Table of Contents
As filed with the Securities and Exchange Commission on October 19, 2011
Registration No. 333-175880
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 2
TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
ARGOS THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 2834 | 56-2110007 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
4233 Technology Drive
Durham, North Carolina 27704
(919) 287-6300
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Jeffrey Abbey
Chief Executive Officer
4233 Technology Drive
Durham, North Carolina 27704
(919) 287-6300
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| David E. Redlick Stuart M. Falber Wilmer Cutler Pickering Hale and Dorr LLP 60 State Street Boston, Massachusetts 02109 (617) 526-6000 |
Mitchell S. Bloom Edward A. King Goodwin Procter LLP 53 State Street Boston, Massachusetts 02109 (617) 570-1000 |
Approximate date of commencement of proposed sale to public: As soon as practicable after this Registration Statement is declared effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||
| Non-accelerated filer | x (Do not check if a smaller reporting company) | Smaller reporting company | ¨ |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities, and we are not soliciting an offer to buy these securities, in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED OCTOBER 19, 2011
PROSPECTUS
Shares

Common Stock
Argos Therapeutics, Inc. is offering shares of its common stock. This is our initial public offering, and no public market currently exists for our common stock. We anticipate that the initial public offering price will be between $ and $ per share.
We have applied to have our common stock approved for listing on The NASDAQ Global Market under the symbol “ARGS.”
Investing in our common stock involves risks. See “Risk Factors” beginning on page 11.
| Per Share | Total | |||||||
| Initial public offering price |
$ | $ | ||||||
| Underwriting discounts and commissions |
$ | $ | ||||||
| Proceeds, before expenses, to us |
$ | $ | ||||||
We have granted the underwriters an option for 30 days from the date of this prospectus to purchase up to additional shares of our common stock at the initial public offering price, less underwriting discounts and commissions, to cover over-allotments.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the shares of common stock on or about .
| Lazard Capital Markets | Canaccord Genuity |
| Needham & Company, LLC | BMO Capital Markets |
The date of this prospectus is , 2011.
Table of Contents
| Page | ||||
| 1 | ||||
| 11 | ||||
| 43 | ||||
| 45 | ||||
| 45 | ||||
| 46 | ||||
| 48 | ||||
| 51 | ||||
| Management’s Discussion and Analysis of Financial Condition and Results of Operations |
53 | |||
| 81 | ||||
| 121 | ||||
| 127 | ||||
| 149 | ||||
| 155 | ||||
| 161 | ||||
| 166 | ||||
| 169 | ||||
| 174 | ||||
| 174 | ||||
| 174 | ||||
| F-1 | ||||
You should rely only on the information contained in this prospectus or in any free writing prospectus that we may authorize to be delivered to you. We have not authorized anyone to provide you with different information. We are offering to sell shares of our common stock, and seeking offers to buy shares of our common stock, only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of our common stock. Our business, financial condition, results of operations and prospects may have changed since that date.
Until , 2011 (25 days after the commencement of this offering), all dealers that buy, sell or trade shares of our common stock, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to the obligation of dealers to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
For investors outside the United States: Neither we nor any of the underwriters have taken any action to permit a public offering of the shares of our common stock or the possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
Table of Contents
This summary highlights information contained elsewhere in this prospectus. This summary does not contain all of the information you should consider before investing in our common stock. You should read this entire prospectus carefully, especially the “Risk Factors” section beginning on page 11 and our consolidated financial statements and the related notes appearing at the end of this prospectus, before making an investment decision.
Our Business
We are a biopharmaceutical company focused on the development and commercialization of fully personalized immunotherapies for the treatment of cancer and infectious diseases based on our proprietary technology platform called Arcelis™. Using biological components obtained from each patient, our Arcelis-based immunotherapies employ a specialized white blood cell, called a dendritic cell, to activate an immune response that is specific to the patient’s disease. Our most advanced product candidate is AGS-003 for the treatment of metastatic renal cell carcinoma, or mRCC. We plan to initiate patient enrollment in a pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib, an oral small molecule drug marketed under the trade name Sutent® that is the current standard of care for mRCC, in the first quarter of 2012.
Renal cell carcinoma, or RCC, is the most common type of kidney cancer. According to the National Cancer Institute, there are approximately 61,000 new cases of kidney cancer each year in the United States. The National Comprehensive Cancer Network and the National Cancer Data Base, or NCDB, estimate that 90% of these new cases are RCC. According to the NCDB, 15% to 20% of these newly diagnosed RCC cases are mRCC. Additionally, patients initially diagnosed with early stage RCC may later present with mRCC.
We are also developing a second Arcelis-based product candidate, AGS-004 for the treatment of HIV. We are studying AGS-004 in a phase 2b clinical trial that is funded entirely by the National Institutes of Health, or NIH. In addition to our Arcelis-based product candidates, we are developing two other product candidates based on our expertise in dendritic cell biology: AGS-009, a monoclonal antibody for the treatment of lupus, which we are studying in a phase 1a clinical trial, and AGS-010, a preclinical biologic compound, which we are developing for organ transplantation and the treatment of autoimmune and inflammatory diseases.
Arcelis Technology Platform
We use our Arcelis platform to generate RNA-loaded dendritic cell immunotherapies using biological components derived from the individual patient to be treated. Specifically, we manufacture these personalized therapies using that patient’s disease sample, either tumor cells in the case of cancer or a blood sample containing virus in the case of infectious disease, and dendritic cells derived from the patient’s white blood cells. By using messenger RNA, or mRNA, from the patient’s own cancer cells or virus, our Arcelis platform yields a fully personalized immunotherapy that is designed to contain all of the patient’s disease-specific antigens and to elicit a rapid, broad and potent immune response that is specific to the patient’s own disease. Our Arcelis-based immunotherapies have been well tolerated in clinical trials in more than 100 patients with no serious adverse events attributed to our immunotherapies. We believe that our Arcelis platform can be used for a wide range of oncology and infectious disease indications.
In October 2011, one of our scientific co-founders, Ralph M. Steinman, M.D., was awarded the Nobel Prize in medicine for the discovery of dendritic cells as critical sentinels of the immune system. Dr. Steinman’s discovery of dendritic cells and his research of dendritic cell biology and the role of dendritic cells in adaptive immunity led to the development of our Arcelis platform.
1
Table of Contents
Our Development Programs
The following table summarizes our four development programs and their status. We currently hold all commercial rights to all four of these programs in all geographies.
| Product Candidate |
Description |
Primary Indication |
Status | |||
| AGS-003 |
Arcelis-based fully personalized immunotherapy | mRCC | Planned pivotal phase 3 trial with initiation of enrollment expected in the first quarter of 2012 | |||
| AGS-004 |
Arcelis-based fully personalized immunotherapy | HIV | Ongoing phase 2b trial with completion of enrollment expected in the first quarter of 2012 | |||
| AGS-009 |
Anti-interferon-a monoclonal antibody | Lupus | Ongoing phase 1a trial with results expected in the fourth quarter of 2011 | |||
| AGS-010 |
CD83 recombinant protein | Organ transplantation |
Preclinical | |||
AGS-003. We have tested AGS-003 in clinical trials in combination with sunitinib and as a monotherapy for the treatment of mRCC. In our phase 2 clinical trial of AGS-003 in combination with sunitinib, the combination therapy showed median progression free survival of 11.9 months in intermediate and poor risk patients that had a time from diagnosis to initiation of systemic therapeutic treatment of less than one year. This represented an approximately 50% extension over the eight month median progression free survival achieved by sunitinib alone in similar intermediate and poor risk patients in the pivotal phase 3 clinical trial of sunitinib as a monotherapy. Progression free survival is the length of time that passes after treatment begins and before the patient’s disease worsens or the patient dies, and has served as the primary endpoint in the pivotal phase 3 trials for five of the six products most recently approved by the FDA for the treatment of mRCC.
We plan to conduct a pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib compared to sunitinib plus placebo under a special protocol assessment, or SPA, with the FDA. A pivotal clinical trial is a trial in humans that is intended to collect the primary evidence of safety and effectiveness to support a filing for marketing approval. In order to achieve the primary endpoint under the protocol for this trial, the data must demonstrate an increase of at least 30% in median progression free survival for the AGS-003 plus sunitinib arm compared to the sunitinib plus placebo control arm. We expect to initiate enrollment in this trial in the first quarter of 2012, complete enrollment in late 2013 and have data on the primary endpoint in the second half of 2014. In addition, under the protocol, the independent data monitoring committee will conduct two interim analyses of data from the trial to assess both safety and futility.
In September 2011, we received a letter from the FDA advising that the FDA had completed its review of our protocol for the pivotal phase 3 clinical trial under the SPA process. In the letter, the FDA stated that it had determined that the protocol sufficiently addressed the trial’s objectives and that the trial was adequately designed to provide the necessary data to support a submission for marketing approval. The FDA noted that the ability of the trial to support an efficacy claim for AGS-003 would depend on a review of the data from the trial and an evaluation of the overall risk and benefit of AGS-003.
AGS-004. We have completed two early stage clinical trials of AGS-004, and are currently conducting a phase 2b clinical trial that is funded entirely by the NIH. We expect to complete enrollment in this trial in the first quarter of 2012 and to complete primary endpoint analysis of the data from this trial in mid-2012. According to the World Health Organization, the number of people living with HIV in the world was approximately
2
Table of Contents
33.3 million in 2009. The Henry J. Kaiser Family Foundation estimates that more than 1.1 million people are currently living with HIV in the United States. According to the Centers for Disease Control and Prevention, the number of new cases of HIV infection in the United States is expected to remain steady at about 55,000 cases per year for the next decade.
AGS-009 and AGS-010. We are developing our other two product candidates based on our expertise in dendritic cell biology. We have completed enrollment in a phase 1a clinical trial of AGS-009, a monoclonal antibody for the treatment of lupus, and expect the results of this trial to be available in the fourth quarter of 2011. Following review of these results, we expect that we will commence enrollment in a further clinical trial of this product candidate in the first half of 2012. We are continuing preclinical testing on AGS-010, a recombinant human soluble CD83 protein, for organ transplantation and the treatment of autoimmune and inflammatory diseases.
Manufacturing
We currently manufacture our Arcelis-based product candidates at our single, centralized manufacturing facility located in Durham, North Carolina. To prepare for the commercial launch of AGS-003 and any other Arcelis-based products we develop, we plan to establish automated manufacturing processes based on existing functioning prototypes of automated devices and disposables and to identify, lease, build out and equip a new U.S. commercial manufacturing facility for this purpose. We have developed proprietary processes to generate Arcelis-based products that we believe will allow us to manufacture the drug product for all of North America at this one centralized facility. Our plan is to file our biologics license application, or BLA, for AGS-003 with the FDA using the automated manufacturing processes that we establish. We rely on contract manufacturers for the production of AGS-009 and AGS-010 and do not plan to build our own manufacturing capacity for these product candidates.
Intellectual Property
We own or exclusively license five U.S. patents and 15 U.S. patent applications, as well as more than 100 foreign counterparts to various of these patents and patent applications. The patents and patent applications we have exclusively licensed include patents and patent applications related to our Arcelis-based products that we have licensed under a patent license agreement with Duke University and patents and patent applications related to AGS-009 that we have licensed under patent license agreements with the Baylor Research Institute.
Investment Highlights
We believe that the following are the key attributes of our Arcelis platform and our pipeline of product candidates:
| • | Regulatory pathway for FDA approval of an active immunotherapy. We believe that the FDA’s approval of Dendreon Corporation’s Provenge® for the treatment of prostate cancer, which represents the first U.S. approval of an active immunotherapy, establishes a regulatory pathway for FDA approval of additional active immunotherapies. |
| • | Proprietary technology platform designed for fully personalized immunotherapies. Using our Arcelis platform, we believe that we have the ability to develop fully personalized active immunotherapies for treating a wide range of cancers and infectious diseases. We also believe that our Arcelis platform provides us with competitive advantages over other immunotherapies in terms of product potency, manufacturing scalability and cost of goods. |
3
Table of Contents
| • | Near-term initiation of pivotal phase 3 trial of AGS-003 combination therapy. Based on encouraging phase 2 progression free survival data in mRCC patients, we plan to commence enrollment in the first quarter of 2012 for our planned pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib for the treatment of mRCC. |
| • | Planned commercial scale manufacturing of our Arcelis-based product candidates using automated manufacturing processes. We have developed detailed plans for a single, centralized U.S. commercial manufacturing facility and automated processes to enable manufacturing with a cost of goods for our fully personalized Arcelis-based products that we expect will be comparable to other biologics. |
| • | Pipeline of additional product candidates that have the potential to be advanced using limited internal funding. We believe that we can advance the development of our three product candidates other than AGS-003, each of which addresses a large market with significant unmet medical needs, through government or other third-party funding, including collaboration or license arrangements. Our current phase 2b clinical trial of our Arcelis-based HIV product candidate, AGS-004, is being funded entirely under a contract with the NIH. |
| • | Experienced management team. Our five most senior executives have a combined experience of over 85 years in the biopharmaceutical industry and, except for our vice president of medical and clinical affairs who we hired in October 2011, each of these executives has worked at our company for at least seven years. |
Our Strategy
Our goal is to build a commercial biopharmaceutical company, founded on our extensive expertise in dendritic cell biology and immunology, primarily through the use of our Arcelis platform. Key elements of our strategy are to:
| • | Complete development of AGS-003 for mRCC, and manufacture and commercialize AGS-003 on our own in North America and collaborate with third parties for the manufacture and commercialization of AGS-003 outside North America, or, if we determine that it is in our best interest, with third parties on a worldwide basis. |
| • | Continue development of AGS-004 for HIV through government or other third-party funding and collaborate with third parties for commercialization on a worldwide basis. |
| • | Continue to build on our Arcelis platform by developing fully personalized immunotherapies for other cancers and infectious diseases. |
| • | Establish automated manufacturing processes based on existing functioning prototypes of automated devices and disposables and, prior to the filing of our BLA for AGS-003, identify, lease, build out and equip a new U.S. facility for the commercial manufacture of products based on our Arcelis platform. |
| • | Seek partners for further development and commercialization of AGS-009 for lupus and AGS-010 for organ transplantation and the treatment of autoimmune and inflammatory diseases. |
| • | Pursue broad intellectual property protection for our Arcelis technology platform, product candidates and proprietary manufacturing processes through U.S. and international patent filings and maintenance of trade secret confidentiality. |
4
Table of Contents
Risks Associated with Our Business
Our business is subject to a number of risks of which you should be aware before making an investment decision. These risks are discussed more fully in the “Risk Factors” section of this prospectus immediately following this prospectus summary. These risks include the following:
| • | We currently have no commercial products and have not received regulatory approval for any of our products. |
| • | We plan to use substantially all of the proceeds of this offering to fund our planned pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib. If the trial fails to demonstrate safety and efficacy to the satisfaction of the FDA, we would incur additional costs and experience delays in completing, or ultimately be unable to complete, the development and commercialization of AGS-003. |
| • | Results of earlier clinical trials of AGS-003 and clinical trials of sunitinib conducted by other parties may not be predictive of the outcome of our planned pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib. To date, we have not conducted a clinical trial of AGS-003 against a placebo or a comparator therapy, including sunitinib. While comparisons to results from other reported clinical trials can assist in evaluating the potential efficacy of our AGS-003 product candidate, there are many factors that affect the outcome for patients in clinical trials, some of which are not apparent in published reports, and results from two different trials often cannot be reliably compared. In addition, because we did not have enough evaluable patients to perform the statistical analysis to determine whether the primary endpoint of complete response rate was achieved in our phase 2 clinical trial of AGS-003, we expect that the data from that trial will have only a limited impact on the FDA’s ultimate assessment of efficacy of AGS-003. |
| • | Only one active immunotherapy has been approved by the FDA to date. Our use of our novel Arcelis technology with our active immunotherapies may raise development issues that we may not be able to resolve and may raise regulatory issues that could delay or prevent approval of our active immunotherapies. |
| • | We will need to obtain additional financing prior to the commercialization of AGS-003 or any of our other product candidates, including for the planned leasing, build-out and equipping of a new U.S. commercial manufacturing facility, and for conducting any future clinical trials of our product candidates. We expect that we will spend approximately $25 million to lease, build out and equip the new commercial manufacturing facility to a level necessary to file a BLA for AGS-003 with the FDA using the automated manufacturing processes that we plan to establish. We expect that we would incur an additional $10 million to $15 million after the filing of the BLA to further build out and equip the manufacturing facility so as to have in place the commercial capacity that we anticipate will be required for commercial launch of AGS-003. |
| • | We do not have experience in manufacturing Arcelis-based products on a commercial scale or using automated processes. If we are unable to successfully manufacture these products on a commercial scale, our business may be materially harmed. |
| • | We plan to fund the clinical development of AGS-004 through government or other third-party funding, to collaborate with a third party for commercialization of AGS-004 on a worldwide basis and to seek partners for further development and commercialization of AGS-009 and AGS-010. If we are unable to obtain such funding or establish such collaborations, we may not be able to commercialize or develop these product candidates. |
| • | We have incurred significant losses since our inception and, as of June 30, 2011, we had an accumulated deficit of $117.8 million. We expect to incur losses for the foreseeable future and may never achieve or maintain profitability. |
5
Table of Contents
Our Corporate Information
We were incorporated under the laws of the State of Delaware in 1997 under the name Dendritix, Inc. We changed our name to Merix Bioscience, Inc. in 1999, and to Argos Therapeutics, Inc. in 2004. Our executive offices are located at 4233 Technology Drive, Durham, North Carolina 27704, and our telephone number is (919) 287-6300. Our website address is www.argostherapeutics.com. The information contained in, or accessible through, our website does not constitute part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
In this prospectus, unless otherwise stated or the context otherwise requires, references to “Argos,” “we,” “us,” “our” and similar references refer to Argos Therapeutics, Inc. and its subsidiaries. Argos Therapeutics®, Argos® and Arcelis™, the Argos Therapeutics logo and other trademarks or service marks of Argos appearing in this prospectus are the property of Argos. The other trademarks, trade names and service marks appearing in this prospectus are the property of their respective owners.
6
Table of Contents
The Offering
| Common stock offered by us |
shares |
| Common stock to be outstanding after this offering |
shares |
| Over-allotment option |
The underwriters have an option for a period of 30 days to purchase up to additional shares of our common stock to cover over-allotments. |
| Use of proceeds |
We intend to devote approximately $ million of the net proceeds from this offering to fund the direct costs of our planned pivotal phase 3 combination therapy clinical trial of AGS-003 and to use the balance for other general corporate purposes. See “Use of Proceeds” for more information. |
| Proposed NASDAQ Global Market symbol |
“ARGS” |
| Risk factors |
You should read the “Risk Factors” section of this prospectus for a discussion of factors to consider carefully before deciding to invest in shares of our common stock. |
The number of shares of our common stock to be outstanding after this offering is based on 16,578,213 actual shares of our common stock outstanding as of August 31, 2011 and also includes:
| • | 157,871,412 shares of our common stock issuable upon the automatic conversion of all outstanding shares of our preferred stock upon the closing of this offering; |
| • | shares of our common stock issuable upon the automatic conversion of all principal and accrued interest on our outstanding convertible notes that we issued and sold in 2010, or the 2010 convertible notes, and our outstanding convertible notes that we issued and sold in July 2011, or the July 2011 convertible notes, upon the closing of this offering, at the initial public offering price, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011; |
| • | shares of our common stock issuable upon the automatic exercise of outstanding warrants to purchase shares of our series C redeemable convertible preferred stock, or series C preferred stock, by net exercise at an exercise price of $0.01 per share upon the closing of this offering, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus; and |
| • | 4,609,457 shares of our common stock issuable to specified holders of the capital stock of our 49.94% owned subsidiary, DC Bio Corp., or DC Bio, in exchange for their shares of DC Bio upon the closing of this offering, which will result in DC Bio becoming our wholly-owned subsidiary. |
7
Table of Contents
The number of shares of common stock to be outstanding after this offering excludes:
| • | 24,189,612 shares of our common stock issuable upon the exercise of stock options outstanding as of August 31, 2011 at a weighted average exercise price of $0.08 per share; |
| • | 13,205,160 additional shares of our common stock available for future issuance as of August 31, 2011 under our 2008 stock incentive plan, or our 2008 plan; |
| • | additional shares of our common stock that will be available for future issuance upon the closing of this offering under our 2011 stock incentive plan, or our 2011 plan; and |
| • | 5,617 shares of common stock issuable upon exercise of warrants outstanding as of August 31, 2011, other than the warrants to purchase shares of our series C preferred stock described above, at a weighted average exercise price of $32.57 per share. |
Unless otherwise indicated, all information in this prospectus reflects and assumes:
| • | no exercise of the outstanding options or warrants described above, other than the automatic exercise of the warrants to purchase shares of our series C preferred stock described above; |
| • | the automatic conversion of all outstanding shares of our preferred stock into 157,871,412 shares of our common stock upon the closing of this offering; |
| • | the automatic conversion of all principal and accrued interest on our outstanding convertible notes, including the 2010 convertible notes and the July 2011 convertible notes, upon the closing of this offering, into an aggregate of shares of our common stock, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011; |
| • | the issuance of an aggregate of shares of our common stock issuable upon the automatic exercise of warrants to purchase shares of our series C preferred stock by net exercise at an exercise price of $0.01 per share upon the closing of this offering, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus; |
| • | the issuance of an aggregate of 4,609,457 shares of our common stock to specified holders of the capital stock of DC Bio in exchange for their shares of DC Bio upon the closing of this offering; |
| • | the restatement of our certificate of incorporation and the amendment and restatement of our by-laws upon the closing of this offering; and |
| • | no exercise by the underwriters of their over-allotment option. |
8
Table of Contents
Summary Consolidated Financial Data
You should read the following summary consolidated financial data together with our consolidated financial statements and the related notes appearing at the end of this prospectus and the “Selected Consolidated Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of this prospectus. We have derived the consolidated statements of operations data for the years ended December 31, 2008, 2009 and 2010 from our audited consolidated financial statements included in this prospectus. We have derived the consolidated statements of operations data for the six months ended June 30, 2010 and 2011 and the consolidated balance sheet data as of June 30, 2011 from our unaudited consolidated financial statements included in this prospectus. The unaudited consolidated financial data include, in the opinion of our management, all adjustments, consisting only of normal recurring adjustments, that are necessary for a fair presentation of our financial position and results of operations for these periods. Our historical results for any prior period are not necessarily indicative of results to be expected in any future period, and our results for any interim period are not necessarily indicative of results to be expected for a full fiscal year.
The unaudited pro forma balance sheet data set forth below give effect to:
| • | the automatic conversion of all outstanding shares of our preferred stock into an aggregate of 157,871,412 shares of our common stock upon the closing of this offering; |
| • | the receipt in July 2011 of gross proceeds of $3.5 million upon the issuance and sale of the July 2011 convertible notes; |
| • | the automatic conversion of all principal and accrued interest on our outstanding convertible notes, including the 2010 convertible notes and the July 2011 convertible notes, upon the closing of this offering, into an aggregate of shares of our common stock, at the initial public offering price, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011; |
| • | the issuance of an aggregate of shares of our common stock upon the automatic exercise of outstanding warrants to purchase shares of our series C preferred stock by net exercise at an exercise price of $0.01 per share upon the closing of this offering, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus; and |
| • | the issuance of an aggregate of 4,609,457 shares of our common stock to specified holders of the capital stock of DC Bio in exchange for their shares of DC Bio upon the closing of this offering. |
The unaudited pro forma as adjusted balance sheet data set forth below give further effect to our issuance and sale of shares of our common stock in this offering at an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
9
Table of Contents
| Year Ended December 31, | Six
Months Ended June 30, 2010 |
Six
Months Ended June 30, 2011 |
Cumulative Period from Inception (May 8, 1997) to June 30, 2011 |
|||||||||||||||||||||
| 2008 | 2009 | 2010 | ||||||||||||||||||||||
| (unaudited) | (unaudited) | (unaudited) | ||||||||||||||||||||||
| Statements of Operations Data: |
||||||||||||||||||||||||
| Revenue |
$ | 4,449,735 | $ | 5,367,989 | $ | 7,272,783 | $ | 1,990,006 | $ | 4,128,206 | $ | 71,851,340 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Operating expenses |
||||||||||||||||||||||||
| Research and development |
22,042,597 | 19,543,966 | 13,927,662 | 6,931,617 | 6,754,657 | 169,735,867 | ||||||||||||||||||
| Net reimbursement under collaboration agreement |
(8,127,428 | ) | (6,626,989 | ) | — | — | — | (47,179,130 | ) | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net research and development |
13,915,169 | 12,916,977 | 13,927,662 | 6,931,617 | 6,754,657 | 122,556,737 | ||||||||||||||||||
| General and administrative |
2,852,375 | 2,931,599 | 2,704,231 | 1,552,170 | 1,383,521 | 34,088,991 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total operating expenses |
16,767,544 | 15,848,576 | 16,631,893 | 8,483,787 | 8,138,178 | 156,645,728 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Operating income (loss) |
(12,317,809 | ) | (10,480,587 | ) | (9,359,110 | ) | (6,493,781 | ) | (4,009,972 | ) | (84,794,388 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Other income (expense), net |
(471,835 | ) | (106,122 | ) | 191,758 | (223,039 | ) | (5,092,611 | ) | (15,719,084 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net income (loss) |
(12,789,644 | ) | (10,586,709 | ) | (9,167,352 | ) | (6,716,820 | ) | (9,102,583 | ) | (100,513,472 | ) | ||||||||||||
| Net income (loss) attributable to noncontrolling interest |
136,643 | (654,760 | ) | (172,598 | ) | (86,271 | ) | (41,768 | ) | (738,828 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net income (loss) attributable to Argos Therapeutics, Inc. |
(12,926,287 | ) | (9,931,949 | ) | (8,994,754 | ) | (6,630,549 | ) | (9,060,815 | ) | (99,774,644 | ) | ||||||||||||
| Less: Accretion of redeemable convertible preferred stock |
(395,666 | ) | (101,206 | ) | (101,206 | ) | (50,604 | ) | (50,604 | ) | (29,172,382 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net income (loss) attributable to common stockholders |
$ | (13,321,953 | ) | $ | (10,033,155 | ) | $ | (9,095,960 | ) | $ | (6,681,153 | ) | $ | (9,111,419 | ) | $ | (128,947,026 | ) | ||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Basic and diluted net loss attributable to common stockholders per share |
$ | (153.81 | ) | $ | (62.78 | ) | $ | (2.23 | ) | $ | (33.92 | ) | $ | (0.71 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Basic and diluted weighted average shares outstanding |
86,615 | 159,825 | 4,081,649 | 196,976 | 12,849,491 | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| As of June 30, 2011 | ||||||||||||||||||||||||
| Actual | Pro Forma | Pro Forma as Adjusted |
||||||||||||||||||||||
| Balance Sheet Data: |
||||||||||||||||||||||||
| Cash, cash equivalents and short-term investments |
|
$ | 1,359,340 | $ | $ | |||||||||||||||||||
| Working capital |
|
(8,872,214 | ) | |||||||||||||||||||||
| Total assets |
|
6,407,725 | ||||||||||||||||||||||
| Convertible term note including accrued interest |
|
5,694,032 | ||||||||||||||||||||||
| Long-term portion of notes payable |
|
— | ||||||||||||||||||||||
| Redeemable convertible preferred stock |
|
80,011,016 | ||||||||||||||||||||||
| Total stockholders’ deficit |
|
(87,914,285 | ) | |||||||||||||||||||||
10
Table of Contents
Investing in our common stock involves a high degree of risk. Before you decide to invest in our common stock, you should consider carefully the risks described below, together with the other information contained in this prospectus, including our financial statements and the related notes appearing at the end of this prospectus. We believe the risks described below are the risks that are material to us as of the date of this prospectus. If any of the following risks occur, our business, financial condition, results of operations and future growth prospects could be materially and adversely affected. In these circumstances, the market price of our common stock could decline, and you may lose all or part of your investment.
Risks Related to Our Financial Position and Need for Additional Capital
We have incurred significant losses since our inception. We expect to incur losses for the foreseeable future and may never achieve or maintain profitability.
Since inception, we have incurred significant operating losses. Our net loss, after attribution to the noncontrolling interest, was $9.1 million for the six months ended June 30, 2011, $9.0 million for the year ended December 31, 2010 and $9.9 million for the year ended December 31, 2009. As of June 30, 2011, we had an accumulated deficit of $117.8 million. As a result of our operating losses and negative cash flows from operations since inception, the report of our independent accountants on our December 31, 2010 financial statements included an explanatory paragraph indicating that there is substantial doubt about our ability to continue as a going concern. To date, we have financed our operations primarily through private placements of our preferred stock, convertible debt financings, bank debt, government contracts, grants and license and collaboration agreements. We have devoted substantially all of our efforts to research and development, including clinical trials. We have not completed development of any product candidates. We expect to continue to incur significant expenses and increasing operating losses for at least the next several years. We anticipate that our expenses will increase substantially if and as we:
| • | initiate or continue our clinical trials of AGS-003 and our other product candidates; |
| • | seek regulatory approvals for our product candidates that successfully complete clinical trials; |
| • | lease, build out and equip a new U.S. commercial facility for the manufacture of AGS-003 and our other Arcelis-based products; |
| • | establish a sales, marketing and distribution infrastructure to commercialize products for which we may obtain regulatory approval; |
| • | maintain, expand and protect our intellectual property portfolio; |
| • | continue our other research and development efforts; |
| • | hire additional clinical, quality control, scientific and management personnel; and |
| • | add operational, financial and management information systems and personnel, including personnel to support our product development and planned commercialization efforts. |
To become and remain profitable, we must develop and eventually commercialize a product or products with significant market potential. This development and commercialization will require us to be successful in a range of challenging activities, including successfully completing preclinical testing and clinical trials of our product candidates, obtaining regulatory approval for these product candidates, leasing, building out and equipping a new U.S. commercial manufacturing facility and manufacturing, marketing and selling those
11
Table of Contents
products for which we may obtain regulatory approval. We are only in the preliminary stages of some of these activities. We may never succeed in these activities and may never generate revenues that are significant or large enough to achieve profitability. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would decrease the value of the company and could impair our ability to raise capital, expand our business, maintain our research and development efforts or continue our operations. A decline in the value of our company could also cause you to lose all or part of your investment.
We will need substantial additional funding. If we are unable to raise capital when needed, we would be forced to delay, reduce or eliminate our product development programs, our plans to lease, build out and equip a new U.S. commercial manufacturing facility or our commercialization efforts.
We expect our expenses to increase in connection with our ongoing activities, particularly as we continue the research, development and clinical trials of, and seek regulatory approval for, our product candidates and lease, build out and equip a new U.S. commercial manufacturing facility. In addition, if we obtain regulatory approval of any of our product candidates, we expect to incur significant commercialization expenses for product sales, marketing, manufacturing and distribution. Furthermore, upon the closing of this offering, we expect to incur additional costs associated with operating as a public company. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we would be forced to delay, reduce or eliminate our product development programs, our plans for a new U.S. commercial manufacturing facility or our commercialization efforts.
As of June 30, 2011, we had cash, cash equivalents and short-term investments of $1.4 million and a working capital deficit of $8.9 million. We expect that the net proceeds from this offering, together with our existing cash, cash equivalents, short-term investments and anticipated funding under our NIH contract, as well as the proceeds from our issuance and sale of the July 2011 convertible notes, will enable us to fund our operating expenses and capital expenditure requirements, other than for the planned build-out and equipping of a new U.S. commercial manufacturing facility, through at least . We intend to devote approximately $ million of the net proceeds from this offering to fund the direct costs of our planned pivotal phase 3 combination therapy clinical trial of AGS-003 and to use the balance for general corporate purposes. We also will use $200,000 of the net proceeds to pay a success fee to a former lender under a loan agreement that we have previously repaid in full.
We will need to obtain additional financing prior to the commercialization of AGS-003 or any of our other product candidates, including for the planned leasing, build-out and equipping of a new U.S. commercial manufacturing facility, and for conducting any future clinical trials of our product candidates. We expect that we will spend approximately $25 million to lease, build out and equip the new commercial manufacturing facility to a level necessary to file a BLA for AGS-003 with the FDA using the automated manufacturing processes that we plan to establish. We expect that we would incur an additional $10 million to $15 million after the filing of the BLA to further build out and equip the manufacturing facility so as to have in place the commercial capacity that we anticipate will be required for commercial launch of AGS-003.
Our future capital requirements will depend on many factors, including:
| • | the progress and results of the planned pivotal phase 3 clinical trial of AGS-003; |
| • | the scope, progress, results and costs of preclinical development, laboratory testing and clinical trials for our other product candidates; |
| • | the costs and timing of our planned leasing, build-out and equipping of a new U.S. commercial manufacturing facility; |
12
Table of Contents
| • | the costs, timing and outcome of regulatory review of our product candidates; |
| • | the costs of commercialization activities, including product sales, marketing, manufacturing and distribution, for any of our product candidates for which we receive regulatory approval; |
| • | revenue, if any, received from commercial sales of our product candidates, should any of our product candidates be approved by the FDA or a similar regulatory authority outside the United States; |
| • | the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property-related claims; |
| • | the extent to which we acquire or invest in other businesses, products and technologies; |
| • | our ability to obtain government or other third-party funding; and |
| • | our ability to establish collaborations on favorable terms, if at all, particularly arrangements to market and distribute oncology product candidates outside North America and arrangements for the development and commercialization of our non-oncology product candidates on a worldwide basis. |
Conducting preclinical testing and clinical trials is a time-consuming, expensive and uncertain process that takes years to complete, and we may never generate the necessary data or results required to obtain regulatory approval and achieve product sales. In addition, our product candidates, if approved, may not achieve commercial success. Our commercial revenues, if any, will be derived from sales of products that we do not expect to be commercially available for several years, if at all. Accordingly, we will need to continue to rely on additional financing to achieve our business objectives. Additional financing may not be available to us on acceptable terms, or at all.
Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity offerings, debt financings, government or other third-party funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements. Except for the NIH’s remaining funding commitment with respect to AGS-004, we do not have any committed external source of funds. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a stockholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends.
We currently intend to collaborate with third parties for the commercialization of AGS-003 outside of North America and of AGS-004 worldwide and to seek partners for the further development and commercialization of AGS-009 and AGS-010. We plan to seek government or other third-party funding for the continued development of AGS-004 following completion of the phase 2b clinical trial of AGS-004 and to seek partners or other sources of third-party funding for the further development of AGS-009 and AGS-010. If we raise additional funds through government or other third-party funding, marketing and distribution arrangements or other collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us.
13
Table of Contents
Risks Related to the Development and Commercialization of Our Product Candidates
We depend heavily on the success of AGS-003 and our other product candidates. All of our product candidates are still in preclinical or clinical development. Clinical trials of our product candidates may not be successful. If we are unable to commercialize our product candidates or experience significant delays in doing so, our business will be materially harmed.
We have invested a significant portion of our efforts and financial resources in the development of AGS-003, AGS-004, AGS-009 and AGS-010. Our ability to generate product revenues, which we do not expect will occur for at least the next several years, if ever, will depend heavily on the successful development and eventual commercialization of these product candidates, especially AGS-003, our most advanced product candidate. The success of these product candidates will depend on several factors, including the following:
| • | successful completion of preclinical studies and clinical trials; |
| • | receipt of marketing approvals from the FDA and similar regulatory authorities outside the United States; |
| • | establishing commercial manufacturing capabilities by identifying, leasing, building out and equipping a commercial manufacturing facility for our Arcelis-based product candidates and making arrangements with third-party manufacturers for our other product candidates; |
| • | maintaining patent and trade secret protection and regulatory exclusivity for our product candidates; |
| • | launching commercial sales of the products, if and when approved, whether alone or in collaboration with others; |
| • | acceptance of the products, if and when approved, by patients, the medical community and third-party payors; |
| • | effectively competing with other therapies; and |
| • | a continued acceptable safety profile of the products following approval. |
If we do not achieve one or more of these factors in a timely manner or at all, we could experience significant delays or an inability to successfully commercialize our product candidates, which would materially harm our business.
If clinical trials of our product candidates, such as our planned pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib, fail to demonstrate safety and efficacy to the satisfaction of the FDA or similar regulatory authorities outside the United States or do not otherwise produce positive results, we may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of our product candidates.
Before obtaining regulatory approval for the sale of our product candidates, we must conduct extensive clinical trials to demonstrate the safety, purity and potency, or efficacy, of our product candidates in humans. Clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome. A failure of one or more of our clinical trials can occur at any stage of testing. The outcome of preclinical testing and early clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval of their products.
14
Table of Contents
In particular, to date, we have not conducted a clinical trial of AGS-003 against a placebo or a comparator therapy. While comparisons to results from other reported clinical trials can assist in evaluating the potential efficacy of our AGS-003 product candidate, there are many factors that affect the outcome for patients in clinical trials, some of which are not apparent in published reports, and results from two different trials often cannot be reliably compared. Our planned pivotal phase 3 clinical trial of AGS-003 is intended to compare directly the combination of AGS-003 and sunitinib to treatment with the combination of sunitinib plus placebo. Based on the design of the trial, the data from the trial will need to demonstrate an increase of at least 30% in median progression free survival for the AGS-003 plus sunitinib arm as compared to the sunitinib plus placebo arm in order to show statistical significance and achieve the primary endpoint of the trial. We will need to show this statistically significant benefit of the combined therapy as compared to treatment with the combination of sunitinib plus placebo as part of a submission for approval of AGS-003. However, demonstration of statistical significance and achievement of the primary endpoint of the trial does not assure approval by the FDA or similar regulatory authorities outside the United States.
Patients in our planned pivotal phase 3 clinical trial who receive treatment with sunitinib plus placebo may not have results similar to patients studied in other clinical trials of sunitinib. If the patients in our planned pivotal phase 3 clinical trial who receive sunitinib plus placebo have results which are better than the results that occurred in those other clinical trials, we may not demonstrate a sufficient benefit from AGS-003 in combination with sunitinib to allow the FDA to approve AGS-003 for marketing. In addition, only 21 patients received the combination of AGS-003 and sunitinib in our phase 2 clinical trial. If the patients in our planned pivotal phase 3 clinical trial who receive the combination of AGS-003 and sunitinib have results which are worse than the results that occurred in our phase 2 clinical trial, we may not demonstrate a sufficient benefit from the combination therapy to allow the FDA to approve AGS-003 for marketing.
For drug and biological products, the FDA typically requires the successful completion of two adequate and well-controlled clinical trials to support marketing approval because a conclusion based on two persuasive studies will be more secure. In the case of AGS-003, which is intended for a life-threatening disease, we intend to seek approval based upon the results of a single pivotal phase 3 trial. As a result, the trial may receive enhanced scrutiny from the FDA. In its September 2011 letter, the FDA informed us that in order for a single trial to support approval of an indication, the trial must be well conducted, and the results of the trial must be internally consistent, clinically meaningful and statistically very persuasive. If the FDA disagrees with our choice of a primary endpoint or the results for the primary endpoint are not robust, are subject to confounding factors, or are not adequately supported by other study endpoints, including overall survival, the FDA may refuse to approve our BLA based upon a single clinical trial. For example, although we are including a control arm crossover in the trial design for our pivotal phase 3 clinical trial for ethical reasons, this may well contribute to the confounding of the overall survival results, thereby reducing the chance that the overall survival results would support approval of AGS-003. In its September 2011 letter, the FDA informed us that it remains concerned that this cross over design may potentially confound the interpretation of overall survival and asked us to make every effort to follow patients for survival per the protocol and record the type, dose and schedule of subsequent therapies taken by the patients. In addition, because only 21 patients received the combination of AGS-003 and sunitinib in our phase 2 clinical trial, and as a result, we did not have enough evaluable patients to perform the statistical analysis to determine whether the primary endpoint of complete response rate was achieved in that trial, we expect that the data from our phase 2 trial will have only a limited impact on the FDA’s ultimate assessment of efficacy of AGS-003. Thus, there can be no guarantee that the FDA will not require additional pivotal clinical trials as a condition for approving AGS-003.
We may experience numerous unforeseen events during, or as a result of, clinical trials that could delay or prevent our ability to receive regulatory approval or commercialize our product candidates. For example, in its September 2011 letter, the FDA placed the protocol for our planned pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib on partial clinical hold due to unresolved questions regarding the planned measurement of the secretion of the cytokine interleukin-12, or IL-12, as part of the specifications for the release of AGS-003. Subsequent to receiving the September 2011 letter, we reached agreement with the FDA regarding
15
Table of Contents
the IL-12 release specifications and the FDA lifted the partial clinical hold. Unforeseen events that could delay or prevent our ability to receive regulatory approval or commercialize our product candidates include:
| • | regulators or institutional review boards may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site; |
| • | we may have delays in reaching or fail to reach agreement on acceptable clinical trial contracts or clinical trial protocols with prospective trial sites; |
| • | clinical trials of our product candidates may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical trials or abandon product development programs; |
| • | the number of patients required for clinical trials of our product candidates may be larger than we anticipate, enrollment in these clinical trials may be slower than we anticipate or participants may drop out of these clinical trials at a higher rate than we anticipate; |
| • | our third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all; |
| • | we might have to suspend or terminate clinical trials of our product candidates for various reasons, including a finding that the participants are being exposed to unacceptable health risks; |
| • | regulators or institutional review boards may require that we or our investigators suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable health risks; |
| • | the cost of clinical trials of our product candidates may be greater than we anticipate; |
| • | the supply or quality of our product candidates or other materials necessary to conduct clinical trials of our product candidates may be insufficient or inadequate; and |
| • | our product candidates may have undesirable side effects or other unexpected characteristics, causing us or our investigators, regulators or institutional review boards to suspend or terminate the trials. |
In addition, the patients recruited for clinical trials of our product candidates may have a disease profile or other characteristics that are different than we expect and different than the clinical trials were designed for, which could adversely impact the results of the clinical trials. For instance, our phase 2 combination therapy clinical trial of AGS-003 in combination with sunitinib was originally designed to enroll subjects with favorable disease risk profiles and intermediate disease risk profiles and with a primary endpoint of complete response rate. However, the actual trial population consisted entirely of patients with intermediate disease risk profiles and poor disease risk profiles. This is a population for which published research has shown that sunitinib alone, as well as other new therapies for mRCC, rarely if ever produce complete responses in mRCC, and in our phase 2 clinical trial in this population the combination therapy of AGS-003 and sunitinib did not show a complete response rate that met the endpoint of the trial.
If we are required to conduct additional clinical trials or other testing of our product candidates beyond those that we currently contemplate, if we are unable to successfully complete clinical trials of our product candidates or other testing, if the results of these trials or tests are not positive or are only modestly positive or if there are safety concerns, we may:
| • | be delayed in obtaining marketing approval for our product candidates; |
16
Table of Contents
| • | not obtain marketing approval at all; |
| • | obtain approval for indications or patient populations that are not as broad as intended or desired; |
| • | obtain approval with labeling that includes significant use restrictions or safety warnings, including boxed warnings; |
| • | have the product removed from the market after obtaining marketing approval; |
| • | be subject to additional post-marketing testing requirements; or |
| • | be subject to restrictions on how the product is distributed or used. |
Our product development costs will also increase if we experience delays in testing or approvals. We do not know whether any clinical trials will begin as planned, will need to be restructured or will be completed on schedule, or at all. For example, in response to our submission of an IND for AGS-004, the FDA raised safety concerns regarding the analytical treatment interruption contemplated by our protocol for our phase 2a clinical trial of AGS-004, and required a one year safety follow-up after the final dose for each patient. This resulted in the need for an amendment to the trial protocol and a four month delay prior to initiating the phase 2a clinical trial in the United States. Significant clinical trial delays also could shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do and impair our ability to commercialize our product candidates and may harm our business and results of operations.
The FDA has reviewed the protocol for our planned pivotal phase 3 trial of AGS-003 in combination with sunitinib under the SPA process. Agreement by the FDA with the protocol under the SPA process does not guarantee that the trial will be successful or that, if successful, the combination therapy will receive marketing approval.
The FDA has reviewed under the SPA process a protocol for our planned pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib compared to sunitinib plus placebo. The SPA process is designed to facilitate the FDA’s review and approval of drug and biological products by allowing the FDA to evaluate the proposed design and size of phase 3 clinical trials that are intended to form the primary basis for determining a drug product’s efficacy. In September 2011, we received a letter from the FDA advising us that the FDA had completed its review of our protocol for the pivotal phase 3 clinical trial under the SPA process. In the letter, the FDA stated that it had determined that the protocol sufficiently addressed the trial’s objectives and that the trial was adequately designed to provide the necessary data to support a submission for marketing approval.
Because we are developing AGS-003 under an SPA based on protocol designs negotiated with the FDA, the trial may receive enhanced scrutiny. In addition, an SPA does not guarantee approval. Notwithstanding the SPA, the combination of AGS-003 and sunitinib may not achieve the primary endpoint of the trial. Even if the primary endpoint in the planned pivotal phase 3 clinical trial is achieved, AGS-003 may not be approved. Many companies which have been granted SPAs have ultimately failed to obtain final approval to market their products. In its September 2011 letter, the FDA noted that the ability of the planned phase 3 clinical trial to support an efficacy claim for AGS-003 would depend on a review of the data from the trial and an evaluation of the overall risk and benefit of AGS-003. In addition, the SPA is not binding on the FDA if public health concerns unrecognized at the time the SPA was entered into become evident; the data, assumptions or information underlying the SPA request change or are called into question; other new scientific concerns regarding product safety or efficacy arise, or if we fail to comply with the agreed upon trial protocols. The FDA may raise issues of safety, study conduct, bias, deviation from the protocol, statistical power, patient completion rates, changes in scientific or medical parameters or internal inconsistencies in the data prior to making its final decision. The FDA may also seek the guidance of an outside advisory committee prior to making its final decision.
17
Table of Contents
If we experience delays or difficulties in the enrollment of patients in our clinical trials, our receipt of necessary regulatory approvals could be delayed or prevented.
We may not be able to initiate or continue clinical trials for our product candidates, including our planned pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib, if we are unable to locate and enroll a sufficient number of eligible patients to participate in these trials as required by the FDA or similar regulatory authorities outside the United States. In addition, many of our competitors have ongoing clinical trials for product candidates that could be competitive with our product candidates, and patients who would otherwise be eligible for our clinical trials may instead enroll in clinical trials of our competitors’ product candidates. For example, during the phase 1/2 monotherapy clinical trial of AGS-003 that we conducted, our ability to enroll patients in the trial was adversely affected by the FDA’s approval of sorafenib and sunitinib during our phase 1/2 trial, because patients did not want to receive, and physicians were reluctant to administer, AGS-003 as a monotherapy once new therapies that showed efficacy in clinical trials were introduced to the market and became widely available.
Patient enrollment is affected by other factors including:
| • | severity of the disease under investigation; |
| • | eligibility criteria for the study in question; |
| • | perceived risks and benefits of the product candidate under study; |
| • | efforts to facilitate timely enrollment in clinical trials; |
| • | patient referral practices of physicians; |
| • | the ability to monitor patients adequately during and after treatment; and |
| • | proximity and availability of clinical trial sites for prospective patients. |
We estimate that we will require approximately two years to fully enroll patients in our planned pivotal phase 3 combination therapy clinical trial of AGS-003. However, the actual amount of time for full enrollment could be longer than planned. Enrollment delays in this planned pivotal phase 3 trial or any of our other clinical trials may result in increased development costs for our product candidates, which would cause the value of the company to decline and limit our ability to obtain additional financing. Our inability to enroll a sufficient number of patients for this planned pivotal phase 3 clinical trial or any of our other clinical trials would result in significant delays or may require us to abandon one or more clinical trials altogether.
If serious adverse or inappropriate side effects are identified during the development of our product candidates, we may need to abandon or limit our development of some of our product candidates.
All of our product candidates are still in preclinical or clinical development and their risk of failure is high. It is impossible to predict when or if any of our product candidates will prove effective or safe in humans or will receive regulatory approval. If our product candidates are associated with undesirable side effects or have characteristics that are unexpected, we may need to abandon their development or limit development to certain uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective.
18
Table of Contents
Our Arcelis-based product candidates are active immunotherapies that are based on a novel technology utilizing patient material. This may raise development issues that we may not be able to resolve, regulatory issues that could delay or prevent approval or personnel issues that may prevent us from further developing and commercializing our product candidates.
Our Arcelis-based product candidates, AGS-003 and AGS-004, are based on our novel Arcelis technology platform. In the course of developing this platform and these product candidates, we have encountered difficulties in the development process. For example, we terminated the development of MB-002, the predecessor to AGS-003, when the results from the initial clinical trial of MB-002 indicated that the product candidate only corrected defects in the production of one of the two critical cytokines required for effective immune response. There can be no assurance that additional development problems will not arise in the future which we may not be able to resolve or which may cause significant delays in development.
In addition, regulatory approval of novel product candidates such as our Arcelis-based product candidates manufactured using novel manufacturing processes such as ours can be more expensive and take longer than for other, more well-known or extensively studied pharmaceutical or biopharmaceutical products, due to our and regulatory agencies’ lack of experience with them. The FDA has only approved one active cellular immunotherapy product to date. This lack of experience may lengthen the regulatory review process, require us to conduct additional studies or clinical trials, increase our development costs, lead to changes in regulatory positions and interpretations, delay or prevent approval and commercialization of these product candidates or lead to significant post-approval limitations or restrictions.
The novel nature of our product candidates also means that fewer people are trained in or experienced with product candidates of this type, which may make it difficult to find, hire and retain capable personnel for research, development and manufacturing positions.
Development of our Arcelis-based product candidates is subject to significant uncertainty because each product candidate is derived from source material that is inherently variable. This variability could reduce the effectiveness of our Arcelis-based product candidates, delay any FDA approval of our Arcelis-based product candidates, cause us to change our manufacturing methods and adversely affect the commercial success of any approved Arcelis-based products.
The disease samples from the patients to be treated with our Arcelis-based products vary from patient to patient. This inherent variability may adversely affect our ability to manufacture our products because each tumor or virus sample that we receive and process will yield a different drug. As a result, we may not be able to consistently produce a product for every patient and we may not be able to treat all patients effectively. Such inconsistency could delay FDA or other regulatory approval of our Arcelis-based product candidates or if approved, adversely affect market acceptance and use of our Arcelis-based products. If we have to change our manufacturing methods to address any inconsistency, we may have to perform additional clinical trials, which would delay FDA or other regulatory approval of our Arcelis-based product candidates and increase the costs of development of our Arcelis-based product candidates.
The inherent variability of the disease samples from the patients to be treated with our Arcelis-based products may further adversely affect our ability to manufacture our products because variability in the source material for our product candidates, such as tumor cells or viruses, may cause variability in the composition of other cells in our product candidates. Such variability in composition or purity could adversely affect our ability to establish acceptable release specifications and the development and regulatory approval processes for our product candidates may be delayed, which would increase the costs of development of our Arcelis-based product candidates.
19
Table of Contents
Even if any of our product candidates, including AGS-003 and our other product candidates, receive regulatory approval, they may fail to achieve the degree of market acceptance by physicians, patients, healthcare payors and others in the medical community necessary for commercial success.
If AGS-003 or any of our other product candidates receive marketing approval, they may nonetheless fail to gain sufficient market acceptance by physicians, patients, healthcare payors and others in the medical community. Gaining market acceptance for our Arcelis-based products may be particularly difficult as, to date, the FDA has only approved one active immunotherapy and our Arcelis-based products are based on a novel technology. If these products do not achieve an adequate level of acceptance, we may not generate significant product revenues and we may not become profitable. The degree of market acceptance of our product candidates, if approved for commercial sale, will depend on a number of factors, including:
| • | efficacy and potential advantages compared to alternative treatments; |
| • | the ability to offer our product candidates for sale at competitive prices; |
| • | convenience and ease of administration compared to alternative treatments; |
| • | the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; |
| • | the strength of marketing and distribution support; |
| • | sufficient third-party coverage or reimbursement; and |
| • | the prevalence and severity of any side effects. |
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to sell and market our product candidates, we may not be successful in commercializing our product candidates if and when they are approved.
We do not have a sales or marketing infrastructure and have no experience in the sale, marketing or distribution of pharmaceutical products. To achieve commercial success for any approved product, we must either develop a sales and marketing organization or outsource these functions to third parties. We plan to market and sell AGS-003 and any future oncology product candidates for which we receive marketing approval in North America ourselves and to establish distribution or other marketing arrangements with third parties for such products in the rest of the world. We plan to establish worldwide collaborations, distribution or other marketing arrangements with third parties for AGS-004 and any of our other product candidates should such candidates receive marketing approval.
There are risks involved with both establishing our own sales and marketing capabilities and entering into arrangements with third parties to perform these services. For example, recruiting and training a sales force is expensive and time consuming and could delay any product launch. If the commercial launch of a product candidate for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel.
Factors that may inhibit our efforts to commercialize our products on our own include:
| • | our inability to recruit and retain adequate numbers of effective sales and marketing personnel; |
| • | the inability of sales personnel to obtain access to or persuade adequate numbers of physicians to prescribe any future products; |
20
Table of Contents
| • | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and |
| • | unforeseen costs and expenses associated with creating an independent sales and marketing organization. |
If we enter into arrangements with third parties to perform sales, marketing and distribution services, our product revenues or the profitability of these product revenues to us are likely to be lower than if we were to market and sell any products that we develop ourselves. In addition, we may not be successful in entering into arrangements with third parties to sell and market our product candidates or doing so on terms that are favorable to us. We likely will have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively. If we do not establish sales and marketing capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our product candidates.
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do.
The development and commercialization of new drug products is highly competitive. We face competition with respect to our current product candidates, and will face competition with respect to any products that we may seek to develop or commercialize in the future, from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide. There are a number of large pharmaceutical and biotechnology companies that currently market and sell products or are pursuing the development of products for the treatment of the disease indications for which we are developing our product candidates. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
Some of these competitive products and therapies are based on scientific approaches that are the same as or similar to our approach, and others are based on entirely different approaches. Many marketed therapies for the indications that we are currently pursuing, or indications that we may in the future seek to address using our Arcelis platform, are widely accepted by physicians, patients and payors, which may make it difficult for us to replace them with any products that we successfully develop and are permitted to market.
There are several FDA-approved therapies for mRCC marketed and sold by large pharmaceutical companies. Approved monotherapies for mRCC include Nexavar® (sorafenib), marketed by Bayer Healthcare Pharmaceuticals, Inc. and Onyx Pharmaceuticals, Inc., Sutent (sunitinib), marketed by Pfizer, Inc., AvastinTM (bevacizumab), marketed by Genentech, Inc., a member of the Roche Group, and VotrientTM (pazopanib), marketed by GlaxoSmithKline plc, Torisel® (temsirolimus), marketed by Pfizer, and Afinitor® (everolimus), marketed by Novartis Pharmaceuticals Corporation. In addition, there are several monotherapies in clinical development for the treatment of mRCC, including product candidates in late-stage clinical development, such as tivozanib and axitinib. In addition, we estimate that there are numerous cancer immunotherapy products in clinical development by many public and private biotechnology and pharmaceutical companies targeting numerous different cancer types. A number of these are in late stage development. One biotechnology company, Immatics Biotechnologies GmbH, or Immatics, is developing a therapeutic cancer vaccine, which is a mixture of defined tumor-associated peptides, for the treatment of RCC. Immatics is conducting a pivotal phase 3 clinical trial comparing its vaccine in combination with sunitinib against treatment with sunitinib alone. If this clinical trial is successful, this combination therapy would be in direct competition with our AGS-003 and sunitinib combination therapy. In addition, if a standalone therapy for mRCC were developed that demonstrated improved efficacy over currently marketed therapies with a favorable safety profile and without the need for combination therapy, such a therapy might pose a significant competitive threat to AGS-003.
21
Table of Contents
In addition, we are planning to conduct a pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib. We elected to study AGS-003 in clinical trials in combination with sunitinib due in part to sunitinib being the current standard of care for first-line treatment of mRCC. Although we do not expect to seek FDA approval of AGS-003 solely in combination with sunitinib, if we obtain approval by the FDA, such FDA approval may be limited to the combination of AGS-003 and sunitinib. In such event, the commercial success of AGS-003 would be linked to the commercial success of sunitinib. As a result, if sunitinib ceases to be the standard of care for first-line treatment of mRCC or another event occurs that adversely affects sales of sunitinib, the commercial success of AGS-003 may be adversely affected.
There are also numerous FDA-approved treatments for HIV, primarily antiretroviral therapies marketed by large pharmaceutical companies. Generic competition has recently developed in this market as patent exclusivity periods for older drugs have expired, with more than 15 generic bioequivalents currently on the market. The presence of these generic drugs is resulting in price pressure in the HIV therapeutics market and could affect the pricing of AGS-004.
Our competitors may develop products that are more effective, safer, more convenient or less costly than any that we are developing or that would render our product candidates obsolete or non-competitive. Our competitors may also obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours.
Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical, biotechnology and device industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
Even if we are able to commercialize any product candidates, the products may become subject to unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives, which would harm our business.
The regulations that govern marketing approvals, pricing and reimbursement for new drug products vary widely from country to country. In the United States, recently passed legislation may significantly change the approval requirements in ways that could involve additional costs and cause delays in obtaining approvals. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we might obtain regulatory approval for a product in a particular country, but then be subject to price regulations that delay our commercial launch of the product, possibly for lengthy time periods, and negatively impact the revenues we are able to generate from the sale of the product in that country. Adverse pricing limitations may hinder our ability to recoup our investment in one or more product candidates, even if our product candidates obtain regulatory approval.
Our ability to commercialize any products successfully also will depend in part on the extent to which reimbursement for these products and related treatments will be available from government health administration authorities, private health insurers and other organizations. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. A primary trend in the U.S. healthcare industry and elsewhere is cost containment. Government authorities and third-party payors have attempted to control costs by limiting coverage
22
Table of Contents
and the amount of reimbursement for particular medications. Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. We cannot be sure that reimbursement will be available for any product that we commercialize and, if reimbursement is available, the level of reimbursement. Reimbursement may impact the demand for, or the price of, any product candidate for which we obtain marketing approval. Obtaining reimbursement for our products may be particularly difficult because of the higher prices often associated with drugs administered under the supervision of a physician. If reimbursement is not available or is available only to limited levels, we may not be able to successfully commercialize any product candidate for which we obtain marketing approval.
There may be significant delays in obtaining reimbursement for newly approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA or similar regulatory authorities outside the United States. Moreover, eligibility for reimbursement does not imply that any drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Interim reimbursement levels for new drugs, if applicable, may also not be sufficient to cover our costs and may not be made permanent. Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost drugs, and may be incorporated into existing payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. Third party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement policies. Our inability to promptly obtain coverage and profitable payment rates from both government-funded and private payors for any approved products that we develop could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products and our overall financial condition.
Our reliance on government funding adds uncertainty to our research and commercialization efforts and may impose requirements that increase the costs of commercialization and production of our government-funded product candidates.
Our phase 2b clinical trial for AGS-004 for HIV is currently being funded entirely by the NIH. We plan to seek further government funding for continued development of AGS-004. Contracts and grants from the U.S. government and its agencies include provisions that give the government substantial rights and remedies, many of which are not typically found in commercial contracts, including provisions that allow the government to:
| • | terminate agreements, in whole or in part, for any reason or no reason; |
| • | reduce or modify the government’s obligations under such agreements without the consent of the other party; |
| • | claim rights, including intellectual property rights, in products and data developed under such agreements; |
| • | impose U.S. manufacturing requirements for products that embody inventions conceived or first reduced to practice under such agreements; |
| • | suspend or debar the contractor or grantee from doing future business with the government or a specific government agency; |
| • | pursue criminal or civil remedies under the False Claims Act, False Statements Act and similar remedy provisions specific to government agreements; and |
| • | limit the government’s financial liability to amounts appropriated by the U.S. Congress on a fiscal-year basis, thereby leaving some uncertainty about the future availability of funding for a program even after it has been funded for an initial period. |
23
Table of Contents
Government agreements normally contain additional terms and conditions that may increase our costs of doing business, reduce our profits, and expose us to liability for failure to comply with these terms and conditions. These include, for example:
| • | specialized accounting systems unique to government contracts and grants; |
| • | mandatory financial audits and potential liability for price adjustments or recoupment of government funds after such funds have been spent; |
| • | public disclosures of certain contract and grant information, which may enable competitors to gain insights into our research program; and |
| • | mandatory socioeconomic compliance requirements, including labor standards, non-discrimination and affirmative action programs and environmental compliance requirements. |
Product liability lawsuits against us could cause us to incur substantial liabilities and to limit commercialization of any products that we may develop.
We face an inherent risk of product liability exposure related to the testing of our product candidates in human clinical trials and will face an even greater risk if we commercially sell any products that we may develop. These risks may be even greater with respect to our Arcelis-based products which are manufactured using a novel technology. None of our product candidates has been widely used over an extended period of time, and therefore our safety data are limited. We derive the raw materials for manufacturing of our Arcelis-based product candidates from human cell sources, and therefore the manufacturing process and handling requirements are extensive and stringent, which increases the risk of quality failures and subsequent product liability claims.
If we cannot successfully defend ourselves against claims that our product candidates or products caused injuries, we will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| • | decreased demand for any product candidates or products that we may develop; |
| • | injury to our reputation and significant negative media attention; |
| • | withdrawal of clinical trial participants; |
| • | significant costs to defend the related litigation; |
| • | substantial monetary awards to trial participants or patients; |
| • | loss of revenue; and |
| • | the inability to commercialize any products that we may develop. |
We currently hold $5.0 million in product liability insurance coverage, which may not be adequate to cover all liabilities that we may incur. We will need to increase our insurance coverage when we begin commercializing our product candidates, if ever. Insurance coverage is increasingly expensive. We may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and
24
Table of Contents
radioactive and biological materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we focus on research programs and product candidates for specific indications. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable products.
We have based our research and development efforts on our Arcelis platform. Notwithstanding our large investment to date and anticipated future expenditures in our Arcelis platform, we have not yet developed, and may never successfully develop, any marketed drugs using this approach. As a result of pursuing the development of product candidates using our Arcelis platform, we may fail to develop product candidates or address indications based on other scientific approaches that may offer greater commercial potential or for which there is a greater likelihood of success.
Our long-term business plan is to develop Arcelis-based products for the treatment of various cancers and infectious diseases. We may not be successful in our efforts to identify or discover additional product candidates that may be manufactured using our Arcelis platform. Research programs to identify new product candidates require substantial technical, financial and human resources. These research programs may initially show promise in identifying potential product candidates, yet fail to yield product candidates for clinical development.
If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate.
25
Table of Contents
Risks Related to Our Dependence on Third Parties
We expect to depend on collaborations with third parties for the development and commercialization of our product candidates. If those collaborations are not successful, we may not be able to capitalize on the market potential of these product candidates.
Although we currently intend to commercialize AGS-003 ourselves in North America, we intend to seek to collaborate with third parties to commercialize AGS-003 and any future oncology product candidates outside North America or, if we decide that it is in our best interest, we may seek to collaborate with third parties to commercialize AGS-003 or any future oncology product candidate on a worldwide basis. We also plan to seek government or other third-party funding for continued development of AGS-004 and to collaborate with third parties to commercialize AGS-004 worldwide. In addition, we plan to seek partners for further development and commercialization of AGS-009 and AGS-010.
Our likely collaborators for any development, distribution, marketing, licensing or broader collaboration arrangements include large and mid-size pharmaceutical companies, regional and national pharmaceutical companies and biotechnology companies. If we do enter into any such arrangements with any third parties, we will likely have limited control over the amount and timing of resources that our collaborators dedicate to the development or commercialization of our product candidates. Our ability to generate revenues from these arrangements will depend on our collaborators’ abilities to successfully perform the functions assigned to them in these arrangements.
Collaborations involving our product candidates would pose the following risks to us:
| • | collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations; |
| • | collaborators may not pursue development and commercialization of our product candidates or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in the collaborator’s strategic focus or available funding, or external factors such as an acquisition that diverts resources or creates competing priorities; |
| • | collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing; |
| • | collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our products or product candidates if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours; |
| • | a collaborator with marketing and distribution rights to one or more products may not commit sufficient resources to the marketing and distribution of such product or products; |
| • | collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our proprietary information or expose us to potential litigation; |
| • | disputes may arise between the collaborators and us that result in the delay or termination of the research, development or commercialization of our products or product candidates or that result in costly litigation or arbitration that diverts management attention and resources; and |
| • | collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates. For example, our collaborations with Kyowa Hakko Kirin Co., Ltd., or Kirin, with respect to AGS-003 and AGS-004 and with Novo Nordisk A/S, or Novo, with respect to AGS-009 were each terminated by our collaborator for convenience. |
26
Table of Contents
Collaboration agreements may not lead to development or commercialization of product candidates in the most efficient manner, or at all. In addition, there have been a significant number of recent business combinations among large pharmaceutical companies that have resulted in a reduced number of potential future collaborators. If a present or future collaborator of ours were to be involved in a business combination, the continued pursuit and emphasis on our product development or commercialization program could be delayed, diminished or terminated.
If we are not able to establish collaborations, we may have to alter our development and commercialization plans.
Our drug development programs and the potential commercialization of our product candidates will require substantial additional cash to fund expenses. For some of our product candidates, we plan to collaborate with pharmaceutical and biotechnology companies for the development and potential commercialization of those product candidates. For example, we currently intend to seek to collaborate with third parties to commercialize AGS-003 and any future oncology product candidates in Europe and other parts of the world and to collaborate with third parties to commercialize AGS-004 worldwide. In addition, we plan to seek partners for the further development and commercialization of AGS-009 and AGS-010.
We face significant competition in seeking appropriate collaborators. Whether we reach a definitive agreement for a collaboration will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration, and the proposed collaborator’s evaluation of a number of factors. Those factors may include the design or results of clinical trials, the likelihood of approval by the FDA or similar regulatory authorities outside the United States, the potential market for the subject product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing products, the existence of uncertainty with respect to our ownership of technology, which can exist if there is a challenge to such ownership without regard to the merits of the challenge and industry and market conditions generally. The collaborator may also consider alternative product candidates or technologies for similar indications that may be available to collaborate on and whether such a collaboration could be more attractive than the one with us for our product candidate. We may also be restricted under existing license agreements from entering into agreements on certain terms with potential collaborators. Collaborations are complex and time-consuming to negotiate and document. We may not be able to negotiate collaborations on a timely basis, on acceptable terms, or at all.
We do not currently plan to develop AGS-004 beyond the ongoing phase 2b clinical trial, AGS-009 beyond the next planned clinical trial or AGS-010 unless we obtain government or other third-party funding to support such development, including through collaboration agreements. If we are not able to obtain such funding or enter into collaborations for any such product candidate, we may have to curtail the development of such product candidate, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of any sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on acceptable terms or at all. If we do not have sufficient funds, we may not be able to further develop these product candidates or bring these product candidates to market and generate product revenue.
We rely on third parties to conduct our clinical trials, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials.
We do not independently conduct clinical trials of our product candidates. We rely on third parties, such as contract research organizations, clinical data management organizations, medical institutions and clinical investigators, to perform this function. Our reliance on these third parties for clinical development activities reduces our control over these activities but does not relieve us of our responsibilities. We remain responsible for
27
Table of Contents
ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the FDA requires us to comply with standards, commonly referred to as Good Clinical Practices, for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. We also are required to register ongoing clinical trials and post the results of completed clinical trials on a government-sponsored database, ClinicalTrials.gov, within certain timeframes. Failure to do so can result in fines, adverse publicity and civil and criminal sanctions. Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols, we will not be able to obtain, or may be delayed in obtaining, regulatory approvals for our product candidates and will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates.
We also rely on other third parties to store and distribute drug supplies for our clinical trials. Any performance failure on the part of our existing or future distributors could delay clinical development or regulatory approval of our product candidates or commercialization of our products, producing additional losses and depriving us of potential product revenue.
Risks Related to the Manufacturing of Our Product Candidates
We plan to identify, lease, build out and equip a new U.S. facility to manufacture our Arcelis-based drug products on a commercial scale using automated processes. We do not have experience in manufacturing Arcelis-based drug products on a commercial scale or using automated processes. If, due to our lack of manufacturing experience, we cannot manufacture our Arcelis-based drug products on a commercial scale successfully or manufacture sufficient drug product to meet our expected commercial requirements, our business may be materially harmed.
We currently have manufacturing suites in our facility located at our corporate headquarters in Durham, North Carolina. We manufacture our Arcelis-based product candidates for research and development purposes and for clinical trials at this facility. We plan to identify, lease, build out and equip a new U.S. facility to manufacture our Arcelis-based drug products on a commercial scale using automated processes. We may be unable to find a suitable space to lease for our new commercial manufacturing facility. Even if we are able to find such a space, in light of our limited financial resources, a landlord may not be willing to lease the space to us on terms acceptable to us, or at all.
We do not have experience in manufacturing products on a commercial scale or using automated processes. In addition, because we are aware of only one company that has manufactured an active immunotherapy product for commercial sale, there are limited precedents from which we can learn. We may encounter difficulties in the manufacture of our Arcelis-based drug products due to our limited manufacturing experience. These difficulties could delay the build-out and equipping of the new facility and regulatory approval of the manufacture of our Arcelis-based drug products using the new facility and the automated processes, increase our costs or cause production delays or result in us not manufacturing sufficient drug product to meet our expected commercial requirements, any of which could damage our reputation and hurt our profitability. If we are unable to successfully increase our manufacturing capacity to commercial scale, our business may be materially adversely affected.
28
Table of Contents
We plan to build out and equip a new U.S. commercial manufacturing facility based on automated manufacturing processes and augment our manufacturing personnel in advance of any regulatory submission for approval of AGS-003. If we fail to build out and equip a new U.S. commercial manufacturing facility in compliance with regulatory requirements, implement our automated processes or augment our manufacturing personnel, we may not be able to initiate commercial operations or produce sufficient drug product to meet our expected commercial requirements.
In order to meet our business plan, which contemplates our manufacturing drug product internally using automated processes for the commercial requirements of AGS-003 and any other Arcelis-based product candidates that might be approved, we will need to lease, build out and equip a new U.S. commercial manufacturing facility and add manufacturing personnel in advance of any regulatory submission for approval of AGS-003. The leasing, build-out and equipping of our facilities will require substantial capital expenditures and additional regulatory approvals. In addition, it will be costly and time consuming to recruit necessary additional personnel.
Prior to implementing the automated manufacturing processes for Arcelis-based products and filing a BLA for approval of AGS-003, we will be required to:
| • | demonstrate that the disposable components and sterilization and packaging methods used in the manufacturing process are suitable for use in manufacturing in accordance with current good manufacturing practice, or cGMP, and current Good Tissue Practices, or cGTP; |
| • | build and validate processing equipment that complies with cGMP and cGTP; |
| • | identify, lease, build out and equip a suitable manufacturing facility to accommodate the automated manufacturing process; |
| • | perform process testing with final equipment, disposable components and reagents to demonstrate that the methods are suitable for use in cGMP and cGTP manufacturing; |
| • | demonstrate consistency and repeatability of the automated manufacturing processes in the production of AGS-003 in our new facility to fully validate the manufacturing and control process using the actual automated cGMP processing equipment; and |
| • | demonstrate comparability between AGS-003 that we produce using existing processes in our current facility and AGS-003 produced using the automated processes in our new facility. |
We expect that this implementation will require approximately two years to complete, but such implementation could take longer, particularly if we are unable to achieve any of the required tasks on a timely basis, or at all.
If we are unable to successfully build out and equip our commercial manufacturing facility in compliance with regulatory requirements, implement the automated processes required, demonstrate comparability between the AGS-003 used in our pivotal trial and the AGS-003 produced using the automated processes in the new facility, or hire additional necessary manufacturing personnel appropriately, our filing for regulatory approval of AGS-003 may be delayed or denied and we may not be able to initiate commercial operations even if any of our product candidates are approved for marketing.
29
Table of Contents
Lack of coordination internally among our employees and externally with physicians, hospitals and third-party suppliers and carriers, could cause manufacturing difficulties, disruptions or delays and cause us to not have sufficient drug product to meet our expected clinical trial requirements or potential commercial requirements.
