UNITED STATES SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2012
OR
| £ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ________________ to ________________
Commission file number: 001-15643

SKYSTAR BIO-PHARMACEUTICAL COMPANY
(Exact name of registrant as specified in its charter)
| Nevada | 33-0901534 | |
| (State or other jurisdiction of incorporation or | (I.R.S. Employer Identification No.) | |
| organization) | ||
| 4/F, Building B, Chuangye Square, No. 48 Keji Rd | ||
| Gaoxin District, Xi’an, Shaanxi Province, P.R. | ||
| China | N/A | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number: (8629) 8819-3188
Securities registered pursuant to Section 12(b) of the Act:
| Common Stock $0.001 Par Value | NASDAQ Capital Market | |
| (Title of Each Class) | (Name of Each Exchange on Which Registered ) |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period than the registrant was required to submit and post such files). Yes þ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained herein, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ¨ | Accelerated filer ¨ | |
| Non-accelerated filer ¨ | Smaller reporting company þ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No þ
As of June 29, 2012, the last day of the registrant’s most recently completed second fiscal quarter, the aggregate market value of the voting stock held by non-affiliates of the registrant was approximately $13.3 million based on a closing price of $2.20 per share of common stock as reported on the NASDAQ Stock Market on such date.
On March 27, 2013, we had 7,604,800 shares of common stock issued and outstanding.
TABLE OF CONTENTS
TO ANNUAL REPORT ON FORM 10-K
FOR YEAR ENDED DECEMBER 31, 2012
| Page | ||||
| CAUTION REGARDING FORWARD-LOOKING INFORMATION | ||||
| PART I | ||||
| Item 1. | Business | 2 | ||
| Item 1A. | Risk Factors | 10 | ||
| Item 1B. | Unresolved Staff Comments | 19 | ||
| Item 2. | Properties | 19 | ||
| Item 3. | Legal Proceedings | 20 | ||
| Item 4. | Mine Safety Disclosures | 21 | ||
| PART II | ||||
| Item 5. | Market Price for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 21 | ||
| Item 7. | Management’s Discussion and Analysis of Financial Conditions and Results of Operations | 22 | ||
| Item 8. | Financial Statements | 28 | ||
| Item 9. | Changes in and Disagreements With Accountants on Accounting and Financial Disclosure | 28 | ||
| Item 9A. | Controls and Procedures | 29 | ||
| Item 9B. | Other Information | 30 | ||
| PART III | ||||
| Item 10. | Directors, Executive Officers and Corporate Governance | 31 | ||
| Item 11. | Executive Compensation | 33 | ||
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 36 | ||
| Item 13. | Certain Relationships and Related Transactions, and Director Independence | 38 | ||
| Item 14. | Principal Accountant Fees and Services | 39 | ||
| PART IV | ||||
| Item 15. | Exhibits and Financial Statement Schedules | 40 | ||
| SIGNATURES | 44 | |||
| 1 |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING INFORMATION
This Report contains forward-looking statements. All forward-looking statements are inherently uncertain as they are based on current expectations and assumptions concerning future events or future performance of the Company. Readers are cautioned not to place undue reliance on these forward-looking statements, which are only predictions and speak only as of the date hereof. Forward-looking statements usually contain the words “estimate,” “anticipate,” “believe,” “expect,” or similar expressions, and are subject to numerous known and unknown risks and uncertainties. In evaluating such statements, prospective investors should carefully review various risks and uncertainties identified in this Annual Report on Form 10-K, including the matters set forth under the captions “Risk Factors” and in our other SEC filings. These risks and uncertainties could cause our actual results to differ materially from those indicated in the forward-looking statements. We undertake no obligation to update or publicly announce revisions to any forward-looking statements to reflect future events or developments.
Although forward-looking statements in this Annual Report on Form 10-K reflect the good faith judgment of our management, such statements can only be based on facts and factors currently known by us. Consequently, forward-looking statements are inherently subject to risks and uncertainties, and actual results and outcomes may differ materially from the results and outcomes discussed in or anticipated by the forward-looking statements. Factors that could cause or contribute to such differences in results and outcomes include, without limitation, the matters described in this report generally. Readers are urged not to place undue reliance on these forward-looking statements, which speak only as of the date of this report.
We file reports with the Securities and Exchange Commission (SEC or Commission). We make available on our website free of charge our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports as soon as reasonably practicable after we electronically file such materials with or furnish them to the SEC. Information appearing at our website is not a part of this Annual Report on Form 10-K. You can also read and copy any materials we file with the Commission at its Public Reference Room at 100 F Street, NE, Washington, DC 20549. You can obtain additional information about the operation of the Public Reference Room by calling the Commission at 1-800-SEC-0330. In addition, the Commission maintains an Internet site (www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the Commission.
Our fiscal year begins on January 1, and ends on December 31, and any references herein to "Fiscal 2012" mean the year ended December 31, 2012, and references to other "Fiscal" years mean the year ending December 31, of the year indicated.
We obtained statistical data, market data and other industry data and forecasts used in this Form 10-K from publicly available information. While we believe that the statistical data, industry data, forecasts and market research are reliable, we have not independently verified the data, and we do not make any representation as to the accuracy of that information.
When used in this Annual Report, the terms the “Company,” “Skystar,” “we,” “us,” “our,” and similar terms refer to Skystar Bio-Pharmaceutical Company, a Nevada corporation, and our subsidiaries and variable interest entity.
PART I
ITEM 1. BUSINESS
Overview and Corporate History
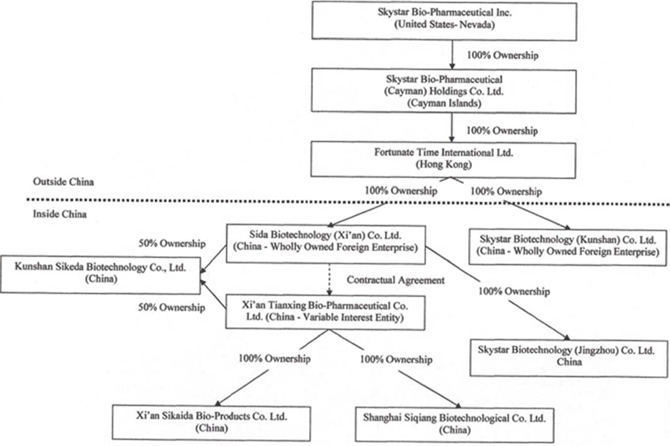
Skystar Bio-Pharmaceutical Company (“Skystar” or the “Company”) was incorporated in Nevada on September 24, 1998. Since its acquisition on November 7, 2005 of Skystar Bio-Pharmaceutical (Cayman) Holdings Co., Ltd. (“Skystar Cayman”), a Cayman Islands company, the Company has been engaged in the research, development, production, marketing, and sales of veterinary healthcare and medical care products. All current operations of the Company are in the People’s Republic of China (“China” or the “PRC”).
All of the Company’s operations are carried out by our subsidiaries in China and Xi’an Tianxing Bio-Pharmaceutical Co., Limited (“Xi’an Tianxing”), a PRC joint stock company that the Company controls through contractual arrangements originally between Skystar Cayman and Xi’an Tianxing. On March 10, 2008, the Company entered into a series of agreements transferring all of the rights and obligations of Skystar Cayman under the contractual arrangements to Sida Biotechnology (Xi’an) Co., Ltd. (“Sida”), a PRC company. Sida is the wholly owned subsidiary of Fortunate Time International Limited (“Fortunate Time”), a Hong Kong company and wholly owned subsidiary of Skystar Cayman. Xi’an Tianxing also has a wholly owned subsidiary, Shanghai Siqiang Biotechnological Co., Ltd. (“Shanghai Siqiang”), a PRC company.
On September 18, 2009, Skystar Bio-Pharmaceutical Inc. (“Skystar California”) was incorporated in California and became a wholly owned subsidiary of Skystar. On December 20, 2010, we dissolved Skystar California.
On April 21, 2010, Kunshan Sikeda Biotechnology Co., Ltd. (“Kunshan Sikeda”) was incorporated in Kunshan, Jiangsu Province, China with a registered capital of $73,970 (RMB 500,000), of which Xi’an Tianxing and Sida each contributed $36,985 (RMB 250,000). Kunshan Sikeda is jointly owned by Xi’an Tianxing and Sida.
| 2 |
On May 7, 2010, Fortunate Time formed Skystar Biotechnology (Kunshan) Co., Limited (“Skystar Kunshan”) in Kunshan, Jiangsu Province, China with a registered capital of $15,000,000, of which $2,250,000 was paid by Fortunate Time in cash, and of which the remaining $12,750,000 is required to be invested in the future. On July 25, 2012, the Company received the approval of extension for payment of the remaining registered capital but a new due date has not been specified by the government. We intend to apply for another extension in 2013 with the government to invest the remaining registered capital or request the government to reduce the total registered capital for this facility. Kunshan was formed in connection with an acquisition of assets to meet part of the registered capital requirements, and was intended to be a micro-organism manufacturing facility for the Company once the acquisition was complete. The asset acquisition was completed in September 2011, and the Company is also in the process of transferring the assets acquired to meet part of the registered capital requirements.
On August 11, 2010, Sida became the parent company of Skystar Biotechnology (Jingzhou) Co., Limited (“Skystar Jingzhou”), a company established in Jingzhou, Hubei Province, China on February 5, 2010, with a registered capital of approximately $4.1 million (RMB 26,000,000), of which approximately $3.7 million (RMB 23,480,000) was paid. On July 5, 2012, Sida paid up the remaining capital of $0.4 million (RMB 2,520,000).
On March 15, 2011, Xi’an Tianxing formed Xi’an Sikaida Bio-products Co., Ltd. (“Xi’an Sikaida”) with registered capital of approximately $1,587,000 (RMB 10,000,000) paid by Xi’an Tianxing.
Hereinafter, Skystar, Skystar Cayman, Fortunate Time, Sida, Xi’an Tianxing, Skystar Kunshan, Kunshan Sikeda, Skystar Jingzhou, Shanghai Siqiang, and Xi’an Sikaida are collectively referred to as the “Company.”
Our current organizational structure is as follows (the percentages depict the current equity interests):
Nasdaq Listing Compliance Matters
On January 12, 2012, we received staff determination from the Nasdaq Stock Market (“Exchange”) indicating that since we did not hold our 2010 annual shareholder meeting by December 31, 2011, we were not in compliance with Nasdaq Listing Rule 5620(a) and (b) relating to the time frame of and proxy solicitation in connection with annual shareholder meetings and, therefore, the Exchange staff determined to initiate proceedings to delist our securities from Nasdaq at the open of business on January 23, 2012. On March 26, 2012, following our presentation at an oral hearing before the Nasdaq Listing Qualifications Panel, we received a letter informing the Company that the Panel had granted our request to remain listed on the Exchange, subject to the condition that by April 28, 2012, we provided evidence that we held our 2011Annual Meeting of Shareholders. On April 27, 2012, we held our 2011 Annual Meeting of Shareholders, at which our shareholders elected all members of our Board and ratified the engagement of Crowe Horwath LLP as our independent registered public accounting firm. On May 1, 2012, the Exchange confirmed that we had met the requirements of the Panel’s decision dated March 26, 2012, and was in compliance with all other applicable requirements for continued listing on Nasdaq. The matter is now closed.
| 3 |
Our Products
We have four major product lines:
| 1. | Our micro-organism products line includes over 16 products and accounted for 45.7% of our revenue in 2012. |
| 2. | Our veterinary medicine line for poultry and livestock includes over 233 products and accounted for 31.7% of our revenue in 2012. |
| 3. | Our feed additives line includes over 16 products and accounted for 12.0% of our revenue in 2012. |
| 4. | Our bio-pharmaceutical veterinary vaccine line includes over 10 products and accounted for 10.6% of our revenue in 2012. |
| 4 |
Industry and Market Overview
China has one of the largest economic animal husbandry industries in the world. According to the Central Government’s 12th 5-year (2011-2015) national livestock development plan, by 2015, the proportion of China's animal husbandry output value will account for 36% of the total output value of agriculture, forestry, animal husbandry and fisheries as compared to 30% in 2010. With continued development of the livestock sector of the national economy, the veterinary medicines, feed additives and veterinary vaccine industry is expected to maintain a sustained and rapid development for the years to come.
Since 2000, with the steady development of animal husbandry in China, China's veterinary medicine industry has entered a fast-growing period. Especially since 2005, annual output value of veterinary medicines grew at a compound annual growth rate of 15%. In recent years, the veterinary medicine industry has been strongly supported by the national industrial policy. The government particularly encourages the development of new green veterinary medicines. In September 2011, the Ministry of Agriculture issued its 12th 5-year feed industry development plan. According to the plan, China's feed production in 2015 is expected to reach 200 million tons; the quality pass rate of feed products will be more than 95%; and the quality and safety of feed products will be further improved. In January 2012, the Ministry of Agriculture and Ministry of Finance developed a national animal disease compulsory vaccination plan, which requires compulsory immunization for certain kinds of animal diseases. In the same month, the State Council issued the modern agriculture development plan (2011-2015). During the 12th 5-year’s period, the state will continue to the increase animal compulsory vaccination subsidy level.
At present, China has become the second largest market after the United States for consumption of animal health products and China’s animal health products industry is expected to continue to maintain sustained growth in the future. However, the growth of China's animal health products industry is largely attached to the development of China's animal husbandry industry. Although the overall trend of development of animal husbandry in China is progressive and promising, there were some unstable periods during recent years due to the impact of food prices, labor costs, land supply, disease hazards, distribution cost, safety of animal products and many other factors and this phenomenon could continue to exist for a long period of time.
Distribution Methods of Our Products and Our Customers
As of December 31, 2012, we had over 3,964 customers in 29 provinces in China, including 2,859 distributors and 1,105 direct customers. Of the 2,859 distributors, 360 are physical stores that have outer signage with our logo and sell products from our four product lines exclusively that are known as “franchise distributors.”
We recognize the importance of branding as well as packaging. All of our products have uniform branding while being specifically designed to also differentiate our four product lines.
We conduct promotional marketing activities within the provinces we operate to publicize and enhance our image as well as to reinforce the recognition of our brand name, including:
| • | sending sales team to rural and urban areas to promote awareness of poultry and livestock conventional disease, educate farmers about prevention and treatment of diseases, and provide demonstrations in the field to them to promote our products; |
| •· | participating in various meetings, seminars, symposiums, exhibitions for bio-pharmaceutical and other related industries; |
| • | organizing cooperative promotional activities with distributors; and |
| • | sending direct mailing to major customers. |
None of our customers accounted for 10% or more of our total revenues in 2012.
Competition
Before the implementation of Good Manufacturing Practices (GMP) Standard in 2005, there were more than 2,600 veterinary drug companies in China. At present, there are approximately 2000 GMP certified veterinary drug manufacturers in China, of which the majority are privately held small businesses with annual output less than $8 million (RMB 50 million) each. We are one of the leading providers and distributors of veterinary healthcare and medical care products in China. In general, we have two major competitors in China – Qilu Animal Health Production Co., Ltd. and China Animal Husbandry Industry Co. Ltd . Each of them has more assets and a larger market share. Individually, Qilu Animal Health Production Co., Ltd. China Animal Husbandry Industry Co. Ltd and Guangdong VTR Bio-Tech Co., Ltd are our primary competitors in the sectors of micro-organism and feed additives products. Qilu Animal Health Production Co., Ltd. and China Animal Husbandry Industry Co. Ltd. are our primary competitors in the sector of veterinary medicine products. Xinjiang Tecon Animal Husbandry Bio-technology Co., Ltd. and Inner Mongolia Jinyu Group Stock Company are our primary competitors in the sector of veterinary vaccine products. We believe we are able to compete with these competitors because of our quick response to market demand, a full range of product offerings, quality customer service, and competitive prices. Other than these named competitors, most of our other competitors are privately held and vary greatly in terms of scale of operations.
| 5 |
Sources and Availability of Raw Materials and Our Principal Suppliers
Xi’an Zhongsen Pharmaceuticals., Ltd, Xi’an Yanghua Chemical Co., Ltd., Xi’an Fandike Chemical Technology Co., Ltd., Xi’an Nanchen Trading Co., Ltd., and Xi’an Chenyue Trading Co., collectively supplied over 85% of the raw materials we used to manufacture our products in 2012. We design, create prototypes for, and manufacture our products at our facilities located at Xi’an city, Shaanxi Province, China and Jingzhou, Hubei Province, China. Our principal raw materials include various chemical compounds including praziquantel, dexamethasone sodium phosphate, stachyose, zeolite, and yeast. We also use Chinese herbs such as Huanglian (Chinese Goldthread) as raw materials, which are supplied to us by Xi'an Wan Shou Bei Lu Longkui Chinese Medicine Store. Our raw materials generally are not scarce and are readily available from a wide range of sources. Accordingly, we do not have any continuing or long-term supply agreements with any of these suppliers, and purchase our raw materials from them on a per purchase order basis. The prices for these raw materials are nevertheless subject to market forces largely beyond our control, including energy costs, organic chemical feedstock, market demand, and freight costs. The prices for these raw materials have varied significantly in the past and may vary significantly in the future.
As a result of our research and development efforts in 2007 in cooperation with research institutes including Shaanxi Microbial Research Institute, Jiangsu Microbial Research Institute, China Northwestern University, and China Northwest A&F University, we now also internally produce microbial strains, which are key components of our micro-organism products. Our ability to produce microbial strains has translated into a significant cost reduction for these raw materials.
Intellectual Property and Licenses
We rely on a combination of trademark, copyright and trade secret protection laws in China and other jurisdictions, as well as confidentiality procedures and contractual provisions to protect our intellectual property and our brand.
We intend to seek other licenses or apply for exclusivity as necessary in order to protect our rights, and we also enter into confidentiality, non-compete and invention assignment agreements with our employees and consultants and nondisclosure agreements with third parties. Skywing, Stardove and the Chinese characters that transliterate as “Jia Teng Jun,” “Hao Shou Yi,” “Lan Yuan Kang”, “Bao Li Jian”, “Bi Ke Ting” are our registered trademarks in the PRC.
Bio-pharmaceutical companies are at times involved in litigation based on allegations of infringement or other violations of intellectual property rights. Furthermore, the application of laws governing intellectual property rights in China and abroad is uncertain and evolving and could involve substantial risks to us.
Approved Drugs and Veterinary Products – Xi’an Tianxing
Xi’an Tianxing is approved by the Chinese Ministry of Agriculture to manufacture and distribute veterinary drugs. Such approvals certify Xi’an Tianxing’s products as conforming to government-mandated standards. The approvals are issued for a period of 5 years and may be renewed 6 months prior to their expiration date. The veterinary drugs and their approval numbers are listed below:
| Veterinary Drug Products | Approval Number | |
| Diclazuril Premix (0.2%) | Veterinary Drug (2007) 270261140 | |
| Metamizole Sodium Injection | Veterinary Drug (2007) 270261152 | |
| Antondine Injection | Veterinary Drug (2007) 270261160 | |
| Mequindox Injection (10ml:0.5g) | Veterinary Drug (2007) 270264645 | |
| Praziquantel Tablets | Veterinary Drug (2007) 270261174 | |
| Amoxicillin Soluble Powder | Veterinary Drug (2007) 270261199 | |
| Kanamycin Sulfate Injection | Veterinary Drug (2007) 270261211 | |
| Salinomycin Sodium Premix | Veterinary Drug (2007) 270261379 | |
| Vitamin B1 Injection | Veterinary Drug (2007) 270261389 | |
| Erythromycin Thiocyanate Soluble Powder | Veterinary Drug (2007) 270261492 | |
| Gentamycin Sulfate Injection | Veterinary Drug (2007) 270261507 | |
| Compound Sulfamethoxydiazine Sodium Injection | Veterinary Drug (2007) 270261608 | |
| Compound Sulfamethoxazole Tablets | Veterinary Drug (2007) 270261612 | |
| Sulfamonomethoxine Sodium Injection | Veterinary Drug (2007) 270261616 | |
| Sulfadiazine Sodium Injection | Veterinary Drug (2007) 270261634 | |
| Pefloxacin Mesylate Granules | Veterinary Drug (2007) 270262042 | |
| Avermectin Powder | Veterinary Drug (2007) 270262066 | |
| Compound Amoxicillin Powder | Veterinary Drug (2007) 270262092 | |
| Florfenicol Powder | Veterinary Drug (2007) 270262110 | |
| Ofloxacin Tablets | Veterinary Drug (2007) 270262123 | |
| Ofloxacin Soluble Powder | Veterinary Drug (2007) 270262124 | |
| Ofloxacin Injection | Veterinary Drug (2007) 270262126 | |
| Ciprofloxacin Hydrochloride Soluble Powder | Veterinary Drug (2007) 270262159 | |
| Ciprofloxacin Hydrochloride Injection | Veterinary Drug (2007) 270262160 | |
| Enrofloxacin Injection | Veterinary Drug (2007) 270262518 | |
| Diclazuril Premix (5%) | Veterinary Drug (2007) 270262528 |
| 6 |
| Dexamethasone Sodium Phosphate Injection | Veterinary Drug (2007) 270262530 | |
| Norfloxacin Nicotinate Injection | Veterinary Drug (2007) 270262593 | |
| Spectinomycin Hydrochloride and Lincomycin Hydrochloride Soluble Powder | Veterinary Drug (2007) 270262602 | |
| Lincomycin Hydrochloride Injection (10ml:0.3g) | Veterinary Drug (2007) 270262614 | |
| Lincomycin Hydrochloride Injection (10ml:1.5g) | Veterinary Drug (2007) 270262616 | |
| Thiamphenicol Powder | Veterinary Drug (2007) 270262722 | |
| Apramycin Sulfate Soluble Powder | Veterinary Drug (2007) 270262745 | |
| Gentamycin Micronomicin Sulfate Injection (10 ml:100,000 parts) | Veterinary Drug (2007) 270262751 | |
| Gentamycin Micronomicin Sulfate Injection (10ml: 200,000 parts) | Veterinary Drug (2007) 270262752 | |
| Neomycin Sulfate Soluble Powder | Veterinary Drug (2007) 270262755 | |
| Colistin Sulfate Soluble Powder | Veterinary Drug (2007) 270262758 | |
| Vitamin C Injection | Veterinary Drug (2007) 270262795 | |
| Compoumd Vitamin B Injection | Veterinary Drug (2007) 270264572 | |
| Mequindox Injection (10ml:0.2g) | Veterinary Drug (2007) 270264644 | |
| Gongying San | Veterinary Drug (2007) 270265028 | |
| Mubin Xiaohuang San | Veterinary Drug (2007) 270265035 | |
| Zhili San | Veterinary Drug (2007) 270265037 | |
| Baitouweng San | Veterinary Drug (2007) 270265053 | |
| Fuzheng Jiedu San | Veterinary Drug (2007) 270265076 | |
| Quchong San | Veterinary Drug (2007) 270265089 | |
| Feizhucai | Veterinary Drug (2007) 270265100 | |
| Yujin San | Veterinary Drug (2007) 270265102 | |
| Baotai Wuyou San | Veterinary Drug (2007) 270265111 | |
| Jingfang Baidu San | Veterinary Drug (2007) 270265127 | |
| Jianji San | Veterinary Drug (2007) 270265133 | |
| Jianwei San | Veterinary Drug (2007) 270265134 | |
| Xiaoji San | Veterinary Drug (2007) 270265146 | |
| Yimu Shenghua San | Veterinary Drug (2007) 270265148 | |
| Tongru San | Veterinary Drug (2007) 270265156 | |
| Qingfei Zhike San | Veterinary Drug (2007) 270265157 | |
| Qingshu San | Veterinary Drug (2007) 270265162 | |
| Qingwen Baidu San | Veterinary Drug (2007) 270265165 | |
| Danjibao | Veterinary Drug (2007) 270265171 | |
| Huanglian Jiedu San | Veterinary Drug (2007) 270265178 | |
| Houyanjing San | Veterinary Drug (2007) 270265179 | |
| Chulijing | Veterinary Drug (2007) 270265192 | |
| Fenbendazole Powder | Veterinary Drug (2008) 270261189 | |
| Enrofloxacin Injection (10ml:250mg) | Veterinary Drug (2008) 270261295 | |
| Gentamycin Sulfate Injection (10ml:0.2g) | Veterinary Drug (2008) 270261506 | |
| Sulfaquinoxaline Sodium Soluble Powder (10%) | Veterinary Drug (2008) 270261624 | |
| Sulfathiazole Sodium Injection | Veterinary Drug (2008) 270261645 | |
| Danofloxacin Mesylate Injection | Veterinary Drug (2008) 270262033 | |
| Danofloxacin Mesylate Powder | Veterinary Drug (2008) 270262036 | |
| Pefloxacin Mesylate Soluble Powder | Veterinary Drug (2008) 270262040 | |
| Kitasamycin Premix | Veterinary Drug (2008) 270262043 | |
| Ciprofloxacin Lactate Soluble Powder | Veterinary Drug (2008) 270262073 | |
| Lomefloxacin Hydrochloride Soluble Powder | Veterinary Drug (2008) 270262166 | |
| Lomefloxacin Hydrochloride Injection | Veterinary Drug (2008) 270262169 | |
| Norfloxacin Nicotinic Soluble Powder | Veterinary Drug (2008) 270262178 | |
| Florfenicol Injection | Veterinary Drug (2008) 270262546 | |
| Sulfaquinoxaline Sodium Soluble Powder (5%) | Veterinary Drug (2008) 270262580 | |
| Lincomycin Hydrochloride Soluble Powder | Veterinary Drug (2008) 270262620 | |
| Pefloxacin Mesylate Injection | Veterinary Drug (2008) 270262665 | |
| Sulfachloropyrazin Sodium Soluble Powder | Veterinary Drug (2008) 270262703 | |
| Tylosin Tartrate Soluble Powder | Veterinary Drug (2008) 270262731 | |
| Berberine Sulfate Injection | Veterinary Drug (2008) 270264595 | |
| Xiaochaihu San | Veterinary Drug (2008) 270265018 | |
| Shengru San | Veterinary Drug (2008) 270265051 | |
| Bailong San | Veterinary Drug (2008) 270265055 | |
| Longdan Xiegan San | Veterinary Drug (2008) 270265057 | |
| Qumai San | Veterinary Drug (2008) 270265067 | |
| Fangji San | Veterinary Drug (2008) 270265072 | |
| Buzhong Yiqi San | Veterinary Drug (2008) 270265082 | |
| Shenling Baishu San | Veterinary Drug (2008) 270265093 | |
| Xiaoshi Pingwei San | Veterinary Drug (2008) 270265145 |
| 7 |
| Yinqiao San | Veterinary Drug (2008) 270265172 | |
| Maxing Shigan San | Veterinary Drug (2008) 270265174 | |
| Cuiqing San | Veterinary Drug (2008) 270265188 | |
| HuoXi’ang Zhengqi San | Veterinary Drug (2008) 270265200 | |
| Qibu San | Veterinary Drug (2008) 270265220 | |
| Ivermectin Premix | Veterinary Drug (2009) 270263059 | |
| Feizhu San | Veterinary Drug (2009) 270265101 | |
| Tilmicosin Premix | Veterinary Drug (2010) 270262263 | |
| Promethazine Hydrochloride Injection (10ml: 0.25g) | Veterinary Drug (2009) 270261360 | |
| Ciprofloxacin hydrochloride soluble powder (5%) | Veterinary Drug (2010) 270262605 | |
| Pefloxacin mesylate soluble powder (10%) | Veterinary Drug (2010) 270262727 | |
| Ivica bacteria injection (10ml: 10 million units) | Veterinary Drug (2010) 270262646 | |
| Ivica bacteria injection (5ml: 0.05g) | Veterinary Drug (2010) 270261126 | |
| Erythromycin thiocyanate soluble powder (2.5%) | Veterinary Drug (2010) 270262740 | |
| The sulfa chloropyrazine sodium soluble powder (30%) | Veterinary Drug (2010) 270261628 | |
| Ciprofloxacin Lactate Soluble Powder (5%) | Veterinary Drug (2010) 270262772 | |
| Alexander Huang at the end | Veterinary Drug (2010) 270265019 | |
| Stimulated egg powder | Veterinary Drug (2010) 270265197 | |
| Wulingsan | Veterinary Drug (2010) 270265026 | |
| Iron dextran injection (10ml: 0.5g) | Veterinary Drug (2010) 270261064 | |
| Iron dextran injection (10ml: 1g) | Veterinary Drug (2010) 270261065 |
Approved Drugs and Veterinary Products - Skystar Jingzhou
Skystar Jingzhou is approved by the Chinese Ministry of Agriculture to manufacture and distribute veterinary drugs. Such approvals certify Skystar Jingzhou’s products as conforming to government-mandated standards. The approvals are issued for a period of 5 years and may be renewed 6 months prior to their expiration date. The veterinary drugs and their approval numbers are listed below:
| Veterinary Drug Products | Approval Number | |
| Povidone-iodine solution | Veterinary Drug (2012)170141575 | |
| Povidone-iodine solution | Veterinary Drug (2012)170141577 | |
| Sulfaquinoxaline sodium soluble powder | Veterinary Drug (2012)170141624 | |
| The sulfa chloropyrazine sodium soluble powder | Veterinary Drug (2012)170141628 | |
| Ciprofloxacin hydrochloride soluble powder | Veterinary Drug (2012)170142159 | |
| Enrofloxacin solution | Veterinary Drug (2012)170141297 | |
| Jing anti-sepsis scattered | Veterinary Drug (2012)170145127 | |
| The multi flavor stomach scattered | Veterinary Drug (2012)170145063 | |
| Aphrodisiac scattered | Veterinary Drug (2012)170145188 | |
| Qingwenbaiduyin scattered | Veterinary Drug (2012)170145165 | |
| Pulsatilla scattered | Veterinary Drug (2012)170145053 | |
| Albendazole tablets | Veterinary Drug (2012)170141194 | |
| Concentrated glutaraldehyde solution | Veterinary Drug (2012)170149073 | |
| Enrofloxacin powder | Veterinary Drug (2012)170149107 | |
| Norfloxacin powder | Veterinary Drug (2012)170149096 | |
| Florfenicol powder | Veterinary Drug (2012)170149014 | |
| Florfenicol powder | Veterinary Drug (2012)170142538 | |
| Vitamin B complex powder | Veterinary Drug (2012)170146071 | |
| Fuzhengjiedu scattered | Veterinary Drug (2012)170145076 | |
| Huanglianjiedu scattered | Veterinary Drug (2012)170145178 | |
| Deworming scattered | Veterinary Drug (2012)170145089 | |
| Heat dispersion | Veterinary Drug (2012)170145161 | |
| Praziquantel tablets | Veterinary Drug (2012)170141174 | |
| Addition and subtraction eliminate yellow scattered | Veterinary Drug (2012)170149240 | |
| Benzalkonium bromide solution | Veterinary Drug (2012)170149004 |
Skystar Jingzhou is currently waiting for the Ministry of Agriculture to renew the approvals of its veterinary products for manufacturing and distribution. The product names that were submitted to Ministry of Agriculture for renewal are listed below and their approval numbers are to be determined.
| Veterinary Drug Products | ||
| Benzalkonium bromide solution | ||
| Albendazole powder |
| 8 |
Skystar Jingzhou is also waiting for the Ministry of Agriculture to approve its application for manufacturing and distribution of its new veterinary products. The product names that were submitted to Ministry of Agriculture for approval are listed below and their approval numbers are to be determined.
| Veterinary Drug Products | ||
| Hydrochloride doxycycline soluble powder | ||
| Of colistin sulfate soluble powder | ||
| Spectinomycin hydrochloride lincomycin hydrochloride soluble powder | ||
| Florfenicol powder | ||
| Tylosin tartrate soluble powder | ||
| Compound amoxicillin powder | ||
| Oral rehydration salts | ||
| Doxycycline injection | ||
| Lincomycin Hydrochloride Injection | ||
| Florfenicol Injection | ||
| Sulfamethoxazole injection of sodium methoxy pyrimidine | ||
| Enrofloxacin Injection | ||
| The size of the sulfuric acid celebrate Connaught mycophenolate injection | ||
| Difloxacin hydrochloride injection | ||
| Composite phenolic | ||
| Longdanxiegan scattered | ||
| Andrographis scattered four flavor | ||
| Of Robenidine powder hydrochloride | ||
| Menadione Sodium bisulfite powder | ||
| Omphalia betel nut powder | ||
| Board yellow powder | ||
| Hepatobiliary Unicorn Powder | ||
| Yinqiao Banlangen scattered | ||
| The green board Treats scattered | ||
| Sodium periodate solution | ||
| Compound iodine |
Government Approval and Regulation of our Principal Products or Services
Government approval is required for the production of bio-pharmaceutical products. The Chinese Ministry of Agriculture has granted Xi’an Tianxing three government permits to produce the following products: Forage Additive Products, Additive and Mixed Forage Products, and Veterinary Medicine Products. For the production of the veterinary medicine, there is a national standard known as the Good Manufacturing Practice (“GMP”) standard. A company must establish its facility according to GMP standards, including both the facility and the production process. After establishing such facility, the company files an application to operate the facility with the PRC Ministry of Agriculture, which then sends a team of specialists to conduct an on-site inspection of the facility. A company cannot start production at the facility until it receives approval from the Ministry of Agriculture to begin operations. The Chinese Ministry of Agriculture has also granted Skystar Jingzhou the government permit to produce veterinary medicine products. As of December 31, 2012, both Xi’an Tianxing and Skystar Jingzhou have the requisite approvals and licenses from the Ministry of Agriculture in order to operate our production facilities.
