false2022FY0001060955http://fasb.org/us-gaap/2022#OtherLiabilitiesCurrenthttp://fasb.org/us-gaap/2022#OtherLiabilitiesCurrent00010609552022-01-012022-12-310001060955dei:BusinessContactMember2022-01-012022-12-3100010609552022-12-31xbrli:sharesiso4217:USD00010609552021-12-31iso4217:EURxbrli:shares00010609552021-01-012021-12-3100010609552020-01-012020-12-31iso4217:USDxbrli:shares0001060955us-gaap:CommonStockMember2019-12-310001060955us-gaap:AdditionalPaidInCapitalMember2019-12-310001060955iclr:OtherUndenominatedCapitalMember2019-12-310001060955us-gaap:AccumulatedOtherComprehensiveIncomeMember2019-12-310001060955us-gaap:RetainedEarningsMember2019-12-3100010609552019-12-310001060955iclr:RedeemableNoncontrollingInterestMember2019-12-310001060955us-gaap:RetainedEarningsMember2020-01-012020-12-310001060955iclr:RedeemableNoncontrollingInterestMember2020-01-012020-12-310001060955us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-01-012020-12-310001060955us-gaap:CommonStockMember2020-01-012020-12-310001060955us-gaap:AdditionalPaidInCapitalMember2020-01-012020-12-310001060955iclr:OtherUndenominatedCapitalMember2020-01-012020-12-310001060955us-gaap:CommonStockMember2020-12-310001060955us-gaap:AdditionalPaidInCapitalMember2020-12-310001060955iclr:OtherUndenominatedCapitalMember2020-12-310001060955us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-12-310001060955us-gaap:RetainedEarningsMember2020-12-3100010609552020-12-310001060955iclr:RedeemableNoncontrollingInterestMember2020-12-310001060955us-gaap:RetainedEarningsMember2021-01-012021-12-310001060955us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-01-012021-12-310001060955us-gaap:CommonStockMember2021-01-012021-12-310001060955us-gaap:AdditionalPaidInCapitalMember2021-01-012021-12-310001060955us-gaap:CommonStockMember2021-12-310001060955us-gaap:AdditionalPaidInCapitalMember2021-12-310001060955iclr:OtherUndenominatedCapitalMember2021-12-310001060955us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-12-310001060955us-gaap:RetainedEarningsMember2021-12-310001060955us-gaap:RetainedEarningsMember2022-01-012022-12-310001060955us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-01-012022-12-310001060955us-gaap:CommonStockMember2022-01-012022-12-310001060955us-gaap:AdditionalPaidInCapitalMember2022-01-012022-12-310001060955iclr:OtherUndenominatedCapitalMember2022-01-012022-12-310001060955us-gaap:CommonStockMember2022-12-310001060955us-gaap:AdditionalPaidInCapitalMember2022-12-310001060955iclr:OtherUndenominatedCapitalMember2022-12-310001060955us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-12-310001060955us-gaap:RetainedEarningsMember2022-12-31iclr:Employeeiclr:Locationiclr:Country0001060955country:USus-gaap:GeographicConcentrationRiskMemberus-gaap:SalesRevenueNetMember2022-01-012022-12-31xbrli:pure0001060955srt:EuropeMemberus-gaap:GeographicConcentrationRiskMemberus-gaap:SalesRevenueNetMember2022-01-012022-12-310001060955iclr:OtherGeographicLocationsMemberus-gaap:GeographicConcentrationRiskMemberus-gaap:SalesRevenueNetMember2022-01-012022-12-310001060955us-gaap:BuildingMember2022-01-012022-12-310001060955srt:MinimumMembericlr:ComputerEquipmentAndCapitalizedSoftwareMember2022-01-012022-12-310001060955srt:MaximumMembericlr:ComputerEquipmentAndCapitalizedSoftwareMember2022-01-012022-12-310001060955us-gaap:FurnitureAndFixturesMember2022-01-012022-12-310001060955us-gaap:EquipmentMember2022-01-012022-12-310001060955us-gaap:VehiclesMember2022-01-012022-12-310001060955us-gaap:CustomerRelationshipsMember2022-01-012022-12-310001060955us-gaap:OrderOrProductionBacklogMember2022-01-012022-12-310001060955us-gaap:TradeNamesMember2022-01-012022-12-310001060955us-gaap:DatabasesMember2022-01-012022-12-310001060955us-gaap:TechnologyBasedIntangibleAssetsMember2022-01-012022-12-310001060955iclr:MeDiNovaResearchMember2020-03-090001060955iclr:TopClientMember2022-01-012022-12-310001060955iclr:TopClientMember2021-01-012021-12-310001060955iclr:TopClientMember2020-01-012020-12-310001060955iclr:Clients25Member2022-01-012022-12-310001060955iclr:Clients25Member2021-01-012021-12-310001060955iclr:Clients25Member2020-01-012020-12-310001060955iclr:Clients610Member2022-01-012022-12-310001060955iclr:Clients610Member2021-01-012021-12-310001060955iclr:Clients610Member2020-01-012020-12-310001060955iclr:Clients1125Member2022-01-012022-12-310001060955iclr:Clients1125Member2021-01-012021-12-310001060955iclr:Clients1125Member2020-01-012020-12-310001060955iclr:OtherCustomersMember2022-01-012022-12-310001060955iclr:OtherCustomersMember2021-01-012021-12-310001060955iclr:OtherCustomersMember2020-01-012020-12-3100010609552023-01-012022-12-3100010609552022-01-012021-12-3100010609552021-07-010001060955iclr:PRAHealthSciencesIncMember2021-07-010001060955iclr:PRAHealthSciencesIncMember2021-07-012021-07-010001060955iclr:PRAHealthSciencesIncMember2022-01-012022-12-310001060955iclr:PRAHealthSciencesIncMember2021-01-012021-12-310001060955iclr:PRAHealthSciencesIncMember2022-12-310001060955iclr:PRAHealthSciencesIncMemberus-gaap:CustomerRelationshipsMember2021-07-010001060955iclr:PRAHealthSciencesIncMemberus-gaap:CustomerRelationshipsMember2021-07-012021-07-010001060955us-gaap:OrderOrProductionBacklogMembericlr:PRAHealthSciencesIncMember2021-07-010001060955us-gaap:OrderOrProductionBacklogMembericlr:PRAHealthSciencesIncMember2021-07-012021-07-010001060955us-gaap:TradeNamesMembericlr:PRAHealthSciencesIncMember2021-07-010001060955us-gaap:TradeNamesMembericlr:PRAHealthSciencesIncMember2021-07-012021-07-010001060955iclr:PRAHealthSciencesIncMemberus-gaap:DatabasesMember2021-07-010001060955iclr:PRAHealthSciencesIncMemberus-gaap:DatabasesMember2021-07-012021-07-010001060955iclr:PRAHealthSciencesIncMemberus-gaap:TechnologyBasedIntangibleAssetsMember2021-07-010001060955iclr:PRAHealthSciencesIncMemberus-gaap:TechnologyBasedIntangibleAssetsMember2021-07-012021-07-010001060955iclr:PRAHealthSciencesIncMember2021-07-012022-12-310001060955us-gaap:AccountingStandardsUpdate202108Membericlr:PRAHealthSciencesIncMember2022-01-012022-12-310001060955us-gaap:FairValueInputsLevel1Member2022-12-310001060955us-gaap:FairValueInputsLevel2Member2022-12-310001060955us-gaap:FairValueInputsLevel3Member2022-12-310001060955us-gaap:FairValueMeasuredAtNetAssetValuePerShareMember2022-12-310001060955us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:FairValueInputsLevel1Membericlr:InterestRateCapAndInterestRateSwapMember2022-12-310001060955us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:FairValueInputsLevel2Membericlr:InterestRateCapAndInterestRateSwapMember2022-12-310001060955us-gaap:DesignatedAsHedgingInstrumentMembericlr:InterestRateCapAndInterestRateSwapMemberus-gaap:FairValueInputsLevel3Member2022-12-310001060955us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:FairValueMeasuredAtNetAssetValuePerShareMembericlr:InterestRateCapAndInterestRateSwapMember2022-12-310001060955us-gaap:DesignatedAsHedgingInstrumentMembericlr:InterestRateCapAndInterestRateSwapMember2022-12-310001060955us-gaap:FairValueInputsLevel1Member2021-12-310001060955us-gaap:FairValueInputsLevel2Member2021-12-310001060955us-gaap:FairValueInputsLevel3Member2021-12-310001060955us-gaap:FairValueMeasuredAtNetAssetValuePerShareMember2021-12-310001060955us-gaap:FairValueMeasurementsNonrecurringMemberus-gaap:FairValueInputsLevel3Member2022-12-310001060955us-gaap:FairValueMeasurementsNonrecurringMember2022-12-3100010609552020-07-240001060955iclr:OncacareMember2022-12-310001060955iclr:OncacareMember2021-01-012021-12-310001060955iclr:OncacareMember2022-01-012022-12-310001060955iclr:OncacareMember2021-01-012021-12-310001060955iclr:OncacareMember2022-01-012022-12-310001060955iclr:OncacareMember2022-12-310001060955us-gaap:CustomerRelationshipsMember2022-12-310001060955us-gaap:CustomerRelationshipsMember2021-12-310001060955us-gaap:OrderOrProductionBacklogMember2022-12-310001060955us-gaap:OrderOrProductionBacklogMember2021-12-310001060955us-gaap:TradeNamesMember2022-12-310001060955us-gaap:TradeNamesMember2021-12-310001060955us-gaap:DatabasesMember2022-12-310001060955us-gaap:DatabasesMember2021-12-310001060955us-gaap:TechnologyBasedIntangibleAssetsMember2022-12-310001060955us-gaap:TechnologyBasedIntangibleAssetsMember2021-12-310001060955iclr:PRAHealthSciencesIncMembersrt:MinimumMember2021-07-012021-07-010001060955iclr:PRAHealthSciencesIncMembersrt:MaximumMember2021-07-012021-07-010001060955iclr:PRAHealthSciencesIncMember2021-07-022022-12-310001060955iclr:TermLoanFacilityMemberus-gaap:SecuredDebtMember2022-12-310001060955iclr:TermLoanFacilityMemberus-gaap:SecuredDebtMember2021-12-310001060955iclr:SeniorSecuredNotesMemberus-gaap:SeniorSubordinatedNotesMember2022-12-310001060955iclr:SeniorSecuredNotesMemberus-gaap:SeniorSubordinatedNotesMember2021-12-310001060955iclr:SeniorSecuredCreditFacilityAndSeniorSecuredNotesMemberus-gaap:SecuredDebtMember2021-07-010001060955iclr:TermLoanFacilityMemberus-gaap:SecuredDebtMember2021-07-010001060955iclr:SeniorSecuredRevolvingLoanFacilityMemberus-gaap:LineOfCreditMemberus-gaap:RevolvingCreditFacilityMember2021-07-010001060955iclr:TermLoanFacilityMemberus-gaap:LondonInterbankOfferedRateLIBORMember2021-07-012021-07-010001060955iclr:TermLoanFacilityMembersrt:MinimumMember2022-01-012022-12-310001060955iclr:SeniorSecuredRevolvingLoanFacilityMembersrt:MaximumMemberus-gaap:BaseRateMembericlr:VariableRateComponentOneMember2022-01-012022-12-310001060955iclr:SeniorSecuredRevolvingLoanFacilityMemberus-gaap:BaseRateMembericlr:VariableRateComponentOneMember2022-01-012022-12-310001060955srt:MinimumMembericlr:SeniorSecuredRevolvingLoanFacilityMemberus-gaap:BaseRateMembericlr:VariableRateComponentOneMember2022-01-012022-12-310001060955us-gaap:LondonInterbankOfferedRateLIBORMembericlr:SeniorSecuredRevolvingLoanFacilityMembersrt:MaximumMembericlr:VariableRateComponentTwoMember2022-01-012022-12-310001060955us-gaap:LondonInterbankOfferedRateLIBORMembericlr:SeniorSecuredRevolvingLoanFacilityMembericlr:VariableRateComponentTwoMember2022-01-012022-12-310001060955us-gaap:LondonInterbankOfferedRateLIBORMembersrt:MinimumMembericlr:SeniorSecuredRevolvingLoanFacilityMembericlr:VariableRateComponentTwoMember2022-01-012022-12-310001060955iclr:SeniorSecuredRevolvingCreditFacilityMemberus-gaap:SecuredDebtMember2022-12-310001060955iclr:TermLoanFacilityMemberus-gaap:SecuredDebtMember2022-12-302022-12-300001060955iclr:TermLoanFacilityMemberus-gaap:SecuredDebtMember2022-09-302022-09-300001060955iclr:TermLoanFacilityMemberus-gaap:SecuredDebtMember2022-06-302022-06-300001060955iclr:TermLoanFacilityMemberus-gaap:SecuredDebtMember2022-03-312022-03-310001060955iclr:TermLoanFacilityMemberus-gaap:SecuredDebtMember2021-12-292021-12-290001060955iclr:TermLoanFacilityMemberus-gaap:SecuredDebtMember2021-09-272021-09-270001060955iclr:SeniorSecuredNotesMemberus-gaap:SeniorSubordinatedNotesMember2021-07-010001060955us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:InterestRateCapMember2022-11-29iclr:instrument0001060955us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:SecuredOvernightFinancingRateSofrOvernightIndexSwapRateMemberus-gaap:InterestRateCapMember2022-11-290001060955us-gaap:InterestRateSwapMember2022-11-290001060955us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:InterestRateCapMember2022-12-310001060955us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:InterestRateSwapMember2022-12-31iclr:tranche0001060955us-gaap:SellingGeneralAndAdministrativeExpensesMember2022-01-012022-12-310001060955us-gaap:CostOfSalesMember2022-01-012022-12-310001060955us-gaap:SellingGeneralAndAdministrativeExpensesMember2021-01-012021-12-310001060955us-gaap:CostOfSalesMember2021-01-012021-12-31iclr:vote0001060955us-gaap:EmployeeStockOptionMember2022-01-012022-12-310001060955us-gaap:RestrictedStockUnitsRSUMember2022-01-012022-12-310001060955iclr:PerformanceShareUnitMember2022-01-012022-12-310001060955us-gaap:EmployeeStockOptionMember2021-01-012021-12-310001060955us-gaap:RestrictedStockUnitsRSUMember2021-01-012021-12-310001060955iclr:PerformanceShareUnitMember2021-01-012021-12-310001060955us-gaap:CommonStockMember2021-07-012021-07-010001060955us-gaap:EmployeeStockOptionMember2020-01-012020-12-310001060955us-gaap:RestrictedStockUnitsRSUMember2020-01-012020-12-310001060955iclr:PerformanceShareUnitMember2020-01-012020-12-310001060955srt:MaximumMembericlr:BuybackProgramMember2016-07-222016-07-220001060955iclr:BuybackProgramMember2016-10-030001060955iclr:BuybackProgramMember2018-01-012018-12-310001060955iclr:BuybackProgramMember2019-01-080001060955iclr:BuybackProgramMember2019-01-012019-12-310001060955iclr:BuybackProgramMember2019-12-312019-12-310001060955iclr:BuybackProgramMember2020-01-012020-12-310001060955iclr:BuybackProgramMember2022-02-182022-02-180001060955iclr:A2021RestructuringPlanMember2022-01-012022-12-310001060955iclr:LeaseLiabilityMember2022-12-310001060955us-gaap:EmployeeSeveranceMember2022-12-310001060955country:IE2022-01-012022-12-310001060955country:IE2021-01-012021-12-310001060955country:IE2020-01-012020-12-310001060955country:US2022-01-012022-12-310001060955country:US2021-01-012021-12-310001060955country:US2020-01-012020-12-310001060955iclr:RestOfEuropeAndOtherCountriesMember2022-01-012022-12-310001060955iclr:RestOfEuropeAndOtherCountriesMember2021-01-012021-12-310001060955iclr:RestOfEuropeAndOtherCountriesMember2020-01-012020-12-310001060955us-gaap:ForeignCountryMembercountry:IE2022-12-310001060955country:USus-gaap:InternalRevenueServiceIRSMember2022-12-310001060955country:USus-gaap:StateAndLocalJurisdictionMember2022-12-310001060955country:US2022-12-310001060955country:USus-gaap:InternalRevenueServiceIRSMembericlr:TaxYear2023To2036Member2022-12-310001060955country:USus-gaap:StateAndLocalJurisdictionMembericlr:TaxYear2023To2036Member2022-12-310001060955iclr:TaxYear20332041Membercountry:USus-gaap:InternalRevenueServiceIRSMember2022-12-310001060955iclr:TaxYear20332041Membercountry:USus-gaap:StateAndLocalJurisdictionMember2022-12-310001060955us-gaap:ForeignCountryMember2022-12-310001060955us-gaap:ForeignCountryMembericlr:TaxYear2022To2028Member2022-12-310001060955us-gaap:ForeignCountryMembericlr:TaxYear2029To2038Member2022-12-310001060955us-gaap:ForeignCountryMembericlr:OtherCountriesMember2022-12-310001060955iclr:IncomeTaxBenefitMember2022-01-012022-12-310001060955us-gaap:OtherComprehensiveIncomeMember2022-01-012022-12-310001060955iclr:AcquiredEntityMember2021-01-012021-12-310001060955iclr:IncomeTaxBenefitMember2021-01-012021-12-310001060955us-gaap:OtherComprehensiveIncomeMember2021-01-012021-12-310001060955country:US2022-01-012022-12-310001060955country:US2021-01-012021-12-310001060955country:US2020-01-012020-12-310001060955country:USus-gaap:PensionPlansDefinedBenefitMember2022-12-310001060955country:USus-gaap:PensionPlansDefinedBenefitMember2021-12-310001060955iclr:OtherRetirementPlansMember2022-12-310001060955iclr:OtherRetirementPlansMember2021-12-310001060955country:GBiclr:ICONDevelopmentSolutionsLimitedPensionPlanMember2022-12-310001060955country:GBiclr:ICONDevelopmentSolutionsLimitedPensionPlanMember2021-12-310001060955country:GBiclr:ICONDevelopmentSolutionsLimitedPensionPlanMember2020-12-310001060955country:GBiclr:ICONDevelopmentSolutionsLimitedPensionPlanMember2022-01-012022-12-310001060955country:GBiclr:ICONDevelopmentSolutionsLimitedPensionPlanMember2021-01-012021-12-310001060955country:GBiclr:ICONDevelopmentSolutionsLimitedPensionPlanMember2020-01-012020-12-310001060955iclr:GovernmentBondsMembericlr:ICONDevelopmentSolutionsLimitedPensionPlanMember2022-12-310001060955iclr:GovernmentBondsMembericlr:ICONDevelopmentSolutionsLimitedPensionPlanMember2021-12-310001060955iclr:DiversifiedBondsMembericlr:ICONDevelopmentSolutionsLimitedPensionPlanMember2022-12-310001060955iclr:DiversifiedBondsMembericlr:ICONDevelopmentSolutionsLimitedPensionPlanMember2021-12-310001060955us-gaap:DefinedBenefitPlanEquitySecuritiesMembericlr:ICONDevelopmentSolutionsLimitedPensionPlanMember2022-12-310001060955us-gaap:DefinedBenefitPlanEquitySecuritiesMembericlr:ICONDevelopmentSolutionsLimitedPensionPlanMember2021-12-310001060955us-gaap:CorporateDebtSecuritiesMembericlr:ICONDevelopmentSolutionsLimitedPensionPlanMember2022-12-310001060955us-gaap:CorporateDebtSecuritiesMembericlr:ICONDevelopmentSolutionsLimitedPensionPlanMember2021-12-310001060955us-gaap:AssetBackedSecuritiesSecuritizedLoansAndReceivablesMembericlr:ICONDevelopmentSolutionsLimitedPensionPlanMember2022-12-310001060955us-gaap:AssetBackedSecuritiesSecuritizedLoansAndReceivablesMembericlr:ICONDevelopmentSolutionsLimitedPensionPlanMember2021-12-310001060955iclr:ICONDevelopmentSolutionsLimitedPensionPlanMember2022-12-310001060955iclr:ICONDevelopmentSolutionsLimitedPensionPlanMember2021-12-310001060955iclr:ICONDevelopmentSolutionsLimitedPensionPlanMember2021-01-012021-12-310001060955iclr:ICONDevelopmentSolutionsLimitedPensionPlanMember2022-01-012022-12-310001060955us-gaap:FairValueInputsLevel1Membericlr:GovernmentBondsMembericlr:ICONDevelopmentSolutionsLimitedPensionPlanMember2022-12-310001060955us-gaap:FairValueInputsLevel1Membericlr:GovernmentBondsMembericlr:ICONDevelopmentSolutionsLimitedPensionPlanMember2021-12-310001060955us-gaap:FairValueInputsLevel1Membericlr:DiversifiedBondsMembericlr:ICONDevelopmentSolutionsLimitedPensionPlanMember2022-12-310001060955us-gaap:FairValueInputsLevel1Membericlr:DiversifiedBondsMembericlr:ICONDevelopmentSolutionsLimitedPensionPlanMember2021-12-310001060955us-gaap:FairValueInputsLevel1Memberus-gaap:DefinedBenefitPlanEquitySecuritiesMembericlr:ICONDevelopmentSolutionsLimitedPensionPlanMember2022-12-310001060955us-gaap:FairValueInputsLevel1Memberus-gaap:DefinedBenefitPlanEquitySecuritiesMembericlr:ICONDevelopmentSolutionsLimitedPensionPlanMember2021-12-310001060955us-gaap:FairValueInputsLevel1Memberus-gaap:CorporateDebtSecuritiesMembericlr:ICONDevelopmentSolutionsLimitedPensionPlanMember2022-12-310001060955us-gaap:FairValueInputsLevel1Memberus-gaap:CorporateDebtSecuritiesMembericlr:ICONDevelopmentSolutionsLimitedPensionPlanMember2021-12-310001060955us-gaap:FairValueInputsLevel1Memberus-gaap:AssetBackedSecuritiesSecuritizedLoansAndReceivablesMembericlr:ICONDevelopmentSolutionsLimitedPensionPlanMember2022-12-310001060955us-gaap:FairValueInputsLevel1Memberus-gaap:AssetBackedSecuritiesSecuritizedLoansAndReceivablesMembericlr:ICONDevelopmentSolutionsLimitedPensionPlanMember2021-12-310001060955us-gaap:FairValueInputsLevel1Membericlr:ICONDevelopmentSolutionsLimitedPensionPlanMember2022-12-310001060955us-gaap:FairValueInputsLevel1Membericlr:ICONDevelopmentSolutionsLimitedPensionPlanMember2021-12-310001060955iclr:ICONDevelopmentSolutionsLimitedPensionPlanMembercountry:GB2022-12-310001060955country:CHiclr:AptivSolutionsMembericlr:AptivSolutionsPensionPlanMember2014-05-070001060955country:CHiclr:AptivSolutionsPensionPlanMember2022-12-310001060955country:CHiclr:AptivSolutionsPensionPlanMember2021-12-310001060955iclr:AptivSolutionsPensionPlanMember2022-12-310001060955iclr:AptivSolutionsPensionPlanMember2021-12-310001060955country:CHiclr:AptivSolutionsPensionPlanMember2020-12-310001060955country:CHiclr:AptivSolutionsPensionPlanMember2022-01-012022-12-310001060955country:CHiclr:AptivSolutionsPensionPlanMember2021-01-012021-12-310001060955iclr:PRASwitzerlandAGPensionPlanMembercountry:CH2022-12-310001060955iclr:PRASwitzerlandAGPensionPlanMembercountry:CH2021-12-310001060955iclr:PRASwitzerlandAGPensionPlanMember2022-12-310001060955iclr:PRASwitzerlandAGPensionPlanMember2021-12-310001060955iclr:PRASwitzerlandAGPensionPlanMembercountry:CH2020-12-310001060955iclr:PRASwitzerlandAGPensionPlanMembercountry:CH2022-01-012022-12-310001060955iclr:PRASwitzerlandAGPensionPlanMembercountry:CH2021-01-012021-12-310001060955iclr:ConsultantsStockPlan2008PlanMember2017-02-130001060955iclr:ConsultantsStockPlan2008PlanMember2017-02-140001060955iclr:EmployeeStockPlanTwentyZeroEightPlanMember2017-02-140001060955iclr:ConsultantsStockPlan2008PlanMember2017-02-142017-02-140001060955iclr:EmployeeStockPlanTwentyZeroEightPlanMember2017-02-142017-02-140001060955iclr:OptionPlans2008Membersrt:MinimumMember2017-02-142017-02-140001060955us-gaap:EmployeeStockOptionMembericlr:AwardDateAllYearsExcluding2018Member1997-01-012017-12-310001060955iclr:The2020LegacyPRAPlanMember2020-05-180001060955iclr:The2018LegacyPRAPlanMember2018-05-310001060955iclr:A2013LegacyPRAPlanMember2013-09-230001060955iclr:StockOptionAndAwardPlansMember2019-12-310001060955iclr:StockOptionAndAwardPlansMember2020-01-012020-12-310001060955iclr:StockOptionAndAwardPlansMember2020-12-310001060955iclr:StockOptionAndAwardPlansMember2021-01-012021-12-310001060955iclr:StockOptionAndAwardPlansMember2021-12-310001060955iclr:StockOptionAndAwardPlansMember2022-01-012022-12-310001060955iclr:StockOptionAndAwardPlansMember2022-12-310001060955iclr:Range1Member2022-01-012022-12-310001060955iclr:Range1Membericlr:StockOptionAndAwardPlansMember2022-12-310001060955iclr:Range1Membericlr:StockOptionAndAwardPlansMember2022-01-012022-12-310001060955iclr:Range2Member2022-01-012022-12-310001060955iclr:Range2Membericlr:StockOptionAndAwardPlansMember2022-12-310001060955iclr:Range2Membericlr:StockOptionAndAwardPlansMember2022-01-012022-12-310001060955iclr:Range3Member2022-01-012022-12-310001060955iclr:Range3Membericlr:StockOptionAndAwardPlansMember2022-12-310001060955iclr:Range3Membericlr:StockOptionAndAwardPlansMember2022-01-012022-12-310001060955iclr:Range4Member2022-01-012022-12-310001060955iclr:Range4Membericlr:StockOptionAndAwardPlansMember2022-12-310001060955iclr:Range4Membericlr:StockOptionAndAwardPlansMember2022-01-012022-12-310001060955us-gaap:EmployeeStockOptionMember2021-07-012021-07-010001060955iclr:RestrictedStockUnitsTwentyThirteenMember2015-05-112015-05-110001060955iclr:RestrictedStockUnitsTwentyThirteenMember2015-05-110001060955iclr:ConsultantsRestrictedStockUnitsTwentyNineteenMember2019-05-160001060955iclr:NonexecutiveDirectorMembericlr:ConsultantsRestrictedStockUnitsTwentyNineteenMember2019-01-012020-12-310001060955iclr:PerformanceShareUnitMember2021-12-310001060955us-gaap:RestrictedStockUnitsRSUMember2021-12-310001060955iclr:PerformanceShareUnitMember2022-12-310001060955us-gaap:RestrictedStockUnitsRSUMember2022-12-310001060955us-gaap:RestrictedStockUnitsRSUMembersrt:MinimumMember2022-12-310001060955us-gaap:RestrictedStockUnitsRSUMembersrt:MaximumMember2022-12-310001060955us-gaap:RestrictedStockUnitsRSUMembersrt:MinimumMember2021-12-310001060955us-gaap:RestrictedStockUnitsRSUMembersrt:MaximumMember2021-12-310001060955srt:MinimumMembericlr:PerformanceShareUnitMember2022-12-310001060955srt:MaximumMembericlr:PerformanceShareUnitMember2022-12-310001060955srt:MinimumMembericlr:PerformanceShareUnitMember2021-12-310001060955srt:MaximumMembericlr:PerformanceShareUnitMember2021-12-310001060955iclr:PerformanceBasedGrantsMembersrt:MaximumMember2022-12-310001060955us-gaap:CostOfSalesMember2020-01-012020-12-310001060955us-gaap:SellingGeneralAndAdministrativeExpensesMember2020-01-012020-12-310001060955iclr:TransactionAndIntegrationExpenseMember2022-01-012022-12-310001060955iclr:TransactionAndIntegrationExpenseMember2021-01-012021-12-310001060955iclr:TransactionAndIntegrationExpenseMember2020-01-012020-12-31iclr:segment0001060955iclr:RestOfEuropeMember2022-01-012022-12-310001060955iclr:RestOfEuropeMember2021-01-012021-12-310001060955iclr:RestOfEuropeMember2020-01-012020-12-310001060955iclr:OtherCountriesMember2022-01-012022-12-310001060955iclr:OtherCountriesMember2021-01-012021-12-310001060955iclr:OtherCountriesMember2020-01-012020-12-310001060955country:IE2022-12-310001060955country:IE2021-12-310001060955iclr:RestOfEuropeMember2022-12-310001060955iclr:RestOfEuropeMember2021-12-310001060955country:US2021-12-310001060955iclr:OtherCountriesMember2022-12-310001060955iclr:OtherCountriesMember2021-12-310001060955us-gaap:SubsidiaryOfCommonParentMembericlr:DSBiopharmaLimitedMember2022-01-012022-12-310001060955us-gaap:SubsidiaryOfCommonParentMembericlr:DSBiopharmaLimitedMember2021-01-012021-12-310001060955us-gaap:SubsidiaryOfCommonParentMembericlr:DSBiopharmaLimitedMember2022-12-310001060955us-gaap:SubsidiaryOfCommonParentMembericlr:DSBiopharmaLimitedMember2021-12-310001060955iclr:AfimmuneLimitedMemberus-gaap:SubsidiaryOfCommonParentMember2022-01-012022-12-310001060955iclr:AfimmuneLimitedMemberus-gaap:SubsidiaryOfCommonParentMember2021-01-012021-12-310001060955iclr:AfimmuneLimitedMemberus-gaap:SubsidiaryOfCommonParentMember2022-12-310001060955iclr:AfimmuneLimitedMemberus-gaap:SubsidiaryOfCommonParentMember2021-12-310001060955us-gaap:SubsidiaryOfCommonParentMembericlr:CorvusPharmaceuticalsMember2022-01-012022-12-310001060955us-gaap:SubsidiaryOfCommonParentMembericlr:CorvusPharmaceuticalsMember2022-12-310001060955iclr:OncacareMemberus-gaap:SubsidiaryOfCommonParentMember2022-12-310001060955iclr:OncacareMemberus-gaap:SubsidiaryOfCommonParentMember2021-12-31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C.20549
______________________________________________________________________
FORM 20-F
(Mark One)
☐ Registration statement pursuant to Section 12(b) or (g) of the Securities Exchange Act of 1934
OR
☒ Annual report pursuant to Section 13 or 15 (d) of the Securities Exchange Act of 1934
For the fiscal year ended: December 31, 2022
OR
☐ Transition report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
OR
☐ Shell company report pursuant to section 13 or 15(d) of the Securities Exchange Act of 1934.