Manufacturing our Arcelis-based product candidates requires coordination internally among our employees and externally with physicians, hospitals and third-party suppliers and carriers. For example, a patient’s physician or clinical site will need to coordinate with us for the shipping of a patient’s disease sample and leukapheresis product to our manufacturing facility, and we will need to coordinate with them for the shipping of the manufactured drug product to them. Such coordination involves a number of risks that may lead to failures or delays in manufacturing our Arcelis-based product candidates, including:
| • | failure to obtain a sufficient supply of key raw materials of suitable quality; |
| • | difficulties in manufacturing our product candidates for multiple patients simultaneously; |
| • | difficulties in obtaining adequate patient-specific material, such as tumor samples, virus samples or leukapheresis product, from physicians; |
| • | difficulties in completing the development and validation of the specialized assays required to ensure the consistency of our product candidates; |
| • | failure to ensure adequate quality control and assurances in the manufacturing process as we increase production quantities; |
| • | difficulties in the timely shipping of patient-specific materials to us or in the shipping of our product candidates to the treating physicians due to errors by third-party carriers, transportation restrictions or other reasons; |
| • | destruction of, or damage to, patient-specific materials or our product candidates during the shipping process due to improper handling by third-party carriers, hospitals, physicians or us; |
| • | destruction of, or damage to, patient-specific materials or our product candidates during storage at our facilities; and |
| • | destruction of, or damage to, patient-specific materials or our product candidates stored at clinical and future commercial sites due to improper handling or holding by clinicians, hospitals or physicians. |
If we are unable to coordinate appropriately, we may encounter delays or additional costs in achieving our clinical and commercialization objectives, including in obtaining regulatory approvals of our product candidates and supplying product, which could materially damage our business and financial position.
If our existing manufacturing facility or the new U.S. commercial manufacturing facility that we plan to lease, build out and equip are damaged or destroyed, or production at one of these facilities is otherwise interrupted, our business and prospects would be negatively affected.
If our existing manufacturing facility or the new U.S. commercial manufacturing facility that we plan to lease, build out and equip, or the equipment in either of these facilities, is damaged or destroyed, we likely would not be able to quickly or inexpensively replace our manufacturing capacity and possibly would not be able to replace it at all. Any new facility needed to replace either our existing manufacturing facility or the planned new U.S. commercial manufacturing facility would need to comply with the necessary regulatory requirements, need
30
Table of Contents
to be tailored to our specialized automated manufacturing requirements and require specialized equipment. We would need FDA approval before selling any products manufactured at a new facility. Such an event could delay our clinical trials or, if any of our product candidates are approved by the FDA, reduce or eliminate our product sales.
We maintain insurance coverage to cover damage to our property and equipment and to cover business interruption and research and development restoration expenses. If we have underestimated our insurance needs with respect to an interruption in our clinical manufacturing of our product candidates, we may not be able to adequately cover our losses.
We expect to contract with third parties for the manufacture of our non-Arcelis-based product candidates for clinical trials and for commercialization. This reliance on third parties increases the risk that we will not have sufficient quantities of our product candidates or products or such quantities at an acceptable cost, which could delay, prevent or impair our development or commercialization efforts.
We currently rely on third-party manufacturers for the production of our non-Arcelis-based product candidates for preclinical testing and clinical trials and expect to rely on third-party manufacturers or third-party collaborators for the manufacture of these product candidates for later stage clinical trials and for commercial supply of any of these product candidates for which we or our collaborators obtain marketing approval.
We do not have any agreements with third-party manufacturers for the clinical or commercial supply of any of our product candidates, and purchase our required drug supply on a purchase order basis. We may be unable to establish any agreements with third-party manufacturers or to do so on acceptable terms. Even if we are able to establish agreements with third-party manufacturers, reliance on third-party manufacturers entails additional risks, including:
| • | reliance on the third party for regulatory compliance and quality assurance; |
| • | the possible breach of the manufacturing agreement by the third party; and |
| • | the possible termination or nonrenewal of the agreement by the third party at a time that is costly or inconvenient for us. |
Third-party manufacturers may not be able to comply with cGMP, cGTP or similar regulatory requirements outside the United States. Our failure, or the failure of our third-party manufacturers, to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of product candidates or products, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect supplies of our products.
Any products that we may develop may compete with other product candidates and products for access to manufacturing facilities. There are a limited number of manufacturers that operate under cGMP regulations and that might be capable of manufacturing for us.
We currently rely on a single manufacturer for the production of AGS-009 and a different single manufacturer for the production of AGS-010. We do not have agreements in place for redundant supply or a second source for the production of these product candidates. Any performance failure on the part of our existing or future manufacturers could delay clinical development or marketing approval. If either of our current contract manufacturers cannot perform as agreed, we may be required to replace that manufacturer. Although we believe that there are a number of potential alternative manufacturers who could manufacture AGS-009 and AGS-010, we may incur added costs and delays in identifying and qualifying any such replacement.
31
Table of Contents
Our potential future dependence upon others for the manufacture of our product candidates or products may adversely affect our future profit margins and our ability to commercialize any products that receive regulatory approval on a timely and competitive basis.
Risks Related to Our Intellectual Property
If we fail to comply with our obligations under our intellectual property licenses with third parties, we could lose license rights that are important to our business.
We are a party to a number of intellectual property license agreements with third parties, including with respect to each of AGS-003, AGS-004 and AGS-009, and expect to enter into additional license agreements in the future. Our existing license agreements impose, and we expect that future license agreements will impose, various diligence, milestone payment, royalty, insurance and other obligations on us. For example, under our license agreement with Duke University which relates to patents and patent applications directed towards the composition of matter of Arcelis-based products, dendritic cells loaded with RNA from tumors or pathogens, methods of manufacture of these products and methods of using these products to treat tumors, we are required to use commercially reasonable efforts to research, develop and market license products and to satisfy other specified obligations. If we fail to comply with our obligations under these licenses, our licensors may have the right to terminate these license agreements, in which event we might not be able to market any product that is covered by these agreements, or to convert the license to a non-exclusive license, which could materially adversely affect the value of the product candidate being developed under the license agreement. Termination of these license agreements or reduction or elimination of our licensed rights may result in our having to negotiate new or reinstated licenses with less favorable terms.
If we are unable to obtain and maintain patent protection for our technology and products, or if our licensors are unable to obtain and maintain patent protection for the technology or products that we license from them, or if the scope of the patent protection obtained is not sufficiently broad, our competitors could develop and commercialize technology and products similar or identical to ours, and our ability to successfully commercialize our technology and products may be adversely affected.
Our success depends in large part on our and our licensors’ ability to obtain and maintain patent protection in the United States and other countries with respect to our proprietary technology and products. We and our licensors have sought to protect our proprietary position by filing patent applications in the United States and abroad related to our novel technologies and products that are important to our business. This process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. Moreover, in some circumstances, we do not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the patents, covering technology or products that we license from third parties and are reliant on our licensors. Therefore, we cannot be certain that these patents and applications will be prosecuted and enforced in a manner consistent with the best interests of our business. If such licensors fail to maintain such patents, or lose rights to those patents, the rights we have licensed may be reduced or eliminated.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our and our licensors’ patent rights are highly uncertain. Our and our licensors’ pending and future patent applications may not result in patents being issued which protect our technology or products or which effectively prevent others from commercializing competitive technologies and products. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection.
The laws of foreign countries may not protect our rights to the same extent as the laws of the United States. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent
32
Table of Contents
applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore we cannot be certain that we or our licensors were the first to make the inventions claimed in our owned or licensed patents or pending patent applications, or that we or our licensors were the first to file for patent protection of such inventions.
Assuming the other requirements for patentability are met, in the United States, the first to invent the claimed invention is entitled to the patent, while outside the United States, the first to file a patent application is generally entitled to the patent. Under the America Invents Act, or AIA, enacted in September 2011, the United States will move to a first inventor to file system in March 2013. We may become involved in opposition or interference proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such proceeding or litigation could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights.
Even if our owned and licensed patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our owned or licensed patents by developing similar or alternative technologies or products in a non-infringing manner. The issuance of a patent is not conclusive as to its scope, validity or enforceability, and our owned and licensed patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in patent claims being narrowed, invalidated or held unenforceable, which could limit our ability to or stop or prevent us from stopping others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. For example, certain of the U.S. patents we exclusively license from Duke University expire in 2016 and the European and Japanese patents exclusively licensed from Duke University expire in 2017. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
We may become involved in lawsuits to protect or enforce our patents, which could be expensive, time consuming and unsuccessful.
Competitors may infringe our patents. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours is invalid or unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation proceeding could put one or more of our patents at risk of being invalidated or interpreted narrowly. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, our licensors may have rights to file and prosecute such claims and we are reliant on them.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a material adverse effect on the success of our business.
Our commercial success depends upon our ability and the ability of our collaborators to develop, manufacture, market and sell our product candidates and use our proprietary technologies without infringing the proprietary rights of third parties. We may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our products and technology, including interference proceedings before the U.S. Patent and Trademark Office. Third parties may assert infringement
33
Table of Contents
claims against us based on existing patents or patents that may be granted in the future. If we are found to infringe a third party’s intellectual property rights, we could be required to obtain a license from such third party to continue developing and marketing our products and technology. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. We could be forced, including by court order, to cease commercializing the infringing technology or product. In addition, we could be found liable for monetary damages. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
We have research licenses to certain reagents and their use in the development of our product candidates. We would need commercial licenses to these reagents for any of our product candidates that receive approval for sale in the United States. We believe that commercial licenses to these reagents will be available. If we are unable to obtain any such commercial licenses, we may be unable to commercialize our product candidates without infringing the patent rights of third parties. If we did seek to commercialize our product candidates without a license, these third parties could initiate legal proceedings against us.
We may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Many of our employees were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these employees have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such employee’s former employer. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
Intellectual property litigation could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patents for some of our technology and products, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive
34
Table of Contents
position. The types of protections available for trade secrets are particularly important with respect to our Arcelis platform’s manufacturing capabilities, which involve significant unpatented know-how. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, sponsored researchers, contract manufacturers, consultants, advisors and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
Risks Related to Regulatory Approval of Our Product Candidates and Other Legal Compliance Matters
If we are not able to obtain, or if there are delays in obtaining, required regulatory approvals, we will not be able to commercialize our product candidates, and our ability to generate revenue will be materially impaired.
Our product candidates, including AGS-003, AGS-004, AGS-009 and AGS-010, and the activities associated with their development and commercialization, including their design, testing, manufacture, safety, efficacy, recordkeeping, labeling, storage, approval, advertising, promotion, sale and distribution, are subject to comprehensive regulation by the FDA and other regulatory agencies in the United States and by comparable authorities in other countries. Failure to obtain regulatory approval for a product candidate will prevent us from commercializing the product candidate. We have not received regulatory approval to market any of our product candidates in any jurisdiction. We have only limited experience in filing and supporting the applications necessary to gain regulatory approvals and expect to rely on third-party contract research organizations to assist us in this process. Securing FDA approval requires the submission of extensive preclinical and clinical data and supporting information to the FDA for each therapeutic indication to establish the product candidate’s safety and efficacy. Securing FDA approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities by, the FDA. Our product candidates may not be effective, may be only moderately effective or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude our obtaining regulatory approval or prevent or limit commercial use.
The process of obtaining regulatory approvals, both in the United States and abroad, is expensive, may take many years if additional clinical trials are required, if approval is obtained at all, and can vary substantially based upon a variety of factors, including the type, complexity and novelty of the product candidates involved. To date, the FDA has only approved one active cellular immunotherapy product. Changes in regulatory approval policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for each submitted product application, may cause delays in the approval or rejection of an application. The FDA has substantial discretion in the approval process and may refuse to accept any application or may decide that our data is insufficient for approval and require additional preclinical, clinical or other studies. In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit or prevent regulatory approval of a product candidate. Any regulatory approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable.
If we experience delays in obtaining approval or if we fail to obtain approval of our product candidates, the commercial prospects for our product candidates may be harmed and our ability to generate revenues will be materially impaired.
35
Table of Contents
Failure to obtain regulatory approval in international jurisdictions would prevent our product candidates from being marketed abroad.
We intend to enter into arrangements with third parties under which they would market our products outside the United States. In order to market and sell our products in the European Union and many other jurisdictions, we or such third parties must obtain separate regulatory approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval may differ substantially from that required to obtain FDA approval. The regulatory approval process outside the United States generally includes all of the risks associated with obtaining FDA approval. In addition, in many countries outside the United States, it is required that the product be approved for reimbursement before the product can be approved for sale in that country. We or these third parties may not obtain approvals from regulatory authorities outside the United States on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the United States does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. We may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize our products in any market.
Any product candidate for which we obtain marketing approval could be subject to restrictions or withdrawal from the market and we may be subject to penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our products, when and if any of them are approved.
Any product candidate for which we obtain marketing approval, along with the manufacturing processes, post-approval clinical data, labeling, advertising and promotional activities for such product, will be subject to continual requirements of and review by the FDA and other regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, registration and listing requirements, cGMP requirements relating to quality control, quality assurance and corresponding maintenance of records and documents, cGTP requirements, requirements regarding the distribution of samples to physicians and recordkeeping. Even if regulatory approval of a product candidate is granted, the approval may be subject to limitations on the indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the product. The FDA closely regulates the post-approval marketing and promotion of drugs to ensure drugs are marketed only for the approved indications and in accordance with the provisions of the approved label. The FDA imposes stringent restrictions on manufacturers’ communications regarding off-label use and if we do not market our products for their approved indications, we may be subject to enforcement action for off-label marketing.
In addition, later discovery of previously unknown problems with our products, manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may yield various results, including:
| • | restrictions on such products, manufacturers or manufacturing processes; |
| • | restrictions on the marketing of a product; |
| • | restrictions on product distribution; |
| • | requirements to conduct post-marketing clinical trials; |
| • | warning or untitled letters; |
| • | withdrawal of the products from the market; |
| • | refusal to approve pending applications or supplements to approved applications that we submit; |
| • | recall of products; |
36
Table of Contents
| • | fines, restitution or disgorgement of profits or revenue; |
| • | suspension or withdrawal of regulatory approvals; |
| • | refusal to permit the import or export of our products; |
| • | product seizure; or |
| • | injunctions or the imposition of civil or criminal penalties. |
Our relationships with customers and third-party payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, program exclusion, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, physicians and third-party payors play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Our future arrangements with third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products for which we obtain marketing approval. Restrictions under applicable federal and state healthcare laws and regulations, include the following:
| • | the federal healthcare anti-kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal and state healthcare programs such as Medicare and Medicaid; |
| • | the federal False Claims Act imposes civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| • | the federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| • | the federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services; |
| • | the federal transparency requirements under the Health Care Reform Law will require manufacturers of drugs, devices, biologics and medical supplies to report to the Department of Health and Human Services information related to physician payments and other transfers of value and physician ownership and investment interests; and |
| • | analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers, and some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines |
37
Table of Contents
| and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any of the physicians or other providers or entities with whom we expect to do business are found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Recently enacted and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and affect the prices we may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any product candidates for which we obtain marketing approval.
In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or Medicare Modernization Act, changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on average sales prices for physician administered drugs. In addition, this legislation provided authority for limiting the number of drugs that will be covered in any therapeutic class in certain cases. Cost reduction initiatives and other provisions of this legislation could decrease the coverage and reimbursement that is provided for any approved products. While the Medicare Modernization Act applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from the Medicare Modernization Act may result in a similar reduction in payments from private payors.
More recently, in March 2010, President Obama signed into law the Health Care Reform Law, a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for health care and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. Effective October 1, 2010, the Health Care Reform Law revises the definition of “average manufacturer price” for reporting purposes, which could increase the amount of Medicaid drug rebates to states. Further, the new law imposes a significant annual fee on companies that manufacture or import branded prescription drug products. Substantial new provisions affecting compliance have also been enacted, which may affect our business practices with health care practitioners. We will not know the full effects of the Health Care Reform Law until applicable federal and state agencies issue regulations or guidance under the new law. Although it is too early to determine the effect of the Health Care Reform Law, the new law appears likely to continue the pressure on pharmaceutical pricing, especially under the Medicare program, and may also increase our regulatory burdens and operating costs.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what
38
Table of Contents
the impact of such changes on the marketing approvals of our product candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
With the enactment of the Biologics Price Competition and Innovation Act of 2009, or BPCIA, as part of the Health Care Reform Law, an abbreviated pathway for the approval of biosimilar and interchangeable biological products was created. The new abbreviated regulatory pathway establishes legal authority for the FDA to review and approve biosimilar biologics, including the possible designation of a biosimilar as “interchangeable” based on its similarity to an existing brand product. Under the BPCIA, an application for a biosimilar product cannot be submitted to the FDA until four years, or approved by the FDA until 12 years, after the original brand product identified as the reference product was approved under a BLA. The new law is complex and is only beginning to be interpreted and implemented by the FDA. As a result, its ultimate impact, implementation and meaning is subject to uncertainty. While it is uncertain when any such processes may be fully adopted by the FDA, any such processes could have a material adverse effect on the future commercial prospects for our biological products.
We believe that if any of our product candidates were to be approved as biological products under a BLA, such approved products should qualify for the four-year and 12-year periods of exclusivity. However, there is a risk that the U.S. Congress could amend the BPCIA to significantly shorten these exclusivity periods as proposed by President Obama, or that the FDA will not consider our product candidates to be reference products for competing products, potentially creating the opportunity for generic competition sooner than anticipated. Moreover, the extent to which a biosimilar, once approved, will be substituted for any one of our reference products in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing.
Risks Related to Employee Matters and Managing Growth
Our future success depends on our ability to retain our chief executive officer and other key executives and to attract, retain and motivate qualified personnel.
We are highly dependent on Jeffrey Abbey, our President and Chief Executive Officer, Charles Nicolette, our Vice President of Research and Development and Chief Scientific Officer, and Fred Miesowicz, our Vice President of Manufacturing and Chief Operating Officer, as well as the other principal members of our management and scientific teams. Although we have formal employment agreements with each of our executive officers, these agreements do not prevent our executives from terminating their employment with us at any time. We do not maintain “key person” insurance for any of our executives or other employees. The loss of the services of any of these persons could impede the achievement of our research, development and commercialization objectives.
Recruiting and retaining qualified scientific, clinical, manufacturing and sales and marketing personnel will also be critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us.
39
Table of Contents
We expect to expand our development, regulatory, manufacturing and sales and marketing capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We expect to experience significant growth in the number of our employees and the scope of our operations, particularly in the areas of drug development, regulatory affairs, manufacturing and sales and marketing. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The physical expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.
Risks Related to Our Common Stock and This Offering
After this offering, our executive officers, directors and principal stockholders will maintain the ability to control all matters submitted to stockholders for approval.
Upon the closing of this offering, our executive officers, directors and stockholders who owned more than 5% of our outstanding common stock before this offering will, in the aggregate, beneficially own shares representing approximately % of our capital stock. As a result, if these stockholders were to choose to act together, they would be able to control all matters submitted to our stockholders for approval, as well as our management and affairs. For example, these persons, if they choose to act together, would control the election of directors and approval of any merger, consolidation or sale of all or substantially all of our assets. This concentration of voting power could delay or prevent an acquisition of our company on terms that other stockholders may desire.
Provisions in our corporate charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our corporate charter and our bylaws that will become effective upon the closing of this offering may discourage, delay or prevent a merger, acquisition or other change in control of us that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. These provisions could also limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock. In addition, because our board of directors is responsible for appointing the members of our management team, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors. Among other things, these provisions:
| • | establish a classified board of directors such that not all members of the board are elected at one time; |
| • | allow the authorized number of our directors to be changed only by resolution of our board of directors; |
| • | limit the manner in which stockholders can remove directors from the board; |
| • | establish advance notice requirements for stockholder proposals that can be acted on at stockholder meetings and nominations to our board of directors; |
| • | require that stockholder actions must be effected at a duly called stockholder meeting and prohibit actions by our stockholders by written consent; |
| • | limit who may call stockholder meetings; |
40
Table of Contents
| • | authorize our board of directors to issue preferred stock without stockholder approval, which could be used to institute a “poison pill” that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by our board of directors; and |
| • | require the approval of the holders of at least 75% of the votes that all our stockholders would be entitled to cast to amend or repeal certain provisions of our charter or bylaws. |
Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner.
If you purchase shares of common stock in this offering, you will suffer immediate dilution of your investment.
The initial public offering price of our common stock is substantially higher than the net tangible book value per share of our common stock. Therefore, if you purchase shares of our common stock in this offering, you will pay a price per share that substantially exceeds our net tangible book value per share after this offering. To the extent outstanding options or warrants are exercised, you will incur further dilution. Based on an assumed initial public offering price of $ per share, which is the midpoint of the price range listed on the cover page of this prospectus, you will experience immediate dilution of $ per share, representing the difference between our pro forma net tangible book value per share after giving effect to this offering and the assumed initial public offering price. In addition, purchasers of common stock in this offering will have contributed approximately % of the aggregate price paid by all purchasers of our stock but will own only approximately % of our common stock outstanding after this offering.
An active trading market for our common stock may not develop.
Prior to this offering, there has been no public market for our common stock. The initial public offering price for our common stock will be determined through negotiations with the underwriters. Although we have applied to have our common stock listed on The NASDAQ Global Market, an active trading market for our shares may never develop or be sustained following this offering. If an active market for our common stock does not develop, it may be difficult for you to sell shares you purchase in this offering without depressing the market price for the shares or at all.
If our stock price is volatile, purchasers of our common stock could incur substantial losses.
Our stock price is likely to be volatile. The stock market in general and the market for biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, you may not be able to sell your common stock at or above the initial public offering price. The market price for our common stock may be influenced by many factors, including:
| • | the success of competitive products or technologies; |
| • | results of clinical trials of our product candidates or those of our competitors; |
| • | regulatory or legal developments in the United States and other countries; |
| • | developments or disputes concerning patents or other proprietary rights; |
| • | the recruitment or departure of key personnel; |
41
Table of Contents
| • | the level of expenses related to any of our product candidates or clinical development programs; |
| • | the results of our efforts to discover, develop, acquire or in-license additional product candidates or products; |
| • | actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts; |
| • | variations in our financial results or those of companies that are perceived to be similar to us; |
| • | changes in the structure of healthcare payment systems; |
| • | market conditions in the pharmaceutical and biotechnology sectors and issuance of new or changed securities analysts’ reports or recommendations; |
| • | general economic, industry and market conditions; and |
| • | the other factors described in this “Risk Factors” section. |
We have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our management will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our common stock. The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse effect on our business, cause the price of our common stock to decline and delay the development of our product candidates. Pending their use, we may invest the net proceeds from this offering in a manner that does not produce income or that loses value.
Because we do not anticipate paying any cash dividends on our capital stock in the foreseeable future, capital appreciation, if any, will be your sole source of gain.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. In addition, the terms of existing or any future debt agreements may preclude us from paying dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
A significant portion of our total outstanding shares are restricted from immediate resale but may be sold into the market in the near future, which could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. After this offering, we will have outstanding shares of common stock based on the number of shares outstanding as of August 31, 2011. This includes the shares that we are selling in this offering, which may be resold in the public market immediately without restriction, unless purchased by our affiliates. Of the remaining shares, shares are currently restricted as a result of securities laws or lock-up agreements but will be able to be sold after the offering as described in the “Shares Eligible for Future Sale” section of this prospectus. Moreover, after this offering, holders of an aggregate of shares of our common stock will have rights, subject to some conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders. We also intend to register all shares of common stock that we may issue under our equity compensation plans. Once we register these shares, they can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates and the lock-up agreements described in the “Underwriting” section of this prospectus.
42
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this prospectus, including statements regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.
The forward-looking statements in this prospectus include, among other things, statements about:
| • | our expectations related to the use of proceeds from this offering; |
| • | the progress and timing of our development and commercialization activities; |
| • | the timing and conduct of our planned pivotal phase 3 combination therapy clinical trial of AGS-003 and sunitinib and of our clinical trials of our other product candidates, including statements regarding the timing of commencement and completion of enrollment in our pivotal phase 3 combination therapy clinical trial of AGS-003 and sunitinib, our phase 2b clinical trial of AGS-004 for the treatment of HIV and our next planned clinical trial of AGS-009, the timing of completion of our pivotal phase 3 clinical trial of AGS-003 and the clinical trials of our other product candidates and the period during which the results of such trials will become available; |
| • | our ability to obtain U.S. and foreign marketing approval for AGS-003 and our other product candidates and the ability of these product candidates to meet existing or future regulatory standards; |
| • | the potential benefits of our Arcelis platform, our Arcelis-based product candidates and our other product candidates; |
| • | our ability to identify, lease, build out and equip a new U.S. commercial manufacturing facility and supply on a commercial scale our Arcelis-based products; |
| • | our intellectual property position; |
| • | the accuracy of our estimates of the size and characteristics of the markets that may be addressed by our product candidates; |
| • | our ability to establish and maintain partnerships for development and commercialization of our product candidates; and |
| • | our estimates regarding expenses, future revenues, capital requirements and needs for additional financing, including the costs of our planned pivotal phase 3 combination therapy clinical trial of AGS-003 and sunitinib, the costs of leasing, building out and equipping a new U.S. commercial manufacturing facility and the costs of producing Arcelis-based products at such facility. |
We have based these forward-looking statements largely on our current plans, intentions, expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. We have included important factors in the cautionary statements included
43
Table of Contents
in this prospectus, particularly in the “Risk Factors” section, that we believe could cause actual results or events to differ materially from the forward-looking statements that we make. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified timeframe, or at all. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make.
You should read this prospectus and the documents that we reference in this prospectus and have filed as exhibits to the registration statement of which this prospectus is a part completely and with the understanding that our actual future results may be materially different from what we expect. We do not assume any obligation to update any forward-looking statements.
This prospectus includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. While we believe these industry publications and third-party research, surveys and studies are reliable, we have not independently verified such data. This prospectus also includes data based on our own internal estimates. While we believe that our internal company research is reliable and that our internal estimates are reasonable, no independent source has verified such research or estimates.
44
Table of Contents
We estimate that the net proceeds from our issuance and sale of shares of our common stock in this offering will be approximately $ million, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. If the underwriters exercise their over-allotment option in full, we estimate that the net proceeds from this offering will be approximately $ million.
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share would increase (decrease) the net proceeds from this offering by approximately $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We intend to devote approximately $ of the net proceeds from this offering to fund the direct costs of our planned pivotal phase 3 combination therapy clinical trial of AGS-003 and to use the balance for other general corporate purposes. We also will use $200,000 of the net proceeds to pay a success fee to a former lender under a loan agreement that we have previously repaid in full.
This expected use of the net proceeds from this offering and our existing cash and cash equivalents represents our intentions based upon our current plans and business conditions. The amounts and timing of our actual expenditures may vary significantly depending on numerous factors, including the progress of our development and commercialization efforts, the status of and results from clinical trials, as well as any collaborations that we may enter into with third parties for our product candidates, and any unforeseen cash needs. As a result, our management will retain broad discretion over the allocation of the net proceeds from this offering. We have no current understandings, agreements or commitments for any material acquisitions or licenses of any products, businesses or technologies.
We expect that the net proceeds from this offering will be sufficient to enable us to substantially complete the planned pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib. However, it is possible that we will not achieve the progress that we expect because the actual costs and timing of development, particularly clinical trials, are difficult to predict and subject to substantial risks and delays. We do not expect that the net proceeds from this offering will be sufficient to enable us to fund our planned leasing, build-out and equipping of a new U.S. commercial manufacturing facility, which we will need to have completed in order to submit to the FDA a BLA for AGS-003 and to manufacture AGS-003 or any of our Arcelis-based products on a commercial scale. Although we do not expect the net proceeds from this offering to be sufficient to enable us to fund any clinical trials of any of our other product candidates, we are planning to commence enrollment in a clinical trial of AGS-009 in the first half of 2012 if the results of our phase 1a clinical trial justify proceeding with further clinical development. We plan to seek a partner for funding the trial and for the further development and commercialization of AGS-009, but would initiate this trial even if we have not secured a partner at the time this trial is scheduled to begin.
Pending our use of the net proceeds from this offering, we intend to invest the net proceeds in a variety of capital preservation investments, including short-term, investment grade, interest bearing instruments and U.S. government securities.
We have never declared or paid cash dividends on our common stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. We do not intend to pay cash dividends to holders of our common stock in the foreseeable future.
45
Table of Contents
The following table sets forth our cash, cash equivalents and short-term investments and capitalization as of June 30, 2011, as follows:
| • | on an actual basis; |
| • | on a pro forma basis to reflect: |
| • | the automatic conversion of all outstanding shares of our preferred stock into an aggregate of 157,871,412 shares of our common stock upon the closing of this offering; |
| • | the receipt in July 2011 of gross proceeds of $3.5 million upon the issuance and sale of the July 2011 convertible notes; |
| • | the automatic conversion of all principal and accrued interest on our outstanding convertible notes, including the 2010 convertible notes and the July 2011 convertible notes, upon the closing of this offering, into an aggregate of shares of our common stock, at the initial public offering price, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011; |
| • | the issuance of an aggregate of shares of our common stock upon the automatic exercise of outstanding warrants to purchase shares of our series C preferred stock by net exercise at an exercise price of $0.01 per share upon the closing of this offering, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus; |
| • | the restatement of our certificate of incorporation upon the closing of this offering; |
| • | the issuance of an aggregate of 4,609,457 shares of our common stock to specified holders of the capital stock of DC Bio in exchange for their shares of DC Bio upon the closing of this offering; and |
| • | on a pro forma as adjusted basis to give further effect to our issuance and sale of shares of common stock in this offering at an assumed initial public offering price of $ per share, which is the midpoint of the price range listed on the cover page of this prospectus, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
46
Table of Contents
Our capitalization following the closing of this offering will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing. You should read this table together with our consolidated financial statements and the related notes appearing at the end of this prospectus and the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section of this prospectus.
| June 30, 2011 | ||||||||||||
| Actual | Pro Forma | Pro Forma As Adjusted |
||||||||||
| (Unaudited) | ||||||||||||
| (In thousands, except share data) | ||||||||||||
| Cash, cash equivalents and short-term investments |
$ | 1,359 | $ | $ | ||||||||
|
|
|
|
|
|
|
|||||||
| Convertible term note including accrued interest |
5,694 | |||||||||||
| Warrant liability |
6,020 | |||||||||||
| Redeemable convertible preferred stock |
80,011 | |||||||||||
| Stockholders’ deficit |
||||||||||||
| Common stock, $0.001 par value, 262,870,000 shares authorized; and 12,849,491 shares issued and outstanding, actual; shares authorized and shares issued and outstanding, pro forma; shares issued and outstanding, pro forma as adjusted |
13 | |||||||||||
| Equity attributable to noncontrolling interest |
2,992 | |||||||||||
| Additional paid-in capital |
26,805 | |||||||||||
| Accumulated other comprehensive income |
88 | |||||||||||
| Deficit accumulated during the development stage |
(117,812 | ) | ||||||||||
|
|
|
|
|
|
|
|||||||
| Total stockholders’ deficit |
(87,914 | ) | ||||||||||
|
|
|
|
|
|
|
|||||||
| Total capitalization |
$ | 3,811 | $ | $ | ||||||||
|
|
|
|
|
|
|
|||||||
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share would increase (decrease) each of cash and cash equivalents, additional paid-in capital, total stockholders’ equity (deficit) and total capitalization on a pro forma as adjusted basis by approximately $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
The table above does not include:
| • | 24,190,022 shares of our common stock issuable upon the exercise of stock options outstanding as of June 30, 2011 at a weighted average exercise price of $0.09 per share; |
| • | 13,205,160 additional shares of our common stock available for future issuance as of June 30, 2011 under our 2008 plan; |
| • | additional shares of our common stock that will be available for future issuance, upon the closing of this offering, under our 2011 plan; and |
| • | 21,617 shares of common stock issuable upon exercise of warrants outstanding as of June 30, 2011, other than the warrants to purchase shares of our series C preferred stock described above, at a weighted average exercise price of $21.49 per share. |
47
Table of Contents
If you invest in our common stock in this offering, your ownership interest will be diluted immediately to the extent of the difference between the initial public offering price per share and the pro forma as adjusted net tangible book value per share of our common stock after this offering.
Our historical net tangible book value as of June 30, 2011 was $ million, or $ per share of our common stock. Historical net tangible book value per share represents the amount of our total tangible assets less total liabilities, divided by the number of shares of our common stock outstanding.
Our pro forma net tangible book value as of June 30, 2011 was $ million, or $ per share of our common stock. Pro forma net tangible book value per share represents the amount of our total tangible assets less our total liabilities, divided by the pro forma number of shares of our common stock outstanding after giving effect to:
| • | the automatic conversion of all outstanding shares of our preferred stock into an aggregate of 157,871,412 shares of our common stock upon the closing of this offering; |
| • | the automatic conversion of all principal and accrued interest on our outstanding convertible notes, including the 2010 convertible notes and the July 2011 convertible notes, upon the closing of this offering, into an aggregate of shares of our common stock, at the initial public offering price, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011; |
| • | the issuance of an aggregate of shares of our common stock upon the automatic exercise of outstanding warrants to purchase shares of our series C preferred stock by net exercise at an exercise price of $0.01 per share upon the closing of this offering, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus; and |
| • | the issuance of an aggregate of 4,609,457 shares of our common stock to specified holders of the capital stock of DC Bio in exchange for their shares of DC Bio upon the closing of this offering. |
After giving effect to our issuance and sale of shares of our common stock in this offering at an assumed initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, our pro forma as adjusted net tangible book value as of June 30, 2011 would have been $ million, or $ per share. This represents an immediate increase in pro forma as adjusted net tangible book value per share of $ to existing stockholders and immediate dilution of $ per share in pro forma as adjusted net tangible book value to new investors purchasing shares of common stock in this offering. Dilution per share to new investors is determined by subtracting pro forma as adjusted net tangible book value per share after this offering from the initial public offering price per share paid by new investors. The following table illustrates this dilution:
| Assumed initial public offering price per share |
$ | |||||||
| Historical net tangible book value per share as of June 30, 2011 |
$ | |||||||
| Increase per share attributable to the assumed conversion of outstanding preferred stock and convertible notes, exercise of warrants to purchase our series C preferred stock and issuance of common stock to shareholders of DC Bio |
||||||||
|
|
|
|||||||
| Pro forma net tangible book value per share as of June 30, 2011 |
||||||||
|
|
|
|||||||
| Increase in net tangible book value per share attributable to new investors |
||||||||
|
|
|
|||||||
| Pro forma as adjusted net tangible book value per share after giving effect to this offering |
$ | |||||||
|
|
|
|||||||
| Dilution per share to new investors |
$ | |||||||
|
|
|
48
Table of Contents
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share would increase (decrease) our pro forma as adjusted net tangible book value by approximately $ , our pro forma as adjusted net tangible book value per share by approximately $ and dilution per share to new investors by approximately $ , assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
If the underwriters exercise their over-allotment option or if any additional shares are issued in connection with outstanding options or warrants, you will experience further dilution.
The following table summarizes, on a pro forma as adjusted basis as of June 30, 2011, the total number of shares purchased from us, the total consideration paid, or to be paid, and the average price per share paid, or to be paid, by existing stockholders and by new investors in this offering at an assumed initial public offering price of $ per share, which is the midpoint of the price range listed on the cover page of this prospectus, before deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. As the table shows, new investors purchasing shares in this offering will pay an average price per share substantially higher than our existing stockholders paid.
| Shares Purchased | Total Consideration | Average Price Per Share |
||||||||||||||||
| Number | Percentage | Amount | Percentage | |||||||||||||||
| Existing stockholders |
% | $ | % | $ | ||||||||||||||
| New investors |
||||||||||||||||||
|
|
|
|
|
|
|
|
||||||||||||
| Total |
100 | % | $ | 100 | % | |||||||||||||
|
|
|
|
|
|
|
|
||||||||||||
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share would increase (decrease) the total consideration paid by new investors by $ million and increase (decrease) the percentage of total consideration paid by new investors by approximately %, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same.
The table and calculations set forth above are based on actual shares outstanding as of June 30, 2011 and include:
| • | 157,871,412 shares of our common stock issuable upon the automatic conversion of all outstanding shares of our preferred stock upon the closing of this offering; |
| • | shares of our common stock issuable upon the automatic conversion of all principal and accrued interest on our outstanding convertible notes, including the 2010 convertible notes and the July 2011 convertible notes, upon the closing of this offering, at the initial public offering price, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011; |
| • | shares of our common stock upon the automatic exercise of outstanding warrants to purchase shares of our series C preferred stock by net exercise at an exercise price of $0.01 per share upon the closing of this offering, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus; and |
| • | 4,609,457 shares of our common stock issuable to specified holders of the capital stock of DC Bio in exchange for their shares of DC Bio upon the closing of this offering. |
The table above excludes:
| • | 24,190,022 shares of our common stock issuable upon the exercise of stock options outstanding as of June 30, 2011 at a weighted average exercise price of $0.09 per share; |
49
Table of Contents
| • | 13,205,160 additional shares of our common stock available for future issuance as of June 30, 2011 under our 2008 plan; |
| • | additional shares of our common stock that will be available for future issuance, upon the closing of this offering, under our 2011 plan; and |
| • | 21,617 shares of common stock issuable upon exercise of warrants outstanding as of June 30, 2011, other than the warrants to purchase shares of our series C preferred stock described above, at a weighted average exercise price of $21.49 per share. |
If the underwriters exercise their over-allotment option in full, the following will occur:
| • | the percentage of shares of our common stock held by existing stockholders will decrease to approximately % of the total number of shares of our common stock outstanding after this offering; and |
| • | the number of shares of our common stock held by new investors will increase to , or approximately % of the total number of shares of our common stock outstanding after this offering. |
50
Table of Contents
SELECTED CONSOLIDATED FINANCIAL DATA
You should read the following selected consolidated financial data together with our consolidated financial statements and the related notes appearing at the end of this prospectus and the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section of this prospectus. We have derived the consolidated statements of operations data for the years ended December 31, 2008, 2009 and 2010 and the consolidated balance sheet data as of December 31, 2009 and 2010 from our audited consolidated financial statements included in this prospectus. We have derived the consolidated statements of operations data for the years ended December 31, 2006 and 2007 and the consolidated balance sheet data as of December 31, 2006, 2007 and 2008 from our audited consolidated financial statements not included in this prospectus. We have derived the consolidated statements of operations data for the six months ended June 30, 2010 and 2011 and the consolidated balance sheet data as of June 30, 2011 from our unaudited consolidated financial statements included in this prospectus. The unaudited consolidated financial data include, in the opinion of our management, all adjustments, consisting only of normal recurring adjustments, that are necessary for a fair presentation of our financial position and results of operations for these periods. Our historical results for any prior period are not necessarily indicative of results to be expected in any future period, and our results for any interim period are not necessarily indicative of results to be expected for a full fiscal year.
| Year Ended December 31, | Six
Months Ended June 30, 2010 |
Six
Months Ended June 30, 2011 |
Cumulative Period from Inception (May 8, 1997) to June 30, 2011 |
|||||||||||||||||||||||||||||
| 2006 | 2007 | 2008 | 2009 | 2010 | ||||||||||||||||||||||||||||
| (unaudited) | (unaudited) | (unaudited) | ||||||||||||||||||||||||||||||
| Statements of Operations Data: |
||||||||||||||||||||||||||||||||
| Revenue |
$ | 19,281,301 | $ | 5,158,789 | $ | 4,449,735 | $ | 5,367,989 | $ | 7,272,783 | $ | 1,990,006 | $ | 4,128,206 | $ | 71,851,340 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Operating expenses |
||||||||||||||||||||||||||||||||
| Research and development |
18,659,920 | 18,378,771 | 22,042,597 | 19,543,966 | 13,927,662 | 6,931,617 | 6,754,657 | 169,735,867 | ||||||||||||||||||||||||
| Net reimbursement under collaboration agreement |
(8,254,848 | ) | (7,615,181 | ) | (8,127,428 | ) | (6,626,989 | ) | — | — | — | (47,179,130 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net research and development |
10,405,072 | 10,763,590 | 13,915,169 | 12,916,977 | 13,927,662 | 6,931,617 | 6,754,657 | 122,556,737 | ||||||||||||||||||||||||
| General and administrative |
2,118,071 | 3,001,774 | 2,852,375 | 2,931,599 | 2,704,231 | 1,552,170 | 1,383,521 | 34,088,991 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total operating expenses |
12,523,143 | 13,765,364 | 16,767,544 | 15,848,576 | 16,631,893 | 8,483,787 | 8,138,178 | 156,645,728 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Operating income (loss) |
6,758,158 | (8,606,575 | ) | (12,317,809 | ) | (10,480,587 | ) | (9,359,110 | ) | (6,493,781 | ) | (4,009,972 | ) | (84,794,388 | ) | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Other income (expense) |
||||||||||||||||||||||||||||||||
| Interest income |
826,747 | 586,829 | 250,698 | 73,746 | 4,976 | 3,452 | 644 | 4,092,735 | ||||||||||||||||||||||||
| Interest expense |
(419,680 | ) | (920,265 | ) | (632,677 | ) | (283,417 | ) | (904,233 | ) | (30,645 | ) | (4,847,037 | ) | (10,409,389 | ) | ||||||||||||||||
| Derivative financing expense |
46,919 | 2,489,108 | (756,789 | ) | — | 298,009 | (231,784 | ) | (246,218 | ) | (1,227,403 | ) | ||||||||||||||||||||
| Investment tax credits |
— | 503,737 | 666,933 | 103,549 | 793,006 | 35,938 | — | 2,067,225 | ||||||||||||||||||||||||
| Loss on sale of marketable securities |
— | — | — | — | — | — | — | (10,242,252 | ) | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Other income (expense), net |
453,986 | 2,659,409 | (471,835 | ) | (106,122 | ) | 191,758 | (223,039 | ) | (5,092,611 | ) | (15,719,084 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net income (loss) |
7,212,144 | (5,947,166 | ) | (12,789,644 | ) | (10,586,709 | ) | (9,167,352 | ) | (6,716,820 | ) | (9,102,583 | ) | (100,513,472 | ) | |||||||||||||||||
| Net income (loss) attributable to noncontrolling interest |
628,188 | 333,744 | 136,643 | (654,760 | ) | (172,598 | ) | (86,271 | ) | (41,768 | ) | (738,828 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net income (loss) attributable to Argos Therapeutics, Inc. |
6,583,956 | (5,613,422 | ) | (12,926,287 | ) | (9,931,949 | ) | (8,994,754 | ) | (6,630,549 | ) | (9,060,815 | ) | (99,774,644 | ) | |||||||||||||||||
| Less: Accretion of redeemable convertible preferred stock |
36,865 | (141,768 | ) | (395,666 | ) | (101,206 | ) | (101,206 | ) | (50,604 | ) | (50,604 | ) | (29,172,382 | ) | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net income (loss) attributable to common stockholders |
$ | 6,620,821 | $ | (5,760,190 | ) | $ | (13,321,953 | ) | $ | (10,033,155 | ) | $ | (9,095,960 | ) | $ | (6,681,153 | ) | $ | (9,111,419 | ) | $ | (128,947,026 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Basic and diluted net loss attributable to common stockholders per share |
$ | 176.00 | $ | (1,433.60 | ) | $ | (153.81 | ) | $ | (62.78 | ) | $ | (2.23 | ) | $ | (33.92 | ) | $ | (0.71 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Basic and diluted weighted average shares outstanding |
4,013 | 4,018 | 86,615 | 159,825 | 4,081,649 | 196,976 | 12,849,491 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
51
Table of Contents
| December 31, | June
30, 2011 |
|||||||||||||||||||||||
| 2006 | 2007 | 2008 | 2009 | 2010 | ||||||||||||||||||||
| (unaudited) | ||||||||||||||||||||||||
| Balance Sheet Data: |
||||||||||||||||||||||||
| Cash, cash equivalents, and short-term investments |
$ | 15,611,833 | $ | 7,623,969 | $ | 23,654,667 | $ | 10,585,433 | $ | 5,141,965 | $ | 1,359,340 | ||||||||||||
| Total assets |
20,105,425 | 13,216,426 | 28,631,528 | 14,262,994 | 10,641,781 | 6,407,725 | ||||||||||||||||||
| Convertible term note including accrued interest |
4,145,115 | 4,387,101 | — | — | 3,669,858 | 5,694,032 | ||||||||||||||||||
| Warrant liability |
— | — | — | — | 3,197,626 | 6,020,386 | ||||||||||||||||||
| Long-term portion of notes payable |
1,996,716 | 3,938,697 | 1,214,780 | 4,317 | — | — | ||||||||||||||||||
| Redeemable convertible preferred stock |
71,023,862 | 71,223,218 | 88,779,980 | 88,881,186 | 79,640,412 | 80,011,016 | ||||||||||||||||||
| Deficit accumulated during the development stage |
(70,743,698 | ) | (76,898,317 | ) | (89,824,604 | ) | (99,756,553 | ) | (108,751,307 | ) | (117,812,122 | ) | ||||||||||||
| Total stockholders’ deficit |
$ | (67,501,166 | ) | $ | (73,797,553 | ) | $ | (69,040,663 | ) | $ | (79,034,936 | ) | $ | (78,936,784 | ) | $ | (87,914,285 | ) | ||||||
52
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and the related notes appearing at the end of this prospectus. In addition to historical information, some of the information contained in this discussion and analysis or set forth elsewhere in this prospectus, including information with respect to our plans and strategy for our business, future financial performance, expense levels and liquidity sources, includes forward-looking statements that involve risks and uncertainties. You should read the “Risk Factors” section of this prospectus for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We are a biopharmaceutical company focused on the development and commercialization of fully personalized immunotherapies for the treatment of cancer and infectious diseases based on our proprietary technology platform called Arcelis. Using biological components obtained from each patient, our Arcelis-based immunotherapies employ a specialized white blood cell, called a dendritic cell, to activate an immune response that is specific to the patient’s disease. Our most advanced product candidate is AGS-003 for the treatment of mRCC. We plan to initiate patient enrollment in a pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib, an oral small molecule drug marketed under the trade name Sutent that is the current standard of care for mRCC, in the first quarter of 2012. We are also developing a second Arcelis-based product candidate, AGS-004 for the treatment of HIV. We are studying AGS-004 in a phase 2b clinical trial that is funded entirely by the NIH. In addition to our Arcelis-based product candidates, we are also developing two other product candidates based on our expertise in dendritic cell biology: AGS-009, a monoclonal antibody for the treatment of lupus, which we are studying in a phase 1a clinical trial, and AGS-010, a preclinical biologic compound, which we are developing for organ transplantation and the treatment of autoimmune and inflammatory diseases.
We have tested AGS-003 in clinical trials as a monotherapy and in combination with sunitinib for the treatment of mRCC. We plan to initiate patient enrollment in a pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib compared to sunitinib plus placebo in the first quarter of 2012. We expect to complete enrollment of this trial in late 2013 and have data on the primary endpoint in the second half of 2014. To prepare for the commercial launch of AGS-003 and any other Arcelis-based products we develop, we plan to establish automated manufacturing processes based on existing functioning prototypes of automated devices and disposables and to identify, lease, build out and equip a new U.S. commercial manufacturing facility for this purpose. Our plan is to file our BLA for AGS-003 with the FDA using the automated manufacturing processes that we establish.
We have devoted substantially all of our resources to our drug development efforts, including advancing our Arcelis platform, conducting clinical trials of our product candidates, protecting our intellectual property and providing general and administrative support for these operations. We have not generated any revenue from product sales and, to date, have funded our operations primarily through the private placement of convertible preferred stock and convertible debt, bank debt, government contracts, grants and license and collaboration agreements. From inception in May 1997 through June 30, 2011, we raised a total of $195.4 million, including:
| • | $88.9 million from the sale of common stock, convertible debt, warrants and convertible preferred stock, including the convertible preferred stock sold by our 49.94% owned subsidiary, DC Bio. A description of DC Bio and the put right held by shareholders of DC Bio is set forth under “— Financial Overview-Derivative Financing Income (Expense);” |
| • | $32.9 million from the licensing of our technology; and |
| • | $73.6 million from government contracts, grants and license and collaboration agreements. |
53
Table of Contents
We have incurred losses in each year since our inception in May 1997. Our net losses, after attribution to the noncontrolling interest in DC Bio, were approximately $12.9 million, $9.9 million and $9.0 million for the years ended December 31, 2008, 2009 and 2010, respectively, and $6.6 million and $9.1 million for the six months ended June 30, 2010 and 2011, respectively. As of June 30, 2011, we had an accumulated deficit of approximately $117.8 million. Substantially all of our operating losses resulted from costs incurred in connection with our development programs and from general and administrative costs associated with our operations.
We expect to continue to incur significant expenses and increasing operating losses for at least the next several years. We anticipate that our expenses will increase substantially as we:
| • | initiate or continue our clinical trials of AGS-003 and our other product candidates; |
| • | seek regulatory approvals for our product candidates that successfully complete clinical trials; |
| • | lease, build out and equip a new U.S. commercial facility for the manufacture of AGS-003 and our other Arcelis-based products; |
| • | establish a sales, marketing and distribution infrastructure to commercialize products for which we may obtain regulatory approval; |
| • | maintain, expand and protect our intellectual property portfolio; |
| • | continue our other research and development efforts; |
| • | hire additional clinical, quality control, scientific and management personnel; and |
| • | add operational, financial and management information systems and personnel, including personnel to support our product development and planned commercialization efforts. |
We do not expect to generate significant funds or product revenue, other than under our contract with the NIH as described below, unless and until we successfully complete development and obtain marketing approval for our product candidates, either alone or in collaboration with third parties, which we expect will take a number of years and is subject to significant uncertainty. Accordingly, we will need to raise additional capital prior to the commercialization of any of AGS-003 or any of our other product candidates. Until such time, if ever, as we can generate substantial product revenues, we expect to finance our operating activities through a combination of equity offerings, debt financings, government funding, grant funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements. However, we may be unable to raise additional funds through equity offerings, debt financings, government funding, grant funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements when needed, on favorable terms or at all.
In September 2006, we entered into a multi-year research contract with the NIH and the National Institute of Allergy and Infectious Diseases, or NIAID, to design, develop and clinically test an autologous HIV immunotherapy capable of eliciting therapeutic immune responses. We are using funds from this contract to develop AGS-004. Under this contract, as amended, the NIH and NIAID have committed to fund up to a total of $33.9 million, including reimbursement of direct expenses and allocated overhead and general and administrative expenses of up to $32.5 million and payment of other specified amounts totaling up to $1.4 million upon our achievement of specified development milestones. This commitment extends until May 2013. We have agreed to a statement of work under the contract, and are obligated to furnish all the services, qualified personnel, material, equipment, and facilities, not otherwise provided by the U.S. government, needed to perform the statement of work. In September 2010, the NIH agreed to make a $2.0 million one-time payment to us as a true-up in connection with an agreement as to our indirect cost rates for allocated overhead and general and administrative expenses. The true-up reflects the difference between the provisional indirect cost rates originally provided for in
54
Table of Contents
the NIH contract and the negotiated indirect cost rates agreed upon in September 2010 for the years 2006 through 2009 and the eight-month period ended August 31, 2010. The $2.0 million true-up is included in the $33.9 million commitment. Since September 2010, we have received reimbursement of our allocated overhead and general and administrative expenses at provisional indirect cost rates equal to negotiated provisional indirect cost rates agreed to with the NIH in September 2010. These provisional indirect cost rates are subject to further true-up based on our actual costs pursuant to the agreement with the NIH. In September 2011, we agreed to a modification of our contract with the NIH and the NIAID under which the NIH and the NIAID agreed to increase their funding commitment to us by an additional $4.0 million in connection with our agreement to add an additional 12 patients to our phase 2b clinical trial of AGS-004. This $4.0 million increase is included in the $33.9 million commitment. We have recorded revenue of $19.9 million through June 30, 2011 under the NIH contract. This contract is the only arrangement under which we currently generate revenue. As of June 30, 2011, there was up to approximately $10.0 million of potential revenue remaining to be earned under the agreement with the NIH.
In July 2011, we issued and sold convertible notes in the aggregate original principal amount of $3.5 million. We refer to these notes as the July 2011 convertible notes. As of June 30, 2011, we had $6.5 million of principal and interest outstanding under convertible notes that we issued in 2010 and that we refer to as the 2010 convertible notes. We expect that the principal and accrued interest under both issues of these convertible notes will convert into common stock upon the closing of this offering.
Financial Overview
Revenue
To date, we have not generated revenue from the sale of any products. All of our revenue has been derived from government contracts, grants and payments from license and collaboration agreements. These government contract payments, grants and payments from license and collaboration agreements have provided us $71.9 million in revenue from inception to June 30, 2011. We may generate revenue in the future from government contracts, grants, payments from future license or collaboration agreements and product sales. We expect that any revenue we generate will fluctuate from quarter to quarter.
Research and Development Expenses
Since our inception, we have focused our resources on our research and development activities, including conducting preclinical studies and clinical trials, manufacturing development efforts and activities related to regulatory filings for our product candidates. We recognize our research and development expenses as they are incurred. Our research and development expenses consist primarily of:
| • | salaries and related expenses for personnel in research and development functions; |
| • | fees paid to consultants and clinical research organizations, or CROs, including in connection with our clinical trials, and other related clinical trial fees, such as for investigator grants, patient screening, laboratory work and statistical compilation and analysis; |
| • | allocation of facility lease and maintenance costs; |
| • | depreciation of leasehold improvements, laboratory equipment and computers; |
| • | costs related to production of product candidates for clinical trials; |
| • | costs related to compliance with regulatory requirements; |
55
Table of Contents
| • | consulting fees paid to third parties related to non-clinical research and development; |
| • | costs related to stock options or other stock-based compensation granted to personnel in research and development functions; and |
| • | acquisition fees, license fees and milestone payments related to acquired and in-licensed technologies. |
From inception through June 30, 2011, we have incurred $169.7 million in research and development expenses. We plan to increase our research and development expenses for the foreseeable future as we seek to complete development of AGS-003 and to further advance our other product candidates.
The table below summarizes our direct research and development expenses by program for the periods indicated. Our direct research and development expenses consist principally of external costs, such as fees paid to investigators, consultants, central laboratories and CROs, including in connection with our clinical trials, and related clinical trial fees. We have been developing AGS-003, AGS-004, AGS-009 and AGS-010 in parallel, and typically use our employee and infrastructure resources across multiple research and development programs. We do not allocate salaries, stock-based compensation, employee benefit or other indirect costs related to our research and development function to specific product candidates. Those expenses are included in “Indirect research and development expense” in the table.
| Years Ended December 31, | Six Months Ended June 30, 2010 |
Six Months Ended June 30, 2011 |
||||||||||||||||||
| 2008 | 2009 | 2010 | ||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Direct research and development expense by program: |
||||||||||||||||||||
| AGS-003 |
$ | 2,531 | $ | 1,890 | $ | 908 | $ | 436 | $ | 474 | ||||||||||
| AGS-004 |
4,060 | 3,624 | 3,117 | 1,158 | 1,445 | |||||||||||||||
| AGS-009 |
— | — | 516 | 196 | 847 | |||||||||||||||
| AGS-010 |
1,785 | 3,309 | 1,117 | 799 | 57 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total direct research and development expense |
8,376 | 8,823 | 5,658 | 2,589 | 2,823 | |||||||||||||||
| Indirect research and development expense |
13,667 | 10,721 | 8,270 | 4,343 | 3,932 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total research and development expense |
$ | 22,043 | $ | 19,544 | $ | 13,928 | $ | 6,932 | $ | 6,755 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
The successful development of our clinical and preclinical product candidates is highly uncertain. At this time, we cannot reasonably estimate the nature, timing or costs of the efforts that will be necessary to complete the remainder of the development of any of our clinical or preclinical product candidates or the period, if any, in which material net cash inflows from these product candidates may commence. This is due to the numerous risks and uncertainties associated with developing drugs, including the uncertainty of:
| • | the scope, rate of progress and expense of our ongoing, as well as any additional clinical trials and other research and development activities; |
| • | future clinical trial results; and |
| • | the timing of regulatory approvals. |
A change in the outcome of any of these variables with respect to the development of a product candidate could mean a significant change in the costs and timing associated with the development of that product candidate. For example, if the FDA or another regulatory authority were to require us to conduct clinical trials beyond those which we currently anticipate will be required for the completion of clinical development of a product candidate or if we experience significant delays in enrollment in any of our clinical trials, we could be required to expend significant additional financial resources and time on the completion of clinical development.
56
Table of Contents
AGS-003. We plan to initiate patient enrollment in a pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib in the first quarter of 2012. We estimate that the direct costs that we will incur in connection with conducting the planned pivotal phase 3 clinical trial will total approximately $ million.
AGS-004. We are currently conducting a phase 2b clinical trial of AGS-004 that is funded entirely by the NIH. We initiated enrollment in this trial in July 2010. We expect to complete enrollment in this trial in the first quarter of 2012 and to complete primary endpoint analysis of the data from this trial in mid-2012. We plan to proceed with further clinical development of AGS-004 following completion of the phase 2b clinical trial if the results of our ongoing phase 2b clinical trial justify proceeding and we are able to obtain government or other third-party funding for such efforts.
AGS-009. In July 2011, we completed enrollment in a phase 1a trial of AGS-009, a monoclonal antibody for the treatment of lupus. We initiated enrollment in this trial in the fourth quarter of 2009. We expect the results of this trial to be available in the fourth quarter of 2011. Following review of these results, we expect that we will commence enrollment in a further clinical trial of this product candidate in the first half of 2012. We plan to seek a partner for funding this trial and for the further development and commercialization of AGS-009, but would initiate this trial even if we have not secured a partner at the time this trial is scheduled to begin.
AGS-010. We are continuing preclinical testing on AGS-010, a recombinant human soluble CD83 protein. We plan to seek a partner for the continued development and commercialization of AGS-010.
Net Reimbursement Under Collaboration Agreement
We have recorded reimbursements of certain research and development costs as a reduction of expense in a separate line item within the statement of operations. We record these reimbursements when received. We received these reimbursements under a collaboration and licensing agreement with Kirin. Under our agreement with Kirin, which we entered into in 2004 and was terminated effective December 31, 2009, we and Kirin had agreed to share all related worldwide research and development costs and profits relating to the development of AGS-003 and AGS-004. Under the Kirin agreement, Kirin had agreed to reimburse us on a quarterly basis for our direct costs and services related to specified development and manufacturing activities and for allocated internal time at a designated full time equivalent rate.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related costs for employees in executive, operational and finance, information technology and human resources functions. Other significant general and administrative expenses include allocation of facilities costs, professional fees for accounting and legal services and expenses associated with obtaining and maintaining patents.
We expect that our general and administrative expenses will increase with the continued development and potential commercialization of our product candidates and as we operate as a public company. We believe that these increases will likely include increased costs for director and officer liability insurance, costs related to the hiring of additional personnel and increased fees for outside consultants, lawyers and accountants. We also expect to incur significant costs to comply with corporate governance, internal controls and similar requirements applicable to public companies.
Interest Income and Interest Expense
Interest income consists of interest earned on our cash and cash equivalents. We expect our interest income to increase following this offering as we invest the net proceeds from this offering pending their use in our operations.
Interest expense consists primarily of cash and non-cash interest costs related to our debt.
57
Table of Contents
As of June 30, 2011, we had $6.5 million in principal and accrued interest outstanding under the 2010 convertible notes that we issued in the original aggregate principal amount of $6.0 million in September and December 2010. These notes bear interest at a rate of 10.0% per annum. These notes had an original maturity date of September 15, 2011. The maturity date was extended to December 31, 2011 in connection with the issuance of the July 2011 convertible notes. We issued warrants to purchase a total of 20,759,953 shares of series C preferred stock at an exercise price of $0.01 per share in connection with the issuance of the 2010 convertible notes. We allocated $3.2 million of the proceeds from the issuance and sale of the convertible notes to the warrants based on the warrants’ fair value and recorded this amount on the balance sheet as warrant liability. We recorded the remaining $2.8 million in proceeds on the balance sheet as the convertible note liability. At June 30, 2011, the 2010 convertible notes are valued at $5.7 million, and the remaining $0.8 million in principal and $0.1 million in interest will be recorded as interest expense such that the value will equal $6.6 million at maturity. We expect that the principal and accrued interest on the 2010 convertible notes and the July 2011 convertible notes will convert to common stock upon the closing of this offering. At such time, the convertible note liability will be converted to additional paid in capital and common stock.
We entered into a loan agreement with a lending institution in December 2000, which we subsequently amended in November 2002, November 2004 and November 2005. We borrowed an aggregate of $5.1 million under the loan agreement at interest rates between 9.76% and 11.00%. We repaid all amounts outstanding under this agreement in April 2011. This agreement has expired. We have no further right or ability to borrow funds under this agreement.
We entered into a loan agreement in April 2007 with two lending institutions under which we borrowed $5.0 million at an interest rate of 11.25%. We repaid all amounts outstanding under this agreement in full in April 2010. This agreement has expired. We have no further right or ability to borrow funds under this agreement.
Accretion of Preferred Stock
Our convertible preferred stock is reflected on our balance sheet at its cost, less associated issuance costs. The convertible preferred stock is redeemable at the option of the holders beginning in March 2013 for an aggregate price equal to $33.1 million. The amount reflected on the balance sheet for our convertible preferred stock is increased by periodic accretions of the issuance costs so that the original amount reflected on the balance sheet will equal the aggregate redemption price.
Derivative Financing Income (Expense)
We own 49.94% of the capital of DC Bio. We consolidate the results of DC Bio in our financial statements. The other shareholders’ ownership in DC Bio is reflected in our financial statements by reference to the equity attributable to noncontrolling interest.
Under an amended and restated put agreement with the other shareholders of DC Bio, holders of Common, Class A preferred and Class B preferred shares of DC Bio have the right to put those shares to us in exchange for shares of our common stock, series B preferred stock and series C preferred stock at any time on or after March 31, 2011. We recorded a liability of $1.2 million on our balance sheet at June 30, 2011 reflecting the fair value of the put, which we calculated based on the estimated fair market value of the shares of our common stock, series B preferred stock and series C preferred stock issuable in exchange for shares of DC Bio.
The shares of DC Bio will be exchanged for shares of our common stock upon the closing of this offering. Upon such conversion, DC Bio will become a wholly-owned subsidiary of our company.
58
Table of Contents
Critical Accounting Policies and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which we have prepared in accordance with generally accepted accounting principles in the United States, or U.S. GAAP. The preparation of these consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported revenues and expenses during the reporting periods. We evaluate these estimates and judgments on an ongoing basis. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Our actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in Note 2 to our consolidated financial statements appearing at the end of this prospectus, we believe that the following accounting policies are the most critical to aid you in fully understanding and evaluating our financial condition and results of operations.
Revenue Recognition
We recognize revenue in accordance with the Financial Accounting Standards Board, or FASB, Accounting Standards Codification 605, Revenue Recognition, or ASC 605. We recognize revenue when the following criteria are met: persuasive evidence of an arrangement exists, services have been rendered, the price is fixed or determinable and collectability is reasonably assured.
We have previously entered into license agreements with collaborators. The terms of these agreements have included nonrefundable signing and licensing fees, as well as milestone payments and royalties on any future product sales developed by the collaborators under our licenses. We assess these multiple elements in accordance with ASC 605, in order to determine whether particular components of the arrangement represent separate units of accounting.
We recognize upfront license payments as revenue upon delivery of the license only if the license has stand-alone value and the fair value of the undelivered performance obligations can be determined. If the fair value of the undelivered performance obligations can be determined, such obligations are accounted for separately as the obligations are fulfilled. If the license is considered to either not have stand-alone value or have stand-alone value but the fair value of any of the undelivered performance obligations cannot be determined, the arrangement is accounted for as a single unit of accounting and the license payments and payments for performance obligations are recognized as revenue over the estimated period of when the performance obligations are performed.
Whenever we determine that an arrangement should be accounted for as a single unit of accounting, we must determine the period over which the performance obligations will be performed and revenue will be recognized. If we cannot reasonably estimate the timing and the level of effort to complete our performance obligations under the arrangement, then we recognize revenue under the arrangement on a straight-line basis over the period that we expect to complete our performance obligations.
Our collaboration agreements may also contain milestone payments. Revenues from milestones, if they are non-refundable and considered substantive, are recognized upon successful accomplishment of the milestones. If not considered substantive, milestones are initially deferred and recognized over the remaining performance obligation.
To date, we have not received any royalty payments and accordingly have not recognized any related revenue. We will recognize royalty revenue upon the sale of the related products, provided we have no remaining performance obligations under the arrangement.
59
Table of Contents
We record deferred revenue when payments are received in advance of the culmination of the earnings process. This revenue is recognized in future periods when the applicable revenue recognition criteria have been met.
Under our NIH contract, we receive reimbursement of our direct expenses and allocated overhead and general and administrative expenses and payment of other specified amounts totaling up to $1.4 million upon our achievement of specified development milestones. We recognize revenue from reimbursements earned in connection with the NIH contract as reimbursable costs are incurred. We recognize revenues from the achievement of milestones under the NIH contract upon the accomplishment of any such milestone.
Accrued Expenses
As part of the process of preparing financial statements, we are required to estimate accrued expenses. This process involves reviewing open contracts and purchase orders, communicating with applicable vendor personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of actual cost. The majority of our service providers invoice us monthly in arrears for services performed. We make estimates of our accrued expenses as of each balance sheet date in our financial statements based on facts and circumstances known to us. We periodically confirm the accuracy of our estimates with the service providers and make adjustments if necessary. Examples of estimated accrued expenses include:
| • | fees paid to CROs in connection with clinical trials; |
| • | fees paid to investigative sites in connection with clinical trials; |
| • | professional service fees; and |
| • | unpaid salaries, wages and benefits. |
We accrue our expenses related to clinical trials based on our estimates of the services received and efforts expended pursuant to contracts with multiple research institutions and CROs that conduct and manage clinical trials on our behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. Payments under some of these contracts depend on factors such as the successful enrollment of patients and the completion of clinical trial milestones. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we will adjust the accrual accordingly. If we do not identify costs that we have begun to incur or if we underestimate or overestimate the level of services performed or the costs of these services, our actual expenses could differ from our estimates. We do not anticipate the future settlement of existing accruals to differ materially from our estimates.
Valuation of Financial Instruments
Preferred Stock Warrant Liability
We account for our preferred stock warrants in accordance with ASC Topic 480-10, Distinguishing Liabilities from Equity, which requires that a financial instrument, other than an outstanding share, that, at inception, includes an obligation to repurchase the issuer’s equity shares regardless of the timing or likelihood of the redemption, shall be classified as a liability. We generally measure the fair value of the warrant liability using an option-pricing model, although, in the case of the warrants to purchase shares of our series C preferred stock, we measured the associated warrant liability based on the fair value of the warrants which we determined based on an allocation of our enterprise value to all classes of equity and preferred stock, including the warrants. We utilized the probability-weighted expected return method, or PWERM method, to determine these values. We record changes in fair value of the warrants as interest income or expense.
60
Table of Contents
The significant assumptions we use in estimating the fair value of the warrant liability include the strike price, estimate for volatility, risk free interest rate, estimated fair value of the preferred stock, and the estimated life of the warrant. Changes to these assumptions will impact the value of the warrant liability and earnings.
Upon the closing of this offering, our warrants to purchase preferred stock will automatically be exercised for shares of our common stock. At such time, the warrant liability will be converted to additional paid in capital and common stock.
Stock-Based Compensation
In accordance with ASC 718, Stock Compensation, we record the fair value of stock options, restricted stock awards and other stock-based compensation issued to employees as of the grant date as compensation expense. We typically recognize compensation expense over the requisite service period, which is the vesting period. For non-employees, we also record stock options, restricted stock awards and other stock-based compensation issued to these non-employees at their fair value as of the grant date. We then periodically remeasure the awards to reflect the current fair value at each reporting period and recognize expense over the related service period.
Stock-based compensation expense includes stock options granted to employees and non-employees and has been reported in our statements of operations as follows:
| Years Ended December 31, | Six Months Ended June 30, 2010 |
Six Months Ended June 30, 2011 |
||||||||||||||||||
| 2008 | 2009 | 2010 | ||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Research and development |
$ | 409 | $ | 286 | $ | 109 | $ | 100 | $ | 60 | ||||||||||
| General and administrative |
265 | 207 | 215 | 109 | 114 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 674 | $ | 493 | $ | 324 | $ | 209 | $ | 174 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
We calculate the fair value of stock-based compensation awards using the Black-Scholes option-pricing model. The Black-Scholes option-pricing model requires the use of subjective assumptions, including stock price volatility, the expected life of stock options, risk free interest rate and the fair value of the underlying common stock on the date of grant.
| • | We do not have sufficient history to estimate the volatility of our common stock price. We calculate expected volatility based on reported data for selected reasonably similar publicly traded companies for which the historical information is available. For the purpose of identifying peer companies, we consider characteristics such as industry, length of trading history, similar vesting terms and in-the-money option status. We plan to continue to use the guideline peer group volatility information until the historical volatility of our common stock is relevant to measure expected volatility for future option grants. |
| • | The assumed dividend yield is based on our expectation of not paying dividends in the foreseeable future. |
| • | We determine the average expected life of stock options based on historical experience of similar awards, giving consideration to the contractual terms, vesting schedules and expectations of future behavior. |
| • | We determine the risk-free interest rate by reference to implied yields available from U.S. Treasury securities with a remaining term equal to the expected life assumed at the date of grant. |
| • | We estimate forfeitures based on our historical analysis of actual stock option forfeitures. |
61
Table of Contents
The assumptions used in the Black-Scholes option-pricing model for the years ended December 31, 2008, 2009, and 2010, and for the six months ended June 30, 2011, are set forth below:
| Years Ended December 31, | Six Months Ended June 30, 2011 |
|||||||||||||||
| 2008 | 2009 | 2010 | ||||||||||||||
| Volatility |
70.00 | % | 81.00 | % | 82.00 | % | 82.00 | % | ||||||||
| Expected term (in years) |
7 | 7 | 7 | 7 | ||||||||||||
| Risk-free interest rate |
3.32 | % | 3.21 | % | 2.64 | % | 2.92 | % | ||||||||
| Dividend yield |
0.00 | % | 0.00 | % | 0.00 | % | 0.00 | % | ||||||||
The following table presents the grant dates, number of underlying shares and related exercise prices of stock options granted to employees from January 1, 2010 through June 30, 2011, as well as the estimated fair value of the stock options and the underlying common stock on the grant date.
| Date of Grant |
Number of Shares Subject To Options Granted |
Exercise Price Per Share |
Estimated Fair Value of Common Stock at Grant Date |
|||||||||
| July 1, 2010 |
188,326 | $ | 0.07 | $ | 0.07 | |||||||
| December 10, 2010 |
9,808,681 | $ | 0.08 | $ | 0.15 | (1) | ||||||
| February 28, 2011 |
1,670,399 | $ | 0.08 | $ | 0.15 | (2) | ||||||
| March 30, 2011 |
53,547 | $ | 0.08 | $ | 0.15 | (2) | ||||||
| (1) | Fair value of common stock at grant date was adjusted in connection with our reassessment of fair value for financial reporting purposes. See “— Stock Option Grants on December 10, 2010.” |
| (2) | Fair value of common stock at grant date was adjusted in connection with our reassessment of fair value for financial reporting purposes. See “—Stock Option Grants on February 28, 2011 and March 30, 2011.” |
The intrinsic value of all outstanding vested and unvested options as of June 30, 2011 is $ based on a per share price of $ for our common stock, which is the midpoint of the price range set forth on the cover page of this prospectus, based on 24,190,022 shares of common stock issuable upon the exercise of options outstanding as of June 30, 2011 and a weighted average exercise price of $0.09 per share.
The estimated fair value of common stock per share in the table above represents the determination by our board of directors of the fair value of our common stock as of the date of grant, taking into consideration various objective and subjective factors, including the conclusions of valuations of our common stock, as discussed below.
