pay dividends out of balance sheet profits or out of distributable reserves. In any event, under the Swiss CO, while the Board may propose that a dividend be paid, we may only pay dividends upon shareholders’ approval at a General Meeting. Furthermore, the Swiss CO requires us to accrue general legal reserves under certain circumstances so long as these reserves amount to less than 20% of our registered share capital, and Swiss law and the Articles permit us to accrue additional reserves beyond the statutory reserves. Our auditors must confirm that the dividend proposal of our Board conforms with the Swiss CO and the Articles. Our Board expects to recommend the payment of a dividend in respect of each financial year. See “Item 6. Directors, Senior Management and Employees—Item 6.C Board Practices—Capital Structure—Limitation on transferability—Per-share information” and “Item 8. Financial Information—Item 8.A. Consolidated statements and other financial information—Dividend policy.”
Dividends are usually due and payable shortly after the shareholders have passed a resolution approving the payment. Dividends that have not been claimed within five years after the due date revert to us and are allocated to our general reserves. For information about deduction of the withholding tax or other duties from dividend payments, see “—Item 10.E Taxation.”
(b) Each share is entitled to one vote at a General Meeting. Voting rights may only be exercised for shares registered with the right to vote on the record date for the applicable General Meeting. To do so, the shareholder must file a share registration form with us, setting forth the shareholder’s name, address and citizenship (or, in the case of a legal entity, its registered office). If the shareholder has not timely registered its shares, then the shareholder may not vote at, or participate in, a General Meeting.
To vote its shares, the shareholder must also explicitly declare that it has acquired the shares in its own name and for its own account. If the shareholder refuses to make such a declaration, the shares may not be voted unless the Board recognizes such shareholder as a nominee.
The Articles provide that no shareholder shall be registered with the right to vote shares comprising more than 2% of the registered share capital. The Board may, upon request, grant an exemption from this restriction. Considerations include whether the shareholder supports our goal of creating sustainable value and has a long‑term investment horizon. Furthermore, the Articles provide that no nominee shall be registered with the right to vote shares comprising more than 0.5% of the registered share capital. The Board may, upon request, grant an exemption from this restriction if the nominee discloses the names, addresses, and number of shares of the persons for whose account it holds 0.5% or more of the registered share capital. The same restrictions indirectly apply to ADR holders. We have in the past granted exemptions from the 2% rule for shareholders and the 0.5% rule for nominees.
For purposes of the 2% rule for shareholders and the 0.5% rule for nominees, groups of companies and groups of shareholders acting in concert are considered to be one shareholder. These rules also apply to shares acquired or subscribed by the exercise of subscription, option or conversion rights.
After hearing the registered shareholder or nominee, the Board may cancel, with retroactive effect as of the date of registration, the registration of the shareholders if the registration was effected based on false information.
Registration restrictions in the Articles may only be removed upon a resolution carrying a two‑thirds majority of the votes represented at a General Meeting.
Except as noted below, shareholders’ resolutions require the approval of an absolute majority of the votes present at a General Meeting. As a result, abstentions have the effect of votes against such resolutions. Some examples of shareholders’ resolutions requiring a vote by such “absolute majority of the votes” are:
• Adoption and amendment of the Articles
• Election and removal of the Board Chair, the Board and Compensation Committee members, the Independent Proxy and the external auditor
• Approval of the management report, the consolidated financial statements and the report on non-financial matters
• Approval of the financial statements of Novartis AG, and the decision on the appropriation of available earnings shown on the balance sheet, in particular with regard to dividends (including any repayment of the statutory capital reserves and the approval of interim dividends and the interim financial statements required for such purpose), if any
• Approval of the maximum aggregate compensation of the Board (from an AGM until the next AGM) and of the Executive Committee (for the financial year following the AGM)
• Discharge of Board and Executive Committee members from liability for matters disclosed to the General Meeting
• Decision on other matters that are reserved by law or by the Articles (e.g., advisory vote on the Compensation Report) to the General Meeting
According to the Articles and Swiss law, the following matters require the approval of a “supermajority” of at least two‑thirds of the votes present at a General Meeting:
• Alteration of the purpose of Novartis AG
• The consolidation of shares, unless the approval of all affected shareholders is required
• Increase of the share capital out of equity, by contributions in kind or by way of set-off against receivable, or the grant of special rights
• Restriction or cancellation of subscription rights
• Introduction of a conditional capital or capital band
• Creation of shares with increased voting powers
• Implementation of restrictions on the transfer of registered shares, and the removal of such restrictions
• Change of the currency of the share capital
• Introduction of the deciding vote for the presiding officer at the General Meeting
• A provision in the Articles allowing the General Meeting to be held abroad
• Delisting of the shares of the Company
• Change of the registered office of Novartis AG
• Introduction of an arbitration clause in the Articles
• Merger, split or transformation of Novartis AG under the Swiss Merger Act (subject to mandatory statutory provisions)
• Dissolution of Novartis AG
Our shareholders are required, on an annual basis, to elect all Directors (including the Board Chair), the Compensation Committee members, the external auditor and the Independent Proxy. The Articles do not provide for cumulative voting of shares.
At a General Meeting, shareholders can be represented by a legal representative or, by means of a written proxy, by a representative of choice. Furthermore, a shareholder may be represented by the Independent Proxy. Votes are taken either by a show of hands or by electronic voting, unless the General Meeting resolves to have a ballot or where a ballot is ordered by the chair of the meeting. ADSs, each representing one Novartis AG share and evidenced by ADRs, are issued by our depositary JPMorgan Chase Bank, N.A., New York, and not by us. The ADR is vested with rights defined and enumerated in the Deposit Agreement (such as the rights to vote, to receive a dividend and to receive a share of Novartis AG in exchange for a certain number of ADRs). The enumeration of rights, including any limitations on those rights in the Deposit Agreement, is final. There are no other rights given to the ADR holders. Only the ADS depositary, holding our shares underlying the ADRs, is registered as shareholder in our share register. An ADR is not a Novartis AG share, and an ADR holder is not a Novartis AG shareholder.
The Deposit Agreement between our depositary, the ADR holder and us has granted certain indirect rights to vote to the ADR holders. ADR holders may not attend a General Meeting in person. ADR holders exercise their voting rights by instructing JPMorgan Chase Bank, N.A., our depositary, to exercise the voting rights attached to the registered shares underlying the ADRs. Each ADR represents one Novartis AG share. JPMorgan Chase Bank, N.A., exercises the voting rights for registered shares underlying ADRs for which no voting instructions have been given by providing a discretionary proxy to an uninstructed independent designee. Such designee has to be a shareholder of Novartis AG. The same voting restrictions apply to ADR holders as to those holding Novartis AG shares (i.e., the right to vote up to 2% of the Novartis AG registered share capital – unless otherwise granted an exemption by the Board – and the disclosure requirement for nominees).
(c) Shareholders have the right to allocate the profit shown on our balance sheet and to distribute dividends by vote taken at the General Meeting, subject to the legal requirements described above.
(d) Under the Swiss CO, any surplus arising out of a liquidation of Novartis AG (i.e., after the settlement of all claims of all creditors) would be distributed to the shareholders in proportion to the paid‑in nominal value of their shares.
(e) The Swiss CO limits a corporation’s ability to hold or repurchase its own shares. We and our subsidiaries may only repurchase shares if we have sufficient freely disposable equity in the amount of the purchase price of the acquired shares. The aggregate nominal value of all Novartis AG shares held by us and our subsidiaries may not exceed 10% of our registered share capital. However, it is accepted that a Swiss corporation may repurchase its own shares beyond the statutory limit of 10% if the repurchased shares are clearly earmarked for cancellation. In addition, we are required to recognize a negative position, or if our subsidiaries acquire our shares, to create a special reserve on our balance sheet in the amount of the purchase price of the acquired shares. Repurchased shares held by us or our subsidiaries do not carry any rights to vote at a General Meeting but are entitled to the economic benefits generally connected with the shares.
Under the Swiss CO, we may not cancel treasury shares without the approval of a capital reduction by our shareholders given that shareholders have not approved the introduction of a capital band.
(f) Not applicable.
(g) Since all of our issued and outstanding shares have been fully paid in, our shareholders are not obliged to make further contributions with respect to their shares.
(h) See “—Item 10.B.3(b) Shareholder rights” and “—Item 10.B.7 Change in control.”
10.B.4 Changes to shareholder rights
Under the Swiss CO, we may not issue new shares without the prior approval of a capital increase by our shareholders. If a capital increase is approved, then our shareholders would generally have certain pre-emptive rights to obtain newly issued shares in an amount proportional to the nominal value of the shares they already hold. These pre-emptive rights could be excluded in certain limited circumstances with the approval of a resolution adopted at a General Meeting by a supermajority of two‑thirds of the votes. In addition, we may not create shares with increased voting powers or place restrictions on the transfer of registered shares without the approval of a resolution adopted at a General Meeting by a supermajority of votes. In addition, see “—Item 10.B.3(b) Shareholder rights” with regard to the Board’s ability to cancel the registration of shares under limited circumstances.
10.B.5 Shareholder meetings
Under the Swiss CO and the Articles, we must hold an AGM within six months after the end of our financial year. A General Meeting may be convened by the Board or, if necessary, by the external auditor. The Board is further required to convene an extraordinary General Meeting if so resolved by a General Meeting, or if so requested by shareholders by signed petition representing at least 5% of the share capital, specifying the items for the agenda and their proposals. Shareholders representing shares with an aggregate nominal value of at least CHF 1 000 000 may request that an item be included in a General Meeting agenda. A General Meeting is convened by publishing a notice in the Swiss Official Gazette
of Commerce (Schweizerisches Handelsamtsblatt) at least 20 days prior to such meeting. Shareholders may also be informed by mail. Neither the Swiss CO nor the Articles require a quorum for a General Meeting. In addition, see “—Item 10.B.3(b) Shareholder rights” regarding conditions for exercising a shareholder’s right to vote at a General Meeting.
10.B.6 Limitations
There are no limitations under the Swiss CO or our Articles on the right of non‑Swiss residents or nationals to own or vote shares other than the restrictions applicable to all shareholders and holders of ADRs described in “—Item 10.B.3(b) Shareholder rights.”
10.B.7 Change in control
The Articles and the Board Regulations contain no provision that would have an effect of delaying, deferring or preventing a change in control of Novartis AG and that would operate only with respect to a merger, acquisition or corporate restructuring involving us or any of our subsidiaries.
According to the Swiss Merger Act, shareholders may pass a resolution to merge with another corporation at any time. Such a resolution would require the consent of at least two‑thirds of all votes present at the necessary General Meeting.
Under the Swiss Financial Market Infrastructure Act, shareholders and groups of shareholders acting in concert who acquire more than 33 1/3% of our shares would be under an obligation to make an offer to acquire all remaining Novartis AG shares. Novartis AG has neither opted out from the mandatory takeover offer obligation nor opted to increase the threshold for mandatory takeover offers in its Articles.
10.B.8 Disclosure of shareholdings
Under the Swiss Financial Market Infrastructure Act, persons who directly, indirectly or in concert with other parties acquire or dispose of our shares or purchase or sale rights relating to our shares are required to notify us and the SIX of the level of their holdings whenever such holdings reach, exceed or fall below certain thresholds – 3%, 5%, 10%, 15%, 20%, 25%, 33 1/3%, 50% and 66 2/3% – of the voting rights represented by our share capital (whether exercisable or not). This also applies to anyone who has discretionary power to exercise voting rights associated with our shares. Following receipt of such notification, we are required to inform the public by publishing the information via the electronic publication platform operated by the SIX.
An additional disclosure obligation exists under the Swiss CO that requires us to disclose once a year in the notes to the financial statements published in our Annual Report, the identity of all of our shareholders (or related groups of shareholders) who have been granted exemption entitling them to vote more than 2% of our registered share capital, as described in “—Item 10.B.3(b) Shareholder rights.”
10.B.9 Differences in the law
See the references to Swiss law throughout this “—Item 10.B Memorandum and articles of association.”
10.B.10 Changes in capital
The requirements of the Articles regarding changes in capital are not more stringent than the requirements of Swiss law.
10.C Material contracts
Sandoz Spin-Off
In connection with the spin-off of Sandoz, we entered into a Separation and Distribution Agreement, a Tax Matters Agreement and several other agreements with Sandoz to effect the separation of the Sandoz business and provide a framework for our relationship with Sandoz after the spin-off.
The Separation and Distribution Agreement sets forth the parties’ agreements regarding the principal actions to be taken in connection with the separation of the Sandoz business and the spin-off, by way of a distribution of shares of Sandoz Group AG by Novartis AG to Novartis shareholders, including the conditions of the spin-off and the rights and obligations of the parties with respect to the separation and distribution. The Separation and Distribution Agreement identifies the assets to be transferred, liabilities to be assumed and contracts to be assigned to each of Novartis and Sandoz as part of the internal transactions effected prior to the distribution and provides for when and how such transfers, assumptions and assignments should occur. Each party agreed to indemnify the other and each of the other’s directors, officers, managers, members, agents and employees against certain liabilities incurred in connection with the spin-off and the parties’ respective businesses (subject to certain exceptions).
The Tax Matters Agreement imposes certain restrictions and indemnity obligations on Sandoz designed to preserve the tax-neutral nature of the spin-off for Swiss tax and US federal income tax purposes.
The Tax Matters Agreement also provides that Sandoz will be liable for any taxes accruing in the ordinary course of business of Novartis and its subsidiaries before the spin-off if such taxes are attributable to entities which are transferred or allocated to the Sandoz Group as part of the spin-off, whereas Novartis will remain liable for any other taxes accruing before the
spin-off in the ordinary course of business, to the extent not attributed to Sandoz.
In connection with the spin-off, we also entered into an employee matters agreement, a claims management agreement, manufacturing and supply agreements, a development and collaboration agreement, a transitional services agreement, an authorized generics agreement, and certain intellectual property agreements, each of which is not material to Novartis.
10.D Exchange controls
There are no Swiss governmental laws, decrees or regulations that affect – in a manner material to Novartis AG – the export or import of capital, including the availability of cash and cash equivalents for use by Novartis or any foreign exchange controls that affect the remittance of dividends, interest or other payments to non‑residents or non‑citizens of Switzerland who hold Novartis AG securities.
10.E Taxation
The taxation discussion set forth below is intended only as a descriptive summary and does not purport to be a complete analysis or listing of all potential tax effects relevant to the ownership or disposition of our shares or ADRs. The statements of US and Swiss tax laws set forth below are based on the laws and regulations in force as of the date of this 20‑F—including the current Convention Between the US and the Swiss Confederation for the Avoidance of Double Taxation with Respect to Taxes on Income, entered into force on December 19, 1997 (the Treaty); the US Internal Revenue Code of 1986, as amended (the Code); Treasury regulations; rulings; judicial decisions; and administrative pronouncements—and may be subject to any changes in US and Swiss law, and in any double taxation convention or treaty between the US and Switzerland occurring after that date, which changes may have retroactive effect.
Swiss taxation
Swiss residents
Withholding Tax on dividends and distributions. Dividends that we pay and similar cash or in‑kind distributions that we may make to a holder of shares or ADRs (including distributions of liquidation proceeds in excess of the nominal value, stock dividends and, under certain circumstances, proceeds from repurchases of shares by us in excess of the nominal value) are generally subject to a Swiss federal withholding tax (the Withholding Tax) at a current rate of 35%. Under certain circumstances, distributions out of capital contribution reserves made by shareholders after December 31, 1996, are exempt from the Withholding Tax. We are required to withhold Withholding Tax due from the gross distribution and to pay the Withholding Tax to the Swiss Federal Tax Administration. The Withholding Tax is refundable in full to Swiss tax residents who are the beneficial owners of the taxable distribution at the time it is resolved and duly report the gross distribution received on their personal tax return or in their financial statements for tax purposes, as the case may be.
Income tax on dividends. A Swiss tax resident who receives dividends and similar distributions (including stock dividends and liquidation surplus) on shares or ADRs is required to include such amounts in the shareholder’s personal income tax return. However, distributions out of qualified capital contribution reserves are not subject to income tax. A corporate shareholder may claim substantial relief from taxation of dividends and similar distributions received if the shares held represent a fair market value of at least CHF 1 million.
Capital gains tax upon disposal of shares. Under current Swiss tax law, the gain realized on shares held by a Swiss resident who holds shares or ADRs as part of his private property is generally not subject to any federal, cantonal or municipal income taxation on gains realized on the sale or other disposal of shares or ADRs. However, gains realized upon a repurchase of shares by us may be characterized as taxable dividend income if certain conditions are met. Book gains realized on shares or ADRs held by a Swiss corporate entity or by a Swiss resident individual as part of the shareholder’s business property are, in general, included in the taxable income of such person. However, the Federal Law on the Direct Federal Tax of December 14, 1990, and several cantonal laws on direct cantonal taxes provide for exceptions for Swiss corporate entities holding more than 10% of our voting stock for more than one year.
Residents of other countries
Recipients of dividends and similar distributions on our shares who are neither residents of Switzerland for tax purposes nor hold shares as part of a business conducted through a permanent establishment situated in Switzerland (Non‑Resident Holders) are not subject to Swiss income taxes in respect of such distributions. Moreover, gains realized by such recipients upon the disposal of shares are not subject to Swiss income taxes.
Non‑Resident Holders of shares are, however, subject to the Withholding Tax on dividends and similar distributions mentioned above and, under certain circumstances, to the Stamp Duty described below. Such Non‑Resident Holders may be entitled to a partial refund
of the Withholding Tax if the country in which they reside has entered into a bilateral treaty for the avoidance of double taxation with Switzerland. Non‑Resident Holders should be aware that the procedures for claiming treaty refunds (and the time frame required for obtaining a refund) may differ from country to country. Non‑Resident Holders should consult their own tax advisors regarding receipt, ownership, purchase, sale or other dispositions of shares or ADRs, and the procedures for claiming a refund of the Withholding Tax.
As of January 1, 2024, Switzerland has entered into bilateral treaties for the avoidance of double taxation with respect to income taxes with the following countries, whereby a part of the above‑mentioned Withholding Tax may be refunded (subject to the limitations set forth in such treaties):
Albania
Algeria
Argentina
Armenia
Australia
Austria
Azerbaijan
Bahrain
Bangladesh
Belarus
Belgium
Brazil
Bulgaria
Canada
Chile
China
Colombia
Croatia
Cyprus
Czechia
Denmark
Ecuador
Egypt
Estonia
Ethiopia
Finland
France
Georgia
Germany
Ghana
Greece
Hong Kong
Hungary
Iceland
India
Indonesia
Iran
Ireland
Israel
Italy
Ivory Coast
Jamaica
Japan
Kazakhstan
Republic of Korea
(South Korea)
Kosovo
Kuwait
Kyrgyzstan
Latvia
Liechtenstein
Lithuania
Luxembourg
Malaysia
Malta
Mexico
Moldova
Mongolia
Montenegro
Morocco
Netherlands
New Zealand
North Macedonia
Norway
Oman
Pakistan
Peru
Philippines
Poland
Portugal
Qatar
Romania
Russia
Saudi Arabia
Serbia
Singapore
Slovak Republic
Slovenia
South Africa
Spain
Sri Lanka
Sweden
Taiwan
Tajikistan
Thailand
Trinidad and Tobago
Tunisia
Türkiye
Turkmenistan
Ukraine
United Arab Emirates
United Kingdom
United States of America
Uruguay
Uzbekistan
Venezuela
Vietnam
Zambia
Tax treaty negotiations are underway, or have been conducted, with Angola, Bosnia and Herzegovina, Cameroon, Costa Rica, Jordan, Kenya, Libya, Nigeria, Rwanda, Senegal, Syria and Zimbabwe. Tax treaty negotiations between Switzerland and some of the countries listed in the immediately preceding sentence have been ongoing for an extended period of time, and we are not certain when or if such negotiations will be completed, and when or if the corresponding treaties will come into effect.
A Non‑Resident Holder of shares or ADRs will not be liable for any Swiss taxes other than the Withholding Tax described above and, if the transfer occurs through or with a Swiss bank or other Swiss securities dealer, the Stamp Duty described below. If, however, the shares or ADRs of Non‑Resident Holders can be attributed to a permanent establishment or a fixed place of business maintained by such person within Switzerland during the relevant tax year, the shares or ADRs may be subject to Swiss income taxes in respect of income and gains realized on the shares or ADRs, and such person may qualify for a full refund of the Withholding Tax based on Swiss tax law.
Residents of the US. A Non‑Resident Holder who is a resident of the US for purposes of the Treaty is eligible for a reduced rate of tax on dividends equal to 15% of the dividend, provided that such holder (i) qualifies for benefits under the Treaty; (ii) is not a company (or, if it is a company, such company directly holds less than 10% of our voting stock); and (iii) does not conduct business through a permanent establishment or fixed base in Switzerland to which the shares or ADRs are attributable. Such an eligible holder must apply for a refund of the amount of the Withholding Tax in excess of the 15% Treaty rate. A Non‑Resident Holder who is a resident of the US for purposes of the Treaty is eligible for a reduced rate of tax on dividends equal to 5% of the dividend, provided that such holder (i) is a company; (ii) qualifies for benefits under the Treaty; (iii) holds directly at least 10% of our voting stock, and (iv) does not conduct business through a permanent establishment or fixed place of business in Switzerland to which the shares or ADRs are attributable. Such an eligible holder must apply for a refund of the amount of the Withholding Tax in excess of the 5% Treaty rate. Claims for refunds must be filed on Swiss Tax Form 82 (82C for corporations; 82I for individuals; 82E for other entities), which may be obtained from any Swiss Consulate General in the US or from the Federal Tax Administration of Switzerland at the address below, together with an instruction form. Four copies of
the form must be duly completed, signed before a notary public of the US, and sent to the Federal Tax Administration of Switzerland, Eigerstrasse 65, CH‑3003 Bern, Switzerland. The form must be accompanied by suitable evidence of deduction of Swiss tax withheld at source, such as certificates of deduction, signed bank vouchers or credit slips. The form may be filed on or after July 1 or January 1 following the date the dividend was payable, but no later than December 31 of the third year following the calendar year in which the dividend became payable. For US resident holders of ADRs, JPMorgan Chase Bank, N.A., as depositary, will comply with these Swiss procedures on behalf of the holders, and will remit the net amount to the holders.
Stamp Duty upon transfer of securities. The sale of shares, whether by Swiss residents or Non‑Resident Holders, may be subject to federal securities transfer Stamp Duty of 0.15%, calculated on the sale proceeds, if the sale occurs through or with a Swiss bank or other Swiss securities dealer, as defined in the Swiss Federal Stamp Duty Act. The Stamp Duty has to be paid by the securities dealer and may be charged to the parties in a taxable transaction who are not securities dealers. Stamp Duty may also be due if a sale of shares occurs with or through a non‑Swiss bank or securities dealer, provided that (i) such bank or dealer is a member of the SIX, and (ii) the sale takes place on the SIX. In addition to this Stamp Duty, the sale of shares by or through a member of the SIX may be subject to a minor stock exchange levy.
US federal income taxation
The following is a general discussion of the material US federal income tax consequences of the ownership and disposition of our shares or ADRs that may be relevant to you if you are a US Holder (as defined below). Because this discussion does not consider any specific circumstances of any particular holder of our shares or ADRs, persons who are subject to US taxation are strongly urged to consult their own tax advisors as to the overall US federal, state and local tax consequences, as well as to the overall Swiss and other foreign tax consequences, of the ownership and disposition of our shares or ADRs. In particular, additional or different rules may apply to US expatriates; banks and other financial institutions; regulated investment companies; traders in securities who elect to apply a mark‑to‑market method of accounting; dealers in securities or currencies; tax‑exempt entities; insurance companies; broker‑dealers; investors liable for alternative minimum tax; investors that hold shares or ADRs as part of a straddle, hedging or conversion transaction; holders whose functional currency is not the US dollar; partnerships or other pass-through entities; persons who acquired our shares pursuant to the exercise of employee stock options or otherwise as compensation; and persons who hold, directly, indirectly or by attribution, 10% or more of our outstanding shares. This discussion generally applies only to US Holders who hold the shares or ADRs as a capital asset (generally, for investment purposes), and whose functional currency is the US dollar. Investors are urged to consult their own tax advisors concerning whether they are eligible for benefits under the Treaty.
For purposes of this discussion, a US Holder is a beneficial owner of our shares or ADRs who is (i) an individual who is a citizen or resident of the US for US federal income tax purposes; (ii) a corporation (or other entity taxable as a corporation for US federal income tax purposes) created or organized in or under the laws of the US or a state thereof or the District of Columbia; (iii) an estate the income of which is subject to US federal income taxation regardless of its source; or (iv) a trust (i) subject to the primary supervision of a US court and the control of one or more US persons; or (ii) that has a valid election in place to be treated as a US person. If a partnership (or other entity treated as a partnership for US federal income tax purposes) holds shares or ADRs, the tax treatment of a partner generally will depend upon the status of the partner and the activities of the partnership. Partners in a partnership that holds shares or ADRs are urged to consult their own tax advisor regarding the specific tax consequences of the owning and disposing of such shares or ADRs by the partnership.
For US federal income tax purposes, a US Holder of ADRs generally will be treated as the beneficial owner of our shares represented by the ADRs. However, see the discussion below under “—Dividends” regarding certain statements made by the US Treasury concerning depositary arrangements.
This discussion assumes that each obligation in the Deposit Agreement and any related agreement will be performed in accordance with its terms.
Dividends. US Holders will be required to include in gross income, as an item of ordinary income, the full amount (without reduction for any Withholding Tax) of the dividend paid with respect to our shares or ADRs at the time that such dividend is received by the US Holder, in the case of shares, or by the depositary, in the case of ADRs. For this purpose, a “dividend” will include any distribution paid by us with respect to our shares or ADRs (other than certain pro rata distributions of our capital stock) paid out of our current or accumulated earnings and profits, as determined under US federal income tax principles. To the extent the amount of a distribution by us exceeds our current and accumulated earnings and profits, such excess will first be treated as a tax‑free return of capital to the extent of a US Holder’s tax basis in the shares or ADRs (with a corresponding reduction in such tax basis), and thereafter will be treated as capital gain, which will be long‑term capital gain if the US Holder held our shares or ADRs for more than one year. Under the Code, dividend payments by us on the shares or ADRs are not eligible for the dividends received deduction generally allowed to corporate shareholders.
Dividend income in respect of our shares or ADRs will constitute income from sources outside the US for US foreign tax credit purposes. Subject to the limitations and conditions provided in the Code, US Holders generally may claim as a credit against their US federal income tax liability, any Withholding Tax withheld from a dividend. The rules governing the foreign tax credit are complex. Each US Holder is urged to consult its own tax advisor concerning whether, and to what extent, a foreign tax credit will be available with respect to dividends received
from us. Alternatively, a US Holder may claim the Withholding Tax as a deduction for the taxable year within which the Withholding Tax is paid or accrued, provided a deduction is claimed for all of the foreign income taxes the US Holder pays or accrues in the particular year. A deduction does not reduce US tax on a dollar‑for‑dollar basis like a tax credit. The deduction, however, is not subject to the limitations applicable to foreign tax credits, but may be subject to other limitations, and each US Holder is urged to consult its own tax advisor.
The US Treasury has expressed concern that parties to whom ADRs are released may be taking actions inconsistent with the claiming of foreign tax credits for US Holders of ADRs. Accordingly, the summary above of the creditability of the Withholding Tax could be affected by future actions that may be taken by the US Treasury.
In general, a US Holder will be required to determine the amount of any dividend paid in Swiss francs, including the amount of any Withholding Tax imposed thereon, by translating the Swiss francs into US dollars at the spot rate on the date the dividend is actually or constructively received by a US Holder, in the case of shares, or by the depositary, in the case of ADRs, regardless of whether the Swiss francs are in fact converted into US dollars. If a US Holder converts the Swiss francs so received into US dollars on the date of receipt, the US Holder generally should not recognize foreign currency gain or loss on such conversion. If a US Holder does not convert the Swiss francs so received into US dollars on the date of receipt, the US Holder will have a tax basis in the Swiss francs equal to the US dollar value on such date. Any foreign currency gain or loss that a US Holder recognizes on a subsequent conversion or other disposition of the Swiss francs generally will be treated as US source ordinary income or loss.
For a non‑corporate US Holder, the US dollar amount of any dividends paid that constitute qualified dividend income generally will be taxable at a maximum rate of 15% (or 20% in the case of taxpayers with annual income that exceeds certain thresholds), provided that the US Holder meets certain holding period and other requirements. In addition, the dividends could be subject to a 3.8% net investment income tax. This tax is applied against the lesser of the US Holder’s net investment income or the amount by which modified adjusted gross income exceeds a statutory threshold amount based on filing status. We currently believe that dividends paid with respect to our shares and ADRs will constitute qualified dividend income for US federal income tax purposes, provided that the US Holder meets certain holding period and other requirements. US Holders of shares or ADRs are urged to consult their own tax advisors regarding the availability to them of the reduced dividend rate in light of their own particular situation and the computations of their foreign tax credit limitation with respect to any qualified dividends paid to them, as applicable.
Sale or other taxable disposition. Upon a sale or other taxable disposition of shares or ADRs, US Holders generally will recognize capital gain or loss in an amount equal to the difference between the US dollar value of the amount realized on the disposition and the US Holder’s tax basis (determined in US dollars) in the shares or ADRs. This capital gain or loss generally will be US source gain or loss and will be treated as long‑term capital gain or loss if the holding period in the shares or ADRs exceeds one year. In the case of a non‑corporate US Holder, any long-term capital gain generally will be subject to US federal income tax at preferential rates, with a maximum rate of 15% (or 20% in the case of taxpayers with annual income that exceeds certain thresholds). In addition, the gains could be subject to a 3.8% investment income tax. This tax is applied against the lesser of the US Holder’s net investment income or the amount by which modified adjusted gross income exceeds a statutory threshold amount based on filing status. The deductibility of capital losses is subject to significant limitations under the Code. Deposits or withdrawals of our shares by US Holders in exchanges for ADRs will not result in the realization of gain or loss for US federal income tax purposes.
US information reporting and backup withholding. Dividend payments with respect to shares or ADRs and proceeds from the sale, exchange or other disposition of shares or ADRs received in the United States or through US‑related financial intermediaries may be subject to information reporting to the US Internal Revenue Service (IRS) and possible US backup withholding. Certain exempt recipients (such as corporations) are not subject to these information reporting and backup withholding requirements. Backup withholding will not apply to a US Holder who furnishes a correct taxpayer identification number and makes any other required certification or who is otherwise exempt from backup withholding. Any US Holders required to establish their exempt status generally must provide a properly executed IRS Form W‑9 (Request for Taxpayer Identification Number and Certification). Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against a US Holder’s US federal income tax liability, and a US Holder may obtain a refund of any excess amounts withheld under the backup withholding rules by timely filing the appropriate claim for refund with the IRS and furnishing any required information.
Tax consequences of the Sandoz spin-off
To implement the Sandoz spin-off, we distributed all of the Sandoz Group AG shares held by Novartis to Novartis AG shareholders, pro rata to their respective holdings. Each Novartis AG shareholder received one Sandoz Group AG share for every five Novartis AG shares or five Novartis AG ADRs they held or had acquired prior to the close of business on October 3, 2023.
The following statements are based on the requirement of the continuing effectiveness and validity of the written confirmations (the Swiss Tax Rulings) from the Swiss Federal Tax Administration and from the tax administration of the Canton of Basel-Stadt, a private letter ruling from the IRS (the IRS Ruling) and a written opinion of Cravath, Swaine & Moore LLP, counsel to Novartis (the Tax Opinion), each to the effect that the Sandoz spin-off qualifies as a tax-neutral transaction.
Material tax consequences to Novartis
The following is a summary of the material tax consequences to Novartis in connection with the Sandoz spin-off that may be relevant to holders of Novartis AG shares.
The Sandoz spin-off was preceded by several internal restructuring steps to separate the Sandoz business from Novartis. Novartis has received the Swiss Tax Rulings, the IRS Ruling and the Tax Opinion, providing that the spin-off should qualify for nonrecognition of gain or loss for US federal income tax purposes or preserve the tax-neutral nature for Swiss tax purposes, as applicable. In addition, the Swiss Tax Rulings provide that no Swiss withholding tax or stamp duty should apply to the distribution of Sandoz Group AG shares in the spin-off. The Tax Opinion and IRS Ruling are subject to the qualifications and limitations set forth below under “—Consequences to US Holders of Novartis AG Shares.” Additionally, Novartis has entered into a tax matters agreement with Sandoz, which restricts Sandoz from taking certain actions that could affect the qualification of the spin-off as tax-neutral.
Consequences to Swiss Holders of Novartis AG shares
General
Subject to the qualifications and limitations set forth herein (including the discussion below relating to the receipt of cash in lieu of fractional shares), for Swiss tax purposes no gain or loss should be recognized by, or be includible in the income of, a Swiss Holder as a result of the tax-neutral spin-off, provided that Swiss Holders who hold Novartis AG shares as business assets accurately maintain the tax and book values of their Novartis AG and Sandoz Group AG shares. This means that for Swiss Holders who hold Novartis AG shares as business assets, the aggregate tax basis of the Novartis AG shares and Sandoz Group AG shares immediately after the distribution should be the same as the aggregate tax basis of the Novartis AG shares held immediately before the distribution, allocated between the Novartis AG shares and Sandoz Group AG shares.
If a Swiss Holder that holds Novartis AG shares as business assets is classified as a “professional securities dealer” or is a legal entity and receives cash in lieu of a fractional share, such Swiss Holder will generally recognize a capital gain or loss measured by the difference between the cash received for such fractional Share and the Swiss Holder’s tax basis in that fractional Share. The same Swiss income tax treatment applies to Swiss Holders of Novartis physical share certificates (Heimverwahrer) held as business assets who receive cash due to non-response by September 19, 2023.
If a Swiss Holder who holds Novartis AG shares as private assets receives cash in lieu of fractional Shares, the receipt of such cash will be tax-free to the holder. The same Swiss income tax treatment applies to Swiss Holders of Novartis physical share certificates (Heimverwahrer) held as private assets who receive cash due to non-response by September 19, 2023.
Novartis has received the Swiss Tax Rulings which cover the relevant Swiss tax aspects of the separation and spin-off. The Swiss Tax Rulings rely upon certain facts, assumptions, representations and undertakings from Sandoz and Novartis regarding the past and future conduct of the businesses of Sandoz and Novartis and other matters. If any of the facts, assumptions, representations or undertakings described therein are incorrect or incomplete or not otherwise satisfied, Novartis may not be able to rely upon the Swiss Tax Rulings. Accordingly, notwithstanding the Swiss Tax Rulings, no assurance can be given that the relevant Swiss tax authorities will not assert, or that a court would not sustain, a position contrary to one or more of the conclusions set forth above.
Consequences to US Holders of Novartis AG shares
The following is a summary of the material US federal income tax consequences to holders of Novartis AG shares or ADRs in connection with the Sandoz distribution. For purposes of the following discussion, any reference to Novartis AG shares includes Novartis ADRs. This summary does not address any US state or local or foreign tax consequences or any estate, gift or other non-income tax consequences.
General
The IRS Ruling and the Tax Opinion, described below, rely upon certain facts, assumptions, representations and undertakings from Novartis and Sandoz regarding the past and future conduct of Novartis and Sandoz businesses and other matters. If any of the facts, assumptions, representations or undertakings described therein are incorrect or not otherwise satisfied, Novartis may not be able to rely upon the IRS Ruling or the Tax Opinion. Accordingly, notwithstanding the Tax Opinion and the IRS Ruling, there can be no assurance that the IRS will not assert, or that a court would not sustain, a position contrary to one or more of the conclusions set forth below.
Novartis has received the IRS Ruling and the Tax Opinion providing, in each case, that the distribution should qualify for nonrecognition of gain or loss under Section 355 of the Code. As a result:
• no gain or loss should be recognized by, or be includible in the income of, a US Holder as a result of the distribution;
• the aggregate tax basis of the Novartis AG shares and the Sandoz Group AG shares held by each US Holder immediately after the distribution should be the same as the aggregate tax basis of the Novartis AG shares held by the US Holder immediately before the distribution, allocated between the Novartis AG shares and the Sandoz Group AG shares in proportion to their relative fair market values on the date of the distribution; and
• the holding period of the Sandoz Group AG shares received by each US Holder should include the holding period of its Novartis AG shares.
Generally, if a Novartis AG shareholder holds different blocks of Novartis AG shares (generally Novartis AG shares purchased or acquired on different dates or at different prices), a US Holder must perform the tax basis allocation described above with respect to each block and will have a holding period in the Sandoz Group AG shares determined with respect to the holding period of such block.
A US Holder that receives cash in lieu of a fractional Share as part of the distribution will be treated as though it first received a distribution of the fractional Share in the distribution and then sold it for the amount of cash
actually received. The US Holder will generally recognize a capital gain or loss measured by the difference between the cash received for such fractional Share and the US Holder’s tax basis in that fractional Share, as determined above. Such capital gain or loss will be a long-term capital gain or loss if the US Holder’s holding period for the Novartis AG shares is more than one year from the date of the distribution. Certain US Holders are eligible for reduced rates of taxation on their long-term capital gains.
A US Holder of Novartis physical share certificates (Heimverwahrer) who receives cash due to non-response by September 19, 2023 will be treated as if the US Holder received the Sandoz Group AG shares with respect to its physical share certificates in the distribution and then sold such Shares for the cash actually received. The deemed receipt and sale of the Sandoz Group AG shares for cash will be subject to the same treatment as the receipt of cash in lieu of a fractional Share for US federal income tax purposes as described above.
Backup Withholding
Payments of cash in lieu of a fractional Share and cash payments to a US Holder of Novartis physical share certificates (Heimverwahrer) who receives cash due to non-response by September 19, 2023 may, under certain circumstances, be subject to “backup withholding”, unless the US Holder provides proof of an applicable exemption or a correct taxpayer identification number, and otherwise complies with the requirements of the backup withholding rules. Corporations will generally be exempt from backup withholding, but may be required to provide a certification to establish their entitlement to the exemption. Backup withholding is not an additional tax, and it may be refunded or credited against a US Holder’s US federal income tax liability if the required information is timely supplied to the IRS.
Information Reporting
Treasury Regulations require each Novartis AG shareholder that, immediately before the distribution, owned 5% or more (by vote or value) of the total outstanding stock of Novartis, to attach to such shareholder’s US federal income tax return for the year in which the distribution occurs a statement setting forth certain information related to the distribution.
10.F Dividends and paying agents
Not applicable.
10.G Statement by experts
Not applicable.
10.H Documents on display
Any statement in this Form 20‑F about any of our contracts or other documents is not necessarily complete. If the contract or document is filed as an exhibit to the Form 20‑F, the contract or document is deemed to modify the description contained in this Form 20‑F. You must review the exhibits themselves for a complete description of the contract or document.
The SEC maintains an internet site at http://www.sec.gov that contains reports and other information regarding issuers that file electronically with the SEC. These SEC filings are also available to the public from commercial document retrieval services.
We are required to file or furnish reports and other information with the SEC under the Exchange Act and regulations under that act. As a foreign private issuer, we are exempt from the rules under the Exchange Act prescribing the form and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act.
10.I Subsidiary information
Not applicable.
Item 11. Quantitative and Qualitative Disclosures About Market Risk
The major financial risks facing us are managed centrally by the Company’s treasury function, which has established processes and procedures to identify, aggregate and manage our financial risk exposure. The Company’s treasury function is included in management’s internal control assessment.
For information about the effects of currency fluctuations and how we manage currency risk, see “Item 5. Operating and Financial Review and Prospects—Item 5.B Liquidity and capital resources.”
The information set forth under “Item 18. Financial Statements—Note 30. Financial instruments - additional disclosures” is incorporated by reference.
Item 12. Description of Securities Other than Equity Securities
12.A Debt securities
Not applicable.
12.B Warrants and rights
Not applicable.
12.C Other securities
Not applicable.
12.D American Depositary Shares
Fees payable by ADR holders
According to the deposit agreement that we entered into with JPMorgan Chase Bank, N.A. (JPMorgan), as depositary (as amended from time to time, the “Deposit Agreement”), holders of our ADRs may have to pay to JPMorgan, either directly or indirectly, fees or charges up to the amounts set forth below:
Category | | Depositary actions | | Associated fee | |
|
Depositing or substituting
underlying shares | | Acceptance of shares surrendered, and issuance of ADSs in exchange,
including surrenders and issuances in respect of:
— Share distributions
— Stock split
— Rights
— Merger
— Exchange of shares or any other transaction or event or other distribution
affecting the ADSs or the deposited shares | | USD 5.00 for each 100 ADSs
(or portion thereof)
| |
|
Withdrawing
underlying shares | | Acceptance of ADSs surrendered for withdrawal of deposited shares or
for ADSs that are cancelled or reduced for any other reason
| | USD 5.00 (or less) for each
100 ADSs (or portion
thereof) surrendered | |
|
Cash distributions | | Distributing cash distributions made or any elective cash/stock dividend offered | | USD 0.05 (or less) per ADS | |
|
Selling or
exercising rights | | Distribution or sale of shares, the fee being in an amount equal to the fee
for the execution and delivery of ADRs that would have been charged
as a result of the deposit of such shares | | USD 5.00 for each 100 ADSs
(or portion thereof)
| |
|
Depositary services | | Services performed by the depositary in administering the ADRs
| | USD 0.05 (or less) per ADS
per calendar year
(or portion thereof) | |
|
Expenses of the
depositary | | Expenses incurred on behalf of holders in connection with:
— Compliance with foreign exchange control regulations or any law or
regulation relating to foreign investment
— The depositary’s or its custodian’s compliance with applicable law,
rule or regulation
— Stock transfer or other taxes and other governmental charges
— Cable, telex and facsimile transmission and delivery
— Expenses of the depositary in connection with the conversion of foreign
currency into US dollars (which are paid out of such foreign currency)
— Any other charge payable by any of the depositary or its agents | | Expenses payable at the sole
discretion of the depositary
by billing holders or by
deducting charges from one
or more cash dividends or
other cash distributions
| |
|
| | | | | |
Fees payable by the depositary to the issuer
Pursuant to a letter agreement effective as of May 11, 2017, as amended from time to time (“the Agreement”), JPMorgan, as our ADS depositary, has agreed to make an annual contribution payment to Novartis at the end of each 12-month period beginning on the effective date of the Agreement and on each subsequent anniversary of the effective date of the Agreement (each such 12-month period is a “Contract Year”). Beginning in the sixth Contract Year, this annual contribution payment will equal: (a)(1) the applicable fixed contribution amount reflected in the table below, based on the average daily balance during such Contract Year of outstanding ADSs backed by ordinary shares less (a)(2) the custody costs, fees and expenses (including, without limitation, any central securities depository fees, charges and expenses) incurred during the applicable Contract Year (the items in (a)(2) collectively are the “Custody Costs”) plus (b) 70% of the gross issuance and cancellation fees collected by JPMorgan under the Deposit Agreement during such Contract Year minus (c) that portion (if any) of JPMorgan’s legal fees, charges and out-of-pocket expenses in excess of USD 50 000 for such Contract Year.
Average Daily Balance Range Start | | Average Daily Balance Range End | | Fixed contribution | |
|
At least 30 000 000 | | Up to 66 999 999 | | USD 340 000 | |
|
At least 67 000 000 | | Up to 133 999 999 | | USD 680 000 | |
|
At least 134 000 000 | | Up to 200 999 999 | | USD 1 020 000 | |
|
At least 201 000 000 | | Up to 267 999 999 | | USD 1 360 000 | |
|
At least 268 000 000 | | | | USD 1 700 000 | |
|
The fixed contribution amount payable under (a)(1) in respect of a given Contract Year shall be zero if the average daily balance of outstanding ADSs backed by ordinary shares is less than 30 000 000 during such Contract Year. If the Custody Costs for a Contract Year exceed the fixed contribution amount for such Contract Year, JPMorgan will reduce the contribution payable to us by an amount equal to such deficit.
