
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
OR
For the fiscal year ended
OR
OR
Date of event requiring this shell company report ______________
For the transition period from ______________ to ______________
Commission file number:
(Exact name of Registrant as specified in its charter)
N/A
(Translation of Registrant's name into English)
British Virgin Islands
(Jurisdiction of incorporation or organization)
(Address of principal executive offices)
c/o Portage Development Services Inc.,
(Name, telephone, e-mail and/or facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol | Name of each exchange on which registered |
Securities registered or to be registered pursuant to Section 12(g) of the Act:
Not applicable
(Title of Class)
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
Not applicable
(Title of Class)
Indicate the number of outstanding shares of each of the Issuer's classes of capital or common stock (ordinary shares) as of the close of the period covered by the annual report. Ordinary shares without par value – as at March 31, 2024
Indicate by check mark if the registrant is a well-known seasoned issuer,
as defined in Rule 405 of the Securities Act. Yes ☐
If this report is an annual or transition report, indicate by check mark
if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. Yes ☐
Indicate by check mark whether the registrant (1) has filed all reports
required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter
period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically
every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the
preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of " large accelerated filer,” large accelerated filer," and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ | Emerging growth company |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act. ☐
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and
attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b)
of the Sarbanes-Oxley Act (15 U.S.C. 7262(b) by the registered public accounting firm that prepared or issued its audit report.
If securities are registered pursuant to Section 12(b) of the Act, indicate
by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously
issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| U.S. GAAP ☐ |
by the International Accounting Standards Board ☒ |
Other ☐ |
If "Other" has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow. Item 17 ☐ Item 18 ☐
If this is an annual report, indicate by check mark whether the registrant
is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No
ii
TABLE OF CONTENTS
iii
FORWARD-LOOKING STATEMENTS
This annual report on Form 20-F (“Annual Report”) includes “forward-looking statements.” All statements, other than statements of historical facts, included herein or incorporated by reference herein, including without limitation, statements regarding our business strategy, plans and objectives of management for future operations and those statements preceded by, followed by or that otherwise include the words “believe,” “expects,” “anticipates,” “intends,” “estimates,” “will,” “may,” “should,” “could,” “targets,” “projects,” “predicts,” “plans,” “potential,” or “continue,” or similar expressions or variations on such expressions are forward-looking statements. We can give no assurances that such forward-looking statements will prove to be correct.
Each forward-looking statement reflects our current view of future events and is subject to risks, uncertainties and other factors that could cause actual results to differ materially from any results expressed or implied by our forward-looking statements.
We have made the decision to discontinue our sponsored trial for the invariant natural killer T-cell (“iNKT”) program and pause further accrual to our sponsored adenosine program for both PORT-6 and PORT-7. In the event that we resume these clinical trials and further development of our programs, our risks and uncertainties include, but are not limited to:
| · | our plans and ability to develop and commercialize product candidates and the timing of these development programs; |
| · | clinical development of our product candidates, including the timing for availability and release of results of current and future clinical trials; |
| · | our expectations regarding regulatory communications, submissions or approvals; |
| · | the potential functionality, capabilities, benefits and risks of our product candidates as compared to others; |
| · | our maintenance and establishment of intellectual property rights in our product candidates; |
| · | our need for financing and our estimates regarding our capital requirements and future revenues and profitability; |
| · | our estimates of the size of the potential markets for our product candidates; and |
| · | our selection and licensing of product candidates. |
Our business focus has been that of a pharmaceutical development business subject to all of the risks of a pharmaceutical development business. In the event that we resume enrollment in the clinical trials and further development of our programs, we do not anticipate directly engaging in the commercialization of the product candidates we develop.
These statements are based on assumptions and analyses made by us in light of our experience and our perception of historical trends, current conditions and expected future developments based on the focus of our business activities on biotechnology, as well as other factors we believe are appropriate in particular circumstances. However, whether actual results and developments will meet our expectations and predictions depends on a number of risks and uncertainties, which could cause actual results to differ materially from our expectations, including the risks set forth in Item 3 “Key Information – Risk Factors.”
Consequently, all of the forward-looking statements made in this Annual Report are qualified by these cautionary statements. We cannot assure you that the actual results or developments anticipated by us will be realized or, even if substantially realized, that they will have the expected effect on us or our business or operations.
Unless the context indicates otherwise the terms “Portage Biotech Inc.,” “the Company,” “our Company,” “Portage,” “we,” “us” or “our” are used interchangeably in this Annual Report and mean Portage Biotech Inc. and its subsidiaries.
| 1 |
FOREIGN PRIVATE ISSUER STATUS AND REPORTING CURRENCY
Foreign Private Issuer Status
Portage Biotech Inc. is a British Virgin Islands (“BVI”) business company pursuant to the Certificate of Continuance issued by the Registrar of Corporate Affairs of the BVI on July 5, 2013. More than 50% of our ordinary shares were held by non-United States residents as of the last measurement date. As a result, we believe that we qualify as a "foreign private issuer" for continuing to report regarding the registration of our ordinary shares using this Form 20-F annual report format.
Currency
The financial information presented in this Annual Report is expressed in United States dollars ("US $"), except where otherwise indicated, and the financial data in this Annual Report is presented in accordance with the International Financial Reporting Standards ("IFRS") as issued by the International Accounting Standards Board ("IASB") and interpretations of the International Financial Reporting Interpretations Committee.
PART I
ITEM 1 – IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISORS
Not required because this is an annual report under the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
ITEM 2 – OFFER STATISTICS AND EXPECTED TIMETABLE
Not required because this is an annual report under the Exchange Act.
ITEM 3 – KEY INFORMATION
| (A) | SELECTED FINANCIAL DATA |
The selected financial data set forth below should be read in conjunction with our Consolidated Financial Statements and Notes thereto appearing elsewhere in this Annual Report. The selected Operations Data for each of the three fiscal years ended March 31, 2024, 2023 and 2022, and the Balance Sheet data as of March 31, 2024 and 2023 are derived from our audited Consolidated Financial Statements appearing elsewhere in this Annual Report. The selected Operations Data for the years ended March 31, 2021 and 2020 and the Balance Sheet data as of March 31, 2022, 2021 and 2020 are derived from our audited Consolidated Financial Statements, which are not included in this Annual Report.
| 2 |
SUMMARY OF FINANCIAL INFORMATION IN THE COMPANY’S FINANCIAL STATEMENTS (U.S. DOLLARS)
Operating Data
| Years ended March 31, | 2024 | 2023 | 2022 | 2021 | 2020 | |||||||||||||||
| All amounts in 000'$ (except for per share amounts) | ||||||||||||||||||||
| Net loss before non-controlling interests | $ | (75,382 | ) | $ | (104,666 | ) | $ | (19,169 | ) | $ | (17,189 | ) | $ | (7,249 | ) | |||||
| Net loss attributable to owners of the Company | $ | (75,339 | ) | $ | (104,611 | ) | $ | (16,870 | ) | $ | (15,833 | ) | $ | (5,333 | ) | |||||
| Comprehensive loss | $ | (75,420 | ) | $ | (109,949 | ) | $ | (19,169 | ) | $ | (17,189 | ) | $ | (6,373 | ) | |||||
| Comprehensive loss attributable to the owners of the Company | $ | (75,377 | ) | $ | (109,894 | ) | $ | (16,870 | ) | $ | (15,833 | ) | $ | (4,457 | ) | |||||
| Working capital | $ | 4,816 | $ | 11,811 | $ | 24,049 | $ | 1,738 | $ | 1,226 | ||||||||||
| Total assets | $ | 7,779 | $ | 99,129 | $ | 194,662 | $ | 174,860 | $ | 173,174 | ||||||||||
| Capital stock | $ | 219,499 | $ | 218,782 | $ | 158,324 | $ | 130,649 | $ | 117,817 | ||||||||||
| Warrant liability | $ | 1,564 | $ | - | $ | 33 | $ | 1,120 | $ | - | ||||||||||
| Stock option reserves | $ | 23,841 | $ | 21,204 | $ | 16,928 | $ | 7,977 | $ | 58 | ||||||||||
| Equity attributable to owners of the Company | $ | 4,022 | $ | 76,045 | $ | 121,205 | $ | 101,449 | $ | 96,531 | ||||||||||
| Weighted average number of shares outstanding - Basic | 19,343 | 16,119 | 13,060 | 11,733 | 10,952 | |||||||||||||||
| Weighted average number of shares outstanding - Diluted | 19,343 | 16,119 | 13,060 | 11,733 | 10,952 | |||||||||||||||
| Net loss per share - Basic | $ | (3.89 | ) | $ | (6.49 | ) | $ | (1.29 | ) | $ | (1.35 | ) | $ | (0.49 | ) | |||||
| Net loss per share - Diluted | $ | (3.89 | ) | $ | (6.49 | ) | $ | (1.29 | ) | $ | (1.35 | ) | $ | (0.49 | ) | |||||
| 1. | The effect of potential share issuances pursuant to the exercise of options and warrants would be anti-dilutive and, therefore, basic and diluted loss per share are the same for the fiscal years presented. |
| 2. | The per share data has been adjusted to reflect the reverse split of the ordinary shares effective June 5, 2020. |
The Company has not declared or paid any dividends in any of the reporting periods presented herein.
Exchange Rates
In this Annual Report on Form 20-F, unless otherwise specified, all monetary amounts are expressed in United States dollars. The Company's subsidiaries have transactions in Canadian dollars, British pound sterling (“GBP”) and European Union (“EU”) euros. Currencies other than the United States dollar have been translated into United States dollars using rates available on Bank of Canada and the Bank of England websites.
On July 31, 2024, the exchange rate, based on the noon buying rates, for the conversion of Canadian dollars into United States dollars (the "Noon Rate of Exchange") was approximately US$1 = CDN$1.38, for the conversion of British pound sterling into United States dollars was approximately US$1 = £0.78 and for the conversion of EU euros into United States dollars was approximately US$1 = €0.92.
The following table sets out the high and low exchange rates in Canadian dollar, British pounds and EU euros for one United States dollar for each of the last six months of the fiscal year.
| Year ended March 31, 2024 | October | November | December | January | February | March | ||||||||||||||||||
| Canadian Dollar | ||||||||||||||||||||||||
| High | 1.39 | 1.39 | 1.36 | 1.35 | 1.36 | 1.36 | ||||||||||||||||||
| Low | 1.36 | 1.36 | 1.32 | 1.33 | 1.34 | 1.35 | ||||||||||||||||||
| British Pounds | ||||||||||||||||||||||||
| High | 0.83 | 0.82 | 0.80 | 0.79 | 0.80 | 0.79 | ||||||||||||||||||
| Low | 0.81 | 0.79 | 0.78 | 0.78 | 0.79 | 0.78 | ||||||||||||||||||
| EU Euros | ||||||||||||||||||||||||
| High | 0.96 | 0.95 | 0.93 | 0.92 | 0.93 | 0.93 | ||||||||||||||||||
| Low | 0.94 | 0.91 | 0.90 | 0.91 | 0.92 | 0.91 | ||||||||||||||||||
The following table sets out the average exchange rates in Canadian dollar, British pounds and EU euros for one United States dollar for the five most recent financial years.
| 3 |
| Years ended March 31, | 2024 | 2023 | 2022 | 2021 | 2020 | |||||||||||||||
| Average for the Fiscal Year | ||||||||||||||||||||
| Canadian Dollar | 1.35 | 1.32 | 1.25 | 1.32 | 1.33 | |||||||||||||||
| British Pounds | 0.80 | 0.83 | 0.73 | 0.77 | 0.79 | |||||||||||||||
| EU Euros | 0.92 | 0.96 | 0.86 | 0.86 | 0.90 | |||||||||||||||
We operate in various jurisdictions and are subject to exchange rates for the Canadian dollar, British pound and the Euro. We are subject to currency risk with respect to certain liabilities settleable in foreign currency, as well as invoices payable in foreign currency. While the rates have changed period to period, the overall effect of exchange rates on our financial statements have historically not been significant.
| (B) | CAPITALIZATION AND INDEBTEDNESS |
Not applicable.
| (C) | REASONS FOR THE OFFER AND USE OF PROCEEDS |
Not applicable.
| (D) | RISK FACTORS |
Risks Related to our Decision to Discontinue our iNKT Program and Pause Further Accrual in our Adenosine Program
After a review of our future funding needs for clinical development of our programs as well as the current capital raising market for biotechnology companies, we made the decision to discontinue our sponsored clinical trial for the iNKT program and pause further accrual to our sponsored adenosine program. We are exploring strategic alternatives, which may include finding a partner for one or more of our assets, a sale of our company, a merger, restructurings, both in and out of court, a company wind down, further financing efforts or other strategic action.
There can be no assurance that our evaluation of strategic alternatives will result in any agreements or transactions, or that, if completed, any agreements or transactions will be successful or on attractive terms. Any potential transaction would be dependent on a number of factors that may be beyond our control, including, among other things, market conditions, industry trends, the interest of third parties in a potential transaction with us and the availability of financing to us or third parties in a potential transaction with us on reasonable terms. The process of reviewing strategic alternatives may require us to incur additional costs and expenses. It could negatively impact our ability to attract, retain and motivate key employees, and expose us to potential litigation in connection with this process or any resulting transaction. If we are unable to effectively manage the process, our financial condition and results of operations could be adversely affected. In addition, any strategic alternative that may be pursued and completed ultimately may not deliver the anticipated benefits or enhance shareholder value. There can be no guarantee that the process of evaluating strategic alternatives will result in our company entering into or completing a potential transaction within the anticipated timing or at all. There is no set timetable for this evaluation and we do not intend to disclose developments with respect to this evaluation unless and until we determine that further disclosure is appropriate or legally required. As of July 31, 2024, we had approximately $3.1 million of cash and cash equivalents on hand, which we expect is only sufficient to cover our operating needs through December 2024.
Additionally, the Nasdaq Stock Market LLC (“Nasdaq”) may take the position that we are a “public shell” under Nasdaq rules, which could have negative consequences, including the potential delisting of our ordinary shares from the Nasdaq Capital Market. We have no current plans to delist our ordinary shares from Nasdaq. However, following the decision to discontinue our sponsored clinical trial for the INKT program and pause further accrual to our sponsored adenosine program, we may be treated as a public shell under Nasdaq rules. Although Nasdaq evaluates whether a listed company is a public shell company based on a facts and circumstances determination, a Nasdaq-listed company with no or nominal operations and either no or nominal assets, assets consisting solely of cash and cash equivalents, or assets consisting of any amount of cash and cash equivalents and nominal other assets is generally considered to be a public shell company.
The following is a brief discussion of the most significant risk factors that are specific to our operations and industry and that may have a material impact on, or constitute the most significant risk factors in respect of, our future financial performance in the event that we were to raise additional capital to fund the clinical development of our programs.
| 4 |
Risks Related to our Business
We have current and future capital needs, and, if we decide to resume enrollment in our clinical trials, there are uncertainties as to our ability to raise additional funding.
Our current cash resources will not cover all of our operational costs and the needs of our subsidiaries to progress towards clinical trials, if we decide to resume enrollment in our clinical programs. Additional capital would be needed to test product candidate in human trials, obtain regulatory approvals and ultimately to commercialize such product candidates if approved.
In addition, our future cash requirements may vary materially from those now expected. For example, our future capital requirements may increase if:
| · | we experience scientific progress sooner than expected in our future discovery, research and development projects, if we expand the magnitude and scope of these activities, or if we modify our focus as a result of our discoveries; |
| · | we experience setbacks in our progress with pre-clinical studies and clinical trials are delayed; |
| · | we experience delays or unexpected increased costs in connection with obtaining regulatory approvals, particularly in light of the current inflationary environment; |
| · | we are required to perform additional pre-clinical studies and/or clinical trials; |
| · | we experience unexpected or increased costs relating to preparing, filing, prosecuting, maintaining, defending and enforcing patent claims; or |
| · | we elect to develop, acquire or license new technologies and products. |
We have incurred, and, if we decide to resume enrollment in our clinical programs, we expect to continue to incur substantial costs related to the development of our product candidates, including costs related to the clinical trials for our adenosine platform. If sufficient capital is not available and we decide to resume enrollment in our clinical programs, we may be required to delay, reduce the scope of, eliminate or divest of one or more of our research or development projects, any of which could have a material adverse effect on our business, financial condition, prospects or results of operations.
Furthermore, under General Instruction I.B.5 to Form F-3 (the “Baby Shelf Rule”), the amount of funds we can raise through primary public offerings of securities in any 12-month period using a registration statement on Form F-3 is limited to one-third of the aggregate market value of the ordinary shares held by non-affiliates of our company, which limitation may change over time based on our stock price, number of ordinary shares outstanding and the percentage of ordinary shares held by non-affiliates. We therefore are limited by the Baby Shelf Rule as of the filing of this Annual Report, until such time as our non-affiliate public float exceeds $75 million.
We have a history of operating losses and may never achieve profitability in the future.
Historically, we have generated only a limited amount of business income, notwithstanding a highly valued asset distribution to our shareholders share ownership of Biohaven.
Prior to our decision to discontinue our iNKT program and pause further accrual in our adenosine program, our objective was to enable research and development so as to create early- to mid-stage, first- and best-in-class therapies for a variety of cancers, by providing funding, strategic business and clinical counsel, and shared services, with the goal of creating viable products that may be monetized through licensing, manufacturing and distribution or outright sale. Our principal activities were engaging in research and development to identify and validate new drug targets that could become marketed drugs in the future. If we decide to resume enrollment in our clinical programs, we will require significant financial resources without any income, and we expect to continue incurring operating losses for the foreseeable future.
| 5 |
Our ability to generate revenue in the future or achieve profitable operations is largely dependent upon our ability to attract and maintain experienced management and know-how to develop new drug candidates and to partner with major pharmaceutical companies to successfully commercialize any successful drug candidates. It takes many years and significant financial resources to successfully develop pre-clinical or early clinical drug candidates into marketable drugs, and we cannot assure you that we will be able to achieve these objectives. Although, we were successful in achieving significant value growth in an investment made in Biohaven, which resulted in the distribution of Biohaven shares as an asset dividend to our shareholders with a then market value of approximately $153 million in fiscal 2018, we cannot guarantee that we will be able to achieve any similar success in our future business activities.
We are in the pharmaceutical development business and will be subject to all of the risks of a pharmaceutical research and development business.
Our business must be evaluated in light of the risks, delays, uncertainties and complications encountered in connection with establishing and carrying on a pharmaceutical research and development business.
If we decide to resume enrollment in our clinical programs, there is a possibility that only a few or none of our drug candidates that may be developed in the future, will be determined to be safe and effective by the governing regulatory bodies, will be able to receive and maintain necessary regulatory approvals in order to be commercialized, or will be commercially viable. Any failure to successfully develop and obtain regulatory approval for our product candidates would have a material adverse effect on our business, financial condition and results of operations.
Rapidly changing medical technology within the life sciences industry could make the product candidates that we may develop in the future obsolete or less attractive to pursue.
The medical industry is characterized by rapid and significant medical technological and therapy changes, frequent new product candidates and product introductions and enhancements and evolving industry standards. If we decide to resume enrollment in our clinical programs, our future success will depend on our ability to continually develop and then improve our product candidates and to develop and introduce new product candidates that address the evolving needs of the physicians and patients on a timely and cost-effective basis. Our new product candidates and products may not be accepted in the intended markets, and our inability to gain market acceptance of new products could harm our future operating results.
Clinical trials for our product candidates will be expensive if we decide to resume enrollment in our clinical programs and will take a considerable amount of time, and the outcomes of such clinical trials are by their nature uncertain.
If we decide to resume enrollment in our clinical programs, we will be required to complete extensive clinical trials to demonstrate safety and efficacy before we can obtain regulatory approval for the commercial sale of any product candidate or attract major pharmaceutical companies to collaborate with us. Clinical trials are very expensive and are difficult to design and implement. The clinical trial process also takes a long time and can often be subject to unexpected delays or have unexpected results.
The timing of the commencement, continuation and completion of clinical trials has been, and may continue to be subject to significant delays relating to various causes, including:
| · | our inability to manufacture or obtain sufficient quantities of materials for use in clinical trials; |
| · | measures related to the COVID-19 pandemic or other similar circumstances; |
| · | delays arising from our collaborative partnerships; |
| · | delays in obtaining regulatory permission to commence a clinical trial, or government intervention to delay, suspend or terminate a clinical trial; |
| · | delays in approving, or refusal to approve, or suspension, or termination of a clinical trial by the institutional review board or independent ethics board responsible for overseeing the trial; |
| · | delays in identifying and reaching agreement on acceptable terms with prospective clinical trial sites, clinical research organizations, laboratories and testing facilities, or other vendors providing clinical trial services; |
| 6 |
| · | slower than expected rates of patient recruitment and enrollment or patients’ early withdrawal from participation; |
| · | uncertain dosing issues; |
| · | inability or unwillingness of medical investigators to follow our clinical protocols; |
| · | variability in the number and types of subjects available for each trial and resulting difficulties in identifying and enrolling subjects who meet trial eligibility criteria; |
| · | scheduling conflicts with participating clinicians and clinical institutions; |
| · | difficulty in maintaining contact with subjects after treatment, which could result in incomplete data; |
| · | unforeseen safety issues or side effects; |
| · | lack of demonstrated efficacy during the clinical trials; |
| · | our reliance on clinical trial sites, clinical research organizations, laboratories and testing facilities and other vendors to conduct clinical trials or provide clinical trial services, which may not conduct those trials in compliance with applicable laws and regulations, or current good clinical or laboratory practices; |
| · | changes in laws or regulations applicable to clinical trial requirements; or |
| · | other regulatory delays. |
If we decide to resume enrollment in our clinical programs, we will rely on third parties to manufacture our preclinical and clinical drug supplies, and we intend to rely on third parties to produce commercial supplies of any product candidate if approved by a regulatory authority.
We have limited personnel with experience in manufacturing, and we do not own facilities for manufacturing product candidates for the potential clinical trials and/or commercial manufacturing of product candidates if approved. If we decide to resume enrollment in our clinical programs, we will depend on our collaboration partners and other third parties to manufacture and provide analytical services with respect to our most advanced product candidates.
If our product candidates are approved, then in order to produce the quantities necessary to meet anticipated market demand, we and our collaboration partners will need to secure sufficient manufacturing capacity with third-party manufacturers. If we and our collaboration partners are unable to produce, or obtain the materials necessary to produce, any approved product in sufficient quantities to meet the requirements for the launch of any such product or to meet future demand, our revenues and gross margins could be adversely affected. To be successful, any approved product must be manufactured in commercial quantities in compliance with regulatory requirements and at acceptable costs. We and our collaboration partners will regularly need to secure access to third-party facilities to manufacture our product candidates commercially. All of this will require additional funds and inspection and approval by the Competent Authorities of the Member States of the European Economic Area (“EEA”), the United States Food and Drug Administration (“FDA”) and other regulatory authorities. If we and our collaboration partners are unable to establish and maintain a manufacturing capacity within our planned time and cost parameters, the development of our product candidates and future sales of any product candidates, if approved, as well as our business, results of operations and prospects, and the value of our ordinary shares could be materially adversely affected.
| 7 |
We and our collaboration partners may encounter problems with aspects of manufacturing our product candidates or any approved products, including the following:
| · | production yields; |
| · | quality control and assurance; |
| · | shortages of qualified personnel; |
| · | compliance with FDA and EEA regulations; |
| · | production costs; and |
| · | development of advanced manufacturing techniques and process controls. |
Prior to our decision to discontinue our iNKT program and pause further accrual in our adenosine program, we evaluated our options for clinical trial supplies and commercial production for our product candidates on a regular basis, including use of third-party manufacturers, or entering into a manufacturing joint venture relationship with a third party. We are aware of only a limited number of companies on a worldwide basis that operate manufacturing facilities in which our product candidates can be manufactured under current Good Manufacturing Practice ("cGMP”) regulations, a requirement for all pharmaceutical products in the U.S. We cannot be certain that we and our collaboration partners will be able to contract with any of these companies on acceptable terms to us, if at all, if we decide to resume enrollment in our clinical programs, which could harm our business, results of operations and prospects, and the value of our ordinary shares.
In addition, if we decide to resume enrollment in our clinical programs, any manufacturing facility that we utilize will be required to be registered with the FDA (and have a U.S. agent for the facility, if outside the United States), the Competent Authorities of the Member States of the EEA, and other regulatory authorities. The facilities will be subject to inspections confirming compliance with the FDA, the Competent Authorities of the Member States of the EEAs, or other regulatory authority cGMP requirements. We have not directly controlled the manufacturing process of our product candidates, and, if we decide to resume enrollment in our clinical programs, we would be dependent on our contract manufacturing partners for compliance with cGMP regulations for the manufacture of both active drug substances and finished drug products. If we or our collaboration partners or any third-party manufacturer fail to maintain regulatory compliance, the FDA, the Competent Authorities of the Member States of the EEA, or other regulatory authorities may take enforcement action that may include issuing a warning letter, instituting a clinical hold, withdrawing regulatory approval, seeking product seizures or injunctions and, where appropriate, pursuing criminal prosecution, any of which could have an adverse effect on our business, financial condition and results of operations.
The results of pre-clinical studies and initial clinical trials may not be predictive of future results, if we decide to resume enrollment in our clinical programs, and our product candidates may not have favorable results in later trials or in the commercial setting.
Pre-clinical tests and Phase 1 and Phase 2 clinical trials are primarily designed to test safety, to study pharmacokinetics and pharmacodynamics, to understand the side effects of product candidates, and to explore efficacy at various doses and schedules. Favorable results in early trials may not be repeated in later trials. Any success that we may experience in pre-clinical or animal studies and early clinical trials if we decide to resume enrollment in our clinical programs does not ensure that later large-scale efficacy trials will be successful, and does not predict final trial results, which could have an adverse effect on our business, financial condition and results of operations.
A number of companies in the life sciences industry have suffered significant setbacks in advanced clinical trials, even after positive results in earlier trials. Clinical results are frequently susceptible to varying interpretations that may delay, limit or prevent regulatory approvals. Negative or inconclusive results or adverse medical events during a clinical trial could cause a clinical trial to be delayed, repeated or terminated. In addition, failure to construct appropriate clinical trial protocols could result in the test or control group experiencing a disproportionate number of adverse events, which could also cause a clinical trial to be repeated or terminated.
There is typically a high rate of attrition for product candidates proceeding through clinical and post-approval trials.
| 8 |
We may face difficulty in enrolling patients in our clinical trials if we decide to resume enrollment in our clinical programs.
If we decide to resume enrollment in our clinical programs, we may find it difficult to enroll qualifying patients in our clinical trials. The timing of our current and future clinical trials depends, in part, on the speed at which we can recruit qualifying patients to participate in testing our therapeutic candidates. If qualifying patients are unwilling to participate in our trials because of negative publicity from adverse reactions or for other reasons, including competitive clinical trials for similar patient populations, then the timeline for recruiting patients, conducting trials and obtaining regulatory approval of potential products may be delayed. These delays could result in increased costs, delays in advancing our product development, delays in testing the effectiveness of our technology or termination of the clinical trials altogether. We may not be able to identify, recruit and enroll a sufficient number of qualifying patients, or those with required or desired characteristics to achieve sufficient diversity in a given trial in order to complete our clinical trials in a timely manner. If we have difficulty enrolling a sufficient number of qualifying patients to conduct our clinical trials as planned, we may need to delay, limit or terminate ongoing or planned clinical trials, any of which could have an adverse effect on our business.
The outcomes of clinical trials are uncertain and our clinical trials may fail to demonstrate adequately the safety or efficacy of a particular therapeutic candidates if we decide to resume enrollment in our clinical programs, which would prevent or delay regulatory approval and commercialization.
There is a risk in any clinical trial that side effects from our product candidates will require a hold on, or termination of, our clinical program(s) or further adjustments to our clinical program(s) in order to progress our product candidates if we decide to resume development of our clinical programs. We will need to demonstrate that the product candidate are safe and effective for use in each target indication. Each product candidate must demonstrate an acceptable risk versus benefit profile in its intended patient population and for its intended use. The risk/benefit profile required for product licensure will vary depending on these factors.
If we decide to resume enrollment in our clinical programs, our success will be dependent upon our collaborations with third parties in connection with services we will need for the development, marketing and commercialization of our products candidates, if approved.
If we decide to resume enrollment in our clinical programs, the success of our business will be largely dependent on our ability to enter into collaborations regarding the development, clinical testing, regulatory approval and commercialization of our product candidates. We may not be able to find collaborative partners to support the future development, marketing and commercialization of our product candidates, which may require us to undertake research and development and/or commercialization activities ourselves and may result in a material adverse effect on our business, financial condition, prospects and results of operations.
Even if we are able to find new collaborative partners, our success is highly dependent upon the performance of these new collaborators. The amount and timing of resources to be devoted to activities by future collaborators, if any, are not within our direct control and, as a result, we cannot assure you that any future collaborators will commit sufficient resources to our research and development projects or the commercialization of our product candidates if approved. Any future collaborators might not perform their obligations as expected and might pursue existing or other development-stage products or alternative technologies in preference to those being developed in collaboration with us, or may terminate particular development programs, or the agreement governing such development programs which could have a material adverse effect on our business, financial condition, prospects and results of operations.
In addition, if any future collaborators fail to comply with applicable regulatory requirements, the FDA, the European Medicines Agency ("EMA"), the Therapeutic Products Directorate of Canada ("TPD") or other authorities could take enforcement action that could jeopardize our ability to develop and commercialize our product candidates. Despite our best efforts to limit them, disputes may arise with respect to ownership of technology developed under any such corporate collaboration which could have a material adverse effect on our business, financial condition, prospects and results of operations.
| 9 |
We will rely on proprietary technology, the protection of which can be unpredictable and costly.
Our success will depend in part upon our ability to obtain and maintain patent protection or patent licenses for our current and future technology related to our product candidates. Obtaining patent protection or patent licenses can be costly and the outcome of any application for patent protection and patent licenses can be unpredictable. In addition, any breach of confidentiality by a third party by premature disclosure may preclude us from obtaining appropriate patent protection, thereby affecting the development and commercial value of our technology and products.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our owned and licensed patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
The patent prosecution process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications in jurisdictions of interest at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. Moreover, in some circumstances, such as with respect to the LICR License described below, we do not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the patents, covering technology that we license from third parties. Therefore, these patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business. If such licensors fail to maintain such patents, or lose rights to those patents, the rights we have licensed may be reduced or eliminated. Moreover, changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection.
Some of our future products rely on licenses of proprietary technology owned by third parties and we may not be able to maintain these licenses on favorable terms or at all.
The development, manufacture and sale of some of the products we develop if we decide to resume enrollment in our clinical programs will involve the use of processes, products, or information, the rights to which are owned by third parties. For example, we rely on certain in-licenses for the development and commercialization of our adenosine receptor antagonists platforms, respectively. If we are unable to obtain and maintain patent protection for technology related to our product candidates, or if our licensors are unable to obtain and maintain patent protection for the technology or products that we license from them, or if the scope of the patent protection obtained is not sufficiently broad, our competitors could develop and commercialize technology and products similar or identical to ours, and our ability to successfully commercialize our technology and products may be impaired. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates. In addition, invalidation of our patent rights by third parties could jeopardize the anticipated revenue streams from current licensees.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our and our licensors’ patent rights are highly uncertain. Any of the abovementioned risks could have a material adverse on us and our business.
| 10 |
We may not be able to successfully identify, consummate or integrate acquisitions or to successfully manage the impacts of such transactions on our operations.
Part of our business strategy has included pursuing synergistic acquisitions, such as our recent acquisition of Tarus Therapeutics. We have expanded, and may plan to continue to expand, our business by making strategic acquisitions and regularly seeking suitable acquisition targets to enhance our growth though we do not have any plans to do so at this time due to our current liquidity. Material acquisitions, dispositions and other strategic transactions involve a number of risks, including: (i) the potential disruption of our ongoing business; (ii) the distraction of management away from the ongoing oversight of our existing business activities; (iii) finding equity funding and incurring additional indebtedness; (iv) issuing additional equity which may have a dilutive effect on our capital, (v) the anticipated benefits and cost savings of those transactions not being realized fully, or at all, or taking longer to realize than anticipated; (vi) an increase in the scope and complexity of our operations; and (vii) the loss or reduction of control over certain of our assets.
The pursuit of acquisitions may pose certain risks to us. We may not be able to identify acquisition candidates that fit our criteria for growth and profitability. Even if we are able to identify such candidates, we may not be able to acquire them on terms or financing satisfactory to us. We will incur expenses and dedicate attention and resources associated with the review of acquisition opportunities, whether or not we consummate such acquisitions.
We rely on information technology and security systems and any damage, interruption or compromise of our information technology and security systems or data could disrupt and harm our business.
We use information technology and security systems to process, transmit and store electronic information in connection with the operation of our business. We also use such systems to protect proprietary and confidential information, including that of physicians, patients, and other individuals involved in clinical trials, suppliers, and employees. We face risks associated with cybersecurity incidents and other significant disruptions of such systems, including denial of service or other similar attacks, to our facilities or systems; unauthorized access to or acquisition of personal information, confidential information or other data we process or maintain; or viruses, loggers, or other malfeasant code, including ransomware, in our data or software. These cybersecurity incidents or other significant disruptions could be caused by persons inside our organization, persons outside our organization with authorized access to systems inside our organization, or by individuals outside our organization. The risk of a cybersecurity incident or disruption, particularly through cyber-attack or cyber-intrusion, has generally increased as the number, intensity and sophistication of attempted attacks and intrusions from around the world have increased. Although we have not experienced any cybersecurity incidents to date, and have not been affected by any incidents incurred by third-party partners, such incidents could have a material adverse effect on our business, financial condition or results of operations in the future. Additionally, future or past business transactions (such as acquisitions or integrations) could expose us to additional cybersecurity risks, as our information technology and systems could be negatively affected by vulnerabilities present in acquired or integrated entities’ systems and technologies. Furthermore, we may learn of cybersecurity issues that were not identified during due diligence of such entities, and it may be difficult to integrate entities into our information technology environment and security program.
We also rely on a number of third-party service providers to host, store or otherwise process information for us, or to provide other facilities or infrastructure that we make use of, including “cloud-based” providers of corporate infrastructure services relating to, among other things, human resources, communication services and some financial functions, and we are therefore dependent on the security systems of these providers. These third-party entities are subject to similar risks as we are relating to cybersecurity, business interruption and systems and employee failures and a cybersecurity incident or other significant disruption affecting such third parties could have a material adverse effect on our business. While we may be entitled to damages if our third-party service providers fail to satisfy their security-related obligations to us, any award may be insufficient to cover our damages, or we may be unable to recover such award.
| 11 |
Because the techniques used to obtain unauthorized access to or sabotage security systems change frequently and are often not recognized until after an attack, we and our third-party service providers may be unable to anticipate the techniques or implement adequate preventative measures, thereby exposing us to material adverse effects on our business, financial condition, results of operations and growth prospects. In order to address risks to our information systems, we continue to make investments in personnel, technologies and training. Data protection laws and regulations around the world, including in jurisdictions where operate, like the U.S. and EU, often require “reasonable,” “appropriate,” or “adequate” technical and organizational security measures, and the interpretation and application of those laws and regulations are often uncertain and evolving; there can be no assurance that our security measures will be deemed adequate, appropriate or reasonable by a regulator or court. Moreover, even security measures that are deemed appropriate, reasonable and/or in accordance with applicable legal requirements may not be able to protect the information we maintain. A cybersecurity incident or other significant disruption impacting us or our third-party service providers could require a substantial level of financial resources to rectify and otherwise respond to, may be difficult to identify or address in a timely manner, may compromise our research, the therapies we are developing or other intellectual property or trade secrets, and may divert management’s attention and require the expenditure of significant time and resources. Such cybersecurity incidents or other significant disruptions could result in claims, increased regulatory scrutiny or investigations, and may cause us to incur substantial fines, penalties or other liability and related legal and other costs. Any actual or perceived cybersecurity incident or significant disruption may also interfere with our ability to comply with financial reporting requirements and harm our reputation and market position, especially given that we handle sensitive information, including clinical trial data. Any of the foregoing matters could harm our operating results and financial condition.
While we have purchased cybersecurity insurance, there are no assurances that the coverage would be adequate in relation to any incurred losses. Moreover, as cyber-attacks increase in frequency and magnitude, we may be unable to obtain cybersecurity insurance in amounts and on terms we view as adequate for our operations.
Any actual or perceived failure by us to comply with government or other obligations related to privacy or data protection could adversely affect our business.
We are subject to compliance risks and uncertainties under a variety of global laws and regulations governing privacy, data protection and the collection, storage, transfer, use, retention, sharing, disclosure, protection and processing of personal data, including personal data of physicians, patients, and other individuals involved in clinical trials. These laws may include sector-specific requirements, including laws or regulations that govern health or clinical trial data. In addition, we may obtain health data from third parties (including research institutions from which we obtain clinical trial data) that is subject to privacy and security requirements. For example, the U.S. Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”) imposes obligations on certain types of individuals and entities, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information. Privacy and data protection laws may be interpreted and applied differently depending on the jurisdiction and continue to evolve, making it difficult to predict how they may develop and apply to us. The regulatory frameworks for these issues worldwide are rapidly evolving and are likely to remain uncertain for the foreseeable future. Federal, state, or non-U.S. government bodies or agencies have in the past adopted, and may in the future adopt, new laws and regulations or may make amendments to existing laws and regulations affecting data privacy or data protection. In addition to government regulation, industry groups have established or may establish new and different self-regulatory standards that may legally or contractually apply to us or our prospective customers. Failure to comply with these varying laws and standards may subject us to investigations, enforcement actions, civil litigation, fines, claims for damages by third parties or affected individuals, damage to our reputation and loss of goodwill, impact our ability to conduct our research and produce therapies and result in other civil or criminal penalties, all of which may generate negative publicity and have a negative impact on our business, financial condition, results of operations or prospects.
In the United States, there are numerous federal and state laws and regulations related to the privacy and security of personal data that may be applicable to our current and future activities. Numerous federal and state laws and regulations protect the confidentiality, privacy, availability, integrity and security of personal data in the United States. Legal requirements vary from state to state, and these laws and regulations in many cases are more restrictive than, and may not be preempted by, federal privacy laws and regulations. These laws and regulations are often uncertain, contradictory, and subject to changing or differing interpretations. Certain state laws may include a private right of action for certain data breaches or noncompliance with privacy obligations, may provide for penalties and other remedies, and may require us to incur substantial costs and expenses and liabilities in connection with our compliance. Other U.S. states and the U.S. federal government are considering or have enacted similar privacy legislation.
| 12 |
Outside the United States, an increasing number of laws, and regulations may govern data privacy and security. As a company doing business in Europe, we are also subject to European data protection laws and regulations. The European Union General Data Protection Regulation (“GDPR”) imposes stringent requirements regarding how we collect and process personal data and provides for significant penalties for noncompliance. Several other countries have passed laws that require personal data relating to their citizens to be maintained on local servers and impose additional data transfer restrictions. In addition, the United Kingdom has adopted a framework similar to the GDPR. The EU has confirmed the UK data protection framework as being “adequate” to receive EU personal data. Although there are currently various mechanisms that may be used to transfer personal data from the EEA and UK to the United States in compliance with law, these mechanisms are subject to legal challenges, and there is no assurance that we can satisfy or rely on these measures to lawfully transfer personal data to the United States. For example, there have been recent updates to laws and regulations governing transfers of EU data, including updates to Standard Contractual Clauses and a proposed EU-US data transfer adequacy agreement. In light of these and other ongoing developments relating to cross-border data transfers, we may experience additional costs associated with increased compliance burdens, and this regulation may impact our ability to transfer personal data across our organization, to clinical trial physicians or patients, to our customers, or to third parties.
We are subject to risks associated with doing business globally.
As a pharmaceutical research and development company, if we decide to resume enrollment in our clinical programs, our operations are likely to expand in the European Union and many other developed countries worldwide, and we will be subject to political, economic, operational, legal, regulatory and other risks that are inherent in conducting business globally. For example, we currently have ongoing clinical operations in the U.K. and are contemplating expanding to other countries. These risks include foreign exchange fluctuations, exchange controls, capital controls, requirements to comply with new laws or regulations or changes in the interpretation or enforcement of existing laws or regulations, political instability, macroeconomic changes, including recessions and inflationary or deflationary pressures, increases in prevailing interest rates by central banks or financial services companies, economic uncertainty, which may adversely affect our research and development, reduce the demand for our potential products and reduce the prices that our potential customers will be willing to pay for our potential products, import or export restrictions, tariff increases, price controls, nationalization and expropriation, changes in taxation, diminished or insufficient protection of intellectual property, lack of access to impartial court systems, violations of law, including the U.S. Foreign Corrupt Practices Act and the United Kingdom (“U.K.”) Bribery Act, disruption or destruction of operations or changes to our business position, regardless of cause, including pandemic, war, terrorism, riot, civil insurrection, social unrest, strikes and natural or man-made disasters, including famine, flood, fire, earthquake, storm or disease. The impact of any of these developments or events, either individually or cumulatively, could have a material adverse effect on our business, financial condition and results of operations.
We may face exposure to adverse movements in foreign currency exchange rates.
We intend to generate revenue and expenses internationally that are likely to be primarily denominated in U.S. dollars, Euros and British pound sterling. Our intended international business will be subject to risks typical of an international business including, but not limited to, differing tax structures, a myriad of regulations and restrictions and general foreign exchange rate volatility. A decrease in the value of such foreign currencies relative to the United States dollar could result in losses in revenues from currency exchange rate fluctuations. Conversely, an increase in the value of such foreign currencies relative to the United States dollar could negatively impact our operating expenses. To date, we have not hedged against risks associated with foreign exchange rate exposure. We cannot be sure that any hedging techniques we may implement in the future will be successful or that our business, results of operations, financial condition and cash flows will not be materially adversely affected by exchange rate fluctuations.
The loss of key personnel could have an adverse effect on our business.
We are highly dependent upon the efforts of our senior management. The loss of the services of one or more members of senior management could have a material adverse effect on us as a small company with a streamlined management structure, and would be potentially disruptive to our business until such time as a suitable replacement is hired. We do not carry any key person insurance on our senior management.
| 13 |
The U.K.’s withdrawal from the EU, commonly referred to as Brexit, continues to result in regulatory uncertainty which may have a negative effect on global economic conditions, financial markets and our business.
Brexit created significant uncertainty concerning the future relationship between the U.K. and the EU. From a regulatory perspective, there is uncertainty about which laws and regulations will apply. A significant portion of the regulatory framework in the U.K. is derived from EU laws. However, it is unclear which EU laws the U.K. will decide to replace or replicate in connection with its withdrawal from the EU. In particular, the regulatory regime applicable to our operations, including with respect to conduct of clinical trials and the approval of our product candidates if we decide to resume development of our clinical programs, may change, potentially significantly, and the impact of these changes is difficult to quantify until new regulation and guidance is published.
A basic requirement related to the grant of a marketing authorization for a medicinal product in the EU is the requirement that the applicant be established in the EU. Following the expiry of the Brexit transitional arrangements, separate applications for marketing authorizations for Great Britain (England, Scotland and Wales) are required to place medicinal products on the market in Great Britain. The European Commission Decision Reliance Procedure, which allowed the U.K. regulatory to “rely” on EU centralized marketing authorization decisions, expired on December 31, 2023. The EU mutual recognition and decentralized procedures no longer apply to Great Britain. From January 1, 2024, under the Windsor Framework, the EU no longer has jurisdiction over medicines placed on the market in Northern Ireland and all medicines intended for Northern Ireland (or the U.K. market more generally) will require a U.K. marketing authorisation. Additional regulation and guidance is anticipated to govern how this new regime will operate, including as to labelling of medicines in Northern Ireland.
To replace EU based mutual recognition procedures, the U.K. has announced plans to introduce an international reliance route for the approval of medicinal products in the U.K. From January 1, 2024, the U.K. intends to recognize approvals of medicinal products from: Australia, Canada, the European Union, Japan, Switzerland, Singapore and the United States. This approach may benefit out strategy and operations if we decide to resume development of our clinical programs as it could lead to approval in the U.K. of “cutting-edge medicines” more quickly and through a more streamlined regulatory process. However, the procedure will not come into effect until new regulations are introduced and these have not yet been published. Delays in implementing this new legislation may lead to regulatory uncertainty and delays.
In addition, the laws and regulations that apply since the U.K.’s withdrawal from the EU may have implications for manufacturing sites that hold certifications issued by the U.K. competent authorities. If batch release and quality control testing sites for our product candidates are located only in the U.K., manufacturers will need to use sites in other EU member states for EU batch release. All of these changes, if they occur, could increase our costs and otherwise adversely affect our business.
Currency exchange rates for the British pound and the Euro, with respect to each other and to the U.S. dollar, were affected by Brexit, and could be affected in the future by other global events.
Risks Related to Ownership of our Shares
We may not be able to regain, or maintain, compliance with the continued listing requirements of The Nasdaq Capital Market.
Our ordinary shares are listed on the Nasdaq Capital Market, and we are therefore subject to its continued listing requirements, including requirements with respect to the market value of our publicly-held shares, market value of our listed shares, minimum bid price per share, and minimum shareholders' equity, among others. If we fail to satisfy one or more of the requirements, we may be delisted from the Nasdaq Capital Market.
On March 7, 2024, we received notice (the “Notice”) from Nasdaq that we are not currently in compliance with the $1.00 minimum bid price requirement for continued listing on the Nasdaq Capital Market, as set forth in Nasdaq Listing Rule 5550(a)(2). The Notice indicated that, consistent with Nasdaq Listing Rule 5810(c)(3)(A), we have 180 calendar days, or until September 3, 2024, to regain compliance with the minimum bid price requirement by having the closing bid price of our ordinary shares meet or exceed $1.00 per share for at least ten consecutive business days. The notification had no immediate effect on the listing of our ordinary shares, and our ordinary shares will continue to trade on the Nasdaq Capital Market under the symbol “PRTG” at this time.
| 14 |
If we do not regain compliance by September 3, 2024, we may be eligible for an additional 180 calendar day grace period. If we fail to regain compliance during the applicable period, we will receive notification from Nasdaq that our ordinary shares are subject to delisting. Such notification will have no immediate effect on our listing on the Nasdaq Capital Market, nor will it have an immediate effect on the trading of our ordinary shares pending such hearing. At that time, we may then appeal the delisting determination to a Nasdaq hearings panel. There can be no assurance, however, that we will be able to regain compliance with Nasdaq’s minimum bid price requirement. If we regain compliance with Nasdaq’s minimum bid price requirement, there can be no assurance that we will be able to maintain compliance with the continued listing requirements for the Nasdaq Capital Market or that our ordinary shares will not be delisted from the Nasdaq Capital Market in the future. In addition, we may be unable to meet other applicable listing requirements of the Nasdaq Capital Market, including maintaining minimum levels of shareholders’ equity or market values of our ordinary shares in which case, our ordinary shares could be delisted notwithstanding our ability to demonstrate compliance with the minimum bid price requirement.
Additionally, Nasdaq Listing Rule 5550(b)(1) requires companies listed on the Nasdaq Capital Market to maintain shareholders’ equity of at least $2.5 million for continued listing. As of March 31, 2024, our shareholders’ equity was $3.3 million and there can be no assurance that we will be able to maintain or increase our shareholders’ equity in the future. If our shareholders’ equity falls below $2.5 million, as a result of operating losses or for other reasons, or if we are unable to demonstrate to Nasdaq’s satisfaction that we subsequently regained compliance with this requirement, Nasdaq will notify us of such non-compliance. If we receive such notice from Nasdaq, in accordance with the Nasdaq Listing Rules, we will have 45 calendar days from the date of the notification to submit a plan to regain compliance with Nasdaq Listing Rule 5550(b)(1). If our compliance plan is accepted, we may be granted up to 180 calendar days from the date of the initial notification to evidence compliance. If our compliance plan is not accepted or we are otherwise unable to evidence compliance within Nasdaq’s allotted timeframe, Nasdaq may take steps to delist our ordinary shares.
Delisting from the Nasdaq Capital Market may adversely affect our ability to raise additional financing through the public or private sale of equity securities, may significantly affect the ability of investors to trade our securities and may negatively affect the value and liquidity of our ordinary shares. Delisting also could have other negative results, including the potential loss of employee confidence, the loss of institutional investors or interest in business development opportunities.
