2014.09.30 10-Q Exhibit 10.2 - Texas Contract Amendment N
Exhibit 10.2
HHSC Contract No. 529-12-0002-00006-N
|
| | |
This Amendment is between the Texas Health and Human Services Commission (HHSC), an administrative agency within the executive department of the State of Texas, having its principal office at 4900 North Lamar Boulevard, Austin, Texas 78751, and Bankers Reserve Life Insurance Company of Wisconsin d.b.a. Superior HealthPlan Network (MCO), an entity organized under the laws of the State of Wisconsin, having its principal place of business at 2100 South IH-35, Suite 202, Austin, Texas 78704. HHSC and MCO may be referred to in this Amendment individually as a “Party” and collectively as the “Parties.”
|
Amendment Effective Date | Contract Expiration Date | Operational Start Date |
October 1, 2014 | August 31, 2015 | March 1, 2012 |
MCO Brand Names |
The MCO will use following brand name(s). The MCO acknowledges that if it requests a change to the brand name(s), it will be responsible for all costs associated with the change(s), including HHSC's costs for modifying its business rules, system identifiers, communications materials, web page, etc. STAR: Superior Health Plan STAR+PLUS: Superior Health Plan CHIP: MRSA: Superior HealthPlan |
Project Managers |
HHSC: Emily Zalkovsky Director, Program Management 4900 North Lamar Boulevard Austin, Texas 78751 Phone: 512-462-6382 Fax: 512-730-7452 MCO: Susan Erickson Plan General Counsel 2100 South IH-35, Suite 202 Austin, Texas 78704 Phone: 512-692-1465 Fax: 866-702-4830 E-mail: serickson@centene.com
|
Legal Notice Delivery Addresses |
HHSC: General Counsel 4900 North Lamar Boulevard, 4th Floor Austin, Texas 78751 Fax: 512-424-6586 MCO: Superior HealthPlan Network 2100 South IH-35, Suite 202 Austin, Texas 78704 Fax: 866-702-4830 |
|
|
|
MCO Programs and Service Areas |
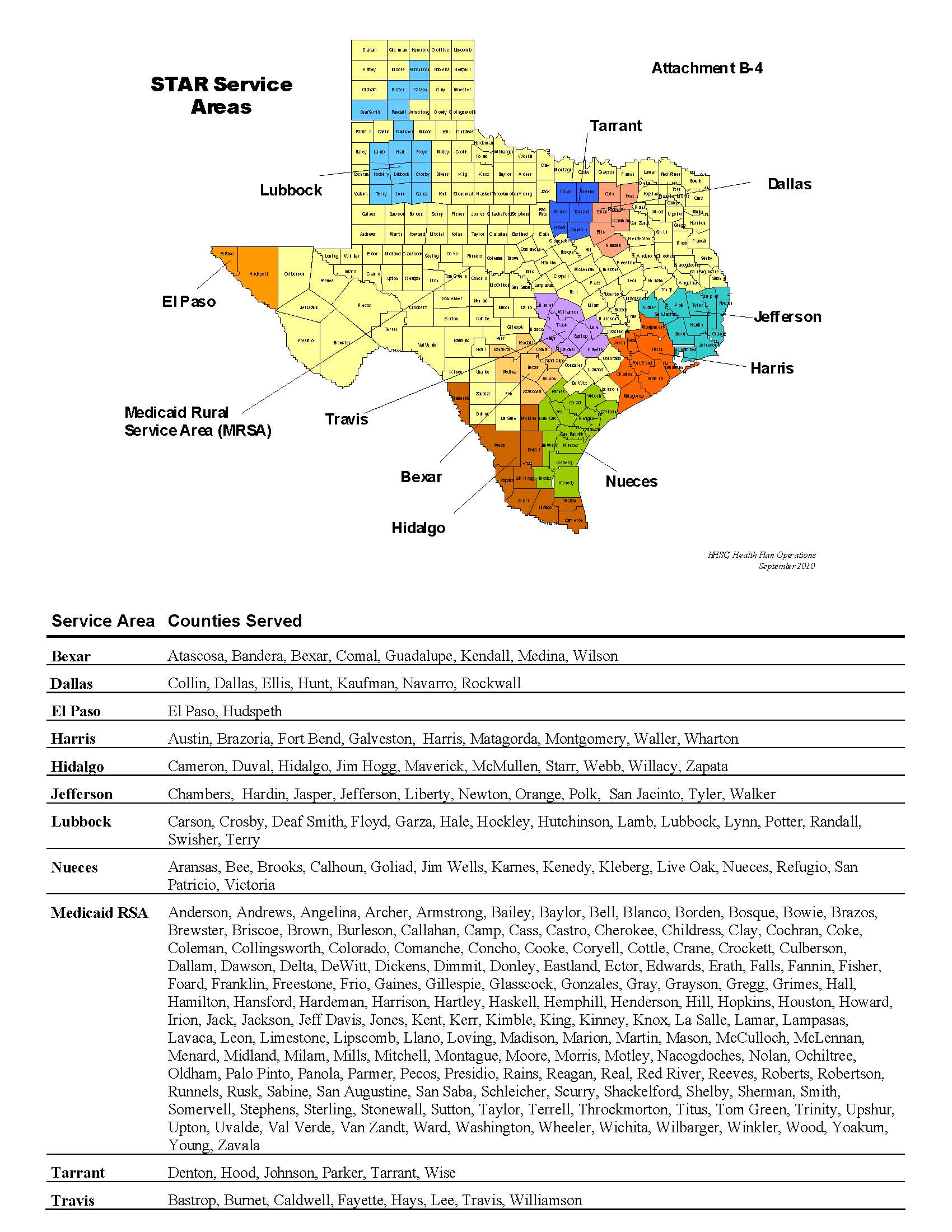
This Amendment applies to the following checked HHSC MCO Programs and Service Areas . All references in the Amendment or the Contract to MCO Programs or Service Areas that are not checked do not apply to the MCO. þ Medicaid STAR MCO Program þ Medicaid STAR + PLUS MCO Program o CHIP MCO Program |
þ Medicaid STAR MCO Program
|
| | | | |
Service Areas: | | o Bexar | | þ Medicaid RSA - Central |
| | o Dallas | | þ Medicaid RSA - Northeast |
| | o El Paso | | þ Medicaid RSA - West |
| | o Harris | | o Nueces |
| | þ Hidalgo | | o Tarrant |
| | o Jefferson | | o Travis |
| | o Lubbock | | |
See Contract Attachment B-4, “Map of Counties with MCO Program Service Areas,” for a list of counties includes within the STAR Service Areas.
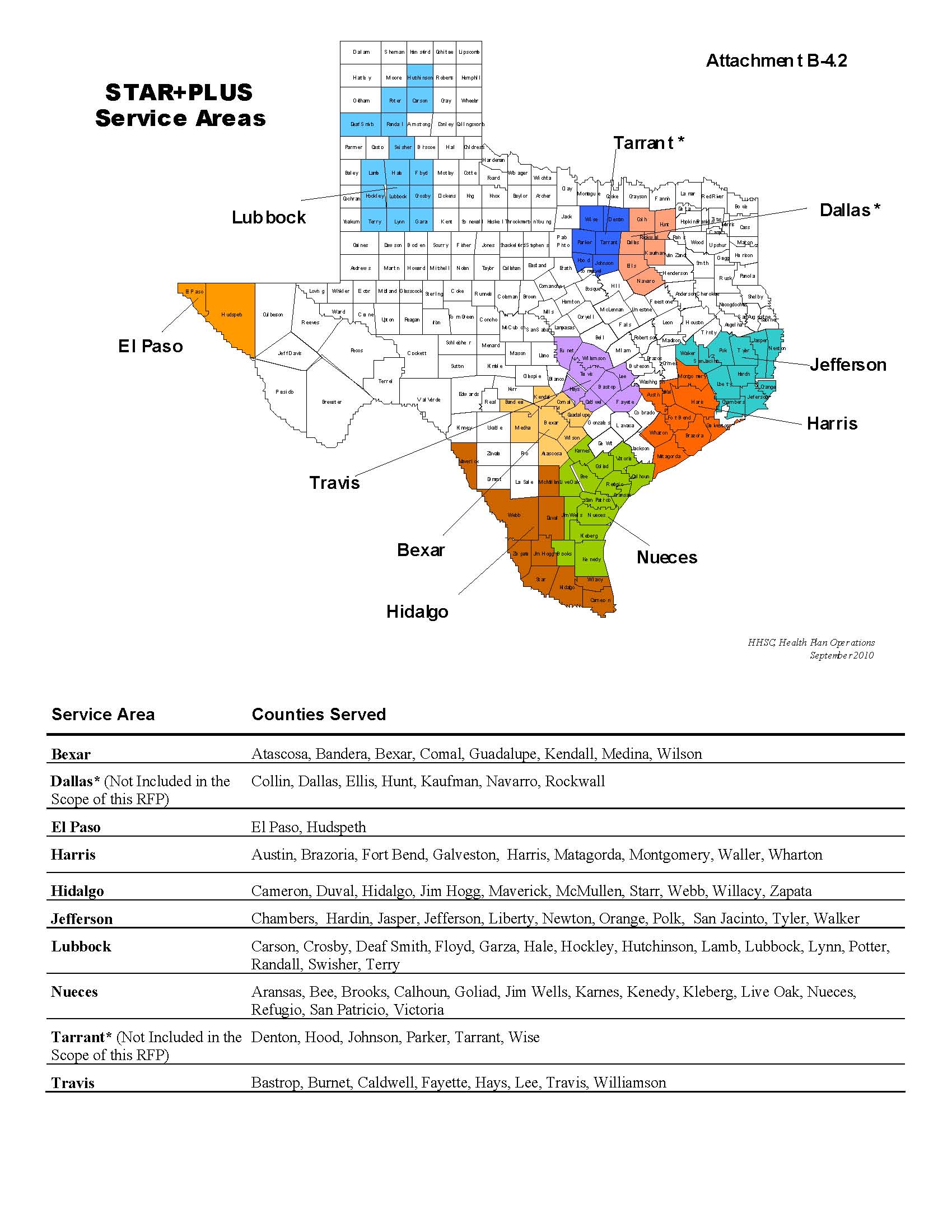
þ Medicaid STAR+PLUS MCO Program
|
| | | | |
Service Areas: | | o Bexar | | o Jefferson |
| | o El Paso | | o Lubbock |
| | o Harris | | o Nueces |
| | þ Hidalgo | | o Travis |
See Contract Attachment B-4.2, “Map of Counties with STAR+PLUS MCO Program Service Areas,” for a list of counties included within the STAR+PLUS Service Areas.
þ Medicaid STAR MCO Program
Capitation: See Attachment A, “Uniform Managed Care Contract Terms and Conditions,” Article 10, for a description of the Capitation Rate-setting methodology and the Capitation Payment requirements for the STAR Program.
|
| | | | | |
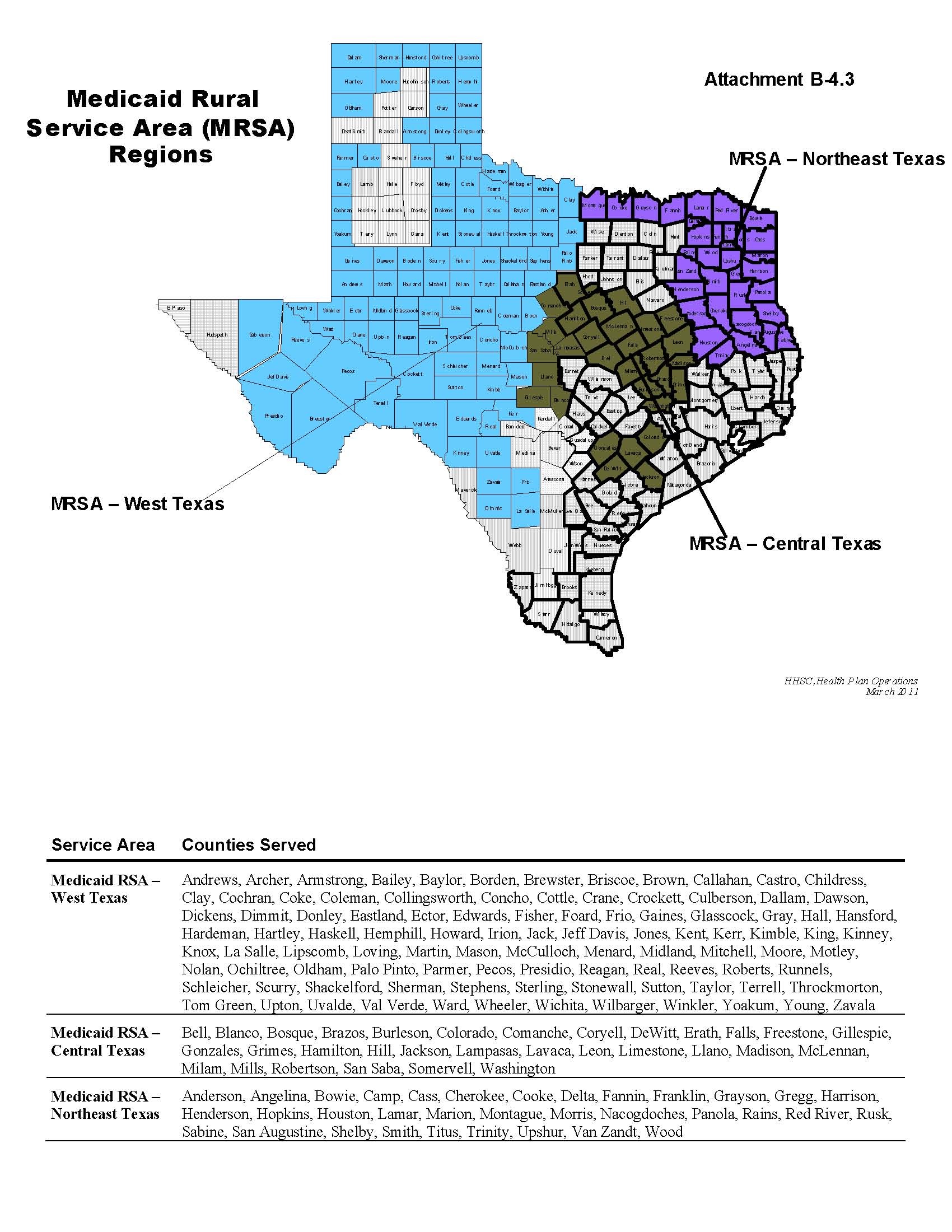
Rate Period 3 Capitation Rates |
| Service Area: | Hidalgo | Medicaid Rural Service Area - Central Texas | Medicaid Rural Service Area - Northeast Texas | Medicaid Rural Service Area - West Texas |
| Rate Cell | | | | |
1 | Under Age 1 Child | $542.61 | $536.31 | $533.97 | $545.64 |
2 | Age 1-5 Child | $234.44 | $138.05 | $139.82 | $123.18 |
3 | Age 6-14 Child | $157.50 | $118.37 | $121.29 | $120.93 |
4 | Age 15-18 Child | $158.46 | $133.80 | $137.30 | $143.34 |
5 | Age 19-20 Child | $317.49 | $317.09 | $346.61 | $366.62 |
6 | TANF Adult | $432.57 | $389.70 | $410.21 | $402.98 |
7 | Pregnant Woman | $372.10 | $434.70 | $435.59 | $429.38 |
Delivery Supplemental Payment: See Contract Attachment A, "Uniform Managed Care Contract Terms and Conditions," Article 10, for a description of the Delivery Supplemental Payment for the STAR Program. The STAR Delivery Supplemental Payments for the Service Areas covered by this contract are listed below.
|
| |
Service Area | Delivery Supplemental Payment |
Hidalgo | $3,409.95 |
Medicaid Rural Service Area - Central Texas | $3,035.27 |
Medicaid Rural Service Area - Northeast Texas | $3,160.40 |
Medicaid Rural Service Area - West Texas | $3,204.07 |
þ Medicaid STAR+PLUS MCO Program
Capitation: See Attachment A, “Uniform Managed Care Contract Terms and Conditions,” Article 10, for a description of the Capitation Rate-setting methodology and the Capitation Payment requirements for the STAR+PLUS Program.
|
| | |
Rate Period 3 Capitation Rates |
| STAR + PLUS Service Area: | Hidalgo |
| Rate Cell | |
1 | Medicaid Only Standard Rate | $1,465.38 |
2 | Medicaid Only HCBS STAR+PLUS Waiver Rate - Above Floor | $3,890.75 |
3 | Medicaid Only HCBS STAR+PLUS Waiver Rate - Below Floor | $3,890.75 |
4 | Dual Eligible Standard Rate | $925.85 |
5 | Dual Eligible HCBS STAR+PLUS Waiver Rate- Above Floor | $1,896.11 |
6 | Dual Eligible HCBS STAR+PLUS Waiver Rate- Below Floor | $1,896.11 |
7 | Nursing Facility - Medicaid Only | $1,465.38 |
8 | Nursing Facility - Dual Eligible | $925.85 |
9 | Individuals with Development Disabilities (IDD) - under age 21 | $3,167.03 |
10 | Individuals with Development Disabilities (IDD) - age 21 and older | $964.64 |
The parties agree to amend their original contract, HHSC contract number 529-12-0002-00006 (Contract). The Parties agree that the terms of the Contract will remain in effect and continue to govern except to the extent modified in this Amendment.
The Parties execute this Amendment in accordance with the authority granted in HHSC Uniform Managed Care Contract Attachment A , "Uniform Managed Care Contract Terms & Conditions," under Article 8, "Amendments & Modifications."
HHSC Uniform Managed Care Contract Version 2.12 is attached.
|
|
Signatures |
The Parties execute this Amendment in their stated capacities with authority to bind their organizations on the dates in this section. Texas Health and Human Services Commission /s/ Chris Traylor Chris Traylor Chief Deputy Commissioner Office of the Chief Deputy Commissioner Date: 8/14/2014 Bankers Reserve Life Insurance Company of Wisconsin d.b.a. Superior HealthPlan Network /s/ Holly Munin By: Holly Munin Title: CEO Date: 7/28/2014 |
Responsible Office: HHSC Office of General Counsel (OGC)
Subject: Attachment A -- HHSC Uniform Managed Care Contract Terms & Conditions Version 2.12
Texas Health & Human Services Commission
Uniform Managed Care Contract Terms & Conditions
|
| | | | |
DOCUMENT HISTORY LOG |
STATUS1 | DOCUMENT REVISION2 | EFFECTIVE DATE | DESCRIPTION3 |
Baseline | n/a | September 1, 2011 | Initial version of the Attachment A, “Medicaid and CHIP Uniform Managed Care Contract Terms & Conditions.” |
Revision | 2.1 | March 1, 2012 | Definition “1915(c) Nursing Facility Waiver” is modified to correct a cross-reference. Definition for Medically Necessary is modified for clarification. The State has determined that all acute care behavioral health and non-behavioral health services for Medicaid children fall within the scope of Texas Health Steps. Note that for LTSS, such as PCS (PAS) services for children in STAR+PLUS, the functional necessity standard for LTSS also applies (see Attachment B-1, Section 8.3.3). Definition for Rate Period 1 is modified. Section 4.04 is modified to clarify the requirements for Medical Director designees, and to clarify that the provision does not apply to prior authorization determinations made by Texas licensed pharmacists. New Section 4.11 “Prohibition Against Performance Outside of the United States” added. Section 5.02(b) is modified to clarify that MCOs may not sell or transfer their Member base. Section 5.06(a)(2) is modified to clarify the exceptions to enrollment in an MCO during an Inpatient Stay. Section 5.06(a)(3) and (4) are modified to clarify that Members cannot move from FFS to an MCO or from one MCO to another during residential treatment or residential detoxification. References to the PCCM program are removed. In addition, Section 5.06(a)(8) is modified to clarify movement requirements for SSI Members in the MRSA. Section 10.06(b) is modified to remove the Perinate Newborn 0% - 185% rate cell. Section 10.10 is modified to consolidate STAR+PLUS with STAR and CHIP for the Experience Rebate calculation. Section 10.10.1 is deleted in its entirety. Section 10.10.2 is modified to consolidate STAR+PLUS into STAR and CHIP for the Experience Rebate calculation. |
|
| | | | |
Revision | 2.2 | June 1, 2012 | Definition for Consolidated FSR Report or Consolidated Basis is added. Definition for Financial Statistical Report is added. Definitions for FSR Reporting Period, FSR Reporting Period 12/13, and FSR Reporting Period 14 are added. Definition for Material Subcontract is modified. Definition for Net Income Before Taxes is modified. Definition for Pre-tax Income is modified. Definition for Program is added. Definition for Rate Period 1 and Rate Period 2 are modified. Section 10.10 is modified to consolidate the Experience Rebate across all contracts and all programs. Section 10.10.2 is modified to consolidate the Administrative Expense Cap across all contracts and all programs. |
Revision | 2.3 | September 1, 2012 | Definition for Case Management for Children and Pregnant Women is modified to remove the acronym “CPW”. Definition for Community-based Long Term Services and Supports is modified to replace references to “1915(c) Nursing Facility Waiver” with “HCBS STAR+PLUS Waiver”. Definition for “1915(c) Nursing Facility Waiver” is modified to change the name to “HCBS STAR+PLUS. Waiver” and to update references to “Texas Healthcare Transformation and Quality Improvement Program 1115 Waiver” and “HCBS STAR+PLUS Waiver”. Definition for “HHSC MCO Programs or MCO Programs” is modified. Definition for “Medically Necessary” is modified. Definition for “Provider Materials” is added. Section 5.06(a)(4) is modified to clarify responsibility for payment. Section 5.11 is deleted in its entirety. Section 7.02 is modified to clarify that only applicable provisions of the listed laws apply to the contract. Section 10.05 is modified to replace references to “1915(c) Nursing Facility Waiver” with “HCBS STAR+PLUS Waiver”.
|
Revision
| 2.4 | March 1, 2013 | All references to the previous Executive Commissioner Suehs are changed to his successor, Executive Commissioner Janek.
Definition for “Electronic Visit Verification” is added.
Section 5.02(e), Subsections (4) and (5) are modified.
Section 10.16 is added to address supplemental payments to MCOs for wrap-around services for outpatient drugs and biological products for STAR-PLUS Members.
|
|
| | | | |
Revision | 2.5 | June 1, 2013 | Contract amendment did not revise Attachment A, Uniform Managed Care Contract Terms and Conditions. |
Revision | 2.6 | September 1, 2013 | Definition for CAHPS is modified to correct the name to which the acronym refers. Definition for “Community Health Worker” is added. Definition for “Court-Ordered Commitment” is modified. Definition for Default Enrollment is modified to add T.A.C. reference. Definition for “DSM” is modified. Definition for “ECI” is modified. Definition for HEDIS is modified to correct the name to which the acronym refers. Definition for Primary Care Physician is modified to remove the list of provider types as being redundant. Definition for Rate Period is modified to include a third sub-period. Section 5.02(e) is modified to remove the language regarding disenrollment for ESRD and ventilator dependency. Section 5.08 is renamed “Modified Default Enrollment Process” and revised to include a process for all Programs. Section 5.09 is deleted and replaced with Section 5.08. Section 5.10 is deleted and replaced with Section 5.08. Section 7.04 is deleted in its entirety and updated within Section 7.02 Section 9.02 is modified for clarification that records must be provided “at no cost.” Section 9.04 is modified for clarification that records must be provided “at no cost.” Section 10.05(a) is modified to comply with the new STAR Risk Groups. Section 10.10.3 is modified to clarify that the Reinsurance Cap impacts only the Experience Rebate calculation. Section 11.01(c) is modified to add the missing word “may.” Section 13.01 is modified to clarify the required certifications. Section 14.08 is modified to delete outdated language
|
|
| | | | |
Revision | 2.7 |
| September 1, 2013 | Section 10.17 “Pass-through Payments for Provider Rate Increases” is added. |
Revision | 2.8 |
| January 1, 2014 | Definition for Expansion Children is removed.
Definition for Federal Poverty Level is updated.
Definition for Former Foster Care Child (FFCC) Member is added.
Section 5.02 is modified to add requirement for default assignment methodologies.
Section 5.04 is modified to clarify that HHSC or the ASC will enroll or disenroll Members.
Section 5.05 is modified to clarify that HHSC or the ASC will transmit new Member information, to remove the FPL limits, to remove the default assignment language, and to clarify the enrollment process when CHIP Perinate coverage expires.
Section 5.06 is modified to add requirements regarding movement from a STAR Health MCO to a STAR MCO.
Section 10.06(b) is modified to clarify the eligibility thresholds.
Section 10.09 is modified to clarify the eligibility thresholds.
Section 11.01(a) is modified to correct an administrative error.
Section 12.03 is modified to delete subsection (b)(8) Termination for Insolvency and all following subsections are renumbered.
|
|
| | | | |
Revision | 2.9 |
| February 1, 2014 | Definition for Capitation Payment is modified to include associated Administrative Services.
Definition for Child (or Children) with Special Health Care Needs (CSHCN) is clarified.
Definition for Clean Claim is clarified to include Nursing Facility Services.
Definition for Cognitive Rehabilitation Therapy is added.
Definition for Community Services Specialist (CSSP) is added.
Definition for Electronic Visit Verification System is added.
Definition for Employment Assistance is added.
Definition for Family Partner is added.
Definition for Fee-for-Service (FFS) is clarified that payment is made after the service is provided.
Definition for ICF-IID Program is added.
Definition for IDD Waiver is added.
Definition for Licensed Medical Personnel is added.
Definition for Licensed Practitioner of the Healing Arts is added.
Definition for Local IDD Authority is added.
Definition for Local Mental Health Authority is modified to reference the legal citation.
Definition for Material Subcontract is modified to clarify excluded subcontractors.
Definition for MCO Administrative Services is modified to include all required deliverables outside of the Covered Services.
Definition for Medical Home is modified to have the meaning assigned in Gov’t Code 533.0029.
Definition for Member with Special Health Care Needs (MSHCN) is modified.
Definition for Mental Health Rehabilitative Services is added.
Definition for Nursing Facility is added.
Definition for PASRR is added.
Definition for PASRR Level I Screening is added.
Definition for PASRR Level II Evaluation is added.
Definition for PASRR Specialized Services is added.
Definition for Peer Provider is added.
Definition for Population Risk Group or Risk Group is modified to add defined criteria.
Definition for SED is modified to remove the reference to LMHAs.
Definition for SPMI is modified to remove the reference to LMHAs.
Definition for Supported Employment is added. |
|
| | | | |
Revision | 2.9 |
| February 1, 2014 | Definition for Targeted Case Management is added.
Definition for Texas Medicaid Bulletin is removed.
Definition for Texas Medicaid Provider Procedures Manual is modified to remove the reference to the Texas Medicaid Bulletin.
Section 4.08 is renamed Subcontractors and Agreements with Third Parties and is modified to include language from Section 4.10 Agreements with Third Parties.
Section 4.10 MCO Agreements with Third Parties is deleted in its entirety.
Section 5.06 Span of Coverage is modified to update the requirements effective through August, 31, 2014 and to add requirements effective September 1, 2014.
Section 10.01 is modified to clarify the calculation of the monthly Capitation Payment.
Section 10.02 is modified to include Liquidated Damages due and unpaid including any associated interest.
Section 10.08 is modified to clarify the requirements for adjustments.
Section 10.10 is modified to include Liquidated Damages assessment.
Section 10.10.2 is modified to clarify the data sources and to update the calculation example.
Section 13.02 is modified to include an obligation to comply with 41 U.S.C. § 423. |
Revision | 2.10 |
| April 1, 2014 | Contract amendment did not revise Attachment A, "Uniform Managed Care Contract Terms and Conditions." |
|
| | | | |
Revision | 2.11 |
| September 1, 2014 | Definition for “Community Health Worker” is modified to conform to formatting of other definitions.
Definition for “FSR Reporting Period 15” is added.
Definition for “ICF-MR” is deleted.
Definition for “Legally Authorized Representative (LAR)” is added.
Definition for Major Systems Change is added.
Definition for “Medical Assistance Only” is revised.
Definition for “Nursing Facility Cost Ceiling” is modified to change TILE to RUG.
Definition for “Nursing Facility Unit Rate” is added.
Definition for “Rate Period 3” is added.
The definition of “Supported Employment” is revised to correct an error.
Definition for “Telehealth” is added.
Definition for “Telemedicine” is added.
Definition for “Telemonitoring” is added.
Definition for “Texas Women’s Health Program” is added. Section 3.01 is modified to add the STAR+PLUS Handbook to the order of documents.
Section 4.04.1 is modified to reflect current terminology.
Section 5.02 is revised to clarify the MCO’s right to request disenrollment.
Section 5.05(c) is deleted in its entirety to maintain consistency with updated policy and rule.
Section 5.06 Span of coverage (Effective through August 31, 2014) is deleted in its entirety and Section 5.06 Span of Coverage (Effective Beginning September 3, 2014) has the parentheses removed. In addition, Section (a) (7) is modified to add movement between STAR MCOs or between STAR+PLUS MCOs during a CDTF stay.
Section 7.07 is modified to clarify the requirement for MCOs to notify HHSC of all breaches or potential breaches of unsecured PHI.
Section 7.09 “Compliance with Fraud, Waste, and Abuse requirements” is added.
Section 10.05(b) is modified to add rate cells for IDD Members.
Section17.01 is amended to exempt Nursing Facilities from the professional liability coverage requirements. |
Revision | 2.12 |
| October 1, 2014 | Section 10.18 "Supplemental Payments for Second Generation Direct Acting Antivirals for Hepatitis C" is added.
|
1 Status should be represented as “Baseline” for initial issuances, “Revision” for changes to the Baseline version, and “Cancellation” for withdrawn versions 2 Revisions should be numbered in accordance according to the version of the issuance and sequential numbering of the revision—e.g., “1.2” refers to the first version of the document and the second revision. 3 Brief description of the changes to the document made in the revision. |
TABLE OF CONTENTS
Article 1. Introduction ................................................................................................................................... 1
Section 1.01 Purpose. ..................................................................................................................................... 1
Section 1.02 Risk-based contract. .................................................................................................................. 1
Section 1.03 Inducements............................................................................................................................... 1
Section 1.04 Construction of the Contract. ..................................................................................................... 1
Section 1.05 No implied authority. .................................................................................................................. 2
Section 1.06 Legal Authority. .......................................................................................................................... 2
Article 2. Definitions ..................................................................................................................................... 2
Article 3. General Terms & Conditions.......................................................................................................16
Section 3.01 Contract elements. ....................................................................................................................16
Section 3.02 Term of the Contract. ................................................................................................................17
Section 3.03 Funding. ....................................................................................................................................17
Section 3.04 Delegation of authority. .............................................................................................................17
Section 3.05 No waiver of sovereign immunity. .............................................................................................17
Section 3.06 Force Majeure. ..........................................................................................................................17 Section 3.07 Publicity. ....................................................................................................................................17
Section 3.08 Assignment. ..............................................................................................................................18
Section 3.09 Cooperation with other vendors and prospective vendors. .......................................................18
Section 3.10 Renegotiation and reprocurement rights. ..................................................................................18
Section 3.11 RFP errors and omissions. ........................................................................................................18
Section 3.12 Enforcement Costs. ...................................................................................................................18
Section 3.13 Preferences under service contracts. ........................................................................................18
Section 3.14 Time of the essence. .................................................................................................................18
Section 3.15 Notice ........................................................................................................................................18
Article 4. Contract Administration & Management ...................................................................................19
Section 4.01 Qualifications, retention and replacement of MCO employees. ................................................19
Section 4.02 MCO's Key Personnel. ..............................................................................................................19
Section 4.03 Executive Director. ....................................................................................................................19
Section 4.04 Medical Director. .......................................................................................................................20
Section 4.05 Responsibility for MCO personnel and Subcontractors. ............................................................20
Section 4.06 Cooperation with HHSC and state administrative agencies. .....................................................21
Section 4.07 Conduct of MCO personnel and Subcontractors. ......................................................................21
Section 4.08 Subcontractors and Agreements with Third Parties. .................................................................22
Section 4.09 HHSC’s ability to contract with Subcontractors. ........................................................................23
Section 4.10 This Section Intentionally Left Blank ........................................................................................23
Section 4.11 Prohibition Against Performance Outside the United States. ....................................................23
Article 5. Member Eligibility & Enrollment .................................................................................................25
Section 5.01 Eligibility Determination .............................................................................................................25
Section 5.02 Member Enrollment & Disenrollment. ........................................................................................25
Section 5.03 STAR enrollment for pregnant women and infants. ...................................................................25
Section 5.04 CHIP eligibility and enrollment. .................................................................................................26
Section 5.05 CHIP Perinatal eligibility, enrollment, and disenrollment ...........................................................26
Section 5.06 Span of Coverage ........................................................................................................................26
Section 5.07 Verification of Member Eligibility. ..............................................................................................28
Section 5.08-Modified Default Enrollment Process ........................................................................................28
Section 5.09-This Section Intentionally Left Blank .........................................................................................29
Section 5.10 This Section Intentionally Left Blank .........................................................................................29
Section 5.11 This Section Intentionally Left Blank .........................................................................................29
Article 6. Service Levels & Performance Measurement ...........................................................................29
Section 6.01 Performance measurement. ......................................................................................................29 Article 7. Governing Law & Regulations ....................................................................................................29
Section 7.01 Governing law and venue. ........................................................................................................29
Section 7.02 MCO responsibility for compliance with laws and regulations. ..................................................29
Section 7.03 TDI licensure/ANHC certification and solvency. ........................................................................30
Section 7.04 This Section Intentionally Left Blank .........................................................................................30
Section 7.05 Compliance with state and federal anti-discrimination laws. .....................................................30
Section 7.06 Environmental protection laws. .................................................................................................31
Section 7.07 HIPAA. ......................................................................................................................................31
Section 7.08 Historically Underutilized Business Participation Requirements ...............................................32
Section 7.09 Compliance with Fraud, Waste, and Abuse requirements ...........................................................32
Article 8. Amendments & Modifications.....................................................................................................32
Section 8.01 Mutual agreement. ....................................................................................................................32
Section 8.02 Changes in law or contract. .......................................................................................................32
Section 8.03 Modifications as a remedy.........................................................................................................32
Section 8.04 Modification Process. ................................................................................................................32
Section 8.05 Modification of the Uniform Managed Care Manual. .................................................................33
Section 8.06 CMS approval of amendments ..................................................................................................33
Section 8.07 Required compliance with amendment and modification procedures. ......................................33
Article 9. Audit & Financial Compliance ....................................................................................................33
Section 9.01 Record retention and audit. .......................................................................................................33
Section 9.02 Access to records, books, and documents. ...............................................................................33
Section 9.03 Audits of Services, Deliverables and inspections. .....................................................................34
Section 9.04 SAO Audit .................................................................................................................................34
Section 9.05 Response/compliance with audit or inspection findings. ...........................................................34
Section 9.06 Notification of Legal and Other Proceedings, and Related Events. ...........................................35
Article 10. Terms & Conditions of Payment ...............................................................................................35
Section 10.01 Calculation of monthly Capitation Payment. ............................................................................35
Section 10.02 Time and Manner of Payment. ................................................................................................35
Section 10.03 Certification of Capitation Rates. .............................................................................................35
Section 10.04 Modification of Capitation Rates. .............................................................................................35
Section 10.05 STAR and STAR+PLUS Capitation Structure. ........................................................................36
Section 10.06 CHIP Capitation Rates Structure. ............................................................................................37
Section 10.07 MCO input during rate setting process. ...................................................................................37
Section 10.08 Adjustments to Capitation Payments. .....................................................................................38
Section 10.09 Delivery Supplemental Payment for CHIP, CHIP Perinatal and STAR MCOs. .......................38
Section 10.10 Experience Rebate ..................................................................................................................38
Section 10.11 Restriction on assignment of fees. ..........................................................................................43
Section 10.12 Liability for taxes. ....................................................................................................................43
Section 10.13 Liability for employment-related charges and benefits. ...........................................................43
Section 10.14 No additional consideration. ....................................................................................................43
Section 10.15 Federal Disallowance ..............................................................................................................43
Section 10.16 Supplemental Payments for Medicaid Wrap-Around Services for Outpatient Drugs and Biological Products ........................................................................................................................................43
Section 10.17 Pass-through Payments for Provider Rate Increases.................................................................44
Section 10.18 Non-Risk Payments for Second Generation Direct Acting Antivirals for Hepatitis C..............44
Article 11. Disclosure & Confidentiality of Information ...........................................................................44
Section 11.01 Confidentiality..........................................................................................................................44
Section 11.02 Disclosure of HHSC's Confidential Information. ......................................................................44
Section 11.03 Member Records .....................................................................................................................45
Section 11.04 Requests for public information. ..............................................................................................45
Section 11.05 Privileged Work Product. .........................................................................................................45
Section 11.06 Unauthorized acts. ..................................................................................................................45
Section 11.07 Legal action. ............................................................................................................................46
Section 11.08 Information Security ................................................................................................................46
Article 12. Remedies & Disputes ................................................................................................................46
Section 12.01 Understanding and expectations. ............................................................................................46
Section 12.02 Tailored remedies. ..................................................................................................................46
Section 12.03 Termination by HHSC. ............................................................................................................48
Section 12.04 Termination by MCO. ..............................................................................................................50
Section 12.05 Termination by mutual agreement. ..........................................................................................50
Section 12.06 Effective date of termination. ...................................................................................................50
Section 12.07 Extension of termination effective date. ..................................................................................50
Section 12.08 Payment and other provisions at Contract termination. ...........................................................50
Section 12.09 Modification of Contract in the event of remedies. ..................................................................51
Section 12.10 Turnover assistance. ...............................................................................................................51
Section 12.11 Rights upon termination or expiration of Contract. ..................................................................51
Section 12.12 MCO responsibility for associated costs. ................................................................................51
Section 12.13 Dispute resolution. ..................................................................................................................51
Section 12.14 Liability of MCO. ......................................................................................................................52
Section 12.15 Pre-termination Process. .........................................................................................................52
Article 13. Assurances & Certifications .....................................................................................................52
Section 13.01 Proposal certifications. ............................................................................................................52
Section 13.02 Conflicts of interest. .................................................................................................................52
Section 13.03 Organizational conflicts of interest. .........................................................................................52
Section 13.04 HHSC personnel recruitment prohibition. ................................................................................53
Section 13.05 Anti-kickback provision. ...........................................................................................................53
Section 13.06 Debt or back taxes owed to State of Texas. ............................................................................53
Section 13.07 Outstanding debts and judgments. ..........................................................................................53
Article 14. Representations & Warranties ..................................................................................................53
Section 14.01 Authorization. ..........................................................................................................................53
Section 14.02 Ability to perform. ....................................................................................................................54
Section 14.03 Minimum Net Worth. ...............................................................................................................54
Section 14.04 Insurer solvency. .....................................................................................................................54
Section 14.05 Workmanship and performance. .............................................................................................54
Section 14.06 Warranty of deliverables. ........................................................................................................54
Section 14.07 Compliance with Contract. ......................................................................................................54
Section 14.08 Technology Access .................................................................................................................54
Section 14.09 Electronic & Information Resources Accessibility Standards ..................................................54
Article 15. Intellectual Property ..................................................................................................................56
Section 15.01 Infringement and misappropriation. .........................................................................................56
Section 15.02 Exceptions...............................................................................................................................56
Section 15.03 Ownership and Licenses .........................................................................................................56
Article 16. Liability .......................................................................................................................................57
Section 16.01 Property damage. ....................................................................................................................57
Section 16.02 Risk of Loss.............................................................................................................................57
Section 16.03 Limitation of HHSC's Liability. .................................................................................................57
Article 17. Insurance & Bonding .................................................................................................................57
Section 17.01 Insurance Coverage. ...............................................................................................................57
Section 17.02 Performance Bond. .................................................................................................................59
Section 17.03 TDI Fidelity Bond .....................................................................................................................59
Article 1. Introduction
Section 1.01 Purpose.
The purpose of this Contract is to set forth the terms and conditions for the MCO’s participation as a managed care organization in one (1) or more of the MCO Programs administered by HHSC. Under the terms of this Contract, MCO will provide comprehensive health care services to qualified Program recipients through a managed care delivery system.
Section 1.02 Risk-based contract.
This is a Risk-based contract.
Section 1.03 Inducements.
In making the award of this Contract, HHSC relied on MCO’s assurances of the following:
(1) MCO is a health maintenance organization, Approved Non-Profit Health Corporation (ANHC), or Exclusive Provider Organization that arranges for the delivery of Health Care Services, and is either (1) has received Texas Department of Insurance (TDI) licensure or approval as such an entity and is fully authorized to conduct business in the Service Areas, or (2) will receive TDI licensure or approval as such an entity and be fully authorized to conduct business in all Service Areas no later than 60 calendar days after HHSC executes this Contract;
(2) MCO and the MCO Administrative Service Subcontractors have the skills, qualifications, expertise, financial resources and experience necessary to provide the Services and Deliverables described in the RFP, MCO’s Proposal, and this Contract in an efficient, cost-effective manner, with a high degree of quality and responsiveness, and has performed similar services for other public or private entities;
(3) MCO has thoroughly reviewed, analyzed, and understood the RFP, has timely raised all questions or objections to the RFP, and has had the opportunity to review and fully understand HHSC’s current program and operating environment for the activities that are the subject of the Contract and the needs and requirements of the State during the Contract term;
(4) MCO has had the opportunity to review and understand the State’s stated objectives in entering into this Contract and, based on such review and understanding, MCO currently has the capability to perform in accordance with the terms and conditions of this Contract;
(5) MCO also has reviewed and understands the risks associated with the MCO Programs as described in the RFP, including the risk of non-appropriation of funds.
Accordingly, on the basis of the terms and conditions of this Contract, HHSC desires to engage MCO to perform the Services and provide the Deliverables described in this Contract under the terms and conditions set forth in this Contract.
Section 1.04 Construction of the Contract.
(a) Scope of Introductory Article.
The provisions of any introductory article to the Contract are intended to be a general introduction and are not intended to expand the scope of the Parties’ obligations under the Contract or to alter the plain meaning of the terms and conditions of the Contract.
(b) References to the “State.”
References in the Contract to the “State” must mean the State of Texas unless otherwise specifically indicated and must be interpreted, as appropriate, to mean or include HHSC and other agencies of the State of Texas that may participate in the administration of the MCO Programs, provided, however, that no provision will be interpreted to include any entity other than HHSC as the contracting agency.
(c) Severability.
If any provision of this Contract is construed to be illegal or invalid, such interpretation will not affect the legality or validity of any of its other provisions. The illegal or invalid provision will be deemed stricken and deleted to the same extent and effect as if never incorporated in this Contract, but all other provisions will remain in full force and effect.
(d) Survival of terms.
Termination or expiration of this Contract for any reason will not release either Party from any liabilities or obligations set forth in this Contract that:
(1) The Parties have expressly agreed must survive any such termination or expiration; or
(2) Arose prior to the effective date of termination and remain to be performed or by their nature would be intended to be applicable following any such termination or expiration.
(e) Headings.
The article, section and paragraph headings in this Contract are for reference and convenience only and may not be considered in the interpretation of this Contract.
(f) Global drafting conventions.
(1) The terms “include,” “includes,” and “including” are terms of inclusion, and where used in this Contract, are deemed to be followed by the words “without limitation.”
(2) Any references to “sections,” “appendices,” “exhibits” or “attachments” are deemed to be references to sections, appendices, exhibits or attachments to this Contract.
(3) Any references to laws, rules, regulations, and manuals in this Contract are deemed references to these documents as amended, modified, or supplemented from time to time during the term of this Contract.
Section 1.05 No implied authority.
The authority delegated to MCO by HHSC is limited to the terms of this Contract. HHSC is the state agency designated by the Texas Legislature to administer the MCO Programs, and no other agency of the State grants MCO any authority related to this program unless directed through HHSC. MCO may not rely upon implied authority, and specifically is not delegated authority under this Contract to:
(1) make public policy;
(2) promulgate, amend or disregard administrative regulations or program policy decisions made by State and federal agencies responsible for administration of HHSC Programs; or
(3) unilaterally communicate or negotiate with any federal or state agency or the Texas Legislature on behalf of HHSC regarding the HHSC Programs.
MCO is required to cooperate to the fullest extent possible to assist HHSC in communications and negotiations with state and federal governments and agencies concerning matters relating to the scope of the Contract and the MCO Program(s), as directed by HHSC.
Section 1.06 Legal Authority.
(a) HHSC is authorized to enter into this Contract under Chapters 531 and 533, Texas Government Code; Section 2155.144, Texas Government Code; and/or Chapter 62, Texas Health & Safety Code. MCO is authorized to enter into this Contract pursuant to the authorization of its governing board or controlling owner or officer.
(b) The person or persons signing and executing this Contract on behalf of the Parties, or representing themselves as signing and executing this Contract on behalf of the Parties, warrant and guarantee that he, she, or they have been duly authorized to execute this Contract and to validly and legally bind the Parties to all of its terms, performances, and provisions.
Article 2. Definitions
As used in this Contract, the following terms and conditions must have the meanings assigned below:
1915(c) Nursing Facility Waiver or 1915(c) STAR+PLUS Waiver (SPW) means the HHSC waiver program that provides home and community based services to aged and disabled adults as cost-effective alternatives to institutional care in nursing homes. Should HHSC begin operating this waiver program under a 1115 Waiver structure, then references to the 1915(c) Nursing Facility Waiver or SPW will mean the home and community based services component of the 1115 Waiver for Members who qualify for the additional services described in Attachment B-2, "STAR+PLUS Covered Services," under the heading “1915(c) STAR+PLUS Waiver Services for those Members who qualify for such services.”
AAP means the American Academy of Pediatrics.
Abuse means provider practices that are inconsistent with sound fiscal, business, or medical practices and result in an unnecessary cost to the Medicaid or CHIP Program, or in reimbursement for services that are not Medically Necessary or that fail to meet professionally recognized standards for health care. It also includes Member practices that result in unnecessary cost to the Medicaid or CHIP Program.
Account Name means the name of the individual who lives with the child(ren) and who applies for the Children’s Health Insurance Program coverage on behalf of the child(ren).
Action (Medicaid only) means:
(1) the denial or limited authorization of a requested Medicaid service, including the type or level of service;
(2) the reduction, suspension, or termination of a previously authorized service;
(3) the denial in whole or in part of payment for service;
(4) the failure to provide services in a timely manner;
(5) the failure of an MCO to act within the timeframes set forth in the Contract and 42 C.F.R. §438.408(b); or
(6) for a resident of a rural area with only one (1) MCO, the denial of a Medicaid Members’ request to obtain services outside of the Network.
An Adverse Determination is one (1) type of Action.
Acute Care means preventive care, primary care, and other medical care provided under the direction of a physician for a condition having a relatively short duration.
Acute Care Hospital means a Hospital that provides Acute Care Services.
Adjudicate means to deny or pay a Clean Claim.
Administrative Services see MCO Administrative Services.
Administrative Services Contractor see HHSC Administrative Services Contractor.
Adverse Determination means a determination by an MCO or Utilization Review agent that the Health Care Services furnished, or proposed to be furnished to a patient, are not Medically Necessary or not appropriate.
Affiliate means any individual or entity that meets any of the following criteria:
(1) owns or holds more than a five percent (5%) interest in the MCO (either directly, or through one (1) or more intermediaries);
(2) in which the MCO owns or holds more than a five percent (5%) interest (either directly, or through one (1) or more intermediaries);
(3) any parent entity or subsidiary entity of the MCO, regardless of the organizational structure of the entity;
(4) any entity that has a common parent with the MCO (either directly, or through one (1) or more intermediaries);
(5) any entity that directly, or indirectly through one (1) or more intermediaries, controls, or is controlled by, or is under common control with, the MCO; or
(6) any entity that would be considered to be an affiliate by any Securities and Exchange Commission (SEC) or Internal Revenue Service (IRS) regulation, Federal Acquisition Regulations (FAR), or by another applicable regulatory body.
Agreement or Contract means this formal, written, and legally enforceable contract and amendments thereto between the Parties.
Allowable Expenses means all expenses related to the Contract between HHSC and the MCO that are incurred during the Contract Period, are not reimbursable or recovered from another source, and that conform with the Uniform Managed Care Manual’s “Cost Principles for Expenses.”
Appeal (CHIP and CHIP Perinatal Program only) means the formal process by which a Utilization Review agent addresses Adverse Determinations.
Appeal (Medicaid only) means the formal process by which a Member or his or her representative request a review of the MCO’s Action, as defined above.
Approved Non-Profit Health Corporation (ANHC) means an organization formed in compliance with Chapter 844 of the Texas Insurance Code and licensed by TDI. See also MCO.
Auxiliary Aids and Services includes:
(1) qualified interpreters or other effective methods of making aurally delivered materials understood by persons with hearing impairments;
(2) taped texts, large print, Braille, or other effective methods to ensure visually delivered materials are available to individuals with visual impairments; and
(3) other effective methods to ensure that materials (delivered both aurally and visually) are available to those with cognitive or other Disabilities affecting communication.
Batch Processing is a billing technique that uses a single program loading to process many individual jobs, tasks, or requests for service. In managed care, batch billing is a technique that allows providers to send billing information all at once in a “batch” rather than in separate individual transactions.
Behavioral Health Services means Covered Services for the treatment of mental, emotional, or chemical dependency disorders.
Benchmark means a target or standard based on historical data or an objective/goal.
Business Continuity Plan or BCP means a plan that provides for a quick and smooth restoration of MIS operations after a disruptive event. BCP includes business impact analysis, BCP development, testing, awareness, training, and maintenance. This is a day-to-day plan.
Business Day means any day other than a Saturday, Sunday, or a state or federal holiday on which HHSC’s offices are closed, unless the context clearly indicates otherwise.
CAHPS means the Consumer Assessment of Healthcare Providers and Systems. This survey is conducted annually by the EQRO.
Call Coverage means arrangements made by a facility or an attending physician with an appropriate level of health care provider who agrees to be available on an as-needed basis to provide medically appropriate services for routine, high risk, or Emergency Medical Conditions or Emergency Behavioral Health Conditions that present without being scheduled at the facility or when the attending physician is unavailable.
Capitation Payment means the aggregate amount paid by HHSC to the MCO on a monthly basis for the provision of Covered Services to enrolled Members (including associated Administrative Services) in accordance with the Capitation Rates in the Contract.
Capitation Payment means the aggregate amount paid by HHSC to the MCO on a monthly basis for the provision of Covered Services to enrolled Members in accordance with the Capitation Rates in the Contract.
Capitation Rate means a fixed predetermined fee paid by HHSC to the MCO each month in accordance with the Contract, for each enrolled Member in a defined Rate Cell, in exchange for the MCO arranging for or providing a defined set of Covered Services to such a Member, regardless of the amount of Covered Services used by the enrolled Member.
Case Head means the head of the household that is applying for Medicaid.
Case Management for Children and Pregnant Women is a Medicaid program for children with a health condition/health risk, birth through 20 years of age and for women with high-risk pregnancies of all ages, in order to help them gain access to medical, social, educational and other health-related services.
C.F.R. means the Code of Federal Regulations.
Chemical Dependency Treatment means treatment provided for a chemical dependency condition by a Chemical Dependency Treatment facility, chemical dependency counselor or Hospital.
Child (or Children) with Special Health Care Needs (CSHCN) means a child (or children) eligible for, and enrolled in, the DSHS CSHCN Program, as further defined in Tex. Health & Safety Code § 35.0022.
Children’s Health Insurance Program or CHIP means the health insurance program authorized and funded pursuant to Title XXI, Social Security Act (42 U.S.C. §§ 1397aa-1397jj) and administered by HHSC. The CHIP Perinatal Program is a subprogram of CHIP.
CHIP MCO Program, or CHIP Program, means the State of Texas program in which HHSC contracts with MCOs to provide, arrange for, and coordinate Covered Services for enrolled CHIP Members.
CHIP MCOs means MCOs participating in the CHIP MCO Program.
CHIP Perinatal MCOs means MCOs participating in the CHIP Perinatal Program, a subprogram of CHIP.
CHIP Perinatal Program means the State of Texas program in which HHSC contracts with MCOs to provide, arrange for, and coordinate Covered Services for enrolled CHIP Perinate and CHIP Perinate Newborn Members. Although the CHIP Perinatal Program is part of the CHIP Program, for Contract administration purposes it is sometimes identified independently in this Contract.
CHIP Perinate means a CHIP Perinatal Program Member identified prior to birth (an unborn child).
CHIP Perinate Newborn means a CHIP Perinate who has been born alive and whose family income meets the criteria for continued participation in the CHIP Perinatal Program (refer to Section 5.04.1 for information concerning eligibility).
Chronic or Complex Condition means a physical, behavioral, or developmental condition which may have no known cure and/or is progressive and/or can be debilitating or fatal if left untreated or under-treated.
Clean Claim means a claim submitted by a physician or provider for health care services rendered to a Member, with the data necessary for the MCO or subcontracted claims processor to adjudicate and accurately report the claim. A Clean Claim other than a Nursing Facility Services Clean Claim must meet all requirements for accurate and complete data as defined in the appropriate claim type encounter guides as follows:
(1) 837 Professional Combined Implementation Guide;
(2) 837 Institutional Combined Implementation Guide;
(3) 837 Professional Companion Guide;
(4) 837 Institutional Companion Guide; or
(5) National Council for Prescription Drug Programs (NCPDP) Companion Guide.
The MCO may not require a physician or provider to submit documentation that conflicts with the requirements of 28 Tex. Admin. Code, Chapter 21, Subchapters C and T.
Claims submitted by a Nursing Facility must meet DADS' criteria for clean claims submission as described in UMCM Chapter 2.3, Nursing Facility Claims Manual.
Clinical Edit means a process for verifying that a Member’s medical condition matches the clinical criteria for dispensing a requested drug. Clinical Edits must be based on evidence-based clinical criteria and nationally recognized peer-reviewed information. If the information about a Member’s medical condition meets the Clinical Edit criteria, the claim can be approved. If a Member's medical condition does not meet the Clinical Edit criteria, then prior authorization is required.
CMS means the Centers for Medicare and Medicaid Services, which is the federal agency responsible for administering Medicare and overseeing state administration of Medicaid and CHIP.
Cognitive Rehabilitation Therapy means an HCBS STAR+PLUS Waiver service that assists a Member in learning or relearning cognitive skills that have been lost or altered as a result of damage to brain cells/chemistry in order to enable the Member to compensate for the lost cognitive functions. Cognitive rehabilitation therapy may be provided when an appropriate professional assesses the Member and determines it is medically necessary. Cognitive rehabilitation therapy it is provided in accordance with the plan of care developed by the assessor, and includes reinforcing, strengthening, or reestablishing previously learned patterns of behavior, or establishing new patterns of cognitive activity or compensatory mechanisms for impaired neurological systems.
COLA means the Cost of Living Adjustment.
Community-based Long Term Services and Supports means services provided to STAR+PLUS Members in their home or other community based settings necessary to provide assistance with activities of daily living to allow the Member to remain in the most integrated setting possible. Community-based Long-term Services and Supports includes services available to all STAR+PLUS Members as well as those services available only to STAR+PLUS Members who qualify for HCBS STAR+PLUS Waiver services.
Community Health Worker means a trusted member of the community who has a close understanding of the ethnicity, language, socio-economic status, and life experiences of the community served. A community health worker, also called a promotor(a), helps people gain access to needed services, increase health knowledge, and become self-sufficient through outreach, patient navigation and follow-up, community health education and information, informal counseling, social support, advocacy, and more.
Community Resource Coordination Groups
(CRCGs)
means a statewide system of local interagency groups, including both public and private providers, which coordinate services for ”multi-need” children and youth. CRCGs develop individual service plans for children and adolescents whose needs can be met only through interagency cooperation. CRCGs address Complex Needs in a model that promotes local decision-making and ensures that children receive the integrated combination of social, medical and other services needed to address their individual problems.
Community Services Specialist (CSSP) means a Mental Health Rehabilitative Service provider who meets the following minimum requirements: (1) high school diploma or high school equivalency, and (2) three continuous years of documented full-time experience in the provisions of Mental Health Rehabilitative Services and demonstrated competency in the provision and documentation of Mental Health Rehabilitative Services.
Complainant means a Member or a treating provider or other individual designated to act on behalf of the Member who filed the Complaint.
Complaint (CHIP Program only) means any dissatisfaction, expressed by a Complainant, orally or in writing to the MCO, with any aspect of the MCO’s operation, including, but not limited to, dissatisfaction with plan administration, procedures related to review or Appeal of an Adverse Determination, as defined in Texas Insurance Code, Chapter 843, Subchapter G; the denial, reduction, or termination of a service for reasons not related to Medical Necessity; the way a service is provided; or disenrollment decisions. The term does not include misinformation that is resolved promptly by supplying the appropriate information or clearing up the misunderstanding to the satisfaction of the CHIP Member.
Complaint (Medicaid only) means an expression of dissatisfaction expressed by a Complainant, orally or in writing to the MCO, about any matter related to the MCO other than an Action. As provided by 42 C.F.R. §438.400, possible subjects for Complaints include, but are not limited to, the quality of care of services provided, and aspects of interpersonal relationships such as rudeness of a provider or employee, or failure to respect the Medicaid Member’s rights.
Complex Need means a condition or situation resulting in a need for coordination or access to services beyond what a PCP would normally provide, triggering the MCO's determination that Care Coordination is required.
Comprehensive Care Program: see definition for Texas Health Steps.
Confidential Information means any communication or record (whether oral, written, electronically stored or transmitted, or in any other form) consisting of:
(1) Confidential Client information, including HIPAA-defined protected health information;
(2) All non-public budget, expense, payment and other financial information;
(3) All Privileged Work Product;
(4) All information designated by HHSC or any other State agency as confidential, and all information designated as confidential under the Texas Public Information Act;
(5) Information utilized, developed, received, or maintained by HHSC, the MCO, or participating State agencies for the purpose of fulfilling a duty or obligation under this Contract and that has not been disclosed publicly.
Consolidated FSR Report or Consolidated Basis, means FSR reporting results for all Programs and all SDAs operated by the MCO or its Affiliates, including those under separate contracts between the MCO or its Affiliates and HHSC. Consolidated FSR Reporting does not include any of the MCO's or its Affiliates' business outside of the HHSC Programs.
Consumer-Directed Services means the Member or his legal guardian is the employer of and retains control over the hiring, management, and termination of an individual providing personal assistance or respite.
Continuity of Care means care provided to a Member by the same PCP or specialty provider to ensure that the delivery of care to the Member remains stable, and services are consistent and unduplicated.
Contract or Agreement means this formal, written, and legally enforceable contract and amendments thereto between the Parties.
Contract Period or Contract Term means the Initial Contract Period plus any and all Contract extensions.
Contractor or MCO means the MCO that is a party to this Contract and is an insurer licensed or approved by TDI as an HMO, ANHC formed in compliance with Chapter 844 of the Texas Insurance Code, or an EPO with an Exclusive Provider Benefit Plan approved by TDI in accordance with 28 T.A.C. §3.9201-3.9212.
Copayment (CHIP only) means the amount that a Member is required to pay when utilizing certain CHIP Covered Services. Once the copayment is made, further payment is not required by the Member.
Corrective Action Plan means the detailed written plan that may be required by HHSC to correct or resolve a deficiency or event causing the assessment of a remedy or damage against MCO.
Court-Ordered Commitment means a commitment of a Member to an inpatient mental health facility for treatment ordered by a court of law pursuant to Texas Health and Safety Code, Chapters 573 or 574.
Covered Services means Health Care Services the MCO must arrange to provide to Members, including all services required by the Contract and state and federal law, and all Value-added Services negotiated by the Parties (see Attachments B-2, B-2.1, B-2.2 and B-3 of the HHSC Managed Care Contract relating to “Covered Services” and “Value-added Services”).
CPW means Case Management for Children and Pregnant Women; a Medicaid program for children with a health condition/health risk, birth through 20 years of age and to women with high-risk pregnancies of all ages, in order to help them gain access to medical, social, educational and other health-related services.
Credentialing means the process of collecting, assessing, and validating qualifications and other relevant information pertaining to a health care provider to determine eligibility and to deliver Covered Services.
Cultural Competency means the ability of individuals and systems to provide services effectively to people of various cultures, races, ethnic backgrounds, and religions in a manner that recognizes, values, affirms, and respects the worth of the individuals and protects and preserves their dignity.
DADS means the Texas Department of Aging and Disability Services or its successor agency (formerly Department of Human Services).
Date of Disenrollment means the last day of the last month for which MCO receives payment for a Member.
Day means a calendar day unless specified otherwise.
Default Enrollment means the processes established by HHSC to assign an enrollee who has not selected an MCO to an MCO. See 1 Tex. Admin. Code § 353.403 for Medicaid default enrollment processes, and 1 Tex. Admin. Code § 370.303 for CHIP default enrollment processes
Deliverable means a written or recorded work product or data prepared, developed, or procured by MCO as part of the Services under the Contract for the use or benefit of HHSC or the State of Texas.
Delivery Supplemental Payment means a one-time per pregnancy supplemental payment for STAR, CHIP and CHIP Perinatal MCOs.
Designated Provider means a physician, clinical practice or clinical group practice, rural clinic, community heath center, community mental health center, home health agency, or any other entity or provider (including pediatricians, gynecologists, and obstetricians) that are determined by the State and approved by the U.S. Secretary of Health and Human Services to be qualified to be a Health Home for Members with chronic conditions on the basis of documentation that the physician practice or clinic (A) has the systems and infrastructure in place to provide Health Home services and (B) satisfies the qualification standards established by the U.S. Secretary of Health and Human Services.
Diagnostic means assessment that may include gathering of information through interview, observation, examination, and use of specific tests that allows a provider to diagnose existing conditions.
Disabled Person or Person with Disability means a person under 65 years of age, including a child, who qualifies for Medicaid services because of a disability.
Disability means a physical or mental impairment that substantially limits one (1) or more of an individual’s major life activities, such as caring for oneself, performing manual tasks, walking, seeing, hearing, speaking, breathing, learning, and/or working.
Disability-related Access means that facilities are readily accessible to and usable by individuals with disabilities, and that auxiliary aids and services are provided to ensure effective communication, in compliance with Title III of the Americans with Disabilities Act.
Disaster Recovery Plan means the document developed by the MCO that outlines details for the restoration of the MIS in the event of an emergency or disaster.
Discharge means a formal release of a Member from an Inpatient Hospital stay when the need for continued care at an inpatient level has concluded. Movement or Transfer from one (1) Acute Care Hospital or Long Term Care Hospital /facility and readmission to another within 24 hours for continued treatment is not a discharge under this Contract.
Disease Management means a system of coordinated healthcare interventions and communications for populations with conditions in which patient self-care efforts are significant.
Disproportionate Share Hospital (DSH) means a Hospital that serves a higher than average number of Medicaid and other low-income patients and receives additional reimbursement from the State.
DSHS means the Texas Department of State Health Services or its successor agency (formerly Texas Department of Health and Texas Department of Mental Health and Mental Retardation).
DSM means the most current edition of the Diagnostic and Statistical Manual of Mental Disorders, which is the American Psychiatric Association's official classification of behavioral health disorders, or its replacement.
Dual Eligibles means Medicaid recipients who are also eligible for Medicare.
ECI means Early Childhood Intervention, a federally mandated program for infants and toddlers under the age of three with developmental delays or disabilities. See 34 C.F.R. § 303.1 et seq. and 40 Tex. Admin. Code § 108.101 et seq. for further clarification.
EDI means electronic data interchange.
Effective Date means the effective date of this Contract, as specified in the HHSC Managed Care Contract document.
Effective Date of Coverage means the first day of the month for which the MCO has received payment for a Member.
Electronic Visit Verification (EVV) is the electronic verification and documentation of visit data, such as the date and time the provider begins and ends the delivery of services, the attendant, the recipient, and the location of services provided.
Eligibles means individuals residing in one (1) of the Service Areas and eligible to enroll in a STAR, STAR+PLUS, CHIP, or CHIP Perinatal MCO, as applicable.
Emergency Behavioral Health Condition means any condition, without regard to the nature or cause of the condition, which in the opinion of a prudent layperson possessing an average knowledge of health and medicine:
(1) requires immediate intervention and/or medical attention without which Members would present an immediate danger to themselves or others, or
(2) renders Members incapable of controlling, knowing or understanding the consequences of their actions.
Emergency Medical Condition means a medical condition manifesting itself by acute symptoms of recent onset and sufficient severity (including severe pain), such that a prudent layperson, who possesses an average knowledge of health and medicine, could reasonably expect the absence of immediate medical care could result in:
(1) placing the patient’s health in serious jeopardy;
(2) serious impairment to bodily functions;
(3) serious dysfunction of any bodily organ or part;
(4) serious disfigurement; or
(5) in the case of a pregnant women, serious jeopardy to the health of a woman or her unborn child.
Emergency Services means covered inpatient and outpatient services furnished by a provider that is qualified to furnish such services under the Contract and that are needed to evaluate or stabilize an Emergency Medical Condition and/or an Emergency Behavioral Health Condition, including Post-stabilization Care Services.
Employment Assistance means assistance provided as an HCBS STAR+PLUS Waiver service to a Member to help the Member locate paid employment in the community. Employment assistance includes:
identifying an individual's employment preferences, job skills, and requirements for a work setting and work conditions;
locating prospective employers offering employment compatible with an individual's identified preferences, skills, and requirements; and contacting a prospective employer on behalf of an individual and negotiating the individual's employment.
Employment Assistance is not available to Members receiving services through a program funded by the Rehabilitation Act of 1973 or the Individuals with Disabilities Education Act. For any Member receiving one of those waiver services, the MCO must document that the Employment Assistance service is not available to the Member in the Member's record.
Encounter means a Covered Service or group of Covered Services delivered by a Provider to a Member during a visit between the Member and Provider. This also includes Value-added Services.
Encounter Data means data elements from Fee-for-Service claims or capitated services proxy claims that are submitted to HHSC by the MCO in accordance with HHSC’s required format for Medicaid and CHIP MCOs.
Enrollment Report/Enrollment File means the daily or monthly list of Eligibles that are enrolled with an MCO as Members on the day or for the month the report is issued.
EPSDT means the federally mandated Early and Periodic Screening, Diagnosis and Treatment program contained at 42 U.S.C. 1396d(r). The name has been changed to Texas Health Steps in the State of Texas.
Exclusive Provider Organization (EPO) means an insurer with an Exclusive Provider Benefit Plan approved by TDI in accordance with 28 T.A.C. §3.9201-3.9212
Expansion Area means a county or Service Area that has not previously provided healthcare to HHSC’s MCO Program Members utilizing a managed care model.
Expansion Service Areas are the Hidalgo and Medicaid Rural Service Areas for the STAR Program; and the El Paso, Hidalgo, and Lubbock Service Areas for the STAR+PLUS Program.
Expedited Appeal means an appeal to the MCO in which the decision is required quickly based on the Member's health status, and the amount of time necessary to participate in a standard appeal could jeopardize the Member's life or health or ability to attain, maintain, or regain maximum function.
Experience Rebate means the portion of the MCO’s Net Income Before Taxes that is returned to the State in accordance with Section 10.10 for the STAR, CHIP and CHIP Perinatal Programs and 10.10.1 for the STAR+PLUS Program (“Experience Rebate”).
Expiration Date means the expiration date of this Contract, as specified in HHSC’s Managed Care Contract document.
External Quality Review Organization (EQRO) means the entity that contracts with HHSC to provide external review of access to and quality of healthcare provided to Members of HHSC’s MCO Programs.
Fair Hearing means the process adopted and implemented by HHSC in 1 T.A.C. Chapter 357, in compliance with federal regulations and state rules relating to Medicaid Fair Hearings.
Family Partner means a Mental Health Rehabilitative Service provider who meets the following minimum requirements: (1) high school diploma or high school equivalency, and (2) one cumulative year of participating in mental health services as the parent or legally authorized representative of a child receiving mental health services.
Farm Worker Child (FWC) means a child birth through age 20 of a Migrant Farm Worker.
Federal Poverty Level (FPL) means the Federal poverty level updated periodically in the Federal Register by the Secretary of Health and Human Services under the authority of 42 U.S.C. § 9902(2) and as in effect for the applicable budget period used to determine an individual’s eligibility in accordance with 42 C.F.R. § 435.603(h).
Fee-for-Service (FFS) means the traditional Medicaid Health Care Services payment system under which providers receive a payment for each unit of service, after the service is provided, according to rules adopted pursuant to Chapter 32, Texas Human Resources Code.
Financial Statistical Report (see FSR below).
Force Majeure Event means any failure or delay in performance of a duty by a Party under this Contract that is caused by fire, flood, hurricane, tornadoes, earthquake, an act of God, an act of war, riot, civil disorder, or any similar event beyond the reasonable control of such Party and without the fault or negligence of such Party.
Former Foster Care Child (FFCC) Member means a young adult who has aged out of the foster care system and has previously received Medicaid while in foster care. FFCC Members may be enrolled in the STAR or STAR Health Program. The FFCC Member may be enrolled until the last day of the month of his or her 26th birthday.
FPL means the Federal Poverty Level.
FQHC means a Federally Qualified Health Center, certified by CMS to meet the requirements of §1861(aa)(3) of the Social Security Act as a federally qualified health center, that is enrolled as a provider in the Texas Medicaid program.
Fraud means an intentional deception or misrepresentation made by a person with the knowledge that the deception could result in some unauthorized benefit to himself or some other person. It includes any act that constitutes fraud under applicable federal or state law.
FSR means Financial Statistical Report. The FSR is a report designed by HHSC, and submitted to HHSC by the MCO in accordance with Contract requirements. The FSR is a form of modified income statement, subject to audit, and contains revenue, cost, and other data, as defined by the Contract. Not all incurred expenses may be included in the FSR.
FSR Reporting Period is the period of months that are measured on a given FSR. Generally, the FSR Reporting Period is a twelve-calendar-month period corresponding to the State Fiscal Year, but it can vary by Contract and by year. If an FSR Reporting Period is not defined in the Contract, then it will be deemed to be the twelve months following the end of the prior FSR Reporting Period.
FSR Reporting Period 12/13 means the 18-month period beginning on March 1, 2012 and ending on August 31, 2013. This is the first FSR Reporting Period under this Contract.
FSR Reporting Period 14 means the 12-month period beginning on September 1, 2013 and ending on August 31, 2014.
FSR Reporting Period 15 means the twelve month period beginning on September 1, 2014 and ending on August 31, 2015.
Functionally Necessary Covered Services means Community-based Long Term Services and Supports services provided to assist STAR+PLUS Members with activities of daily living based on a functional assessment of the Member’s activities of daily living and a determination of the amount of supplemental supports necessary for the STAR+PLUS Member to remain independent or in the most integrated setting possible.
Habilitative and Rehabilitative Services means Health Care Services described in Attachment B-2 that may be required by children who fail to reach (habilitative) or have lost (rehabilitative) age appropriate developmental milestones.
HCBS STAR+PLUS Waiver means the HHSC program that provides home and community based services to aged and disabled adults as cost-effective alternatives to institutional care in nursing homes. Members who qualify for HCBS STAR+PLUS Waiver are eligible to receive the home and community based services component of the Texas Healthcare Transformation and Quality Improvement Program 1115 Waiver as described in Attachment B-2 STAR+PLUS Covered Services, under the heading HCBS STAR+PLUS Waiver services for those Members who qualify for such services.
Health and Human Services Commission or HHSC means the administrative agency within the executive department of Texas state government established under Chapter 531, Texas Government Code, or its designee, including, but not limited to, the HHS Agencies.
Health Care Services means the Acute Care, Behavioral Health Care, and health-related services that an enrolled population might reasonably require in order to be maintained in good health.
Health Home means a Designated Provider (including a provider that operates in coordination with a team of health care professionals) or a Heath Team selected by a Member with chronic conditions to provide Health Home Services.
Health Home Services means comprehensive and timely high-quality services that are provided by a Designated Provider, a Team of Health Care Professionals operating with such a provider, or a Health Team. Health Home Services include:
(1) Comprehensive care management;
(2) Care coordination and health promotion;
(3) Comprehensive transitional care, including appropriate follow-up, from inpatient to other settings;
(4) Patient and family support (including authorized representatives);
(5) Referral to community and social support services, if relevant; and
(6) Use of health information technology to link services, as feasible and appropriate.
Health-related Materials are materials developed by the MCO or obtained from a third party relating to the prevention, diagnosis or treatment of a medical condition.
Health Team means such term as described in Section 3502 of the Patient Protection and Affordable Care Act, P.L. 111-148 (March 23, 2010), as amended or modified.
HEDIS, the Healthcare Effectiveness Data and Information Set, is a registered trademark of NCQA. HEDIS is a set of standardized performance measures designed to reliably compare the performance of managed health care plans. HEDIS is sponsored, supported and maintained by NCQA.
HHS Agency means the Texas health and human service agencies subject to HHSC’s oversight under Chapter 531, Texas Government Code, and their successor agencies.
HHSC Administrative Services Contractor (ASC) means an entity performing MCO administrative services functions, including member enrollment functions, for the STAR, STAR+PLUS, CHIP, or CHIP Perinatal MCO Programs under contract with HHSC.
HHSC MCO Programs or MCO Programs mean the STAR, STAR+PLUS, and CHIP MCO Programs.
HIPAA means the Health Insurance Portability and Accountability Act of 1996, P.L. 104-191 (August 21, 1996), as amended or modified.
Home and Community Support Services Agency or HCSSA means an entity licensed to provide home health, hospice, or personal assistance services provided to individuals in their own home or independent living environment as prescribed by a physician or individualized service plan. Each HCSS must provide clients with a plan of care that includes specific services the agency agrees to perform. The agencies are licensed and monitored by DADS or its successor.
Hospital means a licensed public or private institution as defined by Chapter 241, Texas Health and Safety Code, or in Subtitle C, Title 7, Texas Health and Safety Code.
ICF-IID Program means the Medicaid program serving individuals with intellectual disabilities or related conditions who receive care in intermediate care facilities other than a state supported living center.
IDD Waiver means the Community Living Assistance and Support Services Waiver program (CLASS), the Deaf-Blind with Multiple Disabilities Waiver program (DBMD), the Home and Community-Based Services Waiver program (HCS), or the Texas Home Living Waiver program (TxHmL).
Individual Family Service Plan (IFSP) means the plan for services required by the Early Childhood Intervention (ECI) Program and developed by an interdisciplinary team.
Initial Contract Period means the Effective Date of the Contract through August 31, 2015.
Inpatient Stay means at least a 24-hour stay in a facility licensed to provide Hospital care.
JCAHO means Joint Commission on Accreditation of Health Care Organizations.
Joint Interface Plan (JIP) means a document used to communicate basic system interface information. This information includes: file structure, data elements, frequency, media, type of file, receiver and sender of the file, and file I.D. The JIP must include each of the MCO’s interfaces required to conduct business under this Contract. The JIP must address the coordination with each of the MCO’s interface partners to ensure the development and maintenance of the interface; and the timely transfer of required data elements between contractors and partners.
Key MCO Personnel means the critical management and technical positions identified by the MCO in accordance with Article 4.
Legally Authorized Representative (LAR) means the Member's representative defined by state or federal law, including Tex. Occ. Code § 151.002(6), Tex. Health & Safety Code § 166.164, and Tex. Estates Code Ch. 752.
Licensed Medical Personnel means, in the context of Mental Health Rehabilitative Services day programs, the following provider types: physician; advanced practice registered nurse (APRN); physician assistant (PA); registered nurse (RN); licensed vocational nurse (LVN); or pharmacists.
Licensed Practitioner of the Healing Arts (LPHA) means a person who is:
(1) a physician;
(2) a licensed professional counselor;
(3) a licensed clinical social worker;
(4) a licensed psychologist;
(5) an advanced practice nurse; or
(6) a licensed marriage and family therapist.
Linguistic Access means translation and interpreter services, for written and spoken language to ensure effective communication. Linguistic access includes sign language interpretation, and the provision of other auxiliary aids and services to persons with disabilities.
Local Health Department means a local health department established pursuant to Health and Safety Code, Title 2, Local Public Health Reorganization Act §121.031.
Local IDD Authority has the meaning assigned in Health and Safety Code § 531.002(11).
Local Mental Health Authority (LMHA) has the meaning assigned in Health and Safety Code § 531.002(10).
Major Population Group means any population that represents at least 10% of the Medicaid, CHIP, and/or CHIP Perinatal Program population in the Service Area served by the MCO.
Major Systems Change means a new version of an existing software platform often identified by a new software version number or conversion to an entirely new software platform.
Mandated or Required Services means services that a state is required to offer to categorically needy clients under a state Medicaid plan.
Marketing means any communication from the MCO to a Medicaid or CHIP Eligible who is not enrolled with the MCO that can reasonably be interpreted as intended to influence the Eligible to:
(1) enroll with the MCO; or
(2) not enroll in, or to disenroll from, another MCO.
Marketing Materials means materials that are produced in any medium by or on behalf of the MCO and can reasonably be interpreted as intending to market to potential Members. Health-related Materials are not Marketing Materials.
Material Subcontract means any contract, Subcontract, or agreement between the MCO and another entity that meets any of the following criteria:
- the other entity is an Affiliate of the MCO;
- the Subcontract is considered by HHSC to be for a key type of service or function, including
o Administrative Services (including but not limited to third party administrator, Network administration, and claims processing);
o delegated Networks (including but not limited to behavioral health, dental, pharmacy, and vision);
o management services (including management agreements with parent)
o reinsurance;
o Disease Management;
o pharmacy benefit management (PBM) or pharmacy administrative services; or
o call lines (including nurse and medical consultation); or
- any other Subcontract that exceeds, or is reasonably expected to exceed, the lesser of: a) $500,000 per year, or b) 1% of the MCO’s annual Revenues under this Contract. Any Subcontracts between the MCO and a single entity that are split into separate agreements by time period, Program, or SDA, etc., will be consolidated for the purpose of this definition.
For the purposes of this Agreement, Material Subcontracts do not include contracts with any non-Affiliates for any of the following, regardless of the value of the contract: utilities (e.g., water, electricity, telephone, Internet, trash), mail/shipping, office space, maintenance, security, or computer hardware.
Material Subcontract means any contract, Subcontract, or agreement between the MCO and another entity that meets any of the following criteria:
| |
• | the other entity is an Affiliate of the MCO; |
| |
• | the Subcontract is considered by HHSC to be for a key type of service or function, including |
| |
◦ | Administrative Services (including but not limited to third party administrator, Network administration, and claims processing); |
| |
◦ | delegated Networks (including but not limited to behavioral health, dental, pharmacy, and vision); |
| |
◦ | management services (including management agreements with parent) |
| |
◦ | pharmacy benefit management (PBM) or pharmacy administrative services; or |
| |
◦ | call lines (including nurse and medical consultation); or |
| |
• | any other Subcontract that exceeds, or is reasonably expected to exceed, the lesser of: a) $500,000 per year, or b) 1% of the MCO’s annual Revenues under this Contract. Any Subcontracts between the MCO and a single entity that are split into separate agreements by time period, Program, or SDA, etc., will be consolidated for the purpose of this definition. |
For the purposes of this Agreement, Material Subcontracts do not include contracts with any non-Affiliates for any of the following, regardless of the value of the contract:: utilities (e.g., water, electricity, telephone, Internet), mail/shipping, office space, or computer hardware.
Material Subcontractor or Major Subcontractor means any entity with a Material Subcontract with the MCO. For the purposes of this Agreement, Material Subcontractors do not include providers in the MCO’s Provider Network. Material Subcontractors may include, without limitation, Affiliates, subsidiaries, and affiliated and unaffiliated third parties.
MCO means managed care organization.
MCO or Contractor means the MCO that is a party to this Contract and is an insurer licensed or approved by TDI as an HMO, ANHC formed in compliance with Chapter 844 of the Texas Insurance Code, or an EPO with an Exclusive Provider Benefit Plan approved by TDI in accordance with 28 T.A.C. §3.9201-3.9212.
MCO Administrative Services means the performance of services or functions, other than the direct delivery of Covered Services, necessary for the management of the delivery of and payment for Covered Services, including Network, utilization, clinical or quality management, service authorization, claims processing, management information systems operation, and reporting. This term also includes the infrastructure development for, preparation of, and delivery of, all required Deliverables under the Contract, outside of the Covered Services.
MCO’s Service Area means all the counties included in any HHSC-defined Service Area, as applicable to each MCO Program and within which the MCO has been selected to provide MCO services.
Medicaid means the medical assistance entitlement program authorized and funded pursuant to Title XIX, Social Security Act (42 U.S.C. §1396 et seq.) and administered by HHSC.
Medicaid MCOs means contracted MCOs participating in STAR, STAR+PLUS, and/or STAR Health.
Medical Assistance Only (MAO) means a person that does not receive SSI benefits but qualifies financially and functionally for Medicaid assistance.
Medical Home has the meaning assigned to a patient-centered Medical Home in Texas Government Code § 533.0029(a).
Medically Necessary has the meaning defined in 1 T.A.C. §353.2 for Medicaid and 1 T.A.C. §370.4 for CHIP.
Member means a person who:
(1) is entitled to benefits under Title XIX of the Social Security Act and Medicaid, is in a Medicaid eligibility category included in the STAR or STAR+PLUS Program, and is enrolled in the STAR or STAR+PLUS Program and the MCO’s STAR or STAR+PLUS MCO;
(2) is entitled to benefits under Title XIX of the Social Security Act and Medicaid, is in a Medicaid eligibility category included as a voluntary participant in the STAR or STAR+PLUS Program, and is enrolled in the STAR or STAR+PLUS Program and the MCO’s STAR or STAR+PLUS MCO;
(3) has met CHIP eligibility criteria and is enrolled in the MCO’s CHIP MCO; or
(4) has met CHIP Perinatal Program eligibility criteria and is enrolled in the MCO’s CHIP Perinatal Program.
Member Materials means all written materials produced or authorized by the MCO and distributed to Members or potential members containing information concerning the MCO Program(s). Member Materials include, but are not limited to, Member ID cards, Member handbooks, Provider directories, and Marketing Materials.
Member Month means one (1) Member enrolled with the MCO during any given month. The total Member Months for each month of a year comprise the annual Member Months.
Member(s) with Special Health Care Needs (MSHCN) means a Member, including a Child or Children with Special Health Care Needs (CSHCN), who:
(1) has a serious ongoing illness, a Chronic or Complex Condition, or a Disability that has lasted or is anticipated to last for a significant period of time, and
(2) requires regular, ongoing therapeutic intervention and evaluation by appropriately trained health care personnel.
Mental Health Rehabilitative Services are those age-appropriate services determined by HHSC and Federally-approved protocol as medically necessary to reduce a Member’s disability resulting from severe mental illness for adults, or serious emotional, behavioral, or mental disorders for children, and to restore the Member to his or her best possible functioning level in the community. Services that provide assistance in maintaining functioning may be considered rehabilitative when necessary to help a Member achieve a rehabilitation goal as defined in the Member’s rehabilitation plan.
Migrant Farm Worker means a migratory agricultural worker, generally defined as an individual:
(1) whose principal employment is in agriculture on a seasonal basis;
(2) who has been so employed within the last twenty-four months;
(3) who performs any activity directly related to the production or processing of crops, dairy products, poultry, or livestock for initial commercial sale or as a principal means of personal subsistence; and
(4) who establishes for the purposes of such employment a temporary abode.
MIS means Management Information System.
National Committee for Quality Assurance (NCQA) means the independent organization that accredits MCOs, managed behavioral health organizations, and accredits and certifies disease management programs. HEDIS and the Quality Compass are registered trademarks of NCQA.
Net Income Before Taxes or Pre-tax Income means an aggregate excess of Revenues over Allowable Expenses.
Network or Provider Network means all Providers that have entered into Network Provider agreements with the MCO or its Subcontractor for the delivery of Medicaid or CHIP Covered Services to the MCO’s Members.
Network Provider or Provider means an appropriately credentialed and licensed individual, facility, agency, institution, organization or other entity, and its employees and subcontractors, that has a contract with the MCO for the delivery of Covered Services to the MCO’s Members.
Network Provider Agreement or Provider Agreement means a contract between and MCO and a Network Provider for the delivery of Covered Services to members.
Non-capitated Services means those Medicaid services identified in Attachment B-1, Section 8.2.2.8.
Non-provider Subcontracts means contracts between the MCO and a third party that performs a function, excluding delivery of Health Care Services, that the MCO is required to perform under its Contract with HHSC.
Non-Urban County or Rural County means any county with fewer than 50,000 residents as reported by the Texas Association of Counties at: http://www.county.org/.
Nursing Facility (also called nursing home or skilled nursing facility) means an entity or institution that provides organized and structured nursing care and services, and is subject to licensure under Texas Health and Safety Code, Chapter 242, as defined in 40 Tex. Admin. Code § 19.101 and 1 Tex. Admin. Code § 358.103.
Nursing Facility Cost Ceiling means the annualized cost of serving a client in a nursing facility. A per diem cost is established for each Medicaid nursing facility resident based on the level of care needed. This level of care and associated resource allocation is referred to as the Resource Utilization Group or the RUG. The per diem cost is annualized to achieve the nursing facility ceiling.
Nursing Facility Level of Care means the determination that the level of care required to adequately serve a STAR+PLUS Member is at or above the level of care provided by a nursing facility.
Nursing Facility Unit Rate means the type of services included in the DADS daily rate for Nursing Facility Providers, such as room and board, medical supplies and equipment, personal needs items, social services, and over-the-counter drugs. The Nursing Facility Unit Rate also includes applicable Nursing Facility rate enhancements and professional and general liability insurance. The Nursing Facility Unit Rate excludes Nursing Facility Add-on Services.
OB/GYN means obstetrician-gynecologist.
Open Panel means PCPs who are accepting new patients for the MCO Program(s) served.
Operational Start Date means the first day on which an MCO is responsible for providing Covered Services to MCO Program Members and all related Contract functions in a Service Area. The Operational Start Date may vary per MCO Program and Service Area. The Operational Start Date(s) applicable to this Contract are set forth in the HHSC Managed Care Contract document.
Operations Phase means the period of time when MCO is responsible for providing the Covered Services and all related Contract functions for a Service Area. The Operations Phase begins on the Operational Start Date, and may vary by MCO Program and Service Area.
Out-of-Network (OON) means an appropriately licensed individual, facility, agency, institution, organization or other entity that has not entered into a contract with the MCO for the delivery of Covered Services to the MCO’s Members.
Outpatient Hospital Services means diagnostic, therapeutic, and rehabilitative services that are provided to Members in an organized medical facility, for less than a 24-hour period, by or under the direction of a physician.
Parties means HHSC and MCO, collectively.
Party means either HHSC or MCO, individually.
PASRR means the Preadmission Screening and Resident Review, a federally mandated program applied to all individuals seeking admission to a Medicaid-certified Nursing Facility. PASRR helps ensure that individuals are not inappropriately placed in nursing homes for long-term care and requires that all applicants to a Medicaid-certified nursing facility: (1) be
evaluated for mental illness, intellectual disability, or both; (2) be offered the most appropriate setting for their needs (in the community, a nursing facility, or acute care settings); and (3) receive the services they need in those settings.
PASRR Level I Screening has the meaning assigned in 40 Tex. Admin. Code § 17.102(16).
PASRR Level II Evaluation has the meaning assigned in 40 Tex. Admin. Code § 17.102(24).
PASRR Specialized Services has the meaning assigned in 40 Tex. Admin. Code § 17.102(33).
Peer Provider means a Mental Health Rehabilitative Service provider who meets the following minimum requirements: (1) high school diploma or high school equivalency and (2) one cumulative year of receiving mental health services.
Pended Claim means a claim for payment that requires additional information before the claim can be Adjudicated as a Clean Claim.
Pharmacy Benefit Manager (PBM) is a third party administrator of prescription drug programs.
Population Risk Group means a distinct group of members identified by age, age range, gender, type of program, eligibility category, or other criteria established by HHSC.
Post-stabilization Care Services means Covered Services, related to an Emergency Medical Condition that are provided after a Member is stabilized in order to maintain the stabilized condition, or, for a Medicaid Member, under the circumstances described in 42 §§C.F.R. 438.114(b)&(e) and 42 C.F.R. §422.113(c)(iii) to improve or resolve the Medicaid Member’s condition.
PPACA – means the Patient Protection and Affordable Care Act of 2010 (P.L. 111-148), as amended by the Health Care and Education Reconciliation Act of 2010 (Public Law 111-152), together known as the Affordable Care Act (ACA).
Pre-tax Income or Net Income Before Taxes means an aggregate excess of Revenues over Allowable Expenses.
Primary Care Physician or Primary Care Provider (PCP) means a physician or provider who has agreed with the MCO to provide a Medical Home to Members and who is responsible for providing initial and primary care to patients, maintaining the continuity of patient care, and initiating referral for care.
Program means a managed care program operated by HHSC. Depending on the context, the term may include one or more of the following: STAR, STAR+PLUS, STAR Health, CHIP, Children’s Medicaid Dental Services or CHIP Dental Services.
Proposal means the proposal submitted by the MCO in response to the RFP.
Provider or Network Provider means an appropriately credentialed and licensed individual, facility, agency, institution, organization or other entity, and its employees and subcontractors, that has a contract with the MCO for the delivery of Covered Services to the MCO’s Members.
Provider Agreement or Network Provider Agreement means a contract between and MCO and a Network Provider for the delivery of Covered Services to members.
Provider Materials means all written materials produced or authorized by the MCO or its Administrative Services Subcontractors concerning the MCO Program(s) that are distributed to Network Providers. Provider Materials include the MCO's Provider Manual, training materials regarding MCO Program requirements, and mass communications directed to all or a large group of Network Providers (e-mail or fax blasts). Provider Materials do not include written correspondence between the MCO or its Administrative Services Subcontractors and a provider regarding individual business matters.
Provider Network or Network means all Providers that have contracted with the MCO for the applicable MCO Program.
Proxy Claim Form means a form submitted by Providers to document services delivered to Members under a capitated arrangement. It is not a claim for payment.
Public Health Entity means a HHSC Public Health Region, a Local Health Department, or a Hospital District.
Public Information means information that:
(1) Is collected, assembled, or maintained under a law or ordinance or in connection with the transaction of official business by a governmental body or for a governmental body; and
(2) The governmental body owns or has a right of access to.
Qualified and Disabled Working Individual (QDWI) means an individual whose only Medicaid benefit is payment of the Medicare Part A premium.
Qualified Medicare Beneficiary (QMB) means a Medicare beneficiary whose only Medicaid benefits are payment of Medicare premiums, deductibles, and coinsurance for individuals who are entitled to Medicare Part A, whose income does not exceed 100% of the federal poverty level, and whose resources do not exceed twice the resource limit of the SSI program.
Quality Improvement means a system to continuously examine, monitor and revise processes and systems that support and improve administrative and clinical functions.
Rate Cell means a Population Risk Group for which a Capitation Rate has been determined.
Rate Period 1 means the 18-month period beginning on March 1, 2012 and ending on August 31, 2013. For purposes of rate setting only, Rate Period 1 will be divided into three sub-periods: March 1, 2012 through August 31, 2012, September 1, 2012 to May 31, 2013, and June 1, 2013 to August 31, 2013.
Rate Period 2 means the 12-month period beginning on September 1, 2013 and ending on August 31, 2014.
Rate Period 3 means the 12-month period beginning on September 1, 2014 and ending on August 31, 2015.
Readiness Review means the assurances made by a selected MCO and the examination conducted by HHSC, or its agents, of MCO’s ability, preparedness, and availability to fulfill its obligations under the Contract.
Real-Time Captioning (also known as CART, Communication Access Real-Time Translation) means a process by which a trained individual uses a shorthand machine, a computer, and real-time translation software to type and simultaneously translate spoken language into text on a computer screen. Real Time Captioning is provided for individuals who are deaf, have hearing impairments, or have unintelligible speech. It is usually used to interpret spoken English into text English but may be used to translate other spoken languages into text.
Request for Proposals or RFP means the procurement solicitation instrument issued by HHSC under which this Contract was awarded and all RFP addenda, corrections or modifications, if any.
Revenue means all revenue received by the MCO pursuant to this Contract, including retroactive adjustments made by HHSC. Revenue includes any funds earned on Medicaid or CHIP managed care funds such as investment income and earned interest. Revenue excludes any reinsurance recoveries, which shall be shown as a contra-cost, or reported offset to reinsurance expense. Revenues are reported at gross, and are not netted for any reinsurance premiums paid. See also the Uniform Managed Care Manual’s “Cost Principles for Expenses.”
Risk means the potential for loss as a result of expenses and costs of the MCO exceeding payments made by HHSC under the Contract.
Routine Care means health care for covered preventive and medically necessary Health Care Services that are non-emergent or non-urgent.
Rural County or Non-Urban County means any county with fewer than 50,000 residents as reported by the Texas Association of Counties at: http://www.county.org/.
Rural Health Clinic (RHC) means an entity that meets all of the requirements for designation as a rural health clinic under 1861(aa)(1) of the Social Security Act and approved for participation in the Texas Medicaid Program.
Scope of Work means the description of Services and Deliverables specified in this Contract, the RFP, the MCO’s Proposal, and any attachments and modifications to these documents.
SDX means State Data Exchange.
Security Plan means a document that contains detailed management, operational, and technical information about a system, its security requirements, and the controls implemented to provide protection against risks and vulnerabilities.
SED means severe emotional disturbance.
Service Area means the counties included in any HHSC-defined areas as applicable to each MCO Program.
Service Coordination means a specialized care management service that is performed by a Service Coordinator and that includes but is not limited to:
(1) identification of needs, including physical health, mental health services and for STAR+PLUS Members, long term support services,
(2) development of a Service Plan to address those identified needs;
(3) assistance to ensure timely and a coordinated access to an array of providers and Covered Services;
(4) attention to addressing unique needs of Members; and
(5) coordination of Covered Services with Non-capitated Services, as necessary and appropriate.
Service Coordinator means the person with primary responsibility for providing service coordination and care management to STAR+PLUS Members.
Service Management is an administrative service in the STAR, and CHIP Programs performed by the MCO to facilitate development of a Service Plan and coordination of services among a Member’s PCP, specialty providers and non-medical providers to ensure Members with Special Health Care Needs and/or Members needing high-cost treatment have access to, and appropriately utilize, Medically Necessary Covered Services, Non-capitated Services, and other services and supports.
Service Plan (SP) means an individualized plan developed with and for Members with Special Health Care Needs, including persons with disabilities or chronic or complex conditions.
Services means the tasks, functions, and responsibilities assigned and delegated to the MCO under this Contract.
Significant Traditional Provider or STP means primary care providers, long term services and supports providers, and pharmacy providers identified by HHSC as having provided a significant level of care to Medicaid or CHIP clients. Disproportionate Share Hospitals (DSH) are also Medicaid STPs.
Skilled Nursing Facility Services (CHIP only) Services provided in a facility that provides nursing or rehabilitation services and Medical supplies and use of appliances and equipment furnished by the facility.
Software means all operating system and applications software used by the MCO to provide the Services under this Contract.
Specialty Hospital means any inpatient Hospital that is not a general Acute Care Hospital.
Specified Low-Income Medicare Beneficiary (SLMB) means a Medicare beneficiary whose only Medicaid benefit is payment of the Medicare Part B premium.
SPMI means severe and persistent mental illness.
SSA means the Social Security Administration.
Stabilize means to provide such medical care as to assure within reasonable medical probability that no deterioration of the condition is likely to result from, or occur from, or occur during discharge, transfer, or admission of the Member.
STAR+PLUS or STAR+PLUS Program means the State of Texas Medicaid managed care program in which HHSC contracts with MCOs to provide, arrange, and coordinate preventive, primary, acute and Long-term Services and Supports Covered Services to adult persons with disabilities and elderly persons age 65 and over who qualify for Medicaid through the SSI program and/or the MAO program. Children birth through age 20 who qualify for Medicaid through the SSI program, may voluntarily participate in the STAR+PLUS program.
STAR+PLUS MCOs means contracted MCOs participating in the STAR+PLUS Program.
State Fiscal Year (SFY) means a 12-month period beginning on September 1 and ending on August 31 the following year.
Subcontract means any agreement between the MCO and another party to fulfill the requirements of the Contract.
Subcontractor means any individual or entity, including an Affiliate, that has entered into a Subcontract with MCO.
Subsidiary means an Affiliate controlled by such person or entity directly or indirectly through one (1) or more intermediaries.
Supplemental Security Income (SSI) means a Federal income supplement program funded by general tax revenues (not Social Security taxes) designed to help aged, blind and disabled people with little or no income by providing cash to meet basic needs for food, clothing and shelter.
Supported Employment means assistance provided as an HCBS STAR+PLUS Waiver service, in order to sustain competitive employment, to a Member who, because of a disability, requires intensive, ongoing support to be self-employed, work from home, or perform in a work setting at which Members without disabilities are employed. Supported Employment includes employment adaptations, supervision, and training related to a Member's assessed needs. Individuals receiving supported employment earn at least minimum wage (if not self-employed).
Supported Employment is not available to Members receiving services through a program funded by the Rehabilitation Act of 1973 or the Individuals with Disabilities Education Act. For any Member receiving one of those waiver services, the MCO must document that the Employment Assistance service is not available to the Member in the Member's record.
T.A.C. means Texas Administrative Code.
Targeted Case Management means services designed to assist Members with gaining access to needed medical, social, educational, and other services and supports. Members are eligible to receive these if they have been assessed and diagnosed with a severe and persistent mental illness (SPMI) or a severe emotional disturbance (SED) and they are authorized to receive Mental Health Rehabilitative Services.
TDD means telecommunication device for the deaf. It is interchangeable with the term Teletype machine or TTY.
TDI means the Texas Department of Insurance.
Team of Health Care Professionals means physicians and other professionals, such a a nurse care coordinator, nutritionist, social worker, behavioral health professional, or any professionals deemed appropriate by HHSC and approved by CMS. The team may be free-standing, virtual, or based at a Hospital, community health center, community mental health center, rural clinic, clinical practice or clinical group practice, academic health center, or any entity deemed appropriate by HHSC and approved by CMS.
Telehealth has the meaning defined in 1 Tex. Admin. Code § 354.1430.
Telemedicine has the meaning defined in 1 Tex. Admin. Code § 354.1430.
Telemonitoring has the meaning defined in 1 Tex. Admin. Code § 354.1434.
Temporary Assistance to Needy Families (TANF) means the federally funded program that provides assistance to single parent families with children who meet the categorical requirements for aid. This program was formerly known as the Aid to Families with Dependent Children (AFDC) program.
Texas Health Steps is the name adopted by the State of Texas for the federally mandated Early and Periodic Screening, Diagnosis and Treatment (EPSDT) program. It includes the State’s Comprehensive Care Program extension to EPSDT, which adds benefits to the federal EPSDT requirements contained in 42 U.S.C. §1396d(r), and defined and codified at 42 C.F.R. §§440.40 and 441.56-62. HHSC’s rules are contained in 25 T.A.C., Chapter 33 (relating to Early and Periodic Screening, Diagnosis and Treatment).
Texas Medicaid Provider Procedures Manual means the policy and procedures manual published by or on behalf of HHSC that contains policies and procedures required of all health care providers who participate in the Texas Medicaid program. The manual is published annually and is updated as needed.
Texas Public Information Act refers to the provisions of Chapter 552 of the Texas Government Code.
Texas Women's Health Program means the program that provides primary healthcare services, including family planning services and health screenings, to eligible women under 25 Tex. Admin. Code, Chapter 39, Subchapter B.
Third Party Liability (TPL) means the legal responsibility of another individual or entity to pay for all or part of the services provided to Members under the Contract (see 1 TAC §354.2301 et seq., relating to Third Party Resources).
Third Party Recovery (TPR) means the recovery of payments on behalf of a Member by HHSC or the MCO from an individual or entity with the legal responsibility to pay for the Covered Services.
Transfer means the movement of the Member from one (1) Acute Care Hospital or Long Term Care Hospital/facility and readmission to another Acute Care Hospital or Long Term Care Hospital/facility within 24 hours for continued treatment.
Transition Phase includes all activities the MCO is required to perform between the Contract Effective Date and the Operational Start Date for an MCO Program and all or part of a Service Area.
Turnover Phase includes all activities the MCO is required to perform in order to close out the Contract and/or transition Contract activities and operations to HHSC or a subsequent contractor.
Turnover Plan means the written plan developed by MCO, approved by HHSC, to be employed during the Turnover Phase.
Uniform Managed Care Manual (UMCM) means the manual published by or on behalf of HHSC that contains policies and procedures required of all MCOs participating in the HHSC Programs. The UMCM, as amended or modified, is incorporated by reference into the Contract.
URAC /American Accreditation Health Care Commission means the independent organization that accredits Utilization Review functions and offers a variety of other accreditation and certification programs for health care organizations.
Urban County means any county with 50,000 or more residents as reported by the Texas Association of Counties at: http://www.county.org/.
Urgent Behavioral Health Situation means a behavioral health condition that requires attention and assessment within 24 hours but which does not place the Member in immediate danger to himself or herself or others and the Member is able to cooperate with treatment.
Urgent Condition means a health condition including an Urgent Behavioral Health Situation that is not an emergency but is severe or painful enough to cause a prudent layperson, possessing the average knowledge of medicine, to believe that his or her condition requires medical treatment evaluation or treatment within 24 hours by the Member’s PCP or PCP designee to prevent serious deterioration of the Member’s condition or health.
Utilization Review means the system for retrospective, concurrent, or prospective review of the Medical Necessity and appropriateness of Health Care Services provided, being provided, or proposed to be provided to a Member. The term does not include elective requests for clarification of coverage.
Value-added Services means additional services for coverage beyond those specified in Attachments B-2, B-2.1, and B-2.2. Value-added Services may be actual Health Care Services, benefits, or positive incentives that HHSC determines will promote healthy lifestyles and improve health outcomes among Members. Value-added Services that promote healthy lifestyles should target specific weight loss, smoking cessation, or other programs approved by HHSC. Temporary phones, cell phones, additional transportation benefits, and extra home health services may be Value-added Services, if approved by HHSC. Best practice approaches to delivering Covered Services are not considered Value-added Services.
Waste means practices that are not cost-efficient.
Wrap-Around Services means services for Dual Eligible Members that are covered by Medicaid:
(1) when the Dual Eligible Member has exceeded the Medicare coverage limit; or
(2) that are not covered by Medicare.
Article 3. General Terms & Conditions
Section 3.01 Contract elements.
(a) Contract documentation.
The Contract between the Parties will consist of the HHSC Managed Care Contract document and all attachments and amendments.
(b) Order of documents.
In the event of any conflict or contradiction between or among the contract documents, the documents will control in the following order of precedence:
(1) The final executed HHSC Managed Care Contract document, and all amendments;
(2) HHSC Managed Care Contract Attachment A – “Uniform Managed Care Contract Terms and Conditions,” and all amendments;
(3) HHSC Managed Care Contract Attachment B – “Scope of Work/Performance Measures,” and all amendments;
(4) for STAR+PLUS MCOs only, the STAR+PLUS Handbook and all amendments;
(5) The Uniform Managed Care Manual, and all amendments; and
(6) HHSC Managed Care Contract Attachment C – “MCO’s Proposal.”
Section 3.02 Term of the Contract.
The term of the Contract will begin on the Effective Date and will conclude on the Expiration Date. The Parties may renew the Contract for an additional period or periods, but the Contract Term may not exceed a total of eight (8)
operational years. All reserved contract extensions beyond the Expiration Date will be subject to good faith negotiations between the Parties and mutual agreement to the extension(s).
Section 3.03 Funding.
This Contract is expressly conditioned on the availability of state and federal appropriated funds. MCO will have no right of action against HHSC in the event that HHSC is unable to perform its obligations under this Contract as a result of the suspension, termination, withdrawal, or failure of funding to HHSC or lack of sufficient funding of HHSC for any activities or functions contained within the scope of this Contract. If funds become unavailable, the provisions of
Article 12
, “Remedies and Disputes” will apply. HHSC will use all reasonable efforts to ensure that such funds are available, and will negotiate in good faith with MCO to resolve any MCO claims for payment that represent accepted Services or Deliverables that are pending at the time funds become unavailable. HHSC must make best efforts to provide reasonable written advance notice to MCO upon learning that funding for this Contract may be unavailable.
Section 3.04 Delegation of authority.
Whenever, by any provision of this Contract, any right, power, or duty is imposed or conferred on HHSC, the right, power, or duty so imposed or conferred is possessed and exercised by the Executive Commissioner unless any such right, power, or duty is specifically delegated to the duly appointed agents or employees of HHSC. The Commissioner will reduce any such delegation of authority to writing and provide a copy to MCO on request.
Section 3.05 No waiver of sovereign immunity.
The Parties expressly agree that no provision of this Contract is in any way intended to constitute a waiver by HHSC or the State of Texas of any immunities from suit or from liability that HHSC or the State of Texas may have by operation of law.
Section 3.06 Force Majeure.
Neither Party will be liable for any failure or delay in performing its obligations under the Contract if such failure or delay is due to a Force Majeure Event. The existence of such causes of delay or failure will extend the period of performance in the exercise of reasonable diligence until after the causes of delay or failure have been removed. Each Party must inform the other in writing with proof of receipt within five (5) Business Days of the existence of a Force Majeure Event.
Section 3.07 Publicity.
(a) MCO may use the name of HHSC, the State of Texas, any HHS Agency, and the name of the HHSC MCO Program in any media release, public announcement, or public disclosure relating to the Contract or its subject matter only if, at least seven (7) calendar days prior to distributing the material, the MCO submits the information to HHSC for review and comment. If HHSC has not responded within seven (7) calendar days, the MCO may use the submitted information. HHSC reserves the right to object to and require changes to the publication if, at HHSC’s sole discretion, it determines that the publication does not accurately reflect the terms of the Contract or the MCO’s performance under the Contract. .
(b) MCO will provide HHSC with one (1) electronic copy of any information described in Subsection 3.07(a) prior to public release. MCO will provide additional copies, including hard copies, at the request of HHSC.
(c) The requirements of Subsection 3.07(a) do not apply to:
(1) proposals or reports submitted to HHSC, an administrative agency of the State of Texas, or a governmental agency or unit of another state or the federal government;
(2) information concerning the Contract’s terms, subject matter, and estimated value:
(a) in any report to a governmental body to which the MCO is required by law to report such information, or
(b) that the MCO is otherwise required by law to disclose; and
(3) Member Materials (the MCO must comply with the Uniform Managed Care Manual’s provisions regarding the review and approval of Member Materials).
Section 3.08 Assignment.
(a) Assignment by MCO.
MCO must not assign all or any portion of its rights under or interests in the Contract or delegate any of its duties without prior written consent of HHSC. Any written request for assignment or delegation must be accompanied by written
acceptance of the assignment or delegation by the assignee or delegation by the delegate. Except where otherwise agreed in writing by HHSC, assignment or delegation will not release MCO from its obligations pursuant to the Contract. An HHSC-approved Material Subcontract will not be considered to be an assignment or delegation for purposes of this section.
(b) Assignment by HHSC.
MCO understands and agrees HHSC may in one (1) or more transactions assign, pledge, transfer, or hypothecate the Contract. This assignment will only be made to another State agency or a non-State agency that is contracted to perform agency support.
(c) Assumption.
Each party to whom a transfer is made (an "Assignee") must assume all or any part of MCO’S or HHSC's interests in the Contract, the product, and any documents executed with respect to the Contract.
Section 3.09 Cooperation with other vendors and prospective vendors.
HHSC may award supplemental contracts for work related to the Contract, or any portion thereof. MCO will reasonably cooperate with such other vendors, and will not commit or permit any act that may interfere with the performance of work by any other vendor.
Section 3.10 Renegotiation and reprocurement rights.
(a) Renegotiation of Contract terms.
Notwithstanding anything in the Contract to the contrary, HHSC may at any time during the term of the Contract exercise the option to notify MCO that HHSC has elected to renegotiate certain terms of the Contract. Upon MCO’s receipt of any notice pursuant to this Section, MCO and HHSC will undertake good faith negotiations of the subject terms of the Contract, and may execute an amendment to the Contract in accordance with
Article 8 .
(b) Reprocurement of the services or procurement of additional services.
Notwithstanding anything in the Contract to the contrary, whether or not HHSC has accepted or rejected MCO’s Services and/or Deliverables provided during any period of the Contract, HHSC may at any time issue requests for proposals or offers to other potential contractors for performance of any portion of the Scope of Work covered by the Contract or Scope of Work similar or comparable to the Scope of Work performed by MCO under the Contract.
(c) Termination rights upon reprocurement.
If HHSC elects to procure the Services or Deliverables or any portion of the Services or Deliverables from another vendor in accordance with this Section, HHSC will have the termination rights set forth in Article 12, “Remedies and Disputes.”
Section 3.11 RFP errors and omissions.
MCO will not take advantage of any errors and/or omissions in the RFP or the resulting Contract. MCO must promptly notify HHSC of any such errors and/or omissions that are discovered.
Section 3.12 Enforcement Costs.
In the event of any litigation, appeal, or other legal action to enforce any provision of the Contract, MCO agrees to pay all reasonable expenses of such action, if HHSC is the prevailing Party.
Section 3.13 Preferences under service contracts.
MCO is required in performing the Contract to purchase products and materials produced in the State of Texas when they are available at a price and time comparable to products and materials produced outside the State.
Section 3.14 Time of the essence.
In consideration of the need to ensure uninterrupted and continuous MCO Program performance, time is of the essence in the performance of the Scope of Work under the Contract.
Section 3.15 Notice
(a) Any notice or other legal communication required or permitted to be made or given by either Party pursuant to the Contract will be in writing and in English, and will be deemed to have been given:
(1) Three (3) Business Days after the date of mailing if sent by registered or certified U.S. mail, postage prepaid, with return receipt requested;
(2) When transmitted if sent by facsimile, provided a confirmation of transmission is produced by the sending machine; or
(3) When delivered if delivered personally or sent by express courier service.
(b) The notices described in this Section may not be sent by electronic mail.
(c) All notices must be sent to the Project Manager identified in the HHSC Managed Care Contract document. In addition, legal notices must be sent to the Legal Contact identified in the HHSC Managed Care Contract document.
(d) Routine communications that are administrative in nature will be provided in a manner agreed to by the Parties.
Article 4. Contract Administration & Management
Section 4.01 Qualifications, retention and replacement of MCO employees.
MCO agrees to maintain the organizational and administrative capacity and capabilities to carry out all duties and responsibilities under this Contract. The personnel MCO assigns to perform the duties and responsibilities under this Contract will be properly trained and qualified for the functions they are to perform. Notwithstanding transfer or turnover of personnel, MCO remains obligated to perform all duties and responsibilities under this Contract without degradation and in accordance with the terms of this Contract.
Section 4.02 MCO’s Key Personnel.
(a) Designation of Key Personnel.
MCO must designate key management and technical personnel who will be assigned to the Contract. For the purposes of this requirement, Key Personnel are those with management responsibility or principal technical responsibility for the following functional areas for each MCO Program included within the scope of the Contract:
(1) Member Services;
(2) Management Information Systems;
(3) Claims Processing,
(4) Provider Network Development and Management;
(5) Benefit Administration and Utilization and Care Management;
(6) Quality Improvement;
(7) Behavioral Health Services;
(8) Financial Functions;
(9) Reporting;
(10) Executive Director(s) for applicable HHSC MCO Program(s) as defined in Section 4.03, “Executive Director”;
(11) Medical Director(s) for applicable HHSC MCO Program(s) as defined in Section 4.04 , “Medical Director”; and
(12) Management positions for STAR+PLUS Service Coordinators for STAR+PLUS MCOs as defined in Section 4.04.1, “STAR+PLUS Service Coordinator.”
(b) Support and Replacement of Key Personnel.
The MCO must maintain, throughout the Contract Term, the ability to supply its Key Personnel with the required resources necessary to meet Contract requirements and comply with applicable law. The MCO must ensure project continuity by timely replacement of Key Personnel, if necessary, with a sufficient number of persons having the requisite skills, experience and other qualifications. Regardless of specific personnel changes, the MCO must maintain the overall level of expertise, experience, and skill reflected in the Key MCO Personnel job descriptions and qualifications included in the MCO’s proposal.
(c) Notification of replacement of Key Personnel.
MCO must notify HHSC within 15 Business Days of any change in Key Personnel. Hiring or replacement of Key Personnel must conform to all Contract requirements. If HHSC determines that a satisfactory working relationship cannot be established between certain Key Personnel and HHSC, it will notify the MCO in writing. Upon receipt of HHSC’s notice, HHSC and MCO will attempt to resolve HHSC’s concerns on a mutually agreeable basis.
Section 4.03 Executive Director.
(a) The MCO must employ a qualified individual to serve as the Executive Director for its HHSC MCO Program(s). Such Executive Director must be employed full-time by the MCO, be primarily dedicated to HHSC MCO Program(s), and must hold a Senior Executive or Management position in the MCO’s organization, except that the MCO may propose an alternate structure for the Executive Director position, subject to HHSC’s prior written approval.
(b) The Executive Director must be authorized and empowered to represent the MCO regarding all matters pertaining to the Contract prior to such representation. The Executive Director must act as liaison between the MCO and the HHSC and must have responsibilities that include, but are not limited to, the following:
(1) ensuring the MCO’s compliance with the terms of the Contract, including securing and coordinating resources necessary for such compliance;
(2) receiving and responding to all inquiries and requests made by HHSC related to the Contract, in the timeframes and formats specified by HHSC. Where practicable, HHSC must consult with the MCO to establish timeframes and formats reasonably acceptable to the Parties;
(3) attending and participating in regular HHSC MCO Executive Director meetings or conference calls;
(4) attending and participating in regular HHSC Regional Advisory Committees (RACs) for managed care (the Executive Director may designate key personnel to attend a RAC if the Executive Director is unable to attend);
(5) making best efforts to promptly resolve any issues identified either by the MCO or HHSC that may arise and are related to the Contract;
(6) meeting with HHSC representative(s) on a periodic or as needed basis to review the MCO’s performance and resolve issues, and
(7) meeting with HHSC at the time and place requested by HHSC, if HHSC determines that the MCO is not in compliance with the requirements of the Contract.
Section 4.04 Medical Director.
(a) The MCO must have a qualified individual to serve as the Medical Director for its HHSC MCO Program(s). The Medical Director must be currently licensed in Texas under the Texas Medical Board as an M.D. or D.O. with no restrictions or other licensure limitations. The Medical Director must comply with the requirements of 28 T.A.C. §11.1606 and all applicable federal and state statutes and regulations.
(b) The Medical Director, or his or her designee, must be available by telephone 24 hours a day, seven (7) days a week, for Utilization Review decisions. The Medical Director, and his/her designee, must either possess expertise with Behavioral Health Services, or ready access to such expertise to ensure timely and appropriate medical decisions for Members, including after regular business hours.
(c) The Medical Director, or his or her designee, must be authorized and empowered to represent the MCO regarding clinical issues, Utilization Review and quality of care inquiries. The Medical Director, or his or her designee, must exercise independent medical judgment in all decisions relating to Medical Necessity. The MCO must ensure that its decisions relating to m Medical Necessity are not adversely influenced by fiscal management decisions. HHSC may conduct reviews of decisions relating to Medical Necessity upon reasonable notice.
(d) For purposes of this section, the Medical Director’s designee must be:
(1) a physician that meets the qualifications for a Medical Director, as described in subparts (a) through (c), above; or
(2) for prior authorization determinations for outpatient pharmacy benefits, a Texas-licensed pharmacist working under the direction of the Medical Director, provided such delegation is included in the MCO’s TDI-approved utilization review plan.
(e) The Medical Director, or his or her physician designee, must make determinations regarding Utilization Review appeals, including appeals of prior authorization denials for outpatient pharmacy benefits.
