Exhibit 99.1
©2016 INNOVIVA March 2016

Safe Harbor 2 ©2016 INNOVIVA This presentation contains certain "forward-looking" statements as that term is defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, statements relating to goals, plans, objectives and future events. Innoviva intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. The words “anticipate”, “expect”, “goal”, “intend”, “objective”, “opportunity”, “plan”, “potential”, “target” and similar expressions are intended to identify such forward-looking statements. Such forward-looking statements involve substantial risks, uncertainties and assumptions. Examples of such statements include statements relating to the commercialization of RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA® in the jurisdictions in which these products have been approved, the strategies, plans and objectives of the company, the timing, manner and amount of anticipated potential capital returns to stockholders (including without limitation statements, expectations of future cash dividends or future share repurchases), the status and timing of clinical studies, data analysis and communication of results, the potential benefits and mechanisms of action of product candidates, expectations for product candidates through development and commercialization, the timing of seeking regulatory approval of product candidates, and projections of revenue, expenses and other financial items. These statements are based on the current estimates and assumptions of the management of Innoviva as of the date of this presentation and are subject to risks, uncertainties, changes in circumstances, assumptions and other factors that may cause the actual results of Innoviva to be materially different from those reflected in the forward-looking statements. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include, among others, risks related to: the disruption of operations during the transition period following the spin-off, including the diversion of managements' and employees' attention, disruption of relationships with collaborators and increased employee turnover, lower than expected future royalty revenue from respiratory products partnered with GSK, delays or difficulties in commencing or completing clinical studies, the potential that results from clinical or non-clinical studies indicate product candidates are unsafe or ineffective, dependence on third parties to conduct its clinical studies, delays or failure to achieve and maintain regulatory approvals for product candidates, and risks of collaborating with third parties to discover, develop and commercialize products. Other risks affecting Innoviva are described under the headings "Risk Factors" and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” contained in Innoviva's Annual Report on Form 10-K for the year ended December 31, 2015 which is on file with the Securities and Exchange Commission (SEC). In addition to the risks described above and in Innoviva’s other filings with the SEC, other unknown or unpredictable factors also could affect Innoviva’s results. No forward-looking statements can be guaranteed and actual results may differ materially from such statements. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Innoviva assumes no obligation to update its forward-looking statements on account of new information, future events or otherwise, except as required by law.

Innoviva, Inc. - Investor Overview Bringing Compelling New Medicines to Patients in Areas of Unmet Need Access to large and growing global respiratory market: $20 billion annual sales1 Innovative product portfolio with differentiated features and therapeutic profiles Positioned to enhance capital returns to investors Publicly traded, long-dated, royalty portfolio company Lean staffing, low cost structure organization Quarterly net profit of $4.3 million in Q4 2015 Announced on 10/28/2015 a $150 million stock repurchase program Between October 2015 and January 2016, repurchased $37.3 million of stock Goal: Build recurring revenue business & maximize stockholders return 3 ©2016 INNOVIVA ANORO® ELLIPTA® RELVAR®/BREO® ELLIPTA® 1 Excludes solutions for nebulization. Source: This information is an estimate derived from the use of information under license from the following IMS Health Inc. information service: MIDAS for the period ending September 2013. IMS expressly reserves all rights, including rights of copying, distribution and republication. BREO® ELLIPTA® FDA Approved for COPD and Asthma in adults and ANORO® ELLIPTA® FDA Approved for COPD; BREO® ELLIPTA® and ANORO® ELLIPTA® are not indicated for the relief of acute bronchospasm. Full U.S. Prescribing Information, including BOXED WARNING and Medication Guide for BREO® ELLIPTA® and ANORO® ELLIPTA® are available at us.gsk.com. RELVAR®, BREO®, ANORO® and ELLIPTA® are trademarks of the GlaxoSmithKline group of companies. Approved for COPD/Asthma, launched in 45 countries 356% YoY growth in Global Net Sales between 2014 and 2015 Approved for COPD, launched in 38 countries 432% YoY growth in Global Net Sales between 2014 and 2015
Commercial Respiratory Products 4 ©2016 INNOVIVA ANORO® ELLIPTA® RELVAR®/BREO® ELLIPTA® First and only once-daily ICS/LABA in the U.S. 24 hours efficacy One inhalation once daily Easy-to-use ELLIPTA® inhaler In the U.S., indicated for COPD and for asthma in adults Outside of the U.S., indicated for asthma and COPD; in Japan, indicated for asthma First-in-class LABA/LAMA product in the U.S., combining two long-acting bronchodilators in one inhaler for patients with COPD Provides better lung function than tiotropium 24 hours efficacy One inhalation once daily Easy-to-use ELLIPTA® inhaler BREO® ELLIPTA® FDA Approved for COPD and Asthma in adults and ANORO® ELLIPTA® FDA Approved for COPD; BREO® ELLIPTA® and ANORO® ELLIPTA® are not indicated for the relief of acute bronchospasm. Full U.S. Prescribing Information, including BOXED WARNING and Medication Guide for BREO® ELLIPTA® and ANORO® ELLIPTA® are available at us.gsk.com. RELVAR®, BREO®, ANORO® and ELLIPTA® are trademarks of the GlaxoSmithKline group of companies.