If we are delisted from Nasdaq and we are not able to list our ordinary shares on another exchange, our ordinary shares could be quoted on the OTC Bulletin Board or in the “pink sheets.” As a result, we could face significant adverse consequences including, among others:
| · | a limited availability of market quotations for our securities; |
| · | a determination that our shares are a “penny stock” which will require brokers trading in our ordinary shares to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for our securities; |
| · | a limited amount of news and little or no analyst coverage for us; |
| · | we would no longer qualify for exemptions from state securities registration requirements, which may require us to comply with applicable state securities laws; and |
| · | a decreased ability to issue additional securities (including pursuant to short-form Registration Statements on Form F-3) due to the Baby Shelf Rule or obtain additional financing in the future. |
The issuance of additional ordinary shares, including upon the exercise of our outstanding stock options, will dilute the ownership interest of our existing shareholders and increase the number of ordinary shares eligible for future resale.
As of March 31, 2024, we had 369,340 vested restricted stock units outstanding, which are subject to certain restrictions. Additionally, as of March 31, 2024, we had an aggregate of 1,805,620 stock options to acquire ordinary shares outstanding. During the year ended March 31, 2024 (“Fiscal 2024”), we issued shares as follows: 1,970,000 ordinary shares (along with 1,187,895 pre-funded warrants and four tranches totaling 9,631,580 detachable warrants) relating to the Registered Direct Offering (as defined below); 186,604 ordinary shares sold pursuant to our “at-the-market” (“ATM”) offering program; 15,872 ordinary shares issued to a service provider for services rendered; and 6,165 shares issued pursuant to the exercise of restricted stock units.
| 15 |
Our principal shareholders and senior management own a significant percentage of our ordinary shares and are able to exert significant control over matters subject to shareholder approval.
As of August 14, 2024, our senior management, board members, holders of 5% or more of our share capital and their respective affiliates beneficially owned approximately 41.5% of our outstanding voting securities. As a result, these security holders may have the ability either alone or voting together as a group to determine and/or significantly influence the outcome of matters submitted to our shareholders for approval, including the election and removal of board members, payment of dividends, amendments to our articles of association, including changes to our share capital, or certain mergers, demergers, liquidations and similar transactions. This may prevent or discourage unsolicited acquisition proposals or offers for our ordinary shares that our shareholders may feel are in their best interest as a shareholder. In addition, this group of shareholders generally has the ability to control our management and business affairs and direction of our business. Such control and concentration of ownership may affect the market price of our ordinary shares and may discourage certain types of transactions, including those involving actual or potential change of control of us (whether through merger, consolidation, take-over or other business combination), which might otherwise have a positive effect on the market price of the shares.
We are currently a foreign private issuer, which may limit information about us and legal rights that you as an investor may desire and are different from those of a United States domestic reporting company.
We currently are a "foreign private issuer," as such term is defined in Rule 405 under the U.S. Securities Act 1933, as amended (the “Securities Act”) and, therefore, we are not required to file annual reports on Form 10-K, quarterly reports on Form 10-Q or current reports on Form 8-K with the United States Securities and Exchange Commission (“SEC”). In addition, the proxy rules and Section 16 reporting and short-swing profit rules are not applicable to us. If we lose our status as a foreign private issuer by our election or otherwise and we become subject to the full reporting regime of the United States securities laws, we will be subject to additional reporting obligations and proxy solicitation obligations under the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and our officers, directors and 10% shareholders would become subject to the short-swing profit rules. The imposition of these reporting rules would increase our costs associated with legal and accounting compliance and the obligations of those affected by the short-swing rules.
Complex United States taxation rules apply to holders of our ordinary shares if we have too much passive income compared to ordinary income and we are considered a PFIC.
Generally, if, for any taxable year, at least 75% of our gross income is passive income or at least 50% of the value of our assets is attributable to assets that produce passive income or are held for the production of passive income, including cash, we will be classified as a passive foreign investment company (a “PFIC”), for U.S. federal income tax purposes. For purposes of these tests, passive income includes dividends, interest and gains from the sale or exchange of investment property and rents and royalties other than certain rents and royalties which are received from unrelated parties in connection with the active conduct of a trade or business. We believe that we were a PFIC for our fiscal year ended March 31, 2018 and that we were a PFIC for the year ended March 31, 2024 (“Fiscal 2024”). In addition, we may have been a PFIC in other years and may continue to be a PFIC in the future.
If we are classified as a PFIC, our U.S. tax-resident shareholders could be liable for additional taxes and interest charges upon certain distributions by us and any gain recognized on a sale, exchange or other disposition, including a pledge, of our ordinary shares (and such gain would generally be treated as ordinary income, rather than capital gain, for U.S. federal income tax purposes), whether or not we continue to be a PFIC. In addition, U.S. tax residents who own an interest in a PFIC are required to comply with certain reporting requirements.
A U.S. tax-resident shareholder may in certain circumstances be able to mitigate some of the adverse U.S. federal income tax consequences of us being classified as a PFIC if our ordinary shares qualify as "marketable stock" under the PFIC rules and the shareholder is eligible to make, and successfully makes, a "mark-to-market" election. A U.S. tax-resident shareholder could also mitigate some of the adverse U.S. federal income tax consequences by making a qualified electing fund (“QEF”) election, provided that we provide the information necessary for our U.S. tax-resident shareholders to make such an election, but we are not required to make this information available. We made the information available for the fiscal years 2018 and 2019 to those shareholders who requested it and can make this information available for our fiscal years 2020, 2021, 2022, 2023 or 2024, if requested.
| 16 |
U.S. tax-resident shareholders are strongly urged to consult their tax advisors about the PFIC rules, including tax return filing requirements and the eligibility, manner and consequences to them of making a QEF or mark-to-market election with respect to our ordinary shares if we should be classified as a PFIC.
U.S. shareholders may not be able to enforce civil liabilities against us.
We are a company incorporated under the laws of the British Virgin Islands. Many of our directors and executive officers are non-residents of the United States. Because a substantial portion of their assets and currently most of our assets are located outside the United States, it may be difficult for investors to effect service of process within the United States upon us or those persons.
Our corporate affairs will be governed by our Memorandum and Articles of Association, the BVI Business Companies Act (Revised Edition 2020, as amended) (the "BVI Act"), and the common law of the British Virgin Islands. The rights of shareholders to take action against the directors, actions by minority shareholders and the fiduciary responsibilities of our directors to us under British Virgin Islands law are to a large extent governed by the BVI Act and common law of the British Virgin Islands. The common law of the British Virgin Islands is derived in part from comparatively limited judicial precedent in the British Virgin Islands and from English common law, the decisions of whose courts are considered persuasive authority but are not binding on a court in the British Virgin Islands. The rights of our shareholders and the fiduciary responsibilities of our directors under British Virgin Islands law may not be as clearly established as they would be under statutes or judicial precedent in jurisdictions in the United States or Canada. In particular, the British Virgin Islands has a less developed body of securities laws as compared to the United States, and some states, such as Delaware, have more fully developed and judicially interpreted bodies of corporate law. In addition, British Virgin Islands companies may or may not have standing to initiate a shareholder derivative action in a federal court of the United States.
The British Virgin Islands courts are also unlikely:
| · | to recognize or enforce against us judgments of U.S. courts based on certain civil liability provisions of U.S. securities laws; and |
| · | to impose liabilities against us, in original actions brought in the British Virgin Islands, based on certain civil liability provisions of U.S. securities laws that are penal in nature. |
There is no statutory recognition in the British Virgin Islands of judgments obtained in the United States.
We have been advised by counsel as to British Virgin Islands law, that (i) they are unaware of any proceedings that have been brought in the British Virgin Islands to enforce judgments of the U.S. courts or to impose liabilities based on the civil liability provisions of the U.S. federal or state securities laws; (ii) a final and conclusive judgment in the federal or state courts of the United States under which a sum of money is payable, other than a sum payable in respect of taxes, fines, penalties or similar charges, may be subject to enforcement proceedings as a debt in the courts of the British Virgin Islands under the common law doctrine of obligation; and (iii) because it is uncertain whether a British Virgin Islands court would determine that a judgment of a U.S. court based on the civil liability provisions of the U.S. federal or state securities laws is in the nature of a penalty, it is uncertain whether such a liability judgment would be enforceable in the British Virgin Islands.
As a foreign private issuer, and as permitted by the listing requirements of Nasdaq, we will rely on certain home country governance practices, which are different from the corporate governance requirements that apply to U.S. domestic companies that are listed Nasdaq.
We are a foreign private issuer, and in accordance with Nasdaq Listing Rule 5615(a)(3), we comply with home country governance requirements and certain exemptions thereunder rather than complying with certain of the corporate governance requirements of Nasdaq which may afford less protection to our shareholders than they would otherwise have if we complied fully with Nasdaq’s corporate governance requirements.
| 17 |
British Virgin Islands law does not require that a majority of our board of directors consist of independent directors or that our board committees consist of entirely independent directors. Our board of directors and board committees, therefore, may include fewer independent directors than would be required if we were subject to Nasdaq Listing Rule 5605(b)(1). In addition, we will not be subject to Nasdaq Listing Rule 5605(b)(2), which requires that independent directors must regularly have scheduled meetings at which only independent directors are present.
We also are exempt from the Nasdaq listing rules as to quorum, and instead follow the quorum rules for shareholder meetings under British Virgin Islands law. We also are exempt from the Nasdaq listing rules so as to not be required to obtain shareholder approval for certain issuance of securities, shareholder approval of share option plans and change of control transactions under Nasdaq Listing Rule 5635 and to hold annual shareholder meetings under Nasdaq Listing Rule 5620(a).
If we lose our status as a foreign private issuer, we would be required to fully comply with Nasdaq’s corporate governance requirements, which could have an adverse effect on us. For example, Nasdaq’s director independence requirements could make it more difficult for us to attract directors and Nasdaq’s shareholder approval requirements could make it more difficult and time-consuming to raise capital or engage in certain transactions.
We may lose our foreign private issuer status, which would then require us to comply with the Exchange Act’s domestic reporting regime and cause us to incur significant legal, accounting and other expenses.
We are a foreign private issuer. In order to maintain our current status as a foreign private issuer, at least 50% of our outstanding ordinary shares must continue to be either directly or indirectly owned of record by non-residents of the United States. If more than 50% of our outstanding ordinary shares are instead held by U.S. residents, then in order to continue to maintain our foreign private issuer status, (i) a majority of our executive officers or directors must not be U.S. citizens or residents, (ii) more than 50% of our assets must not be located in the United States, and (iii) our business must be administered principally outside the United States.
Losing our status as a foreign private issuer would require us to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act applicable to U.S. domestic issuers. We also will be required to make changes in our corporate governance practices in accordance with various SEC and Nasdaq rules. The regulatory and compliance costs to us under U.S. securities laws, if we are required to comply with the reporting requirements applicable to a U.S. domestic issuer, would be significantly higher than the cost we would incur as a foreign private issuer. As a result, we would expect that a loss of foreign private issuer status will increase our legal and financial compliance costs and will make some activities highly time consuming and costly. We also expect that if we will be required to comply with the rules and regulations applicable to U.S. domestic issuers, it will make it more difficult and expensive for us to obtain director and officer liability insurance; we may therefore be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These rules and regulations could also make it more difficult for us to attract and retain qualified members of our board of directors.
| 18 |
Macro-economic Risks Related to our Business
The impact of changing economic conditions, including the effects of inflation, may adversely affect our business, financial condition, and results of operations.
As has been widely reported, we are currently operating in a period of economic uncertainty and capital markets disruption, which has been significantly impacted by domestic and global monetary and fiscal policy, geopolitical instability and historically high domestic and global inflation. The U.S. Federal Reserve and other central banks may be unable to contain inflation through more restrictive monetary policy and inflation may increase or continue for a prolonged period of time. Inflationary factors, such as increases in the cost of clinical supplies, interest rates, overhead costs and transportation costs have and may continue to have adversely affect our operating results. We continue to monitor these events and the potential impact on our business.
As a result of inflation and overall economic uncertainty, the cost of capital has dramatically increased in the last 12 months, making capital, if available, very expensive. We will require significant financial resources to complete the current development plans with respect to our assets.
Further, there can be no assurance that further deterioration in credit and financial markets and confidence in economic conditions will not occur. Our general business strategy may be adversely affected by any such economic downturn, volatile business environment or continued unpredictable and unstable market conditions. If the current equity and credit markets deteriorate, or do not improve, it may make any necessary debt or equity financing more difficult, more costly, and more dilutive. Failure to secure any necessary financing in a timely manner and on favorable or acceptable terms could have a material adverse effect on our growth strategy, financial performance and stock price and could require us to delay or abandon some or all of our clinical development plans. In addition, there is a risk that one or more of our current service providers, manufacturers and other partners may not survive these difficult economic times, which could directly affect our ability to attain our operating goals and adversely impact our business, financial condition and results of operations.
ITEM 4 – INFORMATION ON THE COMPANY
(A) HISTORY AND DEVELOPMENT OF THE COMPANY
We were originally incorporated in Ontario, Canada in 1973. We were inactive until 1985. Between 1986 and 2009, we were engaged in a variety of businesses including development of a new technology for the marine propulsion business, distribution and manufacture of a snack food, emerging technology-based businesses and natural resources involving diamond mining and oil & gas exploration. In 2010, we acquired an indirect interest in two drilling licenses in Israel, which were subsequently disposed of in June 2012. During the period 1986 to 2012, we went through several name changes ending with Bontan Corporation Inc.
| 19 |
In December 2012, we decided to change the focus of our business activities from oil and gas to biotechnology mainly due to the increasing difficulty of getting access to viable oil & gas projects and also due to the potentially more profitable business opportunities, which existed in the biotechnology sector. On March 21, 2013, we signed a letter of intent with Portage Pharma Ltd., a biotech private limited company formed under the laws of the British Virgin Islands, to acquire Portage Pharma Ltd. through an exchange of shares. The transaction was completed on June 4, 2013.
On July 5, 2013, we changed our name to Portage Biotech Inc. and moved our jurisdiction to the British Virgin Islands under a certificate of continuance issued by the Registrar of Corporate Affairs of BVI.
We are a BVI business company incorporated under the BVI Act with our registered office located at Clarence Thomas Building, P.O. Box 4649, Road Town, Tortola, British Virgin Islands, VG1110. Our U.S. agent, Portage Development Services Inc. (“PDS”), is located at 59 Wilton Road, Westport, CT 06880. Our telephone number is (203) 221-7378.
We currently are a foreign private issuer under the SEC rules. We are also a reporting issuer under the securities legislation of the provinces of Ontario and British Columbia. Our ordinary shares were listed on the Canadian Securities Exchange (“CSE”) under the symbol “PBT.U”. On February 25, 2021, our ordinary shares began trading on the Nasdaq Capital Market under the symbol “PRTG”. As the principal market for our ordinary shares is Nasdaq, we voluntarily delisted from the CSE on April 23, 2021.
During August 2018, we reached a definitive agreement to acquire 100% of SalvaRx Limited in exchange for 8,050,701 of our ordinary shares. The selling shareholders were SalvaRx Group plc (94.2%), James Mellon (2.9%) and Gregory Bailey (2.9%), the latter two persons being directors of our company. The acquisition of SalvaRx was a "related party transaction" within the meaning of Multilateral Instrument 61-101 Protection of Minority Security Holders in Special Transactions (“MI 61-101”). As a consequence, MI 61-101 required us to seek the approval of a majority of the disinterested shareholders to make this acquisition. On January 8, 2019, the majority of our minority shareholders approved the SalvaRx acquisition on the terms as set out in the signed definitive agreement. At the same time, the SalvaRx Group plc shareholders approved the definitive agreement, all required regulatory approvals were also obtained. The SalvaRx acquisition was completed on January 8, 2019, and we acquired 100% of the equity of SalvaRx, which has full or partial ownership of four immune-oncology companies that are developing nine product candidates.
We filed a shelf registration statement with the SEC in order to sell ordinary shares, debt securities, warrants and units in one or more offerings from time to time, which became effective on March 8, 2021 (the “March 2021 Registration Statement”). In connection with the March 2021 Registration Statement, we have filed with the SEC:
| · | a base prospectus, which covered the offering, issuance and sale by us of up to $200,000,000 in the aggregate of the securities identified above from time to time in one or more offerings; |
| · | a prospectus supplement, which covered the offer, issuance and sale by us in an ATM program of up to a maximum aggregate offering price of $50,000,000 of our ordinary shares that may be issued and sold from time to time under a Controlled Equity Offering Sales Agreement, dated February 24, 2021 (the “Sales Agreement”), with Cantor Fitzgerald & Co., the sales agent (“Cantor Fitzgerald”); |
| · | a prospectus supplement dated June 24, 2021, for the offer, issuance and sale by us of 1,150,000 ordinary shares for gross proceeds of approximately $26.5 million in a firm commitment underwritten public offering with Cantor Fitzgerald; and |
| · | a prospectus supplement dated August 19, 2022, for the resale by us of up to $30,000,000 in ordinary shares that we may sell from time to time to Lincoln and an additional 94,508 shares that were issued to Lincoln and |
| · | a prospectus supplement dated September 29, 2023, for the offer, issuance and sale by us in a registered direct public offering through H.C. Wainwright & Co., the placement agent, to an institutional and accredited investor of (i) 1,970,000 of our ordinary shares at a purchase price of $1.90 per share, (ii) pre-funded warrants to purchase up to 1,187,895 of our ordinary shares, at a purchase price of $1.899 per pre-funded warrant (the “Pre-Funded Warrants”) and (iii) the ordinary shares issuable upon exercise of the pre-funded warrants (each, a “Private Warrant Share”). |
| 20 |
The Sales Agreement permits us to sell in an ATM program up to $50,000,000 of ordinary shares from time to time. The sales under the prospectus will be deemed to be made pursuant to an ATM program as defined in Rule 415(a)(4) promulgated under the Securities Act.
On June 24, 2021, we sold 1,150,000 ordinary shares in a firm commitment public offering, including the underwriters’ option, at a price of $23.00 per share, which generated gross proceeds of approximately $26.5 million and net proceeds of approximately $25.0 million.
During Fiscal 2022, we commenced an ATM program, and we sold 90,888 ordinary shares, generating gross proceeds of approximately $2.6 million ($2.5 million, net of commissions).
During Fiscal 2023, we sold 166,145 ordinary shares under the ATM program, generating net proceeds of approximately $0.9 million.
During Fiscal 2024, we sold 186,604 ordinary shares under the ATM program, generating net proceeds of approximately $0.7 million.
The March 2021 Registration Statement expired on February 24, 2024. In order to issue additional shares under our ATM program or the Committed Purchase Agreement (as defined below) in the future, we would be required to file a new registration statement, which must be declared effective by the SEC prior to use, and to file a prospectus supplement related to the ATM program or the Committed Purchase Agreement, as the case may be.
Furthermore, our ATM program and the Committed Purchase Agreement with Lincoln are generally limited based on, among other things, our Nasdaq trading volume. Under the Baby Shelf Rule, the amount of funds we can raise through primary public offerings of securities in any 12-month period using a registration statement on Form F-3 is limited to one-third of the aggregate market value of the ordinary shares held by our non-affiliates, which limitation may change over time based on our stock price, number of ordinary shares outstanding and the percentage of ordinary shares held by non-affiliates. We are therefore limited by the Baby Shelf Rule as of the filing of this Form 20-F, until such time as our non-affiliate public float exceeds $75 million.
On July 1, 2022, we, our wholly-owned subsidiary, Portage Merger Sub I, Inc., our wholly-owned subsidiary, Portage Merger Sub II, LLC and Tarus Therapeutics, Inc., a Delaware corporation advancing adenosine receptor antagonists for the treatment of solid tumors, entered into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”). Under the structure of the Merger Agreement, Tarus Therapeutics, Inc. was ultimately merged into Portage Merger Sub II, LLC with the surviving entity renamed Tarus Therapeutics, LLC (“Tarus”).
As consideration for Tarus, we issued to the former Tarus shareholders an aggregate of 2,425,999 ordinary shares, calculated on the basis of $18 million divided by the 60-day volume weighted average price per share of ordinary shares. Such ordinary shares have not been registered with the SEC and were subject to lock-up agreements for terms ranging from six to twelve months, which expired on February 1, 2023 and July 1, 2023, respectively. Additionally, the ordinary shares that were subject to a twelve month lock-up period, are also subject to a three month dribble-out period which commenced July 1, 2023. During the dribble out period, each holder could not sell more than 10% of the average trading volume of our ordinary shares for the rolling three month period prior to the date on which the holder executes a trade of our ordinary shares without our prior written consent (which we were permitted to withhold at our sole discretion). Additionally, in the event that we resume further development of our adenosine program, payments of up to $32 million in cash or our ordinary shares (at our discretion) would be triggered upon achievement of future development and sales milestones, as described below. As a result of the transaction:
| · | We also assumed $2 million in short-term debt held by Tarus and deferred license milestones obligations ($1 million plus interest), for an aggregate of $3 million in liabilities. We repaid the short-term debt in July 2022. |
| · | Upon enrolling the first patient in a Phase 2 clinical trial utilizing Tarus’s adenosine receptor antagonists, we would be required to pay an additional one-time payment of $15 million to the former Tarus shareholders. Payment may be in the form of cash or our ordinary shares (at our discretion). The remaining $17 million milestone is based on target commercial sales. |
| 21 |
On July 6, 2022 (the “Signing Date”), we entered into a Purchase Agreement (the “Committed Purchase Agreement”) with Lincoln, pursuant to which we may require Lincoln to purchase our ordinary shares having an aggregate value of up to $30 million over a period of 36 months. For a summary of the Committed Purchase Agreement, please see our Form 20-F for the fiscal year ended March 31, 2023 filed with the SEC on July 31, 2023.
During Fiscal 2023, we sold 480,000 ordinary shares to Lincoln under the Committed Purchase Agreement for net proceeds totaling approximately $2.0 million.
On July 18, 2022, we and our wholly-owned subsidiary, SalvaRx, entered into a Share Exchange Agreement (the “Share Exchange Agreement”) with each of the minority shareholders of iOx (the “Sellers”) resulting in the acquisition of the outstanding non-controlling ownership interest (approximately 22%) of iOx, which had been developing our iNKT engager platform until we decided to pause further development earlier this year.
On March 1, 2023, we, through Tarus, entered into a clinical service agreement with a third-party service provider. The term of the agreement is through the earlier of August 14, 2025 or the completion of provision of services and the payment of contractual obligations. The budgeted costs for the services to be provided is approximately $12.1 million. We are currently negotiating a pause of the clinical service agreement, based upon our decision to pause our clinical programs.
On September 29, 2023, we entered into a Purchase Agreement (the “Purchase Agreement”) with an institutional and accredited investor in connection with the Registered Direct Offering and the Private Placement (collectively, the “Offerings”). The Offerings closed on October 3, 2023.
Pursuant to the Purchase Agreement, in the Registered Direct Offering, we sold (i) 1,970,000 of our ordinary shares at a purchase price of $1.90 per share and (ii) Pre-Funded Warrants to purchase up to 1,187,895 ordinary shares, at a purchase price of $1.899 per Pre-Funded Warrant. All Pre-Funded Warrants, which were immediately exercisable for one ordinary share at an exercise price of $0.001 per share, were exercised in full on May 29, 2024.
| 22 |
In the Private Placement, we issued to such institutional and accredited investor warrants to purchase up to 3,157,895 ordinary shares (the “Series A Warrants”), warrants to purchase up to 3,157,895 ordinary shares (the “Series B Warrants”), and warrants to purchase up to 3,157,895 ordinary shares (the “Series C Warrants,” together with the Series A Warrants and the Series B Warrants, the “Private Warrants”), together exercisable for an aggregate of up to 9,473,685 ordinary shares (the “Private Warrant Shares”). Pursuant to the terms of the Purchase Agreement, for each ordinary share and each Pre-Funded Warrant issued in the Registered Direct Offering, an accompanying Series A Warrant, Series B Warrant and Series C Warrant was issued to such institutional and accredited investor. Each Series A Warrant is exercisable for one Private Warrant Share at an exercise price of $1.90 per share, is immediately exercisable and will expire 18 months from the date of issuance. Each Series B Warrant is exercisable for one Private Warrant Share at an exercise price of $2.26 per share, is immediately exercisable and will expire three years from the date of issuance. Each Series C Warrant is exercisable for one Private Warrant Share at an exercise price of $2.26 per share, is immediately exercisable and will expire five years from the date of issuance. The net proceeds to us from the Offerings were approximately $5.3 million, after deducting placement agent’s fees and estimated offering expenses.
Pursuant to an engagement letter, dated as of August 26, 2023, between us and the Placement Agent, we paid the Placement Agent a total cash fee equal to 6.0% of the aggregate gross proceeds received in the Offerings, or $0.36 million. We paid the Placement Agent in connection with the Offerings a management fee equal to 1.0% of the aggregate gross proceeds raised in the Offerings ($0.06 million), $75,000 for non-accountable expenses and $15,950 for clearing fees. In addition, we issued to the Placement Agent, or its designees, warrants to purchase up to 157,895 ordinary shares (the “Placement Agent Warrants,” and together with the Pre-Funded Warrants and the Private Warrants, the “Warrants”), which represented 5.0% of the aggregate number of ordinary shares and Pre-Funded Warrants sold in the Registered Direct Offering. The Placement Agent Warrants have substantially the same terms as the Series B Warrants and the Series C Warrants, except that the Placement Agent Warrants have an exercise price equal to $2.375, or 125% of the offering price per ordinary share sold in the Registered Direct Offering and will be exercisable for five years from the commencement of the sales pursuant to the Offerings.
We filed a resale registration statement to register for resale the Private Warrant Shares and the ordinary shares issuable upon the exercise of Placement Agent Warrants, which was declared effective by the SEC on November 7, 2023 (the “November 2023 Resale Registration Statement”). Pursuant to the terms of the Purchase Agreement, we are obligated to use commercially reasonable efforts to keep the November 2023 Resale Registration Statement effective at all times until such institutional and accredited investor (and its successors and assigns) no longer owns any Private Warrants or ordinary shares issuable upon exercise thereof.
If a Fundamental Transaction (as defined in the Warrants) occurs, then the successor entity will succeed to, and be substituted for us, and may exercise every right and power that we may exercise and will assume all of our obligations under the Warrants with the same effect as if such successor entity had been named in the Warrants themselves. If holders of ordinary shares are given a choice as to the securities, cash or property to be received in such a Fundamental Transaction, then the holders of the Warrants shall be given the same choice as to the consideration they would receive upon any exercise of the Warrants following such a Fundamental Transaction. Additionally, as more fully described in the Series B Warrants, Series C Warrants and Placement Agent Warrants, in the event of certain Fundamental Transactions, the holders of the Series B Warrants, Series C Warrants and Placement Agent Warrants will be entitled to receive cash consideration in an amount equal to the Black-Scholes value of the Series B Warrants, Series C Warrants and Placement Agent Warrants, as the case may be, upon the consummation of such Fundamental Transaction.
Series A Warrants and Pre-Funded Warrants
The Series A Warrants and the Pre-Funded Warrants are classified as a component of equity because they are freestanding financial instruments that are legally detachable and separately exercisable from the ordinary shares with which they were issued, are immediately exercisable, do not embody an obligation for us to repurchase such shares, and permit the holders to receive a fixed number of ordinary shares upon exercise. In addition, the Series A Warrants and the Pre-Funded Warrants do not provide any guarantee of value or return.
On the October 3, 2023 issue date, the calculated fair value of the Series A Warrants and the Pre-Funded Warrants was $2.968 million ($0.94 per such warrant). Because the fair value of the warrants accounted for as liabilities exceeded the net proceeds from the Registered Direct Offering, the proceeds allocated to our ordinary shares, the Pre-Funded Warrants and the Series A warrants was zero.
| 23 |
Series A Warrants
The inputs associated with calculating the fair value are reflected below.
| October 3, 2023 | ||
| Exercise price | $1.90 | |
| Share price | $1.97 | |
| Expected life | 1.50 years | |
| Expected volatility | 96.0% | |
| Risk-free interest rate | 5.32% | |
| Dividend yield | – |
Capital Expenditures and Divestitures
We had no capital expenditures or divestitures in Fiscal 2024, Fiscal 2023 or during the year ended March 31, 2022 (“Fiscal 2022”).
The SEC maintains an internet site at www.sec.gov that contains reports and information statements and other information regarding registrants like us that file electronically with the SEC.
We routinely post important information on our website at www.portagebiotech.com. This website and the information contained therein or connected thereto shall not be deemed to be incorporated into this Annual Report.
(B) BUSINESS OVERVIEW
Nature of Operations and Overview
Due to our future funding needs for clinical development of our programs as well as the current capital raising market for biotechnology companies, we made the decision to discontinue the IMPORT-201 trial (PORT-2) and to pause further accrual to the ADPORT-601 trial (PORT-6 and PORT-7). The PORT-3 investigator trial is continuing, and all existing patients in the ADPORT-601 study will continue until disease progression. We are continuing to collect and analyze data from these patients. We plan to replace a patient who withdrew and is unevaluable for the 28-day dose limiting toxicity (“DLT”) period. We are exploring strategic alternatives, which may include finding a partner for one or more of our assets, a sale of our company, a merger, restructurings, both in and out of court, company wind down, further financing efforts or other strategic action. The following discussion reflects our operations in the event we were to raise additional capital to fund the clinical development of our programs.
We are a clinical stage immune-oncology company advancing treatments we believe will be first-in-class therapies that target known checkpoint resistance pathways to improve long-term treatment response and quality of life in patients with invasive cancers.
Our access to next-generation technologies provides the capability to identify and understand biological mechanisms, clinical therapies and product development strategies that could accelerate these programs through the translational pipeline.
We source and develop early- to mid-stage treatments that we believe will be first-in-class therapies for a variety of cancers, by funding, implementing viable, cost-effective product development strategies, clinical counsel/trial design, shared services, financial and project management to enable efficient, turnkey execution of commercially informed development plans. Our drug development pipeline portfolio encompasses product candidates or technologies based on biology addressing known resistance pathways/mechanisms of current checkpoint inhibitors with established scientific rationales.
| 24 |
The Portage Approach
Our mission has been to advance and grow a portfolio of innovative, early-stage oncology assets based on the latest scientific breakthroughs focused on overcoming immune resistance and expanding the addressable patient population. Given these foundations, we have managed capital allocation and risk as much as we have overseen drug development. By focusing our efforts on translational medicine and pipeline diversification, we have sought to mitigate overall exposure to many of the inherent risks of drug development.
Our approach has been guided by the following core elements:
| · | Portfolio diversification to mitigate risk and maximize optionality; |
| · | Capital allocation based on risk-adjusted potential, including staged funding to pre-specified scientific and clinical results; |
| · | Virtual infrastructure and key external relationships to maintain a lean operating base; |
| · | Internal development capabilities complemented by external business development; |
| · | Rigorous asset selection for broad targets with disciplined ongoing evaluation; |
| · | Focus on translational medicine and therapeutic candidates with single agent activity; |
| · | Conduct randomized trials early and test non-overlapping mechanisms of action; and |
| · | Improve potential outcomes for patients with invasive cancers. |
We have executed such approach through our internal core team and our network of experts, contract labs and academic partners.
We believe that we are not subject to the regulation of the Investment Company Act of 1940, as amended (“40 Act”), based on the definition of “investment company” and the compositions of our assets. Additionally, as we primarily operate within the biomedical industry as a research and development (“R&D”) business, we believe that we are also able to take advantage of the non-exclusive safe harbor of Rule 3a-8 promulgated under the 40 Act so as not to be characterized as an investment company. We have adopted a capital preservation policy referenced in that rule.
Our Science Strategy
Prior to our decision to discontinue our iNKT IMPORT-201 sponsored study and pause further accrual in the adenosine program, our goal has been to develop immuno-oncology therapeutics that will dramatically improve the standard-of-care for patients with cancer. The key elements of our scientific strategy have been to:
| · | Build a pipeline of differentiated oncology therapeutic candidates that are diversified by mechanism, broad targets, therapeutic approach, modality, stage of development, leading to a variety of deal types that can be executed with partners; |
| · | Expand our pipeline through research collaborations, business development and internally designed programs; |
| · | Continue to advance and evolve our pipeline; and |
| · | Evaluate strategic opportunities to accelerate development timelines and maximize the value of our portfolio. |
| 25 |
Our Pipeline
We have built a pipeline of immuno-oncology therapeutic candidates and programs that are diversified by mechanism, therapeutic approach, modality and stage of development. Prior to our decision to discontinue further development of our iNKT program and pause further accrual to our adenosine program, we rigorously assessed each of our programs on an ongoing basis using internally defined success criteria to justify continued investment and determine proper capital allocation. When certain programs do not meet our de-risking criteria for advancement, we look to monetize or terminate those programs and preserve our capital and resources to invest in programs with greater potential.
The charts below set forth, as of August 14, 2024, the state of our immuno-oncology therapeutic product candidates and programs before development activities were discontinued and/or paused except for PORT-3, which is being evaluated as part of an investigator sponsored study without funding from us. Additionally, notwithstanding our decision to pause further development, PORT-7 remains in Phase 1a from an IND perspective, though we have not commenced dosing patients. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. Before you make an investment decision regarding us, you should make your own analysis of forward-looking statements and our projections about candidate and program development and results.

| 26 |
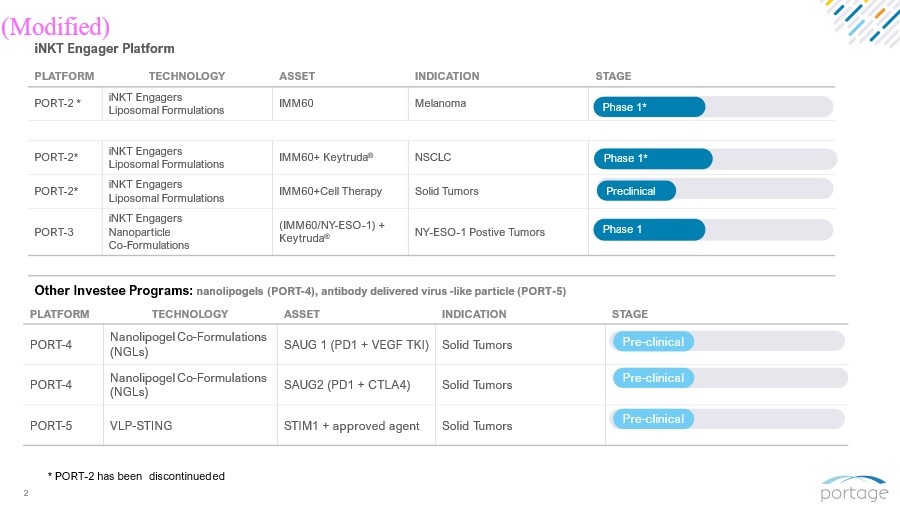
Our Programs and Technology – Recent Developments
After a review of our future funding needs for clinical development of our programs as well as the current capital raising market for biotechnology companies, we made the decision to discontinue our iNKT sponsored trial (the investigator sponsored trial of PORT-3 is ongoing without financial support from the us) and pause further accrual in our sponsored adenosine program. We are exploring strategic alternatives, which may include finding a partner for one or more of our assets, a sale of our company, a merger, restructurings, both in and out of court, a company wind down, further financing efforts or other strategic action. Below is a discussion of our clinical programs and the status of such programs prior to our decision to discontinue our iNKT program and pause further accrual to our sponsored studies.
Adenosine Receptor Antagonist Platform
A critical mechanism of cancer immune evasion is the generation of high levels of immunosuppressive adenosine within the tumor microenvironment (“TME”). Research suggests that the TME has significantly elevated concentrations of extracellular adenosine. Engagement with adenosine receptors type 2A (“A2A”) and type 2B (“A2B”) triggers a dampening effect on the immune response, suppressing effector cell function and stabilizing immunosuppressive regulatory cells. Over-expression of the A2A and A2B receptors leads to a poor prognosis in multiple cancers, including prostate cancer, colorectal cancer and lung adenocarcinoma, driven by a reduced ability to generate an immune response against the tumor.
These findings have made A2A and A2B high-priority targets for immunotherapeutic intervention. Before pausing, we were advancing four adenosine antagonists that we believe to be first-in-class, which together represent a broad suite of adenosine-targeting approaches and were expected to enable a comprehensive exploration of how targeting the adenosine pathway could potentially improve response in multiple cancer and non-cancer indications. By modulating the adenosine pathway in four different ways, we expected to determine the optimal approach to maximize the impact of the mechanism of action on different tumors.
We have designed the ADPORT-601 clinical trial to evaluate the activity and safety of PORT-6 and PORT-7 alone and in combination. If we resume accrual, we would expect this trial to adapt over time and also include safety cohorts for these two agents with other immune activating agents including others from our internal pipeline. Depending on the data, it can be expanded to evaluate either agent as monotherapy or a randomized comparison of either agent plus standard of care versus standard of care alone.
| 27 |
PORT-6 (TT-10)
PORT-6 is an A2A antagonist being studied for the treatment of A2A expressing solid tumors. We believe PORT-6 is more potent, more durable and more selective than other clinical stage A2A agents.
Prior to pausing patient enrollment in the clinical study, the ADPORT-601 Phase 1a trial for PORT-6 dosed its first patient in June 2023. We have fully enrolled and completed the first two dose escalation cohorts. In the third cohort, one patient experienced a serious adverse event (blurry vision and stroke) that the investigator initially determined could possibly be related to PORT-6. With further follow-up, this event was classified as unrelated to treatment. There are two patients who continue on the study for more than six months with prolonged stable disease. The plan is to accrue one more patient to replace a patient who dropped out due to an unrelated adverse event prior to DLT assessment. We originally activated eight sites in the U.S. to complete the Phase 1, and are currently keeping four active while we evaluate strategic alternatives.
PORT-7 (TT-4)
PORT-7 is an A2B antagonist for the treatment of solid tumors. PORT-7 has a very selective profile that focuses on A2B. PORT-7 is in Phase 1a from an IND perspective, though we have not commenced dosing patients.
PORT-8 (TT-53)
PORT-8 is a dual inhibitor of adenosine receptors 2A and 2B (A2A/A2B) to address solid tumors.
PORT-9 (TT-3)
PORT-9 is an A2B antagonist designed to treat colorectal and gastrointestinal cancers. The PORT-9 program is a pre-clinical stage program.
In connection with the adenosine program, we will focus on solid tumor types with high adenosine expression of receptors A2A and A2B and enrich for patients that have high adenosine expression and therefore have potential to benefit most from treatment.
Other Pipeline and Investee Programs
Prior to our decision to discontinue our iNKT program and pause further development of our adenosine program, we were focused on delivering clinical data with the adenosine program described above and prioritizing the allocation of financial resources to that program. Developmental work continued on some of the other developmental assets, through collaborations such as that with the U.S. National Cancer Institute (“NCI”) and other academic groups, as further described below. These developmental assets may be re-evaluated at a future point depending on market conditions, ongoing data, funding priorities and status.
Invariant Natural Killer T-cells (iNKT cells) Platform
iNKT cells play an important role in anti-tumor immune responses and are a distinct class of T lymphocyte displaying a limited diversity of T-cell receptors. They recognize lipid antigens on the surface of tumor cells and produce large amounts of cytokines within hours of stimulation without the need for clonal expansion. Furthermore, iNKT cells activate multiple immune system components, including dendritic cells (“DC”), T-cells and B-cells and stimulate an antigen-specific expansion of these cells. Our operating subsidiary, iOx Therapeutics Ltd. (“iOx”), holds an exclusive license (with the right to sub-license) from the Ludwig Institute for Cancer Research (the “Ludwig Institute”) to use, research, develop and commercialize iNKT cell engagers, for the treatment of various forms of human disease, including cancer, under the Ludwig Institute’s intellectual property and know-how.
PORT-2 (IMM60)
PORT-2 is an iNKT cell engager formulated in a liposome with a six-member carbon head structure that has been shown to activate both human and murine iNKT cells, resulting in DC maturation and the priming of Ag-specific T and B cells.
| 28 |
In animal models, PORT-2 enhanced the frequency of tumor specific immune responses. iNKT cells are unique lymphocytes defined by their co-expression of surface markers associated with NK cells along with a T-cell antigen receptor. They recognize amphipathic ligands such as glycolipids or phospholipids presented in the context of the non-polymorphic, MHC class I-like molecule CD1d. Activated iNKT cells rapidly produce IFN-gamma and IL-4 and induce DC maturation and IL-12 production.
In August 2021, we dosed the first patient in the IMP-MEL PORT-2 clinical trial, a Phase 1/2 dose escalation and randomized expansion trial. Prior to discontinuing the PORT-2 trial, it was expected to enroll up to 88 patients with melanoma or non-small cell lung carcinoma (“NSCLC”) in order to evaluate safety and efficacy. In November 2022, we announced that we had entered into a clinical trial collaboration with Merck to evaluate PORT-2 in combination with pembrolizumab for patients with NSCLC. Under the terms of the collaboration, Merck supplied pembrolizumab for our Phase 1/2 trial of PORT-2 in patients with NSCLC and melanoma. The trial was closed in June 2024. The Merck collaboration terminated in December 2023.
Preliminary Phase 1 data from the IMP-MEL PORT-2 clinical trial, presented at the Society for Immunotherapy of Cancer in November 2023, suggests PORT-2 was well tolerated when administered as a monotherapy, with no related severe or serious adverse events. All possibly related adverse events were mild to moderate and did not limit dosing. Given the favorable safety profile observed in the clinical trials to date, the clinical protocol for the IMP-MEL PORT-2 clinical trial was amended to include a higher Phase 1 dose level as our near-term focus is defining the recommended Phase 2 dose. Prior to our decision to discontinue further development of our iNKT platform, the combination safety cohort with pembrolizumab was being conducted in parallel with the ongoing high dose monotherapy cohort. As of November 2023, two patients had received the combination with pembrolizumab, and no related severe or serious adverse events were reported. The adverse event profile was consistent with pembrolizumab. Previously reported biomarker data confirmed the mechanism of action (i.e., both activation of the innate and adaptive arms of the immune system). The following figure illustrates the different lesion responses. Although these are preliminary results, several lesions showed shrinkage, and the responses in liver metastases were encouraging.
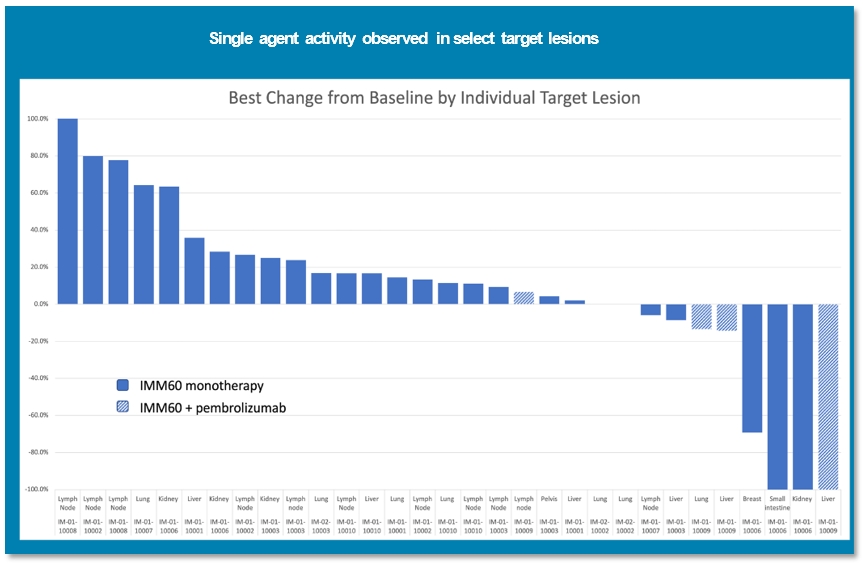
| 29 |
Prior to our decision to discontinue our sponsored iNKT trial, we were encouraged by the patient data set that we believe supports proof of concept for using an iNKT engager in cancer treatment. Preliminary Phase 1 data suggests that PORT-2 has a favorable safety and tolerability profile as a monotherapy at all doses tested to date (as noted above), has demonstrated evidence of single agent activity, and biomarkers confirm mechanistic potential of PORT-2 to activate both the adaptive and innate immune systems.
The clinical trial agreement has been transferred from the University of Oxford to us through our iOx subsidiary and the trial was converted to a program sponsored by iOx.
Prior to our decision to discontinue the iNKT trial, the protocol was being amended, given the safety data shown at the highest planned doses, to escalate patient dosing to include one additional higher dose to identify the recommended Phase 2 dose. We are exploring strategic alternatives, which may include finding a partner for one or more of our assets, a sale of our company, a merger, restructurings, both in and out of court, a company wind down, further financing efforts or other strategic action.
PORT-3 (IMM65)
PORT-3 is a poly(lactide-co-glycolide) (“PLGA”)-nanoparticle formulation of PORT-2 (IMM60) combined with a NY-ESO-1 peptide vaccine. Biodegradable PLGA-nanoparticles function as a delivery platform for immunomodulators and tumor antigens to induce a specific anti-tumor immune response. PLGA has minimal (systemic) toxicity and is used in various drug-carrying platforms as an encapsulating agent. Furthermore, co-formulating an iNKT engager with a peptide vaccine in a particle has shown to be approximately five times more potent in killing cancer cells and generating an antigen-specific CD8 T-cell response than giving the two agents individually.
NY-ESO-1 is a cancer-testis antigen expressed during embryogenesis and in the testis, an immune privileged site. Furthermore, NY-ESO-1 expression is observed in several advanced cancers: Lung (2-32%), melanoma (40%), bladder (32-35%), prostate (38%), ovarian (30%), esophageal (24-33%), and gastric cancers (8-12%). Clinical trials have shown the safety and tolerability of Good Manufacturing Practices-grade NY-ESO-1 peptides in patients with cancer.
PORT-3 is being evaluated as part of an investigator sponsored study without funding from us. The first patient was dosed in 2021 and patients continue to enroll in the PRECIOUS Phase 1 trial of PORT-3 in patients with solid tumors. The Phase 1 portion of the trial is expected to enroll 15 patients. The trial was having difficulty identifying tumors that expressed NY-ESO-1, so the trial protocol was amended to include all solid tumors regardless of expression to facilitate assessment of safety. This platform is designed to demonstrate proof of concept. The combination of NY-ESO-1 and IMM-60 is being evaluated to determine its ability to prime and boost an anti-tumor immune response. Our patent position extends to other known tumor antigens, and, if we resume further development of our iNKT platform, we are prepared to rapidly launch other assets into the clinic if we see strong activity of this formulation. Preliminary safety data for repeat dosing of PORT-3 in the PRECIOUS Phase 1 trial shows a favorable safety profile. The investigators with who we work with have continued to explore next generation targeted nanoparticles.
PORT-4, Nanolipogel (“NLG”) co-formulation Platform
Scientists are interested in novel ways to deliver multiple signals to the immune system in order to better activate an anti-tumor response. We have been impressed with a platform from Yale University that allows different types of agents to be packaged together and will concentrate them in tumors. We have licensed the platform for delivery of DNA aptamers and certain aptamer-small molecule-based combination products. In order to have multiple proprietary agents with known mechanisms of action, we have licensed rights to create DNA aptamers for immune-oncology targets and the first one developed is a proprietary PD1 aptamer, which has been placed in the NLG formulation. Early testing has shown the formulation properly modulates PD1 signaling in vitro similar to a PD1 antibody I. In non-clinical, in vivo experiments, the NLG-PD1 performed favorably compared to a mouse PD1 antibody. The current level of funding is expected to support exploration of multiple PD1 based co-formulations with small molecules and other DNA aptamers. We have conducted further research with the technology licensed from Yale University to co-deliver a PD1 blocking signal with a small molecule vascular endothelial growth factor inhibitor.
As of March 31, 2024, we owned approximately 70% of the outstanding shares of Saugatuck Therapeutics, Ltd., the subsidiary on which the PORT-4 platform is managed.
| 30 |
PORT-5, STING Agonist Platform
Proprietary immune priming and boosting technology (using a STING agonist delivered in a virus-like particle) has shown proof of concept in animal models. This platform was developed to offer multiple ways to target immune stimulation towards the cancer, as well as to co-deliver multiple signals in a single product. The PORT-5 STING platform’s advantage over chemical intratumoral approaches was potent immune priming and boosting pathway within a virus-like particle to enable convenient systemic administration and traffic to the correct targets. This technology would target dendritic cells, which is differentiated from other chemical STING approaches. To that end, Stimunity S.A. (“Stimunity”) received grant funding to study this technology with any COVID-19 vaccine to evaluate if it is possible to boost the immune response for immunocompromised or elderly patients. During April 2022, the American Association for Cancer Research showcased PORT-5 preclinical data at a late-breaking session that shows that one or more targeted immunotherapy agents could be packaged within a virus-like particle to increase potency, while enabling a selective immune activation. Stimunity was unable to raise any outside funding, and activities were scaled back due to our own liquidity issues.
