Exhibit 99.2

JUNE 2023 MEI Pharma & Infinity Pharmaceuticals Merger and Clinical
Program Update

A Transaction with Potential to Create Significant Opportunities and Build
Value 2 TODAY’S AGENDA Welcome and Overview David Urso, President & CEO (MEIP and combined company) Eganelisib Dr. Robert Ilaria, Jr., Chief Medical Officer (INFI and combined company) Dr. Ezra Cohen Chief Medical Officer,
Oncology, Tempus Most recently, Chief, Division of Hematology-Oncology, and Associate Director of Clinical Science at UC San Diego Moores Cancer Center Nick Abbott, PhD. Former sellside analyst with 35 years biotech experience, most
recently at Wells Fargo Voruciclib & ME-344 Dr. Richard Ghalie, Chief Medical Officer (MEIP) Nick Abbott, PhD Conclusion David Urso, President & CEO

3 Cautionary Statement Regarding Forward-Looking Statements Certain
statements contained in this presentation may be considered forward-looking statements within the meaning of the federal securities law. Such statements are based upon current plans, estimates and expectations of the management of MEI and
Infinity that are subject to various risks and uncertainties that could cause actual results to differ materially from such statements. The inclusion of forward-looking statements should not be regarded as a representation that such plans,
estimates and expectations will be achieved. Words such as “anticipate,” “expect,” “project,” “intend,” “believe,” “may,” “will,” “should,” “plan,” “could,” “continue,” “target,” “contemplate,” “estimate,” “forecast,” “guidance,” “predict,”
“possible,” “potential,” “pursue,” “likely,” and words and terms of similar substance used in connection with any discussion of future plans, actions or events identify forward-looking statements. All statements, other than historical
facts, including statements regarding: the expected timing of the closing of the proposed merger; the ability of the parties to complete the proposed merger considering the various closing conditions; the expected benefits of the proposed
merger, including estimations of anticipated cost savings and cash runway; the competitive ability and position of the combined company; the potential, safety, efficacy, and regulatory and clinical progress of the combined company’s product
candidates, including the anticipated timing for initiation of clinical trials and release of clinical trial data and the expectations surrounding potential regulatory submissions, approvals and timing thereof; the sufficiency of the
combined company’s cash, cash equivalents and short-term investments to fund operations; and any assumptions underlying any of the foregoing, are forward-looking statements. Important factors that could cause actual results to differ
materially from MEI’s and Infinity’s plans, estimates or expectations could include, but are not limited to: (i) the risk that the proposed merger may not be completed in a timely manner or at all, which may adversely affect MEI’s and
Infinity’s businesses and the price of their respective securities; (ii) uncertainties as to the timing of the consummation of the proposed merger and the potential failure to satisfy the conditions to the consummation of the proposed
merger, including obtaining stockholder and regulatory approvals; (iii) the proposed merger may involve unexpected costs, liabilities or delays; (iv) the effect of the announcement, pendency or completion of the proposed merger on the
ability of MEI or Infinity to retain and hire key personnel and maintain relationships with customers, suppliers and others with whom MEI or Infinity does business, or on MEI’s or Infinity’s operating results and business generally; (v)
MEI’s or Infinity’s respective businesses may suffer as a result of uncertainty surrounding the proposed merger and disruption of management’s attention due to the proposed merger; (vi) the outcome of any legal proceedings related to the
proposed merger or otherwise, or the impact of the proposed merger thereupon; (vii) MEI or Infinity may be adversely affected by other economic, business, and/or competitive factors; (viii) the occurrence of any event, change or other
circumstances that could give rise to the termination of the merger agreement and the proposed merger; (ix) restrictions during the pendency of the proposed merger that may impact MEI’s or Infinity’s ability to pursue certain business
opportunities or strategic transactions; (x) the risk that MEI or Infinity may be unable to obtain governmental and regulatory approvals required for the proposed merger, or that required governmental and regulatory approvals may delay the
consummation of the proposed merger or result in the imposition of conditions that could reduce the anticipated benefits from the proposed merger or cause the parties to abandon the proposed merger; (xi) risks that the anticipated benefits
of the proposed merger or other commercial opportunities may otherwise not be fully realized or may take longer to realize than expected; (xii) the impact of legislative, regulatory, economic, competitive and technological changes; (xiii)
risks relating to the value of MEI shares to be issued in the proposed merger; (xiv) the risk that integration of the proposed merger post-closing may not occur as anticipated or the combined company may not be able to achieve the benefits
expected from the proposed merger, as well as the risk of potential delays, challenges and expenses associated with integrating the combined company’s existing businesses; (xv) exposure to inflation, currency rate and interest rate
fluctuations, as well as fluctuations in the market price of MEI’s and Infinity’s traded securities; (xvi) the impact of the COVID-19 pandemic on MEI’s and Infinity’s industry and individual companies, including on counterparties, the
supply chain, the execution of clinical development programs, access to financing and the allocation of government resources; (xvii) final data from pre-clinical studies and completed clinical trials may differ materially from reported
interim data from ongoing studies and trials; (xviii) costs and delays in the development and/or U.S. Food and Drug Administration (“FDA”) approval, or the failure to obtain such approval, of the combined company’s product candidates; (xix)
regulatory authorities may not agree with the design or results of clinical studies and as a result future clinical studies may be subject to holds; (xx) uncertainties or differences in interpretation in clinical trial results; (xxi) the
combined company’s inability to maintain or enter into, and the risks resulting from dependence upon, collaboration or contractual arrangements necessary for the development, manufacture, commercialization, marketing, sales and distribution
of any product candidates; and (xxii) the ability of MEI or Infinity to protect and enforce intellectual property rights; and (xxiii) the unpredictability and severity of catastrophic events, including, but not limited to, acts of terrorism
or outbreak of war or hostilities, as well as MEI’s and Infinity’s response to any of the aforementioned factors. Additional factors that may affect the future results of MEI and Infinity are set forth in their respective filings with the
United States Securities and Exchange Commission (the “SEC”), including the section entitled “Risk Factors” in the Registration Statement on Form S-4 that was declared effective by the SEC on June 6, 2023 and each of MEI’s and Infinity’s
most recently filed Annual Reports on Form 10-K, subsequent Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings with the SEC, which are available on the SEC’s website at www.sec.gov. See in particular MEI’s Annual
Report on Form 10-K for the fiscal year ended June 30, 2022 in Part I, Item 1A, “Risk Factors,” and Infinity’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022, in Part I, Item 1A, “Risk Factors.” The risks and
uncertainties described above and in the SEC filings cited above are not exclusive and further information concerning MEI and Infinity and their respective businesses, including factors that potentially could materially affect their
respective businesses, financial conditions or operating results, may emerge from time to time. Readers are urged to consider these factors carefully in evaluating these forward-looking statements, and not to place undue reliance on any
forward-looking statements. Any such forward-looking statements represent management’s reasonable estimates and beliefs as of the date of this presentation. While MEI and Infinity may elect to update such forward-looking statements at some
point in the future, they disclaim any obligation to do so, other than as may be required by law, even if subsequent events cause their views to change. This presentation contains hyperlinks to information that is not deemed to be
incorporated by reference.

Additional Information 4 Important Information about the Merger and Where to
Find It This communication relates to a proposed transaction between Infinity Pharmaceuticals, Inc. (“Infinity”) and MEI Pharma, Inc. (“MEI”). In connection with the proposed merger, MEI filed with the SEC a registration statement on
Form S-4 that includes a joint proxy statement of MEI and Infinity (the “Joint Proxy Statement/Prospectus) that also constitutes a prospectus of MEI. The registration statement on Form S-4 was declared effective by the SEC on June 6, 2023.
MEI and Infinity have each filed and mailed the Joint Proxy Statement/Prospectus to their respective stockholders. INVESTORS AND MEI’S AND INFINITY’S RESPECTIVE STOCKHOLDERS ARE URGED TO READ THE JOINT PROXY STATEMENT/PROSPECTUS IN ITS
ENTIRETY AND ANY OTHER DOCUMENTS FILED BY EACH OF MEI AND INFINITY WITH THE SEC IN CONNECTION WITH THE PROPOSED MERGER OR INCORPORATED BY REFERENCE THEREIN BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED MERGER AND THE
PARTIES TO THE PROPOSED MERGER. Investors and stockholders may obtain a free copy of the Joint Proxy Statement/Prospectus and other documents containing important information about MEI and Infinity from the SEC’s website at www.sec.gov. MEI
and Infinity make available free of charge at www.meipharma.com and www.infi.com, respectively (in the “Investors” and “Investors/Media” sections, respectively), copies of materials they file with, or furnish to, the SEC. Participants
in the Solicitation MEI, Infinity and their respective directors, executive officers and certain employees and other persons may be deemed to be participants in the solicitation of proxies from the stockholders of MEI and Infinity in
connection with the proposed merger. Securityholders may obtain information regarding the names, affiliations and interests of MEI’s and Infinity’s directors and executive officers in the Joint Proxy Statement/Prospectus which may be
obtained free of charge from the SEC’s website at www.sec.gov, MEI’s investor website at https://www.meipharma.com/investors and Infinity’s investor website at https://investors.infi.com/. No Offer or Solicitation This communication
shall not constitute an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or
qualification under the securities laws of any such jurisdiction. No offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the U.S. Securities Act of 1933, as amended.

A Transaction with Potential to Create Significant Opportunities and Build
Value 5 TODAY’S AGENDA Welcome and Overview David Urso, President & CEO (MEIP and combined company) Eganelisib Dr. Robert Ilaria, Jr., Chief Medical Officer (INFI and combined company) Dr. Ezra Cohen Chief Medical Officer,
Oncology, Tempus Most recently, Chief, Division of Hematology-Oncology, and Associate Director of Clinical Science at UC San Diego Moores Cancer Center Nick Abbott, PhD. Former sellside analyst with 35 years biotech experience, most
recently at Wells Fargo Voruciclib & ME-344 Dr. Richard Ghalie, Chief Medical Officer (MEIP) Nick Abbott, PhD Conclusion David Urso, President & CEO

A Combined Company with Significant Opportunities for Value Creation 6 Three
differentiated, promising, clinical candidates based on solid science and data* Pipeline led by planned eganelisib Phase 2 Study in Squamous cell carcinoma of the head & neck (SCCHN) Voruciclib + Venclexta® P1 Study: Initial Results
~YE 2023 ME-344 + Avastin® P1 Study: Initial Results ~YE 2023 Eganelisib + Keytruda® P2 Study: Initial Safety/Efficacy 2H 2024 Utilize understandings of biology to overcome resistance mechanisms of standard of care
therapies Anticipated Cash at closing of ~$100M expected to fund operations to mid-2025 and clinical data over the next ~6-24 months Experienced Leadership Team Advance potential first-in-class programs to value creating transactions or
commercialization *Dates refer to expected timelines.

Leadership with Extensive Industry and Oncology Drug Development
Expertise 7 EXECUTIVE LEADERSHIP David Urso | Chief Executive Officer Robert Ilaria Jr., MD | Chief Medical Officer Stéphane Peluso PhD | Chief Scientific Officer BOARD David Urso Norman C. Selby (Chair) Charles V. Baltic III,
JD Richard Gaynor, MD Daniel Gold, PhD Sujay Kango Adelene Perkins Thomas Reynolds, MD, PhD

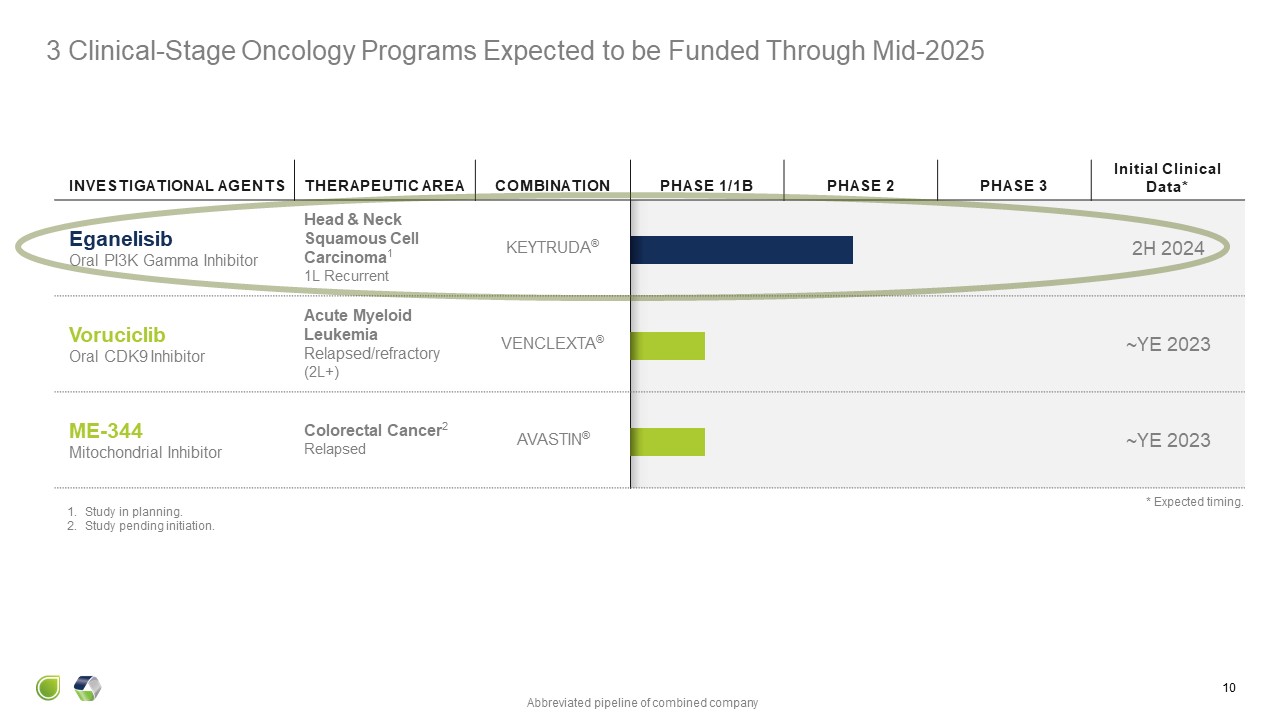
3 Clinical-Stage Oncology Programs Expected to be Funded Through
Mid-2025 8 INVESTIGATIONAL AGENTS THERAPEUTIC AREA COMBINATION PHASE 1/1B PHASE 2 PHASE 3 Initial Clinical Data* Eganelisib Oral PI3K Gamma Inhibitor Head & Neck Squamous Cell Carcinoma1 1L Recurrent KEYTRUDA® 2H
2024 Voruciclib Oral CDK9 Inhibitor Acute Myeloid Leukemia Relapsed/refractory (2L+) VENCLEXTA® ~YE 2023 ME-344 Mitochondrial Inhibitor Colorectal Cancer 2 Relapsed AVASTIN® ~YE 2023 Study in planning. Study pending
initiation. Abbreviated pipeline of combined company * Expected timing.

