Exhibit 99.1
Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI)
and FF over 12 weeks in patients with persistent asthma
Poster #407
Bernstein DI(1), Bateman ED(2), Woodcock A(3), Toler WT(4), Forth R(5), Jacques L(6), Nunn C(6), O’Byrne PM(7)
(1)College of Medicine, University of Cincinnati, Cincinnati, OH, USA; (2)Department of Medicine, University of Cape Town, Cape Town, South Africa; (3)Institute of Inflammation and Repair, University of Manchester, Manchester, UK; (4)Respiratory Medicines Development Center, GlaxoSmithKline, RTP, NC, USA; (5)Quantitative Sciences Division, GlaxoSmithKline, RTP, NC, USA; (6)Respiratory Medicines Development Centre, GlaxoSmithKline, London, UK; (7)Michael G DeGroote School of Medicine, Hamilton, ON, Canada
INTRODUCTION
· Treatment guidelines for persistent asthma in patients not controlled with inhaled corticosteroid (ICS) therapy alone recommend an ICS with a long-acting beta2 agonist (LABA),(1) leading to improved lung function and asthma symptom control compared with ICS monotherapy.(2)
· FF is an ICS in development as a once-daily (OD) monotherapy and available as a combination therapy with the LABA VI (FF/VI) for the treatment of COPD in the USA and for COPD and asthma in the EU and a number of other countries.(3),(4)
· FF/VI 200/25mcg OD significantly improves lung function and symptomatic endpoints compared with FF alone,(5) while numeric benefits have been reported for FF/VI 100/25mcg compared with FF 100mcg.(6)
OBJECTIVES
· To compare the efficacy and safety of OD FF/VI 100/25mcg with FF 100mcg in patients with moderate-to-severe, persistent bronchial asthma over 12 weeks.
· To assess the relative efficacy of FF/VI 100/25mcg with FF/VI 200/25mcg in these patients (descriptive comparison only).
METHODS
· Phase III, multicenter, randomized, double-blind, parallel-group study.
· Patient inclusion criteria: >12 years of age; FEV1 40–80% predicted; demonstrated >12% and >200mL reversibility to albuterol; receiving ICS for >12 weeks pre-study and stable with a mid-to-high dose ICS or mid-dose ICS/LABA for 4 weeks pre-study.
· Following a 4-week run-in without LABA, patients symptomatic or using rescue on >4 of the last 7 days of run-in were randomized 1:1:1 (stratified by baseline forced expiratory volume in one second [FEV1], <65% versus >65%) to FF 100mcg, FF/VI 100/25mcg or FF/VI 200/25mcg administered in the evening via the ELLIPTA™ dry powder inhaler OD for 12 weeks.
· Primary and secondary endpoints were analyzed using ANCOVA (covariates of baseline, region, sex, age and treatment) with a step-down statistical hierarchy for FF 100mcg versus FF/VI 100/25mcg.
RESULTS
· Of 2019 patients screened, 1039 were randomized and received at least one dose of study medication (intent-to-treat [ITT] population; Table 1), with 90% of patients completing the study.
Table 1. Baseline demographics (ITT population)
|
|
|
FF 100mcg |
|
FF/VI 100/25mcg |
|
FF/VI 200/25mcg |
|
|
|
|
N=347 |
|
N=346 |
|
N=346 |
|
|
Age, years |
|
44.7 (15.89) |
|
45.9 (16.14) |
|
46.6 (14.72) |
|
|
Female sex, n (%) |
|
199 (57) |
|
205 (59) |
|
224 (65) |
|
|
Pre-bronchodilator FEV1, L |
|
1.965 (0.5980)(a) |
|
1.985 (0.5563)(a) |
|
1.954 (0.5819)(b) |
|
|
Percent predicted FEV1 |
|
61.13 (10.348)(a) |
|
62.64 (10.148)(a) |
|
62.12 (10.050)(b) |
|
|
Percent reversibility |
|
30.79 (19.153)(c) |
|
29.10 (16.537)(a) |
|
29.33 (15.701)(b) |
|
|
Rescue-free 24-h periods, % |
|
4.4 (12.07) |
|
4.4 (12.58) |
|
5.8 (16.00) |
|
|
Symptom-free 24-h periods, % |
|
4.8 (14.63) |
|
3.8 (14.38) |
|
4.6 (15.44) |
|
Values are mean (SD) unless otherwise stated. (a)n=342; (b)n=344; (c)n=346
Efficacy endpoints
· There were statistically significant improvements with FF/VI 100/25mcg versus FF 100mcg for the primary endpoint of weighted mean 0–24h FEV1 (Figs. 1–2) and the secondary and ‘other’ endpoints, except for AQLQ score (Fig. 1).