Section 4.04.1 STAR+PLUS Service Coordinator
(a) STAR+PLUS MCOs must employ as Service Coordinators persons experienced in meeting the needs of people with disabilities, old and young, and vulnerable populations who have Chronic or Complex Conditions. A Service Coordinator must have an undergraduate and/or graduate degree in social work or a related field, or be a Registered Nurse, Licensed Vocational Nurse, Advanced Nurse Practitioner, or a Physician Assistant.
(b) The STAR+PLUS MCO must monitor the Service Coordinator’s workload and performance to ensure that he or she is able to perform all necessary Service Coordination functions for the STAR+PLUS Members in a timely manner.
(c) The Service Coordinator must be responsible for working with the Member or his or her representative, the PCP and other Providers to develop a seamless package of care in which primary, Acute Care, and Long-term Services and Supports service needs are met through a single, understandable, rational plan. Each Member’s Service Plan must also be well coordinated with the Member’s family and community support systems, including Independent Living Centers, Area Agencies on Aging, Local IDD Authorities, and LMHAs. The Service Plan should be agreed to and signed by the Member or the Member’s representative to indicate agreement with the plan. The plan should promote consumer direction and self-determination and may include information for services outside the scope of Covered Services such as how to access affordable, integrated housing. For Dual Eligible Members, the STAR+PLUS MCO is responsible for meeting the Member’s Community Long- term Services and Supports needs.
(d) The STAR+PLUS MCO must empower its Service Coordinators to authorize the provision and delivery of Covered Services, including Community Long-term Services and Supports Covered Services.
Section 4.05 Responsibility for MCO personnel and Subcontractors.
(a) MCO’s employees and Subcontractors will not in any sense be considered employees of HHSC or the State of Texas, but will be considered for all purposes as the MCO’s employees or its Subcontractor’s employees, as applicable.
(b) Except as expressly provided in this Contract, neither MCO nor any of MCO’s employees or Subcontractors may act in any sense as agents or representatives of HHSC or the State of Texas.
(c) MCO agrees that anyone employed by MCO to fulfill the terms of the Contract is an employee of MCO and remains under MCO’s sole direction and control. MCO assumes sole and full responsibility for its acts and the acts of its employees and Subcontractors.
(d) MCO agrees that any claim on behalf of any person arising out of employment or alleged employment by the MCO (including, but not limited to, claims of discrimination against MCO, its officers, or its agents) is the sole responsibility of MCO and not the responsibility of HHSC. MCO will indemnify and hold harmless the State from any and all claims asserted against the State arising out of such employment or alleged employment by the MCO. MCO understands that any person who alleges a claim arising out of employment or alleged employment by MCO will not be entitled to any compensation, rights, or benefits from HHSC (including, but not limited to, tenure rights, medical and hospital care, sick and annual/vacation leave, severance pay, or retirement benefits).
(e) MCO agrees to be responsible for the following in respect to its employees:
(1) Damages incurred by MCO’s employees within the scope of their duties under the Contract; and
(2) Determination of the hours to be worked and the duties to be performed by MCO’s employees.
(f) MCO agrees and will inform its employees and Subcontractor(s) that there is no right of subrogation, contribution, or indemnification against HHSC for any duty owed to them by MCO pursuant to this Contract or any judgment rendered against the MCO. HHSC’s liability to the MCO’s employees, agents and Subcontractors, if any, will be governed by the Texas Tort Claims Act, as amended or modified (TEX. CIV. PRACT. & REM. CODE §101.001et seq.).
(g) MCO understands that HHSC does not assume liability for the actions of, or judgments rendered against, the MCO, its employees, agents or Subcontractors. MCO agrees that it has no right to indemnification or contribution from HHSC for any such judgments rendered against MCO or its Subcontractors.
Section 4.06 Cooperation with HHSC and state administrative agencies.
(a) Cooperation with Other MCOs.
MCO agrees to reasonably cooperate with and work with the other MCOs in the MCO Programs, Subcontractors, and third-party representatives as requested by HHSC. To the extent permitted by HHSC’s financial and personnel resources, HHSC agrees to reasonably cooperate with MCO and to use its best efforts to ensure that other HHSC contractors reasonably cooperate with the MCO.
(b) Cooperation with state and federal administrative agencies.
MCO must ensure that MCO personnel will cooperate with HHSC or other state or federal administrative agency personnel at no charge to HHSC for purposes relating to the administration of MCO Programs including, but not limited to the following purposes:
(1) The investigation and prosecution of Fraud, Abuse, and Waste in the HHSC programs;
(2) Audit, inspection, or other investigative purposes; and
(3) Testimony in judicial or quasi-judicial proceedings relating to the Services and/or Deliverables under this Contract or other delivery of information to HHSC or other agencies’ investigators or legal staff.
Section 4.07 Conduct of MCO personnel and Subcontractors.
(a) While performing the Scope of Work, MCO’s personnel and Subcontractors must:
(1) Comply with applicable state rules and regulations and HHSC’s requests regarding personal and professional conduct generally applicable to the service locations; and
(2) Otherwise conduct themselves in a businesslike and professional manner.
(b) If HHSC determines in good faith that a particular employee or Subcontractor is not conducting himself or herself in accordance with this Contract, HHSC may provide MCO with notice and documentation concerning such conduct. Upon receipt of such notice, MCO must promptly investigate the matter and take appropriate action that may include:
(1) Removing the employee or Subcontractor from the project;
(2) Providing HHSC with written notice of such removal; and
(3) Replacing the employee or Subcontractor with a similarly qualified individual acceptable to HHSC.
(c) Nothing in the Contract will prevent MCO, at the request of HHSC, from replacing any personnel who are not adequately performing their assigned responsibilities or who, in the reasonable opinion of HHSC’s Project Manager, after consultation with MCO, are unable to work effectively with the members of the HHSC’s staff. In such event, MCO will provide replacement personnel with equal or greater skills and qualifications as soon as reasonably practicable. Replacement of Key Personnel will be subject to HHSC review. The Parties will work together in the event of any such replacement so as not to disrupt the overall project schedule.
(d) MCO agrees that anyone employed or retained by MCO to fulfill the terms of the Contract remains under MCO’s sole direction and control.
(e) MCO must have policies regarding disciplinary action for all employees who have failed to comply with federal and/or state laws and the MCO’s standards of conduct, policies and procedures, and Contract requirements. MCO must have policies regarding disciplinary action for all employees who have engaged in illegal or unethical conduct.
Section 4.08 Subcontractors and Agreements with Third Parties.
(a) MCO remains fully responsible for the obligations, services, and functions performed by its Subcontractors to the same extent as if such obligations, services, and functions were performed by MCO’s employees, and for purposes of this Contract such work will be deemed work performed by MCO. HHSC reserves the right to require the replacement of any Subcontractor found by HHSC to be unacceptable and unable to meet the requirements of the Contract, and to object to the selection of a Subcontractor.
(b) MCO must:
(1) actively monitor the quality of care and services, as well as the quality of reporting data, provided under a Subcontract;
(2) provide HHSC with a copy of TDI filings of delegation agreements;
(3) unless otherwise provided in this Contract, provide HHSC with written notice no later than:
(i) three (3) Business Days after receiving notice from a Material Subcontractor of its intent to terminate a Subcontract;
(ii) 180 calendar days prior to the termination date of a Material Subcontract for MIS systems operation or reporting;
(iii) 90 calendar days prior to the termination date of a Material Subcontract for non-MIS MCO Administrative Services; and
(iv) 30 calendar days prior to the termination date of any other Material Subcontract.
HHSC may grant a written exception to these notice requirements if, in HHSC’s reasonable determination, the MCO has shown good cause for a shorter notice period.
(c) During the Contract Period, Readiness Reviews by HHSC or its designated agent may occur if:
(1) a new Material Subcontractor is employed by MCO;
(2) an existing Material Subcontractor provides services in a new Service Area;
(3) an existing Material Subcontractor provides services for a new MCO Program;
(4) an existing Material Subcontractor changes locations or changes its MIS and or operational functions;
(5) an existing Material Subcontractor changes one (1) or more of its MIS subsystems, claims processing or operational functions; or
(6) a Readiness Review is requested by HHSC.
The MCO must submit information required by HHSC for each proposed Material Subcontractor as indicated in Section 7, Transition Phase Requirements. Refer to Sections 8.1.1.2, Additional Readiness Reviews and Monitoring Efforts, and 8.1.18, Management Information System Requirements for additional information regarding MCO Readiness Reviews during the Contract Period.
(d) MCO must not disclose Confidential Information of HHSC or the State of Texas to a Subcontractor unless and until such Subcontractor has agreed in writing to protect the confidentiality of such Confidential Information in the manner required of MCO under this Contract.
(e) MCO must identify any Subcontractor that is a subsidiary or entity formed after the Effective Date of the Contract, whether or not an Affiliate of MCO. The MCO must substantiate the proposed Subcontractor’s ability to perform the subcontracted Services, and certify to HHSC that no loss of service will occur as a result of the performance of such Subcontractor. The MCO will be the sole point of contact with regard to contractual matters.
(f) Except as provided herein, all Subcontracts must be in writing and must provide HHSC the right to examine the Subcontract and all Subcontractor records relating to the Contract and the Subcontract. This requirement does not apply to agreements with non-Affiliate utility or mail service providers.
If the MCO intends to report compensation or any other payments paid to any third party (including without limitation an Affiliate) as an Allowable Expense under this Contract, and the amounts paid to the third party exceed $200,000, or are reasonably anticipated to exceed $200,000, in a State Fiscal Year (or in any contiguous twelve-month period), then the MCO’s agreement with the third party must be in writing. The agreement must provide HHSC the right to examine the agreement and all records relating to the agreement.
For any third party agreements not in writing valued under $200,000 per State Fiscal Year that are reported as Allowable Expenses , the MCO still must maintain standard financial records and data sufficient to verify the accuracy of those expenses in accordance with the requirements of Article 9, Audit & Financial Compliance. Any agreements that are, or could be interpreted to be, with a single party, must be in writing if the combined total is more than $200,000. This would include payments to individuals or entities that are related to each other.
(g) A Subcontract or any other agreement in which the MCO receives rebates, recoupments, discounts, payments, incentives, fees, free goods, bundling arrangements, retrocession payments (as described in UMCM Chapter 6.1) or any other consideration from a Subcontractor or any other third party (including without limitation Affiliates) as related to this Contract must be in writing and The MCO must allow HHSC and the Office of the Attorney General to examine the Subcontract or agreement and all related records.
(h) All Subcontracts or agreements described in subsections (f) and (g) must show the dollar amount or the value of any consideration that MCO pays to or receives from the Subcontractor or any other third party.
(i) The MCO must submit a copy of each Material Subcontract and any agreement covered under subsection (g) executed prior to the Effective Date of the Contract to HHSC no later than 30 days after the Effective Date of the Contract. For Material Subcontracts or Section 4.08(g) agreements executed or amended after the Effective Date of the Contract, the MCO must submit a copy to HHSC no later than 5 Business Days after execution or amendment.
(j) Network Provider Contracts must include the mandatory provisions included in Uniform Managed Care Manual Chapter 8.1, Provider Contract Checklist.
(k) HHSC reserves the right to reject any Subcontract or require changes to any provisions that do not comply with the requirements or duties and responsibilities of this Contract or create significant barriers for HHSC in monitoring compliance with this Contract.
(l) MCO must comply with the requirements of Section 6505 of the PPACA, entitled Prohibition on Payments to Institutions or Entities Located Outside of the United States.
(m) Provider payment must comply with the requirements of Section 2702 of PPACA, entitled Payment Adjustment for Health Acquired Conditions.
(n) The MCO and its Subcontractors must provide all information required under Section 4.08 to HHSC, or to the Office of the Attorney General, if requested, at no cost.
Section 4.09 HHSC’s ability to contract with Subcontractors.
The MCO may not limit or restrict, through a covenant not to compete, employment contract or other contractual arrangement, HHSC’s ability to contract with Subcontractors or former employees of the MCO.
Section 4.10 This section Intentionally left blank
Section 4.11 Prohibition Against Performance Outside the United States.
(a) Findings.
(1) HHSC finds the following:
(A) HHSC is responsible for administering several public programs that require the collection and maintenance of information relating to persons who apply for and receive services from HHSC programs. This information consists of, among other things, personal financial and medical information and information designated “Confidential Information” under state and federal law and this Agreement. Some of this information may, within the limits of the law and this Agreement, be shared from time to time with MCO or a subcontractor for purposes of performing the Services or providing the Deliverables under this Agreement.
(B) HHSC is legally responsible for maintaining the confidentiality and integrity of information relating to applicants and recipients of HHSC services and ensuring that any person or entity that receives such information—including MCO and any subcontractor—is similarly bound by these obligations.
(C) HHSC also is responsible for the development and implementation of computer software and hardware to support HHSC programs. These items are paid for, in whole or in part, with state and federal funds. The federal agencies that fund these items maintain a limited interest in the software and hardware so developed or acquired.
(D) Some of the software used or developed by HHSC may also be subject to statutory restrictions on the export of technology to foreign nations, including but not limited to the Export Administration Regulations, 15 C.F.R. Parts 730-774.
(2) In view of these obligations, and to ensure accountability, integrity, and the security of the information maintained by or for HHSC and the work performed on behalf of HHSC, HHSC DETERMINES that it is necessary and appropriate to require THAT:
(A) All work performed under this Agreement must be performed exclusively within the United States; and
(B) All information obtained by MCO or a subcontractor under this Agreement must be maintained within the United States.
(3) Further, HHSC finds it necessary and appropriate to forbid the performance of any work or the maintenance of any information relating or obtained pursuant to this Agreement to occur outside of the United States except as specifically authorized or approved by HHSC.
(b) Meaning of “within the United States” and “outside the United States.”
(1) As used in this Section 4.11, the term “within the United States” means any location inside the territorial boundaries comprising the republic of the United States of America, including of any of the 48 coterminous states in North America, the states of Alaska and Hawaii, and the District of Columbia.
(2) Conversely, the phrase “outside the United States” means any location that is not within the territorial boundaries comprising the republic of the United States of America, including of any of the 48 coterminous states in North America, the states of Alaska and Hawaii, and the District of Columbia.
(c) Maintenance of Confidential Information.
(1) MCO and all subcontractors, vendors, agents, and service providers of or for MCO must not allow any Confidential Information that MCO receives from or on behalf of HHSC to leave the United States by any means (physical or electronic) at any time, for any period of time, for any reason.
(2) MCO and all subcontractors, vendors, agents, and service providers of or for MCO must not permit any person to have remote access to HHSC information, systems, or Deliverables from a location outside the United States.
(d) Performance of Work under Agreement.
(1) Unless otherwise approved in advance by HHSC in writing, and subject to the exceptions specified in paragraph (d) of this Section 4.11, MCO and all subcontractors, vendors, agents, and service providers of or for MCO must perform all services under the Agreement, including all tasks, functions, and responsibilities assigned and delegated to MCO under this Agreement, within the United States.
(A) This obligation includes, but is not limited to, all Services, including but not limited to information technology services, processing, transmission, storage, archiving, data center services, disaster recovery sites and services, customer support), medical, dental, laboratory and clinical services.
(B) All custom software prepared for performance of this Agreement, and all modifications of custom, third party, or vendor proprietary software, must be performed within the United States.
(2) Unless otherwise approved in advance by HHSC in writing, and subject to the exceptions specified in paragraph (d) of this Section 4.11, MCO and all subcontractors, vendors, agents, and service providers of or for MCO must not permit any person to perform work under this Agreement from a location outside the United States.
(e) Exceptions.
(1) COTS Software. The foregoing requirements will not preclude the acquisition or use of commercial off-the-shelf software that is developed outside the United States or hardware that is generically configured outside the United States.
(2) Foreign-made Products and Supplies. The foregoing requirements will not preclude MCO from acquiring, using, or reimbursing products or supplies that are manufactured outside the United States, provided such products or supplies are commercially available within the United States for acquisition or reimbursement by HHSC.
(3) HHSC Prior Approval. The foregoing requirements will not preclude MCO from performing work outside the United States that HHSC has approved in writing and that HHSC has confirmed will not involve the sharing of Confidential Information outside the United States.
(f) Disclosure.
MCO must disclose all Services and Deliverables under or related to this Agreement that MCO intends to perform or has performed outside the United States, whether directly or via subcontractors, vendors, agents, or service providers.
(g) Remedy.
(1) MCO’s violation of this Section 4.11 will constitute a material breach in accordance with Article 12. MCO will be liable to HHSC for all monetary damages, in the form of actual, consequential, direct, indirect, special and/or liquidated damages in accordance with this Agreement.
(2) HHSC may terminate the Agreement with notice to MCO at least one calendar day before the effective date of such termination.
Article 5. Member Eligibility & Enrollment
Section 5.01 Eligibility Determination
The State or its designee will make eligibility determinations for each of the HHSC MCO Programs.
Section 5.02 Member Enrollment & Disenrollment.
(a) HHSC or the HHSC Administrative Services Contractor will enroll and disenroll eligible individuals in the MCO Program. The HHSC Administrative Services Contractor will use HHSC’s default assignment methodologies, as described in 1 Tex. Admin. Code § 353.403 and § 370.303, to enroll individuals who do not select an MCO or PCP. To enroll in an MCO, the Member’s permanent residence must be located within the MCO’s Service Area. The MCO is not allowed to induce or accept disenrollment from a Member. The MCO must refer the Member to the HHSC Administrative Services Contractor.
(b) HHSC makes no guarantees or representations to the MCO regarding the number of eligible Members who will ultimately be enrolled into the MCO or the length of time any such enrolling Members remain enrolled with the MCO. The MCO has no ownership interest in its Member base, and therefore cannot sell or transfer this base to another entity.
(c) The HHSC Administrative Services Contractor will electronically transmit to the MCO new Member information and change information applicable to active Members.
(d) As described in the following Sections, depending on the MCO Program, special conditions may also apply to enrollment and span of coverage for the MCO.
(e) A Medicaid MCO has a limited right to request a Member be disenrolled from MCO without the Member’s consent. HHSC must approve any MCO request for disenrollment of a Member for cause. MCO must take reasonable documented
measures to correct Member behavior prior to requesting disenrollment. Reasonable documented measures may include providing education and counseling regarding the offensive acts or behaviors. HHSC may permit disenrollment of a Member under the following circumstances:
(1) Member misuses or loans Member’s MCO membership card to another person to obtain services.
(2) Member's behavior is disruptive or uncooperative to the extent that Member’s continued enrollment in the MCO seriously impairs MCO’s or Provider’s ability to provide services to either the Member or other Members, and Member’s behavior is not related to a developmental, intellectual, or physical disability or behavioral health condition.
(3) Member steadfastly refuses to comply with managed care restrictions (e.g., repeatedly using emergency room in combination with refusing to allow MCO to treat the underlying medical condition).
(f) HHSC must notify the Member of HHSC’s decision to disenroll the Member if all reasonable measures have failed to remedy the problem.
(g) If the Member disagrees with the decision to disenroll the Member from MCO, HHSC must notify the Member of the availability of the Complaint procedure and, for Medicaid Members, HHSC’s Fair Hearing process.
(h) MCO cannot request a disenrollment based on adverse change in the member’s health status or utilization of services that are Medically Necessary for treatment of a member’s condition.
(i) Members taken into conservatorship by the Department of Family and Protective Services (DFPS) will be disenrolled from the MCO effective the date of conservatorship, and enrolled in the STAR Health Program unless otherwise determined by DFPS.
Section 5.03 STAR enrollment for pregnant women and infants.
(a) The HHSC Administrative Services Contractor will retroactively enroll some pregnant Members in a Medicaid MCO based on their date of eligibility.
(b) The HHSC Administrative Services Contractor will enroll newborns born to Medicaid eligible mothers who are enrolled in a STAR MCO in the same MCO for at least 90 days following the date of birth, unless the mother requests a plan change as a special exception. The HHSC Administrative Service Contractor will consider such requests on a case-by-case basis. The HHSC Administrative Services Contractor will retroactively, to date of birth, enroll newborns in the applicable STAR MCO.
Section 5.03.1 Enrollment for infants born to pregnant women in STAR+PLUS.
If a newborn is born to a Medicaid-eligible mother enrolled in a STAR+PLUS MCO, the HHSC Administrative Service Contractor will enroll the newborn into that MCO’s STAR MCO product, if one (1) exists. All rules related to STAR newborn enrollment will apply to the newborn. If the STAR+PLUS MCO does not have a STAR product but the newborn is eligible for STAR, the newborn will be enrolled in traditional Fee-for-Service Medicaid, and given the opportunity to select a STAR MCO.
Section 5.04 CHIP eligibility and enrollment.
(a) Term of coverage.
HHSC or the HHSC Administrative Services Contractor, on HHSC’s behalf, determines CHIP eligibility. HHSC or the HHSC Administrative Services Contractor will enroll and disenroll eligible individuals into and out of CHIP.
(b) Pregnant Members and Infants.
(1) HHSC or the HHSC Administrative Contractor will refer pregnant CHIP Members, with the exception of Legal Permanent Residents and other legally qualified aliens barred from Medicaid due to federal eligibility restrictions, to Medicaid for eligibility determinations. Those CHIP Members who are determined to be Medicaid Eligible will be disenrolled from the MCO’s CHIP plan. Medicaid coverage will be coordinated to begin after CHIP eligibility ends to avoid gaps in health care coverage.
(2) In the event the MCO remains unaware of a CHIP Member’s pregnancy until delivery, the facility and professional costs associated with the delivery will be covered by CHIP in accordance with Attachment B-1.1, CHIP Covered Services. This includes the post-delivery costs for the newborn’s care while in the facility, as described in Attachment B-1.1, CHIP Covered Services., HHSC or the HHSC Administrative Services Contractor will set a pregnant CHIP mother’s eligibility expiration date at the later of (1) the end of the second month following the month of the pregnancy delivery or the pregnancy termination or (2) the Member’s original eligibility expiration date.
HHSC or the Administrative Services Contractor will screen the newborn’s eligibility for Medicaid, and then CHIP (if the newborn is not eligible for Medicaid). If the newborn is eligible for CHIP, the Administrative Services Contractor will enroll the newborn in the mother’s CHIP plan prospectively, following standard cut-off rules. The newborn’s CHIP eligibility ends when the mother’s CHIP eligibility expires, as described above.
Section 5.05 CHIP Perinatal eligibility, enrollment, and disenrollment
(a) HHSC or the HHSC Administrative Contractor will electronically transmit to the MCO new CHIP Perinate Member information based on the appropriate CHIP Perinate or CHIP Perinate Newborn Rate Cell. There is no waiting period for CHIP Perinatal Program Members.
(b) Once born, a CHIP Perinate who lives in a family with an income at or below the Medicaid eligibility threshold will be deemed eligible for 12 months of continuous Medicaid coverage (beginning on the date of birth). A CHIP Perinate will continue to receive coverage through the CHIP Perinatal Program as a CHIP Perinate Newborn after birth if the child’s family income is above the Medicaid eligibility threshold. A CHIP Perinate Newborn is eligible for 12 months continuous enrollment, beginning with the month of enrollment as a CHIP Perinate (month of enrollment as an unborn child plus 11 months). A CHIP Perinate Newborn will maintain coverage in his or her CHIP Perinatal MCO.
(c) Once a CHIP Perinate Newborn Member’s coverage expires, the child will be added to his or her siblings’ active CHIP program case. If there is no active CHIP program case, then in the 10th month of the CHIP Perinate Newborn’s coverage, the family will receive a CHIP renewal form. The family must complete and submit the renewal form, which will be pre-populated to include the CHIP Perinate Newborn’s and the CHIP Program Members’ information.
Section 5.06 Span of Coverage
(a) Medicaid MCOs.
(1) Open Enrollment.
HHSC will conduct continuous open enrollment for Medicaid Eligibles and the MCO must accept all persons who choose to enroll as Members in the MCO or who are assigned as Members in the MCO by HHSC, without regard to the Member’s health status or any other factor.
(2) Enrollment Changes during an Inpatient Stay in a Hospital.
The following table describes payment responsibility for Medicaid enrollment changes that occur during an Inpatient Stay in a Hospital, as of the Member’s Effective Date of Coverage with the receiving MCO (New MCO).
|
| | | | |
| Scenario | Hospital Facility Charge | All Other Covered Services |
1 |
| Member Retroactively Enrolled in STAR or STAR+PLUS | New MCO | New MCO |
2 |
| Member Prospectively Moves from FFS to STAR or STAR+PLUS | FFS | New MCO |
3 |
| Member Moves between STAR MCOs | Former MCO | New MCO |
4 |
| Member Moves between STAR+PLUS MCOs | Former STAR+PLUS MCO | New STAR+PLUS MCO |
5 |
| Member Moves from STAR to STAR Health | Former STAR MCO | New STAR Health MCO |
6 |
| Member Moves from STAR+PLUS to STAR Health | Former STAR+PLUS MCO | New STAR Health MCO |
7 |
| Member Moves from STAR to STAR+PLUS | Former STAR MCO | New STAR+PLUS MCO |
8 |
| Adult Member Moves from STAR Health to STAR | Former STAR Health MCO | New STAR MCO |
The responsible party will pay the Hospital facility charge until the earlier of: (1) date of Discharge from the Hospital, or (2) loss of Medicaid eligibility.
For Members who move from STAR or STAR+PLUS into STAR Health, the date of Discharge from the Hospital for behavioral health stays includes extended stay days, as described in the Texas Medicaid Provider Procedures Manual.
(3) Enrollment Changes Due to SSI Status.
When an adult STAR Member becomes qualified for SSI, the Member will move to STAR+PLUS. When a child STAR Member becomes qualified for SSI, the Member will move to FFS or STAR+PLUS. Section 5.06(c) describes how HHSC will determine the effective date of the Member’s SSI status.
(4) Disenrollment from Managed Care during an Inpatient Stay in a Hospital.
Children who are voluntarily enrolled in STAR+PLUS can move to FFS during an Inpatient Stay in a Hospital.
STAR and STAR+PLUS Members also can move to FFS during an Inpatient Stay in a Hospital under the limited circumstances described in Section 5.02. (e), regarding disenrollment at the MCO’s request.
The following table describes how MCOs should divide payment responsibility between entities, beginning on the effective date of FFS coverage.
|
| | | | |
| Scenario | Hospital Facility Charge | All Other Covered Services |
1 |
| Voluntary Child Member Moves from STAR+PLUS to FFS (Includes Change Based on SSI Status) | Former STAR+PLUS MCO | FFS |
2 |
| Member Moves from STAR to FFS (Disenrolled at MCO’s Request) | Former STAR MCO | FFS |
3 |
| Member Moves from STAR+PLUS to FFS (Disenrolled at MCO’s Request) | Former STAR+PLUS MCO | FFS |
(5) Responsibility for Costs Incurred After Loss of Medicaid Eligibility.
Medicaid MCOs are not responsible for services incurred on or after the effective date of loss of Medicaid eligibility.
(6) Reenrollment after Temporary Loss of Medicaid Eligibility.
Members who are disenrolled because they are temporarily ineligible for Medicaid will be automatically reenrolled into the same MCO, if available. Temporary loss of eligibility is defined as a period of six months or less.
(7) Enrollment Changes during a Chemical Dependency Treatment Facility (CDTF) Stay.
The following table describes payment responsibility for Medicaid enrollment changes that occur during a stay in a residential substance use disorder treatment facility or residential detoxification for substance use disorder treatment facility (collectively, CDTF), beginning on the Member’s Effective Date of Coverage with the New MCO.
|
| | | | |
| Scenario | CDTF Charge | All Other Covered Services |
1 |
| Member Retroactively Enrolled in STAR or STAR+PLUS | New MCO | New MCO |
2 |
| Member Prospectively Moves from FFS to STAR or STAR+PLUS | New MCO | New MCO |
3 |
| Member Moves between STAR MCOs | Former MCO | New MCO |
4 |
| Member Moves between STAR+PLUS MCOs | Former STAR+PLUS MCO | New STAR+PLUS MCO |
5 |
| Member Moves from STAR to STAR Health | Former STAR MCO | New STAR Health MCO |
6 |
| Member Moves from STAR+PLUS to STAR Health | Former STAR+PLUS MCO | New STAR Health MCO |
7 |
| Adult Member Moves from STAR Health to STAR | Former STAR Health MCO | New STAR MCO |
8 |
| Child Member in Non-MRSA STAR Service Area Moves to STAR+PLUS (Based on Change in SSI Status) | Former STAR MCO | New STAR+PLUS MCO |
9 |
| Adult Member in Non-MRSA STAR Service Area Moves to STAR+PLUS (Based on Change in SSI Status) | Former STAR MCO | New STAR+PLUS MCO |
The responsible party will pay the CDTF charge until the earlier of: (1) date of Discharge from the CDTF, or (2) loss of Medicaid eligibility. The New MCO may evaluate for medical necessity of the CDTF stay prior to the end of the authorized services period. For Members who move from STAR or STAR+PLUS into Star Health, the date of Discharge from the CDTF includes extended stay days, as described in the Texas Medicaid Provider Procedures Manual.
(8) Disenrollment from Managed Care during a CDTF Stay.
Children who are enrolled voluntarily in STAR+PLUS can move to FFS during a CDTF Stay. STAR and STAR+PLUS Members also can move to FFS during a CDTF stay under the limited circumstances described in Section 5.02. (e), regarding disenrollment at the MCO’s request.
The following table describes how payment responsibility is divided between entities, beginning on the effective date of the Member’s FFS coverage.
|
| | | | |
| Scenario | CDTF Charge | All Other Covered Services |
1 |
| Voluntary Child Member Moves from STAR+PLUS to FFS (Includes Change Based on SSI Status) | Former STAR+PLUS MCO | FFS |
2 |
| Member Moves from STAR to FFS (Disenrolled at MCO’s Request) | Former STAR MCO | FFS |
3 |
| Member Moves from STAR+PLUS to FFS (Disenrolled at MCO’s Request) | Former STAR+PLUS MCO | FFS |
The responsible party will pay the CDTF charge until the earlier of: (1) date of Discharge from the CDTF, or (2) loss of Medicaid eligibility.
(9) Enrollment Changes during a Nursing Facility Stay (effective March 1, 2015).
The following table describes payment responsibility for Medicaid enrollment changes that occur during a Nursing Facility stay, beginning on the Member’s Effective Date of Coverage with the New MCO.
|
| | | | |
| Scenario | Nursing Facility Charge | All Other Covered Services |
1 |
| Member Moves from FFS to STAR+PLUS | New STAR+PLUS MCO | New STAR+PLUS MCO |
2 |
| Member Moves between STAR+PLUS MCOs | New STAR+PLUS MCO | New STAR+PLUS MCO |
(b) CHIP MCOs.
If a CHIP Program or CHIP Perinatal Program Member’s Effective Date of Coverage occurs while the Member is confined in a Hospital, the MCO is responsible for the Member’s costs of Covered Services beginning on the Effective Date of Coverage. If a Member is disenrolled while the Member is confined in a Hospital, the MCO’s responsibility for the Member’s costs of Covered Services terminates on the Date of Disenrollment.
(c) Effective Date of SSI Status.
In accordance with Section 8.2.12, SSI status is effective on the date HHSC’s eligibility system identifies a STAR, CHIP, or CHIP Perinate Newborn Member as Type Program 13 (TP 13). HHSC is will update the eligibility system within 45 days of official notice of the Member’s Federal SSI status by the Social Security Administration (SSA). Once HHSC has updated the eligibility system to identify the STAR, CHIP, or CHIP Perinate Newborn Member as TP13, following standard eligibility cut-off rules, HHSC will allow the Member to choose to:
(1) prospectively move to Medicaid FFS (if the Member is a child) or
(2) prospectively move to STAR+PLUS (if the Member is a child or adult).
HHSC will not retroactively disenroll a Member from the STAR, CHIP, or CHIP Perinatal Programs.
Section 5.07 Verification of Member Eligibility.
Medicaid MCOs are prohibited from entering into an agreement to share information regarding their Members with an external vendor that provides verification of Medicaid recipients’ eligibility to Medicaid providers. All such external vendors must contract with the State and obtain eligibility information from the State.
Section 5.08 Modified Default Enrollment Process
Under The circumstances described in HHSC's administrative rules at 1 Tex. Admin. Code § 353.403 and 1 Tex. Admin. Code § 370.303, HHSC may implement a modified default enrollment process to equitably assign enrollees who have not selected an MCO. To the extent possible, HHSC will make assignments based on an enrollee's prior history with and geographic proximity to a PCP. HHSC will determine the length of the modified default enrollment period by considering factors such as MCO market share, viability, and Member Choice. HHSC reserves the right to extend the modified default period, or implement additional modified default periods as it determines necessary and with prior written notice to impacted MCOs.
Section 5.09 This Section Intentionally Left Blank
Section 5.10 This Section Intentionally Left Blank
Section 5.11 This Section Intentionally Left Blank
Article 6. Service Levels & Performance Measurement
Section 6.01 Performance measurement.
Satisfactory performance of this Contract will be measured by:
(a) Adherence to this Contract, including all representations and warranties;
(b) Delivery of the Services and Deliverables;
(c) Results of audits performed by HHSC or its representatives in accordance with Article 9, “Audit and Financial Compliance”;
(d) Timeliness, completeness, and accuracy of required reports; and
(e) Achievement of performance measures developed by MCO and HHSC and as modified from time to time by written agreement during the term of this Contract.
Article 7. Governing Law & Regulations
Section 7.01 Governing law and venue.
This Contract is governed by the laws of the State of Texas and interpreted in accordance with Texas law. Provided MCO first complies with the procedures set forth in Section 12.13, “Dispute Resolution,” proper venue for claims arising from this Contract will be in the State District Court of Travis County, Texas.
Section 7.02 MCO responsibility for compliance with laws and regulations.
(a) MCO must comply, to the satisfaction of HHSC, with all provisions set forth in this Contract, all provisions of state and federal laws, rules, regulations, federal waivers, policies and guidelines, and any court-ordered consent decrees, settlement agreements, or other court orders that govern the performance of the Scope of Work including, but not limited to, all applicable provisions of the following:
(1) Titles XIX and XXI of the Social Security Act;
(2) Chapters 62 and 63, Texas Health and Safety Code;
(3) Chapters 531 and 533, Texas Government Code;
(4) 42 C.F.R. Parts 417, 455, and 457, as applicable;
(5) 45 C.F.R. Parts 74 and 92;
(6) 48 C.F.R. Part 31, or OMB Circular A-122, based on whether the entity is for-profit or nonprofit;
(7) 1 T.A.C. Part 15, Chapters 361, 370, 371, 391, and 392;
(8) Consent Decree and Corrective Action Orders, Frew, et al. v. Janek, et al., (applies to Medicaid MCOs only);
(9) partial settlement agreements, Alberto N., et al. v. Janek, et al., (applies to Medicaid MCOs only);
(10) Texas Human Resources Code Chapters 32 and 36;
(11) Texas Penal Code Chapter 35A (Medicaid Fraud);
(12) 1 T.A.C. Chapter 353;
(13) 1 T.A.C. Chapter 354, Subchapters B, J, and F, with the exception of the following provisions in Subchapter F: 1 T.A.C. §354.1865, §354.1867, §354.1873, and Division 6, Pharmacy Claims; and §354.3047:
(14) 1 T.A.C. Chapter 354, Subchapters I and K, as applicable;
(15) The Patient Protection and Affordable Care Act (PPACA; Public Law 111-148);
(16) The Health Care and Education Reconciliation Act of 2010 (HCERA; Public Law 111-152) 42 CFR Part 455;
(17) The Immigration and Nationality Act (8 U.S.C § 1101 et seq.) and all subsequent immigration laws and amendments; and
(18) all State and Federal tax laws, State and Federal employment laws, State and Federal regulatory requirements, and licensing provisions.
(b) The Parties acknowledge that the federal and/or state laws, rules, regulations, policies, or guidelines, and court-ordered consent decrees, settlement agreements, or other court orders that affect the performance of the Scope of Work may change from time to time or be added, judicially interpreted, or amended by competent authority. MCO acknowledges that the MCO Programs will be subject to continuous change during the term of the Contract and, except as provided in Section 8.02, MCO has provided for or will provide for adequate resources, at no additional charge to HHSC, to reasonably accommodate such changes. The Parties further acknowledge that MCO was selected, in part, because of its expertise, experience, and knowledge concerning applicable Federal and/or state laws, regulations, policies, or guidelines that affect the performance of the Scope of Work. In keeping with HHSC's reliance on this knowledge and expertise, MCO is responsible for identifying the impact of changes in applicable Federal or state legislative enactments and regulations that
affect the performance of the Scope of Work or the State's use of the Services and Deliverables. MCO must timely notify HHSC of such changes and must work with HHSC to identify the impact of such changes.
(c) HHSC will notify MCO of any changes in applicable law, regulation, policy, or guidelines that HHSC becomes aware of in the ordinary course of its business.
(d) MCO is responsible for any fines, penalties, or disallowances imposed on the State or MCO arising from any noncompliance with the laws and regulations relating to the delivery of the Services or Deliverables by the MCO, its Subcontractors or agents.
(e) MCO is responsible for ensuring each of its employees, agents or Subcontractors who provide Services under the Contract are properly licensed, certified, and/or have proper permits to perform any activity related to the Services.
(f) MCO warrants that the Services and Deliverables will comply with all applicable Federal, State, and County laws, regulations, codes, ordinances, guidelines, and policies. MCO will indemnify HHSC from and against any losses, liability, claims, damages, penalties, costs, fees, or expenses arising from or in connection with MCO's failure to comply with or violation of any such law, regulation, code, ordinance, or policy
Section 7.03 TDI licensure/ANHC certification and solvency.
(a) Licensure
MCO must receive TDI approval to operate in all counties of the Service Areas included within the scope of the Contract.
(b) Solvency
MCO must maintain compliance with the Texas Insurance Code and rules promulgated and administered by the TDI requiring a fiscally sound operation. MCO must have a plan and take appropriate measures to ensure adequate provision against the risk of insolvency as required by TDI. Such provision must be adequate to provide for the following in the event of insolvency:
(1) continuation of benefits, until the time of discharge, to Members who are confined on the date of insolvency in a Hospital or other inpatient facility;
(2) payment to unaffiliated health care providers and affiliated health care providers whose agreements do not contain member “hold harmless” clauses acceptable to TDI for required services rendered to Members for the duration of the Contract period for which HHSC has paid a Capitation Payment, and
(3) continuation of benefits for the duration of the Contract period for which HHSC has paid a Capitation Payment.
Provision against the risk of insolvency must be made by establishing adequate reserves, insurance or other guarantees in full compliance with all financial requirements of TDI.
Section 7.04 This Section Intentionally Left Blank
Section 7.05 Compliance with state and federal anti-discrimination laws.
(a) MCO agrees to comply with state and federal anti-discrimination laws, including without limitation:
(1) Title VI of the Civil Rights Act of 1964 (42 U.S.C. §2000d et seq.);
(2) Section 504 of the Rehabilitation Act of 1973 (29 U.S.C. §794);
(3) Americans with Disabilities Act of 1990 (42 U.S.C. §12101 et seq.);
(4) Age Discrimination Act of 1975 (42 U.S.C. §§6101-6107);
(5) Title IX of the Education Amendments of 1972 (20 U.S.C. §§1681-1688);
(6) Food Stamp Act of 1977 (7 U.S.C. §200 et seq.); and
(7) The HHS agency’s administrative rules, as set forth in the Texas Administrative Code, to the extent applicable to this Agreement.
MCO agrees to comply with all amendments to the above-referenced laws, and all requirements imposed by the regulations issued pursuant to these laws. These laws provide in part that no persons in the United States may, on the grounds of race, color, national origin, sex, age, disability, political beliefs, or religion, be excluded from participation in or denied any aid, care, service or other benefits provided by Federal or State funding, or otherwise be subjected to discrimination.
(b) MCO agrees to comply with Title VI of the Civil Rights Act of 1964, and its implementing regulations at 45 C.F.R. Part 80 or 7 C.F.R. Part 15, prohibiting a contractor from adopting and implementing policies and procedures that exclude or have the effect of excluding or limiting the participation of clients in its programs, benefits, or activities on the basis of national origin. Applicable state and federal civil rights laws require contractors to provide alternative methods for ensuring access to services for applicants and recipients who cannot express themselves fluently in English. MCO agrees to ensure that its policies do not have the effect of excluding or limiting the participation of persons in its programs, benefits, and activities on the basis of national origin. MCO also agrees to take reasonable steps to provide services and information, both orally and in writing, in appropriate languages other than English, in order to ensure that persons with limited English proficiency are effectively informed and can have meaningful access to programs, benefits, and activities.
(c) MCO agrees to comply with Executive Order 13279, and its implementing regulations at 45 C.F.R. Part 87 or 7 C.F.R. Part 16. These provide in part that any organization that participates in programs funded by direct financial assistance from the United States Department of Agriculture or the United States Department of Health and Human Services must not, in providing services, discriminate against a program beneficiary or prospective program beneficiary on the basis of religion or religious belief.
(d) Upon request, MCO will provide HHSC Civil Rights Office with copies of all of the MCO’s civil rights policies and procedures.
(e) MCO must notify HHSC’s Civil Rights Office of any civil rights complaints received relating to its performance under this Agreement. This notice must be delivered no more than ten (10) calendar days after receipt of a complaint. Notice provided pursuant to this section must be directed to:
HHSC Civil Rights Office
701 W. 51st Street, Mail Code W206
Austin, Texas 78751
Phone Toll Free: (888) 388-6332
Phone: (512) 438-4313
TTY Toll Free: (877) 432-7232
Fax: (512) 438-5885.
Section 7.06 Environmental protection laws.
MCO must comply with the applicable provisions of federal environmental protection laws as described in this Section:
(a) Pro-Children Act of 1994.
MCO must comply with the Pro-Children Act of 1994 (20 U.S.C. §6081 et seq.), as applicable, regarding the provision of a smoke-free workplace and promoting the non-use of all tobacco products.
(b) National Environmental Policy Act of 1969.
MCO must comply with any applicable provisions relating to the institution of environmental quality control measures contained in the National Environmental Policy Act of 1969 (42 U.S.C. §4321 et seq.) and Executive Order 11514 (“Protection and Enhancement of Environmental Quality”).
(c) Clean Air Act and Water Pollution Control Act regulations.
MCO must comply with any applicable provisions relating to required notification of facilities violating the requirements of Executive Order 11738 (“Providing for Administration of the Clean Air Act and the Federal Water Pollution Control Act with Respect to Federal Contracts, Grants, or Loans”).
(d) State Clean Air Implementation Plan.
MCO must comply with any applicable provisions requiring conformity of federal actions to State (Clean Air) Implementation Plans under §176(c) of the Clean Air Act of 1955, as amended (42 U.S.C. §740 et seq.).
(e) Safe Drinking Water Act of 1974.
MCO must comply with applicable provisions relating to the protection of underground sources of drinking water under the Safe Drinking Water Act of 1974, as amended (21 U.S.C. § 349; 42 U.S.C. §§ 300f to 300j-9).
Section 7.07 HIPAA.
(a) MCO must comply with applicable provisions of HIPAA. This includes the requirement that the
MCO’s MIS system comply with applicable certificate of coverage and data specification and reporting requirements promulgated pursuant to HIPAA. MCO must comply with HIPAA EDI requirements.
(b) Additionally, MCO must comply with HIPAA notification requirements, including those set forth in the Health Information Technology for Economic and Clinical Health Act (HITECH Act) at 42 U.S.C. § 17931 et seq. If, in HHSC’s determination, MCO has not provided notice in the manner or format prescribed by the HITECH Act, then HHSC may require the MCO to provide this notice.
(c) MCO must notify HHSC of all breaches or potential breaches of unsecured protected health information, as that term is defined by the HITECH Act. As noted in Article 2, "Definitions," Confidential Information includes HIPAA-defined protected health information. Therefore, any breach of that information is also subject to the requirements, including notice requirements, in Article 11, "Disclosure & Confidentiality of Information."
(d) The MCO must use or disclose protected health information as authorized and in response to another HIPAA-covered entity’s inquiry about a Member for authorized purposes of treatment, payment, healthcare operations, or as required by law under HIPAA.
(e) The MCO must comply with rights of individual access by a Member or a Member’s Legally Authorized Representative to Member’s protected health information. The MCO may permit limited disclosures of protected health information as permissible under HIPAA for a family member, other relative, or close personal friends of the Member or
anyone identified in the Member’s protected health information directly relevant to the Member's involvement with the Member's healthcare or payment related to the Member's healthcare. The MCO should refer to 45 C.F.R. § 164.510(b) and related regulatory guidance for additional information.
Section 7.08 Historically Underutilized Business Participation Requirements
(a) Definitions.
For purposes of this Section:
(1) “Historically Underutilized Business” or “HUB” means a minority or women-owned business as defined by Texas Government Code, Chapter 2161
(2) “HSP” means a HUB Subcontracting Plan.
(b) HUB Requirements.
(1) In accordance with Attachment B-1, Section 8.1.20.2, the MCO must submit an HSP for HHSC’s approval during the Transition Phase, and maintain the HSP thereafter.
(2) MCO must report to HHSC’s contract manager and HUB Office monthly, in the format required by Chapter 5.4.4.5 of the Uniform Managed Care Manual, its use of HUB subcontractors to fulfill the subcontracting opportunities identified in the HSP.
(3) MCO must obtain prior written approval from the HHSC HUB Office before making any changes to the HSP. The proposed changes must comply with HHSC’s good faith effort requirements relating to the development and submission of HSPs.
(i) The MCO must submit a revised HSP to the HHSC HUB Office when it: changes the dollar amount of, terminates, or modifies an existing Subcontract for MCO Administrative Services; or enters into a new Subcontract for MCO Administrative Services. All proposed changes to the HSP must comply with the requirements of this Agreement.
(4) HHSC will determine if the value of Subcontracts to HUBs meet or exceed the HUB subcontracting provisions specified in the MCO's HSP. If HHSC determines that the MCO's subcontracting activity does not demonstrate a good faith effort, the MCO may be subject to provisions in the Vendor Performance and Debarment Program (Title 34, Part 1, Chapter 20, Subchapter C, Rule §20.105), and subject to remedies for Breach.
Section 7.09 Compliance with Fraud, Waste, and Abuse requirements.
MCO, MCO’s personnel, and all Subcontractors must comply with all fraud, waste, and abuse requirements found in HHS Circular C-027. The MCO must comply with Circular C-027 requirements in addition to other fraud, waste, and abuse provisions in the contract and in state and federal law.
Article 8. Amendments & Modifications
Section 8.01 Mutual agreement.
This Contract may be amended at any time by mutual agreement of the Parties. The amendment must be in writing and signed by individuals with authority to bind the Parties.
Section 8.02 Changes in law or contract.
If Federal or State laws, rules, regulations, policies or guidelines are adopted, promulgated, judicially interpreted or changed, or if contracts are entered or changed, the effect of which is to alter the ability of either Party to fulfill its obligations under this Contract, the Parties will promptly negotiate in good faith appropriate modifications or alterations to the Contract. Such modifications or alterations must be in writing and signed by individuals with authority to bind the parties, equitably adjust the terms and conditions of this Contract, and must be limited to those provisions of this Contract affected by the change.
Section 8.03 Modifications as a remedy.
This Contract may be modified under the terms of Article 12, “Remedies and Disputes.”
Section 8.04 Modification Process.
(a) If HHSC seeks modifications to the Contract, HHSC’s notice to MCO will specify those modifications to the Scope of Work, the Contract pricing terms, or other Contract terms and conditions.
(b) MCO must respond to HHSC’s proposed modification within the timeframe specified by HHSC, generally within ten (10) Business Days of receipt. Upon receipt of MCO’s response to the proposed modifications, HHSC may enter into negotiations with MCO to arrive at mutually agreeable Contract amendments. In the event that HHSC determines that the Parties will be unable to reach agreement on mutually satisfactory contract modifications, then HHSC will provide written notice to MCO of its intent terminate the Contract, or not to extend the Contract beyond the current Contract Term.
Section 8.05 Modification of the Uniform Managed Care Manual.
(a) HHSC will provide MCO with at least ten (10) Business Days advance written notice before implementing a substantive and material change in the Uniform Managed Care Manual (a change that materially and substantively alters the MCO’s ability to fulfill its obligations under the Contract). The Uniform Managed Care Manual, and all modifications thereto made during the Contract Term, are incorporated by reference into this Contract. HHSC will provide MCO with a reasonable amount of time to comment on such changes, generally at least five (5) Business Days. HHSC is not required to provide advance written notice of changes that are not material and substantive in nature, such as corrections of clerical errors or policy clarifications.
(b) The Parties agree to work in good faith to resolve disagreements concerning material and substantive changes to the Uniform Managed Care Manual. If the Parties are unable to resolve issues relating to material and substantive changes, then either Party may terminate the agreement in accordance with Article 12, “Remedies and Disputes.”
(c) Changes will be effective on the date specified in HHSC’s written notice, which will not be earlier than the MCO’s response deadline, and such changes will be incorporated into the Uniform Managed Care Manual. If the MCO has raised an objection to a material and substantive change to the Uniform Managed Care Manual and submitted a notice of termination in accordance with Section 12.04(c), HHSC will not enforce the policy change for the objecting MCO during the period of time between the receipt of the notice and the date of Contract termination.
Section 8.06 CMS approval of amendments
Amendments, modifications, and changes to the Contract are subject to the approval of the Centers for Medicare and Medicaid Services (“CMS.”)
Section 8.07 Required compliance with amendment and modification procedures.
No different or additional services, work, or products will be authorized or performed except as authorized by this Article. No waiver of any term, covenant, or condition of this Contract will be valid unless executed in compliance with this Article. MCO will not be entitled to payment for any services, work or products that are not authorized by a properly executed Contract amendment or modification.
Article 9. Audit & Financial Compliance
Section 9.01 Record retention and audit.
MCO agrees to maintain, and require its Subcontractors to maintain, records, books, documents, and information (collectively “records”) that are adequate to ensure that services are provided and payments are made in accordance with the requirements of this Contract, including applicable Federal and State requirements (e.g., 45 CFR §74.53). Such records must be retained by MCO or its Subcontractors for a period of five (5) years after the Contract Expiration Date or until the resolution of all litigation, claim, financial management review or audit pertaining to this Contract, whichever is longer.
Section 9.02 Access to records, books, and documents.
(a) Upon reasonable notice, MCO must provide, and cause its Subcontractors to provide, at no cost to the officials and entities identified in this Section prompt, reasonable, and adequate access to any records that are related to the scope of this Contract.
(b) MCO and its Subcontractors must provide the access described in this Section upon HHSC's request. This request may be for, but is not limited to, the following purposes:
(1) Examination;
(2) Audit;
(3) Investigation;
(4) Contract administration; or
(5) The making of copies, excerpts, or transcripts.
(c) The access required must be provided to the following officials and/or entities:
(1) The United States Department of Health and Human Services or its designee;
(2) The Comptroller General of the United States or its designee;
(3) MCO Program personnel from HHSC or its designee;
(4) The Office of Inspector General;
(5) The Medicaid Fraud Control Unit of the Texas Attorney General's Office or its designee;
(6) Any independent verification and validation contractor, audit firm, or quality assurance contractor acting on behalf of HHSC;
(7) The Office of the State Auditor of Texas or its designee;
(8) A State or Federal law enforcement agency;
(9) A special or general investigating committee of the Texas Legislature or its designee; and
(10) Any other state or federal entity identified by HHSC, or any other entity engaged by HHSC.
(d) MCO agrees to provide the access described wherever MCO maintains such books, records, and supporting documentation. MCO further agrees to provide such access in reasonable comfort and to provide any furnishings, equipment, and other conveniences deemed reasonably necessary to fulfill the purposes described in this Section. MCO will require its Subcontractors to provide comparable access and accommodations.
(e) Upon request, the MCO must provide copies of the information described in this Section free of charge to HHSC and the entities described in subsection (c).
(f) In accordance with Texas Government Code §533.012(e), any information submitted to HHSC or the Texas Attorney General's Office pursuant to Texas Government Code §533.012(a)(1) is confidential and is not subject to disclosure under the Texas Public Information Act.
Section 9.03 Audits of Services, Deliverables and inspections.
(a) Upon reasonable notice from HHSC, MCO will provide, and will cause its Subcontractors to provide, such auditors and inspectors as HHSC may from time to time designate, with access to:
(1) service locations, facilities, or installations;
(2) records; and
(3) Software and Equipment.
(b) The access described in this Section will be for the purpose of examining, auditing, or investigating:
(1) MCO’s capacity to bear the risk of potential financial losses;
(2) the Services and Deliverables provided;
(3) a determination of the amounts payable under this Contract;
(4) a determination of the allowability of costs reported under this Contract;
(5) an examination of Subcontract terms and/or transactions;
(6) an assessment of financial results under this Contract;
(7) detection of Fraud, Waste and/or Abuse; or
(8) other purposes HHSC deems necessary to perform its oversight function and/or enforce the provisions of this Contract.
(c) MCO must provide, as part of the Scope of Work, any assistance that such auditors and inspectors reasonably may require to complete such audits or inspections.
(d) If, as a result of an audit or review of payments made to the MCO, HHSC discovers a payment error or overcharge, HHSC will notify the MCO of such error or overcharge. HHSC will be entitled to recover such funds as an offset to future payments to the MCO, or to collect such funds directly from the MCO. MCO must return funds owed to HHSC within 30 days after receiving notice of the error or overcharge, or interest will accrue on the amount due. HHSC will calculate interest at 12% per annum, compounded daily. In the event that an audit reveals that errors in reporting by the MCO have resulted in errors in payments to the MCO or errors in the calculation of the Experience Rebate, the MCO will indemnify HHSC for any losses resulting from such errors, including the cost of audit. If the interest rate stipulated hereunder is found by a court of competent jurisdiction to be outside the range deemed legal and enforceable, then the rate hereunder will be adjusted as little as possible so as to be deemed legal and enforceable.
Section 9.04 SAO Audit
The MCO understands that acceptance of funds under this Contract acts as acceptance of the authority of the State Auditor's Office (SAO), or any successor agency, to conduct an investigation in connection with those funds. The MCO further agrees to cooperate fully with the SAO or its successor in the conduct of the audit or investigation, including providing all records requested at no cost. The MCO will ensure that this clause concerning the authority to audit funds and the requirement to cooperate is included in any Subcontract, and in any third party agreements described in Section 4.10, "MCO Agreements with Third Parties."
Section 9.05 Response/compliance with audit or inspection findings.
(a) MCO must take action to ensure its or a Subcontractor’s compliance with or correction of any finding of noncompliance with any law, regulation, audit requirement, or generally accepted accounting principle relating to the Services and Deliverables or any other deficiency contained in any audit, review, or inspection conducted under this Article. This action will include MCO’s delivery to HHSC, for HHSC’S approval, a Corrective Action Plan that addresses deficiencies identified in any audit, review, or inspection within 30 calendar days of the close of the audit, review, or inspection.
(b) MCO must bear the expense of compliance with any finding of noncompliance under this Section that is:
(1) Required by Texas or Federal law, regulation, rule, court order, or other audit requirement relating to MCO's business;
(2) Performed by MCO as part of the Scope of Work; or
(3) Necessary due to MCO's noncompliance with any law, regulation, rule, court order, or audit requirement imposed on MCO.
(c) As part of the Scope of Work, MCO must provide to HHSC upon request a copy of those portions of MCO's and its Subcontractors' internal audit reports relating to the Services and Deliverables provided to HHSC under the Contract.
Section 9.06 Notification of Legal and Other Proceedings, and Related Events.
The MCO must notify HHSC of all proceedings, reports, documents, actions, and events as specified in Uniform Managed Care Manual Chapter 5.8, “Report of Legal and Other Proceedings, and Related Events.”
Article 10. Terms & Conditions of Payment
Section 10.01 Calculation of monthly Capitation Payment.
(a) This is a Risk-based contract. For each applicable MCO Program, HHSC will pay the MCO fixed monthly Capitation Payments based on the number of eligible enrolled Members. HHSC will calculate the monthly Capitation Payments by multiplying the number of Members in each Rate Cell category by the Capitation Rate for each Rate Cell. In consideration of the Monthly Capitation Payments, the MCO agrees to provide the Services and Deliverables described in this Contract.
(b) MCO will be required to provide timely financial and statistical information necessary in the Capitation Rate determination process. Encounter Data provided by MCO must conform to all HHSC requirements. Encounter Data containing non-compliant information, including, but not limited to, inaccurate Member identification numbers, inaccurate provider identification numbers, or diagnosis or procedures codes insufficient to adequately describe the diagnosis or medical procedure performed, will not be considered in the MCO’s experience for rate-setting purposes.
(c) Information or data, including complete and accurate Encounter Data, as requested by HHSC for rate-setting purposes, must be provided to HHSC: (1) within 30 days of receipt of the letter from HHSC requesting the information or data; and (2) no later than March 31st of each year.
(d) The fixed monthly Capitation Rate consists of the following components:
(1) an amount for Health Care Services performed during the month;
(2) an amount for administering the MCO Program, and
(3) an amount for the MCO’s Risk margin.
Capitation Rates for each MCO Program may vary by Service Area and MCO. HHSC will employ or retain qualified actuaries to perform data analysis and calculate the Capitation Rates for each Rate Period.
(e) MCO understands and expressly assumes the risks associated with the performance of the duties and responsibilities under this Contract, including the failure, termination or suspension of funding to HHSC, delays or denials of required approvals, and cost overruns not reasonably attributable to HHSC.
Section 10.02 Time and Manner of Payment.
(a) During the Contract Term and beginning after the Operational Start Date, HHSC will pay the monthly Capitation Payments by the 10th Business Day of each month.
(b) The MCO must accept Capitation Payments by direct deposit into the MCO’s account.
(c) HHSC may adjust the monthly Capitation Payment to the MCO in the case of an overpayment to the MCO; for Experience Rebate amounts due and unpaid, including any associated interest; and if monetary damages (including any associated interest) are assessed in accordance with Article 12, Remedies and Disputes.
(d) HHSC’s payment of monthly Capitation Payments is subject to availability of federal and state appropriations. If appropriations are not available to pay the full monthly Capitation Payment, HHSC may:
(1) equitably adjust Capitation Payments for all participating MCOs, and reduce scope of service requirements as appropriate in accordance with Article 8, Amendments and Modifications, or
(2) terminate the Contract in accordance with Article 12, Remedies and Disputes.
Section 10.03 Certification of Capitation Rates.
HHSC will employ or retain a qualified actuary to certify the actuarial soundness of the Capitation Rates, and all revisions or modifications thereto.
Section 10.04 Modification of Capitation Rates.
The Parties expressly understand and agree that the agreed Capitation Rates are subject to modification in accordance with
Article 8, “Amendments and Modifications,” if changes in state or federal laws, rules, regulations, guidelines, policies, or court orders affect the rates or the actuarial soundness of the rates. HHSC will provide the MCO notice of a modification to the Capitation Rates at least 60 days prior to the effective date of the change, unless HHSC determines that circumstances warrant a shorter notice period. If the MCO does not accept the rate change, either Party may terminate the Contract in accordance with Article 12 , “Remedies and Disputes.”
Section 10.05 STAR and STAR+PLUS Capitation Structure.
(a) STAR Rate Cells.
STAR Capitation Rates are defined on a per Member per month basis by Rate Cells and Service Areas. STAR Rate Cells are:
(1) Under Age 1 Child;
(2) Age 1-5 Child;
(3) Age 6-14 Child;
(4) Age 15-18 Child;
(5) Age 19-20 Child;
(6) TANF adults;
(7) Pregnant women; and
(8) SSI (applies to the Medicaid Rural Service Area only).
These Rate Cells are subject to change.
(b) STAR+PLUS Rate Cells.
STAR+PLUS Capitation Rates are defined on a per Member per month basis by Rate Cells. STAR+PLUS Rate Cells are based on client category as follows:
(1) Medicaid Only Standard Rate
(2) Medicaid Only HCBS STAR+PLUS Waiver Rate - Above Floor
(3) Medicaid Only HCBS STAR+PLUS Waiver Rate - Below Floor
(4) Dual Eligible Standard Rate
(5) Dual Eligible HCBS STAR+PLUS Waiver Rate - Above Floor
(6) Dual Eligible HCBS STAR+PLUS Waiver Rate - Below Floor
(7) Nursing Facility - Medicaid only
(8) Nursing Facility - Dual Eligible
(9) Individuals with Developmental Disabilities (IDD) - under age 21
(10) Individuals with Developmental Disabilities (IDD) - age 21 and older
These Rate Cells are subject to change.
(c) STAR and STAR+PLUS Capitation Rate development:
(1) Capitation Rates for Service Areas with historical Medicaid MCO Program participation.
For Service Areas where HHSC operated a Medicaid MCO Program prior to the Effective Date of this Contract, HHSC will develop base Capitation Rates by analyzing the Medicaid MCO Program's historical Encounter Data and financial data for the Service Area (e.g., Capitation Rates for the STAR Program will be based on STAR Program historical Encounter Data and financial data for the Service Area). This analysis will apply to all MCOs in the Service Area, including MCOs that have no historical participation in the Medicaid MCO Program in Service Area. The analysis will include a review of historical enrollment and claims experience information; any changes to Covered Services and covered populations; rate changes specified by the Texas Legislature; and any other relevant information. If the MCO participated in the Medicaid MCO Program in the Service Area prior to the Effective Date of this Contract, HHSC may modify the Service Area base Capitation Rates using diagnosis-based risk adjusters to yield the final Capitation Rates.
(2) Capitation Rates for Rate Periods 1 and 2 for Service Areas with no historical STAR Program participation.
For Service Areas where HHSC has not operated a Medicaid MCO Program prior to the Effective Date of this Contract, HHSC will establish base Capitation Rates for Rate Periods 1 and 2 by analyzing Fee-for-Service claims data for the Medicaid MCO Program and Service Area (e.g., Capitation Rates for the STAR Program will be based fee-for-service data in the Service Area). This analysis will include a review of historical enrollment and claims experience information; any changes to Covered Services and covered populations; rate changes specified by the Texas Legislature; and any other relevant information.
(3) Capitation Rates for subsequent Rate Periods for Service Areas with no historical STAR Program participation.
For Service Areas where HHSC has not operated a Medicaid MCO Program prior to the Effective Date of this Contract, HHSC will establish base Capitation Rates for the Rate Periods following Rate Period 2 by analyzing the Medicaid MCO Program's historical Encounter Data and financial data for the Service Area. This analysis will include a review of historical enrollment and claims experience information; any changes to Covered Services and covered populations; rate changes specified by the Texas Legislature; and any other relevant information.
(d) Acuity adjustment.
HHSC may evaluate and implement an acuity adjustment methodology, or alternative reasonable methodology, that appropriately reimburses the MCO for acuity and cost differences that deviate from that of the community average, if HHSC in its sole discretion determines that such a methodology is reasonable and appropriate. The community average is a uniform rate for all MCOs in a Service Area, and is determined by combining all the experience for all MCOs in a Service Area to get an average rate for the Service Area.
(e) Value-added Services.
Value-added Services will not be included in the rate-setting process.
(f) Delay in Increased STAR+PLUS Capitation Level for Certain Members Receiving Waiver Services.
Once a current STAR+PLUS MCO Member has been certified to receive STAR+PLUS Waiver (SPW) services, there is a two (2) month delay before the MCO will begin receiving the higher capitation payment.
Non-Waiver Members who qualify for STAR+PLUS based on eligibility for SPW services and Waiver recipients who transfer from another region will not be subject to this two (2) month delay in the increased capitation payment.
All SPW recipients will be registered into Service Authorization System Online (SASO). The Premium Payment System (PPS) will process data from the SASO system in establishing a Member's correct capitation payment.
Section 10.06 CHIP Capitation Rates Structure.
(a) CHIP Rate Cells.
CHIP Capitation Rates are defined on a per Member per month basis by the Rate Cells applicable to a Service Area. CHIP Rate Cells are based on the Member’s age group as follows:
(1) under age one (1);
(2) ages one (1) through five (5);
(3) ages six (6) through fourteen (14); and
(4) ages fifteen (15) through eighteen (18).
(b) CHIP Perinatal Program Rate Cells.
CHIP Perinatal Capitation Rates are defined on a per Member per month basis by the Rate Cells applicable to a Service Area. CHIP Perinatal Rate Cells are based on the Member’s birth status and household income as follows:
(1) CHIP Perinate at or Below Medicaid Eligibility Threshold (an unborn child who will qualify for Medicaid once born);
(2) CHIP Perinate Above Medicaid Eligibility Threshold (an unborn child who will not qualify for Medicaid once born); and
(3) CHIP Perinate Newborn Above Medicaid Eligibility Threshold (newborn that does not qualify for Medicaid).
(c) CHIP and CHIP Perinatal Program Capitation Rate development:
HHSC will establish base Capitation Rates by analyzing Encounter Data and financial data for each Service Area. This analysis will include a review of historical enrollment and claims experience information; any changes to Covered Services and covered populations; rate changes specified by the Texas Legislature; and any other relevant information. HHSC may modify the Service Area base Capitation Rate using diagnosis based risk adjusters to yield the final Capitation Rates.
(d) Acuity adjustment.
HHSC may evaluate and implement an acuity adjustment methodology, or alternative reasonable methodology, that appropriately reimburses the MCO for acuity and cost differences that deviate from that of the community average, if HHSC in its sole discretion determines that such a methodology is reasonable and appropriate. The community average is a uniform rate for all MCOs in a Service Area, and is determined by combining all the experience for all MCOs in a Service Area to get an average rate for the Service Area.
(e) Value-added Services.
Value-added Services will not be included in the rate-setting process.
Section 10.07 MCO input during rate setting process.
(a) In Service Areas with historical STAR or STAR+PLUS Program participation, MCO must provide certified Encounter Data and financial data as prescribed in Uniform Managed Care Manual Chapter 5.0, “Deliverable Matrix.” Such information may include, without limitation: claims lag information by Rate Cell, capitation expenses, and stop loss reinsurance expenses. HHSC may request clarification or for additional financial information from the MCO. HHSC will notify the MCO of the deadline for submitting a response, which will include a reasonable amount of time for response.
(b) HHSC will allow the MCO to review and comment on data used by HHSC to determine base Capitation Rates. In Service Areas with no historical STAR or STAR+PLUS Program participation, this will include Fee-for-Service data for Rate Periods 1 and 2. HHSC will notify the MCO of deadline for submitting comments, which will include a reasonable amount of time for response. HHSC will not consider comments received after the deadline in its rate analysis.
(c) During the rate setting process, HHSC will conduct at least two (2) meetings with the MCOs. HHSC may conduct the meetings in person, via teleconference, or by another method deemed appropriate by HHSC. Prior to the first meeting, HHSC will provide the MCO with proposed Capitation Rates. During the first meeting, HHSC will describe the process used to generate the proposed Capitation Rates, discuss major changes in the rate setting process, and receive input from the MCO. HHSC will notify the MCO of the deadline for submitting comments, which will include a reasonable amount of time to review and comment on the proposed Capitation Rates and rate setting process. After reviewing such comments, HHSC will conduct a second meeting to discuss the final Capitation Rates and changes resulting from MCO comments, if any.
Section 10.08 Adjustments to Capitation Payments.
(a) Adjustment.
HHSC may adjust a payment made to the MCO for a Member if:
(1) a Member’s eligibility status or program type is changed, corrected as a result of error, or is retroactively adjusted;
(2) the Member is enrolled into the MCO in error;
(3) the Member moves outside the United States;
(4) the Member dies before the first day of the month for which the payment was made; or
(5) payment has been denied by the CMS in accordance with the requirements in 42 C.F.R. § 438.730.
(b) Appeal of adjustment.
The MCO may appeal the adjustment of capitations in the above circumstances using the HHSC dispute resolution process set forth in Section 12.13, Dispute Resolution.
Section 10.09 Delivery Supplemental Payment for CHIP, CHIP Perinatal and STAR MCOs.
(a) The Delivery Supplemental Payment (DSP) is a function of the average delivery cost in each Service Area. Delivery costs include facility and professional charges.
(b) CHIP and STAR MCOs will receive a Delivery Supplemental Payment (DSP) from HHSC for each live or stillbirth by a Member. CHIP Perinatal MCOs will receive a DSP from HHSC for each live or stillbirth of a CHIP Perinate above the Medicaid eligibility threshold (i.e., a Perinate who does not qualify for Medicaid once born, measured at the time of enrollment in the CHIP Perinatal subprogram). CHIP Perinatal MCOs will not receive a DSP from HHSC for a live or stillbirth of a CHIP Perinate at or below the Medicaid eligibility threshold (i.e., a Perinate who qualifies for Medicaid once born). For STAR and CHIP and CHIP Perinatal Program MCOs, the one-time DSP payment is made in the amount identified in the HHSC Managed Care Contract document regardless of whether there is a single birth or there are multiple births at time of delivery. A delivery is the birth of a live born infant, regardless of the duration of the pregnancy, or a stillborn (fetal death) infant of twenty (20) weeks or more of gestation. A delivery does not include a spontaneous or induced abortion, regardless of the duration of the pregnancy.
(c) MCO must submit a monthly DSP Report as described in, Section 8.1.20.2, Reports to the RFP, in the format prescribed in Uniform Managed Care Manual Chapter 5.3.9, Disproportionate Share Hospital Report.
(d) HHSC will pay the Delivery Supplemental Payment within twenty (20) Business Days after receipt of a complete and accurate report from the MCO.
(e) The MCO will not be entitled to Delivery Supplemental Payments for deliveries that are not reported to HHSC within 210 days after the date of delivery, or within thirty (30) days from the date of discharge from the Hospital for the stay related to the delivery, whichever is later.
(f) MCO must maintain complete claims and adjudication disposition documentation, including paid and denied amounts for each delivery. The MCO must submit the documentation to HHSC within five (5) Business Days after receiving a request for such information from HHSC.
Section 10.10 Experience Rebate
(a) MCO’s duty to pay.
(1) General.
At the end of each FSR Reporting Period beginning with FSR Reporting Period 12/13, , the MCO must pay an Experience Rebate if the MCO’s Net Income Before Taxes is greater than the percentage set forth below of the total Revenue for the period. The Experience Rebate is calculated in accordance with the tiered rebate method set forth below. The Net Income Before Taxes and the total Revenues are as measured by the FSR, as reviewed and confirmed by HHSC. The final amount used in the calculation of the percentage may be impacted by various factors herein, including the Loss Carry Forward, the Admin Cap, and/or the Reinsurance Cap.
(2) Basis of Consolidation.
The percentages are calculated on a Consolidated Basis, and include the consolidated Net Income Before Taxes for all of the MCO’s and its Affiliates’ Texas HHSC Programs and Service Areas.
(b) Graduated Experience Rebate Sharing Method.
|
| | |
Pre-tax Income as a % of Revenues | MCO Share | HHSC Share |
≤ 3% | 100% | —% |
> 3% and ≤ 5% | 80% | 20% |
> 5% and ≤ 7% | 60% | 40% |
> 7% and ≤ 9% | 40% | 60% |
> 9% and ≤ 12% | 20% | 80% |
> 12% | —% | 100% |
HHSC and the MCO will share the consolidated Net Income Before Taxes for its HHSC Programs as follows, unless HHSC provides the MCO an Experience Rebate Reward in accordance with Section 6, “Premium Payment Incentives and Disincentives,” and Uniform Managed Care Manual Chapter 6.2, “Financial Incentive Methodology”:
(1) The MCO will retain all the Net Income Before Taxes that is equal to or less than 3% of the total Revenues received by the MCO;
(2) HHSC and the MCO will share that portion of the Net Income Before Taxes that is over 3% and less than or equal to 5% of the total Revenues received, with 80% to the MCO and 20% to HHSC.
(3) HHSC and the MCO will share that portion of the Net Income Before Taxes that is over 5% and less than or equal to 7% of the total Revenues received, with 60% to the MCO and 40% to HHSC.
(4) HHSC and the MCO will share that portion of the Net Income Before Taxes that is over 7% and less than or equal to 9% of the total Revenues received, with 40% to the MCO and 60% to HHSC.
(5) HHSC and the MCO will share that portion of the Net Income Before Taxes that is over 9% and less than or equal to 12% of the total Revenues received, with 20% to the MCO and 80% to HHSC.
(6) HHSC will be paid the entire portion of the Net Income Before Taxes that exceeds 12% of the total Revenues.
(c) Net income Before taxes.
(1) The MCO must compute the Net Income Before Taxes in accordance with applicable federal regulations and Uniform Managed Care Manual Chapter 6.1 “Cost Principles for Expenses,” Chapter 5.3.1.2, “CHIP FSR Instructions for Completion,” Chapter 5.3.1.4, “STAR FSR Instructions for Completion,” ”Chapter 5.3.1.6, “STAR+PLUS FSR Instructions for Completion,” and similar such instructions for other HHSC Programs. The Net Income Before Taxes will be confirmed by HHSC or its agent for the FSR Reporting Period relating to all Revenues and Allowable Expenses incurred pursuant to the Contract. HHSC reserves the right to modify the “Cost Principles for Expenses” and “FSR Instructions for Completion” found in the Uniform Managed Care Manual in accordance with Section 8.05, “Modification of the Uniform Managed Care Manual.”
(2) For purposes of calculating Net Income Before Taxes certain items are omitted from the calculation, as they are not Allowable Expenses; these include:
(i) the payment of an Experience Rebate;
(ii) any interest expense associated with late or underpayment of the Experience Rebate;
(iii) financial incentives, including without limitation the Quality Challenge Award described in Attachment B-1, Section 6.3.2.3; and
(iv) financial disincentives, including without limitation: the Performance-based Capitation Rate Program described in Attachment B-1, Section 6.3.2.2; and
(v) liquidated damages, and any interest expense associated, as described in Attachment B-5.
See Uniform Managed Care Manual Chapter 6.1, “Cost Principles for Expenses.”
(3) Financial incentives will not be reduced by potential increased Experience Rebate payments. Financial disincentives will not be offset in whole or part by potential decreases in Experience Rebate payments.
(4) For FSR reporting purposes, financial incentives incurred must not be reported as an increase in Revenues or as an offset to costs, and any award of such will not increase reported income. Financial disincentives incurred must not be included as reported expenses, and must not reduce reported income. The reporting or recording of any of these incurred items will be done on a memo basis, which is below the income line, and will be listed as separate items.
(d) Carry forward of prior FSR Reporting Period losses.
(1) General.
Losses incurred on a Consolidated Basis for a given FSR Reporting Period may be carried forward to the next FSR Reporting Period, and applied as an offset against consolidated pre-tax net income for determination of any Experience Rebate due. Any such prior losses may be carried forward for the next two (2) contiguous FSR Reporting Periods.
In the case when a loss in a given FSR Reporting Period is carried forward and applied against profits in either or both of the next two (2) FSR Reporting Periods, the loss must first be applied against the first subsequent FSR Reporting Period such that the profit in the first subsequent FSR Reporting Period is reduced to a zero pre-tax income; any additional loss then remaining unapplied may be carried forward to any profit in the next subsequent FSR Reporting Period. In such case, the revised income in the third FSR Reporting Period would be equal to the cumulative income of the three (3) contiguous FSR Reporting Periods. In no case could the loss be carried forward to the fourth FSR Reporting Period or beyond.
Carrying forward of losses may be impacted by the Admin Cap; see Section 10.10.2 (f) below.
Losses incurred in the last or next-to-last FSR Reporting Period of a prior contiguous contract with HHSC may be carried forward up to two (2) FSR Reporting Periods into the first or potentially second FSR Reporting Period of this Contract, if such losses meet all other requirements of both the prior and current contracts.
(2) Basis of consolidation.
In order for a loss to be eligible for potential carry forward as an offset against future income, the MCO must have a negative Net Income Before Taxes for an FSR Reporting Period on a Consolidated Basis.
(e) Settlements for payment.
(1) There may be one or more MCO payment(s) of the State share of the Experience Rebate on income generated for a given FSR Reporting Period under the applicable Programs. The first scheduled payment (the “Primary Settlement”) will equal 100% of the State share of the Experience Rebate as derived from the FSR, and will be paid on the same day the 90-day FSR Report is submitted to HHSC.
The “Primary Settlement,” as utilized herein, refers strictly to what should be paid with the 90-day FSR, and does not refer to the first instance in which an MCO may tender a payment. For example, an MCO may submit a 90-day FSR indicating no Experience Rebate is due, but then submit a 334-day FSR with a higher income and a corresponding Experience Rebate payment. In such case, this initial payment would be subsequent to the Primary Settlement.
(2) The next scheduled payment will be an adjustment to the Primary Settlement, if required, and will be paid on the same day that the 334-day FSR Report is submitted to HHSC if the adjustment is a payment from the MCO to HHSC. Section 10.10(f) describes the interest expenses associated with any payment after the Primary Settlement.
An MCO may make non-scheduled payments at any time to reduce the accumulation of interest under Section 10.10(f). For any nonscheduled payments prior to the 334-day FSR, the MCO is not required to submit a revised FSR, but is required to submit an Experience Rebate calculation form and an adjusted summary page of the FSR. The FSR summary page is labeled “Summary Income Statements (Dollars), All Coverage Groups Combined (FSR, Part I).”
(3) HHSC or its agent may audit or review the FSRs. If HHSC determines that corrections to the FSRs are required, based on an HHSC audit/review or other documentation acceptable to HHSC, then HHSC will make final adjustments. Any payment resulting from an audit or final adjustment will be due from the MCO within 30 days of the earlier of:
(i) the date of the management representation letter resulting from the audit; or
(ii) the date of any invoice issued by HHSC.
Payment within this 30-day timeframe will not relieve the MCO of any interest payment obligation that may exist under Section 10.10(f).
(4) In the event that any Experience Rebates and/or corresponding interest payments owed to the State are not paid by the required due dates, then HHSC may offset such amounts from any future Capitation Payments, or collect such sums directly from the MCO. HHSC may adjust the Experience Rebate if HHSC determines the MCO has paid amounts for goods or services that are not reasonable, necessary, or allowable in accordance with Uniform Managed Care Manual Chapter 6.1, “Cost Principles for Expenses,” Chapter 5.3.1.2, “CHIP FSR Instructions for Completion,” Chapter 5.3.1.4, “STAR FSR Instructions for Completion,” ”Chapter 5.3.1.6, “STAR+PLUS FSR Instructions for Completion,” and the Federal Acquisition Regulations (FAR), or other applicable federal or state regulations. HHSC has final authority in auditing and determining the amount of the Experience Rebate.
(f) Interest on Experience Rebate.
(1) Interest on any Experience Rebate owed to HHSC will be charged beginning 35 days after the due date of the Primary Settlement, as described in Section 10.10(e)(1). Thus, any Experience Rebate due or paid on or after the Primary Settlement will accrue interest starting at 35 days after the due date for the 90-day FSR Report. For
example, any Experience Rebate payment (s) made in conjunction with the 334-day FSR, or as a result of audit findings, will accrue interest back to 35 days after the due-date for submission of the 90-day FSR.
The MCO has the option of preparing an additional FSR based on 120 days of claims run-out (a “120- day FSR”). If a 120-day FSR, and an Experience Rebate payment based on it, are received by HHSC before the interest commencement date above, then such a payment would be counted as part of the Primary Settlement.
(2) If an audit or adjustment determines a downward revision of income after an interest payment has previously been required for the same State Fiscal Year, then HHSC will recalculate the interest and, if necessary, issue a full or partial refund or credit to the MCO.
(3) Any interest obligations that are incurred pursuant to Section 10.10 that are not timely paid will be subject to accumulation of interest as well,
at the same rate as applicable to the underlying Experience Rebate.
(4) All interest assessed pursuant to Section 10.10 will continue to accrue until such point as a payment is received by HHSC, at which point interest on the amount received will stop accruing. If a balance remains at that point that is subject to interest, then the balance will continue to accrue interest. If interim payments are made, then any interest that may be due will only be charged on amounts for the time period during which they remained unpaid. By way of example only, if $100,000 is subject to interest commencing on a given day, and a payment is received for $75,000 45 days after the start of interest, then the $75,000 will be subject to 45 days of interest, and the $25,000 balance will continue to accrue interest until paid. The accrual of interest as defined under Section 10.10(f) will not stop during any period of dispute. If a dispute is resolved in the MCO’s favor, then interest will only be assessed on the revised unpaid amount.
(5) If the MCO incurs an interest obligation pursuant to Section 10.10 for an Experience Rebate payment HHSC will assess such interest at 12% per annum, compounded daily. If any interest rate stipulated hereunder is found by a court of competent jurisdiction to be outside the range deemed legal and enforceable, then the rate hereunder will be adjusted as little as possible so as to be deemed legal and enforceable.
(6) Any such interest expense incurred pursuant to Section 10.10 is not an Allowable Expense for reporting purposes on the FSR.
Section 10.10.1 This Section Intentionally Left Blank
Section 10.10.2 Administrative Expense Cap.
(a) General requirement.
The calculation methodology of Experience Rebates described in Section 10.10 will be adjusted by an Administrative Expense Cap (“Admin Cap.”) The Admin Cap is a calculated maximum amount of administrative expense dollars that can be deducted from Revenues for purposes of determining income subject to the Experience Rebate. While Administrative Expenses may be limited by the Admin Cap to determine Experience Rebates, all valid Allowable Expenses will continue to be reported on the Financial Statistical Reports (FSRs). Thus, the Admin Cap does not impact FSR reporting, but may impact any associated Experience Rebate calculation.
The calculation of any corresponding Experience Rebate due will be subject to limitations on total deductible administrative expenses.
Such limitations will be calculated as follows:
(b) Calculation methodology.
HHSC will determine the administrative expense component of the applicable Capitation Rate structure for each Program prior to each applicable Rate Period. At the conclusion of an FSR Reporting Period, HHSC will apply that predetermined administrative expense component against the MCO’s actually incurred number of Member Months and aggregate premiums received (monthly Capitation Payments plus any Delivery Supplemental Payments, which excludes any investment income or interest earned), to determine the specific Admin Cap, in aggregate dollars, for a given MCO.
If rates are changed during the FSR Reporting Period, HHSC will use this same methodology of multiplying the predetermined HHSC rates for a given month against the ultimate actual number of member months or Revenues that occurred during that month, such that HHSC will apply each month’s actual results against the rates that were in effect for that month.
(c) Data sources.
In determining the amount of Experience Rebate payment to include in the Primary Settlement (or in conjunction with any subsequent payment or settlement), the MCO will need to make the appropriate calculation, in order to assess the impact, if any, of the Admin Cap.
(1) The total premiums paid by HHSC (received by the MCO), and corresponding Member Months, will be taken from the relevant FSR (or audit report) for the FSR Reporting Period.
(2) There are three components of the administrative expense portion of the Capitation Rate structure:
(i) the percentage rate to apply against the total premiums paid (the “percentage of premium” within the administrative expenses),
(ii) the dollar rate per Member Month (the “fixed amount” within the administrative expenses), and
(iii) the portion incorporated into the pharmacy (prescription expense) rate that pertains to prescription administrative expenses.
These will be taken from the supporting details associated with the official notification of final Capitation Rates, as supplied by HHSC. This notification is sent to the MCOs during the annual rate setting process via email, labeled as “the final rate exhibits for your health plan.” The email has one (1) or more spreadsheet files attached, which are particular to the given MCO. The spreadsheet(s) show the fixed amount and percentage of premium components for the administrative component of the Capitation Rate.
The components of the administrative expense portion of the Capitation Rate can also be found on HHSC’s Medicaid website, under “Rate Analysis for Managed Care Services.” Under each Program, there is a separate Rate Setting document for each Rate Period that describes the development of the Capitation Rates. Within each such document, there is a section entitled “Administrative Fees,” where it refers to “the amount allocated for administrative expenses.”
(3) In cases where the administrative expense portion of the Capitation Rate refers to “the greater of (a) [one (1) set of factors], and (b) [another set of factors],” then the Admin Cap will be calculated each way, and the larger of the two (2) results will be the Admin Cap utilized for the determination of any Experience Rebates due.
(d) Example of calculation.
By way of example only, HHSC will calculate the Admin Cap for an FSR Reporting Period as follows:
(1) Multiply the predetermined administrative expense rate structure “fixed amount,” or dollar rate per Member Month (for example, $8.00), by the actual number of Member Months for a given Program and Service Area during the Rate Period (for example, 70,000):
• $8.00 x 70,000 = $560,000.
(2) Multiply the predetermined percent of premiums in the administrative expense rate structure (for example, 5.75%), by the actual aggregate premiums earned for the Program and Service Area during the Rate Period (for example, $6,000,000).
•5.75% x $6,000,000 = $345,000.
(3) Multiply the predetermined pharmacy administrative expense rate (for example, $1.80), by the actual number of Member Months for the Program during the FSR Reporting Period (for example, 70,000):
•$1.80 x $70,000 = $126,000.
(4) Add the totals of items 1, 2 and 3 plus applicable premium taxes and maintenance taxes (for example, $112,000), to determine the Admin Cap for the Program:
• ($560,000 + $345,000+126,000) + $112,000 = $1,143,000.
In this example, $1,143,000 would be the Admin Cap for a single Program for an MCO in a particular FSR Reporting Period.
(e) Consolidation and offsets.
The Admin Cap will be first calculated individually by Program, and then totaled and applied on a Consolidated Basis. There will be one aggregate amount of dollars determined as the Admin Cap for each MCO, which will cover all of an MCO’s and its Affiliates’ Programs and Service Areas. This consolidated Admin Cap will be applied to the administrative expenses of the MCO on a Consolidated Basis. The net impact of the Admin Cap will be applied to the Experience Rebate calculation. Calculation details are provided in the applicable FSR Templates and FSR Instructions in the Uniform Managed Care Manual.
(f) Impact on Loss carry-forward.
For Experience Rebate calculation purposes, the calculation of any loss carry-forward, as described in Section 10.10(d), will be based on the allowable pre-tax loss as determined under the Admin Cap.
(g) MCOs entering a Service Delivery Area or Program.
If an MCO enters a new Service Area or offers a Program that it did not offer under a previous contract, it may be exempt from the Admin Cap for those Service Areas and Programs for a period of time to be determined by HHSC, up through the first FSR Reporting Period or portion thereof.
(h) Service Delivery Areas with only one (1) MCO in a Program.
In Service Areas operating with only one (1) MCO for a Program, HHSC may, at its sole discretion, revise the Admin Cap if its application would create an undue hardship on the MCO.
(i) Unforeseen events.
If, in HHSC’s sole discretion, it determines that unforeseen events have created significant hardships for one (1) or more MCOs, HHSC may revise or temporarily suspend the Admin Cap as it deems necessary.
Section 10.10.3 Reinsurance Cap
Beginning with FSR Reporting Period 12/13, the MCO is subject to the Reinsurance Cap.
Reinsurance is reported on HHSC's FSR report format as: 1) gross reinsurance premiums paid, and 2) reinsurance recoveries received. The premiums paid are treated as a part of medical expenses, and the recoveries received are treated as an offset to those medical expenses (also known as a contra-cost). The net of the gross premiums paid minus the recoveries received is called the net reinsurance cost. The net reinsurance cost, as measured in aggregate dollars over the FSR Reporting Period, divided by the number of member-months for that same period, is referred to as the net reinsurance cost per-member-per-month (PMPM).
The MCO will be limited to a maximum amount of net reinsurance cost PMPM for purposes of calculating the pre-tax net income that is subject to the Experience Rebate. This limitation does not impact an MCO's ability to purchase or arrange for reinsurance. It only impacts what is factored into the Experience Rebate calculation. The maximum amount of allowed net reinsurance cost PMPM (Reinsurance Cap) varies by MCO Program, and is equal to 110% of the net reinsurance cost PMPM contained in the Capitation Rates for the Program during the FSR Reporting Period.
Regardless of the maximum amounts as represented by the Reinsurance Cap, all reinsurance reported on the FSR is subject to audit, and must comply with the UMCM Cost Principles.
Section 10.11 Restriction on assignment of fees.
During the term of the Contract, MCO may not, directly or indirectly, assign to any third party any beneficial or legal interest of the MCO in or to any payments to be made by HHSC pursuant to this Contract. This restriction does not apply to fees the MCO pays to Subcontractors for the performance of the Scope of Work.
Section 10.12 Liability for taxes.
HHSC is not responsible in any way for the payment of any Federal, state or local taxes related to or incurred in connection with the MCO’s performance of this Contract. MCO must pay and discharge any and all such taxes, including any penalties
and interest. In addition, HHSC is exempt from Federal excise taxes, and will not pay any personal property taxes or income taxes levied on MCO or any taxes levied on employee wages.
Section 10.13 Liability for employment-related charges and benefits.
MCO will perform work under this Contract as an independent contractor and not as agent or representative of HHSC. MCO is solely and exclusively liable for payment of all employment-related charges incurred in connection with the performance of this Contract, including but not limited to salaries, benefits, employment taxes, workers compensation benefits, unemployment insurance and benefits, and other insurance or fringe benefits for Staff.
Section 10.14 No additional consideration.
(a) MCO will not be entitled to nor receive from HHSC any additional consideration, compensation, salary, wages, charges, fees, costs, or any other type of remuneration for Services and Deliverables provided under the Contract, except by properly authorized and executed Contract amendments.
(b) No other charges for tasks, functions, or activities that are incidental or ancillary to the delivery of the Services and Deliverables will be sought from HHSC or any other state agency, nor will the failure of HHSC or any other party to pay for such incidental or ancillary services entitle the MCO to withhold Services and Deliverables due under the Agreement.
(c) MCO will not be entitled by virtue of the Contract to consideration in the form of overtime, health insurance benefits, retirement benefits, disability retirement benefits, sick leave, vacation time, paid holidays, or other paid leaves of absence of any type or kind whatsoever.
Section 10.15 Federal Disallowance
If the federal government recoups money from the state for expenses and/or costs that are deemed unallowable by the federal government, the state has the right to, in turn, recoup payments made to the MCOs for these same expenses and/or costs, even if they had not been previously disallowed by the state and were incurred by the MCO, and any such expenses and/or costs would then be deemed unallowable by the state. If the state retroactively recoups money from the MCOs due to a federal disallowance, the state will recoup the entire amount paid to the MCO for the federally disallowed expenses and/or costs, not just the federal portion.
Section 10.16 Supplemental Payments for Medicaid Wrap-Around Services for Outpatient Drugs and Biological Products
The capitation rates do not include the costs of Medicaid wrap-around services for outpatient drugs and biological products for STAR+PLUS Members, as described in Attachment B-1, Section 8.2.13.1.
HHSC will make supplemental payments to the MCO for these Medicaid wrap-around services, based on
encounter data received by HHSC’s Administrative Services Contractor during an encounter reporting period. The first supplemental payment will cover encounter data received from March 1, 2012, to February 28, 2013. Thereafter, supplemental
payments will cover six-month encounter reporting periods. HHSC will make supplemental payments within a reasonable amount of time after the encounter reporting period, generally no later than 95 calendar days after HHSC’s Administrative Services Contractor has processed the encounter data. Supplemental payments will be limited to the actual amounts paid to pharmacy providers for these Medicaid wrap-around services, as represented in “Net Amount Due” field (Field 281) on the National Council for Prescription Drug Programs (NCPDP) encounter transaction. To be eligible for reimbursement, encounters must contain a Financial Arrangement Code “14” in the “Line of Business” field (Field 270) on the NCPDP encounter transaction.
Section 10.17 Pass-through Payments for Provider Rate Increases
The capitation rates do not include the costs of federally-mandated provider rate increases, per PPACA as amended by Section 1202 of the Health Care and Education Reconciliation Act. HHSC will make supplemental payments to the MCO for these rate increases, and the MCO will pass through the full amount of the supplemental payments to qualified providers no later than 30 calendar days after receipt of HHSC's supplemental payment report, contingent upon the receipt of HHSC's payment allocation. Additional information regarding these requirements is located in Attachment B-1, Section 8.2.16, “Supplemental Payments for Qualified Providers.”
Section 10.18 Non-Risk Payments for Second Generation Direct Acting Antivirals for Hepatitis C
The capitation rates do not include the costs of second generation direct acting antivirals (DAAs) for the treatment of hepatitis C as described in Attachment B-1, Section 8.1.21.17. For providing these drugs to Members, HHSC will make non-risk payments to the MCO based on pharmacy encounter data received by HHSC’s Administrative Services Contractor during an encounter reporting period. The first non-risk payment will cover pharmacy encounter data received from the date the drugs are added to the Medicaid and CHIP formularies through the end of that State Fiscal Quarter. Thereafter, non-risk payments will cover quarterly encounter reporting periods. HHSC will make non-risk payments within a reasonable amount of time after the encounter reporting period, generally no later than 95 calendar days after HHSC’s Administrative Services Contractor has processed the encounter data. Non-risk payments will be limited to the actual amounts paid to pharmacy providers for these drugs as represented in “Net Amount Due” field (Field 281) on the National Council for Prescription Drug Programs (NCPDP) encounter transaction. To be eligible for reimbursement, pharmacy encounters must contain a Financial Arrangement Code “14” in the “Line of Business” field (Field 270) on the NCPDP encounter transaction.
Article 11. Disclosure & Confidentiality of Information
Section 11.01 Confidentiality.
(a) MCO and all Subcontractors, consultants, or agents must treat all information that is obtained through performance of the Services under the Contract, including information relating to applicants or recipients of HHSC Programs, as Confidential Information to the extent that confidential treatment is provided under state and federal law, rules, and regulations.
(b) MCO is responsible for understanding the degree to which information obtained through performance of this Contract is confidential under State and Federal law, rules, and regulations.
(c) MCO and all Subcontractors, consultants, or agents may not use any information obtained through performance of this Contract in any manner except as is necessary for the proper discharge of obligations and securing of rights under the Contract.
(d) MCO must have a system in effect to protect all records and all other documents deemed confidential under this Contract that are maintained in connection with the activities funded under the Contract. Any disclosure or transfer of Confidential Information by MCO, including information required by HHSC, will be in accordance with applicable law. If the MCO receives a request for information deemed confidential under this Contract, the MCO will immediately notify HHSC of such request, and will make reasonable efforts to protect the information from public disclosure.
(e) In addition to the requirements expressly stated in this Section, MCO must comply with any policy, rule, or reasonable requirement of HHSC that relates to the safeguarding or disclosure of information relating to Members, MCO's operations, or MCO's performance of the Contract.
(f) In the event of the expiration of the Contract or termination of the Contract for any reason, all Confidential Information disclosed to and all copies thereof made by the MCOI must be returned to HHSC or, at HHSC's option, erased or destroyed. MCO must provide HHSC certificates evidencing such destruction.
(g) The obligations in this Section must not restrict any disclosure by the MCO pursuant to any applicable law, or by order of any court or government agency, provided that the MCO must give prompt notice to HHSC of such order.
(h) With the exception of confidential Member information, Confidential Information must not be afforded the protection of the Contract if such data was:
(1) Already known to the receiving Party without restrictions at the time of its disclosure by the furnishing Party;
(2) Independently developed by the receiving Party without reference to the furnishing Party's Confidential Information;
(3) Rightfully obtained by the other Party without restriction from a third party after its disclosure by the furnishing Party;
(4) Publicly available other than through the fault or negligence of the other Party; or
(5) Lawfully released without restriction to anyone.
Section 11.02 Disclosure of HHSC’s Confidential Information.
(a) MCO will immediately report to HHSC any and all unauthorized disclosures or uses of HHSC’s Confidential Information of which it or its Subcontractors, consultants, or agents is aware or has knowledge. MCO acknowledges that any publication or disclosure of HHSC’s Confidential Information to others may cause immediate and irreparable harm to HHSC and may constitute a violation of State or federal laws. If MCO, its Subcontractors, consultants, or agents should publish or disclose such Confidential Information to others without authorization, HHSC will immediately be entitled to injunctive relief or any other remedies to which it is entitled under law or equity. HHSC will have the right to recover from MCO all damages and liabilities caused by or arising from MCO’s, its Subcontractors’, consultants’, or agents’ failure to protect HHSC’s Confidential Information. MCO will defend with counsel approved by HHSC, indemnify and hold harmless HHSC from all damages, costs, liabilities, and expenses caused by or arising from MCO’s or its Subcontractors’, consultants’ or agents’ failure to protect HHSC’s Confidential Information. HHSC will not unreasonably withhold approval of counsel selected by the MCO.
(b) MCO will require its Subcontractors, consultants, and agents to comply with the terms of this provision.
Section 11.03 Member Records
(a) MCO must comply with the requirements of state and federal laws, including the HIPAA requirements set forth in Section 7.07, regarding the transfer of Member Records.
(b) If at any time during the Contract Term this Contract is terminated, HHSC may require the transfer of Member Records, upon written notice to MCO, to another entity, as consistent with federal and state laws and applicable releases.
(c) The term “Member Record” for this Section means only those administrative, enrollment, case management and other such records maintained by MCO and is not intended to include patient records maintained by participating Network Providers.
Section 11.04 Requests for public information.
(a) When the MCO produces reports or other forms of information that the MCO believes consist of proprietary or otherwise confidental information, the MCO must clearly mark such information as confidential information or provide written notice to HHSC that it considers the information confidential.
(b) If HHSC receives a request, filed in accordance with the Texas Public Information Act (“Act,”) seeking information that has been identified by the MCO as proprietary or otherwise confidential, HHSC will deliver a copy of the request for public information to MCO, in accordance with the requirements of the Act.
(c) With respect to any information that is the subject of a request for disclosure, MCO is required to demonstrate to the Texas Office of Attorney General the specific reasons why the requested information is confidential or otherwise excepted from required public disclosure under law. MCO will provide HHSC with copies of all such communications.
Section 11.05 Privileged Work Product.
(a) MCO acknowledges that HHSC asserts that privileged work product may be prepared in anticipation of litigation and that MCO is performing the Services with respect to privileged work product as an agent of HHSC, and that all matters related thereto are protected from disclosure by the Texas Rules of Civil Procedure, Texas Rules of Evidence, Federal Rules of Civil Procedure, or Federal Rules of Evidence.
(b) HHSC will notify MCO of any privileged work product to which MCO has or may have access. After the MCO is notified or otherwise becomes aware that such documents, data, database, or communications are privileged work product, only MCO personnel, for whom such access is necessary for the purposes of providing the Services, may have access to privileged work product.
(c) If MCO receives notice of any judicial or other proceeding seeking to obtain access to HHSC’s privileged work product, MCO will:
(1) Immediately notify HHSC; and
(2) Use all reasonable efforts to resist providing such access.
(d) If MCO resists disclosure of HHSC’s privileged work product in accordance with this Section, HHSC will, to the extent authorized under Civil Practices and Remedies Code or other applicable State law, have the right and duty to:
(1) Represent MCO in such resistance;
(2) Retain counsel to represent MCO; or
(3) Reimburse MCO for reasonable attorneys' fees and expenses incurred in resisting such access.
(e) If a court of competent jurisdiction orders MCO to produce documents, disclose data, or otherwise breach the confidentiality obligations imposed in the Contract, or otherwise with respect to maintaining the confidentiality, proprietary nature, and secrecy of privileged work product, MCO will not be liable for breach of such obligation.
Section 11.06 Unauthorized acts.
Each Party agrees to:
(1) Notify the other Party promptly of any unauthorized possession, use, or knowledge, or attempt thereof, by any person or entity that may become known to it, of any HHSC Confidential Information or any information identified by the MCO as confidential or proprietary;
(2) Promptly furnish to the other Party full details of the unauthorized possession, use, or knowledge, or attempt thereof, and use reasonable efforts to assist the other Party in investigating or preventing the reoccurrence of any unauthorized possession, use, or knowledge, or attempt thereof, of Confidential Information;
(3) Cooperate with the other Party in any litigation and investigation against third Parties deemed necessary by such Party to protect its proprietary rights; and
(4) Promptly prevent a reoccurrence of any such unauthorized possession, use, or knowledge such information.
Section 11.07 Legal action.
Neither party may commence any legal action or proceeding in respect to any unauthorized possession, use, or knowledge, or attempt thereof by any person or entity of HHSC’s Confidential Information or information identified by the MCO as confidential or proprietary, which action or proceeding identifies the other Party’s information without such Party’s consent.
Section 11.08 Information Security
The MCO and all Subcontractors, consultants, or agents must comply with all applicable laws, rules, and regulations regarding information security, including without limitation the following:
(1) Health and Human Services Enterprise Information Security Standards and Guidelines;
(2) Title 1, Sections 202.1 and 202.3 through 202.28, Texas Administrative Code;
(3) The Health Insurance Portability and Accountability Act of 1996 (HIPAA); and
(4) The Health Information Technology for Economic and Clinical Health Act (HITECH Act).
Article 12. Remedies & Disputes
Section 12.01 Understanding and expectations.
The remedies described in this Section are directed to MCO’s timely and responsive performance of the Services and production of Deliverables, and the creation of a flexible and responsive relationship between the Parties. The MCO is expected to meet or exceed all HHSC objectives and standards, as set forth in the Contract. All areas of responsibility and all Contract requirements will be subject to performance evaluation by HHSC. Performance reviews may be conducted at the discretion of HHSC at any time and may relate to any responsibility and/or requirement. Any and all responsibilities and/or requirements not fulfilled may be subject to the remedies set forth in the Contract.
Section 12.02 Tailored remedies.
(a) Understanding of the Parties.
MCO agrees and understands that HHSC may pursue tailored contractual remedies for noncompliance with the Contract. At any time and at its discretion, HHSC may impose or pursue one (1) or more remedies for each item of noncompliance and will determine remedies on a case-by-case basis. HHSC’s pursuit or non-pursuit of a tailored remedy does not constitute a waiver of any other remedy that HHSC may have at law or equity.
(b) Notice and opportunity to cure for non-material breach.
(1) HHSC will notify MCO in writing of specific areas of MCO performance that fail to meet performance expectations, standards, or schedules set forth in the Contract, but that, in the determination of HHSC, do not result in a material deficiency or delay in the implementation or operation of the Services.
(2) MCO will, within five (5) Business Days (or another date approved by HHSC) of receipt of written notice of a non-material deficiency, provide the HHSC Project Manager a written response that:
(i) Explains the reasons for the deficiency, MCO’s plan to address or cure the deficiency, and the date and time by which the deficiency will be cured; or
(ii) If MCO disagrees with HHSC’s findings, its reasons for disagreeing with HHSC’s findings.
(3) MCO’s proposed cure of a non-material deficiency is subject to the approval of HHSC. MCO’s repeated commission of non-material deficiencies or repeated failure to resolve any such deficiencies may be regarded by HHSC as a material deficiency and entitle HHSC to pursue any other remedy provided in the Contract or any other appropriate remedy HHSC may have at law or equity.
(c) Corrective action plan.
(1) At its option, HHSC may require MCO to submit to HHSC a written plan (the “Corrective Action Plan”) to correct or resolve a material breach of this Contract, as determined by HHSC.
(2) The Corrective Action Plan must provide:
(i) A detailed explanation of the reasons for the cited deficiency;
(ii) MCO’s assessment or diagnosis of the cause; and
(iii) A specific proposal to cure or resolve the deficiency.
(3) The Corrective Action Plan must be submitted by the deadline set forth in HHSC’s request for a Corrective Action Plan. The Corrective Action Plan is subject to approval by HHSC, which will not unreasonably be withheld.
(4) HHSC will notify MCO in writing of HHSC’s final disposition of HHSC’s concerns. If HHSC accepts MCO’s proposed Corrective Action Plan, HHSC may:
(i) Condition such approval on completion of tasks in the order or priority that HHSC may reasonably prescribe;
(ii) Disapprove portions of MCO’s proposed Corrective Action Plan; or
(iii) Require additional or different corrective action(s).
Notwithstanding the submission and acceptance of a Corrective Action Plan, MCO remains responsible for achieving all written performance criteria.
(5) HHSC’s acceptance of a Corrective Action Plan under this Section will not:
(i) Excuse MCO’s prior substandard performance;
(ii) Relieve MCO of its duty to comply with performance standards; or
(iii) Prohibit HHSC from assessing additional tailored remedies or pursuing other appropriate remedies for continued substandard performance.
(d) Administrative remedies.
(1) At its discretion, HHSC may impose one (1) or more of the following remedies for each item of material noncompliance and will determine the scope and severity of the remedy on a case-by-case basis:
(i) Assess liquidated damages in accordance with Attachment B-3, “Liquidated Damages Matrix;”
(ii) Conduct accelerated monitoring of the MCO. Accelerated monitoring includes more frequent or more extensive monitoring by HHSC or its agent;
(iii) Require additional, more detailed, financial and/or programmatic reports to be submitted by MCO;
(iv) Require additional and/or more detailed financial and/or programmatic audits or other reviews of the MCO;
(v) Decline to renew or extend the Contract;
(vi) Appoint temporary management under the circumstances described in 42 C.F.R. §438.706;
(vii) Initiate disenrollment of a Member or Members;
(viii) Suspend enrollment of Members;
(ix) Withhold or recoup payment to MCO;
(x) Require forfeiture of all or part of the MCO’s bond; or
(xi) Terminate the Contract in accordance with Section 12.03, “Termination by HHSC.”
(2) For purposes of the Contract, an item of material noncompliance means a specific action of MCO that:
(i) Violates a material provision of the Contract;
(ii) Fails to meet an agreed measure of performance; or
(iii) Represents a failure of MCO to be reasonably responsive to a reasonable request of HHSC relating to the Scope of Work for information, assistance, or support within the timeframe specified by HHSC.
(3) HHSC will provide notice to MCO of the imposition of an administrative remedy in accordance with this Section, with the exception of accelerated monitoring, which may be unannounced. HHSC may require MCO to file a written response in accordance with this Section.
(4) The Parties agree that a State or Federal statute, rule, regulation, or Federal guideline will prevail over the provisions of this Section unless the statute, rule, regulation, or guidelines can be read together with this Section to give effect to both.
(e) Damages.
(1) HHSC will be entitled to monetary damages in the form of actual, consequential, direct, indirect, special, and/or liquidated damages resulting from Contractor’s Breach of this Agreement In some cases, the actual damage to
HHSC or State of Texas as a result of MCO’s failure to meet any aspect of the responsibilities of the Contract and/or to meet specific performance standards set forth in the Contract are difficult or impossible to determine with precise accuracy. Therefore, liquidated damages will be assessed in writing against and paid by the MCO in for failure to meet any aspect of the responsibilities of the Contract and/or to meet the specific performance standards identified by the HHSC in Attachment B-3, “Deliverables/Liquidated Damages Matrix.” Liquidated damages will be assessed if HHSC determines such failure is the fault of the MCO (including the MCO’S Subcontractors, agents and/or consultants) and is not materially caused or contributed to by HHSC or its agents. If at any time HHSC determines the MCO has not met any aspect of the responsibilities of the Contract and/or the specific performance standards due to mitigating circumstances, HHSC reserves the right to waive all or part of the liquidated damages. All such waivers must be in writing, contain the reasons for the waiver, and be signed by the appropriate executive of HHSC.
(2) The liquidated damages prescribed in this Section are not intended to be in the nature of a penalty, but are intended to be reasonable estimates of HHSC’s projected financial loss and damage resulting from the MCO’s nonperformance, including financial loss as a result of project delays. Accordingly, in the event MCO fails to perform in accordance with the Contract, HHSC may assess liquidated damages as provided in this Section.
(3) If MCO fails to perform any of the Services described in the Contract, HHSC may assess liquidated damages for each occurrence of a liquidated damages event, to the extent consistent with HHSC's tailored approach to remedies and Texas law.
(4) HHSC may elect to collect liquidated damages:
(i) Through direct assessment and demand for payment delivered to MCO; or
(ii) By deduction of amounts assessed as liquidated damages as set-off against payments then due to MCO or that become due at any time after assessment of the liquidated damages. HHSC will make deductions until the full amount payable by the MCO is collected by HHSC.
(f) Equitable Remedies
(1) MCO acknowledges that, if MCO breaches (or attempts or threatens to breach) its material obligation under this Contract, HHSC may be irreparably harmed. In such a circumstance, HHSC may proceed directly to court to pursue equitable remedies.
(2) If a court of competent jurisdiction finds that MCO breached (or attempted or threatened to breach) any such obligations, MCO agrees that without any additional findings of irreparable injury or other conditions to injunctive relief, it will not oppose the entry of an appropriate order compelling performance by MCO and restraining it from any further breaches (or attempted or threatened breaches).
(g) Suspension of Contract
(1) HHSC may suspend performance of all or any part of the Contract if:
(i) HHSC determines that MCO has committed a material breach of the Contract;
(ii) HHSC has reason to believe that MCO has committed, or assisted in the commission of, Fraud, Abuse, Waste, malfeasance, misfeasance, or nonfeasance by any party concerning the Contract;
(iii) HHSC determines that the MCO knew, or should have known, of Fraud, Abuse, Waste, malfeasance, or nonfeasance by any party concerning the Contract, and the MCO failed to take appropriate action; or
(iv) HHSC determines that suspension of the Contract in whole or in part is in the best interests of the State of Texas or the HHSC Programs.
(2) HHSC will notify MCO in writing of its intention to suspend the Contract in whole or in part. Such notice will:
(i) Be delivered in writing to MCO;
(ii) Include a concise description of the facts or matter leading to HHSC’s decision; and
(iii) Unless HHSC is suspending the contract for convenience, request a Corrective Action Plan from MCO or describe actions that MCO may take to avoid the contemplated suspension of the Contract.
Section 12.03 Termination by HHSC.
This Contract will terminate upon the Expiration Date. In addition, prior to completion of the Contract Term, all or a part of this Contract may be terminated for any of the following reasons:
(a) Termination in the best interest of HHSC.
HHSC may terminate the Contract without cause at any time when, in its sole discretion, HHSC determines that termination is in the best interests of the State of Texas. HHSC will provide reasonable advance written notice of the termination, as it deems appropriate under the circumstances. The termination will be effective on the date specified in HHSC’s notice of termination.
(b) Termination for cause.
HHSC reserves the right to terminate this Contract, in whole or in part, upon the following conditions:
(1) Assignment for the benefit of creditors, appointment of receiver, or inability to pay debts.
HHSC may terminate this Contract at any time if MCO:
(i) Makes an assignment for the benefit of its creditors;
(ii) Admits in writing its inability to pay its debts generally as they become due; or
(iii) Consents to the appointment of a receiver, trustee, or liquidator of MCO or of all or any part of its property.
(2) Failure to adhere to laws, rules, ordinances, or orders.
HHSC may terminate this Contract if a court of competent jurisdiction finds MCO failed to adhere to any laws, ordinances, rules, regulations or orders of any public authority having jurisdiction and such violation prevents or substantially impairs performance of MCO’s duties under this Contract. HHSC will provide at least 30 days advance written notice of such termination.
(3) Breach of confidentiality.
HHSC may terminate this Contract at any time if MCO breaches confidentiality laws with respect to the Services and Deliverables provided under this Contract.
(4) Failure to maintain adequate personnel or resources.
HHSC may terminate this Contract if, after providing notice and an opportunity to correct, HHSC determines that MCO has failed to supply personnel or resources and such failure results in MCO’s inability to fulfill its duties under this Contract. HHSC will provide at least 30 days advance written notice of such termination.
(5) Termination for gifts and gratuities.
(i) HHSC may terminate this Contract at any time following the determination by a competent judicial or quasi-judicial authority and MCO’s exhaustion of all legal remedies that MCO, its employees, agents or representatives have either offered or given any thing of value to an officer or employee of HHSC or the State of Texas in violation of state law.
(ii) MCO must include a similar provision in each of its Subcontracts and must enforce this provision against a Subcontractor who has offered or given any thing of value to any of the persons or entities described in this Section, whether or not the offer or gift was in MCO’s behalf.
(iii) Termination of a Subcontract by MCO pursuant to this provision will not be a cause for termination of the Contract unless:
(a) MCO fails to replace such terminated Subcontractor within a reasonable time; and
(b) Such failure constitutes cause, as described in this Subsection 12.03(b).
(iv) For purposes of this Section, a “thing of value” means any item of tangible or intangible property that has a monetary value of more than $50.00 and includes, but is not limited to, cash, food, lodging, entertainment, and charitable contributions. The term does not include contributions to holders of public office or candidates for public office that are paid and reported in accordance with state and/or federal law.
(6) Termination for non-appropriation of funds.
Notwithstanding any other provision of this Contract, if funds for the continued fulfillment of this Contract by HHSC are at any time not forthcoming or are insufficient, through failure of any entity to appropriate funds or otherwise, then HHSC will have the right to terminate this Contract at no additional cost and with no penalty whatsoever by giving prior written notice documenting the lack of funding. HHSC will provide at least 30 days advance written notice of such termination. HHSC will use reasonable efforts to ensure appropriated funds are available.
(7) Judgment and execution.
(i) HHSC may terminate the Contract at any time if judgment for the payment of money in excess of $500,000.00 that is not covered by insurance, is rendered by any court or governmental body against MCO, and MCO does not:
(a) Discharge the judgment or provide for its discharge in accordance with the terms of the judgment;
(b) Procure a stay of execution of the judgment within 30 days from the date of entry thereof; or
(c) Perfect an appeal of such judgment and cause the execution of such judgment to be stayed during the appeal, providing such financial reserves as may be required under generally accepted accounting principles.
(ii) If a writ or warrant of attachment or any similar process is issued by any court against all or any material portion of the property of MCO, and such writ or warrant of attachment or any similar process is not released or bonded within 30 days after its entry, HHSC may terminate the Contract in accordance with this Section.
(8) Termination for Criminal Conviction
HHSC will have the right to terminate the Contract in whole or in part, or require the replacement of a Material Subcontractor, if the MCO or a Material Subcontractor is convicted of a criminal offense in a state or federal court:
(i) Related to the delivery of an item or service;
(ii) Related to the neglect or abuse of patients in connection with the delivery of an item or service;
(iii) Consisting of a felony related to fraud, theft, embezzlement, breach of fiduciary responsibility, or other financial misconduct, or
(iv) resulting in a penalty or fine in the amount of $500,000 or more in a state or federal administrative proceeding.
(9) Termination for MCO’S material breach of the Contract.
HHSC will have the right to terminate the Contract in whole or in part if HHSC determines, at its sole discretion, that MCO has materially breached the Contract. HHSC will provide at least 30 days advance written notice of such termination, unless HHSC in its reasonable determination finds that a shorter notice period is warranted.
Section 12.04 Termination by MCO.
(a) Failure to pay.
MCO may terminate this Contract if HHSC fails to pay the MCO undisputed charges when due as required under this Contract. Retaining premium, recoupment, sanctions, or penalties that are allowed under this Contract or that result from the MCO’s failure to perform or the MCO’s default under the terms of this Contract is not cause for termination. Termination for failure to pay does not release HHSC from the obligation to pay undisputed charges for services provided prior to the termination date.
If HHSC fails to pay undisputed charges when due, then the MCO may submit a notice of intent to terminate for failure to pay in accordance with the requirements of Subsection 12.04(d). If HHSC pays all undisputed amounts then due within 30 days after receiving the notice of intent to terminate, the MCO cannot proceed with termination of the Contract under this Article.
(b) Change to HHSC Uniform Managed Care Manual.
MCO may terminate this agreement if the Parties are unable to resolve a dispute concerning a material and substantive change to the Uniform Managed Care Manual (a change that materially and substantively alters the MCO’s ability to fulfill its obligations under the Contract). MCO must submit a notice of intent to terminate due to a material and substantive change in the Uniform Managed Care Manual no later than 30 days after the effective date of the policy change. HHSC will not enforce the policy change for the MCO during the period of time between the receipt of the notice of intent to terminate and the effective date of termination.
(c) Change to Capitation Rate.
If HHSC proposes a modification to the Capitation Rate that is unacceptable to the MCO, the MCO may terminate the Contract. MCO must submit a written notice of intent to terminate due to a change in the Capitation Rate no later than 30 days after HHSC’s notice of the proposed change. HHSC will not enforce the rate change against the MCO during the period of time between the receipt of the notice of intent to terminate and the effective date of termination.
(d) Notice of intent to terminate.
In order to terminate the Contract pursuant to this Section, MCO must give HHSC at least 90 days written notice of intent to terminate. The termination date will be calculated as the last day of the month following 90 days from the date the notice of intent to terminate is received by HHSC.