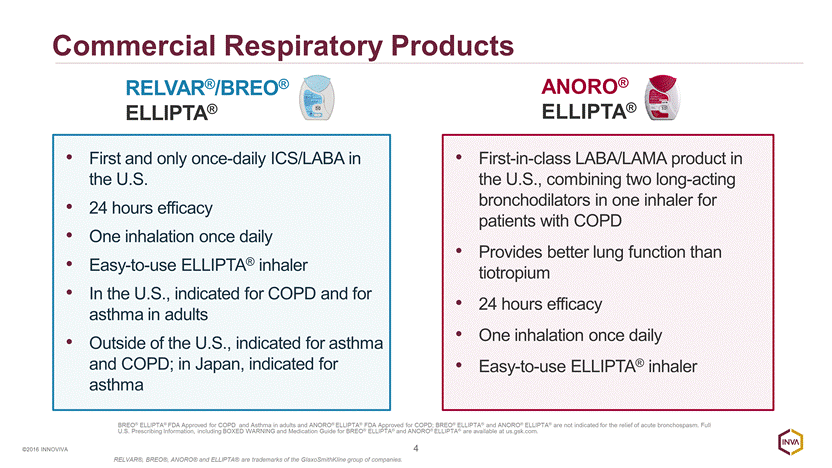
Significant Respiratory Market Opportunity 5 ©2016 INNOVIVA 4.6% CAGR 2012 - 2014 Revenue Long-Acting Bronchodilators Market growth primarily in COPD3 Market Growth Drivers4: Expansion of aging population Patients diagnosed earlier 70% of COPD cases occur in people aged 60 or older4 Medicare Part-D is major U.S. segment BREO® and ANORO® Medicare Part-D U.S. coverage steadily increasing since launch (BREO® 72% and ANORO® 74%) 1 Excludes solutions for nebulization. Source: This information is an estimate derived from the use of information under license from the following IMS Health Inc. information service: MIDAS for the period ending December 2014. IMS expressly reserves all rights, including rights of copying, distribution and republication. 2 © 2015 DR/Decision Resources, LLC. All rights reserved. This data is provided for informational purposes only and is not intended to, and does not, constitute an offer or recommendation to buy or sell securities or investment advice. 3 © Chronic Obstructive Pulmonary Disease (Event Driven), January 2015, Asthma April 2015,. Decision Resources, Inc. All Rights Reserved. Reproduction, distribution, transmission or publication is prohibited. Reprinted with permission. 4 2014 Decision Resources COPD Report, October 2014, All Rights Reserved. Reproduction, distribution, transmission or publication is prohibited. Reprinted with permission. BREO® ELLIPTA® FDA Approved for COPD and Asthma in adults and ANORO® ELLIPTA® FDA Approved for COPD; BREO® ELLIPTA® and ANORO® ELLIPTA® are not indicated for the relief of acute bronchospasm. Full U.S. Prescribing Information, including BOXED WARNING and Medication Guide for BREO® ELLIPTA® and ANORO® ELLIPTA® are available at us.gsk.com. RELVAR®, BREO®, ANORO® and ELLIPTA® are trademarks of the GlaxoSmithKline group of companies. $19.71 $21.29 $22.58 ~21% market growth from 2013 - 2023 2 2012 2013 2014 2023 Global Long - Acting Bronchidilator Sales 1 (in $B USD)
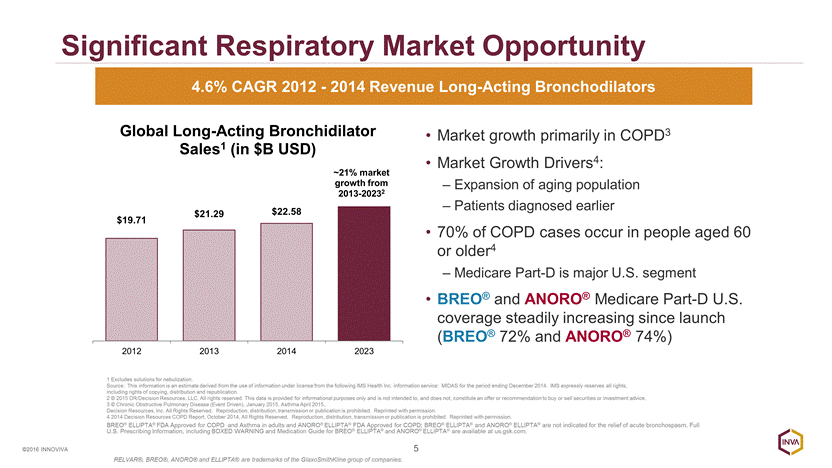
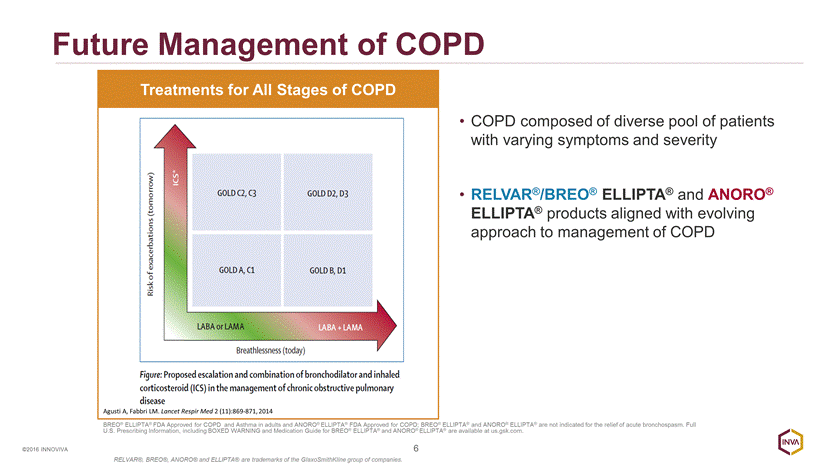
Future Management of COPD 6 ©2016 INNOVIVA COPD composed of diverse pool of patients with varying symptoms and severity RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA® products aligned with evolving approach to management of COPD Treatments for All Stages of COPD Agusti A, Fabbri LM. Lancet Respir Med 2 (11):869-871, 2014 BREO® ELLIPTA® FDA Approved for COPD and Asthma in adults and ANORO® ELLIPTA® FDA Approved for COPD; BREO® ELLIPTA® and ANORO® ELLIPTA® are not indicated for the relief of acute bronchospasm. Full U.S. Prescribing Information, including BOXED WARNING and Medication Guide for BREO® ELLIPTA® and ANORO® ELLIPTA® are available at us.gsk.com. RELVAR®, BREO®, ANORO® and ELLIPTA® are trademarks of the GlaxoSmithKline group of companies.