JPMorgan has further agreed to waive the USD 0.05 per ADS issuance fees that would normally be owed by Novartis in connection with our deposits of shares as part of our employee stock ownership and employee participation plans. Novartis is responsible for reimbursing JPMorgan for all taxes and governmental charges required to have been withheld and/or paid, and not so withheld and/or paid, arising from such waived fees.
PART II
Item 13. Defaults, Dividend Arrearages and Delinquencies
None.
Item 14. Material Modifications to the Rights of Security Holders and Use of Proceeds
None.
Item 15. Controls and Procedures
(a) Novartis AG’s Chief Executive Officer and Chief Financial Officer, after evaluating the effectiveness of our disclosure controls and procedures (as defined in Exchange Act Rule 13a-15(e)) as of the end of the period covered by this Annual Report, have concluded that, as of such date, our disclosure controls and procedures were effective.
(b) Report of Novartis Management on Internal Control Over Financial Reporting: The Board of Directors and management of the Company are responsible for establishing and maintaining adequate internal control over financial reporting. The Company’s internal control over financial reporting was designed to provide reasonable assurance to the Company’s management and Board of Directors regarding the reliability of financial reporting and the preparation and fair presentation of its published consolidated financial statements.
Internal controls over financial reporting, no matter how well designed, have inherent limitations. Therefore, even those internal controls over financial reporting determined to be effective may not prevent or detect misstatements and can provide only reasonable assurance with respect to financial statement preparation and presentation. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The Company’s management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2023. In making this assessment, it used the criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on our assessment, management concluded that, as of December 31, 2023, the Company’s internal control over financial reporting is effective based on those criteria.
KPMG AG, Switzerland, an independent registered public accounting firm, has issued an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting, which is included in this Annual Report under “Item 18. Financial Statements—Report of independent registered public accounting firm.”
(c) See the report of KPMG AG, an independent registered public accounting firm, included under “Item 18. Financial Statements—Report of independent registered public accounting firm.”
(d) There were no changes to our internal control over financial reporting that occurred during the period covered by this Annual Report that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 16A. Audit Committee Financial Expert
Our Audit and Compliance Committee has determined that Elizabeth Doherty and Ana de Pro Gonzalo possess specific accounting and financial management expertise, and that they are “audit committee financial experts” as defined in Item 16A of Form 20-F. The Board of Directors has also determined that each member of the Audit and Compliance Committee is “independent” in accordance with the applicable requirements set forth under the listing standards of the NYSE and Rule 10A‑3 under the Exchange Act, and has sufficient experience and ability in finance and compliance matters to enable them to adequately discharge their responsibilities.
Item 16B. Code of Ethics
In addition to our Code of Ethics and Doing Business Ethically Policy, which are applicable to all of our employees, we have adopted Ethical Conduct Requirements that impose additional obligations on our principal executive officer, principal financial officer, principal accounting officer, and persons performing similar functions. This document is accessible on our internet website at:
https://www.novartis.com/investors/company-overview/corporate-governance
Item 16C. Principal Accountant Fees and Services
The information set forth under “Item 6. Directors, Senior Management and Employees—Item 6.C Board practices—Corporate governance—Auditors” is incorporated by reference.
Item 16D. Exemptions from the Listing Standards for Audit Committees
Not applicable.
Item 16E. Purchases of Equity Securities by the Issuer and Affiliated Purchasers
2023
| |
Total number of
shares purchased
(a)1
| |
Average price
paid per share
in USD
(b)
| |
Total number
of shares
purchased
as part of
publicly
announced
plans or
programs
(c)2
| | Maximum
approximate
value of
shares that
may yet be
purchased
under the
plans or
programs
(CHF millions)
(d) | | Maximum
approximate
value of
shares that
may yet be
purchased
under the
plans or
programs
(USD millions)
(e)3 | |
|
Jan. 1-31 | | 11 383 594 | | 91.91 | | 10 500 000 | | 7 433 | | 8 030 | |
|
Feb. 1-28 | | 10 147 593 | | 87.02 | | 10 000 000 | | 6 628 | | 7 061 | |
|
Mar. 1-31 | | 11 145 700 | | 85.02 | | 11 000 000 | | 15 762 | | 17 252 | |
|
Apr. 1-30 | | 9 092 383 | | 98.58 | | 9 000 000 | | 14 966 | | 16 736 | |
|
May 1-31 | | 10 018 880 | | 102.20 | | 10 000 000 | | 14 050 | | 15 450 | |
|
Jun. 1-30 | | 10 871 925 | | 100.29 | | 10 847 255 | | 13 071 | | 14 537 | |
|
Jul. 1-31 | | 5 026 486 | | 100.98 | | 5 000 000 | | 12 625 | | 14 493 | |
|
Aug. 1-31 | | 4 446 306 | | 102.80 | | 4 400 000 | | 12 228 | | 13 903 | |
|
Sep. 1-30 | | 4 250 523 | | 101.17 | | 4 200 000 | | 11 846 | | 12 998 | |
|
Oct. 1-31 | | 4 494 588 | | 96.85 | | 4 400 000 | | 11 461 | | 12 705 | |
|
Nov. 1-30 | | 4 426 601 | | 95.48 | | 4 400 000 | | 11 087 | | 12 701 | |
|
Dec. 1-31 | | 3 821 670 | | 98.39 | | 3 800 000 | | 10 763 | | 12 793 | |
|
| | | | | | | | | | | |
|
Total | | 89 126 249 | | 95.55 | | 87 547 255 | | | | | |
|
|
1 Column (a) shows shares repurchased on the SIX Swiss Exchange second trading line plus shares we purchased from employees who had obtained the shares through a Novartis Employee Ownership Plan. See "Item 18. Financial Statements - Note 27. Equity-based participation plans for employees." |
2 Column (c) shows shares repurchased on the SIX Swiss Exchange second trading line under the CHF 10 billion share buyback authority approved at the 2022 AGM for transactions in 2023. See "Item 6. Directors, Senior Management and Employees - Item 6C. Board Practices - Our capital structure - Changes in capital." |
3 Column (e) shows the Swiss franc amount from column (d) converted into US dollars as of the month-end, using the Swiss franc/US dollar exchange rate at the applicable month-end. |
Item 16F. Change in Registrant’s Certifying Accountant
Not applicable.
Item 16G. Corporate Governance
Novartis AG is subject to and compliant with the laws and regulations of Switzerland (in particular, Swiss company and securities laws, SIX Swiss Exchange rules and the Swiss Code of Best Practice for Corporate Governance) and the securities laws of the United States, including NYSE listing standards, as applicable to foreign private issuers of securities. The following summarizes some significant ways in which our corporate governance practices differ from those followed by domestic listed US companies under the listing standards of the NYSE:
• Novartis AG shareholders do not receive written reports directly from Board committees.
• External auditors are appointed by shareholders at the Annual General Meeting of Shareholders (AGM), as opposed to being appointed by the Audit and Compliance Committee.
• While shareholders cannot vote on all equity compensation plans, they are entitled to hold separate, yearly binding votes on Board and Executive Committee compensation.
• The Board has set up a separate Risk Committee that oversees the risk management system and processes, as opposed to delegating this responsibility to the Audit and Compliance Committee.
• The full Board is responsible for overseeing the performance evaluation of the Board and Executive Committee.
• The full Board is responsible for setting objectives relevant to the CEO’s compensation and for evaluating his performance.
Item 16H. Mine Safety Disclosure
Not applicable.
Item 16I. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
Item 16J. Insider Trading Policies
Not applicable.
Item 16K. Cybersecurity
Protecting the security and integrity of the IT systems under our control and safeguarding the privacy of our customers, patients and employees is a top priority for us at all levels. Cybersecurity and data privacy risks are among the core enterprise risks evaluated through our annual enterprise risk management assessment.
The Chief Security Officer oversees our cybersecurity risk management program in partnership with our Chief Information Officer and other business leaders. The program was developed to respond to the threat of security breaches and cyberattacks, and to protect and preserve the confidentiality, integrity, and continued availability of information owned by, or in the care of Novartis. Our Chief Security Officer reports to our Chief Information Officer, and is a subject matter expert on information security, privacy, information technology strategy and management with over 20 years of relevant experience across a number of industries, including pharmaceuticals, consumer goods, financial services and consulting. Our Chief Information Officer has nearly 25 years of experience as an IT professional, including 14 years with Novartis, and is responsible for our technology strategy, delivery and operations globally.
To address cybersecurity threats and prevent IT system interruptions, the Information Security & Compliance (ISC) team, which is headed by our Chief Security Officer, has implemented enterprise-wide policies, processes and practices. We follow industry best practices, such as the NIST Cybersecurity Framework and ISO 27001 to manage information security. Novartis has risk-based services continuity and systems recovery plans in place for key business processes, which are tested periodically. We also conduct ongoing internal vulnerability analyses (including simulated hacking) as well as external testing via a third-party to ensure the effectiveness of our cybersecurity controls. We require employees to report IT security incidents to a Cyber Security Operations Center (CSOC) that operates 24 hours a day, 7 days a week. CSOC is a function within ISC that is responsible for investigating all security incidents and alerts including determining the threat type, incident scope and incident severity. Where appropriate, major incidents are escalated to our Chief Executive Officer, who may then inform our Board of the incident pursuant to our internal procedures. Novartis has not experienced any material cybersecurity incidents in the three years through 2023.
As part of its enterprise risk management oversight, the Risk Committee of our Board is responsible for ensuring that the Company has implemented an appropriate and effective risk management system and process, including annually reviewing updates on cybersecurity. The Risk Committee receives updates on cybersecurity risks, which address a wide range of topics, including recent developments, security incidents, evolving standards, vulnerability assessments, third-party and independent reviews, the threat environment, technological trends and information security considerations arising with respect to the peers and vendors of Novartis. At least once each year, the Risk Committee discusses the Company’s approach to cybersecurity risk management with the Chief Security Officer.
PART III
Item 17. Financial Statements
See response to “Item 18. Financial Statements.”
Item 18. Financial Statements
The following financial statements are filed as part of this Annual Report.
Item 19. Exhibits
101.INS XBRL Instance Document
101.SCH XBRL Taxonomy Extension Schema Document
101.CAL XBRL Taxonomy Extension Calculation Linkbase Document
101.DEF XBRL Taxonomy Extension Definition Linkbase Document
101.LAB XBRL Taxonomy Extension Label Linkbase Document
101.PRE XBRL Taxonomy Extension Presentation Linkbase Document
104 Cover Page Interactive Data File (embedded within the Inline XBRL document and included in Exhibit 101).
The total amount of long-term debt securities authorized under any instrument does not exceed 10% of the total assets of the Company and its subsidiaries on a consolidated basis. We hereby agree to furnish to the SEC, upon its request, a copy of any instrument defining the rights of holders of long-term debt of the Company or of its subsidiaries for which consolidated or unconsolidated financial statements are required to be filed.
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this Annual Report on its behalf.
Novartis AG
By: /s/ Harry Kirsch
Name: Harry Kirsch
Title: Chief Financial Officer of Novartis
By: /s/ Karen Hale
Name: Karen Hale
Title: Chief Legal Officer of Novartis
Date: January 31, 2024
Novartis consolidated financial statements
Consolidated income statements
(For the years ended December 31, 2023, 2022 and 2021)
(USD millions unless indicated otherwise) | | Note | | 2023 | | 2022 | | 2021 | |
|
Net sales from continuing operations | | 4 | | 45 440 | | 42 206 | | 42 781 | |
|
Other revenues | | 4 | | 1 220 | | 1 255 | | 1 193 | |
|
Cost of goods sold | | | | -12 472 | | -11 582 | | -11 735 | |
|
Gross profit from continuing operations | | | | 34 188 | | 31 879 | | 32 239 | |
|
Selling, general and administration | | | | -12 517 | | -12 193 | | -12 827 | |
|
Research and development | | | | -11 371 | | -9 172 | | -8 641 | |
|
Other income | | | | 1 772 | | 696 | | 1 620 | |
|
Other expense | | | | -2 303 | | -3 264 | | -2 335 | |
|
Operating income from continuing operations | | | | 9 769 | | 7 946 | | 10 056 | |
|
(Loss)/income from associated companies | | 5 | | -13 | | -11 | | 15 337 | |
|
Interest expense | | 6 | | -855 | | -800 | | -787 | |
|
Other financial income and expense | | 6 | | 222 | | 42 | | -76 | |
|
Income before taxes from continuing operations | | | | 9 123 | | 7 177 | | 24 530 | |
|
Income taxes | | 7 | | -551 | | -1 128 | | -1 625 | |
|
Net income from continuing operations | | | | 8 572 | | 6 049 | | 22 905 | |
|
Net income from discontinued operations before gain on distribution of Sandoz Group AG to Novartis AG shareholders | | 31 | | 422 | | 906 | | 1 113 | |
|
Gain on distribution of Sandoz Group AG to Novartis AG shareholders | | 2 | | 5 860 | | | | | |
|
Net income from discontinued operations | | 31 | | 6 282 | | 906 | | 1 113 | |
|
Net income | | | | 14 854 | | 6 955 | | 24 018 | |
|
Attributable to: | | | | | | | | | |
Shareholders of Novartis AG | | | | 14 850 | | 6 955 | | 24 021 | |
|
Non-controlling interests | | | | 4 | | 0 | | -3 | |
|
| | | | | | | | | |
Basic earnings per share (USD) from continuing operations | | | | 4.13 | | 2.77 | | 10.22 | |
|
Basic earnings per share (USD) from discontinued operations | | | | 3.02 | | 0.42 | | 0.49 | |
|
Total basic earnings per share (USD) | | 8 | | 7.15 | | 3.19 | | 10.71 | |
|
| | | | | | | | | |
Diluted earnings per share (USD) from continuing operations | | | | 4.10 | | 2.76 | | 10.14 | |
|
Diluted earnings per share (USD) from discontinued operations | | | | 3.00 | | 0.41 | | 0.49 | |
|
Total diluted earnings per share (USD) | | 8 | | 7.10 | | 3.17 | | 10.63 | |
|
|
The accompanying Notes form an integral part of the consolidated financial statements. |
Consolidated statements of comprehensive income
(For the years ended December 31, 2023, 2022 and 2021)
(USD millions) | | Note | | 2023 | | 2022 | | 2021 | |
|
Net income | | | | 14 854 | | 6 955 | | 24 018 | |
|
| | | | | | | | | |
Other comprehensive income | | | | | | | | | |
Items that are or may be recycled into the consolidated income statement | | | | | | | | | |
|
Novartis share of other comprehensive income recognized by associated companies, net of taxes | | 5 | | | | | | 46 | |
|
Net investment hedge, net of taxes | | 9 | | -50 | | 91 | | 216 | |
|
Currency translation effects, net of taxes | | 9 | | 1 375 | | -450 | | -4 762 | |
|
Total of items that are or may be recycled | | | | 1 325 | | -359 | | -4 500 | |
|
| | | | | | | | | |
Items that will never be recycled into the consolidated income statement | | | | | | | | | |
|
Actuarial (losses)/gains from defined benefit plans, net of taxes | | 9 | | -160 | | -103 | | 1 809 | |
|
Fair value adjustments on equity securities, net of taxes | | 9 | | 37 | | -382 | | 194 | |
|
Total of items that will never be recycled | | | | -123 | | -485 | | 2 003 | |
|
| | | | | | | | | |
|
Total comprehensive income | | | | 16 056 | | 6 111 | | 21 521 | |
|
Total comprehensive income for the year attributable to: | | | | | | | | | |
Shareholders of Novartis AG | | | | 16 050 | | 6 116 | | 21 528 | |
|
Continuing operations | | | | 10 115 | | 5 181 | | 20 450 | |
|
Discontinued operations | | | | 5 935 | | 935 | | 1 078 | |
|
Non-controlling interests | | | | 6 | | -5 | | -7 | |
|
|
The accompanying Notes form an integral part of the consolidated financial statements. |
Consolidated balance sheets
(At December 31, 2023 and 2022)
(USD millions) | | Note | | 2023 | | 2022 | |
|
Assets | | | | | | | |
Non-current assets | | | | | | | |
Property, plant and equipment | | 10 | | 9 514 | | 10 764 | |
|
Right-of-use assets | | 11 | | 1 410 | | 1 431 | |
|
Goodwill | | 12 | | 23 341 | | 29 301 | |
|
Intangible assets other than goodwill | | 12 | | 26 879 | | 31 644 | |
|
Investments in associated companies | | 5 | | 205 | | 143 | |
|
Deferred tax assets | | 13 | | 4 309 | | 3 739 | |
|
Financial assets | | 14 | | 2 607 | | 2 411 | |
|
Other non-current assets | | 14 | | 1 199 | | 1 110 | |
|
Total non-current assets | | | | 69 464 | | 80 543 | |
|
Current assets | | | | | | | |
Inventories | | 15 | | 5 913 | | 7 175 | |
|
Trade receivables | | 16 | | 7 107 | | 8 066 | |
|
Income tax receivables | | | | 426 | | 268 | |
|
Marketable securities, commodities, time deposits and derivative financial instruments | | 17 | | 1 035 | | 11 413 | |
|
Cash and cash equivalents | | 17 | | 13 393 | | 7 517 | |
|
Other current assets | | 18 | | 2 607 | | 2 471 | |
|
Total current assets | | | | 30 481 | | 36 910 | |
|
Total assets | | | | 99 945 | | 117 453 | |
|
| | | | | | | |
Equity and liabilities | | | | | | | |
Equity | | | | | | | |
Share capital | | 19 | | 825 | | 890 | |
|
Treasury shares | | 19 | | -41 | | -92 | |
|
Reserves | | | | 45 883 | | 58 544 | |
|
Equity attributable to Novartis AG shareholders | | | | 46 667 | | 59 342 | |
|
Non-controlling interests | | | | 83 | | 81 | |
|
Total equity | | | | 46 750 | | 59 423 | |
|
Liabilities | | | | | | | |
Non-current liabilities | | | | | | | |
Financial debts | | 20 | | 18 436 | | 20 244 | |
|
Lease liabilities | | 11 | | 1 598 | | 1 538 | |
|
Deferred tax liabilities | | 13 | | 2 248 | | 2 686 | |
|
Provisions and other non-current liabilities | | 21 | | 4 523 | | 4 906 | |
|
Total non-current liabilities | | | | 26 805 | | 29 374 | |
|
Current liabilities | | | | | | | |
Trade payables | | | | 4 926 | | 5 146 | |
|
Financial debts and derivative financial instruments | | 22 | | 6 175 | | 5 931 | |
|
Lease liabilities | | 11 | | 230 | | 251 | |
|
Current income tax liabilities | | | | 1 893 | | 2 533 | |
|
Provisions and other current liabilities | | 23 | | 13 166 | | 14 795 | |
|
Total current liabilities | | | | 26 390 | | 28 656 | |
|
Total liabilities | | | | 53 195 | | 58 030 | |
|
Total equity and liabilities | | | | 99 945 | | 117 453 | |
|
|
The accompanying Notes form an integral part of the consolidated financial statements. |
Consolidated statements of changes in equity
(For the years ended December 31, 2023, 2022 and 2021)
| | | | | | | | Reserves | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
(USD millions)
| |
Note
| |
Share
capital
| |
Treasury
shares
| |
Retained
earnings
| |
Total value
adjustments
| | Equity
attributable
to Novartis
shareholders | |
Non-
controlling
interests
| |
Total
equity
| |
|
Total equity at January 1, 2021 | | | | 913 | | -53 | | 57 157 | | -1 419 | | 56 598 | | 68 | | 56 666 | |
|
Net income | | | | | | | | 24 021 | | | | 24 021 | | -3 | | 24 018 | |
|
Other comprehensive income | | 9 | | | | | | 46 | | -2 539 | | -2 493 | | -4 | | -2 497 | |
|
Total comprehensive income | | | | | | | | 24 067 | | -2 539 | | 21 528 | | -7 | | 21 521 | |
|
Dividends | | 19.1 | | | | | | -7 368 | | | | -7 368 | | | | -7 368 | |
|
Purchase of treasury shares | | 19.2 | | | | -18 | | -2 902 | | | | -2 920 | | | | -2 920 | |
|
Reduction of share capital | | 19 | | -12 | | 18 | | -6 | | | | | | | | | |
|
Exercise of options and employee transactions | | 19.2 | | | | 0 | | 39 | | | | 39 | | | | 39 | |
|
Equity-based compensation | | 19.2 | | | | 5 | | 740 | | | | 745 | | | | 745 | |
|
Shares delivered to Alcon employees as a result of the Alcon spin-off | | 19.2 | | | | 0 | | 17 | | | | 17 | | | | 17 | |
|
Taxes on treasury share transactions | | | | | | | | 1 | | | | 1 | | | | 1 | |
|
Increase of treasury share repurchase obligation under a share buyback trading plan | | 19.4 | | | | | | -1 040 | | | | -1 040 | | | | -1 040 | |
|
Transaction costs, net of taxes | | 19.8 | | | | | | 12 | | | | 12 | | | | 12 | |
|
Changes in non-controlling interests | | 19.6 | | | | | | | | | | | | -1 | | -1 | |
|
Fair value adjustments on financial assets sold | | 9 | | | | | | 164 | | -164 | | | | | | | |
|
Value adjustments related to divestments | | 9 | | | | | | 65 | | -65 | | | | | | | |
|
Impact of change in ownership of consolidated entities | | 19.5 | | | | | | -5 | | 0 | | -5 | | 107 | | 102 | |
|
Other movements | | 19.7 | | | | | | 48 | | | | 48 | | | | 48 | |
|
Total of other equity movements | | | | -12 | | 5 | | -10 235 | | -229 | | -10 471 | | 106 | | -10 365 | |
|
Total equity at December 31, 2021 | | | | 901 | | -48 | | 70 989 | | -4 187 | | 67 655 | | 167 | | 67 822 | |
|
Net income | | | | | | | | 6 955 | | | | 6 955 | | 0 | | 6 955 | |
|
Other comprehensive income | | 9 | | | | | | | | -839 | | -839 | | -5 | | -844 | |
|
Total comprehensive income | | | | | | | | 6 955 | | -839 | | 6 116 | | -5 | | 6 111 | |
|
Dividends | | 19.1 | | | | | | -7 506 | | | | -7 506 | | | | -7 506 | |
|
Purchase of treasury shares | | 19.2 | | | | -66 | | -10 844 | | | | -10 910 | | | | -10 910 | |
|
Reduction of share capital | | 19 | | -11 | | 15 | | -4 | | | | | | | | | |
|
Exercise of options and employee transactions | | 19.2 | | | | 1 | | 87 | | | | 88 | | | | 88 | |
|
Equity-based compensation | | 19.2 | | | | 6 | | 848 | | | | 854 | | | | 854 | |
|
Shares delivered to Alcon employees as a result of the Alcon spin-off | | 19.2 | | | | 0 | | 5 | | | | 5 | | | | 5 | |
|
Taxes on treasury share transactions | | | | | | | | 14 | | | | 14 | | | | 14 | |
|
Decrease of treasury share repurchase obligation under a share buyback trading plan | | 19.4 | | | | | | 2 809 | | | | 2 809 | | | | 2 809 | |
|
Changes in non-controlling interests | | 19.6 | | | | | | | | | | | | -81 | | -81 | |
|
Fair value adjustments on financial assets sold | | 9 | | | | | | 4 | | -4 | | | | | | | |
|
Value adjustments related to divestments | | 9 | | | | | | -34 | | 34 | | | | | | | |
|
Other movements | | 19.7 | | | | | | 217 | | | | 217 | | | | 217 | |
|
Total of other equity movements | | | | -11 | | -44 | | -14 404 | | 30 | | -14 429 | | -81 | | -14 510 | |
|
Total equity at December 31, 2022 | | | | 890 | | -92 | | 63 540 | | -4 996 | | 59 342 | | 81 | | 59 423 | |
|
Net income | | | | | | | | 14 850 | | | | 14 850 | | 4 | | 14 854 | |
|
Other comprehensive income | | 9 | | | | | | | | 1 200 | | 1 200 | | 2 | | 1 202 | |
|
Total comprehensive income | | | | | | | | 14 850 | | 1 200 | | 16 050 | | 6 | | 16 056 | |
|
Dividends | | 19.1 | | | | | | -7 255 | | | | -7 255 | | | | -7 255 | |
|
Dividend in kind to effect the spin-off of Sandoz Group AG | | 2 | | | | | | -13 962 | | | | -13 962 | | | | -13 962 | |
|
Purchase of treasury shares | | 19.2 | | | | -51 | | -8 466 | | | | -8 517 | | | | -8 517 | |
|
Reduction of share capital | | 19.3 | | -65 | | 94 | | -29 | | | | | | | | | |
|
Exercise of options and employee transactions | | 19.2 | | | | 2 | | 144 | | | | 146 | | | | 146 | |
|
Equity-based compensation | | 19.2 | | | | 6 | | 898 | | | | 904 | | | | 904 | |
|
Shares delivered to Sandoz employees as a result of the Sandoz spin-off | | 19.2 | | | | 0 | | 30 | | | | 30 | | | | 30 | |
|
Taxes on treasury share transactions | | | | | | | | 14 | | | | 14 | | | | 14 | |
|
Transaction costs, net of taxes | | 19.8 | | | | | | -214 | | | | -214 | | | | -214 | |
|
Changes in non-controlling interests | | 19.6 | | | | | | | | | | | | -4 | | -4 | |
|
Fair value adjustments on financial assets sold | | 9 | | | | | | -1 | | 1 | | | | | | | |
|
Value adjustments related to divestments | | 9 | | | | | | -29 | | 29 | | | | | | | |
|
Other movements | | 19.7 | | | | | | 129 | | | | 129 | | | | 129 | |
|
Total of other equity movements | | | | -65 | | 51 | | -28 741 | | 30 | | -28 725 | | -4 | | -28 729 | |
|
Total equity at December 31, 2023 | | | | 825 | | -41 | | 49 649 | | -3 766 | | 46 667 | | 83 | | 46 750 | |
|
|
The accompanying Notes form an integral part of the consolidated financial statements. |
Consolidated statements of cash flows
(For the years ended December 31, 2023, 2022 and 2021)
(USD millions) | | Note | | 2023 | | 2022 | | 2021 | |
|
Net income from continuing operations | | | | 8 572 | | 6 049 | | 22 905 | |
|
Adjustments to reconcile net income from continuing operations to net cash flows from operating activities from continuing operations | | | | | | | | | |
Reversal of non-cash items and other adjustments | | 24.1 | | 10 369 | | 10 631 | | -6 430 | |
|
Dividends received from associated companies and others | | | | 2 | | 1 | | 523 | |
|
Interest received | | | | 645 | | 252 | | 11 | |
|
Interest paid | | | | -751 | | -667 | | -643 | |
|
Other financial receipts | | | | 90 | | 71 | | | |
|
Other financial payments | | | | -17 | | -26 | | -297 | |
|
Income taxes paid | | 24.2 | | -2 787 | | -1 702 | | -1 856 | |
|
Net cash flows from operating activities from continuing operations before working capital and provision changes | | | | 16 123 | | 14 609 | | 14 213 | |
|
Payments out of provisions and other net cash movements in non-current liabilities | | | | -1 534 | | -774 | | -775 | |
|
Change in net current assets and other operating cash flow items | | 24.3 | | -369 | | -796 | | -73 | |
|
Net cash flows from operating activities from continuing operations | | | | 14 220 | | 13 039 | | 13 365 | |
|
Net cash flows from operating activities from discontinued operations | | | | 238 | | 1 197 | | 1 706 | |
|
Total net cash flows from operating activities | | | | 14 458 | | 14 236 | | 15 071 | |
|
Purchases of property, plant and equipment | | | | -1 060 | | -916 | | -1 066 | |
|
Proceeds from sale of property, plant and equipment | | | | 237 | | 158 | | 211 | |
|
Purchases of intangible assets | | | | -1 693 | | -1 323 | | -1 490 | |
|
Proceeds from sale of intangible assets | | | | 1 955 | | 170 | | 686 | |
|
Purchases of financial assets | | | | -106 | | -115 | | -188 | |
|
Proceeds from sale of financial assets | | | | 348 | | 133 | | 440 | |
|
Purchases of other non-current assets | | | | | | -1 | | -59 | |
|
Proceeds from sale of other non-current assets | | | | | | | | 4 | |
|
Acquisitions and divestments of interests in associated companies, net | | 24.4 | | -11 | | -24 | | 20 669 | |
|
Acquisitions and divestments of businesses, net | | 24.5 | | -3 558 | | -840 | | -205 | |
|
Purchases of marketable securities, commodities and time deposits | | | | -641 | | -34 695 | | -16 403 | |
|
Proceeds from sale of marketable securities, commodities and time deposits | | | | 11 248 | | 39 357 | | 2 298 | |
|
Net cash flows from investing activities from continuing operations | | | | 6 719 | | 1 904 | | 4 897 | |
|
Net cash flows used in investing activities from discontinued operations | | 31 | | -1 123 | | -436 | | -689 | |
|
Total net cash flows from investing activities | | | | 5 596 | | 1 468 | | 4 208 | |
|
Dividends paid to shareholders of Novartis AG | | | | -7 255 | | -7 506 | | -7 368 | |
|
Acquisitions of treasury shares | | | | -8 719 | | -10 652 | | -3 057 | |
|
Proceeds from exercised options and other treasury share transactions, net | | | | 153 | | 100 | | 53 | |
|
Repayments of the current portion of non-current financial debts | | 24.6 | | -2 223 | | -2 575 | | -2 162 | |
|
Change in current financial debts | | 24.6 | | 546 | | 252 | | -3 547 | |
|
Payments of lease liabilities | | 24.6 | | -258 | | -262 | | -278 | |
|
Impact of change in ownership of consolidated entities | | | | | | | | -3 | |
|
Other financing cash flows, net | | | | 192 | | -38 | | 72 | |
|
Net cash flows used in financing activities from continuing operations | | | | -17 564 | | -20 681 | | -16 290 | |
|
Net cash flows from financing activities from discontinued operations | | 31 | | 3 286 | | 119 | | 26 | |
|
Total net cash flows used in financing activities | | | | -14 278 | | -20 562 | | -16 264 | |
|
Net change in cash and cash equivalents before effect of exchange rate changes | | | | 5 776 | | -4 858 | | 3 015 | |
|
Effect of exchange rate changes on cash and cash equivalents | | | | 100 | | -32 | | -266 | |
|
Net change in cash and cash equivalents | | | | 5 876 | | -4 890 | | 2 749 | |
|
Cash and cash equivalents at January 1 | | | | 7 517 | | 12 407 | | 9 658 | |
|
Cash and cash equivalents at December 31 | | | | 13 393 | | 7 517 | | 12 407 | |
|
|
The accompanying Notes form an integral part of the consolidated financial statements. |
Notes to the Novartis consolidated financial statements
1. Accounting policies
Novartis is a multinational group of companies (Novartis or Company) specializing in the research, development, manufacturing and marketing of a broad range of innovative pharmaceutical medicines. The Company is headquartered in Basel, Switzerland.
The consolidated financial statements of the Company are prepared in accordance with International Financial Reporting Standards (IFRS) Accounting Standards as issued by the International Accounting Standards Board. They are prepared in accordance with the historical cost convention, except for items that are required to be accounted for at fair value.
The Company’s financial year-end is December 31, which is also the annual closing date of the individual entities’ financial statements incorporated into the Company’s consolidated financial statements.
The preparation of financial statements requires management to make certain estimates and assumptions, either at the balance sheet date or during the year, which affect the reported amounts of revenues, expenses, assets, liabilities, including the distribution liability and the non-cash, non-taxable gain recognized in connection with the distribution of Sandoz Group AG to Novartis AG shareholders, and contingent amounts.
Estimates are based on historical experience and other assumptions that are considered reasonable under the given circumstances and are regularly monitored. Actual outcomes and results could differ from those estimates and assumptions. Revisions to estimates are recognized in the period in which the estimate is revised.
At the Novartis AG Extraordinary General Meeting, held on September 15, 2023, our shareholders approved the spin-off of the Sandoz business. Following the shareholder approval, IFRS Accounting Standards require the Sandoz Division and selected portions of corporate activities attributable to Sandoz’s business, as well as certain expenses related to the spin-off (the “Sandoz business”) to be reported as discontinued operations in the consolidated financial statements. As a result, the Sandoz business has been presented as discontinued operations in the consolidated financial statements. This requires the year ended December 31, 2023 consolidated income statement, consolidated statement of comprehensive income and consolidated statement of cash flows to present separately continuing operations from discontinued operations, with comparative amounts in the prior years restated on a consistent basis. There is no requirement for the restatement of the December 31, 2022 consolidated balance sheet related to the assets and liabilities of the Sandoz business that were derecognized in 2023 as at the October 3, 2023 Distribution date. For further information and disclosures refer to the section Distribution of Sandoz Group AG to Novartis AG shareholders in this Note 1, Note 2 and Note 31.
Listed below are the material accounting policies of significance to Novartis or, in cases where IFRS Accounting Standards provide alternatives, the option adopted by Novartis.
Scope of consolidation
The consolidated financial statements include all entities, including structured entities, over which Novartis AG, Basel, Switzerland, directly or indirectly has control (generally as a result of owning more than 50% of the entity’s voting interest). Consolidated entities are also referred to as “subsidiaries.”
In cases where Novartis does not fully own a subsidiary, it has elected to value any remaining outstanding non-controlling interest at the time of acquiring control of the subsidiary at its proportionate share of the fair value of the net identified assets.
Investments in associated companies (generally defined as investments in entities in which Novartis holds between 20% and 50% of voting shares or over which it otherwise has significant influence) and joint ventures are accounted for using the equity method, except for selected venture fund investments for which the Company has elected to apply the method of fair value through the consolidated income statement.
Foreign currencies
The consolidated financial statements of Novartis are presented in US dollars (USD). The functional currency of a subsidiary is generally the local currency of that entity. The functional currency used for the reporting of certain Swiss and foreign finance entities is USD instead of their respective local currencies. This reflects the fact that the cash flows and transactions of these entities are primarily denominated in this currency.
For subsidiaries using a functional currency other than USD the subsidiary’s results, financial position and cash flows are translated into USD using the following exchange rates:
• Income, expense and cash flows for each month using the average exchange rate, with the US dollar values for each month being aggregated during the year
• Balance sheet using year-end exchange rates
• Resulting exchange rate differences are recognized in other comprehensive income
Distribution of Sandoz Group AG to Novartis AG shareholders
At the Extraordinary General Meeting (EGM) of Novartis AG shareholders, held on September 15, 2023, the Novartis AG shareholders approved a special distribution by way of a dividend in kind to effect the spin-off of Sandoz Group AG.
The September 15, 2023, shareholder approval for the spin-off required the Sandoz Division and selected portions of corporate activities attributable to Sandoz’s business, as well as certain expenses related to the spin-off (the “Sandoz business”) to be reported as discontinued operations.
The shareholder approval on September 15, 2023, for the spin-off the Sandoz business, required the recognition of a distribution liability at the fair value of the Sandoz business. Novartis policy is to measure the distribution liability at the fair value of the Sandoz business net assets taken as a whole. The distribution liability was recognized through a reduction in retained earnings. It was required to be adjusted at each balance sheet date for changes in its estimated fair value, up to the date of the distribution to shareholders through retained earnings. Any resulting impairment of the business assets to be distributed would have been recognized in the consolidated income statements in “Other expense” of discontinued operations, at the date of initial recognition of the distribution liability or at subsequent dates resulting from changes of the distribution liability valuation.
At the October 4, 2023, distribution settlement date, the resulting gain, which is measured as the excess amount of the distribution liability over the then-carrying value of the net assets of the business distributed, was recognized on the line “Gain on distribution of Sandoz Group AG to Novartis AG shareholders” within the income statement of discontinued operations.
The recognition of the distribution liability required the use of valuation techniques for the purposes of impairment testing of the Sandoz business’ assets to be distributed and for the measurement of the fair value of the distribution liability. These valuations required the use of management assumptions and estimates related to the Sandoz business’ future cash flows, market multiples, opening share price of Sandoz Group AG on the first day of trading its shares on the SIX Swiss Exchange, to estimate day one market value, and control premiums to apply in estimating the Sandoz business fair value. These fair value measurements are classified as “Level 3” in the fair value hierarchy. The section “—Goodwill and intangible assets other than goodwill” in this Note 1 provides additional information on key assumptions that are highly sensitive in the estimation of fair values using valuation techniques.
Transaction costs that are directly attributable to the Distribution (spin-off) of the Sandoz business to Novartis AG shareholders by way of a dividend in kind, and that would otherwise have been avoided, were accounted for as a deduction from equity (within retained earnings). Prior to the recognition of the distribution liability, these costs were recorded as prepaid expenses in the consolidated balance sheet.
For additional disclosures, refer to “Note 2. Significant transactions—Significant transactions in 2023—Completion of the spin-off of the Sandoz business through a dividend in kind distribution to Novartis AG shareholders,” and “Note 31. Discontinued operations.”
Acquisition of assets and businesses
Assets separately acquired are recorded at cost, which includes the purchase price and any directly attributable costs for bringing the asset into the condition to operate as intended. Expected costs for obligations to dismantle and remove property, plant and equipment and restore the site when it is no longer used are included in their cost.
Acquired businesses are accounted for by applying the acquisition method, unless the optional concentration test is applied. The optional concentration test allows for an election on a transaction-by-transaction basis to account for the acquired business as an asset separately acquired when substantially all of the fair value of the gross assets acquired is concentrated in a single identifiable asset or group of similar identifiable assets.
The acquisition method requires that the assets acquired and liabilities assumed be recorded at their respective fair values on the date the Company obtains control. The excess of the fair value of the total purchase consideration transferred over the fair value of the acquired assets and assumed liabilities is recognized as goodwill. The valuations are based on information available at the acquisition date. Acquisition related costs are expensed as incurred.
The application of the acquisition method requires certain estimates and assumptions to be made, especially concerning the fair values of the acquired intangible assets, inventories, property, plant and equipment and the liabilities assumed at the acquisition date, and the useful lives of the intangible assets and property, plant and equipment. Estimates of fair value require the use of valuation techniques. These valuations require the use of management assumptions and estimates, including the value of comparable assets in the market, amount and timing of future cash flows, outcomes and costs of research and development activities, probability of obtaining regulatory approval, long-term sales forecasts, actions of competitors, discount rates and terminal growth rates. The section “—Impairment of goodwill and intangible assets” in this Note 1 provides additional information on key assumptions that are highly sensitive in the estimation of fair values using valuation techniques.
Goodwill and intangible assets other than goodwill
Goodwill arises on applying the acquisition method on the acquisition of a business and is the excess of the fair value of the consideration transferred to acquire the business over the underlying fair value of the net identified assets acquired. Goodwill is allocated to groups of cash-generating units (CGUs) that is expected to benefit from the synergies of the combination, which are usually represented by the operating segment. Goodwill is
tested for impairment annually at the level of this group of CGUs, and any impairment charges are recorded under “Other expense” in the consolidated income statement.
Purchased intangible assets other than goodwill are initially recorded at cost. Intangible assets that have been acquired through a business combination are initially recorded at fair value using the acquisition method of accounting.
Intangible assets available for use with a definitive useful life (which includes the categories Currently marketed products and Other intangible assets) are amortized on a straight-line basis and evaluated for potential impairment whenever facts and circumstances indicate that their carrying value may not be recoverable.
Acquired research and development intangible assets that have not yet obtained marketing approval are recognized as in-process research and development (IPR&D). IPR&D is not amortized as it is not yet available for use. It is evaluated for potential impairment on an annual basis or when facts and circumstances warrant. Once a project included in IPR&D has received marketing approval from a regulatory authority, it is transferred to the “Currently marketed products” category of intangible assets.
An asset, a CGU or a grouping of CGUs is considered impaired when its balance sheet carrying amount exceeds its estimated recoverable amount, which is defined as the higher of its fair value less costs of disposal and its value in use. Usually, Novartis applies the fair value less costs of disposal method for its impairment assessment. In most cases, no directly observable market inputs are available to measure the fair value less costs of disposal. An estimate is therefore derived indirectly and is based on net present value techniques utilizing post-tax cash flows and discount rates. In the limited cases where the value-in-use method would be applied, net present value techniques would be applied using pre-tax cash flows and discount rates.
Fair value less costs of disposal reflects estimates of assumptions that market participants would be expected to use when pricing the asset or CGU, and for this purpose, management considers the range of economic conditions that are expected to exist over the remaining useful life of the asset. These valuations are classified as “Level 3” in the fair value hierarchy.
The estimates used in calculating the net present values are highly sensitive and depend on assumptions specific to the nature of the Company’s activities with regard to:
• Amount and timing of projected future cash flows
• Sales forecasts
• Actions of competitors (launch of competing products, marketing initiatives, etc.)
• Sales erosion rates after the end of patent or other intellectual property rights protection, and timing of the entry of generic competition
• Outcome of research and development activities (compound efficacy, results of clinical trials, etc.)
• Amount and timing of projected costs to develop IPR&D into commercially viable products
• Profit margins
• Probability of obtaining regulatory approval
• Future tax rate
• Appropriate terminal growth rate
• Appropriate discount rate
Generally, for intangible assets with a definite useful life, Novartis uses cash flow projections for the whole useful life of these assets. For goodwill, Novartis generally utilizes cash flow projections for a three-year period based on management forecasts, with a terminal value based on cash flow projections usually in line with inflation rates for later periods.
Probability-weighted scenarios are typically used.
Discount rates used consider the Company’s estimated weighted average cost of capital, adjusted for specific asset, country and currency risks associated with cash flow projections, to approximate the discount rate that market participants would use to value the asset.
Due to the above factors, actual cash flows and values could vary significantly from forecasted future cash flows and related values derived using discounting techniques.
Cash and cash equivalents
Cash and cash equivalents include highly liquid investments with original maturities of three months or less, which are readily convertible to known amounts of cash. Bank overdrafts are presented within current financial debts on the consolidated balance sheet.
Marketable securities and non-current financial assets
Marketable securities are financial assets held for short-term purposes that are principally traded in liquid markets and are classified within current assets on the consolidated balance sheet. The financial impacts related to these financial assets are recorded in “Other financial income and expense” in the consolidated income statement. Non-current financial assets held for long-term strategic purposes are classified within non-current assets on the consolidated balance sheet. The financial impacts related to these financial assets are recorded in “Other income” and “Other expense” in the consolidated income statement.
Marketable securities and non-current financial assets are initially recorded at fair value on their trade date, which is different from the settlement date when the transaction is ultimately effected. Quoted securities are remeasured at each reporting date to fair value based on current market prices. If the market for a financial asset is not active or no market is available, fair values are established using valuation techniques. The majority of non-quoted investments are initially valued at fair value through the purchase price established between a willing buyer and seller. Non-quoted investments are subsequently adjusted based on values derived from discounted cash flow analysis or other pricing models. These investment values are classified as “Level 3” in the fair value hierarchy.
The Company classifies and accounts for its marketable securities and non-current financial assets in the following categories:
• Debt securities are valued at fair value through other comprehensive income with subsequent recycling into the consolidated income statement, as they meet both the “solely payment of principal and interest” and the business model criteria. Unrealized gains and losses, except exchange gains and losses, are recorded as a fair value adjustment in the consolidated statement of comprehensive income. They are recognized in the consolidated income statement when the debt instrument is sold, at which time the gain is transferred to “Other financial income and expense.” Exchange gains and losses related to debt instruments are immediately recognized in the consolidated income statement in “Other financial income and expense.”
• Fund investments and equity securities of the Novartis Venture Fund are valued at fair value through profit and loss (FVPL). Unrealized gains and losses, including exchange gains and losses, are recognized in the consolidated income statement in “Other income” for gains and “Other expense” for losses.