Section 12.05 Termination by mutual agreement.
This Contract may be terminated by mutual written agreement of the Parties.
Section 12.06 Effective date of termination.
Except as otherwise provided in this Contract, termination will be effective as of the date specified in the notice of termination.
Section 12.07 Extension of termination effective date.
The Parties may extend the effective date of termination one (1) or more times by mutual written agreement.
Section 12.08 Payment and other provisions at Contract termination.
(a) In the event of termination pursuant to this Article, HHSC will pay the Capitation Payment for Services and Deliverables rendered through the effective date of termination. All pertinent provisions of the Contract will form the basis of settlement.
(b) MCO must provide HHSC all reasonable access to records, facilities, and documentation as is required to efficiently and expeditiously close out the Services and Deliverables provided under this Contract.
(c) MCO must prepare a Turnover Plan, which is acceptable to and approved by HHSC. The Turnover Plan will be implemented during the time period between receipt of notice and the termination date, in accordance with Attachment B-1, RFP Section 9.
Section 12.09 Modification of Contract in the event of remedies.
HHSC may propose a modification of this Contract in response to the imposition of a remedy under this Article. Any modifications under this Section must be reasonable, limited to the matters causing the exercise of a remedy, in writing, and executed in accordance with Article 8, “Amendments and Modifications.” MCO must negotiate such proposed modifications in good faith.
Section 12.10 Turnover assistance.
Upon receipt of notice of termination of the Contract by HHSC, MCO will provide any turnover assistance reasonably necessary to enable HHSC or its designee to effectively close out the Contract and move the work to another vendor or to perform the work itself.
Section 12.11 Rights upon termination or expiration of Contract.
In the event that the Contract is terminated for any reason, or upon its expiration, HHSC will, at HHSC's discretion, retain ownership of any and all associated work products, Deliverables and/or documentation in whatever form that they exist.
Section 12.12 MCO responsibility for associated costs.
If HHSC terminates the Contract for Cause, the MCO will be responsible to HHSC for all reasonable costs incurred by HHSC, the State of Texas, or any of its administrative agencies to replace the MCO. These costs include, but are not limited to, the costs of procuring a substitute vendor and the cost of any claim or litigation that is reasonably attributable to MCO’s failure to perform any Service in accordance with the terms of the Contract
Section 12.13 Dispute resolution.
(a) General agreement of the Parties.
The Parties mutually agree that the interests of fairness, efficiency, and good business practices are best served when the Parties employ all reasonable and informal means to resolve any dispute under this Contract. The Parties express their mutual commitment to using all reasonable and informal means of resolving disputes prior to invoking a remedy provided elsewhere in this Section.
(b) Duty to negotiate in good faith.
Any dispute that in the judgment of any Party to this Contract may materially or substantially affect the performance of any Party will be reduced to writing and delivered to the other Party. The Parties must then negotiate in good faith and use
every reasonable effort to resolve such dispute and the Parties must not resort to any formal proceedings unless they have reasonably determined that a negotiated resolution is not possible. The resolution of any dispute disposed of by Contract between the Parties must be reduced to writing and delivered to all Parties within ten (10) Business Days.
(c) Claims for breach of Contract.
(1) General requirement. MCO’s claim for breach of this Contract will be resolved in accordance with the dispute resolution process established by HHSC in accordance with Chapter 2260, Texas Government Code.
(2) Negotiation of claims. The Parties expressly agree that the MCO’s claim for breach of this Contract that the Parties cannot resolve in the ordinary course of business or through the use of all reasonable and informal means will be submitted to the negotiation process provided in Chapter 2260, Subchapter B, Texas Government Code.
(i) To initiate the process, MCO must submit written notice to HHSC that specifically states that MCO invokes the provisions of Chapter 2260, Subchapter B, Texas Government Code. The notice must comply with the requirements of Title 1, Chapter 392, Subchapter B of the Texas Administrative Code.
(ii) The Parties expressly agree that the MCO’s compliance with Chapter 2260, Subchapter B, Texas Government Code, will be a condition precedent to the filing of a contested case proceeding under Chapter 2260, Subchapter C, of the Texas Government Code.
(3) Contested case proceedings. The contested case process provided in Chapter 2260, Subchapter C, Texas Government Code, will be MCO’s sole and exclusive process for seeking a remedy for any and all alleged breaches of contract by HHSC if the Parties are unable to resolve their disputes under Subsection (c)(2) of this Section.
The Parties expressly agree that compliance with the contested case process provided in Chapter 2260, Subchapter C, Texas Government Code, will be a condition precedent to seeking consent to sue from the Texas Legislature under Chapter 107, Civil Practices & Remedies Code. Neither the execution of this Contract by HHSC nor any other conduct of any representative of HHSC relating to this Contract will be considered a waiver of HHSC’s sovereign immunity to suit.
(4) HHSC rules. The submission, processing and resolution of MCO’s claim is governed by the rules adopted by HHSC pursuant to Chapter 2260, Texas Government Code, found at Title 1, Chapter 392, Subchapter B of the Texas Administrative Code.
(5) MCO’s duty to perform. Neither the occurrence of an event constituting an alleged breach of contract nor the pending status of any claim for breach of contract is grounds for the suspension of performance, in whole or in part, by MCO of any duty or obligation with respect to the performance of this Contract. Any changes to the Contract as a result of a dispute resolution will be implemented in accordance with Article 8, “Amendments and Modifications.”
Section 12.14 Liability of MCO.
(a) MCO bears all risk of loss or damage to HHSC or the State due to:
(1) Defects in Services or Deliverables;
(2) Unfitness or obsolescence of Services or Deliverables; or
(3) The negligence or intentional misconduct of MCO or its employees, agents, consultants, Subcontractors, or representatives.
(b) MCO must, at the MCO’s own expense, defend with counsel approved by HHSC, indemnify, and hold harmless HHSC and State employees, officers, directors, contractors and agents from and against any losses, liabilities, damages, penalties, costs, fees, and expenses from any claim or action for property damage, bodily injury or death, to the extent caused by or arising from the negligence or intentional misconduct of the MCO and its employees, officers, agents, consultants, or Subcontractors. HHSC will not unreasonably withhold approval of counsel selected by MCO.
(c) MCO will not be liable to HHSC for any loss, damages or liabilities attributable to or arising from the failure of HHSC or any state agency to perform a service or activity in connection with this Contract.
Section 12.15 Pre-termination Process.
The following process will apply when HHSC terminates the Agreement for any reason set forth in Section 12.03(b), “Termination for Cause,” other than Subpart 6, “Termination for Non-appropriation of Funds.” HHSC will provide the MCO with reasonable advance written notice of the proposed termination, as it deems appropriate under the circumstances. The notice will include the reason for the proposed termination, the proposed effective date of the termination, and the time and place where the parties will meet regarding the proposed termination. During this meeting, the MCO may present written information explaining why HHSC should not affirm the proposed termination. HHSC’s Associate Commissioner for Medicaid and CHIP will consider the written information, if any, and will provide the MCO with a written notice of HHSC’s final decision affirming or reversing the termination. An affirming decision will include the effective date of termination.
The pre-termination process described herein will not limit or otherwise reduce the parties’ rights and responsibilities under Section 12.13, “Dispute Resolution;” however, HHSC’s final decision to terminate is binding and is not subject to review by the State Office of Administrative Hearings under Chapter 2260, Texas Government Code.
Article 13. Assurances & Certifications
Section 13.01 Proposal certifications.
MCO acknowledges its continuing obligation to comply with the requirements of the certifications contained in its Proposal, and will immediately notify HHSC of any changes in circumstances affecting the certifications.
Section 13.02 Conflicts of interest.
(a) Representation.
MCO agrees to comply with applicable state and federal laws, including 41 U.S.C. § 423, rules, and regulations regarding conflicts of interest in the performance of its duties under this Contract. MCO warrants that it has no interest and will not acquire any direct or indirect interest that would conflict in any manner or degree with its performance under this Contract.
(b) General duty regarding conflicts of interest.
MCO will establish safeguards to prohibit employees from using their positions for a purpose that constitutes or presents the appearance of personal or organizational conflict of interest, or personal gain. MCO will operate with complete independence and objectivity without actual, potential or apparent conflict of interest with respect to the activities conducted under this Contract.
Section 13.03 Organizational conflicts of interest.
(a) Definition.
An organizational conflict of interest is a set of facts or circumstances, a relationship, or other situation under which an MCO or a Subcontractor has past, present, or currently planned personal or financial activities or interests that either directly or indirectly:
(1) Impairs or diminishes the MCO’s or Subcontractor’s ability to render impartial or objective assistance or advice to HHSC; or
(2) Provides the MCO or Subcontractor an unfair competitive advantage in future HHSC procurements (excluding the award of this Contract).
(b) Warranty.
Except as otherwise disclosed and approved by HHSC prior to the Effective Date of the Contract, MCO warrants that, as of the Effective Date and to the best of its knowledge and belief, there are no relevant facts or circumstances that could give rise to an organizational conflict of interest affecting this Contract. MCO affirms that it has neither given, nor intends to give, at any time hereafter, any economic opportunity, future employment, gift, loan, gratuity, special discount, trip, favor, or service to a public servant or any employee or representative of same, at any time during the procurement process or in connection with the procurement process except as allowed under relevant state and federal law.
(c) Continuing duty to disclose.
(1) MCO agrees that, if after the Effective Date, MCO discovers or is made aware of an organizational conflict of interest, MCO will immediately and fully disclose such interest in writing to the HHSC project manager. In addition, MCO must promptly disclose any relationship that might be perceived or represented as a conflict after its discovery by MCO or by HHSC as a potential conflict. HHSC reserves the right to make a final determination regarding the existence of conflicts of interest, and MCO agrees to abide by HHSC’s decision.
(2) The disclosure will include a description of the actions that MCO has taken or proposes to take to avoid or mitigate such conflicts.
(d) Remedy.
If HHSC determines that an organizational conflict of interest exists, HHSC may, at its discretion, terminate the Contract pursuant to Subsection 12.03(b)(9).
If HHSC determines that MCO was aware of an organizational conflict of interest before the award of this Contract and did not disclose the conflict to the contracting officer, such nondisclosure will be considered a material breach of the Contract. Furthermore, such breach may be submitted to the Office of the Attorney General, Texas Ethics Commission, or appropriate State or Federal law enforcement officials for further action.
(e) Flow-down obligation.
MCO must include the provisions of this Section in all Subcontracts for work to be performed similar to the service provided by MCO, and the terms "Contract," "MCO," and "project manager" modified appropriately to preserve the state's rights.
Section 13.04 HHSC personnel recruitment prohibition.
MCO has not retained or promised to retain any person or company, or utilized or promised to utilize a consultant that participated in HHSC’s development of specific criteria of the RFP or who participated in the selection of the MCO for this Contract.
Unless authorized in writing by HHSC, MCO will not recruit or employ any HHSC personnel who have worked on projects relating to the subject matter of this Contract, or who have had any influence on decisions affecting the subject matter of this Contract, for two (2) years following the completion of this Contract.
Section 13.05 Anti-kickback provision.
MCO certifies that it will comply with the Anti-Kickback Act of 1986, 41 U.S.C. §51-58 and Federal Acquisition Regulation 52.203-7, to the extent applicable.
Section 13.06 Debt or back taxes owed to State of Texas.
In accordance with Section 403.055 of the Texas Government Code, MCO agrees that any payments due to MCO under the Contract will be first applied toward any debt and/or back taxes MCO owes State of Texas. MCO further agrees that payments will be so applied until such debts and back taxes are paid in full.
Section 13.07 Outstanding debts and judgments.
MCO certifies that it is not presently indebted to the State of Texas, and that MCO is not subject to an outstanding judgment in a suit by State of Texas against MCO for collection of the balance. For purposes of this Section, an indebtedness is any amount or sum of money that is due and owing to the State of Texas and is not currently under dispute. A false statement regarding MCO’s status will be treated as a material breach of this Contract and may be grounds for termination at the option of HHSC.
Article 14. Representations & Warranties
Section 14.01 Authorization.
(a) The execution, delivery and performance of this Contract has been duly authorized by MCO and no additional approval, authorization or consent of any governmental or regulatory agency is required to be obtained in order for MCO to enter into this Contract and perform its obligations under this Contract.
(b) MCO has obtained all licenses, certifications, permits, and authorizations necessary to perform the Services under this Contract and currently is in good standing with all regulatory agencies that regulate any or all aspects of MCO’s performance of this Contract. MCO will maintain all required certifications, licenses, permits, and authorizations during the term of this Contract.
Section 14.02 Ability to perform.
MCO warrants that it has the financial resources to fund the capital expenditures required under the Contract without advances by HHSC or assignment of any payments by HHSC to a financing source.
Section 14.03 Minimum Net Worth.
The MCO has, and will maintain throughout the life of this Contract, minimum net worth that complies with standards adopted by TDI. Minimum net worth means the excess total admitted assets over total liabilities, excluding liability for subordinated debt issued in compliance with Chapter 843 of the Texas Insurance Code.
Section 14.04 Insurer solvency.
(a) The MCO must be and remain in full compliance with all applicable state and federal solvency requirements for basic-service health maintenance organizations, including but not limited to, all reserve requirements, net worth standards, debt-
to-equity ratios, or other debt limitations. In the event the MCO fails to maintain such compliance, HHSC, without limiting any other rights it may have by law or under the Contract, may terminate the Contract.
(b) If the MCO becomes aware of any impending changes to its financial or business structure that could adversely impact its compliance with the requirements of the Contract or its ability to pay its debts as they come due, the MCO must notify HHSC immediately in writing.
(c) The MCO must have a plan and take appropriate measures to ensure adequate provision against the risk of insolvency as required by TDI. Such provision must be adequate to provide for the following in the event of insolvency:
(1) continuation of Covered Services, until the time of discharge, to Members who are confined on the date of insolvency in a hospital or other inpatient facility;
(2) payments to unaffiliated health care providers and affiliated healthcare providers whose Contracts do not contain Member “hold harmless” clauses acceptable to the TDI;
(3) continuation of Covered Services for the duration of the Contract Period for which a capitation has been paid for a Member;
(4) provision against the risk of insolvency must be made by establishing adequate reserves, insurance or other guarantees in full compliance with all financial requirements of TDI and the Contract.
Should TDI determine that there is an immediate risk of insolvency or the MCO is unable to provide Covered Services to its Members, HHSC, without limiting any other rights it may have by law, or under the Contract, may terminate the Contract.
Section 14.05 Workmanship and performance.
(a) All Services and Deliverables provided under this Contract will be provided in a manner consistent with the standards of quality and integrity as outlined in the Contract.
(b) All Services and Deliverables must meet or exceed the required levels of performance specified in or pursuant to this Contract.
(c) MCO will perform the Services and provide the Deliverables in a workmanlike manner, in accordance with best practices and high professional standards used in well-managed operations performing services similar to the Services described in this Contract.
Section 14.06 Warranty of deliverables.
MCO warrants that Deliverables developed and delivered under this Contract will meet in all material respects the specifications as described in the Contract during the period following its acceptance by HHSC, through the term of the Contract, including any subsequently negotiated by MCO and HHSC. MCO will promptly repair or replace any such Deliverables not in compliance with this warranty at no charge to HHSC.
Section 14.07 Compliance with Contract.
MCO will not take any action substantially or materially inconsistent with any of the terms and conditions set forth in this Contract without the express written approval of HHSC.
Section 14.08 Technology Access
All technological solutions offered by the MCO must comply with the requirements of Texas Government Code § 531.0162. This includes providing technological solutions that meet federal accessibility standards for persons with disabilities, as applicable.
Section 14.09 Electronic & Information Resources Accessibility Standards
(a) Applicability
The following Electronic and Information Resources (EIR) requirements apply to the Contract because the MCO perform services that include EIR that: (i) HHSC employees are required or permitted to access; or (ii) members of the public are required or permitted to access. This Section does not apply to incidental uses of EIR in the performance of a Contract, unless the Parties agree that the EIR will become property of the State or will be used by the HHSC’s clients or recipients after completion of the Contract. Nothing in this section is intended to prescribe the use of particular designs or technologies or to prevent the use of alternative technologies, provided they result in substantially equivalent or greater access to and use of a Product.
(b) Definitions.
For purposes of this Section:
“Accessibility Standards” means the Electronic and Information Resources Accessibility Standards and the Web Site Accessibility Standards/Specifications.
“Electronic and Information Resources” means information resources, including information resources technologies, and any equipment or interconnected system of equipment that is used in the creation, conversion, duplication, or delivery of data or information. The term includes, but is not limited to, telephones and other telecommunications products, information kiosks, transaction machines, Internet websites, multimedia resources, and office equipment, including copy machines and fax machines.
“Electronic and Information Resources Accessibility Standards” means the accessibility standards for electronic and information resources contained in Volume 1 Texas Administrative Code Chapter 213.
“Web Site Accessibility Standards/ Specifications” means standards contained in Volume 1 Texas Administrative Code Chapter 206.
“Product” means information resources technology that is, or is related to, EIR.
(c) Accessibility Requirements.
Under Texas Government Code Chapter 2054, Subchapter M, and implementing rules of the Texas Department of Information Resources, HHSC must procure Products that comply with the Accessibility Standards when such Products are available in the commercial marketplace or when such Products are developed in response to a procurement solicitation. Accordingly, MCO must provide electronic and information resources and associated Product documentation and technical support that comply with the Accessibility Standards.
(d) Evaluation, Testing, and Monitoring.
(1) HHSC may review, test, evaluate and monitor MCO’s Products and associated documentation and technical support for compliance with the Accessibility Standards. Review, testing, evaluation and monitoring may be conducted before and after the award of a contract. Testing and monitoring may include user acceptance testing.
Neither (1) the review, testing (including acceptance testing), evaluation or monitoring of any Product, nor (2) the absence of such review, testing, evaluation or monitoring, will result in a waiver of the State’s right to contest the MCO’s assertion of compliance with the Accessibility Standards.
(2) MCO agrees to cooperate fully and provide HHSC and its representatives timely access to Products, records, and other items and information needed to conduct such review, evaluation, testing and monitoring.
(e) Representations and Warranties.
(1) MCO represents and warrants that: (i) as of the Effective Date of the Contract, the Products and associated documentation and technical support comply with the Accessibility Standards as they exist at the time of entering the Contract, unless and to the extent the Parties otherwise expressly agree in writing; and (ii) if the Products will be in the custody of the state or an HHS Agency’s client or recipient after the Contract expiration or termination, the Products will continue to comply with such Accessibility Standards after the expiration or termination of the Contract Term, unless HHSC and/or its clients or recipients, as applicable, use the Products in a manner that renders it noncompliant.
(2) In the event MCO should have known, becomes aware, or is notified that the Product and associated documentation and technical support do not comply with the Accessibility Standards, MCO represents and warrants that it will, in a timely manner and at no cost to HHSC, perform all necessary steps to satisfy the Accessibility Standards, including but not limited to remediation, replacement, and upgrading of the Product, or providing a suitable substitute.
(3) MCO acknowledges and agrees that these representations and warranties are essential inducements on which HHSC relies in awarding this Contract.
(4) MCO’s representations and warranties under this subsection will survive the termination or expiration of the Contract and will remain in full force and effect throughout the useful life of the Product.
(f) Remedies.
(1) Pursuant to Texas Government Code Sec. 2054.465, neither MCO nor any other person has cause of action against HHSC for a claim of a failure to comply with Texas Government Code Chapter 2054, Subchapter M, and rules of the Department of Information Resources.
(2) In the event of a breach of MCO’s representations and warranties, MCO will be liable for direct, consequential, indirect, special, and/or liquidated damages and any other remedies to which HHSC may be entitled under this Contract and other applicable law. This remedy is cumulative of any and all other remedies to which HHSC may be entitled under this Contract and other applicable law.
Article 15. Intellectual Property
Section 15.01 Infringement and misappropriation.
(a) MCO warrants that all Deliverables provided by MCO will not infringe or misappropriate any right of, and will be free of any claim of, any third person or entity based on copyright, patent, trade secret, or other intellectual property rights.
(b) MCO will, at its expense, defend with counsel approved by HHSC, indemnify, and hold harmless HHSC, its employees, officers, directors, contractors, and agents from and against any losses, liabilities, damages, penalties, costs, and fees from any claim or action against HHSC that is based on a claim of breach of the warranty set forth in the preceding paragraph. HHSC will promptly notify MCO in writing of the claim, provide MCO a copy of all information received by HHSC with respect to the claim, and cooperate with MCO in defending or settling the claim. HHSC will not unreasonably withhold, delay or condition approval of counsel selected by the MCO.
(c) In case the Deliverables, or any one (1) or part thereof, is in such action held to constitute an infringement or misappropriation, or the use thereof is enjoined or restricted or if a proceeding appears to MCO to be likely to be brought, MCO will, at its own expense, either:
(1) Procure for HHSC the right to continue using the Deliverables; or
(2) Modify or replace the Deliverables to comply with the Specifications and to not violate any intellectual property rights.
Section 15.02 Exceptions.
MCO is not responsible for any claimed breaches of the warranties set forth in Section 15.01 to the extent caused by:
(a) Modifications made to the item in question by anyone other than MCO or its Subcontractors, or modifications made by HHSC or its contractors working at MCO’s direction or in accordance with the specifications; or
(b) The combination, operation, or use of the item with other items if MCO did not supply or approve for use with the item; or
(c) HHSC’s failure to use any new or corrected versions of the item made available by MCO.
Section 15.03 Ownership and Licenses
(a) Definitions.
For purposes of this Section 15.03, the following terms have the meanings set forth below:
(1) “Custom Software” means any software developed by the MCO: for HHSC; in connection with the Contract; and with funds received from HHSC. The term does not include MCO Proprietary Software or Third Party Software.
(2) “MCO Proprietary Software” means software: (i) developed by the MCO prior to the Effective Date of the Contract, or (ii) software developed by the MCO after the Effective Date of the Contract that is not developed: for HHSC; in connection with the Contract; and with funds received from HHSC.
(3) “Third Party Software” means software that is: developed for general commercial use; available to the public; or not developed for HHSC. Third Party Software includes without limitation: commercial off-the-shelf software; operating system software; and application software, tools, and utilities.
(b) Deliverables.
The Parties agree that any Deliverable, including without limitation the Custom Software, will be the exclusive property of HHSC.
(c) Ownership rights.
(1) HHSC will own all right, title, and interest in and to its Confidential Information and the Deliverables provided by the MCO, including without limitation the Custom Software and associated documentation. For purposes of this Section 15.03, the Deliverables will not include MCO Proprietary Software or Third Party Software. MCO will take all actions necessary and transfer ownership of the Deliverables to HHSC, including, without limitation, the Custom Software and associated documentation prior to Contract termination.
(2) MCO will furnish such Deliverables, upon request of HHSC, in accordance with applicable State law. All Deliverables, in whole and in part, will be deemed works made for hire of HHSC for all purposes of copyright law, and copyright will belong solely to HHSC. To the extent that any such Deliverable does not qualify as a work for hire under applicable law, and to the extent that the Deliverable includes materials subject to copyright, patent, trade secret, or other proprietary right protection, MCO agrees to assign, and hereby assigns, all right, title, and interest in and to Deliverables, including without limitation all copyrights, inventions, patents, trade secrets, and other proprietary rights therein (including renewals thereof) to HHSC.
(3) MCO will, at the expense of HHSC, assist HHSC or its nominees to obtain copyrights, trademarks, or patents for all such Deliverables in the United States and any other countries. MCO agrees to execute all papers and to give all facts known to it necessary to secure United States or foreign country copyrights and patents, and to
transfer or cause to transfer to HHSC all the right, title, and interest in and to such Deliverables. MCO also agrees not to assert any moral rights under applicable copyright law with regard to such Deliverables.
(d) License Rights
HHSC will have a royalty-free and non-exclusive license to access the MCO Proprietary Software and associated documentation during the term of the Contract. HHSC will also have ownership and unlimited rights to use, disclose, duplicate, or publish all information and data developed, derived, documented, or furnished by MCO under or resulting from the Contract. Such data will include all results, technical information, and materials developed for and/or obtained by HHSC from MCO in the performance of the Services hereunder, including but not limited to all reports, surveys, plans, charts, recordings (video and/or sound), pictures, drawings, analyses, graphic representations, computer printouts, notes and memoranda, and documents whether finished or unfinished, which result from or are prepared in connection with the Scope of Work performed as a result of the Contract.
(e) Proprietary Notices
MCO will reproduce and include HHSC’s copyright and other proprietary notices and product identifications provided by MCO on such copies, in whole or in part, or on any form of the Deliverables.
(f) State and Federal Governments
In accordance with 45 C.F.R. §95.617, all appropriate State and Federal agencies will have a royalty-free, nonexclusive, and irrevocable license to reproduce, publish, translate, or otherwise use, and to authorize others to use for Federal Government purposes all materials, the Custom Software and modifications thereof, and associated documentation designed, developed, or installed with federal financial participation under the Contract, including but not limited to those materials covered by copyright, all software source and object code, instructions, files, and documentation.
Article 16. Liability
Section 16.01 Property damage.
(a) MCO will protect HHSC’s real and personal property from damage arising from MCO’s, its agent’s, employees.’ Consultants’, and Subcontractors’ performance of the Scope of Work, and MCO will be responsible for any loss, destruction, or damage to HHSC’s property that results from or is caused by MCO’s, its agents’, employees’, consultant’s, or Subcontractors’ negligent or wrongful acts or omissions. Upon the loss of, destruction of, or damage to any property of HHSC, MCO will notify the HHSC Project Manager thereof and, subject to direction from the Project Manager or her or his designee, will take all reasonable steps to protect that property from further damage.
(b) MCO agrees to observe and encourage its employees and agents to observe safety measures and proper operating procedures at HHSC sites at all times.
(c) MCO will distribute a policy statement to all of its employees and agents that directs the employee or agent to promptly report to HHSC or to MCO any special defect or unsafe condition encountered while on HHSC premises. MCO will promptly report to HHSC any special defect or an unsafe condition it encounters or otherwise learns about.
Section 16.02 Risk of Loss.
During the period Deliverables are in transit and in possession of MCO, its carriers or HHSC prior to being accepted by HHSC, MCO will bear the risk of loss or damage thereto, unless such loss or damage is caused by the negligence or intentional misconduct of HHSC. After HHSC accepts a Deliverable, the risk of loss or damage to the Deliverable will be borne by HHSC, except loss or damage attributable to the negligence or intentional misconduct of MCO’s agents, employees, consultants, or Subcontractors.
Section 16.03 Limitation of HHSC’s Liability.
HHSC WILL NOT BE LIABLE FOR ANY INCIDENTAL, INDIRECT, SPECIAL, OR CONSEQUENTIAL, EXEMPLARY, OR PUNITIVE DAMAGES UNDER CONTRACT, TORT (INCLUDING NEGLIGENCE), OR OTHER LEGAL THEORY. THIS WILL APPLY REGARDLESS OF THE CAUSE OF ACTION AND EVEN IF HHSC HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES.
HHSC’S LIABILITY TO MCO UNDER THE CONTRACT WILL NOT EXCEED THE TOTAL CHARGES TO BE PAID BY HHSC TO MCO UNDER THE CONTRACT, INCLUDING CHANGE ORDER PRICES AGREED TO BY THE PARTIES OR OTHERWISE ADJUDICATED.
MCO’s remedies are governed by the provisions in Article 12.
Article 17. Insurance & Bonding
Section 17.01 Insurance Coverage.
(a) Statutory and General Coverage
MCO will maintain, at the MCO’s expense, the following insurance coverage:
(1) Business Automobile Liability Insurance for all owned, non-owned, and hired vehicles for bodily injury and property damage;
(2) Comprehensive General Liability Insurance of at least $1,000,000.00 per occurrence and $5,000,000.00 in the aggregate (including Bodily Injury coverage of $100,000.00 per each occurrence and Property Damage Coverage of $25,000.00 per occurrence); and
(3) If MCO’s current Comprehensive General Liability insurance coverage does not meet the above stated requirements, MCO will obtain Umbrella Liability Insurance to compensate for the difference in the coverage amounts. If Umbrella Liability Insurance is provided, it must follow the form of the primary coverage.
(b) Professional Liability Coverage.
(1) MCO must maintain, or cause its Network Providers to maintain, Professional Liability Insurance for each Network Provider of $100,000.00 per occurrence and $300,000.00 in the aggregate, or the limits required by the hospital at which the Network Provider has admitting privileges.
(2) MCO must maintain an Excess Professional Liability (Errors and Omissions) Insurance Policy for the greater of $3,000,000.00 or an amount (rounded to the nearest $100,000.00) that represents the number of Members enrolled in the MCO in the first month of the applicable State Fiscal Year multiplied by $150.00, not to exceed $10,000,000.00.
(c) General Requirements for All Insurance Coverage
(1) Except as provided herein, all exceptions to the Contract’s insurance requirements must be approved in writing by HHSC. HHSC’s written approval is not required in the following situations:
(i) An MCO or a Network Provider is not required to obtain the insurance coverage described in Section 17.01 if the MCO or Network Provider qualifies as a state governmental unit or municipality under the Texas Tort Claims Act, and is required to comply with, and subject to the provisions of, the Texas Tort Claims Act.
(ii) An MCO may waive the Professional Liability Insurance requirement described in Section 17.01(b)(1) for a Network Provider of Community-based Long-term Services and Supports. An MCO may not waive this requirement if the Network Provider provides other Covered Services in addition to Community-based Long Term Services and Supports, or if a Texas licensing entity requires the Network Provider to carry such Professional Liability coverage. An MCO that waives the Professional Liability Insurance requirement for a Network Provider pursuant to this provision is not required to obtain such coverage on behalf of the Network Provider.
(iii) The Professional Liability Insurance requirements described in Section 17.01(b)(1) do not apply to Nursing Facility Providers.
(2) MCO or the Network Provider is responsible for any and all deductibles stated in the insurance policies.
(3) Insurance coverage must be issued by insurance companies authorized to conduct business in the State of Texas.
(4) With the exception of Professional Liability Insurance maintained by Network Providers, all insurance coverage must name HHSC as an additional insured. In addition, with the exception of Professional Liability Insurance maintained by Network Providers and Business Automobile Liability Insurance, all insurance coverage must name HHSC as a loss payee.
(5) Insurance coverage kept by the MCO must be maintained in full force at all times during the Term of the Contract, and until HHSC’s final acceptance of all Services and Deliverables. Failure to maintain such insurance coverage will constitute a material breach of this Contract.
(6) With the exception of Professional Liability Insurance maintained by Network Providers, the insurance policies described in this Section must have extended reporting periods of two (2) years. When policies are renewed or replaced, the policy retroactive date must coincide with, or precede, the Contract Effective Date.
(7) With the exception of Professional Liability Insurance maintained by Network Providers, the insurance policies described in this Section must provide that prior written notice be given to HHSC at least 30 calendar days before coverage is reduced below minimum HHSC contractual requirements, canceled, or non-renewed. MCO must submit a new coverage binder to HHSC to ensure no break in coverage.
(8) The Parties expressly understand and agree that any insurance coverages and limits furnished by MCO will in no way expand or limit MCO’s liabilities and responsibilities specified within the Contract documents or by applicable law.
(9) MCO expressly understands and agrees that any insurance maintained by HHSC will apply in excess of and not contribute to insurance provided by MCO under the Contract.
(10) If MCO, or its Network Providers, desire additional coverage, higher limits of liability, or other modifications for its own protection, MCO or its Network Providers will be responsible for the acquisition and cost of such additional protection. Such additional protection will not be an Allowable Expense under this Contract.
(11) MCO will require all insurers to waive their rights of subrogation against HHSC for claims arising from or relating to this Contract.
(d) Proof of Insurance Coverage
(1) Except as provided in Section 17.01(d)(2), the MCO must furnish the HHSC Project Manager original Certificates of Insurance evidencing the required insurance coverage on or before the Effective Date of the Contract. If insurance coverage is renewed during the Term of the Contract, the MCO must furnish the HHSC Project Manager renewal certificates of insurance, or such similar evidence, within five (5) Business Days of renewal. The failure of HHSC to obtain such evidence from MCO will not be deemed to be a waiver by HHSC and MCO will remain under continuing obligation to maintain and provide proof of insurance coverage.
(2) The MCO is not required to furnish the HHSC Project Manager proof of Professional Liability Insurance maintained by Network Providers on or before the Effective Date of the Contract, but must provide such information upon HHSC’s request during the Term of the Contract.
Section 17.02 Performance Bond.
(a) The MCO must obtain a performance bond with a one (1) year term. The performance bond must be renewable and renewal must occur no later than the first day of each subsequent State Fiscal Year. The performance bond must continue to be in effect for one (1) year following the expiration of the final renewal period. MCO must obtain and maintain the performance bonds in the form prescribed by HHSC and approved by TDI, naming HHSC as Obligee, securing MCO’s faithful performance of the terms and conditions of this Contract. The performance bonds must comply with Chapter 843 of the Texas Insurance Code and 28 T.A.C. §11.1805. At least one (1) performance bond must be issued. The amount of the performance bond(s) should total $100,000.00 for each MCO Program within each Service Area that the MCO covers under this Contract. Performance bonds must be issued by a surety licensed by TDI, and specify cash payment as the sole remedy. MCO must deliver each renewal prior to the first day of the State Fiscal Year.
(b) Since the CHIP Perinatal Program is a subprogram of the CHIP Program, neither a separate performance bond for the CHIP Perinatal Program nor a combined performance bond for the CHIP and CHIP Perinatal Programs is required. The same bond that the MCO obtains for its CHIP Program within a particular Service Area also will cover the MCO’s CHIP Perinatal Program in that same Service Area.
Section 17.03 TDI Fidelity Bond
The MCO will secure and maintain throughout the life of the Contract a fidelity bond in compliance with Chapter 843 of the Texas Insurance Code and 28 T.A.C. §11.1805. The MCO must promptly provide HHSC with copies of the bond and any amendments or renewals thereto.
Subject: Attachment B-1 - HHSC Medicaid/CHIP Managed Care Services RFP, Sections 1-5
|
| | | |
DOCUMENT HISTORY LOG |
STATUS1 | DOCUMENT REVISION2 | EFFECTIVE DATE | DESCRIPTION3 |
Baseline | n/a | September 1, 2011 | Initial version of Attachment B-1, RFP Sections 1 – 5, “Introduction; Procurement Strategy; General Instructions & Requirements; Submission Requirements; and Evaluation Process & Criteria.” |
Revision | 2.1 | March 1, 2012 | Section 1.3 is modified to clarify that Medicaid Wrap Services will become covered services at a future date to be determined by HHSC. Section 1.8.1 is modified to clarify that Medicaid Wrap Services will become covered services at a future date to be determined by HHSC. |
Revision | 2.2 | June 1, 2012 | Contract amendment did not revise Attachment B-1, Sections 1-5, "Introduction; Procurement Strategy; General Instructions & Requirements; Submission Requirements; and Evaluation Process & Criteria." |
Revision
| 2.3 | September 1, 2012
| Section 1.6.1 is modified to replace reference to the 1915(b) waiver with the Texas Healthcare Transformation and Quality Improvement Program 1115 Waiver. Section 1.6.2 is modified to replace references to the 1915(b) and 1915(c) waivers with the Texas Healthcare Transformation and Quality Improvement Program 1115 Waiver. Section 1.8 is modified to reference the Texas Healthcare Transformation and Quality Improvement Program (THTQIP) 1115 Waiver and HHSC”s administrative rules for identification of eligible populations. Section 1.8.1 STAR Program Eligibility is deleted in its entirety. Section 1.8.2 STAR+PLUS Eligibility is deleted in its entirety. Section 1.8.3 CHIP Program Eligibility is deleted in its entirety.
|
Revision | 2.4 | March 1, 2013 | Contract amendment did not revise Attachment B-1, Sections 1-5, “Introduction; Procurement Strategy; General Instructions & Requirements; Submission Requirements; and Evaluation Process & Criteria.”
|
Revision | 2.5 | June 1, 2013 | Contract amendment did not revise Attachment B-1, Sections 1-5, Introduction; Procurement Strategy; General Instructions & Requirements; Submission Requirements; and Evaluation Process & Criteria. |
Revision | 2.6 | September 1, 2013 | Section 2.1 is modified to clarify that HHSC uses two dashboards. Section 4.3.7.2 is modified to correct the name to which the acronym HEDIS refers.
|
Revision | 2.7 | September 1, 2013 | Contract amendment did not revise Attachment B-1, Sections 1-5, "Introduction; Procurement Strategy; General Instructions & Requirements; Submission Requirements; and Evaluation Process & Criteria." |
Revision | 2.8 | January 1, 2014 | Section 1.6.3 is modified to clarify the eligibility thresholds. |
|
| | | |
Revision | 2.9 | February 1, 2014 | Section 1.6.3 is modified to clarify that in this contract CSHCN is defined as a specific DSHS program.
Section 2.1 is modified to add MCO Report Cards.
Section 4.3.10 is modified to clarify that use of the term CSHCN refers to a specific DSHS program. |
Revision | 2.10 | April 1, 2014 | Contract amendment did not revise Attachment B-1, Sections 1-5, "Introduction; Procurement Strategy; General Instructions & Requirements; Submission Requirements; and Evaluation Process & Criteria." |
Revision | 2.11 | September 1, 2014 | Contract amendment did not revise Attachment B-1, Sections 1-5, "Introduction; Procurement Strategy; General Instructions & Requirements; Submission Requirements; and Evaluation Process & Criteria." |
1 Status should be represented as “Baseline” for initial issuances, “Revision” for changes to the Baseline version, and “Cancellation” for withdrawn versions 2 Revisions should be numbered in accordance according to the version of the issuance and sequential numbering of the revision—e.g., “1.2” refers to the first version of the document and the second revision. 3 Brief description of the changes to the document made in the revision. |
Table of Contents
1. Introduction ............................................................................................................. 1-6
1.1 Point-of-Contact ......................................................................................................... 1-6
1.2 Procurement Schedule ............................................................................................... 1-6
1.3 Purpose ...................................................................................................................... 1-7
1.4 Mission Statement ...................................................................................................... 1-8
1.5 Mission Objectives ..................................................................................................... 1-8
1.6 Overview of the HHSC MCO Programs ..................................................................... 1-9
1.6.1 STAR .................................................................................................................... 1-10
1.6.2 STAR+PLUS ........................................................................................................ 1-10
1.6.3 CHIP ..................................................................................................................... 1-10
1.7 Other HHSC Managed Care Programs .................................................................... 1-11
1.8 Eligible Populations for HHSC MCO Programs ........................................................ 1-12
1.9 Authorization ............................................................................................................ 1-12
1.10 Eligible Respondents ................................................................................................ 1-13
1.11 Term of Contract ...................................................................................................... 1-13
1.12 Development of Contracts ........................................................................................ 1-14
1.13 Medicaid and CHIP Service Areas ........................................................................... 1-14
2. Procurement Strategy and Approach ................................................................... 2-16
2.1 HHSC Model Management Strategy ........................................................................ 2-16
2.2 Performance Measures and Associated Remedies ................................................. 2-17
3. General Instructions and Requirements ............................................................... 3-18
3.1 Strategic Elements ................................................................................................... 3-18
3.1.1 Contract Elements ................................................................................................ 3-18
3.1.2 HHSC’s Basic Philosophy: Contracting for Results ............................................. 3-18
3.2 External Factors ....................................................................................................... 3-18
3.3 Legal and Regulatory Constraints ............................................................................ 3-18
3.3.1 Delegation of Authority ......................................................................................... 3-18
3.3.2 Conflicts of Interest ............................................................................................... 3-19
3.3.3 Former Employees of a State Agency .................................................................. 3-19
3.4 HHSC Amendments and Announcements Regarding this RFP ............................... 3-20
3.5 RFP Cancellation/Partial Award/Non-Award ............................................................ 3-20
3.6 Right to Reject Proposals or Portions of Proposals ................................................. 3-20
3.7 Costs Incurred .......................................................................................................... 3-20
3.8 Protest Procedures................................................................................................... 3-20
3.9 Vendor Conference .................................................................................................. 3-21
3.10 Questions and Comments ........................................................................................ 3-21
3.11 Modification or Withdrawal of Proposal .................................................................... 3-21
3.12 News Releases ........................................................................................................ 3-22
3.13 Incomplete Proposals ............................................................................................... 3-22
3.14 State Use of Proposal Information ........................................................................... 3-22
3.15 Property of HHSC ..................................................................................................... 3-22
3.16 Copyright Restriction ................................................................................................ 3-22
3.17 Additional Information ............................................................................................... 3-23
3.18 Multiple Responses .................................................................................................. 3-23
3.19 No Joint Proposals ................................................................................................... 3-23
3.20 Use of Subcontractors .............................................................................................. 3-23
3.21 Texas Public Information Act .................................................................................... 3-24
3.22 Inducements ............................................................................................................. 3-24
3.23 Definition of Terms ................................................................................................... 3-24
4. Submission Requirements .................................................................................... 4-25
4.1 General Instructions ................................................................................................. 4-25
4.1.1 Economy of Presentation ..................................................................................... 4-26
4.1.2 Number of Copies and Packaging ........................................................................ 4-27
4.1.3 Due Date, Time, and Location .............................................................................. 4-27
4.2 Part 1 – Business Proposal ...................................................................................... 4-28
4.2.1 Section 1 – Executive Summary .......................................................................... 4-28
4.2.2 Section 2 – Respondent Identification and Information ........................................ 4-29
4.2.3 Section 3 – Corporate Background and Experience ............................................ 4-31
4.2.4 Section 4 – Material Subcontractor Information ................................................... 4-36
4.2.5 Section 5 – Historically Underutilized Business (HUB) Participation .................... 4-38
4.2.6 Section 6 – Certifications and Other Required Forms .......................................... 4-43
4.3 Part 2 – Programmatic Proposal .............................................................................. 4-43
4.3.1 Section 1 – Proposed Programs, Service Area, and Capacity ............................. 4-45
4.3.2 Section 2 – Experience Providing Covered Services ........................................... 4-46
4.3.3 Section 3 – Value-added Services ....................................................................... 4-47
4.3.4 Section 4 – Access to Care .................................................................................. 4-47
4.3.5 Section 5 – Provider Network Provisions ............................................................. 4-50
4.3.6 Section 6 – Member Services .............................................................................. 4-56
4.3.7 Section 7 – Quality Assessment and Performance Improvement ........................ 4-61
4.3.8 Section 8 – Utilization Management ..................................................................... 4-63
4.3.9 Section 9 – Early Childhood Intervention (ECI) .................................................... 4-64
4.3.10 Section 10 – Services for People with Special Health Care Needs .................. 4-64
4.3.11 Section 11 – Care Management and/or Service Coordination ......................... 4-65
4.3.12 Section 12 – Disease Management (DM)/Health Home Services .................... 4-67
4.3.13 Section 13 – Behavioral Health Services and Network .................................... 4-67
4.3.14 Section 14 – Management Information System (MIS) Requirements .............. 4-70
4.3.15 Section 15 – Fraud and Abuse ......................................................................... 4-71
4.3.16 Section 16 – Pharmacy Services...................................................................... 4-71
4.3.17 Section 17 – Transition Plan ............................................................................ 4-72
4.3.18 Section 18 – Additional Requirements Regarding Dual Eligibles (for STAR+PLUS
only) 4-73
5. Evaluation Process and Criteria ........................................................................... 5-74
5.1 Overview of Evaluation Process ............................................................................... 5-74
5.2 Evaluation Criteria .................................................................................................... 5-74
5.3 Initial Compliance Screening .................................................................................... 5-75
5.4 Competitive Field Determinations ............................................................................ 5-75
5.5 Oral Presentations and Site Visits ............................................................................ 5-75
5.6 Best and Final Offer ................................................................................................. 5-76
5.7 Discussions with Respondents ................................................................................. 5-76
5.8 Contract Awards ....................................................................................................... 5-76
1. Introduction
The sole point of contact for inquiries concerning this RFP is:
Texas Health and Human Services Commission
Enterprise Contracts and Procurement Services
4405 North Lamar Blvd
Austin, Texas 78756-3422
ATT: Alice Hanna, Purchaser
(512) 206-5277
alice.hanna@hhsc.state.tx.us
All communications relating to this RFP must be directed to the HHSC contact person named above. All communications between Respondents and other HHSC staff members concerning this RFP are strictly prohibited. Failure to comply with these requirements may result in proposal disqualification.
The following table documents the critical pre-award events for the procurement. All dates are subject to change at HHSC’s discretion.
|
| |
Procurement Schedule |
Draft RFP Release Date | November 5, 2010 |
Draft RFP Respondent Comments Due | December 6, 2010 |
RFP Release Date | April 8, 2011 |
Vendor Conference | April 18, 2011 1:00pm CDT |
Respondent Questions Due | April 19, 2011 |
Letters Claiming Mandatory Contract Status Due | April 28, 2011 |
HHSC Posts Responses to Respondent Questions | April 29, 2011 |
Proposals Due | May 23, 2011 |
Deadline for Proposal Withdrawal | May 23, 2011 |
Respondent Demonstrations/Oral Presentations (HHSC option) | HHSC will not be holding presentations |
Tentative Award Announcement | August 1, 2011 |
Anticipated Contract Start Date | September 1, 2011 |
Operational Start Date | March 1, 2012 |
1.3 Purpose
The State of Texas, by and through the Texas Health and Human Services Commission (HHSC), is soliciting competitive proposals for managed care services for recipients who participate in the following managed care programs:
|
| |
| Medicaid State of Texas Access Reform Program (STAR); |
|
| |
| Medicaid STAR+PLUS Program; |
|
| |
| Children’s Health Insurance Program (CHIP), including the CHIP Perinatal subprogram. |
In order to ensure that recipients have a choice of health plans in all MCO Programs, HHSC will select at least two (2) managed care organizations (MCOs) per MCO Program and Service Area.
Through this Request for Proposals (RFP), HHSC is expanding both the scope of services and the geographical areas covered by its current managed care programs. New features include:
|
| |
| Expansion of STAR into two (2) new regions, the Hidalgo Service Area and Medicaid Rural Service Area (MRSA). |
|
| |
| Expansion of STAR+PLUS into the El Paso and Lubbock Service Areas, as well as the new Hidalgo Service Area. |
|
| |
| Adjustments to the Service Area boundaries for STAR, STAR+PLUS and CHIP Service Areas, so that the Service Areas are consistent for all Programs. |
|
| |
| The addition of prescription drug benefits to the managed care structure. The prescription drug benefit will no longer be carved-out of managed care and paid through HHSC’s Vendor Drug Program. Medicaid and CHIP MCOs will be responsible for recruiting and maintaining pharmacy providers and paying for pharmacy benefits. |
|
| |
| The addition of inpatient facility services to the managed care structure for STAR+PLUS. |
|
| |
| For Dual Eligible Members in the STAR+PLUS Program, the addition of Medicaid Wrap Services to the scope of Covered Services at a date determined by HHSC. |
Attachments B-5, 5.1, and 5.2 include maps of the planned STAR, STAR+PLUS and CHIP Service Areas.
HHSC’s mission is to create a customer-focused, innovative, and adaptable managed care system that provides the highest quality of care to clients while at the same time ensures access to services. Through this procurement, HHSC seeks to accomplish its mission by contracting for measurable results that improve Member access and satisfaction; maximize program efficiency, effectiveness, and responsiveness; and limit operational costs.
To accomplish the HHSC’s mission, HHSC will prioritize desired outcomes and benefits for the managed care programs, and will focus its monitoring efforts on the MCOs’ ability to provide satisfactory results in the following areas.
|
| |
| 1. Network adequacy and access to care |
All Members must have timely access to quality of care through a Network of Providers designed to meet the needs of the population served. The MCO will be held accountable for creating and maintaining a Network capable of delivering all Covered Services to Members. The MCO must provide Members with access to qualified Network Providers within the travel distance and waiting time for appointment standards defined in this RFP.
HHSC is accountable to Texans for ensuring that all Members receive quality services in the most efficient and effective manner possible. Accordingly, the MCO will be responsible for providing high quality services in a professional and ethical
manner. HHSC expects the MCO to implement new and creative approaches that ensure quality services, cost-effective service delivery, and careful stewardship of public resources.
|
| |
| 3. Timeliness of claim payment |
The MCO’s ability to ensure that Network Providers receive timely and fair payment for services rendered is a key component of their success in the STAR, STAR+PLUS, and CHIP programs. The MCO must have the ability to timely comply with HHSC’s claims adjudication requirements, as set forth in the Uniform Managed Care Manual. Therefore, HHSC will require strict adherence to claims adjudication standards during the term of the Contract. HHSC also encourages MCOs to provide a no-cost alternative for providers to allow billing without the use of a clearinghouse, and to include attendant care payments as part of the regular claims payment process.
|
| |
| 4. Timeliness with which prenatal care is initiated |
STAR Program data has revealed that 83% of pregnant women received prenatal care in the first trimester or within 42 days of enrollment. While this rate approximates the Medicaid managed care national average, HHSC believes that the high prevalence of births in the STAR population warrants efforts to improve timeliness of prenatal care initiation.
|
| |
| 5. Behavioral health services |
Members must have timely access to Medically Necessary Behavioral Health Services, such as mental health counseling and treatment, as well as timely and appropriate follow-up care.
|
| |
| 6. Delivery of health care to diverse populations |
Member populations in Texas are as diverse as those of any state in the nation. Health Care Services must be delivered without regard to racial or ethnic factors. HHSC expects the MCO to implement intervention strategies to avoid disparities in the delivery of Health Care Services to diverse populations and provide services in a culturally competent manner as described in Section 8.1.5.8 of the RFP.
|
| |
| 7. Disease management requirements |
The MCO must provide a comprehensive disease management program or coverage for Disease Management (DM) services for asthma, diabetes, and other chronic diseases identified by the MCO, based upon an evaluation of the prevalence of the diseases within the MCO’s membership. Please refer to the Uniform Managed Care Manual, Chapter 9.1 “Disease Management,” for additional DM requirements.
The integration of Acute Care services and Community-based Long-Term Services and Supports is an essential feature of STAR+PLUS. A STAR+PLUS MCO must demonstrate that there are sufficient levels of qualified and competent personnel devoted to Service Coordination to meet the everyday needs of STAR+PLUS Members, including Dual Eligibles.
HHSC expects that established Member/Provider relationships, existing treatment protocols, and ongoing care plans will not be impacted significantly by this procurement. Transition to the MCO must be as seamless as possible for Members and their Providers.
|
|
1.6 Overview of the HHSC MCO Programs |
House Bill 7 from the 72nd Regular Session of the Texas Legislature mandated the establishment of Medicaid managed care pilot projects that utilized proven approaches for delivering comprehensive health care. In 1991, the Texas Department of Health created the Bureau of Managed Care. Since that time, Texas has administered a comprehensive set of managed care programs to serve low income Texans. These programs, as presently constituted and administered by HHSC, include the STAR, STAR+PLUS, and CHIP Programs as described in this section.
STAR is currently HHSC's primary managed care program for Medicaid Eligibles and operates under the Texas Healthcare Transformation and Quality Improvement Program (THTQIP) 1115 Waiver. It grew out of a pilot project in Travis County in 1993.
STAR is currently available in Bexar, Dallas, El Paso, Harris, Nueces, Jefferson, Lubbock, Tarrant, and Travis regions. Total STAR enrollment as of August 1, 2010 was 1,452,531.
All non-STAR counties in Texas (primarily rural areas) are currently served by the Medicaid Primary Care Case Management Program (PCCM). Total PCCM enrollment as of August 1, 2010 was 840,172. As a result of this procurement, PCCM will be replaced by STAR in the Hidalgo Service Area and the Medicaid Rural Service Area (MRSA). Note, however, that in the Hidalgo Service Area, HHSC will secure legislative direction before including Cameron, Hidalgo, and Maverick Counties in the STAR Program. Refer to the Procurement Library for current and projected STAR enrollment by Service Area.
STAR+PLUS is a Texas Medicaid program integrating the delivery of Acute Care services and Community-based Long-Term Services and Supports to aged, blind, and disabled (ABD) Medicaid recipients through a managed care system. STAR+PLUS began as a Medicaid pilot project in Harris County in 1998. The STAR+PLUS program operates under the Texas Healthcare Transformation and Quality Improvement Program (THTQIP) 1115 Waiver. The waivers allow the state to provide home and community-based services for Supplemental Security Income (SSI) eligible and SSI-related Medicaid clients, and to mandate managed care participation for SSI/SSI-related eligible clients who are 21 years of age and older. Enrollment in STAR+PLUS is voluntary for clients who are 20 years of age and younger.
As of August 1, 2010, STAR+PLUS MCOs served 169,873 Members in the Bexar, Harris, Nueces, and Travis Service Areas. Through this procurement, HHSC intends to expand STAR+PLUS to the El Paso, Hidalgo, and Lubbock Service Areas (see Attachment B-5.2 STAR+PLUS Service Area Map). As in STAR, HHSC will seek legislative direction before including Cameron, Hidalgo, and Maverick Counties in the STAR+PLUS Hidalgo Service Area. Refer to the Procurement Library for current and projected STAR+PLUS enrollment by Service Area.
CHIP is HHSC’s program to help Texas families obtain affordable coverage for their uninsured children (from birth through the month of their 19th birthday). In 1999, the 76th Texas Legislature authorized the state’s participation in the federal CHIP program. The principal objective of the state legislation was to provide primary and preventative health care to low-income, uninsured children of Texas, including children with special health care needs who were not served by or eligible for other state-assisted health insurance programs.
HHSC began operating CHIP in 2000. CHIP Members are currently covered through two (2) types of managed care entities - health maintenance organizations (HMOs) licensed by the Texas Department of Insurance (TDI) and exclusive provider organizations (EPOs) with TDI-approved exclusive provider benefit plans (EPBPs). HMOs serve CHIP Members in eight (8), primarily urban Service Areas. EPOs serve the remaining CHIP Members, who reside primarily in the 174-county rural service area (the CHIP RSA). As of September 1, 2010, 523,895 children were enrolled in CHIP. Of these, 400,243 were enrolled in HMOs. The balance of the CHIP enrollment is in the EPOs serving the CHIP RSA. Refer to the Procurement Library for current and projected CHIP enrollment by Service Area.
The CHIP Perinatal Program, a subprogram of CHIP, is for unborn children of women who are not eligible for Medicaid. The 2006-07 General Appropriations Act (Article II, Health and Human Services Commission, Rider 70, S.B. 1, 79th Legislature, Regular Session, 2005) authorized HHSC to expend funds to provide unborn children with health benefit coverage under CHIP. The result was the CHIP Perinatal Program, which began in January 2007. This benefit allows pregnant women who are ineligible for Medicaid due to income (whose income is greater than the Medicaid eligibility threshold) or immigration status (and whose income is also below the Medicaid eligibility threshold) to receive prenatal care for their unborn children. Upon delivery, newborns in families with income at or below the Medicaid eligibility threshold move from the CHIP Perinatal Program to Medicaid, where they receive 12-months of continuous Medicaid coverage. CHIP Perinatal newborns in families with incomes above the Medicaid eligibility threshold remain in the CHIP Perinatal Program and receive CHIP benefits for a
12-month coverage period, beginning on the date of enrollment as an unborn child. CHIP Perinatal Program Members are exempt from the 90-day waiting period, the asset test, and all cost-sharing that applies to traditional CHIP Members, including enrollment fees and co-pays, for the duration of their coverage period. As of September 1, 2010, 33,860 CHIP Perinates (unborn children) and 19,076 CHIP Perinate Newborns were enrolled in this subprogram.
Throughout this RFP, references to CHIP apply to both the traditional CHIP Program and the CHIP Perinatal subprogram unless the context indicates otherwise.
|
|
1.7 Other HHSC Managed Care Programs |
The following managed care options are not included in the scope of this procurement:
CHIP Rural Service Area (RSA): 174 primarily-rural counties.
Medicaid and CHIP Dental Programs: The Medicaid State Plan encourages eligible individuals to improve and maintain good oral health by providing access to comprehensive dental care. The CHIP Dental Program is a statewide program that provides services such as routine check-ups, cleanings, X-rays, sealants, fillings, tooth removal, crowns/caps and root canals for all CHIP children. HHSC has issued a managed care procurement with an anticipated operational start date of March 1, 2012 for both the Medicaid and CHIP Dental Programs.
STAR+PLUS Program in the Dallas and Tarrant Service Areas: Effective February 1, 2011, STAR+PLUS began serve approximately 78,000 Medicaid clients in the Dallas and Tarrant Service Areas.
STAR Health Program: On April 1, 2008, HHSC launched the STAR Health program as the first comprehensive health and medical network for children who are in the state’s foster care system. The goal is to give children health care services that are coordinated, comprehensive, easy to find, and uninterrupted when the child moves.
NorthSTAR: NorthSTAR is an integrated behavioral health delivery system for Medicaid Eligibles in the Dallas Service Area. It is an initiative of the Texas Department of Mental Health and Mental Retardation and the Texas Commission on Alcohol and Drug Abuse. Behavioral Health Services are provided by a licensed behavioral health organization. Due to the presence of NorthSTAR in the Dallas Service Area, MCOs in the Service Area will not be required to provide Behavioral Health Services to STAR Members.
|
|
1.8 Eligible Populations for HHSC MCO Programs |
The Texas Healthcare Transformation and Quality Improvement Program (THTQIP) 1115 Waiver and HHSC's administrative rules identify the populations that are eligible for STAR and STAR+PLUS, and the CHIP State Plan identifies the populations eligible for CHIP.
Federal law requires a choice of Medicaid managed care health plans in any given Service Area. For the STAR Program, during the period after which the Medicaid eligibility determination has been made, but prior to enrollment in the MCO, Medicaid Eligibles, with the exception of certain newborns and pregnant women will be enrolled under the traditional fee-for-service Medicaid program (see Article 5 of Attachment A, Uniform Managed Care Contract Terms and Conditions of the RFP). All such Medicaid Eligibles will remain in the fee-for-service Medicaid program until enrolled in or assigned to a STAR or STAR+PLUS MCO, as applicable. For the CHIP MCO Program, there is no benefit coverage for CHIP-eligible children prior to enrollment in a CHIP MCO.
The Texas Legislature has designated HHSC as the single state agency to administer the Medicaid and CHIP Programs in the State of Texas. HHSC has authority to contract with MCOs to carry out the duties and functions of the Medicaid Managed Care Program under Title XIX of the Social Security Act; §12.011 and §12.02, Texas Health and Safety Code; and Chapter 533, Texas Government Code. HHSC has the authority to contract with MCOs to carry out the duties of the CHIP Managed Care Program under Title XXI of the Social Security Act, and Chapter 62, Texas Health and Safety Code.
Contracts awarded under this RFP are subject to all necessary federal and state approvals, including, but not limited to, Centers for Medicare and Medicaid Services (CMS) approval.
|
|
1.10 Eligible Respondents |
Except as provided herein, eligible Respondents include insurers that are licensed by the TDI as HMOs in accordance with Chapter 843 of the Texas Insurance Code, or a certified Approved Non-Profit Health Corporation (ANHC), formed in compliance with Chapter 844 of the Texas Insurance Code.
For the STAR and STAR+PLUS Hidalgo Service Area, eligible respondents include HMOs, ANHCs, and EPOs with TDI-approved EPBPs. Note that under current state law, HHSC is precluded from providing services to Medicaid recipients through an HMO model in the following three (3) counties in the Hidalgo Service Area: Cameron, Hidalgo, and Maverick. HHSC will not implement any form of capitated managed care in these three (3) counties in the Hidalgo Service Area without guidance from the Texas Legislature. Respondents who are interested in bidding on the Hidalgo Service Area should nevertheless pursue one or more forms of TDI approval appropriate to these counties.
For the Medicaid Rural Service Area for STAR, eligible respondents include HMOs, ANHCs, EPOs with TDI-approved EPBPs. Note that, for purposes of bidding, HHSC has subdivided the Medicaid Rural Service Area into three (3) areas – West, Central, and Northeast Texas. Respondents may seek TDI approval in one (1) or more of these areas, but should note that HHSC will more favorably evaluate responses that propose to serve all three (3) areas. Should HHSC determine that it is in the state’s best interest to subdivide the Medicaid Rural Service Area for purposes of award, the Medicaid Rural Service Area will still be treated as one (1) Service Area for rate-setting purposes.
Throughout this RFP, the term “MCO” is used to refer to HMOs, ANHCs, and EPOs.
A Respondent that has submitted its application for licensure as an HMO, for certification as an ANHC, or for approval of an EPBP prior to the Proposal due date is also eligible to respond to this RFP; however, the Respondent must receive TDI approval no later than 60 days after HHSC executes the Contract (see Section 1.2, “Procurement Schedule”). Failure to receive the required approval within 60 days after HHSC executes the Contract will result in the cancellation of the award.
For more information on the reasons for HHSC’s disqualification of Respondents, see Section 3.3.2, “Conflicts of Interest,” and Section 3.3.3, “Former Employees of a State Agency.”
The Initial Contract Period will begin on the Contract’s Effective Date (generally the date HHSC signs the contract) and will continue through August 31, 2015 (the “Initial Contract Period”). HHSC may, at its option, extend the Contract for an additional period or periods, not to exceed a total of eight (8) operational years. All reserved Contract extensions beyond the Initial Contract Period will be subject to good faith negotiation between the parties.
|
|
1.12 Development of Contracts |
HHSC intends to execute one (1) Contract per MCO, which will include all awarded MCO Programs and Service Areas. For reference only, HHSC has included a copy of the standard Managed Care Contract in the Procurement Library. The Managed Care Contract identifies an MCO’s awarded MCO Programs and Service Areas, and identifies all documents that will become part of the agreement, including Attachment A, "Uniform Managed Care Contract Terms and Conditions."
|
|
1.13 Medicaid and CHIP Service Areas |
In this RFP, HHSC distinguishes areas of Texas by MCO Program Service Areas. If a Respondent proposes to participate in an HHSC MCO Program Service Area, the Respondent must propose to serve all counties in the HHSC-defined Service Area, with the following exception. As described above, Respondents may chose to serve all or part of the STAR Medicaid Rural Service Area. Maps and tables depicting the Service Area configuration for each of the MCO Programs can be found in
Attachments B-5, 5.1, and 5.2. The tables indicate the counties included in each of the designated Service Areas. The following chart summarizes the MCO Program options included in the scope of this procurement, by Service Area.
|
| | | |
| | | |
Service Areas | STAR | STAR+PLUS | CHIP MCO |
Bexar | √ | √ | √ |
Dallas | √ | | √ |
El Paso | √ | √ | √ |
Harris | √ | √ | √ |
Hidalgo | √ | √ | |
Jefferson | √ | √ | √ |
Lubbock | √ | √ | √ |
Medicaid RSA (Entire Service Area) | √ | | |
West Texas | √ | | |
Central Texas | √ | | |
Northeast Texas | √ | | |
Nueces | √ | √ | √ |
Tarrant | √ | | √ |
Travis | √ | √ | √ |
As described above, HHSC intends to expand the STAR Program to include the Hidalgo Service Area and Medicaid RSA, and the STAR+PLUS MCO Program to include the El Paso, Hidalgo, and Lubbock Service Areas. HHSC reserves the right to change the boundaries for, or otherwise modify, the Service Areas if it determines that such action is in the best interest of the State.
|
|
2. Procurement Strategy and Approach |
HHSC seeks to contract with at least two (2) MCOs for each MCO Program and Service Areas to provide for client choice. It is possible that a Service Area could have more than two (2) MCOs. HHSC reserves the right to enter into Contracts with more than two (2) MCOs in any Service Area based on:
|
| |
| • the number of managed care Eligibles in the Service Area compared to the combined capacity of qualified MCO Respondents, and |
|
| |
| • statutory requirements, such as HHSC’s consideration of Proposals from an MCO owned or operated by a hospital district. |
Section 2155.144, Texas Government Code obligates HHSC to purchase goods and services on the basis of best value. HHSC rules define “best value” as the optimum combination of economy and quality that is the result of fair, efficient, and practical procurement decision-making and that achieves health and human services procurement objectives (see 1 TAC §391.31). HHSC will evaluate proposals using the best value criteria set forth in Section 5 of this RFP.
|
|
2.1 HHSC Model Management Strategy |
HHSC will use two Performance Indicator Dashboards (one for administrative and financial measures and another for quality measures). The Performance Indicator Dashboards are included in the Uniform Managed Care Manual. The Performance Indicator Dashboards are not all-inclusive sets of performance measures; HHSC will measure other aspects of the MCO's performance as well. Rather, the Performance Indicator Dashboards assemble performance indicators that assess many of the most important dimensions of the MCO's performance, and includes measures that, when publicly shared, will also serve to incentivize excellence.
As described in Section 8.1.1.1, "Performance Evaluation," after Rate Year 1 HHSC will also collaborate with each MCO to establish an annual series of performance improvement projects. The MCO will be committed to making its best efforts to achieve the established projects.
HHSC may establish some or all of the annual performance improvement projects. HHSC and each MCO will negotiate any remaining projects. These projects will be highly specified and measurable. The projects will reflect areas that present significant opportunities for performance improvement. Once finalized and approved by HHSC, the projects will become part of each MCO's annual plan for its Quality Assurance and Performance Improvement (QAPI) Program, as defined in Section 8.1.7, "Quality Assessment and Performance Improvement," and will be incorporated by reference into the Contract.
As described in Section 8.1.1.1.1, HHSC will develop MCO report cards to help STAR, STAR+PLUS, and CHIP enrollees to identify and select an MCO.
HHSC recognizes the importance of applying a variety of financial and non-financial incentives and disincentives for demonstrated MCO performance. It is HHSC's objective to recognize and reward both excellence in performance and improvement in performance within existing state and federal financial constraints. It is likely that this approach will be modified over time based on several variables, including accumulated experience by HHSC and the MCO, changes in the status of state finances, and changes in each MCO's performance levels. Section 6.3, "Performance Incentives and Disincentives," describes the incentive and disincentive approach in additional detail.
The incentives and disincentives will be linked to some of the measures in the Performance Indicator Dashboard. The MCO's performance relative to the annual performance improvement projects may be used by HHSC to identify and reward excellence and improvement by the MCO in subsequent years.
Finally, HHSC plans to improve methods for sharing information regarding the Texas Medicaid and CHIP Programs with all of the MCOs through HHSC-sponsored workgroups and other initiatives.
|
|
2.2 Performance Measures and Associated Remedies |
The MCO must provide all services and deliverables under the Contract at an acceptable quality level and in a manner consistent with acceptable industry standard, custom, and practice. Failure to do so may result in HHSC’s assessment of contractual remedies, including liquidated damages, as set forth in Attachment B-4, “Deliverables/Liquidated Damages Matrix.”
|
|
3. General Instructions and Requirements |
The term “Contract” means the contract awarded as a result of this RFP and all exhibits thereto. At a minimum, the following documents will be incorporated into the contract: this RFP and all attachments and exhibits; any modifications, addendum or amendments issued in conjunction with this RFP; HHSC’s “Uniform Managed Care Contract Terms and Conditions;” and the MCO’s Proposal.
Respondents are responsible for reviewing all parts of the Contract, including the “Uniform Managed Care Contract Terms and Conditions,” and noting any exceptions, reservations, and limitations on the Respondent Information and Disclosures Form.
|
|
3.1.2 HHSC’s Basic Philosophy: Contracting for Results |
HHSC’s fundamental commitment is to contract for results. HHSC defines a successful result as the generation of defined, measurable, and beneficial outcomes that satisfy the Contract requirements and support HHSC’s missions and objectives. This
RFP describes what is required of the MCO in terms of services, deliverables, performance measures, and outcomes, and unless otherwise noted in the RFP, places the responsibility for how they are accomplished on the MCO.
External factors may affect the project, including budgetary and resource constraints. Any contract resulting from the RFP is subject to the availability of state and federal funds. As of the issuance of this RFP, HHSC anticipates that budgeted funds will be available to reasonably fulfill the project requirements. If, however, funds are not available, HHSC reserves the right to withdraw the RFP or terminate the resulting contract without penalty.
|
|
3.3 Legal and Regulatory Constraints |
|
|
3.3.1 Delegation of Authority |
State and federal laws generally limit HHSC’s ability to delegate certain decisions and functions to a vendor, including, but not limited to: (1) policy-making authority, and (2) final decision-making authority on the acceptance or rejection of contracted services.
|
|
3.3.2 Conflicts of Interest |
A conflict of interest is a set of facts or circumstances in which either a Respondent or anyone acting on its behalf in connection with this procurement has past, present, or currently planned personal, professional, or financial interests or obligations that, in HHSC’s determination, would actually or apparently conflict or interfere with the Respondent’s contractual obligations to HHSC. A conflict of interest would include circumstances in which a party’s personal, professional, or financial interests or obligations may directly or indirectly:
|
| |
| • make it difficult or impossible to fulfill its contractual obligations to HHSC in a manner that is consistent with the best interests of the State of Texas; |
|
| |
| • impair, diminish, or interfere with that party’s ability to render impartial or objective assistance or advice to HHSC; and/or |
|
| |
| • provide the party with an unfair competitive advantage in future HHSC procurements. |
Neither the Respondent nor any other person or entity acting on its behalf, including, but not limited to subcontractors, employees, agents, and representatives, may have a conflict of interest with respect to this procurement. Before submitting a proposal, Respondents should carefully review Attachment A, “Uniform Managed Care Contract Terms and Conditions,” for additional information concerning conflicts of interests.
A Respondent must certify that it does not have personal or business interests that present a conflict of interest with respect to this RFP and resulting contract (see the Required Certifications form). Additionally, if applicable, the Respondent must disclose all potential conflicts of interest. The Respondent must describe the measures it will take to ensure that there will be no actual conflict of interest and that its fairness, independence, and objectivity will be maintained (see the Respondent Information and Disclosures Form). HHSC will determine to what extent, if any, a potential conflict of interest can be mitigated and managed during the term of the Contract. Failure to identify potential conflicts of interest may result in HHSC’s disqualification of a proposal or termination of the Contract.
|
|
3.3.3 Former Employees of a State Agency |
Respondents must comply with Texas and federal laws and regulations relating to the hiring of former state employees (see e.g., Texas Government Code §572.054 and 45 C.F.R. §74.43). Such “revolving door” provisions generally restrict former agency heads from communicating with or appearing before the agency on certain matters for two (2) years after leaving the
agency. The revolving door provisions also restrict some former employees from representing clients on matters that the employee participated in during state service or matters that were in the employees’ official responsibility.
As a result of such laws and regulations, a Respondent must certify that it has complied with all applicable laws and regulations regarding former state employees (see the Required Certifications Form). Furthermore, a Respondent must disclose any relevant past state employment of the Respondent’s or its subcontractors’ employees and agents in the Respondent Information and Disclosure Form.
|
|
3.4 HHSC Amendments and Announcements Regarding this RFP |
HHSC will post all official communication regarding this RFP on its website, including the notice of tentative award. HHSC reserves the right to revise the RFP at any time. Any changes, amendments, or clarifications will be made in the form of written responses to Respondents’ questions, amendments, or addendum issued by HHSC on its website. Respondents should check the website frequently for notice of matters affecting the RFP. To access the website, go to the “HHSC Contracting Opportunities” page and enter a search for this procurement.
|
|
3.5 RFP Cancellation/Partial Award/Non-Award |
HHSC reserves the right to cancel this RFP, to make a partial award, or to make no award if it determines that such action is in the best interest of the State of Texas.
|
|
3.6 Right to Reject Proposals or Portions of Proposals |
HHSC may, in its discretion, reject any and all proposals or portions thereof.
Respondents understand that issuance of this RFP in no way constitutes a commitment by HHSC to award a contract or to pay any costs incurred by a Respondent in the preparation of a response to this RFP. HHSC is not liable for any costs incurred by a Respondent prior to issuance of or entering into a formal agreement, contract, or purchase order. Costs of developing proposals, preparing for or participating in oral presentations and site visits, or any other similar expenses incurred by a Respondent are entirely the responsibility of the Respondent, and will not be reimbursed in any manner by the State of Texas.
Texas Administrative Code, Title 1, Part 15, Chapter 392, Subchapter C outlines HHSC’s Respondent protest procedures.
HHSC will hold a vendor conference according to the time and date in Section 1.2, “Procurement Schedule” in the Lone Star Conference Room located at 11209 Metric Blvd, Building H, Austin, Texas. Vendor conference attendance is strongly recommended, but is not required.
Respondents may email questions for the conference to the HHSC Point of Contact (see Section 1.1) no later than five (5) days before the conference. HHSC will also give Respondents the opportunity to submit written questions at the conference. All questions should reference the appropriate RFP page and section number. HHSC will attempt to respond to questions at the vendor conference, but responses are not official until posted in final form on the HHSC website. HHSC reserves the right to amend answers prior to the proposal submission deadline.
|
|
3.10 Questions and Comments |
All questions and comments regarding this RFP should be sent to the HHSC Point of Contact (see Section 1.1). Questions should reference the appropriate RFP page and section number, and must be submitted by the deadline set forth in Section 1.2. HHSC will not respond to questions received after the deadline. HHSC’s responses to Respondent questions will be posted to the HHSC website. HHSC reserves the right to amend answers prior to the proposal submission deadline.
Respondents must notify HHSC of any ambiguity, conflict, discrepancy, exclusionary specification, omission, or other error in the RFP by the deadline for submitting questions and comments. If a Respondent fails to notify HHSC of these issues, it will submit a proposal at its own risk, and if awarded a contract:
(1) must have waived any claim of error or ambiguity in the RFP or resulting contract;
(2) must not contest HHSC’s interpretation of such provision(s); and
(3) must not be entitled to additional compensation, relief, or time by reason of the ambiguity, error, or its later correction.
|
|
3.11 Modification or Withdrawal of Proposal |
Prior to the proposal submission deadline set forth in Section 1.2, a Respondent may: (1) withdraw its proposal by submitting a written request to the HHSC Point of Contact, or (2) modify its proposal by submitting a written amendment to the HHSC Point of Contact. HHSC may request proposal modifications at any time.
HHSC reserves the right to waive minor informalities in a proposal and award a contract that is in the best interest of the State of Texas. A “minor informality” is an omission or error that, in HHSC’s determination, if waived or modified when evaluating proposals, would not give a Respondent an unfair advantage over other Respondents or result in a material change in the proposal or RFP requirements. When HHSC determines that a proposal contains a minor informality, it may at its discretion provide the Respondent with the opportunity to correct.
Prior to tentative award, a Respondent may not issue a press release or provide any information for public consumption regarding its participation in the procurement. After tentative award, a Respondent must receive prior written approval from HHSC before issuing a press release or providing information for public consumption regarding its participation in the procurement. Requests should be directed to the HHSC Point of Contact identified in Section 1.1.
Section 3.12 does not preclude business communications necessary for a Respondent to develop a proposal, or required reporting to shareholders or governmental authorities.
|
|
3.13 Incomplete Proposals |
HHSC may reject without further consideration a proposal that does not include a complete, comprehensive, or total solution as requested by this RFP.
|
|
3.14 State Use of Proposal Information |
HHSC reserves the right to use any and all ideas and information presented in a proposal. A Respondent may not object to HHSC’s use of such information.
Except as otherwise provided in this RFP or the resulting Contract, all products produced by a Respondent, including without limitations the proposal, all plans, designs, software, and other contract deliverables, become the sole property of HHSC. See
Attachment A, “Uniform Managed Care Contract Terms and Conditions,” Article 15 for additional information concerning intellectual property rights.
|
|
3.16 Copyright Restriction |
HHSC will not consider any proposal that is copyrighted by the Respondent, in whole or part.
|
|
3.17 Additional Information |
By submitting a proposal, the Respondent grants HHSC the right to obtain information from any lawful source regarding the Respondent’s and its directors’, officers’, and employees’:
(1) past business history, practices, and conduct;
(2) ability to supply the goods and services; and
(3) ability to comply with Contract requirements.
By submitting a proposal, a Respondent generally releases from liability and waives all claims against any party providing HHSC information about the Respondent. HHSC may take such information into consideration in evaluating proposals.
A Respondent may only submit one (1) proposal as a prime contractor. If a Respondent submits more than one (1) proposal, HHSC may reject one or more of the submissions. This requirement does not limit a subcontractor’s ability to collaborate with one (1) or more Respondents submitting proposals.
A Respondent may not entice or require a subcontractor to enter into an exclusive subcontract for the purpose of this procurement. Any subcontract entered into by a Respondent with a third party to meet a requirement of this RFP must not include any provision that would prevent or bar that subcontractor from entering into a comparable contractual relationship with another Respondent submitting a proposal under this procurement. This prohibition against exclusive subcontracts does not apply to professional services that solely pertain to development of the proposal, including gathering of competitive intelligence.
HHSC will not consider joint or collaborative proposals that require it to contract with more than one (1) Respondent.
|
|
3.20 Use of Subcontractors |
Subcontractors providing services under the Contract must meet the same requirements and level of experience as required of the Respondent. No subcontract under the Contract must relieve the Respondent of the responsibility for ensuring the requested services are provided. Respondents planning to subcontract all or a portion of the work to be performed must identify the proposed subcontractors and describe the subcontracted functions in their proposals.
|
|
3.21 Texas Public Information Act |
Proposals will be subject to the Texas Public Information Act (the Act), located in Chapter 552 of the Texas Government Code, and may be disclosed to the public upon request. By submitting a proposal, the Respondent acknowledges that all information and ideas presented in the proposal are public information and subject to disclosure under the Texas Public Information Act, with the limited exception of Social Security Numbers and certain non-public financial reports or information submitted in response to RFP Sections 4.2.3.3 and 4.2.3.4.
If the Respondent asserts that financial reports or information provided in response to RFP Sections 4.2.3.3 and 4.2.3.4 contains trade secret or other confidential information, it must be clearly marked such information in boldface type and include the words “confidential” or “trade secret” at top of the page. Furthermore, the Respondent must identify the financial reports or information, and provide an explanation of why the reports or information are excepted from public disclosure, on the Respondent Information and Disclosures form.
HHSC will process any request from a member of the public in accordance with the procedures outlined in the Act. Respondents should consult the Texas Attorney General’s website (www.oag.state.tx.us) for information concerning the Act’s application to applications and potential exceptions to disclosure.
HHSC submits this RFP setting forth certain information regarding the objectives of the Contract and HHSC’s desire to mitigate risk throughout the life of the Contract by use of expert MCO services.
Therefore, HHSC will consider all representations contained in a Respondent’s proposal, oral or written presentations, correspondence, discussions, and negotiations as representations of the Respondent’s expertise. HHSC accepts these representations as inducements to contract.
Defined terms must have the meaning stated as described in the Attachment A, “Uniform Managed Care Contract Terms and Conditions,” unless the context clearly indicates otherwise. Defined terms are capitalized throughout this RFP. For example, the word “Provider,” when capitalized, refers to Network provider. When the word “provider” is not capitalized, the connotation is all providers, whether Network or Out-of-Network.
|
|
4. Submission Requirements |
To be considered for award, the Respondent must address all applicable RFP specifications to HHSC’s satisfaction. If requested by HHSC, the Respondent must provide HHSC with information necessary to validate any statements made in its Proposal. This includes, but may not be limited to, granting permission or access for HHSC to verify information with third parties, whether identified by the Respondent or HHSC. If any requested information is not provided within the timeframe allotted, HHSC may reject the Proposal.
Respondents must prepare and submit proposals in accordance with the provisions of this section. Proposals received that do not follow these instructions may be evaluated as non-responsive and may not be considered for award.
For Respondents bidding on more than one MCO Program, i.e., STAR, STAR+PLUS, or CHIP Program, HHSC has attempted to minimize the need for Respondents to submit multiple copies of the same information.
Each bid for participation in the STAR Program, the STAR+PLUS Program, and/or the CHIP Program must include the following two (2) components:
|
| |
| 1. Business Specifications; and |
|
| |
| 2. General Programmatic Proposal. |
Respondents proposing to participate in multiple MCO Programs do not need to submit multiple copies of the Business Specifications or the General Programmatic Proposal. However, these Respondents will need to carefully read each submission requirement to ensure that they provide specific information for each MCO Program bid and Service Area, as applicable, when completing any element of their Proposals.
All Proposal information must be submitted on 8 ½ x 11 inch, white bond paper, three (3)-hole punched, and placed in sturdy three (3) ring binders. Text must be no smaller than 11-point font, single-spaced. Figures may not incorporate text smaller than 8-pt font. All pages must have one-inch margins and page numbering must be sequential per section. Where practical, pages should be double-sided. Each binder must be clearly labeled with the title of this RFP, the Respondent’s legal name, and the title of the document contained in the binder, e.g., Business Proposal or Programmatic Proposal.
Proposals must be organized and numbered in a manner that facilitates reference to this RFP and its requirements. Respondents must respond to each item in the order it appears in the RFP. The response must include headings and numbering to match the corresponding section of the RFP. Respondents may place attachments and appendices in a separate section if the RFP provides that such attachments are not included in the section’s specified page limits.
|
|
4.1.1 Economy of Presentation |
Unnecessarily elaborate Proposals beyond those sufficient to provide a complete and effective response to this RFP are not desired and may be construed as an indication of the Respondent’s lack of ability to provide efficient work products.
The Respondent must adhere to page limits where specified. Page limits are listed in parentheses at the end of the title of the section. A three (3) page limit, for example, means that the response should not be in excess of three (3) one-sided pages that meet the size, font, and margin requirements specified in the General Instructions in Section 4.1 above.
Some page limits are identical regardless of the number of MCO Programs in which a Respondent is proposing to participate. If a page limit is listed but does not include the phrase “per MCO Program,” the page limit applies to the entire response regardless of the number of MCO Programs bid. In these cases, the page limit will be indicated as a set number, e.g., “3 pages.”
In some cases, additional pages are provided for Respondents proposing to serve more than one MCO Program. For example, “3 pages plus 1 additional page per additional MCO Program” indicates that a Respondent proposing to serve one (1) MCO Program has a three (3) page limit, a Respondent proposing to serve two (2) MCO Programs has a four (4) page limit, and a Respondent proposing to serve all three (3) MCO Programs has a five (5) page limit. This page limit approach is designed to give Respondents submitting a Proposal for multiple MCO Programs sufficient space to respond to the submission requirement when submission responses differ across MCO Programs. Respondents proposing to serve multiple programs should have similar or identical approaches across MCO Programs where administrative efficiencies are possible and appropriate. Respondents must clearly indicate differences, if any, in their response to each submission requirement for each applicable MCO Program.
In other cases, additional pages may be provided based on certain aspects of the Respondent’s Proposal or organization, such as the number of organizational charts submitted reflecting arrangements with Material Subcontractors, or the number of Key Contract Personnel included in the Proposal for Respondents proposing to serve more than one MCO Program.
Finally, some page limits are by MCO Program, e.g., two (2) pages per MCO Program means that a Respondent proposing to serve all three (3) MCO Programs would have a six (6) page limit for that requirement.
If the Respondent chooses to repeat the RFP question in its Proposal, the question text will be included in the page limit.
In responding to questions in Section 4.2 (“Business Proposal”) and Section 4.3 (“Programmatic Proposal”) for which the Respondent includes information about a Material Subcontractor or Action Plans, up to one (1) page may be used to describe each Material Subcontractor arrangement, and up to one (1) page may be used to describe each Action Plan. These pages are outside of the page limit instructions for the specific submission requirement.
HHSC reserves the right not to review information provided in excess of the page limits. Respondents need not feel compelled to submit unnecessary text in order to reach the page limits.
Attachments required by the RFP, such as certain policies and procedures, are not counted in calculating the Respondent’s page limits. Respondents must not submit information or attachments that are not explicitly requested in the RFP. Elaborate artwork, expensive paper and bindings, and expensive visual or other presentation aids are neither necessary nor desired.
|
|
4.1.2 Number of Copies and Packaging |
Respondents must submit one (1) hardbound original and eight (8) hardbound copies of the Proposal. The original must be clearly labeled “Original” on the outside of the binder. In addition to the hardbound original and copies, Respondents must submit 22 electronic copies of each Proposal component. At the Respondent’s option, it may produce only electronic copies of certain attachments and appendices. This exception applies to attachments and appendices that exceed ten (10) pages, such as GeoAccess tables, Significant Traditional Provider (STP) files, TDI filings, and other financial documents. The exception does not apply to the attachments referenced in Section 4.2, Section 5, “HUB Subcontracting Plan,” or Section 6, “Certifications and Other Required Forms,” which must be included in both the hardbound and electronic copies of the Proposal. If the Respondent produces only an electronic copy of an attachment or appendix, the hardbound Proposals should refer the reader to the electronic Proposal for the required information.
For the electronic copies, the Proposal, attachments, financial documents, signed forms, pamphlets, and all other documents included in the proposal hardcopy must be submitted on CDs compatible with Microsoft Office 2000 files. PDF files should be prepared in a format that allows for OCR text recognition. HHSC will not accept Proposals by facsimile or e-mail.
|
|
4.1.3 Due Date, Time, and Location |
Submit all copies of the Proposal to HHSC’s Enterprise Contracts and Procurement Services (ECPS) no later than 2:00 p.m. Central Time (CT) according to the timeline in Section 1.2, “Procurement Schedule.” All submissions will be date and time stamped when received by ECPS. The clock in the ECPS office is the official timepiece for determining compliance with the deadlines in this procurement. HHSC reserves the right to reject late submissions. It is the Respondent’s responsibility to appropriately mark and deliver the Proposal to HHSC by the specified date and time. The sole point of contact for inquiries concerning this RFP is:
Texas Health and Human Services Commission
Enterprise Contracts and Procurement Services
4405 North Lamar Blvd
Austin, Texas 78756-3422
ATT: Alice Hanna, Purchaser
(512) 206-5277
alice.hanna@hhsc.state.tx.us
|
|
4.2 Part 1 – Business Proposal |
The Business Proposal must include the following:
Section 1 – Executive Summary
Section 2 – Respondent Identification and Information
Section 3 – Corporate Background and Experience
Section 4 – Material Subcontractor Information
Section 5 – HUB Subcontracting Plan
Section 6 – Certifications and Other Required Forms
|
|
4.2.1 Section 1 – Executive Summary |
(2 pages, excluding Table 1)
In this section, condense and highlight the content of the Business Proposal to provide HHSC with a broad understanding of the respondent’s approach to meeting the RFP’s business requirements. The summary must demonstrate an understanding of
HHSC’s goals and objectives for this procurement. Please identify the Respondent’s proposed MCO Program(s) and the Service Areas. The Respondent should complete Table 1 by placing an “X” in all Service Areas and MCO Programs bid. (The Service Areas are described in the Attachments B-5, 5.1, 5.2, and 5.3. A Respondent may elect to bid on some, all, or none of the Service Areas.) Respondents should note that, for purposes of bidding, HHSC has subdivided the Medicaid Rural Service Area into three (3) areas – West, Central, and Northeast Texas. Respondents may bid on one (1) or more of these areas; however, HHSC will more favorably evaluate responses that propose to serve all three (3) areas.
Table 1: Proposed MCO Programs and Service Areas
|
| | | |
| | | |
Service Area | Proposal for STAR | Proposal for STAR+PLUS | Proposal for CHIP |
Bexar | | | |
Dallas | | | |
El Paso | | | |
Harris | | | |
Hidalgo | | | |
Jefferson | | | |
Lubbock | | | |
Medicaid RSA (Entire Service Area) | | | |
West Texas | | | |
Central Texas | | | |
Northeast Texas | | | |
Nueces | | | |
Tarrant | | | |
Travis | | | |
|
|
4.2.2 Section 2 – Respondent Identification and Information |
(no page limit)
Submit the following information:
|
| |
| 1. Respondent identification and basic information. |
|
| |
| a. The Respondent’s legal name, trade name, dba, acronym, and any other name under which the Respondent does business. |
|
| |
| b. The physical address, mailing address, and telephone number of the Respondent’s headquarters office. |
|
| |
| 2. TDI Authority. A copy of the MCO’s licensure, certification, or approval to operate as an HMO, ANHC, or EPBP. If the Respondent has not received TDI approval, then submit a copy of the application filed with TDI. In accordance with RFP Section 7.2.9, the Respondent must receive TDI approval no later than 60 days after HHSC executes the Contract. |
|
| |
| 3. Authorized Counties. Indicate whether the Respondent is currently authorized by TDI to operate as an MCO in each county in the Service Area with a “Yes-MCO,” “No MCO,” or “Partial MCO.” If the Respondent is not authorized to conduct business as an MCO in all or part of a county, it should list those areas in Column C. |
|
| |
| For each county listed in Column C, the Respondent must document that it applied to TDI for such approval prior to the submission of a Proposal for this RFP. The Respondent must indicate the date that it applied for such approval and the status of its application to get TDI approval in the relevant counties in this section of its submission to HHSC. |
Table 2: TDI Authority in Proposed Service Area
|
| | |
| | |
Column A | Column B | Column C |
Service Area | TDI Authority/Status of Approval | Counties/Partial Counties without TDI Authority |
Bexar | | |
Dallas | | |
El Paso | | |
Harris | | |
Hidalgo | | |
Jefferson | | |
Lubbock | | |
Medicaid RSA (Entire Service Area) | | |
West Texas | | |
Central Texas | | |
Northeast Texas | | |
Nueces | | |
Tarrant | | |
Travis | | |
|
| |
| 4. Texas Comptroller Certificate. A current Certificate of Good Standing issued by the Texas Comptroller of Public Accounts, or an explanation for why this form is not applicable to the Respondent. |
|
| |
| 5. Respondent Legal Status and Ownership. |
|
| |
| a. The type of ownership of the Respondent by its ultimate parent: |
|
| |
| • wholly-owned subsidiary of a publicly-traded corporation; |
|
| |
| • wholly-owned subsidiary of a private (closely-held) stock corporation; |
|
| |
| • subsidiary or component of a non-profit foundation; |
|
| |
| • subsidiary or component of a governmental entity such as a County Hospital District; |
|
| |
| • independently-owned member of an alliance or cooperative network; |
|
| |
| • joint venture (describe ultimate owners) |
|
| |
| • stand-alone privately-owned corporation (no parents or subsidiaries); or |
|
| |
| b. The legal status of the Respondent and its parent (any/all that may apply): |
|
| |
| (i.) Respondent is a corporation, partnership, sole proprietor, or other (describe); |
|
| |
| • Respondent is for-profit, or non-profit; |
|
| |
| • the Respondent’s ultimate parent is for-profit, or non-profit; |
|
| |
| • the Respondent’s ultimate parent is privately-owned, listed on a stock exchange, a component of government, or other (describe). |
|
| |
| c. The legal name of the Respondent’s ultimate parent (e.g., the name of a publicly-traded corporation, or a County Hospital District, etc.). |
|
| |
| d. The name and address of any other sponsoring corporation, or others (excluding the Respondent’s parent) who provide financial support to the Respondent, and the type of support, e.g., guarantees, letters of credit, etc. Indicate if there are maximum limits of the additional financial support. |
|
| |
| 6. Hospital District/Non-Profit Corporation. Section 5 of the RFP requires Respondents who believe they qualify for mandatory STAR or STAR+PLUS contracts under Texas Government Code §533.004 to submit notice to HHSC no later than April 28, 2011, explaining the basis for this belief for each proposed Service Area. Please indicate whether the Respondent provided such notice to HHSC. |
|
| |
| 7. The name and address of any health professional that has at least a five percent (5%) financial interest in the Respondent, and the type of financial interest. |
|
| |
| 8. The full names and titles of the Respondent’s officers and directors. |
|
| |
| 9. The state in which the Respondent is incorporated, and the state(s) in which the Respondent is licensed to do business as an MCO. The Respondent must also indicate the state where it is commercially domiciled, if outside Texas. |
|
| |
| 10. The Respondent’s federal taxpayer identification number. |
|
| |
| 11. If any change of ownership of the Respondent’s company or its parent is anticipated during the 12 months following the Proposal Due Date, the Respondent must describe the circumstances of such change and indicate when the change is likely to occur. |
|
| |
| 12. Whether the Respondent or its parent (including other managed care subsidiaries of the parent) had a managed care contract terminated or not renewed for any reason within the past five (5) years. In such instance, the Respondent must describe the issues and the parties involved, and provide the address and telephone number of the principal terminating party. The Respondent must also describe any corrective action taken to prevent any future occurrence of the problem(s) that may have led to the termination or non-renewal. |
|
| |
| 13. Whether the Respondent has ever sought, or is currently seeking, National Committee for Quality Assurance (NCQA) or American Accreditation HealthCare Commission (URAC) accreditation status, and if it has or is, indicate: |
|
| |
| • its current NCQA or URAC accreditation status; |
|
| |
| • if NCQA or URAC accredited, its accreditation term effective dates; and |
|
| |
| • if not accredited, a statement describing whether and when NCQA or URAC accreditation status was ever denied the Contractor. |
|
| |
| 14. The website address (URL) for the homepage(s) of any website(s) operated, owned, or controlled by the Respondent, including any that the Respondent may have contracted to be run by another entity. If the Respondent has a parent, then also provide the same for the parent, and any parent(s) of the parent. If none exist, provide a clear and definitive statement to that effect. |
|
|
4.2.3 Section 3 – Corporate Background and Experience |
(no page limit)
1. Provide the following information on all publicly-funded managed care contracts (if the Respondent does not have publicly-funded managed care contracts, it may include information on privately-funded managed care contracts). Include information for all current contracts, as well as work performed in the past three (3) years:
|
| |
| a. client name and address; |
|
| |
| b. name, telephone, and e-mail address of the person HHSC could contact as a reference that can speak to the Respondent’s performance; |
|
| |
| c. contract size: average monthly covered lives and annual revenues; |
|
| |
| d. whether payments under the contract were capitated or non-capitated; |
|
| |
| e. contract start date and duration; |
|
| |
| f. whether work was performed as a prime contractor or subcontractor; and |
|
| |
| g. a general and brief description of the scope of services provided by the Respondent; including the covered population and services (e.g., Medicaid, CHIP, state-funded program). |
2. With respect to the Respondent and its parent (and including other managed care subsidiaries of the parent), briefly describe any regulatory actions, sanctions, and/or fines imposed by any federal or Texas regulatory entity, or a regulatory entity in another state, within the last three (3) years. Include a description of any letters of deficiencies, corrective actions, findings of non-compliance, and/or sanctions. Please indicate which of these actions or fines, if any, were related to Medicaid or CHIP programs. HHSC may, at its option, contact these clients or regulatory agencies and any other individual or organization whether or not identified by the Respondent.
Respondents should not include letters of support or endorsement from any individual, organization, agency, interest group, or other identified entity in this section or other parts of the Proposal.
When evaluating proposals, HHSC may consider a current or past contractor's performance under an agreement with an HHS agency in Texas, including but not limited to any corrective actions or liquidated damages imposed by HHSC or another HHS agency.
|
|
4.2.3.1 Organizational Chart |
(1 page narrative for each organizational chart, excluding organizational chart itself)
Respondents should submit the following:
|
| |
| 1. an organizational chart (Chart A), showing the corporate structure and lines of responsibility and authority in the administration of the Respondent’s business as a health plan; |
|
| |
| 2. an organizational chart (Chart B) showing the Texas organizational structure and how it relates to the proposed Service Area(s), including staffing and functions performed at the local level. If Chart A represents the entire organizational structure, label the submission as Charts A and B; |
|
| |
| 3. an organizational chart (Chart C) showing the Management Information System (MIS) staff organizational structure and how it relates to the proposed Service Area(s),including staffing and functions performed at the local level; |
|
| |
| 4. if the Respondent is proposing to use one or more Material Subcontractors, the Respondent must include an organizational chart demonstrating how the Material Subcontractor(s) will be managed within the Respondent’s Texas organizational structure, including the primary individuals at the Respondent’s organization and at each Material Subcontractor organization responsible for overseeing such Material Subcontract. This information may be included in Chart B, or in a separate organizational chart(s); and |
|
| |
| 5. submit a brief narrative explaining the organizational charts submitted, and highlighting the key functional responsibilities and reporting requirements of each organizational unit relating to the Respondent’s proposed management of the MCO Program(s), including its management of any proposed Material Subcontractors. |
(1 page per Key Personnel, excluding résumés)
Identify and describe the Respondent’s and its Subcontractor’s proposed labor skill set, years of experience, and provide résumés of all proposed key personnel. Résumés must demonstrate experience germane to the position proposed. Résumés should include work on projects cited under the respondent’s corporate experience, and the specific functions performed on such projects. Each résumé should include at least three (3) references from recent projects, if the projects were performed for unaffiliated parties. References may not be the Respondent’s or Subcontractor’s employees.
Key personnel include: Executive Director (as defined in Attachment A, Article 4), Medical Director (as defined in Attachment A, Article 4), Member Services Manager, Service Coordination Manager (STAR+PLUS only), Management Information Systems Manager, Claims Processing Manager, Provider Network Development Manager, Benefit Administration and Utilization Management Manager, Quality Improvement Manager, Behavioral Health Services Manager, Financial Functions Manager, and Reporting Manager.
STAR+PLUS Service Coordinators. Please refer to Section 8.3.2.1 for a description of Service Coordinator responsibilities. In addition to the Service Coordinator Manager, please submit the following for each Service Coordinator function:
|
| |
| 1. a job description and qualifications; and |
|
| |
| 2. the anticipated maximum caseload for each Service Coordinator (number of Members per Service Coordinator) and the assumptions the Respondent used in developing the maximum caseload estimate. |
|
|
4.2.3.3 Financial Capacity |
(no page limit)
Submit the following financial documents to demonstrate the Respondent’s financial solvency, and its capacity to comply with Section 6, “Premium Payment, Incentives, and Disincentives,” and Section 8, “Operations Phase Requirements,” and Attachment A, “Uniform Managed Care Contract Terms and Conditions”:
|
| |
| 1. Audited Financial Statements covering the two (2) most recent years of the Respondent’s financial results. These statements must include the independent auditor’s report (audit opinion letter to the Board or shareholders), the notes to the financial statements, any written description(s) of legal issues or contingencies, and any management discussion or analysis. |
|
| |
| Make sure that the name and address of the firm that audits the Respondent is shown. State the date of the most-recent audit, and whether the Respondent is audited annually or otherwise. State definitively if there has, or has not, been any of the following: |
|
| |
| • a “going concern” statement was issued by any auditor in the last three (3) years; |
|
| |
| • a qualified opinion was issued by any auditor in the last three (3) years; |
|
| |
| • a change of audit firms in the last three (3) years; and |
|
| |
| • any significant delay (two (2) months or more) in completing the current audit. |
|
| |
| 2. The most recent quarterly and annual financial statements filed with the TDI, and if the Respondent is domiciled in another state, the financial statements filed with the state insurance department in its state of domicile. The annual financial statement must include all schedules, attachments, supplements, management discussion, analysis and actuarial opinions. |
|
| |
| 3. The most recent financial examination report issued by TDI, and also by any state insurance department in states where the Respondent operates a Medicaid, CHIP, or comparable managed care product. If any submitted financial examination report is two (2) or more years old, or if Respondent has never had a financial examination report issued, submit the anticipated approximate date of the next issuance of a TDI or state department of insurance financial examination report. |
|
| |
| 4. The most recent Form B Registration Statement disclosure filed by Respondent with TDI, and any similar form filed with any state insurance department in other states where the Respondent operates a Medicaid, CHIP, or comparable managed care product. If Respondent is exempt from the TDI Form B filing requirement, demonstrate this and explain the nature of the exemption. |
5. Other related documents, as applicable:
|
| |
| a. SEC Form 10-K and 10-Q. If Respondent is a publicly-traded (stock-exchange-listed) corporation, then submit the most recent United States Securities and Exchange Commission (SEC) Form 10K Annual Report, and the most-recent 10-Q Quarterly report. |
|
| |
| b. IRS Form 990. If the Respondent is a non-profit entity, then submit the most recent annual Internal Revenue Service (IRS) Form 990 filing, complete with any and all attachments or schedules. If Respondent is a non-profit entity that is exempt from the IRS 990 filing requirement, demonstrate this and explain the nature of the exemption. |
c. If the Respondent is a non-profit entity that is a component or subsidiary of a County Hospital District, or otherwise an entity of a government, then submit the most recent annual financial statements as prepared under the relevant rules or statutes governing annual financial reporting and disclosure for Respondent, including all attachments, schedules, and supplements.
|
| |
| d. Bond or debt rating analysis. If Respondent has been, in the last three (3) years, the subject of any bond rating analysis, ratings affirmation, write-up, or related report, such as by AM Best, Fitch Ratings, Moody’s, Standard & Poor, etc., submit the most-recent detailed report from each rating entity that has produced such a report. |
|
| |
| e. Annual Report. If Respondent produces any written “annual report” or similar item that is in addition to the above-referenced documents, submit the most recent version. This might be a yearly report or letter to shareholders, the community, regulators, lenders, customers, employees, the Respondent’s owner, or other constituents. |
|
| |
| f. If the Respondent has issued any press releases in the 12 months prior to the submission due date, wherein the press release mentions or discusses financial results, acquisitions, divestitures, new facilities, closures, layoffs, significant contract awards or losses, penalties/fines/sanctions, expansion, new or departing officers or directors, litigation, change of ownership, or other very similar issues, provide a copy of each such press release. HHSC does not wish to receive other types of press releases that are primarily promotional in nature. |
With respect to items 5(a) through (e) above, Respondent must also submit a schedule that shows for each of the five (5) categories: whether there is any applicable filing or report; the name(s) of the entity that does the filing or report; and the regular or estimated filing/distribution date(s).
At a minimum, the financial statements and reports submitted hereunder must include:
|
| |
| 2. statement of income and expense; |
|
| |
| 3. statement of cash flows; |
|
| |
| 4. statement of changes in financial position (capitol & surplus; equity); |
|
| |
| 5. independent auditor’s letter of opinion; |
|
| |
| 6. description of organization and operation, including ownership, markets served, type of entity, number of locations and employees, and, dollar amount and type of any Respondent business outside of that with HHSC; and |
|
| |
| 7. disclosure of any material contingencies, and any current, recent past, or known potential material litigation, regulatory proceedings, legal matters, or similar issues. |
The Respondent must include key non-financial metrics and descriptions, such as facilities, number of covered lives, area of geographic coverage, years in business, material changes in business situation, key risks and prospective issues, etc.
|
|
4.2.3.4 Financial Report of Parent Organization and Corporate Guarantee |
(no page limit)
If another corporation or entity either substantially or wholly owns the Respondent, submit the most recent detailed financial reports (as required above in Section 4.2.3.3) for the parent organization. If there are one (1) or more intermediate owners between the Respondent and the ultimate owner, this additional requirement is applicable only to the ultimate owner.
The Respondent must also include a statement that the parent organization will unconditionally guarantee performance by the Respondent of each and every obligation, warranty, covenant, term and condition of the Contract. This guarantee is not required for Respondents owned by political subdivisions of the State (i.e., hospital districts).
If HHSC determines that an entity does not have sufficient financial resources to guarantee the Respondent’s performance, HHSC may require the Respondent to obtain another acceptable financial instrument or resource from such entity, or to obtain an acceptable guarantee from another entity with sufficient financial resources to guarantee performance.
The Respondent must submit a statement that, if selected as a Contractor, the Respondent agrees to:
1. secure and maintain throughout the life of the Contract, fidelity bonds required by the Texas Department of Insurance in compliance with §843.402, Texas Insurance Code; and
2. secure and maintain throughout the life of the Contract, a performance bond in accordance with the Attachment A, “Uniform Managed Care Contract Terms and Conditions” and 28 T.A.C. §11.1805.
|
|
4.2.4 Section 4 – Material Subcontractor Information |
(no page limit)
See Attachment A, “Uniform Managed Care Contract Terms and Conditions,” for contractual definition of Material Subcontractor. Organize this information by Material Subcontractor, and list them in descending order of estimated annual payments. For each Material Subcontractor, the MCO must provide:
|
| |
| 1. The Material Subcontractor’s legal name, trade name, acronym, d.b.a., and any other name under which the Material Subcontractor does business. |
|
| |
| 2. The Respondent’s estimated annual payments to the Material Subcontractor, by MCO Program. |
|
| |
| 3. The physical address, mailing address, and telephone number of the Material Subcontractor’s headquarters office, and the name of its Chief Executive Officer. |
|
| |
| 4. Whether the Material Subcontractor is an Affiliate of the Respondent or an unrelated third party (see the “Uniform Managed Care Contract Terms and Conditions” for the definition of “Affiliate.”) |
|
| |
| 5. If the Material Subcontractor is an Affiliate, then provide: |
|
| |
| a. the name of the Material Subcontractor’s parent organization, and the Material Subcontractor’s relationship to the Respondent; |
|
| |
| b. the proportion, if any, of the Material Subcontractor’s total revenues that are received from non-Affiliates. If the Material Subcontractor has significant revenues from non-Affiliates, then also indicate the portion, if any, of those external (non-Affiliate) revenues that are for services similar to those that the Respondent would procure under the proposed Subcontract; |
|
| |
| c. a description of the proposed method of pricing under the Subcontract; |
|
| |
| d. indicate if the Respondent presently procures, or has ever procured, similar services from a non-Affiliate; |
|
| |
| e. the number of employees (staff and management) who are dedicated full-time to the Affiliate’s business; |
|
| |
| f. whether the Affiliate’s office facilities are completely separate from the Respondent and the Respondent’s parent. If not, identify the approximate number of square feet of office space that are dedicated solely to the Affiliate’s business; |
|
| |
| g. attach an organization chart for the Affiliate, showing head count, Key Personnel names, titles, and locations; and |
|
| |
| h. indicate if the staff and management of the Affiliate are directly employed by the Affiliate itself, or are they actually, from a technical legal perspective, employed by a different legal entity (such as a parent corporation). What corporation’s name shows up on the employee’s W2 form? |
|
| |
| 6. A description of each Material Subcontractor’s corporate background and experience, including its estimated annual revenues from unaffiliated parties, number of employees, location(s), and identification of three (3) major clients. |
|
| |
| 7. A signed letter of commitment from each Material Subcontractor that states the Material Subcontractor’s willingness to enter into a Subcontractor agreement with the Respondent, and a statement of work for activities to be subcontracted. Letters of Commitment must be provided on the Material Subcontractor’s official company letterhead, signed by an official with the authority to bind the company for the subcontracted work. The Letter of Commitment must state, if applicable, the company’s certified HUB status. |
|
| |
| 8. The type of ownership [e.g., wholly-owned subsidiary of a publicly-traded corporation; wholly-owned subsidiary of a private (closely-held) stock corporation; subsidiary or component of a non-profit foundation; subsidiary or component of a governmental entity such as a County Hospital District; independently-owned member of an alliance or cooperative network; joint venture (describe owners); etc.] Indicate the name of the ultimate owner (e.g., the name of a publicly-traded corporation or a County Hospital District). |
|
| |
| 9. Indicate status (any/all that may apply): sole proprietor, partnership, corporation, for-profit, non-profit, privately owned, and/or listed on a stock exchange. If a Subsidiary or Affiliate, name of the direct and ultimate parent organization. |
|
| |
| 10. The name and address of any sponsoring corporation or others who provide financial support to the Material Subcontractor and the type of support, e.g., guarantees, letters of credit, etc. Indicate if there are maximum limits of the additional financial support. |
|
| |
| 11. The name and address of any health professional that has at least a five percent (5%) financial interest in the Material Subcontractor and the type of financial interest. |
|
| |
| 12. The state in which the Material Subcontractor is incorporated, commercially domiciled, and the state(s) in which the organization is licensed to do business. |
|
| |
| 13. The Material Subcontractor’s federal taxpayer identification number. |
|
| |
| 14. Whether the Material Subcontractor had a managed care contract terminated or not renewed for any reason within the past five (5) years. In such instance, the Respondent must describe the issues, the parties involved, and provide the address and telephone number of the principal terminating party. The Respondent must also describe any corrective action taken to prevent any future occurrence of the problem that may have lead to the termination. |
|
| |
| 15. Whether the Material Subcontractor has ever sought, or is currently seeking, National Committee for Quality Assurance (NCQA) or American Accreditation HealthCare Commission (URAC) accreditation or certification status, and if it has or is, indicate: |
|
| |
| • its current NCQA or URAC accreditation or certification status; |
|
| |
| • if NCQA or URAC accredited or certified, its accreditation or certification term effective dates; and |
|
| |
| • if not accredited, a statement describing whether and when NCQA or URAC accreditation status was ever denied the Material Subcontractor. |
|
| |
| 16. The website address (URL) for the homepage(s) of any website(s) operated, owned, or controlled by the Material Subcontractor, including any websites run by another entity on the Material Subcontractor’s behalf. If the Material Subcontractor has a parent, then also provide the same for the parent organization, and any parent(s) of the parent organization. If none exist, provide a clear and definitive statement to this effect. |
|
|
4.2.5 Section 5 – Historically Underutilized Business (HUB) Participation |
In accordance with Texas Government Code §2162.252, a proposal that does not contain a HUB Subcontracting Plan (HSP) is non-responsive and will be rejected without further evaluation. In addition, if HHSC determines that the HSP was not developed in good faith, it will reject the proposal for failing to comply with material RFP specifications.
HHSC is committed to promoting full and equal business opportunities for businesses in state contracting in accordance with the goals specified in the State of Texas Disparity Study. HHSC encourages the use of HUBs through race, ethnic and gender-neutral means. HHSC has adopted administrative rules relating to HUBs, and a policy on the Utilization of HUBs, which is located on HHSC’s website.
Pursuant to Texas Government Code §2161.181 and §2161.182, and HHSC’s HUB policy and rules, HHSC is required to make a good faith effort to increase HUB participation in its contracts. HHSC may accomplish the goal of increased HUB participation by contracting directly with HUBs or indirectly through subcontracting opportunities.
|
|
4.2.5.2 HHSC’s Administrative Rules |
HHSC has adopted the Comptroller of Public Accounts’ (CPA) HUB rules as its own. HHSC’s rules are located in Title 1, Part 15, Chapter 392, Subchapter J of the Texas Administrative Code, and the CPA rules are located in Title 34, Part 1, Chapter 20, Subchapter C. If there are any discrepancies between HHSC’s administrative rules and this RFP, the rules will take priority.
|
|
4.2.5.3 HUB Participation Goal |
The CPA has established statewide HUB participation goals for different categories of contracts in 34 T.A.C. §20.13. In order to meet or exceed the HUB participation goals, HHSC encourages outreach to certified HUBs. Contractors must make a good faith effort to include certified HUBs in the procurement process.
This contract is classified as an “All Other Services” contract under the CPA rule, and therefore has a HUB Annual Procurement Utilization Goal of 33% per fiscal year. This goal applies to MCO Administrative Services, as defined below.
|
|
4.2.5.4 Required HUB Subcontracting Plan |
HHSC has determined that subcontracting opportunities are probable for this RFP for MCO Administrative Services. MCO Administrative Services are those services or functions other than the direct delivery of medical Covered Services necessary to manage the delivery of and payment for such services. MCO Administrative Services include but are not limited to Network, utilization, clinical and/or quality management, service authorization, claims processing, Management Information System (MIS) operation and reporting. The Respondent must submit an HSP (see the Procurement Library) with its proposal for such MCO Administrative Services. The HSP is required whether or not a Respondent intends to subcontract.
HSP requirements will not apply to Subcontracts with Network Providers (providers who contract directly with the MCO to deliver medical Covered Services to Members). A Respondent therefore should not include Network Providers’ participation in its HSP submissions.
In conjunction with the HSP, a Respondent must indicate whether it is a Texas certified HUB. Being a certified HUB does not exempt a respondent from completing the HSP requirement.
During the good faith effort evaluation, HHSC may, at its discretion, allow clarifications or request additional information to support the Respondent’s good faith effort development of the HSP.
|
|
4.2.5.5 CPA Centralized Master Bidders List |
Respondents may search for HUB subcontractors in the CPA’s Centralized Master Bidders List (CMBL) HUB Directory, which is located on the CPA’s website at http://www2.cpa.state.tx.us/cmbl/cmblhub.html. For this procurement, HHSC has identified the following class and item codes for potential subcontracting opportunities:
NIGP Commodity Codes:
|
| |
| • 948-07: Administration Services, Health |
|
| |
| • 958-56: Health Care Management Services (Including Managed Care Services) |
|
| |
| • 915-49: High Volume, Telephone Call Answering Services (See 915-05 for Low Volume Services) |
Respondents are not required to use, nor limited to using, the class and item codes identified above, and may identify other areas for subcontracting.
HHSC does not endorse, recommend nor attest to the capabilities of any company or individual listed on the CPA’s CMBL. The list of certified HUBs is subject to change, so Respondents are encouraged to refer to the CMBL often to find the most current listing of HUBs.
|
|
4.2.5.6 HUB Subcontracting Procedures – If a Respondent Intends to Subcontract |
An HSP must demonstrate that the Respondent made a good faith effort to comply with HHSC’s HUB policies and procedures. The following subparts outline the items that HHSC will review in determining whether an HSP meets the good faith effort standard. A Respondent that intends to subcontract must complete the HSP to document its good faith efforts.
For step-by-step audio/video instructions on how to complete the HSP, you may also visit the CPA’s website at: http://www.cpa.state.tx.us/procurement/prog/hub/hub-subcontracting-plan/.
1. Identify Subcontracting Areas and Divide Them into Reasonable Lots
A Respondent should first identify each area of the MCO Administrative Service work it intends to subcontract. Then, to maximize HUB participation, it should divide the MCO Administrative Service work into reasonable lots or portions, to the extent consistent with prudent industry practices.
2. Notify Potential HUB Subcontractors
Respondents must notify three (3) or more certified HUBs of each subcontracting opportunity. For example, if a Respondent intends to subcontract two (2) areas of MCO Administrative Service work, then for each class/item code, the Respondent must notify at least three (3) vendors who provide that type of work.
Respondents must provide written notice to potential HUB subcontractors prior to submitting proposals. The notice must include:
|
| |
| 1. a description of the scope of work to be subcontracted; |
|
| |
| 2. information regarding the location to review project plans or specifications; |
|
| |
| 3. information about bonding and insurance requirements; |
|
| |
| 4. required qualifications and other contract requirements; and |
|
| |
| 5. a description of how the subcontractor can contact the Respondent. |
Respondents must give potential HUB subcontractors a reasonable amount of time to respond to the notice, generally no less than five (5) working days from receipt. In rare situations, HHSC will allow a shorter notification period if the Respondent demonstrates: (1) circumstances warranting a shorter notification period, and (2) potential subcontractors still had sufficient time to complete their responses.
Respondents must use the CMBL, the HUB Directory, and Internet resources when searching for HUB subcontractors. Respondents may rely on the services of contractor groups; local, state and federal business assistance offices; and other organizations that provide assistance in identifying qualified applicants for the HUB program. Respondents also must provide written notice to minority or women trade organizations or development centers, which can disseminate notice of subcontracting opportunities to their members/participants. A list of minority and women trade organizations is located on HHSC’s website under the Minority and Women Organization link.
3. Written Justification of the Selection Process
A Respondent must provide written justification of its selection process if it chooses a non-HUB subcontractor. The justification should demonstrate that the Respondent negotiated in good faith with qualified HUB bidders, and did not reject qualified HUBs who were the best value responsive bidders.
|
|
4.2.5.7 Alternatives to Good Faith Effort Requirements (Applies Only to Mentor Protégé and Professional Services Contracts) |
HHSC will accept a Mentor Protégé Agreement that has been entered into by a Respondent (mentor) and a certified HUB (protégé) in accordance with Texas Government Code §2161.065 .