_____________________________________
Commission File Number: 333-08704
| | | | | | | | | | | |
| ICON PUBLIC LIMITED COMPANY | |
| | | |
| | (Exact name of Registrant as Specified in its Charter) | |
| | ICON PLC | |
| | | |
| | (Translation of Registrant’s name into English) | |
| | | |
| | Ireland | |
| | | |
| | (Jurisdiction of Incorporation or Organization) | |
| | | |
| | South County Business Park, | |
| | Leopardstown, | Dublin 18, D18 X5R3 | |
| | Ireland | |
| | | |
| | (Address of principal executive offices) | |
Brendan Brennan, Chief Financial Officer
South County Business Park, Leopardstown, Dublin 18, D18 X5R3, Ireland.
Brendan.Brennan@iconplc.com
+353-1-291-2000
_____________________________________
(Name, telephone number, email and/or facsimile number and address of Company contact person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
| Title of each class | Trading symbol(s) | Name of each exchange on which registered |
| ORDINARY SHARES, PAR VALUE €0.06 EACH | ICLR | NASDAQ Global Select Market |
| Securities registered or to be registered pursuant to section 12(g) of the Act: |
| NONE |
| Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act: |
| NONE |
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report: 81,723,555 Ordinary Shares.
Indicate by check mark if the registrant is a well-known seasoned issuer, as determined in Rule 405 of the Securities Act. Yes ☒ No ☐
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to section 13 or 15(d) of the Securities Exchange Act of 1934. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days: Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non- accelerated filer or an emerging growth company.
Large accelerated filer ☒ Accelerated filer ☐ Non-accelerated filer ☐
Emerging growth company ☐
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Indicate by check mark whether the registrant has filed a report on and attestation to its management's assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
U.S. GAAP ☒ International Financial Reporting Standards as issued Other ☐
by the International Accounting Standards Board ☐
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow. Item 17 ☐ Item 18 ☐
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act) Yes ☐ No ☒
TABLE OF CONTENTS
PART I
PART II
PART III
General Information
As used herein, “ICON plc”, “ICON”, "ICON Group", the “Company” and “we”, "our" or “us” refer to ICON public limited company and its consolidated subsidiaries, unless the context requires otherwise.
Unless otherwise indicated, ICON plc’s financial statements and other financial data contained in this Form 20-F are presented in United States dollars (“$”) and are prepared in accordance with generally accepted accounting principles in the United States (“U.S. GAAP”).
In this Form 20-F, references to "U.S. dollars", "U.S.$" or "$" are to the lawful currency of the United States, references to “euro” or “€” are to the European single currency adopted by nineteen members of the European Union, references to "pound sterling", "sterling", "£", "pence" or "p" are to the lawful currency of the United Kingdom. ICON publishes its consolidated financial statements in U.S. dollars.
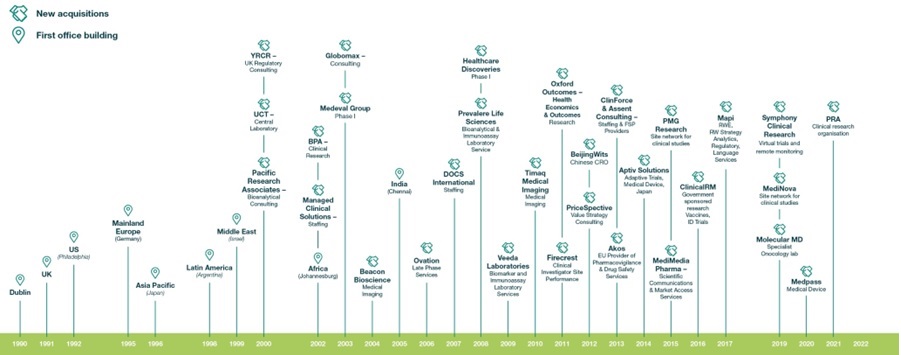
On July 1, 2021, the Company completed the Acquisition of PRA Health Sciences, Inc. ("PRA") by means of a merger whereby Indigo Merger Sub, Inc., a Delaware corporation and subsidiary of ICON, merged with and into PRA, the parent of the PRA Health Sciences ("the Acquisition" and "the Merger"). Upon completion of the Acquisition, PRA and its subsidiaries became wholly owned subsidiaries within the ICON Group. The financial information presented in this Form 20-F reflect the results of the combined Company for the year ended December 31, 2022. The financial information presented for the years ended December 31, 2021 and December 31, 2020 are as previously reported and do not reflect the pre-Merger results of PRA before July 1, 2021, other than where clearly stated and required by GAAP.
Cautionary Statement Regarding Forward-looking Statements
Statements included herein which are not historical facts are forward-looking statements. Such forward-looking statements are made pursuant to the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995 (the “PSLRA”). Forward-looking statements may be identified by the use of future tense or other forward looking words such as “believe”, “expect”, “anticipate”, “should”, “may”, “strategy”, or other variations or comparable terminology. The forward looking statements involve a number of risks and uncertainties and are subject to change at any time. In the event such risks or uncertainties materialize, our results could be materially adversely affected. The risks and uncertainties include, but are not limited to, dependence on the pharmaceutical industry and certain clients, the need to regularly win projects and then to execute them efficiently and correctly, the challenges presented by rapid growth, our expectations concerning the ongoing impact of the novel coronavirus identified as 'COVID-19' on our operational results, the challenges associated with the integration of PRA, competition and the continuing consolidation of the industry, the dependence on certain key executives, changes in the regulatory environment, exchange rate fluctuations, inflation and rising labor costs, the conflict in Ukraine, and other factors identified in the Company’s United States Securities and Exchange Commission filings and in the “Risk Factors” included on pages 3 through 21. The Company has no obligation under the PSLRA to update any forward looking statements and does not intend to do so.
Part I
Item 1. Identity of Directors, Senior Management and Advisers.
Not applicable.
Item 2. Offer Statistics and Expected Timetable.
Not applicable.
Item 3. Key Information.
A.[Reserved]
B. Capitalization and indebtedness
The following table presents our capitalization as of December 31, 2022 and December 31, 2021:
| | | | | | | | |
| December 31, | December 31, |
| 2022 | 2021 |
| Total debt | 4,701,213 | | 5,501,213 | |
| | |
| | |
| | |
Less debt issuance costs and debt discount | (47,026) | | (64,901) | |
| Total debt, net | $ | 4,654,187 | | $ | 5,436,312 | |
| | |
| Share Capital | 6,649 | | 6,640 | |
| Additional paid-in capital | 6,840,306 | | 6,733,910 | |
| Other undenominated capital | 1,162 | | 1,134 | |
| Accumulated other comprehensive income | (171,538) | | (90,937) | |
| Retained earnings | 1,821,384 | | 1,416,080 | |
| Total Shareholders’ Equity | $ | 8,497,963 | | $ | 8,066,827 | |
| | |
| Total Capitalization | $ | 13,152,150 | | $ | 13,503,139 | |
On July 1, 2021, the Company completed the acquisition of PRA by means of a merger whereby Indigo Merger Sub, Inc., a Delaware corporation and subsidiary of ICON, merged with and into PRA Health Sciences, Inc., the parent of PRA Health Sciences ("the Acquisition" and "the Merger"). The Company drew down debt of $6,015 million in order to finance the cash portion of the Merger consideration. ICON entered into a credit agreement providing for a senior secured term loan facility of $5,515 million and a senior secured revolving loan facility in an initial aggregate principal amount of $300 million (the "Senior Secured Credit Facilities") of which, $1,314 million has been repaid as of December 31, 2022 from cash generated by the Company in the period since the completion of the Merger. In addition to the Senior Secured Credit Facilities, the Company, issued $500 million in aggregate principal amount of 2.875% senior secured notes in a private offering ("Senior Secured Notes").
C. Reasons for the offer and use of proceeds
Not applicable.
D. Risk Factors
Various risk factors that are relevant to our business and the services we provide are outlined below. The occurrence of any of these events may materially and adversely affect our business operations, financial condition and results of operations and future prospects.
Summary of Risk Factors
Below is a summary of some of the principal risks that could adversely affect our business, operations and financial results:
Risk Related to Our Business and Operations
•The potential loss or delay of our large contracts, or of multiple contracts, could adversely affect our results.
•If we do not generate new business awards, or if new business awards are delayed, terminated, reduced in scope or fail to go to contract, our business, financial conditions, results of operations or cash flows may be materially adversely affected.
•We depend on a limited number of customers and a loss of, or significant decrease in, business from one or more of them could affect our business.
•The inability of biotechnology customers to raise adequate financing or funding could affect our business.
•Our financial results may be adversely impacted if we underprice our contracts, overrun our cost estimates or fail to receive approval for, or experience delays in, documenting change orders.
•If we are unable to successfully develop and market new services or enter new markets, our growth, results of operations or financial condition could be adversely affected.
•If we fail to attract or retain key personnel, our performance may suffer.
•We may face challenges retaining employees which could cause disruption to our integration plans and day-to-day activities which may result in additional costs to the business.
•Our ability to perform clinical trials is dependent upon the ability to recruit suitable willing patients.
•Our ability to perform clinical trials is dependent upon our ability to recruit suitable willing investigators.
•Climate change, extreme weather events, earthquakes and other natural disasters could adversely affect our business.
•A disease outbreak, epidemic or pandemic such as COVID-19, could adversely affect our business performance.
•Our business depends on the continued effectiveness and availability of our information systems, including the information systems we use to provide our services to our clients, and any system failures of, security breaches of or cyber attacks to these systems may materially limit our operations or have a material adverse effect on our results of operations.
•Upgrading the information systems that support our operating processes and evolving the technology platform for our services pose risks to our business.
•Failure to meet productivity objectives under our business improvement objectives could adversely impact our competitiveness and therefore our operating results.
•We rely on our interactive response technologies to provide accurate information regarding the randomization of patients and the dosage required for patients enrolled in the trials.
•A failure to identify and successfully close and integrate strategic acquisition targets could adversely impact our ongoing business and financial results.
•We may be unable to realize anticipated cost and tax synergies and expect to incur substantial expenses related to the Merger.
•Improper performance of our services could adversely impact our reputation and our financial results.
•Our relationships with existing or potential customers who are in competition with each other may adversely impact the degree to which other customers or potential customers use our services, which may adversely affect our results of operations.
•We have only a limited ability to protect our intellectual property rights and these rights are important to our success.
•The biopharmaceutical industry has a history of patent and other intellectual property litigation and we might be involved in costly intellectual property lawsuits.
•We act as authorized representative or legal representative for some clients pursuant to certain jurisdictional requirements for sponsors of clinical trials to appoint an authorized representative or legal representative with a local presence within the relevant jurisdiction.
•We rely on third parties to provide certain data and other information to us. Our suppliers or providers might increase our cost to obtain, restrict our use of or refuse to license data, which could lead to our inability to access certain data or provide certain services and, as a result, materially and adversely affect our operating results and financial condition.
•We rely on third parties for important products, services and licenses to certain technology and intellectual property rights. If there was failure in delivery by these parties, we might not be able to continue to obtain such products, services and licenses.
Risk Related to Our Industry
•Outsourcing trends in the pharmaceutical, biotechnology and medical device industries and changes in spending on research and development could adversely affect our operating results and growth rates.
•Large pharmaceutical companies are increasingly consolidating their vendor base and entering strategic partnership arrangements with a limited number of outsource providers.
•Increased collaboration amongst pharmaceutical companies in research and development activities may lead to fewer research opportunities.
•We operate in a highly competitive and dynamic market.
•We may be adversely affected by industry, customer or therapeutic concentration.
Risk Related to Our Financial Results and Financial Position
•Our quarterly results are dependent upon a number of factors and can fluctuate from quarter to quarter. They may fall short of prior periods, our projections or the expectations of securities analysts or investors, which may adversely affect the market price of our stock.
•Our exposure to exchange rate fluctuations could adversely affect our future results of operations.
•Inflation and rising labor costs could adversely affect our future results of operations.
•Our effective tax rate may fluctuate from quarter-to-quarter, which may adversely affect our results of operations.
•Our unsatisfied performance obligation may not convert to revenue and the rate of conversion may slow.
•The Company is exposed to various risks in relation to our cash and cash equivalents and short term investments.
•Changes in accounting standards may adversely affect our financial statements.
Risk Related to Our Indebtedness
•We have incurred substantial additional indebtedness in connection with the Merger, which could impair our flexibility and access to capital and could adversely affect the combined Company’s business, financial condition or results of operations.
•Covenants in our credit agreement and the indenture governing the Senior Secured Notes may restrict our business and operations. Our financial condition and results of operations could be adversely affected if we do not comply with those covenants.
•Interest rate fluctuations may materially adversely affect our results of operations and financial conditions due to the variable interest rate on the unhedged balance of our senior secured term loan facility, our revolving credit facility or in respect of any future issuances of debt.
•Borrowings under our Senior Secured Credit Facilities bear interest at a variable rate that is based on the Secured Overnight Financing Rate (“SOFR”), which may have consequences for us that cannot be reasonably predicted and may adversely affect our liquidity, financial condition, and results of operations.
Risk Related to Political, Legal or Regulatory Environment
•We may lose business opportunities as a result of health care reform and the expansion of managed care organizations.
•Healthcare reform legislation, other changes in the healthcare industry and in healthcare spending could adversely affect our business model, financial condition or results of operations.
•The conflict in Ukraine could adversely affect our future results of operations.
•We may lose business as a result of changes in the regulatory environment.
•Failure to comply with the regulations and requirements of the U.S. Food and Drug Administration and other regulatory authorities could result in substantial penalties and/or loss of business.
•We are subject to political, regulatory, operational and legal risks associated with our international operations and any changes or uncertainty in the regulatory regime (including changes in trade compliance laws and regulations as a result of the ongoing conflict in Ukraine) could disrupt our business in the region or the results of our operations as a result of increased compliance costs.
•We operate in many different jurisdictions and we could be adversely affected by violations of anti-corruption laws, including the United States Foreign Corrupt Practices Act of 1977 ("FCPA"), UK Bribery Act of 2010 ("Bribery Act") and similar anti-corruption laws in other jurisdictions as well as laws and regulations relating to trade compliance and economic sanctions.
•Current and proposed laws and regulations regarding the protection of personal data could result in increased risks of liability or increased costs to us or could limit our service offerings.
•Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could have a material adverse effect on our business.
•The failure to comply with our government contracts or applicable laws and regulations could result in, among other things, fines or other liabilities, and changes in procurement regulations could adversely impact our business, results of operations or cash flows.
•Liability claims brought against us could result in payment of substantial damages, costs and liabilities and decrease our profitability.
•Environmental, social and governance matters may impact our business and reputation.
Risk Related to Our Common Stock
•Volatility in the market price of our common stock could lead to losses by investors.
•An investor's return may be reduced if we lose our foreign private issuer status.
•We do not expect to pay any cash dividends for the foreseeable future.
•A future transfer of ICON ordinary shares, other than one effected by means of the transfer of book entry interests in the Depositary Trust Company ("DTC"), may be subject to Irish stamp duty.
Risk Related to Our Business and Operations
The potential loss or delay of our large contracts, or of multiple contracts, could adversely affect our results.
Our clients may discontinue using our services completely or cancel some projects either without notice or upon short notice. The termination or delay of a large contract, or of multiple contracts, could have a material adverse effect on our revenue and profitability. Historically, clients have canceled or discontinued projects and may in the future cancel their contracts with us for reasons including, amongst others:
•the failure of products being tested to satisfy safety or efficacy requirements;
•unexpected or undesired clinical results of the product;
•a decision that a particular study is no longer necessary or viable;
•poor project performance, quality concerns, insufficient patient enrollment or investigator recruitment; and
•production problems resulting in shortages of the drug.
As a result, contract terminations, delays or other changes are part of our clinical services business. In the event of termination, our contracts often provide for fees for winding down the trial but these fees may not be sufficient for us to maintain our margins, and termination may result in lower resource utilization rates. In addition, we may not realize the full benefits of our unsatisfied performance obligation of contractually committed services if our clients cancel, delay or reduce their commitments under our contracts with them. Therefore, the loss, early termination or delay of a large contract or contracts could adversely affect our revenues and profitability.
If we do not generate new business awards, or if new business awards are delayed, terminated, reduced in scope or fail to go to contract, our business, financial conditions, results of operations or cash flows may be materially adversely affected.
Our business is dependent on our ability to generate new business awards from new and existing customers and maintain existing customer contracts. If we were unable to generate new business awards on a timely basis and contract for those awards, that could have a material impact on our business, financial condition, results of operations or cash flows.
We depend on a limited number of customers and a loss of, or significant decrease in, business from one or more of them could affect our business.
While no customers individually contributed more than 10% of our revenues during the years ended December 31, 2022 and December 31, 2021, our top five customers represented 28.3% and 31.6% of our revenues, respectively. The loss of, or a significant decrease in, business from one or more of these key customers could have a material adverse impact on our results of operations and financial results.
The inability of biotechnology customers to raise adequate financing or funding could affect our business.
A portion of our revenue is generated from sales and services to the biotechnology industry. The clients we serve are commonly subject to financial pressures, including, but not limited to, the ability to obtain adequate financing or generate sufficient funding. To the extent our clients face such pressures, or they change how they utilize our offerings, the demand for our services, or the prices our clients are willing to pay for those services, may decline. Any such decline could have a material adverse effect on our business, operating results and financial condition.
Our financial results may be adversely impacted if we underprice our contracts, overrun our cost estimates or fail to receive approval for, or experience delays in, documenting change orders.
Many of our contracts are long-term fixed price or fixed unit price contracts for services. As a result, variations in the timing and progress of large contracts may materially adversely affect our results of operations. Revenue recognized on these service contracts are based on an assessment of progress towards completion being the cost of time and other third party costs as a percentage of total estimated time and other third party costs to deliver our services. As a result, variations in the timing and progress of large contracts may materially adversely affect our results of operations. Estimating time and costs to complete requires judgment and includes consideration of the complexity of the study, the number of geographical sites where trials are to be conducted and the number of patients to be recruited at each site. We regularly review the estimated hours on each contract to determine if the budget accurately reflects the agreed tasks to be performed, taking into account the state of progress at the time of review.
We bear the risk of cost overruns unless the scope of activity is revised from the contract specifications and we are able to negotiate a contract modification. We endeavor to ensure that any changes in scope are appropriately monitored and change orders or contract modifications are promptly negotiated and documented for changes in scope. If we fail to successfully negotiate change orders for changes in the resources required or the scope of the work to be performed, and the costs of performance of these contracts exceeded their fixed fees, it could materially adversely affect our operations and financial results.
If we are unable to successfully develop and market new services or enter new markets, our growth, results of operations or financial condition could be adversely affected.
A key element of our growth strategy is the successful development and marketing of new services or entering new markets that complement or expand our existing business. As we develop new services or enter new markets, we may not have or be able to adequately build the competencies necessary to perform such services satisfactorily, may not receive market acceptance for such services or may face increased competition. If we are unable to succeed in developing new services, entering new markets or attracting a client base for our new services or in new markets, we will be unable to implement this element of our growth strategy, and our future business, reputation, results of operations could be adversely impacted.
If we fail to attract or retain key personnel, our performance may suffer.
Our business, future success and ability to continue to expand operations depends upon our ability to attract, hire, train and retain qualified professional, scientific and technical operating people. We compete for qualified professionals with other Clinical Research Organizations (“CROs”), temporary staffing agencies and the in-house departments of pharmaceutical, biotechnology and medical device companies. An inability to attract and retain a sufficient number of high caliber clinical research professionals (in particular, key personnel and executives) at an acceptable cost would impact our ability to provide our services, our future performance and results of operations.
We may face challenges retaining employees which could cause disruption to our integration plans and day-to-day activities, which may result in additional costs to the business.
The attraction, development and retention of our talent is critical to the success of the Company, and we are working to strengthen processes around these areas to minimize retention risk and support a successful integration. The Company, led by the Chief Human Resource Officer, is taking meaningful action to retain employees. Through our annual Talent Review process we have identified opportunities for improvement as it relates to employee retention. Our People Plans have set specific goals for each functional area in terms of three critical areas: talent attraction, development and retention. However, we can provide no assurances that our efforts in this respect will be successful.
Our leadership and talent programs contribute to the enhanced retention of our employees, better project deliverables for our customers and the enhanced financial performance of the business. We aim to be an industry leader: a company where talented people come to do important work, a place where our employees can shape the future of healthcare, grow their careers, and reach their full potential. We have long held a deep commitment to cultivating strong people practices. This includes competitive total rewards packages along with a focus on continuous learning. Our success depends on the knowledge, capabilities, and quality of our people.
Our ability to perform clinical trials is dependent upon the ability to recruit suitable willing patients.
The successful completion of clinical trials is dependent upon the ability to recruit suitable and willing patients on which to test the drug under study. The availability of suitable patients for enrollment in studies is dependent upon many factors including, amongst others, the size of the patient population, the design of the study protocol, eligibility criteria, the referral practices of physicians, the perceived risks and benefits of the drug under study and the availability of alternative medication, including medication undergoing separate clinical trials. Insufficient or inappropriate patient enrollment may result in the termination or delay of a study which could have a material adverse impact on our results of operations.
The Company is focused on continuing to develop its expertise in patient recruitment with the establishment in 2020 of Accellacare, a global clinical research network, offering patients easier and faster access to innovative treatments and offering customers the option to deploy decentralized trials. The focus is on making it easier for the site and the patient to actively participate in a trial to ensure increased predictability, enrollment and retention. Our site and patient solutions group includes upfront planning of site and patient management including identification, enrollment and engagement.
Improved site selection is achieved through:
•leading technology to identify where the patients are that match the protocol;
•assessment of the qualification of sites based on real data;
•partnerships with leading technology vendors and developing the capability to enable EMR interrogation into clinical insights such as sub-populations and larger pre-screened pool where the technology and regulations are enabled.
The burden on the site, in ensuring patient enrollment and engagement, is achieved through integrated site networks. ICON has a number of site alliance partners. During 2018, we enhanced our site and patient recruitment capabilities with an expansion of the PMG Research network through a partnership with the DuPage Medical Group. During 2019, we further enhanced our site and patient recruitment abilities through the strategic acquisitions of MeDiNova and CRN. In 2020, ICON announced the launch of Accellacare, a global clinical research network offering patients easier and faster access to innovative treatments and offering customers the option to deploy decentralized trials. The site network includes previously acquired PMG Research, MeDiNova and CRN. Also in 2020, we entered into an agreement to jointly establish a new company, Oncacare Limited ("Oncacare"), with a third party. Oncacare operates as a specialized oncology site network in the US and EMEA regions. The new site network is focused on implementing a range of commercial models with specialist oncology healthcare providers in the US and EMEA, to accelerate the recruitment and retention of patients into oncology trials. The oncology site network operates as a joint venture between the Company and a third party company which has extensive experience in developing and running a site network. We also use digital solutions to drive site performance, including pre-screening, eConsent, learning management, document tracking and management with key applications.
Our ability to perform clinical trials is dependent upon our ability to recruit suitable willing investigators.
We contract with physicians located in hospitals, clinics or other similar sites, who serve as investigators in conducting clinical trials to test new drugs on their patients. Investigators supervise administration of the study drug to patients during the course of the clinical trial. The successful conduct of a clinical trial is dependent upon the integrity, experience and capabilities of the investigators conducting the trial. Insufficient investigator recruitment, which in turn may lead to insufficient or inappropriate patient enrollment, may result in the termination or delay of a study which could have a material adverse impact on our results of operations.
Climate change, extreme weather events, earthquakes and other natural disasters could adversely affect our business.
In recent years, extreme weather events and changing weather patterns such as storms, flooding, droughts and temperature changes have become more common. As a result, we are potentially exposed to varying natural disaster or extreme weather risks such as hurricanes, tornadoes, droughts or floods, or other events that may result from the impact of climate change on the environment, such as sea level rise. As a result, we could experience increased costs, business interruptions, destruction of facilities, and loss of life, all of which could have a material adverse effect on our business, financial condition, or results of operations. The potential impacts of climate change may also include increased operating costs associated with additional regulatory requirements and investments in reducing energy, water use and greenhouse gas emissions.
A disease outbreak, epidemic or pandemic such as COVID-19, could adversely affect our business performance.
A disease outbreak, such as influenza or coronavirus, could negatively impact our operations. We could experience restrictions on our ability to travel, or the ability of patients or other service providers to travel, to monitor our clinical trials and to ensure laboratory samples are collected and analyzed on time as a result of an outbreak. The potential impact of an epidemic or pandemic may also result in increased operating costs and result in a requirement to increase investment in impact prevention. COVID-19 has affected, and may continue to affect, our business performance and could adversely affect the economies and financial markets worldwide, resulting in an economic downturn that could impact our business, financial condition and results of operations. We may be required, or choose, to take temporary measures intended to help minimize the risk of infection from the virus for our employees, which could negatively affect our business and cannot presently be predicted with confidence.
Our business depends on the continued effectiveness and availability of our information systems, including the information systems we use to provide our services to our clients, and any system failures of, security breaches of or cyber attacks to these systems may materially limit our operations or have a material adverse effect on our results of operations.