• Equity securities held as strategic investments, typically held outside of the Novartis Venture Fund, are generally designated at the date of acquisition as financial assets valued at fair value through other comprehensive income with no subsequent recycling through profit and loss. Unrealized gains and losses, including exchange gains and losses, are recorded as a fair value adjustment in the consolidated statement of comprehensive income. They are reclassified to retained earnings when the equity security is sold. If these equity securities are not designated at the date of acquisition as financial assets valued at fair value through other comprehensive income, they are valued at FVPL, as described above.
• Other non-current financial assets, such as loans and long-term receivables from customers, advances and other deposits, are valued at amortized cost, which reflects the time value of money less any allowances for expected credit losses.
The Company assesses on a forward-looking basis the expected credit losses associated with its debt securities valued at fair value through other comprehensive income. Impairments on debt securities are recorded in “Other financial income and expense.”
For other financial assets valued at amortized cost, impairments, which are based on their expected credit losses, and exchange rate losses are included in “Other expense” in the consolidated income statement. Exchange rate gains and interest income, using the effective interest rate method, are included in “Other income” or “Other financial income” in the consolidated income statement, depending on the nature of the item.
Derivative financial instruments
Derivative financial instruments are initially recognized in the balance sheet at fair value and are remeasured to their current fair value at the end of each subsequent reporting period. The valuation of a forward exchange rate contract is based on the discounted cash flow model, using interest rate curves and forward rates at the reporting date as observable inputs.
Options are valued based on a modified Black-Scholes model using volatility and exercise prices as major observable inputs.
The Company enters into certain derivative financial instruments for the purpose of hedging to reduce the volatility in the Company’s performance due to the exposure to various business-related risks. The risk mitigation is obtained because the derivative’s value or cash flows are expected, wholly or partly, to offset changes in the value or cash flows of the recognized assets or liabilities. The overall strategy is aiming to mitigate the currency and interest rate risk of positions that are contractually agreed, and to partially mitigate the exposure risk of selected anticipated transactions.
Certain derivative financial instruments meet the criteria for hedge accounting treatment. A prerequisite for obtaining this accounting-hedge relationship is extensive documentation on inception and proving on a regular basis that the economic hedge is effective for accounting purposes. Other derivative financial instruments do not meet the criteria to qualify for hedge accounting or are not designated in a hedge relationship. Changes in the fair value of these derivative instruments are recognized immediately in “Other financial income and expense” in the consolidated income statement.
In addition, the Company has designated certain long-term debt components as hedges of the translation risk arising on certain net investments in foreign operations. On consolidation, foreign currency differences arising on long-term debt designated as net investment hedges of a foreign operation are recognized in other comprehensive income and accumulated in currency translation effects, to the extent that the hedge is effective. The foreign currency differences arising from hedge ineffectiveness are recognized in the income statement in “Other financial income and expense.”
When a hedged net investment is disposed of, the proportionate portion of the cumulative amount recognized in equity in relation to the hedged net investment is transferred to the consolidated income statement as an adjustment to the gain or loss on disposal.
Inventories
Inventory is valued at the lower of acquisition or production cost determined on a first-in, first-out basis and net realizable value. This value is used for the “Cost of goods sold” in the consolidated income statement. Unsaleable inventory is fully written off in the consolidated income statement under “Cost of goods sold.”
Trade receivables
Trade receivables are initially recognized at their invoiced amounts, including any related sales taxes less adjustments for estimated revenue deductions such as rebates, chargebacks and cash discounts.
Provisions for doubtful trade receivables are established using a forward-looking expected credit loss model (ECL). Charges for doubtful trade receivables are recorded as marketing and selling costs recognized in
the consolidated income statement within “Selling, general and administration” expenses.
Legal and environmental liabilities
Novartis and its subsidiaries are subject to contingencies arising in the ordinary course of business, such as patent litigation, environmental remediation liabilities and other product-related and commercial litigation, and governmental investigations and proceedings. A provision is recorded when there is a probable outflow of resources for which a reliable estimate can be made of the outcome of the legal or other disputes against the subsidiary.
Contingent consideration
In the acquisition or divestment of a business, it is necessary to recognize contingent future amounts due to previous owners, representing contractually defined potential amounts as a liability or an asset. Usually for Novartis, these are linked to milestone or royalty payments related to certain assets and are recognized as a financial liability or financial asset at fair value, which is then remeasured at each subsequent reporting date. These estimations typically depend on factors such as technical milestones or market performance, and are adjusted for the probability of their likelihood of payment, and are appropriately discounted to reflect the impact of time.
Changes in the fair value of contingent consideration liabilities in subsequent periods are recognized in the consolidated income statement in “Cost of goods sold” for currently marketed products and in “Research and development” for IPR&D. Changes in contingent consideration assets are recognized in “Other income” or “Other expense,” depending on their nature.
The effect of unwinding the discount over time is recognized for contingent consideration liabilities in “Interest expense” and for contingent consideration assets as interest income recognized in the consolidated income statement within “Other financial income and expense.”
Defined benefit pension plans and other post-employment benefits
The liability in respect of defined benefit pension plans and other post-employment benefits is the defined benefit obligation calculated annually by independent actuaries using the projected unit credit method. The current service cost for defined benefit pension plans and other post-employment benefit plans is included in the personnel expenses of the various functions in which employees are employed, and the net interest on the net defined benefit liability or asset is recognized as “Other expense” or “Other income.”
Revenue recognition
Revenue on the sale of Novartis products and services, which is recorded as “Net sales to third parties” in the consolidated income statement, is recognized when a contractual promise to a customer (performance obligation) has been fulfilled by transferring control over the promised goods and services to the customer, substantially all of which is at the point in time of shipment to or receipt of the products by the customer or when the services are performed. If contracts contain customer acceptance provisions, revenue is recognized upon the satisfaction of the acceptance criteria. If a contract contains more than one performance obligation, the consideration is allocated based on the standalone selling price of each performance obligation. The amount of revenue recognized is based on the consideration Novartis expects to receive in exchange for its goods and services, when it is highly probable that a significant reversal will not occur.
The consideration Novartis receives in exchange for its goods or services may be fixed or variable. Variable consideration is recognized when it is highly probable that a significant reversal will not occur. The most common elements of variable consideration are listed below.
• Rebates and discounts granted to wholesalers, retailers, government agencies (including US Medicaid and US Federal Medicare programs), government supported healthcare systems, private health systems, pharmacy benefit managers, managed healthcare organizations, purchasing organizations and other direct and indirect customers, as well as chargebacks are provisioned and recorded as revenue deductions at the time the related revenues are recorded, or when the incentives are offered. The provision represents estimates of the related obligations, requiring the use of judgment when estimating the effect of these revenue deductions. These rebates and discounts, applied using provision rates, are estimated based on the terms and conditions in the individual government agencies, states, plans and customer agreements (which may be subject to challenge or change in interpretative guidance by government authorities and customers), historical experience, product sales and growth rate, population growth, product pricing including inflation impacts, the mix of contracts and products, the level of inventory in the distribution channel, regulations, contracts, channels and payers, as appropriate to the individual rebate and discount arrangements. These rebate provisions are adjusted based on established processes and experiences, for example from filing data with individual government agencies, states, and plans. There is often a time lag between recording of revenue deductions and the final accounting for them.
• Refunds granted to healthcare providers under innovative pay-for-performance agreements (i.e. outcome based arrangements) are provisioned and recorded as a revenue deduction at the time the related sales are recorded. The provision represents estimates of the related obligations, requiring the use of judgment when estimating the effect of these revenue deductions. They are calculated on the basis of historical experience and clinical data available for the product, as well as specific terms of the individual agreements.
In cases where historical experience and clinical data are not sufficient for a reliable estimation of the outcome, revenue recognition is deferred until the uncertainty is resolved, until such history is available or the period when the refund right has expired. The provisions for revenue deductions under the innovative pay-for-performance agreements are adjusted periodically based on established processes and actual experience, including the products actual outcomes achieved compared with the anticipated predefined targets.
• Cash discounts are offered to customers to encourage prompt payment and are provisioned and recorded as revenue deductions at the time the related sales are recorded.
• Sales returns provisions are recognized and recorded as revenue deductions when there is historical experience of Novartis agreeing to customer returns and Novartis can reasonably estimate expected future returns. The provision represents estimates of the related obligations, requiring the use of judgment when estimating the effect of these revenue deductions. In doing so, the estimated rate of return is applied, determined on the basis of historical experience of customer returns and considering any other relevant factors. This is applied to the amounts invoiced, also considering the amount of returned products to be destroyed versus products that can be placed back in inventory for resale. Where shipments are made on a resale or return basis, without sufficient historical experience for estimating sales returns, revenue is only recorded when there is evidence of consumption or when the right of return has expired. The provisions for sales returns are adjusted periodically based on established processes and actual experience,
Net sales to third parties and provisions for revenue deductions are adjusted periodically to reflect experience and to reflect actual amounts as rebates, refunds, discounts and returns are processed. There is often a time lag between recording of revenue deductions and the final accounting for them. The provision represents estimates of the related obligations, requiring the use of judgment when estimating the effect of these revenue deductions.
“Other revenue” includes income from profit-sharing arrangements with our collaboration partners, and royalty and milestone income from the out-licensing of intellectual property when Novartis retains an interest in the intellectual property through a license. Royalty income earned from a license is recognized when the underlying sales have occurred. Milestone income is recognized at the point in time when it is highly probable that the relevant milestone event criteria are met, and the risk of reversal of revenue recognition is remote. “Other revenue” also includes revenue from activities such as manufacturing or other services rendered, to the extent such revenue is not recorded under net sales, and is recognized when control transfers to the third party and our performance obligations are satisfied.
Research and development
Internal research and development (R&D) costs are fully charged to “Research and development” in the consolidated income statement in the period in which they are incurred. The Company considers that regulatory and other uncertainties inherent in the development of new products preclude the capitalization of internal development expenses as an intangible asset until marketing approval from a regulatory authority is obtained in a major market such as the United States, the European Union, Switzerland or Japan.
Payments made to third parties, such as contract research and development organizations in compensation for subcontracted R&D, that are deemed not to transfer intellectual property to Novartis are expensed as internal R&D expenses in the period in which they are incurred. Such payments are only capitalized if they meet the criteria for recognition of an internally generated intangible asset, usually when marketing approval has been received from a regulatory authority in a major market.
Payments made to third parties to in-license or acquire intellectual property rights, compounds and products, including initial upfront and subsequent milestone payments, are capitalized, as are payments for other assets, such as technologies to be used in R&D activities. If additional payments are made to the originator company to continue performing R&D activities, an evaluation is made as to the nature of the payments. Such additional payments will be expensed if they are deemed to be compensation for subcontracted R&D services not resulting in an additional transfer of intellectual property rights to Novartis. Such additional payments will be capitalized if they are deemed to be compensation for the transfer to Novartis of additional intellectual property developed at the risk of the originator company. Subsequent internal R&D costs in relation to IPR&D and other assets are expensed, since the technical feasibility of the internal R&D activity can only be demonstrated by the receipt of marketing approval for a related product from a regulatory authority in a major market.
Costs for post-approval studies performed to support the continued registration of a marketed product are recognized as marketing expenses. Costs for activities that are required by regulatory authorities as a condition for obtaining marketing approval in a major market are capitalized and recognized as currently marketed products.
Inventory produced ahead of regulatory approval is fully provisioned, and the charge is included in “Other expense” in the consolidated income statement, as its ultimate use cannot be assured. If this inventory can subsequently be sold, the provision is released to “Other income” in the consolidated income statement, either on approval by the appropriate regulatory authority or, exceptionally in Europe, on recommendation by the Committee for Medicinal Products for Human Use (CHMP), if approval is virtually certain.
Share-based compensation
Vested Novartis shares and American Depositary Receipts (ADRs) that are granted as compensation are valued at their market value on the grant date and are immediately expensed in the consolidated income statement.
The fair values of unvested restricted shares (RSs), restricted share units (RSUs) and performance share units (PSUs) in Novartis shares and ADRs granted to employees as compensation are recognized as an expense over the related vesting period. The expense recorded in the consolidated income statement is included in the personnel expenses of the various functions in which the employees are employed.
Unvested restricted shares, restricted ADRs and RSUs are only conditional on the provision of services by the plan participant during the vesting period. They are valued at fair value on the grant date. As RSUs do not entitle the holder to dividends, the fair value is based on the Novartis share price at the grant date adjusted for the net present value of the dividends expected to be paid during the holding period. The fair value of these grants, after making adjustments for assumptions related to forfeiture during the vesting period, is expensed on a straight-line basis over the respective vesting period.
PSUs are subject to the achievement of certain performance criteria based on Novartis internal performance metrics and variables that can be observed in the market, which for Novartis plans is the Novartis total shareholder return (TSR) relative to a specific peer group of companies over the vesting period and require plan participants to provide services during this period The expense is recognized in the consolidated income statement on a straight-line basis over the vesting period, and is determined based on a bifurcation into the components based on the performance criteria related to Novartis internal performance metrics and TSR. The number of equity instruments that finally vest is determined at the vesting date. The following paragraphs provide an overview of the accounting policies for the determination of the components of the PSU share-based compensation plan expense.
The portion of the PSUs expense that is subject to performance criteria based on Novartis internal performance metrics over the vesting period is determined based on assumptions concerning the expected performance against the internal performance metrics throughout the vesting period. The assumptions are based on the Company’s targets for those performance metrics, and the expected forfeitures due to plan participants not meeting their service conditions. The assumptions are periodically adjusted over the vesting period. For this portion of the PSUs expense, any change in estimates for past services is recorded immediately as an expense or income in the consolidated income statement, and amounts for the remaining vesting period are expensed on a straight-line basis. As a result, at the end of the vesting period, the expense during the entire vesting period represents the amount that will finally vest.
The portion of the PSUs expense that is subject to performance criteria based on Novartis TSR relative to a specific peer group of companies over the vesting period is determined based on the total fair value of the grant over the vesting period. IFRS Accounting Standards require that these variables that can be observed in the market are taken into account in determining the fair value of the PSUs at the grant date. Novartis determined the fair value of these PSUs at the date of grant using a Monte Carlo simulation model. For this portion of the PSU expense, adjustments to expense recognized in the consolidated income statement are only made if a plan participant does not fulfill the service conditions.
Measuring the fair values of PSUs granted that include TSR performance criteria requires the use of estimates. The Monte Carlo simulation used to determine the fair value of the PSUs TSR performance criteria requires the probability of factors related to uncertain future events; the term of the award; the grant price of underlying shares or ADRs; expected volatilities; the expected correlation matrix of the underlying equity instruments with those of the peer group of companies; and the risk-free interest rate as input parameters.
If a plan participant leaves Novartis for reasons other than retirement, disability or death, then unvested restricted shares, restricted ADRs, RSUs and PSUs are forfeited, unless determined otherwise by the provision of the plan rules or by the Compensation Committee of the Novartis Board of Directors, for example, in connection with a reorganization or divestment.
Income taxes
Income taxes comprise current income taxes and deferred income taxes and are recognized in the same periods as the revenues and expenses to which they relate. Income taxes include interest and penalties incurred during the period, insofar as they are considered an income tax. Income taxes related to items recognized directly to other comprehensive income or to equity are recognized together with the corresponding item, to which the income tax is attributable, directly in other comprehensive income or in equity.
Deferred income taxes are determined using the comprehensive liability method and are calculated on the temporary differences that arise between the tax base of an asset or liability and its carrying value for financial reporting purposes, except for those temporary differences related to investments in subsidiaries and associated companies, where the timing of their reversal can be controlled and it is probable that the difference will not reverse in the foreseeable future. Since the retained earnings are reinvested, withholding or other taxes on eventual distribution of a subsidiary’s retained earnings are only recognized when a dividend is declared or has been planned. Furthermore, deferred income taxes are recognized for the net tax effects of net operating loss carryforwards and tax credits. The Company applies the IFRS Accounting Standards exception to not recognize or disclose information about deferred tax assets and deferred tax liabilities related to countries that have enacted tax legislation that comply with the Organization for Economic Cooperation and Development (OECD) Pillar Two income taxes.
The carrying amount of deferred tax assets is reduced to the extent that it is not probable that sufficient taxable profits will be available to enable all or part
of the asset to be recovered. In evaluating our ability to recover our deferred tax assets in the jurisdiction from which they arise, we consider all available positive and negative evidence, including scheduled reversals of deferred tax liabilities, projected future taxable income, tax-planning strategies, and results of recent operations.
The estimated amounts for current and deferred tax assets or liabilities, including amounts related to any uncertain tax positions, are based on applicable tax law and regulations in the various tax jurisdictions, in which the Company operates, which are subject to interpretations based on currently known facts and circumstances.
Tax returns are based on an interpretation of tax laws and regulations, and reflect estimates based on these judgments and interpretations. The tax returns are subject to examination by the competent taxing authorities, which may result in an assessment being made requiring payments of additional tax, interest or penalties.
The calculation of income tax assets and liabilities involves dealing with uncertainties in the application of complex tax laws and regulations in a multitude of jurisdictions across our global operations. As a result, inherent uncertainties exist in the estimates of the tax positions. Tax liabilities for uncertain tax provisions are recognized on the consolidated balance sheets within current income tax liabilities.
Impact of new IFRS Accounting Standards, amendments and interpretations in 2023
No new IFRS Accounting Standards were adopted by the Company in 2023. In addition, new IFRS Accounting Standards amendments or interpretations that became effective in 2023 did not have a material impact on the Company’s consolidated financial statements.
Based on the Company’s assessment, there are no IFRS Accounting Standards, amendments or interpretations not yet effective in 2023 that would be expected to have a material impact on the Company’s consolidated financial statements.
Impact of new IFRS Accounting Standards, amendments and interpretations in 2022
No new IFRS Accounting Standards were adopted by the Company in 2022. In addition, new IFRS Accounting Standards amendments or interpretations that became effective in 2022 did not have a material impact on the Company’s consolidated financial statements.
Based on the Company’s assessment, there were no IFRS Accounting Standards, amendments or interpretations not yet effective in 2022 that would have been expected to have a material impact on the Company’s consolidated financial statements.
Impact of adopting significant new IFRS Accounting Standards in 2021
The following new IFRS Accounting Standard was adopted by Novartis from January 1, 2021:
Interest Rate Benchmark Reform – Phase 2, Amendments to IFRS 9, IAS 39, IFRS 7, IFRS 4 and IFRS 16 (Interest Benchmark Reform Amendments)
Interest Benchmark Reform Amendments became effective from January 1, 2021. These amendments address issues that might affect financial reporting when an existing interest rate benchmark (i.e. Interbank offered rate – IBOR) is replaced with an alternative benchmark interest rate. The effects of interest rate benchmark reform on the Company’s financial instruments and risk management strategies did not have a material impact on the Company’s consolidated financial statements.
2. Significant transactions
The Company applied the acquisition method of accounting for businesses acquired, and did not elect to apply the optional concentration test to account for acquired business as an asset separately acquired.
Significant transactions in 2023
Completion of the spin-off of the Sandoz business through a dividend in kind distribution to Novartis AG shareholders
On July 18, 2023, Novartis announced that its Board of Directors had unanimously endorsed the proposed separation of the Sandoz business to create an independent company by way of a spin-off and to seek shareholder approval for the spin-off of the Sandoz business into a separately traded standalone company, following the complete structural separation of the Sandoz business into a standalone company (the Sandoz business or Sandoz Group AG) and subject to the satisfaction of certain conditions and Novartis AG shareholders’ approval.
At the EGM held on September 15, 2023, Novartis AG shareholders approved a special distribution by way of a dividend in kind to effect the spin-off of Sandoz Group AG, subject to the completion of certain conditions precedent to the distribution. Upon shareholder approval, the Sandoz business was reported as discontinued operations and the distribution liability was recognized at its fair value, which exceeded the carrying value of the Sandoz business net assets.
The conditions precedent to the spin-off were met and on October 3, 2023 the spin-off of the Sandoz business was effected by way of a distribution of a dividend in kind of Sandoz Group AG shares to Novartis AG shareholders and American Depositary Receipt (ADR) holders (the Distribution). Through the Distribution, each Novartis AG shareholder received 1 Sandoz Group AG share for every 5 Novartis AG shares and each Novartis ADR holder received 1 Sandoz ADR for every 5 Novartis ADR that they held at the close of business on October 3, 2023. As of October 4, 2023, the shares of Sandoz Group AG have been listed on the SIX Swiss Exchange (SIX) under the stock symbol “SDZ”.
On September 18, 2023, the Sandoz business entered into financing arrangements with a group of banks under which on September 28, 2023, it borrowed a total amount of USD 3.3 billion. These borrowings consisted of a bridge loan in EUR (EUR 2.4 billion) and term loans in EUR (EUR 0.2 billion) and USD (USD 0.5 billion). In addition, the Sandoz business borrowed approximately USD 0.4 billion under a number of local bilateral facilities in different countries. This resulted in a total gross debt of USD 3.7 billion. These outstanding borrowings of the Sandoz business legal entities were recognized in the September 30, 2023 consolidated balance sheet within Liabilities related to discontinued operations and within financing activities cash flows from discontinued operations. Prior to the Distribution on October 3, 2023, Sandoz business legal entities paid approximately USD 3.3 billion in cash to Novartis and its affiliates through a series of intercompany transactions.
At the Distribution date on October 3, 2023, the dividend in kind distribution liability to effect the Distribution (spin-off) of the Sandoz business amounted to USD 14.0 billion, measured by reference to the October 4, 2023 opening Sandoz Group AG share price and applying a control premium. The dividend in kind distribution liability was recorded as a reduction to equity (retained earnings) and remained in excess of the then carrying value of the Sandoz business net assets, which amounted to USD 8.6 billion (see Note 31).
Certain consolidated foundations own Novartis AG dividend-bearing shares that restricts their availability for use by Novartis. These Novartis AG shares are accounted for as treasury shares. Through the Distribution, these foundations received Sandoz Group AG shares representing an approximate 4.31% equity interest in Sandoz Group AG. Upon the loss of control of Sandoz Group AG through the Distribution on October 3, 2023, the financial investment in Sandoz Group AG was recognized at its initial fair value based on the opening traded share price of Sandoz Group AG on October 4, 2023 (a Level 1 hierarchy valuation). At initial recognition, on October 4, 2023, the Sandoz Group AG financial investment had a fair value of USD 0.5 billion, and was reported in the fourth quarter of 2023 on the consolidated balance sheet as a financial asset. Management has designated this investment at fair value through other comprehensive income.
The total non-taxable, non-cash gain recognized at the Distribution date of the spin-off of the Sandoz business amounted to USD 5.9 billion, which consists of:
(USD millions)
| | Oct 3,
2023 | |
|
Net assets derecognized 1 | | -8 647 | |
|
Derecognition of distribution liability | | 13 962 | |
|
Difference between net assets and distribution liability | | 5 315 | |
|
Recognition of Sandoz Group AG shares obtained through consolidated foundations | | 492 | |
|
Currency translation gains recycled into the consolidated income statement | | 357 | |
|
Transaction costs and other items recognized in the consolidated income statement | | -304 | |
|
Gain on distribution of Sandoz Group AG to Novartis AG shareholders | | 5 860 | |
|
|
1 See Note 31 for additional information. |
For additional disclosures on discontinued operations, refer to Note 31.
Acquisition of DTx Pharma Inc.
In the second quarter of 2023, Novartis entered into an agreement to acquire all outstanding shares of DTx Pharma Inc. (DTx), a San-Diego, California US based, pre-clinical stage biotechnology company focused on leveraging its proprietary FALCON platform to develop siRNA therapies for neuroscience indications. DTx’s lead program, DTx-1252 targets the root cause of CMT1A—the overexpression of PMP22, a protein that causes the myelin sheath that supports and insulates nerves in the peripheral nervous system to function abnormally. The
transaction also includes two additional pre-clinical programs for other neuroscience indications. The transaction closed on July 14, 2023.
The purchase price consisted of a cash payment of USD 0.6 billion and potential additional milestones of up to USD 0.5 billion, which the DTx shareholders are eligible to receive upon the achievement of specified milestones.
The fair value of the total purchase consideration was USD 0.6 billion. The amount consisted of a cash payment of USD 0.6 billion and the fair value of contingent consideration of USD 30 million, which DTx shareholders are eligible to receive upon the achievement of specified milestones. The purchase price allocation resulted in net identifiable assets of USD 0.4 billion, consisting primarily of IPR&D intangible assets of USD 0.4 billion, cash of USD 0.1 billion and net deferred tax liabilities of USD 0.1 billion. Goodwill amounted to USD 0.2 billion.
The results of operations since the date of acquisition were not material.
Acquisition of Chinook Therapeutics
On June 12, 2023, Novartis entered into an agreement to acquire all outstanding shares of Chinook Therapeutics, Inc. (Chinook Therapeutics), a Seattle, Washington based clinical stage biopharmaceutical company with two late-stage medicines in development for rare, severe chronic kidney diseases. The acquisition closed on August 11, 2023.
The purchase price consisted of a cash payment of USD 3.2 billion and potential additional payments of up to USD 0.3 billion, which Chinook Therapeutics shareholders are eligible to receive upon the achievement of specified milestones.
The fair value of the total purchase consideration was USD 3.3 billion. The amount consisted of an upfront cash payment of USD 3.2 billion and the fair value of contingent consideration of USD 0.1 billion, which Chinook Therapeutics shareholders are eligible to receive upon achievement of specified milestones. The purchase price allocation resulted in net identifiable assets of USD 2.4 billion, consisting primarily of IPR&D intangible assets of USD 2.5 billion, net deferred tax liabilities of USD 0.4 billion and other net assets of USD 0.3 billion, including cash of USD 0.1 billion. Goodwill amounted to USD 0.9 billion.
The results of operations since the date of acquisition were not material.
Significant transactions in 2022
Acquisition of Gyroscope Therapeutics Holdings plc
On December 22, 2021, Novartis entered into an agreement to acquire all outstanding shares of Gyroscope Therapeutics Holdings plc (Gyroscope), a UK-based ocular gene therapy company. Gyroscope focuses on the discovery and development of gene therapy treatments for retinal indications. The purchase price consisted of a cash payment of USD 0.8 billion, subject to certain customary purchase price adjustments, and potential additional milestone payments of up to USD 0.7 billion, which Gyroscope shareholders are eligible to receive upon the achievement of specified milestones. The acquisition closed on February 17, 2022.
The fair value of the total purchase consideration was USD 1.0 billion. The amount consisted of an upfront cash payment of USD 0.8 billion (including customary purchase price adjustments) and the fair value of contingent consideration of USD 0.2 billion, which Gyroscope shareholders are eligible to receive upon the achievement of specified milestones. The purchase price allocation resulted in net identifiable assets of USD 0.9 billion, consisting primarily of IPR&D intangible assets of USD 1.1 billion and net deferred tax liabilities of USD 0.2 billion. Goodwill amounted to USD 0.1 billion.
The 2022 results of operations since the date of acquisition were not material.
Significant transactions in 2021
Divestment of the investment in Roche Holding AG
On November 3, 2021, Novartis entered into a Share Repurchase Agreement with Roche Holding AG under which Novartis agreed to sell 53.3 million (approximately 33.3%) bearer shares of Roche Holding AG voting shares in a bilateral transaction to Roche Holding AG for a total consideration of USD 20.7 billion. As a result, Novartis discontinued the use of equity method accounting starting from November 3, 2021.
The transaction closed on December 6, 2021. Novartis realized a gain of USD 14.6 billion, recorded in income from associated companies.
3. Operating segment
Prior to the September 15, 2023, shareholders’ approval of the spin-off of the Sandoz business (refer to Note 1 and Note 2 for additional information), the businesses of Novartis were divided operationally on a worldwide basis into two identified reporting segments: Innovative Medicines Division and the Sandoz Division. In addition, we separately reported Corporate activities.
Following the September 15, 2023, shareholders’ approval of the spin-off of the Sandoz business (see Note 1 and Note 2), the Company reported its consolidated financial statements for the current and prior years as “continuing operations” and “discontinued operations” (see Note 1).
Continuing operations include the retained business activities of Novartis, comprising the innovative medicines business (previously the Innovative Medicines Division) and the continuing corporate activities.
Discontinued operations include the Sandoz generic pharmaceuticals and biosimilars business (the Sandoz Division) and certain corporate activities attributable to Sandoz’s business, as well as certain expenses related to the spin-off. Included in 2023 is also the IFRS Accounting Standards non-cash, non-taxable net gain on the Distribution of Sandoz Group AG to Novartis AG shareholders. For further details and disclosures on discontinued operations, refer to Note 1, Note 2 and Note 31.
Effective January 1, 2023, the Sandoz business bio-technology manufacturing services to other companies’ activities and the Coartem brand were transferred to the Novartis continuing operations. The financial information of the Novartis continuing operations and discontinued operations were accordingly adapted in 2023 and prior years, in compliance with IFRS Accounting Standards. This restatement had no impact on the reported financial results and consolidated balance sheet of the total Company.
The Company’s continuing operations is engaged in the research, development, manufacturing, distribution, and commercialization and sale of innovative medicines, with a focus on the core therapeutic areas: cardiovascular, renal and metabolic; immunology; neuroscience; oncology; and established brands.
Following the spin-off of the Sandoz business, on October 3, 2023, Novartis operates as a single global operating segment innovative medicines company that is engaged in the research, development, manufacturing, distribution and commercialization and sale of innovative medicines. The Company’s research, development manufacturing and supply of products and functional activities are managed globally on a vertically integrated basis. Commercial efforts that coordinate marketing, sales and distribution of these products are organized by geographic region, therapeutic area and established brands.
The Executive Committee of Novartis (ECN), chaired by the CEO, is the governance body responsible for allocating resources and assessing the business performance of the operating segment of the Company on a global basis and is the chief operating decision-maker (CODM) for the Company.
The determination of a single operating segment is consistent with the financial information regularly reviewed by the CODM for purposes of assessing performance and allocating resources.
See Note 4 for revenues and geographic information disclosures.
4. Revenues and geographic information
Net sales information
Net sales from continuing operations comprise the following:
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
Net sales to third parties from continuing operations | | 44 635 | | 41 385 | | 41 976 | |
|
Sales to discontinued operations | | 805 | | 821 | | 805 | |
|
Net sales from continuing operations | | 45 440 | | 42 206 | | 42 781 | |
|
Geographic information
The following table shows countries that accounted for more than 5% of net sales from continuing operations or more than 5% of total of selected non-current assets, for the years ended December 31, 2023, 2022 and 2021, and for selected non-current assets for the years ended December 31, 2023 and 2022:
| | Net sales from continuing operations1 | | Total of selected non-current assets2 | |
| | | | | |
| | | | | |
| | | | | |
(USD millions) | | 2023 | | % | | 2022 | | % | | 2021 | | % | | 2023 | | % | | 2022 | | % | |
|
Country | | | | | | | | | | | | | | | | | | | | | |
Switzerland | | 1 308 | | 3 | | 1 036 | | 2 | | 926 | | 2 | | 19 396 | | 32 | | 23 708 | | 32 | |
|
United States | | 17 959 | | 40 | | 15 935 | | 38 | | 14 923 | | 35 | | 34 059 | | 55 | | 35 353 | | 48 | |
|
Germany | | 3 367 | | 7 | | 3 101 | | 7 | | 3 595 | | 8 | | 88 | | | | 2 229 | | 3 | |
|
China | | 3 267 | | 7 | | 2 948 | | 7 | | 2 849 | | 7 | | 547 | | 1 | | 599 | | 1 | |
|
Japan | | 1 924 | | 4 | | 1 883 | | 4 | | 2 259 | | 5 | | 120 | | | | 165 | | | |
|
France | | 1 749 | | 4 | | 1 754 | | 4 | | 1 955 | | 5 | | 3 085 | | 5 | | 3 188 | | 4 | |
|
Other | | 15 866 | | 35 | | 15 549 | | 38 | | 16 274 | | 38 | | 4 269 | | 7 | | 8 241 | | 12 | |
|
Total | | 45 440 | | 100 | | 42 206 | | 100 | | 42 781 | | 100 | | 61 564 | | 100 | | 73 483 | | 100 | |
|
|
1 Net sales from continuing operations by location of customer |
2 Total of property, plant and equipment; right-of-use assets; goodwill; intangible assets; investment in associated companies and other non-current assets excluding post-employment benefit assets |
Net sales from continuing operations by region1
The following table shows net sales from continuing operations by region for the years ended December 31, 2023, 2022 and 2021:
| |
2023
USD m
| |
2022
USD m
| | Change
(2022
to 2023)
USD % | |
2021
USD m
| | Change
(2021
to 2022)
USD % | |
|
US | | 17 959 | | 15 935 | | 13 | | 14 923 | | 7 | |
|
Europe | | 14 997 | | 14 371 | | 4 | | 15 721 | | -9 | |
|
Asia/Africa/Australasia | | 9 308 | | 8 978 | | 4 | | 9 355 | | -4 | |
|
Canada and Latin America | | 3 176 | | 2 922 | | 9 | | 2 782 | | 5 | |
|
Total | | 45 440 | | 42 206 | | 8 | | 42 781 | | -1 | |
|
Of which in established markets | | 33 725 | | 31 386 | | 7 | | 32 183 | | -2 | |
|
Of which in emerging growth markets | | 11 715 | | 10 820 | | 8 | | 10 598 | | 2 | |
|
|
1 Net sales from continuing operations by location of customer. Emerging growth markets comprise all markets other than the established markets of the US, Canada, Western Europe, Japan, Australia and New Zealand. Novartis definition of Western Europe includes Austria, Belgium, Finland, France, Germany, Greece, Iceland, Ireland, Italy, Luxembourg, Malta, The Netherlands, Norway, Portugal, Spain, Sweden, Switzerland, and the United Kingdom. |
|
Information about major customers
The Company’s largest, second-largest and third-largest customers account for approximately 15%, 13% and 8% of net sales from third parties from continuing operations, respectively (2022: 16%, 12% and 8%, respectively; 2021: 14%, 13% and 7%, respectively).
The highest amounts of trade receivables outstanding were for these same three customers and amounted to approximately 17%, 13% and 8%, respectively, of the trade receivables at December 31, 2023 (2022: 16%, 14% and 7%, respectively).
Net sales from continuing operations by core therapeutic area and established brands
| |
2023
USD m
| |
2022
USD m1
| | Change
(2022 to
2023)
USD % | |
2021
USD m1
| | Change
(2021 to
2022)
USD % | |
|
Cardiovascular, renal and metabolic | | | | | | | | | | | |
Entresto | | 6 035 | | 4 644 | | 30 | | 3 548 | | 31 | |
|
Leqvio | | 355 | | 112 | | 217 | | 12 | | nm | |
|
Other | | 1 | | | | nm | | 1 | | nm | |
|
Total cardiovascular, renal and metabolic | | 6 391 | | 4 756 | | 34 | | 3 561 | | 34 | |
|
| | | | | | | | | | | |
Immunology | | | | | | | | | | | |
Cosentyx | | 4 980 | | 4 788 | | 4 | | 4 718 | | 1 | |
|
Xolair 2 | | 1 463 | | 1 365 | | 7 | | 1 428 | | -4 | |
|
Ilaris | | 1 355 | | 1 133 | | 20 | | 1 059 | | 7 | |
|
Other | | | | 1 | | nm | | 1 | | 0 | |
|
Total immunology | | 7 798 | | 7 287 | | 7 | | 7 206 | | 1 | |
|
| | | | | | | | | | | |
Neuroscience | | | | | | | | | | | |
Kesimpta | | 2 171 | | 1 092 | | 99 | | 372 | | 194 | |
|
Zolgensma | | 1 214 | | 1 370 | | -11 | | 1 351 | | 1 | |
|
Mayzent | | 392 | | 357 | | 10 | | 281 | | 27 | |
|
Aimovig | | 266 | | 218 | | 22 | | 215 | | 1 | |
|
Other | | | | 1 | | nm | | 1 | | 0 | |
|
Total neuroscience | | 4 043 | | 3 038 | | 33 | | 2 220 | | 37 | |
|
| | | | | | | | | | | |
Oncology | | | | | | | | | | | |
Promacta/Revolade | | 2 269 | | 2 088 | | 9 | | 2 016 | | 4 | |
|
Kisqali | | 2 080 | | 1 231 | | 69 | | 937 | | 31 | |
|
Tafinlar + Mekinist | | 1 922 | | 1 770 | | 9 | | 1 693 | | 5 | |
|
Tasigna | | 1 848 | | 1 923 | | -4 | | 2 060 | | -7 | |
|
Jakavi | | 1 720 | | 1 561 | | 10 | | 1 595 | | -2 | |
|
Pluvicto | | 980 | | 271 | | 262 | | | | nm | |
|
Lutathera | | 605 | | 471 | | 28 | | 475 | | -1 | |
|
Kymriah | | 508 | | 536 | | -5 | | 587 | | -9 | |
|
Piqray/Vijoice | | 505 | | 373 | | 35 | | 329 | | 13 | |
|
Scemblix | | 413 | | 149 | | 177 | | 7 | | nm | |
|
Votrient | | 390 | | 474 | | -18 | | 577 | | -18 | |
|
Adakveo | | 195 | | 194 | | 1 | | 164 | | 18 | |
|
Tabrecta | | 154 | | 133 | | 16 | | 90 | | 48 | |
|
Other | | 1 | | 2 | | nm | | 2 | | 0 | |
|
Total oncology | | 13 590 | | 11 176 | | 22 | | 10 532 | | 6 | |
|
| | | | | | | | | | | |
|
Total promoted brands | | 31 822 | | 26 257 | | 21 | | 23 519 | | 12 | |
|
| |
2023
USD m
| |
2022
USD m1
| | Change
(2022 to
2023)
USD % | |
2021
USD m1
| | Change
(2021 to
2022)
USD % | |
|
Established brands | | | | | | | | | | | |
Lucentis | | 1 475 | | 1 874 | | -21 | | 2 160 | | -13 | |
|
Sandostatin | | 1 314 | | 1 238 | | 6 | | 1 413 | | -12 | |
|
Gilenya | | 925 | | 2 013 | | -54 | | 2 787 | | -28 | |
|
Exforge Group | | 713 | | 743 | | -4 | | 901 | | -18 | |
|
Galvus Group | | 692 | | 859 | | -19 | | 1 092 | | -21 | |
|
Diovan Group | | 613 | | 652 | | -6 | | 773 | | -16 | |
|
Gleevec/Glivec | | 561 | | 745 | | -25 | | 1 024 | | -27 | |
|
Afinitor/Votubia | | 408 | | 512 | | -20 | | 938 | | -45 | |
|
Contract manufacturing 3 | | 1 490 | | 1 200 | | 24 | | 1 083 | | 11 | |
|
Other 3 | | 5 427 | | 6 113 | | -11 | | 7 091 | | -14 | |
|
Total established brands 3 | | 13 618 | | 15 949 | | -15 | | 19 262 | | -17 | |
|
| | | | | | | | | | | |
|
Total net sales from continuing operations | | 45 440 | | 42 206 | | 8 | | 42 781 | | -1 | |
|
|
1 Reclassified to conform with the 2023 organizational structure. |
2 Net sales from continuing operations reflect Xolair sales for all indications. |
3 Effective January 1, 2023, the discontinued operations Sandoz business transferred to Novartis continuing operations its bio-technology manufacturing services to other companies’ activities (included in Contract manufacturing) and the Coartem brand (included in Other). The financial information of the Novartis continuing operations and discontinued operations were adapted accordingly in 2022 and 2021, in compliance with IFRS Accounting Standards. See Note 3 for additional information. |
|
nm = not meaningful |
|
Net sales from continuing operations of the top 20 brands in 2023
Brands | | Brand classification by therapeutic area or established brands | | Key indications | | US USD m | | Rest of world USD m | | Total USD m | |
|
Entresto | | Cardiovascular, renal and metabolic | | Chronic heart failure, hypertension | | 3 067 | | 2 968 | | 6 035 | |
|
Cosentyx | | Immunology | | Psoriasis (PsO), ankylosing spondylitis (AS), psoriatic arthritis (PsA), non-radiographic axial spondyloarthritis (nr-axSPA), hidradenitis suppurativa (HS) | | 2 636 | | 2 344 | | 4 980 | |
|
Promacta/Revolade | | Oncology | | Immune thrombocytopenia (ITP), severe aplastic anemia (SAA) | | 1 205 | | 1 064 | | 2 269 | |
|
Kesimpta | | Neuroscience | | Relapsing-remitting multiple sclerosis (RRMS) | | 1 528 | | 643 | | 2 171 | |
|
Kisqali | | Oncology | | HR+/HER2- metastatic breast cancer | | 1 032 | | 1 048 | | 2 080 | |
|
Tafinlar + Mekinist | | Oncology | | BRAF V600+ metastatic adjuvant melanoma, advanced non-small cell lung cancer (NSCLC), tumor agnostic with BRAF mutation indication | | 791 | | 1 131 | | 1 922 | |
|
Tasigna | | Oncology | | Chronic myeloid leukemia (CML) | | 884 | | 964 | | 1 848 | |
|
Jakavi | | Oncology | | Myelofibrosis (MF), polycytomia vera (PV), graft-versus-host disease (GvHD) | | | | 1 720 | | 1 720 | |
|
Lucentis 1 | | Established brands | | Age-related macular degeneration (AMD), diabetic macular edema (DME), retinal vein occlusion (RVO) | | | | 1 475 | | 1 475 | |
|
Xolair 2 | | Immunology | | Severe allergic asthma (SAA), chronic spontaneous urticaria (CSU), nasal polyps | | | | 1 463 | | 1 463 | |
|
Ilaris | | Immunology | | Auto-inflammatory (CAPS, TRAPS, HIDS/MKD, FMF, SJIA, AOSD, gout) | | 686 | | 669 | | 1 355 | |
|
Sandostatin | | Established brands | | Carcinoid tumors, acromegaly | | 829 | | 485 | | 1 314 | |
|
Zolgensma | | Neuroscience | | Spinal muscular atrophy (SMA) | | 372 | | 842 | | 1 214 | |
|
Pluvicto | | Oncology | | PSMA-positive mCRPC patients post-ARPI, post-Taxane | | 921 | | 59 | | 980 | |
|
Gilenya 1 | | Established brands | | Relapsing multiple sclerosis (RMS) | | 359 | | 566 | | 925 | |
|
Exforge Group | | Established brands | | Hypertension | | 13 | | 700 | | 713 | |
|
Galvus Group | | Established brands | | Type 2 diabetes | | | | 692 | | 692 | |
|
Diovan Group | | Established brands | | Hypertension | | 52 | | 561 | | 613 | |
|
Lutathera | | Oncology | | GEP-NETs gastroenteropancreatic neuroendocrine tumors | | 427 | | 178 | | 605 | |
|
Gleevec/Glivec | | Established brands | | Chronic myeloid leukemia (CML), gastrointestinal stromal tumors (GIST) | | 150 | | 411 | | 561 | |
|
Top 20 brands total | | | | | | 14 952 | | 19 983 | | 34 935 | |
|
Rest of portfolio | | | | | | 3 007 | | 7 498 | | 10 505 | |
|
Total net sales from continuing operations | | | | | | 17 959 | | 27 481 | | 45 440 | |
|
|
1 In the first quarter of 2023 Lucentis was reclassified from other promoted brands to established brands and Gilenya was reclassified from neuroscience to established brands. |
2 Net sales from continuing operations reflect Xolair sales for all indications. |
| |
| | | | | | | | | | | |
Net sales from continuing operations of the top 20 brands in 2022
Brands | | Brand classification by therapeutic area or established brands1 | | Key indications | | US USD m | | Rest of world USD m | | Total USD m | |
|
Cosentyx | | Immunology | | Psoriasis (PsO), ankylosing spondylitis (AS), psoriatic arthritis (PsA), non-radiographic axial spondyloarthritis (nr-axSPA) | | 2 770 | | 2 018 | | 4 788 | |
|
Entresto | | Cardiovascular, renal and metabolic | | Chronic heart failure, hypertension | | 2 354 | | 2 290 | | 4 644 | |
|
Promacta/Revolade | | Oncology | | Immune thrombocytopenia (ITP), severe aplastic anemia (SAA) | | 1 083 | | 1 005 | | 2 088 | |
|
Gilenya 2 | | Established brands | | Relapsing multiple sclerosis (RMS) | | 1 153 | | 860 | | 2 013 | |
|
Tasigna | | Oncology | | Chronic myeloid leukemia (CML) | | 877 | | 1 046 | | 1 923 | |
|
Lucentis 2 | | Established brands | | Age-related macular degeneration (AMD), diabetic macular edema (DME), retinal vein occlusion (RVO) | | | | 1 874 | | 1 874 | |
|
Tafinlar + Mekinist | | Oncology | | BRAF V600+ metastatic adjuvant melanoma, advanced non-small cell lung cancer (NSCLC), tumor agnostic with BRAF mutation indication | | 678 | | 1 092 | | 1 770 | |
|
Jakavi | | Oncology | | Myelofibrosis (MF), polycytomia vera (PV), graft-versus-host disease (GvHD) | | | | 1 561 | | 1 561 | |
|
Zolgensma | | Neuroscience | | Spinal muscular atrophy (SMA) | | 434 | | 936 | | 1 370 | |
|
Xolair 3 | | Immunology | | Severe allergic asthma (SAA), chronic spontaneous urticaria (CSU), nasal polyps | | | | 1 365 | | 1 365 | |
|
Sandostatin | | Established brands | | Carcinoid tumors, acromegaly | | 800 | | 438 | | 1 238 | |
|
Kisqali | | Oncology | | HR+/HER2- metastatic breast cancer | | 472 | | 759 | | 1 231 | |
|
Ilaris | | Immunology | | Auto-inflammatory (CAPS, TRAPS, HIDS/MKD, FMF, SJIA, AOSD, gout) | | 570 | | 563 | | 1 133 | |
|
Kesimpta | | Neuroscience | | Relapsing-remitting multiple sclerosis (RRMS) | | 921 | | 171 | | 1 092 | |
|
Galvus Group | | Established brands | | Type 2 diabetes | | | | 859 | | 859 | |
|
Gleevec/Glivec | | Established brands | | Chronic myeloid leukemia (CML), gastrointestinal stromal tumors (GIST) | | 205 | | 540 | | 745 | |
|
Exforge Group | | Established brands | | Hypertension | | 14 | | 729 | | 743 | |
|
Diovan Group | | Established brands | | Hypertension | | 55 | | 597 | | 652 | |
|
Kymriah | | Oncology | | r/r pediatric and young adults acute lymphoblastic leukemia (ALL), diffuse large B-cell lymphoma (DLBCL) follicular lymphoma (FL) | | 196 | | 340 | | 536 | |
|
Afinitor/Votubia | | Established brands | | Breast cancer/ tuberous sclerosis complex (TSC) | | 171 | | 341 | | 512 | |
|
Top 20 products total | | | | | | 12 753 | | 19 384 | | 32 137 | |
|
Rest of portfolio 4 | | | | | | 3 182 | | 6 887 | | 10 069 | |
|
Total net sales from continuing operations 4 | | | | | | 15 935 | | 26 271 | | 42 206 | |
|
|
1 Brand classifications have been changed to conform with the 2023 brand classifications. |
2 In the first quarter of 2023 Lucentis was reclassified from other promoted brands to established brands and Gilenya was reclassified from neuroscience to established brands. |
3 Net sales from continuing operations reflect Xolair sales for all indications. |
4 Effective January 1, 2023, the discontinued operations Sandoz business bio-technology manufacturing services to other companies’ activities and the Coartem brand were transferred to the Novartis continuing operations. The financial information of the Novartis continuing operations and discontinued operations were adapted accordingly in 2022, in compliance with IFRS Accounting Standards. See Note 3 for additional information. |
Net sales from continuing operations of the top 20 brands in 2021
Brands | | Brand classification by therapeutic area or established brands1 | | Key indications | | US USD m | | Rest of world USD m | | Total USD m | |
|
Cosentyx | | Immunology | | Psoriasis (PsO), ankylosing spondylitis (AS), psoriatic arthritis (PsA), non-radiographic axial spondyloarthritis (nr-axSPA) | | 2 883 | | 1 835 | | 4 718 | |
|
Entresto | | Cardiovascular, renal and metabolic | | Chronic heart failure | | 1 712 | | 1 836 | | 3 548 | |
|
Gilenya 2 | | Established brands | | Relapsing multiple sclerosis (RMS) | | 1 427 | | 1 360 | | 2 787 | |
|
Lucentis 2 | | Established brands | | Age-related macular degeneration (AMD) | | | | 2 160 | | 2 160 | |
|
Tasigna | | Oncology | | Chronic myeloid leukemia (CML) | | 882 | | 1 178 | | 2 060 | |
|
Promacta/Revolade | | Oncology | | Immune thrombocytopenia (ITP), severe aplastic anemia (SAA) | | 947 | | 1 069 | | 2 016 | |
|
Tafinlar + Mekinist | | Oncology | | BRAF V600+ metastatic adjuvant melanoma, advanced non-small cell lung cancer (NSCLC) | | 606 | | 1 087 | | 1 693 | |
|
Jakavi | | Oncology | | Myelofibrosis (MF), polycythemia vera (PV) | | | | 1 595 | | 1 595 | |
|
Xolair 3 | | Immunology | | Severe allergic asthma (SAA), chronic spontaneous urticaria (CSU), nasal polyps | | | | 1 428 | | 1 428 | |
|
Sandostatin | | Established brands | | Carcinoid tumors, acromegaly | | 843 | | 570 | | 1 413 | |
|
Zolgensma | | Neuroscience | | Spinal muscular atrophy (SMA) | | 469 | | 882 | | 1 351 | |
|
Galvus Group | | Established brands | | Type 2 diabetes | | | | 1 092 | | 1 092 | |
|
Ilaris | | Immunology | | Auto-inflammatory (CAPS, TRAPS, HIDS/MKD, FMF, SJIA, AOSD gout) | | 501 | | 558 | | 1 059 | |
|
Gleevec/Glivec | | Established brands | | Chronic myeloid leukemia (CML), gastrointestinal stromal tumors (GIST) | | 263 | | 761 | | 1 024 | |
|
Afinitor/Votubia | | Established brands | | Breast cancer/ tuberous sclerosis complex (TSC) | | 521 | | 417 | | 938 | |
|
Kisqali | | Oncology | | HR+/HER2- metastatic breast cancer | | 339 | | 598 | | 937 | |
|
Exforge Group | | Established brands | | Hypertension | | 14 | | 887 | | 901 | |
|
Diovan Group | | Established brands | | Hypertension | | 51 | | 722 | | 773 | |
|
Kymriah | | Oncology | | r/r pediatric and young adults acute lymphoblastic leukemia (ALL), diffuse large B-cell lymphoma (DLBCL) | | 230 | | 357 | | 587 | |
|
Ultibro Group | | Established brands | | Chronic obstructive pulmonary disease (COPD) | | | | 584 | | 584 | |
|
Top 20 products total | | | | | | 11 688 | | 20 976 | | 32 664 | |
|
Rest of portfolio 4 | | | | | | 3 235 | | 6 882 | | 10 117 | |
|
Total net sales from continuing operations 4 | | | | | | 14 923 | | 27 858 | | 42 781 | |
|
|
1 Brand classifications have been changed to conform with the 2023 brand classifications. |
2 In the first quarter of 2023 Lucentis was reclassified from other promoted brands to established brands and Gilenya was reclassified from neuroscience to established brands. |
3 Net sales from continuing operations reflect Xolair sales for all indications. |
4 Effective January 1, 2023, the discontinued operations Sandoz business bio-technology manufacturing services to other companies’ activities and the Coartem brand were transferred to the Novartis continuing operations. The financial information of the Novartis continuing operations and discontinued operations were adapted accordingly in 2021, in compliance with IFRS Accounting Standards. See Note 3 for additional information. |
Other revenues
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
Profit-sharing income | | 941 | | 921 | | 873 | |
|
Royalty income | | 87 | | 35 | | 85 | |
|
Milestone income | | 45 | | 145 | | 127 | |
|
Other 1 | | 147 | | 154 | | 108 | |
|
Total other revenues | | 1 220 | | 1 255 | | 1 193 | |
|
|
1 Other includes revenue from activities such as manufacturing or other services rendered, to the extent such revenue is not recorded under net sales to third parties. |
5. Associated companies
| | Net income statement effect | | Other comprehensive income effect 1 | | Total comprehensive income effect | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
(USD millions) | | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 | |
|
Roche Holding AG, Switzerland | | | | | | 15 341 | | | | | | 46 | | | | | | 15 387 | |
|
Others | | -13 | | -11 | | -4 | | | | | | | | -13 | | -11 | | -4 | |
|
Associated companies | | -13 | | -11 | | 15 337 | | | | | | 46 | | -13 | | -11 | | 15 383 | |
|
|
1 In 2021, Novartis share of other comprehensive income recognized by associated companies, net of taxes of USD 3 million was recycled into the consolidated income statement as a result of the divestment of the investment in Roche Holding AG. No Novartis share of other comprehensive income recognized by associated companies was recycled to the consolidated income statement in 2023 and 2022. |
Novartis has certain non-significant investments and had a significant investment in Roche Holding AG, Basel (Roche), which was divested to Roche on December 6, 2021, that are accounted for as associated companies.
