Exhibit
99.2

Management’s
Discussion and Analysis of Financial Condition and Results of Operations
Introduction
This
Management’s Discussion and Analysis (“MD&A”) provides a review of the results of operations, financial condition
and cash flows of Aeterna Zentaris Inc. for the three-months ended March 31, 2022. In this MD&A, “Aeterna Zentaris”,
“Aeterna” the “Company”, “we”, “us” and “our” mean Aeterna Zentaris Inc.
and its subsidiaries. This discussion should be read in conjunction with the information contained in the Company’s unaudited condensed
consolidated financial statements and the accompanying notes thereto as at March 31, 2022 and for the three-months ended March 31, 2022
and 2021 and our audited consolidated financial statements and MD&A for the years ended December 31, 2021 and 2020. The unaudited
condensed interim consolidated financial statements as at March 31, 2022 and for the three-months ended March 31, 2022 and 2021 were
prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board (“IFRS”)
applicable to the preparation of interim financial statements, including IAS 34, Interim Financial Reporting. The Company’s common
shares are listed on both The Nasdaq Capital Market (“Nasdaq”) and on the Toronto Stock Exchange (the “TSX” under
the symbol “AEZS”.
All
amounts in this MD&A are presented in United States (“U.S.”) dollars, except as otherwise noted.
This
MD&A was approved by the Company’s Board of Directors on May 10, 2022. This MD&A is dated May 10, 2022.
Company
Overview
Aeterna
Zentaris is a specialty biopharmaceutical company commercializing and developing therapeutics and diagnostic tests. The Company’s
lead product, Macrilen™ (macimorelin), is the first and only U.S. Food and Drug Administration (“FDA”) and European
Medicines Agency (“EMA”) approved oral test indicated for the diagnosis of patients with adult growth hormone deficiency
(“AGHD”). Macimorelin is currently marketed in the U.S. under the tradename Macrilen™ through the license agreement
and the amended license agreement (collectively the “Novo Amendment”) with Novo Nordisk Healthcare AG (“Novo Nordisk”
or “Novo”), who was granted an exclusive license for the development, manufacturing, registration and commercialization of
Macrilen™ (macimorelin) for the diagnosis of adult and pediatric growth hormone deficiency in the U.S. and Canada.
According
to a commercialization and supply agreement, MegaPharm Ltd. is seeking regulatory approval and plans to subsequently commercialize macimorelin
in Israel and the Palestinian Authority. Additionally, upon receipt of pricing and reimbursement approvals, Aeterna Zentaris expects
that macimorelin will be marketed in Europe and the United Kingdom through license and supply agreements with Consilient Health Ltd.
(“Consilient Health” or “CH”) under which Aeterna Zentaris is entitled to receive: regulatory milestone payments
related to agreed-upon pricing and reimbursement parameters; net sales milestone payments; and royalties, ranging from 10%-20% of net
sales, subject to reduction in certain cases, or sublicense income recorded by Consilient Health. The Company is also leveraging the
clinical success and compelling safety profile of macimorelin to develop it for the diagnosis of childhood-onset growth hormone deficiency
(“CGHD”), an area of significant unmet need. The Company is actively pursuing business development opportunities for the
commercialization of macimorelin in Asia and the rest of the world. We entered into license and supply agreements with NK Meditech Ltd.
(“NK”), a subsidiary of PharmBio Korea, effective November 30, 2021, and a distribution and commercialization agreement with
ER Kim Pharmaceuticals Bulgaria Food (“ER-Kim”), effective February 1, 2022. The agreements with NK are related to the development
and commercialization of macimorelin for the diagnosis of AGHD and CGHD in the Republic of Korea, while the agreement with ER-Kim is
related to the commercialization of macimorelin for the diagnosis of growth hormone deficiency in children and adults in Turkey and some
non-European Union Balkan countries.

The
Company is also dedicated to the development of therapeutic assets and has recently taken steps to establish a pre-clinical pipeline
to potentially address unmet medical needs across a number of indications with a focus on rare or orphan indications.. To date, we have
signed agreements to establish this growing pipeline across a number of indications, including neuromyelitis optica spectrum disorder
(“NMOSD”), Parkinson’s disease, primary hypoparathyroidism and amyotrophic lateral sclerosis (“ALS”, also
known as Lou Gehrig’s disease). Additionally, the Company is developing oral prophylactic bacterial vaccines against each of SARS-CoV-2,
the virus that causes COVID-19, and chlamydia.
About
Forward-Looking Statements
This
document contains statements that may constitute forward-looking statements within the meaning of U.S. and Canadian securities legislation
and regulations, and such statements are made pursuant to the safe-harbor provision of the U.S. Private Securities Litigation Reform
Act of 1995. In some cases, these forward-looking statements can be identified by words or phrases such as “forecast”, “may”,
“will”, “expect”, anticipate”, “estimate”, “intend”, “plan”, “indicate”,
“believe”, “direct”, or “likely”, or the negative of these terms, or other similar expressions intended
to identify forward-looking statements. In addition, any statements that refer to expectations, intentions, projections and other characterizations
of future events or circumstances contain forward-looking information.
Forward-looking
statements are based on the opinions and estimates of the Company as of the date of this MD&A, and they are subject to known and
unknown risks, uncertainties, assumptions and other factors that may cause the actual results, level of activity, performance or achievements
to be materially different from those expressed or implied by such forward-looking statements, including but not limited to the factors
described under “Risk Factors” in our Annual Report on Form 20-F and those relating to: Aeterna’s expectations with
respect to the DETECT-trial (as further defined below) (including regarding the enrollment of subjects in the DETECT-trial, the application
of the Macimorelin growth hormone stimulation tests and the completion of the DETECT-trial); Aeterna’s expectations regarding conducting
pre-clinical research to identify and characterize an AIM Biologicals-based development candidate for the treatment of NMOSD as well
as Parkinson’s disease (as further defined below), and developing a manufacturing process for selected candidates; Aeterna’s
expectations regarding conducting assessments in relevant Parkinson’s disease models; The University of Queensland’s undertaking
a subsequent investigator initiated clinical trial evaluating macimorelin as a potential therapeutic for the treatment of ALS and Aeterna’s
formulating a pre-clinical development plan for same; the commencement of Aeterna’s formal pre-clinical development of AEZS-150
in preparation for a potential investigational new drug (“IND”) filing for conducting the first in-human clinical study of
AEZS-150; Aeterna’s plans to perform challenge experiments, select a development candidate, start clinical development and establish
a manufacturing process for the orally active COVID-19 (SARS-CoV-2) and Chlamydia live-attenuated bacterial vaccine.