TRANSACTION STRUCTURE Stock-for-stock merger: Infinity stockholders will
receive shares of MEI common stock Infinity will become a wholly owned subsidiary of MEI pharma Pro forma outstanding equity of the combined company post-closing: Approximately 58% MEI and approximately 42% Infinity Combined company
will continue to trade on Nasdaq under a new name: Kimbrx Therapeutics Expected transaction closing by mid 2023 Approved by both companies’ boards Projected approximately $100 million in cash, cash equivalents, and short-term investments
at closing Subject to approval by stockholders of both companies, as well as customary closing conditions and regulatory approvals APPROVALS AND CLOSING Transaction Summary 9 SPECIAL MEETING DATES MEI and Infinity Special Meetings
Scheduled for July 14, 2023
INVESTIGATIONAL AGENTS THERAPEUTIC AREA COMBINATION PHASE 1/1B PHASE
2 PHASE 3 Initial Clinical Data* Eganelisib Oral PI3K Gamma Inhibitor Head & Neck Squamous Cell Carcinoma1 1L Recurrent KEYTRUDA® 2H 2024 Voruciclib Oral CDK9 Inhibitor Acute Myeloid Leukemia Relapsed/refractory
(2L+) VENCLEXTA® ~YE 2023 ME-344 Mitochondrial Inhibitor Colorectal Cancer2 Relapsed AVASTIN® ~YE 2023 3 Clinical-Stage Oncology Programs Expected to be Funded Through Mid-2025 10 Study in planning. Study pending
initiation. Abbreviated pipeline of combined company * Expected timing.

Eganelisib: First-in-Class PI3K-�� Inhibitor for Next Generation Macrophage
Reprogramming Cancer Immunotherapy

Cellular IC50 pAKT IC50 (nM) Eganelisib / IPI-549 Eganelisib is a
First-in-Class, Potent and Selective PI3K- Inhibitor with a Strong Scientific Foundation as Next Generation Cancer Immunotherapy Kaneda et al. Nature 2016 539 437 De Henau et al. Nature, 2016 539 443 Evans et al ACS Med Chem Let 2016
7 862 McGovern et al. AACR-NCI-EORTC 2015 #A192

Suppressed T cells M2-like Macrophages Growing Tumor Cells M1-like
Macrophages Activated T Cells Dying Tumor Cells Immunosuppressive M2-like Macrophages Correlate with poor prognosis across most cancers Support tumor immune evasion, metastasis, angiogenesis Key contributors to resistance to
immunotherapy, targeted therapy, chemotherapy Activated, Immunostimulatory M1-like Macrophages Correlate with favorable prognosis across most cancers Favor response to immunotherapy, targeted therapy, chemotherapy Reprogramming Tumor
Associated Macrophages for Cancer Immunotherapy Molgora & Colonna Med 2021 2 666 Cassetta & Pollard Nature Rev Drug Disc 2018 17 887 Macrophage Reprogramming with Eganelisib & PI3K-�� inhibition
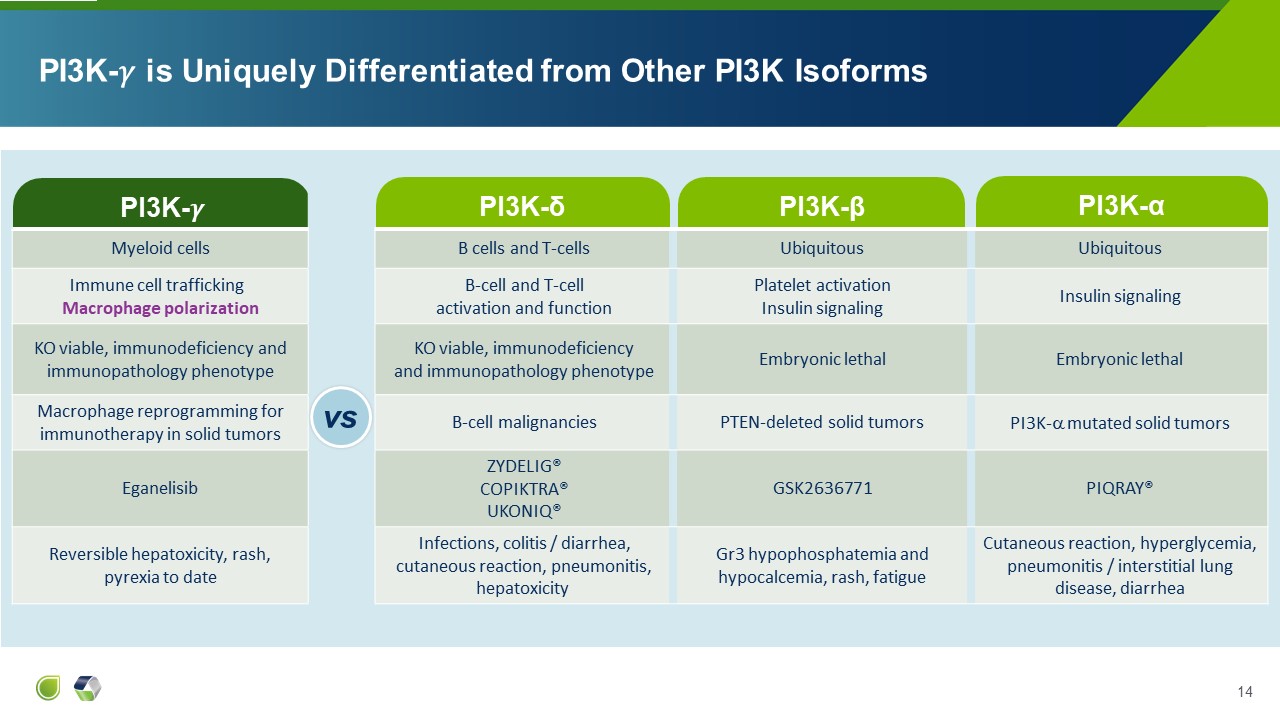
PI3K-�� PI3K-δ PI3K-�� is Uniquely Differentiated from Other PI3K
Isoforms vs PI3K-β PI3K-α Myeloid cells B cells and T-cells Ubiquitous Ubiquitous Immune cell trafficking Macrophage polarization B-cell and T-cell activation and function Platelet activation Insulin signaling Insulin
signaling KO viable, immunodeficiency and immunopathology phenotype KO viable, immunodeficiency and immunopathology phenotype Embryonic lethal Embryonic lethal Macrophage reprogramming for immunotherapy in solid tumors B-cell
malignancies PTEN-deleted solid tumors PI3K- mutated solid tumors Eganelisib ZYDELIG® COPIKTRA® UKONIQ® GSK2636771 PIQRAY® Reversible hepatoxicity, rash, pyrexia to date Infections, colitis / diarrhea, cutaneous reaction,
pneumonitis, hepatoxicity Gr3 hypophosphatemia and hypocalcemia, rash, fatigue Cutaneous reaction, hyperglycemia, pneumonitis / interstitial lung disease, diarrhea vs
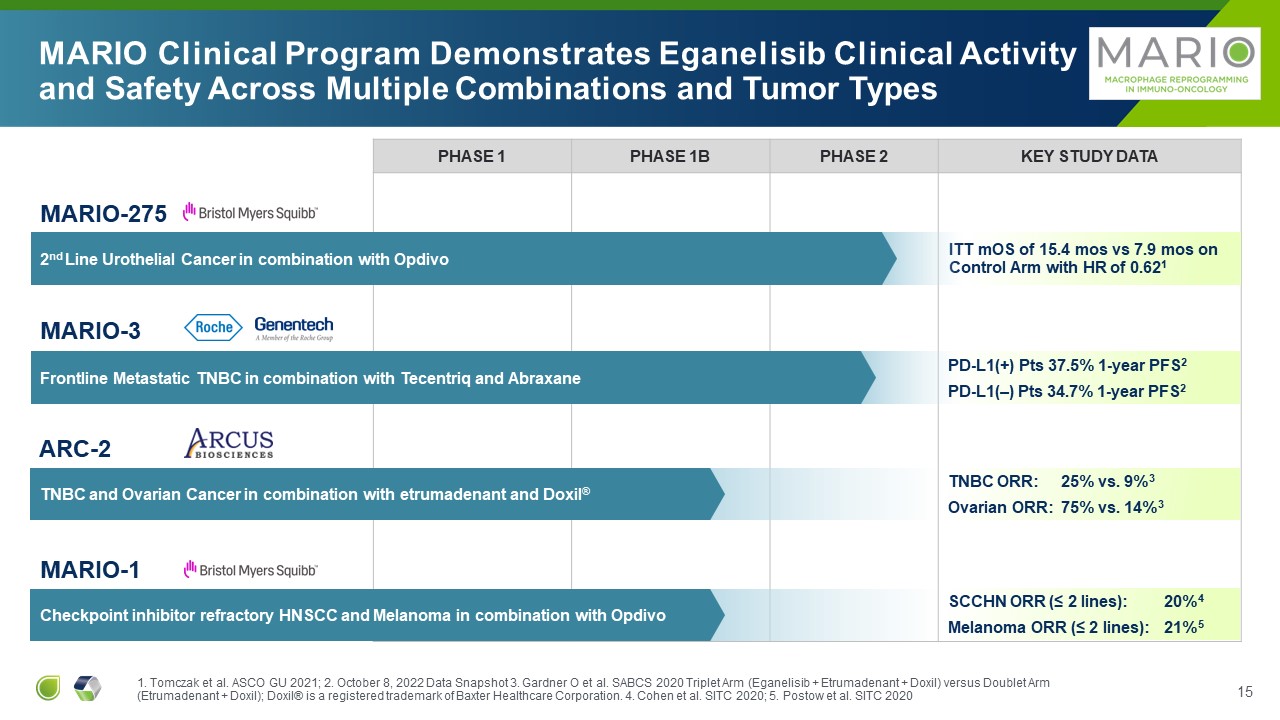
PHASE 1 PHASE 1B PHASE 2 Key Study Data ITT mOS of 15.4 mos vs 7.9 mos on
Control Arm with HR of 0.621 MARIO Clinical Program Demonstrates Eganelisib Clinical Activity and Safety Across Multiple Combinations and Tumor Types MARIO-275 2nd Line Urothelial Cancer in combination with Opdivo ARC-2 TNBC and
Ovarian Cancer in combination with etrumadenant and Doxil® MARIO-1 Checkpoint inhibitor refractory HNSCC and Melanoma in combination with Opdivo MARIO-3 Frontline Metastatic TNBC in combination with Tecentriq and Abraxane PD-L1(+)
Pts 37.5% 1-year PFS2 PD-L1(–) Pts 34.7% 1-year PFS2 TNBC ORR: 25% vs. 9%3 Ovarian ORR: 75% vs. 14%3 SCCHN ORR (≤ 2 lines): 20%4 Melanoma ORR (≤ 2 lines): 21%5 1. Tomczak et al. ASCO GU 2021; 2. October 8, 2022 Data Snapshot 3.
Gardner O et al. SABCS 2020 Triplet Arm (Eganelisib + Etrumadenant + Doxil) versus Doublet Arm (Etrumadenant + Doxil); Doxil® is a registered trademark of Baxter Healthcare Corporation. 4. Cohen et al. SITC 2020; 5. Postow et al. SITC 2020
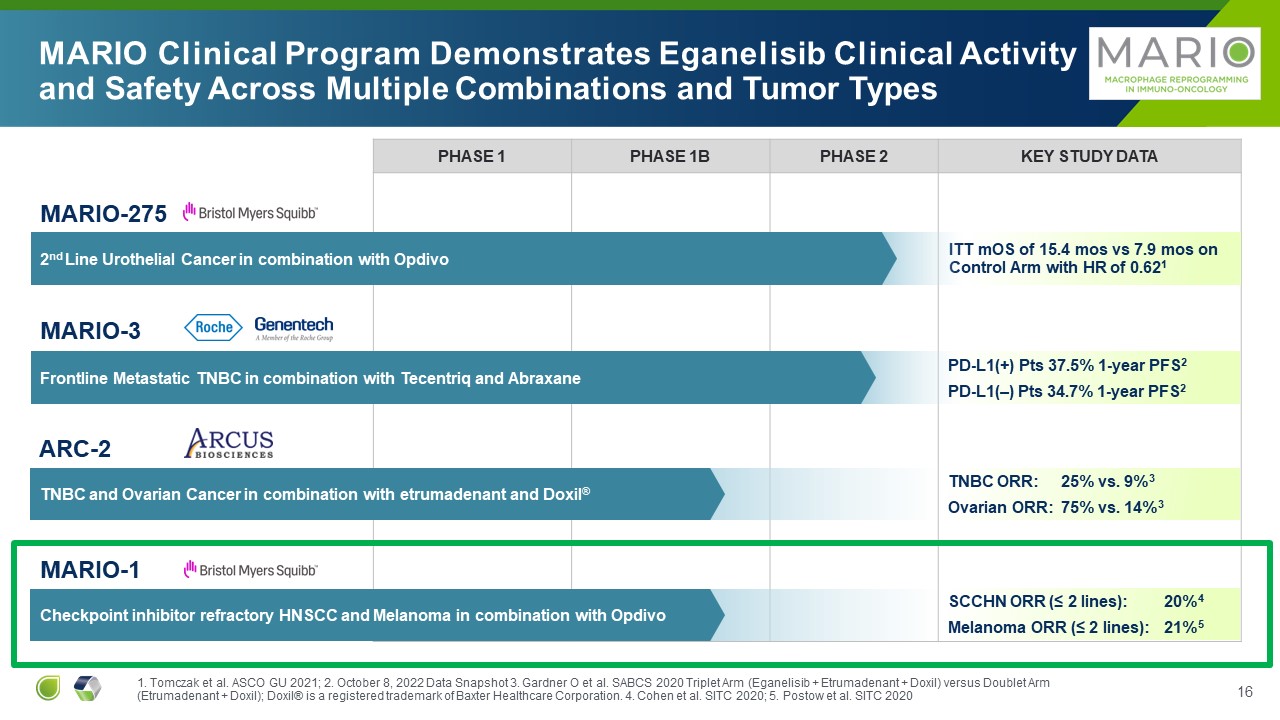
PHASE 1 PHASE 1B PHASE 2 Key Study Data ITT mOS of 15.4 mos vs 7.9 mos on
Control Arm with HR of 0.621 MARIO Clinical Program Demonstrates Eganelisib Clinical Activity and Safety Across Multiple Combinations and Tumor Types MARIO-275 2nd Line Urothelial Cancer in combination with Opdivo ARC-2 TNBC and
Ovarian Cancer in combination with etrumadenant and Doxil® MARIO-1 Checkpoint inhibitor refractory HNSCC and Melanoma in combination with Opdivo MARIO-3 Frontline Metastatic TNBC in combination with Tecentriq and Abraxane PD-L1(+)
Pts 37.5% 1-year PFS2 PD-L1(–) Pts 34.7% 1-year PFS2 TNBC ORR: 25% vs. 9%3 Ovarian ORR: 75% vs. 14%3 SCCHN ORR (≤ 2 lines): 20%4 Melanoma ORR (≤ 2 lines): 21%5 1. Tomczak et al. ASCO GU 2021; 2. October 8, 2022 Data Snapshot 3.
Gardner O et al. SABCS 2020 Triplet Arm (Eganelisib + Etrumadenant + Doxil) versus Doublet Arm (Etrumadenant + Doxil); Doxil® is a registered trademark of Baxter Healthcare Corporation. 4. Cohen et al. SITC 2020; 5. Postow et al. SITC 2020
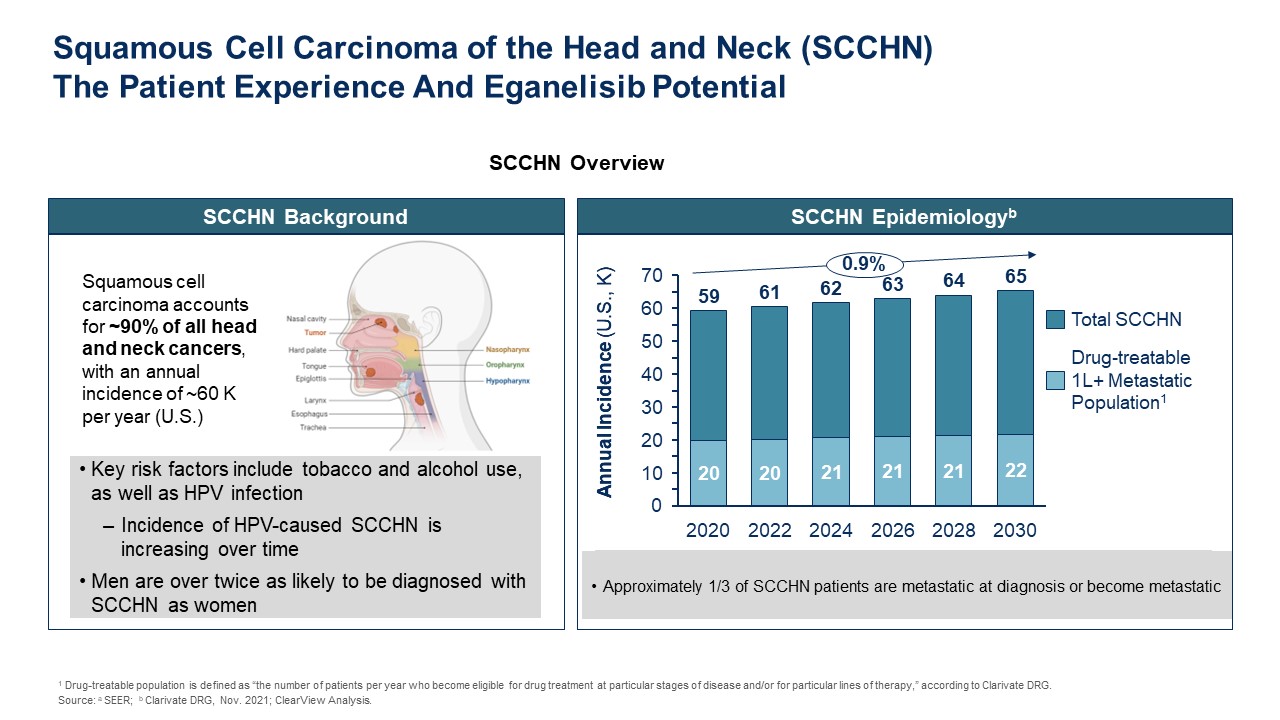
SCCHN Background Key risk factors include tobacco and alcohol use, as well as
HPV infection Incidence of HPV-caused SCCHN is increasing over time Men are over twice as likely to be diagnosed with SCCHN as women Squamous cell carcinoma accounts for ~90% of all head and neck cancers, with an annual incidence of ~60
K per year (U.S.) SCCHN Overview SCCHN Epidemiologyb TBD Annual Incidence (U.S., K) Approximately 1/3 of SCCHN patients are metastatic at diagnosis or become
metastatic 2020 2022 2024 2026 2028 2030 0 50 70 60 40 30 20 10 59 61 62 63 64 65 Annual Incidence (U.S., K) 0.9% Drug-treatable 1L+ Metastatic Population1 Total SCCHN Squamous Cell Carcinoma of the Head and Neck
(SCCHN)The Patient Experience And Eganelisib Potential 1 Drug-treatable population is defined as “the number of patients per year who become eligible for drug treatment at particular stages of disease and/or for particular lines of
therapy,” according to Clarivate DRG. Source: a SEER; b Clarivate DRG, Nov. 2021; ClearView Analysis.