In December 2023, we completed a transfer of our equity in Stimunity and the Stimunity Convertible Note to iOx. In connection with that transfer, the Stimunity Convertible Note was converted into 1,768 Class A shares of Stimunity.
As of March 31, 2024, we owned approximately 48.9% of the outstanding shares of Stimunity, the subsidiary on which the PORT-5 platform is managed. We have made the decision not to further fund Stimunity’s operations and wrote-down the remaining balance of our investment of $1.0 million to nil as of March 31, 2024.
Early-Stage Research and Development Collaborations
We have also been interested in evaluating and testing new antibody targets in the suppressive tumor microenvironment with the goal to down regulate or remove MDSC, TAMs, Tregs and other signals that impede the immune response from clearing cancer cells.
| · | We continue to collaborate with Dr. Robert Negrin and his team at Stanford University in an investigator sponsored trial (“IST”) study to evaluate the use of PORT-2 with iNKT cell therapies in animals. This work was intended to evaluate if an engager co-administered with expanded or transformed iNKT cells can further activate the transplanted and endogenous cells inside the patient. The Stanford collaboration was also expected to study the impact iNKT engagers have on driving an adaptive immune response and correcting the suppressive tumor microenvironment. This IST remains operational as of the date of this report. |
| · | We entered into a Cooperative Research and Development Agreement (“CRADA”) with the NCI. We and NCI planned to advance preclinical and potential clinical development of STING agonists and anti-RAGE agents for cancer vaccines. After the acquisition of Tarus Therapeutics, LLC (“Tarus”), we amended the CRADA to include exploration of the different adenosine compounds. We did not extend the CRADA beyond its current term and made a termination payment of $62,500 in June 2024 as required under the contract. |
| · | We have a collaboration with Dr. Carmela de Santos at University of Birmingham for the use of iNKT agents to treat sarcomas. Dr. de Santos has tested PORT-2 in human sarcoma cell lines and has grant funding to test it in animal models. |
| · | We have a collaboration to study the use of adenosine 2A and adenosine 2B agents in mesothelioma with Drs. Luciano Mutti from Sbarro Institute for Cancer Research and Molecular Medicine, Department of Biology, College of Science and Technology, Temple University and Dr. Steven Gray of St. James Hospital in Dublin. |
| · | There are other collaborations with experts in the products areas for which we provide access to our compounds and collaborates on studies. |
| 31 |
Our Business Model
We are a development organization that is structured to facilitate flexibility in financing and ease of partnering, licensing, and merger/acquisition of individual assets and or technology platforms. The intellectual property (“IP”) for each platform is held in separate private entities. Our employees and consultants work across the pipeline of assets and we believe that this can (i) enhance operational efficiency, (ii) maintain an optimal cost structure, (iii) attract leading collaborators, and (iv) promote asset flexibility, as further described below. If we were to resume enrollment in our clinical programs, we believe our experience and approach would continue to leverage the operating and cost structures that are further described below.
| · | Enhance operational efficiency: We allocate resources while empowering managers to make program-level decisions in order to increase productivity and speed. We believe this model enables a flexible organizational structure that can achieve scale through the addition of programs without increasing burdensome bureaucracy or redundant infrastructure. |
| · | Maintain an optimal cost structure: We have a relatively small number of employees and have partnered with a number of service providers to leverage their infrastructure and expertise as needed instead of embarking on capital-intensive lab, manufacturing, and equipment expenditures. By reducing overhead costs, we believe we can increase the likelihood that we can generate a return on invested capital. |
| · | Attract leading collaborators and licensors: Our pipeline is comprised of therapies we believe will be first-in-class therapies for a variety of cancers sourced via our industry contacts and relationships (including academia and pharmaceutical industry executives). On preclinical programs/technology, we initially established development structures enabling us to keep licensors economically incentivized at the program level. We believe that our experienced drug development leadership team and approach to resource allocation differentiate us from other potential licensees. |
| · | Leverage the commoditized checkpoint marketplace and explore the potential to further enhance long-term clinical benefits for patients with cancer and also expand the eligible population to include those who do not currently receive anti-PD-1 therapy: Presently there are multiple approved checkpoint therapeutics that lack differentiation, resulting in a competitive market dynamic, which will favor combination therapy. There remains opportunity for potential expansion in the PD-1 market with our adenosine antagonists. Studies show that 70-80% of patients do not respond or have a limited response to existing monotherapies, such as PD-1 checkpoint inhibitors. We see potential for our unique approach of using adenosine antagonists to initiate an immune response in tumors that have become refractory to checkpoint therapy or to increase the number of front-line patients achieving more durable responses. Combinations can improve this but often come at the cost of significant additional toxicity. The market is saturated with at least 14 approved PD-1 antibodies, and every major pharmaceutical company competes in this space. Extending the use of PD-1 antibodies could still provide a significant potential upside for companies competing for market share. |
| · | Promote asset flexibility: Our structure is designed to maximize flexibility and cost efficiency. This allows us to efficiently pursue various subsidiary-level transactions, such as stock or asset sales, licensing transactions, strategic partnerships and/or co-development arrangements. It also provides us with the flexibility to terminate programs with minimal costs if results do not meet our de-risking criteria for advancement. |
We are a BVI business company incorporated under the BVI Business Companies Act (Revised Edition 2020, as amended) with our registered office located at Clarence Thomas Building, P.O. Box 4649, Road Town, Tortola, British Virgin Islands, VG1110. Our U.S. agent, Portage Development Services Inc., is located at 59 Wilton Road, Westport, CT 06880.
We currently are a foreign private issuer under SEC rules. We are also a reporting issuer under the securities legislation of the provinces of Ontario and British Columbia. Our ordinary shares were listed on the CSE under the symbol “PBT.U”. On February 25, 2021, our ordinary shares began trading on the Nasdaq Capital Market under the symbol “PRTG”. As the principal market for our ordinary shares is Nasdaq, we voluntarily delisted from the CSE on April 23, 2021.
| 32 |
Competition
We compete in a global marketplace.
Like all companies operating in the pharmaceutical or biotherapeutic development sector, we have faced competition from well-established large pharmaceutical companies as well as innovative new entrants. Due to the prevalence of cancer, there are companies that are focusing their efforts in this space. Some of the smaller entrants in this space with which we may compete over time include Cullinan Oncology, Inc., which develops therapeutics geared toward improving the standard of care for those living with cancer; PureTech Health, which develops medicines for diseases including intractable cancers and lymphatic and GI diseases; and immunotherapy companies such as Black Diamond Therapeutics, Repare Therapeutics, Nuvation Bio, Shattuck Labs, Arcus Biosciences, Syndax Pharmaceuticals Inc. and iTeos Therapeutics S.A.
Nevertheless, we believe our strategic approach is sufficiently differentiated in that we have focused on multiple aspects of resistance to current immunotherapies based on the experience of our management at BMS developing Opdivo and Yervoy. We believe one of our strengths beyond the experience of our management and directors is our ability to understand what technology is potentially attractive to major pharmaceutical companies. We believe our prior collaborations within the research facilities of leading, world class universities and institutes, such as the Department of Investigative Medicine at University of Oxford, Stanford University, The National Cancer Institute, the Institut Curie, the Institut National de la Santé et de la Recherche Médicale, Yale University, Radboud University, and the Ludwig Institute, among others, allow us access to potentially innovative technologies.
(C) ORGANIZATIONAL STRUCTURE
We currently have four diverse oncology technology platforms, the product candidates of which have established scientific rationales, including intra-tumoral, nanoparticles, liposomes, aptamers, cell penetrating peptides, and virus-like particles.
Our significant subsidiaries include:
| (a) | SalvaRx, a wholly-owned subsidiary, incorporated in the British Virgin Islands; |
| (b) | iOx, a wholly-owned subsidiary incorporated in the U.K. In September 2021, we, through SalvaRx, exchanged certain notes, accrued interest, warrants and receivables in exchange for shares of iOx representing 17.83% of the outstanding shares of iOx. As a result of this exchange, we, through SalvaRx, increased our ownership of iOx from 60.49% to 78.32%. On July 18, 2022, we purchased the remaining non-controlling interest of iOx. Our 44% interest in Stimunity was transferred from Portage to iOx in December 2023 and was increased to 48.9% upon the conversion of the convertible note to equity; |
| (c) | Saugatuck, a 70% owned subsidiary incorporated in the British Virgin Islands. “Saugatuck and subsidiary” refers to Saugatuck and Saugatuck Rx LLC; |
| (d) | PDS, a wholly-owned subsidiary incorporated in Delaware, which provides human resources, and other services to each operating subsidiary via a shared services agreement; |
| (e) | SalvaRx LLC, a wholly-owned subsidiary through SalvaRx incorporated in Delaware; |
| (f) | Saugatuck Rx LLC, a wholly-owned subsidiary of Saugatuck incorporated in Delaware; and |
| (g) | Tarus, a wholly-owned subsidiary of Portage incorporated in Delaware. |
(D) PROPERTY, PLANT AND EQUIPMENT
We currently do not have any material tangible fixed assets or leased properties.
ITEM 4A – UNRESOLVED STAFF COMMENTS
None.
| 33 |
ITEM 5 – OPERATING AND FINANCIAL REVIEW AND PROSPECTS
| (A) | OPERATING RESULTS (All Amounts in 000’$) |
The following discussion should be read in conjunction with our Audited Consolidated Financial Statements and notes thereto for the fiscal year ended March 31, 2024, contained elsewhere in this Annual Report.
| Years ended March 31, | 2024 | 2023 | 2022 | |||||||||
| in 000’$ | in 000’$ | in 000’$ | ||||||||||
| Operating expenses | (18,199 | ) | (16,575 | ) | (15,588 | ) | ||||||
| Change in fair value of deferred purchase price payable - Tarus and deferred obligation - iOx milestone | 11,305 | 2,711 | - | |||||||||
| Loss on Registered Direct Offering | (2,432 | ) | - | - | ||||||||
| Offering costs | (662 | ) | - | - | ||||||||
| Change in fair value of warrant liability | 6,868 | 33 | 852 | |||||||||
| Impairment loss - iOx IPR&D | (57,890 | ) | (59,320 | ) | - | |||||||
| Impairment loss - Tarus IPR&D | (23,615 | ) | (4,585 | ) | - | |||||||
| Impairment loss - Goodwill | - | (43,862 | ) | - | ||||||||
| Impairment loss - Stimunity | (1,002 | ) | (818 | ) | - | |||||||
| Impairment loss - Saugatuck | (178 | ) | - | - | ||||||||
| Commitment fee under Committed Purchase Agreement | (839 | ) | - | - | ||||||||
| Share of loss in associate accounted for using equity method | (233 | ) | (260 | ) | (62 | ) | ||||||
| Gain on dissolution of investment in associate | 27 | - | - | |||||||||
| Gain from sale of investment in public company | 725 | - | - | |||||||||
| Foreign exchange transaction gain (loss) | 7 | (53 | ) | 24 | ||||||||
| Depreciation expense | (54 | ) | (1 | ) | - | |||||||
| Interest income (expense), net | 242 | 208 | (43 | ) | ||||||||
| Loss before provision for income taxes | (85,930 | ) | (122,522 | ) | (14,817 | ) | ||||||
| Income tax benefit (expense) | 10,548 | 17,856 | (4,352 | ) | ||||||||
| Net loss | (75,382 | ) | (104,666 | ) | (19,169 | ) | ||||||
| Other comprehensive income (loss) | ||||||||||||
| Net unrealized loss on investments | (38 | ) | (5,283 | ) | - | |||||||
| Total comprehensive loss for year | $ | (75,420 | ) | $ | (109,949 | ) | $ | (19,169 | ) | |||
| Comprehensive loss attributable to: | ||||||||||||
| Owners of the Company | $ | (75,377 | ) | $ | (109,894 | ) | $ | (16,870 | ) | |||
| Non-controlling interest | (43 | ) | (55 | ) | (2,299 | ) | ||||||
| Total comprehensive loss for year | $ | (75,420 | ) | $ | (109,949 | ) | $ | (19,169 | ) | |||
Overview
Due to our future funding needs for clinical development of our programs as well as the current capital raising market for biotechnology companies, we made the decision to discontinue our sponsored iNKT trial and pause further accrual to our sponsored adenosine program for PORT-6 and PORT-7. We are exploring strategic alternatives, which may include finding a partner for one or more of our assets, a sale of our company, a merger, restructurings, both in and out of court, a company wind down, further financing efforts or other strategic action. The following discussion reflects our operations if we were to resume enrollment in our programs.
We are a clinical stage immune-oncology company advancing treatments we believe will be first-in-class therapies that target known checkpoint resistance pathways to improve long-term treatment response and quality of life in patients with invasive cancers.
Our access to next-generation technologies provides the capability to identify and understand biological mechanism, clinical therapies and product development strategies that could accelerate these treatments through the translational pipeline.
We source and develop early- to mid-stage treatments that we believe will be first-in-class therapies for a variety of cancers, by funding, implementing viable, cost-effective product development strategies, clinical counsel/trial design, shared services, financial and project management to enable efficient, turnkey execution of commercially informed development plans. Our drug development pipeline portfolio encompasses product candidates or technologies based on biology addressing known resistance pathways/mechanisms of current checkpoint inhibitors with established scientific rationales.
| 34 |
The Portage Approach
Our mission has been to advance and grow a portfolio of innovative, early-stage oncology assets based on the latest scientific breakthroughs focused on overcoming immune resistance and expanding the addressable patient population. Given these foundations, we have managed capital allocation and risk as much as we have overseen drug development. By focusing our efforts on translational medicine and pipeline diversification, we sought to mitigate overall exposure to many of the inherent risks of drug development.
Our approach has been guided by the following core elements:
| · | Portfolio diversification to mitigate risk and maximize optionality; |
| · | Capital allocation based on risk-adjusted potential, including staged funding to pre-specified scientific and clinical results; |
| · | Virtual infrastructure and key external relationships to maintain a lean operating base; |
| · | Internal development capabilities complemented by external business development; |
| · | Rigorous asset selection for broad targets with disciplined ongoing evaluation; |
| · | Focus on translational medicine and therapeutic candidates with single agent activity; |
| · | Conduct randomized trials early and test non-overlapping mechanisms of action; and |
| · | Improve potential outcomes for patients with invasive cancers. |
We have executed such approach through our internal core team and our network of experts, contract labs and academic partners.
We believe that we are not subject to the regulation of the 40 Act, based on the definition of “investment company” and the compositions of our assets. Additionally, as we primarily operate within the biomedical industry as a R&D business, we believe that we are also able to take advantage of the non-exclusive safe harbor of Rule 3a-8 promulgated under the 40 Act so as not to be characterized as an investment company. We have adopted a capital preservation policy referenced in that rule.
| 35 |
Results of Operations for Fiscal 2024 Compared to Fiscal 2023
We incurred a net loss of approximately $75.4 million during Fiscal 2024, which includes approximately $60.6 million of net non-cash expenses, compared to a net loss of approximately $104.7 million during Fiscal 2023, a decrease in net loss of $29.3 million, year-over-year.
The components of the change in net loss and total comprehensive loss are:
| · | A non-cash gain totaling $11.3 million comprised of the change (decrease) in the fair value of the deferred obligation - iOx milestone of $4.1 million, and the change (decrease) in the fair value of the deferred purchase price payable to the former Tarus shareholders of $7.2 million. This is attributable to the full impairment of the related iOx IPR&D and Tarus IPR&D. |
| · | A $2.4 million loss from our equity financing in October 2023 representing the excess of the fair value of certain warrants over the net proceeds, $0.7 million of offering costs and a $6.9 million non-cash gain from the change in the fair value of certain warrants accounted for as liabilities issued in connection with such equity offering in October 2023. |
| · | A non-cash loss on impairment of $57.9 million with respect to the iOx in-process research and development (“IPR&D”) (PORT-2 platform), based on our IAS 36 fair value analysis triggered by our decision to pause those trials and our decision to explore strategic alternatives, which may include finding a partner for one or more of our assets, a sale of our company, a merger, restructurings, both in and out of court, a company wind down, further financing efforts or other strategic action. This represents a full impairment of the iOx IPR&D. |
| · | A non-cash loss on impairment of $23.6 million with respect to the Tarus IPR&D. This represents a full impairment of the Tarus IPR&D. |
| · | A non-cash impairment loss of $1.0 million with respect of our investment in Stimunity, based upon our IAS 36 analysis predicated on the inability of Stimunity to raise financing and our decision not to finance future operations. |
| · | A $0.8 million commitment fee expense related to the decision not to utilize the Committed Purchase Agreement. |
| · | A $0.7 million gain recognized on the sale of Intensity shares during Fiscal 2024. There was no such sale in Fiscal 2023. |
| · | Operating expenses, which include R&D and general and administrative (“G&A”) expenses, were $18.2 million in Fiscal 2024, compared to $16.6 million in Fiscal 2023, an increase of $1.6 million, which is discussed more fully below. |
| · | Additionally, we reflected a non-cash net deferred income tax benefit of $10.5 million in Fiscal 2024, compared to a non-cash net deferred income tax benefit of $17.9 million in Fiscal 2023, a year-over-year change of $7.4 million reflecting the reduction of deferred tax liability due to the full impairment of the IPR&D for iOx, partially offset by the derecognition of losses previously recognized. Fiscal 2023 reflected the recognition of current tax losses, the change (benefit) in exchange rates on the liability settleable in British pound sterling and the change (benefit) on the change in deferred income tax rates in the U.K. |
Total comprehensive loss in Fiscal 2024 was $75.4 million, compared to $109.9 million in Fiscal 2023, a decrease in total comprehensive loss of $34.5 million. The primary difference between net loss and total comprehensive loss in Fiscal 2024 was substantially due to the recognition of the net change in fair value of an investment of $0.038 million originally in OCI in Fiscal 2024.
Results of Operations for Fiscal 2023 Compared to Fiscal 2022
We generated a net loss of approximately $104.7 million and other comprehensive loss of approximately $109.9 million during Fiscal 2023, which include approximately $88.0 million of non-cash expenses, net, compared to a net loss and comprehensive loss of approximately $19.2 million during Fiscal 2022, an increase in net loss of $85.5 million and an increase in other comprehensive loss of $90.7 million, year-over-year.
| 36 |
The components of the change in net loss and other comprehensive loss are as follows:
| · | Operating expenses, which include R&D and G&A expenses, were $16.6 million in Fiscal 2023, compared to $15.6 million in Fiscal 2022, an increase of $1.0 million, which is discussed more fully below. |
| · | Our other items of income and expense were substantially non-cash in nature and aggregated approximately $105.9 million net loss in Fiscal 2023, compared to approximately $0.8 million net income in Fiscal 2022, a change in other items of income and expense of approximately $106.7 million, year-over-year. The primary reason for the year-over-year difference in other items of income and expense was non-cash impairment adjustments relating to the carrying value of IPR&D for iOx and Tarus of $59.320 million and $4.585 million, respectively, the impairment of goodwill totaling $43.862 million, and the loss on impairment relating to our investment in Stimunity and the Stimunity Convertible Note of $0.607 million and $0.211 million, respectively. The impairment analysis was undertaken as a result of indications of impairment from the overall life sciences market and our market capitalization. We considered a number of factors relating to the fair value analysis of the assets at March 31, 2023, including the cost of capital, discount rates, and the impact of timing delays of obtaining data. These losses were slightly offset by non-cash gains from the change (decrease) in fair value of the deferred purchase price payable to the former Tarus shareholders and the deferred obligation – iOx milestone totaling $2.711 million and net interest income from investments in short-term investments in Fiscal 2023. |
| · | Additionally, we reflected a non-cash net deferred income tax benefit of $17.9 million in Fiscal 2023, compared to a net deferred income tax expense of $4.4 million in Fiscal 2022. Fiscal 2023 includes $11.3 million to recognize the deferred tax effect of loss on impairment recognized with respect to the iOx IPR&D, $0.7 million related to other current year losses, $3.8 million to reflect the change related to the future U.K. tax rates and $2.1 million to reflect the effect of the change in exchange rates on the liability settleable in British pound sterling. Fiscal 2022 reflected recoverable R&D tax credits generated in the U.K., partially offset by the foreign currency effect on deferred tax liability balance settleable in British pound sterling. |
| · | In March 31, 2023, we performed a fair value analysis on its investment in Intensity, and determined a fair value of $2.087 million, which was $5.322 million less than the then-carrying value. Accordingly, we recognized an unrealized loss in value in Intensity of $5.322 million through other comprehensive income (loss) in Fiscal 2023, which was partially offset by an unrealized gain on the change in fair value of the Stimunity Convertible Note (as defined below) of $0.039 million recognized through other comprehensive income (loss). We sold our investment in Intensity in Fiscal 2024, and accordingly, a gain from the sale was recognized in Fiscal 2024. |
We may be required to record additional charges during the period in which there is an indication of impairment and the fair value of any of our intangible assets or other long-lived assets is determined to be less than the then carrying value, which could have a material adverse impact on our results of operations. Even though these charges are non-cash items, do not necessarily reflect the underlying fundamentals of our development programs and may not have an immediate impact on our liquidity, the fact that we report charges of this nature could contribute to negative market perceptions about us or our securities.
| 37 |
Operating Expenses
Total operating expenses (in 000’$) for the last three completed fiscal years are as follows:
| Years ended March 31, | 2024 | 2023 | 2022 | |||||||||
| Research and development | $ | 12,535 | $ | 8,674 | $ | 6,769 | ||||||
| General and administrative expenses | 5,664 | 7,901 | 8,819 | |||||||||
| Total operating expenses | $ | 18,199 | $ | 16,575 | $ | 15,588 | ||||||
Research and Development Costs
Fiscal 2024
R&D costs increased by approximately $3.8 million, or approximately 44%, from approximately $8.7 million in Fiscal 2023, to approximately $12.5 million in Fiscal 2024. The increase was primarily attributable to clinical trial costs (principally CRO-related), which increased by approximately $2.5 million, from $2.7 million in Fiscal 2023 to $5.2 million in Fiscal 2024, as activities ramped up throughout the period until we made the decision to pause enrollment in our sponsored clinical trials in the third and fourth quarters of Fiscal 2024. Manufacturing-related costs increased by $1.0 million from $0.8 million in Fiscal 2023, compared to $1.8 million in Fiscal 2024, related to the iNKT and adenosine clinical trials. Payroll-related expenses decreased by $0.3 million from $1.9 million in Fiscal 2023 to $1.6 million in Fiscal 2024; the increases in salaries effective January 2023 were more than offset by the fact that no annual bonuses were incurred in Fiscal 2024. R&D non-cash share-based compensation expense decreased by $0.8 million, from $2.2 million in Fiscal 2023, compared to $1.4 million in Fiscal 2024. This decrease was due to the continued vesting of options granted in prior years, as well as recent grants having a lower grant date fair value. Additionally, in Fiscal 2024, we incurred a milestone payment of $0.5 million for dosing our first adenosine patients, an increase in consulting fees of approximately $0.4 million from $0.4 million in Fiscal 2023 to $0.8 million in Fiscal 2024 to reflect the increase in activity year-over-year, and, finally, $0.5 million in fees paid with respect to the transition of the iNKT study prior to discontinuing the study in Fiscal 2024.
Fiscal 2023
R&D costs increased by approximately $1.9 million, or approximately 28%, from approximately $6.8 million in Fiscal 2022, to approximately $8.7 million in Fiscal 2023. The increase was primarily attributable to the start-up and manufacturing costs associated with the adenosine assets (PORT-6 and PORT-7) acquired in the Tarus acquisition of $1.1 million and the clinical trial costs of $2.4 million associated with the iNKT clinical trial for PORT-2. There were no such costs incurred in Fiscal 2022. Additionally, we incurred costs of $0.2 million associated with the NCI trial for clinical development of STING agonists and anti-RAGE agents for cancer vaccines in Fiscal 2023, an increase of $0.3 million in other R&D costs relating to services and storage and an increase of $0.3 million in payroll-related expenses. These increases were partially offset by a reduction in non-cash share-based compensation expense of $2.4 million with respect to stock options to purchase ordinary shares granted to employees, which was attributable to (a) the vesting over time of a portion of prior year grants; and (b) the decrease in the fair value of grants of stock options made in Fiscal 2023, as well as the timing of the grants.
| 38 |
Fiscal 2022
R&D costs decreased by approximately $0.5 million, or approximately 7%, from approximately $7.3 million in Fiscal 2021, to approximately $6.8 million in Fiscal 2022. Fiscal 2021 R&D costs were reduced by the receipt of a $0.6 million legal settlement in respect of certain clinical development costs; accordingly, normalized expenses decreased $1.1 million year over year. The decrease was primarily attributable to non-cash share-based compensation expense associated with grants of stock options made under the Company’s Amended and Restated 2021 Equity Incentive Plan (as defined below) of $0.7 million and a decrease in iOx related share-based compensation expense of $0.5 million, a decrease of $0.5 million in other R&D costs relating to outside supplier costs, control activities and medical writing, and a decrease of $0.4 million in other R&D costs relating to services and storage, partially offset by a year over year increase in compensation of $1.0 million for employees and consultants involved in R&D activities.
General and Administrative Expenses
Fiscal 2024
G&A expenses decreased by approximately $2.2 million, or approximately 28%, from approximately $7.9 million in Fiscal 2023, to approximately $5.7 million in Fiscal 2024. Professional fees decreased by $0.7 million, to $2.3 million in Fiscal 2024, compared to $3.0 million in Fiscal 2023, primarily attributable to legal fees associated with the Tarus acquisition and other regulatory filings in Fiscal 2023. Additionally, G&A non-cash share-based compensation expense decreased by $0.8 million, from $2.0 million in Fiscal 2023 to $1.2 million in Fiscal 2024. This decrease of $0.8 million in G&A non-cash share-based compensation expense was attributable to the vesting of certain stock options granted in prior years and lower fair value associated with more recent grants. Insurance expense decreased by $0.5 million from $1.2 million in Fiscal 2023 to $0.7 million in Fiscal 2024, due to the decrease in the D&O premium year-over-year resulting from changes in the insurance markets. Directors’ fees decreased by $0.1 million in Fiscal 2024, compared to Fiscal 2023 as certain directors waived their fees in the quarter ended March 31, 2024. Finally, payroll-related expenses decreased by $0.1 million from $1.0 million in Fiscal 2023 to $0.9 million in Fiscal 2024; the increase in annual salaries effectuated in January 2023 was slightly more than offset by the fact that there were no annual bonuses incurred in Fiscal 2024.
Fiscal 2023
G&A expenses decreased by approximately $0.9 million, or approximately 10%, from approximately $8.8 million in Fiscal 2022, to approximately $7.9 million in Fiscal 2023. Professional fees increased by $1.3 million, of which $0.8 million was attributable to legal fees associated with the Tarus acquisition and $0.3 million was attributable to audit and accounting related expenses in Fiscal 2023 associated with the updating of public filings, as well as costs associated with the Tarus acquisition review. $0.2 million of the increase was attributable to stamp fees in the U.K. related to acquiring the outstanding minority interest of iOx, our subsidiary that manages our iNKT engager platform. Additionally, payroll-related expenses increased by $0.3 million due to the adoption of a compensation program in Fiscal 2023 designed to attract and retain management; along the same lines, we incurred $0.3 million in compensation to our directors in Fiscal 2023. These increases were partially offset by a decrease in non-cash share-based compensation expense of $2.4 million attributable to the vesting of certain stock options granted in prior years and lower fair value associated with more recent grants and the decrease of $0.4 million associated with D&O insurance, which was attributable to a decrease in the D&O premium market year-over-year.
Fiscal 2022
G&A expenses increased by approximately $3.7 million, or approximately 73%, from approximately $5.1 million in Fiscal 2021, to approximately $8.8 million in Fiscal 2022. The principal reason for the increase was the $1.6 million of non-cash share-based compensation expense associated with the Company’s Amended and Restated 2021 Equity Incentive Plan, of which $2.4 million is associated with directors’ compensation and $0.8 million is associated with the new grants of stock options issued in January and February 2022, which was partially offset by a decrease of $1.6 million associated with management compensation; and a decrease in iOx related share-based compensation expense of $0.1 million. Additionally, the Company incurred an increase of $1.0 million in professional fees relating to initiatives associated with a corporate restructuring and public relations and business development. Finally, D&O insurance premiums increased $1.4 million in Fiscal 2022 compared to Fiscal 2021 due to market rate increases in the cost of coverage, partially offset by a decrease in office and general expenses of $0.2 million, attributable to investor related expense, which includes transfer agent fees, Nasdaq fees and investor meeting costs.
| 39 |
| (B) | LIQUIDITY AND CAPITAL RESOURCES |
Capital Resources
We filed the March 2021 Registration Statement with the SEC in order to sell ordinary shares, debt securities, warrants and units in one or more offerings from time to time, which became effective on March 8, 2021. In connection with the March 2021 Registration Statement, we have filed with the SEC:
| · | a base prospectus, which covers the offering, issuance and sale by us of up to $200 million in the aggregate of the securities identified above from time to time in one or more offerings; |
| · | a prospectus supplement, which covers the offer, issuance and sale by us in an ATM offering program of up to a maximum aggregate offering price of $50 million of our ordinary shares that may be issued and sold from time to time under the Sales Agreement with Cantor Fitzgerald & Co., the sales agent; |
| · | a prospectus supplement dated June 24, 2021, for the offer, issuance and sale by us of 1,150,000 ordinary shares for gross proceeds of approximately $26.5 million in a firm commitment underwritten public offering with Cantor Fitzgerald; |
| · | a prospectus supplement dated August 19, 2022, for the resale of up to $30 million in ordinary shares that we may sell from time to time to Lincoln and an additional 94,508 shares that were issued to Lincoln; and |
| · | a prospectus supplement dated September 29, 2023 for the offer, issuance and sale by us in a registered direct public offering through H.C. Wainwright & Co., the placement agent, to an institutional and accredited investor of (i) 1,970,000 of our ordinary shares at a purchase price of $1.90 per share and (ii) Pre-Funded Warrants to purchase up to 1,187,895 of our ordinary shares, at a purchase price of $1.899 per Pre-Funded Warrant), for aggregate gross proceeds of approximately $6 million (the “2023 Equity Financing”). |
The Sales Agreement permits us to sell in an ATM program up to $50 million of ordinary shares from time to time. The sales under the prospectus will be deemed to be made pursuant to an ATM program as defined in Rule 415(a)(4) promulgated under the Securities Act of 1933, as amended (the “Securities Act”).
On June 24, 2021, we completed the sale of 1,150,000 ordinary shares, including the underwriters’ option, at a price of $23.00 per share, which generated gross proceeds of approximately $26.5 million and net proceeds of approximately $25.0 million, and was settled June 28, 2021.
During Fiscal 2022, we commenced an ATM program, and we sold 90,888 ordinary shares, generating gross proceeds of approximately $2.6 million ($2.5 million, net of commissions).
During Fiscal 2023, we sold 166,145 ordinary shares under the ATM program, generating net proceeds of approximately $0.9 million.
During Fiscal 2024, we sold 186,604 ordinary shares under the ATM program, generating net proceeds of approximately $0.7 million.
On July 6, 2022, we entered into the Committed Purchase Agreement with Lincoln, pursuant to which we may require Lincoln to purchase our ordinary shares having an aggregate value of up to $30 million over a period of 36 months. For a summary of the terms of the Committed Purchase Agreement, please see our Form 20-F for the fiscal year ended March 31, 2023 filed with the SEC on July 31, 2023.
During Fiscal 2023, we sold 480,000 ordinary shares to Lincoln under the Committed Purchase Agreement for net proceeds totaling approximately $2.0 million.
The March 2021 Registration Statement expired on February 24, 2024. In order to issue additional shares under our ATM program or the Committed Purchase Agreement in the future, we would be required to file a new registration statement, which must be declared effective by the SEC prior to use, and to file a prospectus supplement related to the ATM program or the Committed Purchase Agreement, as the case may be.
| 40 |
Furthermore, our ATM program and the Committed Purchase Agreement are generally limited based on, among other things, our Nasdaq trading volume. Under the Baby Shelf Rule, the amount of funds we can raise through primary public offerings of securities in any 12-month period using a registration statement on Form F-3 is limited to one-third of the aggregate market value of the ordinary shares held by our non-affiliates, which limitation may change over time based on our stock price, number of ordinary shares outstanding and the percentage of ordinary shares held by non-affiliates. We are therefore limited by the Baby Shelf Rule as of the filing of this Form 20-F, until such time as our non-affiliate public float exceeds $75 million.
On March 1, 2023, we, through Tarus, entered into a clinical service agreement with a third-party service provider. The term of the agreement is through the earlier of August 14, 2025 or the completion of provision of services and the payment of contractual obligations. The agreement provides for budgeted costs totaling approximately $12.1 million.
In connection with the 2023 Equity Financing, on September 29, 2023, we entered into the Purchase Agreement with an institutional and accredited investor in connection with the Registered Direct Offering and the concurrent private placement. The Offerings closed on October 3, 2023.
Pursuant to the Purchase Agreement, in the Registered Direct Offering, we sold (i) 1,970,000 of our ordinary shares, at a purchase price of $1.90 per share and (ii) Pre-Funded Warrants to purchase up to 1,187,895 Pre-Funded Warrant Shares. All Pre-Funded Warrants, which were immediately exercisable for one ordinary share at an exercise price of $0.001 per share, were exercised in full on May 29, 2024.
In the Private Placement, we issued to such institutional and accredited investor unregistered Series A Warrants to purchase up to 3,157,895 ordinary shares, unregistered Series B Warrants to purchase up to 3,157,895 ordinary shares, and unregistered Series C Warrants to purchase up to 3,157,895 ordinary shares, together exercisable for an aggregate of up to 9,473,685 Private Warrant Shares. Pursuant to the terms of the Purchase Agreement, for each ordinary share and Pre-Funded Warrant issued in the Registered Direct Offering, an accompanying Series A Warrant, Series B Warrant and Series C Warrant were issued to such institutional and accredited investor. Each Series A Warrant is exercisable for one Private Warrant Share at an exercise price of $1.90 per share, is immediately exercisable and will expire 18 months from the date of issuance. Each Series B Warrant is exercisable for one Private Warrant Share at an exercise price of $2.26 per share, is immediately exercisable and will expire three years from the date of issuance. Each Series C Warrant is exercisable for one Private Warrant Share at an exercise price of $2.26 per share, is immediately exercisable and will expire five years from the date of issuance. The net proceeds to us from the Offerings were approximately $5.3 million, after deducting placement agent’s fees and estimated offering expenses.
| 41 |
Pursuant to an engagement letter, dated as of August 26, 2023, between us and H.C. Wainwright & Co., LLC (the “Placement Agent”), we paid the Placement Agent a total cash fee equal to 6.0% of the aggregate gross proceeds received in the Offerings, or $0.36 million. We also agreed to pay the Placement Agent in connection with the Offerings a management fee equal to 1.0% of the aggregate gross proceeds raised in the Offerings ($0.06 million), $75,000 for non-accountable expenses and $15,950 for clearing fees. In addition, we agreed to issue to the Placement Agent, or its designees, Placement Agent Warrants to purchase up to 157,895 ordinary shares, which represents 5.0% of the aggregate number of ordinary shares and Pre-Funded Warrants sold in the Registered Direct Offering. The Placement Agent Warrants have substantially the same terms as the Private Warrants, except that the Placement Agent Warrants have an exercise price equal to $2.375, or 125% of the offering price per ordinary share sold in the Registered Direct Offering, and will be exercisable for five years from the commencement of the sales pursuant to the Offerings.
Going Concern
The accompanying consolidated financial statements for the year ended March 31, 2024 have been prepared on a basis that assumes that we will continue as a going concern and that contemplates the continuity of operations, the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. Accordingly, the accompanying consolidated financial statements for the year ended March 31, 2024 do not include any adjustments relating to the recoverability and classification of recorded asset amounts or amounts of liabilities that might result from the outcome of this uncertainty.
As of March 31, 2024, we had cash and cash equivalents of approximately $5.0 million and total current liabilities of approximately $2.9 million. For the year ended March 31, 2024, we are reporting a net loss of approximately $75.4 million (which include approximately $60.6 million of non-cash expenses, net of non-cash income), and cash used in operating activities of approximately $14.3 million. As of July 31, 2024, we had approximately $3.1 million of cash and cash equivalents on hand.
Due to our future funding needs for clinical development of our programs as well as the current capital raising market for biotechnology companies, we made the decision to discontinue our sponsored iNKT trial and pause further accrual to our adenosine program. We are exploring strategic alternatives, which may include finding a partner for one or more of our assets, a sale of our company, a merger, restructurings, both in and out of court, a company wind down, further financing efforts or other strategic action.
There can be no assurance that our evaluation of strategic alternatives will result in any agreements or transactions, or that, if completed, any agreements or transactions will be successful or on attractive terms. Any potential transaction would be dependent on a number of factors that may be beyond our control, including, among other things, market conditions, industry trends, the interest of third parties in a potential transaction with us and the availability of financing to us or third parties in a potential transaction with us on reasonable terms. The process of reviewing strategic alternatives may require us to incur additional costs and expenses. It could negatively impact our ability to attract, retain and motivate key employees, and expose us to potential litigation in connection with this process or any resulting transaction. If we are unable to effectively manage the process, our financial condition and results of operations could be adversely affected. In addition, any strategic alternative that may be pursued and completed ultimately may not deliver the anticipated benefits or enhance shareholder value. There can be no guarantee that the process of evaluating strategic alternatives will result in our company entering into or completing a potential transaction within the anticipated timing or at all. There is no set timetable for this evaluation and we do not intend to disclose developments with respect to this evaluation unless and until we determine that further disclosure is appropriate or legally required. As of July 31, 2024, we had approximately $3.1 million of cash and cash equivalents on hand, which we expect is only sufficient to cover our operating needs through December 2024. These factors raise substantial doubt about our ability to continue as a going concern within one year after the date of the consolidated statement of financial position (March 31, 2024).
| 42 |
We have incurred significant operating losses since inception and expect to continue to incur significant operating losses for the foreseeable future and may never become profitable. The losses result primarily from our conduct of research and development activities. As previously discussed, we have discontinued further development of our iNKT sponsored trial and paused further accrual to our adenosine program in order to preserve cash resources. Additionally, during the fourth quarter of Fiscal 2024, we sold our shares in Intensity on Nasdaq.
We historically have funded our operations principally from proceeds from issuances of equity and debt securities. We will require significant additional capital to make the investments we need to execute our longer-term business plan. Our ability to successfully raise sufficient funds through the sale of debt or equity securities when needed is subject to many risks and uncertainties and, future equity issuances would result in dilution to existing shareholders and any future debt securities may contain covenants that limit our operations or ability to enter into certain transactions. For additional information, see Item 3 Key Information – Risk Factors – “Risks Related to our Decision to Discontinue our iNKT Program and Pause Further Accrual in our Adenosine Program” and “We have current and future capital needs, and, if we decide to resume enrollment in our clinical trials, there are uncertainties as to our ability to raise additional funding.”
As of the date of this filing, we currently anticipate that current cash and cash equivalents, is only sufficient to cover our operating needs through December 2024. These factors raise substantial doubt about our ability to continue as a going concern within one year after the date of the consolidated statement of financial position (March 31, 2024).
Operating Cash Flow
Fiscal 2024
During Fiscal 2024, we used cash of $14.3 million to fund operating activities, which was consistent with running both clinical programs until the decisions were made in the last half of Fiscal 2024 to discontinue and pause further accrual in the respective clinical programs.
Fiscal 2023
During Fiscal 2023, we used cash of $12.1 million to fund operating activities. Operations in Fiscal 2023 were funded by our existing cash and the ATM program and the public offerings in 2022 and 2021 and the ordinary shares issued to Lincoln under the Committed Purchase Agreement, described above under “Capital Resources.”
Fiscal 2022
During Fiscal 2022, we used cash of approximately $6.8 million to fund operating activities, which was provided by our existing cash and the ATM program and the public offering, described above.
Investing Cash Flows
Fiscal 2024
During Fiscal 2024, we generated cash of $2.8 million from investing activities, which reflected the proceeds from the sales of Intensity shares.
Fiscal 2023
During Fiscal 2023, we used cash of $0.6 million to fund investing activities.
| 43 |
On September 12, 2022, we funded a €600,000 convertible note (the “Stimunity Convertible Note”) with a maturity date of September 1, 2023 (the “Maturity Date”). The Stimunity Convertible Note provided for simple interest at 7% per annum for automatic conversion into Series A shares of Stimunity upon Stimunity completing a Series A round for at least €20 million. Also, we were entitled, in certain circumstances, to convert the Stimunity Convertible Note into Series A shares of Stimunity at the subscription share price less 15%, or if Stimunity completed a financing with a new category of shares (other than common shares or Series A shares of Stimunity) for at least €5 million (the “Minimum Raise”), we had the right to convert the Stimunity Convertible Note and the historical Series A shares of Stimunity owned into the new category of shares of Stimunity. Stimunity did not close a financing prior to the Maturity Date. In December 2023, we completed a transfer of our equity in Stimunity and the Stimunity Convertible Note to iOx. In connection with that transfer, the Stimunity Convertible Note was converted into 1,768 Class A shares of Stimunity.
On July 18, 2022, we and our wholly-owned subsidiary, SalvaRx, entered into the Share Exchange Agreement with each of the minority shareholders of iOx resulting in the acquisition of the outstanding non-controlling ownership interest (approximately 22%) of iOx, which is developing the iNKT engager platform. We followed IFRS 3, “Business Combinations,” and IAS 27, “Separate Financial Statements,” (which substantially replaced IAS 3) to account for this transaction. We achieved control of iOx on January 8, 2019 upon the completion of our acquisition of SalvaRx. Further transactions whereby we acquire further equity interests from non-controlling interests, or disposes of equity interests but without losing control, are accounted for as equity transactions (i.e., transactions with owners in their capacity as owners). As such:
| · | the carrying amounts of the controlling and non-controlling interests are adjusted to reflect the changes in their relative interests in the subsidiary; |
| · | any difference between the amount by which the non-controlling interests is adjusted and the fair value of the consideration paid or received is recognized directly in equity and attributed to us; and |
| · | there is no consequential adjustment to the carrying amount of goodwill, and no gain or loss is recognized in profit or loss. |
Fiscal 2022
During Fiscal 2022, the Company did not use any cash for investing activities.
| 44 |
Financing Cash Flows
Fiscal 2024
During Fiscal 2024, we generated $6.0 million in cash from financing activities, of which $5.3 million net proceeds related to the Registered Direct Offering and $0.7 million related to net proceeds from sales under the ATM program.
Fiscal 2023
During Fiscal 2023, we used cash of $0.1 million to fund financing activities.
During Fiscal 2023, as consideration for the Tarus acquisition, we issued to the former Tarus shareholders an aggregate of 2,425,999 of our ordinary shares, calculated on the basis of $18 million divided by the 60-day volume weighted average price per our ordinary share. The ordinary shares have not been registered with the SEC and were subject to lock-up agreements for terms ranging from six to twelve months. We also assumed certain liabilities totaling $3.0 million for short-term debt held by Tarus and deferred license milestones obligations, which were repaid by us in July 2022. Additionally, milestone payments of up to $32 million in cash or our ordinary shares would be triggered upon achievement of future development and sales milestones, as further described above.
In October 2022, we began selling shares pursuant to the ATM program and the Sales Agreement. Through March 31, 2023, we sold 166,145 ordinary shares under the ATM program, generating net proceeds of approximately $0.9 million. Separately, we sold 480,000 ordinary shares to Lincoln under the Committed Purchase Agreement for net proceeds totaling approximately $2.0 million.
Fiscal 2022
During Fiscal 2022, we generated net cash from financing activities of $27.3 million.
During the three months ended June 30, 2021, we commenced an ATM program, under which it sold 90,888 ordinary shares generating gross proceeds of approximately $2.6 million ($2.5 million, net of commissions). On June 24, 2021, we completed a firm commitment underwritten public offering of 1,150,000 ordinary shares at a public offering price of $23.00 per share for gross proceeds of approximately $26.5 million and was settled on June 28, 2021. We incurred aggregate offering expenses for the public offering of approximately $1.8 million, including approximately $1.6 million of management, underwriting and selling expenses.
(C) RESEARCH AND DEVELOPMENT, PATENTS AND LICENSES
From May 23, 2012 to date, we, through our operating subsidiaries, have been engaged in general research and development and clinical and pre-clinical studies as detailed under Item 4 (B) “Business Overview” of this Annual Report. Research and development expenses analysis and details are provided under Item 5 (A) “Operating Results” of this Annual Report. All research and development expenses are expensed as they are incurred.
iOx (iNKT) License
On July 1, 2015, iOx entered into a licensing agreement with Ludwig Institute for Cancer Research Ltd. (“LICR”), which covers certain technology, intellectual property and know-how and development with respect to iNKT cell agonists to treat human diseases. Under the terms of the licensing agreement (“LICR License”), LICR granted to iOx an exclusive worldwide license, with the right to grant sublicenses, under the Licensed Patent and Licensed Technology, each as defined in the LICR License, in each case, to develop, make, have made, use, sell, offer for sale and import Licensed Products, as defined in the LICR License, subject to certain rights retained by LICR for academic and research purposes. The LICR License provides for a royalty term of ten years after the first commercial sale, on a Licensed Product by Licensed Product, country by country basis. Upon the expiration of the applicable royalty term, the license with respect to such Licensed Product in such country will convert to a non-exclusive, fully paid-up license.
| 45 |
LICR is entitled to 15,000 GBP as an annual license fee on each annual anniversary of the effective date of the LICR License until royalties become duly payable and 15,000 GBP as a patent reimbursement fee until LICR has been fully reimbursed for all patent costs incurred prior to the LICR License.
Additionally, LICR is entitled to milestone payments totaling up to 20.45 million GBP based upon the first Licensed Product achieving specific clinical, regulatory and sales-based milestones. LICR is also entitled to milestone payment totaling up to 10.25 million GBP based upon a second Licensed Product achieving specific clinical, regulatory and sales-based milestones.
Finally, LICR is entitled to a low-single digit royalty on net sales of Licensed Products that marginally escalates upon sales levels all determined by territory. LICR is also entitled to a percentage of any sublicensing income that gradually decreases based on the stage of development of the most advanced Licensed Product that is the subject of the applicable sublicense agreement.
Pursuant to the terms and conditions of the LICR License, LICR is responsible for managing the preparation, filing, prosecution and maintenance of all Licensed Patent Rights, as defined in the LICR License. iOx will reimburse LICR for all reasonable patent costs it incurs after the effective date of the LICR License. Further, the LICR License provides that both parties have the right to termination for material breach or default in the performance of obligations under the LICR License by the other party and in the event of insolvency of the other party.
Tarus (adenosine) License
On July 1, 2022, we acquired Tarus Therapeutics, Inc. Pursuant to the license agreement entered into by Tarus Therapeutics, Inc. and Impetis Biosciences Limited (“Impetis”) dated October 29, 2019 (“Impetis License”), Impetis granted to Tarus an exclusive sublicensable worldwide license to develop and commercialize the adenosine receptor antagonists for all indications and certain other assets which were granted upon exercise of a call option on November 5, 2020.
Under the terms of the Impetis License, Impetis is eligible to receive payments totaling up to $38 million on an Impetis Compound (as defined in the Impetis License) based upon achievement of certain clinical and commercial milestones. Milestone payments due in the amount of USD $1 million for achievement of certain regulatory milestones were paid in July 2022 and a $0.5 million milestone was paid upon dosing the first patient in September 2023.
Additionally, commencing upon the First Commercial Sale (as defined in the Impetis License) of a Licensed Product (as defined in the Impetis License), Impetis is entitled to royalties on worldwide net sales that begin in the mid-single digits and escalate through multiple tiers, with net sales over $1 billion receiving low double digit royalties.
Pursuant to the terms and conditions of the Impetis License, Tarus has exclusive and full authority to manage all intellectual property (whether licensed or not) underlying the assets covered by the Impetis License and any other aspects related to exploitation, development and commercialization thereof at its own cost, and Impetis must provide Tarus reasonable assistance as requested at Tarus’ cost and expense. Further, the Impetis License provides that both parties have the right to termination for material breach by the other party and in the event that the other party undergoes certain events such as a voluntary winding-up, a liquidation or entry into receivership.
