UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of
the Securities Exchange Act of 1934 (Amendment No. )
|
Filed by the Registrant x | |||
|
| |||
|
Filed by a Party other than the Registrant o | |||
|
| |||
|
Check the appropriate box: | |||
|
o |
Preliminary Proxy Statement | ||
|
o |
Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) | ||
|
o |
Definitive Proxy Statement | ||
|
o |
Definitive Additional Materials | ||
|
x |
Soliciting Material under §240.14a-12 | ||
|
| |||
|
INNOVIVA, INC. | |||
|
(Name of Registrant as Specified In Its Charter) | |||
|
| |||
|
| |||
|
(Name of Person(s) Filing Proxy Statement, if other than the Registrant) | |||
|
| |||
|
Payment of Filing Fee (Check the appropriate box): | |||
|
x |
No fee required. | ||
|
o |
Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. | ||
|
|
(1) |
Title of each class of securities to which transaction applies: | |
|
|
|
| |
|
|
(2) |
Aggregate number of securities to which transaction applies: | |
|
|
|
| |
|
|
(3) |
Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): | |
|
|
|
| |
|
|
(4) |
Proposed maximum aggregate value of transaction: | |
|
|
|
| |
|
|
(5) |
Total fee paid: | |
|
|
|
| |
|
o |
Fee paid previously with preliminary materials. | ||
|
o |
Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. | ||
|
|
(1) |
Amount Previously Paid: | |
|
|
|
| |
|
|
(2) |
Form, Schedule or Registration Statement No.: | |
|
|
|
| |
|
|
(3) |
Filing Party: | |
|
|
|
| |
|
|
(4) |
Date Filed: | |
|
|
|
| |
Corporate Presentation March 2017

This presentation contains certain “forward-looking” statements as that term is defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, statements relating to goals, plans, objectives and future events. Innoviva intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. The words “anticipate”, “expect”, “goal”, “intend”, “objective”, “opportunity”, “plan”, “potential”, “target” and similar expressions are intended to identify such forward-looking statements. Such forward-looking statements involve substantial risks, uncertainties and assumptions. These statements are based on the current estimates and assumptions of the management of Innoviva as of the date of this presentation and are subject to risks, uncertainties, changes in circumstances, assumptions and other factors that may cause the actual results of Innoviva to be materially different from those reflected in the forward-looking statements. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include, among others, risks related to: lower than expected future royalty revenue from respiratory products partnered with GSK, the commercialization of RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA® in the jurisdictions in which these products have been approved; the strategies, plans and objectives of Innoviva (including Innoviva’s growth strategy and corporate development initiatives beyond the existing respiratory portfolio); the timing, manner, amount and planned growth of anticipated potential capital returns to stockholders (including, without limitation, statements regarding Innoviva’s expectations of future purchases under its capital return programs and future cash dividends); the status and timing of clinical studies, data analysis and communication of results; the potential benefits and mechanisms of action of product candidates; expectations for product candidates through development and commercialization; the timing of regulatory approval of product candidates; and projections of revenue, expenses and other financial items. Other risks affecting Innoviva are described under the headings “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” contained in Innoviva’s Annual Report on Form 10-K for the year ended December 31, 2016, which is on file with the Securities and Exchange Commission (SEC) and available on the SEC’s website at www.sec.gov. In addition to the risks described above and in Innoviva’s other filings with the SEC, other unknown or unpredictable factors also could affect Innoviva’s results. Past performance is not necessarily indicative of future results. No forward-looking statements can be guaranteed and actual results may differ materially from such statements. Given these uncertainties, you should not place undue reliance on these forward-looking statements. The information in this presentation is provided only as of March 6, 2017, and Innoviva assumes no obligation to update its forward-looking statements on account of new information, future events or otherwise, except as required by law. Forward-Looking Statements 2 ©2017 INNOVIVA Use of Non-GAAP Financial Measures In certain