Roche Holding AG
On November 3, 2021, Novartis entered into an agreement with Roche Holding AG to divest its 33.3% of Roche Holding AG (Roche) voting shares, representing approximately 6.2% of Roche’s total outstanding voting and non-voting equity instruments, to Roche for USD 20.7 billion in cash. As a result, Novartis discontinued the use of equity method accounting starting from November 3, 2021.
The divestment transaction closed on December 6, 2021, and Novartis realized a gain of USD 14.6 billion, recorded in income from associated companies. For more information, see Note 2.
Since full-year financial data for Roche is not available when Novartis produces its consolidated financial results, a survey of analyst estimates is used to estimate the Company’s share of Roche’s net income. Any differences between these estimates and actual results were adjusted in the Company’s consolidated financial statements when available. As Novartis discontinued the use of equity method accounting starting from November 3, 2021, and the divestment closed on December 6, 2021, no such adjustment has been made to the 2023 and 2022 Company’s consolidated financial statements.
The consolidated income statement effects from applying Novartis accounting principles for this investment in 2021 are as follows:
(USD millions) | | 2021 | |
|
Novartis share of Roche's estimated current-year consolidated net income | | 815 | |
|
Prior-year adjustment | | 40 | |
|
Amortization of fair value adjustments relating to intangible assets, net of taxes of USD 10 million | | -70 | |
|
Gain on divestment of the investment in Roche 1 | | 14 556 | |
|
Net income effect | | 15 341 | |
|
|
1 The gain on divestment of the investment in Roche includes the recycling of currency translation effects (see Note 9.1) and other comprehensive income effects totaling USD 3.2 billion. |
6. Interest expense and other financial income and expense
Interest expense
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
Interest expense | | -730 | | -642 | | -633 | |
|
Interest expense on lease liabilities | | -62 | | -57 | | -59 | |
|
Expense arising from discounting long-term liabilities and capitalized borrowing costs | | -63 | | -101 | | -95 | |
|
Total interest expense from continuing operations | | -855 | | -800 | | -787 | |
|
Other financial income and expense
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
Interest income | | 627 | | 377 | | 70 | |
|
Other financial income | | 21 | | 19 | | 12 | |
|
Monetary loss from hyperinflation accounting | | -194 | | -137 | | -48 | |
|
Financial expense | | -18 | | -33 | | -41 | |
|
Currency result, net | | -214 | | -184 | | -69 | |
|
Total other financial income and expense from continuing operations | | 222 | | 42 | | -76 | |
|
7. Income taxes
Income before taxes
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
Switzerland 1 | | 9 719 | | 5 751 | | 21 830 | |
|
Foreign 2 | | -596 | | 1 426 | | 2 700 | |
|
Income before taxes from continuing operations | | 9 123 | | 7 177 | | 24 530 | |
|
|
1 The 2021 income before taxes from continuing operations in Switzerland includes a USD 14.6 billion non-taxable gain on the divestment of the Company’s investment in Roche Holding AG (see Note 2 and Note 5). |
2 The 2023 foreign income before taxes from continuing operations is impacted by non-recurring events, including impairment charges on intangible assets other than goodwill. |
Current and deferred income tax expense
The significant components of the provision for income taxes from continuing operations are as follows:
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
Switzerland | | -1 136 | | -598 | | -949 | |
|
Foreign | | -1 290 | | -1 155 | | -973 | |
|
Current income tax expense | | -2 426 | | -1 753 | | -1 922 | |
|
Switzerland | | 355 | | -131 | | 39 | |
|
Foreign | | 1 520 | | 756 | | 258 | |
|
Deferred tax income | | 1 875 | | 625 | | 297 | |
|
Income tax expense from continuing operations | | -551 | | -1 128 | | -1 625 | |
|
Analysis of tax rate
Novartis has a substantial business presence in many countries and is therefore subject to income taxes in different tax jurisdictions. This leads to differences in income and expense items that are non-taxable or non-deductible (permanent differences) or are taxed at different statutory tax rates in those tax jurisdictions. As a result, there is a difference between our applicable tax rate and effective tax rate.
The applicable tax rate changes from year to year due to changes in the mix of the Company’s pre-tax income and changes in statutory tax rates since it is calculated as the weighted average tax rate based on the pre-tax income of each subsidiary.
The main elements contributing to the difference between the Company’s overall applicable tax rate and the effective tax rate are shown in the following table:
(As a percentage) | | 2023 | | 2022 | | 2021 | |
|
Applicable tax rate | | 15.0 | | 15.3 | | 14.2 | |
|
Effect of disallowed expenditures | | 1.4 | | 2.6 | | 1.0 | |
|
Effect of income taxed at reduced rates | | -0.6 | | -0.4 | | -0.1 | |
|
Effect of income not subject to tax 1 | | -2.5 | | -0.1 | | -7.9 | |
|
Effect of tax credits and allowances | | -3.9 | | -4.1 | | -1.5 | |
|
Effect of release of contingent consideration liability | | -0.3 | | -0.5 | | -0.1 | |
|
Effect of tax rate change on current and deferred tax assets and liabilities | | -1.6 | | 0.0 | | 0.0 | |
|
Effect of derecognition and reversals of derecognition of deferred tax assets | | 0.9 | | 1.3 | | 0.0 | |
|
Effect of write-down of investments in subsidiaries | | -3.0 | | 0.0 | | 0.0 | |
|
Effect of prior-year items | | 0.0 | | -0.3 | | 0.1 | |
|
Effect of changes in uncertain tax positions | | 0.1 | | 1.7 | | 1.0 | |
|
Effect of other items | | 0.5 | | 0.2 | | -0.1 | |
|
Effective tax rate from continuing operations | | 6.0 | | 15.7 | | 6.6 | |
|
|
1 2021 includes the effect of income not subject to tax (-7.7%) arising from the non-taxable gain on the divestment of our investment in Roche. See Notes 2 and 5 for further details. |
The effective tax rate of Novartis fluctuates primarily as a result of, among other factors, changes in pre-tax
income between countries with varying statutory tax rates and the effects of disallowed expenditures, income not subject to tax, tax credits and allowances, tax rate changes on current and deferred tax assets and liabilities, write-down of investments in subsidiaries, and changes in uncertain tax positions. The table above provides the details of the significant items that impact the comparability of the effective tax rate between years.
In December 2021, the OECD issued model rules for a new global minimum tax framework (Pillar Two). Novartis is within the scope of the OECD Pillar Two model rules. A number of governments in countries in which Novartis operates are in the process of enacting or have enacted tax legislation to comply with Pillar Two. Of the major countries in which we operate, only the enactment of Pillar Two tax legislation in Switzerland is expected to have an impact to our income tax provision as from 2024. In December 2023, Switzerland decided to partially implement Pillar Two, whereby effective from January 1, 2024, a 15% minimum taxation will be assessed on Pillar Two qualifying profits earned by companies domiciled in Switzerland (Qualified Domestic Minimum Top-Up Tax). This Qualified Domestic Minimum Top-Up Tax will not be applied to the Pillar Two qualifying profits earned by a company’s affiliates domiciled in tax jurisdictions outside of Switzerland. The timing of implementation and the specific provisions of any further Pillar Two tax regulations in Switzerland remains subject to further assessments at both the Federal and Cantonal levels. The Company estimates that the impact of these changes to tax legislation in the respective countries that have (substantively) enacted Pillar Two tax legislation in 2023 would not be material to our consolidated financial position, income statement and cash flows.
8. Earnings per share
| | 2023 | | 2022 | | 2021 | |
|
Net income attributable to shareholders of Novartis AG (USD millions) | | | | | | | |
|
- Continuing operations | | 8 568 | | 6 049 | | 22 908 | |
|
- Discontinued operations | | 6 282 | | 906 | | 1 113 | |
|
Net income attributable to shareholders of Novartis AG (USD millions) | | 14 850 | | 6 955 | | 24 021 | |
|
| | | | | | | |
Number of shares (in millions) | | | | | | | |
Weighted average number of shares outstanding used in basic earnings per share | | 2 077 | | 2 181 | | 2 243 | |
|
Adjustment for vesting of restricted shares, restricted share units and dilutive shares from options | | 15 | | 16 | | 17 | |
|
Weighted average number of shares in diluted earnings per share | | 2 092 | | 2 197 | | 2 260 | |
|
| | | | | | | |
Basic earnings per share (USD) | | | | | | | |
- Continuing operations | | 4.13 | | 2.77 | | 10.22 | |
|
- Discontinued operations | | 3.02 | | 0.42 | | 0.49 | |
|
Total basic earnings per share (USD) | | 7.15 | | 3.19 | | 10.71 | |
|
| | | | | | | |
Diluted earnings per share (USD) | | | | | | | |
- Continuing operations | | 4.10 | | 2.76 | | 10.14 | |
|
- Discontinued operations | | 3.00 | | 0.41 | | 0.49 | |
|
Total diluted earnings per share (USD) | | 7.10 | | 3.17 | | 10.63 | |
|
Basic earnings per share (EPS) is calculated by dividing net income attributable to shareholders of Novartis AG by the weighted average number of shares outstanding in a reporting period. This calculation excludes the average number of issued shares purchased by the Company and held as treasury shares.
For diluted EPS, the weighted average number of shares outstanding is adjusted to assume the vesting of all restricted shares, restricted share units, and in 2022 and 2021 the conversion of all potentially dilutive shares arising from options on Novartis shares that have been issued. At December 31, 2023, there were no options on Novartis shares issued or outstanding.
No options were excluded from the calculation of diluted EPS in 2022 or 2021, as all options were dilutive in both years.
9. Changes in consolidated statements of comprehensive income
The consolidated statements of comprehensive income include the Company’s net income for the year as well as all other valuation adjustments recorded in the Company’s consolidated balance sheet, which under IFRS Accounting Standards are not recorded in the consolidated income statement. These include fair value adjustments on financial instruments, actuarial gains or losses on defined benefit pension plans, and currency translation effects, all net of taxes.
(USD millions)
| |
Note
| |
Fair value
adjustments
on financial
instruments
| |
Actuarial
gains/(losses)
from defined
benefit plans
| |
Cumulative
currency
translation
effects
| | Total value
adjustments
attributable to
Novartis AG
shareholders | |
Non-
controlling
interest
| |
Total value
adjustments
| |
|
Value adjustments at December 31, 2020 | | | | 220 | | -5 773 | | 4 134 | | -1 419 | | -29 | | -1 448 | |
|
Fair value adjustments on equity securities, net of taxes of USD -44 million 1 | | | | 194 | | | | | | 194 | | | | 194 | |
|
Net investment hedge, net of taxes of USD 33 million | | | | | | | | 216 | | 216 | | | | 216 | |
|
Defined benefit plans, net of taxes of USD -323 million | | | | | | 1 808 | | | | 1 808 | | 1 | | 1 809 | |
|
Currency translation effects, net of taxes of USD 17 million | | 9.1 | | | | | | -4 757 | | -4 757 | | -5 | | -4 762 | |
|
Total value adjustments in 2021 | | | | 194 | | 1 808 | | -4 541 | | -2 539 | | -4 | | -2 543 | |
|
Fair value adjustments on equity securities sold, reclassified to retained earnings net of taxes of USD 48 million | | | | -164 | | | | | | -164 | | | | -164 | |
|
Value adjustments related to divestments | | | | -62 | | -3 | | | | -65 | | | | -65 | |
|
Value adjustments at December 31, 2021 | | | | 188 | | -3 968 | | -407 | | -4 187 | | -33 | | -4 220 | |
|
Fair value adjustments on equity securities, net of taxes of USD 81 million 1 | | | | -382 | | | | | | -382 | | | | -382 | |
|
Net investment hedge, net of taxes of USD -30 million | | | | | | | | 91 | | 91 | | | | 91 | |
|
Defined benefit plans, net of taxes of USD -104 million | | | | | | -104 | | | | -104 | | 1 | | -103 | |
|
Currency translation effects, net of taxes of USD 18 million | | 9.1 | | | | | | -444 | | -444 | | -6 | | -450 | |
|
Total value adjustments in 2022 | | | | -382 | | -104 | | -353 | | -839 | | -5 | | -844 | |
|
Fair value adjustments on equity securities sold, reclassified to retained earnings net of taxes of nil | | | | -4 | | | | | | -4 | | | | -4 | |
|
Value adjustments related to divestments, net of taxes of USD -4 million | | | | | | 34 | | | | 34 | | | | 34 | |
|
Value adjustments at December 31, 2022 | | | | -198 | | -4 038 | | -760 | | -4 996 | | -38 | | -5 034 | |
|
Fair value adjustments on equity securities, net of taxes of USD -6 million 1 | | | | 37 | | | | | | 37 | | | | 37 | |
|
Net investment hedge, net of taxes of USD 19 million | | | | | | | | -50 | | -50 | | | | -50 | |
|
Defined benefit plans, net of taxes of USD 16 million | | | | | | -160 | | | | -160 | | | | -160 | |
|
Currency translation effects, net of taxes of USD -6 million | | 9.1 | | | | | | 1 373 | | 1 373 | | 2 | | 1 375 | |
|
Total value adjustments in 2023 | | | | 37 | | -160 | | 1 323 | | 1 200 | | 2 | | 1 202 | |
|
Fair value adjustments on equity securities sold, reclassified to retained earnings net of taxes of USD -7 million | | | | 1 | | | | | | 1 | | | | 1 | |
|
Value adjustments related to divestments, net of taxes of USD -4 million | | | | 2 | | 27 | | | | 29 | | | | 29 | |
|
Value adjustments at December 31, 2023 | | | | -158 | | -4 171 | | 563 | | -3 766 | | -36 | | -3 802 | |
|
|
1 Includes fair value adjustments on equity securities designated as financial assets valued at fair value through other comprehensive income with no subsequent recycling into the consolidated income statement |
9.1) In 2023, net cumulative currency translation gains of USD 358 million were recycled through the income statement, consisting of USD 357 million as a result of the spin-off of the Sandoz business through a dividend in kind distribution to Novartis AG shareholders (see Note 2), and of USD 1 million as a result of the divestment of subsidiaries.
In 2022, net cumulative currency translation gains of USD 13 million were recycled through the income statement as a result of the divestments of subsidiaries.
In 2021, net cumulative currency translation gains of USD 3.2 billion were recycled through the income statement as a result of the divestment of the investment in Roche. See Notes 2 and 5.
10. Property, plant and equipment
The following table summarizes the movements of property, plant and equipment during 2023:
(USD millions)
| |
Land
| |
Buildings
| |
Construction
in progress
| | Machinery
and other
equipment | |
Total
| |
|
At January 1, 2023 | | | | | | | | | | | |
|
Cost | | 451 | | 11 396 | | 1 184 | | 11 842 | | 24 873 | |
|
Accumulated depreciation and impairment | | -9 | | -5 903 | | -27 | | -8 170 | | -14 109 | |
|
Net book value | | 442 | | 5 493 | | 1 157 | | 3 672 | | 10 764 | |
|
| | | | | | | | | | | |
At January 1, 2023 | | 442 | | 5 493 | | 1 157 | | 3 672 | | 10 764 | |
|
Costs and accumulated depreciation/impairments on assets related to discontinued operations 1 | | -54 | | -422 | | -280 | | -588 | | -1 344 | |
|
Impact of acquisitions of businesses | | | | 12 | | 1 | | 5 | | 18 | |
|
Reclassifications | | | | 197 | | -420 | | 223 | | | |
|
Additions | | 1 | | 85 | | 734 | | 245 | | 1 065 | |
|
Disposals and derecognitions | | -16 | | -261 | | -20 | | -63 | | -360 | |
|
Depreciation charge | | | | -343 | | | | -573 | | -916 | |
|
Impairment charge | | -3 | | -36 | | -10 | | -57 | | -106 | |
|
Reversal of impairment charge | | 3 | | 9 | | | | 4 | | 16 | |
|
Currency translation effects | | 25 | | 162 | | 44 | | 146 | | 377 | |
|
At December 31, 2023 | | 398 | | 4 896 | | 1 206 | | 3 014 | | 9 514 | |
|
| | | | | | | | | | | |
At December 31, 2023 | | | | | | | | | | | |
|
Cost | | 403 | | 10 147 | | 1 213 | | 9 630 | | 21 393 | |
|
Accumulated depreciation and impairment | | -5 | | -5 251 | | -7 | | -6 616 | | -11 879 | |
|
Net book value | | 398 | | 4 896 | | 1 206 | | 3 014 | | 9 514 | |
|
Commitments for purchases of property, plant and equipment | | | | | | | | | | 744 | |
|
Capitalized borrowing costs | | | | | | | | | | 3 | |
|
|
1 Represents the cost of assets and accumulated depreciation/impairments at January 1, 2023, related to the Sandoz business reported as discontinued operations, and the net transfers between discontinued and continuing operations from January 1, 2023 to October 3, 2023. Note 31 provides disclosure of discontinued operations additions, depreciation charge, impairment charge and reversals of impairment change. |
The following table summarizes the movements of property, plant and equipment during 2022:
(USD millions)
| |
Land
| |
Buildings
| |
Construction
in progress
| | Machinery
and other
equipment | |
Total
| |
|
At January 1, 2022 | | | | | | | | | | | |
|
Cost | | 492 | | 11 819 | | 1 508 | | 13 328 | | 27 147 | |
|
Accumulated depreciation and impairment | | -7 | | -5 744 | | -65 | | -9 786 | | -15 602 | |
|
Net book value | | 485 | | 6 075 | | 1 443 | | 3 542 | | 11 545 | |
|
| | | | | | | | | | | |
At January 1, 2022 | | 485 | | 6 075 | | 1 443 | | 3 542 | | 11 545 | |
|
Impact of acquisitions of businesses | | | | | | | | 13 | | 13 | |
|
Reclassifications | | | | 297 | | -964 | | 667 | | | |
|
Additions 1 | | 3 | | 124 | | 780 | | 312 | | 1 219 | |
|
Disposals and derecognitions | | -28 | | -49 | | -33 | | -45 | | -155 | |
|
Depreciation charge 2 | | | | -437 | | | | -726 | | -1 163 | |
|
Impairment charge 2 | | -7 | | -351 | | -13 | | -43 | | -414 | |
|
Reversal of impairment charge 2 | | 1 | | | | 1 | | 5 | | 7 | |
|
Currency translation effects | | -12 | | -166 | | -57 | | -53 | | -288 | |
|
At December 31, 2022 | | 442 | | 5 493 | | 1 157 | | 3 672 | | 10 764 | |
|
| | | | | | | | | | | |
At December 31, 2022 | | | | | | | | | | | |
|
Cost | | 451 | | 11 396 | | 1 184 | | 11 842 | | 24 873 | |
|
Accumulated depreciation and impairment | | -9 | | -5 903 | | -27 | | -8 170 | | -14 109 | |
|
Net book value | | 442 | | 5 493 | | 1 157 | | 3 672 | | 10 764 | |
|
Commitments for purchases of property, plant and equipment | | | | | | | | | | 549 | |
|
Capitalized borrowing costs | | | | | | | | | | 5 | |
|
| | | | | | | | | | | |
1 Additions in continuing operations were USD 930 million. Note 31 provides disclosure of discontinued operations additions. |
2 Note 31 provides disclosure of discontinued operations depreciation charge, impairment charge and reversals of impairment charge. |
Government grants obtained for construction activities, including any related equipment, are deducted from the gross acquisition cost to arrive at the balance sheet carrying value of the related assets.
Property, plant and equipment is depreciated on a straight-line basis in the consolidated income statement over the estimated useful life of the individual asset. The related depreciation expense is included in the costs of the functions using the asset.
The following table shows the estimated useful life by major categories for property, plant and equipment:
| | Useful life | |
|
Buildings | | 20 to 40 years | |
|
Machinery and other equipment | | | |
|
Machinery and equipment | | 7 to 20 years | |
|
Furniture and vehicles | | 5 to 10 years | |
|
Computer hardware | | 3 to 7 years | |
|
Property, plant and equipment is assessed for impairment whenever there is an indication that the balance sheet carrying amount may not be recoverable using cash flow projections over the useful life.
The following table shows the property, plant and equipment depreciation charge, impairment charge and reversals of impairment charge for continuing operations for the years ended December 31, 2023, 2022 and 20211:
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
Depreciation charge | | -916 | | -967 | | -1 005 | |
|
Impairment charge | | -106 | | -411 | | -316 | |
|
Impairment reversals | | 16 | | 4 | | 44 | |
|
|
1 Note 31 provides disclosure of discontinued operations depreciation charge, impairment charge and reversals of impairment charge. |
11. Right-of-use assets and lease liabilities
The Company recognizes a right-of-use asset and a corresponding lease liability for all arrangements in which it is a lessee, except for leases with a term of 12 months or less (short-term leases) and low-value leases. For these short-term and low-value leases, the Company recognizes the lease payments as an operating expense on a straight-line basis over the term of the lease. The Company allocates the consideration in the lease contract to the lease and non-lease components on the basis of the relative standalone price of each component.
The portion of the lease payments attributable to the repayment of lease liabilities is recognized in cash flows used in financing activities, and the portion attributable to the payment of interest is included in cash flows from operating activities.
Right-of-use assets are depreciated on a straight-line basis from the commencement date of the lease over the shorter of the useful life of the right-of-use asset or the end of the lease term.
Right-of-use assets are assessed for impairment whenever there is an indication that the balance sheet carrying amount may not be recoverable using cash flow projections for the useful life.
The following table summarizes the movements of the right-of-use assets:
(USD millions) | | 2023 | | 2022 | |
|
Right-of-use assets at January 1 | | 1 431 | | 1 561 | |
|
Costs and accumulated depreciation/impairments on assets related to discontinued operations 1 | | -117 | | | |
|
Impact of acquisitions of businesses | | 16 | | 12 | |
|
Additions 2 | | 421 | | 247 | |
|
Depreciation charge | | -259 | | -300 | |
|
Impairment charge 3 | | -4 | | -3 | |
|
Lease contract terminations 4 | | -93 | | -34 | |
|
Currency translation effects | | 15 | | -52 | |
|
Total right-of-use assets at December 31 | | 1 410 | | 1 431 | |
|
|
1 Represents the cost of assets and accumulated depreciation/impairments at January 1, 2023, related to the Sandoz business reported as discontinued operations, and the net transfers between discontinued and continuing operations from January 1, 2023 to October 3, 2023. Note 31 provides disclosure of discontinued operations additions, depreciation charge, impairment charge and reversals of impairment change. |
2 Additions in continuing operations were USD 216 million in 2022. |
3 Impairment charge in continuing operations was USD 3 million in 2022 and nil in 2021. |
4 Lease contract terminations also includes modifications to existing leases that result in reductions to the right-of-use assets, and reductions due to sub-leasing. |
The following table shows the right-of-use assets carrying value at December 31, 2023 and 2022, and the continuing operations depreciation charge for years 2023, 2022 and 2021, by underlying class of asset1:
(USD millions)
| | December 31,
2023
carrying value | | December 31,
2022
carrying value | | Depreciation
charge
2023 | | Depreciation
charge
2022 | | Depreciation
charge
2021 | |
|
Land | | 483 | | 505 | | 12 | | 16 | | 11 | |
|
Buildings | | 749 | | 745 | | 156 | | 162 | | 174 | |
|
Vehicles | | 112 | | 117 | | 80 | | 82 | | 89 | |
|
Machinery and equipment, and other assets | | 66 | | 64 | | 11 | | 7 | | 5 | |
|
Total right-of-use assets | | 1 410 | | 1 431 | | 259 | | 267 | | 279 | |
|
|
1 Note 31 provides disclosure of discontinued operations depreciation charge. |
The following table shows the lease liabilities by maturity at December 31, 2023 and 2022:
(USD millions)
| |
Lease liabilities
2023
| | Lease liabilities
undiscounted
2023 | |
Lease liabilities
2022
| | Lease liabilities
undiscounted
2022 | |
|
Less than one year | | 230 | | 284 | | 251 | | 297 | |
|
Between one and two years | | 203 | | 248 | | 190 | | 232 | |
|
Between two and three years | | 170 | | 211 | | 167 | | 201 | |
|
Between three and four years | | 149 | | 184 | | 137 | | 172 | |
|
Between four and five years | | 113 | | 142 | | 122 | | 154 | |
|
After five years | | 963 | | 2 173 | | 922 | | 2 149 | |
|
Total lease liabilities | | 1 828 | | 3 242 | | 1 789 | | 3 205 | |
|
Less current portion of lease liabilities | | -230 | | -284 | | -251 | | -297 | |
|
Non-current portion of lease liabilities | | 1 598 | | 2 958 | | 1 538 | | 2 908 | |
|
Commitments for leases not yet commenced | | | | 89 | | | | 83 | |
|
At December 31, 2023, and December 31, 2022, there were no material future cash outflows, including extension options, excluded from the measurement of lease liabilities. The Company’s most material lease with a lease term extension, representing a lease liability value of USD 0.7 billion (2022: USD 0.7 billion), has a determined lease term end date of 2071 (2022: 2071). Non-enforceable extension options of up to 10 years have not been included within the measurement of this lease liability, and do not have a material impact to the carrying value of the lease for both 2023 and 2022. Should the landlord agree to a lease extension, rent will be referenced to the market rates as at the commencement of the extension period.
In 2023, the Company completed two sale and leaseback transactions for certain property, plant and equipment as part of the Company’s strategy. The transactions resulted in net cash inflows of USD 273 million (2022: USD 49 million) and the recognition of USD 146 million of lease liabilities(2022: USD 23 million), and USD 109 million of right-of-use assets (2022: USD 13 million). The right-of-use assets value reflects the proportion of the property, plant and equipment retained. Extension options have been included where management believe that such options will be exercised. The liabilities reflect the net present value of future lease payments. The net gain on the sale and leaseback transactions amounted to USD 18 million (2022: USD 17 million). There were no significant sale and leaseback transactions in 2021.
The following table provides additional disclosures related to continuing operations right-of-use assets and lease liabilities for 2023, 2022 and 2021:
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
Interest expense on lease liabilities 1 | | 62 | | 57 | | 59 | |
|
Expense on short-term leases | | 5 | | 3 | | 6 | |
|
Expense on low-value leases | | 6 | | 6 | | 7 | |
|
Total cash outflows for leases | | 321 | | 319 | | 339 | |
|
Thereof: | | | | | | | |
|
Cash outflows for short-term leases and low-value leases 2 | | 11 | | 9 | | 13 | |
|
Payments of interest 3 | | 52 | | 48 | | 48 | |
|
Payments of lease liabilities 4 | | 258 | | 262 | | 278 | |
|
|
1 The weighted average interest rate is 3.5% (2022: 3.3%, 2021: 3.2%). Interest on lease liabilities as at December 31, 2023, is estimated to be USD 54 million for 2024 and USD 1.4 billion thereafter. |
2 Cash flows from short-term and low-value leases are included within total net cash flows from operating activities. The portfolio of short-term leases to which the Company is committed to at December 31, 2023, 2022 and 2021, is similar to the portfolio of short-term leases the Company entered into during 2023, 2022 and 2021. |
3 Included within total net cash flows from operating activities |
4 Reported as cash outflows in financing activities net of lease incentives received, if any. |
The net investment held and income from subleasing right-of-use assets were not significant for 2023, 2022, and 2021. Income from leasing Novartis property, plant and equipment to third parties for 2023, 2022 and 2021 was not significant.
12. Goodwill and intangible assets other than goodwill
Novartis has the following classes of available for use intangible assets other than goodwill: Currently marketed products and Other intangible assets.
Currently marketed products represent the composite value of acquired intellectual property (IP), patents, distribution rights and product trade names.
Other intangible assets include capitalized internally developed and acquired computer software and technologies, which represent identified and separable acquired know- how used in research, development, and production.
The following table summarizes the movements of goodwill and intangible assets other than goodwill in 2023:
| | Goodwill | | Intangible assets other than goodwill | |
| | | | | |
| | | | | |
| | | | | |
(USD millions)
| |
Total
| | In-process
research and
development | | Currently
marketed
products | | Other
intangible
assets | |
Total
| |
|
At January 1, 2023 | | | | | | | | | | | |
|
Cost | | 29 596 | | 7 092 | | 58 249 | | 4 343 | | 69 684 | |
|
Accumulated amortization and impairment | | -295 | | -2 671 | | -32 736 | | -2 633 | | -38 040 | |
|
Net book value | | 29 301 | | 4 421 | | 25 513 | | 1 710 | | 31 644 | |
|
| | | | | | | | | | | |
At January 1, 2023 | | 29 301 | | 4 421 | | 25 513 | | 1 710 | | 31 644 | |
|
Costs and accumulated amortization/impairments on assets related to discontinued operations 1 | | -7 445 | | -235 | | -1 026 | | -199 | | -1 460 | |
|
Impact of acquisitions of businesses | | 1 094 | | 2 931 | | | | 15 | | 2 946 | |
|
Reclassifications | | | | -235 | | 23 | | 212 | | | |
|
Additions | | | | 770 | | 290 | | 516 | | 1 576 | |
|
Disposals and derecognitions 2 | | | | | | -1 842 | | -3 | | -1 845 | |
|
Amortization charge | | | | | | -3 319 | | -641 | | -3 960 | |
|
Impairment charge | | | | -2 544 | | -310 | | -194 | | -3 048 | |
|
Currency translation effects | | 391 | | 221 | | 688 | | 117 | | 1 026 | |
|
At December 31, 2023 | | 23 341 | | 5 329 | | 20 017 | | 1 533 | | 26 879 | |
|
| | | | | | | | | | | |
At December 31, 2023 | | | | | | | | | | | |
|
Cost | | 23 391 | | 7 822 | | 46 909 | | 3 588 | | 58 319 | |
|
Accumulated amortization and impairment | | -50 | | -2 493 | | -26 892 | | -2 055 | | -31 440 | |
|
Net book value | | 23 341 | | 5 329 | | 20 017 | | 1 533 | | 26 879 | |
|
|
1 Represents the cost of assets and accumulated depreciation/impairments at January 1, 2023, related to the Sandoz business reported as discontinued operations, and the net transfers between discontinued and continuing operations from January 1, 2023 to October 3, 2023. Note 31 provides disclosure of discontinued operations additions, depreciation charge, impairment charge and reversals of impairment change. |
2 Derecognition of assets that are no longer being used or developed and are not considered to have a significant disposal value or other alternative use. Disposals include the divested currently marketed product Xiidra. |
The following table summarizes the movements of goodwill and intangible assets other than goodwill in 2022:
| | Goodwill | | Intangible assets other than goodwill | |
| | | | | |
| | | | | |
| | | | | |
(USD millions)
| |
Total
| | In-process
research and
development | | Currently
marketed
products | | Other
intangible
assets | |
Total
| |
|
At January 1, 2022 | | | | | | | | | | | |
|
Cost | | 29 900 | | 8 013 | | 56 213 | | 3 985 | | 68 211 | |
|
Accumulated amortization and impairment | | -305 | | -2 514 | | -29 107 | | -2 408 | | -34 029 | |
|
Net book value | | 29 595 | | 5 499 | | 27 106 | | 1 577 | | 34 182 | |
|
| | | | | | | | | | | |
At January 1, 2022 | | 29 595 | | 5 499 | | 27 106 | | 1 577 | | 34 182 | |
|
Impact of acquisitions of businesses | | 161 | | 1 209 | | | | | | 1 209 | |
|
Reclassifications 1 | | | | -1 429 | | 1 403 | | 26 | | | |
|
Additions 2 | | | | 330 | | 1 175 | | 588 | | 2 093 | |
|
Disposals and derecognitions 3 | | -28 | | -95 | | -3 | | -2 | | -100 | |
|
Amortization charge 4 | | | | | | -3 603 | | -379 | | -3 982 | |
|
Impairment charge 4 | | | | -917 | | -322 | | -87 | | -1 326 | |
|
Currency translation effects | | -427 | | -176 | | -243 | | -13 | | -432 | |
|
At December 31, 2022 | | 29 301 | | 4 421 | | 25 513 | | 1 710 | | 31 644 | |
|
| | | | | | | | | | | |
At December 31, 2022 | | | | | | | | | | | |
|
Cost | | 29 596 | | 7 092 | | 58 249 | | 4 343 | | 69 684 | |
|
Accumulated amortization and impairment | | -295 | | -2 671 | | -32 736 | | -2 633 | | -38 040 | |
|
Net book value | | 29 301 | | 4 421 | | 25 513 | | 1 710 | | 31 644 | |
|
|
1 Reclassifications between various asset categories as a result of product launches of acquired in-process research and development and completion of software development |
2 Additions in continuing operations were USD 1 930 million. Note 31 provides disclosure of discontinued operations additions. |
3 Derecognition of assets that are no longer being used or developed and are not considered to have a significant disposal value or other alternative use |
4 Note 31 provides disclosure of discontinued operations amortization charge and impairment charge. |
As at December 31, 2023, the most significant intangible assets within currently marketed products category are Leqvio (acquisition of The Medicines Company) and Zolgensma (acquisition of Avexis Inc.). As at December 31, 2023, the carrying value and remaining amortization period for Leqvio is USD 6.8 billion and 12 years, respectively (2022: USD 7.4 billion and 13 years, respectively), and for Zolgensma USD 5.2 billion and 7 years, respectively (2022: USD 5.9 billion and 8 years, respectively).
The following table shows the estimated useful life by category for intangible assets available for use and the line in the consolidated income statement in which the amortization and any potential impairment charge is recognized:
| |
Useful life
| | Income statement line
for amortization and
impairment charges | |
|
Currently marketed products | | 5 to 20 years | | "Cost of goods sold" | |
|
Other (including software and technologies) | | 3 to 15 years | | In the relevant functional expense, and for technologies in "Cost of goods sold" or "Research and Development" | |
|
Any impairment charge for IPR&D is recorded in the consolidated income statement under “Research and development.”
The Company has no indefinite useful life intangible asset other than goodwill.
The Company’s cash-generating units to which goodwill is allocated is at the level of the operating segment, which is comprised of a group of smaller cash-generating units. The valuation method of the recoverable amount of the operating segment to which goodwill is allocated is based on the fair value less costs of disposal. Any impairment charges are recorded under “Other expense” in the consolidated income statement.
The following assumptions were used in the goodwill impairment testing calculation:
(As a percentage) | | | |
|
Terminal growth rate | | 1.3 | |
|
Discount rate (post-tax) | | 8.0 | |
|
The discount rates consider the Company’s weighted average cost of capital, adjusted to approximate the weighted average cost of capital of a comparable market participant.
The fair value less costs of disposal, for all cash-generating units containing goodwill, is reviewed for the impact of reasonably possible changes in key assumptions. In particular, we considered an increase in the discount rate, a decrease in the terminal growth rate, and certain negative impacts on the forecasted cash flows. These reasonably possible changes in key assumptions did not indicate an impairment.