Research and Development
We place great emphasis on product research and development, and we work closely with two research institutes in the veterinary science field.
With Shanghai Poultry Verminosis Institute, which is a part of the Chinese Academy of Agricultural Sciences, we jointly established the Skystar Research and Development Center in Shanghai. We are currently working on the following project at this research center:
| · | Culture isolation and purification of various livestock and poultry common pathogen species. |
We also established an R&D center located on our premises in Huxian with the Shaanxi Microbial Institute, the only microbial research institute in northwest China. We provide for the running and operation of the research center, including research equipment and materials. In exchange, we have exclusive rights to any technology derived from any research project that we solely fund. We and the Shaanxi Microbial Institute mutually staff research personnel at, and joint-appointment of the director for, the research center. The Institute, however, is not obligated to us with respect to a specific amount of time or a specific project. We are currently working on the following projects at this research center:
| 9 |
| · | Development of common fish, livestock, poultry pathogen immunodiagnostic reagents, therapeutic agents and preventive reagents. We have developed a new immunodiagnostic kit and are currently conducting farm field experiments. |
| · | Development of fish, livestock, and poultry disease multi-specific transfer factor. |
| · | Development of mononuclear vaccine biological agents. |
During the first quarter of 2008, Xi’an Tianxing contracted with Northwestern Agricultural Technology University to jointly work on an R&D project concerning the application of nano-technology in the prevention of major milk cow disease. The project reached trial stage in March 2009 and remains in progress as of December 31, 2012. We have submitted an application to obtain veterinary permits for the new product. In 2009, Xi’an Tianxing contracted with the Fourth Military Medical University to jointly work on fish diseases linked immunosorbent detection kit and fish diseases multi-linked monoclonal antibody therapeutic agents. The project remains in progress as of December 31, 2012
On September 23, 2009, we purchased an exclusive aquaculture vaccine technology from and signed a collaborative research and development agreement with China’s Fourth Military Medical University (“FMMU”) for approximately $1.2 million (RMB 8 million), granting the Company exclusive rights to sell and market the patented aquaculture vaccine through 2020. Under this patented technology, and in collaboration with FMMU, we will produce the first vaccine in China designed to safely prevent and treat certain bacterial infection and diseases in marine life without causing harmful side effects. Based on its first-to-market status, the Chinese Ministry of Agriculture has issued a Grade I Veterinary Certificate for our vaccine.
During the first quarter of 2011, Xi’an Tianxing contracted with the Fourth Military Medical University to jointly work on new treatment and diagnosis method for Mycoplasmal pneumonia of swine. During the second quarter of 2011, Xi’an Tianxing launched four in-house R&D projects to develop ceftiofur sodium for injection (powder for injection), a sulfuric acid injection neostigmine, dexamethasone sodium phosphate injection, and houttuynia preparation of compound application in weaning piglets. As of December 31, 2012, Xi’an Tianxing had completed these four in-house R&D projects.
During the second quarter of 2012, Xi’an Tianxing contracted with the Fourth Military Medical University to jointly work on an R&D project of ring disease and pseudo-rabies specific transfer factor and blue-ear disease and Mycoplasma pneumoniae disease specific transfer factor to enhance the livestock and poultry's own immuneforce and prevent the onset of conventional disease. The project is currently in clinical research stage. During the fourth quarter of 2012, Xi’an Tianxing launched eight in-house R&D projects to develop oral liquid product, powder for injection product, Vitamin E injection product, anthelmintic product, three disease grams product, premixes class product, bulk powder product and livestock and fish diseases immunoassay kit. All eight projects remain in progress as of December 31, 2012
In addition, the Company also launched various in house R&D projects in 2011 and 2012 on veterinary products formula adjustment, pet drug development and fermentation engineering design and development.
In 2012, we spent $2,154,241, or approximately 6.4%, of our revenue on R&D. In 2011, we spent $2,814,328, or approximately 5.3%, of our revenue on R&D.
Employees
As of December 31, 2012, we have 317 employees, of which 134 employees are production personnel, 72 employees are sales personnel, 24 employees are research and development personnel, and 87 employees are administrative personnel. None of our employees are covered by collective bargaining agreements. We consider our relationships with our employees to be good. We also employ consultants on an as needed basis.
ITEM 1A. RISK FACTORS
Our business involves a number of risks, many of which are beyond our control. The risks and uncertainties described below could individually or collectively have a material adverse effect on our business, assets, profitability or prospects. While these are not the only risks and uncertainties we face, management believes that the more significant risks and uncertainties are as follows:
Risks Relating to Our Business
Our relatively limited operating history makes it difficult to evaluate our future prospects and results of operations.
We have a relatively limited operating history. Xi’an Tianxing, the variable interest entity through which we operate the majority of our business, commenced operations in 1997 and first achieved profitability in the quarter ended September 30, 1999. We acquired the Jingzhou plant and related assets through bankruptcy court and started production in the third quarter of 2010 and the asset acquisition was completed in August of 2010. We completed Kunshan micro-organism plant’s asset acquisition in September 2011. The facility renovation construction was completed in 2012, and the related inspection was completed and accepted in March 2013. However, the equipment installation, tooling and testing has only partially been completed; thus this facility is not operational at this point. Accordingly, you should consider our future prospects in light of the risks and uncertainties typically experienced by companies such as ours in evolving industries such as the bio-pharmaceutical industry in China. Some of these risks and uncertainties relate to our ability to:
| • | offer new and innovative products to attract and retain a larger customer base; | |
| • | attract additional customers and increase spending per customer; |
| 10 |
| • | increase awareness of our brand and continue to develop user and customer loyalty; | |
| • | raise sufficient capital to sustain and expand our business; |
| • | maintain effective control of our costs and expenses; | |
| • | respond to changes in our regulatory environment; |
| • | respond to competitive market conditions; | |
| • | manage risks associated with intellectual property rights; |
| • | attract, retain and motivate qualified personnel; | |
| • | upgrade our technology to support additional research and development of new products; and |
| • | maintain or improve our position as one of the market leaders in China. |
If we are unsuccessful in addressing any of these risks and uncertainties, our business may be materially and adversely affected.
If we fail to obtain additional financing we may be unable to execute our business plan.
Despite our current financing from banks, we may need additional funds to build new production facilities; pursue further research and development; obtain regulatory approvals; file, prosecute, defend and enforce our intellectual property rights; and market our products. Should such needs arise, we intend to seek additional funds through public or private equity or debt financing, strategic transactions and/or from other sources.
There are no assurances that future funding will be available on favorable terms or at all. If additional funding is not obtained, we may need to reduce, defer or cancel development programs, planned initiatives or overhead expenditures, to the extent necessary. The failure to fund our capital requirements would have a material adverse effect on our business, financial condition and results of operations.
Our business will be materially and adversely affected if our collaborative partners, licensees and other third parties over whom we are very dependent fail to perform as expected.
Due to the complexity of the process of developing bio-pharmaceuticals, our core business depends on arrangements with bio-pharmaceutical institutes, corporate and academic collaborators, licensors, licensees and others for the research, development, clinical testing, technology rights, manufacturing, marketing and commercialization of our products. We have various research collaborations and outsource other business functions. Our license agreements could obligate us to diligently bring potential products to market, make substantial milestone payments and royalties and incur the costs of filing and prosecuting patent applications. There are no assurances that we will be able to establish or maintain collaborations that are important to our business on favorable terms, or at all. We could enter into collaborative arrangements for the development of particular products that may lead to our relinquishing some or all rights to the related technology or products. A number of risks arise from our dependence on collaborative agreements with third parties. Product development and commercialization efforts could be adversely affected if any collaborative partner:
| • | terminates or suspends its agreement or arrangement with us; | |
| • | causes delays; | |
| • | fails to timely develop or manufacture in adequate quantities a substance needed in order to conduct clinical trials; | |
| • | fails to adequately perform clinical trials; | |
| • | determines not to develop, manufacture or commercialize a product to which it has rights; or |
| • | otherwise fails to meet its contractual obligations. |
Our collaborative partners could pursue other technologies or develop alternative products that could compete with the products we are developing.
Our products will be adversely affected if we are unable to protect proprietary rights or operate without infringing the proprietary rights of others.
The profitability of our products will depend in part on our ability to obtain and maintain patents and licenses and preserve trade secrets, and the period our intellectual property remains exclusive. We must also operate without infringing the proprietary rights of third parties and without third parties circumventing our rights. The patent positions of bio-pharmaceutical and biotechnology enterprises, including ours, are uncertain and involve complex legal and factual questions for which important legal principles are largely unresolved. In addition, the scope of the originally claimed subject matter in a patent application can be significantly reduced before a patent is issued. The biotechnology patent situation outside the U.S. is uncertain, is currently undergoing review and revision in many countries, and may not protect our intellectual property rights to the same extent as the laws of the U.S. Because patent applications are maintained in secrecy in some cases, we cannot be certain that we or our licensors are the first creators of inventions described in our pending patent applications or patents or the first to file patent applications for such inventions.
Other companies may independently develop similar products and design around any patented products we develop. We cannot assure you that:
| • | any of our patent applications will result in the issuance of patents; | |
| • | we will develop additional patentable products; |
| • | patents we have been issued will provide us with any competitive advantages; | |
| • | the patents of others will not impede our ability to do business; or |
| 11 |
| • | third parties will not be able to circumvent our patents. |
A number of pharmaceutical, biotechnology, research and academic companies and institutions have developed technologies, filed patent applications or received patents on technologies that may relate to our business. If these technologies, applications or patents conflict with ours, the scope of our current or future patents could be limited or our patent applications could be denied. Our business may be adversely affected if competitors independently develop competing technologies, especially if we do not obtain, or obtain only narrow, patent protection. If patents that cover our activities are issued to other companies, we may not be able to obtain licenses at a reasonable cost, or at all; develop our technology; or introduce, manufacture or sell the products we have planned.
Patent litigation is becoming widespread in the biotechnology industry. Such litigation may affect our efforts to form collaborations, to conduct research or development, to conduct clinical testing or to manufacture or market any products under development. There are no assurances that our patents would be held valid or enforceable by a court or that a competitor’s technology or product would be found to infringe our patents in the event of patent litigation. Our business could be materially affected by an adverse outcome to such litigation. Similarly, we may need to participate in interference proceedings declared by the U.S. Patent and Trademark Office or equivalent international authorities to determine priority of invention. We could incur substantial costs and devote significant management resources to defend our patent position or to seek a declaration that another company’s patents are invalid.
Much of our know-how and technology may not be patentable, though it may constitute trade secrets. There are no assurances that we will be able to meaningfully protect our trade secrets. We cannot assure you that any of our existing confidentiality agreements with employees, consultants, advisors or collaborators will provide meaningful protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure. Collaborators, advisors or consultants may dispute the ownership of proprietary rights to our technology, for example by asserting that they developed the technology independently.
Difficulties in manufacturing our products could have a material adverse effect on our profitability.
Before our products can be profitable, they must be produced in commercial quantities in a cost-effective manufacturing process that complies with regulatory requirements, including China’s Good Manufacturing Practice (GMP), production and quality control regulations. If we cannot arrange for or maintain commercial-scale manufacturing on acceptable terms, or if there are delays or difficulties in the manufacturing process, we may not be able to conduct clinical trials, obtain regulatory approval or meet demand for our products.
Failure or delays in obtaining an adequate amount of raw material or other supplies would materially and adversely affect our revenue.
Production of our products could require raw materials which are scarce or which can be obtained only from a limited number of sources. If we are unable to obtain adequate supplies of such raw materials, the development, regulatory approval and marketing of our products could be delayed.
Our ability to generate more revenue would be adversely affected if we need more clinical trials or take more time to complete our clinical trials than we have planned.
Clinical trials vary in design by factors including dosage, end points, length, and controls. We may need to conduct a series of trials to demonstrate the safety and efficacy of our products. The results of these trials may not demonstrate safety or efficacy sufficiently for regulatory authorities to approve our products. Further, the actual schedules for our clinical trials could vary dramatically from the forecasted schedules due to factors including changes in trial design, conflicts with the schedules of participating clinicians and clinical institutions, and changes affecting product supplies for clinical trials.
We rely on collaborators, including academic institutions, governmental agencies and clinical research organizations, to conduct, supervise, monitor and design some or all aspects of clinical trials involving our products. Since these trials depend on governmental participation and funding, we have less control over their timing and design than trials we sponsor. Delays in or failure to commence or complete any planned clinical trials could delay the ultimate timelines for our product releases. Such delays could reduce investors’ confidence in our ability to develop products, likely causing the price of our common stock to decrease.
If we are unable to obtain the regulatory approvals or clearances that are necessary to commercialize our products, we will have less revenue than expected.
China and other countries impose significant statutory and regulatory obligations upon the manufacture and sale of bio-pharmaceutical products. Each regulatory authority typically has a lengthy approval process in which it examines pre-clinical and clinical data and the facilities in which the product is manufactured. Regulatory submissions must meet complex criteria to demonstrate the safety and efficacy of the ultimate products. Addressing these criteria requires considerable data collection, verification and analysis. We may spend time and money preparing regulatory submissions or applications without assurances as to whether they will be approved on a timely basis or at all.
Our product candidates, some of which are currently in the early stages of development, will require significant additional development and pre-clinical and clinical testing prior to their commercialization. These steps and the process of obtaining required approvals and clearances can be costly and time-consuming. If our potential products are not successfully developed, cannot be proven to be safe and effective through clinical trials, or do not receive applicable regulatory approvals and clearances, or if there are delays in the process:
| 12 |
| • | the commercialization of our products could be adversely affected; | |
| • | any competitive advantages of the products could be diminished; and |
| • | revenues or collaborative milestones from the products could be reduced or delayed. |
Governmental and regulatory authorities may approve a product candidate for fewer indications or narrower circumstances than requested or may condition approval on the performance of post-marketing studies for a product candidate. Even if a product receives regulatory approval and clearance, it may later exhibit adverse side effects that limit or prevent its widespread use or that force us to withdraw the product from the market.
Any marketed product and its manufacturer, including us, will continue to be subject to strict regulation after approval. Results of post-marketing programs may limit or expand the further marketing of products. Unforeseen problems with an approved product or any violation of regulations could result in restrictions on the product, including its withdrawal from the market and possible civil actions.
In manufacturing our products we will be required to comply with applicable good manufacturing practices regulations, which include requirements relating to quality control and quality assurance, as well as the maintenance of records and documentation. If we cannot comply with regulatory requirements, including applicable good manufacturing practice requirements, we may not be allowed to develop or market the product candidates. If we or our manufacturers fail to comply with applicable regulatory requirements at any stage during the regulatory process, we may be subject to sanctions, including fines, product recalls or seizures, injunctions, refusal of regulatory agencies to review pending market approval applications or supplements to approve applications, total or partial suspension of production, civil penalties, withdrawals of previously approved marketing applications and criminal prosecution.
Competitors may develop and market bio-pharmaceutical products that are less expensive, more effective or safer, making our products obsolete or uncompetitive.
Some of our competitors and potential competitors have greater product development capabilities and financial, scientific, marketing and human resources than we do. Technological competition from biopharmaceutical companies and biotechnology companies is intense and is expected to increase. Other companies have developed technologies that could be the basis for competitive products. Some of these products have an entirely different approach or means of accomplishing the desired curative effect than products we are developing. Alternative products may be developed that are more effective, work faster and are less costly than our products. Competitors may succeed in developing products earlier than us, obtaining approvals and clearances for such products more rapidly than us, or developing products that are more effective than ours. In addition, other forms of treatment may be competitive with our products. Over time, our technology or products may become obsolete or uncompetitive.
Our revenue will be materially and adversely affected if our products are unable to gain market acceptance.
Our products may not gain market acceptance in the agricultural community. The degree of market acceptance of any product depends on a number of factors, including establishment and demonstration of clinical efficacy and safety, cost-effectiveness, clinical advantages over alternative products, and marketing and distribution support for the products. Limited information regarding these factors is available in connection with our products or products that may compete with ours.
To directly market and distribute our bio-pharmaceutical products, we or our collaborators require a marketing and sales force with appropriate technical expertise and supporting distribution capabilities. We may not be able to further establish sales, marketing and distribution capabilities or enter into arrangements with third parties on acceptable terms. If we or our partners cannot successfully market and sell our products, our ability to generate revenue will be limited.
Our operations and the use of our products could subject us to damages relating to injuries or accidental contamination and thus reduce our earnings or increase our losses.
Our research and development processes involve the controlled use of hazardous materials. We are subject to federal, provincial and local laws and regulations governing the use, manufacture, storage, handling and disposal of such materials and waste products. The risk of accidental contamination or injury from handling and disposing of such materials cannot be completely eliminated. In the event of an accident involving hazardous materials, we could be held liable for resulting damages. We are not insured with respect to this liability. Such liability could exceed our resources. In the future we could incur significant costs to comply with environmental laws and regulations.
If we were sued for product liability, we could face substantial liabilities that may exceed our resources.
We may be held liable if any product we develop, or any product which is made using our technologies, causes injury or is found unsuitable during product testing, manufacturing, marketing, sale or use. These risks are inherent in the development of agricultural and bio-pharmaceutical products. We currently do not have product liability insurance. If we cannot obtain sufficient insurance coverage at an acceptable cost or otherwise protect against potential product liability claims, the commercialization of products that we develop may be prevented or inhibited. If we are sued for any injury caused by our products, our liability could exceed our total assets, whether or not we are successful.
| 13 |
We have no business liability or disruption insurance coverage and therefore we are susceptible to catastrophic or other events that may disrupt our business.
The insurance industry in China is still at an early stage of development. Insurance companies in China offer limited business insurance products. We do not have any business liability or disruption insurance coverage for our operations in China. Any business disruption, litigation or natural disaster may result in our incurring substantial costs and the diversion of our resources.
We will be unsuccessful if we fail to attract and retain qualified personnel.
We depend on a core management and scientific team. The loss of any of these individuals could prevent us from achieving our business objective of commercializing our product candidates. Our future success will depend in large part on our continued ability to attract and retain other highly qualified scientific, technical and management personnel, as well as personnel with expertise in clinical testing and government regulation. We face competition for personnel from other companies, universities, public and private research institutions, government entities and other organizations. If our recruitment and retention efforts are unsuccessful, our business operations could suffer.
Downturn in the global economy may slow domestic growth in China, which in turn may affect our business.
Due to the slowing of global economy, China may not be able to maintain its recent growth rates mainly due to the lack of demand of exports to countries that are in recessions. Although we do not presently export any of our products, our earnings may become unstable if China’s domestic growth slows significantly and the demand for meats and poultry declines.
Risks Related to Our Corporate Structure
Chinese laws and regulations governing our businesses and the validity of certain of our contractual arrangements are uncertain. If we are found to be in violation, we could be subject to sanctions. In addition, changes in such Chinese laws and regulations may materially and adversely affect our business.
There are substantial uncertainties regarding the interpretation and application of Chinese laws and regulations, including, but not limited to, the laws and regulations governing our business, or the enforcement and performance of our contractual arrangements with our affiliated Chinese entity, Xi’an Tianxing, and its stockholders. We are considered a foreign person or foreign invested enterprise under Chinese law. As a result, we are subject to Chinese law limitations on foreign ownership of Chinese companies. These laws and regulations are relatively new and may be subject to change, and their official interpretation and enforcement may involve substantial uncertainty. The effectiveness of newly enacted laws, regulations or amendments may be delayed, resulting in detrimental reliance by foreign investors. New laws and regulations that affect existing and proposed future businesses may also be applied retroactively.
The Chinese government has broad discretion in dealing with violations of laws and regulations, including levying fines, revoking business and other licenses and requiring actions necessary for compliance. In particular, licenses and permits issued or granted to us by relevant governmental bodies may be revoked at a later time by higher regulatory bodies. We cannot predict the effect of the interpretation of existing or new Chinese laws or regulations on our businesses. We cannot assure you that our current ownership and operating structure would not be found in violation of any current or future Chinese laws or regulations. As a result, we may be subject to sanctions, including fines, and could be required to restructure our operations or cease to provide certain services. Any of these or similar actions could significantly disrupt our business operations or restrict us from conducting a substantial portion of our business operations, which could materially and adversely affect our business, financial condition and results of operations.
We may be adversely affected by complexity, uncertainties and changes in Chinese regulation of bio-pharmaceutical business and companies, including limitations on our ability to own key assets.
The Chinese government regulates the bio-pharmaceutical industry including foreign ownership of, and the licensing and permit requirements pertaining to, companies in the bio-pharmaceutical industry. These laws and regulations are relatively new and evolving, and their interpretation and enforcement involve significant uncertainty. As a result, in certain circumstances it may be difficult to determine what actions or omissions may be deemed to be a violation of applicable laws and regulations. Issues, risks and uncertainties relating to Chinese government regulation of the bio-pharmaceutical industry include the following:
| • | we only have contractual control over Xi’an Tianxing. We do not own it due to the restriction of foreign investment in Chinese businesses; and | |
| • | uncertainties relating to the regulation of the bio-pharmaceutical business in China, including evolving licensing practices, means that permits, licenses or operations at our company may be subject to challenge. This may disrupt our business, or subject us to sanctions, requirements to increase capital or other conditions or enforcement, or compromise enforceability of related contractual arrangements, or have other harmful effects on us. |
The interpretation and application of existing Chinese laws, regulations and policies and possible new laws, regulations or policies have created substantial uncertainties regarding the legality of existing and future foreign investments in, and the businesses and activities of, bio-pharmaceutical businesses in China, including our business.
| 14 |
In order to comply with Chinese laws limiting foreign ownership of Chinese bio-pharmaceutical companies, we conduct our bio-pharmaceutical business primarily through Xi’an Tianxing by means of contractual arrangements. If the Chinese government determines that these contractual arrangements do not comply with applicable regulations, our business could be adversely affected.
The Chinese government restricts foreign investment in bio-pharmaceutical businesses in China. Accordingly, we operate our business in China primarily through Xi’an Tianxing, a Chinese joint stock company. Xi’an Tianxing holds the licenses and approvals necessary to operate our bio-pharmaceutical business in China. We have contractual arrangements with Xi’an Tianxing and its stockholders that allow us to substantially control Xi’an Tianxing. We cannot assure you, however, that we will be able to enforce these contracts.
Although we believe we comply with current Chinese regulations, we cannot assure you that the Chinese government would agree that these operating arrangements comply with Chinese licensing, registration or other regulatory requirements, with existing policies or with requirements or policies that may be adopted in the future. If the Chinese government determines that we do not comply with applicable law, it could revoke our business and operating licenses, require us to discontinue or restrict our operations, restrict our right to collect revenues, require us to restructure our operations, impose additional conditions or requirements with which we may not be able to comply, impose restrictions on our business operations or on our customers, or take other regulatory or enforcement actions against us that could be harmful to our business.
Our contractual arrangements with Xi’an Tianxing and its stockholders may not be as effective in providing control over these entities as direct ownership.
Since Chinese law limits foreign equity ownership in bio-pharmaceutical companies in China, we operate our business through Xi’an Tianxing. We have no equity ownership interest in Xi’an Tianxing and rely on contractual arrangements to control and operate such businesses. These contractual arrangements may not be as effective in providing control over Xi’an Tianxing as direct ownership. For example, Xi’an Tianxing could fail to take actions required for our business despite its contractual obligation to do so. If Xi’an Tianxing fails to perform under their agreements with us, we may have to rely on legal remedies under Chinese law, which may not be effective. In addition, we cannot assure you that either of Xi’an Tianxing’s stockholders will act in our best interests.
Because we rely on the consulting services agreement with Xi’an Tianxing for the majority of our revenue, the termination of this agreement will severely and detrimentally affect our continuing business viability under our current corporate structure.
We are a holding company and do not have any assets or conduct any business operations. As a result, our revenues are primarily dependent on dividend payments from Sida after it receives payments from Xi’an Tianxing pursuant to the consulting services agreement which forms a part of the contractual arrangements between Sida and Xi’an Tianxing. The consulting services agreement may be terminated by written notice of Sida or Xi’an Tianxing in the event that: (a) one party causes a material breach of the agreement, provided that if the breach does not relate to a financial obligation of the breaching party, that party may attempt to remedy the breach within 14 days following the receipt of the written notice; (b) one party becomes bankrupt, insolvent, is the subject of proceedings or arrangements for liquidation or dissolution, ceases to carry on business, or becomes unable to pay its debts as they become due; (c) Sida terminates its operations; (d) Xi’an Tianxing’s business license or any other license or approval for its business operations is terminated, cancelled or revoked; or (e) circumstances arise which would materially and adversely affect the performance or the objectives of the agreement. Additionally, Sida may terminate the consulting services agreement without cause. Because neither we nor our direct and indirect subsidiaries own equity interests of Xi’an Tianxing, the termination of the consulting services agreement would sever our ability to continue receiving payments from Xi’an Tianxing under our current holding company structure. While we are currently not aware of any event or reason that may cause the consulting services agreement to terminate, we cannot assure you that such an event or reason will not occur in the future. In the event that the consulting services agreement is terminated, this may have a severe and detrimental effect on our continuing business viability under our current corporate structure, which in turn may affect the value of your investment.
Members of Xi’an Tianxing’s management have potential conflicts of interest with us, which may adversely affect our business and your ability for recourse.
Weibing Lu, our Chief Executive Officer, is also the Chief Executive Officer and Chairman of the Board of Directors of Xi’an Tianxing. Mr. Wei Wen, who is Xi’an Tianxing’s Vice-General Manager and Director, is a member of Skystar’s board of directors. Conflicts of interests between their respective duties to our company and Xi’an Tianxing may arise. As our directors and executive officer (in the case of Mr. Lu), they have a duty of loyalty and care to us under U.S. and Cayman Islands law when there are any potential conflicts of interests between our company and Xi’an Tianxing. We cannot assure you, however, that when conflicts of interest arise, every one of them will act completely in our interests or that conflicts of interests will be resolved in our favor. For example, they may determine that it is in Xi’an Tianxing’s interests to sever the contractual arrangements with Sida, irrespective of the effect such action may have on us. In addition, any one of them could violate his or her legal duties by diverting business opportunities from us to others, thereby affecting the amount of payment Xi’an Tianxing is obligated to remit to us under the consulting services agreement.
Our board of directors is comprised of a majority of independent directors. These independent directors may be in a position to deter and counteract the actions of our officers or non-independent directors that are against our interests, as the independent directors do not have any position with, or interests in, our affiliate entities, and should therefore not have any conflicts of interests such as those potentially of our officers and directors who are management members of Xi’an Tianxing. Additionally, the independent directors have fiduciary duties to act in our best interests, and failure on their part to do so may subject them to personal liabilities for breach of such duties. We cannot, however, give any assurance as to how the independent directors will act. Further, if we or the independent directors cannot resolve any conflicts of interest between us and those of our officers and directors who are management members of Xi’an Tianxing, we would have to rely on legal proceedings, which could result in the disruption of our business.
| 15 |
In the event that you believe that your rights have been infringed under the securities laws or otherwise as a result of any one of the circumstances described above, it may be difficult or impossible for you to bring an action against Xi’an Tianxing or our officers or directors who are members of its management, the majority of whom reside within China. Even if you are successful in bringing an action, the laws of China may render you unable to enforce a judgment against the assets of Xi’an Tianxing and its management, all of which are located in China.
Risks Related to Doing Business in China
Adverse changes in economic and political policies of the Chinese government could have a material adverse effect on the overall economic growth of China, which could adversely affect our business.
Substantially all of our business operations are conducted in China. Accordingly, our results of operations, financial condition and prospects are subject to a significant degree to economic, political and legal developments in China. China’s economy differs from the economies of most developed countries in many respects, including with respect to the amount of government involvement, level of development, growth rate, control of foreign exchange and allocation of resources. While the Chinese economy has experienced significant growth in the past 20 years, growth has been uneven across different regions and among various economic sectors of China. The Chinese government has implemented various measures to encourage economic development and guide the allocation of resources. Some of these measures benefit the overall Chinese economy, but may also have a negative effect on us. For example, our financial condition and results of operations may be adversely affected by government control over capital investments or changes in tax regulations that are applicable to us. Since early 2004, the Chinese government has implemented certain measures to control the pace of economic growth. Such measures may cause a decrease in the level of economic activity in China, which in turn could adversely affect our results of operations and financial condition.
If Chinese law were to phase out the preferential tax benefits currently being extended to foreign invested enterprises and “new or high-technology enterprises” located in a high-tech zone, we would have to pay more taxes, which could have a material and adverse effect on our financial condition and results of operations.
Under Chinese laws and regulations, a foreign invested enterprise may enjoy preferential tax benefits if it is registered in a high-tech zone and also qualifies as “new or high-technology enterprise”. As a foreign invested enterprise as well as a certified “new or high-technology enterprise” located in a high-tech zone in Xian, Xi’an Tianxing has been approved as a new technology enterprise for a period of three years until October 2015 and thus under Chinese Income Tax Laws, it is entitled to a preferential tax rate of 15%. If the Chinese law were to phase out preferential tax benefits currently granted to “new or high-technology enterprises” and technology consulting services, we would be subject to the standard statutory tax rate, which currently is 25%, and we would be unable to obtain business tax refunds for our provision of technology consulting services. Loss of these preferential tax treatments could have a material and adverse effect on our financial condition and results of operations.
Xi’an Tianxing is subject to restrictions on making payments to us.
We are a holding company incorporated in Nevada and do not have any assets or conduct any business operations other than our investments in our affiliated entity in China. As a result of our holding company structure, we rely heavily on payments from Xi’an Tianxing under our contractual arrangements. The Chinese government also imposes controls on the conversion of the Chinese currency, Renminbi (RMB), into foreign currencies and the remittance of currencies out of China. We may experience difficulties in completing the administrative procedures necessary to obtain and remit foreign currency. See “Government control of currency conversion may affect the value of your investment.” Furthermore, if our affiliated entity in China incurs debt on their own in the future, the instruments governing the debt may restrict their ability to make payments. If we are unable to receive the revenues from our operations through these contractual or dividend arrangements, we may be unable to pay dividends on our shares.
Uncertainties with respect to the Chinese legal system could adversely affect us.
We conduct our business primarily through our affiliated Chinese entity, Xi’an Tianxing. Our operations in China are governed by Chinese laws and regulations. We are generally subject to laws and regulations applicable to foreign investments in China and, in particular, laws applicable to wholly foreign-owned enterprises. The Chinese legal system is based on written statutes. Prior court decisions may be cited for reference but have limited precedential value.
Since 1979, Chinese legislation and regulations have significantly enhanced the protections afforded to various forms of foreign investments in China. However, China has not developed a fully integrated legal system and recently enacted laws and regulations may not sufficiently cover all aspects of economic activities in China. In particular, because these laws and regulations are relatively new, and because of the limited volume of published decisions and their nonbinding nature, the interpretation and enforcement of these laws and regulations involve uncertainties. In addition, the Chinese legal system is based in part on government policies and internal rules (some of which are not published on a timely basis or at all) that may have a retroactive effect. As a result, we may not be aware of our violation of these policies and rules until some time after the violation. In addition, any litigation in China may be protracted and result in substantial costs and diversion of resources and management attention.
| 16 |
Governmental control of currency conversion may affect the value of your investment.
The Chinese government imposes controls on the convertibility of RMB into foreign currencies and, in certain cases, the remittance of currency out of China. We receive substantially all of our revenues in RMB. Under our current structure, our income is primarily derived from payments from Xi’an Tianxing. Shortages in the availability of foreign currency may restrict the ability of our Chinese subsidiaries and our affiliated entity to remit sufficient foreign currency to pay dividends or other payments to us, or otherwise satisfy their foreign currency denominated obligations. Under existing Chinese foreign exchange regulations, payments of current account items, including profit distributions, interest payments and expenditures from trade-related transactions, can be made in foreign currencies without prior approval from China State Administration of Foreign Exchange by complying with certain procedural requirements. However, approval from appropriate government authorities is required where RMB is to be converted into foreign currency and remitted out of China to pay capital expenses such as the repayment of bank loans denominated in foreign currencies. The Chinese government may also at its discretion restrict access in the future to foreign currencies for current account transactions. If the foreign exchange control system prevents us from obtaining sufficient foreign currency to satisfy our currency demands, we may not be able to pay dividends in foreign currencies to our stockholders.
Fluctuation in the value of RMB may have a material adverse effect on your investment.