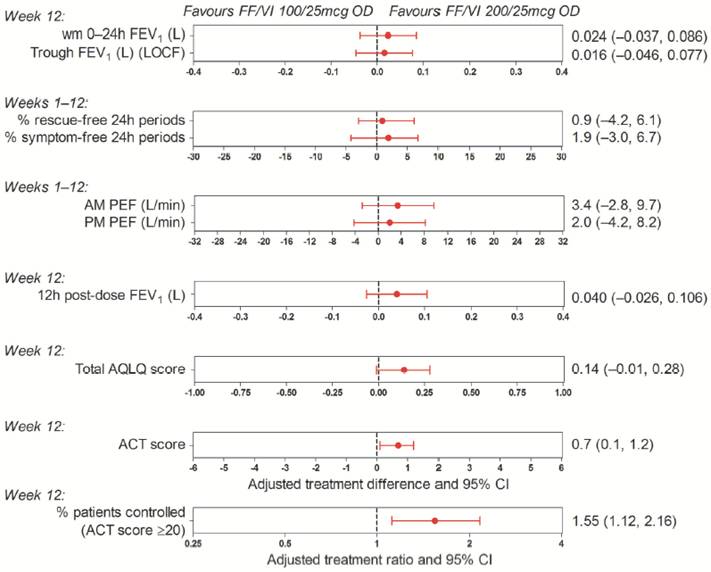
· There were numerical improvements for all efficacy endpoints with FF/VI 200/25mcg versus FF/VI 100/25mcg (Fig. 3).
Figure 1. Adjusted treatment differences and ratios for efficacy
endpoints: FF/VI 100/25mcg versus FF 100mcg (ITT population)
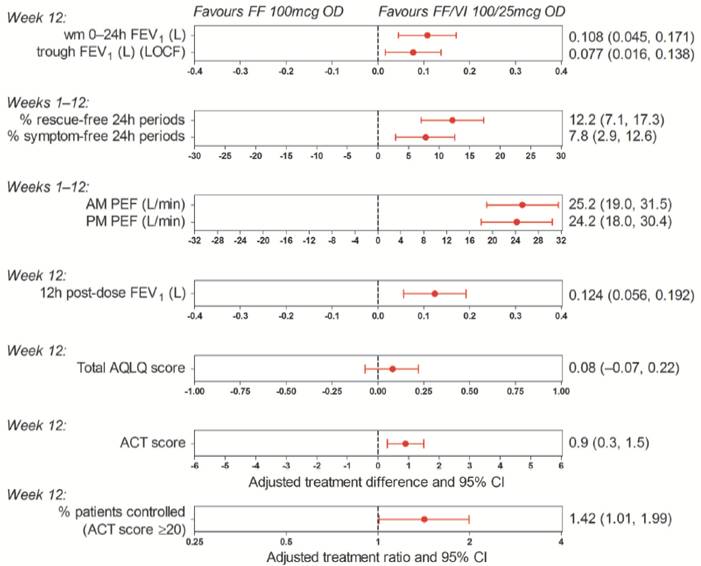
ACT = Asthma Control Test™; AQLQ = Asthma Quality of Life Questionnaire; CI = confidence interval; PEF = peak expiratory flow; wm = weighted mean
Figure 2. Adjusted mean change from baseline of individual
serial FEV1 assessments at Week 12 (ITT population)
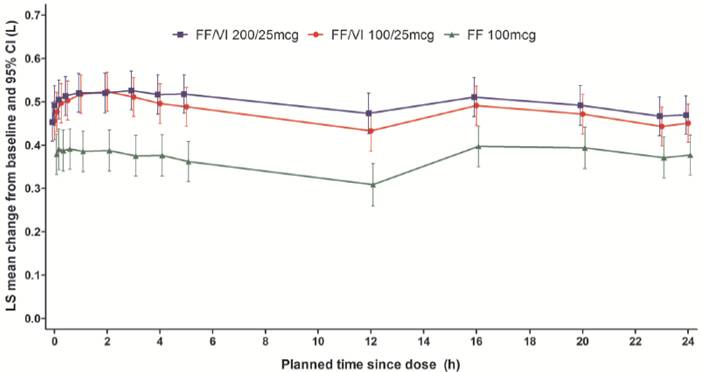
LS = least squares
Figure 3. Adjusted treatment differences and ratios for efficacy
endpoints: FF/VI 200/25mcg versus 100/25mcg (ITT population)
Safety (Table 2)
· Incidence of AEs and serious AEs (SAEs) were similar across treatments; there were no post-treatment SAEs and no fatal AEs.