“Note 1. Accounting policies—Goodwill and intangible assets other than goodwill” provides additional disclosures on how the Company performs goodwill and intangible asset impairment testing.
The following table shows the intangible asset amortization charge and impairment charges for continuing operations for the years ended December 31, 2023, 2022 and 20211:
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
Amortization charge | | -3 960 | | -3 760 | | -3 665 | |
|
Impairment charge 2 | | -3 048 | | -1 301 | | -376 | |
|
|
1 Note 31 provides disclosure of discontinued operations amortization charge and impairment charge. |
2 2023 impairment charge includes the write-down of IPR&D on the cessation of clinical development programs, including PPY988 (USD 1.0 billion), which was acquired with the 2022 acquisition of Gyroscope Therapeutics Holdings plc (see Note 2), VDT482 (USD 0.4 billion), and MBG453 (USD 0.3 billion), and the clinical research program NIZ985 (USD 0.3 billion); as well as the write-down of a currently marketed product by USD 0.3 billion to reflect the reduction in its recoverable amount. |
2022 intangible asset impairment charges include the write-down of IPR&D on the cessation of clinical development programs, including UNR844 (USD 0.6 billion). |
2021 intangible asset impairment charges includes the write down of IPR&D on the cessation of clinical development programs, including GTX312 (USD 0.2 billion). |
In 2023, 2022 and 2021, there were no reversals of impairment charges on intangible assets.
13. Deferred tax assets and liabilities
(USD millions)
| |
Property,
plant and
equipment
| |
Intangible
assets
| | Pensions and
other benefit
obligations
of employees | |
Inventories
| |
Tax loss
carry-
forwards
| |
Other assets,
provisions
and accruals
| |
Total
| |
|
Gross deferred tax assets at January 1, 2023 | | 158 | | 1 726 | | 739 | | 2 214 | | 425 | | 2 789 | | 8 051 | |
|
Gross deferred tax liabilities at January 1, 2023 | | -343 | | -4 785 | | -420 | | -138 | | | | -1 312 | | -6 998 | |
|
Net deferred tax balance at January 1, 2023 | | -185 | | -3 059 | | 319 | | 2 076 | | 425 | | 1 477 | | 1 053 | |
|
| | | | | | | | | | | | | | | |
At January 1, 2023 | | -185 | | -3 059 | | 319 | | 2 076 | | 425 | | 1 477 | | 1 053 | |
|
Net deferred tax balance related to discontinued operations 1 | | 60 | | 120 | | -36 | | -311 | | -13 | | -233 | | -413 | |
|
Credited/(charged) to income | | -13 | | 1 344 | | 32 | | 386 | | 173 | | -47 | | 1 875 | |
|
Credited/(charged) to other comprehensive income | | -3 | | | | 16 | | | | | | -34 | | -21 | |
|
Impact of acquisitions of businesses | | -2 | | -530 | | | | | | 111 | | -19 | | -440 | |
|
Other movements | | -50 | | 85 | | 13 | | -28 | | 17 | | -30 | | 7 | |
|
Net deferred tax balance at December 31, 2023 | | -193 | | -2 040 | | 344 | | 2 123 | | 713 | | 1 114 | | 2 061 | |
|
| | | | | | | | | | | | | | | |
Gross deferred tax assets at December 31, 2023 | | 117 | | 2 188 | | 764 | | 2 200 | | 713 | | 2 206 | | 8 188 | |
|
Gross deferred tax liabilities at December 31, 2023 | | -310 | | -4 228 | | -420 | | -77 | | | | -1 092 | | -6 127 | |
|
Net deferred tax balance at December 31, 2023 | | -193 | | -2 040 | | 344 | | 2 123 | | 713 | | 1 114 | | 2 061 | |
|
| | | | | | | | | | | | | | | |
After offsetting the following amount of deferred tax assets and liabilities within the same tax jurisdiction, the balance amounts to: | | | | | | | | | | | | | | 3 879 | |
|
Deferred tax assets at December 31, 2023 | | | | | | | | | | | | | | 4 309 | |
|
Deferred tax liabilities at December 31, 2023 | | | | | | | | | | | | | | -2 248 | |
|
Net deferred tax balance at December 31, 2023 | | | | | | | | | | | | | | 2 061 | |
|
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
|
Gross deferred tax assets at January 1, 2022 | | 125 | | 1 307 | | 1 026 | | 2 273 | | 374 | | 2 727 | | 7 832 | |
|
Gross deferred tax liabilities at January 1, 2022 | | -381 | | -4 704 | | -591 | | -148 | | | | -1 335 | | -7 159 | |
|
Net deferred tax balance at January 1, 2022 | | -256 | | -3 397 | | 435 | | 2 125 | | 374 | | 1 392 | | 673 | |
|
| | | | | | | | | | | | | | | |
At January 1, 2022 | | -256 | | -3 397 | | 435 | | 2 125 | | 374 | | 1 392 | | 673 | |
|
Credited/(charged) to income 2 | | 69 | | 628 | | -5 | | -43 | | 5 | | 1 | | 655 | |
|
Charged to equity | | | | | | | | | | | | 1 | | 1 | |
|
Credited/(charged) to other comprehensive income 2 | | -2 | | | | -104 | | | | | | 63 | | -43 | |
|
Impact of acquisitions of businesses | | | | -300 | | | | | | 55 | | 1 | | -244 | |
|
Other movements 2 | | 4 | | 10 | | -7 | | -6 | | -9 | | 19 | | 11 | |
|
Net deferred tax balance at December 31, 2022 | | -185 | | -3 059 | | 319 | | 2 076 | | 425 | | 1 477 | | 1 053 | |
|
| | | | | | | | | | | | | | | |
Gross deferred tax assets at December 31, 2022 | | 158 | | 1 726 | | 739 | | 2 214 | | 425 | | 2 789 | | 8 051 | |
|
Gross deferred tax liabilities at December 31, 2022 | | -343 | | -4 785 | | -420 | | -138 | | | | -1 312 | | -6 998 | |
|
Net deferred tax balance at December 31, 2022 | | -185 | | -3 059 | | 319 | | 2 076 | | 425 | | 1 477 | | 1 053 | |
|
| | | | | | | | | | | | | | | |
After offsetting the following amount of deferred tax assets and liabilities within the same tax jurisdiction, the balance amounts to: | | | | | | | | | | | | | | 4 312 | |
|
Deferred tax assets at December 31, 2022 | | | | | | | | | | | | | | 3 739 | |
|
Deferred tax liabilities at December 31, 2022 | | | | | | | | | | | | | | -2 686 | |
|
Net deferred tax balance at December 31, 2022 | | | | | | | | | | | | | | 1 053 | |
|
|
1 Represents the net deferred tax balance at January 1, 2023, related to the Sandoz business reported as discontinued operations. Notes 1, 2 and 31 provide disclosures related to discontinued operations. |
2 In 2022 the total related to continuing operations for the charge to income was USD 625 million, for the charge to other comprehensive income was USD -20 million and for the charge to other movements was USD 8 million. |
Deferred tax liabilities have not been recognized for the withholding tax and other taxes that would be payable on the remittance of earnings of foreign subsidiaries, insofar as the Company has the ability to control any future reversal and the unremitted earnings are retained in the foreign subsidiaries for reinvestment. The total unremitted earnings retained for reinvestment in the Company’s foreign subsidiaries that would be subject to withholding tax or other taxes if remitted to the Company were estimated to be approximately USD 34 billion in 2023, (2022: USD 32 billion).
The gross value of tax-loss carry-forwards that have or have not been recognized as deferred tax assets, with their expiry dates, is as follows:
(USD millions) | | Unrecognized | | Recognized | | 2023 total | |
|
One year | | 23 | | 44 | | 67 | |
|
Two years | | 12 | | 15 | | 27 | |
|
Three years | | 67 | | 79 | | 146 | |
|
Four years | | 22 | | 569 | | 591 | |
|
Five years | | 1 569 | | 580 | | 2 149 | |
|
More than five years | | 2 891 | | 2 975 | | 5 866 | |
|
Not subject to expiry | | 687 | | 2 258 | | 2 945 | |
|
Total | | 5 271 | | 6 520 | | 11 791 | |
|
| | | | | | | |
(USD millions) | | Unrecognized | | Recognized | | 2022 total | |
|
One year | | 18 | | 0 | | 18 | |
|
Two years | | 37 | | 5 | | 42 | |
|
Three years | | 25 | | 5 | | 30 | |
|
Four years | | 138 | | 0 | | 138 | |
|
Five years | | 79 | | 688 | | 767 | |
|
More than five years | | 3 880 | | 2 380 | | 6 260 | |
|
Not subject to expiry | | 433 | | 452 | | 885 | |
|
Total | | 4 610 | | 3 530 | | 8 140 | |
|
|
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
Tax losses carried forward that expired | | 8 | | 6 | | 18 | |
|
Deferred tax assets related to carry-forwards of taxable losses and tax credits of relevant Company entities are recognized to the extent that it is considered probable that future taxable profits will be available in the respective tax jurisdictions against which such losses and credits can be utilized.
14. Financial and other non-current assets
Financial assets
(USD millions) | | 2023 | | 2022 | |
|
Equity securities | | 1 403 | | 1 145 | |
|
Debt securities | | 29 | | 37 | |
|
Fund investments | | 190 | | 281 | |
|
Total financial investments | | 1 622 | | 1 463 | |
|
Long-term receivables from finance subleases | | 104 | | 59 | |
|
Other long-term receivables | | 214 | | 197 | |
|
Contingent consideration receivables 1 | | 553 | | 607 | |
|
Long-term loans, advances and security deposits | | 114 | | 85 | |
|
Total financial assets | | 2 607 | | 2 411 | |
|
|
1 Note 30 provides additional disclosures related to contingent consideration. |
(USD millions) | | 2023 | | 2022 | |
|
Deferred compensation plans | | 439 | | 419 | |
|
Prepaid post-employment benefit plans 1 | | 545 | | 491 | |
|
Other non-current assets | | 215 | | 200 | |
|
Total other non-current assets | | 1 199 | | 1 110 | |
|
|
1 Note 26 provides additional disclosures related to post-employment benefits. |
15. Inventories
(USD millions) | | 2023 | | 2022 | |
|
Raw material, consumables | | 963 | | 934 | |
|
Work in progress | | 3 502 | | 3 673 | |
|
Finished products | | 1 448 | | 2 568 | |
|
Total inventories | | 5 913 | | 7 175 | |
|
The following table shows the amount of inventory recognized as an expense in “Cost of goods sold” in the consolidated income statements from continuing operations:
(USD billions) | | 2023 | | 2022 | | 2021 | |
|
Cost of goods sold | | -5.8 | | -5.2 | | -5.4 | |
|
The following table shows the recognized amount of inventory provision and reversals of inventory provision recorded in the consolidated income statements from continuing operations:
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
Inventory provisions | | -467 | | -373 | | -283 | |
|
Reversals of inventory provisions | | 111 | | 121 | | 97 | |
|
The reversals mainly result from the release of products initially requiring additional quality control inspections and from the reassessment of inventory values manufactured prior to regulatory approval but for which approval was subsequently received.
16. Trade receivables
(USD millions) | | 2023 | | 2022 | |
|
Total gross trade receivables | | 7 158 | | 8 128 | |
|
Provisions for doubtful trade receivables | | -51 | | -62 | |
|
Total trade receivables | | 7 107 | | 8 066 | |
|
The following table shows the trade receivables that are not overdue as specified in the payment terms and conditions established with Novartis customers, as well as an analysis of overdue amounts and related provisions for doubtful trade receivables:
(USD millions) | | 2023 | | 2022 | |
|
Not overdue | | 6 791 | | 7 664 | |
|
Past due for not more than one month | | 146 | | 190 | |
|
Past due for more than one month but less than three months | | 66 | | 110 | |
|
Past due for more than three months but less than six months | | 64 | | 62 | |
|
Past due for more than six months but less than one year | | 38 | | 23 | |
|
Past due for more than one year | | 53 | | 79 | |
|
Provisions for doubtful trade receivables | | -51 | | -62 | |
|
Total trade receivables | | 7 107 | | 8 066 | |
|
Trade receivable balances represent amounts due from our customers, which are mainly drug wholesalers, retailers, private health systems, government agencies, managed care providers, pharmacy benefit managers and government-supported healthcare systems. In particular, we monitor the level of trade receivables in countries deemed to have an elevated credit risk. We consider macroeconomic environment, historical experience, country and political risk, in addition to other relevant information when assessing risk. These risk factors are monitored regularly to determine any adjustments in risk classification. The majority of the past due trade receivables from elevated credit risk countries are due from local governments or from government-funded entities. Deteriorating credit and economic conditions as well as other factors in these elevated credit risk countries have resulted in, and may continue to result in, an increase in the average length of time that it takes to collect these trade receivables, and may require the Company to re-evaluate the expected credit loss amount of these trade receivables in future periods. At December 31, 2023, amounts past due for more than one year are not significant in elevated credit risk countries.
Total trade receivables include amounts denominated in the following major currencies:
(USD millions) | | 2023 | | 2022 | |
|
US dollar (USD) | | 3 520 | | 3 709 | |
|
Euro (EUR) | | 1 138 | | 1 426 | |
|
Japanese yen (JPY) | | 288 | | 177 | |
|
Russian ruble (RUB) | | 240 | | 430 | |
|
Chinese yuan (CNY) | | 231 | | 155 | |
|
British pound (GBP) | | 146 | | 176 | |
|
Brazilian real (BRL) | | 130 | | 145 | |
|
Australian dollar (AUD) | | 96 | | 137 | |
|
Swiss franc (CHF) | | 84 | | 108 | |
|
Canadian dollar (CAD) | | 75 | | 151 | |
|
Other currencies | | 1 159 | | 1 452 | |
|
Total trade receivables | | 7 107 | | 8 066 | |
|
17. Marketable securities, commodities, time deposits, derivative financial instruments, and cash and cash equivalents
Marketable securities, commodities, time deposits and derivative financial instruments
(USD millions) | | 2023 | | 2022 | |
|
Commodities | | 111 | | 111 | |
|
Debt securities | | | | 9 | |
|
Time deposits and short-term investments with original maturity more than 90 days | | 569 | | 11 089 | |
|
Derivative financial instruments | | 355 | | 204 | |
|
Total marketable securities, commodities, time deposits and derivative financial instruments | | 1 035 | | 11 413 | |
|
The vast majority of debt securities, time deposits and short-term investments with an original maturity of more than 90 days was denominated in USD as at December 31, 2023, and 2022.
Cash and cash equivalents
(USD millions) | | 2023 | | 2022 | |
|
Current accounts | | 3 207 | | 2 877 | |
|
Time deposits and short-term investments with original maturity less than 90 days | | 10 186 | | 4 640 | |
|
Total cash and cash equivalents | | 13 393 | | 7 517 | |
|
18. Other current assets
(USD millions) | | 2023 | | 2022 | |
|
VAT receivable | | 462 | | 509 | |
|
Withholding tax recoverable | | 64 | | 50 | |
|
Prepaid expenses | | 764 | | 911 | |
|
Contingent consideration receivable 1 | | 65 | | 43 | |
|
Other receivables and current assets | | 1 252 | | 958 | |
|
Total other current assets | | 2 607 | | 2 471 | |
|
|
1 Note 30 provides additional disclosures related to contingent consideration. |
19. Equity
The following table shows the movement in the share capital:
(USD millions)
| |
Jan 1, 2021
| | Movement
in year | |
Dec 31, 2021
| | Movement
in year | |
Dec 31, 2022
| | Movement
in year | |
Dec 31, 2023
| |
|
Share capital 1 | | 913 | | -12 | | 901 | | -11 | | 890 | | -65 | | 825 | |
|
Treasury shares | | -53 | | 5 | | -48 | | -44 | | -92 | | 51 | | -41 | |
|
Outstanding share capital | | 860 | | -7 | | 853 | | -55 | | 798 | | -14 | | 784 | |
|
|
1 At December 31, 2023, the Novartis AG share capital consists of registered shares with a nominal value of CHF 0.49 each. Prior to the 2023 capital decrease (see Note 19.3), Novartis AG share capital at December 31, 2022 and 2021 consists of registered shares with a nominal value of CHF 0.50 each. No authorized and conditional capital exists. |
The following table shows the movement in the shares:
| | | | 2023 | | 2022 | | 2021 | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
Number of outstanding shares
(in millions)
| |
Note
| | Total
Novartis
shares | | Total
treasury
shares1 | | Total
outstanding
shares | | Total
Novartis
shares | | Total
treasury
shares1 | | Total
outstanding
shares | | Total
Novartis
shares | | Total
treasury
shares1 | | Total
outstanding
shares | |
|
Balance at beginning of year | | | | 2 403.7 | | -284.1 | | 2 119.6 | | 2 434.4 | | -199.5 | | 2 234.9 | | 2 467.0 | | -210.2 | | 2 256.8 | |
|
Shares canceled for capital reduction 2 | | | | -126.2 | | 126.2 | | | | -30.7 | | 30.7 | | | | -32.6 | | 32.6 | | | |
|
Shares acquired to be canceled 3 | | | | | | -87.5 | | -87.5 | | | | -126.2 | | -126.2 | | | | -30.7 | | -30.7 | |
|
Other share purchases 4 | | | | | | -1.6 | | -1.6 | | | | -1.4 | | -1.4 | | | | -1.5 | | -1.5 | |
|
Exercise of options and employee transactions 5 | | 19.9 | | | | 2.8 | | 2.8 | | | | 1.9 | | 1.9 | | | | 0.6 | | 0.6 | |
|
Equity-based compensation 5 | | | | | | 10.4 | | 10.4 | | | | 10.4 | | 10.4 | | | | 9.6 | | 9.6 | |
|
Shares delivered to Alcon employees | | | | | | | | | | | | 0.0 | | 0.0 | | | | 0.1 | | 0.1 | |
|
Shares delivered to Sandoz employees | | | | | | 0.3 | | 0.3 | | | | | | | | | | | | | |
|
Total movements | | | | -126.2 | | 50.6 | | -75.6 | | -30.7 | | -84.6 | | -115.3 | | -32.6 | | 10.7 | | -21.9 | |
|
Balance at end of year | | | | 2 277.5 | | -233.5 | | 2 044.0 | | 2 403.7 | | -284.1 | | 2 119.6 | | 2 434.4 | | -199.5 | | 2 234.9 | |
|
|
1 Approximately 93.8 million treasury shares (2022: 99.0 million; 2021: 102.5 million) are held in Novartis entities that restrict their availability for use. |
2 Novartis reduced its share capital by canceling shares that were repurchased on the SIX Swiss Exchange second trading line during previous years. |
3 Shares repurchased on the SIX Swiss Exchange second trading line under a CHF 10 billion share buyback authority approved at the 2019 Annual General Meeting (AGM) for transactions after February 28, 2019, until March 2, 2021. Transactions after March 2, 2021, were executed under the CHF 10 billion share buyback authority approved at the 2021 AGM and the additional CHF 10 billion authority approved at the 2022 AGM. |
4 Shares acquired from employees, which were previously granted to them under the respective equity-based participation plans |
5 Shares delivered as a result of options being exercised and physical share deliveries related to equity-based participation plans |
|
19.1) The amount available for distribution as a dividend to shareholders is based on the available distributable retained earnings of Novartis AG determined in accordance with the legal provisions of the Swiss Code of Obligations.
| | 2023 | | 2022 | | 2021 | |
|
Dividend per share (in CHF) | | 3.20 | | 3.10 | | 3.00 | |
|
Total dividend payment (in USD billion) | | 7.3 | | 7.5 | | 7.4 | |
|
19.2) Treasury shares are initially recorded at fair value on their trade date, which is different from the settlement date, when the transaction is ultimately effected. Treasury shares are deducted from consolidated equity at their nominal per share value. Differences between the nominal amount and the transaction price on purchases or sales of treasury shares with third parties, or the value of services received for the shares allocated to employees as part of share-based compensation arrangements, are recorded in “Retained earnings” in the consolidated statement of changes in equity.
The following table summarizes the treasury shares movements:
| | | | 2023 | | 2022 | | 2021 | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| |
Note
| | Number of
outstanding
shares
(in millions) | |
Equity impact
USD m
| | Number of
outstanding
shares
(in millions) | |
Equity impact
USD m
| | Number of
outstanding
shares
(in millions) | |
Equity impact
USD m
| |
|
Shares acquired to be canceled 1 | | | | -87.5 | | -8 369 | | -126.2 | | -10 787 | | -30.7 | | -2 775 | |
|
Other share purchases 2 | | | | -1.6 | | -148 | | -1.4 | | -123 | | -1.5 | | -145 | |
|
Purchase of treasury shares | | | | -89.1 | | -8 517 | | -127.6 | | -10 910 | | -32.2 | | -2 920 | |
|
Exercise of options and employee transactions 3 | | 19.9 | | 2.8 | | 146 | | 1.9 | | 88 | | 0.6 | | 39 | |
|
Equity-based compensation 4 | | | | 10.4 | | 904 | | 10.4 | | 854 | | 9.6 | | 745 | |
|
Shares delivered to Alcon employees | | | | | | | | 0.0 | | 5 | | 0.1 | | 17 | |
|
Shares delivered to Sandoz employees | | | | 0.3 | | 30 | | | | | | | | | |
|
Total | | | | -75.6 | | -7 437 | | -115.3 | | -9 963 | | -21.9 | | -2 119 | |
|
|
1 Shares repurchased on the SIX Swiss Exchange second trading line under a CHF 10 billion share buyback authority approved at the 2019 Annual General Meeting (AGM) for transactions after February 28, 2019, until March 2, 2021. Transactions after March 2, 2021, were executed under the CHF 10 billion share buyback authority approved at the 2021 AGM and the additional CHF 10 billion authority approved at the 2022 AGM. |
2 Shares acquired from employees, which were previously granted to them under the respective equity-based participation plans |
3 Shares delivered as a result of options being exercised related to equity-based participation plans and the delivery of treasury shares. The average share price of the shares delivered was significantly below market price, reflecting the strike price of the options exercised. |
4 Equity-settled share-based compensation is expensed in the consolidated income statement in accordance with the vesting period of the share-based compensation plans. The value for the shares and options granted is credited to consolidated equity over the respective vesting period. In addition, tax benefits arising from tax-deductible amounts exceeding the expense recognized in the income statement are credited to equity. |
|
19.3) In 2023, in connection with the Distribution (spin-off) of Sandoz business, Novartis AG shareholders approved at the EGM held on September 15, 2023, a decrease in Novartis AG share capital in the amount of CHF 22.8 million (USD 17.1 million). The capital decrease resulted in a reduction of the nominal value of the Novartis AG shares by CHF 0.01 from CHF 0.50 per share to CHF 0.49 per share.
19.4) In December 2021, Novartis entered into an irrevocable, non-discretionary arrangement with a bank to repurchase Novartis shares on the second trading line under its up-to USD 15.0 billion share buyback. The arrangement was updated in July 2022, December 2022, and May 2023, and concluded in June 2023. Novartis was able to cancel this arrangement at any time but could have been subject to a 90-day waiting period. As of December 31, 2022, these waiting period conditions were not applicable and as a result, there was no requirement to record a liability under this arrangement as of December 31, 2022. The liability under this arrangement amounted to USD 2.8 billion as at December 31, 2021.
In June 2023, Novartis entered into an irrevocable, non-discretionary arrangement with a bank to repurchase 11.7 million Novartis shares on the second trading line, which concluded in July 2023.
In July 2023, Novartis entered into a new irrevocable, non-discretionary arrangement with a bank to repurchase Novartis shares on the second trading line under its new up-to USD 15.0 billion share buyback. Novartis is able to cancel this arrangement but may be subject to a 90-day waiting period under certain conditions. As of December 31, 2023, these waiting period conditions were not applicable and as a result, there was no requirement to record a liability under this arrangement as of December 31, 2023.
In June 2021, Novartis entered into an irrevocable, non-discretionary arrangement with a bank to repurchase Novartis shares to mitigate dilution related to participation plans of employees. Novartis would have been able to cancel this arrangement at any time but would have been subject to a 90-day waiting period. This trading plan commitment was fully executed and expired in June 2021, and as a consequence, there was no liability related to this plan recognized as of December 31, 2021.
In November 2020, Novartis entered into an irrevocable, non-discretionary arrangement with a bank to repurchase Novartis shares on the second trading line under its up-to USD 2.5 billion share buyback. Novartis would have been able to cancel this arrangement at any time, but would have been subject to a 90-day waiting period. This trading plan commitment was fully executed and expired in March 2021, and as a consequence, there was no liability related to this plan recognized as of December 31, 2021.
19.5) The impact of change in ownership of consolidated entities represents the excess of the amount paid to non-controlling interest over their carrying value and equity allocation to non-controlling interest due to change in ownership percentage.
19.6) Changes in non-controlling interests represent the impact on the non-controlling interest of transactions with minority shareholders, such as change in ownership percentage, dividend payments and other equity transactions.
19.7) Other movements include, for subsidiaries in hyperinflationary economies, the impact of the application of IAS 29 “Financial reporting in Hyperinflation Economies”. See Note 30 for additional disclosures.
19.8) Transaction costs in 2023 of USD 214 million, net of tax of USD 29 million, that are directly attributable to the Distribution (spin-off) of Sandoz business to Novartis AG shareholders and that would otherwise have been avoided, are recorded as a deduction from equity (retained earnings). See Note 1.
In 2021, transaction costs that were directly attributable to the distribution (spin-off) of Alcon Inc. to Novartis AG shareholders and that would otherwise have been avoided, were recorded to equity.
19.9) At December 31, 2022, the market maker held 3 million (2021: 3 million) written call options, originally issued as part of the share-based compensation for employees, that have not yet been exercised. The weighted average exercise price of these options at December 31, 2022, was USD 66.07 (2021: USD 61.45), and they had contractual lives of 10 years, with remaining lives less than one year (2021: two years). In the first quarter of 2023, the market maker exercised 3 million written call options and as a result there are no written call options outstanding at December 31, 2023.
20. Non-current financial debt
(USD millions) | | 2023 | | 2022 | |
|
Straight bonds | | 20 585 | | 22 341 | |
|
Liabilities to banks and other financial institutions 1 | | 42 | | 144 | |
|
Total, including current portion of non-current financial debt | | 20 627 | | 22 485 | |
|
Less current portion of non-current financial debt | | -2 191 | | -2 241 | |
|
Total non-current financial debt | | 18 436 | | 20 244 | |
|
|
1 Average interest rate during the year 2023 2.6% (2022: 2.3%) |
All bonds are initially recorded at the amount of proceeds received, net of transaction costs. They are subsequently carried at amortized cost, with the difference between the proceeds, net of transaction costs, and the amount due on redemption being recognized as a charge to the consolidated income statement over the period of the relevant bond. Financial debts, including current financial debts, contain only general default covenants. The Company is in compliance with these covenants.
The percentage of fixed-rate financial debt to total financial debt was 84% as at December 31, 2023, and 86% as at December 31, 2022.
The average interest rate on total financial debt in 2023 was 2.9% (2022: 2.4%).
Note 30 contains a maturity table of the Company’s future contractual interest payments commitments.
The following table provides a breakdown of straight bonds:
Coupon
| |
Currency
| | Notional
amount
(millions) | |
Issuance
year
| |
Maturity
year
| |
Issuer
| |
Issue price
| | 2023
(USD
millions) | | 2022
(USD
millions) | |
|
3.700% | | USD | | 500 | | 2012 | | 2042 | | Novartis Capital Corporation, New York, United States | | 98.325% | | 491 | | 490 | |
|
3.400% | | USD | | 2 150 | | 2014 | | 2024 | | Novartis Capital Corporation, New York, United States | | 99.287% | | 2 150 | | 2 147 | |
|
4.400% | | USD | | 1 850 | | 2014 | | 2044 | | Novartis Capital Corporation, New York, United States | | 99.196% | | 1 828 | | 1 827 | |
|
1.625% | | EUR | | 600 | | 2014 | | 2026 | | Novartis Finance S.A., Luxembourg, Luxembourg | | 99.697% | | 663 | | 638 | |
|
0.250% | | CHF | | 500 | | 2015 | | 2025 | | Novartis AG, Basel, Switzerland | | 100.640% | | 595 | | 541 | |
|
0.625% | | CHF | | 550 | | 2015 | | 2029 | | Novartis AG, Basel, Switzerland | | 100.502% | | 654 | | 595 | |
|
1.050% | | CHF | | 325 | | 2015 | | 2035 | | Novartis AG, Basel, Switzerland | | 100.479% | | 387 | | 352 | |
|
3.000% | | USD | | 1 750 | | 2015 | | 2025 | | Novartis Capital Corporation, New York, United States | | 99.010% | | 1 745 | | 1 742 | |
|
4.000% | | USD | | 1 250 | | 2015 | | 2045 | | Novartis Capital Corporation, New York, United States | | 98.029% | | 1 222 | | 1 221 | |
|
0.125% | | EUR | | 1 250 | | 2016 | | 2023 | | Novartis Finance S.A., Luxembourg, Luxembourg | | 99.127% | | | | 1 330 | |
|
0.625% | | EUR | | 500 | | 2016 | | 2028 | | Novartis Finance S.A., Luxembourg, Luxembourg | | 98.480% | | 549 | | 528 | |
|
3.100% | | USD | | 1 000 | | 2017 | | 2027 | | Novartis Capital Corporation, New York, United States | | 99.109% | | 995 | | 994 | |
|
1.125% | | EUR | | 600 | | 2017 | | 2027 | | Novartis Finance S.A., Luxembourg, Luxembourg | | 99.874% | | 662 | | 638 | |
|
0.500% | | EUR | | 750 | | 2018 | | 2023 | | Novartis Finance S.A., Luxembourg, Luxembourg | | 99.655% | | | | 798 | |
|
1.375% | | EUR | | 750 | | 2018 | | 2030 | | Novartis Finance S.A., Luxembourg, Luxembourg | | 99.957% | | 828 | | 797 | |
|
1.700% | | EUR | | 750 | | 2018 | | 2038 | | Novartis Finance S.A., Luxembourg, Luxembourg | | 99.217% | | 823 | | 792 | |
|
1.750% | | USD | | 1 000 | | 2020 | | 2025 | | Novartis Capital Corporation, New York, United States | | 99.852% | | 999 | | 998 | |
|
2.000% | | USD | | 1 250 | | 2020 | | 2027 | | Novartis Capital Corporation, New York, United States | | 99.909% | | 1 247 | | 1 246 | |
|
2.200% | | USD | | 1 500 | | 2020 | | 2030 | | Novartis Capital Corporation, New York, United States | | 99.869% | | 1 495 | | 1 494 | |
|
2.750% | | USD | | 1 250 | | 2020 | | 2050 | | Novartis Capital Corporation, New York, United States | | 97.712% | | 1 216 | | 1 215 | |
|
0.000% 1 | | EUR | | 1 850 | | 2020 | | 2028 | | Novartis Finance S.A., Luxembourg, Luxembourg | | 99.354% | | 2 036 | | 1 958 | |
|
Total straight bonds | | | | | | | | | | | | | | 20 585 | | 22 341 | |
|
|
1 The EUR 1 850 million bond issued in 2020 features a coupon step-up of 0.25% commencing with the first interest payment date after December 31, 2025, if one or both of the 2025 Patient Access Targets are not met. These 2025 Patient Access Targets are the 2025 Flagship Programs Patient Reach Target and the 2025 Strategic Innovative Therapies Patient Reach Target, as defined in the bond prospectus. As of December 31, 2023, there is no indication that these 2025 Patient Access Targets will not be met. |
The following tables provide a breakdown of total non-current financial debt, including current portion by maturity and currency:
Breakdown by maturity:
(USD millions) | | 2023 | | 2022 | |
|
2023 | | | | 2 241 | |
|
2024 | | 2 191 | | 2 147 | |
|
2025 | | 3 338 | | 3 281 | |
|
2026 | | 663 | | 638 | |
|
2027 | | 2 906 | | 2 909 | |
|
2028 | | 2 585 | | 2 485 | |
|
After 2028 | | 8 944 | | 8 784 | |
|
Total | | 20 627 | | 22 485 | |
|
(USD millions) | | 2023 | | 2022 | |
|
US dollar (USD) | | 13 388 | | 13 376 | |
|
Euro (EUR) | | 5 563 | | 7 478 | |
|
Japanese yen (JPY) | | | | 76 | |
|
Swiss franc (CHF) | | 1 635 | | 1 488 | |
|
Others | | 41 | | 67 | |
|
Total | | 20 627 | | 22 485 | |
|
The following table shows the comparison of balance sheet carrying value and fair value of total non-current financial debt, including current portion:
(USD millions)
| | 2023
Balance
sheet | | 2023
Fair
values | | 2022
Balance
sheet | | 2022
Fair
values | |
|
Straight bonds | | 20 585 | | 19 194 | | 22 341 | | 20 277 | |
|
Others | | 42 | | 42 | | 144 | | 144 | |
|
Total | | 20 627 | | 19 236 | | 22 485 | | 20 421 | |
|
The fair values of straight bonds are determined by quoted market prices. Other financial debts are recorded at notional amounts, which are a reasonable approximation of the fair values.
21. Provisions and other non-current liabilities
(USD millions) | | 2023 | | 2022 | |
|
Accrued liability for employee benefits: | | | | | |
Defined benefit pension plans 1 | | 1 815 | | 1 723 | |
|
Other long-term employee benefits and deferred compensation | | 546 | | 554 | |
|
Other post-employment benefits 1 | | 369 | | 362 | |
|
Environmental remediation provisions | | 518 | | 535 | |
|
Provisions for product liabilities, governmental investigations and other legal matters | | 82 | | 154 | |
|
Contingent consideration 2 | | 389 | | 704 | |
|
Other non-current liabilities | | 804 | | 874 | |
|
Total provisions and other non-current liabilities | | 4 523 | | 4 906 | |
|
|
1 Note 26 provides additional disclosures related to post-employment benefits. |
2 Note 30 provides additional disclosures related to contingent consideration. |
Novartis believes that its total provisions are adequate based upon currently available information. However, given the inherent difficulties in estimating liabilities in this area, Novartis may incur additional costs beyond the amounts provided. Management believes that such additional amounts, if any, would not be material to the Company’s financial condition but could be material to the results of operations or cash flows in a given period.
Environmental remediation provisions
The following table shows the movements in the environmental liability provisions:
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
January 1 | | 588 | | 616 | | 809 | |
|
Provisions related to discontinued operations 1 | | -53 | | | | | |
|
Cash payments 2 | | -4 | | -6 | | -169 | |
|
Releases of provisions 3 | | -54 | | - 18 | | -105 | |
|
Additions to provisions 4 | | 14 | | 6 | | 105 | |
|
Currency translation effects | | 47 | | -10 | | -24 | |
|
December 31 | | 538 | | 588 | | 616 | |
|
Less current provision | | -20 | | -53 | | -49 | |
|
Non-current environmental remediation provisions at December 31 | | 518 | | 535 | | 567 | |
|
|
1 Represents the environmental remediation provision at January 1, 2023, related to the Sandoz business reported as discontinued operations. Notes 1, 2 and 31 provide disclosures related to discontinued operations. |
2 Cash payments from continuing operations were USD 5 million in 2022, and USD 169 million in 2021. |
3 Releases of provisions credited to the consolidated income statement from continuing operations were USD 18 million in 2022, and USD 105 million in 2021. |
4 Additions to provisions charged to the consolidated income statement from continuing operations were USD 6 million in 2022, and USD 105 million in 2021. |
The significant components of the environmental remediation provisions consist of costs to sufficiently clean and refurbish contaminated sites to the extent necessary, and to continue surveillance at sites where the environmental remediation exposure is less significant.
A substantial portion of the environmental remediation provisions relate to the remediation of Basel regional landfills in the adjacent border areas in Switzerland, Germany and France. The provisions are reassessed on an annual basis and adjusted as necessary.
In the United States, Novartis has been named under federal legislation (the Comprehensive Environmental Response, Compensation and Liability Act of 1980, as amended) as a potentially responsible party (PRP) in respect of certain sites. Novartis actively participates in, or monitors, the cleanup activities at the sites in which it is a PRP. The provision takes into consideration the number of other PRPs at each site as well as the identity and financial position of such parties in light of the joint and several nature of the liability.
The expected timing of the related cash outflows as of December 31, 2023, is currently projected as follows:
(USD millions)
| | Expected
cash outflows | |
|
Due within two years | | 82 | |
|
Due later than two years, but within five years | | 158 | |
|
Due later than five years, but within 10 years | | 217 | |
|
Due after 10 years | | 81 | |
|
Total environmental remediation provisions | | 538 | |
|
Provisions for product liabilities, governmental investigations and other legal matters
Novartis has established provisions for certain product liabilities, governmental investigations and other legal matters where a potential cash outflow is probable, and Novartis can make a reliable estimate of the amount of the outflow. These provisions represent the Company’s current best estimate of the total financial effect for the matters described below and for other less significant matters. Potential cash outflows reflected in a provision might be fully or partially offset by insurance in certain circumstances.
Novartis has not established provisions for potential damage awards for certain additional legal claims against its subsidiaries if Novartis currently believes that a payment is either not probable or cannot be reliably estimated. These not-provisioned-for matters include individual product liability cases and certain other legal matters. Plaintiffs have alleged claims in these matters and the Company does not believe that information about the amount sought by plaintiffs, if that is known, would be meaningful with respect to those legal proceedings. This is due to a number of factors, including, but not limited to, the stage of proceedings, the entitlement of parties to appeal a decision and clarity as to theories of liability, damages and governing law. It is therefore, not practicable to provide information about the potential financial impact of these matters. In addition, in some of these matters there are claims for punitive or multiple (treble) damages, civil penalties and disgorgement of profits that in the view of Novartis are either wholly or partially unspecified, or wholly or partially unquantifiable at present; the Company believes that information about these amounts claimed by plaintiffs generally is not meaningful for purposes of determining a reliable estimate of a loss that is probable or more than remote.
A number of other legal matters are in such early stages or the issues presented are such that the Company has not made any provisions since it cannot currently estimate either a potential outcome or the amount of any potential losses. For these reasons, among others, the Company generally is unable to make a reliable estimate of possible loss with respect to such cases. It is therefore not practicable to provide information about the potential financial impact of those cases.
There might also be cases for which the Company was able to make a reliable estimate of the possible loss or the range of possible loss, but the Company believes that publication of such information on a case-by-case basis would seriously prejudice the Company’s position in ongoing legal proceedings or in any related settlement discussions. Accordingly, in such cases, information has been disclosed with respect to the nature of the contingency, but no disclosure is provided as to an estimate of the possible loss or range of possible loss.
Note 29 contains additional information on contingent liabilities.
Summary of significant legal proceedings
The following is a summary of significant legal proceedings to which Novartis or its subsidiaries are currently a party, or were a party and that concluded in 2023.
Investigations and related litigations
Southern District of New York (S.D.N.Y.) Gilenya marketing practices investigation and litigation
In 2013, Novartis Pharmaceuticals Corporation (NPC) received a civil investigative demand from the United States Attorney’s Office (USAO) for the S.D.N.Y. requesting the production of documents and information relating to marketing practices for Gilenya, including the remuneration of healthcare providers in connection therewith. In 2017, the S.D.N.Y. and New York State declined to intervene in claims raised by an individual relator in a qui tam complaint. In 2022, NPC’s motion to dismiss this complaint was granted, which was appealed. The claims are being vigorously contested.
Lucentis/Avastin® matters
In 2019, the French Competition Authority (FCA) issued a Statement of Objections against Novartis entities, alleging anti-competitive practices on the French market for anti-vascular endothelial growth factor treatments for wet age-related macular degeneration from 2008 to 2013. In 2020, the FCA issued a decision finding that the Novartis entities had infringed competition law by abusing a dominant position and imposing a fine equivalent to approximately USD 452 million. Novartis paid the fine, again subject to recoupment, and appealed the FCA’s decision. In February 2023, the Paris Court of Appeal (Court) overturned the FCA’s decision which triggered the reimbursement of the originally paid fine (recorded as “Other income” in the Company’s consolidated income statement), and, in March 2023, the FCA appealed the Court’s decision.
Novartis is the subject of similar investigations and proceedings involving the competition authority in Greece and is currently in an appeal process in Turkey. Novartis continues to vigorously contest all claims in both countries. Novartis is also challenging policies and regulations allowing off-label/unlicensed use and reimbursement for economic reasons in Turkey.
Greece investigation
The Greek authorities are investigating legacy allegations of potentially inappropriate economic benefits to healthcare providers (HCPs), government officials and others in Greece. These authorities include the Greek Coordinating Body for Inspection and Control, and the Greek Body of Prosecution of Financial Crime (SDOE), from which the Company received a summons in 2018 and 2020. Novartis has cooperated in these investigations. In 2021, SDOE imposed on Novartis Hellas a fine equivalent to approximately USD 1.2 million; Novartis Hellas appealed the fine and, in September 2023, the Court overturned the decision and fine. The Greek State filed an appeal. In 2022, the Greek State served a civil lawsuit on Novartis Hellas, seeking approximately USD 225 million for moral damages allegedly arising from the conduct that was the subject of the Company’s 2020 settlement with the US Department of Justice (DOJ) regarding allegations of inappropriate economic benefits in Greece that was disclosed in the 2020 Annual Report and the 2020 Form 20-F. The claims are being vigorously contested.
340B Drug Pricing Program investigations
In 2021, NPC received a notification from the US Health Resources and Services Administration (HRSA) which stated that HRSA believes NPC’s contract pharmacy policy violates the 340B statute, and threatened potential enforcement action. NPC subsequently sued HRSA in the U.S. District Court (USDC) for the District of Columbia to challenge HRSA’s determination and to enjoin HRSA from taking action with respect to NPC’s contract pharmacy policy. HRSA then referred the matter regarding NPC’s contract pharmacy policy to the Office of Inspector General of the US Department of Health and Human Services, which could result in the imposition of civil monetary penalties on NPC. The USDC issued a decision rejecting HRSA’s interpretation of the 340B statute, vacating the violation notification and remanding the matter to HRSA. HRSA appealed, and the United States Court of Appeals for the DC Circuit heard argument on the case in 2022. In addition, in 2021 and 2023, two medical centers filed Administrative Dispute Resolution (ADR) proceedings against NPC, seeking the return of alleged overcharges resulting from NPC’s contract pharmacy policy. NPC has moved to dismiss these proceedings pending resolution of the HRSA litigation. Also in 2021, NPC received a civil investigative subpoena from the Office of the Attorney General of the State of Vermont (Vermont AG) requesting the production of documents and information concerning NPC’s participation in the 340B Drug Pricing Program in Vermont. NPC responded by providing documents and information to the Vermont AG.
Swiss and EU investigation
In September 2022, the Swiss Competition Commission (COMCO) initiated an investigation of the acquisition of certain patents by Novartis from Genentech in April 2020 and their subsequent enforcement against Eli Lilly and other parties, allegedly in an attempt to protect Cosentyx from competing products. COMCO is investigating whether enforcement of the patents violates the Swiss Cartel Act. The European Commission also requested information from Novartis regarding this matter. Novartis is cooperating with the authorities and will vigorously contest any allegations.
Inflation Reduction Act (IRA) litigation
In 2023, following the U.S. government’s selection of Entresto for the first round of the IRA’s “Medicare Drug Price Negotiation Program,” NPC filed a complaint in the USDC for the District of New Jersey on the grounds that those drug price-setting provisions are unconstitutional under the First, Fifth and Eighth Amendments to the U.S. Constitution.
Product liability litigation
Tasigna
NPC is a defendant in more than 400 US product liability actions involving Tasigna, alleging that the product
caused various cardiovascular effects and that NPC failed to provide adequate warnings about those alleged side effects. State court actions are pending in a multicounty litigation in Bergen County, New Jersey, and federal cases are pending in a multidistrict litigation in the Middle District of Florida. The claims are being vigorously contested.