Current Landscape in R/M HNSCC 18 Ezra Cohen,
MD FIRST LINE SECOND LINE THIRD LINE + R/M= Recurrent/Metastatic

Current Landscape in R/M HNSCC 19 Ezra Cohen, MD Anti-PD1
Monotherapy PDL1+ Lesser tumor burden FIRST LINE SECOND LINE THIRD LINE + R/M= Recurrent/Metastatic

Current Landscape in R/M HNSCC 20 Ezra Cohen, MD Anti-PD1
Monotherapy PDL1+ Lesser tumor burden Anti-PD1 + chemotherapy PDL1 nil or unknown Greater tumor burden FIRST LINE SECOND LINE THIRD LINE + R/M= Recurrent/Metastatic

Current Landscape in R/M HNSCC 21 Ezra Cohen, MD Anti-PD1
Monotherapy PDL1+ Lesser tumor burden Anti-PD1 + chemotherapy PDL1 nil or unknown Greater tumor burden Chemotherapy +/- EGFRi aPD-1 unavailable or not preferred FIRST LINE SECOND LINE THIRD LINE + R/M= Recurrent/Metastatic

Current Landscape in R/M HNSCC 22 Ezra Cohen, MD Anti-PD1
Monotherapy PDL1+ Lesser tumor burden Anti-PD1 + chemotherapy PDL1 nil or unknown Greater tumor burden Chemotherapy +/- EGFRi aPD-1 unavailable or not preferred FIRST LINE SECOND LINE THIRD LINE + Standard
UNDEFINED Dependent on 1st line therapy and performance status aPD1 naïve aPD1 Chemotherapy naïve chemotherapy single or doublet aEGFRi naïve cetuximab +/- chemotherapy R/M= Recurrent/Metastatic
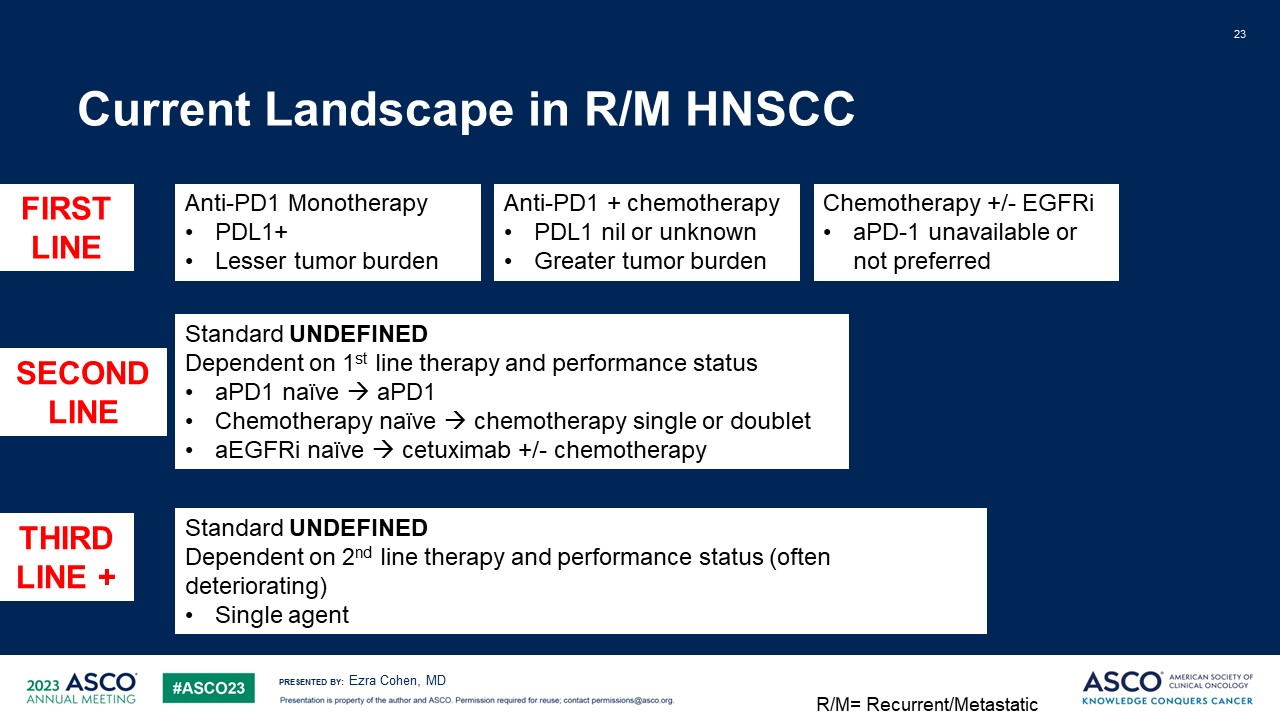
Current Landscape in R/M HNSCC 23 Ezra Cohen, MD Anti-PD1
Monotherapy PDL1+ Lesser tumor burden Anti-PD1 + chemotherapy PDL1 nil or unknown Greater tumor burden Chemotherapy +/- EGFRi aPD-1 unavailable or not preferred FIRST LINE SECOND LINE THIRD LINE + Standard
UNDEFINED Dependent on 1st line therapy and performance status aPD1 naïve aPD1 Chemotherapy naïve chemotherapy single or doublet aEGFRi naïve cetuximab +/- chemotherapy Standard UNDEFINED Dependent on 2nd line therapy and
performance status (often deteriorating) Single agent R/M= Recurrent/Metastatic

Current Landscape in R/M HNSCC 24 Ezra Cohen, MD Anti-PD1
Monotherapy PDL1+ Lesser tumor burden Anti-PD1 + chemotherapy PDL1 nil or unknown Greater tumor burden Chemotherapy +/- EGFRi aPD-1 unavailable or not preferred FIRST LINE SECOND LINE THIRD LINE + Standard
UNDEFINED Dependent on 1st line therapy and performance status aPD1 naïve aPD1 Chemotherapy naïve chemotherapy single or doublet aEGFRi naïve cetuximab +/- chemotherapy Standard UNDEFINED Dependent on 2nd line therapy and
performance status (often deteriorating) Single agent Clinical Trial Molecular Testing R/M= Recurrent/Metastatic
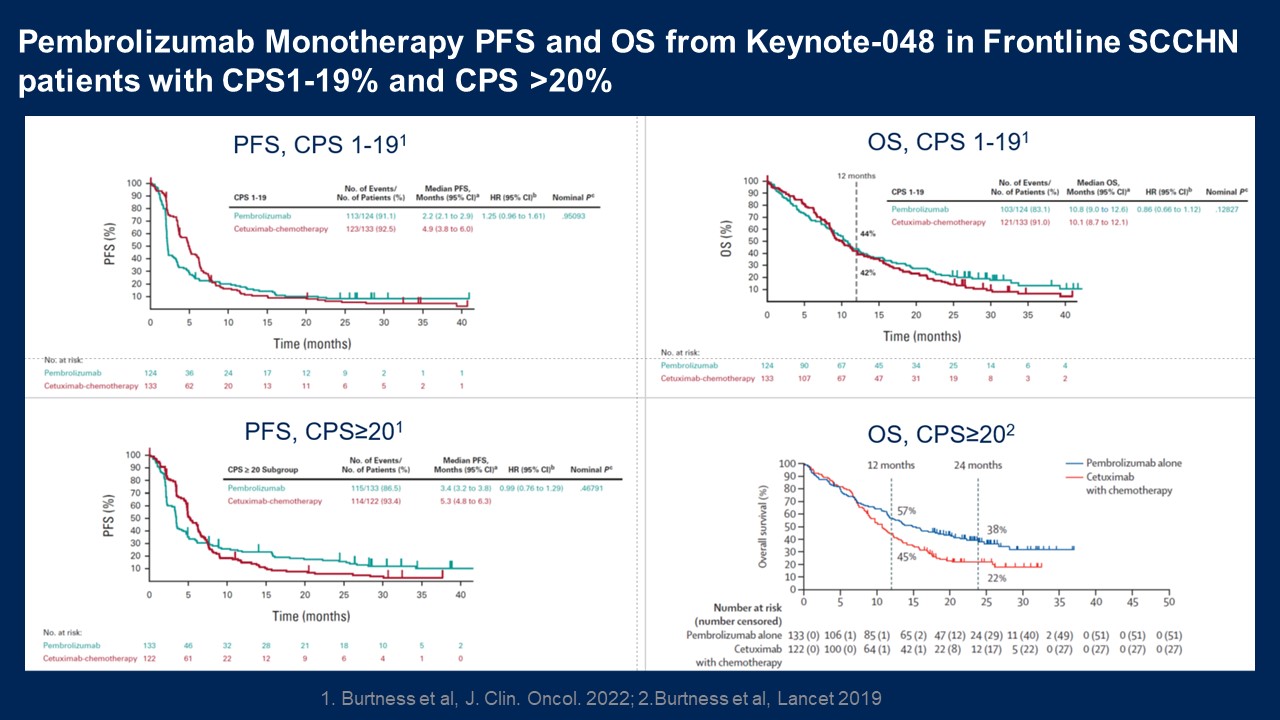
Pembrolizumab Monotherapy PFS and OS from Keynote-048 in Frontline SCCHN
patients with CPS1-19% and CPS >20% PFS, CPS≥201 OS, CPS≥202 OS, CPS 1-191 PFS, CPS 1-191 1. Burtness et al, J. Clin. Oncol. 2022; 2.Burtness et al, Lancet 2019
OS, CPS≥202 OS, CPS 1-191 PFS, CPS 1-191 MARIO-8: Egan + Pembro Potential
IO/IO Chemo Free Regimen in Patients with CPS 1-19% and CPS>20%