Participation in the Mentor Protégé Program, along with the submission of a protégé as a subcontractor in an HSP, constitutes a good faith effort for the particular area subcontracted to the protégé. If a Respondent proposes to subcontract with a protégé, it does not need to provide notice to three (3) vendors for that subcontracted area. To demonstrate that a Respondent meets the good faith requirement for mentor/protégé arrangements, the HSP should:
|
| |
| 1. include a fully executed copy of the Mentor Protégé Agreement, which must be registered with the CPA prior to submission to HHSC; and |
|
| |
| 2. identify areas of the HSP that will be performed by the protégé. |
|
|
4.2.5.8 HUB Subcontracting Procedures – If a Respondent Does Not Intend to Subcontract |
If the Respondent plans to complete all MCO Administrative Service requirements with its own equipment, supplies, materials and/or employees, it is still required to complete an HSP. The Respondent must complete the “Self Performance Justification”
portion of the HSP, and attest that it does not intend to subcontract for any administrative goods or services, including the class and item codes identified in Section 4.2.5.5. In addition, the Respondent must identify the sections of the proposal that describe how it will complete the Scope of Work using its own resources or provide a statement explaining how it will complete the Scope of Work using its own resources. The Respondent must provide the following information regarding self-performance if requested by HHSC:
|
| |
| 1. evidence of sufficient Respondent staffing to meet the RFP requirements; |
|
| |
| 2. monthly payroll records showing the Respondent staff fully dedicated to the contract; and |
|
| |
| 3. documentation proving employment of qualified personnel holding the necessary licenses and certificates required to perform the Scope of Work. |
|
|
4.2.5.9 Post-award HSP Requirements |
After contract award, HHSC will coordinate a post-award meeting with the successful Respondents to discuss HSP reporting requirements. The MCO must maintain business records documenting compliance with the HSP, and must submit monthly reports to HHSC by completing the HUB “Prime Contractor Progress Assessment Report.” This monthly report is required as a condition for payment. In addition, the MCO must allow periodic onsite reviews of the MCO’s headquarters or work site where services are to be performed if requested by HHSC.
Once accepted, the finalized HSP will become part of the Contract with the successful Respondents. The Uniform Managed Care Manual utlines the procedures for changing the HSP, as well as the HSP compliance and reporting requirements. All changes to the approved HSP require prior HHSC approval. In general, if the MCO decides to subcontract any part of the Contract after the award, it must follow the good faith effort procedures outlined in
Section 4.2.5.6 e.g., divide work into reasonable lots, notify at least three (3) vendors per subcontracted area, provide written justification of the selection process, participate in the Mentor Protégé Program, or for professional services contracts meet the 20% goal). For this reason, HHSC encourages Respondents to identify, as part of their HSP, multiple subcontractors who are able to perform the work in each area the Respondent plans to subcontract. Selecting additional subcontractors may help the selected MCO make changes to its original HSP, when needed, and will allow HHSC to approve any necessary changes expeditiously.
Failure to meet the HSP and post-award requirements will constitute a breach of contract, and will be subject to remedial actions. HHSC may also report noncompliance to the CPA in accordance with the CPA’s respondent performance (see 34 T.A.C. §20.108) and debarment program (see 34 T.A.C. §20.105).
|
|
4.2.6 Section 6 – Certifications and Other Required Forms |
Respondents must submit the following required forms with their proposals:
|
| |
| 1. Child Support Certification; |
|
| |
| 2. Debarment, Suspension, Ineligibility, and Voluntary Exclusion of Covered Contracts; |
|
| |
| 3. Federal Lobbying Certification; |
|
| |
| 4. Nondisclosure Statement; |
|
| |
| 5. Required Certifications; and |
|
| |
| 6. Respondent Information and Disclosures. |
The required forms are located on HHSC’s website, under the “Business Opportunities” link. HHSC encourages Respondents to carefully review all of these forms and submit questions regarding their completion prior to the deadline for submitting questions (see Section 1.2, “Procurement Schedule”).
Respondents should note that the “Respondent Information and Disclosures” form asks Respondents to provide information on certain litigation matters. In addition to the information required on this form, Respondents must provide all of the information described in Uniform Managed Care Manual Chapter 5.8, “Report of Legal and Other Proceedings.” Respondents may include this supplemental information on the “Respondent Information and Disclosures” form, or under a separate submission.
|
|
4.3 Part 2 – Programmatic Proposal |
Respondents must provide a detailed description of the proposed programmatic solution, which must support all business activities and requirements described in the RFP. The Programmatic Proposal must reflect a clear understanding of the nature of the work undertaken.
Respondents should carefully read the submission requirement instructions for specific questions in this section. For each applicable programmatic submission requirement, the Respondent must indicate, in addition to the information requested in each subsection, the following information if applicable to the Respondent and its Proposal:
Material Subcontractor: If the Respondent plans to provide the service or perform the function through a Material Subcontractor, the Respondent must detail the services and/or function to be subcontracted, and how the Respondent and the Material Subcontractor will coordinate such service or function. Respondents should describe any prior working relationships with the Material Subcontractor.
Action Plan: This requirement applies to any Respondent who is not currently: (1) providing services or performing functions relating to a specific RFP submission requirement as a current vendor in STAR, STAR+PLUS, and/or CHIP, or (2) meeting the Operations Phase Requirements in Section 8 relating to a specific submission requirement for STAR, STAR+PLUS, and/or CHIP. In the Action Plan, the Respondent must, for each such submission requirement: (1) submit a description of its current comparable experience and abilities, if any; (2) describe how the Respondent will meet the Contract responsibilities, including assigned resources for completing such activities; and (3) and a timeline for completing such activities.
In responding to questions for which the Respondent includes information about a Material Subcontractor or Action Plans, up to one (1) page may be used to describe each Material Subcontractor arrangement and up to one (1) page may be used to describe each Action Plan. These pages are not included in the page limit instructions for the specific submission requirement.
HHSC understands that some Respondents may not have current experience providing managed care services to STAR, STAR+PLUS, and/or CHIP members in Texas. In responding to questions relating to experience, Respondents should clearly indicate if their experience is in Texas, and if their experience is with STAR, STAR+PLUS, CHIP, or other comparable populations of managed care members. For Respondents proposing to serve STAR+PLUS members, the Proposal should describe the Respondent’s experience with elderly and disabled populations, including persons eligible for Medicare.
The Programmatic Proposal must include a detailed description of the following program components, at a minimum:
|
| |
| 1. Section 1 – Proposed Programs, Service Area, and Capacity |
|
| |
| 2. Section 2 – Experience Providing Covered Services |
|
| |
| 3. Section 3 – Value-added Services |
|
| |
| 4. Section 4 – Access to Care |
|
| |
| 5. Section 5 – Provider Network Provisions |
|
| |
| 6. Section 6 – Member Services |
|
| |
| 7. Section 7 – Quality Assessment and Performance Improvement |
|
| |
| 8. Section 8 – Utilization Management |
|
| |
| 9. Section 9 – Early Childhood Intervention (ECI) |
|
| |
| 10. Section 10 – Services for People with Special Health Care Needs |
|
| |
| 11. Section 11 – Care Management/Service Coordination |
|
| |
| 12. Section 12 – Disease Management (DM)/Health Home Services |
|
| |
| 13. Section 13 – Behavioral Health Services and Network |
|
| |
| 14. Section 14 – Management Information Systems Requirements |
|
| |
| 15. Section 15 – Fraud and Abuse |
|
| |
| 16. Section 16 – Pharmacy Services |
|
| |
| 17. Section 17 – Transition Plan |
|
| |
| 18. Section 18 – Additional Requirements Regarding Dual Eligibles |
|
|
4.3.1 Section 1 – Proposed Programs, Service Area, and Capacity |
(3 pages, excluding tables)
The Respondent shall:
|
| |
| 1. complete the MCO Program Proposed Service Area and Capacity table found in the Procurement Library, which must include for each proposed Service Area indicated in Table 1 of the Respondent’s Executive Summary, an estimate of the number of HHSC MCO Members the Bidder has the capacity to serve in each MCO Program bid on the Operational Start Date; |
|
| |
| 2. describe the calculations and assumptions used to arrive at these Service Area capacity projections. In developing these projections, the Respondent should consider the capacity of its Network, including its PCP Network, its Behavioral Health Services Network, its specialty care Network, its Pharmacy Network, and for STAR+PLUS, its home and community-based services Network. Respondents should specify: |
|
| |
| • the anticipated STAR, STAR+PLUS, or CHIP Program enrollment, as applicable; |
|
| |
| • the expected utilization of services, taking into consideration the characteristics and health care needs of specific populations represented in the particular HHSC MCO Program; |
|
| |
| • the numbers and types (in terms of training, experience, and specialization) of providers required to furnish the Covered Services; |
|
| |
| • the numbers of Network Providers and providers with signed contracts, LOAs, or LOIs who are not accepting new patients, by MCO Program; |
|
| |
| • the geographic location of providers and HHSC MCO members, considering travel time, the means of transportation ordinarily used by HHSC MCO members, and whether the location provides physical access for members with disabilities; and |
|
| |
| • generally describe anticipated Service Area capacity changes, if any, for each of the proposed Service Areas over the Initial Contract Period; and |
|
| |
| 3. generally describe methods that the MCO will use to ensure access to all Covered Services upon potential population growth due to changes in law, including growth resulting from the Patient Protection and Affordable Care Act and Health Care and Education Reconciliation Act of 2010. |
|
|
4.3.2 Section 2 – Experience Providing Covered Services |
(3 pages, plus 1 additional page for each additional MCO Program bid, if any.)
Covered Services are described in Section 8.1.2, “Covered Services;” Section 8.2.2, “Provisions Related to Covered Services for Medicaid Members;” and Attachment B-1, “STAR Covered Services,” Attachment B-1.1, “CHIP Covered Services,” and Attachment B-1.2, “STAR+PLUS Covered Services.”
For all MCO Programs bid, the Respondent must:
|
| |
| 1. briefly describe the Respondent’s experience providing, on a capitated basis, Acute Care services, including Behavioral Health Services, equivalent or comparable to Covered Services included in the MCO Programs bid (STAR Covered Services are described in Attachment B-1, CHIP Covered Services are described in Attachment B-1.1, and STAR+PLUS Covered Services are described in Attachment B-1.2). The description should indicate: |
|
| |
| a. the extent to which the Respondent has experience providing such Acute Care services for a managed care population(s) comparable to the population in the MCO Programs bid; and |
|
| |
| b. the Respondent’s experience providing such Acute Care services in Texas, and in the Respondent’s proposed Service Areas, if applicable; |
|
| |
| 2. indicate which STAR or CHIP Covered Service(s) (in whole or in part) the Respondent does not have experience providing on a capitated basis or does not have experience providing to a comparable Medicaid or CHIP population; |
|
| |
| 3. for STAR+PLUS Respondents, briefly describe the Respondent’s experience providing managed Community-based Long-Term Services and Supports and Acute Care services equivalent or comparable to STAR+PLUS Covered Services described in Attachment B-1.2. The description should indicate: |
|
| |
| a. the extent to which the Respondent has experience providing Community-based Long-Term Services and Supports and Acute Care services for a managed care population(s) comparable to the population in STAR+PLUS; and |
|
| |
| b. the Respondent’s experience providing such Community-based Long-Term Services and Supports in Texas, and in the Respondent’s proposed Service Areas, if applicable; |
|
| |
| 4. indicate which STAR+PLUS Covered Service(s) (in whole or in part) the Respondent does not have experience providing on a capitated basis or does not have experience providing to a comparable Medicaid population; |
|
| |
| 5. briefly describe the Respondent’s proposal for providing Covered Services, including any plans for expansions of its Provider Network in any of the proposed Service Areas prior to a Readiness Review. If the Respondent proposes to use a Material Subcontractor to provide or manage Behavioral Health Services, Pharmacy Services, or any other Covered Service, the Respondent must describe its relationship with the Material Subcontractor, as required by Section 4.3; |
|
| |
| 6. for STAR Respondents for the Medicaid Rural Service Area, describe the Respondent’s experience in providing Medicaid wrap-around services for Dual Eligibles entitled to these benefits. If the Respondent does not have experience in providing these services, indicate how the Respondent intends to meet this requirement; and |
|
| |
| 7. for STAR+PLUS Respondents, describe the Respondent’s experience in providing Service Coordination for Dual Eligibles. Respondent should specifically describe the processes and procedures used to coordinate Medicare services with Medicaid Community-based Long-Term Services and Supports and related services. If the Respondent does not have experience coordinating these services, indicate how the Respondent intends to meet this requirement. |
|
|
4.3.3 Section 3 – Value-added Services |
(1 page per Value-added Service)
Respondents may propose to offer Value-added Services as described in Section 8.1.2.1. If offered, the Respondent will not receive additional compensation for Value-added Services, and may not report the costs of Value-added Services as allowable medical or administrative costs.
For each MCO Program and Value-added Service proposed, the Respondent must:
|
| |
| 1. define and describe the Value-added Service; |
|
| |
| 2. specify the applicable Service Areas for the proposed Value-added Services; |
|
| |
| 3. identify the category or group of Members eligible to receive the proposed Value-added Services if it is a type of service that is not appropriate for all Members; |
|
| |
| 4. note any limitations or restrictions that apply to the Value-added Services; |
|
| |
| 5. for each Service Area, identify the types of Providers responsible for providing the Value-added Service, including any limitations on Provider capacity if applicable. |
|
| |
| 6. propose how and when Providers and Members will be notified about the availability of such Value-added Service; |
|
| |
| 7. describe how a Member may obtain or access the Value-added Service; |
|
| |
| 8. include a statement that the Respondent will provide any Value-added Service(s) that are approved by HHSC for at least 12 months after the Operational Start Date of the Contract; and |
|
| |
| 9. describe if, and how, the Respondent will identify the Value-added Service in administrative data (Encounter Data). |
The Respondent may propose different Value-added Services for each MCO Program and Service Area bid.
|
|
4.3.4 Section 4 – Access to Care |
Access to Care standards are described in Section 8.1.3.
(no page limit, should only submit applicable tables)
For each proposed Service Area and for each MCO Program bid (if the proposed Provider Network would be different across MCO Programs within a Service Area), submit tables created using GeoAccess, or a comparable software program, to demonstrate the geographic adequacy of the Respondent’s proposed Provider Network compared to the projected population in each proposed Service Area.
Providers in the demonstrated Provider Network must have an executed contract with the Respondent, a letter of intent (LOI), or a letter of agreement (LOA) indicating the provider intends to contract with the Respondent if HHSC awards the Respondent an MCO Contract. Respondents do not need to submit the signed contracts, LOIs, or LOAs with the Proposal, but HHSC may request to review these documents during its evaluation of the Proposal. Providers who have not signed a Network Provider contract or LOI/LOAs may not be included in the Respondent’s Network for purposes of responding to this RFP submission requirement.
For each proposed Service Area, the Respondent must generate GeoAccess or comparable tables to display the following information on its proposed Provider Network utilizing the Member Files provided by HHSC. For purposes of Geo Mapping, the distribution method will be to place all members at the center of the zip code.
|
| |
| 1. adults with access to PCPs (STAR and STAR+PLUS only): |
|
| |
| a. Percentage and number of adult Members with access to one (1) Open-Panel, age-appropriate Network PCP within 30 miles, and the average number of miles within which adults have such access; |
|
| |
| b. Percentage and number of adult Members with access to two (2) Open-Panel, age-appropriate Network PCPs within 30 miles, and the average number of miles within which adults have such access; |
|
| |
| 2. children with access to PCPs: |
|
| |
| a. Percentage and number of child Members with access to one (1) Open-Panel, age-appropriate Network PCP within 30 miles, and the average number of miles within which children have such access; |
|
| |
| b. Percentage and number of child Members with access to two (2) Open-Panel, age-appropriate Network PCPs within 30 miles, and the average number of miles within which children have such access; |
|
| |
| 3. access to cardiologists (STAR and STAR+PLUS only): |
|
| |
| a. Percentage and number of adult Members with access to one (1) Network cardiologist within 75 miles, and the average number of miles within which adults have such access; |
|
| |
| b. Percentage and number of adult Members with access to two (2) Network cardiologists within 75 miles, and the average number of miles within which adults have such access; |
|
| |
| 4. access to Acute Care Hospitals: |
|
| |
| a. Percentage and number of Members with access to a Network Acute Care Hospital within 30 miles; |
|
| |
| 5. access to outpatient Behavioral Health Services Providers (does not apply to the STAR Dallas Service Area, where Behavioral Health services are provided through NorthSTAR): |
|
| |
| a. Percentage and number of Members with access to one (1) Network outpatient Behavioral Health Service Provider within 75 miles, and the average number of miles within which Members have such access; |
|
| |
| b. Percentage and number of Members with access to two (2) Network outpatient Behavioral Health Providers within 75 miles, and the average number of miles within which Members have such access; |
|
| |
| 6. access to OB/GYNs (does not apply to CHIP Members or CHIP Perinatal Newborn Members – but does apply to CHIP Perinate Members (unborn children)): |
|
| |
| a. Percentage and number of female Members over age 19 with access to one (1) Network OB/GYN within 75 miles, and the average number of miles within which such female Members have such access (applies to Medicaid Members and CHIP Perinate Members in both urban and rural areas); |
|
| |
| b. Percentage and number of female Members over age 19 with access to two (2) Network OB/GYNs within 75 miles, and the average number of miles within which such female Members have such access(applies to Medicaid Members and CHIP Perinate Members in both urban and rural areas); |
|
| |
| c. Percentage and number of CHIP Perinate Members in rural areas with access to one (1) Network OB/GYN within 125 miles, and the average number of miles within which such Members have such access; |
|
| |
| d. Percentage and number of CHIP Perinate Members in rural areas with access to one (1) Network OB/GYN within 125 miles, and the average number of miles within which such Members have such access; |
|
| |
| 7. access to otolaryngologists (STAR and CHIP only): |
|
| |
| a. Percentage and number of child Members with access to one (1) Network otolaryngologist (ENT) within 75 miles, and the average number of miles within which children have such access; and |
|
| |
| b. Percentage and number of child Members with access to two (2) Network otolaryngologists (ENTs) within 75 miles, and the average number of miles within which children have such access; and |
|
| |
| a. Percentage and number Members with access to one (1) Network pharmacy within 15 miles, and the average number of miles within which Members have such access; |
|
| |
| b. Percentage and number Members with access to two (2) Network pharmacies within 15 miles, and the average number of miles within which Members have such access; |
|
| |
| c. Percentage and number Members with access to one (1) 24 hour Network pharmacy within 75 miles, and the average number of miles within which Members have such access; and |
|
| |
| d. Percentage and number Members with access to two (2) 24 hour Network pharmacies within 75 miles, and the average number of miles within which Members have such access. |
Respondents should submit one (1) set of the above tables for each MCO Program and Service Area bid (e.g, one (1) table for the STAR Tarrant Service Area, one (1) table for the STAR Harris Service Area, etc.). Respondents should report the zip code, the city or town associated with the zip code, the percentage and number of eligible Members residing within the zip code, and the percentage and number of eligible Members residing within a zip code who have access to Network Provider addresses within the HHSC-specified travel distance standard. Each table should be sorted in descending order based on zip code-eligible Member population. In addition, each Service Area table should report the aggregate percentage of eligible Members residing within the Service Area who have access within the HHSC-specified travel standard.
|
|
4.3.4.2 Assessing Access to Care |
(3 pages, plus one additional page per additional MCO Program bid if the Respondent’s response is different by MCO Program)
|
| |
| 1. Identify the process(es) by which the Respondent must measure and regularly verify: |
|
| |
| a. Network compliance, including pharmacy, regarding travel distance access in Section 8.1.3.2; |
|
| |
| b. Provider compliance regarding appointment access standards in Section 8.1.3.1, and |
|
| |
| c. PCP compliance with after-hours coverage standards in Section 8.1.4.2. |
|
| |
| 2. Describe the steps the Respondent has taken in the past when it identified: |
|
| |
| a. a deficiency in its compliance with plan or state travel distance access standards; |
|
| |
| b. a Provider that was not meeting plan or state appointment access standards, and |
|
| |
| c. a PCP that was not in compliance with the plan or state after-hours coverage requirements. |
|
| |
| If the Respondent has not taken such steps listed in 2a, b, or c above with regularity, describe how it proposes to take such steps in the future. |
|
| |
| 3. Describe the processes the Respondent implement to accommodate additional Members and to ensure the access standards are met if actual enrollment exceeds projected enrollment. |
|
|
4.3.5 Section 5 – Provider Network Provisions |
Provider Network requirements are primarily described in Section 8.1.4. In addition, the Significant Traditional Provider (STP) requirements applicable to Medicaid MCOs are described in Section 8.2.3.
(1 page, excluding Provider listing and tables)
Network Providers must have an executed contract with the Respondent, a letter of intent (LOI) or a letter of agreement (LOA) indicating the Provider intends to contract with the Respondent should HHSC award the Respondent a contract for the applicable MCO Program. Network Providers must be licensed in the State of Texas to provide the contracted Covered Services. As described in Section 8.1.4.4 , the MCO must credential Network Providers before they may serve Members. Sample LOI/LOA agreements and sample Network Providers tables can be found in the
Procurement Library.
|
| |
| 1. For each Service Area in which the Respondent proposes to participate in the STAR, STAR+PLUS, and/or CHIP Program, the Respondent must submit a complete listing of proposed Network Providers for each of the following Acute Care provider types. Such listing must indicate for each provider type: the name, address, and NPI and/or TPI, if applicable, of the Providers with signed contracts, LOIs or LOAs. If the Respondent’s Provider Network is identical across more than one MCO Program within a Service Area, the Respondent may submit one Excel file worksheet for the Service Area that specifies the applicable MCO Programs. The Respondent must include in an Excel file at least the two (2) nearest Providers meeting each of the following provider type descriptions. The Respondent must also include in the Excel file all Providers in the designated provider type within the Service Area. The listing must include separate lists of each provider type in the order listed below and a separate worksheet for each proposed Service Area: |
Acute Care Services
|
| |
| a. Acute Care Hospitals, inpatient and outpatient services; |
|
| |
| b. Hospitals providing Level 1 trauma care; |
|
| |
| c. Hospitals providing Level 2 trauma care; |
|
| |
| d. Hospitals designated as transplant centers; |
|
| |
| e. Hospitals designated as Children’s Hospitals by the CMS; |
|
| |
| f. other Hospitals with specialized pediatric services; |
|
| |
| g. Psychiatric Hospitals providing mental health services, inpatient and outpatient; |
|
| |
| h. Other facilities or clinics that provide outpatient mental health services; |
|
| |
| i. Hospitals providing substance abuse services, inpatient and outpatient; and |
|
| |
| j. other facilities or clinics providing outpatient substance abuse services. |
|
| |
| 2. For STAR+PLUS only, identify a list of Community-based Long-Term Services and Supports Providers with whom the Respondent has a signed contract, LOI or LOA. These Providers should be listed by type, name, and address. Respondent should also list the array of Community-based Long-Term Services and Supports each of these entities provides. |
Community-based Long-Term Services and Supports (for STAR+PLUS only)
|
| |
| a. Personal Assistance Services (PAS); |
|
| |
| b. Day Activity and Health Services (DAHS); |
|
| |
| c. adaptive aids and medical supplies; |
|
| |
| e. assisted living and residential care services; |
|
| |
| f. emergency response services; |
|
| |
| h. in-home skilled nursing care; |
|
| |
| j. minor home modifications; |
|
| |
| l. therapy – occupational; |
|
| |
| n. therapy – speech, hearing, and/or language pathology services; |
|
| |
| o. consumer directed services; and |
|
| |
| p. transition assistance services. |
|
| |
| 3. Identify the types of Providers the Respondent allows to be PCPs for adults, PCPs for children, OB/GYNs, and outpatient Behavioral Health Service Providers. The Respondent should identify its contract requirements for these provider types and any exceptions. For example, Respondent should note under what circumstances, if any, an internist is allowed to be a PCP for children, or a family practitioner is allowed to be an OB/GYN. |
|
|
4.3.5.2 Significant Traditional Providers |
(No page limit, Respondents should only submit STP tables, not text, with the exception of bidders not meeting the 50 percent threshold described in Section 5.2. These Respondents should provide clear documentation of any problems in meeting this threshold)
The STP requirements in Section 8.2.3 are applicable as follows:
Medicaid STP requirements apply statewide for pharmacy and substance use disorder providers (SUDs) in STAR and STAR+PLUS. For STAR MCOs, STP requirements for other provider types are limited to the following areas: Hidalgo, Jefferson, and Medicaid Rural Service Area(s); and in the following counties: Hudspeth, Carson, Deaf Smith, Hutchinson, Potter, Randall, Swisher, Austin, Wharton, Matagorda, Bandera, Brooks, Goliad, Karnes, Kenedy, Live Oak, and Fayette. For STAR+PLUS MCOs, STP requirements for other provider types apply to Jefferson, El Paso, Lubbock and Hidalgo Service Areas; as well as the following counties: Austin, Wharton, Matagorda, Bandera, Brooks, Goliad, Karnes, Kenedy, Live Oak, and Fayette.
HHSC-designated Medicaid Significant Traditional Providers (STPs) can be found in the Procurement Library. The STP list includes, without limitation, SUD, pharmacy, and State Mental Health Hospitals for all MCO Programs. For STAR+PLUS, STPs also include Community-based Long-Term Services and Supports Providers.
For each STP provider type in the MCO Program(s) and Service Area(s) bid, the Respondent must complete the charts provided in the Procurement Library.
|
|
4.3.5.3 Provider Network Capacity |
(3 pages, plus 1 additional page per additional MCO Program bid if the Respondent’s response differs by MCO Program)
HHSC has targeted improved Network capacity and improved Member access to Covered Services as a priority for the Initial Contract Period.
|
| |
| 1. indicate which, if any, Covered Services are not available from a qualified Provider in the Respondent’s proposed Network in the Service Area and how the Respondent proposes to provide such Covered Services to Members in the Service Area; and |
|
| |
| 2. briefly describe how deficiencies will be addressed when the Provider Network is unable to provide a Member with appropriate access to Covered Services due to lack of a qualified Network Provider within the travel distance of the Member’s residence specified in Section 8.1.3.2. The description should include, but not be limited to, how the Respondent will address deficiencies in the Network related to: |
|
| |
| a. the lack of an age-appropriate Network PCP with an Open-Panel within the required travel distance of the Member’s residence; |
|
| |
| b. for female Members, the lack of an Network OB/GYN with an open practice within the travel distance of the Member’s residence; |
|
| |
| c. the lack of a Network cardiologist within the travel distance of the Member’s residence (STAR and STAR+PLUS only); and |
|
| |
| d. the lack of a Network pharmacy within the travel distance of the Member’s residence. |
|
|
4.3.5.4 Credentialing and Re-credentialing |
(4 pages plus 2 additional pages for Respondents bidding STAR+PLUS)
Provider credentialing and re-credentialing requirements are described in Section 8.1.4.4. For all of the following submission requirements, instead of attaching copies of the Respondent’s credentialing/re-credentialing policies and procedures, the Respondent should provide a brief summary of its policies and procedures.
|
| |
| 1. Describe the Respondent’s minimum credentialing and/or licensure requirements and procedures for Acute Care Providers by type of Provider, and demonstrate how the Respondent ensures, or proposes to ensure, that the minimum credentialing requirements are met. Such description must demonstrate compliance with Section 8.1.4.4. |
|
| |
| 2. Describe the re-credentialing process or process between re-credentialing cycles for Acute Care Providers and how the Respondent will capture and assess the following information: |
|
| |
| a. Member Complaints and Appeals; |
|
| |
| b. results from quality reviews and Provider quality profiling; |
|
| |
| c. utilization management information; and |
|
| |
| d. information from licensing and accreditation agencies. |
|
| |
| 3. For STAR+PLUS only, describe the Respondent’s minimum credentialing and/or licensure requirements and procedures for Providers of Community-based Long-Term Services and Supports by type of Provider, and how Respondent will ensure that the minimum credentialing and licensing requirements are met by any Provider rendering Covered Services. |
|
| |
| 4. For STAR+PLUS only, describe the re-credentialing process for Providers of Community-based Long-Term Services and Supports. The description of the re-credentialing process should include how the Respondent will capture and assesses the following information: |
|
| |
| a. Member Complaints and Appeals; |
|
| |
| b. results from quality reviews and quality Provider profiling; |
|
| |
| c. utilization management information; and |
|
| |
| d. information from licensing and accreditation agencies. |
|
| |
| 5. A Respondent currently operating in Texas must separately report the following information for its Texas Network. A Respondent not currently operating in Texas must separately report the same information for a managed care program it operates in another state that is similar to the MCO Program bid: |
|
| |
| a. the percentage of providers in its Network re-credentialed in the past three (3) years, for the following provider types: primary care physician, specialty care provider, and masters-level outpatient Behavioral Health Service providers; and |
|
| |
| b. the number and percentage of providers in its Network who were subjected to the regularly scheduled re-credentialing process over the past 24 months that were denied continued Network status. |
(3 pages, plus 2 additional pages for each additional MCO Program bid if the Respondent’s response differs by MCO Program; excluding hotline telephone reports)
Describe the proposed Provider Hotline function and how the Respondent would meet the requirements of Section 8.1.4.7. Such description must include:
|
| |
| 1. normal hours of operation of the hotline; |
|
| |
| 2. staffing for the hotline; |
|
| |
| 3. training for the hotline staff on Covered Services and HHSC MCO Program requirements; |
|
| |
| 4. the routing of calls among hotline staff to ensure timely and appropriate response to provider inquiries; |
|
| |
| 5. responsibilities of hotline staff, if any, in addition to responding to HHSC Provider Hotline calls (e.g., responding to non-Network provider calls and/or HHSC Member Hotline calls); |
|
| |
| 6. after-hours procedures and available services; |
|
| |
| 7. provider hotline telephone reports for the most recent four (4) quarters with data that show the monthly call volume, the monthly trends for average speed of answer (where answer is defined by reaching a live voice, not an automated call system) and the monthly trends for the abandonment rate; and |
|
| |
| 8. Whether the Provider Hotline has the capability to administer automated surveys to callers at the end of calls. |
A Respondent currently participating in any of the MCO Programs bid must submit the information in #7 above for each provider hotline operated, and identify any proposed changes to provider hotline functions.
A Respondent not currently participating in any of the MCO Programs bid must submit the information in #7 above for a similar managed care program that it operates. If such a Respondent referenced a non-HHSC managed care program in another submission requirement, the Respondent must submit its provider hotline telephone report for the same managed care program.
A Respondent proposing to participate in more than one (1) MCO Program should note that it is not required to operate separate STAR, STAR+PLUS, and CHIP Provider Hotlines, so long it meets the RFP Provider Hotline requirements for all MCO Programs bid.
If a Respondent is submitting a multi-program response to this RFP, the Respondent should separately describe each proposed Provider Hotline, or if proposing to staff a single Provider Hotline for multiple programs, and should note in its Proposal the differences, if any, in its Provider Hotline and staffing for each MCO Program bid.
|
|
4.3.5.6 Provider Training |
(2 pages, plus 1 additional page per additional MCO Program bid if the Respondent’s response differs by MCO Program)
Provider training requirements are described in Section 8.1.4.6.
|
| |
| 1. Provide a brief description of the proposed Provider training programs for each MCO Program bid. For STAR+PLUS only, distinguish between training programs for Acute Care Providers and Community-based Long-Term Services and Supports Providers. The description should include: |
|
| |
| a. the types of programs to be offered, including the modality of training; |
|
| |
| b. what topics will be covered; |
|
| |
| c. which Providers will be invited to attend; |
|
| |
| d. how the Respondent proposes to maximize Provider participation; |
|
| |
| e. how Provider training programs will be evaluated; |
|
| |
| f. the frequency of Provider training; and |
|
| |
| g. for STAR+PLUS Long Term Services and Supports providers in El Paso, Lubbock, and Hidalgo, who have never submitted traditional claim forms, a brief summary of additional methods to assist these providers. |
|
| |
| 2. Briefly describe two (2) examples of recent Provider training programs relevant to each of the MCO Programs bid. These examples must include: |
|
| |
| a. a description of the training program; |
|
| |
| b. a summary of distributed materials (the actual materials are not to be submitted); |
|
| |
| c. number and type of attendees; and |
|
| |
| d. results of any evaluations from the training. |
A Respondent currently participating in any of the MCO Programs bid must submit the above Provider training examples for each such MCO Program. A Respondent may use the same such Provider education example for more than one (1) MCO Program, provided the education program was given to Providers participating in each MCO Program.
A Respondent not currently participating in one (1) or more of the MCO Programs bid must submit the above provider training examples for a similar managed care program. If the Respondent referenced a non-HHSC managed care program in another submission requirement, the Respondent must submit its provider education information in this submission requirement.
4.3.5.7 Provider Incentives
(2 pages, plus 1 additional page per additional MCO Program bid if the Respondent’s response differs by MCO Program)
The Respondent must submit a proposal for a pilot “gain sharing” program. The program should focus on collaborating with Network physicians and Hospitals in order to allow them to share a portion of the Respondent’s savings resulting from reducing inappropriate utilization of services, including inappropriate admissions and readmissions. The proposal should include mechanisms whereby the Respondent will provide incentive payments to Hospitals and physicians for quality care. The proposal should include quality metrics required for incentives, recruitment strategies of providers, and a proposed structure for payment.
|
|
4.3.6 Section 6 – Member Services |
|
|
4.3.6.1 Member Services Staffing |
(5 pages, plus 1 additional page per additional MCO Program bid if the Respondent’s response differs by MCO Program; excluding organizational chart(s))
The MCO must maintain a Member Services Department to assist Members and Members’ representatives in obtaining Covered Services as described in Section 8.1.5.
|
| |
| 1. Provide an organizational chart of the Member Services Department, showing the placement of Member Services within the Respondent’s organization and showing the key staff within the Member Services Department. |
|
| |
| 2. Explain the functions of the Member Services staff, including brief job descriptions and qualifications. |
|
| |
| 3. Describe the curriculum for training to be provided to Member Services representatives, including when the training is conducted and how the training addresses: |
|
| |
| a. Covered Services, including Behavioral Health Services and Community-based Long Term Services and Supports; |
|
| |
| b. MCO Program requirements; |
|
| |
| c. Cultural Competency; and |
|
| |
| d. providing assistance to Members with limited English proficiency. |
|
| |
| 4. Identify the turnover rate for Member Services staff in the past two (2) years. A Respondent operating any HHSC MCO Program must provide the staff turnover rate for each of its MCO Programs. A Respondent not currently operating an HHSC MCO rogram must provide its Member Services staff turnover rate for a comparable managed care program and identify the managed care program. |
|
| |
| 5. For STAR+PLUS only, identify the number and professional background of Member Services staff that the Respondent intends to dedicate to the Service Coordination function. |
|
| |
| 6. Identify the percentage of Member Services staff who will be physically located in the Service Area. |
A Respondent submitting a multi-program response must clearly indicate any differences in the Respondent’s Member services approach across each of the MCO Program bid.
(3 pages, plus 2 additional pages per additional MCO Program bid if the Respondent’s response differs by MCO Program; excluding hotline telephone reports)
The Member Hotline requirements are described in Section 8.1.5.6.
Describe the proposed Member Hotline function, including:
|
| |
| 1. normal hours of operation; |
|
| |
| 2. number of Member Hotline staff, expressed in the number of full time employees (FTEs) per 1000 Members who are available 8:00 a.m. to 5:00 p.m., local time in the Service Area, Monday through Friday, excluding state-approved holidays; |
|
| |
| 3. routing of calls among Member Hotline staff to ensure timely and accurate response to Member inquiries; |
|
| |
| 4. responsibilities of Member Hotline staff, if any, in addition to responding to HHSC Member Hotline calls, (e.g., responding to non-HHSC Member calls and/or HHSC Provider Hotline or Behavioral Health Hotline calls); |
|
| |
| 5. after-hours procedures and available services, including those provided to non-English speaking Members in Major Population Groups; |
|
| |
| 6. the number and percentage of FTE Member Hotline staff who are bilingual in English and Spanish; |
|
| |
| 7. the number and percentage of FTE Member Hotline staff who are multi-lingual for any additional language, by language spoken; |
|
| |
| 8. for STAR+PLUS only, the number and percentage of FTE Member Hotline staff dedicated to the Service Coordination function; |
|
| |
| 9. Member Hotline telephone reports for the most recent four (4) quarters with data that show the monthly trends for call volume, monthly trends for average speed of answer (where answer is defined by reaching a live voice, not an automated call system) and monthly trends for the abandonment rate; and |
|
| |
| 10. Whether the Member Hotline has the capability to administer automated surveys to callers at the end of calls. |
A Respondent currently participating in any of HHSC’s MCO Programs must submit the information in #9 above for each Member Hotline operated, and identify any proposed changes to hotline functions.
If the Respondent is not currently participating in any of HHSC’s MCO Programs, it should describe its experience and proposed approach in establishing and maintaining an accessible call center for Members that is comparable to the Member Hotline described in Section 8.1.5.6. Such a description must include the information listed in items 1 to 10 above.
A Respondent proposing to participate in more than one (1) MCO Program should note that it is not required to operate separate STAR, STAR+PLUS, and CHIP Member Hotlines, if it meets the RFP Member Hotline requirements for all MCO Program bid.
If a Respondent is submitting a multi-program response to this RFP, the Respondent should separately describe each proposed Member Hotline, or if proposing to staff a single Member Hotline for multiple programs, and should note the differences, if any, in its Member Hotline and staffing for each MCO Program bid.
|
|
4.3.6.3 Member Service Scenarios |
(5 pages)
Describe the procedures a Member Services representative will follow to respond to the following situations:
|
| |
| 1. a Member has received a bill for payment of Covered Services from a Network Provider or Out-of-Network Provider; |
|
| |
| 2. a Member is unable to reach her PCP after normal business hours; |
|
| |
| 3. a Member is having difficulty scheduling an appointment for preventive care with her PCP, |
|
| |
| 4. for STAR+PLUS only, a Member is having difficulty scheduling an appointment for preventive care with her Medicare PCP; |
|
| |
| 5. for STAR+PLUS only, a Member is in urgent need of meals, adaptive aids, or other Community-Based Long- Term Services and Supports and is unable to reach their Service Coordinator or provider, |
|
| |
| 6. a Member becomes ill while traveling outside of the Service Area, and |
|
| |
| 7. a Member has a request for a specific medication that the pharmacy is unable to provide. |
|
|
4.3.6.4 Cultural Competency |
(3 pages)
Provide a high-level description of the processes the Respondent will put in place to the meet the requirements of the cultural competency requirements as described in Section 8.1.5.8, “Cultural Competency Plan.”
|
| |
| 1. Describe how the Respondent will ensure culturally competent services to people of all cultures, races, ethnic backgrounds, and religions as well as those with disabilities in a manner that recognizes values, affirms, and respects the worth of the individuals and protects and preserves the dignity of each. |
|
| |
| 2. Describe how the Respondent will develop intervention strategies and work with Network Providers to avoid disparities in the delivery of medical services to diverse populations. |
|
|
4.3.6.5 Member Complaint and Appeal Processes |
(3 pages per MCO Program, excluding flow chart)
Medicaid Member Complaint and Appeal Processes are described in Section 8.2.6. CHIP Member Complaint and Appeal Processes are described in Section 8.4.2. For each MCO Program bid, a Respondent’s proposal should describe how it intends to meet the applicable Member Complaint and Appeal requirements. A Respondent should not submit detailed Complaint and Appeal policies and procedures as an attachment.
For each MCO Program bid, the Respondent must:
|
| |
| 1. describe the process the Respondent will put in place for the review of Member Complaints and Appeals, including which staff will be involved; |
|
| |
| 2. provide a flowchart that depicts the process the Respondent will employ, from the receipt of a request through each phase of the review to notification of disposition, including providing notice of access to HHSC Fair Hearings; |
|
| |
| 3. document the MCO’s average time for resolution over the past 12 months for Member Complaints and Appeals (excluding Expedited Appeals), from date of receipt to date of notification of disposition; and |
|
| |
| 4. for STAR and STAR+PLUS only, describe the number and job descriptions of Member Advocates, how Members are informed of the availability of Member Advocates, and how Members access Advocates. |
|
|
4.3.6.6 Marketing Activities and Prohibited Practices |
(no page limit)
If the Respondent has been sanctioned or placed under corrective action for prohibited Marketing practices related to managed care products by the CMS, Texas, or by another state:
|
| |
| 1. describe the basis for each sanction or corrective action, and |
|
| |
| 2. explain how the Respondent would ensure that it would not commit any practices prohibited by the CMS or HHSC in its Marketing activities. |
A Respondent should have reported whether it has been sanctioned or been placed under corrective action by the federal government, Texas, or any other state in the past three (3) years as part of its Business Specifications submission.
|
|
4.3.6.7 Continuity of Care (for STAR and STAR+PLUS only) |
(3 pages plus 1 additional page if the Respondent is proposing to participate in both STAR and STAR+PLUS)
Continuity of Care transition requirements for certain new Members with Out-of-Network providers are described in Section 8.2.1.
Describe the proposed Continuity of Care Transition Plan for serving new Members whose current PCP, OB/GYN, specialty care providers (including Behavioral Health Service providers) or Community-based Long-Term Services and Supports are not participants in the Respondent’s Provider Network. Respondents proposing to serve STAR+PLUS Members must also describe the proposed Continuity of Care Transition Plan for serving new Members whose current home health services provider is not a participant in the Respondent’s proposed Provider Network.
If a Respondent is proposing to serve both STAR and STAR+PLUS MCO Members, the Respondent should note the differences, if any, in its Continuity of Care Transition Plan in each MCO Program bid.
|
|
4.3.6.8 Objection to Providing Certain Services |
(1 page)
In accordance with 42 C.F.R. §438.102, the Respondent may file an objection to provide, reimburse for, or provide coverage of, counseling or referral service for a Covered Service based on moral or religious grounds (see Section 8.2.2.7). HHSC reserves the right to make downward adjustments to Capitation Rates for any Respondent that objects to providing certain services based on moral or religious grounds.
Respondent should indicate objections, if any, to providing a Covered Service based on moral or religious grounds. Identify the specific service(s) to which it objects and describe the basis for its objection on moral or religious grounds.
|
|
4.3.6.9 Coordination of Services for Dual Eligibles |
(2 pages)
Coordination of Services for STAR+PLUS Dual Eligibles is described in Section 8.3.7.1, and Medicaid wrap-services are described in Section 8.2.3.
As applicable to the Programs bid, please describe the Respondent’s process for coordinating Medicaid and Medicare care for STAR+PLUS Dual Eligibles, and providing Medicaid wrap-around services to Dual Eligibles in STAR+PLUS and STAR (Medicaid Rural Service Area only).
|
|
4.3.7 Section 7 – Quality Assessment and Performance Improvement |
The Quality Assessment and Performance Improvement (QAPI) requirements of the RFP are described in Section 8.1.7.
|
|
4.3.7.1 Clinical Initiatives |
(3 pages, plus 2 additional pages per additional MCO Program, excluding QA plan)
|
| |
| 1. For each MCO Program bid, describe data-driven clinical initiatives that the Respondent initiated within the past 24 months that have yielded improvement in clinical care for a managed care population comparable to the population bid and document two (2) statistically significant improvements generated by the Respondent’s clinical initiatives. |
|
| |
| 2. For STAR+PLUS only, propose two (2) clinical initiatives focused on Community-based Long-Term Services and Supports for STAR+PLUS Members, including how Members will be involved in such initiatives and the Respondent’s experience implementing similar clinical initiatives. |
|
| |
| 3. For each MCO Program bid, describe two (2) new or ongoing Acute Care clinical initiatives that the Respondent proposes to pursue in the first year of the Contract. Document why each topic warrants quality improvement investment, and describe the Respondent’s measurable goals for the initiative. |
|
| |
| 4. For STAR+PLUS only, describe the planned approach the Respondent will take towards quality assessment and ongoing review of providers with whom it intends to contract, using the following provider types as an example: |
a. Adult Day Health Facilities;
b. Personal Assistance Services providers, and
c. Home and Community Support Services Agencies (HCSSAs).
|
| |
| 5. For Respondents that already participate in an HHSC MCO Program, provide a copy of the most recent QAPI Plan. For Respondents that do not participate in an HHSC MCO Program, provide a copy of a 2009 quality assurance plan for a comparable managed care population. |
|
| |
| 6. Many Texas Medicaid and CHIP children reportedly receive their immunizations through Local Health Departments. Discuss the impact this has on creating a Medical Home for child Members, and what steps, if any, the Respondent proposes to take to improve child preventive services delivery. |
|
|
4.3.7.2 Healthcare Effectiveness Data and Information Set (HEDIS) and Other Quality Data |
(3 pages, plus 2 additional pages per additional MCO Program bid)
HHSC's External Quality Review Organization (EQRO) will perform HEDIS and Consumer Assessment of Healthcare Providers and Systems (CAHPS) calculations required by HHSC for MCO Program management. The following questions are designed to solicit information on a Respondent's proposed approach to generating its own clinical indicator information to identify and address opportunities for improvement, as well as the Respondent's approach to acting on clinical indicator data reported by HHSC's EQRO.
For each MCO Program bid, the Respondent must:
1. identify the MCO-level HEDIS and any other statistical clinical indicator measures the Respondent will generate to identify opportunities for clinical quality improvement;
2. document examples of statistical clinical indicator measures previously generated by the Respondent during 2008-2009 for a managed care population comparable to the population in the MCO Program bid;
3. describe efforts that the Respondent has made to assess member satisfaction during 2008-2009 for a managed care population comparable to the population in the MCO Program bid; and
4. describe management interventions implemented in 2008 or 2009 based on member satisfaction measurement findings for a managed care population comparable to the population in the MCO Program bid, and whether these interventions resulted in measurable improvements in later member satisfaction findings.
|
|
4.3.7.3 Clinical Practice Guidelines |
(2 pages per MCO Program bid)
There is significant evidence that medical professionals are often slow to adopt evidence-based clinical practice guidelines.
|
| |
| 1. For each MCO Program bid, describe two (2) clinical guidelines that are relevant to the enrolled populations and that the Respondent believes are currently not being adhered to at a satisfactory level. |
|
| |
| 2. Describe what steps the Respondent will take to increase compliance with the clinical guidelines noted in its response to question number 1 above. |
|
| |
| 3. Provide a general description of the Respondent’s process for developing and updating clinical guidelines, and for disseminating them to participating Providers. |
|
|
4.3.7.4 Provider Profiling |
(3 pages, excluding sample profile reports)
|
| |
| 1. Describe the Respondent’s practice of profiling the quality of care delivered by Network PCPs, and any other Acute Care Providers (e.g., high volume specialists, Hospitals), including the methodology for determining which and how many Providers will be profiled. |
|
| |
| 2. For STAR+PLUS, describe the Respondent’s method to ensure the quality of care delivered by Long-Term Services and Supports Providers. |
|
| |
| 3. Submit sample quality profile reports used by the Respondent, or proposed for future use (identify which). |
|
| |
| 4. Describe the rationale for selecting the performance measures presented in the sample profile reports. |
|
| |
| 5. Describe the proposed frequency with which the Respondent will distribute such reports to Network Providers, and identify which Providers will receive such profile reports. |
If a Respondent is submitting a multi-program response to this RFP, the Respondent should note in its Proposal the differences, if any, in its provider profiling activities and reports for each MCO Program bid.
|
|
4.3.7.5 Network Management |
(4 pages, plus 1 additional page per additional MCO Program bid if the Respondent’s response differs by MCO Program)
Describe how the Respondent will actively work with Network Providers to ensure accountability and improvement in the quality of care provided by both Acute and Long-Term Services and Supports Providers. The description should include:
|
| |
| 1. the steps the Respondent will take with each profiled Provider following the production of each profile report, including a description of how the Respondent will motivate and facilitate improvement in the performance of each profiled Provider; |
|
| |
| 2. the process and timeline the Respondent proposes for periodically assessing Provider progress on its implementation of strategies to attain improvement goals; |
|
| |
| 3. how the Respondent will reward Providers who demonstrate continued excellence and/or significant performance improvement over time, through non-financial or financial means, including pay-for-performance; |
|
| |
| 4. how the Respondent will share “best practice” methods or programs with Providers of similar programs in its Network; |
|
| |
| 5. how the Respondent will take action with Providers who demonstrate continued unacceptable performance and performance that does not improve over time; |
|
| |
| 6. the steps the Respondent will take with a Provider that specifically is not meeting HHSC contractual access standards; and |
|
| |
| 7. the extent to which the Respondent currently operates a Network management program consistent with HHSC requirements in Section 8.1.7.8, and measurable results it has achieved from such Network management efforts. |
If a Respondent is submitting a multi-program response to this RFP, the Respondent should note in its Proposal the differences, if any, in its Network Management activities and reports for each MCO Program bid.
|
|
4.3.8 Section 8 – Utilization Management |
(3 pages, plus 1 additional page for each additional MCO Program bid if the Respondent’s response differs by MCO Program)
Utilization Management (UM) requirements are described generally in Section 8.1.8 and specifically for Behavioral Health Services in Section 8.1.15. A Respondent’s response to this submission requirement should address UM for all Covered Services.
1. Describe the UM guidelines the Respondent plans to employ, including whether and how the guidelines comply with the standards in Sections 8.1.8 and 8.1.15.
2. If the UM guidelines were developed internally, describe the process by which they were developed and when they were developed or last revised.
3. Describe how the UM guidelines will generally be applied to authorize or retrospectively review services for the spectrum of Covered Services.
If a Respondent is submitting a multi-program response to this RFP, the Respondent should note in its Proposal the differences, if any, in its UM activities for each MCO Program bid.
|
|
4.3.9 Section 9 – Early Childhood Intervention (ECI) |
(3 pages, plus one additional page for each additional MCO Program bid if the Respondent’s response differs by MCO Program)
ECI Services are described in Section 8.1.9.
|
| |
| 1. Describe the Respondent’s experience with, and general approach to, providing ECI services, including how the Respondent will identify such individuals. |
|
| |
| 2. Describe procedures and protocols for using the IFSP information to develop a Member Care Plan and authorize services. |
|
| |
| 3. Describe procedures and protocols for developing and including the interdisciplinary team in the assessment and care planning process. |
|
| |
| 4. Describe the process by which the Respondent will provide the IFSP and other necessary information to the PCP. |
If a Respondent is submitting a multi-program response to this RFP, the Respondent should note in its Proposal the differences, if any, in its services for ECI for each MCO Program bid.
|
|
4.3.10 Section 10 – Services for People with Special Health Care Needs |
(3 pages, plus one additional page for each additional MCO Program bid if the Respondent’s response differs by MCO Program)
Services for people with special health care needs are described in Section 8.1.12. Note: All STAR+PLUS Members are considered to be persons with Special Health Care Needs as defined in Attachment A, "Uniform Managed Care Contract Terms and Conditions.”
|
| |
| 1. Describe the Respondent’s experience with, and general approach to, providing services for adults with Special Health Care Needs (STAR and STAR+PLUS only), including how the Respondent will identify such individuals and the criteria it will use in assessing whether an adult is a Member with Special Health Care Needs (MSHCN). |
|
| |
| 2. Describe the Respondent’s experience with, and general approach to, providing services for children with special health care needs, including how the Respondent will identify such individuals and the criteria it will use in assessing whether a Member has special health care needs. |
|
| |
| 3. Describe the process for initially and periodically assessing Members’ needs for services, and identify the staff performing the assessments and their credentials. |
|
| |
| 4. Describe procedures and protocols for using the assessment information to develop a Member Care Plan and authorize services. |
|
| |
| 5. Describe procedures and protocols for including the Member and/or Member’s Representative in the assessment and care planning process. |
|
| |
| 6. Describe the process by which the Respondent will allow MSHCN to have: |
|
| |
| a. direct access to a specialist as appropriate for the Member’s condition and identified needs, such as a standing referral to a specialty physician; and |
|
| |
| b. access to non-primary care physician specialists as PCPs, as required by 28 T.A.C. § 11.900 and Section 8.1.3. |
If a Respondent is submitting a multi-program response to this RFP, the Respondent should note in its Proposal the differences, if any, in its services for MSHCN for each MCO Program bid.
|
|
4.3.11 Section 11 – Care Management and/or Service Coordination |
(9 pages, plus 1 additional page per additional MCO Program bid if the Respondent’s response differs by MCO Program)
Care Management and/or Service Coordination is described in Sections 8.1.12.2 and 8.1.13. Additional requirements for Service Coordination are described in Section 8.3.2.
|
| |
| 1. Describe the Respondent’s experience providing Care Management and/or Service Coordination to members with high-cost catastrophic situations (e.g., recent spinal cord injury) and the Respondent’s proposal for implementing high-cost catastrophic Care Management and/or Service Coordination, including how the Respondent will identify Members for high cost catastrophic Care Management and/or Service Coordination, and the criteria used to identify such Members. |
|
| |
| 2. Describe the Respondent’s experience providing Care Management and/or Service Coordination services to Members with the following serious health care conditions, as applicable to the MCO Programs bid, and the Respondent’s proposal for offering Care Management and/or Service Coordination services to these Members. Include how Members will be identified for Care Management and/or Service Coordination, and the criteria used to identify such Members: |
|
| |
| a. women with high-risk pregnancies (STAR only); and |
|
| |
| b. individuals with mental illness and co-occurring substance abuse. |
|
| |
| 3. Identify any measurable results in terms of clinical outcomes and program savings that have resulted from the Respondent’s Care Management and/or Service Coordination initiatives. |
|
| |
| 4. For STAR+PLUS only, describe the duties and responsibilities of the Service Coordinator to authorize Community-based Long-Term Services and Supports. The Respondent must describe in detail how the Service Coordinator will function in relation to the Member’s PCP for: |
|
| |
| a. Dual Eligible STAR+PLUS Members receiving both Medicaid and Medicare services from the MCO, and |
|
| |
| b. Dual Eligible STAR+PLUS Members receiving Medicare services through either fee-for-service Medicare or another Medicare MCO. |
|
| |
| 5. For STAR+PLUS only, submit detailed information, including protocols and procedures, for identifying Members requiring Service Coordination, and for providing the Service Coordination function to them. The information should include how the protocols and procedures vary for: |
|
| |
| a. Dual Eligible STAR+PLUS Members receiving both Medicaid and Medicare services from the MCO, and for |
|
| |
| b. Dual Eligible STAR+PLUS Members receiving Medicare services through either fee-for-service Medicare or another Medicare MCO. |
|
| |
| 6. For STAR+PLUS only, describe the circumstances or conditions when the Member would require a licensed nurse or other allied health care provider as a Service Coordinator. |
|
| |
| 7. For STAR+PLUS only, submit criteria for identifying and training certain Members and their Member Representative(s) to coordinate and direct the Member’s own care, to the extent the Member is capable of doing so. Criteria should include those used to enable the Member and family to select, train, and supervise providers of Community-based Long-Term Services and Supports. |
|
| |
| 8. For STAR+PLUS only, describe the criteria and processes for advising Members of, and assisting them to access, the most appropriate, least restrictive home and community-based services as alternatives to institutional care. Additionally, describe how the Respondent will ensure that the Member is given the opportunity to make an informed choice among the options for care settings. |
|
| |
| 9. For STAR+PLUS only, submit a list of the relevant community organizations in each proposed STAR+PLUS Service Area with which the Respondent will coordinate services for Members and to which it will refer Members for services. |
|
| |
| 10. For STAR+PLUS only, describe the process for initially and periodically assessing Members’ needs for services. |
|
| |
| 11. For STAR+PLUS only, describe how the Respondent will identify Members who are at risk of nursing facility placement. |
|
| |
| 12. For STAR+PLUS only, submit all functional assessment instruments proposed for use and describe how the assessment instrument(s) will be employed to identify the Member’s need for Community-based Long-Term Services and Supports. (Note: If the MCO is allowed to modify a functional assessment instrument required by the State, HHSC must approve the proposed instrument prior to implementation. See Section 8.3.3 for more information.) |
|
| |
| 13. For STAR+PLUS only, identify who will perform each assessment and specify their credentials. |
|
| |
| 14. Describe procedures and protocols for using the assessment information to develop a Member Service/Care Plan and authorize services. |
|
| |
| 15. Describe procedures and protocols for including the Member and/or Member’s Representative in the assessment and care planning process. |
|
| |
| 16. For STAR+PLUS only, provide a description of the appropriate staffing ratio of Service Coordinators to Members, and the Respondent’s target ratio of Service Coordinators to Members. |
If a Respondent is submitting a multi-program response to this RFP, the Respondent should note in its Proposal the differences, if any, in its Care Management and/or Service Coordination activities in the applicable MCO Programs.
|
|
4.3.12 Section 12 – Disease Management (DM)/Health Home Services |
(3 pages, plus 1 additional page for each MCO Program bid)
Disease Management/Health Home Services is described in Section 8.1.14.
|
| |
| 1. Describe the Respondent’s experience in implementing Disease Management/Health Home Services programs for populations comparable to the proposed HHSC MCO Program. |
|
| |
| 2. Identify any measurable results in terms of clinical outcomes and program savings that have resulted from the Respondent’s Disease Management/Health Home Services initiatives, and briefly describe the analyses used to identify such outcomes and savings. |
|
| |
| 3. Identify the process by which the Respondent proposes to provide Members with Disease Management/Health Home Services. Describe how the Respondent will identify Members in need of such Disease Management/Health Home Services program, the proposed outreach approach, and the Disease Management/Health Home Services program components for Members of different risk levels. |
|
| |
| 4. Describe the process by which the Respondent will ensure continuity of care with the Member’s previous Disease Management/Health Home Services program(s), if any. |
|
|
4.3.13 Section 13 – Behavioral Health Services and Network |
The Behavioral Health Services and Network requirements are described in Section 8.1.15. Note: STAR Members in the Dallas Service Area will receive Behavioral Health services through the NorthSTAR Program instead of STAR.
|
|
4.3.13.1 Behavioral Health Services Hotline |
(3 pages, plus 2 additional pages per additional MCO Program bid if the Respondent’s response differs by MCO Program; excluding telephone reports)
The Behavioral Health Services Hotline requirements are described in Section 8.1.15.3.
Describe the proposed Behavioral Health Services Hotline function, including:
|
| |
| 1. verification that it is, or will be, staffed 24 hours per day, 365 days per year; |
|
| |
| 2. staffing of Behavioral Health Services Hotline staff, including clinical credentials; |
|
| |
| 3. routing of calls among Behavioral Health Services Hotline staff to ensure timely and accurate response to Member inquiries; |
|
| |
| 4. the curriculum for training to be provided to Behavioral Health Services Hotline representatives, including when the training will be conducted and how the training will address a) Covered Services; b) HHSC MCO Program requirements; c) Cultural Competency; and d) providing assistance to Members with limited English proficiency. |
|
| |
| 5. responsibilities of Behavioral Health Services Hotline staff, if any, in addition to responding to HHSC Member Hotline calls, (e.g., responding to non-HHSC member calls and/or HHSC Provider Hotline or Member Hotline calls ); |
|
| |
| 6. the number and percentage of FTE Behavioral Health Services Hotline staff who are bilingual in English and Spanish; |
|
| |
| 7. the number and percentage of FTE Behavioral Health Services Hotline staff who are multi-lingual for any additional language, by language spoken; |
|
| |
| 8. Behavioral Health Services telephone reports for the most recent four (4) quarters with data that show the monthly trends for call volume, monthly trends for average speed of answer (where answer is defined by reaching a live voice, not an automated call system), and monthly trends for the abandonment rate; and |
|
| |
| 9. whether the Behavioral Health Services Hotline has the capability to administer automated surveys to callers at the end of calls. |
A Respondent currently participating in any of the HHSC MCO Programs bid must submit the information above for each Behavioral Health Services Hotline that it operates, and should provide the monthly call volume for each Service Area by MCO Program. Such a Respondent should also indicate any changes it proposes to its Behavioral Health Services Hotline.
If the Respondent is not currently participating in the STAR, STAR+PLUS, or CHIP MCO Programs, describe its experience and proposed approach in establishing and maintaining an accessible call center for Members that is comparable to the Behavioral Health Services Hotline described in Section 8.1.15.3. Such a description must include the information listed in items 1 to 9 above.
If a Respondent is submitting a multi-program response to this RFP, the Respondent should separately describe each proposed Behavioral Health Services Hotline, or if proposing to staff a single Behavioral Health Services Hotline for multiple programs, shall note in its Proposal the differences, if any, in its Behavioral Health Services Hotline and staffing for each applicable MCO Program.
|
|
4.3.13.2 Behavioral Health Provider Network Expertise |
(no page limit)
|
| |
| 1. For each proposed Service Area, identify Behavioral Health Service Providers with expertise in providing services to each of the following populations, as applicable to the Respondent’s Proposal. |
|
| |
| b. children and adolescents; |
|
| |
| c. persons with a dual diagnosis of mental health and substance abuse; and |
|
| |
| d. services for linguistic and cultural minorities. |
|
| |
| 2. Indicate the criteria the Respondent will use to determine that such Behavioral Health Providers have the requisite expertise. |
|
|
4.3.13.3 Coordination of Behavioral Health Care |
(2 pages, plus 1 additional page per additional MCO Program bid if the Respondent’s response differs by MCO Program)
|
| |
| 1. Describe the Respondent’s approach to coordinating Behavioral Health Service delivery with primary care services delivered by a Member’s PCP, and vice versa. |
|
| |
| 2. Describe or propose innovative programs and identify Network Providers contracted to serve special populations through integrated medical/Behavioral Health Service delivery models. Describe the program model services, treatment approach, special considerations, and expected outcomes for the special populations. |
|
| |
| 3. Describe the process by which the Respondent will ensure the delivery of outpatient Behavioral Health Services within seven (7) days of inpatient discharge for Behavioral Health Services. |
If a Respondent is submitting a multi-program response to this RFP, the Respondent should note in its Proposal the differences, if any, in its coordination of Behavioral Health Services in the applicable MCO Programs.
|
|
4.3.13.4 Behavioral Health Quality Management |
(2 pages per MCO Program bid)
|
| |
| 1. Identify the areas Respondent believes to be the greatest opportunities for clinical quality improvement in behavioral health in each MCO Program bid and provide supporting information. |
|
| |
| 2. Discuss the approaches the Respondent will pursue to realize one such opportunity for each MCO Program bid. |
|
| |
| 3. Describe how the Respondent proposes to integrate behavioral health into its quality assurance program, as described in Section 8.1.7.5. |
If a Respondent is submitting a multi-program response to this RFP, the Respondent should note in its Proposal the differences, if any, in the Respondent’s Behavioral Health quality management activities in each applicable MCO Program.
|
|
4.3.13.5 Behavioral Health Emergency Services |
(2 pages per MCO Program bid)
For each MCO Program bid, describe the Respondent’s experience with, and plans for, providing Behavioral Health Emergency Services, including, emergency screening services, Emergency Services, and short-term crisis stabilization to Medicaid, CHIP, or other similar populations.
|
|
4.3.14 Section 14 – Management Information System (MIS) Requirements |
(10 pages plus an additional 6 pages per additional MCO Program bid if the Respondent’s response differs by MCO Program - Page limit excludes system diagrams and process flow charts.)
For each MCO Program bid, the Respondent must:
|
| |
| 1. describe the Management Information System (MIS) the Respondent will implement, including how the MIS will comply with Health Insurance Portability and Accountability Act of 1996 (HIPAA). The response must address the requirements of Section 8.1.18. At a minimum, the description should address: |
|
| |
| a. hardware and system architecture specifications; |
|
| |
| b. data and process flows for all key business processes in Section 8.1.18.3; and |
|
| |
| c. attest to the availability of the data elements required to produce required management reports; |
|
| |
| 2. if claims processing and payment functions are outsourced, provide the above information for the Material Subcontractor; |
|
| |
| 3. describe how the Respondent would ensure accuracy, timeliness, and completeness of Encounter Data submissions for each of the MCO Programs bid; |
|
| |
| 4. describe the Respondent’s ability and experience in performing coordination of benefits and Third Party Liability/Third Party Recovery (TPL/TPR); |
|
| |
| 5. describe the Respondent’s ability and experience in allowing providers to submit claims electronically and its ability and experience in processing electronic claims payments to providers: |
|
| |
| a. if currently processing claims electronically, generally describe the type and volume of provider claims received electronically in the previous year versus paper claims for each claim type; |
|
| |
| b. if currently making claims payments to providers electronically, generally describe the type and volume of provider claims payment processed electronically; |
|
| |
| c. does the MCO provide a no-cost alternative for providers to allow billing without the use of a clearinghouse? If so please describe; and |
|
| |
| d. does the MCO include attendant care payments as part of the regular claims payment process (for STAR+PLUS only)? If so please describe; |
|
| |
| 6. describe the Respondent’s experience and capability to comply with the Internet website requirements of Section 8.1.5.5, and briefly describe any additional website capabilities that the Respondent proposes to offer to Members or Providers; |
|
| |
| 7. provide acknowledgment and verification that the Respondent’s proposed systems are 5010 compliant by submitting a copy of the 5010 compliancy plan, and proposed timeline for meeting the deadlines for being 5010 compliant; and |
|
| |
| 8. describe the Respondent’s capability to pay providers via direct deposit and its experience in doing so, including the percentage, number, and types of providers paid via direct deposit in the most recent 12 month period for which the Respondent has such statistics. If the Respondent operates in Texas, the Respondent must provide this information related to its experience in Texas. If the Respondent does not currently operate in Texas, the Respondent must provide this information for a state in which the Respondent currently operates a managed care program similar to the MCO Programs bid. |
|
|
4.3.15 Section 15 – Fraud and Abuse |
(3 pages, plus 1 additional page per additional MCO Program bid if the Respondent’s response differs by MCO Program)
The Fraud and Abuse requirements of the RFP are described in Section 8.1.19. The Respondent must describe how it will implement a Fraud and Abuse Plan that will comply with state and federal law and this RFP, including the requirements of §531.113, Texas Government Code. The Respondent must:
1. include detail about what parts of the organization and which key staff will have responsibilities in implementing and carrying out the Fraud and Abuse program; and
2. identify which officer or director of the Respondent organization will have overall responsibility and authority for carrying out the Fraud and Abuse Program provisions.
|
|
4.3.16 Section 16 – Pharmacy Services |
(8 pages plus an additional 2 pages per additional MCO Program bid if the Respondent’s response differs by MCO Program)
The Pharmacy Services requirements are described in Section 8.1.21. For all of the following submission requirements, instead of attaching copies of the Respondent’s policies and procedures, the Respondent should provide a brief summary of its policies and procedures.
|
| |
| 1. The Respondent must describe the processes it will use to manage the pharmacy benefit under both of the following scenarios: |
|
| |
| a. HHSC requires the MCO to implement the Medicaid and CHIP formularies and preferred drug lists (PDLs); and |
|
| |
| b. the MCO is allowed to establish its own formularies and PDLs. |
|
| |
| 2. The Respondent must describe the policies and procedures for how mail-order pharmacies will be available to Members. |
|
| |
| 3. The Respondent must identify the rationale for requiring prior authorizations, identify the types of drugs that normally require prior authorization, and describe the policies and procedures for the prior authorization process. |
|
| |
| 4. The Respondent must describe how rebates will be negotiated (if HHSC determines that the MCO will perform this service), identified, and reported. |
|
| |
| 5. The Respondent must describe the policies and procedures for drug utilization reviews, including ensuring prospective reviews take place at the dispensing pharmacy’s point of sale (POS). |
|
| |
| 6. The Respondent must describe its policies and procedures for targeted interventions for Network Providers over-utilizing certain drugs. |
|
|
4.3.17 Section 17 – Transition Plan |
(4 pages per MCO Program bid)
The Transition Plan Requirements are described in Section 7.
|
| |
| 1. Briefly describe the Respondent’s experience establishing and maintaining electronic interfaces with other contractors responsible for portions of Medicaid and CHIP operations. A Respondent with experience participating in one or more MCO Programs must clearly note its experience in establishing and maintaining such interfaces in Texas. A Respondent without experience establishing and maintaining electronic interfaces with other contractors responsible for Medicaid or CHIP operations must note its experience in establishing and maintaining similar electronic interfaces with similar contractors. |
|
| |
| 2. A Respondent that is proposing to participate in an HHSC MCO Program in a Service Area for the first time must, for each MCO Program bid, briefly describe its Transition Plan for all proposed Service Areas, including major activities related to the System Readiness Review and the Operational Readiness Review, including Network development, internal system testing, and proposed schedule to comply with the anticipated Operational Start Date and other requirements described in Section 7. The Respondent must clearly indicate in which Service Area(s) it currently does not operate as an MCO and any differences in its transition approach by Service Area. |
|
| |
| 3. A Respondent that is currently a contractor for an HHSC MCO Program must, for each such MCO Program, briefly describe its Transition Plan, including major activities related to the System Readiness Review and the Operational Readiness Review, such as Network Development, internal system testing, and schedule to comply with the anticipated Operational Start Date and other requirements described in Section 7. The Respondent must clearly indicate in which Service Area(s) it currently does not operate as an MCO, and any differences in its transition approach by Service Area. |
|
|
4.3.18 Section 18 – Additional Requirements Regarding Dual Eligibles (for STAR+PLUS only) |
(4 pages)
The additional provisions regarding certain categories of Dual Eligibles are described in Section 8.3.7.
|
| |
| 1. Submit evidence of Respondent’s MA Dual SNP contract with CMS if any, including the contract number and counties/zip codes served, or submit documentation showing that an application for such a contract has or will be submitted to CMS. For Respondents that do not already have an MA Dual SNP contract and who intend to obtain one, describe the plans for submitting an application and obtaining such a contract. The description should include the timeline for submitting the application and the proposed counties/zip codes for coverage. |
|
| |
| 2. Describe the Respondent’s experience in providing Medicare encounter data in HIPAA-compliant formats to federal or state authorities. |
|
| |
| 3. Describe how the Respondent intends to coordinate care for Dual Eligible Members, including: |
|
| |
| a. How the Respondent will identify Long-Term Services and Supports providers in the relevant Service Areas. |
|
| |
| b. The processes and procedures Respondent will use to coordinate the delivery of Community-based Long-Term Services and Supports with Medicare benefits for Dual Eligible Members. |
|
| |
| c. The training Respondent will provide to staff and providers regarding Community-based Long-Term Services and Supports and the coordination of those services with Medicare benefits. |
|
| |
| 4. Describe how the Respondent will work with the State to share information regarding Medicare and Medicaid participating providers, Member complaints, and HEDIS data. |
|
|
5. Evaluation Process and Criteria |
|
|
5.1 Overview of Evaluation Process |
HHSC will use a formal evaluation process to select the successful Respondent. HHSC will consider capabilities or advantages that are clearly described in the proposal, which may be confirmed by oral presentations, site visits, demonstrations, and/or references contacted by HHSC. HHSC reserves the right to contact individuals, entities, or organizations that have had dealings with the Respondent or proposed staff, whether or not identified in the proposal.
HHSC will more favorably evaluate proposals that offer no or few exceptions, reservations, or limitations to the terms and conditions of the RFP, including Attachment A, “Uniform Managed Care Contract Terms and Conditions.”
HHSC will evaluate proposals based on the following best value criteria, listed in order of precedence:
|
| |
| • The extent to which the Respondent’s proposal demonstrates an ability to accomplish the missions and objectives for this procurement, including: |
|
| |
| • the extent to which the proposal meets HHSC’s needs, and the MCO Program clients’ needs for high quality and accessible medical care; |
|
| |
| • The degree to which the proposal demonstrates program innovation, adaptability, and exceptional customer service; and |
|
| |
| • the extent to which the Respondent accepts without reservation or exception the RFP’s terms and conditions, including Attachment A, “Uniform Managed Care Contract Terms and Conditions.” |
|
| |
| • Indicators of probable performance under the Contract, including past performance in Texas or comparable experience; financial resources and solvency, including the impact on the Respondent’s and its Subcontractors’ ability to perform, and relevant organizational experience. |
|
| |
| • Effect of the acquisition on agency productivity; including the level of effort and resources required to monitor the Respondent’s performance and maintain a good working relationship with the Respondent. |
Proposals for the STAR Medicaid Rural Service Area that include all three (3) regions will be given preference over proposals that do not include all three (3) regions.
If all other considerations are equal, HHSC will give preference to:
|
| |
| 1. proposals from Texas institutions providing graduate medical education; |
|
| |
| 2. proposals that include substantial participation by Network providers who are Significant Traditional Providers (STP). HHSC defines “substantial participation” as proposals that include at least 50 percent of the STPs in a Service Area. The Respondent must either have a Network Provider agreement in place with the STP, or a Letter of Intent/Letter of Agreement to participate in the Network. A listing of STPs for the new Service Areas can be found in the Procurement Library; and |
|
| |
| 3. proposals that ensure continuity of coverage for Medicaid Members for at least three (3) months beyond the period of Medicaid eligibility. For purposes of this provision, HHSC defines “continuity of coverage” as providing the full set of Covered Services. |
NOTE: Respondents who are licensed as health maintenance organizations pursuant to Chapter 843 of the Texas Insurance Code, and believe they meet the requirements for mandatory contracting under Texas Government Code §533.004, must provide written notice to HHSC’s Point of Contact (see RFP Section 1.1) no later than April 28, 2011. The notice must provide a clear description of why the Respondent believes it is entitled to a mandatory contract under the Texas Government Code.
|
|
5.3 Initial Compliance Screening |
HHSC will perform an initial screening of all proposals received. Unsigned proposals and proposals that do not include all required forms and sections are subject to rejection without further evaluation.
In accordance with Section 3.11, “Modification or Withdrawal of Proposal,” HHSC reserves the right to waive minor informalities in a proposal and award contracts that are in the best interest of the State of Texas.
|
|
5.4 Competitive Field Determinations |
HHSC may determine that certain proposals are within the field of competition for admission to discussions. The field of competition consists of the proposals that receive the highest or most satisfactory evaluations. HHSC may, in the interest of administrative efficiency, place reasonable limits on the number of proposals admitted to the field of competition.
|
|
5.5 Oral Presentations and Site Visits |
HHSC may, at its sole discretion, request oral presentations, site visits, and/or demonstrations from one or more Respondents admitted to the field of competition. HHSC will notify selected Respondents of the time and location for these activities, and may supply agendas or topics for discussion. HHSC reserves the right to ask additional questions during oral presentations, site visits, and or demonstrations to clarify the scope and content of the written proposal.
The Respondent’s oral presentation, site visit, and/or demonstration must substantially represent material included in the written proposal, and should not introduce new concepts or offers unless specifically requested by HHSC.
Respondents will not submit cost proposals for this RFP. HHSC will establish the Capitation Rates for each Program and Service Area in accordance with the methodology described in Attachment A, “Uniform Managed Care Contract Terms and Conditions,” Article 10, “Terms and Conditions of Payment.”
HHSC may, but is not required to, permit Respondents to prepare one or more revised offers for services. For this reason, Respondents are encouraged to treat their original proposals, and any revised offers requested by HHSC, as best and final offers of services.
|
|
5.7 Discussions with Respondents |
HHSC may, but is not required to, conduct discussions with all, some, or none of the Respondents admitted to the field of competition for the purpose of obtaining the best value for the State of Texas. It may conduct discussions for the purpose of:
|
| |
| • obtaining clarification of proposal ambiguities; |
|
| |
| • requesting modifications to a proposal; and/or |
|
| |
| • obtaining a best and final offer of services. |
HHSC may make an award prior to the completion of discussions with all Respondents admitted to the field of competition if HHSC determines that the award represents best value to the State of Texas.
Respondents are allowed to select which MCO Programs and Services Areas to include in their Proposals. It is possible that a Respondent submitting a Proposal for more than one MCO Program in a Service Area could be awarded a Contract for some, but not all, of the MCO Programs. Similarly, a Respondent could be awarded a Contract for some, but not all, of its proposed Service Areas. HHSC reserves the right to change the boundaries for, or otherwise modify, the Service Areas if it determines that such action is in the best interest of the State.
Subject: Attachment B-1 - Medicaid and CHIP Managed Care Services RFP, Section 6
|
| | | |
DOCUMENT HISTORY LOG |
STATUS1 | DOCUMENT REVISION2 | EFFECTIVE DATE | DESCRIPTION3 |
Baseline | n/a | September 1, 2011 | Initial version of Attachment B-1, RFP Section 6, “Incentives & Disincentives.” |
Revision | 2.1 | March 1, 2012 | Contract amendment did not revise Attachment B-1, RFP Section 6, "Incentives & Disincentives." |
Revision | 2.2 | June 1, 2012 | Section 6.3.2.1 is modified to change "Rate Period 1" to "FSR Reporting Period 12/13." Section 6.3.2.2 is modified to change "Rate Period" to "FSR Reporting Period." |
Revision | 2.3 | September 1, 2012 | Section 6.3.2.5 is modified to remove auto-assignment default methodology.
|
Revision | 2.4 | March 1, 2013 | All references to the previous Executive Commissioner Suehs are changed to his successor, Executive Commissioner Janek.
|
Revision | 2.5 | June 1, 2013 | Contract amendment did not revise Attachment B-1, RFP Section 6, "Incentives & Disincentives."
|
Revision | 2.6 | September 1, 2013 | Section 6.2.1 is modified to remove the reference to Bariatric Supplemental Payments. Section 6.3.1.2 is modified to provide HHSC more flexibility to implement reward-based assignment methodologies. Section 6.3.2.2 is modified to add the word “Program” to the section title. Section 6.3.2.3 is renamed “Performance-Incentive Program”. Subsection 6.3.2.3.1 “Quality Challenge Award” is renamed “Quality Challenge Award Program” and to add clarifying language. Subsection 6.3.2.3.2 State-MCO Shared Savings Program is added.
|
Revision | 2.7 | September 1, 2013 | Contract amendment did not revise Attachment B-1, RFP Section 6, "Incentives & Disincentives."
|
Revision | 2.8 | January 1, 2014 | Contract amendment did not revise Attachment B-1, RFP Section 6, "Incentives & Disincentives."
|
|
| | | |
Revision | 2.9 | February 1, 2014 | Section 6.3.2.3.2 is renamed Other Incentive Programs’ and updated. |
Revision | 2.10 | April 1, 2014 | Contract amendment did not revise Attachment B-1, RFP Section 6, "Incentives & Disincentives." |
Revision | 2.11 | September 1, 2014 | Section 6.3.2.1 "Experience Rebate Reward" is deleted in its entirety. |
1 Status should be represented as “Baseline” for initial issuances, “Revision” for changes to the Baseline version, and “Cancellation” for withdrawn versions 2 Revisions should be numbered in accordance according to the version of the issuance and sequential numbering of the revision—e.g., “1.2” refers to the first version of the document and the second revision. 3 Brief description of the changes to the document made in the revision. |
Table of Contents
6. Premium Payment, Incentives, and Disincentives .................................................................................................................. 6-4
6.1 Capitation Rate Development ............................................................................................................................................... 6-4
6.2 Financial Payment Structure and Provisions ......................................................................................................................... 6-4
6.2.1 Capitation Payments ............................................................................................................................................ 6-4
6.3 Performance Incentives and Disincentives ............................................................................................................................ 6-5
6.3.1 Non-financial Incentives ...................................................................................................................................... 6-5
6.3.2 Financial Incentives and Disincentives .............................................................................................................................. 6-5
|
|
6. Premium Payment, Incentives, and Disincentives |
This section describes performance incentives and disincentives related to HHSC’s value-based purchasing approach. For further information, MCOs should refer to Attachment A, “Uniform Managed Care Contract Terms and Conditions.”
Under the MCO Contracts, health care coverage for Members will be provided on a fully insured basis. The MCO must provide the Services and Deliverables, including Covered Services, to enrolled Members in exchange for the monthly Capitation Payments. Section 8, “Operations Phase Requirements” includes the MCO’s financial responsibilities regarding Out-of-Network Emergency Services and Medically Necessary Covered Services that are not available through Network Providers.
|
|
6.1 Capitation Rate Development |
Refer to Attachment A, “Uniform Managed Care Contract Terms and Conditions,” Article 10, “Terms & Conditions of Payment” for information concerning Capitation Rate development.
|
|
6.2 Financial Payment Structure and Provisions |
HHSC will pay the MCO monthly Capitation Payments based on the number of eligible and enrolled Members. HHSC will calculate the monthly Capitation Payments by multiplying the number of Member Months times the applicable monthly Capitation Rate by Member Rate Cell.
The MCO must understand and expressly assume the risks associated with the performance of the duties and responsibilities under the Contract, including the failure, termination, or suspension of funding to HHSC, delays or denials of required approvals, cost of claims incorrectly paid by the MCO, and cost overruns not reasonably attributable to HHSC. The MCO must further agree that no other charges for tasks, functions, or activities that are incidental or ancillary to the delivery of the
Services and Deliverables will be sought from HHSC or any other state agency, nor will the failure of HHSC or any other party to pay for such incidental or ancillary services entitle the MCO to withhold Services or Deliverables due under the Contract.
|
|
6.2.1 Capitation Payments |
The MCO must refer to Attachment A, "Uniform Managed Care Contract Terms and Conditions" for information and Contract requirements on the:
1. time and Manner of Payment,
2. adjustments to Capitation Payments,
3. Delivery Supplemental Payment, and
4. Experience Rebate.
|
|
6.3 Performance Incentives and Disincentives |
HHSC has included several financial and non-financial performance incentives and disincentives on this Contract. These incentives and disincentives are subject to change by HHSC over the course of the Contract. The MCO is prohibited from passing down financial disincentives and/or sanctions imposed on the MCO to health care providers, except on an individual basis and related to the individual provider’s inadequate performance.
|
|
6.3.1 Non-financial Incentives |
|
|
6.3.1.1 Performance Profiling |
HHSC intends to distribute information on key performance indicators to MCOs on a regular basis, identifying an MCO’s performance, and comparing that performance to other MCOs and to HHSC standards and/or external Benchmarks. HHSC may recognize MCOs that attain superior performance and/or improvement by publicizing their achievements. For example, HHSC may post information concerning exceptional performance on its website, where it will be available to both stakeholders and members of the public. Likewise, HHSC may post its final determination regarding poor performance or MCO peer group performance comparisons on its website, where it will be available to both stakeholders and members of the public.
|
|
6.3.1.2 Auto-assignment Methodology for Medicaid MCOs |
HHSC may revise its auto-assignment methodology during the Contract Period for enrollees who do not select an MCO. The new assignment methodology may reward those MCOs that demonstrate superior performance or improvement on one or more key dimensions of performance (see 1 Tex. Admin. Code § 353.403(d)(3)(B) for Medicaid).
HHSC will invite MCO comments on potential approaches prior to implementation of a performance-based auto-assignment algorithm.
|
|
6.3.2 Financial Incentives and Disincentives |
6.3.2.1 This Section Intentionally Left Blank
6.3.2.2 Performance-Based Capitation Rate (5%-at-risk)
HHSC will place each MCO at risk for 5% of the Capitation Payment. HHSC retains the right to reduce the percentage of the Capitation Payment placed at risk in a given FSR Reporting Period.
During the FSR Reporting Period, HHSC will pay the MCO the full monthly Capitation Payments as described in Section 6.2. Then, at the end of each FSR Reporting Period, HHSC will evaluate if the MCO has demonstrated that it has fully met the performance expectations for which the MCO is at risk. If the MCO falls short on some or all of the performance expectations,
HHSC will adjust a future monthly Capitation Payment in accordance with Uniform Managed Care Manual Chapter 6.2, Financial Incentive Methodology, by an appropriate portion of the aggregate at-risk amount. HHSC's objective is that all MCOs achieve performance levels that enable them to retain the full at-risk amount.
HHSC will determine the extent to which the MCO has met the performance expectations by assessing the MCO's performance for each applicable MCO Program relative to performance targets for the FSR Reporting Period. HHSC will conduct separate accounting for each MCO Program's at-risk Capitation Payment amount.
HHSC will identify no more than 10 at-risk performance indicators for each MCO Program. Some of the performance indicators will be standard across all Programs while others may apply to only one (1) Program.
Specific contractual requirements are set forth in the Uniform Managed Care Manual, Chapter 6.2, Financial Incentive Methodology.
Failure to timely provide HHSC with necessary data related to the calculation of the performance indicators will result in HHSC's assignment of a zero percent (0%) performance rate for each related performance indicator.
MCOs will report actual Capitation Payments received on the Financial Statistical Report (FSR) during the FSR Reporting Period that is at risk (i.e., the MCO will not report Revenues at a level equivalent to 95% of the payments received, leaving five percent (5%) as contingent). Actual Capitation Payments received include all of the at-risk Capitation Payment paid to the MCO. Any loss of the at-risk amount that may be realized in a subsequent FSR Reporting Period, via reduction to a monthly payment, will not be reported in the FSR as a reduced amount of capitation revenue, but will instead be reported below the income line, as an informational item, as described in the Uniform Managed Care Manual, Chapter 5.3.1, "Financial Statistical Report and Instructions." Any performance assessment based on performance for a FSR Reporting Period will appear on the final (334-day) FSR for that FSR Reporting Period.
HHSC will evaluate the performance-based Capitation Rate methodology annually in consultation with MCOs. HHSC may then modify the methodology as it deems necessary and appropriate, in order to motivate, recognize, and reward MCOs for superior performance. The methodologies for all FSR Reporting Periods will be included in Uniform Managed Care Manual Chapter 6.2, "Financial Incentive Methodology."
6.3.2.3 Performance Based Incentive Program
HHSC, at its discretion, may implement one or both of the following financial incentive programs in conjunction with provisions listed in 6.3.2.2.
6.3.2.3.1 Quality Challenge Award Program
Should one or more MCOs be unable to earn the full amount of the performance-based at-risk portion of the Capitation Rate, HHSC may reallocate the funds through the MCO Program's Quality Challenge Award. Under this program, HHSC may use these funds to reward MCOs that demonstrate superior clinical quality, service delivery, access to care, or Member satisfaction. HHSC will determine the number of MCOs that will receive Quality Challenge Award funds annually based on the amount of the funds to be reallocated. Separate Quality Challenge Award payments will be made for each of the MCO Programs.
As with the performance-based Capitation Rate, each MCO will be evaluated separately for each MCO Program. HHSC may evaluate MCO performance annually on some combination of performance indicators in order to determine which MCOs demonstrate superior performance. In no event will a distribution from the Quality Challenge Award, plus any other incentive payments made in accordance with the MCO Contract, when combined with the Capitation Rate payments, exceed 105% of the Capitation Rate payments to an MCO. Measures utilized for the Quality Challenge Program may be the same as those used in the Performance-Based Capitation Rate Program, or may be different than those selected for the Performance-Based Capitation Rate Program.
Information about the data collection period to be used and each indicator that will be considered for any specific time period can be found in Uniform Managed Care Manual Chapter 6.2.6, "Quality Challenge Award Performance Indicators."
Failure to provide timely and accurate information may result in HHSC's assignment of a 0% performance rate for each applicable Quality Challenge Award indicator.
HHSC may evaluate the Quality Challenge Award methodology annually in consultation with MCOs. HHSC may make methodology modifications annually as it deems necessary and appropriate to motivate, recognize, and reward MCOs for superior performance based on available Quality Challenge Award funds and/or other performance incentives applicable to the award. HHSC may include the Quality Challenge Award methodology and risk adjustment factors, or any other modifications in Uniform Managed Care Manual Chapter 6.2.6, "Quality Challenge Award Performance Indicators."
6.3.2.3.2 Other Incentive Programs
HHSC may incentivize MCOs to achieve superior performance through other incentive programs. Opportunities for these incentives will depend upon whether the MCO meets or exceeds performance measures identified by HHSC. HHSC reserves the right to modify the methodologies used to determine the incentives in these programs. HHSC will consult with the MCOs in the development of incentive programs.
Programs identified in 6.3.2.3.1 and 6.3.2.3.2 could be operated concurrently, at HHSC’s discretion.
|
|
6.3.2.4 Remedies and Liquidated Damages |
All areas of responsibility and all requirements in the Contract will be subject to performance evaluation by HHSC. Any and all responsibilities or requirements not fulfilled will be subject to contractual remedies, including without limitation liquidated damages. Refer to Attachment A, “Uniform Managed Care Contract Terms and Conditions,” and Attachment B-3, “Deliverables/Liquidated Damages Matrix” for performance standards that carry liquidated damage values.
|
|
6.3.2.5 Frew Incentives and Disincentives |
As required by the "Frew vs. Janek Corrective Action Order: Managed Care," this Contract includes a system of incentives and disincentives associated with the Medicaid Managed Care Texas Health Steps Medical Checkups Reports and Children of Migrant Farm Workers Reports. These incentives and disincentives apply to Medicaid MCOs.
The incentives and disincentives and corresponding methodology are set forth in the Uniform Managed Care Manual, Chapter 12 "Frew."
|
|
6.3.2.6 Nursing Facility Utilization Disincentive |
HHSC has developed the nursing facility utilization disincentive to prevent inappropriate admission to nursing facilities. The rate of nursing facility admissions for Medicaid-only STAR+PLUS Members will be part of the Performance Indicator Dashboard (see Section 6.3.2.2).
|
|
6.3.2.7 Additional Incentives and Disincentives |
HHSC will evaluate all performance-based incentives and disincentive methodologies annually and in consultation with the MCOs. HHSC may then modify the methodologies as needed, as funds become available, or as mandated by court decree, statute, or rule, in an effort to motivate, recognize, and reward MCOs for performance.
Information about the data collection period to be used, performance indicators selected or developed, or MCO ranking methodologies used for any specific time period will be found in the Uniform Managed Care Manual.
Subject: Attachment B-1 - Medicaid and CHIP Managed Care Services RFP, Section 7
DOCUMENT HISTORY LOG
|
| | | |
STATUS1 | DOCUMENT REVISION2 | EFFECTIVE DATE | DESCRIPTION3 |
Baseline | n/a | September 1, 2011 | Initial version of Attachment B-1, RFP Section 7, “Transition Phase Requirements.” |
Revision | 2.1 | March 1, 2012 | Section 7.1 is modified to add termination of the contract to the list of remedies for failure to timely satisfy Readiness Review requirements. |
Revision | 2.2 | June 1, 2012 | Contract amendment did not revise Attachment B-1, Section 7, "Transition Phase Requirements." |
Revision | 2.3 | September 1, 2012 | Contract amendment did not revise Attachment B-1, Section 7, "Transition Phase Requirements." |
Revision | 2.4 | March 1, 2013 | Contract amendment did not revise Attachment B-1, Section 7, “Transition Phase Requirements.”
|
Revision | 2.5 | June 1, 2013 | Contract amendment did not revise Attachment B-1, Section 7, “Transition Phase Requirements.”
|
Revision | 2.6 | September 1, 2013 | Section 7.2.8.1 is modified for clarification and to comply with requirements of SB 7, 83R.
|
Revision | 2.7 | September 1, 2013 | Contract amendment did not revise Attachment B-1, Section 7, “Transition Phase Requirements.”
|
Revision | 2.8 | January 1, 2014 | Contract amendment did not revise Attachment B-1, Section 7, “Transition Phase Requirements.”
|
Revision | 2.9 | February 1, 2014 | Contract amendment did not revise Attachment B-1, Section 7, “Transition Phase Requirements.”
|
Revision | 2.10 | April 1, 2014 | Contract amendment did not revise Attachment B-1, Section 7, "Transition Phase Requirements." |
Revision | 2.11 | September 1, 2014 | Section 7.2.7 is modified to update SAS70 to SSAE16. Section 7.2.10 is revised to include reference to a Dual Eligible Medicare-Medicaid Plan (MMP). |
1 Status should be represented as “Baseline” for initial issuances, “Revision” for changes to the Baseline version, and “Cancellation” for withdrawn versions 2 Revisions should be numbered in accordance according to the version of the issuance and sequential numbering of the revision—e.g., “1.2” refers to the first version of the document and the second revision. 3 Brief description of the changes to the document made in the revision. |
Table of Contents
7. Transition Phase Requirements.........................................................................................7-4
7.1 Introduction.................................................................................................................7-4
7.2 Transition Phase Schedule and Tasks.......................................................................7-4
7.2.1 Contract Start-Up and Planning.............................................................................7-4
7.2.2 Administration and Key MCO Personnel................................................................7-5
7.2.3 Organizational Readiness Review.........................................................................7-5
7.2.4 Financial Readiness Review..................................................................................7-6
7.2.5 System Testing and Transfer of Data.....................................................................7-7
7.2.6 System Readiness Review.....................................................................................7-7
7.2.7 Demonstration and Assessment of System Readiness.........................................7-8
7.2.8 Operations Readiness............................................................................................7-8
7.2.9 Assurance of System and Operational Readiness...............................................7-12
7.2.10 TDI and Centers for Medicare and Medicaid Services (CMS) Licensure,
Certification or Approval...................................................................................................7-12
7.2.11 Post-Transition.................................................................................................7-12
|
|
7. Transition Phase Requirements |
This Section presents the scope of work for the Transition Phase of the Contract, which includes those activities that must take place between the time of Contract award and the Operational Start Date.
The Transition Phase will include all activities that must be completed successfully prior to a MCO’s Operational Start Date for each applicable MCO Program and Service Area, including all Readiness Review activities. HHSC will conduct Readiness Reviews to determine whether the MCO has implemented all systems and processes necessary to begin serving Members. MCOs must satisfy all Readiness Review requirements no later than 60 days prior to the Operational Start Date for each applicable MCO Program and Service Area, with the exception of HHSC’s review of the Service Coordination function. HHSC may, at its discretion, terminate the contract, postpone the MCO’s Operational Start Date(s) and assess contractual remedies if an MCO fails to timely satisfy all Readiness Review requirements. Refer to Attachment A, “Uniform Managed Care Contract Terms and Conditions” and the Attachment B-3, “Deliverables/Liquidated Damages Matrix” for additional information.
The MCO is required to promptly provide a Corrective Action Plan and/or Risk Mitigation Plan as requested by HHSC in response to Transition Phase deficiencies identified by theMCO, HHSC, or its agent. The MCO must promptly alert HHSC of deficiencies, and must correct a deficiency or provide a Corrective Action Plan and/or Risk Mitigation Plan no later than ten (10) calendar days after HHSC’s notification of deficiencies. If the MCO documents to HHSC’s satisfaction that the deficiency has been corrected within ten (10) calendar days of such deficiency notification by HHSC, no Corrective Action Plan is required.
|
|
7.2 Transition Phase Schedule and Tasks |
The MCO has overall responsibility for the timely and successful completion of each of the Transition Phase tasks. The MCO is responsible for clearly specifying and requesting information needed from HHSC, other HHSC contractors, and Providers in a manner that does not delay the schedule or work to be performed.
|
|
7.2.1 Contract Start-Up and Planning |
HHSC and the MCO will work together during the initial Contract start-up phase to:
|
| |
| • define project management and reporting standards; |
|
| |
| • establish communication protocols between HHSC and the MCO; |
|
| |
| • establish contacts with other HHSC contractors; |
|
| |
| • establish a schedule for key activities and milestones; and |
|
| |
| • clarify expectations for the content and format of Contract Deliverables. |
The MCO will be responsible for developing a written work plan, referred to as the “Transition/Implementation Plan,” which will be used to monitor progress throughout the Transition Phase. The MCO must update the Transition/Implementation Plan provided with its proposal no later than 30 days after the Contract’s Effective Date, then provide monthly implementation progress reports through the sixth month of MCO Program operations. HHSC may require more frequent reporting as it determines necessary.
|
|
7.2.2 Administration and Key MCO Personnel |
No later than the Effective Date of the Contract, the MCO must designate and identify Key MCO Personnel that meet the requirements in Attachment A, “Uniform Managed Care Contract Terms and Conditions,” Article 4, “Contract Administration and Management.” The MCO will supply HHSC with resumes of each Key MCO Personnel as well as any organizational information that has changed relative to the MCO’s Proposal, such as updated job descriptions and updated organizational charts (including updated Management Information System (MIS) job descriptions and an updated MIS staff organizational chart), if applicable. If the MCO is using a Material Subcontractors, the MCO must also provide the organizational chart for these Material Subcontractors.
|
|
7.2.3 Organizational Readiness Review |
In order to complete an organizational review and assess the most current corporate environment, the MCO must submit an Organization Update Report no later than 60 days prior to the Operational Start Date that updates the organizational information submitted in its proposal (see Section 4.2, “Business Proposal”). For each of the numbered items below, the report must describe whether the information provided in MCO’s proposal has changed. If so, the report must include relevant portions of the proposal with changes highlighted.
1. Respondent identification and information, Section 4.2.2.
2. Corporate background and experience:
a. Item #1, concerning publicly-funded managed care contracts, under Section 4.2.3;
b. Item # 2, concerning regulatory actions, sanctions, and/or fines, under Section 4.2.3;
c. Section 4.2.3.1, concerning organizational charts; and
d. Section 4.2.3.2, concerning resumes; and
3. Material Subcontractor information, Section 4.2.4.
|
|
7.2.4 Financial Readiness Review |
To complete a financial review, the MCO must submit a Financial Update Report no later than 60 days prior to the Operational Start Date. At a minimum, the report must include the following:
1. Material change in financial condition.
For both the MCO and its ultimate parent, the report must identify whether either entity has experienced any material financial deterioration following proposal submission. The report must identify and briefly describe any changes to the financial statements, including changes to net worth; cash flow; loss of contracts; credit, audit, regulatory, and/or legal issues; major contingencies, etc. The report must also describe any known potential issues, and any issues with respect to change of ownership or control.
2. Updated financial statements.
The report must include the most recently updated financial statements, which should be more current than those provided in the proposal. The updated financial statements should include the most recent quarterly (or monthly) internal financial statements, the most-recently completed annual statements, and the most-recent audited statements. The statements should generally include the notes, management discussion, and where appropriate, the audit letter. Internal most-recent-month statements are not expected to include these items.
The report must include any of the following new or updated reports (as referenced under Sections 4.2.3.3 and 4.2.3.4) that have become available since proposal submission: TDI financial examination report (or similar report from another state); Form B Registration statement filing; IRS Form 990; and bond or debt rating analysis. It is not necessary to submit updated SEC 10-K or 10-Q filings with the report.
In addition to the Financial Update Report, the MCO must submit documentation demonstrating it has secured all required bonds in accordance with TDI requirements, Section 8, “Operations Phase Requirements,” and Attachment A, “Uniform Managed Care Terms and Conditions,” Article 17. Such documentation is due no later than ten (10) business days after the Contract Effective Date.
|
|
7.2.4.1 Employee Bonus and/or Incentive Payment Plan |
If the MCO intends to include Employee Bonus or Incentive Payments as allowable administrative expenses, the MCO must furnish a written Employee Bonus and/or Incentive Payments Plan to HHSC. The written plan must include a description of the MCO’s criteria for establishing bonus and/or incentive payments, the methodology to calculate bonus and/or incentive payments, and the timing of bonus and/or incentive payments. The Bonus and/or Incentive Payment Plan and description must be submitted during the Transition Phase, no later than 30 days after the Effective Date of the Contract. If the MCO substantively revises the Employee Bonus and/or Incentive Payment Plan during the Operations Phase, the MCO must submit the revised plan to HHSC at least 30 days in advance of its effective date.
HHSC reserves the right to disallow all or part of a plan that it deems inappropriate. Any such payments are subject to audit, and must conform with the Uniform Managed Care Manual, Chapter 6.1, “Cost Principles for Expenses.”
|
|
7.2.5 System Testing and Transfer of Data |
The MCO must have hardware, software, network and communications systems with the capability and capacity to handle and operate all MIS systems and subsystems identified in Section 8.1.18, “Management Information System Requirements.” For example, the MCO’s MIS system must comply with the Health Insurance Portability and Accountability Act of 1996 (HIPAA) as indicated in Section 8.1.18.4, “HIPAA Compliance.”
During this Readiness Review task, the MCO will accept into its system any and all necessary data files and information available from HHSC or its contractors. The MCO will install and test all hardware, software, and telecommunications required to support the Contract. The MCO will define and test modifications to the MCO’s systems required to support the business functions of the Contract.
The MCO will produce data extracts and receive all electronic data transfers and transmissions.
If any errors or deficiencies are evident, the MCO will develop resolution procedures to address problems identified. The MCO will provide HHSC, or a designated vendor, with test data files for systems and interface testing for all external interfaces. This includes testing of the required telephone lines for Providers and Members and any necessary connections to the HHSC Administrative Services Contractor. The HHSC Administrative Services Contractor will provide enrollment test
files to new MCOs that do not have previous HHSC enrollment files. The MCO will demonstrate its system capabilities and adherence to Contract specifications during Readiness Review.
|
|
7.2.6 System Readiness Review |
The MCO must assure that systems services are not disrupted or interrupted during the Operations Phase of the Contract. The MCO must coordinate with HHSC and other contractors to ensure the business and systems continuity for the processing of all health care claims and data as required under this contract.
The MCO must submit descriptions of interface and data and process flow for each key business processes described in Section 8.1.18.3, “System-wide Functions.”
The MCO must clearly define and document the policies and procedures that will be followed to support day-to-day systems activities. No later than 90 days prior to the Operational Start Date, new MCOs must develop and incumbent MCOs must update the following plans:
|
| |
| 1. Disaster Recovery Plan;* |
|
| |
| 2. Business Continuity Plan*; |
|
| |
| 5. Risk Management Plan; and |
|
| |
| 6. Systems Quality Assurance Plan. |
*The Business Continuity Plan and the Disaster Recovery Plan may be combined into one document.
|
|
7.2.7 Demonstration and Assessment of System Readiness |
The MCO must provide documentation on systems and facility security and provide evidence or demonstrate that it is compliant with HIPAA. The MCO must also provide HHSC with a summary of all recent external audit reports, including findings and corrective actions, relating to the MCO’s proposed systems, including any SSAE16 audits that have been conducted in the past three years. The MCO must promptly make additional information on the detail of such system audits available to HHSC upon request.
In addition, HHSC will provide to the MCO a test plan that will outline the activities that need to be performed by the MCO prior to the Operational Start Date(s). The MCO must be prepared to assure and demonstrate system readiness. The MCO must execute system readiness test cycles to include all external data interfaces, including those with the MCO’s Pharmacy Benefits Manager (PBM) and other Material Subcontractors.
HHSC, or its agents, may independently test whether the MCO’s MIS has the capacity to administer the STAR, STAR+PLUS, and/or CHIP business. This Readiness Review may include a desk review and/or an onsite review. HHSC may request additional documentation to support the provision of STAR, STAR+PLUS, and/or CHIP MCO Services. Based in part on the MCO’s assurances of systems readiness, information contained in the Proposal, additional documentation submitted by the MCO, and any review conducted by HHSC or its agents, HHSC will assess the MCO’s understanding of its responsibilities and the MCO’s capability to assume the MIS functions required under the Contract.
|
|
7.2.8 Operations Readiness |
The MCO must clearly define and document the policies and procedures that will be followed to support day-to-day business activities related to the provision of STAR, STAR+PLUS, and/or CH IP MCO Services, including coordination with
Subcontractors and HHSC’s contractors. The MCO will be responsible for developing and documenting its approach to quality assurance.
7.2.8.1 Readiness Review
Readiness Review includes all activities that the MCO must complete prior to the Operational Start Date. At a minimum, the MCO must, for each MCO Program
1. Develop new, or revise existing, operations procedures and associated documentation to support the MCO's proposed approach to conducting operations activities in compliance with the contracted Scope of Work.
2. Submit a comprehensive plan for Network adequacy that includes a list of all contracted and credentialed Providers, in an HHSC-approved format. At a minimum, the list must include the acute care and long-term care Provider types identified in Texas Government Code § 533.005(20)(A). The plan must include a description of additional contracting and credentialing activities scheduled to be completed before the Operational Start Date. The MCO must submit a listing of all contracted and credentialed providers to be included in the first Provider Directory 90 days prior to the first enrollment kit mail out, or as otherwise directed by HHSC.
3. Inform all Network Providers about the information required to submit a claim: (1) at least 30 days prior to the Operational Start Date, and (2) as a provision within the Network Provider agreement.
4. Prepare and implement a Member Services staff training curriculum and a Provider training curriculum.
5. Prepare a Coordination Plan documenting how the MCO will coordinate its business activities with those activities performed by HHSC's contractors, the MCO's PBM and other Material Subcontractors, if any. The Coordination Plan will include identification of coordinated activities and protocols for the Transition Phase.
6. Develop and submit the following draft materials: Member Handbook, Provider Manual, Provider Directory, and Member Identification Card for HHSC's. The materials must at a minimum meet the requirements specified in Section 8.1.5, "Member Services" and include the Critical Elements defined in Uniform Managed Care Manual Chapter 3, "Critical Elements."
7. Develop and submit the MCO's proposed Member Complaint and Appeals processes for STAR, STAR+PLUS, and CHIP, as applicable to the MCO.
8. Provide sufficient copies of the final Provider Directory to the HHSC Administrative Services Contractor in sufficient time to meet the enrollment schedule.
9. Demonstrate toll-free telephone systems and reporting capabilities for the Member Services Hotline, the Behavioral Health Hotline, and the Provider Services Hotline.
10. Submit a written plan for providing pharmacy services, including proposed policies and procedures for:
| |
• | routinely updating formulary data following receipt of HHSC's daily files (no less frequently than weekly, and off-cycle upon HHSC's request); |
| |
• | prior authorization of drugs, including how HHSC's preferred drug lists (PDLs) will be incorporated into prior authorization systems and processes. The MCO must adopt HHSC's prior authorization policies unless HHSC grants a written exception, and HHSC's approval is required for all Clinical Edit policies; |
| |
• | implementing drug utilization review; |
| |
• | overriding standard drug utilization review criteria and clinical edits when Medically Necessary based on the individual Member's circumstances (e.g, overriding quantity limitations, drug-drug interactions, refill too soon, etc.); |
| |
• | call center operations, including how the MCO will ensure that staff for all appropriate hotlines are trained to respond to prior authorization inquiries and other inquiries regarding pharmacy services, and |
| |
• | monitoring the PBM Subcontractor. |
The plan must also include a written description of the assurances and procedures that must be put in place under the proposed PBM Subcontract, such as an independent audit, to ensure no conflicts of interest exist and ensure the confidentiality of proprietary information.
Additionally, the MCO must include a written attestation by the PBM Subcontractor in the plan stating, in the three (3) years preceding the Contract's Effective Date, the PBM Subcontractor has not been: (1) convicted of an offense involving a material misrepresentation or any act of fraud or of another violation of state or federal criminal law; (2) adjudicated to have committed a breach of contract, or (3) assessed a penalty or fine of $500,000 or more in a state or federal administrative proceeding. If the PMB Subcontractor cannot affirmatively attest to any of these items, then it must provide a comprehensive description of the matter and all related corrective actions.
11. Between the date of Contract award and the Operational Start date, the MCO must identify a list of Pharmacy Providers with whom the MCO's PBM has successfully contracted and credentialed for inclusion in the first Provider Directory. These providers should be listed by name and address with an indicator for pharmacies that are open 24-hours.
12. No later than 30 days after the Contract Effective Date, new MCOs must develop and incumbent MCOs must update their written Fraud and Abuse Compliance Plans. See Section 8.1.19, Fraud and Abuse for the requirements of the plan, including new requirements for special investigation units. As part of the Fraud and Abuse Compliance Plan, the MCO must:
| |
• | Designate executive and essential personnel to attend mandatory training in fraud and abuse detection, prevention and reporting. Executive and essential fraud and abuse personnel means MCO staff persons who: (1) are directly involved in the decision-making and administration of the fraud and abuse detection program within the MCO, and (2) who supervise staff in the following areas: data collection, Provider enrollment or disenrollment, Encounter Data, claims processing, Utilization Review, Appeals or Grievances, quality assurance and marketing. The training will be conducted by the Office of Inspector General, Health and Human Services Commission, and will be provided free of charge. The MCO must schedule and complete training no later than 90 days after the Contract's Effective Date. |
| |
• | Designate an officer or director within the organization responsible for carrying out the provisions of the Fraud and Abuse Compliance Plan. |
| |
• | For STAR+PLUS MCOs, complete hiring and training of Service Coordination staff no later than 45 days prior to the Operational Start Date. |
If this function is subcontracted to another entity, the Subcontractor also meets all the requirements in this section and the Fraud and Abuse section as stated in Section 8, "Operations Phase Requirements."
13. The MCO must submit a copy of each Material Subcontract in accordance with the timeframes identified in Attachment A, "Uniform Managed Care Contract Terms and Conditions," Section 4.08, "Subcontractors."
14. No later than ten (10) days after the Contract Effective Date, the MCO must submit documentation demonstrating that it has secured all required insurance, in accordance with TDI requirements and Section 8, "Operations Phase Requirements," and Attachment A, "Uniform Managed Care Contract Terms and Conditions," Article 17.
HHSC may require the MCO to resubmit one or more of the above items if the MCO begins providing a new service or benefit, expands into a new Program or Service Area, or implements a major systems change after the Contract's Effective Date.
During the Readiness Review, HHSC may request additional information, including more detailed or updated information regarding the MCO's operating procedures and documentation. HHSC will assess the MCO's understanding of its responsibilities and the MCO's capability to assume the functions required under the Contract, based in part on the MCO's assurances of operational readiness, information contained in the Proposal, and in Transition Phase documentation submitted by the MCO.
|
|
7.2.8.2 Value-Added Services |
The MCO must use HHSC’s template for submitting proposed Value-added Services. (See Uniform Managed Care Manual Chapter 4.4) Once approved by HHSC, this document is incorporated by reference into the Contract.
During the Transition Phase, HHSC will offer a one-time opportunity for the MCO to propose two (2) additional Value-added Services to its list of current, approved Value-added Services HHSC will establish the requirements and the timeframes for submitting the two (2) additional proposed Value-added Services.
During this HHSC-designated opportunity, the MCO may propose either to add new Value-added Services or to enhance its approved Value-added Services. The MCO may propose two (2) additional Value-added Services per MCO Program, which will be effective on the Operational Start Date. The services do not have to be the same for each Program. The Contract will be amended to include any additional Value-added Services approved by HHSC.
The MCO does not have to add Value-added Services during the HHSC-designated opportunity, but this will be the only time during the Transition Phase for the MCO to add Value- added Services. At no time during the Transition Phase will the MCO be allowed to delete, limit or restrict any of its approved Value-added Services.
|
|
7.2.9 Assurance of System and Operational Readiness |
In addition to successfully providing the Deliverables described in the preceding sections, the MCO must assure HHSC that all processes, MIS systems, and staffed functions are ready and able to successfully assume responsibilities for operations prior to the Operational Start Date. In particular, the MCO must assure that Key MCO Personnel, Member Services staff, Provider Services staff, and MIS staff are hired and trained, MIS systems and interfaces are in place and functioning properly, communications procedures are in place, Provider Manuals have been distributed, and that Provider training sessions have occurred according to an HHSC-approved schedule.
|
|
7.2.10 TDI and Centers for Medicare and Medicaid Services (CMS) Licensure, Certification or Approval |
The MCO must receive TDI licensure, certification or approval (as applicable) for all zip codes in the awarded Service Areas no later than 60 days after HHSC executes the Contract. In addition, HHSC encourages STAR+PLUS MCO to contract with the CMS to provide a Medicare Advantage Special Needs Plan for Dual Eligibles in the most populous counties in the STAR+PLUS Service Area(s) no later than January 1, 2013, or as a Dual Eligible Medicare-Medicaid Plan (MMP) in the designated demonstration counties no later than January 1, 2015.
The MCO will work with HHSC, Providers, and Members to promptly identify and resolve problems identified after the Operational Start Date and to communicate to HHSC, Providers, and Members, as applicable, the steps the MCO is taking to resolve the problems.
Subject: Attachment B-1 - Medicaid and CHIP Managed Care Services RFP, Section 8
DOCUMENT HISTORY LOG
|
| | | |
STATUS1 | DOCUMENT REVISION2 | EFFECTIVE DATE | DESCRIPTION3 |
Baseline | n/a | September 1, 2011 | Initial version of Attachment B-1, RFP Section 8, “Operations Phase Requirements.” |
Revision | 2.1 | March 1, 2012 | Section 8.1.1.1 is modified to change the timeframes for PIPs from SFY to calendar year and to revise the due dates. Section 8.1.3 is modified to clarify PCP requirement’s application (does not apply to CHIP Perinates (unborn children) and add a requirement regarding timely access to Network Providers, as required by 42 CFR §438.206(c)(1)(ii). Section 8.1.3.2 is modified to add pharmacy access requirements effective 9/1/12. These standards are derived from Medicare Part D access standards, and the standards currently being met in the fee-for-service program. Section 8.1.4 is modified to require MCOs to enter into network provider agreements with any willing State Hospital and to clarify requirements for contracting with specialty pharmacies. Section 8.1.5.5 is modified to require the MCOs to include a link to financial literacy information on the OCCC web page as required by HB 2615. Section 8.1.8 is modified to add prior authorizations by pharmacists. Section 8.1.17 is modified to remove the requirement to submit an accounting policy manual. Section 8.1.17.1 “Financial Disclosure Report” is renamed “MCO Disclosure Statement” and the submission date is updated. Section 8.1.18.1 is modified to require MCOs to submit pharmacy encounter data no later than 25 calendar days after the date of adjudication. Section 8.1.18.4 is modified to clarify claims transaction formats for pharmacy claims. Section 8.1.18.5 is modified to require MCOs to maintain a mechanism to receive claims in addition to the HHSC claims portal. Section 8.1.19 is modified to require MCOs to designate a primary and secondary contact for all OIG requests and to outline the process and timeframes for responding to the OIG, to change the 60 day timeline for submitting the annual plan to 90 days, and to require MCOs to ensure their subcontractors receiving or making annual Medicaid payments of at least $5 million comply with 1902(a)(68)(A) of the Social Security Act. Section 8.1.20.2 is modified to add DUR reporting requirements. Section 8.1.21 is revised to delete MCO developed PDLs and to clarify the reimbursement process. Section 8.1.21.1 is revised to clarify legal references and Clinical Edit requirements, and to add requirements regarding 340B drugs. Section 8.1.21.4 is modified to add requirements for the rebate dispute resolution process.
|
|
| | | |
| | | Section 8.1.21.5 is modified to clarify that HHSC will provide up to 1 year of medication history to the MCOs for new Members with previous Medicaid eligibility. Section 8.1.21.9 is modified to clarify requirements for contracting with specialty pharmacies.
|
| | | Section 8.1.21.10 is deleted in its entirety.
Section 8.1.23.1 is modified that copayment amounts are capped at the MCO’s cost and that CHIP copayments do not apply to preventive services or pregnancy-related services.
Section 8.1.24 is modified to clarify that MCOs must notify Medicaid and CHIP Providers of availability of vaccines through Texas Vaccines for Children Program and work with HHSC and Providers to improve the reporting of immunizations to the statewide ImmTrac Registry.
Section 8.2.2.3.4 is modified to require MCOs to use standard Texas Health Steps language in their Member Materials as provided in the UMCM.
Section 8.2.2.8 is amended to clarify the requirements regarding non-capitated dental services and to add “Texas Health Steps environmental lead investigation (ELI)”. Remainder of list is renumbered.
Section 8.2.4.2 is modified to add a reference to Gov’t Code §533.005(a)(19).
Section 8.2.8 is modified to add the phrase “unless an exception applies under federal law” to the first sentence.
Section 8.2.13 is modified to specify that MCOs may be required to provide other wrap-around services at a date to be determined by HHSC.
Section 8.3.2 is modified to require the MCO to consider the availability of the PACE program when considering whether to refer a member to a nursing facility or other long-term care facility.
Section 8.3.7.1 is modified to clarify the MA Dual SNP requirements.