Due to the absence of an active market for our common stock, the fair value of our common stock for purposes of determining the exercise price for stock option grants was determined by our board of directors, with the assistance and upon the recommendation of management, in good faith based on a number of objective and subjective factors consistent with the methodologies outlined in the American Institute of Certified Public Accountants Practice Aid, Valuation of Privately-Held-Company Equity Securities Issued as Compensation, or the Practice Aid, including:
| • | the prices at which we most recently sold our convertible preferred stock and the rights, preferences and privileges of the convertible preferred stock as compared to those of our common stock, including the liquidation preferences of the convertible preferred stock; |
| • | our results of operations, financial position and the status of our research and development efforts, including the status of clinical trials for our product candidates under development; |
62
Table of Contents
| • | the composition of, and changes to, our management team and board of directors; |
| • | the material risks related to our business; |
| • | achievement of enterprise milestones, including the results of clinical trials and our entry into or termination of collaboration and license agreements; |
| • | the market performance of publicly traded companies in the life sciences and biotechnology sectors, and recently completed mergers and acquisitions of companies comparable to us; |
| • | external market conditions affecting the life sciences and biotechnology industry sectors; |
| • | the likelihood of achieving a liquidity event for the holders of our common stock and stock options, such as an initial public offering, or IPO, given prevailing market conditions; and |
| • | contemporaneous valuations. |
Stock Option Grants on July 1, 2010
Our board of directors granted stock options on July 1, 2010, with each option having an exercise price of $0.07 per share. In establishing this exercise price, in addition to the objective and subjective factors discussed above, our board of directors also considered input from management, as well as a contemporaneous valuation as of December 31, 2009, which valued our common stock at $0.07 per share.
In the December 31, 2009 valuation, we used the PWERM method, to value our common stock. Although we did not rely on the market approach to determine our enterprise value, we did review the performance of a set of guideline comparable companies. Under the PWERM approach, share value is derived from the probability-weighted present value of expected future investment returns, considering each of the possible outcomes available to us, as well as the economic and control rights of each share class. We calculated the implied enterprise value using the PWERM approach. The fair value of our common stock was estimated using a probability-weighted analysis of the present value of the returns afforded to common stockholders under several future stockholder exit or liquidity event scenarios:
| • | an IPO on or before December 31, 2012; |
| • | a strategic merger or sale of our company at a high valuation on or before December 31, 2012, which assumed no further clinical trials for our product candidates; |
| • | a strategic merger or sale of our company at a lower valuation on or before December 31, 2012, which assumed no further clinical trials for our product candidates; and |
| • | the failure or dissolution of our company with no value to common stockholders on or before December 31, 2010. |
In the December 31, 2009 valuation, probability weightings of 10.0% were used for the IPO scenario, 15.0% for a strategic merger or sale of our company at a high valuation, 15.0% for a strategic merger or sale of our company at a lower valuation and 60.0% for the failure or dissolution of our company with no value to common stockholders. The probability weightings assigned to the respective exit scenarios were primarily based on consideration of our various drug development programs, industry clinical success rates, our expected near-term
63
Table of Contents
and long-term funding requirements, and an assessment of the current financing and biotechnology industry environments at the time of the valuation. These probability weightings represented our best estimate of the respective exit scenarios at December 31, 2009 and considered our drug development programs in process, industry clinical success rates, our expected near-term and long-term funding requirements and the anticipation that two of our strategic partners, Novo and Kirin, would be terminating their agreements with us. Also at this time, our board of directors implemented a reduction in force involving seven of our employees, including three members of management, which was carried out in the first quarter of 2010.
In the December 31, 2009 valuation, we applied a discount for lack of marketability of 20.2% to reflect the fact that our shares of common stock represented a minority interest in our company and there is no market mechanism to sell these shares. A common stockholder would have to wait for a liquidity event such as an IPO or a sale of our company to enable the sale of the common stock. We used an option pricing model to determine the value of this lack of marketability.
The board of directors then determined that the events and circumstances that occurred between December 31, 2009 and July 1, 2010 did not indicate a significant change in the fair value of our common stock during that period. The specific facts and circumstances considered by our board of directors for the July 1, 2010 valuation included the following:
| • | Jeffrey Abbey was named chief executive officer in February 2010. |
| • | We completed dosing in our phase 2a trial of AGS-004 in the first half of 2010 and obtained trial results that justified proceeding with a phase 2b trial of this product candidate. |
| • | We had ceased enrollment in our phase 2 clinical trial of AGS-003 in combination with sunitinib in October 2009 in anticipation of Kirin’s termination of our collaboration agreement in December 2009. |
| • | We assumed sponsorship of the phase 1a clinical trial of AGS-009 from our then partner Novo following the termination of our agreement with Novo in June 2010. |
| • | In April 2010, the FDA approved the first active immunotherapy for the treatment of cancer — Dendreon Corporation’s Provenge (sipuleucel-T) for metastatic castrate-resistant prostate cancer. |
Based on all of these factors, the board determined that the fair value of our common stock at July 1, 2010 was $0.07 per share.
Stock Option Grants on December 10, 2010
Our board of directors granted stock options on December 10, 2010, with each option having an exercise price of $0.08 per share. In connection with the preparation of our consolidated financial statements included in this prospectus, we determined that the fair value of the common stock subject to the option awards granted on December 10, 2010, as determined by our board of directors at the time of grant, was less than the valuations that prospective underwriters in this offering estimated could be obtained in an initial public offering in the later half of 2011, based on market and other conditions at the time. As a result, we determined in July 2011, subsequent to the date of these option grants and prior to filing the registration statement of which this prospectus is a part, that the awards granted on December 10, 2010 had a compensatory element. Accordingly, we reassessed the fair value of our common stock at December 10, 2010 for financial reporting purposes. In reassessing the fair value of our common stock, the board of directors considered the factors used in determining the July 1, 2010 fair value of our common stock, including the December 31, 2009 valuation, as well as the following facts and circumstances:
| • | In July 2010, we commenced enrollment of patients in a phase 2b clinical trial of AGS-004. |
| • | In July 2010, the NIH and NIAID extended our contract to May 2013, with a total contract value of up to $27.9 million. |
64
Table of Contents
| • | We re-started clinical testing of AGS-009 in a phase 1a trial in adult patients with mild to moderate lupus in the fourth quarter of 2010. |
| • | We sold convertible notes in the aggregate original principal amount of $6.0 million in September and December 2010. |
| • | We obtained final median progression free survival data from our phase 2 clinical trial of AGS-003 in combination with sunitinib. |
Based on all of these factors, the board determined that the fair value of our common stock for financial reporting purposes at December 10, 2010 was $0.15 per share.
Stock Option Grants on February 28, 2011 and March 30, 2011
Our board of directors granted stock options on February 28, 2011 and March 30, 2011, with each option having an exercise price of $0.08 per share. In establishing this exercise price, in addition to the objective and subjective factors discussed above, our board of directors also considered input from management, as well as a contemporaneous valuation as of January 31, 2011, which valued our common stock at $0.05 per share. In the January 31, 2011 valuation, we used the PWERM to value our common stock. Under the PWERM approach, share value is derived from the probability weighted present value of expected future investment returns, considering each of the possible outcomes available to us, as well as the economic and control rights of each share class. We calculated the implied enterprise value using the PWERM approach. The fair value of our common stock was estimated using a probability-weighted analysis of the present value of the returns afforded to common stockholders under several future stockholder exit or liquidity event scenarios:
| • | an IPO on or before December 31, 2011; |
| • | a strategic merger or sale of company at a high valuation on or before December 31, 2011, which assumed no further clinical trials for our products; |
| • | a strategic merger or sale of our company at a lower valuation on or before December 31, 2011, which assumed no further clinical trials for our products; |
| • | a strategic sale of our intellectual property on or before December 31, 2011, which assumed no further clinical trials for our products; and |
| • | the failure or dissolution of our company with no value to our common stockholders |
In the January 31, 2011 valuation, probability weightings of 10% were used for the IPO scenario, 10% for a strategic merger or sale of our company at a high valuation, 65% for a strategic merger or sale of our company at a lower valuation, 5% for a strategic sale of our intellectual property and 10% for the failure or dissolution of our company with no value to common stockholders. These probability weightings assigned to the respective exit scenarios were based primarily on our considerable need for additional financing, indications that we had received from third parties who were interested in purchasing us as well as discussions with the FDA regarding our planned pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib. In addition, our board hired an investment banker to assist with the sale of our company with the expectation that a Phase 3 clinical trial would progress under the acquirer and eliminate our expected near-term funding requirements.
In the January 31, 2011 valuation, we applied a discount for lack of marketability of 12.4% to reflect the fact that our shares of common stock represented a minority interest in our company and there is no market mechanism to sell these shares. A common stockholder would have to wait for a liquidity event such as an IPO or a sale of our company to enable the sale of the common stock. We used an option pricing model to determine the value of this lack of marketability. The discount for lack of marketability used in the January 31, 2011 valuation was lower than the discount for lack of marketability used in the December 31, 2009 valuation because the January 31, 2011 valuation assumed that a liquidity event would occur in a shorter period from the date of the valuation than the December 31, 2009 valuation did.
65
Table of Contents
In establishing the exercise price of the February 28, 2011 and March 30, 2011 stock options, our board of directors recognized that the January 31, 2011 valuation described above had valued our common stock at a price that was less than the price at which our common stock had been valued in the December 31, 2009 valuation, but determined not to grant stock options at an exercise price that was less than the $0.08 per share exercise price used for the December 10, 2010 option grants.
Furthermore, in connection with the preparation of our consolidated financial statements included in this prospectus, we determined that the fair value of the common stock subject to the option awards granted on February 28, 2011 and March 30, 2011, as determined by our board of directors at their respective times of grant, was less than the valuations that prospective underwriters in this offering estimated could be obtained in an initial public offering in the later half of 2011, based on market and other conditions at the time. As a result, we determined in July 2011, subsequent to the date of these option grants and prior to filing the registration statement of which this prospectus is a part, that the awards granted on these dates had a compensatory element. Accordingly, we reassessed the fair value of our common stock at February 28, 2011 and March 30, 2011 for financial reporting purposes. In reassessing the fair value of our common stock, the board of directors considered the factors used in determining the December 10, 2010 fair value of our common stock and determined that, overall, no significant events or other circumstances had occurred between December 10, 2010 and March 30, 2011 that would indicate that there was a significant change in the fair value of our common stock during that period.
Based on all of these factors, the board determined that the fair value of our common stock for financial reporting purposes at each of February 28, 2011 and March 30, 2011 was $0.15 per share.
Stock Option Grants on September 6, 2011
Our board of directors granted stock options on September 6, 2011, with each option having an exercise price of $0.26 per share. In establishing this exercise price, in addition to the objective and subjective factors discussed above, our board of directors also considered input from management, as well as a contemporaneous valuation as of June 30, 2011, which valued our common stock at $0.26 per share. In the June 30, 2011 valuation, we used the PWERM approach to value our common stock, consistent with our previous valuations. Under the PWERM approach, share value is derived from the probability weighted present value of expected future investment returns, considering each of the possible outcomes available to us, as well as the economic and control rights of each share class. We calculated the implied enterprise value using the PWERM approach. The fair value of our common stock was estimated using a probability-weighted analysis of the present value of the returns afforded to common stockholders under several future stockholder exit or liquidity event scenarios:
| • | an IPO on or before December 31, 2011; |
| • | a strategic merger or sale of our company at a high valuation on or before December 31, 2014, which assumed an additional fundraising of at least $75 million and a completed, successful phase 3 clinical trial for AGS-003, our lead compound, as well as continued development on the remaining trials; |
| • | a strategic merger or sale of our company at a low valuation on or before December 31, 2014, which assumed an additional fundraising of at least $25 million and a completed successful phase 3 clinical trial for AGS-003, as well as continued development on the remaining trials; and |
| • | a strategic sale of our intellectual property on or before December 31, 2011, which assumed no further clinical trials for our products. |
In the June 30, 2011 valuation, probability weightings of 70% were used for the IPO scenario, 10% for a strategic merger or sale of our company at a high valuation, 10% for a strategic merger or sale of our company at a low valuation and 10% for a strategic sale of our intellectual property. These probability weightings assigned to the respective exit scenarios were based on consideration of our various drug development programs, industry
66
Table of Contents
clinical success rates, our expected near-term and long-term funding requirements and an assessment of the current financing and biotechnology industry environments at the time of the valuation. Specifically, in May 2011, we initiated formal discussions with the underwriters of this offering to pursue an IPO, established a timeline for the filing of the registration statement of which this prospectus forms a part and obtained approval from our board of directors to initiate an IPO process. Based on these factors and the market success of other comparable biotechnology IPOs in the second quarter of 2011, we determined that a 70% probability of a successful IPO before December 31, 2011 was appropriate. The probability assigned to the merger at a high valuation and merger at a low valuation was based on indications from our investors that they would continue to fund our operations through our planned pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib and their expression of a desire to access public markets rather than completing a private placement.
In the June 30, 2011 valuation, we applied a discount for lack of marketability of 11.4% to reflect the fact that our shares of common stock represented a minority interest in our company and there is no market mechanism to sell these shares. A common stockholder would have to wait for a liquidity event such as an IPO or a sale of our company to enable the sale of the common stock. We used a Black-Scholes option pricing model to determine the value of this lack of marketability. The discount for lack of marketability used in the June 30, 2011 valuation was lower than the discount for lack of marketability used in the January 31, 2011 valuation because the June 30, 2011 valuation assumed that a liquidity event would occur in a shorter period from the date of the valuation than did the January 31, 2011 valuation.
In addition, on September 21, 2011, our board of directors granted options to certain employees and a consultant with an exercise price equal to the price to the public in this offering if this offering is completed prior to March 31, 2012 and equal to the fair market value of the common stock as of December 31, 2011 as determined by a contemporaneous valuation if the offering is not completed prior to March 31, 2012.
The compensation charges reflected in our consolidated financial statements included in this prospectus reflect the reassessments of fair value that we conducted with respect to the December 10, 2010, February 28, 2011 and March 30, 2011 option grants.
The compensatory element of $0.07 per share for the stock option grants on December 10, 2010 were recorded in our statements of operations as follows:
| Year Ended December 31, |
Six Months Ended June 30, |
|||||||
| 2010 | 2011 | |||||||
| (in thousands) | ||||||||
| Research and Development |
$ | 3 | $ | 23 | ||||
| General and Administrative |
6 | 54 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 9 | $ | 77 | ||||
|
|
|
|
|
|||||
The compensatory element of $0.07 per share for the stock option grants on February 28, 2011 and March 30, 2011 were recorded in our statements of operations as follows:
| Six Months Ended June 30, |
||||
| 2011 | ||||
| (In thousands) | ||||
| Research and Development |
$ | 6 | ||
| General and Administrative |
3 | |||
|
|
|
|||
| Total |
$ | 9 | ||
|
|
|
|||
There are significant judgments and estimates inherent in the determination of these valuations. These judgments and estimates include assumptions regarding our future performance; the time to completing an IPO, a
67
Table of Contents
strategic merger or sale, or other liquidity event; and the timing and probability of continuing to successfully progress our various product candidates toward commercialization, as well as determinations of the appropriate valuation methods. If different assumptions had been applied in the valuations, our stock-based compensation expense, net loss and net loss per share could have been significantly different. While the assumptions used to calculate and account for stock-based compensation awards represents management’s best estimates, these estimates involve inherent uncertainties and the application of management’s judgment. As a result, if revisions are made to the underlying assumptions and estimates, our stock-based compensation expense could vary significantly from period to period.
Results of Operations
Comparison of the Six Months Ended June 30, 2011 and the Six Months Ended June 30, 2010
The following table summarizes the results of our operations for each of the six months ended June 30, 2010 and 2011, together with the changes in those items in dollars and as a percentage:
| Six Months Ended June 30, |
Increase/ (decrease) |
% Change |
||||||||||||||
| 2010 | 2011 | |||||||||||||||
| (in thousands) | ||||||||||||||||
| Revenue |
$ | 1,990 | $ | 4,128 | $ | 2,138 | 107.4 | % | ||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
6,932 | 6,755 | (177 | ) | (2.6 | )% | ||||||||||
| General and administrative |
1,552 | 1,384 | (168 | ) | (10.8 | )% | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
8,484 | 8,139 | (345 | ) | (4.1 | )% | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Income (loss) from operations |
(6,494 | ) | (4,011 | ) | 2,483 | (38.2 | )% | |||||||||
| Interest income |
3 | 1 | (2 | ) | (66.7 | )% | ||||||||||
| Interest expense |
(30 | ) | (4,847 | ) | (4,817 | ) | 16,056.7 | % | ||||||||
| Derivative financing expense |
(232 | ) | (246 | ) | 14 | 6.0 | % | |||||||||
| Investment tax credits |
36 | — | (36 | ) | (100.0 | )% | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net income (loss) |
$ | (6,717 | ) | $ | (9,103 | ) | $ | (2,386 | ) | 35.5 | % | |||||
|
|
|
|
|
|
|
|
|
|||||||||
Revenue
Revenue was $4.1 million for the six months ended June 30, 2011, compared to $2.0 million for the six months ended June 30, 2010, an increase of approximately $2.1 million, or 107.4%. The $2.1 million increase for the six months ended June 30, 2011 reflects increased reimbursement under our NIH contract associated with increased activity with respect to our phase 2b clinical trial of AGS-004 as we commenced patient enrollment in the trial in the third quarter of 2010 and an increase in the rates paid to us for allocated overhead and general and administrative costs under the NIH contract that was agreed to in September 2010. The increase also reflected our recognition of milestone revenue under the NIH contract totaling $0.5 million in the six months ended June 30, 2011. We did not recognize any milestone revenue in the six months ended June 30, 2010.
Research and Development Expenses
Research and development expenses were $6.8 million for the six months ended June 30, 2011, compared to $6.9 million for the six months ended June 30, 2010, a decrease of 2.6%. This decrease in research and development expense reflects a $0.4 million decrease in indirect research and development expense, partially offset by an increase in direct research and development expense of $0.2 million. The decrease in indirect
68
Table of Contents
research and development expense was due to a reduction in force that we conducted in February 2010 involving seven of our employees, including three members of management. Direct research and development expense remained relatively constant over the periods.
| • | Direct research and development expense for AGS-003 increased from $0.4 million the six months ended June 30, 2010 to $0.5 million in the six months ended June 30, 2011. This increase reflects increased clinical expenses in the six months ended June 30, 2011 related to the preparation and submission to the FDA of a protocol for our planned pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib. |
| • | Direct research and development expense with respect to AGS-004 increased from $1.2 million in the six months ended June 30, 2010 to $1.4 million in the six months ended June 30, 2011 due to the continued enrollment in our phase 2b clinical trial of AGS-004 that we commenced in the third quarter of 2010. |
| • | Direct research and development expense with respect to AGS-009 increased from $0.2 million in the six months ended June 30, 2010 to $0.8 million in the six months ended June 30, 2011, reflecting the continued enrollment in our phase 1a clinical trial of AGS-009 that we commenced in the third quarter of 2010. |
| • | Direct research and development expense with respect to AGS-010 decreased from $0.8 million in the six months ended June 30, 2010 to $0.1 million in the six months ended June 30, 2011, reflecting our determination to seek a partner for the further development and commercialization of AGS-010. |
General and Administrative Expenses
General and administrative expenses were $1.4 million for the six months ended June 30, 2011, compared to $1.6 million for the six months ended June 30, 2010, a decrease of 10.9%. This decrease reflects $0.3 million of severance expense in the six months ended June 30, 2010 related to our 2010 reduction in force. This decrease was partially offset by an increase in legal patent expense in the six months ended June 30, 2011.
Interest Expense
Interest expense was $4.8 million for the six months ended June 30, 2011, compared to $30,000 for the six months ended June 30, 2010. This increase was due to the amortization of a warrant liability recorded to represent the fair market value of warrants and accrual of interest on the 2010 convertible notes issued in September and December 2010. We expect to incur an additional $1.1 million in interest expense in respect of the 2010 convertible notes from June 30, 2011 through December 31, 2011, the maturity date of the 2010 convertible notes. We also expect to incur additional interest expense in 2011 in respect of the July 2011 notes and the associated warrants.
Derivative Financing Expense
Derivative financing expense was $0.2 million for the six months ended June 30, 2011 and the six months ended June 30, 2010. Derivative financing expense in these periods reflected increases in the fair market value of the put held by the other shareholders of DC Bio.
Investment Tax Credits
Investment tax credits were $36,000 for the six months ended June 30, 2011. We recorded no investment tax credits in the six months ended June 30, 2011. Under Canadian and Ontario law, DC Bio is entitled to refunds on scientific research and experimental development, or SR&ED investment tax credits. Because the investment tax credits are subject to a claim review and audit by the Canadian Revenue Agency, we recognize these tax credit when they are received.
69
Table of Contents
Comparison of the Year Ended December 31, 2010 and the Year Ended December 31, 2009
The following table summarizes the results of our operations for the years ended December 31, 2009 and 2010, together with the changes in those items in dollars and as a percentage:
| Year Ended December 31, |
Increase/ (decrease) |
% Change |
||||||||||||||
| 2009 | 2010 | |||||||||||||||
| (in thousands) | ||||||||||||||||
| Revenue |
$ | 5,368 | $ | 7,273 | $ | 1,905 | 35.5 | % | ||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
19,544 | 13,928 | (5,616 | ) | (28.7 | )% | ||||||||||
| Net reimbursement under collaboration |
(6,627 | ) | — | 6,627 | (100.0 | )% | ||||||||||
| General and administrative |
2,932 | 2,704 | (228 | ) | (7.8 | )% | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
15,849 | 16,632 | 783 | 4.9 | % | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Income (loss) from operations |
(10,481 | ) | (9,359 | ) | 1,122 | (10.7 | )% | |||||||||
| Interest income |
74 | 5 | (69 | ) | (93.2 | )% | ||||||||||
| Interest expense |
(283 | ) | (904 | ) | (621 | ) | 219.4 | % | ||||||||
| Derivative financing income |
— | 298 | 298 | 100.0 | % | |||||||||||
| Investment tax credits |
103 | 793 | 690 | 669.9 | % | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net income (loss) |
$ | (10,587 | ) | $ | (9,167 | ) | $ | 1,420 | (13.4 | )% | ||||||
Revenue
Revenue was $7.3 million for the year ended December 31, 2010, compared to $5.4 million for the year ended December 31, 2009. The $1.9 million, or 35.5%, increase for 2010, as compared to 2009 reflects an increase in the revenue we received under our NIH contract from $3.3 million in 2009, to $7.2 million in 2010. This increased NIH revenue reflects the increased reimbursement associated with increased activity with respect to our phase 2b clinical trial of AGS-004 in 2010 and the $2.0 million true-up of our indirect cost rates for overhead and general and administrative costs that we recognized in 2010. The increase in revenue in 2010 as compared to 2009 was offset by a decrease in milestone revenue reflecting a 2009 milestone payment of $2.0 million that we received under the license agreement to which we were a party with Novo. Under this license agreement, which we and Novo entered into in February 2006 and terminated in June 2010, we licensed Novo rights to technologies for use in the development and commercialization of human antibodies against interferon-a, including AGS-009. We received no milestone revenue in 2010.
Research and Development Expenses
Research and development expenses were $13.9 million for the year ended December 31, 2010, compared to $19.5 million for the year ended December 31, 2009. Research and development expenses in 2009 were offset by reimbursements of $6.6 million that we received in 2009 under our collaboration and licensing agreement with Kirin.
Research and development expense for 2010 was $5.6 million, or 28.7%, lower than for 2009. The decrease in research and development expense was due primarily to less research and clinical trial activity in 2010 as compared to 2009.
| • | Direct research and development expense relating to AGS-003 decreased from $1.9 million in 2009 to $0.9 million in 2010, reflecting that we ceased enrollment in our phase 2 clinical trial of AGS-003 in combination with sunitinib in October 2009. |
70
Table of Contents
| • | Direct research and development expense relating to AGS-004 decreased from $3.6 million in 2009 to $3.1 million in 2010, reflecting the completion of the enrollment of the AGS-004 phase 2a clinical trial in June 2009 and that we did not begin enrolling patients in the AGS-004 phase 2b clinical trial until the third quarter of 2010. |
| • | Direct research and development expense with respect to AGS-009 increased from $0 in 2009 to $0.5 million in 2010, reflecting the commencement of enrollment in our phase 1a clinical trial of AGS-009 late in the third quarter 2010. Prior to June 30, 2010, Novo was responsible for conducting research and development activities for AGS-009 under our agreement with Novo. During that period, we did not incur any direct costs for AGS-009. |
| • | Direct research and development expense with respect to AGS-010 decreased from $3.3 million in 2009 to $1.1 million in 2010 due to a decrease in preclinical testing and our determination to seek a partner for further development and commercialization of AGS-010. |
In addition, indirect research and development expense decreased by $2.4 million from 2009 to 2010. Lower personnel costs, including stock-based compensation expense, accounted for $1.4 million of the decrease as our average headcount during 2010 decreased from 67 to 57. The lower headcount was in part due to our 2010 reduction in force. The lower headcount also contributed to a $0.1 million decrease in miscellaneous other expenses. Costs related to the development of our automated manufacturing processes decreased by $0.2 million from 2009 to 2010 as we completed the final stages of development of our automated manufacturing devices and disposables in 2009. Costs for laboratory supplies that were not allocated to any of our product candidates decreased by $0.7 million from 2009 to 2010 due to lower levels of research activity in 2010.
General and Administrative Expenses
General and administrative expenses were $2.7 million for the year ended December 31, 2010, compared to $2.9 million for the year ended December 31, 2009. General and administrative expenses were $0.2 million, or 7.8%, lower in 2010 as compared to 2009 due to a decrease in consulting costs.
Interest Income
Interest income was $5,000 for the year ended December 31, 2010, compared to $74,000 for the year ended December 31, 2009. The $69,000 decrease in interest income for 2010 as compared to 2009 reflected lower cash balances in 2010.
Interest Expense
Interest expense was $0.4 million for the year ended December 31, 2010, compared to $0.3 million for the year ended December 31, 2009. The $0.1 million increase in interest expense for 2010 as compared to 2009 was due to the amortization of warrant liability recorded to represent the fair market value of warrants and accrual of interest on the 2010 convertible notes issued in September and December 2010.
Derivative Financing Income
Derivative financing income was $0.3 million for the year ended December 31, 2010. We recorded no derivative financing income or expense in the year ended December 31, 2009. Derivative financing income in 2010 reflected a decrease in the fair market value of the put held by other shareholders of DC Bio. This decrease reflected a decrease in the fair market value of the shares of our stock issuable upon the put in 2010 as certain shareholders of DC Bio elected not to purchase their pro rata share of the 2010 convertible notes. As a result,
71
Table of Contents
50% of the shares of DC Bio held by such shareholders became exchangeable for shares of our common stock rather than our preferred stock, which had a lower fair value per share. There was no change in the fair market value of the put during 2009, as the value of the preferred stock issuable upon the exercise of the put and the value of the DC Bio shares did not change materially during the year.
Investment Tax Credits
Investment tax credits were $0.1 million for the year ended December 31, 2010 and for the year ended December 31, 2009. In addition, we recorded $0.7 million in other income awarded under the Qualifying Therapeutic Discovery Project Program in 2010.
Comparison of the Year Ended December 31, 2009 and the Year Ended December 31, 2008
The following table summarizes the results of our operations for each the years ended December 31, 2008 and 2009, together with the changes in those items in dollars and as a percentage:
| Year Ended December 31, |
Increase/ (decrease) |
% Change |
||||||||||||||
| 2008 | 2009 | |||||||||||||||
| (in thousands) | ||||||||||||||||
| Revenue |
$ | 4,450 | $ | 5,368 | $ | 918 | 20.6 | % | ||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
22,043 | 19,544 | (2,499 | ) | (11.3 | )% | ||||||||||
| Net reimbursement under collaboration |
(8,128 | ) | (6,627 | ) | 1,501 | (18.5 | )% | |||||||||
| General and administrative |
2,853 | 2,932 | 79 | 2.8 | % | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
16,768 | 15,849 | (919 | ) | (5.5 | )% | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Income (loss) from operations |
(12,318 | ) | (10,481 | ) | 1,837 | (14.9 | )% | |||||||||
| Interest income |
251 | 74 | (177 | ) | (70.5 | )% | ||||||||||
| Interest expense |
(633 | ) | (283 | ) | 350 | (55.3 | )% | |||||||||
| Derivative financing expense |
(756 | ) | — | (756 | ) | (100.0 | )% | |||||||||
| Investment tax credits |
667 | 103 | (564 | ) | (84.6 | )% | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net income (loss) |
$ | (12,789 | ) | $ | (10,587 | ) | $ | 2,202 | (17.2 | )% | ||||||
Revenue
Revenue was $5.4 million for the year ended December 31, 2009, compared to $4.4 million for the year ended December 31, 2008. The $1.0 million, or 20.6%, increase in revenue for 2009, as compared to 2008 primarily results from an increase in milestone revenue, reflecting a milestone payment of $2.0 million under our agreement with Novo in 2009 as compared to a milestone payment under our agreement with Novo of $1.0 million in 2008. The increase in revenue in 2009, as compared to 2008, was partially offset by a slight decrease in revenue under our NIH contract from $3.4 million in 2008 to $3.3 million in 2009.
Research and Development Expense
Research and development expenses were $19.5 million for the year ended December 31, 2009, compared to $22.0 million for the year ended December 31, 2008. Research and development expenses were offset by reimbursements under the Kirin agreement of $6.6 million in 2009 and $8.1 million in 2008.
Research and development expense for 2009 was $2.5 million, or 11.3%, lower than for 2008. The decrease in research and development expense in 2009 reflects a $2.9 million decrease in indirect research and development expense partially offset by a $0.4 million increase in direct research and development costs.
72
Table of Contents
The decrease in indirect research and development expense in 2009, as compared to 2008, was due to a $1.3 million decrease in costs for laboratory supplies that were not allocated to any of our product candidates, reflecting lower levels of research activity in 2009. Also, personnel expense, including stock-based compensation expense, in 2009 decreased by $0.9 million reflecting severance pay in 2008 in connection with a small reduction of our workforce at the beginning of 2008. The decrease in personnel also contributed to lower miscellaneous other expenses of $0.3 million in 2009. Costs related to the development of our automated processes decreased $0.4 million in connection with the anticipated termination of the Kirin agreement.
The increase in direct research and development expense was due primarily to a higher level of research and clinical trial activity in 2009 as compared to 2008.
| • | Direct research and development expense relating to AGS-003 decreased from $2.5 million in 2008 to $1.9 million in 2009, reflecting that in September 2009 we ceased enrollment in our phase 2 combination therapy trial of AGS-003 after accruing six patients. In comparison, during 2008, we completed the phase 1/2 clinical trial of AGS-003 and initiated and enrolled 15 patients in the phase 2 combination therapy trial of AGS-003. |
| • | Direct research and development expense relating to AGS-004 decreased from $4.0 million in 2008 to $3.6 million in 2009 due to the completion of the phase 2a trial in 2009. |
| • | Direct research and development expense with respect to AGS-010 increased from $1.8 million in 2008 to $3.3 million in 2009, reflecting costs of preclinical animal testing of AGS-010 in 2009. |
General and Administrative Expenses
General and administrative expenses were $2.9 million for the year ended December 31, 2009 and for the year ended December 31, 2008. There was no change in general and administrative expenses between 2008 and 2009, although consulting expense was $0.3 million higher in 2009 and personnel and patent expenses were $0.1 million and $0.2 million higher, respectively, in 2008.
Interest Income
Interest income was $74,000 for the year ended December 31, 2009, compared to $0.3 million for the year ended December 31, 2008. The $0.2 million decrease in interest income for 2009 as compared to 2008 reflected lower cash balances in 2009.
Interest Expense
Interest expense was $0.3 million for the year ended December 31, 2009, compared to $0.6 million for the year ended December 31, 2008. The $0.3 million decrease in interest expense for 2009 as compared to 2008 was due to the repayment of loans in 2008 under loan agreements we had with lending institutions.
Derivative Financing Expense
Derivative financing expense was $0.8 million for the year ended December 31, 2008. There was no derivative financing income or expense in the year ended December 31, 2009. No derivative financing expense was recognized in 2009 as the fair market value of the shares of our series B preferred stock and series C preferred stock did not change materially from 2008 to 2009. Derivative financing expense in 2008 reflected the increase in the fair market value of the put as the fair market value of our series B preferred stock and series C preferred stock issuable upon the put increased in 2008.
73
Table of Contents
Investment Tax Credits
SR&ED investment tax credits were $0.1 million for the year ended December 31, 2009, compared to $0.7 million for the year ended December 31, 2008.
Liquidity and Capital Resources
Sources of Liquidity
Since our inception in May 1997 through June 30, 2011, we have funded our operations principally with $88.9 million from the sale of common stock, convertible debt, warrants and convertible preferred stock, including the convertible preferred stock sold by DC Bio, $32.9 million from the licensing of our technology, and $73.6 million from government contracts, grants and license and collaboration agreements.
The gross proceeds we have received from the issuance and sale of our convertible preferred stock are as follows:
| Issue |
Year | Number of Shares |
Gross Proceeds |
|||||||||
| (in thousands) | ||||||||||||
| Series A |
2000 | 2,408,749 | $ | 1,594 | ||||||||
| Series B |
2001 | 22,511,556 | 39,382 | |||||||||
| Series B-1 |
2004 | 2,840,909 | 5,000 | |||||||||
| Series C |
2008 | 123,099,041 | 33,362 | |||||||||
As of June 30, 2011, we had cash and cash equivalents of approximately $1.4 million. Of this $1.4 million, $43,000 was held by DC Bio. In July 2011, we raised an additional $3.5 million through the issuance of the July 2011 convertible notes. The July 2011 convertible notes bear interest at the rate of 10.00% per annum and mature on December 31, 2011.
Cash Flows
The following table sets forth the major sources and uses of cash for the periods set forth below:
| Years Ended December 31, | Six Months Ended June 30, 2010 |
Six Months Ended June 30, 2011 |
||||||||||||||||||
| 2008 | 2009 | 2010 | ||||||||||||||||||
| (in thousands) |
||||||||||||||||||||
| Net cash provided by (used in): |
||||||||||||||||||||
| Operating activities |
$ | (10,920 | ) | $ | (10,407 | ) | $ | (10,147 | ) | $ | (5,919 | ) | $ | (3,752 | ) | |||||
| Investing activities |
3,788 | (1,956 | ) | 3,154 | 3,234 | (6 | ) | |||||||||||||
| Financing activities |
27,442 | (2,718 | ) | 4,802 | (1,052 | ) | (27 | ) | ||||||||||||
| Effect of exchange rate changes |
(254 | ) | 141 | 18 | 5 | 2 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net increase (decrease) in cash and cash equivalents |
$ | 20,056 | $ | (14,940 | ) | $ | (2,173 | ) | $ | (3,732 | ) | $ | (3,783 | ) | ||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Operating Activities. Cash used in operating activities of $10.9 million during the year ended December 31, 2008 was primarily a result of our $12.8 million net loss coupled with changes in operating assets and liabilities of $0.5 million, partially offset by non-cash items of $2.4 million. Cash used in operating activities of $10.4 million during the year ended December 31, 2009 was primarily a result of our $10.6 million net loss,
74
Table of Contents
coupled with changes in operating assets and liabilities of $1.2 million, partially offset by non-cash items of $1.4 million. Cash used in operating activities of $10.1 million during the year ended December 31, 2010 was primarily a result of our $9.2 million net loss, coupled with changes in operating assets and liabilities of $(2.7) million which reflects an increase in other receivables, primarily the cash due from NIH under our agreement. Receivables from NIH significantly increased in 2010 as NIH agreed to extend the contract and to increase our provisional reimbursement rates. These uses of cash were partially offset by non-cash items of $1.7 million. Cash used in operating activities of $5.9 million during the six month period ended June 30, 2010 was primarily a result of our net loss of $6.7 million, coupled with changes in operating assets and liabilities of $0.1 million, partially offset by non-cash items of $0.9 million. Cash used in operating activities of $3.8 million during the six month period ended June 30, 2011 was primarily a result of our $9.1 million net loss, coupled with changes in operating assets and liabilities of $0.3 million, partially offset by non-cash items of $5.6 million.
Investing Activities. Net cash provided by (used in) investing activities amounted to $3.8 million for the year ended December 31, 2008, $(2.0) million for the year ended December 31, 2009 and $3.2 million for the year ended December 31, 2010. Investing activities provided cash of $3.2 million for the six month period ended June 30, 2010 and used cash of $6,000 for the six month period ended June 30, 2011. Cash used in investing activities during all of these periods primarily reflected our purchases of equipment or our purchases of short-term investments. Cash provided by investment activities during each of these periods, if applicable, was primarily due to sales of short-term investments.
Financing Activities. Net cash provided by (used in) financing activities amounted to $27.4 million for the year ended December 31, 2008, $(2.7) million for the year ended December 31, 2009, $4.8 million for the year ended December 31, 2010, $(1.1) million for the six months ended June 30, 2010 and $(27,000) for the six months ended June 30, 2011. The cash provided by financing activities in 2008 consisted primarily of $30.7 million of net proceeds associated with the issuance of series C preferred stock, including the sale of Class B preferred shares of DC Bio which may be put to us in exchange for shares of our common stock, series B preferred stock and series C preferred stock, partially offset by payments of $2.8 million on certain of our notes payable. The cash used in financing activities in 2009 consisted primarily of payments on certain of our notes payable of $2.7 million. Cash provided by financing activities for the year ended December 31, 2010 consisted primarily of $6.0 million of proceeds from the issuance and sale of the 2010 convertible notes, partially offset by payments on certain of our notes payable of $1.2 million. The cash used in financing activities for the six months ended June 30, 2010 and 2011 consisted primarily of payments on certain of our notes payable.
Funding Requirements
To date, we have not generated any product revenue from our development stage product candidates. We do not know when, or if, we will generate any product revenue. We do not expect to generate significant product revenue unless or until we obtain marketing approval of, and commercialize, AGS-003 or any of our other product candidates. At the same time, we expect our expenses to increase in connection with our ongoing activities, particularly as we continue the research, development and clinical trials of, and seek regulatory approval for, our product candidates and lease, build out and equip our new U.S. commercial manufacturing facility. Furthermore, upon the closing of this offering, we expect to incur additional costs associated with operating as a public company. In addition, subject to obtaining regulatory approval of any of our product candidates, we expect to incur significant commercialization expenses for product sales, marketing, manufacturing and distribution. We will need substantial additional funding in connection with our continuing operations.
We expect that the net proceeds from this offering, together with our existing cash, cash equivalents, short-term investments and anticipated funding under our NIH contract, as well as the proceeds from our issuance and sale of the July 2011 convertible notes, will enable us to fund our operating expenses and capital expenditure requirements, other than for the planned leasing, build-out and equipping of a new U.S. commercial
75
Table of Contents
manufacturing facility, through at least . We intend to devote approximately $ million of the net proceeds from this offering to fund the direct costs of our planned pivotal phase 3 combination therapy clinical trial of AGS-003 and to use the balance for general corporate purposes. We also will use $200,000 of the net proceeds of this offering to pay a success fee to a former lender under a loan agreement that we have previously repaid in full.
We will need to obtain additional financing prior to the commercialization of AGS-003 or any of our other product candidates, including for the planned leasing, build-out and equipping of a new U.S. commercial manufacturing facility, and for conducting any future clinical trials of our product candidates. We expect that we will spend approximately $25 million to lease, build out and equip the new commercial manufacturing facility to a level necessary to file a BLA for AGS-003 with the FDA using the automated manufacturing processes that we plan to establish. We expect that we would incur an additional $10 million to $15 million after the filing of the BLA to further build out and equip the manufacturing facility so as to have in place the commercial capacity that we anticipate will be required for commercial launch of AGS-003.
We have based our estimates on assumptions that may prove to be wrong, and we may use our available capital resources sooner than we currently expect. Because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates, we are unable to estimate the amounts of increased capital outlays and operating expenditures necessary to complete the development of our product candidates.
Our future capital requirements will depend on many factors, including:
| • | the progress and results of the planned pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib; |
| • | the scope, progress, results and costs of preclinical development, laboratory testing and clinical trials for our other product candidates; |
| • | the costs and timing of our planned leasing, build-out and equipping of a new U.S. commercial manufacturing facility; |
| • | the costs, timing and outcome of regulatory review of our product candidates; |
| • | the costs of commercialization activities, including product sales, marketing, manufacturing and distribution, for any of our product candidates for which we receive regulatory approval; |
| • | revenue received from sales of our product candidates, if approved by the FDA; |
| • | the costs of preparing, filing and prosecuting patent applications and maintaining, enforcing and defending intellectual property-related claims; |
| • | the extent to which we acquire or invest in businesses, products and technologies; |
| • | our ability to obtain government or other third-party funding; and |
| • | our ability to establish collaborations on favorable terms, particularly marketing and distribution arrangements for oncology product candidates outside North America and for the development and commercialization of our non-oncology product candidates on a worldwide basis. |
Until such time, if ever, as we can generate substantial product revenue, we expect to finance our cash needs through a combination of equity offerings, debt financings, government or other third-party funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements. Except for
76
Table of Contents
the NIH’s remaining funding commitment with respect to AGS-004, we do not have any committed external source of funds. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a stockholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through government or other third-party funding, marketing and distribution arrangements or other collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us.
We plan to seek government or other third-party funding for the continued development of AGS-004 following completion of the phase 2b clinical trial of AGS-004 and to seek partners or other sources of third- party funding for the further development of AGS-009 and AGS-010. We will also need to raise funds to pay for the proposed leasing, build-out and equipping of a new U.S. commercial manufacturing facility. If we are unable to raise additional funds through equity or debt financings, government or other third-party funding when needed, we may be required to delay, limit, reduce or terminate our development of our product candidates, our planned leasing, build-out and equipping of our new U.S. commercial manufacturing facility or our commercialization efforts, or to grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
Our recurring operating losses raise substantial doubt about our ability to continue as a going concern. As a result, our independent registered public accounting firm included an explanatory paragraph in its report on our financial statements as of and for the year ended December 31, 2010 with respect to this uncertainty. We have no current source of revenue to sustain our present activities, and we do not expect to generate product revenue until, and unless, the FDA or other regulatory authorities approve AGS-003 or another one of our product candidates and we successfully commercialize such product candidate. Accordingly, our ability to continue as a going concern will require us to obtain additional financing to fund our operations.
Contractual Obligations and Commitments
The following table summarizes our significant contractual obligations and commercial commitments at December 31, 2010 and the effects such obligations are expected to have on our liquidity and cash flows in future periods (in thousands):
| Total | Less than 1 year |
1 - 3 years |
3 - 5 years |
More than 5 years |
||||||||||||||||
| Operating lease |
$ | 214 | $ | 211 | $ | 3 | — | — | ||||||||||||
| Convertible notes |
6,100 | 6,100 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 6,314 | $ | 6,311 | $ | 3 | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
In June 2011, we renewed our operating lease agreement for office space in Durham, North Carolina to extend the term of the lease to November 2016. As part of the lease renewal, we retained an option to terminate the lease in December 2014. Under this lease renewal, we are obligated to pay $1.4 million in total over the term of the lease, a total of $0.9 million in 2012, 2013 and 2014 and a total of $0.5 million in 2015 and 2016.
In September and December 2010 we issued and sold our 2010 convertible notes in the original aggregate principal amount of $6.0 million. These notes bear interest at a rate of 10.00% per year, and have an original maturity date of December 31, 2011. At December 31, 2010, our accrued interest on the 2010 convertible notes totaled approximately $0.1 million. In July 2011, we issued and sold our July 2011 convertible notes in the aggregate original principal amount of $3.5 million. The July 2011 convertible notes accrue interest at a rate
77
Table of Contents
equal to 10.00% per year, and have a maturity date of December 31, 2011. The July 2011 convertible notes are not reflected in the table. We expect that the principal and accrued interest on the 2010 convertible notes and the July 2011 convertible notes will convert to common stock upon the closing of this offering.
In addition, we plan to lease a new U.S. commercial manufacturing facility for the manufacture of our Arcelis-based products. We expect that we will incur a significant long term financial commitment in connection with the lease of such facility.
We are a party to license agreements with universities and other third parties, as well as patent assignment agreements, under which we have obtained rights to patents, patent applications and know-how. Under these agreements, we have agreed to pay the other parties milestone payments upon the achievement of specified clinical, regulatory and commercialization events and royalties based on future sales of products. We have not included these payments in the table as we cannot estimate if, when or in what amounts such payments will become due under these agreements.
Net Operating Losses
As of December 31, 2010, we had U.S. Federal and state, and Canadian federal and provincial net operating loss carryforwards of approximately $63.7 million, $83.6 million, $4.2 million, and $4.2 million, respectively. These net operating loss carryforwards begin to expire in 2012, 2017, 2014 and 2014, respectively. As of December 31, 2010, we had U.S. Federal and state tax credit carryforwards of approximately $2.8 million and $340,000, respectively. These credit carryforwards begin to expire in 2020 and 2019, respectively. The Tax Reform Act of 1986 provides for a limitation on the annual use of net operating loss and research and development tax credit carryforwards following certain ownership changes that could limit our ability to utilize these carryforwards. We have not completed a study to assess whether an ownership change has occurred, or whether there have been multiple ownership changes since our inception, due to the significant costs and complexities associated with such study. Accordingly, our ability to utilize the aforementioned carryforwards may be limited. Additionally, U.S. tax laws limit the time during which these carryforwards may be utilized against future taxes. As a result, we may not be able to take full advantage of these carryforwards for federal and state tax purposes.
As of December 31, 2010, we have received $1.3 million in refunds through scientific research and experimental development tax credits through our consolidated subsidiary in Canada.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements as defined under Securities and Exchange Commission, or SEC, rules.
Recent Accounting Pronouncements
On January 1, 2010, we adopted new accounting and disclosure guidance for the consolidation of variable interest entities, or VIE’s, which required enhanced disclosures intended to provide users of financial statements with more transparent information about an enterprise’s involvement in a VIE. We performed an assessment of our VIE, DC Bio, and determined that continued consolidation under the new guidance is appropriate. Accordingly, there was no impact on our financial position and results of operations. The impact of this pronouncement on future transactions will be evaluated under the new criteria when and if encountered.
In October 2009, the FASB issued new guidance for revenue recognition with multiple deliverables, which is effective for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010, although early adoption is permitted. This guidance eliminates the residual method under the
78
Table of Contents
current guidance and replaces it with the “relative selling price” method when allocating revenue in a multiple deliverable arrangement. The selling price for each deliverable shall be determined using vendor specific objective evidence of selling price, if it exists, otherwise third-party evidence of selling price shall be used. If neither exists for a deliverable, the vendor shall use its best estimate of the selling price for that deliverable. After adoption, this guidance will also require expanded qualitative and quantitative disclosures. We will evaluate the impact of adoption on future transactions and material modifications of existing agreements when and if encountered.
In April 2010, the FASB issued authoritative guidance on defining a milestone and determining when it may be appropriate to apply the milestone method of revenue recognition for research or development transactions. Consideration that is contingent on achievement of a milestone in its entirety may be recognized as revenue in the period in which the milestone is achieved only if the milestone is judged to meet certain criteria to be considered substantive. Milestones should be considered substantive in their entirety and may not be bifurcated. An arrangement may contain both substantive and non substantive milestones, and each milestone should be evaluated individually to determine if it is substantive. The guidance is effective on a prospective basis for milestones achieved in fiscal years, and interim periods within those years, beginning on or after June 15, 2010, with early adoption permitted. We adopted this guidance on January 1, 2011. The adoption of this guidance did not have a material impact on our consolidated financial statements.
In January 2010, the FASB amended the existing disclosure guidance on fair value measurements, which is effective January 1, 2010, except for disclosures about purchases, sales, issuances, and settlements in the roll forward of activity in Level 3 fair value measurements, which is effective January 1, 2011. Among other things, the updated guidance requires additional disclosure for the amounts of significant transfers in and out of Level 1 and Level 2 measurements and requires certain Level 3 disclosures on a gross basis. Additionally, the updates amend existing guidance to require a greater level of disaggregated information and more robust disclosures about valuation techniques and inputs to fair value measurements. Since the amended guidance requires only additional disclosures, the adoption of the provisions effective January 1, 2010 did not, and for the provisions effective in 2011 will not, impact our financial position or results of operations.
In June 2011, the FASB issued authoritative guidance related to the Presentation of Comprehensive Income. This standard eliminates the current option to report other comprehensive income and its components in the statement of changes in equity. The new U.S. GAAP requirements are effective for public entities for fiscal years beginning after December 15, 2011 and interim and annual periods thereafter, with early adoption permitted. As this accounting standard only requires enhanced disclosure, the adoption of this standard will not impact our financial position or results of operations.
In May 2011, the FASB issued amended guidance on fair value measurements. This newly issued accounting standard clarifies the application of certain existing fair value measurement guidance and expands the disclosures for fair value measurements that are estimated using significant unobservable (Level 3) inputs. This accounting standard is effective on a prospective basis for annual and interim reporting periods beginning on or after December 15, 2011. We do not expect that adoption of this standard will have a material impact on our financial position or results of operations.
Quantitative and Qualitative Disclosure about Market Risk
Our primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of U.S. interest rates.
Due to the short-term duration of our investment portfolio and the low risk profile of our investments, an immediate 10.0% change in interest rates would not have a material effect on the fair market value of our portfolio. Accordingly, we would not expect our operating results or cash flows to be affected to any significant degree by the effect of a sudden change in market interest rates on our investment portfolio.
79
Table of Contents
We do not believe that our cash, cash equivalents and available-for-sale investments have significant risk of default or illiquidity. While we believe our cash, cash equivalents and available-for-sale investments do not contain excessive risk, we cannot provide absolute assurance that in the future our investments will not be subject to adverse changes in market value. In addition, we maintain significant amounts of cash and cash equivalents at one or more financial institutions that are in excess of federally insured limits.
Inflation generally affects us by increasing our cost of labor and clinical trial costs. We do not believe that inflation has had a material effect on our results of operations during 2008, 2009, 2010 or through June 30, 2011.
80
Table of Contents
Overview
We are a biopharmaceutical company focused on the development and commercialization of fully personalized immunotherapies for the treatment of cancer and infectious diseases based on our proprietary technology platform called Arcelis. Using biological components obtained from each patient, our Arcelis-based immunotherapies employ a specialized white blood cell, called a dendritic cell, to activate an immune response that is specific to the patient’s disease. Our most advanced product candidate is AGS-003 for the treatment of metastatic renal cell carcinoma, or mRCC. We plan to initiate patient enrollment in a pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib, an oral small molecule drug marketed under the trade name Sutent that is the current standard of care for mRCC, in the first quarter of 2012. We are also developing a second Arcelis-based product candidate, AGS-004 for the treatment of HIV. We are studying AGS-004 in a phase 2b clinical trial that is funded entirely by the National Institutes of Health, or NIH. In addition to our Arcelis-based product candidates, we are developing two other product candidates based on our expertise in dendritic cell biology: AGS-009, a monoclonal antibody for the treatment of lupus, which we are studying in a phase 1a clinical trial, and AGS-010, a preclinical biologic compound, which we are developing for organ transplantation and the treatment of autoimmune and inflammatory diseases.
We use our Arcelis platform to generate RNA-loaded dendritic cell immunotherapies using biological components derived from the individual patient to be treated. Specifically, we manufacture these fully personalized therapies using that patient’s disease sample, either tumor cells in the case of cancer or a blood sample containing virus in the case of infectious disease, and dendritic cells derived from the patient’s white blood cells. By using messenger RNA, or mRNA, from the patient’s own cancer cells or virus, our Arcelis platform yields a fully personalized immunotherapy that is designed to contain all of the patient’s disease-specific antigens, which are foreign or mutated proteins associated with the cancer cells or virus, and to elicit a rapid, broad and potent immune response that is specific to the patient’s own disease. Our Arcelis-based immunotherapies have been well tolerated in clinical trials in more than 100 patients with no serious adverse events attributed to our immunotherapies. We believe that our Arcelis platform can be used for a wide range of oncology and infectious disease indications.
In October 2011, one of our scientific co-founders, Ralph M. Steinman, M.D., was awarded the Nobel Prize in medicine for the discovery of dendritic cells as critical sentinels of the immune system. Dr. Steinman’s discovery of dendritic cells and his research of dendritic cell biology and the role of dendritic cells in adaptive immunity led to the development of our Arcelis platform.
We have tested AGS-003 in clinical trials in combination with sunitinib and as a monotherapy for the treatment of mRCC. In our phase 2 clinical trial of AGS-003 in combination with sunitinib, the combination therapy showed median progression free survival of 11.9 months in intermediate and poor risk patients that had a time from diagnosis to initiation of systemic therapeutic treatment of less than one year. This represented an approximately 50% extension over the eight month median progression free survival achieved by sunitinib alone in similar intermediate and poor risk patients in the pivotal phase 3 clinical trial of sunitinib as a monotherapy. Progression free survival is the length of time that passes after treatment begins and before the patient’s disease worsens or the patient dies. We plan to conduct a pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib compared to sunitinib plus placebo under a special protocol assessment, or SPA, with the FDA. Under our protocol, in order to achieve the primary endpoint of the trial, the data must demonstrate an increase of at least 30% in median progression free survival for the AGS-003 plus sunitinib arm compared to the sunitinib plus placebo control arm. We expect to complete enrollment of this trial in late 2013 and have data on the primary endpoint in the second half of 2014. In addition, under the protocol, the independent data monitoring committee will conduct two interim analyses of data from the trial to assess both safety and futility.
In September 2011, we received a letter from the FDA advising that the FDA had completed its review of our protocol for the pivotal phase 3 clinical trial under the SPA process. In the letter, the FDA stated that it had
81
Table of Contents
determined that the protocol sufficiently addressed the trial’s objectives and that the trial was adequately designed to provide the necessary data to support a submission for marketing approval. The FDA noted that the ability of the trial to support an efficacy claim for AGS-003 would depend on a review of the data from the trial and an evaluation of the overall risk and benefit of AGS-003.
Renal cell carcinoma, or RCC, is the most common type of kidney cancer. According to the National Cancer Institute, or NCI, there are approximately 61,000 new cases of kidney cancer each year in the United States. The National Comprehensive Cancer Network, or NCCN, and the National Cancer Data Base, or NCDB, estimate that 90% of these new cases are RCC. According to the NCDB, 15% to 20% of these newly diagnosed RCC cases are mRCC. Additionally, patients initially diagnosed with early stage RCC may later present with mRCC.
We have completed two early stage clinical trials of AGS-004 for the treatment of HIV, and are currently conducting a phase 2b clinical trial that is funded entirely by the NIH. We expect to complete enrollment in this trial in the first quarter of 2012 and to complete primary endpoint analysis of the data from this trial in mid-2012. According to the World Health Organization, the number of people living with HIV in the world was approximately 33.3 million in 2009. The Henry J. Kaiser Family Foundation estimates that more than 1.1 million people are currently living with HIV in the United States. According to the Centers for Disease Control and Prevention, the number of new cases of HIV infection in the United States is expected to remain steady at about 55,000 cases per year for the next decade.
We are developing our other two product candidates based on our expertise in dendritic cell biology. We have completed enrollment in a phase 1a clinical trial of AGS-009, a monoclonal antibody for the treatment of lupus, and expect to have the results of the trial in the fourth quarter of 2011. Following review of these results, we expect that we will commence enrollment in a further clinical trial of this product candidate in the first half of 2012. We are continuing preclinical testing on AGS-010, a recombinant human soluble CD83 protein, for organ transplantation and the treatment of autoimmune and inflammatory diseases.
We currently manufacture our Arcelis-based product candidates at our single, centralized manufacturing facility located in Durham, North Carolina. To prepare for the commercial launch of AGS-003 and any other Arcelis-based products we develop, we plan to establish automated manufacturing processes based on existing functioning prototypes of automated devices and disposables, to identify and lease suitable space for a new U.S. commercial manufacturing facility and to build out and equip the new facility for this purpose. We have developed proprietary processes to generate Arcelis-based products that we believe will allow us to manufacture the drug product for all of North America at this one centralized facility. Our plan is to file our biologics license application, or BLA, for AGS-003 with the FDA using the automated manufacturing processes that we establish. We rely on contract manufacturers for the production of AGS-009 and AGS-010 and do not plan to build our own manufacturing capacity for those product candidates.
Immunotherapy to Treat Cancer and Infectious Diseases
According to the NCI, cancer is the second leading cause of death in the United States and is responsible for nearly one quarter of all deaths in the United States each year. Cancer is characterized by aberrant cells that multiply uncontrollably, resulting in tumors that may invade other tissues throughout the body and producing additional tumors, called metastases. Cancer growth can cause tissue damage, organ failure and, ultimately, death.
Many immunologists believe that cancer cells occur frequently in the human body, yet are effectively controlled by the immune system because the cancer cells, or the antigens that are associated with the cancer cells, are recognized by the immune system as abnormal and then killed. Specifically, the cancer cells or antigens are recognized by cytotoxic T lymphocytes, or T-cells, which are the cells responsible for killing the cancer cells.
82
Table of Contents
Cancer cells utilize several strategies to escape detection by the immune system and T-cells. Typically, cancer cells secrete factors that act systemically to prevent T-cells from responding to activation signals, resulting in the T-cells not acting and remaining quiescent. In addition, large tumor masses are associated with a greater level of suppression of immune responses.
Chronic viral infections such as HIV or hepatitis C present the same challenges to the immune system as cancer in terms of the immune system needing to overcome disease-induced immune dysfunction to recognize and respond to virus-infected cells.
Immunotherapy Approaches
Immunotherapy is intended to stimulate and enhance the body’s natural mechanism for killing cancer cells and virus-infected cells. Immunotherapeutic approaches to treat disease can be separated into two broad classes, passive and active, based on their mechanism of action.
Passive Immunotherapy. Passive immunotherapies do not rely on or actively stimulate the body’s immune system to initiate the attack on the disease. Instead, the attack is made by the therapy which is manufactured ex vivo, or outside of the body. These therapies are not considered to be personalized and consist mainly of monoclonal antibodies directed at a single disease-specific enzyme or protein on the surface of targeted cells. The goal of these passive immunotherapies is to prevent targeted cells from dividing or to cause their death.
Passive immunotherapy is well established in cancer and infectious disease therapy. In 1997, Rituxan was the first passive immunotherapy to receive FDA approval when it was approved for the treatment of non-Hodgkin lymphoma. Since then, numerous other monoclonal antibody-based products have been approved for use in a variety of different cancer types. These products are typically positioned for use as second-line or third-line therapies in advanced disease settings at a time when the patient’s immune system has been weakened by prior therapy and by the disease. Most recently, in March 2011, the FDA approved Bristol-Myers Squibb’s passive immunotherapy Yervoy™ (ipilimumab), a monoclonal antibody, for the treatment of patients with unresectable or metastatic melanoma. Passive immunotherapies have also been approved for the treatment of infectious diseases, including the use of immunoglobulins to both prevent and treat viral infections such as hepatitis A and B.
Active Immunotherapy. Active immunotherapies, on the other hand, are designed to trigger or stimulate the body’s own immune system to fight the disease. Active immunotherapy is a more specific approach to immunotherapy than passive immunotherapy because active immunotherapies contain a particular tumor-associated antigen or set of antigens that are designed to activate the patient’s own immune system to seek out and kill cells that carry the same antigen. Active immunotherapies have no direct therapeutic action, but rather rely on the patient’s immune system to recognize and kill the intended target. Most active immunotherapies utilize off-the-shelf, also referred to as defined, antigens, rather than antigens that are patient specific, and are frequently paired with adjuvants, which are agents that non-specifically activate the cells of the immune system to enhance tumor-specific immune responses.
In April 2010, the FDA approved the first active immunotherapy for the treatment of cancer – Dendreon Corporation’s Provenge (sipuleucel-T) for metastatic castrate-resistant prostate cancer. Sipuleucel-T is a partially personalized cellular immunotherapy that consists of white blood cells obtained from the patient combined with a fusion protein consisting of two parts: the antigen prostatic acid phosphatase, which is present in many prostate cancer cells, and an adjuvant, granulocyte-macrophage colony stimulating factor (GM-CSF).
A number of active cancer immunotherapies are currently being tested in late stage clinical development. For example, GlaxoSmithKline plc is conducting the largest ever phase 3 clinical trial in lung cancer with its investigational active cancer immunotherapy, MAGE-3. In addition, Amgen, Inc., through its March 2011
83
Table of Contents
acquisition of Biovex, is conducting a multinational phase 3 clinical trial in metastatic melanoma of an active cancer immunotherapy that utilizes a virus to stimulate anti-tumor immunity and kill cancer cells.
Shortcomings of Immunotherapies. Although passive and active immunotherapies have been proven to be incrementally effective in malignant and infectious diseases, both approaches have shortcomings, which include:
| • | Due to tumor-induced or virus-induced immunosuppression, even with the administration of immunotherapies, many patients may not be able to mount effective immune responses against the intended target antigens due to the reliance of the immunotherapy on accessory cells such as CD4+ helper T-cells, which are functionally impaired by the patient’s disease. |
| • | Most passive and active immunotherapies target one or only a few antigens, which increases the probability that the cancer or virus-infected cells will escape detection by the immune system. The antigens used in most active immunotherapies are normal, non-mutated, self-antigens which are generally poor at stimulating immune responses, even from healthy immune systems, as the natural protection against autoimmune disease is that the human immune system generally does not generate immune responses against self-antigens. |
| • | Most active immunotherapies employing defined antigens are not directly applicable to multiple types of cancer and require significant preclinical development work to identify relevant antigens that address the targeted cancer. |
| • | We believe that most active immunotherapies do not impact early clinical endpoints, such as progression free survival, but rather manifest their clinical effects after a protracted period of time, with benefit generally seen only in overall survival. |
| • | Toxicity is frequently associated with these therapies as the presence of the target antigen on some normal cells can result in collateral damage to healthy tissues. |
| • | Therapies involving cells derived from the patient’s own cells present challenges to commercialization due to the difficulty of producing such therapies at commercial scale using manual processes, producing multiple doses from a limited amount of patient material, storing and shipping individual doses and keeping costs as low as possible. |
Another shortcoming of these immunotherapies is highlighted by a study recently published in Nature Medicine, which provides evidence that, even in the same type of cancer, the genetic makeup of the tumor is dramatically different from patient to patient. In the study, scientists sequenced the whole genomes of 50 patients’ breast cancer tumors alongside matching DNA from the same patients’ healthy cells in order to identify genomic alterations present only in the cancerous cells. The results revealed that the genetic makeup of each of the tumors was highly diverse. Of the approximately 1,700 gene mutations found in total, most were unique to individual patients’ tumors, and only three occurred in 10% or more of the patients. These results demonstrate that, even within the same indication, each patient’s tumor contains distinct antigens and suggests that immunotherapies that rely on defined, off-the-shelf antigens or a single targeted antigen may have limited effectiveness.
Although many of the immunotherapies currently in clinical development have shown promising results, we do not believe that any of them utilizes a technology that employs the patient’s own cancer or virus-infected cells to create a fully personalized immunotherapy that is directly targeted to the patient’s unique genetic disease.
Arcelis Technology Platform
Arcelis is our proprietary active immunotherapy technology platform for generating fully personalized RNA-loaded dendritic cell immunotherapies. We use our Arcelis platform to manufacture AGS-003, which we
84
Table of Contents
are developing for the treatment of mRCC, and AGS-004, which we are developing for the treatment of HIV. We believe that Arcelis is potentially applicable to a wide range of cancers and infectious diseases.
Our Arcelis platform is focused on dendritic cells that present antigens to the attention of the immune system and are critical to the human immune system’s recognition of the presence of proteins derived from cancer cells or virus-infected cells. Dendritic cells are capable of internalizing cancer protein antigens or virus protein antigens and displaying fragments of these protein antigens on their surface as small peptides. The dendritic cells then present these peptide antigens to T-cells capable of binding to these peptide antigens and producing a large complement of molecular factors that, in the case of cancer, lead to direct cancer cell death and, in the case of infectious disease, kill virus-infected cells to control the spread of infectious pathogens.
The following graphic illustrates the processes comprising our Arcelis platform:
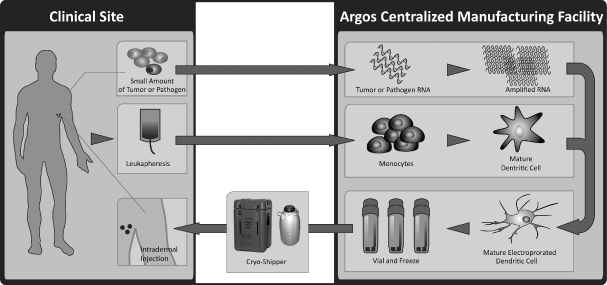
As shown in the graphic above, our Arcelis platform requires two components derived from the particular patient to be treated, specifically:
| • | a disease sample from the patient — tumor cells in the case of cancer or a blood sample containing virus in the case of infectious disease — which is generally collected at the time of diagnosis or initial treatment, and |
| • | dendritic cells derived from the patient’s monocytes, a particular type of white blood cell, which are obtained from the patient through a laboratory procedure called leukapheresis that occurs after diagnosis and at least four weeks prior to the initiation of our immunotherapy. |
The tumor cells, or the blood sample containing the virus, and the leukapheresis product are shipped separately following collection from the clinical site to our single, centralized manufacturing facility in Durham, North Carolina.
At our facility, we use standard methods to isolate the patient’s mRNA, which is a key component of the genetic code, from the disease sample and amplify the mRNA. In parallel, we take the monocytes from the leukapheresis product and culture them using a proprietary process to create matured dendritic cells. We then immerse the matured dendritic cells in a solution of the patient’s isolated mRNA and a synthetic RNA that encodes a protein known as CD40 ligand, or CD40L, and apply a brief electric pulse to the solution, in a process
85
Table of Contents
referred to as electroporation. This process enables the patient’s isolated mRNA and the CD40L protein to pass into, or load, the dendritic cells. We then further culture the mRNA-loaded dendritic cells so that these cells allow for antigen expression from the patient’s mRNA and presentation in the form of peptides on the surface of the dendritic cells. These mature, loaded dendritic cells are formulated into the patient’s plasma that was collected during the leukapheresis to become the Arcelis-based drug product. We then vial, freeze and ship the drug product to the clinic, which thaws the drug product and administers it to the patient by intradermal injection.
Upon injection into the skin of the patient, the antigen-loaded dendritic cells in the drug product migrate to the lymph nodes near the site of the injection. It is at these lymph nodes that the drug product comes into contact with T-cells. We believe that through this interaction the loaded dendritic cells orchestrate the differentiation, expansion and education, of antigen-specific T-cells. A unique property of our dendritic cells is that they result in the generation of a special kind of T-cell known to be associated with good clinical outcome. These are known as CD8+ central effector and memory T-cells which are long-lived and provide durable immune responses. Once activated and expanded, these T-cells are able to seek out and kill cancer or virus-infected cells that express the identical antigens as those displayed on the surface of the dendritic cells. Because the generation of these T-cells is dependent on secretion of IL-12 from the dendritic cells, measurement of IL-12 is a marker for potency of AGS-003 and potentially other Arcelis-based products.
We believe that our Arcelis platform addresses important shortcomings of both passive and other active immunotherapies:
| • | We believe that the inclusion of the CD40L protein in our Arcelis platform results in Arcelis-based products not requiring CD4+ helper T-cells or adjuvants with potential adverse side effects to mount an immune response with strong anti-tumor or anti-viral reactivity. Our Arcelis-based products have demonstrated the ability to completely reverse tumor-induced immunosuppression with as few as three doses. |
| • | Our Arcelis platform, by using mRNA from the patient’s own cancer or virus, yields a fully personalized immunotherapy that is designed to contain all of the patient’s disease-specific antigens, including mutated antigens, and to elicit a broad and potent immune response that is specific to the patient’s own disease. Accordingly, we believe our Arcelis platform limits the probability that cancer or virus-infected cells will escape detection by the immune system. |
| • | Our Arcelis technology is a platform technology that can potentially be used to treat any cancer or virus with essentially the same manufacturing process and equipment. |
| • | In clinical trials, our Arcelis-based products have been shown in a majority of patients to rapidly establish anti-tumor or anti-viral immunity. We believe that this is due to the unique mechanism of action of our Arcelis-based products, which mimics the natural immune response to foreign antigens. This rapid immune response may allow achievement of early clinical endpoints, such as progression free survival. |
| • | Our Arcelis-based product candidates have been well tolerated in clinical trials in more than 100 patients with no serious adverse events attributed to our immunotherapies. |
| • | Our Arcelis technology, though fully personalized, requires only a small disease sample and a single leukapheresis to produce several years of therapy for each patient — five years of therapy in the case of AGS-003 based on our current dosing regimen. In addition, Arcelis-based products can be stored and shipped frozen, which we expect will allow the use of a single, centralized manufacturing facility for North America. We believe that this facility, when built and equipped to use the automated manufacturing processes that we establish, will enable manufacturing with a cost of goods for our fully personalized Arcelis-based products that we expect will be comparable to other biologics. |
86
Table of Contents
Strategy
Our goal is to build a commercial biopharmaceutical company, founded on our extensive expertise in dendritic cell biology and immunology, primarily through the use of our Arcelis platform. Key elements of our strategy are to:
| • | Complete development of AGS-003 for mRCC, and manufacture and commercialize AGS-003 on our own in North America and collaborate with third parties for the manufacture and commercialization of AGS-003 outside North America, or, if we determine that it is in our best interest, with third parties on a worldwide basis. |
| • | Continue development of AGS-004 for HIV through government or other third-party funding and collaborate with third parties for commercialization on a worldwide basis. |
| • | Continue to build on our Arcelis platform by developing fully personalized immunotherapies for other cancers and infectious diseases. |
| • | Establish automated manufacturing processes based on existing functioning prototypes of automated devices and disposables and, prior to the filing of our BLA for AGS-003, identify, lease, build out and equip a new U.S. facility for the commercial manufacture of products based on our Arcelis platform. |
| • | Seek partners for further development and commercialization of AGS-009 for lupus and AGS-010 for organ transplantation and the treatment of autoimmune and inflammatory diseases. |
| • | Pursue broad intellectual property protection for our Arcelis technology platform, product candidates and proprietary manufacturing processes through U.S. and international patent filings and maintenance of trade secret confidentiality. |
Our Development Programs
The following table summarizes our four development programs and their development status. We currently hold all commercial rights to all four of these programs in all geographies.
| Product |
|
Primary Indication | Status | |||
| AGS-003 |
Arcelis-based fully personalized immunotherapy | mRCC | Planned pivotal phase 3 with initiation of enrollment expected in the first quarter of 2012 | |||
| AGS-004 |
Arcelis-based fully personalized immunotherapy | HIV | Ongoing phase 2b with completion of enrollment expected in the first quarter of 2012 | |||
| AGS-009 |
Anti-interferon-a monoclonal antibody | Lupus | Ongoing phase 1a with results expected in the fourth quarter of 2011 | |||
| AGS-010 |
CD83 recombinant protein | Organ transplantation |
Preclinical | |||
AGS-003 for Metastatic Renal Cell Carcinoma
Our most advanced product candidate, AGS-003, is based on our Arcelis platform. We are developing AGS-003 for use in combination with sunitinib and potentially other therapies for the treatment of mRCC.
87
Table of Contents
Sunitinib is an oral small molecule drug sold under the trade name Sutent and is the current standard of care for first-line treatment of mRCC. First-line treatment means the initial treatment of a disease following diagnosis. We have conducted multiple clinical trials of AGS-003 for the treatment of mRCC, including a phase 2 clinical trial of AGS-003 in combination with sunitinib. We plan to conduct a pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib compared to sunitinib plus placebo under an SPA with the FDA. We expect to commence patient enrollment in this pivotal phase 3 clinical trial in the first quarter of 2012.
Renal Cell Carcinoma. Renal cell carcinoma is the most common type of kidney cancer. The NCI estimated that there would be approximately 61,000 new cases of kidney cancer and that approximately 13,000 people would die from this disease in the United States in 2011. According to the NCCN and the NCDB, it is estimated that 90% of new kidney cancer cases each year are RCC. Relative to the general population, the NCI estimates that the five-year survival rate from 2001 to 2007 for patients with RCC was approximately 70%; however, for patients with RCC that had metastasized by the time RCC was first diagnosed, which condition we refer to as newly diagnosed mRCC, the five-year survival rate has historically been approximately 10%.