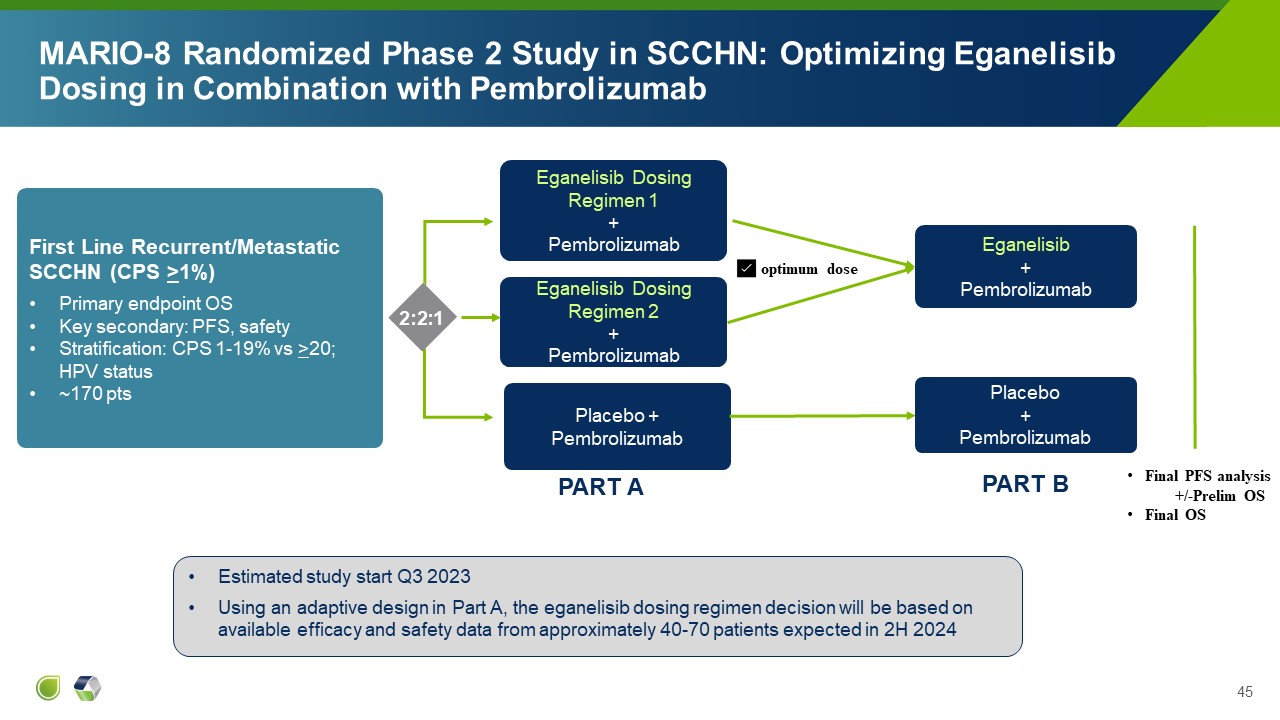
MARIO-8 Randomized Phase 2 Study in SCCHN: Optimizing Eganelisib Dosing in
Combination with Pembrolizumab Eganelisib Dosing Regimen 1 + Pembrolizumab Placebo + Pembrolizumab 2:2:1 Using an adaptive design in Part A, the eganelisib dosing regimen decision will be based on available efficacy and safety data
from approximately 40-70 patients Peripheral blood biomarker and PK data may also influence dose choice First Line Recurrent/Metastatic SCCHN (CPS >1%) Primary endpoint OS Key secondary: PFS, safety Stratification: CPS 1-19% vs
>20; HPV status ~170 pts Eganelisib Dosing Regimen 2 + Pembrolizumab Eganelisib + Pembrolizumab ✅ optimum dose Placebo + Pembrolizumab Final PFS analysis +/-Prelim OS Final OS PART A PART B

MARIO-1: Phase 1 Clinical Study

MARIO-1: Phase 1/1b Study of Eganelisib Alone and in Combination With
Nivolumab in Advanced Solid Tumors (N=224 Patients) Checkpoint inhibitor-naïve Immediate prior checkpoint inhibitor-resistant Checkpoint inhibitor-independent Checkpoint non-responsive
tumors NSCLC Melanoma SCCHN TNBC Mesothelioma Adrenocortical Carcinoma MDSC High Combination Dose Escalation Eganelisib+ Nivolumab Monotherapy Dose Escalation Monotherapy Expansion All Solid Tumors Combination Expansion

Sustained inhibition above the PI3K-g EC90 and below PI3K-d EC50 at Eganelisib
doses up to 40 mg Cycle 2 Day 1 EC90 PI3Kg EC50 PI3Kd Eganelisib concentration (mg/mL) Time (hr) Note: EC50/EC90 from ex-vivo whole blood PD assay PI3Kd:pAKT (S473) in CD19+ B cells PI3Kg:pAKT (T308) in CD14+ Monocytes Error bars
indicate standard deviation

Eganelisib Monotherapy Leads to Immune Activation in Peripheral Blood T Cell
Reinvigoration Day 0 Day 28 Ki67+ of PD1+ memory CD8 (%) log2FC over baseline * N=35 all dose groups combined for monotherapy *p<.05 T-Test IFN-�� Responsive Cytokines CXCL10 Similar results for CXCL9 (not shown) IFN-��
Responsive Genes Genes p-value log2FC at Day 28 GBP5 3.0 x 10-6 1.2 GBP1 1.4 x 10-4 .98 GBP4 3.9 x 10-4 .73 GBP6 5.3 x 10-4 1.2 STAT1 2.3 x 10-3 .58 FCGR1A 2.9 x 10-3 1.1 ICAM1 1.5 x 10-2 .45 IRF1 3.3 x
10-2 .31 TRIM21 4.7 x 10-2 .30 N=18 Concentration (pg/mL) log2FC from baseline Day 0 Day 28 N=35 *

Treatment-related Adverse Events Occurring in at Least 5% of Patients or with
Any Event of Grade 3 or Highera in the Eganelisib Monotherapy Cohort Eganelisib dose escalation (Part A) n (%) Eganelisib dose expansion (Part D: 60 mg)(n=20) n (%) 10–30 mg (n=12) 40 mg (n=4) 60 mg (n=3) Any grade G≥3 Any
grade G≥3 Any grade G≥3b Any grade G≥3b Any treatment-related TEAE 6 (50) - 3 (75) - 2 (67) 2 (67) 14 (70) 8 (40) AST increased 1 (8) - 2 (50) - 1 (33) 1 (33) 9 (45) 6 (30) ALT increased 2 (50) - 1 (33) 1
(33) 8 (40) 6 (30) Pruritus 1 (25) - 4 (20) - Fatigue 1 (8) - 1 (25) - 3 (15) - Rash maculopapular 1 (8) - 1 (25) - 3 (15) 1 (5) Headache 2 (17) - 1 (5) - Blood ALP
increased 1 (33) - 2 (10) 2 (10) Dyspnea 2 (10) 1 (5) Amylase increased 1 (8) - 1 (5) - Lipase increased 1 (8) - 1 (5) - WBC decreased 1 (25) - 1
(5) - Blood bilirubin increased 1 (5) 1 (5) Rash 1 (5) 1 (5) Hypercalcemia 1 (33) 1 (33) aAll events were grade 3 except for grade 4 increases in ALT and bilirubin that both
occurred in the same patient. bNo grade ≥3 events occurred during the DLT observation period (first treatment cycle). Eganelisib Monotherapy Well Tolerated with No Grade ≥3 Treatment Related Adverse Events up Through 40 mg Dose
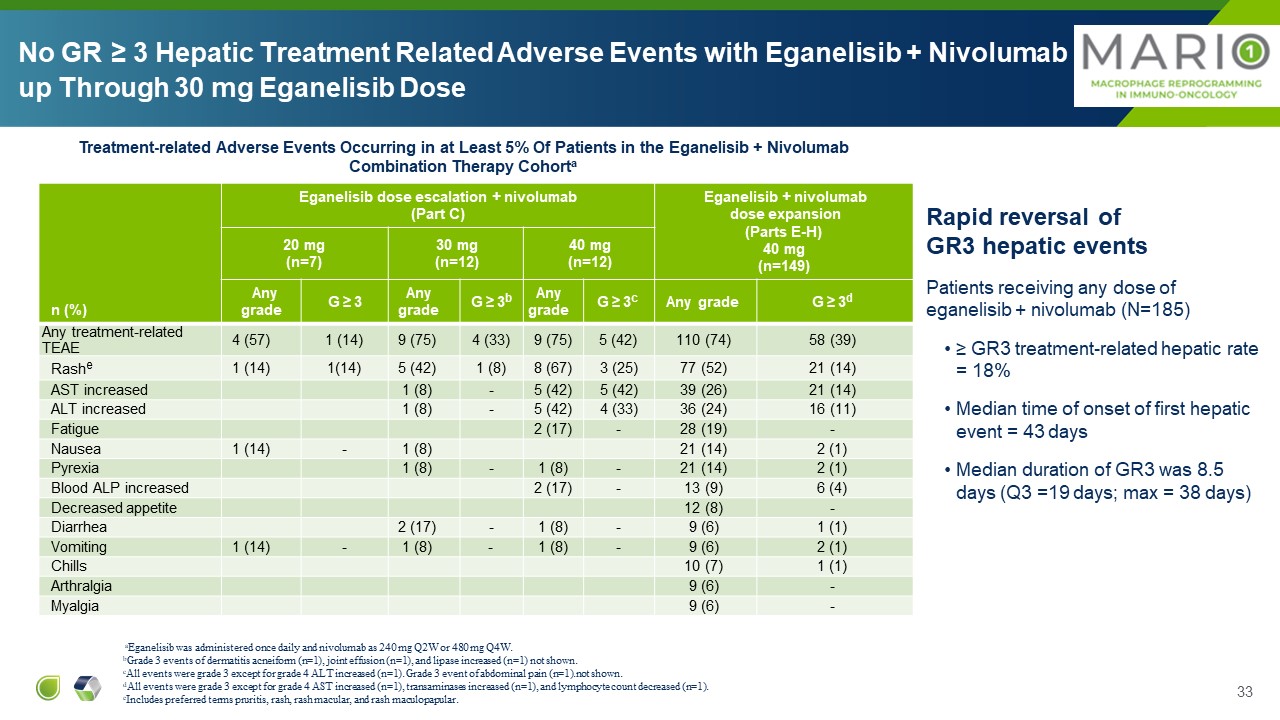
n (%) Eganelisib dose escalation + nivolumab (Part C) Eganelisib +
nivolumab dose expansion (Parts E-H) 40 mg (n=149) 20 mg (n=7) 30 mg (n=12) 40 mg (n=12) Any grade G ≥ 3 Any grade G ≥ 3b Any grade G ≥ 3c Any grade G ≥ 3d Any treatment-related TEAE 4 (57) 1 (14) 9 (75) 4 (33) 9
(75) 5 (42) 110 (74) 58 (39) Rashe 1 (14) 1(14) 5 (42) 1 (8) 8 (67) 3 (25) 77 (52) 21 (14) AST increased 1 (8) - 5 (42) 5 (42) 39 (26) 21 (14) ALT increased 1 (8) - 5 (42) 4 (33) 36 (24) 16 (11) Fatigue 2
(17) - 28 (19) - Nausea 1 (14) - 1 (8) 21 (14) 2 (1) Pyrexia 1 (8) - 1 (8) - 21 (14) 2 (1) Blood ALP increased 2 (17) - 13 (9) 6 (4) Decreased appetite 12 (8) - Diarrhea 2 (17) - 1 (8) - 9 (6) 1
(1) Vomiting 1 (14) - 1 (8) - 1 (8) - 9 (6) 2 (1) Chills 10 (7) 1 (1) Arthralgia 9 (6) - Myalgia 9 (6) - aEganelisib was administered once daily and nivolumab as 240 mg Q2W or 480 mg Q4W. bGrade 3 events of dermatitis
acneiform (n=1), joint effusion (n=1), and lipase increased (n=1) not shown. cAll events were grade 3 except for grade 4 ALT increased (n=1). Grade 3 event of abdominal pain (n=1).not shown. dAll events were grade 3 except for grade 4 AST
increased (n=1), transaminases increased (n=1), and lymphocyte count decreased (n=1).eIncludes preferred terms pruritis, rash, rash macular, and rash maculopapular. Treatment-related Adverse Events Occurring in at Least 5% Of Patients in
the Eganelisib + Nivolumab Combination Therapy Cohorta Rapid reversal of GR3 hepatic events Patients receiving any dose of eganelisib + nivolumab (N=185) ≥ GR3 treatment-related hepatic rate = 18% Median time of onset of first
hepatic event = 43 days Median duration of GR3 was 8.5 days (Q3 =19 days; max = 38 days) No GR ≥ 3 Hepatic Treatment Related Adverse Events with Eganelisib + Nivolumab up Through 30 mg Eganelisib Dose

MARIO-275Addition of Eganelisib to Standard of Care Opdivoin I/O Naïve
Urothelial Cancer Patients