Compliance in COPD Patients COPD patients with higher adherence experienced fewer hospitalizations and lower Medicare costs than those with lower adherence behavior3 Ongoing Salford Lung Studies aimed at identifying the effectiveness of once-a-day therapy with RELVAR®/BREO® ELLIPTA® versus standard of care therapy 7 ©2016 INNOVIVA DiMatteo, et al. Med Care. 2004;42:200-9 Toy, et al. Resp Med 2011;105:435-41 Simoni-Wastila, et al. Am J Geriatr Pharmacother 2012;10:201-10 Mean Proportion of Days Covered (%) after 12 months Dosing Frequency and Adherence in Patients with COPD Adherence is poor in the treatment of chronic conditions1,2 BREO® ELLIPTA® FDA Approved for COPD and Asthma in adults and ANORO® ELLIPTA® FDA Approved for COPD; BREO® ELLIPTA® and ANORO® ELLIPTA® are not indicated for the relief of acute bronchospasm. Full U.S. Prescribing Information, including BOXED WARNING and Medication Guide for BREO® ELLIPTA® and ANORO® ELLIPTA® are available at us.gsk.com. RELVAR®, BREO®, ANORO® and ELLIPTA® are trademarks of the GlaxoSmithKline group of companies. 23% 30% 37% 43% Four Times Daily (QID) Three Times Daily (TID) Twice-Daily (BID) Once-Daily (QD)

ELLIPTA® – A Familiar and Easy To Use Inhaler “Single-Step Dose Activation” Open Breathe in Close Simple, large font dose counter Same familiar inhaler for all patients 8 ©2016 INNOVIVA * These programs, partnered with GSK, will be held & managed by a limited liability company subsidiary of Innoviva, Inc., (the “LLC”) and 85% of the LLC’s economic interests in these programs will accrue to Theravance Biopharma and 15% will accrue to Innoviva, Inc.. RELVAR®/BREO® ELLIPTA® ANORO® ELLIPTA® RELVAR®/ BREO® + INCRUSE® FF/UMEC/VI* MABA/ICS* BREO® ELLIPTA® FDA Approved for COPD and Asthma in adults and ANORO® ELLIPTA® FDA Approved for COPD; BREO® ELLIPTA® and ANORO® ELLIPTA® are not indicated for the relief of acute bronchospasm. Full U.S. Prescribing Information, including BOXED WARNING and Medication Guide for BREO® ELLIPTA® and ANORO® ELLIPTA® are available at us.gsk.com. RELVAR®, BREO®, ANORO® and ELLIPTA® are trademarks of the GlaxoSmithKline group of companies.