Other matters
Shareholder derivative lawsuit
In 2021, NPC, Sandoz Inc., Novartis Capital Corporation and certain present and former directors and officers of Novartis were named as defendants, and Novartis was named as a nominal defendant, in a purported shareholder derivative lawsuit filed in New York State Court. The plaintiffs, derivatively as purported Novartis shareholders on behalf of Novartis, seek damages and other remedies based on alleged conduct by the corporate and individual defendants. In 2022, the court granted Novartis motion to dismiss the lawsuit, which the plaintiffs have appealed.
Concluded legal matters
Exforge – Concluded matter
Since 2018, Novartis companies as well as other pharmaceutical companies were sued by various direct and indirect purchasers of Exforge in multiple US individual and putative class action complaints. They claimed that Novartis made a reverse payment in the form of an agreement not to launch an authorized generic, alleging violations of federal antitrust law and state antitrust, consumer protection and common laws, and sought damages as well as injunctive relief. The cases were consolidated in the S.D.N.Y. In 2022, Novartis agreed to pay USD 245 million to resolve these cases, and this resolution was completed in 2023.
Lucentis/Avastin® (Italian and Belgian Competition Authorities) – Concluded matter
In connection with an investigation into whether Novartis entities, F. Hoffmann-La Roche AG, Genentech Inc. and Roche S.p.A. colluded to artificially preserve the market positions of Avastin® and Lucentis, in 2014 the Italian Competition Authority (ICA) imposed a fine equivalent to USD 125 million on the Novartis entities. Novartis paid the fine, subject to the right to later claim recoupment, and appealed the decision. In 2023, the final appeal by Novartis was denied, and the ICA decision is now final. In 2014 and 2015, following the ICA’s fine, the Italian Ministry of Health and the Lombardia region sent letters with payment requests for a total equivalent of approximately USD 1.3 billion in damages from Novartis and Roche entities based on these allegations, and several additional Italian regions and hospitals subsequently sent letters in 2019 claiming damages for an aggregate amount of approximately USD 330 million. None of these claims have been asserted in legal proceedings. A similar matter involving the competition authority in Belgium is concluded.
South Korea investigation – Concluded matter
In 2016, the Seoul Western District Prosecutor initiated a criminal investigation into, among other things, allegations that Novartis Korea utilized medical journals to provide inappropriate economic benefits to HCPs. This resulted in a non-material fine, which the prosecutor appealed. In 2021, the appellate court upheld the fine, and the prosecutor appealed that decision. In 2023, the Supreme Court dismissed the appeal. This matter is now concluded.
U.S. Government Foreign Corrupt Practices Act (FCPA) investigations – Concluded matter
As previously disclosed in Note 20 to the Consolidated Financial Statements in our 2020 Annual Report, Novartis reached settlements with the US Department of Justice (DOJ) and the US Securities and Exchange Commission (SEC) that resolved all FCPA investigations into historical conduct by Novartis and its subsidiaries. To resolve the DOJ investigation, Novartis Hellas S.A.C.I. entered into a deferred prosecution agreement (DPA) with the DOJ. To resolve the SEC investigation, Novartis AG reached an agreement that resulted in an Order issued by the SEC. The DPA and the Order each contained certain reporting and compliance obligations for a three-year term, which ended on June 26, 2023. On December 21, 2023 the court formally dismissed the Information filed against Novartis Hellas S.A.C.I at the request of the DOJ. This matter is now concluded.
Summary of product liability, governmental investigations and other legal matters provision movements
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
January 1 | | 702 | | 397 | | 487 | |
|
Provisions related to discontinued operations 1 | | -97 | | | | | |
|
Impact of acquisitions of businesses | | | | 4 | | | |
|
Cash payments 2 | | -448 | | -105 | | -292 | |
|
Releases of provisions 3 | | -219 | | -52 | | -44 | |
|
Additions to provisions 4 | | 170 | | 466 | | 251 | |
|
Currency translation effects | | 16 | | -8 | | -5 | |
|
December 31 | | 124 | | 702 | | 397 | |
|
Less current portion | | -42 | | -548 | | -56 | |
|
Non-current product liabilities, governmental investigations and other legal matters provisions at December 31 | | 82 | | 154 | | 341 | |
|
|
1 Represents the provisions for product liability, governmental investigations and other legal matters at January 1, 2023, related to the Sandoz business reported as discontinued operations. Notes 1, 2 and 31 provide disclosures related to discontinued operations. |
2 Cash payments from continuing operations were USD 67 million in 2022, and USD 64 million in 2021. |
3 Releases of provisions credited to the consolidated income statement from continuing operations were USD 38 million in 2022, and USD 18 million in 2021. |
4 Additions to provisions charged to the consolidated income statement from continuing operations were USD 435 million in 2022, and USD 190 million in 2021. |
Novartis believes that its total provisions for investigations, product liability, arbitration and other legal matters are adequate based upon currently available information. However, given the inherent difficulties in estimating liabilities, there can be no assurance that additional liabilities and costs will not be incurred beyond the amounts provided.
Discontinued operations
On October 4, 2023, the separation and spin-off of the Sandoz business was completed (Note 2). Pursuant to the Separation and Distribution Agreement that Novartis and Sandoz entered into in connection with that separation and spin-off, Sandoz and Novartis agreed, subject to certain limitations, exclusions and conditions, that Sandoz would retain or assume (as applicable) liabilities, including pending and future claims that relate to the spun-off Sandoz business (whether arising prior to, at or after the date of execution of the Separation and Distribution Agreement). Additionally, pursuant to the Separation and Distribution Agreement, Sandoz agreed to indemnify Novartis and each of its directors, officers, managers, members, agents and employees against liabilities incurred in connection with the spun-off Sandoz business.
22. Current financial debt and derivative financial instruments
(USD millions) | | 2023 | | 2022 | |
|
Bank and other financial debt 1 | | 624 | | 863 | |
|
Commercial paper | | 3 269 | | 2 772 | |
|
Current portion of non-current financial debt | | 2 191 | | 2 241 | |
|
Derivative financial instruments | | 91 | | 55 | |
|
Total current financial debt and derivative financial instruments | | 6 175 | | 5 931 | |
|
|
1 Weighted average interest rate during the year 2023 13.2% (2022: 9.7%) |
The carrying amounts of current financial debt, other than the current portion of non-current financial debt, approximate the estimated fair value due to the short-term nature of these instruments.
Details on commercial papers and short-term borrowings are provided under “Liquidity risk” in Note 30.
23. Provisions and other current liabilities
(USD millions) | | 2023 | | 2022 | |
|
Taxes other than income taxes | | 516 | | 836 | |
|
Restructuring provisions | | 703 | | 1 131 | |
|
Accrued expenses for goods and services received but not invoiced | | 1 026 | | 1 059 | |
|
Accruals for royalties | | 844 | | 767 | |
|
Accrued interests on financial debt | | 116 | | 116 | |
|
Provisions for deductions from revenue | | 6 315 | | 6 732 | |
|
Accruals for compensation and benefits, including social security | | 2 330 | | 2 321 | |
|
Environmental remediation provisions | | 20 | | 53 | |
|
Deferred income | | 98 | | 123 | |
|
Provisions for product liabilities, governmental investigations and other legal matters 1 | | 42 | | 548 | |
|
Accrued share-based payments | | 322 | | 235 | |
|
Contingent consideration 2 | | 14 | | 131 | |
|
Other payables | | 820 | | 743 | |
|
Total provisions and other current liabilities | | 13 166 | | 14 795 | |
|
|
1 Note 21 provides additional disclosures related to legal provisions. |
2 Note 30 provides additional disclosures related to contingent consideration. |
|
Provisions are based upon management’s best estimate and adjusted for actual experience. Such adjustments to historic estimates have not been material.
Provisions for deductions from revenue
The following table shows the movement of the provisions for deductions from revenue:
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
January 1 | | 6 732 | | 6 481 | | 6 256 | |
|
Provisions related to discontinued operations 1 | | -1 415 | | | | | |
|
Effect of currency translation, business combinations | | 68 | | -210 | | -218 | |
|
Payments/utilizations 2 | | -16 703 | | -22 261 | | -19 838 | |
|
Adjustments of prior years charged to income statement 3 | | -206 | | -322 | | -245 | |
|
Current year income statement charge 4 | | 17 798 | | 23 072 | | 20 413 | |
|
Change in provisions offset against gross trade receivables 5 | | 41 | | -28 | | 113 | |
|
December 31 | | 6 315 | | 6 732 | | 6 481 | |
|
|
1 Represents the provision for deductions from revenue at January 1, 2023, related to the Sandoz business reported as discontinued operations. Notes 1, 2 and 31 provide disclosures related to discontinued operations. |
2 Payments/utilizations from continuing operations were USD 14 691 million in 2022 and USD 12 473 million in 2021. |
3 Adjustments of prior years charged to income statement from continuing operations were USD 218 million in 2022, and USD 251 million in 2021. |
4 Current year income statement charge from continuing operations were USD 15 231 million in 2022 and USD 13 084 million in 2021. |
5 Change in provisions offset against gross trade receivables from continuing operations were USD 2 million in 2022 and USD -44 million in 2021. |
The provisions for deductions from revenue include specific healthcare plans and program rebates as well as non-healthcare plans and program-related rebates, returns and other deductions. The provisions for deductions from revenue are adjusted to reflect experience and to reflect actual amounts as rebates, refunds, discounts and returns are processed. The provision represents estimates of the related obligations, requiring the use of judgment when estimating the effect of these deductions from revenue.
Restructuring provisions movements
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
January 1 | | 1 131 | | 345 | | 459 | |
|
Provisions related to discontinued operations 1 | | -51 | | | | | |
|
Additions to provisions 2 | | 658 | | 1 368 | | 328 | |
|
Cash payments 3 | | -816 | | -468 | | -344 | |
|
Releases of provisions 4 | | -193 | | -42 | | -54 | |
|
Transfers 5 | | -57 | | -53 | | - 27 | |
|
Currency translation effects | | 31 | | -19 | | -17 | |
|
December 31 | | 703 | | 1 131 | | 345 | |
|
|
1 Represents the restructuring provisions at January 1, 2023, related to the Sandoz business reported as discontinued operations. Notes 1, 2 and 31 provide disclosures related to discontinued operations. |
2 Additions to provisions charged to the consolidated income statement from continuing operations were USD 1.3 billion in 2022 and USD 266 million in 2021. |
3 Cash-payments from continuing operations were USD 421 million in 2022 and USD 259 million in 2021. |
4 Releases of provisions credited to the consolidated income statement from continuing operations were USD 33 million in 2022 and USD 29 million in 2021. |
5 Transfers from continuing operations were USD 53 million in 2022 and USD 24 million in 2021. |
| |
Restructuring provisions are recognized for the direct expenditure arising from the restructuring, where the plans are sufficiently detailed and where appropriate communication to those affected has been made.
Charges to increase restructuring provisions are included in “Other expense” in the consolidated income statements.
In 2023, additions to provisions of USD 658 million were mainly related to the continuation of the initiative announced in April 2022 to implement a new streamlined organizational model designed to support innovation, growth and productivity.
In 2022, additions to provisions of USD 1.4 billion were mainly related to the following reorganizations:
• Initiative announced in April 2022 to implement a new streamlined organizational model designed to support innovation, growth and productivity.
• The continuation of the 2021 restructuring initiatives.
In 2021, additions to provisions of USD 328 million were mainly related to the following reorganizations:
• The commencement of a plan to restructure the field force and supporting functions in response to changes in the Company’s go-to-market structure with increased utilization of digital technology.
• Company-wide initiatives to streamline manufacturing platforms and manufacturing functions and implement new technologies continued. In addition, the Operations unit (formerly Customer & Technology Solutions) continued the phased implementation of the new operating model to transition activities to service centers.
24. Details to the consolidated statements of cash flows
24.1) Non-cash items and other adjustments from continuing operations
The following table shows the reversal of non-cash items and other adjustments in the consolidated statements of
cash flows.
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
Depreciation, amortization and impairments on: | | | | | | | |
Property, plant and equipment | | 1 006 | | 1 374 | | 1 277 | |
|
Right-of-use assets | | 263 | | 270 | | 279 | |
|
Intangible assets | | 7 008 | | 5 061 | | 4 041 | |
|
Financial assets 1 | | 106 | | 260 | | -38 | |
|
Change in provisions and other non-current liabilities | | 61 | | 1 318 | | 806 | |
|
Gains on disposal and other adjustments on property, plant and equipment; intangible assets; financial assets and other non-current assets, net | | -180 | | -308 | | -646 | |
|
Equity-settled compensation expense | | 865 | | 791 | | 700 | |
|
Loss/(income) from associated companies 2 | | 13 | | 11 | | -15 337 | |
|
Income taxes | | 551 | | 1 128 | | 1 625 | |
|
Net financial expense | | 633 | | 758 | | 863 | |
|
Other | | 43 | | -32 | | | |
|
Total | | 10 369 | | 10 631 | | -6 430 | |
|
|
1 Includes fair value changes |
2 2021 included the gain of USD 14.6 billion recognized from the divestment of the Company's investment in Roche (see Notes 2 and 5). |
In 2023, other than through business combinations, there were no additions to intangible assets with deferred payments (2022: USD 635 million, 2021: nil).
In 2023, there were USD 421 million (2022: USD 216 million, 2021: USD 295 million) additions to right-of-use assets recognized.
24.2) Total amount of income taxes paid
In 2023, the total amount of income taxes paid by continuing operations was USD 2 787 million and by discontinued operations was USD 162 million, which was included within “Net cash flows from operating activities from discontinued operations.” In 2023, the total amount of income taxes paid by the Company was USD 2 949 million.
In 2022, the total amount of income taxes paid by continuing operations was USD 1 702 million and by discontinued operations was USD 273 million, which was included within “Net cash flows from operating activities from discontinued operations.” In 2022, the total amount of income taxes paid by the Company was USD 1 975 million.
In 2021, the total amount of income taxes paid by continuing operations was USD 1 856 million and by discontinued operations was USD 486 million, which was included within “Net cash flows from operating activities from discontinued operations.” In 2021, the total amount of income taxes paid by the Company was USD 2 342 million.
24.3) Cash flows from changes in working capital and other operating items included in the net cash flows from operating activities from continuing operations
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
Increase in inventories | | -546 | | -560 | | -102 | |
|
Increase in trade receivables | | -1 504 | | -397 | | -352 | |
|
Increase/(decrease) in trade payables | | 479 | | -181 | | -111 | |
|
Change in other current and non-current assets | | -125 | | -84 | | -179 | |
|
Change in other current liabilities | | 1 327 | | 426 | | 671 | |
|
Total | | -369 | | -796 | | -73 | |
|
24.4) Cash flows arising from acquisitions and divestments of interests in associated companies, net
In 2021, acquisitions and divestments of interests in associated companies, net included USD 20.7 billion proceeds from the divestment of the Company’s investment in Roche (see Notes 2 and 5).
24.5) Cash flows arising from acquisitions and divestments of businesses, net
The following table is a summary of the cash flow impact of acquisitions and divestments of businesses. The most significant transactions are described in Note 2.
(USD millions) | | Note | | 2023 | | 2022 | | 2021 | |
|
Net assets recognized as a result of acquisitions of businesses | | 25 | | -3 699 | | -1 077 | | -320 | |
|
Fair value of previously held equity interests | | | | 26 | | 21 | | 42 | |
|
Contingent consideration payables, net | | | | 146 | | 224 | | 18 | |
|
Payments, deferred consideration and other adjustments, net | | | | -34 | | 0 | | 1 | |
|
Cash flows used for acquisitions of businesses | | | | -3 561 | | -832 | | -259 | |
|
Cash flows from/(used for) divestments of businesses, net 1 | | | | 3 | | -8 | | 54 | |
|
Cash flows used for acquisitions and divestments of businesses, net | | | | -3 558 | | -840 | | -205 | |
|
|
1 In 2023, USD 3 million represented net cash inflows from divestments in previous years. |
In 2022, USD 8 million net cash outflows from divestments of businesses included USD 20 million reduction to cash and cash equivalents due to the derecognized cash and cash equivalents following a loss of control of a company upon expiry of an option to purchase the company, partly offset by USD 12 million net cash inflows from business divestments in 2022 and in prior years. |
In 2022, the net identifiable assets of divested businesses amounted to USD 139 million, comprised of non-current assets of USD 127 million, current assets of USD 70 million, including USD 62 million cash and cash equivalents and of non-current and current liabilities of USD 58 million. The deferred sale price receivable and other adjustments amounted to USD 19 million. |
In 2021, USD 54 million included net cash inflows from divestments in previous years. |
Notes 2 and 25 provide further information regarding acquisitions and divestments of businesses. All acquisitions were for cash.
24.6) Reconciliation of liabilities arising from financing activities
| | 2023 | | 2022 | | 2021 | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
(USD millions)
| |
Financial
debts
| | Derivative
financial
instruments | |
Lease
liabilities
| |
Financial
debts
| | Derivative
financial
instruments | |
Lease
liabilities
| |
Financial
debts
| | Derivative
financial
instruments | |
Lease
liabilities
| |
|
January 1 | | 26 120 | | 55 | | 1 789 | | 29 129 | | 68 | | 1 896 | | 35 850 | | 194 | | 2 005 | |
|
Financial debts, derivative financial instruments and lease liabilities related to discontinued operations 1 | | -214 | | -1 | | -98 | | | | | | | | | | | | | |
|
Increase in non-current financial debts 2 | | | | | | | | 16 | | | | | | 16 | | | | | |
|
Repayments of the current portion of non-current financial debts 3 | | -2 223 | | | | | | -2 575 | | | | | | -2 162 | | | | | |
|
Change in current financial debts 4 | | 546 | | | | | | 295 | | | | | | -3 524 | | | | | |
|
Payments of lease liabilities 5 | | | | | | -258 | | | | | | -295 | | | | | | -316 | |
|
Interest payments for amounts included in lease liabilities classified as cash flows from operating activities 6 | | | | | | -52 | | | | | | -51 | | | | | | -52 | |
|
New, modified and terminated leases, net | | | | | | 349 | | | | | | 222 | | | | | | 253 | |
|
Impact of acquisitions and divestments of businesses, net | | | | | | 51 | | | | | | 12 | | 1 | | | | | |
|
Changes in fair values, lease interest and other changes, net | | -2 | | 37 | | 28 | | | | -13 | | 60 | | 1 | | -125 | | 62 | |
|
Amortization of bonds discount | | 17 | | | | | | 22 | | | | | | 29 | | | | | |
|
Currency translation effects | | 276 | | | | 19 | | -767 | | | | -55 | | -1 082 | | -1 | | -56 | |
|
December 31 | | 24 520 | | 91 | | 1 828 | | 26 120 | | 55 | | 1 789 | | 29 129 | | 68 | | 1 896 | |
|
Non-current 7 | | 18 436 | | | | 1 598 | | 20 244 | | | | 1 538 | | 22 902 | | | | 1 621 | |
|
Current 7 | | 6 084 | | 91 | | 230 | | 5 876 | | 55 | | 251 | | 6 227 | | 68 | | 275 | |
|
|
1 Represents the financial debts, derivative financial instruments and lease liabilities at January 1, 2023 related to the Sandoz business reported as discontinued operations. Notes 1, 2 and 31 provide disclosures related to discontinued operations. |
2 Increases in non-current financial debts included in the consolidated statements of cash flows from continuing operations were nil in 2022 and 2021. |
3 Repayments of the current portion of non-current financial debts were only recorded in the consolidated statements of cash flows from continuing operations. |
4 Changes in current financial debts included in the consolidated statements of cash flows from continuing operations were USD 252 million in 2022 (2021: USD 3 547 million) which included net cash outflows from interest-bearing accounts of employees payable on demand amounting to USD 1.7 billion. |
5 Payments of lease liabilities included in the consolidated statements of cash flows from continuing operations were USD 262 million in 2022 (2021: USD 278 million). |
6 Interest payments for amounts included in lease liabilities classified as cash flows from operating activities within the consolidated statements of cash flows from continuing operations were USD 48 million in 2022 (2021: USD 48 million). |
7 Note 11 provides additional disclosures related to lease liabilities, Note 20 provides additional disclosures related to non-current financial debt, and Note 22 provides additional disclosures related to current financial debt and derivative financial instruments. |
25. Acquisitions of businesses
Fair value of assets and liabilities arising from acquisitions of businesses:
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
Property, plant and equipment | | 18 | | 13 | | | |
|
Right-of-use assets | | 16 | | 12 | | | |
|
Currently marketed products | | | | | | 292 | |
|
Acquired research and development | | 2 931 | | 1 209 | | 262 | |
|
Other intangible assets | | 15 | | | | 98 | |
|
Deferred tax assets | | 34 | | 56 | | 28 | |
|
Non-current financial and other assets | | 164 | | | | | |
|
Trade receivables and financial and other current assets | | 183 | | 5 | | 1 | |
|
Cash and cash equivalents | | 226 | | 89 | | 10 | |
|
Deferred tax liabilities | | -474 | | -300 | | -74 | |
|
Current and non-current financial debts | | | | | | -1 | |
|
Current and non-current lease liabilities | | -51 | | -12 | | | |
|
Trade payables and other liabilities | | -231 | | -67 | | -4 | |
|
Net identifiable assets acquired | | 2 831 | | 1 005 | | 612 | |
|
Acquired cash and cash equivalents | | -226 | | -89 | | -10 | |
|
Non-controlling interests | | | | | | -105 | |
|
Goodwill | | 1 094 | | 161 | | 238 | |
|
Net assets recognized as a result of acquisitions of businesses 1 | | 3 699 | | 1 077 | | 735 | |
|
|
1 In 2023 and 2022 all net assets recognized relate to business combinations of continuing operations. In 2021, net assets recognized as a result of acquisitions of businesses from continuing operations were USD 320 million. |
Note 2 details significant acquisitions of businesses by continuing operations, specifically the acquisition of DTx Pharma and Chinook Therapeutics in 2023, and of Gyroscope in 2022. Note 31 details significant acquisitions by discontinued operations, specifically the cephalosporin antibiotics business from GSK by Sandoz in 2021. The goodwill arising out of these acquisitions is attributable to the synergies, the accounting for deferred tax liabilities on the acquired assets and the assembled workforce. In 2023, no goodwill (2022: nil; 2021: USD 107 million) is tax deductible.
26. Post-employment benefits for employees
Defined benefit plans
In addition to the legally required social security schemes, the Company has numerous independent pension and other post-employment benefit plans. In most cases, these plans are externally funded in entities that are legally separate from the Company. For certain Company entities, however, no independent plan assets exist for the pension and other post-employment benefit obligations of employees. In these cases, the related unfunded liability is included in the balance sheet. The defined benefit obligations (DBOs) of all major pension and other post-employment benefit plans are reappraised annually by independent actuaries using the projected unit credit method. Plan assets are recognized at fair value.
The major plans are based in Switzerland, the United States, the United Kingdom, Germany and Japan, which represent 96% of the Company’s total DBO for pension plans. Details of the plans in the two most significant countries, Switzerland and the United States, which represent 84% of the Company’s total DBO for post-employment benefit plans, are provided below.
Swiss-based pension plans represent the most significant portion of the Company’s total DBO and plan assets. For the active insured members the benefits are linked to contributions paid into the plan, interest credits granted and conversion rates applied.
All benefits granted under Swiss-based pension plans are vested, and Swiss legislation prescribes that the employer has to contribute a fixed percentage of an employee’s pay to an external pension fund. Additional employer contributions may be required whenever the plan’s statutory funding ratio falls below a certain level. The employee also contributes to the plan. The pension plans are run by separate legal entities, each governed by a board of trustees that – for the principal plans – consists of representatives nominated by Novartis and the
active insured employees. The boards of trustees are responsible for the plan design and asset investment strategy.
The United States pension plans represent the second-largest component of the Company’s total DBO and plan assets. The principal plans (Qualified Plans) are funded, whereas plans providing additional benefits for executives (Restoration Plans) are unfunded. Employer contributions are required for Qualified Plans whenever the statutory funding ratio falls below a certain level.
Furthermore, in certain countries, employees are covered under other post-employment benefit plans and post-retirement medical plans.
In the US, other post-employment benefit plans consist primarily of post-employment healthcare benefits, which have been closed to new members since 2015. Part of the costs of these plans is reimbursable under the Medicare Prescription Drug, Improvement, and Modernization Act of 2003. There is no statutory funding requirement for these plans. The Company is funding these plans to the extent that it is tax efficient.
The following tables are a summary of the funded and unfunded defined benefit obligation for pension and other post-employment benefit plans of employees at December 31, 2023 and 2022:
| | Pension plans | | Other post-employment benefit plans | |
| | | | | |
| | | | | |
| | | | | |
(USD millions) | | 2023 | | 2022 | | 2023 | | 2022 | |
|
Benefit obligation at January 1 | | 17 533 | | 23 583 | | 422 | | 560 | |
|
Benefit obligations related to discontinued operations 1 | | -529 | | | | -26 | | | |
|
Current service cost | | 260 | | 348 | | 9 | | 12 | |
|
Interest cost | | 504 | | 249 | | 22 | | 17 | |
|
Past service costs and settlements | | 28 | | -40 | | | | 1 | |
|
Administrative expenses | | 25 | | 23 | | | | | |
|
Remeasurement gains arising from changes in financial assumptions 2 | | 1 350 | | -5 046 | | 13 | | -94 | |
|
Remeasurement (gains)/losses arising from changes in demographic assumptions | | -303 | | -53 | | -14 | | | |
|
Experience-related remeasurement losses/(gains) | | 23 | | 199 | | 44 | | -28 | |
|
Currency translation effects | | 1 304 | | -650 | | 4 | | -2 | |
|
Benefit payments | | -1 384 | | -1 253 | | -34 | | -44 | |
|
Contributions of employees | | 174 | | 174 | | | | | |
|
Effect of acquisitions, divestments or transfers | | 52 | | -1 | | | | | |
|
Benefit obligation at December 31 | | 19 037 | | 17 533 | | 440 | | 422 | |
|
Fair value of plan assets at January 1 | | 18 945 | | 22 420 | | 60 | | 73 | |
|
Plan assets related to discontinued operations 1 | | -386 | | | | | | | |
|
Interest income | | 514 | | 220 | | 2 | | 2 | |
|
Return on plan assets excluding interest income | | 175 | | -2 500 | | 10 | | -12 | |
|
Currency translation effects | | 1 524 | | -539 | | | | | |
|
Novartis contributions | | 408 | | 424 | | 33 | | 41 | |
|
Contributions of employees | | 174 | | 174 | | | | | |
|
Settlements | | -35 | | -1 | | | | | |
|
Benefit payments | | -1 384 | | -1 253 | | -34 | | -44 | |
|
Effect of acquisitions, divestments or transfers | | -1 | | | | | | | |
|
Fair value of plan assets at December 31 | | 19 934 | | 18 945 | | 71 | | 60 | |
|
Funded status | | 897 | | 1 412 | | -369 | | -362 | |
|
Limitation on recognition of fund surplus at January 1 | | -2 644 | | -62 | | | | | |
|
Limitation on recognition of fund surplus at January 1, related to discontinued operations | | 6 | | | | | | | |
|
Change in limitation on recognition of fund surplus | | 740 | | -2 504 | | | | | |
|
Currency translation effects | | -209 | | -76 | | | | | |
|
Interest income on limitation of fund surplus | | -60 | | -2 | | | | | |
|
Limitation on recognition of fund surplus at December 31 3 | | -2 167 | | -2 644 | | | | | |
|
Net liability in the balance sheet at December 31 | | -1 270 | | -1 232 | | -369 | | -362 | |
|
|
1 Represents the benefit obligation, respectively the plan assets at January 1, 2023, related to the Sandoz business reported as discontinued operations. Notes 1, 2 and 31 provide disclosures related to discontinued operations. |
2 The remeasurement gains arising from changes in financial assumptions is driven mainly by changes in the actuarial discount rates used to determine the benefit obligation. |
3 The most significant pension plans where the asset ceiling was required to be applied were in Switzerland and amounted to USD 2 112 million (2022: USD 2 587 million). |
The reconciliation of the net liability from January 1 to December 31 is as follows:
| | Pension plans | | Other post-employment benefit plans | |
| | | | | |
| | | | | |
| | | | | |
(USD millions) | | 2023 | | 2022 | | 2023 | | 2022 | |
|
Net liability at January 1 | | -1 232 | | -1 225 | | -362 | | -487 | |
|
Less: net liability related to discontinued operations 1 | | 149 | | | | 26 | | | |
|
Current service cost | | -260 | | -348 | | -9 | | -12 | |
|
Net interest expense | | -50 | | -31 | | -20 | | -15 | |
|
Administrative expenses | | -25 | | -23 | | | | | |
|
Past service costs and settlements | | -63 | | 39 | | | | -1 | |
|
Remeasurements | | -895 | | 2 400 | | -33 | | 110 | |
|
Currency translation effects | | 11 | | 35 | | -4 | | 2 | |
|
Novartis contributions | | 408 | | 424 | | 33 | | 41 | |
|
Effect of acquisitions, divestments or transfers | | -53 | | 1 | | | | | |
|
Change in limitation on recognition of fund surplus | | 740 | | -2 504 | | | | | |
|
Net liability at December 31 | | -1 270 | | -1 232 | | -369 | | -362 | |
|
| | | | | | | | | |
Amounts recognized in the consolidated balance sheet | | | | | | | | | |
|
Prepaid benefit cost | | 545 | | 491 | | | | | |
|
Accrued benefit liability | | -1 815 | | -1 723 | | -369 | | -362 | |
|
|
1 Represents the net liability at January 1, 2023, related to the Sandoz business reported as discontinued operations. Notes 1, 2 and 31 provide disclosures related to discontinued operations |
The following table shows a breakdown of the DBO for pension plans by geography and type of member, and the breakdown of plan assets into the geographical locations in which they are held:
| | 2023 | | 2022 | |
| | | | | |
| | | | | |
| | | | | |
(USD millions)
| |
Switzerland
| | United
States | | Rest of
the world | |
Total
| |
Switzerland
| | United
States | | Rest of
the world | |
Total
| |
|
Benefit obligation at December 31 | | 13 453 | | 2 574 | | 3 010 | | 19 037 | | 11 824 | | 2 746 | | 2 963 | | 17 533 | |
|
Thereof unfunded | | | | 538 | | 390 | | 928 | | | | 556 | | 363 | | 919 | |
|
By type of member | | | | | | | | | | | | | | | | | |
|
Active | | 5 557 | | 389 | | 847 | | 6 793 | | 4 799 | | 431 | | 931 | | 6 161 | |
|
Deferred pensioners | | | | 770 | | 912 | | 1 682 | | | | 830 | | 861 | | 1 691 | |
|
Pensioners | | 7 896 | | 1 415 | | 1 251 | | 10 562 | | 7 025 | | 1 485 | | 1 171 | | 9 681 | |
|
Fair value of plan assets at December 31 | | 15 892 | | 1 835 | | 2 207 | | 19 934 | | 14 701 | | 1 978 | | 2 266 | | 18 945 | |
|
Funded status | | 2 439 | | -739 | | -803 | | 897 | | 2 877 | | -768 | | -697 | | 1 412 | |
|
The following table shows a breakdown of the DBO for other post-employment benefit plans by geography and type of member, and the breakdown of plan assets into the geographical locations in which they are held:
| | 2023 | | 2022 | |
| | | | | |
| | | | | |
| | | | | |
(USD millions)
| | United
States | | Rest of
the world | |
Total
| | United
States | | Rest of
the world | |
Total
| |
|
Benefit obligation at December 31 | | 356 | | 84 | | 440 | | 346 | | 76 | | 422 | |
|
Thereof unfunded | | 285 | | 84 | | 369 | | 286 | | 76 | | 362 | |
|
By type of member | | | | | | | | | | | | | |
|
Active | | 30 | | 10 | | 40 | | 30 | | 18 | | 48 | |
|
Deferred pensioners | | 1 | | 0 | | 1 | | 8 | | 0 | | 8 | |
|
Pensioners | | 325 | | 74 | | 399 | | 308 | | 58 | | 366 | |
|
Fair value of plan assets at December 31 | | 71 | | 0 | | 71 | | 60 | | 0 | | 60 | |
|
Funded status | | -285 | | -84 | | -369 | | -286 | | -76 | | -362 | |
|
The following table shows the principal weighted average actuarial assumptions used for calculating defined benefit plans and other post-employment benefits of employees:
| | Pension plans | | Other post-employment benefit plans | |
| | | | | |
| | | | | |
| | | | | |
| | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 | |
|
Weighted average assumptions used to determine benefit obligations at December 31 | | | | | | | | | | | | | |
Discount rate | | 2.2% | | 3.0% | | 0.9% | | 5.5% | | 6.3% | | 3.3% | |
|
Expected rate of pension increase | | 0.3% | | 0.4% | | 0.5% | | | | | | | |
|
Expected rate of salary increase | | 3.0% | | 2.9% | | 2.7% | | | | | | | |
|
Interest on savings account | | 1.3% | | 2.2% | | 0.5% | | | | | | | |
|
Current average life expectancy for a 65-year-old male in years | | 22 | | 22 | | 22 | | 21 | | 21 | | 21 | |
|
Current average life expectancy for a 65-year-old female in years | | 24 | | 24 | | 24 | | 23 | | 23 | | 23 | |
|
Changes in the aforementioned actuarial assumptions can result in significant volatility in the accounting for the Company’s pension plans in the consolidated financial statements. This can result in substantial changes in the Company’s other comprehensive income, long-term liabilities and prepaid pension assets.
The DBO is significantly impacted by assumptions regarding the rate that is used to discount the actuarially determined post-employment benefit liability. This rate is based on yields of high-quality corporate bonds in the country of the plan. Decreasing corporate bond yields decrease the discount rate, so that the DBO increases and the funded status decreases.
In Switzerland, an increase in the DBO due to lower discount rates is slightly offset by lower future benefits expected to be paid on the employee’s savings account where the assumption on interest accrued often changes broadly in line with the discount rate.
The impact of decreasing interest rates on a plan’s assets is more difficult to predict. A significant part of the plan assets is invested in bonds. Bond values usually rise when interest rates decrease and may therefore partially compensate for the decrease in the funded status. Furthermore, pension assets also include significant holdings of equity instruments. Share prices usually tend to rise when interest rates decrease and therefore often counteract the negative impact of the rising defined benefit obligation on the funded status (although the correlation of interest rates with equities is not as strong as with bonds, especially in the short term).
The expected rate for pension increases significantly affects the DBO of most plans in Switzerland, Germany and the United Kingdom. Such pension increases also decrease the funded status, although there is no strong correlation between the value of the plan assets and pension/inflation increases.
Assumptions regarding life expectancy significantly impact the DBO. An increase in longevity increases the DBO. There is no offsetting impact from the plan assets, as no longevity bonds or swaps are held by the pension funds. The Company’s actuaries use mortality tables that take into account historic patterns and expected changes, such as further increases in longevity.
In 2023 the mortality assumptions used for the pension plans in Switzerland were based on BVG 2020 tables with future improvements based on the Continuous Mortality Investigation (‘CMI’) model (2022: based on the BVG generational model). For the pension and postretirement medical benefit plans in the US, the Society of Actuaries Pri-2012 mortality tables with generational improvements based on Scale MP-2021 are used.
The following table shows the sensitivity of the defined benefit pension obligation to the principal actuarial assumptions for the major plans in Switzerland, the United States, the United Kingdom, Germany and Japan on an aggregated basis:
(USD millions)
| | Change in 2023
year-end defined
benefit pension
obligation | | Change in 2022
year-end defined
benefit pension
obligation | |
|
25 basis point increase in discount rate | | -528 | | -466 | |
|
25 basis point decrease in discount rate | | 557 | | 491 | |
|
One-year increase in life expectancy | | 644 | | 535 | |
|
25 basis point increase in rate of pension increase | | 366 | | 316 | |
|
25 basis point decrease in rate of pension increase | | -61 | | -63 | |
|
25 basis point increase of interest on savings account | | 43 | | 38 | |
|
25 basis point decrease of interest on savings account | | -42 | | -37 | |
|
25 basis point increase in rate of salary increase | | 41 | | 37 | |
|
25 basis point decrease in rate of salary increase | | -42 | | -37 | |
|
The healthcare cost trend rate assumptions used for other post-employment benefits are as follows:
| | 2023 | | 2022 | | 2021 | |
|
Healthcare cost trend rate assumed for next year | | 6.3% | | 6.5% | | 6.0% | |
|
Rate to which the cost trend rate is assumed to decline | | 4.5% | | 4.5% | | 4.5% | |
|
Year that the rate reaches the ultimate trend rate | | 2031 | | 2031 | | 2028 | |
|
The following table shows the weighted average plan asset allocation of funded defined benefit pension plans at December 31, 2023 and 2022:
| | Pension plans | |
| | | |
| | | |
| | | |
(as a percentage)
| | Long-term
target
minimum | | Long-term
target
maximum | |
2023
| |
2022
| |
|
Equity securities | | 15 | | 40 | | 25 | | 24 | |
|
Debt securities | | 20 | | 60 | | 34 | | 31 | |
|
Real estate | | 5 | | 30 | | 19 | | 21 | |
|
Alternative investments | | 0 | | 20 | | 17 | | 18 | |
|
Cash and other investments | | 0 | | 15 | | 5 | | 6 | |
|
Total | | | | | | 100 | | 100 | |
|
Cash and most of the equity and debt securities have a quoted market price in an active market. Real estate and alternative investments, which include hedge fund, private equity, infrastructure and commodity investments, usually have a quoted market price or a regularly updated net asset value.
The strategic allocation of assets of the different pension plans is determined with the objective of achieving an investment return that, together with the contributions paid by the Company and its employees, is sufficient to maintain reasonable control over the various funding risks of the plans. Based upon local requirement, the market and economic environments, actual asset allocations may be permitted to deviate from policy targets. The asset allocation currently includes investments in shares of Novartis AG as per the below table:
| | December 31,
2023 | | December 31,
2022 | |
|
Investment in shares of Novartis AG | | | | | |
|
Number of shares (in millions) | | 2.3 | | 2.3 | |
|
Market value (in USD billions) | | 0.2 | | 0.2 | |
|
The weighted average duration of the defined benefit pension obligation is 12.3 years (2022: 11.8 years).
The Company’s ordinary contribution to the various pension plans is based on the rules of each plan. Additional contributions are made whenever this is required by statute or law (i.e., usually when statutory funding levels fall below predetermined thresholds). The only significant plan that requires additional funding is in Germany.
The expected future cash flows over the upcoming 10 years in respect of pension and other post-employment benefit plans at December 31, 2023, were as follows:
(USD millions)
| |
Pension plans
| | Other post-
employment
benefit plans | |
|
Company contributions | | | | | |
|
2024 (estimated) | | 388 | | 39 | |
|
Expected future benefit payments | | | | | |
|
2024 | | 1 434 | | 40 | |
|
2025 | | 1 317 | | 40 | |
|
2026 | | 1 186 | | 40 | |
|
2027 | | 1 159 | | 39 | |
|
2028 | | 1 132 | | 38 | |
|
2029–2033 | | 5 316 | | 175 | |
|
Defined contribution plans
In many subsidiaries, employees are covered by defined contribution plans. Contributions charged to the consolidated income statement for continuing operations for the defined contribution plans were:
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
Contributions for defined contribution plans continuing operations | | 477 | | 483 | | 484 | |
|
The Company’s total personnel costs for continuing operations amounted to USD 12.7 billion in 2023 (2022: USD 13.1 billion).
27. Equity-based participation plans for employees
The equity-based compensation expense from continuing operations related to all equity-based participation plans and the liabilities arising from equity-based payment transactions were as follows:
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
Expense related to equity-based participation plans | | 1 142 | | 982 | | 910 | |
|
Liabilities arising from equity-based payment transactions | | 322 | | 235 | | 253 | |
|
Equity-based participation plans can be separated into the following plans:
Annual Incentive
The Annual Incentive for the Novartis Company CEO and other Executive Committee members (ECN) is paid 50% in cash and 50% in Novartis restricted shares (RSs) or restricted share units (RSUs). For a select portion of Novartis management leadership team, the Annual Incentive is paid 70% in cash and 30% in RSs or RSUs. Both the ECN and a select portion of Novartis management leadership team can opt to invest up to the maximum cash portion of their Annual Incentive to receive further RSs or RSUs. Any cash is paid out during March in the year following the end of the performance period, and the shares are granted during January in the year following the end of the performance period.
Employee share savings plan
Novartis operates employee share savings and purchase plans in certain countries. The most significant is described below.
The Employee Share Ownership Plan (ESOP) in Switzerland offers participants to choose to receive their Annual Incentive (i) 100% in shares, (ii) 50% in shares and 50% in cash, or (iii) 100% in cash. After the expiration of a three-year holding period for Novartis shares invested under the ESOP, participants receive one matching share for every two invested shares. Employees eligible for the equity plan “Select” are not eligible to receive ESOP matching shares. The Novartis Company CEO, the other Executive Committee members and a select portion of Novartis management leadership team are not eligible to participate in this plan.
Novartis Employee share purchase plan
In 2022 Novartis started to grant shares under the Employee Share Purchase Plan (ESPP). The ESPP enables employees to voluntarily purchase Novartis AG shares through payroll deductions at a discounted price. While the ESPP is global in scope, the first phase covers employees in North America (the US, Puerto Rico and Canada). Other countries employees will become eligible to participate in the ESPP commencing in 2024, according to a multi-year phased implementation plan. The shares are not subject to a vesting period.
Novartis equity plan “Select”
The equity plan “Select” is a global equity incentive plan under which eligible employees may annually be awarded a grant subject to a three-year, and for eligible employees of selected Company units a four-year, staggered vesting period. No awards are granted for performance ratings below a certain threshold. Executive Committee members and a select portion of Novartis management leadership team are not eligible to participate in the equity plan “Select.”
The equity plan “Select” currently allows participants employed and living in Switzerland to choose the form of their equity compensation in RSs or RSUs. In all other jurisdictions, RSs or RSUs are granted unilaterally. Until 2013, participants could also choose to receive part or the entire grant in the form of tradable share options.
All tradable share options expired on their 10th anniversary from the grant date, which was in January 2023. Each tradable share option entitled the holder to purchase after vesting (and before the 10th anniversary from the grant date) one Novartis share at a stated exercise price that equals the closing market price of the underlying share at the grant date. As the exercise price did not reflect the decrease in the Novartis share due to the Alcon spin, one-fifth of an Alcon share was also awarded to the option holder upon exercise.
Options under Novartis equity plan “Select” outside North America
The following table shows the activity associated with the share options during the period. The weighted average prices in the table below are translated from Swiss francs into USD at historical rates.
| | 2023 | | 2022 | |
| | | | | |
| | | | | |
| | | | | |
| |
Options
(millions)
| | Weighted
average
exercise
price
(USD) | |
Options
(millions)
| | Weighted
average
exercise
price
(USD) | |
|
Options outstanding at January 1 | | 0.5 | | 66.0 | | 1.7 | | 63.6 | |
|
Sold or exercised | | -0.5 | | 66.0 | | -1.2 | | 62.6 | |
|
Outstanding at December 31 | | 0.0 | | | | 0.5 | | 66.0 | |
|
Exercisable at December 31 | | 0.0 | | | | 0.5 | | 66.0 | |
|
|
All share options were granted at an exercise price that was equal to the closing market price of the Company’s shares at the grant date. The weighted average share price at the dates of sale or exercise was USD 92.61.