FDA Fast-Track Designation Eganelisib 40/30mg QD** + Nivolumab 480 mg
Q4W Placebo + Nivolumab 480 mg Q4W Advanced Platinum Refractory 2nd Line Urothelial Cancer Patients MDSC all comers (pre-specified and stratified) PD-L1* status all comers (pre-specified) Primary objective: ORR in MDSC
High Secondary objectives: DOR, PFS, OS, ORR in Total population + MDSC subset MARIO-275: Addition of Eganelisib to Standard of Care Nivolumab in I/O Naïve Urothelial Cancer Patients, Including PD-L1(-) Patients Cross-over R PD DOR,
duration of response; MDSC, myeloid-derived suppressor cells; ORR, overall response rate; OS, overall survival; PD-L1, programmed death-ligand 1; PFS, progression-free survival; Q4W, once every four weeks; QD, once a day *PD-L1 expression
measured in baseline/archival tumor biopsies with Dako PD-L1 immunohistochemical 28-8 pharmDx kit approved for nivolumab in UC, except 2 biopsies tested with 22C3 PD-L1 antibody prior to study (Tumor Proportion Score ≥1% cutoff for
PD-L1(+)) **Infinity voluntarily paused enrollment in May 2020 and implemented a dose reduction of eganelisib from 40mg QD to 30mg QD to address reversible liver enzyme elevations. 2:1 randomization

log2FC from baseline Concentration (pg/mL) Eganelisib + Nivolumab Placebo +
Nivolumab (n=28) (n=12) Day 0 Day 15 Day 0 Day 15 * Increased Immune Activation with Eganelisib + Nivolumab vs Nivolumab Alone in Peripheral Blood * NS *p<.05 Two-Way ANOVA Eganelisib + Nivolumab Placebo +
Nivolumab (n=20) (n=9) Day 0 Day 15 Day 0 Day 15 Ki67+ PD1+ memory CD8 (cells/mL) log2FC over baseline * * * IFN-�� Responsive Cytokines CXCL10 T Cell Reinvigoration Similar results for CXCL9 (not shown)

TEAEs Leading to Treatment Discontinuation of Eganelisib/Placebo and/or
Nivolumab Hepatic TEAEs Non-Hepatic TEAEs Preferred Term (PT) Egan + Nivo N=33, n (%) Placebo + Nivo N=16 , n (%) Total N=49, n (%) Patients with >=1 non-hepatic TEAE 5 (15.2) 2 (12.5) 7 (14.3) Asthenia 2 (6.1) 0 2
(4.1) Amylase increased 1 (3.0) 0 1 (2.0) Lipase increased 1 (3.0) 0 1 (2.0) Cardiac failure chronic 1 (3.0) 0 1 (2.0) Diarrhoea 1 (3.0) 0 1 (2.0) Decreased appetite 1 (3.0) 0 1 (2.0) Hyperglycaemia 0 1 (6.3) 1
(2.0) Ketoacidosis 0 1 (6.3) 1 (2.0) Pemphigoid 0 1 (6.3) 1 (2.0) Preferred Term (PT) Egan + Nivo N=33, n (%) Placebo + Nivo N=16 , n (%) Total N=49, n (%) Patients with >=1 hepatic TEAE 7 (21.2) 0 7
(14.3) Alanine aminotransferase increased 2 (6.1) 0 2 (4.1) Aspartate aminotransferase increased 1 (3.0) 0 1 (2.0) Hypertransaminasaemia 2 (6.1) 0 2 (4.1) Hepatotoxicity 2 (6.1) 0 2 (4.1) Hepatic cytolysis 1 (3.0) 0 1
(2.0) Hepatic TEAEs No Hy’s Law No grade 5 hepatic TEAE All hepatic Grade ≥3 TEAEs resolved in the combination arm except 2 One patient had grade 3 hepatotoxicity and subsequently died due to disease progression One patient had grade
3 non-treatment -related ALP increased after treatment discontinuation for disease progression. Mitigation: Dose reduced to 30 mg for MARIO-275 (same dose as used for MARIO-3 (combo with atezo/nab-pac)) Increased, earlier LFT monitoring
to allow earlier intervention Database lock 15 Nov 2021
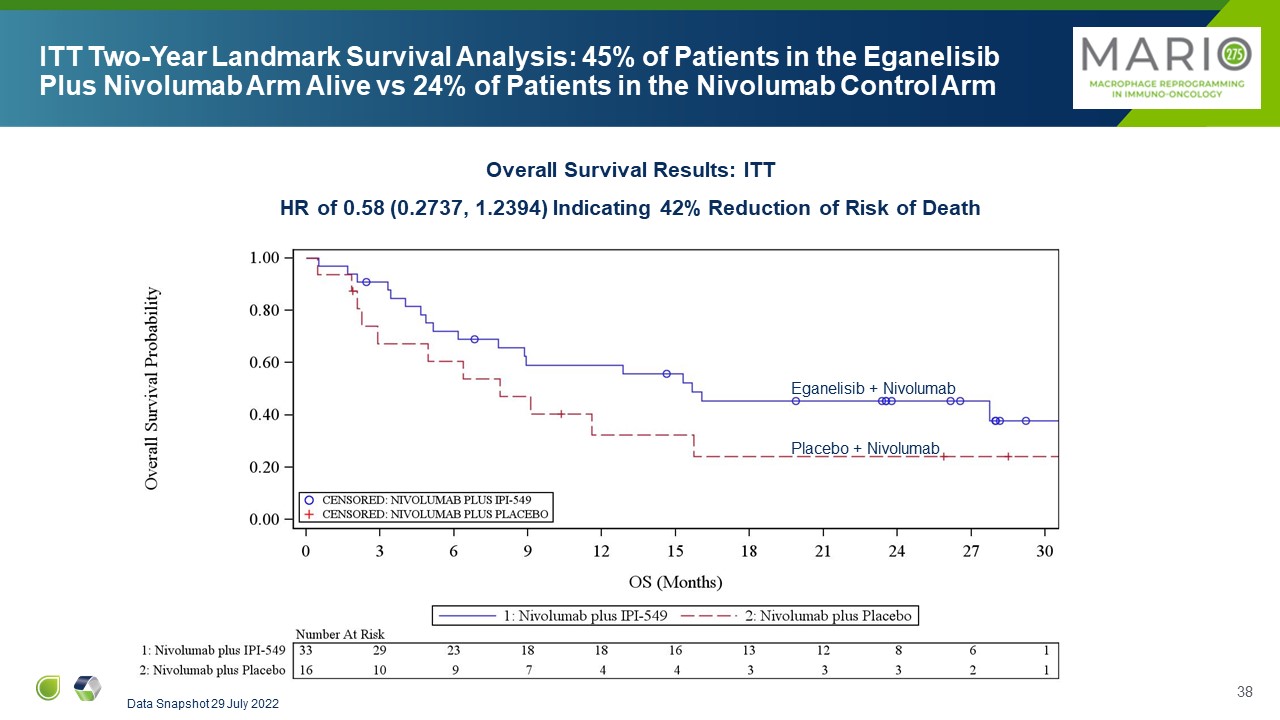
ITT Two-Year Landmark Survival Analysis: 45% of Patients in the Eganelisib
Plus Nivolumab Arm Alive vs 24% of Patients in the Nivolumab Control Arm Overall Survival Results: ITT HR of 0.58 (0.2737, 1.2394) Indicating 42% Reduction of Risk of Death Data Snapshot 29 July 2022 Eganelisib + Nivolumab Placebo +
Nivolumab
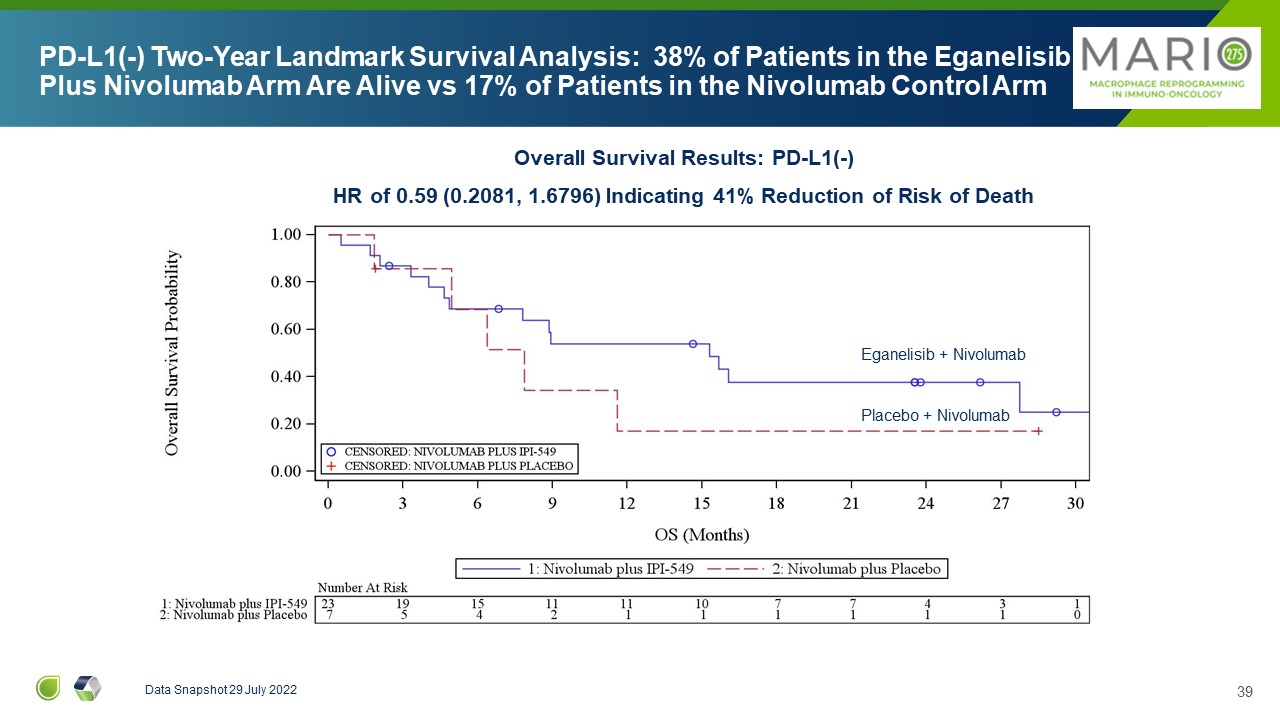
PD-L1(-) Two-Year Landmark Survival Analysis: 38% of Patients in the
Eganelisib Plus Nivolumab Arm Are Alive vs 17% of Patients in the Nivolumab Control Arm Data Snapshot 29 July 2022 Eganelisib + Nivolumab Placebo + Nivolumab Overall Survival Results: PD-L1(-) HR of 0.59 (0.2081, 1.6796) Indicating
41% Reduction of Risk of Death

SCCHN Clinical Data (MARIO-1) and Key Elements of the Planned Randomized Trial
of Eganelisib + Pembro vs Pembro as First line treatment for Relapsed/metastatic SCCHN (MARIO-8)

1st Line Chemo “X” 2nd Line Chemo “Y” 3rd Line Immediate Prior Checkpoint
Inhibitor IPI-549 + Checkpoint Treatment Stable Disease Partial Response Progressive Disease Eganelisib Response MARIO-1 Study Start PD Progressive Disease PD PD PD Tumor Burden Study Concept: Design examines the activity of
eganelisib in patients not expected to respond to checkpoint inhibitor due to immediate prior therapeutic failure MARIO-1: Eganelisib + Nivolumab Combination in Patients Having Progressed on Immediate Prior CPI Therapy

Patient A: stage IV disease at study entry Refractory to pembrolizumab after
15 months (best response PR) 63% tumor reduction PFS: 11 months Patient B: stage IV disease at study entry Refractory to pembrolizumab after 5 months (best response SD) 36% tumor reduction PFS: 7 months Start of MARIO-1 Therapy
After Progression on Immediately Prior CPI Start of MARIO-1 therapy After Progression on Immediately Prior CPI Overcoming Resistance to CPI in MARIO-1 SCCHN Patients Who Progressed on Immediate Prior Checkpoint Inhibitor Therapy Cohen et
al, SITC 2020 Data Snapshot 30 November 2020 Individual patient results may not be representative of overall trial results
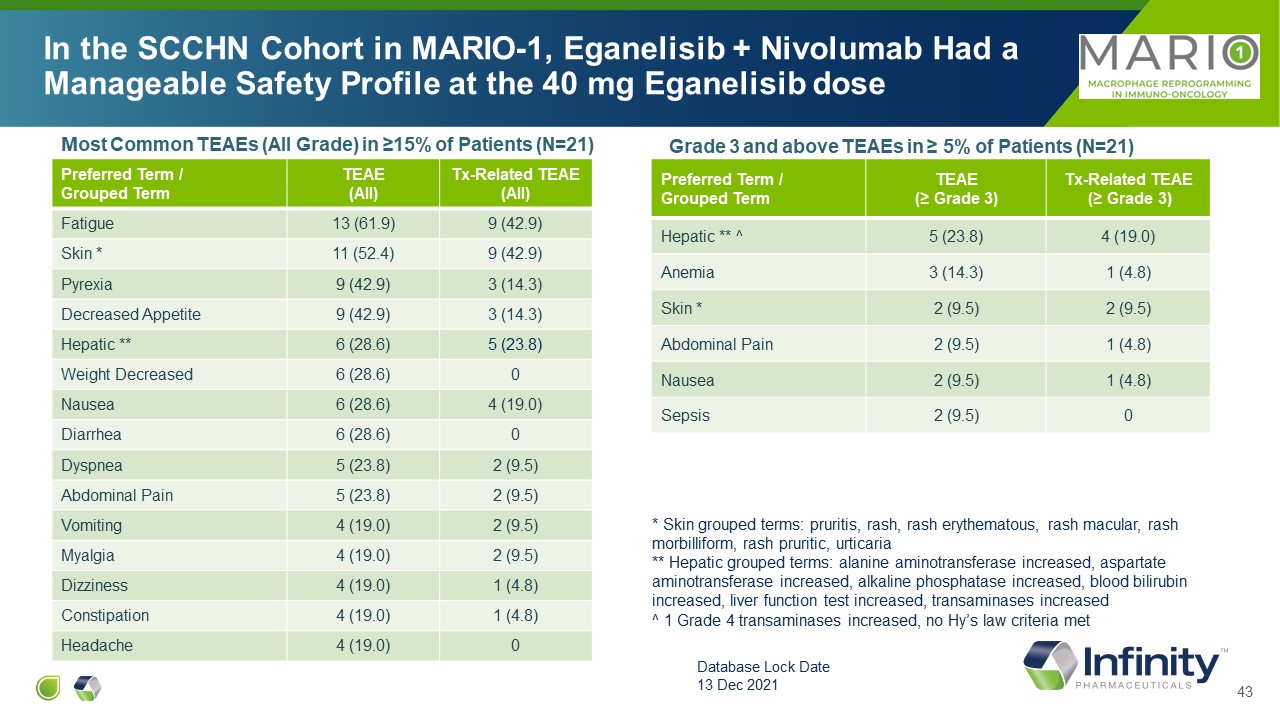
Most Common TEAEs (All Grade) in ≥15% of Patients (N=21) Preferred Term /
Grouped Term TEAE (All) Tx-Related TEAE (All) Fatigue 13 (61.9) 9 (42.9) Skin * 11 (52.4) 9 (42.9) Pyrexia 9 (42.9) 3 (14.3) Decreased Appetite 9 (42.9) 3 (14.3) Hepatic ** 6 (28.6) 5 (23.8) Weight Decreased 6
(28.6) 0 Nausea 6 (28.6) 4 (19.0) Diarrhea 6 (28.6) 0 Dyspnea 5 (23.8) 2 (9.5) Abdominal Pain 5 (23.8) 2 (9.5) Vomiting 4 (19.0) 2 (9.5) Myalgia 4 (19.0) 2 (9.5) Dizziness 4 (19.0) 1 (4.8) Constipation 4 (19.0) 1
(4.8) Headache 4 (19.0) 0 Preferred Term / Grouped Term TEAE (≥ Grade 3) Tx-Related TEAE (≥ Grade 3) Hepatic ** ^ 5 (23.8) 4 (19.0) Anemia 3 (14.3) 1 (4.8) Skin * 2 (9.5) 2 (9.5) Abdominal Pain 2 (9.5) 1
(4.8) Nausea 2 (9.5) 1 (4.8) Sepsis 2 (9.5) 0 Grade 3 and above TEAEs in ≥ 5% of Patients (N=21) In the SCCHN Cohort in MARIO-1, Eganelisib + Nivolumab Had a Manageable Safety Profile at the 40 mg Eganelisib dose Database Lock
Date 13 Dec 2021 * Skin grouped terms: pruritis, rash, rash erythematous, rash macular, rash morbilliform, rash pruritic, urticaria ** Hepatic grouped terms: alanine aminotransferase increased, aspartate aminotransferase increased,
alkaline phosphatase increased, blood bilirubin increased, liver function test increased, transaminases increased ^ 1 Grade 4 transaminases increased, no Hy’s law criteria met