Due to the global nature of our business and our reliance on information systems to provide our services, we use web-enabled and other integrated information systems in delivering our services. We will continue to increase the use of these systems and such systems will either be developed internally or provided in conjunction with third parties. We also provide access to similar information systems to certain clients in connection with the services we provide them. As the use, scope and complexity of our information systems continue to grow, we are exposed to, and will increasingly be exposed to, the risks inherent in the development, integration and ongoing operation of evolving information systems, including:
•disruption or failure of data centers, telecommunications facilities or other key infrastructure platforms;
•security breaches, cyber attacks or other failures or malfunctions in our application or information systems or their associated hardware or other systems that we have access to, or that we rely upon, or that have access to our systems;
•security breaches, cyber attacks or malfunctions with key suppliers or partners who we rely on to provide services to customers; and
•excessive costs, excessive delays or other deficiencies in, or problems with, systems development and deployment.
The materialization of any of these risks may impede our ability to provide services, the processing of data, the delivery of databases and services and the day-to-day management of our business and could result in the corruption, loss or unauthorized disclosure of proprietary, confidential or other data, as well as reputational harm.
While we have cybersecurity controls and disaster recovery plans in place, they might not adequately protect us in the event of a system failure, security breach or cyber attack. To date, no cyber attacks have had a material impact on operations or financial reporting. Additionally, despite any precautions we take, damage from fire, floods, hurricanes, power loss, telecommunications failures, computer viruses, information system security breaches, cyber attacks and similar events that impact our various computer facilities could result in interruptions in the flow of data to our servers and from our servers to our clients. Corruption or loss of data may result in the need to repeat a trial at no cost to the client, but at significant cost to us, or result in the termination of one or more contracts, legal proceedings or claims against us or damage to our reputation. Additionally, significant delays in system enhancements or inadequate performance of new or upgraded systems once completed could damage our reputation and harm our business. Long-term disruptions in the infrastructure caused by events such as security breaches, cyber attacks, natural disasters, the outbreak of war, the escalation of hostilities and acts of terrorism, particularly involving cities in which we have offices, could adversely affect our business.
Unauthorized disclosure of sensitive or confidential data, whether through system failure or employee negligence, fraud or misappropriation, could damage our reputation and cause us to lose clients. Similarly, despite investing in information and cybersecurity controls, there is a risk that unauthorized access to our information systems or those we develop for our clients, whether by our employees or third parties, including a cyber attack by computer programmers and hackers who may attack ICON systems, develop and deploy viruses, worms, ransomware or other malicious software programs, could result in negative publicity, significant remediation costs, legal liability, loss of customers and damage to our reputation and could have a material adverse effect on our results of operations and financial results. In addition, our liability insurance might not be sufficient in type, the cover provided or amount to adequately cover us against claims related to security breaches, cyber attacks and other related breaches.
Our information systems, and those of third parties which we utilize, may face increased cybersecurity risks due to the COVID-19 pandemic, including from the significant number of employees that are working remotely or otherwise impacted by stay-at-home orders. Additional remote access points provide new potential vulnerabilities to cybercriminals. Employees of ICON and third parties may be more susceptible to social engineering efforts, and to phishing attempts which can disguise malware as a legitimate effort to circulate important information relating to COVID-19.
Additionally, ICON completed the Merger with PRA on July 1, 2021 and, as a result, the IT landscape and physical footprint of the Company has increased significantly. As the organization invests in the consolidation of offices, data centers, IT systems and business services a significant amount of due diligence has been completed to understand the IT landscape and increased attack surface. While the organization continues with substantial integration efforts, a failure to effectively manage these activities in a timely and cost-effective manner may result in disruption to our business and negatively affect our operations.
Upgrading the information systems that support our operating processes and evolving the technology platform for our services pose risks to our business.
Continued efficient operation of our business requires that we implement standardized global business processes and evolve our information systems to enable this implementation. We have continued to undertake significant programs to optimize business processes with respect to our services. A failure to effectively manage the implementation and adapt to new processes designed into these new or upgraded systems in a timely and cost-effective manner may result in disruption to our business and negatively affect our operations.
We have entered into agreements with certain vendors to provide systems development and integration services that develop or license to us the IT platform for programs to optimize our business processes. If such vendors fail to perform as required or if there are substantial delays in developing, implementing and updating the IT platform, our customer delivery may be impaired and we may have to make substantial further investments, internally or with third parties, to achieve our objectives. Additionally, our progress may be limited by parties with existing or claimed patents who seek to prevent us from using preferred technology or seek license payments from us.
Meeting our objectives is dependent on a number of factors which may not take place as we anticipate, including obtaining adequate technology-enabled services, creating IT-enabled services that our customers will find desirable and implementing our business model with respect to these services. We are continuing to develop opportunities for automation across ICON using state of the art automation tools including Robotic Process Automation (RPA), the development of new applications and capabilities, and enabling deeper integration across our digital ecosystem. To remain competitive within our industry and keep pace with the rapid evolution of the technological landscape, it is critical that we continue to innovate and expand the capabilities of our current technologies. This applies in particular to our ICONIK, Firecrest, ADDPLAN, Integrated Dataverse (IDV®) and One Search services. Also, increased requirements for investment in information technology may negatively impact our financial condition, including profitability.
Failure to meet productivity objectives under our business improvement objectives could adversely impact our competitiveness and therefore our operating results.
We continue to pursue business transformation initiatives to embed technology and innovation and deliver operational efficiencies. As part of these initiatives, we seek to improve our productivity, flexibility, quality, functionality and cost savings by our on-going investment in global technologies, continuous improvement of our business processes and functions to deliver economies of scale. These initiatives may not deliver their intended gains or be completed in a timely manner which may adversely impact our competitiveness and our ability to meet our growth objectives and therefore, could adversely affect our business and operating results, including profitability.
We rely on our interactive response technologies to provide accurate information regarding the randomization of patients and the dosage required for patients enrolled in the trials.
We develop and maintain computer run and web based interactive response technologies to automatically manage the randomization of patients in trials, assign the study drug and adjust the dosage when required for patients enrolled in trials we support. An error in the design, programming or validation of these systems could lead to inappropriate assignment or dosing of patients, which could give rise to patient safety issues and invalidation of the trial and/or liability claims against the Company, amongst other things, any of which could have a material effect on our financial condition and operations.
A failure to identify and successfully close and integrate strategic acquisition targets could adversely impact our ongoing business and financial results.
We have made a number of acquisitions, including the Merger, and continue to review new acquisition opportunities. If we are unable to identify suitable acquisition targets, complete an acquisition or successfully integrate an acquired company or business, our business may be disrupted. The success of an acquisition will depend upon, among other things, our ability to:
•effectively and quickly assimilate the operations and services or products of the acquired company or business;
•integrate acquired personnel;
•retain and motivate key employees;
•retain customers; and
•minimize the diversion of management's attention from other business concerns.
In the event that the operations of an acquired company or business do not meet our performance expectations, we may have to restructure the acquired company or business or write-off the value of some, or all, of the assets of the acquired company or business.
We may be unable to realize anticipated cost and tax synergies and expect to incur substantial expenses related to the Merger.
We expect to generate run rate cost synergies of approximately $150 million and tax savings from the combined target effective tax rate; both to be realized within approximately four years after completion of the Merger. Since completion of the Merger, we have progressed many of our key integration plans including rationalization of our office footprint, targeted headcount reductions to improve efficiency and key system integrations. The actions completed to date are ahead of our original plan such that we now anticipate realizing our run rate cost synergies by the end of 2023. Our ability to achieve such cost and tax synergies in the timeframe described, is subject to various assumptions by management, which may or may not prove to be accurate, as well as the incurrence of costs in our operations that offset all or a portion of such cost synergies. As a consequence, we may not be able to realize all of these cost and tax synergies within the timeframe expected. Failure to achieve the expected cost and tax synergies could significantly reduce the expected benefits associated with the Merger.
We expect to continue to incur non-recurring costs associated with the combination of the two companies and achieving the desired cost synergies. These fees and costs have been, and will continue to be, substantial. Such costs, as well as other unanticipated costs and expenses, could have a material adverse effect on our financial condition and operating results.
Improper performance of our services could adversely impact our reputation and our financial results.
The performance of clinical development services is complex and time-consuming. We may make mistakes in conducting a clinical trial that could negatively impact or damage the usefulness of the clinical trial or cause the results to be reported improperly. If the clinical trial results are compromised, we could be subject to significant costs or liability, which could have an adverse impact on our ability to perform our services. Large clinical trials are costly, and while we endeavor to contractually limit our exposure to such risks, improper performance of our services could have an adverse effect on our financial condition, damage our reputation and result in the cancellation of current contracts or failure to obtain new contracts from affected or other clients.
Our relationships with existing or potential customers who are in competition with each other may adversely impact the degree to which other customers or potential customers use our services, which may adversely affect our results of operations.
The biopharmaceutical industry is highly competitive, with biopharmaceutical companies each seeking to persuade payers, providers and patients that their drug therapies are better and more cost-effective than competing therapies marketed or being developed by competing companies. In addition to the adverse competitive interests that biopharmaceutical companies have with each other, biopharmaceutical companies also have adverse interests with respect to drug selection and reimbursement with other participants in the healthcare industry, including payers and providers. Biopharmaceutical companies also compete to be first to market with new drug therapies. We regularly provide services to biopharmaceutical companies who compete with each other and we sometimes provide services to such customers regarding competing drugs in development. Our existing or future relationships with our biopharmaceutical customers may therefore deter other biopharmaceutical customers from using our services or may result in our customers seeking to place limits on our ability to serve other biopharmaceutical industry participants. In addition, our further expansion into the broader healthcare market may adversely impact our relationships with biopharmaceutical customers and such customers may elect not to use our services, reduce the scope of services that we provide to them or seek to place restrictions on our ability to serve customers in the broader healthcare market with interests that are adverse to theirs. Any loss of customers or reductions in the level of revenues from a customer could have a material adverse effect on our results of operations, business and prospects.
We have only a limited ability to protect our intellectual property rights and these rights are important to our success.
Our success depends, in part, upon our ability to develop, use and protect our proprietary methodologies, analytics, systems, technologies and other intellectual property. Existing laws of the various countries in which we provide services or solutions offer only limited protection of our intellectual property rights, and the protection in some countries may be very limited. We rely upon a combination of trade secrets, confidentiality policies, non-disclosure, invention assignment and other contractual arrangements and patent, copyright and trademark laws, to protect our intellectual property rights. These laws are subject to change at any time and certain agreements may not be fully enforceable, which could further restrict our ability to protect our innovations. Intellectual property rights may not prevent competitors from independently developing services similar to, or duplicative of, ours. Further, the steps we take in this regard might not be adequate to prevent or deter infringement or other misappropriation of our intellectual property by competitors, former employees or other third parties and we might not be able to detect unauthorized use of, or take appropriate and timely steps to enforce our intellectual property rights. Enforcing our rights might also require considerable time, money and oversight and we may not be successful in enforcing our rights.
The biopharmaceutical industry has a history of patent and other intellectual property litigation and we might be involved in costly intellectual property lawsuits.
The biopharmaceutical industry has a history of intellectual property litigation, and these lawsuits will likely continue in the future. Accordingly, we may face patent infringement legal proceedings by companies that have patents for similar business processes or other legal proceedings alleging infringement of their intellectual property rights. Legal proceedings relating to intellectual property could be expensive, take significant time and divert management’s attention from other business concerns, regardless of the outcome of the litigation. If we do not prevail in an infringement lawsuit brought against us, we might have to pay substantial damages and we could be required to stop the infringing activity or obtain a license to use technology on unfavorable terms. Any infringement or other legal processing related to intellectual property could have a material adverse effect on our operations and financial condition.
We act as authorized representative or legal representative for some clients pursuant to certain jurisdictional requirements for sponsors of clinical trials to appoint an authorized representative or legal representative with a local presence within the relevant jurisdiction.
We act as authorized representative pursuant to Medical Devices Directive 93/42/EEC (“MDD”), Medical Devices Regulation 2017/745 (“MDR”) and Active Implantable Medical Devices Directive 90/385/EEC (“AIMD”) for certain clients who are located outside of the European Union. As authorized representative, we act on behalf of medical device manufacturers in relation to specified tasks with regard to their obligations under MDR.
We also act as legal representative pursuant to European Clinical Trials Directive (2021/20/EC) (“CTD”), EU Clinical Trials Regulation (No.536/2014) (“CTR”), MDD, MDR and AIMD, for certain clients who are located outside of the European Union with respect to clinical trials being carried out by those clients in the European Union. We also perform similar legal representative services for certain clients in other non-EU jurisdictions where client is located outside the relevant local jurisdiction, ICON has an established local legal entity and analogous local regulations have a similar requirement for a local legal representative for clinical trials being carried out in those jurisdictions. As legal representative, we are responsible for ensuring compliance with the client’s obligations pursuant to CTD, CTR and MDR or analogous local legislation and we are the addressee for all communications with the client provided for under CTD, CTR and MDR or analogous local legislation.
We provide these services subject to certain terms and conditions which are contained in our agreements with clients pertaining to these services. We aim to reduce any potential liability associated with these activities by seeking contractual
indemnification from our clients and by maintaining an appropriate level of insurance cover. However, there is no guarantee that the specific insurance will be available or that a client will fulfill its obligations in relation to their indemnity.
We rely on third parties to provide certain data and other information to us. Our suppliers or providers might increase our cost to obtain, restrict our use of or refuse to license data, which could lead to our inability to access certain data or provide certain services and, as a result, materially and adversely affect our operating results and financial condition.
Our services are derived from, or include, the use of data we collect from third parties. We have several data suppliers that provide us with a broad and diverse scope of information that we collect, use in our business and sell.
We generally enter into long-term contractual arrangements with many of our data suppliers. At the time we enter into a new data supply contract or renew an existing contract, suppliers may increase our cost to obtain and use the data provided by such supplier, increase restrictions on our ability to use or sell such data, or altogether refuse to license the data to us. Also, our data suppliers may fail to meet or adhere to our quality control standards or fail to deliver the data to us. Although no single supplier is material to our business, if suppliers that collectively provide a significant amount of the data we receive or use were to increase our costs to obtain or use such data, further restrict our access to or use of such data, fail to meet or adhere to our quality control standards, refuse to provide or fail to deliver data to us, our ability to provide data-dependent services to our clients may be adversely impacted, which could have a material adverse effect on our business, results of operations, financial condition or cash flow.
We rely on third parties for important products, services and licenses to certain technology and intellectual property rights. If there was failure in delivery by these parties, we might not be able to continue to obtain such products, services and licenses.
We depend on certain third parties to provide us with products and services critical to our business. Such services include, among others, suppliers of drugs for patients participating in trials, suppliers of kits for use in our laboratories, suppliers of reagents for use in our testing equipment and providers of maintenance services for our equipment. The failure of any of these third parties to adequately provide the required products or services, or to do so in compliance with applicable regulatory requirements, could have a material adverse effect on our business.
Some of our services rely on intellectual property, technology and other similar property owned and/or controlled by third parties. Our licenses to this property and technology could terminate or expire and we might not be able to replace these licenses in a timely manner. Also, we might not be able to renew these licenses on similar terms and conditions. Failure to renew these licenses, or renewals of these licenses on less advantageous terms, could have a material adverse effect on our business, results of operations, financial condition or cash flow.
Risk Related to Our Industry
Outsourcing trends in the pharmaceutical, biotechnology and medical device industries and changes in spending on research and development could adversely affect our operating results and growth rates.
We are dependent upon the ability and willingness of the pharmaceutical, biotechnology and medical device companies to continue to spend on research and development and to outsource the services that we provide. We are therefore subject to risks, uncertainties and trends that affect companies in these industries that we do not control. We have benefited to date from the tendency of pharmaceutical, biotechnology and medical device companies to outsource clinical research projects. Any downturn in these industries or reduction in spending or outsourcing could materially adversely affect our business. The following could each result in such a downturn:
•if pharmaceutical, biotechnology or medical device companies expanded upon their in-house clinical or development capabilities, they would be less likely to utilize our services;
•if governmental regulations were changed, it could affect the ability of our clients to operate profitably, which may lead to a decrease in research spending and therefore this could have a material adverse effect on our business; and
•if unfavorable economic conditions or disruptions in the credit and capital markets negatively impacted our clients.
Large pharmaceutical companies are increasingly consolidating their vendor base and entering strategic partnership arrangements with a limited number of outsource providers.
Large pharmaceutical companies are continually seeking to drive efficiencies in their development processes to both reduce costs associated with the development of new drug candidates and accelerate time to market. As a result, large pharmaceutical companies, in particular, are increasingly looking to consolidate the number of outsource providers with which they engage, with many entering strategic partnership arrangements with a limited number of outsource providers. The failure to enter strategic partnership arrangements with customers or the loss of existing customers as a result of them entering strategic partnership arrangements with our competitors could have a material adverse impact on our results of operations.
Increased collaboration amongst pharmaceutical companies in research and development activities may lead to fewer research opportunities.
Certain pharmaceutical companies have begun to collaborate in seeking to develop new drug candidates. Increased collaboration amongst pharmaceutical companies may lead to fewer research opportunities, which in turn may lead to fewer outsource opportunities for companies within the CRO industry. A reduction in outsource opportunities as a result of this increased collaboration could have a material adverse impact on our results of operations.
We operate in a highly competitive and dynamic market.
The CRO industry is highly competitive. In particular, we compete with other large global CROs for strategic relationships with large pharmaceutical companies. If we are unable to retain and renew existing strategic relationships and win new strategic relationships, there could be a material adverse impact on our results. Similarly, we compete with other CROs for work which comes outside of these strategic relationships and being unable to win work outside of these strategic relationships would have a material adverse impact on our results.
The type and depth of services provided by CROs has changed in recent years. Failure to develop and market new services or expand existing service offerings could adversely affect our business and operations.
New entrants may also enter the market which would further increase competition and could adversely affect our business and operations.
We may be adversely affected by industry, customer or therapeutic concentration.
We provide services to biopharmaceutical, biotechnology, medical device and government organizations and our revenue is dependent on expenditures by these customers. Our business could therefore be adversely impacted by mergers, consolidation, business failures, distress in financial markets or other factors resulting in a decrease in the number of potential customers or therapeutic products being developed through the drug development progress. There has been consolidation in the biopharmaceutical market in recent years. If the number of our potential customers were to decline in the future, they may be able to negotiate price discounts or other terms for services that are less favorable to us than they have been historically.
Risk Related to Our Financial Results and Financial Position
Our quarterly results are dependent upon a number of factors and can fluctuate from quarter to quarter. They may fall short of prior periods, our projections or the expectations of securities analysts or investors, which may adversely affect the market price of our stock.
Our results of operations in any quarter can fluctuate or differ from expected or forecast results depending upon or due to, among other things, the number and scope of ongoing client projects, the commencement, postponement, variation, cancellation or termination of projects in a quarter, the mix of activity, cost overruns, employee hiring, employee attrition and other factors. Our revenue in any period is directly related to the number of employees who were working on billable projects together with investigator activity during that period. We may be unable to compensate for periods of under-utilization during one part of a fiscal period by earning revenue during another part of that period. We believe that operating results for any particular quarter are not necessarily a meaningful indicator of future results.
Also, if in future quarters, we are unable to continue to deliver operational efficiencies and our expenses grow faster than our revenues, our operating margins, profitability and overall financial condition may be materially adversely impacted.
Our exposure to exchange rate fluctuations could adversely affect our future results of operations.
Our contracts with clients are sometimes denominated in currencies other than the currency in which we incur expenses related to such contracts. Where expenses are incurred in currencies other than those in which contracts are priced, fluctuations in the relative value of those currencies could have a material adverse effect on our results of operations.
In addition, we are also subject to translation exposures as our consolidated financial results are presented in U.S. dollars, while the local results of a certain number of our subsidiaries are prepared in currencies other than U.S. dollars, including, amongst others, the pound sterling and the euro. Accordingly, changes in exchange rates between the U.S. dollar and those other currencies will affect the translation of subsidiary companies' financial results into U.S. dollars in reporting our consolidated financial results.
Inflation and rising labor costs could adversely affect our future results of operations.
Inflation and rising labor costs may result in significant increases to the cost of our services, which we may not be able to recover from our customers. Our contracts with clients are often fixed price or fixed price-per-unit contracts. If macroeconomic forces, such as inflation, cause the cost of inputs required to deliver these contracts to increase significantly, we may be unable to pass along these cost to our customers. A sustained increase in these costs may require us to increase the price of future service offerings. These actions could adversely affect our future revenue, gross margin, or both.
Our effective tax rate may fluctuate from quarter-to-quarter, which may adversely affect our results of operations.
Our quarterly effective tax rate has depended and will continue to depend on the geographic distribution of our taxable earnings amongst the multiple tax jurisdictions (such as Ireland, United States and United Kingdom) in which we operate and the tax law in those jurisdictions. Changes in the geographic mix of our results of operations amongst these jurisdictions may have a significant impact on our effective tax rate from quarter-to-quarter. Changes in tax law in one or more jurisdictions could also have a significant impact on our tax rate and results. In addition, as we operate in multiple tax jurisdictions, we may be subject to audits in certain jurisdictions. These audits may involve complex issues which could require an extended time period before being resolved. The resolution of audit issues may lead to additional taxes, interest as well as fines and/or penalties being imposed which could have a material adverse impact on our effective tax rate and our consolidated financial results.
Our unsatisfied performance obligation may not convert to revenue and the rate of conversion may slow.
Our unsatisfied performance obligation is the amount of awards that has not yet converted to revenue. This value is not necessarily a meaningful predictor of future results due to the potential for the cancellation or delay of projects included in the unsatisfied performance obligation. No assurances can be given that we will be able to realize this unsatisfied performance obligation in full as revenue. A failure to realize these awards could have a material adverse impact on our results of operations. In addition, as the length and complexity of projects increases, the rate at which awards convert to revenue may be slower than in the past. A significant reduction in the rate of conversion could have a material impact on our results of operations.
The Company is exposed to various risks in relation to our cash and cash equivalents and short term investments.
The Company’s treasury function manages our available cash resources and invests significant cash balances in various financial institutions to try to ensure optimum returns for our surplus cash balances. These balances are classified as cash and cash equivalents or short term investments depending on the maturity of the related investment. Cash and cash equivalents comprise cash and highly liquid investments with maturities of three months or less. Short term investments comprise highly liquid investments with maturities of greater than three months and minimum “A-” rated fixed and floating rate securities.
Given the global nature of our business, we are exposed to various risks in relation to these balances including liquidity risk, credit risk associated with the counterparties with whom we invest, interest rate risk on floating rate securities, sovereign risk (our principle sovereign risk relates to investments in U.S. Treasury funds) and other factors.
Although we have not recognized any significant losses to date on our cash and cash equivalents or short term investments, any significant declines in their market values could have a material adverse effect on our financial position and operating results.
Changes in accounting standards may adversely affect our financial statements.
We prepare our financial statements in accordance with generally accepted accounting principles in the United States of America ("US GAAP") which are revised on an on-going basis by the authoritative bodies. It is possible that future accounting standard updates may require changes to the accounting treatment that we apply in preparation of our financial statements. These changes may also require significant changes to our reporting systems. These updates may result in unexpected variability in the timing of recognition of revenue or expenses and therefore in our operating results.
Risk Related to Our Indebtedness
We have incurred substantial additional indebtedness in connection with the Merger, which could impair our flexibility and access to capital and could adversely affect the combined Company’s business, financial condition or results of operations.
Following completion of the Merger and the other transactions contemplated by the Merger Agreement, the Company has a substantial amount of debt. ICON borrowed approximately $6,015 million in order to pay PRA stockholders the cash consideration due to them as merger consideration under the Merger Agreement, pay related fees and transaction costs in connection with the transactions, and refinance existing indebtedness. The total remaining transaction related debt balance at December 31, 2022 was $4,701 million. This level of borrowings could adversely affect the Company in a number of ways, including, but not limited to, by placing us at a competitive disadvantage compared to our competitors that have less debt, causing us to incur substantial fees from time to time in connection with debt amendments or refinancing, making it more difficult for the Company to satisfy its obligations with respect to its debt or to its trade or other creditors, requiring a substantial portion of the Company’s cash flows from operations for the payment of interest on the Company’s debt, reducing the Company’s flexibility to respond to changing business and economic conditions, and reducing funds available for the Company’s investments in research and development, capital expenditures and other activities. If ICON cannot service its debt, it may have to take actions such as selling assets, seeking additional debt or equity, or reducing or delaying capital expenditures, strategic acquisitions, investments and alliances.
In addition, ICON’s increased level of indebtedness could adversely affect ICON’s credit rating, which could result in increased borrowing costs for the Company in the future. No assurances can be made that ICON will be able to refinance any indebtedness incurred in connection with the Merger on terms acceptable to it or at all.
Covenants in our credit agreement and the indenture governing the Senior Secured Notes may restrict our business and operations. Our financial condition and results of operations could be adversely affected if we do not comply with those covenants.
The Senior Secured Credit Facilities and the indenture include certain customary covenants that limit our ability to, amongst other things, subject to certain exceptions:
•make dividends, investments and other restricted payments;
•enter into sale and leaseback transactions;
•engage in share buybacks;
•incur or assume liens or additional debt;
•engage in mergers or reorganizations; or
•enter into certain types of transactions with affiliates.
The revolving credit facility also includes a financial covenant that requires us to comply with a maximum consolidated leverage ratio. Our ability to comply with this financial covenant may be affected by events beyond our control.
Interest rate fluctuations may materially adversely affect our results of operations and financial conditions due to the variable interest rate on our senior secured term loan facility, our revolving credit facility or in respect of any future issuances of debt.
Borrowings under the senior secured term loan facility amortize in equal quarterly installments in an amount equal to 1.00% per annum of the original principal amount, with the remaining balance due at final maturity. The interest rate margin applicable to borrowings under the senior secured term loan facility is USD Term SOFR and a Term SOFR Adjustment depending on the interest period chosen plus an applicable margin of 2.25%. The senior secured term loan facility is subject to a floor of 0.50%.
The interest rate margin applicable to borrowings under the revolving loan facility will be, at the option of the borrower, either (i) the applicable base rate plus an applicable margin of 1.00%, 0.60% or 0.25% based on ICON’s current corporate family rating assigned by S&P of BB- (or lower), BB or BB+ (or higher), respectively, or (ii) Term SOFR plus a Term SOFR Adjustment on the interest period chosen plus an applicable margin of 2.00%, 1.60% or 1.25% based on ICON’s current corporate family rating assigned by S&P of BB- (or lower), BB or BB+ (or higher), respectively. In addition, lenders under the revolving loan facility are entitled to commitment fees as a percentage of the applicable margin at the time of drawing and utilization fees dependent on the proportion of the facility drawn. At December 31, 2022, no amounts were outstanding under the revolving loan facility with the exception of $4.5 million letters of credit given to landlords to guarantee lease arrangements.
Because the Company has variable rate debt, fluctuations in interest rates affect our business. We attempt to minimize interest rate risk and lower our overall borrowing costs through the utilization of interest rate cap and interest rate swap derivative financial instruments. We have entered into certain interest rate cap and interest rate swap agreements with three financial institutions with respect to a portion of our outstanding debt. Accordingly, any change in market value associated with these agreements is offset by the opposite market impact on the portion of the debt covered by such agreements. See Note 14 - Derivatives.
Borrowings under our Senior Secured Credit Facilities bear interest at a variable rate that is based on the Secured Overnight Financing Rate (“SOFR”), which may have consequences for us that cannot be reasonably predicted and may adversely affect our liquidity, financial condition, and results of operations.
On July 27, 2017, the U.K. Financial Conduct Authority (the “FCA”) announced that it intended to end the use of LIBOR effective after December 31, 2021 as the benchmark rate that many banks and issuers use to set interest rates for loans, securities, derivative contracts and other financial instruments. Recognizing the need to replace LIBOR, authorities in the United States convened the Alternative Reference Rates Committee (“ARRC”) in 2014 to identify a replacement for LIBOR with respect to indebtedness denominated in U.S. Dollars. In 2017, the ARRC identified SOFR, and in April 2018, the Federal Reserve Bank of New York began publishing SOFR.