NCDB statistics indicate that from 2000 through 2008 between 15% and 20% of newly diagnosed RCC patients presented with mRCC annually in the United States. Additional patients that were initially diagnosed with earlier stage RCC may also present with mRCC as these patients suffer relapses. The NCCN estimates that between 20% to 30% of patients with early stage RCC will relapse within three years of surgical excision of the primary tumor. Although the NCI does not provide prevalence of RCC by stage, we believe that the estimated three-year relapse rate suggests that there may be an additional 10,000 to 15,000 cases of mRCC identified annually in the United States. Combining newly diagnosed mRCC patients with patients who relapse, we estimate that there may be between 20,000 to 25,000 new cases of mRCC in the United States each year.
The diagnosis of RCC is generally made by examination of a tumor biopsy under a microscope. Upon evaluation of the visual appearance of the tumor cells, the pathologist will classify the RCC into clear cell or non-clear cell types. According to NCCN, approximately 85% of all RCC diagnoses are clear cell RCC. Because clear cell types are the most common type of tumor cell and are more likely to respond favorably to treatment, most of the more recently approved therapies for mRCC have limited their clinical trials to patients with the clear cell type of tumor cell. However, approvals for these therapies have not been limited to clear cell types of RCC, and these therapies may be used for both clear cell and non-clear cell types.
Diagnosis and Current Treatment of mRCC. The initial treatment for most patients with RCC, including mRCC, is surgical removal of the tumor, usually requiring partial or complete removal of the affected kidney, referred to as nephrectomy. In the absence of metastatic disease, the NCCN recommends observation after nephrectomy, although systemic therapies are recommended for patients who are believed to be at higher risk of relapse. Notably, patients whose tumors have metastasized to other organs beyond the primary kidney at the time of diagnosis are considered to have newly diagnosed mRCC and have the poorest overall prognosis and survival. For patients who present with mRCC upon diagnosis or as a result of a relapse from an earlier stage of RCC, the NCCN recommends systemic treatment with currently available therapies, except in the rare instances where metastatic lesions can be removed by surgery alone.
Upon diagnosis, the prognosis for patients with mRCC is classified into three overall disease risk profiles—favorable, intermediate and poor—using objective prognostic risk factors developed by researchers at Memorial Sloan-Kettering Cancer Center, or MSKCC. These risk factors have been correlated to reduced progression-free and overall survival in mRCC and include:
| • | time from diagnosis to the initiation of systemic therapeutic treatment of less than one year, which is indicative of more aggressive disease. We refer to this risk factor as the less than one year to treatment risk factor; |
| • | low levels of hemoglobin, a protein in the blood that carries oxygen; |
88
Table of Contents
| • | elevated corrected calcium levels; |
| • | diminished patient performance status or physical functioning; and |
| • | elevated levels of the enzyme lactate dehydrogenase in the blood. |
Patients exhibiting zero risk factors at the time of treatment are included in the favorable risk group; patients exhibiting one or two risk factors are included in the intermediate risk group; and patients exhibiting three or more risk factors are included in the poor risk group. Patients in the intermediate and poor risk groups, even when treated with standard of care therapies such as sunitinib, generally have an expected survival of less than one to two years.
mRCC is generally resistant to conventional systemic approaches such as chemotherapy, radiation and hormonal therapies. Although mRCC has been treated with cytokine-based immunotherapies such as interferon-a and IL-2, which have demonstrated a clinical benefit in a small number of mRCC patients, these therapies have been shown to have severe toxicities which limit their use, including cardiopulmonary, neuropsychiatric, dermatologic, renal, hepatic and hematologic side effects. Although high-dose IL-2 has demonstrated durable complete mRCC remissions, its toxicity restricts its use to a small minority of patients.
In the past few years, several new agents, such as Sutent (sunitinib), Votrient (pazopanib), Torisel (temsirolimus), Nexavar (sorafenib), Avastin (bevacizumab) plus interferon-a, and Afinitor (everolimus) have been approved for the treatment of mRCC. While the majority of these new agents have been evaluated in first-line treatment of mRCC, we do not believe that any of them demonstrated in their pivotal phase 3 clinical trials progression free survival or overall survival greater than the progression free survival and overall survival demonstrated in the pivotal phase 3 clinical trial of sunitinib. According to Datamonitor, in 2007 Sutent controlled a majority of the market share for systemic therapies for first-line treatment of mRCC. Although multiple other agents have been approved for first- or second-line mRCC since 2007, in 2010 Sutent continued to control a majority of the market share for first-line treatment of mRCC. Additional agents, such as axitinib and tivozanib, are in late-stage development for mRCC and could be approved by or during 2012. We estimate, based on publicly available information, including 2010 quarterly and annual reports of companies that market drugs approved for mRCC, that the current worldwide mRCC market for these new agents is over $1.5 billion.
Although most of these new agents have demonstrated prolonged progression free survival as compared to interferon-a, they are rarely associated with durable remissions or survival, particularly in patients who are not classified as favorable risk at the time of treatment. In addition, each of these new agents has shortcomings that limit its use in the treatment of mRCC, including significant toxicities, such as neutropenia and other hematologic toxicities, fatigue, diarrhea, hand-foot syndrome, hypertension and other cardiovascular effects. For example:
| • | Sunitinib is limited in ability to achieve long-term disease control and overall survival in intermediate and poor risk patients, including in particular those with the less than one year to treatment risk factor. In addition, approximately 19% of patients in the pivotal phase 3 clinical trial of sunitinib discontinued treatment prior to disease progression due to the toxicities associates with the drug. These toxicities have prevented other new agents for mRCC from being readily combined with sunitinib for first-line treatment of mRCC. |
| • | In the pivotal phase 3 clinical trial of pazopanib, 16% of patients discontinued treatment prior to disease progression due to toxicities. Although pazopanib demonstrated median progression free survival of 11.1 months and median overall survival of 22.9 months in 155 first-line mRCC patients in its pivotal phase 3 trial, we do not believe that, since its approval in 2009, it has substantially reduced the use of sunitinib in first-line treatment. |
| • | Temsirolimus is administered by IV infusion, which is inconvenient for patients. Temsirolimus has been evaluated in clinical trials primarily in poor risk patients and was approved by the FDA for first-line |
89
Table of Contents
| treatment in poor risk mRCC patients. However, its benefits in this population have been shown to be short-lived. For example, in the pivotal phase 3 trial of temsirolimus, the median progression free survival in poor risk mRCC patients was 5.5 months and median overall survival was 10.9 months. |
| • | Sorafenib has shown lower efficacy in first-line therapy for mRCC than sunitinib and other new agents, as demonstrated by the median progression free survival of 5.7 months that it achieved in a randomized phase 2 trial in first-line treatment. Sorafenib is primarily used as a second-line or third-line treatment for mRCC. |
| • | Bevacizumab has been approved for use in combination with interferon-a as a first-line treatment for mRCC. However, the combined toxicities of the two therapies and the multiple weekly injections of interferon-a appear to make this a less appealing option for mRCC patients and oncologists. In two separate phase 3 studies of this combination, 23% and 28% of patients discontinued treatment due to toxicities. |
| • | Everolimus has been approved for mRCC patients who have progressed after initial treatment with sunitinib, sorafenib or both. While its activity in this setting is improved over placebo, it only demonstrated an overall response rate of 2% and median progression free survival of 4.9 months in its pivotal phase 3 clinical trial. |
AGS-003 Opportunity. The overlapping and combined toxicities of the new agents described above have prevented their use in combination therapies. For instance, a phase 1 clinical trial of the combination of sunitinib and temsirolimus was discontinued due to toxicities. Furthermore, in a phase 2 clinical trial of the combination of temsirolimus with bevacizumab, 41% of patients discontinued treatment early due to toxicities. We believe that the lack of success to date in combining these therapies without significant toxicity and the lack of durable remissions and prolonged survival in subjects with intermediate and poor risk disease, suggest that there remains an unmet need for novel therapeutic approaches for mRCC that can improve efficacy without adding any appreciable toxicity. We believe that the safety profile that AGS-003 has demonstrated to date, which may enable its use in combination with other therapies with little or no additional toxicities, gives it the potential to address this unmet need.
Development Status. The following table summarizes our clinical development of AGS-003 and its predecessor product, MB-002.
| Clinical Trial |
Enrollment Time Frame |
Number
of Patients Receiving Therapy |
Status | |||
| Pivotal Phase 3 Combination Therapy Clinical Trial of AGS-003 in Combination with Sunitinib (planned) |
2012 – 2013 (planned) |
325 planned (650 planned to be randomized) |
Planned to commence enrollment in the first quarter of 2012 | |||
| Phase 2 Combination Therapy Clinical Trial of AGS-003 in Combination with Sunitinib |
2008 – 2009 | 21 | Completed (4 patients ongoing as of August 31, 2011) | |||
| Phase 1/2 Monotherapy Clinical Trial of AGS-003 |
2006 – 2008 | 22 | Completed (2 patients ongoing as of August 31, 2011)* | |||
| Monotherapy Rollover Clinical Trial of AGS-003 |
2006 – 2010 | 2 | Completed | |||
| Phase 1/2 Monotherapy Clinical Trial of MB-002 |
2004 – 2005 | 20 | Completed | |||
| * | One of these two patients is receiving AGS-003 after progression per the investigator’s discretion as allowed under the protocol for the trial. |
90
Table of Contents
Efficacy Endpoints. There are a number of standard efficacy endpoints that clinicians use to measure outcomes for clinical trials for cancer therapies. The following are explanations of the meanings of the various efficacy endpoints that we have used and will be using in our clinical trials of AGS-003 and MB-002. Each is determined in accordance with Response Criteria in Solid Tumors, or RECIST, measurement guidelines.
| • | Progression free survival (PFS): the length of time that passes after treatment begins and before the patient’s disease worsens or the patient dies. |
| • | Overall survival (OS): the time interval from treatment to death. |
| • | Overall response rate: the number of patients in the trial who achieve a partial or complete response. |
| • | Complete response: disappearance of all measurable target lesions and non-target lesions. |
| • | Partial response: overall tumor regression based on a decrease of at least 30% in the overall amount of measurable tumor mass in the body and improvement or no change in non-target lesions. |
| • | Stable disease: neither sufficient decrease in tumor size to qualify as a partial response nor sufficient increase in tumor size to qualify as disease progression. |
| • | Clinical benefit rate: the number of patients in the trial who achieve stable disease or a partial or complete response. |
| • | Disease progression: growth of at least 20% in the size of the measurable tumor mass, appearance of new tumor(s) or growth of non-target lesions since beginning of treatment. |
Planned Pivotal Phase 3 Combination Therapy Clinical Trial. We plan to conduct a pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib for the treatment of newly diagnosed mRCC under an SPA with the FDA. We expect to initiate patient enrollment in this trial in the first quarter of 2012.
We have designed this trial to be a randomized, multicenter, double-blind, placebo-controlled trial of AGS-003 in combination with sunitinib compared to sunitinib plus placebo. We plan to enroll approximately 650 patients in the trial to generate 600 evaluable patients. We plan to enroll these patients at approximately 100 to 120 clinical sites in North America and Europe. Under the protocol, these patients will be randomized between the two arms on a one-to-one basis. We anticipate that we will require approximately two years to fully enroll patients in the trial. We expect to monitor these patients for approximately 11 months following randomization, at which time we believe we will have data from at least 460 patients that reach disease progression during the trial, which would provide us with sufficient data to determine whether the primary endpoint of the trial has been achieved. If we complete enrollment as planned, we expect to have primary endpoint data in the second half of 2014.
We have designed this study with a primary endpoint of progression free survival, which has served as the primary endpoint in the pivotal phase 3 trials for five of the six products most recently approved by the FDA for the treatment of mRCC. Secondary endpoints will include overall survival, overall response rate and safety. In order to achieve the primary endpoint, results from the trial must demonstrate an increase of at least 30% in progression free survival for the AGS-003 plus sunitinib arm compared to the sunitinib plus placebo arm. Such a result would be statistically significant (p £ 0.05). The statistical significance of clinical trial results is determined by statistical methods that establish the p-value of the results. Typically, clinical trial results are statistically significant if they have a p-value of 0.05 or less, meaning that there is less than a one-in-20 likelihood that the observed results occurred by chance. There will be an interim analysis of data from the trial at 30% and 50% information by the independent data monitoring committee to assess both safety and futility.
91
Table of Contents
Our design for this trial requires patients to be adults who have been newly diagnosed with mRCC with primary tumor intact (no prior nephrectomy) and measurable metastatic disease, who are histologically confirmed to have a component of clear cell RCC based upon the tumor collected at nephrectomy, who are suitable for sunitinib therapy and who are poor risk or intermediate risk patients with the less than one year to treatment risk factor and not more than three MSKCC risk factors in total. The protocol provides for exclusion of patients from the trial if they have brain metastases or autoimmune conditions requiring chronic immunosuppressive therapy or if they have received any prior therapies for RCC. As part of the trial design, the two arms of the trial will be balanced based upon known prognostic risk factors and enrollment location. Patients who are randomized will be stratified by overall performance status (0, 1), number of MSKCC risk factors (1, 2 or 3) and geographic region (North America, Western Europe and other).
Under the protocol, patients in the AGS-003-sunitinib arm would be dosed with AGS-003 once every three weeks for five doses, followed by a booster dose every three months until disease progression. Sunitinib would be dosed in six-week cycles, consisting of four weeks on drug and two weeks on drug holiday. A total of four six-week cycles of sunitinib, combined with five AGS-003 doses would comprise the 24-week induction phase. AGS-003 dosing would be initiated at the end of the initial six-week sunitinib cycle. The first dose of AGS-003 would be administered prior to the start of sunitinib dosing in the second sunitinib cycle. Patients in the placebo-sunitinib arm would receive placebo on the same dosing schedule as AGS-003 in the AGS-003-sunitinib arm and sunitinib on the same dosing schedule as sunitinib in the AGS-003-sunitinib arm. This dosing regimen is identical to the dosing regimen used in our phase 2 combination therapy clinical trial of AGS-003 and sunitinib. We also expect the patient population in the planned pivotal phase 3 clinical trial to be comparable to the patient population treated in our phase 2 combination therapy clinical trial. In addition, we intend to submit to the FDA a separate protocol under which, following disease progression, patients in the placebo-sunitinib arm will be able to participate in an optional extension trial of AGS-003 in combination with other compatible second-line agents.
In September 2011, we received a letter from the FDA advising us that the FDA had completed its review of our protocol for the pivotal phase 3 clinical trial under the SPA process. In the letter, the FDA stated that it had determined that the protocol sufficiently addressed the trial’s objectives and that the trial was adequately designed to provide the necessary data to support a submission for marketing approval. The FDA noted that the ability of the trial to support an efficacy claim for AGS-003 would depend on a review of the data from the trial and an evaluation of the overall risk and benefit of AGS-003.
Phase 2 Combination Therapy Clinical Trial. From July 2008 to October 2009, we enrolled 21 newly diagnosed mRCC patients in a single arm, multicenter, open label phase 2 trial of AGS-003 in combination with sunitinib. We conducted this clinical trial at nine clinical sites in the United States and Canada. In selecting an agent to study with AGS-003 in combination therapy, we chose to evaluate the combination of AGS-003 with sunitinib for the following reasons:
| • | Sunitinib was quickly establishing itself as the first-line standard of care for the treatment of mRCC in our targeted patient population. |
| • | In our laboratory tests measuring the potency of the immune responses generated by the combination of AGS-003 and sunitinib as measured by CD8+ central effector and memory T-cell responses, we observed that sunitinib had not demonstrated any detrimental effect on the ability of AGS-003 to generate the desired immune response. |
| • | Due to the anti-cancer activity of sunitinib, by initiating dosing of AGS-003 following the first treatment cycle of sunitinib, patients were likely to have less metastatic tumor burden, or at least a slowing of tumor progression, at the time of initiation of AGS-003 therapy, making it more likely that AGS-003 would have the opportunity to elicit immunologic responses and an effect on the tumor. |
92
Table of Contents
| • | In its pivotal phase 3 clinical trial, sunitinib had demonstrated a significant improvement in median progression free survival over interferon-a, the previous standard of care. However, because of sunitinib’s toxicity, oncologists have not been able to improve upon these results by trying to combine sunitinib with other approved targeted agents as a result of the overlapping toxicities and inability to combine full doses of these various targeted agents. Because of AGS-003’s relative lack of toxicity, we were comfortable that we could combine AGS-003 with sunitinib and administer a full dose of both compounds without added toxicity. |
In addition, although we only learned this after starting the phase 2 combination therapy trial, sunitinib decreases regulatory T-cells and myeloid-derived suppressor cells, or MDSC’s, both of which are known to expand during cancer and suppress T-cell responses. As a result, we hypothesized that AGS-003 would be particularly potent in combination with sunitinib because of the ability of AGS-003 to generate T-cell responses in patients who may have experienced a reduction in these immunosuppressive regulatory T-cells and MDSC’s after the initiation of sunitinib therapy.
The original design for this trial called for the recruitment of 50 patients to generate 38 fully evaluable patients. The primary endpoint of the trial was complete response rate. Secondary endpoints included progression free survival, overall survival, safety, clinical benefit rate and immune response.
Our design for the trial required patients to be adults with previously untreated mRCC with no prior nephrectomy or at least one accessible lesion for biopsy, a histologically confirmed predominantly clear cell tumor and suitability for sunitinib therapy. Patients with brain metastases or autoimmune conditions requiring chronic immunosuppressive therapy, and those who had received prior RCC therapy, were excluded from the trial.
Patients in the trial generally received one initial six-week cycle of sunitinib, consisting of four weeks on drug and two weeks on drug holiday, prior to initiating the combined treatment with AGS-003. Patients then received a dose of AGS-003 every three weeks for a total of five doses, while also continuing three additional six-week cycles of sunitinib. This 24-week induction phase was followed by a booster phase during which patients received a dose of AGS-003 once every three months and continued to receive sunitinib in six-week cycles until disease progression.
In October 2009, we terminated enrollment in this trial early in connection with the anticipated termination of our former collaboration involving AGS-003 with Kyowa Hakko Kirin Co., Ltd., or Kirin, and the resulting termination of funding for the trial by Kirin. As a result, only a total of 21 patients were enrolled and received at least one dose of AGS-003 in this trial. In addition, the trial population evaluated in the trial differed significantly from the intended population. The trial was originally designed to enroll subjects with favorable and intermediate risk disease profiles. Instead, the actual population enrolled consisted entirely of patients with intermediate or poor risk disease profiles that had the less than one year to treatment risk factor. Based on published research, the expected complete response rate, progression free survival rate and overall survival rate for patients treated with sunitinib alone with intermediate and poor risk factors is greatly diminished compared to the expectations for patients treated with sunitinib alone who present with a favorable risk disease profile at the time of diagnosis. Primarily for these reasons, we could not and did not perform the statistical analysis to determine whether the primary endpoint of complete response rate was achieved, as we did not have sufficient number of evaluable patients nor any favorable risk patients included at the time of early trial closure. For this reason, we expect the data from this trial to be considered by the FDA for the purpose of evaluating the safety and feasibility of AGS-003, but that it will only have a limited impact on the FDA’s ultimate assessment of the efficacy of AGS-003.
As a result, we concluded that the initial trial design and expectation of complete responses in patients with mRCC was inappropriate for our immunotherapy, and particularly inappropriate for the enrolled patient population of intermediate and poor risk patients with the less than one year to treatment risk factor. We also concluded that the secondary endpoints in the trial, progression free survival and overall survival, along with immunologic response, were the appropriate endpoints to consider for measuring the efficacy of AGS-003 in combination with sunitinib in patients with mRCC.
93
Table of Contents
The following table summarizes certain key data from the 21 patients (15 intermediate risk; 6 poor risk) enrolled in the trial:
| Outcome |
(N=21) | |
| Median PFS |
11.9 months | |
| Median OS |
Not yet reached | |
| Complete response |
0 patients | |
| Partial response |
6 patients | |
| Stable disease |
7 patients | |
| Immune response |
IL-2 and interferon-g (IFN-g) recovery | |
Particular observations from these data and the trial, which have informed our further clinical development of AGS-003, include:
| • | To date, four patients have not progressed and continue to be dosed. |
| • | Median progression free survival was 6.0 months for poor risk patients and 14.9 months for intermediate risk patients. |
| • | As of August 31, 2011, we had not yet reached median overall survival for patients in the trial. Median overall survival was 7.9 months for poor risk patients and, although not yet reached, at least 29 months for intermediate risk patients. |
| • | Of the six patients that exhibited a partial response, one partial response was observed during the 24-week induction phase. The other five partial responses were observed after prolonged dosing with AGS-003 in combination with sunitinib during the booster phase. We do not believe that these late occurring partial responses have been routinely observed in clinical trials of sunitinib alone in intermediate and poor risk patients. As a result, we believe that these late responses may relate to the immunologic effects of AGS-003 and AGS-003’s effect on CD8+ central effector and memory T-cells. |
| • | AGS-003 was found to have positive impact on immune cell function and restoration of cellular immunity, including an increase in levels of IL-2 and IFN-g in a majority of patients. |
| • | In the trial, we observed a trend towards correlation between the increase in percentage expansion or proliferation of CD8+ central effector and memory T-cells and prolonged progression-free survival. These results were accepted for presentation during the 2011 annual American Society of Clinical Oncology (ASCO) meeting. |
| • | In this trial, we observed a correlation between the increase in percentage expansion or proliferation of CD8+ central effector and memory T-cells and prolonged overall survival. These results were presented at the 2011 annual Kidney Cancer Association, or KCA, meeting and have been submitted for presentation at the 2012 ASCO Genitourinary Cancers Symposium. |
| • | In the trial, we observed a correlation between increased progression-free survival and prolonged overall survival. |
| • | The adverse events in this trial associated with AGS-003 were generally only mild injection site reactions, while the toxicities with sunitinib were consistent with those expected from treatment with sunitinib alone. |
94
Table of Contents
AGS-003 Phase 2 Combination Therapy Clinical Trial, as Compared to Sunitinib Pivotal Phase 3 Clinical Trial and Sunitinib EFFECT Clinical Trial. Our phase 2 combination therapy clinical trial used similar patient inclusion and exclusion criteria as the pivotal phase 3 clinical trial of the use of sunitinib as a monotherapy in mRCC conducted between 2004 and 2006 and a more recently reported 2011 “EFFECT” clinical trial of sunitinib at two different doses and schedules as a monotherapy in mRCC. A summary comparison of the results of these two sunitinib monotherapy trials and of our phase 2 clinical trial of AGS-003 in combination with sunitinib is set forth in the graph below. The results of the sunitinib monotherapy clinical trials were presented by Dr. Robert Motzer of MSKCC in oral presentations at the 2007 ASCO annual meeting and the 2011 ASCO Genitourinary annual meeting. Although we believe this comparison is useful in evaluating the overall results of our phase 2 clinical trial, the three studies were separate trials conducted at different sites and at different times. Moreover, the patient population with mRCC is heterogeneous and the differences in patient population in the trials could have had a significant impact on the results of the trials. We have not conducted a head-to-head comparison of sunitinib and AGS-003 as a combination therapy against sunitinib as a monotherapy in a clinical trial. This is the design of the protocol for our planned pivotal phase 3 combination therapy clinical trial of AGS-003. Results of this head-to-head comparison may differ significantly from those set forth in the following graph. In addition, because the three studies were separate trials and because only 21 patients received AGS-003 in our phase 2 combination therapy clinical trial, differences between and among the results of the three trials may not be statistically meaningful.
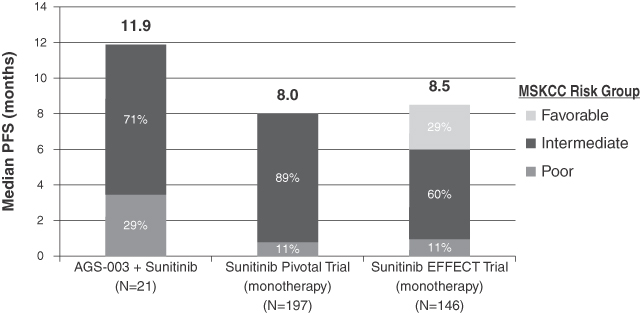
The results from the sunitinib phase 3 pivotal trial set forth in the graph above represent the results of sunitinib monotherapy in intermediate or poor risk patients with the less than one year to treatment risk factor. We have excluded the results for the 38% favorable risk patients in the sunitinib phase 3 pivotal trial and any intermediate and poor risk patients who did not have the less than one year to treatment risk factor, as we do not believe that these patients are comparable to the patient population that we evaluated in our phase 2 combination therapy clinical trial. Median progression free survival was 3.7 months for poor risk patients in the sunitinib phase 3 pivotal trial and 10.6 months for intermediate risk patients in the sunitinib phase 3 pivotal trial. The EFFECT trial results shown in the above graph show median progression free survival of 8.5 months for all three patient groups (favorable, intermediate and poor) because the results of the trial were not analyzed separately by risk group or by MSKCC risk factors. As a result, the stated EFFECT median progression free survival includes the results from the 29% favorable risk patients enrolled in the trial. In the sunitinib pivotal trial, favorable risk patients exhibited a substantially longer period of progression free survival than either intermediate or poor risk
95
Table of Contents
patients. We did not enroll any favorable risk patients in our phase 2 combination therapy clinical trial of AGS-003. In addition, results from sunitinib monotherapy in the sunitinib phase 3 pivotal trial showed a median overall survival of 5.3 months in the poor risk patients in the trial and 20.7 months in the intermediate risk patients in the trial.
Phase 1/2 Monotherapy Clinical Trial. From April 2006 through October 2008, we enrolled 22 newly diagnosed mRCC patients in a single arm, multicenter, open label phase 1/2 clinical trial of AGS-003 as a monotherapy. These patients were enrolled at six sites in the United States and Canada, including 13 at one site. The trial was designed as a two-stage trial. In stage 1, we would recruit 24 patients to generate at least 18 evaluable patients, and in stage 2, we would recruit an additional 22 patients to generate at least a total of 35 evaluable patients. In order to advance to stage 2 of the trial, we were required to achieve the primary endpoint for stage 1, which was three patients with a complete response or partial response. Secondary endpoints included progression free survival, overall survival, safety and immunogenicity.
Our design for the trial required patients to be asymptomatic or symptomatic but fully functional adults with mRCC, whether or not displaying symptoms, with no prior nephrectomy and with sufficient renal function in the remaining kidney. Patients with brain metastases, those who had received prior RCC therapy and those with active autoimmune disease were excluded from the trial. The trial was designed to enroll subjects with favorable and intermediate risk disease profiles.
In the trial, patients were administered a dose of AGS-003 every two weeks for a total of five doses, followed by a dose of AGS-003 every month for an additional four doses during the 24-week induction phase of the trial. These doses were followed by booster doses every three months until disease progression. However, due to the approval of sunitinib and sorafenib at the time we were beginning to enroll patients in the trial and the changing standard of care for mRCC as a result of the approvals, we experienced delays in enrolling patients and were unable to enroll any favorable risk patients in the trial. For these reasons, we shifted our development focus for subsequent trials to AGS-003 as a combination therapy.
To date, one patient in the trial has continued to be dosed for more than three years, and two other patients in the trial continue to be monitored for overall survival.
The following table summarizes certain key data from the 22 patients (13 intermediate risk; 9 poor risk) enrolled in the trial:
| Outcome |
(N=22) | |
| Median PFS |
5.6 months | |
| Median OS |
13.6 months | |
| Complete response |
0 patients | |
| Partial response |
1 patient | |
| Stable disease |
7 patients | |
| Immune response |
IL-2 and IFN-g recovery | |
Particular observations from these data and the trial, which have informed our further clinical development of AGS-003, include:
| • | Despite the intermediate and poor risk population, seven of the evaluable patients experienced some degree of tumor regression at one point during the induction phase of treatment with AGS-003. |
| • | AGS-003 was found to have positive impact on immune cell function and restoration of cellular immunity, including an increase in levels of IL-2 and IFN-g in a majority of patients. |
| • | AGS-003 was well-tolerated, with adverse events limited to mild injection site reactions, transient flu-like symptoms and tenderness in the lymph nodes. |
96
Table of Contents
Phase 1/2 Monotherapy Clinical Trial of MB-002. Prior to developing AGS-003, we developed a predecessor RNA-loaded dendritic cell drug product candidate, MB-002, for the treatment of mRCC. From April 2004 through March 2005, we enrolled 20 newly diagnosed mRCC patients in a single arm, multicenter, open label phase 1/2 clinical trial of MB-002 as a monotherapy. The overall endpoints for this trial included safety, feasibility of developing and supplying patient-specific product to clinical sites from a central facility, clinical benefit, progression free survival and immunologic response. In order to achieve success for this trial, we had to demonstrate the product was safe, could be readily manufactured and supplied from a central facility, generated anti-tumor activity and had the ability to induce an immunologic response capable of restoring IL-2 and IFN-g based T-cell responses in treated subjects.
This trial was designed to enroll patients with newly diagnosed mRCC, whether or not displaying symptoms, with no prior nephrectomy and with sufficient renal function in the remaining kidney. Patients with brain metastases, those who had received prior RCC therapy and those with active autoimmune disease were excluded from the trial.
In the trial, patients were administered a dose of MB-002 every two weeks for five doses, followed by a dose of MB-002 every month for four doses, during the 24-week induction phase. This dosing of MB-002 was followed by booster doses every three months until disease progression.
The following table summarizes certain key data from the 20 patients (15 intermediate risk; 5 poor risk) enrolled in the trial:
| Outcome |
(N=20) | |
| Median PFS |
6.3 months | |
| Complete response |
0 patients | |
| Partial response |
0 patients | |
| Immune response |
IL-2 recovery only | |
Particular observations from these data and the trial, which informed our further development of AGS-003, include:
| • | Four patients experienced some tumor regression at one point during the induction phase of treatment with MB-002. |
| • | MB-002 was well-tolerated, with adverse events limited to mild injection site reactions, transient flu-like symptoms and tenderness in the lymph nodes. |
| • | MB-002 was feasible to manufacture and deliver from a central facility to clinical trial sites in the United States and Canada. |
| • | The immunologic response data suggested that MB-002 was only able to partially overcome the immune suppression observed in mRCC. While IL-2 recovery was observed, IFN-g recovery was not observed in the majority of patients. |
Because MB-002 corrected defects in the production of only one of the two critical cytokines - IL-2, but not IFN-g - required for an effective immune response, we conducted further laboratory research and developed an optimized product candidate, AGS-003. AGS-003 differs from MB-002 in the way we mature the dendritic cells prior to administration. Based on in vitro experiments that simulate immunization in the laboratory, we observed that AGS-003 could overcome both critical cytokine defects in RCC patient cells and fully restore immune response. We subsequently confirmed this observation in vivo in the phase 1/2 monotherapy clinical trial of AGS-003 and in the phase 2 combination therapy clinical trial of AGS-003 and sunitinib.
97
Table of Contents
Monotherapy Rollover Clinical Trial. In 2006, we enrolled two intermediate risk patients who had completed dosing in the phase 1/2 monotherapy clinical trial of MB-002 and who had experienced at least two years of stable disease in a separate, open label rollover trial of AGS-003 monotherapy. As this trial was an extension trial and observational in nature, the endpoints for this trial consisted of continued safety assessments and immunologic assessments to denote any differences between MB-002 and AGS-003. In this trial, the two patients received an induction phase of AGS-003 doses, followed by continued booster doses, consistent with the AGS-003 dosing schedule in the phase 1/2 monotherapy clinical trial of AGS-003, and were treated with AGS-003 for an additional four and four and a quarter years, respectively, until disease progression occurred.
Other Development Activities. We are conducting preclinical testing of AGS-003 in combination with some of the new mRCC therapies and the mRCC therapies in late-stage development with the goal of determining whether AGS-003 may be used in combination with multiple approved therapies and therapies in late-stage development in addition to sunitinib.
AGS-004 for Human Immunodeficiency Virus
We are developing AGS-004, our second Arcelis-based product candidate, for the treatment of HIV. We completed two early stage clinical trials of AGS-004 and are currently conducting a phase 2b clinical trial. Our phase 2b clinical trial of AGS-004 is currently being funded entirely by the NIH as part of a $29.9 million contract awarded to us as part of a competitive submission process in 2006. We plan to proceed with further clinical development of AGS-004 following completion of the phase 2b trial if we are able to obtain government or other third-party funding for such efforts.
Human Immunodeficiency Virus. HIV is characterized by a chronic viral infection and an associated deterioration of immune function. Specifically, the virus disables and kills crucial human immune cells called CD4+ T-cells. Over time, this viral impact on an infected person’s immune system outpaces the body’s natural ability to replace CD4+ T-cells and immunodeficiency results. CD4+ T-cells are a sub-group of lymphocytes, a type of white blood cell. CD4+ T-cells play an important role in establishing and maximizing the capabilities of the immune system. These cells provide signals for orchestrating an immune response by activating and directing other immune cells, which ultimately leads to the cytotoxic activity.
According to the World Health Organization, the number of people living with HIV in the world was approximately 33.3 million in 2009. The Henry J. Kaiser Family Foundation estimates that more than 1.1 million people are currently living with HIV in the United States. According to the Centers for Disease Control and Prevention, the number of new cases of HIV infection in the United States is expected to remain steady at about 55,000 cases per year for the next decade. The number of new cases of HIV in Western Europe in 2009 was 30,000 according to the World Health Organization.
Existing Therapies. In 1996, a combination of oral medications known as highly-active antiretroviral therapy, or HAART, was introduced to treat patients with HIV. Since then, the introduction of new therapeutic drug classes and combination drug treatment strategies has enhanced treatment for HIV. Datamonitor estimates 2008 global sales of HIV treatments to be $10.7 billion.
New drugs for the treatment of HIV are continually being introduced because current treatments are imperfect. The requirement of existing therapies for lifelong daily treatment has made population-wide adherence difficult. Poor compliance has led to drug resistant HIV variants that are ineffectively controlled by the available armamentarium of approved drugs. In addition, although some current drugs used for the treatment of HIV are relatively benign, few combination regimens are wholly non-toxic. The most recent U.S. guidelines on antiretroviral treatment contain a number of tables of adverse effects of combination regimens and how to manage them. Some combinations present potentially life-threatening complications and other complications that are chronic, cumulative and overlapping, and sometimes irreversible. As a result, although HAART and these new therapeutic drug classes and drug treatment strategies have saved many lives, there is a need to develop new therapies that address these shortcomings.
98
Table of Contents
Description and Development Status. AGS-004 is a fully personalized immunotherapy based on our Arcelis platform. It is produced by electroporating dendritic cells with RNA encoding for patient-specific HIV antigens that have been derived from a patient’s virus-infected blood and with the CD40L protein. The process for producing AGS-004 is the same process as is used to produce AGS-003, with the one key difference being that AGS-003 contains all of the antigens from a patient’s tumor cells and AGS-004 contains all variants unique to each individual patient of four selected HIV antigens.
Phase 2b Clinical Trial. In July 2010, we began enrolling patients in a randomized, placebo-controlled, double-blind phase 2b clinical trial of AGS-004. We plan to enroll 54 patients in the trial at approximately nine sites in the United States and Canada. These patients will be randomized between AGS-004 treatment and a placebo control on a two-to-one basis. As of August 31, 2011, we had enrolled 28 patients in this trial. We expect to complete enrollment of this trial in the first quarter of 2012. We expect to complete primary endpoint analysis of the data from this trial in mid-2012.
The primary endpoint of the trial is a comparison against placebo of the anti-HIV effects of AGS-004 as measured by viral load after the discontinuation of standard HIV antiretroviral drug therapy. Secondary endpoints include change in viral load from pre-antiretroviral therapy to the end of the antiretroviral treatment interruption, changes in CD4+ T-cell counts, assessment of plasma viral load kinetics and safety. In order to achieve the primary endpoint, the trial must demonstrate a 1.1 log 10 reduction in the median viral load in patients in the AGS-004 arm from the point of discontinuation of antiretroviral drug therapy to the date that is 12 weeks later as compared to the placebo control arm. Such a result would be statistically significant. A log 10 reduction means a decrease in virus concentration to 10% of the original concentration. A 1.1 log 10 reduction means a decrease to 8% of the original concentration. Viral load is reported as copies of the HIV virus in a milliliter (mL) of blood. Changes in viral load are reported as a log change.
To be included in this trial, patients must be adults with HIV-1 infection that was virally suppressed with a stable antiretroviral therapy regimen, adequate CD4+ T-cell counts and a pre-antiretroviral therapy plasma viral sample to be used to manufacture AGS-004. Patients with HIV Type 2, or HIV-2, active viral infection other than HIV Type 1, or HIV-1, active syphilis infection or a history of hepatic, renal or cardiac impairment which would increase risks associated with prolonged interruption of antiretroviral therapy are being excluded.
In this trial, patients will receive intradermal doses of AGS-004 or placebo every four weeks for a total of four doses, together with their standard antiretroviral therapy. Following the fourth dose of AGS-004 or placebo, the patient will discontinue their antiretroviral therapy but continue to receive AGS-004 or placebo every four weeks for 12 weeks. Patients in the AGS-004 arm that demonstrate control of viral replication and maintain adequate CD4+ T-cell counts can remain off their antiretroviral therapy and continue their treatment interruption past 12 weeks, which we expect will enable us to assess the durability of the clinical benefit to these patients.
Phase 2a Clinical Trial. From 2008 to 2009, we enrolled 29 patients in a single arm, multicenter, open label phase 2a clinical trial of AGS-004. These patients were enrolled at eight clinical sites in Canada. Eight of these patients had previously participated in our phase 1/2 clinical trial of AGS-004. We are still following two patients post-treatment to evaluate safety and durability.
The primary endpoint of this trial was to measure the ability of AGS-004 to improve immune control of HIV as measured by the proportion of patients with HIV viral loads of less than 1,000 copies/mL on at least three time points during a 12-week antiretroviral treatment interruption. Secondary endpoints included safety, change in viral load from pre-antiretroviral therapy to the end of the antiretroviral treatment interruption and changes in CD4+ T-cell counts.
To be included in this trial, patients were required to be adults with HIV-1 infection that was virally suppressed with a stable antiretroviral therapy regimen, adequate CD4+ T-cell counts and a pre-antiretroviral therapy plasma viral sample to be used to manufacture AGS-004. Patients with HIV-2, active viral infection other than HIV-1, active syphilis infection or active autoimmune disease were excluded.
99
Table of Contents
Patients initially received four monthly doses of AGS-004, together with their standard antiretroviral therapy. After receiving those doses, antiretroviral therapy was discontinued and two additional monthly doses of AGS-004 were administered. Patients who maintained plasma viral loads under 10,000 copies/mL and CD4+ T-cell counts above 350 were eligible to remain off of their antiretroviral drugs and receive up to two additional booster doses of AGS-004 at eight weeks after the second monthly dose administered during the period of antiretroviral treatment interruption and at twelve weeks after the first booster dose.
In this clinical trial, eight of the 24 patients that continued the trial during the 12-week period of antiretroviral treatment interruption had HIV viral loads of less than 1,000 copies/mL on at least three time points during the 12-week period of antiretroviral treatment interruption. In addition, we observed the following other results:
| • | AGS-004 was safe and well-tolerated, with no additional risk observed during antiretroviral treatment interruption, no subjects experienced serious adverse events that were related to treatment with AGS-004 and no subjects reported an adverse event leading to the discontinuation of AGS-004. |
| • | Sixteen patients had reduced HIV viral loads at the end of the antiretroviral treatment interruption period as compared to their pre-antiretroviral therapy viral loads. |
| • | Similar to the trend we observed in our phase 2 combination therapy trial of AGS-003, we observed a trend towards correlation between the percentage expansion or proliferation of CD8+ central effector and memory T-cells and improved virologic control. |
| • | The median CD4+ T-cell counts three months after the antiretroviral treatment interruption did not demonstrate a statistically significant decrease from the median of the last three CD4+ T-cell counts for the period during which the patients received AGS-004 and antiretroviral drug therapy. |
Phase 1/2 Clinical Trial. From 2006 to 2007, we enrolled 10 patients in a single arm, single center, open label phase 1/2 clinical trial of AGS-004. The primary endpoint of this trial was to examine the immunogenicity of AGS-004 monotherapy through CD8+ central effector and memory T-cell response. The secondary endpoint was the safety of AGS-004 in combination with antiretroviral therapy. The inclusion and exclusion criteria were substantially similar to those for the phase 2a clinical trial of AGS-004. Patients received four doses of AGS-004 one month apart.
This trial met its predefined endpoint of demonstrating multifunctional CD8+ central effector and memory T-cell response against multiple HIV antigens in four of the nine patients whose materials were available for testing. AGS-004 was well-tolerated, with adverse events limited to mild injection site reactions, transient flu-like symptoms and tenderness in the lymph nodes.
Future Clinical Development. Our ongoing phase 2b clinical trial of AGS-004 may support a similar phase 3 trial design with viral load control as the primary endpoint. However, we are considering other potential endpoints, such as maintenance of CD4+ T-cell counts or reduction in inflammatory markers. Alternatively, we may target HIV-infected individuals who have not received any prior treatment with the goal of significantly delaying the need for antiretroviral therapy. We are working with key opinion leaders to determine the appropriate future clinical development plan for AGS-004. We expect to make this determination based, in part, on the outcome of our ongoing phase 2b clinical trial. We do not plan to conduct a phase 3 trial or other clinical trials of AGS-004 unless we obtain government or other third-party funding for such efforts.
NIH Contract. Our development of AGS-004 has received significant funding from the U.S. federal government. In September 2006, we entered into a multi-year research contract with the NIH and the National Institute of Allergy and Infectious Diseases, or NIAID, to design, develop and clinically test an autologous HIV immunotherapy capable of eliciting therapeutic immune responses. We are using funds from this contract to
100
Table of Contents
develop AGS-004. Under this contract, as it has been amended, the NIH and NIAID have committed to fund up to $33.9 million, including reimbursement of our direct expenses and allocated overhead and general and administrative expenses and payment of specified amounts totaling up to $1.4 million upon our achievement of specified development milestones. This commitment extends until May 2013. As of August 31, 2011, there was up to approximately $10.0 million of potential revenue remaining to be earned under the agreement with the NIH. In September 2011, we agreed to a modification of our contract with the NIH and the NIAID under which the NIH and the NIAID agreed to increase their funding commitment to us by an additional $4.0 million in connection with our agreement to add an additional 12 patients to our phase 2b clinical trial of AGS-004. This $4.0 million increase is included in the $33.9 million commitment.
We have agreed to a statement of work under the contract, and are obligated to furnish all the services, qualified personnel, material, equipment, and facilities, not otherwise provided by the U.S. government, needed to perform the statement of work. In accordance with the laws applicable to government intellectual property rights under federal contracts, we have a right under our contract with the NIH to elect to retain title to inventions conceived or first reduced to practice under the NIH contract, subject to the right of the U.S. government to a royalty-free license to practice or have practiced for or on behalf of the United States the subject invention throughout the world. The government also has special statutory “march-in” rights to license or to require us to license such inventions to third parties under limited circumstances. In addition, we may not grant to any person the exclusive right to use or sell any such inventions in the United States unless such person agrees that any products embodying the subject invention or produced through the use of the subject invention will be manufactured substantially in the United States.
AGS-009 for Lupus
AGS-009 is a monoclonal antibody that we are developing for the treatment of systemic lupus erythematosus, or lupus. We discovered AGS-009 through our dendritic cell biology research. We are currently conducting a phase 1a clinical trial of AGS-009 in lupus patients.
Lupus. Lupus is a chronic and disabling autoimmune disorder, characterized by inflammation that can affect a person’s skin, joints, kidneys and other organs. Most patients suffer from joint pain and swelling. Other common symptoms include fever, rash, malaise, myalgia, fatigue, temporary cognitive impairment, anemia and other hematologic complications. The Lupus Foundation of America estimates that there are approximately 1.5 million people living with lupus in the United States.
Existing Therapies. No permanent cure is available for lupus. Physicians typically use steroids, non-steroidal anti-inflammatory drugs, anti-malaria agents and immunosuppressive drugs to treat the symptoms of lupus. In 2011, the FDA approved Benlysta® (belimumab) as the first new drug regimen for lupus in over 50 years. In clinical trials, patients treated with belimumab and standard therapies experienced less disease activity than those who received a placebo and standard therapies. Although belimumab has the potential to improve the quality of life for many lupus patients, it appears to have limited benefit to patients with certain severe variations of lupus. In addition, African American patients and patients of African heritage participating in two belimumab studies did not appear to respond to treatment. Overall, lupus treatments have proven inadequate to control the disease and there remains a need for new lupus treatments.
Description and Development Status. AGS-009 is a humanized monoclonal antibody with the ability to bind and neutralize human interferon-a. Interferon-a is a protein that induces inflammation and which, when uncontrolled, has been suggested, to be involved in a range of chronic inflammatory diseases, including lupus. Interferon-a is activated and produced by dendritic cells. In preclinical testing, AGS-009 demonstrated high binding affinity and broad specificity against all subtypes of interferon-a, which we believe enables it to target a cytokine hypothesized to be a major factor in lupus symptoms and progression. We expect that inhibiting interferon-a in lupus patients may control the disease and mitigate its debilitating side effects.
101
Table of Contents
Phase 1a Clinical Trial. From September 2009 to July 2011, we enrolled 24 patients in a randomized, double-blind, placebo-controlled, single dose escalation phase 1a clinical trial of AGS-009 in adult patients with mild to moderate lupus. These patients were enrolled at seven clinical sites in North America. Patients enrolled in the trial had laboratory confirmed lupus for six months or more. There were six dose levels involving four patients at each level. The dose levels in each cohort were 0.01, 0.1, 1, 3, 10 and 30 mg/kg. Within each cohort of four patients, three were randomized to receive AGS-009 and one received placebo. Each patient was administered a single intravenous dose of AGS-009 or placebo. Each patient was then monitored continuously for 12 weeks for safety, pharmacokinetics, pharmacodynamics and preliminary indications of bioactivity.
The primary endpoint for safety and tolerability in this trial is dose-limiting toxicity. Secondary endpoints include pharmacodynamic and pharmacokinetic parameters, bioactivity and immunogenicity assays, including changes in disease status. This clinical trial was initially conducted by Novo Nordisk A/S, or Novo, with which we had a partnership related to this program. The partnership was terminated in June 2010, and we assumed sponsorship of the trial.
Future Clinical Development. We expect results of our phase 1a clinical trial will be available in the fourth quarter of 2011. Following review of these results, we expect that we will commence enrollment in a further clinical trial of this product candidate in the first half of 2012. We plan to seek a partner for funding the trial and for the further development and commercialization of AGS-009, but plan to commence the clinical trial even if we have not entered into a funding arrangement with a partner.
AGS-010 for Organ Transplantation and the Treatment of Autoimmune and Inflammatory Diseases
We are developing AGS-010, a recombinant human soluble CD83 protein, for organ transplantation and the treatment of autoimmune and inflammatory diseases. Our current preclinical research is primarily directed at organ transplantation. This drug candidate is the result of our research efforts into how the ability to turn off specific undesirable immune responses can have a therapeutic effect on all immune mediated diseases, including organ transplant rejection and autoimmune and inflammatory diseases.
In preclinical testing, AGS-010 has demonstrated strong immunomodulatory attributes in vitro and in vivo. In these tests, AGS-010 was active in disease models of multiple sclerosis, Type 1 diabetes, inflammatory bowel disease and solid organ transplant rejection. Specifically, in animal models, nine or fewer daily bolus intravenous doses conferred complete clinical resolution of disease and, therefore, did not require chronic administration or the maintenance of concomitant drug therapies. The effects were complete and permanent, and treated animals were not globally immunosuppressed.
We believe these data suggest that a limited course of AGS-010 treatment may be able to permanently turn off any immune responses that are active during the time of administration of AGS-010. Thus, we believe that AGS-010 could be used in organ transplantation and may potentially have broad utility in a wide spectrum of autoimmune and inflammatory diseases.
Organ Transplantation. In connection with organ transplantation, transplant rejection and failure of the transplant therapy can occur when the transplant recipient’s body recognizes the new organ as foreign and attacks it. As a result, transplant recipients must take drugs, generally for the duration of the patient’s life, to suppress the immune system and prevent such rejection.
Autoimmune and Inflammatory Diseases. Autoimmune and inflammatory conditions such as type 1 diabetes, lupus and Crohn’s disease are characterized by inappropriate chronic immune activation directed against either an individual’s own tissues or normally benign pathogens such as bacteria found in the digestive tract. Over time, chronic immune activation can result in permanent organ or tissue damage and can ultimately be fatal. Treatments developed for these indications are largely palliative in nature, meaning that they are designed to treat the symptoms but do not alter the underlying pathology of the diseases.
102
Table of Contents
Planned Clinical Development. We plan to seek government or other third-party funding for continued development of AGS-010. If we obtain such funding, we plan to advance the development of AGS-010 and conduct the necessary studies to enable the submission of an investigational new drug application, or IND, for AGS-010.
Manufacturing
We manufacture our Arcelis-based products, AGS-003 and AGS-004, for use in our clinical trials of those product candidates and rely on third-party contract manufacturers for clinical supply of AGS-009 and preclinical supply of AGS-010.
Manufacturing of AGS-003 and AGS-004
Our facility in Durham, North Carolina includes manufacturing suites for the production of drug products using our Arcelis technology platform. We have designed these suites to comply with the FDA’s current good manufacturing practice, or cGMP, requirements. We have manufactured the drug product for our development and clinical trial activities associated with AGS-003 and AGS-004 to date, and plan to manufacture the drug product for our planned pivotal phase 3 combination therapy trial of AGS-003 using our current processes at our current facility. We do not believe that our current manufacturing processes and current facility can be used to produce AGS-003 on a commercial scale. Accordingly, we plan to establish automated manufacturing processes based on existing functioning prototypes of automated devices and disposables, to identify and lease suitable space for a new U.S. commercial manufacturing facility and build out and equip the new facility for manufacture of our Arcelis-based products. As part of our BLA for AGS-003, we will need to demonstrate comparability between AGS-003 that we produce using our current processes in our current facility and AGS-003 produced using the automated processes in our planned new facility.
Arcelis Manufacturing Processes. Our Arcelis platform requires two components derived from the particular patient to be treated, specifically:
| • | a disease sample from the patient — tumor cells in the case of cancer or a blood sample containing virus in the case of infectious disease — which is generally collected at the time of diagnosis or initial treatment, and |
| • | dendritic cells derived from the patient’s monocytes, a particular type of white blood cell, which are obtained from the patient through a laboratory procedure called leukapheresis that occurs after diagnosis and at least four weeks prior to the initiation of our immunotherapy. |
Monocytes are obtained from the patient’s blood at his or her clinical site through leukapheresis. The tumor cells or blood sample containing the virus and the leukapheresis product, including plasma from the patient, are separately shipped from the clinical site following collection to our manufacturing facility in Durham, North Carolina. The tumor cells or blood sample containing the virus are shipped to us in a kit that we provide to the clinical site. The shipping containers that we provide for the leukapheresis product are temperature-controlled. The leukapheresis product is viable for manufacture if it arrives at our facility within four days after being taken from the patient.
At our facility, we isolate the mRNA from the disease sample. Using standard methods, we amplify the mRNA so that only small disease samples are required to manufacture drug product. This process allows us to manufacture additional drug product at a later date without requiring new disease samples from the patient. In parallel, we take the monocytes from the leukapheresis product and use a proprietary process to culture them to create mature dendritic cells.
We then take the mature dendritic cells and immerse them in a solution of the patient’s isolated mRNA and a synthetic RNA that encodes CD40L. The CD40L protein enables the dendritic cells to secrete IL-12, a cytokine
103
Table of Contents
required to induce new immune responses. We then apply a brief electric pulse to the solution, which is a process referred to as electroporation. This process generates pores in the dendritic cell membranes and permits the patient’s isolated mRNA and the CD40L protein to pass into the cells. We refer to this process as loading the dendritic cells.
We then culture the mRNA-loaded dendritic cells to allow for antigen expression from the patient’s mRNA in the form of peptides on the surface of the dendritic cells in order to interact with the patient’s immune system upon administration. These mature, loaded dendritic cells are formulated into the patient’s plasma that was collected during leukapheresis. We also add cryoprotectants to protect the cells during freezing and thawing. The resulting mixture is the Arcelis-based drug product. We then vial, freeze and cryogenically store the drug product using liquid nitrogen. We ship the drug product to the clinic using containers that can maintain a constant temperature for up to three weeks. Following receipt of the drug product, the clinic thaws the drug product and administers it to the patient by intradermal injection.
Commercial Manufacturing Facility and Automated Process. We plan to establish automated manufacturing processes based on existing functioning prototypes of automated devices and disposables for the production of commercial volumes of our Arcelis-based drug product. These devices and disposables can be used to perform all steps required for the manufacture of our Arcelis-based product candidates. We intend to identify and lease suitable space for the build-out and equipping of a new U.S. commercial manufacturing facility for the manufacture of AGS-003, AGS-004 and other Arcelis-based products.
In the new facility, we plan to use the automated equipment to perform Arcelis drug product processing in closed, single-use disposable components. A patient’s disease sample would only be in contact with these disposable sets or components and not with the balance of our manufacturing equipment. These disposable components would be discarded as biological waste upon completion of the manufacturing process for the particular patient’s drug product. Because our equipment would never be in direct contact with patient material, we believe that the time required to prepare the equipment between batches would be minimal. We also believe that processing material in disposable components will reduce the complexity and size of the facility by reducing the amount of required labor. We expect that automation will help ensure consistency of the manufacturing processes and facilitate compliance with cGMP.
Specifically, we estimate that using our automated manufacturing processes in a new facility would enable a 70% reduction in the personnel required for commercial scale production as compared to our existing processes, resulting in the need for a facility only approximately one-third the size of what would be required for commercial scale production using our existing processes. Furthermore, we estimate that our cost of goods would be reduced by approximately 75% by manufacturing our Arcelis-based drug products using automated processes, as compared to our existing processes.
Prior to implementing automated manufacture of AGS-003, we will be required to demonstrate that the new facility and the automated equipment are constructed and operated in accordance with cGMP and the comparability between AGS-003 that we produce using the processes in our current facility and AGS-003 produced using the automated processes in our new facility. We expect that it will require approximately two years from the time we begin the build-out and an approximately $25 million investment to build out and equip the facility to a level necessary to file a BLA for AGS-003 with the FDA using the automated manufacturing processes. We expect that we would incur an additional $10 million to $15 million after the filing of the BLA to further build out and equip the facility so as to have in place the commercial capacity that we anticipate will be required for commercial launch of AGS-003.
We believe that this facility and automated process will provide us key advantages, including:
| • | manufacturing methods which are consistent and reproducible across patients and indications; |
| • | centralized manufacturing capable of delivering AGS-003 to clinical sites throughout North America; |
104
Table of Contents
| • | commercial scale manufacturing of AGS-003; |
| • | manufacturing with a cost of goods for our fully personalized Arcelis-based products that we expect will be comparable to other biologics; and |
| • | an automated manufacturing platform that is scalable for large disease indications such as HIV and other cancer indications. |
Manufacturing of AGS-009 and AGS-010
We use contract manufacturers for the production of our AGS-009 and AGS-010 product candidates and purchase drug product from these manufacturers on a purchase order basis. AGS-009 is produced in Chinese hamster ovary cells using conventional recombinant, cell culture and antibody purification methods. AGS-010 is produced in bacteria and purified to a sufficient level for use in laboratory research and animal studies. We are currently working with a contract manufacturing organization to further refine this process for AGS-010 and scale it in order to provide sufficient material for our planned preclinical studies and early stage clinical trials. We believe that a number of suppliers are available for the production of AGS-009 and AGS-010. We do not plan to build our own manufacturing capacity for these products.
Sales and Marketing
We own exclusive worldwide commercial rights to all of our product candidates. We currently intend to retain North American marketing rights for AGS-003 and any future oncology products that we may develop. To exploit these rights, we would expect to build a commercial infrastructure for such products comprised of a targeted specialty sales force led by several sales management personnel, an internal marketing and medical affairs staff and a specialty distribution team. Our sales infrastructure would also include personnel to establish and direct reimbursement activities with third-party payors, such as managed care organizations, group purchasing organizations, oncology group networks and government accounts. We would need to hire selected personnel to fill key positions in advance of the approval of AGS-003. We currently have no sales and marketing or distribution capabilities or in-house personnel specializing in these functions. Outside North America, we plan to seek to enter into collaboration agreements with other pharmaceutical or biotechnology firms to commercialize AGS-003. We may, however, decide to collaborate with third parties to commercialize AGS-003 or any of our future oncology product candidates on a worldwide basis if we determine such arrangement to be in our best interest.
For AGS-004, AGS-009 and AGS-010, we plan to seek to enter into collaboration agreements with other pharmaceutical or biotechnology firms to commercialize these product candidates on a worldwide basis.
Competition
The biotechnology and pharmaceutical industries are highly competitive. There are many pharmaceutical companies, biotechnology companies, public and private universities and research organizations actively engaged in the research and development of products that may be similar to or competitive with our products. There are a number of multinational pharmaceutical companies and large biotechnology companies currently marketing or pursuing the development of products or product candidates targeting the same indications as our product candidates. It is probable that the number of companies seeking to develop products and therapies for the treatment of unmet needs in these indications will increase. Some of these competitive products and therapies are based on scientific approaches that are the same as or similar to our approaches, and others are based on entirely different approaches.
Many of our competitors, either alone or with their strategic partners, have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development
105
Table of Contents
of product candidates, obtaining FDA and other regulatory approvals of products and the commercialization of those products. Accordingly, our competitors may be more successful than we may be in obtaining approval for drugs and achieving widespread market acceptance. Our competitors’ drugs may be more effective, or more effectively marketed and sold, than any drug we may commercialize and may render our product candidates obsolete or non-competitive before we can recover the expenses of developing and commercializing any of our product candidates. We anticipate that we will face intense and increasing competition as new drugs enter the market and advanced technologies become available. We expect any products that we develop and commercialize to compete on the basis of, among other things, efficacy, safety, convenience of administration and delivery, price, the level of generic competition and the availability of reimbursement from government and other third-party payors.
mRCC
Historically, mRCC was treated with chemotherapy, radiation and hormonal therapies, as well as cytokine-based therapies such as interferon-a and IL-2. More recently, the FDA has approved several new agents as monotherapies for mRCC, including Nexavar, marketed by Bayer Healthcare Pharmaceuticals, Inc. and Onyx Pharmaceuticals, Inc., Sutent, marketed by Pfizer, Inc., Avastin, marketed by Genentech, Inc., a member of the Roche Group, and Votrient, marketed by GlaxoSmithKline. Other recently approved agents for the treatment of mRCC are Torisel, marketed by Pfizer, and Afinitor, marketed by Novartis Pharmaceuticals Corporation. In addition, there are product candidates in late-stage clinical development for the treatment of mRCC, such as tivozanib and axitinib. We believe that each of these existing therapies has efficacy or safety limitations and, as a result, that there remains an unmet need for novel therapeutic approaches for mRCC that can improve efficacy without adding appreciable toxicity. We believe that the safety profile that AGS-003 has demonstrated to date, which may enable it to be used in combination with these therapies with little or no additional toxicities, gives AGS-003 the potential to address this unmet need. Accordingly, existing therapies with which AGS-003 would be used as part of a combination would not be competitive with AGS-003. However, a standalone therapy for mRCC that demonstrated improved efficacy over currently marketed therapies with a favorable safety profile and without the need for combination therapy might pose a significant competitive threat to AGS-003.
Immatics Biotechnologies GmbH is developing a therapeutic cancer vaccine, IMA-901, which is a mixture of defined tumor-associated peptides, for the treatment of RCC. Immatics is conducting a pivotal phase 3 clinical trial comparing IMA-901 in combination with sunitinib against sunitinib alone. If this clinical trial is successful, IMA-901 and sunitinib combination therapy would be in direct competition with AGS-003 and sunitinib combination therapy. Immatics could have a competitive advantage if it is able to introduce its product to the market before the time, if any, at which we receive marketing approval for AGS-003.
We estimate that there are numerous other cancer immunotherapy products in clinical development by many public and private biotechnology and pharmaceutical companies targeting numerous different cancer types. A number of these product candidates are in late-stage clinical development.
HIV
There are numerous FDA-approved treatments for HIV, primarily antiretroviral therapies, marketed by large pharmaceutical companies. In addition, generic competition has recently developed as patent exclusivity periods for older drugs have expired, with more than 15 generic bioequivalents currently on the market. The presence of these generic drugs is resulting in price pressure in the HIV therapeutics market.
Lupus
Several biopharmaceutical companies are marketing or developing products for the treatment of lupus. In March 2011, the FDA approved Benlysta (belimumab) for marketing by Human Genome Sciences, Inc. and GlaxoSmithKline for the treatment of lupus. Two other monoclonal antibodies that employ a similar mechanism of action as AGS-009, Genentech’s rontalizumab and Medimmune, Inc.’s sifalimumab, are currently in phase 2 clinical trials.
106
Table of Contents
Organ Transplantation
The standard of care for immunosuppression in organ transplantation is cyclosporine, which is marketed as a generic by a number of manufacturers. The FDA has approved numerous other therapies for the prophylaxis of organ rejection, and several more are in clinical development.
Intellectual Property
Our success depends in part on our ability to obtain and maintain proprietary protection for our products and product candidates, technology and know-how, to operate without infringing the proprietary rights of others and to prevent others from infringing our proprietary rights. We are seeking a range of patent and other protections for our product candidates and platform technology. We also rely on trade secrets, know-how, continuing technological innovation and in-licensing opportunities to develop and maintain our proprietary position.
Patents
We own or exclusively license five U.S. patents and 15 U.S. patent applications, as well as more than 100 foreign counterparts to various of these patents and patent applications.
Arcelis Technology Platform and Arcelis-Based Products. We use our Arcelis technology platform to generate fully personalized RNA-loaded dendritic cell immunotherapies. As described above, the process of obtaining a disease sample and dendritic cells from a patient, using those materials to manufacture a fully personalized drug product and shipping the drug product to the clinical site for use in the treatment of the patient involves many important steps. These steps include:
| • | amplifying mRNA from a disease sample obtained from the patient; |
| • | differentiating dendritic cell precursors (monocytes) isolated from the patient into immature dendritic cells; |
| • | maturing the immature dendritic cells in culture and loading the mature dendritic cells with the amplified mRNA and CD40L protein; and |
| • | formulating the matured, loaded dendritic cells in the patient’s plasma with cryoprotectants to protect the cells in the resulting drug product when the drug product is frozen and thawed. |
We have sought to protect these steps or the equipment related to carrying out one or more of these steps through patents and/or trade secrets. We have also sought to protect the resultant drug product through patents.
Five of the U.S. patents and ten of the U.S. patent applications that we own or exclusively license, as well as European and Japanese counterparts to various of these patents and patent applications, are directed to one or more aspects of our Arcelis technology platform or Arcelis-based products. Specifically, these patents and patent applications are collectively directed to:
| • | Arcelis-based compositions of matter and products; |
| • | methods of manufacturing Arcelis-based products; |
| • | methods of using Arcelis-based products for treatment of tumors; |
| • | equipment that we intend to use for assisting the automated manufacture of Arcelis-based products; and |
| • | equipment that we may use for the frozen storage and delivery of Arcelis-based products. |
107
Table of Contents
The U.S. patents expire in 2016 and 2027, the U.S. patent applications, if issued, would expire between 2016 and 2028, the counterpart patents in Europe and Japan expire between 2017 and 2027, and the counterpart patent applications in Europe and Japan, if issued, would expire between 2017 and 2027. Included in these patents and patent applications are:
| • | four U.S. patents and one U.S. patent application that are collectively directed toward the composition of matter of Arcelis-based products (dendritic cells loaded with RNA from tumors or pathogens), methods of manufacture of these products and methods of using these products to treat tumors. Each of the four U.S. patents encompass the AGS-003 composition of matter. One of the four U.S. patents encompasses the AGS-004 composition of matter. The U.S. patents expire in 2016 and the U.S. patent application, if issued, would expire in 2016. Corresponding patents in Europe and Japan and pending corresponding patent applications in Europe, if issued, would expire in 2017. |
| • | one U.S. patent, one U.S. patent application and corresponding patent applications in Europe and Japan collectively directed towards an automated apparatus for the manipulation of nucleic acids in a closed container and related methods of use. The U.S. patent expires in 2027, and the patent applications in the United States, Europe and Japan, if issued, would expire in 2027. |
| • | one U.S. patent application and corresponding European and Japanese patents directed towards methods of manufacture of cryoconserved dendritic cells. The U.S. patent application, if issued, would expire in 2021, and the European and Japanese patents expire in 2021. |
| • | three U.S. patent applications, two corresponding European patents and corresponding patent applications in Europe and Japan collectively directed towards methods of maturing dendritic cells and the composition of matter of dendritic cells that have undergone this maturation process. The U.S. patent applications, if issued, would expire in 2025, the European patents expire in 2025 and 2027 and the patent applications in Europe and Japan, if issued, would expire in 2025 and 2027. |
| • | one U.S. patent application and corresponding patent applications in Europe and Japan collectively directed towards methods of manufacture of dendritic cells from monocytes stored for more than six hours and up to four days without freezing and the composition of matter of dendritic cells that have been manufactured from these monocytes. The U.S. patent application, if issued, and the patent applications in Europe and Japan, if issued, would expire in 2026. |
| • | one U.S. patent application and patent applications in Europe and Japan are directed towards the composition of matter of AGS-004. The U.S. patent application and the patent applications in Europe and Japan, if issued, would expire in 2025. |
| • | one U.S. patent application is directed towards the composition of matter of some of the primers that we use in the manufacture of AGS-004. The U.S. patent application, if issued, would expire in 2028. |
AGS-009. We own or exclusively license two U.S. patent applications, and European and Japanese counterparts to these patent applications, which are directed toward the composition of matter of AGS-009. The U.S. patent applications and patent applications in Europe and Japan, if issued, would expire between 2026 and 2029.
AGS-010. We own three U.S. patent applications, and European and Japanese counterparts to these patent applications, which collectively are directed toward the AGS-010 composition of matter, potential methods of treatment, and compositions for use in potential methods of treatment. The U.S. patent applications and corresponding patent applications in Europe and Japan, if issued, would expire between 2023 and 2029.
In addition, if the use of Arcelis-based products for the treatment of RCC and HIV, our anti-interferon-a monoclonal antibody AGS-009 and/or our recombinant human soluble CD83 protein AGS-010 are approved by
108
Table of Contents
the FDA, then, depending upon factors such as the timing and duration of FDA review and the timing and conditions of FDA approval, as well as factors such as patent claim scope, some of our issued U.S. patents (or patents that may issue from our pending U.S. patent applications) may be eligible for limited patent term extension under the Hatch-Waxman Act.
Trade Secrets
In addition to patents, we rely on trade secrets and know-how to develop and maintain our competitive position. For example, significant aspects of the process by which we manufacture our Arcelis-based drug product candidates are based on unpatented trade secrets and know-how. Trade secrets and know-how can be difficult to protect. We seek to protect our proprietary technology and processes, in part, by confidentiality agreements and invention assignment agreements with our employees, consultants, scientific advisors, contractors and commercial partners. These agreements are designed to protect our proprietary information and, in the case of the invention assignment agreements, to grant us ownership of technologies that are developed through a relationship with a third party. We also seek to preserve the integrity and confidentiality of our data, trade secrets and know-how by maintaining physical security of our premises and physical and electronic security of our information technology systems. Although we have confidence in these individuals, organizations and systems, agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our consultants, contractors or collaborators use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Key Licenses and Other Agreements
We are party to a number of license and other agreements that are important to our business.
Duke University. Pursuant to a 2000 agreement with Duke University, we hold an exclusive worldwide license to specified patents, patent applications and know-how owned or otherwise controlled by Duke, including for use in the development, manufacture and commercialization of dendritic cells loaded with tumor or pathogen RNA. Under the agreement, we:
| • | issued 5,000 shares of our common stock to Duke upon execution of the agreement; |
| • | must pay all costs of prosecution and maintenance of the licensed patent rights; |
| • | must pay an annual minimum royalty to Duke beginning with the calendar year beginning the second January 1 after first approval of a licensed product approved by the FDA or a comparable regulatory authority in a foreign country or any sale of a licensed product that does not require regulatory approval; and |
| • | must pay low single-digit percentage royalties, subject to reduction in specified circumstances, to Duke on net sales of licensed products, which are creditable against the annual minimum royalty. |
We are required to use reasonable commercial diligence to research, develop and market licensed products, to develop manufacturing capabilities, and to sublicense those patent rights for applications which we are not pursuing. If we fail to satisfy these obligations and do not cure such failure after receiving written notice from Duke, Duke may terminate the agreement or convert it to a nonexclusive license. Pursuant to these requirements, we have sublicensed rights under this agreement to Geron Corporation for the treatment or prophylaxis of cancer, as discussed below.
We may terminate our agreement with Duke at any time upon three months’ written notice. The agreement will terminate upon expiration of the last to expire of the patent rights licensed under the agreement. The U.S.
109
Table of Contents
patents licensed under the agreement expire in 2016 and the patents licensed under the agreement in Europe and Japan expire in 2017. Either party may terminate the agreement upon written notice for fraud, willful misconduct or illegal conduct of the other party that materially adversely affects the terminating party. If either party fails to fulfill any of its material obligations under the agreement, subject to a cure process specified in the agreement, the non-breaching party may terminate the agreement. A party’s ability to cure a breach will only apply to the first two breaches. In addition, the agreement will terminate if we become insolvent, bankrupt or placed in the hands of a receiver or trustee.
Baylor Research Institute. Pursuant to two agreements with Baylor Research Institute, which we refer to as Baylor, one from 2002 and the other from 2005, we hold exclusive worldwide licenses in specified fields of use under specified patent rights and technical information directed toward treating autoimmune diseases and anti-interferon-a antibody technologies. Under these agreements, we must pay:
| • | all costs of prosecution and maintenance of licensed patent rights, including reimbursement for patent costs incurred by Baylor prior to the effective date of the agreement; |
| • | low- to mid-single-digit percentage royalties on net sales of licensed products by us, our affiliates or sublicensees; and |
| • | up to an aggregate of $1.5 million (payable in cash or capital stock, in our discretion) based on the achievement of specified regulatory milestones. |
We were required under the 2002 agreement to use best commercial efforts to file a BLA with the FDA by January 2007. We are also required under the 2005 agreement to use commercially reasonable efforts to file a BLA with the FDA by a specified date in the future, and under both agreements to use commercially reasonable efforts to develop, evaluate, test and commercialize licensed products. Baylor may terminate the applicable agreement if we fail to meet these diligence obligations following written notice from Baylor and an opportunity to cure.
We may terminate these agreements at any time upon 60 days’ notice. Each agreement will terminate upon the later of the expiration of the last to expire of the patent rights licensed under the agreement or 20 years after its effective date. The latest dates of expiration of the patent applications licensed under the agreements, if issued, would be 2027 in the United States and would be 2026 in Europe and Japan. Baylor may terminate the applicable agreement if we are more than 30 days late paying Baylor any monies due under the agreement and we do not pay all uncontested amounts with ten days after Baylor’s written demand, if we become insolvent, bankrupt or generally fail to pay our debts when due or under similar specified circumstances, or if we materially breach the agreement and fail to cure the breach within specified periods.
Celldex Therapeutics, Inc. In July 2011, we entered into an agreement with Celldex Therapeutics, Inc., or Celldex, pursuant to which Celldex granted us a non-exclusive license to specified patents and patent applications directed to compositions and/or methods for processing dendritic cells. Upon the execution of the agreement, we paid Celldex $50,000 of a $100,000 upfront license fee. We agreed to pay the balance of this fee upon the earlier of the initiation of the next clinical trial of AGS-003 or AGS-004, or January 31, 2012. Under this agreement, we must pay:
| • | a $75,000 annual license fee; |
| • | a specified milestone payment based on the achievement of a specified regulatory milestone; and |
| • | a specified dollar amount per dose of AGS-003 we sell. |
We may terminate our agreement with Celldex at any time upon notice to Celldex. We or Celldex may terminate the agreement, subject to a cure period specified in the agreement, upon a material breach of the other
110
Table of Contents
party by providing written notice and waiting a specified period. The agreement will terminate upon the expiration of the last to expire of the patent rights licensed under the agreement on country-by-country basis. The latest date of expiration of the licensed Celldex patents is 2016.