Forward-looking
statements involve known and unknown risks and uncertainties and other factors which may cause the actual results, performance or achievements
stated herein to be materially different from any future results, performance or achievements expressed or implied by the forward-looking
information. Such risks and uncertainties include, among others: our reliance on the success of the pediatric clinical trial in the European
Union and U.S. for Macrilen™ (macimorelin); the commencement of the DETECT-trial may be delayed or we may not obtain regulatory
approval to initiate that study; we may be unable to enroll the expected number of subjects in the DETECT-trial and the result of the
DETECT-trial may not support receipt of regulatory approval in CGHD; the coronavirus vaccine platform technology (and any vaccine candidates
using that technology) licensed from the University of Wuerzburg has never been tested in humans, and as such, further pre-clinical or
clinical studies of that technology and any vaccine developed using that technology may not be effective as a vaccine against COVID-19
(SARS-CoV-2) or against any other coronavirus disease; the timeline to develop a vaccine may be longer than expected; such technology
or vaccines may not be capable of being used orally or may not have the same characteristics as vaccines previously approved using the
Salmonella Typhi Ty21a carrier strain; results from ongoing or planned pre-clinical studies of macimorelin by the University of Queensland
or for our other products under development may not be successful or may not support advancing the product to human clinical trials;
our ability to raise capital and obtain financing to continue our currently planned operations; our dependence on the success of Macrilen™
(macimorelin) and related out-licensing arrangements and the continued availability of funds and resources to successfully commercialize
the product, including our reliance on the success of the Novo Amendment; the global instability due to the global pandemic of COVID-19
and COVID-19’s unknown potential effect on our operations; our ability to enter into out-licensing, development, manufacturing,
marketing and distribution agreements with other pharmaceutical companies and to keep such agreements in effect; and our ability to continue
to list our common shares on the Nasdaq or the TSX. These risk factors are not intended to represent a complete list of the risk factors
that could affect the Company. These factors and assumptions, however, should be considered carefully. More detailed information about
these and other factors is included under “Risk Factors” in our Annual Report on Form 20-F. Although the Company has attempted
to identify important factors that could cause actual results to differ materially from those contained in forward-looking statements,
there may be other factors that cause results not to be as anticipated, estimated or intended. Many of these factors are beyond our control.
There can be no assurance that such statements will prove to be accurate, as actual results and future events could differ materially
from those anticipated in such statements, particularly in light of the ongoing and developing COVID-19 pandemic and its impact on the
global economy and its uncertain impact on the Company’s business. Accordingly, readers should not place undue reliance on forward-looking
statements. The Company does not undertake to update any forward-looking statements contained herein, except as required by applicable
securities laws. New factors emerge from time to time, and it is not possible for the Company to predict all of these factors, or to
assess in advance the impact of each such factor on the Company’s business or the extent to which any factor, or combination of
factors, may cause actual results to differ materially from those contained in any forward-looking statement.
Certain
forward-looking statements contained herein about prospective results of operations, financial position or cash flows may constitute
a financial outlook. Such statements are based on assumptions about future events, are given as of the date hereof and are based on economic
conditions, proposed courses of action and management’s assessment of the relevant information currently available. Management
of the Company has approved the financial outlook as of the date hereof. Readers are cautioned that such financial outlook information
contained herein should not be used for purposes other than for which it is disclosed herein.
About
Material Information
This
MD&A includes information that we believe to be material to investors after considering all circumstances. We consider information
and disclosures to be material if they result in, or would reasonably be expected to result in, a significant change in the market price
or value of our securities, or where it is likely that a reasonable investor would consider the information and disclosures to be important
in making an investment decision.
We
are a reporting issuer under the securities legislation of all of the provinces of Canada, and our securities are registered with the
SEC. We are therefore required to file or furnish continuous disclosure information, such as interim and annual financial statements,
management’s discussion and analysis, proxy or information circulars, annual reports on Form 20-F, material change reports and
press releases with the appropriate securities regulatory authorities. Additional information about the Company and copies of these documents
may be obtained free of charge upon request from our Corporate Secretary or on the Internet at the following addresses: www.zentaris.com,
www.sedar.com and www.sec.gov.

Diagnostic
Commercial and Development Pipeline

Macimorelin
Clinical Program
On
January 28, 2020, we announced the successful completion of patient recruitment for the first pediatric study of macimorelin as a growth
hormone stimulation test for the evaluation of GHD in children. This study, AEZS-130-P01 (“Study P01”), was the first of
two studies as agreed with the EMA in our Pediatric Investigation Plan (the “PIP”) for macimorelin as a GHD diagnostic. Macimorelin,
a ghrelin agonist, is an orally active small molecule that stimulates the secretion of growth hormone from the pituitary gland into the
circulatory system. The goal of Study P01 was to establish a dose that can both be safely administered to pediatric patients and cause
a clear rise in growth hormone concentration in subjects ultimately diagnosed as not having GHD. The recommended dose derived from Study
P01 is being evaluated in the pivotal second study, DETECT-trial, on diagnostic efficacy and safety.
In
late 2020, Aeterna entered into the start-up phase for the clinical safety and efficacy study, AEZS-130-P02 (“DETECT-trial”),
evaluating macimorelin for the diagnosis of CGHD. The DETECT-trial is an open-label, single dose, multicenter and multinational study
expected to enroll approximately 100 subjects worldwide, with at least 40 pre-pubertal and 40 pubertal subjects, and a minimum of 25
subjects expected to be enrolled in the U.S. The study design is expected to be suitable to support a claim for potential stand-alone
testing, if successful. In addition, under the Novo Amendment, Novo and Aeterna agreed that Novo will fund DETECT-trial costs up to $11
million (€9 million) which includes reimbursement of Aeterna’s budgeted internal labor costs. Any additional external jointly
approved DETECT-trial costs incurred over $11 million (€9 million) will be shared equally between Novo and Aeterna. On April 22,
2021, the U.S. FDA Investigational New Drug Application associated with this clinical trial became active, see: https://clinicaltrials.gov/ct2/show/NCT04786873
and on May 13, 2021, we announced the opening of the first clinical site in the U.S. On January 26, 2022, the Company announced that
it had experienced unavoidable delays in site initiation and patient enrollment due to the rise of the Omicron variant in the COVID-19
pandemic. Our team is diligently working to get more clinical sites up and running with the goal of building momentum and bringing this
study across the finish line while navigating as best as possible through this challenge. However, we have engaged a Contract Research
Organization (CRO) to conduct the DETECT-trial in the United States and European countries, including Russia and Ukraine. The clinical
trial sites in Russia and Ukraine were halted in February 2022 due to the Russian invasion of Ukraine. To date, no patients have been
enrolled in these countries’ clinical sites. Russia’s invasion of Ukraine may also impact our ability to conduct our trials
in the region. This could delay or hinder the completion of our clinical trials and/or analyses of clinical results, which could materially
harm our business.

Macimorelin
Pre-clinical Program
On
January 13, 2021, we entered into a material transfer agreement with Queensland University to provide macimorelin for the conduct of
preclinical and clinical studies evaluating macimorelin as a therapeutic for the treatment of amyotrophic lateral sclerosis (“ALS”
and commonly known as Lou Gehrig’s disease). Queensland University researchers have filed funding applications to dedicated organizations
in Australia to finance parts of the abbreviated preclinical development program and to conduct a subsequent investigator-initiated clinical
trial to evaluate the safety, tolerability and efficacy of macimorelin as a potential new treatment option for ALS patients. The Company
expects to continue work with Queensland University but also to conduct independent proof-of-concept studies with macimorelin in disease
specific ALS animal models, assess alternative formulations and formalize a preclinical development plan.
The
Company is developing additional alternative formulations for administration routes more suitable for ALS with the goal of ensuring sufficient
bioavailability and expects to provide updates on its progress as results become available.
Macimorelin
Commercialization Program
On
June 25, 2020, we announced that we entered into an exclusive distribution and related quality agreement with MegaPharm Ltd., a leading
Israel-based biopharmaceutical company, for the commercialization in Israel and in the Palestinian Authority of macimorelin, to be used
in the diagnosis of patients with AGHD and in clinical development for the diagnosis of CGHD. Under the terms of the agreement, MegaPharm
Ltd. will be responsible for obtaining registration to market macimorelin in Israel and the Palestinian Authority, while the Company
will be responsible for manufacturing, product supply, quality assurance and control, regulatory support, and maintenance of the relevant
intellectual property. In June 2021, MegaPharm Ltd. filed an application to the Ministry of Health of Israel for regulatory approval
of macimorelin in Israel
On
November 16, 2020, the Company announced that it had entered into the Novo Amendment related to the development and commercialization
of macimorelin. Novo is currently marketing macimorelin in the U.S. under the tradename Macrilen™ for the diagnosis of AGHD. Aeterna,
in collaboration with Novo, is currently developing the expanded use of macimorelin for the diagnosis of CGHD, an area of significant
unmet medical need.
On
December 7, 2020, the Company entered into an exclusive licensing agreement with Consilient Health Limited (“CH” or “Consilient”)
for the commercialization of macimorelin (the “Licensed Product”) in the European Economic Area and the United Kingdom (the
“CH License Agreement”). In December 2021, the Department of Health and Social Care in the United Kingdom approved a list
price which triggered a $226 (€0.2 million) pricing milestone payment from CH to the Company. We have shipped an initial batch of
macimorelin (Ghryvelin®) to Consilient in Q1-2022 as they prepare to launch in the United Kingdom later in 2022.
We
entered into license and supply agreements with NK Meditech Ltd. (“NK”), a subsidiary of PharmBio Korea, effective November
30, 2021, and a distribution and commercialization agreement with ER Kim Pharmaceuticals Bulgaria Food (“ER-Kim”), effective
February 1, 2022. The agreements with NK are related to the development and commercialization of macimorelin for the diagnosis of AGHD
and CGHD in the Republic of Korea, while the agreement with ER-Kim is related to the commercialization of macimorelin for the diagnosis
of growth hormone deficiency in children and adults in Turkey and some non-European Union Balkan countries.
On
April 19, 2022, we announced that European Patent Office (EPO) had issued a patent providing intellectual property protection of macimorelin
in 27 countries within the European Union as well as additional European non-EU countries, such as the UK and Turkey, for macimorelin
(Ghryvelin®; Macrilen™) for use to diagnose GHD in adults.