Options under Novartis equity plan “Select” for North America
The following table shows the activity associated with the ADR options during the period:
| | 2023 | | 2022 | |
| | | | | |
| | | | | |
| | | | | |
| |
ADR
options
(millions)
| | Weighted
average
exercise
price
(USD) | |
ADR
options
(millions)
| | Weighted
average
exercise
price
(USD) | |
|
Options outstanding at January 1 | | 1.1 | | 66.1 | | 4.0 | | 64.4 | |
|
Sold or exercised | | -1.1 | | 66.1 | | -2.9 | | 63.7 | |
|
Outstanding at December 31 | | 0.0 | | | | 1.1 | | 66.1 | |
|
Exercisable at December 31 | | 0.0 | | | | 1.1 | | 66.1 | |
|
|
All ADR options were granted at an exercise price that was equal to the closing market price of the ADRs at the grant date. The weighted average share price at the dates of sale or exercise was USD 91.68.
Long-Term Performance Plan
The Long-Term Performance Plan (LTPP) is an equity plan for the ECN, a select portion of Novartis management leadership team and employees of Company units with specific targets.
Participants are granted a target number of performance share units (PSUs) at the beginning of every performance period, which are converted into unrestricted Novartis shares after the performance period. The actual payout depends on the achievement of the performance measures and ranges between 0% and 200% of the granted amount. PSUs granted under the LTPP do not carry voting rights, but do carry dividend equivalents that are paid in unrestricted Novartis shares at the end of the performance period.
The LTPP awards are subject to a three-year performance and vesting period. The performance criteria for the ECN are based on both Novartis internal performance metrics and variables that can be observed in the market, which is the ranking of the Novartis total shareholder return (TSR) relative to a global healthcare peer group of 14 other companies, over rolling three-year performance periods. Only ECN members, as from performance cycle 2023, are subject to the TSR performance metric under the LTPP.
TSR for Novartis and the peer companies is calculated as the change in the company share price, which is translated to USD at the relevant exchange rate, including the reinvestment return of dividends, over the three-year performance period. The calculation is based on Bloomberg standard published TSR data, which is publicly available. The position of Novartis in the peer group determines the payout range based on a payout matrix.
Other share awards
Selected employees may exceptionally receive Special Share Awards of RSs or RSUs. These Special Share Awards provide an opportunity to reward outstanding achievements or exceptional performance, and aim to retain key contributors. They are based on a formal internal selection process, through which the individual performance of each candidate is thoroughly assessed at several management levels. Special Share Awards had a minimum three-year vesting period before 2021 and mainly three years thereafter. In exceptional circumstances, Special Share Awards may be awarded to attract special expertise and new talents to the organization. Externally recruited ECN members are eligible only for special awards that are “buyouts” in the case that it is to replace equity forfeited with their former employer. The equity is provided on a like-for-like basis as the forfeited equity, at the same value with the same vesting period, and with or without a performance condition.
Worldwide, employees at different levels in the organization were awarded RSs and RSUs in 2023, 2022 and 2021.
In addition, in 2023, 2022 and 2021, Board members received unrestricted shares as part of their regular compensation.
Summary of share grants
The table below provides a summary of share grants (shares, RSs, RSUs and PSUs) for all plans. At the Sandoz Distribution date, all RSU and PSU holders, who were not entitled to the dividend in kind in the form of Sandoz Group AG shares received keep whole awards in Novartis AG shares to compensate for the loss of the Sandoz value from their Novartis AG shares. These keep whole awards were accounted for as a modification, which did not significantly change the fair value of the original grant. The change in fair value was measured by comparing the fair value of the grant before the spin against the fair value of the grant plus keep whole award right after spin.
| | 2023 | | 2022 | |
| | | | | |
| | | | | |
| | | | | |
| |
Number
of shares
in millions
| | Weighted
average fair
value at grant
date in USD | |
Number
of shares
in millions
| | Weighted
average fair
value at grant
date in USD | |
|
Annual Incentive | | | | | | | | | |
|
– RSU | | 0.3 | | 74.2 | | 0.2 | | 74.7 | |
|
– Restricted shares | | 0.1 | | 92.3 | | 0.1 | | 85.0 | |
|
Share savings plans | | | | | | | | | |
|
– RSU | | 0.4 | | 71.3 | | 0.4 | | 75.0 | |
|
– Shares | | 1.0 | | 92.3 | | 1.2 | | 85.0 | |
|
Novartis Employee Share Purchase Plan | | 0.9 | | 96.2 | | 0.8 | | 82.8 | |
|
Select North America (RSU) | | 4.5 | | 73.9 | | 4.9 | | 74.5 | |
|
Select outside North America | | | | | | | | | |
|
– RSU | | 1.9 | | 73.3 | | 2.0 | | 75.1 | |
|
– Restricted shares | | 0.6 | | 92.3 | | 0.7 | | 85.0 | |
|
Long-Term Performance Plan (PSU) | | 1.8 | | 80.6 | | 1.7 | | 82.0 | |
|
Other share awards | | | | | | | | | |
|
– RSU | | 0.6 | | 75.9 | | 0.5 | | 76.3 | |
|
– Restricted shares | | | | | | 0.1 | | 86.9 | |
|
– Shares | | | | | | 0.1 | | 86.1 | |
|
28. Transactions with related parties
Roche Holding AG
Novartis has two agreements with Genentech, Inc., United States (Genentech), and one agreement with Spark Therapeutics, Inc., United States (Spark). Both companies are subsidiaries of Roche Holding AG (Roche), which were indirectly included in the consolidated financial statements using equity accounting until November 3, 2021, when Novartis entered into an agreement with Roche to divest its 33.3% of Roche voting shares. On December 6, 2021, Novartis divested its investment in Roche, on which date Roche ceased to be a related party (see Notes 2 and 5).
Lucentis
Novartis has licensed from Genentech/Roche the exclusive rights to develop and market Lucentis outside the United States for indications related to diseases of the eye. Novartis pays royalties on the net sales to third parties of Lucentis products outside the United States. From January 1, 2021 until December 6, 2021, Lucentis sales of USD 2.0 billion were recognized by Novartis.
Xolair
Novartis and Genentech/Roche are co-promoting Xolair in the United States, where Genentech/Roche records all sales. Novartis records sales outside the United States.
Novartis markets Xolair and records all sales and related costs outside the United States as well as co-promotion costs in the US. Genentech/Roche and Novartis share the resulting profits from sales in the United States, Europe and other countries, according to agreed profit-sharing percentages. From January 1, 2021 until December 6, 2021, Novartis recognized total sales of Xolair of USD 1.3 billion, including sales to Genentech/Roche for the United States market.
Luxturna
In 2018, Novartis entered into an exclusive licensing and commercialization agreement and a supply agreement with Spark for Luxturna outside the United States. The agreements include regulatory and sales milestones as well as royalties payable to Spark on ex-US sales. On December 17, 2019, Roche acquired Spark.
The net income for royalties, cost sharing and profit sharing arising out of the Lucentis, Xolair and Luxturna agreements with Roche totaled USD 188 million from January 1, 2021 until December 6, 2021.
Furthermore, Novartis has several patent license, supply and distribution agreements with Roche.
Novartis Pension Fund
In 2018, a Company subsidiary provided an uncommitted overnight credit facility to the Novartis Pension Fund, Switzerland, for up to USD 500 million with interest at the US Federal Funds Rate. This credit facility was not utilized during the current and past years.
Executive Officers and Non-Executive Directors compensation
As at December 31, 2023, there were 11 Executive Committee members (“Executive Officers”). During 2023, 1 Executive Officer stepped down.
As at December 31, 2022, there were 11 Executive Officers. During 2022, 5 Executive Officers stepped down.
As at December 31, 2021, there were 12 Executive Officers. During 2021, 3 Executive Officers stepped down.
The total compensation for Executive Committee members and the 14 Non-Executive Directors (15 in 2022 and 14 in 2021) using the Company’s accounting policies for equity-based compensation and pension benefits was as follows:
| | Executive Officers | | Non-Executive Directors | | Total | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
(USD millions) | | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 | |
|
Cash and other compensation | | 18.0 | | 25.0 | | 20.3 | | 4.9 | | 4.6 | | 4.7 | | 22.9 | | 29.6 | | 25.0 | |
|
Post-employment benefits | | 2.1 | | 2.8 | | 2.5 | | | | | | | | 2.1 | | 2.8 | | 2.5 | |
|
Equity-based compensation | | 62.2 | | 42.6 | | 37.3 | | 5.0 | | 4.8 | | 5.2 | | 67.2 | | 47.4 | | 42.5 | |
|
Total | | 82.3 | | 70.4 | | 60.1 | | 9.9 | | 9.4 | | 9.9 | | 92.2 | | 79.8 | | 70.0 | |
|
During 2023, there was an increase in the IFRS Accounting Standards compensation expense for Executive Officers compared to 2022, primarily driven by higher equity-based compensation, mainly due to higher realized and expected payouts on the achievement of the defined performance criteria, partly offset by lower cash
and other compensation, due to lower accelerated expenses from stepped down Executive Officers compared to 2022.
During 2022, there was an increase in the IFRS Accounting Standards compensation expense for Executive Officers compared to 2021, driven by accelerated expenses (cash and other compensation and equity-based compensation) required under IFRS Accounting Standards for the executive members who stepped down in 2022, in accordance with their employment contracts and the relevant incentive plan terms, compared to the accelerated expenses due to executive officers who stepped down in 2021.
During 2021, the IFRS Accounting Standards compensation expense decreased due to one role less at the ECN, and lower cash and equity compensation attributable to former ECN members, partially offset by the net increase of the IFRS Accounting Standards compensation expense of current ECN members.
The Annual Incentive award, which is fully included in equity-based compensation even when paid out in cash, is granted in January in the year following the reporting period.
The disclosures on Board and executive compensation required by the Swiss Code of Obligations and in accordance with the Swiss Ordinance against Excessive Compensation in Stock Exchange Listed Companies are shown in the Compensation Report of the Company.
Transactions with a former member of the Board of Directors
Dr. Alex Krauer, was an Honorary Chairman of Novartis and was entitled to an amount of CHF 60 000 for annual periods from one AGM to the next. This amount was fixed in 1998 upon his departure from the Board in 1999. The last payment under this arrangement was in 2021.
29. Commitments and contingent liabilities
Research and development commitments
The Company has entered into long-term research and development agreements with various institutions related to intangible assets. These agreements provide for potential milestone payments by Novartis, which are dependent on successful clinical development, or meeting specified sales targets, or other conditions that are specified in the agreements.
As of December 31, 2023, the amount and estimated timing of the Company’s commitments to make payments under those agreements, which are shown without risk adjustment and on an undiscounted basis, were as follows:
(USD millions) | | 2023 | |
|
2024 | | 202 | |
|
2025 | | 269 | |
|
2026 | | 493 | |
|
2027 | | 316 | |
|
2028 | | 597 | |
|
Thereafter | | 4 206 | |
|
Total | | 6 083 | |
|
Commitments for capital calls
The Company holds investments in funds in which it has committed to invest further upon future capital calls. As at December 31, 2023, the total uncalled capital commitments for the Company’s investments in funds amounts to USD 80 million. Note 30 contains further information on the Company’s investments in funds.
Other commitments
The Company has entered into various purchase commitments for services and materials as well as for equipment in the ordinary course of business. These commitments are generally entered into at current market prices and reflect normal business operations. For the disclosure of property, plant and equipment purchase commitments, see Note 10. The Company routinely acquires businesses and interests in intellectual property focused on key disease areas and indications that the Company expects to be growth drivers in the future. The Company has commitments through to the date the consolidated financial statements were approved for publication (see Note 32), totaling USD 3.8 billion (of which USD 3.4 billion may become payable in 2024) related to the acquisition of businesses and interests in intellectual property, the majority of which is subject to the satisfaction of conditions precedent in the arrangements.
Guarantees issued
The Company has issued guarantees to third parties in the ordinary course of business, mostly for tax, customs or other governmental agencies.
Contingent liabilities
Novartis companies have to observe the laws, government orders and regulations of the country in which they operate.
A number of Novartis companies are, and will likely continue to be, subject to various legal proceedings and investigations that arise from time to time, including
proceedings pertaining to: pricing; bribery and corruption; trade regulation and embargo legislation; product liability; commercial disputes; employment and wrongful discharge; antitrust and competition; securities; government benefit programs; reimbursement; rebates; healthcare fraud; sales and marketing practices; insider trading; occupational health and safety; environmental regulations; tax; cyber and data security; use of technologies, including AI; data privacy; regulatory interactions; disclosure compliance; and intellectual property. As a result, the Company may become subject to substantial liabilities that may not be covered by insurance and that could affect our business, financial position and reputation. While Novartis does not believe that any of these legal proceedings will have a material adverse effect on its financial position, litigation is inherently unpredictable and large judgments sometimes occur. Consequently, we may in the future incur judgments that could involve large payments, including the potential repayment of amounts allegedly obtained improperly, and other penalties, including treble damages, any of which could have a material adverse effect on our results of operations or cash flow.
Governments and regulatory authorities around the world have been stepping up their compliance and law enforcement activities in recent years in key areas, including marketing practices, pricing, corruption, trade restrictions, embargo legislation, insider trading, antitrust, cyber security and data privacy. Furthermore, when a government or regulatory authority undertakes an investigation, it is not uncommon for other governments or regulators to undertake investigations regarding the same or similar matters. Responding to such investigations is costly and requires an increasing amount of management’s time and attention. In addition, such investigations may affect our reputation, create a risk of potential exclusion from government reimbursement programs in the United States and other countries, and lead to (or arise from) litigation. These factors have contributed to decisions by Novartis and other companies in the healthcare industry, when deemed in their interest, to enter into settlement agreements with governmental authorities around the world prior to any formal decision by the authorities or a court. These government settlements have involved and may in the future involve large cash payments, sometimes in the hundreds of millions of dollars or more, including the potential repayment of amounts allegedly obtained improperly and other penalties, including treble damages. In addition, settlements of government healthcare fraud cases and antitrust cases often require companies to enter into corporate integrity agreements, which are intended to regulate company behavior for a period of years. Our affiliate Novartis Corporation is party to such an agreement, which will expire in 2025. In addition, matters underlying governmental investigations and settlements may be the subject of separate private litigation.
While provisions have been made for probable outflows of economic resources, which management deems to be reasonable or appropriate, there are uncertainties connected with these estimates.
Note 21 contains additional information on these matters.
A number of Novartis companies are involved in legal proceedings concerning intellectual property rights. The inherent unpredictability of such proceedings means that there can be no assurances as to their ultimate outcome. A negative result in any such proceeding could potentially adversely affect the ability of certain Novartis companies to sell their products, or require the payment of substantial damages or royalties. The timing and the outcome of legal proceedings and their potential financial effect are not predictable.
In the opinion of management, however, the outcome of these actions will not materially affect the Company’s financial position but could be material to the results of operations or cash flow in a given period.
The Company’s potential environmental remediation liability is assessed based on a risk assessment and investigation of the various sites identified by the Company as at risk for environmental remediation exposure. The Company’s future remediation expenses are affected by a number of uncertainties. These uncertainties include, but are not limited to, the method and extent of remediation, the percentage of material attributable to the Company at the remediation sites relative to that attributable to other parties, and the financial capabilities of the other potentially responsible parties.
Note 21 contains additional information on environmental liabilities.
30. Financial instruments – additional disclosures
The following tables show the carrying values of financial instruments by measurement category as at December 31, 2023 and 2022. Except for straight bonds (see Note 20), the carrying values are equal to, or a reasonable approximation of, the fair values.
| | | | 2023 | |
| | | | | |
| | | | | |
| | | | | |
(USD millions)
| |
Note
| |
Financial
instruments at
amortized
costs
| |
Financial
instruments at
fair value
through other
comprehensive
income
| | Financial
instruments at
fair value
through the
consolidated
income
statement | |
Other
financial
liabilities at
amortized
costs
| |
|
Cash and cash equivalents | | 17 | | 13 343 | | 50 | | | | | |
|
Time deposits and short-term investments with original maturity more than 90 days | | 17 | | 569 | | | | | | | |
|
Trade receivables | | 16 | | 7 107 | | | | | | | |
|
Other receivables and current assets | | 18 | | 1 127 | | 124 | | 1 | | | |
|
Long-term financial investments - equity securities | | 14 | | | | 1 086 | | 317 | | | |
|
Long-term financial investments - debt securities | | 14 | | | | 29 | | | | | |
|
Long-term financial investments - fund investments | | 14 | | | | | | 190 | | | |
|
Long-term loans, advances, security deposits and other long-term receivables | | 14 | | 432 | | | | | | | |
|
Associated companies at fair value through profit and loss | | | | | | | | 101 | | | |
|
Derivative financial instruments | | 17 | | | | | | 355 | | | |
|
Contingent consideration receivables | | 14/18 | | | | | | 618 | | | |
|
Total financial assets | | | | 22 578 | | 1 289 | | 1 582 | | | |
|
| | | | | | | | | | | |
|
Bank and other short-term financial debt | | 22 | | 624 | | | | | | | |
|
Commercial paper | | 22 | | 3 269 | | | | | | | |
|
Straight bonds | | 20 | | 20 585 | | | | | | | |
|
Long-term liabilities to banks and other financial institutions | | 20 | | 42 | | | | | | | |
|
Trade payables | | | | 4 926 | | | | | | | |
|
Contingent consideration liabilities (see Note 21/23) and other financial liabilities | | | | | | | | 491 | | | |
|
Derivative financial instruments | | 22 | | | | | | 91 | | | |
|
Lease liabilities | | 11 | | | | | | | | 1 828 | |
|
Total financial liabilities | | | | 29 446 | | | | 582 | | 1 828 | |
|
| | | | 2022 | |
| | | | | |
| | | | | |
| | | | | |
(USD millions)
| |
Note
| |
Financial
instruments at
amortized
costs
| |
Financial
instruments at
fair value
through other
comprehensive
income
| | Financial
instruments at
fair value
through the
consolidated
income
statement | |
Other
financial
liabilities at
amortized
costs
| |
|
Cash and cash equivalents | | 17 | | 7 517 | | | | | | | |
|
Time deposits and short-term investments with original maturity more than 90 days | | 17 | | 11 089 | | | | | | | |
|
Trade receivables | | 16 | | 8 066 | | | | | | | |
|
Other receivables and current assets | | 18 | | 958 | | | | | | | |
|
Marketable securities - debt securities | | 17 | | | | 9 | | | | | |
|
Long-term financial investments - equity securities | | 14 | | | | 828 | | 317 | | | |
|
Long-term financial investments - debt securities | | 14 | | | | 37 | | | | | |
|
Long-term financial investments - fund investments | | 14 | | | | | | 281 | | | |
|
Long-term loans, advances, security deposits and other long-term receivables | | 14 | | 341 | | | | | | | |
|
Associated companies at fair value through profit and loss | | | | | | | | 129 | | | |
|
Derivative financial instruments | | 17 | | | | | | 204 | | | |
|
Contingent consideration receivables | | 14 | | | | | | 650 | | | |
|
Total financial assets | | | | 27 971 | | 874 | | 1 581 | | | |
|
| | | | | | | | | | | |
|
Bank and other short-term financial debt | | 22 | | 863 | | | | | | | |
|
Commercial paper | | 22 | | 2 772 | | | | | | | |
|
Straight bonds | | 20 | | 22 341 | | | | | | | |
|
Long-term liabilities to banks and other financial institutions | | 20 | | 144 | | | | | | | |
|
Trade payables | | | | 5 146 | | | | | | | |
|
Contingent consideration liabilities (see Note 21/23) and other financial liabilities | | | | | | | | 1 067 | | | |
|
Derivative financial instruments | | 22 | | | | | | 55 | | | |
|
Lease liabilities | | 11 | | | | | | | | 1 789 | |
|
Total financial liabilities | | | | 31 266 | | | | 1 122 | | 1 789 | |
|
|
|
Derivative financial instruments
The following tables show the contract or underlying principal amounts and fair values of derivative financial instruments analyzed by type of contracts as at December 31, 2023 and 2022. Contract or underlying principal amounts indicate the gross volume of business outstanding at the consolidated balance sheet date and do not represent amounts at risk. The fair values are determined by reference to market prices or standard pricing models that use observable market inputs as at December 31, 2023 and 2022.
| | Contract or underlying principal amounts | | Positive fair values | | Negative fair values | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
(USD millions) | | 2023 | | 2022 | | 2023 | | 2022 | | 2023 | | 2022 | |
|
Forward foreign exchange rate contracts | | 11 944 | | 7 907 | | 335 | | 189 | | -91 | | -41 | |
|
Commodity purchase contracts | | 76 | | 97 | | 20 | | 15 | | | | | |
|
Options on equity securities | | | | 39 | | | | | | | | -14 | |
|
Total derivative financial instruments included in marketable securities and in current financial debts | | 12 020 | | 8 043 | | 355 | | 204 | | -91 | | -55 | |
|
The following table shows a breakdown by currency of the contract or underlying principal amounts of derivative financial instruments as at December 31, 2023 and 2022:
| | 2023 | |
| | | |
| | | |
| | | |
(USD millions) | | EUR | | USD | | Other | | Total | |
|
Forward foreign exchange rate contracts | | 1 629 | | 8 980 | | 1 335 | | 11 944 | |
|
Commodity purchase contracts | | 61 | | 15 | | | | 76 | |
|
Total derivative financial instruments | | 1 690 | | 8 995 | | 1 335 | | 12 020 | |
|
| | 2022 | |
| | | |
| | | |
| | | |
(USD millions) | | EUR | | USD | | Other | | Total | |
|
Forward foreign exchange rate contracts | | 687 | | 5 659 | | 1 561 | | 7 907 | |
|
Commodity purchase contracts | | 80 | | 17 | | | | 97 | |
|
Options on equity securities | | | | 39 | | | | 39 | |
|
Total derivative financial instruments | | 767 | | 5 715 | | 1 561 | | 8 043 | |
|
Derivative financial instruments effective for hedge accounting purposes
At the end of 2023 and 2022, there were no open hedging instruments for anticipated transactions.
Fair value by hierarchy
As required by the IFRS Accounting Standards, financial assets and liabilities recorded at fair value in the consolidated financial statements are categorized based upon the level of judgment associated with the inputs used to measure their fair value. There are three hierarchical levels, based on increasing subjectivity associated with the inputs to derive fair valuation for these assets and liabilities, which are as follows:
The assets carried at Level 1 fair value are equity and debt securities as well as fund investments listed in active markets.
The assets generally included in Level 2 fair value hierarchy are derivatives, and certain debt securities. The liabilities generally included in this fair value hierarchy consist of derivatives. These are valued using corroborated market data.
Level 3 inputs are unobservable for the asset or liability. The assets generally included in Level 3 fair value hierarchy are various investments in funds and unquoted equity security investments. Contingent consideration and other financial liabilities carried at fair value are included in this category.
| | 2023 | |
| | | |
| | | |
| | | |
(USD millions) | | Level 1 | | Level 2 | | Level 3 | | Total | |
|
Financial assets | | | | | | | | | |
Cash and cash equivalents | | | | | | | | | |
Debt securities 1 | | 50 | | | | | | 50 | |
|
Total cash and cash equivalents at fair value | | 50 | | | | | | 50 | |
|
Marketable securities | | | | | | | | | |
Derivative financial instruments | | | | 355 | | | | 355 | |
|
Total marketable securities and derivative financial instruments at fair value | | | | 355 | | | | 355 | |
|
Fund investments and equity securities current | | 94 | | | | 31 | | 125 | |
|
Current contingent consideration receivables | | | | | | 65 | | 65 | |
|
Long-term financial investments | | | | | | | | | |
Debt and equity securities | | 796 | | 20 | | 616 | | 1 432 | |
|
Fund investments | | 7 | | | | 183 | | 190 | |
|
Non-current contingent consideration receivables | | | | | | 553 | | 553 | |
|
Total long-term financial investments at fair value | | 803 | | 20 | | 1 352 | | 2 175 | |
|
Associated companies at fair value through profit and loss | | | | | | 101 | | 101 | |
|
| | | | | | | | | |
Financial liabilities | | | | | | | | | |
Current contingent consideration liabilities | | | | | | -14 | | -14 | |
|
Other financial liabilities current | | | | | | -88 | | -88 | |
|
Derivative financial instruments | | | | -91 | | | | -91 | |
|
Total current financial liabilities at fair values | | | | -91 | | -102 | | -193 | |
|
Non-current contingent consideration liabilities | | | | | | -389 | | -389 | |
|
Total non-current financial liabilities at fair value | | | | | | -389 | | -389 | |
|
|
1 Includes short-term highly rated government-backed debt securities, with an original maturity of three months or less |
| | 2022 | |
| | | |
| | | |
| | | |
(USD millions) | | Level 1 | | Level 2 | | Level 3 | | Total | |
|
Financial assets | | | | | | | | | |
Marketable securities and derivative financial instruments | | | | | | | | | |
Debt securities | | | | 9 | | | | 9 | |
|
Derivative financial instruments | | | | 204 | | | | 204 | |
|
Total marketable securities and derivative financial instruments at fair value | | | | 213 | | | | 213 | |
|
Current contingent consideration receivables | | | | | | 43 | | 43 | |
|
Long-term financial investments | | | | | | | | | |
Debt and equity securities | | 473 | | 10 | | 699 | | 1 182 | |
|
Fund investments | | 20 | | | | 261 | | 281 | |
|
Non-current contingent consideration receivables | | | | | | 607 | | 607 | |
|
Total long-term financial investments at fair value | | 493 | | 10 | | 1 567 | | 2 070 | |
|
Associated companies at fair value through profit and loss | | | | | | 129 | | 129 | |
|
| | | | | | | | | |
Financial liabilities | | | | | | | | | |
Contingent consideration liabilities | | | | | | -131 | | -131 | |
|
Derivative financial instruments | | | | -55 | | | | -55 | |
|
Total current financial liabilities at fair value | | | | -55 | | -131 | | -186 | |
|
Non-current contingent consideration liabilities | | | | | | -704 | | -704 | |
|
Other financial liabilities | | | | | | -232 | | -232 | |
|
Total non-current financial liabilities at fair value | | | | | | -936 | | -936 | |
|
| | | | | | | | | |
The change in carrying values associated with Level 3 financial instruments, using significant unobservable inputs during the year ended December 31, is set forth below:
| | 2023 | |
| | | |
| | | |
| | | |
(USD millions)
| | Associated
companies at
fair value through
profit and loss | |
Fund
investments
| |
Debt and
equity
securities
| |
Contingent
consideration
receivables
| |
Contingent
consideration
liabilities
| |
Other
financial
liabilities
| |
|
January 1 | | 129 | | 261 | | 699 | | 650 | | -835 | | -232 | |
|
Impact from discontinued operations 1 | | | | | | | | | | 101 | | | |
|
Fair value gains and other adjustments, including from divestments recognized in the consolidated income statement | | 4 | | 1 | | 11 | | 48 | | 552 | | | |
|
Fair value losses (including impairments and amortizations) and other adjustments recognized in the consolidated income statement | | -28 | | -48 | | -63 | | -31 | | -65 | | -9 | |
|
Fair value adjustments recognized in the consolidated statement of comprehensive income, including currency translation effects | | 2 | | 3 | | 71 | | | | -32 | | | |
|
Purchases | | 9 | | 14 | | 82 | | | | -180 | | | |
|
Cash receipts and payments | | | | | | | | -49 | | 20 | | 153 | |
|
Disposals | | -6 | | -47 | | -80 | | | | 36 | | | |
|
Reclassification | | -9 | | | | -73 | | | | | | | |
|
December 31 | | 101 | | 184 | | 647 | | 618 | | -403 | | -88 | |
|
| | | | | | | | | | | | | |
Total of fair value gains and losses recognized in the consolidated income statement for assets and liabilities held at December 31, 2023 | | -24 | | -47 | | -52 | | 17 | | 487 | | -9 | |
|
|
1 Represents the carrying values associated with Level 3 financial instruments at January 1, 2023, related to the Sandoz business reported as discontinued operations. Notes 1, 2 and 31 provide disclosures related to discontinued operations. |
| | 2022 | |
| | | |
| | | |
| | | |
(USD millions)
| | Associated
companies at
fair value through
profit and loss | |
Fund
investments
| |
Debt and
equity
securities
| |
Contingent
consideration
receivables
| |
Contingent
consideration
liabilities
| |
Other
financial
liabilities
| |
|
January 1 | | 192 | | 338 | | 617 | | 641 | | -1 075 | | -19 | |
|
Fair value gains and other adjustments, including from divestments recognized in the consolidated income statement | | | | 4 | | 35 | | 53 | | 530 | | 15 | |
|
Fair value losses (including impairments and amortizations) and other adjustments recognized in the consolidated income statement | | -63 | | -78 | | -84 | | | | -114 | | -18 | |
|
Fair value adjustments recognized in the consolidated statement of comprehensive income, including currency translation effects | | | | | | 24 | | | | 11 | | | |
|
Purchases | | 4 | | 11 | | 160 | | | | -231 | | -238 | |
|
Cash receipts and payments | | | | | | | | -44 | | 44 | | 28 | |
|
Disposals | | | | -12 | | -13 | | | | | | | |
|
Reclassification | | -4 | | -2 | | -40 | | | | | | | |
|
December 31 | | 129 | | 261 | | 699 | | 650 | | -835 | | -232 | |
|
| | | | | | | | | | | | | |
Total of fair value gains and losses recognized in the consolidated income statement for assets and liabilities held at December 31, 2022 | | -63 | | -74 | | -49 | | 53 | | 416 | | -3 | |
|
|
During 2023, there were three individually immaterial transfers of equity securities from Level 3 to Level 1 for USD 63 million (2022: USD 44 million), due to the Initial Public Offering of the invested company or lift of certain restrictions.
Realized gains and losses associated with Level 3 long-term financial investments measured at fair value through the consolidated income statement are recorded in the consolidated income statement under “Other income” or “Other expense,” respectively. Realized gains and losses associated with Level 3 long-term financial investments measured at fair value through other comprehensive income are not recycled through the consolidated income statement but are instead reclassified to retained earnings.
During the year, the net loss and net gain recorded on associated companies, fund investments and long-term financial investments at fair value through profit and loss were USD 145 million and USD 39 million, respectively.
To determine the fair value of a contingent consideration, various unobservable inputs are used. A change in these inputs might result in a significantly higher or lower fair value measurement. The inputs used are, among others, the probability of success, sales forecast and assumptions regarding the discount rate and timing and different scenarios of triggering events. The inputs are interrelated. The significance and usage of these inputs to each contingent consideration may vary due to differences in the timing and triggering events for payments or in the nature of the asset related to the contingent consideration.
If the most significant parameters for the Level 3 input were to change by 10% positively or negatively, or where the probability of success (POS) is the most significant input parameter 10% were added or deducted from the applied probability of success, for contingent consideration payables and contingent consideration receivables, this would change the amounts recorded in the 2023 consolidated income statement by USD 57 million and USD 53 million, respectively.
Equity securities measured at fair value through other comprehensive income
Equity securities held as strategic investments, typically held outside the Novartis Venture Fund, are generally designated at date of acquisition as financial assets valued at fair value through other comprehensive income with no subsequent recycling through profit and loss. Except for the investment in Sandoz Group AG with a fair value of USD 595 million as at December 31, 2023, these are made up of individually non-significant investments. As at December 31, 2023, the Company holds 61 non-listed equity securities (December 31, 2022: 65) and 28 listed equity securities (December 31, 2022: 46) in this category with the following fair values:
(USD millions) | | 2023 | | 2022 | |
|
Listed equity securities | | 861 | | 438 | |
|
Non-listed equity securities | | 349 | | 390 | |
|
Total equity securities | | 1 210 | | 828 | |
|
|
|
During 2023 and 2022, dividends received from these equity securities were insignificant. In 2023, equity securities that were no longer considered strategic, with a fair value of USD 279 million (2022: USD 4 million), were sold, and the USD 1 million gain on disposal (2022: USD 4 million loss) was transferred from other comprehensive income to retained earnings (see Note 9).
Nature and extent of risks arising from financial instruments
Market risk
Market risk in general comprises currency risk, interest rate risk and price risk, such as commodity and equity prices. Novartis is exposed to market risk, primarily related to foreign currency exchange rates, interest rates and the market value of the investments. The Company actively monitors and seeks to reduce, where it deems it appropriate to do so, fluctuations in these exposures. It is the Company’s policy and practice to enter into a variety of derivative financial instruments to manage the volatility of these exposures. It does not enter into any financial transactions containing a risk that cannot be quantified at the time the transaction is concluded. In addition, it does not sell short assets it does not have, or does not know it will have, in the future. The Company only sells existing assets or enters into transactions and future transactions (in the case of anticipatory hedges) that it confidently expects it will have in the future, based on past experience.
Foreign currency exchange rate risk
The Company uses the US dollar as its reporting currency. As a result, the Company is exposed to foreign currency exchange movements, primarily in European, Japanese and emerging market currencies. Fluctuations in the exchange rates between the US dollar and other currencies can have a significant effect on both the Company’s results of operations, including reported sales and earnings, as well as on the reported value of our assets, liabilities and cash flows. This, in turn, may significantly affect the comparability of period-to-period results of operations.
Because our expenditures in Swiss francs are significantly higher than our revenues in Swiss francs, volatility in the value of the Swiss franc can have a significant impact on the reported value of our earnings, assets and liabilities, and the timing and extent of such volatility can be difficult to predict.
There is also a risk that certain countries could experience a devaluation of their currency. If this occurs, it could impact the effective prices we would be able to charge for our products and also have an adverse impact on both our consolidated income statement and balance sheet.
Subsidiaries whose functional currencies have experienced a cumulative inflation rate of more than 100% over the past three years apply the principles of IAS 29 “Financial reporting in Hyperinflationary Economies.” The hyperinflationary economies in which Novartis operates are Argentina, Venezuela and Turkey. Venezuela and Argentina were hyperinflationary for all periods presented, and Turkey became hyperinflationary effective May 1, 2022, requiring retroactive implementation of hyperinflation accounting as of January 1, 2022. The impacts of applying IAS 29 are recorded in “Other financial income and expense” and are presented in Note 6.
The Company manages its global currency exposure by engaging in hedging transactions where management deems appropriate. Novartis may enter into various contracts that reflect the changes in the value of foreign currency exchange rates to preserve the value of assets, commitments and anticipated transactions. Novartis also uses forward contracts and may enter into foreign currency option contracts to hedge.
Net investments in subsidiaries in foreign countries are long-term investments. Their fair value changes through movements of foreign currency exchange rates. The Company has designated a certain portion of its long-term euro-denominated straight bonds, maturing in 2028, as hedges of the translation risk arising on certain of these net investments in foreign operations with euro functional currency. As of December 31, 2023, long-term financial debt with a carrying amount of EUR 1.8 billion (USD 2.0 billion; December 31, 2022: USD 2.0 billion), has been designated as a hedge instrument. During 2023, USD 50 million of net of taxes unrealized losses (2022: USD 91 million income) was recognized in other comprehensive income and accumulated in currency translation effects in relation with this net investment hedge. The hedge remained effective since inception, and no amount was recognized in the consolidated income statement in 2022 and 2021. In 2023, USD 8 million of accumulated net investment hedge reserve was recognized in the consolidated income statement at the time of the Sandoz spin off.
Commodity price risk
The Company has only a very limited exposure to price risk related to anticipated purchases of certain commodities used as raw materials by the Company’s businesses. A change in those prices may alter the gross margin of a specific business, but generally by not more than 10% of the margin and thus below the Company’s risk management tolerance levels. Accordingly, the Company does not enter into significant commodity futures, forward or option contracts to manage fluctuations in prices of anticipated purchases.
Interest rate risk
The Company addresses its net exposure to interest rate risk mainly through the ratio of its fixed-rate financial debt to variable-rate financial debt contained in its total financial debt portfolio. To manage this mix, Novartis may enter into interest rate swap agreements, in which it exchanges periodic payments based on a notional amount and agreed-upon fixed and variable interest rates.
Equity risk
The Company may purchase equities as investments of its liquid funds. As a policy, it limits its holdings in an unrelated company to less than 5% of its liquid funds. Potential investments are thoroughly analyzed. Call options are written on equities that the Company owns, and put options are written on equities that the Company wants to buy and for which cash is available.
Credit risk
Credit risks arise from the possibility that customers may not be able to settle their obligations as agreed. To manage this risk, the Company periodically assesses country and customer credit risk, assigns individual credit limits, and takes actions to mitigate credit risk where appropriate (for example payment guarantees, credit insurance and factoring).
The provisions for expected credit losses for customers are based on a forward-looking expected credit loss, which includes possible default events on the trade receivables over the entire holding period of the trade receivables.
In measuring the expected credit losses, trade receivables are grouped based on shared credit risk characteristics (such as private versus public receivables) and days past due. In determining the expected credit loss rates, the Company considers current and forward-looking macroeconomic factors that may affect the ability of customers to settle the receivables, and historical loss rates for each category of customers.
The Company’s largest customer accounted for approximately 15% of net sales to third parties, and the second largest and third largest customers accounted for 13% and 8% of net sales to third parties, respectively (2022: 16%, 12% and 8%, respectively; 2021: 14%, 13% and 7%, respectively).
The highest amounts of trade receivables outstanding were for these same three customers and amounted to 17%, 13% and 8%, respectively, of the Company’s trade receivables as at December 31, 2023 (2022: 16%, 14% and 7%, respectively). There is no other significant concentration of customer credit risk.
Counterparty risk
Counterparty risk encompasses issuer risk on marketable securities and money market instruments; credit risk on cash, time deposits and derivatives; as well as settlement risk for different instruments. Issuer risk is reduced by only buying securities that are at least A- rated. Counterparty credit risk and settlement risk are reduced by a policy of entering into transactions with counterparties (banks or financial institutions) that feature a strong credit rating. Exposure to these risks is closely monitored and kept within predetermined parameters. The limits are regularly assessed and determined based upon credit analysis, including financial statement and capital adequacy ratio reviews. In addition, reverse repurchasing agreements are contracted, and Novartis has entered into credit support agreements with various banks for derivative transactions. To further reduce the settlement risk, the Company has implemented a multi-currency payment system, Continuous Linked Settlement (CLS), which provides multilateral netting (payment-versus-payment settlement) of cash flows from foreign exchange transactions.
The Company’s cash and cash equivalents are held with major regulated financial institutions, the three largest of which hold approximately 8.3%, 7.5% and 7.4%, respectively (2022: 13.2%, 9.2% and 6.8%, respectively).
The Company does not expect any losses from non-performance by these counterparties and does not have any significant grouping of exposures to financial sector or country risk.
Liquidity risk
Liquidity risk is defined as the risk that the Company could not be able to settle or meet its obligations associated with financial liabilities that are settled by delivering cash or another financial asset. Treasury is responsible for liquidity, funding and settlement management. In addition, liquidity and funding risks, and related processes and policies, are overseen by management. Novartis manages its liquidity risk on a consolidated basis according to business needs and tax, capital or regulatory considerations, if applicable, through numerous sources of financing in order to maintain flexibility.
Certain countries have legal or economic restrictions on the ability of subsidiaries to transfer funds to the Company in the form of cash dividends, loans or advances, but these restrictions do not have an impact on the ability of the Company to meet its cash obligations.
Management monitors the Company’s net debt or liquidity position through rolling forecasts on the basis of expected cash flows.
Novartis has two US commercial paper programs under which it can issue up to USD 9.0 billion in the aggregate of unsecured commercial paper notes. Novartis also has one Japanese commercial paper program under which it can issue up to JPY 150 billion (approximately USD 1.1 billion) of unsecured commercial paper notes. Commercial paper notes totaling USD 3.3 billion under these three programs were outstanding as per December 31, 2023 (2022: USD 2.8 billion). Novartis further has a committed credit facility of USD 6.0 billion. This credit facility is provided by a syndicate of banks and is intended to be used as a backstop for the US commercial paper programs. The facility matures in September 2025 and was undrawn as at December 31, 2023, and December 31, 2022.
The following table sets forth how management monitors net debt or liquidity based on details of the remaining contractual maturities of selected financial assets and liabilities as at December 31, 2023, and December 31, 2022:
| | 2023 | |
| | | |
| | | |
| | | |
(USD millions)
| |
Due within
one month
| | Due later than
one month
but less than
three months | | Due later than
three months
but less than
one year | | Due later than
one year
but less than
five years | |
Due after
five years
| |
Total
| |
|
Current assets | | | | | | | | | | | | | |
Marketable securities, time deposits and short-term investments with original maturity more than 90 days and accrued interest | | 12 | | 516 | | 41 | | | | | | 569 | |
|
Commodities | | | | | | | | | | 111 | | 111 | |
|
Derivative financial instruments | | 24 | | 310 | | 1 | | | | 20 | | 355 | |
|
Cash and cash equivalents | | 7 641 | | 5 752 | | | | | | | | 13 393 | |
|
Total current financial assets | | 7 677 | | 6 578 | | 42 | | | | 131 | | 14 428 | |
|
| | | | | | | | | | | | | |
Non-current liabilities | | | | | | | | | | | | | |
Financial debt | | | | | | | | -9 492 | | -8 944 | | -18 436 | |
|
Financial debt - undiscounted | | | | | | | | -9 522 | | -9 050 | | -18 572 | |
|
Total non-current financial debt | | | | | | | | -9 492 | | -8 944 | | -18 436 | |
|
| | | | | | | | | | | | | |
Current liabilities | | | | | | | | | | | | | |
Financial debt | | -3 328 | | -372 | | -2 384 | | | | | | -6 084 | |
|
Financial debt - undiscounted | | -3 328 | | -372 | | -2 384 | | | | | | -6 084 | |
|
Derivative financial instruments | | -43 | | -39 | | -9 | | | | | | -91 | |
|
Total current financial debt | | -3 371 | | -411 | | -2 393 | | | | | | -6 175 | |
|
| | | | | | | | | | | | | |
|
Net debt | | 4 306 | | 6 167 | | -2 351 | | -9 492 | | -8 813 | | -10 183 | |
|
| | 2022 | |
| | | |
| | | |
| | | |
(USD millions)
| |
Due within
one month
| | Due later than
one month
but less than
three months | | Due later than
three months
but less than
one year | | Due later than
one year
but less than
five years | |
Due after
five years
| |
Total
| |
|
Current assets | | | | | | | | | | | | | |
Marketable securities, time deposits and short-term investments with original maturity more than 90 days and accrued interest | | 4 142 | | 6 911 | | 36 | | | | 9 | | 11 098 | |
|
Commodities | | | | | | | | | | 111 | | 111 | |
|
Derivative financial instruments | | 23 | | 147 | | 19 | | | | 15 | | 204 | |
|
Cash and cash equivalents | | 4 011 | | 3 506 | | | | | | | | 7 517 | |
|
Total current financial assets | | 8 176 | | 10 564 | | 55 | | | | 135 | | 18 930 | |
|
| | | | | | | | | | | | | |
Non-current liabilities | | | | | | | | | | | | | |
Financial debt | | | | | | | | -8 975 | | -11 269 | | -20 244 | |
|
Financial debt - undiscounted | | | | | | | | -9 002 | | -11 394 | | -20 396 | |
|
Total non-current financial debt | | | | | | | | -8 975 | | -11 269 | | -20 244 | |
|
| | | | | | | | | | | | | |
Current liabilities | | | | | | | | | | | | | |
Financial debt | | -3 215 | | -146 | | -2 515 | | | | | | -5 876 | |
|
Financial debt - undiscounted | | -3 215 | | -146 | | -2 517 | | | | | | -5 878 | |
|
Derivative financial instruments | | -38 | | -13 | | -4 | | | | | | -55 | |
|
Total current financial debt | | -3 253 | | -159 | | -2 519 | | | | | | -5 931 | |
|
| | | | | | | | | | | | | |
|
Net debt | | 4 923 | | 10 405 | | -2 464 | | -8 975 | | -11 134 | | -7 245 | |
|
The carrying amounts of financial liabilities included in the above analysis are not materially different to the contractual amounts due on maturity. The positive and negative fair values on derivative financial instruments represent the net contractual amounts to be exchanged at maturity.