Total N = 21 ≤ 2 Prior Lines N = 11 ≥ 3 Prior Lines N = 10 Best Overall
Response Partial Response (PR), n Stable Disease (SD), n Progressive Disease (PD), n Not evaluable, n 2 7 10 2 2 2 5 2 0 5 5 0 Overall Response Rate (ORR) (PR), n (%) 2 (9.5) 2 (18.2) 0
(0) Disease Control Rate (DCR) (PR + SD), n (%) 9 (42.9) 4 (36.4) 5 (50.0) Progression Free Survival (PFS in Months), Median (95%) 3.7 (1.9, 5.5) 5.3 (1.9, 11.1) 3.6 (0.5, 4.5) Best % Change in Target Lesion Size from
Baseline Encouraging PFS in Heavily Pretreated SCCHN Patients Who Had Progressed on Immediate Prior CPI Therapy MARIO-1 SCCHN Cohort 2 or fewer prior therapies 3 or more prior therapies Cohen et al, SITC 2020 Data Snapshot 30 November
2020 Keynote-048 (Burtness et al Lancet 2019) mPFS for pembro monotherapy in recurrent/metastatic pts = 2.3 months mPFS for pembro monotherapy in recurrent/metastatic pts with CPS ≥ 1 = 3.2 months MARIO-1 Database lock 13 Dec 2021
MARIO-8 Randomized Phase 2 Study in SCCHN: Optimizing Eganelisib Dosing in
Combination with Pembrolizumab Eganelisib Dosing Regimen 1 + Pembrolizumab Placebo + Pembrolizumab 2:2:1 Estimated study start Q3 2023 Using an adaptive design in Part A, the eganelisib dosing regimen decision will be based on
available efficacy and safety data from approximately 40-70 patients expected in 2H 2024 First Line Recurrent/Metastatic SCCHN (CPS >1%) Primary endpoint OS Key secondary: PFS, safety Stratification: CPS 1-19% vs >20; HPV
status ~170 pts Eganelisib Dosing Regimen 2 + Pembrolizumab Eganelisib + Pembrolizumab ✅ optimum dose Placebo + Pembrolizumab Final PFS analysis +/-Prelim OS Final OS PART A PART B

Summary Encouraging data from heavily pretreated patients with
advanced/metastatic head & neck squamous cell cancer, whose tumors had progressed on immediate prior ICI treatment, supports further development of eganelisib in this tumor type Data supports potentially greater activity in earlier
lines of treatment: first line recurrent/metastatic SCCHN in combination with pembrolizumab FDA feedback has been received and Infinity plans to move forward with a randomized Phase 2 study in this indication that includes eganelisib
dosing optimization* Estimated Study start in Q3 2023, with dosing decision expected in 2H 2024 46 *Subject to submission of the final study protocol to the FDA and responses to FDA comments.

Q&A Participants 47 Ezra Cohen, MD, FRCPSC, FASCO Chief Medical
Officer, Oncology, Tempus Most recently, Chief, Division of Hematology-Oncology, and Associate Director of Clinical Science at UC San Diego Moores Cancer Center Robert Ilaria, Jr, MD Chief Medical Officer, Infinity Pharmaceuticals
Previously, BMS and Celgene, with leading roles on the CTLA-4 and PD-1 drug development teams Nick Abbott, PhD Principal, Abbott Biotech Consultancy Most recently, Senior Analyst Equity Research, Wells Fargo Corporate and
Investment Banking

Q & A

Voruciclib: Oral CDK9 Inhibitor

Scientific Rationale & Mechanism of Action
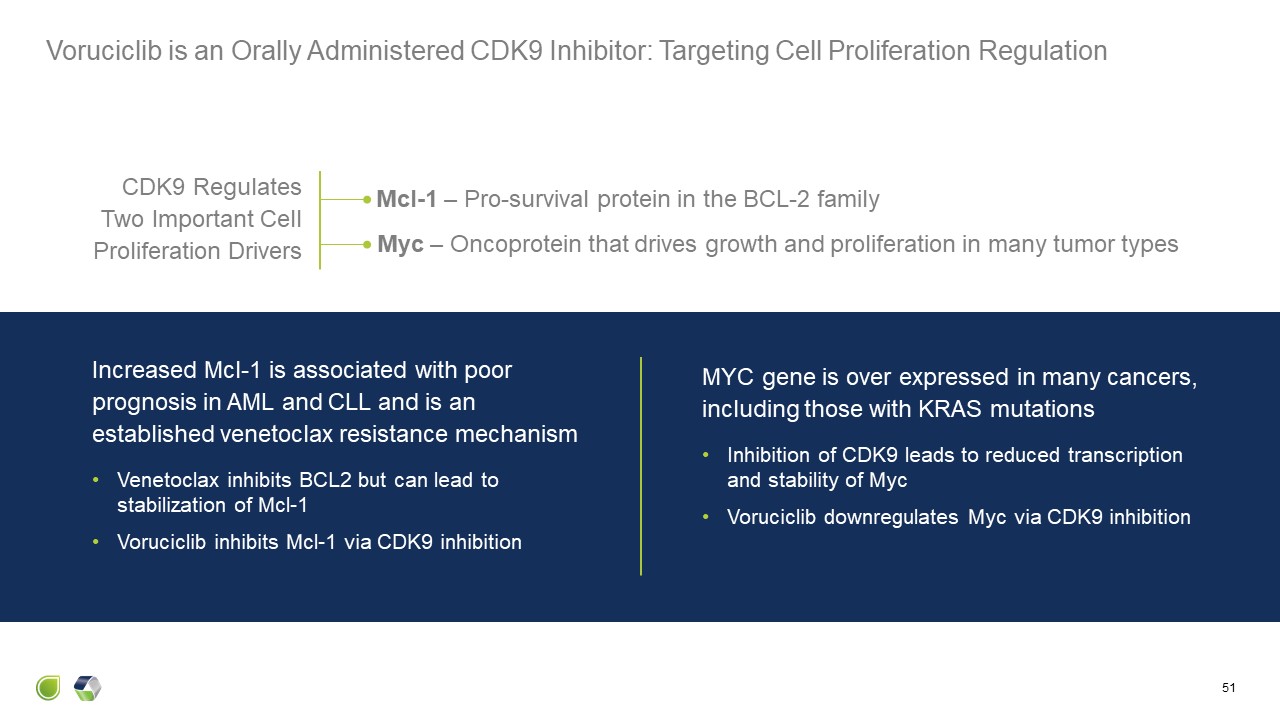
Voruciclib is an Orally Administered CDK9 Inhibitor: Targeting Cell
Proliferation Regulation 51 Increased Mcl-1 is associated with poor prognosis in AML and CLL and is an established venetoclax resistance mechanism Venetoclax inhibits BCL2 but can lead to stabilization of Mcl-1 Voruciclib inhibits Mcl-1
via CDK9 inhibition CDK9 Regulates Two Important Cell Proliferation Drivers MYC gene is over expressed in many cancers, including those with KRAS mutations Inhibition of CDK9 leads to reduced transcription and stability of
Myc Voruciclib downregulates Myc via CDK9 inhibition Myc – Oncoprotein that drives growth and proliferation in many tumor types Mcl-1 – Pro-survival protein in the BCL-2 family

Voruciclib is an Oral, Selective and Specific CDK9 Inhibitor Cyclin dependent
kinases (CDK) bind with cyclins to regulate the cell cycle and transcription Voruciclib inhibits CDK9 Higher specificity and longer residence time on target vs CDK 4, 6 &1 Greater selectivity against CDKs relative to other
kinases 52

Preclinical Studies Show Synergy with Venetoclax
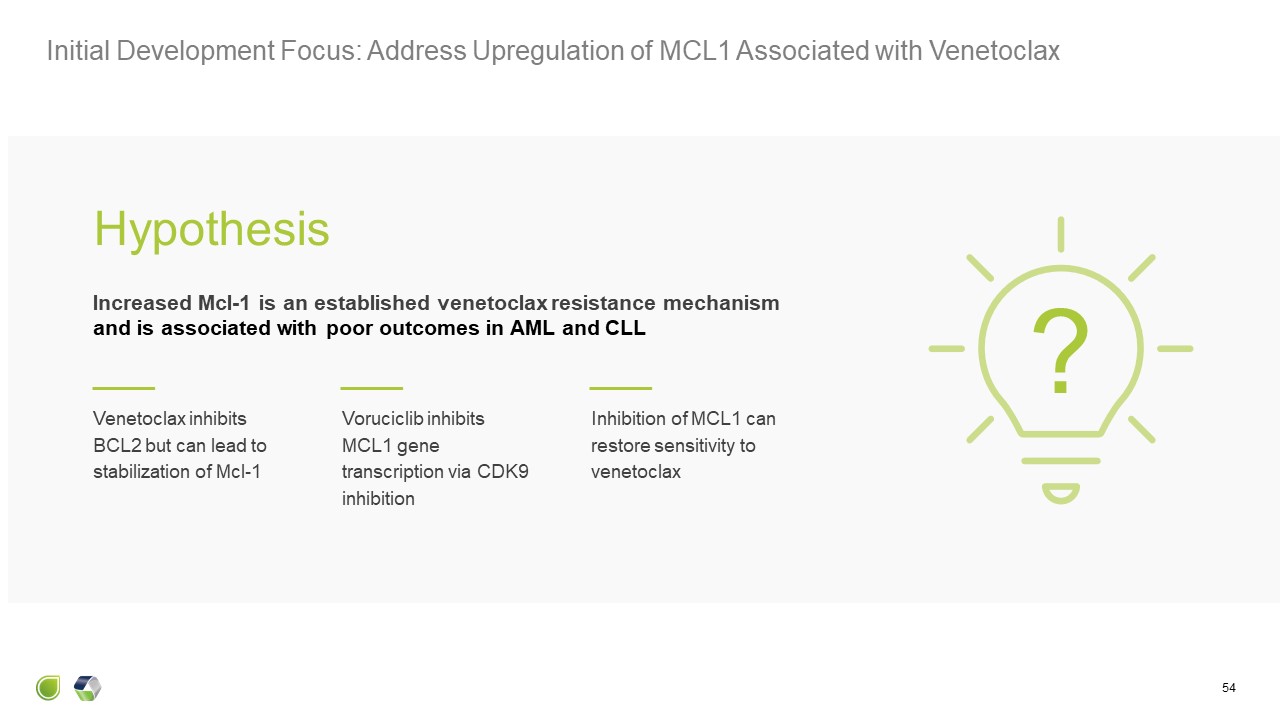
Initial Development Focus: Address Upregulation of MCL1 Associated with
Venetoclax 54 Increased Mcl-1 is an established venetoclax resistance mechanismand is associated with poor outcomes in AML and CLL Hypothesis ? Venetoclax inhibits BCL2 but can lead to stabilization of Mcl-1 Voruciclib inhibits MCL1
gene transcription via CDK9 inhibition Inhibition of MCL1 can restore sensitivity to venetoclax

Voruciclib Synergizes with Venetoclax in AML Murine Xenograft
Model 55 Luedtke, et al. Signal Transduct Ther (2020) Suppresses Mcl-1 Level Extends Survival in MV4-11 Tumor Increases Apoptosis VOR (nM) VEN
(nM) 0 0 0 12.5 0 25 0 50 500 0 1000 0 2000 0 1000 12.5 1000 25 2000 12.5 2000 25 VEN VOR VOR + VEN a. b. c.

Voruciclib Synergizes with Venetoclax in Multiple Models, Including High Risk
DLBCL Murine Xenograft Models 56 Dey et al. Nature Sci Rep 2017 Suppresses Mcl-1 Level Inhibits Tumor Growth a. b.