Section 8.4.3 is modified to correct a cross-reference. |
Revision | 2.2 | June 1, 2012 | Section 8.1.21 is modified to add pharmaceutical delivery requirements. |
|
| | | |
Revision | 2.3 | September 1, 2012 | Section 8.1.1.1 is modified to conform to the timelines in the UMCM. Section 8.1.3 is modified to replace references to “1915(c) STAR+PLUS Waiver” with “HCBS STAR+PLUS Waiver”. Section 8.1.3.2 is modified to clarify language regarding additional benchmark performance standards. Section 8.1.4 is modified to correct reference to TMPPM. Section 8.1.4.6 is modified to require HHSC review of all provider materials relating to Medicaid managed care or CHIP. Section 8.1.4.8 is modified to clarify the applicable federal regulations. Section 8.1.5.1 is modified to prohibit the MCOs from including any language in their member materials which limits the members' ability to contest or appeal denial of a benefit. Section 8.1.5.2 is modified to clarify that PCP name is not required for Dual Eligible STAR+PLUS Members or CHIP Perinates. Section 8.1.5.7 is modified to remove the acronym “CPW”. Section 8.1.9 is modified to clarify the requirements regarding IFSPs. Section 8.1.12.2 is modified to remove the acronym “CPW”. Section 8.1.14 is renamed and modified to remove all references to Health Home Services. Section 8.1.14.1 is renamed and modified to remove all references to Health Home Services. Section 8.1.14.2 is renamed and modified to remove all references to Health Home Services. Section 8.1.19 is modified to update the time frames for responding to the OIG and to add language regarding Credible Allegation of Fraud notices. Section 8.1.20.2 items (j) and (l) are modified to correct UMCM references. Items (n) and (o) are modified to include pharmacy providers. Item (s) “Medicaid Managed Care Texas Health Steps Medical Checkups Quarterly Utilization Reports” is added. Section 8.1.20.2 is modified to add STAR+PLUS LTSS Utilization reporting requirements. Section 8.1.24 is modified to change the Texas Health Steps Periodicity Schedule to ACIP Immunization Schedule. Section 8.1.25 is modified to replace references to “1915(c) STAR+PLUS Waiver” with “HCBS STAR+PLUS Waiver”. Section 8.1.26 Health Home Services is added. Section 8.1.26.1 Health Home Services and Participating Providers is added. Section 8.1.26.2 MCO Health Home Services Evaluation is added Section 8.2.2.3.2 is modified to correct the acronym for Oral Evaluation and Fluoride Varnish.
|
|
| | | |
| | | Section 8.2.2.3.3 is modified to clarify statutory authority. Section 8.2.2.3.5 is modified to add training requirements for pharmacy and DME. Section 8.2.2.8 is modified to remove the acronym “CPW”. Section 8.2.2.11 is modified to replace the acronym CPW with “Case Management for Children and Pregnant Women” and the acronym THSteps with “Texas Health Steps”. Section 8.2.7.1 is modified to correct URL for UM guidelines. Section 8.2.8 is modified to clarify the pay and chase requirements for prenatal and preventative care, and recoveries in the context of state child support enforcement actions (SSA §1902(a)(25)(E) and (F); and to correct contract cross reference. Section 8.2.10 is modified to remove the acronym “CPW” and to replace it with Case Management for Children and Pregnant Women. Section 8.3.1.1 is modified to clarify eligibility for DAHS. Section 8.3.1.2 is modified to replace references to “1915(c) STAR+PLUS Waiver” with “HCBS STAR+PLUS Waiver” and to add DAHS to the list of Community Based LTSS under the HCBS STAR+PLUS Waiver. Section 8.3.2.6 is modified to replace references to “1915(c) Nursing Facility Waiver” with “HCBS STAR+PLUS Waiver”. Section 8.3.2.8 is modified to update the MAO reference. Section 8.3.3 is modified to replace references to “1915(c) Nursing Facility Waiver” with “HCBS STAR+PLUS Waiver”. Section 8.3.4 is modified to replace references to “1915(c) Nursing Facility Waiver” with “HCBS STAR+PLUS Waiver” and to increase the cost of care threshold from 200% to 202%. Section 8.3.4.1 is modified to replace references to “1915(c) STAR+PLUS Waiver” and “SPW” with “HCBS STAR+PLUS Waiver”. In addition, risk criteria language is removed. Section 8.3.4.2 is modified to change the section name from “For Medical Assistance Only (MAO) Non-Member Applicants” to “For 217-Like Group Applicants' and to replace references to “1915(c) STAR+PLUS Waiver” and “SPW” with “HCBS STAR+PLUS Waiver”. In addition, risk criteria language is removed. Section 8.3.4.3 is modified to replace references to “1915(c) Nursing Facility Waiver” with “HCBS STAR+PLUS Waiver”. Section 8.3.5 is modified to replace references to “1915(c) STAR+PLUS Waiver” with “HCBS STAR+PLUS Waiver”. Section 8.3.6.4 is modified to replace references to the 1915(b) and 1915(c) waivers with the Texas Healthcare Transformation and Quality Improvement Program 1115 Waiver. Section 8.4.3 is modified for consistency with the Medicaid pay and chase requirements.
|
|
| | | |
Revision | 2.4 | March 1, 2013 | All references to the previous Executive Commissioner Suehs are changed to his successor, Executive Commissioner Janek.
Section 8.1.2.1 is modified to add language regarding reducing or deleting Value-added Services.
Section 8.1.3.2 is modified to clarify network provider access and compliance rating.
Section 8.1.4.11 Provider Advisory Groups is added.
Section 8.1.5.10 Member Advisory Groups is added.
Section 8.1.18.5 is modified to add new language modeled off of insurance code requirements.
Section 8.2.3 is modified to add new language regarding terminating Significant Traditional Providers.
Section 8.2.13 is modified to address supplemental payments to MCOs for wrap-around services for outpatient drugs and biological products for STAR+PLUS Members.
Section 8.2.13.1 Medicaid Wrap-Around Services for Outpatient Drugs and Biological Products is added.
Section 8.3.1.1 is modified to delete Personal Attendant Services and delete language after (DAHS) is the service column.
Section 8.3.1.2 is modified to delete DAHS service description and Licensure and Certification Requirements and modify Personal Assistance Services.
8.3.6.6 Electronic Visit Verification is added.
|
Revision
| 2.5 | June 1, 2013 | Contract amendment did not revise Attachment B-1, |
Revision | 2.6 | September 1, 2013
| Section 8.1.1.1 is modified to remove references to overarching goals and to clarify that HHSC will provide the PIP topics. Section 8.1.2.1 is modified to clarify that MCOs may not charge copayments for Value-added Services, but may offer discounts for non-covered services as Value-added Services as required by SB 632.
Section 8.1.3.1 is modified to clarify timeframes for PCP referrals.
Section 8.1.3.2 is modified to add a requirement for 2 PCPs within 30 miles for Medicaid child Members to comply with the Frew Corrective Action order.
Section 8.1.4 is modified to add new pharmacy requirements as required by SB 1106 and HB 1358.
Section 8.1.4.2 is modified for clarification and to comply with requirements of SB 406, 83R.
Section 8.1.4.4 is modified to add timeframes for completing the credentialing process and to comply with requirements of SB 365, 83R.
|
|
| | | |
| | | Section 8.1.4.8 is modified to clarify the MCO's obligations for payment and Network Provider agreements and to comply with requirements of SB 7, 83R. Section 8.1.4.8.1 is modified to correct “Provider Preventable Conditions” to “Potentially Preventable Complications”. Section 8.1.4.8.2 is modified to clarify provider incentives.
Section 8.1.4.10 is modified for clarification and to comply with requirements of SB 1401, 83R.
Section 8.1.4.12 Provider Protection Plan is added as required by SB 1150, 83R.
Section 8.1.5.5 is modified to allow MCOs to offer provider search functionality on their websites instead of PDF versions of the Provider Directory. In addition, duplicative language is removed.
Section 8.1.5.6 is modified to require the MCO's Member Services representatives to be trained regarding the override process for Members in the HHSC-OIG Lock-in Program.
Section 8.1.5.6.1 is modified to require the MCO's nurseline staff to be trained regarding the override process for Members in the HHSC-OIG Lock-in Program.
Section 8.1.5.7 is modified to allow MCOs to use certified community health workers/promotoras to conduct outreach and member education activities.
Section 8.1.5.9 is modified to correct cross references.
Section 8.1.8 is modified to update the URL for UM guidelines.
Section 8.1.8.1 “Compliance with State and Federal Prior Authorization Requirements” is added as required by SB8, SB 644, and SB1216, 83R.
Section 8.1.9 is modified to update the T.A.C. references and to align the age reference with the definition.
Section 8.1.14 is modified to add a new Subsection 8.1.14.1 Special Populations. Subsequent subsections are renumbered.
Section 8.1.14.3 is modified to add requirements for special populations.
Section 8.1.15 is modified to clarify which DSM edition is referenced.
Section 8.1.15.7 is modified to delete the duplicative definition. The term “Court-Ordered Commitment” is defined in Attachment A.
Section 8.1.18.1 is modified to require MCO Provider Agreements to comply with Texas Gov't. Code regarding reimbursement of claims based on orders or referrals by supervising providers.
Section 8.1.18.5 is modified for clarification, for consistency with Section 1213.005 of the Insurance Code, and to comply with requirements of House Bill 15, 83R
|
|
| | | |
| | | Section 8.1.19 is modified to include the HHSC-OIG Lock-in Program.
Section 8.1.20 is modified for clarification that records must be provided “at no cost.”
Section 8.1.20.1 is modified to correct the name to which the acronym HEDIS refers.
Section 8.1.20.2 is modified to add Service Coordination reporting requirements.
Section 8.1.21 Pharmacy Services is modified to reorganize the section and to add requirements as required by SB 644, HB 1358, 83R.
Section 8.1.21.1 Formulary and Preferred Drug List (PDL) is added.
Section 8.1.21.2 Prior Authorization for Prescription Drugs is modified to add “and 72-hour Emergency Supplies” to the title and to add requirements as required by SB 644, HB 1358, 83R
Section 8.1.21.3 Coverage Exclusions is modified for clarity.
Section 8.1.21.5 Pharmacy Rebate Program is modified to require MCOs to include NDCs on all encounters.
Section 8.1.21.6 Drug Utilization Review (DUR) Program is modified to add requirements as required by SB 644, HB 1358, 83R
Section 8.1.21.7 Pharmacy Benefit manager (PBM) is modified to add requirements as required by SB 644, HB 1358, 83R
Section 8.1.21.8 Financial Disclosures for Pharmacy Services is modified for clarity.
Section 8.1.21.9 Limitations Regarding Registered Sex Offenders is modified for clarity
Section 8.1.21.10 Specialty Drugs is modified to add requirements as required by SB 644, HB 1358, 83R
Section 8.1.21.11 Maximum Allowable Cost (MAC) Requirements is added.
Section 8.1.21.12 Mail-order and Delivery is added.
Section 8.1.21.13 Health Resources and Services Administration 340B Discount Drug Program is added.
Section 8.1.21.14 Pharmacy Claims and File Processing is added.
Section 8.1.21.15 Pharmacy Audits is added.
Section 8.1.21.16 E-prescribing is added.
Section 8.1.22 is modified to add more detail regarding FQHC/RHC payments.
Section 8.1.27 Cancellation of Product Orders is added.
Section 8.2.2.4 is modified to include education and care coordination for Members who are at high risk for pre-term labor.
|
|
| | | |
| | | Section 8.2.2.8 is modified to add ECI Specialized Skills Training, to clarify the requirements for DADS hospice services, and to add court-ordered commitments to inpatient mental health facilities as a condition of probation.
Section 8.2.4.2 is modified for clarification and to comply with requirements of SB 7, 83R.
Section 8.2.13 is modified to clarify the language.
Section 8.2.13.1 is modified to clarify the language.
Section 8.3.2 is modified to add new subsections 8.3.2.1 “Service Coordination Plan Requirements,” and 8.3.2.2 “Service Coordination Structure.” Subsequent subsections are renumbered.
Section 8.3.2.3 is modified to include minimum requirements for Service Coordinators.
Section 8.3.4.3 is modified to require the MCO to inform the Member about CDS during the annual reassessment.
Section 8.3.4.4 STAR+PLUS Utilization Reviews is added as required by SB 348, 83R.
Section 8.3.7.2 is modified to remove the reference to Attachment B-6.
Section 8.3.8 Minimum Wage Requirements for STAR+PLUS Attendants in Community Settings Reviews is added as required by Article II, Rider 61 of the General Appropriations Act (83R).
|
Revision
| 2.7 | September 1, 2013 | Section 8.2.16 “Supplemental Payments for Qualified Providers” is added. Additional detail regarding the process, including payment and reporting requirements will be added to the UMCM.
|
Revision
| 2.8 | January 1, 2014 | Section 8.1.4.4 is modified to clarify the timeframes for completing the credentialing process.
Section 8.1.12.2 is modified to add Former Foster Care Child (FFCC) Members.
Section 8.1.13 is modified to add Former Foster Care Child (FFCC) Members.
Section 8.1.21.6 is modified to add requirements for assessing prescribing patterns for psychotropic medications.
Section 8.1.21.14 is modified to clarify timeframes.
Section 8.3.6.6 Cost Reporting for LTSS Providers is added. |
|
| | | |
Revision
| 2.9 | February 1, 2014 | Section 8.1.1.1 is modified to clarify that absent HHSC’s direction the MCO may choose to collaborate with other MCOs in the Service Area on one PIP per year.
Section 8.1.1.1.1 MCO Report Cards is added.
Section 8.1.2 is modified to remove the reference to Texas Medicaid Bulletins.
Section 8.1.3 is modified to clarify Member payment responsibilities for services in a 24-hour setting as an alternative to Nursing Facility or hospitalization and for services in a Nursing Facility.
Section 8.1.3.2 is modified to remove the definition of Qualified Mental Health Provider from Outpatient Behavioral Health Service Provider Access. In addition, Nursing Facility Access and Mental Health Rehabilitative Service Provider Access are added. |
|
| | | |
| | | Section 8.1.4 is modified to clarify licensure or certification requirements for all providers. In addition, Nursing Facility Services, Hospice Services, and Mental Health Rehabilitative Services are added.
Section 8.1.4.2 is modified to include physicians serving Members residing in Nursing Facilities.
Section 8.1.4.4 is modified to require MCOs to use state-identified credentialing criteria for Nursing Facilities. In addition, a sub-section heading is added for 8.1.4.4.1 Expedited Credentialing Process.
Section 8.1.4.6 is modified to require STAR+PLUS MCOs to assign a provider relations specialist proficient in Nursing Facility billing to each Nursing Facility. In addition, the role of Service Coordinators and early notification of and participation in discharge planning are added to the required Provider training. In addition, requirements for Mental health Rehabilitative Services are added.
Section 8.1.4.8 is modified to update the UMCM chapter reference.
Section 8.1.4.8.1 is modified to include CHIP.
Section 8.1.4.8.3 Nursing Facility Incentives is added.
Section 8.1.4.10 is modified to add TAC reference for pharmacy.
Section 8.1.4.12 is modified to update the UMCM chapter reference.
Section 8.1.5.2 is modified to clarify that the PCP’s name and telephone number are not required for Nursing Facility residents.
Section 8.1.5.7 is modified to add Service Coordination for Cognitive Rehabilitation Therapy, Nursing Facility residents; Nursing Facility Services; discharge planning, transitional care, and other education programs for Nursing Facility residents; and supported employment and employment services.
Section 8.1.5.11 Member Eligibility is added.
Section 8.1.8 is modified to add that compensation to individuals or entities conducting UM activities cannot be structured to provide incentives for the individual or entity to deny, limit, or discontinue medically necessary services as required by 42 C.F.R. 438.210(e). Section 8.1.12.1 is modified to delete unnecessary information and clarify use of the term CSHCN. Section 8.1.12.2 is modified to clarify use of the term CSHCN. Section 8.1.15.8 is modified to remove the requirement to comply with additional BH requirements as described in Section 8.2.8. Section 8.1.18.5 is modified to add timeframes for Nursing Facility claims and to clarify the MCO must provide a web portal at no cost to the Provider and its functionality. Section 8.1.19 is modified to require the MCOs to meet all requirements in Texas Government Code § 531.105. Section 8.1.20.2 is modified to add Nursing Facility Reports. Section 8.1.23 is modified to allow STAR+PLUS MCOs to assist with the collection of applied income from Nursing Facility Members. Section 8.1.28 Preadmission Screening and Resident Review (PASRR) Referring Entity Requirements is added. Section 8.2.1 is modified to clarify timeframes for prior authorizations for transitioning Members. Section 8.2.2.8 is modified to add PASRR Evaluations; and to clarify DSHS Targeted Case Management, Personal Care Services and Nursing Facility Services. Section 8.2.3 is modified to add Nursing Facilities as STPs for STAR+PLUS. Section 8.2.7.1 Local Mental Health Authority (LMHA) will be deleted in its entirety effective September 1, 2014. Section 8.2.7.3 Mental Health Rehabilitative |
|
| | | |
| | | Services and Targeted Case Management Services is added. Section 8.3.1 is clarified that LTSS providers must be licensed or certified. Section 8.3.1.1 is modified to clarify that MCOs must ensure access to PAS and DAHS for qualified STAR+PLUS Members. Section 8.3.1.2 is modified to add licensure, certification and other minimum qualification requirements for Employment Assistance,
Supported Employment, Support Consultation, and Cognitive Rehabilitation Therapy. In addition, Consumer Directed Services (CDS) is renamed Financial Management Services and the requirements for Adult Foster Care are clarified. Section 8.3.2.1 is modified to add Level 1 requirements for Members in Nursing Facilities. Section 8.3.2.2 is modified to add Behavioral Health outpatient services and Mental Health Rehabilitative Services, and Employment Assistance/Supported Employment. Section 8.3.2.3 is modified to clarify Member needs, and to add Employment Assistance/Supported Employment and Targeted Case management for Members receiving Mental Health Rehabilitative Services. Section 8.3.2.5 is modified to require the MCO to provide discharge planning, transition care, and other education programs to Network Providers regarding all available long term care settings and options. In addition Nursing Facilities are added. Section 8.3.2.6 is modified to include Nursing Facility Services and to change Service Plan to transition plan. Section 8.3.2.8 Nursing Facilities will be deleted in its entirety effective September 1, 2014. Section 8.3.2.9 MCO Four-Month Liability for Nursing Facility Care will be deleted in its entirety effective September 1, 2014. Section 8.3.3 is modified to add assessment requirements for Members in Nursing Facilities. Section 8.3.6.3 is modified to include Nursing Facility Providers. Section 8.3.6.7 Electronic Visit Verification is added. The UMCM chapter is under development. Section 8.3.9 Nursing Facility Services Available to All Members is added. Section 8.3.9.1 Preadmission Screening and Resident Review (PASRR) is added. Section 8.3.9.2 Participation in Texas Promoting Independence Initiative’ is added. Section 8.3.9.3 Nursing Facilities Training is added. Section 8.3.9.4 Nursing Facility Claims Adjudication, Payment, and File Processing is added. Section 8.3.10 Acute Care Services for Recipients of ICF-IID Program and IDD Waiver services is added. Section 8.3.11 Cognitive Rehabilitation Therapy is added.
|
Revision | 2.10 | April 1, 2014 | Section 8.1.4 is amended to include any willing provider language for Nursing Facilities.
Section 8.2.17 Electronic Visit Verification is added to include both STAR and STAR+PLUS.
Section 8.3.6.7 is deleted in its entirety and the language is moved to Section 8.2.17. |
|
| | | |
Revision | 2.11 | September 1, 2014 | Section 8.1.1.1 is modified to change the due date for PIP projects and to require the MCOs to complete a mid-year review process.
Section 8.1.3 is amended to clarify that a STAR+PLUS Member receiving Adult Foster Care in his or her home is not required to pay room and board to the provider of that care and to remove duplicative language.
Section 8.1.3.2 is modified to update the mileage requirements for Outpatient Behavioral Health Service Provider Access.
Section 8.1.4 is modified to add a reference to utilization standards for CHIP (the Rule will be effective in December 2014), to clarify licensure requirements for all Providers, and include updated Nursing Facility dates.
Section 8.1.4.2 is modified to change the date by which the MCO’s network may include physicians serving Nursing Facilities.
Section 8.1.4.4 is modified to specifically refer to anti-discrimination requirements.
Section 8.1.4.6 is modified to add training materials pertaining to ADHD.
Section 8.1.4.8 is modified to include language requiring compliance with Tex. Ins. Code § 1458.051 and §§ 1458.101-102.
Section 8.1.4.8.1 is modified to add the UMCM chapter reference and to remove the HHSC approved methodology.
Section 8.1.4.8.2 is modified to change the name from “Provider Incentives” to “MCO Value Based Contracting.” In addition, the language is clarified.
Section 8.1.4.12 is modified to include notice requirements for changes to the prior authorization process.
Section 8.1.5.7 is revised to reflect the accurate date of Nursing Facility carve-in.
Section 8.1.5.8 is modified to remove reference to Section 7.
Section 8.1.12.2 is modified to add a reference to women’s health and family planning programs.
Section 8.1.14.1 is modified to update the requirements.
Section 8.1.18 is revised to define Major Systems Changes and to outline notice requirements.
Section 8.1.18.4 is revised to clarify notice requirements.
Attachment B-1, Section 8.1.18.5 is modified to clarify notice requirements and reflect updated Nursing Facility date.
Section 8.1.19 is modified to include language related to requirements regarding a provider in the MCO’s network who is under investigation by HHSC OIG.
Section 8.1.20.2 is modified to remove the Medicaid Disproportionate Share Hospital (DSH) Report. In addition the Provider Referral and Perinatal Risk Reports are added |
|
| | | |
| | | Section 8.1.21.2 is modified to require the MCOs to have an automated PA process.
Section 8.1.21.7 is modified to add language prohibiting spread pricing. Section 8.1.21.11 is modified to clarify the process for making the MAC list accessible to Providers.
Section 8.1.23.1 is modified to clarify requirements with respect to CHIP copayments.
Section 8.2.1 is revised to clarify prior authorization requirements with respect to new Members.
Section 8.2.2.2 is revised to update family planning requirements.
Section 8.2.2.4 is updated to include requirements regarding outreach, education, and care coordination for Members at risk of a preterm birth. Section 8.2.2.8 is modified to remove DSHS Targeted Case management and DSHS mental health rehabilitation and to update Nursing Facility services.
Section 8.2.3 is revised to reflect updated dates for Nursing Facilities.
Section 8.2.4.2 is revised to include a requirement for the physician resolving the claims dispute.
Section 8.2.7.1 Local Mental Health Authority (LMHA) is deleted in its entirety.
Section 8.2.10 is revised to include a reference to women’s health and family planning programs.
Section 8.2.13 is modified to reference newly added 8.2.13.2.
Section 8.2.13.2 is added to set out coinsurance obligations for Members in Nursing Facilities.
Section 8.2.17 is revised to reflect the modified date for EVV.
Section 8.2.18 “Telemedicine, Telehealth, and Telemonitoring Access” is added.
Section 8.3.1.2 is modified to remove the effective date and correct the experience requirements for Employment Assistance and Supported Employment. In addition, the effective date is removed for Cognitive Rehabilitation Therapy.
Section 8.3.2.1 is modified to reflect Nursing Facility date.
Section 8.3.2.2 is revised to reflect Nursing Facility date.
Section 8.3.2.3 is revised to reflect Nursing Facility date.
Section 8.3.2.4 is revised to use updated terminology.
Section 8.3.2.6 is revised to reflect Nursing Facility date.
Section 8.3.2.8 Nursing Facilities is modified to change the deletion date.
Section 8.3.2.9 MCO four-Month Liability for Nursing Facility Care is revised to reflect updated Nursing Facility dates.
Section 8.3.3 is modified to change the DADS Form 2060 to Form H2060 and any applicable addendums; and a form 3671 to Form H1700. In addition, section is modified to require assessments for Members receiving DAHS and HCBS waiver services.
|
|
| | | |
| | | Section 8.3.6.2 is modified to remove the reference to UMCM Chapter 2.1.2 and replace it with the STAR+PLUS Handbook.
Section 8.3.6.3 is revised to reflect updated Nursing Facility date.
Section 8.3.7.1 is modified to add a reference to a Dual Eligible Medicare-Medicaid (MMP) Plan.
Section 8.3.9 is revised to reflect updated Nursing Facility date.
Section 8.3.9.4 is revised to include requirements for retroactive rate adjustments.
Section 8.3.9.5 "Nursing Facility Direct Care Rate Enhancement" is added. |
Revision | 2.12 | October 1, 2014 | Section 8.1.21.17 “Second Generation Direct Acting Antivirals for Hepatitis C” is added. |
1 Status should be represented as “Baseline” for initial issuances, “Revision” for changes to the Baseline version, and “Cancellation” for withdrawn versions 2 Revisions should be numbered in accordance according to the version of the issuance and sequential numbering of the revision—e.g., “1.2” refers to the first version of the document and the second revision. 3 Brief description of the changes to the document made in the revision. |
Table of Contents
8. OPERATIONS PHASE REQUIREMENTS ......................................................................................................................... 8-22
8.1 General Scope of Work ....................................................................................................................................................... 8-22
8.1.1 Administration and Contract Management ...................................................................................................................... 8-22
8.1.2 Covered Services ............................................................................................................................................................. 8-25
8.1.3 Access to Care .................................................................................................................................................................. 8-27
8.1.4 Provider Network ............................................................................................................................................................. 8-33
8.1.5 Member Services .............................................................................................................................................................. 8-48
8.1.6 Marketing and Prohibited Practices ................................................................................................................................. 8-56
8.1.7 Quality Assessment and Performance Improvement ........................................................................................................ 8-56
8.1.8 Utilization Management ................................................................................................................................................... 8-60
8.1.9 Early Childhood Intervention (ECI) ................................................................................................................................. 8-62
8.1.10 Special Supplemental Nutrition Program for Women, Infants, and Children
(WIC) - Specific Requirements ................................................................................................................................................. 8-63
8.1.11 Coordination with Texas Department of Family and Protective Services ...................................................................... 8-63
8.1.12 Services for People with Special Health Care Needs ..................................................................................................... 8-64
8.1.13 Service Management for Certain Populations ................................................................................................................ 8-67
8.1.14 Disease Management (DM) ............................................................................................................................................ 8-67
8.1.15 Behavioral Health (BH) Network and Services ............................................................................................................. 8-69
8.1.16 Financial Requirements for Covered Services ............................................................................................................... 8-73
8.1.17 Accounting and Financial Reporting Requirements ....................................................................................................... 8-73
8.1.18 Management Information System Requirements ........................................................................................................... 8-79
8.1.19 Fraud, Waste and Abuse ................................................................................................................................................. 8-86
8.1.20 General Reporting Requirements ................................................................................................................................... 8-89
8.1.21 Pharmacy Services ......................................................................................................................................................... 8-94
8.1.22 Federally Qualified Health Centers (FQHCs) and Rural Health Clinics
(RHCs) ..................................................................................................................................................................................... 8-101
8.1.23 Payment by Members. .................................................................................................................................................. 8-102
8.1.24 Immunizations .............................................................................................................................................................. 8-103
8.1.25 Dental Coverage ........................................................................................................................................................... 8-104
8.1.26 Health Home Services .................................................................................................................................................. 8-104
8.1.27 Cancellation of Product Orders .................................................................................................................................... 8-105
8.1.28 Preadmission Screening and Resident Review (PASRR) Referring Entity Requirements ...................................................................................................................................................................8-106
8.2 Additional Medicaid MCO Scope of Work ....................................................................................................................... 8-106
8.2.1 Continuity of Care and Out-of-Network Providers ........................................................................................................ 8-106
8.2.2 Provisions Related to Covered Services for Medicaid Members ................................................................................... 8-108
8.2.3 Medicaid Significant Traditional Providers .................................................................................................................... 8-118
8.2.4 Provider Complaints and Appeals .................................................................................................................................. 8-119
8.2.5 Member Rights and Responsibilities .............................................................................................................................. 8-120
8.2.6 Medicaid Member Complaint and Appeal System ......................................................................................................... 8-121
8.2.7 Additional Medicaid Behavioral Health Provisions ....................................................................................................... 8-129
8.2.8 Third Party Liability and Recovery and Coordination of Benefits ................................................................................ 8-131
8.2.9 Coordination with Public Health Entities ....................................................................................................................... 8-132
8.2.10 Coordination with Other State Health and Human Services (HHS) Programs..............................................................8-133
8.2.11 Advance Directives ....................................................................................................................................................... 8-134
8.2.12 SSI Members ................................................................................................................................................................ 8-135
8.2.13 Medicaid Wrap-Around Services ................................................................................................................................. 8-136
8.2.14 Medical Transportation ................................................................................................................................................. 8-136
8.2.15 This Section Intentionally Left Blank............................................................................................................................8-137
8.2.16 Supplemental Payments for Qualified Providers ......................................................................................................... 8-137
8.2.17 Electronic Visit Verification.......................................................................................................................................... 8-138
8.2.18 Telemedicine, Telehealth, and Telemonitoring Access ..................................................................................................8-138
8.3 Additional STAR+PLUS Scope of Work .......................................................................................................................... 8-138
8.3.1 Covered Community-Based Long-Term Services and Supports ................................................................................... 8-138
8-143
8.3.2 Service Coordination ...................................................................................................................................................... 8-143
8.3.3 STAR+PLUS Assessment Instruments .......................................................................................................................... 8-151
8.3.4 HCBS STAR+PLUS Waiver Service Eligibility ............................................................................................................ 8-152
8.3.5 Consumer Directed Services Options ..............................................................................................................................8-155
8.3.6 Community Based Long-term Services and Supports Providers ................................................................................... 8-157
8.3.7 Additional Requirements Regarding Dual Eligibles ...................................................................................................... 8-159
8.3.8 Minimum Wage Requirements for STAR+PLUS Attendants in Community Settings................................................... 8-159
8.3.9 Nursing Facility Services Available to All Members ..................................................................................................... 8-160
8.3.10 Acute Care Services for Recipients of ICF-IID Program and IDD Waiver Services ................................................... 8-163
8.3.11 Cognitive Rehabilitation Therapy ................................................................................................................................ 8-163
8.4 Additional CHIP Scope of Work ....................................................................................................................................... 8-163
8.4.1 CHIP Provider Complaint and Appeals .......................................................................................................................... 8-163
8.4.2 CHIP Member Complaint and Appeal Process .............................................................................................................. 8-163
8.4.3 Third Party Liability and Recovery, and Coordination of Benefits ................................................................................ 8-164
8.4.4 Perinatal Services for Traditional CHIP Members ......................................................................................................... 8-165
|
|
8. OPERATIONS PHASE REQUIREMENTS |
This Section describes Scope of Work requirements for the Operations Phase of the Contract.
Section 8.1 includes the general Scope of Work that applies to all MCO Programs (STAR, STAR+PLUS, and CHIP).
Section 8.2 includes the additional Medicaid Scope of Work that applies only to the STAR and STAR+PLUS MCOs.
Section 8.3 includes the additional Scope of Work that applies only to STAR+PLUS MCOs.
Section 8.4 includes the additional CHIP Scope of Work that applies only to CHIP MCOs.
The CHIP Perinatal Program is a CHIP subprogram. CHIP Program requirements apply to the CHIP Perinatal Program, unless the Contract otherwise indicates.
Additional information regarding the STAR, STAR+PLUS, and CHIP Program requirements, such as reporting timeframes and formats is included in Attachment A, "Uniform Managed Care Contract Terms and Conditions," and the Uniform Managed Care Manual. HHSC reserves the right to modify these documents as it deems necessary using the procedures set forth in the Attachment A, “Uniform Managed Care Contract Terms and Conditions.”
|
|
8.1 General Scope of Work |
In each MCO Program and Service Area, HHSC will select MCOs to provide Health Care Services and prescription drug benefits to Members. The MCO must have approval from the Texas Department of Insurance (TDI) to operate as an HMO, ANHC, and/or an EPO in all zip codes in the respective Service Area(s).
Coverage for benefits will be available to enrolled Members effective on the Operational Start Date. The Operational Start Date is March 1, 2012, for all MCO Programs and Service Areas.
|
|
8.1.1 Administration and Contract Management |
The MCO must comply, to the satisfaction of HHSC, with: (1) all provisions set forth in this Contract, and (2) all applicable provisions of state and federal laws, rules, regulations, and waiver agreements with the Centers for Medicare and Medicaid Services (CMS).
|
|
8.1.1.1 Performance Evaluation |
On an annual basis, HHSC will provide the MCO with two Performance Improvement Project (PIP) topics per Program. The MCO must develop one PIP per topic. At HHSC’s direction, the MCO must conduct one PIP per program in collaboration with other MCOs in the Service Area. Absent HHSC’s direction, the MCO may choose to collaborate with other MCOs in the Service Area on one PIP per program. The MCO must send PIP projects to HHSC no later than September 8 each year. Each MCO must also complete a mid-year review process as outlined in the Uniform Managed Care Manual Chapter 10.2.9. PIPs will follow CMS protocol, as described below. The purpose of health care quality PIPs is to assess and improve processes, and thereby outcomes, of care. In order for these projects to achieve real improvements in care and for interested parties to have confidence in the reported improvements, PIPs must be designed, conducted, and reported in a methodologically sound manner.
MCOs must use the following ten (10) step CMS protocol when conducting PIPs:
1. select the study topic(s);
2. define the study question(s);
3. select the study indicator(s);
4. use a representative and generalizable study population;
5. use sound sampling techniques (if sampling is used);
6. collect reliable data;
7. implement intervention and improvement strategies;
8. analyze data and interpret study results;
9. plan for real improvement; and
10. achieve sustained improvement.
(See Uniform Managed Care Manual Chapter 10.2.4, Performance Improvement Project Submission Instructions and 10.2.5, Performance Improvement Project Template).
The MCO must participate in semi-annual Contract Status Meetings (CSMs) with HHSC for the primary purpose of reviewing progress toward the achievement of annual PIPs and Contract requirements. HHSC may request additional CSMs as it deems necessary to address areas of noncompliance. HHSC will provide the MCO with reasonable advance notice of additional CSMs, generally at least five (5) Business Days.
The MCO must provide to HHSC, no later than 14 Business Days prior to each semi-annual CSM, an electronic report detailing the MCO's progress toward and any barriers in meeting the annual PIPs.
HHSC will track MCO performance on PIPs. It will also track other key facets of MCO performance through the use of a Performance Indicator Dashboard for Quality Measures (see Uniform Managed Care Manual Chapter 10.1.7). HHSC will compile the Performance Indicator Dashboard based on MCO submissions, data from the External Quality Review Organization (EQRO), and other data available to HHSC. HHSC will share the Performance Indicator Dashboard with the MCO on an annual basis.
8.1.1.1.1 MCO Report Cards
Texas Government Code § 536.051 requires HHSC to provide information to Medicaid and CHIP Members regarding MCO performance on outcome and process measures during the enrollment process. To comply with this requirement, HHSC will develop annual MCO report cards. HHSC will develop a separate report card for each Program Service Area to allow enrollees to easily compare the MCOs on quality measures. HHSC may publish the report cards on its website, and include them in the enrollment packets. HHSC will provide a copy of the report card to the MCO before publication and the MCO will have the opportunity to review and provide comments. However, HHSC reserves the right to publish the results while awaiting MCO feedback.
HHSC may charge the MCO any costs related to recalculating the report card measures if the EQRO determines the original data was valid.
8.1.1.2 Additional Readiness Reviews and Monitoring Efforts
During the Operations Phase, HHSC may conduct desk and/or onsite reviews as part of its normal Contract monitoring efforts. Additionally, an MCO that chooses to make a change to any operational system or undergo any major transition may be subject to an additional Readiness Review(s). HHSC will determine whether the proposed changes will require a desk review and/or an onsite review. The MCO is responsible for all reasonable travel costs incurred by HHSC or its authorized agent for onsite reviews conducted as part of Readiness Review or HHSC’s normal Contract monitoring efforts. For purposes of this section, “reasonable travel costs” include airfare, lodging, meals, car rental and fuel, taxi, mileage, parking and other incidental travel expenses incurred by HHSC or its authorized agent in connection with the onsite reviews. This provision does not limit HHSC’s ability to collect other costs as damages in accordance with Attachment A, Section 12.02(e), “Damages.”
Refer to Section 7, “Transition Phase Requirements,” and Section 8.1.18, “Management Information System Requirements,” for additional information regarding MCO Readiness Reviews. Refer to Attachment A, "Uniform Managed Care Contract Terms and Conditions," Section 4.08(c) for information regarding Readiness Reviews of the MCO’s Material Subcontractors.
The MCO is responsible for authorizing, arranging, coordinating, and providing Covered Services in accordance with the requirements of the Contract. The MCO must provide Medically Necessary Covered Services to all Members beginning on the Member’s date of enrollment regardless of pre-existing conditions, prior diagnosis and/or receipt of any prior Health Care Services. STAR+PLUS MCOs must also provide Functionally Necessary Community Long-term Services and Supports to all Members beginning on the Member’s date of enrollment regardless of pre-existing conditions, prior diagnosis and/or receipt of any prior Health Care Services. The MCO must not impose any pre-existing condition limitations or exclusions or require Evidence of Insurability to provide coverage to any Member.
The MCO must provide full coverage for Medically Necessary Covered Services to all Members and, for STAR+PLUS Members, Functionally Necessary Community Long-term Services and Supports, without regard to the Member’s:
|
| |
| 1. previous coverage, if any, or the reason for termination of such coverage; |
|
| |
| 3. confinement in a health care facility; or |
The MCO must not practice discriminatory selection, or encourage segregation among the total group of eligible Members by excluding, seeking to exclude, or otherwise discriminating against any group or class of individuals.
Covered Services for all Medicaid MCO Members are listed in Attachments B-2, STAR Covered Services, and B-2.2, STAR+PLUS Covered Services. Medicaid MCOs are responsible for providing all services and benefits available to clients of the Medicaid Fee-for-Service Program to the MCO’s Medicaid Members, with the exception of Non-Capitated Services (Section 8.2.2.8). Medicaid MCOs must provide the services and benefits described in the most recent Texas Medicaid Provider
Procedures Manual and any updates to the Manual. A description of CHIP Covered Services and exclusions is provided in Attachment B-2.1, CHIP Covered Services. Covered Services are subject to change due to changes in federal and state law; changes in Medicaid, CHIP or CHIP Perinatal Program policy; and changes in medical practice, clinical protocols, or technology.
8.1.2.1 Value-added Services
MCOs may propose additional services for coverage. These are referred to as "Value-added Services." Value-added Services may be actual Health Care Services, benefits, or positive incentives that HHSC determines will promote healthy lifestyles and improved health outcomes among Members. Value-added Services that promote healthy lifestyles should target specific weight loss, smoking cessation, or other programs approved by HHSC. Temporary phones, cell phones, additional transportation benefits, and extra home health services may be Value-added Services, if approved by HHSC. Best practice approaches to delivering Covered Services are not considered Value-added Services.
The MCO generally must offer Value-added Services to all MCO Program Members in a Service Area. For Medicaid Acute Care services, the MCO may distinguish between the Dual Eligible and non-Dual Eligible populations. The MCO is not required to offer the same Value-added Services to CHIP Perinate Members as traditional CHIP Members and CHIP Perinate Newborn Members. Value-added Services do not need to be consistent across more than one (1) MCO Program or across more than one (1) Service Area. Value-added Services that are approved by HHSC during the contracting process will be included in the Contract's scope of services.
Any Value-added Services that a MCO elects to provide must be provided at no additional cost to HHSC. The costs of Value-added Services are not reportable as allowable medical or administrative expenses, and therefore are not factored into the rate setting process. In addition, the MCO must not pass on the cost of the Value-added Services to Members or Providers.
The MCO may offer discounts on non-covered benefits to Members as Value-added Services, provided that the MCO complies with Texas Insurance Code § 1451.155 and § 1451.2065. The MCO must ensure that Providers do not charge Members for any other cost-sharing for a Value-added Service (including copayments or deductibles).
The MCO must specify the conditions and parameters regarding the delivery of the Value-added Services in the MCO's Marketing Materials and Member Handbook, and must clearly describe any limitations or conditions specific to the Value-added Services.
During the Operations Phase, Value-added Services can be added or removed only by written amendment of the Contract. MCOs will be given the opportunity to add or enhance Value-added Services twice per State Fiscal Year, with changes to be effective September 1 and March 1. MCOs will also be given the opportunity to delete or reduce Value-added Services once per State Fiscal Year, with changes to be effective September 1. HHSC may allow additional modifications to Value-added Services if Covered Services are amended by HHSC during a State Fiscal Year. This approach allows HHSC to coordinate biannual revisions to HHSC's MCO Comparison Charts for Members. A MCO's request to add, enhance, delete, or reduce a Value-added Service must be submitted to HHSC by April 1 of each year to be effective September 1 for the following contract period. The MCOs cannot reduce or delete any Value-added Services until September 1 of the next SFY. A second request to add or enhance Value-added Services must be submitted to HHSC by October 1 each year to be effective March 1. (See Uniform Managed Care Manual Chapter 4.5 "Physical and Behavioral Health Value-Added Services Template.")
A MCO's request to add a Value-added Service must:
a. define and describe the proposed Value-added Service;
b. specify the Service Areas and MCO Programs for the proposed Value-added Service;
c. identify the category or group of Members eligible to receive the Value-added Service if it is a type of service that is not appropriate for all mandatory Members;
d. note any limits or restrictions that apply to the Value-added Service;
e. identify the Providers responsible for providing the Value-added Service;
f. Describe how the MCO will identify the Value-added Service in administrative data (Encounter Data);
g. propose how and when the MCO will notify Providers and Members about the availability of such Value-added Service;
h. describe how a Member may obtain or access the Value-added Service; and
i. include a statement that the MCO will provide such Value-added Service for at least 12 months from the September 1 effective date.
A MCO cannot include a Value-added Service in any material distributed to Members or prospective Members until the Parties have amended the Contract to include that Value-added Service. If a Value-added Service is deleted by amendment, the MCO must notify each Member that the service is no longer available through the MCO. The MCO must also revise all materials distributed to prospective Members to reflect the change in Value-added Services.
8.1.2.2 Case-by-Case Added Services
Except as provided below, the MCO may offer additional benefits that are outside the scope of services to individual Members on a case-by-case basis. Case-by-case services may be based on Medical Necessity, cost-effectiveness, the wishes of the Member/Member’s family, the potential for improved health status of the Member, and for STAR+PLUS Members based on Functional Necessity.
Section 8.1.2.2, “Case-by-Case Added Services,” does not apply to the CHIP Perinate Members (unborn children).
All Covered Services must be available to Members on a timely basis in accordance the Contract's requirements and medically appropriate guidelines, and consistent with generally accepted practice parameters. The MCO must comply with the access requirements as established by the Texas Department of Insurance (TDI) for all MCOs doing business in Texas, except as otherwise required by this Contract. Medicaid MCOs must be responsive to the possibility of increased Members due to the phase-out of the PCCM model in Service Areas where HHSC has determined that adequate MCO coverage exists.
The MCO must provide coverage for Emergency Services to Members 24 hours a day and seven (7) days a week, without regard to prior authorization or the Emergency Service provider's contractual relationship with the MCO. The MCO's policy and procedures, Covered Services, claims adjudication methodology, and reimbursement performance for Emergency Services must comply with all applicable state and federal laws and regulations, whether the provider is Network or Out-of-Network. A MCO is not responsible for payment for unauthorized non-emergency services provided to a Member by Out-of-Network providers.
The MCO must also have a toll-free emergency and crisis Behavioral Health Services Hotline available 24 hours a day, seven (7) days a week. The Behavioral Health Services Hotline must meet the requirements described in Section 8.1.15.3. For Medicaid Members, a MCO must provide coverage for Emergency Services in compliance with 42 C.F.R. §438.114, and as described in more detail in Section 8.2.2.1. The MCO may arrange Emergency Services and crisis Behavioral Health Services through mobile crisis teams.
For CHIP Members, Emergency Covered Services, including emergency Behavioral Health Services, must be provided in accordance with the requirements of the Texas Insurance Code and TDI regulations.
MCO must require, and make best efforts to ensure, that PCPs are accessible to STAR, STAR+PLUS, CHIP, and CHIP Perinate Newborn Members 24 hours a day, seven (7) days a week and that its Network Primary Care Providers (PCPs) have after-hours telephone availability that is consistent with Section 8.1.4. The MCO must ensure that Network Providers offer office hours to Members that are at least equal to those offered to the MCO's commercial lines of business or Medicaid fee-for-service participants, if the provider accepts only Medicaid patients.
CHIP MCOs are not required to establish PCP Networks for CHIP Perinates (Unborn Child).
The MCO must provide that if Medically Necessary Covered Services are not available through Network Providers, the MCO must, upon the request of a Network Provider, allow a referral to a non-network physician or provider within the time appropriate to the circumstances relating to the delivery of the services and the condition of the patient, but in no event to exceed five (5) Business Days after receipt of reasonably requested documentation. The MCO must fully reimburse the non-network provider in accordance with the Out-of-Network methodology for Medicaid as defined by HHSC in 1 T.A.C. §353.4, and for CHIP, at the usual and customary rate defined by TDI in 28 T.A.C. Section 11.506.
The MCO may not require the Member to pay for any Medically Necessary or Functionally Necessary Covered Services other than:
(1) HHSC-specified copayments for CHIP Members, where applicable;
(2) HHSC-specified copayments for Medicaid Members, where applicable (if HHSC implements Medicaid cost sharing after the Effective Date of the Contract); and
(3) STAR+PLUS Members who enter a 24-hour setting are required to pay the provider of care room and board costs and any income in excess of the personal needs allowance, as established by HHSC. Therefore, the MCO is not required to pay the provider of care room and board costs and any income in excess of the personal needs allowance for these Members. However, the MCO is responsible for notifying HHSC when it becomes aware that a Member is not paying the provider of care. Neither the MCO nor the Member are required to pay the provider of care room and board costs for a Member receiving Adult Foster Care in his or her home.
(4) STAR+PLUS Members who enter a Nursing Facility on or after March 1, 2015, will be required to pay the provider of care room and board costs and any income in excess of the personal needs allowance, as established by HHSC.
8.1.3.1 Waiting Times for Appointments
Through its Provider Network composition and management, the MCO must ensure that the following standards are met. In all cases below, "day" is defined as a calendar day, and the standards are measured from the date of presentation or request, whichever occurs first.
1. Emergency Services must be provided upon Member presentation at the service delivery site, including at non-network and out-of-area facilities;
2. urgent care, including urgent specialty care, must be provided within 24 hours;
3. routine primary care must be provided within 14 days;
4. initial outpatient behavioral health visits must be provided within 14 days;
5. PCPs must make referrals for specialty care on a timely basis, based on the urgency of the Member's medical condition, but no later than 30 days;
6. pre-natal care must be provided within 14 days, except for high-risk pregnancies or new Members in the third trimester, for whom an appointment must be offered within five days, or immediately, if an emergency exists;
7. preventive health services for adults must be offered within 90 days; and
8. preventive health services for children, including well-child checkups should be offered to CHIP Members in accordance with the American Academy of Pediatrics (AAP) periodicity schedule. Medicaid MCOs should utilize the Texas Health Steps periodicity schedule. For a New Member birth through age 20, overdue or upcoming well-child checkups, including Texas Health Steps medical checkups, should be offered as soon as practicable, but in no case later than 14 days of enrollment for newborns, and no later than 90 days of enrollment for all other eligible child Members. The Texas Health Steps annual medical checkup for an Existing Member age 36 months and older is due on the child's birthday. The annual medical checkup is considered timely if it occurs no later than 364 calendar days after the child's birthday. For purposes of this requirement, the terms "New Member" and "Existing Member" are defined in Chapter 12.4 of the Uniform Managed Care Manual.
8.1.3.2 Access to Network Providers
The MCO's Network must include all of the provider types described in this section in sufficient numbers, and with sufficient capacity, to provide timely access to all Covered Services in accordance with the waiting times for appointments in Section 8.1.3.1. The MCO's Network must provide timely access to regular and preventive care to all Members, and Texas Health Steps services to all child Members in Medicaid.
This section includes distance standards for each provider type. For each provider type, the MCO must provide access to at least 90 percent of members in each Program and Service Area within the prescribed distance standard for each State Fiscal Quarter. This 90-percent benchmark does not apply to pharmacy providers (refer to the "Pharmacy Access" heading for applicable benchmarks).
HHSC will consider requests for exceptions to the distance standards for all provider types under limited circumstances. Each exception request must be supported by information and documentation as specified in HHSC's exception request template.
Medicaid PCP Access: At a minimum, the MCO must ensure that all adult Members have access to one age-appropriate Network PCP with an Open Panel within 30 miles of the Member's residence. Child Members must have access to two age-appropriate Network PCPs with an Open Panel within 30 miles of the Member's residence.
CHIP PCP Access: At a minimum, the MCO must ensure that all Members have access to one age-appropriate PCP in the Provider Network with an Open Panel within 30 miles of the Member's residence. This provision does not apply to CHIP Perinates, but it does apply to CHIP Perinate Newborns.
For the purpose of assessing compliance with the Medicaid and CHIP PCP access requirements, an internist who provides primary care to adults only is not considered an age-appropriate PCP choice for a Member birth through age 20, and a pediatrician is not considered an age-appropriate choice for a Member age 21 and over.
As described above, the MCO can request a special exception if no appropriate provider types are located within the mileage standards.
OB/GYN Access: STAR, STAR+PLUS and CHIP Program Networks: with the following exception, STAR, STAR+PLUS and CHIP MCOs must ensure that all female Members have access to an OB/GYN in the Provider Network within 75 miles of the Member's residence. CHIP MCOs must ensure that CHIP Perinate Members (unborn children) in rural areas have access to Network OB/GYNs within 125 miles of the Member's residence.
If an OB/GYN is acting as the Member's PCP, the MCO must follow the access requirements for the PCP (within 30 miles of the Member's residence).
The MCO must allow female Members to select an OB/GYN within its Provider Network. A female Member who selects an OB/GYN must be allowed direct access to the OB/GYN's Health Care Services without a referral from the Member's PCP or a prior authorization. The MCO must allow pregnant Member who is past the 24th week of pregnancy to remain under the Member's current OB/GYN care though the Member's post-partum checkup, even if the OB/GYN provider is, or becomes, Out-of-Network.
Outpatient Behavioral Health Service Provider Access: At a minimum, the MCO must ensure that all Members have access to a covered outpatient Behavioral Health Service Provider in the Network within 30 miles of the Member's residence for Members in an Urban County, or within 75 miles of the Member's residence for Members in a Rural County. Outpatient Behavioral Health Service Providers must include Masters and Doctorate-level trained practitioners practicing independently or at community mental health centers, other clinics or at outpatient Hospital departments.
The MCO must ensure that a Member has Network access to an entity within 75 miles of the Member’s residence that provides Covered Services, including Mental Health Rehabilitative Services for Medicaid and rehabilitative day treatment for CHIP, through Qualified Mental Health Professionals for Community Services (QMHPs-CS). QMHPs-CS include Licensed Practitioners of the Healing Arts (LPHAs). QMHP-CS can also include Community Services Specialists (CSSP), Peer Providers, or Family Partners if acting under the supervision of an LPHA. In addition, day program providers who address pharmacology issues must be certified as Licensed Medical Personnel.
Other Specialist Physician Access: At a minimum, the MCO must ensure that all Members have access to a Network specialist physician for all covered services within 75 miles of the Member's residence for common medical specialties. For adult Members, common medical specialties must include general surgery, cardiology, orthopedics, urology, and ophthalmology. For child Members, common medical specialties must include orthopedics and otolaryngology. In addition, all Members must be allowed to: 1) select a Network ophthalmologist or therapeutic optometrist to provide eye Health Care Services, other than surgery, and 2) have access without a PCP referral to eye Health Care Services from a Network specialist who is an ophthalmologist or therapeutic optometrist for non-surgical services.
Hospital Access: The MCO must ensure that all Members have access to an Acute Care Hospital in the Provider Network within 30 miles of the Member's residence. For MCOs participating in the CHIP Program, exceptions to this access standard must be approved by HHSC on a case-by-case basis for Perinate Members (unborn children). MCOs participating in the Medicaid Rural Service Area may also request exceptions on a case-by-case basis.
Pharmacy Access: Effective March 1, 2012, the MCO must meet the following minimum requirements. The MCO must ensure that all Members have access to at least one (1) Network Pharmacy within 15 miles of the Member's residence, and access to at least one (1) pharmacy with 24-hour coverage within 75 miles of the Member's residence. MCOs may request exceptions to this requirement on a case-by-case basis.
Effective September 1, 2012, HHSC will apply additional benchmark performance standards. For purposes of this requirement only, the terms urban, suburban, and rural counties have the following meaning:
Urban - Counties that have been designated as metropolitan by the Office of Management and Budget (OMB), and that contain the most populated city within a metropolitan area, also known as Metropolitan Statistical Area. HHSC Strategic Decision Support (SDS) classifies these counties as Metro Central City counties. A county meets the definition of metropolitan if it has a central city, or pair of twin cities in it, with a minimum population of 50,000.
Suburban - Counties that have been designated as metropolitan by the OMB, and that are adjacent (share a boundary) to a Metro Central City county. The SDS classifies these counties as Metro Suburban counties.
Rural - Non-metropolitan counties of the state, regardless of whether they are adjacent or non-adjacent to a metropolitan county.
For counties included in the Medicaid Rural Service Area, the following standard applies to STAR effective September 1, 2012:
| |
• | In urban counties, at least 75 percent of Members must have access to a Network Pharmacy within 2 miles of the Member's residence; |
| |
• | In suburban counties, at least 55 percent of Members must have access to a Network Pharmacy within 5 miles of the Member's residence; |
| |
• | In rural counties, at least 90 percent of Members must have access to a Network Pharmacy within 15 miles of the Member's residence; and |
| |
• | In urban, suburban, and rural counties, at least 90 percent of Members must have access to a 24-hour pharmacy within 75 miles of the Member's residence. |
For all other counties and Programs, the following standard applies effective September 1, 2012:
| |
• | In urban counties, at least 80 percent of Members must have access to a Network Pharmacy within 2 miles of the Member's residence; |
| |
• | In suburban counties, at least 75 percent of Members must have access to a Network Pharmacy within 5 miles of the Member's residence; |
| |
• | In rural counties, at least 90 percent of Members must have access to a Network Pharmacy within 15 miles of the Member's residence; and |
| |
• | In urban, suburban, and rural counties, at least 90 percent of Members must have access to a 24-hour pharmacy within 75 miles of the Member's residence. |
Note: MCOs may request exceptions to these requirements on a case-by-case basis. Mail order pharmacies, including specialty pharmacies that only mail prescriptions, will not be included when calculating these percentages. However, MCOs will be required to report on the number of prescriptions filled and number of clients served through mail order/specialty pharmacies by MCO Program and Service Area.
Nursing Facility Access: Effective March 1, 2015, STAR+PLUS MCOs must ensure that Members have access to a Nursing Facility in the Provider Network within 75 miles of the Member’s residence.
All other Covered Services, except for services provided in the Member's residence: At a minimum, the MCO must ensure that all Members have access to at least one (1) Network Provider for each of the remaining Covered Services described in Attachments B-2, "STAR Covered Services," B-2.1 "CHIP Covered Services," and B-2.2, "STAR+PLUS Covered Services," within 75 miles of the Member's residence. This access requirement includes, but is not limited to, specialists, specialty Hospitals, psychiatric Hospitals, diagnostic and therapeutic services, and single or limited service health care physicians or Providers, as applicable to the MCO Program.
The MCO is not precluded from making arrangements with physicians or providers outside the MCO's Service Area for Members to receive a higher level of skill or specialty than the level available within the Service Area, including but not limited to, treatment of cancer, burns, and cardiac diseases. HHSC may consider exceptions to the above access-related requirements when an MCO has established, through utilization data provided to HHSC, that a normal pattern for securing Health Care Services within an area does not meet these standards, or when an MCO is providing care of a higher skill level or specialty than the level which is available within the Service Area.
8.1.3.3 Monitoring Access
The MCO is required to systematically and regularly verify that Covered Services furnished by Network Providers are available and accessible to Members in compliance with the standards described in Sections 8.1.3.1 and 8.1.3.2, and for Covered Services furnished by PCPs, the standards described in Section 8.1.4.2.
The MCO must enforce access and other Network standards required by the Contract and take appropriate action with noncompliant Providers.
The MCO must enter into written contracts with properly credentialed Providers as described in this Section. The Provider contracts must comply with the Uniform Managed Care Manual's requirements, and include reasonable administrative and professional terms.
The MCO must maintain a Provider Network sufficient to provide all Members with access to the full range of Covered Services required under the Contract. The MCO must ensure its Providers and Subcontractors meet all current and future state and federal eligibility criteria, reporting requirements, and any other applicable rules and/or regulations related to the Contract.
The Provider Network must be responsive to the linguistic, cultural, and other unique needs of any minority, elderly, or disabled individuals, or other special populations served by the MCO. This includes the capacity to communicate with Members in languages other than English, when necessary, as well as with those who are deaf or hearing impaired.
The MCO must seek to obtain the participation in its Provider Network of qualified providers currently serving the Medicaid and CHIP Members in the MCO's proposed Service Area(s). MCOs utilizing Out-of-Network providers to render services to their Members must not exceed the utilization standards established in 1 Tex. Admin. Code §353.4. for Medicaid and 1 Tex. Admin. Code § 370.604 for CHIP, effective December 2014. HHSC may modify this requirement for MCOs that demonstrate good cause for noncompliance, as set forth in §353.4(e)(3) for Medicaid and § 370.604(d) for CHIP, effective December 2014.
The MCO must seek participation in the Provider Network from the following types of entities that may serve American Indian and Alaskan Native children:
1. health clinics operated by a federally-recognized tribe in the Service Area;
2. Federally Qualified Health Centers (FQHC) operated by a federally-recognized tribe in the Service Area; and
3. Urban Indian organizations in the Service Area.
All Providers: Except as provided in Section 8.1.4.10, all Providers must comply with State of Texas licensure requirements and all state and federal laws governing the provision of Covered Services. Providers may not be under sanction or exclusion from the Medicaid program. All Acute Care Providers serving Medicaid Members must be enrolled as Medicaid providers and have a Texas Provider Identification Number (TPIN). All Pharmacy Providers must be enrolled with HHSC’s Vendor Drug Program. Long-term Services and Supports Providers are not required to have a TPIN but must have a LTSS Provider number. Providers must also have a National Provider Identifier (NPI) in accordance with the timelines established in 45 C.F.R. Part 162, Subpart D.
Inpatient Hospital and medical services: The MCO must ensure access to Acute Care Hospitals and Specialty Hospitals in the MCO's Network. Covered Services provided by such Hospitals must be available and accessible 24 hours per day, seven (7) days per week. The MCO must enter into a Network Provider Agreement with any willing State Hospital that meets the MCO's credentialing requirements and agrees to the MCO's contract rates and terms.
Children's Hospitals/Hospitals with specialized pediatric services: The MCO must ensure Members access to Hospitals designated as Children's Hospitals by Medicare and Hospitals with specialized pediatric services, such as teaching Hospitals and Hospitals with designated children's wings. Covered Services provided by such Hospitals must be available and accessible 24 hours per day, seven (7) days per week. If the MCO does not have a designated Children's Hospital and/or Hospital with specialized pediatric services in proximity to the Member's residence in its Network, the MCO must enter into written arrangements for services with Out-of-Network Hospitals. Provider Directories, Member Materials, and Marketing Materials must clearly distinguish between Hospitals designated as Children's Hospitals and Hospitals that have designated children's units.
Trauma: The MCO must ensure Members access to Texas Department of State Health Services (TDSHS)-designated Level I and Level II trauma centers within the State, or Hospitals meeting the equivalent level of trauma care in the MCO's Service Area or in close proximity to such Service Area. The MCO must make written Out-of-Network reimbursement arrangements with the DSHS-designated Level I and Level II trauma centers or Hospitals meeting equivalent levels of trauma care if the MCO does not include such a trauma center in its Network.
Transplant centers: The MCO must ensure Member access to HHSC-designated transplant centers or centers meeting equivalent levels of care. A list of HHSC-designated transplant centers can be found in the Procurement Library. If the MCO's Network does not include a designated transplant center or center meeting equivalent levels of care in proximity to the Member's residence, the MCO must make written arrangements with Out-of-Network providers for such care.
Hemophilia centers: The MCO must ensure Member access to hemophilia centers supported by the Centers for Disease Control (CDC). A list of these hemophilia centers can be found at http://www.cdc.gov/ncbddd/hemophilia/HTC.html. If the MCO's Network does not include CDC-supported hemophilia centers in proximity to the Member's residence, the MCO must make written arrangements with Out-of-Network providers for such care.
Physician services: The MCO must ensure that Primary Care Providers are available and accessible 24 hours per day, seven (7) days per week, within the Provider Network. The MCO must contract with a sufficient number of participating physicians and specialists within each Service Area to comply with Section 8.1.3's access requirements and meet Members' needs for all Covered Services.
The MCO must ensure that an adequate number of participating physicians have admitting privileges at one (1) or more participating Acute Care Hospitals in the Provider Network to ensure that necessary admissions are made. In no case may there be less than one Network PCP with admitting privileges available and accessible 24 hours per day, seven (7) days per week for each Acute Care Hospital in the Provider Network.
The MCO must ensure that an adequate number of participating specialty physicians have admitting privileges at one or more participating Hospitals in the MCO's Provider Network to ensure necessary admissions are made. The MCO must require that all physicians who admit to Hospitals maintain Hospital access for their patients through appropriate call coverage.
Urgent Care Clinics: The MCO must ensure that Urgent Care Clinics, including multi-specialty clinics serving in this capacity, are included within the Provider Network.
Laboratory services: The MCO must ensure that Network reference laboratory services are of sufficient size and scope to meet Members' non-emergency and emergency needs and the access requirements in Section 8.1.3. Reference laboratory specimen procurement services must facilitate the provision of clinical diagnostic services for physicians, Providers, and Members through the use of convenient reference satellite labs in each Service Area, strategically located specimen collection areas in each Service Area, and the use of a courier system under the management of the reference lab. For Medicaid Members, Texas Health Steps requires Providers to use the DSHS Laboratory Services for specimens obtained as part of a Texas Health Steps medical checkup, including Texas Health Steps newborn screens; blood lead testing; hemoglobin electrophoresis; and total hemoglobin tests that are processed at the Austin Laboratory; and Pap Smear, gonorrhea and chlamydia screening processed at the Women's Health Laboratories in San Antonio. Providers may submit specimens for glucose, cholesterol, HDL, lipid profile, HIV and RPR to the DSHS Laboratory or to a laboratory of the provider's choice. Hematocrit may be performed at the provider's clinic if the provider needs an immediate result for anemia screening. Providers should refer to the Texas Health Steps Online Provider Training Modules referencing specimen collection on the DSHS website and the Texas Medicaid Provider Procedures Manual, Children's Services Handbook for the most current information and any updates.
Pharmacy Providers: The MCO must ensure that all Pharmacy Network Providers meet all requirements under 1 Tex. Admin. Code § 353.909. Providers must not be under sanction or exclusion from the Medicaid or CHIP Programs. The MCO must enter into a Network Provider Agreement with any willing pharmacy provider that meets the MCO's credentialing requirements and agrees to the MCO's contract rates and terms. However, the MCO may enter into selective contracts for specialty pharmacy services with one or more pharmacy provider, subject to the following conditions. These arrangements must comply with Texas Government Code § 533.005(a)(23)(G) and 1 Tex. Admin. Code § 353.905, § 354.1853, and § 370.701.
Diagnostic imaging: The MCO must ensure that diagnostic imaging services are available and accessible to all Members in each Service Area in accordance with the access standards in Section 8.1.3. The MCO must ensure that diagnostic imaging procedures that require the injection or ingestion of radiopaque chemicals are performed only under the direction of physicians qualified to perform those procedures.
Home health services: All Members living within the MCO's Service Area must have access to at least one (1) Network Provider of home health Covered Services. (These services are provided as part of the Acute Care Covered Services, not the Community Long Term Services and Supports.)
Community Long Term Services and Supports: All Members living within a STAR+PLUS MCO's Service Area must have access to Medically Necessary and Functionally Necessary Covered Services.
Nursing Facility Services: The STAR+PLUS MCO must ensure Members have access to Nursing Facility services effective March 1, 2015. Nursing facilities that are licensed, certified, and have a valid DADS contract on September 1, 2013, will be included on the STP list. PCPs associated with Nursing Facilities must have admitting privileges to Network Hospitals. If any willing Nursing Facility Provider is not on the STP list but will agree to the MCO’s contract rates and terms, the MCO must also enter into a Network Provider Agreement with that provider if the Nursing Facility is licensed, certified, and has a valid DADS contract. Any willing Nursing Facility Provider includes Nursing Facility STPs who have gone through a change in ownership after September 1, 2013, and new Nursing Facility providers.
Hospice Services: Effective March 1, 2015, Nursing Facility residents in STAR+PLUS MCOs must have access to Hospice Services as Non-capitated Services.
Ambulance providers: The MCO must enter into a Network Provider Agreement with any willing ambulance provider that meets the MCO's credentialing requirements and agrees to the MCO's contract rates and terms.
Mental Health Rehabilitative Services: Effective September 1, 2014, the MCO must ensure Members have access to Mental Health Rehabilitative Services.
8.1.4.1 Provider Contract Requirements
The MCO is prohibited from requiring a provider or provider group to enter into an exclusive contracting arrangement with the MCO as a condition for Network participation.
The MCO’s contract with health care Providers must be in writing, must be in compliance with applicable federal and state laws and regulations, and must include minimum requirements specified in Attachment A, "Uniform Managed Care Contract Terms and Conditions," and Uniform Managed Care Manual Chapter 8.1 “Provider Contract Checklist.”
As described in Section 7, the MCO must submit model Provider contracts to HHSC for review during Readiness Review. The MCO must resubmit the model Provider contracts any time it makes substantive modifications to such agreements. HHSC retains the right to reject or require changes to any Provider contract that does not comply with MCO Program requirements or the HHSC-MCO Contract.
8.1.4.2 Primary Care Providers
The MCO’s PCP Network may include Providers from any of the following practice areas: General Practice; Family Practice; Internal Medicine; Pediatrics; Obstetrics/Gynecology (OB/GYN); Advanced Practice Registered Nurses (APRNs) and Physician Assistants (PAs) (when APRNs and PAs are practicing under the supervision of a physician specializing in Family Practice, Internal Medicine, Pediatrics or Obstetrics/Gynecology who also qualifies as a PCP under this contract); Federally Qualified Health Centers (FQHCs), Rural Health Clinics (RHCs), and similar community clinics; physicians serving Members residing in Nursing Facilities effective March 1, 2015; and specialist physicians who are willing to provide a Medical Home to selected Members with special needs and conditions. Texas Government Code Section 533.005(a)(13) and Texas Health and Safety Code Section 62.1551 require the MCO to use advance practice registered nurses (APRNs) and physician assistants (PAs) practicing under the supervision of a Network physician. The MCO must treat APRNs and PAs in the same manner as other Network PCPs with regard to: (1) selection and assignment as PCPs, (2) inclusion as PCPs in the MCO’s Provider Network, and (3) inclusion as a PCP in any Provider Directory maintained by the MCO.
An internist or other Provider who provides primary care to adults only is not considered an age-appropriate PCP choice for a Member birth through age 20. An internist or other Provider who provides primary care to adults and children may be a PCP for children if:
1. the Provider assumes all MCO PCP responsibilities for such child Members in a specific age range from birth through age 20,
2. the Provider has a history of practicing as a PCP for the specified age range, as evidenced by the Provider's primary care practice including an established patient population within the specified age range, and
3. the Provider has admitting privileges to a local Hospital that includes admissions to pediatric units.
A pediatrician is not considered an age-appropriate choice for a Member age 21 and over.
The PCP for a Member with disabilities, Special Health Care Needs, Chronic or Complex Conditions, or in a Nursing Facility may be a specialist physician who agrees to provide PCP services to the Member. The specialist physician must agree to perform all PCP duties required in the Contract, and PCP duties must be within the scope of the specialist’s license. Any interested person may initiate the request through the MCO for a specialist to serve as a PCP for a Member with disabilities, Special Health Care Needs, or Chronic or Complex Conditions. The MCO must handle these requests in accordance with 28 Tex. Admin. Code Chapter 11, Subchapter J.
PCPs who provide Covered Services for STAR and CHIP newborns must either have admitting privileges at a Hospital that is part of the MCO's Provider Network, or make referral arrangements with a Provider who has admitting privileges to a Network Hospital. STAR+PLUS PCPs must either have admitting privileges at a Network Hospital, or make referral arrangements with a Provider who has admitting privileges to a Network Hospital.
The MCO must require, through contract provisions, that PCPs are accessible to Members 24 hours a day, seven (7) days a week. The MCO is encouraged to enter into Network Provider agreements with sites that offer primary care services during evening and weekend hours. The following are acceptable and unacceptable telephone arrangements for contacting PCPs after their normal business hours.
Acceptable after-hours coverage:
1. the office telephone is answered after-hours by an answering service that meets language requirements of the Major Population Groups and that can contact the PCP or another designated medical practitioner. All calls answered by an answering service must be returned within 30 minutes;
2. the office telephone is answered after normal business hours by a recording in the language of each of the Major Population Groups served, directing the patient to call another number to reach the PCP or another provider designated by the PCP. Someone must be available to answer the designated provider's telephone. Another recording is not acceptable; and
3. the office telephone is transferred after office hours to another location where someone will answer the telephone and be able to contact the PCP, or another designated medical provider, who can return the call within 30 minutes.
Unacceptable after-hours coverage:
1. the office telephone is only answered during office hours;
2. the office telephone is answered after-hours by a recording that tells patients to leave a message;
3. the office telephone is answered after-hours by a recording that directs patients to go to an Emergency Room for any services needed; and
4. returning after-hours calls outside of 30 minutes.
The CHIP MCOs must require PCPs, through contract provisions, to provide children birth through age 20 with preventive services in accordance with the AAP recommendations. Medicaid MCOs must require PCPs, through contract provisions, to provide children birth through age 20 with preventive services in accordance with the Texas Health Steps periodicity schedule. The MCO must require PCPs, through contract provisions, to provide adults with preventive services in accordance with the U.S. Preventive Services Task Force requirements. The MCO must make best efforts to ensure that PCPs follow these periodicity requirements for children and adult Members. Best efforts must include, but not be limited to, Provider education, Provider profiling, monitoring, and feedback activities.
The MCO must require PCPs, through contract provisions, to assess the medical needs of Members for referral to specialty care providers and provide referrals as needed. PCPs must coordinate Members' care with specialty care providers after referral. The MCO must make best efforts to ensure that PCPs assess Member needs for referrals and make such referrals. Best efforts must include, but not be limited to, Provider education activities and review of Provider referral patterns.
8.1.4.3 PCP Notification
The MCO must furnish each PCP with a current list of Members enrolled or assigned to that Provider no later than five (5) Business Days after the MCO receives the Enrollment File from the HHSC Administrative Services Contractor each month. The MCO may offer and provide such enrollment information in alternative formats, such as through access to a secure Internet site, when such format is acceptable to the PCP.
8.1.4.4 Provider Credentialing and Re-credentialing
The MCO must review, approve, and periodically recertify the credentials of all participating physician Providers and all other licensed Providers who participate in the MCO’s Network. The MCO may subcontract with another entity to which it delegates credentialing activities if the delegated credentialing is maintained in accordance with the National Committee for Quality Assurance (NCQA) delegated credentialing requirements and any comparable requirements defined by HHSC.
At a minimum, the scope and structure of an MCO’s credentialing and re-credentialing processes must be consistent with recognized MCO industry standards and relevant state and federal regulations including 28 Tex. Admin. Code §§ 11.1902 and 11.1402(c), relating to provider credentialing and notice. Medicaid MCOs must also comply with 42 C.F.R. § 438.12 and § 438.214(b-e). The re-credentialing process must occur at least every three years.
The MCOs must use state-identified credentialing criteria for Nursing Facilities and may only contract with a Nursing Facility with a valid certification, license, and contract with DADS. The MCO may not discriminate for the participation, reimbursement, or indemnification of any provider who is acting within the scope of his or her license or certification under applicable State law, solely on the basis of that license or certification. Additionally, if the MCO declines to include individual or groups of providers in its Network, it must give the affected providers written notice of the reasons for its decision.
The re-credentialing process must take into consideration Provider performance data including Member Complaints and Appeals, quality of care, and utilization management.
8.1.4.4.1 Expedited Credentialing Process
MCOs must comply with the requirements of Texas Insurance Code Chapter 1452, Subchapters C, D, and E, regarding expedited credentialing and payment of physicians, podiatrists, and therapeutic optometrists who have joined established medical groups or professional practices that are already contracted with the MCO. Additionally, the MCO must comply with the Subchapters’ hold harmless requirements for Members.
The MCO must complete the credentialing process for a new provider and its claim systems must be able to recognize the provider as a Network Provider no later than 90 calendar days after receipt of a complete application.
If an application does not include required information, the MCO must provide the provider written notice of all missing information no later than 5 Business Days after receipt.
Additionally, if a provider qualifies for expedited credentialing, the MCO’s claims system must be able to process claims from the provider as if the Provider was a Network Provider no later than 30 calendar days after receipt of a complete application, even if the MCO has not yet completed the credentialing process.
8.1.4.5 Board Certification Status
The MCO must maintain a policy with respect to board certification for PCPs and specialty physicians that encourages participation of board certified PCPs and specialty physicians in the Provider Network. The MCO must make information on the percentage of board-certified PCPs in the Provider Network and the percentage of board-certified specialty physicians, by specialty, available to HHSC upon request.
8.1.4.6 Provider Relations Including Manual, Materials and Training
The MCO must maintain a provider relations presence in each Service Area or, for the Medicaid Rural Service Area, in regions approved by HHSC.
The STAR+PLUS MCOs must assign a provider relations specialist to each Network Nursing Facility. The assigned provider relations specialist may be assigned to more than one Nursing Facility in a Service Area. The specialist must be proficient in
Nursing Facility billing and able to resolve provider billing and payment inquiries. The MCO must notify the Nursing Facility within 10 days of any change to the assigned provider relations specialist.
The MCO must prepare and issue Provider Manual(s) to all Network Providers, including any necessary specialty manuals (e.g., behavioral health, Nursing Facility Services). For newly contracted Providers, the MCO must issue copies of the Provider Manual(s) no later than five Business Days after inclusion in the Network. The Provider Manual must contain sections relating to special requirements of the MCO Program(s) and the enrolled populations in compliance with the requirements of this Contract, including Uniform Managed Care Manual Chapter 3.3.
HHSC or its designee must approve the Provider Manual, and any substantive revisions to the Provider Manual, prior to publication and distribution to Providers. The Provider Manual must contain the critical elements defined in Uniform Managed Care Manual Chapter 3, Critical Elements. HHSC’s initial review of the Provider Manual is part of the Operational Readiness Review described in Section 7, Transition Phase Requirements.
The MCO must provide training to all Providers and their staff regarding the requirements of the Contract and special needs of Members. The MCO’s STAR, STAR+PLUS, CHIP and/or CHIP Perinatal Program training must be completed within 30 days of placing a newly contracted Provider on active status. The MCO must provide ongoing training to new and existing Providers as required by the MCO, or as required by HHSC to comply with the Contract. The MCO must maintain and make available
upon request enrollment or attendance rosters dated and signed by each attendee, or other written evidence of training of each Provider and his or her staff.
The MCO must establish ongoing Provider training that includes the following issues:
1. Covered Services and the Provider’s responsibilities for providing and coordinating these services.
a. Special emphasis must be placed on areas that vary from commercial coverage rules (e.g., Early Childhood Intervention services, therapies and DME/Medical Supplies); pharmacy services and processes, including information regarding outpatient drug benefits, HHSC’s drug formulary, preferred drugs, prior authorization processes, and 72 hour emergency supplies of prescription drugs;
b. For Medicaid, the MCO should also place special emphasis on Mental Health Rehabilitative Services and the availability of Targeted Case Management for qualified Members, and the processes for making referrals and coordination with Non-capitated Services;
2. relevant requirements of the Contract;
3. the MCO’s quality assurance and performance improvement program and the Provider’s role in such a program;
4. the MCO’s policies and procedures, especially regarding Network and Out-of-Network referrals;
5. Member cost-sharing obligations, benefit limitations, Value-added Services, and prohibitions on balance-billing Members for Covered Services;
6. Cultural Competency Training;
7. Texas Health Steps benefits, periodicity, and required elements of a checkup;
8. Medical Transportation Program services available to Medicaid members such as rides to services by bus, taxi, van, airfare, etc., gas money, mileage reimbursement, and meals and lodging when away from home;
9. the importance of updating contact information to ensure accurate Provider Directories and the Medicaid Online Provider Lookup;
10. information about the MCO’s process for acceleration of Texas Health Steps services for Children of Migrant Farm Workers;
11. missed appointment referrals and assistance provided by the Texas Health Steps Outreach and Informing Unit;
12. for STAR in the Medicaid Rural Service Area, the process for continuing up to six months of Community-based Long Term Care Services for Members receiving those services as of the Operational Start Date, including provider billing practices for these services and whom to contact at the MCO for assistance with this process;
13. for STAR+PLUS, the role of the MCO Service Coordinators;
14. for STAR+PLUS, information on discharge planning, transitional care, and other educational programs related to long-term care settings;
15. administrative issues such as claims filing and services available to Members;
13. requirements of the Frew v. Janek Consent Decree and Corrective Action Orders; and
16. For Medicaid, specific information in training materials (such as in the MCO's Provider Manual) pertaining to Attention Deficit Hyperactivity Disorder (ADHD) Covered Services for children including reimbursement for ADHD and availability of follow-up care for children who have been prescribed ADHD medications.
Provider Materials must comply with state and federal laws; Attachment A, Uniform Managed Care Contract Terms and Conditions; and Uniform Managed Care Manual Chapter 3, Critical Elements.
As described above, HHSC must approve the MCO’s Provider Manual and all revisions. Additionally, the MCO must submit, for HHSC’s review, all other Provider Materials relating to Medicaid or CHIP prior to use or mailing. If HHSC has not responded to MCO’s request for review within 15 Business Days, the MCO may use the submitted materials. HHSC reserves the right to require discontinuation or correction of any Provider Materials that are not in compliance with State and Federal laws or the Contract’s requirements.
8.1.4.7 Provider Hotline
The MCO must operate a toll-free telephone line for Provider inquiries from 8 a.m. to 5 p.m. local time for the Service Area, Monday through Friday, except for State-approved holidays. The State-approved holiday schedule is updated annually and can be found at http://sao.hr.state.tx.us/compensation/holidays.html. The Provider Hotline must be staffed with personnel who are knowledgeable about Covered Services, each applicable MCO Program, and for Medicaid, about Non-capitated Services.
The MCO must ensure that after regular business hours the line is answered by an automated system with the capability to provide callers with operating hours information and instructions on how to verify enrollment for a Member with an Urgent Condition or an Emergency Medical Condition. The MCO must have a process in place to handle after-hours inquiries from Providers seeking to verify enrollment for a Member with an Urgent Condition or an Emergency Medical Condition, provided, however, that the MCO and its Providers must not require such verification prior to providing Emergency Services.
The MCO must ensure that the Provider Hotline meets the following minimum performance requirements for all MCO Programs and Service Areas:
|
| |
| 1. 99% of calls are answered by the fourth ring or an automated call pick-up system is used; |
|
| |
| 2. no more than one percent (1%) of incoming calls receive a busy signal; |
|
| |
| 3. the average hold time is two (2) minutes or less; and |
|
| |
| 4. the call abandonment rate is seven percent (7%) or less. |
The MCO must conduct ongoing call quality assurance to ensure these standards are met. The Provider Hotline may serve multiple MCO Programs if Hotline staff is knowledgeable about all of the MCO’s Programs. The Provider Hotline may serve multiple Service Areas if the Hotline staff is knowledgeable about all Service Areas, including the Provider Network in each Service Area.
The MCO must monitor Provider Hotline performance and submit reports summarizing call center performance as required by Section 8.1.20. If the MCO subcontracts with a Behavioral Health Organization (BHO) that is responsible for Provider Hotline functions related to Behavioral Health Services, the BHO’s Provider Hotline must meet the requirements in Section 8.1.4.7.
If HHSC determines that it is necessary to conduct onsite monitoring of the MCO’s Provider Hotline functions, the MCO is responsible for all reasonable travel costs incurred by HHSC or its authorized agent(s) relating to such monitoring. For purposes of this section, “reasonable travel costs” include airfare, lodging, meals, car rental and fuel, taxi, mileage, parking and other incidental travel expenses incurred by HHSC or its authorized agent in connection with the onsite monitoring.
8.1.4.8 Provider Reimbursement
The MCO must pay for all Medically Necessary Covered Services provided to Members. A STAR+PLUS MCO must also pay for all Functionally Necessary Covered Services provided to Members. The MCO's Network Provider Agreement must include a complete description of the payment methodology or amount, as described in Uniform Managed Care Manual Chapter 8.1.
The MCO must ensure claims payment is timely and accurate as described in Section 8.1.18.5, "Claims Processing Requirements," and UMCM Chapters 2.0 through 2.3. The MCO must require tax identification numbers from all participating Providers. The MCO is required to do back-up withholding from all payments to Providers who fail to give tax identification numbers or who give incorrect numbers.
Provider payments must comply with all applicable state and federal laws, rules, and regulations, including the following sections of the Patient Protection and Affordable Care Act (PPACA) and, upon implementation, corresponding federal regulations:
| |
• | Section 2702 of PPACA, entitled "Payment Adjustment for Health Care-Acquired Conditions;" |
| |
• | Section 6505 of PPACA, entitled "Prohibition on Payments to Institutions or Entities Located Outside of the United States;" and |
| |
• | Section 1202 of the Health Care and Education Reconciliation Act as amended by PPACA, entitled "Payments to Primary Care Physicians." |
The MCO must comply with registration requirements in Tex. Ins. Code § 1458.051 and with reimbursement and fee schedule requirements in Tex. Ins. Code § 1451.451 and 1458.101–102.
As required by Texas Government Code § 533.005(a)(25), the MCO cannot implement across-the-board Provider reimbursement rate reductions unless: (1) it receives HHSC's prior approval, or (2) the reductions are based on changes to the Medicaid fee schedule or cost containment initiatives implemented by HHSC. For purposes of this requirement an across-the-board rate reduction is a reduction that applies to all similarly-situated providers or types of providers. The MCO must submit a request for an across-the-board rate reduction to HHSC's Director of Program Operations, if the reduction is not based on a change in the Medicaid fee schedule or cost containment initiative implemented by HHSC. The MCO must submit the request at least 90 days prior to the planned effective date of the reduction, and provide a copy to the Health Plan Manager. If HHSC does not issue a written statement of disapproval within 45 days of receipt, then the MCO may move forward with the reduction on the planned effective date.
Further, the MCO must give Providers at least 30 days’ notice of changes to the MCO’s fee schedule, excluding changes derived from changes to the Medicaid fee schedule, before implementing the change. If the MCO fee schedule is derived from the Medicaid fee schedule, the MCO must implement fee schedule changes no later than 60 days after the Medicaid fee schedule change, and any retroactive claim adjustments must be completed within 60 days after HHSC retroactively adjusts the Medicaid fee schedule.
8.1.4.8.1 Potentially Preventable Complications
MCOs must identify Present on Admission (POA) indicators as required in the Uniform Managed Care Manual Chapter 2.0, "Claims Manual," and MCOs must reduce or deny payments for Potentially Preventable Complications that were not POA.
8.1.4.8.2 MCO Value-Based Contracting (Expansion of Alternative Payment Structures for Providers)
The MCO must develop and submit to HHSC a written plan for expansion of alternative payment structures with its Providers that encourages innovation and collaboration, as well as increases quality and efficiency. Payment structures should be focused on incentivizing quality outcomes, shared savings, or both resulting from the reduction of inappropriate utilization of services, including inappropriate admissions and readmissions rather than be based on volume. The plan will include mechanisms by which the MCO will provide incentive payments to hospitals, physicians and other health care providers for quality care resulting in reductions of inappropriate services. The plan will include quality metrics required for incentives, recruitment strategies of providers, and a proposed structure for incentive payments, shared savings, or both. Beginning with SFY15, the MCO must submit its state fiscal year plan to HHSC no later than November 1 of each year using UMCM Chapter 8.4, "Plan for Expansion of Alternative Payment Structures for Providers." HHSC will evaluate the plan and provide feedback to the MCO. Upon HHSC's approval of the plan, HHSC will retrospectively evaluate the MCO on its execution of the written plan. Modifications can be made to the plan after submission, but are subject to HHSC review and approval. Plan approval is based on the following criteria: the number of providers, diversity of selected providers, geographic representation, and the methodology of the shared savings, data sharing strategy with providers, and other factors. Each year, the annual plan must show a measurable increase in the percent of business (providers, dollars, or other) being incentivized from the previous year.
HHSC's retrospective review of the execution of the plan may include a review of encounter data, MCO financial statistical reports, and surveys or interviews with MCO representatives or providers. HHSC may ask the MCO to submit additional information upon request. HHSC may delay or reduce payments to the MCO if it does not submit a plan by the required deadline or does not execute a plan as approved.
8.1.4.8.3 Nursing Facility Incentives
HHSC will develop a Nursing Facility payment system that incentivizes the reduction of potentially preventable events, including potentially preventable Hospital admissions, Hospital readmissions, unnecessary institutionalization, and Acute Care costs. HHSC will also develop payment incentives that encourage Nursing Facility culture change, including the development of resident-centered service delivery and improvements to Nursing Facility physical plant features. HHSC will consult with the MCOs in the development of this incentive program.
8.1.4.9 Termination of Provider Contracts
Unless prohibited or limited by applicable law, the MCO must make a good faith effort to give written notice of termination of a Network Provider, within 15 calendar days after receipt or issuance of the termination notice, to each Member who receives his or her primary care from, or who is seen on a regular basis by, the Network Provider. The MCO must send notice to: (1) all Members in a PCP’s panel, and (2) all Members who have had two or more visits with the Network Provider for home-based or office-based care in the past 12 months. The MCO must notify HHSC of provider terminations in accordance with UMCM Chapter 5.4.1.1, “Provider Termination Report.”
The MCO’s process for terminating CHIP Provider contracts must comply with the Texas Insurance Code and TDI regulations.
|
|
8.1.4.10 Out-of-State Providers |
To participate in Medicaid, the provider must be enrolled with HHSC as a Medicaid provider. The MCO may enroll out-of-state providers in its Medicaid and CHIP Networks in accordance with 1 Tex. Admin. Code § 352.17 and Pharmacy Network Providers in accordance with 1 Tex. Admin. Code § 353.909 as effective on September 1, 2014.
The MCO may enroll out-of-state diagnostic laboratories in its Medicaid and CHIP Networks under the circumstances described in Texas Government Code § 531.066.
8.1.4.11 Provider Advisory Groups
The MCO must establish and conduct quarterly meetings with Network Providers. Membership in the Provider Advisory Group(s) must include, at a minimum, acute, community-based LTSS (STAR+PLUS only), and pharmacy providers. The MCO must maintain a record of Provider Advisory Group meetings, including agendas and minutes, for at least three years.
8.1.4.12 Provider Protection Plan
The MCO must comply with HHSC's provider protection plan requirements for reducing the administrative burdens placed on Network Providers, and ensuring efficiency in Network enrollment and reimbursement. At a minimum, the plan must comply with the requirements of Texas Government Code § 533.0055, and:
| |
• | Provide for timely and accurate claims adjudication and proper claims payment in accordance with UMCM Chapters 2.0 through 2.3. |
| |
• | Include Network Provider training and education on the requirements for claims submission and appeals, including the MCO's policies and procedures (see also Section 8.1.4.6, "Provider Relations Including Manual, Materials and Training.") |
| |
• | Ensure Member access to care, in accordance with Section 8.1.3, "Access to Care," and the UMCM's Geo-Mapping requirements (see UMCM Chapters 5.14.1 through 5.14.4.) |
| |
• | Ensure prompt credentialing, as required by Section 8.1.4.4, "Provider Credentialing and Re-credentialing." |
| |
• | Ensure compliance with state and federal standards regarding prior authorizations, as described in Sections 8.1.8, "Utilization Management," and 8.1.21.2, "Prior Authorization for Prescription Drugs and 72-Hour Emergency Supplies." |
| |
• | Provide 30 days’ notice to Providers before implementing changes to policies and procedures affecting the prior authorization process. However, in the case of suspected fraud, waste, or abuse by a single Provider, the MCO may |
implement changes to policies and procedures affecting the prior authorization process without the required notice period.
| |
• | Include other measures developed by HHSC or a provider protection plan workgroup, or measures developed by the MCO and approved by HHSC. |
Additionally, the MCO must participate in HHSC's work group, which will develop recommendations and proposed timelines for other components of the provider protection plan.
The MCO must maintain a Member Services Department to assist Members and their family members or guardians in obtaining Covered Services for Members. The MCO must maintain employment standards and requirements (e.g., education, training, and experience) for Member Services Department staff and provide a sufficient number of staff for the Member Services Department to meet the requirements of this Section.
8.1.5.1 Member Materials
The MCO must design, print and distribute Member identification (ID) cards and a Member Handbook to Members. Within five (5) Business Days following the receipt of an Enrollment File from the HHSC Administrative Services Contractor, the MCO must mail a Member's ID card and Member Handbook to the Case Head or Account Name for each new Member. When the Case Head or Account Name represents two (2) or more new Members, the MCO is only required to send one (1) Member Handbook. The MCO is responsible for mailing materials only to those households for whom valid address data are contained in the Enrollment File.
The MCO must design, print and deliver Provider Directories to the HHSC Administrative Services Contractor as described in Section 8.1.5.4.
Member Materials must be at or below a 6th grade reading level as measured by the appropriate score on the Flesch reading ease test. Member Materials must be available in English, Spanish, and the languages of other Major Population Groups. HHSC will provide the MCO with reasonable notice when the enrolled population reaches the 10% threshold for a Major Population Group in the MCO's Service Area. All Member Materials must be available in a format accessible to the visually impaired, which may include large print, Braille, and audiotapes.
The MCO must submit member materials to HHSC for approval prior to use or mailing. HHSC will identify any required changes to the Member materials within 15 Business Days. If HHSC has not responded to a request for review by the fifteenth Business Day, the Contractor may proceed to use the submitted materials. HHSC reserves the right to require discontinuation of any Member materials that violate the terms of this Contract, including but not limited to Marketing Policies and Procedures as described in Uniform Managed Care Manual Chapter 4.3, "Uniform Managed Care Marketing Policies and Procedures."
If the MCO distributes HHSC-approved Member Materials groups of Members or all Members (i.e., "mass communications,") it also must post a copy of the materials on its website.
The MCO's Member Materials and other communications cannot contain discretionary clauses, as described in Section 1271.057(b) of the Texas Insurance Code. For CHIP MCOs, this restriction also applies to the MCO's Evidence of Coverage or Certificate of Coverage documents.
8.1.5.2 Member Identification (ID) Card
All Member ID cards must, at a minimum, include the following information:
1. the Member's name;
2. the Member's Medicaid or CHIP Program number;
3. the effective date of the PCP assignment (excluding CHIP Perinates);
4. the PCP’s name (not required for Dual Eligible STAR+PLUS Members, CHIP Perinates, and Nursing Facility residents), address (optional for all products), and telephone number (not required for Dual Eligible STAR+PLUS Members, CHIP Perinates, and Nursing Facility residents);
5. the name of the MCO;
6. the 24-hour, seven (7) day a week toll-free Member services telephone number and BH Hotline number operated by the MCO; and
7. any other critical elements identified in Uniform Managed Care Manual Chapter 3, Critical Elements.
The MCO must reissue the Member ID card if a Member reports a lost card or name change, if the Member requests a new PCP, or for any other reason that results in a change to the information disclosed on the ID card.
8.1.5.3 Member Handbook
HHSC must approve the Member Handbook, and any substantive revisions, prior to publication and distribution. As described in Section 7, “Transition Phase Requirements,” the MCO must develop and submit to HHSC the draft Member Handbook for approval during the Readiness Review and must submit a final Member Handbook incorporating changes required by HHSC prior to the Operational Start Date.
The Member Handbook for each applicable MCO Program must, at a minimum, meet the Member materials requirements specified by Section 8.1.5.1 and must include critical elements in Uniform Managed Care Manual Chapter 3, “Critical Elements.” CHIP MCOs must issue Member Handbooks to both CHIP Perinates and CHIP Perinate Newborns. The Member Handbook for CHIP Perinate Newborns may be the same as that used for CHIP.
The MCO must produce a revised Member Handbook, or an insert informing Members of changes to Covered Services, upon HHSC notification and at least 30 days prior to the effective date of such change in Covered Services. In addition to modifying the Member Materials for new Members, the MCO must notify all existing Members of the Covered Services change during the timeframe specified in this subsection.
8.1.5.4 Provider Directory
The Provider Directory for each MCO Program, and any substantive revisions, must be approved by HHSC prior to publication and distribution, with the exception of PCP information changes or clerical corrections. The MCO is responsible for submitting draft Provider Directory updates to HHSC for prior review and approval.
As described in Section 7, “Transition Phase Requirements,” during Readiness Review the MCO must develop and submit to HHSC the draft Provider Directory template for approval and must submit a final Provider Directory incorporating changes required by HHSC prior to the Operational Start Date. Such draft and final Provider Directories must be submitted according to the deadlines established in Section 7, “Transition Phase Requirements.”
The Provider Directory for each applicable MCO Program must, at a minimum, meet the Member Materials requirements specified by Section 8.1.5.1 above and must include critical elements in Uniform Managed Care Manual Chapter 3. The Provider Directory must include only Network Providers credentialed by the MCO in accordance with Section 8.1.4.4. If the MCO contracts with limited Provider Networks, the Provider Directory must comply with the requirements of 28 T.A.C. §11.1600(b)(11), relating to the disclosure and notice of limited Provider Networks.
At a minimum, the MCO must update the Provider Directory on a quarterly basis. The MCO must make such updates available to existing Members on request, and must provide such updates to the HHSC Administrative Services Contractor at the beginning of each State Fiscal Quarter. Weight limits for the Provider Directories are included in Uniform Managed Care Manual Chapter 3.1, “MMC Provider Directory” and Chapter 3.2, “CHIP Provider Directory”. HHSC will require MCOs that exceed the weight limits to compensate HHSC for postage fees in excess of the weight limits.
The MCO must send the most recent Provider Directory, including any updates, to Members upon request. The MCO must, at least annually, include written and verbal offers of such Provider Directory in its Member outreach efforts and education materials.
8.1.5.5 Internet Website
The MCO must develop and maintain, consistent with HHSC standards and Texas Insurance Code Section 843.2015 and other applicable state laws, a website to provide general information about the MCO's Program(s), its Provider Network, its customer services, and its Complaints and Appeals process. The website must contain a link to financial literacy information on the Office of Consumer Credit Commissioner's webpage. The MCO may develop a page within its existing website to meet the requirements of this section.
For each Program operated by the MCO, the MCO's website must include either a Provider Directory in text-searchable format, or Network Provider search functionality. This information must be accurate and the MCO must update it at least twice a
month. The online Provider Directory or online Provider search functionality must designate PCPs with open versus closed panels. The online Provider Directory or online Provider search functionality must also identify Providers that provide Long-Term Services and Supports (LTSS). The MCO must list Home Health Ancillary providers on its website, with an indicator for pediatric services if provided.
8.1.5.6 Member Hotline
The MCO must operate a toll-free hotline that Members can call 24 hours a day, seven (7) days a week. The Member Hotline must be staffed with personnel who are knowledgeable about its MCO Program(s) and Covered Services between the hours of 8:00 a.m. to 5:00 p.m. local time for the Service Area, Monday through Friday, excluding state-approved holidays. The State-approved holiday schedule is updated annually and can be found at http://sao.hr.state.tx.us/compensation/holidays.html.
The MCO must ensure that after hours, on weekends, and on holidays the Member Services Hotline is answered by an automated system with the capability to provide callers with operating hours and instructions on what to do in cases of emergency. All recordings must be in English, Spanish, and the languages of other Major Population Groups in the Service Area. A voice mailbox must be available after hours for callers to leave messages. The MCO's Member Services representatives must return calls received by the automated system from Members or their representatives on the next Business Day.
If the Member Hotline does not have a voice-activated menu system, the MCO must have a menu system that will accommodate Members who cannot access the system through other physical means, such as pushing a button.
The MCO must ensure that its Member Service representatives treat all callers with dignity and respect the callers' need for privacy. At a minimum, the MCO's Member Service representatives must be:
1. knowledgeable about Covered Services;
2. able to answer non-technical questions about the role of the PCP, as applicable;
3. able to answer non-clinical questions about referrals or the process for receiving authorization for procedures or services;
4. able to give information about Providers in a particular area;
5. knowledgeable about Fraud, Abuse, and Waste including the Lock-in Program and the requirements to report any conduct that, if substantiated, may constitute Fraud, Abuse, or Waste;
6. trained regarding Cultural Competency;
7. trained regarding the process used to confirm the status of persons with Special Health Care Needs;
8. for Medicaid Members, able to answer non-clinical questions about accessing Non-capitated Services.
9. for Medicaid Members, trained regarding: a) the emergency prescription process and what steps to take to immediately address problems when pharmacies do not provide a 72-hour supply of emergency medicines; b) how Members in the Lock-in Program can fill prescriptions at a non-designated pharmacy in an emergency situation; and c) DME processes for obtaining services and how to address common problems;
10. for CHIP Members, able to give correct cost-sharing information relating to premiums, co-pays or deductibles, as applicable. (Cost-sharing does not apply to CHIP Perinates (unborn child), CHIP Perinate Newborns, and some Members in the traditional CHIP Program. See Uniform Managed Care Manual Chapter 6.3, for additional information regarding CHIP cost-sharing; and
11. hotlines must meet Cultural Competency requirements and must appropriately handle calls from non-English speaking (and particularly, Spanish-speaking) callers, as well as calls from individuals who are deaf or hard-of-hearing. To meet these requirements, the MCO must employ bilingual Spanish-speaking Member Services representatives and must secure the services of other contractors as necessary to meet these requirements. The MCO must provide such oral interpretation services to all Hotline callers free of charge.
The MCO must process all incoming Member correspondence and telephone inquiries in a timely and responsive manner. The MCO cannot impose maximum call duration limits and must allow calls to be of sufficient length to ensure adequate information is provided to the Member. The MCO must ensure that the toll-free Member Hotline meets the following minimum performance requirements for all MCO Programs and Service Areas:
1. 99% of calls are answered by the fourth ring or an automated call pick-up system;
2. no more than one percent (1%) of incoming calls receive a busy signal;
3. at least 80% of calls must be answered by Hotline staff within 30 seconds; measured from the time the call is placed in queue after selecting an option;
4. the call abandonment rate is seven percent (7%) or less; and
5. the average hold time is two (2) minutes or less.
The MCO must conduct ongoing quality assurance to ensure these standards are met.
The Member Services Hotline may serve multiple MCO Programs if Hotline staff is knowledgeable about all of the MCO's Medicaid and/or CHIP Programs. The Member Services Hotline may serve multiple Service Areas if the Hotline staff is knowledgeable about all Service Areas, including the Provider Network in each Service Area.
The MCO must monitor its performance regarding HHSC Member Hotline standards and submit performance reports summarizing call center performance for the Member Hotline as indicated in Section 8.1.20 and Uniform Managed Care Manual Chapter 5.4.3, "Hotline Reports."
If HHSC determines that it is necessary to conduct onsite monitoring of the MCO's Member Hotline functions, the MCO is responsible for all reasonable travel costs incurred by HHSC or its authorized agent(s) relating to such monitoring. For purposes of this section, "reasonable travel costs" include airfare, lodging, meals, car rental and fuel, taxi, mileage, parking and other incidental travel expenses incurred by HHSC or its authorized agent in connection with the onsite monitoring.
If the MCO provides a 24-hour nurse hotline, it must train hotline staff about: a) the emergency prescription process and what steps to take to immediately address Medicaid Members' problems when pharmacies do not provide a 72-hour supply of emergency medicines; b) the HHSC-OIG Lock-in Program pharmacy override process to ensure Member access to Medically Necessary outpatient drugs; and c) DME processes for obtaining services and how to address common problems. The 24-hour Nurse Hotline will attempt to respond immediately to problems concerning emergency medicines by means at its disposal, including explaining the rules to Medicaid Members so that they understand their rights and, if need be, by offering to contact the pharmacy that is refusing to fill the prescription to explain the 72-hour supply policy, Lock-in Program override procedure, and DME processes.
8.1.5.7 Member Education
The MCO must, at a minimum, develop and implement health education initiatives that educate Members about:
1. how the MCO system operates, including the role of the PCP;
2. Covered Services, limitations and any Value-added Services offered by the MCO;
3. the value of screening and preventive care; and
4. how to obtain Covered Services, including:
a. Emergency Services;
b. accessing OB/GYN and specialty care;
c. Behavioral Health Services;
d. Disease Management programs;
e. Service Coordination, treatment for pregnant women, Members with Special Health Care Needs, including Children with Special Health Care Needs, Nursing Facility residents in STAR+PLUS, and other special populations;
f. Early Childhood Intervention (ECI) Services;
g. screening and preventive services, including well-child care (Texas Health Steps medical checkups for Medicaid Members);
h. for CHIP Members, Member copayments responsibilities (note that copayments to do not apply to CHIP Perinates (unborn child) and CHIP Perinate Newborn Members);
i. for Medicaid Members, Member copayment responsibilities (if HHSC implements Medicaid cost sharing after the Effective Date of the Contract);
j. suicide prevention;
k. identification and health education related to Obesity;
l. obtaining 72-hour supplies of emergency prescriptions from Network pharmacies;
m. how Members in the Lock-in Program can receive outpatient drugs in an emergency situation;
n. Case Management for Children and Pregnant Women;
o. Cognitive Rehabilitation Therapy for STAR+PLUS Members (effective March 6, 2014);
p. Nursing Facility Services for STAR+PLUS Members (available March 1, 2015);
q. Discharge planning, transitional care, and other education programs on all available long term care settings for Nursing Facility residents in STAR+PLUS;
r. Supported Employment and Employment Assistance for STAR+PLUS Members(effective September 1, 2014); and
5. Medical Transportation Program for Medicaid Members.
The MCO must provide a range of health promotion and wellness information and activities for Members in formats that meet the needs of all Members. The MCO must propose, implement, and assess innovative Member education strategies for wellness care and immunization, as well as general health promotion and prevention. The MCO must conduct wellness promotion programs to improve the health status of its Members. The MCO may cooperatively conduct health education classes with one or more of the contracted MCOs in the Service Area. The MCO must work with its Providers to integrate health education, wellness, and prevention training into each Member's care.
The MCO also must provide condition and disease-specific information and educational materials to Members, including information on its Service Management and Disease Management programs as described in Sections 8.1.13 and 8.1.14. Condition- and disease-specific information must be oriented to various groups of Members, such as children, the elderly, persons with disabilities and non-English speaking Members, as appropriate to the MCO's Medicaid or CHIP Programs.
Per Texas Health and Safety Code § 48.052(c), MCOs may use certified Community Health Workers to conduct outreach and Member education activities.
8.1.5.8 Cultural Competency Plan
The MCO must have a comprehensive written Cultural Competency Plan describing how it will ensure culturally competent services, and provide Linguistic Access and Disability- related Access. The Cultural Competency Plan must describe how the individuals and systems within the MCO will effectively provide services to people of all cultures, races, ethnic backgrounds, and religions as well as those with disabilities in a manner that recognizes, values, affirms, and respects the worth of the individuals and protects and preserves the dignity of each. The MCO must submit the Cultural Competency Plan to HHSC for Readiness Review. During the Operations Phase, the MCO must submit modifications and amendments to the Plan to HHSC no later than 30 days prior to implementation of a change. The MCO must also make the Plan available to its Network Providers.
8.1.5.9 Member Complaint and Appeal Process
The MCO must develop, implement and maintain a system for tracking, resolving, and reporting Member Complaints regarding its services, processes, procedures, and staff. The MCO must ensure that Member Complaints are resolved within 30 calendar days after receipt. The MCO is subject to remedies, including liquidated damages, if at least 98 percent of Member Complaints are not resolved within 30 days of the MCO's receipt. Please see Attachment A, "Uniform Managed Care Contract Terms and Conditions," and Attachment B-3, "Deliverables/Liquidated Damages Matrix."
The MCO must develop, implement and maintain a system for tracking, resolving, and reporting Member Appeals regarding the denial or limited authorization of a requested service, including the type or level of service and the denial, in whole or in part, of payment for service. Within this process, the MCO must respond fully and completely to each Appeal and establish a tracking mechanism to document the status and final disposition of each Appeal.
The MCO must ensure that Member Appeals are resolved within 30 calendar days, unless the MCO can document that the Member requested an extension or the MCO shows there is a need for additional information and the delay is in the Member's interest. The MCO is subject to liquidated damages if at least 98 percent of Member Appeals are not resolved within 30 days of the MCO's receipt. Please see Attachment A, "Uniform Managed Care Contract Terms and Conditions," and Attachment B-3, "Deliverables/Liquidated Damages Matrix."
Medicaid MCOs must follow the Member Complaint and Appeal Process described in Section 8.2.6. CHIP MCOs must comply with the CHIP Complaint and Appeal Process described in Sections 8.4.2.
8.1.5.10 Member Advisory Groups
The MCO must establish and conduct quarterly meetings with Members in each service area in which it operates. Membership in the Member Advisory Group(s) must include at least three Members attending each meeting and allow for member
advocates to participate. The MCO must maintain a record of Member Advisory Group meetings, including agendas and minutes, for at least three years.
8.1.5.11 Member Eligibility
The MCO may provide eligibility renewal assistance for Members whose eligibility is about to expire.
|
|
8.1.6 Marketing and Prohibited Practices |
The MCO and its Subcontractors must adhere to the Marketing Policies and Procedures as set forth in Uniform Managed Care Manual Chapter 4.3, “Uniform Managed Care Marketing Policies and Procedures.”
|
|
8.1.7 Quality Assessment and Performance Improvement |
The MCO must provide for the delivery of quality care with the primary goal of improving the health status of Members and, where the Member’s condition is not amenable to improvement, maintain the Member’s current health status by implementing measures to prevent any further decline in condition or deterioration of health status. The MCO must work in collaboration with Providers to actively improve the quality of care provided to Members, consistent with the Quality Improvement Goals and all other requirements of the Contract. The MCO must provide mechanisms for Members and Providers to offer input into the MCO’s quality improvement activities.
8.1.7.1 QAPI Program Overview
The MCO must develop, maintain, and operate a Quality Assessment and Performance Improvement (QAPI) Program consistent with the Contract and TDI requirements, including 28 T.A.C. §11.1901(a)(5) and §11.1902. Medicaid MCOs must also meet the requirements of 42 C.F.R. §438.240.
The MCO must have on file with HHSC an approved plan describing its QAPI Program, including how the MCO will accomplish the activities required by this section. The MCO must submit a QAPI Program Annual Summary in a format and timeframe specified by HHSC or its designee. The MCO must keep participating physicians and other Network Providers informed about the QAPI Program and related activities. The MCO must include in Provider contracts a requirement securing cooperation with the QAPI.
The MCO must approach all clinical and non-clinical aspects of quality assessment and performance improvement based on principles of Continuous Quality Improvement (CQI)/Total Quality Management (TQM) and must:
|
| |
| 1. evaluate performance using objective quality indicators; |
|
| |
| 2. foster data-driven decision-making; |
|
| |
| 3. recognize that opportunities for improvement are unlimited; |
|
| |
| 4. solicit Member and Provider input on performance and QAPI activities; |
|
| |
| 5. support continuous ongoing measurement of clinical and non-clinical effectiveness and Member satisfaction; |
|
| |
| 6. support programmatic improvements of clinical and non-clinical processes based on findings from ongoing measurements; and |
|
| |
| 7. support re-measurement of effectiveness and Member satisfaction, and continued development and implementation of improvement interventions as appropriate. |
8.1.7.2 QAPI Program Structure
The MCO must maintain a well-defined QAPI structure that includes a planned systematic approach to improving clinical and non-clinical processes and outcomes. The MCO must designate a senior executive responsible for the QAPI Program and the
Medical Director must have substantial involvement in QAPI Program activities. At a minimum, the MCO must ensure that the QAPI Program structure:
|
| |
| 1. is organization-wide, with clear lines of accountability within the organization; |
|
| |
| 2. includes a set of functions, roles, and responsibilities for the oversight of QAPI activities that are clearly defined and assigned to appropriate individuals, including physicians, other clinicians, and non-clinicians; |
|
| |
| 3. includes annual objectives and/or goals for planned projects or activities including clinical and non-clinical programs or initiatives and measurement activities; and |
|
| |
| 4. evaluates the effectiveness of clinical and non-clinical initiatives. |
8.1.7.3 Clinical Indicators
The MCO must engage in the collection of clinical indicator data. The MCO must use such clinical indicator data in the development, assessment, and modification of its QAPI Program.
8.1.7.4 QAPI Program Subcontracting
If the MCO subcontracts any of the essential functions or reporting requirements contained within the QAPI Program to another entity, the MCO must maintain detailed files documenting work performed by the Subcontractor. The file must be available for review by HHSC or its designee upon request.
8.1.7.5 Behavioral Health Integration into QAPI Program
The MCO must integrate behavioral health into its QAPI Program and include a systematic and ongoing process for monitoring, evaluating, and improving the quality and appropriateness of Behavioral Health Services provided to Members. Except for the Members identified below, the MCO must collect data, and monitor and evaluate for improvements to physical health outcomes resulting from behavioral health integration into the Member’s overall care.
STAR Members in the Dallas Service Area receive Behavioral Health Services through the NorthSTAR Program, and Behavioral Health Services are not a covered benefit for CHIP Perinates (unborn children).
8.1.7.6 Clinical Practice Guidelines
The MCO must adopt not less than two (2) evidence-based clinical practice guidelines for each applicable MCO Program. Such practice guidelines must be based on valid and reliable clinical evidence, consider the needs of the MCO’s Members, be adopted in consultation with Network Providers, and be reviewed and updated periodically, as appropriate. The MCO must develop practice guidelines based on the health needs and opportunities for improvement identified as part of the QAPI Program.
The MCO may coordinate the development of clinical practice guidelines with other HHSC MCOs in a Service Area to avoid providers receiving conflicting practice guidelines from different MCOs.
The MCO must disseminate the practice guidelines to all affected Providers and, upon request, to Members and potential Members.
The MCO must take steps to encourage adoption of the guidelines, and to measure compliance with the guidelines, until such point that 90% or more of the Providers are consistently in compliance, based on MCO measurement findings. The MCO must employ substantive Provider motivational incentive strategies, such as financial and non-financial incentives, to improve Provider compliance with clinical practice guidelines. The MCO’s decisions regarding utilization management, Member education, coverage of services, and other areas included in the practice guidelines must be consistent with the MCO’s clinical practice guidelines.
8.1.7.7 Provider Profiling
The MCO must conduct PCP and other Provider profiling activities at least annually. As part of its QAPI Program, the MCO must describe the methodology it uses to identify which and how many Providers to profile and to identify measures to use for profiling such Providers.
Provider profiling activities must include, without limitation:
|
| |
| 1. developing PCP and Provider-specific reports that include a multi-dimensional assessment of a PCP or Provider’s performance using clinical, administrative, and Member satisfaction indicators of care that are accurate, measurable, and relevant to the enrolled population; |
|
| |
| 2. establishing PCP, Provider, group, Service Area or regional Benchmarks for areas profiled, where applicable, including STAR, STAR+PLUS, and CHIP Program-specific Benchmarks, where appropriate; and |
|
| |
| 3. providing feedback to individual PCPs and Providers regarding the results of their performance and the overall performance of the Provider Network. |
8.1.7.8 Network Management
The MCO must:
|
| |
| 1. use the results of its Provider profiling activities to identify areas of improvement for individual PCPs and Providers, and/or groups of Providers; |
|
| |
| 2. establish Provider-specific quality improvement goals for priority areas in which a Provider or Providers do not meet established MCO standards or improvement goals; |
|
| |
| 3. develop and implement incentives, which may include financial and non-financial incentives, to motivate Providers to improve performance on profiled measures; and |
|
| |
| 4. at least annually, measure and report to HHSC on the Provider Network and individual Providers’ progress, or lack of progress, towards such improvement goals. |
If the MCO implements a physician incentive plan, the plan must comply with the requirements of 42 C.F.R. §438.6(h), §422.208 and §422.210. The MCO cannot make payments under a physician incentive plan if the payments are designed to induce providers to reduce or limit Medically Necessary Covered Services to Members.
If the physician incentive plan places a physician or physician group at a substantial financial risk for services not provided by the physician or physician group, the MCO must ensure adequate stop-loss protection and conduct and submit annual Member surveys no later than five (5) Business Days after the MCO finalizes the survey results (refer to 42 C.F.R. §422.208 for information concerning “substantial financial risk” and “stop-loss protection”).
The MCO must make information regarding physician incentive plans available to Members upon request, in accordance with the Uniform Managed Care Manual’s requirements. The MCO must provide the following information to the Member:
|
| |
| 1. whether the Member’s PCP or other Providers are participating in the MCO’s physician incentive plan; |
|
| |
| 2. whether the MCO uses a physician incentive plan that affects the use of referral services; |
|
| |
| 3. the type of incentive arrangement; and |
|
| |
| 4. whether stop-loss protection is provided. |
No later than five (5) Business Days prior to implementing or modifying a physician incentive plan, the MCO must provide the following information to HHSC:
|
| |
| 1. Whether the physician incentive plan covers services that are not furnished by a physician or physician group. The MCO is only required to report on items 2-4 below if the physician incentive plan covers services that are not furnished by a physician or physician group. |
|
| |
| 2. The type of incentive arrangement (e.g., withhold, bonus, capitation); |
|
| |
| 3. The percent of withhold or bonus (if applicable); |
|
| |
| 4. The panel size, and if patients are pooled, the method used (HHSC approval is required for the method used); and |
If the physician or physician group is at substantial financial risk, the MCO must report proof that the physician or group has adequate stop-loss coverage, including the amount and type of stop-loss coverage.
8.1.7.9 Collaboration with the EQRO
The MCO will collaborate with HHSC’s external quality review organization (EQRO) to develop studies, surveys, or other analytical approaches that will be carried out by the EQRO. The purpose of the studies, surveys, or other analytical approaches is to assess the quality of care and service provided to Members and to identify opportunities for MCO improvement. To facilitate this process, the MCO will supply claims data to the EQRO in a format identified by HHSC in consultation with MCOs, and will supply medical records for focused clinical reviews conducted by the EQRO. The MCO must also work collaboratively with HHSC and the EQRO to annually measure selected HEDIS measures that require chart reviews. During the first year of operations, HHSC anticipates that the selected measures will include, at a minimum, well-child visits and immunizations, appropriate use of asthma medications, measures related to Members with diabetes, and control of high blood pressure.
|
|
8.1.8 Utilization Management |
The MCO must have a written utilization management (UM) program description, which includes, at a minimum:
1. procedures to evaluate the need for Medically Necessary Covered Services;
2. the clinical review criteria used, the information sources, the process used to review and approve the provision of Covered Services;
3. the method for periodically reviewing and amending the UM clinical review criteria; and
4. the staff position functionally responsible for the day-to-day management of the UM function.
The MCO must make best efforts to obtain all necessary information, including pertinent clinical information, and consult with the treating physician as appropriate in making UM determinations. When making UM determinations, the MCO must comply with the requirements of 42 C.F.R. §456.111 (Hospitals) and 42 CFR §456.211 (Mental Hospitals), as applicable.
The MCO must issue coverage determinations, including adverse determinations, according to the following timelines:
1. within three (3) Business Days after receipt of the request for authorization of services;
2. within one (1) Business Day for concurrent Hospitalization decisions; and
3. within one (1) hour for post-stabilization or life-threatening conditions, except that for Emergency Medical Conditions and Emergency Behavioral Health Conditions, the MCO must not require prior authorization.
The MCO's UM Program must include written policies and procedures to ensure:
1. consistent application of review criteria that are compatible with Members' needs and situations;
2. determinations to deny or limit services are made by physicians under the direction of the Medical Director;
3. at the HMO's discretion, pharmacy prior authorization determinations may be made by pharmacists, subject to the limitations described in Attachment A, Section 4.04, "Medical Director;"
4. appropriate personnel are available to respond to utilization review inquiries 8:00 a.m. to 5:00 p.m., Monday through Friday, with a telephone system capable of accepting utilization review inquiries after normal business hours. The MCO must respond to calls within one (1) Business Day;
5. confidentiality of clinical information; and
6. compensation to individuals or entities conducting UM activities is not structured to provide incentives for the individual or entity to deny, limit, or discontinue medically necessary services as required by 42 C.F.R. § 438.210(e), and quality is not adversely impacted by financial and reimbursement-related processes and decisions.