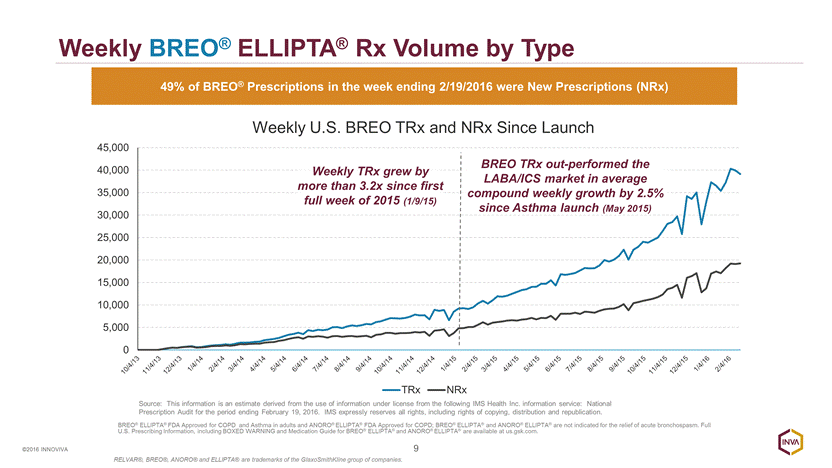
Weekly BREO® ELLIPTA® Rx Volume by Type 9 ©2016 INNOVIVA 49% of BREO® Prescriptions in the week ending 2/19/2016 were New Prescriptions (NRx) BREO TRx out-performed the LABA/ICS market in average compound weekly growth by 2.5% since Asthma launch (May 2015) Source: This information is an estimate derived from the use of information under license from the following IMS Health Inc. information service: National Prescription Audit for the period ending February 19, 2016. IMS expressly reserves all rights, including rights of copying, distribution and republication. Weekly TRx grew by more than 3.2x since first full week of 2015 (1/9/15) BREO® ELLIPTA® FDA Approved for COPD and Asthma in adults and ANORO® ELLIPTA® FDA Approved for COPD; BREO® ELLIPTA® and ANORO® ELLIPTA® are not indicated for the relief of acute bronchospasm. Full U.S. Prescribing Information, including BOXED WARNING and Medication Guide for BREO® ELLIPTA® and ANORO® ELLIPTA® are available at us.gsk.com. RELVAR®, BREO®, ANORO® and ELLIPTA® are trademarks of the GlaxoSmithKline group of companies. 0 5,000 10,000 15,000 20,000 25,000 30,000 35,000 40,000 45,000 Weekly U.S. BREO TRx and NRx Since Launch TRx NRx
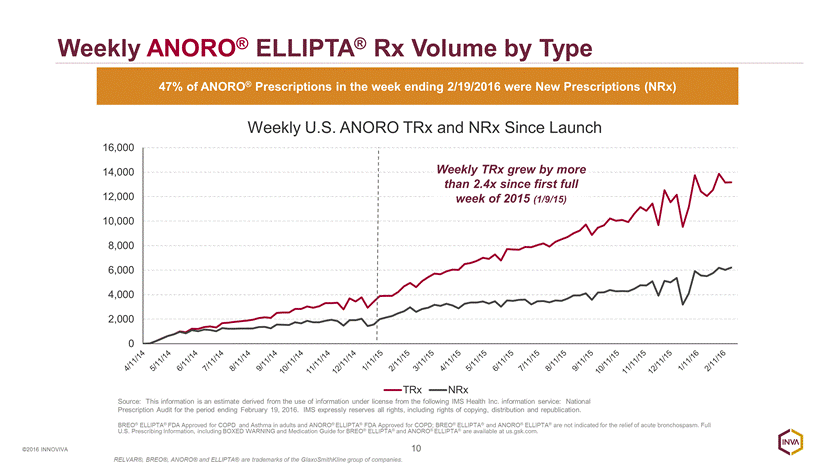
Weekly ANORO® ELLIPTA® Rx Volume by Type 10 ©2016 INNOVIVA 47% of ANORO® Prescriptions in the week ending 2/19/2016 were New Prescriptions (NRx) Source: This information is an estimate derived from the use of information under license from the following IMS Health Inc. information service: National Prescription Audit for the period ending February 