(D) TREND INFORMATION
There are no other trends, commitments, events or uncertainties presently known to management that are reasonably expected to have a material effect on the Company's business, financial condition or results of operation other than as disclosed elsewhere in this Annual Report (refer to the heading entitled "Risk Factors") under Item 3 (D) and Item 4 (B) “Business Overview” and elsewhere in this Item 5).
| 46 |
(E) CRITICAL ACCOUNTING ESTIMATES
Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Significant areas where estimates are made include valuation of financial instruments (including the Stimunity Convertible Note (as defined below), deferred tax assets and liabilities, warrant liabilities, research and development costs, fair value used for acquisition of intangible assets, contingent consideration assumed and measurement of share-based compensation. Significant areas where critical judgments are applied include assessment of impairment of investments, in-process research and development and warrant liabilities.
ITEM 6 – DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
(A) DIRECTORS AND SENIOR MANAGEMENT
At March 31, 2024, we had seven members on the Board of Directors – Dr. Gregory Bailey, Mr. Steven Mintz, Dr. Ian Walters, Mr. James Mellon, Ms. Linda Kozick, Mr. Mark Simon and Dr. Robert Glassman. Dr. Walters is our Chairman of the Board and Chief Executive Officer (“CEO”), Dr. Bailey is our Lead Director.
On April 25, 2024, Mr. Simon resigned from the Board and all committees thereof.
On April 26, 2024, Ms. Kozick and Dr. Glassman resigned from the Board and all committees thereof.
On April 30, 2024, the Board appointed Jean-Christophe Renondin, M.D. and Justin Stebbing, M.D., Ph.D. as directors. Both Dr. Renondin and Dr. Stebbing have been appointed to the Nominating Committee of the Board and the Audit Committee of the Board.
Biographical information of the key people in our organization is provided below.
Ian B. Walters, MD, MBA – Chairman of the Board and CEO
Ian B. Walters, M.D., M.B.A., is the Chairman of the Board and CEO of Portage Biotech Inc. Over his 26-year plus career, he has demonstrated both leadership and expertise in drug development, including the advancement of multiple cancer compounds from research stages through regulatory approval.
Ian specializes in the evaluation, prioritization, and the innovative development of new therapies for the treatment of severe diseases. He has worked at PDL Biopharma, Inc., Millenium Pharmaceuticals, Inc. and Sorrento Therapeutics, Inc., leading corporate development, translational medicine, clinical development and medical affairs.
Ian spent seven years at Bristol Myers Squibb, where he managed physicians overseeing the international development of more than eight oncology compounds (including Nivolimab (anti-PD-1), Ipilimumab (anti-CTLA-4), brivanib (anti VEGF/FGF), anti-IGF/IR, VEGFR2 biologic, Elotuzimab (antiCS1), as well as biomarker and companion diagnostic work. He was a core member of Bristol Myers Squibb’s Strategic Transactions Group evaluating and executing licensing agreements, mergers and acquisitions, clinical collaborations and the company’s immuno-oncology strategy.
Before entering the private sector, Ian was a lead investigator at the Rockefeller University and initiated advanced immunology research to understand the mechanism of action of several compounds. Ian received his MD from the Albert Einstein College of Medicine and an MBA from the Wharton School of The University of Pennsylvania. Ian is also a member of the board of directors of Enzo Biochem, Inc., a Nasdaq listed company, and BoKo Therapeutics. Ian is also a part-time consultant to Intensity Therapeutics, Inc.
| 47 |
Gregory Bailey MD – Lead Director
Dr. Bailey is the Lead Director of the Company. Gregory Bailey is a co-founder and managing partner of MediqVentures. Previously he was a managing partner of Palantir Group, Inc., a merchant bank involved in a number of biotech company startups and financings. Palantir was also involved in acquiring intellectual property assets and founding companies around such IP.
Greg was the co-founder of Portage, Ascent Healthcare Solutions, VirnetX Inc. (VHC: AMEX) and DuraMedic Inc. He was the initial financier and an independent director of Medivation, Inc. (MDVN: Nasdaq), from 2005 to December 2012. Dr. Bailey served as the Managing Director and co-Head of Life Sciences at MDB Capital Group LLC from May 2004 to December 2006. Greg has served on the board of directors of multiple public companies. His current company board positions include Biohaven, Culminant Reinsurance, Chelsea Avondale, Agex, Manx Financial, and Portage. He is also a director and the CEO of Juvanescence Ltd.
Dr. Bailey is a Member of the Compensation Committee and Nominating Committee of our Board of Directors.
Greg practiced emergency medicine for 10 years before entering finance. He received his medical degree from the University of Western Ontario.
Steven Mintz – Director
Steven Mintz C.A. graduated from University of Toronto in 1989 and went into public accounting, working at a large accounting firm from 1989 until 1992. He obtained his C.A. designation in June of 1992. In June 1992 he became employed by a boutique bankruptcy and insolvency firm where he was employed until January 1997. He obtained his Trustee in Bankruptcy license in 1995.
Since January 1997, he has been a self-employed financial consultant serving both private individuals and companies, as well as public companies in a variety of industries including mining, oil and gas, real estate and investment strategies. He is currently President of St. Germain Capital Corp., a private consulting and investment firm. He is also a principal and CFO of the Minkids Group, a family investment, and development company. Steven is currently a director of Pool Safe, Inc. (since December 2009), Everton Resources, Inc. (since May 2023) and IM Cannabis (since April 2018, formerly Navasota Resources).
Mr. Mintz is the Chair of the Audit Committee and a Member of the Compensation Committee of our Board of Directors.
Mr. James Mellon – Director
Jim Mellon is an entrepreneur, investor and author. He was one of the founders of Portage Biotech and is the co-author of five books, all written with a view toward identifying emerging thematic trends leading to investment opportunities. He is a founder and Executive Chair of Agronomics Limited (LSE:ANIC), an investment vehicle for cultivated meat. He has a particular interest in longevity research and is currently the co-founder and Director of anti-aging biopharma company, Juvenescence Limited. He is also a non-executive Chair of Condor Gold plc (LSE:CNR), the Executive Chair of Manx Financial Group plc (LSE:MFX), co-founder and Director of Bradda Head Lithium Limited (LSE:BHL.L), and the non-executive Chairman of the Board of SalvaRx Group plc. He is also the co-founder and Chair of Regent Pacific Group Limited (XHKG:575).
Mr. Mellon is the Chair of the Compensation Committee of our Board of Directors.
Mr. Mellon holds a Master’s Degree in Philosophy, Politics and Economics from Oxford University.
| 48 |
Jean-Christophe Renondin, MD – Director
Dr. Jean-Christophe is an accomplished and seasoned healthcare investor with more than 20 years of experience in financing and investing in healthcare asset globally.
Dr. Renondin is currently Managing Partner at Vesalius Biocapital IV, a well-established European life-sciences venture capital firm. Previously, he was Senior Healthcare Manager at the Sovereign Fund of Oman since 2015, the Oman Investment Authority, (OIA), in charge of the healthcare investment practice at the Oman Investment Authority. Prior to joining the Oman Investment Authority, Dr. Renondin was Managing Director at Bryan Garnier & Co., a pan-european investment bank in charge of the healthcare practice leading ECM and Private Placement transactions for European healthcare clients.
Dr. Renondin was General Partner at CDC Innovation investing in various European healthcare assets. From 1999 to 2005, Dr. Renondin was consequently Vice President at Sofinov, a subsidiary of the Caisse de Depot et Placement of Quebec (CDPQ), leading the Healthcare investment fund, and Managing Director at MDS Capital, one of the leading healthcare investment firms in Canada. Prior to 1999, Dr. Renondin was the General Manager of different international subsidiaries for Servier laboratories, in Ireland and in South Africa where he successfully restructured some departments. Prior to working at Servier, Dr. Renondin was at JP Morgan in the Healthcare equity research and Corporate Finance department at the NY office.
Dr. Renondin is the Chair of the Nominating Committee and a Member of the Audit Committee of our Board of Directors.
Dr. Renondin earned his Medical’s Doctorate degree (MD) from Paris V Descartes University and his MBA from the Amos Tuck Scholl of Business Administration in 1991.
Justin Stebbing, MD, PhD – Director
Justin Stebbing, PhD, M.D., is Editor-in-Chief of Oncogene and a member of the American Society for Clinical Investigation. Dr. Stebbing holds the distinct honor of being appointed the British government’s first oncology professor funded by the National Institute for Health Research (NIHR). He has published over 700 papers, and during the COVID-19 pandemic his work starting with artificial intelligence led to an FDA EUA for baricitinib, now a fully approved rheumatoid arthritis treatment.
Dr. Stebbing is a Member of the Audit Committee and Nominating Committee of our Board of Directors.
Dr. Stebbing has combined his medical career with healthcare investing. He has worked with Atticus Capital, Lansdowne, and Vitruvian Partners, and previously chaired the board of BB Healthcare Trust.
| 49 |
Prior to a residency at The Johns Hopkins Hospital, Dr. Stebbing gained a medical degree from Trinity College Oxford. He trained at the Royal Marsden and Barts and he has been principal investigator on numerous studies through all phases of development. He was previously the first NIHR Research Translational Oncology Professor in the U.K., and he is also a Professor of Biomedical Sciences at ARU, Cambridge.
Allan L. Shaw – Chief Financial Officer
Allan brings more than two decades of public company financial, operational, and strategic global business leadership. Allan serves as our Chief Financial Officer (“CFO”) and is a five-time public company CFO with proven skills across multiple finance disciplines: corporate finance, capital markets and strategic transactions as well as a broad base of expertise in corporate governance and risk management. He structured, directed, negotiated and closed over $4 billion in public and private financings for several companies. Mr. Shaw has served on five public boards including chairing two audit committees, two compensation committees, and is currently involved with a portfolio of healthcare activities. Mr. Shaw is the founder and since 2005, has served as senior managing director, of Shaw Strategic Capital LLC, an international financial advisory firm focused on providing strategic financial counsel on a wide variety of issues such as general corporate finance, mergers and acquisitions, capital structuring, licensing and capital markets, and serving as financial consultant to private and public companies. Mr. Shaw was the CFO and Treasurer of Syndax Pharmaceuticals, Inc. from January 2016 to February 2017 and from December 2011 to September 2015 was Managing Director of Alvarez & Marsal LLC, a global professional services firm, where he led their biopharmaceutical consulting practice. Additional prior experience includes serving as the CFO of Serono S.A. from November 2002 to May 2004, NewLead Holdings Ltd from October 2009 to July 2011 and Viatel, Inc. from November 1994 to June 2002. He currently serves on the board of directors of Calcimedica (Nasdaq: CALC) and Evecxia Therapeutics, Inc. as an independent director and Edith & Carl Marks JCH of Bensonhurst, a non-profit organization, and chairs their finance committee. Mr. Shaw is a certified public accountant in the State of New York. Mr. Shaw received a B.S. from the State University of New York at Oswego College.
Robert Kramer, PhD – Chief Scientific Officer
Robert has 26 years of experience in the pharmaceutical industry and is the former Head of Oncology Discovery Research at both Bristol Myers Squibb and Janssen Pharmaceuticals, part of the Johnson & Johnson group of companies. He has been responsible for enabling the transition of 35 drugs from initial discovery into the clinic. Robert championed immunotherapy at Bristol Myers Squibb, which led, in 2009 to the acquisition of Medarex, Inc. and its portfolio of immune therapeutics that included Ipilimumab and Nivolumab. He received his PhD in pharmacology from the University of Vermont and undertook his post doctorate studies at the U.S. National Cancer Institute. Robert has also held an Assistant Professorship at the Harvard Medical School.
Brian Wiley – Chief Business Officer
Brian Wiley has nearly 30 years of experience in the biopharmaceutical industry, with over 25 years dedicated to oncology. His experience includes licensing deals, collaborations, M&A, both public and private financings and multiple product launches in oncology. He founded Boston BioConsulting, LLC, a consulting firm that specializes in corporate strategy, business development and pre-commercial planning for the biopharmaceutical industry. Additionally, he served as Chief Commercial Officer and Head of Business Development at NewLink Genetics and also served in various leadership and management roles at Celgene, Gloucester Pharmaceuticals, Millennium and Aventis.
Brian has a B.A. in Marketing from Pennsylvania State University.
| 50 |
The following sets forth the names and province or state and country of residence of our directors and executive officers, the offices held by them as of the date of this Annual Report, and the month and year in which they became directors or executive officers.
| Name, Province/State and Country of Residence and Present Position with Portage (1) | Date became Director/Officer | Principal Occupation Last five years |
|
Dr. Gregory Bailey (2) London, U.K. Lead Director effective August 16, 2022 (formerly Chairman of the Board) |
June 4, 2013 | See Item 6 (A) above |
|
Mr. Steven Mintz (3) Ontario, Canada Director |
April 6, 2016 | See Item 6 (A) above |
|
Mr. James Mellon (4) Isle of Man Director |
February 15, 2022 | See Item 6 (A) above |
|
Jean-Christophe Renondin, MD (5) Paris, France Director |
April 30, 2024 | See Item 6 (A) above |
|
Justin Stebbing, MD, PhD (6) London, U.K. Director |
April 30, 2024 | See Item 6 (A) above |
|
Dr. Ian Walters Connecticut, USA Chairman of the Board effective August 16, 2022 and CEO effective May 1, 2019 (formerly Director) |
August 1, 2016 | See Item 6 (A) above |
|
Mr. Allan Shaw New York, USA CFO |
May 12, 2020 | See Item 6 (A) above |
|
Mr. Robert Kramer (7) Utah, USA Chief Scientific Officer |
January 8, 2019 | See Item 6 (A) above |
|
Mr. Brian Wiley Massachusetts, USA Chief Business Officer |
February 15, 2022 | See Item 6 (A) above |
| 51 |
| (1) | Neither age nor date of birth of directors or executive officers is required to be reported in our home country nor otherwise publicly disclosed. |
| (2) | Lead Director of the Company and Member of the Nominating Committee and Compensation Committee (formerly Chair of the Company). |
| (3) | Chair of the Audit Committee and Member of the Compensation Committee. |
| (4) | Chair of the Compensation Committee. |
| (5) | Chair of the Nominating Committee and Member of the Audit Committee. |
| (6) | Member of the Nominating Committee and Audit Committee. |
| (7) | Reflects the date of the SalvaRx acquisition by the Company. Prior to that, this individual was contracted by SalvaRx. |
Family Relationships
There are no family relationships between or among the directors and executive officers.
Other Relationships
There are no arrangements or understandings between or among any major shareholder, customer, supplier or others, pursuant to which any of the above-named persons were selected as directors or as members of senior management.
Board Diversity Matrix of Portage Biotech Inc.
The below chart is intended to disclose, to the extent legally permitted, the board of director diversity of Portage Biotech Inc., pursuant to Rule 5606(f) of the Nasdaq listing rules.
| Board Diversity Matrix (As of August 14, 2024) | ||||
| Country of Principal Executive Offices: | British Virgin Islands | |||
| Foreign Private Issuer | Yes | |||
| Disclosure Prohibited under Home Country Law | No | |||
| Total Number of Directors | 6 | |||
| Female | Male | Non-Binary | Did Not Disclose Gender | |
| Part I: Gender Identity | ||||
| Directors | 0 | 6 | – | – |
| Part II: Demographic Background | ||||
| Under-represented person in Home Country | 2 | |||
| LGBTQ+ | – | |||
| Did not Disclose Demographic Background | 1 | |||
| 52 |
(B) COMPENSATION
| 1. | General |
During Fiscal 2023 and Fiscal 2022, the Company engaged two separate third-party compensation consultants to review the Company’s compensation structure and provide recommendations to make the Company competitive for the purpose of recruiting and retaining board members, key management and staff. The review included benchmarking and other analytical tools.
As a result of the compensation consultant’s studies and resulting recommendations, in November 2021, the Board, as recommended by the Compensation Committee, approved cash fees and stock options to be paid and awarded, as applicable, to the Company’s non-employee Board members for both participation as a member of the Board, as well as membership on Board committees. Cash fees to board members commenced as of January 1, 2022.
As a result of the compensation consultants’ studies and resulting recommendations, in December 2022, the Board approved, and as recommended by the Compensation Committee, salaries for officers and employees commencing January 1, 2023 and approved achievement of 73% of the target bonus for Fiscal 2023, of which 25% was paid in January 2023 and the balance of which accrued as of March 31, 2023 and is payable upon achievement of a successful financing. Additionally, as part of this effort, on March 30, 2023, the Board approved grants of stock options for all employees and directors.
Additionally, as a result of the compensation consultant’s studies and resulting recommendations, in December 2022, the Compensation Committee recommended and the Board approved a compensation regime based upon targeted goals and other metrics.
The Company does not have any plans that provide for pensions, retirement or similar benefits.
| 2. | Statement of Director and Executive Compensation |
The following tables and accompanying notes set forth all compensation paid or payable by the Company to its directors and senior management for the fiscal years ended March 31, 2024, 2023 and 2022:
| 53 |
| 2. | Statement of Director and Executive Compensation (Cont’d) |
| Name & Principal Position | Year | Fee and Salary (2) | Bonus | Other |
Securities Under Options / SARs Granted (1) |
Shares or Units Subject to Resale Restrictions | Other (12) | Total Compensation | |||||||||
| $ | $ | $ | $ | $ | $ | $ | |||||||||||
| Gregory Bailey – Lead Director (Former Chairman of the Board of Directors), Compensation Committee Member and Nominating Committee Member | |||||||||||||||||
| 2024 | 49,500 | – | – | – | – | – | 49,500 | ||||||||||
| 2023 | 68,500 | – | – | 35,478 | (4) | – | – | 103,978 | |||||||||
| 2022 | 21,667 | – | – | 57,063 | (8) | – | – | 78,730 | |||||||||
| James Mellon – Independent Director and Compensation Committee Member | |||||||||||||||||
| 2024 | 35,242 | – | – | – | – | – | 35,242 | ||||||||||
| 2023 | 51,500 | – | – | 35,478 | (4) | – | – | 86,978 | |||||||||
| 2022 | 5,208 | – | – | 99,360 | (9) | – | – | 104,568 | |||||||||
| Steven Mintz – Independent Director and Chairman of the Audit Committee and Compensation Committee Member | |||||||||||||||||
| 2024 | 45,750 | – | – | – | – | – | 45,750 | ||||||||||
| 2023 | 61,000 | – | – | 35,478 | (4) | – | – | 96,478 | |||||||||
| 2022 | 18,750 | – | – | 57,063 | (8) | – | – | 75,813 | |||||||||
| Linda Kozick – Former Director | |||||||||||||||||
| 2024 | 42,000 | – | – | – | – | – | 42,000 | ||||||||||
| 2023 | 56,000 | – | – | 35,478 | (4) | – | – | 91,478 | |||||||||
| 2022 | 5,000 | – | – | 99,360 | (9) | – | – | 104,360 | |||||||||
| Mark Simon – Former Director | |||||||||||||||||
| 2024 | 55,500 | – | – | – | – | – | 55,500 | ||||||||||
| 2023 | 55,500 | – | – | 35,478 | (4) | – | – | 90,978 | |||||||||
| 2022 | 5,208 | – | – | 99,360 | (9) | – | – | 104,568 | |||||||||
| Robert Glassman – Former Director | |||||||||||||||||
| 2024 | 45,258 | – | – | – | – | – | 45,258 | ||||||||||
| 2023 | 30,000 | – | – | 168,743 | (5) | – | – | 198,743 | |||||||||
| Ian Walters – Chairman of the Board and CEO | |||||||||||||||||
| 2024 | 642,700 | – | – | – | – | 75,185 | 717,885 | ||||||||||
| 2023 | 624,175 | 267,996 | (3) | – | 748,186 | (6) | – | 75,480 | 1,715,837 | ||||||||
| 2022 | 459,195 | 375,000 | – | 1,101,132 | (10) | 879,942 | (11) | 13,952 | 2,829,221 | ||||||||
| Allan Shaw – CFO and Secretary | |||||||||||||||||
| 2024 | 469,000 | – | – | – | – | 49,205 | 518,205 | ||||||||||
| 2023 | 378,250 | 102,312 | (3) | – | 349,308 | (6) | – | 43,519 | 873,389 | ||||||||
| 2022 | 256,000 | 161,000 | – | 294,336 | (10) | 235,469 | (11) | 13,952 | 960,757 | ||||||||
| 54 |
| 2. | Statement of Director and Executive Compensation (Cont’d) |
| Name & Principal Position | Year | Fee and Salary (2) | Bonus | Other |
Securities Under Options / SARs Granted (1) |
Shares or Units Subject to Resale Restrictions | Other (12) | Total Compensation | |||||||||
| $ | $ | $ | $ | $ | $ | $ | |||||||||||
| Robert Kramer - Chief Scientific Officer | |||||||||||||||||
| 2024 | 168,750 | – | – | – | – | 17,141 | 185,891 | ||||||||||
| 2023 | 218,628 | 63,504 | (3) | – | 137,454 | (6) | – | 28,025 | 447,611 | ||||||||
| 2022 | 195,501 | 83,000 | – | 219,876 | (10) | 175,784 | (11) | – | 674,161 | ||||||||
| Steven Innaimo – Former Vice President of Project Management & Operations (13) | |||||||||||||||||
| 2024 | 267,712 | – | – | – | – | 56,363 | 324,075 | ||||||||||
| 2023 | 313,875 | 68,355 | (3) | – | 76,632 | (6) | – | 61,827 | 520,689 | ||||||||
| 2022 | 310,000 | 93,000 | – | 120,888 | (10) | 96,068 | (11) | 13,952 | 633,908 | ||||||||
| Brian Wiley - Chief Business Officer | |||||||||||||||||
| 2024 | 153,125 | – | – | – | – | 2,297 | 155,422 | ||||||||||
| 2023 | 177,185 | 38,588 | (3) | – | 118,343 | (6) | – | 4,915 | 339,031 | ||||||||
| 2022 | 84,057 | – | – | 525,600 | (10) | – | – | 609,657 | |||||||||
| Justin Fairchild – Former Vice President of Development (14) | |||||||||||||||||
| 2024 | 270,000 | – | – | – | – | 55,301 | 325,301 | ||||||||||
| 2023 | 190,000 | 25,358 | (3) | – | 544,632 | (7) | – | 30,473 | 790,463 | ||||||||
Notes:
| 1. | “SAR” means stock appreciation rights. The Company never issued any SARs. |
| 2. | Represents base salary earned by officers in accordance with their respective contracts and Director’s fees earned by directors, as applicable, in accordance with the directors’ fee structure established by the Compensation Committee of the Board. Mr. Bailey, Mr. Mellon, Mr. Mintz and Ms. Kozick waived their director’s fees with respect to the three months ended March 31, 2024. |
| 3. | Represents the bonus for Fiscal 2023 approved by the Board, as recommended for approval by the Compensation Committee. Such amount represents 73% of the target bonus for Fiscal 2023, of which 25% was paid in January 2023 and the balance of which is accrued as of March 31, 2024. |
| 4. | Represents aggregate grant date fair value of 14,600 options to purchase ordinary shares granted March 30, 2023, which vested on the first anniversary of the date of grant. |
| 5. | Represents aggregate grant date fair value of 15,900 options to purchase ordinary shares granted July 27, 2022, which vest monthly on the grant date anniversary over three years following the date grant and the grant date fair value of 14,600 options to purchase ordinary shares granted March 30, 2023, which vested on the first anniversary of the date of grant. |
| 55 |
| 2. | Statement of Director and Executive Compensation (Cont’d) |
| 6. | Represents aggregate grant date fair value of options to purchase ordinary shares granted March 30, 2023, which were scheduled to vest ratably on each of the first four anniversaries of the date of grant. See “Outstanding Equity Awards at Fiscal Year-End” below for additional information. |
| 7. | Represents aggregate grant date fair value of 50,000 options to purchase ordinary shares granted June 8, 2022, and 30,900 options to purchase ordinary shares granted March 30, 2023, which vest ratably on each of the first four anniversaries of the respective date of grant. |
| 8. | Represents aggregate grant date fair value of 6,900 options to purchase ordinary shares granted January 19, 2022, which vested on the first anniversary of the date of grant. |
| 9. | Represents aggregate grant date fair value of 13,800 options to purchase ordinary shares granted February 15, 2022, which were scheduled to vest monthly on the grant date anniversary over the first three years following the date of grant. |
| 10. | Represents aggregate grant date fair value of options to purchase ordinary shares granted January 19, 2022, which were scheduled to vest ratably on each of the first four anniversaries of the date of grant. See “Outstanding Equity Awards at Fiscal Year-End” below for additional information. |
| 11. | Represents aggregate grant date fair value (market value) of restricted stock units granted January 19, 2022, which were vested on grant date and are subject to certain restrictions. |
| 12. | Represents employee benefits paid by the Company. |
| 13. | Mr. Innaimo resigned his position effective January 26, 2024. |
| 14. | Mr. Fairchild resigned his positive effective May 31, 2024. |
| 56 |
Outstanding Equity Awards at Fiscal Year-End
The following table and related notes provide information regarding all outstanding equity awards for our executive officers as of March 31, 2024:
| Option Awards (1) | Stock Awards (6) (7) (8) | |||||||||||||||||||||||||||||
| Name | Number
of Securities Underlying Unexercised Options (#) Exercisable (1) | Equity
Incentive Unearned Options (#) (1) |
Option |
Option | Number
of Shares or Units of Stock That Have Not Vested (#) | Market
Value of Shares or Units of Stock That Have Not Vested (#) | Equity
Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | Equity
Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested (#) | ||||||||||||||||||||||
| Ian B Walters | 75,422 | 226,266 | (2) | $ | 2.92 | March 30, 2033 | - | - | - | - | ||||||||||||||||||||
| Ian B. Walters | 62,850 | (3) | 62,850 | (3) | $ | 10.22 | January 19, 2032 | - | - | - | - | |||||||||||||||||||
| Ian B. Walters | 151,000 | (4) | - | (4) | $ | 17.75 | January 13, 2031 | - | - | - | - | |||||||||||||||||||
| Allan Shaw | 35,212 | 105,638 | (2) | $ | 2.92 | March 30, 2033 | - | - | - | - | ||||||||||||||||||||
| Allan Shaw | 16,800 | (3) | 16,800 | (3) | $ | 10.22 | January 19, 2032 | - | - | - | - | |||||||||||||||||||
| Allan Shaw | 131,000 | (4) | - | (4) | $ | 17.75 | January 13, 2031 | - | - | - | - | |||||||||||||||||||
| Steven Innaimo | 6,900 | (6) | - | $ | 10.22 | April 26, 2024 | (6) | - | - | - | - | |||||||||||||||||||
| Steven Innaimo | 175,000 | (6) | - | $ | 17.75 | April 26, 2024 | (6) | - | - | - | - | |||||||||||||||||||
| Robert Kramer | 13,856 | 41,569 | (2) | $ | 2.92 | March 30, 2033 | - | - | - | - | ||||||||||||||||||||
| Robert Kramer | 12,550 | (3) | 12,550 | (3) | $ | 10.22 | January 19, 2032 | - | - | - | - | |||||||||||||||||||
| Robert Kramer | 61,000 | (4) | - | (4) | $ | 17.75 | January 13, 2031 | - | - | - | - | |||||||||||||||||||
| Brian Wiley | 11,930 | 35,789 | (2) | $ | 2.92 | March 30, 2033 | - | - | - | - | ||||||||||||||||||||
| Brian Wiley | 30,000 | (3) | 30,000 | (3) | $ | 10.22 | January 19, 2032 | - | - | - | - | |||||||||||||||||||
| Justin Fairchild | 7,725 | 23,175 | (2) | $ | 2.92 | March 30, 2033 | - | - | - | - | ||||||||||||||||||||
| Justin Fairchild | 12,500 | 37,500 | (5) | $ | 11.00 | June 8, 2032 | - | - | - | - | ||||||||||||||||||||
| 57 |
Outstanding Equity Awards at Fiscal Year-End (Cont’d)
| (1) |
Amounts represent options to purchase ordinary shares.
|
| (2) |
These options to purchase ordinary shares were granted on March 30, 2023, have a ten-year term and vest ratably on each of the first four anniversaries of the grant date.
|
| (3) |
These options to purchase ordinary shares were granted on January 19, 2022, have a ten-year term and vest ratably on each of the first four anniversaries of the grant date.
|
| (4) |
These options to purchase ordinary shares were granted on January 13, 2021, have a ten-year term and vest ratably on each of the first three anniversaries of the grant date.
|
| (5) |
These options to purchase ordinary shares were granted on June 8, 2022, have a ten-year term and vest ratably on each of the first four anniversaries of the grant date.
|
| (6) |
Mr. Innaimo resigned his position effective January 26, 2024 accordingly, 6,900 unvested options to purchase ordinary shares granted January 19, 2022 and 30,900 options to purchase ordinary shares granted March 30, 2023 were forfeited. On April 26, 2024, 175,000 vested options to purchase ordinary shares originally granted January 13, 2021 and 6,900 vested options to purchase ordinary shares originally granted January 19, 2022, expired unexercised in accordance with the terms of the option agreements.
|
| (7) |
The above table excludes 152,000 restricted stock units to Mr. Walters granted January 13, 2021 with a grant day value of $2,698,000, which vested on grant date but are subject to certain restrictions and 86,100 restricted stock units granted January 19, 2022 with a grant day value of $879,942, which vested on grant date but are subject to certain restrictions.
|
| (8) |
The above table excludes 23,040 restricted stock units to Mr. Shaw granted January 19, 2022 with a grant day value of $235,469, which vested on grant date but are subject to certain restrictions.
|
| (9) |
The above table excludes 91,000 restricted stock units to Mr. Kramer granted January 13, 2021, with a grant day value of $1,615,250, which vested on grant date but are subject to certain restrictions and 17,200 restricted stock units granted January 19, 2022 with a grant day value of $175,784, which vested on grant date but are subject to certain restrictions.
|
Directors' and Officers' Liability Insurance
We have purchased, at our expense, directors' and officers' liability insurance policy to provide insurance against possible liabilities incurred by them in their capacity as our directors and officers.
| 58 |
EXECUTIVE COMPENSATION
For the year ended March 31, 2024, our members of senior management were:
| · | Ian B. Walters, Chairman of the Board and CEO |
| · | Allan Shaw, CFO |
| · | Steven Innaimo, Former Vice President of Project Management & Operations (resigned his position effective January 26, 2024) |
| · | Robert Kramer, Chief Scientific Officer |
| · | Brian Wiley, Chief Business Officer |
| · | Justin Fairchild, Former Vice President of Development (accepted a reduced role beginning February 1, 2024 and resigned effective May 31, 2024). |
Executive Compensation Overview
Through November 30, 2021, the compensation of our members of senior management primarily consisted of consulting fees (and in some cases bonuses), and share-based compensation. During Fiscal 2022 and Fiscal 2023, the Company entered into Employment contracts with Dr. Walters, Mr. Shaw, Mr. Innaimo, Mr. Kramer, Mr. Wiley and Mr. Fairchild that provide for a combination of base salary, bonuses and long-term incentive compensation in the form of restricted stock units and options to purchase ordinary shares. Our members of senior management, like all full-time employees, are eligible to participate in our health and dental benefit plans and 401(k) plan matching program. At a minimum, we expect to review executive compensation annually with input from a compensation consultant, if necessary. As part of this review process, we expect the Board and the Compensation Committee to apply our values and philosophy, while considering the compensation levels needed to ensure our executive compensation program remains competitive. We will also review whether we are meeting our retention objectives and the potential cost of replacing a key employee.
Components of Executive Compensation
Annual Base Salary
Our members of senior management each receive a base salary to compensate them for services rendered to our company. The base salary payable to each member of senior management is intended to provide a fixed component of compensation reflecting the executive’s skill set, experience, role and responsibilities. Base salaries are reviewed annually, typically in connection with our annual performance review process, approved by our board of directors and the compensation committee, and may be adjusted from time to time to realign salaries with market levels after taking into account individual responsibilities, performance, and experience.
Annual Bonus
In December 2022, the Board approved executive performance bonuses, as recommended by the Compensation Committee, totaling $0.6 million, which is equivalent to 73.5% of original annual targets established by the Board in December 2021. The bonuses were approved based upon the original performance targets established. The Board further approved a payment structure of 25% of approved bonuses, which were paid in January 2023, with the balance of amounts due payable upon a new financing, which was completed in October 2023. The bonuses have not yet been paid. The Board did not approve performance bonuses for Fiscal 2024.
Equity-Based Compensation
In Fiscal 2024, the Compensation Committee did not approve the granting of any equity awards to our directors or members of senior management.
In Fiscal 2023, the Compensation Committee approved the granting of options to purchase ordinary shares as follows:
On June 8, 2022, the Company granted 50,000 options to purchase shares to an executive of the Company. The options have an exercise price of $11.00, the average price of the stock on that date, vest ratably on each of the first four anniversaries of the grant date and will expire, if unexercised, on June 8, 2032.
| 59 |
On July 27, 2022, the Company granted 15,900 options to purchase shares to a member of the Board. The options have an exercise price of $10.06, the average price of the stock on that date, vest ratably on each monthly anniversary of the grant date in the three-year period following the grant date and will expire, if unexercised, on July 27, 2032.
On March 30, 2023, the Board unanimously approved to increase the maximum number of ordinary shares reserved for issuance under the Amended and Restated 2021 Equity Incentive Plan. The aggregate number of shares available for awards under the Amended and Restated 2021 Equity Incentive Plan was increased to 2,880,992, which represented a 5% increase (or 879,180 shares) based on ordinary shares outstanding on March 29, 2023, which is equal to 16% of the issued and outstanding ordinary shares in the capital of the Company as of this date.
On March 30, 2023, the Company granted an aggregate of 746,120 stock options exercisable at a price of $2.92 per share, representing the average price of the shares on the grant date (March 30, 2023), which expire on March 30, 2033, to various directors, officers and a consultant of the Company. 14,600 options to purchase ordinary shares (total 87,600), were granted to each non-executive member of the Board and vest on the first anniversary of the grant date. A total of 651,020 stock options were granted to employees (including Mr. Walters, who is Chairman of the Board of Directors), and a consultant, and such stock options vest ratably on each of the first four annual anniversaries of the grant date. The balance of 7,500 stock options were also granted to a consultant, which was fully vested as of the grant date.
Employment Agreements
PDS entered into a Services Agreement with our CEO effective December 15, 2021 (the “CEO Services Agreement”). The CEO Services Agreement originally provided for a base salary of $618,000, plus cost-of-living increases. On December 19, 2022, the Compensation Committee approved the CEO’s compensation of $642,700 for Fiscal 2024. The CEO Services Agreement provides for annual increases based upon the review of the base salary by the Board prior to the anniversary of the CEO Services Agreement provided that the annual increase cannot be less than the cost-of-living increase. The CEO Services Agreement also provides that the CEO is eligible to receive an annual performance-based bonus targeted at 59% (subject to annual increase by the Board in its sole discretion), of the applicable year’s base salary, which bonus is earned based on the achievement of performance targets, as determined annually by the Board and communicated to the CEO in the first quarter of the year. Any annual bonus, to the extent earned, is to be paid no later than March 15 of the following year. The CEO Services Agreement is for an initial term of three years, after which it will automatically renew annually unless terminated in accordance with the CEO Services Agreement.
Under the CEO Services Agreement, the CEO may terminate his employment with PDS at any time for Good Reason (as defined in the CEO Services Agreement). PDS may terminate the CEO’s employment immediately upon his death, upon a period of disability or without Just Cause (as defined in the CEO Services Agreement). In the event that the CEO’s employment is terminated due to his death or Disability (as defined in the CEO Services Agreement), for Good Reason or without Just Cause, he will be entitled to accrued obligations (accrued unpaid portion of base salary, accrued unused vacation time and any unpaid expenses). Additionally, he may be entitled to Severance Benefits (as defined in the CEO Services Agreement), which include his then current base salary and the average of his annual bonus for the prior two completed performance years, paid over 12 monthly installments. Additionally, the CEO will be entitled to life insurance benefits and medical and dental benefits for a period of 12 months at the same rate the CEO and PDS shared such costs during his period of employment.
Additionally, all stock options (and any other unvested equity incentive award) held by the CEO relating to shares of the Company will be deemed fully vested and exercisable on the Termination Date (as defined in the CEO Services Agreement), and the exercise period for such stock options will be increased by a period of two years from the Termination Date.
If the CEO’s employment by PDS is terminated by PDS or any successor entity without Just Cause (not including termination by virtue of the CEO’s death or Disability) or by the CEO for Good Reason within 12 months following the effective date of a Change in Control (as defined in the CEO Services Agreement), then, in addition to paying or providing the CEO with the Accrued Obligations (as defined in the CEO Services Agreement), the Company will provide the following Change in Control Severance Benefits (as defined in the CEO Services Agreement):
| 60 |
| (1) | PDS will pay the base salary continuation benefit for 18 months; |
| (2) | PDS will pay the life insurance benefit for 18 months; |
| (3) | PDS will pay an additional amount equivalent to the CEO’s target annual bonus calculated using the bonus percentage for the performance year in which the CEO’s termination occurs. This bonus will be paid in 12 equal installments commencing on the first payroll date that is more than 60 days following the date of termination of the CEO’s employment, with the remaining installments occurring on the first day of the month for the 11 months thereafter; |
| (4) | PDS will provide the CEO with continued medical and dental benefits, as described above, for 18 months; and |
| (5) | All stock options (and any other unvested equity incentive award) held by the CEO relating to shares of the Company or its parent will be deemed fully vested and exercisable on the Termination Date, as defined, and the exercise period for such stock options will be increased by a period of two years from the Termination Date. |
PDS entered into services agreements (individually, an “Executive Service Agreement,” and collectively, the “Executive Service Agreements”) with each of our other members of senior management (individually, “Executive” and collectively, “Executives”), three of which are dated as of December 1, 2021, one of which is dated December 15, 2021 and one of which is dated June 1, 2022. Each of the Executive Services Agreements provides for an initial term of two years that is automatically renewed for one-year periods (except two of the Executive Services Agreement, which provides for an initial term of one year and that is automatically renewed for one-year periods). The Executive Services Agreements initially provide for annual base salaries ranging from $175,000 to $348,000 (pro-rated for services rendered) and annual bonus targets ranging from 30% to 40%. They also provide for long-term incentives in the form of equity awards from time to time under the Portage Biotech Inc. Amended and Restated 2021 Equity Incentive Plan.
On December 19, 2022, the Compensation Committee approved executive compensation for Fiscal 2024, as set forth below. Compensation for Fiscal 2024 and Fiscal 2023 is also set forth below:
| FISCAL 2024 (***) | FISCAL 2023 (***) | |||||||||||||||
BASE SALARY | TARGET BONUS | BASE SALARY | TARGET BONUS | |||||||||||||
| Ian Walters | $ | 642,700 | 60 | % | $ | 618,000 | 59 | % | ||||||||
| Allan Shaw | $ | 469,000 | 40 | % | $ | 348,000 | 40 | % | ||||||||
| Robert Kramer | $ | 225,000 | 40 | % | $ | 216,000 | 40 | % | ||||||||
| Steven Innaimo (*) | $ | 325,500 | 30 | % | $ | 310,000 | 30 | % | ||||||||
| Brian Wiley | $ | 183,750 | 40 | % | $ | 175,000 | 30 | % | ||||||||
| Justin Fairchild (**) | $ | 300,000 | 30 | % | $ | 300,000 | 30 | % | ||||||||
| (*) | Mr. Innaimo resigned his position effective January 26, 2024. | |
| (**) | Mr. Fairchild’s Executive Service Agreement took effect on June 1, 2022 and his base salary for Fiscal 2023 was pro-rated based upon the contract terms. Additionally, Mr. Fairchild accepted a reduced role at 40% of his base salary commencing February 1, 2024. Mr. Fairchild resigned on May 31, 2024. | |
| (***) | The base salaries for officers aggregated $2,399,950 and $2,195,000 in Fiscal 2024 and Fiscal 2023, respectively. |
The Executive Services Agreements can be terminated by PDS without Just Cause, by death or Disability, or by the Executive (except Mr. Fairchild) for Good Reason (each as defined in the respective Executive Services Agreements). In such instances, the Executive Services Agreements provide for the payment of accrued obligations (accrued unpaid portion of base salary, accrued unused vacation time and any unpaid expenses). Additionally, the Executives (except Messrs. Wiley and Fairchild) are entitled to 50% of base salary plus 50% of average annual bonus earned over the prior two performance years, as well as prevailing life insurance benefits for a period of six months and medical and dental benefits for a period of six months at the prevailing rate PDS and the Executive were sharing such expenses.
Additionally, all stock options (and any other unvested equity incentive award) held by the Executives relating to shares of the Company will be deemed fully vested and exercisable on the Termination Date (as defined in the respective Executive Services Agreements), and the exercise period for such stock options will be increased by a period of two years from the Termination Date.
| 61 |
If an Executive’s employment by PDS is terminated by the Company or any successor entity without Just Cause (not including termination by virtue of the Executive’s death or Disability) or by the Executive (except Mr. Fairchild) for Good Reason within 12 months following the effective date of a Change in Control (as defined in the respective Executive Services Agreements), then, in addition to paying or providing the Executive with the Accrued Obligations (as defined in the respective Executive Services Agreements), the Company will provide the following Change in Control Severance Benefits (as defined in the respective Executive Services Agreements), except in two cases in which the Executive is entitled to item (5) and 50% of items (1) and (3) below:
| (1) | PDS will pay the Base Salary continuation benefit for 12 months; |
| (2) | PDS will pay the life insurance benefit for 12 months; |
| (3) | The Company will pay an additional amount equivalent to the Executive’s target annual bonus calculated using the bonus percentage for the performance year in which the Executive’s termination occurs. This bonus will be payable in 12 equal installments commencing on the first payroll date that is more than 60 days following the date of termination of the Executive’s employment, with the remaining installments occurring on the first day of the month for the 11 months thereafter; |
| (4) | PDS will provide the Executive with continued medical and dental benefits, as described above, for 12 months; and |
| (5) | All stock options (and any other unvested equity incentive award) held by the Executive relating to shares of the Company will be deemed fully vested and exercisable on the Termination Date and the exercise period for such stock options will be increased by a period of two years from the Termination Date. |
The Executive Services Agreements also include customary confidentiality, as well as provisions relating to assignment of inventions. The Executive Services Agreements also includes non-competition and non-solicitation of employees and customers provisions that run during the Executive’s employment with PDS and for a period of one year after termination of employment.
Director Compensation
Non-Employee Director Compensation Policy
Effective January 1, 2022, our board of directors adopted a non-employee director compensation policy that is designed to enable us to attract and retain, on a long-term basis, highly qualified non-employee directors. Under the policy, each director who is not an employee will be paid cash compensation quarterly in arrears based upon the following table:
| ANNUAL RETAINER | ||||
| Board of Directors: | ||||
| Chair (if applicable) | $ | 30,000 | ||
| Lead | $ | 20,000 | ||
| All non-employee members | $ | 40,000 | ||
| Audit Committee: | ||||
| Chair | $ | 15,000 | ||
| Members | $ | 7,500 | ||
| Compensation Committee: | ||||
| Chair | $ | 12,000 | ||
| Members | $ | 6,000 | ||
| Nominating Committee: | ||||
| Chair | $ | 8,000 | ||
| Members | $ | 4,000 |
| 62 |
Effective January 1, 2022, each non-employee Board member is entitled to receive cash Board fees of $40,000 per annum, payable quarterly in arrears. Additionally, each non-employee Board member is entitled to an annual grant of 6,900 options to purchase our ordinary shares, which vest the first annual anniversary of the grant date. The Company incurred Board fees totaling $273,250 and $322,500 during the years ended March 31, 2024 and 2023, respectively. Mr. Bailey, Mr. Mellon, Mr. Mintz and Ms. Kozick waived their Board fees for the three months ended March 31, 2024.
Non-executive Board chairpersons are entitled to an annual cash fee of $30,000, payable quarterly in arrears. In lieu of a non-executive chairperson, the lead director is entitled to an annual cash fee of $20,000 per annum paid quarterly in arrears. Additionally, the chairperson of each of the Audit Committee, Compensation Committee and Nominating Committee is entitled to annual fees of $15,000, $12,000 and $8,000, respectively, payable quarterly in arrears. Members of those committees is entitled to annual fees of $7,500, $6,000 and $4,000, respectively, payable quarterly in arrears.
(C) BOARD PRACTICES
Audit Committee
During Fiscal 2024, our audit committee consisted of Mr. Steven Mintz, Dr. Robert Glassman and Mr. Mark Simon, with Mr. Steven Mintz serving as Chairperson. In connection with the resignations of Mr. Simon and Dr. Glassman in April 2024, our audit committee was reconstituted, effective April 30, 2024, as follows: Mr. Mintz (Chair), Dr. Renondin and Dr. Stebbing. Each member of our audit committee meets the financial literacy requirements of Nasdaq listing standards. In addition, our board of directors has determined that Mr. Steven Mintz is an audit committee financial expert within the meaning of Item 407(d) of Regulation S-K under the Exchange Act. Mr. Steven Mintz is a Canadian Chartered Professional Accountant. He has over sixteen years of international experience in corporate financial analysis, mergers and acquisitions. He has been on the board of directors of several private and public corporations, operating in various sectors, including technology, oil & gas and biotechnology.
Our audit committee will, among other things:
| · | review our consolidated financial statements and our critical accounting policies and practices; |
| · | select a qualified firm to serve as the independent registered public accounting firm to audit our consolidated financial statements; |
| · | help to ensure the independence and performance of the independent registered public accounting firm; |
| · | discuss the scope and results of the audit with the independent registered public accounting firm and review, with management and the independent registered public accounting firm, our interim and year-end results of operations; |
| · | pre-approve all audit and all permissible non-audit services to be performed by the independent registered public accounting firm; |
| · | oversee the performance of our internal audit function when established; |
| · | review the adequacy of our internal controls; |
| · | develop procedures for employees to submit concerns anonymously about questionable accounting or audit matters; |
| · | review our policies on risk assessment and risk management; and |
| · | review related party transactions. |
Pre-Approval Policies and Procedures
In the event that we plan to retain the services of the external auditors to the Company for tax compliance, tax advice or tax planning, the CFO of the Company must consult with the chair of the Audit Committee, who has the authority to approve or disapprove on behalf of the committee, those non-audit services. All other permissible non-audit services shall be approved or disapproved by the ACC as a whole.
Our external auditors are prohibited from performing for the Company non-audit services of the following nature: (a) bookkeeping or other services related to the accounting records or financial statements; (b) financial information systems design and implementation; (c) appraisal or valuation services, fairness opinions or contribution in-kind reports; (d) actuarial services; (e) internal audit outsource services; (f) management functions; (g) human resources; (h) broker or dealer, investment adviser or investment banking services; (i) legal services; (j) expert services unrelated to the audit; and (k) any other service that the Canadian and the United States Public Company Accounting Oversight Board determines is impermissible.
| 63 |
The Audit Committee Charter relating to compensation matters sets forth the evaluation and review requirements for incentive and equity-based compensation plans for the executives based on their periodic performance evaluation.