The Company’s contractual undiscounted potential cash flows from derivative financial instruments to be settled on a gross basis are as follows:
| | 2023 | |
| | | |
| | | |
| | | |
(USD millions)
| |
Due within
one month
| | Due later than
one month
but less than
three months | | Due later than
three months
but less than
one year |
|
Total
| |
|
Derivative financial instruments and accrued interest on derivative financial instruments | | | | | | | | | |
Potential outflows in various currencies - from financial derivative liabilities | | -4 329 | | -6 604 | | -556 | | -11 489 | |
|
Potential inflows in various currencies - from financial derivative assets | | 4 311 | | 6 841 | | 623 | | 11 775 | |
|
| | 2022 | |
| | | |
| | | |
| | | |
(USD millions)
| |
Due within
one month
| | Due later than
one month
but less than
three months | | Due later than
three months
but less than
one year |
|
Total
| |
|
Derivative financial instruments and accrued interest on derivative financial instruments | | | | | | | | | |
Potential outflows in various currencies - from financial derivative liabilities | | -2 029 | | -4 598 | | -316 | | -6 943 | |
|
Potential inflows in various currencies - from financial derivative assets | | 2 029 | | 4 712 | | 321 | | 7 062 | |
|
Other contractual liabilities that are not part of management’s monitoring of the net debt or liquidity consist of the following items:
| | 2023 | |
| | | |
| | | |
| | | |
(USD millions)
| |
Due within
three months
| | Due later than
three months
but less than
one year | | Due later than
one year
but less than
five years | |
Due after
five years
| |
Total
| |
|
Contractual interest on non-current financial debt, including current portion | | -64 | | -372 | | -1 258 | | -3 376 | | -5 070 | |
|
Lease liabilities 1 | | -65 | | -165 | | -635 | | -963 | | -1 828 | |
|
Trade payables | | -4 793 | | -133 | | | | | | -4 926 | |
|
Contingent consideration liabilities | | | | -14 | | -205 | | -184 | | -403 | |
|
|
1 Note 11 provides additional disclosures related to lease liabilities. |
| | 2022 | |
| | | |
| | | |
| | | |
(USD millions)
| |
Due within
three months
| | Due later than
three months
but less than
one year | | Due later than
one year
but less than
five years | |
Due after
five years
| |
Total
| |
|
Contractual interest on non-current financial debt, including current portion | | -64 | | -412 | | -1 432 | | -3 624 | | -5 532 | |
|
Lease liabilities 1 | | -71 | | -180 | | -616 | | -922 | | -1 789 | |
|
Trade payables | | -5 020 | | -126 | | | | | | -5 146 | |
|
Contingent consideration liabilities | | -16 | | -115 | | -437 | | -267 | | -835 | |
|
|
1 Note 11 provides additional disclosures related to lease liabilities. |
Capital risk management
Novartis strives to maintain a strong credit rating. In managing its capital, Novartis focuses on maintaining a strong balance sheet. As at December 31, 2023, Moody’s Investors Service rated the Company A1 for long-term maturities and P-1 for short-term maturities, and S&P Global Ratings rated the Company AA- for long-term maturities and A-1+ for short-term maturities.
Sensitivity analysis
The Company uses sensitivity analysis disclosures to provide quantitative information about market risks to which it is exposed.
The sensitivity analysis disclosures are in line with the Company’s financial risk management policy, and are based on a one-parameter risk model that considers a one-factor linear relationship between risk factors and exposures. They consider aggregated risk exposures arising from the most significant risk factors (currency risk, interest rate risk and equity price risk) and include
all financial assets and financial liabilities as set forth in the table on page F-59.
The disclosures below illustrate the potential impact on the Company’s consolidated financial statements as a result of hypothetical market movements in foreign currency exchange rates, interest rates and equity prices. The range of variables chosen reflects management’s view of changes that are reasonably possible over a one-year period.
Foreign currency exchange rate sensitivity
The Company uses the US dollar as its reporting currency. As a result, the Company is exposed to foreign currency exchange movements, primarily in European, Japanese and emerging market currencies, as well as in the Swiss franc. A strengthening (weakening) of the US dollar against these currencies as at December 31, 2023 and 2022 would have affected the measurement of financial instruments denominated in these foreign currencies. This analysis assumes that all other variables, in particular interest rates, remain constant. A hypothetical 5% increase or decrease in the foreign currency exchange rates against the US dollar would have impacted the Company’s consolidated income statement as presented below:
(USD millions) | | 2023 | | 2022 | |
|
5% increase in foreign currency exchange rates against USD | | 3 | | -6 | |
|
5% decrease in foreign currency exchange rates against USD | | -3 | | 7 | |
|
As of December 31, 2023, the Company designated EUR 1.8 billion (December 31, 2022: EUR 1.8 billion) of its long-term euro-denominated straight bonds as hedges of the translation risk arising on certain net investments in foreign operations with euro functional currency. This analysis assumes that all other variables, in particular interest rates, remain constant. A hypothetical 5% increase, or decrease, in the foreign currency exchange rates against the US dollar, without considering the translation effect of these net investments, would have impacted the Company’s consolidated equity as presented below:
(USD millions) | | 2023 | | 2022 | |
|
5% increase in foreign currency exchange rates against USD | | 97 | | 93 | |
|
5% decrease in foreign currency exchange rates against USD | | -102 | | -98 | |
|
Interest rate sensitivity
Our portfolio of fixed-income instruments as at December 31, 2023, was mainly composed of time deposits and debt securities.
Novartis uses duration models to approximate the possible change in the value of fixed-income instruments. Based on these models, management believes that a 100-basis point change in interest is deemed a reasonable possible change over a one-year period.
Based on exposures in 2023 and 2022, a hypothetical 100-basis point increase (decrease) in interest rates would not have resulted in a significant increase (decrease) in the fair values of the fixed-income instruments nor in a significant increase (decrease) of cash flows attributable to such instruments.
The vast majority of our outstanding financial debts are straight bonds with fixed interest rates and are therefore not affected by movements in interest rates.
Equity price sensitivity
Fund investments and equity securities held by the Novartis Venture Fund are valued at fair value through profit and loss. Equity securities held as strategic investments, typically held outside the Novartis Venture Fund, are generally designated at date of acquisition as financial assets valued at fair value through other comprehensive income with no subsequent recycling through profit and loss.
The fair value of these fund investments and equity securities was USD 1.8 billion as at December 31, 2023 (December 31, 2022: USD 1.6 billion). The fair values of these investments are impacted by the volatility of the stock market, valuation parameters applied (for non-listed equities) and changes in general economic factors. This analysis assumes that all other variables, in particular interest rates, remain constant. A hypothetical increase or decrease of 15% in the risk factors would have impacted the Company’s consolidated income statement as presented below:
(USD millions) | | 2023 | | 2022 | |
|
15% increase in equity prices | | 91 | | 109 | |
|
15% decrease in equity prices | | -91 | | -109 | |
|
A hypothetical increase or decrease of 15% in the risk factors would have impacted the Company’s consolidated equity as presented below:
(USD millions) | | 2023 | | 2022 | |
|
15% increase in equity prices | | 182 | | 124 | |
|
15% decrease in equity prices | | -182 | | -124 | |
|
31. Discontinued operations
Discontinued operations include the operational results from the Sandoz generic pharmaceuticals and biosimilars division and certain corporate activities attributable to the Sandoz business, as well as certain other expenses related to the spin-off. Included in 2023 is also the IFRS Accounting Standards non-cash, non-taxable net gain on the distribution of Sandoz Group AG to Novartis AG shareholders (refer to Notes 1 and 2 for further details).
The Sandoz business operates in the off-patent medicines segment and specializes in the development, manufacturing, and marketing of generic pharmaceuticals and biosimilars. The Sandoz business is organized globally into two franchises: Generics and Biosimilars.
Net income from discontinued operations
(USD millions) | | 20231 | | 2022 | | 2021 | |
|
Net sales to third parties from discontinued operations | | 7 128 | | 9 160 | | 9 650 | |
|
Sales to continuing segments | | 300 | | 212 | | 184 | |
|
Net sales from discontinued operations | | 7 428 | | 9 372 | | 9 834 | |
|
Other revenues | | 19 | | 28 | | 58 | |
|
Cost of goods sold | | -4 044 | | -4 937 | | -5 121 | |
|
Gross profit from discontinued operations | | 3 403 | | 4 463 | | 4 771 | |
|
Selling, general and administration | | -1 728 | | -2 060 | | -2 059 | |
|
Research and development | | -671 | | -824 | | -899 | |
|
Other income | | 56 | | 109 | | 232 | |
|
Other expense | | -795 | | -437 | | -412 | |
|
Operating income from discontinued operations | | 265 | | 1 251 | | 1 633 | |
|
Income from associated companies | | 2 | | 2 | | 2 | |
|
Interest expense | | -33 | | -37 | | -24 | |
|
Other financial income and expense | | -20 | | -22 | | -4 | |
|
Income before taxes from discontinued operations | | 214 | | 1 194 | | 1 607 | |
|
Income taxes 2 | | 208 | | -288 | | -494 | |
|
Net income from discontinued operations before gain on distribution of Sandoz Group AG to Novartis AG shareholders | | 422 | | 906 | | 1 113 | |
|
Gain on distribution of Sandoz Group AG to Novartis AG shareholders 3 | | 5 860 | | | | | |
|
Net income from discontinued operations | | 6 282 | | 906 | | 1 113 | |
|
|
1 The net income from discontinued operations for 2023 is for the period from January 1, 2023, to the October 3, 2023, Distribution date. |
2 The tax rate in 2023 was impacted by non-recurring items such as tax benefits arising from intercompany transactions to effect the spin-off of the Sandoz business, net decreases in uncertain tax positions of the Sandoz business and the favorable settlement of a tax matter related to the Alcon business, which was spun-off in 2019. Excluding these impacts, the tax rate would have been 31.2% in 2023, compared to 24.1% and 30.7% in 2022 and 2021, respectively. The tax rate in 2023 is higher than 2022 primarily due to a change in profit mix between years. |
3 See Note 2 for further details on the non-taxable, non-cash gain on distribution of Sandoz Group AG to Novartis AG shareholders. |
Net assets derecognized
The following table presents the Sandoz business net assets derecognized as at October 3, 2023 Distribution (spin-off) date:
(USD millions)
| | Oct 3,
2023 | |
|
Property, plant and equipment | | 1 447 | |
|
Right-of-use assets | | 133 | |
|
Goodwill | | 7 424 | |
|
Intangible assets other than goodwill | | 1 481 | |
|
Deferred tax assets | | 624 | |
|
Financial assets, investments in associated companies and other non-current assets | | 142 | |
|
Inventories | | 2 565 | |
|
Trade receivables and other current assets | | 2 935 | |
|
Cash and cash equivalents | | 686 | |
|
Deferred tax liabilities | | -270 | |
|
Current and non-current lease liabilities | | -139 | |
|
Current and non-current financial debts | | -3 691 | |
|
Trade payables, provisions, current income tax liabilities and other liabilities | | -4 690 | |
|
Net assets derecognized | | 8 647 | |
|
| | | |
Supplemental disclosures related to discontinued operations
Revenue
In addition to the elements of variable consideration listed in the revenue accounting policy described in Note 1, the Sandoz business grants shelf stock adjustments to customers to cover the inventory held by them at the time a price decline becomes effective. Revenue deduction provisions for shelf stock adjustments are recorded when the price decline is anticipated, based on the impact of the price decline on the customer’s estimated inventory levels.
Significant transactions in 2021
On February 10, 2021, Sandoz entered into an agreement with certain subsidiaries of GlaxoSmithKline plc (GSK) for the acquisition of the GSK’s cephalosporin antibiotics business.
Under the agreement, Sandoz acquired the global rights to three established brands (Zinnat®, Zinacef® and Fortum®) in more than 100 markets. It excluded the rights in the US, Australia and Germany to certain of those brands, which were previously divested by GSK, and the rights in India, Pakistan, Egypt, Japan (to certain of the brands) and China, which will be retained by GSK. The transaction closed on October 8, 2021.
The purchase price consisted of a USD 350 million upfront payment paid at closing and potential milestone payments up to USD 150 million, which GSK is eligible to receive upon the achievement of certain annual sales milestones for the portfolio.
The fair value of the total purchase consideration was USD 415 million. The amount consisted of a payment of USD 351 million, including purchase price adjustments, and the fair value of contingent consideration of USD 64 million, which GSK is eligible to receive upon the achievement of specified milestones. The purchase price allocation resulted in net identifiable assets of USD 308 million, consisting of USD 292 million intangible assets and USD 16 million deferred tax assets. Goodwill amounted to USD 107 million.
The 2021 results of operations since the date of acquisition were not material.
Net income from discontinued operations
Included in net income from discontinued operations are:
(USD millions unless indicated otherwise) | | 20231 | | 2022 | | 2021 |
|
Interest income | | 2 | | 2 | | 1 |
|
Depreciation of property, plant and equipment | | -144 | | -196 | | -203 |
|
Depreciation of right-of-use assets | | -32 | | -33 | | -39 |
|
Amortization of intangible assets | | -171 | | -222 | | -238 |
|
Impairment charges on property, plant and equipment | | -5 | | -3 | | -68 |
|
Impairment charges on right-of-use assets | | -8 | | | | |
|
Impairment charges on intangible assets | | -44 | | -25 | | -27 |
|
Impairment reversals of property, plant and equipment | | 1 | | 3 | | 59 |
|
Additions to restructuring provisions | | -27 | | -40 | | -62 |
|
Equity-based compensation expense related to Novartis equity-based participation plans | | -60 | | -66 | | -69 |
|
|
1 2023 amounts are for the period from January 1, 2023, to the October 3, 2023, Distribution date. |
In 2023, 2022 and 2021, there were no reversals of impairment charges on right-of-use assets or on intangible assets of discontinued operations.
Balance sheet
The following table shows for discontinued operations the additions to property, plant and equipment, right-of-use assets and to goodwill and intangible assets:
(USD millions) | | 20231 | | 2022 | |
|
Additions to property, plant and equipment | | 245 | | 289 | |
|
Additions to right-of-use assets | | 66 | | 32 | |
|
Additions to goodwill and intangible assets | | 221 | | 163 | |
|
|
1 The additions for 2023 are for the period from January 1, 2023, to the October 3, 2023, Distribution date. |
Financial debt
The Sandoz business entered into financing agreements with a group of banks under which it borrowed on September 28, 2023 a total amount of USD 3.3 billion. See Note 2 for further disclosures.
Net cash flows used in investing activities from discontinued operations
Net cash flows used in investing activities from discontinued operations include the investing activities of the Sandoz business. In 2023, other cash flows used in investing activities, net includes cash outflows of USD 22 million (2022: USD 39 million, 2021: USD 362 million, including the acquisition of GSK’s cephalosporin antibiotics business) for the acquisitions and divestments of business, net.
(USD millions) | | 2023 | | 2022 | | 2021 | |
|
Payments out of provision for transaction cost attributable to the spin-off of the Sandoz business | | -52 | | | | | |
|
Derecognized cash and cash equivalents attributable to the spin-off of the Sandoz business | | -686 | | | | | |
|
Other cash flows used in investing activities, net | | -385 | | -436 | | -689 | |
|
Net cash flows used in investing activities from discontinued operations | | -1 123 | | -436 | | -689 | |
|
Net cash flows from financing activities from discontinued operations
In 2023, the net cash inflows from financing activities from discontinued operations of USD 3.3 billion (2022: USD 119 million, 2021: USD 26 million) were mainly driven by USD 3.6 billion cash inflows from bank borrowings (including the USD 3.3 billion Sandoz business borrowings from a group of banks on September 28, 2023) in connection with the Distribution (spin-off) of the Sandoz business to Novartis AG shareholders, partly offset by transaction cost payments of USD 0.2 billion (2022: nil, 2021: nil) directly attributable to the Distribution (spin-off) of the Sandoz business (see Notes 1 and 2).
For additional information related to the October 3, 2023 Distribution (spin-off) of the Sandoz business to Novartis AG shareholders, effected through a dividend in kind distribution of Sandoz Group AG shares to Novartis AG shareholders and ADR holders, refer to Note 1 and Note 2.
32. Events subsequent to the December 31, 2023, consolidated balance sheet date
Dividend proposal for 2023 and approval of Novartis 2023 consolidated financial statements
On January 30, 2024, the Novartis AG Board of Directors proposed the acceptance of the 2023 consolidated financial statements of Novartis for approval by the Annual General Meeting on March 5, 2024. Furthermore, also on January 30, 2024, the Board proposed a dividend of CHF 3.30 per share to be approved at the Annual General Meeting on March 5, 2024. If approved, the total dividend payments would amount to approximately USD 8.0 billion (2022: USD 7.3 billion), using the CHF/USD December 31, 2023, exchange rate.
33. Novartis principal subsidiaries and associated companies
The following table lists the principal subsidiaries controlled by Novartis, associated companies in which Novartis is deemed to have significant influence, and foundations required to be consolidated under IFRS Accounting Standards. It includes all subsidiaries, associated companies and consolidated foundations with total assets or net sales to third parties in excess of USD 25 million. The equity interest percentage shown in the table also represents the share in voting rights in those entities.
As at December 31, 2023
| | | | | Share
capital1 | | | Equity
interest | |
|
Argentina | | | | | | | | | |
Novartis Argentina S.A. | | Buenos Aires | | ARS | 906.1 | m | | 100% | |
|
Australia | | | | | | | | | |
Novartis Australia Pty Ltd | | Macquarie Park, NSW | | AUD | 2 | | | 100% | |
Novartis Pharmaceuticals
Australia Pty Ltd | | Macquarie Park, NSW | | AUD | 3.8 | m | | 100% | |
|
Austria | | | | | | | | | |
Novartis Holding GmbH | | Vienna | | EUR | 35 000 | | | 100% | |
Novartis Pharmaceutical Manufacturing GmbH | | Langkampfen | | EUR | 763 070 | | | 100% | |
Novartis Pharma GmbH | | Vienna | | EUR | 1.1 | m | | 100% | |
|
Bangladesh | | | | | | | | | |
Novartis (Bangladesh) Limited | | Gazipur | | BDT | 162.5 | m | | 60% | |
|
Belgium | | | | | | | | | |
Novartis Pharma NV | | Vilvoorde | | EUR | 7.1 | m | | 100% | |
Alcon - Couvreur NV | | Puurs | | EUR | 110.6 | m | | 100% | |
|
Bermuda | | | | | | | | | |
Novartis Investment Ltd. | | Hamilton 2 | | USD | 12 000 | | | 100% | |
Novartis Securities Investment Ltd. | | Hamilton | | CHF | 30 000 | | | 100% | |
Novartis Finance Services Ltd. | | Hamilton | | CHF | 20 000 | | | 100% | |
Triangle International Reinsurance Limited | | Hamilton | | CHF | 1.0 | m | | 100% | |
Trinity River Insurance Co Ltd. | | Hamilton | | USD | 370 000 | | | 100% | |
|
Brazil | | | | | | | | | |
Novartis Biociências S.A. | | São Paulo | | BRL | 507.1 | m | | 100% | |
|
Canada | | | | | | | | | |
Novartis Pharmaceuticals Canada Inc. | | Montreal, Quebec | | CAD | 420 717 | | | 100% | |
|
Chile | | | | | | | | | |
Novartis Chile S.A. | | Santiago de Chile | | CLP | 2.0 | bn | | 100% | |
|
China | | | | | | | | | |
Beijing Novartis Pharma Co., Ltd. | | Beijing | | USD | 30.0 | m | | 100% | |
Novartis Pharmaceuticals (HK) Limited | | Hong Kong | | HKD | 200 | | | 100% | |
China Novartis Institutes for
BioMedical Research Co., Ltd. | | Shanghai | | USD | 320.0 | m | | 100% | |
Suzhou Novartis Technical
Development Co., Ltd. | | Changshu | | USD | 12.0 | m | | 100% | |
Shanghai Novartis Trading Ltd. | | Shanghai | | USD | 3.2 | m | | 100% | |
|
Colombia | | | | | | | | | |
Novartis de Colombia S.A. | | Santafé de Bogotá | | COP | 7.9 | bn | | 100% | |
|
Czech Republic | | | | | | | | | |
Novartis s.r.o. | | Prague | | CZK | 51.5 | m | | 100% | |
|
Denmark | | | | | | | | | |
Novartis Healthcare A/S | | Copenhagen | | DKK | 14.0 | m | | 100% | |
|
Dominican Republic | | | | | | | | | |
Novartis Caribe, S.A. | | Santo Domingo | | DOP | 20.0 | m | | 100% | |
|
Ecuador | | | | | | | | | |
Novartis Ecuador S.A. | | Quito | | USD | 4.0 | m | | 100% | |
|
Egypt | | | | | | | | | |
Novartis Pharma S.A.E. | | Cairo | | EGP | 2.1 | bn | | 99.98% | |
|
Finland | | | | | | | | | |
Novartis Finland Oy | | Espoo | | EUR | 459 000 | | | 100% | |
|
France | | | | | | | | | |
Novartis Groupe France S.A.S. | | Rueil-Malmaison | | EUR | 903.0 | m | | 100% | |
Novartis Pharma S.A.S. | | Rueil-Malmaison | | EUR | 43.4 | m | | 100% | |
Advanced Accelerator Applications S.A. | | Rueil-Malmaison | | EUR | 9.6 | m | | 99.23% | |
Advanced Accelerator Applications
Molecular Imaging France | | Saint-Genis-Pouilly | | EUR | 7.5 | m | | 99.23% | |
|
Germany | | | | | | | | | |
Novartis Business Services GmbH | | Wehr | | EUR | 25 000 | | | 100% | |
Novartis Pharma GmbH | | Nuremberg | | EUR | 25.6 | m | | 100% | |
Novartis Pharma Produktions GmbH | | Wehr | | EUR | 2.0 | m | | 100% | |
Novartis Pharma Vertriebs GmbH | | Nuremberg | | EUR | 25 000 | | | 100% | |
|
Greece | | | | | | | | | |
Novartis (Hellas) S.A.C.I. | | Metamorphosis / Athens | | EUR | 233.9 | m | | 100% | |
|
As at December 31, 2023
| | | | | Share
capital1 | | | Equity
interest | |
|
Hungary | | | | | | | | | |
Novartis Hungary Healthcare Limited Liability
Company | | Budapest | | HUF | 545.6 | m | | 100% | |
|
India | | | | | | | | | |
Novartis India Limited | | Mumbai | | INR | 123.5 | m | | 70.68% | |
Novartis Healthcare Private Limited | | Mumbai | | INR | 60.0 | m | | 100% | |
|
Indonesia | | | | | | | | | |
PT. Novartis Indonesia | | Jakarta | | IDR | 7.7 | bn | | 100% | |
|
Ireland | | | | | | | | | |
Novartis Ireland Limited | | Dublin | | EUR | 25 000 | | | 100% | |
Novartis Integrated Services Limited | | Cork City | | EUR | 100 | | | 100% | |
|
Israel | | | | | | | | | |
Novartis Israel Ltd. | | Tel Aviv | | ILS | 1 000 | | | 100% | |
|
Italy | | | | | | | | | |
Novartis Farma S.p.A. | | Milan | | EUR | 18.2 | m | | 100% | |
Advanced Accelerator Applications (Italy) S.r.l. | | Pozzilli | | EUR | 119 000 | | | 99.23% | |
|
Japan | | | | | | | | | |
Novartis Pharma K.K. | | Tokyo | | JPY | 100.0 | m | | 100% | |
Ciba-Geigy Japan Limited | | Tokyo | | JPY | 100.0 | m | | 100% | |
|
Latvia | | | | | | | | | |
Novartis Baltics SIA | | Riga | | EUR | 3.0 | m | | 100% | |
|
Luxembourg | | | | | | | | | |
Novartis Investments S.à r.l. | | Luxembourg City 2 | | USD | 100.0 | m | | 100% | |
Novartis Finance S.A. | | Luxembourg City | | USD | 100 000 | | | 100% | |
|
Malaysia | | | | | | | | | |
Novartis Corporation (Malaysia) Sdn. Bhd. | | Petaling Jaya | | MYR | 3.3 | m | | 100% | |
|
Mexico | | | | | | | | | |
Novartis Farmacéutica, S.A. de C.V. | | Mexico City | | MXN | 206.7 | m | | 100% | |
|
Morocco | | | | | | | | | |
Novartis Pharma Maroc SA | | Casablanca | | MAD | 80.0 | m | | 100% | |
|
Netherlands | | | | | | | | | |
Novartis Netherlands B.V. | | Amsterdam | | EUR | 1.4 | m | | 100% | |
Novartis Pharma B.V. | | Amsterdam | | EUR | 4.5 | m | | 100% | |
Aduro Netherlands Coöperatief U.A. | | Rosmalen 4 | | -- | -- | | | -- | |
Aduro Biotech Holdings Europe B.V. | | Rosmalen | | EUR | 46 216 | | | 100% | |
IDB Holland BV | | Baarle-Nassau | | EUR | 18 000 | | | 99.23% | |
|
New Zealand | | | | | | | | | |
Novartis New Zealand Ltd | | Auckland | | NZD | 820 000 | | | 100% | |
|
Norway | | | | | | | | | |
Novartis Norge AS | | Oslo | | NOK | 1.5 | m | | 100% | |
|
Pakistan | | | | | | | | | |
Novartis Pharma (Pakistan) Limited | | Karachi | | PKR | 6.7 | bn | | 99.99% | |
|
Panama | | | | | | | | | |
Novartis Pharma (Logistics), Inc. | | Panama City | | USD | 10 000 | | | 100% | |
|
Philippines | | | | | | | | | |
Novartis Healthcare Philippines, Inc. | | Makati City | | PHP | 298.8 | m | | 100% | |
|
Poland | | | | | | | | | |
Novartis Poland Sp. z o.o. | | Warsaw | | PLN | 44.2 | m | | 100% | |
|
Portugal | | | | | | | | | |
Novartis Portugal, S.G.P.S., Lda. | | Porto Salvo | | EUR | 500 000 | | | 100% | |
Novartis Farma - Produtos Farmacêuticos, S.A. | | Porto Salvo | | EUR | 2.4 | m | | 100% | |
|
Romania | | | | | | | | | |
Novartis Pharma Services Romania S.R.L. | | Bucharest | | RON | 3.0 | m | | 100% | |
Sandoz S.R.L. | | Targu-Mures | | RON | 119.5 | m | | 100% | |
|
Russian Federation | | | | | | | | | |
Novartis Pharma LLC | | Moscow | | RUB | 20.0 | m | | 100% | |
Novartis Neva LLC | | St. Petersburg | | RUB | 500 | m | | 100% | |
|
Saudi Arabia | | | | | | | | | |
Novartis Saudi Company | | Riyadh | | SAR | 30.0 | m | | 100% | |
|
As at December 31, 2023
| | | | | Share
capital1 | | | Equity
interest | |
|
Singapore | | | | | | | | | |
Novartis (Singapore) Pte Ltd. | | Singapore | | SGD | 100 000 | | | 100% | |
Novartis Singapore Pharmaceutical
Manufacturing Pte Ltd | | Singapore | | SGD | 45.0 | m | | 100% | |
Novartis Asia Pacific Pharmaceuticals
Pte Ltd | | Singapore | | SGD | 39.0 | m | | 100% | |
|
Slovakia | | | | | | | | | |
Novartis Slovakia s.r.o. | | Bratislava | | EUR | 2.0 | m | | 100% | |
|
Slovenia | | | | | | | | | |
Novartis farmacevtska proizvodnja d.o.o. | | Ljubljana | | EUR | 7 500 | | | 100% | |
|
South Africa | | | | | | | | | |
Novartis South Africa (Pty) Ltd | | Midrand | | ZAR | 86.3 | m | | 100% | |
|
South Korea | | | | | | | | | |
Novartis Korea Ltd. | | Seoul | | KRW | 24.5 | bn | | 100% | |
|
Spain | | | | | | | | | |
Novartis Farmacéutica, S.A. | | Barcelona | | EUR | 63.0 | m | | 100% | |
Advanced Accelerator Applications
Iberica, S. L. U. | | Esplugues de Llobregat | | EUR | 22.6 | m | | 99.23% | |
Abadia Retuerta S.A. | | Sardón de Duero / Valladolid | | EUR | 6.0 | m | | 100% | |
|
Sweden | | | | | | | | | |
Novartis Sverige AB | | Stockholm | | SEK | 5.0 | m | | 100% | |
|
Switzerland | | | | | | | | | |
Novartis International AG | | Basel | | CHF | 10.0 | m | | 100% | |
Novartis Holding AG | | Basel 2 | | CHF | 100.2 | m | | 100% | |
Novartis International Pharmaceutical Investment AG | | Basel | | CHF | 100 000 | | | 100% | |
Novartis Bioventures AG | | Basel | | CHF | 100 000 | | | 100% | |
Novartis Forschungsstiftung | | Basel 3 | | -- | -- | | | -- | |
Novartis Stiftung für Kaderausbildung | | Basel 3 | | -- | -- | | | -- | |
Novartis-Mitarbeiterbeteiligungsstiftung | | Basel 3 | | -- | -- | | | -- | |
Novartis Stiftung für Mensch und Umwelt | | Basel 3 | | -- | -- | | | -- | |
Stiftung der Novartis AG für Erziehung,
Ausbildung und Bildung | | Basel 3 | | -- | -- | | | -- | |
Novartis Overseas Investments AG | | Basel | | CHF | 1.0 | m | | 100% | |
Japat AG | | Basel | | CHF | 100 000 | | | 100% | |
Novartis Pharma AG | | Basel 2 | | CHF | 350.0 | m | | 100% | |
Novartis Pharma Services AG | | Basel | | CHF | 20.0 | m | | 100% | |
Novartis Pharma Schweizerhalle AG | | Muttenz | | CHF | 18.9 | m | | 100% | |
Novartis Pharma Stein AG | | Stein | | CHF | 251 000 | | | 100% | |
Novartis Pharma Schweiz AG | | Risch | | CHF | 5.0 | m | | 100% | |
Cellerys AG | | Schlieren | | CHF | 129 630 | | | 20% | |
Novartis Innovative Therapies AG | | Risch | | CHF | 100 000 | | | 100% | |
Advanced Accelerator Applications International SA | | Geneva | | CHF | 9.3 | m | | 99.23% | |
|
Taiwan | | | | | | | | | |
Novartis (Taiwan) Co., Ltd. | | Taipei | | TWD | 170.0 | m | | 100% | |
|
Thailand | | | | | | | | | |
Novartis (Thailand) Limited | | Bangkok | | THB | 302.0 | m | | 100% | |
|
Turkey | | | | | | | | | |
Novartis Saglik, Gida ve Tarim Ürünleri Sanayi
ve Ticaret A.S. | | Istanbul | | TRY | 448.0 | m | | 100% | |
|
As at December 31, 2023
| | | | | Share
capital1 | | | Equity
interest | |
|
United Arab Emirates | | | | | | | | | |
Novartis Middle East FZE | | Dubai | | AED | 7.0 | m | | 100% | |
|
United Kingdom | | | | | | | | | |
Novartis UK Limited | | London | | GBP | 25.5 | m | | 100% | |
Novartis Pharmaceuticals UK Limited | | London | | GBP | 5.4 | m | | 100% | |
Novartis Grimsby Limited | | London | | GBP | 250.0 | m | | 100% | |
Advanced Accelerator Applications (UK & Ireland) | | London | | GBP | 100 | | | 99.23% | |
Neutec Pharma Limited | | London | | GBP | 7.7 | m | | 100% | |
Gyroscope Therapeutics Limited | | London | | GBP | 1 492 | | | 100% | |
|
United States of America | | | | | | | | | |
Novartis Corporation | | East Hanover, NJ | | USD | 72.2 | m | | 100% | |
Novartis Finance Corporation | | East Hanover, NJ 2 | | USD | 1 000 | | | 100% | |
Novartis Capital Corporation | | East Hanover, NJ | | USD | 1 | | | 100% | |
Novartis Services, Inc. | | East Hanover, NJ | | USD | 1 | | | 100% | |
Novartis US Foundation | | East Hanover, NJ 3 | | -- | -- | | | -- | |
Novartis Pharmaceuticals Corporation | | East Hanover, NJ 2 | | USD | 650 | | | 100% | |
Advanced Accelerator Applications USA, Inc. | | Millburn, NJ | | USD | 1 | | | 99.23% | |
Novartis Gene Therapies, Inc. | | Bannockburn, IL | | USD | 1 | | | 100% | |
Novartis Technology LLC | | East Hanover, NJ 4 | | -- | -- | | | -- | |
Novartis Institutes for BioMedical
Research, Inc. | | Cambridge, MA | | USD | 1 | | | 100% | |
Novartis Manufacturing LLC | | East Hanover, NJ 4 | | -- | -- | | | -- | |
Cadent Therapeutics, Inc. | | Cambridge, MA | | USD | 0.1 | | | 100% | |
Endocyte, Inc. | | East Hanover, NJ | | USD | 1 | | | 100% | |
Navigate BioPharma Services, Inc. | | Carlsbad, CA | | USD | 1 | | | 100% | |
The Medicines Company | | East Hanover, NJ | | USD | 1 000 | | | 100% | |
DTX Pharma, Inc. | | San Diego, CA | | USD | 1 | | | 100% | |
Chinook Therapeutics, Inc. | | Seattle, WA | | USD | 1 | | | 100% | |
Chinook Therapeutics U.S., Inc. | | Seattle, WA | | USD | 1 | | | 100% | |
|
Venezuela | | | | | | | | | |
Novartis de Venezuela, S.A. | | Caracas | | VES | 0 | | | 100% | |
|
Vietnam | | | | | | | | | |
Novartis Vietnam Company Limited | | Ho Chi Minh City | | VND | 70 | bn | | 100% | |
|
|
In addition, the Company is represented by subsidiaries and associated companies with total assets or net sales to third parties below USD 25 million in the following countries: Bosnia and Herzegovina, Bulgaria, Cameroon, Croatia, Ghana, Guatemala, Ivory Coast, Kenya, Kuwait, Nigeria, Peru, Senegal, Ukraine and Uruguay. |
1 Share capital may not reflect the taxable share capital and does not include any paid-in surplus. |
2 Significant subsidiary under SEC Regulation S-X Rule 1-02(w) |
3 Fully consolidated Foundation |
4 Fully consolidated entity |
m = million; bn = billion |
Report of Independent Registered Public Accounting Firm
To the shareholders and the Board of Directors of Novartis AG
Opinions on the Consolidated Financial Statements and Internal Control Over Financial Reporting
We have audited the accompanying consolidated balance sheets of Novartis AG and its consolidated subsidiaries (the Company) as of December 31, 2023 and 2022, the related consolidated income statements, consolidated statements of comprehensive income, consolidated statements of changes in equity, and consolidated statements of cash flows for each of the years in the two-year period ended December 31, 2023 and the related notes (collectively, the consolidated financial statements). We also have audited the Company’s internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2023, in conformity with International Financial Reporting Standards (IFRS) Accounting Standards as issued by the International Accounting Standards Board. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2023 based on criteria established in Internal Control – Integrated Framework (2013) issued by the COSO.
Basis for Opinions
The Company’s management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Report of Novartis Management on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s consolidated financial statements and an opinion on the Company’s internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with IFRS Accounting Standards. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Assessment of the recoverable amount for the Leqvio intangible asset
As discussed in Note 1 to the consolidated financial statements, the Company determined the recoverable amount of the intangible assets other than goodwill based on the fair value less costs of disposal method for which no directly observable market inputs were available. As discussed in Note 12, the Company has intangible assets other than goodwill totaling USD 26 879 million as of December 31, 2023, of which USD 6.8 billion related to the currently marketed product Leqvio.
We identified the assessment of the recoverable amount, specifically the sales forecasts, of the Leqvio intangible asset, as a critical audit matter. Significant auditor judgment and specialist skills and knowledge were required to assess the sales forecasts assumptions due to the high degree of subjectivity and estimation uncertainty involved. These sales forecasts assumptions were a significant input in the determination of the recoverable amount of the Leqvio intangible asset.
The following are the primary procedures we performed to address this critical audit matter:
• We evaluated the design and tested the operating effectiveness of a certain internal control related to the Company’s intangible asset impairment process for Leqvio, including the development of the sales forecasts;
• We evaluated the reasonableness of management’s sales forecasts for Leqvio by (1) comparing the sales forecasts assumptions to company-specific operational information and management’s communications to the Board of Directors, (2) comparing the most recent sales performance to previous drug launches, and (3) comparing the sales forecasts assumptions to available external market and industry data;
• We involved professionals with specialized skills and knowledge, who assisted in evaluating the reasonableness and appropriateness of certain inputs to the sales forecasts (in particular, the epidemiological inputs); and
• We assessed management’s ability to accurately forecast sales by comparing historical sales forecasts for Leqvio to actual results.
Provisions for deductions from revenue related to US Managed Care, Medicare Part D and Medicaid rebate programs
As discussed in Note 1 to the consolidated financial statements, the Company records provisions for estimated rebates as a deduction from revenue when the related revenue is recognized. Rebates involve the use of assumptions and judgements in the determination of the provision rates at the time revenues are recorded. Provision rates are influenced by the terms and conditions in the individual agreements, historical experience, product sales and growth rate, population growth, product pricing, the mix of contracts and products, the level of inventory in the distribution channel, regulations, contracts, and channels and payers. As discussed in Note 23, provisions for deductions from revenue totaled USD 6 315 million as of December 31, 2023, a significant portion of which related to US Managed Care, Medicare Part D and Medicaid rebate programs (hereafter “US rebates”).
We identified the evaluation of the US rebates provisions a critical audit matter. The evaluation of the rebate provision rates required a high degree of subjective auditor judgment as it involved estimating the portion of the Company’s consolidated revenue which will ultimately be subject to a related rebate.
The following are the primary audit procedures we performed to address this critical audit matter:
• We evaluated the design and tested the operating effectiveness of certain internal controls over the Company’s US rebates process related to the development of the rebate provision rates;
• We developed our own independent expectation of the US rebates provisions, by using internal and external information, including historical experience and trend analysis of actual rebate claims paid, and comparing it to management’s actual recorded balances; and
• We assessed management’s ability to accurately estimate the US rebates provisions by comparing historically recorded provisions to the actual amount that was ultimately paid by the Company.
Valuation of the dividend-in-kind distribution liability to effect the spin-off of Sandoz Group AG
As discussed in Notes 1 and 2 to the consolidated financial statements, the dividend-in-kind to effect the spin-off of Sandoz Group AG (the Sandoz business) required the Company to recognize a distribution liability representing the fair value of the Sandoz business distributed of USD 13 962 million. The Company measured the distribution liability at the fair value of the Sandoz business net assets as a whole. Fair value was measured using the opening share price of Sandoz Group AG on the first day of trading its shares on the SIX Swiss Exchange and an estimated control premium.
We identified the valuation of the dividend-in-kind distribution liability, specifically the determination of a reasonable control premium, as a critical audit matter. Significant auditor judgment and specialist skills and knowledge were required to assess the control premium, which was sensitive to variation, such that minor changes in the assumption can cause significant changes in the valuation of the dividend-in-kind distribution liability and therefore on the resulting gain on distribution.
The following are the primary procedures we performed to address this critical audit matter:
• We evaluated the design and tested the operating effectiveness of certain internal controls related to the Company’s valuation of the dividend-in-kind distribution liability, including controls relating to selecting the control premium estimate; and
• We involved valuation professionals with specialized skills and knowledge, who assisted in developing an independent control premium range by utilizing the premiums paid in historic transactions to acquire controlling interests in comparable companies.
/s/ KPMG AG
We have served as the Company’s auditor since 2022.
Basel, Switzerland
January 30, 2024
Report of Independent Registered Public Accounting Firm
To the shareholders and Board of Directors of Novartis AG
Opinion on the Financial Statements
We have audited the consolidated income statement, consolidated statement of comprehensive income, consolidated statement of cash flows, and consolidated statement of changes in equity of Novartis AG and its subsidiaries (the “Company”) for the year ended December 31, 2021, including the related notes appearing under Item 18 (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the results of operations and cash flows of the Company for the year ended December 31, 2021 in conformity with International Financial Reporting Standards (“IFRS”) Accounting Standards as issued by the International Accounting Standards Board.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit of these consolidated financial statements in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud.
Our audit included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ PricewaterhouseCoopers AG
Basel, Switzerland
February 1, 2022, except for the effects of discontinued operations discussed in Note 1 to the consolidated financial statements, as to which the date is January 30, 2024
We served as the Company’s auditor from at least 1940 to 2022. We have not been able to determine the specific year we began serving as auditor of the Company’s predecessors.




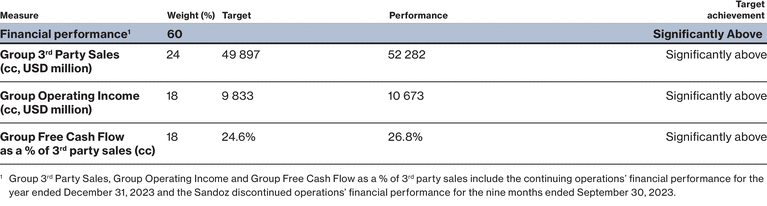










 On or ahead of target
On or ahead of target 



























 Propose
Propose  Endorse
Endorse  Approve
Approve





 Medicine/healthcare/R&D
Medicine/healthcare/R&D  Environmental, social and governance (ESG)
Environmental, social and governance (ESG)  Leadership/management
Leadership/management  Law/regulatory/risk management
Law/regulatory/risk management 
 Medicine/healthcare/R&D
Medicine/healthcare/R&D  Environmental, social and governance (ESG)
Environmental, social and governance (ESG)  Leadership/management
Leadership/management  Law/regulatory/risk management
Law/regulatory/risk management 
 Medicine/healthcare/R&D
Medicine/healthcare/R&D  Leadership/management
Leadership/management 
 Environmental, social and governance (ESG)
Environmental, social and governance (ESG)  Leadership/management
Leadership/management  Finance/accounting
Finance/accounting  Law/regulatory/risk management
Law/regulatory/risk management 
 Environmental, social and governance (ESG)
Environmental, social and governance (ESG)  Data/digital
Data/digital  Leadership/management
Leadership/management  Finance/accounting
Finance/accounting 
 Leadership/management
Leadership/management  Finance/accounting
Finance/accounting  Law/regulatory/risk management
Law/regulatory/risk management
 Medicine/healthcare/R&D
Medicine/healthcare/R&D  Environmental, social and governance (ESG)
Environmental, social and governance (ESG)  Data/digital
Data/digital  Leadership/management
Leadership/management  Finance/accounting
Finance/accounting  Law/regulatory/risk management
Law/regulatory/risk management
 Law/regulatory/risk management
Law/regulatory/risk management
 Medicine/healthcare/R&D
Medicine/healthcare/R&D  Environmental, social and governance (ESG)
Environmental, social and governance (ESG)  Data/digital
Data/digital  Leadership/management
Leadership/management  Finance/accounting
Finance/accounting  Law/regulatory/risk management
Law/regulatory/risk management 
 Environmental, social and governance (ESG)
Environmental, social and governance (ESG)  Data/digital
Data/digital  Leadership/management
Leadership/management  Finance/accounting
Finance/accounting  Law/regulatory/risk management
Law/regulatory/risk management 
 Medicine/healthcare/R&D
Medicine/healthcare/R&D 
 Environmental, social and governance (ESG)
Environmental, social and governance (ESG)  Data/digital
Data/digital  Leadership/management
Leadership/management  Finance/accounting
Finance/accounting  Law/regulatory/risk management
Law/regulatory/risk management 
 Medicine/healthcare/R&D
Medicine/healthcare/R&D  Leadership/management
Leadership/management  Finance/accounting
Finance/accounting  Law/regulatory/risk management
Law/regulatory/risk management