circumstances, results have been presented that are not generally accepted accounting principles measures (“Non-GAAP”) and should be viewed in addition to, and not as a substitute for, Innoviva’s reported results. Innoviva believes that the non-GAAP financial information provided in this presentation can assist investors in understanding and assessing Innoviva's on-going operations and prospects for the future and provides an additional tool for investors to use in comparing Innoviva's financial results with other companies in Innoviva's industry or with similar operating profiles. Investors are encouraged to review the reconciliation of Innoviva's non-GAAP financial measures to their most directly comparable GAAP financial measures. Please see the Appendix provided at the end of this presentation entitled “Reconciliation of Non-GAAP Financial Measures to GAAP” for additional information and the reconciliations of these non-GAAP financial measures to the closest GAAP financial measures.
Company Mission & Investment Highlights 3 ©2017 INNOVIVA * Non-GAAP Financial Measure, please refer to Appendix for reconciliation to GAAP Measures Platform for growth anchored by a major product portfolio Existing portfolio partnered with GSK addresses a $20+ billion market in respiratory treatments Innovative Asthma/COPD medicines with differentiated features and therapeutic profiles 32% quarterly CGR in royalty revenues in last 10 quarters Long duration royalty portfolio Strong patent estate Royalty Term - Greater of 15 years from launch or last valid patent Royalties extend into late 2020s Capital return commitment to investors Returned more than $210 million to investors since Q1 2015 Flexible approach through stock/debt repurchases & repayments Solid cash generating capacity & strong balance sheet Q4 Global Net Sales: RELVAR®/ BREO® ELLIPTA® $273 million; ANORO® ELLIPTA® $91 million $44 million Q4 Adjusted EBITDA* $0.26 adjusted earnings per share* in Q4 2016 1.1 x effective Net Debt / LTM Adjusted EBITDA *

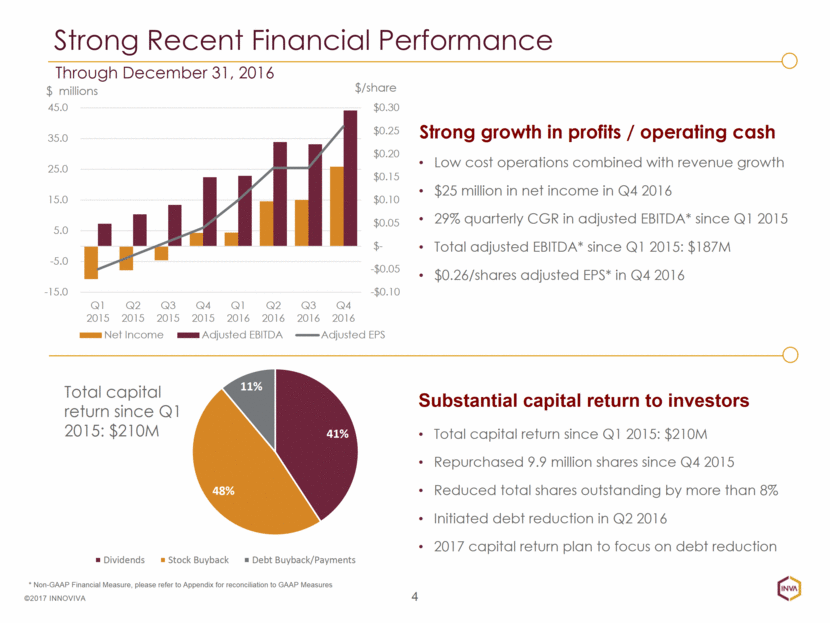
Total capital return since Q1 2015: $210M Repurchased 9.9 million shares since Q4 2015 Reduced total shares outstanding by more than 8% Initiated debt reduction in Q2 2016 2017 capital return plan to focus on debt reduction 4 ©2017 INNOVIVA Strong growth in profits / operating cash Substantial capital return to investors Low cost operations combined with revenue growth $25 million in net income in Q4 2016 29% quarterly CGR in adjusted EBITDA* since Q1 2015 Total adjusted EBITDA* since Q1 2015: $187M $0.26/shares adjusted EPS* in Q4 2016 Strong Recent Financial Performance Through December 31, 2016 * Non-GAAP Financial Measure, please refer to Appendix for reconciliation to GAAP Measures Total capital return since Q1 2015: $210M -$0.10 -$0.05 $- $0.05 $0.10 $0.15 $0.20 $0.25 $0.30 -15.0 -5.0 5.0 15.0 25.0 35.0 45.0 Q1 2015 Q2 2015 Q3 2015 Q4 2015 Q1 2016 Q2 2016 Q3 2016 Q4 2016 $/ share $ millions Net Income Adjusted EBITDA Adjusted EPS 41% 48% 11% Dividends Stock Buyback Debt Buyback/Payments
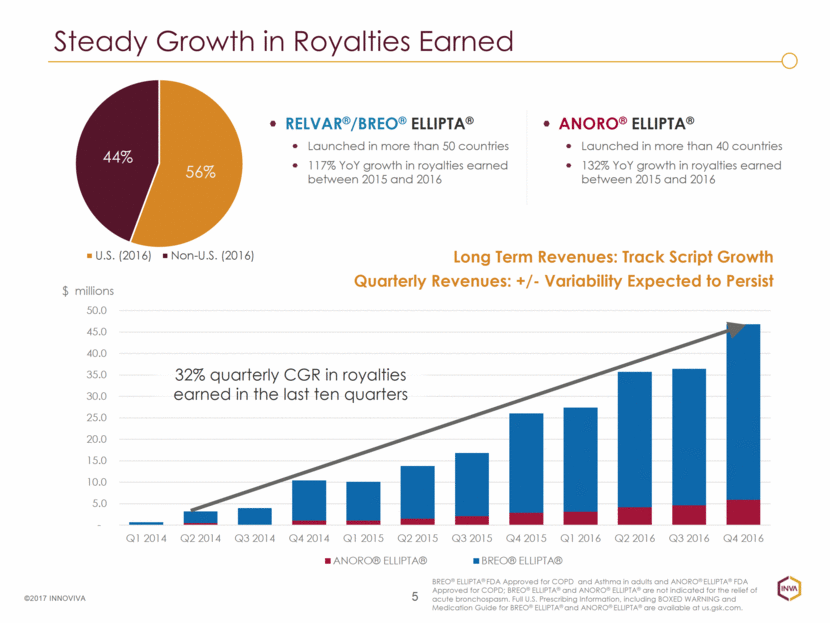
Steady Growth in Royalties Earned RELVAR®/BREO® ELLIPTA® Launched in more than 50 countries 117% YoY growth in royalties earned between 2015 and 2016 5 ©2017 INNOVIVA ANORO® ELLIPTA® Launched in more than 40 countries 132% YoY growth in royalties earned between 2015 and 2016 Long Term Revenues: Track Script Growth Quarterly Revenues: +/- Variability Expected to Persist BREO® ELLIPTA® FDA Approved for COPD and Asthma in adults and ANORO® ELLIPTA® FDA Approved for COPD; BREO® ELLIPTA® and ANORO® ELLIPTA® are not indicated for the relief of acute bronchospasm. Full U.S. Prescribing Information, including BOXED WARNING and Medication Guide for BREO® ELLIPTA® and ANORO® ELLIPTA® are available at us.gsk.com. 32% quarterly CGR in royalties earned in the last ten quarters - 5.0 10.0 15.0 20.0 25.0 30.0 35.0 40.0 45.0 50.0 Q1 2014 Q2 2014 Q3 2014 Q4 2014 Q1 2015 Q2 2015 Q3 2015 Q4 2015 Q1 2016 Q2 2016 Q3 2016 Q4 2016 $ millions ANORO® ELLIPTA® BREO® ELLIPTA® 56% 44% U.S. (2016) Non-U.S. (2016)
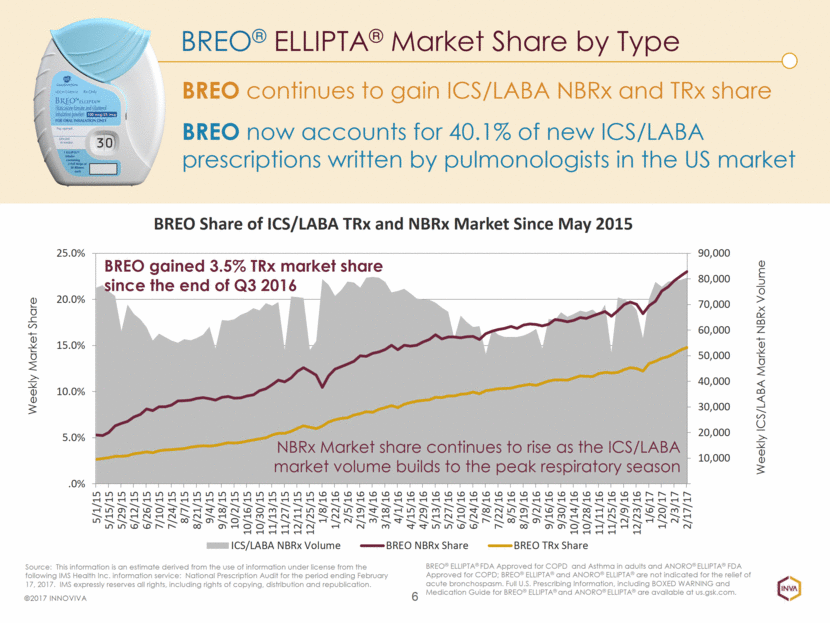
BREO® ELLIPTA® Market Share by Type 6 ©2017 INNOVIVA BREO continues to gain ICS/LABA NBRx and TRx share BREO® ELLIPTA® FDA Approved for COPD and Asthma in adults and ANORO® ELLIPTA® FDA Approved for COPD; BREO® ELLIPTA® and ANORO® ELLIPTA® are not indicated for the relief of acute bronchospasm. Full U.S. Prescribing Information, including BOXED WARNING and Medication Guide for BREO® ELLIPTA® and ANORO® ELLIPTA® are available at us.gsk.com. Source: This information is an estimate derived from the use of information under license from the following IMS Health Inc. information service: National Prescription Audit for the period ending February 17, 2017. IMS expressly reserves all rights, including rights of copying, distribution and republication. Weekly Market Share Weekly ICS/LABA Market NBRx Volume BREO now accounts for 40.1% of new ICS/LABA prescriptions written by pulmonologists in the US market NBRx Market share continues to rise as the ICS/LABA market volume builds to the peak respiratory season BREO gained 3.5% TRx market share since the end of Q3 2016 10,000 20,000 30,000 40,000 50,000 60,000 70,000 80,000 90,000 .0% 5.0% 10.0% 15.0% 20.0% 25.0% 5/1/15 5/15/15 5/29/15 6/12/15 6/26/15 7/10/15 7/24/15 8/7/15 8/21/15 9/4/15 9/18/15 10/2/15 10/16/15 10/30/15 11/13/15 11/27/15 12/11/15 12/25/15 1/8/16 1/22/16 2/5/16 2/19/16 3/4/16 3/18/16 4/1/16 4/15/16 4/29/16 5/13/16 5/27/16 6/10/16 6/24/16 7/8/16 7/22/16 8/5/16 8/19/16 9/2/16 9/16/16 9/30/16 10/14/16 10/28/16 11/11/16 11/25/16 12/9/16 12/23/16 1/6/17 1/20/17 2/3/17 2/17/17 BREO Share of ICS/LABA TRx and NBRx Market Since May 2015 ICS/LABA NBRx Volume BREO NBRx Share BREO TRx Share
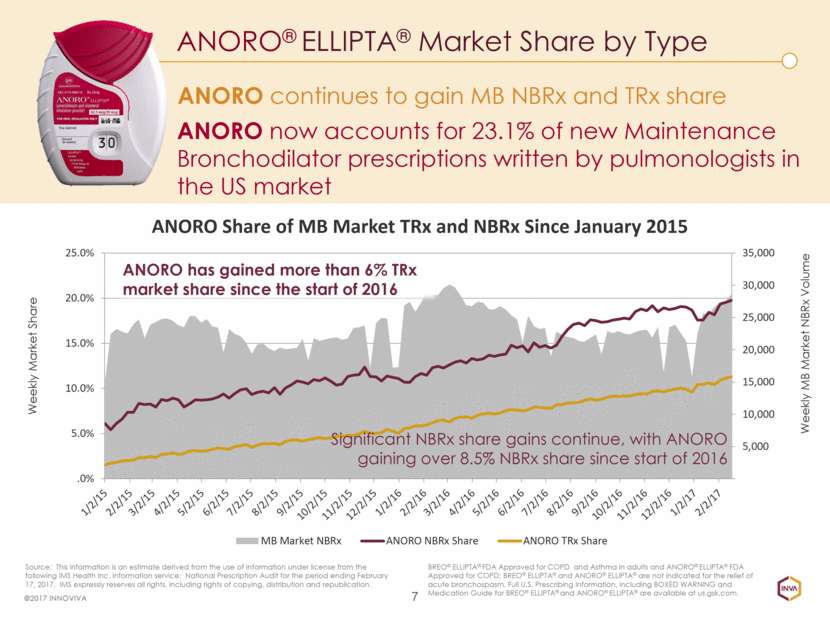
ANORO continues to gain MB NBRx and TRx share ANORO® ELLIPTA® Market Share by Type 7 ©2017 INNOVIVA BREO® ELLIPTA® FDA Approved for COPD and Asthma in adults and ANORO® ELLIPTA® FDA Approved for COPD; BREO® ELLIPTA® and ANORO® ELLIPTA® are not indicated for the relief of acute bronchospasm. Full U.S. Prescribing Information, including BOXED WARNING and Medication Guide for BREO® ELLIPTA® and ANORO® ELLIPTA® are available at us.gsk.com. Source: This information is an estimate derived from the use of information under license from the following IMS Health Inc. information service: National Prescription Audit for the period ending February 17, 2017. IMS expressly reserves all rights, including rights of copying, distribution and republication. Weekly Market Share Weekly MB Market NBRx Volume ANORO now accounts for 23.1% of new Maintenance Bronchodilator prescriptions written by pulmonologists in the US market Significant NBRx share gains continue, with ANORO gaining over 8.5% NBRx share since start of 2016 ANORO has gained more than 6% TRx market share since the start of 2016 5,000 10,000 15,000 20,000 25,000 30,000 35,000 .0% 5.0% 10.0% 15.0% 20.0% 25.0% ANORO Share of MB Market TRx and NBRx Since January 2015 MB Market NBRx ANORO NBRx Share ANORO TRx Share
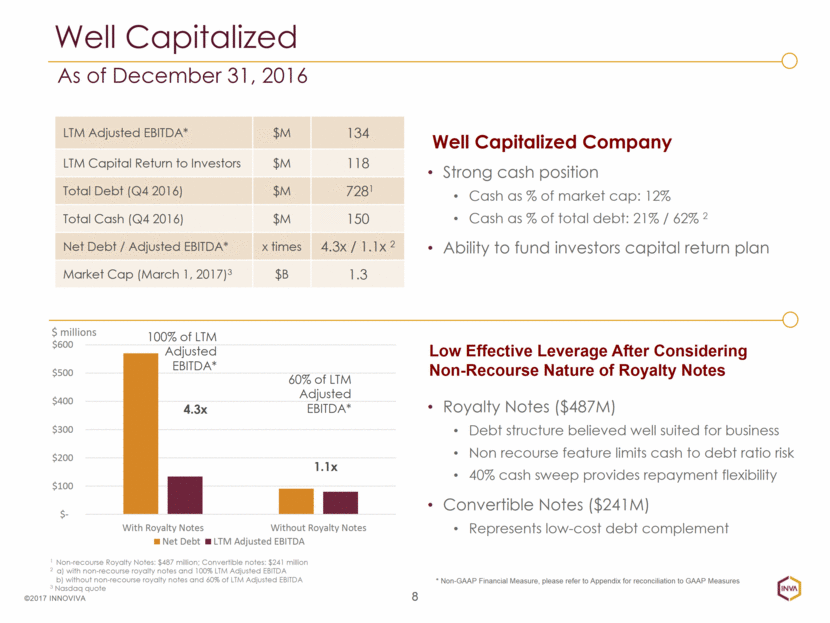
8 ©2017 INNOVIVA LTM Adjusted EBITDA* $M 134 LTM Capital Return to Investors $M 118 Total Debt (Q4 2016) $M 7281 Total Cash (Q4 2016) $M 150 Net Debt / Adjusted EBITDA* x times 4.3x / 1.1x 2 Market Cap (March 1, 2017)3 $B 1.3 Well Capitalized Company Low Effective Leverage After Considering Non-Recourse Nature of Royalty Notes Strong cash position Cash as % of market cap: 12% Cash as % of total debt: 21% / 62% 2 Ability to fund investors capital return plan 100% of LTM Adjusted EBITDA* 4.3x 60% of LTM Adjusted EBITDA* 1.1x Well Capitalized As of December 31, 2016 Royalty Notes ($487M) Debt structure believed well suited for business Non recourse feature limits cash to debt ratio risk 40% cash sweep provides repayment flexibility Convertible Notes ($241M) Represents low-cost debt complement 1 Non-recourse Royalty Notes: $487 million; Convertible notes: $241 million 2 a) with non-recourse royalty notes and 100% LTM Adjusted EBITDA b) without non-recourse royalty notes and 60% of LTM Adjusted EBITDA 3 Nasdaq quote * Non-GAAP Financial Measure, please refer to Appendix for reconciliation to GAAP Measures $- $100 $200 $300 $400 $500 $600 With Royalty Notes Without Royalty Notes $ millions Net Debt LTM Adjusted EBITDA
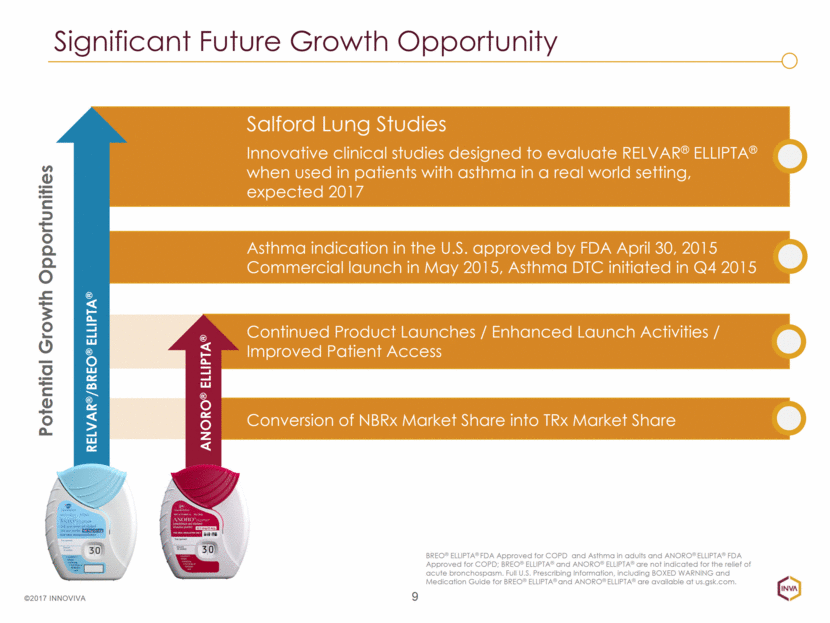
Significant Future Growth Opportunity 9 ©2017 INNOVIVA Salford Lung Studies Innovative clinical studies designed to evaluate RELVAR® ELLIPTA® when used in patients with asthma in a real world setting, expected 2017 Asthma indication in the U.S. approved by FDA April 30, 2015 Commercial launch in May 2015, Asthma DTC initiated in Q4 2015 Conversion of NBRx Market Share into TRx Market Share Continued Product Launches / Enhanced Launch Activities / Improved Patient Access Potential Growth Opportunities RELVAR®/BREO® ELLIPTA® ANORO® ELLIPTA® BREO® ELLIPTA® FDA Approved for COPD and Asthma in adults and ANORO® ELLIPTA® FDA Approved for COPD; BREO® ELLIPTA® and ANORO® ELLIPTA® are not indicated for the relief of acute bronchospasm. Full U.S. Prescribing Information, including BOXED WARNING and Medication Guide for BREO® ELLIPTA® and ANORO® ELLIPTA® are available at us.gsk.com.
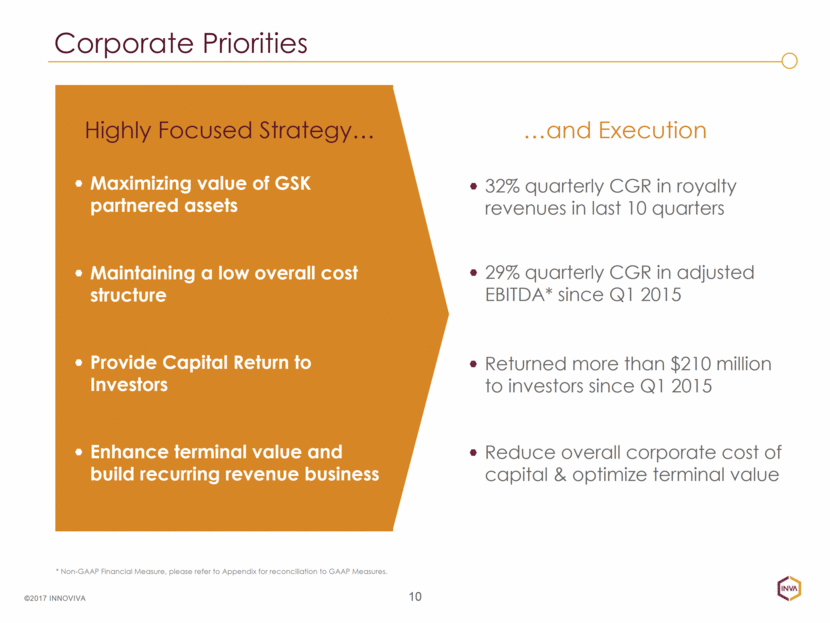
Corporate Priorities 10 Highly Focused Strategy Maximizing value of GSK partnered assets Maintaining a low overall cost structure Provide Capital Return to Investors Enhance terminal value and build recurring revenue business 32% quarterly CGR in royalty revenues in last 10 quarters * Non-GAAP Financial Measure, please refer to Appendix for reconciliation to GAAP Measures. ©2017 INNOVIVA and Execution 29% quarterly CGR in adjusted EBITDA* since Q1 2015 Returned more than $210 million to investors since Q1 2015 Reduce overall corporate cost of capital & optimize terminal value
Appendix ©2017 INNOVIVA

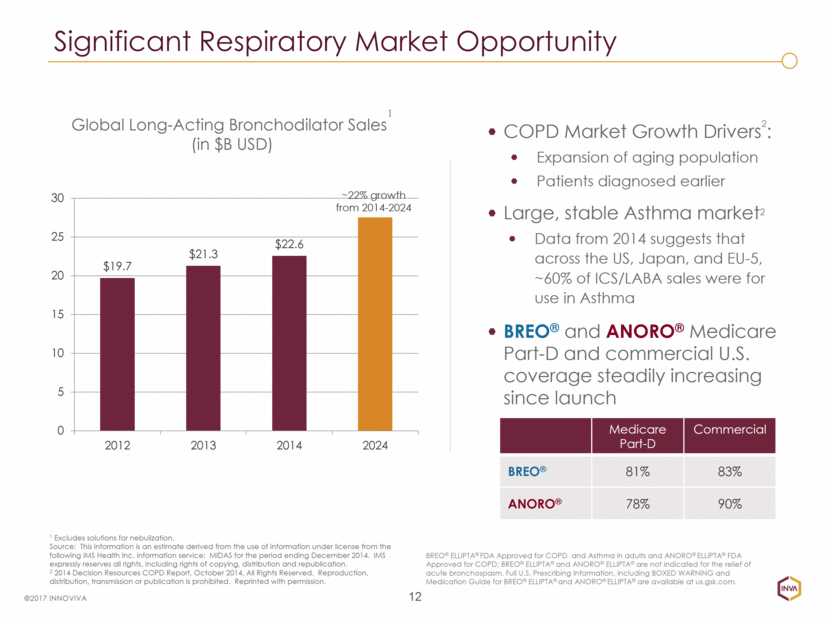
12 COPD Market Growth Drivers2: Expansion of aging population Patients diagnosed earlier Large, stable Asthma market2 Data from 2014 suggests that across the US, Japan, and EU-5, ~60% of ICS/LABA sales were for use in Asthma BREO® and ANORO® Medicare Part-D and commercial U.S. coverage steadily increasing since launch 1 Excludes solutions for nebulization. Source: This information is an estimate derived from the use of information under license from the following IMS Health Inc. information service: MIDAS for the period ending December 2014. IMS expressly reserves all rights, including rights of copying, distribution and republication. 2 2014 Decision Resources COPD Report, October 2014, All Rights Reserved. Reproduction, distribution, transmission or publication is prohibited. Reprinted with permission. Significant Respiratory Market Opportunity ©2017 INNOVIVA BREO® ELLIPTA® FDA Approved for COPD and Asthma in adults and ANORO® ELLIPTA® FDA Approved for COPD; BREO® ELLIPTA® and ANORO® ELLIPTA® are not indicated for the relief of acute bronchospasm. Full U.S. Prescribing Information, including BOXED WARNING and Medication Guide for BREO® ELLIPTA® and ANORO® ELLIPTA® are available at us.gsk.com. ~22% growth from 2014-2024 Global Long-Acting Bronchodilator Sales1 (in $B USD) Medicare Part-D Commercial BREO® 81% 83% ANORO® 78% 90% $19.7 $21.3 $22.6 0 5 10 15 20 25 30 2012 2013 2014 2024
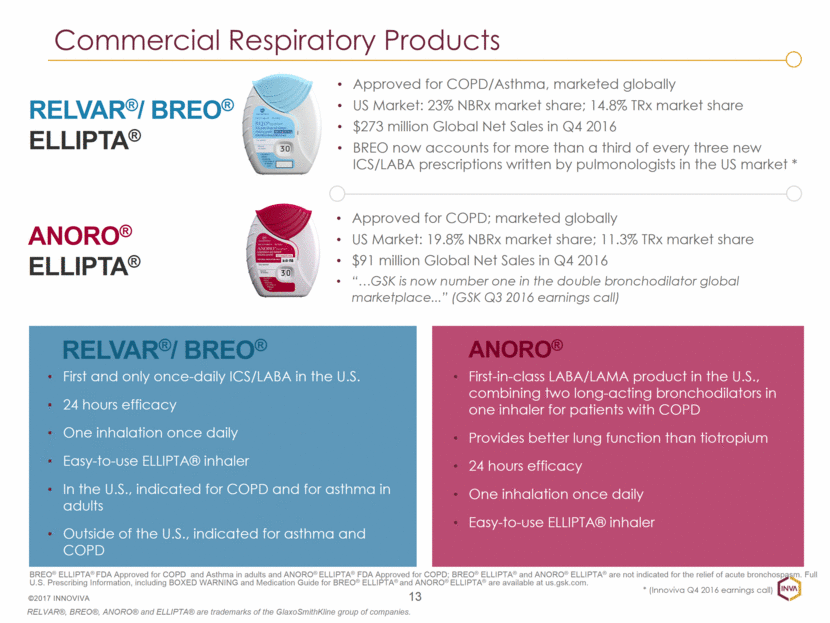
ANORO® RELVAR®/ BREO® First and only once-daily ICS/LABA in the U.S. 24 hours efficacy One inhalation once daily Easy-to-use ELLIPTA® inhaler In the U.S., indicated for COPD and for asthma in adults Outside of the U.S., indicated for asthma and COPD 13 ©2017 INNOVIVA ANORO® ELLIPTA® RELVAR®/ BREO® ELLIPTA® First-in-class LABA/LAMA product in the U.S., combining two long-acting bronchodilators in one inhaler for patients with COPD Provides better lung function than tiotropium 24 hours efficacy One inhalation once daily Easy-to-use ELLIPTA® inhaler BREO® ELLIPTA® FDA Approved for COPD and Asthma in adults and ANORO® ELLIPTA® FDA Approved for COPD; BREO® ELLIPTA® and ANORO® ELLIPTA® are not indicated for the relief of acute bronchospasm. Full U.S. Prescribing Information, including BOXED WARNING and Medication Guide for BREO® ELLIPTA® and ANORO® ELLIPTA® are available at us.gsk.com. RELVAR®, BREO®, ANORO® and ELLIPTA® are trademarks of the GlaxoSmithKline group of companies. Commercial Respiratory Products Approved for COPD/Asthma, marketed globally US Market: 23% NBRx market share; 14.8% TRx market share $273 million Global Net Sales in Q4 2016 BREO now accounts for more than a third of every three new ICS/LABA prescriptions written by pulmonologists in the US market * Approved for COPD; marketed globally US Market: 19.8% NBRx market share; 11.3% TRx market share $91 million Global Net Sales in Q4 2016 “ GSK is now number one in the double bronchodilator global marketplace...” (GSK Q3 2016 earnings call) * (Innoviva Q4 2016 earnings call)
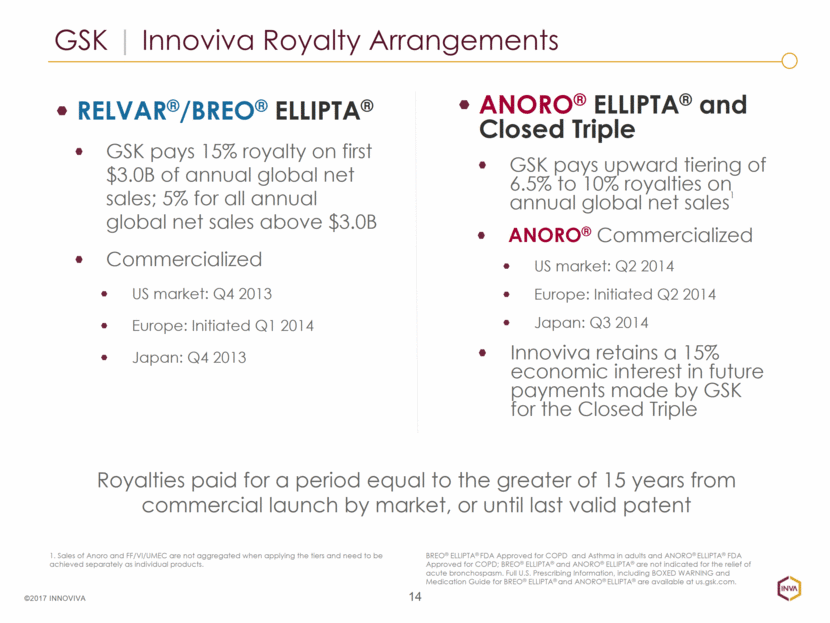
GSK Innoviva Royalty Arrangements 14 ©2017 INNOVIVA ANORO® ELLIPTA® and Closed Triple GSK pays upward tiering of 6.5% to 10% royalties on annual global net sales1 ANORO® Commercialized US market: Q2 2014 Europe: Initiated Q2 2014 Japan: Q3 2014 Innoviva retains a 15% economic interest in future payments made by GSK for the Closed Triple BREO® ELLIPTA® FDA Approved for COPD and Asthma in adults and ANORO® ELLIPTA® FDA Approved for COPD; BREO® ELLIPTA® and ANORO® ELLIPTA® are not indicated for the relief of acute bronchospasm. Full U.S. Prescribing Information, including BOXED WARNING and Medication Guide for BREO® ELLIPTA® and ANORO® ELLIPTA® are available at us.gsk.com. 1. Sales of Anoro and FF/VI/UMEC are not aggregated when applying the tiers and need to be achieved separately as individual products. RELVAR®/BREO® ELLIPTA® GSK pays 15% royalty on first $3.0B of annual global net sales; 5% for all annual global net sales above $3.0B Commercialized US market: Q4 2013 Europe: Initiated Q1 2014 Japan: Q4 2013 Royalties paid for a period equal to the greater of 15 years from commercial launch by market, or until last valid patent
ELLIPTA® - A Familiar and Easy To Use Inhaler “Single-Step Dose Activation” Open Breathe in Close Simple, large font dose counter Same familiar inhaler for all patients 15 ©2017 INNOVIVA BREO® ELLIPTA® FDA Approved for COPD and Asthma in adults and ANORO® ELLIPTA® FDA Approved for COPD; BREO® ELLIPTA® and ANORO® ELLIPTA® are not indicated for the relief of acute bronchospasm. Full U.S. Prescribing Information, including BOXED WARNING and Medication Guide for BREO® ELLIPTA® and ANORO® ELLIPTA® are available at us.gsk.com. * These programs, partnered with GSK, will be held & managed by a limited liability company subsidiary of Innoviva, Inc., (the “LLC”) and 85% of the LLC’s economic interests in these programs will accrue to Theravance Biopharma and 15% will accrue to Innoviva, Inc.
SALFORD Lung Study (SLS) SALFORD Lung Study: Phase IIIb multi-centre, open label randomized controlled trial (RCT) Compared the effectiveness and safety profile of FF/VI 100/25mcg with existing COPD usual care 2802 patients randomised 1:1 to receive FF/VI 100/25mcg or continue to receive usual care Patients located at 80 primary care sites in and around Salford and South Manchester (UK) Initial Study Results: Primary Endpoint Statistically significant reduction of 8.4% (CI 1.12,15.17) in the rate of moderate or severe exacerbations in patients treated with Relvar® Ellipta® compared with those receiving usual care (p=0.025) Safety Within the intent-to-treat (ITT) population, the incidence of serious adverse events (SAE) was similar between the groups (29% FF/VI, 27% usual care) For pneumonia, an SAE of special interest, FF/VI demonstrated non-inferiority versus usual care (7% FF/VI versus 6% usual care) 16 ©2017 INNOVIVA BREO® ELLIPTA® FDA Approved for COPD and Asthma in adults and ANORO® ELLIPTA® FDA Approved for COPD; BREO® ELLIPTA® and ANORO® ELLIPTA® are not indicated for the relief of acute bronchospasm. Full U.S. Prescribing Information, including BOXED WARNING and Medication Guide for BREO® ELLIPTA® and ANORO® ELLIPTA® are available at us.gsk.com. COPD patients treated with RELVAR® ELLIPTA® achieve superior reduction in exacerbations compared to ‘usual care’ Primary Endpoint Achieves Superiority
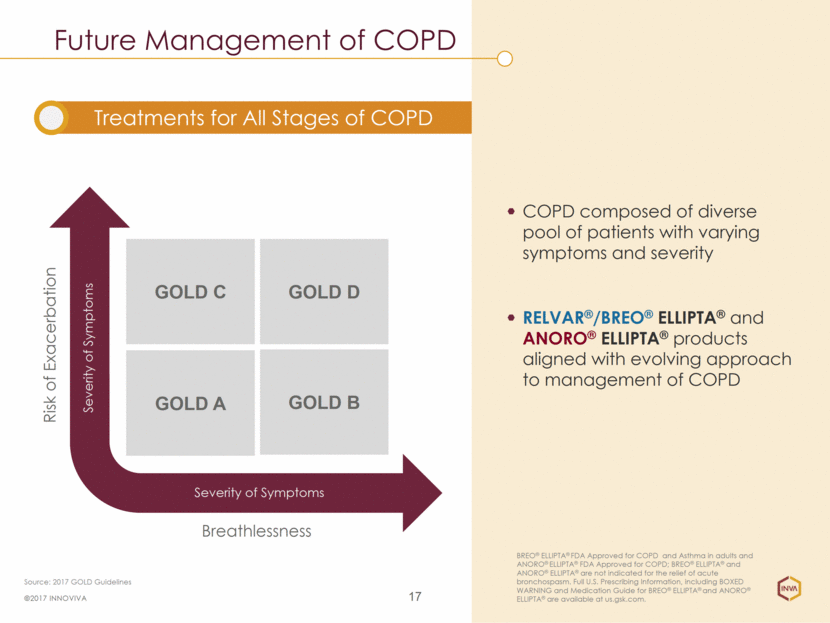
Future Management of COPD 17 COPD composed of diverse pool of patients with varying symptoms and severity RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA® products aligned with evolving approach to management of COPD Treatments for All Stages of COPD ©2017 INNOVIVA BREO® ELLIPTA® FDA Approved for COPD and Asthma in adults and ANORO® ELLIPTA® FDA Approved for COPD; BREO® ELLIPTA® and ANORO® ELLIPTA® are not indicated for the relief of acute bronchospasm. Full U.S. Prescribing Information, including BOXED WARNING and Medication Guide for BREO® ELLIPTA® and ANORO® ELLIPTA® are available at us.gsk.com. Source: 2017 GOLD Guidelines Breathlessness Risk of Exacerbation Severity of Symptoms Severity of Symptoms GOLD C GOLD A GOLD B GOLD D
©2017 INNOVIVA Aligning Corporate Governance With Our Strategic Mission

19 Innoviva’s Engaged, Experienced Board of Directors ©2017 INNOVIVA A Strong Board with the Experience, Diversity and Fresh Perspectives to Guide Innoviva Critical Expertise Pharmaceuticals leadership Significant financial experience Sophisticated capital markets understanding Public company executive experience Diverse Backgrounds Healthcare: pharmaceuticals, biopharmaceutical and medical devices Financial services Investing / private equity Operations Capital markets and M&A Fresh Perspectives Five new independent directors added since 2014 bring new and valuable insight to the board Innoviva is constantly evaluating potential director candidates to enhance skills and experience mix Formerly held Chairman and CEO roles at Technology Solutions Company, Bausch & Lomb and Biggers Brothers as well as CEO and director roles at Purolator Courier Other Board experience includes: Thomas & Betts and Charles River Laboratories William H. Waltrip President & CEO, Innoviva Formerly held executive positions at Gilead Sciences, Immunex and Honeywell International Michael W. Aguiar Formerly CFO at Intercept Pharmaceuticals and CEO of Dov Pharmaceuticals Financial services experience at Lehman Brothers and SBC Warburg Dillon Read Serves on the Board of Adaptimmune Therapeutics, Aevi Genomic, Jounce Therapeutics and ObsEva Barbara Duncan Financial services expertise including former Head of West Coast Healthcare and Co-Head of Biotechnology, Morgan Stanley Serves on the Board of GSV Capital, Radius Health and Yahoo! Catherine J. Friedman Managing Director, Wells Fargo Former Managing Director at Citadel; Head of Americas Corporate Finance at Bank of America; and Co-Head of Equity Linked Capital Markets and Head of Equity Derivatives at Merrill Lynch Paul A. Pepe Former Chairman and CEO of Par Pharmaceuticals, President of Cardinal Health’s healthcare marketing group and founder of Boron LePore and Associates Serves on the Board of PharMerica Patrick G. Lepore Co-founder and Managing Partner of Tyree and D'Angelo Partners Held numerous executive roles at Abbott Former Chairman of the Illinois Biotechnology Industry Organization Serves on the Board of ChemoCentryx and Depomed James L. Tyree
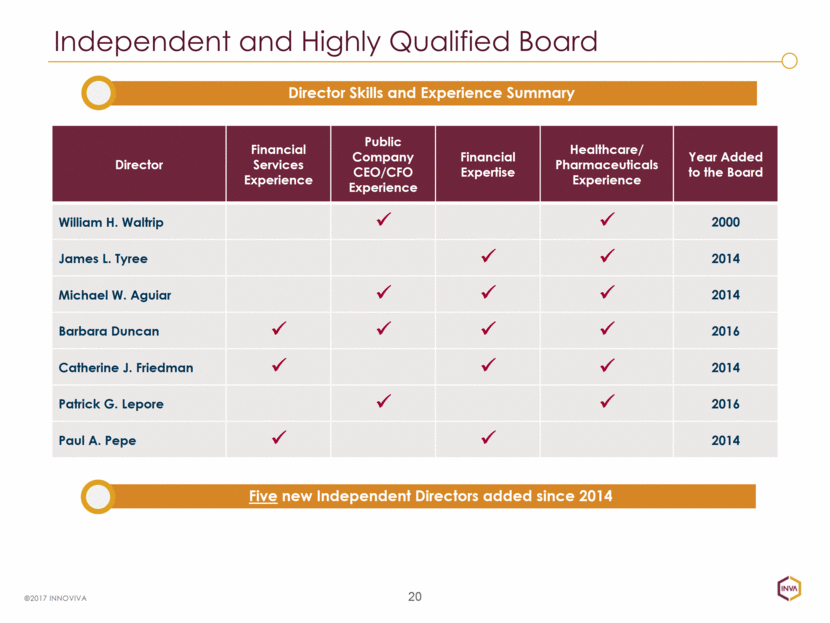
20 Independent and Highly Qualified Board ©2017 INNOVIVA Director Financial Services Experience Public Company CEO/CFO Experience Financial Expertise Healthcare/ Pharmaceuticals Experience Year Added to the Board William H. Waltrip 2000 James L. Tyree 2014 Michael W. Aguiar 2014 Barbara Duncan 2016 Catherine J. Friedman 2014 Patrick G. Lepore 2016 Paul A. Pepe 2014 Director Skills and Experience Summary Five new Independent Directors added since 2014
21 Governance Practices ©2017 INNOVIVA Our Objectives Highly-qualified Board of Directors Independent oversight Active shareholder engagement Emphasis on shareholder rights Compensation policies and practices aligned with shareholder interests Board Independence 6 of 7 Directors are independent Independent Chairman and Vice Chairman No over-boarded directors Board Tenure Added six directors since 2014, including five independent directors Average tenure of ~4 years (vs S&P average of 8.3), providing fresh perspective Best Practices No classified board – all directors elected annually Majority vote standard in director elections Track record of proactive, ongoing shareholder dialogue Directors are significant owners, aligning interest with stockholders

22 Compensation Practices and Policies ©2017 INNOVIVA Compensation Highlights 100% of the compensation committee members are independent Recent implementation of performance-based RSA program Equity plans expressly forbid option repricing without shareholder approval Active equity plans expressly forbid exchanges of underwater options for cash The CEO's stock ownership guidelines are equivalent to 600% of salary Strong Say on Pay support

RELVAR®/BREO® ELLIPTA® Important Safety Information (U.S.) The following ISI is based on the Highlights section of the US Prescribing Information for Breo Ellipta Please consult the full Prescribing Information for all the labelled safety information for Breo Ellipta. Long-acting beta2-adrenergic agonists (LABA), such as vilanterol, increase the risk of asthma-related death. A placebo-controlled trial with another LABA (salmeterol) showed an increase in asthma-related deaths. This finding with salmeterol is considered a class effect of all LABA. Currently available data are inadequate to determine whether concurrent use of inhaled corticosteroids (ICS) or other long-term asthma control drugs mitigates the increased risk of asthma-related death from LABA. Available data from controlled clinical trials suggest that LABA increase the risk of asthma-related hospitalisation in paediatric and adolescent patients. When treating patients with asthma, only prescribe Breo Ellipta for patients not adequately controlled on a long-term asthma control medication, such as an ICS, or whose disease severity clearly warrants initiation of treatment with both an ICS and a LABA. Once asthma control is achieved and maintained, assess the patient at regular intervals and step down therapy (e.g., discontinue Breo Ellipta) if possible without loss of asthma control and maintain the patient on a long-term asthma control medication, such as an ICS. Do not use Breo Ellipta for patients whose asthma is adequately controlled on low- or medium-dose ICS. Breo Ellipta is contraindicated for primary treatment of status asthmaticus or other acute episodes of COPD or asthma where intensive measures are required and in patients with severe hypersensitivity to milk proteins or who have demonstrated hypersensitivity to either fluticasone furoate, vilanterol, or any of the excipients. Breo Ellipta should not be initiated in patients during rapidly deteriorating or potentially life-threatening episodes of COPD or asthma, or used for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. Acute symptoms should be treated with an inhaled, short-acting beta2-agonist. Breo Ellipta should not be used more often than recommended, at higher doses than recommended, or in conjunction with other medications containing LABAs, as an overdose may result. Oropharyngeal candidiasis has occurred in patients treated with Breo Ellipta. Patients should be advised to rinse their mouth with water without swallowing after inhalation to help reduce this risk. An increase in the incidence of pneumonia has been observed in subjects with COPD receiving the fluticasone furoate/vilanterol combination, including Breo Ellipta 100 mcg/25 mcg, in clinical trials. There was also an increased incidence of pneumonias resulting in hospitalisation. In some incidences these pneumonia events were fatal. Patients who use corticosteroids are at risk for potential worsening of existing tuberculosis; fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex. A more serious or even fatal course of chickenpox or measles may occur in susceptible patients. Particular care is needed for patients who have been transferred from systemically active corticosteroids to inhaled corticosteroids because deaths due to adrenal insufficiency have occurred in patients with asthma during and after transfer from systemic corticosteroids to less systemically available inhaled corticosteroids. Hypercorticism and adrenal suppression may occur with very high dosages or at the regular dosage of inhaled corticosteroids in susceptible individuals. Caution should be exercised when considering the coadministration of Breo Ellipta with long-term ketoconazole and other known strong CYP3A4 inhibitors because increased systemic corticosteroid and cardiovascular adverse effects may occur. Breo Ellipta can produce paradoxical bronchospasm which may be life-threatening. Hypersensitivity reactions such as anaphylaxis, angioedema, rash, and urticaria may occur after administration of Breo Ellipta. Vilanterol, the LABA in Breo Ellipta, can produce clinically significant cardiovascular effects in some patients as measured by increases in pulse rate, systolic or diastolic blood pressure, and also cardiac arrhythmias. Breo Ellipta should be used with caution in patients with cardiovascular disorders. Decreases in bone mineral density have been observed with long-term administration of products containing inhaled corticosteroids, as have glaucoma, increased intraocular pressure, and cataracts. Breo Ellipta should be used with caution in patients with convulsive disorders, thyrotoxicosis, diabetes mellitus, ketoacidosis, and in patients who are unusually responsive to sympathomimetic amines. Beta-adrenergic agonist medicines may produce significant hypokalemia in some patients. Beta-adrenergic agonist medicines may produce transient hyperglycemia in some patients. Orally inhaled corticosteroids may cause a reduction in growth velocity when administered in children and adolescents. For COPD, the most common adverse reactions (>3% and more common than in placebo) reported in two 6-month clinical trials with Breo Ellipta 100/25 (and placebo) were nasopharyngitis, 9% (8%); upper respiratory tract infection, 7% (3%); headache, 7% (5%); and oral candidiasis, 5% (2%). In addition to the reactions reported in the 6-month studies, adverse reactions occurring in >3% of the subjects treated with Breo Ellipta 100/25 in two 1-year studies included back pain, pneumonia, bronchitis, sinusitis, cough, oropharyngeal pain, arthralgia, influenza, pharyngitis, and pyrexia. For asthma, the most common adverse reactions in a 12-week trial (incidence >2% and more common than placebo) reported with Breo Ellipta 100/25 (and placebo) were nasopharyngitis 10% (7%), headache 5% (4%), oropharyngeal pain 2% (1%), oral candidiasis 2% (0%), and dysphonia 2% (0%). In a separate 12-week trial the most common adverse reactions (>2% incidence) reported with Breo Ellipta 100/25 or 200/25 were headache, nasopharyngitis, influenza, upper respiratory tract infection, oropharyngeal pain, sinusitis, bronchitis, and cough. In addition to adverse reactions reported in the 12 week studies, adverse reactions (>2% incidence) reported with Breo Ellipta 200/25 in a 24-week trial included viral respiratory tract infection, pharyngitis, pyrexia, and arthralgia, and with Breo Ellipta 100/25 or 200/25 in a 12-month trial included pyrexia, back pain, extrasystoles, upper abdominal pain, respiratory tract infection, allergic rhinitis, pharyngitis, rhinitis, arthralgia, supraventricular extrasystoles, ventricular extrasystoles, acute sinusitis, and pneumonia. ©2017 INNOVIVA 23

ANORO® ELLIPTA® Important Safety Information (U.S.) The following Important Safety Information (ISI) is based on the Highlights section of the Prescribing Information for Anoro Ellipta. Please consult the full Prescribing Information for all the labeled safety information for Anoro Ellipta. Long-acting beta2-adrenergic agonists (LABAs), such as vilanterol, one of the active ingredients in Anoro Ellipta, increase the risk of asthma-related death. A placebo-controlled trial with another LABA (salmeterol) showed an increase in asthma-related deaths in subjects receiving salmeterol. This finding with salmeterol is considered a class effect of all LABAs, including vilanterol. The safety and efficacy of Anoro Ellipta in patients with asthma have not been established. Anoro Ellipta is not indicated for the treatment of asthma. Anoro Ellipta is contraindicated in patients with severe hypersensitivity to milk proteins or who have demonstrated hypersensitivity to either umeclidinium, vilanterol, or any of the other ingredients. Anoro Ellipta should not be initiated in patients during rapidly deteriorating or potentially life-threatening episodes of COPD, or as rescue therapy for the treatment of acute episodes of bronchospasm, which should be treated with an inhaled, short-acting beta2-agonist. Anoro Ellipta should not be used more often than recommended, at higher doses than recommended, or in conjunction with additional medicine containing a LABA, as an overdose may result. Anoro Ellipta should be used with caution when considering coadministration with long-term ketoconazole and other known strong cytochrome P450 3A4 inhibitors because increased cardiovascular adverse effects may occur. As with other inhaled medicines, Anoro Ellipta can produce paradoxical bronchospasm, which may be life-threatening. Anoro Ellipta should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension. Anoro Ellipta should be used with caution in patients with convulsive disorders, thyrotoxicosis, diabetes mellitus, ketoacidosis, and in patients who are unusually responsive to sympathomimetic amines. Anoro Ellipta should be used with caution in patients with narrow-angle glaucoma. Instruct patients to contact a physician immediately should any signs or symptoms of narrow-angle glaucoma occur. Anoro Ellipta should be used with caution in patients with urinary retention, especially in patients with prostatic hyperplasia or bladder neck obstruction. Instruct patients to contact a physician immediately should any signs or symptoms of urinary retention occur. Beta-adrenergic agonist medicines may produce significant hypokalemia and transient hyperglycemia in some patients. The most common adverse reactions (incidence >1% and more common than placebo) reported in four 6-month clinical trials with Anoro Ellipta (and placebo) were pharyngitis, 2% (<1%); sinusitis 1% (<1%); lower respiratory tract infection, 1% (<1%); constipation, 1% (<1%); diarrhea, 2% (1%); pain in extremity 2% (1%); muscle spasms, 1% (<1%); neck pain, 1% (<1%); and chest pain 1% (<1%). In addition to the 6-month efficacy trials with Anoro Ellipta, a 12-month trial evaluated the safety of umeclidinium/vilanterol 125 mcg/25 mcg in subjects with COPD. Adverse reactions (incidence >1% and more common than placebo) in subjects receiving umeclidinium/vilanterol 125 mcg/25 mcg were: headache, back pain, sinusitis, cough, urinary tract infection, arthralgia, nausea, vertigo, abdominal pain, pleuritic pain, viral respiratory tract infection, toothache, and diabetes mellitus. Use of beta2-agonists, such as vilanterol should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors, tricyclic antidepressants, or drugs known to prolong the QTc interval or within 2 weeks of discontinuation of such agents, because the effect of adrenergic agonists on the cardiovascular system may be potentiated. Use beta-blockers with caution as they not only block the pulmonary effect of beta-agonists, such as vilanterol, but may produce severe bronchospasm in patients with COPD. Use with caution in patients taking non–potassium-sparing diuretics, as electrocardiographic changes and/or hypokalemia associated with non–potassium-sparing diuretics may worsen with concomitant beta-agonists. Avoid co-administration of Anoro Ellipta with other anticholinergic-containing drugs as this may lead to an increase in anticholinergic adverse effects such as cardiovascular effects, worsening of narrow-angle glaucoma, and worsening of urinary retention. ©2017 INNOVIVA 24

To supplement the consolidated financial statements presented in accordance with generally accepted accounting principles in the United States, or GAAP, Innoviva uses the non-GAAP financial measures of adjusted EBITDA and adjusted earnings per share. Generally, a non-GAAP financial measure is a numerical measure of a company's operating performance or financial position that either excludes or includes amounts that are not normally included or excluded in the most directly comparable measure calculated and presented in accordance with GAAP. A reconciliation of these non-GAAP financial measures to the closest GAAP financial measure is presented in the accompanying financial table under the headings “Reconciliation of Non-GAAP Financial Measures to GAAP.” Innoviva believes that the non-GAAP financial information provided in this presentation can assist investors, research analysts and others in understanding and assessing Innoviva's on-going operations, financial performance and prospects for the future and provides an additional tool to use in comparing Innoviva's financial results with other companies in Innoviva's industry or with similar operating profiles, without regard to financing or capital structures. Adjusted EBITDA and adjusted earnings per share are used as supplemental financial operating measures by Innoviva's management and frequently discussed with external users of its financial statements. Adjusted EBITDA is determined by taking GAAP net income (loss) and adding back interest expense (income), taxes, stock-based compensation expense, depreciation expense and amortization of capitalized fees paid to a related party. Innoviva believes the non-GAAP measure of adjusted EBITDA is important as it measures the Company's ability to generate cash to pay interest costs and support its indebtedness, and it is also used currently in the Company's annual performance review process. Innoviva's method of computing adjusted EBITDA may not be the same method used to compute similar measures reported by other companies. Adjusted earnings per share is determined by taking Adjusted net income (loss) and dividing the total by the fully diluted number of shares outstanding used to calculate the GAAP diluted EPS. Adjusted net income (loss) is determined by taking GAAP net income (loss) and adding back stock-based compensation expense, depreciation expense and amortization of capitalized fees paid to a related party, Innoviva believes the non-GAAP measure of adjusted earnings per share provides useful information about the Company's core operating performance, and enhances the overall understanding of the Company's past financial performance and its prospects for the future. Innoviva's method of computing adjusted earnings per share may not be the same method used to compute similar measures reported by other companies. Adjusted EBITDA, adjusted net income (loss) and adjusted earnings per share should not be considered in isolation or as a substitute to net income/loss, income/loss from operations, cash flows from operating activities, earnings per share or any other measure of financial performance presented in accordance with GAAP. Adjusted earnings per share is not intended to represent cash flow per share and does not represent a measure of liquidity or cash available for distribution. The principal limitation of these non-GAAP financial measures is that it excludes significant elements that are required by GAAP to be recorded in Innoviva's consolidated financial statements. In addition, it is subject to inherent limitations as it reflects the exercise of judgments by management in determining these non-GAAP financial measures. In order to compensate for these limitations, management of Innoviva presents its non-GAAP financial measures in connection with its GAAP results. Investors are encouraged to review the reconciliation of Innoviva's non-GAAP financial measures to their most directly comparable GAAP financial measure. 25 ©2017 INNOVIVA Reconciliation of Non-GAAP Financial Measures to GAAP
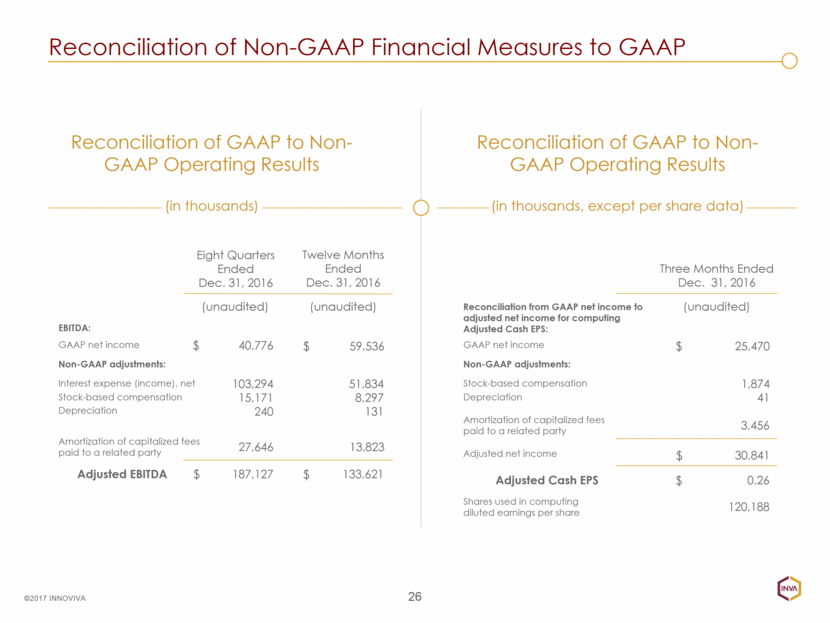
GAAP net income Reconciliation of Non-GAAP Financial Measures to GAAP 26 ©2017 INNOVIVA Reconciliation of GAAP to Non-GAAP Operating Results (in thousands) Reconciliation of GAAP to Non-GAAP Operating Results (in thousands, except per share data) Eight Quarters Ended Dec. 31, 2016 Twelve Months Ended Dec. 31, 2016 (unaudited) (unaudited) 40,776 59,536 103,294 15,171 240 51,834 8,297 131 27,646 13,823 187,127 133,621 $ $ $ $ Adjusted EBITDA Interest expense (income), net Amortization of capitalized fees paid to a related party Stock-based compensation Depreciation Non-GAAP adjustments: EBITDA: Three Months Ended Dec. 31, 2016 (unaudited) 25,470 1,874 41 3,456 0.26 $ $ Adjusted Cash EPS Stock-based compensation Amortization of capitalized fees paid to a related party Depreciation Non-GAAP adjustments: Reconciliation from GAAP net income to adjusted net income for computing Adjusted Cash EPS: GAAP net income Adjusted net income 30,841 $ Shares used in computing diluted earnings per share 120,188
Innoviva, its directors and certain of its executive officers and employees may be deemed to be participants in the solicitation of proxies from stockholders in connection with Innoviva’s 2017 annual meeting of stockholders (the “2017 Annual Meeting”). On March 7, 2017, Innoviva filed a preliminary proxy statement with the U.S. Securities and Exchange Commission (the “SEC”) in connection with the solicitation of proxies for the 2017 Annual Meeting. Prior to the 2017 Annual Meeting, Innoviva will furnish a definitive proxy statement to its stockholders (the “2017 Proxy Statement”), together with a WHITE proxy card. STOCKHOLDERS ARE URGED TO READ THE 2017 PROXY STATEMENT (INCLUDING ANY AMENDMENTS OR SUPPLEMENTS THERETO) AND ANY OTHER RELEVANT DOCUMENTS THAT INNOVIVA WILL FILE WITH THE SEC CAREFULLY IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION. Additional information regarding the identity of these potential participants and their direct or indirect interests, by security holdings or otherwise, is set forth in the preliminary proxy statement for the 2017 Annual Meeting and will be set forth in the 2017 Proxy Statement and other materials to be filed with the SEC in connection with the 2017 Annual Meeting. Stockholders will be able to obtain, free of charge, copies of the 2017 Proxy Statement, any amendments or supplements thereto and any other documents (including the WHITE proxy card) when filed by Innoviva with the SEC in connection with the 2017 Annual Meeting at the SEC’s website (http://www.sec.gov), at Innoviva’s website (http://investor.inva.com/sec.cfm), by email at investor.relations@inva.com or by mail at Innoviva, Inc., Attn: Investor Relations, 2000 Sierra Point Parkway, Suite 500, Brisbane, California 94005. In addition, copies of the proxy materials, when available, may be requested from Innoviva’s proxy solicitor, Innisfree M&A Incorporated at 501 Madison Ave, 20th Floor, New York, NY 10022 or toll-free at (888) 750-5834. 14a-12 LEGEND 27 ©2017 INNOVIVA

©2017 INNOVIVA INVA.COM