SOFR is a measure of the cost of borrowing cash overnight, collateralized by U.S. Treasury securities, and is based on directly observable U.S. Treasury-backed repurchase transactions. Although the U.S. Treasury-backed overnight repo market is highly liquid, there is currently no robust market for determining forward-looking SOFR term rates. Because SOFR is an overnight risk-free rate, whereas LIBOR has various terms and an embedded credit charge, the transition from LIBOR to SOFR will require adjustments, which may continue to vary for certain forms of indebtedness and financial instruments as the relevant markets adapt to SOFR’s implementation. Similar alternative benchmark replacements will be required to be implemented in respect of indebtedness and other financial instruments that are currently based on LIBOR quotes for currencies other than the U.S. Dollar.
The credit agreement governing the Senior Secured Credit Facilities provides that borrowings denominated in U.S. Dollars will bear interest based on LIBOR or other base rate (as elected by the borrower), plus an applicable margin. The credit agreement also provides that LIBOR may be replaced by a SOFR-based rate for borrowings in U.S. Dollars upon (i) the FCA ceasing to provide LIBOR for U.S. Dollars or announcing that LIBOR is no longer representative or (ii) an early election by the Company and the administrative agent under our credit agreement to transition from LIBOR. On November 29, 2022, the Company agreed with its lenders to the adoption of SOFR as the benchmark rate within the Credit Agreement. The possible volatility of and uncertainty around SOFR as a LIBOR replacement rate and the applicable credit adjustment could result in higher borrowing costs for us, which would adversely affect our liquidity, financial condition, and results of operations.
Risk Related to Political, Legal or Regulatory Environment
We may lose business opportunities as a result of healthcare reform and the expansion of managed care organizations.
Numerous governments, including the U.S. government, have undertaken efforts to control growing healthcare costs through legislation, regulation and voluntary agreements with medical care providers and drug companies. If these efforts are successful, pharmaceutical, biotechnology and medical device companies may react by spending less on research and development and therefore this could have a material adverse effect on our business.
In addition to healthcare reform proposals, the expansion of managed care organizations in the health care market may result in reduced spending on research and development. Managed care organizations' efforts to cut costs by limiting expenditures on pharmaceuticals and medical devices could result in pharmaceutical, biotechnology and medical device companies spending less on research and development. If this were to occur, we would have fewer business opportunities and our revenues could decrease, possibly materially.
Healthcare reform legislation, other changes in the healthcare industry and in healthcare spending could adversely affect our business model, financial condition or results of operations.
Our results of operations and financial conditions could be affected by changes in healthcare spending and policy. The healthcare industry is subject to changing political, regulatory and other influences. It is possible that legislation will be introduced and passed in the United States repealing, modifying or invalidating the current healthcare reform legislation, in whole or in part, and signed into law. Because of the continued uncertainty about the implementation of the current healthcare reform legislation, including the potential for further legal challenges or repeal of that legislation, we cannot quantify or predict with any certainty the likely impact of the current healthcare reform legislation or its repeal on the healthcare sector, on our customers and ultimately on our financial condition or results of operations.
On August 16, 2022, the U.S. government enacted the Inflation Reduction Act of 2022, which, among other things, implements a 15% minimum tax on book income of certain large corporations, a 1% excise tax on net stock repurchases and
several tax incentives to promote clean energy, which will go into effect in 2023. The Company is continuing to assess the potential impact of these changes.
The conflict in Ukraine could adversely affect our future results of operations.
The current conflict in Ukraine has led to, among other things, hardship and the imposition of international economic sanctions aimed at the region. While the situation is subject to change, there remains the possibility of additional and harsher sanctions if the conflict intensifies. If that were to happen, our operations in the region may be severely curtailed or eliminated, which could adversely affect our results of operations. In addition, if the current unrest broadens or further escalates, our operations may be severely curtailed, which could adversely affect our results of operations.
We may lose business as a result of changes in the regulatory environment.
Various regulatory bodies throughout the world may enact legislation, rules and guidance which could introduce changes to the regulatory environment for drug development and research. The adoption and implementation of such legislation, rules and guidance is difficult to predict and therefore could have a material adverse effect on our business.
Failure to comply with the regulations and requirements of the U.S. Food and Drug Administration and other regulatory authorities could result in substantial penalties and/or loss of business.
The U.S. Food and Drug Administration, ("FDA"), and other regulatory and government authorities and agencies inspect and audit us from time to time to ensure that we comply with their regulations and guidelines, including environmental, health and safety matters, and other requirements imposed in connection with the performance of government contracts. We must comply with the applicable regulatory requirements governing the conduct of clinical trials and contracting with the government in all countries in which we operate.
If we fail to comply with any of these requirements we could suffer some or all of:
•termination of or delay in any research;
•disqualification of data;
•denial of the right to conduct business;
•criminal penalties;
•financial penalties;
•other enforcement actions including debarment from government contracts;
•loss of clients and/or business; and
•litigation from clients and/or patients and/or regulatory authorities and/or other affected third parties, and resulting material penalties, damages and costs.
We are subject to political, regulatory, operational and legal risks associated with our international operations.
We are one of a small group of organizations with the capability and expertise to conduct clinical trials on a global basis. We believe that this capability to provide our services globally in most major and developing pharmaceutical markets enhances our ability to compete for new business from large multinational pharmaceutical, biotechnology and medical device companies. We have expanded geographically in the past and intend to continue expanding in regions that have the potential to increase our client base or increase our investigator and patient populations. We expect that revenues earned in emerging markets will continue to account for an increasing portion of our total revenues. However, emerging market operations may present several risks, including civil disturbances, health concerns, cultural differences such as employment, regulatory and business practices, compliance with economic sanctions, laws and regulations, volatility in gross domestic product, economic and governmental instability, the potential for nationalization of private assets and the imposition of exchange controls. In addition, operating globally means the Company faces the challenges associated with coordinating its services across different countries, time zones and cultures.
Changes in the political and regulatory environment in the international markets in which we operate such as price or exchange controls could impact our revenue and profitability and could lead to penalties, sanctions and reputational damages if we are not compliant with those regulations. Political uncertainty and a lack of institutional continuity in some of the emerging, developing or other countries in which we operate could affect the orderly operation of markets in these economies. In addition, in countries with a large and complicated structure of government and administration, national, regional, local and other governmental bodies may issue inconsistent decisions and opinions that could increase our cost of regulatory compliance and/or have a material adverse effect on our business. The ongoing conflict in Ukraine has resulted in an increasingly complex economic sanctions and export controls environment applicable to our business operations in the region (including Russia and Belarus) as a result of additional trade compliance measures enacted by the United States, United Kingdom and European Union member states. These economic sanctions and export controls restrict our ability to do business with sanctioned entities, require additional compliance resources, and could have a material adverse effect on the results of our operations.
Uncertainty of the legal environment in some emerging countries could also limit our ability to enforce our rights. In certain emerging and developing countries we enjoy less comprehensive protection for some of our rights, including intellectual
property rights, which could undermine our competitive position. Proceedings to enforce our future patent rights, if any, in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
If any of the above risks or similar risks associated with our international operations were to materialize, our results of operations and financial condition could be materially adversely affected.
We operate in many different jurisdictions and we could be adversely affected by violations of anti-corruption laws, including the United States Foreign Corrupt Practices Act of 1977 ("FCPA"), UK Bribery Act of 2010 ("Bribery Act") and similar anti-corruption laws in other jurisdictions as well as laws and regulations relating to trade compliance and economic sanctions.
The FCPA, UK Bribery Act of 2010 and similar anti-corruption laws in other jurisdictions prohibit us and our officers, directors, employees and third parties acting on our behalf, including agents, from corruptly offering, promising, authorizing, or providing anything of value to a "foreign official" for the purposes of influencing official decisions or obtaining or retaining business or otherwise obtaining favorable treatment. In addition, the FCPA imposes certain books, records and accounting control obligations on public companies and other issuers. The UK Bribery Act also prohibits "commercial" bribery and accepting bribes.
Our global business operations also must be conducted in compliance with applicable export controls and economic sanctions laws and regulations, including those administered by the U.S. Department of the Treasury’s (the “U.S. Treasury”) Office of Foreign Assets Control, the U.S. Department of State, the U.S. Department of Commerce, the United Nations Security Council, the European Union, His Majesty’s Treasury and other relevant trade compliance authorities.
Our internal policies mandate compliance with these anti-corruption and trade compliance laws and regulations. We also operate in many jurisdictions in which bribery or corruption can be common and compliance with anti-bribery laws may conflict with local customs and practices. Despite our training and compliance program safeguards, we cannot assure that our internal control policies, procedures and safeguards will protect us from acts in violation of anti-corruption and trade compliance laws and regulations committed by employees or other third parties associated with us and our continued expansion, including in developing countries, could increase such risk in the future. Violations of anti-corruption, economic sanctions and trade control laws and regulations, or even allegations of such violations, could disrupt our business and result in a material adverse effect on our financial condition, results of operations, cash flows and reputation. For example, violations of anti-corruption and trade compliance laws can result in restatements of, or irregularities in, our financial statements, disgorgement of profits, related stockholder lawsuits as well as severe criminal or civil sanctions. In some cases, companies that violate anti-corruption and trade compliance laws might be debarred by the U.S. government and/or lose their U.S. export privileges. In addition, the U.S. government or other governments may seek to hold us liable based on successor liability for violations of anti-corruption and trade compliance laws committed by companies that we acquire or in which we invest. Changes in anti-corruption and trade compliance laws or enforcement priorities could also result in increased compliance requirements and related costs which could materially adversely affect our business, financial condition, results of operations and cash flows. The recent increase in economic sanctions and trade controls, particularly relating to our ongoing operations in Russia, Ukraine and Belarus, has increased the amount of resources necessary to ensure compliance in this area.
Current and proposed laws and regulations regarding the protection of personal data could result in increased risks of liability or increased costs to us or could limit our service offerings.
ICON has a strong privacy posture, driven by the implementation of a core privacy governance strategy and the adoption of policies and procedures designed to help ensure that ICON, including our employees and contractors, can comply with applicable data protection laws (including, but not limited to, the General Data Protection Regulation (“GDPR”) (EU) 2016/679). Notwithstanding these measures, failure to comply with applicable data protection laws may occur and could result in increased risk of liability or increased costs to us or could limit our service offerings.
Administrative fines. The GDPR introduced a new regime of administrative fines for data protection infringements and provided for a tiered penalty structure based on the nature of the infringement. The EU supervisory authorities for the GDPR can directly impose fines on organizations found to be in breach of the GDPR. Lower tier administrative fines allow for fines of up to 2% of worldwide turnover of the group in the preceding financial year. Higher tier administrative fines allow for fines of up to 4% of worldwide turnover of the group in the preceding financial year. Higher tier administrative fines are more likely to be levied for major infringements of the GDPR and core data protection principles (e.g. transparency, data retention, accountability).
Penalties. The GDPR also permits Member States to implement rules on other penalties applicable to infringements of the GDPR, in particular, for infringements which are not subject to administrative fines under the GDPR itself. Therefore, Member States may legislate for further fines or penalties that may be criminal in nature.
Any fines levied under the GDPR must be effective, proportionate, and dissuasive. Supervisory authorities have been strengthening enforcement activities across the EU in recent years in respect of breaches of GDPR. The risk of fines and
penalties under the GDPR carries increased risk of liability to ICON and can result in increased costs and disruption to the delivery of our services.
Right to compensation of data subjects. In addition to the risk of administrative and criminal penalties, the GDPR also provides that any person who has suffered material or non-material damage as a result of an infringement of the GDPR shall have the right to receive compensation for the damage suffered, from the controller or processor responsible for the infringement. The level of award of damages is set by the competent court in the applicable EU Member State. This carries increased risk of liability for ICON.
Corrective Powers of the supervisory authorities. Each supervisory authority across the Member States of the EU also has corrective powers. Supervisory authorities have the power to order ICON to bring processing operations into compliance with the provisions of the GDPR in a specified manner within a specified time period, or to impose a temporary or definitive limitation including a ban on processing, and to order the suspension of data flows to a recipient in a third country or to an international organization. Supervisory authorities also have powers to conduct audits and investigations of ICON and instruct ICON to take certain actions. The exercise of these powers by supervisory authorities has the potential to increase costs for ICON and cause disruption to the business and delivery of our services.
From a US perspective, the confidentiality, collection, use and disclosure of personal data, including clinical trial patient-specific information, is subject to governmental regulation generally in the country that the personal data was collected or used. For example, United States federal regulations under the Health Insurance Portability and Accountability Act of 1996, or ("HIPAA"), and as amended in 2014 by the Health Information Technology for Economic and Clinical Health (“HITECH”) Act, require individuals’ written authorization, in addition to any required informed consent, before Protected Health Information may be used for research. HIPAA specifies standards for de-identifications and for limited data sets. We are both directly and indirectly affected by the privacy provisions surrounding individual authorizations because many investigators and organizations with whom we are involved in clinical trials and in our services are directly subject to them as a HIPAA “covered entity” and because we obtain identifiable health information from third parties that are subject to such regulations. As there are some instances where we are a HIPAA “business associate” of a “covered entity”, we can also be directly liable to the covered entity contractually for mishandling protected health information and, under HIPAA’s enforcement scheme, we can be subject to up to $1.9 million per year in civil money penalties for identical HIPAA violations. The per violation penalties and calendar year cap on penalties are adjusted annually for inflation under the Federal Civil Penalties Inflation Adjustment Act.
The foundational principles of the GDPR have helped shape the development of many other privacy laws globally. Internationally, data protection laws continue to be introduced at a rapid rate, with greater protections afforded to personal data than ever before, and greater risk of liability to organizations processing that personal data. As a global organization, ICON must ensure that our privacy posture continues to adapt to these new laws and regulations.
Additional legislation or regulation of this type might, among other things, require us to implement new security measures and processes which may require substantial expenditures or limit our ability to offer some of our services. Additionally, if we violate applicable laws, regulations or duties relating to the use, processing or security of personal data, we could be subject to civil liability or criminal prosecution, be forced to alter our business practices or suffer reputational harm.
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could have a material adverse effect on our business.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to comply with governmental regulations, comply with federal and state healthcare fraud and abuse laws and regulations, report financial information or data accurately or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of information obtained in the course of clinical studies or data or documentation fraud or manipulation, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent misconduct may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions.
The failure to comply with our government contracts or applicable laws and regulations could result in, among other things, fines or other liabilities, and changes in procurement regulations could adversely impact our business, results of operations or cash flows.
Revenues from our government customers are derived from sales to federal, state and local governmental departments and agencies through various contracts. Sales to public segment customers are highly regulated. Noncompliance with contract provisions, government procurement regulations or other applicable laws or regulations (including but not limited to the False Claims Act) could result in civil, criminal and administrative liability, including substantial monetary fines or damages, termination of government contracts or other public segment customer contracts, and suspension, debarment or ineligibility from doing business with the government and other customers in the public segment. In addition, generally contracts in the public segment are terminable at any time for convenience of the contracting agency or upon default. The effect of any of these possible actions by any governmental department or agency could adversely affect our business, results of operations or cash flows. In addition, the adoption of new or modified procurement regulations and other requirements may increase our compliance costs and reduce our gross margins, which could have a negative effect on our business, results of operations or cash flows.
Liability claims brought against us could result in payment of substantial damages, costs and liabilities and decrease our profitability.
We may face legal claims involving stockholders, consumers, clinical trial subjects, competitors, regulators and other parties. See 'Legal Proceedings' in Part A, Item 8 of this Form 20-F. Litigation and other legal proceedings are inherently uncertain, and adverse rulings could occur, including monetary damages, or an injunction stopping us from engaging in business practices, or requiring other remedies, including, but not limited to, compulsory licensing of patents. In addition, the combined Company may be exposed to increased litigation from stockholders, customers, suppliers, consumers and other third parties due to the combination of ICON’s business and PRA’s business following the Merger.
Customer Claims
If we breach the terms of an agreement with a customer (for example if we fail to comply with the agreement, all applicable regulations or Good Clinical Practice) this could result in claims against us for substantial damages which could have a material adverse effect on our business. As we provide staff to deliver our services, there is a risk that our management, quality and control structures fail to quickly detect a failure by one or more employees or contractors to comply with all applicable regulations and Good Clinical Practice and our internal requirements and standard operating procedures thereby exposing us to the risk of claims by customers.
Claims relating to Investigators
We contract with physicians who serve as investigators in conducting clinical trials to test new drugs on their patients. These patients will generally have underlying health conditions and this testing creates the risk of liability for personal injury to the patient or the risk of a serious adverse event occurring. Although investigators are generally required by law to maintain their own liability insurance, we could be named in lawsuits and incur expenses arising from any professional malpractice or other actions brought against the investigators with whom we contract.
Indemnification from Customers
Indemnifications provided by our customers against the risk of liability for personal injury to or death of the patients arising from a study drug vary from customer to customer and from trial to trial and may not be sufficient in scope or amount, or our customer may not have the financial ability to fulfill their indemnification obligations. Furthermore, we would be liable for our own negligence and negligence of our employees which could lead to litigation from customers or action or enforcement by regulatory authorities.
Insurance
We maintain what we believe is an appropriate level of worldwide Professional Liability/Error and Omissions Insurance. In the future we may be unable to maintain or continue our current insurance coverage on the same or similar terms. If we are liable for a claim or settlement that is beyond the level of insurance coverage, we may be responsible for paying all or part of any award or settlement amount. Also, the insurance policies contain exclusions which mean that the policy will not respond or provide cover in certain circumstances.
Claims to Date
To date, we have not been subject to any liability claims that are expected to have a material effect on our business; however, there can be no assurance that we will not become subject to such claims in the future or that such claims will not have a material effect on our business.
Environmental, social and governance matters may impact our business and reputation.
Increasingly, in addition to the importance of their financial performance, companies are being judged by their performance on a variety of environmental, social and governance ('ESG') matters, which are considered to contribute to the long-term sustainability of companies’ performance. A variety of organizations measure the performance of companies on such ESG topics, and the results of these assessments are widely publicized. Customers may have specific ESG related requirements or targets and if we fail to meet these targets we may lose business. In addition, investment in funds that specialize in companies that perform well in such assessments are increasingly popular, and major institutional investors have publicly emphasized the importance of such ESG measures to their investment decisions. Topics taken into account in such assessments include, among others, the Company’s efforts and impacts on climate change and human rights, ethics and compliance with law, and the role of the Company’s board of directors in supervising various sustainability issues. We actively manage a broad range of such ESG matters, taking into consideration their expected impact on the sustainability of our business over time, and the potential impact of our business on society and the environment. However, in light of investors’ increased focus on ESG matters, there can be no certainty that we will manage such issues successfully, or that we will successfully meet society’s perceived expectations as to our proper role. Any failure or perceived failure by us in this regard could have a material adverse effect on our reputation and on our business, share price, financial condition, or results of operations, including the sustainability of our business over time.
Risk Related to Our Common Stock
Volatility in the market price of our common stock could lead to losses by investors.
The market price of our common stock has experienced volatility in the past and may experience volatility in the future which could lead to losses for investors. Factors impacting volatility in the market price of our common stock include, amongst others:
•general market and economic conditions;
•our results of operations;
•issuance of new or changed securities analysts’ reports or recommendations;
•developments impacting the industry or our competitors;
•declines in the market prices of stocks generally;
•strategic actions by us or our competitors;
▪announcements by us or our competitors of significant contracts, new products, acquisitions, joint marketing relationships, joint ventures, other strategic relationships or capital commitments;
•the public's reaction to press releases, other public announcements by us or third parties, including our filings with the SEC;
•guidance, if any, that we provide to the public, any changes in this guidance or failure to meet this guidance;
•changes in the credit rating of our debt;
•sale, or anticipated sale, of large blocks of our stock;
•additions or departures of key personnel;
•regulatory or political developments;
•our performance on ESG matters
•litigation and governmental investigations;
•changing economic conditions;
•exchange rate fluctuations;
•changes in accounting principles; and
•other events or factors, including those resulting from natural disasters, war, acts of terrorism or responses to those events.
In addition, stock markets have from time to time experienced significant price and volume fluctuations unrelated to the operating performance of particular companies. Future fluctuations in stock markets may lead to volatility in the market price of our common stock which could lead to losses by investors.
Investment returns may be reduced if we lose our foreign private issuer status.
We are a “foreign private issuer,” as such term is defined in Rule 405 under the U.S. Securities Act 1933, and, therefore, we are not required to file quarterly reports on Form 10-Q or current reports on Form 8-K with the SEC. In addition, the proxy rules and Section 16 reporting and short-swing profit recapture rules are not applicable to us. If we lose our status as a foreign private issuer by our election or otherwise and we become subject to the full reporting regime of the United States securities laws, we will be subject to additional reporting obligations and proxy solicitation obligations under the Exchange Act and our officers, directors and 10% shareholders would become subject to the short-swing profit rules. The imposition of these reporting rules would increase our costs and the obligations of those affected by the short-swing rules.
We do not expect to pay any cash dividends for the foreseeable future.
We currently do not expect to declare dividends on our common stock and have not done so in the past. We continue to anticipate that our earnings will be used to provide working capital, to support operations, to make debt repayments and to finance the growth and development of our business. They may also be used to continue our share repurchase program. Any determination to declare or pay dividends in the future will be at the discretion of our board of directors, subject to relevant laws and dependent on a number of factors, including our earnings, capital requirements and overall financial condition. Therefore, the only opportunity for stockholders to achieve a return on their investment may be if the market price of our common stock appreciates and shares are sold at a profit. The market price for our common stock may not appreciate and may fall below the price stockholders paid for such common stock.
A future transfer of ICON ordinary shares, other than one effected by means of the transfer of book entry interests in the Depositary Trust Company ("DTC"), may be subject to Irish stamp duty.
Transfers of ICON ordinary shares effected by means of the transfer of book entry interests in the Depositary Trust Company ("DTC") should not be subject to Irish stamp duty where ICON ordinary shares are traded through DTC, either directly or through brokers that hold such shares on behalf of customers through DTC. However, if ICON ordinary shares are held as of record rather than beneficially through DTC, any transfer of ICON ordinary shares could be subject to Irish stamp duty (currently at the rate of 1% of the higher of the price paid or the market value of the shares acquired). Payment of Irish stamp duty is generally a legal obligation of the transferee. The potential for Irish stamp duty to arise could adversely affect the price of ICON ordinary shares.
Item 4. Information on the Company.
A.History and development
ICON public limited company (“ICON plc”) is a clinical research organization (“CRO”), founded in Dublin, Ireland in 1990. Over thirty years we have grown significantly to become a leading global provider of outsourced development and services to pharmaceutical, biotechnology, medical device and government and public health organizations. Our mission is to improve the lives of patients by accelerating the development of our customers’ drugs and devices through innovative solutions.
We are a public limited company in Ireland and operate under the Irish Companies Acts. Our principal executive office is located at: South County Business Park, Leopardstown, Dublin 18, Republic of Ireland. The contact telephone number of this office is +353 1 2912000. Our website is www.iconplc.com. Additionally, the SEC maintains a website (www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC.
Our service offering includes clinical development, functional outsourcing and laboratory services. Our clinical development services include all phases of development (Phases I-IV), peri and post approval, data solutions and site and patient access services. Our laboratory services include a range of high value testing services, including bionanalytical, biomarker, vaccine, good manufacturing practice ('GMP') and central laboratory services. We also offer full-service and functional service partnerships to our customers.
Since ICON was founded, the Company has expanded through organic growth, together with a number of strategic acquisitions to enhance its expertise and capabilities in certain areas of the clinical development process and to broaden the service portfolio and add scale to existing services.
Recent investments, which continue to strengthen our service offerings to meet the needs of our customers include:
On July 1, 2021, the Company completed the acquisition of PRA by means of a merger whereby Indigo Merger Sub, Inc., a Delaware corporation and subsidiary of ICON, merged with and into PRA Health Sciences, Inc., the parent of PRA Health Sciences ("the Acquisition" and "the Merger"). Upon completion of the Acquisition, PRA became a wholly owned subsidiary within the ICON Group. The Acquisition has transformed the scale and capabilities of the Company. The combined Company leverages its enhanced operations to transform clinical trials and accelerate biopharma customers’ commercial success through the development of much needed medicines and medical devices. The combined Company retained the name ICON and brought together approximately 38,000 (as at the Merger date) employees across the globe, creating one of the world’s most advanced healthcare intelligence and clinical research organizations.
The Acquisition has brought together two high-quality, innovative, growing organizations with similar cultures and values to create the world's leading clinical research organization with a singular focus on clinical research and commercialization. Under the terms of the Merger, PRA shareholders received per share $80 in cash and 0.4125 shares of ICON stock. The total value of the Merger consideration is $12.0 billion and has resulted in the recognition of goodwill of $8.1 billion, intangible assets of $4.9 billion and an associated deferred tax liability of $1.1 billion. The accounting for the Merger was final as of June 30, 2022.
In conjunction with the completion of the Merger, on July 1, 2021, ICON entered into a credit agreement providing for a senior secured term loan facility of $5,515 million and a senior secured revolving loan facility in an initial aggregate principal
amount of $300 million (the "Senior Secured Credit Facilities"). The proceeds of the senior secured term loan facility were used to repay the outstanding amount of (i) PRA’s existing credit facilities and (ii) the Company's private placement notes outstanding and fund, in part, the Merger. The senior secured term loan facility will mature in July 2028 and the revolving loan facility will mature in July 2026. The credit agreement governing the Senior Secured Credit Facilities provides that borrowings denominated in U.S. Dollars will bear interest based on the London Interbank Offered Rate ("LIBOR") or other base rate (as elected by the borrower), plus an applicable margin. On November 29, 2022, the Company agreed with its lenders to the adoption of the Secured Overnight Financing Rate ("SOFR") as the benchmark rate within the Senior Secured Credit Facilities, thus LIBOR is no longer the benchmark rate available to the Company under the terms of the Senior Secured Credit Facilities.
The Company repaid $800 million and $513.8 million of the senior secured term loan facility for the years ended December 31, 2022 and 2021, respectively. At December 31, 2022 and 2021, no amounts were outstanding under the senior secured revolving loan facility with the exception of $4.5 million letters of credit given to landlords to guarantee lease arrangements.
The financial information presented in this Form 20-F reflect the results of the combined Company for the year ended December 31, 2022. The financial information presented for the years ended December 31, 2021 and December 31, 2020 are as previously reported and do not reflect the pre-Merger results of PRA before July 1, 2021, other than where clearly stated and required by GAAP.
During 2019, we further enhanced our site and patient recruitment abilities through the strategic acquisitions of MeDiNova and CRN. In 2020, ICON announced the launch of Accellacare, a global clinical research network offering patients easier and faster access to innovative treatments and offering customers the option to deploy decentralized trials. The site network includes previously acquired PMG Research, MeDiNova and CRN. Also in 2020, we entered into an agreement to jointly establish a new company, Oncacare Limited ("Oncacare"), with a third party. Oncacare operates as a specialized oncology site network in the US and EMEA regions.
With approximately 41,100 employees across the globe, the new ICON has established relationships with a majority of the world’s top pharmaceutical and biotech companies. We believe the Company now has the expertise, technology, and data assets to lead the industry into a new paradigm for bringing clinical research to more patients and enabling expanded capabilities for customers. We believe the Merger will deliver a transformational effect on ICON through:
Scale: With a deeper clinical, commercialization and consulting services portfolio, a broader geographic footprint, depth in therapeutic expertise, and data-driven healthcare technology, the Company can deliver enhanced globally scaled expertise & solutions for all customers and patients.
Focus: The Company will have a singular focus on clinical research and commercialization, leveraging transformational technology and innovation to execute clinical trials from Phase 1 to post-approval studies with the highest quality, expertise and speed.
Speed to market: Our extensive services portfolio, digital and data technology capabilities, and enhanced access to more diverse patient populations, have been combined with flexible delivery approaches and partnership models – all with the aim of reducing development time and costs.
Flexible partnership models: ICON has partnerships with a majority of world’s top biopharma and biotech companies worldwide. ICON is a global leader in Functional Service Provision and a top global provider of full service clinical research.