Compensation Committee
During Fiscal 2024, our compensation committee consisted of Ms. Linda Kozick, Mr. Gregory Bailey and Mr. Steven Mintz, with Ms. Linda Kozick serving as Chairperson. In connection with the resignations of Ms. Kozick and Mr. Simon in April 2024, our compensation committee was reconstituted, effective April 30, 2024, as follows: Mr. Mellon (Chair), Dr. Bailey (Member) and Mr. Mintz (Member). Each member of the compensation committee is also a non-employee director, as defined pursuant to Rule 16b-3 promulgated under the Exchange Act. The purpose of our compensation committee is to discharge the responsibilities of our board of directors relating to compensation of our executive officers. Our compensation committee will, among other things:
| · | review annually our compensation strategy, including base salary, incentive compensation and equity-based plans, including whether to adopt, amend and terminate compensation plans or arrangements |
| · | review and approve, or recommend to the Board for review and approval, annually our corporate goals and objectives, including those applicable to the compensation of the CEO and to the extent applicable, other executive officers; |
| · | review, approve and determine, or make recommendations to our board of directors regarding, the compensation of our executive officers; |
| · | administer our stock and equity incentive plans; |
| · | review and approve, or make recommendations to our board of directors regarding, incentive compensation and equity plans; |
| · | evaluate the efficacy of our compensation policy and strategy in achieving gender and minority pay parity, positive social impact and attracting a diverse workforce; and |
| · | establish and review general policies relating to compensation and benefits of our employees. |
Nominating Committee
During Fiscal 2024, our nominating committee consisted of Mr. Mark Simon, Ms. Linda Kozick and Mr. James Mellon with Mr. Mark Simon as Chairperson. In connection with the resignations of Mr. Simon and Ms. Kozick in April 2024, our nominating committee was reconstituted, effective April 30, 2024, as follows: Dr. Renondin (Chair), Mr. Bailey (Member) and Dr. Stebbing (Member). Our nominating committee will, among other things:
| · | identify, evaluate and select, or make recommendations to our board of directors regarding, nominees for election to our board of directors and its committees; |
| · | evaluate the performance of our board of directors and of individual directors; |
| · | consider and make recommendations to our board of directors regarding the size and composition of our board of directors and its committees; |
| · | review developments in corporate governance practices; |
| · | oversee environmental, social and governance (ESG) matters; |
| · | evaluate the adequacy of our corporate governance practices and reporting; and |
| · | develop and make recommendations to our board of directors regarding corporate governance guidelines and matters. |
Role of Board of Directors in Risk Oversight Process
Our board of directors has responsibility for the oversight of our risk management processes and, either as a whole or through its committees, regularly discusses with management our major risk exposures, their potential impact on our business and the steps we take to manage them. The risk oversight process includes receiving regular reports from board committees and members of senior management to enable our board of directors to understand our risk identification, risk management and risk mitigation strategies with respect to areas of potential material risk, including operations, finance, legal, regulatory, cybersecurity, strategic and reputational risk.
| 64 |
Code of Business Conduct
The Company has established a Code of Conduct applicable to our directors, officers and employees. The Code of Conduct is accessible on our website at www.portagebiotech.com. If we make any substantive amendments to the Code of Conduct or grant any waiver, including any implicit waiver, from a provision of the Code of Conduct to our officers, we will disclose the nature of such amendment or waiver on that website or in a report on Form 6-K.
Compensation Committee Interlocks and Insider Participation
All compensation and related matters are reviewed by our Compensation Committee. None of the members of our compensation committee is or has at any time during the past year been an officer or employee of ours. None of our executive officers currently serves or in the past year has served as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving on our board of directors or on our compensation committee.
(D) EMPLOYEES
We had seven full-time employees as of March 31, 2024, as compared to seven employees as of March 31, 2023. The employees are located in the United States. Four employees oversee business operations and management of clinical development, one employee provides business development, one employee is the CFO and one employee is the executive chairman and CEO. We also use the services of consultants from time to time.
(E) SHARE OWNERSHIP
The objective of the Company's and our subsidiaries equity-based incentive plans is to provide for and encourage ownership of our ordinary shares by our directors, officers, consultants and employees, if any and those of any subsidiary companies so that such persons may increase their stake in our company and benefit from increases in the value of the ordinary shares. The plans are designed to be competitive with the benefit programs of other companies in the biotechnology sector and enable the Company and its subsidiaries to attract and retain directors, officers and employees of the Company and its subsidiaries and to consultants and management company employees of exceptional skill. It is the view of management that the plans are a significant incentive for the directors, officers, consultants and employees to continue and to increase their efforts in promoting our operations to the mutual benefit of both our company and such individuals and also allows us to avail of the services of experienced persons with minimum cash outlay.
On June 25, 2020, at the annual meeting of shareholders, the Company’s incentive stock option plan (the “2020 Stock Option Plan”) was approved, which authorized the Company’s directors to fix the option exercise price and to issue stock options under the plan as they see fit. The Company’s 2020 Stock Option Plan was a 10% rolling stock option plan under which the Company’s directors were authorized to grant up to a maximum of 10% of the issued and outstanding ordinary shares on the date of grant.
Effective January 13, 2021, the Company amended and restated its 2020 Stock Option Plan to permit the grant of additional types of equity compensation securities, including restricted stock units (“RSUs”) and dividend equivalent rights (the “2021 Equity Incentive Plan”). Pursuant to the 2021 Equity Incentive Plan, on January 13, 2021, the Company granted an aggregate of 868,000 stock options exercisable at a price of $17.75 per share, representing the closing price of the shares on the day immediately preceding the grant date, which expire on January 13, 2031 to various directors, officers and consultants of the Company. 350,000 options granted to members of the Board vest 1/3 on grant date, 1/3 on the first anniversary of the grant and 1/3 on the second anniversary of the grant. 518,000 options granted to consultants (one of whom is also a director of the Company) vest 1/3 on each of the first three anniversaries of the grant date.
Additionally, the Company granted 243,000 RSUs on January 13, 2021, with a grant date fair value of $17.75 per share, which was the closing price on the day immediately preceding the grant date. The RSUs vested on the date of grant, but underlying shares cannot be sold until one of four of the following conditions are met: (1) a Change in Control (as defined in the Amended and Restated 2021 Equity Incentive Plan), (2) the participant’s Separation from Service (as defined in the Amended and Restated 2021 Equity Incentive Plan), (3) the participant’s death, or (4) the participant’s Disability (as defined in the Amended and Restated 2021 Equity Incentive Plan).
| 65 |
On January 19, 2022, the Board of Directors unanimously approved the Amended and Restated 2021 Equity Incentive Plan. The Amended and Restated 2021 Equity Incentive Plan provides for:
| (1) | An increase of aggregate number of ordinary shares available for awards to 2,001,812, which is equal to 15% of the issued and outstanding ordinary shares of the Company as of January 19, 2022 subject to discretionary annual increases (on a cumulative basis) as may be approved by the Board in future years by a number of ordinary shares not to exceed an additional 5% of the aggregate number of shares then outstanding; |
| (2) | The authorization of incentive stock options under the Amended and Restated 2021 Equity Incentive Plan; and |
| (3) | The provision of dividend equivalent rights to be issued when authorized. |
Pursuant to the Amended and Restated 2021 Equity Incentive Plan, on January 19, 2022, the Company granted an aggregate of 302,000 stock options exercisable at a price of $10.22 per share, representing the average price of the Company’s ordinary shares on the grant date (January 19, 2022), which expire on January 19, 2032, to various directors, officers and consultants of the Company. A total of 13,800 of the 302,000 stock options were granted to two members of the Board and vest on the first anniversary of the grant date. The balance of 288,200 stock options were granted to employees (one of whom is also a director of the Company), and a consultant, and such stock options vest ratably on each of the first four annual anniversaries of the grant date.
Additionally, the Company granted 135,740 RSUs to employees (one of whom is also a director of the Company) on January 19, 2022, with a fair value of $10.22 per share, representing the average price of the shares on the grant date (January 19, 2022). The RSUs were fully vested and nonforfeitable as of the grant date and will expire on January 19, 2032.
On February 15, 2022, James Mellon, Linda Kozick and Mark Simon were appointed to the Board. Mr. Mellon owned approximately 23.9% of the Company’s outstanding shares at that date. Additionally, Mr. Mellon had previously served as a member of the Board from 2016 to August 14, 2020. On February 15, 2022, in connection with the appointments, each of these directors were granted 13,800 non-qualified stock options, which vest ratably monthly over a three-year period. The options have an exercise price of $8.59 per share, the average price of the stock on February 15, 2022, the day immediately preceding the grant date, and will expire, if unexercised, on February 15, 2032.
On June 8, 2022, the Company granted 50,000 options to purchase shares to an executive of the Company. The options have an exercise price of $11.00, the average price of the stock on that date, vest ratably on each of the first four anniversaries of the grant date and will expire, if unexercised, on June 8, 2032.
On July 27, 2022, the Company granted 15,900 options to purchase shares to a member of the Board. The options have an exercise price of $10.06, the average price of the stock on that date, vest ratably on each monthly anniversary of the grant date in the three-year period following the grant date and will expire, if unexercised, on July 27, 2032.
On March 30, 2023, the Board unanimously approved to increase the maximum number of ordinary shares reserved for issuance under the Amended and Restated 2021 Equity Incentive Plan. The aggregate number of shares available for awards under the Amended and Restated 2021 Equity Incentive Plan was increased to 2,880,992, which represented a 5% increase (or 879,180 shares) based on ordinary shares outstanding on March 29, 2023, which is equal to 16% of the issued and outstanding ordinary shares in the capital of the Company as of this date.
On March 30, 2023, the Company granted an aggregate of 746,120 stock options exercisable at a price of $2.92 per share, representing the average price of the shares on the grant date (March 30, 2023), which expire on March 30, 2033, to various directors, officers and a consultant of the Company. 14,600 options to purchase ordinary shares, totaling 87,600, were granted to each non-executive member of the Board and vest on the first anniversary of the grant date. A total of 651,020 stock options were granted to employees (including Mr. Walters, who is Chairman of the Board of Directors), and a consultant, and such stock options vest ratably on each of the first four annual anniversaries of the grant date. The balance of 7,500 stock options were also granted to a consultant, which was fully vested as of the grant date.
| 66 |
The following table sets forth the share ownership of our executive officers and directors as at March 31, 2024:
| Ordinary Shares Beneficially Owned | ||||||||
| Name | Number | Percentage * | ||||||
| Gregory Bailey | 3,508,695 | (1) (8) | 17.64 | % | ||||
| Steven Mintz | 161,811 | (1) (2) | 0.81 | % | ||||
| James Mellon | 3,056,671 | (6) (8) | 15.43 | % | ||||
| Ian Walters | 504,362 | (3) | 2.51 | % | ||||
| Allan Shaw | 183,013 | (4) | 0.92 | % | ||||
| Robert Kramer | 228,140 | (5) | 1.15 | % | ||||
| Brian Wiley | 41,930 | (7) | 0.21 | % | ||||
* Based on issued and outstanding ordinary shares at August 14, 2024, plus vested stock options and stock options that vest in the following 60 days.
| (1) | Includes 85,000, 6,900 and 14,600 vested stock options to purchase ordinary shares granted January 13, 2021, January 19, 2022 and March 30, 2023, respectively. |
| (2) | Excludes 57,473 shares for which Mr. Mintz has shared investment control and disclaims beneficial ownership. |
| (3) | Includes 151,000 vested stock options to purchase ordinary shares and excludes 152,000 vested restricted stock units subject to certain restrictions granted January 13, 2021. Includes 62,850 vested stock options to purchase ordinary shares and excludes 86,100 vested restricted stock units subject to certain restrictions and 62,850 unvested stock options granted January 19, 2022. Includes 75,422 vested stock options to purchase ordinary shares and excludes 226,266 unvested stock options granted March 30, 2023. Additionally, excludes 87,519 shares held in trusts for the benefit of his children for which Mr. Walters disclaims beneficial ownership. |
| (4) | Includes 131,000 vested stock options to purchase ordinary shares granted January 13, 2021. Includes 16,800 vested stock options to purchase ordinary shares and excludes 23,040 vested restricted stock units subject to certain restrictions and 16,800 unvested stock options granted January 19, 2022. Includes 35,212 vested stock options to purchase ordinary shares and excludes 105,638 unvested stock options granted March 30, 2023. |
| (5) | Includes 61,000 vested stock options to purchase ordinary shares and excludes 91,000 vested restricted stock units subject to certain restrictions granted January 13, 2021. Includes 12,550 vested stock options to purchase ordinary shares and excludes 17,200 vested restricted stock units subject to certain restrictions and 12,550 unvested stock options granted January 19, 2022. Includes 13,856 vested stock options to purchase ordinary shares and excludes 41,569 unvested stock options granted March 30, 2023. |
| (6) | Includes 9,583 vested stock options to purchase ordinary shares and excludes 4,217 unvested stock options granted February 15, 2022. Includes 14,600 vested stock options to purchase ordinary shares granted March 30, 2023. |
| (7) | Includes 30,000 vested stock options to purchase ordinary shares and excludes 30,000 unvested stock options granted January 19, 2022. Includes 11,930 vested stock options to purchase ordinary shares and excludes 35,789 unvested stock options granted March 30, 2023. |
| (8) | These shares for Mr. Bailey and Mr. Mellon exclude 713,191 shares owned by SalvaRx Group plc. Mr. Bailey and Mr. Mellon own 36.91% and 35.07% of SalvaRx Group plc, respectively. |
All shares held by the above persons carry the same rights as the other holders of the ordinary shares of the Company.
| 67 |
(F) DISCLOSURE OF A REGISTRANT’S ACTION TO RECOVER ERRONEOUSLY AWARDED COMPENSATION.
None.
ITEM 7 – MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
(A) MAJOR SHAREHOLDERS
Our ordinary shares are recorded on the books of our transfer agent in registered form. A large number of the ordinary shares are, however, registered in the name of intermediaries such as brokerage houses and clearing firms on behalf of their respective clients. We do not have knowledge of all the beneficial owners of our ordinary shares. Intermediaries like CDS & Co, Toronto, Canada and Cede & Co., New York, USA held approximately 17% of the issued and outstanding ordinary shares of the company on behalf of beneficial shareholders whose individual holdings details were not available as of August 14, 2024.
At March 31, 2024, we had 19,784,390 ordinary shares issued and outstanding and at August 14, 2024, we had 20,972,285 ordinary shares issued and oustanding.
The following table sets forth persons known by us to be beneficial owners of more than 5% of our ordinary shares as of August 14, 2024. Beneficial ownership of shares is determined under rules of the SEC and generally includes any shares over which a person exercises sole or shared voting or investment power. Shares subject to options and warrants that are currently exercisable or exercisable within 60 days of the date indicated above are deemed to be beneficially owned by the person holding the option and warrant and included in the holding. These beneficially held ordinary shares, however, are not deemed outstanding for the purpose of computing the percentage ownership of any other person.
| Name of Beneficial Owner | No. of Shares (1) | Percentage of Shares (2) | ||||||
| Gregory Bailey | 3,508,695 | 16.65 | % | |||||
| James Mellon | 3,056,671 | 14.56 | % | |||||
| (1) | The share counts below exclude 713,191 shares of our stock owned by SalvaRx Group plc, in which Mr. Bailey and Mr. Mellon own interests of 36.91% and 35.07%, respectively. |
| (2) | Based on ordinary shares issued and outstanding as of August 14, 2024. |
There were no changes to the holdings of major shareholders in Fiscal 2024. All shares have the same voting rights. For details on Mr. Bailey and Mr. Mellon’s holdings for the last three years, see our Annual Report on Form 20-F for the fiscal year ended March 31, 2023, our Annual Report on Form 20-F for the fiscal year ended March 31, 2022 and our Annual Report on Form 20-F for the fiscal year ended March 31, 2021.
We are a publicly owned BVI business company. We are not owned or controlled directly or indirectly by another corporation or any foreign government. There are no arrangements, known to us, the operation of which may at a subsequent date result in a change of control of us.
Exemption from Insider Report Filings
We are a reporting issuer under the Securities Acts of each of the province of Ontario and British Columbia in Canada, which would normally require certain "insiders" of the Company (including its directors, certain executive officers, and persons who directly or indirectly beneficially own, control or direct more than 10% of its ordinary shares) to file insider reports of changes in their ownership of the Company's ordinary shares under National Instrument 55-104 - Insider Reporting Requirements and Exemptions (“NI 55-104”). Under section 4.12 of National Instrument 71-102 Continuous Disclosure and Other Exemptions Relating to Foreign Issuers however, as the Company is deemed to be an SEC Foreign Issuer, the insider reporting requirements of NI 55-104 do not apply provided the insiders comply with the requirements of U.S. federal securities law relating to insider reporting.
| 68 |
The United States also has rules governing public reporting of the ownership of securities held in public companies. Section 13 of the Exchange Act imposes reporting requirements on persons who acquire beneficial ownership (as such term is defined in the Rule 13d-3 under the Exchange Act) of more than five per cent of a class of an equity security registered under Section 12 of the Exchange Act. Subject to certain exceptions, these persons must file, within 10 days after such acquisition, a report of beneficial ownership with the United States Securities and Exchange Commission containing the information prescribed by the regulations under Section 13 of the Exchange Act. This information is also required to be sent to the issuer of the securities and to each exchange where the securities are traded.
As a foreign private issuer, the reporting and short-swing profit re-capture rules of Section 16 of the Exchange Act are not applicable to our directors, offices and holders of 10% or more of our issued and outstanding ordinary shares, calculated on a beneficial basis under Rule 13d-3 of the Exchange Act.
(B) RELATED PARTY TRANSACTIONS
Investments
We have entered into related party transactions and certain services agreements with our investees. Key management personnel of ours have also entered into related party transactions with investees. Key management personnel are those persons having the authority and responsibility for planning, directing and controlling the activities of us, including directors and senior management of ours.
The following subsidiaries and associates are considered related parties:
| (a) | Stimunity. The CEO of Portage is one of three members of the board of directors of Stimunity. We wrote-off our investment in Stimunity to nil in Fiscal 2024. |
| (b) | iOx. Upon execution of the iOx Share Exchange on July 18, 2022, the non-Portage director resigned from the iOx board leaving two Portage insiders as directors. The CEO of Portage is also the CEO of iOx, and the management team of Portage comprises the management team of iOx. See below for a discussion of the Company’s purchase of the non-controlling interest in iOx through its wholly-owned subsidiary SalvaRx. |
| (c) | Saugatuck. One of the three directorships on the board of directors of Saugatuck is controlled by Portage. Additionally, the CEO of Portage is also the CEO of Saugatuck, and the management team of Portage comprises the management team of Saugatuck. |
| (d) | Intensity. The CEO of Portage previously served as a part-time officer of Intensity until becoming a consultant in 2023. Additionally, Intensity provided services (primarily rent) to Portage through April 2023. We sold all of our shares of Intensity in Fiscal 2024 and no longer hold any ownership interest in Intensity. |
| (e) | Portage Development Services Inc. PDS provides human resources and other services to each operating subsidiary of Portage through a shared services agreement. |
| 69 |
The following are related party balances and transactions other than those disclosed elsewhere in the consolidated financial statements:
Transactions between the parent company and its subsidiaries, which are related parties, have been eliminated in consolidation and are not disclosed in this note.
On September 8, 2021, the Company, through SalvaRx, completed a settlement of loans (including interest) to and receivables from iOx for services rendered in exchange for 23,772 ordinary shares of iOx at a price of £162. Simultaneously, the Company entered into an agreement with OSI, the holder of $0.15 million notes plus accrued interest under which OSI exchanged the notes plus accrued interest for 820 shares of iOx. Additionally, no profit or loss was recorded in connection with the exchange. As a result of these transactions, the Company, through SalvaRx, increased its ownership of iOx from 60.49% to 78.32%.
Share Exchange Agreement – iOx
On July 18, 2022, the Company and SalvaRx entered into a Share Exchange Agreement with each of the minority shareholders of iOx resulting in the acquisition of the outstanding non-controlling ownership interest (approximately 22%) of iOx, which is developing the iNKT engager platform.
We now own the worldwide rights to its small molecule iNKT engagers, including lead programs PORT-2 and PORT-3. Under the terms of the Share Exchange Agreement, each Seller sold to the Company, and the Company acquired from each Seller, legal and beneficial ownership of the number of iOx shares held by each Seller, free and clear of any share encumbrances, in exchange for the issuance in an aggregate of 1,070,000 of our ordinary shares to be allocated among the Sellers based upon their relative ownership. As a result of the Share Exchange Agreement, the Company owns 100% of the issued and outstanding shares of iOx through its wholly-owned subsidiary, SalvaRx.
As additional consideration for the sale of the iOx shares to the Company under the Share Exchange Agreement, the Sellers have the contingent right to receive additional shares (“Earnout Shares”) from the Company having an aggregate value equal to $25 million calculated at the Per Share Earnout Price, as defined in the Share Exchange Agreement, upon the achievement of certain milestones defined as the dosing of the first patient in a Phase 3 clinical trial for either PORT-2 (IMM60 iNKT cell activator/engager) or PORT-3 (PLGA-nanoparticle formulation of IMM60 combined with a NY-ESO-1 peptide vaccine). This liability was accounted for as deferred contingent consideration on the consolidated balance sheet of the Company and adjusted to its fair value quarterly. During Fiscal 2024, it was determined that the likelihood of achieving the milestone was remote and wrote down the liability to zero.
| 70 |
Employment Agreements
For a description of compensation arrangements and employment agreements between the Company and its members of senior management as well as director compensation arrangements, see Part I Item 6 (B) “Compensation” of this Annual Report.
(C) INTERESTS OF EXPERTS AND COUNSEL
Not applicable.
ITEM 8 – FINANCIAL INFORMATION
(A) CONSOLIDATED STATEMENTS AND OTHER FINANCIAL INFORMATION
Financial Statements
Information regarding our financial statements is contained under Item 18 of this Annual Report.
Dividend Policy
Since its incorporation, the Company has not declared or paid, and has no present intention to declare or to pay in the foreseeable future, any cash dividends with respect to its ordinary shares. Earnings will be retained to finance further growth and development of the business of the Company. However, if the Board of Directors declares dividends; all the ordinary shares will participate equally in the dividends, and, in the event of liquidation, in the net assets, of the Company.
In January 2018, the Company declared and distributed its then holdings of common shares of Biohaven Pharmaceuticals Holding Company Ltd. as stock dividend. Whether or not the Board of Directors will determine to do any other distributions of property of the Company in the future is in their sole discretion and will depend on their determination at the future time.
(B) SIGNIFICANT CHANGES
There were no significant events or changes to report that happened subsequent to March 31, 2024, to the date of this report.
ITEM 9 – THE OFFER AND LISTING
(A) OFFER AND LISTING DETAILS
Our ordinary shares have been listed for trading on Nasdaq on the Nasdaq Capital Market under the symbol “PRTG” since February 25, 2021.
| 71 |
The following table outlines the annual high and low market prices for an ordinary share for the five most recent fiscal years. Except as noted, reflects share price prior to the 100 to 1 reverse stock split effective June 5, 2020:
| High | Low | |||||||||||||||
| Nasdaq | CSE | Nasdaq | CSE | |||||||||||||
| Year ended March 31, | US$ | US$ | US$ | US$ | ||||||||||||
| 2024* | 3.87 | N/A | 0.41 | N/A | ||||||||||||
| 2023* | 11.95 | N/A | 2.60 | N/A | ||||||||||||
| 2022* | 42.81 | N/A | 6.57 | N/A | ||||||||||||
| 2021* | 39.50 | 38.99 | 8.88 | 0.09 | ||||||||||||
| 2020 | 0.15 | 0.14 | 0.07 | 0.08 | ||||||||||||
* Reflects share price subsequent to the 100 to 1 reverse stock split effective June 5, 2020.
The following table outlines the high and low market prices for an ordinary share for each fiscal financial quarter for the two most recent fiscal periods and subsequent periods:
| High | Low | |||||||
| Nasdaq | Nasdaq | |||||||
| Quarter ended: | US$ | US$ | ||||||
| 31-Mar-24* | 1.89 | 0.41 | ||||||
| 31-Dec-23* | 2.65 | 1.09 | ||||||
| 30-Sep-23* | 3.68 | 2.09 | ||||||
| 30-Jun-23* | 3.87 | 2.73 | ||||||
| 31-Mar-23* | 7.20 | 2.60 | ||||||
| 31-Dec-22* | 7.53 | 4.42 | ||||||
| 30-Sep-22* | 10.12 | 5.89 | ||||||
| 30-Jun-22* | 11.95 | 5.16 | ||||||
| 31-Mar-22* | 12.00 | 6.57 | ||||||
* Reflects share price subsequent to the 100 to 1 reverse stock split effective June 5, 2020.
The following table outlines the high and low market prices for each of the most recent six months:
| High | Low | |||||||
| Nasdaq | Nasdaq | |||||||
| Month | US$ | US$ | ||||||
| July 2024 | 0.23 | 0.15 | ||||||
| June 2024 | 0.29 | 0.21 | ||||||
| May 2024 | 0.48 | 0.23 | ||||||
| April 2024 | 0.55 | 0.22 | ||||||
| March 2024 | 0.59 | 0.45 | ||||||
| February 2024 | 0.75 | 0.41 | ||||||
| January 2024 | 1.89 | 0.84 | ||||||
| 72 |
(B) PLAN OF DISTRIBUION
Not applicable.
(C) MARKETS
The Company's ordinary shares currently trade in one place. The Company’s shares have been listed for trading on Nasdaq on the Nasdaq Capital Market under the symbol “PRTG” since February 25, 2021. Before April 23, 2021, the Company’s ordinary shares were traded in two places.
| 1. | Since February 25, 2021, the ordinary shares of the Company began trading on Nasdaq under the trading symbol “PRTG”. Before then, the ordinary shares had been traded in the OTC market since 2000 under the trading symbol "PTGEF”. |
| 2. | Effective October 28, 2013, the Company's ordinary shares were also listed for trading in United States currency on the Canadian Securities Exchange (formerly, Canadian National Stock Exchange) under the symbol "PBT.U". The Company voluntarily delisted its ordinary shares from the CSE at the market close on April 23, 2021, since the Company’s shares were trading on Nasdaq from February 2021. |
(D) SELLING SHAREHOLDERS
Not applicable.
(E) DILUTION
Not applicable.
(F) EXPENSES OF THE ISSUE
Not applicable.
ITEM 10 – ADDITIONAL INFORMATION
(A) SHARE CAPITAL
This Form 20-F is being filed as an Annual Report under the Exchange Act and, as such, there is no requirement to provide any information under this section.
(B) MEMORANDUM AND ARTICLES OF ASSOCIATION
General
We amended our Memorandum of Association and Articles of Association (“M&A”) on September 20, 2022 and filed an updated version thereof with the Registrar of Companies in the British Virgin Islands on September 20, 2022.
Pursuant to our M&A, we are authorized to issue an unlimited number of ordinary shares of no-par value.
| 73 |
The following are summaries of material terms and provisions of our M&A and the BVI Act, insofar as they relate to the material terms applicable to our ordinary shares. Unless otherwise stated, the following summaries are of the terms of our shares as of the date of this Annual Report. This summary is not intended to be complete, and you should read the form of our Memorandum and Articles of Association, which has been filed as an exhibit to this report.
Meetings of shareholders
If our shareholders want us to hold a meeting of shareholders of the company, they may requisition the directors to hold one upon the written request of shareholders entitled to exercise at least 10% of the voting rights in respect of the matter for which the meeting is requested. Under British Virgin Islands law, this 10% threshold may only be increased to a maximum of 30% and any such increase would require an amendment to the M&A.
The directors may decide whether a meeting of the shareholders will be held as a Physical Meeting, a Virtual Meeting or a Hybrid Meeting as those terms are defined in the M&A.
Subject to our M&A, a meeting of shareholders of the company will be called by not less than ten days' written notice and no more than 60 days’ notice. Notice of every meeting of shareholders may be delivered electronically and will be given to all of our shareholders. However, the inadvertent failure of the convener or conveners of a meeting of shareholders to give notice of the meeting to a shareholder, or the fact that a shareholder has not received the notice, does not invalidate the meeting.
A meeting of shareholders is duly constituted if, at the commencement of the meeting, there are present in person or by proxy two or more shareholders entitled to vote at the meeting.
Rights attaching to shares
Voting rights
Holders of our ordinary shares have identical rights, including dividend and liquidation rights, provided that, except as otherwise expressly provided in our M&A or required by applicable law, on any matter that is submitted to a vote of our shareholders, holders of our ordinary shares are entitled to one vote per ordinary share.
Under the BVI Act, the ordinary shares are deemed to be issued when the name of the shareholder is entered in our register of members. Our register of members is maintained by our transfer agent, TSX Trust Company, which enters the names of our shareholders in our register of members. If (a) information that is required to be entered in the register of shareholders is omitted from the register or is inaccurately entered in the register, or (b) there is unreasonable delay in entering information in the register, a shareholder of the company, or any person who is aggrieved by the omission, inaccuracy or delay, may apply to the British Virgin Islands courts for an order that the register be rectified, and the court may either refuse the application or order the rectification of the register, and may direct us to pay all costs of the application and any damages the applicant may have sustained.
Subject to any rights or restrictions attached to any shares, at any general meeting on a show of hands every shareholder of record who is present in person (or, in the case of a shareholder being a corporation, by its duly authorized representative) or by proxy shall have one vote and on a poll every shareholder present in person (or, in the case of a shareholder being a corporation, by its duly appointed representative) or by proxy shall have one vote for each share which such shareholder is the holder. Voting at any meeting of the shareholders is by show of hands unless a poll is demanded. A poll may be demanded by shareholders present in person or by proxy if the shareholder disputes the outcome of the vote on a proposed resolution and the chairman shall cause a poll to be taken. In the case of a tie vote at a meeting of shareholders, the chairman shall be entitled to a second or casting vote.
No shareholder shall be entitled to vote or be reckoned in a quorum, in respect of any share, unless such shareholder is registered as our shareholder at the applicable record date for that meeting. Shareholders of record may also pass written resolutions without a meeting by a majority vote.
| 74 |
Protection of minority shareholders
Under the laws of the British Virgin Islands, there is little statutory law for the protection of minority shareholders other than the provisions of the BVI Act dealing with shareholder remedies. The principal protection under statutory law is that shareholders may bring an action to enforce the BVI Act or the constituent documents of the Company, our M&A. Shareholders are entitled to have our affairs conducted in accordance with the BVI Act and the M&A.
There are common law rights for the protection of shareholders that may be invoked, largely dependent on English company law, since the common law of the British Virgin Islands is limited. Under the general rule pursuant to English company law known as the rule in Foss v. Harbottle, a court will generally refuse to interfere with the management of a company at the insistence of a minority of its shareholders who express dissatisfaction with the conduct of the company's affairs by the majority or the board of directors. However, every shareholder is entitled to have the affairs of the company conducted properly according to British Virgin Islands law and the constituent documents of the company. As such, if those who control the company have persistently disregarded the requirements of the BVI Act or the provisions of the company's M&A, then the courts may grant relief. Generally, the areas in which the courts will intervene are the following: (1) an act complained of which is outside the scope of the authorized business or is illegal or not capable of ratification by the majority; (2) acts that constitute fraud on the minority where the wrongdoers control the company; (3) acts that infringe or are about to infringe on the personal rights of the shareholders, such as the right to vote; and (4) where the company has not complied with provisions requiring approval of a special or extraordinary majority of shareholders, which are more limited than the rights afforded minority shareholders under the laws of many states in the U.S.
Pre-emption rights
British Virgin Islands law does not make a distinction between public and private companies and some of the protections and safeguards (such as statutory pre-emption rights) that investors may expect to find in relation to a public company are not provided for under British Virgin Islands law, save to the extent they are expressly provided for in the M&A. There are no pre-emption rights applicable to the issuance of new shares by us under either British Virgin Islands law generally or our M&A more specifically.
Modification of rights
As permitted by British Virgin Islands law, and our M&A, we may vary the rights attached to our ordinary shares.
Transfer of shares
Subject to any applicable restrictions set forth in our M&A, any of our shareholders may transfer all or any of his or her shares by a written instrument of transfer in the usual or common form or in a form prescribed by the Designated Stock Exchange or by means of a Relevant System (as defined in our M&A) or in any other form which our directors may approve. Shares may be held electronically and transferred electronically.
The registration of transfers may be suspended at such times and for such periods as the directors may from time to time determine.
| 75 |
Changes in authorized ordinary shares
By resolution of our directors, we may (i) consolidate and divide all or any of our unissued authorized shares into shares of larger amount than our existing shares; (ii) sub-divide our existing ordinary shares, or any of them into shares of smaller amount than is fixed by our memorandum of association, subject nevertheless to the provisions of the BVI Act; or (iii) create new classes of shares with preferences to be determined by the board of directors at the time of authorization.
Dividends
Subject to the BVI Act and our M&A, our directors may, by resolution, authorize a distribution to shareholders at such time and of such an amount as they think fit, if they are satisfied, on reasonable grounds, that, immediately after the distribution, we will satisfy the 'solvency test'. A company will satisfy the solvency test if (i) the value of the company's assets exceeds its liabilities; and (ii) the company is able to pay its debts as they fall due. Where a distribution is made to a shareholder at a time when the company did not, immediately after the distribution, satisfy the solvency test, it may be recovered by the company from the shareholder unless (i) the shareholder received the distribution in good faith and without knowledge of the company's failure to satisfy the solvency test; (ii) the shareholder has altered his position in reliance on the validity of the distribution; and (iii) it would be unfair to require repayment in full or at all.
Share repurchases
As permitted by the BVI Act and our M&A, shares may be repurchased, redeemed or otherwise acquired by us provided that, immediately following the repurchase or redemption, we are satisfied we will pass the aforementioned solvency test.
We will require member consent before any share can be purchased, redeemed or otherwise acquired by us, save where such redemption is pursuant to certain statutory provisions, such as pursuant to section 179 of the BVI Act (redemption of minority shares) which allows for the holders of 90% or more of the votes to instruct the company to redeem the shares of the company held by the remaining shareholders.
Liquidation rights
As permitted by British Virgin Islands law and our M&A, a voluntary liquidator may be appointed under Part XII of the BVI Act if we satisfy the solvency test (as aforementioned save that it is satisfied if assets equal or exceed liabilities).
Board of directors
We are managed by a board of directors, which consisted of seven directors at March 31, 2024. Our M&A provide that the board of directors may be established by the board of directors up to a maximum of 15 members.
Our shareholders may, pursuant to our M&A, by resolution of shareholders passed at a meeting of shareholders called for the purpose of removing the director or for purposes including the removal of the director or by a written resolution of shareholders at any time remove any director before the expiration of his or her period of office with or without cause, and may, pursuant to our M&A, elect another person in his or her stead. Subject to our M&A, the directors will have power at any time and from time to time to appoint any person to be a director, either as an addition to the existing directors or to fill a vacancy as long as the total number of directors does not at any time exceed the maximum number fixed by or in accordance with our M&A (if any).
| 76 |
Our M&A do not provide for alternate directors.
There are no share ownership qualifications for directors, unless otherwise decided by a resolution of shareholders. Meetings of our board of directors may be convened at any time deemed necessary by any of our directors.
Unless the quorum has been otherwise fixed by the board, a meeting of our board of directors will be competent to make lawful and binding decisions if a majority of the directors are present or represented. At any meeting of our directors, each director, whether by his or her presence or by his or her alternate, is entitled to one vote.
Questions arising at a meeting of our board of directors are required to be decided by simple majority votes of the directors' present or represented at the meeting. In the case of a tie vote, the chairman of the meeting shall not have a second or deciding vote. Our board of directors may also pass written resolutions without a meeting by a majority vote.
The remuneration to be paid to the directors shall be such remuneration as the directors or shareholders shall determine through a resolution.
Issuance of additional ordinary shares
Our M&A authorize our board of directors to issue additional ordinary shares from time to time as our board of directors shall determine, to the extent of available authorized but unissued shares.
Our M&A authorize our board of directors from time to time to issue ordinary shares to the extent permitted by the BVI Act.
Changes in authorized shares
We are authorized to issue unlimited number of ordinary shares without par value, which will be subject to the same provisions with reference to the payment of calls, liens, transfers, transmissions, forfeitures and otherwise as the shares in issue. We may by resolution:
| ● | consolidate and divide all or any of our unissued authorized shares into shares of a larger amount than our existing shares; |
| ● | sub-divide our existing ordinary shares, or any of them into shares of smaller amount than is fixed by our memorandum of association, subject nevertheless to the provisions of the BVI Act; or |
| ● | create new classes of shares with preferences to be determined by the board of directors at the time of authorization. |
| 77 |
Inspection of books and records
Under British Virgin Islands law holders of our ordinary shares will be entitled, on giving written notice to us, to inspect and make copies or take extracts of our: (a) M&A; (b) register of shareholders; (c) register of directors; and (d) minutes of meetings and resolutions of shareholders and those classes of shareholders of which he is a shareholder.
Subject to our M&A, our board of directors may, if they are satisfied that it would be contrary to our interest to allow a shareholder to inspect any document, or part of a document as referenced above, refuse to permit the shareholder to inspect the document or limit the inspection of the document, including limiting the making of copies or the taking of extracts from the records. Where our directors exercise their powers in these circumstances, they shall notify the shareholder as soon as reasonably practicable.
Conflicts of interest
Pursuant to the BVI Act and the Company's M&A, a director of the Company who has an interest in a transaction and who has declared such interest to the other directors, may:
| ● | vote on a matter relating to the transaction; |
| ● | attend a meeting of directors at which a matter relating to the transaction arises and be included among the directors present at the meeting for the purposes of a quorum; and |
| ● | sign a document on behalf of the company or do any other thing in his capacity as a director, which relates to the transaction. |
Anti-money laundering laws
In order to comply with legislation or regulations aimed at the prevention of money laundering we are required to adopt and maintain anti-money laundering procedures and may require subscribers to provide evidence to verify their identity. Where permitted, and subject to certain conditions, we may also delegate the maintenance of our anti-money laundering procedures (including the acquisition of due diligence information) to a suitable person.
We reserve the right to request such information as is necessary to verify the identity of a subscriber for our ordinary shares. In the event of delay or failure on the part of the subscriber in producing any information required for verification purposes, we may refuse to accept the application, in which case any funds received will be returned without interest to the account from which they were originally debited.
If any person resident in the British Virgin Islands knows or suspects that another person is engaged in money laundering or terrorist financing and the information for that knowledge or suspicion came to their attention in the course of their business, the person will be required to report his belief or suspicion to the Financial Investigation Agency of the British Virgin Islands, pursuant to the Proceeds of Criminal Conduct Act (Revised Edition 2020, as amended). Such a report shall not be treated as a breach of confidence or of any restriction upon the disclosure of information imposed by any enactment or otherwise.
Duties of directors
British Virgin Islands law provides that every director of the company in exercising his powers or performing his duties shall act honestly and in good faith and in what the director believes to be in the best interests of the company. Additionally, the director shall exercise the care, diligence, and skill that a reasonable director would exercise in the same circumstances taking into account the nature of the company, the nature of the decision and the position of the director and his responsibilities. In addition, British Virgin Islands law provides that a director shall exercise his powers as a director for a proper purpose and shall not act, or agree to the company acting, in a manner that contravenes British Virgin Islands law or the memorandum and articles of association of the company.
| 78 |
Anti-takeover provisions
The BVI Act does not prevent companies from adopting a wide range of defensive measures, such as staggered boards, blank check preferred shares, removal of directors only for cause and provisions that restrict the rights of shareholders to call meetings and submit shareholder proposals.
Voting rights and quorum requirements
Under British Virgin Islands law, the voting rights of shareholders are regulated by the company's memorandum and articles of association and, in certain circumstances, the BVI Act. The memorandum and articles of association will govern matters such as quorum for the transaction of business, rights of shares, and majority votes required to approve any action or resolution at a meeting of the shareholders or board of directors. Unless the articles of association otherwise provide, the requisite majority is usually a simple majority of votes cast. Under the M&A, a resolution of shareholders requires a majority vote of those persons voting at a meeting or in the case of a written resolution of shareholders, the vote of a majority of the shareholders.
Mergers and similar arrangements
Under the BVI Act, two or more companies may merge or consolidate in accordance with the statutory provisions. A merger means the merging of two or more constituent companies into one of the constituent companies, and a consolidation means the uniting of two or more constituent companies into a new company. In order to merge or consolidate, the directors of each constituent company must approve a written plan of merger or consolidation which must be authorized by a resolution approved, at a duly convened and constituted meeting of the shareholders of the Company, by the affirmative vote of a majority of those persons voting at a meeting or in the case of a written resolution of shareholders, the vote of a majority of the shareholders.
Shareholders not otherwise entitled to vote on the merger or consolidation may still acquire the right to vote if the plan or merger or consolidation contains any provision which, if proposed as an amendment to the memorandum of association and articles of association, would entitle them to vote as a class or series on the proposed amendment. In any event, all shareholders must be given a copy of the plan of merger or consolidation irrespective of whether they are entitled to vote at the meeting or consent to the written resolution to approve the plan of merger or consolidation.
Shareholder suits
We are not aware of any reported class action or derivative action having been brought against the company in a British Virgin Islands court.
Under the BVI Act, if a company or a director of a company engages in, or proposes to engage in, conduct that contravenes the BVI Act or the memorandum of association or articles of the company, the BVI Court may, on the application of a shareholder or a director of the company, make an order directing the company or director to comply with, or restraining the company or director from engaging in that conduct.
In addition, under the BVI Act, the BVI Court may, on the application of a shareholder of a company, grant leave to that shareholder to bring proceedings in the name and on behalf of that company or to intervene in proceedings to which the company is a party for the purpose of continuing, defending or discontinuing the proceedings on behalf of the company. In determining whether to grant leave for such derivative actions, the Court must take into account certain matters, including whether the shareholder is acting in good faith, whether the derivative action is in the interests of the company taking account of the views of the company's directors on commercial matters and whether an alternative remedy to the derivative claim is available.
A shareholder of a company may bring an action against the company for breach of a duty owed by the company to him as a shareholder. The BVI Act also includes provisions for actions based on oppression, and for representative actions where the interests of the claimant are substantially the same as those of other shareholders.
Corporate governance
British Virgin Islands laws do not restrict transactions between a company and its directors, requiring only that directors exercise a duty to act honestly, in good faith and in what the directors believe to be in the best interests to the companies for which they serve.
| 79 |
Indemnification
British Virgin Islands law does not limit the extent to which a company's memorandum and articles of association may provide for indemnification of officers and directors, except to the extent any such provision may be held by the British Virgin Islands courts to be contrary to public policy, such as to provide indemnification against civil fraud or the consequences of committing a crime. Our M&A provide for the indemnification of our directors against all losses or liabilities incurred or sustained by a director as a director of our company in defending any proceedings, whether civil or criminal and this indemnity only applies if he or she acted honestly and in good faith with a view to our best interests and, with respect to any criminal action, he or she must have had no reasonable cause to believe his or her conduct was unlawful.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted for directors, officers or persons controlling us under the foregoing provisions, we have been advised that, in the opinion of the U.S. Securities and Exchange Commission, such indemnification is against public policy as expressed in the Securities Act and therefore is unenforceable.
Staggered board of directors
The BVI Act does not contain statutory provisions that require staggered board arrangements for a British Virgin Islands company and our M&A do not provide for a staggered board.
(C) MATERIAL CONTRACTS
We had no material contracts, other than contracts entered into in the ordinary course of business, to which we or any of our subsidiaries is a party, for the two fiscal years immediately preceding the filing of this report that are not otherwise disclosed in this Annual Report (including the Exhibits).
(D) EXCHANGE CONTROLS
There is no income or other tax of the British Virgin Islands imposed by withholding or otherwise on any payment to be made by us.
We are free to acquire, hold and sell foreign currency and securities without restriction. There is no exchange control legislation under British Virgin Islands law and accordingly there are no exchange control regulations imposed under British Virgin Islands law that would prevent us from paying dividends to shareholders in United States Dollars or any other currencies, and all such dividends may be freely transferred out of the British Virgin Islands, clear of any income or other tax of the British Virgin Islands imposed by withholding or otherwise without the necessity of obtaining any consent of any government or authority of the British Virgin Islands.
(E) TAXATION
British Virgin Islands Tax Consequences
Under the law of the British Virgin Islands as currently in effect, a holder of our ordinary shares who is not a resident of the British Virgin Islands is not liable for British Virgin Islands income tax on dividends paid with respect to our ordinary shares, and all holders of our ordinary shares are not liable to the British Virgin Islands for income tax on gains realized on the sale or disposal of securities. The British Virgin Islands does not impose a withholding tax on dividends paid by a company incorporated or continued under the BVI Act.
There are no capital gains, gift or inheritance taxes levied by the British Virgin Islands on companies incorporated under the BVI Act. In addition, securities of companies incorporated under the BVI Act are not subject to transfer taxes, stamp duties (unless such companies hold land in the BVI) or similar charges.
There is no income tax treaty or convention currently in effect between (i) the United States and the British Virgin Islands or (ii) Canada and the British Virgin Islands, although a Tax Information Exchange Agreement is in force between the United States and the BVI and Canada and the BVI.
| 80 |
The BVI Economic Substance (Companies and Limited Partnership) Act (Revised Edition 2020) (the “ESA”)
The above legislation aimed at addressing concerns raised by the Council of the European Union as to offshore structures engaged in certain activities, which attract profits without real economic activity provides (among other things) that BVI companies that carry out certain defined activities, need to take steps to establish substance in the British Virgin Islands. We have filed all our economic substance declarations when due in accordance with the requirements of the legislation. We are not currently subject to any requirements to establish economic substance in the BVI and given the nature of our business and assets at the current time the ESA has little material impact on us and our operations. However, the legislation is still in its early stages and therefore remains subject to further clarification and interpretation.
U.S. Federal Income Tax Consequences
The discussion below is for general information only and is not, and should not be interpreted to be, tax advice to any holder of our ordinary shares. Each holder or a prospective holder of our ordinary shares is urged to consult his, her or its own tax advisor.
General
This section is a general summary of the material United States federal income tax consequences of the ownership and disposition of our ordinary shares. This summary is based on the provisions of the Internal Revenue Code of 1986, as amended (the “Code”), the applicable Treasury regulations promulgated and proposed thereunder, judicial decisions and current administrative rulings and practice, all of which are subject to change, possibly on a retroactive basis. The summary applies to you only if you hold our ordinary shares as a capital asset within the meaning of Section 1221 of the Code. The United States Internal Revenue Service (the “IRS”) may challenge the tax consequences described below, and we have not requested, nor will we request, a ruling from the IRS or an opinion of counsel with respect to the United States federal income tax consequences of ownership or disposition of our ordinary shares. This summary does not purport to be a comprehensive description of all the tax considerations that may be relevant to the ownership of our ordinary shares. In particular, the discussion below does not cover tax consequences that depend upon your particular tax circumstances nor does it cover any state, local or non-United States law, or the possible application of the United States federal estate or gift tax. You are urged to consult your own tax advisors regarding the application of the United States federal income tax laws to your particular situation as well as any state, local, non-United States and United States federal estate and gift tax consequences of the ownership and disposition of our ordinary shares. In addition, this summary does not take into account any special United States federal income tax rules that may apply to a particular holder of our ordinary shares, including, without limitation, the following:
| ● | a dealer in securities; |
| ● | a trader in securities that elects to use a mark-to-market method of accounting for its securities holdings; |
| ● | a financial institution or a bank; |
| ● | an insurance company; |
| ● | a tax-exempt organization; |
| ● | a person that holds our ordinary shares in a hedging transaction or as part of a straddle or a conversion transaction; |
| ● | a person whose functional currency for United States federal income tax purposes is not the U.S. dollar; |
| ● | a person liable for alternative minimum tax; |
| ● | a person that owns, or is treated as owning, 10% or more, by voting power or value, of our ordinary shares; |
| ● | certain former U.S. citizens and residents who have expatriated; or |
| ● | a person who receives our ordinary shares pursuant to the exercise of employee stock options or otherwise as compensation. |
| 81 |
U.S. Holders
For purposes of the discussion below, you are a “U.S. Holder” if you are a beneficial owner of our ordinary shares who or which is:
| ● | an individual United States citizen or resident alien of the United States (as specifically defined for United States federal income tax purposes); |
| ● | a corporation, or other entity treated as a corporation for United States federal income tax purposes, created or organized in or under the laws of the United States, any State or the District of Columbia; |
| ● | an estate whose income is subject to United States federal income tax regardless of its source; or |
| ● | a trust (x) if a United States court can exercise primary supervision over the trust's administration and one or more United States persons are authorized to control all substantial decisions of the trust or (y) if it was in existence on August 20, 1996, was treated as a United States person prior to that date and has a valid election in effect under applicable Treasury regulations to be treated as a United States person. |
If a partnership holds our ordinary shares, the tax treatment of a partner will generally depend upon the status of the partner and upon the activities of the partnership. If you are a partner of a partnership holding our ordinary shares, you should consult your tax advisor.
Distributions
In general, subject to the PFIC rules discussed below, the gross amount of any distribution received by a U.S. Holder with respect to our ordinary shares will be included in the gross income of the U.S. Holder as a dividend to the extent attributable to our current and accumulated earnings and profits, as determined under U.S. federal income tax principles. Unless we maintain calculations of our earnings and profits in accordance with U.S. federal income tax principles, U.S. Holders should expect that any distribution will generally be treated as a dividend for U.S. federal income tax purposes. Any dividends from us will not be eligible for the dividends-received deduction generally allowed to corporations in respect of dividends received from U.S. corporations. For U.S. foreign tax credit purposes, dividends received on our ordinary shares by a U.S. Holder will generally be treated as income from sources outside the United States and will generally constitute “passive category income.” A portion of such dividends, however, will be treated as U.S. source income, subject to certain exceptions, in proportion to our U.S. source earnings and profits if U.S. persons collectively own, directly or indirectly, 50% or more of the voting power or value of our ordinary shares.