Clinical Studies in Solid Tumors

58 68 PTS ENROLLED IN 2 DOSE ESCALATION/EXPANSION STUDIES EVALUATING 2 DOSING
SCHEDULES SAFETY Maximum Tolerated Dose (MTD) 600 mg on intermittent dosing of 14 days on/7 days off 350 mg on continuous daily dosing Most common adverse events (AE) involved the gastrointestinal tract No neutropenia No pulmonary
toxicity No effect on QTc EFFICACY Intermittent dosing: 1 patient with partial response and 8 with stable diseases lasting 2 to 6 months Daily dosing: 12 patients with stable disease lasting a median of 15 weeks Key Findings in 2
Monotherapy Phase 1 Studies in Solid Tumors MEIP Anticipates Therapeutic Voruciclib Dose in Combination with Venetoclax is 100-200 mg Intermittently Solid tumor studies by prior sponsor (Piramal) as a CDK 4-6 inhibitor

Decreased c-MYC Expression Observed in Phase 1 Study in Solid Tumors 59 10
gene biomarkers evaluated in blood in daily dosing study c-MYC expression decreased in ~60% patients tested (n=25)

Current Clinical Study in Hematologic Malignancies

Voruciclib Pivot to Hematologic Malignancies Based on Clear Scientific
Evidence and Medical Need Rationale Recognition that voruciclib is primarily a CDK9 inhibitor Evidence of effect in CLL patient samples and synergy with venetoclax in preclinical models Goal is to overcome the most common mechanism of
resistance to venetoclax Focus on diseases where venetoclax is approved and clear medical need identified Acute Myeloid Leukemia (AML) Chronic Lymphocytic Leukemia (CLL) 61

62 Ongoing Phase 1 Study of Voruciclib Alone and in Combination with
Venetoclax in AML and B-cell Malignancies Voruciclib monotherapy dose escalation in AML and B-cell Malignancies Completed (N = 40) Voruciclib + Venetoclaxdose escalation in AML Enrolling V2 Study population Relapsed/Refractory B-cell
malignancies Relapsed/Refractory AML Dose escalation with standard 3+3 design Single agent In combination with venetoclax 100 mg 150 mg 200 mg 50 mg 50 mg 100 mg 150 mg 50 qod 200 mg Endpoints Safety and
tolerability Pharmacokinetics Biologic correlative studies BH3 profiling, MCL-1 expression Molecular mutations analysis Preliminary efficacy
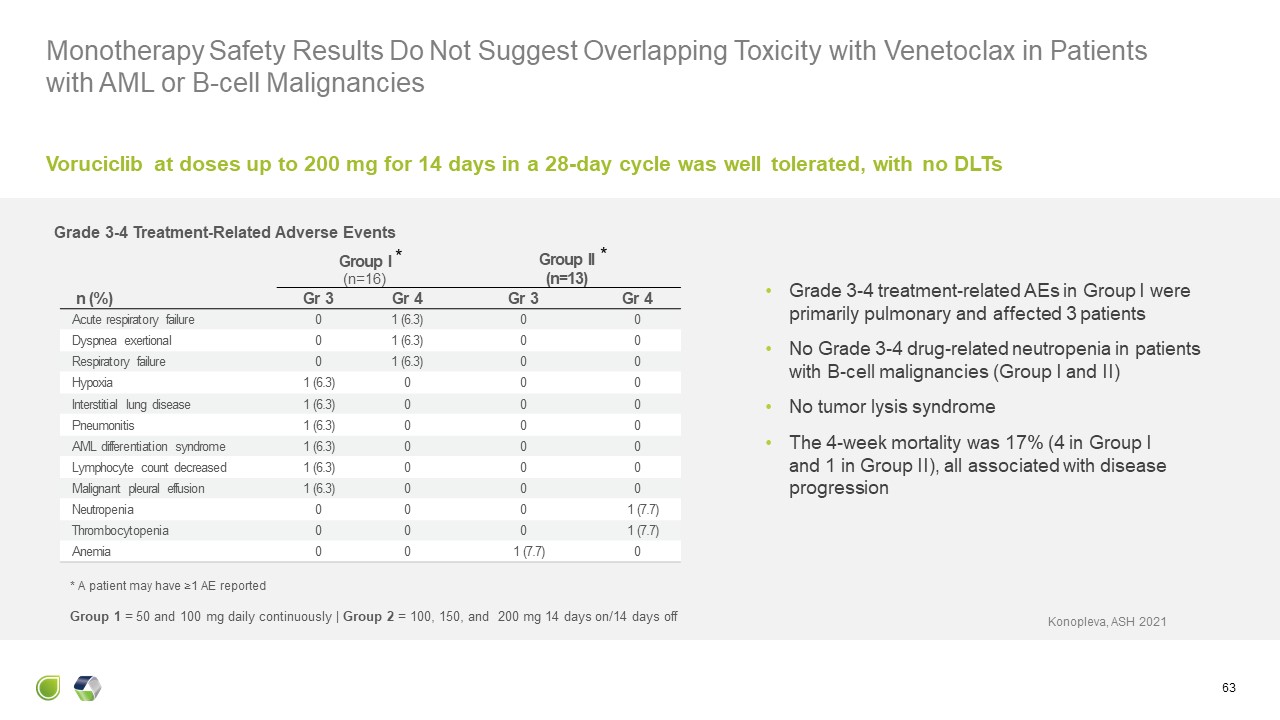
Monotherapy Safety Results Do Not Suggest Overlapping Toxicity with Venetoclax
in Patients with AML or B-cell Malignancies 63 Group I(n=16) Group II(n=13) n (%) Gr 3 Gr 4 Gr 3 Gr 4 Acute respiratory failure 0 1 (6.3) 0 0 Dyspnea exertional 0 1 (6.3) 0 0 Respiratory failure 0 1
(6.3) 0 0 Hypoxia 1 (6.3) 0 0 0 Interstitial lung disease 1 (6.3) 0 0 0 Pneumonitis 1 (6.3) 0 0 0 AML differentiation syndrome 1 (6.3) 0 0 0 Lymphocyte count decreased 1 (6.3) 0 0 0 Malignant pleural effusion 1
(6.3) 0 0 0 Neutropenia 0 0 0 1 (7.7) Thrombocytopenia 0 0 0 1 (7.7) Anemia 0 0 1 (7.7) 0 Grade 3-4 Treatment-Related Adverse Events Grade 3-4 treatment-related AEs in Group I were primarily pulmonary and affected 3
patients No Grade 3-4 drug-related neutropenia in patients with B-cell malignancies (Group I and II) No tumor lysis syndrome The 4-week mortality was 17% (4 in Group I and 1 in Group II), all associated with disease progression * A
patient may have ≥1 AE reported Voruciclib at doses up to 200 mg for 14 days in a 28-day cycle was well tolerated, with no DLTs Konopleva, ASH 2021 Group 1 = 50 and 100 mg daily continuously | Group 2 = 100, 150, and 200 mg 14 days on/14
days off * *

Key Findings From Monotherapy Ph 1 Study in Hematologic Malignancies (N = 40
pts) Safety/Tolerability Dose limiting toxicity (DLT) of respiratory failure at 100 mg daily in 2 pts with AML Confounded by prior allogeneic transplant and AML differentiation syndrome No DLTs on intermittent dosing at 100, 150 and 200
mg Dose escalation stopped without reaching maximum tolerated dose (MTD) 150 - 200 mg expected to inhibit CDK9 based on preclinical studies Konopleva et al, ASH 2021 64 Clinical Activity Evidence of single agent antitumor activity 1
patient with follicular lymphoma achieved a near partial response (46% reduction in tumor size) lasting 6 months 1 patient with diffuse large B-cell lymphoma had stable disease lasting 4 months 1 patient with AML achieved a Morphology
Leukemia Free State Disease Control Rate = 50% in 24 patients administered voruciclib on 14 days on/14 days off schedule

Ongoing Evaluation of Voruciclib + Venetoclax in Relapsed/Refractor (R/R) AML
Demonstrates Encouraging Signs of Clinical Activity at Low Dose Dose escalation began from a very low dose of 50 mg every-other-day Requested by FDA due to the introduction of a new formulation Encouraging results in 2 patients in 6
patient cohort at 50 mg every-other-day Partial remission after 1 cycle in a patient who had received 4 prior therapies including standard induction chemotherapy, stem cell transplant and venetoclax-azacitidine Decreased transfusion
requirement in 1 patient No new safety findings compared to single-agent results Enrollment ongoing at higher dose levels 65

Clinical Development Plan

Acute Myeloid Leukemia Venetoclax in combination with a hypomethylating agent
(e.g. azacitidine) or low-dose cytarabine is standard of care in unfit patients with previously untreated AML Median overall survival of 15 months with venetoclax-azacitidine1 indicates further improvement is needed Voruciclib in
combination with venetoclax-azacitidine may improve response rate and overall survival and represents a significant medical need An additional medical need is in patients with AML after failure of standard therapies Median overall
survival of <6 months with current approaches indicates further improvement needed Voruciclib Clinical Development Strategy 67 1. DiNardo, NEJM 2020 Chronic Lymphocytic Leukemia Venetoclax-rituximab is an approved combination for
the treatment of relapsed CLL Voruciclib in combination with venetoclax-rituximab may improve response rate and Progression Free Survival (PFS) and represents a medical need in this disease

Voruciclib Summary Oral CDK9 inhibitor: Pre-clinical data demonstrate down
regulation of Mcl-1 and synergy with venetoclax in multiple hematologic malignancy models Increased Mcl-1 is clinically established as a venetoclax resistance mechanism Early clinical data demonstrates encouraging initial tolerability and
activity No overlapping toxicity with venetoclax predicted and no significant myelosuppression observed as monotherapy The ongoing Phase 1b trial is expected to report initial results from the combination regimen around the end of
2023 Proof of principle of combination will support voruciclib value in combination where venetoclax is standard of care 68

ME-344: Mitochondrial Inhibitor

Scientific Rationale & Mechanism of Action

ME-344: A Potential Novel Mechanism of Action to Address Multiple Cancers in
Combination with Anti-angiogenic Therapies Like Avastin® ME-344 blocks the production of adenosine triphosphate (ATP), a source of cellular energy, by inhibiting the OXPHOS pathway. Anti-angiogenic therapies, like Avastin, result in
reducing glycolysis, another source of energy for cells Cancer cells need significant amounts of energy to grow, and can switch between mitochondria and glycolytic metabolic pathways to escape the blocking of either energy source The
potential to inhibit both mitochondrial energy production via ME-344 and glycolytic energy production via VEGF inhibition (e.g., Avastin) is intended to result in synthetic lethality of cancer cells 71 3 Energy for cancer cells
growth 2 Glycolytic Energy 1 Mitochondrial Energy 4 Tumor cell proliferation and growth ME-344 Avastin X X

Non-Clinical Studies
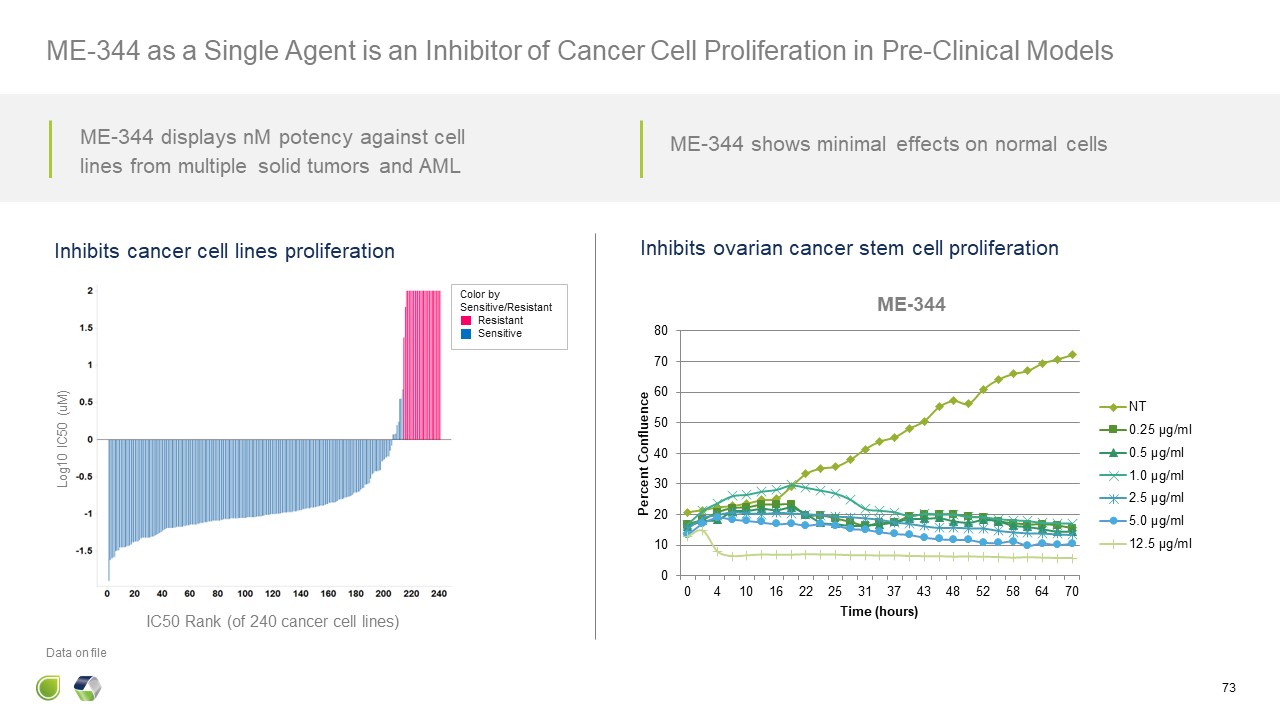
Log10 IC50 (uM) IC50 Rank (of 240 cancer cell lines) Inhibits cancer cell
lines proliferation Inhibits ovarian cancer stem cell proliferation 73 ME-344 as a Single Agent is an Inhibitor of Cancer Cell Proliferation in Pre-Clinical Models Color by Sensitive/Resistant Resistant Sensitive Data on
file ME-344 displays nM potency against cell lines from multiple solid tumors and AML ME-344 shows minimal effects on normal cells

Decreases Tumor Volume Data on file ME-344 + REGORAFENIB DECREASES MEAN
TUMOR VOLUMES AND EXTENDS SURVIVAL Improves Survival 74 ME-344 Synergizes with Anti-angiogenic TKI to Enhance Antitumor Effect in Colorectal Cancer Xenograft Model

Clinical Studies in Solid Tumors

ME-344 Initial Studies in Solid Tumors 76
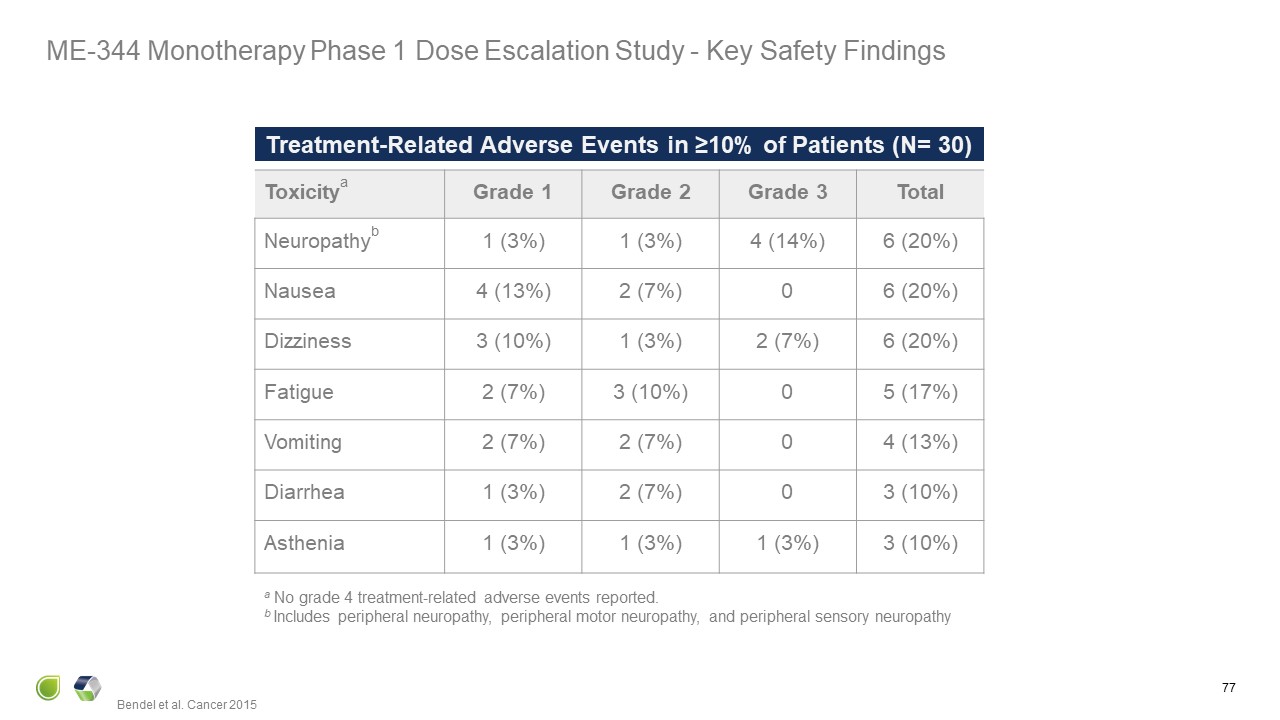
Treatment-Related Adverse Events in ≥10% of Patients (N= 30) Toxicitya Grade
1 Grade 2 Grade 3 Total Neuropathyb 1 (3%) 1 (3%) 4 (14%) 6 (20%) Nausea 4 (13%) 2 (7%) 0 6 (20%) Dizziness 3 (10%) 1 (3%) 2 (7%) 6 (20%) Fatigue 2 (7%) 3 (10%) 0 5 (17%) Vomiting 2 (7%) 2 (7%) 0 4
(13%) Diarrhea 1 (3%) 2 (7%) 0 3 (10%) Asthenia 1 (3%) 1 (3%) 1 (3%) 3 (10%) a No grade 4 treatment-related adverse events reported. b Includes peripheral neuropathy, peripheral motor neuropathy, and peripheral sensory
neuropathy Bendel et al. Cancer 2015 77 ME-344 Monotherapy Phase 1 Dose Escalation Study - Key Safety Findings
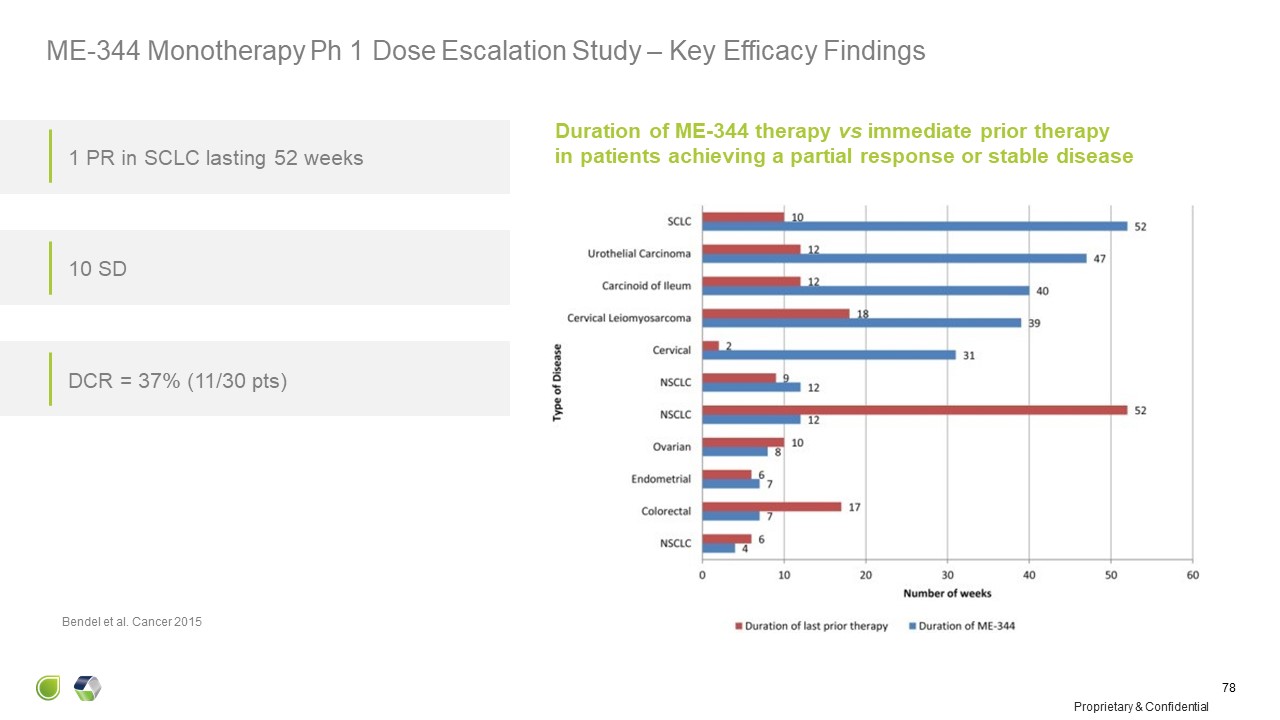
Duration of ME-344 therapy vs immediate prior therapyin patients achieving a
partial response or stable disease Bendel et al. Cancer 2015 1 PR in SCLC lasting 52 weeks 10 SD DCR = 37% (11/30 pts) Proprietary & Confidential 78 ME-344 Monotherapy Ph 1 Dose Escalation Study – Key Efficacy Findings

Clinical Study Objectives: Assess ability of bevacizumab to shift tumor
reliance from glycolysis to mitochondrial metabolism Assess ability of ME-344 + Avastin to inhibit tumor proliferation compared to Avastin + placebo Arm A Arm B Bevacizumab 15 mg/kg day 1 ME-344 10mg/kg days 8, 15, 21 Bevacizumab 15
mg/kg day 1 Saline 500cc days 8, 15, 21 Treatment-naïve HER2-negative breast cancer Analysis: FDG-PET: days 1 and 28 Biopsy: days 1 and 28 Sponsored by Spanish National Cancer Research Centre Demonstrating Clinical Proof of Concept
of ME-344 as a Novel Mitochondrial Inhibitor with the Potential to Prevent Anti-Angiogenic Escape in Combination with Bevacizumab 79 Qunitela-Fandino, Clin Cancer Res (2020) 26 (1): 35–45.

ME-344 in combination with bevacizumab in Her2-negative breast cancer patients
demonstrated anti-tumor activity as evidenced by decreased Ki67 ME-344: A Novel Mitochondrial Inhibitor with the Potential to Prevent Anti-Angiogenic Escape in Combination with Bevacizumab 80 Qunitela-Fandino, Clin Cancer Res (2020) 26
(1): 35–45.

Current Clinical Study in Colorectal Cancer
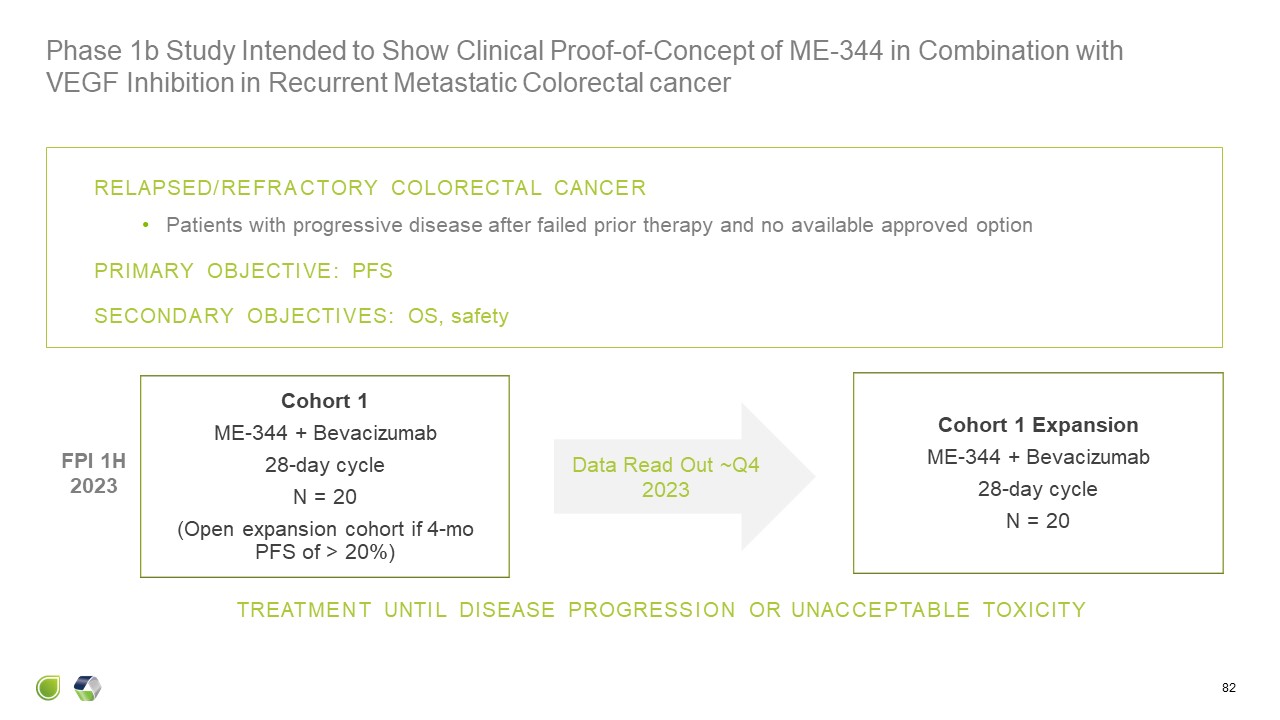
Cohort 1 ME-344 + Bevacizumab 28-day cycle N = 20 (Open expansion cohort
if 4-mo PFS of > 20%) Cohort 1 Expansion ME-344 + Bevacizumab 28-day cycle N = 20 TREATMENT UNTIL DISEASE PROGRESSION OR UNACCEPTABLE TOXICITY RELAPSED/REFRACTORY COLORECTAL CANCER Patients with progressive disease after failed
prior therapy and no available approved option PRIMARY OBJECTIVE: PFS SECONDARY OBJECTIVES: OS, safety FPI 1H 2023 Phase 1b Study Intended to Show Clinical Proof-of-Concept of ME-344 in Combination with VEGF Inhibition in Recurrent
Metastatic Colorectal cancer 82 Data Read Out ~Q4 2023

Clinical Development Plan

Colorectal Cancer Oral TKI VGEF inhibitors and trifluridine-tipiracilare ±
bevacizumab are standard treatment options for patients with colorectal cancer after failures of standard therapies Median overall survival of 6-12 months indicates a significant medical need ME-344 in combination with bevacizumab may
improve response rate and overall survival and represents an attractive registration strategy ME-344 Clinical Development Strategy 84 Other Cancers VGEF inhibitors like Avastin and regorafenib are approved in multiple solid tumor
indications, providing opportunities to expand combinatorial use of ME-344 beyond colorectal cancer patients. Examples include gastrointestinal stromal tumors, hepatocellular carcinoma, ovarian cancer and renal cell carcinoma

ME-344 Summary 85 ME-344 demonstrated Ki67 decrease in combination with
Avastin compared to placebo in HER2-negative breast cancer study, indicative of antitumor activity Pharmacodynamics supports on-target effect Normalized tumor vasculature and hypoxia correction correlate with enhanced antitumor
activity Phase 1b trial evaluating ME-344 + bevacizumab in patients with relapsed metastatic colorectal cancer intended to show proof of principle: Data to support ME-344 value in combination with bevacizumab/VEGF inhibition Data from
the Phase 1b study to support opening enrollment in an expansion cohort are expected around the end of 2023 ME-344 demonstrated potential to prevent anti-angiogenic escape with Avastin in patients and with VGEF TKIs in multiple
pre-clinical models

Q&A Participants 86 Nick Abbott, PhD Principal, Abbott Biotech
Consultancy Most recently, Senior Analyst Equity Research, Wells Fargo Corporate and Investment Banking Richard Ghalie, MD Chief Medical Officer, MEI Pharma Formerly Ligand, Favrille and others, and practicing oncologist

Q & A

A Combined Company with Significant Opportunities for Value
Creation 88 Three differentiated, promising, clinical candidates based on solid science and data* Pipeline led by planned eganelisib Phase 2 Study in Squamous cell carcinoma of the head & neck (SCCHN) Voruciclib + Venclexta® P1
Study: Initial Results ~YE 2023 ME-344 + Avastin® P1 Study: Initial Results ~YE 2023 Eganelisib + Keytruda® P2 Study: Initial Safety/Efficacy 2H 2024 Utilize understandings of biology to overcome resistance mechanisms of standard of
care therapies Anticipated Cash at closing of ~$100M expected to fund operations to mid-2025 and clinical data over the next ~6-24 months Experienced Leadership Team Advance potential first-in-class programs to value creating
transactions or commercialization *Dates refer to expected timelines.