Geron Corporation. In 2004, in exchange for an equity interest in Geron Corporation, we granted Geron a worldwide, fully paid-up license, under specified patent rights and other technology that we then owned or otherwise controlled, to develop, manufacture and sell products using dendritic cells with one or more defined antigens, but expressly excluding any use or incorporation of exogenously added uncharacterized antigens, in a field limited to the treatment or prophylaxis of cancer. The sublicense does not include the freedom to produce AGS-003, because AGS-003 incorporates undefined antigens (encoded by total tumor mRNA). The sublicense also does not include the freedom to produce AGS-004 because AGS-004 is outside the licensed field of treatment or prophylaxis of cancer. The agreement also includes non-exclusive, fully paid-up cross licenses under patent rights to certain improvements owned or controlled by either party during the first three years of the agreement. Under the agreement, we may only grant further licenses of the patent rights and technology that are the subject of the agreement for research and development purposes to academic or other non-profit organizations, or pursuant to a bona fide active development or commercialization collaboration with one or more commercial entities, or for products and in fields other than those licensed to Geron under the agreement.
The agreement will terminate upon expiration of all patent rights licensed under the agreement. The latest date of expiration of the U.S., European and Japanese patents and patent applications, if issued, that we have licensed to Geron would be in 2027. Either party may terminate the agreement for the other party’s uncured material breach or if specified conditions occur relating to the other party’s insolvency or bankruptcy.
Terminated Collaboration and License Agreements and Assignment of Patent Rights. We were a party to two collaboration and license agreements that have been terminated. In each case, our former collaborative partner has assigned to us its patent rights under the terminated agreement, and we have agreed to pay the partner future royalty and sublicense income payments.
Kirin. In 2004, we entered into a Collaboration and License Agreement, or the Kirin agreement, with Kirin. We and Kirin agreed to terminate the agreement in 2009. Upon termination, Kirin assigned to us its ownership interest in specified jointly owned patent rights. In return, we must pay Kirin low single-digit percentage royalties on net sales of AGS-003 and AGS-004 products or comparable products beginning with their first commercial sale and continuing for 15 years thereafter.
Novo Nordisk. In 2010, we entered into an agreement with Novo to terminate a 2006 license agreement in which we had granted Novo certain rights. Pursuant to the 2010 agreement, Novo granted us an exclusive worldwide license to patent rights directed to technologies for use in the development and commercialization of humanized antibodies against human interferon-a, including AGS-009 for use in treating autoimmune diseases, as well as a non-exclusive license to specified know-how for use in treating autoimmune diseases. Novo also granted us an option to obtain a non-exclusive worldwide license to a specified downstream manufacturing patent family. Under the termination agreement, we made a $100,000 upfront payment, and we must also pay:
| • | a low single-digit percentage royalty on net sales of licensed products; and |
| • | a percentage in the twenties of any additional consideration paid to us in excess of the single-digit royalty by third parties for a sublicense of licensed patent rights and/or know-how, up to a maximum payment to Novo of $8,000,000. |
Under the termination agreement, we are required to use commercially reasonable efforts to develop, commercialize and exploit licensed products. The agreement expires concurrently with the obligation to pay royalties, which occurs on the expiration of the last to expire patent covered by the license, at which time we receive a perpetual, fully paid-up license to the licensed patent rights and know-how. The latest date of expiration
111
Table of Contents
of the patent applications licensed under the agreement, if issued, in the United States, Europe and Japan, would be in 2029. We have the right to terminate the agreement upon six months prior written notice. Novo may terminate the agreement upon 180 days written notice if we decide to abandon our anti-interferon-a antibody program. Either party may terminate the agreement if the other party fails to remedy a material breach within specified periods. In addition, the agreement may be terminated by either party if specified events of insolvency occur involving the other party. In 2011, Novo assigned its rights in the previously licensed patent rights to us.
Government Regulation
Government authorities in the United States, at the federal, state and local level, and in other countries extensively regulate, among other things, the research, development, testing, manufacture, including any manufacturing changes, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, import and export of pharmaceutical and biological products such as those we market and are developing. The processes for obtaining regulatory approvals in the United States and in foreign countries, along with subsequent compliance with applicable statutes and regulations, require the expenditure of substantial time and financial resources.
U.S. Drug and Biological Product Approval Process
In the United States, the FDA regulates drugs and biological products under the Federal Food, Drug, and Cosmetic Act, or FDCA, the Public Health Service Act, or PHSA, and implementing regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to a variety of administrative or judicial sanctions, such as the FDA’s refusal to approve pending applications, withdrawal of an approval, imposition of a clinical hold, issuance of warning letters, product recalls, product seizures, total or partial suspension of production or distribution injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties.
The process required by the FDA before a drug or biological product may be marketed in the United States generally involves the following:
| • | completion of preclinical laboratory tests, animal studies and formulation studies in compliance with the FDA’s good laboratory practice, or GLP, regulations; |
| • | submission to the FDA of an IND which must become effective before human clinical trials may begin; |
| • | approval by an independent institutional review board, or IRB, at each clinical site before each trial may be initiated; |
| • | performance of adequate and well-controlled human clinical trials in accordance with good clinical practices, or GCP, to establish the safety and efficacy of the proposed drug or biological product for each indication; |
| • | submission to the FDA of a new drug application, or NDA, or a BLA; |
| • | satisfactory completion of an FDA advisory committee review, if applicable; |
| • | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with cGMP, and to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; and |
| • | FDA review and approval of the NDA or BLA. |
112
Table of Contents
Preclinical Studies. Preclinical studies include laboratory evaluation of product chemistry, toxicity and formulation, as well as animal studies to assess its potential safety and efficacy. An IND sponsor must submit the results of the preclinical tests, together with manufacturing information, analytical data and any available clinical data or literature, among other things, to the FDA as part of an IND. Some preclinical testing may continue even after the IND is submitted. An IND automatically becomes effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions related to one or more proposed clinical trials and places the clinical trial on a clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. As a result, submission of an IND may not result in the FDA allowing clinical trials to commence.
Clinical Trials. Clinical trials involve the administration of the investigational new drug to human subjects under the supervision of qualified investigators in accordance with GCP requirements, which include the requirement that all research subjects provide their informed consent in writing for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. In addition, an IRB at each institution participating in the clinical trial must review and approve the plan for any clinical trial before it commences at that institution. Information about certain clinical trials must be submitted within specific timeframes to the National Institutes of Health, or NIH, for public dissemination on their ClinicalTrials.gov website.
Human clinical trials are typically conducted in three sequential phases, which may overlap or be combined:
| • | Phase 1: The drug or biological product is initially introduced into healthy human subjects or patients with the target disease or condition and tested for safety, dosage tolerance, absorption, metabolism, distribution, excretion and, if possible, to gain an early indication of its effectiveness. |
| • | Phase 2: The drug or biological product is administered to a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage. |
| • | Phase 3: The drug or biological product is administered to an expanded patient population, generally at geographically dispersed clinical trial sites, in well-controlled clinical trials to generate enough data to statistically evaluate the efficacy and safety of the product for approval, to establish the overall risk-benefit profile of the product, and to provide adequate information for the labeling of the product. |
Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and more frequently if serious adverse events occur. Phase 1, phase 2 and phase 3 clinical trials may not be completed successfully within any specified period, or at all. Furthermore, the FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients.
Special Protocol Assessment. The SPA process is designed to facilitate the FDA’s review and approval of drug and biological products by allowing the FDA to evaluate the proposed design and size of phase 3 clinical trials that are intended to form the primary basis for determining a drug product’s efficacy. Upon specific request by a clinical trial sponsor, the FDA will evaluate the protocol and respond to a sponsor’s questions regarding, among other things, primary efficacy endpoints, trial conduct and data analysis, within 45 days of receipt of the request. The FDA ultimately assesses whether the protocol design and planned analysis of the trial adequately address objectives in support of a regulatory submission. All agreements and disagreements between the FDA and the sponsor regarding an SPA must be clearly documented in an SPA letter or the minutes of a meeting between the sponsor and the FDA.
113
Table of Contents
Even if the FDA agrees to the design, execution and analyses proposed in protocols reviewed under an SPA, the FDA may revoke or alter its agreement under the following circumstances:
| • | public health concerns emerge that were unrecognized at the time of the protocol assessment; |
| • | a sponsor fails to follow a protocol that was agreed upon with the FDA; |
| • | the relevant data, assumptions, or information provided by the sponsor in a request for SPA change are found to be false statements or misstatements or are found to omit relevant facts; or |
| • | the FDA and the sponsor agree in writing to modify the protocol and such modification is intended to improve the study. |
Marketing Approval. Assuming successful completion of the required clinical testing, the results of the preclinical and clinical studies, together with detailed information relating to the product’s chemistry, manufacture, controls and proposed labeling, among other things, are submitted to the FDA as part of an NDA or BLA requesting approval to market the product for one or more indications. In most cases, the submission of an NDA or BLA is subject to a substantial application user fee.
In addition, under the Pediatric Research Equity Act of 2003, or PREA, as amended and reauthorized by the Food and Drug Administration Amendments Act of 2007, or FDAAA, an NDA, BLA or supplement to an NDA or BLA must contain data that are adequate to assess the safety and effectiveness of the drug or biological product for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers from the pediatric data requirements. Unless otherwise required by regulation, the pediatric data requirements do not apply to products with orphan designation.
The FDA also could require submission of a risk evaluation and mitigation strategy, or REMS, plan to mitigate any identified or suspected serious risks. The REMS plan could include medication guides, physician communication plans, assessment plans, and elements to assure safe use, such as restricted distribution methods, patient registries, or other risk minimization tools.
The FDA conducts a preliminary review of all NDAs and BLAs within the first 60 days after submission before accepting them for filing to determine whether they are sufficiently complete to permit substantive review. The FDA may request additional information rather than accept an NDA or BLA for filing. In this event, the application must be resubmitted with the additional information. The resubmitted application is also subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review. The FDA reviews an NDA or BLA to determine, among other things, whether the product is safe, pure and potent and the facility in which it is manufactured, processed, packaged or held meets standards designed to assure the product’s continued safety, purity and potency. The FDA is required to refer an application for a novel drug or biological product to an advisory committee or explain why such referral was not made. An advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Before approving an NDA or BLA, the FDA typically will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA or BLA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP.
114
Table of Contents
The testing and approval process requires substantial time, effort and financial resources, and each may take several years to complete. Data obtained from clinical activities are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all. We may encounter difficulties or unanticipated costs in our efforts to develop our product candidates and secure necessary governmental approvals, which could delay or preclude us from marketing our products.
If the FDA’s evaluation of the NDA or BLA and inspection of the manufacturing facilities are favorable, the FDA may issue an approval letter, or, in some cases, a complete response letter. A complete response letter generally contains a statement of specific conditions that must be met in order to secure final approval of the NDA or BLA and may require additional clinical or preclinical testing in order for FDA to reconsider the application. If and when those conditions have been met to the FDA’s satisfaction, the FDA will typically issue an approval letter. An approval letter authorizes commercial marketing of the drug or biological product with specific prescribing information for specific indications. Even with submission of this additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
Even if the FDA approves a product, it may limit the approved indications for use for the product, require that contraindications, warnings or precautions be included in the product labeling, require that post-approval studies, including phase 4 clinical trials, be conducted to further assess a drug’s safety after approval, require testing and surveillance programs to monitor the product after commercialization, or impose other conditions, including distribution restrictions or other risk management mechanisms, which can materially affect the potential market and profitability of the product. The FDA may prevent or limit further marketing of a product based on the results of post-marketing studies or surveillance programs. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes, and additional labeling claims, are subject to further testing requirements and FDA review and approval.
Special FDA Expedited Review and Approval Programs. The FDA has various programs, including fast track designation, accelerated approval and priority review, that are intended to expedite or simplify the process for the development and FDA review of drug and biological products that are intended for the treatment of serious or life threatening conditions and demonstrate the potential to address unmet medical needs. The purpose of these programs is to provide important new drugs to patients earlier than under standard FDA review procedures.
To be eligible for a fast track designation, the FDA must determine, based on the request of a sponsor, that a product is intended to treat a serious aspect of a serious or life threatening condition and will fill an unmet medical need. The FDA will determine that a product will fill an unmet medical need if it will provide a therapy where none exists or provide a therapy that may be potentially superior to existing therapy based on efficacy or safety factors.
In addition, the FDA may give a priority review designation to drugs or biological products that offer major advances in treatment, or provide a treatment where no adequate therapy exists. For products regulated by the Center for Biologics Evaluation and Research, or CBER, the product must be intended to treat a serious or life-threatening disease or condition. A priority review means that the targeted time for the FDA to review an application is six months, rather than ten months. Most products that are eligible for fast track designation are also likely to be considered appropriate to receive a priority review.
Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or decide that the time period for FDA review or approval will not be shortened.
Post-Approval Requirements. Any drug or biological products manufactured or distributed by us pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things,
115
Table of Contents
requirements relating to recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion and reporting of adverse experiences with the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims are subject to prior FDA review and approval. There also are continuing, annual user fee requirements for any marketed products and the establishments at which such products are manufactured, as well as new application fees for supplemental applications with clinical data.
The FDA may impose a number of post-approval requirements as a condition of approval of an NDA or BLA. For example, the FDA may require post-marketing testing, including phase 4 clinical trials, and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization. Regulatory approval of oncology products often requires that patients in clinical trials be followed for long periods to determine the overall survival benefit of the drug or biologic.
In addition, drug manufacturers and other entities involved in the manufacture and distribution of approved drugs and biological products are required to register their establishments with the FDA and state agencies, and are subject to periodic unannounced inspections by the FDA and these state agencies for compliance with cGMP requirements. Changes to the manufacturing process are strictly regulated and often require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon us and any third-party manufacturers that we may decide to use. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance.
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, among other things:
| • | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
| • | fines, warning letters or holds on post-approval clinical trials; |
| • | refusal of the FDA to approve pending applications or supplements to approved applications, or suspension or revocation of product license approvals; |
| • | product seizure or detention, or refusal to permit the import or export of products; or |
| • | injunctions or the imposition of civil or criminal penalties. |
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off label uses, and a company that is found to have improperly promoted off label uses may be subject to significant liability.
In addition, the distribution of prescription pharmaceutical and biological products is subject to the Prescription Drug Marketing Act, or PDMA, which regulates the distribution of drugs and drug samples at the federal level, and sets minimum standards for the registration and regulation of drug distributors by the states. Both the PDMA and state laws limit the distribution of prescription pharmaceutical product samples and impose requirements to ensure accountability in distribution.
116
Table of Contents
Exclusivity and Approval of Competing Products
Non-Patent Exclusivity. Under the Patient Protection and Affordable Care Act, or PPACA, newly-approved biological products may benefit from statutory periods of non-patent data and marketing exclusivity. The PPACA, among other things, permits the FDA to approve biosimilar or interchangeable versions of biological products through an abbreviated approval pathway following periods of data and marketing exclusivity. Biological products that are considered to be “reference products” are granted two overlapping periods of data and marketing exclusivity: a four-year period during which no abbreviated biologics license application, or abbreviated BLA, relying upon the reference product may be submitted, and a twelve-year period during which no abbreviated BLA relying upon the reference product may be approved by FDA. For purposes of the PPACA, a reference product is defined as the single biological product licensed under a full BLA against which a biological product is evaluated in an application submitted under an abbreviated BLA.
We believe that our investigational products, if approved via full BLAs, will be considered “reference products” that are entitled to both four-year and twelve-year exclusivity under the PPACA. The FDA, however, has not issued any regulations or guidance explaining how it will implement the PPACA, including the exclusivity provisions for reference products. In November 2010, the FDA held a two-day public hearing to obtain feedback on how to interpret and implement the abbreviated BLA provisions of the PPACA. The FDA subsequently received letters from several U.S. Senators and members of the U.S. House of Representatives regarding the proper interpretation of PPACA exclusivity provisions. It is thus possible that the FDA will decide to interpret the PPACA in such a way that our products are not considered to be reference products for purposes of the PPACA or be entitled to any period of data or marketing exclusivity. Even if our products are considered to be reference products and obtain exclusivity under the PPACA, another company nevertheless could also market a competing version of any of our biological products if such company can complete, and the FDA permits the submission of and approves, a full BLA. Although protection under PPACA will not prevent the submission or approval of another “full” BLA, the applicant would be required to conduct its own preclinical and adequate and well-controlled clinical trials to demonstrate safety, purity, and potency (i.e., effectiveness).
Pediatric Exclusivity. Pediatric exclusivity is another type of non-patent marketing exclusivity in the United States and, if granted, provides for the attachment of an additional six months of marketing protection to the term of any existing regulatory exclusivity, including the four- and 12-year non-patent exclusivity periods described above. This six-month exclusivity may be granted based on the voluntary completion of a pediatric study or studies in accordance with an FDA-issued “Written Request” for such a study or studies.
Orphan Drug Designation and Exclusivity. Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug (including a biologic) intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making available in the United States a drug for this type of disease or condition will be recovered from sales in the United States for that drug. Orphan drug designation must be requested before submitting an NDA or BLA. After the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA.
If a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications, including a full NDA or full BLA, to market the same drug for the same indication for seven years. For purposes of small molecule drugs, the FDA defines “same drug” as a drug that contains the same active moiety and is intended for the same use as the previously approved orphan drug. For purposes of large molecule drugs, the FDA defines “same drug” as a drug that contains the same principal molecular structural features, but not necessarily all of the same structural features, and is intended for the same use as the drug in question. Notwithstanding the above definitions, a drug that is clinically superior to an orphan drug will not be considered the “same drug” and thus will not be blocked by orphan drug exclusivity.
117
Table of Contents
A designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. In addition, orphan drug exclusive marketing rights in the United States may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the drug to meet the needs of patients with the rare disease or condition.
The FDA also administers a clinical research grants program, whereby researchers may compete for funding to conduct clinical trials to support the approval of drugs, biologics, medical devices, and medical foods for rare diseases and conditions. An application for an orphan grant should propose one discrete clinical study to facilitate FDA approval of the product for a rare disease or condition. The study may address an unapproved new product or an unapproved new use for a product already on the market.
Foreign Regulation
Although we do not currently market any of our products outside the United States and have no current plans to engage in product commercialization outside the United States, we may decide to do so in the future. In order to market any product outside of the United States, we would need to comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy and governing, among other things, clinical trials, marketing authorization, commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we would need to obtain the necessary approvals by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country and can involve additional product testing and additional administrative review periods, and may be otherwise complicated by some of our products and product candidates being controlled substances. The time required to obtain approval in other countries might differ from and be longer than that required to obtain FDA approval. Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may negatively impact the regulatory process in others.
To date, we have not initiated any discussions with the European Medicines Agency, or EMEA, or any other foreign regulatory authorities with respect to seeking regulatory approval for any of our products in Europe or in any other country outside the United States.
Pharmaceutical Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any drug products for which we obtain regulatory approval. Sales of any of our product candidates, if approved, will depend, in part, on the extent to which the costs of the products will be covered by third-party payors, including government health programs such as Medicare and Medicaid, commercial health insurers and managed care organizations. The process for determining whether a payor will provide coverage for a drug product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the drug product once coverage is approved. Third party payors may limit coverage to specific drug products on an approved list, or formulary, which might not include all of the approved drugs for a particular indication.
In order to secure coverage and reimbursement for any product that might be approved for sale, we may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of the product, in addition to the costs required to obtain FDA or other comparable regulatory approvals. Our product candidates may not be considered medically necessary or cost-effective. A payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Third party reimbursement may not be sufficient to enable us to maintain price levels high enough to realize an appropriate return on our investment in product development.
118
Table of Contents
The containment of healthcare costs has become a priority of federal, state and foreign governments, and the prices of drugs have been a focus in this effort. Third party payors are increasingly challenging the prices charged for medical products and services and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. If these third-party payors do not consider our products to be cost-effective compared to other available therapies, they may not cover our products after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow us to sell our products at a profit. The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost-containment programs to limit the growth of government-paid health care costs, including price controls, restrictions on reimbursement and requirements for substitution of generic products for branded prescription drugs. Adoption of such controls and measures, and tightening of restrictive policies in jurisdictions with existing controls and measures, could limit payments for pharmaceuticals such as the drug product candidates that we are developing and could adversely affect our net revenue and results.
Pricing and reimbursement schemes vary widely from country to country. Some countries provide that drug products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost-effectiveness of a particular product candidate to currently available therapies. For example, the European Union provides options for its member states to restrict the range of drug products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. European Union member states may approve a specific price for a drug product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the drug product on the market. Other member states allow companies to fix their own prices for drug products, but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become intense. As a result, increasingly high barriers are being erected to the entry of new products. In addition, in some countries, cross-border imports from low-priced markets exert competitive pressure that may reduce pricing within a country. There can be no assurance that any country that has price controls or reimbursement limitations for drug products will allow favorable reimbursement and pricing arrangements for any of our products.
The marketability of any products for which we receive regulatory approval for commercial sale may suffer if the government and third-party payors fail to provide adequate coverage and reimbursement. In addition, emphasis on managed care in the United States has increased and we expect will continue to increase the pressure on drug pricing. Coverage policies, third-party reimbursement rates and drug pricing regulation may change at any time. In particular, the PPACA and a related reconciliation bill, which we collectively refer to as the Affordable Care Act or ACA, contain provisions that may reduce the profitability of drug products, including, for example, increased rebates for covered outpatient drugs sold to Medicaid programs, extension of Medicaid rebates to Medicaid managed care plans, mandatory discounts for certain Medicare Part D beneficiaries, and annual fees based on pharmaceutical companies’ share of sales to federal health care programs. Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
New Legislation and Regulations
From time to time, legislation is drafted, introduced and passed in Congress that could significantly change the statutory provisions governing the testing, approval, manufacturing and marketing of products regulated by the FDA. For example, the FDAAA and PPACA provisions discussed above were enacted in 2007 and 2010, respectively. In addition to new legislation, FDA regulations and policies are often revised or interpreted by the agency in ways that may significantly affect our business and our products. It is impossible to predict whether further legislative changes will be enacted, or FDA regulations, guidance, policies or interpretations changed or what the impact of such changes, if any, may be.
119
Table of Contents
Facilities
Our only facility is located in Durham, North Carolina, where we occupy approximately 20,000 square feet of office, laboratory and cellular processing space. Our lease expires in November 2016, but we may terminate it in December 2014. We are in the process of identifying suitable space to lease for our planned new commercial manufacturing facility.
Employees
As of August 31, 2011, we had 58 employees, including 13 in research and development, seven in clinical development, 27 in manufacturing and 11 in general and administrative functions. None of our employees is subject to a collective bargaining agreement or represented by a labor or trade union. We believe that our relations with our employees are good.
Legal Proceedings
From time to time, we have been and may again become involved in legal proceedings arising in the ordinary course of our business. We are not presently a party to any material litigation.
120
Table of Contents
The following table sets forth the name, age and position of each of our executive officers and directors as of August 31, 2011.
| Name |
Age | Position | ||||
| Jeffrey D. Abbey |
49 | President, Chief Executive Officer and Director | ||||
| Lori R. Harrelson |
41 | Vice President of Finance | ||||
| Frederick M. Miesowicz, Ph.D. |
62 | Chief Operating Officer and Vice President of Manufacturing | ||||
| Charles A. Nicolette, Ph.D. |
48 | Chief Scientific Officer and Vice President of Research and Development | ||||
| Douglas C. Plessinger |
43 | Vice President of Medical and Clinical Affairs | ||||
| Hubert Birner, Ph.D.(1) |
45 | Chairman of the Board of Directors | ||||
| B. Jefferson Clark(2) |
55 | Director | ||||
| Michel Gréco(3) |
68 | Director | ||||
| Monique Laliberté |
53 | Director | ||||
| Philip R. Tracy(2)(3) |
69 | Director | ||||
| Brian J. Underdown, Ph.D.(1)(3) |
70 | Director | ||||
| Sander van Deventer M.D., Ph.D.(1)(3) |
56 | Director | ||||
| (1) | Member of the nominating and corporate governance committee. |
| (2) | Member of the audit committee. |
| (3) | Member of the compensation committee. |
Jeffrey D. Abbey has served as our President and Chief Executive Officer and a member of our board since February 2010. Mr. Abbey served in various other positions at our company from September 2002 to February 2010, including as our Vice President of Business Development from February 2004 to January 2009 and as our Chief Business Officer from January 2009 to February 2010. Prior to joining us, Mr. Abbey was Vice President of Business Development and Finance for Internet Appliance Network, an information technology company, from 1999 to 2001. Mr. Abbey was also formerly a partner at Eilenberg and Krause, LLP, a corporate law firm, from 1994 to 1999. Mr. Abbey received his A.B. in mathematical economics from Brown University and received an M.B.A. and J.D. from the University of Virginia. We believe that Mr. Abbey is qualified to serve on our board of directors due to his extensive knowledge of our company and our industry.
Lori R. Harrelson has served as our Vice President of Finance since July 2011. Ms. Harrelson served as our Director of Finance and Accounting from January 2007 to June 2011 and as our Director of Accounting and Financial Reporting from September 2004 to January 2007. Prior to joining us, Ms. Harrelson was Finance Manager at LipoScience, Inc., a diagnostic company, from 2001 to 2004. Ms. Harrelson received her B.S. in finance from East Carolina University and is a C.P.A.
Frederick M. Miesowicz, Ph.D. has served as our Chief Operating Officer and Vice President of Manufacturing since February 2005. Dr. Miesowicz served as our Vice President of Manufacturing from May 2003 to February 2005. Prior to joining us, Dr. Miesowicz was Vice President of U.S. Operations for Gamida-Cell Ltd., a stem cell company, from 2000 to 2003; Senior Vice President and General Manager of Hybridon Specialty Products, a manufacturing division of a biotechnology company, from 1998 to 2000; and Vice President and General Manager of Cellcor, a subsidiary of Cytogen Corporation, a biopharmaceutical company, from 1995 to 1998. Dr. Miesowicz received his B.S. in chemistry from Siena College and his Ph.D. in chemistry from Harvard University.
Charles A. Nicolette, Ph.D. has served as our Chief Scientific Officer since December 2007 and as our Vice President of Research and Development since December 2004. Dr. Nicolette served as our Vice President of
121
Table of Contents
Research from July 2003 to December 2004. Prior to joining us, Dr. Nicolette served in various positions at Genzyme Molecular Oncology, Inc., a biotechnology company, from 1997 to 2003, most recently as Director of Antigen Discovery. Dr. Nicolette received his B.S. from the State University of New York at Stony Brook and his Ph.D. in biochemistry and cellular and developmental biology from the State University of New York at Stony Brook, completing his doctoral dissertation and post-doctoral fellowship at Cold Spring Harbor Laboratory.
Douglas C. Plessinger, RPh, joined as our Vice President of Medical and Clinical Affairs effective October 2011, after serving as a clinical consultant for Argos since October 2007. Prior to joining us, Mr. Plessinger was Executive Vice President and Managing Director of Axcelo MSL Solutions, LLC, a consulting company, from August 2008 to September 2011 and founding partner and Managing Director of its predecessor company, venn5 BioConsulting, LLC, from 2007 to 2008. Prior to this, he served as Senior Vice President, Account Services for The Navicor Group, LLC, a healthcare advertising agency and a division of inVentiv Health, Inc., a healthcare services company, from 2004 to 2007; Oncology Medical Science Liaison for Millennium Pharmaceuticals, Inc., from 2002 to 2003; and in various Medical Affairs and Product Development roles for Bristol-Myers Squibb Oncology, a pharmaceutical company, from 1997 to 2002. Mr. Plessinger graduated summa cum laude and received his B.S. in pharmacy from Northeastern University.
Hubert Birner, Ph.D. has been the Chairman of our board of directors since 2005 and a member of our board of directors since 2001. Dr. Birner joined the Munich office of TVM Capital, a venture capital firm, in 2000 and currently serves as a general partner for the firm’s life sciences group. From 1998 to 2000, Dr. Birner served as head of European business development and director of marketing for Germany at Zeneca Agrochemicals, a biopharmaceutical company. Prior to joining Zeneca Agrochemicals, Dr. Birner was a management consultant in McKinsey & Company’s European healthcare and pharmaceutical practice. Dr. Birner serves on the board of directors of Horizon Pharma, Inc. and several private biopharmaceutical companies. Dr. Birner received his M.B.A. from Harvard Business School and his doctorate in biochemistry from Ludwig-Maximillians University in Munich, Germany. His doctoral thesis was honored with the Hoffmann-La Roche prize for outstanding basic research in metabolic diseases. We believe that Dr. Birner is qualified to serve as the Chairman of our board of directors due to his extensive experience with biopharmaceutical companies and his years of experience providing strategic and advisory services to pharmaceutical and biotechnology companies.
B. Jefferson Clark has served as a member of our board since 1998. Mr. Clark co-founded the Aurora Funds, Inc., a venture capital firm, in 1994 and currently serves as the firm’s Managing General Partner. Prior to forming Aurora, Mr. Clark worked in development and external affairs for Duke University, including the Duke Comprehensive Cancer Center, Duke Medical Center and the School of Engineering, from 1981 to 1994. Mr. Clark serves and has served on the boards of several private biopharmaceutical companies. Mr. Clark received his B.S. in mechanical engineering from Duke University and an M.B.A. from the Fuqua School of Business at Duke University. We believe that Mr. Clark is qualified to serve on our board of directors due to his extensive experience serving as a director on a wide range of companies within the biopharmaceutical field, as well his expertise in venture capital financing and development.
Michel Gréco has served as a member of our board since 2004. Prior to retiring from Aventis Pasteur in 2003, Mr. Gréco served as deputy Chief Executive Officer and member of the board of Aventis Pasteur. Mr. Gréco serves and has served on the boards of several private and foreign public biopharmaceutical companies. Mr. Gréco received his master’s degree from Institut d’Etudes Politiques de Paris and an M.B.A. from the University of Western Ontario, Richard Ivey School of Business. We believe that Mr. Gréco is qualified to serve on our board of directors due to his extensive experience as an executive in the pharmaceutical industry and his years serving on boards of biotechnology and pharmaceutical companies.
Monique Laliberté has served as a member of our board since March 2011. Ms. Laliberté has served as Investment Manager, Private Equity of Caisse de dépôt et placement du Québec, a fund management company, since May 2002. She has also served on the board of directors of Pharmaxis Ltd. (formerly Topigen
122
Table of Contents
Pharmaceuticals Inc.) since 2008. Ms. Laliberté received her M.Sc. from Université Laval and an M.B.A. from HEC Montréal. We believe that Ms. Laliberté is qualified to serve on our board of directors due to her financial expertise and her extensive experience with biotechnology and pharmaceutical companies.
Philip R. Tracy has served as a member of our board since 2011. Mr. Tracy has been a Venture Partner at Intersouth Partners since 1998. He is also counsel to the Raleigh, North Carolina law firm Smith, Anderson, Blount, Dorsett, Mitchell & Jernigan, L.L.P. Previously, Mr. Tracy was employed by Burroughs Wellcome Co. from 1974 to 1995 and served as President and Chief Executive Officer from 1989 to 1995. Mr. Tracy has served on the board of Alimera Sciences, Inc. since 2004. Mr. Tracy received his L.L.B. from George Washington University and his B.A. from the University of Nebraska. We believe that Mr. Tracy is qualified to serve on our board of directors because he has served on the board of directors of three publicly traded companies in the biotechnology and pharmaceutical industries, has experience as president and chief executive officer of Burroughs Wellcome Co. with full responsibility for its North American pharmaceutical business, has legal training and experience as a lawyer including his service as general counsel to Burroughs Wellcome Co., and has over 10 years of experience in the venture capital industry as a venture partner with Intersouth Partners.
Brian J. Underdown, Ph.D. has served as a member of our board since 1999. Dr. Underdown joined Lumira Capital Corp. (formerly MDS Capital Corp.), a venture capital firm, in 1997, and currently serves as a Managing Director. Before joining Lumira, Dr. Underdown was Assistant Vice President of Research at Pasteur Merieux Connaught from 1994 to 1997. Dr. Underdown has been a member of the board of directors of Vistagen Therapeutics, Inc. since 2009. Dr. Underdown received his Ph.D. from McGill University and undertook post-doctoral studies at Washington University School of Medicine. We believe that Dr. Underdown is qualified to serve on our board of directors due to his experience in the biopharmaceutical industry and his scientific background.
Sander van Deventer, M.D., Ph.D. has served as a member of our board since 2001. Dr. van Deventer has been a General Partner of Forbion Capital Partners (formerly ABN AMRO Capital) since 2006. From 2008 to 2009, he served as the Chief Executive Officer of Amsterdam Molecular Therapeutics, or AMT, a gene therapy company that he co-founded in 1998. He has also served as a member of AMT’s board of directors since 2007. Dr. van Deventer has also been a Professor of Translational Gastroenterology at Leiden University since 2008. He received his M.D. and Ph.D. from the University of Amsterdam. We believe that Dr. van Deventer is qualified to serve on our board of directors due to his experience as a founder of a biopharmaceutical company and his expertise in clinical development.
Composition of the Board of Directors
Our board of directors is currently authorized to have up to ten members. In accordance with the terms of our certificate of incorporation and bylaws that will become effective upon the closing of this offering, our board of directors will be divided into three classes, class I, class II and class III, with members of each class serving staggered three-year terms. Upon the closing of this offering, the members of the classes will be divided as follows:
| • | the class I directors will be Mr. Clark, Ms. Laliberté and Mr. Tracy, and their term will expire at the annual meeting of stockholders to be held in 2012; |
| • | the class II directors will be Dr. Birner, Dr. van Deventer and Dr. Underdown, and their term will expire at the annual meeting of stockholders to be held in 2013; and |
| • | the class III directors will be Mr. Abbey and Mr. Gréco, and their term will expire at the annual meeting of stockholders to be held in 2014. |
Upon the expiration of the term of a class of directors, directors in that class will be eligible to be elected for a new three-year term at the annual meeting of stockholders in the year in which their term expires. In
123
Table of Contents
accordance with the terms of the bylaws that will become effective upon the closing of this offering, our directors will only be able to be removed for cause by the affirmative vote of the holders of 75% or more of our voting stock.
Our board of directors has determined that each of our directors, other than Mr. Abbey, are independent directors, as defined by the applicable NASDAQ Marketplace Rules.
There are no family relationships among any of our directors or executive officers.
Board Leadership Structure
The positions of chairman of the board and chief executive officer are presently separated and have generally been separated at our company. The duties of the chairman of the board include the following:
| • | chairing meetings of the board and of the independent directors in executive session; |
| • | meeting with any director who is not adequately performing his or her duties as a member of our board or any committees; |
| • | facilitating communications between other members of our board and the chief executive officer; |
| • | determining the frequency and length of board meetings and recommending when special meetings of our board should be held; |
| • | preparing or approving the agenda for each board meeting; and |
| • | reviewing and, if appropriate, recommending action to be taken with respect to written communications from stockholders submitted to our board. |
Our board of directors decided to separate the roles of chairman and chief executive officer because it believes that a bifurcated leadership structure offers the following benefits:
| • | increasing the independent oversight of our company and enhancing our board’s objective evaluation of our chief executive officer; |
| • | freeing the chief executive officer to focus on company operations instead of board administration; |
| • | providing the chief executive officer with an experienced sounding board; |
| • | providing greater opportunities for communication between stockholders and our board; |
| • | enhancing the independent and objective assessment of risk by our board; and |
| • | providing an independent spokesman for our company. |
Board Committees and Independence
Our board of directors has established an audit committee, a compensation committee and a nominating and corporate governance committee. Upon the closing of this offering, each of these committees will operate under a charter that has been approved by our board.
124
Table of Contents
Our board of directors has determined that all of the members of the audit committee, the compensation committee and the nominating and corporate governance committee are independent as defined under The NASDAQ Marketplace Rules, including, in the case of all the members of our audit committee, the independence requirements contemplated by Rule 10A-3 under the Exchange Act.
Audit Committee
The members of our audit committee are Messrs. Clark and Tracy. Mr. Clark chairs the audit committee. Upon the closing of this offering, our audit committee’s responsibilities will include:
| • | appointing, approving the compensation of, and assessing the independence of our registered public accounting firm; |
| • | overseeing the work of our registered public accounting firm, including through the receipt and consideration of reports from such firm; |
| • | reviewing and discussing with management and the registered public accounting firm our annual and quarterly financial statements and related disclosures; |
| • | monitoring our internal control over financial reporting, disclosure controls and procedures and code of business conduct and ethics; |
| • | overseeing an internal audit function; |
| • | overseeing our risk assessment and risk management policies; |
| • | establishing policies regarding hiring employees from the registered public accounting firm and procedures for the receipt and retention of accounting related complaints and concerns; |
| • | meeting independently with our internal auditing staff, registered public accounting firm and management; |
| • | reviewing and approving or ratifying any related person transactions; and |
| • | preparing the audit committee report required by SEC rules. |
All audit and non-audit services, other than de minimis non-audit services, to be provided to us by our independent registered public accounting firm must be approved in advance by our audit committee.
Our board of directors has determined that is an “audit committee financial expert” as defined in applicable SEC rules. We believe that, upon the closing of this offering, the composition of our audit committee will meet all applicable requirements with respect to audit committee composition under the current NASDAQ Global Market and SEC rules and regulations.
Compensation Committee
The members of our compensation committee are Messrs. Gréco and Tracy and Drs. Underdown and van Deventer. Mr. Tracy chairs the compensation committee. Upon the closing of this offering, our compensation committee’s responsibilities will include:
| • | annually reviewing and approving corporate goals and objectives relevant to chief executive officer compensation; |
125
Table of Contents
| • | determining our chief executive officer’s compensation; |
| • | reviewing and approving, or making recommendations to our board with respect to, the compensation of our other executive officers; |
| • | overseeing an evaluation of our senior executives; |
| • | overseeing and administering our cash and equity incentive plans; |
| • | reviewing and making recommendations to our board with respect to director compensation; |
| • | reviewing and discussing annually with management our “Compensation Discussion and Analysis” disclosure required by SEC rules; and |
| • | preparing the annual compensation committee report required by SEC rules. |
Nominating and Corporate Governance Committee
The members of our nominating and corporate governance committee are Drs. Birner, Underdown and van Deventer. Dr. Underdown chairs the nominating and corporate governance committee. Upon the closing of this offering, our nominating and corporate governance committee’s responsibilities will include:
| • | identifying individuals qualified to become members of our board; |
| • | recommending to our board the persons to be nominated for election as directors and to each of our board’s committees; |
| • | reviewing and making recommendations to the board with respect to management succession planning; |
| • | developing and recommending to our board corporate governance principles; and |
| • | overseeing an annual evaluation of our board. |
Compensation Committee Interlocks and Insider Participation
None of our executive officers serves as a member of the board of directors or compensation committee, or other committee serving an equivalent function, of any other entity that has one or more of its executive officers serving as a member of our board of directors or our compensation committee. None of the members of our compensation committee has ever been our employee.
Code of Ethics and Code of Conduct
Effective upon the closing of this offering, we plan to adopt a written code of business conduct and ethics that will apply to our directors, officers and employees, including our principal executive officer, principal financial officer, or persons performing similar functions. We will post a current copy of the code on our website, www.argostherapeutics.com. In addition, we intend to post on our website all disclosures that are required by law or NASDAQ stock market listing standards concerning any amendments to, or waivers from, any provision of the code.
126
Table of Contents
Compensation Discussion and Analysis
The following discussion relates to the compensation of our named executive officers for the fiscal year ended December 31, 2010 and provides an overview of the material elements of our executive compensation policies and programs. It provides qualitative information regarding the manner and context in which compensation is awarded to and earned by our named executive officers and should be read together with the related tables and disclosures that follow.
Our named executive officers in 2010 were:
| • | Jeffrey D. Abbey, our president and chief executive officer; |
| • | Lori R. Harrelson, our vice president of finance, who became our principal financial officer on February 24, 2010; |
| • | Charles A. Nicolette, Ph.D., our vice president of research and development and chief scientific officer; |
| • | Frederick M. Miesowicz, Ph.D., our vice president of manufacturing and chief operating officer; |
| • | John N. Bonfiglio, Ph.D., our former president and chief executive officer, who served in that position until February 23, 2010; and |
| • | Timothy W. Trost, our former chief financial officer, who served in that position until February 23, 2010. |
The discussion that follows contains forward-looking statements that are based on current plans, considerations, expectations and determinations regarding future compensation programs. The actual compensation programs that we adopt may differ materially from the current or planned programs summarized in this discussion.
Overview of Our Compensation Philosophy and Objectives
Our primary objective with respect to executive compensation is to attract, retain and motivate highly talented individuals with skills and experience that can help us successfully execute our business strategy. Our executive compensation program is designed to:
| • | attract and retain high quality and experienced executive officers; |
| • | motivate and reward executive officers whose knowledge and skills are critical to our business; |
| • | recognize individual contributions; |
| • | achieve accountability for performance by linking company performance and individual achievement to compensation; and |
| • | align the interests of our executive officers and our stockholders by providing our executive officers with long-term incentives to increase stockholder value. |
To achieve these objectives, our executive compensation program ties a portion of each executive’s overall compensation to key corporate objectives. We also provide a portion of our executive compensation in the form
127
Table of Contents
of stock option awards that vest over time. We believe that the grant of stock options subject to vesting helps to retain our executive officers and serves to align their interests with those of our stockholders by allowing them to participate in our long-term performance as reflected in the value of shares of our common stock.
Overview of Our Executive Compensation Process
Roles of Our Board, Chief Executive Officer and Compensation Committee
As a private company, our compensation committee and board of directors, with input from our chief executive officer and chief financial officer, have historically overseen our executive compensation program. We initially established each of our executive officer’s compensation arrangements when negotiating their employment at the time they joined our company. We typically include these initial compensation terms in an offer letter or employment agreement with the executive. We have reviewed each executive officer’s compensation on an annual basis since they joined our company.
Our compensation committee, either as a committee or together with the other non-management directors, makes all compensation decisions regarding our chief executive officer. Our chief executive officer has historically reviewed compensation decisions relating to our executive officers other than himself. He has annually reviewed the performance of our other executive officers, and, based on these reviews, has made recommendations to our compensation committee regarding salary adjustments, annual incentive bonus payments and equity incentive awards for our executive officers. Our chief executive officer does not participate in the final decision-making discussions regarding his own compensation.
Based on the evaluation of our chief executive officer’s recommendations regarding the compensation of executive officers other than himself, our compensation committee, acting under authority delegated by our board of directors, either makes all compensation decisions itself regarding our executive officers, including the chief executive officer, or makes formal recommendations concerning executive compensation to the board of directors, with the non-management directors making the final compensation decisions.
Use of Competitive Data
From time to time, the compensation committee has reviewed surveys of compensation practices of similarly situated companies. To date, we have not identified a specified group of peer companies against which we would compare our compensation practices nor have we formally benchmarked our executive compensation against that of our competitors. In setting compensation for 2010, the compensation committee did not rely on any survey data.
Role of Compensation Consultant
Our compensation committee and board of directors did not engage the services of outside consultants or advisors to review and provide advice with respect to our executive officer compensation policies and program for 2010, although they have done so from time to time in prior years. In reviewing compensation levels of our executive officers for 2010, our compensation committee and board of directors considered our financial condition and the contributions that the management team had made to our business, and relied on their members’ collective knowledge of compensation trends and standards in our industry and their experiences and business judgment. Based on a review of our executive compensation program for 2010, our board of directors determined that our existing program satisfies our compensation philosophy and objectives and will remain generally unchanged for 2011.
Following the closing of this offering, our compensation committee will continue to be primarily responsible for developing and implementing our compensation policies and establishing and approving the
128
Table of Contents
compensation for all of our executive officers. In the future, our compensation committee may choose to retain the services of an independent compensation consultant to assist it in evaluating and reviewing executive compensation and to advise it regarding executive compensation practices in our industry.
We expect that, following the completion of this offering, our compensation committee will continue to review and evaluate our existing compensation programs, objectives and philosophy to determine whether such programs, objectives and philosophy are appropriate after we have become a public company. In addition, as we gain experience as a public company, we expect that the specific direction, emphasis and components of our executive compensation program will continue to evolve.
Elements of Our Executive Compensation Program
The primary elements of our executive compensation program are:
| • | base salaries; |
| • | annual incentive and other bonuses; |
| • | equity incentive awards; |
| • | other employee benefits; and |
| • | severance and change in control benefits. |
We strive to achieve an appropriate mix between the various elements of our compensation program to meet our compensation objectives and philosophy; however, we have not adopted any formal policies or guidelines for allocating compensation among these elements.
Base Salaries
We use competitive base salaries to attract and retain qualified candidates to help us achieve our business objectives and performance goals. We believe that a competitive salary is a necessary element of any compensation program that is designed to attract and retain qualified talent. Our base salaries are intended to recognize an executive officer’s level of responsibilities and immediate contribution to our organization, as well as his or her prior experience, knowledge and responsibilities.
Upon joining our company, each of our executive officers either entered into an offer letter or an executive employment agreement that provided for an initial base salary. These initial salaries are typically the product of negotiation with the executive, but we generally seek to establish salaries that we believe are commensurate with salaries being paid to executive officers serving in similar roles at comparable biopharmaceutical companies. Although we have not engaged in formal benchmarking of salaries, we have from time to time reviewed compensation surveys, conferred with compensation consultants and relied on our board of directors and compensation committee members’ collective experience in the marketplace for determining salaries for executives of comparable companies.
Historically, base salaries are reviewed annually by the compensation committee in conjunction with annual assessments of performance and bonus opportunities. Our board of directors has delegated authority for any annual salary adjustments to the compensation committee. Shortly before the end of each calendar year, the compensation committee reviews company and individual performance, as well as our financial status, to, among other things, determine whether adjustments in base salary are necessary or appropriate. Any annual adjustments to base salary adopted by the compensation committee typically take effect at the beginning of the following year. We may also adjust the base salary of an executive officer at other times due to market conditions or if a change in the scope of the officer’s responsibilities justifies such consideration.
129
Table of Contents
In December 2009, in light of our financial condition, our compensation committee determined not to make any adjustments to the base salaries for our chief executive officer or any of our other executive officers for 2010. In April 2010, the compensation committee recommended and the non-management directors approved an increase in the base salary of our current chief executive officer, Mr. Abbey, effective as of February 24, 2010, from $220,835 to $265,000, to reflect his promotion at that time to the position of chief executive officer. In December 2010, in light of our financial condition, our compensation committee determined that no adjustments to the base salaries of our chief executive officer or any of our other named executive officers would be made for 2011. However, Ms. Harrelson’s base salary was increased effective January 1, 2011 from $119,319 to $131,250, in recognition of the additional responsibilities and leadership she assumed since becoming our principal financial officer in February 2010.
In July 2011, the compensation committee recommended and the board of directors approved a further increase in Ms. Harrelson’s base salary, from $131,250 to $150,000, in connection with her promotion at that time to the position of vice president of finance. In September 2011, the compensation committee recommended and the board of directors approved an increase in Mr. Abbey’s base salary from $265,000 to $300,000, and in Dr. Nicolette’s base salary from $235,872 to $250,000, in light of the relative salaries of the other executive officers at our company and based on the directors’ views as to compensation trends and standards in our industry.
Annual Incentive and Other Bonuses
In addition to base salaries, our executive officers are eligible to receive annual discretionary cash bonuses based on the achievement of corporate objectives and individual performance. Bonuses are typically prorated on a monthly basis, as applicable, for executive officers who commence employment after the beginning of the year. Our executive officers’ annual bonus opportunities are generally set in their offer letters as a specified percentage of annual base salary. The current annual target bonus amount for each of our current executive officers other than Mr. Abbey is 20% of base salary. The target bonus for Mr. Abbey was increased from 20% to 40% of his base salary in September 2011. The target bonus for our former chief executive officer, Dr. Bonfiglio, was set forth in his employment agreement at 40% of base salary, and the target bonus for our former chief financial officer, Mr. Trost, was set forth in his offer letter at 20% of base salary. In determining annual bonuses, we have generally attributed 85% of the target bonus to the achievement of specified corporate objectives and 15% to the individual’s effectiveness in helping us achieve our corporate objectives or other individual performance criteria. The annual corporate objectives are recommended by our chief executive officer and approved by the compensation committee and the board of directors. Annual bonuses have been determined by the compensation committee and ratified by the non-management directors in December of each year and paid by the end of December of the year in which they were determined.
At the end of the year, our chief executive officer develops bonus recommendations for each of our executive officers based on our corporate objectives and the individual’s performance and contributions to those objectives during that year. These recommendations are generally based on an assessment of the extent to which we have achieved each of the specified annual corporate objectives which typically include goals tied to factors such as development and progression of our existing product candidates, achievement of clinical and regulatory milestones, operational goals and financial factors such as raising and maintaining capital. These recommendations may reflect achievement in whole or in part of each of the specified corporate objectives. Our compensation committee then assesses the bonuses recommended by the chief executive officer and make its bonus recommendations to the board of directors. Based on its consideration of the recommendations of the compensation committee, our board then makes the final decision in its discretion regarding cash bonus payments, if any, for the year.
Early in 2010, the board authorized the termination of the employment of seven of our employees, including three members of management. Taking into account a number of factors including prevailing market conditions and our financial position, the newly constituted executive management team presented, and our board of directors approved, a 2010 budget that did not provide for any bonuses for our executive officers for 2010
130
Table of Contents
performance. Thereafter, our executive officers sought to achieve our 2010 corporate objectives without the additional incentive of a formal annual bonus program. Our compensation committee did not establish formal annual bonus goals based on 2010 corporate objectives, and at the end of the year made the decision not to pay annual bonuses for 2010 based on the need to manage expenses and allocate resources to our clinical, business development and other strategic initiatives. However, our compensation committee did award a $7,000 discretionary bonus to Ms. Harrelson in December 2010 in recognition of her performance in 2010 and her assumption in February 2010 of responsibility as our principal financial officer.
Our board of directors determined to reinstate our annual bonus program for 2011. The compensation committee and board of directors have approved corporate objectives for 2011 which will serve as the basis for any annual cash bonuses to be awarded for performance in 2011. We expect that our compensation committee and our board of directors will evaluate each executive officer’s performance against these goals, consider their individual performance and determine and pay annual bonus amounts, if any, in December 2011, consistent with our historical practice.
Equity Incentive Awards. Our equity award program is the primary vehicle for offering long-term incentives to our executives. We believe that equity awards provide our executives with a strong link to our long-term performance, create an ownership culture and help to align the interests of our executives and our stockholders. To date, we have used stock option grants for this purpose because we believe they are an effective means by which to align the long-term interests of our executive officers with those of our stockholders. The use of options also can provide tax and other advantages to our executive officers relative to other forms of equity compensation. We believe that our equity awards are an important retention tool for our executive officers, as well as for our other employees.
We award stock options broadly to our employees, including to our non-executive employees. Grants to our executives and other employees are made at the discretion of our board of directors and are not made at any specific time period during a fiscal year. All of our named executive officers have received stock option grants under our 1999 stock option plan or our 2008 stock incentive plan, both of which are described below. The 1999 stock option plan has expired and no further options may be granted under that plan. Our board of directors intends to adopt a new equity incentive plan described under “— Stock Option and Other Equity Compensation Plans” below, effective upon the closing of this offering, and has determined not to grant any additional awards under our 2008 stock incentive plan after the effectiveness of such plan. Our new 2011 plan will afford our compensation committee continued flexibility in making a wide variety of equity awards.
Initial option grants to our executive officers are generally set forth in their offer letters. These initial grants are the product of negotiation with the executive officer, but we generally seek to establish equity ownership levels that we believe are commensurate with the equity stakes held by executive officers serving in similar roles at comparable biopharmaceutical companies. In addition, from time to time in connection with corporate finance transactions and at other times as our compensation committee and board of directors deem appropriate, we provide subsequent option grants to those executive officers determined to be performing well.
The majority of the stock option grants we have made to our executive officers vest over four years. However, from time to time, our board of directors has approved grants with different and sometimes shorter vesting provisions. Our historical practice has been to provide for 100% acceleration of vesting of outstanding stock options in the event of a change of control. Additional information regarding the effect of accelerated vesting upon a change in control with respect to our named executive officers is discussed below under “— Agreements with our Named Executive Officers.”
We have not adopted stock ownership guidelines for our executive officers. Our board of directors’ grants of stock options have not been formula-based but rather have been based on the recommendation of the compensation committee, which generally takes into account the compensation committee’s determination of relevant qualitative factors, including the executive’s position and level of responsibility, the competitive market
131
Table of Contents
for the executive’s position and the executive’s contribution to the advancement of our clinical and business development programs, as well as the individual’s recent performance. Equity awards are not generally based on specific performance objectives, however. Typically, larger awards have been made to the executive officers who have areas of responsibility and functions that are more likely to build long-term stockholder value as determined by how directly linked their areas of responsibility are to our growth and success. We expect that after we become a public company our compensation committee will grant equity awards, at least in part, with reference to market surveys and based on input from third-party consultants.
In December 2010, based on the recommendations of our compensation committee, our board of directors granted an option to purchase 1,984,850 shares of our common stock to Dr. Nicolette, an option to purchase 6,845,926 shares of our common stock to Mr. Abbey and an option to purchase 924,555 shares of our common stock to Dr. Miesowicz. These options were granted as a retention mechanism with the goal of maintaining equity ownership for each of these executives at what our board believed to be reasonable levels following the issuance of the 2010 convertible notes. The options granted to Dr. Nicolette and Mr. Abbey were also awarded, in part, to recognize the additional responsibilities assumed by Dr. Nicolette and Mr. Abbey after our management reorganization in February 2010, and in the case of Mr. Abbey, to reflect his promotion in February 2010 to the position of chief executive officer. In February 2011, our board of directors granted an option to Ms. Harrelson to purchase 375,883 shares of our common stock in recognition of her assumption of additional management responsibilities following the company’s management reorganization in February 2010. On September 6, 2011, our board of directors granted an additional option to Ms. Harrelson to purchase 1,081,760 shares of our common stock in recognition of her promotion to the position of vice president of finance in July 2011.
The exercise price of each stock option granted under our 1999 stock option plan and our 2008 stock incentive plan is based on the fair market value of our common stock on the date of the grant. Prior to 2006, the fair market value of our common stock for purposes of determining the exercise price of stock options was determined by our board of directors based on its analysis of a number of factors it considered relevant. Beginning in 2006, our board of directors also obtained contemporaneous valuations to assist it in determining the fair market value of our common stock.
Following this offering, we expect that we will continue to grant all stock options with an exercise price equal to the fair market value of our common stock on the date of grant, but fair market value will be defined as the closing market price of a share of our common stock on the date of grant. We do not currently have any program, plan or practice of setting the exercise price based on a date or price other than the fair market value of our common stock on the date of grant of the option.
Additional Executive Benefits and Other Compensation.
We maintain broad-based benefits that are provided to all employees, including our 401(k) retirement plan, medical and dental care plans, life insurance, short- and long-term disability policies, vacation and company holidays. These benefits are provided to our executive officers on the same general terms as our non-executive employees. The compensation committee has the authority to match all or a portion of our employees’ 401(k) plan contributions. Each year since 2006, our compensation committee has approved matching contributions to our 401(k) plan in aggregate amounts of between $50,000 and $75,000 per year.
We do not view perquisites or other personal benefits as a significant component of our executive compensation program and did not provide perquisites to our executive officers during 2010.
Severance and Change of Control Arrangements.
Pursuant to offer letters, certain of our current executive officers are entitled to specified benefits in the event of the termination of their employment under specified circumstances, including termination following a change of control of our company. The terms of these arrangements are more fully described below under
132
Table of Contents
“— Agreements with our Named Executive Officers.” We have historically included these arrangements among the employment terms negotiated at the time each executive is hired. We have amended these provisions of the offer letters to increase these benefits from time to time to reflect promotions or the assumption of increased responsibilities. For example, in March 2011, we approved increased benefits for Mr. Abbey and Dr. Nicolette in the event of a termination of employment in the event of a change in control and in September 2011 we approved severance benefits in the event of the termination of Ms. Harrelson’s employment without cause.
We have included these benefits in our arrangements with each of our executive officers because we believe they are necessary to induce these individuals to forego other opportunities or leave their existing positions to join our company, and we believe these protections also serve an important retention function for retention of executives. Further, we believe that providing such benefits in the event of a change of control of our company allows our executive officers to focus their attention on the requirements of the business rather than on the implications of the transaction for them personally.
In December 2008, our board of directors adopted a management incentive plan that provided for a potential payment to specified executive officers in the event of a sale of our company that satisfied specified criteria. The purpose of the 2008 management incentive plan was to align the interests of our executive officers with those of our stockholders in connection with a sale transaction and to pay a portion of the sale proceeds to the executive officers in cases in which the executive’s outstanding stock options would not provide competitive compensation. The 2008 management incentive plan was not triggered during its term and expired by its terms at the end of 2010. Our compensation committee and board of directors have considered adopting a similar incentive plan to replace the 2008 management incentive plan, but do not currently intend to establish such a replacement plan.
Risk Considerations in our Compensation Program
When determining our compensation policies and practices, our board of directors and compensation committee consider various factors related to the development of a reasonable and prudent compensation program, including whether the policies and practices are reasonably likely to have a material adverse effect on us. We believe that the mix and design of our executive compensation plans and policies do not encourage management to assume excessive risks and are not reasonably likely to have a material adverse effect on us. We believe that our compensation program offers an appropriate balance of short and long-term incentives and fixed and variable amounts of compensation. The mix between fixed and variable, annual and long-term and cash and equity compensation are designed to encourage strategies and actions that balance the company’s short-term and long-term best interests. Our stock option awards vest over a period of time, which we believe encourages executives to take a long-term view of our business. In addition, our compensation committee and board of directors retains discretionary authority to adjust certain aspects of variable and other compensation as appropriate.
Federal Tax Considerations under Sections 162(m)
Section 162(m) of the Internal Revenue Code of 1986, as amended, disallows a federal income tax deduction to any publicly traded corporation for any remuneration in excess of $1.0 million of compensation paid to specified executive officers in a calendar year. Compensation in excess of $1.0 million may be deducted if, among other things, it qualifies as performance-based compensation within the meaning of Section 162(m). We expect that once we are a publicly traded company our compensation committee will periodically review the potential consequences of Section 162(m) on the various elements of our executive compensation program and where reasonably practicable, will seek to structure the equity incentives component of our executive compensation program to comply with exemptions in Section 162(m). However, our board of directors or compensation committee may, in their judgment, authorize compensation payments that do not comply with the exemptions in Section 162(m) in situations where they believe that such payments are appropriate to attract and retain qualified executive talent.
133
Table of Contents
Accounting Considerations
We have adopted the fair value provisions of ASC 718 for our stock-based compensation awards. Under ASC 718, we are required to estimate and record an expense for each award of equity compensation, including stock options, over the vesting period of the award. We expect to retain for the foreseeable future our stock option program as the principal component of our long-term compensation program, and, therefore, to record this expense on an ongoing basis in accordance with ASC 718. Our compensation committee may in the future consider the grant of restricted stock or other equity-based awards to our executive officers in lieu, in whole or in part, of stock option grants, in light of the accounting impact of ASC 718 with respect to stock option grants.
Summary Compensation Table
The following table sets forth information regarding compensation awarded to, paid to or earned by our named executive officers during the year ended December 31, 2010.
| Name and Principal Position |
Year | Salary ($) |
Bonus ($) |
Option Awards(1) ($) |
All
Other Compensation(2) ($) |
Total ($) |
||||||||||||||||||
| Jeffrey D. Abbey(3) President and Chief Executive Officer |
2010 | $ | 261,828 | — | $ | 846,469 | $ | 4,539 | $ | 1,112,836 | ||||||||||||||
| Lori R. Harrelson(4) Vice President of Finance |
2010 | $ | 120,972 | $ | 7,000 | — | $ | 2,374 | $ | 130,346 | ||||||||||||||
| Charles A. Nicolette, Ph.D. Chief Scientific Officer and Vice President of Research and Development |
2010 | $ | 238,828 | — | $ | 245,418 | $ | 3,592 | $ | 487,838 | ||||||||||||||
| Frederick M. Miesowicz, Ph.D. Chief Operating Officer and Vice President of Manufacturing |
2010 | $ | 264,502 | — | $ | 114,305 | $ | 3,650 | $ | 705,101 | ||||||||||||||
| John N. Bonfiglio, Ph.D.(5) Former President and Chief Executive Officer |
2010 | $ | 52,118 | (5) | — | — | $ | 266,221 | (6) | $ | 318,339 | |||||||||||||
| Timothy W. Trost(5) Former Chief Financial Officer |
2010 | $ | 41,932 | (5) | — | — | $ | 133,745 | (7) | $ | 175,677 | |||||||||||||
| (1) | Amounts reported represent the grant date fair value of the stock options granted during 2010 over the entire term of the options, computed in accordance with ASC 718, formerly Statement of Financial Accounting Standards No. 123R. Our named executive officers will only realize compensation to the extent that the trading price of our common stock is greater than the exercise price of such stock options. The valuation assumptions used in calculating the fair value of the stock options are set forth in note 2 to our financial statements included elsewhere in this prospectus. |
| (2) | Unless otherwise indicated, consists of a company matching contribution to our 401(k) plan and payment of life insurance premiums, as itemized below, and excludes medical, dental and certain other benefits received by the named executive officers that are available generally to all of our employees. |
| Name |
401(k) Matching Contribution | Insurance Premium Payment | ||||||
| Jeffrey D. Abbey |
$ | 3,675 | $ | 864 | ||||
| Lori R. Harrelson |
$ | 1,920 | $ | 454 | ||||
| Charles A. Nicolette, Ph.D. |
$ | 2,948 | $ | 644 | ||||
| Frederick M. Miesowicz, Ph.D. |
$ | 3,000 | $ | 650 | ||||
| (3) | Mr. Abbey became our president and chief executive officer on February 24, 2010. |
| (4) | Ms. Harrelson became our principal financial officer on February 24, 2010. She was promoted to vice president of finance in July 2011. |
134
Table of Contents
| (5) | Our employment of Dr. Bonfiglio and Mr. Trost terminated on February 23, 2010. Salary information represents base salary paid through February 23, 2010. |
| (6) | Represents payment of life insurance premiums in the amount of $150.86, payment of $3,106 in unused accrued paid time off, or PTO, and amounts paid to Dr. Bonfiglio, in accordance with his employment agreement with us and pursuant to a release and settlement agreement entered into in connection with his termination on February 23, 2010, which consist of cash payments representing continued base salary and the value of continued employee benefits. In addition, in connection with his termination we transferred to Dr. Bonfiglio ownership of a company owned cell phone. |
| (7) | Represents payment of life insurance premiums in the amount of $65.17, payment of $7,400 in unused accrued PTO and amounts paid to Mr. Trost, in accordance with his offer letter with us and pursuant to a release and settlement agreement entered into in connection with his termination on February 23, 2010, which consist of cash payments representing continued base salary and the value of continued employee benefits. In addition, in connection with his termination, we transferred to Mr. Trost ownership of a company owned Blackberry. |
The terms of our employment agreements and offer letters with our named executive officers are described below under “— Agreements with our Named Executive Officers.”
Grants of Plan-Based Awards
The following table sets forth, for each of our named executive officers, information regarding each grant of a plan-based award made during the fiscal year ended December 31, 2010. This information supplements the information about these awards set forth in the Summary Compensation Table.
| Name |
Grant Date | All Other Option Awards: Number of Securities Underlying Options (#)(1) |
Exercise or Base Price of Option Awards ($/Sh) |
Grant Date Fair Value of Option Awards(2) |
||||||||||||
| Jeffrey D. Abbey |
12/10/10 | 6,845,926 | $ | 0.08 | $ | 846,469 | ||||||||||
| Lori R. Harrelson |
— | — | — | — | ||||||||||||
| Charles A. Nicolette, Ph.D. |
12/10/10 | 1,984,850 | $ | 0.08 | $ | 245,418 | ||||||||||
| Frederick M. Miesowicz, Ph.D. |
12/10/10 | 924,555 | $ | 0.08 | $ | 114,305 | ||||||||||
| John N. Bonfiglio, Ph.D. |
— | — | — | — | ||||||||||||
| Timothy W. Trost |
— | — | — | — | ||||||||||||
| (1) | The vesting schedules for these options are described in the footnotes to the Outstanding Equity Awards at 2010 Fiscal Year End table below. |
| (2) | Amounts reported represent the grant date fair value of the stock options granted during 2010 over the entire term of the options, computed in accordance with ASC 718, formerly Statement of Financial Accounting Standards No. 123R. The valuation assumptions used in calculating the fair value of the stock options are set forth in note 2 to our financial statements included elsewhere in this prospectus. |
135
Table of Contents
Outstanding Equity Awards at 2010 Fiscal Year End
The following table provides information about outstanding stock options held by each of our named executive officers at December 31, 2010. All of the listed options were granted under our 2008 stock incentive plan.
| Name |
Number of
Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Option
Exercise Price ($) |
Option Expiration Date |
||||||||||||
| Jeffrey D. Abbey |
|
1,924,518 774,710 1,426,234 |
(1) (2) (3) |
|
— — 5,419,692 |
(3) |
$ $ $ |
0.08 0.08 0.08 |
|
|
7/02/18 12/05/18 12/10/20 |
| ||||
| Lori R. Harrelson |
|
236,621 95,251 |
(4) (5) |
|
— — |
|
$ $ |
0.08 0.08 |
|
|
7/02/18 12/05/18 |
| ||||
| Charles A. Nicolette, Ph.D. |
|
1,987,617 800,110 413,510 |
(6) (7) (8) |
|
— — 1,571,340 |
(8) |
$ $ $ |
0.08 0.08 0.08 |
|
|
7/02/18 12/05/18 12/10/20 |
| ||||
| Frederick M. Miesowicz, Ph.D. |
|
1,892,969 762,009 192,594 |
(9) (10) (11) |
|
— — 731,861 |
(11) |
$ $ $ |
0.08 0.08 0.08 |
|
|
7/02/18 12/05/18 12/10/20 |
| ||||
| John N. Bonfiglio, Ph.D. |
|
5,652,615 2,275,444 |
(12) (13) |
|
— — |
|
$ $ |
0.08 0.08 |
|
|
12/31/10 12/31/10 |
| ||||
| Timothy W. Trost |
— | — | — | — | ||||||||||||
| (1) | This option was granted on July 2, 2008 and vested as to 50% of the shares on the date of grant, with the remaining 50% of the shares vesting in equal amounts per month over the next two years commencing on April 1, 2008, provided that Mr. Abbey continued to provide services to us over such period. |
| (2) | This option was granted on December 5, 2008 and vested in specified increments over a two-year period ending on April 1, 2010, provided that Mr. Abbey continued to provide services to us over such period. |
| (3) | This option was granted on December 10, 2010. This option vests in equal monthly installments over a four year period, with the first installment vesting on February 24, 2010, provided that Mr. Abbey continues to provide services to us over such period. |
| (4) | This option was granted on July 2, 2008 and vested as to 50% of the shares on the date of grant, with the remaining 50% of the shares vesting in equal amounts per month over the next two years commencing on April 1, 2008, provided that Ms. Harrelson continued to provide services to us over such period. |
| (5) | This option was granted on December 5, 2008 and vested in specified increments over the period ending on April 1, 2010, provided that Ms. Harrelson continued to provide services to us over such period. |
| (6) | This option was granted on July 2, 2008 and vested as to 50% of the shares on the date of grant, with the remaining 50% of the shares vesting in equal amounts per month over the next two years commencing on April 1, 2008, provided that Dr. Nicolette continued to provide services to us over such period. |
| (7) | This option was granted on December 5, 2008 and vested in specified increments over the period ending on April 1, 2010, provided that Dr. Nicolette continued to provide services to us over such period. |
| (8) | This option was granted on December 10, 2010. This option vests in equal monthly installments over a four year period, with the first installment vesting on February 24, 2010, provided that Dr. Nicolette continues to provide services to us over such period. |
136
Table of Contents
| (9) | This option was granted on July 2, 2008 and vested as to 50% of the shares on the date of grant, with the remaining 50% of the shares vesting in equal amounts per month over the next two years commencing on April 1, 2008, provided that Dr. Miesowicz continued to provide services to us over such period. |
| (10) | This option was granted on December 5, 2008 and vested in specified increments over the period ending on April 1, 2010, provided that Dr. Miesowicz continued to provide services to us over such period. |
| (11) | This option was granted on December 10, 2010. This option vests in equal monthly installments over a four year period, with the first installment vesting on February 24, 2010, provided that Dr. Miesowicz continues to provide services to us over such period. |
| (12) | Represents the vested portion of a stock option held by Dr. Bonfiglio as of the date his employment was terminated. Pursuant to the terms of the release and settlement agreement entered into in connection with the termination of Dr. Bonfiglio’s employment, the period of time within which this vested option could be exercised following his termination was extended until December 31, 2010. Dr. Bonfiglio did not exercise this option, and the option expired on December 31, 2010. |
| (13) | Represents the vested portion of a stock option held by Dr. Bonfiglio as of the date his employment was terminated. Pursuant to the terms of the release and settlement agreement entered into in connection with the termination of Dr. Bonfiglio’s employment, the period of time within which this vested option could be exercised following termination was extended until December 31, 2010. Dr. Bonfiglio did not exercise this option, and the option expired on December 31, 2010. |
Options Exercised and Stock Vested
Our named executive officers did not exercise any stock options during 2010, and they do not hold any other stock awards that vested in 2010.
Pension Benefits and Nonqualified Deferred Compensation
Our named executive officers did not participate in or have account balances in any qualified or nonqualified defined benefit plans sponsored by us during 2010 and did not earn any nonqualified deferred compensation benefits from us during 2010.
Agreements with our Named Executive Officers
We have entered into offer letters with each of our named executive officers and Mr. Plessinger, our vice president of medical and clinical affairs. We entered into these letters with our executive officers upon the commencement of their employment with us and have amended them in certain cases since that time. These offer letters set forth the terms of the executive officer’s compensation package, including the executive officer’s initial base salary, the terms of a recommended initial stock option grant, severance arrangements, if applicable, in the event of a termination of employment and an annual incentive cash bonus target percentage. In addition, the offer letters provide that as a regular employee, the named executive officer is eligible to participate in company-sponsored benefit programs that are available generally to all salaried employees and entitled to a specified amount of paid vacation, in accordance with our general vacation policy. These offer letters also provide that the executive officer’s employment can be terminated by either party for any reason. In connection with the execution of these offer letters and commencing employment with us, we required our named executive officers to execute our standard confidential information, invention assignment and noncompetition agreement. Information regarding potential payments and benefits due upon a termination of employment or a change of control transaction are described below under “— Agreements with our Named Executive Officers.”
Jeffrey D. Abbey. Under the terms of his offer letter with us, Mr. Abbey is eligible to receive an annual cash bonus of up to 20% of his annual base salary, subject to corporate and individual performance. In September 2011, our board of directors increased his target cash bonus to 40% of his annual base salary. Mr. Abbey’s
137
Table of Contents
current base salary is $300,000. In addition, under the terms of his original offer letter, we agreed that if we terminate Mr. Abbey’s employment without cause, as determined by the board of directors or the compensation committee, we would pay Mr. Abbey an amount equal to six months of his then annual base salary, payable monthly in accordance with our payroll practice for a period of six months, and continue to provide his standard health benefits for a period of six months, subject to such benefits being available to non-employees.
In March 2011, we amended Mr. Abbey’s offer letter with us to provide for enhanced benefits in the event of termination. Specifically, we agreed that if we terminate Mr. Abbey’s employment without cause, as determined by the board of directors or the compensation committee, we would pay Mr. Abbey an amount equal to nine months of his then annual base salary, payable monthly in accordance with our payroll practice for a period of nine months, and continue to provide his standard health benefits for the nine month severance period, subject to such benefits being available to non-employees. In addition, we agreed that if we terminated Mr. Abbey’s employment without cause within 90 days prior to or six months after a change of control, as defined in the amended offer letter, then in lieu of the nine months of severance and nine months of health benefits, we would provide Mr. Abbey 15 months of severance and 15 months of health benefits, subject to such benefits being available to non-employees. In the event that we were unable to provide Mr. Abbey health benefits as a non-employee post-termination, we would pay him the cash equivalent of such health benefits.