The value of RMB against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in political and economic conditions. Our revenues and costs are mostly denominated in RMB, while a significant portion of our financial assets are denominated in U.S. dollars. We rely entirely on fees paid to us by our affiliated entity in China. Any significant fluctuation in the value of RMB may materially and adversely affect our cash flows, revenues, earnings and financial position, and the value of, and any dividends payable on, our stock in U.S. dollars. For example, an appreciation of RMB against the U.S. dollar would make any new RMB denominated investments or expenditures more costly to us, to the extent that we need to convert U.S. dollars into RMB for such purposes. An appreciation of RMB against the U.S. dollar would also result in foreign currency translation losses for financial reporting purposes when we translate our U.S. dollar denominated financial assets into RMB, as RMB is our functional currency.
We face risks related to health epidemics and other outbreaks.
Our business could be adversely affected by the effects of an epidemic outbreak, such as the SARS epidemic in April 2004. Any prolonged recurrence of such adverse public health developments in China may have a material adverse effect on our business operations. For instance, health or other government regulations adopted in response may require temporary closure of our production facilities or of our offices. Such closures would severely disrupt our business operations and adversely affect our results of operations. We have not adopted any written preventive measures or contingency plans to combat any future outbreak of SARS or any other epidemic.
The audit report included in this filing are prepared by auditors who are not inspected by the Public Company Accounting Oversight Board and, as such, you may not have the benefits of such inspection.
Our independent registered public accounting firm that issues the audit reports included in our Annual Reports filed with the U.S. Securities and Exchange Commission, as auditors of companies that are traded publicly in the United States and a firm registered with the U.S. Public Company Accounting Oversight Board (United States) (“the “PCAOB”), is required by the laws of the United States to undergo regular inspections by the PCAOB to assess its compliance with the laws of the United States and professional standards. Because we have substantial operations within the Peoples' Republic of China and the PCAOB is currently unable to conduct inspections of the work of our auditors as it relates to those operations without the approval of the Chinese authorities, our auditors are not currently inspected fully by the PCAOB. Inspections of other firms that the PCAOB has conducted outside China have identified deficiencies in those firms’ audit procedures and quality control procedures, which may be addressed as part of the inspection process to improve future audit quality. This lack of PCAOB inspections in China prevents the PCAOB from regularly evaluating our auditor's audits and its quality control procedures. As a result, investors may be deprived of the benefits of PCAOB inspections. The inability of the PCAOB to conduct full inspections of auditors in China makes it more difficult to evaluate the effectiveness of our auditor’s audit procedures or quality control procedures as compared to auditors outside of China that are subject to PCAOB inspections. Investors may lose confidence in our reported financial information and procedures and the quality of our financial statements.
Risks Related to the Common Stock
Techniques employed by manipulative short sellers in Chinese small cap stocks may drive down the market price of our common stock
Short selling is the practice of selling securities that the seller does not own but rather has, supposedly, borrowed from a third party with the intention of buying identical securities back at a later date to return to the lender. The short seller hopes to profit from a decline in the value of the securities between the sale of the borrowed securities and the purchase of the replacement shares, as the short seller expects to pay less in that purchase than it received in the sale. As it is therefore in the short seller’s best interests for the price of the stock to decline, many short sellers (sometime known as “disclosed shorts”) publish, or arrange for the publication of, negative opinions regarding the relevant issuer and its business prospects in order to create negative market momentum and generate profits for themselves after selling a stock short. While traditionally these disclosed shorts were limited in their ability to access mainstream business media or to otherwise create negative market rumors, the rise of the Internet and technological advancements regarding document creation, videotaping and publication by weblog (“blogging”) have allowed many disclosed shorts to publicly attack a company’s credibility, strategy and veracity by means of so-called research reports that mimic the type of investment analysis performed by large Wall Street firm and independent research analysts. These short attacks have, in the past, led to selling of shares in the market, on occasion in large scale and broad base. Issuers with business operations based in the PRC and who have limited trading volumes and are susceptible to higher volatility levels than U.S. domestic large-cap stocks, can be particularly vulnerable to such short attacks.
| 17 |
These short seller publications are not regulated by any governmental, self-regulatory organization or other official authority in the U.S., are not subject to the certification requirements imposed by the Securities and Exchange Commission in Regulation AC (Regulation Analyst Certification) and, accordingly, the opinions they express may be based on distortions of actual facts or, in some cases, fabrications of facts. In light of the limited risks involved in publishing such information, and the enormous profit that can be made from running just one successful short attack, unless the short sellers become subject to significant penalties, it is more likely than not that disclosed shorts will continue to issue such reports.
While we intend to strongly defend our public filings against any such short seller attacks, often times we are constrained, either by principles of freedom of speech, applicable state law (often called “Anti-SLAPP statutes”), or issues of commercial confidentiality, in the manner in which we can proceed against the relevant short seller. You should be aware that in light of the relative freedom to operate that such persons enjoy – oftentimes blogging from outside the U.S. with little or no assets or identity requirements – should we be targeted for such an attack, our stock will likely suffer from a temporary, or possibly long term, decline in market price should the rumors created not be dismissed by market participants.
The market price for our securities may be subject to wide fluctuations
The securities of a number of Chinese companies and companies with substantial operations in China have experienced wide fluctuations in their stock price. Among the factors that could affect the price of our common stock are risk factors described in this section and other factors, including:
| • | announcements of competitive developments, by our competitors; |
| • | regulatory developments of our industry affecting us, our customers or our competitors; |
| • | actual or anticipated fluctuations in our quarterly operating results; |
| • | failure of our quarterly financial and operating results to meet market expectations or failure to meet our previously announced guidance, if any; |
| • | changes in financial estimates by securities research analysts; |
| • | changes in the economic performance or market valuations of our competitors; |
| • | additions or departures of our executive officers and other key personnel; |
| • | announcements regarding intellectual property litigation (or potential litigation) involving us or any of our directors and officers; |
| • | fluctuations in the exchange rates between the U.S. dollar and the Renminbi; and |
| • | release or expiration of the underwriters’ post-offering lock-up or other transfer restrictions on our outstanding common stock. |
In addition, the securities markets have from time to time experienced significant price and volume fluctuations that are not related to the operating performance of particular industries or companies. For example, the capital and credit markets have been experiencing volatility and disruption for more than 12 months. Starting in September 2008, the volatility and disruption have reached extreme levels, developing into a global crisis. As a result, stock prices of a broad range of companies worldwide, whether or not they are related to financial services, have declined significantly. These market fluctuations may also have a material adverse effect on the market price of our securities.
We may need additional capital and may sell additional securities or other equity securities or incur indebtedness, which could result in additional dilution to our shareholders or increase our debt service obligations.
We may require additional cash resources due to changed business conditions or other future developments, including any investments or acquisitions we may decide to pursue. If our cash resources are insufficient to satisfy our cash requirements, we may seek to sell additional equity or debt securities or obtain a credit facility. The sale of additional equity securities or equity-linked debt securities could result in additional dilution to our shareholders. The incurrence of indebtedness would result in increased debt service obligations and could result in operating and financing covenants that would restrict our operations. We cannot assure you that financing will be available in amounts or on terms acceptable to us, if at all.
Substantial future sales of our securities in the public market, or the perception that these sales could occur, could cause the price of our securities to decline.
Additional sales of our securities in the public market or the perception that these sales could occur, could cause the market price of our securities to decline. As of March 12, 2013 there were 7,604,800 shares of our common stock outstanding. Of that amount, 5,675,660 shares of common stock are freely transferable without restriction upon resale. None of our shares are subject to any lock-up restrictions, although 1,929,140 of them are subject to volume and other restrictions as applicable under Rule 144 under the Securities Act. In addition, we may grant or sell additional options, restricted shares or other share-based awards in the future under our share incentive plan to our management, employees and other persons, the settlement and sale of which may further dilute our shares and drive down the price of our securities.
| 18 |
If NASDAQ were to delist our securities from trading on its exchange, such action could limit investors’ ability to make transactions in our securities and subject us to additional trading restrictions.
Our common stock is currently listed on the NASDAQ Capital Market. We cannot assure you that our securities will meet the continued listing requirements be listed on NASDAQ in the future.
If NASDAQ delists our common stock from trading on its exchange, we could face significant material adverse consequences including:
| · | a limited availability of market quotations for our securities; |
• a determination that our common stock is a “penny stock” which will require brokers trading in our common stock to adhere to more stringent rules and possibly resulting in a reduced level of trading activity in the secondary trading market for our common stock;
| • | a limited amount of news and analyst coverage for our company; and |
| • | a decreased ability to issue additional securities or obtain additional financing in the future. |
If our shares of common stock become subject to the SEC’s penny stock rules, broker-dealers may experience difficulty in completing customer transactions and trading activity in our securities may be adversely affected.
If our common stock were removed from listing with the Nasdaq Capital Market, it may be subject to the so-called “penny stock” rules. The SEC has adopted regulations that define a “penny stock” to be any equity security that has a market price per share of less than $5.00, subject to certain exceptions, such as any securities listed on a national securities exchange. For any transaction involving a “penny stock,” unless exempt, the rules impose additional sales practice requirements on broker-dealers, subject to certain exceptions. If our common stock were delisted and determined to be a “penny stock,” a broker-dealer may find it more difficult to trade our common stock and an investor may find it more difficult to acquire or dispose of our common stock on the secondary market. Investors in penny stocks should be prepared for the possibility that they may lose their whole investment.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
Our administrative headquarters is located at 4/F, Building B, Chuangye Square, No. 48 Keji Road, Gaoxin District, Xi’an, Shaanxi Province, China. This property is owned by the Company’s subsidiary Sida, and has a total office space of 17,174 square feet. Our sales department is located at Rm. 10601, Jiezuo Plaza, No.4, Fenghui Road South, Gaoxin District, Xi’an, Shaanxi Province, China, and has an office space of 3,700 square feet. This property is owned by our Chairman of the Board and Chief Executive Officer, Mr. Weibing Lu, and we were under a 5-year lease with Mr. Lu from January 1, 2007 to December 31, 2011 at rent of RMB 165,600 (approximately $26,281) per year. In January 2012, the Company renewed the lease with Mr. Lu for another 5 years from January 1, 2012 to December 31, 2016 at a rent of RMB 180,000 (approximately $28,566) per year.
Shanghai Siqiang, Xi’an Tianxing’s wholly owned subsidiary, leases its office and facility space in Shanghai from Mr. Lu pursuant to a 10-year lease agreement from August 1, 2007 to July 31, 2017 at rent of RMB 144,000 (approximately $22,853) per year.
Skystar Jingzhou, a subsidiary we acquired in August 2010, owns the land use right and buildings located at No. 10 Yingxing Road, Songzi Economic Development Zone, East City Industrial Park, Jingzhou, Hubei Province, China. Total land area is 301,688 square feet. The total building space including office and plant facilities is 63,217 square feet.
Skystar Kunshan owns the land use right and buildings and is located at Kunshan, Jiangsu Province, China. The Company received land use rights in October 2010 and building ownership certificates in June 2011. Total land area is 403,887 square feet. The total building space including office and plant facilities is 81,809 square feet.
Production Facilities
The Company currently has four manufacturing facilities. Two of them are located in Xi’an, Shaanxi Province, China, of which one site is located in the town of Sanqiao and the other site is located in the town of Huxian. The third manufacturing facility is located in Songzi, Jingzhou City, Hubei Province, China and the fourth manufacturing facility is located in Kunshan, Jiangsu Province, China.
The Sanqiao Plant
The Company entered into a ten-year lease agreement for the factory premises underlying its Sanqiao plant from October 1, 2004 to September 30, 2014. The annual rent has been adjusted to about RMB 116,000 (approximately $18,409) and subject to a 10% increase every two years starting October 1, 2009. The Sanqiao plant has two facilities. The Micro-Organism facility is run in cooperation with experts Microbiology Institute of Shaanxi Province and Northwest Agro-Forestry Sci-tech University. This facility is approximately 21,500 square feet and has a production permit and certain product approval numbers issued by the Chinese Ministry of Agriculture. The Feed Additive facility occupies approximately 10,700 square feet.
| 19 |
| Description | Approx. Size | Status | ||
| Micro-organism facility | 21,500 sq. ft. | Complete | ||
| Feed additive facility | 10,700 sq. ft. | Complete |
The Huxian Plant
In 2003, the Company received approval from the State Council of China to expand its production facilities and construct a new GMP standard plant. In connection with the approval, the Company acquired a long-term land use right for the land now underlying its Huxian plant. Total land area is approximately 335,000 square feet. The total building space including office and plant facilities is 151,700 square feet. The development of Huxian plant is supported by both the Shaanxi provincial government and the Xi’an municipal government as Xi’an Tianxing is an accredited high-tech enterprise.
Construction of the Huxian plant commenced in late 2004 and portions of the plant were fully operational since the end of the second quarter of 2007. The veterinary medicine facility is currently fully operational. In June 2012, China's Ministry of Agriculture (MOA) inspected Huxian veterinary medications facility for its GMP re-examination. On September 26, 2012, China's Ministry of Agriculture (MOA) renewed our GMP certificate for this facility which is valid for another five years. . The construction of the vaccine facility was completed in June 2010 and equipment installation, tooling and testing were completed in the third quarter of 2010. In September 2012, the MOA physically inspected this facility and deemed the facility GMP compliant. Following physical inspection, the MOA’s inspection team recommended that the Office of the Working Committee proceed with Stage 2 of its GMP certification process. The management expects the GMP certification process of the vaccine facility to be completed by the third quarter of 2013. When the vaccine facility becomes operational, the Huxian plant will occupy approximately 7.7 acres and have a total plant and office area of approximately 151,700 square feet. The table below lists the primary facilities at the plant and the status of each facility:
| Description | Approx. Size | Status | ||
| GMP standard veterinary medicine facility | 45,200 sq. ft. | Complete | ||
| Quality control, research and development, and administration building | 36,600 sq. ft. | Complete | ||
| Warehouse | 10,400 sq. ft | Complete | ||
| GMP standard bio-pharmaceutical facility with the production lines for active bacteria, inactivated vaccines, coccidiosis vaccines and aquaculture vaccines. | 48,800 sq. ft. | In progress of completing GMP certification | ||
| Animal laboratory complying with Animal Bio-safety Level 2 (ABSL-2) requirements | 10,700 sq. ft. | In progress of completing GMP certification |
The Jingzhou Plant
The Company acquired the Jingzhou plant and related assets through bankruptcy court for RMB 24,600,000 (approximately $3.9 million) and completed the acquisition in August of 2010. Skystar Jingzhou holds a 30-year land use right for the land now underlying the Jinzhou plant. Total land area is 301,688 square feet. The total building space including office and plant facilities is 63,217 square feet. The Jingzhou plant is GMP certified to produce veterinary medicines including aqua culture medicines. The table below lists the primary facilities at the plant and their status.
| Description | Approx. Size | Status | ||
| GMP standard veterinary medicine facility and warehouse | 39,095 sq. ft. | Complete | ||
| Administration building, dorm and other | 24,122 sq. ft. | Complete |
The Kunshan Plant
The Company acquired the Kunshan plant and related assets for RMB 50,000,000 (approximately $7.9 million) and completed the acquisition in September 2011. Skystar Kunshan holds a 41-year land use right for the land of the Kunshan plant. Total land area is 403,887 square feet. The table below lists the primary facilities at the plant and their statuses. As of the end of 2012, the facility renovation construction was completed, and inspection was completed and accepted in March 2013. However, the equipment installation, tooling and testing has only partially completed; thus this facility is not operational at this point.
| Description | Approx. Size | Status | ||
| Micro-organism manufacturing facility | 35,654 sq. ft. | In progress | ||
| Boiler room | 6,712 sq. ft. | Complete | ||
| Administration building | 39,443 sq. ft. | Complete |
ITEM 3. LEGAL PROCEEDINGS
From time to time, the Company is involved in legal matters arising in the ordinary course of business. Except as set forth below our management is currently not aware of any legal matters or pending litigation that would have a significant effect on the Company’s results of operation of financial statements.
| 20 |
In May 2007, Andrew Chien filed suit against the Company, R. Scott Cramer, Steve Lowe, David Wassung and Weibing Lu in United States District Court for the District of Connecticut, alleging causes of action for violation of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934. On July 17, 2008, in a decision that is now published, the court granted defendants’ motion to dismiss and subsequently dismissed the lawsuit, entering judgment on behalf of the defendants. Chien appealed the dismissal. Defendants filed a post judgment motion for sanctions against Chien. On February 5, 2009, the court found the action filed by Chien to have been frivolous, and to have constituted a “substantial” violation of Rule 11, and imposed monetary sanctions on both Chien and his former attorney. Chien appealed the award of sanctions. All appeals, including the one referenced below concerning Chien’s second lawsuit, were subsequently consolidated. The Court of Appeals issued a Mandate upholding the decision granting defendant’s motion to dismiss and found that the District Court did not “abuse its discretion” in issuing sanctions against Chien in light of the circumstances and facts on record. This Mandate was entered on or about November 8, 2010.
Andrew Chien, proceeding pro se (i.e., he represented himself without an attorney), filed his second lawsuit against the Company, R. Scott Cramer, Steve Lowe, David Wassung and Weibing Lu in Connecticut Superior Court (which was removed to U.S. District Court) alleging causes of action similar to those alleged in his federal complaint described above as well as state law causes of action. The court held that all claims asserted against the defendants were barred and failed to state a claim on a multiplicity of grounds, including on the basis of res judicata. Defendants filed a second Motion for Sanctions under Rule 11 and the PSLRA, which was granted. Chien appealed the dismissal. The Court of Appeals for the Second Circuit consolidated all of Chien’s appeals from both of his lawsuits. On November 8, 2010, the Court of Appeals affirmed the dismissals and the awards of sanctions. On January 22, 2011, Chien filed a petition with the Supreme Court of the United States, appealing the lower court’s ruling. The Supreme Court denied review of the petition.
Andrew Chien, again proceeding pro se, commenced his third lawsuit against the Company, Scott Cramer, Steve Lowe, David Wassung, Weibing Lu (and also Weinberg & Company, P.A., Moore Stephens Wirth Frazer & Torbet, LLP, Frazer Frost, LLP, Crowe Horwath LLP, Richardson & Patel LLP, Kevin K. Leung, Harvey Kesner, and Jody M. Borrelli) alleging the same facts and circumstances as set forth in the above two matters, although adding the aforementioned accountants and lawyers as defendants to the claims. The matter commenced on August 8, 2011, and shortly thereafter was removed to U.S. District Court. On October 5, 2011, the court dismissed the entire action as to all defendants. As in the two previous lawsuits, sanctions were issued against Chien. Moreover, the District Court issued an order prohibiting Chien from filing any more lawsuits against the defendants without prior approval from the court. Chien appealed the Court’s ruling to the United States Court of Appeals for the Second Circuit, where all defendants have filed motions to dismiss.
The United States Court of Appeals for the Second Circuit recently affirmed the judgments and orders of the United States District Court and dismissed all of Mr. Chien’s pending appeals.
Although the Second Circuit declined to issue sanctions against Chien, it expressly warned Chien “that the continued filing of duplicative, vexatious, or frivolous appeals regarding issues already resolved by this Court may result in the imposition of sanctions, including a leave-to-file sanction requiring Chien to obtain permission from this Court prior to filing further submissions in this Court.” Mr. Chien has filed a petition for writs of certiorari with the Supreme Court of the United States. The Supreme Court has not yet acted on Chien’s petitions.
Other than the above described legal proceedings, the Company is not aware of any other legal matters in which any director, officer, or any owner of record or beneficial owner of more than five percent of any class of voting securities of the Company, or any affiliate of any such director, officer, affiliate of the Company, or security holder, is a party adverse to the Company or has a material adverse interest to the Company. No provision has been made in the consolidated financial statements for the above contingencies.
ITEM 4. MINE SAFETY DISCLOSURES
NOT APPLICABLE.
PART II
ITEM 5. MARKET PRICE FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASE OF EQUITY SECURITIES
Market Information
Since June 2009, our common stock has traded on the Nasdaq Global Select Market under the symbol “SKBI.” Prior to that, our common stock was quoted on the Over-The-Counter Bulletin Board, or OTCBB, under the symbol “SKBO.OB”.
The following table sets forth the high and low bid prices for our common stock on the Nasdaq Capital Market for the periods indicated. The high and low bid prices reflect inter-dealer prices, without retail markups, markdowns, or commissions, and may not represent actual transactions. The closing price of the Company’s securities on March 12, 2013 was $1.63.
| The Nasdaq Capital Market Sales Price per Share | ||||||||
| Quarter ended | High | Low | ||||||
| December 31, 2012 | $ | 1.95 | $ | 1.55 | ||||
| September 30, 2012 | $ | 2.31 | $ | 1.52 | ||||
| June 30, 2012 | $ | 3.06 | $ | 1.93 | ||||
| March 31, 2012 | $ | 3.20 | $ | 2.13 | ||||
| December 31, 2011 | $ | 3.09 | $ | 1.64 | ||||
| September 30, 2011 | $ | 4.42 | $ | 1.77 | ||||
| June 30, 2011 | $ | 5.64 | $ | 3.15 | ||||
| March 31, 2011 | $ | 10.53 | $ | 5.28 | ||||
| 21 |
Holders
As of March 12, 2013, we had approximately 425 registered stockholders of our common stock on record.
Dividends
We have not paid, and do not currently intend to pay, cash dividends on our common stock in the foreseeable future. Our policy is to retain all earnings, if any, to provide funds for operation and expansion of our business. The declaration of dividends, if any, will be subject to the discretion of our board of directors, which may consider such factors as our results of operations, financial condition, capital needs, and acquisition strategy, among others.
Securities Authorized for Issuance under Equity Compensation Plans
Please see the discussion in Item 12 titled “Equity Compensation Plan Information” below.
Recent Sales of Unregistered Securities
The Company has not sold any unregistered securities during the year ended December 31, 2012 which has not been previously disclosed in its public filings in 2012. The Company did not repurchase any of its equity securities during the fiscal year ended December 31, 2012.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our results of operations and financial condition for the fiscal years ended December 31, 2012 and 2011 should be read in conjunction with our financial statements and the notes thereto that are included elsewhere in this report. Our discussion includes forward-looking statements based upon current expectations that involve risks and uncertainties, such as our plans, objectives, expectations, and intentions. Please see the section in this report titled “Caution Regarding Forward-Looking Information.”
Overview
Our financial statements are prepared in U.S. Dollars and in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). See “Exchange Rates” below for information regarding the exchanges rates at which Renminbi (“RMB”) were translated into U.S. Dollars (“USD”) at various pertinent dates and for pertinent periods.
Critical Accounting Policies
In preparing the consolidated financial statements in accordance with U.S. GAAP, we make estimates and assumptions about the effect of matters that are inherently uncertain and may change in subsequent periods. The resulting accounting estimates will, by definition, vary from the related actual results. We consider the following to be the most critical accounting policies:
Principles of consolidation
The accompanying consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America. The consolidated financial statements include the financial statements of our company, our wholly-owned subsidiaries, and our VIEs. All significant inter-company transactions and balances between our company, its subsidiaries, and its VIEs have been eliminated in consolidation.
We have evaluated the relationship with Xi’an Tianxing and Xi’an Tianxing’s wholly owned subsidiaries, Xi’an Sikaida and Shanghai Siqiang. As a result of the contractual arrangements which obligate Sida to absorb all of the risk of loss from Xi’an Tianxing’s activities and enable Sida to receive all of its expected residual returns, the Company accounts for Xi’an Tianxing, Xi’an Sikaida and Shanghai Siqiang as VIEs under the Financial Accounting Standards Board’s (“FASB”) interpretation on consolidation of variable interest entities. Accordingly, the Company consolidates the results, assets, and liabilities of Xi’an Tianxing, Xi’an Sikaida and Shanghai Siqiang.
Revenue recognition
Our revenue is primarily from the sales of veterinary healthcare and medical care products in China. Sales are recognized when the following four revenue criteria are met: persuasive evidence of an arrangement exists, delivery has occurred, the selling price is fixed or determinable, and collectability is reasonably assured. Sales are presented net of value added tax (“VAT”). No estimated allowance for sales returns is reflected in these consolidated financial statements as sales returns are de minimus based on historical experience.
| 22 |
There are two types of sales upon which revenue is recognized:
| a. | Credit sales: revenue is recognized when the products have been delivered to the customers. |
| b. | Full payment before delivering: revenue is recognized when the products have been delivered to the customers. |
Accounts receivable
Accounts receivable is stated at cost less an allowance for uncollectible accounts, as needed. We use the aging method to estimate the allowance for anticipated uncollectible receivable balances. Under the aging method, a bad debt percentage determined by management, based on historical experience and current economic climate, is applied to customers’ balances categorized by the number of months the underlying invoices have remained outstanding. At each reporting period, the allowance balance is adjusted to reflect the amount computed as a result of the aging method. When facts subsequently become available to indicate that the allowance provided requires an adjustment, a corresponding adjustment is made to the allowance account as a change in estimate. The ultimate collection of our accounts receivable may take one year. Delinquent account balances are reserved after management determines that the likelihood of collection is not probable, and known bad debts are written-off against the allowance for doubtful accounts when identified.
Stock based compensation
We record and report stock-based compensation by measuring the cost of services received in exchange for an award of equity instruments based on the grant-date fair value of the award. That cost is recognized over the period during which services are received. Stock compensation for stock granted to non-employees is determined as the fair value of the consideration received or the fair value of equity instruments issued, whichever is more reliably measured.
Recently Issued Accounting Pronouncements
In July 2012, the FASB issued 2012-02 Intangibles — Goodwill and Other (Topic 350): The amendments in this update will allow an entity to first assess qualitative factors to determine whether it is necessary to perform a quantitative impairment test. Under these amendments, an entity would not be required to calculate the fair value of an indefinite-lived intangible asset unless the entity determines, based on a qualitative assessment, that it is not more likely than not, the indefinite-lived intangible asset is impaired. The amendments include a number of events and circumstances for an entity to consider in conducting the qualitative assessment. The amendments are effective for annual and interim impairment tests performed for fiscal years beginning after September 15, 2012. Early adoption is permitted, including for annual and interim impairment tests performed as of a date before July 27, 2012, if a public entity’s financial statements for the most recent annual or interim period have not yet been issued. We do not expect to have a material impact on our consolidated financial statements.
In February 2013, the FASB issued Accounting Standards Update No. 2013-02 Comprehensive Income (Topic 220): The objective of this update is to improve the reporting of reclassifications out of accumulated other comprehensive income. The amendments in this update seek to attain that objective by requiring an entity to report the effect of significant reclassifications out of accumulated other comprehensive income on the respective line items in net income if the amount being reclassified is required under U.S. generally accepted accounting principles (GAAP) to be reclassified in its entirety to net income. For other amounts that are not required under U.S. GAAP to be reclassified in their entirety to net income in the same reporting period, an entity is required to cross-reference other disclosures required under U.S. GAAP that provide additional detail about those amounts. This would be the case when a portion of the amount reclassified out of accumulated other comprehensive income is reclassified to a balance sheet account (for example, inventory) instead of directly to income or expense in the same reporting period. For public entities, the amendments are effective prospectively for reporting periods beginning after December 15, 2012. For nonpublic entities, the amendments are effective prospectively for reporting periods beginning after December 15, 2013. Early adoption is permitted. We do not expect the adoption of the provisions in this update will have a significant impact on our consolidated financial statements.
Other accounting standards that have been issued or proposed by the FASB or other standards-setting bodies that do not require adoption until a future date are not expected to have a material impact on the Company’s consolidated financial statements upon adoption.
Results of Operations – Comparison of Two Years Ended December 31, 2012 and 2011
The following table summarizes our results of operations for the years ended December 31, 2012 and 2011.
| Years Ended December 31, | ||||||||||||||||
| 2012 | 2011 | |||||||||||||||
| Amount | Percentage of total revenue | Amount | Percentage of total revenue | |||||||||||||
| Revenues | $ | 33,586,791 | 100.0 | % | $ | 52,788,279 | 100.0 | % | ||||||||
| Gross Profit | $ | 18,491,787 | 55.1 | % | $ | 26,225,531 | 49.7 | % | ||||||||
| Operating Expense | $ | 9,940,806 | 29.6 | % | $ | 10,975,607 | 20.8 | % | ||||||||
| Income from Operations | $ | 8,550,981 | 25.5 | % | $ | 15,249,924 | 28.9 | % | ||||||||
| Other Income (Expenses) | $ | (170,428 | ) | (0.5 | )% | $ | 1,279,015 | 2.4 | % | |||||||
| Income Tax Expenses | $ | 2,168,125 | 6.5 | % | $ | 2,871,299 | 5.4 | % | ||||||||
| Net Income | $ | 6,212,428 | 18.5 | % | $ | 13,657,640 | 25.9 | % | ||||||||
| 23 |
Revenues. All of our revenues are derived from the sale of veterinary healthcare and medical care products in the PRC. For the year ended December 31, 2012, we had revenues of $33,586,791 as compared to revenues of $52,788,279 for the year ended December 31, 2011, a decrease of $19,201,488 or 36.4% due to temporary suspension of production at the Huxian veterinary medication plant during the first three quarters as a result of the GMP re-examination. We generate revenue from sales of four product lines: veterinary medications, micro-organism, feed additives, and vaccines. The selling prices of our products increased more than 4.3% on average in the year ended December 31, 2012 compared to 2011. The decrease in revenue was primarily due to the decrease of sales volume.
Revenue — Veterinary Medications. Revenue from sales of our veterinary medications decreased by $23,102,865 or 68.4% from $33,761,224 for the year ended December 31, 2011 to $10,658,359 for the year ended December 31, 2012. The decrease of revenue was primarily due to temporary suspension of production at Huxian plant for the first three quarters of 2012. On January 7, 2012, the GMP certification of Huxian veterinary medications facility expired and its production was temporarily suspended. On September 26, 2012, China's Ministry of Agriculture (MOA) renewed our GMP certificate which is valid for another five years until September 2017 and we resumed the production at Huxian veterinary medications facility in October 2012. We currently have two veterinary medications plants located in Huxian and Jingzhou with Huxian historically being our main facility. The Jingzhou plant completed its GMP re-examination in 2011 and resumed its normal production in 2012. Of the total revenues from veterinary medications during the year ended December 31, 2012, approximately 63.7% of total revenue resulted from the sale of products from the Huxian facility. The selling prices of our veterinary medication products for the year ended December 31, 2012 increased approximately 13.7% on average from last year. The decrease in revenue was primarily due to the decrease of sales volume.
Revenue — Micro-Organism. Revenue from sales of our micro-organism products increased by $1,208,528 or 8.5% from $14,146,939 for the year ended December 31, 2011 to $15,355,467 for the year ended December 31, 2012. The increase was primarily due to a growing concern for food safety and increasing market demand for organic and green food products in China resulting in increasing the utilization of non-drug feed additives in the animal husbandry industry and our increased sales efforts to respond to this market trend. Currently we have a micro-organism plant located in Sanqiao. The revenue from sales of our micro-organism products was the largest revenue contributor for the entire company and contributed 45.7% of total revenue during the year ended December 31, 2012. The selling prices of our micro-organism products for the year ended December 31, 2012 have not materially changed on average from last year. The increase in revenue was mainly due to the increase of sales volume.
Revenue — Feed Additives. Revenue from sales of our feed additives product line increased by $1,461,653 or 57.1% from $2,559,914 for the year ended December 31, 2011 to $4,021,567 for the year ended December 31, 2012. The increase was primarily the result of increased sales efforts despite the slowing economy in China. Due to the temporary reduction of veterinary medication production in the first nine months of 2012, we were able to shift some of our veterinary medication production and sales forces to focus on the manufacturing and selling of feed additives products and penetrate the market. Currently we have a feed additive plant located in Sanqiao. The selling prices of our feed additives products for the year ended December 31, 2012 have not materially changed on average from last year. The increase in revenue was primarily due to the increase of sales volume.
Revenue — Vaccines. Revenue from sales of our vaccines increased by $1,231,196 or 53.1% from $2,320,202 for the year ended December 31, 2011 to $3,551,398 for the year ended December 31, 2012. The increase was primarily the result of increased sales efforts despite the slowing economy in China. We completed the construction of a new vaccine facility at our Huxian plant in 2010. On September 20 and 21, 2012, MOA physically inspected this new facility and deemed the facility GMP compliant. Following physical inspection, the MOA’s inspection team recommended that the Office of the Working Committee proceed with Stage 2 of its GMP certification process. The Company expects the GMP certification process to be completed by the third quarter of 2013, and to commence production shortly thereafter. The selling prices of our vaccine products increased approximately 0.3% on average for the year ended December 31, 2012 compared to those in 2011. The increase in revenue was primarily due to the increase of sales volume.