Differentiated DCT platform, healthcare intelligence & technology: The new ICON can deliver differentiated decentralized and hybrid trial solutions through a suite of capabilities, including mobile health, commercial connected health platforms, real world data and information solutions, a global site network, home health services and wearables expertise.
Access to patients: The new ICON offers customers enhanced access to a larger global pool of more diverse patients through its global site network (Accellacare), specialized oncology network (Oncacare), a pediatric site network, in-home clinical services and a network of six Phase I clinical research units across the United States and Europe.
Interest Rate Risk Management
The Company has entered into derivative financial instruments to manage exposures to interest rate fluctuations. On November 29, 2022, the Company entered into two interest rate cap agreements and one interest rate swap agreement with notional amounts of $2,100.6 million and $1,100.6 million, respectively. These derivative financial instruments are used to limit the Company's exposure to changes in the variable interest rate on its Senior Secured Credit Facilities by fixing the interest rates on approximately 48% of ICON's term loan facility at that date.
Share Repurchase Program
On February 18, 2022, the Board authorized the repurchase of up to $100 million of the Company's common stock. The share repurchase program was completed during the year ended December 31, 2022 with a total of 420,530 ordinary shares redeemed by the Company for a total consideration of $100.0 million. All ordinary shares that were redeemed under the buyback program were canceled in accordance with the Constitution of the Company and the nominal value of these shares transferred to other undenominated capital as required by Irish Company law.
Ukraine
On February 24, 2022 Russia invaded Ukraine, creating significant instability and unrest in the region. Since that time, the Company's key focus has been on the safety of our employees and their families, patients, investigators and on the mitigation of adverse impacts on ongoing clinical trials. The Company has worked to ensure the safety of employees and their families based in Ukraine through the implementation of a number of employee assistance programs. These programs aim to provide affected employees and their families with transportation, accommodation in neighboring countries, financial assistance, communications and other support services as needed.
The Company's operations in these affected regions have been significantly curtailed as a result of these events. The ongoing conflict in Ukraine has resulted in an increasingly complex economic sanctions and export controls environment applicable to our business operations in the region (including Russia and Belarus) as a result of additional trade compliance measures enacted by the United States, United Kingdom and European Union member states. These economic sanctions and export controls restrict our ability to do business with sanctioned entities, require additional compliance resources, and could have a material adverse effect on the results of our operations. The financial impact of the conflict was not material to the Company during the year ended December 31, 2022.
Foreign exchange
The Company prepares its financial statements in United States dollars while the local results of a certain number of our subsidiaries are prepared in currencies other than United States dollars, including, amongst others, the pound sterling and the euro. In addition, the Company's contracts with clients are sometimes denominated in currencies other than the United States dollar. Finally, the Company is exposed to a wider variety of currencies in the expenses line due to most expenses being incurred in the local currencies of where our global operations are based. Accordingly, changes in exchange rates between the United States dollar and those other currencies could have a material adverse effect on the Company’s financial results. In the year ended December 31, 2022, the Company recorded foreign currency gains of $26.0 million.
B.Business Overview
ICON is a leading global provider of outsourced development and commercialization services to pharmaceutical, biotechnology, medical device, and government and public health organizations.
We offer a full range of clinical, consulting and commercial services that range from clinical development strategy, planning and trial design, to full study execution, and post-market commercialization.
ICON provides its services across a range of clinical outsourcing operating models including strategic partnerships, preferred provider, full-service delivery to functional service provision and stand-alone services.
We specialize in the strategic development, management and analysis of programs that support all stages of the clinical development process, from compound selection to Phase I-IV clinical studies. We earn revenue by providing a number of different services to our customers. Those services are integral components of the clinical development process and include clinical trial management, consulting, contract staffing, data solutions and laboratory services.
Our vision is to be the healthcare intelligence partner of choice by delivering industry leading solutions and best in class performance in clinical development. We believe that we are one of a select group of CROs with the expertise and capability to conduct clinical trials in the major therapeutic areas on a global basis and have the operational flexibility to provide development services on a stand-alone basis or as part of an integrated full-service solution. In order to achieve this vision, we are capitalizing on the enhanced scale, technology and data analytics capabilities that the recent acquisition of PRA Health Sciences delivered.
ICON maintains a sustained focus on research and development and developing industry-leading technologies and processes to support our clients. We continue to enhance our portfolio of data solutions and decentralized clinical trial technology. ICON is leading the industry transformation through four key levers: transforming clinical trials, site and patient centricity, healthcare intelligence and applied innovation, and seamless, integrated service delivery.
At December 31, 2022, we employed approximately 41,100 employees in 111 locations in 53 countries. During the year ended December 31, 2022, we derived approximately 46.2%, 46.5% and 7.3% of our revenue in the United States, Europe and Rest of World, respectively.
The ICON strategy
We have achieved strong growth since our foundation in 1990, as a global provider of outsourced development and commercialization services to pharmaceutical, biotechnology, medical device and government and public health organizations. We focus our innovation on those factors that are critical to our clients - reducing time to market, reducing cost and increasing quality. Our global team has extensive experience in a broad range of therapeutic areas. ICON has been recognized as one of the world's leading Contract Research Organizations (''CROs") through a number of high-profile industry awards (see www.iconplc.com/awards).
As our market has evolved, biopharmaceutical companies are tackling productivity challenges, increasing budget constraints and greater demands to demonstrate product value; all of which are placing increased pressure on their revenues and levels of profitability. However, these trends have generally been positive for CROs, as increased outsourcing has been adopted by these companies as they seek to create greater efficiencies in their development processes, convert previously fixed costs to variable, and accelerate time to market for new treatments.
Regulatory and reimbursement pressures will increase the emphasis on late stage (post marketing) research, while increasing requirements to demonstrate the economic value of new treatments. As a result, outcomes and comparative effectiveness research will most likely be required in order to secure on-going product reimbursement. Furthermore, we believe advances in molecular biology and genetics will drive further growth in innovation in the long term which in turn should create further growth opportunities for both biopharma companies and their outsource development partners.
We expect that continued outsourcing will be a core strategy of clients in the near term as they respond to the increased pressures on their revenues and profitability. Larger clients were the first to form strategic partnerships with global CROs in an effort to reduce the number of outsource partners with whom they engage and to reduce inefficiencies in their current drug
development models. More recently we have seen the increasing adoption of this partner model with mid-tier pharmaceutical and biotechnology firms as they also seek to drive development efficiencies. As outsourcing penetration increases, we believe clients may seek a greater level of integration of service offerings from CROs, although some will continue to purchase services on a stand-alone basis. Creating greater connectivity and “seamlessness” between our services and the sharing of “real-time” clinical, operational and “real world” data with clients will therefore become increasingly important for CROs. ICON will seek to benefit from this increased outsourcing by clients to grow our business by increasing market share with our existing client base and adding new clients within the Phase I-IV outsourced development services market; the aim being to ensure we will be considered for all major Phase I-IV projects.
Delivery of our mission and strategy is focused on our four strategic pillars, being (i) Patient Access & Engagement (ii) Career Development & Employer of choice (iii) Enduring Customer Partnerships and (iv) Healthcare Intelligence & Applied Innovation.
Patient Access & Engagement
ICON has a focused patient, site and data strategy, which is helping us to improve site identification, study placement and patient recruitment and retention.
Accellacare is ICON's global clinical research network offering customers a wide range of stand-alone and integrated solutions at the site or in patients' homes as part of decentralized trials. Our patient centric approach accelerates study start-up and increases patient recruitment and retention for pharmaceutical, biotechnology and medical device industries.
The Accellacare Site Network encompasses more than 76 sites across 6 countries covering the United States and Europe. Accellacare offers a quality focused clinical research infrastructure delivering value and benefits to sponsors. Accellacare supports customers with faster start-up. The time from site selected to site initiation visit is on average 30% faster when compared to other sites. Also, Accellacare achieves an average of 40% more patients per site when compared to other sites.
Accellacare In-Home Services takes study visits directly to patients where they live, work, study or play in all phases and therapeutic areas of clinical trials. By bringing trial visits directly to patients, we ease the burden of participating in clinical research to increase patient recruitment, retention and diversity. Accellacare In-Home Services has experience in more than 400 clinical trials, tailoring our services to fit each study's specific requirements across more than 55 countries. This cohesive approach is leading to higher patient recruitment and retention rates. Accellacare is also achieving faster study start-up for its customers through efficiencies gained in central process management including budget and contracting, which can otherwise be a source of delay. This combined with a finely tuned feasibility approach allows the network to identify and recruit more patients to studies, in a wide range of therapeutic areas, in a shorter time frame. Accellacare is an important part of the integrated patient, site and data strategy, helping us to improve patient recruitment and retention. Through Accellacare we are committed to delivering on the promise of patient centricity in clinical research. It is also providing investigators with innovative treatments for their patients with a quality-focused clinical research infrastructure supported by experienced professionals globally.
In 2021, Accellacare entered new partnerships with six research sites across four countries, expanding its global footprint and capabilities. Agreements with Asclepes Research and Olympian Clinical Research in the U.S., Curiositas ad Sanum and Intermed in Germany, Quironsalud in Spain, and KO-MED in Poland. Through these new partnerships, Accellacare is also enhancing its capability in the central nervous system (CNS) and immune-inflammation therapeutic areas.
In 2022 the continued expansion of the Accellacare Site Network increases access and engagement with investigative sites and its patients, with the goal of faster recruitment and reducing the overall time and cost associated with drug development for customers. Accellacare now has access to more than 9 million patients.
Finding and engaging suitable patients to conduct clinical trials is one of the biggest issues facing the drug development industry today. Less than 1% of the US population participates in clinical trials and the performance of investigative sites that do take part in research is uneven, hard to predict and many trials do not meet the initial recruitment goals. The current market challenge in patient enrollment creates an opportunity for ICON to differentiate its service offering and we are working to reduce patient recruitment times through enhanced site and investigator selection based on key performance metrics and through use of our proprietary FIRECREST technology which is used to train and support sites during the development process. Our Accellacare and Oncacare site network alliances enhances our ability to enroll patients onto the clinical studies we perform. We have also developed strategic alliances with investigator site groups and healthcare systems in all major global research markets. In partnership with others, we are pioneering patient recruitment solutions that leverage cognitive computing to transform clinical trial matching and allow a data-driven approach to deliver the right patients for trials. One Search is our intuitive, integrated workflow and interrogation tool that enables access to multiple data sources and provides the visualization and tools necessary for optimum site identification based on ICON and industry data of capability, experience and performance. Scoring on enrollment performance, speed of start-up and quality supports better site selection.
Career development and employer of choice
People have long been central to our mission to improve the lives of patients by accelerating the development of our customers’ drugs and devices through innovative solutions. We encourage our people to bring flexibility, innovation, and determination to every situation. By doing so, our people can build exciting and rewarding careers, and deliver results to bring life-changing medicines to market and to maintain our success as an industry leader.
Our leadership and talent programs contribute to the enhanced retention of our employees, better project deliverables for our customers and the enhanced financial performance of the business.
We aim to be an industry leader: a company where talented people come to do important work, a place where our employees can shape the future of healthcare, grow their careers, and reach their full potential. We have long held a deep commitment to cultivating strong people practices. This includes competitive total rewards packages along with a focus on continuous learning. We nurture a culture of development and aim to boost engagement by supporting our people’s growth, both personally and professionally. We are dedicated to finding opportunities for our employees to grow and develop.
Our success depends on the knowledge, capabilities, and quality of our people. To improve their skills, we are committed to providing continuous learning. This commitment is underpinned by clearly defined competencies, which offer employees a clear path along which to develop skills and advance their careers.
To support employees at every stage of their career journeys, training and development programs are aimed at advancing scientific, technical, and business knowledge. Programs include tailored CRA academies and a range of project management curricula, therapeutic-focused programs, and people leader development programs.
Enduring customer partnerships
We continue to focus on expanding and deepening our partnerships with existing customers, while also developing new customer relationships.
Strategic client relationships will increasingly manifest themselves in many different forms. Many of these relationships will require innovative forms of collaboration across ICON service areas and departments and will therefore require increased flexibility to offer services on both a standalone functional basis and as part of a fully integrated service solution. To support this objective, we continue to evolve our collaboration and delivery models, invest in technology that will enable closer data integration across our service areas and enhance our project and program management capabilities.
To meet the evolving needs of both our existing and new clients we continue to enhance our capabilities through both organic service development and targeted acquisitions.
During the year, we continued to enhance our scientific and therapeutic expertise to support our customers in specific areas including oncology, orphan and rare diseases, CNS, dermatology, infectious disease and women's health.
ICON has extensive experience in vaccine clinical development for commercial businesses, governments and NGOs, having participated in over 160 vaccine studies in the past five years. This experience enabled us to play a significant role in the search for vaccines and treatments for COVID-19. ICON is currently or has already conducted a total of over 130 COVID-19 related trials.
Of particular note was our work in partnering with Pfizer and BioNTech on their investigational COVID-19 vaccine program - the first to announce positive efficacy results from a Phase 3, late-stage study of a COVID-19 vaccine and to receive Emergency Use Authorization in individuals 16 years of age or older from the U.S. Food and Drug Administration.
ICON mobilized a large global team of therapeutic and operational specialists to partner on the implementation of Pfizer‘s and BioNTech’s strategic plan and framework for the monitoring of the trial, which included a high level of remote clinical monitoring and source data verification in addition to on-site monitoring, safeguarding data quality and integrity in the evolving pandemic environment. The team combined the benefits of full service and functional service provider clinical operating models to increase efficiency and ensure rapid study start-up.
ICON worked with 153 sites in the US, Europe, South Africa and Latin America to ensure the recruitment of more than 44,000 trial participants over a four-month period in late 2020. ICON provided site training, document management and operational support for patient Informed Consent Form review, coordinated eConsent in most countries, and assisted with clinical supply management services. Achieving the unprecedented trial timelines, while maintaining high standards of quality, undertaken in response to the pandemic required collaboration and strong communication between the ICON and companies’ project teams.
We continue to target growth in under-penetrated CRO market segments. Penetration within medical device companies has lagged that of bio-pharma firms but is beginning to accelerate. EU regulatory reform enacted in 2017 is a further catalyst to growth in this segment as it included stricter requirements to perform clinical evaluations and post-sale surveillance. In early 2020, ICON acquired MedPass which has further enhanced our value offering in this area.
We also invested significantly in our site and patient network (Accellacare), and consider our expertise and offering in this area as one of our strategic pillars effective from 2021.
Healthcare intelligence and applied innovation
Innovation at ICON is focused on the factors that are critical to our clients. We develop integrated technologies to significantly enhance the efficiency and productivity of clients’ drug and device development programs, providing true transparency across all areas of a study.
ICON is focused on applying innovation that can help our customers improve their development outcomes. We are focusing this innovation in three critical areas: improving clinical trial design and execution; faster and more predictable patient recruitment; and evolving clinical trials to be more patient centric which includes data collection and analysis directly from patient’s digital devices. Our approach to developing solutions to these challenges incorporates partnering with best-in-class technology providers but is also supported by a suite of differentiated ICON proprietary technologies.
We have continued to invest in building our capabilities in the gathering, analysis and application of real world patient data within both the clinical trial and post-trial observational study environments. Alongside expanding internal capabilities, we continue to develop innovative partnerships with providers of real world data including TriNetX. During 2018, we signed an agreement with Intel to deploy the Intel® Pharma Analytics Platform for use in clinical trials. The Intel platform is an artificial intelligence solution that enables remote monitoring and continuous capture of clinical data from study subjects using sensors and wearable devices and can apply machine learning techniques to objectively measure symptoms and quantify the impact of new therapies.
ICON’s proprietary One Search tool helps identify optimum trial sites the first time. It synthesizes multiple data sources, applying AI machine learning and rich data visualization for optimum site identification, resulting in improved study start-up and site cycle times, significant reductions in the percentage of low performing sites and increasing the percentage of studies meeting planned First Patient In (FPI).
FIRECREST is ICON’s proprietary comprehensive site performance management system. It is a web-based solution which enables accurate study information, including protocol information, training manuals and case report forms, to be rolled out quickly and simultaneously to investigative sites. It allows site behavior to be tracked to ensure training is understood, procedures are being followed and that timelines and study parameters are met. It can significantly reduce the number of data queries originated from investigator sites. FIRECREST is now integrated into the ICON Safety Reporting Solution and provides a Site Question Management Tool.
ICON has also developed a patient engagement platform to support improved patient experience and enrollment in clinical trials. The web-based patient engagement platform, provides patients with study specific information and connectivity with the nearest investigative site. The solution supplements patient recruitment outreach by sites and increases visibility of potential
study participants for sponsors and sites. An easy to navigate, user friendly interface guides the patient to new and ongoing studies in their particular indication and a pre-qualification questionnaire helps to determine if the study is a right fit for them. If the patient decides to register interest, they are given the option to select their nearest investigative site. This establishes connection with the site and the patient can then choose to contact the site or ask to be contacted for pre-screening.
We positively impact patients’ lives by understanding their journeys and how they can benefit from drugs currently in development and on the market. We do this by developing a holistic, global data environment across pharmaceutical and biotech companies (development to commercial) that gives insights into patients, and how best to serve them. Alongside the application of these technology solutions we are also focused on innovation through the redesign and where appropriate the automation of current clinical trial processes.
Operational excellence, quality and delivery
Quality is the foundation of our success. The quality of our work is vital to our mission of bringing better medications to patients around the world. We are committed to maintaining, supporting, checking and improving our quality systems to meet or exceed the quality standards demanded by our clients, patients and regulatory authorities. We focus our innovation on the factors that are critical to our clients – reducing time to market, reducing cost and increasing quality – and our global team of experts has extensive experience in a broad range of therapeutic areas.
Quality project execution underpins all that we do and we have an ongoing focus on developing our people and processes to continue to enhance our service delivery. We also deploy supporting technologies which we believe will enable faster and deeper insights into the quality of trial data.
We are focused on operational excellence across our support functions, and we operate a global business support infrastructure across functions including finance, information technology, facilities, human resources and legal. This enables us to enhance the service levels across these support areas whilst driving down the costs of the service provision.
Capabilities and service offerings
ICON plc is a world-leading healthcare intelligence and clinical research organization. From molecule to medicine, we advance clinical research providing outsourced services to pharmaceutical, biotechnology, medical device and government and public health organizations. We develop new innovations, drive emerging therapies forward and improve patient lives.
With an expansive portfolio of integrated clinical, commercialization and consulting services, global presence, depth in therapeutic expertise, and data-driven healthcare technology, we deliver globally scaled expertise & solutions for all customers and patients. Solutions span the Clinical Development lifecycle from compound selection to Phase I-IV clinical studies and post approval outcome research and market access consulting solutions, and can be adapted to suit local trials or large global programs: 
Industry overview
The CRO industry provides independent product development solutions and services for the pharmaceutical, biotechnology and medical device industries. Companies in these industries outsource services to CROs in order to manage the drug and device development process more efficiently and to bring both patent-protected bio-similars and medical devices to market faster to enhance patient well-being and maximize their return on investment. The CRO industry has evolved since the 1970s from a small number of companies that provided limited clinical development services to a larger number of CROs that offer a range of services that encompass the entire research and development process, including pre-clinical development, clinical trials management, clinical data management, study design, bio statistical analyses, post market surveillance, regulatory affairs, central laboratory and market access services. CROs are required to provide services in accordance with good clinical and laboratory practices, as governed by the applicable regulatory authorities.
The CRO industry is highly fragmented, consisting of several hundred small, limited-service providers, medium sized CROs and a small number of large CROs with global operations. Although there are few barriers to entry for small, specialist service providers, we believe there are significant barriers to becoming a CRO with global capabilities and expertise. These barriers include the infrastructure and experience necessary to serve the global demands of clients (sponsors), the ability to recruit sites and patients globally, the simultaneous management of complex clinical trials, the ability to offer customers a variety of delivery models, broad therapeutic expertise and the development and maintenance of the complex information technology systems required to integrate these capabilities. In recent years, the CRO industry has experienced consolidation, resulting in the emergence of a select group of CROs that have the capital, technical resources, integrated global capabilities, data and expertise to manage the development programs of pharmaceutical, biotechnology and medical device companies. We believe that large and medium-sized pharmaceutical companies are selecting a limited number of CRO service providers with which they deal rather than utilizing many, in order to form strategic partnerships with global CROs in an effort to drive incremental development efficiencies and leverage the scientific and medical expertise. We believe that this trend will continue to concentrate the market share among the larger CROs with a track record of quality, speed, flexibility, responsiveness, global capabilities and access to patients and overall development experience and expertise.
New drug development overview – ethical pharmaceuticals and biologics
Before a new drug or biologic may be marketed, it must undergo extensive testing and regulatory review to determine that it is safe and effective. The following discussion primarily relates to the FDA approval process for such products. Similar procedures must be followed for product development with other global regulatory agencies. The stages of this development process are as follows:
Preclinical Research “In vitro” (test tube) and animal studies must be conducted in accordance with applicable regulations to establish the relative toxicity of the drug over a wide range of doses and to detect any potential to cause birth defects, affect vital organs, cause mutations or cancer. Many of these tests must be performed before a new investigational therapy can progress into human studies. If results warrant continuing development of the drug or biologic, the sponsor or owner of the asset will file for an Investigational New Drug Application, or ("IND"), which must be approved by the FDA before starting the proposed clinical trials. However, preclinical studies will continue to be conducted in parallel with the clinical trials, some of which can take up to 3 years to complete. Preclinical research is not commonly provided by ICON as a service to its customers.
Clinical trials (approximately 3.5 to 7 years)
Exploratory development
Phase I (approximately 6 months to 1 year) consists of basic safety and tolerability testing in small numbers of human subjects, initially in healthy volunteers, and includes studies which may show the drug is having an effect on the body, if it is safe, how it is affected by other drugs, where it goes in the body, how long it remains active and how it is broken down by and eliminated from the body. After single and multiple dose studies have been conducted, the asset can progress into Phase II, however, Phase I studies will continue to be done to help support the development of the asset in new populations such as children or the elderly.
Phase II (approximately 2 to 3 years) includes basic efficacy and dose-range testing in a limited patient population (usually) 100 to 200 patients to help provide preliminary safety and evidence that the drug is likely to be effective in the target disease. If the Phase II results are satisfactory the sponsor may decide to proceed to Phase III studies.
Confirmatory development
Phase III (2 years or greater) consists of efficacy and safety studies in several hundred to a few thousand patients at multiple investigational sites (hospitals and clinics), often in multiple geographies.
FDA approval, through submission of an IND, is necessary for all clinical trials, regardless of the phase of development. In addition, parallel independent committee approval is also required.
NDA or BLA Preparation and Submission. Upon completion of Phase III trials, the sponsor assembles the statistically analyzed data from all phases of development into a single large submission along with the Chemistry, Manufacturing and Controls (CMC) and preclinical data and the proposed labelling into the New Drug Application (NDA), or Biologics License Application (BLA) and submits them for assessment and approval by the relevant division of the FDA.
Expanded Access Programs (EAPs). Sometimes a study drug may continue to be provided to subjects after completion of a clinical trial, also called compassionate use. EAPs refer to the regulated use of a study drug outside of a clinical trial by patients with serious or life-threatening conditions where there is no alternative therapy available. In this context the FDA may allow the sponsor to make the study drug available to a larger number of patients for treatment use.
FDA review and approval of NDA or BLA (1 to 1.5 years). Data from all phases of development is scrutinized to confirm that the applicant company has complied with all applicable regulations and that the benefit to risk ratio for the drug or biologic is positive for the specific use (or “indication”) under study. The FDA may refuse to accept the NDA or BLA if the application has administrative or content criteria which do not meet FDA standards. The FDA may also deny approval of the drug or biologic product if applicable regulatory requirements are not satisfied, if the drug has not adequately shown to be effective or if there are safety concerns. Often a company will be required to conduct specific studies after the approval of a drug. These are called post approval commitments.
Post-market surveillance, Phase IV studies and health outcomes. Once approved by the FDA, it requires the drug or biologic license holder to collect and periodically report to them additional safety (and perhaps efficacy) data on the drug or biologic for as long as the license holder markets it (post-market surveillance, including pharmacovigilance). If the product is marketed outside the U.S., these reports must include data from all countries in which the drug is sold. Additional studies (Phase III and Phase IV) may be undertaken after initial approval to find new uses for the drug, to test new dosage formulations, or to confirm selected non-clinical benefits, e.g., increased cost-effectiveness or improved quality of life. Additionally, the FDA and other regulatory agencies require license holders of drugs or biologics to prepare risk management plans that are aimed at assessing areas of product risk and actively managing such risks throughout the product lifecycle.
Key trends affecting the CRO industry
CROs derive substantially all their revenue from the research and development expenditures of pharmaceutical, biotechnology and medical device companies. We believe that the following trends create further growth opportunities for global CROs, although there is no assurance that growth will materialize.
Continued innovation and development of enabling technologies
Innovation driving new drug development activity
New technologies together with improved understanding of disease pathology (driven by scientific advances such as the mapping of the human genome) have increased the number of new drug candidates being investigated in early development. This has greatly broadened the number of biological mechanisms being targeted, which increasingly include rare or orphan diseases that currently have no effective treatments.
These developments should lead to increased activity in both preclinical and Phase I development and in turn lead to more treatments in Phase II-III clinical trials. As the number of trials that need to be performed increases and these trials become focused in indications where finding suitable patients is increasingly challenging, we believe that drug developers will increasingly rely on CROs to manage these trials to leverage their global expertise and to continue to focus their own competences on drug discovery and sales and marketing.
Decentralized and hybrid trials
Decentralized and hybrid trials have existed for quite some time, but the coronavirus pandemic accelerated the demand when the pharma industry was challenged to move to remote models to protect patient safety and ensure data integrity for COVID-19 vaccine trials and other ongoing trials. The pandemic has provided an opportunity to move many technologies and remote patient care solutions from pilot phase to supporting patients and research.
As an industry, we have an opportunity to make decentralized and hybrid the standard moving forward. The ways the industry have been conducting clinical research in a traditional site-based approach need to flex so we can implement these tools, techniques and processes in everyday research to bring about a more patient-centric approach. Each new element needs to be evaluated to assess the impact for the individual patients and study sites. Recent experiences in the industry have shown:
•Using fewer countries and fewer sites can reduce costs, decrease timelines for start-up and minimize the risk of disruption during and post pandemic.
•Hybrid studies, utilizing digital health, in-home health, and telehealth, can reduce the number and frequency of onsite patient visits and therefore reduce patient burden.
•Home-based patient visits and direct-to-patient contacts can increase patient satisfaction, compliance and retention, providing greater trial resilience.
•Harmonizing data from disparate data sources will provide real-time access and consistent data visibility, helping to improve safety monitoring and enabling the visualization of data trends.
Regulatory easement has resulted in a number of positive changes to clinical trial procedures, enabling studies to continue and in some case progress at a faster pace, and improvements to the process of CTA and IND approvals despite the restrictions imposed by the current COVID-19 pandemic. However, the regulatory authorities have been clear that regulatory easement will be discontinued once the pandemic recedes so it remains to be seen how many of these improvements will endure and become standard practice in the longer term. It is hoped that we can hold on to some of the improvements for the benefit of the patient and healthcare advancement.
Ultimately, we are trying to increase the speed at which drugs can meet approval guidelines and help treatable populations. By using decentralized tools, technologies and processes, we will reduce the burden on patients, increase satisfaction and provide them with the same standard of care during a virtual or home visit that they would receive in a clinic, thereby fulfilling the promise of clinical research as a care option. While reduced costs may not be seen in the early phase of adoption (in fact investment may be required initially) choosing the right solution for the specific study characteristics has the potential to increase patient recruitment and retention which can result in reduced overall research costs and quicker time to market. To find the best clinical trial design to suit their needs, sponsors will need to take patient centricity into consideration from the outset and at every step along the way, because what benefits the patient will ultimately benefit the sponsor in outcomes.
New technology enabling more efficient development
Technology innovation is playing an increasingly important role in helping to support more efficient drug development. Leveraging differentiated technology solutions and data collaborations drives better execution in clinical trials. The larger CROs have been at the forefront of this innovation developing technology solutions that support the integration of trial data across multiple systems, data repositories that enable sponsors to get real time clinical insights on the performance of their drugs and tools that support better trial designs and operation.