Our obligation to make the severance payments and provide the benefits is contingent upon Mr. Abbey’s execution of a release in a form reasonably acceptable to us. If required by Section 409A of the Internal Revenue Code, the payments we are required to make to Mr. Abbey in the first six months following the termination of his employment under his agreement will be made as a lump sum on the date that is six months and one day following such termination.
Lori R, Harrelson. In July 2011, Ms. Harrelson was promoted to the position of vice president of finance and in connection with that promotion became eligible to receive an annual cash bonus of up to 20% of her annual base salary, subject to corporate and individual performance. Ms. Harrelson’s current annual base salary is $150,000. In September 2011, we amended Ms. Harrelson’s offer letter with us to provide for the annual cash bonus eligibility described above and to provide severance benefits in the event of termination. Specifically, we agreed that if we terminate Ms. Harrelson’s employment without cause, as determined by the board of directors or the compensation committee, we would pay Ms. Harrelson an amount equal to six months of her then annual base salary, payable monthly in accordance with our payroll practice for a period of six months, and continue to provide her standard health benefits for a period of six months, subject to such benefits being available to non-employees.
Our obligation to make the severance payments and provide the benefits to Ms. Harrelson is contingent upon her execution of a release in a form reasonably acceptable to us. If required by Section 409A of the Internal Revenue Code, the payments we are required to make to Ms. Harrelson in the first six months following the termination of her employment under her agreement will be made as a lump sum on the date that is six months and one day following such termination.
Charles A. Nicolette, Ph.D. Under the terms of his offer letter with us, Dr. Nicolette is eligible to receive an annual cash bonus of up to 20% of his annual base salary, subject to corporate and individual performance. Dr. Nicolette’s current base salary is $250,000. In addition, under the terms of his original offer letter, we agreed that if we terminate Dr. Nicolette’s employment without cause, as determined by the board of directors or the compensation committee, we would pay Dr. Nicolette an amount equal to six months of his then annual base salary, payable monthly in accordance with our payroll practice for a period of six months, and continue to provide his standard health benefits for a period of six months, subject to such benefits being available to non-employees.
In March 2011, we amended Dr. Nicolette’s offer letter with us to provide for enhanced benefits in the event of termination. Specifically, we agreed that if we terminate Dr. Nicolette’s employment without cause, as determined by the board of directors or the compensation committee, we would pay Dr. Nicolette an amount
138
Table of Contents
equal to nine months of his then annual base salary, payable monthly in accordance with our payroll practice for a period of nine months, and continue to provide his standard health benefits for the nine month severance period, subject to such benefits being available to non-employees. In addition, we agreed that if we terminated Dr. Nicolette’s employment without cause within 90 days prior to or six months after a change of control, as defined in the amended offer letter, then in lieu of the nine months of severance and nine months of health benefits, we would provide Dr. Nicolette 15 months of severance and 15 months of health benefits, subject to such benefits being available to non-employees. In the event that we were unable to provide Dr. Nicolette health benefits as a non-employee post-termination, we would pay him the cash equivalent of such health benefits.
Our obligation to make the severance payments and provide the benefits is contingent upon Dr. Nicolette’s execution of a release in a form reasonably acceptable to us. If required by Section 409A of the Internal Revenue Code, the payments we are required to make to Dr. Nicolette in the first six months following the termination of his employment under his agreement will be made as a lump sum on the date that is six months and one day following such termination.
Frederick M. Miesowicz, Ph.D. Under the terms of his offer letter with us, Dr. Miesowicz is eligible to receive an annual cash bonus of up to 20% of his annual base salary, subject to corporate and individual performance. Dr. Miesowicz’s current base salary is $261,380. In addition, under the terms of his offer letter, we agreed that if we terminate Dr. Miesowicz’s employment without cause, as determined by the board of directors or the compensation committee, we would pay Dr. Miesowicz an amount equal to nine months of his then annual base salary, payable monthly in accordance with our payroll practice for a period of nine months, and continue to provide his standard health benefits for a period of nine months, subject to such benefits being available to non-employees. In July 2011, we amended Dr. Miesowicz’s offer letter to provide that in the event that we were unable to provide Dr. Miesowicz health benefits as a non-employee post-termination, we would pay Dr. Miesowicz the cash equivalent of such health benefits.
Our obligation to make the severance payments and provide the benefits to Dr. Miesowicz is contingent upon Dr. Miesowicz’s execution of a release in a form reasonably acceptable to us. If required by Section 409A of the Internal Revenue Code, the payments we are required to make to Dr. Miesowicz in the first six months following the termination of his employment under his agreement will be made as a lump sum on the date that is six months and one day following such termination.
Douglas C. Plessinger, RPh. Effective October 4, 2011, we hired Mr. Plessinger as our vice president medical and clinical affairs. Under the terms of his offer letter with us, Mr. Plessinger is eligible to receive an annual cash bonus of up to 20% of his annual base salary (pro rated for 2011), subject to corporate and individual performance. Mr. Plessinger’s current base salary is $240,000. Mr. Plessinger is also entitled to reimbursement of certain relocation expenses, up to $30,000, which amounts must be repaid if he resigns within the first year of his employment. In addition, under the terms of his offer letter, we agreed that if we terminate Mr. Plessinger’s employment without cause, as determined by the board of directors or the compensation committee, we would pay Mr. Plessinger an amount equal to six months of his then annual base salary, payable monthly in accordance with our payroll practice for a period of six months, and continue to provide his standard health benefits for a period of six months, subject to such benefits being available to non-employees. Our obligation to make the severance payments and provide the benefits to Mr. Plessinger is contingent upon his execution of a release in a form reasonably acceptable to us. If required by Section 409A of the Internal Revenue Code, the payments we are required to make to Mr. Plessinger in the first six months following the termination of his employment under his agreement will be made as a lump sum on the date that is six months and one day following such termination.
John N. Bonfiglio, Ph.D. We were a party to an employment agreement with Dr. Bonfiglio which we entered into with him in December 2006. Under the terms of his employment agreement, Dr. Bonfiglio was entitled to an initial annual base salary of $300,000 and was eligible to receive an annual cash bonus of up to 40% of his annual base salary based on corporate and individual performance. The initial term of the employment agreement was two years, but under the agreement the term would renew automatically for
139
Table of Contents
additional one year terms unless either party provided notice of non-renewal. Other than non-renewal, if we terminated Dr. Bonfiglio’s employment other than for cause or he terminated his employment for good reason, as defined in his employment agreement, we would pay Dr. Bonfiglio as severance his annual base salary for a period equal to the longer of nine months or the period left in the term of his employment agreement and continue to provide his standard health benefits for a period of nine months or the cash equivalent if such benefits were not available to non-employees. In addition, his employment agreement provided that he would receive an additional nine months of accelerated vesting under each of his stock options if his employment were terminated other than for cause or he terminated his employment for good reason as well as full acceleration of vesting under all of his stock options in the event of a change of control.
In February 2010, we terminated the employment of Dr. Bonfiglio. In connection with his termination, Dr. Bonfiglio entered into a release and settlement agreement with us, pursuant to which we agreed to pay him the severance and benefits contemplated by his employment agreement and extended the period within which he was entitled to exercise his vested stock options to December 31, 2010.
Timothy W. Trost. We were a party to an offer letter with Mr. Trost which we entered into with him in June 2002. Under the terms of his offer letter, Mr. Trost was entitled to an initial annual base salary of $220,000 and was eligible to receive an annual cash bonus of up to 20% of his annual base salary, subject to corporate and individual performance. If we terminated Mr. Trost’s employment other than for cause, we would pay Mr. Trost an amount equal to six months of his then annual base salary, payable monthly in accordance with our payroll practice for a period of six months, and continue to provide his standard health benefits for a period of six months, subject to such benefits being available to non-employees.
In February 2010, we terminated the employment of Mr. Trost. In connection with his termination, Mr. Trost entered into a release and settlement agreement with us, pursuant to which we agreed to pay him the severance and benefits contemplated by his offer letter.
Potential Payments upon Termination or Change of Control
As described under “— Agreements with our Named Executive Officers,” each of our named executive officers is entitled to severance and benefits in connection with the termination of their employment under their offer letters or employment agreements with us.
Under the terms of the stock options granted to our named executive officers under our 2008 stock incentive plan, upon a change of control transaction all unvested portions of any outstanding options held by them will vest in full.
In addition, eligible participants under our 2008 management incentive plan, which expired on December 31, 2010, would have been entitled to potential payments in the event of a qualifying change of control transaction that occurred during the term of the plan. See discussion above under “— 2008 Management Incentive Plan.” As of December 31, 2010, the eligible participants in the plan were Mr. Abbey, Dr. Miesowicz and Dr. Nicolette.
140
Table of Contents
The following table sets forth the estimated potential severance and healthcare benefits that our named executive officers would be entitled to receive upon their termination of employment with our company assuming the named executive officers’ employment terminated on December 31, 2010. Except as provided in note (1) below the table, the named executive officers would not be entitled to any additional severance and healthcare benefits if the termination of their employment occurred in connection with a change in control of our company. The following table also sets forth the estimated other potential benefits that our named executive officers would be entitled to receive in connection with a change in control of our company, assuming that the change in control occurred on December 31, 2010. Dr. Bonfiglio’s and Mr. Trost’s employment with us terminated on February 23, 2010. This table represents estimates only and does not necessarily reflect the actual amounts that would be paid to our named executive officers, which would only be known at the time that they become eligible for payment following their termination.
| Name |
Benefit |
Termination Without Cause |
Change of Control Event |
|||||||
| Jeffrey D. Abbey |
Salary Continuation | $ | 132,500 | (1) | — | |||||
| Continuation of Benefits | $ | 6,249 | (1) | — | ||||||
| Vesting of Options | — | $ | (2) | |||||||
| Management Incentive Plan(3) | — | $ | ||||||||
|
|
|
|
|
|||||||
| Total | $ | 138,749 | ||||||||
| Lori R. Harrelson |
Salary Continuation | — | — | |||||||
| Continuation of Benefits | — | — | ||||||||
| Vesting of Options | — | $ | (2) | |||||||
| Management Incentive Plan(3) | — | — | ||||||||
|
|
|
|
|
|||||||
| Total | ||||||||||
| Charles A. Nicolette, Ph.D. |
Salary Continuation | $ | 117,936 | (1) | — | |||||
| Continuation of Benefits | $ | 4,397 | (1) | — | ||||||
| Vesting of Options | — | $ | (2) | |||||||
| Management Incentive Plan(3) | — | $ | ||||||||
|
|
|
|
|
|||||||
| Total | $ | 122,333 | ||||||||
| Frederick W. Miesowicz, Ph.D. |
Salary Continuation | $ | 196,035 | — | ||||||
| Continuation of Benefits | $ | 6,595 | — | |||||||
| Vesting of Options | — | $ | (2) | |||||||
| Management Incentive Plan(3) | — | $ | ||||||||
|
|
|
|
|
|||||||
| Total | $ | 202,630 | ||||||||
| (1) | In March 2011 we amended Mr. Abbey’s and Dr. Nicolette’s offer letters to provide that their severance and healthcare benefits would increase from six to nine months in the event of termination without cause and from nine months to 15 months in the event of termination without cause within 90 days prior to or six months after a change of control. In September 2011, we amended Ms. Harrelson’s offer letter to provide that if we terminate Ms. Harrelson’s employment without cause, as determined by the board of directors or the compensation committee, we would pay Ms. Harrelson an amount equal to six months of her then annual base salary, payable monthly in accordance with our payroll practice for a period of six months, and continue to provide her standard health benefits for a period of six months. |
| (2) | Represents the in-the-money value of the unvested portions of Mr. Abbey’s, Ms. Harrelson’s, Dr. Nicolette’s and Dr. Miesowicz’s stock options as of December 31, 2010. The value is calculated by multiplying (x) the amount, if any, by which the assumed initial public offering price of $ per share, which is the midpoint of the range listed on the cover page of this prospectus, exceeds the exercise price of the options, by (y) the number of shares subject to the unvested portion of such options. |
| (3) | Under our 2008 management incentive plan, payments to executives would be calculated based on the sale proceeds from the change in control transaction and cannot be determined. |
141
Table of Contents
Director Compensation
We currently do not have a formal non-employee director compensation policy. However, we do reimburse our non-employee directors for their reasonable expenses incurred in connection with attending our board of directors and committee meetings, and we have historically granted stock options to our independent directors who are not affiliated with one of our investors when they initially joined our board of directors. Other than reimbursement of out-of-pocket expenses as described above, we did not provide any cash or equity compensation to our non-employee directors during the year ended December 31, 2010.
Our board of directors has approved a compensation policy for our non-employee directors that will become effective upon the closing of this offering. This policy is intended to provide a total compensation package that enables us to attract and retain qualified and experienced individuals to serve as directors and to align our directors’ interests with those of our stockholders. The policy provides for the following compensation to our non-employee directors beginning upon the closing of this offering:
Equity Compensation
| • | Upon initial election to our board of directors, each newly elected non-employee director will receive an option to purchase 24,000 shares of common stock with an exercise price equal to the fair market value of our common stock on the date of grant. Each of our current non-employee directors will be granted a similar option to purchase 24,000 shares of common stock upon the closing of this offering. |
| • | Our non-employee directors will receive annual grants of options to purchase 7,500 shares of common stock, commencing at the first regular board meeting following the first anniversary of the closing of this offering, such options will be granted with an exercise price equal to the fair market value of our common stock on the date of each such grant. |
These initial and annual option grants will vest in equal quarterly installments over a three year period from the date of grant, subject to the director’s continued service on our board of directors. Director options will be granted pursuant to our 2011 stock incentive plan. The 2011 stock incentive plan is described under “— Stock Option and Other Equity Compensation Plans — 2011 Stock Incentive Plan.”
Cash Compensation
| • | Each non-employee director will receive an annual retainer of $20,000 for service on the board, and the chairman of the board will receive an additional $10,000 annual retainer for such service; |
| • | The chair of our audit committee will receive an additional annual retainer of $10,000, and the chairs of our compensation committee and our nominating and corporate governance committee will each receive an additional annual retainer of $5,000; and |
| • | Each non-employee director will receive an attendance fee of $1,000 for each board or committee meeting attended in person and $500 for each board or committee meeting attended by teleconference. |
Each annual retainer is payable in arrears in four equal quarterly installments. In the event of resignation from the board, the retainers will be pro-rated based on the number of calendar days served by such director during the quarter.
Reimbursement of Expenses
Each non-employee director will also be reimbursed for reasonable travel and other expenses incurred in connection with attending meetings of the board of directors and any committee on which he or she serves.
142
Table of Contents
Stock Option and Other Equity Compensation Plans
We believe that equity-based awards are an important vehicle by which to align the interest of our employees with the financial interests of our stockholders, and we historically have awarded stock options broadly to our employees, including our executive officers. The material terms and conditions of our stock option and other equity compensation plans are described below.
2011 Stock Incentive Plan
We expect our board of directors to adopt and our stockholders to approve the 2011 stock incentive plan, which will become effective immediately prior to the closing of this offering. The 2011 stock incentive plan provides for the grant of incentive stock options, non-statutory stock options, stock appreciation rights, restricted stock awards, restricted stock units and other stock-based awards. Upon effectiveness of the plan, the number of shares of our common stock that will be reserved for issuance under the 2011 stock incentive plan will be the sum of shares plus (1) the number of shares of our common stock then available for issuance under the 2008 stock incentive plan, both described below, up to shares, (2) the number of shares of our common stock subject to outstanding awards under the 2008 stock incentive plan that expire, terminate or are otherwise surrendered, cancelled, forfeited or repurchased by us at their original issuance price pursuant to a contractual repurchase right, and (3) an annual increase, to be added on the first day of each fiscal year beginning in fiscal year 2013 and each subsequent anniversary until the expiration of the 2011 stock incentive plan, equal to the lowest of (a) shares of our common stock, (b) % of the number of shares of our common stock outstanding on the first day of the fiscal year and (c) an amount determined by our board of directors.
Our employees, officers, directors, consultants and advisors are eligible to receive awards under the 2011 stock incentive plan. However, incentive stock options may only be granted to our employees. The maximum number of shares of our common stock with respect to which awards may be granted to any participant under the 2011 stock incentive plan is per calendar year. For purposes of this limit on the maximum number of shares that may be awarded to any participant, the combination of an option in tandem with a stock appreciation right will be treated as a single award.
Pursuant to the terms of the 2011 stock incentive plan, our board of directors administers the plan and, subject to any limitations in the plan, selects the recipients of awards and determines:
| • | the number of shares of our common stock covered by options and the dates upon which the options become exercisable; |
| • | the type of options to be granted; |
| • | the duration of options, which may not be in excess of ten years; |
| • | the exercise price of options, which may not be less than the fair market value of our common stock on the date of grant of the options; and |
| • | the number of shares of our common stock subject to any stock appreciation rights, restricted stock awards, restricted stock units or other stock-based awards and the terms and conditions of such awards, including conditions for repurchase, issue price and repurchase price. |
If our board of directors delegates authority to an executive officer to grant awards under the 2011 stock incentive plan, the executive officer has the power to make awards to all of our employees, except executive officers. Our board of directors will fix the terms of the awards to be granted by such executive officer, including
143
Table of Contents
the exercise price of such awards, and the maximum number of shares subject to awards that such executive officer may make.
Upon a merger or other reorganization event, our board of directors may, in its sole discretion, take any one or more of the following actions pursuant to the 2011 stock incentive plan as to some or all outstanding awards other than restricted stock:
| • | provide that all outstanding awards shall be assumed, or substantially equivalent awards shall be substituted, by the acquiring or successor corporation (or an affiliate thereof); |
| • | upon written notice to a participant, provide that all of the participant’s unexercised awards will terminate immediately prior to the consummation of such reorganization event unless exercised by the participant; |
| • | provide that outstanding awards shall become exercisable, realizable or deliverable, or restrictions applicable to an award shall lapse, in whole or in part, prior to or upon such reorganization event; |
| • | in the event of a reorganization event pursuant to which holders of shares of our common stock will receive a cash payment for each share surrendered in the reorganization event, make or provide for a cash payment to the participants with respect to each award held by a participant equal to (1) the number of shares of common stock subject to the vested portion of the award (after giving effect to any acceleration of vesting that occurs upon or immediately prior to such reorganization event) multiplied by (2) the excess, if any, of the cash payment for each share surrendered in the reorganization event over the exercise, measurement or purchase price of such award and any applicable tax withholdings, in exchange for the termination of such award; and |
| • | provide that, in connection with a liquidation or dissolution, awards shall convert into the right to receive liquidation proceeds. |
In the case of certain restricted stock units, no assumption or substitution is permitted, and the restricted stock units will instead be settled in accordance with the terms of the applicable restricted stock unit agreement.
Upon the occurrence of a reorganization event other than a liquidation or dissolution, the repurchase and other rights with respect to outstanding restricted stock will continue for the benefit of the successor company and will, unless the board of directors may otherwise determine, apply to the cash, securities or other property into which shares of our common stock are converted or exchanged pursuant to the reorganization event. Upon the occurrence of a reorganization event involving a liquidation or dissolution, all restrictions and conditions on each outstanding restricted stock award will automatically be deemed terminated or satisfied, unless otherwise provided in the agreement evidencing the restricted stock award.
At any time, our board of directors may, in its sole discretion, provide that any award under the 2011 stock incentive plan will become immediately exercisable in full or in part, free of some or all restrictions or conditions, or otherwise realizable in full or in part.
No award may be granted under the 2011 stock incentive plan on or after , 2021. Our board of directors may amend, suspend or terminate the 2011 stock incentive plan at any time, except that stockholder approval will be required to comply with applicable law or stock market requirements.
2008 Stock Incentive Plan
In February 2008, our board of directors adopted our 2008 stock incentive plan. Our stockholders approved our 2008 stock incentive plan in March 2008. Following the completion of this offering, our board of directors
144
Table of Contents
will not grant any further awards under the 2008 stock incentive plan but all outstanding awards will continue to be governed by their existing terms. We expect to make future equity-based awards under our new 2011 stock incentive plan described below.
Types of Awards. The 2008 stock incentive plan provides for the grant of incentive stock options within the meaning of Section 422 of the Internal Revenue Code, nonstatutory stock options, restricted stock awards, consisting of restricted stock and restricted stock units, and other forms of stock-based awards. Awards under the plan may be granted to our employees, directors and individual consultants and advisors. Only our employees are eligible to receive incentive stock options.
Share Reserve. When initially adopted, an aggregate of 27,326,263 shares were reserved for issuance under the 2008 stock incentive plan. In December 2008, the 2008 stock incentive plan was amended by our board of directors, and approved by our stockholders, to increase to 37,612,814 the total number of shares available for issuance under the plan. As of August 31, 2011, options to purchase 24,183,826 shares of common stock at a weighted average exercise price per share of $0.08 were outstanding under the 2008 stock incentive plan. As of August 31, 2011, 13,205,160 shares of common stock remained available for future issuance under the plan.
Administration. Our board of directors, or a duly authorized committee thereof, is authorized to administer our 2008 stock incentive plan. Our board of directors has delegated certain authority to administer the 2008 stock incentive plan to our compensation committee; however, our general practice has been that awards are approved by the board of directors. Our board of directors or its authorized committee has the authority under the plan to interpret and adopt rules and procedures relating to the 2008 stock incentive plan, as well as to determine the terms of any award or amend the terms of any award made under the plan. No amendment to any award made under the plan may materially and adversely affect the rights of a participant under any outstanding award without the participant’s consent.
Stock Options. Each stock option awarded under the 2008 stock incentive plan is granted pursuant to a notice of stock option and stock option agreement. The board of directors determines the exercise price for a stock option, within the terms and conditions of the 2008 stock incentive plan, provided that the exercise price of a stock option generally cannot be less than 100% of the fair market value of our common stock on the date of grant. The vesting and other terms of each grant under the 2008 stock incentive plan is determined by the board of directors in its discretion; however, shares subject to stock options granted under the 2008 stock incentive plan generally vest in installments over a specified period of service, typically four years.
The board of directors determines the term of stock options granted under the 2008 stock incentive plan, subject to limitations in the case of some incentive stock options, as described below. In general, if an optionee’s service relationship with us, or any of our affiliates, ceases for any reason other than disability, death or cause, the optionee may generally exercise the vested portion of any option for a period of three months following the cessation of service. If an optionee’s service relationship with us, or any of our affiliates, ceases due to disability or death or if an optionee dies within a specified period following cessation of service, the optionee or a beneficiary generally may exercise the vested portion of any option for a period of 12 months following the death or disability. If an optionee’s services are terminated for cause, options generally terminate immediately upon such termination. In no event may an option be exercised beyond the expiration of its term.
Stock purchased upon the exercise of a stock option may, depending on the terms of the particular option agreement, be paid for using any of the following: (1) cash or check, (2) a broker-assisted cashless exercise, (3) when our common stock is registered under the Securities Exchange Act of 1934, the tender of common stock previously owned by the optionee, (4) delivery of a promissory note, (5) payment of other lawful consideration as determined by the plan administrator, or (6) any combination of the above.
Tax Limitations on Incentive Stock Options. Incentive stock options are subject to certain restrictions contained in the Internal Revenue Code. Among such restrictions, incentive stock options may be granted only to
145
Table of Contents
our employees. The maximum term of an incentive stock option is ten years from the date of grant. Any incentive stock option granted to any person who, at the time of the grant, owns or is deemed to own stock possessing more than 10% of our total combined voting power or that of any of our affiliates must have an exercise price equal at least to 110% of the fair market value of the stock subject to the option on the date of grant, and the term of the incentive stock option may not exceed five years from the date of grant. The aggregate fair market value, determined at the time of grant, of shares of our common stock with respect to incentive stock options that are exercisable for the first time by an optionee during any calendar year under all of our stock plans may not exceed $100,000.
Restricted Stock Awards. Each restricted stock award granted under the 2008 stock incentive plan is granted pursuant to a summary of restricted stock purchase and a restricted stock purchase agreement. An award of restricted stock entitles a participant to acquire shares of our common stock that are subject to specified restrictions, which may include a repurchase right or forfeiture right, if the shares are issued at no cost, in our favor that lapses in accordance with a vesting schedule or as conditions specified in the award are satisfied. The board of directors determines the terms and conditions of restricted stock awards, including the conditions for repurchase or forfeiture and the purchase price, if any. Unless the board of directors determines otherwise, participants holding shares of restricted stock are entitled to all ordinary cash dividends paid with respect to such shares.
Other Stock-Based Awards. The board of directors may grant other awards based in whole or in part on our common stock. The board of directors sets the number of shares under the equity award, as well as the purchase price applicable to the equity award and all other terms and conditions of the award.
Term; Amendment. No awards may be granted under the 2008 stock incentive plan after the expiration of ten years from the earlier of the date the plan was adopted by our board of directors or the date the plan was approved by our stockholders, but awards granted prior to such expiration may extend beyond that date. The board of directors may amend, suspend or terminate the plan at any time, subject to approval of the stockholders in certain circumstances if required by the Internal Revenue Code to ensure that incentive stock options are tax-qualified and to a participant’s consent to the extent that any amendment to the plan may materially and adversely affect the rights of a participant under any outstanding award.
Effect of Certain Corporate Transactions. In the event of specified changes of control of our company, our board of directors may take any one or more actions as to outstanding equity award, or as to a portion of any outstanding equity award, including:
| • | providing that such awards will be assumed, or substantially equivalent awards substituted, by the acquiring or succeeding corporation or an affiliate thereof; |
| • | providing, upon notice to the participant, that all unexercised awards will terminate immediately prior to the consummation of such transaction unless exercised within a specified period of time; |
| • | providing that all or any outstanding awards will become vested or exercisable, or restrictions applicable to such awards will lapse, in full or in part, at or immediately prior to such event; |
| • | in the event of a consolidation, merger, combination, reorganization or similar transaction under the terms of which holders of our common stock will receive a cash payment per share surrendered in the transaction, making or providing for an equivalent cash payment in exchange for the termination of such equity awards; or |
| • | providing that in the event of a liquidation or dissolution awards will convert into the right to receive liquidation proceeds. |
146
Table of Contents
Transferability. Awards made under the 2008 stock incentive plan are not transferable except by will or by the laws of descent or distribution or, other than in the case of an incentive stock option, pursuant to a domestic relations order.
1999 Stock Option Plan
Our board of directors adopted, and our stockholders approved, the 1999 stock option plan in December 1999. In 2008, our employees who then held options under the 1999 stock option plan agreed to the cancellation and termination of all of their options previously granted under the 1999 stock option plan in exchange for the grant of new options under our 2008 stock incentive plan described below, and the board of directors determined at that time to discontinue making awards under the 1999 stock option plan. As of August 31, 2011, options to purchase 5,786 shares of common stock at a weighted average exercise price per share of $19.25 were outstanding under the 1999 stock option plan. The 1999 stock option plan has expired and no shares of common stock are available for issuance under that plan, although all outstanding options remain outstanding in accordance with their terms.
Administration. Our board of directors administered the 1999 stock option plan and had the authority to interpret the terms of the 1999 stock option plan and the options granted under it.
Eligibility. The 1999 stock option plan provided for the grant of incentive stock options within the meaning of Section 422 of the Code and nonstatutory stock options. Our employees, including officers, non-employee directors, advisors and independent consultants, were eligible to receive options under the 1999 stock option plan, provided that incentive stock options could be granted only to employees.
Effect of Certain Corporate Transactions. The 1999 stock option plan provided that, unless otherwise determined by our board of directors at the time of grant, in the event of a merger, consolidation, corporate reorganization or any transaction in which all or substantially all of our assets or stock were sold, leased, transferred or otherwise disposed of, unless otherwise provided in an individual’s option agreement, any unvested portion of a stock option granted under the 1999 stock option plan would become fully vested unless the surviving or acquiring corporation assumed or substituted comparable options for the outstanding options granted under the plan or replaced the options with a cash incentive program that preserved the intrinsic value of the options at the time of the transaction and provided for subsequent payout over the same vesting schedules as the options being replaced.
Limitation of Liability and Indemnification
Our certificate of incorporation, which will become effective upon the closing of this offering, limits the personal liability of directors for breach of fiduciary duty to the maximum extent permitted by the Delaware General Corporation Law and provides that no director will have personal liability to us or to our stockholders for monetary damages for breach of fiduciary duty or other duty as a director. However, these provisions do not eliminate or limit the liability of any of our directors for:
| • | any breach of the director’s duty of loyalty to us or our stockholders; |
| • | any act or omission not in good faith or which involves intentional misconduct or a knowing violation of law; |
| • | voting or assenting to any unlawful payments of dividends, stock repurchases or other distributions; or |
| • | any transaction from which the director derived an improper personal benefit. |
Any amendment to or repeal of these provisions will not eliminate or reduce the effect of these provisions in respect of any act, omission or claim that occurred or arose prior to such amendment or repeal. If the Delaware
147
Table of Contents
General Corporation Law is amended to provide for further limitations on the personal liability of directors of corporations, then the personal liability of our directors will be further limited to the greatest extent permitted by the Delaware General Corporation Law.
In addition, our certificate of incorporation, which will become effective upon the closing of this offering, provides that we must indemnify our directors and officers and we must advance expenses, including attorneys’ fees, to our directors and officers in connection with legal proceedings, subject to very limited exceptions.
We maintain a general liability insurance policy that covers certain liabilities of our directors and officers arising out of claims based on acts or omissions in their capacities as directors or officers. In addition, we plan to enter into indemnification agreements with all of our directors and executive officers. These indemnification agreements may require us, among other things, to indemnify each such director for some expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by him in any action or proceeding arising out of his service as one of our directors.
Certain of our non-employee directors may, through their relationships with their employers, be insured or indemnified against certain liabilities incurred in their capacity as members of our board of directors.
148
Table of Contents
TRANSACTIONS WITH RELATED PERSONS
Since January 1, 2008, we have engaged in the following transactions, in which the amount involved in the transaction exceeds $120,000, with our directors, executive officers and holders of more than 5% of our voting securities, and affiliates or immediate family members of our directors, executive officers and holders of more than 5% of our voting securities. We believe that all of these transactions were on terms as favorable as could have been obtained from unrelated third parties.
Series C Preferred Stock Financing
During March, April and November 2008, we issued and sold an aggregate of 123,099,041 shares of our series C preferred stock for an aggregate purchase price of $30,004,666. We issued and sold these shares at a price per share of $0.289018 in the case of shares of our series C preferred stock issued and sold for cash or upon the cancellation of interest then outstanding on our then outstanding convertible promissory notes, and at a price per share of $0.2167635 in the case of shares of our series C preferred stock issued and sold upon the cancellation of principal then outstanding under our then outstanding convertible promissory notes. Holders of our preferred stock that purchased their pro rata share of our series C preferred stock in the series C preferred stock financing also received an aggregate of 651,728 shares of our series A preferred stock, 9,314,522 shares of our series B preferred stock and 830,177 shares of our series B-1 preferred stock, in exchange for, in part, the cancellation of accrued but unpaid dividends on such shares of preferred stock.
The following table sets forth the number of shares of each series of our preferred stock that were issued to our directors, executive officers and holders of more than 5% of our voting securities, and affiliates or immediate family members of our directors, executive officers and holders of more than 5% of our voting securities in connection with the series C preferred stock financing, the amount of principal and interest cancelled on our then outstanding convertible notes, if any, held by such persons and entities and the aggregate cash purchase price paid by such persons and entities.
| Name(1) |
Shares of Series A Preferred Stock |
Shares of Series B Preferred Stock |
Shares of Series B-1 Preferred Stock |
Shares of Series C Preferred Stock |
Cancelled Convertible Note Principal Amount |
Cancelled Convertible Note Interest Amount |
Aggregate Cash Purchase Price(2) |
|||||||||||||||||||||
| Entities affiliated with The Aurora Funds(3) |
113,905 | 743,130 | — | 4,124,833 | $ | 255,874 | $ | 71,346 | $ | 779,640 | ||||||||||||||||||
| Caisse de dépôt et de placement du Québec(4) |
— | 1,255,556 | — | 12,301,263 | $ | 344,435 | $ | 96,040 | $ | 3,000,000 | ||||||||||||||||||
| Coöperatieve AAC LS U.A. (Forbion)(5) |
— | 1,212,122 | — | 13,650,847 | $ | 586,379 | $ | 163,502 | $ | 3,000,000 | ||||||||||||||||||
| Entities affiliated with Intersouth Partners(6) |
227,738 | 2,265,605 | — | 11,688,457 | $ | 652,176 | $ | 181,848 | $ | 2,326,758 | ||||||||||||||||||
| Kyowa Hakko Kirin Co., Ltd. |
— | — | 830,177 | 5,189,988 | — | — | $ | 1,500,000 | ||||||||||||||||||||
| Entities affiliated with Lumira Capital(7) |
— | 1,098,612 | — | 21,342,351 | $ | 175,126 | $ | 48,830 | $ | 5,000,000 | ||||||||||||||||||
| TVM V Life Science Ventures GmbH & Co. KG(8) |
— | 1,191,919 | — | 26,322,097 | $ | 873,086 | $ | 243,445 | $ | 6,200,000 | ||||||||||||||||||
| Jeffrey D. Abbey |
— | — | — | 69,200 | — | — | $ | 20,000 | ||||||||||||||||||||
| Frederick M. Miesowicz, Ph.D. |
— | — | — | 34,600 | — | — | $ | 10,000 | ||||||||||||||||||||
| Charles A. Nicolette, Ph.D. |
— | — | — | 69,200 | — | — | $ | 20,000 | ||||||||||||||||||||
| (1) | See “Principal Stockholders” for more information about shares held by these persons and entities. |
| (2) | Excludes any purchase price attributable to the cancellation of principal and interest under our then outstanding convertible promissory notes. |
149
Table of Contents
| (3) | These amounts are distributed among the entities set forth in the table below. Mr. Clark, one of our directors, is the Managing General Partner of Aurora Funds, Inc. and is affiliated with these entities. |
| Name |
Shares of Series A Preferred Stock |
Shares of Series B Preferred Stock |
Shares of Series C Preferred Stock |
Cancelled Convertible Note Principal Amount |
Cancelled Convertible Note Interest Amount |
Cash Purchase Price |
||||||||||||||||||
| Aurora Ventures II, LLC |
113,905 | 115,352 | 679,950 | — | — | $ | 196,518 | |||||||||||||||||
| Harbinger/Aurora QP Venture Fund, LLC |
— | 371,035 | 2,036,026 | $ | 151,229 | $ | 42,167 | $ | 344,642 | |||||||||||||||
| Harbinger/Aurora Venture Fund, LLC |
— | 256,743 | 1,408,857 | $ | 104,645 | $ | 29,178 | $ | 238,480 | |||||||||||||||
| (4) | Ms. Laliberté, one of our directors, is the Investment Manager, Private Equity of Caisse de dépôt et de placement du Québec. |
| (5) | Dr. van Deventer, one of our directors, is a General Partner of Forbion Capital Partners and is affiliated with this entity. |
| (6) | These amounts are distributed among the entities set forth in the table below. Mr. Tracy, one of our directors, is a Venture Partner at Intersouth Partners and is affiliated with these entities. |
| Name |
Shares of Series A Preferred Stock |
Shares of Series B Preferred Stock |
Shares of Series C Preferred Stock |
Cancelled Convertible Note Principal Amount |
Cancelled Convertible Note Interest Amount |
Cash Purchase Price |
||||||||||||||||||
| Intersouth Affiliates V, L.P. |
— | 52,834 | 262,747 | $ | 15,479 | $ | 4,316 | $ | 50,984 | |||||||||||||||
| Intersouth Partners IV, L.P. |
227,738 | 1,057,019 | 5,678,164 | $ | 298,093 | $ | 83,118 | $ | 1,160,516 | |||||||||||||||
| Intersouth Partners V, L.P. |
— | 1,155,752 | 5,747,546 | $ | 338,604 | $ | 94,414 | $ | 1,115,258 | |||||||||||||||
| (7) | These amounts are distributed among the entities set forth in the table below. Dr. Underdown, one of our directors, is the Managing Director of Lumira Capital Corp. and is affiliated with these entities. |
| Name |
Shares of Series B Preferred Stock |
Shares of Series C Preferred Stock |
Cancelled Convertible Note Principal Amount |
Cancelled Convertible Note Interest Amount |
Cash Purchase Price |
|||||||||||||||
| Lumira Capital Corp. |
470,834 | 976,868 | $ | 175,126 | $ | 48,830 | — | |||||||||||||
| Lumira Capital I Limited Partnership |
155,273 | 15,059,663 | — | — | $ | 3,697,350 | ||||||||||||||
| Lumira Capital I Quebec Limited Partnership |
424,375 | 5,305,820 | — | — | $ | 1,302,650 | ||||||||||||||
| (8) | Dr. Birner, one of our directors, is a general partner of TVM Capital and is affiliated with this entity. |
2010 Convertible Note and Warrant Financing
In September and December 2010, we sold convertible promissory notes in the aggregate principal amount of $6.0 million in a private placement to certain of our existing holders of preferred stock. We refer to these notes as the 2010 convertible notes. The 2010 convertible notes accrue interest at a rate equal to 10% per year, and have a maturity date of September 15, 2011, unless converted prior thereto. No payments of principal or interest have been made under these notes. In July 2011, we amended the 2010 convertible notes to extend the maturity date to December 31, 2011 and to provide that all principal and accrued interest on the 2010 convertible notes will automatically convert into shares of our common stock upon the closing of this offering, at a conversion price per share equal to the price per share at which our common stock is sold to the public in this offering, if investors holding at least a majority-in-interest of the aggregate principal amount of the then outstanding 2010 convertible notes so elect.
150
Table of Contents
In connection with the issuance and sale of the 2010 convertible notes, we issued warrants to the purchasers of the 2010 convertible notes to purchase an aggregate of 20,759,953 shares of our series C preferred stock with an exercise price of $0.01 per share. The warrants have a term of seven years and will be automatically exercised by net exercise upon the closing of this offering.
The following table sets forth the aggregate original principal amount of the 2010 convertible notes purchased by our directors, executive officers and holders of more than 5% of our voting securities, and affiliates or immediate family members of our directors, executive officers and holders of more than 5% of our voting securities, and the number of shares of series C preferred stock underlying the warrants issued in connection with the purchase of the 2010 convertible notes.
| Name |
Original Principal Amount of Notes |
Number of Warrant Shares | ||||||
| Entities affiliated with The Aurora Funds(1) |
$ | 264,541 | (1) | 915,310 | ||||
| Caisse de dépôt et de placement du Québec(2) |
$ | 605,433 | 2,094,795 | |||||
| Coöperatieve AAC LS U.A. (Forbion)(3) |
$ | 1,023,048 | 3,539,740 | |||||
| Entities affiliated with Intersouth Partners(4) |
$ | 772,509 | (2) | 2,672,877 | ||||
| Entities affiliated with Lumira Capital(5) |
$ | 1,243,939 | (3) | 4,304,017 | ||||
| TVM V Life Science Ventures GmbH & Co. KG(6) |
$ | 1,436,319 | 4,969,652 | |||||
| Jeffrey D. Abbey |
$ | 2,263 | 7,831 | |||||
| Frederick M. Miesowicz, Ph.D. |
$ | 1,132 | 3,916 | |||||
| Charles A. Nicolette, Ph.D |
$ | 22,263 | 77,031 | |||||
| (1) | These amounts are distributed among the entities set forth in the table below. Mr. Clark, one of our directors, is the Managing General Partner of Aurora Funds, Inc. and is affiliated with these entities. |
| Name |
Original Principal Amount of Notes |
Number of Warrant Shares | ||||||
| Aurora Ventures II, LLC |
$ | 20,000 | 69,200 | |||||
| Harbinger/Aurora QP Venture Fund, LLC |
$ | 144,531 | 500,075 | |||||
| Harbinger/Aurora Venture Fund, LLC |
$ | 100,010 | 346,034 | |||||
| (2) | Ms. Laliberté, one of our directors, is the Investment Manager, Private Equity of Caisse de dépôt et de placement du Québec. |
| (3) | Dr. van Deventer, one of our directors, is a General Partner of Forbion Capital Partners and is affiliated with this entity. |
| (4) | These amounts are distributed among the entities set forth in the table below. Mr. Tracy, one of our directors, is a Venture Partner at Intersouth Partners and is affiliated with these entities. |
| Name |
Original Principal Amount of Notes |
Number of Warrant Shares | ||||||
| Intersouth Affiliates V, L.P. |
$ | 17,354 | 60,045 | |||||
| Intersouth Partners IV, L.P. |
$ | 375,536 | 1,299,352 | |||||
| Intersouth Partners V, L.P. |
$ | 379,619 | 1,313,479 | |||||
| (5) | These amounts are distributed among the entities set forth in the table below. Dr. Underdown, one of our directors, is the Managing Director of Lumira Capital Corp. and an affiliate of these entities. |
| Name |
Original Principal Amount of Notes |
Number of Warrant Shares | ||||||
| Lumira Capital Corp. |
$ | 108,113 | 374,070 | |||||
| Lumira Capital I Limited Partnership |
$ | 839,909 | 2,906,078 | |||||
| Lumira Capital I Quebec Limited Partnership |
$ | 295,917 | 1,023,869 | |||||
| (6) | Dr. Birner, one of our directors, is a general partner of TVM Capital and is affiliated with this entity. |
151
Table of Contents
2011 Convertible Note and Warrant Financing
In July 2011, we sold convertible promissory notes in the aggregate principal amount of $3.5 million in a private placement to certain of our existing holders of preferred stock. We refer to these notes as the July 2011 convertible notes. The July 2011 convertible notes accrue interest at a rate equal to 10% per year, and have a maturity date of December 31, 2011, unless converted prior thereto. No payments of principal or interest have been made under these notes. All principal and accrued interest on the July 2011 convertible notes will automatically convert into shares of our common stock, at a conversion price per share equal to the price per share at which our common stock is sold to the public in this offering, if investors holding at least a majority-in-interest of the aggregate principal amount of the then outstanding July 2011 convertible notes so elect.
In connection with the issuance and sale of the July 2011 convertible notes, we issued warrants to the purchasers of the July 2011 convertible notes to purchase an aggregate of 12,109,975 shares of our series C preferred stock with an exercise price of $0.01 per share. The warrants have a term of seven years and will be automatically exercised upon the closing of this offering by net exercise.
The following table sets forth the aggregate original principal amount of the July 2011 convertible notes purchased by entities affiliated with our directors and our 5% stockholders and their affiliates, and the number of shares of series C preferred stock underlying the warrants issued in connection with the purchase of the July 2011 convertible notes.
| Name |
Original Principal Amount of Notes |
Number of Warrant Shares | ||||||
| Entities affiliated with The Aurora Funds(1) |
$ | 171,139 | 592,140 | |||||
| Caisse de dépôt et de placement du Québec(2) |
$ | 391,672 | 1,355,182 | |||||
| Coöperatieve AAC LS U.A. (Forbion)(3) |
$ | 458,610 | 1,586,787 | |||||
| Entities affiliated with Intersouth Partners(4) |
$ | 499,758 | 1,729,159 | |||||
| Entities affiliated with Lumira Capital(5) |
$ | 689,300 | 2,384,973 | |||||
| TVM V Life Science Ventures GmbH & Co. KG(6) |
$ | 697,786 | 2,414,332 | |||||
| Jeffrey D. Abbey |
$ | 1,464 | 5,066 | |||||
| Frederick M. Miesowicz, Ph.D. |
$ | 732 | 2,533 | |||||
| Charles A. Nicolette, Ph.D. |
$ | 20,000 | 69,200 | |||||
| (1) | These amounts are distributed among the entities set forth in the table below. Mr. Clark, one of our directors, is the Managing General Partner of Aurora Funds, Inc. and is affiliated with these entities. |
| Name |
Original Principal Amount of Notes |
Number of Warrant Shares | ||||||
| Harbinger/Aurora QP Venture Fund, LLC |
$ | 101,148 | 349,972 | |||||
| Harbinger/Aurora Venture Fund, LLC |
$ | 69,991 | 242,168 | |||||
| (2) | Ms. Laliberté, one of our directors, is the Investment Manager, Private Equity of Caisse de dépôt et de placement du Québec. |
| (3) | Dr. van Deventer, one of our directors, is a General Partner of Forbion Capital Partners and is affiliated with this entity. |
| (4) | These amounts are distributed among the entities set forth in the table below. Mr. Tracy, one of our directors, is a Venture Partner at Intersouth Partners and is affiliated with these entities. |
| Name |
Original Principal Amount of Notes |
Number of Warrant Shares | ||||||
| Intersouth Affiliates V, L.P. |
$ | 11,227 | 38,845 | |||||
| Intersouth Partners IV, L.P. |
$ | 242,945 | 840,588 | |||||
| Intersouth Partners V, L.P. |
$ | 245,586 | 849,727 | |||||
152
Table of Contents
| (5) | These amounts are distributed among the entities set forth in the table below. Dr. Underdown, one of our directors, is the Managing Director of Lumira Capital Corp. and is affiliated with these entities. |
| Name |
Original Principal Amount of Notes |
Number of Warrant Shares | ||||||
| Lumira Capital Corp. |
$ | 85,784 | 296,812 | |||||
| Lumira Capital I Limited Partnership |
$ | 445,838 | 1,542,597 | |||||
| Lumira Capital I Quebec Limited Partnership |
$ | 157,678 | 545,564 | |||||
| (6) | Dr. Birner, one of our directors, is a general partner of TVM Capital and is affiliated with this entity. |
Kirin Collaboration and License Agreement
In 2004, we entered into the Kirin agreement with Kirin, which is also a stockholder. Under this agreement, we and Kirin agreed to share all related worldwide research and development costs and profits relating to the development of AGS-003 and AGS-004. Pursuant to the Kirin agreement Kirin agreed to reimburse us on a quarterly basis for our direct costs and services related to specified development and manufacturing activities and for allocated internal time at a designated full time equivalent rate. In 2008 and 2009, we received reimbursements under the Kirin agreement of $8.1 million and $6.6 million, respectively. We and Kirin agreed to terminate the Kirin agreement effective December 31, 2009. Under the termination agreement, Kirin assigned to us its ownership interest in specified jointly owned patent rights. In return, we agreed to pay Kirin low single-digit percentage royalties on net sales of specified AGS-003 and AGS-004 products beginning with their first commercial sale and continuing for a specified period thereafter. We have not paid any such amounts to date.
Agreements With Our Stockholders
We have entered into a registration rights agreement with purchasers of our series A preferred stock, series B preferred stock, series B-1 preferred stock and series C preferred stock. The registration rights agreement provides those holders with the right to demand that we file a registration statement, subject to certain limitations, and to request that their shares be covered by a registration statement that we are otherwise filing. See “Description of Capital Stock — Registration Rights” for additional information.
We have also entered into a stockholders’ agreement with certain purchasers of our common stock, series A preferred stock, series B preferred stock, series B-1 preferred stock and series C preferred stock. The stockholders’ agreement provides for rights of first refusal in respect of sales of securities by certain holders of our common stock and preferred stock. The stockholders’ agreement also provides holders of our series C preferred stock with co-sale rights and with a participation right to purchase their pro rata share of new securities that the Company may propose to sell and issue, subject to specified exceptions. The stockholders’ agreement also contains provisions with respect to the election of our board of directors and its composition. The rights under this agreement will terminate upon the closing of this offering.
Indemnification Agreements
Our certificate of incorporation provides that we will indemnify our directors and officers to the fullest extent permitted by Delaware law. In addition, we plan to enter into indemnification agreements with our directors and executive officers. See “Executive Compensation — Limitation of Liability and Indemnification” for additional information regarding these agreements.
Policies and Procedures for Related Person Transactions
Our board of directors has adopted written policies and procedures for the review of any transaction, arrangement or relationship in which Argos is a participant, the amount involved exceeds $120,000, and one of our executive officers, directors, director nominees or 5% stockholders, or their immediate family members, each of whom we refer to as a “related person,” has a direct or indirect material interest.
153
Table of Contents
If a related person proposes to enter into such a transaction, arrangement or relationship, which we refer to as a “related person transaction,” the related person must report the proposed related person transaction to our chief executive officer. The policy calls for the proposed related person transaction to be reviewed and, if deemed appropriate, approved by our audit committee. Whenever practicable, the reporting, review and approval will occur prior to entry into the transaction. If advance review and approval is not practicable, the committee will review, and, in its discretion, may ratify the related person transaction. The policy also permits the chairman of the committee to review and, if deemed appropriate, approve proposed related person transactions that arise between committee meetings, subject to ratification by the committee at its next meeting. Any related person transactions that are ongoing in nature will be reviewed annually.
A related person transaction reviewed under the policy will be considered approved or ratified if it is authorized by the committee after full disclosure of the related person’s interest in the transaction. As appropriate for the circumstances, the committee will review and consider:
| • | the related person’s interest in the related person transaction; |
| • | the approximate dollar value of the amount involved in the related person transaction; |
| • | the approximate dollar value of the amount of the related person’s interest in the transaction without regard to the amount of any profit or loss; |
| • | whether the transaction was undertaken in the ordinary course of our business; |
| • | whether the terms of the transaction are no less favorable to us than terms that could have been reached with an unrelated third party; |
| • | the purpose of, and the potential benefits to us of, the transaction; and |
| • | any other information regarding the related person transaction or the related person in the context of the proposed transaction that would be material to investors in light of the circumstances of the particular transaction. |
The committee may approve or ratify the transaction only if the committee determines that, under all of the circumstances, the transaction is in, or is not inconsistent with, Argos’ best interests. The committee may impose any conditions on the related person transaction that it deems appropriate.
In addition to the transactions that are excluded by the instructions to the SEC’s related person transaction disclosure rule, our board of directors has determined that the following transactions do not create a material direct or indirect interest on behalf of related persons and, therefore, are not related person transactions for purposes of this policy:
| • | interests arising solely from the related person’s position as an executive officer of another entity (whether or not the person is also a director of such entity), that is a participant in the transaction, where (a) the related person and all other related persons own in the aggregate less than a 10% equity interest in such entity, (b) the related person and his or her immediate family members are not involved in the negotiation of the terms of the transaction and do not receive any special benefits as a result of the transaction, and (c) the amount involved in the transaction equals less than the greater of $200,000 dollars or 5% of the annual gross revenues of the other entity that is a party to the transaction; and |
| • | a transaction that is specifically contemplated by provisions of our charter or bylaws. |
The policy provides that transactions involving compensation of executive officers shall be reviewed and approved by the compensation committee in the manner specified in its charter.
154
Table of Contents
The following table sets forth information with respect to the beneficial ownership of our common stock, as of August 31, 2011 by:
| • | each of our directors; |
| • | each of our named executive officers; |
| • | all of our directors and executive officers as a group; and |
| • | each person, or group of affiliated persons, who is known by us to beneficially own more than 5% of our common stock. |
The column entitled “Percentage of Shares Beneficially Owned — Before Offering” is based on a total of shares of our common stock outstanding as of August 31, 2011, assuming:
| • | the automatic conversion of all outstanding shares of our preferred stock into an aggregate of 157,871,412 shares of our common stock upon the closing of this offering; |
| • | the automatic conversion of all principal and accrued interest on the 2010 convertible notes and the July 2011 convertible notes, upon the closing of this offering, into an aggregate of shares of our common stock, at the initial public offering price, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011; |
| • | the issuance of an aggregate of shares of our common stock upon the automatic exercise of outstanding warrants to purchase shares of our series C preferred stock by net exercise at an exercise price of $0.01 per share upon the closing of this offering, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus; and |
| • | the issuance of an aggregate of 4,609,457 shares of our common stock to specified holders of the capital stock of DC Bio in exchange for their shares of DC Bio upon the closing of this offering. |
The column entitled “Percentage of Shares Beneficially Owned — After Offering” also gives effect to the shares of our common stock that we are selling in this offering.
Beneficial ownership is determined in accordance with the rules and regulations of the SEC and includes voting or investment power with respect to our common stock. Shares of our common stock subject to options or warrants that are currently exercisable or exercisable within 60 days of August 31, 2011 are considered outstanding and beneficially owned by the person holding the options or warrants for the purpose of calculating the percentage ownership of that person but not for the purpose of calculating the percentage ownership of any other person. Except as otherwise noted, the persons and entities in this table have sole voting and investing power with respect to all of the shares of our common stock beneficially owned by them, subject to community property laws, where applicable.
155
Table of Contents
Except as otherwise set forth in the footnotes below, the address of the beneficial owner is c/o Argos Therapeutics, Inc., 4233 Technology Drive, Durham, North Carolina 27704.
| Name and Address of Beneficial Owner |
Number of Shares Beneficially Owned |
Percentage of
Shares Beneficially Owned | ||||
| Before Offering |
After Offering | |||||
| 5% Stockholders: |
||||||
| Entities affiliated with The Aurora Funds(1) |
||||||
| Caisse de dépôt et placement du Québec(2) |
||||||
| Entities affiliated with Intersouth Partners(3) |
||||||
| Entities affiliated with Lumira Capital(4) |
||||||
| Coöperatieve AAC LS U.A. (Forbion)(5) |
||||||
| TVM V Life Science Ventures GmbH &Co. KG(6) |
||||||
| Directors and Named Executive Officers |
||||||
| Jeffrey D. Abbey(7) |
||||||
| John N. Bonfiglio, Ph.D.(8) |
||||||
| Lori R. Harrelson(9) |
||||||
| Timothy W. Trost(10) |
||||||
| Frederick M. Miesowicz, Ph.D.(11) |
||||||
| Charles A. Nicolette, Ph.D.(12) |
||||||
| Hubert Birner, Ph.D.(6) |
||||||
| B. Jefferson Clark(1) |
||||||
| Michel Gréco(13) |
||||||
| Monique Laliberté (2) |
||||||
| Philip R. Tracy (3) |
||||||
| Brian J. Underdown, Ph.D.(4) |
||||||
| Sander van Deventer, M.D., Ph.D.(14) |
||||||
| All current executive officers and directors as a group (11 persons)(15) |
||||||
| * | Represents beneficial ownership of less than one percent of our outstanding common stock. |
| (1) | The address for the Aurora Funds is 3100 Tower Blvd, Ste 1600, Durham, North Carolina 27077. Consists of (i) 288,071 shares of common stock issuable upon conversion of shares of series A preferred stock held by Aurora Ventures II, LLC, (ii) 570,511 shares of common stock issuable upon conversion of shares of series B preferred stock held by Aurora Ventures II, LLC, (iii) 679,950 shares of common stock issuable upon conversion of shares of series C preferred stock held by Aurora Ventures II, LLC, (iv) shares of common stock issuable upon exercise of warrants held by Aurora Ventures II, LLC, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, (v) shares of common stock issuable upon conversion of convertible notes held by Aurora Ventures II, LLC, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011, (vi) 1,835,079 shares of common stock issuable upon conversion of shares of series B preferred stock held by Harbinger/Aurora QP Venture Fund, LLC, (vii) 2,036,026 shares of common stock issuable upon conversion of shares of series C preferred stock held by Harbinger/Aurora QP Venture Fund, LLC, (viii) shares of common stock issuable upon exercise of warrants held by Harbinger/Aurora QP Venture Fund, LLC, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, (ix) shares of common stock issuable upon conversion of convertible notes held by Harbinger/Aurora QP Venture Fund, LLC assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on September 30, 2011, (x) 1,269,810 shares of common stock issuable upon conversion of shares of series B preferred stock held by Harbinger/ |
156
Table of Contents
| Aurora Venture Fund, LLC, (xi) 1,408,857 shares of common stock issuable upon conversion of shares of series C preferred stock held by Harbinger/Aurora Venture Fund, LLC, (xii) shares of common stock issuable upon exercise of warrants held by Harbinger/Aurora Venture Fund, LLC, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and (xiii) shares of common stock issuable upon conversion of convertible notes held by Harbinger/Aurora Venture Fund, LLC, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011. Aurora Management II, LLC is the managing member of Aurora Ventures II, LLC and has voting and investment power with respect to all shares held by Aurora Ventures II, LLC, which is exercised by an investment committee consisting of B. Jefferson Clark and M. Scott Albert, none of whom has individual voting and investment power with respect to such shares and each of whom disclaims beneficial ownership of such shares except to the extent of such person’s pecuniary interest therein. Harbinger/Aurora Ventures, LLC is the managing member of Harbinger/Aurora QP Venture Fund, LLC and Harbinger/Aurora Venture Fund, LLC and has voting and investment power with respect to all shares held by those entities, which is exercised by an investment committee consisting of Mr. Clark, Mr. Albert and William W. Brooke, none of whom has individual voting and investment power with respect to such shares and each of whom disclaims beneficial ownership of such shares except to the extent of such person’s pecuniary interest therein. Mr. Clark, a member of our board of directors, is also the president of Harbinger/Aurora Ventures, LLC and the president of Aurora Management II, LLC. Mr. Clark disclaims beneficial ownership of all of the shares held by the foregoing funds except to the extent of his pecuniary interest therein. |
| (2) | The address for Caisse de dépôt et placement du Québec is 1000, place Jean-Paul-Riopelle Montréal, Québec, H2Z 2B3. Consists of (i) 6,209,779 shares of common stock issuable upon conversion of shares of series B preferred stock, (ii) 12,301,263 shares of common stock issuable upon conversion of shares of series C preferred stock, (iii) shares of common stock issuable upon exercise of warrants, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and (iv) shares of common stock issuable upon conversion of convertible notes, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011. An investment committee consisting of Robert Côté, Pierre Fortier, Marcel Gagnon, Luc Houle, Normand Provost and Cyrille Vittecoq has voting and dispositive power over the shares held by Caisse de dépôt et placement du Québec. None of these individuals have individual voting and investment power with respect to these shares and each disclaims beneficial ownership of such shares. Monique Laliberté, a member of our board of directors, is Investment Manager, Private Equity of Caisse de dépôt et placement du Québec. Ms. Laliberté disclaims beneficial ownership of these shares except to the extent of her pecuniary interest therein. |
| (3) | The address for Intersouth Partners is 406 Blackwell Street, Suite 200, Durham, North Carolina 27701. Consists of (i) 267,850 shares of common stock issuable upon conversion of shares of series B preferred stock held by Intersouth Affiliates V, L.P., (ii) 262,747 shares of common stock issuable upon conversion of shares of series C preferred stock held by Intersouth Affiliates V, L.P., (iii) shares of common stock issuable upon exercise of warrants held by Intersouth Affiliates V, L.P., assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, (iv) shares of common stock issuable upon conversion of convertible notes held by Intersouth Affiliates V, L.P., assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011, (v) 575,961 shares of common stock issuable upon conversion of shares of series A preferred stock held by Intersouth Partners IV, L.P., (vi) 5,227,845 shares of common stock issuable upon conversion of shares of series B preferred stock held by Intersouth Partners IV, L.P., (vii) 5,678,164 shares of common stock issuable upon conversion of shares of series C preferred stock held by Intersouth Partners IV, L.P., (viii) shares of common stock issuable upon exercise of warrants held by Intersouth Partners IV, L.P., assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, (ix) shares of common stock issuable upon conversion of convertible notes held by Intersouth Partners IV, L.P., assuming an initial public |
157
Table of Contents
| offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011, (x) 5,859,261 shares of common stock issuable upon conversion of shares of series B preferred stock held by Intersouth Partners V, L.P., (xi) 5,747,546 shares of common stock issuable upon conversion of shares of series C preferred stock held by Intersouth Partners V, L.P., (xii) shares of common stock issuable upon exercise of warrants held by Intersouth Affiliates V, L.P., assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and (xiii) shares of common stock issuable upon conversion of convertible notes held by Intersouth Partners V, L.P., assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011. Intersouth Associates V LLC, the general partner of each of Intersouth Partners V, L.P. and Intersouth Affiliates V, L.P., and Intersouth Associates IV LLC, the general partner of Intersouth Partners IV, L.P., may be deemed to share voting and dispositive power over the shares held by each of Intersouth Affiliates V, L.P. and Intersouth Partners V, L.P. and Intersouth Partners IV, L.P., respectively. Dennis Dougherty and Mitch Mumma are both Member Managers of Intersouth Associates V, LLC, and Intersouth Associates IV, LLC and share voting and investment power over the shares held by Intersouth Partners V, L.P. and Intersouth Affiliates V, L.P. and Intersouth Partners IV, L.P. Philip R. Tracy, a member of our board of directors, is a member of Intersouth Associates V LLC and Intersouth Associates IV LLC and, pursuant to powers of attorney granted by each of Intersouth Associates V LLC and Intersouth Associates IV LLC, shares voting and investment power with respect to the shares held by Intersouth Partners V, L.P., Intersouth Affiliates V, L.P., and Intersouth Partners IV, L.P. Mr. Tracy disclaims beneficial ownership of all of the shares held by the foregoing funds except to the extent of his pecuniary interest therein. |
| (4) | The address of Lumira Capital is 141 Adelaide Street West, Suite 770, Toronto, Ontario M5H 3L5. Consists of (i) 2,328,668 shares of common stock issuable upon conversion of shares of series B preferred stock held by Lumira Capital Corp., (ii) 976,868 shares of common stock issuable upon conversion of shares of series C preferred stock held by Lumira Capital Corp., (iii) shares of common stock issuable upon exercise of warrants held by Lumira Capital Corp., assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, (iv) shares of common stock issuable upon conversion of convertible notes held by Lumira Capital Corp., assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011, (v) 2,119,948 shares of common stock issuable upon conversion of shares of series B preferred stock held by Lumira Capital I Limited Partnership, (vi) 15,059,663 shares of common stock issuable upon conversion of shares of series C preferred stock held by Lumira Capital I Limited Partnership, (vii) shares of common stock issuable upon exercise of warrants held by Lumira Capital I Limited Partnership, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, (viii) shares of common stock issuable upon conversion of convertible notes held by Lumira Capital I Limited Partnership, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011, (ix) 746,900 shares of common stock issuable upon conversion of shares of series B preferred stock held by Lumira Capital I Quebec Limited Partnership, (x) 5,305,820 shares of common stock issuable upon conversion of shares of series C preferred stock held by Lumira Capital I Quebec Limited Partnership, (xi) shares of common stock issuable upon exercise of warrants held by Lumira Capital I Quebec Limited Partnership, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and (xii) shares of common stock issuable upon conversion of convertible notes held by Lumira Capital I Quebec Limited Partnership, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011. Lumira Capital I Limited Partnership, or CI, and Lumira Capital I Quebec Limited Partnership, or CQ, are investment funds, or Lumira Funds, Lumira Capital Corp., or LCC, and its wholly-owned subsidiaries provide investment management services to the Lumira Funds. In the case of CQ: Lumira Capital I (QGP) Inc., which is the general partner of CQ and a wholly-owned subsidiary of LCC, has voting and investment power over the shares held by CQ. Such investment and voting power is |
158
Table of Contents
| exercised, based on the recommendations of the LCC investment committee (members listed below), by the board of directors of Lumira Capital I (QGP) Inc., being: Bernard Coupal; Jean Page; Maurice Forget; Murray Ducharme; Peter van der Velden. None of the foregoing persons has individual voting or investment power with respect to such shares and each disclaims beneficial ownership of such shares except to the extent of such person’s pecuniary interest therein. In the case of CI: LCC, acting as the Manager, has voting and investment power over the securities held by CI, which is exercised by the LCC investment committee (members listed below). In the case of LCC: voting and investment power over the securities held by LCC is exercised by the LCC investment committee (members listed below). The LCC investment committee is currently composed of the following persons, none of whom has individual voting or investment power with respect to the shares held by Funds or LCC, and each of whom disclaims beneficial ownership of such shares except to the extent of such person’s interest therein: Gerald Brunk; Stephen Cummings; Daniel Hetu; Benjamin Rovinski; Nandini Tandon; Brian Underdown; Peter van der Velden. Brian J. Underdown, a member of our board of directors, is a Managing Director of Lumira Capital Corp and a member of its investment committee. Dr. Underdown disclaims beneficial ownership of all of the shares held by Lumira Funds except to the extent of his pecuniary interest therein. |
| (5) | The address of Forbion is Gooimeer 2-35 1411 DC Naarden, Netherlands. Consists of (i) 6,133,336 shares of common stock issuable upon conversion of shares of series B preferred stock, (ii) 13,650,847 shares of common stock issuable upon conversion of shares of series C preferred stock, (iii) shares of common stock issuable upon exercise of warrants, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and (iv) shares of common stock issuable upon conversion of convertible notes, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011. The director of Coöperatieve AAC LS U.A., Forbion I Management B.V., has voting and investment power over the shares held by Coöperatieve AAC LS U.A., which is exercised through Forbion I Management B.V.’s investment committee, consisting of P. A. Bergstein, H. A. Slootweg, M. A. van Osch, G. J. Mulder and S. J. H. van Deventer. None of the members of the investment committee has individual voting and investment power with respect to such shares, and the members disclaim beneficial ownership of such shares except to the extent of their proportionate pecuniary interests therein. Sander van Deventer, is also a member of our board of directors. |
| (6) | The address of TVM V Life Science Ventures GmbH &Co. KG is 101 Arch Street, Suite 1950, Boston, Massachusetts 02110. Consists of (i) 6,097,778 shares of common stock issuable upon conversion of shares of series B preferred stock, (ii) 26,322,097 shares of common stock issuable upon conversion of shares of series C preferred stock, (iii) shares of common stock issuable upon exercise of warrants, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and (iv) shares of common stock issuable upon conversion of convertible notes, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011. TVM V Life Sciences Venture Management GmbH & Co. KG is the managing limited partner of TVM V Life Science Ventures GmbH & Co. KG. TVM V Life Sciences Venture Management GmbH & Co. KG, acting through its investment committee, consisting of limited partners Hubert Birner, who serves as a member of our board of directors, Mark Cipriano, Stefan Fischer, Axel Polack, John DiBello, Alexandra Goll and Helmut Schühsler, has voting and investment power over the shares held by TVM V Life Science Ventures GmbH & Co. KG. None of the members of the investment committee have individual voting and investment power with respect to such shares and each of the members, including Dr. Birner, disclaims beneficial ownership of such shares except to the extent of such person’s pecuniary interest therein. |
| (7) | Consists of (i) 69,200 shares of common stock issuable upon conversion of shares of series C preferred stock, (ii) shares of common stock issuable upon exercise of warrants, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, (iii) shares of common stock issuable upon conversion of convertible notes, assuming an initial public offering price of $ per share, which is the midpoint of the price range |
159
Table of Contents
| set forth on the cover page of this prospectus, and that the closing occurs on , 2011, and (iv) 5,551,697 shares of common stock issuable upon exercise of options that are exercisable as of August 31, 2011 or will become exercisable within 60 days after such date. |
| (8) | Consists of (i) 193,760 shares of common stock issuable upon conversion of shares of series C preferred stock, (ii) shares of common stock issuable upon exercise of warrants, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and (iii) shares of common stock issuable upon conversion of convertible notes, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011. |
| (9) | Consists of 488,489 shares of common stock issuable upon exercise of options that are exercisable as of August 31, 2011 or will become exercisable within 60 days after such date. |
| (10) | Consists of (i) 69,200 shares of common stock issuable upon conversion of shares of series C preferred stock, (ii) shares of common stock issuable upon exercise of warrants, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and (iii) shares of common stock issuable upon conversion of convertible notes, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011. |
| (11) | Consists of (i) 34,600 shares of common stock issuable upon conversion of shares of series C preferred stock, (ii) shares of common stock issuable upon exercise of warrants, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, (iii) shares of common stock issuable upon conversion of convertible notes, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011, and (iv) 3,040,167 shares of common stock issuable upon exercise of options that are exercisable as of August 31, 2011 or will be come exercisable within 60 days after such date. |
| (12) | Consists of (i) 69,200 shares of common stock issuable upon conversion of shares of series C preferred stock, (ii) shares of common stock issuable upon exercise of warrants, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, (iii) shares of common stock issuable upon conversion of convertible notes, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011, and (iv) 3,614,747 shares of common stock issuable upon exercise of options that are exercisable as of August 31, 2011 or will be come exercisable within 60 days after such date. |
| (13) | Consists of 221,248 shares of common stock issuable upon exercise of options that are exercisable as of August 31, 2011 or will become exercisable within 60 days after such date. |
| (14) | Includes 1,260 shares of common stock issuable upon exercise of options that are exercisable as of August 31, 2011 or will become exercisable within 60 days after such date. Sander van Deventer, a member of our board of directors, is general partner of Coöperatieve AAC LS U.A. Dr. van Deventer disclaims beneficial ownership of these shares except to the extent of his pecuniary interest therein. Also includes the securities described in note 5 above. |
| (15) | Includes (i) 89,602,848 shares of common stock issuable upon conversion of shares of series C preferred stock, (ii) shares of common stock issuable upon exercise of warrants, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, (iii) shares of common stock issuable upon conversion of convertible notes, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011, and (iv) 12,917,608 shares of common stock issuable upon exercise of options that are exercisable as of August 31, 2011 or will become exercisable within 60 days after such date. |
160
Table of Contents
The following description of our capital stock and provisions of our certificate of incorporation and bylaws are summaries and are qualified by reference to the certificate of incorporation and the bylaws that will be in effect upon the closing of this offering and to the applicable provisions of the Delaware General Corporation Law. We have filed copies of these documents with the SEC as exhibits to our registration statement of which this prospectus forms a part. The description of the capital stock reflects changes to our capital structure that will occur upon the closing of this offering.
Upon the closing of this offering, our authorized capital stock will consist of shares of our common stock, par value $0.001 per share, and shares of our preferred stock, par value $0.001 per share, all of which preferred stock will be undesignated.
As of August 31, 2011, we had issued and outstanding:
| • | 16,578,213 shares of our common stock outstanding and held of record by 43 stockholders; |
| • | 1,276, 103 shares of our series A preferred stock that are convertible into 1,276,103 shares of our common stock and held of record by four stockholders; |
| • | 24,326,574 shares of our series B preferred stock that are convertible into 42,814,762 shares of our common stock and held of record by 16 stockholders; |
| • | 917,771 shares of our series B-1 preferred stock that are convertible into 1,615,276 shares of our common stock and held of record by one stockholder; and |
| • | 112,165,271 shares of our series C preferred stock that are convertible into 112,165,271 shares of our common stock and held of record by 32 stockholders. |
As of August 31, 2011, we also had outstanding:
| • | options to purchase 24,189,162 shares of our common stock at a weighted average exercise price of $0.08 per share; |
| • | warrants to purchase 5,617 shares of our common stock at a weighted average exercise price of $32.57 per share held by three holders; and |
| • | warrants to purchase 32,869,928 shares of our series C preferred stock at an exercise price of $0.01 per share. |
Upon the closing of this offering:
| • | all outstanding shares of our preferred stock will automatically convert into an aggregate of 157,871,412 shares of our common stock; |
| • | all principal and accrued interest on our outstanding convertible notes, including the 2010 convertible notes and the July 2011 convertible notes, will convert into an aggregate of shares of our common stock, at the initial public offering price, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011; |
| • | shares of our common stock will be issued upon the automatic exercise of outstanding warrants to purchase shares of our series C preferred stock by net exercise at an exercise price of $0.01 per share, |
161
Table of Contents
| assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus; and |
| • | 4,609,457 shares of our common stock will be issued to specified holders of the capital stock of DC Bio in exchange for their shares of DC Bio upon the closing of this offering, which will result in DC Bio becoming our wholly-owned subsidiary. |
Upon the closing of this offering and after giving effect to the automatic exercise of the Series C warrants by net exercise, warrants to purchase an aggregate of 5,617 shares of common stock will remain outstanding.
Common Stock
Holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders and do not have cumulative voting rights. An election of directors by our stockholders shall be determined by a plurality of the votes cast by the stockholders entitled to vote on the election. Holders of common stock are entitled to receive proportionately any dividends as may be declared by our board of directors, subject to any preferential dividend rights of outstanding preferred stock.
In the event of our liquidation or dissolution, the holders of common stock are entitled to receive proportionately all assets available for distribution to stockholders after the payment of all debts and other liabilities and subject to the prior rights of any outstanding preferred stock. Holders of common stock have no preemptive, subscription, redemption or conversion rights. The rights, preferences and privileges of holders of common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of preferred stock that we may designate and issue in the future.