For MCOs with preauthorization or concurrent review programs, qualified medical professionals must supervise preauthorization and concurrent review decisions.
The MCO UM Program must include policies and procedures to:
1. routinely assess the effectiveness and the efficiency of the UM Program;
2. evaluate the appropriate use of medical technologies, including medical procedures, drugs and devices;
3. target areas of suspected inappropriate service utilization;
4. detect over- and under-utilization;
5. routinely generate Provider profiles regarding utilization patterns and compliance with utilization review criteria and policies;
6. compare Member and Provider utilization with norms for comparable individuals;
7. routinely monitor inpatient admissions, emergency room use, ancillary, and out-of-area services;
8. ensure that when Members are receiving Behavioral Health Services from the Local Mental Health Authority, the MCO is using the same UM guidelines as those prescribed for use by Local Mental Health Authorities by MHMR which are published at: http://www.dshs.state.tx.us/MHSA/UMGUIDELINES/; and
9. refer suspected cases of Network Provider, Out-of-Network provider, or Member Fraud, Abuse, or Waste to the Office of Inspector General (OIG) as required by Section 8.1.19 .
8.1.8.1 Compliance with State and Federal Prior Authorization Requirements
The MCO must adopt prior authorization (PA) requirements that comply with state and federal laws governing authorization of health care services and prescription drug benefits, including 42 U.S.C. § 1396r-8 and Texas Government Code §§ 531.073 and 533.005(a)(23). In addition, the MCO must comply with Texas Human Resources Code § 32.073 and Texas Insurance Code §§ 1217.004 and 1369.256, which require MCOs to use national standards for electronic prior authorization of prescription drug and health care benefits no later than two years after adoption, and accept PA requests submitted using the Texas Department of Insurance's (TDI's) standard form, once adopted.
|
|
8.1.9 Early Childhood Intervention (ECI) |
The MCO must ensure Network Providers are educated regarding the federal laws on child find and referral procedures (e.g., 20 U.S.C. § 1435 (a)(5); 34 C.F.R. § 303.303). The MCO must require Network Providers to identify and refer any Member under the age of three suspected of having a developmental delay or disability or otherwise meeting eligibility criteria for ECI services in accordance with 40 Tex. Admin. Code Chapter 108 to the designated ECI program for screening and assessment within seven calendar days from the day the Provider identifies the Member. The MCO must use written educational materials developed or approved by the Department of Assistive and Rehabilitative Services- Division for Early Childhood Intervention Services for these child find activities. The local ECI program will determine eligibility for ECI services using the criteria contained in 40 Tex. Admin. Code Chapter 108.
ECI Providers must submit claims for all physical, occupational, speech, and language therapy to the MCO.
ECI Targeted Case Management services and Early Childhood Intervention Specialized Skills Training are Non-capitated Services, as described in Section 8.2.2.8.
The MCO must contract with qualified ECI Providers to provide ECI Covered Services to Members under the age of three who are eligible for ECI services. The MCO must permit Members to self-refer to local ECI Service Providers without requiring a referral from the Member's PCP. The MCO's policies and procedures, including its Provider Manual, must include written policies and procedures for allowing a self-referral to ECI providers.
The MCO will implement the Individual Family Service Plan (IFSP) and other services, including ongoing case management and other Covered Services required by the Member's IFSP. Ongoing case management does not include ECI Targeted Case Management services. The IFSP is an agreement developed by the interdisciplinary team that consists of the MCO, ECI Case
Manager/Service Coordinator, the Member/family, and other professionals who participated in the Member's evaluation or are providing direct services to the Member. The interdisciplinary team may include the Member's Primary Care Physician (PCP) with parental consent. The IFSP identifies the Member's present level of development based on assessment, describes the services to be provided to the child to meet the needs of the child and the family, and identifies the person or persons responsible for each service required by the plan. The IFSP must be maintained by the MCO and, with parental consent, provided to the PCP to enhance coordination of the plan of care. The IFSP may be included in the Member's medical record.
The ECI program includes covering medical diagnostic procedures and providing medical records required to perform developmental assessments and developing the IFSP within the 45-day timeline established in federal rule (34 C.F.R. §303.342(a)). The MCO must require compliance with these requirements through Provider contract provisions. The MCO must not withhold authorization for the provision of such medical diagnostic procedures. The MCO must promptly provide relevant medical records available as needed.
The MCO must require, through contract provisions, that all Medically Necessary health and Behavioral Health Services contained in the Member's IFSP are provided to the Member in the amount, duration, scope and service setting established by the IFSP. The MCO must allow services to be provided by an Out-of-Network provider if a Network Provider is not available to provide the services in the amount, duration, scope and service setting as required by the IFSP. The IFSP will serve as authorization for services and the MCO cannot create unnecessary barriers for the Member to obtain IFSP services, including requiring prior authorization for the ECI assessment or additional authorization for services. For STAR Members in the Dallas Service Area, Behavioral Health Services will be provided through NorthSTAR and will not be included on the IFSP.
|
|
8.1.10 Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) - Specific Requirements |
The MCO must, by contract, require its Providers to coordinate with the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) to provide medical information necessary for WIC eligibility determinations, such as height, weight, hematocrit or hemoglobin. The MCO must make referrals to WIC for Members who are potentially eligible for WIC. The MCO may use the nutrition education provided by WIC to satisfy certain health education requirements of the Contract.
|
|
8.1.11 Coordination with Texas Department of Family and Protective Services |
The MCO must cooperate and coordinate with the Texas Department of Family and Protective Services (TDFPS) for the care of a child who is receiving services from or has been placed in the conservatorship of TDFPS.
The MCO must comply with all provisions related to Covered Services, including Behavioral Health Services, in the following documents:
|
| |
| 1. a court order (Order) entered by a Court of Continuing Jurisdiction placing a child under the protective custody of TDFPS; |
|
| |
| 2. a TDFPS Service Plan entered by a Court of Continuing Jurisdiction placing a child under the protective custody of TDFPS; and |
|
| |
| 3. a TDFPS Service Plan voluntarily entered into by the parents or person having legal custody of a Member and TDFPS. |
The MCO cannot deny, reduce, or controvert the Medical Necessity of any health or Behavioral Health Services included in the above-referenced Orders of TDFPS Service Plans. The MCO may participate in the preparation of the medical and behavioral care plan prior to TDFPS submitting the health care plan to the Court. Any modification or termination of court-ordered services must be presented and approved by the court having jurisdiction over the matter.
A Member or the parent or guardian whose rights are subject to an Order or TDFPS Service Plan cannot use the MCO’s Complaint or Appeal processes, or the HHSC Fair Hearing process to Appeal the necessity of the Covered Services.
The MCO must include information in its Provider Manuals and training materials regarding:
|
| |
| 1. providing medical records to TDFPS; |
|
| |
| 2. scheduling medical and Behavioral Health Services appointments within 14 days unless requested earlier by TDFPS; and |
|
| |
| 3. recognition of abuse and neglect, and appropriate referral to TDFPS. |
The MCO must continue to provide all Covered Services to a Member receiving services from, or in the protective custody of, TDFPS until the Member has been (1) disenrolled from the MCO due to loss of Medicaid managed care eligibility; or (2) enrolled in STAR Health, HHSC’s managed care program for children in foster care.
|
|
8.1.12 Services for People with Special Health Care Needs |
8.1.12.1 Identification
The MCO must develop and maintain a system and procedures for identifying Members with Special Health Care Needs (MSHCN).
The MCO must contact Members pre-screened by the HHSC Administrative Services Contractor as MSHCN to determine whether they meet the MCO’s MSHCN assessment criteria, and to determine whether the Member requires special services described in this section. The MCO must implement mechanisms to assess each Member that has been prescreened by the Administrative Services Contractor, or identified by the MCO as having special health care needs, in order to identify ongoing special conditions requiring a course of treatment or regular care monitoring. The MCO’s assessment mechanisms must use appropriate health care professionals.
The MCO must provide information to the HHSC Administrative Services Contractor that identifies Members who the MCO has assessed to be MSHCN, including any Members pre-screened by the HHSC Administrative Services Contractor and confirmed by the MCO as a MSHCN. The information must be provided in a format and on a timeline as determined by HHSC. The information must be updated with newly identified MSHCN by the 10th day of each month. In the event that a MSHCN changes MCOs, the MCO must provide the receiving MCO information concerning the results of the MCO’s identification and assessment of that Member’s needs to prevent duplication of those activities.
8.1.12.2 Access to Care and Service Management
Once identified, the MCO must have effective systems to ensure the provision of Covered Services to meet the special preventive, primary Acute Care, and specialty health care needs appropriate for treatment of a Member’s condition(s). All STAR+PLUS and Former Foster Care Child (FFCC) Members are considered MSHCN.
The MCO must provide access to identified PCPs and specialty care Providers with experience serving MSHCN. Such Providers must be board-qualified or board-eligible in their specialty. The MCO may request exceptions from HHSC for approval of traditional providers who are not board-qualified or board-eligible but who otherwise meet the MCO’s credentialing requirements.
The MCO must have Network PCPs and specialty care Providers that have demonstrated experience with children who have special health needs in pediatric
specialty centers such as children’s Hospitals, teaching Hospitals, and tertiary care centers.
The MCO is responsible for working with MSHCN, their health care providers, their families and, if applicable, legal guardians to develop a seamless package of care in which primary, Acute Care, and specialty service needs are met through a Service Plan that is understandable to the Member, and his or her representatives.
The Service Plan includes, but is not limited to, the following:
1. the Member’s history;
2. summary of current medical and social needs and concerns;
3. short and long term needs and goals;
4. a list of services required, their frequency, and
5. a description of who will provide the services.
The Service Plan should incorporate as a component of the plan the Individual Family Service Plan (IFSP) for members in the Early Childhood Intervention (ECI) Program. The Service Plan may include information regarding non-covered services, such as Non-Capitated Services (see below), community and other resources, and information on how to access affordable, integrated housing.
The MCO is responsible for providing Service Management, developing a Service Plan, and ensuring MSHCN have access to treatment by a multidisciplinary team when the Member’s PCP determines the treatment is Medically Necessary, or to avoid separate and fragmented evaluations and service plans. The team must include both physician and non-physician providers that the PCP determines are necessary for the comprehensive treatment of the Member. The team must:
1. participate in Hospital discharge planning;
2. participate in pre-admission Hospital planning for non-emergency Hospitalizations;
3. develop specialty care and support service recommendations to be incorporated into the Service Plan; and
4. provide information to the Member, or when applicable, the Member’s representatives concerning the specialty care recommendations.
MSHCN, their families, legal guardians, or their health providers may request Service Management from the MCO. The MCO must make an assessment of whether Service Management is needed and furnish Service Management when appropriate. The MCO may also recommend to MSHCNs or their families or legal guardians that Service Management be furnished if the MCO determines that Service Management would benefit the Member.
The MCO must provide information and education in its Member Handbook and Provider Manual about the care and treatment available in the MCO’s plan for Members with Special Health Care Needs, including the availability of Service Management.
The MCO must have a mechanism in place to allow Members with Special Health Care Needs to have direct access to a specialist as appropriate for the Member’s condition and identified needs, such as a standing referral to a specialty physician.
The MCO must also provide MSHCN with access to non-primary care physician specialists as PCPs, as required by 28 T.A.C. §11.900, and Section 8.1.4.2, Primary Care Providers.
The MCO must implement a systematic process to coordinate Non-capitated Services, and enlist the involvement of community organizations that may not be providing Covered Services but are otherwise important to the health and wellbeing of Members. The MCO also must make a best effort to establish relationships with State and local programs and community organizations, such as those listed below, in order to make referrals for MSHCN and other Members who need community services:
1. Community Resource Coordination Groups (CRCGs);
2. Early Childhood Intervention (ECI) Program;
3. local school districts (Special Education);
4. Health and Human Services Commission’s Medical Transportation Program (MTP);
5. Texas Department of Assistive and Rehabilitative Services (DARS) Blind Children’s Vocational Discovery and Development Program;
6. Texas Department of State Health (DSHS) services, including Title V Maternal and Child Health, Children with Special Health Care Needs (CSHCN) Programs;
7. other state and local agencies and programs such as food stamps, and the Women, Infants, and Children’s (WIC) Program, and Case Management for Children and Pregnant Women;
8. family planning programs including the Texas Women's Health Program and DSHS Family Planning, Primary Health Care, and Expanded Primary Health Care programs; and
8. civic and religious organizations and consumer and advocacy groups, such as United Cerebral Palsy, which also work on behalf of the MSHCN population.
|
|
8.1.13 Service Management for Certain Populations |
The MCO must have service management programs and procedures for the following populations, as applicable to the MCO:
1. high-cost catastrophic cases;
2. women with high-risk pregnancies (STAR and STAR+PLUS Programs only);
3. individuals with mental illness and co-occurring substance abuse;
4. Farmworker Children (FWC) (STAR and STAR+PLUS Programs only); and
5. Former Foster Care Child (FFCC) Members (STAR Program only).
|
|
8.1.14 Disease Management (DM) |
The MCO must provide or arrange the provision of comprehensive disease management (DM) programs consistent with state and federal statutes and regulations. The program design of these DM programs must focus on the whole person, typically high-risk enrollees with complex chronic or co-morbid conditions rather than traditionally-designed programs with restricted diagnoses or disease silos. These programs must identify enrollees at highest risk of utilization of medical services, tailor interventions to better meet enrollees' needs, encourage provider input in care plan development, and apply clinical evidence-based practice protocols for individualized care.
MCOs must focus their DM programs on 3 main components:
• client self-management;
• provider practice/delivery system design; and
• technological support.
Under client self-management, a client becomes an informed and active participant in the management of physical and mental health conditions and co-morbidities. Under the provider practice/delivery system design approach, medical home providers take an active role in helping their patients make informed healthcare decisions. Technology, such as the use of predictive modeling, helps identify potential program patients and providers.
8.1.14.1 Special Populations
The MCO is also required to have a specialized program for targeting, outreach, education and intervention for Members who have excessive utilization patterns that indicate typical DM approaches are not effective. For the purposes of this contract, this group of Members is called "super-utilizers." The MCO must have the following infrastructure in place to address super-utilizers' needs, using, at a minimum, the following criteria.
1. Methodology for identification of super-utilizers on an ongoing basis, based on cost, utilization of the ER, utilization of inpatient or pharmacy, services, physical and behavioral health comorbidities, or other specified basis.
2. Resources dedicated to ongoing targeting and identification of super-utilizers such as staff, specialized analytical tools, etc.
3. Staff resources for effective outreach and education of Providers and super-utilizers.
4. Specialized intervention strategies for super-utilizers. The interventions must include an option for in-person interactions with the Member that occur outside of a standard clinical setting. This in-person intervention may be performed by medical care providers or other non-medical providers that are employed by the MCO or are subcontracted with the MCO.
5. Evaluation process to determine effectiveness of super-utilizer program. As part of the annual evaluation of effectiveness, the MCO should include a description or example of an intervention it found effective. It can be a member case study with a description of the interventions and improvements or a specific project with demonstrated effectiveness.
On or before November 1, 2014, the MCO must provide its plan for management of super-utilizers including the criteria listed above using UMCM Chapter 9.4, "Plan for Special Populations Program." HHSC will evaluate the plan and provide feedback to the MCO. Upon HHSC's approval of the plan, each MCO will be retrospectively evaluated on their execution of the written plan, as described in 8.1.14.3. An MCO may reuse elements of the same plan from year to year as long as submission reflects the current state of their special population program and is updated as necessary on evaluation methodologies and key findings. HHSC may request updates to the plan during the state fiscal year as part of ongoing quality improvement efforts.
The disease management requirements do not apply to CHIP Perinate Members.
8.1.14.2 DM and Participating Providers
At a minimum, the MCO must:
1. implement a system for Providers to request specific DM interventions;
2. give Providers information, including differences between recommended prevention and treatment and actual care received by Members enrolled in a DM program, and information concerning such Members' adherence to a service plan; and
3. for Members enrolled in a DM program, provide reports on changes in a Member's health status to his or her PCP.
8.1.14.3 MCO DM Evaluation
HHSC or its EQRO will evaluate the MCO's DM program.
HHSC or its EQRO will also evaluate DM as it relates to specialized populations identified in 8.1.14.1. These evaluations will be on a retrospective basis, and will include an analysis of MCO Encounter Data and other relevant data (e.g., reports). Evaluations could also include interviews with MCO staff that oversee the program as well as identified Providers. Based on HHSC's retrospective evaluation, MCOs may be required to submit a Corrective Action Plan if directed by HHSC.
It is HHSC's intent to hold quarterly collaborative calls or webinars with MCO medical directors to discuss plan implementation, barriers, successful strategies, etc.
|
|
8.1.15 Behavioral Health (BH) Network and Services |
The requirements in this subsection pertain to all MCOs except: (1) the STAR MCOs in the Dallas Service Area, whose Members receive Behavioral Health Services through the NorthSTAR Program, and (2) the CHIP Perinatal Program MCOs with respect to their Perinate Members (unborn children).
The MCO must provide, or arrange to have provided, to Members all Medically Necessary Behavioral Health (BH) Services as described in Attachments B-2, "STAR Covered Services," B-2.1, "CHIP Covered Services," and B-2.2, "STAR+PLUS Covered Services," All BH Services must comply with the access standards included in Section 8.1.3. For Medicaid MCOs, BH Services are described in more detail in the Texas Medicaid Provider Procedures Manual. When assessing Members for BH Services, the MCO and its Network Behavioral Health Service Providers must use the DSM multi-axial classification in effect at the time of service. HHSC may require use of other assessment instrument/outcome measures in addition to the DSM. Providers must document DSM and assessment/outcome information in the Member's medical record.
8.1.15.1 BH Provider Network
The MCO must maintain a Behavioral Health Services Provider Network that includes psychiatrists, psychologists, and other Behavioral Health Service Providers. To ensure accessibility and availability of qualified Providers to all Members in the Service Area, the Provider Network must include Behavioral Health Service Providers with experience serving special populations among the MCO Program(s)’ enrolled population, including, as applicable, children and adolescents, persons with disabilities, the elderly, and cultural or linguistic minorities.
8.1.15.2 Member Education and Self-referral for Behavioral Health Services
The MCO must maintain a Member education process to help Members know where and how to obtain Behavioral Health Services.
The MCO must permit Members to self refer to any Network Behavioral Health Services Provider without a referral from the Member’s PCP. The MCOs’ policies and procedures, including its Provider Manual, must include written policies and procedures for allowing such self-referral to Behavioral Health Services.
The MCO must permit Members to participate in the selection of the appropriate behavioral health providers, and must provide the Member with information on accessible Network Providers with relevant experience.
8.1.15.3 Behavioral Health Services Hotline
This Section includes Member Hotline requirements. Requirements for Provider Hotlines are found in Section 8.1.4.7.
The MCO must have an emergency and crisis Behavioral Health Services Hotline staffed by trained personnel 24 hours a day, seven (7) days a week, toll-free throughout the Service Area. Crisis hotline staff must include or have access to qualified Behavioral Health Services professionals to assess Behavioral Health emergencies. Emergency and crisis Behavioral Health Services may be arranged through mobile crisis teams. It is not acceptable for an emergency intake line to be answered by an answering machine.
The MCO must operate a toll-free hotline as described in Section 8.1.5.6 to handle Behavioral Health-related calls. The MCO may operate one hotline to handle emergency and crisis calls and routine Member calls. The MCO cannot impose maximum call duration limits and must allow calls to be of sufficient length to ensure adequate information is provided to the Member. Hotline services must meet Cultural Competency requirements and provide linguistic access to all Members, including the interpretive services required for effective communication.
The Behavioral Health Services Hotline may serve multiple MCO Programs if the Hotline staff is knowledgeable about all of the MCO Programs. The Behavioral Health Services Hotline may serve multiple Service Areas if the Hotline staff is knowledgeable about all such Service Areas, including the Behavioral Health Provider Network in each Service Area. The MCO must ensure that the toll-free Behavioral Health Services Hotline meets the following minimum performance requirements for all MCO Programs and Service Areas:
|
| |
| 1. 99% of calls are answered by the fourth ring or an automated call pick-up system; |
|
| |
| 2. no incoming calls receive a busy signal; |
|
| |
| 3. at least 80% of calls must be answered by toll-free line staff within 30 seconds measured from the time the call is placed in queue after selecting an option; |
|
| |
| 4. the call abandonment rate is seven percent (7%) or less; and |
|
| |
| 5. the average hold time is two (2) minutes or less. |
The MCO must conduct ongoing quality assurance to ensure these standards are met.
The MCO must monitor the MCO’s performance against the Behavioral Health Services Hotline standards and submit performance reports summarizing call center performance as indicated in Section 8.1.20 and the Uniform Managed Care Manual.
As a component of quality monitoring, HHSC may require the MCO to implement a system where callers are given the option of participating in an automated survey at the end of a call.
If HHSC determines that it is necessary to conduct onsite monitoring of the MCO’s Behavioral Health Services Hotline functions, the MCO is responsible for all reasonable travel costs incurred by HHSC or its authorized agent(s) relating to such monitoring. For purposes of this section, “reasonable travel costs” include airfare, lodging, meals, car rental and fuel, taxi, mileage, parking and other incidental travel expenses incurred by HHSC or its authorized agent in connection with the onsite monitoring.
8.1.15.4 Coordination between the BH Provider and the PCP
The MCO must require, through Provider contract provisions, that PCPs have screening and evaluation procedures for the detection and treatment of, or referral for, any known or suspected Behavioral Health problems and disorders. PCPs may provide any clinically appropriate Behavioral Health Services within the scope of their practice.
The MCO must provide training to Network PCPs on how to screen for and identify behavioral health disorders, the MCO’s referral process for Behavioral Health Services, and clinical coordination requirements for such services. The MCO must include training on coordination and quality of care such as behavioral health screening techniques for PCPs and new models of behavioral health interventions.
The MCO must develop and disseminate policies regarding clinical coordination between Behavioral Health Service Providers and PCPs. The MCO must require that Behavioral Health Service Providers refer Members with known or suspected and untreated physical health problems or disorders to their PCP for examination and treatment, with the Member’s or the Member’s legal guardian’s consent. Behavioral Health Providers may only provide physical Health Care Services if they are licensed to do so. This requirement must be specified in all Provider Manuals.
The MCO must require that behavioral health Providers send initial and quarterly (or more frequently if clinically indicated) summary reports of a Members’ behavioral health status to the PCP, with the Member’s or the Member’s legal guardian’s consent. This requirement must be specified in all Provider Manuals.
8.1.15.5 Follow-up after Hospitalization for Behavioral Health Services
The MCO must require, through Provider contract provisions, that all Members receiving inpatient psychiatric services are scheduled for outpatient follow-up and/or continuing treatment prior to discharge. The outpatient treatment must occur within seven (7) days from the date of discharge. The MCO must ensure that Behavioral Health Service Providers contact Members who have missed appointments within 24 hours to reschedule appointments.
8.1.15.6 Chemical Dependency
The MCO must comply with 28 T.A.C. §3.8001 et seq., regarding utilization review for Chemical Dependency Treatment. Chemical Dependency Treatment must comply with the standards set forth in 28 T.A.C. Part 1, Chapter 3, Subchapter HH.
8.1.15.7 Court-Ordered Services
The MCO must provide inpatient psychiatric services to Members birth through age 20, up to the annual limit, who have been ordered to receive the services by a court of competent jurisdiction under Texas Health and Safety Code Chapters 573 and 574, relating to Court-Ordered Commitments to inpatient mental health facilities. The MCO is not obligated to cover placements as a condition of probation, authorized by the Texas Family Code. These placements are Non-capitated services.
The MCO cannot deny, reduce, or controvert the Medical Necessity of inpatient mental health services provided pursuant to a Court-ordered Commitment for Members birth through age 20. Any modification or termination of services must be presented to the court with jurisdiction over the matter for determination.
A Member who has been ordered to receive treatment under Texas Health and Safety Code Chapter 573 or 574 can only Appeal the commitment through the court system.
8.1.15.8 Local Mental Health Authority (LMHA)
The MCO must coordinate with the Local Mental Health Authority (LMHA) and state psychiatric facility regarding admission and discharge planning, treatment objectives and projected length of stay for Members committed by a court of law to the state psychiatric facility.
|
|
8.1.16 Financial Requirements for Covered Services |
The MCO must pay for or reimburse Providers for all Medically Necessary Covered Services provided to all Members. STAR+PLUS MCOs must also provide Functionally Necessary Community Long-term Services and Supports to Members. The MCO is not liable for cost incurred in connection with health care rendered prior to the date of the Member’s Effective Date of Coverage in that MCO.
Coverage under Medicaid and CHIP is secondary to all other insurance coverage. A Member may receive collateral health benefits under a different type of insurance such as workers compensation or personal injury protection under an automobile policy. If a Member is entitled to coverage for specific services payable under another insurance plan and the MCO paid for such Covered Services, the MCO may obtain reimbursement from the responsible insurance entity not to exceed 100% of the value of Covered Services paid. See Sections 8.2.9 and 8.4.5 for additional information regarding coordination of benefits and recoveries from third parties.
|
|
8.1.17 Accounting and Financial Reporting Requirements |
The MCO’s accounting records and supporting information related to all aspects of the Contract must be accumulated in accordance with Federal Acquisition Regulations (“FAR”), Generally Accepted Accounting Principles (GAAP), Attachment A, "Uniform Managed Care Contract Terms and Conditions," and the cost principles contained in the Cost Principles Document in Uniform Managed Care Manual Chapter 6.1. HHSC will not recognize or pay services that cannot be properly substantiated by the MCO and verified by HHSC.
The MCO must:
|
| |
| 1. maintain accounting records for each applicable MCO Program separate and apart from other corporate accounting records; |
|
| |
| 2. maintain records for all claims payments, refunds and adjustment payments to providers, Capitation Payments, interest income and payments for administrative services or functions and must maintain separate records for medical and administrative fees, charges, and payments; |
|
| |
| 3. ensure and provide access to HHSC and/or its auditors or agents to the detailed records and supporting documentation for all costs incurred by the MCO. The MCO must ensure such access to its Subcontractors, including Affiliates, for any costs billed to or passed to the MCO with respect to an MCO Program; |
|
| |
| 4. maintain an accounting system that provides an audit trail containing sufficient financial documentation to allow for the reconciliation of billings, reports, and financial statements with all general ledger accounts; and |
The MCO agrees to pay for all reasonable costs incurred by HHSC to perform an examination, review or audit of the MCO’s books relating to this Contract.
8.1.17.1 Financial Reporting Requirements
HHSC will require the MCO to provide financial reports by MCO Program and by Service Area to support Contract monitoring as well as State and Federal reporting requirements. All financial information and reports submitted by the MCO become the property of HHSC. HHSC may, at its discretion, release such information and reports to the public at any time and without notice to the MCO. In accordance with state and federal laws regarding Member confidentiality, HHSC will not release any Member-identifying information contained in such reports.
CHIP Perinatal Program data will be integrated into the CHIP Program financial reports. Except for the Financial Statistical Report, no separate CHIP Perinatal Program reports are required. For all other CHIP financial reports, where appropriate, HHSC will designate specific attributes within the CHIP Program financial reports that CHIP MCOs must complete to allow HHSC to extract financial data particular to the CHIP Perinatal Program.
Any data submitted with respect to the required financial reports or filings that is in PDF (or similar file format such as TIF) must be generated in a text-searchable format.
Due dates, content, and formats for the following deliverables and reports may be referenced herein or in Uniform Managed Care Manual Chapter 5.0 “Consolidated Deliverables Matrix.”
(a) Financial-Statistical Report (FSR) – The MCO must file four (4) quarterly and two (2) annual Financial-Statistical Reports (FSR) for each complete State Fiscal Year, in the format and timeframe specified by HHSC. HHSC will include FSR format and directions in Uniform Managed Care Manual Chapter 5.3.1. The MCO must incorporate financial and statistical data of delegated networks (e.g., IPAs, ANHCs, Limited Provider Networks), if any, in its FSR Reports. The FSR is one (1) of the primary financial reports used by HHSC to monitor Contract financial results. It is a modified (HHSC-defined) form of an income statement, with some other elements added. Not all expenses incurred may be included on the FSR.
All amounts reported in the FSRs must be reported in accordance with Uniform Managed Care Manual Chapter 6.1, “Cost Principles for Expenses.” Each FSR must provide amounts by month, with a year-to-date total (based on the SFY, or other Contract period as designated by HHSC). Each successive FSR will show the most current amounts for each month in the SFY; thus, a given month’s amount may change in future FSRs as more claims run-out is experienced for the month. Quarterly FSRs are generally due 30 days after the end of each State Fiscal Quarter. The MCO must transmit these reports electronically, in a locked MS Excel file.
After the 4 th Quarter FSR, the first annual FSR for a given SFY (the “90-day FSR”) must reflect claims run-out and accruals through the 90 th calendar day after the end of the Contract Year. This report must be filed on or before the 120 th calendar day after the end of the Contract Period. If the MCO has made a pre-tax profit in excess of the thresholds as established in the Contract with respect to the Experience Rebate, then a payment for any amounts to be refunded to HHSC is due in conjunction with filing the 90-day FSR. The second annual report for a given SFY (the “334-day FSR”) must reflect data completed through the 334 th calendar day after the end of the Contract Period, and must be filed on or before the 365 th calendar day following the end of the Contract Period. The 334-day FSR is routinely audited by HHSC and/or its independent auditors.
HHSC will post all or part of an FSR on the HHSC website.
As set forth above, CHIP MCOs are required to submit separate FSRs for the CHIP Perinatal Program, in accordance with Uniform Managed Care Manual Chapters 5.3.1.7 and 5.3.1.8.
(b) Delivery Supplemental Payment (DSP) Report - The MCO must submit a monthly DSP Report in accordance with Uniform Managed Care Manual Chapter 5.3.5. The Report must include only unduplicated deliveries and only deliveries for which the MCO has made a payment to either a Hospital or other provider.
(c) Claims Lag Report - The MCO must submit a Claims Lag Report on a quarterly basis, by the last day of the month following the reporting period. The report must disclose the amount of incurred claims each month and the amount paid each month, on a contract-to-date basis. The report must be submitted in accordance with Uniform Managed Care Manual Chapter 5.6.2.
(d) Third Party Liability and Recovery (TPL/TPR) Report – The MCO must file TPL/TPR Reports in accordance with Uniform Managed Care Manual Chapter 5.3.4. MCOs must submit TPL/TPR reports quarterly, by MCO Program and Service Area. TPL/TPR reports must include total dollars costs avoided, and total dollars recovered from third party payers through the MCO’s coordination of benefits and subrogation efforts during the Quarter.
(e) Report of Legal and Other Proceedings and Related Events - The MCO must comply with the Uniform Managed Care Manual Chapter 5.8, regarding the disclosure of certain matters involving either the MCO, its Affiliates, and/or its Material Subcontractors. Reports are due both on an as-occurs basis and annually each August 31st. The as-occurs report is due no later than 30 days after the event that triggered the notification requirement.
(f) Audit Reports - The MCO must comply with the Uniform Managed Care Manual Chapter 5.3.11 regarding notification and/or submission of certain internal and external audit reports.
(g) Affiliate Report – The MCO must submit an Affiliate Report on an as-occurs basis and annually by August 31st of each year in accordance with the Uniform Managed Care Manual. The “as-occurs” update is due within 30 days of the event that triggered the change. Note that “Affiliate” is a defined term (see Attachment A, "Uniform Managed Care Contract Terms and Conditions").
(h) MCO Disclosure Statement - The MCO must file:
1. an updated MCO Disclosure Statement by September 1st of each Contract Year;
and
2. a “change notification” abbreviated version of the report, no later than 30 days
after any of the following events:
a. entering into, renewing, modifying, or terminating a relationship with an
affiliated party;
b. after any change in control, ownership, or affiliations; or,
c. after any material change in, or need for addition to, the information
previously disclosed.
The MCO Disclosure Statement will include, at a minimum, a listing of the MCO’s control, ownership, and any affiliations, and information regarding Affiliate transactions. This report will replace, and be in lieu of, the former “Section 1318 Financial Disclosure Report” and the “Form CMS 1513,” and will disclose the same information, plus other information as may be required by HHSC and/or CMS Program Integrity requirements. Minor quarterly adjustments in stock holdings for publicly-traded corporations are excluded from the reporting requirements. The reporting format is included in the Uniform Managed Care Manual.
(i) TDI Filings – The MCO must provide HHSC with a copy of the following information no later than 30 calendar days after the MCO’s submission to TDI:
|
| |
| 1. the “Health Annual Statement” and the “Annual Audited Financial Report” including all schedules, attachments, exhibits, supplements, management discussion, supplemental filings, etc., and any other annual financial filings (including any filings that may take the place of the above-named annual financial filings, and any financial filings that occur less frequently than on a quarterly basis); |
|
| |
| 2. the annual figures for controlled risk-based capital; and |
|
| |
| 3. the quarterly financial statements. |
Additionally, if the MCO is a foreign carrier (i.e., domiciled in another state), copies of any filings with the National Association of Insurance Commissioners (NAIC), as well as the financial statements filed with the state insurance department in its state of domicile, must be submitted to HHSC no later than 30 calendar days after submission to NAIC or the state of domicile.
Notwithstanding the 30 calendar day deadlines described above, the MCO must notify HHSC if it cannot provide the most recent Annual Statements by March 31st each year, and the Annual Audited Financial Report by June 30th each year. The notice should include an expected submission date.
(j) Registration Statement (also known as the “Form B”) –
With the following exceptions, MCOs must submit a complete state insurance department registration statement, also known as Form B, and all annual and other amendments to this form, and any other related or similar information filed by the MCO with the insurance regulatory authority of its domiciliary jurisdiction. The exceptions to this requirement are those MCOs that are either (i) part of a County Hospital District or other governmental entity, or (ii) a stand-alone entity with no parent or other Affiliates. If the MCO is excepted from the TDI Form B filing requirement, the MCO must demonstrate this and explain the nature of the exemption.
The Form B is filed in three (3) forms: (i) the initial registration; (ii) the annual amendment; and (iii) the every-five-years complete restatement of registration. For purposes herein, the MCO must submit:
|
| |
| 1. the complete registration restatement that was due to TDI by approximately May 2010; |
|
| |
| 2. each annual registration amendment form (which is due to TDI within 120 days of the end of the MCO’s parent’s fiscal year), commencing with the most recent one that the MCO has filed after May 2010; |
|
| |
| 3. future complete five-year registration re-statements (the first of which will be due to TDI by approximately May 2015); and |
|
| |
| 4. any other registration statement amendments or re-statements that may be submitted to TDI, per TDI regulations. |
If the MCO was not yet subject to TDI requirements with respect to the May 2010 registration re-statement, it must submit its initial registration
If the MCO anticipates that the registration statement annual amendment form will be filed at some other date than approximately 120 days after the end of the parent’s fiscal year, then the MCO must notify HHSC of the anticipated filing date.
All registration statement submission items herein are due to HHSC by the later of: (i) 30 calendar days after the MCO’s submission of the item to TDI, or (ii) the date identified in this section.
(k) TDI Examination Report - The MCO must furnish HHSC with a full and complete copy of any examination report issued by TDI, including the financial, market conduct, target exam, quality of care components, and corrective action plans and responses. The MCO must submit this information to HHSC no later than 30 calendar days after the MCO receives the final version of the examination report from TDI.
The MCO must furnish HHSC with a copy of any similar examination report issued by a state insurance department in any other states where the MCO operates a Medicaid, CHIP, or other managed care product. These reports are also due no later than 30 calendar days after the MCO receives the final version of the examination report.
Each September 1 st , the MCO must notify HHSC of the anticipated date of the next issuance of a state department of insurance financial examination report, unless the last submitted financial examination report is less than two (2) years old. This annual notification should include a list of any other states in which the MCO is potentially subject to such examination reports, or a statement that there are no other states.
(l) Employee Bonus and/or Incentive Payment Plan – If a MCO intends to include Employee Bonus or Incentive Payments as allowable administrative expenses, the MCO must furnish a written Employee Bonus and/or Incentive Payments Plan to HHSC. The written plan must include a description of the MCO’s criteria for establishing bonus and/or incentive payments, the methodology to calculate bonus and/or incentive payments, and the timing of bonus and/or incentive payments. The Bonus and/or Incentive Payment Plan and description must be submitted during the Transition Phase, no later than 30 days after the Effective Date of the Contract. If the MCO substantively revises the Employee Bonus and/or Incentive Payment Plan, the MCO must submit the revised plan to HHSC at least 30 days in advance of its effective date.
HHSC reserves the right to disallow all or part of a plan that it deems inappropriate. Any such payments are subject to audit, and must comply with Uniform Managed Care Manual Chapter 6.1, “Cost Principles for Expenses.”
(m) Filings with other entities, and other existing financial reports – The MCO must submit an electronic copy of the following reports or filings pertaining to the MCO, or its parent, or its parent’s parent:
|
| |
| 1. SEC Form 10-K. For publicly-traded (stock-exchange-listed) for-profit corporations, submit the most-recent annual SEC Form 10K filing. |
|
| |
| 2. IRS Form 990. For nonprofit entities, submit the most recent annual IRS Form 990 filing, complete with any and all attachments or schedules. If a nonprofit entity is exempt from the IRS 990 filing requirement, demonstrate this and explain the nature of the exemption. |
|
| |
| 3. If the MCO is a nonprofit entity that is a component or subsidiary of a County Hospital District, or otherwise an entity of a government, then submit the annual financial statements as prepared under the relevant rules or statutes governing annual financial reporting and disclosure for the MCO and/or its parent, including all attachments, schedules, and supplements. |
|
| |
| 4. Annual Report. The MCO must submit this report if it is different than or supplementary to the audited financial statements or Form 10-K required herein, and if it is distributed to either shareholders, customers, employees, owner(s), parent, bank or creditor(s), donors, the community, or to any regulatory body or constituents, or is otherwise externally distributed or posted. |
|
| |
| 5. Bond or debt rating analysis. If the MCO or its ultimate parent has been the subject of any bond rating analysis, ratings affirmation, write-up, or related report, such as by AM Best, Fitch Ratings, Moody’s, Standard & Poor, etc., submit the most recent complete detailed report from each rating entity that has produced such a report. |
All of the above such reports or filings are due to HHSC no later than 30 calendar days after such report is filed or otherwise initially distributed. Each report should include all exhibits, attachments, notes, supplemental data, management letters, auditor letters, etc., and any updates, revisions, clarifications, or supplemental filings. If the reporting entity has a regular required due date for any of the above reports, and receives an extension on the filing deadline, then the MCO should notify HHSC of any such extension and the estimated revised filing date.
|
|
8.1.18 Management Information System Requirements |
The MCO must maintain a Management Information System (MIS) that supports all functions of the MCO’s processes and procedures for the flow and use of MCO data. If the MCO subcontracts a MIS function, the Subcontractor’s MIS must comply with the requirements of this section.
The MCO must have hardware, software, and a network and communications system with the capability and capacity to handle and operate all MIS subsystems for the following operational and administrative areas:
|
| |
| 1. Enrollment/Eligibility Subsystem; |
|
| |
| 3. Encounter/Claims Processing Subsystem; |
|
| |
| 5. Utilization/Quality Improvement Subsystem; |
|
| |
| 7. Interface Subsystem; and |
|
| |
| 8. TPL/TPR Subsystem, as applicable to each MCO Program. |
The MIS must enable the MCO to meet the Contract requirements, including all applicable state and federal laws, rules, and regulations. The MIS must have the capacity and capability to capture and utilize various data elements required for MCO administration.
The MCO must have a system that can be adapted to changes in Business Practices/Policies within the timeframes negotiated by the Parties. The MCO is expected to cover the cost of such systems modifications over the life of the Contract.
The MCO is required to participate in the HHSC Systems Work Group.
The MCO must provide HHSC written notice of Major Systems Changes and implementations no later than 180 days prior to the planned change or implementation, including any changes relating to Material Subcontractors, in accordance with the requirements of this Contract and Attachment A, "Uniform Managed Care Contract Terms and Conditions."
The MCO must notify HHSC of Major Systems Changes in writing, as well as by e-mail to HPM staff. The notification must detail the following.
| |
• | The aspects of the system that will be changed and date of implementation |
| |
• | How these changes will affect the Provider and Member community, if applicable |
| |
• | The communication channels that will be used to notify these communities, if applicable |
| |
• | A contingency plan in the event of downtime of system(s) |
Major Systems Changes are subject to HHSC desk review and onsite review of the MCO's facilities as necessary to test readiness and functionality prior to implementation. Prior to HHSC approval of the Major Systems Change, the MCO may not implement any changes to its operating systems. Failure to comply will result in contractual remedies, including damages. HHSC retains the right to modify or waive the notification requirement contingent upon the nature of the request from the MCO.
The MCO must provide HHSC any updates to the MCO’s organizational chart relating to MIS and the description of MIS responsibilities at least 30 days prior to the effective date of the change. The MCO must provide HHSC official points of contact for MIS issues on an ongoing basis.
HHSC, or its agent, may conduct a Systems Readiness Review to validate the MCO’s ability to meet the MIS requirements as described in Section 7, “Transition Phase Requirements.” The System Readiness Review may include a desk review and/or an onsite review and must be conducted for the following events:
|
| |
| 1. a new plan is brought into the MCO Program; |
|
| |
| 2. an existing plan begins business in a new Service Area or a Service Area expansion; |
|
| |
| 3. an existing plan changes location; |
|
| |
| 4. an existing plan changes its processing system, including changes in Material Subcontractors performing MIS or claims processing functions; and |
|
| |
| 5. an existing plan in one (1) or two (2) HHSC MCO Programs is initiating a Contract to participate in any additional MCO Programs. |
If HHSC determines that it is necessary to conduct an onsite review, the MCO is responsible for all reasonable travel costs associated with such onsite reviews. For purposes of this section, “reasonable travel costs” include airfare, lodging, meals, car rental and fuel, taxi, mileage, parking, and other incidental travel expenses incurred by HHSC or its authorized agent in connection with the onsite reviews. This provision does not limit HHSC’s ability to collect other costs as damages in accordance with Attachment A, Section 12.02(e), “Damages.”
If for any reason an MCO does not fully meet the MIS requirements, then the MCO must, upon request by HHSC, either correct such deficiency or submit to HHSC a Corrective Action Plan and Risk Mitigation Plan to address such deficiency. Immediately upon identifying a deficiency, HHSC may impose contractual remedies according to the severity of the deficiency. Refer to Attachment A, "Uniform Managed Care Contract Terms and Conditions," Article 12 and Attachment B-3, “Deliverables/Liquidated Damages Matrix,” for additional information regarding remedies and damages. Refer to Section 7, “Transition Phase Requirements,” and Section 8.1.1.2, “Additional Readiness Reviews and Monitoring Efforts,” for additional information regarding MCO Readiness Reviews. Refer to Attachment A, "Uniform Managed Care Contract Terms and Conditions," Section 4.08(c) for information regarding Readiness Reviews of the MCO’s Material Subcontractors.
8.1.18.1 Encounter Data
The MCO must provide complete Encounter Data for all Covered Services, including Value-added Services. Encounter Data must follow the format and data elements as described in the HIPAA-compliant 837 Companion Guides and Encounter Submission Guidelines. HHSC will specify the method of transmission, the submission schedule, and any other requirements in Uniform Managed Care Manual Chapter 5.0, "Consolidated Deliverables Matrix." The MCO must submit Encounter Data transmissions at least monthly, and include all Encounter Data and Encounter Data adjustments processed by the MCO. In addition, Pharmacy Encounter Data must be submitted no later than 25 calendar days after the date of adjudication and include all Encounter Data and Encounter Data adjustments processed by the MCO. Encounter Data quality validation must incorporate assessment standards developed jointly by the MCO and HHSC. The MCO must submit complete and accurate Encounter Data not later than the 30th calendar day after the last day of the month in which the claim was adjudicated. The MCO must make original records available for inspection by HHSC for validation purposes. Encounter Data that does not meet quality standards must be corrected and returned within a time period specified by HHSC.
For reporting claims processed by the MCO and submitted on Encounter 837 and NCPDP format, the MCO must use the procedure codes, diagnosis codes, provider identifiers, and other codes as directed by HHSC. Any exceptions will be considered on a code-by-code basis after HHSC receives written notice from the MCO requesting an exception.
The MCO's Provider Agreements must require Network Providers to comply with the requirements of Texas Government Code § 531.024161, regarding reimbursement of claims based on orders or referrals by supervising providers.
|
|
8.1.18.2 MCO Deliverables related to MIS Requirements |
At the beginning of each State Fiscal Year, the MCO must submit the following documents and corresponding checklists for HHSC’s review and approval:
1. Disaster Recovery Plan;*
2. Business Continuity Plan;* and
3. Security Plan.
* The Business Continuity Plan and the Disaster Recovery Plan may be combined into one document.
Additionally, at the beginning of each State Fiscal Year, if the MCO modifies the following documents, it must submit the revised documents and corresponding checklists for HHSC’s review and approval:
|
| |
| 2. Risk Management Plan; and |
|
| |
| 3. Systems Quality Assurance Plan. |
The MCO must submit plans and checklists in accordance with the Uniform Managed Care Manual Chapter 5.2, “Information Concerning MIS Deliverables;” Chapter 7, “Management Information Systems;” and Chapter 5.0, “Consolidated Deliverables Matrix.” Additionally, if a Systems Readiness Review is triggered by one of the events described in Section 8.1.18, the MCO must submit all of the deliverables identified in this Section 8.1.18.2 in accordance with an HHSC-approved timeline.
The MCO must follow all applicable Joint Interface Plans (JIPs) and all required file submissions for HHSC’s Administrative Services Contractor, External Quality Review Organization (EQRO), and HHSC Medicaid Claims Administrator. The JIPs can be accessed through Uniform Managed Care Manual Chapter 7.1, “Joint Interface Plans (JIP).”
8.1.18.3 System-wide Functions
The MCO’s MIS system must include key business processing functions and/or features, which must apply across all subsystems as follows:
|
| |
| 1. process electronic data transmission or media to add, delete or modify membership records with accurate begin and end dates; |
|
| |
| 2. track Covered Services received by Members through the system, and accurately and fully maintain those Covered Services as HIPAA-compliant Encounter transactions; |
|
| |
| 3. transmit or transfer Encounter Data transactions on electronic media in the HIPAA format to the contractor designated by HHSC to receive the Encounter Data; |
|
| |
| 4. maintain a history of changes and adjustments and audit trails for current and retroactive data; |
|
| |
| 5. maintain procedures and processes for accumulating, archiving, and restoring data in the event of a system or subsystem failure; |
|
| |
| 6. employ industry standard medical billing taxonomies (procedure codes, diagnosis codes, NDC codes) to describe services delivered and Encounter transactions produced; |
|
| |
| 7. accommodate the coordination of benefits; |
|
| |
| 8. produce standard Explanation of Benefits (EOBs) for providers; |
|
| |
| 9. Pay financial transactions to Network Providers and Out-of-Network providers in compliance with federal and state laws, rules and regulations; |
|
| |
| 10. ensure that all financial transactions are auditable according to GAAP guidelines; |
|
| |
| 11. ensure that Financial Statistical Reports (FSRs) comply with Uniform Managed Care Manual Chapter 6.1, “Cost Principles for Expenses,” with respect to segregating costs that are allowable for inclusion in HHSC-designed financial reports; |
|
| |
| 12. relate and extract data elements to produce report formats (provided within the Uniform Managed Care Manual) or otherwise required by HHSC; |
|
| |
| 13. ensure that written process and procedures manuals document and describe all manual and automated system procedures and processes for the MIS; and |
|
| |
| 14. maintain and cross-reference all Member-related information with the most current Medicaid, or CHIP Program Provider number. |
8.1.18.4 Health Insurance Portability and Accountability Act (HIPAA) Compliance
The MCO’s MIS system must comply with applicable certificate of coverage and data specification and reporting requirements promulgated pursuant to the Health Insurance Portability and Accountability Act (HIPAA) of 1996, P.L. 104-191 (August 21, 1996), as amended or modified. The MCO must comply with HIPAA Electronic Data Interchange (EDI) requirements, including the HIPAA-compliant format version. MCO’s enrollment files must be in the 834 HIPAA-compliant format. Eligibility inquiries must be in the 270/271 HIPAA-compliant format, with the exception of pharmacy services. Pharmacies may submit eligibility inquiries in the NCPDP E1 HIPAA-compliant format. Claim transactions for pharmacy services must be in the NCPDP B1/B2 HIPAA-compliant formats; all others must be in the 837/835 HIPAA-compliant format.
The MCO must also be 5010 compliant by January 2012. The following website includes the final rules for 5010 Compliancy and ICD-10 Compliancy: www.cms.hhs.gov/TransactionCodeSetsStands/02_TransactionsandCodeSetsRegulations.asp.
The MCO must provide its Members with a privacy notice as required by HIPAA, including 45 C.F.R. § 164.520. The MCO must provide HHSC with a copy of its privacy notice during Readiness Review and any changes to the notice prior to distribution.
8.1.18.5 Claims Processing Requirements
The MCO must process and adjudicate all provider claims for Medically Necessary health care Covered Services that are filed within the timeframes specified in Uniform Managed Care Manual Chapter 2.0, Claims Manual, and pharmacy claims in that are filed in accordance with the timeframes specified in Uniform Managed Care Manual Chapter 2.2, Pharmacy Claims Manual, and beginning March 1, 2015, Nursing Facility claims that are filed in accordance with the timeframes specified in Uniform Managed Care Manual Chapter 2.3, Nursing Facility Claims Manual. The MCO is subject to contractual remedies, including liquidated damages and interest, if the MCO does not process and adjudicate claims in accordance with the procedures and the timeframes listed in Uniform Managed Care Manual Chapters 2.0, 2.1, 2.2, and 2.3.
The MCO must administer an effective, accurate, and efficient claims payment process in compliance with federal laws and regulations, applicable state laws and rules, and the Contract, including Uniform Managed Care Manual Chapters 2.0, 2.1, 2.2, and 2.3. In addition, a Medicaid MCO must be able to accept and process provider claims in compliance with the Texas Medicaid Provider Procedures Manual. The MCO and its Subcontractors cannot directly or indirectly charge or hold a Member or Provider responsible for claims adjudication or transaction fees.
The MCO must maintain an automated claims processing system that registers the date a claim is received by the MCO the detail of each claim transaction (or action) at the time the transaction occurs, and has the capability to report each claim transaction by date and type to include interest payments. The claims system must maintain information at the claim and line detail level. The claims system must maintain adequate audit trails and report accurate claims performance measures to HHSC.
The MCO's claims system must maintain online and archived files. The MCO must keep online automated claims payment history for the most current 18 months. The MCO must retain other financial information and records, including all original claims forms, for the time period established in Attachment A, "Uniform Managed Care Contract Terms and Conditions," Section 9.01, "Record Retention and Audit." All claims data must be easily sorted and produced in formats as requested by HHSC.
The MCO must offer its Providers/Subcontractors the option of submitting and receiving claims information through electronic data interchange (EDI) that allows for automated processing and adjudication of claims. EDI processing must be offered as an alternative to the filing of paper claims. Electronic claims must use HIPAA-compliant electronic formats.
HHSC reserves the right to require the MCO to receive initial electronic claims through an HHSC-contracted vendor at a future date. This function will allow Providers to send claims to one location, which will then identify where the claim should be submitted. The MCO will be expected to have an interface that allows receipt of these electronic submissions. If HHSC implements this requirement, then the MCO must maintain a mechanism to receive claims in addition to the HHSC claims portal. Providers must be able to send claims directly to the MCO or its Subcontractor.
The MCO must provide a provider portal that supports functionality to reduce administrative burden on Network Providers at no cost to the Providers. A provider portal brings information together from diverse sources in a uniform way. The provider portal functionality must include the following.
| |
• | Client eligibility verification |
| |
• | Submission of electronic claims |
| |
• | Prior Authorization requests |
| |
• | Claims appeals and reconsiderations |
| |
• | Exchange of clinical data and other documentation necessary for prior authorization and claim processing |
To the extent possible, the provider portal should support both online and batch processing as applicable to the information being exchanged. Batch Processing is a billing technique that uses a single program loading to process many individual jobs, tasks, or requests for service. Specifically in managed care, batch billing is a technique that allows Providers to send billing information all at once in a batch rather than in separate individual transactions. To facilitate the exchange of clinical data and other relevant documentation, the Provider Portal must provide a secure exchange of information between the Provider and MCO, including, as applicable, a Subcontractor of the MCO.
The MCO must make an electronic funds transfer (EFT) payment process (for direct deposit) available to Network Providers.
The MCO may deny a claim submitted by a provider for failure to file in a timely manner as provided for in Uniform Managed Care Manual Chapters 2.0, 2.1, 2.2, and 2.3. The MCO must not pay any claim submitted by a provider:
(1) excluded or suspended from the Medicare, Medicaid, or CHIP programs for Fraud, Abuse, or Waste;
(2) on payment hold under the authority of HHSC or its authorized agent(s);
(3) with pending accounts receivable with HHSC;
(4) for neonatal services provided on or after September 1, 2017, if submitted by a Hospital that does not have a neonatal level of care designation from HHSC;
(5) for maternal services provided on or after September 1, 2019, if submitted by a Hospital that does not have a maternal level of care designation from HHSC; or
(6) for a Nursing Facility Service provided on or after March 1, 2015, that does not comply with DADS' criteria for Clean Claims.
In accordance with Texas Health and Safety Code § 241.186, the restrictions on payment identified in items 4-5 above do not apply to emergency services that must be provided or reimbursed under state or federal law.
With the following exceptions, the MCO must complete all audits of a provider claim no later than two years after receipt of a clean claim, regardless of whether the provider participates in the MCO's Network. This limitation does not apply in cases of provider Fraud, Waste, or Abuse that the MCO did not discover within the two-year period following receipt of a claim. In addition, the two-year limitation does not apply when the officials or entities identified in Attachment A, Section 9.02(c), conclude an examination, audit, or inspection of a provider more than two years after the MCO received the claim. Finally, the two-year limitation does not apply when HHSC has recovered a capitation from the MCO based on a Member's ineligibility. If an exception to the two-year limitation applies, then the MCO may recoup related payments from providers.
If an additional payment is due to a provider as a result of an audit, the MCO must make the payment no later than 30 days after it completes the audit. If the audit indicates that the MCO is due a refund from the provider, the MCO must send the provider written notice of the basis and specific reasons for the recovery no later than 30 days after it completes the audit. If the provider disagrees with the MCO's request, the MCO must give the provider an opportunity to appeal, and may not attempt to recover the payment until the provider has exhausted all appeal rights.
The MCO's provider agreement must specify that program violations arising out of performance of the contract are subject to administrative enforcement by the Health and Human Services Commission Office of Inspector General (OIG) as specified in 1 Tex. Admin. Code, Chapter 371, Subchapter G.
The MCO is subject to the requirements related to coordination of benefits for secondary payors in the Texas Insurance Code Section 843.349(e-f).
The MCO must notify HHSC of major claim system changes in writing no later than 180 days prior to implementation. The MCO must provide an implementation plan and schedule of proposed changes. HHSC reserves the right to require a desk or onsite Readiness Review of the changes.
The MCO must any policies affecting claims adjudication and claims coding and processing guidelines available to Providers for the applicable provider type. Providers must receive 90 days notice prior to the MCO's implementation of changes to these claims policies and guidelines.
|
|
8.1.18.6 National Correct Coding Initiative |
MCOs must comply with the requirements of Section 6507 of the Patient Protection and Affordable Care Act of 2010 (P.L. 111-148), regarding “Mandatory State Use of National Correct Coding Initiatives,” including all applicable rules, regulations, and methodologies implemented as a result of this initiative.
|
|
8.1.19 Fraud, Waste and Abuse |
An MCO is subject to all state and federal laws and regulations relating to Fraud, Waste, and Abuse, in health care and the Medicaid and CHIP programs. The MCO must cooperate and assist HHSC and any state or federal agency charged with the duty of identifying, investigating, sanctioning or prosecuting suspected Fraud, Waste, or Abuse. In order to facilitate cooperation with the Office of Inspector General (OIG) at HHSC, the MCO must have staff available for Special Investigative Unit (SIU) representation located in Texas. The MCO must allow access to all premises and provide originals or copies of all records and information requested free of charge to the HHSC OIG, HHSC or its authorized agent(s), the Centers for Medicare and Medicaid Services (CMS), the U.S. Department of Health and Human Services (DHHS), Federal Bureau of Investigation, the Office of the Attorney General, the Texas Department of Insurance (TDI), or other units of state government.
Each MCO must designate one primary and one secondary contact person for all HHSC OIG records requests. HHSC OIG records requests will be sent to the designated MCO contact person(s) in writing by email, fax or mail, and will provide the specifics of the information being requested (see below). The MCO will respond to the appropriate HHSC OIG staff member within the timeframe designated in the request. If the MCO is unable to provide all of the requested information with in the designated timeframe, an extension may be granted and must be requested in writing (e-mail) by the MCO no less than two Business Days prior to the due date. When a request for data is provided to the MCO, the MCO's response must include data for all data fields, as available. If any data field is left blank, an explanation must accompany the response. The data must be provided in the order and format requested. The MCO must not include any additional data fields in its response. All requested information must be accompanied by a notarized Business Records Affidavit unless indicated otherwise in HHSC OIG's record request.
The most common requests will include:
Ø 1099 data and other financial information - 3 Business Days.
ØClaims data for sampling - 5 Business Days.
Ø Urgent claims data requests - 3 Business Days (with OIG manager's approval).
Ø Provider education information - 10 Business Days.
Ø Files associated with an HMO conducted investigation - 15 Business Days.
Ø Other time-sensitive requests - as needed.
The MCO must submit a written Fraud and Abuse compliance plan to the HHSC OIG for approval each year. The plan must be submitted 90 days prior to the start of the State Fiscal Year. (See Section 7, Transition Phase Requirements. for requirements regarding timeframes for submitting the original plan.) If an MCO has not made any changes to its plan from the previous year, it may notify the HHSC OIG that: (1) no changes have been made to the previously-approved plan, (2) the plan will remain in place for the upcoming State Fiscal Year. The notification must be signed and certified by an officer or director of the MCO that is responsible for carrying out the Fraud and Abuse compliance plan. Upon receipt of a written request from the HHSC OIG, the MCO must submit the complete Fraud and Abuse compliance plan.
The MCO is subject to and must meet all requirements in Texas Government Code § 531.113, Texas Government Code § 533.012, 1 Tex. Admin. Code, Subchapter F, Rule §§ 353.501-353.505, and 1 Tex. Admin. Code §§ 370.501-370.505 as well as all laws specified in the contract. Additionally, the MCO must require all employees who process Medicaid claims, including
Subcontractors, to attend annual training as provided by HHSC per Texas Government Code § 531.105. Failure to comply with any requirement of Sections 8.1.19 and 8.1.20.2(c) and (d) subjects the MCO to enforcement pursuant to 1 TEX. ADMIN. CODE Chapter 371 Subchapter G in addition to any other legal remedy.
42 C.F.R. § 455.23 requires the State Medicaid agency to suspend all Medicaid payments to a provider after the agency determines there is a credible allegation of fraud for which an investigation is pending under the Medicaid program against an individual or entity unless the agency has good cause to not suspend payments or suspend payment only in part. In Texas, HHSC OIG is responsible for evaluating allegations of fraud and imposing payment suspensions when appropriate. The rules governing payment suspensions based upon pending investigations of credible allegations of fraud apply to Medicaid managed care entities. Managed care capitation payments may be included in a suspension when an individual network provider is under investigation based upon credible allegations of fraud, depending on the allegations at issue.
The MCO must cooperate with HHSC OIG when HHSC OIC imposes payment suspensions. When HHSC OIG sends notice that payments to a provider have been suspended, the MCO must also suspend payments to the provider within 1 business day. When this notice is received, the MCO must respond to the notice within 3 business days and inform HHSC OIG whether the MCO has implemented the suspension.
The MCO must follow the requirements set forth in a settlement agreement involving a MCO’s provider and HHSC OIG. The MCO must withhold the designated percentage of funds to be paid toward an identified overpayment. Upon HHSC OIG request, the MCO must forward the held funds to HHSC OIG, Attn: Sanctions Division, along with an itemized spreadsheet detailing the provider’s claims paid so that the claims data can be reconciled with the monthly Remittance & Status statements.
The MCO must also report all of the following information to HHSC OIG after it suspends payments to the provider: date the suspension was imposed, date the suspension was discontinued, reason for discontinuing the suspension, outcome of any appeals, amount of payments held, and, if applicable, the good cause rationale for not suspending payment (for example, the provider is not enrolled in the MCO's network) or imposing a partial payment suspension. If the MCO does not suspend payments to the provider, HHSC may impose contractual or other remedies.
For payment suspensions initiated by the MCO, the MCO must report the following information to HHSC OIG: the nature of the suspected fraud, basis for the suspension, date the suspension was imposed, date the suspension was discontinued, reason for discontinuing the suspension, outcome of any appeals, the amount of payments held, and, if applicable, the good cause rationale for imposing a partial payment suspension.
MCOs must maintain all documents and claim data on Providers who are under HHSC OIG investigation or any internal investigations that are referred to HHSC OIG for recoupment. The MCO’s failure to comply with this Section 8.1.19 and all state and federal laws and regulations relating to Fraud, Waste, and Abuse in healthcare and the Medicaid and CHIP programs are subject to administrative enforcement by HHSC OIG as specified in 1 Tex. Admin. Code, Chapter 371, Subchapter G.
Additional Requirements for STAR and STAR+PLUS MCOs:
In accordance with Section 1902(a)(68) of the Social Security Act, STAR and STAR+PLUS MCOs and their Subcontractors that receive or make annual Medicaid payments of at least $5 million must:
1. Establish written policies for all employees, managers, officers, contractors, Subcontractors, and agents of the MCO or Subcontractor. The policies must provide detailed information about the False Claims Act, administrative remedies for false claims and statements, any state laws about civil or criminal penalties for false claims, and whistleblower protections under such laws, as described in Section 1902(a)(68)(A).
2. Include as part of such written policies detailed provisions regarding the MCO's or Subcontractor's policies and procedures for detecting and preventing Fraud, Waste, and Abuse.
3. Include in any employee handbook a specific discussion of the laws described in Section 1902(a)(68)(A), the rights of employees to be protected as whistleblowers, and the MCO's or Subcontractor's policies and procedures for detecting and preventing Fraud, Waste, and Abuse.
HHSC OIG's Lock-in Program (OIG-LP) restricts, or locks in, a Medicaid Member to a designated provider or pharmacy if it finds that the Member used Medicaid services, including drugs, at a frequency or amount that is duplicative, excessive, contraindicated, or conflicting; or that the Member's actions indicate abuse, misuse, or fraud. The MCO is required to maintain, and provide to OIG upon request, written policies for all employees, managers, officers, contractors, subcontractors, and agents
of the MCO or Subcontractor. The policies must provide detailed information related to the "HHSC OIG Lock-in Program MCO Policies and Procedures" about overutilization of prescription medications.
|
|
8.1.20 General Reporting Requirements |
The MCO must provide and must require its Subcontractors to provide at no cost to the Texas Health and Human Services Commission (HHSC):
1. all information required under the Contract, including but not limited to, the reporting requirements or other information related to the performance of its responsibilities hereunder as reasonably requested by the HHSC; and
2. any information in its possession sufficient to permit HHSC to comply with the Federal Balanced Budget Act of 1997 or other federal or state laws, rules, and regulations. All information must be provided in accordance with the timelines, definitions, formats and instructions as specified by HHSC. Where practicable, HHSC may consult with MCOs to establish timeframes and formats reasonably acceptable to both parties.
Any deliverable or report in Section 8.1.20 without a specified due date is due quarterly on the last day of the month following the end of the reporting period. Where the due date states 30 days, the MCO is to provide the deliverable by the last day of the month following the end of the reporting period. Where the due date states 45 days, the MCO is to provide the deliverable by the 15th day of the second month following the end of the reporting period. (See Uniform Managed Care Manual Chapter 5.0, "Consolidated Deliverables Matrix.")
8.1.20.1 Healthcare Effectiveness Data and Information Set (HEDIS) and Other Statistical Performance Measures
The MCO must provide to HHSC or its designee all information necessary to analyze the MCO's provision of quality care to Members using measures to be determined by HHSC in consultation with the MCO. These measures must be consistent with HEDIS or other externally based measures or measurement sets, and involve collection of information beyond that present in Encounter Data. The Performance Indicator Dashboards, found in Uniform Managed Care Manual Chapter 10.1 provides additional information on the role of the MCO and the EQRO in the collection and calculation of HEDIS, Consumer Assessment of Healthcare Providers and Systems (CAHPS), and other performance measures.
8.1.20.2 Reports
The MCO must provide the following reports, in addition to the Financial Reports described in Section 8.1.17 and the reporting requirements listed elsewhere in the Contract. Uniform Managed Care Manual Chapter 5.0, "Consolidated Deliverables Matrix," includes a list of all required reports, and a description of the format, content, file layout and submission deadlines for each report.
For the following reports, MCO must integrate CHIP Perinatal Program data into CHIP Program reports. With the exception of FSR reporting, separate CHIP Perinatal Program reports generally are not required. Where appropriate, HHSC will designate specific attributes within the CHIP Program reports that the CHIP MCOs must complete to allow HHSC to extract data particular to the CHIP Perinatal population.
(a) Claims Summary Report - The MCO must submit quarterly Claims Summary Reports by MCO Program, Service Area and claim type by the 30th day following the end of the reporting period unless otherwise specified. Claim Types include facility and/or professional services for Acute Care, Behavioral Health, Vision, Pharmacy, and Long Term Services and Supports. Within each claim type, claims data must be reported separately by applicable claim form. The format for the Claims Summary Report is contained in Uniform Managed Care Manual Chapter 5.6.1.
(b) QAPI Program Annual Summary Report - The MCO must submit a QAPI Program Annual Summary in a format and timeframe as specified in Uniform Managed Care Manual Chapter 5.7, "Quality Reports."
(c) Fraudulent Practices Report - Utilizing the HHSC-Office of Inspector General (OIG) fraud referral form, the MCO's assigned officer or director must report and refer all possible acts of Waste, Abuse, or Fraud to the HHSC-OIG within 30 Business Days of receiving the reports of possible acts of Waste, Abuse, or Fraud from the MCO's Special Investigative Unit (SIU). The report and referral must include: an investigative report identifying the allegation, statutes/regulations violated or considered, and the results of the investigation; copies of program rules and regulations violated for the time period in question; copies of any HMO contractual provisions, policies, published HMO program bulletins, policy notification letters, or provider policy or procedure manuals that apply to the alleged conduct for the
time period in question; the estimated overpayment identified; a summary of the interviews conducted; the Encounter Data submitted by the provider for the time in question; and all supporting documentation obtained as the result of the investigation. This requirement applies to all reports of possible acts of Waste, Abuse, and Fraud.
Additional reports required by the Office of the Inspector General relating to Waste, Abuse, or Fraud are listed in Uniform Managed Care Manual Chapter 5.5, "Fraud Deliverable/Report Formats."
(d) Provider Termination Report: (CHIP, STAR, and STAR+PLUS) - MCO must submit a quarterly report that identifies any Providers who cease to participate in MCO's Provider Network, either voluntarily or involuntarily. The report must be submitted in the format specified by HHSC, no later than 30 days after the end of the reporting period.
(e) PCP Network & Capacity Report: (CHIP only) - For the CHIP Program, MCO must submit a quarterly report listing all unduplicated PCPs in the MCO's Provider Network. For the CHIP Perinatal Program, the Perinate Newborn Members are
assigned PCPs that are part of the CHIP PCP Network. Perinate Members are not assigned PCPs. The report must be submitted in the format specified by HHSC no later than 30 days after the end of the reporting quarter.
(f) Summary Report of Member Complaints and Appeals - The MCO must submit quarterly Member Complaints and Appeals reports. The MCO must include in its reports Complaints and Appeals submitted to its subcontracted risk groups (e.g., IPAs) and any other Subcontractor that provides Member services. The MCO must submit the Complaint and Appeals reports electronically on or before 45 days following the end of the State Fiscal Quarter, using the format specified in Uniform Managed Care Manual Chapter 5.4.2, "Complaints and Appeals Report."
HHSC may direct the CHIP MCOs to provide segregated Member Complaints and Appeals reports for the CHIP Perinatal Program on an as-needed basis.
(g) Summary Report of Provider Complaints - The MCO must submit Provider complaints reports on a quarterly basis. The MCO must include in its reports complaints submitted by providers to its subcontracted risk groups (e.g., IPAs) and any other Subcontractor that provides provider services. The complaint reports must be submitted electronically on or before 45 days following the end of the State Fiscal Quarter, using the format specified by HHSC in the Uniform Managed Care Manual Chapter 5.4.2, "Complaints and Appeals Report."
HHSC may direct the CHIP MCOs to provide segregated Provider Complaints and Appeals reports for the CHIP Perinatal Program on an as-needed basis.
(h) Hotline Reports - The MCO must submit quarterly status reports of the Member Hotline, the Behavioral Health Services Hotline, and the Provider Hotline performance compared to the performance standards set out in Sections 8.1.4.7, 8.1.5.6, and 8.1.15.3, using the format specified by HHSC in Uniform Managed Care Manual Chapter 5.4.3, "Hotline Reports."
If the MCO is not meeting a hotline performance standard, HHSC may require the MCO to submit monthly hotline performance reports and implement corrective actions until the hotline performance standards are met. If a MCO has a single hotline serving multiple Service Areas, multiple MCO Programs, or multiple hotline functions, (i.e. Member, Provider, Behavioral Health Services hotlines), HHSC may request on an annual basis that the MCO submit certain hotline response information by MCO Program, Service Area, and hotline function, as applicable to the MCO. HHSC may also request additional hotline information if a MCO is not meeting a hotline performance standard.
(i) Historically Underutilized Business (HUB) Reports - Upon contract award, the MCO must attend a post award meeting, which will be scheduled by the HHSC HUB Program Office, to discuss the development and submission of a HUB Subcontracting Plan (HSP) Progress Assessment Report (PAR) for the inclusion of HUBs. The MCO must maintain its original HSP and submit monthly PAR reports documenting the MCO's good faith effort to comply with the originally submitted HSP. The report must be in the format included in Uniform Managed Care Manual Chapter 5.4.4.4 for the HUB monthly reports. The MCO must comply with the HUB Program's HSP and PAR requirements for all Subcontractors.
(j) Medicaid Managed Care Texas Health Steps Medical Checkups Reports - Medicaid MCOs must submit reports identifying the number of New Members and Existing Members receiving Texas Health Steps medical checkups, or refusing to obtain the medical checkups. Medicaid MCOs must also document and report those Members refusing to obtain the medical checkups. The documentation must include the reason the Member refused the checkup or the reason the checkup was not received.
The definitions, timeframe, format, and details of the reports are contained and described in Uniform Managed Care Manual Chapters 12.4, 12.5, and 12.6.
(k) Children of Migrant Farm Workers Annual Plan - Medicaid MCOs must submit an annual plan in the timeframe and format described in Uniform Managed Care Manual Chapters 12.1 and 12.2 that describes how the MCO will identify and provide accelerated services to Children of Migrant Farm Workers (FWC).
(l) Children of Migrant Farm Workers Annual Report (FWC Annual Report) - Medicaid MCOs must submit an annual report, in the timeframe and format described in Uniform Managed Care Manual Chapters 12.1, 12.3, 12.25, and 12.26 about the identification of and delivery of services to Children of Migrant Farm Workers (FWC).
(m) Frew Quarterly Monitoring Report - Each calendar year quarter, HHSC prepares a report for the court that addresses the status of the Consent Decree paragraphs of the Frew v. Janek lawsuit. Medicaid MCOs must prepare responses to questions posed by HHSC on the Frew Quarterly Monitoring Report template. The timeframe, format, and details of the report are set forth in Uniform Managed Care Manual Chapter 12.
(n) Frew Annual Provider Training Report - Per the Frew v. Janek "Corrective Action Order: Health Care Provider Training," HHSC must compile a summary of the training health care and pharmacy providers receive throughout the year for the October Quarterly Monitoring Report for the court. Medicaid MCOs must report to HHSC health care and pharmacy provider training conducted throughout the year to be included in this report. The training report must include, at a minimum, the number of Medicaid enrolled healthcare and pharmacy providers that received the training and a description of provider feedback received on the subject matter and methodology of the training. The timeframe, format, and details of the report are contained and described in Uniform Managed Care Manual Chapter 12.
(o) Frew Provider Recognition Report - Per the Frew v. Janek "Corrective Action Order: Health Care Provider Training," HHSC must recognize Medicaid enrolled healthcare and pharmacy providers who complete Frew, Texas Health Steps, and/or pharmacy benefit education training. Medicaid MCOs must collect and track provider training recognition information for all Frew, Texas Health Steps, and/or pharmacy benefit education trainings conducted and report the names of those Medicaid enrolled healthcare and pharmacy providers who consent to being recognized to HHSC quarterly. The timeframe, format, and details of the report are contained and described in Uniform Managed Care Manual Chapter 12.
(p) Out-of-Network Utilization Reports - The MCO must file quarterly Out-of Network Utilization Reports in accordance with Uniform Managed Care Manual Chapter 5.3.8, "Out Of Network (OON) Utilization Report." Quarterly reports are due 30 days after the end of each quarter.
(q) Drug Utilization Review (DUR) Reports - MCOs must submit the DUR reports in accordance with the requirements of HHSC's Uniform Managed Care Manual.
(r) Medicaid Managed Care Texas Health Steps Medical Checkups Quarterly Utilization Reports - For each State Fiscal Quarter, Medicaid MCOs must submit a report of the number and percent of Members birth through age 20 receiving at least one Texas Health Steps medical checkup in total and broken down by various age groups. The time frame, format, and details of the report are contained and described in Uniform Managed Care Manual Chapter 12.
(s) STAR+PLUS Long Term Services and Supports (LTSS) Utilization Quarterly Reports - The STAR+PLUS MCO must file quarterly LTSS Utilization Reports in accordance with Uniform Managed Care Manual Chapter 5.4.5.1, "STAR+PLUS LTSS Utilization Report." Quarterly reports are due 30 days after the end of each quarter.
(t) Service Coordination Report - STAR+PLUS MCOs must submit annual reports regarding the number and types of visits conducted by Service Coordinators, as described in the Uniform Managed Care Manual. The reports are due 30 days after the end of each State Fiscal Year.
(u) Nursing Facility Reports - Beginning in SFY 2015, the STAR+PLUS MCO must file quarterly Nursing Facility Utilization Reports in accordance with Uniform Managed Care Manual Chapter 5.4.5.2., STAR+PLUS Nursing Facility Report. Quarterly reports are due 30 days after the end of each quarter.
(v) Provider Referral Report - MCOs must submit reports containing the information required in Texas Government Code § 533.005(a)(20)(B) in accordance with Uniform Managed Care Manual Chapter 5.
(w) Perinatal Risk Reports - The MCO must submit a quarterly perinatal risk report as described in Uniform Managed Care Manual Chapter 5. Quarterly reports are due 30 days after the end of each quarter.
8.1.21 Pharmacy Services
The MCO must provide pharmacy-dispensed prescriptions as a Covered Service.
The MCO must submit pharmacy clinical guidelines and prior authorization policies and for review and approval during Readiness Review, then after the Operational Start Date prior to any changes. In determining whether to approve these materials, HHSC will review factors such as the clinical efficacy and Members' needs.
The MCO must allow pharmacies to fill prescriptions for covered drugs ordered by any licensed provider regardless of Network participation and must encourage Network pharmacies to also become Medicaid-enrolled durable medical equipment (DME) providers.
The MCO is responsible for negotiating reasonable pharmacy provider reimbursement rates, including individual MCO maximum allowable cost (MAC) rates, as described in Section 8.1.21.11, "Maximum Allowable Cost Requirements." The MCO must ensure that, as an aggregate, rates comply with 42 C.F.R. Part 50, Subpart E, regarding upper payment limits.
8.1.21.1 Formulary and Preferred Drug List
The MCO must provide access to covered outpatient drugs and biological products through formularies and a preferred drug list (PDL) developed by HHSC. HHSC will maintain separate Medicaid and CHIP formularies, and a Medicaid PDL. The MCO must administer the PDL in a way that allows access to all non-preferred drugs that are on the formulary through a structured PA process.
The MCO must educate Network Providers about how to access HHSC's formularies and the Medicaid PDL on HHSC's website. In addition, no later than November 1, 2013, the MCO must allow Network Providers access to the formularies and Medicaid PDL through a free, point-of-care web-based application accessible on smart phones, tablets, or similar technology. The application must also identify preferred/non-preferred drugs, Clinical Edits, and any preferred drugs that can be substituted for non-preferred drugs. The MCO must update this information at least weekly.
8.1.21.2 Prior Authorization for Prescription Drugs and 72-Hour Emergency Supplies
The MCO must adopt PA policies and procedures that are consistent with Section 8.1.8.1, "Compliance with State and Federal Prior Authorization Requirements."
The MCO must adhere to HHSC's PDL for Medicaid. Preferred drugs must adjudicate as payable without PA, unless they are subject to Clinical Edits. HHSC will identify Clinical Edits that the MCO must implement on the Vendor Drug Program website, and HHSC approval is required for all other Clinical Edit policies and any revisions. HHSC will respond to Clinical Edit approval requests within 30 calendar days. If a requested drug is subject to more than one edit (e.g., the drug is both non-preferred and subject to a Clinical Edit), the MCO must process all edits concurrently.
HHSC's Medicaid PA, PDL, Clinical Edit, and other policies for the fee-for-service Vendor Drug Program are available on HHSC's Vendor Drug Program website at http://www.txvendordrug.com/index.shtml. HHSC's website also includes exception criteria for each drug class included on HHSC's Medicaid PDL. These exception criteria describe the circumstances under which a non-preferred drug may be dispensed without a PA. If HHSC modifies the policies described above on the Vendor Drug Program website, HHSC will notify MCOs.
The MCO may require a prescriber's office to request a PA as a condition of coverage or pharmacy payment if the PA request is approved or denied within 24 hours of receipt. If a prescription cannot be filled when presented to the pharmacist due to a PA requirement and the prescriber's office cannot be reached, then the MCO must instruct the pharmacy to dispense a 72-hour emergency supply of the prescription. The pharmacy is not required to dispense a 72-hour supply if the dispensing pharmacist determines that taking the prescribed medication would jeopardize the Member's health or safety, and he or she has made good faith efforts to contact the prescriber. The pharmacy may fill consecutive 72-hour supplies if the prescriber's office remains unavailable. The MCO must reimburse the pharmacy for dispensing the temporary supply of medication.
The MCO must provide access to a toll-free call center for prescribers to call to request a PA for non-preferred drugs or drug that are subject to Clinical Edits. If the prescriber's office calls the MCO's PA call center, the MCO must provide a PA approval
or denial immediately. For all other PA requests, the MCO must notify the prescriber's office of a PA denial or approval no later than 24 hours after receipt. If the MCO cannot make a timely PA determination, the MCO must allow the Member to receive a sufficient supply (e.g., a 72-hour supply) of the medication pending resolution of the PA request.
The MCOs must have an automated process that may be used to assess a Medicaid recipient's medical and drug claim history to determine whether the recipient's medical condition satisfies the applicable criteria for dispensing a drug without an additional prior authorization request. (See Texas Government Code § 531.073(h).) This process must automatically evaluate whether a submitted pharmacy claim meets Prior Authorization criteria for both PDL and Clinical Edits. (See UMCM, Chapter 2.2., Section V for the definition of an Automated Prior Authorization Request.) The MCO's PA system must accept PA requests from prescribers that are sent electronically, by phone, fax, or mail. The MCO may not charge pharmacies for PA transaction, software, or related costs for processing PA requests.
If the MCO or its PBM operates a separate call center for PA requests, the PA call center must meet the provider hotline performance standards set forth in Section 8.1.4.7, "Provider Hotline." The MCO must train all PA, provider hotline, and pharmacy call center staff on the requirements for dispensing 72-hour emergency supplies of medication.
The MCO may not require a PA for any drug exempted from PA requirements by federal law.
For drug products purchased by a pharmacy through the Health Resources Services Administration (HRSA) 340B discount drug program, the MCO may only impose Clinical Edit PA requirements. These drugs must be exempted from all PDL PA requirements.
A provider may appeal PA denials on a Member's behalf, in accordance with Sections 8.2.6 (Medicaid) and 8.4.2 (CHIP).
If a Member changes Medicaid or CHIP health plans, the MCO must provide the new health plan information about the Member's PA and medication history at no cost and upon request. The MCO, in consultation with HHSC, will develop a standard process and timeline for implementing a standard format for sharing member medication and PA history. HHSC expects the former MCO to respond with the requested information within 72-hours of the new MCO's request.
8.1.21.3 Coverage Exclusions
In accordance with 42 U.S.C. § 1396r-8, the MCO must exclude coverage for any drug marketed by a drug company (or labeler) that does not participate in the federal drug rebate program. The MCO is not permitted to provide coverage for any drug product, brand name or generic, legend or non-legend, sold or distributed by a company that did not sign an agreement with the federal government to provide Medicaid rebates for that product.
8.1.21.4 DESI Drugs
The MCO must not provide coverage under any circumstances for drug products that have been classified as less-than-effective by the Food and Drug Administration (FDA) Drug Efficacy Study Implementation (DESI).
8.1.21.5 Pharmacy Rebate Program
Under the provisions of, 42 U.S.C. §1396r-8, drug companies that wish to have their products covered through the Texas Medicaid Program must sign an agreement with the federal government to provide the pharmacy claims information that is necessary to return federal rebates to the state.
The MCO is not authorized to negotiate rebates with drug companies for preferred pharmaceutical products. HHSC or its designee will negotiate rebate agreements. If the MCO or its PBM has an existing rebate agreement with a manufacturer, all Medicaid and CHIP outpatient drug claims, including provider-administered drugs, must be exempt from such rebate agreements. The MCO must include National Drug Codes (NDCs) on all encounters for outpatient drugs and biological products, including physician-administered drugs.
The MCO must implement a process to timely support HHSC's Medicaid and CHIP rebate dispute resolution processes.
a. The MCO must allow HHSC or its designee to contact Network pharmacy Providers to verify information submitted on claims, and upon HHSC's request, assist with this process.
b. The MCO must establish a single point of contact where HHSC's designee can send information or request clarification.
c. HHSC will notify the MCO of claims submitted with incorrect information. The MCO must correct this information on the next scheduled pharmacy encounter data transmission.
8.1.21.6 Drug Utilization Review Program
The MCO must have a process in place to conduct prospective and retrospective utilization review of prescriptions that is consistent with Medicare Part D drug utilization review standards (see 42 C.F.R. § 423.153). Prospective review should take place at the dispensing pharmacy’s point-of-sale (POS). The prospective review at the POS should compare the prescribed medication against previous drug history for drug-to-drug, interactions, ingredient duplication, therapeutic duplication, age or gender contraindications, drug-allergy contraindications, overutilization or underutilization, incorrect dosage, and high dose situations. The MCO’s retrospective review should monitor prescriber and contracted pharmacies for outlier activities. Retrospective reviews should also determine whether services were delivered as prescribed and consistent with the MCO’s payment policies and procedures. The MCO must provide a summary of the quarterly retrospective reviews, including outcomes, as described in UMCM Chapter 5.13.1, MCO Drug Utilization Review (DUR) Quarterly Report Template.
The MCO’s Drug Utilization Review should specifically assess prescribing patterns for psychotropic medications as defined by Texas Family Code § 266.001(7). If the MCO identifies patterns outside of the MCO’s parameters for psychotropic medications, or if HHSC notifies the MCO of outlier prescribing patterns, then the MCO must conduct a peer-to-peer discussion on the appropriateness of the drug regimen with the prescriber. For children, the MCO must model its parameters on DFPS’s Psychotropic Medication Utilization Parameters for Foster Children. (See DFPS’s website for more information: http://www.dfps.state.tx.us/Child_Protection/Medical_Services/guide-psychotropic.asp).For adults, the MCO must base its parameters for psychotropic medications on approved compendia, such as peer-reviewed academic literature or nationally accepted guidelines.
8.1.21.7 Pharmacy Benefit Manager (PBM)
The MCO must use a PBM to process prescription claims.
The MCO must identify the proposed PBM and the ownership of the proposed PBM. If the PBM is owned wholly or in part by a retail pharmacy provider, chain drug store or pharmaceutical manufacturer, the MCO will submit a written description of the assurances and procedures that must be put in place under the proposed PBM Subcontract, such as an independent audit, to ensure no conflicts of interest exist and ensure the confidentiality of proprietary information. The MCO must provide a plan documenting how it will monitor these Subcontractors. These assurances and procedures must be submitted for HHSC's review during Readiness Review (see Section 7, "Transition Phase Requirements") then prior to initiating any PBM Subcontract after the Operational Start Date.
The MCO must ensure its subcontracted PBM follows all pharmacy-related Contract, UMCM, state, and federal law requirements related to the provision of pharmacy services.
Further, the MCO’s reimbursement methodology for the PBM must be based on the actual amount paid by the PBM to a pharmacy for dispensing and ingredient costs. However, this prohibition on the industry practice known as “spread pricing” is not intended to prohibit the MCO from paying the PBM reasonable administrative and transactional costs for services, as described in Uniform Managed Care Manual Chapter 6.1, “Cost Principles for Expenses.”
8.1.21.8 Financial Disclosures for Pharmacy Services
The MCO must disclose all financial terms and arrangements for remuneration of any kind that apply between the MCO and any prescription drug manufacturer or labeler, including formulary management, drug-switch programs, educational support, claims processing, pharmacy network fees, data sales fees, and any other fees. Article 9 of Attachment A, "Uniform Managed Care Contract Terms and Conditions," provides HHSC with the right to audit this information at any time. HHSC agrees to maintain the confidentiality of information disclosed by the MCO pursuant to this section, to the extent that the information is confidential under state or federal law.
|
|
8.1.21.9 Limitations Regarding Registered Sex Offenders |
HHSC's Medicaid and CHIP formularies do not include sexual performance enhancing medications. If these medications are added to the Medicaid or CHIP formulary, then the MCO must comply with the requirements of Texas Government Code §531.089 prohibiting the provision of sexual performance enhancing medication to persons required to register as sex offenders under Chapter 62, Texas Code of Criminal Procedure.
8.1.21.10 Specialty Drugs
The MCO must develop policies and procedures for reclassifying prescription drugs from retail to specialty drugs for purposes of entering into selective contracting arrangements for specialty drugs. The MCO's policies and procedures must comply with 1 Tex. Admin. Code § 353.905 and § 354.1853 and include processes for notifying Network Pharmacy Providers.
8.1.21.11 Maximum Allowable Cost Requirements
The MCO must develop maximum allowable cost (MAC) prices and lists that comply with state and federal laws, including Texas Government Code § 533.005(a)(23)(K). To place an outpatient drug on a MAC list, the MCO must ensure that:
| |
• | the drug is listed as "A" or "B" rated in the most recent version of the United States Food and Drug Administration's Approved Drug Products with Therapeutic Equivalence Evaluations, also known as the Orange Book, has an "NR" or "NA" rating or similar rating by a nationally recognized reference; and |
| |
• | the drug is generally available for purchase by pharmacies in Texas from national or regional wholesalers and is not obsolete. |
The MCO cannot set a MAC on a drug that is both preferred on HHSC's PDL and a brand name drug.
The MCO must provide a Network pharmacy the sources used to determine the MAC pricing at contract execution, renewal, and upon request. When determining MAC prices, the MCO may only compare drugs listed as therapeutically equivalent in the most recent version of the Orange Book to formulate the MAC price.
The MCO must review and update MAC prices at least once every seven days to reflect any modifications of MAC pricing, and establish a process for eliminating products from the MAC list or modifying MAC prices in a timely manner to remain consistent with pricing changes and product availability in the Service Area.
The MCO must implement a process for allowing Network pharmacies to challenge a MAC price no later than September 1, 2013. The MCO must submit the process for HHSC's review and approval prior to implementation and modification. The MCO must respond to a challenge by the 15th day after it is made. If the challenge is successful, the MCO must adjust the drug price, effective on the date the challenge is resolved, and apply the new price to all similarly situated Network pharmacies, as appropriate and determined by the MCO. If the challenge is denied, the MCO must provide the pharmacy the reasons for the denial. The MCO must provide a quarterly report regarding MAC price challenges in the manner and format specified in the UMCM.
The MCOs or PBMs, as applicable, must provide a process for each of its network pharmacy providers to readily access the MAC list specific to that provider. At a minimum, MCOs and PBMs must allow a network pharmacy to download a searchable file of the MAC list specific to that pharmacy from the MCO or PBM website. Alternatively, MCOs or PBMs may allow a network pharmacy to view and search the MAC list specific to that pharmacy on the website. The list provided on the website must be searchable by drug name. The MCO must submit the process for HHSC's review and approval prior to implementation and modification. As described in Texas Government Code § 533.005(a-2), a MAC price list that is specific to a Network pharmacy is confidential for all other purposes.
The MCO must inform HHSC no later than 21 days after implementing a MAC price list for drugs dispensed at retail pharmacies but not by mail.
8.1.21.12 Mail-Order and Delivery
The MCO may include mail-order pharmacies in its pharmacy Network, but cannot require Members to use a mail-order pharmacy. The MCO cannot charge a Member who opts to use a mail order pharmacy any fees for using this service, including postage or handling for standard or expedited deliveries.
In Medicaid fee-for-service, the Vendor Drug Program pays qualified community retail pharmacies for pharmaceutical delivery services. The MCO must implement a process to ensure that Medicaid and CHIP Members receive free outpatient pharmaceutical deliveries from community retail pharmacies in their Service Areas, or through other methods approved by HHSC. Mail order delivery is not an appropriate substitute for delivery from a qualified community retail pharmacy unless requested by the Member. The MCO's process must be approved by HHSC, submitted using HHSC's template, and include all qualified community retail pharmacies identified by HHSC.
8.1.21.13 Health Resources and Services Administration 340B Discount Drug Program
The MCO must use a shared-savings approach for reimbursing Network Providers that participate in the federal Health Resources and Services Administration's (HRSA's) 340B discount drug program. The MCO cannot require a Network Provider to submit its actual acquisition cost (AAC) on outpatient drugs and biological products purchased through the 340B program, consistent with UMCM Chapter 2.2, "Pharmacy Claims Manual." In addition, the MCO cannot impose PA requirements based on non-preferred status ("PDL PAs") for these drugs and products.
8.1.21.14 Pharmacy Claims and File Processing
The MCO must process claims in accordance with UMCM Chapter 2.2, Pharmacy Claims Manual, and Texas Insurance Code § 843.339. This law requires the MCO to pay clean claims that are submitted electronically no later than 18 days after adjudication, and no later than 21 days after adjudication if the claim is not submitted electronically. In addition, the MCO must comply with Sections 8.2.1 (Medicaid) and 8.4.3 (CHIP) regarding payment of out-of-network pharmacy claims.
HHSC will provide the MCO or its designee with pharmacy interface files, including formulary, PDL, third party liability, master provider, and drug exception files. Due to the point-of-sale nature of outpatient pharmacy benefits, the MCO must ensure all applicable MIS systems (including pharmacy claims adjudication systems) are updated to include the data provided in the pharmacy interface files. The MCO must update within two business days of the files becoming available through HHSC’s file transfer process, unless clarification is needed or data/ file exceptions are identified. If clarification is needed, the MCO must notify HHSC within the same two business days. Additionally, the MCO must be able to perform off-cycle formulary and PDL updates at HHSC’s request.
The MCO must ensure that all daily enrollment and eligibility files in the Joint Interface Plan are loaded into the pharmacy claims adjudication system within two calendar days of receipt.
8.1.21.15 Pharmacy Audits
The MCO must comply with the requirements of Texas Insurance Code § 843.3401, regarding audits of pharmacists and pharmacies, including the prohibition on the use of extrapolation.
8.1.21.16 E-Prescribing
The MCO must provide the appropriate data to the national e-prescribing network, which at a minimum will support: eligibility confirmation, PDL benefit confirmation, identification of preferred drugs that can be used in place of non-preferred drugs ("alternative drugs"), medication history, and prescription routing.
8.1.21.17 Second Generation Direct Acting Antivirals for Hepatitis C
The MCO will provide second generation direct acting antivirals (DAAs) for the treatment of hepatitis C to Members under a non-risk, cost settlement basis, as described in Attachment A, Section 10.18, “Non-risk Payments for Second Generation Direct Acting Antivirals for Hepatitis C.” The MCO must follow HHSC’s clinical review criteria located at www.txvendordrug.com to approve the provision of these drugs. Reimbursement by HHSC may be up to the Medicaid fee-for-service rate that HHSC would have paid for the drug on the date of service for a valid claim. The non-risk payments will cover only the cost of the drugs. Reasonable administrative costs associated with coverage of these drugs as well as adjunctive therapies associated with
the DAA treatment for hepatitis C will be part of the existing Capitation Rate. The MCO may not include the cost of the drugs in the FSR.
|
|
8.1.22 Federally Qualified Health Centers (FQHCs) and Rural Health Clinics (RHCs) |
The MCO must make reasonable efforts to include FQHCs and RHCs (freestanding and Hospital-based) in its Provider Network. If a Member visits an FQHC or RHC (or a Municipal Health Department's public clinic for Health Care Services) at a time that is outside of regular business hours (as defined by HHSC in rules, including weekend days or holidays), the MCO is obligated to reimburse the FQHC, RHC, or public clinic for Medically Necessary Covered Services. The MCO must do so at a rate that is equal to the allowable rate for those services as determined under Section 32.028 of the Human Resources Code. The Member does not need a referral from his/her PCP.
The MCO must pay full encounter rates to FQHCs and RHCs for Medically Necessary Covered Services provided to Medicaid and CHIP Members using the prospective payment methodology described in Sections 1902(bb) and 2107(e)(1) of the Social Security Act. Because the MCO is responsible for the full payment amount in effect on the date of service, HHSC cost settlements (or "wrap payments") will not apply.
|
|
8.1.23 Payment by Members. |
Except as provided in Section 8.1.23.1, MCOs, Network Providers, and Out-of-Network Providers are prohibited from billing or collecting any amount from a Member for Covered Services.
However, the STAR+PLUS MCO will work with Members or their representatives to help facilities collect applied income where applicable.
MCOs must also inform Members of their responsibility to pay the costs for non-covered services, and must require its Network Providers to:
1. inform Members of costs for non-covered services prior to rendering such services; and
2. obtain a signed private pay form from such Members.
CHIP Network Providers and Out-of-Network Providers may collect copayments authorized in the CHIP State Plan from CHIP Members.
CHIP families that meet the enrollment period cost share limit requirement must report it to the HHSC Administrative Services Contractor. The HHSC Administrative Service Contractor notifies the MCO that a family’s cost share limit has been reached. Upon notification from the HHSC Administrative Services Contractor that a family has reached its cost-sharing limit for the term of coverage, the MCO will generate and mail to the CHIP Member a new Member ID card within five calendar days, showing that the CHIP Member’s cost-sharing obligation for that term of coverage has been met. No cost-sharing may be collected from these CHIP Members for the balance of their term of coverage.
Providers are responsible for collecting all Member copayments at the time of service. Copayments that families must pay vary according to their income level.
Copayments do not apply, at any income level, to Covered Services that qualify as well-baby and well-child care services, preventive services, or pregnancy-related services as defined by 42 C.F.R. §457.520 and SSA § 2103(e)(2).
Except for costs associated with unauthorized non-emergency services provided to a Member by Out-of-Network providers and for non-covered services, the copayments outlined in the CHIP Cost Sharing Table in Uniform Managed Care Manual Chapter 6.3, “CHIP Cost Sharing,” are the only amounts that an MCO may impose and a provider may collect from a CHIP-eligible family. If the cost of a Covered Service is less than the Member's CHIP copayment for that Covered Service, the copayment amount the Member pays will be capped at the cost of the Covered Service.
As required by 42 C.F.R. §457.515, this includes, without limitation, Emergency Services that are provided at an Out-of-Network facility. Cost sharing for such Emergency Services is limited to the copayment amounts set forth in the CHIP Cost Sharing Table. If the MCO would have paid a lesser amount than the CHIP copayment in the absence of a CHIP copayment, then the copayment amount will be capped at the lesser amount.
Federal law prohibits charging premiums, deductibles, coinsurance, copayments, or any other cost-sharing to Members of Native Americans or Alaskan Natives. The HHSC Administrative Services Contractor will notify the MCO of Members who are not subject to cost sharing requirements. The MCO is responsible for educating Providers regarding the cost sharing waiver for this population.
An MCO’s monthly Capitation Payment will not be adjusted for a family’s failure to make its CHIP premium payment. There is no relationship between HHSC’s Capitation Payment to the MCO for coverage provided during a month and the family’s payment of its CHIP premium obligation for that month.
Cost sharing does not apply to CHIP Perinatal Program Members. The exemption from cost sharing applies through the end of the enrollment period.
As of the Effective Date of the Contract, cost sharing does not apply to Medicaid Members. If HHSC implements cost-sharing for Medicaid Members after the Effective Date of this Contract, the requirements of this section will apply, and HHSC will amend the Uniform Managed Care Manual to include Medicaid Cost Sharing Tables. Except for costs associated with unauthorized non-emergency services provided to a Member by Out-of-Network providers and for non-covered services, the Medicaid copayments outlined in the Uniform Managed Care Manual will be the only amounts that an MCO may impose and a provider may collect from a Medicaid-eligible family.
The MCO must educate Providers on the Immunization Standard Requirements set forth in Chapter 161, Health and Safety Code; the standards in the Advisory Committee on Immunization Practices (ACIP) Immunization Schedule; the AAP Periodicity Schedule for CHIP Members; and the ACIP Immunization Schedule for Medicaid Members. The MCO must educate Providers that Medicaid Members birth through age 20 must be immunized during the Texas Health Steps checkup according to the ACIP routine immunization schedule. The MCO shall also educate Providers that the screening provider is responsible for administration of the immunization and should not refer children to Local Health Departments to receive immunizations.
The MCO must educate Providers about, and require Providers to comply with, the requirements of Chapter 161, Health and Safety Code, relating to the Texas Immunization Registry (ImmTrac), to include parental consent on the Vaccine Information Statement.
The MCO must notify Medicaid and CHIP Providers that they may enroll, as applicable, as Texas Vaccines for Children Providers. In addition, the MCO must work with HHSC and Providers to improve the reporting of immunizations to the statewide ImmTrac Registry.
The MCO is not responsible for reimbursing dental providers for preventive and therapeutic dental services obtained by Medicaid or CHIP Members, with the exception of the dental services available to STAR+PLUS Members in the enrolled in the HCBS STAR+PLUS Waiver. However, medical and/or Hospital charges, such as anesthesia, that are necessary in order for Medicaid or CHIP Members to access standard therapeutic dental services, are Covered Services for Medicaid or CHIP Members. The MCO must provide access to facilities and physician services that are necessary to support the dentist who is providing dental services to a Medicaid or CHIP Member under general anesthesia or intravenous (IV) sedation.
The MCO must inform Network facilities, anesthesiologists, and PCPs what authorization procedures are required, and how Providers are to be reimbursed for the preoperative evaluations by the PCP and/or anesthesiologist and for the facility services. For dental-related medical Emergency Services, the MCO must reimburse Network and Out-of-Network providers in accordance with federal and state laws, rules, and regulations.
|
|
8.1.26 Health Home Services |
The MCO must provide Health Home Services. The MCOs must include a designated Provider to serve as the health home. The designated provider must meet the qualifications as established by the U.S. Secretary of Health and Human Services. The designated provider may be a provider operating with a team of health professionals, or a health team selected by the enrollee. The Health Home Services must be part of a person-based approach and holistically address the needs of persons with multiple chronic conditions or a single serious and persistent mental or health condition.
Health Home Services must include:
1. patient self-management education;
2. provider education;
3. evidence-based models and minimum standards of care;
4. standardized protocols and participation criteria;
5. provider-directed or provider-supervised care;
6. a mechanism to incentivize providers for provision of timely and quality care;
7. implementation of interventions that address the continuum of care;
8. mechanisms to modify or change interventions that are not proven effective;
9. mechanisms to monitor the impact of the Health Home Services over time, including both the clinical and the financial impact.
10. comprehensive care management;
11. care coordination and health promotion;
12. comprehensive traditional care, including appropriate follow-up, from inpatient to other settings;
13. patient and family support (including authorized representatives);
14. referral to community and social support services, if relevant, and;
15. use of health information technology to link services, as feasible and appropriate.
The Health Home Services requirements do not apply to Dual Eligible Members unless HHSC enters into a Dual Eligible Demonstration Project with the CMS. Under a demonstration project, STAR+PLUS MCOs will be required to coordinate health home initiatives with their affiliated Medicare Advantage/Special Needs Plans.
8.1.26.1 Health Home Services and Participating Providers
HHSC encourages MCOs to develop provider incentive programs for designated Providers who meet the requirements for patient-centered medical homes found in Texas Government Code §533.0029.
At a minimum, the MCO must:
1. maintain a system to track and monitor all Health Home Services participants for clinical, utilization, and cost measures;
2. implement a system for Providers to request specific Health Home interventions;
3. inform Providers about differences between recommended prevention and treatment and actual care received by Members enrolled in a Health Home Services program and Members' adherence to a service plan; and
4. provide reports on changes in a Member's health status to his or her PCP for Members enrolled in a Health Home Services program,.
8.1.26.2 MCO Health Home Services Evaluation
HHSC or its EQRO will evaluate the MCO's Health Home Services program.
8.1.27 Cancellation of Product Orders
If a Network Provider offers delivery services for covered products, such as durable medical equipment (DME), home health supplies, or outpatient drugs or biological products, then the MCO's Network Provider Agreement must require the Provider to reduce, cancel, or stop delivery at the Member's or the Member's authorized representative's written or oral request. The Provider must maintain records documenting the request.
8.1.28 Preadmission Screening and Resident Review (PASRR) Referring Entity Requirements
The MCO must follow any PASRR requirements when acting as a referring entity for Members as required by 40 Tex. Admin. Code §§ 17.101, 17.102(25), and 17.301.
|
|
8.2 Additional Medicaid MCO Scope of Work |
The following provisions apply to any MCO participating in the STAR or STAR+PLUS MCO Program.
|
|
8.2.1 Continuity of Care and Out-of-Network Providers |
The MCO must ensure that the care of newly enrolled Members is not disrupted or interrupted. The MCO must take special care to provide continuity in the care of newly enrolled Members whose health or behavioral health condition has been treated by specialty care providers or whose health could be placed in jeopardy if Medically Necessary Covered Services are disrupted or interrupted. Upon notification from a Member or Provider of the existence of a prior authorization, the new MCO must ensure Members receiving services through a prior authorization from either another MCO or FFS receive continued authorization of those services for the same amount, duration, and scope for the shortest period of one of the following: (1) 90 calendar days after the transition to a new MCO, (2) until the end of the current authorization period, or (3) until the MCO has evaluated and assessed the Member and issued or denied a new authorization. See Section 8.1.14, "Disease Management (DM)." for specific requirements for new Members transferring to the MCO’s Disease Management (DM) Program.
The MCO is required to ensure that Expansion Service Area clients receiving acute care services through a prior authorization as of the STAR and STAR+PLUS Operational Start Date receive continued authorization of those services for the shorter period of one of the following: 90 calendar days after Operational Start Date, or (2) until the expiration date of the prior authorization. The MCO is also required to ensure that Expansion Service Area clients receiving Community-based Long Term Care Services as of the STAR+PLUS Operational Start Date receive continued authorization of those services for up to 6 months after the Operational Start Date, unless a new assessment has been completed and new authorizations issued as described in Section 8.3.2.4. During transition, an HHSC’s Administrative Services Contractor or an HHS Agency will provide the MCO with files identifying Members with prior authorizations for acute care services and Members receiving Community-based Long Term Care Services. The MCO is required to work with HHSC, its Administrative Services Contractor, and DADS to ensure that all necessary authorizations are in place within the MCO’s system(s) for the continuation of Community-based Long Term Care Services and prior authorized acute care services. The MCO must describe the process it will use to ensure continuation of these services in its Transition/Implementation Plan for the Expansion Service Areas as noted in Section 7.2.1 Contract Start-Up and Planning. The MCO is also required to ensure that Community-based Long Term Care Services Providers in the Expansion Service Areas are educated about and trained regarding the process for continuing these services prior to the Operational Start Date (see Section 8.3.6.1 Training).
As described in Section 8.1.3.2, the MCO must allow pregnant Members past the 24th week of pregnancy to remain under the care of the Member’s current OB/GYN through the Member’s postpartum checkup, even if the provider is Out-of-Network. If a Member wants to change her OB/GYN to one who is in the Network, she must be allowed to do so if the Provider to whom she wishes to transfer agrees to accept her in the last trimester of pregnancy.
The MCO must pay a Member’s existing Out-of-Network providers for Medically Necessary Covered Services until the Member’s records, clinical information and care can be transferred to a Network Provider, or until such time as the Member is no longer enrolled in that MCO, whichever is shorter. Payment to Out-of-Network providers must be made within the time period required for Network Providers. The MCO must comply with Out-of-Network provider reimbursement rules as adopted by HHSC.
With the exception of pregnant Members who are past the 24th week of pregnancy, this Article does not extend the obligation of the MCO to reimburse the Member’s existing Out-of-Network providers for ongoing care for:
|
| |
| 1. more than 90 days after a Member enrolls in the MCO’s Program, or |
|
| |
| 2. for more than nine (9) months in the case of a Member who, at the time of enrollment in the MCO, has been diagnosed with and receiving treatment for a terminal illness and remains enrolled in the MCO. |
The MCO’s obligation to reimburse the Member’s existing Out-of-Network provider for services provided to a pregnant Member past the 24th week of pregnancy extends through delivery of the child, immediate postpartum care, and the follow-up checkup within the first six (6) weeks of delivery.
If a Member moves out of a Service Area, the MCO must provide or pay Out-of-Network providers in the new Service Area who provide Medically Necessary Covered Services to Members through the end of the period for which the MCO received a Capitation Payment for the Member.
If Covered Services are not available within the MCO’s Network, the MCO must provide Members with timely and adequate access to Out-of-Network services for as long as those services are necessary and not available in the Network, in accordance with 42 C.F.R. §438.206(b)(4). The MCO will not be obligated to provide a Member with access to Out-of-Network services if such services become available from a Network Provider.
The MCO must ensure that each Member has access to a second opinion regarding the use of any Medically Necessary Covered Service. A Member must be allowed access to a second opinion from a Network Provider or Out-of-Network provider if a Network Provider is not available, at no cost to the Member, in accordance with 42 C.F.R. §438.206(b)(3).
|
|
8.2.2 Provisions Related to Covered Services for Medicaid Members |
8.2.2.1 Emergency Services
MCO policy and procedures, Covered Services, claims adjudication methodology, and reimbursement performance for Emergency Services must comply with all applicable state and federal laws, rules, and regulations including 42 C.F.R. §438.114, whether the provider is Network or Out-of-Network. MCO policies and procedures must be consistent with the prudent layperson definition of an Emergency Medical Condition and the claims adjudication processes required under the Contract and 42 C.F.R. §438.114.
The MCO must pay for professional, facility, and ancillary services provided in a Hospital emergency department that are Medically Necessary to perform the medical screening examination and stabilization of a Member presenting with an Emergency Medical Condition or an Emergency Behavioral Health Condition, whether rendered by Network Providers or Out-of-Network providers.
The MCO cannot require prior authorization as a condition for payment for an Emergency Medical Condition, an Emergency Behavioral Health Condition, or labor and delivery. The MCO cannot limit what constitutes an Emergency Medical Condition on the basis of lists of diagnoses or symptoms. The MCO cannot refuse to cover Emergency Services based on the emergency room provider, Hospital, or fiscal agent not notifying the Member’s PCP or the MCO of the Member’s screening and treatment within ten (10) calendar days of presentation for Emergency Services. The MCO may not hold the Member who has an Emergency Medical Condition liable for payment of subsequent screening and treatment needed to diagnose the specific condition or stabilize the patient. The MCO must accept the emergency physician or provider’s determination of when the Member is sufficiently stabilized for transfer or discharge.
A medical screening examination needed to diagnose an Emergency Medical Condition must be provided in a Hospital based emergency department that meets the requirements of the Emergency Medical Treatment and Active Labor Act (EMTALA) (42 C.F.R. §§489.20, 489.24 and 438.114(b)&(c)). The MCO must pay for the emergency medical screening examination, as required by 42 U.S.C. §1395dd. The MCO must reimburse for both the physician's services and the Hospital's Emergency Services, including the emergency room and its ancillary services.
When the medical screening examination determines that an Emergency Medical Condition exists, the MCO must pay for Emergency Services performed to stabilize the Member. The emergency physician must document these services in the Member's medical record. The MCO must reimburse for both the physician's and Hospital's emergency stabilization services including the emergency room and its ancillary services.
The MCO must cover and pay for Post-Stabilization Care Services in the amount, duration, and scope necessary to comply with 42 C.F.R. §438.114(b)&(e) and 42 C.F.R. §422.113(c)(iii). The MCO is financially responsible for post-stabilization care services obtained within or outside the Network that are not pre-approved by a Provider or other MCO representative, but administered to maintain, improve, or resolve the Member’s stabilized condition if:
|
| |
| 1. the MCO does not respond to a request for pre-approval within one (1) hour; |
|
| |
| 2. the MCO cannot be contacted; or |
|
| |
| 3. the MCO representative and the treating physician cannot reach an agreement concerning the Member’s care and a Network physician is not available for consultation. In this situation, the MCO must give the treating physician the opportunity to consult with a Network physician and the treating physician may continue with care of the patient until an Network physician is reached. The MCO’s financial responsibility ends as follows: the Network physician with privileges at the treating Hospital assumes responsibility for the Member’s care; the Network physician assumes responsibility for the Member’s care through transfer; the MCO representative and the treating physician reach an agreement concerning the Member’s care; or the Member is discharged. |
8.2.2.2 Family Planning - Specific Requirements
The MCO must provide access to confidential family planning services.
The MCO must require, through Provider contract provisions, that Members requesting contraceptive services or family planning services are also provided counseling and education about the family planning and family planning services available to Members. The MCO must develop outreach programs to increase community support for family planning and encourage Members to use available family planning services.
The MCO must ensure that Members have the right to choose any Medicaid-enrolled family planning provider, whether the provider chosen by the Member is in or outside the Provider Network. The MCO must provide Members access to information about available providers of family planning services and the Member’s right to choose any Medicaid-enrolled family planning provider. As described in Sections 8.1.12.2 and 8.2.10, the MCO must also have procedures in place to educate the following Members about family planning programs, including the Texas Women's Health Program and DSHS Family Planning, Primary Health Care, and Expanded Primary Health Care programs.
| |
• | Pregnant Women in Medicaid who will lose eligibility after delivery |
| |
• | Young pregnant adults in Children's Medicaid who will have aged out of Children's Medicaid by the time of delivery |
The MCO must provide, at a minimum, the full scope of services available under the Texas Medicaid program for family planning services. The MCO will reimburse family planning agencies no less than the Medicaid fee-for service amounts for family planning services, including Medically Necessary medications, contraceptives, and supplies and will reimburse Out-of-Network family planning providers in accordance with HHSC’s administrative rules. The MCO cannot require prior authorization for family planning services whether rendered by a Network or Out-of-Network provider.
The MCO must provide medically approved methods of contraception to Members, provided that the methods of contraception are Covered Services. Contraceptive methods must be accompanied by verbal and written instructions on their correct use. The MCO must establish mechanisms to ensure all medically approved methods of contraception are made available to the Member, either directly or by referral to a Subcontractor.
The MCO must develop, implement, monitor, and maintain standards, policies and procedures for providing information regarding family planning to Providers and Members, specifically regarding State and federal laws governing Member confidentiality (including minors). Providers and family planning agencies cannot require parental consent for minors to receive family planning services. The MCO must require, through contractual provisions, that Subcontractors have mechanisms in place to ensure Member’s (including minor’s) confidentiality for family planning services.
8.2.2.3 Texas Health Steps (EPSDT)
8.2.2.3.1 Medical Checkups
The MCO must develop effective methods to ensure that children birth through age 20 receive Texas Health Steps services when due and according to the recommendations established by the Texas Health Steps periodicity schedule for children. The MCO must arrange for Texas Health Steps services for all eligible Members, except when Members or their representatives knowingly and voluntarily decline or refuse services after receiving sufficient information to make an informed decision.
For New Members birth through age 20, overdue or upcoming Texas Health Steps medical checkups should be offered as soon as practicable, but in no case later than 14 days of enrollment for newborns, and no later than 90 days of enrollment for all other eligible child Members. A Texas Health Steps annual medical checkup for an Existing Member age 36 months and older is due beginning on the child’s birthday and is considered timely if it occurs no later than 364 calendar days after the child’s birthday. For purposes of this requirement, the terms “New Member” and “Existing Member” are defined in Chapter 12.4 of the Uniform Managed Care Manual.
The MCO must have mechanisms in place to ensure that all newborn Members have an initial newborn checkup before discharge from the Hospital and in accordance with the Texas Health Steps periodicity schedule.
8.2.2.3.2 Oral Evaluation and Fluoride Varnish
The MCO must educate Providers on the availability of the Oral Evaluation and Fluoride Varnish (OEFV) Medicaid benefit that can be rendered and billed by certified Texas Health Steps providers when performed on the same day as the Texas Health Steps medical checkup. The Provider education must include information about how to assist a Member with referral to a dentist to establish a dental home.
8.2.2.3.3 Lab
The MCO must require Providers to send all Texas Health Steps newborn screens to the DSHS Laboratory Services Section or to a laboratory approved by the department under Section 33.016 of the Health and Safety Code. Providers must include detailed identifying information for all screened newborn Members and the Member's mother to allow DSHS to link the screens performed at the Hospital with screens performed at the newborn follow up Texas Health Steps medical checkup.
All laboratory specimens collected as a required component of a Texas Health Steps checkup (see Texas Medicaid Provider Procedures Manual for age-specific requirements) must be submitted to the DSHS Laboratory Services Section or to a laboratory approved by the department under Section 33.016 of the Health and Safety Code for analysis unless the Texas Medicaid Provider Procedures Manual, Children’s Services Handbook provides otherwise. The MCO must educate Providers about Texas Health Steps Program requirements for submitting laboratory tests to the DSHS Laboratory Services Section.
8.2.2.3.4 Education/Outreach
The MCO must ensure that Members are provided information and educational materials about the services available through the Texas Health Steps Program, and how and when they may obtain the services. The information should tell the Member how they can obtain dental benefits, services through the Medical Transportation Program, and advocacy assistance from the MCO. Standard language describing Texas Health Steps services, including medical, dental and case management services is provided in the UMCM. The MCO should use this language for Member Materials. Any additions to or deviations from the standard language must be reviewed and approved by HHSC prior to publication and distribution to Members.
The MCO will encourage Network pharmacies to also become Medicaid-enrolled durable medical equipment (DME) providers.
The MCO must provide outreach to Members to ensure they receive prompt services and are effectively informed about available Texas Health Steps services. Each month, the MCO must retrieve from the HHSC Administrative Services Contractor Bulletin Board System a list of Members who are due and overdue Texas Health Steps services. Using these lists and its own internally generated list, the MCO will contact such Members to schedule the service as soon as possible. The MCO outreach staff must coordinate with Texas Health Steps outreach unit to ensure that Members have access to the Medical Transportation Program, and that any coordination with other agencies is maintained.