U.S. Holders that are individuals and certain other non-corporate U.S. Holders will be subject to tax on dividend income from a “qualified foreign corporation” at preferential rates of taxation provided that certain holding period and other requirements are met. For this purpose, a foreign corporation (other than a corporation that is classified as a PFIC (as discussed below) for the taxable year in which the dividend is paid or the preceding taxable year) will generally be considered to be a qualified foreign corporation (i) if it is eligible for the benefits of a comprehensive tax treaty with the United States which the Secretary of Treasury of the United States determines is satisfactory for purposes of this provision and which includes an exchange of information program, or (ii) with respect to any dividend it pays on stock which is readily tradable on an established securities market in the United States. Our ordinary shares are listed on Nasdaq, which is an established securities market in the United States, and are expected to be readily tradable. Thus, we expect that dividends paid on its ordinary shares will meet the conditions above required for the preferential tax rates, provided we are not a PFIC in the year such dividend is paid or the preceding taxable year.
| 82 |
Sale, Exchange or Other Taxable Disposition
Subject to the PFIC rules discussed below, upon a sale, exchange or other taxable disposition of our ordinary shares, a U.S. Holder will generally recognize a capital gain or loss equal to the difference between the amount realized on such sale, exchange or other taxable disposition and the adjusted tax basis of such ordinary shares. As discussed above, a U.S. Holder’s initial tax basis in our ordinary shares will generally equal the fair market value on the distribution date of such shares. Such gain or loss will be a long-term capital gain or loss if our ordinary shares have been held for more than one year and will be a short-term gain or loss if the holding period is equal to or less than one year. Such gain or loss will generally be considered U.S. source gain or loss for U.S. foreign tax credit purposes. Long-term capital gains of certain non-corporate U.S. Holders are eligible for reduced rates of taxation. For both corporate and non-corporate U.S. Holders, limitations apply to the deductibility of capital losses.
Passive Foreign Investment Company (PFIC)
Under the Code, we will be a PFIC for any taxable year in which, after the application of certain "look-through" rules with respect to related companies, either (i) 75% or more of our gross income consists of "passive income," or (ii) 50% or more of the average quarterly value of our assets consist of assets that produce, or are held for the production of, "passive income." Passive income generally includes interest, dividends, rents, rents and royalties other than certain rents and royalties which are received from unrelated parties in connection with the active conduct of a trade or business, and capital gains. Whether we will be a PFIC in any year depends on the composition of our income and assets, and the relative fair market value of our assets from time to time, which we expect may vary substantially over time. We must make a separate determination each year as to whether we are a PFIC. As a result, our PFIC status may change from year to year based on our income and assets. We believe that we were a PFIC in the fiscal year ended in 2018 and that we were a PFIC for the fiscal years ended March 31, 2023 and 2024. We may have been a PFIC in other years and we may be a PFIC in the future.
If we are a PFIC for any fiscal year during which a U.S. Holder holds our ordinary shares, we generally will continue to be treated as a PFIC with respect to that U.S. Holder for all succeeding fiscal years during which the U.S. Holder holds our ordinary shares, unless we cease to meet the threshold requirements for PFIC status and that U.S. Holder makes a qualifying "deemed sale" election with respect to the ordinary shares. If such an election is made, the U.S. Holder will be deemed to have sold the ordinary shares it holds at their fair market value on the last day of the last fiscal year in which we qualified as a PFIC, and any gain from such deemed sale will be subject to the consequences described below. After the deemed sale election, the ordinary shares with respect to which the deemed sale election was made will not be treated as shares in a PFIC unless we subsequently become a PFIC.
If we are a PFIC for any taxable year during which a U.S. Holder holds our ordinary shares, the U.S. Holder may be subject to adverse tax consequences. Generally, gain recognized upon a disposition of our ordinary shares by the U.S. Holder would be allocated ratably over the U.S. Holder's holding period for such ordinary shares. The amounts allocated to the taxable year of disposition and to years before we became a PFIC would be taxed as ordinary income. The amount allocated to each other taxable year would be subject to tax at the highest rate in effect for that taxable year for individuals or corporations, as appropriate, and would be increased by an additional tax equal to interest on the resulting tax deemed deferred with respect to each such other taxable year. Further, to the extent that any distribution received by a U.S. Holder on our ordinary shares exceeds 125% of the average of the annual distributions on such ordinary shares received during the preceding three years or the U.S. Holder's holding period, whichever is shorter, that distribution would be subject to taxation in the same manner described immediately above with respect to gain on disposition.
| 83 |
If we are a PFIC for any fiscal year during which any of our non-U.S. subsidiaries is also a PFIC, a U.S. Holder of our ordinary shares during such year will be treated as owning a proportionate amount (by value) of the shares of the lower-tier PFIC for purposes of the application of these rules to such subsidiary. U.S. Holders should consult their tax advisers regarding the tax consequences if the PFIC rules apply to any of our subsidiaries. Alternatively, if we are a PFIC and if our ordinary shares are "regularly traded" on a “qualified exchange,” a U.S. Holder may be eligible to make a mark-to-market election that would result in tax treatment different from the general tax treatment described above. Our ordinary shares would be treated as “regularly traded” in any calendar year in which more than a de minimis quantity of the ordinary shares are traded on a qualified exchange on at least 15 days during each calendar quarter. Nasdaq is a qualified exchange for this purpose. However, because a mark-to-market election cannot be made for equity interests in any lower-tier PFIC that we may own, a U.S. Holder that makes a mark-to-market election with respect to us may continue to be subject to the PFIC rules with respect to any indirect investments held by us that are treated as an equity interest in a PFIC for U.S. federal income tax purposes. If a U.S. Holder makes the mark-to-market election, the U.S. Holder generally will recognize as ordinary income any excess of the fair market value of the ordinary shares at the end of each taxable year over their adjusted tax basis, and will recognize an ordinary loss in respect of any excess of the adjusted tax basis of the ordinary shares over their fair market value at the end of the taxable year (but only to the extent of the net amount of income previously included as a result of the mark-to-market election). If a U.S. Holder makes the election, the U.S. Holder's tax basis in the ordinary shares will be adjusted to reflect these income or loss amounts. Any gain recognized on the sale or other disposition of our ordinary shares in a year when we are a PFIC will be treated as ordinary income and any loss will be treated as an ordinary loss (but only to the extent of the net amount of income previously included as a result of the mark-to-market election). If a U.S. Holder makes a mark-to-market election it will be effective for the taxable year for which the election is made and all subsequent taxable years unless our ordinary shares are no longer regularly traded on a qualified exchange or the IRS consents to the revocation of the election. U.S. Holders are urged to consult their tax advisers about the availability of the mark-to-market election, and whether making the election would be advisable in their particular circumstances.
Alternatively, a U.S. Holder of stock in a PFIC may make a so-called “Qualified Electing Fund” election to avoid the PFIC rules regarding distributions and gain described above. The PFIC taxation regime would not apply to a U.S. Holder who makes a QEF election for all taxable years that such U.S. Holder has held our ordinary shares while we are a PFIC, provided that we comply with specified reporting requirements. Instead, each U.S. Holder who has made a valid and effective QEF election is required for each taxable year that we are a PFIC to include in income such U.S. Holder’s pro rata share of our ordinary earnings as ordinary income and such U.S. Holder’s pro rata share of our net capital gains as long-term capital gain, regardless of whether we make any distributions of such earnings or gain. In general, a QEF election is effective only if we make available certain required information. U.S. Holders should be aware, however, that we are not required to make this information available but have agreed to do so for prior fiscal years for those U.S. Holders who ask for it. The QEF election is made on a shareholder-by-shareholder basis and generally may be revoked only with the consent of the IRS. U.S. Holders should consult with their own tax advisors regarding eligibility, manner and advisability of making a QEF election if we are treated as a PFIC.
In addition, if we are a PFIC or, with respect to particular U.S. Holders, are treated as a PFIC for the taxable year in which we paid a dividend or for the prior taxable year, the preferential rates discussed above with respect to dividends paid to certain non-corporate U.S. Holders would not apply.
If a U.S. Holder owns our ordinary shares during any year in which we are a PFIC, the U.S. Holder generally will be required to file an IRS Form 8621 (Information Return by a Shareholder of a Passive Foreign Investment Company or Qualified Electing Fund) with respect to us, generally with the U.S. Holder’s federal income tax return for that year. If we are a PFIC for a given taxable year, you should consult your tax advisor concerning your annual filing requirements.
The U.S. federal income tax rules relating to PFICs are complex. U.S. Holders are urged to consult their own tax advisers with respect to the ownership and disposition of our ordinary shares, the consequences if we are or become a PFIC, any elections available with respect to our ordinary shares, and the IRS information reporting obligations with respect to the ownership and disposition of our ordinary shares.
Foreign asset reporting
Certain U.S. Holders, who are individuals, are required to report information relating to an interest in ordinary shares, subject to certain exceptions (including an exception for ordinary shares held in accounts maintained by U.S. financial institutions). U.S. Holders are urged to consult their tax advisors regarding their information reporting obligations, if any, with respect to their ownership and disposition of ordinary shares.
| 84 |
Non-U.S. Holders
If you are not a U.S. Holder, you are a “Non-U.S. Holder.”
Distributions on Our Ordinary Shares
You generally will not be subject to U.S. federal income tax, including withholding tax, on distributions made on our ordinary shares unless:
| ● | you conduct a trade or business in the United States; and |
| ● | the distributions are effectively connected with the conduct of that trade or business (and, if an applicable income tax treaty so requires as a condition for you to be subject to U.S. federal income tax on a net income basis in respect of income from our ordinary shares, such distributions are attributable to a permanent establishment that you maintain in the United States). |
If you meet the two tests above, you generally will be subject to tax in respect of such dividends in the same manner as a U.S. Holder, as described above. In addition, any effectively connected dividends received by a non-U.S. corporation may also, under certain circumstances, be subject to an additional “branch profits tax” at a 30 percent rate or such lower rate as may be specified by an applicable income tax treaty.
Sale, Exchange or Other Disposition of Our Ordinary Shares
Generally, you will not be subject to U.S. federal income tax, including withholding tax, in respect of gain recognized on a sale or other taxable disposition of our ordinary shares unless:
| ● | your gain is effectively connected with a trade or business that you conduct in the United States (and, if an applicable income tax treaty so requires as a condition for you to be subject to U.S. federal income tax on a net income basis in respect of gain from the sale or other disposition of our ordinary shares, such gain is attributable to a permanent establishment maintained by you in the United States); or |
| ● | you are an individual Non-U.S. Holder and are present in the United States for at least 183 days in the taxable year of the sale or other disposition, and certain other conditions exist. |
If you meet one of tests above, you generally will be subject to tax in respect of any gain effectively connected with your conduct of a trade or business in the United States in the same manner as a U.S. Holder, as described above. Effectively connected gains realized by a non-U.S. corporation may also, under certain circumstances, be subject to an additional “branch profits tax” at a rate of 30 percent or such lower rate as may be specified by an applicable income tax treaty.
Backup Withholding and Information Reporting
Payments, including dividends and proceeds of sales, in respect of our ordinary shares that are made in the United States or by a United States related financial intermediary may be subject to United States information reporting rules. In addition, U.S. Holders may be subject to United States federal backup withholding tax. U.S. Holders will not be subject to backup withholding provided that:
| ● | you are a corporation or other exempt recipient; or |
| ● | you provide your correct United States federal taxpayer identification number and certify, under penalties of perjury, that you are not subject to backup withholding. |
Amounts withheld under the backup withholding rules may be credited against your United States federal income tax, and you may obtain a refund of any excess amounts withheld under the backup withholding rules by filing the appropriate claim for refund with the IRS in a timely manner.
| 85 |
(F) DIVIDEND AND PAYING AGENTS
Not applicable.
(G) STATEMENT BY EXPERTS
Not applicable.
(H) DOCUMENTS ON DISPLAY
We are currently subject to the informational requirements of the Exchange Act applicable to foreign private issuers. To fulfill these requirements, we file with the Securities and Exchange Commission, within four months after the end of our fiscal year an annual report on Form 20-F containing financial statements that will be examined and reported on, with an opinion expressed, by an independent public accounting firm. We also file current reports on Form 6-K for significant corporate events throughout the year. As a foreign private issuer, we are exempt from the rules under the Exchange Act relating to the furnishing of proxy statements. Also, because we are a foreign private issuer our officers, directors and principal shareholders are exempt from the reporting and short swing profit provisions contained in Section 16 of the Exchange Act.
You may read and copy any document we file with the SEC without charge at the SEC's public reference room at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may also obtain copies of the documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1 800 SEC 0330 for further information on the public reference room. The SEC also maintains an Internet site that contains reports and other information regarding issuers that file electronically with the SEC. Our filings with the SEC are also available to the public through this web site at http://www.sec.gov.
(I) SUBSIDIARY INFORMATION
Not applicable.
(J) ANNUAL REPORT TO SECURITY HOLDERS
If we are required to provide an annual report to security holders in response to the requirements of Form 6-K, we will submit the annual report to security holders in electronic format in accordance with the EDGAR Filer Manual.
ITEM 11 – QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed in varying degrees to a number of risks arising from financial instruments. Management's close involvement in the operations allows for the identification of risks and variances from expectations. We do not participate in the use of financial instruments to mitigate these risks and we have no designated hedging transactions. The Board approves and monitors the risk management processes. The Board's main objectives for managing risks are to ensure liquidity, the fulfilment of obligations, the continuation of our search for new business participation opportunities, and limited exposure to credit and market risks while ensuring greater returns on the surplus funds on hand. There were no changes to the objectives or the process from the prior year.
| 86 |
A summary of our risk exposures as it relates to financial instruments are reflected below.
Fair value of Financial Instruments
Our financial assets and liabilities are comprised of cash and cash equivalents, receivables and investments in equities and public entities, accounts payable and accrued liabilities, lease liability, warrant liability, deferred purchase price payable and deferred obligation.
We classify the fair value of these transactions according to the following fair value hierarchy based on the amount of observable inputs used to value the instrument:
| · | Level 1 – Values are based on unadjusted quoted prices available in active markets for identical assets or liabilities as of the reporting date. |
| · | Level 2 – Values are based on inputs, including quoted forward prices for commodities, time value and volatility factors, which can be substantially observed or corroborated in the marketplace. Prices in Level 2 are either directly or indirectly observable as of the reporting date. |
| · | Level 3 – Values are based on prices or valuation techniques that are not based on observable market data. Investments are classified as Level 3 financial instrument. |
Assessment of the significance of a particular input to the fair value measurement requires judgment and may affect the placement within the fair value hierarchy.
Management has assessed that the fair values of cash and cash equivalents, other receivables and accounts payable approximate their carrying amounts largely due to the short-term maturities of these instruments.
Our financial instruments are exposed to certain financial risks: Credit Risk, Liquidity Risk and Foreign Currency Risk.
Credit Risk
Credit risk is the risk of loss associated with a counterparty’s inability to fulfill its payment obligations. The credit risk is attributable to various financial instruments, as noted below. The credit risk is limited to the carrying value as reflected in our consolidated statements of financial position.
Cash and cash equivalents: Cash and cash equivalents comprise cash on hand and amounts invested in underlying treasury and money market funds that are readily convertible to a known amount of cash with three months or less from date of acquisition and are subject to an insignificant risk of change in value. As of March 31, 2024 and 2023, cash equivalents was comprised of a money market account with maturities less than 90 days from the date of purchase. Cash and cash equivalents are held with major international financial institutions and therefore the risk of loss is minimal.
Liquidity Risk
Liquidity risk is the risk that we will encounter difficulty in satisfying financial obligations as they become due.
Our approach to managing liquidity is to ensure, as far as possible, that we will have sufficient liquidity to meet our liabilities when due, under both normal and stressed conditions without incurring unacceptable losses or risking harm to our reputation. We hold sufficient cash and cash equivalents to satisfy current obligations under accounts payable and accruals.
| 87 |
We monitor our liquidity position regularly to assess whether we have the funds necessary to meet our operating needs and needs for investing in new projects.
As a biotech company at an early stage of development and without significant internally generated cash flows, there are inherent liquidity risks, including the possibility that additional financing may not be available to us, or that actual drug development expenditures may exceed those planned. The current uncertainty in global capital markets could have an impact on our future ability to access capital on terms that are acceptable us. There can be no assurance that required financing will be available to us.
Foreign Currency Risk
While we operate in various jurisdictions, substantially all of our transactions are denominated in the U.S. Dollar, except the deferred tax liability in the U.K. settleable in British pound sterling and the Stimunity Convertible Note receivable settleable in euros.
ITEM 12 – DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
Not applicable.
PART II
ITEM 13 – DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
None.
ITEM 14 – MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
None.
ITEM 15 – CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
Our disclosure controls and procedures, as such term is defined in Rules 13(a)-15(e) and 15(d)-15(e) of the Exchange Act, are designed to provide reasonable assurance that all relevant information is communicated to senior management, including the CEO and the CFO, to allow timely decisions regarding required disclosure. We carried out an evaluation, under the supervision and with the participation of our management, including our CEO and CFO. Based on this evaluation, these officers concluded that as of the end of the period covered by this Annual Report on Form 20-F, our disclosure controls and procedures were not effective to ensure that the information required to be disclosed by our company in reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the Securities and Exchange Commission. These disclosure controls and procedures include controls and procedures designed to ensure that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, to allow timely decisions regarding required disclosure. The conclusion that the disclosure controls and procedures were not effective was due to the presence of material weaknesses in internal control over financial reporting as identified below under the heading " Management's Annual Report on Internal Control over Financial Reporting". Management anticipates that such disclosure controls and procedures will not be effective until the material weakness is remediated.
| 88 |
Management's Annual Report on Internal Control over Financial Reporting (ICFR)
Our management, including the CEO and CFO, is responsible for establishing and maintaining adequate internal controls over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act). Our internal control system was designed to provide reasonable assurance to our management and our Board regarding the reliability of financial reporting and preparation and fair presentation of published financial statements for external purposes in accordance with IFRS. Internal control over financial reporting includes those policies and procedures that:
| 1. | pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; |
| 2. | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with IFRS, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and |
| 3. | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that have a material effect on the financial statements. |
All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of our internal control over financial reporting as of March 31, 2024. In making this assessment, it used the criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on the evaluation under these criteria, management identified material weaknesses in our internal controls over financial reporting and, as a result, management concluded that our internal control over financial reporting was not effective as of March 31, 2024.
Management identified the following material weaknesses in our internal control over financial reporting.
| · | Management was unable to perform an effective risk assessment or monitor internal controls over financial reporting; |
| · | Management lacks the number of skilled persons that it requires given the complexity of the reporting requirements that it has to make, which more specifically include the staff and expertise to (i) properly segregate duties and perform oversight of work performed and to perform compensating controls over the finance and accounting functions, (ii) establish and perform fair value estimates or subsequently monitor fluctuations in fair value estimates, and (iii) apply complex accounting principles, including those relating to business combination accounting, income taxes, warrant liabilities and fair value estimates; and |
| · | There are insufficient written policies and procedures in place to ensure the correct application of accounting and financial reporting with respect to the current requirements of IFRS and SEC disclosure requirements, some of which specifically relate to investment accounting and fair value measures, assessment of in-process R&D assets, share-based payments, carrying amounts of goodwill and intangible assets and business combination accounting. |
| 89 |
Attestation Report of the Registered Public Accounting Firm
This Annual Report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management's report is not subject to attestation by our registered public accounting firm pursuant to rules of the SEC that permit us to provide only management's report in this Annual Report.
Changes in Internal Control over Financial Reporting and Planned Remediation Activities
Management continues to improve its control over financial reporting and leverages experienced personnel, including consultants to perform ongoing IFRS and SEC compliance requirements. There have been no significant changes to our internal controls in Fiscal 2024.
ITEM 16A – AUDIT COMMITTEE FINANCIAL EXPERT
Our Board has determined that Mr. Steven Mintz is an “audit committee financial expert,” as such term is defined in Part II Item 16A.(b) of the General Instructions to Form 20-F, and is “independent,” as such term is defined in Rule 10A-3(b)(1) under the Exchange Act.
ITEM 16B – CODE OF ETHICS
We have adopted a Code of Ethics, which applies to all consultants, officers and directors. A copy of our current code of ethics was included in the exhibits to the fiscal 2014 annual report on Form 20-F and is incorporated by reference into the exhibit index to this Annual Report on Form 20-F.
During the most recently completed fiscal year, we neither: (a) amended its Code of Ethics; nor (b) granted any waiver (including any implicit waiver) from any provision of its Code of Ethics.
ITEM 16C – PRINCIPAL ACCOUNTANT FEES AND SERVICES
The following outlines the expenditures for accounting fees paid or accrued to our independent auditing firm for the last two fiscal periods ended:
| March 31, | 2024 | 2023 | ||||||
| Audit fees | $ | 345,750 | $ | 292,075 | ||||
| Audit-related fees | $ | – | $ | – | ||||
| All other fees | $ | 56,250 | $ | 109,160 | ||||
Included in audit fees are $171,655 and $157,075 with respect to the three quarterly reviews performed in Fiscal 2024 and Fiscal 2023, respectively. We also incurred fees of $56,650 in Fiscal 2024 with respect to comfort letters issued relating to the October 2023 Registered Direct Offering, the November 2023 Resale Registration Statement, and a Form S-8 registration statement filed in December 2023. In Fiscal 2023, we also incurred fees of $74,160 with respect to comfort letters on the registration statement and $35,000 with respect to a review of the financial statements of an acquired company. We did not have any engagement with our independent accounting firm during the Fiscal 2024 and Fiscal 2023 with respect to professional services for tax compliance, tax advice or tax planning or for any audit-related services.
Under our existing policies, the audit committee must approve all audit and non-audit related services provided by our independent accounting firm.
| 90 |
ITEM 16D – EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
Not applicable.
ITEM 16E – PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
We did not, nor did any affiliated purchaser, purchase any of our equity securities during Fiscal 2024.
ITEM 16F – CHANGE IN REGISTRANT'S CERTIFYING ACCOUNTANT
Not applicable.
ITEM 16G – CORPORATE GOVERNANCE
We are incorporated under the BVI Act. Our ordinary shares are registered with the SEC and are listed on the Nasdaq Capital Market. As a result, our corporate governance framework is subject to laws of the British Virgin Islands, or BVI, the securities laws and regulations of the United States and the listing requirements of the Nasdaq Marketplace Rules.
Under Rule 5615 of the Nasdaq Marketplace Rules, a foreign private issuer may follow its home country practice in lieu of the requirements of the Nasdaq Marketplace Rules. We follow the exemptions provide under the Nasdaq Marketplace Rules as described below.
British Virgin Islands law does not require that a majority of our board of directors consist of independent directors or that our board committees consist of entirely independent directors. Our board of directors and board committees, therefore, may include fewer independent directors than would be required if we were subject to Nasdaq Listing Rule 5605(b)(1). In addition, we will not be subject to Nasdaq Listing Rule 5605(b)(2), which requires that independent directors must regularly have scheduled meetings at which only independent directors are present.
We also are exempt from the Nasdaq listing rules as to quorum, and instead to follow the quorum rules for shareholder meetings under British Virgin Islands law. We also are exempt from the Nasdaq listing rules so as to not be required to obtain shareholder approval for certain issuance of securities, shareholder approval of share option plans and change of control transactions under Nasdaq Listing Rule 5635 and to hold annual shareholder meetings under Nasdaq Listing Rule 5620(a).
As a foreign private issuer, we are exempt from the proxy rules set forth in Sections 14(a), 14(b), 14(c) and 14(f) of the Securities Exchange Act of 1934. We solicit proxies in accordance with applicable rules and regulations in the British Virgin Islands.
ITEM 16H – MINE SAFETY DISCLOSURE
Not applicable.
ITEM 16I – DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS.
Not applicable.
ITEM 16J – INSIDER TRADING POLICIES
We have adopted an insider trading policy governing the purchase, sale, and other dispositions of our securities by directors, senior management, and employees that are reasonably designed to promote compliance with applicable insider trading laws, rules and regulations, and any applicable listing standards. A copy of our insider trading policy is included in the exhibits to this Annual Report on Form 20-F.
| 91 |
ITEM 16K – CYBERSECURITY
We are increasingly dependent on software applications and computing infrastructure to conduct key operations. We depend on both our own record retention and technology as well as the systems, networks and technology of our contractors, consultants, vendors and other business partners.
Cybersecurity Program
Given the importance of cybersecurity to our business, we maintain a cybersecurity program to support both the effectiveness of our systems and our preparedness for information security risks. This program includes a number of safeguards, such as: password protection; multi-factor authentication; continuous monitoring and alerting systems for internal and external threats; regular evaluations of our cybersecurity program, including retention of outsourced experts.
We use a risk-based approach with respect to our use and oversight of third-party service providers, tailoring processes according to the nature and sensitivity of the data accessed, processed, or stored by such third-party service provider.
Process for Assessing, Identifying and Managing Material Risks from Cybersecurity Threats
In the event of a cybersecurity incident, we maintain a regularly tested incident response program. Pursuant to the program and its escalation protocols, designated personnel are responsible for assessing the severity of an incident and associated threat, containing the threat, remediating the threat, including recovery of data and access to systems, analyzing any reporting obligations associated with the incident, and performing post-incident analysis and program enhancements.
We have relationships with a number of third-party service providers to assist with cybersecurity containment and remediation efforts.
Governance
We currently engage a qualified IT consultant who reports to our Chief Executive Officer. This consultant has robust experience with cybersecurity, information technology development and deployment and information technology risk assessment and management, including information security management.
Our IT consultant regularly monitors our information technology systems and monitors the prevention, detection, mitigation and remediation of cybersecurity incidents in consultation with our Chief Executive Officer. To the extent necessary, our Chief Executive Officer reports such risks to our Board, which has overall responsibility for risk oversight.
Over the last two years, we have not experienced any cybersecurity incidents that have materially affected or are reasonably likely to materially affect is, including our business, results of operations, or financial condition.
| 92 |
Board Oversight
While the Board of Directors has overall responsibility for risk oversight, our Audit Committee oversees cybersecurity risk matters. The Audit Committee is responsible for reviewing, discussing with management, and overseeing the Company’s data privacy, information technology and security and cybersecurity risk exposures, including: (i) the potential impact of those exposures on the Company’s business, financial results, operations and reputation; (ii) the programs and steps implemented by management to monitor and mitigate any exposures; (iii) the Company’s information governance and cybersecurity policies and programs; and (iv) major legislative and regulatory developments that could materially impact the Company’s data privacy and cybersecurity risk exposure.
In the event of a cybersecurity incident, the Chief Financial Officer would apprise the full Board promptly with respect to any incident.
Cybersecurity Risks
Our cybersecurity risk management processes are integrated into our overall Enterprise Risk Management (“ERM”) process. As part of our ERM process, we identify, assess and evaluate risks impacting our operations across the Company, including those risks related to cybersecurity. We consider the severity and likelihood of certain risk factors, drawing upon our industry experience and company knowledge. While we maintain a robust cybersecurity program, the techniques used to infiltrate information technology systems continue to evolve. Accordingly, we may not be able to timely detect threats or anticipate and implement adequate security measures. For additional information, see Item 3 (D) “Risk Factors.”
We also maintain cybersecurity insurance providing coverage for certain costs related to cybersecurity-related incidents that impact our own systems, networks, and technology or the systems, networks and technology of our contractors, consultants, vendors and other business partners.
In the last three years, we did not experience any material cybersecurity incidents or threats.
PART III
ITEM 17 – FINANCIAL STATEMENTS
The financial statements are provided pursuant to Item 18.
ITEM 18 – FINANCIAL STATEMENTS
See the Financial Statements and Exhibits listed in Item 19 hereof and filed as part of this Annual Report.
ITEM 19 – EXHIBITS
(a) Financial Statements
| 93 |
PORTAGE BIOTECH INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED MARCH 31, 2024, 2023 AND 2022
(U.S. Dollars in thousands)
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of
Portage Biotech Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated statements of financial position of Portage Biotech Inc. (the “Company”) as of March 31, 2024 and 2023, the related consolidated statements of operations and other comprehensive income (loss), changes in equity and cash flows for each of the three years in the period ended March 31, 2024, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of March 31, 2024 and 2023, and the results of its operations and its cash flows for each of the three years in the period ended March 31, 2024, in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Explanatory Paragraph – Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As more fully described in Note 2, the Company has incurred significant losses and needs to raise additional funds to meet its obligations and sustain its operations. These conditions raise substantial doubt about the Company's ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters.
/s/ Marcum llp
We have served as the Company’s auditor since 2019.
August 14, 2024
| F-1 |
PORTAGE BIOTECH INC.
Consolidated Statements of Financial Position
(U.S. Dollars in thousands)
| March 31, | ||||||||||
| Notes | 2024 | 2023 | ||||||||
| Assets | ||||||||||
| Current assets | ||||||||||
| Cash and cash equivalents | 4 | $ | $ | |||||||
| Prepaid expenses and other receivables | 5 | |||||||||
| Convertible note receivable | 6 | |||||||||
| Total current assets | ||||||||||
| Non-current assets | ||||||||||
| Investment in associate | 6 | |||||||||
| Investment in public company | 7 | |||||||||
| In-process research and development | 9, 11 | |||||||||
| Deferred commitment fee, net of amortization of $ | 18 | |||||||||
| Right to use asset | 8 | |||||||||
| Other assets, including equipment, net | ||||||||||
| Total non-current assets | ||||||||||
| Total assets | $ | $ | ||||||||
| Liabilities and Equity | ||||||||||
| Current liabilities | ||||||||||
| Accounts payable and accrued liabilities | 12 | $ | $ | |||||||
| Lease liability - current, including interest | 8 | |||||||||
| Other current liabilities | ||||||||||
| Total current liabilities | ||||||||||
| Non-current liabilities | ||||||||||
| Lease liability - non-current | 8 | |||||||||
| Warrant liability | 13 | |||||||||
| Deferred tax liability | 11, 14 | |||||||||
| Deferred purchase price payable - Tarus | 9, 20 | |||||||||
| Deferred obligation - iOx milestone | 19, 20 | |||||||||
| Total non-current liabilities | ||||||||||
| Total liabilities | ||||||||||
| Shareholders’ Equity | ||||||||||
| Capital stock | 15 | |||||||||
| Stock option reserve | 16 | |||||||||
| Accumulated other comprehensive loss | ( | ) | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||||
| Total equity attributable to owners of the Company | ||||||||||
| Non-controlling interest | 22 | ( | ) | ( | ) | |||||
| Total equity | ||||||||||
| Total liabilities and equity | $ | $ | ||||||||
| Commitments and Contingent Liabilities (Note 18) | ||||||||||
| /s/Allan Shaw | Chief Financial Officer | /s/Ian Walters |
Chairman of the Board and Chief Executive Officer |
| Allan Shaw | Ian Walters |
The accompanying notes are an integral part of these consolidated financial statements.
| F-2 |
PORTAGE BIOTECH INC.
Consolidated Statements of Operations and Other Comprehensive Income (Loss)
(U.S. Dollars in thousands, except per share amounts)
| Years Ended March 31, | ||||||||||||||
| Notes | 2024 | 2023 | 2022 | |||||||||||
| Expenses | ||||||||||||||
| Research and development | $ | $ | $ | |||||||||||
| General and administrative expenses | ||||||||||||||
| Loss from operations | ( | ) | ( | ) | ( | ) | ||||||||
| Change in fair value of deferred purchase price payable - Tarus and deferred obligation - iOx milestone | 9, 19, 20 | |||||||||||||
| Loss on Registered Direct Offering | 13 | ( | ) | |||||||||||
| Offering costs | 13 | ( | ) | |||||||||||
| Change in fair value of warrant liability | 13 | |||||||||||||
| Impairment loss - iOx IPR&D | 11 | ( | ) | ( | ) | |||||||||
| Impairment loss - Tarus IPR&D | 9, 11 | ( | ) | ( | ) | |||||||||
| Impairment loss - Goodwill | 10 | ( | ) | |||||||||||
| Impairment loss - Stimunity | 6 | ( | ) | ( | ) | |||||||||
| Impairment loss - Saugatuck | 11 | ( | ) | |||||||||||
| Commitment fee under Committed Purchase Agreement | 18 | ( | ) | |||||||||||
| Share of loss in associate accounted for using equity method | 6 | ( | ) | ( | ) | ( | ) | |||||||
| Gain on dissolution of investment in associate | 6 | |||||||||||||
| Gain from sale of investment in public company | 7 | |||||||||||||
| Foreign exchange transaction gain (loss) | 14 | ( | ) | |||||||||||
| Depreciation expense | ( | ) | ( | ) | ||||||||||
| Interest income | 4 | |||||||||||||
| Interest expense | ( | ) | ( | ) | ( | ) | ||||||||
| Loss before provision for income taxes | ( | ) | ( | ) | ( | ) | ||||||||
| Income tax benefit (expense) | 14 | ( | ) | |||||||||||
| Net loss | ( | ) | ( | ) | ( | ) | ||||||||
| Other comprehensive income (loss) | ||||||||||||||
| Net unrealized loss on investments | 6, 7 | ( | ) | ( | ) | |||||||||
| Total comprehensive loss for year | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||||
| Net loss attributable to: | ||||||||||||||
| Owners of the Company | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||||
| Non-controlling interest | 22 | ( | ) | ( | ) | ( | ) | |||||||
| Net loss | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||||
| Comprehensive loss attributable to: | ||||||||||||||
| Owners of the Company | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||||
| Non-controlling interest | 22 | ( | ) | ( | ) | ( | ) | |||||||
| Total comprehensive loss for year | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||||
| Loss per share | 17 | |||||||||||||
| Basic and diluted | $ | ) | $ | ) | $ | ) | ||||||||
| Weighted average shares outstanding | 17 | |||||||||||||
| Basic and diluted | ||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-3 |
PORTAGE BIOTECH INC.
Consolidated Statements of Changes in Equity
(U.S. Dollars in thousands)
| Accumulated | Equity | |||||||||||||||||||||||||||||||
| Number | Stock | Other | Attributable | Non- | ||||||||||||||||||||||||||||
| of | Capital | Option | Comprehensive | (Accumulated | to Owners | Controlling | Total | |||||||||||||||||||||||||
| Shares | Stock | Reserve | Income (Loss) | Deficit) | of Company | Interest | Equity | |||||||||||||||||||||||||
| Balance, April 1, 2021 | $ | $ | $ | $ | ( | ) | $ | $ | $ | |||||||||||||||||||||||
| Share-based compensation expense | - | |||||||||||||||||||||||||||||||
| Shares issued under ATM | ||||||||||||||||||||||||||||||||
| Shares issued under offering | ||||||||||||||||||||||||||||||||
| Share issuance costs | - | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||||
| Shares issued or accrued for services | ||||||||||||||||||||||||||||||||
| Warrants exercised | ||||||||||||||||||||||||||||||||
| Exchange of notes payable and accrued interest for iOx shares | - | |||||||||||||||||||||||||||||||
| Net loss for year | - | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||
| Balance, March 31, 2022 | $ | $ | $ | $ | ( | ) | $ | $ | $ | |||||||||||||||||||||||
| Share-based compensation expense | - | |||||||||||||||||||||||||||||||
| Shares issued in Tarus acquisition | ||||||||||||||||||||||||||||||||
| Shares issued in iOx exchange | ( | ) | ||||||||||||||||||||||||||||||
| Deferred obligation - iOx milestone | - | ( | ) | ( | ) | |||||||||||||||||||||||||||
| Excess of non-controlling interest acquired over consideration - iOx | - | ( | ) | |||||||||||||||||||||||||||||
| Shares issued to Lincoln for commitment fee under Committed Purchase Agreement | ||||||||||||||||||||||||||||||||
| Shares issued under ATM | ||||||||||||||||||||||||||||||||
| Purchase of shares issued under Committed Purchase Agreement | ||||||||||||||||||||||||||||||||
| Share issuance costs | - | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||||
| Shares issued or accrued for services | ||||||||||||||||||||||||||||||||
| Net unrealized loss on investments | - | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||||
| Net loss for year | - | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||
| Balance, March 31, 2023 | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||
| Share-based compensation expense | - | |||||||||||||||||||||||||||||||
| Shares issued under Registered Direct Offering | ||||||||||||||||||||||||||||||||
| Shares issued under ATM | ||||||||||||||||||||||||||||||||
| Share issuance costs under ATM | - | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||||
| Shares issued or accrued for services | ||||||||||||||||||||||||||||||||
| Shares issued pursuant to distribution of restricted stock units | ||||||||||||||||||||||||||||||||
| Derecognition of investment in public company | - | ( | ) | |||||||||||||||||||||||||||||
| Net unrealized loss on investments | - | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||||
| Net loss for year | - | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||
| Balance, March 31, 2024 | $ | $ | $ | $ | ( | ) | $ | $ | ( | ) | $ | |||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-4 |
PORTAGE BIOTECH INC.
Consolidated Statements of Cash Flows
(U.S. Dollars in thousands)
| Years Ended March 31, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Cash flows from operating activities: | ||||||||||||
| Net loss for the year | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Adjustments for non-cash items: | ||||||||||||
| Share-based compensation expense | ||||||||||||
| Change in fair value of deferred purchase price payable - Tarus and deferred obligation - iOx milestone | ( | ) | ( | ) | ||||||||
| Loss on Registered Direct Offering | ||||||||||||
| Offering costs | ||||||||||||
| Change in fair value of warrant liability | ( | ) | ( | ) | ( | ) | ||||||
| Impairment loss - iOx IPR&D | ||||||||||||
| Impairment loss - Tarus IPR&D | ||||||||||||
| Impairment loss - Stimunity | ||||||||||||
| Impairment loss - Saugatuck | ||||||||||||
| Impairment loss - Goodwill | ||||||||||||
| Commitment fee under Committed Purchase Agreement | ||||||||||||
| Gain on dissolution of investment in associate | ( | ) | ||||||||||
| Gain from sale of investment in public company | ( | ) | ||||||||||
| (Decrease) increase in deferred tax liability | ( | ) | ( | ) | ||||||||
| Share of loss in associate | ||||||||||||
| Fair value of shares issued for services | ||||||||||||
| Depreciation | ||||||||||||
| Foreign exchange transaction gain | ( | ) | ||||||||||
| Changes in operating working capital: | ||||||||||||
| Prepaid expenses and other receivables | ( | ) | ( | ) | ||||||||
| Other assets | ( | ) | ||||||||||
| Accounts payable and accrued liabilities | ( | ) | ||||||||||
| Other | ||||||||||||
| Net cash used in operating activities | ( | ) | ( | ) | ( | ) | ||||||
| Cash flows from investing activities: | ||||||||||||
| Proceeds from sale of investment in public company | ||||||||||||
| Purchase of convertible note receivable | ( | ) | ||||||||||
| Purchase of equipment | ( | ) | ||||||||||
| Net cash used in investing activities | ( | ) | ||||||||||
| Cash flows from financing activities: | ||||||||||||
| Proceeds from Registered Direct Offering | ||||||||||||
| Proceeds from shares issued under ATM and Committed Purchase Agreement | ||||||||||||
| Share issuance costs | ( | ) | ( | ) | ( | ) | ||||||
| Repayment of lease liability | ( | ) | ||||||||||
| Shares from RSU distribution | ||||||||||||
| Repayment of notes payable assumed in Tarus acquisition | ( | ) | ||||||||||
| Repayment of milestone obligation assumed in Tarus acquisition | ( | ) | ||||||||||
| Proceeds from shares issued under registered offering | ||||||||||||
| Proceeds from exercise of stock purchase warrants | ||||||||||||
| Net cash (used in) provided by financing activities | ( | ) | ||||||||||
| (Decrease) increase in cash and cash equivalents during year | ( | ) | ( | ) | ||||||||
| Cash and cash equivalents at beginning of year | ||||||||||||
| Cash and cash equivalents at end of year | $ | $ | $ | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-5 |
PORTAGE BIOTECH INC.
Consolidated Statements of Cash Flows (Cont’d)
(U.S. Dollars in thousands)
| Years Ended March 31, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Supplemental disclosure of cash flow information: | ||||||||||||
| Cash paid for interest | $ | $ | $ | |||||||||
| Increase in accounts payable for stock issuance costs | $ | $ | $ | |||||||||
| Supplemental disclosure of non-cash investing and financing activities: | ||||||||||||
| Exchange of Stimunity Convertible Note for Stimunity shares at fair value | $ | $ | $ | |||||||||
| Right to use asset acquired | $ | $ | $ | |||||||||
| Lease liability incurred | $ | $ | $ | |||||||||
| Fair value of shares issued for Tarus | $ | $ | $ | |||||||||
| Fair value of shares issued for non-controlling interest purchase of iOx | $ | $ | $ | |||||||||
| Fair value of deferred purchase price payable - Tarus | $ | $ | $ | |||||||||
| Fair value of deferred obligation - iOx milestone | $ | $ | $ | |||||||||
| Liabilities assumed in Tarus acquisition | $ | $ | $ | |||||||||
| Fair value of shares issued for commitment fees - Committed Purchase | $ | $ | $ | |||||||||
| Net unrealized loss on investments in Intensity and Stimunity Convertible Note | $ | $ | ( | ) | $ | |||||||
| Exchange of iOx shares for settlement of notes payable, accrued interest and warrants | $ | $ | $ | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-6 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 1. NATURE OF OPERATIONS
Portage Biotech Inc. (the “Company” or “Portage”) is incorporated in the British Virgin Islands (“BVI”) with its registered office located at Clarence Thomas Building, P.O. Box 4649, Road Town, Tortola, BVI. Its USA agent, Portage Development Services Inc. (“PDS”), is located at 59 Wilton Road, Westport, CT, 06880, USA.
The Company is a foreign private issuer under the Securities and Exchange Commission (the “SEC”) rules. It is also a reporting issuer under the securities legislation of the provinces of Ontario and British Columbia. Its ordinary shares were listed on the Canadian Securities Exchange (“CSE”) under the symbol “PBT.U”. On February 25, 2021, the ordinary shares of the Company began trading on the Nasdaq Capital Market (“Nasdaq”) under the symbol “PRTG”. As the principal market for the Company’s ordinary shares is Nasdaq, the Company voluntarily delisted from the CSE on April 23, 2021.
Portage is a clinical-stage immuno-oncology company advancing treatments the Company believes will be first-in class therapies that target known checkpoint resistance pathways to improve long-term treatment response and quality of life in patients with invasive cancers. Portage’s access to next-generation technologies coupled with a deep understanding of biological mechanisms enables the identification of clinical therapies and product development strategies that accelerate these medicines through the translational pipeline. After a review of the Company’s future funding needs for clinical development of its programs as well as the current capital raising market for biotechnology companies, the Company made the decision to discontinue the Company sponsored trial for the invariant natural killer T-cell (“iNKT”) program and pause further accrual to the Company sponsored adenosine program for both PORT-6 and PORT-7. The Company is exploring strategic alternatives, which may include finding a partner for one or more of its assets, a sale of the company, a merger, restructurings, both in and out of court, a company wind down, further financing efforts or other strategic action.
On August 13, 2018, the Company reached a definitive agreement to acquire
In September 2021, the Company, through SalvaRx, exchanged certain notes,
accrued interest, warrants and receivables in exchange for shares of iOx representing % of the outstanding shares of iOx. As a result
of this exchange, the Company, through SalvaRx, increased its ownership of iOx from
NOTE 2. GOING CONCERN
As of March 31, 2024, the Company had cash and cash equivalents of approximately
$
| F-7 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 2. GOING CONCERN (Cont’d)
The Company’s cash and cash equivalents balance is decreasing, and the Company did not generate positive cash flows from operations for the fiscal year ended March 31, 2024 (“Fiscal 2024”).
Due to the Company’s future funding needs for clinical development of its programs as well as the current capital raising market for biotechnology companies, the Company made the decision to discontinue further clinical development of its iNKT program and pause further accrual to its sponsored adenosine program for both PORT-6 and PORT-7. The Company is exploring strategic alternatives, which may include finding a partner for one or more of its assets, a sale of our company, a merger, restructurings, both in and out of court, company wind down, further financing efforts or other strategic action. These factors raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date of the consolidated statement of financial position (March 31, 2024).
The Company has incurred significant operating losses since inception and expects to continue to incur significant operating losses for the foreseeable future and may never become profitable. The losses result primarily from its conduct of research and development activities. As previously discussed, the Company has discontinued the Company sponsored iNKT study and paused further accrual to the Company sponsored adenosine program in order to preserve cash resources. Additionally, during the fourth quarter of Fiscal 2024, the Company sold its shares in Intensity on Nasdaq.
The Company historically has funded its operations principally from proceeds from issuances of equity and debt securities. The Company will require significant additional capital to make the investments it needs to execute its longer-term business plan, beyond the potential proceeds that could be reasonably generated from its ATM program and Committed Purchase Agreement with Lincoln given the Company’s current trading volume on Nasdaq. The Company’s ability to successfully raise sufficient funds through the sale of debt or equity securities when needed is subject to many risks and uncertainties and, future equity issuances would result in dilution to existing stockholders and any future debt securities may contain covenants that limit the Company's operations or ability to enter into certain transactions. See Note 15, “Capital Stock and Reserves,” for a further discussion.
There can be no assurance that the Company’s evaluation of strategic
alternatives will result in any agreements or transactions, or that, if completed, any agreements or transactions will be successful or
on attractive terms. Any potential transaction would be dependent on a number of factors that may be beyond the Company’s control,
including, among other things, market conditions, industry trends, the interest of third parties in a potential transaction with the Company
and the availability of financing to the Company or third parties in a potential transaction with the Company on reasonable terms. The
process of reviewing strategic alternatives may require the Company to incur additional costs and expenses. It could negatively impact
the Company’s ability to attract, retain and motivate key employees, and expose the Company to potential litigation in connection
with this process or any resulting transaction. If the Company is unable to effectively manage the process, the Company’s financial
condition and results of operations could be adversely affected. In addition, any strategic alternative that may be pursued and completed
ultimately may not deliver the anticipated benefits or enhance shareholder value. There can be no guarantee that the process of evaluating
strategic alternatives will result in the Company entering into or completing a potential transaction within the anticipated timing or
at all. There is no set timetable for this evaluation and the Company does not intend to disclose developments with respect to this evaluation
unless and until the Company determines that further disclosure is appropriate or legally required. As of July 31, 2024, the Company had
approximately $
| F-8 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 3. BASIS OF PRESENTATION
Statement of Compliance and Basis of Presentation
These consolidated financial statements have been prepared in accordance with International Financial Reporting Standards (“IFRS”) issued by the International Accounting Standards Board (“IASB”), International Accounting Standards (“IAS”) 34 Interim Financial Reporting and interpretations of the International Financial Reporting Interpretations Committee.
These consolidated financial statements have been prepared on an historical cost basis except for items disclosed herein at fair value (see Note 20, “Financial Instruments and Risk Management”). In addition, these consolidated financial statements have been prepared using the accrual basis of accounting, except for cash flow information.
The Company has only one reportable operating segment.
These consolidated financial statements were approved and authorized for issuance by the Audit Committee (the “Audit Committee”) of the Board of Directors (the “Board”) on August 14, 2024.
Consolidation
The consolidated financial statements include the accounts of the Company and:
| (a) | SalvaRx, a wholly-owned subsidiary, incorporated on May 6, 2015 in the British Virgin Islands; |
| (b) | iOx, a wholly-owned subsidiary incorporated in the U.K. on February 10, 2015. In September 2021, the Company, through SalvaRx, exchanged
certain notes, accrued interest, warrants and receivables in exchange for shares of iOx representing |
| (c) | Saugatuck, a |
| (d) | PDS, a |
| (e) | SalvaRx LLC, a wholly-owned subsidiary through SalvaRx incorporated in Delaware; |
| (f) | Saugatuck Rx LLC, a wholly-owned subsidiary of Saugatuck incorporated in Delaware; and |
| (g) | Tarus Therapeutics, LLC (“Tarus”), a wholly-owned subsidiary of Portage incorporated in Delaware. |
All inter-company balances and transactions have been eliminated in consolidation.
| F-9 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 3. BASIS OF PRESENTATION (Cont’d)
Consolidation (Cont’d)
Non-controlling interest in the equity of a subsidiary
is accounted for and reported as a component of stockholders’ equity. As of March 31, 2024, non-controlling interest represents
the
Functional and Presentation Currency
The Company’s functional and presentation currency is the U.S. Dollar.
Use of Estimates and Judgments
The preparation of the consolidated financial statements in conformity with IFRS requires management to make judgments, estimates and assumptions that affect the application of accounting policies and the reported amounts of assets, liabilities, income and expenses. Actual results may differ from these estimates.
Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Significant areas where estimates are made include valuation of financial instruments (including the Stimunity Convertible Note) (as defined below), deferred tax assets and liabilities, warrant liabilities, research and development costs, fair value used for acquisition of intangible assets, contingent consideration assumed and measurement of share-based compensation. Significant areas where critical judgments are applied include assessment of impairment of investments, in-process research and development and warrant liabilities.
Reclassifications
Certain prior year amounts between research and development costs and general and administrative expenses have been reclassified for consistency with the current year presentation. These reclassifications had no effect on the reported results of operations.
| F-10 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 4. SIGNIFICANT ACCOUNTING POLICIES
The accounting policies set out below have been applied consistently to all periods presented in these consolidated financial statements, which have, in management's opinion, been properly prepared within reasonable limits of materiality and within the framework of the significant accounting policies summarized below:
Financial Instruments
i) Financial Assets
Classification
Upon the initial recognition of financial assets, the financial assets are classified as one of the following measurement methodologies: (a) amortized cost, (b) fair value through other comprehensive income (“FVTOCI”), or (c) fair value through profit or loss (“FVTPL”). Subsequent measurement will be based on the initial classification of the financial assets.
The classification of a financial asset at initial recognition depends on the Company's business model for managing the financial asset and the financial asset's contractual cash flow characteristics.
In order for a financial asset to be measured at amortized cost or fair value through other comprehensive income (“OCI”), it needs to give rise to cash flows that are solely payments of principal and interest (“SPPI”) on the principal amount outstanding. This assessment is referred to as the SPPI test and is performed at an instrument level.
The Company's business model for managing financial assets refers to how it manages its financial assets in order to generate cash flows. The business model determines whether cash flows will result from collecting contractual cash flows, selling the financial assets, or both.
Measurement
For purposes of subsequent measurement, financial assets are classified in three categories:
| · | Financial assets at amortized cost (debt instruments); |
| · | Financial assets at FVTOCI (equity instruments); and |
| · | Financial assets at FVTPL. |
| F-11 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 4. SIGNIFICANT ACCOUNTING POLICIES (Cont'd)
Financial Assets at Amortized Cost (Debt Instruments)
The Company measures financial assets at amortized cost if both of the following conditions are met:
| · | The financial asset is held within a business model with the objective of holding the financial asset in order to collect contractual cash flows; and |
| · | The contractual terms of the financial asset give rise on specified dates to cash flows that are solely payments of principal and interest on the principal amount outstanding. |
Financial assets at amortized cost are subsequently measured using the effective interest rate method and are subject to a period impairment review. Gains and losses are recognized in profit or loss when the asset is derecognized, modified or impaired.
The Company's financial assets classified at amortized cost includes other receivables.
Financial Assets designated at Fair Value through OCI (Equity Instruments)
Upon initial recognition, the Company can elect to classify irrevocably its equity investments as equity instruments designated at FVTOCI when they meet the definition of equity under IAS 32, “Financial Instruments: Presentation,” and are not held for trading. The classification is determined on an instrument-by-instrument basis.
Gains and losses on these financial assets are never recycled to profit or loss. Dividends are recognized as other income in the statement of profit or loss when the right of payment has been established, except when the Company benefits from such proceeds as a recovery of part of the cost of the financial asset, in which case, such gains are recorded in OCI. Equity instruments designated at fair value through OCI are not subject to impairment assessment.
The Company irrevocably elected to classify its investment in Intensity as FVTOCI.
Financial Assets at Fair Value through Profit or Loss
Financial assets at FVTPL include financial assets held for trading, financial assets designated upon initial recognition at fair value through profit or loss, or financial assets mandatorily required to be measured at fair value. Financial assets are classified as held for trading if they are acquired for the purpose of selling or repurchasing in the near term. Derivatives, including separated embedded derivatives, are also classified as held for trading unless they are designated as effective hedging instruments. Financial assets with cash flows that are not solely payments of principal and interest are classified and measured FVTPL, irrespective of the business model.
Financial assets at fair value through profit or loss are carried in the consolidated statements of financial position at fair value with net changes in fair value recognized in the statement of profit or loss. The investment in associate (Stimunity) and the Stimunity Convertible Note receivable are accounted for as FVTPL.
ii) Financial Liabilities
The Company’s financial liabilities include accounts payable and accrued liabilities, which approximate fair value due to their short maturity, lease liability, warrant liability, deferred purchase price payable and deferred obligation.
| F-12 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 4. SIGNIFICANT ACCOUNTING POLICIES (Cont'd)
Warrant Liability
In connection with the SalvaRx Acquisition, the Company acquired notes
payable and associated warrants, which were recorded at fair value on the date of the acquisition.
On September 29, 2023, the Company entered into a securities purchase agreement (the “Purchase Agreement”) with an institutional and accredited investor in connection with a registered direct offering (the “Registered Direct Offering”) and a concurrent private placement (the “Private Placement,” and together with the Registered Direct Offering, the “Offerings”). The Offerings closed on October 3, 2023.
Pursuant to the Purchase Agreement, in the Registered Direct Offering,
the Company sold
In the Private Placement, the Company issued to such institutional and
accredited investor unregistered warrants to purchase up to ordinary shares (the “Series A Warrants”), unregistered
warrants to purchase up to ordinary shares (the “Series B Warrants”), and unregistered warrants to purchase up to
ordinary shares (the “Series C Warrants,” together with the Series A Warrants and the Series B Warrants, the “Private
Warrants”), together exercisable for an aggregate of up to ordinary shares (the “Private Warrant Shares”).
Pursuant to the terms of the Purchase Agreement, for each ordinary share and each Pre-Funded Warrant issued in the Registered Direct Offering,
an accompanying Series A Warrant, Series B Warrant and Series C Warrant were issued to such institutional and accredited investor. Each
Series A Warrant is exercisable for one Private Warrant Share at an exercise price of $ per share, is immediately exercisable and
will expire months from the date of issuance. Each Series B Warrant is exercisable for one Private Warrant Share at an exercise price
of $ per share, is immediately exercisable and will expire three years from the date of issuance. Each Series C Warrant is exercisable
for one Private Warrant Share at an exercise price of $ per share, is immediately exercisable and will expire five years from the
date of issuance. The net proceeds to the Company from the Offerings were approximately $
The Company filed the Resale Registration Statement (as defined below) to register for the resale of the Private Warrant Shares and the ordinary shares issuable upon the exercise of the Placement Agent Warrants, which was declared effective by the SEC on November 7, 2023. Pursuant to the terms of the Purchase Agreement, the Company is obligated to use its commercially reasonable efforts to keep the Resale Registration Statement effective at all times until such institutional and accredited investor (and its successors and assigns) no longer owns any Private Warrants or ordinary shares issuable upon exercise thereof.
| F-13 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 4. SIGNIFICANT ACCOUNTING POLICIES (Cont'd)
Series B Warrants, Series C Warrants and Placement Agent Warrants
The Company accounts for the Series B Warrants, the Series C Warrants and the Placement Agent Warrants (defined below) under IAS 9, “Financial Instruments” and IAS 32, “Financial Instruments: Presentation”.
An equity instrument is any contract that evidences a residual interest in the assets of an entity after deducting all of its liabilities.
The Series B Warrants, the Series C Warrants and the Placement Agent Warrants (defined below) include the obligation, in the event of a Fundamental Transaction, as defined in such warrants, for the Company or the successor entity to purchase the warrants from the holder at the discretion of the holder and at the Black-Scholes value, as defined in the warrant agreements. As a result, management concluded that such warrants met the criteria of paragraphs 16A and 16B of IAS 32 and should be reflected as a liability on the consolidated statement of financial position with the changes in fair value recognized in the consolidated statement of operations and other comprehensive income (loss).
Series A Warrants and Pre-Funded Warrants
The Series A Warrants and the Pre-Funded Warrants are classified as a component of equity because they are freestanding financial instruments that are legally detachable and separately exercisable from the ordinary shares with which they were issued, are immediately exercisable, do not embody an obligation for the Company to repurchase such warrants, and permit the holders to receive a fixed number of ordinary shares upon exercise. In addition, the Series A Warrants and the Pre-Funded Warrants do not provide any guarantee of value or return. See Note 15, “Capital Stock and Reserves,” for a further discussion.
| F-14 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 4. SIGNIFICANT ACCOUNTING POLICIES (Cont'd)
Impairment of Financial Assets and Intangible Assets
IFRS 9, “Financial Instruments,” requires the Company to recognize an allowance for expected credit losses ("ECLs") for all debt instruments and investments not held at fair value through profit or loss and contract assets. For intangible assets, at the end of each reporting period and whenever there is an indication that the intangible asset may be impaired, the Company reviews the carrying amounts of its intangible assets to determine whether there is any indication that those assets have suffered an impairment loss.
At the end of each reporting period, the Company assesses whether there was objective evidence that a financial asset was impaired. The Company recognizes an allowance for ECLs for all debt instruments not held at fair value through profit or loss. ECLs are based on the difference between the contractual cash flows due in accordance with the contract and all the cash flows that the Company expects to receive, discounted at an approximation of the original effective interest rate. The expected cash flows will include cash flows from the sale of collateral held or other credit enhancements that are integral to the contractual terms.
ECLs are recognized in two stages. For credit exposures for which there has not been a significant increase in credit risk since initial recognition, ECLs are provided for credit losses that result from default events that are possible within the next 12-months (a 12-month ECL). For those credit exposures for which there has been a significant increase in credit risk since initial recognition, a loss allowance is required for credit losses expected over the remaining life of the exposure, irrespective of the timing of the default (a lifetime ECL).
Foreign Currencies
The functional and presentation currency of the Company and its subsidiaries (see Note 3, “Basis of Presentation”) is the U.S. dollar. Monetary assets and liabilities are translated at exchange rates in effect at the consolidated statement of financial position date. Non-monetary assets are translated at exchange rates in effect when they were acquired. Revenue and expenses are translated at the approximate average rate of exchange for the period. Foreign currency differences arising on retranslation are recognized in income or loss.
The effect of exchange rates on the Company’s foreign currency-denominated asset and liability balances are recorded as foreign currency transaction losses in the determination of net income (loss).
| F-15 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 4. SIGNIFICANT ACCOUNTING POLICIES (Cont'd)
Cash and Cash Equivalents
Cash and cash equivalents comprise cash on hand and amounts invested
in underlying Treasury and money market funds that are readily convertible to a known amount of cash with three months or less from date
of acquisition and are subject to an insignificant risk of change in value. As of March 31,
2024 and 2023, cash equivalents was comprised of a money market account with maturities less than 90 days from the date of purchase.
Interest income earned on such investments totaled $
Intangible Assets acquired in Business Combinations
Intangible assets acquired in business combinations that are separable from goodwill are recorded at their acquisition date fair value. Subsequent to initial recognition, intangible assets acquired in business combinations are reported net of accumulated amortization and any impairment losses.
Impairment of Indefinite Life Intangible Assets other than Goodwill
At the end of each annual reporting period and whenever there is an indication that an indefinite life intangible asset may be impaired, the Company reviews the carrying amounts of such intangible assets to determine whether there is any indication that those assets have suffered an impairment loss. If any such indication exists, the recoverable amount of the asset is estimated to determine the extent of impairment loss (if any). When it is not possible to estimate the recoverable amount of any individual asset, the Company estimates the recoverable amount of the cash-generating unit to which the asset belongs. When a reasonable and consistent basis of allocation can be identified, corporate assets are also allocated to individual cash-generating units ("CGU" or "CGUs"), or the smallest group of cash-generating units for which a reasonable and consistent allocation basis can be identified.
Recoverable amount is the higher of fair value less costs of disposal and value in use. In assessing value in use, the estimated future cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset for which the estimates of future cash flows have not been adjusted.
The Company determines the fair value of share-based payments granted to directors, officers, employees and consultants using the Black-Scholes option-pricing model at the grant date. Assumptions for the Black-Scholes model are determined as follows:
| · | Expected Volatility. The expected volatility rate used to value stock option grants is based on the Company’s historical volatility. |
| · | Expected Term. The Company used historical experience. |
| · | Risk-free Interest Rate. The risk-free interest rate assumption was based on zero-coupon U.S. Treasury instruments that had terms consistent with the expected term of the Company's stock option grants. |
| · | Expected Dividend Yield. The Company has never declared or paid any cash dividends and does not presently plan to pay cash dividends in the foreseeable future. |
| F-16 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 4. SIGNIFICANT ACCOUNTING POLICIES (Cont'd)
Share-based payments to employees, officers and directors are recorded and reflected as an expense over the vesting period with a corresponding increase in the stock option reserve. On exercise, the associated amounts previously recorded in the stock option reserve are transferred to common share capital.
Basic loss per share is calculated by dividing net loss (the numerator) by the weighted average number of ordinary shares outstanding (the denominator) during the period. Diluted loss per share reflects the dilution that would occur if outstanding stock options and share purchase warrants were exercised into ordinary shares using the treasury stock method and convertible debt instruments were converted into ordinary shares using the if-converted method. Diluted loss per share is calculated by dividing net loss applicable to ordinary shares by the sum of the weighted average number of ordinary shares outstanding and all additional ordinary shares that would have been outstanding if potentially dilutive common shares had been issued. The share and per share information has been retroactively adjusted to reflect the impact of the stock dividend.
The inclusion of the Company's stock options, restricted stock units and share purchase warrants in the computation of diluted loss per share would have an anti-dilutive effect on loss per share and are therefore excluded from the computation. Consequently, there is no difference between basic loss per share and diluted loss per share for the years ended March 31, 2024, 2023 and 2022. The following table reflects the outstanding securities by year that would have an anti-dilutive effect on loss per share, and accordingly, were excluded from the calculation (see Note 17, “Loss Per Share”).
| As of March 31, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Warrants | ||||||||||||
| Stock options | ||||||||||||
| Restricted stock units | ||||||||||||
Investment in Public Company
The investment is comprised of shares of private/public companies that have been acquired through a private placement. The investment is initially recorded at fair value. Following acquisition, the Company evaluates whether control or significant influence is exerted by the Company over the affairs of the investee company. Based on the evaluation, the Company accounts for the investment using either the consolidation, equity accounting or fair value method (see Note 7, “Investment in Public Company”). The Company sold the entire investment in Fiscal 2024.
Investment in Associate
An associate is an entity over which the Company has significant influence.
Significant influence is the power to participate in the financial and operating policy decisions of the investee but is not control
or joint control over those policies. The investment in associate was written down to nil
| F-17 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 4. SIGNIFICANT ACCOUNTING POLICIES (Cont'd)
The results and assets and liabilities of associates are incorporated in these consolidated financial statements using the equity method of accounting, except when the investment, or a portion thereof, is classified as held for sale, in which case it is accounted for in accordance with IFRS 5, “Non-current Assets Held for Sale and Discontinued Operations”. Under the equity method, an investment in an associate is initially recognized in the consolidated statement of financial position at cost from the date the investee becomes an associate and adjusted thereafter to recognize the Company's share of the profit or loss and other comprehensive income of the associate. When the Company's share of losses of an associate exceed the Company's interest in that associate (which includes any long-term interests that, in substance, form part of the Company's net investment in the associate), the Company discontinues recognizing its share of further losses. Additional losses are recognized only to the extent that the Company has incurred legal or constructive obligations or made payments on behalf of the associate.
After application of the equity method, the Company determines whether it is necessary to recognize an impairment loss on its investment in its associate. At each reporting date, the Company determines whether there is objective evidence that the investment in the associate is impaired. If there is such evidence, the Company calculates the amount of impairment as the difference between the recoverable amount of the associate and its carrying value, and then recognizes the loss within 'share of (loss) income in associate' in the consolidated statements of operations.
Research and Development Expenses
(i) Research and Development
Expenditure on research activities, undertaken with the prospect of gaining new scientific or technical knowledge and understanding, is expensed as incurred.
Development activities involve a plan or design for the production of new or substantially improved products and processes. Development expenditures are capitalized only if development costs can be measured reliably, the product or process is technically, and commercially feasible, future economic benefits are probable, and the Company intends to and has sufficient resources to complete development and to use or sell the asset. Following initial recognition of the development expenditure as an asset, the asset is carried at cost less any accumulated amortization. Amortization of the asset begins when development is complete and the asset is available for use. It is amortized over the period of expected future benefit. During the period of development, the asset is tested for impairment annually.
Research and development expenses include all direct and indirect operating expenses supporting the products in development.
(ii) Subsequent Expenditure
Subsequent expenditure is capitalized only when it increases the future economic benefits embodied in the specific asset to which it relates. All other expenditures are recognized in income or loss as incurred.
(iii) Clinical Trial Expenses
Clinical trial expenses are a component of the Company's research and development costs. These expenses include fees paid to contract research organizations, clinical sites, and other organizations who conduct development activities on the Company's behalf. The amount of clinical trial expenses recognized in a period related to clinical agreements is based on estimates of the work performed using an accrual basis of accounting. These estimates incorporate factors such as patient enrolment, services provided, contractual terms, and prior experience with similar contracts.
| F-18 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 4. SIGNIFICANT ACCOUNTING POLICIES (Cont'd)
Contingent Liability
A contingent liability is a possible obligation that arises from past events and of which the existence will be confirmed only by the occurrence or non-occurrence of one or more uncertain future events not within the control of the Corporation; or a present obligation that arises from past events (and therefore exists), but is not recognized because it is not probable that a transfer or use of assets, provision of services or any other transfer of economic benefits will be required to settle the obligation; or the amount of the obligation cannot be estimated reliably.
Determination of Fair Value
A number of the Company's accounting policies and disclosures required the determination of fair value, both for financial and non-financial assets and liabilities. Fair values have been determined for measurement and/or disclosure purposes based on assumptions that market participants would use when pricing the asset or liability, assuming that market participants act in their economic best interest. When applicable, further information about the assumptions made in determining fair values is disclosed in Note 20, “Financial Instruments and Risk Management” and other footnotes that specifically relate to assets or liabilities measured at fair value.
Income Tax
The Company uses the asset and liability method to account for income taxes. Deferred income tax assets and liabilities are recognized for the future tax consequences attributable to differences between the carrying amounts of existing assets and liabilities for accounting purposes, and their respective tax bases.
Deferred income tax assets and liabilities are measured using tax rates that have been enacted or substantively enacted and applied to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred income tax assets and liabilities of a change in statutory tax rates is recognized in profit or loss in the year of change. Deferred income tax assets are recorded when their recoverability is considered probable and are reviewed at the end of each reporting period.
Business Combinations
Business combinations are accounted for using the acquisition method as of the date when control transfers to the Company. The total purchase price less the fair value of non-controlling interest is allocated to the acquired net tangible and intangible assets and liabilities assumed at fair value.
Transaction costs that the Company incurs in connection with a business combination are expensed as incurred.
Goodwill
Goodwill represents the excess of the purchase price paid for the acquisition of an entity and the amount recognized for non-controlling interests over the fair value of the net identifiable assets acquired and liabilities assumed. Goodwill is allocated to the CGUs, which are expected to benefit from the synergies of the combination. Goodwill is not subject to amortization and is tested annually for impairment, or more frequently if events or changes in circumstances indicate that they might be impaired.
Impairment is determined for goodwill by assessing if the carrying value of a CGU, including the allocated goodwill, exceeds its recoverable amount determined as the greater of the estimated fair value less costs to sell and the value in use. Impairment losses recognized in respect of a CGU are first allocated to the carrying value of goodwill and any excess is allocated to the carrying amount of assets in the CGU. Any goodwill impairment is recorded in income in the period in which the impairment is identified. Impairment losses on goodwill are not subsequently reversed.
| F-19 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 4. SIGNIFICANT ACCOUNTING POLICIES (Cont'd)
Recent Accounting Pronouncements
IFRS Pronouncements Issued
Impact of Adoption of Significant New IFRS Standards in Fiscal 2024
| (a) | Annual Improvements to IFRS Standards 2018-2020 |
The annual improvements process addresses issues in the 2018-2020 reporting cycles including changes to IFRS 9, “Financial Instruments,” IFRS 1, “First Time Adoption of IFRS,” IFRS 16, “Leases,” and IAS 41, “Biological Assets”.
| i) | The amendment to IFRS 9 addresses which fees should be included in the 10% test for derecognition of financial liabilities. |
| ii) | The amendment to IFRS 1 allows a subsidiary adopting IFRS at a later date than its parent to also measure cumulative translation differences using the amounts reported by the parent based on the parent’s date of transition to IFRS. |
| iii) | The amendment to IFRS 16’s illustrative example 13 removes the illustration of payments from the lessor related to leasehold improvements. |
These amendments were effective for annual periods beginning on or after January 1, 2022. The adoption of these amendments did not have a material effect on the Company’s annual consolidated financial statements.
New Accounting Standards, Interpretations and Amendments
Standards issued but not yet effective up to the date of issuance of the Company’s consolidated financial statements are listed below. This listing is of standards and interpretations issued, which the Company reasonably expects to be applicable at a future date. The Company intends to adopt those standards when they become effective.
| (a) | IAS 1: Presentation of Financial Statements |
The amendment to IAS 1 clarifies how to classify debt and other liabilities as either current or non-current. The amendment is effective for annual periods beginning on or after January 1, 2024. The Company is currently evaluating the new guidance and impacts on its consolidated financial statements.
| (b) | Amendments to IFRS 10 and IAS 28: Sale or Contribution of Assets between an Investor and Its Associate or Joint Venture |
The amendment addresses the conflict between IFRS 10, “Consolidated Financial Statements,” and IAS 28, “Investments in Associates and Joint Ventures,” in dealing with the loss of control of a subsidiary that is sold or contributed to an associate or joint venture. The amendments clarify that the gain or loss resulting from the sale or contribution of assets that constitute a business, as defined in IFRS 3, “Business Combinations,” between an investor and its associate or joint venture, is recognized in full. Any gain or loss resulting from the sale or contribution of assets that do not constitute a business, however, is recognized only to the extent of unrelated investors’ interests in the associate or joint venture. The IASB has deferred the effective date of these amendments indefinitely, but an entity that early adopts the amendments must apply them prospectively. The Company is evaluating whether the adoption of the above amendment will have a material impact on its consolidated financial statements.
| F-20 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 5. PREPAID EXPENSES AND OTHER RECEIVABLES
| As of March 31, | ||||||||
| (In thousands) | 2024 | 2023 | ||||||
| Prepaid clinical research costs | $ | $ | ||||||
| Prepaid insurance | ||||||||
| Other prepaid expenses | ||||||||
| Tax deposits | ||||||||
| Other receivables | ||||||||
| Research & development tax credits | ||||||||
| Total prepaid expenses and other receivables | $ | $ | ||||||
NOTE 6. INVESTMENT IN ASSOCIATE AND CONVERTIBLE NOTE RECEIVABLE
Details of the Company’s associate, Stimunity S.A. (“Stimunity”), as of March 31, 2024 and 2023 are as follows:
| Name | Principal Activity |
Place of Incorporation and Principal Place of Business |
Voting Rights Held as of March 31, 2024 |
Voting Rights Held as of March 31, 2023 |
||||
The following table is a roll-forward of the Company’s investment in Stimunity as of and for the years ended March 31, 2024, 2023 and 2022:
| As of and for the Years Ended March 31, | ||||||||||||
| (In thousands) | 2024 | 2023 | 2022 | |||||||||
| Balance, beginning of year | $ | $ | $ | |||||||||
| Share of loss | ( | ) | ( | ) | ( | ) | ||||||
| Conversion of Stimunity Convertible Note | ||||||||||||
| Impairment loss | ( | ) | ( | ) | ||||||||
| Balance, end of year | $ | $ | $ | |||||||||
The Company accounted for its investment in Stimunity under the equity
method and, accordingly, recorded its share of Stimunity’s earnings or loss based on its ownership percentage. The Company recorded
loss in equity in Stimunity of $
| F-21 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 6. INVESTMENT IN ASSOCIATE AND CONVERTIBLE NOTE RECEIVABLE (Cont'd)
On September 12, 2022,
The Stimunity Convertible Note was initially recorded at $
As of each of March 31, 2024, and 2023, the Company determined that there
were indications of impairment of both the investment in associate and the Stimunity Convertible Note receivable, based upon the inability
of Stimunity to obtain financing. The Company performed an IAS 36 fair value analyses and recorded provisions of impairment of $
| F-22 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 7. INVESTMENT IN PUBLIC COMPANY
The following table is a roll-forward of the investments in Intensity as of March 31, 2024 and 2023:
| (In thousands) | Intensity | |||
| Balance as of April 1, 2022 (including unrealized gain of $) | $ | |||
| Unrealized loss on investment | ( |
) | ||
| Balance as of March 31, 2023 | ||||
| Proceeds received from sale of Intensity shares | ( |
) | ||
| Gain on sale of Intensity | ||||
| Balance as of March 31, 2024 | $ |
The following is a discussion of the Company’s investment in Intensity Therapeutics, Inc. (“Intensity”) as of March 31, 2024 and 2023.
Intensity Therapeutics, Inc.
In connection with the SalvaRx Acquisition in fiscal 2019, the Company
acquired a $
On July 11, 2019, the Company entered into an agreement with Fast Forward
Innovations Limited (“Fast Forward”) to purchase Intensity Holdings Limited (“IHL”), a wholly-owned subsidiary
of Fast Forward. The Company paid $
On October 28, 2021, Intensity filed a Form S-1 Registration Statement with the SEC to register shares for an IPO, which was declared effective by the SEC, but subsequently withdrawn prior to closing.
Subsequently, Intensity amended its Form S-1 Registration Statement and
continued to complete its IPO. As of March 31, 2023, the Company undertook an IAS 36 fair value analysis based on the continued existence
of external indications of impairment, which resulted in an aggregate $
In April 2023, Intensity completed a 1:2 reverse stock split, which reduced the Company’s holdings to shares.
On July 5, 2023, Intensity completed an IPO of its common stock selling
shares at a price of $ per share generating net proceeds of approximately $
| F-23 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 7. INVESTMENT IN PUBLIC COMPANY (Cont’d)
The Company owned approximately
NOTE 8. LEASE
The Company entered into a lease of office space, which commenced on May
1, 2023 (the “Original Lease”). The Original Lease provided for an original term of two years with an option to renew the
Original Lease for an additional term of three years. The Company has included the extension option in the Original Lease analysis under
IFRS 16, based upon management’s intentions. The Company calculated the Original Lease liability using its incremental borrowing
rate of 13%. The Company provided a $
During the quarter ended March 31, 2024, the Company determined a change
of circumstances had occurred and that it was not likely to renew the lease. On February 20, 2024, the Company entered into an amendment
of the Original Lease (the “Amended Lease”), which commenced on March 1, 2024. The Amended Lease provides for a smaller lease
space and a lower base rent per month, subject to similar escalations as the Original Lease. The term of the Amended Lease is the same
as the Original Lease’s expiration date of May 25, 2025. The Amended Lease provides for a term of one year with an option to renew
the Amended Lease for an additional term of three years. The effect of the change in circumstance reduced the asset and liability by $
The Amended Lease liability is payable as follows (in thousands):
| Twelve Months Ended March 31, | Amount | |||
| 2025 | $ | |||
| 2026 | ||||
| Total | ||||
| Less: Interest | ( | ) | ||
| Total lease liability | ||||
| Lease liability - current | ||||
| Lease liability - non-current | $ |
| F-24 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 9. ACQUISITION OF TARUS
On July 1, 2022, the Company, its wholly-owned subsidiary, Portage Merger Sub I, Inc., its wholly-owned subsidiary, Portage Merger Sub II, LLC and Tarus Therapeutics, Inc., a Delaware corporation advancing adenosine receptor antagonists for the treatment of solid tumors, entered into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”). Per the Merger Agreement, Tarus Therapeutics, Inc. was ultimately merged into Portage Merger Sub II, LLC, with the surviving entity renamed “Tarus Therapeutics, LLC”. The Tarus merger entitles the Company to the rights, know-how and/or ownership related to the assets developed by Tarus (the “Adenosine Compounds”), including:
| 1. | All rights and obligations related to the License Agreement between Tarus and Impetis Biosciences Limited, dated October 29, 2019 (“Tarus License Agreement”), and the call option under the Tarus License Agreement, which was exercised on November 5, 2020; |
| 2. | All intellectual property and related documents owned or controlled by Tarus, including issued or pending patents, patent applications and trade secrets. Additionally, any draft submissions and/or correspondence with patent authorities; |
| 3. | All documents and supplies related to Adenosine Compounds (as defined in the Tarus License Agreement) including inventory, reagents, data, assays, reports, vendor agreements and other information related to the preclinical development; |
| 4. | All clinical supplies, manufacturing know-how, batch records, regulatory documents pertaining to the Adenosine Compounds, certain reservations for manufacturing campaigns and any related agreements; |
| 5. | All regulatory documents and correspondence pertaining to the Adenosine Compounds; |
| 6. | All contract research organization (“CRO”) agreements and protocol related documents for Adenosine Compounds; |
| 7. | All current documents related to market research, forecasting, budgets and competitive intelligence; and |
| 8. | Rights to the use of Tarus Therapeutics’ name for regulatory purposes. |
As consideration for Tarus, the Company issued to former Tarus shareholders an aggregate of ordinary shares of Portage, calculated on the basis of $ million divided by the 60-day volume weighted average price per share of ordinary shares of Portage. Such ordinary shares have not been registered with the SEC and were subject to lock-up agreements for terms ranging from six to twelve months, which expired on February 1, 2023 and July 1, 2023, respectively. Additionally, the ordinary shares that were subject to a twelve-month lock-up period, are also subject to a three-month dribble-out period, which commenced July 1, 2023. During the dribble out period, each holder may not sell more than 10% of the average trading volume of the Company’s ordinary shares for the rolling three-month period prior to the date on which the holder executes a trade of the Company’s ordinary shares without its prior written consent (which the Company is permitted to withhold at its sole discretion). Additionally, in the event that the Company resumes the clinical trial and further accrual of its adenosine program, milestone payments of up to $ million in cash or Portage ordinary shares (at the discretion of the Company) would be triggered upon achievement of future development and sales milestones, as described below. As a result of the transaction:
| · | The Company also assumed $ |
| · | Upon enrolling the first patient in a Phase 2 clinical trial utilizing Tarus’s adenosine receptor antagonists,
the Company will pay an additional one-time milestone payment of $ |
| F-25 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 9. ACQUISITION OF TARUS (Cont’d)
In connection with the acquisition of Tarus, the Company performed a
fair value analysis of the assets acquired and liabilities assumed. The Company based the analysis on its clinical plan and timing
of development events, and the probabilities of success determined primarily based upon empirical third-party data and Company
experience as well as the relevant cost of capital. In its fair value analysis, the Company used the Multi-Period Excess Earnings
Method for PORT-6 and PORT-7 and the Replacement Cost Method for PORT-8 and PORT-9, determined based upon the maturity of the assets
and the availability of sufficient data to measure fair value. The Company recorded the ordinary shares issued at $
million, which represented the aggregate market value of the ordinary shares issued on July 1, 2022. The Company followed the
guidance of IAS 3 and IAS 32 and recorded a deferred purchase price payable - Tarus of $
The following table summarizes the original purchase price allocation to the fair value of assets acquired and liabilities assumed for Tarus:
| Assets: | (In thousands) | |||
| Identifiable intangible assets | $ | |||
| Goodwill | ||||
| Total assets | $ | |||
| Consideration: | ||||
| Fair value of shares issued | $ | |||
| Liabilities assumed | ||||
| Deferred purchase consideration at fair value | ||||
| Total liabilities | $ |
Pro forma Information
Summary unaudited pro forma condensed results of operations for the years ended March 31, 2023 and 2022, assuming the Tarus acquisition had occurred at the beginning of the earliest period presented, are as follows:
| Years Ended March 31, | ||||||||
| (In thousands) | 2023 | 2022 | ||||||
| Loss from operations | $ | ( | ) | $ | ( | ) | ||
| Loss before provision for income taxes | $ | ( | ) | $ | ( | ) | ||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Total comprehensive loss for year | $ | ( | ) | $ | ( | ) | ||
| Loss per share | $ | ( | ) | $ | ( | ) | ||
There are no pro forma adjustments for the year ended March 31, 2024 since the operating results for the year ended March 31, 2024 include the results of operations of Tarus.
These pro forma results are not necessarily indicative of what would have occurred if the acquisition had been in effect for the period presented, and they may not be indicative of results expected in the future.
| F-26 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 10. GOODWILL
The following is a roll-forward of goodwill:
| As of March 31, | ||||
| (In thousands) | 2023 | |||
| Balance, beginning of year | $ | |||
| Tarus goodwill | ||||
| Loss on impairment | ( | ) | ||
| Balance, end of year | $ | |||
The Company’s goodwill arose primarily from the acquisition of SalvaRx and its portfolio of several projects and investments.
As a result of the acquisition of Tarus in July 2022, the Company recorded
$
Under purchase accounting as of July 1, 2022 (the acquisition date), the assets and liabilities of Tarus Therapeutics, Inc., was recorded at their respective fair values and the excess of the acquisition consideration is goodwill. The purchase was in the form of a merger in which Tarus Therapeutics, Inc. was merged into Tarus Therapeutics, LLC., which is a wholly-owned subsidiary of Portage. All of the consideration for Tarus Therapeutics, LLC was paid or assumed by Portage and Portage will control the voting rights, the Board and the operations of Tarus Therapeutics, LLC.
As of March 31, 2024, the Company determined that it has only one CGU, the consolidated Portage Biotech Inc.
Impairment Review
On an annual basis, pursuant to IAS 36, the Company assesses its long-lived assets with definite lives, which are not yet available for use, for potential indicators of impairment.
If any such indication exists, the Company estimates the recoverable amount of the asset or CGU and compares it to the carrying value.
The Company performed its annual impairment test in each of Fiscal 2023 and the year ended March 31, 2022 (“Fiscal 2022”) and estimated the recoverable amount of the above-noted CGU based on its value in use, which was determined using a capitalized cash flow methodology and categorized within level 3 of the fair market value hierarchy.
The recoverable amount of the CGU has been determined based on its value in use. The recoverable amount considered assumptions based on probabilities of technical, regulatory and clinical acceptances and financial support. Further, management uses risk-adjusted cash flow projections based on financial budgets. Management believes that any reasonably possible change in the key assumptions on which the recoverable amount is based would not cause the carrying amount to exceed its recoverable amount. The discount rate has been determined based on the Company’s best estimate of a risk adjusted discount rate.
| F-27 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 10. GOODWILL (Cont’d)
The key assumptions used in the calculation of the recoverable amount include forecasts of the following:
(a) revenues;
(b) normalized operating expenses;
(c) income taxes; and
(d) capital expenditures.
Each asset and liability analyzed was valued independently, as required.
Certain assets were valued under the discounted cash flow method and discount rates ranged from
As of March 31, 2023, management determined that there were external
factors, including the fact that the Company’s market capitalization was less than its net assets. As a result, the Company completed
an IAS 36 fair value analyses for all assets required to be tested. The studies evaluated current market conditions, costs of capital,
the Company’s development plans and recorded losses on impairment aggregating $
NOTE 11. IN-PROCESS RESEARCH AND DEVELOPMENT AND DEFERRED TAX LIABILITY
In-process research and development (“IPR&D”) consists of the following projects (in thousands):
| Value as of March 31, | ||||||||||
| Project # | Description | 2024 | 2023 | |||||||
| iOx: | ||||||||||
| PORT 2 (IMM60) | Melanoma & Lung Cancers | $ | $ | |||||||
| PORT 3 (IMM65) | Ovarian/Prostate Cancers | |||||||||
| Oncomer/Saugatuck | DNA Aptamers | |||||||||
| Tarus: | ||||||||||
| PORT-6 & PORT-7 | Adenosine Receptors | |||||||||
| PORT-8 | Adenosine Receptors | |||||||||
| PORT-9 | Adenosine Receptors | |||||||||
| In-process research and development | $ | $ | ||||||||
| Deferred tax liability | $ | $ | ||||||||
| F-28 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 11. IN-PROCESS RESEARCH AND DEVELOPMENT AND DEFERRED TAX LIABILITY (Cont’d)
As indicated in the goodwill discussion above, the Company identified
external indicators of potential impairment as of March 31, 2024 and March 31, 2023. Pursuant to IAS 36, the Company evaluated the current
capital markets, the increasing costs of capital, and the delays in the timing of asset development and concluded that provisions for
impairment were required during the years ended March 31, 2024 and 2023 with respect to the iOx IPR&D and the Tarus IPR&D. During
the year ended March 31, 2023, the Company recognized an impairment of $
NOTE 12. ACCOUNTS PAYABLE AND ACCRUED LIABILITIES
| As of March 31, | ||||||||
| (In thousands) | 2024 | 2023 | ||||||
| Accrued CRO | $ | $ | ||||||
| Accrued bonuses and other payroll-related expenses | ||||||||
| Accounts payable | ||||||||
| Accrued legal fees | ||||||||
| Accrued accounting and auditing fees | ||||||||
| Accrued other professional fees | ||||||||
| Accrued clinical and R&D services | ||||||||
| Other | ||||||||
| Accrued rent and administration | ||||||||
| Total accounts payable and accrued liabilities | $ | $ | ||||||
| F-29 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 13. WARRANT LIABILITY
The following table summarizes the changes in the warrant liability during the year ended March 31, 2024:
| Exercise Price | Warrants | Fair Value Balance |
||||||||||
| In 000’$ | ||||||||||||
| Warrant liability as of April 1, 2023 | $ | $ | ||||||||||
| Fair value of warrants at issuance on October 3, 2023: | ||||||||||||
| Class B Warrants | $ | |||||||||||
| Class C Warrants | $ | |||||||||||
| Placement Agent Warrants | $ | |||||||||||
| Change in fair value of warrant liability | ( | ) | ||||||||||
| Warrant liability as of March 31, 2024 | $ | |||||||||||
On September 29, 2023, the Company entered into the Purchase Agreement with an institutional and accredited investor in connection with the Registered Direct Offering and the Private Placement. The Offerings closed on October 3, 2023.
Pursuant to the Purchase Agreement, in the Registered Direct Offering,
the Company sold (i)
In the Private Placement, the Company issued to such institutional and
accredited investor Series A Warrants to purchase up to ordinary shares, Series B Warrants to purchase up to ordinary
shares, and Series C Warrants to purchase up to ordinary shares, together exercisable for an aggregate of up to 9,473,685 ordinary
shares. Pursuant to the terms of the Purchase Agreement, for each ordinary share and Pre-Funded Warrant issued in the Registered Direct
Offering, an accompanying Series A Warrant, Series B Warrant and Series C Warrant were issued to such institutional and accredited investor.
Each Series A Warrant is exercisable for one Private Warrant Share at an exercise price of $
| F-30 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 13. WARRANT LIABILITY (Cont’d)
Pursuant to an engagement letter, dated as of August 26, 2023, between the Company and H.C. Wainwright & Co., LLC (the “Placement Agent”), the Company paid the Placement Agent a total cash fee equal to % of the aggregate gross proceeds received in the Offerings, or $ million. The Company also paid the Placement Agent in connection with the Offerings a management fee equal to % of the aggregate gross proceeds raised in the Offerings ($ million), $ for non-accountable expenses and $ for clearing fees. In addition, the Company issued to the Placement Agent, or its designees, warrants to purchase up to ordinary shares (the “Placement Agent Warrants,” and together with the Pre-Funded Warrants and the Private Warrants, the “Warrants”), which represented % of the aggregate number of ordinary shares and Pre-Funded Warrants sold in the Registered Direct Offering. The Placement Agent Warrants have substantially the same terms as the Series B Warrants and the Series C Warrants, except that the Placement Agent Warrants have an exercise price equal to $, or 125% of the offering price per ordinary share sold in the Registered Direct Offering and will be exercisable for five years from the commencement of the sales pursuant to the Offerings. The Private Warrants, Private Warrant Shares, Placement Agent Warrants and ordinary shares underlying the Placement Agent Warrants were registered for resale under the Securities Act of 1933, as amended (the “Securities Act”) pursuant to a registration statement on Form F-1 that was declared effective by the SEC on November 7, 2023 (the “Resale Registration Statement”).
The Series B Warrants, the Series C Warrants and the Placement Agent Warrants
include the obligation, in the event of a Fundamental Transaction, as defined in the Series B Warrants, the Series C Warrants and the
Placement Agent Warrants, for the Company or the successor entity to purchase the warrants from the holder at the discretion of the holder
and at the Black-Scholes value, as defined in the warrant agreements. As a result, management concluded that, in line with IAS 9, “Financial
Instruments” and IAS 32, “Financial Instruments: Presentation,” such warrants will be accounted for as financial liabilities
on the consolidated statement of financial position with the changes in fair value recognized in the consolidated statement of operations
and other comprehensive income (loss). The Company allocated the net proceeds of $
The Company filed the Resale Registration Statement to register for the resale of the Private Warrant Shares and the ordinary shares issuable upon the exercise of the Placement Agent Warrants, which was declared effective by the SEC on November 7, 2023. Pursuant to the terms of the Purchase Agreement, the Company is obligated to use its commercially reasonable efforts to keep the Resale Registration Statement effective at all times until such institutional and accredited investor (and its successors and assigns) no longer owns any Private Warrants or ordinary shares issuable upon exercise thereof.
The accounting for the Series A Warrants and the Pre-Funded Warrants is detailed below in Note 15, “Capital Stock and Reserves.”
| F-31 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 13. WARRANT LIABILITY (Cont’d)
Series B Warrants
A fair value of $ per each Series B Warrant was identified at the issue date of October 3, 2023. A fair value of $ per each warrant has been identified as of March 31, 2024.
The inputs associated with calculating the fair value are reflected below.
| October 3, 2023 | March 31, 2024 | |||||||
| Exercise price | $ | $ | ||||||
| Share price | $ | $ | ||||||
| Expected life (in years) | ||||||||
| Expected volatility | % | % | ||||||
| Risk-free interest rate | % | % | ||||||
| Dividend yield |
Series C Warrants
A fair value of $ per each Series C Warrant was identified at the issue date of October 3, 2023. A fair value of $ per each warrant has been identified as of March 31, 2024.
The inputs associated with calculating the fair value are reflected below.
| October 3, 2023 | March 31, 2024 | |||||||
| Exercise price | $ | $ | ||||||
| Share price | $ | $ | ||||||
| Expected life (in years) | ||||||||
| Expected volatility | % | % | ||||||
| Risk-free interest rate | % | % | ||||||
| Dividend yield |
Placement Agent Warrants
A fair value of $ per each Placement Agent Warrant was identified at the issue date of October 3, 2023. A fair value of $ per each warrant has been identified as of March 31, 2024.
The inputs associated with calculating the fair value are reflected below.
| October 3, 2023 | March 31, 2024 | |||||||
| Exercise price | $ | $ | ||||||
| Share price | $ | $ | ||||||
| Expected life (in years) | ||||||||
| Expected volatility | % | % | ||||||
| Risk-free interest rate | % | % | ||||||
| Dividend yield |
| F-32 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 14. INCOME TAXES
The Company is a BVI business company. The BVI government does not, under existing legislation, impose any income or corporate tax on corporations.
PDS is a U.S. corporation and is subject to U.S. federal, state and local income taxes, as applicable.
iOx is subject to U.K. taxes.
The (expense) benefit from income taxes consists of the following for the years ended March 31, 2024 and 2023 (U.S. Dollars in thousands):
| Years Ended March 31, | ||||||||
| (In thousands) | 2024 | 2023 | ||||||
| Current: | ||||||||
| Federal | $ | ( | ) | $ | ( | ) | ||
| State and local | ||||||||
| Foreign | ||||||||
| Total current | ( | ) | ( | ) | ||||
| Deferred: | ||||||||
| Federal | ||||||||
| State and local | ||||||||
| Foreign | ||||||||
| Total deferred | ||||||||
| Benefit from income taxes | $ | $ | ||||||
The following is a reconciliation of the U.S. taxes to the effective income tax rates for the years ended March 31, 2024 and 2023 (U.S. Dollars in thousands):
| Years Ended March 31, | ||||||||
| 2024 | 2023 | |||||||
| Loss on ordinary activities before tax | $ | ( | ) | $ | ( | ) | ||
| Statutory U.S. income tax rate | % | % | ||||||
| Income tax benefit at statutory income tax rate | ||||||||
| Share-based compensation expense recognized for financial statement purposes | ( | ) | ||||||
| Other losses (unrecognized) | ( | ) | ( | ) | ||||
| Utilization of losses not previously benefitted | ||||||||
| Income tax (expense) | $ | ( | ) | $ | ( | ) | ||
As of March 31, 2024, the Company had $
indefinitely but are limited to 80% of taxable income when utilized and $0.4 million of items
deducted for financial statements but not for federal income tax purposes, excluding share-based compensation. As of March 31, 2024 and
2023, the Company had U.S. deferred tax assets of $
| F-33 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 14. INCOME TAXES (Cont’d)
The following is a reconciliation of the U.K. taxes to the effective income tax rates for the years ended March 31, 2024 and 2023 (U.S. Dollars in thousands):
| Years Ended March 31, | ||||||||
| 2024 | 2023 | |||||||
| Loss on ordinary activities before tax | $ | ( | ) | $ | ( | ) | ||
| Statutory U.K. income tax rate | % | % | ||||||
| Loss at statutory income tax rate | ||||||||
| Change from increase in deferred income tax rate | ||||||||
| Losses for which no benefit was taken | ( | ) | ||||||
| Derecognition of deferred tax assets | ( | ) | ||||||
| Foreign currency effect and other | ( | ) | ||||||
| Income tax benefit | $ | $ | ||||||
Research and development credit receivables of $0.2 million was included in prepaid expenses and other receivables on the consolidated statement of financial position as of March 31, 2023. The receivable was collected in July 2023.
The following is a reconciliation of financial statement income (loss) to tax basis income (loss) (in thousands):
| Years Ended March 31, | ||||||||||||||||||||||||||||||||
| 2024 | 2023 | |||||||||||||||||||||||||||||||
| United States |
BVI | United Kingdom |
Total | United States |
BVI | United Kingdom | Total | |||||||||||||||||||||||||
| Pre-tax loss | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||||
| Share-based compensation expense for financial statement purposes for which no benefit was taken | ||||||||||||||||||||||||||||||||
| Loss for which no benefit was taken | ||||||||||||||||||||||||||||||||
| Losses not subject to tax | ||||||||||||||||||||||||||||||||
| Utilization of losses not previously benefitted | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||
| Other | ||||||||||||||||||||||||||||||||
| Taxable income (loss) | $ | $ | $ | ( | ) | $ | ( | ) | $ | $ | $ | ( | ) | $ | ( | ) | ||||||||||||||||
As of March 31, 2024 and 2023, the Company’s deferred tax assets and liabilities in the U.K. consisted of the effects of temporary differences attributable to the following (U.S. Dollars in thousands):
| As of March 31, | ||||||||
| 2024 | 2023 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss | $ | ( | ) | $ | ( | ) | ||
| Deferred tax asset (unrecognized) | ||||||||
| Deferred tax asset | ( | ) | ||||||
| Deferred tax liabilities: | ||||||||
| In-process research and development | ||||||||
| Deferred tax liability | ||||||||
| Net deferred tax liability | $ | $ | ||||||
| F-34 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 14. INCOME TAXES (Cont’d)
iOx generated
As of March 31, 2024 and 2023, iOx had a net deferred tax liability of
nil
There is no expiration date for accumulated tax losses in the U.K. entities.