Table 2. Summary of AE incidence and most commonly
reported on-treatment AEs (ITT population)
|
|
|
FF |
|
FF/VI |
|
FF/VI |
|
|
|
|
100mcg |
|
100/25mcg |
|
200/25mcg |
|
|
n (%) |
|
N=347 |
|
N=346 |
|
N=346 |
|
|
AEs |
|
|
|
|
|
|
|
|
Any on-treatment |
|
127 (37) |
|
127 (37) |
|
123 (36) |
|
|
Drug-related(a),(b) |
|
11 (3) |
|
7 (2) |
|
8 (2) |
|
|
Leading to permanent discontinuation or withdrawal(b) |
|
4 (1) |
|
3 (1) |
|
3 (<1) |
|
|
Serious AEs |
|
|
|
|
|
|
|
|
Any on-treatment |
|
3 (<1)(c) |
|
4 (1)(d) |
|
1 (<1)(e) |
|
|
Drug-related(a),(b) |
|
1 (<1) |
|
0 |
|
0 |
|
|
Most common (>3%) on-treatment AEs in any treatment group |
|
|
|
|
|
|
|
|
Headache |
|
32 (9) |
|
29 (8) |
|
29 (8) |
|
|
Nasopharyngitis |
|
26 (7) |
|
22 (6) |
|
25 (7) |
|
|
Upper respiratory tract infection |
|
12 (3) |
|
8 (2) |
|
7 (2) |
|
|
Influenza |
|
4 (1) |
|
10 (3) |
|
9 (3) |
|
(a)investigator’s judgement of causality; (b)includes on-treatment and post-treatment AEs;
(c)2 pneumonia, 1 borderline mucinous ovarian tumour; (d)biliary colic, acute pancreatitis, thermal burn, occipital neuralgia; (e)abortion threatened
CONCLUSIONS
· There were statistically significant improvements in lung function and symptoms with FF/VI 100/25mcg versus FF 100mcg after 12 weeks of treatment in patients with moderate-to-severe persistent bronchial asthma.
· Numerical improvements were seen for FF/VI 200/25mcg versus FF/VI 100/25mcg across all endpoints with patients on FF/VI 200/25mcg being 55% more likely to be well controlled than those on FF/VI 100/25mcg.
· No new safety concerns were identified for any of the study treatments.
REFERENCES
(1) Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention 2012. http://www.ginasthma.com/Guidelines/guidelines-resources.html (accessed 3/20/2014).
(2) Ducharme FM, et al. Cochrane Database Syst Rev 2010;10:CD005533.
(3) Breo Ellipta Medication Guide. Food and Drug Administration. www.fda.gov/downloads/drugs/drugsafety/ucm352347.pdf (accessed 4/08/2014).
(4) EPAR Summary For The Public. European Medicines Agency. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/ 002673/WC500157636.pdf (accessed 4/08/2014).
(5) O’Byrne PM, et al. Eur Respir J 2014;43:773–82.
(6) Bleecker ER, et al. J Allergy Clin Immunol Pract 2014; ePub ahead of print (25 April 2014).
ACKNOWLEDGEMENTS
· The presenting author, WT Toler, is an employee of and holds stock in GlaxoSmithKline.
· This study was funded by GlaxoSmithKline (HZA116863, NCT01686633).
· Editorial support (in the form of writing assistance, assembling tables and figures, collating author comments, grammatical editing and referencing) was provided by David Cutler, PhD at Gardiner-Caldwell Communications (Macclesfield, UK) and was funded by GlaxoSmithKline.
ELLIPTA™ is a trade mark of GlaxoSmithKline


Presented at the American Thoracic Society Annual Congress, San Diego, CA, USA, 16–21 May 2014