The MCO must cooperate and coordinate with the State, outreach programs and Texas Health Steps regional program staff and agents to ensure prompt delivery of services to Children of Migrant Farm Workers and other migrant populations who may transition into and out of the MCO’s Program more rapidly and/or unpredictably than the general population.
The MCO must make an effort to coordinate and cooperate with existing community and school-based health and education programs that offer services to school-aged children in a location that is both familiar and convenient to the Members. The MCO must make a good faith effort to comply with Head Start’s requirement that Members participating in Head Start receive their Texas Health Steps checkup no later than 45 days after enrolling into either program.
8.2.2.3.5 Training
The MCO must provide appropriate training to all Network Providers and Provider staff in the Providers' area of practice regarding the scope of benefits available and the Texas Health Steps Program. Training must include:
1. Texas Health Steps benefits;
2. the periodicity schedule for Texas Health Steps medical checkups and immunizations;
3. the required elements of Texas Health Steps medical checkups;
4. providing or arranging for all required lab screening tests (including leadscreening), and Comprehensive Care Program (CCP) services available under the Texas Health Steps program to Members birth through age 20 years,
5. Medical Transportation services available to Members such as rides to healthcare service by bus, taxi, van, airfare, etc., gas money, mileage reimbursement, meals and lodging when away from home;
6. importance of updating contact information to ensure accurate Provider Directories and the Medicaid Online Provider Lookup;
7. information about MCO's process for acceleration of Texas Health Steps services for Children of Migrant Farm Workers;
8. missed appointment referrals and assistance provided by the Texas Health Steps Outreach and Informing Unit; and
9. administrative issues such as claims filing and services available to Members.
10. 72-hour emergency supply prescription policy and procedures;
11. outpatient prescription drug prior authorization process;
12. how to access the Medicaid formulary and preferred drug list (PDL) on HHSC's website;
13. how to use HHSC's free subscription service for accessing the Medicaid formulary and PDL through the Internet or hand-held devices; and
14. scope of Durable Medical Equipment (DME) and other items commonly found in a pharmacy that are available for Members birth through age 20 years.
MCO must also educate and train Providers regarding the requirements imposed on HHSC and contracting MCOs under the Consent Decree and Corrective Action Orders entered in Frew v. Janek, et. al. Providers should be educated and trained to treat each Texas Health Steps visit as an opportunity for a comprehensive assessment of the Member.
8.2.2.3.6 Data Validation
The MCO must require all Texas Health Steps Providers to submit claims for services paid (either on a capitated or fee-for service basis) on the CMS 1500 claim form and use the HIPAA compliant code set required by HHSC.
Encounter Data will be validated by chart review of a random sample of Texas Health Steps eligible enrollees against monthly Encounter Data reported by the MCO. HHSC or its designee will conduct chart reviews to validate that all screens are performed when due and as reported, and that reported data is accurate and timely. Substantial deviation between reported and charted Encounter Data could result in the MCO and/or Network Providers being investigated for potential Fraud, Abuse, or Waste without notice to the MCO or the Provider.
8.2.2.4 Perinatal Services
The MCO's perinatal Health Care Services must ensure appropriate care is provided to women and infant Members from the preconception period through the infant's first year of life. The MCO's perinatal health care system must comply with the requirements of the Texas Health and Safety Code, Chapter 32 (the Maternal and Infant Health Improvement Act) and administrative rules codified at 25 T.A.C. Chapter 37, Subchapter M.
The MCO must have a perinatal health care system in place that, at a minimum, provides the following services:
1. pregnancy planning and perinatal health promotion and education for reproductive-age women and adolescents;
2. perinatal risk assessment of non-pregnant women, pregnant and postpartum women, and infants up to one year of age;
3. access to appropriate levels of care based on risk assessment, including emergency care;
4. transfer and care of pregnant women, newborns, and infants to tertiary care facilities when necessary;
5. availability and accessibility of OB/GYNs, anesthesiologists, and neonatologists capable of dealing with complicated perinatal problems;
6. availability and accessibility of appropriate outpatient and inpatient facilities capable of dealing with complicated perinatal problems; and
7. education and care coordination for Members who are at high-risk for preterm labor, including education on the availability of medication regimens to prevent preterm birth, such as hydroxyprogesterone caproate. The MCO should also educate Providers on the prior authorization processes for these benefits and services.
On a monthly basis, HHSC will supply the MCO with a file containing birth record data. The MCO must use this file to identify reproductive-age Members with a previous preterm birth. The MCO must provide outreach to, education to, and care coordination for identified Members as described in this section to prevent preterm births. Care coordination may include service management under Section 8.1.13 and Member referrals to Providers to assess the need for the use of
hydroxyprogesterone caproate. The MCO must report on use of the data file as specified Section 8.1.20.2, “Reports” and in the Uniform Managed Care Manual Chapter 5.
The MCO must have a process to expedite scheduling a prenatal appointment for an obstetrical exam for a Member that meets the eligibility criteria to be designated in the Pregnant Woman Risk Group no later than two weeks after receiving the daily Enrollment File verifying the Member's enrollment into the MCO or has a confirmed diagnosis indicating pregnancy.
The MCO must have procedures in place to contact and assist a pregnant/delivering Member in selecting a PCP for her baby either before the birth or as soon as the baby is born.
The MCO must provide inpatient care and professional services relating to labor and delivery for its pregnant/delivering Members for up to 48 hours following an uncomplicated vaginal delivery and 96 hours following an uncomplicated Caesarian delivery. The MCO must provide neonatal care for its newborn Members until the time of discharge.
The MCO must Adjudicate provider claims for services provided to a newborn Member in accordance with HHSC's claims processing requirements using the proxy ID number or State-issued Medicaid ID number. The MCO cannot deny claims based on a provider's non-use of State-issued Medicaid ID number for a newborn Member. The MCO must accept provider claims for newborn services based on mother's name and/or Medicaid ID number with accommodations for multiple births, as specified by the MCO.
The MCO must notify providers involved in the care of pregnant/delivering women and newborns (including Out-of-Network providers and Hospitals) of the MCO's prior authorization requirements. The MCO cannot require a prior authorization for services provided to a pregnant/delivering Member or newborn Member for a medical condition that requires Emergency Services, regardless of when the emergency condition arises.
8.2.2.5 Sexually Transmitted Diseases (STDs) and Human Immunodeficiency Virus (HIV)
The MCO must provide STD services that include STD/HIV prevention, screening, counseling, diagnosis, and treatment. The MCO is responsible for implementing procedures to ensure that Members have prompt access to appropriate services for STDs, including HIV. The MCO must allow Members access to STD services and HIV diagnosis services without prior authorization or referral by a PCP.
The MCO must comply with Texas Family Code Section 32.003, relating to consent to treatment by a child. The MCO must provide all Covered Services required to form the basis for a diagnosis by the Provider as well as the STD/HIV treatment plan.
The MCO must make education available to Providers and Members on the prevention, detection and effective treatment of STDs, including HIV.
The MCO must require Providers to report all confirmed cases of STDs, including HIV, to the local or regional health authority according to 25 T.A.C. §§97.131 - 97.134, using the required forms and procedures for reporting STDs. The MCO must require the Providers to coordinate with the HHSC regional health authority to ensure that Members with confirmed cases of syphilis, chancroid, gonorrhea, chlamydia and HIV receive risk reduction and partner elicitation/notification counseling.
The MCO must have established procedures to make Member records available to public health agencies with authority to conduct disease investigation, receive confidential Member information, and provide follow up activities.
The MCO must require that Providers have procedures in place to protect the confidentiality of Members provided STD/HIV services. These procedures must include, but are not limited to, the manner in which medical records are to be safeguarded, how employees are to protect medical information, and under what conditions information can be shared. The MCO must inform and require its Providers who provide STD/HIV services to comply with all state laws relating to communicable disease reporting requirements. The MCO must implement policies and procedures to monitor Provider compliance with confidentiality requirements.
The MCO must have policies and procedures in place regarding obtaining informed consent and counseling Members provided STD/HIV services.
8.2.2.6 Tuberculosis (TB)
The MCO must provide Members and Providers with education on the prevention, detection and effective treatment of tuberculosis (TB). The MCO must establish mechanisms to ensure all procedures required to screen at-risk Members and to form the basis for a diagnosis and proper prophylaxis and management of TB are available to all Members, except services
referenced in Section 8.2.2.8 as Non-Capitated Services. The MCO must develop policies and procedures to ensure that Members who may be or are at risk for exposure to TB are screened for TB. An at-risk Member means a person who is susceptible to TB because of the association with certain risk factors, behaviors, drug resistance, or environmental conditions. The MCO must consult with the local TB control program to ensure that all services and treatments are in compliance with the guidelines recommended by the American Thoracic Society (ATS), the Centers for Disease Control and Prevention (CDC), and DSHS policies and standards.
The MCO must implement policies and procedures requiring Providers to report all confirmed or suspected cases of TB to the local TB control program within one (1) Business Day of identification, using the most recent DSHS forms and procedures for reporting TB. The MCO must provide access to Member medical records to DSHS and the local TB control program for all confirmed and suspected TB cases upon request.
The MCO must coordinate with the local TB control program to ensure that all Members with confirmed or suspected TB have a contact investigation and receive Directly Observed Therapy (DOT). The MCO must require, through contract provisions, that Providers report to DSHS or the local TB control program any Member who is non-compliant, drug resistant, or who is or may be posing a public health threat. The MCO must cooperate with the local TB control program in enforcing the control measures and quarantine procedures contained in Chapter 81 of the Texas Health and Safety Code.
The MCO must have a mechanism for coordinating a post-discharge plan for follow-up DOT with the local TB program. The MCO must coordinate with the DSHS South Texas Hospital and Texas Center for Infectious Disease for voluntary and court-ordered admission, discharge plans, treatment objectives and projected length of stay for Members with multi-drug resistant TB.
8.2.2.7 Objection to Provide Certain Services
In accordance with 42 C.F.R. §438.102, the MCO may file an objection based on moral or religions grounds to providing, reimbursing for, or providing coverage of a Covered Service or a counseling or referral service related to the Covered Service. The MCO must work with HHSC to develop a work plan to complete the necessary tasks and determine an appropriate date for implementation of the requested changes to the requirements related to Covered Services. The work plan will include timeframes for completing the necessary Contract and waiver amendments, adjustments to Capitation Rates, identification of the MCO and enrollment materials needing revision, and notifications to Members.
In order to meet the requirements of this section, no less than 120 days prior to the proposed effective date of a policy change, the MCO must notify HHSC of grounds for and provide detail concerning its moral or religious objections and the specific services covered under the objection.
8.2.2.8 Medicaid Non-capitated Services
The following Texas Medicaid programs, services, or benefits have been excluded from MCO Covered Services. Medicaid Members are eligible to receive these Non-capitated Services on a Fee-for-Service basis, or through a Dental MCO (for most dental services). MCOs should refer to relevant chapters in the Texas Medicaid Provider Procedures Manual for more information.
1. Texas Health Steps dental (including orthodontia);
2. Texas Health Steps environmental lead investigation (ELI);
3. Early Childhood Intervention (ECI) case management/service coordination;
4. Early Childhood Intervention Specialized Skills Training;
5. Case Management for Children and Pregnant Women;
6. Texas School Health and Related Services (SHARS);
7. Department of Assistive and Rehabilitative Services Blind Children's Vocational Discovery and Development Program;
8. tuberculosis services provided by DSHS-approved providers (directly observed therapy and contact investigation);
9. Health and Human Services Commission's Medical Transportation;
10. DADS hospice services;
11. Court-Ordered Commitments to inpatient mental health facilities as a condition of probation;
12. for STAR, Texas Health Steps Personal Care Services for Members birth through age 20;
13. for STAR+PLUS, Nursing Facility services (until February 28, 2015);
14. PASRR screenings, evaluations, and specialized services for STAR+PLUS Members; and
15. for Members who are enrolled in STAR or STAR+PLUS during an Inpatient Stay under one of the exceptions identified in Attachment A, Section 5.06(a)(2), Hospital facility charges associated with the Inpatient Stay are Non-Capitated Services under the circumstances described in Attachment A, Section 5.06(a)(2).
8.2.2.9 Referrals for Non-capitated Services
Although Medicaid MCOs are not responsible for paying or reimbursing for Non-capitated Services, MCOs are responsible for educating Members about the availability of Non-capitated Services, and for providing appropriate referrals for Members to obtain or access these services. The MCO is responsible for informing Providers that bills for all Non-capitated Services must be submitted to HHSC’s Claims Administrator for reimbursement.
8.2.2.10 Cooperation with Immunization Registry
The MCO must work with HHSC and health care providers to improve the immunization rate of Medicaid clients and the reporting of immunization information for inclusion in the Texas Immunization Registry, called “ImmTrac.”
8.2.2.11 Case Management for Children and Pregnant Women
The MCO must coordinate services with Case Management for Children and Pregnant Women. This coordination includes, but is not limited to, client education, outreach, case collaboration and referrals to Case Management for Children and Pregnant Women. The MCO is required to follow referral procedures as outlined by the State. Referrals to Case Management for Children and Pregnant Women are to be based upon guidelines provided by the State, assessment, plan of care, change in client's physical, mental or psychosocial condition, or at client's request.
Annually, all MCO Care Coordination/Case Management Staff must complete the Texas Health Steps Online module titled: Case Management Services in Texas and maintain proof of completion.
8.2.2.12 Children of Migrant Farm Workers (FWC)
The MCO must cooperate and coordinate with the State, outreach programs, and Texas Health Steps regional program staff and agents to ensure prompt delivery of services, in accordance with the Contract’s timeframes, to FWC Members and other migrant populations who may transition into and out of the MCO more rapidly and/or unpredictably than the general population.
The MCO must provide accelerated services to FWC Members. For purposes of this section, “accelerated services” are services that are provided to FWC Members prior to their leaving Texas for work in other states. Accelerated services include the provision of preventive Health Care Services that will be due during the time the FWC Member is out of Texas. The need for accelerated services must be determined on a case-by-case and according to the FWC Member’s age, periodicity schedule and health care needs.
The MCO must develop an annual plan identifying the process and methods it will use to identify/validate FWC and provide accelerated services to such Members in accordance with Chapter 12 of the Uniform Managed Care Manual.
|
|
8.2.3 Medicaid Significant Traditional Providers |
In the first three operational years of a Medicaid MCO Program, the MCO must offer Network Provider agreements to all Medicaid Significant Traditional Providers (STPs) identified by HHSC. Medicaid STPs are defined as pharmacy providers and providers of Acute and Long Term Services and Supports and, for STAR+PLUS, Community-based Long Term Care providers in a county that provided a significant level of care to Medicaid clients. Effective September 1, 2014, Medicaid STPs also include LMHAs. Effective March 1, 2015 Medicaid STPs must also include Nursing Facilities.
Medicaid STP requirements apply statewide for pharmacy and substance use disorder providers (SUDs). For STAR MCOs, the STP requirements for other provider types apply only in the Hidalgo, Jefferson, and Medicaid Rural Service Area(s); and in the following counties: Hudspeth, Carson, Deaf Smith, Hutchinson, Potter, Randall, Swisher, Austin, Wharton, Matagorda, Bandera, Brooks, Goliad, Karnes, Kenedy, Live Oak, and Fayette. For STAR+PLUS MCOs, the STP requirements for other types of providers apply to the Jefferson, El Paso, Lubbock, and Hidalgo Service Areas; as well as the following counties:
Austin, Wharton, Matagorda, Bandera, Brooks, Goliad, Karnes, Kenedy, Live Oak, and Fayette. The RFP documents included a list of Medicaid STPs by Service Area.
Beginning September 1, 2014, Medicaid STP requirements apply statewide for LMHAs in both STAR and STAR+PLUS. Beginning March 1, 2015, Medicaid STP requirements apply statewide for Nursing Facilities in STAR+PLUS. The MCO must treat a Nursing Facility as an STP if it holds a valid certification and license and it contracts with DADS as of September 1, 2013.
The MCO must comply with the STP enrollment requirements through February 28, 2018, for Nursing Facilities August 31, 2017, for LMHAs, and, with the exception of SUD providers, February 28, 2015 for all other STP Providers. The MCO must comply with the STP enrollment requirements for SUD providers in accordance with Section 8.2.7.2.2. Network Providers and non-network providers who believe they meet the STP requirements may contact HHSC to request HHSC’s consideration for STP status.
The MCO must give STPs the opportunity to participate in its Network for at least three years. However, the STP must:
1. agree to accept the MCO’s Provider reimbursement rate for the provider type; and
2. meet the standard credentialing requirements of the MCO, provided that lack of board certification or accreditation by the Joint Commission on Accreditation of Health Care Organizations (JCAHO) is not the sole grounds for exclusion from the Provider Network.
The MCO may terminate a Network Provider agreement with an STP after demonstrating, to the satisfaction of HHSC, good cause for the termination. Good cause may include evidence of provider fraud, waste, or abuse.
|
|
8.2.4 Provider Complaints and Appeals |
8.2.4.1 Provider Complaints
MCOs must develop, implement, and maintain a system for tracking and resolving all Medicaid Provider complaints. Within this process, the MCO must respond fully and completely to each complaint and establish a tracking mechanism to document the status and final disposition of each Provider complaint. The MCO must resolve Provider complaints within 30 days from the date the complaint is received. The HMO is subject to remedies, including liquidated damages, if at least 98 percent of Provider Complaints are not resolved within 30 days of receipt of the Complaint by the HMO. Please see the Attachment A “Uniform Managed Care Contract Terms & Conditions” and Attachment B-3 , “Deliverables/Liquidated Damages Matrix.”
MCOs must also resolve Provider complaints received by HHSC and referred to the MCOs no later than the due date indicated on HHSC’s notification form. HHSC will generally provide MCOs ten (10) Business Days to resolve such complaints. If an MCO cannot resolve a complaint by the due date indicated on the notification form, it may submit a request to extend the deadline. HHSC may, in its reasonable discretion, grant a written extension if the MCO demonstrates good cause.
Unless HHSC has granted a written extension as described above, the MCO is subject to contractual remedies, including liquidated damages if Provider complaints are not resolved by the timeframes indicated herein.
8.2.4.2 Appeal of Provider Claims
MCOs must develop, implement, and maintain a system for tracking and resolving all Medicaid Provider appeals related to claims payment, as required by Texas Government Code § 533.005(a)(15). Within this process, the MCO must respond fully and completely to each Medicaid Provider's claims payment appeal and establish a tracking mechanism to document the status and final disposition of each appeal. The MCO must allow Community-based Long Term Services and Supports providers to appeal claims that the MCO has not paid or denied by the 31st day following receipt.
In addition, the MCO's process must comply with Texas Government Code § 533.005(a)(19).
The MCO is subject to liquidated damages if at least 98 percent of Provider Appeals are not resolved within 30 calendar days of the MCO's receipt.
MCOs must contract with non-network physicians to resolve claims disputes related to denial on the basis of Medical Necessity that remain unresolved subsequent to a provider appeal. The physician resolving the dispute must not be an employee of the MCO's Medicaid or CHIP business but may be an employee in the MCO's Medicare or commerical lines of business. The determination of the physician resolving the dispute must be binding on the MCO and a Network Provider. The physician resolving the dispute must be licensed in the State of Texas and hold the same specialty or a related specialty as the appealing provider. HHSC reserves the right to amend this process to include an independent review process established by HHSC for final determination on these disputes.
|
|
8.2.5 Member Rights and Responsibilities |
In accordance with 42 C.F.R. §438.100, MCOs must maintain written policies and procedures for informing Members of their rights and responsibilities, and must notify
Members of their right to request a copy of these rights and responsibilities. The Member Handbook must include a notice that complies with Uniform Managed Care Manual Chapter 3.4.
|
|
8.2.6 Medicaid Member Complaint and Appeal System |
The MCO must develop, implement, and maintain a Member Complaint and Appeal system that complies with the requirements in applicable federal and state laws and regulations, including 42 C.F.R. §431.200; 42 C.F.R. Part 438, Subpart F, “Grievance System”; and the provisions of 1 T.A.C. Chapter 357, relating to Medicaid managed care organizations.
The Complaint and Appeal system must include a Complaint process, an Appeal process, and access to HHSC’s Fair Hearing System. The procedures must be the same for all Members and must be reviewed and approved in writing by HHSC or its designee. Modifications and amendments to the Member Complaint and Appeal system must be submitted for HHSC’s approval at least 30 days prior to the implementation.
8.2.6.1 Member Complaint Process
The MCO must have written policies and procedures for receiving, tracking, responding to, reviewing, reporting and resolving Complaints by Members or their authorized representatives. For purposes of Section 8.2.6 an “authorized representative” is any person or entity acting on behalf of the Member and with the Member’s written consent. A Provider may be an authorized representative.
MCOs also must resolve Member Complaints received by HHSC and referred to the MCOs no later than the due date indicated on HHSC’s notification form. HHSC will provide MCOs up to ten (10) Business Days to resolve such Complaints, depending on the severity and/or urgency of the Complaint. HHSC may, in its reasonable discretion, grant a written extension if the MCO demonstrates good cause.
Unless the HHSC has granted a written extension as described above, the MCO is subject to contractual remedies, including liquidated damages, if Member Complaints are not resolved by the timeframes indicated herein.
The MCO must resolve Complaints within 30 days from the date the Complaint is received. The MCO is subject to remedies, including liquidated damages, if at least 98 percent of Member Complaints are not resolved within 30 days of receipt of the Complaint by the MCO. Please see the Attachment A, "Uniform Managed Care Contract Terms and Conditions," and Attachment B-3, “Deliverables/Liquidated Damages Matrix.” The Complaint procedure must be the same for all Members. The Member or Member’s authorized representative may file a Complaint either orally or in writing. The MCO must also inform Members how to file a Complaint directly with HHSC, once the Member has exhausted the MCO’s Complaint process.
The MCO must designate an officer of the MCO who has primary responsibility for ensuring that Complaints are resolved in compliance with written policy and within the required timeframe. For purposes of Section 8.2.6.2, an “officer” of the MCO means a president, vice president, secretary, treasurer, or chairperson of the board for a corporation, the sole proprietor, the managing general partner of a partnership; or a person having similar executive authority in the organization.
The MCO must have a routine process to detect patterns of Complaints. Management, supervisory, and quality improvement staff must be involved in developing policy and procedure improvements to address the Complaints.
The MCO’s Complaint procedures must be provided to Members in writing and through oral interpretive services. A written description of the MCO’s Complaint procedures must be available in prevalent non-English languages for Major Population Groups identified by HHSC, at no more than a 6th grade reading level.
The MCO must include a written description of the Complaint process in the Member Handbook. The MCO must maintain and publish in the Member Handbook at least one toll-free telephone number with TeleTypewriter/Telecommunications Device for the Deaf (TTY/TDD) and interpreter capabilities for making Complaints. The MCO must provide such oral interpretive service to callers free of charge.
The MCO’s process must require that every Complaint received in person, by telephone, or in writing must be acknowledged and recorded in a written record and logged with the following details:
|
| |
| 2. identification of the individual filing the Complaint; |
|
| |
| 3. identification of the individual recording the Complaint; |
|
| |
| 4. nature of the Complaint; |
|
| |
| 5. disposition of the Complaint (i.e., how the MCO resolved the Complaint); |
|
| |
| 6. corrective action required; and |
For Complaints that are received in person or by telephone, the MCO must provide Members or their representatives with written notice of resolution if the Complaint cannot be resolved within one working day of receipt.
The MCO is prohibited from discriminating or taking punitive action against a Member or his or her representative for making a Complaint.
If the Member makes a request for disenrollment, the MCO must give the Member information on the disenrollment process and direct the Member to the HHSC Administrative Services Contractor. If the request for disenrollment includes a Complaint by the Member, the Complaint will be processed separately from the disenrollment request, through the Complaint process.
The MCO will cooperate with the HHSC’s Administrative Services Contractor and HHSC or its designee to resolve all Member Complaints. Such cooperation may include, but is not limited to, providing information or assistance to internal Complaint committees.
The MCO must provide designated Member Advocates, as described in Section 8.2.6.9, to assist Members in understanding and using the MCO’s Complaint system. The MCO’s Member Advocates must assist Members in writing or filing a Complaint and monitoring the Complaint through the MCO’s Complaint process until the issue is resolved.
8.2.6.2 Medicaid Standard Member Appeal Process
The MCO must develop, implement and maintain an Appeal procedure that complies with state and federal laws and regulations, including 42 C.F.R.§ 431.200 and 42 C.F.R. Part 438, Subpart F, “Grievance System.” An Appeal is a disagreement with an MCO Action as defined in Attachment A, "Uniform Managed Care Contract Terms and Conditions." The Appeal procedure must be the same for all Members. When a Member or his or her authorized representative expresses orally or in writing any dissatisfaction or disagreement with an Action, the MCO must regard the expression of dissatisfaction as a request to Appeal an Action.
A Member must file a request for an Appeal with the MCO within 30 days from receipt of the notice of the Action. The MCO is subject to remedies, including liquidated damages, if at least 98 percent of Member Appeals are not resolved within 30 days of receipt of the Appeal by the MCO. Please see the Attachment A, "Uniform Managed Care Contract Terms and
Conditions," and Attachment B-3, “Deliverables/Liquidated Damages Matrix.” To ensure continuation of currently authorized services, however, the Member must file the Appeal on or before the later of: (1) ten (10) days following the MCO’s mailing of the notice of the Action, or (2) the intended effective date of the proposed Action. The MCO must designate an officer who has primary responsibility for ensuring that Appeals are resolved in compliance with written policy and within the 30-day time limit.
The provisions of Chapter 4201, Texas Insurance Code, relating to a Member’s right to Appeal an Adverse Determination made by the MCO or a utilization review agent to an independent review organization, do not apply to a Medicaid recipient. Chapter 4201 is preempted by federal Fair Hearings requirements.
The MCO must have policies and procedures in place outlining the Medical Director’s role in an Appeal of an Action. The Medical Director must have a significant role in monitoring, investigating and hearing Appeals. In accordance with 42 C.F.R.§ 438.406, the MCO’s policies and procedures must require that individuals who make decisions on Appeals are not involved in any previous level of review or decision-making, and are health care professionals who have the appropriate clinical expertise in treating the Member’s condition or disease.
The MCO must provide designated Member Advocates, as described in Section 8.2.6.9 , to assist Members in understanding and using the Appeal process. The MCO’s Member Advocates must assist Members in writing or filing an Appeal and monitoring the Appeal through the MCO’s Appeal process until the issue is resolved.
The MCO must have a routine process to detect patterns of Appeals. Management, supervisory, and quality improvement staff must be involved in developing policy and procedure improvements to address the Appeals.
The MCO’s Appeal procedures must be provided to Members in writing and through oral interpretive services. A written description of the Appeal procedures must be available in prevalent non-English languages identified by HHSC, at no more than a 6th grade reading level. The MCO must include a written description of the Appeals process in the Member Handbook. The MCO must maintain and publish in the Member Handbook at least one toll-free telephone number with TTY/TDD and interpreter capabilities for requesting an Appeal of an Action. The MCO must provide such oral interpretive service to callers free of charge.
The MCO’s process must require that every oral Appeal received must be confirmed by a written, signed Appeal by the Member or his or her representative, unless the Member or his or her representative requests an expedited resolution. All Appeals must be recorded in a written record and logged with the following details:
|
| |
| 2. effective date of the Action; |
|
| |
| 3. date the Member or his or her representative requested the Appeal; |
|
| |
| 4. date the Appeal was followed up in writing; |
|
| |
| 5. identification of the individual filing; |
|
| |
| 6. nature of the Appeal; and |
|
| |
| 7. disposition of the Appeal, including a copy of the notice of disposition and the date it was sent to Member. |
The MCO must send a letter to the Member within five (5) Business Days acknowledging receipt of the Appeal request. Except for the resolution of an Expedited Appeal as provided in Section 8.2.6.3, the MCO must complete the entire standard Appeal process within 30 calendar days after receipt of the initial written or oral request for Appeal. The timeframe for a standard Appeal may be extended up to 14 calendar days if the Member or his or her representative requests an extension, or the MCO shows that there is a need for additional information and how the delay is in the Member’s interest. If the timeframe is extended, the MCO must give the Member written notice of the reason for delay if the Member had not requested the delay. The MCO must designate an officer who has primary responsibility for ensuring that Appeals are resolved within these timeframes and in accordance with the MCO’s written policies.
During the Appeal process, the MCO must provide the Member a reasonable opportunity to present evidence and any allegations of fact or law in person as well as in writing. The MCO must inform the Member of the time available for providing this information and that, in the case of an expedited resolution, limited time will be available.
The MCO must provide the Member and his or her representative opportunity, before and during the Appeal process, to examine the Member’s case file, including medical records and any other documents considered during the Appeal process. The MCO must include, as parties to the Appeal, the Member and his or her representative, including the legal representative of a deceased Member’s estate.
In accordance with 42 C.F.R.§ 438.420, the MCO must continue the Member’s benefits currently being received by the Member, including the benefit that is the subject of the Appeal, if all of the following criteria are met:
|
| |
| 1. the Member or his or her representative files the Appeal timely as defined in this Contract: |
|
| |
| 2. the Appeal involves the termination, suspension, or reduction of a previously authorized course of treatment; |
|
| |
| 3. the services were ordered by an authorized provider; |
|
| |
| 4. the original period covered by the original authorization has not expired; and |
|
| |
| 5. the Member requests an extension of the benefits. |
If, at the Member’s request, the MCO continues or reinstates the Member’s benefits while the Appeal is pending, the benefits must be continued until one of the following occurs:
1. the Member withdraws the Appeal;
2. ten (10) days pass after the MCO mails the notice resolving the Appeal against the Member, unless the Member, within the 10-day timeframe, has requested a Fair Hearing with continuation of benefits. In such a case, the benefits will continue until a Fair Hearing decision can be reached; or
3. a State Fair Hearing Officer issues a hearing decision adverse to the Member or the time period or service limits of a previously authorized service has been met.
In accordance with 42 C.F.R.§ 438.420(d), if the final resolution of the Appeal is adverse to the Member and upholds the MCO’s Action, then to the extent that the services were furnished to comply with the Contract, the MCO may recover such costs from the Member.
If the MCO or State Fair Hearing Officer reverses a decision to deny, limit, or delay services that were not furnished while the Appeal was pending, the MCO must authorize or provide the disputed services promptly and as expeditiously as the Member’s health condition requires.
If the MCO or State Fair Hearing Officer reverses a decision to deny authorization of services and the Member received the disputed services while the Appeal was pending, the MCO is responsible for the payment of services.
The MCO is prohibited from discriminating or taking punitive action against a Member or his or her representative for making an Appeal.
8.2.6.3 Expedited Medicaid MCO Appeals
In accordance with 42 C.F.R. §438.410, the MCO must establish and maintain an expedited review process for Appeals. Such expedited process will apply when the MCO determines (for a request from a Member) or the provider indicates (in making the request on the Member’s behalf or supporting the Member’s request) that taking the time for a standard resolution could seriously jeopardize the Member’s life or health. The MCO must follow all Appeal requirements for standard Member Appeals as set forth in Section 8.2.6.2), except where differences are specifically noted. The MCO must accept oral or written requests for Expedited Appeals.
Members must exhaust the MCO’s Expedited Appeal process before making a request for an expedited Fair Hearing. After the MCO receives the request for an Expedited Appeal, it must hear an approved request for a Member to have an Expedited Appeal and notify the Member of the outcome of the Expedited Appeal within three (3) Business Days, except that the MCO must complete investigation and resolution of an Appeal relating to an ongoing emergency or denial of continued
Hospitalization: (1) in accordance with the medical or dental immediacy of the case; and (2) not later than one (1) Business Day after receiving the Member’s request for Expedited Appeal.
Except for an Appeal relating to an ongoing emergency or denial of continued hospitalization, the timeframe for notifying the Member of the outcome of the Expedited Appeal may be extended up to 14 calendar days if the Member requests an extension or the MCO shows (to the satisfaction of HHSC, upon HHSC’s request) that there is a need for additional information and how the delay is in the Member’s interest. If the timeframe is extended, the MCO must give the Member written notice of the reason for delay if the Member had not requested the delay.
If the decision is adverse to the Member, the MCO must follow the procedures relating to the notice in Section 8.2.6.5. The MCO is responsible for notifying the Member of his or her right to access an expedited Fair Hearing from HHSC. The MCO will be responsible for providing documentation to HHSC and the Member, indicating how the decision was made, prior to HHSC’s expedited Fair Hearing.
The MCO is prohibited from discriminating or taking punitive action against a Member or his or her representative for requesting an Expedited Appeal. The MCO must ensure that punitive action is neither taken against a provider who requests an expedited resolution or supports a Member’s request.
If the MCO denies a request for expedited resolution of an Appeal, it must:
|
| |
| 1. transfer the Appeal to the timeframe for standard resolution, and |
|
| |
| 2. make a reasonable effort to give the Member prompt oral notice of the denial, and follow up within two (2) calendar days with a written notice. |
8.2.6.4 Access to Fair Hearing for Medicaid Members
The MCO must inform Members that they have the right to access the Fair Hearing process at any time during the Appeal system provided by the MCO, with the following exception. In the case of an expedited Fair Hearing process, the MCO must inform the Member that he or she must first exhaust the MCO’s internal Expedited Appeal process prior to filing an Expedited Fair Hearing request. The MCO must notify Members that they may be represented by an authorized representative in the Fair Hearing process.
If a Member requests a Fair Hearing, the MCO will complete the request for Fair Hearing and submit the form via facsimile to the appropriate Fair Hearings office, within five (5) calendar days of the Member's request for a Fair Hearing.
Within five (5) calendar days of notification that the Fair Hearing is set, the MCO will prepare an evidence packet for submission to the HHSC Fair Hearings staff and send a copy of the packet to the Member. The evidence packet must comply with HHSC’s Fair Hearings requirements.
8.2.6.5 Notices of Action and Disposition of Appeals for Medicaid Members
The MCO must notify the Member, in accordance with 1 T.A.C. Chapter 357, whenever the MCO takes an Action. The notice must, at a minimum, include any information required by the Uniform Managed Care Manual Chapters 3.21 and 3.22 regarding notices of actions and incomplete prior authorization requests.
8.2.6.6 Timeframe for Notice of Action
In accordance with 42 C.F.R.§ 438.404(c), the MCO must mail a notice of Action within the following timeframes:
|
| |
| 1. for termination, suspension, or reduction of previously authorized Medicaid-covered services, within the timeframes specified in 42 C.F.R.§§ 431.211, 431.213, and 431.214; |
|
| |
| 2. for denial of payment, at the time of any Action affecting the claim; |
|
| |
| 3. for standard service authorization decisions that deny or limit services, within the timeframe specified in 42 C.F.R.§ 438.210(d)(1); |
|
| |
| 4. if the MCO extends the timeframe in accordance with 42 C.F.R. §438.210(d)(1), it must: |
|
| |
| a. give the Member written notice of the reason for the decision to extend the timeframe and inform the Member of the right to file an Appeal if he or she disagrees with that decision; and |
|
| |
| b. issue and carry out its determination as expeditiously as the Member’s health condition requires and no later than the date the extension expires; |
|
| |
| 5. for service authorization decisions not reached within the timeframes specified in 42 C.F.R.§ 438.210(d) (which constitutes a denial and is thus an Adverse Action), on the date that the timeframes expire; and |
|
| |
| 6. for expedited service authorization decisions, within the timeframes specified in 42 C.F.R. 438.210(d). |
8.2.6.7 Notice of Disposition of Appeal
In accordance with 42 C.F.R.§ 438.408(e), the MCO must provide written notice of disposition of all Appeals including Expedited Appeals. The written resolution notice must include the results and date of the Appeal resolution. For decisions not wholly in the Member’s favor, the notice must contain:
|
| |
| 1. the right to request a Fair Hearing; |
|
| |
| 2. how to request a Fair Hearing; |
|
| |
| 3. The circumstances under which the Member may continue to receive benefits pending a Fair Hearing; |
|
| |
| 4. how to request the continuation of benefits; |
|
| |
| 5. if the MCO’s Action is upheld in a Fair Hearing, the Member may be liable for the cost of any services furnished to the Member while the Appeal is pending; and |
|
| |
| 6. any other information required by 1 T.A.C. Chapter 357 that relates to a managed care organization’s notice of disposition of an Appeal. |
8.2.6.8 Timeframe for Notice of Resolution of Appeals
In accordance with 42 C.F.R.§ 438.408, the MCO must provide written notice of resolution of Appeals, including Expedited Appeals, as expeditiously as the Member’s health condition requires, but the notice must not exceed the timeframes provided in this Section for standard Appeals or Expedited Appeals. For expedited resolution of Appeals, the MCO must make reasonable efforts to give the Member prompt oral notice of resolution of the Appeal, and follow up with a written notice within the timeframes set forth in this Section. If the MCO denies a request for expedited resolution of an Appeal, the MCO must transfer the Appeal to the timeframe for standard resolution as provided in this Section, make reasonable efforts to give the Member prompt oral notice of the denial, and follow up within two (2) calendar days with a written notice.
8.2.6.9 Medicaid Member Advocates
The MCO must provide Member Advocates to assist Members. Member Advocates must be physically located within the Service Area unless an exception is approved by HHSC. Member Advocates must inform Members of the following:
|
| |
| 1. their rights and responsibilities, |
|
| |
| 2. the Complaint process, |
|
| |
| 4. Covered Services available to them, including preventive services, and |
|
| |
| 5. Non-capitated Services available to them. |
Member Advocates must assist Members in writing Complaints and are responsible for monitoring the Complaint through the MCO’s Complaint process.
Member Advocates are responsible for making recommendations to the MCO’s management on any changes needed to improve either the care provided or the way care is delivered. Member Advocates are also responsible for helping or referring Members to community resources that are available to meet Members’ needs if services are not available from the MCO as Covered Services.
|
|
8.2.7 Additional Medicaid Behavioral Health Provisions |
8.2.7.1 This Section Intentionally Left Blank
8.2.7.2 Substance Abuse Benefit
8.2.7.2.1 Substance Abuse and Dependency Treatment Services
The requirements in this subsection apply to STAR+PLUS MCOs in all Service Areas and to STAR MCOs in all Service Areas except the Dallas Service Area. Members in the Dallas Service Area receive Behavioral Health Services through the NorthSTAR Program.
Substance use disorder includes substance abuse and dependence as defined by the current Diagnostic and Statistical Manual of Mental Disorders (DSM).
8.2.7.2.2 Providers
Providers for the substance abuse and dependency treatment benefit include: Hospitals, chemical dependency treatment facilities licensed by the Department of State Health Services, and practitioners of the healing arts.
MCOs must include Significant Traditional Providers (STPs) of these benefits in its Network, and provide such STPs with expedited credentialing. Medicaid MCOs must enter into provider agreements with any willing Significant Traditional Provider (STP) of these benefits that meets the Medicaid enrollment requirements, MCO credentialing requirements and agrees to the MCO’s contract terms and rates. For purposes of this section, STPs are providers who meet the Medicaid enrollment requirements and have a contract with the Department of State Health Services (DSHS) to receive funding for treatment under the Federal Substance Abuse Prevention and Treatment block grant. The STP requirements described herein apply to all Service Areas, and unlike other STP requirements are not limited to the first three (3) years of operations.
MCOs must maintain a provider education process to inform substance abuse treatment Providers in the MCO’s Network on how to refer Members for treatment.
8.2.7.2.3 Care Coordination
MCOs must ensure care coordination is provided to Members with a substance use disorder. MCOs must work with providers, facilities, and Members to coordinate care for Members with a substance use disorder and to ensure Members have access to the full continuum of Covered Services (including without limitation assessment, detoxification, residential treatment, outpatient services, and medication therapy) as Medically Necessary and appropriate. MCOs must also coordinate services with the DSHS, DFPS, and their designees for Members requiring Non-Capitated Services. Non-Capitated Services includes, without limitation, services that are not available for coverage under the Contract, State Plan or Waiver that are available under the Federal Substance Abuse and Prevention and Treatment block grant when provided by a DSHS-funded provider or covered by the DFPS under direct contract with a treatment provider. MCOs must work with DSHS, DFPS, and providers to ensure payment for Covered Services is available to Out-of-Network Providers who also provide related Non-capitated Services when the Covered Services are not available through Network Providers.
8.2.7.2.4 Member Education and Self-Referral for Substance Abuse and Dependency Treatment Services
MCOs must maintain a Member education process (including hotlines, manuals, policies and other Member Materials) to inform Members of the availability of and access to substance abuse treatment services, including information on self-referral.
8.2.7.3 Mental Health Rehabilitative Services and Targeted Case Management Services
This section is effective September 1, 2014.
For Members with severe and persistent mental illness (SPMI) and severe emotional disturbance (SED), Mental Health Rehabilitative Services and Targeted Case Management must be available to eligible STAR and STAR+PLUS Members. The MCO must maintain a qualified Network of entities, such as Local Mental Health Authorities (LMHAs) and multi-specialty groups, that employ providers of these services.
Mental Health Rehabilitative Services include training and services that help the Member maintain independence in the home and community, such as the following.
1. Medication training and support - curriculum-based training and guidance that serves as an initial orientation for the Member in understanding the nature of his or her mental illnesses or emotional disturbances and the role of medications in ensuring symptom reduction and the increased tenure in the community.
2. Psychosocial rehabilitative services - social, educational, vocational, behavioral, or cognitive interventions to improve the Member’s potential for social relationships, occupational or educational achievement, and living skills development.
3. Skills training and development - skills training or supportive interventions that focus on the improvement of communication skills, appropriate interpersonal behaviors, and other skills necessary for independent living or, when age appropriate, functioning effectively with family, peers, and teachers.
4. Crisis intervention - intensive community-based one-to-one service provided to Members who require services in order to control acute symptoms that place the Member at immediate risk of hospitalization, incarceration, or placement in a more restrictive treatment setting.
5. Day program for acute needs - short-term, intensive, site-based treatment in a group modality to an individual who requires multidisciplinary treatment in order to stabilize acute psychiatric symptoms of prevent admission to a more restrictive setting or reduce the amount of time spent in the more restrictive setting.
The MCO must authorize Mental Health Rehabilitative Services and Targeted Case Management in accordance with UMCM Chapter 15. As described in the UMCM, from September 1, 2014, to August 31, 2015, the MCO must authorize Mental Health Rehabilitative Services and Targeted Case Management using the DSHS Resiliency and Recovery Utilization Management Guidelines (RRUMG). During this year, the MCO must also ensure that a provider review a Member’s plan of care for Mental Health Rehabilitative Services in accordance with the RRUMG to determine whether a change in the Member’s condition or needs warrants a reassessment or change in service. If the Member’s condition warrants a change in service, the provider must submit a new plan of care to the MCO for authorization. Additionally, the MCO must ensure that from September 1, 2014, to August 31, 2015, providers of Mental Health Rehabilitative Services and Targeted Case Management use, and are trained and certified to use, the Adult Needs and Strengths Assessment (ANSA) and Child and Adolescent Needs and Strengths (CANS) tools for assessing a Member’s needs.
The MCO must ensure that STAR Service Management units and STAR+PLUS Service Coordinators coordinate with providers of TCM to ensure integration of behavioral and physical health needs of Members. Additionally, the MCO must ensure that if a Member loses Medicaid eligibility, STAR Service Management units and STAR+PLUS Service Coordinators refer the Member to Local Mental Health Authorities that can provide indigent mental health care.
|
|
8.2.8 Third Party Liability and Recovery and Coordination of Benefits |
Medicaid coverage is secondary when coordinating benefits with all other insurance coverage, unless an exception applies under federal law. Coverage provided under Medicaid will pay benefits for Covered Services that remain unpaid after all other insurance coverage has been paid. For Network Providers and Out-of Network providers with written reimbursement arrangements with the MCO, the MCO must pay the unpaid balance for Covered Services up to the agreed rates. For Out-of-Network providers with no written reimbursement arrangement, the MCO must pay the unpaid balance for Covered Services in accordance with HHSC's administrative rules regarding Out-of-Network payment (1 T.A.C. §353.4).
MCOs are responsible for establishing a plan and process for avoiding or recovering costs for services that should have been paid through a third party. The plan and process must be in accordance with state and federal law and regulations, including Section 1902(a)(25)(E) and (F) of the Social Security Act, which require MCOs to pay and later seek recovery from liable third
parties: (1) for prenatal and preventive pediatric care, and (2) in the context of a state child support enforcement action. The projected amount of TPR that the MCO is expected to recover may be factored into the rate setting process.
HHSC will provide the MCO, by Plan code, a monthly Member file (also known as a TPR client file). The file is an extract of those Medicaid Members who are known or believed to have other insurance. The file contains any Third Party Recovery (TPR) data that HHSC's claims administration agent has on file for individual Medicaid clients, organized by name and client number, and adding additional relevant information where available, such as the insured's name/contact information, type of coverage, the insurance carrier, and the effective dates.
The MCO must provide related reports to HHSC, as stated in Section 8.1.17.1, "Financial Reporting Requirements."
After 120 days from the date of adjudication of a claim that is subject to TPR, HHSC has the right to attempt recovery, independent of any MCO action. HHSC will retain, in full, all funds received as a result of any state-initiated TPR or subrogation action.
|
|
8.2.9 Coordination with Public Health Entities |
8.2.9.1 Reimbursed Arrangements with Public Health Entities
The MCO must make a good faith effort to enter into a Subcontract for Covered Services with Public Health Entities. Possible Covered Services that could be provided by Public Health Entities include, but are not limited to, the following services:
|
| |
| 1. Sexually Transmitted Diseases (STDs) services; |
|
| |
| 2. confidential HIV testing; |
|
| |
| 4. tuberculosis (TB) care; |
|
| |
| 5. Family Planning services; |
|
| |
| 6. Texas Health Steps medical checkups, and |
If the MCO is unable to enter into a contract with Public Health Entities, the MCO must document efforts to contract with Public Health Entities, and make such documentation available to HHSC upon request.
MCO Contracts with Public Health Entities must specify the scope of responsibilities of each party, the methodology and agreements regarding billing and reimbursements, reporting responsibilities, Member and Provider educational responsibilities, and the methodology and agreements regarding sharing of confidential medical record information between the Public Health Entity and the MCO or PCP.
The MCO must:
|
| |
| 1. identify care managers who will be available to assist public health providers and PCPs in efficiently referring Members to the public health providers, specialists, and health-related service providers either within or outside the MCO’s Network; and |
|
| |
| 2. inform Members that confidential healthcare information will be provided to the PCP, and educate Members on how to better utilize their PCPs, public health providers, emergency departments, specialists, and health-related service providers. |
8.2.9.2 Non-Reimbursed Arrangements with Local Public Health Entities
The MCO must coordinate with Public Health Entities in its Service Area(s) regarding the provision of essential public Health Care Services. In addition to the requirements listed above in Section 8.2.2, or otherwise required under state law or the Contract, the MCO must meet the following requirements:
|
| |
| 1. report to Public Health Entities regarding communicable diseases and/or diseases that are preventable by immunization as defined by state law; |
|
| |
| 2. notify the local Public Health Entity of communicable disease outbreaks involving Members; and |
|
| |
| 3. educate Members and Providers regarding WIC services available to Members. |
To follow-up on suspected or confirmed cases of childhood lead exposure, the MCO must coordinate with local Public Health Entities that have a child lead program, or with the DSHS Childhood Lead Poisoning Prevention Program when the local Public Health Entity does not have a child lead program.
In addition, the MCO must make a good faith effort to establish an effective working relationship with all state and local public health entities in its Service Area(s) to identify issues and promote initiatives addressing public health concerns.
|
|
8.2.10 Coordination with Other State Health and Human Services (HHS) Programs |
The MCO must coordinate with other state HHS Programs in each Service Area regarding the provision of essential public Health Care Services. In addition to the requirements listed above in Section 8.2.2. or otherwise required under state law or the Contract, the MCO must meet the following requirements:
1. require Providers to use the DSHS Bureau of Laboratories for specimens obtained as part of a Texas Health Steps medical checkup, as indicated in Section 8.1.4 under Laboratory Services;
2. notify Providers of the availability of vaccines through the Texas Vaccines for Children Program;
3. work with HHSC and Providers to improve the reporting of immunizations to the statewide ImmTrac Registry;
4. educate Providers and Members about services available through the Department of State Health Services (DSHS) Case Management for Children and Pregnant Women program;
5. coordinate with Case Management for Children and Pregnant Women for health care needs that are identified by Case Management for Children and Pregnant Women and referred to the MCO;
6. participate, to the extent practicable, in the community-based coalitions with the Medicaid-funded case management programs in the Department of Assistive and Rehabilitative Services (DARS), the Department of Aging and Disability Services (DADS), and DSHS;
7. cooperate with activities required of state and local public health authorities necessary to conduct the annual population and community based needs assessment;
8. report all blood lead results, coordinate and follow-up on suspected or confirmed cases of childhood lead exposure with the Childhood Lead Poisoning Prevention Program in DSHS, and follow the Centers for Disease Control and Prevention guidelines for testing children for lead and follow-up actions for children with elevated lead levels located at http://www.dshs.state.tx.us/lead/pdf_files/pb_109_physician_reference.pdf;
9. coordinate with Texas Health Steps Outreach Unit;
10. coordination of care protocols for working with Dental Contractors, as well as protocols for reciprocal referral and communication of data and clinical information regarding the Member's Medically Necessary dental Covered Services;
11. develop a coordination plan to share with local entities regarding clients identified as requiring special needs or assistance during a disaster; and
12. for STAR MCOs, educate Providers and Members about primary and family planning services available through the Texas Women's Health Program and DSHS Family Planning, Primary Health Care, and Expanded Primary Health Care programs.
|
|
8.2.11 Advance Directives |
Federal and state laws require MCOs and providers to maintain written policies and procedures for informing all adult Members 18 years of age and older about their rights to refuse, withhold or withdraw medical treatment and mental health treatment through advance directives (see Social Security Act §1902(a)(57) and §1903(m)(1)(A)). The MCO’s policies and procedures must include written notification to Members and comply with provisions contained in 42 C.F.R. § 489, Subpart I, relating to advance directives for all Hospitals, critical access Hospitals, skilled nursing facilities, home health agencies,
providers of home health care, providers of personal care services and hospices. The MCO’s policies and procedures must comply with state laws and rules regarding:
|
| |
| 1. a Member’s right to self-determination in making health care decisions; |
|
| |
| 2. the Advance Directives Act, Chapter 166, Texas Health and Safety Code, which includes: |
|
| |
| a. a Member’s right to execute an advance written directive to physicians and family or surrogates, or to make a non-written directive to administer, withhold or withdraw life-sustaining treatment in the event of a terminal or irreversible condition; |
|
| |
| b. a Member’s right to make written and non-written out-of-Hospital do-not-resuscitate (DNR) orders; |
|
| |
| c. a Member’s right to execute a Medical Power of Attorney to appoint an agent to make health care decisions on the Member’s behalf if the Member becomes incompetent; and |
|
| |
| 3. Chapter 137, Texas Civil Practice and Remedies Code, which includes a Member’s right to execute a Declaration for Mental Health Treatment in a document making a declaration of preferences or instructions regarding mental health treatment. |
The MCO must maintain written policies for implementing a Member’s advance directive. Those policies must include a clear and precise statement of limitation if a Provider cannot or will not implement a Member’s advance directive.
The MCO cannot require a Member to execute or issue an advance directive as a condition of receiving Health Care Services.
The MCO cannot discriminate against a Member based on whether or not the Member has executed or issued an advance directive.
The MCO’s policies and procedures must require the MCO and Subcontractors to comply with the requirements of state and federal law relating to advance directives. The MCO must provide education and training to employees and Members on issues concerning advance directives.
All materials provided to Members regarding advance directives must be written at a 7th - 8th grade reading comprehension level, except where a provision is required by state or federal law and the provision cannot be reduced or modified to a 7th - 8th grade reading level because it is a reference to the law or is required to be included “as written” in the state or federal law.
The MCO must notify Members of any changes in state or federal laws relating to advance directives within 90 days from the effective date of the change, unless the law or regulation contains a specific time requirement for notification.
A Member’s SSI status is effective the date the State’s eligibility system identifies the Member as Type Program 13 (TP13). The State is responsible for updating the State's eligibility system within 45 days of official notice of the Member’s Federal SSI eligibility by the Social Security Administration (SSA).
|
|
8.2.13 Medicaid Wrap-Around Services |
The MCO may be required to supplement Medicare coverage for STAR+PLUS Members by providing services, supplies, and outpatient drugs and biologicals that are available under the Texas Medicaid program. There are 3 categories of Medicaid wrap-around services:
1. Medicaid Only Services (i.e., services that do not have a corresponding Medicare service);
2. Medicare Services that become a Medicaid expense due to a benefit limitation on the Medicare side being met; and
3. Medicare Services that become a Medicaid expense due to coinsurance (True Cross-over Claims).
Section 8.2.13.1 includes requirements for Medicaid wrap-around services for outpatient drugs and biological products, and Section 8.2.13.2 includes requirements for Medicaid wrap-around services for coinsurance for Members in a Nursing Facility.
HHSC will provide advance written notice to the MCOs identifying other types of Medicaid wrap-around services that will become Covered Services, and the effective date of coverage.
8.2.13.1 Medicaid Wrap-Around Services for Outpatient Drugs and Biological Products
Effective March 1, 2012, STAR+PLUS MCOs will provide Medicaid wrap-around services for outpatient drugs and biological products to STAR+PLUS Members under a non-risk, cost settlement basis, as described in Attachment A, Section 10.16, "Supplemental Payments for Medicaid Wrap-Around Services for Outpatient Drugs and Biological Products." Refer to HHSC's Uniform Managed Care Manual, Chapter 2.2, "Pharmacy Claims Manual," for additional information regarding the claims processing requirements for these Medicaid wrap-around services.
8.2.13.2 Coinsurance for Members in Nursing Facilities
Effective March 1, 2015, The MCO will pay the State's Medicare coinsurance obligation for a qualified Dual Eligible Member's Medicare-covered stay in a Nursing Facility. The MCO is not responsible for the State's Medicare cost-sharing obligation for a Dual Eligible Member's Medicare-covered Nursing Facility Add-on Services, which are adjudicated by either the State’s fee-for-service claims administrator or the Dual Eligible Member’s Medicare plan, as applicable to the Member.
|
|
8.2.14 Medical Transportation |
HHSC reserves the right to amend the scope of the Contract to include medical transportation services (MTP) for Medicaid Members. For additional information regarding the MTP Program, the MCO should refer to the Nonemergency Medical Transportation (NEMT) Full Risk Broker Services RFP. MCOs should note that the MTP Program includes numerous Frew v. Janek requirements, including enhanced call center performance standards. If MTP services are added to the scope of the Contract, HHSC will provide advance written notice and conduct appropriate Readiness Review.
8.2.15 This Section Intentionally Left Blank
8.2.16 Supplemental Payments for Qualified Providers
In accordance with PPACA as amended by Section 1202 of the Health Care and Education Reconciliation Act and corresponding federal regulations at 42 C.F.R §§ 438.6 and 438.804, the MCO will make supplemental payments to qualified Medicaid providers for dates of service beginning on January 1, 2013, and ending on December 31, 2014. The Uniform Managed Care Manual will identify the types of providers and services that qualify for the supplemental payments.
HHSC or its Administrative Services Contractor will conduct the provider self-attestation process, and determine which providers and services are eligible for supplemental payments. HHSC will use encounter and other data provided by the MCO to calculate supplemental payments, and will provide the MCO with detailed reports identifying qualified providers, claims, and supplemental payment amounts. The MCO will use this information to respond to provider inquiries and complaints regarding supplemental payments, and will refer all cases for resolution as directed by HHSC.
The MCO will pay claims from qualified Network Providers at the MCO's contracted rates, and out-of-network providers in accordance with 1 Tex. Admin. Code § 353.4. The MCO's encounter data should reflect the actual amount paid to providers, and should not be adjusted to include supplemental payment amounts.
As described in Attachment A, Section 10.17, "Pass-through Payments for Provider Rate Increases," the MCO must pay the full amount of supplemental payments to qualified providers no later than 30 calendar days after receipt of HHSC's supplemental payment report, contingent upon MCO's receipt of payment of the allocation. The MCO must submit a report and certification, in the form and manner identified in the Uniform Managed Care Manual, to validate that payments have been made to qualified providers in accordance with HHSC's calculations. In addition, the MCO must provide reports, in the manner and frequency prescribed in the Uniform Managed Care Manual, documenting all claims adjustments that alter the supplemental payment amounts, including documentation of recoupments of overpaid amounts. The MCO must collect and refund all overpayments of supplemental payments to HHSC in the format and manner prescribed in the Uniform Managed Care Manual. In cases where a third party is responsible for all or part of a Covered Service and the MCO recovers only part of the amount paid by the MCO, then the amount recovered must be applied first to the supplemental payment and returned to HHSC. If the amount recovered is less than the supplemental payment, then the MCO will return the full amount of the recovery to HHSC.
8.2.17 Electronic Visit Verification
On or after June 1, 2014, HHSC will require STAR+PLUS MCOs to use an EVV system to verify nursing services, attendant care services, and other services identified by HHSC, including:
(1) the provider's name;
(2) the recipient's name;
(3) the service location; and
(4) the date and time the provider begins and ends each service delivery visit.
Similarly, on or after September 1, 2014, HHSC may require STAR MCOs to use an EVV system to verify nursing services and other services identified by HHSCMCOs must contract with EVV Vendors for the provision of EVV services in a manner consistent with the UMCM. The MCO may not pass EVV transaction costs to providers.
8.2.18 Telemedicine, Telehealth, and Telemonitoring Access
Telemedicine, telehealth, and telemonitoring are Covered Services and are benefits of Texas Medicaid as provided in the Texas Medicaid Provider Procedures Manual. MCOs are encouraged to contract with Providers offering these services to provide better access to healthcare for its Members. In addition, a Medicaid MCO must be able to accept and process Provider claims for these services in conformity with the Texas Medicaid benefit.
|
|
8.3 Additional STAR+PLUS Scope of Work |
|
|
8.3.1 Covered Community-Based Long-Term Services and Supports |
The MCO must ensure that STAR+PLUS Members needing Community Long-term Services and Supports are identified, and that services are referred and authorized in a timely manner. The MCO must ensure that Providers of Community Long-term Services and Supports are licensed or certified to deliver the services they provide. The inclusion of Community Long-term Services and Supports in a managed care model presents challenges, opportunities and responsibilities.
Community Long-term Services and Supports may be necessary as a preventive service to avoid more expensive hospitalizations, emergency room visits, or institutionalization. Community Long-term Services and Supports should also be made available to Members to assure maintenance of the highest level of functioning possible in the least restrictive setting. Community Long-term Services and Supports to assist with the activities of daily living must be considered as important as needs related to a medical condition. MCOs must provide both Medically Necessary and Functionally Necessary Covered Services to Community Long-term Services and Supports Members.
8.3.1.1 Community Based Long-Term Services and Supports Available to All Members
The MCO must contract with Providers of Personal Assistance Services (PAS) and Day Activity and Health Services (DAHS) to ensure access to these services for all STAR+PLUS Members. At a minimum, these Providers must meet all of the following state licensure and certification requirements for providing the services in Attachment B-2.2, STAR+PLUS Covered Services.
|
| |
Community-based Long-Term Services and Supports Available to All Members |
Service | Licensure and Certification Requirements |
Primary Home Care | The Provider must be licensed by DADS as a Home and Community Support Services Agency (HCSSA). The level of licensure required depends on the type of service delivered. NOTE: For primary home care and client managed attendant care, the agency may have only the Personal Assistance Services level of licensure.
|
Day Activity and Health Services (DAHS)
| The Provider must be licensed by the DADS Regulatory Division as an adult day care provider. To provide DAHS, the Provider must provide the range of services required for DAHS.
|
8.3.1.2 HCBS STAR+PLUS Waiver Services Available to Qualified Members
The HCBS STAR+PLUS Waivers provides Community Long-term Services and Supports to Medicaid Eligibles who are elderly and to adults with disabilities as a cost-effective alternative to living in a nursing facility. These Members must be age 21 or older, be a Medicaid recipient or be otherwise financially eligible for waiver services. To be eligible for HCBS STAR+PLUS Waiver Services, a Member must meet income and resource requirements for Medicaid nursing facility care, and receive a determination from HHSC on the medical necessity/level of care of the nursing facility care. The MCO must make available to STAR+PLUS Members who meet these eligibility requirements the array of services allowable through HHSC's CMS-approved HCBS STAR+PLUS Waiver (see Attachment B-2.2, “STAR+PLUS Covered Services”).
|
| |
Community-based Long-Term Services and Supports under the HCBS STAR+PLUS Waiver |
Service | Licensure and Certification Requirements |
Personal Assistance Services
| The Provider must be licensed by DADS as a Home and Community Support Services Agency (HCSSA). The level of licensure required depends on the type of service delivered. For Primary Home Care and Client Managed Attendant Care, the agency may have only the Personal Assistance Services level of licensure.
|
|
| |
Employment Assistance | The Provider must meet all of the criteria in one of these three options.
Option 1: a bachelor's degree in rehabilitation, business, marketing, or a related human services field; and six months of documented experience providing services to people with disabilities in a professional or personal setting.
Option 2: an associate's degree in rehabilitation, business, marketing, or a related human services field; and one year of documented experience providing services to people with disabilities in a professional or personal setting.
Option 3: a high school diploma or GED; and two years of documented experience providing services to people with disabilities in a professional or personal setting. |
Supported Employment | The Provider must meet all of the criteria in one of these three options.
Option 1: a bachelor's degree in rehabilitation, business, marketing, or a related human services field; and six months of documented experience providing services to people with disabilities in a professional or personal setting.
Option 2: an associate's degree in rehabilitation, business, marketing, or a related human services field; and one year of documented experience providing services to people with disabilities in a professional or personal setting.
Option 3: a high school diploma or GED; and two years of documented experience providing services to people with disabilities in a professional or personal setting. |
Assisted Living Services | The Provider must be licensed by the Texas Department of Aging and Disability Services, Long Term Care Regulatory Division in accordance with 40 T.A.C., Part 1, Chapter 92. The type of licensure determines what services may be provided. |
|
| |
Emergency Response Service Provider | Licensed by the Texas Department of State Health Services as a Personal Emergency Response Services Agency under 25 T.A.C., Part 1, Chapter 140, Subchapter B. |
Nursing Services | Licensed Registered Nurse by the Texas Board of Nursing under 22 T.A.C., Part 11, Chapter 217. The registered nurse must comply with the requirements for delivery of nursing services, which include requirements such as compliance with the Texas Nurse Practice Act and delegation of nursing tasks. The licensed vocational nurse must practice under the supervision of a registered nurse, licensed to practice in the State. |
Cognitive Rehabilitation Therapy | Psychologist must be licensed under Texas Occupations Code Chapter 501.
Speech and language pathologists must be licensed under Texas Occupations Code Chapter 401. Occupational Therapist must be licensed under Texas Occupations Code Chapter 454. |
Adult Foster Care | Adult foster care homes must meet the minimum standards described in the STAR+PLUS Handbook Section 7100 found at http://www.dads.state.tx.us/handbooks/sph/. Adult foster care homes serving four or more participants must be licensed by DADS under 40 Tex. Admin. Code Chapter 92. |
Dental | Licensed by the Texas State Board of Dental Examiners as a Dentist under 22 T.A.C., Part 5, Chapter 101. |
Respite Care | Licensed by DADS as a Home and Community Support Services Agency (HCSSA) under 40 T.A.C., Part 1, Chapter 97. |
Home Delivered Meals | Providers must comply with requirement of 40 T.A.C., Part 1, Chapter 55 for providing home delivered meal services, which include requirements such as dietary requirements, food temperature, delivery times, and training of volunteers and others who deliver meals. |
Physical Therapy (PT) Services | Licensed Physical Therapist through the Texas Board of Physical Therapy Examiners, Chapter 453 of the Texas Occupations Code. |
Occupational Therapy (OT) Services | Licensed Occupational Therapist through the Texas Board of Occupational Therapy Examiners, Chapter 454 of the Texas Occupations Code. |
Speech, Hearing, and Language Therapy Services | Licensed Speech Therapist through the Department of State Health Services. |
Financial Management Services | The Providers must complete DADS’ required training. Current FMSAs contracted by DADS are assumed to have completed the training. |
Support Consultation | Providers must be certified by the Department of Aging and Disability Services. |
|
| |
Transition Assistance Services (TAS) | The Provider must comply with the requirements for delivery of TAS, which include requirements such as allowable purchases, cost limits, and timeframes for delivery. TAS providers must demonstrate knowledge of, and experience in, successfully serving individuals who require home and community-based services |
Minor Home Modification | No licensure or certification requirements. |
Adaptive Aids and Medical Equipment | No licensure or certification requirements. |
Medical Supplies | No licensure or certification requirements. |
|
|
8.3.2 Service Coordination |
8.3.2.1 Service Coordination Plan Requirements
The MCO must implement an HHSC-approved service coordination plan no later than October 1, 2013. At a minimum, the service coordination plan must address:
| |
• | how outreach to Members will be conducted; |
| |
• | how Members are assessed and their service plans developed (the initial identification of Members' needed services and supports); |
| |
• | how Members will be identified as needing an assessment when changes in their health or life circumstances occur; |
| |
• | the Member's needs and preferences; |
| |
• | the minimum number of service coordination contacts a Member will receive per year; |
| |
• | how service coordination will be provided (face-to-face, telephone contact, etc.); and |
| |
• | how these service coordination services will be tracked by the MCO. |
The service coordination plan must address service planning for Members in the following categories.
| |
• | Level 1 Members: Highest level of utilization |
| |
• | Includes HCBS SPW, Nursing Facility, and other Members with complex medical needs. |
| |
• | MCOs must provide Level 1 Members with a single identified person as their assigned Service Coordinator. Beginning March 1, 2015, all Members within a Nursing Facility must have the same assigned Service Coordinator. HHSC must provide written approval for any exceptions. |
| |
• | At a minimum, beginning March 1, 2015, all Level 1 Members in a Nursing Facility must receive quarterly face-to-face visits, including Nursing Facility care planning meetings or other interdisciplinary team meetings. |
| |
• | All other Level 1 Members must receive a minimum of two face-to-face service coordination contacts annually. |
•
| |
• | Level 2 Members: Lower risk/utilization |
| |
• | MCOs must provide Level 2 Members with a single identified person as their assigned Service Coordinator. Members and required assessments are as follows. |
| |
• | Members receiving LTSS for Personal Assistance Services or Day Activity and Health Services (PAS and DAHS) must receive a minimum of one face-to-face and one telephonic service coordination contact annually. |
| |
• | Members with a history of behavioral health issues (multiple outpatient visits, hospitalization, or institutionalization within the past year) must receive a minimum of one face-to-face and one telephonic service coordination contact annually. |
| |
• | Members with a history of substance abuse (multiple outpatient visits, hospitalization, or institutionalization within the past year) must receive a minimum of one face-to-face and one telephonic service coordination contact annually. |
| |
• | Dual Eligibles who do not meet Level 1 requirements must receive a minimum of two telephonic service coordination contacts annually. |
| |
• | Level 3 Members: Members who do not qualify as Level 1 or Level 2 |
| |
• | MCO must make at least two telephonic service coordination outreach contacts yearly. |
| |
• | Level 3 Members are not required to have a named Service Coordinator, unless they request service coordination services. |
MCOs must provide written notice to all STAR+PLUS Members (including Level 3 Members who do not have a named Service Coordinator) that includes:
| |
• | A description of service coordination; and |
| |
• | The MCO's Service Coordination phone number. |
MCOs must notify all STAR+PLUS Members receiving service coordination of:
| |
• | The name of their Service Coordinator; |
| |
• | The phone number of their Service Coordinator; |
| |
• | The minimum number of contacts they will receive every year; and |
| |
• | The types of contacts they will receive. |
8.3.2.2 Service Coordination Structure
Individuals receiving Level 1 or Level 2 Service Coordination must have a single, identified person as their assigned Service Coordinator and the MCO must notify Members within 15 Business Days of the name and phone number of their new Service Coordinator, if their Service Coordinator changes. The MCO must also post the new Service Coordinator's information on the portal within the same time period.
Service coordination teams must be led by at least one Service Coordinator. Team members must have the following expertise or access within the MCO to identified subject matter experts in the following areas.
| |
• | Behavioral health, including outpatient services and Mental Health Rehabilitative Services (Mental Health Rehabilitative Services become Covered Services September 1, 2014) |
| |
• | Local resources (such as basic needs like housing, food, utility assistance) |
| |
• | End of life/advanced illness |
| |
• | Texas Promoting Independence strategies |
| |
• | Consumer Directed Services options |
| |
• | Person-directed planning |
| |
• | Employment Assistance and Supported Employment (become Covered Services September 1, 2014) |
| |
• | PASRR requirements (effective March 1, 2015) |
Service Coordination teams will have an overarching philosophy of independent living, self-determination, and community integration.
All STAR+PLUS MCOs must provide dedicated toll-free service coordination phone numbers. These numbers, if not regional, must have the capabilities of warm transferring to the MCO's regional office.
The MCO must furnish a Service Coordinator to all STAR+PLUS Members who request one. The MCO should also furnish a Service Coordinator to a STAR+PLUS Member when the MCO determines one is required through an assessment of the Member's health and support needs. If the Member refuses Service Coordination, the MCO should document the refusal in the Member's case file.
At a minimum, the MCO will have three tiers of Service Coordination for all Members.
The MCO must ensure that each STAR+PLUS Member has a qualified PCP who is responsible for overall clinical direction and, in conjunction with the Service Coordinator, serves as a central point of integration and coordination of Covered Services, including primary, Acute Care, Long-term Services and Supports, and Behavioral Health Services.
The Service Coordinator must work with the Member's PCP to coordinate all STAR+PLUS Covered Services and any applicable Non-capitated Services. This requirement applies regardless of whether the PCP is in the MCO's Network particularly for Dual Eligible Members. In order to integrate the Member's care while remaining informed of the Member's needs and condition, the Service Coordinator must actively involve the Member's primary and specialty care Providers, including Behavioral Health Service Providers, Providers of Non-capitated Services, and Medicare Advantage health plans for qualified Dual Eligible Members. When considering whether to refer a Member to a nursing facility or other long-term care facility, the MCO must consider the availability of the Program of All-Inclusive Care for the Elderly (PACE) for that Member.
Dual Eligible Members receive most Acute Care services through Medicare, rather than Medicaid.
The MCO must identify and train Members or their families to coordinate their own care, to the extent of the Member's or the family's capability and willingness to coordinate care.
8.3.2.3 Service Coordinators
The MCO must employ as Service Coordinators persons experienced in meeting the needs of vulnerable populations who have Chronic or Complex Conditions. Service Coordinators are Key MCO Personnel as described in Attachment A, "Uniform Managed Care Contract Terms and Conditions," Section 4.02, and must meet the requirements set forth in Section 4.04.1 of Attachment A.
Service Coordinators must meet the following minimum requirements:
| |
• | A Service Coordinator for a Level 1 Member must be a registered nurse (RN) or nurse practitioner (NP). Licensed vocational nurses (LVNs) employed as Service Coordinators before March 1, 2013 will be allowed to continue in that role. |
| |
• | A Service Coordinator for a Level 2 or 3 Member must have an undergraduate or graduate degree in social work or a related field or be an LVN, RN, NP, or physician's assistant (PA); or have a minimum of a high school diploma or GED and direct experience with the ABD/SSI population in three of the last five years. |
| |
• | Service Coordinators for Level 3 Members must have experience in meeting the needs of the member population served (for example, people with disabilities). |
| |
• | Service Coordinators must possess knowledge of the principles of most integrated settings, including federal and state requirements. |
| |
• | Service Coordinators must complete 16 hours of service coordination training every two years. MCOs must administer the training, which must include: |
o information related to the population served;
o how to assess Member’s medical, behavioral health, and social needs and concerns;
o how to assess and provide information to Members related to Employment Assistance and Supported Employment (become Covered Services September 1, 2014);
o how to provide Targeted Case Management for Members receiving Mental Health Rehabilitative Services (become Covered Services September 1, 2014);
o PASRR requirements (effective March 1, 2015)
o person-directed planning;
o refresher of available local and statewide resources; and
o respect for cultural, spiritual, racial, and ethnic beliefs of others.
8.3.2.4 Referral to Community Organizations
The MCO must provide information about and referral to community organizations that may not be providing STAR+PLUS Covered Services, but are otherwise important to the health and wellbeing of Members. These organizations include, but are not limited to:
1. state/federal agencies (e.g., those agencies with jurisdiction over aging, public health, substance abuse, mental health, intellectual or development disabilities, rehabilitation, income support, nutritional assistance, family support agencies, etc.);
2. social service agencies (e.g., area agencies on aging, residential support agencies, independent living centers, supported employment agencies, etc.);
3. city and county agencies (e.g., welfare departments, housing programs, etc.);
4. civic and religious organizations; and
5. consumer groups, advocates, and councils (e.g., legal aid offices, consumer/family support groups, permanency planning, etc.).
8.3.2.5 Discharge Planning
The MCO must provide discharge planning, transition care, and other education programs to Network Providers regarding all available long-term care settings and options. The MCO must have a protocol for quickly assessing the needs of Members discharged from a Hospital, Nursing Facility, or other care or treatment facility.
The MCO’s Service Coordinator must work with the Member’s PCP, the Hospital, or Nursing Facility discharge planner(s), the attending physician, the Member, and the Member’s family to assess and plan for the Member’s discharge. When Long-term Services and Supports are needed, the MCO must ensure that the Member’s discharge plan includes arrangements for receiving community-based care whenever possible. The MCO must ensure that the Member, the Member’s family, and the Member’s PCP are all well informed of all service options available to meet the Member’s needs in the community.
8.3.2.6 Transition Plan for New STAR+PLUS Members
The MCO must provide a transition plan for Members enrolled in the STAR+PLUS Program. HHSC, or the previous STAR+PLUS MCO contractor, will provide the MCO with information such as detailed care plans and names of current providers, for newly enrolled Members already receiving Long-term Services and Supports, including Nursing Facility Services, at the time of enrollment in the MCO. The MCO must ensure that current providers are paid for Medically Necessary and Functionally Necessary Covered Services that are delivered in accordance with the Member’s existing care plan after the Member is enrolled in the MCO and until the transition plan is developed.
The transition planning process must include the following:
1. review of existing care plans prepared by DADS or another STAR+PLUS MCO;
2. preparation of a transition plan that ensures continuous care under the Member’s existing Care Plan during the transfer into the MCO’s Network while the MCO conducts an appropriate assessment and development of a new plan, if needed;
3. if durable medical equipment or supplies had been ordered prior to enrollment but have not been received by the time of enrollment, coordination and follow-through to ensure that the Member receives the necessary supportive equipment and supplies without undue delay; and
4. payment to the existing provider of service under the existing authorization for up to six months, until the MCO has completed the assessment and Service Plans and issued new authorizations.
Except as provided below, the MCO must review any existing care plan and develop a transition plan within 30 days of receiving notice of the Member’s enrollment. For all existing care plans received prior to the Operational Start Date, the MCO will have additional time to complete this process, not-to-exceed 120 days after the Member’s enrollment. The transition plan will remain in place until the MCO contacts the Member or the Member’s representative and coordinates modifications to the Member’s current plan. The MCO must ensure that the existing services continue and that there are no breaks in services. For initial implementation of the STAR+PLUS program in a Service Area, the MCO must honor existing LTSS authorizations for up to six months following the Operational Start Date, or until the MCO has evaluated and assessed the Member and issued new authorizations. For the carve-in of Nursing Facility services effective March 1, 2015, the MCO must honor existing authorizations for the earliest of (1) six months after the carve-in of Nursing Facility services, (2) until the expiration date of the prior authorization, or (3) until the MCO has evaluated and assessed the Member and issued or denied a new authorization.
The transition plan must include:
1. the Member’s history;
2. summary of current medical, behavioral health, and social needs and concerns;
3. short-term and long term needs and goals;
4. a list of services required, their frequency, and
5. a description of who will provide these services.
The transition plan may include information for services outside the scope of covered benefits such as how to access affordable, integrated housing.
The MCO must ensure that the Member or the Member’s representative is involved in the assessment process and fully informed about options, is included in the development of the transition plan, and is in agreement with the plan when completed.
8.3.2.7 Centralized Medical Record and Confidentiality
The Service Coordinator must be responsible for maintaining a centralized record related to Member contacts, assessments and service authorizations. The MCO must ensure that the organization of and documentation included in the centralized Member record meets all applicable professional standards ensuring confidentiality of Member records, referrals, and documentation of information.
The MCO must have a systematic process for generating or receiving referrals and sharing confidential medical, treatment, and planning information across providers.
8.3.2.8 Nursing Facilities
This section is deleted effective February 28, 2015.
Nursing facility care, although a part of the care continuum, presents a challenge for managed care. Because of the process for becoming eligible for Medicaid assistance in a nursing facility, there is frequently a significant time gap between entry into the nursing home and determination of Medicaid eligibility. During this gap, it is likely that the resident will have "nested" in the facility and many of the community supports are no longer available. To require participation of all nursing facility residents would result in the MCO maintaining a Member in the nursing facility without many options for managing their health. For this reason, persons who qualify for Medicaid as a result of nursing facility residency are not enrolled in STAR+PLUS.
The STAR+PLUS MCO must participate in the Promoting Independence (PI) initiative for such individuals. PI is a philosophy that aged and disabled individuals remain in the most integrated setting to receive Long-term Services and Supports. PI is Texas' response to the U.S. Supreme Court ruling in Olmstead v. L.C., which requires states to provide community-based services for persons with disabilities who would otherwise be entitled to institutional services, when:
1. the state's treatment professionals determine that such placement is appropriate;
2. the affected persons do not oppose such treatment; and
3. the placement can be reasonably accommodated, taking into account the resources available to the state and the needs of others who are receiving state supported disability services.
In accordance with legislative direction, the MCO must designate a point of contact to receive referrals for nursing facility residents who may potentially be able to return to the community through the use of HCBS STAR+PLUS Waiver services. To be eligible for this option, an individual must reside in a nursing facility until a written plan of care for safely moving the resident back into a community setting has been developed and approved.
A STAR+PLUS Member who enters a nursing facility will remain a STAR+PLUS Member for a total of four (4) months. The nursing facility will bill the state directly for covered nursing facility services delivered while the Member is in the nursing facility. See Section 8.3.2.7 for further information.
The MCO is responsible for the Member at the time of nursing facility entry and must utilize the Service Coordinator staff to complete an assessment of the Member within 30 days of entry in the nursing facility, and develop a plan of care to transition the Member back into the community if possible. If at this initial review, return to the community is possible, the Service Coordinator will work with the resident and family to return the Member to the community using HCBS STAR+PLUS Waiver Services.
If the initial review does not support a return to the community, the Service Coordinator will conduct a second assessment 90 days after the initial assessment to determine any changes in the individual's condition or circumstances that would allow a return to the community. The Service Coordinator will develop and implement the transition plan.
The MCO will provide these services as part of the PI initiative. The MCO must maintain the documentation of the assessments completed and make them available for state review at any time.
It is possible that the STAR+PLUS MCO will be unaware of the Member's entry into a nursing facility. It is the responsibility of the nursing facility to review the Member's Medicaid card upon entry into the facility and notify the MCO. The nursing facility is also required to notify HHSC of the entry of a new resident.
8.3.2.9 MCO Four-Month Liability for Nursing Facility Care
This section is deleted effective February 28, 2015.
A STAR+PLUS Member who enters a nursing facility will remain a STAR+PLUS Member for a total of four months. The four months do not have to be consecutive. Upon completion of four months of nursing facility care, the individual will be disenrolled from the STAR+PLUS Program and the Medicaid Fee-for-Service program will provide Medicaid benefits. Effective October 1, 2014, HHSC will turn off the four-month liability counter described above. STAR+PLUS Members who enter a Nursing Facility and who reach the four-month liability between October and February 28, 2015, will remain in managed care.
Tracking the four months of liability is done through a counter system. The four-month counter starts with the earlier of: (1) the date of the Medicaid admission to the nursing facility, or (2) on the 21st day of a Medicare stay, if applicable. A partial month counts as a full month. In other words, the month in which the Medicaid admission occurs or the month on which the 21st day of the Medicare stay occurs is counted as one of the four months.
The MCO will not be responsible for the cost of care provided in a nursing facility. For Medicaid-only Members, the MCO is responsible for cost of Covered Services provided outside of the nursing facility. The MCO will not maintain nursing facilities in its Provider Network, and will not reimburse the nursing facilities for Covered Services provided in such facilities. Nursing facilities will use the traditional Fee-for-Service (FFS) system of billing HHSC rather than billing the MCO.
8.3.2.10 Prioritization Plan
Prior to the 3/1/2012 Operational Start Date of the STAR+PLUS Program in the Expansion Service Areas, HHSC and DADS will provide the MCO a plan that outlines a priority of populations and special handling procedures that the MCO must implement to help ensure timely assessments for potential enrollees and incoming Members as well as continuity of care for incoming Members. The populations that will be part of the priority list will include but are not limited to Money Follows the Person (MFP); Medically Dependent Children Program (MDCP), Comprehensive Care Program -Personal Care Services (CCP-PCS) and Comprehensive Care Program-Private Duty Nursing (CCP-PDN) aging out consumers; 217-Like Group Interest List consumers; and Supplemental Security Income (SSI) consumer. HHSC and/or DADS will also provide the MCO with information concerning Members who will be enrolled through manual processes and will need expedited access to services.
|
|
8.3.3 STAR+PLUS Assessment Instruments |
The MCO must have and use functional assessment instruments to identify Members with significant health problems, Members requiring immediate attention, and Members who need or are at risk of needing Long-term Services and Supports. The MCO, a Subcontractor, or a Provider may complete assessment instruments, but the MCO remains responsible for the data recorded.
Effective January 1, 2015, the MCOs must complete HHSC's Form H2060, Needs Assessment Questionnaire and Task/Hour Guide, including any applicable addendums, to assess or reassess a Member's need for or a change in Functionally Necessary Personal Attendant Services. The MCO may adapt the form to reflect the MCO's name or distribution instructions, but the elements must be the same and instructions for completion must be followed without amendment. The MCO may not add or delete questions from the form or change the questions in any way. The MCO must use Form H2060 any time there is an assessment of the need for or a change in Personal Attendant Services, including the initial contact with the Member, the Member’s annual reassessment, the Member’s request for services or a change in services, and the MCO ‘s determination that there is a need for a change in the Member's services.
MCOs must use the Texas Medicaid Personal Care Assessment Form (PCAF Form) in lieu of Form H2060 for children under the age of 21 when assessing the Member's need for Functional Necessary Personal Attendant Services. MCOs may adapt the PCAF Form to reflect the MCO's name or distribution instructions, but the elements must be the same and instructions for
completion must be followed without amendment. Reassessments using the PCAF Form must be completed every 12 months and as requested by the Member's parent or other legal guardian. The PCAF Form must also be completed at any time the MCO determines the Member may require a change in the number of authorized Personal Attendant Service hours.
For Members and applicants seeking or needing the HCBS STAR+PLUS Waiver services, the MCOs must use the Community Medical Necessity and Level of Care Assessment Instrument, as amended or modified, to assess Members and to supply current medical information for Medical Necessity determinations. The MCO must also complete the Individual Service Plan (ISP), Form H1700, including all H1700 series addendums, for each Member receiving HCBS STAR+PLUS Waiver Services. The ISP is established for a one-year period. After the initial ISP is established, the ISP must be completed on an annual basis and the end date or expiration date does not change. These forms (Community Medical Necessity and Level of Care Assessment Instrument, Form H2060 and addendums, and Form H1700 series addendums) must be completed annually at reassessment. The MCO is responsible for tracking the end dates of the ISP to ensure all Member reassessment activities have been completed and posted on the LTC online portal prior to the expiration date of the ISP. Note that the MCO cannot submit its initial Community Medical Necessity and Level of Care Assessment Instrument earlier than 120 days prior to the expiration date of the ISP. An Initial Community Medical Necessity and Level of Care determination will expire 120 days after it is approved by the HHSC Claims Administrator. The MCO cannot submit a renewal of the Community Medical Necessity and Level of Care Assessment Instrument earlier than 90 days prior to the expiration date of the ISP. The renewal will expire 90 days after it is approved by the HHSC Claims Administrator.
For Members needing Nursing Facility Services on or after March 1, 2015, the MCO's Network Provider Agreement must require that the Nursing Facility use the state and federally-required assessment instrument, as amended or modified, to assess Members and to supply current medical information for Medical Necessity determinations. The MCO's Network Provider Agreement must require the Nursing Facility to supply these assessments to the MCO.
|
|
8.3.4 HCBS STAR+PLUS Waiver Service Eligibility |
Recipients of HCBS STAR+PLUS Waiver services must meet level of care criteria for participation in the waiver and must have a plan of care at initial determination of eligibility in which the plan's annualized cost is equal to or less than 202% of the annualized cost of care if the individual were to enter a nursing facility. If the MCO determines that the recipient's cost of care will exceed the 202% limit, the MCO will submit to HHSC's Health Plan Operations Unit a request to consider the use of State General Revenue Funds to cover costs over the 202% allowance, as per HHSC's policy and procedures related to use of general revenue for HCBS STAR+PLUS Waiver participants. If HHSC approves the use of State General Revenue Funds, the MCO will be allowed to provide waiver services as per the Individual Service Plan, and non-waiver services (services in excess of the 202% allowance) utilizing State General Revenue Funds. Non-waiver services are not Medicaid Allowable Expenses, and may not be reported as such on the FSRs. The MCO will submit reports documenting expenses for non-waiver services in an HHSC-approved format. HHSC will reimburse the MCO for such expenses.
8.3.4.1 For Members
Members can request to be tested for eligibility into the HCBS STAR+PLUS Waiver. The MCO can also initiate HCBS STAR+PLUS Waiver eligibility testing on a STAR+PLUS Member if the MCO determines that the Member would benefit from the HCBS STAR+PLUS Waiver services.
To be eligible for the HCBS STAR+PLUS Waiver, the Member must meet Medical Necessity/Level of Care and the cost of the Individual Service Plan (ISP) cannot exceed 202% of cost of providing the same services in a nursing facility. The MCO must be able to demonstrate that that Member has a minimum of one (1) unmet need for at least one (1) HCBS STAR+PLUS Waiver service.
The MCO must complete the Community Medical Necessity and Level of Care Assessment Instrument for Medical Necessity/Level of Care determination, and submit the form to HHSC's Administrative Services Contractor. The MCO is also responsible for completing the assessment documentation, and preparing a HCBS STAR+PLUS ISP for identifying the needed HCBS STAR+PLUS Waiver services. The ISP is submitted to the State to ensure that the total cost does not exceed the 202% cost limit. The MCO must complete these activities within 45 days of receiving the State's authorization form for eligibility testing.
HHSC will notify the Member and the MCO of the eligibility determination, which will be based on results of the assessments and the information provided by the MCO. If the STAR+PLUS Member is eligible for HCBS STAR+PLUS Waiver services, HHSC will notify the Member of the effective date of eligibility. If the Member is not eligible for HCBS STAR+PLUS Waiver
services, HHSC will provide the Member information on right to Appeal the Adverse Determination. The MCO is responsible for preparing any requested documentation regarding its assessments and ISPs, and if requested by HHSC, attending the Fair Hearing. Regardless of the HCBS STAR+PLUS Waiver eligibility determination, HHSC will send a copy of the Member notice to the MCO.
8.3.4.2 For 217-Like Group Non-Member Applicants
Non-member persons who are not eligible for Medicaid in the community may apply for participation in the HCBS STAR+PLUS Waiver under the financial and functional eligibility requirements for the 217-Like Group (this group is described in the Texas Healthcare Transformation and Quality Improvement Program 1115 Waiver). HHSC will inform the non-member applicant that services are provided through an MCO and allow the applicant to select the MCO. HHSC will provide the selected MCO an authorization form to initiate pre-enrollment assessment services required under the HCBS STAR+PLUS Waiver for the applicant. The MCO's initial home visit with the applicant must occur within 14 days of the receipt of the referral. To be eligible for HCBS STAR+PLUS Waiver, the applicant must meet financial eligibility and Medical Necessity/Level of Care, and the cost of the Individual Service Plan (ISP) cannot exceed 202% of cost of providing the same services in a nursing facility. The MCO must be able to demonstrate that the applicant has a minimum of one (1) unmet need for at least one (1) HCBS STAR+PLUS Waiver service.
The MCO must complete the Community Medical Necessity and Level of Care Assessment Instrument for Medical Necessity/Level of Care determination, and submit the form to HHSC's Administrative Services Contractor. The MCO is also responsible for completing the assessment documentation, and preparing a HCBS STAR+PLUS ISP for identifying the needed HCBS STAR+PLUS Waiver services. The ISP is submitted to the State to ensure that the total cost does not exceed the 202% cost ceiling. The MCO must complete these activities within 45 days of receiving the State's authorization form for eligibility testing.
HHSC will notify the applicant and the MCO of the results of its eligibility determination. If the applicant is eligible, HHSC will notify the applicant and the MCO will be notified of the effective date of eligibility, which will be the first day of the month following the determination of eligibility. The MCO must initiate the Individual Service Plan (ISP) on the date of enrollment.
If the applicant is not eligible, the HHSC notice will provide information on the applicant's right to Appeal the Adverse Determination. HHSC will also send notice to the MCO if the applicant is not eligible for HCBS STAR+PLUS Waiver services. The MCO is responsible for preparing any requested documentation regarding its assessments and service plans, and if requested by HHSC, attending the Fair Hearing.
8.3.4.3 Annual Reassessment
Prior to the end date of the annual ISP, the MCO must initiate an annual reassessment to determine and validate continued eligibility for HCBS STAR+PLUS Waiver services for each Member receiving these services. As part of the assessment, the MCO must inform the Member about Consumer Directed Services options. The MCO will be expected to complete the same activities for each annual reassessment as required for the initial eligibility determination.
8.3.4.4 STAR+PLUS Utilization Reviews
HHSC will conduct STAR+PLUS utilization reviews, as described in Texas Government Code § 533.00281. The reviews will include the MCO's assessment processes used to determine HCBS waiver eligibility. If HHSC recoups money from the MCO as a result of a utilization review conducted under this section, the MCO cannot hold a Network service provider liable for the good faith provision of services based on the MCO's authorization.
|
|
8.3.5 Consumer Directed Services Options |
There are three (3) options available to STAR+PLUS Members desiring to self-direct the delivery of:
1. Primary Home Care (PHC) (which is available to all STAR+PLUS Members), and
2. Personal Attendant Services (PAS); in-home or out-of-home respite; nursing; physical therapy (PT); occupational therapy (OT); and/or speech/language therapy (SLT) for (which are available to Members in the HCBS STAR+PLUS Waivers).
These three (3) options are: 1) Consumer-Directed; 2) Service Related; and 3) Agency. The MCO must provide information concerning the three (3) options to all Members: (1) who meet the functional requirements for PHC Services and the requirements for PAS (the functional criteria for these services are described in the Form 2060), (2) who are eligible for in-home or out-of-home respite services in the SPW; and (3) who are eligible for nursing, PT, OT and/or SLT in the SPW. In addition to providing information concerning the three (3) options, the MCO must provide Member orientation in the option selected by the Member. The MCO must provide the information to any STAR+PLUS Member receiving PHC/PAS and/or in-home or out-of-home respite:
1. at initial assessment;
2. at annual reassessment or annual contact with the STAR+PLUS Member;
3. at any time when a STAR+PLUS Member receiving PHC/PAS/Respite/Nursing/PT/TO/SLT requests the information; and
4. in the Member Handbook.
The MCO must contract with providers who are able to offer PHC/PAS in-home or out-of-home respite, nursing, PT, TO, and/or SLT and must also educate/train the MCO Network Providers regarding the three (3) PAS options. Network Providers must meet licensure/certification requirements as indicated in Attachment B-1, Sections 8.3.11 and 8.3.1.2 of the Uniform Managed Care Contract.
In all three (3) options, the Service Coordinator and the Member work together in developing the Individual Service Plan.
A more comprehensive description of Consumer Directed Services is found in the STAR+PLUS Handbook: http://www.dads.state.tx.us/handbooks/sph/8000/8000.htm#sec8120
8.3.5.1 Consumer-Directed Option Model
In the Consumer-Directed Model, the Member or the Member's legal guardian is the employer of record and retains control over the hiring, management, and termination of an individual providing PHC/PAS in-home or out-of-home respite; nursing, PT, TO, and/or SLT. The Member is responsible for assuring that the employee meets the requirements for PHC/PAS; in-home or out-of-home respite; nursing, PT, TO, and/or SLT, including the criminal history check. The Member uses a Consumer Directed Services agency (CDSA) to handle the employer-related administrative functions such as payroll, substitute (back-up), and filing tax-related reports of PHC/PAS; in-home or out-of-home respite; nursing, PT, TO, and/or SLT.
8.3.5.2 Service Related Option Model
In the Service Related Option Model, the Member or the Member's legal guardian is actively involved in choosing their personal attendant, respite provider, nurse, physical therapist, occupational therapist and/or speech/language therapist but is not the employer of record. The Home and Community Support Services agency (HCSSA) in the MCO Provider Network is the employer of record for the personal attendant employee and respite provider. In this model, the Member selects the personal attendant and/or respite provider from the HCSSA's personal attendant employees. The personal attendant's/respite provider's schedule is set up based on the Member input, and the Member manages the PHC/PAS, in-home or out-of-home respite. The Member retains the right to supervise and train the personal attendant. The Member may request a different personal attendant and the HCSSA would be expected to honor the request as long as the new attendant is a Network Provider. The HCSSA establishes the payment rate, benefits, and provides all administrative functions such as payroll, substitute (back-up), and filing tax-related reports of PHC/PAS and/or in-home or out-of-home respite. In this model, the Member selects the nurse, physical therapist, occupational therapist, and/or speech/language therapist from the MCO's Provider Network. The nurse, physical therapist, occupational therapist, and/or speech/language therapist's schedule is set up based on the Member's input, and the Member manages the nursing, PT, OT, and/or SLT services. The Member retains the right to supervise and train the nurse, physical therapist, occupational therapist, and/or speech/language therapist. The Member may request a different nurse, physical therapist, occupational therapist, and/or speech/language therapist and the MCO must honor the request as long as the nurse, physical therapist, occupational therapist, and/or speech/language therapist is a Network Provider. The MCO establishes the payment rate, benefits, and provides all administrative functions such as payroll, substitute (back-up), and filing tax-related reports of nursing, PT, OT, and/or SLT services.
8.3.5.3 Agency Model
In the Agency Model, the MCO contracts with a Home and Community Support Services agency (HCSSA) for the delivery of waiver services. The HCSSA is the employer of record for the personal attendant, respite provider, nurse, physical therapist,
occupational therapist, and speech language therapist. The HCSSA establishes the payment rate, benefits, and provides all administrative functions such as payroll, substitute (back-up), and filing tax-related reports of PHC/PAS and/or in-home or out-of home respite.
|
|
8.3.6 Community Based Long-term Services and Supports Providers |
8.3.6.1 Training
The MCO must comply with Section 8.1.4.6 regarding Provider Manual and Provider training specific to the STAR+PLUS Program. The MCO must train all Community Long-term Services and Supports Providers regarding the requirements of the Contract and special needs of STAR+PLUS Members. The MCO must establish ongoing STAR+PLUS Provider training addressing the following issues at a minimum:
|
| |
| 1. Covered Services and the Provider’s responsibilities for providing such services to STAR+PLUS Members and billing the MCO. The MCO must place special emphasis on Community Long-term Services and Supports and STAR+PLUS requirements, policies, and procedures that vary from Medicaid Fee-for-Service and commercial coverage rules, including payment policies and procedures; |
|
| |
| 2. relevant requirements of the STAR+PLUS Contract, including the role of the Service Coordinator; |
|
| |
| 3. processes for making referrals and coordinating Non-capitated Services; |
|
| |
| 4. the MCO’s quality assurance and performance improvement program and the Provider’s role in such programs; and |
|
| |
| 5. the MCO’s STAR+PLUS policies and procedures, including those relating to Network and Out-of-Network referrals. |
|
| |
| 6. For STAR+PLUS in the El Paso, Hidalgo and Lubbock Service Areas with an Operational Start Date of 3/1/2012, the process for continuing up to six (6) months of Community-based Long Term Care Services for Members receiving those services as of the Operational Start Date, including provider billing practices for these services and whom to contact at the MCO for assistance with this process. |
8.3.6.2 LTSS Provider Billing
Long-term Services and Supports providers serving clients in the traditional Fee-for-Service Medicaid program have not been required to utilize the billing systems that most medical facilities use on a regular basis. For this reason, the MCO must make accommodations to the claims processing system for such providers to allow for a smooth transition from traditional Medicaid to STAR+PLUS.
HHSC has developed a standardized method for Long-term Services and Supports billing. All STAR+PLUS MCOs are required to utilize the standardized method, as found in Uniform Managed Care Manual Chapter 2.1.1 and the STAR+PLUS Handbook.
8.3.6.3 Rate Enhancement Payments for Agencies Providing Attendant Care
All MCOs participating in the STAR+PLUS Program must allow their Long-term Services and Supports Providers to participate in the STAR+PLUS Attendant Care Enhancement Program.
Uniform Managed Care Manual Chapter 2.1.3, “STAR+PLUS Attendant Care Enhanced Payment Methodology,” includes the methodology that the STAR+PLUS MCO will use to implement and pay the enhanced payments, including a description of the timing of the payments. Such methodology must comply with the requirements in the Uniform Managed Care Manual and the intent of 1 Tex. Admin. Code. §355.112. In addition to the requirements in UMCM Chapter 2.1.3, the MCO must apply vendor holds to participating Providers in accordance with 1 Tex. Admin. Code § 355.101and recoup enhancement payments made to Providers at HHSC’s direction. Additionally, upon HHSC’s request, the MCO must provide HHSC with a current list of Network Providers of the following attendant services: Day Activity Health Care Services (DAHS), Primary Home Care (PHC), Personal Assistance Services (PAS), Texas Health Steps Personal Care Services (PCS), and Community Based Alternative Services (CBA).
8.3.6.4 STAR+PLUS Handbook
The STAR+PLUS Handbook contains HHSC-approved policies and procedures related to the STAR+PLUS Program, including policies and procedures relating to the Texas Healthcare Transformation and Quality Improvement Program 1115 waiver. The STAR+PLUS Handbook includes additional requirements regarding the STAR+PLUS Program and guidance for the MCOs, the STAR+PLUS Support Units at DADS, and HHSC staff for administrating and managing STAR+PLUS Program operations. The STAR+PLUS Handbook is incorporated by reference into the Contract.
8.3.6.5 Annual Contact with STAR+PLUS Members
The MCO is required to contact each STAR+PLUS Member a minimum of two (2) times per calendar year. This contact can be written, telephonic, or an onsite visit to the Member’s residence, depending upon the Member’s level of need. The MCO must document the mechanisms, number and method of contacts, and outcomes within the MCO’s Service Coordination system.
8.3.6.6 Cost Reporting for LTSS Providers
MCOs must require that LTSS Providers submit periodic cost reports and supplemental reports to HHSC in accordance with 1 Tex. Admin. Code Chapter 355, including Subchapter A (Cost Determination Process) and 1 Tex. Admin. Code § 355.403 (Vendor Hold). If an LTSS Provider fails to comply with these requirements, HHSC will notify the MCO to hold payments to the LTSS provider until HHSC instructs the MCO to release the payments.
|
|
8.3.7 Additional Requirements Regarding Dual Eligibles |
|
|
8.3.7.1 Coordination of Services for Dual Eligibles |
The STAR+PLUS MCOs must coordinate Medicare and Medicaid services for Dual Eligible recipients. To facilitate such coordination, the MCO must be contracted with the CMS and operating as a MA Dual SNP in the most populous counties in the Service Area(s), as identified by HHSC, no later than January 1, 2013, or as a Dual Eligible Medicare-Medicaid Plan (MMP) in the designated demonstration counties no later than January 1, 2015. After these dates, the MCO must maintain its status as an MA DUAL SNP or an MMP contractor throughout the term of the Contract. Failure to do so may result in HHSC’s assessment of contractual remedies, including Contract termination.
|
|
8.3.7.2 MA Dual SNP Agreement |
As part of the integrated care initiative for Dual Eligible STAR+PLUS Members, the MCO may maintain a separate capitation agreement with HHSC whereby the MCO’s MA Dual SNP plan reimburses Medicare providers for the cost-sharing obligations that the State would otherwise be required to pay on behalf of qualified STAR+PLUS Dual Eligible Members. The final Texas MA Dual SNP Agreement, as amended or modified, will be incorporated by reference into the STAR+PLUS Contract. The MCO will be required to provide all enrolled STAR+PLUS Dual Eligible Members with the coordinated care and other services described in the Texas MA Dual SNP Agreement, and any violations of the Texas MA Dual SNP Agreement with respect to STAR+PLUS Members will also be a violation of the STAR+PLUS Contract. Note that, for STAR+PLUS Members who are also enrolled in the MA Dual SNP’s Medicare plan, the Parties may develop alternative methods for verifying Member eligibility and submitting encounter data. Any modifications to these processes or other requirements identified in the Texas MA Dual SNP Agreement will be included in the Texas MA Dual SNP Agreement.
8.3.8 Minimum Wage Requirements for STAR+PLUS Attendants in Community Settings
The MCO must ensure that facilities and agencies that provide attendant services in community settings pay attendants at or above the minimum rates described below. This requirement applies to the following types of services, whether or not the Member chooses to self-direct these services (see Section 8.3.5, "Consumer Directed Services Options:")
| |
• | Day Activity Health Care Services (DAHS); |
| |
• | Primary Home Care (PHC); |
| |
• | Personal Assistance Services (PAS); and |
| |
• | Texas Health Steps Personal Care Services (PCS). |
This requirement does not apply to attendant services provided by non-institutional facilities, such as assisted living, adult foster care, residential care, and nursing facilities.
8.3.8.1 State Fiscal Year 2014
The MCO must ensure that attendants are paid no less than $7.50 per hour for dates of service in SFY 2014 (September 1, 2013 to August 31, 2014).
8.3.8.2 State Fiscal Year 2015 and After
The MCO must ensure that attendants are paid no less than $7.86 per hour for dates of service on or after September 1, 2014.
8.3.9 Nursing Facility Services Available to All Members
This section is effective March 1, 2015, when STAR+PLUS MCOs will begin providing Nursing Facility Services.
Nursing Facilities provide institutional care to Members whose physician has certified that the Member has a medical condition that requires daily skilled nursing care that meets Medical Necessity requirements. The need for custodial care solely does not constitute Medical Necessity for a Nursing Facility placement.
Institutional care includes coverage for the medical, social, and psychological needs of each resident, including room and board, social services, medications not covered by Medicare Part B or D, medical supplies and equipment, rehabilitative services, and personal needs items.
Beginning March 1, 2015, MCOs must provide access to Nursing Facility services for all qualified STAR+PLUS Members.
At a minimum, Nursing Facility Providers must meet all of the following state licensure, certification, and contracting requirements for providing the services in Attachment B-2.2, STAR+PLUS Covered Services.
|
| |
Nursing Facility Services Available to All Members |
Service | Licensure and Certification Requirements |
Nursing Facility | The MCOs must use state-identified credentialing criteria for Nursing Facilities. At a minimum, the Nursing Facility must hold a valid certification and license and must contract with DADS. Credentialing documentation of the Nursing Facilities in the STAR+PLUS MCO’s Provider Network meets all licensure requirements as established in 40 Tex. Admin. Code Chapter 19. Credentialing documentation must be submitted to HHSC upon request. |
8.3.9.1 Preadmission Screening and Resident Review (PASRR)
The MCO must fulfill PASRR requirements when providing services for STAR+PLUS Members as required by 40 Tex. Admin. Code §§ 17.101-17.401. MCO participation includes coordinating with the Local MH or IDD Authority, nursing facility, Member, and interdisciplinary team to develop the Member's service plan and ensure PASRR specialized services are provided in compliance with the Member’s service plan.
8.3.9.2 Participation in Texas Promoting Independence Initiative
The STAR+PLUS MCO must participate in the Texas Promoting Independence (PI) initiative. The PI initiative is Texas's response to Olmstead v. L.C. ex rel. Zimring, 527 U.S. 581 (1999), which requires states to provide community-based services for persons with disabilities who would otherwise be entitled to institutional services when:
1. the state's treatment professionals determine that such placement is appropriate;
2. the affected persons do not oppose such treatment; and
3. the placement can be reasonably accommodated, taking into account the resources available to the state and the needs of others who are receiving state supported disability services.
In accordance with legislative direction, the MCO must designate a point of contact to receive referrals for Nursing Facility Members who may be able to return to the community through the use of HCBS STAR+PLUS Waiver services. To be eligible for this option, a Member must reside in a Nursing Facility until the Member meets the eligibility criteria for entry into the HCBS STAR+PLUS Waiver. This includes the development and approval of a written plan of care for safely moving into a community setting.
The MCO Service Coordinator must complete an assessment of the Member within 30 days of the MCO’s notification of a Member's Medicaid-covered stay and develop a plan of care to transition the Member back into the community, if possible. If the initial review/assessment supports a return to the community, the Service Coordinator will work with the Member and his/her family to return the Member to the community using HCBS STAR+PLUS Waiver services.
If the initial review does not support a return to the community, the Service Coordinator will conduct a second assessment 90 days after the initial assessment, and quarterly thereafter, to determine if the individual’s condition or circumstances have changed that would allow a return to the community. If a return to the community is possible and appropriate, the Service Coordinator will develop and implement the transition plan with the Member and his/her family.
The MCO must maintain documentation of the assessments completed as part of this initiative and make them available for state review at any time.
8.3.9.3 Nursing Facilities Training
In addition to Section 8.1.4.6, the MCO must train all Nursing Facility Providers regarding the requirements of the Contract and special needs of STAR+PLUS Members. The MCO must establish ongoing Provider training addressing the following issues at a minimum.
| |
• | Covered Services and the Provider’s responsibilities for providing services to Members and billing the MCO for the services. The MCO must place special emphasis on Nursing Facility Services and STAR+PLUS requirements, policies, and procedures that vary from Medicaid Fee-for-Service and commercial coverage rules, including payment policies and procedures. |
| |
• | The transition process of up to six (months for the continuation of Nursing Facility for Members receiving those services at the time of program implementation, including provider billing practices for these services and who to contact at the MCO for assistance with this process. |
| |
• | Relevant requirements of the STAR+PLUS Contract, including the role of the Service Coordinator; |
| |
• | Processes for making referrals and coordinating Non-capitated Services; |
| |
• | The MCO’s quality assurance and performance improvement program and the Provider’s role in these programs; and |
| |
• | The MCO’s STAR+PLUS policies and procedures, including those relating to Network and Out-of-Network referrals. |
8.3.9.4 Nursing Facility Claims Adjudication, Payment, and File Processing
The MCO must process claims in accordance with UMCM Chapter 2.3, Nursing Facility Claims Manual. The MCO must pay clean claims, as defined in Texas Gov't. Code § 533.00251(a)(2), no later than 10 calendar days after submission of the clean claim.
Effective March 1, 2015, the MCO must ensure that Network Nursing Facility Providers are paid Nursing Facility Unit Rates at or above the minimum rates established by HHSC for the dates of service. HHSC will post this information on the HHSC website at http://www.hhsc.state.tx.us/rad/rate-packets.shtml. If HHSC makes a retroactive rate adjustment to a Nursing Facility Unit Rate, the MCO must retroactively adjust payment to a Nursing Facility no later than 30 days after receipt of HHSC notification. Further, the MCO must provide a list of Network Nursing Facilities upon HHSC's request and must withhold payments to a Nursing Facility in accordance with 1 Tex. Admin. Code § 355.101.
The MCO must ensure that all enrollment and eligibility files in the Joint Interface Plan are loaded into the claims adjudication system before the first day of the month following receipt.
8.3.9.5 Nursing Facility Direct Care Rate Enhancement
All MCOs participating in the STAR+PLUS Program must allow their Nursing Facility Providers to participate in the STAR+PLUS Direct Care Staff Rate Enhancement Program in accordance with 1Tex. Admin. Code § 355.308. HHSC will determine enhancement payments, which will be included in the Nursing Facility Unit Rates. HHSC will post information regarding Nursing Facility enhanced payments on the HHSC website at http://www.hhsc.state.tx.us/rad/long-term-svcs/nursing-facility/index.shtml. HHSC will determine Nursing Facility compliance with Direct Care Rate Enhancement spending and staffing requirements. If HHSC makes a retroactive rate adjustment to a Nursing Facility’s Unit Rate due to non-compliance with Direct Care Rate Enhancement spending or staffing requirements, the MCO must retroactively adjust payment to a Nursing Facility no later than 30 days after receipt of HHSC notification in accordance with Section 8.3.9.4.
8.3.10 Acute Care Services for Recipients of ICF-IID Program and IDD Waiver Services
Effective September 1, 2014, individuals with intellectual disabilities or related conditions who do not qualify for Medicare and who receive services through the ICF-IID Program or an IDD Waiver are eligible for Acute Care services through STAR+PLUS. These individuals will not be eligible for the HCBS STAR+PLUS Waiver Services while enrolled in the ICF-IID Program or an IDD Waiver.
8.3.11 Cognitive Rehabilitation Therapy
The MCO may only authorize Cognitive Rehabilitation Therapy if one of the following Texas Medicaid-covered assessment tests, as listed in the Texas Medicaid Provider Procedures Manual, shows that the therapy can benefit the Member and is Medically Necessary:
• Neurobehavioral Test (CPT Code 96116); or
• Neuropsychological Test (CPT Code 96118).
|
|
8.4 Additional CHIP Scope of Work |
The following provisions only apply to MCOs participating in CHIP.
|
|
8.4.1 CHIP Provider Complaint and Appeals |
CHIP Provider complaints and claims payment appeals are subject to disposition consistent with the Texas Insurance Code and any applicable TDI regulations. The MCO must resolve Provider complaints and claims payment appeals within 30 days from the date of receipt.
|
|
8.4.2 CHIP Member Complaint and Appeal Process |
CHIP Member Complaints and Appeals are subject to disposition consistent with the Texas Insurance Code and any applicable TDI regulations. HHSC will require the MCO to resolve Member Complaints and Appeals (that are not elevated to TDI) within 30 days from the date the Member Complaint or Appeal is received. The MCO is subject to remedies, including liquidated damages, if at least 98 percent of Member Complaints and Member Appeals are not resolved within 30 days of receipt of the Complaint or Appeal by the MCO. Please see the Attachment A , "Uniform Managed Care Contract Terms and Conditions," Article 12, and Attachment B-3, “Deliverables/Liquidated Damages Matrix.” Any person, including those dissatisfied with a MCO’s resolution of a Member Complaint or Appeal, may report an alleged violation to TDI.
|
|
8.4.3 Third Party Liability and Recovery, and Coordination of Benefits |
CHIP coverage is secondary when coordinating benefits with all other insurance coverage. Coverage provided under CHIP will pay benefits for Covered Services that remain unpaid after all other insurance coverage has been paid. For Network Providers and Out-of Network providers with written reimbursement arrangements with the MCO, the MCO must pay the unpaid balance for Covered Services up to the agreed rates. For Out-of-Network providers with no written reimbursement arrangement, the MCO must pay the unpaid balance for Covered Services in accordance with TDI's rules regarding usual and customary payment.
MCOs are responsible for establishing a plan and process for avoiding or recovering costs for services that should have been paid through a third party. The plan and process must comply with state and federal law and regulations. Consistent with Medicaid requirements, MCOs must pay and later seek recovery from liable third parties: (1) for prenatal and preventive pediatric care, and (2) in the context of a state child support enforcement action.
If a Member visits an FQHC or RHC (or a Municipal Health Department's public clinic for Health Care Services) at a time that is outside of regular business hours (as defined by HHSC in rules, including weekend days or holidays), the MCO is obligated to reimburse the FQHC, RHC, or public clinic for Medically Necessary Covered Services. The MCO must do so at a rate that is equal to the allowable rate for those services as determined under Section 32.028 of the Human Resources Code. The Member does not need a referral from his/her PCP.
The MCO must provide related reports to HHSC, as stated in Section 8.1.17.1, Financial Reporting Requirements.
After 120 days from the date of adjudication (on any claim, encounter, or other Medicaid related payment made by the MCO, wherein the claim, encounter, or payment is subject to Third Party Recovery), HHSC may attempt recovery, independent of any MCO action. HHSC will retain, in full, all funds received as a result of any state-initiated recovery or subrogation action.
|
|
8.4.4 Perinatal Services for Traditional CHIP Members |
The MCO’s perinatal Health Care Services must ensure appropriate care is provided to women and infant Members of the MCO from the preconception period through the infant’s first year of life. The MCO’s perinatal health care system must comply with the requirements of the Texas Health and Safety Code, Chapter 32 (the Maternal and Infant Health Improvement Act), and administrative rules codified at 25 T.A.C. Chapter 37, Subchapter M.
The MCO must have a perinatal health care system in place that, at a minimum, provides the following services:
|
| |
| 1. pregnancy planning and perinatal health promotion and education for reproductive-age women; |
|
| |
| 2. perinatal risk assessment of non-pregnant women, pregnant and postpartum women, and infants up to one year of age; |
|
| |
| 3. access to appropriate levels of care based on risk assessment, including emergency care; |
|
| |
| 4. transfer and care of pregnant women, newborns, and infants to tertiary care facilities when necessary; |
|
| |
| 5. availability and accessibility of OB/GYNs, anesthesiologists, and neonatologists capable of dealing with complicated perinatal problems; and |
|
| |
| 6. availability and accessibility of appropriate outpatient and inpatient facilities capable of dealing with complicated perinatal problems. |
The MCO must have a process to expedite scheduling a prenatal appointment for an obstetrical exam for a Member with a confirmed diagnosis indicating pregnancy.
The MCO must have procedures in place to contact and assist a pregnant/delivering Member in selecting a PCP for her baby either before the birth or as soon as the baby is born.
Except as provided in Attachment A, Section 5.06, the MCO must provide inpatient care and professional services relating to labor and delivery for its pregnant/delivering Members for up to 48 hours following an uncomplicated vaginal delivery and 96
hours following an uncomplicated caesarian delivery. The MCO must provide neonatal care for its newborn Members until the time of discharge.
The MCO must notify providers involved in the care of pregnant/delivering women and newborns (including Out-of-Network providers and Hospitals) of the MCO’s prior authorization requirements. The MCO cannot require a prior authorization for services provided to a pregnant/delivering Member or newborn Member for a medical condition that requires Emergency Services, regardless of when the emergency condition arises.