The emergence of modern healthcare technologies ("mHealth") that build on the global prevalence of mobile and digital technologies also have an influence on drug development. It is now possible to capture health data using mobile devices and wearables. This enables sponsors to gather new clinical and “real-world” patient insights and will also be used to enhance patient engagement and adherence throughout the development process. As these devices mature it will also be possible to complete more “decentralized and hybrid trials” based on remote monitoring of patients in their home environment which may drive further efficiencies in the trial process.
Social media is also becoming an important platform for life sciences companies to strengthen patient engagement programs and collaborate with other stakeholders in the healthcare system. Many sufferers of specific diseases are forming patient groups and actively collaborating using social media. These groups represent an important potential source of patients for new clinical studies but can also provide valuable insights into effectiveness and safety of new treatments.
As the influence of technology on drug development grows, it broadens the potential number of partners that CROs will work with in the future.
Expanded use of new patient data sources
Pharmaceutical companies are looking to access a variety of new healthcare data sources containing medical and prescribing records to help improve development programs and to get better evidence of the value their treatments are bringing to patients once they are launched in the market. The larger global CROs have significant data management experience which can be leveraged to support these efforts and have invested in analytics capabilities to help deliver better insights for customers during the product lifecycle. Global CROs are also forging collaborations to access specific data sets that can provide further patient insights to support better matching of patients to the clinical trial process.
Improving productivity and operating efficiencies
Continuing focus on productivity within research and development programs
Pharmaceutical and biotechnology companies continue to seek ways to improve the productivity of their development efforts and increasingly see the use of CROs as a strategic component of these efforts. They are leveraging the expertise with CROs to help identify the most promising drug candidates in early development and discontinue developing those that have safety issues, limited efficacy or that will have significant reimbursement challenges. These companies are also initiating programs to drive more efficiency in their development programs. One example of this has been the efforts to achieve a more seamless transition across development phases, particularly Phase I-III. In parallel, regulatory initiatives such as the 21st Century Cures Act and the emergence of clinical trial techniques such as adaptive trial design, risk based clinical trial monitoring, decentralized and hybrid trials are enhancing development, allowing effective treatments to get to patients quicker at reduced development costs.
Cost containment pressures
Over the past several years, drug companies have sought more efficient ways of conducting business due to margin pressures stemming from patent expirations, greater acceptance of generic drugs, pricing pressures caused by the impact of managed care, purchasing alliances and regulatory consideration of the economic benefit of new drugs. Consequently, drug companies are centralizing research and development, streamlining their internal structures and outsourcing certain functions to CROs, thereby converting previously fixed costs to variable costs. Larger companies (and more recently medium sized companies) are actively entering strategic partnerships with a limited number of CROs in an effort to drive increased efficiencies. The CRO industry and, in particular, large CROs with global capabilities, considerable scientific knowledge and expertise are often able to perform the needed services with greater focus and at a lower cost than the client could perform internally, although CRO companies themselves are facing increased cost containment pressures as drug companies seek to further reduce their cost base.
Global trends influencing the CRO industry
Pressure to accelerate time to markets and globalization of the marketplace
Reducing product development time maximizes the client’s potential period of patent exclusivity, which in turn maximizes potential economic returns. We believe that clients are increasingly using CROs that have the appropriate expertise and innovation to improve the speed of product development to assist them in improving economic returns. In addition, applying for regulatory approval in multiple markets and for multiple indications simultaneously, rather than sequentially, reduces product development time and thereby maximizes economic returns. We believe that CROs with global capabilities, considerable knowledge and experience in a broad range of therapeutic areas are key resources to support a global regulatory approval strategy. Alongside this, the increasing need to access pools of new patients is leading to the conduct of clinical trials in new “emerging regions” such as Eastern Europe, Latin America, Asia-Pacific and South America. We believe that having access to both traditional and emerging clinical research markets gives global CROs a competitive advantage.
Growth within the biotechnology sector
The nature of the drugs being developed is continuing to change. Biotechnology is enabling the development of targeted drugs with diagnostic tests to determine whether a drug will be effective given a patient’s genomic profile. An increasing proportion of research and development expenditure is being spent on the development of highly technical drugs to treat very specific therapeutic areas in sectors of unmet medical need. Much of this discovery expertise is found in biotechnology firms. We believe that it is to these organizations that the large pharmaceutical companies will look for an increasing proportion of their new drug pipelines. Whether it is through licensing agreements, joint ventures or equity investment, we believe we may see the emergence of more strategic relationships between small discovery firms and the larger pharmaceutical groups. As the majority of these biotechnology companies do not have a clinical development infrastructure, we believe that the services offered by CROs will continue to be in demand from such companies providing they have the necessary funding. ICON has over 8,000 staff exclusively dedicated to working with biotech clients.
Increasing number of large long-term studies and an increasing requirement to show the economic value of new treatments
We believe that to establish competitive claims and demonstrate product value, to obtain reimbursement authorization from bodies such as the National Institute for Health and Clinical Excellence in the UK, and to encourage drug prescription by physicians in some large and competitive categories, more clients need to conduct outcome studies to demonstrate, for example, that mortality rates are reduced by certain drugs. To verify such outcomes, very large patient numbers are required, and they must be monitored over long time periods. We believe that as these types of studies increase there will be a commensurate increase in demand for the services of CROs who have the ability to quickly assemble large patient populations, globally if necessary, and manage this complex process throughout its duration.
The rising costs of healthcare in most developed countries also means there is an increasing pressure to show that new medical treatments are more cost effective and deliver better patient outcomes than existing treatment regimes. This also means that sponsors need to increasingly generate outcome data both as part of the product approval submissions and as part of post-approval research programs. This is creating opportunities for CROs who can offer support in developing and interpreting this data.
A focus on long-term product safety
The clinical trial approval process can only detect major and common adverse side effects of drugs; less common but no less serious side effects may only become apparent after many years of use. As a result, there is an increase in the number of drugs given “conditional approvals” where further ‘post-approval’ studies are being mandated. In addition, prudent sponsors undertake similar studies to detect early warning signs of any potential problems with their products. Such studies may take the form of prospective long-term safety studies, simpler observational studies or registries where patients meeting specific criteria for disease or drug use are followed for long periods to detect any safety issues. CROs are well positioned to perform these studies on behalf of sponsors.
Increasing regulatory demands
Regulatory agencies increasingly require more data to support new drug approvals and are seeking more evidence that new drugs are safer and more effective than existing products. As a result, the complexity of clinical trials, the number of procedures required to be conducted in these trials and the size of regulatory submissions are driving the demand for services provided by CROs.
Environment, Social and Governance ('ESG')
Our mission is to improve the lives of patients by accelerating the development of our customers’ drugs and devices through innovative solutions. We are passionate about providing innovative solutions for customers, we are better together working as one team, we value diversity and care about the success of our people, and we care about doing the right thing. We are advancing clinical research while offering customers broader and deeper experience, scale, and focus, complemented by continuity of delivery and speed to market. Our business model is described in the preceding sections. Consistent with our values, we seek to not only operate in compliance with applicable laws but also to positively influence our global workforce, the communities that we operate in, the environment and society as a whole. Doing so makes us a stronger, more resilient organization by every measure.
Our core values underpin our mission and drive a culture and mind-set of ownership at ICON. "OwnIt@ICON", as set out below, is a statement of values that has remained at the very heart of ICON’s culture, encouraging our people to seize the opportunity and bring flexibility, innovation, and determination to every situation. We believe our culture of ownership personifies who we are as a company — it also helps us apply our expertise, collaborate to get things done, and succeed at our mission.
Our values underpin how we work together to deliver on our mission to improve the lives of patients by accelerating the development of our customers’ drugs and devices through innovative solutions. These values and our Code of Ethical Conduct, which underpins these values, form the core of what we do, and how we do it. It applies to all of our officers, directors, employees, consultants and agents globally. All employees and temporary workers are mandated to complete global ethics training.
At ICON, we care about conducting business sustainably. We care about our people, patients, and the communities in which we live. We care about doing the right thing and we are committed to working to the highest ethical standards and demonstrating our commitment to honesty, transparency, and quality. As a testament to our commitment, we launched our “ICON Cares” program at the start of 2023 which incorporates all our Environment, Social and Governance (ESG), Diversity, Inclusion and Belonging (DIB), and CSR initiatives into one program. ICON’s Environment, Social, and Governance Committee ('ESG Committee') brings together all these initiatives and efforts under one umbrella to ensure consistency, enhance monitoring, reveal areas for development and facilitate reporting to the Board. The ESG Committee is chaired by the Chief Administrative Officer and General Counsel (CAO), who is responsible for reporting to the ICON executive leadership team, the Nominating, Sustainability and Governance Committee and the Board on ESG matters. In February 2022, the Board delegated oversight responsibilities of the Company's strategies, activities, and risks in respect to ESG matters to the Nominating and Governance Committee, which was renamed the “Nominating, Sustainability and Governance Committee” in April 2022. Accordingly, the CAO reports on ESG matters to the Nominating, Sustainability and Governance Committee and reports to the Board on an annual basis whilst also providing periodic ESG updates to the executive leadership team.
The ESG Committee is focused on developing our strategy and initiatives relating to the environment, social matters, health and safety, community engagement, corporate governance, sustainability, and other public policy matters relevant to the Company. The ESG Committee is a cross-functional management committee of the Company including representation from facilities, health and safety, corporate communications, finance, legal, investor relations, procurement, commercial, marketing, and human resources departments. The Committee assists and supports executive management and the Nominating, Sustainability and Governance Committee of the Company in:
•determining and setting the strategy relating to ESG matters;
•developing, implementing and monitoring initiatives and policies based on that strategy; and
•communicating these strategies, initiatives, and their results.
We are committed to building and developing our ESG strategies and reporting. In 2020 we launched our ESG page on the ICON website and have an internal ESG page on our MyICON intranet portal to engage with our employees and provide information and updates relating to ESG matters and our commitment to sustainability. In 2021, as a testament to our commitment to managing ICON responsibly and sustainably, we became a participant in the United Nations Global Compact (UNGC), a set of Ten Principles covering the areas of human rights, labor, environment, and anti-corruption. In our 2021 ESG report, released in 2022, we reported under the newest Global Reporting Initiative (GRI) standards and the Task Force on Climate-Related Financial Disclosures (TCFD). Our report summarizes our current policies, priorities, and commitments in respect to ESG matters. During 2022, ICON received a silver medal from EcoVadis in recognition of our environment, social and governance efforts throughout ICON. The ESG page on our website is available at https://www.iconplc.com/about/esg/.
The global landscape in respect to regulatory and legislative requirements relating to ESG reporting and disclosure requirements is rapidly evolving, and we are monitoring potential requirements so that we are positioned to adhere to any additional requirements in due course.
Building a sustainable future – our commitment to the United Nations Sustainable Development Goals
As a global company, we maintain an ethical and sustainable presence in hundreds of locations worldwide. At its core, ICON’s mission is to improve health and lives. We are also committed to contributing to the 2030 United Nations Sustainable Development Goals (SDGs) and are proud that our work contributes to their advancement.
Our research, our work with customers and patients and our on-the-ground efforts to meet the diverse needs across our communities align with the SDGs. These efforts, however, focus on a subset of themes where we have the greatest opportunity to effect change and further details are set out in our ESG Report.
Environment: Conducting business sustainably
ICON is committed to delivering excellence in care to our communities. To improve our overall sustainability, this commitment means tracking and improving our environmental performance across all business activities. We achieve this by pursuing sustainability strategies that recognize the impact of our operations as a CRO on the environment, addressing greenhouse gas (GHG) emissions, energy use, waste generation and procurement-related activities. Our employees, directors, officers, contractors, and temporary workers are expected to support our sustainability objectives.
Our Global Environmental Management Policy and Environmental Management Plan is our program for managing environmental sustainability initiatives. The implementation of the program is led by our facilities team, reporting to our Chief Administrative Officer and General Counsel (CAO). The CAO is responsible for reporting on the program to the ICON executive leadership team and Nominating, Sustainability and Governance Committee and the Board.
ICON set environmental goals around the use of renewable energy and carbon emissions in 2019 and we are working towards achieving these goals which are as follows:
•100% renewable electricity by 2025
•20% reduction in kilowatt hours (kWh) of electricity by 2030
•Net zero carbon emissions on Scope 1 & 2 by 2030
We have programs in place to manage and minimize climate impacts of business activities. To continue to improve processes and reduce our environmental impact, we track, calculate, and report our GHG footprint. We apply the GHG Protocol Corporate Standard, which is the global corporate accounting and reporting standard for calculating carbon emissions. Carbon Trust provides annual verification of our emissions data.
In line with carbon reduction targets, ICON’s combined Scope 1 and 2 GHG emissions, relative to revenue and the number of employees, have fallen year on year since 2018. Since 2018, following our reduction efforts, the pandemic-related closure of many of our facilities and a reduction in business travel, GHG emissions across our operations declined significantly. Since 2021, as more normal operations resumed, we have seen a decrease in combined Scope 1 and 2 emissions with an overall increase in our total GHG emissions due to an increase in business travel (Scope 3). As the pandemic recovery continues, and we have resumed more normal operations, we are evaluating additional opportunities to continue to reduce our carbon emissions across our organization to develop and improve our environment program.
CDP (formerly the Carbon Disclosure Project) provides a globally recognized system that enables companies to measure and manage their environmental impacts. ICON continues to be committed to improving its current scoring of a C.
We are focused on reducing energy use across our global operations. For example, reducing energy use and shifting to renewable energy are components of our specific environment goals. Waste reduction is embedded into our environment policies and practices and is one of the objectives of ICON’s Environmental Management Policy. We will seek new opportunities to reduce waste by increasing recycling volumes, reducing consumption of primary materials, and decreasing use of disposable products in our offices and facilities.
The majority of our sites are leased, and we work closely with our landlords and leasing agents to implement measures to ensure we operate in an environmentally sustainable manner. The Acquisition of PRA expanded our global real estate footprint and our real estate group worked with other business leaders to understand the sustainability implications and opportunities of this new footprint and find ways to continue to advance our collective sustainability goals. During 2022, we continued to integrate offices and reduce our footprint which resulted in downsizing or closing 31 locations to align with new working styles and business needs. When selecting new locations for offices and planning building modifications, experts from our real estate team factor in environmental considerations. In addition, we have implemented a series of measures globally to reduce the local footprint of our offices, such as installing energy-efficient LED lighting, using motion detectors to reduce energy use, purchasing recycled office supplies, and reducing paper consumption by promoting paperless office processes, or where printing is necessary, enabling double-sided output.
Our office design has efficiency in mind, utilizing space to provide the maximum number of desks and functional provisions while still providing comfortable, safe spaces for our employees. Our strategies include:
•Perimeter glazing of meeting rooms, offices, and other spaces which allow in natural light.
•Recycling areas built into business centers and kitchen/ canteens which reduce waste sent to landfills.
•Planted green spaces which contribute to internal air quality, temperature, and humidity.
•Building materials and vendors which we select for low environmental impact.
We also require our suppliers to abide by our Global Supplier Code of Conduct which includes a commitment to comply with applicable environment laws and regulations, our expectations around waste management and sustainable use of resources.
Community Engagement
We are committed to making a positive impact on the communities in which we work and live and we have aligned our community efforts to a broader vision for social impact, including by aligning priorities with our organizational goals of diversity, inclusion, and belonging.
Our community engagement activities are focused on two core areas:
•Supporting education and building closer ties between industry and academia.
•Improving the welfare of people in the communities in which we live.
Supporting education and building closer ties between industry and academia
A core area of community support includes building ties between industry and academia to inspire the next generation of leaders in business and science. Our existing partnerships continue with the following organizations:
▪The ICON-McKeon Research Fellowship in Motor Neuron Disease ('MND') in honor of Mr. Declan McKeon, former Board member, acting Chairman, Lead Independent Director and Chair of the ICON Audit committee. The ICON-McKeon Research Fellow in MND carries out research in the areas of machine-learning and artificial intelligence to derive insights from multimodal clinical, imaging neuro-electric signaling, in the context of the neurodegenerative disease of ALS.
▪Partnership with Trinity Centre for People with Intellectual Disabilities ('TCPID') - TCPID situated within the School of Education, Trinity College Dublin, aims to promote the inclusion of people with intellectual disabilities in education and society. The Centre provides people who have intellectual disabilities with the opportunity to participate in a higher education program designed to enhance their capacity to fully participate in society as independent adults. The 2-year education program includes work placements and internships to enable students to experience and participate in the work environment. In 2022, ICON facilitated a 12-month internship in our Laboratory and Facilities departments for one of the TCPID graduates and our aim is to continue to offer work placements and internships as part of this partnership in 2023.
•Partnership with Junior Achievement to inspire schoolchildren. Junior Achievement encourages young people to remain in education and teaches them the skills they need to succeed in a changing world. ICON volunteers take time out of their working day to deliver Junior Achievement programs, teaching primary and secondary-level students valuable business, STEM and entrepreneurship skills that will serve them throughout their professional lives. ICON expanded its partnership with Junior Achievement to include an additional three locations in the United States and the United Kingdom in 2022.
In 2022, we further expanded our industry-academia partnerships through the creation of a new ICON scholarship program to provide increased opportunities for underrepresented groups to study STEM (Science, Technology, Engineering & Mathematics) courses and to build a more diverse graduate pool of talented and ambitious STEM professionals who can help to ensure the future success of the life sciences industry. Through the program, ICON is partnering with three universities in Ireland – Dublin City University (DCU), Trinity College Dublin (TCD) and the University of Limerick (UL) – as well as with the Thurgood Marshall College Fund (TMCF) in the US, to fund 33 scholarships for students of STEM courses. TMCF is a not-for-profit organization that supports nearly 300,000 students attending its 47 member-schools that include publicly-supported Historically Black Colleges and Universities (HBCUs).
Improving the welfare of people in the communities in which we live
Through volunteering, donations and other charitable initiatives, our employees across the world are making a positive difference to their communities. We support causes that are important to our employees and have several programs that support the welfare of people in our local communities. In 2022, 540 employees across the world participated in Run in the Dark, an annual event organized by the Mark Pollock Foundation to bring people together and fundraise to help find a cure for paralysis. ICON was the largest global corporate team to participate in the event and helped to raise $10,000 for the cause. A team of 90 ICON cyclists from 19 countries also participated in our annual ICON cycle challenge, which raised funds for the International Committee for the Red Cross.
Since 2012, ICON’s annual employee-nominated charity donation program has supported over 90 charities, donating $10,000 to each organization. The selected organizations focus on a range of critical issues, from relieving poverty and homelessness to improving child welfare through education and enhancing the lives of people living with a variety of diseases. The organizations were chosen to align with our ESG goals. In addition, to support ongoing humanitarian efforts in Ukraine, we also made a donation to the Children of Heroes charity fund in Ukraine to support children who have been severely impacted by the war in that region.
Talent and People
Our people are core to our ability to deliver our services and drive better patient outcomes. Through diversity, inclusion and belonging, industry-leading talent management practices, a sincere attention to our employees’ needs, well-being and health and safety, we continue to power the potential of together.
At the core of our strategy is our people
People have long been central to our mission to improve the lives of patients by accelerating the development of our customers’ drugs and devices through innovative solutions. We encourage our people to bring flexibility, innovation, and determination to every situation. By doing so, our people can build exciting and rewarding careers, and deliver results to bring life-changing medicines to market and to maintain our success as an industry leader.
Learning and development of our staff is a key focus for us
Our leadership and talent programs contribute to the enhanced retention of our employees, better project deliverables for our customers and the enhanced financial performance of the business.
We aim to be an industry leader where talented people come to do important work and where our employees can shape the future of healthcare, grow their careers, and reach their full potential. We have long held a deep commitment to cultivating strong people practices. This includes competitive total rewards packages along with a focus on continuous learning. We nurture a culture of development and aim to boost engagement by supporting our people’s growth, both personally and professionally. We are dedicated to finding opportunities for our employees to grow and develop.
Our success depends on the knowledge, capabilities, and quality of our people. To improve their skills, we are committed to providing continuous learning. This commitment is underpinned by clearly defined competencies, which offer employees a clear path along which to develop skills and advance their careers.
To support employees at every stage of their career journeys, training and development programs are aimed at advancing scientific, technical, and business knowledge. Programs include tailored CRA academies and a range of project management curricula, therapeutic-focused programs, and people leader development programs. During 2022, this also included diversity, inclusion and belonging training for everyone on our talent acquisition team and many of our People Leaders.
Our People Leader development program focuses on providing our People Leaders with the relevant skills to effectively manage themselves, their team and their business, including psychometrics to raise their awareness of their behavioral preferences and the preference of others. ICON also invested in Harvard Manage Mentor, an online learning platform providing People Leaders with access to learning available at any time with topics ranging from change management, diversity & inclusion, retaining employees and developing employees.
We provide our people with a personalized and flexible learning experience, delivered through a combination of in-person and technology-driven programs that suit their learning styles and can flex to suit their schedules. Through our industry leading CareerHub, ICON employees are encouraged to broaden their scientific, technical, leadership, and business knowledge. By tapping into development programs and partnerships with leading academic institutions, team members can use the hub to develop competencies that advance their careers. We also collaborate with UCD Smurfit School Executive Development to deliver customized leadership development programs for global employees.
As an organization we are keen to hear directly from our employees
To attract and retain the best talent, we must listen and respond to employees’ needs. This begins with a focus on diversity, inclusion and belonging, and extends to every aspect of our work, from recruitment and onboarding, to training, engagement, enablement, and reward. We pursue best-in-class approaches to building employee engagement and these include, among others:
•Comprehensive global employee surveys, which measure how people feel about their work and whether they feel they have the tools to do their jobs well. Feedback from these studies informs detailed action plans at the group, function, and team level.
•Pulse check surveys, which are smaller-scale studies designed to measure employee sentiment on specific topics and initiatives.
•Fostering an environment of diversity, inclusion and belonging where everyone is valued.
•Stay interviews to help managers understand why staff stay and to uncover what might put them at risk of departing.
•Skip-level meetings to develop trust and rapport between senior leaders and employees.
Our listening strategy supports our efforts to reduce employee turnover, which we monitor closely through analytics. Qualitative information is collected through formal exit interviews and, where we believe they’ll make an impact, we intervene via retention plans and related efforts.
Employee well-being
ICON’s commitment to improving health and enriching lives extends beyond the work we do with our customers. Employees across the globe have direct access to locally relevant information and resources to support every facet of their well-being, including physical, social, psychological, and environmental.
Our global Employee Assistance Program (EAP) ensures that all employees, and their families, have access to a range of different, confidential resources and experts to help them better manage their working life and personal life. The EAP also delivers a web-based and mobile app platform with access to toolkits providing advice and resources in local languages.
Health and safety
At ICON, the health and safety of our employees, customers and clinical trial patients are our most important priorities. We take guidance from global and regional health authorities and governments to protect the safety and welfare of employees, as well as abide by government directives. Our global health and safety management system ensures we deliver on all local and national requirements. Our priority objectives are the safety of our staff, clinical trial patients, protecting the environment, maintaining business continuity, and ensuring all sensitive health and safety data is protected.
We are committed to providing a safe working environment for our people. We achieve this goal by working in ways that protect the safety, health, and welfare of all our employees, clinical trial patients, and visitors. Risk assessment is the basis of the safety management system, and we work to identify, mitigate, and monitor existing and emerging health or environment risks that may be associated with our business activities. We work to identify, mitigate, and monitor existing and emerging health or environment risks that may be associated with our business activities.
Fostering diversity, inclusion and belonging
We believe in a workplace culture that embraces diverse perspectives and empowers our team members to grow, whether at work, at home or in their communities. The diversity of our teams is critical to our success. As a global operation, we deliberately structure teams to be diverse to support the delivery of our customers’ clinical development programs across multiple geographies and communities.
Diversity, inclusion, and belonging (DIB) are fundamental to our culture and values. Diversity makes us more innovative and more creative, which helps us better serve our patients, our customers, and our communities. We recognize the critical importance of diversity in clinical trials ensuring all types of patients who will eventually receive therapies are represented on clinical trials, as well as offering clinical trials as a care option for those who may not otherwise have access to medical treatment.
We are committed to being a workplace where all employees are included and feel a sense of belonging. To achieve this, we acknowledge and celebrate our differences in gender, ethnicity, culture, and abilities. As a values-driven organization, respect for diverse points is foundational to how we interact with each other, as well as with customers, patients, and suppliers.
ICON’s approach to DIB is a key focus area. To reflect this, inclusion is one of our core values and our DIB strategy is now organized into four key ambitions:
ICON’s DIB practices and programs are viewed through the lens of our four DIB ambitions. Each ambition has sponsors from our executive leadership team to support and drive the agenda and provide leadership.
We believe in a workplace culture that embraces diverse perspectives and empowers our team members to grow — at work, at home and in their communities. The key areas of focus for our diversity, inclusion, and belonging agenda include talent management, country-level inclusion policies, rewards, training, and communications.
We established a Diversity, Inclusion & Belonging Steering Committee in 2019 which brings together individuals from across ICON to develop and execute work streams under each of the four ambitions, with a central team overseeing the overarching efforts. ICON has Diversity, Inclusion & Belonging advocates from across our global employee population to better understand local needs, build local presence and awareness, and to give a voice to every corner of the company across the world. These individuals play a key role in supporting the Diversity, Inclusion & Belonging Steering Committee and in aligning activities across the organization.
The DIB community groups we have at ICON are:
| | | | | | | | |
| DAWN: | The Disability Awareness Network is a community group striving to develop and foster a mindset towards creating an inclusive workplace and working environment where everyone is treated equally with respect and dignity, irrespective of any visible or hidden disabilities. |
| EmbRACE: | Supporting all race and ethnic backgrounds in creating an inclusive workplace culture. |
| NOW@ICON: | Networking Organization for Women at ICON is committed to inspiring and connecting current and potential leaders through an inclusive environment of targeted initiatives and supportive mentorship. |
| Pride@ICON: | Supporting LGBTQ+ colleagues and allies, ensuring that no matter where employees are in the world, our offices are a safe space where they are welcomed, respected, and valued. |
| SPACE: | Supporting Parents and Carers Everywhere to create a workplace where stepping out of careers due to personal commitments for a period is wholly accepted and not career limiting, and where stepping back into their career is an organic and positive process. |
As a testament to our commitment to diversity, ICON are aiming for gender parity at the VP level and above by 2025. As at February 24, 2023, women represent 44% of the positions at the VP level and above.
ICON is focused on building an inclusive culture where employees feel supported by a fair system supporting pay equity. We have a long track record of developing talent and filling vacancies through internal hires. Using best-in-class analysis, we conduct regular reviews of salary ranges to ensure fair pay, irrespective of gender, race, or ethnicity whilst considering legitimate business factors that explain differences such as performance, tenure, and experience.
We continuously monitor and seek to maintain pay equity for our employees. We have structured our pay principles so that individual differences not related to tenure, experience or performance criteria are not a factor in how we deliver rewards. ICON has made significant investments in organizational design structures, tools and education that uphold and support our pay principles.
We are committed to ensuring fair employment practices. For every jurisdiction in which we operate, we act in compliance with relevant laws relating to labor rights and labor relations as well as market competitive benefits. We believe in fair and equal treatment for all our people, without regard to gender, race, ethnicity, sexual orientation, marital status, physical or mental disability, age, pregnancy, veteran status, nationality, religion, or any other legally protected status. We do not tolerate our employees being subjected to physical, sexual, racial, psychological, verbal, or any other form of harassment. We encourage our
employees to report any issues of harassment or discrimination. We prohibit retaliation against any employee who rejects, protests, or complains about unlawful discrimination or harassment.
Human rights
ICON is committed to human rights and in 2021, ICON became a participant in the UN Global Compact (UNGC), signaling our commitment to uphold the UNGC’s 10 Principles, including those related to human rights across our global operations. Our business model and our policies, including our Global Code of Ethical Conduct and Global Supplier Code of Conduct, are intended to fully comply with applicable human rights legislation in the countries where we operate. Our zero-tolerance policy on forced labor, slavery, and human trafficking is defined clearly in these policies, which are available to employees, suppliers, customers, and the public.