NOTE 15. CAPITAL STOCK AND RESERVES
Authorized ordinary shares: Unlimited number of Portage ordinary shares without par value.
| 1. | The following is a roll-forward of Portage’s ordinary shares for the years ended March 31, 2024 and 2023: |
| Years Ended March 31, | ||||||||||||||||
| 2024 | 2023 | |||||||||||||||
| Ordinary Shares |
Amount | Ordinary Shares |
Amount | |||||||||||||
| In 000’ | In 000’$ | In 000’ | In 000’$ | |||||||||||||
| Balance, beginning of year | $ | $ | ||||||||||||||
| Shares issued under Registered Direct Offering, net of issue costs | ||||||||||||||||
| Shares issued under ATM, net of issue costs | ||||||||||||||||
| Shares issued or accrued for services | ||||||||||||||||
| Shares issued pursuant to distribution of restricted stock units | ||||||||||||||||
| Shares issued in Tarus acquisition | ||||||||||||||||
| Shares issued in iOx exchange | ||||||||||||||||
| Excess of non-controlling interest acquired over consideration – iOx | ||||||||||||||||
| Shares issued to Lincoln for commitment fee under Committed Purchase Agreement | ||||||||||||||||
| Purchase of shares issued under Committed Purchase Agreement, net of issue costs | ||||||||||||||||
| Balance, end of year | $ | $ | ||||||||||||||
| F-35 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 15. CAPITAL STOCK AND RESERVES (Cont’d)
Portage filed a shelf registration statement with the SEC in order to sell ordinary shares, debt securities, warrants and units in one or more offerings from time to time, which became effective on March 8, 2021 (“March 2021 Registration Statement”). In connection with the March 2021 Registration Statement, Portage has filed with the SEC:
| · | a base prospectus, which covered the offering, issuance and sale by Portage of up to $200 million in the aggregate of the securities identified above from time to time in one or more offerings; |
| · | a prospectus supplement, which covered the offer, issuance and sale by Portage in its ATM offering of up to a maximum aggregate offering
price of $ |
| · | a prospectus supplement dated June 24, 2021, for the offer, issuance and sale by Portage of ordinary shares for gross proceeds
of approximately $ |
| · | a prospectus supplement dated August 19, 2022, for the resale of up to $ million in ordinary shares that Portage may sell from time to time to Lincoln and an additional shares that were issued to Lincoln; and |
| · | a prospectus supplement dated September 29, 2023 for the offer, issuance and sale by Portage in a registered direct public offering
through H.C. Wainwright & Co., the placement agent, to an institutional and accredited investor of (i) ordinary shares at
a purchase price of $ |
During Fiscal 2022, the Company sold ordinary shares under the
ATM program, generating gross proceeds of approximately $
The Company has issued ordinary shares in connection with the acquisition of Tarus Therapeutics, Inc. and in connection with the Tarus Therapeutics, Inc.’s acquisition it may issue additional ordinary shares. See Note 9, “Acquisition of Tarus,” for a further discussion.
On July 18, 2022, the Company entered into the iOx Share Exchange Agreement
under which it exchanged ordinary shares of the Company for the remaining minority interest of
| F-36 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 15. CAPITAL STOCK AND RESERVES (Cont’d)
On July 6, 2022, the Company entered into a Purchase Agreement (the “Committed Purchase Agreement”) with Lincoln, under which it may require Lincoln to purchase ordinary shares of the Company having an aggregate value of up to $30 million (the “Purchase Shares”) over a period of 36 months. Upon execution of the Committed Purchase Agreement, the Company issued to Lincoln ordinary shares, representing a 3% commitment fee. Pursuant to the Committed Purchase Agreement, Lincoln will be obligated to purchase the Purchase Shares in three different scenarios that are based on various market criteria and share amounts. The Company has the right to terminate the Committed Purchase Agreement for any reason, effective upon one business day prior written notice to Lincoln. Lincoln has no right to terminate the Committed Purchase Agreement. The requirement that Lincoln must make a purchase will be suspended based on various criteria such as there not being an effective registration statement for Lincoln to be able to resell the ordinary shares it is committed to purchase and market criteria such as the Company continuing to be Depository Trust Company eligible, among other things. The Committed Purchase Agreement does not impose any financial or business covenants on the Company, and there are no limitations on the use of proceeds. The Company may raise capital from other sources in its sole discretion; provided, however, that the Company shall not enter into any similar agreement for the issuance of variable priced equity-like securities until the three-year anniversary of the date of the Committed Purchase Agreement, excluding, however, an ATM transaction with a registered broker-dealer, which includes any sales under the Sales Agreement with Cantor Fitzgerald.
During Fiscal 2023, the Company sold ordinary shares to Lincoln
under the Committed Purchase Agreement for net proceeds totaling approximately $
During Fiscal 2022, the Company commenced an ATM program, and it sold
ordinary shares, generating gross proceeds of approximately $
During Fiscal 2023, the Company sold ordinary shares under the
ATM program, generating net proceeds of approximately $
During Fiscal 2024, the Company sold ordinary shares under the
ATM program, generating net proceeds of approximately $
The Company’s March 2021 Registration Statement expired on February 24, 2024. In order to issue additional shares under its ATM program or the Committed Purchase Agreement (as defined below) in the future, the Company would be required to file a new registration statement, which must be declared effective by the SEC prior to use, and to file a prospectus supplement related to the ATM program and the Committed Purchase Agreement, as the case may be.
Furthermore, the ATM program and the Committed Purchase Agreement with
Lincoln are generally limited based on, among other things, the Company’s Nasdaq trading volume. Under the Baby Shelf Rule, the
amount of funds the Company can raise through primary public offerings of securities in any 12-month period using a registration statement
on Form F-3 is limited to one-third of the aggregate market value of the ordinary shares held by the Company’s non-affiliates, which
limitation may change over time based on its stock price, number of ordinary shares outstanding and the percentage of ordinary shares
held by non-affiliates. Accordingly, the Company is limited by the Baby Shelf Rule as of the filing of this Form 20-F, until such time
as its non-affiliate public float exceeds $
On September 29, 2023, the Company entered into the Purchase Agreement with an institutional and accredited investor in connection with the Registered Direct Offering and the Private Placement. The Offerings closed on October 3, 2023.
See Note 13, “Warrant Liability,” for a discussion of the Registered Direct Offering.
| F-37 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 15. CAPITAL STOCK AND RESERVES (Cont’d)
| 2. | Nature and Purpose of Reserves |
| (a) | Stock Option Reserve |
The stock option reserve reflects the reserve of compensation expense recognized over the vesting period based upon the grant date fair value of the Company’s equity settled grants calculated in accordance with IFRS 2, “Share-based Payment”. See Note 16, “Stock Option Reserve,” below for a further discussion.
| (b) | Accumulated Other Comprehensive Income (Loss) |
| As of March 31, | ||||||||
| (In thousands) | 2024 | 2023 | ||||||
| Balance, beginning of year | $ | ( | ) | $ | ||||
| Investment in public company at FVTOCI - net change in fair value | ( | ) | ||||||
| Derecognition of investment in public company at FVTOCI | ||||||||
| Investment in associate at FVTPL - net change in fair value | ( | ) | ||||||
| Investment in associate at FVTPL - recognition of net change in fair value | ( | ) | ||||||
| Other investments - derecognition of investment at FVTOCI | ||||||||
| Balance, end of year | $ | $ | ( | ) | ||||
| (a) | The following table provides the activity for the Company’s stock option reserve for the years ended March 31, 2024 and 2023: |
| Years Ended March 31, | ||||||||||||||||
| 2024 | 2023 | |||||||||||||||
| (In thousands) | Non-Controlling Interest | Stock Option Reserve | Non-Controlling Interest | Stock Option Reserve | ||||||||||||
| Balance, beginning of year | $ | $ | $ | $ | ||||||||||||
| Share-based compensation expense | ||||||||||||||||
| Settled in iOx exchange | ( | ) | ||||||||||||||
| Balance, end of year | $ | $ | $ | $ | ||||||||||||
Stock Options and Restricted Stock Units
On June 25, 2020, at the annual meeting of shareholders, the Company’s incentive stock option plan (the “2020 Stock Option Plan”) was approved, which authorized the Company’s directors to fix the option exercise price and to issue stock options under the plan as appropriate. The Company’s 2020 Stock Option Plan was a 10% rolling stock option plan under which the Company’s directors were authorized to grant up to a maximum of 10% of the issued and outstanding ordinary shares on the date of grant.
Effective January 13, 2021, the Company amended and restated its 2020 Stock Option Plan to permit the grant of additional types of equity compensation securities, including restricted stock units (“RSUs”) and dividend equivalent rights (the “2021 Equity Incentive Plan”).
| F-38 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 16. STOCK OPTION RESERVE (Cont’d)
Amended and Restated 2021 Equity Incentive Plan and Grants of Stock Options and Restricted Stock Units
On January 19, 2022, the Board unanimously approved the Amended and Restated 2021 Equity Incentive Plan (the “Amended and Restated 2021 Equity Incentive Plan”). The Amended and Restated 2021 Equity Incentive Plan provides for:
| (1) | An increase of aggregate number of ordinary shares available for awards to 2,001,812, which is equal to 15% of the issued and outstanding ordinary shares of the Company as of January 19, 2022 subject to discretionary annual increases (on a cumulative basis) as may be approved by the Board in future years by a number of ordinary shares not to exceed an additional 5% of the aggregate number of shares then outstanding; |
| (2) | The authorization of incentive stock options under the Amended and Restated 2021 Equity Incentive Plan; and |
| (3) | The provision of dividend equivalent rights to be issued when authorized. |
Pursuant to the Amended and Restated 2021 Equity Incentive Plan, on January
19, 2022, the Company granted an aggregate of
Additionally, the Company granted
On February 15, 2022, James Mellon, Linda Kozick and Mark Simon were appointed
to the Board. Mr. Mellon owned approximately 23.9% of the Company’s outstanding shares at that date. Additionally, Mr. Mellon had
previously served as a member of the Board from 2016 to August 14, 2020. On February 15, 2022, in connection with the appointments, each
of these directors were granted
On June 8, 2022, the Company granted
| F-39 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 16. STOCK OPTION RESERVE (Cont’d)
On July 27, 2022, the Company granted
On March 30, 2023, the Board unanimously approved to increase the maximum number of ordinary shares reserved for issuance under the Amended and Restated 2021 Equity Incentive Plan by shares, which represented % of ordinary shares outstanding on March 29, 2023, to shares.
On March 30, 2023, the Company granted an aggregate of stock options exercisable at a price of $ per share, representing the average price of the shares on the grant date, which expire on , to various directors, officers and a consultant of the Company. options to purchase ordinary shares, totaling , were granted to each non-executive member of the Board and vest on the first anniversary of the grant date. A total of stock options were granted to employees (including Mr. Walters, who is Chairman of the Board of Directors), and a consultant, and such stock options vest ratably on each of the first four annual anniversaries of the grant date. The balance of stock options were also granted to a consultant, which was fully vested as of the grant date.
As of March 31, 2024, shares had been issued (including shares bought back in cashless exercise), shares were reserved for awards previously granted, shares were issued or bought back into treasury and shares were available for future awards under the Amended and Restated 2021 Equity Incentive Plan.
Following are the weighted average assumptions used in connection with the January 13, 2021 option grants, with respect to the Company’s Amended and Restated 2021 Equity Incentive Plan:
| Assumption | Unvested Options | Vested Options | ||
| Risk free interest rate | % | % | ||
| Expected dividend | Nil | Nil | ||
| Expected volatility | % | % | ||
| Expected life (in years) | ||||
| Fair value of Portage stock | US$ | US$ |
Following are the weighted average assumptions used in connection with the January 19, 2022 option grants, with respect to the Company’s Amended and Restated 2021 Equity Incentive Plan:
| Assumption | Unvested Options | |
| Risk free interest rate | % | |
| Expected dividend | Nil | |
| Expected volatility | % | |
| Expected life (in years) | ||
| Fair value of Portage stock | US$ |
Following are the weighted average assumptions used in connection with the February 15, 2022 option grants, with respect to the Company’s Amended and Restated 2021 Equity Incentive Plan:
| Assumption | Unvested Options | |
| Risk free interest rate | % | |
| Expected dividend | Nil | |
| Expected volatility | % | |
| Expected life (in years) | ||
| Fair value of Portage stock | US$ |
| F-40 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 16. STOCK OPTION RESERVE (Cont’d)
The following is the weighted average assumptions used in connection with the June 8, 2022 option grant with respect to the Company’s Amended and Restated 2021 Equity Incentive Plan:
| Assumption | Unvested Options | |
| Risk free interest rate | % | |
| Expected dividend | Nil | |
| Expected volatility | % | |
| Expected life (in years) | ||
| Fair value of Portage option | US$ |
The following is the weighted average assumptions used in connection with the July 27, 2022 option grant with respect to the Company’s Amended and Restated 2021 Equity Incentive Plan:
| Assumption | Unvested Options | |
| Risk free interest rate | % | |
| Expected dividend | Nil | |
| Expected volatility | % | |
| Expected life (in years) | ||
| Fair value of Portage option | US$ |
The following is the weighted average assumptions used in connection with the March 30, 2023 option grants with respect to the Company’s Amended and Restated 2021 Equity Incentive Plan:
| Unvested Options | ||||||||
| Assumption | (1 Year Grants) | (4 Year Grants) | ||||||
| Risk free interest rate | % | % | ||||||
| Expected dividend | Nil | Nil | ||||||
| Expected volatility | % | % | ||||||
| Expected life (in years) | ||||||||
| Fair value of Portage option | US$ | US$ | ||||||
| (b) | The changes in the number of options issued for the years ended March 31, 2024 and 2023 were: |
| Years Ended March 31, | ||||||||||||||||
| 2024 | 2023 | 2024 | 2023 | |||||||||||||
| PBI Amended and Restated 2021 Equity Incentive Plan |
iOx Option Plan (Subsidiary Plan) |
|||||||||||||||
| Balance, beginning of year | ||||||||||||||||
| Granted | ||||||||||||||||
| Expired or forfeited | ( | ) | ( | ) | ||||||||||||
| Balance, end of year | ||||||||||||||||
| Exercisable, end of year | ||||||||||||||||
| (c) | The following is the weighted average exercise price and the remaining contractual life for outstanding options by plan as of March 31, 2024 and 2023: |
| As of March 31, | ||||||||||||||||
| 2024 | 2023 | 2024 | 2023 | |||||||||||||
| PBI Amended and Restated 2021 Equity Incentive Plan |
iOx Option Plan (Subsidiary Plan) |
|||||||||||||||
| Weighted average exercise price | $ | $ | $ | |||||||||||||
| Weighted average remaining contractual life (in years) | — | — | ||||||||||||||
| F-41 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 16. STOCK OPTION RESERVE (Cont’d)
The vested options can be exercised at any time in accordance with the applicable option agreement. The exercise price was greater than the market price for all options outstanding as of March 31, 2024 and 2023, except for vested options and unvested options as of March 31, 2023.
The Company recorded approximately $ million, $ million and $ million of share-based compensation expense with respect to the Amended and Restated 2021 Equity Incentive Plan in the years ended March 31, 2024, 2023 and 2022, respectively.
Basic earnings per share (“EPS”) is calculated by dividing the net loss attributable to ordinary equity holders of the Company by the weighted average number of ordinary shares outstanding during the period.
Diluted EPS is calculated by dividing the net loss attributable to ordinary equity holders of the Company by the weighted average number of ordinary shares outstanding during the period plus the weighted average number of ordinary shares that would be issued on conversion of all the dilutive potential ordinary shares into ordinary shares. Shares issuable under Pre-Funded Warrants are considered outstanding for this purpose.
The calculation of Basic and Diluted EPS reflects the Pre-Funded Warrants as outstanding shares.
The following table reflects the loss and share data used in the basic and diluted EPS calculations (U.S. Dollars in thousands, except per share amounts):
| Years Ended March 31, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Numerator (in 000’$) | ||||||||||||
| Net loss attributable to owners of the Company | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Denominator (in 000’) | ||||||||||||
| Weighted average number of shares – Basic and Diluted | ||||||||||||
| Basic and diluted loss per share | $ | ) | $ | ) | $ | ) | ||||||
The inclusion of the Company’s share purchase warrants (other than Pre-Funded Warrants, as described above), stock options and RSUs in the computation of diluted loss per share would have an anti-dilutive effect on loss per share and are therefore excluded from the computation. Consequently, there is no difference between basic loss per share and diluted loss per share for the years ended March 31, 2024, 2023 and 2022. The following table reflects the Company’s outstanding securities by year that would have an anti-dilutive effect on loss per share and, accordingly, were excluded from the calculation.
| As of March 31, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Warrants | ||||||||||||
| Stock options | ||||||||||||
| Restricted stock units | ||||||||||||
| F-42 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 18. COMMITMENTS AND CONTINGENT LIABILITIES
Effective March 15, 2022, iOx entered into a Master Services Agreement
(the “MSA”) with Parexel International (IRL) Limited (“Parexel”) under which Parexel agreed to act as clinical
service provider (CRO) pursuant to a work order (“Work Order”) effective June 1, 2022. Pursuant to such Work Order, Parexel
will operate a Phase 2 trial of IMM60 and pembrolizumab in advanced melanoma and non-small lung cancer. The MSA provides for a five-year
term, and the Work Order provides for a term to be ended upon the completion of the services required. The budget provides for service
fees and pass-through expenses and clinical sites totaling $11.5 million. During Fiscal 2023, the Company executed two change orders resulting
in a $0.6 million increase in the overall estimated budgeted costs. As a result of the Company’s decision to discontinue the development
with respect to this program, the Company provided a notice of termination under the MSA. The Company is in negotiations to settle all
obligations with respect to the MSA. The Company reflected $
On March 1, 2023, Tarus entered into a clinical service agreement with
Fortrea Inc. (formerly Labcorp Drug Development Inc.), a third-party CRO. The term of the agreement is through the earlier of August 14,
2025 or the completion of provision of services and the payment of contractual obligations. The budgeted costs for the services to be
provided is approximately $
Stimunity Convertible Note
On September 12, 2022, the Company funded €
Committed Purchase Agreement
On July 6, 2022 (the “Signing Date”), the Company entered
into the Committed Purchase Agreement with Lincoln, pursuant to which the Company may require Lincoln to purchase ordinary shares having
an aggregate value of up to $
Upon execution of the Committed Purchase Agreement, the Company
issued to Lincoln ordinary
shares, representing a 3% commitment fee valued at $
| F-43 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 18. COMMITMENTS AND CONTINGENT LIABILITIES (Cont’d)
The Committed Purchase Agreement does not impose any financial or business covenants on the Company and there are no limitations on the use of proceeds received by the Company from Lincoln. The Company may raise capital from other sources in its sole discretion; provided, however, that the Company shall not enter into any similar agreement for the issuance of variable priced equity-like securities until the three-year anniversary of the Signing Date, excluding, however, an at-the-market transaction with a registered broker-dealer.
In connection with the Committed Purchase Agreement, the Company and Lincoln entered into a Registration Rights Agreement, dated July 6, 2022 (the “Registration Rights Agreement”). Pursuant to the Registration Rights Agreement, the Company agreed to file with the SEC the prospectus supplement to the Company’s shelf registration statement pursuant to Rule 424(b) for the purpose of registering for resale the ordinary shares to be issued to Lincoln under the Committed Purchase Agreement. The prospectus supplement was filed on August 19, 2022.
Access to the Committed Purchase Agreement with Lincoln is generally limited based on, among other things, the Company’s Nasdaq trading volume. Furthermore, under the Baby Shelf Rule, the amount of funds the Company can raise through primary public offerings of securities in any 12-month period using a registration statement on Form F-3 is limited to one-third of the aggregate market value of the ordinary shares held by the Company’s non-affiliates, which limitation may change over time based on the Company’s stock price, number of ordinary shares outstanding and the percentage of ordinary shares held by non-affiliates. The Company is therefore limited by the Baby Shelf Rule as of the filing of this Form 6-K, until such time as its non-affiliate public float exceeds $75 million.
The Company is obligated under the Merger Agreement and the iOx Share Exchange Agreement to pay certain third-party earnouts based on the achievement of certain milestones. See Note 9, “Acquisition of Tarus,” and Note 19, “Related Party Transactions – Share Exchange Agreement – iOx,” respectively, for further discussions.
NOTE 19. RELATED PARTY TRANSACTIONS
SalvaRx Acquisition
Two of the Company’s directors are also directors of SalvaRx Group plc, a company which owns approximately 4.1% of the Company’s issued and outstanding ordinary shares as of March 31, 2024.
Investments
The Company has entered into related party transactions and certain services agreements with its investees. Key management personnel of the Company have also entered into related party transactions with investees. Key management personnel are those persons having the authority and responsibility for planning, directing and controlling the activities of the Company, including directors and senior management of the Company.
The following subsidiaries and associates are considered related parties:
| (a) | Stimunity. The Chief Executive Officer (“CEO”) of Portage is one of three members of the board of directors of Stimunity (see Note 6, “Investment in Associate and Convertible Note Receivable,” and Note 18, “Commitments and Contingent Liabilities – Stimunity Convertible Note”). The Company wrote-off its investment in Stimunity to nil in the Fiscal 2024 period. |
| (b) | iOx. Upon execution of the iOx Share Exchange on July 18, 2022, the non-Portage director resigned from the iOx board leaving two Portage insiders as directors. The CEO of Portage is also the CEO of iOx, and the management team of Portage comprises the management team of iOx. See below for a discussion of the Company’s purchase of the non-controlling interest in iOx through its wholly-owned subsidiary SalvaRx. |
| (c) | Saugatuck. The Chairman and CEO of the Company is the sole director of Saugatuck. Saugatuck is 70% owned by the Company and is controlled by Portage. |
| F-44 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 19. RELATED PARTY TRANSACTIONS (Cont’d)
| (d) | Intensity. The CEO of Portage previously served as a
part-time officer of Intensity until becoming a consultant in 2023. Additionally, Intensity
provided services (primarily rent) to Portage through April 2023. For the years ended March
31, 2024, 2023 and 2022, the Company paid $ |
| (e) | Portage Development Services Inc. PDS provides human resources and other services to each operating subsidiary of Portage through shared services agreements. |
Transactions between the parent company and its subsidiaries, which are related parties, have been eliminated in consolidation and are not disclosed in this note.
On September 8, 2021,
Share Exchange Agreement – iOx
On July 18, 2022, the Company and SalvaRx entered into a Share Exchange Agreement (the “Share Exchange Agreement”) with each of the minority shareholders of iOx (the “Sellers”) resulting in the acquisition of the outstanding non-controlling ownership interest (approximately 22%) of iOx, which is developing the iNKT engager platform. The Company followed IFRS 3 and IAS 27, “Separate Financial Statements,” (which substantially replaced IAS 3) to account for this transaction. The Company achieved control of iOx, as defined, on January 8, 2019 upon the completion of the SalvaRx Acquisition. Further transactions whereby the parent entity acquires further equity interests from non-controlling interests, or disposes of equity interests but without losing control, are accounted for as equity transactions (i.e., transactions with owners in their capacity as owners). As such:
| · | the carrying amounts of the controlling and non-controlling interests are adjusted to reflect the changes in their relative interests in the subsidiary; |
| · | any difference between the amount by which the non-controlling interests is adjusted and the fair value of the consideration paid or received is recognized directly in equity and attributed to the owners of the parent; and |
| · | there is no consequential adjustment to the carrying amount of goodwill, and no gain or loss is recognized in profit or loss. |
The Company now owns the worldwide rights to its small molecule iNKT engagers, including lead programs PORT-2 and PORT-3. Under the terms of the Share Exchange Agreement, each Seller sold to the Company, and the Company acquired from each Seller, legal and beneficial ownership of the number of iOx shares held by each Seller, free and clear of any share encumbrances, in exchange for the issuance in an aggregate of Portage ordinary shares to be allocated among the Sellers based upon their relative ownership. As a result of the Share Exchange Agreement, the Company owns 100% of the issued and outstanding shares of iOx through its wholly-owned subsidiary, SalvaRx.
| F-45 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 19. RELATED PARTY TRANSACTIONS (Cont’d)
As additional consideration for the sale of the iOx shares to the Company under the Share Exchange Agreement, the Sellers shall have the contingent right to receive additional shares (“Earnout Shares”) from the Company having an aggregate value equal to $25 million calculated at the Per Share Earnout Price (as defined in the Share Exchange Agreement) upon the achievement of certain milestones defined as the dosing of the first patient in a Phase 3 clinical trial for either PORT-2 (IMM60 iNKT cell activator/engager) or PORT-3 (PLGA-nanoparticle formulation of IMM60 combined with a NY-ESO-1 peptide vaccine). The Company shall have the option, in its sole and absolute discretion, to settle the Earnout Shares in cash. The Company followed IFRS 3 and IAS 32, “Financial Instruments: Presentation,” to account for the fair value of the Earnout Shares. The principal assumptions for determining the fair value include the timing of development events, the probabilities of success and the discount rate used. The fundamental principle of IAS 32 is that a financial instrument should be classified as either a financial liability or an equity instrument according to the substance of the contract, not its legal form, and the definitions of financial liability and equity instrument. A financial instrument is an equity instrument if, and only if, both conditions (a) and (b) below are met:
| (a) | the instrument includes no contractual obligation to deliver cash or another financial asset to another entity, and |
| (b) | if the instrument will or may be settled in the Company’s own equity instruments, it is either: |
| (i) | a non-derivative that includes no contractual obligation for the Company to deliver a variable number of its own equity instruments; or |
| (ii) | a derivative that will be settled only by the issuer exchanging a fixed amount of cash or another financial asset for a fixed number of its own equity instruments. |
When a derivative financial instrument gives one party a choice over how it is settled (for instance, the Company or the holder can choose settlement net in cash or by exchanging shares for cash), it is a financial asset or a financial liability unless all of the settlement alternatives would result in it being an equity instrument. The financial instrument includes the exclusive right of the Company to settle the obligation with cash or equity and, accordingly, accounted for the fair value of the Earnout Shares as a non-current liability.
The Company originally recorded $5.478 million as the fair value estimate
of the Earnout Shares, which is reflected as deferred obligation - iOx milestone on the consolidated statement of financial position as
of March 31, 2023. The Company determined the fair value of the Earnout Shares at each consolidated statement of financial position date.
Any changes to the fair value were recorded in the Company’s statements of operations and other comprehensive income (loss). The
Company recorded a gain from the change (decrease) in the fair value of the liability of $
Employment Agreements
PDS entered into a Services Agreement with the Company’s CEO effective
December 15, 2021 (the “CEO Services Agreement”). The CEO Services Agreement originally provided for a base salary of $
| F-46 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 19. RELATED PARTY TRANSACTIONS (Cont’d)
Under the CEO Services Agreement, the CEO may terminate his employment with PDS at any time for Good Reason (as defined in the CEO Services Agreement). PDS may terminate the CEO’s employment immediately upon his death, upon a period of disability or without Just Cause (as defined in the CEO Services Agreement). In the event that the CEO’s employment is terminated due to his death or Disability (as defined in the CEO Services Agreement), for Good Reason or without Just Cause, he will be entitled to accrued obligations (accrued unpaid portion of base salary, accrued unused vacation time and any unpaid expenses). Additionally, he may be entitled to Severance Benefits (as defined in the CEO Services Agreement), which include his then current base salary and the average of his annual bonus for the prior two completed performance years, paid over 12 monthly installments. Additionally, the CEO will be entitled to life insurance benefits and medical and dental benefits for a period of 12 months at the same rate the CEO and PDS shared such costs during his period of employment.
Finally, all stock options (and any other unvested equity incentive award) held by the CEO relating to shares of the Company will be deemed fully vested and exercisable on the Termination Date (as defined in the CEO Services Agreement), and the exercise period for such stock options will be increased by a period of two years from the Termination Date.
If the CEO’s employment by PDS is terminated by PDS or any successor entity without Just Cause (not including termination by virtue of the CEO’s death or Disability) or by the CEO for Good Reason within 12 months following the effective date of a Change in Control (as defined in the CEO Services Agreement), then, in addition to paying or providing the CEO with the Accrued Obligations (as defined in the CEO Services Agreement), the Company will provide the following Change in Control Severance Benefits (as defined in the CEO Services Agreement):
| (1) | PDS will pay the base salary continuation benefit for 18 months; |
| (2) | PDS will pay the life insurance benefit for 18 months; |
(3) PDS will pay an additional amount equivalent to the CEO’s target annual bonus calculated using the bonus percentage for the performance year in which the CEO’s termination occurs. This bonus will be paid in 12 equal installments commencing on the first payroll date that is more than 60 days following the date of termination of the CEO’s employment, with the remaining installments occurring on the first day of the month for the 11 months thereafter;
| (4) | PDS will provide the CEO with continued medical and dental benefits, as described above, for 18 months; and |
| (5) | All stock options (and any other unvested equity incentive award) held by the CEO relating to shares of the Company will be deemed fully vested and exercisable on the Termination Date, as defined, and the exercise period for such stock options will be increased by a period of two years from the Termination Date. |
| F-47 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 19. RELATED PARTY TRANSACTIONS (Cont’d)
PDS entered into services agreements (individually, an “Executive
Service Agreement,” and collectively, the “Executive Service Agreements”) with each of the Company’s five other
members of senior management (individually, “Executive” and collectively, “Executives”), three of which are dated
as of December 1, 2021, one of which is dated December 15, 2021 and one of which is dated June 1, 2022. Each of the Executive Services
Agreements provides for an initial term of two years that is automatically renewed for one-year periods (except two of the Executive Services
Agreement, which provides for an initial term of one year and that is automatically renewed for one-year periods). The Executive Services
Agreements initially provide for annual base salaries ranging from $
Two of the Executive Service Agreements were terminated on a voluntary basis during the year ended March 31, 2024.
On December 19, 2022, the Compensation Committee approved executive compensation
(other than for the CEO) for Fiscal Year 2024 for annual base salaries ranging from $
The Executive Services Agreements can be terminated by PDS without Just Cause, by death or Disability, or by the Executive (except one) for Good Reason (each as defined in the respective Executive Services Agreements). In such instances, the Executive Services Agreements provide for the payment of accrued obligations (accrued unpaid portion of base salary, accrued unused vacation time and any unpaid expenses). Additionally, the Executives (except two) are entitled to 50% of base salary plus 50% of average annual bonus earned over the prior two performance years, as well as prevailing life insurance benefits for a period of six months and medical and dental benefits for a period of six months at the prevailing rate PDS and the Executive were sharing such expenses.
Additionally, all stock options (and any other unvested equity incentive award) held by the Executives relating to shares of the Company will be deemed fully vested and exercisable on the Termination Date (as defined in the respective Executive Services Agreements), and the exercise period for such stock options will be increased by a period of two years from the Termination Date.
If an Executive’s employment by PDS is terminated by the Company or any successor entity without Just Cause (not including termination by virtue of the Executive’s death or Disability) or by the Executive (except one) for Good Reason within 12 months following the effective date of a Change in Control (as defined in the respective Executive Services Agreements), then, in addition to paying or providing the Executive with the Accrued Obligations (as defined in the respective Executive Services Agreements), the Company will provide the following Change in Control Severance Benefits (as defined in the respective Executive Services Agreements), except in two cases in which the Executive is entitled to Item (5) and 50% of Items (1) and (3) below:
| (1) | PDS will pay the base salary continuation benefit for 12 months; |
| (2) | PDS will pay the life insurance benefit for 12 months; |
| (3) | The Company will pay an additional amount equivalent to the Executive’s target annual bonus calculated using the bonus percentage for the performance year in which the Executive’s termination occurs. This bonus will be payable in 12 equal installments commencing on the first payroll date that is more than 60 days following the date of termination of the Executive’s employment, with the remaining installments occurring on the first day of the month for the 11 months thereafter; |
| (4) | PDS will provide the Executive with continued medical and dental benefits, as described above, for 12 months; and |
| F-48 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 19. RELATED PARTY TRANSACTIONS (Cont’d)
| (5) | All stock options (and any other unvested equity incentive award) held by the Executive relating to shares of PDS or the Company will be deemed fully vested and exercisable on the Termination Date and the exercise period for such stock options will be increased by a period of two years from the Termination Date. |
The Executive Services Agreements also include customary confidentiality, as well as provisions relating to assignment of inventions. The Executive Services Agreements also includes non-competition and non-solicitation of employees and customers provisions that run during the Executive’s employment with PDS and for a period of one year after termination of employment.
Bonuses & Board Compensation Arrangements
In December 2022, the Board approved
executive performance bonuses, as recommended by the Compensation Committee, totaling $
Effective January 1, 2022, each non-employee Board member is entitled
to receive cash Board fees of $40,000 per annum, payable quarterly in arrears. Additionally, each non-employee Board member is entitled
to an annual grant of 6,900 options to purchase Portage ordinary shares, which would vest the first annual anniversary of the grant date.
The Company incurred Board fees totaling $
Non-employee Board chairpersons are entitled to an annual cash fee of
$30,000, payable quarterly in arrears. In lieu of a non-executive chairperson, the lead director is entitled to an annual cash fee of
$20,000 per annum paid quarterly in arrears.
NOTE 20. FINANCIAL INSTRUMENTS AND RISK MANAGEMENT
The Company’s financial instruments recognized in the Company’s consolidated statements of financial position consist of the following:
Fair value estimates are made at a specific point in time, based on relevant market information and information about financial instruments. These estimates are subject to and involve uncertainties and matters of significant judgment; and therefore, these estimates cannot be determined with precision. Changes in assumptions could significantly affect these estimates.
| F-49 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 20. FINANCIAL INSTRUMENTS AND RISK MANAGEMENT (Cont'd)
The following table summarizes the Company’s financial instruments as of March 31, 2024 and 2023:
| Years Ended March 31, | ||||||||||||||||||||||||
| 2024 | 2023 | |||||||||||||||||||||||
| Amortized Cost |
FVTOCI | FVTPL | Amortized Cost |
FVTOCI | FVTPL | |||||||||||||||||||
| Financial assets | ||||||||||||||||||||||||
| Cash and cash equivalents | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
| Prepaid expenses and other receivables | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
| Convertible note receivable, including accrued interest, net of impairment | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
| Investment in associate | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
| Investment in public company | $ | $ | $ | $ | $ | |||||||||||||||||||
| Years Ended March 31, | ||||||||||||||||
| 2024 | 2023 | |||||||||||||||
| Amortized Cost |
FVTPL | Amortized Cost |
FVTPL | |||||||||||||
| Financial liabilities | ||||||||||||||||
| Accounts payable and accrued liabilities | $ | $ | $ | $ | ||||||||||||
| Warrant liability | $ | $ | $ | $ | ||||||||||||
| Deferred purchase price payable - Tarus | $ | $ | $ | $ | ||||||||||||
| Deferred obligation - iOx milestone | $ | $ | $ | $ | ||||||||||||
A summary of the Company’s risk exposures as it relates to financial instruments are reflected below.
Fair value of Financial Instruments
The Company’s financial assets and liabilities are comprised of cash and cash equivalents, receivables and investments in equities and public entities, accounts payable and accrued liabilities, lease liability, warrant liability, deferred purchase price payable and deferred obligation.
The Company classifies the fair value of these transactions according to the following fair value hierarchy based on the amount of observable inputs used to value the instrument:
| · | Level 1 – Values are based on unadjusted quoted prices available in active markets for identical assets or liabilities as of the reporting date. |
| · | Level 2 – Values are based on inputs, including quoted forward prices for commodities, time value and volatility factors, which can be substantially observed or corroborated in the marketplace. Prices in Level 2 are either directly or indirectly observable as of the reporting date. |
| · | Level 3 – Values are based on prices or valuation techniques that are not based on observable market data. Investments are classified as Level 3 financial instrument. |
Assessment of the significance of a particular input to the fair value measurement requires judgment and may affect the placement within the fair value hierarchy.
| F-50 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 20. FINANCIAL INSTRUMENTS AND RISK MANAGEMENT (Cont’d)
Management has assessed that the fair values of cash and cash equivalents, other receivables and accounts payable approximate their carrying amounts largely due to the short-term maturities of these instruments.
The following methods and assumptions were used to estimate their fair values:
Convertible Note Receivable: The fair value of the Stimunity Convertible
Note receivable denominated in euros at initial recognition is the transaction price for the instrument adjusted for the effect of the
currency translation rate on the reporting date (Level 3) (see Note 16, “Commitments and Contingent Liabilities – Stimunity
Convertible Note”). The Stimunity Convertible Note was initially recorded at $
The Stimunity Convertible Note matured on September 1, 2023 and was not settled. In December 2023, the Company completed a transfer of the investment in Stimunity and the Stimunity Convertible Note to iOx. Simultaneously, the Convertible Note was converted into Class A shares of Stimunity, which increased the Company’s interest in Stimunity to %. Accordingly, the Convertible Note Receivable was included in the investment in associate, described below.
Investment in Associate: The fair value of the Stimunity investment
was determined based on an IAS 36 fair value analysis evaluating the likelihood of various scenarios given the then-current market conditions,
the increasing cost of capital and development delays associated with Stimunity’s lack of liquidity (Level 3). The Company recorded
a provision of impairment of $
Investment in Public Company: Upon Intensity’s IPO effective June 30, 2023, the fair value of the investment was determined by the quoted market price (Level 1).The Company sold this investment in the year ended March 31, 2024.
Warrant Liability: The fair value is estimated using a Black-Scholes model and in certain cases, a Monte Carlo simulation (Level 3) (see Note 13, “Warrant Liability”).
| F-51 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 20. FINANCIAL INSTRUMENTS AND RISK MANAGEMENT (Cont'd)
Deferred Purchase Price Payable - Tarus: The fair value is the
estimated value of a future contingent obligation based upon a fair value analysis performed in accordance with IFRS 3 at acquisition
date, adjusted at each reporting date for any change in fair value (Level 3) (see Note 9, “Acquisition of Tarus”). The fair
value was determined using the Income Approach and was primarily based upon the analysis on the Tarus clinical plan, the timing of development
events and the probabilities of success determined primarily based upon empirical third-party data and Company experience, as well as
the relevant cost of capital. These are primarily unobservable inputs within Level 3 of the fair value hierarchy, which can be further
impacted by other unobservable inputs such as market changes and unknown timing delays. The Company is also impacted by its liquidity
constraints. The deferred purchase price payable is correlated to the value of the Tarus in-process research and development and will
increase or decrease based upon the amounts realized, if any, on the sale or licensing of the IPR&D. The Company has determined the
probability of events based upon the clinical work performed to date, the current capital and biotech markets and the Company’s
remaining liquidity. During the year ended March 31, 2024, the Company fully impaired its investment in Tarus IPR&D and accordingly,
wrote-off the related deferred purchase price payable. The Company recorded a gain from the change (decrease) in the fair value of the
liability of $
Deferred Obligation - iOx Milestone: The fair value is the estimated
value of a future contingent obligation based upon a fair value analysis performed in accordance with IFRS 3 as of July 18, 2022, the
date of the Share Exchange Agreement, adjusted at each reporting date for any change in fair value (Level 3) (see Note 19, “Related
Party Transactions – Share Exchange Agreement – iOx”). The fair value was determined using the Income Approach and based
on factors including the clinical plan, the timing of development events and the probabilities of success determined primarily based upon
empirical third-party data and Company experience, as well as the relevant cost of capital. In connection with the decision by the Company
to pause further clinical development of its iNKT program, the Company performed an impairment analysis which resulted in an impairment
loss of $
Fair Value Hierarchy
The investment in public company (Intensity) was transferred from Level 3 to Level 1 of the fair value hierarchy for the year ended March 31, 2024 as the result of Intensity’s IPO. For Fiscal 2023, the fair value of the investment was determined based on an IAS 36 impairment analysis after determining there were external indications of impairment (Level 3). See Note 7, “Investment in Public Company,” for a further discussion.
The Company’s financial instruments are exposed to certain financial risks: Credit Risk, Liquidity Risk and Foreign Currency Risk.
Credit Risk
Credit risk is the risk of loss associated with a counterparty’s inability to fulfill its payment obligations. The credit risk is attributable to various financial instruments, as noted below. The credit risk is limited to the carrying value as reflected in the Company’s consolidated statements of financial position.
Cash and cash equivalents: Cash and cash equivalents comprise cash on hand and amounts invested in underlying treasury and money market funds that are readily convertible to a known amount of cash with three months or less from date of acquisition and are subject to an insignificant risk of change in value. As of March 31, 2024 and 2023, cash equivalents was comprised of a money market account with maturities less than 90 days from the date of purchase. Cash and cash equivalents are held with major international financial institutions and therefore the risk of loss is minimal.
| F-52 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 20. FINANCIAL INSTRUMENTS AND RISK MANAGEMENT (Cont'd)
Liquidity Risk
Liquidity risk is the risk that the Company will encounter difficulty in satisfying financial obligations as they become due.
The Company’s approach to managing liquidity is to ensure, as far as possible, that it will have sufficient liquidity to meet its liabilities when due, under both normal and stressed conditions without incurring unacceptable losses or risking harm to the Company’s reputation. The Company holds sufficient cash and cash equivalents to satisfy current obligations under accounts payable and accruals.
The Company monitors its liquidity position regularly to assess whether it has the funds necessary to meet its operating needs and needs for investing in new projects.
As a biotech company at an early stage of development and without significant internally generated cash flows, there are inherent liquidity risks, including the possibility that additional financing may not be available to the Company, or that actual drug development expenditures may exceed those planned. The current uncertainty in global capital markets could have an impact on the Company’s future ability to access capital on terms that are acceptable to the Company. There can be no assurance that required financing will be available to the Company. See Note 2, “Going Concern,” and Note 15, “Capital Stock and Reserves,” for a discussion of the Company’s share offering and Note 18, “Commitments and Contingent Liabilities – Committed Purchase Agreement,” for a further discussion.
Foreign Currency Risk
While the Company operates in various jurisdictions, substantially all of the Company’s transactions are denominated in the U.S. Dollar, except the deferred tax liability in the U.K. settleable in British pound sterling and the Stimunity Convertible Note receivable settleable in euros.
NOTE 21. CAPITAL MANAGEMENT
The Company considers the items included in shareholders’ equity
as capital. The Company had accounts payable and accrued liabilities of approximately $
The Company manages the capital structure and makes adjustments to it in light of changes in economic conditions and the risk characteristics of the underlying assets.
As of March 31, 2024, shareholders’ equity attributable to the owners of the Company was approximately $.0 million (approximately $.0 million as of March 31, 2023).
The Company is not subject to any externally imposed capital requirements and does not presently utilize any quantitative measures to monitor its capital. There have been no changes to the Company’s approach to capital management during the years ended March 31, 2024 and 2023.
| F-53 |
PORTAGE BIOTECH INC.
Notes to Consolidated Financial Statements
(U.S. Dollars)
March 31, 2024 and 2023
NOTE 22. NON-CONTROLLING INTEREST
| (In thousands) | iOx | Saugatuck and subsidiary |
Total | |||||||||
| Non-controlling interest as of April 1, 2022 | $ | ( | ) | $ | ||||||||
| Net income (loss) attributable to non-controlling interest | ( | ) | ( | ) | ||||||||
| Purchase of non-controlling interest pursuant to Share Exchange Agreement | ( | ) | ( | ) | ||||||||
| Non-controlling interest as of March 31, 2023 | ( | ) | ( | ) | ||||||||
| Net loss attributable to non-controlling interest | ( | ) | ( | ) | ||||||||
| Non-controlling interest as of March 31, 2024 | $ | ( | ) | ( | ) |
On September 8, 2021, the Company, through SalvaRx, completed a settlement of loans (including interest) to and receivables from iOx for services rendered in exchange for 23,772 ordinary shares of iOx at a price of £162. On July 18, 2022, the Company completed the acquisition of the remaining non-controlling interest in iOx, by issuing 1,070,000 shares of its ordinary shares and assuming certain milestone obligations. See Note 19, “Related Party Transactions – Share Exchange Agreement – iOx,” for a discussion of the Company’s purchase of the balance of the non-controlling interest in iOx.
Saugatuck and subsidiary includes Saugatuck and its wholly-owned subsidiary, Saugatuck Rx LLC.
NOTE 23. EVENT AFTER THE STATEMENT OF FINANCIAL POSITION DATE
| (a) | Board Resignations and Appointments |
On April 25, 2024, Mark Simon resigned all of his positions on the Board of Directors, and on April 26, 2024, Linda Kozick and Dr. Robert Glassman resigned all of their positions on the Board of Directors. On April 30, 2024, Dr. Jean -Christophe Renondin and Dr. Justin Stebbing were elected to the Board.
| (b) | Exercise of Pre-Funded Warrrants |
On May 29, 2024, Armistice Capital Master Fund Ltd. exercised its Pre-Funded
Warrants at an exercise price of $
| (c) | 1-for-20 Reverse Stock Split |
The Company’s Board of Directors approved a reverse stock split of its ordinary shares at a ratio of 1-for-20. Beginning with the opening of trading on August 15, 2024, the Company’s ordinary shares are expected to begin trading on Nasdaq on a split-adjusted basis under the existing trading symbol “PRTG”.
The reverse stock split is being implemented to increase the per share trading price of the Company’s ordinary shares for the purpose of ensuring a share price high enough to comply with the minimum $1.00 bid price requirement for continued listing on Nasdaq.
As a result of the reverse stock split, every twenty (20) pre-split ordinary shares will be converted into one (1) post-split ordinary share. Any fractional shares resulting from the reverse stock split will be rounded up to the nearest whole post-split ordinary share. The reverse stock split affects all shareholders uniformly and will not alter any shareholder’s percentage interest in the Company’s ordinary shares, except for adjustments that may result from the treatment of fractional shares. All outstanding options and warrants entitling their holders to purchase the Company’s ordinary shares will be adjusted as a result of the reverse stock split, in accordance with the terms of each such security. In addition, the number of ordinary shares reserved for future issuance pursuant to the Company’s equity incentive plans will also be appropriately adjusted. The number of authorized ordinary shares will not be proportionately reduced because the Company has an unlimited number of authorized ordinary shares available for issuance, as permitted under the laws of the British Virgin Islands.
The following table reflects the unaudited pro forma share data used in the basic and diluted EPS calculations (U.S. Dollars in thousands, except per share amounts) based upon the 1-for-20 reverse stock split having occurred at March 31, 2024:
| Schedule of proforma shares | ||||||||||||
| Years Ended March 31, | ||||||||||||
| Numerator (in 000’$) | 2024 | 2023 | 2022 | |||||||||
| Net loss attributable to owners of the Company | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Denominator (in 000’) | ||||||||||||
| Weighted average number of shares – Basic and Diluted | ||||||||||||
| Basic and diluted loss per share | $ | ) | $ | ) | $ | ) | ||||||
| F-54 |
(b) EXHIBITS
The following documents are filed as part of this Annual Report on Form 20-F.
| 94 |
(b) EXHIBITS (Cont’d)
| 95 |
(b) EXHIBITS (Cont’d)
| 96 |
(b) EXHIBITS (Cont’d)
| Exhibit No. | Description of Exhibit | |
| 11.5* | Insider Trading Policy. | |
| 12.1* | Certifications of Chief Executive Officer Pursuant to Rule 13a-14(a) or 15d-14(a) under the Securities Exchange Act of 1934, as amended. | |
| 12.2* | Certifications of Chief Financial Officer Pursuant to Rule 13a-14(a) or 15d-14(a) under the Securities Exchange Act of 1934, as amended. | |
| 13.1* | Certification of Chief Executive Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
| 13.2* | Certification of Chief Financial Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
| 15.1* | Consent of Marcum LLP. | |
| 97.1* | Incentive Compensation Recovery Policy. | |
| 101 | The following financial information from our Annual Report on Form 20-F for the year ended March 31, 2024 has been formatted in Extensible Business Reporting Language (XBRL): (i) Consolidated Statements of Financial Position, (ii) Consolidated Statements of Operations and Other Comprehensive Income, (iii) Consolidated Statements of Cash Flows, and (iv) Notes to Consolidated Financial Statements. | |
| 101.INS* | XBRL Instance Document. | |
| 101.SCH* | XBRL Taxonomy Extension Schema Document. | |
| 101.CAL* | XBRL Taxonomy Extension Calculation Linkbase Document. | |
| 101.DEF* | XBRL Taxonomy Extension Definition Linkbase Document. | |
| 101.LAB * | XBRL Taxonomy Extension Label Linkbase Document. | |
| 101.PRE* | XBRL Taxonomy Extension Presentation Linkbase Document. | |
| 104 | Cover Page Interactive Data File (formatted in Inline XBRL and contained in Exhibit 101) |
_________________
* Filed herewith
◊ Schedules have been omitted pursuant to the Instructions to Exhibits of Form 20-F. The registrant undertakes to furnish supplemental copies of any of the omitted schedules upon request by the SEC.
+ Portions of this exhibit have been omitted pursuant to Instruction 4(a)(ii) to Exhibits of Form 20-F.
| 97 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
DATED at Toronto, Ontario, Canada, this 14th day of August, 2024
| PORTAGE BIOTECH INC. | ||
| By: | /s/ Ian Walters | |
| Title: | Chairman of the Board and Chief Executive Officer | |
| By: | /s/ Allan Shaw | |
| Title: | Chief Financial Officer | |
98