Subject: Attachment B-1 - Medicaid and CHIP Managed Care Services RFP, Section 9
DOCUMENT HISTORY LOG
|
| | | |
STATUS1 | DOCUMENT REVISION2 | EFFECTIVE DATE | DESCRIPTION3 |
Baseline | n/a | September 1, 2011 | Initial version of Attachment B-1, RFP Section 9, “Turnover Requirements.” |
Revision | 2.1 | March 1, 2012 | Contract amendment did not revise Attachment B-1, RFP Section 9, "Turnover Requirements." |
Revision | 2.2 | June 1, 2012 | Contract amendment did not revise Attachment B-1, Section 9, "Turnover Requirements." |
Revision | 2.3 | September 1, 2012 | Contract amendment did not revise Attachment B-1, Section 9, "Turnover Requirements." |
Revision | 2.4 | March 1, 2013 | Contract amendment did not revise Attachment B-1, Section 9, “Turnover Requirements.”
|
Revision | 2.5 | June 1, 2013 | Contract amendment did not revise Attachment B-1, Section 9, “Turnover Requirements.”
|
Revision | 2.6 | September 1, 2013 | Contract amendment did not revise Attachment B-1, Section 9, “Turnover Requirements.”
|
Revision | 2.7 | September 1, 2013 | Contract amendment did not revise Attachment B-1, Section 9, “Turnover Requirements.”
|
Revision | 2.8 | January 1, 2014 | Contract amendment did not revise Attachment B-1, Section 9, “Turnover Requirements.”
|
Revision | 2.9 | February 1, 2014 | Contract amendment did not revise Attachment B-1, Section 9, “Turnover Requirements.”
|
Revision | 2.10 | April 1, 2014 | Contract amendment did not revise Attachment B-1, Section 9, “Turnover Requirements.”
|
Revision | 2.11 | September 1, 2014 | Contract amendment did not revise Attachment B-1, Section 9, "Turnover Requirements." |
1 Status should be represented as “Baseline” for initial issuances, “Revision” for changes to the Baseline version, and “Cancellation” for withdrawn versions 2 Revisions should be numbered in accordance according to the version of the issuance and sequential numbering of the revision—e.g., “1.2” refers to the first version of the document and the second revision. 3 Brief description of the changes to the document made in the revision. |
Table of Contents
9. Turnover Requirements.....................................................................................................9-4
9.1 Introduction.................................................................................................................9-4
9.2 Turnover Plan.............................................................................................................9-4
9.3 Transfer of Data.........................................................................................................9-4
9.4 Turnover Services......................................................................................................9-5
9.5 Post-Turnover Services..............................................................................................9-5
This section presents the Turnover Requirements. Turnover is defined as those activities that the MCO is required to perform prior to or upon termination of the Contract in situations where the MCO will transition data and documentation acquired under the Contract to HHSC or a subsequent contractor.
Twelve (12) months after the Effective Date of the Contract, the MCO must provide a Turnover Plan covering the turnover of the records and information maintained to either HHSC or a subsequent contractor. The Turnover Plan will be a comprehensive document detailing the proposed schedule, activities, and resource requirements associated with the turnover tasks.
The Turnover Plan must describe the MCO’s policies and procedures that will assure:
|
| |
| 1. The least disruption in the delivery of Covered Services to Members during the transition to a subsequent contractor. |
|
| |
| 2. Cooperation with HHSC and a subsequent contractor in notifying Members of the transition, as requested and in the form required or approved by HHSC. |
|
| |
| 3. Cooperation with HHSC and a subsequent contractor in transferring information to HHSC or a subsequent contractor, as requested and in the form required or approved by HHSC. |
The Turnover Plan must be approved by HHSC, and include at a minimum:
|
| |
| 1. The MCO’s approach and schedule for the transfer of data and information, as described above. |
|
| |
| 2. The quality assurance process that the MCO will use to monitor Turnover activities. |
|
| |
| 3. The MCO’s approach to training HHSC or a subsequent contractor’s staff in the operation of its business processes. |
HHSC is not limited or restricted in the ability to require additional information from the MCO or modify the Turnover Plan as necessary.
The MCO must transfer to HHSC or a subsequent contractor all data and information necessary to transition operations, including: data and reference tables; data entry software; third-party software and modifications; documentation relating to software and interfaces; functional business process flows; and operational information, including correspondence, documentation of ongoing or outstanding issues, operations support documentation, and operational information regarding Subcontractors. For purposes of this provision, "documentation" means all operations, technical and user manuals used in conjunction with the software, Services and Deliverables, in whole or in part, that HHSC determines are necessary to view and
extract application data in a proper format. The MCO must provide the documentation in the formats in which such documentation exists at the expiration or termination of the Contract. See Attachment A , “Uniform Managed Care Contract Terms and Conditions,” Section 15.03, “Ownership and Licenses” for additional information concerning intellectual property rights.
In addition, the MCO will provide to HHSC the following:
|
| |
| 1. Data, information and services necessary and sufficient to enable HHSC to map all Texas data from the MCO's system(s) to the replacement system(s) of HHSC or a successor contractor, including a comprehensive data dictionary as defined by HHSC. |
|
| |
| 2. All necessary data, information and services will be provided in the format defined by HHSC, and must be HIPAA compliant. |
|
| |
| 3. All of the data, information and services mentioned in this section must be provided and performed in a manner by the MCO using its best efforts to ensure the efficient administration of the contract. The data and information must be supplied in media and format specified by HHSC and according to the schedule approved by HHSC in the Turnover Plan. The data, information and services provided pursuant to this section must be provided at no additional cost to HHSC. |
All relevant data and information must be received and verified by HHSC or a subsequent contractor. If HHSC determines that data or information are not accurate, complete, nor HIPAA compliant, HHSC reserves the right to hire an independent contractor to assist HHSC in obtaining and transferring all the required data and information and to ensure that all the data are HIPAA compliant. The reasonable cost of providing these services will be the responsibility of the MCO.
Six (6) months prior to the end of the Contract Period, including any extensions, the MCO must revise its Turnover Plan. If HHSC terminates the Contract prior to the expiration of the Contract Period, then HHSC may require the MCO to submit an updated Turnover Plan sooner than six (6) months prior to the termination date. In such cases, HHSC’s notice of termination will include the date the Turnover Plan is due.
|
|
9.5 Post-Turnover Services |
Thirty (30) days following Turnover of operations, the MCO must provide HHSC with a Turnover Results Report documenting the completion and results of each step of the Turnover Plan. Turnover will not be considered complete until this document is approved by HHSC. HHSC may withhold up to 20% of the last month’s Capitation Payment until the Turnover activities are complete and the Turnover Plan is approved by HHSC.
If the MCO does not provide the required data or information necessary for HHSC or a subsequent contractor to assume the operational activities successfully, the MCO agrees to reimburse HHSC for all reasonable costs and expenses, including, but not limited to: transportation, lodging, and subsistence to carry out inspection, audit, review, analysis, reproduction and transfer functions at the location(s) of such records; and attorneys’ fees and costs. This section does not limit HHSC’s ability to impose remedies or damages as set forth in the Contract.
Subject: Attachment B-2 - STAR Covered Services
DOCUMENT HISTORY LOG
|
| | | |
STATUS1 | DOCUMENT REVISION2 | EFFECTIVE DATE | DESCRIPTION3 |
Baseline | n/a | September 1, 2011 | Initial version of Attachment B-2, “STAR Covered Services.” |
Revision | 2.1 | March 1, 2012 | Attachment B-2 is modified to reinstate the waiver of the three prescription limit for adults language and to clarify the waiver of the $200,000 individual annual limit on inpatient services. STAR Covered Services is modified to add “Cancer screening, diagnostic, and treatment services” and “Prenatal care services rendered in a birthing center” as clarification items and to clarify the requirements for services provided in free-standing psychiatric hospitals and chemical dependency treatment facilities in lieu of the acute care hospital setting. |
Revision | 2.2 | June 1, 2012 | Contract amendment did not revise Attachment B-2, "STAR Covered Services." |
Revision | 2.3 | September 1, 2012 | STAR Covered Services is modified to remove the reference to Dual Eligible STAR Members in the MRSA
|
Revision | 2.4 | March 1, 2013 | Contract amendment did not revise Attachment B-2, “STAR Covered Services.”
|
Revision | 2.5 | June 1, 2013 | Contract amendment did not revise Attachment B-2, “STAR Covered Services.”
|
Revision | 2.6 | September 1, 2013 | STAR Covered Services is modified to remove the reference to the Texas Medicaid Bulletin.
|
Revision | 2.7 | September 1, 2013 | Contract amendment did not revise Attachment B-2, “STAR Covered Services.”
|
|
| | | |
Revision | 2.8 | January 1, 2014 | Inpatient General Acute and Inpatient Rehabilitation Hospital Services (CHIP Perinatal Coverage) is modified to clarify the eligibility thresholds.
Birthing Center Services (CHIP Perinatal Coverage) is modified to clarify the eligibility thresholds.
Exclusions for CHIP Perinatal is modified to clarify the eligibility thresholds. |
Revision | 2.9 | February 1, 2014 | STAR Covered Services include Medically Necessary: is modified to add telemedicine and telemonitoring. |
Revision | 2.10 | April 1, 2014 | Contract amendment did not revise Attachment B-2, "STAR Covered Services." |
Revision | 2.11 | September 1, 2014 | "STAR Covered Services include Medically Necessary" is modified to add Telehealth. |
1 Status should be represented as “Baseline” for initial issuances, “Revision” for changes to the Baseline version, and “Cancellation” for withdrawn versions 2 Revisions should be numbered in accordance according to the version of the issuance and sequential numbering of the revision—e.g., “1.2” refers to the first version of the document and the second revision. 3 Brief description of the changes to the document made in the revision. |
STAR Covered Services
The following is a non-exhaustive, high-level listing of Acute Care Covered Services included under the Medicaid STAR Program.
STAR MCOs are responsible for providing a benefit package to Members that includes all Medically Necessary services covered under the traditional, fee-for-service Medicaid programs except for Non-capitated Services. Non-capitated Services are listed in Attachment B-1, RFP Section 8.2.2.8. Non-capitated services are not included in the STAR MCOs’ Capitation Rates;
however, STAR MCOs must coordinate care these Non-capitated Services so that Members have access to a full range of Medically Necessary Medicaid services, both capitated and noncapitated.
STAR MCOs may also elect to include Value-added Services in their benefit packages, if approved by HHSC (see UMCM Chapter 4.5 “Physical and Behavioral Health Value-Added Services Template”).
STAR Program benefits are subject to the same benefit limits and exclusions that apply to the traditional, fee-for-service Medicaid programs, with the following three (3) exceptions. Adult STAR Members are provided with three (3) enhanced benefits compared to the traditional, fee for-service Medicaid coverage:
1 waiver of the three (3) prescription per-month limit;
2 waiver of the 30-day spell-of-illness limitation; and
3 waiver of the $200,000 individual annual limit on inpatient services.
For a complete listing of the limitations and exclusions that apply to each Medicaid benefit category, STAR MCOs should refer to the current Texas Medicaid Provider Procedures Manual, which can be accessed online at: http://www.tmhp.com.
The services listed in this Attachment are subject to modification based on changes in Federal and State laws, regulations, and policies.
STAR Covered Services include Medically Necessary:
|
| |
| • Audiology services, including hearing aids, for adults and children |
|
| |
| • Behavioral Health Services*, including: |
|
| |
| o Inpatient mental health services for Children (birth through age 20) |
|
| |
| o Acute inpatient mental health services for Adults |
|
| |
| o Outpatient mental health services |
|
| |
| o Counseling services for adults (21 years of age and over) |
|
| |
| o Outpatient substance use disorder treatment services including: |
|
| |
| o Detoxification services |
|
| |
| o Medication assisted therapy |
|
| |
| o Residential substance use disorder treatment services including: |
|
| |
| o Detoxification services |
|
| |
| o Substance use disorder treatment (including room and board) |
|
| |
| *These services are not subject to the quantitative treatment limitations that apply under traditional, fee-for-service Medicaid coverage. The services may be subject to the MCO’s non-quantitative treatment limitations, provided such limitations comply with the requirements of the Mental Health Parity and Addiction Equity Act of 2008. |
|
| |
| • Birthing services provided by a physician and certified nurse midwife (CNM) in a licensed birthing center |
|
| |
| • Birthing services provided by a licensed birthing center |
|
| |
| • Cancer screening, diagnostic, and treatment services |
|
| |
| • Durable medical equipment and supplies |
|
| |
| • Early Childhood Intervention (ECI) services |
|
| |
| • Family planning services |
|
| |
| • Home health care services |
|
| |
| • Hospital services, including inpatient and outpatient |
|
| |
| o The MCO may provide inpatient services for acute psychiatric conditions in a free-standing psychiatric hospital in lieu of an acute care inpatient hospital setting. |
|
| |
| o The MCO may provide substance use disorder treatment services in a chemical dependency treatment facility in lieu of an acute care inpatient hospital setting. |
|
| |
| • Mastectomy, breast reconstruction, and related follow-up procedures, including: |
|
| |
| • inpatient services; outpatient services provided at an outpatient hospital and ambulatory health care center as clinically appropriate; and physician and professional services provided in an office, inpatient, or outpatient setting for: |
|
| |
| o all stages of reconstruction on the breast(s) on which medically necessary mastectomy procedure(s) have been performed; |
|
| |
| o surgery and reconstruction on the other breast to produce symmetrical appearance; |
|
| |
| o treatment of physical complications from the mastectomy and treatment of lymphedemas; and |
|
| |
| o prophylactic mastectomy to prevent the development of breast cancer. |
|
| |
| • external breast prosthesis for the breast(s) on which medically necessary mastectomy procedure(s) have been performed. |
|
| |
| • Medical checkups and Comprehensive Care Program (CCP) Services for children (birth through age 20) through the Texas Health Steps Program |
|
| |
| • Oral evaluation and fluoride varnish in the Medical Home in conjunction with Texas Health Steps medical checkup for children 6 months through 35 months of age. |
|
| |
| • Outpatient drugs and biologicals; including pharmacy-dispensed and provider-administered outpatient drugs and biologicals |
|
| |
| • Drugs and biologicals provided in an inpatient setting |
|
| |
| • Prenatal care provided by a physician, certified nurse midwife (CNM), nurse practitioner (NP), clinical nurse specialist (CNS), and physician assistant (PA) in a licensed birthing center |
|
| |
| • Preventive services including an annual adult well check for patients 21 years of age and over |
|
| |
| • Radiology, imaging, and X-rays |
|
| |
| • Specialty physician services |
|
| |
| • Therapies – physical, occupational and speech |
|
| |
| • Transplantation of organs and tissues |
|
| |
| • Vision (Includes optometry and glasses. Contact lenses are only covered if they are medically necessary for vision correction that can not be accomplished by glasses.) |
|
| |
| • Telemonitoring (effective October 1, 2013, through August 31, 2015)
|
| • Telehealth
|
Subject: Attachment B-2.1 - CHIP Covered Services
|
| | | |
DOCUMENT HISTORY LOG |
STATUS1 | DOCUMENT REVISION2 | EFFECTIVE DATE | DESCRIPTION3 |
Baseline | n/a | September 1, 2011 | Initial version of Attachment B-2.1, “CHIP Covered Services.” |
Revision | 2.1 | March 1, 2012 | “Birthing Center Services” is added as a clarification item. “Services Rendered by a Certified Nurse Midwife or physician in a licensed birthing center” is added as a clarification item. Attachment B-2.1 is modified to clarify Drug Benefits for CHIP Perinate Members. CHIP Exclusions from Covered Services is modified to clarify that over the counter drugs, contraceptives, and medications prescribed for weight loss or gain are not a covered benefit. CHIP Exclusions from Covered Services for CHIP Perinates is modified to clarify that over the counter drugs contraceptives, and medications prescribed for weight loss or gain are not a covered benefit. |
Revision | 2.2 | June 1, 2012 | Contract amendment did not revise Attachment B-2.1, "CHIP Covered Services." |
Revision | 2.3 | September 1, 2012 | Contract amendment did not revise Attachment B-2.1, “CHIP Covered Services.” |
Revision | 2.4 | March 1, 2013 | CHIP Exclusions from Covered Services is modified to add Coverage while traveling outside of the United States and U.S. Territories.
|
Revision | 2.5 | June 1, 2013 | Contract amendment did not revise Attachment B-2.1, “CHIP Covered Services.” |
Revision | 2.6 | September 1, 2013 | Contract amendment did not revise Attachment B-2.1, “CHIP Covered Services.”
|
Revision | 2.7 | September 1, 2013 | Contract amendment did not revise Attachment B-2.1, “CHIP Covered Services.”
|
Revision | 2.8 | January 1, 2014 | Inpatient General Acute and Inpatient Rehabilitation Hospital Services (CHIP Perinatal Coverage) is modified to clarify the eligibility thresholds.
Birthing Center Services (CHIP Perinatal Coverage) is modified to clarify the eligibility thresholds.
Exclusions for CHIP Perinatal is modified to clarify the eligibility thresholds. |
Revision | 2.9 | February 1, 2014 | Contract amendment did not revise Attachment B-2.1, “CHIP Covered Services.”
|
Revision | 2.10 | April 1, 2014 | Contract amendment did not revise Attachment B-2.1, “CHIP Covered Services.”
|
|
| | | |
Revision | 2.11 | September 1, 2014 | Durable Medical Equipment (DME), Prosthetic Devices and Disposable Medical Supplies is modified to add a limited set of disposable medical supplies when they are obtained from an authorized pharmacy provider.
CHIP Perinatal Program Exclusions From Covered Services For CHIP Perinates is modified to add a limited set of disposable medical supplies when they are obtained from an authorized pharmacy provider.
CHIP & CHIP Perinatal Program DME/Supplies is modified to add a limited set of disposable medical supplies when they are obtained from an authorized pharmacy provider. |
1 Status should be represented as “Baseline” for initial issuances, “Revision” for changes to the Baseline version, and “Cancellation” for withdrawn versions 2 Revisions should be numbered in accordance according to the version of the issuance and sequential numbering of the revision—e.g., “1.2” refers to the first version of the document and the second revision. 3 Brief description of the changes to the document made in the revision. |
CHIP Covered Services
Covered CHIP services must meet the CHIP definition of Medically Necessary Covered Services. There is no lifetime maximum on benefits; however, 12-month period or lifetime limitations do apply to certain services, as specified in the following chart. Co-pays apply until a family reaches its specific cost-sharing maximum.
Covered CHIP Perinatal services must meet the definition of Medically Necessary Covered Services. There is no lifetime maximum on benefits; however, 12-month period or lifetime limitations do apply to certain services, as specified in the following chart. Co-pays do not apply to CHIP Perinatal Members. CHIP Perinate Newborns are eligible for 12-months continuous coverage, beginning with the month of enrollment as a CHIP Perinate.
|
| | |
Covered Benefit | CHIP Members and CHIP Perinate Newborn Members | CHIP Perinate Members (Unborn Child) |
Inpatient General Acute and Inpatient Rehabilitation Hospital Services | Services include, but are not limited to, the following:
¤ Hospital-provided Physician or Provider services
¤ Semi-private room and board (or private if medically necessary as certified by attending)
¤ General nursing care
¤ Special duty nursing when medically necessary
¤ ICU and services
¤ Patient meals and special diets
¤ Operating, recovery and other treatment rooms
¤ Anesthesia and administration (facility technical component)
¤ Surgical dressings, trays, casts, splints
¤ Drugs, medications and biologicals
| For CHIP Perinates in families with income at or below the Medicaid eligibility threshold (Perinates who qualify for Medicaid once born), the facility charges are not a covered benefit; however, professional services charges associated with labor with delivery are a covered benefit.
For CHIP Perinates in families with income above the Medicaid eligibility threshold (Perinates who do not qualify for Medicaid once born), benefits are limited to professional service charges and facility charges associated with labor with delivery until birth, and services related to miscarriage or a non-viable pregnancy. Services include: ¤ Operating, recovery and other treatment rooms ¤ Anesthesia and administration (facility technical component
|
|
| | |
| ¤ Blood or blood products that are not provided free-of-charge to the patient and their administration
¤ X-rays, imaging and other radiological tests (facility technical component)
¤ Laboratory and pathology services (facility technical component)
¤ Machine diagnostic tests (EEGs, EKGs, etc.)
¤ Oxygen services and inhalation therapy
¤ Radiation and chemotherapy
¤ Access to DSHS-designated Level III perinatal centers or Hospitals meeting equivalent levels of care
¤ In-network or out-of-network facility and Physician services for a mother and her newborn(s) for a minimum of 48 hours following an uncomplicated vaginal delivery and 96 hours following an uncomplicated delivery by caesarian section.
¤ Hospital, physician and related medical services, such as anesthesia, associated with dental care
¤ Inpatient services associated with (a) miscarriage or (b) a non-viable pregnancy (molar pregnancy, ectopic pregnancy, or a fetus that expired in utero). Inpatient services associated with miscarriage or non-viable pregnancy include, but are not limited to:
| Medically necessary surgical services are limited to services that directly relate to the delivery of the unborn child, and services related to miscarriage or non-viable pregnancy (molar pregnancy, ectopic pregnancy, or a fetus that expired in utero).
Inpatient services associated with (a) miscarriage or (b) a non-viable pregnancy (molar pregnancy, ectopic pregnancy, or a fetus that expired in utero) are a covered benefit. Inpatient services associated with miscarriage or non-viable pregnancy include, but are not limited to:
¤ dilation and curettage (D&C) procedures;
¤ appropriate provider-administered medications;
¤ ultrasounds, and
¤ histological examination of tissue samples.
|
|
| | |
| ¤ dilation and curettage (D&C) procedures;
¤ appropriate provider-administered medications;
¤ ultrasounds, and
¤ histological examination of tissue samples.
¤ Surgical implants
¤ Other artificial aids including surgical implants
¤ Inpatient services for a mastectomy and breast reconstruction include:
¤ all stages of reconstruction on the affected breast;
¤ external breast prosthesis for the breast(s) on which medically necessary mastectomy procedure(s) have been performed
¤ surgery and reconstruction on the other breast to produce symmetrical appearance; and
¤ treatment of physical complications from the mastectomy and treatment of lymphedemas.
¤ Implantable devices are covered under Inpatient and Outpatient services and do not count towards the DME 12-month period limit
¤ Pre-surgical or post-surgical orthodontic services for medically necessary treatment of craniofacial anomalies requiring surgical intervention and delivered as part of a proposed and clearly outlined treatment plan to treat:
¤ cleft lip and/or palate; or
¤ severe traumatic skeletal and/or congenital craniofacial deviations; or
severe facial asymmetry secondary to skeletal defects, congenital syndromal conditions and/or tumor growth or its treatment.
| |
Skilled Nursing Facilities (Includes Rehabilitation Hospitals) | Services include, but are not limited to, the following: ¤ Semi-private room and board ¤ Regular nursing services ¤ Rehabilitation services ¤ Medical supplies and use of appliances and equipment furnished by the facility | Not a covered benefit. |
|
| | |
Outpatient Hospital, Comprehensive Outpatient Rehabilitation Hospital, Clinic (Including Health Center) and Ambulatory Health Care Center | Services include, but are not limited to, the following services provided in a hospital clinic or emergency room, a clinic or health center, hospital-based emergency department or an ambulatory health care setting: ¤ X-ray, imaging, and radiological tests (technical component) ¤ Laboratory and pathology services (technical component) ¤ Machine diagnostic tests ¤ Ambulatory surgical facility services ¤ Drugs, medications and biologicals ¤ Casts, splints, dressings ¤ Preventive health services ¤ Physical, occupational and speech therapy ¤ Renal dialysis ¤ Respiratory services - Radiation and chemotherapy ¤ Blood or blood products that are not provided free-of-charge to the patient and the administration of these products ¤ Outpatient services associated with (a) miscarriage or (b) a non-viable pregnancy (molar pregnancy, ectopic pregnancy, or a fetus that expired in utero). Outpatient services associated with miscarriage or non-viable pregnancy include, but are not limited to: ¤ dilation and curettage (D&C) procedures; ¤ appropriate provider-administered medications; ¤ ultrasounds, and ¤ histological examination of tissue samples. ¤ Facility and related medical services, such as anesthesia, associated with dental care, when provided in a licensed ambulatory surgical facility. ¤ Surgical implants ¤ Other artificial aids including surgical implants ¤ Outpatient services provided at an outpatient hospital and ambulatory health care center for a mastectomy and breast reconstruction as clinically appropriate, include: ¤ all stages of reconstruction on the affected breast; ¤ external breast prosthesis for the breast(s) on which medically necessary mastectomy procedure(s) have been performed ¤ surgery and reconstruction on the other breast to produce symmetrical appearance; and ¤ treatment of physical complications from the mastectomy and treatment of lymphedemas. ¤ Implantable devices are covered under Inpatient and Outpatient services and do not count towards the DME 12-month period limit ¤ Pre-surgical or post-surgical orthodontic services for medically necessary treatment of craniofacial anomalies requiring surgical intervention and delivered as part of a proposed and clearly outlined treatment plan to treat: ¤ cleft lip and/or palate; or ¤ severe traumatic skeletal and/or congenital craniofacial deviations; or severe facial asymmetry secondary to skeletal defects, congenital syndromal conditions and/or tumor growth or its treatment. | Services include, the following services provided in a hospital clinic or emergency room, a clinic or health center, hospital-based emergency department or an ambulatory health care setting: ¤ X-ray, imaging, and radiological tests (technical component) ¤ Laboratory and pathology services (technical component) ¤ Machine diagnostic tests ¤ Drugs, medications and biologicals that are medically necessary prescription and injection drugs. ¤ Outpatient services associated with (a) miscarriage or (b) a non-viable pregnancy (molar pregnancy, ectopic pregnancy, or a fetus that expired in utero). Outpatient services associated with miscarriage or non-viable pregnancy include, but are not limited to: ¤ dilation and curettage (D&C) procedures; ¤ appropriate provider-administered medications; ¤ ultrasounds, and ¤ histological examination of tissue samples. (1) Laboratory and radiological services are limited to services that directly relate to ante partum care and/or the delivery of the covered CHIP Perinate until birth. (2) Ultrasound of the pregnant uterus is a covered benefit when medically indicated. Ultrasound may be indicated for suspected genetic defects, high-risk pregnancy, fetal growth retardation, gestational age confirmation or miscarriage or non-viable pregnancy. (3) Amniocentesis, Cordocentesis, Fetal Intrauterine Transfusion (FIUT) and Ultrasonic Guidance for Cordocentesis, FIUT are covered benefits with an appropriate diagnosis. (4) Laboratory tests are limited to: nonstress testing, contraction, stress testing, hemoglobin or hematocrit repeated once a trimester and at 32-36 weeks of pregnancy; or complete blood count (CBC), urinanalysis for protein and glucose every visit, blood type and RH antibody screen; repeat antibody screen for Rh negative women at 28 weeks followed by RHO immune globulin administration if indicated; rubella antibody titer, serology for syphilis, hepatitis B surface antigen, cervical cytology, pregnancy test, gonorrhea test, urine culture, sickle cell test, tuberculosis (TB) test, human immunodeficiency virus (HIV) antibody screen, Chlamydia test, other laboratory tests not specified but deemed medically necessary, and multiple marker screens for neural tube defects (if the client initiates care between 16 and 20 weeks); screen for gestational diabetes at 24-28 weeks of pregnancy; other lab tests as indicated by medical condition of client. (5) Surgical services associated with (a) miscarriage or (b) a non-viable pregnancy (molar pregnancy, ectopic pregnancy, or a fetus that expired in utero) are a covered benefit. |
|
| | |
Physician/Physician Extender Professional Services | Services include, but are not limited to, the following: ¤ American Academy of Pediatrics recommended well-child exams and preventive health services (including, but not limited to, vision and hearing screening and immunizations) ¤ Physician office visits, inpatient and outpatient services ¤ Laboratory, x-rays, imaging and pathology services, including technical component and/or professional interpretation ¤ Medications, biologicals and materials administered in Physician’s office ¤ Allergy testing, serum and injections ¤ Professional component (in/outpatient) of surgical services, including: ¤ Surgeons and assistant surgeons for surgical procedures including appropriate follow-up care ¤ Administration of anesthesia by Physician (other than surgeon) or CRNA ¤ Second surgical opinions ¤ Same-day surgery performed in a Hospital without an over-night stay ¤ Invasive diagnostic procedures such as endoscopic examinations ¤ Hospital-based Physician services (including Physician-performed technical and interpretive components) ¤ Physician and professional services for a mastectomy and breast reconstruction include: ¤ all stages of reconstruction on the affected breast; ¤ external breast prosthesis for the breast(s) on which medically necessary mastectomy procedure(s) have been performed
| Services include, but are not limited to the following: ¤ Medically necessary physician services are limited to prenatal and postpartum care and/or the delivery of the covered unborn child until birth ¤ Physician office visits, inpatient and outpatient services ¤ Laboratory, x-rays, imaging and pathology services including technical component and /or professional interpretation ¤ Medically necessary medications, biologicals and materials administered in Physician’s office ¤ Professional component (in/outpatient) of surgical services, including: ¤ Surgeons and assistant surgeons for surgical procedures directly related to the labor with delivery of the covered unborn child until birth. ¤ Administration of anesthesia by Physician (other than surgeon) or CRNA ¤ Invasive diagnostic procedures directly related to the labor with delivery of the unborn child. ¤ Surgical services associated with (a) miscarriage or (b) a non-viable pregnancy (molar pregnancy, ectopic pregnancy, or a fetus that expired in utero.) ¤ Hospital-based Physician services (including Physician performed technical and interpretive components)
|
|
| | |
| ¤ surgery and reconstruction on the other breast to produce symmetrical appearance; and
¤ treatment of physical complications from the mastectomy and treatment of lymphedemas.
¤ In-network and out-of-network Physician services for a mother and her newborn(s) for a minimum of 48 hours following an uncomplicated vaginal delivery and 96 hours following an uncomplicated delivery by caesarian section.
¤ Physician services associated with (a) miscarriage or (b) a non-viable pregnancy (molar pregnancy, ectopic pregnancy, or a fetus that expired in utero). Physician services associated with miscarriage or non-viable pregnancy include, but are not limited to:
¤ dilation and curettage (D&C) procedures;
- appropriate provider-administered
- medications;
- ultrasounds, and
- histological examination of tissue samples.
¤ Physician services medically necessary to support a dentist providing dental services to a CHIP member such as general anesthesia or intravenous (IV) sedation.
¤ Pre-surgical or post-surgical orthodontic services for medically necessary treatment of craniofacial anomalies requiring surgical intervention and delivered as part of a proposed and clearly outlined treatment plan to treat:
¤ cleft lip and/or palate; or
¤ severe traumatic skeletal and/or congenital craniofacial deviations; or
severe facial asymmetry secondary to skeletal defects, congenital syndromal conditions and/or tumor growth or its treatment. | ¤ Professional component of the ultrasound of the pregnant uterus when medically indicated for suspected genetic defects, high-risk pregnancy, fetal growth retardation, or gestational age confirmation.
¤ Professional component of Amniocentesis, Cordocentesis, Fetal Intrauterine Transfusion (FIUT) and Ultrasonic Guidance for Amniocentesis, Cordocentrsis, and FIUT.
¤ Professional component associated with (a) miscarriage or (b) a non-viable pregnancy (molar pregnancy, ectopic pregnancy, or a fetus that expired in utero). Professional services associated with miscarriage or non-viable pregnancy include, but are not limited to:
¤ dilation and curettage (D&C) procedures;
¤ appropriate provider-administered medications;
¤ ultrasounds, and
histological examination of tissue samples |
|
| | |
Prenatal Care and Pre-Pregnancy Family Services and Supplies | Covered, unlimited prenatal care and medically necessary care related to diseases, illness, or abnormalities related to the reproductive system, and limitations and exclusions to these services are described under inpatient, outpatient and physician services. Primary and preventive health benefits do not include pre-pregnancy family reproductive services and supplies, or prescription medications prescribed only for the purpose of primary and preventive reproductive health care. | Services are limited to an initial visit and subsequent prenatal (ante partum) care visits that include: (1) One (1) visit every four (4) weeks for the first 28 weeks or pregnancy; (2) one (1) visit every two (2) to three (3) weeks from 28 to 36 weeks of pregnancy; and (3) one (1) visit per week from 36 weeks to delivery. More frequent visits are allowed as Medically Necessary. Benefits are limited to: Limit of 20 prenatal visits and two (2) postpartum visits (maximum within 60 days) without documentation of a complication of pregnancy. More frequent visits may be necessary for high-risk pregnancies. High-risk prenatal visits are not limited to 20 visits per pregnancy. Documentation supporting medical necessity must be maintained in the physician’s files and is subject to retrospective review. Visits after the initial visit must include: ¤ interim history (problems, marital status, fetal status); ¤ physical examination (weight, blood pressure, fundalheight, fetal position and size, fetal heart rate, extremities) and laboratory tests (urinanalysis for protein and glucose every visit; hematocrit or hemoglobin repeated once a trimester and at 32-36 weeks of pregnancy; multiple marker screen for fetal abnormalities offered at 16-20 weeks of pregnancy; repeat antibody screen for Rh negative women at 28 weeks followed by Rho immune globulin administration if indicated; screen for gestational diabetes at 24-28 weeks of pregnancy; and other lab tests as indicated by medical condition of client). |
Birthing Center Services
| Covers birthing services provided by a licensed birthing center. Limited to facility services (e.g., labor and delivery) Limitation: Applies only to CHIP members.
| Covers birthing services provided by a licensed birthing center. Limited to facility services related to labor with delivery. Applies only to CHIP Perinate Members (unborn child) with income above the Medicaid eligibility threshold (who will not qualify for Medicaid once born). |
|
| | |
Services Rendered by a Certified Nurse Midwife or physician in a licensed birthing center
| CHIP Members: Covers prenatal services and birthing services rendered in a licensed birthing center. CHIP Perinate Newborn Members: Covers services rendered to a newborn immediately following delivery.
| Covers prenatal services and birthing services rendered in a licensed birthing center. Prenatal services subject to the following limitations: Services are limited to an initial visit and subsequent prenatal (ante partum) care visits that include: (1) one (1) visit every four (4) weeks for the first 28 weeks or pregnancy; (2) one (1) visit every two (2) to three (3) weeks from 28 to 36 weeks of pregnancy; and (3) one (1) visit per week from 36 weeks to delivery.
More frequent visits are allowed as Medically Necessary. Benefits are limited to: Limit of 20 prenatal visits and two (2) postpartum visits (maximum within 60 days) without documentation of a complication of pregnancy. More frequent visits may be necessary for high-risk pregnancies. High-risk prenatal visits are not limited to 20 visits per pregnancy. Documentation supporting medical necessity must be maintained and is subject to retrospective review. Visits after the initial visit must include: interim history (problems, marital status, fetal status); physical examination (weight, blood pressure, fundalheight, fetal position and size, fetal heart rate, extremities) and laboratory tests (urinanalysis for protein and glucose every visit; hematocrit or hemoglobin repeated once a trimester and at 32-36 weeks of pregnancy; multiple marker screen for fetal abnormalities offered at 16-20 weeks of pregnancy; repeat antibody screen for Rh negative women at 28 weeks followed by Rho immune globulin administration if indicated; screen for gestational diabetes at 24-28 weeks of pregnancy; and other lab tests as indicated by medical condition of client).
|
|
| | |
Durable Medical Equipment (DME), Prosthetic Devices and Disposable Medical Supplies | $20,000 12-month period limit for DME, prosthetic devices and disposable medical supplies (diabetic supplies and equipment are not counted against this cap). Services include DME (equipment which can withstand repeated use and is primarily and customarily used to serve a medical purpose, generally is not useful to a person in the absence of Illness, Injury, or Disability, and is appropriate for use in the home), including devices and supplies that are medically necessary and necessary for one or more activities of daily living and appropriate to assist in the treatment of a medical condition, including: ¤ Orthotic braces and orthotics ¤ Dental devices ¤ Prosthetic devices such as artificial eyes, limbs, braces, and external breast prostheses ¤ Prosthetic eyeglasses and contact lenses for the management of severe ophthalmologic disease ¤ Hearing aids Diagnosis-specific disposable medical supplies, including diagnosis-specific prescribed specialty formula and dietary supplements. (See Attachment A) | Not a covered benefit, with the exception of a limited set of disposable medical supplies, published at http://www.txvendordrug.com/formulary/limited-hhs.shtml and only when they are obtained from a CHIP-enrolled pharmacy provider. |
Home and Community Health Services | Services that are provided in the home and community, including, but not limited to: ¤ Home infusion ¤ Respiratory therapy ¤ Visits for private duty nursing (R.N., L.V.N.) ¤ Skilled nursing visits as defined for home health purposes (may include R.N. or L.V.N.). ¤ Home health aide when included as part of a plan of care during a period that skilled visits have been approved. ¤ Speech, physical and occupational therapies. ¤ Services are not intended to replace the CHILD'S caretaker or to provide relief for the caretaker ¤ Skilled nursing visits are provided on intermittent level and not intended to provide 24-hour skilled nursing services Services are not intended to replace 24-hour inpatient or skilled nursing facility services | Not a covered benefit. |
|
| | |
Inpatient Mental Health Services | Mental health services, including for serious mental illness, furnished in a free-standing psychiatric hospital, psychiatric units of general acute care hospitals and state-operated facilities, including, but not limited to: ¤ Neuropsychological and psychological testing. ¤ When inpatient psychiatric services are ordered by a court of competent jurisdiction under the provisions of Chapters 573 and 574 of the Texas Health and Safety Code, relating to court ordered commitments to psychiatric facilities, the court order serves as binding determination of medical necessity. Any modification or termination of services must be presented to the court with jurisdiction over the matter for determination ¤ Does not require PCP referral | Not a covered benefit. |
Outpatient Mental Health Services | Mental health services, including for serious mental illness, provided on an outpatient basis, including, but not limited to: ¤ The visits can be furnished in a variety of community-based settings (including school and home-based) or in a state-operated facility • Neuropsychological and psychological testing • Medication management • Rehabilitative day treatments • Residential treatment services • Sub-acute outpatient services (partial hospitalization or rehabilitative day treatment) ¤ Skills training (psycho-educational skill development) ¤ When outpatient psychiatric services are ordered by a court of competent jurisdiction under the provisions of Chapters 573 and 574 of the Texas Health and Safety Code, relating to court ordered commitments to psychiatric facilities, the court order serves as binding determination of medical necessity. Any modification or termination of services must be presented to the court with jurisdiction over the matter for determination ¤ A Qualified Mental Health Provider – Community Services (QMHP-CS), is defined by the Texas Department of State Health Services (DSHS) in Title 25 T.A.C., Part I, Chapter 412, Subchapter G, Division 1, §412.303(48). QMHP-CSs shall be providers working through a DSHS-contracted Local Mental Health Authority or a separate DSHS-contracted entity. QMHP-CSs shall be supervised by a licensed mental health professional or physician and provide services in accordance with DSHS standards. Those services include individual and group skills training (which can be components of interventions such as day treatment and in-home services), patient and family education, and crisis services Does not require PCP referral | Not a covered benefit. |
|
| | |
Inpatient Substance Abuse Treatment Services | Services include, but are not limited to: ¤ Inpatient and residential substance abuse treatment services including detoxification and crisis stabilization, and 24-hour residential rehabilitation programs ¤ Does not require PCP referral | Not a covered benefit. |
Outpatient Substance Abuse Treatment Services | Services include, but are not limited to, the following: ¤ Prevention and intervention services that are provided by physician and non-physician providers, such as screening, assessment and referral for chemical dependency disorders. ¤ Intensive outpatient services ¤ Partial hospitalization ¤ Intensive outpatient services is defined as an organized non-residential service providing structured group and individual therapy, educational services, and life skills training which consists of at least 10 hours per week for four to 12 weeks, but less than 24 hours per day ¤ Outpatient treatment service is defined as consisting of at least one to two hours per week providing structured group and individual therapy, educational services, and life skills training ¤ Does not require PCP referral | Not a covered benefit. |
Rehabilitation Services | Services include, but are not limited to, the following: ¤ Habilitation (the process of supplying a child with the means to reach age-appropriate developmental milestones through therapy or treatment) and rehabilitation services include, but are not limited to the following: ¤ Physical, occupational and speech therapy ¤ Developmental assessment | Not a covered benefit. |
Hospice Care Services | Services include, but are not limited to: ¤ Palliative care, including medical and support services, for those children who have six (6) months or less to live, to keep patients comfortable during the last weeks and months before death ¤ Treatment services, including treatment related to the terminal illness ¤ Up to a maximum of 120 days with a 6 month life expectancy ¤ Patients electing hospice services may cancel this election at anytime ¤ Services apply to the hospice diagnosis | Not a covered benefit. |
|
| | |
Emergency Services, including Emergency Hospitals, Physicians, and Ambulance Services | MCO cannot require authorization as a condition for payment for emergency conditions or labor and delivery. Covered services include, but are not limited to, the following: ¤ Emergency services based on prudent layperson definition of emergency health condition ¤ Hospital emergency department room and ancillary services and physician services 24 hours a day, seven (7) days a week, both by in-network and out-of-network providers ¤ Medical screening examination ¤ Stabilization services ¤ Access to DSHS designated Level 1 and Level II trauma centers or hospitals meeting equivalent levels of care for emergency services ¤ Emergency ground, air and water transportation ¤ Emergency dental services, limited to fractured or dislocated jaw, traumatic damage to teeth, removal of cysts, and treatment relating to oral abscess of tooth or gum origin. | MCO cannot require authorization as a condition for payment for emergency conditions related to labor with delivery. Covered services are limited to those emergency services that are directly related to the delivery of the unborn child until birth. ¤ Emergency services based on prudent lay person definition of emergency health condition ¤ Medical screening examination to determine emergency when directly related to the delivery of the covered unborn child. ¤ Stabilization services related to the labor with delivery of the covered unborn child. ¤ Emergency ground, air and water transportation for labor and threatened labor is a covered benefit ¤ Emergency ground, air and water transportation for an emergency associated with (a) miscarriage or (b) a non-viable pregnancy (molar pregnancy, ectopic pregnancy, or a fetus that expired in utero) is a covered benefit. Benefit limits: Post-delivery services or complications resulting in the need for emergency services for the mother of the CHIP Perinate are not a covered benefit. |
Transplants | Services include, but are not limited to, the following: ¤ Using up-to-date FDA guidelines, all non-experimental human organ and tissue transplants and all forms of non-experimental corneal, bone marrow and peripheral stem cell transplants, including donor medical expenses. | Not a covered benefit. |
Vision Benefit | The health plan may reasonably limit the cost of the frames/lenses. Services include: ¤ One (1) examination of the eyes to determine the need for and prescription for corrective lenses per 12-month period, without authorization ¤ One (1) pair of non-prosthetic eyewear per 12-month period | Not a covered benefit. |
Chiropractic Services | Services do not require physician prescription and are limited to spinal subluxation | Not a covered benefit. |
Tobacco Cessation Program | Covered up to $100 for a 12-month period limit for a plan- approved program ¤ Health Plan defines plan-approved program. ¤ May be subject to formulary requirements. | Not a covered benefit. |
|
| | |
Case Management and Care Coordination Services | These services include outreach informing, case management, care coordination and community referral. | Covered benefit. |
Drug Benefits | Services include, but are not limited to, the following: ¤ • Outpatient drugs and biologicals; including pharmacy-dispensed and provider-administered outpatient drugs and biologicals; and ¤ • Drugs and biologicals provided in an inpatient setting. | Not a covered benefit unless identified elsewhere in this table. |
[Value-added services] | See RFP Attachment B-2.1 | |
CHIP Exclusions from Covered Services
|
| |
| Inpatient and outpatient infertility treatments or reproductive services other than prenatal care, labor and delivery, and care related to disease, illnesses, or abnormalities related to the reproductive system |
|
| |
| Contraceptive medications prescribed only for the purpose of primary and preventive reproductive health care (i.e., cannot be prescribed for family planning)
|
|
| |
| Personal comfort items including but not limited to personal care kits provided on inpatient admission, telephone, television, newborn infant photographs, meals for guests of patient, and other articles which are not required for the specific treatment of sickness or injury |
|
| |
| Experimental and/or investigational medical, surgical or other health care procedures or services which are not generally employed or recognized within the medical community |
|
| |
| Treatment or evaluations required by third parties including, but not limited to, those for schools, employment, flight clearance, camps, insurance or court |
|
| |
| Private duty nursing services when performed on an inpatient basis or in a skilled nursing facility. |
|
| |
| Mechanical organ replacement devices including, but not limited to artificial heart |
|
| |
| Hospital services and supplies when confinement is solely for diagnostic testing purposes, unless otherwise pre-authorized by Health Plan |
|
| |
| Prostate and mammography screening |
|
| |
| Elective surgery to correct vision |
|
| |
| Gastric procedures for weight loss |
|
| |
| Cosmetic surgery/services solely for cosmetic purposes |
|
| |
| Dental devices solely for cosmetic purposes |
|
| |
| Out-of-network services not authorized by the Health Plan except for emergency care and physician services for a mother and her newborn(s) for a minimum of 48 hours following an uncomplicated vaginal delivery and 96 hours following an uncomplicated delivery by caesarian section |
|
| |
| Services, supplies, meal replacements or supplements provided for weight control or the treatment of obesity, except for the services associated with the treatment for morbid obesity as part of a treatment plan approved by the Health Plan |
|
| |
| Medications prescribed for weight loss or gain |
|
| |
| Acupuncture services, naturopathy and hypnotherapy |
|
| |
| Immunizations solely for foreign travel |
|
| |
| Routine foot care such as hygienic care |
|
| |
| Diagnosis and treatment of weak, strained, or flat feet and the cutting or removal of corns, calluses and toenails (this does not apply to the removal of nail roots or surgical treatment of conditions underlying corns, calluses or ingrown toenails) |
|
| |
| Replacement or repair of prosthetic devices and durable medical equipment due to misuse, abuse or loss when confirmed by the Member or the vendor |
|
| |
| Corrective orthopedic shoes |
|
| |
| Over-the-counter medications
|
|
| |
| Orthotics primarily used for athletic or recreational purposes |
|
| |
| Custodial care (care that assists a child with the activities of daily living, such as assistance in walking, getting in and out of bed, bathing, dressing, feeding, toileting, special diet preparation, and medication supervision that is usually self-administered or provided by a parent. This care does not require the continuing attention of trained medical or paramedical personnel.) This exclusion does not apply to hospice services. |
|
| |
| Public facility services and care for conditions that federal, state, or local law requires be provided in a public facility or care provided while in the custody of legal authorities |
|
| |
| Services or supplies received from a nurse, which do not require the skill and training of a nurse |
|
| |
| Vision training and vision therapy |
|
| |
| Reimbursement for school-based physical therapy, occupational therapy, or speech therapy services are not covered except when ordered by a Physician/PCP |
|
| |
| Donor non-medical expenses |
|
| |
| Charges incurred as a donor of an organ when the recipient is not covered under this health plan |
|
| |
| Coverage while traveling outside of the United States and U.S. Territories (including Puerto Rico, U.S. Virgin Islands, Commonwealth of Northern Mariana Islands, Guam, and American Samoa)
|
EXCLUSIONS FROM COVERED SERVICES FOR CHIP PERINATES
|
| |
| For CHIP Perinates in families with income at or below the Medicaid eligibility threshold (Perinates who qualify for Medicaid once born), inpatient facility charges are not a covered benefit if associated with the initial Perinatal Newborn admission. "Initial Perinatal Newborn admission" means the hospitalization associated with the birth. |
|
| |
| Contraceptive medications prescribed only for the purpose of primary and preventive reproductive health care (i.e. cannot be prescribed for family planning)
|
|
| |
| Inpatient and outpatient treatments other than prenatal care, labor with delivery, services related to (a) miscarriage and (b) a non-viable pregnancy, and postpartum care related to the covered unborn child until birth. |
|
| |
| Inpatient mental health services. |
|
| |
| Outpatient mental health services. |
|
| |
| Durable medical equipment or other medically related remedial devices. |
|
| |
| Disposable medical supplies, with the exception of a limited set of disposable medical supplies, published at http://www.txvenordrug.com/formulary/limited-hhs.shtml, when they are obtained from an authorized pharmacy provider. |
|
| |
| Home and community-based health care services. |
|
| |
| Inpatient substance abuse treatment services and residential substance abuse treatment services. |
|
| |
| Outpatient substance abuse treatment services. |
|
| |
| Physical therapy, occupational therapy, and services for individuals with speech, hearing, and language disorders. |
|
| |
| Skilled nursing facility and rehabilitation hospital services. |
|
| |
| Emergency services other than those directly related to the labor with delivery of the covered unborn child. |
|
| |
| Tobacco Cessation Programs. |
|
| |
| Medical transportation not directly related to labor or threatened labor, miscarriage or non-viable pregnancy, and/or delivery of the covered unborn child. |
|
| |
| Personal comfort items including but not limited to personal care kits provided on inpatient admission, telephone, television, newborn infant photographs, meals for guests of patient, and other articles which are not required for the specific treatment related to labor with delivery or post partum care. |
|
| |
| Experimental and/or investigational medical, surgical or other health care procedures or services which are not generally employed or recognized within the medical community |
|
| |
| Treatment or evaluations required by third parties including, but not limited to, those for schools, employment, flight clearance, camps, insurance or court |
|
| |
| Private duty nursing services when performed on an inpatient basis or in a skilled nursing facility. |
|
| |
| Coverage while traveling outside of the United States and U.S. Territories (including Puerto Rico, U.S. Virgin Islands, Commonwealth of Northern Mariana Islands, Guam, and American Samoa). |
|
| |
| Mechanical organ replacement devices including, but not limited to artificial heart |
|
| |
| Hospital services and supplies when confinement is solely for diagnostic testing purposes and not a part of labor with delivery |
|
| |
| Prostate and mammography screening |
|
| |
| Elective surgery to correct vision |
|
| |
| Gastric procedures for weight loss |
|
| |
| Cosmetic surgery/services solely for cosmetic purposes |
|
| |
| Out-of-network services not authorized by the Health Plan except for emergency care related to the labor with delivery of the covered unborn child. |
|
| |
| Services, supplies, meal replacements or supplements provided for weight control or the treatment of obesity |
|
| |
| Acupuncture services, naturopathy and hypnotherapy |
|
| |
| Immunizations solely for foreign travel |
|
| |
| Routine foot care such as hygienic care |
|
| |
| Diagnosis and treatment of weak, strained, or flat feet and the cutting or removal of corns, calluses and toenails (this does not apply to the removal of nail roots or surgical treatment of conditions underlying corns, calluses or ingrown toenails) |
|
| |
| Corrective orthopedic shoes |
|
| |
| Orthotics primarily used for athletic or recreational purposes |
|
| |
| Custodial care (care that assists with the activities of daily living, such as assistance in walking, getting in and out of bed, bathing, dressing, feeding, toileting, special diet preparation, and medication supervision that is usually self-administered or provided by a caregiver. This care does not require the continuing attention of trained medical or paramedical personnel.) |
|
| |
| Public facility services and care for conditions that federal, state, or local law requires be provided in a public facility or care provided while in the custody of legal authorities |
|
| |
| Services or supplies received from a nurse, which do not require the skill and training of a nurse |
|
| |
| Vision training, vision therapy, or vision services |
|
| |
| Reimbursement for school-based physical therapy, occupational therapy, or speech therapy services are not covered |
|
| |
| Donor non-medical expenses |
|
| |
| Charges incurred as a donor of an organ |
CHIP DME/SUPPLIES
Note: DME/SUPPLIES are not a covered benefit for CHIP Perinate Members (Unborn Child), with the exception of a limited set of disposable medical supplies, published at http://www.txvendordrug.com/formulary/limited-hhs.shtml, when they are obtained from an authorized pharmacy provider.
|
| | | | | | |
SUPPLIES | | COVERED | | EXCLUDED | | COMMENTS / MEMBER CONTRACT PROVISIONS |
Ace Bandages | | | | X | | Exception: If provided by and billed through the clinic or home care agency it is covered as an incidental supply. |
Alcohol, rubbing | | | | X | | Over-the-counter supply. |
Alcohol, swabs (diabetic) | | X | | | | Over-the-counter supply not covered, unless RX provided at time of dispensing. |
Alcohol, swabs | | X | | | | Covered only when received with IV therapy or central line kits/supplies. |
Ana Kit Epinephrine | | X | | | | A self-injection kit used by patients highly allergic to bee stings. |
Arm Sling | | X | | | | Dispensed as part of office visit. |
Attends (Diapers) | | X | | | | Coverage limited to children age 4 or over only when prescribed by a physician and used to provide care for a covered diagnosis as outlined in a treatment care plan |
Bandages | | | | X | | |
Basal Thermometer | | | | X | | Over-the-counter supply. |
Batteries – initial | | X | | . | | For covered DME items |
Batteries – replacement | | X | | | | For covered DME when replacement is necessary due to normal use. |
Betadine | | | | X | | See IV therapy supplies. |
Books | | | | X | | |
Clinitest | | X | | | | For monitoring of diabetes. |
Colostomy Bags | | | | | | See Ostomy Supplies. |
Communication Devices | | | | X | | |
Contraceptive Jelly | | | | X | | Over-the-counter supply. Contraceptives are not covered under the plan. |
Cranial Head Mold | | | | X | | |
Dental Devices | | X | | | | Coverage limited to dental devices used for treatment of craniofacial anomalies requiring surgical intervention. |
Diabetic Supplies | | X | | | | Monitor calibrating solution, insulin syringes, needles, lancets, lancet device, and glucose strips. |
Diapers/Incontinent Briefs/Chux | | X | | | | Coverage limited to children age 4 or over only when prescribed by a physician and used to provide care for a covered diagnosis as outlined in a treatment care plan |
|
| | | | | | |
Diaphragm | | | | X | | Contraceptives are not covered under the plan. |
Diastix | | X | | | | For monitoring diabetes. |
Diet, Special | | | | X | | |
Distilled Water | | | | X | | |
Dressing Supplies/Central Line | | X | | | | Syringes, needles, Tegaderm, alcohol swabs, Betadine swabs or ointment, tape. Many times these items are dispensed in a kit when includes all necessary items for one dressing site change. |
Dressing Supplies/Decubitus | | X | | | | Eligible for coverage only if receiving covered home care for wound care. |
Dressing Supplies/Peripheral IV Therapy | | X | | | | Eligible for coverage only if receiving home IV therapy. |
Dressing Supplies/Other | | | | X | | |
Dust Mask | | | | X | | |
Ear Molds | | X | | | | Custom made, post inner or middle ear surgery |
Electrodes | | X | | | | Eligible for coverage when used with a covered DME. |
Enema Supplies | | | | X | | Over-the-counter supply. |
Enteral Nutrition Supplies | | X | | | | Necessary supplies (e.g., bags, tubing, connectors, catheters, etc.) are eligible for coverage. Enteral nutrition products are not covered except for those prescribed for hereditary metabolic disorders, a non-function or disease of the structures that normally permit food to reach the small bowel, or malabsorption due to disease |
Eye Patches | | X | | | | Covered for patients with amblyopia. |
|
| | | | | | |
Formula | | | | X | | Exception: Eligible for coverage only for chronic hereditary metabolic disorders a non-function or disease of the structures that normally permit food to reach the small bowel; or malabsorption due to disease (expected to last longer than 60 days when prescribed by the physician and authorized by plan.) Physician documentation to justify prescription of formula must include: • Identification of a metabolic disorder, dysphagia that results in a medical need for a liquid diet, presence of a gastrostomy, or disease resulting in malabsorption that requires a medically necessary nutritional product Does not include formula: • For members who could be sustained on an age-appropriate diet. • Traditionally used for infant feeding • In pudding form (except for clients with documented oropharyngeal motor dysfunction who receive greater than 50 percent of their daily caloric intake from this product) • For the primary diagnosis of failure to thrive, failure to gain weight, or lack of growth or for infants less than twelve months of age unless medical necessity is documented and other criteria, listed above, are met. Food thickeners, baby food, or other regular grocery products that can be blenderized and used with an enteral system that are not medically necessary, are not covered, regardless of whether these regular food products are taken orally or parenterally. |
Gloves | | | | X | | Exception: Central line dressings or wound care provided by home care agency. |
Hydrogen Peroxide | | | | X | | Over-the-counter supply. |
Hygiene Items | | | | X | | |
Incontinent Pads | | X | | | | Coverage limited to children age 4 or over only when prescribed by a physician and used to provide care for a covered diagnosis as outlined in a treatment care plan |
Insulin Pump (External) Supplies | | X | | | | Supplies (e.g., infusion sets, syringe reservoir and dressing, etc.) are eligible for coverage if the pump is a covered item. |
Irrigation Sets, Wound Care | | X | | | | Eligible for coverage when used during covered home care for wound care. |
Irrigation Sets, Urinary | | X | | | | Eligible for coverage for individual with an indwelling urinary catheter. |
|
| | | | | | |
IV Therapy Supplies | | X | | | | Tubing, filter, cassettes, IV pole, alcohol swabs, needles, syringes and any other related supplies necessary for home IV therapy. |
K-Y Jelly | | | | X | | Over-the-counter supply. |
Lancet Device | | X | | | | Limited to one device only. |
Lancets | | X | | | | Eligible for individuals with diabetes. |
Med Ejector | | X | | | | |
Needles and Syringes/Diabetic | | | | | | See Diabetic Supplies |
Needles and Syringes/IV and Central Line | | | | | | See IV Therapy and Dressing Supplies/Central Line. |
Needles and Syringes/Other | | X | | | | Eligible for coverage if a covered IM or SubQ medication is being administered at home. |
Normal Saline | | | | | | See Saline, Normal |
Novopen | | X | | | | |
Ostomy Supplies | | X | | | | Items eligible for coverage include: belt, pouch, bags, wafer, face plate, insert, barrier, filter, gasket, plug, irrigation kit/sleeve, tape, skin prep, adhesives, drain sets, adhesive remover, and pouch deodorant. Items not eligible for coverage include: scissors, room deodorants, cleaners, rubber gloves, gauze, pouch covers, soaps, and lotions. |
Parenteral Nutrition/Supplies | | X | | | | Necessary supplies (e.g., tubing, filters, connectors, etc.) are eligible for coverage when the Health Plan has authorized the parenteral nutrition. |
Saline, Normal | | X | | | | Eligible for coverage: a) when used to dilute medications for nebulizer treatments; b) as part of covered home care for wound care; c) for indwelling urinary catheter irrigation. |
Stump Sleeve | | X | | | | |
Stump Socks | | X | | | | |
Suction Catheters | | X | | | | |
Syringes | | | | | | See Needles/Syringes. |
Tape | | | | | | See Dressing Supplies, Ostomy Supplies, IV Therapy Supplies. |
Tracheostomy Supplies | | X | | | | Cannulas, Tubes, Ties, Holders, Cleaning Kits, etc. are eligible for coverage. |
Under Pads | | | | | | See Diapers/Incontinent Briefs/Chux. |
Unna Boot | | X | | | | Eligible for coverage when part of wound care in the home setting. Incidental charge when applied during office visit. |
Urinary, External Catheter & Supplies | | | | X | | Exception: Covered when used by incontinent male where injury to the urethra prohibits use of an indwelling catheter ordered by the PCP and approved by the plan |
Urinary, Indwelling Catheter & Supplies | | X | | | | Cover catheter, drainage bag with tubing, insertion tray, irrigation set and normal saline if needed. |
|
| | | | | | |
Urinary, Intermittent | | X | | | | Cover supplies needed for intermittent or straight catherization. |
Urine Test Kit | | X | | | | When determined to be medically necessary. |
Urostomy supplies | | | | | | See Ostomy Supplies. |
Subject: Attachment B-2.2 - STAR+PLUS Covered Services
DOCUMENT HISTORY LOG
|
| | | |
STATUS1 | DOCUMENT REVISION2 | EFFECTIVE DATE | DESCRIPTION3 |
Baseline | n/a | September 1, 2011 | Initial version of Attachment B-2.2, “STAR+PLUS Covered Services.” |
Revision | 2.1 | March 1, 2012 | Attachment B-2.2 is modified to reinstate the waiver of the three prescription limit for adults language and to add the waiver of the $200,000 individual annual limit on inpatient services. STAR+PLUS Covered Services is modified to clarify the requirements regarding services provided in free-standing psychiatric hospitals and chemical dependency treatment facilities in lieu of the acute care hospital setting. Services included under the HMO capitation payment is modified to clarify the requirements for "Prenatal care services rendered in a birthing center." |
Revision | 2.2 | June 1, 2012 | Contract amendment did not revise Attachment B-2.2, "STAR+PLUS Covered Services." |
Revision | 2.3 | September 1, 2012 | Community Based Long Term Care Services is modified to replace references to “1915(c) STAR+PLUS Waiver” and “1915(c) Nursing Facility Waiver” with “HCBS STAR+PLUS Waiver”.
|
Revision | 2.4 | March 1, 2013 | Contract amendment did not revise Attachment B-2.2, “STAR+PLUS Covered Services.”
|
Revision | 2.5 | June 1, 2013 | Contract amendment did not revise Attachment B-2.2, “STAR+PLUS Covered Services.”
|
Revision | 2.6 | September 1, 2013 | Acute Care Services is modified to remove the waiver of the 30-day spell of illness as required by Article II, Rider 51 of the General Appropriations Act (83R), and to remove the reference to the Texas Medicaid Bulletin.
|
Revision | 2.7 | September 1, 2013 | Contract amendment did not revise Attachment B-2.2, “STAR+PLUS Covered Services.”
|
Revision | 2.8 | January 1, 2014 | Contract amendment did not revise Attachment B-2.2, “STAR+PLUS Covered Services.”
|
|
| | | |
Revision | 2.9 | February 1, 2014 | Services included under the MCO capitation payment is modified for consistency with the STAR Covered Services Attachment. The vision benefits have not changed. In addition, telemedicine and telemonitoring are added.
Nursing Facility Services is added.
HCBS STAR+PLUS Waiver Services is modified to add Dental Services, Financial Management Services, Support Consultation, Employment Assistance, Supported Employment, and Cognitive Rehabilitation Therapy. |
Revision | 2.10 | April 1, 2014 | Contract amendment did not revise Attachment B-2.2, “STAR+PLUS Covered Services.”
|
Revision | 2.11 | September 1, 2014 | Services included under the MCO capitation payment is revised to add Telehealth.
Nursing Facility Services is revised to reflect Nursing Facility effective date.
HCBS STAR+PLUS Waiver Services for those Members who qualify for these services is modified to reflect updated Cognitive Rehabilitation Therapy effective date. |
1 Status should be represented as “Baseline” for initial issuances, “Revision” for changes to the Baseline version, and “Cancellation” for withdrawn versions 2 Revisions should be numbered in accordance according to the version of the issuance and sequential numbering of the revision—e.g., “1.2” refers to the first version of the document and the second revision. 3 Brief description of the changes to the document made in the revision. |
STAR+PLUS Covered Services
Acute Care Services
The following is a non-exhaustive, high-level listing of Acute Care Covered Services included under the Medicaid STAR+PLUS Program.
STAR+PLUS MCOs are responsible for providing a benefit package to Members that includes all Medically Necessary services covered under the traditional, fee-for-service Medicaid programs except for Non-capitated Services. Non-capitated Services are listed in Attachment B-1, RFP Section 8.2.2.8. Non-capitated Services are not included in the STAR+PLUS MCOs’ Capitation Rates; however, STAR+PLUS MCOs must coordinate care for Members for these Non-capitated Services so that Members have access to a full range of Medically Necessary Medicaid services, both capitated and non-capitated.
STAR+PLUS MCOs may also elect to include Value-added Services in their benefit packages, if approved by HHSC (see UMCM Chapter 4.5 “Physical and Behavioral Health Value-Added Services Template”).
STAR+PLUS Program benefits are subject to the same benefit limits and exclusions that apply to the traditional, fee-for-service Medicaid programs, with the following two exceptions. Adult STAR+PLUS Members are provided with two enhanced benefits compared to the traditional, fee-for-service Medicaid coverage:
1. waiver of the three prescription per month limit, for members not covered by
Medicare; and
2. waiver of the $200,000 individual annual limit on inpatient services.
For a complete listing of the limitations and exclusions that apply to each Medicaid benefit category, STAR+PLUS MCOs should refer to the current Texas Medicaid Provider Procedures Manual, which can be accessed online at:
http://www.tmhp.com.
The services listed in this Attachment are subject to modification based on changes in Federal and State laws, regulations, and policies.
Services included under the MCO capitation payment
|
| |
| • Audiology services, including hearing aids, for adults and children |
|
| |
| • Behavioral Health Services*, including: |
|
| |
| o Inpatient mental health services for Adults and Children |
|
| |
| o Outpatient mental health services for Adults and Children |
|
| |
| o Counseling services for adults (21 years of age and over) |
|
| |
| o Substance use disorder treatment services, including |
|
| |
| o Outpatient services, including: |
|
| |
| Medication assisted therapy |
|
| |
| o Residential services, including |
|
| |
| Substance use disorder treatment (including room and board) |
|
| |
| *These services are not subject to the quantitative treatment limitations that apply under traditional, fee-for-service Medicaid coverage. The services may be subject to the MCO’s non-quantitative treatment limitations, provided such limitations comply with the requirements of the Mental Health Parity and Addiction Equity Act of 2008. |
|
| |
| • Birthing services provided by a physician or Advanced Practice Nurse in a licensed birthing center |
|
| |
| • Birthing services provided by a licensed birthing center |
|
| |
| • Cancer screening, diagnostic, and treatment services |
|
| |
| • Durable medical equipment and supplies |
|
| |
| • Early Childhood Intervention (ECI) services |
|
| |
| • Family planning services |
|
| |
| • Home health care services |
|
| |
| • Hospital services, inpatient and outpatient |
|
| |
| • Mastectomy, breast reconstruction, and related follow-up procedures, including: |
|
| |
| o outpatient services provided at an outpatient hospital and ambulatory health care center as clinically appropriate; and physician and professional services provided in an office, inpatient, or outpatient setting for: |
|
| |
| o all stages of reconstruction on the breast(s) on which medically necessary mastectomy procedure(s) have been performed; |
|
| |
| o surgery and reconstruction on the other breast to produce symmetrical appearance; |
|
| |
| o treatment of physical complications from the mastectomy and treatment of lymphedemas; and |
|
| |
| o prophylactic mastectomy to prevent the development of breast cancer. |
|
| |
| o external breast prosthesis for the breast(s) on which medically necessary mastectomy procedure(s) have been performed. |
|
| |
| • Medical checkups and Comprehensive Care Program (CCP) Services for children (birth through age 20) through the Texas Health Steps Program |
|
| |
| • Oral evaluation and fluoride varnish in the Medical Home in conjunction with Texas Health Steps medical checkup for children six (6) months through 35 months of age. |
|
| |
| • Outpatient drugs and biologicals; including pharmacy-dispensed and provider-administered outpatient drugs and biologicals |
|
| |
| • Drugs and biologicals provided in an inpatient setting |
|
| |
| • Preventive services including an annual adult well check for patients 21 years of age and over |
|
| |
| • Radiology, imaging, and X-rays |
|
| |
| • Specialty physician services |
|
| |
| • Therapies – physical, occupational and speech |
|
| |
| • Transplantation of organs and tissues |
|
| |
| • Vision (Includes optometry and glasses. Contact lenses are only covered if they are medically necessary for vision correction that cannot be accomplished by glasses.) |
|
| |
| • Telemonitoring (effective October 1, 2013, through August 31, 2015) |
Nursing Facility Services (effective March 1, 2015)
Nursing Facility Services are included under the STAR+PLUS Medicaid managed care program.
Community Based Long Term Care Services
The following is a non-exhaustive, high-level listing of Community Based Long Term Care Covered Services included under the STAR+PLUS Medicaid managed care program.
• Community Based Long Term Care Services for all Members
o Personal Attendant Services - All Members of a STAR+PLUS MCO may receive medically and functionally necessary Personal Attendant Services (PAS).
o Day Activity and Health Services - All Members of a STAR+PLUS MCO may receive medically and functionally necessary Day Activity and Health Care Services (DAHS).
• HCBS STAR+PLUS Waiver Services for those Members who qualify for these services The state provides an enriched array of services to clients who would otherwise qualify for nursing facility care through a Home and Community Based Medicaid Waiver. In traditional Medicaid, this is known as the Community Based Alternatives (CBA) waiver. The STAR+PLUS MCO must also provide medically necessary services that are available to clients through the CBA waiver in traditional Medicaid to those clients that meet the functional and financial eligibility for the HCBS STAR+PLUS Waiver.
o Personal Attendant Services (including the three service delivery options: Self-Directed; Agency Model, Self-Directed; and Agency Model)
o In-Home or Out-of-Home Respite Services
o Nursing Services (in home)
o Emergency Response Services (Emergency call button)
o Home Delivered Meals
o Minor Home Modifications
o Adaptive Aids and Medical Equipment
o Medical Supplies not available under the Texas Medicaid State Plan/ Texas Healthcare Transformation and Quality Improvement Program (THTQIP) 1115 Waiver
o Physical Therapy, Occupational Therapy, Speech Therapy
o Day Activity Health Services (DAHS) (for members in 217-Like STAR+PLUS eligibility group, as identified in the Texas Healthcare Transformation and Quality Improvement Program 1115 Waiver, whose income exceeds 150% FPL)
o Adult Foster Care
o Assisted Living
o Transition Assistance Services (These services are limited to a maximum of $2,500.00. If the MCO determines that no other resources are available to pay for the basic services/items needed to assist a Member, who is leaving a nursing facility, with setting up a household, the MCO may authorize up to $2,500.00 for Transition Assistance Services (TAS). The $2,500.00 TAS benefit is part of the expense ceiling when determining the Total Annual Individual Service Plan (ISP) Cost.)
o Dental Services (The annual cost cap of this service is $5,000 per waiver plan year. The $5,000 cap may be waived by the managed care organization upon request of the member only when the services of an oral surgeon are required. Exceptions to the $5,000 cap may be made up to an additional $5,000 per waiver plan year when the services of an oral surgeon are required.)
o Cognitive Rehabilitation Therapy (effective March 6, 2014)
o Financial Management Services
o Support Consultation
o Employment Assistance (effective September 1, 2014)
o Supported Employment (effective September 1, 2014)
Subject: Attachment B-3 - Deliverables/Liquidated Damages Matrix
DOCUMENT HISTORY L |
| | | |
STATUS1 | DOCUMENT REVISION2 | EFFECTIVE DATE | DESCRIPTION3 |
Baseline | n/a | September 1, 2011 | Initial version of Attachment B-3, "Deliverables/Liquidated Damages Matrix." |
Revision | 2.1 | March 1, 2012 | Contract amendment did not revise Attachment B-3, "Deliverables/Liquidated Damages Matrix." |
Revision | 2.2 | June 1, 2012 | Contract amendment did not revise Attachment B-3, "Deliverables/Liquidated Damages Matrix." |
Revision | 2.3 | September 1, 2012 | Item 27 is modified to remove the quarterly reports for item (a), add pharmacy to items (d) and (e), and to add item (f) Medicaid Managed Care Texas Health Steps Medical Checkups Quarterly Utilization Reports. Item 28 is modified to replace references to “1915 (c) Waiver” with “HCBS STAR +PLUS Waiver”. |
Revision | 2.4 | March 1, 2013 | Item 19 is modified to clarify liquidated damage assessment and variance. |
Revision | 2.5 | June 1, 2013 | Contract amendment did not revise Attachment B-3, "Deliverables/Liquidated Damages Matrix." |
Revision | 2.6 | September 1, 2013 | Items 4, 6, 7, 16, 23, 24, 26, 27, 28, 29, 30, and 31 are modified to add “not submitted” to the LD. Items 10 and 21 are modified and items 28-31 are added to include pharmacy requirements. All subsequent items are renumbered. Items 21 and 22 are modified to include pharmacy claims.
Item 24 is modified to change the name of the report.
Item 27 is modified to remove quarterly from the measurement period.
|
Revision | 2.7 | September 1, 2013 | Contract amendment did not revise Attachment B-3, "Deliverables/Liquidated Damages Matrix." |
Revision | 2.8 | January 1, 2014 | Contract amendment did not revise Attachment B-3, "Deliverables/Liquidated Damages Matrix."
|
Revision | 2.9 | February 1, 2014 | Item 9 Geo-Mapping is added. All subsequent items are renumbered. |
Revision | 2.10 | April 1, 2014 | Contract amendment did not revise Attachment B-3, "Deliverables/Liquidated Damages Matrix." |
Revision | 2.11 | September 1, 2014 | Item 6 is modified to add "Security Plan."
Items 11,12, and 16 "Hotlines" are modified to add busy signal standard for consistency with the Dental contract.
Items 11.1, 13.1, and 18.1 through 18.9 are added for consistency with the Dental contract.
Item 14 is modified to conform to the other contracts. |
1 Status should be represented as “Baseline” for initial issuances, “Revision” for changes to the Baseline version, and “Cancellation” for withdrawn versions 2 Revisions should be numbered in accordance according to the version of the issuance and sequential numbering of the revision—e.g., “1.2” refers to the first version of the document and the second revision. 3 Brief description of the changes to the document made in the revision. |
Deliverables/Liquidated Damages Matrix
|
| | | | | |
# | Service/ Component1 | Performance Standard2 | Measurement Period3 | Measurement Assessment4 | Liquidated Damages |
1. | General Requirement: Failure to Perform an Administrative Service Contract Attachment A, "Uniform Managed Care Contract Terms and Conditions", Contract Attachment B-1, RFP §§ 6, 7, 8 and 9 | The MCO fails to timely perform an MCO Administrative Service that is not otherwise associated with a performance standard in this matrix and, in the determination of HHSC, such failure either: (1) results in actual harm to a Member or places a Member at risk of imminent harm, or (2) materially affects HHSC’s ability to administer the Program(s). | Ongoing | Each incident of non-compliance per MCO Program and SA. | HHSC may assess up to $5,000.00 per calendar day for each incident of non-compliance per MCO Program and SA. |
2. | General Requirement: Failure to Provide a Covered Service Contract Attachment A, "Uniform Managed Care Contract Terms and Conditions", Contract Attachment B-1, RFP §§ 6, 7, 8 and 9 | The MCO fails to timely provide a MCO Covered Service that is not otherwise associated with a performance standard in this matrix and, in the determination of HHSC, such failure results in actual harm to a Member or places a Member at risk of imminent harm. | Ongoing | Each calendar day of non-compliance | HHSC may assess up to $ 7,500.00 per day for each incident of non-compliance. |
3. | Contract Attachment A, "Uniform Managed Care Contract Terms and Conditions", Section 4.08 Subcontractors | (i) three (3) Business Days after receiving notice from a Material Subcontractor of its intent to terminate a Subcontract; (ii) 180 calendar days prior to the termination date of a Material Subcontract for MIS systems operation or reporting; (iii) 90 calendar days prior to the termination date of a Material Subcontract for non-MIS MCO Administrative Services; and (iv) 30 calendar days prior to the termination date of any other Material Subcontract. | Transition, Measured Quarterly during the Operations Period | Each calendar day of non-compliance, per MCO Program, per SA. | HHSC may assess up to $5,000 per calendar day of non-compliance. |
|
| | | | | |
4. | Contract Attachment B-1, RFP §§ 6, 7, 8 and 9 Uniform Managed Care Manual | All reports and deliverables as specified in Sections 6, 7, 8 and 9 of Attachment B-1, must be submitted according to the timeframes and requirements stated in the Contract (including all attachments) and the Uniform Managed Care Manual. (Specific Reports or deliverables listed separately in this matrix are subject to the specified liquidated damages.) | Transition Period, Quarterly during Operations Period | Each calendar day of non-compliance, per MCO Program, per SA. | HHSC may assess up to $250 per calendar day if the report/deliverable is not submitted, late, inaccurate, or incomplete. |
5. | Contract Attachment B-1, RFP §7.2 Transition Phase Schedule Contract Attachment B-1, RFP §7.2.1 Contract Start-Up and Planning Contract Attachment B-1, RFP §8.1 General Scope | The MCO must be operational no later than the agreed upon Operations Start Date. HHSC, or its agent, will determine when the MCO is considered to be operational based on the requirements in Section 7 and 8 of Attachment B-1. | Operations Start Date | Each calendar day of non-compliance, per MCO Program, per Service Area (SA). | HHSC may assess up to $10,000 per calendar day for each day beyond the Operations Start date that the MCO is not operational until the day that the MCO is operational, including all systems. |
6. | Contract Attachment B-1, RFP §7.2.5 System Readiness Review | The MCO must submit to HHSC or to the designated Readiness Review Contractor the following plans for review, no later than 120 days prior to Operational Start Date: • Joint Interface Plan; • Disaster Recovery Plan; • Business Continuity Plan; • Risk Management Plan; • Systems Quality Assurance Plan; and • Security Plan. | Transition Period | Each calendar day of non-compliance, per report, per MCO Program, and per SA. | HHSC may assess up to $1,000 per calendar day for each day a deliverable is not submitted, late, inaccurate or incomplete. |
7. | Contract Attachment B-1, RFP §7.2.7 Operations Readiness | Final versions of the Provider Directory must be submitted to the Administrative Services Contractor no later than 95 days prior to the Operational Start Date. | Transition Period | Each calendar day of non-compliance, per directory, per MCO Program and per SA. | HHSC may assess up to $1,000 per calendar day for each day the directory is not submitted, late, inaccurate or incomplete. |
8. | Attachment B-1, RFP Sections 7.2.8.1 and 8.1.19 | The MCO must submit or comply with the requirements of the HHSC-approved Fraud and Abuse Compliance Plan. | Transition, Operations, and Turnover | Each incident of noncompliance, per MCO Program | HHSC may assess up to $250 per calendar day for each incident of noncompliance, per MCO Program. |
|
| | | | | |
9. | Attachment B-1, Section 8.1.3 Access to Care UMCM Chapter 5.14 Geo-Mapping | The MCO must comply with the contract’s mileage standards and benchmarks for member access. | Quarterly | Per incident of noncompliance, per Program, Service Area, and Provider type | HHSC may assess up to $1,000 per quarter, per Program, per Service Area, and per Provider type. |
10. | Contract Attachment B-1, RFP §8.1.4 Provider Network UMCM Chapter 5.38 Out of Network Utilization Report | (1) No more than 15 percent of an MCO's total hospital admissions, by service delivery area, may occur in out-of-network facilities. (2) No more than 20 percent of an MCO's total emergency room visits, by service delivery area, may occur in out-of-network facilities (3) No more than 20 percent of total dollars billed to an MCO for "other outpatient services" may be billed by out-of-network providers. | Measured Quarterly beginning March 1, 2010. | Per incident of non-compliance, per Medicaid MCO, per Service Area. | HHSC may assess up to $25,000 per quarter, per standard, per Medicaid MCO, per Service Area. |
11. | Contract Attachment B-1, RFP §8.1.4.7 Provider Hotline; §8.1.21.1 Prior Authorization for Prescription Drugs and 72-Hour Emergency Supplies
| A. The MCO must operate a toll-free Provider telephone hotline for Provider inquiries from 8 AM – 5 PM, local time for the Service Area, Monday through Friday, excluding State-approved holidays. B. Performance Standards: 1. Call pickup rate – At least 99% of calls are answered on or before the fourth ring or an automated call pick up system is used. 2. No more than 1% of incoming calls receive a busy signal. 3. Call abandonment rate— Call abandonment rate is 7% or less. C. Average hold time is 2 minutes or less. | Operations and Turnover | A. Each incident of non-compliance per MCO Program and SA. B. Each percentage point below the standard for 1 and each percentage point above the standard for 2 per MCO Program and SA. C. Per month, for each 30 second time increment, or portion of it, by which the average hold time exceeds the maximum acceptable hold time. | HHSC may assess: A. Per MCO Program and SA, up to $100.00 for each hour or portion thereof that appropriately staffed toll-free lines are not operational. If the MCO’s failure to meet the performance standard is caused by a Force Majeure Event, HHSC will not assess liquidated damages unless the MCO fails to implement its Disaster Recovery Plan. B. Up to $100.00 per MCO Program and SA for each percentage point for each standard that the MCO fails to meet the requirements for a monthly reporting period for any MCO operated toll-free lines. C. Up to $100.00 may be assessed for each 30 second time increment, or portion thereof, by which the MCO’s average hold time exceeds the maximum acceptable hold time. |
|
| | | | | |
11.1 | RFP §8.1.5.1 Member Materials | No later than the 5th Business Day following the receipt of the enrollment file from the Administrative Services Contractor, the MCO must mail a Member's ID card and Member Handbook to the Account Name or Case Head for each new Member. | Transition, Operations, Turnover | Each incident of noncompliance | HHSC may assess up to $500 per incident of the MCO's failure to mail Member Materials. |
12. | Contract Attachment B-1, RFP §8.1.5.6 Member Services Hotline | A. The MCO must operate a toll-free hotline that Members can call 24 hours a day, 7 days a week. B. Performance Standards. 1. Call pickup rate—At least 99% of calls are answered on or before the forth ring or an automated call pick up system is used. 2. No more than 1% of incoming calls receive a busy signal; 3. Call hold rate—At least 80% of calls must be answered by toll-free line staff within 30 seconds 4. Call abandonment rate—Call abandonment rate is 7% or less. C. Average hold time 2 minutes or less. | Ongoing during Operations and Turnover | A. Each incident of non-compliance per. MCO Program and SA. B. Each percentage point below the standard for 1 and 2 and each percentage point above the standard for 3 per MCO Program and SA. C. Per month, for each 30 second time increment, or portion thereof, by which the average hold time exceeds the maximum acceptable hold time. | HHSC may assess: A. Per MCO Program and SA, up to $100.00 for each hour or portion thereof that toll-free lines are not operational. If the MCO’s failure to meet the performance standard is caused by a Force Majeure Event, HHSC will not assess liquidated damages unless the MCO fails to implement its Disaster Recovery Plan. B. Per MCO Program and SA, up to $100.00 for each percentage point for each standard that the MCO fails to meet the requirements for a monthly reporting period for any MCO operated toll-free lines. C. Up to $100.00 may be assessed for each 30 second time increment, or portion thereof, by which the MCO’s average hold time exceeds the maximum acceptable hold time. |
13. | Contract Attachment B-1, RFP §8.1.5.9 Member Complaint and Appeal Process Contract Attachment B-1, RFP §8.2.7.1 Member Complaint Process Contract Attachment B-1, RFP §8.4.3 CHIP Member Complaint and Appeal Process Contract Attachment B-1, RFP §8.2.4.1 Provider Complaints | The MCO must resolve at least 98% of Member and Provider Complaints within 30 calendar days from the date the Complaint is received by the MCO. | Measured Quarterly during the Operations Period | Per reporting period, per MCO Program, per SA. | HHSC may assess up to $250 per reporting period if the MCO fails to meet the performance standard. |
13.1 | RFP §8.2.4.2, Appeal of Provider Claims | The MCO must resolve at least 98% of Provider Appeals within 30 calendar days of the MCO's receipt. | Operations, Turnover | Per reporting period, per MCO Program, per SA | HHSC may assess up to $500 per reporting period if the MCO fails to meet the performance standard. |
|
| | | | | |
14. | Contract Attachment B-1, RFP §8.1.5.9 Member Complaint and Appeal Process Contract Attachment B-1, RFP §8.2.7.2 Medicaid Standard Member Appeal Process Contract Attachment B-1, RFP § 8.4.3 CHIP Member Complaint and Appeal Process
| The MCO must resolve at least 98% of Member Appeals within 30 calendar days of the MCO's receipt. | Measured Quarterly during the Operations Period | Per reporting period, per MCO Program, per SA. | HHSC may assess up to $500 per reporting period if the MCO fails to meet the performance standard. |
15. | Contract Attachment B-1, RFP §8.1.6 Marketing & Prohibited Practices Uniform Managed Care Manual Chapter 4.3 | The MCO may not engage in prohibited marketing practices. | Transition, Measured Quarterly during the Operations Period | Per incident of non-compliance. | HHSC may assess up to $1,000 per incident of non-compliance. |
|
| | | | | |
16. | Contract Attachment B-1, RFP §8.1.15.3 Behavioral Health Services Hotline | A. The MCO must have an emergency and crisis Behavioral Health services Hotline available 24 hours a day, seven (7) days a week, toll-free throughout the Service Area(s). B. Crisis hotline staff must include or have access to qualified Behavioral Health Services professionals to assess behavioral health emergencies. C. The MCO must ensure that the toll-free Behavioral Health Services Hotline meets the following minimum performance requirements for the MCO Program: 1. Call pickup rate: 99% of calls are answered by the fourth ring or an automated call pick-up system: 2. No more than one percent 1% of incoming calls receive a busy signal; 3. Call hold rate: At least 80% of calls must be answered by toll-free line staff within 30 seconds. 4. Call abandonment rate: The call abandonment rate is seven percent (7%) or less. D. Average hold time is 2 minutes or less. | Operations and Turnover | A. Each incident of non-compliance per MCO Program and SA. B. Each incident of non-compliance per MCO Program and SA. C. Per MCO Program, and SA, per month, each percentage point below the standard for 1 and 2 and each percentage point above the standard for 3. D. Per month, for each 30 second time increment, or portion thereof, by which the average hold time exceeds the maximum acceptable hold time. | HHSC may assess: A. Up to $100.00 for each hour or portion thereof that appropriately staffed toll-free lines are not operational If the MCO’s failure to meet the performance standard is caused by a Force Majeure Event, HHSC will not assess liquidated damages unless the MCO fails to implement its Disaster Recovery Plan. B. Up to $100.00 per incident for each occurrence that HHSC identifies through its recurring monitoring processes that toll-free line staff were not qualified or did not have access to qualified professionals to assess behavioral health emergencies. C. Up to $100.00 for each percentage point for each standard that the MCO fails to meet the requirements for a monthly reporting period for any MCO operated toll-free lines. D. Up to $100.00 may be assessed for each 30 second time increment, or portion thereof, by which the MCO’s average hold time exceeds the maximum acceptable hold time. |
|
| | | | | |
17. | Contract Attachment B-1, RFP §8.1.17.1 Financial Reporting Requirements Uniform Managed Care Manual Chapter 5.0 | Financial Statistical Reports (FSR): For each MCO Program and SA, the MCO must file quarterly and annual FSRs. Quarterly reports are due no later than 30 days after the conclusion of each State Fiscal Quarter (SFQ). The first annual report is due no later than 120 days after the end of each Contract Year and the second annual report is due no later than 365 days after the end of each Contract Year. | Quarterly during the Operations Period | Per calendar day of non-compliance, per MCO Program, per SA. | HHSC may assess up to $1,000 per calendar day a quarterly or annual report is not submitted, late, inaccurate or incomplete. |
18. | Contract Attachment B-1, RFP §8.1.17.1 Financial Reporting Requirements: Uniform Managed Care Manual Chapter 5.0 | Medicaid Disproportionate Share Hospital (DSH) Reports: The Medicaid MCO must submit, on an annual basis, preliminary and final DSH Reports. The Preliminary report is due no later than June 1st after each reporting year, and the final report is due no later than July 1st after each reporting year. This standard does not apply to CHIP or CHIP Perinatal Programs. Any claims added after July 1st shall include supporting claim documentation for HHSC validation. | Measured during 4th Quarter of the Operations Period (6/1–8/31) | Per calendar day of non-compliance, per MCO Program, per SA. | HHSC may assess up to $1,000 per calendar day, per program, per service area, for each day the report is late, incorrect, inaccurate or incomplete. |
18.1 | RFP §8.1.17.1 financial Reporting Requirements; Uniform Managed Care Manual Chapters 5.6.2 and 5.6.1 | Claims lag Report must be submitted by the last day of the month following the reporting period. | Operations, Turnover | Per calendar day of non-compliance | HHSC may assess up to $1,000 per calendar day/per program the report is not submitted, late, inaccurate, or incomplete. |
18.2 | RFP §8.1.17.1, Financial Reporting Requirements | Financial Disclosure Report: an annual submission no later than 30 days after the end of each calendar year and update after any change, no later than 30 days after the change. | Operations, Turnover | Per calendar day of non-compliance | HHSC may assess up to $1,000 per calendar day the report is not submitted, late, inaccurate, or incomplete. |
|
| | | | | |
18.3 | RFP §8.1.17.1, Financial Reporting Requirements | Affiliate report: on an as-occurs basis and annually by August 31 of each year in accordance with the Uniform Managed Care Manual. The "as-occurs" update is due within 30 days of the event triggering the change. | Operations, Turnover | Per calendar day of non-compliance | HHSC may assess up to $1,000 per calendar day the report is not submitted, late, inaccurate, or incomplete. |
18.4 | RFP §8.1.17.1, Financial Reporting Requirements | TDI Examination Report: Furnish HHSC with a full and complete copy of any TDI Examination Report issued by TDI no later than 30 calendar days after the receipt of the final version from TDI. | Operations, Turnover | Per calendar day of non-compliance | HHSC may assess up to $1,000 per calendar day the report is not submitted, late, inaccurate, or incomplete. |
18.5 | RFP §8.1.17.1, Financial Reporting Requirements | TDI Financial Filings: Submit copies to HHSC of reports submitted to TDI, as specified in §8.1.11.1. | Operations, Turnover | Per calendar day of non-compliance | HHSC may assess up to $500 per calendar day the report is not submitted, late, inaccurate, or incomplete. |
18.6 | RFP §8.1.17.1, Financial Reporting Requirements | Filings with Other Entities, and Other Annual Financial Reports: submit an electronic copy of reports or filings identified in §8.1.11.1 pertaining to the MCO, or its parent, or its parent's parent. | Operations, Turnover | Per calendar day of non-compliance | HHSC may assess up to $500 per calendar day the report is not submitted, late, inaccurate, or incomplete. |
18.7 | RFP §8.1.17.1, Financial Reporting Requirements; UMCM Ch. 5.3.11 | Audit Reports - comply with UMCM requirements regarding notification or submission of audit reports. | Operations, | Per calendar day of non-compliance | HHSC may assess up to $500 per calendar day the report is not submitted, late, inaccurate, or incomplete. |
18.8 | RFP §8.1.17.1, Financial Reporting Requirements; UMCM Ch. 5.8 | Report of Legal and Other Proceedings and Related Events - comply with UMCM requirements regarding the disclosure of certain matters involving the MCO, its Affiliates, or its Material Subcontractors, as specified. This requirement is both on an as-occurs basis and an annual report due annually on August 31. | Transition, Operations, | Per calendar day of non-compliance | HHSC may assess up to $1,000 per calendar day the report is not submitted, late, inaccurate, or incomplete. |
|
| | | | | |
18.9 | RFP §8.1.17.1, Financial Reporting Requirements | Employee Bonus and/or Incentive Payment Plan, Registration Statement (aka "Form B"), and Third Party Recovery (TPR) Reports: due as specified in §8.1.17.1. | Operations | Per calendar day of non-compliance | HHSC may assess up to $500 per calendar day the report is not submitted, late, inaccurate, or incomplete. |
19. | Contract Attachment B-1, RFP §8.1.18 Management Information System (MIS) Requirements | The MCO’s MIS must be able to resume operations within 72 hours of employing its Disaster Recovery Plan. | Measured Quarterly during the Operations Period | Per calendar day of non-compliance, per MCO Program, per SA. | HHSC may assess up to $5,000 per calendar day of non-compliance |
20. | Contract Attachment B-1, RFP §8.1.18.1 Encounter Data
| The MCO must submit Encounter Data transmissions and include all Encounter Data and Encounter Data adjustments processed by the MCO on a monthly basis, not later than the 30th calendar day after the last day of the month in which the claim(s) are adjudicated. Pharmacy Encounter Data must be submitted no later than 25 calendar days after the date of adjudication and include all Encounter Data and Encounter Data adjustments. Additionally, the MCO will be subject to liquidated damages if the Quarterly Encounter Reconciliation Report (which reconciles the yearto- date paid claims reported in the Financial Statistical Report (FSR) to the appropriate paid dollars reported in the Texas Encounter Data (TED) Warehouse) includes more than a 2% variance.
| Measured Quarterly during Operations Period | Per incident of non-compliance, per MCO Program, per Service Area (SA) | Liquidated Damages: a) Failure to submit Encounter Data: 1. HHSC may assess up to $2,500 per Financial Arrangement Code, per month (or every 25 days for Pharmacy Encounter Data), per Program, per SA if the MCO fails to submit encounter data in a quarter. 2. HHSC may assess up to $5,000 per Financial Arrangement Code, per month (or every 25 days for Pharmacy Encounter Data), per Program, per SA for each month in any subsequent quarter that the MCO fails to submit Encounter Data. b) Encounter Data Reconciliation: Additionally, HHSC may assess up to $2,500 per Quarter, per Program, per SA if the MCO is not within the 2% variance. HHSC may assess up to $5,000 per Quarter, per Program, per SA for each additional Quarter that the MCO is not within the 2% variance.
|
21. | Contract Attachment B-1, RFP §8.1.18.3 System-Wide Functions | The MCO’s MIS system must meet all requirements in Section 8.1.18.3 of Attachment B-1. | Measured Quarterly during the Operations Period | Per calendar day of non-compliance, per MCO Program, per SA. | HHSC may assess up to $5,000 per calendar day of non-compliance. |
|
| | | | | |
22. | Contract Attachment B-1, RFP §8.1.18.5 Claims Processing Requirements and §8.1.21.14 Pharmacy Claims and File Processing Uniform Managed Care Manual Chapter 2.0 and 2.2
| The MCO must adjudicate all provider Clean Claims within 30 days of receipt by the MCO. The MCO must pay providers interest at 18% per annum, calculated daily for the full period in which the Clean Claim remains unadjudicated beyond the 30-day claims processing deadline. Interest owed to the provider must be paid on the same date as the claim. The MCO must adjudicate all Clean Claims for outpatient pharmacy benefits within (1) 18 days after receipt by the MCO if submitted electronically, or (2) 21 days after receipt by the MCO if submitted non-electronically. The MCO must pay providers interest at 18% per annum, calculated daily for the full period in which the Clean Claim remains unadjudicated beyond the 18-day or 21-day claims-processing deadline. Interest owed to the provider must be paid on the same date as the claim. | Measured Quarterly during the Operations Period | Per incident of non-compliance. | HHSC may assess up to $1,000 per claim if the MCO fails to pay interest timely. |
23. | Contract Attachment B-1, RFP §8.1.18.5 Claims Processing Requirements Uniform Managed Care Manual Chapters 2.0 and 2.2
| The MCO must comply with the claims processing requirements and standards as described in Section 8.1.18.5 of Attachment B-1 and in Chapters 2.0 and 2.2 of the Uniform Managed Care Manual.
| Measured Quarterly during the Operations Period | Per quarterly reporting period, per MCO Program, per Service Area, per claim type. | HHSC may assess liquidated damages of up to $5,000 for the first quarter that an MCO’s Claims Performance percentages by claim type, by Program, and by service area, fall below the performance standards. HHSC may assess up to $25,000 per quarter for each additional quarter that the Claims Performance percentages by claim type, by Program, and by service area, fall below the performance standards. |
|
| | | | | |
24. | Attachment B-1, RFP Section 8.1.19 | The MCO must respond to Office of Inspector General request for information in the manner and format requested. | Transition, Operations, and Turnover | Each calendar day of noncompliance, per MCO Program. | HHSC may assess up to $250 per calendar day, per MCO Program, that the report is not submitted, late, inaccurate, or incomplete. |
25. | Attachment B-1, RFP Section 8.1.20.2, UMCM Chapter 5.5
| The MCO must submit a Fraudulent Practices Report to the HHSC-OIG within 30 Business Days of receiving a report of possible Waste, Abuse, or Fraud from the MCO’s Special Investigative Unit (SIU). The MCO must submit quarterly MCO Open Case List Reports.
| Transition, Operations, and Turnover | Each calendar day of noncompliance, per MCO Program. | HHSC may assess up to $250 per calendar day, per MCO Program, that the report is not submitted, late, inaccurate, or incomplete. |
26. | Attachment B-1, RFP §8.1.20.2 Reports Attachment B-1, RFP §8.2.5.1 Provider Complaints Attachment B-1, RFP §8.2.7.1 Member Complaint Process | The MCO fails to submit a timely response to an HHSC Member or Provider Complaint received by HHSC and referred to the MCO by the specified due date. The MCO response must be submitted according to the timeframes and requirements stated within the MCO Notification Correspondence (letter, email, etc). | Measured on a Quarterly Basis | Each incident of non-compliance per MCO Program and SA | HHSC may assess up to $250 per calendar day for each day beyond the due date specified within the MCO Notification Correspondence. |
27. | Contract Attachment B-1, RFP §8.1.20.2 Reports Uniform Managed Care Manual Chapters 2.0 and 5.0 | Claims Summary Report: The MCO must submit quarterly, Claims Summary Reports to HHSC by MCO Program, by Service Area, and by claim type, by the 30th day following the reporting period unless otherwise specified. | Measured Quarterly during the Operations Period | Per calendar day of non-compliance, per MCO Program, per Service Area, per claim type. | HHSC may assess up to $1,000 per calendar day the report is not submitted, late, inaccurate, or incomplete. |
|
| | | | | |
28. | Contract Attachment B-1, RFP §8.1.20.2 Reports; Uniform Managed Care Manual Chapter 12 Frew | (a) Medicaid Managed Care Texas Health Steps Medical Checkups Reports - The MCO must submit an annual report of the number of New Members and Existing Members that receive timely Texas Health Steps (THSteps) medical checkups or refuse to obtain medical checkups. (b) Children of Migrant Farm Workers Annual Plan and Children of Migrant Farm Workers Annual Report - The MCO must submit an annual plan that describes how the MCO will identify and provide accelerated services to Children of Migrant Farm Workers and an annual report that summarizes the MCO's migrant efforts as stated in its annual plan. (c) Frew Quarterly Monitoring Report - The MCO must submit each quarter responses to questions on this report's template addressing the status of Frew Consent Decree paragraphs.
| (a) Annually (b) Annually (c) Quarterly (d) Annually (e) Quarterly (f) Quarterly
| (a) Per calendar day of non-compliance per Program. (b) Plan: Per calendar day of non-compliance. Report: Per calendar day of non-compliance per Program and Service Area. (c) Per calendar day of non-compliance per MCO. (d) Per calendar day of non-compliance per MCO. (e) Per calendar day of non-compliance per MCO. (f) Per calendar day of non-compliance per Program.
| HHSC may assess up to $1,000 per calendar day for the first measurement period the reports are not submitted, late, inaccurate, or incomplete.
HHSC may assess up to $5,000 per calendar day for each consecutive measurement period that a subsequent report is not submitted, late, inaccurate, or incomplete.
In addition, HHSC may assess up to $2,500 per calendar day for any report resubmissions that are not submitted, late, inaccurate, or incomplete within each measurement period. |
|
| | | | | |
| | (d) Frew Annual Provider Training Report - The MCO must submit an annual report of health care and pharmacy provider training conducted throughout the year on Texas Health Steps, Frew, and/or pharmacy benefit education topics that includes the number of Medicaid providers that received training and feedback received on the subject matter and methodology of the training. (e) Frew Provider Recognition Report - The MCO must submit a quarterly report of Medicaid enrolled healthcare and pharmacy providers who attended the MCO's training on Frew, Texas Health Steps, and/or pharmacy benefit education topics and consented to being recognized as having attended training on the HHSC website. (f) Medicaid Managed Care Texas Health Steps Medical Checkups Quarterly Utilization Reports - Each State Fiscal Quarter, the MCO must submit a report of the number and percent of Members birth through age 20 receiving at least one Texas Health Steps medical checkup in total and broken down by various age groups.
| | | |
|
| | | | | |
29. | Contract Attachment B-1, §8.1.21.1 Formulary and Preferred Drug List
| The MCO fails to allow Network Providers free access to a point-of- care web-based application accessible to smart phones, tablets, or similar technology. The application must also identify preferred/non-preferred drugs; Clinical Edits, and any preferred drugs that can be substituted for non-preferred drugs. The MCO must update this information at least weekly.
| Ongoing
| Each calendar day of non-compliance
| HHSC may assess up to $5,000 per calendar day for each incident of non-compliance per MCO Program.
|
30. | Contract Attachment B-1, §8.1.21.2 Prior Authorization (PA) for Prescription Drugs and 72-Hour Emergency Supplies
| The MCO fails to reimburse a pharmacy for providing a 72-hour emergency supply as outlined in this section or fails to make a prior authorization determination within 24 hours of the request.
| Ongoing
| Each incident of noncompliance
| HHSC may assess up to $5,000 per incident of non-compliance per MCO Program.
|
31. | Contract Attachment B-1, §8.1.21.5 Pharmacy Rebate Program Uniform Managed Care Manual, Chapters 2.0 and 2.2
| The MCO fails to include valid national drug codes (NDCs) on encounters for outpatient prescription drugs, including physician-administered drugs.
| Ongoing
| Each incident of noncompliance
| HHSC may assess up to $500 for each incident of non-compliance per MCO Program.
|
32. | Contract Attachment B-1, §8.1.21.16 E-Prescribing
| The MCO fails to provide timely data updates to the national e-prescribing network
| Ongoing
| Each calendar day of Non compliance | HHSC may assess up to $5,000 per calendar day of non-compliance per MCO Program.
|
|
| | | | | |
33. | Contract Attachment B-1, RFP §8.3.3 STAR+PLUS Assessment Instruments Attachment B-1, RFP §8.3.4.1 For Members Attachment B-1, RFP §8.3.4.2 217-Like Group Non-Member Applicants
| The Community Medical Necessity and Level of Care (MN LOC) Assessment Instrument must be completed and electronically submitted via the TMHP portal in the specified format within 45 days: 1) from the date of referral for HCBS STAR+PLUS Waiverservices for 217-Like Group applicants; 2) from the date of the Member's request for HCBS STAR+PLUS Waiver services for current Members requesting an upgrade; or 3) prior to the annual ISP expiration date for all Members receiving HCBS STAR+PLUS Waiver services as specified in Section 8.3.3.
| Operations, Turnover | Per calendar day of non-compliance, per Service Area. | HHSC may assess up to $500 per calendar day per Service Area, for each day a report is not submitted, late, inaccurate or incomplete. |
34. | Contract Attachment B-1, RFP §9.3 Transfer of Data | The MCO must transfer all data regarding the provision of Covered Services to Members to HHSC or a new MCO, at the sole discretion of HHSC and as directed by HHSC. All transferred data must comply with the Contract requirements, including HIPAA. | Measured at Time of Transfer of Data and ongoing after the Transfer of Data until satisfactorily completed | Per incident of non-compliance (failure to provide data and/or failure to provide data in required format), per MCO Program, per SA. | HHSC may assess up to $10,000 per calendar day the data is not submitted,late, inaccurate or incomplete. |
35. | Contract Attachment B-1, RFP §9.4 Turnover Services | Six (6) months prior to the end of the contract period or any extension thereof, the MCO must propose a Turnover Plan covering the possible turnover of the records and information maintained to either the State (HHSC) or a successor MCO. | Measured at Six (6) Months prior to the end of the contract period or any extension thereof and ongoing until satisfactorily completed | Each calendar day of non-compliance, per MCO Program, per SA. | HHSC may assess up to $1,000 per calendar day the Plan is not submitted, late, inaccurate, or incomplete. |
36. | Contract Attachment B-1, RFP §9.5 Post-Turnover Services | The MCO must provide the State (HHSC) with a Turnover Results report documenting the completion and results of each step of the Turnover Plan 30 days after the Turnover of Operations. | Measured 30 days after the Turnover of Operations | Each calendar day of non-compliance, per MCO program, per SA. | HHSC may assess up to $250 per calendar day the report is not submitted, late, inaccurate or incomplete. |
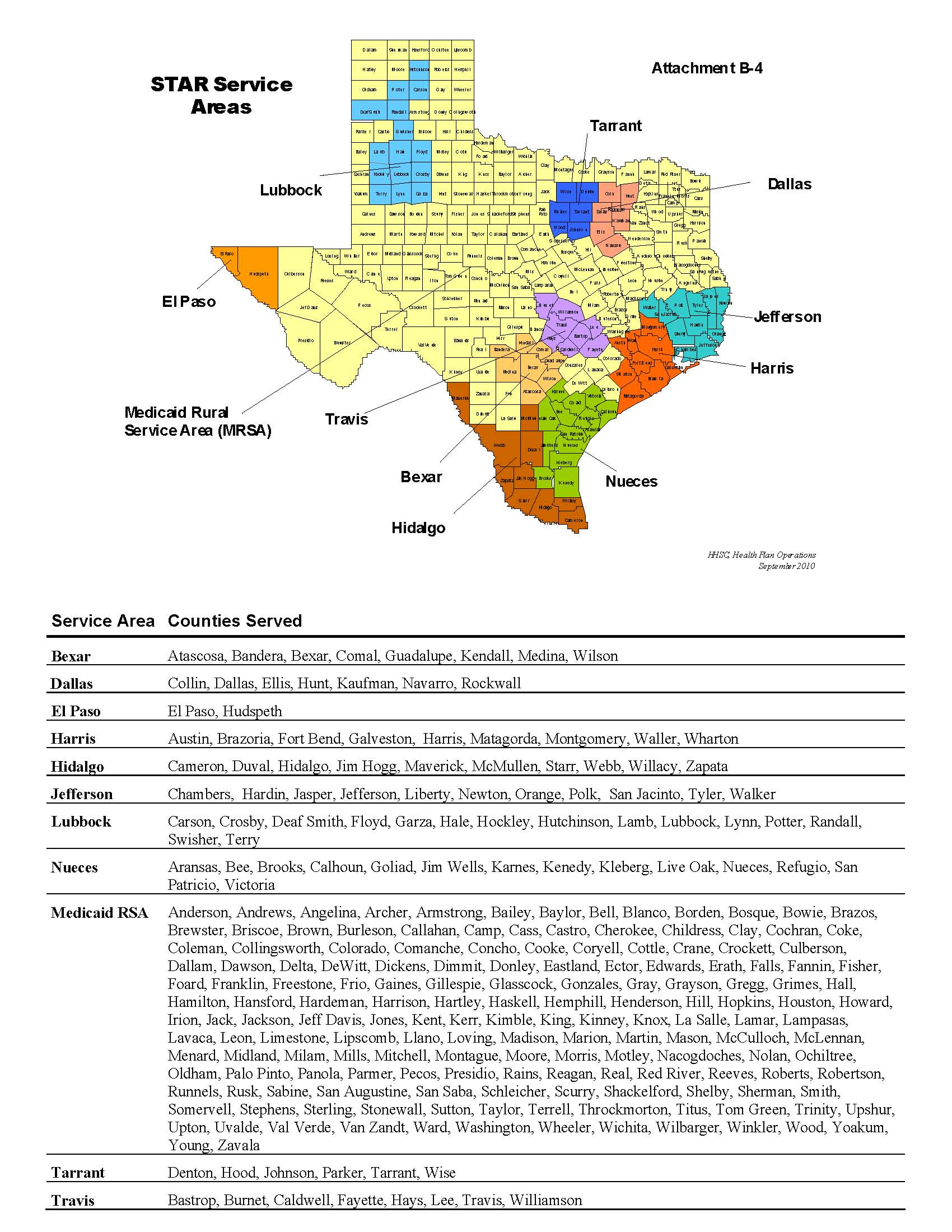

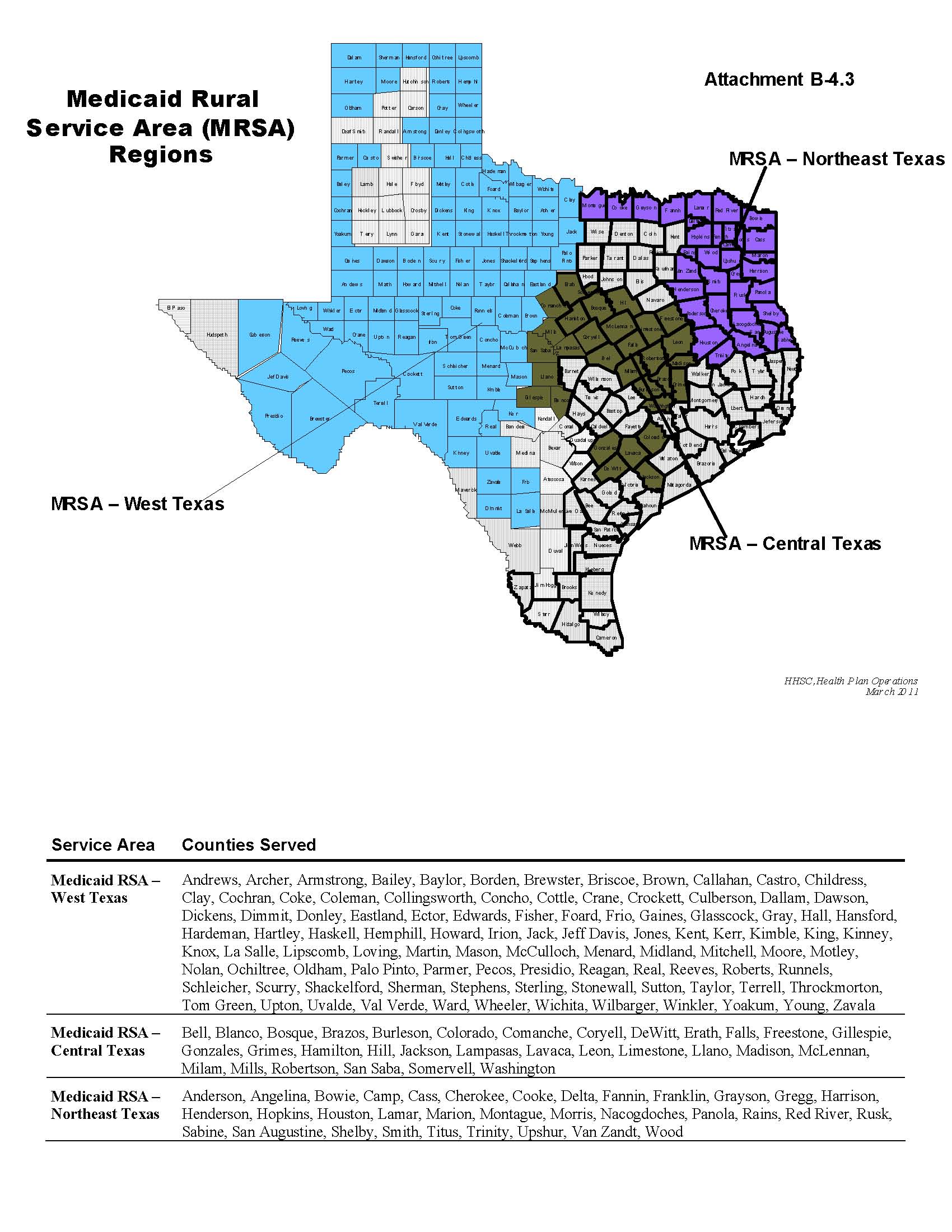
Attachment D
CORPORATE GUARANTEE
In consideration of the execution by the Texas Health & Human Services Commission (“Beneficiary”) of the (HHSC Contract No. 529-12-0002-000__, as amended, hereinafter the "Contract") with ________________________ ("Subsidiary"), _______________________________________________________________ ("Parent") unconditionally and irrevocably guarantees to Beneficiary, on the terms and conditions herein, the full and faithful performance by Subsidiary of all of the obligations undertaken by Subsidiary pursuant to the Contract and as it may hereafter be amended, modified, or extended from time to time, by work authorizations or otherwise.
If Subsidiary fails or refuses to complete any of its obligations, Parent shall complete, or cause to be completed, the obligation that Subsidiary failed or refused to complete, or be considered to be in breach of the Contract to the same extent as Subsidiary, pursuant to the terms and conditions of the Contract. The obligations of Parent under this Guarantee (i) are joint and several obligations made for the benefit of Beneficiary, and (ii) are direct and unconditional obligations to Beneficiary, independent of obligations of Subsidiary or any other guarantor, and may be the basis of a separate action by Beneficiary against any or all guarantors that may be asserted without first bringing an action against Subsidiary.
Parent authorizes Beneficiary, without notice or demand and without affecting its liability hereunder, from time to time to: (a) waive or delay the exercise of any rights or remedies of Beneficiary against Subsidiary and/or any guarantor; (b) release or substitute any guarantor; (c) renew, amend, extend, compromise or waive any obligation of any guarantor; and (d) renew, compromise, extend, waive, or amend any term of the Contract pursuant to its terms.
Parent agrees that, until its obligations hereunder have been performed and/or paid in full, Parent shall not be released by or because of the taking, or failure to take, any action by Subsidiary or Beneficiary that might in any manner or to any extent vary the risks of Parent under this Guarantee or that, but for this paragraph, might discharge or otherwise reduce, limit, or modify Parent's obligations under this Guarantee. Parent waives and surrenders any defense to any liability under this Guarantee based upon any such action, including but not limited to any action of Beneficiary described in the immediately preceding paragraph of this Guarantee, provided, however, Parent does not waive any defenses, remedies, or offsets to which Subsidiary is entitled under or with respect to the Contract. It is the express intent of Parent that Parent’s obligations under this Guarantee are and shall be absolute, irrevocable and unconditional guarantees of performance and payment of Subsidiary and are not merely guarantees of collection.
Parent waives:
|
| |
| (a) the right to require Beneficiary to proceed against Subsidiary; |
|
| |
| (b) all requirements of presentment, protest or default and notices of presentment, protest or default; |
|
| |
| (c) any right to require Beneficiary to proceed against Subsidiary or to pursue any other remedy in Beneficiary's power whatsoever; |
|
| |
| (d) notice of acceptance of this Guarantee; |
|
| |
| (e) notice of any amendments, work authorizations, extensions of time for performance, changes in the work, or other acts by Beneficiary affecting Subsidiary's rights or obligations under the Contract; |
|
| |
| (f) notice of any breach or claim of breach by Subsidiary, provided Beneficiary has complied with any required notice provisions to Subsidiary under the Contract; |
|
| |
| (g) any defense arising out of the exercise by Beneficiary of any right or remedy it may have with respect to the Contract, including the right to amend or modify the Contract and the right to waive or delay the exercise of any rights it may otherwise have against Subsidiary; |
|
| |
| (h) notice of the settlement or compromise of any claim of Beneficiary against Subsidiary relating to any of Subsidiary’s obligations under the Contract; and |
|
| |
| (i) the benefit of suretyship defenses generally. |
No provision or waiver in this Guarantee shall be construed as limiting the generality of any other waiver contained in this Guarantee.
Parent hereby irrevocably waives all claims it has or may acquire against Subsidiary in respect of Parent’s obligations under this Guarantee, including rights of exoneration, reimbursement and subrogation but excluding any rights it may have under any surety bonds. Parent agrees to indemnify Beneficiary, and hold it harmless from and against all loss and expense, including legal fees, suffered or incurred by Beneficiary as the prevailing party in the enforcement of the Contract and/or this Guarantee.
Parent represents and warrants that the execution and delivery of, and performance of the obligations contained in this Guarantee have been authorized by all appropriate action and will not constitute a breach of or contravene any agreement or instrument to which Parent is a party, and that this Guarantee is a valid and binding obligation of Parent enforceable against Parent in accordance with its terms.
Parent consents to all of the terms and conditions of the Contract, as they may be amended or modified from time-to-time by the Beneficiary and Subsidiary. Such Contract terms and conditions are incorporated herein by reference, except that all references to the parties shall mean Beneficiary and Parent, all references to Subsidiary shall mean Parent, all references to the Contract shall be to this Guarantee, and notices to Parent shall be sent to the address set forth below instead of to the address set forth in the Contract.
Parent may not directly or indirectly assign or otherwise transfer (except as a result of a merger or acquisition of or involving Parent) or delegate any rights or obligations hereunder, including any claim arising by subrogation, and any attempt by Parent to assign or delegate any of its rights or obligations hereunder shall be void. This Guarantee shall be binding on the successors and assigns of Parent, and shall inure to the benefit of the successors and assigns of Beneficiary.
If any provision of this Guarantee should be held invalid, illegal or unenforceable in any respect in any jurisdiction, then, to the fullest extent permitted by law:
|
| |
| (a) all other provisions hereof shall remain in full force and effect in such jurisdiction and shall be liberally construed in favor of Beneficiary in order to carry out the intentions of the parties hereto as nearly as may be possible; and |
|
| |
| (b) such invalidity, illegality or unenforceability shall not affect the validity or enforceability of such provision in any other jurisdiction. |
This Guarantee shall be governed by and interpreted in accordance with the laws of the State of Texas. Parent hereby irrevocably submits to the jurisdiction of any State district court sitting in Travis County, State of Texas, in any action or proceeding brought to enforce or otherwise arising out of or relating to this Guarantee and irrevocably waives to the fullest extent permitted by law any defense asserting an inconvenient forum in connection therewith. Service of process by Beneficiary in connection with such action or proceeding shall be binding on Parent if sent to Parent by registered or certified mail at its address specified below. Parent agrees to pay all expenses of Beneficiary in connection with the lawful enforcement of this Guarantee, including, without limitation, costs of collection incurred as the prevailing party in any such action.
PARENT
Name of Parent: _____________________________
By:
Printed Name:
Title:
Address:
Date: