____________________________________________________________________________________________
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
Form 10-K
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2023
of
A Tennessee Corporation
IRS Employer Identification No. 62-1765329
Commission file number 001-33637
(615 ) 255-0068
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||||||||
Securities registered pursuant to Section 12(g) of the Act: None
Cumberland Pharmaceuticals Inc. is not a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Cumberland Pharmaceuticals Inc. is required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Cumberland Pharmaceuticals Inc. (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months, and (2) has been subject to such filing requirements for the past 90 days.
Cumberland Pharmaceuticals Inc. has submitted electronically every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months.
Cumberland Pharmaceuticals Inc. is a non-accelerated filer and a smaller reporting company as defined in Rule 12b-2 of the Exchange Act and is not a shell company.
Cumberland Pharmaceuticals Inc. has not filed a report on and attestation to its management's assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared and issued its audit report.
The aggregate market value of common stock held by non-affiliates as of June 30, 2023 was $12,061,328 . The number of shares of the registrant’s Common Stock, no par value, outstanding as of March 8, 2024 was 14,176,529 .
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant's executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
DOCUMENTS INCORPORATED BY REFERENCE
Certain information required in Part III of Form 10-K is incorporated by reference from the registrant’s Proxy Statement for its 2024 annual meeting of shareholders.
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Index
| Page Number | |||||
PART I
Item 1. Business.
THE COMPANY
Cumberland Pharmaceuticals Inc. (“Cumberland,” the “Company,” or as used in the context of “we,” “us,” or “our”), is a specialty pharmaceutical company focused on the acquisition, development and commercialization of branded prescription pharmaceutical products. We are dedicated to providing innovative products that improve the quality of care for patients and address poorly met medical needs.
Our primary target markets are hospital acute care, gastroenterology and oncology. These medical specialties are characterized by relatively concentrated prescriber bases that we believe can be served effectively by small, targeted sales forces. We promote our approved products through our hospital, field and oncology sales divisions in the United States while we continue to build a network of international partners to register and provide our medicines to patients in their countries.
Our portfolio of brands approved for marketing by the U.S. Food and Drug Administration (“FDA”) includes:
•Acetadote® (acetylcysteine) injection, for the treatment of acetaminophen poisoning;
•Caldolor® (ibuprofen) injection, for the treatment of pain and fever;
•Kristalose® (lactulose) for oral solution, a prescription laxative, for the treatment of constipation;
•Omeclamox®-Pak, (omeprazole, clarithromycin, amoxicillin) oral, for the treatment of Helicobacter pylori (H. pylori) infection and related duodenal ulcer disease;
•Sancuso® (granisetron) transdermal, for the prevention of nausea and vomiting in patients receiving certain types of chemotherapy treatment;
•Vaprisol® (conivaptan) injection, to raise serum sodium levels in hospitalized patients with euvolemic and hypervolemic hyponatremia; and
•Vibativ® (telavancin) injection, for the treatment of certain serious bacterial infections including hospital-acquired and ventilator-associated bacterial pneumonia, as well as complicated skin and skin structure infections.
In addition to these commercial brands, we have Phase II clinical programs underway evaluating our ifetroban product candidate in 1) Systemic Sclerosis (“SSc”) or scleroderma, a debilitating autoimmune disorder characterized by diffuse fibrosis of the skin and internal organs, 2) patients with cardiomyopathy associated with Duchenne Muscular Dystrophy (“DMD”), a rare, fatal, genetic neuromuscular disease results in deterioration of the skeletal, heart and lung muscles and 3) patients with Idiopathic Pulmonary Fibrosis (“IPF”), the most common form of progressive fibrosing interstitial lung disease. Investigational new study applications have been cleared by the FDA enabling us to launch clinical studies in each of these areas.
Cumberland has built core competencies for the acquisition, development and commercialization of pharmaceutical products in the U.S. – and we believe we can leverage this existing infrastructure to support our continued growth. Our management team consists of pharmaceutical industry veterans with experience in business development, product development, regulatory, manufacturing, sales, marketing and finance.
Our business development team identifies, evaluates, and negotiates product acquisition, licensing and co- promotion agreements. Our product development team creates proprietary formulations, manages our clinical studies, prepares our FDA submissions and staffs our medical call center. Our quality and manufacturing professionals oversee the manufacturing, release and shipment of our products. Our marketing and sales organization is responsible for our commercial activities, and we work closely with our distribution partners to ensure the availability and delivery of our products.
1
New Mission
During the year we took a fresh look at our mission statement and refined it to better capture the spirit of what we do each day at Cumberland. Our mission is now: working together to provide unique products that improve the quality of patient care.
In designing this statement, we considered several factors.
First, we wanted our mission to address the constituencies we serve, which include patients in need of care, as well as health care providers, our employees, shareholders, partners and community.
We also sought to reflect Cumberland’s culture, where teamwork is prized, emphasized and expected – in order to achieve our goals.
Next, it needed to demonstrate our focus on developing, acquiring and distributing differentiated brands.
And finally, we wanted to emphasize that the patient is at the core of everything we do. Our collective efforts are directed at providing unique products that serve as better alternatives for poorly met medical needs.
Sustainability Metrics
We issued our inaugural Sustainability Report in 2019, detailing Cumberland’s activities pertaining to our environmental, social and governance matters, and we remain committed to sustainability and to maintaining transparency of our corporate operations. As one of the largest biopharmaceutical companies founded and headquartered in the Mid-South, we hold ourselves to the highest standards of ethical practices and understand the importance of recognizing and addressing our impact on our constituents, our community and the environment.
Our 2023 sustainability metrics reveal that during that year we provided 3 million patient doses of our products, while safely disposing of nearly 6,000 pounds of expired and damaged products. We had no product recalls and no clinical trials terminated due to failure to practice good clinical standards.
In 2023, we also highlighted our investment in our employees through our continuing education programs, employee development initiatives and employee recognition awards. We reported that women represented 44% of Cumberland’s workforce – and 15% of our employees were minorities.
Through our sustainability initiatives, we will continue to identify and address critical industry issues, monitor relevant guidelines and utilize best practices.
Additional Information
We were incorporated in 1999 and have been headquartered in Nashville, Tennessee since inception. During 2009, we completed an initial public offering of our common shares and listing on the Nasdaq stock exchange. Our website address is www.cumberlandpharma.com. Our Annual Reports (on Form 10-K), Quarterly Reports (on Form 10-Q), Current Reports (on Form 8-K) and all material press releases are available on our website as soon as reasonably practicable after their filing with the U.S. Securities and Exchange Commission (“SEC”). These filings are also available to the public at www.sec.gov.
2
PRODUCTS
| Products | Indication | Status | ||||||||||||
Acetadote® | Acetaminophen Poisoning | Marketed | ||||||||||||
Caldolor® | Pain and Fever | Marketed | ||||||||||||
Kristalose® | Chronic and Acute Constipation | Marketed | ||||||||||||
Omeclamox®-Pak | H. pylori Infection and Related Duodenal Ulcer Disease | Approved | ||||||||||||
Sancuso® | Nausea and Vomiting Associated with Chemotherapy | Marketed | ||||||||||||
Vaprisol® | Euvolemic and Hypervolemic Hyponatremia | Approved | ||||||||||||
Vibativ® | Serious Bacterial Infections | Marketed | ||||||||||||
Acetadote®
Acetadote is an intravenous formulation of N-acetylcysteine, indicated for the treatment of liver toxicity associated with acetaminophen poisoning. Cumberland developed and obtained U.S. FDA approval for Acetadote, and then introduced the product through our hospital sales force.
Acetadote is typically used in hospital emergency departments to prevent or lessen potential liver damage resulting from an overdose of acetaminophen, a common ingredient in many over-the-counter and prescription pain- relieving and fever-reducing products. Acetaminophen overdose continues to be a leading cause of poisonings reported by hospital emergency departments in the U.S., and Acetadote has become a standard of care for treating this potentially life-threatening condition.
Acetadote received U.S. FDA approval as an orphan drug, which provided seven years of marketing exclusivity from the date of approval. That exclusivity has since expired.
In connection with the FDA’s approval of Acetadote, we committed to certain post-marketing activities for the product. Completion of our first Phase IV commitment resulted in the FDA’s approval of expanded labeling for the product for use in pediatric patients. Completion of our second Phase IV commitment resulted in further revised labeling for the product with FDA approval of additional safety data.
Completion of our third and final Phase IV commitment culminated in the FDA’s approval of a new formulation for the product. The next generation formulation contains no ethylene diamine tetracetic acid (“EDTA”) or other stabilization agent, chelating agent or preservative. Cumberland introduced this new Acetadote formulation replacing the original form of the product which we no longer manufacture.
The FDA subsequently approved updated labeling for Acetadote revising the product’s indication and providing new dosing guidance for specific patient populations. As a result, dosing guidance is now included for patients weighing over 100 kg, and new language was added to alert health care providers that, in certain clinical situations, therapy should be extended for some patients.
The United States Patent and Trademark Office (the “USPTO”) issued us a series of patents associated with our Acetadote product. These patents are discussed in Part I, Item I – “Business - Trademarks and Patents” – of this Form 10-K. The FDA has approved several abbreviated new drug applications (“ANDA”) filed by various generics companies referencing Acetadote. Those products all possess the old formulation containing EDTA.
We entered into an agreement with Perrigo Company resulting in the distribution of our Authorized Generic acetylcysteine injection (our “Authorized Generic”) product. Both Acetadote and our Authorized Generic utilize the new, EDTA-free formulation.
An Illinois judge issued a final ruling in favor of Cumberland Pharmaceuticals Inc. in a patent case associated with Acetadote. By ruling in Cumberland’s favor, the court upheld the validity of the patent that encompasses our EDTA-free formulation. The court also granted a permanent injunction preventing challengers from marketing a generic version of our proprietary Acetadote product formulation before the expiration of Cumberland’s patent in
3
August 2025. An Appeals Court affirmed the District Court ruling in the Company’s favor upholding Cumberland’s Acetadote patent and expressly rejected the validity challenge.
During 2023, we continued to distribute our Acetadote brand, however our Authorized Generic product is now distributed through Padagis US LLC (formerly a division of Perrigo Company).
Caldolor®
Caldolor, our intravenous formulation of ibuprofen, was the first injectable product approved in the U.S. for the treatment of both pain and fever. We conducted a series of clinical studies in over 900 adult patients to develop the data to support our FDA submission for the product’s registration. Following a priority review, the FDA approved Caldolor for marketing in the U.S.
A non-steroidal anti-inflammatory drug (“NSAID”), the product was indicated for use in adults as a sole treatment for the management of mild to moderate pain and for the management of moderate to severe pain as an adjunct to opioid analgesics. It was also the first FDA-approved intravenous therapy for treating fever.
We then launched Caldolor and continue to promote the product in the U.S. through our hospital sales force.
We completed a series of Phase IV studies to gather additional data to support our Caldolor product. Those clinical trials involved another 1,000 adult and pediatric patients. These studies included data on a shortened infusion time and pre-surgical administration of the product. To address our Phase IV commitment to the FDA, these studies also included evaluation of the product for the reduction of fever in hospitalized children and the treatment of pain in children undergoing tonsillectomy surgeries.
We then received FDA approval for the use of Caldolor in pediatric patients 6 months of age and older.
In early 2018, we completed and filed the application for FDA approval of a next generation Caldolor product featuring an improved presentation and formulation which was approved in January 2019. The new, premixed presentation provides health care professionals a formulation that is easy to administer, helping manage the treatment of patient pain and fever, while reducing opioid consumption. It is provided in a pre-mixed bag containing 800 mg of ibuprofen in a 200 mL patented low sodium formulation for injection that is ready to use. It is the first and only FDA-approved pre-mixed bag of ibuprofen. Caldolor is still available as an 800 mg/8mL single–dose vial for dilution in addition to the ready-to-use bag.
In November 2021, the FDA approved our submission to expand the labeling for Caldolor to include administration of the product prior to surgery. During our clinical studies we found that the product delivered its best results when dosed prior to surgery, reducing both patient pain as well as their need for opiates.
During 2022, we distributed both the vial and the ready-to-use premixed bag presentations of Caldolor. We also announced an agreement with PiSA Pharmaceutical for the registration and commercialization of Caldolor in Mexico. Under the terms of the agreement, Cumberland will be responsible for providing the product dossier and supplies. PiSA will be responsible for obtaining regulatory approval for the product in Mexico and introducing it to the new market. PiSA expects to provide the product in both 400- and 800-milligram vials.
In 2023, the FDA approved expanded labeling for Caldolor to include use in infants 3 to 6 months old. The safety and efficacy of Caldolor has now been established for the treatment of pain and fever in pediatric patients aged 3 months and older. With this newly approved labeling, Caldolor is the only non-opioid product approved to treat pain in infants that is delivered through injection.
Also in 2023, we announced the publication of positive results from a clinical study investigating the safety and pharmacokinetics of Caldolor in infants. Published in the journal Pediatric Drugs, the results support the FDA’s approval of Caldolor in patients 3 to 6 months of age.
In addition, we reported that we expect Caldolor will be eligible for special Medicare reimbursement under the new Non-Opioids Prevent Addiction in the Nation Act (the “NOPAIN Act”), which was enacted as part of the Consolidated Appropriations Act of 2023.
4
The NOPAIN Act requires Medicare to provide separate and more favorable reimbursement for non-opioid products that are used to manage pain during surgeries conducted in hospital outpatient departments or ambulatory surgical centers. In the Medicare Hospital Outpatient Prospective Payment System Proposed Rule, the Centers for Medicare and Medicaid Services (“CMS”) requested that manufacturers with potentially applicable non-opioid products submit comments and supporting clinical evidence regarding products that should be eligible for separate payment. We submitted a comment letter along with the requisite clinical information to the CMS in September of 2023 explaining why Caldolor should be included and separately reimbursed.
We now await further information from CMS expected this year, including the potential new reimbursement level for Caldolor. The Act is scheduled to go into effect in early 2025 and will initially apply to those products that are furnished between January 1st, 2025 and January 1st, 2028.
Kristalose®
Kristalose is a prescription laxative administered orally for the treatment of acute and chronic constipation. An innovative, dry powder crystalline formulation of lactulose, Kristalose is designed to enhance patient acceptance and compliance. It is the only prescription laxative available in pre-measured powder packets.
Kristalose dissolves easily in 4 ounces of water, offering patients a virtually taste-free, grit-free and essentially calorie-free alternative to lactulose syrups. We conducted a preference study which indicated that 77% of patients surveyed prefer the taste, consistency and portability of Kristalose over similar products in syrup forms.
We acquired the assets and exclusive rights to Kristalose through a series of transactions, then assembled a dedicated field sales force which re-launched the product as a Cumberland brand. We directed our sales efforts to physicians who are the most prolific writers of prescription laxatives, including gastroenterologists and internists. We supplemented this personal promotion with telemarketing campaigns to expand our reach and support of the product. Using preference data as a cornerstone of our marketing efforts, we repositioned the brand, enhancing patient affordability through a coupon program and expanded managed care coverage for the product.
We added a co-promotion partner, Poly Pharmaceuticals, who is promoting Kristalose to physician targets not covered by our field sales forces. We then added another partner, Foxland Pharmaceuticals, Inc., who is repackaging Kristalose and featuring it with additional new physician targets.
The Kristalose award-winning marketing campaign was refreshed for 2022 to support increased engagement with our customers. We also expanded patient support for the brand – and it was added to the GoodRx platform in 2022.
During 2023, we continued to support Kristalose through our field sales force as well as our partnerships with Poly Pharmaceuticals and Foxland Pharmaceuticals, Inc.
We have found that the brand performs best in states where we have Medicaid coverage. New York state recently added Kristalose to its Medicaid formulary, and we are implementing a special initiative to increase our presence and share of voice in the state. We believe that this new coverage is contributing to the growth of the product.
Omeclamox®-Pak
Many ulcers of the gastrointestinal tract are caused by an infection from the Helicobacter pylori (“H. pylori”) bacterium. Omeclamox-Pak is a branded prescription product used for the treatment of these infections and the related duodenal ulcer disease. This innovative product combines three well-known and widely prescribed medications: omeprazole, clarithromycin and amoxicillin.
Omeclamox-Pak was the first FDA-approved triple therapy combination medication to contain omeprazole as the proton pump inhibitor, which works to decrease the amount of acid the stomach produces. Clarithromycin and amoxicillin are both antibiotic agents that hinder the growth of the H. pylori bacteria. Interaction of these agents allows the stomach lining to heal effectively. The medications are packaged together on convenient daily dosing cards, making it simple to follow the twice a day dosing before meals.
5
We acquired the assets and exclusive rights to Omeclamox-Pak through a series of transactions and re-launched the product as a Cumberland brand supported by our field sales force.
The packager for Omeclamox-Pak encountered financial difficulties in 2020 due to the impact of COVID-19, and their operations are currently suspended. As a result, we depleted our inventory of the product and notified the FDA that the product is currently unavailable. That facility is now under new ownership and new management. We are awaiting resumption of their operations, while also exploring other alternatives to restart the product’s packaging.
Given the delay in identifying a packager for Omeclamox-Pak, as a precaution, the Company has taken a non-cash write down of the related intangible asset of $3.3 million. The financial statements reflect this adjustment.
Sancuso®
At the end of 2021, we entered into an agreement with Kyowa Kirin to acquire the U.S. assets and rights to Sancuso (granisetron transdermal system), an FDA-approved oncology supportive care medicine. This transaction closed in January 2022.
Sancuso is the first and only FDA-approved prescription patch for the prevention of nausea and vomiting in patients receiving certain types of chemotherapy treatment for their cancer. The active drug in Sancuso, granisetron, slowly dissolves in the thin layer of adhesive that sticks to the patient’s skin and is released into their bloodstream over several days, working continuously to prevent chemotherapy-induced nausea and vomiting (“CINV”). It is applied 24 to 48 hours before receiving chemotherapy and can prevent CINV for up to five consecutive days. Alternative oral treatments must be taken several times (day and night) to deliver the same therapeutic doses.
In early 2022, we assumed full commercial responsibility for the product in the U.S. – including its marketing, promotion, distribution, manufacturing and medical support activities. Kyowa Kirin retains international rights, continuing to deliver the product to address oncology patients’ needs throughout the rest of the world. In January 2022, we began shipments of the product and formed a new sales force, Cumberland Oncology, to support the brand. During 2023, we completed the transition of Sancuso from Kyowa Kirin to Cumberland, including the NDA transfer, and expanded our oncology sales division to further support the brand. We also successfully transferred manufacture of the product to a new facility. Following FDA approval of that site, we have now received new supplies of Cumberland-packaged product, which we will begin shipping this year.
Vaprisol®
We acquired the assets and rights to Vaprisol, a prescription brand indicated to raise serum sodium levels in hospitalized patients with euvolemic and hypervolemic hyponatremia. It is one of two branded prescription products indicated for the treatment of hyponatremia, and the only intravenously administered branded treatment.
Hyponatremia, an imbalance of serum sodium to body water, is the most common electrolyte disorder among hospitalized patients. These electrolyte disturbances occur when the sodium ion concentration in the plasma is lower than normal and are often associated with a variety of critical care conditions including congestive heart failure, liver failure, kidney failure and pneumonia. Vaprisol raises serum sodium to appropriate levels and promotes free water secretion. Our Vaprisol product has a proven day-one response rate to normalize serum sodium levels in hyponatremic patients and move them out of the Intensive Care Unit as efficiently as possible.
Vaprisol is supported by our hospital sales division. Demand for the product increased in 2020 during the pandemic, and we worked to support the expanded use of the product in hospitals and clinics during the health care crisis. During 2021, we shipped all remaining inventory of the product and notified the FDA that supplies of the product are not currently available. We have since transferred the product’s manufacturing to a new facility. Our new manufacturing partner is working with the FDA to address several Form 483 and warning letter issues in a timely manner. Meanwhile, we have been working with them to support a special, interim supply of compounded product for critically ill patients, which we introduced to the market in late 2023 and will begin selling in early 2024. We then expect to file for the approval to manufacture branded Vaprisol once all FDA issues at the new site are resolved.
6
Vibativ®
In November 2018, the Company announced an agreement to acquire the Vibativ assets and assume global responsibility for the brand including the related marketing, distribution, manufacturing and regulatory activities. In early 2021, we introduced the Cumberland-packaged product, which is supported by our hospital sales force.
Vibativ is a patented, FDA-approved injectable anti-infective. It is designed to treat serious infections due to Staphylococcus aureus (“S. Aureus") and other Gram-positive bacteria, including Methicillin-resistant Staphylococcus aureus (“MRSA”) and Methicillin-sensitive Staphylococcus aureus (“MSSA”). Vibativ addresses a range of Gram-positive bacterial pathogens, including those that are considered difficult-to-treat and multidrug-resistant.
Vibativ can serve as a potentially life-saving treatment in patients with hospital-acquired and ventilator-associated pneumonia resulting from infections including the flu, RSV and COVID-19.
Pneumonia caused by secondary bacterial infections is common among patients with viral respiratory infections. Research shows that hospital-acquired pneumonia (“HAP”) and ventilator-associated pneumonia (“VAP”) have historically accounted for 22% of common hospital-acquired infections. MSSA and MRSA are important disease-causing pathogens in these cases.
While many recently introduced antibiotics are quickly losing the battle to fight the bacteria they were designed to kill because those bacteria have become drug-resistant, Vibativ was specifically designed to kill drug-resistant bacteria.
The molecule of an existing antibiotic to which bacteria had developed a resistance, vancomycin, was altered by adding a lipophilic (fat-loving) component and a hydrophilic (water-loving) component. The lipophilic addition increases Vibativ’s ability to penetrate the cell wall and inhibits the formation of new cell walls (the development of new and/or additional cell walls is the most common way that bacteria become resistant to drugs). The hydrophilic addition increases Vibativ’s penetration into tissue – so it is able to attack infections that are not reachable by other antibiotics. In comparison to vancomycin, Vibativ is 32 times more potent against MRSA strains when tested under in vitro conditions. Further, in clinical trials, Vibativ demonstrated superior cure rates of patients with hospital-acquired bacterial pneumonia.
In 2023, we announced a new publication in Antimicrobial Agents and Chemotherapy detailing the results of the first clinical study investigating the safety and pharmacokinetics of our Vibativ product in children 2 to 17 years of age. The results of the study suggest that a single dose of Vibativ is safe in children and they experience reduced exposure to Vibativ, compared with the same body weight-based dosing in adults.
While we remain focused on promoting Vibativ in the U.S. market, we are building a network of other established companies to bring Vibativ to patients in their countries and territories. In 2022, we announced a new partnership with Saudi Arabia-based Tabuk Pharmaceutical to introduce Vibativ into the Middle East. The arrangement provides Tabuk exclusive rights to distribute Vibativ in Saudi Arabia and Jordan, with the option to expand into other countries in the region. Additionally, D.B. Pharm, our partner in South Korea who also distributes Caldolor, is awaiting the approval of Vibativ in their country.
Meanwhile, our Vibativ partner for the Chinese market, SciClone Pharmaceuticals, had their approval application in China accepted for review in September 2021. We have since been supporting SciClone and their requests associated with review of that submission. They are working toward the approval and believe that there is significant potential for Vibativ in their country.
7
PIPELINE
Ifetroban Clinical Studies
Ifetroban is a selective thromboxane-prostanoid receptor (“TPr”) antagonist dosed in nearly 1,400 subjects and found to be safe and well tolerated in healthy volunteers and various patient populations. We have been evaluating our ifetroban product candidate in a series of clinical studies. We have three Phase II clinical programs underway evaluating our ifetroban product candidate in 1) Systemic Sclerosis or scleroderma, a debilitating autoimmune disorder characterized by diffuse fibrosis of the skin and internal organs, 2) patients with cardiomyopathy associated with Duchenne Muscular Dystrophy, a rare, fatal, genetic neuromuscular disease results in deterioration of the skeletal, heart and lung muscles and 3) patients with Idiopathic Pulmonary Fibrosis, the most common form of progressive fibrosing interstitial lung disease. Investigational new study applications have been cleared by the FDA enabling us to launch clinical studies in each of these areas.
We also completed a pilot Phase II study involving 1) patients suffering from Hepatorenal Syndrome, a life-threatening condition involving liver and kidney failure 2) patients with Portal Hypertension associated with chronic liver disease and 3) patients with Aspirin-Exacerbated Respiratory Disease, a severe form of asthma. There were no significant safety issues identified with the use of ifetroban in these patients.
Additional pilot studies of ifetroban are underway, including several investigator-initiated trials.
We are awaiting results from the studies underway before deciding on the best development path for the registration of ifetroban, our first new chemical entity.
Following is more information about the clinical programs in which we are evaluating ifetroban:
Systemic Sclerosis (“SSc”)
We have initiated the clinical development of ifetroban oral capsules under the brand name Vasculan® for the treatment of Systemic sclerosis, also called scleroderma. It’s a debilitating autoimmune disorder characterized by diffuse fibrosis of the skin and internal organs, including the heart, as well as vascular dysfunction. SSc has a high morbidity and the highest case-specific mortality of any rheumatic disorder, with 50% of patients dying or developing major internal organ complications within three years of diagnosis. Although several medications are used to treat the skin disease associated with SSc, there is no universally effective treatment to improve the function of affected internal organs including the cardiovascular system.
Cardiac involvement associated with SSc is often underestimated due to its subtle and atypical presentation. Despite the cardiovascular events associated with its elevated mortality at later stages of the disease, overt signs are suggestive of advance disease including myocardial or pericardial inflammation, heart failure and pulmonary arterial hypertension (“PAH”).
Our Vanderbilt collaborators completed preclinical studies demonstrating TPr blockade with ifetroban prevents cardiac fibrosis and can restore cardiac function in animal models of PAH.
The FDA cleared our IND application to evaluate 12 months of oral ifetroban (Vasculan) in a 34-subject phase II trial entitled, A Phase II Randomized, Double-Blind, Placebo-Controlled Study to Assess the Safety and Efficacy of Ifetroban in Patients with Diffuse Cutaneous Systemic Sclerosis or Systemic Sclerosis-Associated Pulmonary Arterial Hypertension. This study is well underway and includes patients with diffuse cutaneous SSc, as well as those with pulmonary arterial hypertension associated with their SSc.
Duchenne Muscular Dystrophy (“DMD”)
We also initiated the clinical development of oral ifetroban under the brand name Dyscorban® for the treatment of cardiomyopathy associated with Duchenne Muscular Dystrophy, a rare and fatal disease caused by a genetic defect which leads to inexorable muscle damage. Cardiomyopathy is the leading cause of death in DMD patients. TPr and its ligand, isoprostanes, are found to have increased in DMD patients.
8
Preclinical studies by our Vanderbilt collaborators demonstrated TPr blockade by ifetroban prevented cardiac dysfunction and improved mortality in several animal models of muscular dystrophy. These results, published in the Journal of the American Heart Association, suggest TPr activation contributes to DMD cardiomyopathy and blockade with ifetroban may serve as a novel therapeutic for DMD patients.
The FDA cleared Cumberland’s IND application to evaluate 12 months of oral ifetroban (Dyscorban) in a Phase II study entitled, A Randomized, Double-Blind, Placebo-Controlled, Multiple Dose Study with an Open-Label Extension to Determine the Safety, Pharmacokinetics and Efficacy of Oral Ifetroban in Subjects with Duchenne Muscular Dystrophy. With medical centers across the U.S. screening patients, our clinical study is enrolling those with 48 ambulatory and non-ambulatory DMD, 7 years of age and older with stable cardiac function.
Cumberland was awarded just over $1 million in federal funding to support this clinical trial, which is the first DMD clinical study awarded FDA Orphan Product Development funding. As a result of the COVID-19 pandemic and its global impact on clinical research in 2020, the FDA awarded a supplemental grant in support of our Phase II DMD study.
Idiopathic Pulmonary Fibrosis (“IPF”)
In September 2021, our Board of Directors approved a new clinical program for the use of ifetroban to treat Progressive Fibrosing Interstitial Lung Diseases (“PF-ILDs”). Nonclinical studies are complete, and the resulting manuscript was prepared and submitted for publication in 2021.
In May 2023, we announced that the FDA cleared the IND application for a Phase II study in patients with Idiopathic Pulmonary Fibrosis, the most common form of progressive fibrosing interstitial lung disease. As a result, we are in the process of initiating our FIGHTING FIBROSIS trial designed to enroll 128 patients in over 20 medical centers of excellence across the U.S. This Phase II clinical trial will study the safety, tolerability and efficacy of oral ifetroban in patients with IPF. Recent studies have shown ifetroban can both prevent and enhance resolution of lung fibrosis in multiple preclinical models.
Other Ifetroban Programs
We also completed a pilot Phase II study involving 1) patients suffering from Hepatorenal Syndrome, a life-threatening condition involving liver and kidney failure 2) patients with Portal Hypertension associated with chronic liver disease and 3) patients with Aspirin-Exacerbated Respiratory Disease, a severe form of asthma. There were no significant safety issues identified with the use of ifetroban in these patients. Additional pilot studies of ifetroban are underway, including several investigator-initiated trials.
Our plan going forward is to complete each of our Company-sponsored studies, analyze their final data, announce top-line results and decide on the best development path for the registration of ifetroban, which we continue to believe has the potential to benefit many patients with orphan diseases that represent unmet medical needs.
FDA Fee Waivers
During 2023, the FDA informed us that it had granted two barrier-to-innovation waivers that would result in refunds totaling approximately $2.8 million that we had previously paid for prescription drug program fees.
The FDA granted each waiver after concluding that Cumberland met the statutory criteria based on the innovation associated with its ifetroban clinical development programs, as the funds could be better used to advance those studies. We received both refunds in June 2023.
9
GROWTH STRATEGY
Cumberland’s growth strategy involves maximizing the potential of our existing brands while continuing to build a portfolio of differentiated products. We currently feature seven products approved by the FDA in the United States. We are also continuing to explore international partnerships to bring our medicines to patients in other countries. Additionally, we look for opportunities to expand our products into additional patient populations through clinical trials, new presentations and our support of select, investigator-initiated studies. We actively pursue opportunities to acquire additional marketed products as well as late-stage development product candidates in our target medical specialties. Our clinical team is developing a pipeline of new product candidates to address poorly met medical needs.
We are supplementing these activities with the earlier-stage drug development at Cumberland Emerging Technologies (“CET”), our majority-owned subsidiary. CET partners with academic research institutions to identify and support the progress of promising new product candidates, which Cumberland could further develop and commercialize.
Specifically, we are seeking long-term, sustainable growth by:
•Supporting and expanding the use of our marketed products. We continue to evaluate our products following their FDA approval to determine if additional clinical data could expand their market and use. For example, we have secured pediatric approval of Acetadote and Caldolor and expanded the labeling for both brands accordingly. We also added pre-surgery dosing for Caldolor, and recently included newborns to the patients who can benefit from the product. We will continue to explore such opportunities to bring our products to new patient populations.
•Selectively adding complementary brands. In addition to our product development activities, we are also seeking to acquire products or late-stage development product candidates to continue to build a portfolio of complementary brands. We focus on under-promoted, FDA-approved drugs as well as late-stage development products that can improve patient care. We will continue to target product acquisition candidates that are competitively differentiated, have valuable intellectual property or other protective features, and allow us to leverage our existing infrastructure. Our acquisitions of Vibativ and Sancuso are examples of the implementation of this strategy.
•Progressing our clinical pipeline and incubating future product opportunities at CET. We believe it is important to build a pipeline of innovative new product opportunities, as we are doing through our ifetroban Phase II development programs. We are also supplementing our acquisitions and late-stage development activities with early-stage drug development activities with CET.
•Leveraging our infrastructure through co-promotion partnerships. We believe that our commercial infrastructure can help drive prescription volume and product sales. We look for strategic partners that can complement our capabilities and enhance opportunities for our brands. For example, our co-promotion partnerships have allowed us to expand the support for Kristalose and Sancuso across the U.S.
•Building an international contribution to our business. We have established our own commercial capabilities, including three sales divisions, to cover the U.S. market for our products. We are also building a network of select international partners to register our products and make them available to patients in their countries. We will continue to develop and expand our network of international partners while supporting our partners’ registration and commercialization efforts in their respective territories. The acquisition of Vibativ resulted in several new international partners and market opportunities.
•Managing our operations with financial discipline. We continually work to manage our expenses in line with our revenues to deliver positive cash flow from operations. We remain in a strong financial position, with favorable gross margins and a strong balance sheet.
10
SALES AND MARKETING
Cumberland’s sales and marketing team has broad industry experience in selling branded pharmaceuticals. Our sales and marketing executives direct our national marketing campaigns and maintain key national account relationships. They also manage our dedicated hospital, field and oncology sales forces, which are comprised of approximately 60 sales professionals.
•Hospital market: We promote Caldolor, Vaprisol, Acetadote and Vibativ through our dedicated hospital sales division. This organization targets key hospitals across the U.S. and is comprised of sales professionals with substantial experience in the hospital market. Independent market data continues to indicate that the majority of pharmaceutical promotional spending is directed toward large, outpatient markets on drugs intended for chronic use rather than short-term, hospital use.
We believe the hospital market is under-served and highly concentrated, and that it can be penetrated effectively by a small, dedicated sales force without large-scale promotional activity. Our established position in the hospital market provided the rationale for adding Vibativ as our first infectious disease product that complements our hospital product line. Our strategy has been to focus our hospital sales team on select, high-priority accounts.
•Gastroenterology market: We promote Kristalose and Omeclamox-Pak through a dedicated field sales team addressing a targeted group of physicians who are large prescribers of the products. Because the market for gastrointestinal diseases is broad in patient scope, yet relatively narrow in physician base, we believe it provides opportunities that can be penetrated with a modest-sized sales force. We also believe that we can increase market share for these products through our sales and marketing activities.
•Oncology market: In early 2022, we formed a new oncology sales force to promote our Sancuso brand. This organization targets key oncologists and clinics across the U.S. and is comprised of both inside and field-based sales professionals. During 2023, we expanded the sales division as we work to deliver Sancuso to cancer patients, helping them tolerate their chemotherapy treatments. The division is comprised of 12 individuals, including four inside reps. This group can be expanded through additional personnel or augmented through co-promotion partners.
Our commercial executives conduct ongoing analyses to evaluate marketing campaigns and promotional programs in support of our brands. The evaluations include development of product profiles, testing of the profiles against the needs of the market, determining what additional product information or development work is needed to effectively market the products and preparing financial forecasts.
We utilize professional branding and packaging as well as promotional items to support our products, including direct mail, sales brochures, journal advertising, educational and reminder leave-behinds, patient educational pieces, coupons and product sampling. We also regularly attend select medical meetings and trade shows to expand the awareness of our products.
Our national accounts team is responsible for key large buyers and related marketing programs. This team maintains relationships with our wholesaler customers as well as with third-party payors, such as group purchasing organizations, pharmacy benefit managers, hospital buying groups, outpatient centers, state and federal government purchasers, and health insurance companies.
MATERIAL CUSTOMERS
Our primary customers are wholesale pharmaceutical distributors in the United States. Total revenue by customer for each customer representing 10% or more of consolidated gross revenues are summarized below for the year ended December 31, 2023:
| 2023 | ||||||||||||||
| Customer 1 | 29% | |||||||||||||
| Customer 2 | 26% | |||||||||||||
| Customer 3 | 24% | |||||||||||||
11
INTERNATIONAL PARTNERSHIPS
We have established our own capabilities to support the commercialization of our products in the U.S. Our international strategy is to identify and partner with other companies that have the appropriate capabilities to support our products in their respective countries. We have entered into a series of agreements to establish an international network, which is summarized in the table below and includes information on our primary partners:
| International Partner | Product(s) | Territory | Status | ||||||||||||||||||||
| Phebra Pty Ltd | Acetadote & Caldolor | Australia | Marketed | ||||||||||||||||||||
| D.B. Pharm Korea Co., Ltd. | Caldolor | South Korea | Marketed | ||||||||||||||||||||
| Sandor Medicaids Pvt. Ltd. | Caldolor | India | Marketed | ||||||||||||||||||||
| R-Pharm JSC | Vibativ | Russia and CIS | Marketed | ||||||||||||||||||||
| Tabuk Pharmaceuticals | Vibativ | Saudi Arabia and Jordan | Registration | ||||||||||||||||||||
| PiSA Pharmaceutical | Caldolor | Mexico | Registration | ||||||||||||||||||||
| SciClone Pharmaceuticals, Inc. | Vibativ | China and Hong Kong | Registration | ||||||||||||||||||||
| D.B. Pharm Korea Co., Ltd. | Vaprisol & Vibativ | South Korea | Registration | ||||||||||||||||||||
| WinHealth Pharma Group Co. | Acetadote | China and Hong Kong | Development | ||||||||||||||||||||
Our international commercialization agreements include a license to one or more Cumberland products for a specific territory as noted in the table above. We seek partners who have the local infrastructure to support the registration and commercialization of our products in their territory.
Under the terms of our agreements our partners are responsible for:
•Seeking regulatory approvals for the products;
•Launching the brand;
•Managing the ongoing marketing, sales and product distribution;
•Addressing the ongoing regulatory requirements in the international territories;
•Remitting any upfront, regulatory and sales milestone payments;
•Providing the transfer price for supplies of the product; and
•Calculating and paying any royalties, as applicable.
Our responsibilities include:
•Providing a dossier of relevant information to support product registration;
•Maintaining our intellectual property associated with the product;
•Sharing our marketing strategy, experience and materials for the brand; and
•Manufacturing and providing the finished product for sale.
During 2023, we worked to support our existing international partners, conclude unproductive arrangements and identify new companies to represent our products in select additional territories.
12
BUSINESS DEVELOPMENT
Since inception, we have had an active business development initiative focused on acquiring rights to marketed products and product candidates that fit our strategy and target markets. We source business development opportunities through our international network of advisory firms and individual pharmaceutical industry and medical advisors. A multi-disciplinary internal management team reviews these opportunities on a regular basis using a group of selection criteria. We have historically focused on product opportunities that are a strategic fit with our commercial organization, development expertise and medical focus, employing a variety of transaction structures.
We have continued to build our product portfolio of complementary, niche brands largely through product acquisitions and late-stage development of product candidates.
Our primary targets are under-promoted, FDA - approved drugs with existing brand recognition and late-stage development product candidates that address unmet or poorly met medical needs in the hospital acute care, gastroenterology and oncology markets. We believe that by focusing mainly on approved or late-stage products, we can minimize the significant risk, cost and time associated with drug development.
We continue to strategically review our brands, pipeline and capabilities, as well as our international partners. We believe that it is prudent to continually evaluate our product portfolio, partners, and organization in order to ensure a proper focus and the needed supporting capabilities.
International Partners
D.B. Pharm Korea Co., Ltd. (“D.B. Pharm”) has licensed our Caldolor product for the South Korean market, and they obtained regulatory approval for Caldolor in their country. During 2023, D.B. Pharm continued to purchase supplies of Caldolor and distributed the brand in South Korea. We have also entered into agreements with D.B. Pharm to register and commercialize our Vaprisol and Vibativ brands in their country. During 2022, we worked with them to prepare the submissions for the approval of each brand there. They are currently awaiting the approval of Vibativ in their country.
We have executed a license and distribution agreement with HongKong WinHealth Pharma Group Co. Limited (“WinHealth”) for our Caldolor and Acetadote brands in China and Hong Kong. Under the terms of the agreement, WinHealth will provide development milestone payments and purchase supplies of the products following their registration in China.
We also entered into a strategic alliance agreement with WinHealth to explore future business opportunities that will further the mission and goals of each organization. Founded in Hangzhou, China and currently headquartered in Hong Kong, WinHealth has developed a wide breadth of capabilities, including drug licensing, product development and registration, and has established a strong network of distribution and sales promotional capabilities for the Chinese market. WinHealth has established partnerships with international companies that include Boehringer-Ingelheim, Janssen, Novartis, Pfizer and Roche, generating several hundred million dollars in sales annually.
In August 2020, we entered into an agreement with WinHealth Investment (Singapore) Ltd. creating WHC Biopharmaceuticals, Pte. Ltd. The joint venture will focus on acquiring, developing, registering, and commercializing development stage and commercial stage biopharmaceuticals for China, Hong Kong and other Asian markets.
R-Pharma JSC (“R Pharma”) has licensed our Vibativ product for a territory that includes Russia and a number of adjacent countries in Eastern Europe. R-Pharma is one of the leading multinational pharmaceutical organizations based in Russia. Headquartered in Moscow and focusing on a wide breadth of therapeutic areas in the specialty and hospital care markets, R-Pharma generates $1 billion in annual revenue. R-Pharma has registered Vibativ in Russia and during 2023, continued to purchase supplies of the product for that market.
SciClone Pharmaceuticals (Holdings) Limited (“SciClone”) has licensed our Vibativ product for sale and distribution in China and several adjacent countries. In February 2021, SciClone completed an initial public offering and listing of their shares on the Hong Kong stock exchange. In June 2021, SciClone submitted an application to the
13
Chinese regulatory authority for the approval of Vibativ in that country. In October 2021, SciClone informed us that the filing was accepted by the regulatory agency for review. We have since been supporting SciClone and their requests associated with review of that submission. They are working toward the approval, responding to regulatory inquiries, and believe that there is significant potential for Vibativ in their country.
In March 2022, we established distribution for our Vibativ product in the Middle East through a partnership with Tabuk Pharmaceutical Manufacturing Company (“Tabuk”). Through our partnership, Tabuk will introduce the product in Saudi Arabia, Jordan and potentially other countries in the Middle East. They are currently updating their product approval in Saudi Arabia with new manufacturing information.
In October 2022, we announced an agreement with PiSA Pharmaceutical for the registration and commercialization of Caldolor in Mexico. Under the terms of the agreement, Cumberland will be responsible for providing the product dossier and supplies. PiSA will be responsible for obtaining regulatory approval for the product in Mexico and introducing it to the new market. They are currently preparing their submission for the approval of Caldolor in their country.
Poly Co-Promotion Agreement
We entered into a co-promotion arrangement with Poly Pharmaceuticals, Inc. (“Poly”) for our Kristalose product in 2017. Poly is a privately held U.S. specialty pharmaceutical company that is featuring Kristalose to an expanded number of physicians. Poly’s sales organization is more than doubling the number of nationwide physicians that are reached with the Kristalose brand message. In 2019, we extended our co-promotion arrangement with Poly.
2R and Foxland Agreements
In 2018, we entered into another co-promotion arrangement related to our Kristalose product. We have agreements with 2R Investments, LLC and with Foxland Pharmaceuticals, Inc. (“Foxland”) to package, distribute and promote an unbranded generic form of our Kristalose product to physician targets that we do not cover. We renewed our agreement with Foxland in 2022.
Nordic License Agreement
In July 2022, we entered into an amendment to our agreement with Nordic Pharma (“Nordic”) that addressed the responsibilities and financial arrangements regarding our license to Nordic’s methotrexate line of products for the U.S. Our line of prefilled methotrexate syringes, marketed under the brand name RediTrex in the U.S., was covered by the license. As of July 1, 2023, Nordic has assumed responsibility for the product in the U.S.
Following the return of the license, Nordic will provide us with a royalty on their future sales of the products through April 2035. The companies will continue to collaborate on any transition and the ongoing commercialization of the product line.
Cumberland transferred the marketing authorization associated with the RediTrex product line to Nordic. They returned the 180,000 shares we issued to them that were associated with the license and refunded the $1 million we paid them following the brand’s approval in the U.S. Nordic has also issued a credit note in favor of Cumberland in the amount of $1 million for the unpaid milestone payment due from us which was associated with our launch of the product line.
CET University Collaboration Agreements
Through CET, we collaborate with a select group of academic research institutions located in the Mid-South region of the U.S. to identify, co-develop and seek grant funding for promising biomedical technologies emerging from those research institutions. CET is collaborating with Vanderbilt University and other regional academic research institutions and has entered into a series of agreements to access and collaborate on the development of innovative product candidates. These arrangements enable CET to team with university-based researchers to advance their scientific discoveries and breakthroughs by designing new product candidates to improve patient care and address unmet medical needs. CET has been able to help secure federal small business grant funding to support these various projects.
14
CET has also established and manages the Nashville Life Sciences Center, which serves as an incubator for Middle Tennessee’s emerging biomedical industry. The Life Sciences Center provides offices, laboratory space, and equipment to early-stage companies looking to develop their technologies and products. We maintain the Cumberland Pharmaceuticals formulation and testing laboratory at the CET Life Sciences Center.
Nearly 30 life science companies exist today because of CET, including a vibrant group of current tenants at the Life Sciences Center as well as a growing number of successful graduates.
CLINICAL AND REGULATORY AFFAIRS
We have in-house capabilities for the management of our clinical, professional and regulatory affairs. Our team develops and manages our clinical trials, prepares regulatory submissions, manages ongoing product-related regulatory responsibilities and manages our medical information call center. Team members have been responsible for devising the regulatory and clinical strategies for all our products as well as obtaining FDA approvals for Acetadote and Caldolor brands.
Clinical Development
Our clinical development personnel are responsible for:
•creating clinical development strategies;
•designing, implementing and monitoring our clinical trials;
•creating case report forms and other study-related documents; and
•analyzing efficacy and safety data obtained from clinical trials for subsequent submissions and approval of new drugs.
Regulatory and Quality Affairs
Our internal regulatory and quality affairs team is responsible for:
•preparing and submitting INDs for clearance to begin patient studies;
•preparing and submitting NDAs and fulfilling post-approval marketing commitments;
•maintaining investigational and marketing applications through the submission of appropriate reports;
•submitting supplemental applications for additional label indications, product line extensions and manufacturing improvements;
•evaluating regulatory risk profiles for product acquisition candidates, including compliance with manufacturing, labeling, distribution and marketing regulations;
•monitoring applicable third-party service providers for quality and compliance with current Good Manufacturing Practices (“GMPs”), Good Laboratory Practices (“GLPs”), and Good Clinical Practices (“GCPs”), and performing periodic audits of such vendors; and
•maintaining systems for document control, product and process change control and customer complaints.
15
PROFESSIONAL AND MEDICAL AFFAIRS
Our medical team provides in-house medical information support for our marketed products. This includes interacting directly with health care professionals to address any product or medical inquiries through our medical information call center and medical science liaisons. In addition to coordinating the call center, our clinical/regulatory group generates medical information letters, provides informational memos to our sales forces and assists with ongoing training for the sales forces.
CLINICAL DEVELOPMENT AND STUDY RESULTS
Vibativ Clinical Manuscripts
Vibativ is a patented, FDA-approved injectable anti-infective for the treatment of certain serious bacterial infections including hospital-acquired and ventilator-associated bacterial pneumonia and complicated skin and skin structure infections. It addresses a range of Gram-positive bacterial pathogens, including those that are considered difficult-to-treat and multidrug-resistant.
In October 2023, Cumberland announced a new study published in Antimicrobial Agents and Chemotherapy detailing the results of the first clinical study investigating the safety and pharmacokinetics of our Vibativ (telavancin) product in children 2 to 17 years of age. The study found a single 10 mg/kg dose of Vibativ was safe with no serious adverse events or renal concerns. Drug exposure to Vibativ was lower in children compared with the same body weight-based dosing in adults. The study suggests Vibativ is a safe and viable option for pediatric patients ages 2 to 17 who require systemic antibiotics for the treatment of a known or suspected bacterial infection, including those with MRSA or another S. aureus pathogen.
Caldolor Newborn Study and Clinical Manuscripts
We previously received FDA approval for the use of Caldolor in pediatric patients 6 months of age and older. Caldolor is the first and only injectable NSAID approved for use in children. We then initiated a study to collect data on the use of Caldolor in children ranging in age from birth up to 6 months of age. Enrollment in that multi-center study was completed in 2019, and topline results were announced in 2020, indicating that Caldolor was well tolerated in this patient population, with no safety concerns noted.
In May 2023, we announced the FDA approval of Caldolor in pediatric patients 3 to 6 months of age. With this newly approved labeling, Caldolor is the only non-opioid injectable product approved to treat pain in infants that is delivered through injection.
Additionally, in June 2023, we shared the positive results from a clinical study investigating the safety and pharmacokinetics of Caldolor in newborns, published in the journal Pediatric Drugs. The clinical study evaluated the safety and drug exposure profile of Caldolor in 24 hospitalized infants between the ages of 1 and 6 months who required treatment for pain or fever. Of the 24 patients included in the study, three were under 3 months of age, and the remaining 21 patients were 3 to 6 months of age. Twenty patients received a single dose, and four patients received multiple doses. In this study, single and multiple 10 mg/kg doses of Caldolor were reported safe, with no drug-related adverse events or renal concerns. Drug exposure following a single dose of Caldolor in infants 1 to 6 months of age was similar to what was previously reported in older children. The results of this study support the growing body of evidence that demonstrates Caldolor is a safe therapeutic option available to practitioners for the treatment of fever and pain in infants, children and adults.
Ifetroban Phase II Studies
We have been evaluating our ifetroban product candidate in a series of clinical studies. We have three Phase II clinical programs underway evaluating our ifetroban product candidate in 1) Systemic Sclerosis or scleroderma, a debilitating autoimmune disorder characterized by diffuse fibrosis of the skin and internal organs, 2) patients with cardiomyopathy associated with Duchenne Muscular Dystrophy, a rare, fatal, genetic neuromuscular disease results in deterioration of the skeletal, heart and lung muscles and 3) patients with Idiopathic Pulmonary Fibrosis, the most
16
common form of progressive fibrosing interstitial lung disease. Investigational new study applications have been cleared by the FDA enabling us to launch clinical studies in each of these areas.
We also completed a pilot Phase II study involving 1) patients suffering from Hepatorenal Syndrome, a life-threatening condition involving liver and kidney failure 2) patients with Portal Hypertension associated with chronic liver disease and 3) patients with Aspirin-Exacerbated Respiratory Disease, a severe form of asthma. There were no significant safety issues identified with the use of ifetroban in these patients.
Additional pilot studies of ifetroban are underway, including several investigator-initiated trials.
We are awaiting results from the studies underway before deciding on the best development path for the registration of ifetroban, our first new chemical entity.
Additional Testing Program
Cumberland entered into a non-clinical evaluation agreement, to test one of our products against bacterial strains and subsequently, in vivo animal models utilizing the preclinical services program funded by the Division of Microbiology and Infectious Diseases (“DMID”), part of the National Institute of Allergy and Infectious Diseases (“NIAID”), an institute of the National Institutes of Health (“NIH”), which is part of the Department of Health and Human Services (“HHS”), an agency of the U.S. government. The NIAID contractors completed these studies and prepared a manuscript for submission to a scientific journal for publication.
CORPORATE DEVELOPMENT
Cumberland Pharma Foundation
We have formed the Cumberland Pharma Foundation (the “Foundation”) to provide the ongoing philanthropic endeavors of Cumberland Pharmaceuticals Inc.
The Foundation was formed as an independent, nonprofit corporation designed to qualify as a tax-exempt organization pursuant to Section 501(a) of the Internal Revenue Code. The Foundation’s Board of Directors is comprised of Cumberland Pharmaceuticals executives who are responsible for overseeing the Foundation’s ongoing activities, including charitable contributions.
We initially provided a grant of 50,000 shares of our common stock to the Foundation. The shares will address the ongoing financial needs of the Foundation, with most of the shares expected to be held for the opportunity to realize long-term appreciation to support the Foundation’s future.
The Foundation maintains independent financial statements and its contributions will not impact the financial statements of Cumberland Pharmaceuticals. Initial annual grants by the Foundation have been and remain consistent with the historic level of contributions made by Cumberland Pharmaceuticals.
Cumberland Health and Wellness Political Action Committee
We have also formed the Cumberland Health and Wellness Political Action Committee (the “PAC”). The objective of the PAC is to support candidates and policies that are consistent with Cumberland’s mission of advancing patient care. The PAC’s activities are held at a local, state, and federal level and conducted in a bi-partisan manner.
The initial committee membership is comprised of Cumberland Pharmaceuticals employees. The PAC received initial funding from us, and future funding will include voluntary individual contributions from Cumberland Pharmaceuticals directors and employees.
17
MANUFACTURING AND DISTRIBUTION
Manufacturing
We partner with third parties for certain non-core, capital-intensive capabilities, including the manufacturing and distribution of our products. We manage these third-party relationships and are responsible for the quality review and release of each lot of our products.
Acetadote®
We have an agreement with one manufacturer, who provided commercial supplies of Acetadote in 2023.
Caldolor®
We have agreements with multiple manufacturers for the supply of Caldolor and during 2023, we obtained commercial supplies from three of these manufacturers for our international and domestic Caldolor requirements.
Kristalose®
We have an agreement for the purchase of Kristalose API with an international supplier. We also had manufacturing arrangements with a packager who provided finished supplies of the product for commercial and sampling purposes during 2023.
Omeclamox®-Pak
The packager for Omeclamox-Pak encountered financial difficulties in 2020 due to the impact of COVID-19, and their operations are currently suspended. As a result, we depleted our inventory of the product and notified the FDA that the product is currently unavailable. That facility is now under new ownership and new management. We are awaiting resumption of their operations, while also exploring other alternatives to restart the product’s packaging. With uncertain future cash flows, the Board of Directors approved the write-down of the intangible assets related to the product.
Sancuso®
As part of the acquisition of Sancuso, we obtained an initial supply of finished goods inventory. The agreement with the manufacturer of Sancuso was assigned to us and there were additional goods supplied to us during 2022. The production was moved to one of the manufacturer’s other facilities. During 2022, that new facility was approved by the FDA to manufacture and supply Sancuso. We received supplies of the product from the new facility in 2023 and will begin shipping the Cumberland-packaged product during 2024.
Vaprisol®
As part of the acquisition of Vaprisol, we obtained a significant existing supply of raw material inventory. We reached an agreement during 2020 with a new manufacturer to provide us with long-term supplies of the product. We subsequently completed the transfer of the product’s manufacturing to the new facility in 2021. We informed the FDA that supplies of the product are not currently available and are awaiting approval for that new facility. Our new manufacturing partner is working with the FDA to address several Form 483 and warning letter issues in a timely manner. Meanwhile, we have been working with them to support a special, interim supply of compounded product for critically ill patients, which we introduced to the market in late 2023 and will begin selling in early 2024. We then expect to file for the approval to manufacture branded Vaprisol once all FDA issues at the new site are resolved.
Vibativ®
Through our acquisition of Vibativ, we obtained a multi-year supply of raw material, work in process and finished goods inventory. As a result of the agreement, we are now responsible for the future manufacturing of the product. We completed the transfer of the product’s manufacturing activities to a new supplier and received FDA approval for that facility.
18
Distribution
Like many pharmaceutical companies, we engage a third-party with appropriate facilities and logistical expertise to support the U.S. distribution of our products. In 2023, Cardinal Health Specialty Solutions exclusively handled our U.S. product logistics activities, including warehousing, shipping, and various other customer activities. Our primary customers are the wholesalers of pharmaceuticals who provide our products to hospitals, clinics and retail pharmacies in the U.S.
PATENTS, TRADEMARKS AND OTHER INTELLECTUAL PROPRIETARY RIGHTS
We own the trademarks for each of our branded pharmaceutical products as well as for our corporate name and logo. We have applied for trademark registration for other various names and logos. Over time, we intend to maintain registrations on trademarks that remain valuable to our business.
We seek to protect our products from competition through a combination of patents, trademarks, trade secrets, FDA exclusivity and contractual restrictions on disclosure. Proprietary rights, including patents, are an important element of our business. We seek to protect our proprietary information by requiring our employees, consultants, contractors and other advisors to execute agreements providing for protection of our confidential information upon commencement of their employment or engagement. We also require confidentiality agreements from entities to which we provide our confidential information or materials.
Acetadote®
We developed a new formulation of Acetadote (acetylcysteine) injection as part of a Phase IV commitment in response to a request by the FDA to evaluate the reduction of ethylene diamine tetraacetic acid (“EDTA”) from the product’s formulation. In April 2012, the USPTO issued U.S. Patent number 8,148,356 (the “356 Acetadote Patent”) which is assigned to us. The claims of the 356 Acetadote Patent encompass the new Acetadote formulation and include composition of matter claims. Following its issuance, the 356 Acetadote Patent was listed in the FDA Orange Book. The 356 Acetadote Patent is scheduled to expire in May 2026, which includes a 270-day patent term adjustment granted by the USPTO.
Following the issuance of the 356 Acetadote Patent, we received separate Paragraph IV certification notices from InnoPharma, Inc. (“InnoPharma”), Paddock Laboratories, LLC (“Paddock”), Mylan Institutional LLC (“Mylan”), Sagent Agila LLC (“Sagent”) and Perrigo Company (“Perrigo”) challenging the 356 Acetadote Patent on the basis of non-infringement and/or invalidity. We responded by filing five separate infringement lawsuits, in the appropriate United States District Courts, to contest each of the challenges.
On November 12, 2012, we entered into a Settlement Agreement (the “Settlement Agreement”) with Paddock and Perrigo to resolve the challenges and the pending litigation with those two companies.
On November 1, 2013, the United States District Courts filed opinions granting Sagent’s and InnoPharma’s motions to dismiss our suits and we agreed not to file an appeal or motion to reconsider, thereby resolving the challenges and the pending litigation with those two companies.
Under the Settlement Agreement, Paddock and Perrigo admit that the 356 Acetadote Patent is valid and enforceable and that any Paddock or Perrigo generic version of Acetadote (with or without EDTA) would infringe upon the 356 Acetadote Patent. In addition, Paddock and Perrigo will not challenge the validity, enforceability, ownership or patentability of the 356 Acetadote Patent through its expiration currently scheduled for May 2026. On November 12, 2012, in connection with the execution of the Settlement Agreement, we entered into a License and Supply Agreement with Paddock and Perrigo (the “License and Supply Agreement”).
Under the terms of the License and Supply Agreement, if a third party receives final approval from the FDA for an ANDA to sell a generic Acetadote product and such third party made such generic version available for purchase in commercial quantities in the United States, we are to supply Perrigo with an Authorized Generic version of our Acetadote product.
19
On May 18, 2012, we also submitted a Citizen Petition to the FDA requesting that the FDA refrain from approving any applications for acetylcysteine injection that contain EDTA, based in part on the FDA’s request that we evaluate the reduction or removal of EDTA from our original Acetadote formulation.
On November 7, 2012, the FDA responded to the Citizen Petition denying our request and on November 8, 2012, we learned that the FDA approved the ANDA referencing Acetadote filed by InnoPharma, Inc. We brought suit against the FDA contesting the FDA’s decision to approve the InnoPharma generic on November 13, 2012.
On September 30, 2013, the United States District Court filed an opinion granting a summary judgment in favor of the FDA regarding this suit.
As noted above, during 2012 the FDA approved the ANDA referencing Acetadote filed by InnoPharma, Inc. Upon this condition, in accordance with the License and Supply agreement with Perrigo, we began to supply Perrigo with our Authorized Generic. On January 7, 2013, Perrigo announced initial distribution of our Authorized Generic acetylcysteine injection product.
On March 19, 2013, the USPTO issued U.S. Patent number 8,399,445 (the “445 Acetadote Patent”) which is assigned to us. The claims of the 445 Acetadote Patent encompass the use of the 200 mg/ml Acetadote formulation to treat patients with acetaminophen overdose. On April 8, 2013, the 445 Acetadote Patent was listed in the FDA Orange Book. The 445 Acetadote Patent is scheduled to expire in August 2025. Following the issuance of the 445 Acetadote Patent we received separate Paragraph IV certification notices from Perrigo, Sagent Pharmaceuticals, Inc., and Mylan challenging the 445 Acetadote Patent on the basis of non-infringement, unenforceability and/or invalidity.
On June 10, 2013, we became aware of a Paragraph IV certification notice from Akorn, Inc. challenging the 445 Acetadote Patent and the 356 Acetadote Patent on the basis of non-infringement. On July 12, 2013, we filed a lawsuit for infringement of the 356 Acetadote Patent against Akorn, Inc. in United States District Court.
On February 18, 2014, the USPTO issued U.S. Patent number 8,653,061 (the “061 Acetadote Patent”) which is assigned to us. The claims of the 061 Acetadote Patent encompass the use of the 200 mg/ml Acetadote formulation to treat patients with acetaminophen overdose. Following its issuance, the 061 Acetadote Patent was listed in the FDA Orange Book. The 061 Acetadote Patent is scheduled to expire in August 2025.
On May 13, 2014, the USPTO issued U.S. Patent number 8,722,738 (the “738 Acetadote Patent”) which is assigned to us. The claims of the 738 Acetadote Patent encompass administration methods of acetylcysteine injection, without specification of the presence or lack of EDTA in the injection. Following its issuance, the 738 Acetadote Patent was listed in the FDA Orange Book and it is scheduled to expire in April 2032.
On December 11, 2014 and March 3, 2015, we became aware of Paragraph IV certification notices from Aurobindo Pharma Limited and Zydus Pharmaceuticals (USA) Inc., respectively, challenging the 356, 445, 061 and 738 Acetadote Patents on the basis of non-infringement.
On February 10, 2015, the USPTO issued U.S. Patent number 8,952,065 (the “065 Acetadote Patent”) which is assigned to us. The claims of the 065 Acetadote Patent encompass the use of the 200 mg/ml Acetadote formulation to treat patients with acute liver failure. The 065 Acetadote Patent is scheduled to expire in August 2025.
On September 30, 2015, the United States District Court for the Northern District of Illinois, Eastern Division (“District Court”) ruled in our favor in our lawsuit against Mylan for infringement of the 445 Acetadote Patent. The opinion upheld our 445 Acetadote Patent and expressly rejected Mylan’s validity challenge. The District Court ruled that Mylan is liable to us for infringement of the 445 Acetadote patent in light of Mylan’s Abbreviated New Drug Application in which Mylan sought to market a generic version of Acetadote.
On November 17, 2015, the District Court entered an order enjoining Mylan and its affiliates from selling or using its generic version of Acetadote until August 2025, the date of expiration of the 445 Acetadote Patent. On October 30, 2015, Mylan filed a notice of appeal to the U.S. Court of Appeals for the Federal Circuit (the “Appeals Court”).
20
On May 3, 2016, the USPTO issued U.S. Patent number 9,327,028 (the “028 Acetadote Patent”) which is assigned to us. The claims of the 028 Acetadote Patent encompass administration methods of acetylcysteine injection, without specification of the presence or lack of EDTA in the injection. Following its issuance, the 028 Acetadote Patent was listed in the FDA Orange Book and it is scheduled to expire in July 2031.
On January 26, 2017, the Appeals Court affirmed the District Court ruling in our favor in our lawsuit against Mylan for infringement of the 445 Acetadote Patent. The Appeals Court opinion affirmed the District Court’s ruling upholding our 445 Acetadote Patent and expressly rejected Mylan’s validity challenge.
On November 3, 2017, we became aware of a Paragraph IV certification notice from Exela Pharma Sciences, LLC challenging the 356, 445, 061, 738 and 028 Acetadote Patents on the basis of non-infringement.
Caldolor®
We have an exclusive, worldwide license to clinical data for intravenous ibuprofen from Vanderbilt University, in consideration for royalty obligations related to Caldolor. During 2014, we obtained additional patents for the brand. On May 27, 2014, the USPTO issued U.S. Patent number 8,735,452 (the “452 Caldolor Patent”) which is assigned to us. The claims of the 452 Caldolor Patent encompass methods of treating pain using intravenous ibuprofen. Following its issuance, the 452 Caldolor Patent was listed in the FDA Orange Book and is scheduled to expire in September 2029.
On October 28, 2014, the USPTO issued U.S. Patent number 8,871,810 (the “810 Caldolor Patent”) which is assigned to us. The claims of the 810 Caldolor Patent encompass methods of treating pain using intravenous ibuprofen. Following its issuance, the 810 Caldolor Patent was listed in the FDA Orange Book and is scheduled to expire in September 2029.
During the third quarter of 2015, we obtained four additional patents for Caldolor. On July 7, 2015, the USPTO issued U.S. Patent number’s 9,072,710 (the “710 Caldolor Patent”) and 9,072,661 (the “661 Caldolor Patent”) which are assigned to us. The claims of the 710 Caldolor Patent and the 661 Caldolor Patent include composition and methods of treating pain, inflammation and fever using intravenous ibuprofen. These Caldolor Patents are listed in the FDA Orange Book and are scheduled to expire in March 2032.
On April 21, 2015, the USPTO issued U.S. Patent No. 9,012,508 (the “508 Caldolor Patent”) which is assigned to us.
The claims of the 508 Caldolor Patent include methods of treating pain using intravenous ibuprofen. Following its issuance, the 508 Caldolor Patent was listed in the FDA Orange Book and is scheduled to expire in September 2030.
On August 25, 2015, the USPTO issued U.S. Patent number 9,114,068 (the “068 Caldolor Patent”) which is assigned to us. The claims of the 068 Caldolor Patent include methods of treating pain using intravenous ibuprofen.
Following its issuance, the 068 Caldolor Patent was listed in the FDA Orange Book and is scheduled to expire in September 2029.
On September 22, 2015, the USPTO issued U.S. Patent number 9,138,404 (the “404 Caldolor Patent”) which is assigned to us.
The claims of the 404 Caldolor Patent include methods of treating pain in critically ill patients with intravenous ibuprofen. Following its issuance, the 404 Caldolor Patent was listed in the FDA Orange Book and is scheduled to expire in September 2029.
On March 29, 2016, the USPTO issued U.S. Patent number 9,295,639 (the “639 Caldolor Patent”) which is assigned to us. The claims of the 639 Caldolor Patent include methods of treating pain in critically ill patients with intravenous ibuprofen. Following its issuance, the 639 Caldolor Patent was listed in the FDA Orange Book and is scheduled to expire in September 2029.
21
On May 16, 2017, the USPTO issued U.S. Patent number 9,649,284 (the “284 Caldolor Patent”) which is assigned to us. The claims of the 284 Caldolor Patent include methods of treating pain in critically ill patients with intravenous ibuprofen. Following its issuance, the 284 Caldolor Patent was listed in the FDA Orange Book and is scheduled to expire in September 2029. We also have additional patent applications related to Caldolor pending with the USPTO.
Vibativ®
We own numerous U.S. patents and related international patents for Vibativ. These patents were acquired in our November 2018 acquisition of certain product rights, intellectual property and related assets of Vibativ from Theravance. Two Vibativ patents are listed in the FDA Orange Book. U.S. Patent number 7,531,623 (the “623 Vibativ Patent”) is scheduled to expire in January 2027 and includes composition of matter claims that encompass the Vibativ drug substance as well as methods for preparing the Vibativ drug substance.
Sancuso®
We are the owner of U.S. Patent number 7,608,282 (the “282 Sancuso Patent”) for Sancuso. This patent was acquired in our December 2021 acquisition, which closed in January 2022. The patent is of certain product rights, intellectual property and related assets of Sancuso from Kyowa Kirin, Inc. The 282 Sancuso Patent is listed in the FDA Orange Book and is scheduled to expire in January 2025. The 282 Sancuso Patent includes composition of matter claims that encompass the Sancuso drug product as well as methods of using Sancuso for treatment and/or prophylaxis.
Remaining Products
We have no issued patents for our Vaprisol, Omeclamox-Pak or Kristalose products. We have multiple granted patents relating to our ifetroban products and patent applications pending with the USPTO.
COMPETITION
The pharmaceutical industry is characterized by intense competition and rapid innovation. Our continued success in developing and commercializing pharmaceutical products will depend, in part, upon our ability to compete against existing and future products in our target markets. Competitive factors directly affecting our markets include but are not limited to:
•product attributes such as efficacy, safety, ease-of-use and cost-effectiveness;
•brand awareness and recognition driven by sales, marketing and distribution capabilities;
•intellectual property and other exclusivity rights;
•availability of resources to build and maintain developmental and commercial capabilities;
•successful business development activities;
•extent of third-party reimbursements, insurance coverage; and
•establishment of advantageous collaborations to conduct development, manufacturing or commercialization efforts.
A number of our competitors possess research and development and sales and marketing capabilities as well as financial resources greater than ours. These competitors, in addition to emerging companies and academic research institutions, may be developing, or in the future could develop, new technologies that could compete with our current and future products or render our products obsolete.
Our products face competition from other branded products, generics, and alternate medical treatments. Our task is to position each brand to feature its competitive advantages, implement a well thought out marketing plan and provide focused sales, field based medical and other tactical support.
22
Acetadote®
Acetadote is our injectable formulation of N-acetylcysteine (“NAC”) for the treatment of acetaminophen overdose. NAC is accepted worldwide as the standard of care for acetaminophen overdose. Our competitors in the acetaminophen overdose market are those companies selling orally administered NAC including, but not limited to, Geneva Pharmaceuticals, Inc., Bedford Laboratories division of Hikma Pharmaceuticals, Roxane Laboratories, Inc., InnoPharma Inc. and Hospira Inc.
In November 2012, InnoPharma Inc. was granted approval by the FDA to distribute their generic form of the old formulation of Acetadote containing EDTA. In late 2012, we entered into the Settlement Agreement with Paddock and Perrigo that included the right to distribute our Authorized Generic Acetadote injection product. Our branded Acetadote now competes with both the EDTA free Authorized Generic Acetadote distributed by Paddock and Perrigo along with generic Acetadote products that contain EDTA.
Manufacturers of the old Acetadote formulation include: Akorn, AuroMedics Pharma, Fresenius Kabi and Sagent Pharmaceuticals.
Caldolor®
Caldolor is marketed for the treatment of pain and fever, primarily in a hospital or surgery center setting. A variety of other products address the acute pain market:
•Morphine, the most commonly used product for the treatment of acute, post-operative pain, is manufactured and distributed by several generic pharmaceutical companies;
•Other generic injectable opioids, including fentanyl, meperidine and hydromorphone, address this market;
•Ketorolac tromethamine (brand name Toradol®), an injectable NSAID, is also manufactured and distributed by several generic pharmaceutical companies;
•IV acetaminophen (brand name Ofirmev®), an injectable analgesic product is sold by Mallinckrodt plc, and there are also generic versions from different manufacturers available;
•Bupivacaine and meloxicam extended release solution (brand name Zynrelef®), is a dual-acting anesthetic for postoperative pain sold by Heron Therapeutics;
•Oliceridine injection (brand name Olinvyk®), is an opioid agonist, a new chemical entity in the acute surgical postoperative pain market, sold by Trevena, Inc.;
•Bupivacaine injectable suspension (brand name Exparel®), product sold by Pacira Pharmaceuticals, Inc., along with two additional bupivacaine products, Xaracoll and Posimir,which were more recently approved; and
•Acetaminophen and ibuprofen combination injection (brand name Combogesic®), is a combination of acetaminophen and ibuprofen approved by the U.S. FDA in October 2023, and is sold by Hikma Pharmaceuticals PLC.
We are aware of other product candidates in development to treat acute pain including injectable NSAIDs, novel opioids, new formulations of existing therapies and extended release anesthetics. We believe non-narcotic analgesics for the treatment of post-surgical pain are the primary potential competitors to Caldolor.
In addition to the injectable analgesic products above, many companies are developing analgesics for specific indications such as migraine and neuropathic pain, oral extended-release forms of existing narcotic and non-narcotic products, as well as those with new methods of delivery such as transdermal. We are not aware of any approved injectable products indicated for the treatment of fever in the U.S. other than Caldolor and Ofirmev.
There are, however, numerous drugs available to physicians to reduce fevers in hospital settings via oral and rectal administration to the patient, including ibuprofen, acetaminophen and aspirin. These drugs are manufactured by numerous pharmaceutical companies.
23
Kristalose®
Kristalose is a dry powder crystalline prescription formulation of lactulose indicated for the treatment of constipation. The U.S. constipation therapy market includes various prescription and over the counter, or OTC, products. The branded prescription products which we believe are our primary competitors are:
•Lubiproston (brand name Amitiza®), an oral product indicated for the treatment of chronic idiopathic constipation, irritable bowel syndrome with constipation in adults, is manufactured and sold by Mallinckrodt Pharmaceuticals.
•Naloxegol (brand name Movantik®), an oral product indicated for the treatment of opioid-induced constipation in adults with chronic non-cancer pain. It was acquired by RedHill Biopharma in the first quarter of 2020.
•Linaclotide (brand name Linzess®), an oral product indicated for the treatment of irritable bowel syndrome with constipation and chronic idiopathic constipation. It is sold by Allergan, Inc. and Ironwood Pharmaceuticals, Inc.
•Plecanatide (brand name Trulance®), an oral product indicated for the treatment of irritable bowel syndrome with constipation and chronic idiopathic constipation. It is sold by Synergy Pharmaceuticals.
•Generic and branded liquid lactulose products are marketed by a number of pharmaceutical companies.
•Lactitol for oral solution (brand name Pizensy®), an oral, osmotic laxative indicated for the treatment of chronic idiopathic constipation. It is distributed by Braintree Laboratories, Inc. and was recently approved by the FDA.
There are several hundred OTC products used to treat constipation marketed by numerous pharmaceutical and consumer health companies. MiraLax (polyethylene glycol 3350), previously a prescription product, was indicated for the treatment of constipation and manufactured and marketed by Bayer. MiraLax was converted to an OTC product in February 2007 and recently, the FDA rescinded the approval of the generic prescription polyethylene glycol 3350 products.
Sancuso®
Sancuso is the only transdermal patch FDA approved for the management of chemotherapy induced nausea and vomiting (“CINV”). Each patch delivers up to five days of treatment with granisetron, a standard of care for CINV, through the skin. Recommended treatment suggests the patch be applied 24 to 48 hours prior to chemotherapy treatment and remain in place for five days.
While there are no other transdermal products available to treat CINV, there is a large number of generic and branded oral products as well as a limited number of injectables. Cumberland considers the oral branded products to be the most important competition including Akynzeo; Emend® Oral (manufactured by Merck); Varubi® branded NK1 receptor antagonist sold by Tesaro Inc. a division of GSK); Zuplenz® (a branded version of Ondansetron marketed by Par Pharmaceuticals, Inc.) and Kytril® (a branded version of injectable granisetron hydrochloride manufactured by Roche Pharmaceuticals Inc.).
Vaprisol®
Vaprisol is a patented, prescription brand indicated to raise serum sodium levels in hospitalized patients with euvolemic and hypervolemic hyponatremia. The product was developed and registered by Astellas and then launched in 2006. It is one of two branded prescription products indicated for the treatment of hyponatremia, and the first and only intravenously administered branded treatment. The other competing product is Samsca®(an oral tolvaptan product sold by Otsuka Pharmaceutical Company). There are also several generic versions of tolvaptan sold by other companies.
24
Vibativ®
Vibativ is a potent, once-daily, injectable antibiotic for the treatment of certain gram-positive infections. Vibativ is approved for the treatment of complicated skin and skin structure infections and hospital-acquired or ventilator-associated bacterial pneumonia caused by susceptible isolates of Staphylococcus aureus when alternative treatments are not suitable. There are several generic and branded antibiotics that compete for these indications.
The major generic competitors are vancomycin, linezolid, and daptomycin. Vancomycin is by far the most widely used agent. Newer branded agents are also available including:
•Ceftaroline fosamil (brand name Teflaro® ), an injectable antibiotic manufactured and sold by Allergan;
•Dalbavancin (brand name Dalvance® ), an injectable antibiotic manufactured and sold by Allergan; and
•Oritavancin (brand name Orbactiv® ), an injectable antibiotic manufactured and sold by Melinta
We are also aware of a number of other novel antibiotics that are currently in development.
Antibiotic drug selection is based both on an empiric and susceptibility proven basis. In the hospital setting, cost is an important factor which favors the use of generic agents if they are effective. Newer agents are often reserved for two reasons: they are valuable in the treatment of patients that fail to respond to generics and it is considered good practice to conserve the use of these agents to reduce the risk of resistance.antimicrobial resistance, which is considered a global public health threat by the Center for Disease Control and Prevention (“CDC”).
GOVERNMENT REGULATION
The development of new pharmaceutical products can be a long, expensive and risky process. There is no assurance we will obtain successful study results or secure the needed market approvals for our pipeline product candidates. Governmental authorities in the U.S. and other countries extensively regulate the research, development, testing, manufacturing, distribution, marketing and sale of pharmaceutical products. For more information, see "Risks Relating to Government Regulation" in Part I, Item 1A of this Form 10-K.
In the U.S., the FDA under the Federal Food, Drug, and Cosmetic Act, (“FDCA”), the Public Health Service Act, and other federal statutes and regulations, subjects pharmaceutical products to rigorous review. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending New Drug Applications (“NDAs”) or biologics license applications, (“BLAs”), warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties, and criminal prosecution.
We, our manufacturers and contract research organizations may also be subject to regulations under other federal, state and local laws, including the Occupational Safety and Health Act, (“OSHA”), the Resource Conservation and Recovery Act, the Clean Air Act and import, export and customs regulations as well as the laws and regulations of other countries.
FDA Approval Process
The FDA is a regulatory agency within the Department of Health and Human Services. One of their key responsibilities is to regulate the safety and effectiveness of drugs sold in the United States. The FDA manages this responsibility in two phases: pre-approval (pre-market) and post approval (post-market). The FDA reviews manufacturers' applications to market drugs in the United States; a drug may not be sold unless it has FDA approval. The FDA continues its oversight of drug safety and effectiveness as long as the drug is on the market.
To market a prescription drug in the United States, a manufacturer needs FDA approval. To get that approval, the manufacturer must demonstrate the drug's safety and effectiveness according to criteria specified in law and agency regulations, ensure that its manufacturing plant passes FDA inspection, and obtain FDA approval for the drug's labeling, a term that includes all written material about the drug, including, for example, packaging, prescribing information for physicians and patient brochures.
25
The progression to drug approval begins before FDA involvement. First, scientists work in the laboratory to discover and develop a new compound. Next, basic safety questions are answered by nonclinical testing with animals and then, a drug or biotechnology company develops a prototype drug. That company must seek clearance from the FDA by way of an IND application to test the product with human subjects.
Those tests, called clinical trials, are carried out sequentially in Phase I, II, and III studies, which involve increasing numbers of subjects. The manufacturer then compiles the resulting data and analyses in an NDA. The FDA reviews the NDA with three major concerns: (1) safety and effectiveness in the drug's proposed use; (2) appropriateness of the proposed labeling; and (3) adequacy of manufacturing methods to assure the drug's identity, strength, quality and purity.
The FDA and associated regulations detail the requirements at each step. The FDA uses a few special mechanisms to expedite drug development and the review process when a drug might address an unmet need or a serious disease or condition. Those mechanisms include accelerated approval, fast track and priority reviews and the newer designation, breakthrough therapy.
The sponsor of the drug typically conducts human clinical trials in three sequential phases, but the phases may overlap. Phase I clinical trials are generally conducted in a small number of healthy volunteers, primarily to collect and assess pharmacokinetics and safety data at one or more dosages prior to proceeding into patients.
In Phase II clinical trials, the sponsor evaluates the early efficacy of the product in short term trials on the targeted indication and identifies possible adverse effects and safety risks in a patient population.
Phase III clinical trials typically involve testing for patients in long term trials examining safety and clinical efficacy in an expanded population at geographically-dispersed test sites.
The FDA requires that clinical trials be conducted in accordance with the FDA’s Good Clinical Practice (“GCP”) requirements. The FDA may order the partial, temporary or permanent discontinuation of a clinical trial at any time or impose other sanctions if it believes that the clinical trial is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. The institutional review board ("IRB"), or ethics committee (outside of the U.S.), of each clinical site generally must approve the clinical trial design and patient informed consent and may also impose other conditions or require the clinical trial at that site to be halted, either temporarily or permanently, for failure to comply with the IRB's requirements.
The results of the nonclinical and clinical trials, together with detailed information on the manufacturing and composition of the product and proposed labeling, are submitted to the FDA in the form of an NDA for marketing approval. The NDA undergoes a 60-day validation review period before it is accepted for filing.
If the NDA is found to be incomplete, it will not be accepted. Once the NDA is validated and accepted for filing, the FDA begins an in-depth review of the NDA.
Under policies agreed to by the FDA under the Prescription Drug User Fee Act, or PDUFA (currently PDUFA VII - effective September 30, 2022), the FDA has a target timeline of 10 months in which to complete its initial review of a standard NDA and respond to the applicant. The review process and the PDUFA goal date may be extended by two months to address deficiencies, or by three months if the FDA requests or if the NDA sponsor otherwise provides additional information or clarification regarding information already provided in the submission at any time during the review clock period. If the FDA’s evaluations of the NDA and the clinical and manufacturing procedures and facilities are favorable and meet all regulations, the FDA will issue an approval letter. Priority review is reserved for drugs that represent a “significant improvement in safety or efficacy” over existing treatments. The FDA endeavors to complete priority reviews in six months.
If the NDA meets with FDA approval, a letter will be sent out indicating approval and final labeling recommendations. If not, a complete response letter will be sent to applicants indicating that the review cycle for an application is complete and that the application is not ready for approval.
The complete response letter will describe the specific deficiencies that the agency has identified in an application and what changes must be made before the application can be approved, with no implication regarding
26
whether the application will ultimately be approved. An approval letter authorizes commercial marketing of the drug for the proposed indication(s) under study. While the FDA’s PDUFA 2021 Performance Report showed a continued increase in the percentage of first-cycle approval letters for new molecular entities rising from 56% for FY 2009 to 84% for FY 2023, we cannot be certain that timely first-cycle approvals will be maintained by the FDA.
While the time and cost of completing these steps and obtaining FDA approval can vary dramatically depending on the drug, it can take many years and cost millions of dollars for a novel drug.
Section 505(b) New Drug Applications
An NDA may be submitted under different methods, a 505(b)(1), 505(b)(2) or 505(j). Section 505(b) provides for the submission of an NDA to support the approval of a drug. Upon approval, a drug may be marketed only for the FDA-approved indication(s) in the approved dosage form. Further clinical trials may be necessary to gain approval for the use of the product for any additional indications or dosage forms.
The FDA also requires post market safety surveillance reporting to monitor the side effects of the drug, which may result in withdrawal of approval after marketing begins if significant adverse safety findings are found.
Section 505(b)(1), or the ‘full’ NDA, is used for new chemical entities (“NCEs”) and requires full clinical and nonclinical development of a compound. Marketing exclusivity assigned to a 505(b)(1) approval is five years. A 505(b)(2) NDA permits the submission of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant using previously reported safety and efficacy data, and for which the applicant has not obtained a right of reference. Generally new studies are required to provide data on the proposed change.
Some examples of products that may be allowed to follow a 505(b)(2) path to approval are drugs that have a new dosage form, strength, route of administration, formulation or indication or combination drugs. Marketing exclusivity for a 505(b)(2) submission is three years.
Both 505 (b)(1) and (b)(2) are eligible for seven years of exclusivity for orphan drugs and/or six months for pediatric exclusivity. Any marketing exclusivity is independent of patent exclusivity. We successfully secured FDA approvals for Acetadote in January 2004 and for Caldolor in June 2009 pursuant to the 505(b)(2) pathway.
Orphan drug designation
The Orphan Drug Act of 1983 (the “Orphan Drug Act”) encourages manufacturers to seek approval of products intended to treat “rare diseases and conditions” with a prevalence of fewer than 200,000 patients in the U.S. or for which there is no reasonable expectation of recovering the development costs for the product. For products that receive orphan drug designation by the FDA, the Orphan Drug Act provides tax credits for clinical research, FDA assistance with protocol design, eligibility for FDA grants to fund clinical studies, waiver of the FDA application fee and a period of seven years of marketing exclusivity for the product following FDA marketing approval.
Acetadote received orphan drug designation in October 2001 and in 2004 the FDA approved the product to prevent or lessen hepatic injury after ingestion of a potentially hepatotoxic quantity of acetaminophen. Acetadote was entitled to marketing exclusivity until January 2011 for the treatment of this approved indication.
Section 505(j) abbreviated new drug applications
An abbreviated new drug application (“ANDA”) is a type of NDA where approval of a generic drug is based on demonstrating comparability to an innovator drug product (the Reference Listed Drug, or RLD). Applications are "abbreviated" because they generally do not include pre-clinical and clinical data to establish safety and effectiveness. Generics must demonstrate that the product is bioequivalent (i.e., performs in the same manner and is comparable to the 'innovator' product in active ingredient, dosage form, strength, route of administration, labeling, quality, performance characteristics and intended use).
27
Abbreviated applications may be submitted for drug products that are the same as a listed drug and must be identical in active ingredient(s), form, strength, route of administration and in conditions of use (non-exclusive uses). Products are declared suitable based on a suitability petition to the FDA. If the petition is approved, the sponsor may then submit the ANDA.
The Hatch-Waxman Act
The Drug Price Competition and Patent Term Restoration Act, informally known as the “Hatch-Waxman Act”, is a 1984 United States federal law that established the modern system of generic drugs.
The Hatch-Waxman Act amended the Federal Food, Drug, and Cosmetic Act. Section 505(j) 21 U.S.C. 355(j) sets forth the process by which would-be marketers of generic drugs can file ANDAs to seek FDA approval of the generic. Section 505(j)(2)(A)(vii)(IV), the so-called Paragraph IV, allows 180-day exclusivity to companies that are the "first-to-file" an ANDA against holders of patents for branded counterparts.
These Hatch-Waxman Act amendments grant generic manufacturers the ability to mount a validity challenge without incurring the cost of entry or risking enormous damages flowing from any possible infringement. The Hatch-Waxman Act essentially redistributes the relative risk assessments and explains the flow of settlement funds and their magnitude. The Hatch-Waxman Act gives generics considerable leverage in patent litigation.
Health care legislation
On March 23, 2010, former President Obama signed into law the Patient Protection and Affordable Care Act, (“PPACA”). On March 30, 2010, the Health Care and Education Reconciliation Act of 2010 (“HCERA”), was enacted into law, which modified the revenue provisions of the PPACA. The PPACA as amended by the HCERA constitutes the healthcare reform legislation. The following highlights certain provisions of the legislation that may affect us.
Pharmaceutical Industry Fee: Beginning in calendar-year 2011, an annual fee was imposed on pharmaceutical manufacturers and importers that sell branded prescription drugs to specified government programs (e.g., Medicare Part D, Medicare Part B, Medicaid, Department of Veterans Affairs programs, Department of Defense programs and TRICARE).
The annual fee is allocated to companies based on their previous calendar-year market share using sales data that the government agencies that purchase the pharmaceuticals will provide to the Treasury Department. Although we participate in governmental programs that subject us to this fee, our sales volume in such programs is less than $10 million, with the first $5 million of sales being exempt from the fee. This fee has not had a material impact and is not expected to have a material impact on our results of operations.
In addition, PDUFA imposes annual program fees. An applicant will be assessed annual prescription drug program fees for prescription drug products, incurring a fee for each strength of a drug product. An applicant may not be assessed more than five prescription drug program fees for a fiscal year for prescription drug products identified in a single approved application.
Physician Payments Sunshine Act: The PPACA also includes provisions known as the Physician Payments Sunshine Act (“Sunshine Act”), which requires manufacturers of pharmaceuticals and medical devices covered under Medicare and Medicaid to record any transfers of value to physicians and teaching hospitals and to report this data to the Centers for Medicare and Medicaid Services (“CMS”), for aggregation and subsequent public disclosure. Under the Sunshine Act, beginning August 1, 2013, we have collected data regarding reportable transfers of value and have reported such data to CMS. Failure to report appropriate data may result in civil or criminal fines and/or penalties. In addition to the Federal Sunshine Act, similar reporting requirements have also been enacted on the state level requiring transparency of interactions with health care professionals.
Medicaid Rebate Rate: Under the Medicaid Drug Rebate program we currently are required to provide rebates for covered outpatient drugs that are dispensed to Medicaid beneficiaries. In addition, we also are required to participate in the Public Health Service’s 340B drug pricing program, which requires us to agree to
28
charge no more than a designated ceiling price for covered outpatient drugs that are dispensed to community health clinics and other entities that receive health services grants from the Public Health Service, as well as hospitals that serve a disproportionate share of low-income patients.
Product Serialization: In November of 2013, the FDA passed the Drug Supply Chain Security Act (“DSCSA”). The DSCSA was created to strengthen the security of the drug distribution supply chain by adding controls such as a national pharmaceutical track and trace system and establishing national standards for licensing of prescription drug wholesale distributors and third-party logistics providers. DSCSA requires trading partners, including manufacturers, repackagers, wholesale distributors and dispensers to provide transaction information to subsequent purchasers for certain prescription drugs. We have taken necessary steps to implement this program and are in compliance with all requirements.
21st Century Cures Act: The 21st Century Cures Act (“Cures Act”), signed into law on December 13, 2016, is designed to help accelerate medical product development and bring new innovations and advances to patients who need them faster and more efficiently. The law builds on the FDA’s ongoing work to incorporate the perspectives of patients into the development of drugs, biological products and devices in the FDA’s decision-making process. The Cures Act enhances the FDA’s ability to modernize clinical trial designs and clinical outcome assessments, which will speed the development and review of novel medical products, including medical countermeasures.
Specifically, the Cures Act enables us to work with the FDA in the development of new biomarkers, clinical outcome assessments, surrogate endpoints and patient reported outcomes. It allows for the use of data summaries (rather than full clinical trials for approval) as well as the use of real-world evidence to support approval of new indications of approved medical products, or to help satisfy post-approval study requirements for marketed products.
Build Back Better Act and Other Proposed Legislation: The Build Back Better Act (“BBBA”) was introduced in the 117th Congress and included provisions that were intended to lower the price of prescription drugs, including granting the Medicare program the authority to negotiate prescription drug prices and imposing tax penalties on drug manufacturers if the price of drugs increases too rapidly. Ultimately the BBBA was not enacted, however, future legislative initiatives are likely to include provisions targeted at containing costs in the prescription drug market.
Post Approval Activities
Once a drug is on the U.S. market (following FDA approval of the NDA), the FDA continues to address drug production, distribution and use. FDA activities are based on ensuring drug safety and effectiveness. They address product integrity, labeling, reporting of research and adverse events, surveillance, drug studies, risk management, information dissemination, off-label use, physician advertising and direct-to-consumer advertising.
If we amend the NDA for an FDA approved product, such as adding safety or efficacy labeling claims, promoting those new claims, making certain manufacturing changes or product enhancements, we will need FDA review and approval before the change can be implemented. While physicians may use products for indications that have not been approved by the FDA, we may not label or promote the product for an indication that has not been approved.
Securing FDA approval for new indications, product enhancements and manufacturing and labeling changes may require us to conduct additional clinical trials under the FDA’s IND regulations. Even if such studies are conducted, they are still subject to the same requirements and timelines as an original NDA.
The FDA continuously gathers information about possible adverse reactions to the products it has approved for use. The FDA requires all manufacturers to report adverse events. It also provides a procedure for consumers and physicians to voluntarily report their concerns about drugs. The agency collects those reports through MedWatch and uses its FDA Adverse Event Reporting System (FAERS) to store and analyze them. Because some events may occur after the use of a drug for reasons unrelated to the product, the FDA reviews the events to assess which ones may indicate a problem with that particular drug.
29
They then use information gleaned from the surveillance data to determine a course of action. They might recommend a change in drug labeling to alert users to a potential problem, or perhaps, require the manufacturer to study the observed association between the drug and the adverse event.
In addition to FDA restrictions on marketing of pharmaceutical products, several other types of state and federal laws have been applied to restrict certain marketing practices in the pharmaceutical industry in recent years. These laws include anti-kickback statutes and false claims statutes.
The federal health care program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any health care item or service reimbursable under Medicare, Medicaid or other federally financed health care programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers and prescribers, purchasers or formulary managers. Violations of the anti-kickback statute are punishable by imprisonment, criminal fines, civil monetary penalties and exclusion from participation in federal health care programs.
In addition to these U.S. laws, we are subject to similar laws that govern our marketing practices and financial arrangements with health care providers and otherwise are intended to prohibit illicit kickbacks and bribery, including the Foreign Corrupt Practices Act.
Federal False Claims Act
The federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to have a false claim paid.
A number of pharmaceutical and other health care companies have been prosecuted under these laws for allegedly inflating drug prices they report to pricing services, which in turn were used by the government to set Medicare and Medicaid reimbursement rates, and for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product.
HIPAA and Other Data Protection Laws
In the United States, we and our collaborators are subject to numerous federal and state privacy and security laws and regulations, including the Health Insurance Portability and Accountability Act of 1996. These laws include obligations related to protecting the privacy and security of health-related personal information, such as information that we may obtain through the clinical trial process. In addition, similar laws and regulations exist in Europe and other jurisdictions, including the European Union’s General Data Protection Regulation.
ICH - International Committee on Harmonization
Outside of the U.S., our ability to market our products will depend on receiving marketing authorizations from the appropriate regulatory authorities. The International Committee on Harmonization (“ICH”) provides a set of standards that most regulatory authorities adhere to (e.g. U.S., Europe, and Japan) allowing greater harmonization in the interpretation and application of technical guidelines and requirements for pharmaceutical product registration, thereby reducing or obviating duplication of testing carried out during the research and development of new human medicines. Regulatory harmonization offers many direct benefits to both regulatory authorities and the pharmaceutical industry with beneficial impact for the protection of public health.
30
ENVIRONMENTAL MATTERS
We are subject to federal, state and local environmental laws and regulations and we believe that our operations comply with such regulations. We anticipate that the effects of compliance with federal, state and local laws and regulations relating to the discharge of materials into the environment will not have any material effect on our capital expenditures, earnings or competitive position.
SEASONALITY
There are no significant seasonal aspects to our business.
BACKLOG
Due to the relatively short lead-time required to fill orders for our products, backlog of orders is not considered material to our business.
EMPLOYEES
As of December 31, 2023, we had 91 employees. We believe that our future will depend in part on our continued ability to attract, hire, and retain qualified personnel, including hospital oncology and field sales personnel in particular. To that end, we work with qualified search firms to identify talent, we measure and adjust compensation levels to remain competitive and we work closely with team members to support their success.
31
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
We make statements in this Annual Report on Form 10-K that are “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. All statements other than statement of historical facts may be forward-looking statements. In particular, forward-looking statements include, among other things, statements regarding our intent, belief or expectations, and can be identified by the use of terminology such as “may,” “will,” “expect,” “believe,” “intend,” “plan,” “estimate,” “should,” “seek,” “anticipate” and other comparable terms or the negative thereof. In addition, we, through our senior management, from time to time make forward-looking oral and written public statements concerning our expected future operations and other developments. While forward-looking statements reflect our good-faith beliefs and best judgment based upon current information, they are not guarantees of future performance and are subject to known and unknown risks and uncertainties, including those mentioned in Item 1A, “Risk Factors,” Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere in this Form 10-K. Accordingly, investors are cautioned not to place undue reliance on any forward-looking statements. Actual results may differ materially from the expectations contained in the forward-looking statements as a result of various factors. Such factors include, but are not limited to:
•The possible or assumed future results of operations, including the accuracy of our estimates regarding expenses, future revenues, capital requirements and needs for additional financing;
•The impact of macroeconomic conditions, including inflationary pressures, rising interest rates, general economic slowdown or a recession, changes in monetary policy, volatile market conditions, financial institution instability, as well as geopolitical instability and the ongoing conflicts outside the U.S., on our operations;
•Our competitive position and competitors, including the size and growth potential of the markets for our products and product candidates;
•The success, cost and timing of our product acquisition and development activities and clinical trials; and our ability to successfully commercialize our product candidates;
•Product efficacy or safety concerns, whether or not based on scientific evidence, resulting in product withdrawals, recalls, regulatory action on the part of the FDA (or international counterparts) or declining sales;
•The performance of our third-party suppliers and manufacturers which impacts our supply chain and could create business shutdowns or product shortages; and the retention of key scientific and management personnel;
•The impact on our business, financial condition and results of operations from the effects of a pandemic or the outbreak of an infectious disease in the United States and worldwide and resulting governmental and societal responses;
•Challenges to our patents and the introduction of generic versions of our products and product candidates, which could negatively impact our ability to commercialize and sell our products and product candidates and decrease sales a result of market exclusivity;
•Changes in reimbursement available to us, including changes in Medicare and Medicaid payment levels and availability of third-party insurance coverage and the effects of future legislation or regulations, including changes to regulatory approval of new products, licensing and patent rights, environmental protection and possible drug re-importation legislation;
•Interruptions and breaches of our computer and communications systems, and those of our vendors, including computer viruses, hacking and cyber-attacks, that could impair our ability to conduct business and communicate internally and with our customers, or result in the theft of trade secrets or other misappropriation of assets, or otherwise compromise privacy of sensitive information belonging to us, our customers or other business partners; and
•Issuance of new or revised accounting standards by the Financial Accounting Standards Board and the Securities and Exchange Commission.
32
The list above contains many, but not all, of the factors that could impact our ability to achieve results described in any forward-looking statements. Investors should understand that it is not possible to predict or identify all such factors and should not consider this list to be a complete statement of all potential risks and uncertainties. For more information about the risks, uncertainties, and other factors that could affect our future results, please refer to Item 1A, Risk Factors, included herein.
33
Item 1A. Risk Factors.
Risk Factor Summary
Investing in our common stock involves a high degree of risk. You should carefully consider all information in this Annual Report on Form 10-K prior to investing in our common stock. These risks are discussed more fully in the section titled “Risk Factors.” These risks and uncertainties include, but are not limited to, the following:
•Global and national conditions and events, including, but not limited to, rising interest rates, increased inflation, supply chain disruptions, labor conditions, pandemics and public health crises and international conflict, may adversely affect our business, revenues, results of operations and financial condition.
•Failure to implement strategies to enhance our performance could have a material adverse effect on our business, results of operations and financial conditions.
•Our ability to perform depends on keeping and hiring exceptionally talented management and employees, and our failure to do so could have a material adverse effect on our business, revenues, results of operations and financial condition.
•Our success depends, in part, on our ability to successfully obtain or retain high-performing third-party performers on commercially acceptable terms, and the failure to do so can have a material adverse effect on our business, financial conditions and results of operations.
•Our business is subject to stringent government regulations, it must adhere to numerous complex pieces of legislation, and all of our products face regulatory challenges.
•Our business depends on the successful protection of our intellectual property rights and our product candidates becoming approved by regulatory agencies, commercially viable, and accepted by the market.
•Our business faces a serious financial risk if generic products that compete with any of our branded pharmaceutical products are approved and sold because sales of our products will be adversely-affected and our business may not recover the capital costs of bringing that product to market.
•Our business faces an inherent risk of product liability lawsuits related to the testing of our product candidates and the commercial sale of our products, and if we cannot successfully defend ourselves against the product liability claim, we may incur substantial liabilities.
•We may attempt to develop internationally and license our products globally, as well as invest in other businesses or joint ventures, all of which may be unsuccessful, divert our management’s attention and harm our operating results and prospects.
The risk factors described below and throughout this report should be carefully considered and could materially affect our business. There are also risks that are not presently known or not presently material, as well as the other information set forth in this report that could materially affect our business. In addition, in our periodic filings with the SEC, press releases and other statements, we discuss estimates and projections regarding our future performance and business outlook. By their nature, such “forward-looking statements” involve known and unknown risks, uncertainties and other factors that in some cases are out of our control. For a further discussion of forward-looking statements, please refer to the section entitled “Special Note Regarding Forward-Looking Statements.” These factors could cause our actual results to differ materially from our historical results or our present expectations and projections. These risk factors and uncertainties include, but are not limited to the following:
34
RISKS RELATED TO OUR BUSINESS
Global and national economic conditions and events, including, but not limited to increased inflation, rising interest rates, supply chain disruptions, labor conditions, pandemics and public health crises and international conflicts, could affect our future access to liquidity and materially adversely affect our results of operations and financial condition.
Our business and results of operations could be adversely affected by changes in global or national economic conditions. These conditions include, but are not limited to, increased inflation, high and rising interest rates, supply chain disruptions, labor conditions, the negative impacts from pandemics and public health crises (including any lingering or recurring adverse impacts from the COVID-19 pandemic) and the negative impacts resulting from the ongoing conflicts in Eastern Europe and the Middle East. These conditions have had a significant adverse impact on economic and market conditions around the world, including the United States. While the economic impact brought by, and the duration of, such global events is difficult to assess or predict, such events could result in additional disruption of global financial markets, reducing our ability to access capital in the future, which could negatively affect our liquidity in the future and in ways that cannot be predicted potentially including a prolonged recessionary environment in the United States. In the longer term, there could be significant new regulatory actions and other events that could limit our activities and investment opportunities or change the functioning of the capital markets, and there is the possibility of a severe worldwide economic downturn. Inflation rates have increased recently to levels not seen in decades. If our costs, in particular costs related to clinical trial expenses and/or employee-related expenses, were to become subject to significant inflationary pressures, it may adversely impact our business, operating results and financial condition. In response to inflationary pressures, the Federal Reserve raised interest rates in 2022 and 2023, and these increases may continue in 2024 and beyond. Increases in interest rates, especially if coupled with reduced government spending and volatility in financial markets, may further increase economic uncertainty and heighten these risks. Although we do not believe that inflation has had a material impact on our financial position or results of operations to date, we may experience increases in the near future (especially if inflation rates continue to rise) on our operating costs, including our labor costs and research and development costs, due to supply chain constraints, ongoing effects of the COVID-19 pandemic, the ongoing conflicts in Eastern Europe and the Middle East and employee availability and wage increases.
An adverse development regarding our products could have a material and adverse impact on our future revenues and profitability.
Our product portfolio currently includes seven brands: Acetadote, Caldolor, Kristalose, Vaprisol, Omeclamox-Pak, Vibativ and Sancuso. A product contamination or other safety or regulatory issues, such as a failure to meet certain FDA reporting requirements involving our products, could negatively impact us and possibly lead to a product recall. In addition, changes impacting any of our products in areas such as competition, lack of market acceptance or demand, government regulation, intellectual property, reimbursement and manufacturing could have an adverse impact on our future revenues and profitability including:
•Changes in intellectual property protection available for our products or competing treatments;
•Any unfavorable publicity concerning us, our products, or the markets for these products such as information concerning product contamination or other safety issues in any of our product markets, whether or not directly involving our products;
•Perception by physicians and other members of the healthcare community of the safety or efficacy of our products or competing products;
•Regulatory developments related to our marketing and promotional practices or the manufacture or continued use of our products;
•The prices of our products relative to other drugs or competing treatments;
•The impact of current or additional generic competitors;
•The availability and level of third-party reimbursement for sales of our products;
35
•The continued availability of adequate supplies of our products to meet demand:
•Weakened demand for our products; and
•Unforeseen or serious adverse effects outside of those specified in current product labeling being attributed to any of our approved products.
Acetadote may be used to treat acetaminophen overdoses. The FDA has previously requested prescribers and manufacturers of prescription combination products that contain acetaminophen to limit the amount of acetaminophen to no more than 325 milligrams (mg) in each tablet or capsule. The FDA requested this action to protect consumers from the risk of severe liver damage which can result from excess acetaminophen which may reduce the number of acetaminophen overdoses which could result in a lower demand for Acetadote. If the demand for Acetadote decreases, it could have an adverse impact on our future revenues and profitability.
The commercial success of Caldolor is dependent on many third-parties, including physicians, pharmacists, hospital pharmacy and therapeutics committees, or P&T committees, suppliers and distributors, all of whom we have little or no control over. We expect Caldolor to continue to be administered primarily to hospital and surgery center patients who are unable to receive oral therapies for the treatment of pain or fever. Before we can distribute Caldolor to any new hospital customers, Caldolor must be approved for addition to the hospitals’ formulary lists by their P&T committees. A hospital’s P&T committee generally governs all matters pertaining to the use of medications within the institution, including review of medication formulary data and recommendations of drugs to the medical staff. We cannot guarantee that we will be successful in getting the approvals we need from enough P&T committees to be able to optimize hospital sales of Caldolor. Even if we obtain hospital approval for Caldolor, we must still convince individual hospital physicians to prescribe Caldolor repeatedly. The commercial success of Caldolor also depends on our ability to coordinate supply, distribution, marketing, sales and education efforts. As with our other products, if Caldolor is not accepted in the marketplace, it could have an adverse impact on our future revenues and profitability.
Disruptions at the FDA and other government agencies caused by funding shortages or global health concerns could hinder their ability to review and approve new products and otherwise affect the FDA’s ability to perform routine functions. Disruptions at the FDA and other agencies may also slow the time necessary for new drugs or modifications to approved drugs to be reviewed and/or approved by necessary government agencies. Such disruptions could significantly impact the ability of the FDA or other regulatory authorities to timely review and process our regulatory submissions, which could have a material adverse effect on our business.
If any manufacturer or partner we rely upon fails to supply our products in the amounts we require on a timely basis, or fails to comply with stringent regulations applicable to pharmaceutical drug manufacturers, we may be unable to meet demand for our products and may lose potential revenues.
We do not manufacture any of our products, and we do not currently plan to develop any capacity to do so. Our dependence upon third parties for the manufacture of our products could adversely affect our profit margins or our ability to develop and deliver products on a timely and competitive basis. If for any reason we are unable to obtain or retain third-party manufacturers on commercially acceptable terms, we may not be able to sell our products as planned. Furthermore, if we encounter delays or difficulties with contract manufacturers in producing our products, the distribution, marketing and subsequent sales of these products could be adversely affected. A long-term inability to meet demand for our products could result in impairment of our brands overall future and the carrying value of the assets associated with our brands.
Acetadote: We have an agreement with one manufacturer to provide commercial supply of Acetadote. If this manufacturer is unable to produce marketable inventory in sufficient quantities, in the agreed upon time period, we could suffer an inability to meet demand for Acetadote.
Caldolor: We have agreements with multiple manufacturers for the supply of Caldolor. If the manufacturers of Caldolor are unable to produce marketable inventory in sufficient quantities, in the agreed upon time period, we could suffer an inability to meet demand for Caldolor.
36
Kristalose: The active pharmaceutical ingredient for Kristalose is manufactured at a single facility through a complex process. It would be particularly difficult to find a new manufacturer of the Kristalose active pharmaceutical ingredient on an expedited basis. We have manufacturing relationship with one packager who provided finished supplies of Kristalose for commercial and sampling purposes during 2022. If the manufacturing or packaging facilities are unable to produce useable or marketable inventory in sufficient quantities, in the agreed upon time period, we could suffer an inability to meet demand for Kristalose.
Omeclamox-Pak: Our packager for Omeclamox-Pak encountered financial difficulties due to the impact of COVID-19, and their operations are currently suspended. Cumberland is awaiting resumption of those operations while also exploring other alternatives to restart the product’s packaging. In October 2020, we informed the FDA of a shortage of Omeclamox-Pak which continues. If we are unable to obtain marketable inventory in the future, we could continue to suffer an inability to meet demand for Omeclamox-Pak. With uncertain future cash flows, the Board of Directors approved the write-down of the intangible assets related to the product.
Vaprisol: In 2018, the manufacturer of Vaprisol informed us that they would no longer be able to provide the product following the manufacturing of one final batch which is providing us with a multi-year supply. We are currently working with a new manufacturer to provide us with long term supplies of the product. In February 2022, we notified the FDA of a shortage of Vaprisol. If we are unable to produce additional marketable inventory in sufficient quantities, in the required time frame, we could suffer an inability to meet demand for Vaprisol.
Vibativ: Through our acquisition of Vibativ, we acquired a multi-year supply of raw material, work in process and finished goods inventory. In 2020, we completed the transfer of Vibativ manufacturing activities to a new supplier. If we are unable to continue to obtain marketable inventory in sufficient quantities, in the agreed upon time period, we could suffer an inability to meet demand for Vibativ.
Sancuso: As part of the acquisition of Sancuso in January 2022, we obtained an initial supply of finished goods inventory and work in progress. On December 6, 2022, we received notification from Kyowa Kirin, Inc. that the FDA approved a supplemental new drug application associated with a new site at Kindeva Drug Delivery L.P., for the manufacturing and primary packaging of the Sancuso brand.
In addition, all manufacturers of our products and product candidates must comply with current good manufacturing practices, ("GMPs"), enforced by the FDA through its facilities inspection program. These requirements include quality control, quality assurance, and the maintenance of records and documentation. Manufacturers of our products must be unable to comply with GMP requirements and with other FDA, state, and foreign regulatory requirements.
We have no control over our manufacturers’ compliance with these regulations and standards. If our third-party manufacturers do not comply with these requirements, we could be subject to fines and civil penalties; suspension of production or distribution; suspension or delay in product approval; product seizure or recall; and withdrawal of product approval.
We are dependent on a variety of other third parties. If these third parties fail to perform as we expect, our operations could be disrupted and our financial results could suffer.
We have a relatively small internal infrastructure. We rely on a variety of third parties, in addition to our manufacturers, to help us operate our business. If these third parties do not continue to provide services to us, or collaborate with us, we might not be able to obtain others who can serve these functions. This could disrupt our business operations, increase our operating expenses or otherwise adversely affect our operating results.
Specifically, we depend and will continue to depend upon independent investigators and collaborators, such as universities, medical institutions, contract research organizations (CROs) and strategic partners to conduct our preclinical and clinical trials. We negotiate budgets and contracts with CROs and study sites, which may result in delays to our development timelines and increased costs. We will rely heavily on these third parties over the course of our clinical trials, and we control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our studies is conducted in accordance with applicable protocol, legal, regulatory and scientific standards, and our reliance on third parties does not relieve us of our regulatory responsibilities. We and these third parties are required to comply with Good Clinical Practice (GCPs), which are regulations and guidelines enforced by
37
the FDA and comparable foreign regulatory authorities for therapeutic candidates in clinical development. Regulatory authorities enforce these GCPs through periodic inspections of trial sponsors, principal investigators and trial sites. If we or any of these third parties fail to comply with applicable GCP regulations, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that, upon inspection, such regulatory authorities will determine that any of our clinical trials comply with the GCP regulations. Moreover, our business may be implicated if any of these third parties violates federal or state fraud and abuse or false claims laws and regulations or healthcare privacy and security laws. Any third parties conducting our clinical trials are not and will not be our employees and, except for remedies available to us under our agreements with such third parties, we cannot control whether or not they devote sufficient time and resources to our ongoing preclinical, clinical and nonclinical programs.
Further, these third parties may have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical studies or other drug development activities, which could affect their performance. If these third parties do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated and we may not be able to complete development of, obtain regulatory approval of or successfully commercialize our therapeutic candidates. As a result, our financial results and the commercial prospects for our therapeutic candidates would be harmed, our costs could increase and our ability to generate revenue could be delayed. If any of our relationships with trial sites, or any CRO that we may use in the future, terminates, we may not be able to enter into arrangements with alternative trial sites or CROs or do so on commercially reasonable terms. Switching or adding third parties to conduct our clinical trials involves substantial cost and requires extensive management time and focus. In addition, there is a natural transition period when a new third party commences work. As a result, delays occur, which can materially impact our ability to meet our desired clinical development timelines.
Competitive pressures could reduce our revenues and profits.
The pharmaceutical industry is intensely competitive. Our strategy is to target differentiated products in specialized markets. However, this strategy does not relieve us from competitive pressures and can entail distinct competitive risks. Certain of our competitors do not aggressively promote their products in our markets. An increase in promotional activity in our markets could result in large shifts in market share, adversely impacting us.
Our competitors may sell or develop drugs that are more effective and useful or less costly than ours, and they may be more successful in manufacturing and marketing their products. Many of our competitors have significantly greater financial and marketing resources than we do. Additional competitors may enter our markets.
The pharmaceutical industry is characterized by constant and significant investment in new product development, which can result in rapid technological change. The introduction of new products could substantially reduce our market share or render our products obsolete. The selling prices of pharmaceutical products tend to decline as competition increases, through new product introduction or otherwise, which could reduce our revenues and profitability.
If generic products that compete with any of our branded pharmaceutical products are approved and sold, sales of our products will be adversely affected.
Generic equivalents for branded pharmaceutical products are typically sold at lower costs than the branded products. The regulatory approval process in the United States exempts generic products from costly and time-consuming clinical trials to demonstrate their safety and efficacy and rely instead on the safety and efficacy of prior products, manufacturers of generic products can invest far less in research and development. After the introduction of a competing generic product, a significant percentage of the prescriptions previously written for the branded product are often written for the generic version. In addition, legislation enacted in most U.S. states allows or, in some instances mandates, that a pharmacist dispense an available generic equivalent when filling a prescription for a branded product, in the absence of specific instructions from the prescribing physician. Governmental and private healthcare payors also emphasize substitution of branded pharmaceuticals with less expensive generic equivalents.
38
Pursuant to the provisions of the Hatch-Waxman Act, manufacturers of branded products often bring lawsuits to enforce their patent rights against generic products released prior to the expiration of branded products’ patents, but it is possible for generic manufacturers to offer generic products while such litigation is pending. As a result, branded products typically experience a significant loss in revenues following the introduction of a competing generic product, even if subject to an existing patent. Our branded pharmaceutical products are or may become subject to competition from generic equivalents because there is no proprietary protection for some of the branded pharmaceutical products we sell, because our patent protection expires or because our patent protection is not sufficiently broad or enforceable. In addition, we may not be successful in our efforts to extend the proprietary protection afforded our branded products through the development and commercialization of proprietary product improvements. Competition from generic equivalents could result in a decrease in revenues of our branded pharmaceuticals or result in a material impairment of our intangible assets or the acceleration of amortization on our non-impaired intangible assets and may have a material adverse impact on our revenues, financial condition, results of operations and cash flows.
Any attempt by us to expand the potential market for any of our products is subject to limitations.
Expansion of the market for our products may be subject to certain limitations. In the past, these limitations have included FDA required Phase IV commitments. We may also experience delays associated with future required Phase IV clinical studies potentially resulting from, among other factors, difficulty enrolling patients. We may not be able to initiate or continue clinical trials for our product candidates if we are unable to identify and enroll a sufficient number of eligible patients to participate in these trials as required by the FDA. Subject enrollment, a significant factor in the timeline of clinical trials, is affected by many factors including the size and characteristics of the patient population, the proximity of patients to clinical sites, the eligibility and exclusion criteria for the trial, the design of the clinical trial, the risk that enrolled patients will not complete a clinical trial, our ability to recruit clinical trial investigators with the appropriate competencies and experience, our ability to obtain and maintain patient consents, patient referral practices of physicians, ability to monitor patients adequately during and after treatment, competing clinical trials and clinicians’ and patients’ perceptions as to the potential advantages and risks of the product candidate being studied in relation to other available therapies, including any new products that may be approved for the indications we are investigating as well as any product candidates under development. We will be required to identify and enroll a sufficient number of subjects for each of our clinical trials. Delays that may result from difficulty enrolling patients could impact our ability to explore opportunities for label expansion and limit our ability to bring our products to new patient populations.
In addition, we have largely obtained regulatory approval to market our products in the United States. Not all foreign jurisdictions may represent attractive opportunities for our products due to pricing, competitive, regulatory or other factors. In certain foreign jurisdictions, we have licensed the right to market some of our products to third parties. These third parties are responsible for seeking and maintaining regulatory approval for the products in their respective jurisdictions. We have no control over these third parties and cannot be sure that marketing approval for our products will be obtained outside the United States.
Our future growth depends on our ability to identify, acquire rights and successfully integrate new brands into our operations. If we do not successfully identify and acquire rights to products or if we do not successfully integrate acquired product brands into our operations, our growth opportunities may be limited.
We have added six products to our portfolio of brands through acquisitions. Our business strategy is to continue to acquire rights to FDA-approved products as well as pharmaceutical product candidates in the late stages of development. We do not plan to conduct basic research or preclinical product development, except to the extent of our investment in CET. As compared to large multi-national pharmaceutical companies, we have limited resources to acquire third-party products, businesses and technologies and integrate them into our current infrastructure. Many acquisition opportunities involve competition among several potential purchasers including large multi-national pharmaceutical companies and other competitors that have access to greater financial resources than we do. With future acquisitions, we may face financial and operational risks and uncertainties. We may not be able to engage in future product acquisitions, and those we do complete may not be beneficial to us in the long term.
39
Furthermore, other products in development may encounter unforeseen issues during their clinical trials. Any unforeseen issues or lack of FDA approval will negatively affect marketing and development plans for those products.
If we are unable to successfully integrate the marketing, sale and distribution of any other potential products into our current infrastructure or if they require significantly greater resources than originally anticipated, we may face financial and operational risks and uncertainties. If we are unable to successfully integrate any acquired brands, both current and future, these product acquisitions may not be beneficial to us in the long term.
Our ifetroban product candidates have not been approved for sale and may never be successfully commercialized.
We anticipate that a portion of our future revenue growth may come from sales of our ifetroban product candidates. However, none of these products have been approved by the FDA for marketing, and these product candidates are still subject to risks associated with their development. Drug development is a long, expensive and inherently uncertain process with a high risk of failure at every stage of development, and results of earlier studies and trials may not be predictive of future trial results
The FDA has cleared our IND's for the ifetroban product candidates as we evaluate them as treatments for these conditions. Delays in the enrollment and completion of the clinical studies could significantly delay commercial launch and affect our product development costs. Moreover, results from the clinical studies may not be favorable.
Even if they are eventually developed and approved by the FDA, they may never gain significant acceptance in the marketplace and therefore never generate substantial revenue or profits for us. Physicians may determine that existing drugs are adequate to address patients’ needs. The extent to which these product candidates will be reimbursed by the U.S. government or third-party payors is also currently unknown.
As a result of the foregoing and other factors, we do not know the extent to which our product candidates will contribute to our future growth.
If we are unable to maintain, train and build an effective sales and marketing infrastructure, we will not be able to commercialize and grow our products and product candidates successfully.
As we grow, we may not be able to secure sales personnel or organizations that are adequate in number or expertise to successfully market and sell our products. This risk would be accentuated if we acquire products in areas outside of our current focus areas since our sales forces specialize in our existing areas. If we are unable to expand our sales and marketing capability, train our sales force effectively or provide any other capabilities necessary to commercialize our products and product candidates, we will need to contract with third parties to market and sell our products. We must train our employees on proper regulatory compliance, including, but not limited to, “fair balance” promotion of our products and anti-kickback laws. If we are unable to establish and maintain compliant and adequate sales and marketing capabilities, we may not be able to increase our product revenue, may generate increased expenses and may experience regulatory compliance issues.
If governmental or third-party payors do not provide adequate reimbursement for our products, our revenue and prospects for profitability may be limited.
Our financial success depends, in part, on the availability of adequate reimbursement from third-party healthcare payors. Such third-party payors include governmental health programs such as Medicare and Medicaid, managed care providers and private health insurers. Third-party payors are increasingly challenging the pricing of medical products and services, while governments continue to propose and pass legislation designed to reduce the cost of healthcare. Adoption of such legislation could further limit reimbursement for pharmaceuticals. In addition, as part of the Inflation Reduction Act legislation, provisions intended to lower the price of prescription drugs, including permitting Medicare to negotiate the price of prescription drugs once they have been on the market for a fixed number of years, and imposing a tax penalty on drug manufacturers if the price of their drugs increase faster than the rate of inflation. At this time no assurances can be given that these measures, or subsequent legislative proposals, will not have an adverse effect on our revenues in the future. Future cost control initiatives, legislation, and regulations could decrease the price that we receive for our products, which would limit our revenue and profitability.
40
Also, reimbursement practices of third-party payors might preclude us from achieving market acceptance for our products or maintaining price levels sufficient to realize an appropriate return on our investment in product acquisition and development. If we cannot obtain adequate reimbursement levels, our business, financial condition and results of operations would be materially and adversely affected.
Our employees have been trained to submit accurate and correct pricing information to payors. If, despite the training, our employees provide incorrect or fraudulent information, then we will be subject to various administrative and judicial investigations and litigation.
“Formulary” practices of third-party payors could adversely affect our competitive position.
Many managed healthcare organizations control the pharmaceutical products included on their formulary lists. Having products listed on these formulary lists creates competition among pharmaceutical companies which, in turn, has created a trend of downward pricing pressure in our industry. In addition, many managed care organizations are pursuing various ways to reduce pharmaceutical costs and are considering formulary contracts primarily with those pharmaceutical companies that can offer a full line of products for a given therapy sector or disease state. Our products might not be included on the formulary lists of managed care organizations, and downward pricing pressure in our industry generally could negatively impact our operations.
Continued consolidation of distributor networks in the pharmaceutical industry as well as increases in retailer concentration may limit our ability to profitably sell our products.
We sell most of our products to large pharmaceutical wholesalers, who in turn sell to hospitals, surgery centers and retail pharmacies. The distribution network for pharmaceutical products has become increasingly consolidated in recent years. Further consolidation or financial difficulties could also cause our customers to reduce the amounts of our products that they purchase, adversely impacting our business, financial condition and results of operations.
Our CET joint initiative may not result in our gaining access to commercially viable products.
Our CET joint initiative with Vanderbilt University, WinHealth and Tennessee Technology Development Corporation is designed to help us investigate, in a cost-effective manner, early-stage products and technologies. However, we may never gain access to commercially viable products from CET for a variety of reasons, including:
•CET investigates early-stage products, which have risk of failure prior to FDA approval and commercialization;
•In some programs, we do not have pre-set rights to product candidates developed by CET. We would need to agree with CET and its collaborators on the terms of any product licensed or acquired by us;
•We rely principally on government grants to fund CET’s research and development programs. If these grants were no longer available, we or our co-owners might be unable or unwilling to fund CET operations at current levels or at all;
•We may become involved in disputes with our co-owners regarding CET policy or operations, such as how best to deploy CET assets or which product opportunities to pursue. Disagreement could disrupt or halt product development; and
•CET may disagree with one of the various universities with which CET is collaborating on research. A disagreement could disrupt or halt product development.
We depend on our key personnel, the loss of whom would adversely affect our operations. If we fail to attract and retain the talent required for our business, our business will be materially harmed.
We are a relatively small company, and we depend to a great extent on principal members of our management, scientific staff, and sales representatives and managers. If we lose the services of any key personnel, in particular, A.J. Kazimi, our Chief Executive Officer, or other members of senior management it could have a material adverse effect on our business prospects. Mr. Kazimi, plays a key role in several operational and strategic decisions such that any loss of his services due to death or disability would adversely impact our day-to-day operations. We have a life insurance policy covering the life of Mr. Kazimi. We have entered into agreements with each of our employees that
41
contain restrictive covenants relating to non-competition and non-solicitation of our customers and suppliers for one year after termination of employment. Nevertheless, each of our officers and key employees may terminate his or her employment at any time without notice and without cause or good reason, and so as a practical matter these agreements do not guarantee the continued service of these employees. Our success depends on our ability to attract and retain highly qualified scientific, technical, sales and managerial personnel and research partners. Competition among pharmaceutical companies for qualified employees is intense, and we may not be able to retain existing personnel or attract and retain qualified staff in the future. If we experience difficulties in hiring and retaining personnel in key positions, we could suffer from delays in product development, loss of customers and sales and diversion of management resources, which could adversely affect operating results.
In addition, we have recently observed an overall tightening and increasingly competitive labor market. Our business could be adversely affected by an inability to retain personnel or upward pressure on wages as a result of the competitive labor market.
The size of our organization and our potential growth may lead to difficulties in managing operations.
As of December 31, 2023, we had 91 employees. We may need to continue to expand our managerial, operational, financial and other resources in order to increase our marketing efforts with regard to our currently marketed products, continue our business development and product development activities and commercialize our product candidates. We have experienced, and may continue to experience, growth and increased expenses in the scope of our operations in connection with the continued marketing and development of our products. Our financial performance will depend, in part, on our ability to manage any such growth and expenses of the current organization effectively.
We face potential product liability exposure, and if successful claims are brought against us, we may incur substantial liability for a product or product candidate and may have to limit its commercialization.
We face an inherent risk of product liability lawsuits related to the testing of our product candidates and the commercial sale of our products. An individual may bring a liability claim against us if one of our product candidates or products causes, or appears to have caused, an injury. If we cannot successfully defend ourselves against the product liability claim, we may incur substantial liabilities. Liability claims may result in decreased demand for our products; injury to our reputation; withdrawal of clinical trial participants; significant litigation costs; substantial monetary awards to or costly settlement with patients; product recalls; loss of revenue; and the inability to commercialize our product candidates.
We have product liability insurance that covers our clinical trials, the marketing and sale of our products up to a $10 million annual aggregate limit, subject to specified deductibles. Our current or future insurance coverage may prove insufficient to cover any liability claims brought against us.
Because of the increasing costs of insurance coverage, we may not be able to maintain insurance coverage at a reasonable cost or obtain insurance coverage that will be adequate to satisfy any liability that may arise.
Regulatory approval for any approved product is limited by the FDA to those specific indications and conditions for which clinical safety and efficacy have been demonstrated.
Any regulatory approval is limited to those specific diseases and indications for which a product is deemed to be safe and effective by the FDA. In addition to the FDA approval required for new formulations, any new indication for an approved product also requires FDA approval. If we are not able to obtain FDA approval for any desired future indications for our products, our ability to effectively market and sell our products may be reduced and our business may be adversely affected.
Our failure to follow FDA rules and guidelines relating to promotion and advertising may cause the FDA to suspend or withdraw an approved product from the market, require a recall or payment of fines, or could result in disgorgement of money, operating restrictions, injunctions or criminal prosecution, any of which could harm our business.
42
Our business and operations would suffer in the event of system failures, security breaches, including any cybersecurity incidents, adverse events or other disruptions within our information technology infrastructure at our corporate headquarters; or in the event of intellectual property infringement.
Our business depends on effective, secure and operational information systems which include systems provided by external contractors and other service providers. Despite the implementation of security measures, our computer systems and information technology infrastructure, including those resources at our corporate headquarters, are vulnerable to damage from cyber-attacks, computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Our business is at risk from and may be impacted by information security incidents, including ransomware, malware, phishing, social engineering, and other security events. Such incidents can range from individual attempts to gain unauthorized access to information technology systems to more sophisticated security threats. These events can also result from internal compromises, such as human error or malicious acts. These events can occur on our systems or on the systems of our partners and subcontractors.
In the ordinary course of our business, we store sensitive data, including intellectual property, our proprietary business information and that of our customers. We also maintain personally identifiable information of our employees in our data centers and on our networks. The secure processing and maintenance of this information is critical to our operations. Problems with, or the failure of, our technology and systems or any system upgrades or programming changes associated with such technology and systems would have a substantial and material negative effect on our operations. Furthermore, any system failure, accident or security breach that causes interruptions in our operations could result in a material disruption of our drug development programs.
While we continue to invest in data protection and information technology, our information technology and infrastructure may be vulnerable to attacks by hackers or breached due to employee error, malfeasance or other disruptions. Any such breach could compromise our networks and the information stored there could be accessed, publicly disclosed, lost or stolen. If we are subject to cyber-attacks or security breaches, this could result in business interruptions and delays; the loss, misappropriation, corruption or unauthorized access of data; litigation and potential liability under privacy, security and consumer protection laws or other applicable laws; reputational damage and federal and state governmental inquiries. Any such problems or failures and the costs incurred in correcting any such problems or failures, could have a material adverse effect on our business and financial condition, results of operations and cash flows. To the extent that any disruption or security breach results in a loss or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we may incur liability and the further development of our products or product candidates may be delayed. A failure to restore our information systems after the occurrence of any of these events could have a material adverse effect on our business and financial condition, results of operations and cash flows.
Our information systems and applications also require maintenance, upgrading and enhancement to meet our operational needs. We regularly upgrade and expand our information systems’ capabilities. If we experience difficulties with the transition and integration of information systems or are unable to implement, maintain, or expand our systems properly, we could suffer from, among other things, operational disruptions, regulatory problems and increases in administrative expenses.
As cyber threats continue to evolve, we may be required to expend significant capital and other resources to protect against the threat of security breaches or to mitigate and alleviate problems caused by breaches, including unauthorized access to proprietary information and personally identifiable information stored in our information systems, and the introduction of computer viruses or other malicious software programs to our systems. Our security measures may be inadequate to prevent security breaches and our business operations could be materially adversely affected by federal and state fines and penalties, legal claims or proceedings, cancellation of contracts and loss of customers if security breaches are not prevented.
We believe that our subcontractors and vendors take precautionary measures to prevent problems that could affect our business operations as a result of failure or disruption to their information systems. However, there is no guarantee such efforts will be successful in preventing a disruption, and it is possible that we may be impacted by information system failures. The occurrence of any information system failures could result in interruptions, delays, loss or corruption of data and cessations or interruptions in the availability of these systems. All of these events or
43
circumstances, among others, could have an adverse effect on our business, results of operations, financial position and cash flows, and they could harm our business reputation.
We believe we have all the necessary licenses from third parties to use technology and software that we do not own. A third party could, however, allege that we are infringing its rights, which may deter our ability to obtain licenses on commercially reasonable terms from the third party, if at all, or cause the third party to commence litigation against us. In addition, we may find it necessary to initiate litigation to protect our trade secrets, to enforce our intellectual property rights and to determine the scope and validity of any proprietary rights of others. Any such litigation, or the failure to obtain any necessary licenses or other rights, could materially and adversely affect our business.
We license our products globally; therefore, we may have exposure to foreign regulatory requirements and fluctuations in foreign currency exchange rates.
Continued foreign licensure inherently subjects us to a number of risks and uncertainties, including:
•longer payment cycles and difficulties in enforcing agreements and collecting receivables through certain foreign legal systems;
•political and economic instability or sanctions in areas in which we operate;
•potentially adverse tax consequences, tariffs, customs charges, bureaucratic requirements and other trade barriers;
•regulations related to customs and import/export matters (including sanctions);
•tax issues, such as tax law changes and variations in tax laws;
•challenges in collecting accounts receivable from customers in the jurisdictions in which we operate;
•complying with laws, rules and regulations relating to the manufacturing, marketing, distribution and sale of pharmaceutical products in the jurisdictions in which we do or will operate;
•operating under regulations in jurisdictions related to obtaining eligibility for government or private payor reimbursement for our products at the wholesale/retail level;
•competition from local, regional and international competitors;
•difficulties and costs of staffing and managing foreign operations, including cultural and language differences and additional employment regulations, union workforce negotiations and potential disputes in the jurisdictions in which we operate;
•difficulties associated with compliance with a variety of laws and regulations governing international trade, including the Foreign Corrupt Practices Act;
•difficulties protecting or procuring intellectual property rights; and
•fluctuations in foreign currency exchange rates.
Any of these factors may, individually or as a group, have a material adverse effect on our business and results of operations. These or other similar risks could adversely affect our revenue and profitability. As we continue to develop internationally, our exposure to these factors will increase.
We may decide not to commercialize one of our drug candidates once it obtains regulatory approval if we determine that commercialization of that product would require more capital and time than we are willing to invest.
Even if any of our drug candidates receives regulatory approval, it could be subject to matters such as post-regulatory surveillance, additional clinical trials or testing, reformulation, changes in labeling, warnings to the public, recall, competition from similar or superior products, and lack of sufficient payor reimbursement by
44
insurance companies or Medicare. As a result, we may not commercialize or continue to commercialize a product that has obtained regulatory approval.
Any approved drug product that we bring to the market may not gain market acceptance by physicians, patients, healthcare payors and others in the medical community.
Even if we are successful in gaining regulatory approval of any of our drug candidates or acquire rights to approved drug products, we may not generate significant product revenues and we may not become profitable if these drug products do not achieve an adequate level of acceptance. Physicians may not recommend our drug products until longer-term clinical data or other factors demonstrate the safety and efficacy of our drug products as compared to other alternative treatments. Even if the clinical safety and efficacy of our drug products is established, physicians may elect not to prescribe these drug products for a variety of reasons, including the reimbursement policies of government and other third-party payors and the effectiveness of our competitors in marketing their products.
Market acceptance of our drug products, if approved for commercial sale, will depend on a number of factors, including:
•the willingness and ability of patients and the healthcare community to use our drug products;
•the ability to manufacture our drug products in sufficient quantities with acceptable quality and to offer our drug products for sale at competitive prices;
•the perception of patients and the healthcare community, including third-party payors, regarding the safety, efficacy and benefits of our drug products compared to those of competing products or therapies;
•the label and promotional claims allowed by the FDA; and
•the pricing and reimbursement of our drug products relative to existing treatments.
We may acquire businesses or assets, form joint ventures or make investments in other companies that may be unsuccessful, divert our management’s attention and harm our operating results and prospects.
As part of our business strategy, we may pursue additional acquisitions of what we believe to be complementary businesses or assets or seek to enter into joint ventures. We also may pursue strategic alliances in an effort to leverage our existing infrastructure and industry experience to expand our product offerings or distribution, or make investments in other companies. The success of our acquisitions, joint ventures, strategic alliances and investments will depend on our ability to identify, negotiate, complete and, in the case of acquisitions, integrate those transactions and, if necessary, obtain satisfactory debt or equity financing to fund those transactions. We may not realize the anticipated benefits of any acquisition, joint venture, strategic alliance or investment. We may not be able to integrate acquisitions successfully into our existing business, maintain the key business relationships of businesses we acquire, or retain key personnel of an acquired business, and we could assume unknown or contingent liabilities or incur unanticipated expenses. Integration of acquired companies or businesses also may require management resources that otherwise would be available for ongoing development of our existing business. Any acquisitions or investments made by us also could result in significant write-offs or the incurrence of debt and contingent liabilities, any of which could harm our operating results. In addition, if we choose to issue shares of our stock as consideration for any acquisition, dilution to our shareholders could result.
We may be required to modify our business practices, pay fines and significant expenses or experience other losses due to governmental investigations or other enforcement activities.
We may become subject to litigation or governmental investigations in the United States and foreign jurisdictions that may arise from the conduct of our business. Like many companies in our industry, we have from time to time received inquiries and other types of information requests from government authorities.
45
While the ultimate outcomes of investigations and legal proceedings are difficult to predict, adverse resolutions or settlements of those matters could result in, among other things:
•significant damage awards, fines, penalties or other payments, and administrative remedies, such as exclusion and/or debarment from government programs, or other rulings that preclude us from operating our business in a certain manner;
•changes and additional costs to our business operations to avoid risks associated with such litigation or investigations;
•product recalls;
•reputational damage and decreased demand for our products; and
•expenditure of significant time and resources that would otherwise be available for operating our business.
RISKS RELATING TO GOVERNMENT REGULATION
Virtually all aspects of our business activities are regulated by government agencies. The manufacturing, processing, formulation, packaging, labeling, distribution, promotion and sampling, advertising of our products, and disposal of waste products arising from such activities are subject to governmental regulation. These activities are regulated by one or more of the FDA, the Federal Trade Commission, ("FTC"), the Consumer Product Safety Commission, the U.S. Department of Agriculture and the U.S. Environmental Protection Agency, ("EPA"), as well as by comparable agencies in foreign countries. These activities are also regulated by various agencies of the states and localities in which our products are sold. For more information, see “ Business—Government Regulation" in Part I, Item 1 of this Form 10-K.
Like all pharmaceutical manufacturers, we are subject to regulation by the FDA under the Federal Food, Drug and Cosmetic Act ("FDCA"). All new drugs must be the subject of an FDA-approved new drug application, ("NDA"), before they may be marketed in the United States. The FDA has the authority to withdraw existing NDA approvals and to review the regulatory status of products marketed under the enforcement policy. The FDA may require an approved NDA for any drug product marketed under the enforcement policy if new information reveals questions about the drug’s safety and effectiveness. All drugs must be manufactured in conformity with GMP, and drug products subject to an approved NDA must be manufactured, processed, packaged, held and labeled in accordance with information contained in the NDA. Since we rely on third parties to manufacture our products, GMP requirements directly affect our third party manufacturers and indirectly affect us. The manufacturing facilities of our third-party manufacturers are continually subject to inspection by such governmental agencies, and manufacturing operations could be interrupted or halted in any such facilities if such inspections prove unsatisfactory. Our third-party manufacturers are subject to periodic inspection by the FDA to assure such compliance.
Even after regulatory approval, certain developments may decrease demand for our products, including the following:
•the re-review of products that are already marketed;
•new scientific information and evolution of scientific theories;
•the recall or loss of marketing approval of products that are already marketed;
•changing government standards or public expectations regarding safety, efficacy or labeling changes; and
•greater scrutiny in advertising and promotion.
Certain regulatory changes or decisions could make it more difficult for us to sell our products and could have a material adverse effect on our business, results of operations, financial condition and cash flows. Manufacturers of drug products and their facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with GMP and other applicable regulations. If we or a regulatory agency discovers previously unknown problems with a product, such as adverse events of unanticipated severity or
46
frequency, or problems with a facility where the product is manufactured, a regulatory agency may impose restrictions on that product or the manufacturer, including withdrawal of the product from the market or suspension of manufacturing. If we, our partners or the manufacturing facilities for our products fail to comply with applicable regulatory requirements or violate healthcare laws, a regulatory agency may take the following actions, among others:
•issue warning letters or untitled letters;
•impose civil or criminal penalties
•suspend or withdraw regulatory approval;
•suspend any ongoing clinical trials;
•refuse to approve pending applications or supplements to applications submitted by us;
•impose restrictions on operations, including costly new manufacturing requirements; or
•seize or detain products or require us to initiate a product recall.
Any change in the FDA’s enforcement policy could have a material adverse effect on our business, financial condition and results of operations. We cannot determine what effect changes in regulations or statutes or legal interpretation, when and if promulgated or enacted, may have on our business in the future. Such changes, or new legislation, could have a material adverse effect on our business, financial condition and results of operations.
Proposed legislation may permit re-importation of drugs from other countries into the U.S., including foreign countries where the drugs are sold at lower prices than in the U.S., which could materially and adversely affect our operating results and our overall financial condition.
In previous years, legislation has been introduced in Congress that, if enacted, would permit more widespread re-importation of drugs from foreign countries into the U.S., which may include re-importation from foreign countries where the drugs are sold at lower prices than in the U.S. Such legislation, or similar regulatory changes, if enacted, could decrease the price we receive for any approved products which, in turn, could materially and adversely affect our operating results and our overall financial condition.
We must comply with the CREATES Act.
There have been a number of recent regulatory and legislative initiatives designed to encourage generic competition for pharmaceutical products, including expedited review procedures for generic manufacturers and incentives designed to spur generic competition of branded drugs. In particular, FDA and FTC have been focused on brand companies’ denial of drug supply to potential generic competitors for testing. In December 2019, the Creating and Restoring Equal Access to Equivalent Samples Act, or the CREATES Act, was enacted, which provides a legislatively defined private right of action under which eligible product developers can bring suit against companies who refuse to sell sufficient quantities of their branded products on commercially reasonable, market-based terms to support such eligible product developers’ marketing applications. We cannot currently predict the specific outcome or impact on our business of such regulatory and legislative initiatives.
We must comply with the Foreign Corrupt Practices Act.
We are required to comply with the United States Foreign Corrupt Practices Act, which prohibits U.S. companies from engaging in bribery or other prohibited payments to foreign officials for the purpose of obtaining or retaining business. Foreign companies, including some of our competitors, are not subject to these prohibitions. If our competitors engage in these practices, they may receive preferential treatment from officials or agencies in some countries, giving our competitors an advantage in securing business from government officials who might give them priority in obtaining new licenses, which would put us at a disadvantage. We have established formal policies or procedures for prohibiting or monitoring this conduct, but we cannot assure you that our employees or other agents will not engage in such conduct for which we might be held responsible. If our employees or other agents are found to have engaged in such practices, we could suffer severe penalties.
47
We must comply with the Physician Payment Sunshine Act.
We are required to comply with the United States Physician Payment Sunshine Act, which requires manufacturers of drugs, medical devices and biologicals that participate in U.S. federal healthcare programs to report certain payments and items of value given to physicians and teaching hospitals. Manufacturers are required to report this information annually to The Centers for Medicare & Medicaid Services ("CMS"). In addition, some states require reporting information concerning payments to health care providers or other transfers of value by drug manufacturers beyond the requirements of the Federal Sunshine Act. Cumberland has implemented a series of policies and procedures for every employee involved in the data collection process, and has systems in place to capture the data, which is verified by an outside firm that specializes in reporting the payments. Cumberland has also established a system to ensure that data was reported completely, in the correct format, and on time. Despite these policies, procedures and systems, we cannot assure you that we will collect and report all data accurately. If we fail to accurately report this information, we could suffer severe penalties.
If we fail to comply with our reporting and payment obligations under the Medicaid Drug Rebate program or other governmental pricing programs, we could be subject to additional reimbursement requirements, penalties, sanctions and fines, which could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
We participate in the Medicaid Drug Rebate program, the 340B program, and other governmental pricing programs and have obligations to report the average sales price for certain of our drugs to CMS. These programs and rebate calculations vary across products and programs, are complex, and are often subject to interpretation by us, governmental or regulatory agencies and the courts, which can change over time.
In the case of our Medicaid pricing data, if we become aware that our reporting for a prior quarter was incorrect or has changed as a result of recalculation of the pricing data, we are obligated to resubmit the corrected data for up to three years after those data originally were due. Such restatements and recalculations increase our costs for complying with the laws and regulations governing the Medicaid Drug Rebate program and could result in an overage or underage in our rebate liability for past quarters. Price recalculations also may affect the ceiling price at which we are required to offer our products under the 340B program.
Civil monetary penalties can be applied if we are found to have knowingly submitted any false price or product information to the government, if we are found to have made a misrepresentation in the reporting of our average sales price, if we fail to submit the required price data on a timely basis, or if we are found to have charged 340B covered entities more than the statutorily mandated ceiling price. CMS, could also decide to terminate our Medicaid drug rebate agreement, in which case federal payments may not be available under Medicaid or Medicare Part B for our covered outpatient drugs. We cannot assure you that our submissions will not be found by CMS to be incomplete or incorrect. Our failure to comply with our reporting and payment obligations under the Medicaid Drug Rebate program and other governmental programs could negatively impact our financial results.
We may be subject to foreign, federal, and state data privacy and security laws, and failure to protect our information systems against security breaches, service interruptions, or misappropriation of data could disrupt operations, compromise sensitive data, and expose us to liability, possibly causing our business and reputation to suffer.
In the United States, numerous federal and state laws and regulations govern the collection, use, disclosure and protection of health-related and other personal information and could apply to our operations or the operations of our collaborators and third-party providers. Certain of these laws grant individual rights with respect to their information, and we may be required to expend significant resources to comply with these laws. For example, the California Consumer Privacy Act, or CCPA, was enacted in 2020. These laws and regulations are evolving and subject to interpretation and may impose limitations on our activities or otherwise adversely affect our business. Similarly, there are a number of legislative proposals in the European Union, the United States, at both the federal and state level, as well as other jurisdictions that could impose new obligations or limitations in areas affecting our business. These changes may lead to additional costs and increase our overall risk exposure.
48
RISKS RELATING TO INTELLECTUAL PROPERTY
Our strategy to secure and extend marketing exclusivity or patent rights may provide only limited or no protection from competition.
We seek to secure and extend marketing exclusivity for our products through a variety of means, including FDA exclusivity and patent rights. Additional barriers for competitors seeking to enter the market include the time and cost associated with the development, regulatory approval and manufacturing of a similar product formulation.
As discussed in Part I, Item 1, Business - Patents, Trademarks, and Other Intellectual Proprietary Rights, of this report on Form 10-K, we have several patents for formulations of Acetadote, and have previously engaged in litigation to enforce our patent rights.
We intend to continue to vigorously defend and protect our Acetadote product and related intellectual property rights. If we are unsuccessful in protecting our Acetadote intellectual property rights, our competitors may be able to introduce products into the marketplace that reduce the sales and market share of our Acetadote product which may require us to take measures such as reducing prices or increasing our marketing expense, any of which may result in a material adverse effect to our financial condition and results of operations.
While we consider patent protection when evaluating product acquisition opportunities, any products we acquire in the future may not have significant patent protection. Neither the USPTO nor the courts have a consistent policy regarding the breadth of claims allowed or the degree of protection afforded under many pharmaceutical patents. Patent applications in the U.S. and many foreign jurisdictions are typically not published until 18 months following the filing date of the first related application, and in some cases not at all. In addition, publication of discoveries in scientific literature often lags significantly behind actual discoveries. Therefore, neither we nor our licensors can be certain that we or they were the first to make the inventions claimed in our issued patents or pending patent applications, or that we or they were the first to file for protection of the inventions set forth in these patent applications. Obtaining and enforcing patents in the biopharmaceutical industry involves both technological and legal complexity and is therefore costly, time consuming and inherently uncertain. In addition, changes in either patent laws or in interpretations of patent laws in the U.S. and other countries may increase the uncertainties and costs, diminish the value of our intellectual property, or narrow the scope of our patent protection. Furthermore, our competitors may independently develop similar technologies or duplicate technology developed by us in a manner that does not infringe our patents or other intellectual property. As a result of these factors, our patent rights may not provide any commercially valuable protection from competing products.
If we are unable to protect the confidentiality of our proprietary information and know-how, the value of our technology and products could be adversely affected.
In addition to patents, we rely upon trade secrets, unpatented proprietary know-how and continuing technological innovation where we do not believe patent protection is appropriate or attainable. For example, the manufacturing process for Kristalose involves substantial trade secrets and proprietary know-how. We have entered into confidentiality agreements with certain key employees and consultants pursuant to which such employees and consultants must assign to us any inventions relating to our business if made by them while they are our employees, as well as certain confidentiality agreements relating to the acquisition of rights to products. Confidentiality agreements can be breached, though, and we might not have adequate remedies for any breach. Also, others could acquire or independently develop similar technology.
We may depend on certain licensors for the maintenance and enforcement of intellectual property rights and have limited, if any, control over the amount or timing of resources that our licensors devote on our behalf.
When we license products, we often depend on our licensors to protect the proprietary rights covering those products. We have limited, if any, control over the amount or timing of resources that our licensors devote on our behalf or the priority they place on maintaining patent or other rights and prosecuting patent applications to our advantage. While any such licensor is expected to be contractually obligated to diligently pursue its patent applications and allow us the opportunity to consult, review and comment on patent office communications, we cannot be sure that it will perform as required. If a licensor does not perform and if we do not assume the
49
maintenance of the licensed patents in sufficient time to make required payments or filings with the appropriate governmental agencies, we risk losing the benefit of all or some of those patent rights.
If the use of our technology conflicts with the intellectual property rights of third parties, we may incur substantial liabilities, and we may be unable to commercialize products based on this technology in a profitable manner or at all.
If our products conflict with the intellectual property rights of others, they could bring legal action against us or our licensors, licensees, manufacturers, customers or collaborators. If we were found to be infringing a patent or other intellectual property rights held by a third party, we could be forced to seek a license to use the patented or otherwise protected technology. We might not be able to obtain such a license on terms acceptable to us or at all. If legal action involving an alleged infringement or misappropriation were to be brought against us or our licensors, we would incur substantial costs in defending the action. If such a dispute were to be resolved against us, we could be subject to significant damages, and the manufacturing or sale of one or more of our products could be enjoined.
We may be involved in lawsuits to protect or enforce our patents or the patents of our collaborators or licensors, which could be costly and time consuming.
We have been involved in lawsuits for infringement of the Acetadote Patents as previously described. Because of their nature, these lawsuits can be costly and time-consuming, and we only experience limited benefits and patent protection. A significant adverse ruling in any such lawsuit could put our patents at risk of being invalidated or interpreted narrowly and could compromise the issuance of our existing patent applications.
Competitors may infringe on our patents or the patents of our collaborators or licensors. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceeding could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing.
Interference proceedings brought by the USPTO may be necessary to determine the priority of inventions with respect to our patent applications or those of our collaborators or licensors. Litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distraction of our management. We may not be able, alone or with our collaborators and licensors, to prevent misappropriation of our proprietary rights, particularly in countries where the laws may not protect such rights as fully as in the United States.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, some of our confidential information could be disclosed during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments.
We may be involved in lawsuits to protect or enforce our trademarks or for allegedly infringing the trademark rights of others, which could be costly and time consuming.
We own certain trademark registrations for each of our branded pharmaceutical products as well as for our corporate name and logo. We have applied for trademark registration for other various names and logos. We also may have common law trademark rights in unregistered names, phrases, and logos under which we market or offer certain products and services. Over time, we intend to obtain and maintain registrations on trademarks that remain valuable to our business.
Third parties may oppose registration of our federal trademark applications. Further, we could be involved in lawsuits for allegedly infringing the rights of others with respect to their prior-existing trademarks. These lawsuits or opposition proceedings can be costly and time-consuming. A significant adverse ruling in any such lawsuit could put our trademarks at risk of being invalidated and could compromise the issuance of our existing trademark applications.
50
Competitors may infringe on our trademarks or the trademarks of our collaborators or licensors. To counter infringement or unauthorized use, we may be required to file trademark infringement claims, which can be expensive and time-consuming. In addition, in a trademark infringement proceeding, a court may decide that a trademark registration of ours is not valid or is unenforceable, or may refuse to stop the other party from using the mark or a mark that is similar to our registered mark at issue on the grounds that the competitor’s use of the mark is not confusingly similar to our registered trademark. An adverse result in any litigation or defense proceeding could put one or more of our trademark registrations at risk of being invalidated or interpreted narrowly and could put our trademark applications at risk of not registering.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, some of our confidential information could be disclosed during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments.
If we breach any of the agreements under which we license rights to our products and product candidates from others, we could lose the ability to continue commercialization of our products and development and commercialization of our product candidates.
We have exclusive licenses for the marketing and sale of certain products and may acquire additional licenses. Such licenses may terminate prior to expiration if we breach our obligations under the license agreement related to these pharmaceutical products. For example, the licenses may terminate if we fail to meet specified quality control standards, including GMP with respect to the products, or commit a material breach of other terms and conditions of the licenses. Such early termination could have a material adverse effect on our business, financial condition and results of operations.
We may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As is common in the biotechnology and pharmaceutical industry, we employ individuals who were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject to claims that we or these employees have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management. We are subject to stringent government regulation. All of our products face regulatory challenges.
51
RISKS RELATED TO OUR FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Our operating results are likely to fluctuate from period to period.
We are a company actively seeking to deliver significant growth. As we execute our business strategy of adding new products, increasing market share in our existing growth products and striving to maintain market share in our other products, we anticipate that there may be fluctuations in our future operating results. We may not be able to maintain or improve our current levels of revenue or income. Potential causes of future fluctuations in our operating results may include:
•New product launches, which could increase revenues but also increase sales and marketing expenses;
•Acquisition activity and other charges;
•Increases in research and development expenses resulting from the acquisition of a product candidate that requires significant additional studies and development;
•Ability to utilize unrecognized federal and state net operating loss carryforwards as a result of the exercise of nonqualified options
•Changes in the competitive, regulatory or reimbursement environment, which could drive down revenues or drive up sales and marketing or compliance costs; and
•Unexpected product liability or intellectual property claims and lawsuits.
See also “Management’s discussion and analysis of financial condition and results of operations—Liquidity and capital resources.” Fluctuation in operating results, particularly if not anticipated by investors and other members of the financial community, could add to volatility in our stock price.
Our focus on acquisitions as a growth strategy has created intangible assets whose amortization could negatively affect our results of operations.
Our total assets include intangible assets related to our acquisitions. As of December 31, 2023, intangible assets relating to products, which are being amortized, represented approximately 28 percent of our total assets. We may never realize the value of these assets. U.S. Generally Accepted Accounting Principles ("GAAP") require that we evaluate on a regular basis whether events and circumstances have occurred that indicate that all or a portion of the carrying amount of the asset may no longer be recoverable, in which case we would write down the value of the asset and take a corresponding charge to earnings. Any determination requiring the write-off of a significant portion of unamortized intangible assets would adversely affect our results of operations.
We may need additional funding and may be unable to raise capital when needed, which could force us to delay, reduce or eliminate our product development or commercialization and marketing efforts.
We may need to raise additional funds in order to meet the capital requirements of running our business and acquiring and developing new pharmaceutical products. If we require additional funding, we may seek to sell common stock or other equity or equity-linked securities, which could result in dilution to our shareholders. We may also seek to raise capital through a debt financing, which would result in ongoing debt-service payments and increased interest expense. Furthermore, the terms of any additional debt securities we may issue in the future may impose restrictions on our operations, which may include limiting our ability to incur additional indebtedness, pay dividends on or repurchase our common shares, or make certain acquisitions or investments. In addition, we may be subject to covenants requiring us to satisfy certain financial tests and ratios, and our ability to satisfy such covenants may be affected by events outside of our control. Any financings would also likely involve operational and financial restrictions being imposed on us. If we are unable to obtain any needed additional funding, we may be required to reduce the scope of, delay, or eliminate some or all of, our planned research, development and commercialization activities or to license to third parties the rights to develop and/or commercialize products or technologies that we would otherwise seek to develop and/or commercialize ourselves or on terms that are less attractive than they might otherwise be, any of which could materially harm our business.
52
We might also seek to sell assets or rights in one or more commercial products or product development programs. Additional capital might not be available to us when we need it. We are unable to predict the impact of global credit market trends, and if economic conditions deteriorate, our business, results of operations and ability to raise needed capital could be materially and adversely affected. If we are unable to raise additional capital when needed due to the reasons listed above and lack of creditworthiness, bank failures, or price decline in market investments, we could be forced to scale back our operations to conserve cash.
We may incur losses in the future and we may not achieve or maintain profitability.
We intend to continue to spend significant amounts on our efforts to discover and develop drugs. As a result, we may incur losses in future periods.
We anticipate that our drug discovery and development efforts and related expenditures will increase as we focus on the studies, including clinical trials prior to seeking regulatory approval, that are required before we can sell a drug product.
The development of drug products will require us to spend significant funds on research, development, testing, obtaining regulatory approvals, manufacturing and marketing.
We cannot be certain whether or when we will achieve profitability because of the significant uncertainties relating to our ability to generate commercially successful drug products. Even if we are successful in obtaining regulatory approvals for manufacturing and commercializing additional drug products, we may incur losses if our drug products do not generate significant revenues. If we achieve profitability, we may not be able to sustain or increase profitability.
Our officers, directors, and principal shareholders, acting as a group, could significantly influence corporate actions.
As of December 31, 2023, our officers and directors control approximately 43.3 percent of our common stock. Acting together, these shareholders could significantly influence any matter requiring approval by our shareholders, including the election of directors and the approval of mergers or other business combinations. The interests of this group may not always coincide with our interests or the interests of other shareholders and may prevent or delay a change in control. This significant concentration of share ownership may adversely affect the trading price of our common stock because many investors perceive disadvantages to owning stock in companies with controlling shareholders.
Research analysts may not continue to provide or initiate coverage of our common stock or may issue negative reports.
The market for our common stock may be affected by the reports financial analysts publish about us. If one of the analysts covering us downgrades our stock, its price could decline rapidly and significantly. Securities analysts covering our common stock may discontinue coverage. A lack of research coverage may adversely affect our stock’s market price.
53
RISKS RELATED TO OWNING OUR STOCK
The market price of our common stock may fluctuate substantially.
The price for the shares of our common stock sold in our initial public offering was determined by negotiation between the representatives of the underwriters and us. This price may not have reflected the market price of our common stock following our initial public offering. Moreover, the market price of our common stock might decline below current levels. In addition, the market price of our common stock is likely to be highly volatile and may fluctuate substantially. Sales of a substantial number of shares of our common stock in the public market or the perception that these sales may occur could cause the market price of our common stock to decline.
The realization of any of the risks described in these “Risk Factors” could have a dramatic and material adverse impact on the market price of our common stock. In addition, securities class action litigation has often been instituted against companies whose securities have experienced periods of volatility in market price. Any such securities litigation brought against us could result in substantial costs and a diversion of management’s attention and resources, which could negatively impact our business, operating results and financial condition.
Unstable market conditions may have serious adverse consequences on our business.
Our general business strategy may be adversely affected by unpredictable and unstable market conditions. While we believe we have adequate capital resources to meet current working capital and capital expenditure requirements, a radical economic downturn or increase in our expenses could require additional financing on less than attractive rates or on terms that are dilutive to existing shareholders. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our growth strategy, financial performance and stock price and could require us to delay or abandon clinical developments plans. There is a risk that one or more of our current service providers, manufacturers and other partners may encounter difficult economic circumstances, which would directly affect our ability to attain our operating goals on schedule and on budget. The equity and lending markets have been and will most likely continue to be negatively impacted for an unknown period of time due to global events such as the COVID-19 pandemic, increased inflation and the U.S. government’s response thereto.
We may not be able to maintain our listing on the NASDAQ Global Select Market (“NASDAQ”), which could have a material adverse effect on us and our stockholders.
The standards for continued listing on NASDAQ include, among other things, that the minimum bid price for the listed securities not fall below $1.00 for a period in excess of thirty consecutive business days. If the closing bid price of our common stock were to fail to meet NASDAQ’s minimum closing bid price requirement, or if we otherwise fail to meet any other applicable requirements of NASDAQ and we are unable to regain compliance, NASDAQ may make a determination to delist our common stock. The delisting of our common stock from NASDAQ could negatively impact us by (i) reducing the liquidity and market price of our common stock; (ii) reducing the number of investors willing to hold or acquire our common stock, which could negatively impact our ability to raise equity financing; (iii) impacting our ability to use a registration statement to offer and sell freely tradable securities, thereby preventing or limiting us from accessing the public capital markets; and (iv) impairing our ability to provide equity incentives to our employees.
54
Some provisions of our third amended and restated charter, bylaws and Tennessee law may inhibit potential acquisition bids that you may consider favorable.
Our corporate documents contain provisions that may enable our board of directors to resist a change in control of our company even if a change in control were to be considered favorable by you and other shareholders. These provisions include:
•The authorization of undesignated preferred stock, the terms of which may be established and shares of which may be issued without shareholder approval;
•Advance notice procedures required for shareholders to nominate candidates for election as directors or to bring matters before an annual meeting of shareholders;
•Limitations on persons authorized to call a special meeting of shareholders;
•A staggered board of directors;
•A restriction prohibiting shareholders from removing directors without cause;
•A requirement that vacancies in directorships are to be filled by a majority of the directors then in office and the number of directors is to be fixed by the board of directors; and
•No cumulative voting.
These and other provisions contained in our third amended and restated charter and bylaws could delay or discourage transactions involving an actual or potential change in control of us or our management, including transactions in which our shareholders might otherwise receive a premium for their shares over then current prices, and may limit the ability of shareholders to remove our current management or approve transactions that our shareholders may deem to be in their best interests and, therefore, could adversely affect the price of our common stock.
In addition, we are subject to control share acquisitions provisions and affiliated transaction provisions of the Tennessee Business Corporation Act, the applications of which may have the effect of delaying or preventing a merger, takeover or other change in control of us and therefore could discourage attempts to acquire our company.
We have never paid cash dividends on our capital stock.
We have never paid cash dividends on our capital stock. The availability of funds for distributions to shareholders will depend on our financial performance and assets. Any future decision to declare or pay dividends will be at the sole discretion of our Board of Directors.
DEBT-RELATED RISKS
Our Revolving Credit Agreement impose restrictive and financial covenants on us. Our failure to comply with these covenants could trigger events that would have a material adverse effect on our business.
Our Revolving Credit Agreement contains covenants that restrict the way we conduct business and require us to satisfy certain financial tests in order to incur debt or take other actions. Additionally, our Revolving Credit Agreement contains financial covenants that, for example, require us to maintain certain financial ratios which are measured at the end of each fiscal quarter.
Our Revolving Credit Agreement contains specified quarterly financial maintenance covenants. As of December 31, 2023, we were in compliance with the Maximum Funded Debt Ratio financial covenant of the Revolving Credit Agreement. However, we can make no assurance that we will be able to comply with the restrictive and financial covenants contained in the Revolving Credit Agreement in the future.
Our inability to comply with the covenants in our debt instruments could lead to a default or an event of default under the terms thereof, for which we may need to seek relief from our lender in order to waive the associated default or event of default and avoid a potential acceleration of the related indebtedness or cross-default or cross-
55
acceleration to other debt. There can be no assurance that we would be able to obtain such relief on commercially reasonable terms or otherwise and we may be required to incur significant additional costs. In addition, the lender under our Revolving Credit Agreement may impose additional operating and financial restrictions on us as a condition to granting any such waiver. If an event of default is not cured or is not otherwise waived, the lender under our Revolving Credit Agreement may accelerate the maturity of the related debt, foreclose upon any collateral securing the debt and terminate any commitments to lend, any of which would have a material adverse effect on our business, financial condition, cash flows and results of operations and would cause the market value of our securities to decline.
We have risks related to interest rates.
Our revolving credit facility bears interest based on variable interest. Thus, a change in the short-term interest rate environment (especially a material change) could have a material adverse effect on our business, financial condition, cash flows and results of operations. As of December 31, 2023, we did not have any outstanding interest rate swap contracts.
Item 1B. Unresolved Staff Comments.
None.
Item 1C. Cybersecurity
Risk Management and Strategy. We rely on information technology and data to operate our business and develop, market, and deliver our products to our customers. We have implemented and maintain various information security processes designed to identify, assess and manage material risks from cybersecurity threats to our communication systems, and our critical data which includes confidential, personal, proprietary, and other sensitive information (collectively “Information Assets”).
Accordingly, we maintain certain risk assessment processes intended to identify cybersecurity threats, determine their likelihood of occurring, and assess potential material impact to our business. Based on our assessment, we implement and maintain risk management processes designed to protect the confidentiality, integrity, and availability of our Information Assets and mitigate harm to our business.
Our company’s general risk management program is designed to manage potential material risks, which includes material cybersecurity risks to our Information Assets. We engage in processes designed to identify such threats by, among other things, monitoring the threat environment using manual and automated tools, subscribing to services that identify cybersecurity threats, analyzing reports of threats and actors, conducting scans of the threat environment, evaluating threats reported to us, coordinating with law enforcement concerning threats, and conducting threats and vulnerability assessments. We rely on a multidisciplinary team to assess cybersecurity threats and a potential impact to our business. These assessments leverage industry tools and metrics designed to assist in the assessment of risks from such cybersecurity threats.
We also implement and maintain various technical, physical and organizational measures designed to manage and mitigate material risks from cybersecurity threats to our Information Assets. The cybersecurity risk management and mitigation measures we implement include policies and procedures designed to address cybersecurity threats, including an incident response plan and a disaster recovery/business continuity plan.
To address the company’s cybersecurity risk, we utilize incident detection and response tools, internal and third-party assessments of our exposure to cybersecurity threats and compliance with risk mitigation procedures, and testing of our relevant controls including data segregation, insurance and assignment of cybersecurity responsibilities.
We also work with third parties from time to time to assist us in identification, assessment and management of cybersecurity risks.
For additional informant and a description of the risks from cybersecurity threats that may materially affect us and how they may do so, refer to Part I, Item 1A. Risk Factors.
56
Governance. Our cybersecurity risk assessment and management processes are implemented and maintained by certain company employees. Management is responsible for hiring appropriate personnel, integrating cybersecurity considerations into our company’s overall risk management strategy, and for communicating key priorities to employees, helping prepare for cybersecurity incidents, approving cybersecurity processes, and reviewing security assessments and other security-related reports. Our cybersecurity incident response and vulnerability assessments processes involve management, who participates in our disclosure controls and procedures.
Our cybersecurity processes are designed to escalate certain incidents and vulnerabilities to members of management depending on the circumstances, including cooperation with our company’s incident response team to help mitigate and remediate cybersecurity incidents. In addition, these processes include reporting to the board of directors for certain cybersecurity incidents.
Management including information technology, legal and accounting executives are involved with our company’s efforts to prevent, detect, and mitigate cybersecurity incidents by overseeing preparation of cybersecurity policies and procedures, testing of incident response plans, and engaging vendors with appropriate expertise. They participates in cybersecurity incident response efforts and directs the company’s response to cybersecurity incidents.
Our Board of Directors addresses the company’s cybersecurity risk management as part of its general oversight function. The Board of Directors has access to various reports, summaries or presentations related to cybersecurity threats, risk, and mitigation.
Item 2. Properties.
As of December 31, 2023, we leased approximately 16,903 rentable square feet of space at the new Broadwest development in Nashville, Tennessee for our corporate headquarters. The lease commencement date occurred in October 2022 with a term of 157 months leased through November 2035. We believe these facilities are adequate to meet our current needs for office space. Manufacturing, packaging or warehousing services are provided to us through contracts with third-party organizations.
The laboratory space at CET, under an agreement amended in July 2012, is now leased through April 2028. During 2023, Cumberland exercised the 2nd Extension option to extend the lease for five more years though April 2028. CET leases approximately 14,200 square feet of office and wet laboratory space in Nashville, Tennessee to operate the CET Life Sciences Center. Cumberland’s product formulation and testing laboratories are located at this facility, along with CET’s offices. The CET Life Sciences Center also provides laboratory and office space, equipment and infrastructure to early-stage life sciences companies and university spin-outs.
Item 3. Legal Proceedings.
Please see discussion of Melinta Litigation in Note 19 Commitments and Contingencies contained in the Notes to Consolidated Financial Statements, which is incorporated herein by reference.
Item 4. Mine Safety Disclosures.
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Our common stock, no par value, has been traded on the Nasdaq Global Select Market since August 11, 2009 under the symbol “CPIX.” As of March 8, 2024, we had 97 shareholders of record of our common stock. This excludes shareholders whose shares are held by brokers and other institutions on behalf of shareholders. The closing price of our common stock on the Nasdaq Global Select Market on March 8, 2024 was $2.03 per share.
57
Dividend Policy
We have not declared or paid any cash dividends on our common stock. Any future decision to declare or pay dividends will be at the sole discretion of our Board of Directors.
Performance Graph
The stock performance graph below illustrates a comparison of the total cumulative stockholder return on our common stock since December 31, 2018 to the Nasdaq Composite and a composite of ten Nasdaq Pharmaceutical and Specialty Pharmaceutical Stocks which most closely compare to our Company - Avadel Pharmaceuticals plc, Harrow Health, Inc., Eagle Pharmaceuticals, Inc., Assertio Holdings, Inc., HLS Therapeutics Inc., EyePoint Pharmaceuticals, Inc., Eton Pharmaceuticals, Inc., Theratechnologies Inc., Acorda Therapeutics Inc. and Talphera, Inc. The graph assumes an initial investment of $100 on December 31, 2018, and that all dividends were reinvested.
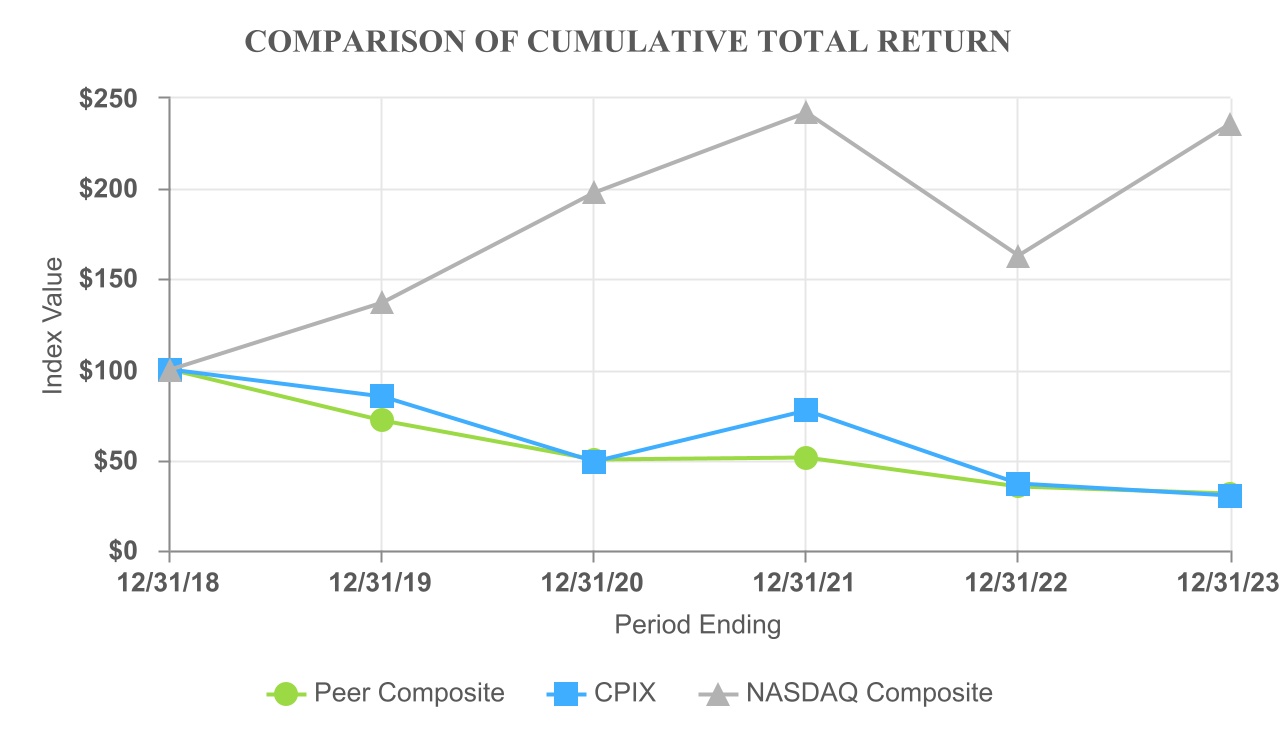
58
Purchases of Equity Securities
The Company currently has a share repurchase program to repurchase up to $10.0 million of our common stock pursuant to Rule 10b-18 of the Securities Exchange Act of 1934, as amended. In January 2019, the Company’s Board of Directors established the current $10.0 million repurchase program to replace the prior authorizations. We repurchased 402,143 shares and 367,793 shares of common stock for approximately $0.7 and $1.0 million during the years ended December 31, 2023 and 2022, respectively. The following table summarizes the activity, by month, during the fourth quarter of 2023:
| Period | Total Number of Shares (or Units) Purchased | Average Price Paid per Share (or Unit) | Total Number of Shares (or Units) Purchased as Part of Publicly Announced Plans or Programs | Maximum Number (or Approximate Dollar Value) of Shares (or Units) that May Yet Be Purchased Under the Plans or Programs | ||||||||||||||||||||||
| October | 28,114 | $2.04 | 28,114 | $3,135,727 | ||||||||||||||||||||||
| November | 50,220 | (1) | $1.81 | 50,220 | $3,044,578 | |||||||||||||||||||||
| December | 21,359 | (2) | $1.83 | 21,359 | $3,005,385 | |||||||||||||||||||||
| Total | 99,693 | |||||||||||||||||||||||||
(1) Of this amount, 25,000 shares were repurchased directly in private purchases at the then-current fair market value of common stock.
(2) Of this amount, 971 shares were repurchased directly in private purchases at the then-current fair market value of common stock.
Item 6. Reserved.
None.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis of our financial position and results of operations should be read together with our audited consolidated financial statements and related notes appearing elsewhere in this Form 10-K. This discussion and analysis may contain forward-looking statements that involve risks and uncertainties – please refer to the section entitled, “Special Note Regarding Forward-Looking Statements,” contained in Part I, Item 1A, “Risk Factors,” of this Form 10-K. You should review the “Risk Factors” section of this Form 10-K for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements described in the following discussion and analysis.
59
EXECUTIVE SUMMARY
We are a specialty pharmaceutical company focused on the acquisition, development and commercialization of branded prescription pharmaceutical products. We are dedicated to our mission of working together to provide unique products that improve the quality of patient care.
Our commercial portfolio includes seven branded products approved for marketing by the FDA. In addition to these commercial brands, we have Phase II clinical programs underway evaluating our ifetroban product candidate for patients with 1) cardiomyopathy associated with Duchenne Muscular Dystrophy, a fatal, genetic neuromuscular disease and 2) Systemic Sclerosis or scleroderma, a debilitating autoimmune disorder characterized by fibrosis of the skin and internal organs. We are also developing an oral capsule to treat Idiopathic Pulmonary Fibrosis, the most common form of progressive fibrosing interstitial lung disease. Following FDA clearance of our Investigational New Drug application in May 2023, we are in the process of initiating our Phase II study in patients in centers of excellence across the U.S.
Our primary target markets are hospital acute care, gastroenterology and oncology. These medical specialties are characterized by relatively concentrated prescriber bases that we believe can be served effectively by small, targeted sales forces. We promote our approved products through our hospital, field and oncology sales divisions in the United States and are building a network of international partners to register and provide our medicines to patients in their countries.
We have established the capabilities needed to acquire, develop and commercialize branded pharmaceuticals in the U.S. We believe we can leverage this existing infrastructure to support new products and our expected growth.
Our management team consists of pharmaceutical industry veterans with significant experience in their areas of responsibility. Our business development team identifies, evaluates and negotiates product acquisition, licensing and co-promotion agreements. Our product development team creates proprietary formulations, manages our clinical studies, prepares our FDA submissions and staffs our medical call center. Our quality and manufacturing professionals oversee the manufacturing, release and shipment of our products. Our marketing and sales organization is responsible for our commercial activities, and we work closely with our distribution partners to ensure the availability of our brands.
60
2023 Highlights
Below is a list of our Company’s highlights from 2023. For more information, please see Part I, Item I, Business of this Form 10-K.
•Announced our newly refined mission statement: Working together to provide unique products that improve the quality of patient care.
•Obtained FDA approval for our Caldolor® product, to include its use in infants. The non-narcotic agent may now be administered for the treatment of pain and fever in patients 3 to 6 months of age. With this newly approved labeling, Caldolor is the only non-opioid product approved to treat pain in infants that is delivered through injection.
•Shared the publication of positive results from a clinical study investigating the safety and pharmacokinetics of Caldolor in newborns. Published in the journal Pediatric Drugs, the results of the study support the growing body of evidence that demonstrates Caldolor is a safe therapeutic option available to practitioners for the treatment of fever and pain in infants, children and adults.
•Announced a new publication in Antimicrobial Agents and Chemotherapy detailing the results of the first clinical study investigating the safety and pharmacokinetics of our Vibativ® product in children 2 to 17 years of age. The results of the study suggest that a single dose of Vibativ is safe in children.
•Completed the Sancuso® transition from Kyowa Kirin to Cumberland, including the NDA transfer, and initiation of the product’s manufacture at a new facility. We also expanded our oncology sales division to further support the brand.
•Helped advance the submissions for the approval of Vibativ in China, South Korea and Saudi Arabia.
•Completed the transition of our former RediTrex® product to Nordic Pharma.
•Obtained FDA clearance of the Investigational New Drug application for a new ifetroban Phase II program in patients with Idiopathic Pulmonary Fibrosis, the most common form of progressive fibrosing interstitial lung disease.
•Shared our fourth annual Sustainability Metrics, which detail the Company’s activities pertaining to our environmental, social and governance matters.
•Entered into a new revolving credit loan agreement with Pinnacle Bank for a $20 million facility, expandable to $25 million over a three-year term.
•Continued our corporate share repurchase initiative, with a group of our board members also purchasing shares through Rule 10b5-1 trading plans in order to add to their holdings in the Company.
61
CRITICAL ACCOUNTING POLICIES AND SIGNIFICANT JUDGMENTS AND ESTIMATES
Accounting Estimates and Judgments
The preparation of condensed consolidated financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates, judgments and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the period. We base our estimates on past experience and on other factors we deem reasonable given the circumstances. Past results help form the basis of our judgments about the carrying value of assets and liabilities that cannot be determined from other sources. Actual results could differ from these estimates. The Company’s most significant estimates include: (1) its allowances for chargebacks and accruals for rebates and product returns, (2) the allowances for obsolescent or unmarketable inventory, (3) valuation of contingent consideration liabilities associated with business combinations and (4) valuation of continuing utility of intangible assets.
Revenue Recognition
We recognize revenue in accordance with the Accounting Standards Codification (ASC) Topic 606. Effective January 1, 2018, we adopted the Financial Accounting Standards Board’s (“FASB”) amended guidance in the form of Accounting Standards Update (“ASU”) No. 2014-09, "Revenue from Contracts with Customers," (ASC 606).
Our revenue is derived primarily from the product sales of our FDA approved pharmaceutical brands. Revenue from sales of products is recognized at the point where the customer obtains control of the goods and we satisfy our performance obligation, which occurs upon either shipment of the product or arrival at its destination, depending upon the shipping terms of the transaction. Payment terms typically range from 30 to 60 days from date of shipment. Our net product revenue reflects the reduction from gross product revenue for estimated allowances for chargebacks, and discounts and reflects sales related accruals for rebates, coupons, product returns, and certain administrative and service fees. Significant judgments must be made in determining the transaction price for our sales of products related to these adjustments. Other revenue, which is a component of net revenues, includes non-refundable upfront payments and milestone payments under licensing agreements along with grant and rental income. Other revenue was approximately 5.2% percent of net revenues in 2023 and 3.2% in 2022.
Our financial statements reflect accounts receivable allowances of $0.6 million at December 31, 2023 and 2022, for chargebacks and early pay discounts for products.
The following table reflects our sales-related accrual activity for the periods indicated below:
| 2023 | 2022 | |||||||||||||
| Balance, January 1 | $ | 8,347,214 | $ | 3,680,677 | ||||||||||
| Current provision | 22,184,661 | 24,426,431 | ||||||||||||
| Actual product returns and credits issued | (22,952,092) | (19,759,894) | ||||||||||||
| Balance, December 31 | $ | 7,579,783 | $ | 8,347,214 | ||||||||||
The allowances for chargebacks and discounts and sales related accruals for rebates, fee for service and product returns are determined on a product-by-product basis. We establish them using our best estimate at the time of sale based on:
•Each product’s historical experience adjusted to reflect known changes in the factors that impact such allowances;
•The contractual terms with direct and indirect customers;
•Analyses of historical levels of chargebacks, discounts and returns of product;
•Communications with customers;
62
•Purchased information about the rate of prescriptions being written and the level of inventory remaining in the distribution channel, if known; and
•Expectations about the market for each product, including any anticipated introduction of competitive products.
Other organizations, such as managed care providers, pharmacy benefit management companies and government agencies, may receive rebates from us based on either negotiated contracts to carry our products or reimbursements for filled prescriptions. These entities are considered our indirect customers. When recognizing a sale to a wholesaler, sales revenues are reduced and accrued liabilities are increased by our estimate of the rebate that may be claimed.
The allowances for chargebacks and accruals for rebates and product returns are the most significant estimates used in the recognition of our revenue from product sales. Of the accounts receivable allowances and our sales related accruals, our accrual for product returns and rebates represents the majority of the balance. Sales related accrued liabilities for rebates, product returns, service fees, and administrative fees totaled $7.6 million and $8.3 million as of December 31, 2023 and 2022 , respectively. Of these amounts, our estimated liability for fee for services represented $1.4 million and $1.5 million, respectively, while our accrual for product returns totaled $2.6 million and $2.7 million, respectively. If the actual amount of cash discounts, chargebacks, rebates, and product returns differs from the amounts estimated by management, material differences may result from the amount of our revenue recognized from product sales. A change in our rebate estimate of one percentage point would have impacted net sales by approximately $0.6 million for the years ended December 31, 2023 and 2022. A change in our product return estimate of one percentage point would have impacted net sales by $0.4 million for the years ended December 31, 2023 and 2022.
Inventories
We record amounts for estimated obsolescence or unmarketable inventory in an amount equal to the difference between the cost of inventory and the net realizable value based upon assumptions about remaining shelf life, future demand and market conditions. The estimated inventory obsolescence amounts are calculated based upon specific review of the inventory expiration dates and the quantity on-hand at December 31, 2023, in comparison to our expected inventory usage. The amount of actual inventory obsolescence and unmarketable inventory could differ (either higher or lower) in the near term from the estimated amounts. Changes in our estimates would be recorded in our statement of operations in the period of the change.
Non-current inventories consist of active pharmaceutical ingredients which typically has an extended life and selected finished good products with extended life longer than one year.
Income Taxes
We provide for deferred taxes using the asset and liability approach. Under this method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to operating loss and tax credit carry-forwards and differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Our principal differences are related to the timing of deductibility of certain items such as depreciation, amortization and expense for options issued to nonemployees. Deferred tax assets and liabilities are measured using management’s estimate of tax rates expected to apply to taxable income in the years in which management believes those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in our results of operations in the period that includes the enactment date.
In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income and tax planning strategies in making this assessment.
The Company’s accounting policy with respect to interest and penalties arising from income tax settlements is to recognize them as part of the provision for income taxes.
63
Share-Based Payments
We recognize compensation expense for all share-based payments based on the fair value of the award on the date of grant. In addition, incremental compensation expense is recognized upon the modification of equity awards.
We issue restricted stock and incentive stock option awards to employees, directors and consultants. Compensation expense for restricted equity awards granted to employees and directors is generally equal to the fair market value of the underlying common stock on the date of grant. If a sufficient disincentive for nonperformance does not exist at the date of grant, the compensation cost is remeasured at each reporting date at the then-current fair market value of the underlying common stock until the award vests.
Research and Development
We accrue for and expense research and development costs based on estimates of work performed, patient enrollment or fixed-fee-for-services. As work is performed and/or invoices are received, we adjust our estimates and accruals. To date, our accruals have not differed materially from our estimates. Total research and development costs are a function of studies being conducted and will increase or decrease based on the level of activity in any particular year.
Intangible Assets and Goodwill
Intangible assets include product rights, license agreements, other identifiable intangible assets and goodwill associated with the Vibativ acquisition. We assess the impairment of goodwill at least annually. We assess the impairment of identifiable intangible assets subject to amortization whenever events or changes in circumstances indicate the carrying value may not be recoverable. In determining the recoverability of our intangible assets, we make assumptions regarding estimated future cash flows and other factors. If the estimated undiscounted future cash flows do not exceed the carrying value of the intangible assets, we must determine the fair value of the intangible assets. If the fair value of the intangible assets is less than the carrying value, an impairment loss will be recognized in an amount equal to the difference. Fair value is determined through various valuation techniques including quoted market prices, third-party independent appraisals and discounted cash flow models, as considered necessary.
64
RESULTS OF OPERATIONS
Year ended December 31, 2023 compared to year ended December 31, 2022
The following table presents the statements of operations for the years ended December 31, 2023 and 2022:
| Years ended December 31, | |||||||||||||||||
| 2023 | 2022 | Change | |||||||||||||||
| Net revenues | $ | 39,552,507 | $ | 42,010,949 | $ | (2,458,442) | |||||||||||
| Costs and expenses: | |||||||||||||||||
| Cost of products sold | 6,066,611 | 9,118,521 | (3,051,910) | ||||||||||||||
| Selling and marketing | 18,451,765 | 16,660,945 | 1,790,820 | ||||||||||||||
| Research and development | 5,834,229 | 6,688,924 | (854,695) | ||||||||||||||
| General and administrative | 10,651,915 | 10,180,120 | 471,795 | ||||||||||||||
| Amortization and impairment | 8,102,648 | 5,067,368 | 3,035,280 | ||||||||||||||
| Total costs and expenses | 49,107,168 | 47,715,878 | 1,391,290 | ||||||||||||||
| Operating loss | (9,554,661) | (5,704,929) | (3,849,732) | ||||||||||||||
| Interest income | 286,854 | 98,405 | 188,449 | ||||||||||||||
| Other income | 2,828,871 | — | 2,828,871 | ||||||||||||||
| Other income - settlement | 475,000 | — | 475,000 | ||||||||||||||
| Other income - insurance proceeds | 346,800 | 611,330 | (264,530) | ||||||||||||||
| Interest expense | (667,861) | (585,995) | (81,866) | ||||||||||||||
| Loss before income taxes | (6,284,997) | (5,581,189) | (703,808) | ||||||||||||||
| Income tax (expense) benefit | (45,769) | (68,850) | 23,081 | ||||||||||||||
| Net loss | $ | (6,330,766) | $ | (5,650,039) | $ | (680,727) | |||||||||||
The following table summarizes net revenues for the years presented:
| Years ended December 31, | |||||||||||||||||
| 2023 | 2022 | Change | |||||||||||||||
| Products: | |||||||||||||||||
| Kristalose | $ | 15,981,850 | $ | 15,205,155 | $ | 776,695 | |||||||||||
| Vibativ | 8,812,692 | 7,487,462 | 1,325,230 | ||||||||||||||
| Sancuso | 8,096,788 | 13,205,603 | (5,108,815) | ||||||||||||||
| Caldolor | 4,333,923 | 4,827,200 | (493,277) | ||||||||||||||
| Acetadote | 458,759 | 501,040 | (42,281) | ||||||||||||||
| Omeclamox-Pak | 20,030 | 29,145 | (9,115) | ||||||||||||||
| Vaprisol | 151,336 | (447,697) | 599,033 | ||||||||||||||
| RediTrex | (341,886) | (126,726) | (215,160) | ||||||||||||||
| Other | 2,039,015 | 1,329,767 | 709,248 | ||||||||||||||
| Total net revenues | $ | 39,552,507 | $ | 42,010,949 | $ | (2,458,442) | |||||||||||
Net revenues. Net revenues for the year ended December 31, 2023, were approximately $39.6 million compared to $42.0 million for the year ended December 31, 2022. As detailed in the table above, net revenue increased during 2023 for two of our marketed products: Kristalose and Vibativ. These increases were mainly offset by decreased net product sales of Sancuso and Caldolor.
65
Kristalose revenue increased by $0.8 million, or 5.1%, compared to December 31, 2022, primarily as a result of increased shipments of the product.
Vibativ revenue increased to $8.8 million for the year ended December 31, 2023, compared to $7.5 million in the same prior year period. The increase of $1.3 million, or 17.7%, reflected fewer product returns in the current year.
Sancuso revenue was $8.1 million compared to $13.2 million in the prior year. This decrease in net revenue was primarily a result of an inordinate amount of product returns and higher commercial rebates.
Caldolor revenue was $4.3 million during the year ended December 31, 2023, compared to $4.8 million in the same period last year. This decrease in Caldolor revenue for the year ended December 31, 2023, was the result of the timing of international shipments.
There was no Vaprisol revenue for the year ended December 31, 2023, as Cumberland is currently out of commercial inventory of the product. Net revenue was positively impacted by various sales deduction adjustments.
Acetadote revenue included net sales of our branded product and our share of net sales from our Authorized Generic. For the year ended December 31, 2023, the Acetadote net revenue was $0.5 million, similar to the prior year period.
Omeclamox-Pak had no sales for the year ended December 31, 2023, as Cumberland is currently out of commercial inventory of this product. The packager for our Omeclamox-Pak product encountered financial difficulties and currently is under new management and is reorganizing.
Reditrex revenue decreased $0.2 million in 2023 compared to 2022. We discontinued sale of the product in 2023.
Other Revenue. Other revenue increased due to the litigation settlement based on two $500,000 milestone payments due to us for the license associated with our Vibativ product.
Cost of products sold. Cost of products sold for the year ended December 31, 2023, were $6.1 million compared to $9.1 million in the prior year, a decrease of $3.1 million. These cost savings were due to the availability of lower cost inventory and fewer inventory write downs.
Selling and marketing. Selling and marketing expense for the year ended December 31, 2023, were $18.5 million compared to $16.7 million in the prior year, which was an increase of $1.8 million. This increase was primarily a result of an increase in marketing expenses associated with the Sancuso acquisition including royalty costs, promotional spending and the cost associated with our expanded Oncology division.
Research and development. Research and development costs for the year ended December 31, 2023, were $5.8 million, compared to $6.7 million last year, representing a decrease of $0.9 million due to reduced FDA fees. A portion of our research and development costs is variable based on the number of trials, study sites, number of patients and the cost per patient in each of our clinical programs. We continue to fund our ongoing clinical initiatives associated with our pipeline products.
General and administrative. General and administrative expenses for the year ended December 31, 2023, were $10.7 million compared to $10.2 million in the prior year. The change resulted from an increase in hiring costs and deferred compensation expenses.
66
The components of the statements of operations discussed above reflect the following impacts from Vibativ:
| Financial Impact of Vibativ | Years ended December 31, | |||||||||||||
| 2023 | 2022 | |||||||||||||
Net revenue (1) | $ | 9,812,692 | $ | 7,637,462 | ||||||||||
Cost of products sold (2) | 1,423,399 | 3,535,851 | ||||||||||||
| Royalty and operating expenses | 2,379,939 | 83,145 | ||||||||||||
| Vibativ contribution | $ | 6,009,354 | $ | 4,018,466 | ||||||||||
(1) 2023 net revenue includes a $1,000,000 payment to Cumberland related to a settlement agreement of milestone payments.
(2) The Vibativ inventory included in the costs of product sold during the period was acquired and paid for by Cumberland as part of the acquisition of the brand during 2018.
The components of the statements of operations discussed above reflect the following impacts from Sancuso:
| Financial Impact of Sancuso | Years ended December 31, | |||||||||||||
| 2023 | 2022 | |||||||||||||
| Net revenue | $ | 8,096,788 | $ | 13,555,603 | ||||||||||
Cost of products sold (1) | 1,214,826 | 1,543,600 | ||||||||||||
| Royalty and operating expenses | 3,375,823 | 4,202,026 | ||||||||||||
| Sancuso contribution | $ | 3,506,139 | $ | 7,809,977 | ||||||||||
(1) The Sancuso inventory included in the costs of product sold during the period was acquired and paid for by Cumberland as part of the acquisition of the brand during 2022.
Amortization and impairment. Amortization and impairment expenses represent the ratable use of our capitalized intangible assets including product and license rights, patents, trademarks and patent defense costs. Amortization and impairment for 2023 totaled approximately $8.1 million which is an increase of $3.0 million due to a $3.3 million write down of our Omeclamox intangible assets.
Income taxes. Income taxes totaled $45,769 for the year ended December 31, 2023, and $68,850 for the year ended December 31, 2022.
Other income. In 2023, we recognized a $2.8 million refund of FDA fees for the periods of 2023 and 2022 to be used to further our product research efforts. In addition, in 2023 we recognized a gain of $0.5 million to settle a manufacturing dispute and a gain of $0.3 million for a payout earned on a company owned insurance policy.
67
LIQUIDITY AND CAPITAL RESOURCES
Our primary sources of liquidity are cash flows provided by our operations, the amounts borrowed and available under our line of credit and the cash proceeds from our initial public offering of common stock that was completed in August 2009. We believe that our internally generated cash flows, existing working capital and our line of credit will be adequate to finance internal growth, finance business development initiatives, and fund capital expenditures for the foreseeable future.
At December 31, 2023 and December 31, 2022, all our investments had original maturities of less than ninety days and as a result were classified as cash equivalents.
The following table summarizes our liquidity and working capital as of the years ended December 31:
| 2023 | 2022 | ||||||||||
| Cash and cash equivalents | $ | 18,321,624 | $ | 19,757,970 | |||||||
| Total cash and cash equivalents | $ | 18,321,624 | $ | 19,757,970 | |||||||
| Working capital (current assets less current liabilities) | $ | 7,732,161 | $ | 17,290,378 | |||||||
| Current ratio (multiple of current assets to current liabilities) | 1.3 | 1.6 | |||||||||
| Revolving line of credit availability | $ | 7,215,856 | $ | 3,800,000 | |||||||
The following table summarizes our net changes in cash and cash equivalents for the years ended December 31:
| 2023 | 2022 | ||||||||||
| Cash provided by (used in): | |||||||||||
| Operating activities | $ | 6,093,821 | $ | 8,453,396 | |||||||
| Investing activities | (105,695) | (13,674,456) | |||||||||
| Financing activities | (7,424,472) | (2,061,786) | |||||||||
Net decrease in cash and cash equivalents | $ | (1,436,346) | $ | (7,282,846) | |||||||
The net $1.4 million decrease in cash and cash equivalents for the year ended December 31, 2023, was attributable to cash provided by operating activities offset by cash used in investing and financing activities. Cash provided by operating activities of $6.1 million is primarily due to an increase in accounts payable and other accrued liabilities of $3.7 million, and a $3.4 million decrease in accounts receivable, partially offset by a decrease in non-cash contingent consideration of $1.3 million. Cash used in investing activities of $0.1 million was the result of additions of intangibles and property and equipment offset by life insurance proceeds received. Our financing activities included payments of $3.3 million of contingent consideration for Vibativ and Sancuso, a pay down on our line of credit of $3.4 and $0.7 million in cash used to repurchase shares of our common stock.
As noted above, we continue to repurchase shares of our common stock, as discussed in Part II, Item 5, "Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities", of this Form 10-K.
The net $7.3 million decrease in cash and cash equivalents for the year ended December 31, 2022, was attributable to cash provided by operating activities offset by cash used in investing and financing activities. Cash provided by operating activities of $8.5 million includes a decrease of inventory of $0.9 million, most of which was Vibativ and Sancuso related, an increase in accounts payable and other accrued liabilities of $14.5 million, partially offset by a $6.1 million increase in accounts receivable and a decrease in non-cash contingent consideration of $2.1 million. Cash used in investing activities of $13.7 million was the result of the acquisition of Sancuso of $13.5
68
million, additions to intangibles of $2.0 million, additions to property and equipment of $0.1 million and the proceeds of officer life insurance proceeds of $0.9 million. Our financing activities included payments of $2.2 million of contingent consideration for Vibativ and Sancuso and $1.1 million in cash used to repurchase shares of our common stock.
Shelf Registration
In December 2023, the Company filed a Shelf Registration on Form S-3 with the SEC associated with the sale of up to $100 million in corporate securities which also was declared effective in December 2023. The Company intends to enter into an At the Market Sales Agreement in March 2024, in order to allow the Company to sell shares at market prices. The Company did not issue any shares under its ATM program during the year ended December 31, 2023.
Debt Agreement
On September 5, 2023, the Company entered into a new Revolving Credit Loan Agreement with Pinnacle Bank. This facility provides for an aggregate principal funding amount of up to $25 million. The initial revolving line of credit is up to $20 million, with the ability for Cumberland to increase the amount to $25 million, under certain conditions. It has a three year term expiring on October 1, 2026. The interest rate is based on Benchmark (Term SOFR) plus a spread of 2.75%. Cumberland is subject to one financial covenant, the maintenance of a Funded Debt Ratio, determined on a quarterly basis. Borrowings under the line of credit are collateralized by substantially all of our assets.
Minimum Product Purchase Requirements
Our manufacturing and supply agreements do not require minimum annual purchase obligations.
Contractual cash obligations
The following table summarizes our contractual cash obligations as of December 31, 2023:
| Payments Due by Year | |||||||||||||||||||||||||||||||||||||||||
Contractual obligations(1) | Total | 2024 | 2025 | 2026 | 2027 | 2026 and thereafter | |||||||||||||||||||||||||||||||||||
Line of credit(2) | $ | 12,784,144 | $ | — | $ | — | $ | 12,784,144 | $ | — | $ | — | |||||||||||||||||||||||||||||
Estimated interest on debt (2) | 2,856,458 | 1,038,712 | 1,038,712 | 779,034 | — | — | |||||||||||||||||||||||||||||||||||
Vibativ contingent consideration liability payments (3) | 4,033,273 | 245,361 | 514,476 | 508,997 | 492,696 | 2,271,743 | |||||||||||||||||||||||||||||||||||
Sancuso contingent consideration liability payments (4) | 2,306,000 | 124,565 | 387,316 | 365,174 | 344,064 | 1,084,881 | |||||||||||||||||||||||||||||||||||
Operating leases(5) | 9,131,702 | 863,320 | 836,100 | 909,911 | 934,180 | 5,588,191 | |||||||||||||||||||||||||||||||||||
Total (1) | $ | 31,111,577 | $ | 2,271,958 | $ | 2,776,604 | $ | 15,347,260 | $ | 1,770,940 | $ | 8,944,815 | |||||||||||||||||||||||||||||
1.The sum of the individual amounts may not agree due to rounding.
2.The line of credit payments represent the estimated unused line of credit payments and the amount due at maturity. The estimated interest on debt represents the interest on the principal outstanding on the line of credit. These amounts are based on the $12.8 million line of credit assuming the current $12.8 million balance outstanding on December 31, 2023 is consistently outstanding through maturity of October 2026. Interest and unused line of credit payments are due and payable quarterly in arrears.
3.The contingent consideration liability represents the fair value of the royalty payments of up to 20% of future net sales as part of the Vibativ acquisition.
69
4.The contingent consideration liability represents the fair value of the royalty payments of up to 10% of future net sales as part of the Sancuso acquisition.
5.The Broadwest contractual cash obligation began upon commencement in October 2022 and CET began May 2023.
OFF-BALANCE SHEET ARRANGEMENTS
During 2023 and 2022 we did not engage in any off-balance sheet arrangements.
RECENT ACCOUNTING PRONOUNCEMENTS
Recent Accounting Pronouncements
In June 2016, the FASB issued ASU No. 2016-13, “Financial Instruments-Credit Losses,” which changes the impairment model for most financial assets and certain other instruments. For trade and other receivables, held-to-maturity debt securities, loans and other instruments, companies will be required to use a new forward-looking “expected loss” model that generally will result in the earlier recognition of allowances for losses. For available-for-sale debt securities with unrealized losses, companies will measure credit losses in a manner similar to what they do today, except that the losses will be recognized as allowances rather than as reductions in the amortized cost of the securities. Companies will have to disclose additional information, including information they use to track credit quality by year of origination for most financing receivables. Companies will apply the ASU’s provisions as a cumulative-effect adjustment, if any, to accumulated deficit as of the beginning of the first reporting period in which the guidance is adopted.
Related to ASU No. 2016-13 discussed above, in May 2019, the FASB issued ASU 2019-05, "Financial Instruments-Credit Losses (Topic 326): Targeted Transition Relief" which provides transition relief for ASU 2016-13 by providing entities with an alternative to irrevocably electing the fair value option for eligible financial assets measured at amortized cost upon adoption of the new credit losses standard. Certain eligibility requirements must be met and the election must be applied on an instrument-by-instrument basis. The election is not available for either available-for-sale or held-to-maturity debt securities. We adopted both ASU 2016-13 and ASU 2019-05 on January 1, 2023. The adoption of ASU 2016-13 and ASU 2019-05 did not have a material impact on the Company’s consolidated financial statements.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
Interest Rate Risk
We are exposed to market risk related to changes in interest rates on our cash on deposit in highly-liquid money market accounts and revolving credit facility. We do not utilize derivative financial instruments or other market risk-sensitive instruments to manage exposure to interest rate changes. The main objective of our cash investment activities is to preserve principal while maximizing interest income through low-risk investments. Our investment policy focuses on principal preservation and liquidity.
We believe that our interest rate risk related to our cash and cash equivalents is not material. The risk related to interest rates for these accounts would produce less income than expected if market interest rates fall. Based on current interest rates, we do not believe we are exposed to significant downside risk related to a change in interest on our money market accounts. The Company did not have any investments in marketable securities at December 31, 2023.
The interest rate risk related to borrowings under our line of credit is based on a benchmark (Term SOFR) plus a spread of 2.75%. As of December 31, 2023, we had $12.8 million in borrowings outstanding under our revolving line of credit.
70
Exchange Rate Risk
While we operate primarily in the U.S., we are exposed to foreign currency risk. A portion of our research and development is performed abroad.
Currently, we do not utilize financial instruments to hedge exposure to foreign currency fluctuations. We believe our exposure to foreign currency fluctuation is minimal as our purchases in foreign currency have a maximum exposure of 90 days based on invoice terms with a portion of the exposure being limited to 30 days based on the due date of the invoice. Foreign currency exchange losses were immaterial for 2023 and 2022. Neither a five percent increase nor decrease from current exchange rates would have had a material effect on our operating results or financial condition.
Item 8. Financial Statements and Supplementary Data.
See consolidated financial statements, including the reports of the independent registered public accounting firm, starting on page F-1, which is incorporated herein by reference.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Our Chief Executive Officer and Chief Financial Officer, with the participation of other members of management, have evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the "Exchange Act") as of December 31, 2023. Based on such evaluations, our Chief Executive Officer and Chief Financial Officer concluded that, as of such date, our disclosure controls and procedures were effective (at the reasonable assurance level) to ensure that the information required to be disclosed by the Company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized, and reported, within the time periods specified in the SEC’s rules and forms and to ensure that such information is accumulated and communicated to the Company’s management, including its Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
Management’s report on internal control over financial reporting is included on page F-1 of this annual report on Form 10-K, and incorporated herein by reference. During our fourth quarter of 2023, there were no changes in our internal control over financial reporting (as defined in Rule 13a-15(f) or 15d-15(f)) that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
71
Item 9B. Other Information.
(a) Recent Developments
Form 8-K, Item 5.02(e). Compensatory Arrangements of Certain Officers
On March 7, 2024, the Company entered into new employment agreements with each of A.J. Kazimi, our Chief Executive Officer (the “Kazimi Employment Agreement”), Jim Herman, our Executive Vice President National Accounts and Chief Compliance Officer (the “Herman Employment Agreement”), Todd Anthony, our Vice President Organizational Development (the “Anthony Employment Agreement”), Chris T. Bitterman, Vice President Sales and Marketing (the “Bitterman Employment Agreement”) and John M. Hamm, our Vice President, Chief Financial Officer (the “Hamm Employment Agreement”), and together with the Kazimi Employment Agreement, the Herman Employment Agreement, the Anthony Employment Agreement, the Bitterman Employment Agreement and the Hamm Employment Agreement, (the “Employment Agreements”). The Employment Agreements were effective as of January 1, 2024.
Employment Agreements
Each Employment Agreement provides for a salary for services performed, a potential annual bonus and a grant of restricted incentive stock options pursuant to a restricted stock agreement. Under the terms of each Employment Agreement, employment is at-will and may be terminated by the Company at any time, with or without notice and with or without cause. Similarly, each of Mr. Kazimi, Mr. Herman, Mr. Anthony, Mr. Bitterman, and Mr. Hamm may terminate his respective employment with us at any time, with or without notice. The Employment Agreements do not provide for any severance payments in the event employment is terminated for cause nor any severance benefits in the event employment is terminated as a result of death or permanent disability. The Employment Agreements include non-competition, non-solicitation and non-disclosure covenants on the part of employees. The Employment Agreements impose obligations regarding confidential information and state that any discoveries or improvements conceived, developed or otherwise made by the employees, or with others, are deemed our sole property. The Employment Agreements do not contain any termination or change in control provisions.
Kazimi Employment Agreement
Pursuant to the Kazimi Employment Agreement, Mr. Kazimi will serve as the Company’s Chief Executive Officer and will receive a base salary of $702,000.
The foregoing description of the Kazimi Employment Agreement is qualified in its entirety by reference to the Kazimi Employment Agreement, which is included as Exhibit 10.11 to this Annual Report on Form 10-K and is incorporated by reference into this Item. The foregoing description of the Kazimi Employment Agreement does not purport to be complete and is qualified in its entirety by reference to such exhibit.
Herman Employment Agreement
Pursuant to the Herman Employment Agreement, Mr. Herman will serve as the Company’s Senior Vice President, National Accounts and Chief Compliance Officer and will receive a base salary of $310,000.
The foregoing description of the Herman Employment Agreement is qualified in its entirety by reference to the Herman Employment Agreement, which is included as Exhibit 10.12 to this Annual Report on Form 10-K and is incorporated by reference into this Item. The foregoing description of the Herman Employment Agreement does not purport to be complete and is qualified in its entirety by reference to such exhibit.
72
Anthony Employment Agreement
Pursuant to the Anthony Employment Agreement, Mr. Anthony will serve as the Company’s Vice President Organizational Development and will receive a base salary of $286,125.
The foregoing description of the Anthony Employment Agreement is qualified in its entirety by reference to the Anthony Employment Agreement, which is included as Exhibit 10.13 to this Annual Report on Form 10-K and is incorporated by reference into this Item. The foregoing description of the Anthony Employment Agreement does not purport to be complete and is qualified in its entirety by reference to such exhibit.
Bitterman Employment Agreement
Pursuant to the Bitterman Employment Agreement, Mr. Bitterman will serve as the Company’s Vice President Sales and Marketing and will receive a base salary of $268,840.
The foregoing description of the Bitterman Employment Agreement is qualified in its entirety by reference to the Bitterman Employment Agreement, which is included as Exhibit 10.14 to this Annual Report on Form 10-K and is incorporated by reference into this Item. The foregoing description of the Bitterman Employment Agreement does not purport to be complete and is qualified in its entirety by reference to such exhibit.
Hamm Employment Agreement
Pursuant to the Hamm Employment Agreement, Mr. Hamm will serve as the Company’s Vice President, Chief Financial Officer and will receive a base salary of $246,240.
The foregoing description of the Hamm Employment Agreement is qualified in its entirety by reference to the Hamm Employment Agreement, which is included as Exhibit 10.15 to this Annual Report on Form 10-K and is incorporated by reference into this Item. The foregoing description of the Hamm Employment Agreement does not purport to be complete and is qualified in its entirety by reference to such exhibit.
(b) Insider Trading Arrangements and Policies
During the three months ended December 31, 2023, no “director” or “officer” (as defined in Rule 16a-1(f) under the Exchange Act) of the Company adopted terminated
Item 9C: Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not applicable.
PART III
The information called for by Part III of Form 10-K (Item 10 – Directors, Executive Officers and Corporate Governance, Item 11 – Executive Compensation, Item 12 – Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters, Item 13 – Certain Relationships and Related Transactions, and Director Independence, Item 14 – Principal Accountant Fees and Services), is incorporated by reference from our proxy statement related to our 2024 annual meeting of shareholders, which is expected to be filed with the SEC on or around March 13, 2024.
73
PART IV
Item 15. Exhibits, Financial Statement Schedules.
a.Documents filed as part of this report:
(1) Financial Statements
| Page Number | ||||||||
F-1 | ||||||||
Report of Independent Registered Public Accounting Firm - Consolidated Financial Statements - Carr, Riggs & Ingram; Nashville, TN; PCAOB ID: | ||||||||
F-2 | ||||||||
F-8 | ||||||||
F-9 | ||||||||
F-10 | ||||||||
F-12 | ||||||||
F-13 | ||||||||
(2) Financial Statement Schedule
F-39 | ||||||||
b. Exhibits
Exhibit Number | Description | |||||||
| 2.1 | ||||||||
| 3.1 | ||||||||
| 3.2 | ||||||||
| 4.1 | ||||||||
| 4.2 | ||||||||
74
| 4.3 | ||||||||
| 4.4 | ||||||||
| 4.5# | ||||||||
| 4.6.1# | ||||||||
| 4.6.2# | ||||||||
| 4.7# | ||||||||
| 4.8 | ||||||||
| 4.9 | ||||||||
| 4.10 | ||||||||
| 4.11 | ||||||||
| 10.7† | ||||||||
| 10.7.1† | ||||||||
| 10.10† | ||||||||
| Registrant's Quarterly Report on Form 10-Q (File No. 001-33637) as filed with the SEC on May13, 2022 | ||||||||
| 10.11# | ||||||||
| 10.12# | ||||||||
75
| 10.13# | ||||||||
| 10.14# | ||||||||
| 10.15# | ||||||||
| 10.17# | ||||||||
| 10.18# | ||||||||
| 10.19# | ||||||||
| 10.20 | ||||||||
| 10.23† | ||||||||
| 10.24 | ||||||||
| 10.24.1 | ||||||||
| 10.24.2† | ||||||||
| 10.25† | ||||||||
| 10.28† | ||||||||
| 10.30# | ||||||||
76
| 10.31† | ||||||||
| 10.32† | ||||||||
| 10.38# | ||||||||
| 10.39# | ||||||||
| 10.44 | ||||||||
| 16 | ||||||||
| 21 | ||||||||
| 23.1 | ||||||||
| 23.2 | ||||||||
| 31.1 | ||||||||
| 31.2 | ||||||||
| 97.1 | ||||||||
| 32.1** | ||||||||
| 101.INS | INLINE XBRL INSTANCE DOCUMENT - THE INSTANCE DOCUMENT DOES NOT APPEAR IN THE INTERACTIVE DATA FILE BECAUSE ITS XBRL TAGS ARE EMBEDDED WITHIN THE INLINE XBRL DOCUMENT. | |||||||
| 101.SCH | INLINE XBRL TAXONOMY EXTENSION SCHEMA DOCUMENT | |||||||
| 101.CAL | INLINE XBRL TAXONOMY EXTENSION CALCULATION LINKBASE DOCUMENT | |||||||
| 101.DEF | INLINE XBRL TAXONOMY EXTENSION DEFINITION LINKBASE DOCUMENT | |||||||
| 101.LAB | INLINE XBRL TAXONOMY EXTENSION LABEL LINKBASE DOCUMENT | |||||||
77
| 101.PRE | INLINE XBRL TAXONOMY EXTENSION PRESENTATION LINKBASE DOCUMENT | |||||||
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document). | |||||||
| # | Indicates a management contract or compensatory plan. | |||||||
| † | Confidential treatment has been granted for portions of this exhibit. These portions have been omitted from the Registration Statement and submitted separately to the Securities and Exchange Commission. | |||||||
| †† | Confidential treatment has been requested for portions of this exhibit. These portions have been omitted from the Registration Statement and submitted separately to the Securities and Exchange Commission. | |||||||
| * | Schedules have been omitted pursuant to Item 601(b)(2) of Regulation S-K. A copy of any omitted schedule will be furnished supplementally to the U.S. Securities and Exchange Commission upon request, provided, however, that the parties may request confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended for any document so furnished. | |||||||
| ** | Furnished herewith. | |||||||
Item 16. Form 10-K Summary
Registrants may voluntarily include a summary of information required by Form 10-K under this Item 16. The Company has elected not to include such summary information.
78
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on March 12, 2024.
| Cumberland Pharmaceuticals, Inc. | ||||||||
| /s/ A. J. Kazimi | ||||||||
| By: | A. J. Kazimi | |||||||
| Chief Executive Officer | ||||||||
| (Principal Executive Officer) | ||||||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||||||||||||
| /s/ A. J. Kazimi | Chairman and CEO | March 12, 2024 | ||||||||||||
| A. J. Kazimi | (Principal Executive Officer and Director) | |||||||||||||
| /s/ John M. Hamm | Vice President, Chief Financial Officer | March 12, 2024 | ||||||||||||
| John M. Hamm | (Principal Financial and Accounting Officer | |||||||||||||
| /s/ Gordon R. Bernard | Director | March 12, 2024 | ||||||||||||
| Gordon R. Bernard | ||||||||||||||
| /s/ James R. Jones | Director | March 12, 2024 | ||||||||||||
| James R. Jones | ||||||||||||||
| /s/ Caroline R. Young | Director | March 12, 2024 | ||||||||||||
| Caroline R. Young | ||||||||||||||
| /s/ Kenneth J. Krogulski | Director | March 12, 2024 | ||||||||||||
| Kenneth J. Krogulski | ||||||||||||||
| /s/ Joseph C. Galante | Director | March 12, 2024 | ||||||||||||
| Joseph C. Galante | ||||||||||||||
| /s/ Martin S. Brown, Jr. | Director | March 12, 2024 | ||||||||||||
| Martin S. Brown, Jr. | ||||||||||||||
79
MANAGEMENT’S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
The management of Cumberland Pharmaceuticals Inc. and its subsidiaries (the "Company") is responsible for establishing and maintaining adequate internal control over financial reporting. The Company’s internal control system was designed to provide reasonable assurance to the Company’s management and board of directors regarding the preparation and fair presentation of published financial statements. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
The Company’s management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2023. In making this assessment, it used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control – Integrated Framework (2013).
Based on its assessment, management has concluded that, as of December 31, 2023, the Company’s internal control over financial reporting was effective based on those criteria.
| /s/ A. J. Kazimi | ||
| A. J. Kazimi | ||
| Chief Executive Officer | ||
| March 12, 2024 | ||
| /s/ John M. Hamm | ||
| John M. Hamm | ||
| Chief Financial Officer | ||
| March 12, 2024 | ||
F-1
Report of Independent Registered Public Accounting Firm
To the Shareholders, Board of Directors and Audit Committee of
Cumberland Pharmaceuticals Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheet of Cumberland Pharmaceuticals Inc. and subsidiaries (the "Company") as of December 31, 2023, the related consolidated statements of operations, equity, and cash flows for the year then ended, and the related notes and schedule (collectively referred to as the “financial statements”). In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2023, and the results of its operations and its cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit.
We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
F-2
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current-period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which it relates.
Customer Allowances for Chargebacks, Discounts and Damaged Goods, and Accruals for Rebates, Coupons, Product Returns, and Certain Fees
As described in Note 2 to the consolidated financial statements, the allowances against accounts receivable and accrued liabilities for chargebacks, discounts, service fees and expired product returns are determined on a product-by-product basis and established by management as the Company’s best estimate at the time of sale based on each product’s historical experience adjusted to reflect known changes in the factors that impact such allowances. These allowances are established based on the contractual terms with direct and indirect customers and analyses of historical levels of chargebacks, discounts and returns of expired product.
As of December 31, 2023, allowances in accounts receivable for chargebacks, cash discounts, and damaged goods were $0.6 million and the estimated liability for rebates, coupons, product returns, and certain fees were $7.6 million. These provisions are recognized concurrently with the sales of products. Provisions for chargebacks involve estimates of usage by retailers and other indirect buyers with varying contract prices for multiple wholesalers. The provision for chargebacks varies in relation to changes in sales volume, product mix, pricing, and the level of inventory at the wholesalers. Provisions are calculated using historical chargeback experience, and/or expected chargeback levels for new products and anticipated pricing changes. Provisions for rebates are recognized based on contractual obligations in place at the time of sales with consideration given to relevant factors that may affect the payment, as well as historical experience for estimated market activity. Provisions for product returns are calculated based on the expiration dates of products sold, the window where customers are permitted to return products, and the history of returns for individual products in relation to the sales volume for each product.
We identified the customer allowances for chargebacks, discounts, and damaged goods and accruals for rebates, coupons, product returns, and certain fees as a critical audit matter. The principal consideration for our determination was the significant measurement uncertainty involved in developing the reserves. Management exercises judgment in computing the amount of sales subject to the allowances and tracks the amount of allowances taken over time. All of this in turn led to a high degree of auditor judgment, subjectivity, and effort in performing procedures and evaluating management’s significant assumptions, which includes the assumption that historical activity is a good predictor of future allowance activity and modifications made to calculations based on more recent history.
The primary procedures we performed to address this critical audit matter included:
•Tested management’s process for calculating certain allowances and developed an independent expectation of the reserve balance for the remaining allowances.
•Performed a look back analysis of prior year reserves compared to actual experience in the current year.
•Tested the completeness and accuracy of underlying data used to estimate the accrual by evaluating a service organization from which data was obtained, agreeing sales data used in the calculations to reports that were reconciled to the consolidated financial statements, reconciling various allowance percentages to signed customer contracts, and tracing allowance amounts used by various customers during the year to supporting documentation.
F-3
•Evaluated the reasonableness of significant assumptions used by management in the computation of selected allowances, including comparison to historical results and considering recent changes in factors that could influence claims, such as changes in the shelf life of the products.
•Tested the clerical accuracy of individual customer allowances computed by management and agreeing the total of all estimated allowances to the respective accounts on the consolidated financial statements.
•Compared actual activity for chargebacks, discounts, and damaged goods and rebates, coupons, product returns, and certain fees reported after December 31, 2023, to estimated reserves and accruals on the December 31, 2023, consolidated balance sheet.
/s/ Carr, Riggs & Ingram, LLC
We have served as the Company’s auditor since 2023.
March 12, 2024
F-4
Report of Independent Registered Public Accounting Firm
To the Shareholders, Board of Directors and Audit Committee
Cumberland Pharmaceuticals Inc.
Nashville, Tennessee
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheet of Cumberland Pharmaceuticals Inc. (“Company”) as of December 31, 2022, the related consolidated statements of operations, equity, and cash flows for the year ended December 31, 2022, and the related notes and schedule (collectively referred to as the financial statements). In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2022, and the results of its operations and its cash flows for the year ended December 31, 2022, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit.
We are a public accounting firm registered with the Public Company Accounting Oversight Board (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission (SEC) and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures include examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current-period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
F-5
Customer Allowances for Chargebacks, Discounts, and Damaged Goods and Accruals for Rebates, Coupons, Product Returns, and Certain Fees
As described in Note 2 to the consolidated financial statements, the allowances against accounts receivable and accrued liabilities for chargebacks, discounts, service fees and expired product returns are determined on a product-by-product basis and established by management as the Company’s best estimate at the time of sale based on each product’s historical experience adjusted to reflect known changes in the factors that impact such allowances. These allowances are established based on the contractual terms with direct and indirect customers and analyses of historical levels of chargebacks, discounts and returns of expired product.
As of December 31, 2022, allowances in accounts receivable for chargebacks, cash discounts, and damaged goods were $0.6 million and the estimated liability for rebates, coupons, product returns, and certain fees were $8.3 million. These provisions are recognized concurrently with the sales of products. Provisions for chargebacks involve estimates of usage by retailers and other indirect buyers with varying contract prices for multiple wholesalers. The provision for chargebacks varies in relation to changes in sales volume, product mix, pricing, and the level of inventory at the wholesalers. Provisions are calculated using historical chargeback experience, and/or expected chargeback levels for new products and anticipated pricing changes. Provisions for rebates are recognized based on contractual obligations in place at the time of sales with consideration given to relevant factors that may affect the payment, as well as historical experience for estimated market activity. Provisions for product returns are calculated based on the expiration dates of products sold, the window where customers are permitted to return products, and the history of returns for individual products in relation to the sales volume for each product.
We identified the customer allowances for chargebacks, discounts, and damaged goods and accruals for rebates, coupons, product returns, and certain fees as a critical audit matter. The principal consideration for our determination was the significant measurement uncertainty involved in developing the reserves. Management exercises judgment in computing the amount of sales subject to the allowances and tracks the amount of allowances taken over time. All of this in turn led to a high degree of auditor judgment, subjectivity, and effort in performing procedures and evaluating management’s significant assumptions, which includes the assumption that historical activity is a good predictor of future allowance activity and modifications made to calculations based on more recent history.
The primary procedures we performed to address this critical audit matter included:
•Tested management’s process for calculating the allowances, including a look back analysis of prior year reserves compared to actual experience in the current year.
•Tested the completeness and accuracy of underlying data used to estimate the accrual by agreeing sales data used in the calculations to reports that were reconciled to the consolidated financial statements, reconciling various allowance percentages to signed customer contracts, and tracing allowance amounts used by various customers during the year to supporting documentation.
•Evaluated the reasonableness of significant assumptions used by management in the computation of allowances, including comparison to historical results and considering recent changes in factors that could influence the future allowances to be claimed, such as changes in the shelf life of the products.
•Tested the clerical accuracy of individual customer allowances computed by management and agreeing the total of all estimated allowances to the respective accounts on the consolidated financial statements.
•Developed an independent expectation of the reserve balance for certain allowances and comparing that to the balance recorded on the December 31, 2022, consolidated balance sheet.
•Compared actual activity for chargebacks, discounts, and damaged goods and rebates, coupons, product returns, and certain fees reported after December 31, 2022, to estimated reserves and accruals on the December 31, 2022, consolidated balance sheet.
F-6
Valuation of Contingent Consideration, Acquired Intangible Assets, and Inventory Associated with Business Combinations
As described in Note 3 to the consolidated financial statements, the Company acquired the U.S. rights to the FDA approved oncology-supportive care medicine Sancuso from Kyowa Kirin, Inc., the U.S. affiliate of Japan-based Kyowa Kirin Co., Ltd. during the year ended December 31, 2022, resulting in the expansion of the Company’s product offerings. This acquisition resulted in additional goodwill of $0.03 million, additional intangible assets of $14.1 million, and contingent liabilities of $5.1 million being recognized on the Company’s consolidated balance sheet. As part of this acquisition, management assessed that the acquisition qualified as a business combination and all identifiable assets and liabilities acquired were recorded at fair value as part of the purchase price allocation as of the asset date. The identification and valuation of such acquired assets and assumed liabilities requires management to exercise significant judgment and consider the use of outside vendors to estimate the fair value allocations.
We identified the consummated acquisition and the valuation of acquired assets and assumed liabilities as a critical audit matter. Our principal consideration for this determination included high degree of audit effort, including utilizing individuals with specialized skills, in evaluating management’s significant assumptions including its forecasts of future performance of the component, discount rates and other inputs into the model used in computing the fair value of the intangible asset, acquired inventory, and contingent consideration liability.
The primary procedures we performed to address this critical audit matter included:
a.We obtained an understanding of management’s process for determining the fair value measurements of the contingent consideration, acquired intangible assets and inventory, including reviewing the executed Asset Purchase Agreement documents to gain an understanding of the underlying terms of the consummated acquisition.
b.Evaluated forward-looking assumptions, such as forecasted revenue and earnings used by management by performing procedures that included, but not limited to, comparisons to industry and historical performance data, and sensitivity analysis to assess their reasonableness.
c.Utilizing a valuation specialist, we evaluated the significant assumptions and methods utilized in developing the fair value of the contingent consideration, acquired intangible assets, and inventory including:
i.Evaluated the reasonableness of the Company’s third-party valuation models and methodologies, expected cash flow calculations, and reviewed significant assumptions.
ii.Developed an independent calculation of the discount rates used and compared our rates to those used by management.
iii.Prepared an independent calculation of the fair value of the contingent consideration and the intangible assets to test the accuracy of management’s valuation models.
i.Reviewing and evaluating the adequacy of the disclosures made in the Company’s SEC filings.
/s/ FORVIS, LLP
We served as the Company’s auditor from 2020 to 2023.
March 13, 2023
F-7
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Consolidated Balance Sheets
December 31, 2023 and 2022
| 2023 | 2022 | |||||||||||||
| ASSETS | ||||||||||||||
| Current assets: | ||||||||||||||
| Cash and cash equivalents | $ | $ | ||||||||||||
| Accounts receivable, net | ||||||||||||||
| Inventories, net | ||||||||||||||
| Prepaid and other current assets | ||||||||||||||
| Total current assets | ||||||||||||||
| Non-current inventories | ||||||||||||||
| Property and equipment, net | ||||||||||||||
| Intangible assets, net | ||||||||||||||
| Goodwill | ||||||||||||||
| Operating lease right-of-use assets | ||||||||||||||
| Other assets | ||||||||||||||
| Total assets | $ | $ | ||||||||||||
| LIABILITIES AND EQUITY | ||||||||||||||
| Current liabilities: | ||||||||||||||
| Accounts payable | $ | $ | ||||||||||||
| Operating lease current liabilities | ||||||||||||||
| Other current liabilities | ||||||||||||||
| Total current liabilities | ||||||||||||||
| Revolving line of credit | ||||||||||||||
| Operating lease non-current liabilities | ||||||||||||||
| Other long-term liabilities | ||||||||||||||
| Total liabilities | ||||||||||||||
| Commitments and contingencies | ||||||||||||||
| Equity: | ||||||||||||||
| Shareholders’ equity: | ||||||||||||||
Common stock – | ||||||||||||||
| Accumulated deficit | ( | ( | ||||||||||||
| Total shareholders’ equity | ||||||||||||||
| Noncontrolling interests | ( | ( | ||||||||||||
| Total equity | ||||||||||||||
| Total liabilities and equity | $ | $ | ||||||||||||
See accompanying notes to consolidated financial statements.
F-8
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Consolidated Statements of Operations
Years ended December 31, 2023 and 2022
| 2023 | 2022 | |||||||||||||
| Revenues: | ||||||||||||||
| Net product revenue | $ | $ | ||||||||||||
| Other revenue | ||||||||||||||
| Net revenues | ||||||||||||||
| Costs and expenses: | ||||||||||||||
| Cost of products sold | ||||||||||||||
| Selling and marketing | ||||||||||||||
| Research and development | ||||||||||||||
| General and administrative | ||||||||||||||
| Amortization and impairment | ||||||||||||||
| Total costs and expenses | ||||||||||||||
| Operating loss | ( | ( | ||||||||||||
| Interest income | ||||||||||||||
| Other income | ||||||||||||||
| Other income - settlement | ||||||||||||||
| Other income - gain on insurance proceeds | ||||||||||||||
| Interest expense | ( | ( | ||||||||||||
| Loss before income taxes | ( | ( | ||||||||||||
| Income tax expense | ( | ( | ||||||||||||
| Net loss | ( | ( | ||||||||||||
| Net loss at subsidiary attributable to noncontrolling interests | ||||||||||||||
| Net loss attributable to common shareholders | $ | ( | $ | ( | ||||||||||
| Loss per share attributable to common shareholders: | ||||||||||||||
| Basic | $ | ( | $ | ( | ||||||||||
| Diluted | $ | ( | $ | ( | ||||||||||
| Weighted-average common shares outstanding: | ||||||||||||||
| Basic | ||||||||||||||
| Diluted | ||||||||||||||
See accompanying notes to consolidated financial statements.
F-9
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Consolidated Statements of Cash Flows
Years ended December 31, 2023 and 2022
| 2023 | 2022 | |||||||||||||
| Cash flows from operating activities: | ||||||||||||||
| Net loss | $ | ( | $ | ( | ||||||||||
| Adjustments to reconcile net loss to net cash flows provided by operating activities: | ||||||||||||||
| Depreciation and amortization expense | ||||||||||||||
| Impairment loss on intangible assets | ||||||||||||||
| Amortization of operating lease right-of-use asset | ||||||||||||||
| Disposal of assets | ||||||||||||||
| Stock-based compensation | ||||||||||||||
| Decrease in non-cash contingent consideration | ( | ( | ||||||||||||
| Decrease (increase) in cash surrender value of life insurance policies over premiums paid | ( | |||||||||||||
| Noncash interest expense | ||||||||||||||
| Noncash gain on RediTrex transaction | ( | |||||||||||||
| Gain on receipt of life insurance policies | ( | ( | ||||||||||||
| Net changes in assets and liabilities affecting operating activities: | ||||||||||||||
| Accounts receivable | ( | |||||||||||||
| Inventories, net | ( | |||||||||||||
| Other current assets and other assets | ||||||||||||||
| Operating lease liabilities | ( | |||||||||||||
| Accounts payable and other current liabilities | ||||||||||||||
| Other long-term liabilities | ( | |||||||||||||
| Net cash provided by operating activities | ||||||||||||||
| Cash flows from investing activities: | ||||||||||||||
| Additions to property and equipment | ( | ( | ||||||||||||
| Additions to intangible assets | ( | ( | ||||||||||||
| Return of RediTrex | ||||||||||||||
| Life insurance policy proceeds received | ||||||||||||||
| Settlement of patent litigation | ||||||||||||||
| Cash paid for acquisition | ( | |||||||||||||
| Net cash used in investing activities | ( | ( | ||||||||||||
F-10
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Consolidated Statements of Cash Flows
Years ended December 31, 2023 and 2022
| 2023 | 2022 | |||||||||||||
| Cash flows from financing activities: | ||||||||||||||
| Borrowings on line of credit | ||||||||||||||
| Payments on line of credit | ( | ( | ||||||||||||
| Payments made in connection with repurchase of common shares | ( | ( | ||||||||||||
| Cash settlement of contingent consideration | ( | ( | ||||||||||||
| Net cash used in financing activities | ( | ( | ||||||||||||
| Net decrease in cash and cash equivalents | ( | ( | ||||||||||||
| Cash and cash equivalents, beginning of year | ||||||||||||||
| Cash and cash equivalents, end of year | $ | $ | ||||||||||||
| Supplemental disclosure of cash flow information: | ||||||||||||||
| Net cash paid during the year for: | ||||||||||||||
| Interest | $ | $ | ||||||||||||
| Income taxes | ||||||||||||||
| Total cash paid included in measurement of lease liability | ||||||||||||||
| Noncash investing and financing activities: | ||||||||||||||
| Change in unpaid invoices for intangible asset additions | $ | ( | $ | ( | ||||||||||
| Change in unpaid invoices for offering costs | ( | |||||||||||||
| Lease liability obtained from right-of-use assets | ||||||||||||||
| RediTrex forgiveness of milestone payable | ( | |||||||||||||
| Return of shares related to RediTrex | ||||||||||||||
See accompanying notes to consolidated financial statements
F-11
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Consolidated Statements of Equity
Years ended December 31, 2023 and 2022
| Cumberland Pharmaceuticals Inc. Shareholders | ||||||||||||||||||||||||||||||||
| Common stock | Accumulated deficit | Non-controlling interest | Total equity | |||||||||||||||||||||||||||||
| Shares | Amount | |||||||||||||||||||||||||||||||
| Balance, December 31, 2021 | $ | $ | ( | $ | ( | $ | ||||||||||||||||||||||||||
| Net loss | — | — | ( | ( | ( | |||||||||||||||||||||||||||
| Return of common stock - Methotrexate | ( | ( | — | — | ( | |||||||||||||||||||||||||||
| Share-based compensation | — | — | ||||||||||||||||||||||||||||||
| Repurchase of common shares | ( | ( | — | — | ( | |||||||||||||||||||||||||||
| Balance, December 31, 2022 | $ | $ | ( | $ | ( | |||||||||||||||||||||||||||
| Net loss | — | $ | — | ( | ( | ( | ||||||||||||||||||||||||||
| Share-based compensation | $ | — | — | |||||||||||||||||||||||||||||
| Repurchase of common shares | ( | $ | ( | — | — | ( | ||||||||||||||||||||||||||
| Balance, December 31, 2023 | $ | $ | ( | $ | ( | $ | ||||||||||||||||||||||||||
See accompanying notes to consolidated financial statements
F-12
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
(1) Organization
Cumberland Pharmaceuticals Inc. (“Cumberland,” the “Company,” or as used in the context of “we,” “us,” or “our”) is a specialty pharmaceutical company focused on the acquisition, development and commercialization of branded prescription products. The Company’s primary target markets are hospital acute care, gastroenterology and oncology. These medical specialties are characterized by relatively concentrated prescriber bases that the Company believes can be penetrated effectively by small, targeted sales forces. Cumberland is dedicated to providing innovative products that improve quality of care for patients and address unmet or poorly met medical needs. The Company promotes its approved products through its hospital, field and oncology sales forces in the United States and is establishing a network of international partners to bring its medicines to patients in their countries.
Cumberland focuses its resources on maximizing the commercial potential of its products, as well as developing new product candidates, and has both internal development and commercial capabilities. The Company’s products are manufactured by third parties, which are overseen by Cumberland’s quality and manufacturing professionals. The Company works closely with its third-party distribution partners to make its products available in the United States.
In order to build a pipeline of early-stage product candidates, the Company formed a subsidiary, Cumberland Emerging Technologies, Inc. ("CET"), which teams with universities and other research organizations to help advance scientific discoveries from the laboratory to the marketplace.
In April, 2019, CET entered into an agreement with HongKong WinHealth Pharma Group Co. Limited ("WinHealth") whereby WinHealth made a $1.0 million investment through the purchase of shares of CET stock. As part of the agreement, WinHealth obtained a board position at CET and the first opportunity to license CET products for the Chinese market. In connection with WinHealth's investment in CET, Cumberland also made an additional $1.0 million investment in CET.
The Company’s ownership in CET is now 85 % while the remaining interest is owned by WinHealth, Vanderbilt University and the Tennessee Technology Development Corporation. The operating results of CET allocated to noncontrolling interests in the consolidated statements of operations were $51,446 and $79,798 for the years ended December 31, 2023 and 2022, respectively.
Effective January 1, 2007, the Company formed a wholly-owned subsidiary, Cumberland Pharma Sales Corp. ("CPSC"). CPSC is the subsidiary that employs the Company's hospital and field sales force personnel.
In December 2017, the Company formed the Cumberland Pharma Foundation (the "Foundation") to serve as a vehicle to facilitate the ongoing philanthropic endeavors of Cumberland Pharmaceuticals Inc.
The Foundation was formed as a nonprofit corporation designed to qualify as a tax-exempt organization pursuant to Section 501(a) of the Internal Revenue Code. The Foundation’s Board of Directors is comprised of Cumberland Pharmaceuticals executives who are responsible for overseeing the Foundation’s ongoing activities including charitable contributions.
In 2018, Cumberland provided a grant of 50,000 shares of the Company’s common stock to the Foundation. The shares will address the ongoing financial needs of the Foundation. The organization also plans to hold a portion of the shares for long-term appreciation. The Foundation maintains separate financial statements and its ongoing operations do not impact the financial statements of Cumberland Pharmaceuticals. Initial annual grants by the Foundation have been and are expected to remain consistent with the historic level of contributions made by Cumberland Pharmaceuticals. Since 2019, Cumberland has made annual cash contributions to the Foundation. During the years 2023 and 2022, the Company made cash contributions of $40,000 and $25,000 , respectively.
F-13
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
(2) Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements of the Company are stated in U.S. dollars and are prepared using U.S. generally accepted accounting principles. These financial statements include the accounts of the Company and its wholly and majority-owned subsidiaries. All significant intercompany transactions and accounts have been eliminated in consolidation.
Use of Estimates
The preparation of the consolidated financial statements in conformity with U.S. generally accepted accounting principles requires management of the Company to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the period. Actual results could differ from those estimates under different assumptions and conditions. The Company’s most significant estimates include: (1) its allowances for chargebacks and accruals for rebates and product returns, (2) the allowances for obsolescent or unmarketable inventory, (3) valuation of contingent consideration liability associated with business combinations and (4) evaluation of continuing utility of intangible assets.
Segment Reporting
Fair Value of Financial Instruments
Fair value of financial assets and liabilities is the price the Company would receive to sell an asset or pay to transfer a liability in an orderly transaction with a market participant at the measurement date. The Company’s fair value measurements follow the appropriate rules as well as the fair value hierarchy that prioritizes the information used to develop the measurements. It applies whenever other guidance requires (or permits) assets or liabilities to be measured at fair value and gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements).
A summary of the fair value hierarchy that prioritizes observable and unobservable inputs used to measure fair value into three broad levels is described below:
Level 1 - Quoted prices for identical instruments in active markets.
Level 2 - Quoted prices for similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations whose inputs are observable or whose significant value drivers are observable.
Level 3 - Significant inputs to the valuation model are unobservable.
We maintain policies and procedures to value instruments using the best and most relevant data available. The following section describes the valuation methodologies we use to measure different financial instruments at fair value on a recurring basis.
F-14
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
The Company’s financial instruments include cash and cash equivalents, accounts receivable, accounts payable, accrued liabilities, contingent consideration liability and a revolving line of credit. The carrying values for cash and cash equivalents, accounts receivable, accounts payable and accrued liabilities approximate their fair values due to their short-term nature. The revolving line of credit has a variable interest rate, which approximates the current market rate.
Cash and Cash Equivalents
Cash and cash equivalents include highly liquid investments with original maturities of three months or less. As of December 31, 2023 and 2022, cash equivalents consist primarily of money market funds. The Company monitors concentration of credit risk with the financial institutions in which it conducts business. The Company has cash deposits which fluctuate in excess of federally insured limits throughout the year.
Accounts Receivable
Trade accounts receivable are recorded at the invoiced amount. The Company records allowances for amounts that could become uncollectible in the future based on historical experience, as well as amounts related to chargebacks and cash discounts. The Company reviews each customer balance to assess collection status.
The majority of the Company’s products are distributed through independent pharmaceutical wholesalers. The allowances against accounts receivable for chargebacks and discounts are determined on a product-by-product basis, and established by management as the Company’s best estimate at the time of sale based on each product’s historical experience adjusted to reflect known changes in the factors that impact such allowances. These allowances are established based on the contractual terms with direct and indirect customers and analyses of historical levels of chargebacks and discounts. The allowances in accounts receivable for chargebacks and cash discounts were $0.6 million at December 31, 2023 and 2022.
Other organizations, such as managed care providers, pharmacy benefit management companies and government agencies, may receive rebates from the Company based on either negotiated contracts to carry the Company’s products or reimbursements for filled prescriptions. These entities are considered indirect customers of the Company. In conjunction with recognizing a sale to a wholesaler, revenues are reduced and accrued liabilities are increased by the Company’s estimate of the rebate that may be claimed. Cash discounts are reductions to invoiced amounts offered to customers for payment within a specified period of time from the date of the invoice.
Inventories
The Company works closely with third parties to manufacture and package finished goods for sale. Based on the customer relationship with the manufacturer or packager, the Company will either take title to finished goods at the time of shipment or at the time of arrival from the manufacturer. The Company then warehouses such goods until distribution and sale at third party facilities located in the U.S. and international locations. Periodic inventory counts are made by the warehouse teams and by the Company on a regular basis. In addition, the Company re-tests API inventory prior to use to confirm product expiration. Inventories are stated at the lower of cost or net realizable value with cost determined using the first-in, first-out method.
The Company continually evaluates inventories for potential losses due to expired, short-dated or slow-moving inventory by comparing sales history and sales projections to the inventory on hand. When evidence indicates the carrying value of a product may not be recoverable, a charge is recorded to reduce the inventory to its current net realizable value.
F-15
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
Prepaid and Other Current Assets
Prepaid and other current assets consist of deferred offering costs, prepaid insurance premiums, prepaid consulting services, deposits and annual fees paid to the U.S. Food and Drug Administration ("FDA"). The Company expenses all prepaid and other current asset amounts as used or over the period of benefit primarily on a straight-line basis, as applicable.
In November 2017, the Company filed its Shelf Registration on Form S-3 with the SEC associated with the sale of up to $100 million in corporate securities. The Shelf Registration was declared effective in January 2018. It also included an At the Market ("ATM") feature that allows the Company to sell common shares at market prices. The Company filed an updated Form S-3 with the SEC in December 2020 (the “Prior Registration Statement”), which was declared effective in January 2021.
On December 27, 2021, the Company filed a related prospectus supplement in connection with the sale and issuance of shares having an aggregate gross sales price of up to $19 million. The Company amended the ATM Sales Agreement on December 27, 2021, in order to allow the Company to continue using its ATM feature to sell shares at market prices. This ATM program was under the Prior Registration Statement and is no longer active.
The Company filed an updated Form S-3 with the SEC on December 14, 2023, which was declared effective on December 26, 2023 (the “Current Registration Statement”). The Company intends to enter into an agreement with H.C. Wainwright & Co., LLC to establish a new ATM program under the Current Registration Statement.
The Company has recorded deferred offering costs for payments directly related to the current Shelf Registration on Form S-3 that was completed during December 2021 and December 2023. These costs consist of legal and accounting fees that the Company has capitalized. Deferred costs associated with the Shelf Registration will be reclassified to additional paid in capital on a pro-rata basis as the Company completes sales of shares under the Shelf Registration. The Company did not issue any shares under this ATM during the year ended December 31, 2023.
Property and Equipment
Property and equipment, including leasehold improvements, are stated at cost. Depreciation is recognized using the straight-line method over the estimated useful lives of the assets. Leasehold improvements are amortized over the shorter of the initial lease term plus renewal options, if reasonably assured, or the remaining useful life of the asset. Upon retirement or disposal of assets, any gain or loss is reflected as a component of operating loss in the consolidated statement of operations. Improvements that extend an asset’s useful life are capitalized. Repairs and maintenance costs are expensed as incurred.
Intangible Assets and Goodwill
The Company’s intangible assets and goodwill consist of capitalized costs related to product and license rights, patents, trademarks and goodwill obtained in the Vibativ and Sancuso acquisitions. Goodwill is not amortized for financial reporting purposes, but is subject to impairment analysis at least annually.
The cost of acquiring product and license rights are capitalized at fair value at the date of acquisition for products that are approved by the FDA for commercial use. These costs are amortized ratably over the estimated economic life of the product. The economic life is estimated based upon several factors. This includes the term of the license agreement, the patent life or market exclusivity of the product and as well as management’s expectations of continued involvement with the product and the assessment of future sales, the future periods under which the product will be sold and the profitability of the product. This estimate is evaluated on a regular basis during the amortization period and adjusted if appropriate. If there are any changes made to the useful life of the product and license rights, the costs associated with such a change, if any, will be capitalized and amortized over the revised useful life.
Given the delay in identifying a packager for Omeclamox-Pak, the Company has taken a non-cash write down of the related intangible asset of $3.3 million. The financial statements reflect this adjustment as efforts to identify a packager continue.
F-16
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
Amortization expense is recognized ratably over the following periods:
| Product rights | Estimated economic life | |||||||
| License rights | Term of license agreement | |||||||
| Patents | Life of patent | |||||||
Trade and Note Receivables
Current Expected Credit Losses (“CECL”) is applicable to all financial assets measured at amortized cost. All financial instruments are evaluated, which principally relates to trade receivables and two notes receivable. CECL requires the measurement of expected credit losses on a collective (pool) basis when similar risk characteristics exist. This may include, either individually or in combination, some of the following characteristics of Accounting Standards Codification ("ASC") 326-20-55-5:
•Internal or external credit score/rating
•Risk ratings or classification
•Financial asset type
•Size
•Effective interest rate
•Term
•Geographical location
•Historical or expected credit loss patterns
•Reasonable and supportable forecast periods
The standard requires entities to pool financial assets but allows them to choose which risk characteristics to use. Under the requirements of the guidance, the Company reassesses at the end of each reporting period whether the pool of assets continue to display similar risk characteristics.
With twenty years of experience, Cumberland has experienced virtually no write downs of receivables as most of our receivables are due from large successful pharmaceutical, healthcare or government customers, consistently making payments on account. Although the payment behaviors of all of our customers are consistently reliable, for the sake of transparency, we have separated our customer base into seven separate pools. The Company performs a monthly analysis of aged accounts receivable to determine how much, if any, of the accounts receivable balance should be reserved as potential bad debt. The Company reviews all balances over 90 days past due for a possible reserve and considers any specific factors or information for balances aged under 90 days if there are indicators that the balance should be reserved, such as other aged balances with the customer or bankruptcy as well as any economic issues with a customer, industry or region. The adoption of ASC 326 did not result in a material impact to the Company.
Impairment of Long-Lived Assets
F-17
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
Goodwill and other indefinite lived intangible assets that are not subject to amortization are tested at least annually for impairment. The impairment analysis for goodwill requires a comparison of fair value to the carrying value of the reporting unit. The Company’s goodwill was acquired in November 2018 with the Vibativ acquisition and in January 2022 with the Sancuso acquisition. As a result, the Vibativ and Sancuso components of the Company are the reporting units evaluated for goodwill impairment. Cumberland determined the fair value of the reporting units through current and future estimated revenue and profitability of the product. The Company recorded no 3.3 million, which is included in amortization expense in the statement operations. These intangible assets consisted of product and license rights and trademarks.
Joint Venture Agreement
In August 2020, Cumberland entered into an agreement with WinHealth Investment (Singapore) Ltd creating WHC Biopharmaceuticals, Pte. Ltd. The joint venture, as a limited liability company, focuses on acquiring, developing, registering, and commercializing development stage and commercial stage biopharmaceuticals for China, Hong Kong and other Asian markets. The agreement provided for initial investment from WinHealth in the form of a $0.2 million equity contribution and an initial investment from Cumberland in the form of $0.2 million convertible note. The joint venture will seek additional future capital from additional investors and has entered into exclusive option agreements to license intellectual property from both Cumberland Pharmaceuticals Inc. and Cumberland Emerging Technologies.
Net Product Revenue
Revenues from product sales are recognized in the amount that reflects the consideration that we expect to receive for these goods. Depending upon the shipping terms of the transaction, the revenue is recognized at the point where the customer obtains control of the goods and we satisfy our performance obligation. This occurs upon either shipment of the product or arrival at its ship to destination. Payment terms typically range from 30 to 60 days from date of shipment. The Company’s net product revenue reflects the reduction from gross product revenue for estimated allowances for chargebacks, discounts and damaged goods, and reflects sales related accruals for rebates, coupons, product returns, and certain administrative and service fees. Significant judgments must be made in determining the transaction price for our sales of products related to these adjustments.
During the year ended December 31, 2023, the Company revised its estimate for rebates related to Sancuso revenues based on additional information related to actual product activities. As a result, the Company recognized additional revenue allowances of $0.6 million for rebates as a change of estimate.
Sales Rebates and Discounts
The allowances against accounts receivable and accrued liabilities for chargebacks, discounts, service fees and expired product returns are determined on a product-by-product basis, and established by management as the Company’s best estimate at the time of sale based on each product’s historical experience adjusted to reflect known changes in the factors that impact such allowances. These allowances are established based on the contractual terms with direct and indirect customers and analyses of historical levels of chargebacks, discounts and returns of expired product.
Other organizations, such as managed care providers, pharmacy benefit management companies and government agencies, may receive rebates from the Company based on either negotiated contracts to carry the Company’s products or reimbursements for filled prescriptions. These entities are considered indirect customers of the Company. In conjunction with recognizing a sale to a wholesaler, sales revenues are reduced and accrued liabilities are increased by the Company’s estimate of the rebate that may be claimed.
Sales Returns
Consistent with industry practice, the Company maintains a return policy that allows customers to return product within a specified period prior to and subsequent to the expiration date. The Company’s estimate of the provision for returns is based upon historical experience, expiration date by product as well as any other factor
F-18
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
expected to impact future returns. Any changes in the assumptions used to estimate the provision for returns are recognized in the period those assumptions are changed.
Other Revenues
Other revenues primarily consist of income from grant funding programs, licensing agreements, leases and contract services. Revenue related to grants is recognized when all conditions related to such grants have been met. All other revenue is recognized when earned.
Cost of Products Sold
Cost of products sold consists principally of the cost to acquire each unit of product sold, including in-bound freight expense as well as any adjustment in the net realizable value of inventory acquired in acquisitions. Cost of products sold also includes expenses associated with the reduction in the net realizable value of slow-moving or expired product.
Selling and Marketing Expense
Selling and marketing expense consists primarily of expenses relating to the advertising, promotion, distribution and sale of products, including royalty expense, salaries and related costs.
Distribution Costs
| 2023 | 2022 | |||||||||||||
| Distribution costs | $ | $ | ||||||||||||
Advertising Costs
| 2023 | 2022 | |||||||||||||
| Advertising costs | $ | $ | ||||||||||||
Research and Development
Research and development costs are expensed in the period incurred. Research and development costs are comprised mainly of clinical trial expenses, salaries, wages and other related costs such as materials and supplies. Research and development expense includes activities performed by third-party providers participating in the Company’s clinical studies. The Company accounts for these costs based on estimates of work performed, patients enrolled or fixed fees for services over the period of time the clinical trials are performed.
Income Taxes
The Company provides for deferred taxes using the asset and liability approach. Under this method, deferred tax assets and liabilities are recognized for future tax consequences attributable to operating loss and tax credit carryforwards, as well as differences between the carrying amounts of existing assets and liabilities and their respective tax bases. The Company’s principal differences are related to the timing of deductibility of certain items, such as inventory, depreciation, amortization and share-based compensation. Deferred tax assets and liabilities are measured using enacted statutory tax rates that are expected to apply to taxable income in the years such temporary differences are anticipated to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period of enactment. The Company only recognizes income tax benefits
F-19
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
associated with an income tax position in which it is “more likely than not” that the position would be sustained upon examination by the taxing authorities.
In assessing the realizability of deferred tax assets, management considers whether some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of existing temporary differences, projected future taxable income and tax planning strategies in making this assessment.
The Company’s accounting policy with respect to interest and penalties arising from income tax settlements is to recognize them as part of the provision for income taxes.
Loss per Share
Basic loss per share is calculated by dividing net loss attributable to common shareholders by the weighted-average number of shares outstanding. Except where the result would be antidilutive, diluted loss per share is calculated by assuming the vesting of unvested restricted stock and the exercise of stock options and warrants and unrecognized compensation costs.
Share-Based Payments
The Company recognizes compensation cost for all share-based payments issued, modified, repurchased or canceled. Depending on the nature of the vesting provisions, restricted stock awards are measured using either the fair value on the grant date or the fair value of common stock on the date the vesting provisions lapse. Prior to the lapse for those equity grants not valued on the grant date, the fair value is measured on the last day of the reporting period.
Collaborative Agreements
The Company is a party to several collaborative arrangements with research institutions to identify and pursue promising pharmaceutical product candidates. The funding for these programs is primarily provided through Federal Small Business Administration (SBIR/STTR) and other grant awards. The Company has determined that these collaborative agreements, with the exception of the collaborative payment discussed in Note 3, related to the Vibativ and Sancuso contingent consideration payments, do not meet the criteria for accounting under ASC Topic 808, Collaborative Agreements. The agreements do not specifically designate each party’s rights and obligations to each other under the collaborative arrangements. Except for patent defense costs, expenses incurred by one party are not required to be reimbursed by the other party. Expenses incurred under these collaborative agreements are included in research and development expenses and funding received from grants are recorded as net revenues in the consolidated statements of operations.
Related Party Transactions
In July 2023, Cumberland named Martin S. Brown Jr. to our Board of Directors. Mr. Brown is an attorney with over 30 years of corporate law experience who brings significant legal, public company, health care and civic experience to our board. The Company relies on several law firms for legal advice, including the firm with which Mr. Brown is affiliated. In 2023 and 2022, the Company paid Mr. Brown’s law firm $0.05 million and $0.04 million, respectively.
Recent Accounting Guidance
Recent Accounting Pronouncements Adopted
In June 2016, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") No. 2016-13, “Financial Instruments-Credit Losses,” which changes the impairment model for most financial assets and certain other instruments. For trade and other receivables, held-to-maturity debt securities, loans and other instruments, companies are required to use a new forward-looking “expected loss” model that generally results in an earlier recognition of allowances for losses. For available-for-sale debt securities with unrealized losses, companies measure credit losses in a manner similar with previous guidance, except that the losses are recognized as
F-20
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
allowances rather than as reductions in the amortized cost of the securities. Companies have to disclose additional information, including information they use to track credit quality by year of origination for most financing receivables. Companies apply the ASU’s provisions as a cumulative-effect adjustment, if any, to the accumulated deficit as of the beginning of the first reporting period in which the guidance is adopted.
Recently Issued Accounting Standards Not Yet Adpoted
In November 2023, the FASB issued final guidance intended to improve transparency of segment disclosures, primarily through expanded disclosures for significant segment expenses. The guidance is effective for annual periods beginning in 2024 and interim periods beginning in 2025. Early adoption is permitted. This new guidance will result in incremental disclosures in the notes to the Company’s segment reporting disclosures.
In December 2023, the FASB issued final guidance to improve transparency of income tax disclosures. The final guidance requires enhanced disclosures primarily related to existing rate reconciliation and income taxes paid information. The guidance is effective for 2025 annual reporting. Early adoption is permitted. This new guidance will result in incremental disclosures in the notes to the Company’s income tax disclosures.
(3) Vibativ ®, Sancuso® and RediTrex® Products
Vibativ
During November 2018, the Company executed an agreement with Theravance Biopharma ("Theravance") to acquire the assets and global rights to Vibativ including responsibility for the marketing, distribution, manufacturing and regulatory activities associated with the brand. Vibativ is a patented, FDA approved injectable anti-infective for the treatment of certain serious bacterial infections including hospital-acquired and ventilator-associated bacterial pneumonia and complicated skin and skin structure infections. It addresses a range of Gram-positive bacterial pathogens, including those that are considered difficult-to-treat and multidrug-resistant.
Cumberland accounted for the transaction as a business combination in accordance with ASC 805 and the product sales are included in the results of operations subsequent to the acquisition date. The Company made an upfront payment of $20 million at the closing of the transaction and a $5 million milestone payment in early April 2019. In addition, Cumberland has agreed to pay royalties of up to 20 % of on-going net sales of the product in the U.S. after a $2.5 million threshold is met. The future royalty payments were recognized at their acquisition-date fair value as a contingent consideration liability, as part of the contingent consideration transferred in the business combination. Cumberland prepared the valuations of the contingent consideration liability utilizing significant unobservable inputs. As a result, the valuation is classified as Level 3 fair value measurement.
The following table presents the changes in the fair value of the contingent consideration liability that is remeasured on a recurring basis. The contingent consideration earned and accrued in operating expenses is paid to Theravance annually.
F-21
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
| Contingent Consideration Liability | |||||
| Balance at December 31, 2021 | $ | ||||
| Cash payment of royalty during the period | ( | ||||
| Change in fair value | ( | ||||
| Contingent consideration earned and accrued | |||||
| Balance at December 31, 2022 | $ | ||||
| Cash payment of royalty during the period | ( | ||||
| Change in fair value | ( | ||||
| Contingent consideration earned and accrued | |||||
| Balance at December 31, 2023 | $ | ||||
The current portion of the contingent consideration liability is $1.7 million and the non-current portion is $2.3 million, as of December 31, 2023.
Sancuso
On January 3, 2022, Cumberland acquired the U.S. rights to the FDA-approved oncology-supportive care medicine Sancuso from Kyowa Kirin, Inc. ("Kyowa Kirin"), the U.S. affiliate of Japan-based Kyowa Kirin Co., Ltd.
Sancuso is the first and only FDA-approved prescription patch for the prevention of nausea and vomiting in patients receiving certain types of chemotherapy treatment. The active drug in Sancuso, granisetron, slowly dissolves in the thin layer of adhesive that sticks to the patient’s skin and is released into their bloodstream over several days, working continuously to prevent chemotherapy-induced nausea and vomiting (“CINV”). It is applied 24 to 48 hours before receiving chemotherapy and can prevent CINV for up to five consecutive days. Alternative oral treatments must be taken several times (day and night) to deliver the same therapeutic doses.
Cumberland acquired U.S. rights to Sancuso and assumed full commercial responsibility for the product in the U.S. – including its marketing, promotion, distribution, manufacturing and medical support activities. The product’s FDA registration was subsequently transferred from Kyowa Kirin to Cumberland in August 2023.
Cumberland has accounted for the transaction as a business combination in accordance with ASC 805 and the product sales are included in the results of operations subsequent to the acquisition date. The Company made an upfront payment of $13.5 million at the closing of the transaction. The Agreement calls for milestone payments of up to $3.5 million based on the attainment of various approvals and sales performance. In January 2023, Cumberland made a $1.0 million milestone payment to Kwoya Kirin based on the FDA approval of a manufacturing site for the product. In October 2023, Cumberland made a $0.5 million milestone payment based on the successful transfer of the product’s FDA registration from Kyowa Kirin to Cumberland.
The remaining $2 million in milestone payments are tied to achievement of certain annual sales levels for the product.
In addition, Cumberland has agreed to pay a royalty of up to 10 % of on-going net sales of the product. The future royalty payments were required to be recognized at their acquisition-date fair value as a contingent consideration liability, as part of the contingent consideration transferred in the business combination. Cumberland has prepared a valuation of the contingent consideration liability utilizing significant unobservable inputs. As a result, the valuation is classified as Level 3 fair value measurement.
The acquisition was funded by cash and the Company's revolving credit facility. The fair value for the assets and liabilities assumed using Level 3 fair value inputs were as follows: prepaid expenses of $1.8 million, inventory $2.6 million, goodwill $0.03 million, intangible assets $14.1 million, milestone payable $1.7 million and contingent liability $3.4 million.
F-22
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
The following table presents the changes in the fair value of the contingent consideration liability that is remeasured on a recurring basis.
| Contingent Consideration Liability | Contingent Consideration Liability Including Milestone | |||||||
Balance at January 3, 2022 | $ | $ | 3,946,716 | |||||
Cash payment of royalty during the period | ( | (1,075,631) | ||||||
Change in fair value | 1,210,678 | |||||||
Contingent consideration earned and accrued | 675,237 | |||||||
Balance at December 31, 2022 | $ | $ | 4,757,000 | |||||
Cash payment of royalty during the period | ( | (2,249,632) | ||||||
Change in fair value | ( | (983,355) | ||||||
Contingent consideration earned and accrued | 781,987 | |||||||
Balance at December 31, 2023 | $ | $ | 2,306,000 | |||||
The current portion of the contingent consideration liability is $1.3 million and the non-current portion is $1.0 million, as of December 31, 2023.
RediTrex
In November 2016, the Company announced an agreement with the Nordic Group B.V. ("Nordic") to acquire the exclusive U.S. rights to Nordic’s injectable methotrexate product line designed for the treatment of active rheumatoid arthritis, juvenile idiopathic arthritis, severe psoriatic arthritis, and severe disabling psoriasis.
As consideration for the license Cumberland paid a deposit of $0.1 million at closing. The Company provided $0.9 million in consideration through a grant of 180,000 restricted shares of Cumberland common stock to be vested upon the FDA approval of the first Nordic product. Cumberland also agreed to provide Nordic a series of payments tied to the products’ FDA approval, launch and achievement of certain sales milestones. Under the terms of the agreement, Cumberland is responsible for the product registration and commercialization in the U.S. Nordic is responsible for product manufacturing and supply.
On November 27, 2019, Cumberland received FDA approval for the first Nordic injectable product and authorization to market them under the RediTrex brand name. The 180,000 shares of restricted Cumberland common stock previously provided to Nordic vested upon approval and were valued at $0.9 million on the vesting date. The FDA approval also resulted in a $1.0 million milestone payment due to Nordic. This milestone payment was paid in July 2020. During December 2020, Cumberland began distributing RediTrex which also resulted in a $1.0 million milestone payment due to Nordic. The full launch of RediTrex occurred in October 2021.
F-23
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
(4) Revenues
Product Revenue
The Company’s net product revenues consisted of the following for the years ended December 31:
| 2023 | 2022 | |||||||||||||
| Products: | ||||||||||||||
| Kristalose | $ | $ | ||||||||||||
| Vibativ | ||||||||||||||
| Sancuso | ||||||||||||||
| Caldolor | ||||||||||||||
| Acetadote | ||||||||||||||
| Omeclamox-Pak | ||||||||||||||
| Vaprisol | ( | |||||||||||||
| RediTrex | ( | ( | ||||||||||||
| Total net product revenues | $ | $ | ||||||||||||
Other Revenues
The Company has agreements with international partners for commercialization of the Company’s products with associated payments included in other revenues. Those agreements provide that each of the partners is responsible for seeking regulatory approvals for the product, and following approval, each partner will be responsible for the ongoing distribution and sales in the respective international territories. The Company provides a dossier for product registration and maintains responsibility for the relevant intellectual property. Cumberland is typically entitled to receive a non-refundable, up-front payment at the time each agreement is executed as consideration for the product dossier and for the rights to the distinct intellectual property rights in the respective international territory. These agreements also typically provide for additional payments upon a partner’s achievement of a defined regulatory approval and sales milestones. The Company may also be entitled to receive royalties on future sales of the products and a transfer price on supplies. The contractual payments associated with the partner’s achievement of regulatory approvals, sales milestones and royalties on future sales are recognized as revenue upon occurrence, or at such time that the Company has a high degree of confidence that the revenue would not be reversed in a subsequent period.
In the second quarter of 2023, the Company received $1 million relating to a litigation settlement based on two $500,000
Other revenues includes funding from federal grant programs including those secured from the FDA and from those secured by CET through the Small Business Administration. Grant revenue from these federal grant programs totaled approximately $0.4 million and $0.2 million for the year ended December 31, 2023 and 2022,respectively.
Other revenues also includes lease income generated by CET’s Life Sciences Center. It is a research facility that provides scientists with access to flexible lab space and other resources to develop biomedical products. This lease income, as noted in Footnote 15 - Leases, was approximately $0.5 million for the years ended December 31, 2023 and 2022.
F-24
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
(5) Inventories, Net
The Company's net inventories consisted of the following as of December 31:
| 2023 | 2022 | |||||||||||||
| Raw materials | $ | $ | ||||||||||||
| Consigned inventory | ||||||||||||||
| Finished goods, net of reserve | ||||||||||||||
| Total inventories | ||||||||||||||
| less non-current inventories | ( | ( | ||||||||||||
| Total inventories classified as current | $ | $ | ||||||||||||
The Company's non-current inventories consisted of the following as of December 31:
| 2023 | 2022 | |||||||||||||
| Vibativ - raw materials | $ | $ | ||||||||||||
| Vibativ - finished goods | ||||||||||||||
| Kristalose - raw materials | ||||||||||||||
| Vaprisol - raw materials | ||||||||||||||
| Caldolor - finished goods | ||||||||||||||
| Sancuso - raw materials | ||||||||||||||
| Study drug - raw materials | ||||||||||||||
| Study drug - finished goods | ||||||||||||||
| Other products | ||||||||||||||
| Total non-current inventory | $ | $ | ||||||||||||
The Company purchases the active pharmaceutical ingredient (“API”) for Kristalose and maintains the inventory of that raw material. API for the Company's Vaprisol and Vibativ brands were included in the assets associated with the acquisition of those brands and are also included in the raw materials inventory. As part of the Vibativ acquisition, the Company acquired API and work in process inventories of $15.6 million that were all initially classified as non-current inventories at the date of acquisition. Consigned inventory represents Authorized Generic inventory stored with Padagis until shipment.
F-25
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
(6) Property and Equipment
Property and equipment consisted of the following at December 31:
Range of useful lives | 2023 | 2022 | ||||||||||||||||||
| Computer equipment | $ | $ | ||||||||||||||||||
| Office equipment | ||||||||||||||||||||
| Furniture and fixtures | ||||||||||||||||||||
| Leasehold improvements | ||||||||||||||||||||
Total property and equipment, gross | ||||||||||||||||||||
Less: accumulated depreciation and amortization | ( | ( | ||||||||||||||||||
Total property and equipment, net | $ | $ | ||||||||||||||||||
Depreciation expense, including amortization expense related to leasehold improvements, is included in general and administrative expense in the consolidated statements of operations. Depreciation expense was as follows for the years ended December 31:
| 2023 | 2022 | |||||||||||||
| Depreciation expense | $ | $ | ||||||||||||
(7) Intangible Assets and Goodwill
Intangible assets and Goodwill consisted of the following at December 31, 2023 and 2022.
| 2023 | 2022 | |||||||||||||
| Product and license rights | $ | $ | ||||||||||||
| Less: accumulated amortization | ( | ( | ||||||||||||
| Total product and license rights | ||||||||||||||
| Patents | ||||||||||||||
| Less: accumulated amortization | ( | ( | ||||||||||||
| Total patents | ||||||||||||||
| Trademarks | ||||||||||||||
| Less: accumulated amortization | ( | ( | ||||||||||||
| Total trademarks | ||||||||||||||
| Total intangible assets | $ | $ | ||||||||||||
| Goodwill | $ | $ | ||||||||||||
Product and license rights include assets associated with the Company’s acquired products, including those discussed in Note 3, Vibativ and Sancuso.
F-26
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
During 2023 and 2022, the Company recorded an additional $0.1 million and $0.2 million, respectively, in intangible assets for patents, trademarks and capitalized patent costs, including amounts incurred in the protection of the Company's intellectual property.
Amortization expense related to product and license rights, trademarks and patents were as follows for the years ended December 31:
| 2023 | 2022 | |||||||||||||
| Amortization and impairment expense | $ | $ | ||||||||||||
The expected amortization expense for the Company's current balance of intangible assets are as follows:
| Year ending December 31: | ||||||||
| 2024 | $ | |||||||
| 2025 | ||||||||
| 2026 | ||||||||
| 2027 | ||||||||
| 2028 and thereafter | ||||||||
| $ | ||||||||
(8) Other Current and Other Long-term Liabilities
Other current liabilities consisted of the following at December 31:
| 2023 | 2022 | |||||||||||||
Rebates, product returns, administrative fees and service fees | $ | $ | ||||||||||||
| Employee wages and benefits | ||||||||||||||
| Sancuso related liabilities | ||||||||||||||
| Current portion of accrued contingent consideration | ||||||||||||||
| Studies accrual | ||||||||||||||
| Accrued inventory purchases | ||||||||||||||
| Current deferred charges | ||||||||||||||
| Other | ||||||||||||||
| Total other current liabilities | $ | $ | ||||||||||||
Other long-term liabilities consisted of the following at December 31:
| 2023 | 2022 | |||||||||||||
| Non-current portion of accrued contingent consideration | $ | $ | ||||||||||||
| Deferred compensation | ||||||||||||||
| Other | ||||||||||||||
| Total other long-term liabilities | $ | $ | ||||||||||||
F-27
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
(9) Debt
On September 5, 2023, the Company entered into a new Revolving Credit Loan Agreement with Pinnacle Bank. This facility provides for an aggregate principal funding amount of up to $25 million. The initial revolving line of credit is up to $20 million, with the ability for Cumberland to increase the amount to $25 million, under certain conditions. It has a three year term expiring on October 1, 2026. The interest rate is based on a benchmark (Term SOFR) plus a spread of 2.75 %. Cumberland is subject to one financial covenant, the maintenance of a Funded Debt Ratio, determined on a quarterly basis. Borrowings under the line of credit are collateralized by substantially all of our assets.
As of December 31, 2023 and December 31, 2022, the Company had $12.8 million and $16.2 million, respectively, in borrowings outstanding under its revolving credit facility. The applicable interest rate under the Pinnacle Agreement was 8.125 % at December 31, 2023. In 2022, under a previous agreement with Pinnacle Bank, the applicable interest rate was based on LIBOR plus an interest rate spread and was 6.75 % at December 31, 2022.
(10) Shareholders’ Equity
(a) Initial Public Offering
On August 10, 2009 , the Company completed its initial public offering of 5,000,000 shares of common stock at a price of $17.00 per share, raising gross proceeds of $85.0 million. After deducting underwriting discounts of approximately $6.0 million and offering costs incurred of approximately $4.2 million, the net proceeds to the Company were approximately $74.8 million.
(b) Preferred Stock
The Company is authorized to issue 20,000,000 shares of preferred stock. The Board of Directors is authorized to divide these shares into classes or series, and to fix and determine the relative rights, preferences, qualifications and limitations of the shares of any class or series so established. At December 31, 2023 and 2022, there was no
(c) Common Stock
During 2023 and 2022, the Company issued 157,360 shares and 171,655 shares of common stock, respectively, as a result of restricted shares vesting as well as other common share issuances. There were no option exercise transactions during 2023 and 2022.
(d) Share Repurchases
The Company currently has a share repurchase program available to repurchase up to $10 million of its common stock pursuant to Rule 10b-18 of the Securities Exchange Act of 1934. The Company repurchased 402,143 shares and 367,793 shares of common stock for approximately $0.7 million and $1.0 million during the years ended December 31, 2023 and 2022, respectively. There remains approximately $3 million available under the current repurchase program available for share repurchases at December 31, 2023.
F-28
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
(11) Loss Per Share
The following table shows the computation of the numerator and the denominator used to calculate diluted loss per share for the years ended December 31:
| 2023 | 2022 | |||||||||||||
| Numerator: | ||||||||||||||
| Net loss | ( | ( | ||||||||||||
| Net loss at subsidiary attributable to noncontrolling interests | ||||||||||||||
| Net loss attributable to common shareholders | $ | ( | $ | ( | ||||||||||
| Denominator: | ||||||||||||||
| Weighted-average shares outstanding – basic | ||||||||||||||
| Weighted-average shares outstanding – diluted | ||||||||||||||
The Company's anti-dilutive restricted shares and stock options outstanding were as follows for the years ended December 31:
| 2023 | 2022 | |||||||||||||
| Anti-dilutive shares and options | ||||||||||||||
F-29
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
(12) Income Taxes
The components of the Company's net deferred tax assets at December 31 are as follows:
| 2023 | 2022 | |||||||||||||
| Deferred Tax Assets | ||||||||||||||
| Net operating loss and tax credits | $ | $ | ||||||||||||
| Property and equipment and intangibles | ||||||||||||||
| Operating lease liabilities | ||||||||||||||
| Intangible assets | ||||||||||||||
| Capitalized research cost | ||||||||||||||
| Allowance for accounts receivable | ||||||||||||||
| Reserve for expired product | ||||||||||||||
| Inventory | ||||||||||||||
| Deferred charges | ||||||||||||||
| Cumulative compensation costs incurred on deductible equity awards | ||||||||||||||
| Total deferred tax assets | ||||||||||||||
| Deferred Tax Liabilities | ||||||||||||||
| Operating lease right-of-use assets | ( | ( | ||||||||||||
| Intangible assets | ||||||||||||||
| Net deferred tax assets, before valuation allowance | ||||||||||||||
| Less: deferred tax asset valuation allowance | ( | ( | ||||||||||||
| Net deferred tax assets | $ | $ | ||||||||||||
The following table summarizes the amount and year of expiration of the Company's federal and state net operating loss carryforwards as of December 31, 2023:
| Years of expiration | Federal | State | ||||||||||||
| 2024 | $ | $ | ||||||||||||
| 2025-2042 | ||||||||||||||
| Indefinite Period | ||||||||||||||
| Total federal and state net operating loss carryforwards | $ | $ | ||||||||||||
F-30
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
Income tax expense includes the following components for the years ended December 31:
| 2023 | 2022 | |||||||||||||
| Current: | ||||||||||||||
| Federal | $ | $ | ||||||||||||
| State and other | ||||||||||||||
| Total current income tax expense | ||||||||||||||
| Deferred: | ||||||||||||||
| Federal | ||||||||||||||
| State | ||||||||||||||
| Total deferred income tax expense | ||||||||||||||
| Total income tax expense | $ | $ | ||||||||||||
The Company’s effective income tax rate for 2023 and 2022 reconciles with the federal statutory tax rate as follows:
| 2023 | 2022 | |||||||||||||
| Federal tax expense at statutory rate | $ | $ | ||||||||||||
| State income tax expense (net of federal income tax benefit) | ( | |||||||||||||
| Permanent differences associated with general business credits | ||||||||||||||
| Change in valuation allowance | ( | ( | ||||||||||||
| Other permanent differences | ( | ( | ||||||||||||
| Expiring tax credits | ( | |||||||||||||
| Deferred True-ups | ( | |||||||||||||
| Net loss tax expense | $ | ( | $ | ( | ||||||||||
The Company believes that it is not more likely than not that its net deferred tax assets will be realized. As such, the net deferred tax assets are fully offset with a valuation allowance as of the periods ended December 31, 2023 and 2022.
As of December 31, 2023, the Company has general business credit carryforwards of $1.3 million. These credit carryforwards will expire in years 2024 through 2044.
| Years of expiration | Federal | |||||||
| 2024 | $ | |||||||
| 2025-2044 | ||||||||
| Total federal and state credit carryforwards | $ | |||||||
The Company expects it will continue to pay minimal taxes in future periods through the continued utilization of net operating loss carryforwards, as it is able to achieve taxable income through its operations.
F-31
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
(13) Stock-Based Compensation Plans
The Company has grants outstanding under two equity compensation plans. The 2007 Long-Term Incentive Compensation Plan (the "2007 Plan") and the 2007 Directors’ Incentive Plan (the "Directors’ Plan"), which were approved by shareholders, superseded the 1999 Stock Option Plan. Both plans are available for future grants of equity compensation awards to employees, consultants and directors. The 2007 Plan and the Directors’ Plan provide for the issuance of stock options, stock appreciation rights and restricted stock. Vesting is determined on a grant-by-grant basis in accordance with the terms of the plans and the related grant agreements. The Company has reserved 3.2 million shares of common stock for issuance under the 2007 Plan and 250,000 shares for issuance under the Directors’ Plan. As of December 31, 2023, the Company had 933,517 shares available for future grants under the 2007 Plan and 130,507 shares available under the Directors’ Plan.
The exercise price of stock options is generally 100 % of the fair market value of the underlying common stock on the grant date, except for incentive stock options granted to 10 % shareholders, which the exercise price is no less than 110 % of the fair market value. The maximum contractual term of stock options is ten years from the grant date, except for incentive stock options granted to 10 % shareholders, which is no more than five years .
During 2011, the Company began issuing shares of restricted stock with no exercise price to employees and directors. Restricted stock issued to employees generally cliff-vests on the fourth anniversary of the grant date. Restricted stock issued to directors vests on the one year anniversary of the grant date.
Stock compensation expense is presented as a component of general and administrative expense in the consolidated statements of operations. Stock compensation expense was $0.4
At December 31, 2023, there was approximately $0.5 million of unrecognized compensation cost related to share-based payments, which is expected to be recognized over a weighted-average period of 1.85 years.
Stock Options
Stock options activity was as follows:
Number of option shares | Weighted- average exercise price | Weighted-average remaining contractual term | ||||||||||||||||||
Outstanding, December 31, 2021 | ||||||||||||||||||||
| Granted | ||||||||||||||||||||
| Exercised | ||||||||||||||||||||
| Forfeited/canceled | ( | |||||||||||||||||||
Outstanding, December 31, 2022 | $ | |||||||||||||||||||
| Granted | ||||||||||||||||||||
| Exercised | $ | |||||||||||||||||||
| Forfeited/canceled | ( | $ | ||||||||||||||||||
Outstanding, December 31, 2023 | $ | |||||||||||||||||||
The weighted-average grant-date fair value of options granted during the years 2023 and 2022 was 1.16 and 1.29 , respectively. No
F-32
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
The fair value of stock options is calculated using the Black-Scholes (“Black-Scholes-Merton”, or “BSM”) option-pricing model on the grant date. Since 2012, the Company had been issuing Restricted Share Awards where the grant date Fair Value (“FV”) equaled the closing share price. The assumptions used in the Black-Scholes valuation to determine the FV of stock options were as follows at December 31:
| 2023 | 2022 | ||||||||||
| Expected term (in years) | |||||||||||
| Expected volatility | % | % | |||||||||
| Risk free interest rate | % | % | |||||||||
| Expected dividend yield | |||||||||||
•Expected Term - The Company has estimated the expected life of its stock options using the simplified method, whereby the expected life equals the average of the vesting term and the original contractual term of the option.
•Expected volatility - Based on the Company’s historical stock price volatility.
•Risk Free rate - The Company bases the risk-free interest rate assumption for equity awards on the rates for U.S. Treasury zero-coupon bonds with maturities similar to those of the expected term of the award being valued.
•Expected dividend yield - The Company’s expected dividend yield assumption is zero as it has never paid dividends and has no present intention to do so in the future.
Restricted Stock Awards
Restricted stock activity was as follows:
Number of shares | Weighted-average grant-date fair value | ||||||||||
Nonvested, December 31, 2021 | |||||||||||
| Granted | |||||||||||
| Vested/released | ( | ||||||||||
| Forfeited/canceled | ( | ||||||||||
Nonvested, December 31, 2022 | $ | ||||||||||
| Granted | |||||||||||
| Vested/released | ( | ||||||||||
| Forfeited/canceled | ( | ||||||||||
Nonvested, December 31, 2023 | $ | ||||||||||
The fair value of restricted stock granted was based on the closing market price of the Company’s common stock on the grant date. The fair value of restricted stock awards that vested during the years 2023 and 2022 was $0.3 million and $0.5 million, respectively. At December 31, 2023, there was approximately $0.2 million of unrecognized compensation costs related to restricted stock awards, which is expected to be recognized over a weighted-average period of less than a year. The restricted stock grants are included in the diluted weighted shares outstanding computation until they cliff-vest. Once vested they are included in the basic weighted shares outstanding computation.
F-33
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
(14) Employee Benefit Plans
The Company sponsors an employee benefit plan that was established on January 1, 2006, the Cumberland Pharmaceuticals 401(k) Plan (the "Plan"), under Section 401(k) of the Internal Revenue Code of 1986, as amended, for the benefit of all employees over the age of 21 , having been employed by the Company for at least six months . The Plan provides that participants may contribute up to the maximum amount of their compensation as set forth by the Internal Revenue Service each year. Employee contributions are invested in various investment funds based upon elections made by the employees. During 2023 and 2022, the Company contributed approximately $0.08 million and $0.06 million, respectively, in each year to the Plan as an employer match of participant contributions.
In 2012 and 2013, the Company established non-qualified unfunded deferred compensation plans that allow participants to defer receipt of a portion of their compensation. The liability under the plans, reflected in other long term liabilities in the consolidated balance sheets, was $2.9 million and $2.5 million as of December 31, 2023 and 2022, respectively. The Company had assets consisting of company-owned life insurance contracts generally designated to pay benefits of the deferred compensation plans reflected in other assets in the consolidated balance sheet of $2.4 million and $2.3 million as of December 31, 2023 and 2022, respectively.
(15) Leases
On November 15, 2021, Cumberland entered into a lease, pursuant to which the Company leases approximately 16,903 rentable square feet of space at the new development Broadwest located in Nashville, Tennessee with 1600 West End Avenue Partners, LLC. The leased premise serves as the Company's new corporate headquarters. The initial term of the lease is one hundred fifty-seven (157 ) months, with two consecutive options to renew for a period of five years each, with the commencement date of October 25, 2022. This lease currently expires in November 2035.
The Company is responsible for paying rent to the Landlord under the lease beginning three months after the commencement date. The Company pays a base rent of $33.06 per square foot of rentable space with a gradual rental rate increase of 2.5 % for each year period thereafter of the prior year's base rental. In addition to the monthly base rent, the Company is responsible for its percentage share of the operating expenses of the building. The lease also provides for a tenant improvement allowance for the space.
On October 24, 2022, the CET lease with The Gateway to Nashville, LLC provided the notice of exercise to extend the lease for five years . The lease is for approximately 14,200 square feet of wet laboratory and office space in Nashville, Tennessee where CET operates the CET Life Sciences Center.The wet laboratory and office space is leased through April 2028. The Company also subleases a portion of the space under this lease.
Also included within the right-of-use assets are start up expenditures related to a new supply agreement with Nephron Pharmaceuticals Corporation (“Nephron”). These expenditures are classified as an embedded lease resulting in a right-of-use asset to be amortized over the life of the Nephron contract. As of December 31, 2023, the value of this asset was $1.0 million.
Rent expense is recognized over the expected term of the lease on a straight-line basis as a component of general and administrative expense. Rent expense and sublease income as follows for the years ended December 31:
| 2023 | 2022 | |||||||||||||
| Rent expense | $ | $ | ||||||||||||
| Sublease income | $ | $ | ||||||||||||
In March 2016, the FASB issued ASU 2016-02. ASU 2016-02’s core principle is to increase transparency and comparability among organizations by recognizing lease assets and liabilities on the balance sheet and disclosing key information. The primary effect of adopting ASU 2016-02 to the Company was to record right-of-use assets and obligations for the leases currently classified as operating leases.
F-34
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
Operating lease liabilities were recorded as the present value of remaining lease payments not yet paid for the lease term discounted using the incremental borrowing rate associated with each lease. Operating lease right-of-use assets represent operating lease liabilities adjusted for lease incentives and initial direct costs. As the Company’s leases do not contain implicit borrowing rates, the incremental borrowing rates were calculated based on information available at October 25, 2022 and May 1, 2023. Incremental borrowing rates reflect the Company’s estimated interest rates for collateralized borrowings over similar lease terms.
The weighted-average remaining lease term for the Broadwest and Gateway leases is 10.42 years and 12.69 years for the years ended December 31, 2023 and 2022, respectively.. The weighted-average incremental borrowing rate used to discount the present value of the remaining lease payments of both leases is 9.40 % and 9.24 % for the years ended December 31, 2023 and 2023, respectively.
Lease Position
At December 31, 2023 and 2022, the Company recorded the following on the Consolidated Balance Sheet:
| Right-of-Use Assets | December 31, 2023 | December 31, 2022 | ||||||||||||
| Operating lease right-of-use assets | $ | $ | ||||||||||||
| Lease Liabilities | December 31, 2023 | December 31, 2022 | ||||||||||||
| Operating lease current liabilities | $ | $ | ||||||||||||
| Operating lease non-current liabilities | ||||||||||||||
| Total | $ | $ | ||||||||||||
Cumulative future minimum sublease income under non-cancelable operating subleases totals approximately $0.3 million and will be paid through the leases ending in April 2024. Future minimum lease payments under non-cancelable operating leases (with initial or remaining lease terms in excess of one year) are as follows:
Maturity of Leases Liabilities at December 31, 2023 | Operating Leases | |||||||
| 2024 | $ | |||||||
| 2025 | ||||||||
| 2026 | ||||||||
| 2027 | ||||||||
| 2028 | ||||||||
| After 2028 | ||||||||
| Total minimum lease payments | ||||||||
| Less: Interest | ( | |||||||
| Present value of lease liabilities | $ | |||||||
F-35
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
(16) Market Concentrations
The Company’s principal financial instruments subject to potential concentration of credit risk are accounts receivable, which are unsecured, and cash equivalents. The Company’s cash equivalents consist primarily of money market funds. Certain bank deposits may be in excess of the insurance limits provided by the Federal Deposit Insurance Corporation.
The Company’s primary customers are wholesale pharmaceutical distributors in the U.S. Total revenues by customer for each customer representing 10% or more of consolidated revenues are summarized below for the years ended December 31:
| 2023 | 2022 | |||||||||||||
| Customer 1 | ||||||||||||||
| Customer 2 | ||||||||||||||
| Customer 3 | ||||||||||||||
The Company’s accounts receivable, net of allowances, due from the customers representing 10% or more of consolidated revenue was 62.3 % and 51.8 % at December 31, 2023 and 2022, respectively.
(17) Manufacturing and Supply Agreements
The Company utilizes one or two primary suppliers to manufacture each of its products and product candidates. Although there are a limited number of manufacturers of pharmaceutical products, the Company believes it could utilize other suppliers to manufacture its prescription products on comparable terms. A change in suppliers, problems with its third-party manufacturing operations or related production capacity, or contract disputes with suppliers could cause a delay in manufacturing or shipment of finished goods and possible loss of sales, which could adversely affect operating results.
(18) Employment Agreements
The Company has entered into employment agreements with all its full-time employees. Each employment agreement provides for a salary for services performed, a potential annual bonus and, if applicable, a grant of restricted common shares pursuant to a restricted stock and incentive stock option agreement.
(19) Commitments and Contingencies
Commitments
In connection with its licensing agreements for Caldolor, the Company is required to pay royalties based on net sales over the life of the product. Royalty expense is recognized as a component of selling and marketing expense in the period that revenue is recognized.
In connection with the acquisition of Vibativ, the Company is required to pay royalties based on net sales of the product. At the purchase date, Cumberland recorded the fair value of this liability and will continue to evaluate the liability each period and the royalty expense is recognized as a component of selling and marketing expense in the period that the change in fair value is recognized.
In connection with the acquisition of Sancuso, the Company is required to pay up to $3.5 million in milestones and tiered royalties ranging from 10 % to 5 % on U.S. net product sales for ten years .
F-36
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
Legal Matters
Cumberland has a number of patents issued through the United States Patent and Trademark Office (the “USPTO”) including U.S. Patent number 8,148,356 (the “356 Acetadote Patent”) which is assigned to the Company. The claims of the 356 Acetadote Patent encompass the new Acetadote formulation and include composition of matter claims. Following its issuance, the 356 Acetadote Patent was listed in the FDA Orange Book. The 356 Acetadote Patent is scheduled to expire in May 2026, which time period includes a 270-day patent term adjustment granted by the USPTO.
Since 2012, Cumberland has continued to vigorously defend and protect its Acetadote product and related intellectual property rights including the use of all its legal options.
Melinta Litigation
On February 2, 2022, the Company filed an action for breach of contract against Melinta Therapeutics, LLC and Targanta Therapeutics Corporation (collectively, the “Defendants”) in the United States District Court for the Southern District of New York (Case No. 1:22-cv-00915-VM). The Company and the Defendants are parties to an agreement (the “Agreement”), pursuant to which the Defendants have a license to develop and commercialize products under certain Company patents, in exchange for the Defendants paying the Company certain milestone payments and royalties on net sales of the licensed products.
Specifically, the Agreement requires the Defendants to, among other things, make a $500,000 payment to the Company within 30 days following the first filing of an sNDA in relation to the Product (as defined by the Agreement) and a $500,000 payment to the Company following the approval of the first sNDA in relation to the Product.
After Defendants disclosed the domiciles of its limited partners to the Company, as required by the Court, on October 24, 2022, the action for breach of contract was refiled in the Supreme Court of the State of New York, County of New York (Index No. 654234/2022) on November 7, 2022.
The complaint alleges that, despite the Defendants filing an NDA and sNDA for the Product and receiving FDA approval for both applications, the Defendants failed to make the required total of $1 million in milestone payments to the Company. The Company was seeking damages in the amount of no less than $1 million, pre- and post-judgment interest under N.Y. C.P.L.R. § 5001, costs, and such further relief as the court deems just and proper.
On April 18, 2023 the state court ruled in favor of the Company. On June 16, 2023, the Company received consideration related to the breach of contract action with the Defendants that finalized a settlement agreement that was entered into by the Company and the Defendants to close the case.
F-37
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements (Continued)
(20) Quarterly Financial Information (Unaudited)
The following table sets forth the unaudited operating results for each fiscal quarter of 2023 and 2022:
First Quarter | Second Quarter | Third Quarter | Fourth Quarter | Total | ||||||||||||||||||||||||||||
| 2023: | ||||||||||||||||||||||||||||||||
| Net revenues | $ | $ | $ | $ | $ | |||||||||||||||||||||||||||
| Operating income (loss) | ( | ( | ( | ( | ||||||||||||||||||||||||||||
| Net income (loss) | ( | ( | ( | |||||||||||||||||||||||||||||
| Net income (loss) attributable to common shareholders | ( | ( | ( | |||||||||||||||||||||||||||||
Earnings (loss) per share attributable to common shareholders (1) | ||||||||||||||||||||||||||||||||
| Basic | $ | $ | $ | ( | $ | ( | $ | ( | ||||||||||||||||||||||||
| Diluted | $ | $ | $ | ( | $ | ( | $ | ( | ||||||||||||||||||||||||
| 2022: | ||||||||||||||||||||||||||||||||
| Net revenues | $ | $ | $ | $ | $ | |||||||||||||||||||||||||||
| Operating loss | ( | ( | ( | ( | ( | |||||||||||||||||||||||||||
| Net loss | ( | ( | ( | ( | ( | |||||||||||||||||||||||||||
| Net loss attributable to common shareholders | ( | ( | ( | ( | ( | |||||||||||||||||||||||||||
Loss per share attributable to common shareholders (1) | ||||||||||||||||||||||||||||||||
| Continuing operations - basic | $ | ( | $ | ( | $ | ( | $ | ( | $ | ( | ||||||||||||||||||||||
| Continuing operations - diluted | $ | ( | $ | ( | $ | ( | $ | ( | $ | ( | ||||||||||||||||||||||
(1) Due to the nature of interim earnings per share calculations, the sum of the quarterly earnings (loss) per share amounts may not equal the reported earnings (loss) per share for the full year.
F-38
Schedule II
CUMBERLAND PHARMACEUTICALS INC. AND SUBSIDIARIES
Valuation and Qualifying Accounts
Years ended December 31, 2023 and 2022.
| Description | Balance at beginning of period | Charged to costs and expenses | Charged to other accounts | Deductions | Balance at end of period | |||||||||||||||||||||||||||
| Allowance for uncollectible amounts, cash discounts, chargebacks, and credits issued for damaged products: | ||||||||||||||||||||||||||||||||
For the years ended December 31: | ||||||||||||||||||||||||||||||||
| 2022 | ( | |||||||||||||||||||||||||||||||
| 2023 | ( | (1) | ||||||||||||||||||||||||||||||
| Valuation allowance for deferred tax assets: | ||||||||||||||||||||||||||||||||
For the years ended December 31: | ||||||||||||||||||||||||||||||||
| 2022 | ||||||||||||||||||||||||||||||||
| 2023 | ||||||||||||||||||||||||||||||||
(1) Composed of actual returns and credits for chargebacks and cash discounts.
F-39