Preferred Stock
Under the terms of our certificate of incorporation that will be effective upon the closing of this offering, our board of directors is authorized to issue shares of preferred stock in one or more series without stockholder approval. Our board of directors has the discretion to determine the rights, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences, of each series of preferred stock.
The purpose of authorizing our board of directors to issue preferred stock and determine its rights and preferences is to eliminate delays associated with a stockholder vote on specific issuances. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions, future financings and other corporate purposes, could have the effect of making it more difficult for a third party to acquire, or could discourage a third party from seeking to acquire, a majority of our outstanding voting stock. Upon the closing of this offering, there will be no shares of preferred stock outstanding, and we have no present plans to issue any shares of preferred stock.
Options
As of August 31, 2011, options to purchase 24,189,162 shares of our common stock at a weighted average exercise price of $0.08 per share were outstanding.
162
Table of Contents
Warrants
As of August 31, 2011, we had outstanding warrants to purchase an aggregate of 5,100 shares of our common stock at an exercise price of $18.00 per share held by two holders and warrants to purchase an aggregate of 517 shares of our common stock at an exercise price of $176.00 per share held by General Electric Capital Corporation.
Upon the closing of this offering:
| • | the warrants to purchase an aggregate of 5,100 shares of our common stock will remain outstanding and exercisable to purchase shares of our common stock at a weighted average exercise price of $18.00; and |
| • | the warrants to purchase an aggregate of 517 shares of our common stock will remain outstanding and exercisable to purchase shares of our common stock at a weighted average exercise price of $176.00. |
We have additional outstanding warrants to purchase an aggregate of 32,869,928 shares of series C preferred stock with an exercise price of $0.01 per share. We have assumed throughout this prospectus that these warrants will automatically be exercised by net exercise for shares of common stock upon the closing of this offering, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus.
Registration Rights
We have entered into a second amended and restated registration rights agreement, dated March 31, 2008, as amended, which we refer to as the registration rights agreement, with certain holders of shares of our registrable securities as described in the registration rights agreement. Upon the completion of this offering, holders of a total of shares of our common stock as of August 31, 2011, including shares of our common stock issuable upon conversion of our preferred stock and our convertible notes and upon exercise of our warrants to purchase shares of series C preferred stock, will have the right to require us to register these shares under the Securities Act under specified circumstances and will have incidental registration rights. After registration pursuant to these rights, these shares will become freely tradable without restriction under the Securities Act.
Demand Registration Rights
Beginning on the earlier of (i) March 31, 2012 or (ii) the 180th day after the effective date of the registration statement of which this prospectus forms a part, subject to specified limitations set forth in the registration rights agreement, at any time the holders of at least 25% of the then outstanding registrable securities, acting together, may demand in writing that we register the registrable securities under the Securities Act so long as the total amount of registrable securities registered have an aggregate proposed offering price of at least $3.0 million. We are not obligated to file a registration statement pursuant to this demand provision until 180 days after the completion of this offering, and on more than two occasions, subject to specified exceptions.
In addition, at any time after we become eligible to file a registration statement on Form S-3 under the Securities Act, subject to specified limitations, the holders of at least 15% of the registrable securities may demand in writing that we register on Form S-3 the registrable securities held by them so long as the total amount of registrable securities being registered have an aggregate offering price of at least $1.0 million. We are not obligated to file a Form S-3 pursuant to this provision within six months of the effective date of any other registration statement that we may file.
Incidental Registration Rights
If, at any time after the completion of this offering, we propose to file a registration statement to register any of our securities under the Securities Act, either for our own account or for the account of any of our
163
Table of Contents
stockholders, other than pursuant to the demand registration rights and Form S-3 registration rights described above, the holders of our registrable securities will be entitled to notice of registration and, subject to specified exceptions, we will be required to use our best efforts to register the registrable securities then held by them that they request that we register.
Expenses
Pursuant to the registration rights agreement, we are required to pay all registration expenses, including registration and filing fees, printing expenses, fees and disbursements of our counsel and accountants, fees and expenses incurred in connection with complying with state securities or “blue sky” laws, fees of the Financial Industry Regulatory Authority, Inc., transfer taxes, fees of transfer agents and registrations, any insurance costs and reasonable fees and disbursements of one counsel representing the selling stockholders, other than any underwriting discounts and commissions, related to any demand or incidental registration. The registration rights agreement contains customary cross-indemnification provisions, pursuant to which we are obligated to indemnify the selling stockholders in the event of material misstatements or omissions in the registration statement attributable to us, and they are obligated to indemnify us for material misstatements or omissions in the registration statement attributable to them.
Delaware Anti-Takeover Law and Certain Charter and Bylaws Provisions
Delaware Law
We are subject to Section 203 of the Delaware General Corporation Law. Subject to certain exceptions, Section 203 prevents a publicly held Delaware corporation from engaging in a “business combination” with any “interested stockholder” for three years following the date that the person became an interested stockholder, unless either the interested stockholder attained such status with the approval of our board of directors, the business combination is approved by our board of directors and stockholders in a prescribed manner or the interested stockholder acquired at least 85% of our outstanding voting stock in the transaction in which it became an interested stockholder. A “business combination” includes, among other things, a merger or consolidation involving us and the “interested stockholder” and the sale of more than 10% of our assets. In general, an “interested stockholder” is any entity or person beneficially owning 15% or more of our outstanding voting stock and any entity or person affiliated with or controlling or controlled by such entity or person. The restrictions contained in Section 203 are not applicable to any of our existing stockholders that will own 15% or more of our outstanding voting stock upon the closing of this offering.
Staggered Board
Our certificate of incorporation and our bylaws that will become effective upon the closing of this offering divides our board of directors into three classes with staggered three-year terms. In addition, our certificate of incorporation and our bylaws provide that directors may be removed only for cause and only by the affirmative vote of the holders of 75% of our shares of capital stock present in person or by proxy and entitled to vote. Under our certificate of incorporation and bylaws, any vacancy on our board of directors, including a vacancy resulting from an enlargement of our board of directors, may be filled only by vote of a majority of our directors then in office. Furthermore, our certificate of incorporation provides that the authorized number of directors may be changed only by the resolution of our board of directors. The classification of our board of directors and the limitations on the ability of our stockholders to remove directors, change the authorized number of directors and fill vacancies could make it more difficult for a third party to acquire, or discourage a third party from seeking to acquire, control of our company.
The classification of our board of directors and the limitations on the removal of directors and filling of vacancies could make it more difficult for a third party to acquire, or discourage a third party from seeking to acquire, control of our company.
164
Table of Contents
Super-Majority Voting
The Delaware General Corporation Law provides generally that the affirmative vote of a majority of the shares entitled to vote on any matter is required to amend a corporation’s certificate of incorporation or bylaws, unless a corporation’s certificate of incorporation or bylaws, as the case may be, requires a greater percentage. Our bylaws may be amended or repealed by a majority vote of our board of directors or the affirmative vote of the holders of at least 75% of the votes that all our stockholders would be entitled to cast in any annual election of directors. In addition, the affirmative vote of the holders of at least 75% of the votes that all our stockholders would be entitled to cast in any election of directors is required to amend or repeal or to adopt any provisions inconsistent with any of the provisions of our certificate of incorporation described above.
Stockholder Action; Special Meeting of Stockholders; Advance Notice Requirements for Stockholder Proposals and Director Nominations
Our certificate of incorporation and our bylaws provide that any action required or permitted to be taken by our stockholders at an annual meeting or special meeting of stockholders may only be taken if it is properly brought before such meeting and may not be taken by written action in lieu of a meeting. Our certificate of incorporation and our bylaws also provide that, except as otherwise required by law, special meetings of the stockholders can only be called by our chairman of the board, our president or chief executive officer or our board of directors. In addition, our bylaws establish an advance notice procedure for stockholder proposals to be brought before an annual meeting of stockholders, including proposed nominations of candidates for election to our board of directors. Stockholders at an annual meeting may only consider proposals or nominations specified in the notice of meeting or brought before the meeting by or at the direction of our board of directors, or by a stockholder of record on the record date for the meeting, who is entitled to vote at the meeting and who has delivered timely written notice in proper form to our secretary of the stockholder’s intention to bring such business before the meeting. These provisions could have the effect of delaying until the next stockholder meeting stockholder actions that are favored by the holders of a majority of our outstanding voting securities. These provisions also could discourage a third party from making a tender offer for our common stock, because even if it acquired a majority of our outstanding voting stock, it would be able to take action as a stockholder, such as electing new directors or approving a merger, only at a duly called stockholders meeting and not by written consent.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock will be .
NASDAQ Global Market Listing
We have applied to have our common stock listed on The NASDAQ Global Market under the symbol “ARGS.”
165
Table of Contents
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no public market for our common stock, and a liquid trading market for our common stock may not develop or be sustained after this offering. Future sales of substantial amounts of our common stock in the public market, including shares issued upon exercise of outstanding options and warrants or in the public market after this offering, or the anticipation of these sales, could adversely affect market prices prevailing from time to time and could impair our ability to raise capital through sales of equity securities.
Upon the closing of this offering, we will have outstanding an aggregate of shares of our common stock, after giving effect to:
| • | the automatic conversion of all outstanding shares of our preferred stock into an aggregate of 157,871,412 shares of our common stock upon the closing of this offering; |
| • | the automatic conversion of all principal and accrued interest on the 2010 convertible notes and the July 2011 convertible notes, upon the closing of this offering, into an aggregate of shares of our common stock, at the initial public offering price, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and that the closing occurs on , 2011; |
| • | the issuance of an aggregate of shares of our common stock upon the automatic exercise of outstanding warrants to purchase shares of our series C preferred stock by net exercise at an exercise price of $0.01 per share upon the closing of this offering, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus; and |
| • | the issuance of an aggregate of 4,609,457 shares of our common stock to specified holders of the capital stock of DC Bio in exchange for their shares of DC Bio upon the closing of this offering |
Of the shares to be outstanding immediately after the closing of this offering, we expect that the shares to be sold in this offering will be freely tradable without restriction under the Securities Act unless purchased by our “affiliates,” as that term is defined in Rule 144 under the Securities Act. The remaining shares of our common stock outstanding after this offering will be “restricted securities” under Rule 144, and we expect that substantially all of these restricted securities will be subject to the 180-day lock-up period under the lock-up agreements as described below. These restricted securities may be sold in the public market only if registered or pursuant to an exemption from registration, such as Rule 144 or Rule 701 under the Securities Act.
Rule 144
In general, under Rule 144, beginning 90 days after the date of this prospectus, any person who is not our affiliate and has held their shares for at least six months, including the holding period of any prior owner other than one of our affiliates, may sell shares without restriction, subject to the availability of current public information about us. In addition, under Rule 144, any person who is not our affiliate and has held their shares for at least one year, including the holding period of any prior owner other than one of our affiliates, would be entitled to sell an unlimited number of shares immediately upon the closing of this offering without regard to whether current public information about us is available.
Beginning 90 days after the date of this prospectus, a person who is our affiliate or who was our affiliate at any time during the preceding three months and who has beneficially owned restricted securities for at least six months, including the holding period of any prior owner other than one of our affiliates, is entitled to sell a number of shares within any three-month period that does not exceed the greater of:
| • | 1% of the number of shares of our common stock then outstanding; and |
166
Table of Contents
| • | the average weekly trading volume of our common stock on The NASDAQ Global Market during the four calendar weeks preceding the filing of a Notice of Proposed Sale of Securities Pursuant to Rule 144 with respect to the sale. |
Sales under Rule 144 by our affiliates are also subject to manner of sale provisions and notice requirements and to the availability of current public information about us.
Upon expiration of the 180-day lock-up period described below, approximately shares of our common stock will be eligible for sale under Rule 144, including shares eligible for resale immediately upon the closing of this offering as described above. We cannot estimate the number of shares of our common stock that our existing stockholders will elect to sell under Rule 144.
Rule 701
In general, under Rule 701 of the Securities Act, any of our employees, consultants or advisors, other than our affiliates, who purchased shares from us in connection with a qualified compensatory stock plan or other written agreement, is eligible to resell these shares 90 days after the date of this prospectus in reliance on Rule 144, but without compliance with the holding period requirements of Rule 144 and without regard to the volume of such sales or the availability of public information about us. Subject to the 180-day lock-up period described below, approximately shares of our common stock will be eligible for sale in accordance with Rule 701.
Lock-Up Agreements
We and each of our directors and executive officers and holders of substantially all of our outstanding stock and securities convertible into or exercisable for our stock, who collectively own shares of our common stock, based on shares outstanding as of August 31, 2011, have agreed that, without the prior written consent of Lazard Capital Markets LLC and Canaccord Genuity Inc. on behalf of the underwriters, we and they will not, subject to limited exceptions, during the period ending 180 days after the date of this prospectus, subject to extension in specified circumstances:
| • | offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of, any shares of common stock or any securities convertible into or exercisable or exchangeable for common stock or publicly disclose the intention to do any of the foregoing; |
| • | enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of our common stock or publicly disclose the intention to do any of the foregoing; or |
| • | make any demand for or exercise any right with respect to, the registration of any shares of common stock or any security convertible into or exercisable or exchangeable for common stock. |
The lock-up restrictions, specified exceptions and the circumstances under which the 180-day lock-up period may be extended are described in more detail under “Underwriting.”
Equity Plans
We intend to file one or more registration statements on Form S-8 under the Securities Act to register all shares of common stock subject to outstanding stock options and common stock issued or issuable under our stock plans. We expect to file the registration statement covering shares offered pursuant to our stock plans shortly after the date of this prospectus, permitting the resale of such shares by nonaffiliates in the public market without restriction under the Securities Act and the sale by affiliates in the public market, subject to compliance with the resale provisions of Rule 144.
167
Table of Contents
Registration Rights
Upon the closing of this offering, the holders of shares of our common stock as of August 31, 2011, including shares of our common stock issuable upon conversion of our preferred stock and our convertible notes and upon exercise of our warrants to purchase series C preferred stock, or their transferees will be entitled to various rights with respect to the registration of these shares under the Securities Act. Registration of these shares under the Securities Act would result in these shares becoming fully tradable without restriction under the Securities Act immediately upon the effectiveness of the registration, except for shares purchased by affiliates. See “Description of Capital Stock — Registration Rights” for additional information. Shares covered by a registration statement will be eligible for sale in the public market upon the expiration or release from the terms of the lock-up agreement.
168
Table of Contents
Subject to the terms and conditions set forth in an underwriting agreement dated the date of this prospectus among us and the underwriters named below, we have agreed to sell to the underwriters, and each underwriter has severally agreed to purchase from us, the number of shares of common stock listed next to its name in the following table. Lazard Capital Markets LLC and Canaccord Genuity Inc. are acting as joint book-running managers for the offering and as representatives of the underwriters.
| Underwriters |
Number of Shares | |
| Lazard Capital Markets LLC |
||
| Canaccord Genuity Inc. |
||
| Needham & Company, LLC |
||
| BMO Capital Markets Corp. |
||
| Total |
The underwriters are committed to purchase all the shares of common stock offered by us if they purchase any shares. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of nondefaulting underwriters may be increased or the offering may be terminated. The underwriters are not obligated to purchase the shares of common stock covered by the underwriters’ over-allotment option described below. The underwriters are offering the shares, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, and other conditions contained in the underwriting agreement, such as the receipt by the underwriters of officer’s certificates and legal opinions. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Discounts and Commissions
The underwriters propose initially to offer the shares to the public at the public offering price set forth on the cover page of this prospectus and to dealers at that price less a concession not in excess of $ per share. After the initial offering of the shares, the public offering price and other selling terms may be changed by the representatives.
The following table shows the public offering price, underwriting discounts and commissions and proceeds before expenses to us. The information assumes either no exercise or full exercise by the underwriters of their over-allotment option.
| Per Share | Without Option | With Option | ||||
| Public offering price |
||||||
| Underwriting discounts and commissions |
||||||
| Proceeds, before expenses, to us |
The total estimated expenses of the offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding underwriting discounts and commissions, are approximately $ and are payable by us.
Lazard Frères & Co. LLC referred this transaction to Lazard Capital Markets LLC and will receive a referral fee from Lazard Capital Markets LLC in connection therewith.
Over-Allotment Option
We have granted the underwriters an option to purchase up to additional shares of common stock at the public offering price, less underwriting discounts and commissions. The underwriters may exercise this option for 30 days from the date of this prospectus solely to cover sales of shares of common stock by the
169
Table of Contents
underwriters in excess of the total number of shares set forth in the table above. If any shares are purchased pursuant to this over-allotment option, the underwriters will purchase the additional shares in approximately the same proportions as shown in the table above. If any of these additional shares are purchased, the underwriters will offer the additional shares on the same terms as those on which the shares are being offered. We will pay the expenses associated with the exercise of the over-allotment option.
Initial Public Offering Pricing
Prior to this offering, there has been no public market for our common stock. The initial public offering price will be negotiated between us and the representatives of the underwriters. Among the factors considered in these negotiations are:
| • | the prospects for our company and the industry in which we operate; |
| • | our past and present financial and operating performance; |
| • | financial and operating information and market valuations of publicly traded companies engaged in activities similar to ours; |
| • | the prevailing conditions of U.S. securities markets at the time of this offering; and |
| • | other factors deemed relevant. |
The estimated initial public offering price range set forth on the cover of this preliminary prospectus is subject to change as a result of market conditions and other factors.
Lock-Up Agreements
We, our officers and directors and holders of substantially all of our outstanding stock, options and warrants have entered into lock-up agreements with the underwriters. Under these agreements, we and these other individuals have agreed, subject to specified exceptions, not to sell or transfer any common stock or securities convertible into, or exchangeable or exercisable for, common stock, during a period ending 180 days after the date of this prospectus, without first obtaining the written consent of Lazard Capital Markets LLC and Canaccord Genuity Inc. Specifically, we and these other individuals have agreed not to:
| • | offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of, any shares of common stock or any securities convertible into or exercisable or exchangeable for common stock or publicly disclose the intention to do any of the foregoing; |
| • | enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of our common stock or publicly disclose the intention to do any of the foregoing; or |
| • | make any demand for or exercise any right with respect to, the registration of any shares of common stock or any security convertible into or exercisable or exchangeable for common stock. |
The restrictions described above do not apply to:
| • | the sale of shares of common stock to the underwriters pursuant to the underwriting agreement; |
| • | the issuance by us of shares of common stock upon the exercise of an option or warrant or the conversion of a security outstanding on the date of this prospectus of which the underwriters have been advised in writing or that is described in this prospectus; |
170
Table of Contents
| • | the grant by us of stock options or other stock-based awards, or the issuance of shares of common stock upon exercise thereof, to eligible participants pursuant to employee benefit or equity incentive plans described in this prospectus, provided that, prior to the grant of any such stock options or other stock-based awards that vest within the restricted period, each recipient of such grant shall sign and deliver a lock-up agreement agreeing to be subject to the restrictions on transfer described above; |
| • | transactions by security holders relating to any shares of common stock or other securities acquired in open market transactions after the closing of this offering; |
| • | the exercise of stock options to purchase shares of common stock granted under an equity incentive plan or any warrants to purchase shares of common stock or any securities convertible into or exercisable or exchangeable for common stock; provided that any shares of common stock or such other securities obtained by such exercise shall remain subject to the terms of this letter; |
| • | the establishment of a 10b5-1 trading plan under the Exchange Act by a security holder for the sale of shares of common stock, provided that such plan does not provide for the transfer of common stock during the restricted period; |
| • | transfers by security holders of shares of common stock or other securities as a bona fide gift or by will or intestacy; |
| • | transfers by security holders of shares of common stock or other securities to any trust for the direct or indirect benefit of the security holder or the immediate family of the security holder; or |
| • | transfers by distribution by security holders of shares of common stock or other securities to partners, members, or shareholders of the security holder. |
provided that in the case of each of the preceding three types of transactions, the transfer does not involve a disposition for value and each transferee or distributee signs and delivers a lock-up agreement agreeing to be subject to the restrictions on transfer described above.
The 180-day restricted period is subject to extension if (1) during the last 17 days of the restricted period we issue an earnings release or material news or a material event relating to us occurs or (2) prior to the expiration of the restricted period, we announce that we will release earnings results during the 16-day period beginning on the last day of the restricted period, in which case the restrictions imposed in the lock-up agreements will continue to apply until the expiration of the 18-day period beginning on the issuance of the earnings release or the occurrence of the material news or material event.
Indemnification
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make for these liabilities.
NASDAQ Global Market Listing
We have applied to have our common stock listed on The NASDAQ Global Market under the symbol “ARGS.”
Price Stabilization, Short Positions and Penalty Bids
In order to facilitate the offering of our common stock, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of our common stock. In connection with the offering, the underwriters may purchase and sell our common stock in the open market. These transactions may include short
171
Table of Contents
sales, purchases on the open market to cover positions created by short sales and stabilizing transactions. Short sales involve the sale by the underwriters of a greater number of shares of common stock than they are required to purchase in the offering. “Covered” short sales are sales made in an amount not greater than the underwriters’ option to purchase additional shares of common stock in the offering. The underwriters may close out any covered short position by either exercising their over-allotment option or purchasing shares of common stock in the open market. In determining the source of shares of common stock to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. “Naked” short sales are sales in excess of the over-allotment option. The underwriters must close out any naked short position by purchasing shares of common stock in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of our common stock in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of shares of common stock made by the underwriters in the open market prior to the completion of the offering.
Similar to other purchase transactions, the underwriters’ purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As result, the price of our common stock may be higher than the price that might otherwise exist in the open market.
The underwriters have advised us that, pursuant to Regulation M of the Securities Act, they may also engage in other activities that stabilize, maintain or otherwise affect the price of our common stock, including the imposition of penalty bids. This means that if the representatives of the underwriters purchase common stock in the open market in stabilizing transactions or to cover short sales, the representatives can require the underwriters that sold those shares as part of this offering to repay the underwriting discount received by them.
The underwriters make no representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. In addition, neither we nor the underwriters make any representation that the underwriters will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
Electronic Offer, Sale and Distribution of Shares
A prospectus in electronic format may be made available on the websites maintained by one or more underwriters or selling group members, if any, participating in the offering. The underwriters may agree to allocate a number of shares of common stock to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the representatives to underwriters and selling group members that may make Internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on the underwriters’ websites and any information contained in any other website maintained by the underwriters is not part of this prospectus or the registration statement of which this prospectus forms a part.
Notice to Non-U.S. Investors
In relation to each member state of the European Economic Area that has implemented the Prospectus Directive, each of which we refer to as a relevant member state, with effect from and including the date on which the Prospectus Directive is implemented in that relevant member state, or the relevant implementation date, an offer of securities described in this prospectus may not be made to the public in that relevant member state other than:
| • | to legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities; |
172
Table of Contents
| • | to any legal entity that has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance sheet of more than €43,000,000 and (3) an annual net turnover of more than €50,000,000, as shown in its last annual or consolidated accounts; |
| • | to fewer than 100 natural or legal persons (other than qualified investors as defined in the Prospectus Directive) subject to obtaining the prior consent of representatives for any such offer; or |
| • | in any other circumstances that do not require the publication of a prospectus pursuant to Article 3 of the Prospectus Directive; |
provided that no such offer of securities shall require us or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Directive.
For the purposes of this provision, the expression an “offer of shares to the public” in relation to any shares of common stock in any relevant member state means the communication in any form and by any means of sufficient information on the terms of the offer and the shares to be offered so as to enable an investor to decide to purchase or subscribe for the shares, as the same may be varied in that member state by any measure implementing the Prospectus Directive in that member state and the expression “Prospectus Directive” means Directive 2003/71/EC and includes any relevant implementing measure in each relevant member state.
Other Relationships
From time to time, certain of the underwriters and their affiliates have provided, and may provide in the future, various advisory, investment and commercial banking and other services to us in the ordinary course of business, for which they have received and may continue to receive customary fees and commissions.
173
Table of Contents
The validity of the shares of common stock offered hereby is being passed upon for us by Wilmer Cutler Pickering Hale and Dorr LLP, Boston, Massachusetts. Goodwin Procter LLP is acting as counsel for the underwriters in connection with this offering.
The consolidated financial statements as of December 31, 2009 and December 31, 2010 and for each of the three years in the period ended December 31, 2010, included in this prospectus have been so included in reliance on the report of PricewaterhouseCoopers LLP, an Independent Registered Public Accounting Firm, given on the authority of said firm as experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of common stock we are offering to sell. This prospectus, which constitutes part of the registration statement, does not include all of the information contained in the registration statement and the exhibits, schedules and amendments to the registration statement. For further information with respect to us and our common stock, we refer you to the registration statement and to the exhibits and schedules to the registration statement. Statements contained in this prospectus about the contents of any contract, agreement or other document are not necessarily complete, and, in each instance, we refer you to the copy of the contract, agreement or other document filed as an exhibit to the registration statement. Each of theses statements is qualified in all respects by this reference.
You may read and copy the registration statement of which this prospectus is a part at the SEC’s public reference room, which is located at 100 F Street, N.E., Room 1580, Washington, DC 20549. You can request copies of the registration statement by writing to the Securities and Exchange Commission and paying a fee for the copying cost. Please call the SEC at 1-800-SEC-0330 for more information about the operation of the SEC’s public reference room. In addition, the SEC maintains an Internet website, which is located at http://www.sec.gov, that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. You may access the registration statement of which this prospectus is a part at the SEC’s Internet website. Upon completion of this offering, we will be subject to the information reporting requirements of the Securities Exchange Act of 1934, and we will file reports, proxy statements and other information with the SEC.
174
Table of Contents
(A Development Stage Company)
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Page | ||||
| F-2 | ||||
| Consolidated Financial Statements: |
||||
| F-3 | ||||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-8 | ||||
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Argos Therapeutics, Inc.
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of operations, of changes in stockholders’ deficit and of cash flows present fairly, in all material respects, the financial position of Argos Therapeutics, Inc. and its subsidiaries (a development stage company) at December 31, 2009 and 2010 and the results of their operations and their cash flows for each of the three years ended December 31, 2010 in conformity with accounting principles generally accepted in the United States of America. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As more fully described in Note 1, the Company has incurred operating losses and negative cash flows from operations since inception and will be required to obtain additional financing, alternative means of financial support or both prior to December 31, 2011 in order to fund its operations. These factors raise substantial doubt about its ability to continue as a going concern. Management’s plans in regards to these matters are also described in Note 1. The accompanying financial statements do not include any adjustments that might result from the outcome of this uncertainty.
July 29, 2011
/s/ PricewaterhouseCoopers LLP
Raleigh, North Carolina
F-2
Table of Contents
(A Development Stage Company)
Consolidated Balance Sheets
| December 31, | June
30, 2011 |
|||||||||||
| 2009 | 2010 | |||||||||||
| (unaudited) | ||||||||||||
| Assets |
||||||||||||
| Current assets |
||||||||||||
| Cash and cash equivalents |
$ | 7,314,499 | $ | 5,141,965 | $ | 1,359,340 | ||||||
| Short-term investments |
3,270,934 | — | — | |||||||||
| Prepaid expenses and interest receivable |
372,987 | 371,877 | 198,355 | |||||||||
| Other receivables |
1,241,445 | 3,776,151 | 3,881,085 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current assets |
12,199,865 | 9,289,993 | 5,438,780 | |||||||||
| Furniture, fixtures and equipment, net |
2,045,912 | 1,344,571 | 966,728 | |||||||||
| Other assets |
17,217 | 7,217 | 2,217 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total assets |
$ | 14,262,994 | $ | 10,641,781 | $ | 6,407,725 | ||||||
|
|
|
|
|
|
|
|||||||
| Liabilities, Redeemable Convertible Preferred Stock and Stockholders’ Deficit |
||||||||||||
| Current liabilities |
||||||||||||
| Accounts payable |
$ | 1,020,110 | $ | 1,115,040 | $ | 771,471 | ||||||
| Accrued expenses |
940,418 | 667,087 | 637,152 | |||||||||
| Current portion of notes payable |
1,212,155 | 26,807 | — | |||||||||
| Convertible term note including accrued interest |
— | 3,669,858 | 5,694,032 | |||||||||
| Warrant liability |
— | 3,197,626 | 6,020,386 | |||||||||
| Derivative liabilities |
1,239,744 | 941,735 | 1,187,953 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current liabilities |
4,412,427 | 9,618,153 | 14,310,994 | |||||||||
| Long-term portion of notes payable |
4,317 | — | — | |||||||||
| Commitments |
||||||||||||
| Series A redeemable convertible preferred stock, $0.001 par value; 1,648,253 shares designated; 1,648,253, 1,400,153, and 1,400,153 shares issued and outstanding at December 31, 2009, 2010, and June 30, 2011 (unaudited), respectively; liquidation preference of $1,400,153 at December 31, 2010 and June 30, 2011 (unaudited) |
1,648,253 | 1,400,153 | 1,400,153 | |||||||||
| Series B redeemable convertible preferred stock, $0.001 par value; 29,799,083 shares designated; 26,520,010, 24,767,610, and 24,767,610 shares issued and outstanding at December 31, 2009, 2010, and June 30, 2011, (unaudited), respectively; liquidation preference of $43,590,985 at December 31, 2010 and June 30, 2011 (unaudited) |
46,675,218 | 43,590,985 | 43,590,985 | |||||||||
| Series B-1 redeemable convertible preferred stock, $0.001 par value; 3,671,086 shares designated; 3,671,086, 1,835,543, and 1,835,543 shares issued and outstanding at December 31, 2009, 2010, and June 30, 2011, (unaudited), respectively; liquidation preference of $3,230,555 at December 31, 2010 and June 30, 2011 (unaudited) |
6,461,111 | 3,230,555 | 3,230,555 | |||||||||
| Series C redeemable convertible preferred stock, $0.001 par value; 159,271,445 shares designated; 123,099,041, 114,411,138, and 114,411,138 shares issued and outstanding at December 31, 2009, 2010 and June 30, 2011 (unaudited), respectively; liquidation preference of $33,066,878 at December 31, 2010 and June 30, 2011 (unaudited) |
34,096,604 | 31,738,719 | 31,789,323 | |||||||||
| Stockholders’ deficit |
||||||||||||
| Common stock, $0.001 par value; 262,870,000 shares authorized; 196,976, 12,849,491 and 12,849,491 shares issued and outstanding at December 31, 2009, 2010, and June 30, 2011 (unaudited), respectively |
197 | 12,849 | 12,849 | |||||||||
| Accumulated other comprehensive income |
77,464 | 89,494 | 88,061 | |||||||||
| Additional paid in capital |
17,440,214 | 26,681,856 | 26,804,930 | |||||||||
| Equity attributable to noncontrolling interest |
3,203,742 | 3,030,324 | 2,991,997 | |||||||||
| Deficit accumulated during the development stage attributable to Argos Therapeutics, Inc.’s stockholders |
(99,756,553 | ) | (108,751,307 | ) | (117,812,122 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total stockholders’ deficit |
(79,034,936 | ) | (7,936,784 | ) | (87,914,285 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities, redeemable convertible preferred stock and stockholders’ deficit |
$ | 14,262,994 | $ | 10,641,781 | $ | 6,407,725 | ||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Table of Contents
(A Development Stage Company)
Consolidated Statements of Operations
| Year Ended December 31, | Six Months Ended June 30, 2010 |
Six Months Ended June 30, 2011 |
Cumulative Period from Inception (May 8, 1997) to June 30, 2011 |
|||||||||||||||||||||
| 2008 | 2009 | 2010 | ||||||||||||||||||||||
| (unaudited) | (unaudited) | (unaudited) | ||||||||||||||||||||||
| Revenue |
$ | 4,449,735 | $ | 5,367,989 | $ | 7,272,783 | $ | 1,990,006 | $ | 4,128,206 | $ | 71,851,340 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Operating expenses |
||||||||||||||||||||||||
| Research and development |
22,042,597 | 19,543,966 | 13,927,662 | 6,931,617 | 6,754,657 | 169,735,867 | ||||||||||||||||||
| Net reimbursement under collaboration agreement |
(8,127,428 | ) | (6,626,989 | ) | — | — | — | (47,179,130 | ) | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net research and development |
13,915,169 | 12,916,977 | 13,927,662 | 6,931,617 | 6,754,657 | 122,556,737 | ||||||||||||||||||
| General and administrative |
2,852,375 | 2,931,599 | 2,704,231 | 1,552,170 | 1,383,521 | 34,088,991 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total operating expenses |
16,767,544 | 15,848,576 | 16,631,893 | 8,483,787 | 8,138,178 | 156,645,728 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Operating income (loss) |
(12,317,809 | ) | (10,480,587 | ) | (9,359,110 | ) | (6,493,781 | ) | (4,009,972 | ) | (84,794,388 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Other income (expense) |
||||||||||||||||||||||||
| Interest income |
250,698 | 73,746 | 4,976 | 3,452 | 644 | 4,092,735 | ||||||||||||||||||
| Interest expense |
(632,677 | ) | (283,417 | ) | (904,233 | ) | (30,645 | ) | (4,847,037 | ) | (10,409,389 | ) | ||||||||||||
| Derivative financing expense |
(756,789 | ) | — | 298,009 | (231,784 | ) | (246,218 | ) | (1,227,403 | ) | ||||||||||||||
| Investment tax credits |
666,933 | 103,549 | 793,006 | 35,938 | — | 2,067,225 | ||||||||||||||||||
| Loss on sale of marketable securities |
— | — | — | — | — | (10,242,252 | ) | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Other income (expense), net |
(471,835 | ) | (106,122 | ) | 191,758 | (223,039 | ) | (5,092,611 | ) | (15,719,084 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net income (loss) |
(12,789,644 | ) | (10,586,709 | ) | (9,167,352 | ) | (6,716,820 | ) | (9,102,583 | ) | (100,513,472 | ) | ||||||||||||
| Net income (loss) attributable to noncontrolling interest |
136,643 | (654,760 | ) | (172,598 | ) | (86,271 | ) | (41,768 | ) | (738,828 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net income (loss) attributable to Argos Therapeutics, Inc. |
(12,926,287 | ) | (9,931,949 | ) | (8,994,754 | ) | (6,630,549 | ) | (9,060,815 | ) | (99,774,644 | ) | ||||||||||||
| Less: Accretion of redeemable convertible preferred stock |
(395,666 | ) | (101,206 | ) | (101,206 | ) | (50,604 | ) | (50,604 | ) | (29,172,382 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net income (loss) attributable to common stockholders |
$ | (13,321,953 | ) | $ | (10,033,155 | ) | $ | (9,095,960 | ) | $ | (6,681,153 | ) | $ | (9,111,419 | ) | $ | (128,947,026 | ) | ||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Basic and diluted net loss attributable to common stockholders per share |
$ | (153.81 | ) | $ | (62.78 | ) | $ | (2.23 | ) | $ | (33.92 | ) | $ | (0.71 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Basic and diluted weighted average shares outstanding |
86,615 | 159,825 | 4,081,649 | 196,976 | 12,849,491 | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Table of Contents
(A Development Stage Company)
Consolidated Statements of Changes in Stockholders’ Deficit
| Common Stock | Additional Paid - In Capital |
Stock Subscription Receivable |
Deferred Compensation |
Accumulated Other Comprehensive Income |
Deficit Accumulated during the Development Stage |
Noncontrolling Interest |
Total | |||||||||||||||||||||||||||||
| Shares | Amount | |||||||||||||||||||||||||||||||||||
| Balance at Inception, May 8, 1997 |
— | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | |||||||||||||||||||
| Exercise of common stock warrants |
3,179 | 3 | 31,786 | — | — | — | — | — | 31,789 | |||||||||||||||||||||||||||
| Exercise of common stock options |
1,511 | 1 | 31,533 | — | — | — | — | — | 31,534 | |||||||||||||||||||||||||||
| Issuance of common stock to founders |
20,000 | 20 | 1,980 | (2,000 | ) | — | — | — | — | — | ||||||||||||||||||||||||||
| Issuance of shares for technology license |
5,350 | 5 | 56,295 | — | — | — | — | — | 56,300 | |||||||||||||||||||||||||||
| Issuance of warrants and options to non-employees |
— | — | 2,494,925 | — | — | — | — | — | 2,494,925 | |||||||||||||||||||||||||||
| Issuance of preferred stock warrants |
— | — | 7,499,776 | — | — | — | — | — | 7,499,776 | |||||||||||||||||||||||||||
| Issuance of restricted stock |
22,860 | 23 | 329,152 | — | (329,175 | ) | — | — | — | — | ||||||||||||||||||||||||||
| Issuance of shares in noncontrolling interest |
— | — | — | — | — | — | 2,988,048 | 2,988,048 | ||||||||||||||||||||||||||||
| Vesting of restricted stock |
— | — | 13,830 | — | — | — | — | — | 13,830 | |||||||||||||||||||||||||||
| Repurchase of common stock |
(8,112 | ) | (8 | ) | (97,509 | ) | — | — | — | — | — | (97,517 | ) | |||||||||||||||||||||||
| Forfeiture of unvested restricted stock |
(4,572 | ) | (4 | ) | 4 | — | — | — | — | — | — | |||||||||||||||||||||||||
| Amortization of deferred compensation |
— | — | — | — | 329,175 | — | — | — | 329,175 | |||||||||||||||||||||||||||
| Stock based compensation |
— | — | 132,547 | — | — | — | (8,097 | ) | — | 124,450 | ||||||||||||||||||||||||||
| Accretion of preferred stock |
— | — | (10,494,319 | ) | — | — | — | (18,029,381 | ) | — | (28,523,700 | ) | ||||||||||||||||||||||||
| Payment of stock subscription |
— | — | — | 2,000 | — | — | — | — | 2,000 | |||||||||||||||||||||||||||
| Cumulative translation adjustment |
— | — | — | — | — | 112,676 | — | — | 112,676 | |||||||||||||||||||||||||||
| Net loss from inception to December 31, 2007 |
— | — | — | — | — | — | (58,860,839 | ) | — | (58,860,839 | ) | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Balance at December 31, 2007 |
40,216 | 40 | — | — | — | 112,676 | (76,898,317 | ) | 2,988,048 | (73,797,553 | ) | |||||||||||||||||||||||||
| Exercise of common stock options |
625 | 1 | 49 | — | — | — | — | — | 50 | |||||||||||||||||||||||||||
| Issuance of shares to noncontrolling interest |
— | — | — | — | — | — | — | 714,742 | 714,742 | |||||||||||||||||||||||||||
| Stock based compensation |
— | — | 674,488 | — | — | — | — | — | 674,488 | |||||||||||||||||||||||||||
| Repurchase of common stock |
(5,682 | ) | (6 | ) | 5 | — | — | — | — | — | (1 | ) | ||||||||||||||||||||||||
| Accretion of preferred stock |
— | — | (395,666 | ) | — | — | — | — | 7,508 | (388,158 | ) | |||||||||||||||||||||||||
| Preferred shares converted to common |
67,185 | 67 | 16,761,757 | — | — | — | — | — | 16,761,824 | |||||||||||||||||||||||||||
| Cumulative translation adjustment |
— | — | — | — | — | (216,411 | ) | — | — | (216,411 | ) | |||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | (12,926,287 | ) | 136,643 | (12,789,644 | ) | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Balance at December 31, 2008 |
102,344 | 102 | 17,040,633 | — | — | (103,735 | ) | (89,824,604 | ) | 3,846,941 | (69,040,663 | ) | ||||||||||||||||||||||||
| Exercise of common stock options |
94,632 | 95 | 7,476 | — | — | — | — | — | 7,571 | |||||||||||||||||||||||||||
| Stock based compensation |
— | — | 493,311 | — | — | — | — | — | 493,311 | |||||||||||||||||||||||||||
| Accretion of preferred stock |
— | — | (101,206 | ) | — | — | — | — | 11,561 | (89,645 | ) | |||||||||||||||||||||||||
| Cumulative translation adjustment |
— | — | — | — | — | 181,199 | — | — | 181,199 | |||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | (9,931,949 | ) | (654,760 | ) | (10,586,709 | ) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Balance at December 31, 2009 |
196,976 | 197 | 17,440,214 | — | — | 77,464 | (99,756,553 | ) | 3,203,742 | (79,034,936 | ) | |||||||||||||||||||||||||
| Issuance of restricted stock |
128,571 | 128 | 8,872 | — | — | — | — | — | 9,000 | |||||||||||||||||||||||||||
| Stock based compensation |
— | — | 324,520 | — | — | — | — | — | 324,520 | |||||||||||||||||||||||||||
| Accretion of preferred stock |
— | — | (101,206 | ) | — | — | — | — | (820 | ) | (102,026 | ) | ||||||||||||||||||||||||
| Preferred shares converted to common |
12,523,944 | 12,524 | 9,009,456 | — | — | — | — | — | 9,021,980 | |||||||||||||||||||||||||||
| Cumulative translation adjustment |
— | — | — | — | — | 12,030 | — | — | 12,030 | |||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | (8,994,754 | ) | (172,598 | ) | (9,167,352 | ) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Balance at December 31, 2010 |
12,849,491 | 12,849 | 26,681,856 | — | — | 89,494 | (108,751,307 | ) | 3,030,324 | (78,936,784 | ) | |||||||||||||||||||||||||
| Stock based compensation (unaudited) |
— | — | 173,678 | — | — | — | — | — | 173,678 | |||||||||||||||||||||||||||
| Accretion of preferred stock (unaudited) |
— | — | (50,604 | ) | — | — | — | — | 3,441 | (47,163 | ) | |||||||||||||||||||||||||
| Cumulative translation adjustment (unaudited) |
— | — | — | — | — | (1,433 | ) | — | — | (1,433 | ) | |||||||||||||||||||||||||
| Net loss (unaudited) |
— | — | — | — | — | — | (9,060,815 | ) | (41,768 | ) | (9,102,583 | ) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Balance at June 30, 2011 (unaudited) |
12,849,491 | $ | 12,849 | $ | 26,804,930 | — | — | $ | 88,061 | $ | (117,812,122 | ) | $ | 2,991,997 | $ | (87,914,285 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Table of Contents
(A Development Stage Company)
Consolidated Statement of Cash Flows
| Year Ended December 31, | Six Months Ended June 30, 2010 |
Six Months Ended June 30, 2011 |
Cumulative Period from Inception (May 8, 1997) to June 30, 2011 |
|||||||||||||||||||||
| 2008 | 2009 | 2010 | ||||||||||||||||||||||
| (unaudited | (unaudited) | (unaudited) | ||||||||||||||||||||||
| Cash flows from operating activities |
||||||||||||||||||||||||
| Net income (loss) |
$ | (12,789,644 | ) | $ | (10,586,709 | ) | $ | (9,167,352 | ) | $ | (6,716,820 | ) | $ | (9,102,583 | ) | $ | (100,513,472 | ) | ||||||
| Adjustments to reconcile net income (loss) to net cash used in operating activities |
||||||||||||||||||||||||
| Depreciation and amortization |
973,947 | 871,848 | 818,448 | 421,646 | 383,982 | 8,062,973 | ||||||||||||||||||
| Non-cash licensing revenue |
— | — | — | — | — | (42,775,697 | ) | |||||||||||||||||
| Compensation expense related to stock options and warrants |
674,488 | 493,311 | 324,520 | 208,841 | 173,678 | 5,283,156 | ||||||||||||||||||
| Issuance of warrants recorded as legal expense |
— | — | — | — | — | 1,703,466 | ||||||||||||||||||
| Amortization of debt issuance costs |
1,692 | 1,692 | 1,692 | — | — | 1,368,761 | ||||||||||||||||||
| Derivative expense |
751,521 | — | (298,009 | ) | 231,784 | 246,218 | 1,187,953 | |||||||||||||||||
| Non cash interest expense |
— | — | 867,492 | — | |
4,846,934 |
|
|
5,714,426 |
| ||||||||||||||
| Issuance of common stock for technology license |
— | — | — | — | — | 56,300 | ||||||||||||||||||
| Issuance of restricted stock recorded as consulting expense |
— | — | 9,000 | — | — | 9,000 | ||||||||||||||||||
| Loss on sale of marketable securities |
— | — | — | — | — | 10,242,252 | ||||||||||||||||||
| Loss on disposal of equipment |
— | — | — | — | — | 96,289 | ||||||||||||||||||
| Changes in operating assets and liabilities |
||||||||||||||||||||||||
| Prepaid expenses and other receivables |
(214,186 | ) | 532,091 | (2,534,052 | ) | 562,821 | 68,588 | (4,043,931 | ) | |||||||||||||||
| Other assets |
15,000 | 10,000 | 10,000 | 5,000 | 5,000 | (2,489 | ) | |||||||||||||||||
| Accounts payable |
297,642 | (1,302,905 | ) | 94,930 | (556,811 | ) | (343,579 | ) | 1,328,431 | |||||||||||||||
| Accrued expenses |
(630,105 | ) | (426,004 | ) | (273,425 | ) | (75,080 | ) | (29,924 | ) | 1,971,203 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net cash used in operating activities |
(10,919,645 | ) | (10,406,676 | ) | (10,146,756 | ) | (5,918,619 | ) | (3,751,686 | ) | (110,311,279 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Cash flows from investing activities |
||||||||||||||||||||||||
| Purchase of furniture, fixtures and equipment |
(237,307 | ) | (85,167 | ) | (117,005 | ) | (36,342 | ) | (6,088 | ) | (7,852,874 | ) | ||||||||||||
| Proceeds from sale of fixed assets |
— | — | — | — | — | 302,519 | ||||||||||||||||||
| Purchases of short-term investments |
(106,159,964 | ) | (8,394,934 | ) | — | — | — | (246,201,490 | ) | |||||||||||||||
| Sales of short-term investments |
110,184,964 | 1,400,000 | — | — | — | 213,559,964 | ||||||||||||||||||
| Proceeds from maturity of short-term investments |
— | 5,124,000 | 3,270,934 | 3,270,934 | — | 32,509,687 | ||||||||||||||||||
| Proceeds from sale of marketable securities |
— | — | — | — | — | 32,533,445 | ||||||||||||||||||
| Investment in affiliate |
— | — | — | — | — | (6,500 | ) | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net cash provided by investing activities |
3,787,693 | (1,956,101 | ) | 3,153,929 | 3,234,592 | (6,088 | ) | 24,844,751 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
F-6
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Consolidated Statements of Cash Flows — (Continued)
| Six Months Ended June 30, 2010 |
Six Months Ended June 30, 2011 |
Cumulative Period from Inception (May 8, 1997) to June 30, 2011 |
||||||||||||||||||||||
| Year Ended December 31, | ||||||||||||||||||||||||
| 2008 | 2009 | 2010 | ||||||||||||||||||||||
| (unaudited) | (unaudited) | (unaudited) | ||||||||||||||||||||||
| Cash flows from financing activities |
||||||||||||||||||||||||
| Proceeds from sale of redeemable convertible preferred stock |
29,904,666 | — | — | — | — | 73,671,416 | ||||||||||||||||||
| Stock issuance costs |
(502,603 | ) | — | — | — | — | (812,432 | ) | ||||||||||||||||
| Proceeds from bridge loan |
— | — | 5,999,991 | — | — | 9,456,937 | ||||||||||||||||||
| Proceeds from notes payable |
— | — | — | — | — | 10,773,532 | ||||||||||||||||||
| Payments on notes payable |
(2,787,302 | ) | (2,725,608 | ) | (1,197,817 | ) | (1,052,304 | ) | (26,807 | ) | (10,242,414 | ) | ||||||||||||
| Payments on debentures |
— | — | — | — | — | (3,509,947 | ) | |||||||||||||||||
| Proceeds from issuance of common stock |
50 | 7,571 | — | — | — | 72,944 | ||||||||||||||||||
| Repurchase of common stock |
(1 | ) | — | — | — | — | (97,518 | ) | ||||||||||||||||
| Distributions to noncontrolling interest |
— | — | — | — | — | 22,413 | ||||||||||||||||||
| Consolidation of DC Bio cash and cash equivalents |
827,248 | — | — | — | — | 7,163,141 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net cash provided by financing activities |
27,442,058 | (2,718,037 | ) | 4,802,174 | (1,052,304 | ) | (26,807 | ) | 86,498,072 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Effect of exchange rates changes on cash |
(254,408 | ) | 140,646 | 18,119 | 4,578 | 1,956 | 327,896 | |||||||||||||||||
| Net increase (decrease) in cash and cash equivalents |
20,055,698 | (14,940,168 | ) | (2,172,534 | ) | (3,731,753 | ) | (3,782,625 | ) | 1,359,340 | ||||||||||||||
| Cash and cash equivalents, beginning of year |
2,198,969 | 22,254,667 | 7,314,499 | 7,314,499 | 5,141,965 | — | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Cash and cash equivalents, end of year |
$ | 22,254,667 | $ | 7,314,499 | $ | 5,141,965 | $ | 3,582,746 | $ | 1,359,340 | $ | 1,359,340 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Supplemental disclosure of cash flow information |
||||||||||||||||||||||||
| Cash paid for interest |
$ | 611,610 | $ | 301,101 | $ | 41,278 | $ | 35,009 | $ | 333 | $ | 2,263,029 | ||||||||||||
| Supplemental disclosure of non-cash investing and financing activities |
||||||||||||||||||||||||
| Conversion of notes payable and accrued interest into preferred stock |
$ | 4,420,858 | $ | — | $ | — | $ | — | $ | — | $ | 6,929,815 | ||||||||||||
| Conversion of accounts payable into preferred stock |
$ | 100,000 | $ | — | $ | — | $ | — | $ | — | $ | 853,375 | ||||||||||||
| Conversion of accounts payable into stock options |
$ | — | $ | — | $ | — | $ | — | $ | — | $ | 28,333 | ||||||||||||
| Conversion of preferred stock into common stock |
$ | 16,761,757 | $ | — | $ | 9,021,980 | $ | — | $ | — | $ | 9,021,980 | ||||||||||||
| Preferred stock accretion |
$ | 395,666 | $ | 101,206 | $ | 101,206 | $ | 50,604 | $ | 50,604 | $ | 29,172,382 | ||||||||||||
| Issuance of restricted stock |
$ | — | $ | — | $ | 9,000 | $ | — | $ | — | $ | 338,175 | ||||||||||||
| Marketable securities received under licensing agreement |
$ | — | $ | — | $ | — | $ | — | $ | — | $ | (42,775,697 | ) | |||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
Table of Contents
(A Development Stage Company)
Notes to Consolidated Financial Statements
| 1. | Organization |
Argos Therapeutics, Inc. (the “Company”), was incorporated in the State of Delaware on May 8, 1997. The Company is a biopharmaceutical company focused on the development and commercialization of fully personalized immunotherapies for the treatment of cancer and infectious diseases based on its proprietary technology platform called Arcelis™. The Company’s most advanced product candidate is AGS-003 for the treatment of metastatic renal cell carcinoma, or mRCC. The Company is developing a second Arcelis-based product candidate, AGS-004 for the treatment of HIV. In addition to its Arcelis-based product candidates, the Company is also developing two other product candidates: AGS-009, a monoclonal antibody for the treatment of lupus, and AGS-010, a preclinical biologic compound for organ transplantation and the treatment of autoimmune and inflammatory diseases.
The Company is in its development stage as defined by the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 915, Development Stage Entities. The Company’s activities since inception have consisted principally of acquiring product and technology rights, raising capital and performing research and development activities. Since inception, the Company has incurred significant losses from operations and expects losses to continue for the foreseeable future during its development phase. The Company’s success depends primarily on the successful development and regulatory approval of its product candidates.
The Company’s consolidated financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates the realization of assets and the settlement of liabilities and commitments in the normal course of business. From May 8, 1997 (inception) through June 30, 2011, the Company had a deficit accumulated during the development stage of $117,812,122 (unaudited). Also, at June 30, 2011, the Company’s current assets totaled approximately $5,438,780 (unaudited) compared to current liabilities of $14,310,994 (unaudited) resulting in a lack of sufficient liquidity. These factors raise substantial doubt about its ability to continue as a going concern. The financial statements for the year ended December 31, 2010, do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from uncertainty related to the Company’s ability to continue as a going concern.
The Company needs to raise additional funds through equity or debt financings or generate revenues from collaborative partners prior to the commercialization of AGS-003 or any of the Company’s other product candidates. There can be no assurance that the Company will be able to obtain additional debt or equity financing or generate revenues from collaborative partners, on terms acceptable to the Company, on a timely basis or at all. The failure of the Company to obtain sufficient funds on acceptable terms when needed could have a material adverse effect on the Company’s business, results of operations and financial condition.
In December 2007, the FASB issued new guidance on accounting for noncontrolling interests in the consolidated financial statements. The Company adopted the provisions of the new guidance effective January 1, 2009. All periods presented in these consolidated financial statements have been reclassified in accordance with this new guidance. The new guidance changes the accounting and reporting standards for the noncontrolling interests in a subsidiary (commonly referred to previously as minority interests). The amount of consolidated net income attributable to both the Company and the noncontrolling interests are shown on the Company’s consolidated statements of operations. The noncontrolling interests related to the Company’s subsidiary DC Bio Corp. (“DC Bio”) were $3,846,941 as of December 31, 2008. In accordance with the guidance, this amount has been reclassified as noncontrolling interests in the equity section of the Company’s consolidated balance sheets. Payments made to acquire non-controlling interests of and distribution to noncontrolling interests from the period of inception through December 31, 2008 have been reclassified to the financing section of the Company’s consolidated statements of cash flows.
F-8
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
The Company has evaluated subsequent events through the date that the financial statements were issued on July 29, 2011, based on the accounting guidance for subsequent events. Adjustments and disclosures resulting from this evaluation, if any, are reflected in these consolidated financial statements.
| 2. | Summary of Significant Accounting Policies |
Unaudited Interim Financial Information
The accompanying consolidated interim balance sheet as of June 30, 2011, the consolidated statements of operations and cash flows for the six months ended June 30, 2010 and 2011 and the consolidated statement of changes in stockholders’ deficit for the six months ended June 30, 2011 are unaudited. The unaudited interim consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles. The unaudited interim consolidated financial statements have been prepared on the same basis as the audited consolidated financial statements. In the opinion of management, the unaudited interim consolidated financial statements include all adjustments, consisting of normal recurring adjustments, necessary for the fair statement of the Company’s financial position at June 30, 2011 and the Company’s results of operations and cash flows for the six months ended June 30, 2010 and 2011. The results for the six months ended June 30, 2011 are not necessarily indicative of the results to be expected for the year ending December 31, 2011 or for any future period. All references to June 30 in these footnotes are also unaudited.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Principles of Consolidation
The accompanying consolidated financial statements include the accounts and results of operations of the Company and DC Bio, formerly Merix Canada Corp., an unlimited liability corporation incorporated in the Province of Nova Scotia, a variable interest entity (”VIE”) for which the Company is subject to a majority of the risk of loss from the variable interest’s activities, or entitled to receive a majority of the entity’s residual returns. DC Bio was consolidated effective January 1, 2004. Beginning on January 1, 2010, the Company updated its assessment of consolidation of the VIE under ASC 810-10-50 including a qualitative assessment of power and economics that considered which entity had the power to direct activities that most significantly impact the VIE’s economic performance, primarily research and development, and had the obligation to absorb losses of, or the benefit that could potentially be significant to the VIE and determined that consolidation was still appropriate. All intercompany balances and transactions have been eliminated in consolidation. In prior periods, the Company also consolidated its wholly-owned subsidiaries, Argos Therapeutics B.V. and Argos Therapeutics GmbH, formerly Merix Bioscience B.V. and Merix Germany GmbH, respectively. Argos Therapeutics B.V. and Argos Therapeutics GmbH were dissolved effective November 6, 2006 and October 22, 2008, respectively.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less at the date of purchase to be cash equivalents. Cash deposits are all in financial institutions in the United States of America or Canada. The Company maintains cash in accounts which are in excess of federally insured limits.
F-9
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
Short-term Investments
All marketable investments are classified as available-for-sale and therefore carried at fair market value. All investments with original maturities less than one year from the balance sheet date are considered short-term investments. The Company primarily invests in commercial paper.
Fair Value of Financial Instruments
The carrying values of cash equivalents, short-term investments, accounts receivable, and accounts payable at December 31, 2009, 2010 and for the six months ended June 30, 2011 (unaudited) approximated their fair values due to the short-term nature of these items.
At December 31, 2009, 2010 and for the six months ended June 30, 2011 (unaudited), the Company holds certain assets and liabilities that are required to be measured at fair value on a recurring basis. These assets and liabilities include available for sale securities classified as cash equivalents and short-term investments, a derivative liability and a warrant liability. The valuation of these financial instruments uses a three tiered approach, which requires that fair value measurements be classified and disclosed in one of three tiers. These tiers are: Level 1, defined as quoted prices in active markets for identical assets or liabilities; Level 2, defined as valuations based on observable inputs other than those included in Level 1, such as quoted prices for similar assets and liabilities in active markets, or other inputs that are observable or can be corroborated by observable input data; and Level 3, defined as valuations based on unobservable inputs reflecting the Company’s own assumptions, consistent with reasonably available assumptions made by other market participants.
F-10
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
At December 31, 2009, 2010 and June 30, 2011 (unaudited), theses financial instruments and respective fair values have been classified as follows:
| Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Balance at December 31, 2009 |
|||||||||||||
| Assets: |
||||||||||||||||
| Money Market Funds |
$ | 6,359,401 | $ | — | $ | — | $ | 6,359,401 | ||||||||
| Short-term Investments |
3,270,934 | — | — | 3,270,934 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total assets at fair value: |
$ | 9,630,335 | $ | — | $ | — | $ | 9,630,335 | ||||||||
| Liabilities: |
||||||||||||||||
| Derivative |
$ | — | $ | — | $ | 1,239,744 | $ | 1,239,744 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Liabilities at Fair Value: |
$ | — | $ | — | $ | 1,239,744 | $ | 1,239,744 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Balance at December 31, 2010 |
|||||||||||||
| Assets: |
||||||||||||||||
| Money Market Funds |
$ | 4,017,701 | $ | — | $ | — | $ | 4,017,701 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total assets at fair value: |
$ | 4,017,701 | $ | — | $ | — | $ | 4,017,701 | ||||||||
| Liabilities: |
||||||||||||||||
| Derivative |
$ | — | $ | — | $ | 941,735 | $ | 941,735 | ||||||||
| Warrants |
— | — | 3,197,626 | 3,197,626 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Liabilities at Fair Value: |
$ | — | $ | — | $ | 4,139,361 | $ | 4,139,361 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Balance at June 30, 2011 |
|||||||||||||
| (unaudited) | ||||||||||||||||
| Assets: |
||||||||||||||||
| Money Market Funds |
$ | 1,217,563 | $ | — | $ | — | $ | 1,217,563 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total assets at fair value: |
$ | 1,217,563 | $ | — | $ | — | $ | 1,217,563 | ||||||||
| Liabilities: |
||||||||||||||||
| Derivative |
$ | — | $ | — | $ | 1,187,953 | $ | 1,187,953 | ||||||||
| Warrants |
— | — | 6,020,386 | 6,020,386 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Liabilities at Fair Value: |
$ | — | $ | — | $ | 7,208,339 | $ | 7,208,339 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-11
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
The changes in the balances of Level 3 liabilities for the year ended December 31, 2010 were as follows:
| Fair Value Measurements Using Significant Unobservable Inputs (Level 3) |
||||||||||||||||
| Beginning Balance |
Net Change in Realized (Gains) Losses |
Additions | Ending Balance |
|||||||||||||
| Derivative Liability |
$ | 1,239,744 | $ | (298,009 | ) | $ | — | $ | 941,735 | |||||||
| Warrant Liability |
— | — | 3,197,626 | 3,197,626 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Totals |
$ | 1,239,744 | $ | (298,009 | ) | $ | 3,197,626 | $ | 4,139,361 | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
For the year ended December 31, 2010, there were no transfers between Levels 1 and 2 assets or liabilities.
The changes in the balances of Level 3 liabilities for the six months ended June 30, 2011 were as follows:
| Fair Value Measurements Using Significant Unobservable Inputs (Level 3) |
||||||||||||||||
| Beginning Balance |
Net Change in Realized (Gains) Losses |
Additions | Ending Balance |
|||||||||||||
| Derivative Liability |
$ | 941,735 | $ | 246,218 | $ | — | $ | 1,187,953 | ||||||||
| Warrant Liability |
3,197,626 | 2,822,760 | 6,020,386 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Totals |
$ | 4,139,361 | $ | 3,068,978 | $ | — | $ | 7,208,339 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
For the six months ended June 30, 2011 there were no transfers between Levels 1 and 2 assets or liabilities.
Credit Risk
Financial instruments which potentially subject the Company to concentrations of credit risk consist principally of cash and cash equivalents and short-term investments. Historically, the Company primarily invested in AAA-rated auction rate investments backed by student loan programs. However, effective in 2008 it no longer invests in these securities. Total cash and cash equivalent balances have exceeded insured balances by the Federal Depository Insurance Company (“FDIC”).
Furniture, Fixtures and Equipment
Furniture, fixtures and equipment are recorded at cost and depreciated over their estimated useful lives using the straight-line method. Furniture, fixtures and equipment held under capital leases and leasehold improvements are amortized over the shorter of the lease term or the estimated useful life of the related asset. Upon retirement or sale, the cost of assets disposed of and the related accumulated depreciation are removed from the accounts and any resulting gain or loss is credited or charged to income. Repairs and maintenance costs are expensed as incurred.
Impairment of Long-Lived Assets
The Company periodically assesses the impairment of long lived assets in accordance with ASC Topic 360, Property Plant and Equipment. When indicators of impairment are present, the Company evaluates the carrying value of these assets in relation to the operating performance of the business and future undiscounted cash flows expected to result from the use of these assets. No such impairments have been recognized from inception (May 8, 1997) through June 30, 2011 (unaudited).
F-12
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
Revenue Recognition
For all periods prior to January 1, 2011, the Company recognizes revenue in accordance with ASC Topic 605, Revenue Recognition. The Company recognizes revenue when the following criteria are met: persuasive evidence of an arrangement exists, services have been rendered, the price is fixed or determinable, and collectability is reasonably assured.
License fees are earned and recognized in accordance with the provisions of each agreement. License fees with ongoing involvement or performance obligations are deferred and recognized over the performance term of the agreement.
The Company has entered into license agreements with collaborators. The terms of these agreements have included nonrefundable signing and licensing fees, as well as milestone payments and royalties on any future product sales developed by the collaborators under such licenses. The Company assesses these multiple elements in accordance with ASC 605, to determine whether particular components of the arrangement represent separate units of accounting.
Upfront license payments are recognized as revenue upon delivery of the license only if the license has stand-alone value and the fair value of the undelivered performance obligations can be determined. If the fair value of the undelivered performance obligations can be determined, such obligations are accounted for separately as the obligations are fulfilled. If the license is considered to either not have stand-alone value or have stand-alone value but the fair value of any of the undelivered performance obligations cannot be determined, the arrangement is accounted for as a single unit of accounting and the license payments and payments for performance obligations are recognized as revenue over the estimated period of when the performance obligations are performed.
When an arrangement is accounted for as a single unit of accounting, the Company determines the period over which the performance obligations will be performed and revenue will be recognized, to the extent this is determinable. If the timing and the level of effort to complete performance obligations under the arrangement is not estimable, then revenue is recognized under the arrangement on a straight-line basis over the expected period to complete such performance obligations.
Royalty revenue is recognized upon the sale of the related products, provided there are no remaining performance obligations under the arrangement. To date, the Company has not received any royalty payments.
The Company is studying AGS-004 in a phase 2b clinical trial that is funded entirely by the National Institutes of Health (“NIH”). Under the NIH contract, the Company is entitled to receive reimbursement of its direct expenses and allocated overhead and general and administrative expenses and payment of other specified amounts totaling up to $1,395,099 upon its achievement of specified development milestones. These revenues are directly related to the development of novel HIV immunotherapy candidates and are recognized as revenue based on the reimbursable expenses that have accrued in accordance with the contractual terms in the arrangement. The Company recognizes revenues from the achievement of milestones under the NIH contract upon the accomplishment of any such milestone.
Income Taxes
The Company provides for income taxes using the liability method. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax bases of assets and liabilities, and are measured using the enacted tax rates and laws that will be in effect when the differences are
F-13
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
expected to reverse. Valuation allowances are provided if, based upon the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
Segment and Geographic Information
Operating segments are defined as components of an enterprise engaging in business activities for which discrete financial information is available and regularly reviewed by the chief operating decision maker in deciding how to allocate resources and in assessing performance. The Company views its operations and manages its business in one operating segment and all of the Company operations are in North America.
Research and Development
Research and development costs include all direct costs related to the development of the Company’s technology, including salaries and related benefits of research and development (“R&D”) personnel, depreciation of laboratory equipment, fees paid to consultants and contract research organizations, stock-based compensation for R&D personnel, sponsored research payments and license fees. R&D costs are expensed as incurred.
Reimbursements of certain R&D costs have been recorded as a reduction of expense and presented in a separate line item within the statement of operations. These reimbursements are recorded when received. Such reimbursements are related to the collaboration agreement with Kyowa Hakko Kirin Co., Ltd. (“Kirin”), as further described in Note 15.
Redeemable Convertible Preferred Stock
The carrying value of redeemable convertible preferred stock is increased by periodic accretions so that the carrying amount will equal the redemption amount at the redemption date. These increases are affected through charges against additional paid-in capital, to the extent it is available, or the deficit accumulated during the development stage. All of the redeemable convertible preferred stock will be converted to common stock at the time of the initial public offering (“IPO”), assuming the IPO meets certain thresholds as defined under the Certificate of Incorporation as summarized in Note 10.
Preferred Stock Warrant Liability
Warrants to purchase the Company’s convertible preferred stock are classified as liabilities and are recorded at their estimated fair value. At each reporting period, any change in fair value of the freestanding warrants are recorded as interest expense. The preferred stock warrants will be automatically exercised for shares of common stock at the time of the IPO and, accordingly, the preferred stock warrant liability will be converted to common stock and additional paid in capital.
Stock-Based Compensation
Effective January 1, 2006, the Company adopted the provisions of ASC Topic 718, Compensation-Stock Compensation, using the prospective-transition method. Under the prospective-transition method, nonvested awards outstanding at the date of adoption continue to be accounted for in the same manner as they had been accounted for prior to adoption. All awards granted, modified or settled after the date of adoption are recognized in the Company’s statements of operations on a straight-line basis over their requisite service periods based on their grant fair values as calculated using the measurement and recognition provisions of ASC 718.
F-14
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
Other Receivables
The Company has recorded other receivables of $1,241,445, $3,776,151 and $3,881,085 at December 31, 2009, 2010 and June 30, 2011, respectively. These receivables are primarily related to amounts due from the NIH arrangement as described in Note 13. The Company assesses the recoverability of other receivables at each balance sheet date.
The Company recorded the following stock-based compensation expense as a result of the adoption of ASC Topic 718:
| Years Ended December 31, |
||||||||||||
| 2008 | 2009 | 2010 | ||||||||||
| Research and development |
$ | 409,460 | $ | 285,810 | $ | 109,196 | ||||||
| General and administrative |
265,028 | 207,501 | 215,324 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total stock-based compensation expense |
$ | 674,488 | $ | 493,311 | $ | 324,520 | ||||||
|
|
|
|
|
|
|
|||||||
| Six Months Ended June 30, |
Cumulative Period from Inception (May 8, 1997) to June 30, |
|||||||||||
| 2010 | 2011 | 2011 | ||||||||||
| (unaudited) | (unaudited) | (unaudited) | ||||||||||
| Research and development |
$ | 99,767 | $ | 60,102 | $ | 899,929 | ||||||
| General and administrative |
109,074 | 113,576 | 890,518 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total stock-based compensation expense |
$ | 208,841 | $ | 173,678 | $ | 1,790,447 | ||||||
|
|
|
|
|
|
|
|||||||
Allocations to research and development and general and administrative expense are based upon the department to which the associated employee reported. No related tax benefits of the stock-based compensation expense have been recognized. Share-based payments issued to nonemployees are recorded at their fair values, and are periodically revalued as the equity instruments vest and are recognized as expense over the related service period.
In 2010, the Company revised its previously reported stock based compensation expense for 2008 and 2009 due to an error in the period of expense recognition for employee stock options as required by ASC 718. Amounts related to prior periods are not considered material to the financial statements taken as a whole, but were revised for purposes of comparability. Such amounts for the year ended December 31, 2008 and 2009 were $590,161 and $361,868, respectively.
All employee stock options were granted at an exercise price equal to or above the fair value of the stock at the date of grant in 2008 and 2009. In December 2010, February 2011 (unaudited) and March 2011 (unaudited), options were granted to employees with exercise prices of $0.08 per share, which was less than the deemed fair value of $0.15 per share, resulting an intrinsic value of $0.07 per share. The weighted average fair value at the date of grant for options granted during the years ended December 31, 2008, 2009 and 2010 was $0.05, $0.06 and $0.12, respectively. The weighted average fair value at the date of grant for options during the six months ended June 30, 2010 and 2011 (unaudited), was $0.06 and $0.12, respectively.
F-15
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
Valuation Assumptions for Stock Option Plans
The employee stock-based compensation expense recognized was determined using the Black-Scholes option valuation model. Option valuation models require the input of subjective assumptions and these assumptions can vary over time. The weighted average assumptions used are as follows:
| 2008 | 2009 | 2010 | 2011 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Risk-free interest rate |
3.32 | % | 3.21 | % | 2.64 | % | 2.92 | % | ||||||||
| Dividend yield |
0 | % | 0 | % | 0 | % | 0 | % | ||||||||
| Expected option term (in years) |
7 | 7 | 7 | 7 | ||||||||||||
| Volatility |
70 | % | 81 | % | 82 | % | 82 | % | ||||||||
The risk-free interest rate is based on the U.S. Treasury yield curve in effect on the date of grant. The dividend yield percentage is zero because the Company neither currently pays dividends nor intends to do so during the expected option term. The expected option term represents the estimated period of time until exercise and is based on historical experience of similar awards, giving consideration to the contractual terms, vesting schedules and expectations of future employee behavior. Expected stock price volatility is based on an average of several peer public companies. For purposes of identifying peer companies, the Company considered characteristics such as industry, length of trading history, similar vesting terms and in-the-money option status. The Company used an average of several peer companies with the characteristics described above to calculate our expected term given our limited history. Upon the adoption of ASC 718, the Company was also required to estimate the level of forfeitures expected to occur and record compensation expense only for those awards that ultimately expect to vest. The Company performed a historical analysis of option awards that were forfeited prior to vesting and recorded total stock option expense that reflected this estimated forfeiture rate.
Investment Tax Credits
Other income was recognized for the Qualifying Therapeutic Discovery Project Program (“QTDP”) in 2010. The QTDP program was created by the United States Congress as part of the Patient Protection and Affordable Care Act and provided for reimbursement of certain costs paid or incurred during 2009 and 2010 directly related to the conduct of a Qualifying Therapeutic Discovery Project, as defined. The Department of Health and Human Services designated such projects based on the potential for them to result in new therapies to treat areas of unmet medical need, the potential to create and sustain jobs in the United States and to advance U.S. competitiveness.
Other income was recognized in 2008 and 2009 for scientific research and experimental development (“SR&ED”) investment tax credits in Canada. Under Canadian and Ontario law, DC Bio is entitled to SR&ED. Because these credits are subject to a claims review, the Company recognizes such credits when received.
Comprehensive Income (Loss)
ASC 220, Comprehensive Income, establishes standards for reporting and display of comprehensive income and its components in a full set of financial statements. The Company’s other comprehensive income (loss) is related to foreign currency translation adjustments.
F-16
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
Foreign Currency Translation
Gains and losses from foreign currency transactions are reflected in income currently.
The Company has identified the functional currency of its subsidiaries with foreign operations as the applicable local currency. The translation from the applicable local currency to United States dollars is performed using the exchange rate in effect at the balance sheet date. Revenue and expense accounts are translated using the average exchange rate experienced during the period. Adjustments resulting from the translation of the Company’s subsidiaries’ financial statements from its functional currency to the United States dollar are not included in determining net income (loss), but are reported as accumulated other comprehensive income, a separate component of stockholders’ deficit.
Derivatives
The Company issued freestanding warrants to purchase common stock and a put option to exchange certain shareholders’ stock in DC Bio for stock in the Company. Derivatives are recorded in the balance sheet at fair value at each balance sheet date utilizing pricing models for non-exchange traded contracts (see Note 9). The Company does not use derivative financial instruments for speculative purposes.