Pipeline
Expansion Opportunities
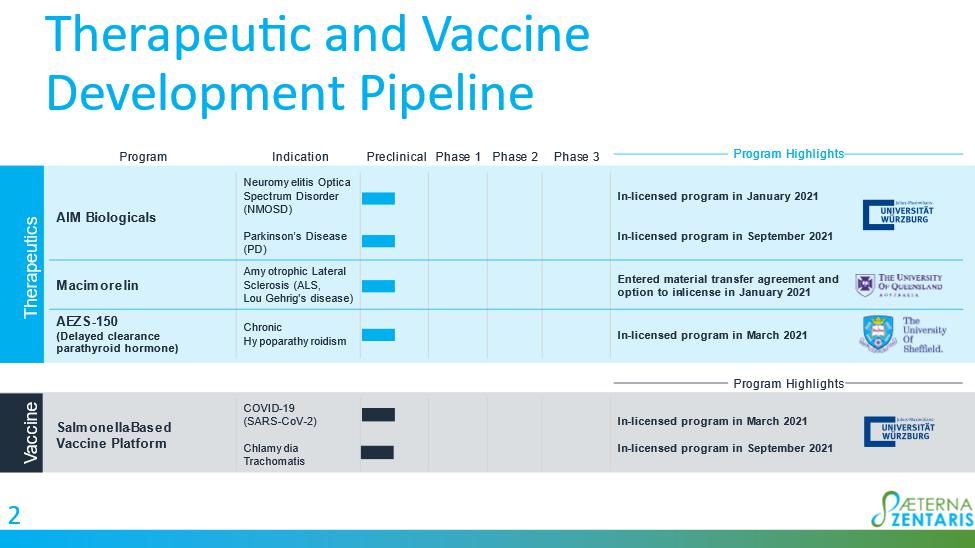
Bacterial
Vaccine Platform: Orally active, live-attenuated bacterial vaccine platform with potential application against viruses and bacteria,
such as coronaviruses and chlamydia bacteria
On
February 2, 2021, the Company announced that it had entered into an exclusive option agreement to evaluate a preclinical potential COVID-19
vaccine developed at the University of Wuerzburg. On March 14, 2021, the Company exercised the option to enter into a license agreement
with the University. Pursuant to the terms of the University License Agreement, the Company has been granted an exclusive, world-wide,
license to certain patent applications and know-how owned by the University to research and develop, manufacture, and sell a potential
COVID-19 vaccine using the University’s bacterial vaccine platform technology. The Company has paid an up-front payment under the
University License Agreement and will conduct milestones payments upon achievement of certain development, regulatory, and sales milestones,
as well as a percentage of any sub-licensing revenue received by the Company as well as royalty payments on net sales of the licensed
vaccine products (including for by the Company or its sub-licensees). Pursuant to the University License Agreement, the University granted
the Company an exclusive option for the exclusive use of the Licensed Rights in an undisclosed field. In September 2021, the Company
exercised this option and disclosed the field to be Chlamydia trachomatis. Additionally, the Company has entered into the Research
Agreement under which the Company has engaged the University on a fee-for-service basis to conduct supplementary research activities
and preclinical development studies on the potential vaccines.
The
vaccine technology developed at the University is based on the active live-attenuated bacterial typhoid fever vaccine Salmonella Typhi
Ty21a with an excellent safety profile, as a carrier strain. Our vaccines have the potential to be administered orally (topically),
induce mucosal immunity, believed to be critical to suppress infection, induce a response to more than one antigen (similar to the spike
protein), and be stored and distributed at 2 to 8°C. We believe that, if there is sufficient data to advance into human clinical
trials, the development program for these vaccines is expected to be abbreviated, as clinical safety data and manufacturing technology
is already available for the underlying vaccine strain.
The
Coronavirus outbreak began in the end of 2019 and in early 2022 was reaching its fourth infection peak worldwide with vaccinated people
getting infected and with booster vaccinations being needed. The competition is large with 11 vaccines currently being in clinical studies
only in Europe. Our COVID-19 vaccine candidate is unique in stimulating the mucosal immune system giving the potential to eliminate the
virus when it enters the body, before an infection can occur, and drastically reducing the risk of vaccinated people getting infected
and spreading the virus. In addition, the oral application and its expected storage stability should greatly facilitate distribution
and administration. Our next development steps include evaluating the administration route, dose and immunization scheme; initiating
in-vivo immunology experiments with antigen variant candidates in relevant mice models; conducting virus challenge experiments
in immunized transgenic animals; starting the manufacturing process assessment / development; and conducting pre-clinical safety and
toxicology assessments.