Cost of Sales. Cost of sales was $15,095,004 for the year ended December 31, 2012, as compared to $26,562,748 for the year ended December 31, 2011, a decrease of $11,467,744 or 43.2%, as a result of decreased sales of our veterinary medications product line. For the year ended December 31, 2012, raw material costs comprised the majority or approximately 77.3% of total cost of sales, packing material costs comprised approximately 13.8% of total cost of sales, and labor costs and manufacturing overhead comprised approximately 8.9% of total cost of sales. The increased gross margins for the year ended December 31, 2012 was mainly because the majority of our revenue during the year came from highly profitable micro-organism and vaccine product lines, while the relatively less profitable veterinary medications product line significantly reduced its production, and therefore sales, during the year, as described above.
Cost of Sales — Veterinary Medications. Cost of sales of our veterinary medications product line decreased from $20,684,170 for the year ended December 31, 2011 to $6,087,300 for the year ended December 31, 2012, a decrease of $14,596,870 or 70.6%. This decrease was mainly due to the decrease in corresponding sales as a result of temporary suspension of production at the Huxian facility for the first three quarters of 2012.
| 24 |
Cost of Sales — Micro-Organism. Cost of sales of our micro-organism product line increased from $4,034,629 for the year ended December 31, 2011 to $5,498,463 for the year ended December 31, 2012, an increase of $1,463,834 or 36.3%. This increase was mainly due to the corresponding increased sales. The changes of formula to manufacture some of our micro-organism products in 2012 also contributed to an increase in raw material costs.
Cost of Sales — Feed Additives. Cost of sales of our feed additives product line increased from $1,542,022 for the year ended December 31, 2011 to $3,074,301 for the year ended December 31, 2012, an increase of $1,532,279 or 99.4%. This increase in cost of sales was mainly due to the corresponding increase in feed additive sales and a significant increase in raw material costs of yeast extract, the main raw material used in the manufacture of feed additive products. We are searching for potential substitutes for certain main raw materials to manufacture our feed additives products in order to control rising costs.
Cost of Sales — Vaccines. Cost of sales of our vaccines product line increased from $301,927 for the year ended December 31, 2011 to $434,940 for the year ended December 31, 2012, an increase of $133,013 or 44.1%. This increase was the result of the corresponding increase of vaccine product sales.
Operating Expenses
| Years Ended December 31, | ||||||||||||||||
| 2012 | 2011 | |||||||||||||||
| Amount | Percentage of total revenue | Amount | Percentage of total revenue | |||||||||||||
| Research and Development Costs | $ | 2,154,241 | 6.4 | % | $ | 2,814,328 | 5.3 | % | ||||||||
| Selling Expenses | 3,040,036 | 9.1 | 4,062,126 | 7.7 | ||||||||||||
| General and Administrative Expenses | 4,746,529 | 14.1 | 4,099,153 | 7.8 | ||||||||||||
| Total Operating Expenses | $ | 9,940,806 | 29.6 | % | $ | 10,975,607 | 20.8 | % | ||||||||
Research and Development Costs. Research and development costs totaled $2,154,241 for the year ended December 31, 2012 as compared to $2,814,328 for the year ended December 31, 2011, a decrease of $660,087 or 23.5%. The decrease was primarily due to less R&D efforts undertaken during 2012.
Selling Expenses. Selling expenses totaled $3,040,036 for the year ended December 31, 2012 as compared to $4,062,126 for the year ended December 31, 2011, a decrease of $1,022,090 or 25.2%. This decrease is mainly due to decreased sales commission expenses as the Huxian veterinary medication facility was temporarily closed for the first three quarters of 2012 resulting in a significant decrease in veterinary medication sales in 2012. Sales commission expenses totaled $904,279 and $1,558,013 during the year ended December 31, 2012 and 2011, respectively, a decrease of $653,734 or 42.0%. Shipping and handling costs decreased $130,508 or 6.8% as the number of product units shipped to our customers dropped during the year. However, the rising unit cost for transportation and delivery services partially offset the impact of the decrease in shipping volume as we continued to expand our market to remote areas. Travelling expenses also decreased $145,210 or 77.5% as we temporarily closed the Huxian veterinary medication facility for its GMP re-certification resulting in less veterinary medicine sales activities during the year.
General and Administrative Expenses. General and administrative expenses totaled $4,746,529 for the year ended December 31, 2012 as compared to $4,099,153 for the year ended December 31, 2011, an increase of $647,376 or 15.8%. The increase was mainly due to the stock based compensation expense of $1,037,911 for the stock grants on May 4, 2012 to the Company’s employees and members of the Board of Directors, all of which grants were made pursuant to the terms and provisions of the 2010 Stock Incentive Plan.
Other Income (Expenses) Other income net of expenses were $(170,428) for 2012 as compared to $1,279,015 for 2011. The net gain in 2011 was mainly a result of a gain in fair value change of warrant/purchase option liability of $1,346,239, and such gain for 2012 was $37,800. Our common stock price experienced a volatile downward movement in 2011, thus we recorded a higher gain on these derivative liabilities. All of our warrants expired in February 2012. On the other hand, interest expenses were $706,842 in 2012, as compared to $140,920 in 2011 due to average higher interest rates on borrowing in 2012.
Provision for Income Taxes Other tax expenses were $2,168,125 for 2012 as compared to $2,871,299 for 2011, representing an effective tax rate of 25.9% and 17.4% in 2012 and 2011, respectively. In 2012 and 2011, Xi’an Tianxing was subject to a preferential income tax rate of 15% while other PRC subsidiaries were subject to statutory tax rate of 25%. In 2012 and 2011, Xi’an Tianxing contributed 91.5% and 100.0% of consolidated profits in China, respectively. In 2012, Xi’an Tianxing’s Huxian veterinary medication production had been temporarily suspended for nine months for GMP re-certification.
Liquidity
For the year ended December 31, 2012, cash provided by operating activities was $5,612,124 compared to cash used in operating activities of $1,658,937 for the year ended December 31, 2011. The major operating activities that provided cash during the year ended December 31, 2012 were net income of $6,212,428, a decrease in prepayment to suppliers of $6,027,718, and an increase in accounts payable of $2,307,066. The major operating activities that used cash during the year ended December 31, 2012 were an increase of accounts receivable of $6,394,244, and a build-up in inventory that used $7,985,875. The inflation rate in China was 2.5% in December 2012 as compared to the 2011 peak of over 4%. According to our observation of the raw material marketplace, the raw material prices in our sector were becoming more stable in 2012 as compared to the same period of last year. However, Chinese inflation rose to 3.2% in February 2013, hitting a 10-month high. We will monitor the market situation closely and continue the strategy of prepaying our suppliers to ensure the supply of raw materials at relatively lower cost levels. As of December 31, 2012, we had 59 suppliers as compared to 55 suppliers as of December 31, 2011 that we made advances to secure our raw material needs and to obtain favorable pricing. We will continue to closely manage these advances to balance the need for lower materials cost and sufficient cash flow.
| 25 |
Cash used in investing activities for the year ended December 31, 2012 was $544,950, as compared to cash used in investing activities of $1,654,637 for the year ended December 31, 2011. Cash provided by investing activities for the year ended December 31, 2012 was primarily the result of collection of loans made to third parties of $1,916,651. Cash used in investing activities for the year ended December 31, 2012 was primarily the result of loans made to third parties of $2,023,393.
Cash used in financing activities for the year ended December 31, 2012 was $779,285, as compared to cash provided by financing activities of $4,085,714 for the year ended December 31, 2011. Cash provided by financing activities for the year ended December 31, 2012, was primarily the result of proceeds from short-term and long-term loans of $5,774,860. Cash used in financing activities for the year ended December 31, 2012 was primarily the result of repayment of short-term loans of $7,488,280.
As of December 31, 2012, we had cash of $11,321,848. Our total current assets were $71,652,265, and our total current liabilities were $17,205,920, which resulted in a net working capital of $54,446,345.
Capital Resources
We finance our ongoing operating activities by using funds from our operations and external credit or financing arrangements. We routinely monitor current and expected operational requirements and financial market conditions to evaluate the use of available financing sources. We secured $5,774,860 in short-term and long term loans and repaid short-term loans of $7,488,280 during the year ended December 31, 2012. Considering our existing working capital position and our ability to access debt funding sources, we believe that our operations and borrowing resources are sufficient to provide for our current and foreseeable capital requirements to support our ongoing operations for at least the next twelve months.
Plan of Operations
Over the next 12 months, we plan to continue to market and sell our current products and to develop new products.
Xi’an Tianxing completed the veterinary medicine facility in Huxian during 2007 and the facility was certified by GMP standard and has been fully operational since then. According to MOA’s regulation, our facility is required to have a GMP re-examination every five years. On January 7, 2012, the GMP certification of Huxian veterinary medications facility expired after five years and its production was temporarily suspended. In June 2012, the MOA inspected Huxian veterinary medications facility and published its recommendation to renew its GMP certificate on the China Institute of Veterinary Drug Control's website. On September 26, 2012, the MOA renewed our GMP certificate which is valid for another five years and we resumed the production at Huxian facility in October, 2012 and expect to get back to full production in 2013. We currently have two veterinary medications factories located in Huxian and Jingzhou with Huxian being our main facility. Skystar Jingzhou veterinary medicine plant started production in the third quarter of 2010 after the asset acquisition was completed. During 2011, the Jingzhou plant completed its GMP re-examination and resumed its normal production in 2012. We expect that Jingzhou will continue its normal production in 2013 and generate more revenue as more production permits were received. Jingzhou’s veterinarian medicine products include aquaculture medicines.
We completed the construction of a new vaccine facility at our Huxian plant in 2010. On September 20 and 21, 2012, the MOA physically inspected this new facility and deemed the facility GMP compliant. Following physical inspection, the MOA’s inspection team recommended that the Office of the Working Committee proceed with Stage 2 of its GMP certification process. The Company expects the GMP certification process to be completed by the third quarter of 2013, and to commence production shortly thereafter.
Xi’an Tianxing also leases a manufacturing facility in Sanqiao to produce feed additive and micro-organism products and the facility is currently fully operational.
Skystar Kunshan micro-organism plant’s asset acquisition was completed in September 2011. Its facility renovation construction project was completed in 2012, and inspection was completed and accepted in March 2013. However, the facility has only completed partial equipment installation, tooling and testing; thus is not operational at this point. We anticipate that Kunshan plant will start small-scale production later 2013. However, we do not expect that will bring significant impact to our revenue in 2013 as a whole.
Skystar intends to continue developing new products, including animal immunization products, non-pathogenic micro-organisms for the cure and prevention of livestock disease, complex enzyme preparations as animal feed additives, pet drugs, animal disease specific transfer factor, vaccine biological agents and other veterinary medicine products within the next 12 months. If we succeed in developing new products and are able to obtain the regulatory approvals or clearances that are necessary to commercialize our products, we will have additional revenue than expected.
| 26 |
Contractual Obligations and Off-Balance Sheet Arrangements
Contractual Obligations
| Payments Due by Period | ||||||||||||||||||||
| Contractual Obligations | Total | Less than 1 year | 1 – 3 years | 3 – 5 years | More than 5 years | |||||||||||||||
| R&D Project Obligation | $ | 302,356 | $ | 302,356 | $ | - | $ | - | $ | - | ||||||||||
| Operating Lease Obligations | 318,449 | 112,075 | 141,624 | 64,750 | - | |||||||||||||||
| Government Grant Obligation | 158,700 | 158,700 | - | - | - | |||||||||||||||
| Total | $ | 779,505 | $ | 573,131 | $ | 141,624 | $ | 64,750 | $ | - | ||||||||||
In addition to the contractual obligations listed above, we have a future registered capital commitment related to our subsidiary Skystar Kunshan. Skystar Kunshan has a registered capital of $15,000,000, of which we invested $2,250,000 in cash. The remaining $12,750,000 of capital was originally required to be invested prior to May 7, 2012. In 2011, the asset acquisition of Kunshan facility was completed. The Company is in the process of getting the government’s approval to transfer the asset purchased to satisfy some of our registered capital commitment. On July 25, 2012, the Company received the approval of extension for payment of the remaining registered capital but a new due date has not been specified by the government. We intend to apply for another extension in 2013 with the government to invest the remaining registered capital or request the government to reduce the total registered capital for this facility.
Short-term Loans:
| December 31, 2012 | December 31, 2011 | Interest | ||||||||||||||||||||
| Bank | Amt RMB | Amt USD | Amt RMB | Amt USD | Due Date | Rate | ||||||||||||||||
| Chang’an Bank | - | $ | - | 5,000,000 | $ | 787,000 | 07/07/12 | 8.203 | % | |||||||||||||
| Shaanxi Agricultural Yanta Credit Union | - | - | 5,000,000 | 787,000 | 08/24/12 | 9.411 | % | |||||||||||||||
| Shaanxi Agricultural Yanta Credit Union | - | - | 3,000,000 | 472,200 | 12/21/12 | 9.411 | % | |||||||||||||||
| ICBC Songzi Branch | - | - | 3,000,000 | 472,200 | 05/04/12 | (1) | ||||||||||||||||
| Third-party Individual | - | - | 800,000 | 125,920 | Various | 0 | % | |||||||||||||||
| Bank of Chengdu | - | - | 30,000,000 | 4,722,000 | 11/30/12 | (1) | ||||||||||||||||
| Xi'an High-tech Zone Guoxin Microcredit Ltd. | 15,000,000 | 2,380,500 | - | - | 02/12/13 | 22.400 | % | |||||||||||||||
| Chang’an Bank | 10,000,000 | 1,587,000 | - | - | 08/26/13 | (1) | ||||||||||||||||
| ICBC Songzi Branch | 3,000,000 | 476,100 | - | - | 06/18/13 | (1) | ||||||||||||||||
| Total | 28,000,000 | $ | 4,443,600 | 46,800,000 | $ | 7,366,320 | ||||||||||||||||
| (1) | People's Bank of China floating benchmark lending rate over the same period plus 30%, which was 7.8% at December 31, 2012. |
Long-term Loans:
| December 31, 2012 | December 31, 2011 | Interest | ||||||||||||||||||||
| Bank | Amt RMB | Amt USD | Amt RMB | Amt USD | Due Date | Rate | ||||||||||||||||
| Shaanxi Agricultural Yanta Credit Union | 8,000,000 | $ | 1,269,600 | - | $ | - | 09/20/14 | 9.446 | % | |||||||||||||
Off-Balance Sheet Arrangements
We do not have any outstanding financial guarantees or commitments to guarantee the payment obligations of any third parties. We have not entered into any derivative contracts that are indexed to our shares and classified as stockholders’ equity or that are not reflected in our consolidated financial statements. Furthermore, we do not have any retained or contingent interest in assets transferred to an unconsolidated entity that serves as credit, liquidity, or market risk support to such entity. We do not have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk, or credit support to us or engages in leasing, hedging, or research and development services with us.
| 27 |
Exchange Rates
Our operating subsidiaries in China maintain their books and records in Renminbi (“RMB”), the currency of China. In general, for consolidation purposes, we translate the subsidiaries’ assets and liabilities into US Dollars using the applicable exchange rates prevailing at the balance sheet date, and the statement of comprehensive income is translated at average exchange rates during the reporting period. Adjustments resulting from the translation of the financial statements of the Company’s Chinese subsidiaries are recorded as accumulated other comprehensive income.
The exchange rates used to translate amounts in RMB into US Dollars for the purposes of preparing the consolidated financial statements or otherwise stated in this MD&A were as follows:
| December 31, 2012 |
December 31, 2011 |
||||
| Assets and liabilities | 1 RMB = 0.1587 USD | 1 RMB = 0.1574 USD |
|
Years Ended December 31, |
|||||
| 2012 | 2011 | ||||
| Statements of operations and cash flows | 1 RMB = 0.15865 USD | 1 RMB = 0.15496 USD | |||
Inflation
Inflation accelerated in China to a seven-month peak of 2.5% in December 2012 as unusually cold winter weather pushed up food prices around the country. Chinese inflation rose again to 3.2% in February 2013, hitting a ten-month high. For the full year of 2012, China's consumer-price index rose by 2.6%, compared with 5.4% in 2011 and 3.3% in 2010, highlighting that the overall inflation level in 2012 is still moderate by historical standards. China's economy has been picking up steam in the past few months after a prolonged slowdown, but China's factory growth cooled to multi-month lows in February 2013 as domestic demand dipped, weighing on firms already hit by slack foreign sales and underlining the patchiness of the country's economic recovery. The weak economy is likely to continue for the rest of 2013. For the year ended December 31, 2012, we were able to secure favorable pricing by prepaying certain suppliers to lock in prices ahead of time. As a result, we did not experience as much cost pressure as evidenced in the spot market prices of raw materials. To take precautions to minimize the negative impact of cost increases that erode our profit margin, we will continue the practice of prepayments to suppliers but at a reduced scale in 2013.
ITEM 8. FINANCIAL STATEMENTS
Our consolidated financial statements begin on page 45 after the signature page to this report.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURES
Effective as of June 29, 2012, Crowe Horwath LLP resigned as independent auditors of Skystar Bio-Pharmaceutical Company (the “Company”).
The reports of Crowe Horwath LLP on the registrant’s financial statements as of December 31, 2011 and 2010 and for the years ended December 31, 2011 and 2010 did not contain an adverse opinion or a disclaimer of opinion, and were not qualified or modified as to uncertainty, audit scope, or accounting principles. In connection with the audits of the registrant’s financial statements for the fiscal periods ended December 31, 2011 and 2010, and through June 29, 2012, there were: (i) no disagreements between the registrant and Crowe Horwath LLP on any matters of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of Crowe Horwath LLP, would have caused Crowe Horwath LLP to make reference to the subject matter of the disagreement in its reports on the registrant’s financial statements for such periods, and (ii) no reportable events within the meaning set forth in Item 304(a)(1)(v) of Regulation S-K.
The registrant provided Crowe Horwath LLP a copy of the disclosures contained herein and requested that Crowe Horwath LLP furnish it with a letter addressed to the Securities and Exchange Commission stating whether or not Crowe Horwath LLP agrees with its statements in this Item 4.01. A copy of the letter dated July 6, 2012, furnished by Crowe Horwath LLP in response to such request, is filed as Exhibit 16 to this Form 8-K.
On June 29, 2012, the Audit Committee of the Board of Directors of the Company engaged Crowe Horwath (HK) CPA Limited (“Crowe HK”) as the Company’s independent registered accounting firm.
During its two most recent fiscal years ended December 31, 2011 and 2010, and the subsequent interim period through the engagement of Crowe HK on June 29, 2012, the registrant did not consult with Crowe HK on (i) the application of accounting principles to a specified transaction, either completed or proposed, or the type of audit opinion that might be rendered on the registrant’s financial statements, and Crowe HK did not provide either a written report or oral advice to the registrant that was an important factor considered by the registrant in reaching a decision as to any accounting, auditing, or financial reporting issue; or (ii) the subject of any disagreement, as defined in Item 304 (a)(1)(iv) of Regulation S-K and the related instructions, or a reportable event within the meaning set forth in Item 304(a)(1)(v) of Regulation S-K.
| 28 |
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our Exchange Act reports is recorded, processed, summarized, and reported within the time periods specified in the Commission’s rules and forms, and that such information is accumulated and communicated to our management, including our principal executive officer (Chief Executive Officer) and principal financial officer (Chief Financial Officer) (together, the “Certifying Officers”), as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management necessarily is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
As of December 31, 2012, the end of the fiscal year covered by this report, our management, under the supervision and with the participation of the Certifying Officers, performed an evaluation of the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act). Based on the evaluation, the Certifying Officers concluded that, as of December 31, 2012, our disclosure controls and procedures were not effective.
The conclusion that our disclosure controls and procedures were not effective was due to the presence of material weaknesses in internal control over financial reporting, as identified below under the heading “Management’s Annual Report on Internal Control over Financial Reporting.” Management anticipates that such disclosure controls and procedures will not be effective until the material weakness described below is remediated.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rule 13a-15(f) and 15d-15(f) promulgated under the Exchange Act. Our internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America. Our internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles in the United States of America, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on the financial statements.
Any system of internal control, no matter how well designed, has inherent limitations, including the possibility that a control can be circumvented or overridden and misstatements due to error or fraud may occur and not be detected in a timely manner. Also, because of changes in conditions, internal control effectiveness may vary over time. Accordingly, even an effective system of internal control will provide only reasonable assurance with respect to financial statement preparation.
Our management assessed the effectiveness of our internal control over financial reporting based on criteria in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. In our assessment of the effectiveness of our internal control over financial reporting as of December 31, 2012, we determined that we had a material weakness related to the lack of requisite U.S. GAAP experience of our finance team, including those primarily responsible for the preparation of our books and records and financial statements. A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our company’s annual or interim financial statements will not be prevented or detected on a timely basis. We intend to remedy the material weakness as discussed below.
As indicated in Item 9A(T) of our Annual Report on Form 10-K for the year ended December 31, 2009 (the “2009 Form 10-K”), we concluded that the Company’s personnel lacked the requisite U.S. GAAP experience. Management’s assessment of the control deficiency over accounting and finance personnel as of December 31, 2012 and 2011 considered the same factors, which included:
| a. | the number of adjustments proposed by our independent auditors during our quarterly review and annual audit processes; |
| b. | the significance of the audit adjustments impact on the overall financial statements; |
| c. | how appropriately we complied with U.S. GAAP on transactions; and |
| d. | how accurately we prepared supporting information to provide to our independent auditors on a quarterly and annual basis. |
Based on the above factors, management concluded that the control deficiency regarding our accounting and finance personnel’s lack of U.S. GAAP experience continues to constitute a material weakness as of December 31, 2012.
| 29 |
Remediation of Material Weaknesses in Internal Control over Financial Reporting
Since 2010, we carried out a number of measures to address and remediate our previously identified material weaknesses related to the insufficient number of our U.S. reporting accounting and finance personnel and overall lack of requisite experience in U.S. GAAP. In particular, the Company changed financial leadership and hired industry veterans to oversee U.S. GAAP accounting and SEC reporting; reengineered period-end financial reporting procedures, increased supervision and review, and consolidated various accounting operations to enhance accuracy and controls over processing of financial data. In addition, the management improved communications with the Audit Committee and external independent auditors, and provided necessary training and mentorship to key staff to improve their U.S. GAAP knowledge and encourage their professional growth. As the result, the Company saw a steady improvement in its internal controls over financial reporting as evidenced by gradually decreased number and significance of adjustments proposed by the Company’s independent auditors during their periodical financial reviews; and noticeable improvement in the accounting staff’s abilities to handle U.S. GAAP-based reporting. In 2012, the Company hired additional accounting staff, realigned the responsibilities of key personnel involved in financial management, and continued the efforts that we initiated previously to streamline and standardize accounting processes.
Although we made significant efforts and achieved progressive improvement to address and remediate our previously identified material weakness, the Company believes that, such efforts have not yet yielded the results required to fully remediate the material weakness.
In the first quarter of 2013, besides the remediation measures described above, we engaged a third party consulting firm which is a global leading provider of consulting and internal audit services and the only consulting firm represented on the COSO advisory board guiding the development of a conceptual framework on Enterprise Risk Management to assist us in our efforts to adequately address and remedy the identified material weakness. With their assistance, we are going to improve our accounting policies and procedures manual in accordance to US GAAP; provide proper training on the manual for our accounting team; continue to standardize our accounting treatments in compliance with US GAAP, especially non-routine transactions; and improve our accounting team’s US GAAP competence.
We intend to implement these steps and measures during the first half of 2013 and will conduct quarterly assessments of the state of the Company’s financial reporting measures and systems, as a whole.
This Annual Report does not include an attestation report of our independent registered public accounting firm regarding internal control over financial reporting.
Our management’s report was not subject to attestation by our independent registered public accounting firm pursuant to the rules of the SEC that permit us to provide only management’s report in this Annual Report.
Changes in Internal Controls over Financial Reporting
Other than the changes described above, there were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) promulgated under the Exchange Act) during the year ended December 31, 2012 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
Not applicable.
| 30 |
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The following table sets forth the names and ages of our directors and executive officers as of March 15, 2013:
| Name | Age | Position(s) with the Company | ||
| Weibing Lu | 49 | Chairman of the Board of Directors and Chief Executive Officer | ||
| Wei Wen | 44 | Secretary, Director | ||
| Mark D. Chen | 44 | Independent Director(1)(2)(3) | ||
| R. Scott Cramer | 48 | Director | ||
| Qiang Fan | 56 | Independent Director(1)(3) | ||
| Chengtun Qu | 46 | Independent Director | ||
| Bing Mei | 48 | Chief Financial Officer(4) | ||
| Weirong Shen | 46 | Independent Director(1)(3) |
| (1) | Member of the Audit Committee. |
| (2) | Audit Committee financial expert. |
| (3) | Member of the Compensation Committee. |
| (4) | Appointed in July 2011 following Michael Lan’s resignation as the Company’s Chief Financial Officer. |
Board of Directors
Our Board oversees our business affairs and monitors the performance of our management. Each director and executive officer holds office until his successor is duly elected and qualified, his resignation or he is removed in the manner provided by our Bylaws. All officers are appointed and serve at the discretion of the Board. All of our officers devote their full-time attention to our business. Our bylaws provide that our directors will hold office until the Annual Meeting of shareholders or until their successors have been elected and qualified. Our Board is responsible for the business and affairs of our Company and considers various matters that require its approval.
Biographical information with respect to the Company’s current executive officers and directors is provided below.
Weibing Lu. Mr. Weibing Lu received his Bachelor’s degree in science from Wuhan University of Mapping Science and Technology (now known as Wuhan University) in 1985. In 1986, he was a teacher of College of Xi’an Geology. Mr. Lu attended Xi’an Jiao Tong University in 1999 where he received a Master’s degree in Business Administration in 2002. Mr. Lu has vast experience in the biotechnology field and in enterprise management. In 1992, he founded the Xi’an Xingji Electronic Engineering Company and served as its Chairman and President until 1997. In 2002, he was awarded as the title of “Outstanding Enterpriser of Xi’an Feed Industry” and appointed as a director of Xi’an Institute of Feed Industry. In July 1997, he founded Xi’an Tianxing Science and Technology Development Co., Ltd. In December 2003, Xi’an Tianxing Science and Technology Development Co., Ltd., was reorganized and became Xi’an Tianxing Bio-Pharmaceutical Co., Ltd. Since December 2003, Mr. Lu has served as Chairman of the Board and General Manager of Xi’an Tianxing Bio-Pharmaceutical Co., Ltd. Weibing Lu’s substantial experience in the pharmaceutical industry and his day to day leadership as Chief Executive Officer of our Company provides him with intimate knowledge of our business and operations.
Wei Wen. Mr. Wei Wen graduated from Xi’an University of Science and Industry (also known as Xi’an University of Technology) in 1989. From 1990 to 1994, Mr. Wen was the manager of Sales Department of Xi’an Zhongtian Science and Technology Development Co., Ltd. From 1994 to 1997, Mr. Wen served as Vice General Manager and Manager of Sales Department of Xi’an Xingji Electronic Engineering Company. In 1997, Mr. Wen was appointed as the Vice General Manager of Xi’an Tianxing Science and Technology Development Co., Ltd. which he served until December 2003. After the reorganization of the company in December 2003, Mr. Wen was appointed and continues to serve as Vice General Manager and a Director of Xi’an Tianxing Bio-Pharmaceutical Co., Ltd. (including as secretary of the Board of Directors). Wei Wen’s strong technology and organizational background provides an important addition to the Company’s Board and the Company’s operations, in general.
Mark D. Chen. Mr. Chen is the chairman and chief executive officer of Pantheon China Acquisition Corp., a U. S. publicly traded acquisition company he founded in 2006 focusing on pre-IPO Chinese companies. Since 1998, Mr. Chen has been a founding general partner and has served in various positions, including managing director and currently a venture partner, with Easton Capital Investment Group and its various affiliated funds, a New York based private equity investment firm. Mr. Chen has also worked extensively in China and was a founder and senior executive of SureData Inc., a marketing and distribution company in China in 1997. Mr. Chen received a B.S. from the Shanghai Jiao Tong University in Shanghai, China, an M.S. from Pennsylvania State University and an M.B.A. from the Columbia Business School at Columbia University. Mr. Chen’s extensive international corporate and private company experience represents a valuable addition to the Company’s Board of Directors.
| 31 |
R. Scott Cramer. Mr. R. Scott Cramer was previously the Chairman from November 2001 to November 2005, Chief Executive Officer from March 2002 to November 2005, and Chief Financial Officer from April 2003 to November 2005, of The Cyber Group Network Corporation. He is currently a member of our Board of Directors. Mr. Cramer is the founder and President of Cramer & Rauchegger, Inc., a firm specializing in retirement management, estate planning and wealth management. He has been a Registered Investment Advisor since August 2001, a Securities Selling Representative since May 1999, and a General Securities Representative (Registered Representative) since July 2002. Mr. Cramer is a graduate of Seminole State College. He received certification as a Chartered Retirement Planning Counselor from the College of Financial Planning in 2001, as a Certified Estate Planning Professional from the Abts Institute for Estate Preservation in 2001, and as a Certified Senior Advisor from the Society of Senior Advisors in 2002. Mr. Cramer’s extensive financial, corporate finance and capital markets experience and background provide valuable additions to the Company’s Board.
Qiang Fan. Mr. Qiang Fan also serves as chairman of the compensation committee and member of the audit committee. Mr. Fan is the President and Founder of MIC Consulting Group, U.S.A., which he established in 1992 to provide operational and financial related problem solving services to privately owned companies. Since 2007, Mr. Fan is the exclusive representative of North America operation for China Venture Capital Research Institute, and the head analyst at Power Partner Institute focusing on IT trends since 2001. From 2006 to 2007, Mr. Fan was a Vice-president of Operation at Kantan Inc., a privately-held boutique technology company focused on wireless solutions for device manufacturers. From 2005 to 2006, he was a Vice-president at Third Wave Ventures, which provides corporate venturing-related advisory, consulting and management services. From 1998 to 2000, Mr. Fan was the exclusive representative in China for PowerQuest, a Utah based international software company that focused on computer data storage management, as well as for ChipCoolers, a U.S. CPU cooler manufacturer. Mr. Fan received his B.A. degree from the Business School of California State University at San Francisco. Qiang Fan provides ongoing expertise and insights in the areas of corporate organization, marketing and consulting.
Chengtun Qu. Since March 2003, Dr. Chengtun Qu has been the Vice Dean of the College of Chemistry and Chemical Engineering at Xi’an Shi You University, where he also teaches and heads the environmental engineering department. Dr. Qu is a board member of both the Shaanxi Province Environmental Protection Association and the Shaanxi Province Chemical Engineering Association. As a principal researcher, Dr. Qu has participated in various projects at both national and provincial levels, including ones sponsored by the Chinese Ministry of Science and Technology, and is the recipient of numerous accolades from the Shaanxi provincial government in recognition of his contributions. Dr. Qu has three patents issued by the Chinese State Intellectual Property Office. He has also been extensively published in various scientific journals both in China and abroad. Dr. Qu has a B.S. degree in chemistry from Northwest University in Xi’an, a master’s degree in applied chemistry from Southwest Petroleum University in July 1993 and a doctorate degree in biochemistry from Xi’an Jiaotong University in July 2006. Chengtun Qu’s technical expertise and background constitute valuable additions to the Board of the Company.
Weirong Shen. Mr. Weirong Shen currently serves as Deputy Director of Shaanxi Province Institute of Microbiology and Director of Microorganism Resources Research Center, Vice Chairman of Shaanxi Province Biochemistry and Molecular Biology Institute, Vice Chairman of Shaanxi Province Food Technology Institute, Deputy Director of Professional Committee of Shaanxi Province Bio-Chemical, Executive Director of Shaanxi Province Society for Microbiology Research Federation, Adjudicator of Food Safety Standards for Shaanxi Province Department of Health, and Member of Committee of Experts for Xi'an Quality Inspection Department. Mr. Shen also serves as Chairman of the Board for Shaanxi Boao Pharmaceutical Co., Ltd. since 2010. Previously, he served as Board Director of Xi’an Strong Biological Engineering Company. Mr. Shen has nearly thirty years of distinguished career in research and development of resources of microbial strains, fermentation engineering, food, feed additives, and genetically engineered drugs and received numerous science and technology awards throughout his career. He led more than thirty major R&D projects in Shaanxi Province and oversaw the design and technology transfer for a number of fermentation of calcium lactate, lactate health beverage, and microecological feed additives production lines. Mr. Shen is a graduate of Northwest University Department of Microbiology. The Company believes that Mr. Shen will bring his substantial academic, R&D and scientific expertise to the Board to enhance the Company’s skill set in those areas.
There are no material proceedings to which any director, executive officer or affiliate of the Company, any owner of record or beneficial owner of more than five percent of any class of voting securities of the Company, or any associate of any such director, executive officer, affiliate or security holder is a party adverse to the Company or has a material interest adverse to the Company. There were no material changes to the procedures by which shareholders may recommend nominees to the Board since the Company’s last disclosure of such policies.
No director or officer of the Company has, during the last 10 years, been subject to or involved in any legal proceedings described under Item 401(f) of Regulation S-K, been convicted of any criminal proceeding (excluding traffic violations or similar misdemeanors), or been a party to a civil proceeding of a judicial or administrative body of competent jurisdiction and as a result of such proceeding was or is subject to a judgment, decree or final order enjoining future violations of, or prohibiting or mandating activities subject to, United States federal or state securities laws or finding any violations with respect to such laws.
Other than the proceedings described in Item 3 of this Annual Report, we are not involved in any material legal proceedings, and we are not aware of any material legal proceedings pending or threatened against us. We are also not aware of any material legal proceedings involving any of our directors, officers, or affiliates or any owner of record or beneficially of more than 5% of any class of our voting securities.
Code of Ethics
Our Board has adopted a Code of Ethics within the meaning of Item 406 of Regulation S-K that applies to all of our officers and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. Our Code codifies the business and ethical principles that govern our business. The Code is designed to deter wrongdoing and to promote: (i) honest and ethical conduct, including the ethical handling of actual or apparent conflicts of interest between personal and professional relationships; (ii) full, fair, accurate, timely, and understandable disclosure in reports and documents that we file with, or submit to, the SEC and in other public communications made by us; (iii) compliance with applicable governmental laws, rules and regulations; (iv) the prompt internal reporting of violations of the ethics code to an appropriate person or persons identified in the code; and (v) accountability for adherence to the Code. A copy of this Code is available at the Company’s website at http://www.skystarbio-pharmaceutical.com and is available at no charge by contacting the Company at its headquarters as listed on the cover page of this report. We will provide to any person, without charge and upon request, a copy of the code of ethics. Any such request must be made in writing to the Company, Attn: Corporate Secretary, 4/F Building B, Chuangye Square, No. 48 Keji Road, Gaoxin District, Xi’an, Shaanxi Province, P.R. China.
| 32 |
Audit Committee
The Audit Committee assists the Board in the oversight of the audit of our consolidated financial statements and the quality and integrity of our accounting, auditing and financial reporting processes. The Audit Committee is responsible for making recommendations to the Board concerning the selection and engagement of independent registered public accountants and for reviewing the scope of the annual audit, audit fees, results of the audit and auditor independence. The Audit Committee also reviews and discusses with management and the Board such matters as accounting policies, internal accounting controls and procedures for preparation of financial statements. Our Board has determined that each of the members of the Audit Committee meets the criteria for independence under the standards provided by Nasdaq. All members of the Audit Committee are “independent” as defined under Rule 10A-3 under the Exchange Act. The Board has determined that Mr. Chen is an “audit committee financial expert” as defined under the Securities Act.
The Audit Committee is entrusted with:
(i) reviewing whether or not management has maintained the reliability and integrity of the accounting policies and financial reporting and disclosure practices of the Company;
(ii) reviewing whether or not management has established and maintained processes to ensure that an adequate system of internal controls is functioning within the Company;
(iii) reviewing whether or not management has established and maintained processes to ensure compliance by the Company with legal and regulatory requirements that may impact its financial reporting and disclosure obligations;
(iv) overseeing the selection and retention of the Company’s independent registered public accounting firm, and their qualifications and independence;
(v) preparing a report of the Audit Committee for inclusion in the proxy statement for the Company’s annual meeting of shareholders;
(vi) reviewing the scope and cost of the audit, the performance of the independent registered public accounting firm, and their report on the annual financial statements of the Company; and
(vii) performing all other duties as the Board may from time to time designate.
Section 16(a) Beneficial Ownership Compliance during 2012
Section 16(a) of the Exchange Act requires our officers, directors and persons who own more than 10% of a registered class of our equity securities within specified time periods to file certain reports of ownership and changes in ownership with the SEC. Based solely upon a review of Forms 3 and Forms 4 furnished to the Company pursuant to Rule 16a-3 under this Exchange Act during the Company’s most recent fiscal year, and Forms 5 with respect to the most recent fiscal year, the Company believes that, except for Forms 3 and 4 for Weirong Shen filed on May 17, 2012, respectively, Form 4 for Qiang Fan filed on May 17, 2012, and Form 3 for Scott Cramer filed on June 18, 2012, which were inadvertently filed late, all such forms required to be filed pursuant to Section 16(a) were timely filed as necessary by the executive officers, directors and security holders required to file same during the fiscal year ended December 31, 2012.
ITEM 11. EXECUTIVE COMPENSATION
Summary Compensation Table
The following table sets forth information concerning the compensation for the fiscal years ended December 31, 2012 and 2011, respectively, of our (i) Chief Executive Officer, (ii) Chief Financial Officer, and (iii) all other executive officers (collectively, the “named executive officers”):
| 33 |
| Name and Principal Position | Year | Salary
($) | Bonus
($) | Stock Awards ( $) | Option Awards ($) | Non-
Equity Incentive Plan Compensation ($) | Non- qualified Deferred Compensation Earnings ($) | All
Other Compensation ($) | Total
($) | |||||||||||||||||||||||||||
| Weibing
Lu, CEO (1) | 2012 | $ | 115,763 | $ | — | $ | 601,999 | $ | — | $ | — | $ | — | $ | — | $ | 717,762 | |||||||||||||||||||
| 2011 | $ | 110,250 | $ | — | $ | $ | — | $ | — | $ | — | $ | — | $ | 110,250 | |||||||||||||||||||||
| Bing
Mei CFO (2) | 2012 | $ | 135,000 | $ | — | $ | 63,740 | $ | — | $ | — | $ | — | $ | — | $ | 198,740 | |||||||||||||||||||
| 2011 | $ | 45,975 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 45,975 | ||||||||||||||||||||
| Michael
H. Lan Former CFO (3) | 2011 | $ | 85,900 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 85,900 | |||||||||||||||||||
| (1) | The Company entered into a five-year employment agreement with Mr. Lu on May 5, 2008. Under the agreement, Mr. Lu is entitled to receive an initial annual compensation of $100,000 as our Chief Executive Officer. The agreement provides that the Company intends to increase his annual salary by 5% each year. Additionally, at the discretion of the Board’s Compensation Committee, Mr. Lu may be eligible for an annual bonus, which amount, if any, and payment will be determined by the Compensation Committee. |
| (2) | Mr. Mei was appointed as our CFO on July 29, 2011. On the same day, the Company entered into a one-year employment agreement with Mr. Mei. Under the agreement, Mr. Mei is entitled to receive an annual compensation of $110,000 and an aggregate 8,000 shares of common stock, 4,000 shares of which shall be issuable on the 6 month anniversary and the remainder 4,000 shares of which shall be issuable on the 12 month anniversary. On July 29, 2012, we executed an employment agreement to continue Mr. Mei’s employment as our Chief Financial Officer. The term of this engagement is one year, during which time Mr. Mei will be entitled to receive an annual salary in the amount of USD$170,000 and 8,000 shares of our common stock, payable in four equal installments of 2,000 shares per quarter. |
| (3) | Mr. Lan was our CFO from April 16, 2010 until July 29, 2011. |
Employment Agreement with Weibing Lu, CEO
We have a five-year Employment Agreement with Mr. Lu dated May 5, 2008. Under the agreement, Mr. Lu’s annual salary in 2009 was $100,000 and in 2010 was $105,000. His agreement provides that the company intends to increase his annual salary by 5% each year. Additionally, at the discretion of the Board’s Compensation Committee, Mr. Lu may be eligible for an annual bonus, which amount, if any, and payment will be determined by the Compensation Committee. Mr. Lu is entitled to medical, disability, and life insurance, as well as 4 weeks of vacation annually and reimbursement of all reasonable or authorized business expenses. During its term, the Employment Agreement terminates upon Mr. Lu’s death, in which event we are obligated to pay Mr. Lu’s estate his base salary amount through the first anniversary of his death (or the expiration of the agreement if earlier than the anniversary date), as well as pro rata allocation of any bonus based on the days of service during the year of death, and all amounts owing to Mr. Lu at the time of termination, including for previously accrued but unpaid bonuses, expense reimbursements, and accrued but unused vacation pay. If Mr. Lu is unable to perform his obligations under the agreement for over 180 consecutive days during any consecutive 12 month period, we may terminate the agreement by written notice to Mr. Lu delivered prior to the date that he resumes his duties. Upon receipt of such written notice, Mr. Lu may request a medical examination under which if he is certified to be incapable of performing his obligations for over 2 additional months, the agreement is terminated. We are obligated to pay Mr. Lu his base salary through the second anniversary of our notice to him of his termination, less any amount Mr. Lu may receive for such period from any Company-sponsored or Company-paid for source of insurance, disability compensation, or governmental program. We will also pay Mr. Lu pro rata allocation of any bonus based on the days of service during the year our notice is issued, and all amounts owing to Mr. Lu at the time of termination, including for previously accrued but unpaid bonuses, expense reimbursements, and accrued but unused vacation pay.
We may also terminate the agreement for cause upon notice if at any time Mr. Lu: (a) refuses in bad faith to carry out specific written directions of our board of directors; (b) intentionally takes fraudulent or dishonest action in his relations with us; (c) is convicted of a crime involving an act of significant moral turpitude; or (d) knowingly commits an act or omits to act in violation of our written policies, the agreement or any agreements that we may have with third parties and that is materially damaging to our business or reputation. Termination, however, for cause described in (a), (b), or (d) is predicated first on Mr. Lu receiving a 5-day written notice and a reasonable opportunity to present his position, then a subsequent written notice of the termination, with the termination to take effect 20 business days thereafter if Mr. Lu does not dispute the cause for the termination or fails to take corrective actions in good faith. Thereafter, if Mr. Lu takes corrective actions, he may be terminated for the same misconduct upon a 5-day written notice. On the other hand, Mr. Lu may terminate the agreement upon written notice if: (w) there is a material adverse change in the nature of his title, duties, or obligations; (x) we materially breach the agreement; (y) we fail to make any payment to Mr. Lu (excepting any payment that is not material and that we are contesting in good faith); or (z) there is a change of control of the Company. Termination, however, for cause described in (w), (x), or (y) is predicated on our receiving a written notice from Mr. Lu specifying the cause, with the termination to take effect if we fail to take corrective action within 20 business days thereafter. If Mr. Lu terminates the agreement for any one of these reasons, or if we terminate the agreement without cause, we are obligated to pay to Mr. Lu (or in the case of his death, his estate), his base salary and any bonus, without any offset, as well as all amounts owing to Mr. Lu at the time of termination, including for previously accrued but unpaid bonuses, expense reimbursements, and accrued but unused vacation pay.
| 34 |
The agreement also contains restrictive covenants: (i) preventing the use and/or disclosure of confidential information during or at any time after termination; (ii) preventing competition with the Company during his employment and for a period of 3 years after termination (including contact with or solicitation of our customers, employees, or suppliers), provided that Mr. Lu may make investments of up to 2% in the publicly-traded equity securities of any competitor of the Company; (iii) requiring Mr. Lu to refer any business opportunities to the Company during his employment and for a period of 1 year after termination. Mr. Lu, however, shall have no further obligations with respect to competition and business opportunities if his employment is terminated without cause or if he terminates his employment for cause. We are obligated under the agreement to indemnify Mr. Lu for any claims made against him in his capacity as our Chief Executive Officer and, in connection with such obligation, we have included him under a directors’ and officers’ insurance policy that is in effect during his employment as our officer, director, or consultant.
Employment Agreement with Bing Mei, CFO
Upon his appointment as the Company’s Chief Financial Officer in July 2011, we entered into an employment agreement with Mr. Mei, dated July 29, 2011. For his services as the Company’s CFO, we agreed to compensate Mr. Mei with a base salary of $110,000 for a term of 1 year and a total 8,000 shares of common stock, 4,000 of which will be issued on the 6-month anniversary of his employment and the other 4,000 of which will be issued on the 1-year anniversary of his employment. While employed as the registrant’s CFO, Mr. Mei may continue to devote his business time to operating Bing Mei, CPA. On July 29, 2012, we executed an employment agreement to continue Mr. Mei’s employment as our Chief Financial Officer. The term of this engagement is one year, during which time Mr. Mei will be entitled to receive an annual salary in the amount of USD$170,000 and 8,000 shares of our common stock, payable in four equal installments of 2,000 shares per quarter.
Employment Agreement with Michael H. Lan (former CFO)
On April 16, 2010, we entered into an employment agreement with Mr. Lan pursuant to which we have engaged his service as our Chief Financial Officer for a period of one year for annual compensation of $100,000 as well as reimbursement for reasonable expenses incurred in connection with the performance of his duties, including travel expenses. We have also included Mr. Lan under a directors’ and officers’ insurance policy. On July 29, 2011, Mr. Lan resigned from the CFO office.
Outstanding Equity Awards in 2012
Except as set forth below, there were no outstanding equity awards in 2012. On July 29, 2012, we executed an employment agreement to continue Mr. Mei’s employment as our Chief Financial Officer. The term of this engagement is one year, during which time Mr. Mei will be entitled to receive 8,000 shares of our common stock, payable in four equal installments of 2,000 shares per quarter. For the year ended December 31, 2012, there were no options exercised and 2,000 shares of stock that vested for our named executive officers.
Director Compensation in 2012
| Name | Fees
Earned or Paid in Cash ($) | Stock
Awards ($) | Option Awards ($) | Non-Equity
Incentive Plan Compensation ($) | Non-
Qualified Deferred Compensation Earnings ($) | All
Other Compensation ($) | Total
($) | |||||||||||||||||||||
| Weibing Lu | $ | 115,763 | $ | 601,999 | $ | — | $ | — | $ | — | $ | — | $ | 717,762 | ||||||||||||||
| Wei Wen | $ | $ | 108,360 | $ | — | $ | — | $ | — | $ | — | $ | 108,360 | |||||||||||||||
| R. Scott Cramer | $ | 30,000 | $ | 49,020 | $ | — | $ | — | $ | — | $ | — | $ | 79,020 | ||||||||||||||
| Qiang Fan | $ | 30,000 | $ | 25,800 | $ | — | $ | — | $ | — | $ | — | $ | 55,800 | ||||||||||||||
| Weirong, Shen | $ | 2,387 | $ | 4,902 | $ | — | $ | — | $ | — | $ | — | $ | 7,289 | ||||||||||||||
| Chengtun Qu | $ | 3,178 | $ | $ | — | $ | — | $ | — | $ | — | $ | 3,178 | |||||||||||||||
| Shouguo Zhao | $ | 1,978 | $ | $ | — | $ | — | $ | — | $ | — | $ | 1,978 | |||||||||||||||
| Mark D. Chen | $ | 14,000 | $ | 50,802 | $ | — | $ | — | $ | — | $ | — | $ | 64,802 | ||||||||||||||
| 35 |
Agreements with Directors
On March 30, 2010, we entered into an agreement with R. Scott Cramer to memorialize the terms under which he has been acting as our United States representative since November 2006. Under the terms of the agreement, we agreed to compensate Mr. Cramer from January 1, 2010 through March 31, 2010 in the amount of $7,500 per year and a stock grant of 2,500 shares of our restricted common stock. On April 16, 2010, we entered into a services agreement for Mr. Cramer to continue to act in this capacity. The services agreement renewed Mr. Cramer’s term for an additional 1-year period beginning on April 1, 2010, for an annual fee of $30,000 payable in four quarterly installments of $7,500 at the end of each quarter. Additionally, Mr. Cramer is entitled to receive 10,000 restricted shares of common stock under our 2010 Incentive Stock Plan. The shares will vest in four installments of 2,500 shares at the end of each quarter with the first vesting date on June 30, 2010. The number and the original value of the shares will be proportionately adjusted for any increase or decrease in the number of issued shares resulting from a recapitalization, subdivision, or consolidation of shares or the payment of a stock dividend, or any other increase or decrease in the number of such shares effected without receipt of consideration by the Company. We have the right to repurchase all or any portion of the shares at a price equal to the original amount paid for the shares by Mr. Cramer upon the termination of the services agreement or any attempted transfer of shares in violation of the services agreement. We have also included Mr. Cramer under a directors’ and officers’ insurance policy.
Under our agreement with Qiang Fan, Mr. Fan receives annual compensation of $30,000 for his services as a member of the Board, Chairman of the Compensation Committee, and a member of the Audit Committee. Mr. Fan’s annual compensation will be paid in cash, but, at the discretion of the Board, up to $8,000 of his annual compensation may be paid in the form of shares of our common stock under our Stock Incentive Plan #2. During his term as a director, we have included Mr. Fan under a directors’ and officers’ insurance policy. In addition, we agreed to reimburse Mr. Fan for reasonable expenses incurred in connection with the performance of duties as a director of the Company, including travel expenses.
Under our agreement with Chengtun Qu, Mr. Qu receives annual compensation of RMB 20,000 or about $3,200 for his services as a member of the Board. In addition, we agreed to reimburse Mr. Qu for reasonable expenses incurred in connection with the performance of duties as a director of the Company, including travel expenses. We have also included Mr. Qu under a directors’ and officers’ insurance policy.
Under our agreement with Shouguo Zhao, Mr. Zhao receives annual compensation of RMB 50,000 or about $7,900 for his services as a member of the Board, Audit Committee, and Compensation Committee. In addition, we agreed to reimburse Mr. Zhao for reasonable expenses incurred in connection with the performance of duties as a director of the Company, including travel expenses. We have also included Mr. Zhao under a directors’ and officers’ insurance policy. During the Annual Meeting of Shareholders held on April 27, 2012, Mr. Zhao was not reelected as a member of the Board, Audit Committee, and Compensation Committee.
Under our agreement with Mark D. Chen, Mr. Chen receives annual compensation of $14,000 for his services as a member of the Board, Chairman of the Audit Committee, and a member of the Compensation Committee. Additionally, Mr. Chen has the right to receive 5,556 shares of our restricted common stock at the beginning of each term of his directorship. We have included Mr. Chen under a directors’ and officers’ insurance policy, and will reimburse him for reasonable expenses incurred in connection with the performance of duties as a director of the Company, including travel expenses.
Under our agreement with Weirong Shen, Mr. Shen receives annual compensation of RMB 20,000 or about $3,200 for his services as a member of the Board. In addition, we agreed to reimburse Mr. Shen for reasonable expenses incurred in connection with the performance of duties as a director of the Company, including travel expenses. We have also included Mr. Shen under a directors’ and officers’ insurance policy.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Security Ownership of Certain Beneficial Owners and Management
Set forth below is information regarding the beneficial ownership of our common stock as of March 15, 2013 by:
| • | each person known to us that beneficially owns more than 5% of our outstanding shares of common stock; |
| • | each of our directors; |
| • | each of our named executive officers; and |
| • | all of our current directors and executive officers as a group. |
We believe that, except as otherwise noted below, each named beneficial owner has sole voting and investment power with respect to the shares listed. Unless otherwise indicated herein, beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission, and includes voting or investment power with respect to shares beneficially owned. Shares of common stock underlying options or warrants currently exercisable or exercisable on or within 60 days of the date hereof are deemed outstanding for computing the percentage ownership of the person holding the options or warrants, but are not deemed outstanding for computing the percentage ownership of any other person. Unless otherwise indicated in the footnotes, the address for each principal shareholder is in the care of Skystar, 4/F Building B Chuangye Square, No. 48 Keji Road, Gaoxin District, Xi’an, Shaanxi Province, P.R. China. There are no arrangements, known to the Company, including any pledge by any person of securities of the registrant, the operation of, which may, at a subsequent date, result in a change of control of the registrant.
| 36 |
| Name and Address of Beneficial Owners | Amount of Beneficial Ownership | % of Class (1) | ||||||
| Weibing Lu, Director and Chief Executive Officer (2) | 1,172,459 | 15.4 | % | |||||
| Wei Wen, Director (3) | 83,544 | 1.1 | % | |||||
| Bing Mei, Chief Financial Officer (4) | 18,000 | * | ||||||
| R. Scott Cramer, Director (5) | 262,266 | 3.4 | % | |||||
| Qiang Fan, Director (6) | 10,000 | * | ||||||
| Chengtun Qu, Director (7) | — | — | ||||||
| Mark D. Chen, Director (8) | 26,668 | * | ||||||
| Weirong Shen , Director (9) | 1,900 | * | ||||||
| Upform Group Limited (2) | 1,172,459 | 15.4 | % | |||||
| All officers and directors as a group (8 total) | 1,574,837 | 20.7 | % | |||||
| * | Less than 1%. |
| (1) | Unless otherwise noted, the number and percentage of outstanding shares of our common stock is based upon 7,604,800 shares outstanding as of record date. |
| (2) | Weibing Lu and Xinya Zhang are directors of Upform Group Limited. Mr. Lu is the majority stockholder and Chairman of the Board of Directors of Upform Group, and indirectly owns the shares held by Upform Group through his majority ownership. Thus, the number of shares reported herein as beneficially owned by Mr. Lu includes the shares held by Upform Group. Similarly, because Xinya Zhang is a director of Upform Group, he may be deemed to have or share investment control over Upform Group’s portfolio, and the number of shares reported herein as beneficially owned by Mr. Zhang also include the shares held by Upform Group. Upform Group’s address is Sea Meadow House, Blackburne Highway, P.O. Box 116, Road Town, Tortola, British Virgin Islands. |
| (3) | The number of shares reported herein as beneficially owned by Wei Wen are held by Clever Mind International Limited, which address is Sea Meadow House, Blackburne Highway, P.O. Box 116, Road Town, Tortola, British Virgin Islands. Mr. Wen is Chairman of the Board of Directors of Clever Mind and owns approximately 2.3% of the issued and outstanding shares of Clever Mind. As Mr. Wen is a director of Clever Mind, he may be deemed to have or share investment control over Clever Mind’s portfolio. |
| (4) | Appointed on July 29, 2011 as the Company’s Chief Financial Officer. |
| (5) | This number includes 260,098 shares held by the Cramer Family Trust, of which Mr. Cramer is the sole trustee and sole primary beneficiary. This shareholder’s address is 1012 Lewis Dr., Winter Park, FL 32789. |
| (6) | This shareholder’s address is 9176 West Laguna Way, Elk Grove, CA 95758. |
| (7) | This shareholder’s address is No. 18 Dian Zi 2 nd Road, School of Chemistry & Chemical Engineering, Xi'an Shiyou University, Xi'an, Shaanxi Province, People’s Republic of China. |
| (8) | This shareholder’s address is 10-64 #9 Jianguomenwai Avenue, Beijing, China 100600. |
| (9) | This shareholder’s address is No. 229 North Tai Bai Road, School of Economics and Management, Northwest University, Xi'an, Shaanxi Province, People’s Republic of China. |
Securities Authorized for Issuance under Equity Compensation Plans
As discussed above, our 2010 Stock Incentive Plan authorizes awards representing up to 700,000 shares of common stock. As of the date hereof, we have issued 542,881 options pursuant to the Plan. 442,881shares were issued under the Plan in 2012.
Equity Compensation Plan Information as of December 31, 2012
| Plan Category | Number
of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted-average exercise price of outstanding options, warrants and rights | Number
of securities remaining available for future issuance under equity compensation plans | |||||||||
| Equity compensation plans approved by security holders (1) | 0 | 0 | 247,119 | |||||||||
| Equity compensation plans not approved by security holders (2) (3) | 0 | 0 | 538,620 | |||||||||
| TOTAL | 0 | 0 | 785,739 | |||||||||
| 37 |
| (1) | On December 8, 2009, our Board approved a stock incentive plan for officers, directors, employees, and consultants entitled the “Skystar Bio-Pharmaceutical Company 2010 Stock Incentive Plan” (the “2010 Plan”). The maximum number of shares that may be issued under the 2010 Plan is 700,000 shares of our common stock. The 2010 Plan was approved by our stockholders on December 31, 2009, and awards may be granted under the 2010 Plan until December 7, 2019. Under the 2010 Plan, we may issue common stock and/or options to purchase common stock to certain officers, directors, and employees and consultants of the Company and our subsidiaries. The 2010 Plan is administered either by the Board or a committee appointed by the Board, which is comprised of two or more independent directors. The Board has full and complete authority, in its discretion, but subject to the express provisions of the 2010 Plan, to approve the eligible persons nominated by the management of the Company to be granted awards of common stock or stock options and to determine specific terms of awards or option grants. |
| (2) | On February 22, 2006, we adopted a stock incentive plan for consultants entitled the “2006 Consultant Stock Plan” (the “2006 Plan”). The maximum number of shares that may be issued under the 2006 Plan is 1,199,648 shares of our common stock. The 2006 Plan has not previously been approved by security holders and awards may be granted under this Plan until February 21, 2016. Under the 2006 Plan, we may issue common stock to certain consultants of the Company who are crucial to the future growth and success of the Company and our subsidiaries and affiliates. The 2006 Plan is administered by either a committee appointed by the Board, which is comprised of one or more members of the Board who is not serving on another plan committee, or the Board. The Board has full and complete authority, in its discretion, but subject to the express provisions of the Plan, to designate the persons or classes of persons eligible to receive awards of common stock awards and to determine specific terms of awards or option grants. As of December 31, 2011, there are 119,930 shares of our common stock remaining available for future issuance under the 2006 Plan. |
| (3) | On October 16, 2002, we adopted a stock incentive plan for officers, directors, employees, and consultants entitled the “Cyber Group Network Corporation Stock Incentive Plan # 2” (hereinafter the “2002 Plan”). The maximum number of shares that may be issued under the 2002 Plan is 40,000,000 shares of our common stock. The 2002 Plan has not previously been approved by security holders and awards may be granted under this Plan until October 15, 2012. Under the 2002 Plan, we may issue common stock and/or options to purchase common stock to certain officers, directors, and employees and consultants of the Company and our subsidiaries. The 2002 Plan is administered either by the compensation committee or a committee appointed by the Board, which is comprised of a combination of two or more officers and/or members of the Board. The board has full and complete authority, in its discretion, but subject to the express provisions of the Plan to approve the eligible persons nominated by the management of the Company to be granted awards of common stock awards or stock options and to determine specific terms of awards or option grants. As of December 31, 2011, there are 418,690 shares of our common stock remaining available for future issuance under the 2002 Plan. |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Review and Approval Policies and Procedures for Related Party Transactions
Except as disclosed below, during the 2012 fiscal year, we have not been a participant in any transaction that is reportable under Item 404(a) of Regulation S-K. We know of no proposed transaction in which it will be a participant that would be reportable under Item 404(a) of Regulation S-K. If and to the extent there are such transactions, all material related party transactions will be made or entered into on terms that are no less favorable to use than can be obtained from unaffiliated third parties. Related party transactions that we have previously entered into were not approved by independent directors, as we had no independent directors at that time. The Audit Committee consisting solely of independent and disinterested directors of the Board has reviewed, approved and ratified the terms of such transactions.
| December 31, 2012 | December 31, 2011 | |||||||
| Shares to be issued to related parties | ||||||||
| Scott Cramer – non-executive director | $ | - | $ | 149,235 | ||||
| Mark D. Chen – non-executive director | 25,002 | 50,004 | ||||||
| Bing Mei – CFO | 4,340 | - | ||||||
| Total | $ | 29,342 | $ | 199,239 | ||||
| Amounts due to (from) related parties | ||||||||
| Weibing Lu – CEO (1) | $ | 608,282 | $ | (64,333 | ) | |||
| Scott Cramer – non-executive director and shareholder (1) | 138,169 | 147,877 | ||||||
| Officers, shareholders and other related parties (2) | 52,474 | (27,271 | ) | |||||
| Total | $ | 798,925 | $ | 56,273 | ||||
| (1) |
As of December 31, 2012 and 2011, the Company had unpaid reimbursement, compensation, and advances for business expenses due to Weibing Lu valued at $608,282 and due from Weibing Lu valued at $64,333, respectively.
As of December 31, 2012 and 2011, the Company had unpaid reimbursement and compensation due to Scott Cramer valued at $138,169 and $147,877, respectively. |
| 38 |
| (2) | The amounts due to (from) officers, shareholders and other related parties at December 31, 2012 and 2011 include unpaid reimbursement and compensation and, advances to other related parties for business expenses. |
For a description of the company's leased office spaces in Xi’an and Shanghai from Mr. Weibing Lu, the Company’s Chairman and CEO in 2011 and 2012 please refer to Note 20 to the financial statements included in this Annual Report. For a description of short-term loans from financial institutions in 2011 and 2012 which loans were personally guaranteed by the Company’s Chairman and his wife please refer to Note 13 to the financial statements included in this Annual Report.
Director Independence
Our Board is subject to the independence requirements of the NASDAQ Stock Market. Pursuant to the requirements, the Board undertakes an annual review of director independence. During this review, the Board considers transactions and relationships between each director or any member of his immediate family and Skystar and its affiliates, including those transactions that are contemplated under Item 404(a) of Regulation S-K. The purpose of this review is to determine whether any such relationships or transactions exist that are inconsistent with a determination that the director is independent. Our Board has determined that Mark D. Chen, Chengtun Qu, Qiang Fan and Weirong Shen who comprise a majority of our directors and all current members of the Audit Committee and the Compensation Committee are “independent” under the standards provided by the Nasdaq and that the members of the Audit Committee are also “independent” for purposes of Section 10A-3 of the Exchange Act. The Board based its independent determinations primarily on a review of the responses of the directors and executive officers to questions regarding employment and transaction history, affiliations and family and other relationships and on discussions with the directors. None of our directors engages in any transaction, relationship, or arrangement contemplated under section 404(a) of Regulation S-K.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
In April 2011, upon the Audit Committee’s review and approval we engaged Crowe Horwath LLP as our independent registered public accounting firm (“Crowe Horwath”) to audit our financial statements and to perform reviews of interim financial statements. Effective as of June 29, 2012, Crowe Horwath resigned as independent auditors of the Company. The reports of Crowe Horwath on our financial statements as of December 31, 2011 and 2010 and for the years ended December 31, 2011 and 2010 did not contain an adverse opinion or a disclaimer of opinion, and were not qualified or modified as to uncertainty, audit scope, or accounting principles. In connection with the audits of our financial statements for the fiscal periods ended December 31, 2011 and 2010, and through June 29, 2012, there were: (i) no disagreements between our Company and Crowe Horwath on any matters of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of Crowe Horwath, would have caused Crowe Horwath to make reference to the subject matter of the disagreement in its reports on our financial statements for such periods, and (ii) no reportable events within the meaning set forth in Item 304(a)(1)(v) of Regulation S-K.
On June 29, 2012, the Audit Committee of our Board engaged Crowe Horwath (HK) CPA Limited (“Crowe HK”) as our independent registered accounting firm. During its two most recent fiscal years ended December 31, 2011 and 2010, and the subsequent interim period through the engagement of Crowe HK on June 29, 2012, the Company did not consult with Crowe HK on (i) the application of accounting principles to a specified transaction, either completed or proposed, or the type of audit opinion that might be rendered on our financial statements, and Crowe HK did not provide either a written report or oral advice to the registrant that was an important factor considered by us in reaching a decision as to any accounting, auditing, or financial reporting issue; or (ii) the subject of any disagreement, as defined in Item 304 (a)(1)(iv) of Regulation S-K and the related instructions, or a reportable event within the meaning set forth in Item 304(a)(1)(v) of Regulation S-K.
Principal Accountant Fees and Services
The following table presents fees for professional services rendered by our auditors for the fiscal year 2012 and 2011:
| Fiscal Year ended December 31 | ||||||||
| 2012 | 2011 | |||||||
| Audit Fees (1) | $ | 210,000 | $ | 308,000 | ||||
| Audit-Related Fees (2) | $ | 0 | $ | 0 | ||||
| Tax Fees (3) | $ | 0 | $ | 0 | ||||
| All Other Fees (4) | $ | 0 | $ | 0 | ||||
| Total | $ | 210,000 | $ | 308,000 | ||||
| 39 |
(1) Audit Fees. The aggregate fees billed for the audit of our annual financial statements, review of our interim financial statements or services that are normally provided in connection with statutory and regulatory filings or engagements for those fiscal years.
(2) Audited-Related Fees. For the years ended December 31, 2012, and 2011, there were no fees billed by our independent auditors for services reasonably related to the performance of the audit or review of the financial statements outside of those fees disclosed above under “Audit Fees.”
(3) Tax Fees. For the years ended December 31, 2012, and 2011, there were no fees billed by our independent auditors for services rendered for tax compliance, tax advice, and tax planning work to the Company.
(4) All Other Fees. For the years ended December 31, 2012 and 2011, there were no fees billed by our independent auditors for products and services outside of those fees disclosed above under “Audit Fees,” “Audit-Related Fees,” and “Tax Fees.”
Pre-Approval Policies and Procedures of the Audit Committee
Our Audit Committee approves the engagement of our independent auditors and is also required to pre-approve all audit and non-audit expenses. During the fiscal years ended December 31, 2012 and 2011, all of our Audit-Related Fees, Tax Fees, and All Other Fees, respectively, were pre-approved by the Audit Committee. Prior to engaging its accountants to perform particular services, our Board obtains an estimate for the service to be performed. All of the services described above were approved by the Board in accordance with its procedure.
ITEM 15. EXHIBITS
Financial Statements
The following consolidated financial statements are included in Part II, Item 8 of this Report:
Report of Independent Registered Public Accounting Firm – Crowe Horwath (HK) CPA Limited
Report of Independent Registered Public Accounting Firm – Crowe Horwath, LLP
Consolidated Balance Sheets at December 31, 2012 and 2011
Consolidated Statements of Comprehensive Income for the Years Ended December 31, 2012 and 2011
Consolidated Statements of Shareholders’ Equity for the Years Ended December 31, 2012 and 2011
Consolidated Statements of Cash Flows for the Years Ended December 31, 2012 and 2011
Notes to Consolidated Financial Statements
Financial Statement Schedules
Schedules are omitted because the required information is not present or is not present in amounts sufficient to require submission of the schedule or because the information required is given in the consolidated financial statements or the notes thereto.
Exhibits
EXHIBIT INDEX
|
Exhibit Number |
Description | |
| 3.1 | Certificate of Incorporation, as amended (15) | |
| 3.2 | Amended and Restated Bylaws (9) | |
| 4.1 | Certificate of Designation of Series B Convertible Preferred Stock (1) | |
| 4.2 | Form of Class A Convertible Debenture (2) | |
| 4.3 | Form of Class B Convertible Debenture (2) | |
| 4.4 | Form of Class A Warrant (2) | |
| 4.5 | Form of Class B Warrant (2) |
| 40 |
| 4.6 | Form of Common Stock Certificate (12) | |
| 4.7 | Form of Common Stock Purchase Option to be granted to the representative of the underwriters (12) | |
| 10.1 | Form of Securities Purchase Agreement, dated as of February 26, 2007 by and among the Company and eight accredited investors (2) | |
| 10.2 | Form of Registration Rights Agreement, dated as of February 26, 2007 by and among the Company and eight accredited investors (2) | |
| 10.3 | Form of Company Principal Lockup Agreement in connection with the Securities Purchase Agreement dated as of February 26, 2007 (2) | |
| 10.4 | Form of the Amendment, Exchange and Waiver Agreement between the Company and certain accredited investors dated November 9, 2007 (3) | |
| 10.5 | Form of the Amendment and Waiver Agreement between Skystar Bio-Pharmaceutical Company and two institutional and accredited investors dated March 31, 2008 (6) | |
| 10.6 | Amendment to Consulting Services Agreement among Skystar Cayman, Xi’an Tianxing and Sida Biotechnology (Xi’an) Co., Ltd. (“Sida”) dated March 10, 2008 (4) | |
| 10.7 | Agreement to Transfer of Operating Agreement among Skystar Cayman, Xi’an Tianxing, Xi’an Tianxing’s Majority Stockholders, Weibing Lu and Sida dated March 10, 2008 (4) | |
| 10.8 | Amendment to Equity Pledge Agreement among Skystar Cayman, Xi’an Tianxing, Xi’an Tianxing’s Majority Stockholders, and Sida dated March 10, 2008 (4) | |
| 10.9 | Designation Agreement among Skystar Cayman, Xi’an Tianxing, Xi’an Tianxing’s Majority Stockholders, Weibing Lu and Sida dated March 10, 2008 (4) | |
| 10.10 | Agreement to Transfer of Option Agreement among Skystar Cayman, Xi’an Tianxing, Xi’an Tianxing Majority Stockholders, Weibing Lu and Sida dated March 10, 2008 (4) | |
| 10.11 | Employment Agreement with Weibing Lu dated May 5, 2008 (7) ** | |
| 10.13 | Form of Director Offer Letter with Mr. Qiang Fan and Mr. Winston Yen (9) ** | |
| 10.14 | Form of Director Offer Letter with Chengtun Qu and Shouguo Zhao (9) ** | |
| 10.16 | Form of Director Offer Letter with Mr. Bennet P. Tchaikovsky (11) ** | |
| 10.17 | Agreement with R. Scott Cramer dated March 30, 2010 (13) ** | |
| 10.18 | Services Agreement with R. Scott Cramer dated April 16, 2010 (14) ** | |
| 10.19 | Employment Agreement with Michael Lan dated April 16, 2010 (14) ** | |
| 10.20 | Employment Agreement with Bing Mei dated July 29, 2011 (14) ** | |
| 10.21 | Services Agreement with R. Scott Cramer dated August 31, 2011 (14) ** | |
| 10.22 | Lease Agreement between Xi’an Tianxing and Weibing Lu dated June 1, 2007 (5) | |
| 10.23 | Lease Agreement between Shanghai Siqiang Biotechnological Co., Ltd. and Weibing Lu dated June 17, 2007 (8) | |
| 10.24 | Summary of Research Arrangement between Shanghai Poultry Verminosis Institute and Xi’an Tianxing (8) | |
| 10.25 | Cooperation Agreement between Shaanxi Microbial Institute and Xi’an Tianxing (8) | |
| 10.26 | Technology Cooperation Agreement with Fourth Military Medical University (13) | |
| 14.1 | Code of Ethics (10) | |
| 21.1 | List of Subsidiaries (10) |
| 41 |
| 23.1 | Consent of Crowe Horwath, LLP * | |
| 23.2 | Consent of Crowe Horwath (HK) CPA Limited* | |
| 31.1 | Certifications of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 * | |
| 31.2 | Certifications of Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 * | |
| 32.1 | Certifications of Chief Executive Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 * | |
| 32.2 | Certifications of Chief Financial Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 * |
| 101.INS** | XBRL Instance Document † | |
| 101.SCH** | XBRL Taxonomy Extension Schema † | |
| 101.CAL** | XBRL Taxonomy Extension Calculation Linkbase † | |
| 101.DEF** | XBRL Taxonomy Extension Definition Linkbase † | |
| 101.LAB** | XBRL Taxonomy Extension Label Linkbase † | |
| 101.PRE** | XBRL Taxonomy Extension Presentation Linkbase † |
* Filed herewith.
** Indicates a management contract or compensatory plan or an agreement.
† Pursuant to Rule 406T of Regulation S-T, these interactive data files are deemed not filed or part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933 or Section 18 of the Securities Exchange Act of 1934 and otherwise are not subject to liability.
| (1) | Incorporated by reference from the Registrant’s Current Report on Form 8-K filed on November 14, 2005. |
| (2) | Incorporated by reference from the Registrant’s Current Report on Form 8-K filed on March 5, 2007. |
| (3) | Incorporated by reference from the Registrant’s Current Report on Form 8-K filed on December 11, 2007. |
| (4) | Incorporated by reference from the Registrant’s Current Report on Form 8-K on March 11, 2008. |
| (5) | Incorporated by reference from the Registrant’s Annual Report on Form 10-K on April 2, 2008. |
| (6) |
Incorporated by reference from the Registrant’s Current Report on Form 8-K on April 23, 2008.
|
| (7) | Incorporated by reference from the Registrant’s Current Report on Form 8-K on May 5, 2008. |
| (8) | Incorporated by reference from the Registrant’s Registration Statement on Form S-1/A filed on June 26, 2008. |
| (9) | Incorporated by reference from the Registrant’s Current Report on Form 8-K on July 15, 2008. |
| (10) | Incorporated by reference from the Registration’s Annual Report on Form 10-K on April 15, 2009. |
| (11) | Incorporated by reference from the Registration’s Current Report on Form 8-K on May 27, 2009. |
| (12) | Incorporated by reference from the Registration’s Amendment to Registration Statement on Form S-1/A on June 26, 2009. |
| (13) | Incorporated by reference from the Registration’s Annual Report on Form 10-K on March 31, 2010. |
| (14) | Incorporated by reference from the Registration’s Current Report on Form 8-K on April 19, 2010. |
| (15) | Incorporated by reference from the Registration’s Quarterly Report on Form 10-Q on November 15, 2010. |
| (16) | Incorporated by reference from the Registration’s Current Report on Form 8-K on December 17, 2010. |
| (17) | Incorporated by reference from the Registration’s Current Report on Form 8-K on January 4, 2011. |
| 42 |
| (18) | Incorporated by reference from the Registration’s Notification of inability to timely file Form 10-K on Form NT 10-K on April 1, 2011. |
| (19) | Incorporated by reference from the Registration’s Annual Report on Form 10-K on April 11, 2011. |
| (20) | Incorporated by reference from the Registration’s Notification of inability to timely file Form 10-Q on Form NT 10-Q on May 16, 2011. |
| (21) | Incorporated by reference from the Registration’s Quarterly Report on Form 10-Q on May 23, 2011. |
| (22) | Incorporated by reference from the Registration’s Current Report on Form 8-K on May 24, 2011. |
| (23) | Incorporated by reference from the Registration’s Current Report on Form 8-K on August 4, 2011. |
| (24) | Incorporated by reference from the Registration’s Notification of inability to timely file Form 10-Q on Form NT 10-Q on August 16, 2011. |
| (25) | Incorporated by reference from the Registration’s Quarterly Report on Form 10-Q on August 22, 2011. |
| (26) | Incorporated by reference from the Registration’s Current Report on Form 8-K on August 23, 2011. |
| (27) | Incorporated by reference from the Registration’s Amend Quarterly Report on Form 10-Q/A on September 21, 2011. |
| (28) | Incorporated by reference from the Registration’s Quarterly Report on Form 10-Q on November 14, 2011. |
| (29) | Incorporated by reference from the Registration’s Current Report on Form 8-K on November 15, 2011. |
| 43 |
SIGNATURES
Pursuant to the requirements of section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
SKYSTAR BIO-PHARMACEUTICAL COMPANY (Registrant) | |||
| Date: April 1, 2013 | By: | /s/ Weibing Lu | |
| Weibing Lu | |||
| Chief Executive Officer (Principal Executive Officer) | |||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Name | Title | Date | ||
| /s/ Weibing Lu | Chief Executive Officer (Principal Executive Officer), Director | April 1, 2013 | ||
| Weibing Lu | ||||
| /s/ Bing Mei | Chief Financial Officer (Principal Financial and Accounting Officer) | April 1, 2013 | ||
| Bing Mei | ||||
| /s/ Wei Wen | Secretary, Director | April 1, 2013 | ||
| Wei Wen | ||||
| /s/ R. Scott Cramer | Director | April 1, 2013 | ||
| R. Scott Cramer | ||||
| /s/ Qiang Fan | Director | April 1, 2013 | ||
| Qiang Fan | ||||
| /s/ Chengtun Qu | Director | April 1, 2013 | ||
| Chengtun Qu | ||||
| /s/ Mark D. Chen | Director | April 1, 2013 | ||
| Mark D. Chen | ||||
| /s/ Weirong Shen | Director | April 1, 2013 | ||
| Weirong Shen |
| 44 |
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of
Skystar Bio-Pharmaceutical Company
We have audited the accompanying consolidated balance sheet of Skystar Bio-Pharmaceutical Company, its subsidiaries and its variable interest entities (the “Company”) as of December 31, 2012, and the related consolidated statements of comprehensive income, shareholders' equity, and cash flows for the year then ended. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of the Company’s internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of the Company, its subsidiaries and its variable interest entities as of December 31, 2012, and the consolidated results of their operations and their cash flows for the year then ended, in conformity with U.S. generally accepted accounting principles.
As discussed in Note 2 to the consolidated financial statements, such statements were prepared in accordance with Financial Accounting Standards Board Accounting Standard Codification (ASC) Topic 810 and related subtopics related to the consolidation of variable interest entities.
/s/ Crowe Horwath (HK) CPA Limited
Hong Kong, China
April 1, 2013
| 45 |
Report of Independent Registered Public Accounting Firm
Board of Directors
Skystar Bio-Pharmaceutical Company and Subsidiaries
Gaoxin District, Xian, Shaanxi Province, P.R. China
We have audited the accompanying consolidated balance sheet of Skystar Bio-Pharmaceutical Company and Subsidiaries as of December 31, 2011, and the related consolidated statements of comprehensive income, shareholders' equity, and cash flows for the year then ended. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2011, and the results of its operations and its cash flows for the year then ended, in conformity with U.S. generally accepted accounting principles.
/s/ Crowe Horwath, LLP
Sherman Oaks, California
March 30, 2012
| 46 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
CONSOLIDATED BALANCE SHEETS
(Amounts in US. Dollars, except share and amounts)
| December 31, | December 31, | |||||||
| 2012 | 2011 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash | $ | 11,321,848 | $ | 7,048,968 | ||||
| Accounts receivable, net of allowance for doubtful accounts of $247,269 and $438,678, respectively | 10,010,796 | 3,391,493 | ||||||
| Inventories | 22,962,209 | 14,851,159 | ||||||
| Deposits, prepaid expenses and other receivables | 2,839,850 | 3,421,487 | ||||||
| Prepayments to suppliers | 23,438,735 | 29,226,961 | ||||||
| Loans receivable | 1,078,827 | 964,088 | ||||||
| Total current assets | 71,652,265 | 58,904,156 | ||||||
| PROPERTY, PLANT AND EQUIPMENT, NET | 28,867,816 | 28,376,559 | ||||||
| CONSTRUCTION-IN-PROGRESS | 8,691,360 | 8,839,055 | ||||||
| OTHER ASSETS: | ||||||||
| Long-term prepayments | 1,050,327 | 1,512,817 | ||||||
| Long-term prepayments for acquisitions | 177,744 | 569,788 | ||||||
| Intangible assets, net | 5,319,831 | 5,674,206 | ||||||
| Total other assets | 6,547,902 | 7,756,811 | ||||||
| Total assets | $ | 115,759,343 | $ | 103,876,581 | ||||
| LIABILITIES AND SHAREHOLDERS' EQUITY | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts payable | $ | 4,017,530 | $ | 1,047,067 | ||||
| Other payable and accrued expenses | 4,374,047 | 5,274,598 | ||||||
| Short-term loans | 4,443,600 | 7,366,320 | ||||||
| Deposits from customers | 1,621,061 | 1,432,529 | ||||||
| Taxes payable | 1,950,757 | 160,081 | ||||||
| Due to related parties | 798,925 | 56,273 | ||||||
| Total current liabilities | 17,205,920 | 15,336,868 | ||||||
| OTHER LIABILITIES: | ||||||||
| Long-term loan | 1,269,600 | - | ||||||
| Deferred government grants | 1,063,290 | 393,500 | ||||||
| Warrant/purchase option liability | 5,600 | 43,400 | ||||||
| Total other liabilities | 2,338,490 | 436,900 | ||||||
| Total liabilities | 19,544,410 | 15,773,768 | ||||||
| COMMITMENTS AND CONTINGENCIES | ||||||||
| SHAREHOLDERS' EQUITY | ||||||||
| Preferred stock, $0.001 par value, 50,000,000 shares authorized, No Series "A" shares authorized, 48,000,000 Series "B" shares authorized, No Series "B" shares issued and outstanding | - | - | ||||||
| Common stock, $0.001 par value, 40,000,000 shares authorized, 7,604,800 and 7,161,919 shares issued and outstanding as of December 31, 2012 and 2011, respectively | 7,605 | 7,162 | ||||||
| Paid-in capital | 37,021,085 | 35,784,378 | ||||||
| Statutory reserves | 5,897,298 | 5,708,135 | ||||||
| Retained earnings | 44,515,296 | 38,492,031 | ||||||
| Accumulated other comprehensive income | 8,773,649 | 8,111,107 | ||||||
| Total shareholders' equity | 96,214,933 | 88,102,813 | ||||||
| Total liabilities and shareholders' equity | $ | 115,759,343 | $ | 103,876,581 | ||||
THE ACCOMPANYING NOTES ARE AN INTEGRAL PART OF THESE CONSOLIDATED FINANCIAL STATEMENTS
| 47 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(Amounts in US. Dollars, except share and amounts)
| Years ended December 31, | ||||||||
| 2012 | 2011 | |||||||
| REVENUE, net | $ | 33,586,791 | $ | 52,788,279 | ||||
| COST OF REVENUE | 15,095,004 | 26,562,748 | ||||||
| GROSS PROFIT | 18,491,787 | 26,225,531 | ||||||
| OPERATING EXPENSES: | ||||||||
| Research and development | 2,154,241 | 2,814,328 | ||||||
| Selling expenses | 3,040,036 | 4,062,126 | ||||||
| General and administrative | 4,746,529 | 4,099,153 | ||||||
| Total operating expenses | 9,940,806 | 10,975,607 | ||||||
| INCOME FROM OPERATIONS | 8,550,981 | 15,249,924 | ||||||
| OTHER INCOME (EXPENSE): | ||||||||
| Other income, net | 498,614 | 43,696 | ||||||
| Interest expense, net | (706,842 | ) | (140,920 | ) | ||||
| Change in fair value of warrant/purchase option liability | 37,800 | 1,376,239 | ||||||
| Total other income (expense), net | (170,428 | ) | 1,279,015 | |||||
| INCOME BEFORE PROVISION FOR INCOME TAXES | 8,380,553 | 16,528,939 | ||||||
| PROVISION FOR INCOME TAXES | 2,168,125 | 2,871,299 | ||||||
| NET INCOME | 6,212,428 | 13,657,640 | ||||||
| OTHER COMPREHENSIVE INCOME : | ||||||||
| Foreign currency translation adjustment | 662,542 | 2,955,344 | ||||||
| COMPREHENSIVE INCOME | $ | 6,874,970 | $ | 16,612,984 | ||||
| EARNINGS PER SHARE: | ||||||||
| Basic | $ | 0.83 | $ | 1.90 | ||||
| Diluted | $ | 0.83 | $ | 1.90 | ||||
| WEIGHTED AVERAGE NUMBER OF COMMON SHARES: | ||||||||
| Basic | 7,471,350 | 7,182,969 | ||||||
| Diluted | 7,471,350 | 7,184,484 | ||||||
THE ACCOMPANYING NOTES ARE AN INTEGRAL PART OF THESE CONSOLIDATED FINANCIAL STATEMENTS
| 48 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
CONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY
(Amounts in US. Dollars, except share and amounts)
| Accumulated | ||||||||||||||||||||||||||||
| Retained earnings | other | |||||||||||||||||||||||||||
| Common stock | Paid-in | Statutory | comprehensive | |||||||||||||||||||||||||
| Shares | Amount | capital | reserves | Unrestricted | income | Total | ||||||||||||||||||||||
| BALANCE, January 1, 2011 | 7,161,919 | $ | 7,162 | $ | 35,784,378 | $ | 5,695,236 | $ | 24,847,290 | $ | 5,155,763 | $ | 71,489,829 | |||||||||||||||
| Foreign currency translation | - | - | - | - | - | 2,955,344 | 2,955,344 | |||||||||||||||||||||
| Net income | - | - | - | - | 13,657,640 | - | 13,657,640 | |||||||||||||||||||||
| Appropriation to statutory reserves | - | - | - | 12,899 | (12,899 | ) | - | — | ||||||||||||||||||||
| BALANCE, December 31, 2011 | 7,161,919 | $ | 7,162 | $ | 35,784,378 | $ | 5,708,135 | $ | 38,492,031 | $ | 8,111,107 | $ | 88,102,813 | |||||||||||||||
| Share issued under 2010 stock incentive plan | 442,881 | 443 | 1,236,707 | - | - | - | 1,237,150 | |||||||||||||||||||||
| Foreign currency translation | - | - | - | - | - | 662,542 | 662,542 | |||||||||||||||||||||
| Net income | - | - | - | - | 6,212,428 | - | 6,212,428 | |||||||||||||||||||||
| Appropriation to statutory reserves | - | - | - | 189,163 | (189,163 | ) | - | - | ||||||||||||||||||||
| BALANCE, December 31, 2012 | 7,604,800 | $ | 7,605 | $ | 37,021,085 | $ | 5,897,298 | $ | 44,515,296 | $ | 8,773,649 | $ | 96,214,933 | |||||||||||||||
THE ACCOMPANYING NOTES ARE AN INTEGRAL PART OF THESE CONSOLIDATED FINANCIAL STATEMENTS
| 49 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Amounts in US. Dollars, except share and amounts)
| Years ended December 31, | ||||||||
| 2012 | 2011 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net income | $ | 6,212,428 | $ | 13,657,640 | ||||
| Adjustments to reconcile net income to net cash provided by (used in) operating activities: | ||||||||
| Depreciation | 1,035,829 | 1,347,090 | ||||||
| Amortization | 401,114 | 524,662 | ||||||
| (Reversal of) provision for doubtful accounts | (194,971 | ) | 85,561 | |||||
| Provision for obsolete inventories | - | 7,562 | ||||||
| Common stock to be issued to related parties for compensation | 86,162 | 146,189 | ||||||
| Common stock issued under 2010 stock incentive plan | 1,037,911 | - | ||||||
| Change in fair value of warrant/purchase option liability | (37,800 | ) | (1,376,239 | ) | ||||
| Change in operating assets and liabilities | ||||||||
| Accounts receivable | (6,394,244 | ) | 1,660,343 | |||||
| Inventories | (7,985,875 | ) | (7,482,193 | ) | ||||
| Deposits, prepaid expenses and other receivables | 1,651,478 | 162,188 | ||||||
| Prepayments to suppliers | 6,027,718 | (12,298,451 | ) | |||||
| Accounts payable | 2,307,066 | 724,579 | ||||||
| Other payables and accrued expenses | (500,127 | ) | 1,667,267 | |||||
| Deposits from customers | 176,645 | 123,215 | ||||||
| Taxes payable | 1,788,790 | (608,350 | ) | |||||
| Net cash provided by (used in) operating activities | 5,612,124 | (1,658,937 | ) | |||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Purchases of property, plant and equipment | (163,049 | ) | (524,574 | ) | ||||
| Payments on construction-in-progress | (393,032 | ) | (7,028,129 | ) | ||||
| Long-term prepayments | (358,077 | ) | (1,365,671 | ) | ||||
| Refund of long-term prepayments | 475,950 | - | ||||||
| Loans to third parties | (2,023,393 | ) | (3,849,994 | ) | ||||
| Collection of loans to third parties | 1,916,651 | 11,113,731 | ||||||
| Net cash used in investing activities | (544,950 | ) | (1,654,637 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Proceeds from short-term and long-term loans | 5,774,860 | 10,856,498 | ||||||
| Repayment of short-term loans | (7,488,280 | ) | (6,695,279 | ) | ||||
| Proceeds from government grants | 666,330 | - | ||||||
| Repayment of government grants | (475,950 | ) | - | |||||
| Due to (from) related parties | 743,755 | (75,505 | ) | |||||
| Net cash (used in) provided by financing activities | (779,285 | ) | 4,085,714 | |||||
| EFFECT OF EXCHANGE RATE CHANGES ON CASH | (15,009 | ) | 388,997 | |||||
| INCREASE IN CASH | 4,272,880 | 1,161,137 | ||||||
| CASH, beginning of year | 7,048,968 | 5,887,831 | ||||||
| CASH, end of year | $ | 11,321,848 | $ | 7,048,968 | ||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | ||||||||
| Cash paid for interest | $ | 647,249 | $ | 362,713 | ||||
| Cash paid for income taxes | $ | 972,739 | $ | 5,366,046 | ||||
| Non-cash investing and financing activities | ||||||||
| Long-term prepayment transferred to construction-in-progress | $ | 1,004,635 | $ | 3,895,580 | ||||
| Long term prepayment transferred to plant and equipment | $ | - | $ | 4,752,513 | ||||
| Construction-in-progress transferred to property, plant and equipment | $ | 1,129,588 | $ | 813,562 | ||||
| Share issued to settle payables to related parties | $ | 199,239 | $ | - | ||||
THE ACCOMPANYING NOTES ARE AN INTEGRAL PART OF THESE CONSOLIDATED FINANCIAL STATEMENTS
| 50 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
Note 1 – ORGANIZATION
Organization and description of business
Skystar Bio-Pharmaceutical Company (“Skystar” or the “Company”) was incorporated in Nevada on September 24, 1998. Since its acquisition on November 7, 2005 of Skystar Bio-Pharmaceutical (Cayman) Holdings Co., Ltd. (“Skystar Cayman”), a Cayman Islands company, the Company has been engaged in the research, development, production, marketing, and sales of veterinary healthcare and medical care products. All current operations of the Company are in the People’s Republic of China (“China” or the “PRC”).
All of the Company’s operations are carried out by our subsidiaries in China and Xi’an Tianxing Bio-Pharmaceutical Co., Limited (“Xi’an Tianxing”), a PRC joint stock company that the Company controls through contractual arrangements originally between Skystar Cayman and Xi’an Tianxing. On March 10, 2008, the Company entered into a series of agreements transferring all of the rights and obligations of Skystar Cayman under the contractual arrangements to Sida Biotechnology (Xi’an) Co., Ltd. (“Sida”), a PRC company. Sida is the wholly owned subsidiary of Fortunate Time International Limited (“Fortunate Time”), a Hong Kong company and wholly owned subsidiary of Skystar Cayman. Xi’an Tianxing also has a wholly owned subsidiary, Shanghai Siqiang Biotechnological Co., Ltd. (“Shanghai Siqiang”), a PRC company.
On September 18, 2009, Skystar Bio-Pharmaceutical Inc. (“Skystar California”) was incorporated in California and became a wholly owned subsidiary of Skystar. On December 20, 2010, we dissolved Skystar California.
On April 21, 2010, Kunshan Sikeda Biotechnology Co., Ltd. (“Kunshan Sikeda”) was incorporated in Kunshan, Jiangsu Province, China with a registered capital of $73,970 (RMB 500,000), of which Xi’an Tianxing and Sida each contributed $36,985 (RMB 250,000). Kunshan Sikeda is jointly owned by Xi’an Tianxing and Sida.
On May 7, 2010, Fortunate Time formed Skystar Biotechnology (Kunshan) Co., Limited (“Skystar Kunshan”) in Kunshan, Jiangsu Province, China with a registered capital of $15,000,000, of which $2,250,000 was paid by Fortunate Time in cash, and of which the remaining $12,750,000 is required to be invested in the future. Skystar Kunshan was formed in connection with an acquisition of assets to meet part of the registered capital requirements, and was intended to be a micro-organism manufacturing facility for the Company once the acquisition was complete. The asset acquisition was completed in September 2011, and the Company is in the process of transferring the assets acquired to meet part of the registered capital requirements.
On August 11, 2010, Sida became the parent company of Skystar Biotechnology (Jingzhou) Co., Limited (“Skystar Jingzhou”), a company established in Jingzhou, Hubei Province, China on February 5, 2010, with registered capital of approximately $4.1 million (RMB 26,000,000), of which approximately $3.7 million (RMB 23,480,000) was paid. On July 5, 2012, Sida paid up the remaining capital of $0.4 million (RMB 2,520,000).
On March 15, 2011, Xi’an Tianxing formed Xi’an Sikaida Bio-products Co., Ltd. (“Xi’an Sikaida”) with a registered capital of approximately $1,587,000 (RMB 10,000,000) paid by Xi’an Tianxing.
Hereinafter, Skystar, Skystar Cayman, Fortunate Time, Sida, Xi’an Tianxing, Skystar Kunshan, Kunshan Sikeda, Skystar Jingzhou, Shanghai Siqiang, and Xi’an Sikaida are sometimes collectively referred to as the “Company”.
| 51 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
Note 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of consolidation
The accompanying consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America. The consolidated financial statements include the financial statements of the Company, its wholly-owned subsidiaries, and its variable interest entities (“VIEs”). All significant inter-company transactions and balances between the Company, its subsidiaries, and its VIEs have been eliminated in consolidation.
The Company has evaluated the relationship with Xi’an Tianxing and Xi’an Tianxing’s wholly owned subsidiaries, Xi’an Sikaida and Shanghai Siqiang. As a result of the contractual arrangements which obligate Sida to absorb all of the risk of loss from Xi’an Tianxing’s activities and enable Sida to receive all of its expected residual returns, the Company is the primary beneficiary of these VIEs and thus it accounts for Xi’an Tianxing, Xi’an Sikaida and Shanghai Siqiang as VIEs under the Financial Accounting Standards Board’s (“FASB”) interpretation on consolidation of variable interest entities. Accordingly, the Company consolidates the results, assets, and liabilities of Xi’an Tianxing. Xi’an Sikaida and Shanghai Siqiang.
Use of estimates
The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. The level of uncertainty in estimates and assumptions increases with the length of time to complete the underlying transactions. Actual results may differ from these estimates in amounts that may be material to the consolidated financial statements and accompanying notes. Significant estimates and assumptions made by the Company are used for, but not limited to the allowance for uncollectible receivables, obsolescence reserve against inventory, useful lives of property, plant and equipment and intangible assets, assumptions used in assessing impairment for long-lived assets and the fair value for derivative instruments.
Foreign currency translation
The Company uses the United States dollar (“U.S. dollar”) for financial reporting purposes and the Chinese Renminbi (“RMB”) as its functional currency. The Company’s subsidiaries and VIEs maintain their books and records in their functional currency, being the primary currency of the economic environment in which their operations are conducted.
The Company translates the subsidiaries’ and VIEs’ assets and liabilities into U.S. dollars using the applicable exchange rates prevailing at the balance sheet dates, and the statements of comprehensive income and cash flows are translated at average exchange rates during the reporting period. As a result, amounts related to assets and liabilities reported on the consolidated statements of cash flows will not necessarily agree with changes in the corresponding balances on the consolidated balance sheets. Equity accounts are translated at historical rates. Adjustments resulting from the translation of the subsidiaries’ and VIEs’ financial statements are recorded as accumulated other comprehensive income.
The quotation of the exchange rates does not imply free convertibility of RMB to other foreign currencies. All foreign exchange transactions continue to take place either through the People’s Bank of China or other banks authorized to buy and sell foreign currencies at the exchange rate quoted by the People’s Bank of China.
Approval of foreign currency payments by the People’s Bank of China or other institutions requires submitting a payment application form together with invoices, shipping documents, and signed contracts. Transaction gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency are included in the results of operations as incurred.
| 52 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
Fair values of financial instruments
ASC Topic 820, Fair Value Measurement and Disclosures, defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. This topic also establishes a fair value hierarchy, which requires classification based on observable and unobservable inputs when measuring fair value. Certain current assets and current liabilities are financial instruments. Management believes their carrying amounts are a reasonable estimate of fair value because of the short period of time between the origination of such instruments and their expected realization and, if applicable, their current interest rates are equivalent to interest rates currently available. The three levels of valuation hierarchy are defined as follows:
| • | Level 1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. |
| • | Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments. |
| • | Level 3 inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
| 53 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
The following table sets forth by level within the fair value hierarchy our financial assets and liabilities that were accounted for at fair value on a recurring basis as of December 31, 2012 and 2011:
| Carrying Value at December 31, | Fair Value Measurement at December 31, 2012 | |||||||||||||||
| 2012 | Level 1 | Level 2 | Level 3 | |||||||||||||
| Purchase option liability | $ | 5,600 | $ | — | $ | 5,600 | $ | — | ||||||||
| Carrying Value at December 31, | Fair Value Measurement at December 31, 2011 | |||||||||||||||
| 2011 | Level 1 | Level 2 | Level 3 | |||||||||||||
| Warrant/purchase option liability | $ | 43,400 | $ | — | $ | 43,400 | $ | — | ||||||||
Below is the reconciliation for the warrant/purchase option liability:
| December 31, 2012 | December 31, 2011 | |||||||
| Balance, beginning of year | $ | 43,400 | $ | 1,419,639 | ||||
| Change in fair value | (37,800 | ) | (1,376,239 | ) | ||||
| Balance, end of year | $ | 5,600 | $ | 43,400 | ||||
Revenue recognition
Revenue of the Company is primarily derived from the sales of veterinary healthcare and medical care products in China. Sales are recognized when the following four revenue criteria are met: persuasive evidence of an arrangement exists, delivery has occurred, the selling price is fixed or determinable, and collectability is reasonably assured. Sales are presented net of value added tax (“VAT”). No estimated allowance for sales returns is reflected in these consolidated financial statements as sales returns historically have been insignificant.
There are two types of sales upon which revenue is recognized:
| a. | Credit sales: revenue is recognized when the products have been delivered to the customers. | |
| b. | Full payment before delivery: Cash received is recorded as “deposits from customers” and revenue is recognized when the products have been delivered to the customers. |
The Company’s revenues and cost of revenues by product line were as follows:
| Years Ended December 31, | ||||||||
| 2012 | 2011 | |||||||
| Revenues | ||||||||
| Micro-organism | $ | 15,355,467 | $ | 14,146,939 | ||||
| Veterinary Medications | 10,658,359 | 33,761,224 | ||||||
| Feed Additives | 4,021,567 | 2,559,914 | ||||||
| Vaccines | 3,551,398 | 2,320,202 | ||||||
| Total Revenues | $ | 33,586,791 | $ | 52,788,279 | ||||
| Cost of Revenues | ||||||||
| Micro-organism | $ | 5,498,463 | $ | 4,034,629 | ||||
| Veterinary Medications | 6,087,300 | 20,684,170 | ||||||
| Feed Additives | 3,074,301 | 1,542,022 | ||||||
| Vaccines | 434,940 | 301,927 | ||||||
| Total Cost of Revenues | 15,095,004 | 26,562,748 | ||||||
| Gross Profit | $ | 18,491,787 | $ | 26,225,531 | ||||
As the Company primarily generates its revenues from customers in the PRC, no geographical segments are presented.
| 54 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
Cash
Cash includes currency on hand, demand deposits with banks, and liquid investments with an original maturity of three months or less.
Accounts receivable
Accounts receivable is stated at cost less an allowance for uncollectible accounts, as needed. The Company uses the aging method to estimate the allowance for anticipated uncollectible receivable balances. Under the aging method, a bad debt percentage is estimated by management based on historical experience and current economic climate. The resulting percentage is applied to customers’ balances categorized by the number of months the underlying invoices have remained outstanding. At each reporting period, the allowance balance is adjusted to reflect the amount computed as a result of the aging method. When facts subsequently become available to indicate that the allowance provided requires an adjustment, a corresponding adjustment is made to the allowance account as a change in estimate. The ultimate collection of the Company’s accounts receivable may take one year. Delinquent account balances are reserved after management determines that the likelihood of collection is not probable, and known bad debts are written-off against the allowance for doubtful accounts when identified.
Inventories
Inventories are stated at the lower of cost as determined on a weighted-average basis, or market. Inventories include purchases and related costs incurred in bringing the inventories to their present location and condition. Management reviews inventories for obsolescence and cost in excess of net realizable value and records a reserve against the inventory and additional cost of goods sold when the carrying value exceeds net realizable value.
Property, plant and equipment
Property, plant and equipment are stated at cost less accumulated depreciation and impairment losses, if any. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. Expenditures for maintenance and repairs that do not improve or extend the useful lives of the assets are charged to operations as incurred, while renewals and betterments are capitalized. Gains and losses on disposals are included in the results of operations. Estimated useful lives of the assets are as follows:
| Estimated useful life | ||
| Buildings and improvements | 10-40 years | |
| Machinery and equipment | 5-10 years | |
| Office equipment and furniture | 5-10 years | |
| Vehicles | 5-10 years |
Management assesses the carrying value of property, plant and equipment annually or more often when factors indicating impairment are present, and reduce the carrying value of such assets by the amount of the impairment. The Company determines the existence of such impairment by measuring the expected future cash flows (undiscounted) and comparing such amount to the net asset carrying value. An impairment loss, if it exists, is measured as the amount by which the carrying amount of the asset exceeds the fair value of the asset. Based on its review, management believes that, as of December 31, 2012 and 2011, there was no impairment for its property, plant and equipment.
Construction-in-progress
Construction-in-progress includes direct costs of construction of a factory building. Interest incurred during the period of construction, if significant, is capitalized. All other interest is expensed as incurred. Construction-in-progress is not depreciated until such time as the asset is completed and put into service.
Intangible assets
Land use rights — Land use rights represent the amounts paid to acquire a long-term interest to utilize the land underlying the Company’s facilities. This type of arrangement is common for the use of land in the PRC. Land use rights are amortized on the straight-line method over the contractual lease terms. The land use right granted to the Company’s Huxian facility was for 50 years. The land use right granted to the Company’s Jingzhou facility was 30 years. The land use right granted to the Company’s Kunshan facility was for 41 years.
Technological know-how — Purchased technological know-how includes confidential formulas, manufacturing processes, and technical and procedural manuals, and is amortized using the straight-line method over an estimated useful life of between five to eleven years that reflects the period over which such confidential formulas, manufacturing processes, and technical and procedural manuals are kept confidential by the Company as agreed between the Company and the selling parties.
| 55 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
Impairment of Intangible assets — the Company evaluates the carrying value of intangible assets at least annually when factors indicating impairment are present. The Company determines the existence of such impairment by measuring the estimated future cash flows (undiscounted) and comparing such amount to the net asset carrying value. If the undiscounted cash flow estimated to be generated by any such intangible asset is less than its carrying amount, a loss is recognized based on the amount by which the carrying amount exceeds the intangible asset’s fair market value. Loss on intangible assets to be disposed of is determined in a similar manner, except that fair market values are reduced by the cost of disposal. Based on its review, the Company believes that, as of December 31, 2012 and 2011, there was no impairment of its intangible assets.
Comprehensive income
Comprehensive income is defined as the change in equity of a business enterprise during a period from transactions and other events and circumstances from non-owner sources. Accumulated other comprehensive income is comprised of the foreign currency translation adjustments.
Shipping and handling costs
Shipping and handling costs related to costs of goods sold are included in selling expenses, which totaled $1,802,654 and $1,933,162 for the years ended December 31, 2012 and 2011, respectively.
Advertising costs
Advertising costs are charged to selling expenses as incurred. Advertising costs were insignificant for the years ended December, 2012 and 2011.
Research and development costs
Research and development costs are charged to operations as incurred and include salaries, professional fees, and technical support fees related to such efforts.
Income taxes
The Company accounts for income taxes using an asset and liability method. Under this method, deferred income taxes are recognized for the tax consequences of temporary differences by applying enacted statutory tax rates applicable to future years to differences between the financial statement carrying amounts and the tax bases of existing assets and liabilities. The effect on deferred income taxes of a change in tax rates is recognized in income in the period that includes the enactment date. A valuation allowance is recognized if it is more likely than not that some portion or all of a deferred tax asset will not be realized.
A tax position is recognized as a benefit only if it is “more likely than not” that the tax position would be sustained in a tax examination, with a tax examination being presumed to occur. The amount recognized is the largest amount of tax benefit that is greater than 50% likely of being realized on examination. For tax positions not meeting the “more likely than not” test, no tax benefit is recorded. As of December 31, 2012 and 2011, there are no unrecognized tax benefits, and the Company does not expect a significant change in tax benefits in the next 12 months. Penalties and interest levied by taxing authorities, if any, are classified as income tax expense in the year incurred. No significant penalties or interest relating to income taxes have been incurred during the years ended December 31, 2012 and 2011.
According to the PRC Tax Administration and Collection Law, the statute of limitations is three years if the underpayment of taxes is due to computational or other errors made by the taxpayer or the withholding agent. The statute of limitations extends to five years under special circumstances. In the case of transfer pricing issues, the statute of limitations is ten years. There is no statute of limitations in the case of tax evasion. Accordingly, the income tax returns of the Company’s PRC operating subsidiaries for the years ended December 31, 2007 through 2012 are open to examination by the PRC state and local tax authorities.
Stock-based compensation
The Company records and reports stock-based compensation by measuring the cost of services received in exchange for an award of equity instruments based on the grant-date fair value of the award. That cost is recognized over the period during which services are received. Stock compensation for stock granted to non-employees is determined as the fair value of the consideration received or the fair value of equity instruments issued, whichever is more reliably measured.
| 56 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
Earnings per share
Basic earnings per share is based upon the weighted-average number of common shares outstanding. Diluted earnings per share is based on the assumption that all dilutive convertible shares, including convertible preferred shares, warrants and stock options were converted or exercised. Further, the method requires that stock dividends or stock splits be accounted for retroactively if the stock dividends or stock splits occur during the period or after the end of the period but before the release of the financial statements, by considering it outstanding for the entirety of each period presented. Diluted earnings per share is computed by applying the treasury stock method. Under this method, options and warrants are assumed to be exercised at the beginning of the period (or at the time of issuance, if later), and as if funds obtained thereby were used to purchase common stock at the average market price during the period.
Related parties
Parties are considered to be related to the Company if the parties, directly or indirectly, through one or more intermediaries, control, are controlled by, or are under common control with the Company. Related parties also include principal owners of the Company, its management, members of the immediate families of such principal owners and management, and other parties with which the Company may deal if one party controls or can significantly influence the management or operating policies of the other to an extent that one of the transacting parties might be prevented from fully pursuing its own separate interests.
Operating segments
While the chief operating decision-makers monitor the revenue streams of the various products lines, operations are managed and financial performance is evaluated on a Company-wide basis. Product lines are aggregated into one as operating results for all product lines are similar. Accordingly, all of the major product lines (micro-organism, veterinary medicine, feed additives and vaccines) are considered by management to be aggregated in one reportable operating segment.
Recently issued accounting pronouncements
In July 2012, the FASB issued 2012-02 Intangibles — Goodwill and Other (Topic 350): The amendments in this update will allow an entity to first assess qualitative factors to determine whether it is necessary to perform a quantitative impairment test. Under these amendments, an entity would not be required to calculate the fair value of an indefinite-lived intangible asset unless the entity determines, based on a qualitative assessment, that it is not more likely than not, the indefinite-lived intangible asset is impaired. The amendments include a number of events and circumstances for an entity to consider in conducting the qualitative assessment. The amendments are effective for annual and interim impairment tests performed for fiscal years beginning after September 15, 2012. Early adoption is permitted, including for annual and interim impairment tests performed as of a date before July 27, 2012, if a public entity’s financial statements for the most recent annual or interim period have not yet been issued. The Company does not expect the adoption of the provisions in this update will have a material impact on its consolidated financial statements.
In February 2013, the FASB issued Accounting Standards Update No. 2013-02 Comprehensive Income (Topic 220): The objective of this update is to improve the reporting of reclassifications out of accumulated other comprehensive income. The amendments in this update seek to attain that objective by requiring an entity to report the effect of significant reclassifications out of accumulated other comprehensive income on the respective line items in net income if the amount being reclassified is required under U.S. generally accepted accounting principles (GAAP) to be reclassified in its entirety to net income. For other amounts that are not required under U.S. GAAP to be reclassified in their entirety to net income in the same reporting period, an entity is required to cross-reference other disclosures required under U.S. GAAP that provide additional detail about those amounts. This would be the case when a portion of the amount reclassified out of accumulated other comprehensive income is reclassified to a balance sheet account (for example, inventory) instead of directly to income or expense in the same reporting period. For public entities, the amendments are effective prospectively for reporting periods beginning after December 15, 2012. For nonpublic entities, the amendments are effective prospectively for reporting periods beginning after December 15, 2013. Early adoption is permitted. The Company does not expect the adoption of the provisions in this update will have a significant impact on its consolidated financial statements.
Other accounting standards that have been issued or proposed by the FASB or other standards-setting bodies that do not require adoption until a future date are not expected to have a material impact on the Company’s consolidated financial statements upon adoption.
| 57 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
Note 3 - CONCENTRATIONS AND CREDIT RISK
The Company’s operations are all carried out in the PRC. Accordingly, the Company’s business, financial condition, and results of operations may be influenced by the political, economic, and legal environments in the PRC, and by the general state of the PRC’s economy. The Company’s operations in the PRC are subject to specific considerations and significant risks not typically associated with companies in North America and Western Europe. These include risks associated with, among others, the political, economic, and legal environments and foreign currency exchange. The Company’s results may be adversely affected by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
Financial instruments which subject the Company to concentration of credit risk consist of cash and accounts receivable. Balances at financial institutions or state-owned banks within the PRC are not covered by insurance. The Company has not experienced any losses in such accounts. The Company provides unsecured credit terms for sales to certain customers. As a result, there are credit risks with the accounts receivable balances. The Company constantly re-evaluates the credit worthiness of customers buying on credit and maintains an allowance for doubtful accounts.
For the years ended December 31, 2012 and 2011, all of the Company’s sales occurred in the PRC. No major customers accounted for more than 10% of the Company’s total revenues in 2012 and 2011. All accounts receivable at December 31, 2012 and 2011 are from customers located in the PRC.
The Company’s six largest vendors accounted for approximately 82% and 70% of the Company’s total purchases for the years ended December 31, 2012 and 2011, respectively. As of December 31, 2012 and 2011, prepayments totaled $20,092,101 and $24,720,862 were made to the six largest vendors, respectively, for raw materials purchases.
The Company had one product that accounted for 15% and 23% of the Company’s total revenues for the years ended December 31, 2012 and 2011, respectively.
Note 4 - ACCOUNTS RECEIVABLE, NET
Accounts receivable consisted of the following:
| December 31, 2012 | December 31, 2011 | |||||||
| Accounts receivable | $ | 10,258,065 | $ | 3,830,171 | ||||
| Allowance for doubtful accounts | (247,269 | ) | (438,678 | ) | ||||
| Accounts receivable, net | $ | 10,010,796 | $ | 3,391,493 | ||||
The following table presents the movement of the allowance for doubtful accounts:
| December 31, 2012 | December 31, 2011 | |||||||
| Beginning of year | $ | 438,678 | $ | 339,031 | ||||
| Addition | 166,276 | 86,908 | ||||||
| Reversal | (361,247 | ) | - | |||||
| Translation adjustment | 3,562 | 12,739 | ||||||
| End of year | $ | 247,269 | $ | 438,678 | ||||
| 58 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
Note 5 – INVENTORIES
Inventories consist of the following:
| December 31, 2012 | December 31, 2011 | |||||||
| Raw materials | $ | 20,652,278 | $ | 12,646,663 | ||||
| Packaging materials | 220,078 | 181,304 | ||||||
| Work-in-process | 219 | 22,559 | ||||||
| Finished goods | 2,243,226 | 2,171,238 | ||||||
| Other | 69,929 | 51,085 | ||||||
| 23,185,730 | 15,072,849 | |||||||
| Less: Allowance for obsolete inventories | (223,521 | ) | (221,690 | ) | ||||
| Total | $ | 22,962,209 | $ | 14,851,159 | ||||
The Company periodically reviews its reserves for slow-moving and obsolete inventories. During the years ended December 31, 2012 and 2011, the Company recorded write-downs of inventories of of $16,026 (RMB 100,982) and $774,165 (RMB 4,918,456) respectively, which were predominantly the result of expired raw material and outdated packing material.
Note 6 - DEPOSITS, PREPAID EXPENSES AND OTHER RECEIVABLES
Deposits, prepaid expenses and other receivables are comprised of the following:
| December 31, 2012 | December 31, 2011 | |||||||
| Prepaid income taxes | $ | 1,263,845 | $ | 2,162,070 | ||||
| Other prepayments | 828,676 | 751,025 | ||||||
| Other receivables | 747,329 | 508,392 | ||||||
| Total | $ | 2,839,850 | $ | 3,421,487 | ||||
Note 7 – PREPAYMENTS TO SUPPLIERS
Prepayments to suppliers are comprised of the following:
| December 31, 2012 | December 31, 2011 | |||||||
| Prepayments for raw materials | $ | 23,053,753 | $ | 28,824,123 | ||||
| Prepayments for packaging materials | 384,982 | 402,838 | ||||||
| Total | $ | 23,438,735 | $ | 29,226,961 | ||||
As part of the Company’s strategy to reduce inventory costs, the Company maintains a balance for prepayments to suppliers in order to secure favorable pricing for raw materials. As inventory is received throughout the period, this balance will fluctuate with the business operations.
| 59 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
Note 8 - CONSTRUCTION-IN-PROGRESS
Construction-in-progress (“CIP”) relates to factilities being built in Xi’an, Jingzhou and Kunshan.
Xi’an facility
Xi’an Tianxing has a vaccine facility and animal laboratory being built in Huxian, Xi’an.
In 2011, the Company started two projects at the vaccine facility to modify air filtration, water treatment, and other facility changes based on recommendations by outside experts hired by the Company to advise on the GMP qualification process for the vaccine facility. As of December 31, 2012, this vaccine facility had a total construction-in-progress of $2,233,860 (RMB 14,075,992). On September 20 and 21, 2012, China’s Ministry of Agriculture (MOA) physically inspected this new facility and deemed the facility GMP compliant. Following physical inspection, the MOA’s inspection team recommended that the Office of the Working Committee proceed with Stage 2 of its GMP certification process. The Company expects the GMP certification process to be completed by the third quarter of 2013.
In 2011, the Company started a facility improvement project in the amount of approximately $317,400 (RMB 2,000,000) for the Huxian Animal Laboratory. The facility is a supporting project to the Huxian vaccine facility and is currently in Stage 2 of its GMP certification process. The Company expects the GMP certification process to be completed by the third quarter of 2013.
Jingzhou facility
In 2011, the Company started a facility improvement project to expand veterinary medicine production capacity at the Jingzhou facility. The project includes plant construction and water supply and drainage and has an estimated total cost of $1,666,350 (RMB 10,500,000). The Company expects the project will be completed by the end of the fourth quarter of 2013.
Kunshan facility
In 2011, the Company started a supporting project at the Kunshan micro-organism facility that includes the construction and installation of plumbing, sewer, electrical, HVAC, fire protection and alarm system, drainage, office, lab, road construction, parking, and landscaping. As of December 31, 2012, the construction and installation were completed with a construction-in-progress of $4,963,657 (RMB 31,276,982). The project was inspected and accepted in March 2013.
No depreciation is provided for construction-in-progress until such time as the assets are completed and placed into service.
The construction projects the Company was in the progress of completing are as follows:
| Total in CIP as of | ||||||||||||||
| Project | December 31, 2012 | Estimate Cost to Complete | Estimated Total Cost | Estimated Completion Date | ||||||||||
| Xi'an vaccine facility | $ | 2,233,860 | $ | - | $ | 2,233,860 | Third quarter of 2013 | |||||||
| Xi'an animal laboratory | 317,400 | - | 317,400 | Third quarter of 2013 | ||||||||||
| Jingzhou veterinary medication facility | 1,176,443 | 489,907 | 1,666,350 | Fourth quarter of 2013 | ||||||||||
| Kunshan micro-organism facility | 4,963,657 | - | 4,963,657 | First quarter of 2013 | ||||||||||
| Total | $ | 8,691,360 | $ | 489,907 | $ | 9,181,267 | ||||||||
As of December 31, 2012 and 2011, the Company had construction in progress amounting to $8,691,360 and $8,839,055, respectively. No interest expense had been capitalized for construction in progress for the years ended December 31, 2012 and 2011 as management determined the amount of capitalized interest would be insignificant.
| 60 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
Note 9 – LOANS RECEIVABLE
In November 2010, the Company provided an unsecured non-interest bearing loan to Xi’an Tiantai Investment, Ltd., the Company’s acquisition advisor, in the amount of $190,440 (RMB 1,200,000) for two years from November 26, 2010 through November 25, 2012. On January 1, 2012, the Company changed this loan to an unsecured interest bearing loan at an annual interest rate of 12.0% for eighteen months from January 1, 2012 to June 30, 2013.
On April 1, 2012, the Company provided a secured interest bearing loan to Shaanxi Jiali Pharmaceutical Co., Ltd., an unrelated third party, in the amount of $793,500 (RMB 5,000,000) at an annual interest rate of 12.0% for eight months from April 1, 2012 through November 30, 2012. This loan receivable was secured by an irrecoverable corporate guarantee from Xi’an Lantech Pharmaceutical Co. Ltd., an unrelated third party. On December 1, 2012, the Company extended this loan for another five months from December 1, 2012 to April 30, 2013.
On April 6, 2012, the Company provided an unsecured interest bearing loan to an unrelated third party, in the amount of $872,850 (RMB 5,500,000) at an annual interest rate of 12.0% for six months from April 6, 2012 through October 5, 2012. On October 31, 2012, the entire loan was repaid.
As of December 31, 2012 and 2011, the Company had other unsecured non-interest bearing short-term loans in the amount of $94,887 (RMB 597,900) and $775,208 (RMB 4,925,082), respectively, due from the Company’s employees and unrelated third parties.
Note 10 – PROPERTY, PLANT AND EQUIPMENT, NET
Property, plant and equipment consist of the following:
| December 31, 2012 | December 31, 2011 | |||||||
| Buildings and improvements | $ | 27,210,008 | $ | 25,866,428 | ||||
| Machinery and equipment | 5,876,467 | 5,675,995 | ||||||
| Office equipment and furniture | 332,653 | 320,498 | ||||||
| Vehicles | 593,296 | 588,436 | ||||||
| Total | 34,012,424 | 32,451,357 | ||||||
| Less: accumulated depreciation | (5,144,608 | ) | (4,074,798 | ) | ||||
| Property, plant and equipment, net | $ | 28,867,816 | $ | 28,376,559 | ||||
Depreciation expense was $1,035,829 and $1,347,090 for the years ended December 31, 2012 and 2011, respectively.
A line of credit agreement with Industrial and Commercial Bank of China (ICBC) Songzi Branch is secured by the Company’s buildings in Jingzhou, Hubei Province.
A two-year line of credit agreement with Shaanxi Agricultural Yanta Credit Union is secured by the Company’s office buildings located in Xi’an City.
As of December 31, 2012 and 2011, property, plant and equipment with a carrying amount of $2,180,382 and $19,055,906, respectively, were pledged against the Company’s short-term and long-term loans.
| 61 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
Note 11 - LONG-TERM PREPAYMENTS
Long-term prepayments consist of the following:
| December 31, 2012 | December 31, 2011 | |||||||
| Prepayments for R&D project | $ | 355,488 | $ | 314,800 | ||||
| Construction deposits | 377,657 | 920,790 | ||||||
| Deposits for equipment purchase and land use rights | 311,227 | 277,227 | ||||||
| Deposits for other | 5,955 | - | ||||||
| Long-term prepayments - total | $ | 1,050,327 | $ | 1,512,817 | ||||
| Long-term prepayments for acquisitions | $ | 177,744 | $ | 569,788 | ||||
As of December 31, 2012 and 2011, deposits for potential acquisitions totaled $177,744 and $569,788, respectively, all of which was held by an unrelated third party engaged to facilitate potential acquisition projects.
| 62 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
Note 12 – INTANGIBLE ASSETS
Intangible assets consist of the following:
| December 31, 2012 | December 31, 2011 | |||||||
| Land use rights | $ | 4,800,677 | $ | 4,761,352 | ||||
| Technological know-how | 2,221,800 | 2,203,600 | ||||||
| Patents | 317,400 | 314,800 | ||||||
| Total | 7,339,877 | 7,279,752 | ||||||
| Less: accumulated amortization | (2,020,046 | ) | (1,605,546 | ) | ||||
| Intangible assets, net | $ | 5,319,831 | $ | 5,674,206 | ||||
For the years ended December 31, 2012 and 2011, the amortization expense for intangibles assets amounted to $401,114 and $524,662, respectively.
Amortization expense expected for the next five years and thereafter is as follows:
| Years ending December 31, | Amount | |||
| 2013 | $ | 242,541 | ||
| 2014 | 242,541 | |||
| 2015 | 242,541 | |||
| 2016 | 242,541 | |||
| 2017 | 242,541 | |||
| Thereafter | 4,107,126 | |||
| Total | $ | 5,319,831 | ||
A line of credit agreement with Industrial and Commercial Bank of China (ICBC) Songzi Branch is secured by the Company’s land use rights in Jingzhou, Hubei Province.
As of December 31, 2012 and 2011, land use rights with a carrying amount of $1,199,456 and $1,574,819, respectively, were pledged against the Company’s short-term loans.
| 63 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
Note 13 – SHORT-TERM AND LONG-TERM LOANS
Short-term loans
On May 5, 2011, the Company obtained a one year loan with Industrial and Commercial Bank of China (ICBC) Songzi Branch for $472,200 (RMB 3,000,000) at an annual interest rate determined by using the People's Bank of China floating benchmark lending rate over the same period plus 30% of that rate. This loan was secured by the Company’s land use rights in Jingzhou, Hubei Province and guaranteed by the legal representative of Skystar Jingzhou. This loan was fully repaid on May 2, 2012.
On July 8, 2011, the Company obtained a one year loan with Chang’an Bank for $787,000 (RMB 5,000,000) at an annual interest rate of 8.203%. This loan was secured by the Company’s office buildings and its Chairman and CEO’s personal property located in Xi’an City, which includes an office building contributed by a shareholder in 2005 as additional capital of Xi’an Tianxing. The title to this property has not been passed to the Company. This loan was also personally guaranteed by the Company’s Chairman and CEO and his wife. This loan was fully repaid on July 11, 2012.
On August 25, 2011, the Company obtained a one year loan with Shaanxi Agricultural Yanta Credit Union for $787,000 (RMB 5,000,000) at an annual interest rate of 9.411%. This loan was secured by the Company’s office buildings located in Xi’an City and was personally guaranteed by the Company’s Chairman and CEO. This loan was fully repaid on August 24, 2012.
On December 22, 2011, the Company obtained a one year loan with Shaanxi Agricultural Yanta Credit Union for $472,200 (RMB 3,000,000) at an annual interest rate of 9.411%. This loan was secured by the Company’s office buildings located in Xi’an City and was also personally guaranteed by the Company’s Chairman and CEO and his wife. This loan was fully repaid on September 20, 2012.
In 2011, the Company obtained two three-month loans with a third-party individual for a total amount of $125,920 (RMB 800,000). During the first quarter of 2012, the Company borrowed another two short-term loans for a total amount of RMB 400,000 with this third-party individual. These four loans were non-interest bearing and were unsecured. On April 6, 2012, the Company repaid one loan of RMB 300,000. On April 9, 2012, the Company repaid the other three remaining loans totaling RMB 900,000. As of December 31, 2012, there were no loans from the third party individual.
On December 1, 2011, the Company obtained a one year loan with Bank of Chengdu for $4,722,000 (RMB 30,000,000) at an annual interest rate determined by using the People's Bank of China floating benchmark lending rate over the same period plus 30% of that rate. This loan was guaranteed by Shaanxi Province Credit Re-guarantee LLC. For this guarantee, the Company paid RMB 675,000 of fees and provided counter-guarantee to Shaanxi Province Credit Re-guarantee LLC, which was secured by the Company’s land use right and manufacturing plant located in Huxian County. This loan was fully repaid on November 30, 2012.
On June 27, 2012, the Company entered into a line of credit agreement with Industrial and Commercial Bank of China (ICBC) Songzi Branch that allows the Company to borrow up to RMB 3,000,000. This line of credit agreement expires on June 18, 2013. The Company withdrew $476,100 (RMB 3,000,000) on July 3, 2012 at an annual interest rate determined by using the People's Bank of China floating benchmark lending rate over the same period plus 30% of that rate, which was 7.8% at December 31, 2012. This loan is secured by the Company’s buildings and land use rights in Jingzhou, Hubei Province.
On August 13, 2012, the Company obtained fifteen six-month loans with Xi'an High-tech Zone Guoxin Microcredit Ltd. for a total amount of $2,380,500 (RMB 15,000,000) to meet short-term cash needs. Each loan is in the amount of $158,700 (RMB 1,000,000) at an annual interest rate of 22.4%. These loans are guaranteed by Sida. On February 5, 2013, the loans were fully repaid.
On August 27, 2012, the Company obtained a one year loan with Chang’an Bank for $1,587,000 (RMB 10,000,000) at an annual interest rate determined by using the People's Bank of China floating benchmark lending rate over the same period plus 30% of that rate, which was 7.8% at December 31, 2012. This loan is guaranteed by Xi’an Taixin Investment Guarantee Co., Ltd. For this guarantee, the Company is required to pay $31,740 (RMB 200,000) of fees to Xi’an Taixin Investment Guarantee Co., Ltd. and 12.5% of the loan or $198,375 (RMB 1,250,000) is required to be kept by Xi’an Taixin Investment Guarantee Co., Ltd. to serve as collateral until the loan is repaid. This loan is also personally guaranteed by the Company’s Chairman and CEO.
| 64 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
Outstanding short-term loans consisted of the following:
| December 31, 2012 | December 31, 2011 | Interest | ||||||||||||||||||||
| Bank | Amt RMB | Amt USD | Amt RMB | Amt USD | Due Date | Rate | ||||||||||||||||
| Chang’an Bank | - | $ | - | 5,000,000 | $ | 787,000 | 07/07/12 | 8.203 | % | |||||||||||||
| Shaanxi Agricultural Yanta Credit Union | - | - | 5,000,000 | 787,000 | 08/24/12 | 9.411 | % | |||||||||||||||
| Shaanxi Agricultural Yanta Credit Union | - | - | 3,000,000 | 472,200 | 12/21/12 | 9.411 | % | |||||||||||||||
| ICBC Songzi Branch | - | - | 3,000,000 | 472,200 | 05/04/12 | (1) | ||||||||||||||||
| Third-party Individual | - | - | 800,000 | 125,920 | Various | 0 | % | |||||||||||||||
| Bank of Chengdu | - | - | 30,000,000 | 4,722,000 | 11/30/12 | (1) | ||||||||||||||||
| Xi'an High-tech Zone Guoxin Microcredit Ltd. | 15,000,000 | 2,380,500 | - | - | 02/12/13 | 22.400 | % | |||||||||||||||
| Chang’an Bank | 10,000,000 | 1,587,000 | - | - | 08/26/13 | (1) | ||||||||||||||||
| ICBC Songzi Branch | 3,000,000 | 476,100 | - | - | 06/18/13 | (1) | ||||||||||||||||
| Total | 28,000,000 | $ | 4,443,600 | 46,800,000 | $ | 7,366,320 | ||||||||||||||||
| (1) | People's Bank of China floating benchmark lending rate over the same period plus 30%, which was 7.8% at December 31, 2012. |
Long-term loan
On September 17, 2012, the Company entered into a two-year line of credit agreement with Shaanxi Agricultural Yanta Credit Union that allows the Company to borrow up to RMB 8,000,000. This line of credit agreement expires September 20, 2014. The Company withdrew $1,269,600 (RMB 8,000,000) on September 26, 2012 at an annual interest rate of 9.446% for the current year. The interest rate is determined annually by using the People's Bank of China floating benchmark lending rate over the same period plus the lender’s floating rate. The new interest rate becomes effective on December 21 of each year. This line of credit is secured by the Company’s office buildings located in Xi’an City. This line of credit is also personally guaranteed by the Company’s Chairman and CEO and his wife.
Outstanding long-term loan consisted of the following:
| December 31, 2012 | December 31, 2011 | Interest | ||||||||||||||||||||||
| Bank | Amt RMB | Amt USD | Amt RMB | Amt USD | Due Date | Rate | ||||||||||||||||||
| Shaanxi Agricultural Yanta Credit Union | 8,000,000 | $ | 1,269,600 | - | $ | - | 09/20/14 | 9.446 | % | |||||||||||||||
Interest expense incurred and associated with the short-term and long-term loans amounted to $647,249 and $362,713 for the years ended December 31, 2012 and 2011, respectively, none of which has been capitalized as part of construction-in-progress in 2012 and 2011, respectively.
Note 14 - DEFERRED GOVERNMENT GRANT
The subsidies for Good Manufacturing Practice projects were granted by Shaanxi provincial government and Xi’an municipal government which may require the subsidies to be repaid. As of December 31, 2012 and 2011, the subsidies were RMB 2,500,000, equivalent to $396,750 and $393,500, respectively.
The subsidies granted by Xi’an City Science and Technology Bureau and Xi’an City High-Tech Industrial Development Zone are required to be used exclusively for the fish disease multi associated anti-idiotypic monoclonal antibody vaccine project during the development period from January 2012 through December 2014. As of December 31, 2012, 70% of the subsidies or $666,540 (RMB 4,200,000) had been received and $Nil has been expensed on this project.
On June 3, 2003 and January 16, 2006, the Company received demand payment from China Go Xin Investment Group Company of $634,800 (RMB 4,000,000) and $158,700 (RMB 1,000,000), respectively. The Company repaid 158,850 (RMB 1,000,000) on December 7, 2010 and $475,950 (RMB 3,000,000) in 2012 and the remaining balance of $158,700 (RMB1,000,000) was recorded in other payables and accrued expenses and was repaid on January 30 and February 28, 2013.
| 65 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
Note 15 - CAPITAL TRANSACTIONS
Stock-based compensation
On March 30, 2010, the Company agreed to issue 2,500 shares of common stock to a non-executive director in exchange for services unrelated to his services as a director at the fair market value of $11.74 per share based on the closing price on March 30, 2010. On April 16, 2010, the Company entered into another agreement to grant 10,000 shares of common stock to that director for his one year service from April 1, 2010. The closing price per share on the grant date was $10.61. The common stock compensation vests in four equal quarterly installments of 2,500 shares. Shares owed were accrued at the end of each quarter at the fair market value on the grant date of $10.61 per share. On August 31, 2011, the Company entered into another agreement to grant 36,000 shares of common stock to that director for his one year service from April 1, 2011. The closing price per share on the grant date was $2.58. The common stock compensation vests in four equal quarterly installments of 9,000 shares. Shares owed were accrued at the end of each quarter at the fair market value on the grant date of $2.58 per share. Currently, the Company is working on completing the contract with this director for his service beginning April 1, 2012. As of December 31, 2012 and 2011, Nil and 34,500 shares were to be issued to this non-executive director and were included in the accrued expenses and were valued at $Nil and $149,235, respectively.
On May 26, 2009, the Company agreed to issue 5,556 shares of common stock to a director at the beginning of each term of his directorship. The trading value of the common stock on May 26, 2009 was $4.50 per share. In accordance with the agreement between the Company and this director, this director must continue to serve as a member of the Board until his successor is duly elected and qualified in order to receive the shares. As of December 31, 2012 and 2011, 5,556 shares and 11,112 shares to be issued to this non-executive director were included in the accrued expenses and amounted to $25,002 and $50,004, respectively.
On July 29, 2011, the Company entered into a one-year employment agreement with its CFO. Under the agreement, he is entitled to receive an aggregate 8,000 shares of common stock, 4,000 shares of which shall be issuable on the 6 month anniversary and the remaining 4,000 shares of which shall be issuable on the 12 month anniversary. The closing price per share on the grant date was $4.20. On July 29, 2012, the Company entered into another one-year employment agreement with the CFO. Under the agreement, he is entitled to receive an aggregate 8,000 shares of common stock, vested in four equal quarterly installments of 2,000 shares. The closing price per share of the stock on the next business day of the grant date was $2.17. As of December 31, 2012 and 2011, 2,000 shares and Nil to be issued to this non-executive director were included in the accrued expenses and amounted to $4,340 and $Nil, respectively.
Equity Compensation Plan
On December 8, 2009, the Company’s board of directors approved a stock incentive plan for officers, directors, employees and consultants entitled the “Skystar Bio-Pharmaceutical Company 2010 Stock Incentive Plan” (the “2010 Plan”). The maximum number of shares that may be issued under the 2010 Plan is 700,000 shares of common stock. The 2010 Plan was approved by the Company’s stockholders on December 31, 2009, and awards may be granted thereunder until December 7, 2019. On May 4, 2012, the Board approved common stock grants in the total amount of 442,881 shares to the Company’s employees and members of the Board of Directors, all of which grants were made pursuant to the terms and provisions of the Plan. As of December 31, 2012, there are 247,119 shares of the Company’s common stock remaining available for future issuance under the Plan. Of the 442,881 shares issued in May 2012, 62,612 shares were issued to settle grants to directors and CFO of $256,056, and the remaining 380,269 shares, valued at $981,094 based on the grant date fair value of the common stock of $2.58 per share. A total of $1,037,911 was charged to general and administrative expenses for the year ended December 31, 2012.
| 66 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
A summary of changes in the Company’s non-vested shares for the year follows:
| Non-vested Shares | ||||
| Non-vested at January 1, 2011 | - | |||
| Granted | 55,112 | |||
| Vested | (38,112 | ) | ||
| Forfeited | - | |||
| Non-vested at December 31, 2011 | 17,000 | |||
| Granted | 13,556 | |||
| Vested | (24,556 | ) | ||
| Forfeited | - | |||
| Non-vested at December 31, 2012 | 6,000 | |||
As of December 31, 2012, there was $13,020 of total unrecognized compensation cost related to non-vested shares granted. The total fair value of shares vested during the years ended December 31, 2012 and 2011 was $86,162 and $146,189, respectively, based on the closing price of the Company’s common stock on the grant date.
Warrants and Purchase Options
On February 28, 2007, the Company issued warrants to the placement agent, exercisable for 114,100 shares of the Company’s common stock at a price of $5.00 per share for a five-year term (number of warrants and exercise price adjusted for 1-for-10 reverse stock split on May 12, 2009 and 2-for-1 forward stock split on November 16, 2009). Up to December 31, 2011, 79,870 warrants were exercised. All the remaining 34,230 warrants expired on February 28, 2012.
In connection with the 2009 equity offering, the Company granted 140,000 common stock purchase options to five designees of the Underwriters with a vesting date of June 30, 2010. The options are exercisable from June 30, 2010 to June 30, 2014, and each option is exercisable for one share of the Company’s common stock at an exercise price at $8.11 per share. All options were provided for services performed. As of December 31, 2012 and 2011, 140,000 common stock purchase options remained outstanding.
| 67 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
Outstanding warrants and purchase options do not trade in an active securities market, and as such, the Company estimates the fair value of these warrants and purchase options using the Black-Scholes Option Pricing Model (“Black-Scholes Model”) using the following assumptions:
| Warrants – (1) | Purchase Options – (2) | |||||||||||||||
| December 31, 2012 | December 31, 2011 | December 31, 2012 | December 31, 2011 | |||||||||||||
| Stock price | $ | - | $ | 2.74 | $ | 1.60 | $ | 2.74 | ||||||||
| Exercise price | $ | - | $ | 5.00 | $ | 8.11 | $ | 8.11 | ||||||||
| Annual dividend yield | - | - | - | - | ||||||||||||
| Expected term (years) | - | 0.16 | 1.50 | 2.50 | ||||||||||||
| Risk-free interest rate | - | 0.01 | % | 0.16 | % | 0.25 | % | |||||||||
| Expected volatility | - | 68 | % | 73 | % | 64 | % | |||||||||
| (1) | As of December 31, 2011, 34,230 warrants with an exercise price of $5.00 were outstanding. All of these warrants expired on February 28, 2012, and none of these warrants were outstanding as of December 31, 2012. |
| (2) | As of December 31, 2011 and 2012, 140,000 purchase options with an exercise price of $8.11 were outstanding. |
Expected volatility is based on historical volatility. Historical volatility was computed using daily pricing observations for recent periods that correspond to the term of the warrants. The Company believes this method produces an estimate that is representative of future volatility over the expected term of these warrants and purchase options. The Company has no reason to believe future volatility over the expected remaining life of these warrants and purchase options is likely to differ materially from historical volatility. The expected life is based on the remaining term of the warrants and purchase options. The risk-free interest rate is based on U.S. Treasury securities according to the remaining term of the warrants and purchase options.
As required by the FASB’s accounting standards, financial assets and liabilities are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. Depending on the nature and the terms of the transaction, the fair values of the warrant/purchase option liability were modeled using a series of techniques, including closed-form analytic formula, such as the Black-Scholes Model, which does not entail material subjectivity because the methodology employed does not necessitate significant judgment, and the pricing inputs are observed from actively quoted markets.
The fair value of the 140,000 purchase options outstanding as of December 31, 2012 and 2011 was determined using the Black-Scholes Model, utilizing level 2 inputs, and the change was recorded in earnings. The Company recognized gains of $37,800 and $1,376,239 from the change in fair value of derivative liability for the year ended December 31, 2012 and 2011, respectively.
Following is an activity summary of the Company’s outstanding warrants and purchase options:
| Number of warrants/purchase options | Weighted – average exercise price | Weighted- average remaining contractual term (Year) | ||||||||||
| Outstanding at January 1, 2011 | 174,230 | $ | 7.50 | 2.04 | ||||||||
| Granted | - | |||||||||||
| Forfeited | - | |||||||||||
| Exercised | - | |||||||||||
| Outstanding at December 31, 2011 | 174,230 | $ | 7.50 | 2.04 | ||||||||
| Granted | - | - | ||||||||||
| Forfeited | (34,230 | ) | 5.00 | |||||||||
| Exercised | - | |||||||||||
| Outstanding at December 31, 2012 | 140,000 | $ | 8.11 | 1.50 | ||||||||
| Exercisable at December 31, 2012 | 140,000 | $ | 8.11 | 1.50 | ||||||||
| 68 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
Note 16 - STATUTORY RESERVES
Statutory reserves represent restricted retained earnings. Based on the legal formation of the entities, all PRC entities are required to set aside 10% of net income as reported in their statutory accounts on an annual basis to the statutory surplus reserve fund. Once the total statutory surplus reserve reaches 50% of the entity’s registered capital, further appropriations are discretionary. The statutory surplus reserve can be used to increase the entity’s registered capital (upon approval by relevant government authorities) and eliminate its future losses under PRC regulatory requirements (upon a resolution by the board of directors). The statutory surplus reserve is not distributable to shareholders except in the event of liquidation.
Appropriations to the above statutory reserves are accounted for as a transfer from unrestricted earnings to statutory reserves. There are no legal requirements in the PRC to fund these statutory reserves by the transfer of cash to any restricted accounts, and as such, the Company has not transferred any cash to these accounts. These reserves are not distributable as cash dividends.
| 69 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
Note 17 – TAXES
Skystar is subject to United States federal income tax. Skystar Cayman is a tax-exempt company incorporated in the Cayman Islands. Fortune Time did not have any assessable profits arising in or derived from Hong Kong for the years ended December 31, 2012 and 2011, and accordingly no provision for Hong Kong profits tax was made in these periods.
The Company’s subsidiaries and VIEs are subject to the PRC’s Enterprise Income Tax. Pursuant to the PRC Income Tax Laws, Enterprise Income Tax is generally imposed at a statutory rate of 25%. Xi’an Tianxing has been approved as a new technology enterprise, and under PRC Income Tax Laws is entitled to a preferential tax rate of 15%.
The following table reconciles the U.S. statutory rates to the Company’s effective tax rate for the years ended December 31, 2012 and 2011:
| Years ended December 31, | ||||||||
| 2012 | 2011 | |||||||
| U.S. Statutory rate | 34.0 | % | 34.0 | % | ||||
| Foreign income not recognized in the U.S. | (34.0 | ) | (34.0 | ) | ||||
| China income tax rate | 25.0 | 25.0 | ||||||
| China income tax exemption | (10.0 | ) | (10.0 | ) | ||||
| Other item (1) | 10.9 | 2.4 | ||||||
| Total provision for income taxes | 25.9 | % | 17.4 | % | ||||
| (1) |
Other items are primarily for operating expenses (income) incurred by Skystar that are not deductible (taxable) in the PRC, expenses incurred by other subsidiaries that are not deductible on the consolidated level, common stock issued under 2010 stock incentive plan, the difference of taxable income under US GAAP rather than Chinese GAAP, and the difference of Enterprise Income Tax imposed at a statutory rate of 25% rather than preferential tax rate of 15% on the income from the subsidiaries other than Xi’an Tianxing, which resulted in an increase in the effective tax rate of 10.9% and 2.4% for the years ended December 31, 2012 and 2011, respectively. As Xi’an Tianxing’s Huxian veterinary medication production was temporarily suspended for nine months for GMP re-certification in 2012 but Skystar Jingzhou resumed its normal production during the year, Xi’an Tianxing had relatively less income but Jingzhou generated more income for the year, resulting in more of our income taxed at standard rate of 25% rather than preferential tax rate of 15% in 2012 than 2011.
Other items consisted of the following: |
| Years ended December 31, | ||||||||
| 2012 | 2011 | |||||||
| Income taxed at Chinese statutory rate of 25% | 1.8 | % | 0.3 | % | ||||
| Expenses not deductible on the consolidated level | 2.8 | 1.0 | ||||||
| Non-deductible (non-taxable) operating expenses (income) | 1.8 | (0.3 | ) | |||||
| Common stock issued under 2010 stock incentive plan | 1.9 | 0.0 | ||||||
| Others | 2.6 | 1.4 | ||||||
| Total other items | 10.9 | % | 2.4 | % | ||||
Taxes payable consisted of the following:
| December 31, 2012 | December 31, 2011 | |||||||
| Income taxes | $ | 279,680 | $ | - | ||||
| Value added taxes | 1,409,762 | 110,880 | ||||||
| Other taxes | 261,315 | 49,201 | ||||||
| Total | $ | 1,950,757 | $ | 160,081 | ||||
| 70 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
As of December 31, 2012 and 2011, the estimated net operating loss carry forwards of Skystar for U.S. income tax purposes amounted to $5,896,749 and $4,531,058, which may be available to reduce future years’ taxable income. These carry forwards will expire, if not utilized, beginning in 2027 and through 2031. Management believes that the realization of the benefits arising from this loss appears to be uncertain due to the Company’s limited operating history and continuing losses for U.S. income tax purposes. Accordingly, the Company has provided a 100% valuation allowance at December 31, 2012 and 2011. The valuation allowance at December 31, 2012 and 2011 was $2,004,895 and $1,540,560, respectively. The Company’s management reviews this valuation allowance periodically and makes adjustments as necessary. As of December 31, 2012 and 2011, the Company has no other deferred tax amounts.
The Company did not provide for deferred income taxes and foreign withholding taxes on the cumulative undistributed earnings of foreign subsidiaries as of December 31, 2012 and 2011 of approximately $35.6 million and $25.6 million, respectively. The cumulative undistributed earnings of foreign subsidiaries were included in consolidated retained earnings and will continue to be indefinitely reinvested in international operations. Accordingly, no provision has been made for U.S. deferred taxes or applicable withholding taxes, related to future repatriation of these earnings, nor is it practicable to estimate the amount of income taxes that would have to be provided if we concluded that such earnings will be remitted in the future.
Note 18 - EARNINGS PER SHARE
The following is the calculation of earnings per share:
| Years ended December 31, | ||||||||
| 2012 | 2011 | |||||||
| Net income | $ | 6,212,428 | $ | 13,657,640 | ||||
| Weighted average shares used in basic computation | 7,471,350 | 7,182,969 | ||||||
| Diluted effect of stock warrants | - | 1,515 | ||||||
| Weighted average shares used in diluted computation | 7,471,350 | 7,184,484 | ||||||
| Earnings per share: | ||||||||
| Basic | $ | 0.83 | $ | 1.90 | ||||
| Diluted | $ | 0.83 | $ | 1.90 | ||||
For the years ended December 31, 2012 and 2011, the outstanding 140,000 options were excluded from the diluted earnings per share calculation as they were anti-dilutive.
For the year ended December 31, 2011, the average stock price was greater than the exercise prices of warrants, which resulted in additional weighted-average common stock equivalents of 1,515.
For the years ended December 31, 2012 and 2011, the outstanding 6,000 and 17,000 non-vested shares were excluded from the diluted earnings per share calculation as they were anti-dilutive.
| 71 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
Note 19 - RELATED PARTY TRANSACTIONS AND ARRANGEMENTS
Amounts receivable from and payable to related parties are summarized as follows:
| December 31, 2012 | December 31, 2011 | |||||||
| Shares to be issued to related parties (Note 15) | ||||||||
| Scott Cramer – non-executive director | $ | - | $ | 149,235 | ||||
| Mark D. Chen – non-executive director | 25,002 | 50,004 | ||||||
| Bing Mei – CFO | 4,340 | - | ||||||
| Total | $ | 29,342 | $ | 199,239 | ||||
| Amounts due to (from) related parties | ||||||||
| Weibing Lu – CEO (1) | $ | 608,282 | $ | (64,333 | ) | |||
| Scott Cramer – non-executive director and shareholder (1) | 138,169 | 147,877 | ||||||
| Officers, shareholders and other related parties (2) | 52,474 | (27,271 | ) | |||||
| Total | $ | 798,925 | $ | 56,273 | ||||
| (1) |
As of December 31, 2012 and 2011, the Company had unpaid reimbursement, compensation, and advances for business expenses due to Weibing Lu valued at $608,282 and due from Weibing Lu valued at $64,333, respectively. |
| (2) | As of December 31, 2012 and 2011, the Company had unpaid reimbursement and compensation due to Scott Cramer valued at $138,169 and $147,877, respectively. |
| (3) | The amounts due to (from) officers, shareholders and other related parties at December 31, 2012 and 2011 include unpaid reimbursement and compensation and, advances to other related parties for business expenses. |
Also refer to Notes 13, 15 and 20 for other related party transactions and arrangements.
| 72 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
Note 20 - COMMITMENTS AND CONTINGENCIES
(a) Lease commitments
The Company recognizes lease expense on the straight-line basis over the term of the lease.
The Company entered into a tenancy agreement for the lease of factory premises of Xi’an Tianxing in Sanqiao for a period of ten years from October 1, 2004 to December 31, 2014. The annual rent for the factory premises is subject to a 10% increase every two years starting October 1, 2009. As of December 31, 2012, the annual rent for the factory premises was adjusted to approximately $20,250 (RMB 127,600).
The Company leased office space in Xi’an from Mr. Weibing Lu, the Company’s Chairman and CEO, for a period of five years from January 1, 2007 to December 31, 2011, with an annual rent of approximately $26,281 (RMB 165,600). In January 2012, the Company renewed the lease with Mr. Lu for another 5 years from January 1, 2012 to December 31, 2016 at a rent of approximately $28,566 (RMB 180,000) per year.
The Company also entered into a tenancy agreement with Mr. Weibing Lu for the lease of Shanghai Siqiang’s office in Shanghai for a period of ten years from August 1, 2007 to August 1, 2017 with an annual rent of approximately $22,853 (RMB 144,000).
The Company entered into a one year tenancy agreement for an office lease in Kunshan, Jiangsu Province from April 15, 2011 to April 14, 2012 with an annual rent of approximately $3,047 (RMB 19,200). On April 15, 2012, the Company entered into a six-month tenancy agreement for this office from April 15, 2012 to October 14, 2012 with six-month rent of approximately $1,651 (RMB 10,405). On October 15, 2012, the Company entered into a another six-month tenancy agreement for this office from October 15, 2012 to April 14, 2013 with a six-month rent of approximately $1,651 (RMB 10,405).
The Company entered into a tenancy agreement for the lease of warehouse premises in Xi’an for a period of three years from July 20, 2011 to July 19, 2014 with an annual rent of approximately $37,295 (RMB 235,000) subject to a 10% increase every two years starting July 20, 2013.
On February 21, 2012, the Company entered into a six-month tenancy agreement for an office lease in Jingzhou, Hubei Province from February 21, 2012 to August 20, 2012 with a six-month rent of approximately $286 (RMB 1,800). On August 21, 2012, the Company entered into another three-month tenancy agreement for this office from August 21, 2012 to November 20, 2012 with three-month rent of approximately $143 (RMB 900).
The minimum future lease payments for the next five years and thereafter are as follows:
| Period | Unrelated third parties | Related parties | Total | |||||||||
| Year ending December 31, 2013 | $ | 60,656 | $ | 51,419 | $ | 112,075 | ||||||
| Year ending December 31, 2014 | 38,786 | 51,419 | 90,205 | |||||||||
| Year ending December 31, 2015 | - | 51,419 | 51,419 | |||||||||
| Year ending December 31, 2016 | - | 51,419 | 51,419 | |||||||||
| Year ending December 31, 2017 and thereafter | - | 13,331 | 13,331 | |||||||||
| Total | $ | 99,442 | $ | 219,007 | $ | 318,449 | ||||||
Rental expense to unrelated third parties for the years ended December 31, 2012 and 2011 amounted to $62,620 and $40,584, respectively. Rental expense to related third parties for the years ended December 31, 2012 and 2011 amounted to $51,403 and $47,976, respectively.
| 73 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
(b) Legal proceedings
From time to time, the Company is involved in legal matters arising in the ordinary course of business. Management currently is not aware of any legal matters or pending litigation that would have a significant effect on the Company’s consolidated financial statements as of December 31, 2012.
In May 2007, Andrew Chien filed suit against the Company, R. Scott Cramer, Steve Lowe, David Wassung and Weibing Lu in United States District Court for the District of Connecticut, alleging causes of action for violation of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934. On July 17, 2008, in a decision that is now published, the court granted defendants’ motion to dismiss and subsequently dismissed the lawsuit, entering judgment on behalf of the defendants. Chien appealed the dismissal. Defendants filed a post judgment motion for sanctions against Chien. On February 5, 2009, the court found the action filed by Chien to have been frivolous, and to have constituted a “substantial” violation of Rule 11, and imposed monetary sanctions on both Chien and his former attorney. Chien appealed the award of sanctions. All appeals, including the one referenced below concerning Chien’s second lawsuit, were subsequently consolidated. The Court of Appeals issued a Mandate upholding the decision granting defendant’s motion to dismiss and found that the District Court did not “abuse its discretion” in issuing sanctions against Chien in light of the circumstances and facts on record. This Mandate was entered on or about November 8, 2010.
Andrew Chien, proceeding pro se (i.e., he represented himself without an attorney), filed his second lawsuit against the Company, R. Scott Cramer, Steve Lowe, David Wassung and Weibing Lu in Connecticut Superior Court (which was removed to U.S. District Court) alleging causes of action similar to those alleged in his federal complaint described above as well as state law causes of action. The court held that all claims asserted against the defendants were barred and failed to state a claim on a multiplicity of grounds, including on the basis of res judicata. Defendants filed a second Motion for Sanctions under Rule 11 and the PSLRA, which was granted. Chien appealed the dismissal. The Court of Appeals for the Second Circuit consolidated all of Chien’s appeals from both of his lawsuits. On November 8, 2010, the Court of Appeals affirmed the dismissals and the awards of sanctions. On January 22, 2011, Chien filed a petition with the Supreme Court of the United States, appealing the lower court’s ruling. The Supreme Court denied review of the petition.
Andrew Chien, again proceeding pro se, commenced his third lawsuit against the Company, Scott Cramer, Steve Lowe, David Wassung, Weibing Lu (and also Weinberg & Company, P.A., Moore Stephens Wirth Frazer & Torbet, LLP, Frazer Frost, LLP, Crowe Horwath LLP, Richardson & Patel LLP, Kevin K. Leung, Harvey Kesner, and Jody M. Borrelli) alleging the same facts and circumstances as set forth in the above two matters, although adding the aforementioned accountants and lawyers as defendants to the claims. The matter commenced on August 8, 2011, and shortly thereafter was removed to U.S. District Court. On October 5, 2011, the court dismissed the entire action as to all defendants. As in the two previous lawsuits, sanctions were issued against Chien. Moreover, the District Court issued an order prohibiting Chien from filing any more lawsuits against the defedentants without prior approval from the court. Chien appealed the Court’s ruling to the United States Court of Appeals for the Second Circuit, where all defendants have filed motions to dismiss.
The United States Court of Appeals for the Second Circuit recently affirmed the judgments and orders of the United States District Court and dismissed all of Mr. Chien’s pending appeals.
Although the Second Circuit declined to issue sanctions against Chien, it expressly warned Chien “that the continued filing of duplicative, vexatious, or frivolous appeals regarding issues already resolved by this Court may result in the imposition of sanctions, including a leave-to-file sanction requiring Chien to obtain permission from this Court prior to filing further submissions in this Court.” Mr. Chien has filed a petition for writs of certiorari with the Supreme Court of the United States. The Supreme Court has not yet acted on Chien’s petitions.
(c) Ownership of leasehold property
In 2005, a shareholder contributed a leasehold office building as additional capital of Xi’an Tianxing. However, as of December 31, 2012, title to this leasehold property has not passed to the Company. The Company does not believe there are any legal barriers for the shareholder to transfer the ownership to the Company. However, in the event that the Company fails to obtain the ownership certificate for the leasehold property, there is a risk that we may be required to vacate the building. Management believes that this possibility is remote, and, as such, no provision has been made in the consolidated financial statements for this potential occurrence.
| 74 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
(d) R&D project
In 2008, Xi’an Tianxing contracted with Northwestern Agricultural Technology University to work jointly on an R&D project concerning the application of nano-technology in the prevention of major milk cow disease. The total projected budget for this project is approximately $634,800 (RMB 4,000,000), which is to be paid according to completed stages of the project. The project reached trial stage in September 2009. As of December 31, 2012, the Company incurred approximately $555,275 (RMB 3,500,000) of cumulative expenses relating to this project.
In 2009, Xi’an Tianxing contracted with the Fourth Military Medical University to jointly work on an R&D project on fish diseases linked immunosorbent detection kit and fish diseases multi-linked monoclonal antibody therapeutic agents. The contracted amount for this project is approximated $952,200 (RMB 6,000,000). As of December 31, 2012, the Company has incurred approximately $824,980 (RMB 5,200,000) of cumulative expenses relating to this project.
During the first quarter of 2011, Xi’an Tianxing contracted with the Fourth Military Medical University to jointly work on an R&D project to develop new treatment and diagnosis method for Mycoplasmal pneumonia of swine. The project term is from January 2011 through September 2013. The cost to the Company for the initial phase is approximately $317,400 (RMB 2,000,000). As of December 31, 2012, the Company has incurred approximately $237,975 (RMB 1,500,000).
During the second quarter of 2011, Xi’an Tianxing launched four in-house R&D projects as set forth in the following table. As of December 31, 2012, all of these projects were completed.
During the second quarter of 2012, Xi’an Tianxing contracted with the Fourth Military Medical University to jointly work on an R&D project of ring disease and pseudo-rabies specific transfer factor and blue-ear disease and Mycoplasma pneumoniae disease specific transfer factor to enhance the livestock and poultry's own immuneforce and prevent the onset of conventional disease. The project term is from April 2012 through March 2013. The cost to the Company for the initial phase is approximately $49,182 (RMB 310,000). As of December 31, 2012, the project was in clinical research stage and the Company has incurred approximately $49,182 (RMB 310,000).
During the fourth quarter of 2012, Xi’an Tianxing launched eight in-house R&D projects as set forth in in the following table. As of December 31, 2012, the Company incurred all the budget expenses and these projects were still under development.
In addition, the Company also launched various R&D projects on veterinary products formula adjustment, pet drug development and fermentation engineering design and development, and lab tests. As of December 31, 2012, approximately $1,002,828 (RMB 6,321,009) has been incurred related to these completed projects and lab tests.
| 75 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
R&D projects are summarized as follows:
| Project | Amount incurred as of 12/31/2012 | Amount expected to be incurred | Total amount of project | |||||||||
| Project with Northwestern Agricultural Technology University | ||||||||||||
| Application of nano-technology in the prevention of major milk cow disease | $ | 555,275 | $ | 79,525 | $ | 634,800 | ||||||
| Project with the Fourth Military Medical University | ||||||||||||
| Fish diseases linked immunosorbent detection kit and fish diseases Multi-linked monoclonal antibody therapeutic agents | 824,980 | 127,220 | 952,200 | |||||||||
| New treatment and diagnosis method for Mycoplasmal pneumonia of swine | 237,975 | 79,425 | 317,400 | |||||||||
| Transfer factor test | 49,182 | - | 49,182 | |||||||||
| In-house R&D project during 2011 | ||||||||||||
| Ceftiofur sodium for injection (powder for injection) | 555,275 | - | 555,275 | |||||||||
| Sulfuric acid injection neostigmine | 530,328 | - | 530,328 | |||||||||
| Dexamethasone sodium phosphate injection | 556,470 | - | 556,470 | |||||||||
| Houttuynia preparation of compound application in weaning piglets | 691,543 | - | 691,543 | |||||||||
| In-house R&D project during 2012 | ||||||||||||
| Oral liquid products R&D and field trials | 158,650 | - | 158,650 | |||||||||
| Powder for injection products R&D and field trials | 158,650 | - | 158,650 | |||||||||
| Vitamin E injection product development | 79,325 | - | 79,325 | |||||||||
| Anthelmintic product development | 79,325 | - | 79,325 | |||||||||
| Three disease grams product development | 79,325 | - | 79,325 | |||||||||
| Premixes class product development | 79,325 | - | 79,325 | |||||||||
| Bulk powder products development | 79,325 | - | 79,325 | |||||||||
| Livestock and fish diseases immunoassay kit development | 396,625 | - | 396,625 | |||||||||
| Other projects for veterinary products formula adjustment, pet drug development and fermentation engineering design and development and lab tests | 1,002,828 | 16,186 | 1,019,014 | |||||||||
| Total | $ | 6,114,406 | $ | 302,356 | $ | 6,416,762 | ||||||
All payments made for R&D projects have been expensed as incurred.
(e) Registered capital commitment
Skystar Kunshan’s remaining registered capital of $12,750,000 was originally required to be invested by May 7, 2012. In 2011, the asset acquisition of Kunshan facility was completed. The Company is in the process of getting the government’s approval to transfer the asset purchased to satisfy some of our registered capital commitment. On July 25, 2012, the Company received the approval of extension for payment of the remaining registered capital but a new due date has not been specified by the government.
| 76 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2012
(Amounts in U.S. dollars, except share and amounts)
Note 21 - RESTRICTED NET ASSETS
Schedule I of Article 5-04 of Regulation S-X requires the condensed financial information of registrant (Parent Company) shall be filed when the restricted net assets of consolidated subsidiaries exceed 25 percent of consolidated net assets as of the end of the most recently completed fiscal year. For purposes of this test, restricted net assets of consolidated subsidiaries shall mean that amount of the registrant’s proportionate share of net assets of consolidated subsidiaries (after intercompany eliminations) which as of the end of the most recent fiscal year may not be transferred to the parent company by subsidiaries in the form of loans, advances or cash dividends without the consent of a third party (i.e., lender, regulatory agency, foreign government, etc.).
The condensed parent company financial statements have been prepared in accordance with Rule 12-04, Schedule I of Regulation S-X as the restricted net assets of the subsidiaries of Skystar Bio-Pharmaceutical Company exceed 25% of the consolidated net assets of Skystar Bio-Pharmaceutical Company. The ability of our Chinese operating affiliates to pay dividends may be restricted due to the foreign exchange control policies and availability of cash balances of the Chinese operating subsidiaries. Because a significant portion of our operations and revenues are conducted and generated in China, all of our revenues being earned and currency received are denominated in Renminbi (RMB). RMB is subject to the exchange control regulation in China, and, as a result, we may be unable to distribute any dividends outside of China due to PRC exchange control regulations that restrict our ability to convert RMB into US Dollars.
| 77 |
SCHEDULE I – CONDENSED FINANCIAL INFORMATION OF REGISTRANT
SKYSTAR BIO-PHARMACEUTICAL COMPANY
PARENT COMPANY STATEMENTS OF OPERATIONS
| Years ended December 31, | ||||||||
| 2012 | 2011 | |||||||
| REVENUE, net | $ | - | $ | - | ||||
| OPERATING EXPENSES: | ||||||||
| Salaries and consulting expenses | 272,660 | 361,972 | ||||||
| General and administrative | 1,819,709 | 713,770 | ||||||
| Total operating expenses | 2,092,369 | 1,075,742 | ||||||
| LOSS FROM OPERATIONS | (2,092,369 | ) | (1,075,742 | ) | ||||
| OTHER INCOME (EXPENSE): | ||||||||
| Interest expenses | - | (2,608 | ) | |||||
| Change in fair value of warrant/purchase option liability | 37,800 | 1,376,239 | ||||||
| Other income | 13,819 | 21,426 | ||||||
| Total other income, net | 51,619 | 1,395,057 | ||||||
| (LOSS) INCOME ATTRIBUTABLE TO PARENT ONLY | (2,040,750 | ) | 319,315 | |||||
| EQUITY IN EARNINGS OF SUBSIDIARIES | 8,253,178 | 13,338,325 | ||||||
| NET INCOME AVAILABLE TO SHAREHOLDERS | $ | 6,212,428 | $ | 13,657,640 | ||||
| 78 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
PARENT COMPANY BALANCE SHEETS
| December 31, | December 31, | |||||||
| 2012 | 2011 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash | $ | 968 | $ | 12,508 | ||||
| Prepaid expenses and other current assets | 283,836 | 256,000 | ||||||
| Total current assets | 284,804 | 268,508 | ||||||
| Interests in subsidiaries | 96,750,650 | 88,953,961 | ||||||
| Total assets | $ | 97,035,454 | $ | 89,222,469 | ||||
| LIABILITIES AND SHAREHOLDERS' EQUITY | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts payable and accrued expenses | $ | 126,062 | $ | 440,977 | ||||
| Salary and consulting fees payable | 71,838 | 344,888 | ||||||
| Due to related parties | 617,021 | 290,391 | ||||||
| Total current liabilities | 814,921 | 1,076,256 | ||||||
| OTHER LIABILITIES: | ||||||||
| Warrant/purchase option liability | 5,600 | 43,400 | ||||||
| Total liabilities | 820,521 | 1,119,656 | ||||||
| SHAREHOLDERS' EQUITY | 96,214,933 | 88,102,813 | ||||||
| Total liabilities and shareholders' equity | $ | 97,035,454 | $ | 89,222,469 | ||||
| 79 |
SKYSTAR BIO-PHARMACEUTICAL COMPANY
PARENT COMPANY STATEMENTS OF CASH FLOWS
| Years ended December 31, | ||||||||
| 2012 | 2011 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net income available to shareholders | $ | 6,212,428 | $ | 13,657,640 | ||||
| Adjustments to reconcile net income to net cash used in operating activities: | ||||||||
| Equity in earnings of subsidiaries | (8,253,178 | ) | (13,338,325 | ) | ||||
| Common stock issued under 2010 stock incentive plan | 1,037,911 | - | ||||||
| Change in fair value of warrant/purchase option liability | (37,800 | ) | (1,376,239 | ) | ||||
| Change in operating assets and liabilities | ||||||||
| Prepaid expenses and other current assets | (27,836 | ) | (256,000 | ) | ||||
| Accounts payable and accrued expenses | (314,915 | ) | 282,300 | |||||
| Salary and consulting fee payable | (273,050 | ) | 182,030 | |||||
| Net cash used in operating activities | (1,656,440 | ) | (848,594 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Decrease in interest in subsidiaries | 1,318,270 | 517,276 | ||||||
| Net cash used in investing activities | 1,318,270 | 517,276 | ||||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Increase in due to related parties | 326,630 | 119,454 | ||||||
| Net cash provided by financing activities | 326,630 | 119,454 | ||||||
| DECREASE IN CASH | (11,540 | ) | (211,864 | ) | ||||
| CASH, beginning of year | 12,508 | 224,372 | ||||||
| CASH, end of year | $ | 968 | $ | 12,508 | ||||
The condensed parent company financial statements have been prepared using the equity method to account for its subsidiaries. Refer to the consolidated financial statements and notes presented above for additional information and disclosures with respect to these financial statements.
| 80 |