Interest Expense
Interest expense is comprised of the interest associated with the change in fair market value of the warrant liability, the accrual of interest associated with the 2010 convertible notes issued in September and December 2010 and interest associated with notes payable.
Recently Issued Accounting Pronouncements
On January 1, 2010, the Company adopted new accounting and disclosure guidance for the consolidation of variable interest entities (“VIE”), which required enhanced disclosures intended to provide users of financial statements with more transparent information about an enterprise’s involvement in a VIE. The company performed an assessment of its VIE, DC Bio, and determined that continued consolidation under the new guidance is appropriate. Accordingly, there was no impact on the Company’s financial position and results of operations. The impact of this pronouncement on future transactions will be evaluated under the new criteria when and if encountered.
In October 2009, the FASB issued new guidance for revenue recognition with multiple deliverables, which is effective for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010, although early adoption is permitted. This guidance eliminates the residual method under the current guidance and replaces it with the “relative selling price” method when allocating revenue in a multiple deliverable arrangement. The selling price for each deliverable shall be determined using vendor specific objective evidence of selling price, if it exists, otherwise third-party evidence of selling price shall be used. If neither exists for a deliverable, the vendor shall use its best estimate of the selling price for that deliverable. After adoption, this guidance will also require expanded qualitative and quantitative disclosures. The Company will assess the impact of adoption on future transactions.
In April 2010, the FASB issued authoritative guidance on defining a milestone and determining when it may be appropriate to apply the milestone method of revenue recognition for research or development transactions.
F-17
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
Consideration that is contingent on achievement of a milestone in its entirety may be recognized as revenue in the period in which the milestone is achieved only if the milestone is judged to meet certain criteria to be considered substantive. Milestones should be considered substantive in their entirety and may not be bifurcated. An arrangement may contain both substantive and non substantive milestones, and each milestone should be evaluated individually to determine if it is substantive. The guidance is effective on a prospective basis for milestones achieved in fiscal years, and interim periods within those years, beginning on or after June 15, 2010, with early adoption permitted. The Company adopted this guidance on January 1, 2011. The adoption of this guidance did not have a material impact on the consolidated financial statements.
In January 2010, the FASB amended the existing disclosure guidance on fair value measurements, which is effective January 1, 2010, except for disclosures about purchases, sales, issuances, and settlements in the roll forward of activity in Level 3 fair value measurements, which is effective January 1, 2011. Among other things, the updated guidance requires additional disclosure for the amounts of significant transfers in and out of Level 1 and Level 2 measurements and requires certain Level 3 disclosures on a gross basis. Additionally, the updates amend existing guidance to require a greater level of disaggregated information and more robust disclosures about valuation techniques and inputs to fair value measurements. Since the amended guidance requires only additional disclosures, the adoption of the provisions effective January 1, 2010 did not, and for the provisions effective in 2011 will not, impact the Company’s financial position or results of operations.
In June 2011, the FASB issued authoritative guidance related to the Presentation of Comprehensive Income. This standard eliminates the current option to report other comprehensive income and its components in the statement of changes in equity. The new requirements are effective for public entities for fiscal years beginning after December 15, 2011 and interim and annual periods thereafter, with early adoption permitted. As this accounting standard only requires enhanced disclosure, the adoption of this standard will not impact the Company’s financial position or results of operations.
In May 2011, the FASB issued amended guidance on fair value measurements. This newly issued accounting standard clarifies the application of certain existing fair value measurement guidance and expands the disclosures for fair value measurements that are estimated using significant unobservable (Level 3) inputs. This accounting standard is effective on a prospective basis for annual and interim reporting periods beginning on or after December 15, 2011. The Company does not expect that adoption of this standard will have a material impact on its financial position or results of operations.
| 3. | Furniture, Fixtures and Equipment |
Furniture, fixtures and equipment consist of the following:
| Useful
Life (Years) |
December 31, | |||||||||||
| 2009 | 2010 | |||||||||||
| Office furniture and equipment |
7 | $ | 436,617 | $ | 435,333 | |||||||
| Computer equipment |
3 | 659,613 | 693,570 | |||||||||
| Laboratory equipment |
7 | 5,153,844 | 5,206,924 | |||||||||
| Leasehold improvements |
5 | 2,178,227 | 2,178,227 | |||||||||
|
|
|
|
|
|||||||||
| 8,428,301 | 8,514,054 | |||||||||||
| Less: Accumulated depreciation and amortization |
(6,382,389 | ) | (7,169,483 | ) | ||||||||
|
|
|
|
|
|||||||||
| Furniture, fixtures and equipment, net |
$ | 2,045,912 | $ | 1,344,571 | ||||||||
|
|
|
|
|
|||||||||
F-18
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
Depreciation and amortization expense was as follows:
| December 31, 2008 |
$ | 973,947 | ||
| December 31, 2009 |
871,848 | |||
| December 31, 2010 |
818,448 | |||
| June 30, 2010 (unaudited) |
421,646 | |||
| June 30, 2011 (unaudited) |
383,982 | |||
| Cumulative from Inception to June 30, 2011 (unaudited) |
8,062,973 |
| 4. | Noncontrolling Interest |
In December 2002, the Company invested $6,500 in DC Bio. This investment was made in anticipation of an additional future financing by the Company into this newly formed entity. In December 2002, DC Bio sold approximately $5,200,000 of convertible subordinated debentures to certain of the other shareholders of DC Bio. In December 2004, these debentures were cancelled in exchange for 360,000 Class A preferred shares of DC Bio, warrants to purchase 72,000 Class A preferred shares of DC Bio and new nonconvertible debentures of DC Bio with an aggregate initial principal amount of approximately $3,652,000 (see Note 7). Also in December 2004, the Company licensed to DC Bio certain intellectual property rights in exchange for 360,000 Class A preferred shares of DC Bio and warrants to purchase 72,000 Class A preferred shares of DC Bio. These warrants were cancelled in conjunction with the issuance of Class B preferred shares of DC Bio.
In 2007, DC Bio issued a note to the Company for CDN $500,000 that bore interest at 7%. In March 2008 and November 2008, DC Bio issued and sold 75,446 Class B preferred shares to a shareholder for a purchase price of $754,460. In connection with this issuance and sale, DC Bio issued and sold 75,446 Class B preferred shares to the Company for a combination of cash in the amount of $257,254 and the cancellation of the 2007 note payable of CDN$500,000 including accrued interest.
The Company owns 49.94% of the total capital stock of DC Bio, and initially accounted for its investment in DC Bio using the equity method of accounting in 2002 and 2003. DC Bio did not have any operating activities in 2002 or 2003. Effective January 1, 2004, the Company consolidated the operations of DC Bio based on the determination that DC Bio is a VIE. The other shareholders’ ownership interests in DC Bio are represented on the accompanying consolidated balance sheets by noncontrolling interest as a separate component of stockholders’ deficit.
F-19
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
| 5. | Income Taxes |
No provision for U.S. Federal, state or foreign income taxes has been recorded as the Company has incurred net operating losses since inception.
Significant components of the Company’s deferred tax assets and liabilities consist of the following:
| December 31, 2009 |
December 31, 2010 |
|||||||
| U.S. Federal and state net operating loss carryforwards |
$ | 22,157,457 | $ | 25,456,702 | ||||
| Foreign net operating loss carryforwards |
1,138,177 | 1,057,400 | ||||||
| Capital loss carryforward |
3,246,049 | 3,246,049 | ||||||
| Contribution carryforwards |
— | 65,790 | ||||||
| Research and development credits |
2,781,065 | 2,970,996 | ||||||
| Investment tax credits |
— | 40,832 | ||||||
| Stock-based compensation |
714 | 714 | ||||||
| Patents and other intangibles |
47,886 | 13,464 | ||||||
| Deferred rent |
8,168 | 4,111 | ||||||
| Fixed assets |
176,958 | 309,380 | ||||||
| Other accruals |
(21,428 | ) | 64,813 | |||||
|
|
|
|
|
|||||
| Total deferred tax assets |
29,535,046 | 33,230,251 | ||||||
| Valuation allowance for deferred assets |
(29,535,046 | ) | (33,230,251 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax assets |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
At December 31, 2010 and 2009, the Company provided a full valuation allowance against its net deferred tax assets since at that time, the Company could not assert that it was more likely than not that these deferred tax assets would be realized. There was an increase in the valuation allowance in the current year in the amount of $3,695,205, all of which was allocable to current operating activities.
As of December 31, 2010, the Company had U.S. Federal and state, and Canadian federal and provincial net operating loss carryforwards of approximately $63,691,063, $83,554,755, $4,229,601, and $4,229,601, respectively. These net operating loss carryforwards begin to expire in 2012, 2017, 2014 and 2014, respectively. As of December 31, 2010, the Company had U.S. Federal and state tax credit carryforwards of approximately $2,746,350 and $340,372, respectively. These credit carryforwards begin to expire in 2020 and 2019, respectively. The utilization of the net operating loss and tax credit carryforwards may be subject to limitation under the rules regarding a change in stock ownership as determined by the Internal Revenue Code, and state and foreign tax laws. As of December 31, 2010, the Company had Canadian investment tax credit carryforwards of approximately $40,832 that begin to expire in 2024. These carryforwards may be subject to limitations at the time of an IPO or other future private placements.
For the year ended December 31, 2010, the Company maintains that all Canadian earnings from DC Bio have been indefinitely re-invested and, as such, a deferred tax liability with respect to the unremitted earnings has not been recognized.
F-20
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
Taxes computed at the statutory U.S. Federal income tax rate of 34.0% are reconciled to the provision for income taxes as follows:
| December
31, 2008 |
December
31, 2009 |
December
31, 2010 |
||||||||||||||||||||||
| Amount | % of Pretax Earnings |
Amount | % of Pretax Earnings |
Amount | % of Pretax Earnings |
|||||||||||||||||||
| U.S. Federal tax at statutory rate |
$ | (4,147,824 | ) | 34.0 | % | $ | (3,476,445 | ) | 34.0 | % | $ | (3,438,328 | ) | 34.0 | % | |||||||||
| State taxes (net of Federal benefit) |
(497,660 | ) | 4.1 | % | (429,152 | ) | 4.2 | % | (444,438 | ) | 4.4 | % | ||||||||||||
| U.S. Federal tax credits |
(442,728 | ) | 3.6 | % | (197,629 | ) | 1.9 | % | (204,394 | ) | 2.0 | % | ||||||||||||
| Expiration of capital loss carryforward |
— | (0.0 | %) | 3,482,366 | (34.1 | %) | — | (0.0 | %) | |||||||||||||||
| Change in valuation reserves |
4,708,988 | (38.6 | %) | (1,051,796 | ) | 10.3 | % | 3,695,205 | (36.5 | %) | ||||||||||||||
| Deferred tax asset true-ups |
— | (0.0 | %) | 1,388,171 | (13.5 | %) | 171,123 | (1.7 | %) | |||||||||||||||
| Other nondeductible expenses |
379,224 | (3.1 | %) | 284,485 | (2.8 | %) | 130,460 | (1.3 | %) | |||||||||||||||
| Other |
— | (0.0 | %) | — | (0.0 | %) | 90,372 | (0.9 | %) | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Provision for income taxes |
$ | — | 0.0 | % | $ | — | 0.0 | % | $ | — | 0.0 | % | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
Effective January 1, 2007, the Company adopted the provisions of the FASB guidance on accounting for uncertainty in income taxes. These provisions provide a comprehensive model for the recognition, measurement and disclosure in financial statements of uncertain income tax positions that a company has taken or expects to take on a tax return. Under these provisions, a company can recognize the benefit of an income tax position only if it is more likely than not (greater than 50%) that the tax position will be sustained upon tax examination, based solely on the technical merits of the tax position. Otherwise, no benefit can be recognized. Assessing an uncertain tax position begins with the initial determination of the sustainability of the position and is measured at the largest amount of benefit that is greater than 50% likely of being realized upon ultimate settlement. As of each balance sheet date, unresolved uncertain tax positions must be reassessed. Additionally, companies are required to accrue interest and related penalties, if applicable, on all tax exposures for which reserves have been established consistent with jurisdictional tax laws. The cumulative effect of this adoption is recorded as an adjustment to the opening balance of its retained deficit on the adoption date.
As a result of adopting this guidance, the Company reduced its deferred tax assets by approximately $645,000 and reduced its valuation allowance against the deferred tax assets by the same amount. Accordingly, the Company did not record a contingent tax liability as a result of the implementation of these provisions. At the adoption date of January 1, 2007 and as of December 31, 2010, the Company had no accrued interest or penalties related to the tax contingencies. The Company’s policy for recording interest and penalties is to record them as a component of provision for income taxes.
The Company had gross unrecognized tax benefits of approximately $1,089,400 as of January 1, 2010. As of December 31, 2010, the total gross unrecognized tax benefits were approximately $1,177,000 and of this total amount, none would affect the Company’s effective tax rate if recognized. The Company does not anticipate a significant change in total unrecognized tax benefits or the Company’s effective tax rate due to the settlement of audits or the expiration of statutes of limitations within the next 12 months.
The Company has analyzed its filing positions in all significant federal and state jurisdictions where it is required to file income tax returns, as well as open tax years in these jurisdictions. With few exceptions, the
F-21
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
Company is no longer subject to United States Federal, state, and local tax examinations by tax authorities for years before 2007, although carryforward attributes that were generated prior to 2007 may still be adjusted upon examination by the Internal Revenue Service if they either have been or will be used in a future period. No income tax returns are currently under examination by taxing authorities.
The following is a tabular reconciliation of the Company’s change in gross unrecognized tax positions:
| 2008 | 2009 | 2010 | ||||||||||
| Beginning balance |
$ | 821,200 | $ | 1,010,900 | $ | 1,089,400 | ||||||
| Gross increase for tax positions related to current periods |
189,700 | 78,500 | 87,600 | |||||||||
|
|
|
|
|
|
|
|||||||
| Ending balance |
$ | 1,010,900 | $ | 1,089,400 | $ | 1,177,000 | ||||||
|
|
|
|
|
|
|
|||||||
| 6. | Convertible Term Notes |
On February 27, 2004, the Company entered into a convertible term note and warrant purchase agreement with a group of existing preferred stockholders, which provided for up to $12,000,000 in borrowings to the Company. On February 27, 2004, the Company received aggregate proceeds under the first of the convertible term notes in the amount of $3,456,946. The convertible term notes bore interest at a rate of 7% and provided for conversion into equity securities of the Company. The agreement also provided for warrant coverage, with such warrants also exercisable for redeemable convertible preferred stock of the Company (see Note 11). On March 31, 2008, the principal and interest on the convertible term notes totaling $4,420,858 converted into an aggregate of 19,283,135 shares of Series C Preferred Stock of the Company (see Note 10).
On September 9, 2010, the Company entered into a convertible note and warrant purchase agreement with a group of existing preferred stockholders, which provided for a total of up to $6,000,000 in borrowing by the Company. On September 9, 2010 and December 15, 2010, the Company received the proceeds under the agreement in two separate tranches of $4,872,066 and $1,127,925, respectively. The convertible notes issued pursuant to the agreement bear interest at a rate of 10% and provide for conversion into Series C Preferred Stock of the Company. The note purchasers were also issued warrants to purchase Series C Preferred Stock of the Company (see Note 11). The proceeds from the convertible note were allocated among the notes and the associated warrants based on the fair value of the warrants with the residual balance assigned to the convertible notes. The value assigned to the convertible notes is increased through interest expense using the effective interest method such that the value of the convertible notes will equal the accrued principal and interest of $6,603,333 at maturity. The Company recognized $867,484 and $2,027,507 respectively, of interest expense related to the convertible notes during the year ended December 31, 2010 and the six months ended June 30, 2011 (unaudited).
| 7. | Non-Controlling Debentures |
In connection with the financing transactions described in Note 4, DC Bio issued approximately $3,652,000 of debentures. Approximately $2,708,000 of debentures were paid in 2005 leaving a balance of approximately $943,000. The remaining debentures and accrued interest were paid in January 2007.
F-22
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
| 8. | Notes Payable |
During December 2000 (and amended in November 2002, November 2004, and November 2005), the Company entered into a loan agreement with a lending institution, which provides for the Company to borrow funds for the acquisition of furniture, fixtures and equipment. The lending institution’s commitment under this line of credit expired in April 2007. Through December 31, 2010, the Company has borrowed $5,129,782 under this agreement, of which $551,497, $26,807 and $0 was outstanding at December 31, 2009, 2010 and June 30, 2011 (unaudited), respectively. Borrowings for the acquisition of furniture, fixtures and equipment are collateralized by substantially all of the tangible assets of the Company, bear interest at rates between 9.76% and 11.00% and are to be repaid in 42 or 48 equal monthly installments commencing on the date of borrowing. In connection with the loan agreement, warrants to purchase 727 shares of common stock were issued at an exercise price of $176 per share. The warrants expire seven or ten years from the date of issuance (see Note 11).
The Company entered into a Loan and Security Agreement with two lending institutions in April 2007 for the purpose of borrowing $5,000,000 to be used for working capital. As of December 31, 2009, 2010 and June 30, 2011 (unaudited), $666,667, $0, and $0, respectively was outstanding on the loan. The loan term was six months of interest only followed by 30 months of principal and interest at 11.25%. The Company is required to pay a success fee of $200,000 upon consummation of a liquidity event, including an IPO.
| 9. | Derivatives |
The Company has issued the following derivative instruments: (a) freestanding warrants to purchase 3,500 shares of the Company’s common stock (see Note 11) and (b) a put option to exchange certain shareholders’ stock in DC Bio for cash or stock in the Company. The Company does not use derivative financial instruments for speculative or trading purposes.
The freestanding warrants to purchase 3,500 shares of the Company’s common stock were issued with an exercise price of $18.00 to an academic institution and allow the institution to choose to receive cash upon exercise under a net cash settlement feature. No quoted price is available for the Company’s freestanding warrants and, as such, the Company uses the Black-Scholes option-pricing model to value the warrants. Factors affecting the valuation include the current price of the underlying asset, exercise price, expiration date, estimated price volatility of the underlying asset over the life of the warrants and restrictions on the transferability or ability to exercise the warrants. As of December 31, 2009, 2010, and June 30, 2011 (unaudited) a liability of $0 was recorded to reflect the estimated fair value of the warrants. These warrants expired unexercised on July 3, 2011.
In conjunction with the DC Bio transaction on December 7, 2004 (see Note 4), the Company issued a put option whereby in the event DC Bio has not been acquired or has not completed an IPO by December 7, 2007, the other shareholders of DC Bio would have the right to put their DC Bio Class A preferred shares and Class B preferred shares to the Company for shares of the Company’s stock. As of June 30, 2011, the Company has reserved 1,305,216 shares of Series C Preferred Stock, 839,536 shares of Series B Preferred Stock and 2,145,682 shares of common stock for issuance upon the exchange of Class A and Class B preferred shares of DC Bio should such put option be exercised. A liability of $1,239,744, $941,735, and $1,187,953, respectively, was recorded as of December 31, 2009, December 31, 2010 and June 30, 2011 (unaudited) based on the fair market value of the Company’s stock issuable upon exercise of the put option, less the fair market value of the DC Bio shares held by such shareholders, as described above. For purposes of this calculation, the Company determined that the fair market value of the Company’s stock issuable upon exercise of the put option was $2,000,000,
F-23
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
$1,400,000 and $1,600,000 at December 31, 2009, December 31, 2010 and June 30, 2011, respectively, and that the fair market value of the DC Bio shares was $800,000, $400,000 and $400,000 at December 31, 2009, December 31, 2010 and June 30, 2011, respectively. The value of the DC Bio shares held by the other shareholders is valued under the income approach utilizing a discounted cash flow model. The discount rate reflects the risk in the cash flows.
The change in fair value of the put option is recorded as derivative financing expense. There was no change in the fair market value of the put option during 2009 as the value of the stock of the Company issuable upon the exercise of the put option and the value of the DC Bio shares subject to the put option did not change materially during 2009.
| 10. | Stockholders’ Deficit and Redeemable Convertible Preferred Stock |
At December 31, 2010, the Company was authorized to issue 250,760,000 shares of common stock and 182,279,894 of shares of preferred stock.
Common Stock
In July 1997, the Company issued 20,000 shares of common stock to its founders for subscription receivable of $2,000 or $0.001 per share. The receivable, including interest, was repaid in December 1999. In January 2000, the Company issued 5,000 shares of common stock to Duke University in exchange for a technology license. As a result, the Company recorded $50,000 of expense in 2000. In October 2002, the Company issued 350 shares of common stock to Duke University in exchange for technology licenses. As a result, the Company recorded $6,300 of expense in 2002.
In connection with the filing of the Company’s Fourth Amended and Restated Certificate of Incorporation in March 2008, the outstanding shares of common stock were subject to a 100:1 reverse stock split. All references to common stock have been retroactively restated to reflect the stock split. Also in March 2008, an aggregate of 1,412,224 shares of Series A Preferred Stock and an aggregate of 5,306,068 shares of Series B Preferred Stock converted into an aggregate of 67,185 shares of common stock in connection with the election not to participate in the Series C Preferred Stock financing.
In connection with the issuance of the convertible term notes in September 2010, an aggregate of 248,100 shares of Series A Preferred Stock, an aggregate of 1,752,400 shares of Series B Preferred Stock, an aggregate of 1,835,543 shares of Series B-1 Preferred Stock and an aggregate of 8,687,903 Series C Preferred Stock, converted into an aggregate of 12,523,944 shares of common stock as a result of the failure of certain shareholders to participate in the financing.
Dividends
The holders of common stock shall be entitled to receive dividends from time to time as may be declared by the Board of Directors. No cash dividend may be declared or paid to common stockholders until paid on each series of outstanding preferred stock in accordance with its terms.
Voting
The holders of shares of common stock are entitled to one vote for each share held with respect to all matters voted on by the stockholders of the Company.
F-24
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
Liquidation
After payment to the preferred stockholders of their liquidation preferences, holders of common stock shall be entitled, together with holders of preferred stock, to share ratably in all remaining assets of the Company.
Restricted Stock
During September 2002, the Company issued 22,860 shares of restricted common stock to one of its officers, of which 18,288 shares vested based on continuous service to the Company. The shares had an estimated fair value of $18.00 per share, which was recorded as deferred compensation and was being amortized over the vesting period. The Company recorded $140,585 of expense during 2005. During August 2005, all of the 18,288 time-vested shares became vested upon the officer’s departure from the Company. Of these, 8,112 shares were surrendered to the Company as a partial settlement of certain promissory notes issued by the officer in favor of the Company. The 4,572 non time-vested shares were forfeited upon the officer’s departure.
During December 2010, the Company issued 128,571 share of restricted stock to a consultant. The shares had an estimated fair value of $0.07 per share, which was recorded as consulting expense during the year ended December 31, 2010.
Redeemable Convertible Preferred Stock
During January 2000, the Company issued 1,893,874 shares of Series A Preferred Stock of the Company for cash proceeds of $1,500,000 or $1.00 per share, less related issuance costs of $65,741, and conversion of convertible notes payable of $374,998 and related accrued interest of $18,876. In addition, the Company issued warrants to purchase 70,833 shares of Series A Preferred Stock for $1.00 per share (see Note 11). These warrants were cancelled on March 31, 2008.
During January 2001, the Company issued 514,875 shares of Series A Preferred Stock for payment of accounts payable of $514,875 representing a price of $1.00 per share.
During April, July and August 2001, the Company issued 22,376,045 shares of Series B Preferred Stock of the Company at $1.76 per share for cash proceeds of $37,266,756, less related issuance costs of $157,594, and conversion of convertible notes payable of $2,000,000 and related accrued interest of $115,083. Also during July 2001, the Company issued 135,511 shares of Series B Preferred Stock for payment of accounts payable of $238,500 representing a price of $1.76 per share. In addition, the Company issued warrants to purchase 4,331,856 shares of Series B Preferred Stock at $1.76 per share (see Note 11). These warrants were cancelled on March 31, 2008 in connection with the Series C Preferred Stock financing.
During June 2004, the Company issued 2,840,909 shares of Series B-1 Preferred Stock of the Company at $1.76 per share for cash proceeds of $5,000,000, less related issuance costs of $86,494. In addition, the Company issued warrants to purchase 1,704,546 shares of Series B-1 Preferred Stock at $1.76 per share (see Note 11). These warrants were cancelled on March 31, 2008 in connection with the Series C Preferred Stock financing.
During March, April and November 2008, the Company issued 122,753,041 shares of Series C Preferred Stock at $0.289018 per share for cash proceeds of $29,904,666, less related issuance costs of $502,603, and
F-25
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
conversion of convertible term notes of $3,456,946 and related accrued interest of $963,912. Also during March and November 2008, the Company issued 346,000 shares of Series C Preferred Stock for settlement of accrued expenses of $100,000 or $0.289018 per share.
During March 2008, holders of shares of Series A and B Preferred Stock that failed to purchase their respective pro rata share of Series C Preferred Stock had 1,412,224 shares of Series A Preferred and 5,306,068 shares of Series B Preferred Stock converted into an aggregate of 67,185 shares of common stock and also forfeited their right to receive any accrued but unpaid dividends thereon. Holders of Series A, B and B-1 Preferred Stock that purchased their pro rata share of Series C Preferred Stock were issued an aggregate of 651,728 shares of Series A Preferred Stock, 9,314,522 shares of Series B Preferred Stock and 830,177 shares of Series B-1 Preferred Stock, respectively, in exchange for, in part, the cancellation of accrued but unpaid dividends on their shares of Preferred Stock. In 2010, the Company revised its previously reported additional paid in capital and deficit accumulated during the development stage for 2008 and 2009 with respect to the March 2008 Preferred Stock conversion into common stock. These revisions are not considered material to the financial statements.
The Company’s Series A Preferred Stock, Series B Preferred Stock, Series B-1 Preferred Stock and Series C Preferred Stock have the following characteristics:
Voting
The holders of the Preferred Stock are entitled to vote, together with the holders of common stock, on all matters submitted to stockholders for a vote. Each preferred stockholder is entitled to the number of votes equal to the number of shares of common stock into which each preferred share is convertible at the time of such vote.
Dividends
The holders of the Preferred Stock are entitled to receive, when and as declared by the Board of Directors and out of funds legally available, noncumulative dividends at the rate of 8% per annum, payable in preference and priority to any payment of any dividend on common stock. No dividends or other distributions shall be made with respect to the common stock, until all declared dividends on the Preferred Stock have been paid. Since inception (May 8, 1997) through December 31, 2010 no dividends have been declared or paid by the Company. As part of the recapitalization in March 2008, the Company amended its Certificate of Incorporation to remove the cumulative feature of dividends on Preferred Stock. In 2008, holders of Series A, B and B-1 Preferred Stock that purchased their pro-rata share of Series C Preferred Stock were issued additional shares of Series A, B and B-1 Preferred Stock, as applicable in exchange for, in part, the cancellation of accrued but unpaid dividends on their shares of Preferred Stock, which had been accrued based on the cumulative dividend feature, prior to the removal of such feature.
Liquidation
In the event of liquidation, dissolution, or winding up of the Company, holders of Preferred Stock shall be entitled, before any distribution is made to the holders of common stock, to be paid an amount equal to $1.00 per share of Series A Preferred Stock, $1.76 per share of Series B Preferred Stock, $1.76 per share of Series B-1 Preferred Stock and $0.289018 per share of Series C Preferred Stock plus any declared but unpaid dividends. In the event that assets of the Company are insufficient to permit payment of the above mentioned amounts, holders shall share ratably in any distribution of the remaining assets and funds of the Company in proportion to the respective amounts which would otherwise be payable under these circumstances in the order of liquidation preference.
F-26
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
Conversion
Each share of Preferred Stock, at the option of the holder, is convertible into a number of fully paid shares of common stock as determined by dividing the respective Preferred Stock issue price by the conversion price in effect at the time. The initial conversion price of Series A Preferred Stock, Series B Preferred Stock, Series B-1 Preferred Stock and Series C Preferred Stock are $1.00, $1.76, $1.76 and $0.289018 per share, respectively, and is subject to adjustment in accordance with antidilution provisions contained in the Company’s Amended and Restated Certificate of Incorporation, as amended. Conversion is automatic immediately upon the closing of a firm commitment underwritten public offering in which the public offering price equals or exceeds $0.867054 per share (adjusted to reflect subsequent stock dividends, stock splits or recapitalization) and the aggregate proceeds raised equal or exceed $30,000,000.
Participation Rights
After payment to the preferred stockholders of their liquidation preferences, holders of Preferred Stock shall be entitled, together with holders of common stock to share ratably in all remaining assets of the Company.
Redemption
The holders of the outstanding Preferred Stock may, by written request delivered five years after the initial closing of the Series C Preferred Stock, require the Company to redeem the outstanding shares of the Preferred Stock by paying in cash a sum equal to the original purchase price of the Preferred Stock plus any declared but unpaid dividends. If the Company does not have sufficient funds legally available to redeem all shares of Preferred Stock to be redeemed at the redemption date, then the Company shall redeem such shares ratably to the extent possible and shall redeem the remaining shares as soon as sufficient funds are legally available.
Carrying Value
The Preferred Stock shares were initially recorded at the total net proceeds received by the Company upon issuance, after reduction for the value of detachable warrants and issuance costs. The difference between the total net proceeds at issuance and the total redemption price at the redemption date (March 31, 2013) will be charged to additional paid-in capital, to the extent it is available, or the deficit accumulated during the development stage over the period from issuance until redemption first becomes available. The amount of accretion recognized during each period is determined by using the effective interest rate method.
F-27
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
The table below represents a rollforward of the Preferred Stock:
| Series A Preferred | Series B Preferred | Series B-1 Preferred | Series C Preferred | |||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||
| Balance at Inception |
— | $ | — | — | $ | — | — | $ | — | — | $ | — | ||||||||||||||||||||
| Issuance of shares |
1,893,874 | 1,942,179 | 21,174,293 | 33,295,602 | 2,840,909 | 5,000,000 | — | — | ||||||||||||||||||||||||
| Conversion of notes payable |
— | — | 1,201,752 | 2,115,083 | — | — | — | — | ||||||||||||||||||||||||
| Stock issuance costs |
— | — | — | (157,594 | ) | — | — | — | — | |||||||||||||||||||||||
| Issuance of shares as payment for accounts payable |
514,875 | 514,875 | 135,511 | 238,500 | — | — | — | — | ||||||||||||||||||||||||
| Accretion |
— | 1,782,345 | — | 25,085,105 | — | 1,493,617 | — | — | ||||||||||||||||||||||||
| Stock issuance costs |
— | — | — | — | — | (86,494 | ) | — | — | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2007 |
2,408,749 | 4,239,399 | 22,511,556 | 60,576,696 | 2,840,909 | 6,407,123 | — | — | ||||||||||||||||||||||||
| Issuance of shares |
— | — | — | — | — | — | 103,469,906 | 29,904,666 | ||||||||||||||||||||||||
| Conversion of bridge note |
— | — | — | — | — | — | 19,283,135 | 4,420,858 | ||||||||||||||||||||||||
| Conversion of accrued services |
— | — | — | — | — | — | 346,000 | 100,000 | ||||||||||||||||||||||||
| Stock issuance costs |
— | — | — | — | — | — | — | (502,603 | ) | |||||||||||||||||||||||
| Reversal of accrued dividends |
(651,728 | ) | — | (16,393,559 | ) | — | (1,461,111 | ) | — | — | ||||||||||||||||||||||
| Accrued dividends converted into preferred |
651,728 | 651,728 | 9,314,522 | 16,393,559 | 830,177 | 1,461,111 | — | — | ||||||||||||||||||||||||
| Shares converted to Common |
(1,412,224 | ) | (1,412,224 | ) | (5,306,068 | ) | (9,338,680 | ) | — | — | — | — | ||||||||||||||||||||
| Unconverted accrued dividends |
— | (1,178,922 | ) | — | (4,831,999 | ) | — | — | — | — | ||||||||||||||||||||||
| Accretion |
— | — | — | 269,201 | — | 53,988 | — | 72,477 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2008 |
1,648,253 | 1,648,253 | 26,520,010 | 46,675,218 | 3,671,086 | 6,461,111 | 123,099,041 | 33,995,398 | ||||||||||||||||||||||||
| Accretion |
— | — | — | — | — | — | — | 101,206 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2009 |
1,648,253 | 1,648,253 | 26,520,010 | 46,675,218 | 3,671,086 | 6,461,111 | 123,099,041 | 34,096,604 | ||||||||||||||||||||||||
| Shares converted to Common |
(248,100 | ) | (248,100 | ) | (1,752,400 | ) | (3,084,233 | ) | (1,835,543 | ) | (3,230,556 | ) | (8,687,903 | ) | (2,459,091 | ) | ||||||||||||||||
| Accretion |
— | — | — | — | — | — | — | 101,206 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2010 |
1,400,153 | 1,400,153 | 24,767,610 | 43,590,985 | 1,835,543 | 3,230,555 | 114,411,138 | 31,738,719 | ||||||||||||||||||||||||
| Accretion (unaudited) |
— | — | — | — | — | — | — | 50,604 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at June 30, 2011 (unaudited) |
1,400,153 | $ | 1,400,153 | 24,767,610 | $ | 43,590,985 | 1,835,543 | $ | 3,230,555 | 114,411,138 | $ | 31,789,323 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Redemption value |
$ | 1,400,153 | $ | 43,590,985 | $ | 3,230,555 | $ | 33,066,878 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 11. | Warrants |
Series A Preferred
In conjunction with the sale of Series A Preferred Stock during January 2000, the Company issued fully vested warrants to purchase 70,833 shares of Series A Preferred Stock at $1.00 per share. The estimated fair value of these warrants, as determined using the Black-Scholes valuation model, was $49,866. The Company allocated $45,587 of the total proceeds from the sale of Series A Preferred Stock to the warrants based on their fair value. These warrants were cancelled on March 31, 2008 in conjunction with the issuance of Series C Preferred Stock.
Series B Preferred
In conjunction with the issuance of convertible promissory notes in October and December 2000, the Company issued fully vested warrants to purchase 240,000 shares of Series B Preferred Stock at $1.25 per share. The estimated fair value of these warrants, as determined using the Black-Scholes valuation model, was $281,760. The Company allocated $246,967 of the proceeds from the issuance of the convertible notes to the warrants based on the fair value of the warrants. These warrants were cancelled on March 31, 2008 in conjunction with the issuance of Series C Preferred Stock.
F-28
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
In conjunction with the sale of Series B Preferred Stock in 2001, the Company issued warrants to purchase 4,331,856 shares of Series B Preferred Stock at $1.76 per share. The warrants were exercisable immediately and expired seven years from the date of issuance. The estimated fair value of these warrants, as determined using the Black-Scholes valuation model, was $4,414,161. The Company allocated $3,971,148 of the proceeds from the sale of Series B Preferred Stock to the warrants based on their fair value. These warrants were cancelled on March 31, 2008 in conjunction with the issuance of Series C Preferred Stock.
Series B-1 Preferred
At the time of the sale of Series B-1 Preferred Stock in June 2004, the Company issued warrants to purchase 1,704,546 shares of Series B-1 Preferred Stock at $1.76 per share. The warrants were exercisable immediately and expired seven years from the date of issuance. The estimated fair value of these warrants, as determined using the Black-Scholes valuation model, was $1,703,466 which the Company recognized as legal expense during 2004. These warrants were cancelled on March 31, 2008 in conjunction with the issuance of Series C Preferred Stock.
Convertible Term Notes
In conjunction with the issuance of convertible term notes, the Company entered into a note and warrant purchase agreement with the note purchasers on February 27, 2004. The agreement authorized the purchase of an aggregate amount of warrant securities equal to 40% of the note principal divided by the applicable price in the event of a future preferred stock financing, or into common stock at $18.00 per share. The purchase price for the bridge notes and associated warrants was the principal amount of the bridge note. The warrants had a term of seven years from the date of issuance and permitted exercise on a net exercise basis. The Company allocated $1,112,909 of the proceeds from the issuance of the convertible term notes to the warrants. The warrants were cancelled on March 31, 2008 in conjunction with the issuance of Series C Preferred Stock.
The Company entered into a convertible note and warrant purchase agreement with the note purchasers on September 9, 2010. The warrants issued under the agreement entitle the purchasers of the convertible term notes to purchase an aggregate of 20,759,953 shares of Series C Preferred Stock with an exercise price of $0.01 per share. The warrants have a term of seven years and will be automatically exercised upon the closing of the IPO by net exercise. The Company allocated $3,197,626 of the proceeds from the issuance of the convertible term notes to the warrants based on their fair value. The warrants have been recorded as a liability at December 31, 2010 and June 30, 2011 (unaudited) and are marked to market at each reporting period based on any underlying change in the fair market value of such warrants. The Company based its valuation of the fair value of the warrants on an allocation of the enterprise value of the Company to all classes of equity and preferred stock including the warrants. The Company utilized the probability-weighted expected return method (“PWERM”) to determine these values. Under the PWERM method share value is derived from the probability-weighted present value of expected future investment returns, considering each of the possible outcomes available to the Company, as well as the economic and control rights of each share class. The Company calculated the implied enterprise value using the PWERM approach. The fair value of the warrants was estimated using a probability-weighted analysis of the present value of the returns afforded to holders of warrants under several scenarios, including dissolution of the Company, a strategic merger or sale of the Company and an IPO.
In conjunction with entering into research and development agreements with two universities in June and August 2001 (see Note 14), the Company issued warrants to purchase 9,000 shares of common stock at $18.00 per share. The warrants are exercisable immediately and expire ten years from the date of issuance. The estimated fair
F-29
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
value of these warrants, as determined using the Black-Scholes valuation model, was $106,560. The Company recognized the fair value of the warrants as research and development expense when the warrants were issued.
In conjunction with entering into license, research and development agreements with a German university in July 2001 (see Note 14), the Company issued fully vested warrants to purchase 3,500 shares of common stock at $18 per share. The warrants are considered a derivative instrument since the warrants contain a net cash settlement feature. As a result, the warrants are measured at each balance sheet date and any increase or decrease in the fair value is recorded as research and development expense or a reduction in research and development expense, respectively. As of December 31, 2009 and December 31, 2010, a liability of $0 was recorded to reflect the estimated fair value of the warrants.
In conjunction with entering into a loan agreement with a bank in December 2000 (and amended in November 2002, November 2004, and November 2005), the Company issued fully vested warrants to purchase 225 shares of common stock at $100 per share, which had expired as of December 31, 2008, and 727 shares of common stock at $176 per share, of which 209 had expired as of December 31, 2009. The Company determines the estimated fair value of the warrants using the Black-Scholes valuation model and recognizes the value of the warrants as interest expense over the loan agreement term. During the years ended December 31, 2009, 2010 and the six months ended June 30, 2011 (unaudited), the Company recognized $1,692, $1,692 and $0, respectively, of expense related to these warrants.
In conjunction with entering into consulting agreements with scientists and other advisors, the Company has issued warrants to purchase 47,398 shares of common stock of the Company, of which 30,600 remain outstanding as of December 31, 2010. During 2000, the Company issued warrants to purchase 12,596 shares of common stock with an exercise price of $10.00 per share, of which 3,179 were exercised in June 2001 and 9,417 terminated in January 2010. In 2001, warrants to purchase 25,500 shares of common stock with exercise prices ranging from $10.00 to $18.00 per share were issued of which 1,250 were cancelled in March 2011. During 2002, the Company issued warrants to purchase 1,302 shares of common stock with exercise prices ranging from $1.00 to $18.00 per share of which 1,002 were cancelled in March 2008. During 2003, the Company issued warrants to purchase 8,000 shares of common stock with an exercise price of $18.00 per share of which 3,200 were later terminated in 2005. As of December 31, 2010, the warrants are fully vested. The Company determines the estimated fair value of the warrants using the Black-Scholes valuation model and recognizes the compensation expense over the vesting period.
Outstanding warrants to purchase the Company’s preferred and common stock at December 31, 2010 are as follows:
| Type of Warrant |
Number of Warrants |
Exercise Price |
Expiration Date(s) | |||||||
| Series C Preferred |
20,759,953 | $ | 0.01 | 9/9/17; 12/15/17 | ||||||
| Series B Preferred |
16,000 | $ | 176.00 | 4/10/11 | ||||||
| Common |
250 | $ | 10.00 | 2/1/11 | ||||||
| Common |
37,750 | $ | 17.60 | 3/5/11–8/31/11 | ||||||
| Common |
5,100 | $ | 18.00 | 10/31/12; 2/5/13 | ||||||
| Common |
518 | $ | 176.00 | 11/8/11–3/30/17 | ||||||
| 12. | Stock Options |
During December 1999, the Company adopted the Argos Therapeutics, Inc. 1999 Stock Option Plan (the “1999 Plan”), which provides for the granting of up to 23,712 stock options to employees, directors and consultants of the Company. During April 2001, the 1999 Plan was amended to allow for the granting of up to
F-30
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
34,703 options and during August 2001, the 1999 Plan was further amended to allow for the granting of up to 67,737 options. Effective January 2007, the 1999 Plan was amended once more to allow for the granting of up to 102,000 options. During February 2008, the Company adopted the Argos Therapeutics, Inc. 2008 Stock Incentive Plan (the “2008 Plan”) which provides for the granting of up to 27,326,263 stock options to employees, directors and consultants of the Company. All options held by employees and directors of the Company under the 1999 Plan were cancelled upon issuance of options to those same employees and directors under the 2008 Plan. Under the 1999 Plan, 9,489 and 8,202 options remain outstanding as of December 31, 2009 and 2010, respectively. During December 2008, the 2008 Plan was amended to allow for the granting of up to 37,612,814 options. Under the 2008 Plan, both incentive and non-qualified stock options may be granted. The Board of Directors shall determine the exercise price, term and dates of the exercise of all options at their grant date. The Board of Directors, after consideration of an independent appraisal, determines the estimated fair value of the common stock. During 2008 and 2009 all options granted to employees were granted with exercise prices equal to or above the deemed fair value of the common stock on the date of grant. During 2010, certain options were granted to employees with exercise prices of $0.08 per share, which was less than the deemed fair value of $0.15 per share. Under the 2008 Plan, some options vested immediately upon grant and some become vested over variable periods, generally ranging from two to four years, and expire not more than ten years after the date of grant.
The following table summarizes the Company’s stock option activity:
| December 31, 2010 |
||||||||||||||||
| Number of Shares |
Weighted Average Exercise Price |
Weighted Average Contractual Term |
Aggregate Intrinsic Value(a) |
|||||||||||||
| Outstanding — December 31, 2007 |
94,016 | $ | 27.00 | |||||||||||||
| Granted |
29,186,179 | $ | 0.08 | |||||||||||||
| Exercised |
(625 | ) | $ | 0.08 | ||||||||||||
| Cancelled |
(101,719 | ) | $ | 22.38 | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding — December 31, 2008 |
29,177,851 | $ | 0.09 | |||||||||||||
|
|
|
|||||||||||||||
| Granted |
1,480,203 | $ | 0.08 | |||||||||||||
| Exercised |
(94,632 | ) | $ | 0.08 | ||||||||||||
| Cancelled |
(3,517,194 | ) | $ | 0.08 | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding — December 31, 2009 |
27,046,228 | $ | 0.09 | |||||||||||||
|
|
|
|||||||||||||||
| Granted |
9,997,007 | $ | 0.08 | |||||||||||||
| Exercised |
— | $ | — | |||||||||||||
| Cancelled |
(14,168,982 | ) | $ | 0.08 | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding — December 31, 2010 |
22,874,253 | $ | 0.09 | 8.64 | $ | 1,372,455 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Exercisable — December 31, 2010 |
14,748,399 | $ | 0.10 | 6.23 | $ | 737,420 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Vested and expected to vest at December 31, 2010(b) |
21,507,554 | $ | 0.08 | 9.13 | $ | 1,505,529 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (a) | Intrinsic value is the excess of the fair value of the underlying common stock over the weighted average exercise price. |
| (b) | The number of stock options expected to vest takes into account an estimate of expected forfeitures. |
F-31
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
The following table summarizes information about the Company’s stock options at December 31, 2010:
| Exercise Price |
Options Outstanding |
Weighted Average Contractual Life (Years) |
Options Exercisable |
|||||||||
| $0.08 |
22,866,051 | 8.64 | 14,740,317 | |||||||||
| $1.00 |
162 | 4.17 | 162 | |||||||||
| $10.00 |
86 | 0.04 | 26 | |||||||||
| $18.00 |
5,448 | 1.17 | 5,448 | |||||||||
| $34.00 |
786 | 4.95 | 786 | |||||||||
| $50.00 |
1,720 | 1.15 | 1,660 | |||||||||
|
|
|
|
|
|||||||||
| 22,874,253 | 8.64 | 14,748,399 | ||||||||||
|
|
|
|
|
|||||||||
As of December 31, 2010, the Company had a total of $1,428,730 in unrecognized compensation expense from non-vested stock option awards, of which $357,439 is expected to be recognized in 2011, $362,378 in 2012, $359,695 in 2013, $340,210 in 2014, and $9,008 in 2015.
| 13. | Revenue |
In March 2004, the Company entered into a license agreement with Geron Corporation (“Geron”) under which the Company licensed to Geron co-exclusive rights with the Company to the use of the Company’s platform technology with a defined antigen in exchange for 5,000,000 shares of Geron common stock which were valued at $42,775,697. As part of the licensing transaction, the Company granted to Geron rights to both the Company’s existing intellectual property as well as certain improvements to the intellectual property during the first three years following the effective date of the agreement. The licensing revenue was recognized on a straight-line basis over the period of performance, which was three years.
During 2005, the Company was awarded a grant of $499,918 from the Alliance for Lupus Research. The Company received notification during 2006 that this grant was extended for an additional $499,527. Revenue under this contract was recognized as the Company performed the related services, as there were no other performance obligations of the Company. As such, the Company recorded these grants as revenue during the years ended December 31, 2005 and 2006. As of December 31, 2006, all obligations under the grant had been satisfied.
In February 2006, the Company entered into a license agreement with Novo Nordisk A/S (“Novo”) under which Novo acquired antibody technology for the research and development of a treatment for systemic immune disorders, including systemic lupus erythematosus. The Company was paid and recorded revenue of $4,000,000 during 2006 when the license agreement was signed with Novo, as there were no other performance obligations of the Company. The Company received an additional $1,000,000 and $2,000,000 in 2008 and 2009, respectively in accordance with the terms of the agreement when non-refundable cash payments were received related to milestones achieved by Novo. Under the terms of the Novo agreement, the Company had no performance obligations and accordingly revenue was recorded upon receipt. Novo terminated the license agreement effective June 2010. As a result, the Company regained the rights to the acquired antibody technology.
In September 2006, the Company entered into a multi-year research contract with the NIH and the National Institute of Allergy and Infectious Diseases (“NIAID”) to design, develop and clinically test an autologous HIV immunotherapy capable of eliciting therapeutic immune responses. Under this contract, as it has been amended,
F-32
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
the NIH and NIAID have committed to fund up to a total of $29,862,329, including reimbursement of direct expenses and allocated overhead and general and administrative expenses of up to $28,467,230 and payment of other specified amounts totaling up to $1,395,099 upon achievement of specified development milestones. This commitment extends until May 2013. In September 2010, the NIH agreed to make a $1,985,104 one-time payment to us as a true-up in connection with an agreement as to the Company’s indirect cost rates for allocated overhead and general and administrative expenses. The true-up reflects the difference between the provisional indirect cost rates originally provided for in the NIH contract and the negotiated indirect cost rates agreed upon in September 2010 for the years 2006 through 2009 and the eight-month period ended August 31, 2010. The $1,985,104 true-up is included in the $29,862,329 commitment.
For the years ended December 31, 2008, 2009, 2010 and the six months ended June 30, 2010 and 2011 (unaudited), the Company recorded revenue under this agreement of $3,449,735, $3,278,849, $7,219,838, $1,976,691 and $4,088,261, respectively. As of December 31, 2009, 2010, and June 30, 2011 (unaudited), the Company recorded a receivable from the NIH of $985,902, $3,776,043 and $3,881,085, respectively, and a payable to subcontractors of $316,767, $459,036 and $139,090, respectively.
The Company entered into an exclusive option agreement with Therakos, Inc. in December 2006 and received an option fee of $200,000 for granting Therakos the option to explore the use and development of certain intellectual property. As there were no performance obligations under the option agreement, the Company recognized revenue upon payment during the year ended December 31, 2006. On September 18, 2007, the Company granted to Therakos, Inc. an exclusive license to this intellectual property pursuant to a license agreement and received a license fee of $800,000. As there were no performance obligations under the license agreement, the Company recognized revenue upon payment during the year ended December 31, 2007.
The Company entered into a manufacturing agreement in November 2009 with Yale University’s School of Medicine to manufacture autologous mature dendritic cells. Under the terms of the agreement, the Company has the potential to earn $281,740. For the years ended December 31, 2009, 2010, and the six months ended June 30, 2011 (unaudited), the Company recorded revenue under this agreement of $89,140, $51,945 and $39,945, respectively. As of April 2011, the Company is no longer providing manufacturing services under this agreement. Revenue under this arrangement was recorded as services were performed as there were no other performance obligations of the Company.
| 14. | Research and Development Agreements |
The Company has entered into certain research support and option agreements with certain universities. These agreements were for varying terms through 2009. Additionally, these agreements name various scientists as the principal investigator. The separation of the principal investigator from the research project for any reason could cause the early termination of an agreement.
Under these agreements, the Company is required to fund certain research and development projects performed at such university or research institution. The Company receives an option to obtain an exclusive license for any intellectual property resulting from the research. If the Company exercises its option, it must also fund all costs related to obtaining and maintaining patents related to the research. During the years ended December 31, 2009, 2010 and the six months ended June 30, 2011 (unaudited) the Company paid $175,576, $0 and $0, respectively, in costs related to these research agreements. As of December 31, 2009, 2010 and June 30, 2011 (unaudited), the Company has $40,000 of accrued costs related to these agreements.
F-33
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
Under certain circumstances, the Company may terminate these research and development agreements with 90 days written notice. Either party may terminate the agreements under conditions of default.
| 15. | Collaboration Agreements |
The Company entered into a Collaboration and License Agreement with Kirin in June 2004 for the development of therapies using the Company’s monocyte-derived dendritic cell technology. The Company recognized a net reimbursement of $6,626,989, $0, and $47,179,130 of research and development costs for the years ended December 31, 2009, 2010 and for the cumulative period from May 8, 1997 (date of inception) to June 30, 2011 (unaudited), respectively. Simultaneous with entering into the agreement, Kirin invested $5,000,000 in Series B-1 Preferred Stock of the Company. In addition, the Company issued a warrant to Kirin to purchase 1,704,546 shares of Series B-1 Preferred Stock at $1.76 per share (see Note 11). These warrants were cancelled on March 31, 2008. The Company recognized a receivable from Kirin of $255,543, $108, and $0, as of December 31, 2009, 2010, and June 30, 2011 (unaudited), respectively, which is included in other receivables on the balance sheet. The agreement was terminated effective December 31, 2009. The Company elected to continue the programs independently.
| 16. | Commitments |
The Company rents laboratory and office space and equipment under operating leases that expire in various years through 2012. Future minimum lease payments under non-cancelable operating leases as of December 31, 2010 are as follows:
| Year Ending December 31, |
Operating Leases |
|||
| 2011 |
$ | 211,493 | ||
| 2012 |
2,996 | |||
|
|
|
|||
| Total minimum lease payments |
$ | 214,489 | ||
|
|
|
|||
On June 21, 2011 (unaudited), the Company signed a lease agreement to renew its rented laboratory and office space through 2016. The amount of obligation associated with this operating lease agreement over the term of the lease is $1,488,116.
Rent expense related to operating leases for the years ended December 31, 2008, 2009, 2010, the six months ended June 30, 2011 (unaudited) and the period from inception (May 8, 1997) through June 30, 2011 (unaudited) was $312,677, $370,817, $362,619, $174,934 and $2,940,109, respectively.
The Company has entered into various licensing agreements with universities and other research institutions under which the Company receives substantially all rights of the inventors or co-assignee to produce and market technology protected by certain patents and patent applications. The Company also entered into various assignment agreements with a scientist under which the Company receives exclusive rights to produce and market technology protected by certain patents and patent applications.
The Company is generally required to make royalty payments ranging from 1 - 4 % of future sales of products employing the technology or falling under claims of a patent. If future sales require the use of technology licensed from multiple different sources, the total royalty rates could be higher. As royalty payments are directly related to future sales volume, future commitments cannot be determined.
F-34
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
In connection with the Loan and Security Agreement with two lending institutions in April 2007, the Company is required to pay a success fee of $200,000 upon consummation of a liquidity event, including an IPO.
| 17. | Concentration of Credit Risk |
The Company’s grant revenue is earned under a contract with NIH. The concentration of credit risk is equal to the outstanding accounts receivable and unbilled balances and such risk is subject to the credit worthiness of the NIH. There have been no credit losses under this arrangement.
| 18. | Employee Benefit Plan |
The Company provides a retirement plan qualified under section 401(k) of the Internal Revenue Code of 1986, as amended (“IRC”). Participants may elect to contribute a portion of their annual compensation to the plan, after complying with certain limitations set by the IRC. All employees are eligible to participate in the plan after attaining the age of 21. The Company matched 25% of the first 6% contributed by eligible participants in the plan during the years ended December 31, 2009, 2010, and the six months ended June 30, 2010 (unaudited) and 2011 (unaudited), or $74,409, $59,073, $0 and $0, respectively, for a total of $390,095 for the period from inception (May 8, 1997) through June 30, 2011.
| 19. | Net Loss Per Share |
Basic and diluted net loss per common share was determined by dividing net loss by the weighted average common shares outstanding during the period. The Company’s potentially dilutive shares, which include redeemable convertible preferred stock, common stock options and warrants, have not been included in the computation of diluted net loss per share for all periods as the result would be antidilutive.
The following table presents the computation of basic and diluted net loss per share of common stock:
| Year Ended December 31, | Six Months Ended June 30, | |||||||||||||||||||
| 2008 | 2009 | 2010 | 2010 | 2011 | ||||||||||||||||
| (unaudited) | ||||||||||||||||||||
| As Reported: |
||||||||||||||||||||
| Net loss attributable to Argos Therapeutics, Inc. |
$ | (12,926,287 | ) | $ | (9,931,949 | ) | $ | (8,994,754 | ) | $ | (6,630,549 | ) | $ | (9,060,815 | ) | |||||
| Accretion of redeemable convertible preferred stock |
(395,666 | ) | (101,206 | ) | (101,206 | ) | (50,604 | ) | (50,604 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss attributable to common stockholders |
$ | (13,321,953 | ) | $ | (10,033,155 | ) | $ | (9,095,960 | ) | $ | (6,681,153 | ) | $ | (9,111,419 | ) | |||||
| Weighted average common shares outstanding, basic and diluted |
86,615 | 159,825 | 4,081,649 | 196,976 | 12,849,491 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss per share attributable to common stockholders, basic and diluted |
$ | (153.81 | ) | $ | (62.78 | ) | $ | (2.23 | ) | $ | (33.92 | ) | $ | (0.71 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-35
Table of Contents
Argos Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements — (Continued)
The following potentially dilutive securities have been excluded from the computation of diluted weighted average shares outstanding, as they would be antidilutive.
| Year Ended December 31, | Six Months Ended June 30, | |||||||||||||||||||
| 2008 | 2009 | 2010 | 2010 | 2011 | ||||||||||||||||
| (unaudited) | ||||||||||||||||||||
| Redeemable convertible preferred stock |
88,778,536 | 154,938,390 | 151,061,113 | 154,938,390 | 142,414,444 | |||||||||||||||
| Stock options outstanding |
10,573,607 | 26,518,989 | 24,220,208 | 12,259,455 | 19,456,599 | |||||||||||||||
| Warrants outstanding |
58,368 | 59,361 | 5,449,539 | 59,618 | 20,811,161 | |||||||||||||||
| 20. | Subsequent Events |
July 2011 Convertible Notes
On July 27, 2011, the Company sold convertible notes in the aggregate principal amount of $3,500,000 in a private placement. The convertible notes accrue interest at a rate equal to 10% per year, and have a maturity date of December 31, 2011, unless converted prior thereto. All principal and accrued interest on these convertible notes will automatically convert into shares of the Company’s common stock, at a conversion price per share equal to the price per share at which the Company’s common stock is sold to the public in the IPO, if investors holding at least a majority-in-interest of the aggregate principal amount of the then outstanding July 2011 convertible notes so elect.
In connection with the issuance and sale of the convertible notes, the Company issued warrants to the purchasers of the July 2011 convertible notes to purchase an aggregate of 12,109,975 shares of Series C Preferred Stock with an exercise price of $0.01 per share. The warrants have a term of seven years and will be automatically exercised upon the closing of the IPO by net exercise.
License Agreement (unaudited)
In July 2011, the Company entered into an agreement with Celldex Therapeutics, Inc. (“Celldex”), pursuant to which Celldex granted the Company a non-exclusive license to specified patents and patent applications regarding actions necessary or helpful for processing dendritic cells. Upon the execution of the agreement, the Company paid Celldex $50,000 of a $100,000 upfront license fee. The Company agreed to pay the balance of this fee upon the earlier of the initiation of the next clinical trial of AGS-003, or January 31, 2012. Under this agreement, the Company must pay:
| • | a $75,000 annual license fee; |
| • | a specified milestone payment based on the achievement of a specified regulatory milestone; and |
| • | a specified dollar amount per dose of AGS-003 the Company sells. |
F-36
Table of Contents
Shares

Common Stock
Lazard Capital Markets
Canaccord Genuity
Needham & Company, LLC
BMO Capital Markets
, 2011
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the expenses to be incurred in connection with the offering described in this Registration Statement, other than underwriting discounts and commissions, all of which will be paid by us. All amounts are estimates except the Securities and Exchange Commission, or SEC, registration fee and the Financial Industry Regulatory Authority, Inc. filing fee.
| Amount | ||||
| Securities and Exchange Commission registration fee |
$ | 10,014 | ||
| Financial Industry Regulatory Authority, Inc. filing fee |
9,125 | |||
| NASDAQ listing fee |
* | |||
| Accountants’ fees and expenses |
* | |||
| Legal fees and expenses |
* | |||
| Blue sky fees and expenses |
* | |||
| Transfer agent’s fees and expenses |
* | |||
| Printing and engraving expenses |
* | |||
| Miscellaneous |
* | |||
|
|
|
|||
| Total expenses |
$ | * | ||
|
|
|
|||
| * | To be filed by amendment. |
Item 14. Indemnification of Directors and Officers
Section 102 of the Delaware General Corporation Law permits a corporation to eliminate the personal liability of its directors or its stockholders for monetary damages for a breach of fiduciary duty as a director, except where the director breached his or her duty of loyalty, failed to act in good faith, engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase in violation of Delaware corporate law or obtained an improper personal benefit. Our certificate of incorporation provides that no director shall be personally liable to us or our stockholders for monetary damages for any breach of fiduciary duty as a director, notwithstanding any provision of law imposing such liability, except to the extent that the Delaware General Corporation Law prohibits the elimination or limitation of liability of directors for breaches of fiduciary duty.
Section 145 of the Delaware General Corporation Law provides that a corporation has the power to indemnify a director, officer, employee or agent of the corporation and certain other persons serving at the request of the corporation in related capacities against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlements actually and reasonably incurred by the person in connection with an action, suit or proceeding to which he or she is or is threatened to be made a party by reason of such position, if such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of the corporation, and, in any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful, except that, in the case of actions brought by or in the right of the corporation, no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or other adjudicating court determines that, despite the adjudication of liability but in view of all of the circumstances of the case, such person is fairly and reasonably entitled to indemnify for such expenses which the Court of Chancery or such other court shall deem proper.
II-1
Table of Contents
Our certificate of incorporation that will be effective upon the closing of the offering our charter provides that we will indemnify each person who was or is a party or threatened to be made a party to any threatened, pending or completed action, suit or proceeding whether civil, criminal, administrative or investigative (other than an action by or in the right of us) by reason of the fact that he or she is or was, or has agreed to become, our director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding and any appeal therefrom, if such indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests, and, with respect to any criminal action or proceeding, he or she had no reasonable cause to believe his or her conduct was unlawful.
Our charter also provides that we will indemnify any such indemnitee who was or is a party to an action or suit by or in the right of us to procure a judgment in our favor by reason of the fact that the Indemnitee is or was, or has agreed to become, our director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee or, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise, or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys’ fees) and, to the extent permitted by law, amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding, and any appeal therefrom, if such indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests, except that no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to us, unless a court determines that, despite such adjudication but in view of all of the circumstances, he or she is entitled to indemnification of such expenses. Notwithstanding the foregoing, to the extent that any such indemnitee has been successful, on the merits or otherwise, he or she will be indemnified by us against all expenses (including attorneys’ fees) actually and reasonably incurred by him or her or on his or her behalf in connection therewith. If we don’t assume the defense, expenses must be advanced to an Indemnitee under certain circumstances.
We plan to enter into indemnification agreements with our directors and executive officers. In general, these agreements will provide that we will indemnify the director or executive officer to the fullest extent permitted by law for claims arising in his or her capacity as a director or officer of our company or in connection with their service at our request for another corporation or entity. The indemnification agreements will also provide for procedures that will apply in the event that a director or executive officer makes a claim for indemnification and establish certain presumptions that are favorable to the director or executive officer.
We maintain a general liability insurance policy which covers certain liabilities of our directors and officers arising out of claims based on acts or omissions in their capacities as directors or officers.
The underwriting agreement we will enter into in connection with the offering of common stock being registered hereby provides that the underwriters will indemnify, under certain conditions, our directors and officers (as well as certain other persons) against certain liabilities arising in connection with such offering.
Item 15. Recent Sales of Unregistered Securities
Set forth below is information regarding shares of capital stock, and options and warrants granted, issued by us within the past three years that were not registered under the Securities Act of 1933, as amended or Securities Act. Also included is the consideration, if any, received by us for such shares, options and warrants and information relating to the section of the Securities Act or rule of the SEC under which exemption from registration was claimed.
(a) Issuance of Capital Stock
During March, April and November 2008, we issued and sold an aggregate of 123,099,041 shares of our series C preferred stock for an aggregate purchase price of $30,004,666. We issued and sold these shares at a
II-2
Table of Contents
price per share of $0.289018 to existing stockholders that were accredited investors in the case of shares of our series C preferred stock issued and sold for cash or upon the cancellation of interest then outstanding on our then outstanding convertible promissory notes, and at a price per share of $0.2167635 in the case of shares of our series C preferred stock sold upon the cancellation of principal on our then outstanding convertible promissory notes. Holders of our preferred stock that purchased their pro rata share of our series C preferred stock in the series C preferred stock financing also received an aggregate of 651,728 shares of our series A preferred stock, 9,314,522 shares of our series B preferred stock and 830,177 shares of our series B-1 preferred stock, in exchange for, in part, the cancellation of accrued but unpaid dividends on such shares of preferred stock.
On December 10, 2010, we issued and sold 128,571 shares of restricted common stock to a consultant pursuant to a restricted stock purchase agreement under our 2008 stock incentive plan. The restricted common stock had an estimated fair value of $0.07 per share and was fully vested upon the purchase date.
No underwriters were involved in the foregoing sales of securities. The securities described in this section (a) of Item 15 were issued to investors in reliance upon the exemption from the registration requirements of the Securities Act, as set forth in Section 4(2) under the Securities Act and Regulation D promulgated thereunder relative to transactions by an issuer not involving any public offering, to the extent an exemption from such registration was required. All purchasers of shares of convertible preferred stock described above represented to us in connection with their purchase that they were accredited investors and were acquiring the shares for their own account for investment purposes only and not with a view to, or for sale in connection with, any distribution thereof and that they could bear the risks of the investment and could hold the securities for an indefinite period of time. The purchasers received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration statement or an available exemption from such registration.
(b) Stock Option Grants
Between January 1, 2008 and August 31, 2011, we issued to certain employees, directors and consultants options to purchase an aggregate of 42,387,335 shares of common stock. As of August 31, 2011, options to purchase 95,257 shares of common stock had been exercised and options to purchase 24,189,612 shares of common stock remained outstanding at a weighted average exercise price of $0.08 per share.
The issuance of stock options and the common stock issuable upon the exercise of such options as described in this section (b) of Item 15 were issued pursuant to written compensatory plans or arrangements with our employees, directors and consultants, in reliance on the exemption from the registration requirements of the Securities Act provided by Rule 701 promulgated under the Securities Act or the exemption set forth in Section 4(2) under the Securities Act and Regulation D promulgated thereunder relative to transactions by an issuer not involving any public offering. All recipients either received adequate information about us or had access, through employment or other relationships, to such information.
(c) Warrants
In September and December 2010, in connection with the issuance and sale of the 2010 convertible notes, we issued warrants to the purchasers of the 2010 convertible notes to purchase an aggregate of 20,759,953 shares of our series C preferred stock with an exercise price of $0.01 per share.
In July 2011, in connection with the issuance and sale of the July 2011 convertible notes, we issued warrants to the purchasers of the 2011 convertible notes to purchase an aggregate of 12,109,975 shares of our series C preferred stock with an exercise price of $0.01 per share.
The sale and issuance of these warrants were made in reliance on the exemption provided by Section 4(2) of the Securities Act and Regulation D promulgated thereunder. The recipients of warrants in the transaction described above represented that they were accredited investors and were acquiring the warrants for their own
II-3
Table of Contents
account for investment purposes only and not with a view to, or for sale in connection with, any distribution thereof and that they could bear the risks of the investment and could hold the warrants for an indefinite period of time and appropriate legends were affixed to the instruments representing such warrants issued in such transactions. Such recipients either received adequate information about us or had, through its relationship with us, access to such information.
All of the foregoing securities are deemed restricted securities for purposes of the Securities Act. All certificates representing the issued shares of capital stock described in this Item 15 included appropriate legends setting forth that the securities had not been registered and the applicable restrictions on transfer.
Item 16. Exhibits and Financial Statement Schedules
The exhibits to the registration statement are listed in the Exhibit Index attached hereto and incorporated by reference herein.
Item 17. Undertakings
(a) The undersigned registrant hereby undertakes to provide to the underwriter at the closing specified in the underwriting agreements, certificates in such denominations and registered in such names as required by the underwriter to permit prompt delivery to each purchaser.
(b) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
(c) The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-4
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Amendment No. 2 to the Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Durham, State of North Carolina, on this 19th day of October, 2011.
| ARGOS THERAPEUTICS, INC. | ||
| By: |
/s/ Jeffrey D. Abbey | |
| Jeffrey D. Abbey President and Chief Executive Officer | ||
Pursuant to the requirements of the Securities Act of 1933, this Amendment No. 2 to the Registration Statement has been signed by the following persons in the capacities held on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ Jeffrey D. Abbey Jeffrey D. Abbey |
President, Chief Executive Officer and Director (Principal executive officer) |
October 19, 2011 | ||
| /s/ Lori Harrelson Lori Harrelson |
Vice President of Finance (Principal financial and accounting officer) |
October 19, 2011 | ||
| * Hubert Birner |
Director | October 19, 2011 | ||
| * B. Jefferson Clark |
Director | October 19, 2011 | ||
| * Michel Gréco |
Director | October 19, 2011 | ||
| * Monique Laliberté |
Director | October 19, 2011 | ||
| * Philip R. Tracy |
Director | October 19, 2011 | ||
| * Brian J. Underdown |
Director | October 19, 2011 | ||
| * Sander van Deventer |
Director | October 19, 2011 | ||
| *By: | /s/ Jeffrey D. Abbey | |
| Jeffrey D. Abbey Attorney-in-fact | ||
II-5
Table of Contents
EXHIBIT INDEX
| Exhibit |
Description of exhibit | |
| 1.1* | Underwriting Agreement | |
| 3.1+ | Third Amended and Restated Certificate of Incorporation of the Registrant, as amended | |
| 3.2* | Restated Certificate of Incorporation of the Registrant to be effective upon the closing of this offering | |
| 3.3+ | Bylaws of the Registrant | |
| 3.4* | Amended and Restated Bylaws of the Registrant to be effective upon the closing of this offering | |
| 4.1* | Specimen certificate evidencing shares of common stock | |
| 5.1* | Opinion of Wilmer Cutler Pickering Hale and Dorr LLP | |
| 10.1+ | Second Amended and Restated Registration Rights Agreement, dated as of March 31, 2008, as amended | |
| 10.2+ | Warrant to purchase shares of Common Stock issued by the Registrant to Eckhart Kämpgen expiring on October 31, 2012 | |
| 10.3+ | Warrant to purchase shares of Common Stock issued by the Registrant to Johannes W.G. Vieweg, M.D. expiring on February 5, 2013 | |
| 10.4+ | 1999 Stock Option Plan | |
| 10.5+ | 2008 Stock Incentive Plan | |
| 10.6+ | Form of Incentive Stock Option Agreement under 2008 Stock Incentive Plan | |
| 10.7+ | Form of Nonstatutory Stock Option Agreement under 2008 Stock Incentive Plan | |
| 10.8* | 2011 Stock Incentive Plan | |
| 10.9* | Form of Incentive Stock Option Agreement under 2011 stock incentive plan | |
| 10.10* | Form of Nonstatutory Stock Option Agreement under 2011 stock incentive plan | |
| 10.11+ | Offer letter, dated as of July 18, 2003, by and between the Registrant and Charles A. Nicolette, as amended | |
| 10.12+ | Offer letter, dated as of August 19, 2002, by and between the Registrant and Jeffrey D. Abbey, as amended | |
| 10.13+ | Offer letter, dated as of March 24, 2003, by and between the Registrant and Frederick M. Miesowicz, as amended | |
| 10.14+ | Offer letter, dated as of August 11, 2004, by and between the Registrant and Lori R. Harrelson | |
| 10.15* | Form of Indemnification Agreement between the Registrant and each director and executive officer | |
| 10.16+ | Lease Agreement, dated as of January 16, 2001, by and between the Registrant and HCP MOP, as amended on July 31, 2005, August 7, 2006 and June 21, 2011 | |
| 10.17†+ | License Agreement, dated as of January 10, 2000, by and between the Registrant and Duke University, as amended on July 28, 2003 | |
| 10.18†+ | Exclusive License Agreement, dated as of January 15, 2002, by and between the Registrant and Baylor Research Institute | |
Table of Contents
| Exhibit |
Description of exhibit | |||
| 10.19†+ | Exclusive License Agreement, dated as of November 17, 2005, by and between the Registrant and Baylor Research Institute | |||
| 10.20†+ | License Agreement, dated as of March 6, 2004, by and between the Registrant and Geron Corporation | |||
| 10.21†+ | License Agreement, dated as of July 28, 2011, by and between the Registrant and Celldex Therapeutics, Inc. | |||
| 10.22†+ | Collaboration Termination Agreement, dated as of December 31, 2009, by and between the Registrant and Kyowa Hakko Kirin Co., Ltd. | |||
| 10.23† | Contract No. HHSN266200600019C, effective September 30, 2006, by and among the Registrant, the National Institutes of Health and the National Institutes of Allergy and Infectious Diseases, as amended | |||
| 10.24+ | Release and Settlement Agreement, dated as of February 23, 2010, by and between the Registrant and John Bonfiglio | |||
| 10.25+ | Release and Settlement Agreement, dated as of February 23, 2010, by and between the Registrant and Timothy W. Trost | |||
| 10.26 | Letter Agreement, dated as of August 26, 2011, by and between the Registrant and Lori R. Harrelson | |||
| 10.27 | Offer letter, dated as of September 8, 2011, by and between the Registrant and Doug Plessinger | |||
| 21.1+ | Subsidiaries of the Registrant | |||
| 23.1 | Consent of PricewaterhouseCoopers LLP | |||
| 23.2* | Consent of Wilmer Cutler Pickering Hale and Dorr LLP (included in Exhibit 5.1) | |||
| 24.1+ | Power of Attorney (included on signature page) | |||
| * | To be filed by amendment. |
| † | Confidential treatment requested as to portions of the exhibit. Confidential materials omitted and filed separately with the Securities and Exchange Commission. |
| + | Previously filed. |