Chlamydia
trachomatis is a sexually transmitted bacterium infecting over 130 million subjects annually. In US, the prevalence 2.4 million per
year, the incidence is 4 million per year, and the associated yearly health cost $691 million. The disease can spread to the reproductive
tract eventually inducing infertility, miscarriage, or ectopic pregnancy, which is a life-threatening condition. Additionally, ocular
infections can lead to inclusion conjunctivitis or trachoma, which is the primary source of visual impairment or infectious blindness.
While diagnosed infections can be treated with antibiotics, three quarters of all infections are asymptomatic and currently no vaccine
exists to protect against chlamydia. The potential strengths of our chlamydia vaccine candidate are the mucosal immunity, oral administration,
good stability, and inexpensive production. Our next development steps include designing and preparing candidate vaccine strains; evaluating
administration route, dose and immunization scheme; and initiating in-vivo immunology experiments with candidate strains in relevant
mouse models.
On
March 10, 2022, the Company announced the expansion of its research program with the University of Wuerzburg to include the development
of human 3D intestinal tissue models to study infection biology in the gut, the site of Salmonella primary action.
Delayed
Clearance Parathyroid Hormone (“DC-PTH”) Fusion Polypeptides: Potential treatment for chronic hypoparathyroidism
On
March 11, 2021, the Company entered into an exclusive license agreement with The University of Sheffield, United Kingdom, for the intellectual
property relating to parathyroid hormone (“PTH”) fusion polypeptides covering the field of human use, which is being initially
studied by Aeterna for the potential therapeutic treatment of chronic hypoparathyroidism (“HypoPT”). Under the terms of the
exclusive patent and know-how license agreement entered into with the University of Sheffield, Aeterna obtained worldwide rights to develop,
manufacture and commercialize PTH fusion polypeptides covered by the licensed patent applications for all human uses for an up-front
cash payment, and milestone payments to be paid upon the achievement of certain development, regulatory and sales milestones, as well
as low single digit royalty payments on net sales of those products and certain fees payable in connection with sublicensing. Aeterna
will be responsible for the further development, manufacturing, approval, and commercialization of the licensed products. Aeterna has
also engaged the University of Sheffield under a research contract to conduct certain research activities to be funded by Aeterna, the
results of which will be included within the scope of the license granted to Aeterna.
The
researchers at the University of Sheffield have developed a method to increase the serum clearance time of peptides, which the Company
is applying to the development of a treatment for HypoPT, an orphan disease where the PTH level is abnormally low or absent. In consultation
with The University of Sheffield, Aeterna has selected AEZS-150 as the lead candidate in its DC-PTH program. AEZS-150 is being developed
to provide a weekly treatment option of chronic hypoparathyroidism in adults and our next steps include working with The University of
Sheffield to conduct in depth characterization of development candidate (in-vitro and in-vivo); selecting and engaging
a contract development and manufacturing company for developing the manufacturing process; and formalizing the pre-clinical development
of AEZS-150 in preparation for a potential IND filing for conducting the first in-human clinical study.
AIM
Biologicals: Targeted, highly specific autoimmunity modifying therapeutics for the potential treatment of neuromyelitis optica spectrum
disorder and Parkinson’s disease
In
January 2021, Aeterna entered into an exclusive patent license and research agreement with the University of Wuerzburg, Germany, for
worldwide rights to develop, manufacture, and commercialize AIM Biologicals for the potential treatment of NMOSD. Additionally, the Company
has engaged Prof. Dr. Joerg Wischhusen from the University Hospital in Wuerzburg as well as neuro-immunologist Dr. Michael Levy from
the Massachusetts General Hospital in Boston as consultants for scientific support and advice in the field of inflammatory CNS disorders,
autoimmune diseases of the nervous system, and NMOSD. In September 2021, the Company entered into an additional exclusive license with
the University of Wuerzburg for early pre-clinical development towards the potential treatment of Parkinson’s disease.
AIM
Biologicals is based on a feto-maternal-tolerance, natural process during pregnancy, which induces immunogenic tolerance of the maternal
immune system to the partially foreign fetal antigens. Fetal proteins are processed and presented on certain immunosuppressive major
histocompatibility complex class I molecules to induce this tolerance. In an autoimmune disease, the immune system is misdirected and
targets the body’s own protein. With AIM Biologicals, we aim to restore the tolerance against such proteins to selectively treat
autoimmune diseases.

NMOSD
is an autoimmune disease targeting the protein aquaporin 4, primarily found in optic nerves and the spinal cord, and is a disease which
leads to blindness and paralysis, more commonly in persons with Asian or African ancestors as compared to European ancestors, and more
prevalent among women. NMOSD progresses in often life-threatening relapses, which are aggressively treated with high-dose steroids and
plasmapheresis. Our pre-clinical plans include conducting in-vitro and in-vivo assessments to select an AIM Biologicals-based
development candidate; and manufacturing process development for the selected candidate.
Parkinson’s
disease is a neurological disease commonly associated with motoric problems with a slow and fast progression form. Dopaminergic medication
is the mainstay treatment of PD symptoms, but currently there is no pharmacological therapy to prevent or delay disease progression leading
to alternate treatments, such as deep brain stimulation with short electric bursts, being investigated for the treatment of symptoms.
For the development of AIM Biologicals as potential PD therapeutics, Aeterna plans to utilize, among others, an innovative animal model
on neurodegeneration by α-synuclein-specific T cells in AAV-A53T-α-synuclein Parkinson’s disease mice, which has recently
been published by University of Wuerzburg researchers. Our next steps include designing and producing antigen-specific AIM Biologics
molecules for the potential treatment of Parkinson’s disease; and conducting in-vitro and in-vivo assessments in
relevant Parkinson’s disease models.
Macimorelin
Therapeutic: Ghrelin agonist in development for the treatment of amyotrophic lateral sclerosis (Lou Gehrig’s disease)
In
January 2021, the Company entered into a material transfer agreement with the University of Queensland, Australia, to provide macimorelin
for the conduct of pre-clinical and subsequent clinical studies, evaluating macimorelin as a potential therapeutic for the treatment
of ALS. ALS is a rare progressive neurological disease primarily affecting the neurons controlling voluntary movement, leading to the
disability to control movements such as walking, talking, and chewing. Most people with ALS die from respiratory failure, usually between
3-5 years after diagnosis. Currently there is no cure for ALS and no effective treatment to halt or reverse the progression of the disease.
Ghrelin is a hormone with wide-ranging biological actions, most known for stimulating growth hormone release, which is demonstrating
emerging evidence as therapeutic for ALS. As a ghrelin agonist, macimorelin has the potential as a treatment for ALS, which is evaluated
in this research collaboration. The University of Queensland researchers have filed for supportive grants to conduct such clinical studies.
AEZS and the University are currently negotiating a research and development agreement. Our next steps include working with the University
of Queensland to conduct proof-of-concept studies with macimorelin in disease-specific animal models, assessing alternative formulations
and formalizing a pre-clinical development plan.
Changes
in personnel and advisors
Effective
January 24, 2022, Mr. Giuliano La Fratta joined the Company as the Senior Vice President, Chief Financial Officer, replacing Ms. Leslie
Auld.
Nasdaq
Letters
On
July 28, 2021, we received a letter from the Listing Qualifications Staff of the Nasdaq, notifying us that during the 30 consecutive
business days prior to the date of the letter, the closing bid price of our common shares was below $1.00 per share and, therefore, we
did not meet the requirement for continued listing on Nasdaq as required by Nasdaq Listing Rule 5550(a)(2) (the “Bid Price Rule”).
In accordance with Nasdaq Listing Rule 5810(c)(3)(A), we were granted a grace period of 180 calendar days, through January 24, 2022.
On January 26, 2022, we announced that the Listing Qualifications Staff of the Nasdaq had notified the Company that it has been granted
an additional 180 calendar day period, through July 26, 2022, to comply with the US$1.00 minimum bid price requirement for continued
listing on the Nasdaq. If at any time before July 26, 2022, the bid price for the Company’s common shares closes at or above US$1.00
per share for a minimum of 10 consecutive business days (and generally not more than 20 consecutive business days, in Nasdaq’s
discretion), it is expected that Nasdaq would provide formal notice that the Company has regained compliance with the bid price requirement.
In the event the Company does not provide, during the 180-day grace period, evidence to demonstrate compliance with Bid Price Rule, it
is expected that Nasdaq would notify the Company that its shares are subject to delisting. At such time, the Company may appeal such
determination to a Nasdaq Hearings Panel (the “Panel”) and it is expected that the Company’s securities would continue
to be listed and available to trade on Nasdaq at least pending the completion of the appeal process. There can be no assurance that any
such appeal would be successful or that the Company would be able to comply with the terms of any extension that may be granted by the
Panel. There is no assurance that we will regain compliance with the Bid Price Rule in the future, and therefore there can be no assurance
that our common shares will remain listed on Nasdaq. The aforementioned notification from the Nasdaq does not impact the Company’s
listing status on the TSX.
Exposure
to Epidemic or Pandemic Outbreak
Coronavirus,
or COVID-19, a contagious disease that was characterized by the World Health Organization as a pandemic in early 2020, continues to affect
the global community. The significant spread of COVID-19 resulted in a widespread health crisis and has had adverse effects on national
economies generally, on the markets that we serve on our operations and on the market price of our common shares.