19, 2016. IMS expressly reserves all rights, including rights of copying, distribution and republication. Weekly TRx grew by more than 2.4x since first full week of 2015 (1/9/15) BREO® ELLIPTA® FDA Approved for COPD and Asthma in adults and ANORO® ELLIPTA® FDA Approved for COPD; BREO® ELLIPTA® and ANORO® ELLIPTA® are not indicated for the relief of acute bronchospasm. Full U.S. Prescribing Information, including BOXED WARNING and Medication Guide for BREO® ELLIPTA® and ANORO® ELLIPTA® are available at us.gsk.com. RELVAR®, BREO®, ANORO® and ELLIPTA® are trademarks of the GlaxoSmithKline group of companies. 0 2,000 4,000 6,000 8,000 10,000 12,000 14,000 16,000 Weekly U.S. ANORO TRx and NRx Since Launch TRx NRx
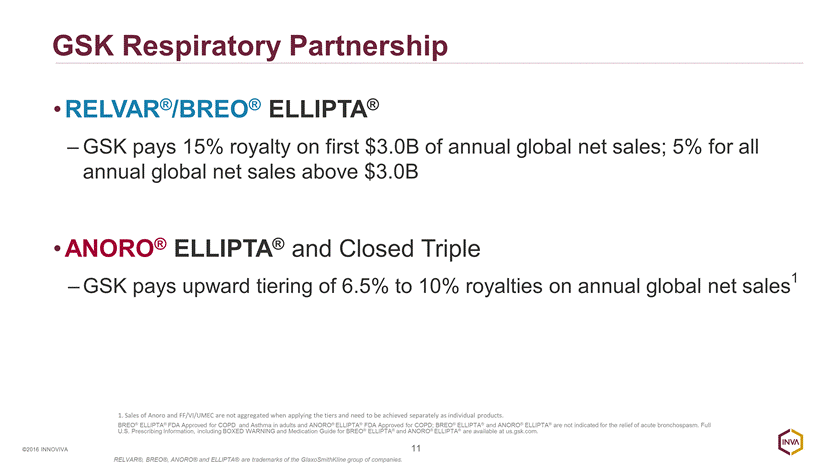
GSK Respiratory Partnership RELVAR®/BREO® ELLIPTA® GSK pays 15% royalty on first $3.0B of annual global net sales; 5% for all annual global net sales above $3.0B ANORO® ELLIPTA® and Closed Triple GSK pays upward tiering of 6.5% to 10% royalties on annual global net sales1 11 ©2016 INNOVIVA 1. Sales of Anoro and FF/VI/UMEC are not aggregated when applying the tiers and need to be achieved separately as individual products. BREO® ELLIPTA® FDA Approved for COPD and Asthma in adults and ANORO® ELLIPTA® FDA Approved for COPD; BREO® ELLIPTA® and ANORO® ELLIPTA® are not indicated for the relief of acute bronchospasm. Full U.S. Prescribing Information, including BOXED WARNING and Medication Guide for BREO® ELLIPTA® and ANORO® ELLIPTA® are available at us.gsk.com. RELVAR®, BREO®, ANORO® and ELLIPTA® are trademarks of the GlaxoSmithKline group of companies.
Quarterly Royalties Earned RELVAR®/BREO® ELLIPTA® Launched in 45 countries 356% YoY growth in royalties earned between 2014 and 2015 ANORO® ELLIPTA® Launched in 38 countries 432% YoY growth in royalties earned between 2014 and 2015 12 ©2016 INNOVIVA 42% CAGR in total royalties earned in the last six quarters BREO® ELLIPTA® FDA Approved for COPD and Asthma in adults and ANORO® ELLIPTA® FDA Approved for COPD; BREO® ELLIPTA® and ANORO® ELLIPTA® are not indicated for the relief of acute bronchospasm. Full U.S. Prescribing Information, including BOXED WARNING and Medication Guide for BREO® ELLIPTA® and ANORO® ELLIPTA® are available at us.gsk.com. RELVAR®, BREO®, ANORO® and ELLIPTA® are trademarks of the GlaxoSmithKline group of companies. - 5.0 10.0 15.0 20.0 25.0 30.0 Q1 2014 Q2 2014 Q3 2014 Q4 2014 Q1 2015 Q2 2015 Q3 2015 Q4 2015 $ millions ANORO® ELLIPTA® BREO® ELLIPTA® 55% 45% Non-U.S. (2015) U.S. (2015)
Significant Future Growth Opportunity 13 ©2016 INNOVIVA POTENTIAL GROWTH OPPORTUNITY Continued Product Launches / Enhanced Launch Activities / Improved Patient Access Asthma indication in the U.S. approved by FDA April 30, 2015 Commercial launch in May 2015, Asthma DTC Initiated in Q4 2015 Salford Lung Studies: Innovative clinical studies designed to evaluate RELVAR® ELLIPTA® when used in patients with COPD and asthma in a real world setting, first half 2016 BREO® ELLIPTA® FDA Approved for COPD and Asthma in adults and ANORO® ELLIPTA® FDA Approved for COPD; BREO® ELLIPTA® and ANORO® ELLIPTA® are not indicated for the relief of acute bronchospasm. Full U.S. Prescribing Information, including BOXED WARNING and Medication Guide for BREO® ELLIPTA® and ANORO® ELLIPTA® are available at us.gsk.com. RELVAR®, BREO®, ANORO® and ELLIPTA® are trademarks of the GlaxoSmithKline group of companies.
Corporate Priorities 3 ©2016 INNOVIVA Highly Focused Strategy & Execution Maximizing value of GSK partnered assets BREO Asthma DTC campaign launched in Q4 2015 BREO TRx out-performing LABA/ICS market since Asthma launch ANORO Weekly TRx grew by more than 2.6x since beginning of 2015 Maintaining a low overall cost structure Quarterly net profit of $4.3 million in Q4 2015 Positive and growing cash from operations in 2015 2016 Opex guidance of $18-20 million (bef. stock comp.) Reduce overall corporate cost of capital & long term tax rate Evaluating optimization of corporate structure (e.g. inversion, partnership structures, others) Provide capital returns to investors Repurchased $37.3 million of stock from October 2015 to January 2016 Enhance terminal value and build recurring revenue business
Innoviva, Inc. - Investor Overview Bringing Compelling New Medicines to Patients in Areas of Unmet Need Access to large and growing global respiratory market: $20 billion annual sales1 Innovative product portfolio with differentiated features and therapeutic profiles Positioned to enhance capital returns to investors Publicly traded, long-dated, royalty portfolio company Lean staffing, low cost structure organization Quarterly net profit of $4.3 million in Q4 2015 Announced on 10/28/2015 a $150 million stock repurchase program Between October 2015 and January 2016, repurchased $37.3 million of stock Goal: Build recurring revenue business & maximize stockholders return 15 ©2016 INNOVIVA ANORO® ELLIPTA® RELVAR®/BREO® ELLIPTA® 1 Excludes solutions for nebulization. Source: This information is an estimate derived from the use of information under license from the following IMS Health Inc. information service: MIDAS for the period ending September 2013. IMS expressly reserves all rights, including rights of copying, distribution and republication. BREO® ELLIPTA® FDA Approved for COPD and Asthma in adults and ANORO® ELLIPTA® FDA Approved for COPD; BREO® ELLIPTA® and ANORO® ELLIPTA® are not indicated for the relief of acute bronchospasm. Full U.S. Prescribing Information, including BOXED WARNING and Medication Guide for BREO® ELLIPTA® and ANORO® ELLIPTA® are available at us.gsk.com. RELVAR®, BREO®, ANORO® and ELLIPTA® are trademarks of the GlaxoSmithKline group of companies. Approved for COPD/Asthma, launched in 45 countries 356% YoY growth in Global Net Sales between 2014 and 2015 Approved for COPD, launched in 38 countries 432% YoY growth in Global Net Sales between 2014 and 2015
©2016 INNOVIVA INVA.COM

RELVAR®/BREO® ELLIPTA® Important Safety Information (U.S.) The following ISI is based on the Highlights section of the US Prescribing Information for Breo Ellipta Please consult the full Prescribing Information for all the labelled safety information for Breo Ellipta. Long-acting beta2-adrenergic agonists (LABA), such as vilanterol, increase the risk of asthma-related death. A placebo-controlled trial with another LABA (salmeterol) showed an increase in asthma-related deaths. This finding with salmeterol is considered a class effect of all LABA. Currently available data are inadequate to determine whether concurrent use of inhaled corticosteroids (ICS) or other long-term asthma control drugs mitigates the increased risk of asthma-related death from LABA. Available data from controlled clinical trials suggest that LABA increase the risk of