The
spread of COVID-19 may continue to impact our operations, including the potential interruption of our clinical trial activities and of
our supply chain. For example, the rise in the Omicron variant in the COVID-19 pandemic has caused delays in site initiation and patient
enrollment in our Phase 3 DETECT clinical trial for diagnostic use in childhood-onset growth hormone deficiency. Additionally, sales
activities for Macrilen™ in the US may be impacted due to delays of diagnostic activities on AGHD in the US. Further, the COVID-19
pandemic may also cause some patients to be unwilling to enroll in our trials or be unable to comply with clinical trial protocols if
quarantines impede patient movement or interrupt healthcare services, which would delay our ability to conduct clinical trials or release
clinical trial results on a timely basis and could delay our ability to obtain regulatory approval and commercialize our product candidates.
The spread of an infectious disease, including COVID-19, may also result in the inability of our suppliers to deliver components or raw
materials on a timely basis or at all. In addition, hospitals may reduce staffing and reduce or postpone certain treatments in response
to the spread of an infectious disease. Such events may result in a period of business disruption and, in reduced operations, doctors
or medical providers may be unwilling to participate in our clinical trials, any of which could materially affect our business, financial
condition or results of operations.
Given
this rapidly evolving situation, the duration, scope and impact on our business operations, clinical studies and financial results cannot
at this time be fully determined or quantified. Aeterna Zentaris has developed protocols and procedures should they be required to deal
with any potential epidemics and pandemics and has implemented these protocols and procedures to address the current COVID-19 pandemic.
Despite appropriate steps being taken to mitigate such risks, there can be no assurance that existing policies and procedures will ensure
that the Company’s operations will not be adversely affected. The COVID-19 pandemic has resulted in a widespread health crisis
that has adversely affected the economies and financial markets of many regions and countries. There can be no assurance that a disruption
in financial markets, regional economies and the world economy would not negatively affect Aeterna Zentaris’ access to capital
or its financial performance.
Uncertain
factors, including the duration of the outbreak, the severity of the disease and the actions to contain or treat its impact, could impair
our operations including, among other things, employee mobility and productivity, availability of our facilities, conduct of our clinical
trials and the availability and the productivity of third-party product and service suppliers. Please see the risk factor in our Annual
Report on 20-F entitled “The economic effects of a pandemic, epidemic or outbreak of an infectious disease could adversely affect
our operations or the market price of our Common Shares”.
Russia/Ukraine
Conflict
Conducting
clinical trials in foreign countries, as in our ongoing DETECT-trial, presents additional risks that may delay completion of our clinical
trials. These risks include the failure of enrolled patients in foreign countries to adhere to clinical protocol as a result of differences
in healthcare services or cultural customs, managing additional administrative burdens associated with foreign regulatory schemes, as
well as political and economic risks, including war, relevant to such foreign countries. For example, we have engaged a CRO to conduct
the DETECT-trial outside the United States, including in Russia and Ukraine and clinical trial sites in those countries are being halted
due to the conflict in Ukraine. To date, no patients have been enrolled in these clinical trials. Russia’s invasion of Ukraine
in February 2022 may impact our ability to conduct certain of our trials in the region. This could hinder the completion of our clinical
trials and/or analyses of clinical results, which could materially harm our business
Furthermore,
the United States and its European allies have imposed significant new sanctions against Russia, including regional embargoes, full blocking
sanctions, and other restrictions targeting major Russian financial institutions. Our ability to conduct clinical trials in Russia, parts
of Ukraine and elsewhere in the region may become restricted under applicable sanctions laws, which would require us to identify alternative
trial sites, which may increase our development costs and delay the clinical development of our product candidates. All of the foregoing
could impede the execution of our clinical development plans, which could materially harm our business.

Condensed
Interim Consolidated Statements of Comprehensive Income (Loss) Data
(in
thousands, except share and per share data)
| | |
Three months ended | |
| | |
March 31 | |
| | |
2022 | | |
2021 | |
| | |
| | |
(As restated) (1) | |
| | |
$ | | |
$ | |
| Revenues | |
| | | |
| | |
| License fees | |
| 432 | | |
| 524 | |
| Development services | |
| 966 | | |
| 1,095 | |
| Product sales | |
| 57 | | |
| — | |
| Royalties | |
| 19 | | |
| 8 | |
| Supply chain | |
| 43 | | |
| 41 | |
| Total revenues | |
| 1,517 | | |
| 1,668 | |
| Operating expenses | |
| | | |
| | |
| Cost of sales | |
| 79 | | |
| 29 | |
| Research and development expenses | |
| 2,390 | | |
| 1,458 | |
| General and administrative expenses | |
| 1,558 | | |
| 1,264 | |
| Selling expenses | |
| 303 | | |
| 246 | |
| Total operating expenses | |
| 4,330 | | |
| 2,997 | |
| Loss from operations | |
| (2,813 | ) | |
| (1,329 | ) |
| Gains (loss) due to changes in foreign currency exchange rates | |
| 174 | | |
| (248 | ) |
| Other finance costs | |
| (1 | ) | |
| (10 | ) |
| Net finance income (costs) | |
| 173 | | |
| (258 | ) |
| Loss before income taxes | |
| (2,640 | ) | |
| (1,587 | ) |
| Income tax recovery | |
| — | | |
| 129 | |
| Net loss | |
| (2,640 | ) | |
| (1,458 | ) |
| Other comprehensive income (loss): | |
| | | |
| | |
| Items that may be reclassified subsequently to profit or loss: | |
| | | |
| | |
| Foreign currency translation adjustments | |
| 37 | | |
| 547 | |
| Items that will not be reclassified to profit or loss: | |
| | | |
| | |
| Actuarial gain on defined benefit plans | |
| 2,749 | | |
| 882 | |
| Comprehensive income (loss) | |
| 146 | | |
| (29 | ) |
| Net loss per share [basic and diluted] | |
| (0.02 | ) | |
| (0.02 | ) |
| Weighted average number of shares outstanding: | |
| | | |
| | |
| Basic | |
| 121,397,007 | | |
| 95,444,990 | |
| Diluted | |
| 121,397,007 | | |
| 95,444,990 | |
(1)
This restatement is discussed below under “Restatement of Comparative Period Figures” in the Results of operations
section of this MD&A. The interim financial statements for the period ended March 31, 2021 have not been refiled, but the comparatives
are corrected in the interim financial statements for the period ended March 31, 2022 filed with this MD&A.

Condensed
Interim Consolidated Statements of Financial Position Data
(in thousands) | |
As at March 31, 2022 | | |
As at December 31, 2021 | |
| | |
| $ | | |
| $ | |
| Cash and cash equivalents | |
| 63,596 | | |
| 65,300 | |
| Trade and other receivables and other current assets | |
| 3,223 | | |
| 5,447 | |
| Inventory | |
| 278 | | |
| 73 | |
| Restricted cash equivalents | |
| 330 | | |
| 335 | |
| Property, plant and equipment | |
| 45 | | |
| 42 | |
| Right of use assets | |
| 153 | | |
| 150 | |
| Other non-current assets | |
| 8,719 | | |
| 8,755 | |
| Total assets | |
| 76,344 | | |
| 80,102 | |
| Payables and accrued liabilities and income taxes payable | |
| 2,907 | | |
| 2,787 | |
| Current portion of provisions | |
| 34 | | |
| 34 | |
| Current portion of deferred revenues | |
| 2,582 | | |
| 4,815 | |
| Lease liabilities | |
| 165 | | |
| 161 | |
| Non-financial non-current liabilities (1) | |
| 17,497 | | |
| 19,319 | |
| Total liabilities | |
| 23,185 | | |
| 27,116 | |
| Shareholders’ equity | |
| 53,159 | | |
| 52,986 | |
| Total liabilities and shareholders’ equity | |
| 76,344 | | |
| 80,102 | |
| (1) | Comprised
mainly of employee future benefits, provisions and non-current portion of deferred revenues. |
Critical
Accounting Policies, Estimates and Judgments
The
preparation of condensed interim consolidated financial statements in accordance with IFRS requires management to make judgments, estimates
and assumptions that affect the reported amounts of the Company’s assets, liabilities, revenues, expenses and related disclosures.
Judgments, estimates and assumptions are based on historical experience, expectations, current trends and other factors that management
believes to be relevant at the time at which the Company’s condensed interim consolidated financial statements are prepared.
Management
reviews, on a regular basis, the Company’s accounting policies, assumptions, estimates and judgments in order to ensure that the
consolidated financial statements are presented fairly and in accordance with IFRS applicable to interim financial statements. Revisions
to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Critical
accounting estimates and assumptions, as well as critical judgments used in applying accounting policies in the preparation of the Company’s
condensed interim consolidated financial statements, were the same as those applied to the Company’s annual consolidated financial
statements for the year ended December 31, 2021.
The
rise in COVID-19 variants has caused delays in site initiation and patient enrollment in our DETECT-trial and may be impacting sales
activities for Macrilen™ in the US. Further, the continuation of the COVID-19 pandemic and the Russia/Ukraine conflict may also
cause some patients to be unwilling to enroll in our trials or be unable to comply with clinical trial protocols if such events impede
patient movement or interrupt healthcare services, both of which would delay our ability to conduct clinical trials or release clinical
trial results on a timely basis and could delay our ability to obtain regulatory approval and commercialize our product candidates. For
the three-month period ended March 31, 2022, the Company assessed the impact of the uncertainties around the COVID-19 pandemic and the
Russia/Ukraine conflict on its judgments, estimates, accounting policies and amounts recognized in these unaudited condensed interim
consolidated financial statements. Management determined that the recruitment for the DETECT-trial may now continue until later into
2023 compared to the end of the 2022 year as anticipated at the end of the previous fiscal year. . As such, an amount of $1.2 million
of deferred revenue has been reclassified from current to long-term portion as of March 31, 2022 to reflect the revised timeline. We
are currently assessing with Novo the impact on timelines and study cost

Financial
Risk Factors and Other Instruments
The
nature and extent of our exposure to risks arising from financial instruments, including credit risk, liquidity risk and market risk
and how we manage those risks are described in note 24 to the Company’s audited consolidated financial statements for the year
ended December 31, 2021.There were no significant changes in the three months of 2022 as compared to the December 31, 2021 disclosures.
Results
of operations for the three-month period ended March 31, 2022
Restatement
of comparative period figures
In
the fourth quarter of 2021, the Company restated its previously reported condensed consolidated interim financial statements for the
three-month period ended March 31, 2021 and the three-month and six-month periods ended June 30, 2021 and three-month and nine-month
periods ended September 30, 2021 with respect to the recognition of revenue for the Novo Amendment, signed in November 2020. During the
fourth quarter of 2021, management reassessed the classification of the development activities associated with the DETECT-trial and concluded
that subsequent to the Novo Amendment the parties no longer shared joint control of these activities and, as such, these development
activities no longer met the definition of a joint operation, as defined in IFRS 11, Joint Arrangements. Therefore, pursuant to
the guidance in IFRS 15, Revenue from Contracts with Customers, the Company reclassified the charges to Novo, from research and
development expenses to development services revenue, in the related periods. In addition, the license fees related to the pediatric
indication were adjusted to reflect the revised pattern of recognition as the performance obligation for the development services has
now been combined with the pediatric license. In addition, the accounting for prepaid expenses and other assets and deferred revenues
related the DETECT-trial expenses incurred was restated.
The
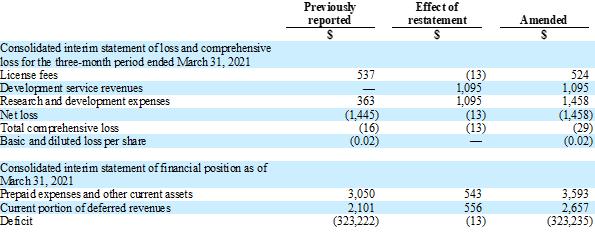
impacts of the March 31, 2021 restatements are as follows (amounts in thousands, except for basic and diluted loss per share):
| | |
Previously reported | | |
Effect of restatement | | |
Amended | |
| | |
| $ | | |
| $ | | |
| $ | |
| Consolidated interim statement of loss and comprehensive loss for the three-month period ended March 31, 2021 | |
| | | |
| | | |
| | |
| License fees | |
| 537 | | |
| (13 | ) | |
| 524 | |
| Development service revenues | |
| — | | |
| 1,095 | | |
| 1,095 | |
| Research and development expenses | |
| 363 | | |
| 1,095 | | |
| 1,458 | |
| Net loss | |
| (1,445 | ) | |
| (13 | ) | |
| (1,458 | ) |
| Total comprehensive loss | |
| (16 | ) | |
| (13 | ) | |
| (29 | ) |
| Basic and diluted loss per share | |
| (0.02 | ) | |
| — | | |
| (0.02 | ) |
| | |
| | | |
| | | |
| | |
| Consolidated interim statement of financial position as of March 31, 2021 | |
| | | |
| | | |
| | |
| Prepaid expenses and other current assets | |
| 3,050 | | |
| 543 | | |
| 3,593 | |
| Current portion of deferred revenues | |
| 2,101 | | |
| 556 | | |
| 2,657 | |
| Deficit | |
| (323,222 | ) | |
| (13 | ) | |
| (323,235 | ) |

These
restatements did not impact the Company’s cash and cash equivalent amounts and reported amounts of operating, investing and financing
activities within the consolidated interim statements of cash flows for the three-month period ended March 31, 2021.
Revenues
Our
total revenue for the three-month period ended March 31, 2022 was $1.5 million as compared with $1.7 million for the same period in 2021,
representing a decrease of $0.2 million as the Company earned $1.4 million in revenue from its agreement with Novo (2021 - $1.7 million)
and $0.1 million from its agreement with CH (2021 - $nil). The decrease in revenue earned from Novo in the period relates to a decrease
in development services provided by the Company for the DETECT-trial impacted mainly by the delays in enrollment in the trials due to
the Omicron wave early in the quarter. During the first quarter of 2022, the Company shipped an initial batch of macimorelin (Ghryvelin®)
to Consilient as they prepare to launch in the United Kingdom later in 2022.
Operating
expenses
Our
total operating expenses for the three-month period ended March 31, 2022 was $4.3 million as compared with $3.0 million for the same
period in 2021, representing an increase of $1.3 million. This increase arose primarily from a $0.9 million increase in research and
development costs, $0.3 million increase in general and administrative expenses and an increase of $0.1 million in selling expenses,
as further discussed below.
Research
and development expenses
The
following table summarizes our research and development expenses incurred during the periods indicated (amounts in thousands, except
percentages):
| | |
QUARTER ENDED
MARCH 31, | | |
| |
| | |
2022 | | |
2021 | | |
$ CHANGE | | |
% CHANGE | |
| Macrilen™ (macimorelin) pediatric trial (DETECT-trial) direct research and development expenses | |
$ | 896 | | |
$ | 1,066 | | |
$ | (170 | ) | |
| (15.9 | )% |
| AEZS-130 direct research and development expenses | |
| 277 | | |
| — | | |
| 277 | | |
| 100.0 | % |
| DC-PTH direct research and development expenses | |
| 268 | | |
| — | | |
| 268 | | |
| 100.0 | % |
| Parkinson’s direct research and development expenses | |
| 189 | | |
| — | | |
| 189 | | |
| 100.0 | % |
| Covid-19 direct research and development expenses | |
| 48 | | |
| 68 | | |
| (20 | ) | |
| (29.4 | )% |
| NMOSD direct research and development expenses | |
| 89 | | |
| 17 | | |
| 72 | | |
| 423.5 | % |
| Chlamydia direct research and development expenses | |
| 118 | | |
| — | | |
| 118 | | |
| 100.0 | % |
| Additional programs’ direct research and development expenses | |
| 136 | | |
| 139 | | |
| (3 | ) | |
| (2.2 | )% |
| Total direct research and development expenses | |
| 2,021 | | |
| 1,290 | | |
| 731 | | |
| 56.7 | % |
| Employee-related expenses | |
| 324 | | |
| 136 | | |
| 188 | | |
| 138.2 | % |
| Facilities, depreciation, and other expenses | |
| 45 | | |
| 32 | | |
| 13 | | |
| 40.6 | % |
| Total | |
$ | 2,390 | | |
$ | 1,458 | | |
$ | 932 | | |
| 63.9 | % |
Research
and development expenses increased $0.9 million for the quarter ended March 31, 2022 compared to the quarter ended March 31, 2021 primarily
due to $0.7 million increase in direct research and development expenses, which was primarily due to a $0.9 million increase in the initiation
of our new pre-clinical projects with universities offset by a $0.2 million decrease in costs for the DETECT-trial. The Company had its
six pre-clinical projects fully initiated in the quarter ended March 31, 2022 as compared to having started only two such projects in
the same period in 2021.

Employee-related
expenses have increased in 2022 by $0.2 million for the quarter ended March 31, 2022 as compared to the quarter ended March 31, 2021
primarily from the impact of the addition of Dr. Michael Teifel as our Chief Scientific Officer in May 2021 and of our Head of Quality
Control and CMC-Regulatory in March 2021, to better support our new pre-clinical initiatives.
General
and administrative expenses
General
and administrative expenses increased by $0.3 million for the quarter ended March 31, 2022 compared to the quarter ended March 31, 2021,
primarily due to increased directors’ and officers’ insurance coverage of $0.2 million and increased salary costs of $0.1
million.
Net
finance income
Our
net finance income for the three-month period ended March 31, 2022 was $0.2 million as compared with net finance costs of ($0.3) million
for the same period in 2021, representing an increase in net finance income of $0.5 million. This is primarily due to a $0.5 million
increase in the gain from changes in foreign currency exchange rates.
Net
loss
For
the three-month period ended March 31, 2022, we reported a consolidated net loss of $2.6 million, or $0.02 loss per common share (basic),
as compared with a consolidated net loss of $1.5 million, or $0.02 loss per common share (basic) for the three-month period ended March
31, 2021. The $1.1 million increase in net loss is primarily from an increase of $1.3 million in total operating expenses and a decline
of $0.2 million in total revenues, partially offset by an increase of $0.5 million in net finance income, as previously discussed above.
Selected
quarterly financial data
| | |
Three months ended | |
| (in thousands, except for per share data) | |
March 31,
2022 | | |
December 31,
2021 | | |
September 30,
2021(1) | | |
June 30,
2021(1) | |
| | |
| | | |
| | | |
| $ | | |
| $ | |
| Revenues | |
| 1,517 | | |
| 956 | | |
| 1,052 | | |
| 1,584 | |
| Net loss | |
| (2,640 | ) | |
| (2,894 | ) | |
| (1,932 | ) | |
| (2,084 | ) |
| Net loss per share (basic and diluted)(2) | |
| (0.02 | ) | |
| (0.02 | ) | |
| (0.02 | ) | |
| (0.02 | ) |
| | |
Three months ended | |
| (in thousands, except for per share data) | |
March 31,
2021(1) | | |
December 31,
2020 | | |
September 30,
2020 | | |
June 30,
2020 | |
| | |
| | | |
| | | |
| $ | | |
| $ | |
| Revenues | |
| 1,668 | | |
| 2,366 | | |
| 128 | | |
| 68 | |
| Net (loss) income | |
| (1,458 | ) | |
| (1,311 | ) | |
| (1,136 | ) | |
| (3,450 | ) |
| Net loss per share (basic and diluted)(2) | |
| (0.02 | ) | |
| (0.02 | ) | |
| (0.02 | ) | |
| (0.15 | ) |
| (1) |
The
restatements are discussed above under “Revenues” in the Results from operations section of this MD&A. The interim
financial statements for the periods ended March 31, 2021, June 30, 2021 and September 30, 2021 have not been refiled but the comparatives
will be corrected when the interim financial statements for the periods ended March 31, 2022, June 30, 2022 and September 30, 2022
are filed. These restatements did not have any impact on 2020 results. |
| |
|
| (2) |
Net
loss per share is based on the weighted average number of shares outstanding during each reporting period, which may differ on a
quarter-to-quarter basis. As such, the sum of the quarterly net loss per share amounts may not equal full-year net loss per share. |

Historical
quarterly results of operations and net loss cannot be taken as reflective of recurring revenue or expenditure patterns of predictable
trends, largely given the non-recurring nature of certain components of our historical revenues, the impact of costs associated with
launching a number of significant preclinical research and development programs in 2021, and of foreign exchange gains and losses. In
addition, we cannot predict what the revenues from royalties will be earned from the Novo Amendment.
Use
of cash and cash equivalents
We
began 2022 with $65.3 million in cash and cash equivalents. During the three-month period ended March 31, 2022, our operating activities
consumed $1.5 million, our financing activities consumed $0.03 million and our investing activities used $0.006 million. As at
March 31, 2022 we had $63.6 million of cash and cash equivalents.
Liquidity
and capital reserves
Our
operations and capital expenditures have generally been financed through certain transactions impacting our cash flows from operating
activities, public equity offerings, registered direct offerings and issuances. A portion of the Company’s cash is held in AEZS
Germany, which is the counterparty to various license, supply and distribution agreements for the Company’s only approved product
Macrilen™ (macimorelin).
Via
public and private financings between September 2019 and February 2021, the Company has received $55.9 million in total funding (net
of transaction costs) and, throughout 2021, holders exercised 35.1 million warrants resulting in proceeds to the Company of $20.1 million
| | |
Three months ended March 31, | |
| (in thousands) | |
2022 | | |
2021 | |
| | |
| | |
| |
| Cash and cash equivalents - beginning of period | |
| 65,300 | | |
| 24,271 | |
| Cash used in operating activities | |
| (1,461 | ) | |
| (1,045 | ) |
| Cash flows (used in) provided by financing activities | |
| (34 | ) | |
| 50,933 | |
| Cash flows used in investing activities | |
| (6 | ) | |
| (507 | ) |
| Effect of exchange rate changes on cash and cash equivalents | |
| (203 | ) | |
| (281 | ) |
| Cash and cash equivalents - end of period | |
| 63,596 | | |
| 73,371 | |
Operating
Activities
Cash
used by operating activities totaled $1.5 million for the three months ended March 31, 2022, as compared to $1.0 million in the same
period in 2021. This $0.5 million increase in use of cash in operating activities is attributed primarily to increased research and development
and general and administrative expenses and offset by the reimbursement of income taxes in the first quarter of 2022.

Financing
Activities
Cash
used by financing activities totaled $0.03 million for the three months ended March 31, 2022, as compared with cash provided by financing
activities of $50.9 million in the same period in 2021. On February 21, 2021, the Company completed the February 2021 Financing with
net cash proceeds of $31.0 million and, throughout the first quarter of 2021, the warrant exercises contributed cash of approximately
$20.0 million.
Investing
Activities
Cash
used by investing activities totaled $0.01 million for the three months ended March 31, 2022, as compared to cash used by investing activities
of $0.5 in the same period in 2021. During the first three months of 2021, the Company executed various agreements including in-licensing
and similar arrangements with development partners reflecting the purchase of separately acquired intangible assets of $0.5 million.
Capital
stock
As
at May 10, 2022, we had 121,397,007 common shares issued and outstanding, as well as 1,136,368 stock options, 423,000 deferred share
units and 11,441,213 warrants outstanding. Each stock option, deferred share unit and warrant is exercisable for one common share.
Adequacy
of financial resources
Since
inception, the Company has incurred significant expenses in its efforts to develop and co-promote products. Our current business focus
is to: investigate further therapeutic uses of Macrilen™, expand pipeline development activities, further expand the commercialization
of macimorelin in available territories and fund our 50% share of the DETECT-trial costs which exceed €9 million. Consequently,
the Company has incurred operating losses and has generated negative cash flow from operations historically and in each of the last several
years except for the year ended December 31, 2018 when the Company earned revenue from the sale of a license for the adult indication
of Macrilen™ (macimorelin) in the U.S. and Canada. The Company expects to incur significant expenses and operating losses for the
foreseeable future as it advances its product candidates through preclinical and clinical development, seeks regulatory approval and
pursues commercialization of any approved product candidates. We expect that our research and development costs will increase in connection
with our planned research and development activities.
As
of March 31, 2022, the Company had cash and cash equivalents of $63.6 million and an accumulated deficit of $334.5 million. The Company
also had a net loss of $2.6 million and negative cash flows from operations of $1.5 million for the three months ended March 31, 2022.
We believe that our existing cash on hand will be sufficient to fund our anticipated operating and capital expenditure requirements through
2023. We have based this estimate on assumptions that may prove to be wrong, and we could exhaust our capital resources sooner than we
expect. We may also require additional capital to pursue in-licenses or acquisitions of other product candidates.
Contractual
obligations and commitments as at March 31, 2022
| (in thousands) | |
Service and manufacturing | | |
R&D
contracts | | |
TOTAL | |
| | |
| $ | | |
| $ | | |
| $ | |
| Less than 1 year | |
| 518 | | |
| 2,056 | | |
| 2,574 | |
| 1 - 3 years | |
| 638 | | |
| 630 | | |
| 1,268 | |
| 4 - 5 years | |
| 1 | | |
| — | | |
| 1 | |
| More than 5 years | |
| — | | |
| — | | |
| — | |
| Total | |
| 1,157 | | |
| 2,686 | | |
| 3,843 | |

During
2021, the Company executed various agreements including in-licensing and similar arrangements with development partners with $0.6 million
in additions of separately acquired intangible assets recognized in the condensed interim consolidated statements of financial position.
Such agreements may require the Company to make payments on achievement of stages of development, launch or revenue milestones, although
the Company generally has the right to terminate these agreements at no penalty. The Company recognizes research and development milestones
as an intangible asset once it is committed to the payment, which is generally when the Company reaches a set point in the development
cycle.
Based
on the closing exchange rates, the Company expects to pay $2.7 million, including $2.5 million (€2.3 million), and $0.1 million
(£0.1 million), in R&D contracts and up to $8.8 million, including $7.2 million (€6.5 million) and $1.5 million (£1.2
million), in R&D milestone payments and up to $32.3 million, including $30.7 million (€27.6 million) and $1.6 million (£1.3
million), in revenue related milestone payments. The table below contains all potential R&D and revenue-related milestone payments
that the Company may be required to make under such agreements:
| (in thousands) | |
Future potential R&D milestone payments | | |
Future potential revenue milestone payments | | |
TOTAL | |
| | |
| $ | | |
| $ | | |
| $ | |
| Less than 1 year | |
| 28 | | |
| — | | |
| 28 | |
| 1 - 3 years | |
| 111 | | |
| — | | |
| 111 | |
| 4 - 5 years | |
| 909 | | |
| — | | |
| 909 | |
| More than 5 years | |
| 7,709 | | |
| 32,309 | | |
| 40,018 | |
| Total | |
| 8,757 | | |
| 32,309 | | |
| 41,066 | |
The
future payments that are disclosed represent contract payments and are not discounted and are not risk-adjusted. The development of any
pharmaceutical product candidates is a complex and risky process that may fail at any stage in the development process due to a number
of factors. The timing of the payments is based on the Company’s current best estimate of achievement of the relevant milestone.
Contingencies
In
the normal course of operations, the Company may become involved in various claims and legal proceedings related to, for example, contract
terminations and employee-related and other matters.
Related
Party Transactions and Off-Balance Sheet Arrangements
Other
than employment agreements and indemnification agreements with our management, there are no related party transactions.
As
at March 31, 2022, we did not have any interests in special purpose entities or any other off-balance sheet arrangements.
Risk
Factors and Uncertainties
An
investment in our securities involves a high degree of risk. In addition to the other information included in this MD&A and in the
related consolidated financial statements, investors are urged to carefully consider the risks described under the caption “Risk
Factors” in our most recent Annual Report on Form 20-F for the year ended December 31, 2021 for a discussion of the various risks
that may materially affect our business. The risks and uncertainties not presently known to us or that we currently deem immaterial may
also materially harm our business, operating results and financial condition and could result in a complete loss of your investment.

Our
most recent Annual Report on Form 20-F was filed with the relevant Canadian and U.S. securities’ regulatory authorities at www.sedar.com
and with the SEC at www.sec.gov. Investors are urged to consult the risk factors in these documents.
Disclosure
Controls and Procedures
Under
the supervision and with the participation of our management, including the Chief Executive Officer and the Chief Financial Officer,
we have evaluated the effectiveness of our disclosure controls and procedures as at March 31,2022 Based on that evaluation, the Chief
Executive Officer and Chief Financial Officer have concluded that these disclosure controls and procedures were not effective as of that
date due to a material weakness in internal control over financial reporting disclosed in our Annual Report on Form 20-F for the year
ended December 31, 2021 available on SEDAR at www.sedar.con and on EDGAR at sec.gov and as described below.
Management’s
Report on Internal Control over Financial Reporting
Our
management is responsible for establishing and maintaining adequate internal control over financial reporting. Our internal control over
financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation
of financial statements for external purposes in accordance with IFRS as issued by the IASB.
Our
internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that,
in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of Aeterna Zentaris; (ii) provide
reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with IFRS,
and that receipts and expenditures of the Company are being made only in accordance with authorizations of Company management; and (iii)
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of Company assets
that could have a material effect on the financial statements.
Because
of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of
any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions,
or that the degree of compliance with the policies or procedures may deteriorate.
Material
weakness
The
material weakness that we identified in our internal controls over financial reporting as of December 31, 2021 was as follows: our review
control was not sufficiently designed to adequately review and assess an accounting analysis for revenue recognition for complex revenue
arrangements. This resulted in a restatement of our previously issued condensed interim consolidated financial statements as at and for
the quarters and year-to-date periods ended March 31, 2021, June 30, 2021 and September 30, 2021, with respect to revenue recognition
on one agreement.
We
have developed and commenced implementation of a remediation plan to address the material weakness discussed above and to improve our
internal control over financial reporting. The remediation plan includes:
| ● |
strengthening
our revenue recognition and financial reporting controls by adding new or additional resources with adequate technical knowledge
and training, including the hiring of a new Chief Financial Officer in January 2022, and utilizing the services of an external professional
with requisite knowledge and experience in the area of revenue recognition and of IFRS more broadly. |
| |
|
| ● |
designing
and implementing effective internal controls related to the involvement of appropriate finance and accounting staff in the review
of strategic and complex transactions, such as license and collaboration agreements, including as those transactions are negotiated
and executed, to ensure that any matters with accounting ramifications are addressed on a timely basis; and |
| |
|
| ● |
ensuring
that all non-routine transactions, including those requiring the application of significant judgment or analysis, are thoroughly
researched at the appropriate level and are sufficiently documented by qualified accounting and finance personnel (including third-party
subject matter experts as necessary), with such documentation to be approved in a timely manner by the Company’s Chief Financial
Officer. |
This
material weakness will continue to be addressed through 2022.
Changes
in Internal Controls over Financial Reporting
There
have been no significant changes to our internal controls over financial reporting for the three-month period ended March 31, 2022 that
have materially affected, or are reasonably likely to materially affect, internal controls over financial reporting.