asthma-related hospitalisation in paediatric and adolescent patients. When treating patients with asthma, only prescribe Breo Ellipta for patients not adequately controlled on a long-term asthma control medication, such as an ICS, or whose disease severity clearly warrants initiation of treatment with both an ICS and a LABA. Once asthma control is achieved and maintained, assess the patient at regular intervals and step down therapy (e.g., discontinue Breo Ellipta) if possible without loss of asthma control and maintain the patient on a long-term asthma control medication, such as an ICS. Do not use Breo Ellipta for patients whose asthma is adequately controlled on low- or medium-dose ICS. Breo Ellipta is contraindicated for primary treatment of status asthmaticus or other acute episodes of COPD or asthma where intensive measures are required and in patients with severe hypersensitivity to milk proteins or who have demonstrated hypersensitivity to either fluticasone furoate, vilanterol, or any of the excipients. Breo Ellipta should not be initiated in patients during rapidly deteriorating or potentially life-threatening episodes of COPD or asthma, or used for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. Acute symptoms should be treated with an inhaled, short-acting beta2-agonist. Breo Ellipta should not be used more often than recommended, at higher doses than recommended, or in conjunction with other medications containing LABAs, as an overdose may result. Oropharyngeal candidiasis has occurred in patients treated with Breo Ellipta. Patients should be advised to rinse their mouth with water without swallowing after inhalation to help reduce this risk. An increase in the incidence of pneumonia has been observed in subjects with COPD receiving the fluticasone furoate/vilanterol combination, including Breo Ellipta 100 mcg/25 mcg, in clinical trials. There was also an increased incidence of pneumonias resulting in hospitalisation. In some incidences these pneumonia events were fatal. Patients who use corticosteroids are at risk for potential worsening of existing tuberculosis; fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex. A more serious or even fatal course of chickenpox or measles may occur in susceptible patients. Particular care is needed for patients who have been transferred from systemically active corticosteroids to inhaled corticosteroids because deaths due to adrenal insufficiency have occurred in patients with asthma during and after transfer from systemic corticosteroids to less systemically available inhaled corticosteroids. Hypercorticism and adrenal suppression may occur with very high dosages or at the regular dosage of inhaled corticosteroids in susceptible individuals. Caution should be exercised when considering the coadministration of Breo Ellipta with long-term ketoconazole and other known strong CYP3A4 inhibitors because increased systemic corticosteroid and cardiovascular adverse effects may occur. Breo Ellipta can produce paradoxical bronchospasm which may be life-threatening. Hypersensitivity reactions such as anaphylaxis, angioedema, rash, and urticaria may occur after administration of Breo Ellipta. Vilanterol, the LABA in Breo Ellipta, can produce clinically significant cardiovascular effects in some patients as measured by increases in pulse rate, systolic or diastolic blood pressure, and also cardiac arrhythmias. Breo Ellipta should be used with caution in patients with cardiovascular disorders. Decreases in bone mineral density have been observed with long-term administration of products containing inhaled corticosteroids, as have glaucoma, increased intraocular pressure, and cataracts. Breo Ellipta should be used with caution in patients with convulsive disorders, thyrotoxicosis, diabetes mellitus, ketoacidosis, and in patients who are unusually responsive to sympathomimetic amines. Beta-adrenergic agonist medicines may produce significant hypokalemia in some patients. Beta-adrenergic agonist medicines may produce transient hyperglycemia in some patients. Orally inhaled corticosteroids may cause a reduction in growth velocity when administered in children and adolescents. For COPD, the most common adverse reactions (>3% and more common than in placebo) reported in two 6-month clinical trials with Breo Ellipta 100/25 (and placebo) were nasopharyngitis, 9% (8%); upper respiratory tract infection, 7% (3%); headache, 7% (5%); and oral candidiasis, 5% (2%). In addition to the reactions reported in the 6-month studies, adverse reactions occurring in >3% of the subjects treated with Breo Ellipta 100/25 in two 1-year studies included back pain, pneumonia, bronchitis, sinusitis, cough, oropharyngeal pain, arthralgia, influenza, pharyngitis, and pyrexia. For asthma, the most common adverse reactions in a 12-week trial (incidence >2% and more common than placebo) reported with Breo Ellipta 100/25 (and placebo) were nasopharyngitis 10% (7%), headache 5% (4%), oropharyngeal pain 2% (1%), oral candidiasis 2% (0%), and dysphonia 2% (0%). In a separate 12-week trial the most common adverse reactions (>2% incidence) reported with Breo Ellipta 100/25 or 200/25 were headache, nasopharyngitis, influenza, upper respiratory tract infection, oropharyngeal pain, sinusitis, bronchitis, and cough. In addition to adverse reactions reported in the 12 week studies, adverse reactions (>2% incidence) reported with Breo Ellipta 200/25 in a 24-week trial included viral respiratory tract infection, pharyngitis, pyrexia, and arthralgia, and with Breo Ellipta 100/25 or 200/25 in a 12-month trial included pyrexia, back pain, extrasystoles, upper abdominal pain, respiratory tract infection, allergic rhinitis, pharyngitis, rhinitis, arthralgia, supraventricular extrasystoles, ventricular extrasystoles, acute sinusitis, and pneumonia. ©2016 INNOVIVA

ANORO® ELLIPTA® Important Safety Information (U.S.) The following Important Safety Information (ISI) is based on the Highlights section of the Prescribing Information for Anoro Ellipta. Please consult the full Prescribing Information for all the labeled safety information for Anoro Ellipta. Long-acting beta2-adrenergic agonists (LABAs), such as vilanterol, one of the active ingredients in Anoro Ellipta, increase the risk of asthma-related death. A placebo-controlled trial with another LABA (salmeterol) showed an increase in asthma-related deaths in subjects receiving salmeterol. This finding with salmeterol is considered a class effect of all LABAs, including vilanterol. The safety and efficacy of Anoro Ellipta in patients with asthma have not been established. Anoro Ellipta is not indicated for the treatment of asthma. Anoro Ellipta is contraindicated in patients with severe hypersensitivity to milk proteins or who have demonstrated hypersensitivity to either umeclidinium, vilanterol, or any of the other ingredients. Anoro Ellipta should not be initiated in patients during rapidly deteriorating or potentially life-threatening episodes of COPD, or as rescue therapy for the treatment of acute episodes of bronchospasm, which should be treated with an inhaled, short-acting beta2-agonist. Anoro Ellipta should not be used more often than recommended, at higher doses than recommended, or in conjunction with additional medicine containing a LABA, as an overdose may result. Anoro Ellipta should be used with caution when considering coadministration with long-term ketoconazole and other known strong cytochrome P450 3A4 inhibitors because increased cardiovascular adverse effects may occur. As with other inhaled medicines, Anoro Ellipta can produce paradoxical bronchospasm, which may be life-threatening. Anoro Ellipta should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension. Anoro Ellipta should be used with caution in patients with convulsive disorders, thyrotoxicosis, diabetes mellitus, ketoacidosis, and in patients who are unusually responsive to sympathomimetic amines. Anoro Ellipta should be used with caution in patients with narrow-angle glaucoma. Instruct patients to contact a physician immediately should any signs or symptoms of narrow-angle glaucoma occur. Anoro Ellipta should be used with caution in patients with urinary retention, especially in patients with prostatic hyperplasia or bladder neck obstruction. Instruct patients to contact a physician immediately should any signs or symptoms of urinary retention occur. Beta-adrenergic agonist medicines may produce significant hypokalemia and transient hyperglycemia in some patients. The most common adverse reactions (incidence >1% and more common than placebo) reported in four 6-month clinical trials with Anoro Ellipta (and placebo) were pharyngitis, 2% (<1%); sinusitis 1% (<1%); lower respiratory tract infection, 1% (<1%); constipation, 1% (<1%); diarrhea, 2% (1%); pain in extremity 2% (1%); muscle spasms, 1% (<1%); neck pain, 1% (<1%); and chest pain 1% (<1%). In addition to the 6-month efficacy trials with Anoro Ellipta, a 12-month trial evaluated the safety of umeclidinium/vilanterol 125 mcg/25 mcg in subjects with COPD. Adverse reactions (incidence >1% and more common than placebo) in subjects receiving umeclidinium/vilanterol 125 mcg/25 mcg were: headache, back pain, sinusitis, cough, urinary tract infection, arthralgia, nausea, vertigo, abdominal pain, pleuritic pain, viral respiratory tract infection, toothache, and diabetes mellitus. Use of beta2-agonists, such as vilanterol should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors, tricyclic antidepressants, or drugs known to prolong the QTc interval or within 2 weeks of discontinuation of such agents, because the effect of adrenergic agonists on the cardiovascular system may be potentiated. Use beta-blockers with caution as they not only block the pulmonary effect of beta-agonists, such as vilanterol, but may produce severe bronchospasm in patients with COPD. Use with caution in patients taking non–potassium-sparing diuretics, as electrocardiographic changes and/or hypokalemia associated with non–potassium-sparing diuretics may worsen with concomitant beta-agonists. Avoid co-administration of Anoro Ellipta with other anticholinergic-containing drugs as this may lead to an increase in anticholinergic adverse effects such as cardiovascular effects, worsening of narrow-angle glaucoma, and worsening of urinary retention. ©2016 INNOVIVA