We are opposed to forced labor, slavery, and human trafficking. We will not knowingly support or conduct business with any organization involved in such activities. We do not employ anyone below the minimum employment age in the jurisdictions in which we operate.
Our Global Supplier Code of Conduct incorporates the Pharmaceutical Supply Chain Initiative (PSCI) principles for responsible supply chain management, including for labor. Before doing business with ICON, suppliers must certify that they will comply with the ICON Global Supplier Code of Conduct or their own materially equivalent internal code, which includes human rights protections. We perform pre-engagement due diligence on our suppliers, including in relation to labor issues, which we support through periodic re-screening. We hold our suppliers accountable for meeting their contractual obligations. Contract non-compliance can result in termination of the business relationship with the supplier and exclusion from future business.
Ethics and Compliance
ICON’s commitment to ethics and integrity is embedded in our company values. We act with integrity and integrate ethical principles into our business practices and culture. ICON’s Global Code of Ethical Conduct (the Code) establishes our core principles and standards for honest, fair, and ethical behavior. This Code addresses the core values expected of our people in our internal interactions with each other as well as in external dealings with patients, customers, healthcare professionals, regulators, investors, vendors and other third parties.
Our Ethics and Compliance program builds on the principles established in the Code to define and drive business conduct consistent with company values and the laws, rules and regulations that apply to our business. The Ethics & Compliance Team (E&C) provides day-to-day independent oversight for the program. The team is independent of the business and reports to the Chief Administrative Officer and General Counsel (CAO). The CAO reports on the program to ICON’s executive leadership team, the Nominating, Sustainability and Governance Committee and the Board. The program supports all functional areas globally and is dedicated to the implementation of standardized global policies, procedures, training, guidance, communications, monitoring, investigations, issue management, assessing compliance-related risk and mitigations, and reporting to ensure the overall compliance program is effectively functioning.
ICON has incorporated a third-party system, Ethics Line, for employees and third parties to confidentially report ethics and compliance questions, as well as concerns, and to track reports through follow-up and resolution. These tools also provide visibility into our risks while highlighting opportunities to address them. ICON’s compliance and ethics program will continue to grow and evolve in response to changes in our business and in the global business climate.
All employees are required to complete mandatory training in key areas which support our values and our ways of working. The training incorporates the key principles of our policies and codes and includes interactive scenarios where applicable.
At ICON, we promote a Speak Up culture that encourages compliance, openness, and accountability without retaliation. The Speak Up Policy aims to support our culture and values and seeks to encourage the prompt reporting or surfacing of concerns or violations. Reported ethics concerns and other ethics and compliance-related data are reported via the CAO to the Board as appropriate.
Anti-bribery and Corruption
ICON is guided by the foundational principle that we do not tolerate bribery or any other form of corruption or fraud. Our anti-bribery and anti-corruption (ABAC) program is a key element of our Ethics and Compliance Program. ICON and all ICON directors, employees, consultants, agents and all third parties acting on ICON's behalf must act in compliance with international laws and regulations relating to bribery, corruption, and illicit payments, including the US Foreign Corrupt Practices Act and the UK Bribery Act 2010.
ICON maintains the ISO 37001:2016 certification for our Anti-Bribery Management System, which establishes the framework for the controls that prevent, detect and mitigate the risk of bribery. Our program is designed to ensure our compliance with anti-corruption laws, including due diligence, training, policies, procedures, and internal controls.
Bribery and corruption remain a business risk as we conduct our business across the globe and enter partnerships and collaborations. There is no certainty that all employees and third-party business partners (including our vendors, suppliers, agents, contractors, and other partners) will comply with anti-bribery laws. When working with third parties, we are committed to working with only those who embrace high standards of ethical behavior consistent with our own. Bribery and corruption risks are a focus of our third-party diligence and management process. We hold our suppliers accountable for meeting their contractual obligations with ICON, including commitments that are made with regard to our Global Supplier Code of Conduct and regulatory compliance. Contract non-compliance can result in termination of the business relationship with the supplier and exclusion from future business with ICON.
ICON's internal audit teams conduct ABAC Program audits. Internal Audit focuses on testing for compliance and design effectiveness of the overall ABAC Program. Internal Audit incorporates an assessment of ABAC measures in all audits, as appropriate. In this approach, bribery and corruption risks are incorporated into the risk assessment and scoping process of each audit.
Information Security and Privacy
Data privacy and information security are fundamental to our business and key to retaining customers, building investors’ trust, protecting patients, and complying with global and regional regulations. We recognize and respect that our customers, employees, patients, and all those who do business with us expect that we will protect their personal information in accordance with our legal obligations and policy commitments.
Our cybersecurity strategy and program protect our systems and data from an evolving threat landscape. The cybersecurity program, overseen by the Chief Information Officer (CIO), has the support of executive leadership and the Board, and we have invested heavily in cybersecurity technologies to protect our environment. Our processes and range of information security policies are certified to ISO 27001 and are independently audited twice annually. ICON also maintains the Cyber Essentials certification. During an acquisition process, we conduct security and privacy due diligence and risk assessments, implement policies, deliver employee training, and securely integrate IT systems.
Our Global Data Protection Policy regulates the processing of personal data in accordance with the applicable data protection laws of the countries where we operate, including Europe’s General Data Protection Regulation (GDPR) framework. This policy governs ICON’s and its employees’ obligations concerning the processing of personal data, including core privacy issues such as how we address data subject rights, data protection impact assessments and our obligations to maintain records of processing activities (ROPAs).
ICON has a separate Personal Data Incident and Breach Response Policy and Process that governs the management of personal data incidents and breaches within ICON. The policy requires incidents to be reported to ICON’s Global Data Protection Officer (DPO) and Privacy Team, who manage them in collaboration with relevant internal stakeholders (e.g., IT Security, Quality & Compliance), to ensure we comply with our legal and contractual obligations, including our reporting obligations. Our privacy program is overseen by the CAO.
Our people and partners play a critical role in safeguarding data. ICON has training in place for all employees and contingent workers on information security and privacy practices so that they understand their responsibilities with respect to data security and privacy.
Sustainable procurement
ICON maintains policies and practices to support responsible, sustainable, and ethical business practices. Our goal is to source from suppliers whose values align with our own, including suppliers who are committed to diversity and inclusion, and are socially and environmentally responsible and conscious.
We manage our suppliers through our Global Procurement department. The onboarding of new suppliers is completed through a centrally managed due diligence process. Environmental sustainability, bribery, and corruption risks are a focus of our collective third-party diligence and management process. We require our suppliers to abide by our Global Supplier Code of Conduct which incorporates the Pharmaceutical Supply Chain Initiative (PSCI) principles for Responsible Supply Chain Management and sets out our standards and expectations regarding:
•Ethics and compliance
•Labor and human rights
•Health and safety
•Environmental stewardship
ICON performs pre-engagement due diligence on our suppliers. This includes screening of sanctions lists, debarment, and adverse media. Suppliers are continuously monitored against sanctions and debarment lists and are periodically re-screened. Suppliers deemed higher risk are subject to enhanced due diligence and controls, which may include periodic training, auditing, and assessments.
We hold our suppliers accountable for meeting their contractual obligations, including commitments relating to our Global Supplier Code of Conduct and regulatory compliance. Contract non-compliance can result in termination of the business relationship and exclusion from future business with our company.
We have also engaged with EcoVadis, CDP and Supplier IO to assess our key suppliers and allow us to take ESG status into our decision making on vendor selection.
Sales and Marketing
Our marketing strategy is focused on building a differentiated brand position for ICON and supporting our business development efforts to develop and build relationships with pharmaceutical, biotechnology, medical device, and government and public health organizations. Our marketing activities are coordinated centrally to ensure a consistent and differentiated market positioning for ICON and to ensure all marketing efforts align to the overall strategic objectives of the business. Our business development teams are located throughout the Americas, Europe and Asia Pacific regions. Business development activities are carried out by account executives with assigned territories and global account directors supporting our large accounts. Specialized business development teams focus on growing each of our business areas. Collectively, our business development team, senior executives and project team leaders share responsibility for the maintenance of key client relationships. Our aim is to develop deeper relationships within our client base to gain repeat business and enable us new opportunities to penetrate into other therapeutic indications and adjacent service lines.
Competition
The CRO industry is fragmented, consisting of many small, niche service providers, a declining number of medium-sized providers and a smaller number of large CROs, including ICON, that are differentiated by the scale of their global operations, breadth of service portfolios and supporting technology infrastructure. The need to conduct complex research and access patients on a global basis is driving market share to these global CROs. When competing for large development programs, ICON competes primarily with IQVIA, PAREXEL, the PPD clinical research services brand of Thermo Fisher Scientific Inc., the Covance Drug Development business of LabCorp and Syneos Health. In some specific markets, for example biotech and mid-tier pharma, ICON may also compete against mid-tier CROs. Competition also exists for acquisition candidates in addition to competition for customers.
CROs generally compete on the basis of previous product experience, the ability to recruit patients on a global basis, the depth of therapeutic and scientific expertise, the strength of project teams, price and increasingly on the ability to apply new innovation that can drive significant time and cost savings throughout the development process. An evolving area of competition is the need to provide services that can help generate the evidence of the economic value of new treatments that payers and regulators require. This requires access to new data sources which includes information to support the identification of suitable investigator sites and patient populations as well as data on the value delivered by new products following marketing approval.
We believe that we compete favorably in all these areas and we continue to invest in our capabilities to ensure that we remain competitive in the future.
Customers
During the year ended December 31, 2022, revenue was earned from a wide range of clients. During the year ended December 31, 2022, 28.3% of our revenues were derived from our top five customers, with no one customer individually contributing more than 10% of our revenues during the period. Our largest customer represented a strategic partnership with a large global pharmaceutical company and contributed 8.8% of revenue for the year.
During the year ended December 31, 2021, 31.6% of our revenues were derived from our top five customers, with no one customer individually contributing more than 10% of our revenues during the period. Our largest customer represented a strategic partnership with a large global pharmaceutical company and contributed 8.0% of revenue for the year ended December 31, 2022.
During the year ended December 31, 2020, 39.1% of our revenues were derived from our top five customers, with one customers individually contributing more than 10% of our revenues during the period (12.1%). No other customer contributed more than 10% of our revenues during this period.
The loss of, or a significant decrease in business from one or more of these key customers could have a material adverse impact on our results of operations.
Unsatisfied Performance Obligation
Our unsatisfied performance obligation consists of contracted revenue yet to be earned from projects awarded by clients. At December 31, 2022 we had contracted unsatisfied performance obligations of $13.7 billion. We believe that our unsatisfied performance obligation as of any date is not necessarily a meaningful predictor of future results due to the potential for cancellation or delay of the projects included in the unsatisfied performance obligation, and no assurances can be given on the extent to which we will be able to realize this unsatisfied performance obligation as revenue.
Information Systems
Having access to accurate and timely information is critical in the management, delivery and quality of all aspects of drug development. ICON utilizes an extensive range of both on premise and cloud based applications that support its services including clinical trial design and planning, site and patient identification and recruitment, site start-up, patient consent, site payments, content management, clinical data analysis and real world evidence generation, customer relationship management (CRM), performance management, compliance and safety reporting and master data management. These solutions are to allow healthcare companies to manage, optimize and execute their clinical and commercial strategies in an orchestrated manner while addressing their regulatory obligations.
ICON has developed an informatics strategy built around key platforms including ICONIK and Health Cloud, web-based information platforms that enable the management, reporting, analysis and visualization of all data relating to drug development. The ICONIK and Health Cloud platforms collect, manage and standardize study data from multiple sources, including Electronic Data Capture (EDC), patient engagement, EMR/EHR, mobile health, Telehealth, Wearables, central laboratories, Symphony Health and Imaging platforms to provide a single view of study information. ICONIK and Health Cloud enable ICON to deliver services such as Risk Based Monitoring (RBM) which uses near-real time clinical data to drive monitoring visit schedules, enabling better decision making and the successful implementation of clinical trial strategies that significantly improve efficiency in clinical trials thereby reducing overall cost and time to market whilst better protecting patient safety.
In addition to managing clinical data, ICONIK and Health Cloud collect operational data, such as project management, clinical trials management system (CTMS) and metrics information to drive trial efficiency and transparency. Investigator data, such as payments, site details and performance, can also be incorporated. ICONIK and Health Cloud can be accessed via a portal that allows clients access to study-related information via a secure web-based environment. Data analysis from ICONIK and Health Cloud Informatics Hubs and CDRP allows us to enhance the design and delivery of our projects, through stronger engagement with investigators and patients.
Data management and collection is a key business process for Symphony Health through its Integrated Dataverse (IDV®) platform. Integrated Dataverse (IDV®) is a comprehensive and longitudinal source of healthcare data in the industry, bringing together our vast claims resources – medical, hospital, and prescription – with our rich point-of-sale prescription data, non-retail invoice data, and demographic data.
Firecrest, our site management and training technology, is another important component of our informatics strategy. Firecrest provides an on-line web-based portal to access visit by visit study guides which drive site performance and quality.
ICON also utilizes a range of enterprise applications that enable the delivery of our business services in a global environment. The focus is to provide ease of access and capture of study information for our staff and clients globally. Our current information systems are built on open standards and leading commercial business applications from vendors including Microsoft, Amazon, Oracle, Dell, SAS, Veeva, Dassault, Salesforce and BOX. IT expenditure is authorized by strict IT governance policies requiring senior level approval of all strategic IT expenditure based on defined business strategy and measurable business benefits.
In Clinical Operations, we have deployed a suite of software applications that assist in the management and tracking of our clinical trial activities. These software applications are both internally developed and commercially available applications from external vendors. These include a clinical trial management application that tracks all relevant data in a trial and automates all management and reporting processes. In our Data Management function, we have both leading clinical data management solutions including EDC and Clinical Data Warehouse solutions from external vendors as well as our proprietary EDC capability NEXTrials Prism eClinical. This allows us to guarantee the integrity of client data and provide consolidated information across client studies.
Within Clinical Operations, we also provide the ICON Digital Platform enabling the delivery of Decentralized and Hybrid Clinical Trial services, maximizing patient recruitment and retention and at the same time expanding access to diverse and remote patient populations. We develop strategic, flexible approaches that leverage clinical informatics, state-of-the-art technologies, and our global reach to maximize safety and efficiency and make data-driven decisions for every study.
In our clinical trials management area, Firecrest Clinical provides a comprehensive site performance management system that improves compliance, consistency and efficient execution of activities at investigative sites. The web-based solution enables accurate study information, including protocol information, training manuals and case report forms, to be rolled out
quickly and simultaneously to sites. Site behavior can then be tracked to ensure training is understood, procedures are being followed, timelines are met and study parameters are maintained. As well as meeting day to day operational requirements, this system feeds data into the ICONIK and Health Cloud platforms.
We provide interactive response technology (IXR) to enable centralized patient randomization, drug inventory management, patient diary collection, providing our clients with a fully flexible multi-channel data retrieval solution which can be utilized via telephone, internet browser or a mobile device. In our central laboratory business, we utilize a comprehensive suite of software, including a laboratory information management system (LIMS), a kit / sample management system and a web interface system to allow clients to review results online. Our Laboratory also utilizes IMRA, a web based laboratory review application that allows global access to the latest laboratory data on a study. IMRA facilitates detailed analysis of any trends, signals, alerts or patient-specific data on a real-time basis. ICON provides imaging services through the use of its internally developed MIRA platform and also utilizes Medidata’s Rave Commercial Imaging for collecting, managing and processing data to support its imaging capabilities.
ICON provides its Pharmacovigilance Services using Oracle’s ARGUS safety database. The system is FDA regulation 21 CFR Part 11 compliant and generates all the standard regulatory required reports, as well the periodic reports required to support operations.
ICON supports Population Pharmacokinetics and Pharmacokinetic Pharmacodynamic modeling through the use of its proprietary software NONMEM®. NONMEM® is a nonlinear mixed effects modeling tool that can be used to fit models to many different types of data. Statistical analysis with NONMEM® using the appropriate model helps pharmaceutical companies determine appropriate dosing strategies for their products, and increase their understanding of drug mechanisms and interactions. NONMEM® can also be accompanied with PDx-Pop proprietary software. PDx-Pop software is a graphical interface for NONMEM® which has its own automation methodology which expedites the iterative process of population pharmacokinetic modeling and analysis. ICON also utilizes PREDPP, which is a powerful package of subroutines handling population PK data, as well as general linear and nonlinear models, which can free the user from coding standard kinetic-type equations while simultaneously allowing complicated patient-type data to be easily analyzed. Finally, ICON utilizes NM-TRAN, which is a preprocessor allowing control and other needed inputs to be specified in a user-friendly manner.
ICON's configurable Real World Data “Evidence” platform is a fit-for-purpose solution to support and enhance observational research. The platform gathers disparate real world data assets into a common data model, provides analytics to support multiple audiences across the product lifecycle, and serves as a central repository and analytic platform for all Real World Data assets.
ICON’s Integrated Dataverse (IDV®) is one of the largest integrated repositories of healthcare data consisting of 280 million patient lives, 1.8 million prescribers and 16 thousand health plans provides powerful data, applications, analytics, and consulting to help companies gain deep insight into the pharmaceutical market. We transform data into decisions and give deeper insight into the relationships that sponsor brands have with the market by allowing a holistic view of the impacts of payer, prescriber, and patient behavior. Our proprietary Tokenisation technology Synoma® simplifies the anonymization, exchange and connection of industry data sources to provide an integrated view of a patient’s data.
The Company’s strategy of using technology to enhance our global processes is evident from our deployment of platforms like ICONIK, Health Cloud, Veeva EDMS/QMS (our global SOP Document Management system), iLearn and Cornerstone (our Web-based training delivery solution), ServiceNow (workflow and automation platform), Sailpoint (identity management and governance), and Pega and ARGUS (pharmacovigilance). The Electronic Trial Master File is delivered via ICON’s proprietary software ICOMaster or the Wingspan and PhlexGlobal software platforms.
The Company’s global finance operations utilize Oracle’s eBusiness suite, with the integrated Excel4Apps reporting tool, to serve the organization’s financial and project accounting requirements. Workday is used to fulfill our HR people management requirements. Our business development and contracting teams use Salesforce CRM.
Our IT systems are operated from two data center hubs in Europe (Dublin, Ireland; Groningen, Netherlands), four in North America (Philadelphia, Pennsylvania; Lenexa, Kansas; Charlottesville, Virginia; Dallas, Texas) and one in Asia (Singapore). These hubs reside within purpose-built data center facility locations. Other offices are linked to these hubs through a network managed by Verizon, a tier one global telecommunications provider. This network provides global connectivity for our applications and allows collaboration and communication using tools like Microsoft Teams, Cisco Jabber, Sharepoint and Box. Mobile staff can also access all systems via secure remote access facilities. A global corporate intranet portal provides access to all authorized data and applications for our internal staff as well as providing an internal platform for company-wide communication. IT systems are protected with robust information security controls which are independently audited biannually as part of maintaining ICON’s ISO27001:2013 certification.
ICON enables its patient site and data strategy through the services delivered via Accellacare and through our partnerships with Oncacare and Veradigm Allscripts. We also work with biopharmaceutical companies and other life science providers (e.g. medical devices companies) to develop and deploy bespoke stakeholder engagement solutions. ICON’s patient
engagement services enable site staff to engage directly with patients to help improve their disease and medication understanding through interventional and non-interventional support.
ICON provides molecular diagnostic laboratory capabilities that enables the development and commercialization of precision medicines in oncology.
ICON is the leading provider of Functional Service Provision (FSP) globally. Our team of operational, functional and therapeutic specialists offer a range of FSP models. We offer FSP solutions across all major functions from clinical monitoring and project management through data management, statistical programming and beyond. Our teams leverage either Sponsor or ICON’s IT Infrastructure to deliver services. Our team has extensive experience managing the migration of systems and can support system upgrades as part of the ramp-up phase.
ICON provides molecular diagnostic laboratory capabilities that enables the development and commercialization of precision medicines in oncology.
Other key innovations and new technologies include:
•FLEX ADVANTAGE, our interactive response technology platform (accessible through the web and web-enabled mobile devices) for managing patient randomization, investigator sites and clinical suppliers.
•PubsHub brings speed and efficiency to medical teams by delivering easy-to-use, web-based solutions that bridge process gaps for system harmonization across companies. ICON utilizes PubsHub to automate medical and scientific communications and publications management.
•The ICON Patient Engagement Platform features an easy to navigate, user friendly website enabling patients to explore new and ongoing studies available, opt-in and connect with their nearest clinical research site.
•One Search, an intuitive, integrated workflow and interrogation tool from ICON, enables access to multiple data sources and provides the visualization and tools necessary for optimum site identification based on ICON and industry data of capability, experience and performance. Scoring on enrollment performance, speed of start-up and quality supports better site selection.
•ADDPLAN for simulation and design of exploratory/pilot and confirmatory/pivotal adaptive clinical trials (ADDPLAN® DF (Dose Finder), ADDPLAN® Base, ADDPLAN® MC (Multiple Comparison) and ADDPLAN® PE (Population Enrichment)).
•AptivAdvantage which is an integrated platform comprising EDC, randomization and drug supply management specifically created for execution of adaptive clinical trials and used to deliver risk-based monitoring; and Aptiv Insite which is a novel approach to risk-based monitoring, using Verification by Statistical Sampling (VSS) to manage data quality and site related risks.
•Sample Inventory Management System (SIMS) is an interactive reporting module in ICOLabs for use by sponsors and study teams. It offers near real time, high level traceability of all patient samples in a clinical trial as they move from accessioning through disposition. SIMS provides detailed sample inventory reports and summaries of sample status and location with drill down capabilities. It helps locate samples more rapidly, particularly at critical study junctures.
•APECS for Investigator Payments ensures timely and accurate payments to sites for the work performed in the care and management of patients as they participate within clinical trials.
•The PredictivvTM platform is a fully integrated solution for designing, planning, managing and optimizing the execution of global clinical studies. Designed around a unified Sales Force platform that harmonizes data, processes, and people across every aspect of a clinical study, PredictivvTM enables unprecedented adaptive intelligence and decision support for the ever-increasing complexities of the clinical development process.
•EXACT™ allows users quickly to construct re-usable programs for data extraction, data transformation, statistical reporting and electronic publishing, in a visual environment with limited code writing. The EXACT™ system is used to simplify and automate the production of multiple Clinical Data Interchange Standards Consortium (CDISC) guidelines as well as tables, figures and listings in trial reports.
Contractual Arrangements
We are generally awarded projects based upon our responses to requests for proposals received from companies in the pharmaceutical, biotechnology and medical device industries, or through strategic partnership agreements.
Revenues on long term contracts are recognized based on an assessment of progress towards completion. Payment terms usually provide either for payments based on the delivery of certain identified milestones, units delivered or monthly payments, according to a contracted payment schedule over the life of the contract. Where there are changes in the scope of a trial or in the services to be provided by us, a change order or amendment is issued which may result either in an increase or decrease in the contract value. We also contract on a "fee-for-service" or "time and materials" basis.
Contract periods may range from several weeks to several years depending on the nature of the work to be performed. In most cases, an upfront portion of the contract fee is paid at the time the study or trial is started. The balance of the contract fee is generally payable in installments over the study or trial duration and may be based on the completion of certain performance targets or "milestones", on units delivered, or on a fixed monthly payment schedule. For instance, installment payments may be based on patient enrollment progress or delivery of the study database.
The progress towards completion for clinical service contracts is measured based on total project costs (direct fees are therefore inclusive of third party costs). Reimbursable costs include payments to investigators, travel and accommodation costs and various other expenses incurred over the course of the clinical trial which are fully reimbursable by the client. Reimbursable expenses are included within the contract and are invoiced on a monthly basis based on actual expenses incurred. Expenses incurred are determined by reference to activity.
As the currency in which contracts are priced can be different from the currencies in which costs relating to those contracts are incurred, we usually negotiate currency fluctuation clauses in our contracts which allow for price adjustments if changes in the relative value of those currencies exceed predetermined tolerances.
Most of our contracts are terminable immediately by the client with justifiable cause or with 30 to 90 days’ notice without cause. In the event of termination, we are usually entitled to all sums owed for work performed and expenses incurred through the notice of termination and certain costs associated with termination of the study. Termination or delay in the performance of a contract occurs for various reasons, including, but not limited to, unexpected or undesired results, production problems resulting in shortages of the drug, adverse patient reactions to the drug, the client's decision to de-emphasize a particular trial, inadequate patient enrollment or investigator recruitment.
Risk Management
Our Chief Executive Officer and other members of the executive management team are responsible for day-to-day risk management of the Company and our Board oversees management's activities through both the full Board and its committees. Our Chief Executive Officer and other members of the executive management team are members of ICON’s Quality and Risk Forum, which reviews risk. Our executive management team regularly reports to the Board and its Committees to ensure effective and efficient oversight of our activities and to assist in proper risk management and the ongoing evaluation of management controls. The Board oversees general business and market risk management, our Audit Committee oversees risk management with respect to financial statements, accounting and financial controls and our Compensation and Organization Committee oversees risk management with respect to our compensation plans, policies and procedures and our Nominating, Sustainability and Governance Committee oversees risks relating to ESG matters. Internal audit reports functionally and administratively to our Chief Financial Officer and directly to the Audit Committee. With respect to non-financial risk management, including cybersecurity, legal compliance, privacy and enterprise risk, the Board and its Committees receive updates from the appropriate executives on the primary risks facing the Company and the measures the Company is taking to mitigate such risks.
Government Regulation
The clinical investigation of new drugs is highly regulated by government agencies. The standard for the conduct of clinical research and development studies is Good Clinical Practice (“GCP”), which stipulates procedures designed to ensure the quality and integrity of data obtained from clinical testing and to protect the rights and safety of clinical subjects.
The FDA and other prominent regulators have promulgated regulations and guidelines that pertain to applications to initiate trials of products, the approval and conduct of studies, report and record retention, informed consent, applications for the approval of drugs and post-marketing requirements. Pursuant to these regulations and guidelines, service providers that assume the obligations of a drug sponsor are required to comply with applicable regulations and are subject to regulatory action for failure to comply with such regulations and guidelines. In the United States and Europe, the trend has been in the direction of increased regulation and enforcement by the applicable regulatory authority.
In providing services in the United States, we are obligated to comply with FDA requirements governing such activities. These include ensuring that the study is approved by an appropriate Independent Review Board (“IRB”) and Ethics Committee, obtaining patient informed consents, verifying qualifications of investigators, reporting patients’ adverse reactions to drugs and maintaining thorough and accurate records. We must maintain critical documents for each study for specified periods, and such documents may be reviewed by the study sponsor and the FDA.
The services we provide outside the United States are ultimately subject to similar regulation by the relevant regulatory authority. In addition, our activities in Europe are affected by the European Medicines Agency.
We must retain records for each study for specified periods for inspection by the client and by the applicable regulatory authority during audits. If we fail to comply with applicable regulations and guidelines, it could result in a material adverse effect. In addition, our failure to comply with applicable regulations and guidelines, depending on the extent of the failure, could result in fines, debarment, termination or suspension of ongoing research, the disqualification of data or litigation by clients, any of which could also result in a material adverse effect.
Potential Liability and Insurance
The nature of our business exposes us to potential liability including, but not limited to, potential liability for (i) breach of contract or negligence claims by our customers; and, (ii) third party (such as patients) claims in respect of our performance of services.
In addition, although we do not believe we are legally responsible for acts of third party investigators (physicians running trials), we could be subject to claims arising as a result of the actions of these investigators.
We try to reduce this potential liability by:
•Seeking contractual indemnification from customers in relation to certain activities. However, the terms and scope of indemnification varies from customer to customer and project to project and the performance of these indemnities is not secured. As a result, we bear the risk that indemnification may not be relevant or sufficient or that the indemnifying party may not have the financial ability to fulfill its indemnification obligations. This indemnification does not protect us against our own acts or omissions such as our negligence or where our performance does not reach the required contractual, industry or regulatory standard.
•Maintaining worldwide professional liability insurance. While we maintain the types and amounts of insurance we view as customary in the industries and countries in which we operate, there is no guarantee that we will continue to be able to maintain such insurance coverage on terms acceptable to us, if at all, or that the relevant policy will respond and provide cover when we want it to.
We could be materially adversely affected if ICON is required to pay damages or bear the costs of defending or settling any claim which a) is outside the scope of or in excess of a contractual indemnification provision, b) an indemnifying party does not fulfill its indemnification obligations, c) the claim is in excess of the level of our insurance coverage or d) the relevant circumstances are not covered by our insurance policies.
C.Organizational Structure
Details of the Company’s significant subsidiaries or entities under the Company's control at December 31, 2022 are as follows:
| | | | | | | | |
| Company | Country | Group ownership |
| ICON Clinical Research S.A. | Argentina | 100% |
| RPS Research S.A. | Argentina | 100% |
| ICON Clinical Research PTY Limited | Australia | 100% |
| Medpass International Pty Ltd | Australia | 100% |
| Pharmaceutical Research Associates Pty Limited | Australia | 100% |
| ICON Clinical Research Austria GmbH | Austria | 100% |
| RPS Research Austria GmbH | Austria | 100% |
| IMP-Logistics Bel, FLLC | Belarus | 100% |
| DOCS International Belgium N.V. | Belgium | 100% |
| Pharmaceutical Research Associates Belgium B.V. | Belgium | 100% |
| RPS Bermuda, Ltd. | Bermuda | 100% |
| ICON Pesquisas Clínicas LTDA. | Brazil | 100% |
| Pharmaceutical Research Associates Ltda. | Brazil | 100% |
| RPS do Brasil Serviços de Pesquisas LTDA. | Brazil | 100% |
| RPS China Inc. | British Virgin Islands | 100% |
| ICON Clinical Research EOOD | Bulgaria | 100% |
| Pharmaceutical Research Associates Bulgaria EOOD | Bulgaria | 100% |
| ICON Clinical Research (Canada) Inc. | Canada | 100% |
| 3065613 Nova Scotia Company | Canada | 100% |
| Pharmaceutical Research Associates ULC | Canada | 100% |
| Services de Recherche Pharmaceutique Srl | Canada | 100% |
| Oxford Outcomes LTD. | Canada | 100% |
| ICON Life Sciences Canada Inc. | Canada | 100% |
| ICON Chile Limitada | Chile | 100% |
| PRA Health Sciences Chile SpA | Chile | 100% |
| ICON Clinical Research (Beijing No.2) Co., Ltd | China | 100% |
| ICON Clinical Research (Beijing) Co., Ltd | China | 100% |
| PRA Health Sciences China, Inc. | China | 100% |
| PRA Health Sciences Colombia Ltda. | Colombia | 100% |
| Research Pharmaceutical Services Costa Rica, LTDA. | Costa Rica | 100% |
Ispitivanja ICON d.o.o
ICON Research Ltd. | Croatia | 100% |
| Pharm Research Associates d.o.o. za klinicka ispitivanja | Croatia | 100% |
| ICON Clinical Research s.r.o. | Czech Republic | 100% |
| Pharmaceutical Research Associates CZ, s.r.o. | Czech Republic | 100% |
| DOCS International Nordic Countries A/S | Denmark | 100% |
| Pharmaceutical Research Associates Denmark ApS | Denmark | 100% |
| RPS Egypt (Limited Liability Company) | Egypt | 100% |
| RPS Estonia OÜ | Estonia | 100% |
| DOCS International Finland Oy | Finland | 100% |
| Pharmaceutical Research Associates Finland Oy | Finland | 100% |
| ICON Clinical Research S.A.R.L. | France | 100% |
| Mapi Research Trust* | France | 100% |
| ReSearch Pharmaceutical Services France S.A.S. | France | 100% |
| IMP Logistics Georgia LLC | Georgia | 100% |
| | | | | | | | |
| Company | Country | Group ownership |
| Pharmaceutical Research Associates Georgia LLC | Georgia | 100% |
| Averion Europe GmbH | Germany | 100% |
| ICON Clinical Research Germany GmbH | Germany | 100% |
| ICON Clinical Research GmbH | Germany | 100% |
| Pharmaceutical Research Associates GmbH | Germany | 100% |
| Pharmaceutical Research Associates Greece A.E. | Greece | 100% |
| RPS Guatemala, S.A. | Guatemala | 100% |
| ICON Clinical Research Hong Kong Limited | Hong Kong | 100% |
| PRA Health Sciences (Hong Kong) Limited | Hong Kong | 100% |
| ICON Klinikai Kutató Korlátolt Felelősségű Társaság (ICON Clinical Research Limited Liability Company) | Hungary | 100% |
| Pharmaceutical Research Associates Magyarország Kutatás-Fejlesztési Korlátolt Felelősségű Társaság (Pharmaceutical Research Associates Hungary Research and Development Ltd.) | Hungary | 100% |
| RPS Iceland ehf. | Iceland | 100% |
| ICON Clinical Research India Private Limited | India | 100% |
| Pharmaceutical Research Associates India Private Limited | India | 100% |
| Accellacare Limited | Ireland | 100% |
| DOCS Resourcing Limited | Ireland | 100% |
| ICON (LR) Limited | Ireland | 100% |
| ICON Clinical Global Holdings Unlimited Company | Ireland | 100% |
| ICON Clinical International Unlimited Company | Ireland | 100% |
| ICON Clinical Research Limited | Ireland | 100% |
| ICON Clinical Research Property Development (Ireland) Limited | Ireland | 100% |
| ICON Clinical Research Property Holdings (Ireland) Limited | Ireland | 100% |
| ICON Holdings Clinical Research International Limited | Ireland | 100% |
| ICON Holdings Unlimited Company | Ireland | 100% |
| ICON Investments Five Unlimited Company | Ireland | 100% |
| ICON Investments Four Unlimited Company | Ireland | 100% |
| ICON Operational Financing Unlimited Company | Ireland | 100% |
| ICON Operational Holdings Unlimited Company | Ireland | 100% |
| Research Pharmaceutical Services (Outsourcing Ireland) Limited | Ireland | 100% |
| ICON Global Treasury Unlimited Company | Ireland | 100% |
| PRA Clinical Limited | Ireland | 100% |
| ICON Clinical Research Holdings (Ireland) Unlimited Company | Ireland | 100% |
| ICON Clinical Research Israel LTD. | Israel | 100% |
| Pharmaceutical Research Associates Israel Ltd. | Israel | 100% |
| Pharmaceutical Research Associates Italy S.r.l. | Italy | 100% |
| PRA Development Center KK | Japan | 100% |
| PRA Health Sciences KK | Japan | 100% |
| ICON Japan K.K. | Japan | 100% |
| ICON Investments Limited | Jersey | 100% |
| PRA Health Sciences Kenya Limited | Kenya | 100% |
| RPS Latvia SIA | Latvia | 100% |
| UAB RPS Lithuania | Lithuania | 100% |
| ICON Luxembourg S.à r.l. | Luxembourg | 100% |
| ICON CRO Malaysia SDN. BHD. | Malaysia | 100% |
| RPS Malaysia Sdn. Bhd. | Malaysia | 100% |
| ICON Clinical Research México, S.A. de C.V. | México | 100% |
| | | | | | | | |
| Company | Country | Group ownership |
| Pharmaceutical Research Associates Mexico S. de R.L. de C. V. | México | 100% |
| RPS Research México, S. de R.L. de C.V. | México | 100% |
| RPS Research Servicios, S. de R.L. de C.V. | México | 100% |
| DOCS International B.V. | Netherlands | 100% |
| Pharmaceutical Research Associates Group B.V. | Netherlands | 100% |
| PRA International Operations B.V. | Netherlands | 100% |
| ReSearch Pharmaceutical Services Netherlands B.V. | Netherlands | 100% |
| ICON Clinical Research (New Zealand) Limited | New Zealand | 100% |
| Pharmaceutical Research Associates New Zealand Limited | New Zealand | 100% |
| RPS Research Norway AS | Norway | 100% |
| RPS Panama Inc. | Panama | 100% |
| ICON Clinical Research Perú S.A. | Perú | 100% |
| RPS Perú S.A.C. | Perú | 100% |
| ICON Clinical Research Services Philippines, Inc. | Philippines | 100% |
| RPS Research Philippines, Inc. | Philippines | 100% |
| DOCS International Poland Sp. z o.o. | Poland | 100% |
| Symphony Clinical Research Sp zoo | Poland | 100% |
| Pharmaceutical Research Associates Sp. z o.o. | Poland | 100% |
| PRA International Portugal, Unipessoal, Lda. | Portugal | 100% |
| Research Pharmaceutical Services Puerto Rico, Inc. | Puerto Rico | 100% |
| ICON Clinical Research S.R.L. | Romania | 100% |
| Pharmaceutical Research Associates Romania S.R.L. | Romania | 100% |
| ICON Clinical Research (Rus) LLC | Russia | 100% |
| Joint Stock Company IMP Logistics | Russia | 100% |
| ICON Clinical Research d.o.o. Beograd | Serbia | 100% |
| Pharmaceutical Research Associates doo Belgrade | Serbia | 100% |
| ICON Clinical Research (Pte) Limited | Singapore | 100% |
| Mapi Life Sciences Singapore Pte. Ltd. | Singapore | 100% |
| Pharmaceutical Research Associates Singapore Pte. Ltd. | Singapore | 100% |
| ICON Clinical Research Slovakia, s.r.o. | Slovakia | 100% |
| Pharmaceutical Research Associates SK s.r.o. | Slovakia | 100% |
| PRA Pharmaceutical S A (Proprietary) Limited | South Africa | 100% |
| RPS Research South Africa (Proprietary) Limited | South Africa | 100% |
| Accellacare South Africa (PTY) LTD | South Africa | 100% |
| ICON Clinical Research Korea Yuhan Hoesa/ ICON Clinical Research Korea Ltd. | South Korea | 100% |
| Pharmaceutical Research Associates Korea Limited | South Korea | 100% |
| ICON Clinical Research España, S.L. | Spain | 100% |
| Pharmaceutical Research Associates España, S.A.U. | Spain | 100% |
| RPS ReSearch Ibérica, S.L.U. | Spain | 100% |
| RPS Spain S.L. | Spain | 100% |
| Accellacare España S.L. | Spain | 100% |
| DOCS International Sweden AB | Sweden | 100% |
| PRA International Sweden AB | Sweden | 100% |
| DOCS International Switzerland GmbH | Switzerland | 100% |
| ICON Clinical Research (Switzerland) GmbH | Switzerland | 100% |
| | | | | | | | |
| Company | Country | Group ownership |
| PRA Switzerland AG | Switzerland | 100% |
| ICON Clinical Research Taiwan Limited | Taiwan | 100% |
| Pharmaceutical Research Associates Taiwan, Inc. | Taiwan | 100% |
| ICON Clinical Research (Thailand) Limited | Thailand | 100% |
| RPS Research (Thailand) Co., Ltd. | Thailand | 100% |
| ICON Ankara Klinik Arastirma Dis Ticaret Anonim Sirketi | Turkey | 100% |
| Pra Turkey Sağlik Araştirma Ve Geliştirme Limited Şirketi | Turkey | 100% |
| DOCS Ukraine LLC | Ukraine | 100% |
| ICON Clinical Research LLC | Ukraine | 100% |
| IMP-Logistics Ukraine, LLC | Ukraine | 100% |
| Pharmaceutical Research Associates Ukraine, LLC | Ukraine | 100% |
| Accellacare UK Limited | United Kingdom | 100% |
| Aptiv Solutions (UK) Ltd | United Kingdom | 100% |
| DOCS International UK Limited | United Kingdom | 100% |
| ICON (LR) Limited | United Kingdom | 100% |
| ICON Clinical Research (U.K.) Limited | United Kingdom | 100% |
| ICON Clinical Research (U.K.) No. 2 Limited | United Kingdom | 100% |
| ICON Clinical Research (U.K.) No. 3 Limited | United Kingdom | 100% |
| ICON Clinical Research (U.K.) No. 4 Limited | United Kingdom | 100% |
| ICON Clinical Research (U.K.) No. 5 Limited | United Kingdom | 100% |
| ICON Development Solutions Limited | United Kingdom | 100% |
| ICON Investments (UK) Ltd | United Kingdom | 100% |
| Improving Treatments Limited | United Kingdom | 100% |
| Medeval Group Limited | United Kingdom | 100% |
| MeDiNova Lakeside Clinical Research Limited | United Kingdom | 100% |
| MeDiNova Merc (UK) Limited | United Kingdom | 100% |
| VSK (Kenilworth) Limited | United Kingdom | 100% |
| IMP Logistics UK Limited | United Kingdom | 100% |
| Pharm Research Associates (UK) Limited | United Kingdom | 100% |
| Sterling Synergy Systems Limited | United Kingdom | 100% |
| ICON Clinical Research Holdings (U.K.) Limited | United Kingdom | 100% |
| ICON Clinical Research (U.K.) No. 6 Limited | United Kingdom | 100% |
| RPS Global S.A. | Uruguay | 100% |
| RPS Latin America S.A | Uruguay | 100% |
| ICON Early Phase Services, LLC | USA | 100% |
| Pharmaceutical Research Associates, Inc. | USA | 100% |
| ClinStar LLC | USA | 100% |
| Nextrials, Inc. | USA | 100% |
| Pharmaceutical Research Associates CIS, LLC | USA | 100% |
| Pharmaceutical Research Associates Eastern Europe, LLC | USA | 100% |
| CRN North America, LLC | USA | 100% |
| ICON Clinical Research, LP | USA | 100% |
| Addplan, Inc. | USA | 100% |
| Beacon Bioscience, Inc | USA | 100% |
| C4 MedSolutions, LLC | USA | 100% |
| CHC Group, LLC | USA | 100% |
| | | | | | | | |
| Company | Country | Group ownership |
| CRN Holdings, LLC | USA | 100% |
| Global Pharmaceutical Strategies Group, LLC | USA | 100% |
| ICON Clinical Investments, LLC | USA | 100% |
| ICON Clinical Research LLC | USA | 100% |
| ICON Laboratory Services, Inc. | USA | 100% |
| ICON Tennessee, LLC | USA | 100% |
| ICON US Holdings Inc. | USA | 100% |
| MMMM Consulting, LLC | USA | 100% |
| MMMM Group, LLC | USA | 100% |
| MolecularMD Corp. | USA | 100% |
| PriceSpective LLC | USA | 100% |
| PubsHub LLC | USA | 100% |
| Care Innovations, Inc. | USA | 100% |
| Care Innovations, LLC | USA | 100% |
| CRI NewCo, Inc. | USA | 100% |
| CRI Worldwide, LLC | USA | 100% |
| International Medical Technical Consultants, LLC | USA | 100% |
| Parallel 6, Inc. | USA | 100% |
| PRA Early Development Research, Inc. | USA | 100% |
| PRA Health Sciences, Inc. | USA | 100% |
| PRA Holdings, Inc. | USA | 100% |
| PRA International, LLC | USA | 100% |
| PRA Receivables, LLC | USA | 100% |
| ReSearch Pharmaceutical Services, LLC | USA | 100% |
| ReSearch Pharmaceutical Services, Inc. | USA | 100% |
| Roy RPS Holdings LLC | USA | 100% |
| RPS Global Holdings, LLC | USA | 100% |
| RPS Parent Holding LLC | USA | 100% |
| Source Healthcare Analytics, LLC | USA | 100% |
| Sunset Hills, LLC | USA | 100% |
| Symphony Health Solutions Corporation | USA | 100% |
| Accellacare of Christie Clinic, LLC | USA | 100% |
| Clinical Resource Network, LLC | USA | 100% |
| DOCS Global, Inc. | USA | 100% |
| Managed Care Strategic Solutions, L.L.C. | USA | 100% |
| CRI International, LLC | USA | 100% |
| Accellacare of Charlotte, LLC | USA | 100% |
| Accellacare of Hickory, LLC | USA | 100% |
| Accellacare of Raleigh, LLC | USA | 100% |
| Accellacare of Rocky Mount, LLC | USA | 100% |
| Accellacare of Salisbury, LLC | USA | 100% |
| Accellacare of Wilmington, LLC | USA | 100% |
| Accellacare of Winston-Salem, LLC | USA | 100% |
| Accellacare US Inc. | USA | 100% |
| Complete Healthcare Communications LLC | USA | 100% |
| Complete Publication Solutions, LLC | USA | 100% |
| | | | | | | | |
| Company | Country | Group ownership |
| Accellacare of Charleston, LLC | USA | 100% |
| Accellacare of Bristol, LLC | USA | 100% |
| Lifetree Clinical Research, LC | USA | 100% |
| ICON Government and Public Health Solutions, Inc. | USA | 100% |
| *Mapi Research Trust is an association, its members are ICON Subsidiary entities. |
D. Description of Property
Our principal executive offices are located in South County Business Park, Leopardstown, Dublin, Republic of Ireland, where we own an office facility of approximately 15,000 square meters. We lease all other properties.
We maintain fifty-three offices in Europe; ten of our offices are in the UK, seven in Germany, five in The Netherlands, four in Spain, two in each of the Czech Republic, France, Hungary, Ireland, Poland, Russia, Sweden, Turkey and Ukraine, and one in each of Belarus, Belgium, Bulgaria, Georgia, Israel, Italy, Latvia, Romania, and Slovakia. We maintain thirty-three offices in North America; twenty-nine in the United States, two in Canada and two in Mexico. We have sixteen offices in Asia; five in China (including one in Hong Kong), three in India, two in each of Japan and Singapore, and one in each of South Korea, Taiwan, Thailand and The Philippines. We have two offices in Australia and one in New Zealand. We have five offices in South America; one in each of Argentina, Brazil, Chile, Columbia and Peru. We maintain one office in South Africa.
Item 4A. Unresolved Staff Comments.
Not applicable.
Item 5. Operating and Financial Review and Prospects.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis should be read in conjunction with our consolidated financial statements, accompanying notes and other financial information, appearing in Item 18. The consolidated financial statements have been prepared in accordance with U.S. GAAP. The information included in the discussion and analysis below provides details on the information for the years ended December 31, 2022 and December 31, 2021. Information related to the year ended December 31, 2020 has not been included. It can be found in the Company's filing of the form 20-F for the year ended December 31, 2021.
Overview
We are a CRO, providing outsourced development services on a global basis to the pharmaceutical, biotechnology and medical device industries. We specialize in the strategic development, management and analysis of programs that support all stages of the clinical development process - from compound selection to Phase I-IV clinical studies. Our vision is to be the healthcare intelligence partner of choice by delivering industry leading solutions and best in class performance in clinical development.
We believe that we are one of a select group of CROs with the expertise and capability to conduct clinical trials in most major therapeutic areas on a global basis and have the operational flexibility to provide development services on a stand-alone basis or as part of an integrated “full-service” solution. At December 31, 2022, we employed approximately 41,100 employees, in 111 locations in 53 countries. During the year ended December 31, 2022 we derived approximately 46.2%, 46.5% and 7.3% of our revenue in the United States, Europe, and the rest of the world, respectively.
Revenue consists of fees earned under contracts with third-party clients. In most cases, a portion of the contract fee is paid at the time the study or trial is started, with the balance of the contract fee generally payable in installments over the study or trial duration, based on the delivery of certain performance targets or milestones. Revenue from long term contracts is recognized on a proportional performance method based on the relationship between cost incurred and the total estimated costs of the trial or on a fee-for-service basis according to the particular circumstances of the contract. As is customary in the CRO industry, we contract with third party investigators in connection with clinical trials. Investigator costs and certain other third party costs are included in our assessment of progress towards completion and costs incurred in measuring revenue. Where these costs are reimbursed by clients, they are included in the total contract value recognized over time, based on our assessment of progress towards completion.
As the nature of our business involves the management of projects, the majority of which have a duration of one to four years, the commencement or completion of projects in a fiscal year can have a material impact on revenues earned with the relevant clients in such years. In addition, as we typically work with some, but not all divisions of a client, fluctuations in the number and status of available projects within such divisions can also have a material impact on revenues earned from such clients from year to year.
Termination or delay in the performance of an individual contract may occur for various reasons, including, but not limited to, unexpected or undesired results, production problems resulting in shortages of the drug, adverse patient reactions to the drug, the client’s decision to de-emphasize a particular trial or inadequate patient enrollment or investigator recruitment. In the event of termination the Company is usually entitled to all sums owed for work performed through the notice of termination and certain costs associated with the termination of the study. In addition, contracts generally contain provisions for renegotiation in the event of changes in the scope, nature, duration, or volume of services of the contract.
Our unsatisfied performance obligation comprises our assessment of contracted revenue yet to be earned from projects awarded by clients. At December 31, 2022 we had unsatisfied performance obligations of approximately $13.7 billion. We believe that our unsatisfied performance obligation as of any date is not necessarily a meaningful predictor of future results, due to the potential for cancellation or delay of the projects included in the unsatisfied performance obligation, and no assurances can be given on the extent to which we will be able to realize the unsatisfied performance obligation.
On July 1, 2021, ICON announced the completion of its Merger with PRA creating one of the world’s most advanced healthcare intelligence and clinical research organizations. The management's discussion and analysis below reflects the operating results of the Group for the year ended December 31, 2022 which incorporates the results of PRA and results in large variances when comparing to the year ended December 31, 2021. The results of PRA prior to July 1, 2021 are not reflected. Where applicable, management have included commentary on specific one-time charges related to the Merger in order to provide an understanding of the normal operations of the Group.
Although we are domiciled in Ireland, we report our results in U.S. dollars. As a consequence, the results of our non-U.S. based operations, when translated into U.S. dollars, could be materially affected by fluctuations in exchange rates between the U.S. dollar and the currencies of those operations.
In addition to translation exposures, we are also subject to transaction exposures because the currency in which contracts are priced can be different from the currencies in which costs relating to those contracts are incurred. Our operations in the United States are not materially exposed to such currency differences as the majority of our revenues and costs are in U.S. dollars. However, outside the United States the multinational nature of our activities means that contracts are usually priced in a single currency, most often U.S. dollars or euros, while costs arise in a number of currencies, depending, among other things, on which of our offices provide staff for the contract and the location of investigator sites. Although many such contracts benefit from some degree of natural hedging, due to the matching of contract revenues and costs in the same currency, where costs are incurred in currencies other than those in which contracts are priced, fluctuations in the relative value of those currencies could have a material effect on our results of operations. We regularly review our currency exposures.
As we conduct operations on a global basis, our effective tax rate has depended and will depend on the geographic distribution of our revenue and earnings among locations with varying tax rates. Our results therefore may be affected by changes in the tax rates of the various jurisdictions. In particular, as the geographic mix of our results of operations among various tax jurisdictions changes, our effective tax rate may vary significantly from period to period.
A. Operating Results
The following table sets forth, for the periods indicated, certain financial data as a percentage of revenue and the percentage change in these items compared to the prior comparable period. The trends illustrated in the following table may not be indicative of future results.
| | | | | | | | | | | |
| Year Ended December 31, |
| 2022 | 2021 | 2022 |
| Percentage of Revenue | Percentage Increase/(Decrease) |
| Revenue | 100.0 | % | 100.0 | % | 41.2 | % |
| Costs and expenses: | | | |
| Direct costs | 71.4 | % | 72.5 | % | 39.1 | % |
| Selling, general and administrative expense | 10.0 | % | 10.7 | % | 33.0 | % |
| Depreciation | 1.4 | % | 1.4 | % | 41.0 | % |
| Amortization | 6.0 | % | 4.4 | % | 93.4 | % |
| Transaction and integration-related expenses | 0.5 | % | 3.6 | % | (80.0) | % |
| Restructuring | 0.4 | % | 0.5 | % | 0.1 | % |
| Income from operations | 10.3 | % | 6.9 | % | 110.1 | % |
Year ended December 31, 2022 compared to year ended December 31, 2021
Revenue
| | | | | | | | | | | | | | |
| Year Ended
December 31, | Change |
| (dollars in thousands) | 2022 | 2021 | $ | % |
| Revenue | $ | 7,741,386 | | $ | 5,480,826 | | $ | 2,260,560 | | 41.2 | % |
Revenue for the year ended December 31, 2022 increased by $2,260.6 million, or 41.2%, to $7,741.4 million, compared to $5,480.8 million for the year ended December 31, 2021. Revenue increased by 45.4% in constant currency terms. The increase in revenues in the year ended December 31, 2022 is due to the Merger and continued organic growth across the Company's markets.
During the year ended December 31, 2022 the Company derived approximately 46.2%, 46.5% and 7.3% of our revenue in the United States, Europe and Rest of World respectively. Revenues from our top five customers amounted to $2,189.6 million in the year ended December 31, 2022 compared to $1,733.1 million in the year ended December 31, 2021 or 28.3% and 31.6% respectively.
Revenue in Ireland increased by $618.7 million in the year ended December 31, 2022, to $1,984.6 million, compared to $1,365.9 million for the year ended December 31, 2021. Revenue in Ireland during the year ended December 31, 2022 increased by 45.3% compared to an overall increase in Group revenue of 41.2%. Revenue in Ireland is principally a function of our global contracting model.
Revenue in the Rest of Europe increased by $442.8 million or 37.7%, to $1,618.4 million, compared to $1,175.5 million for the year ended December 31, 2021. Revenue in the U.S. increased by $993.6 million or 38.5%, to $3,574.6 million, compared to $2,581.0 million for the year ended December 31, 2021. Revenue in our Rest of World (‘Other’) region increased by $205.5 million or 57.3%, to $563.9 million, compared to $358.4 million for the year ended December 31, 2021. Revenue has increased across all regions due to the completion of the Merger and continued organic growth across the Company's markets.
Direct costs
| | | | | | | | | | | |
| Year Ended
December 31, | |
| (dollars in thousands) | 2022 | 2021 | Change |
| Direct costs | $ | 5,527,045 | | $ | 3,972,612 | | $ | 1,554,433 | |
| % of revenue | 71.4 | % | 72.5 | % | 39.1 | % |
Direct costs for the year ended December 31, 2022 increased by $1,554.4 million, or 39.1%, to $5,527.0 million, compared to $3,972.6 million for the year ended December 31, 2021. Direct costs consist primarily of investigator and other reimbursable costs, compensation, associated fringe benefits and share based compensation expense for project-related employees and other direct project driven costs. The increase in direct costs during the year ended December 31, 2022 arose due to an increase in headcount and an increase in personnel related expenditure of $926.5 million combined with an increase in other direct project related costs of $179.7 million, an increase in laboratory costs of $10.0 million and an increase in travel related costs of $5.6 million. There was an increase in third party investigator and other reimbursable costs of $434.5 million. The decrease in direct costs as a percentage of revenue reflect the different activity mix for the year ended December 31, 2022 compared to the year ended December 31, 2021, as well as the realization of integration synergies.
Selling, general and administrative expenses
| | | | | | | | | | | |
| Year Ended
December 31, | |
| (dollars in thousands) | 2022 | 2021 | Change |
| Selling, general and administrative expenses | $ | 778,753 | | $ | 585,330 | | $ | 193,423 | |
| % of revenue | 10.0 | % | 10.7 | % | 33.0 | % |
Selling, general and administrative expenses for the year ended December 31, 2022 increased by $193.4 million, or 33.0%, to $778.8 million, compared to $585.3 million for the year ended December 31, 2021. Selling, general and administrative expenses comprise primarily of compensation, related fringe benefits and routine share based compensation expense for non-project-related employees, recruitment expenditures, professional service costs, advertising costs and all costs related to facilities and information systems. As a percentage of revenue, selling, general and administrative expenses decreased to 10.0% of revenue during the year ended December 31, 2022, compared to 10.7% of revenue for the year ended December 31, 2021. During the year ended December 31, 2022, the increase in selling, general and administrative expenses relates to an increase in general overhead costs of $47.3 million, an increase of $42.9 million in facilities related costs, an increase of $88.0 million in personnel related expenditure, an increase of $24.0 million in professional fees and an increase of $3.7 million in marketing fees. These increases were partly offset by foreign exchange gains for the year ended December 31, 2022 of $26.0 million ($14.3 million for the year ended December 31, 2021) driven mainly by the movement in the Euro to U.S. dollar exchange rate. Finally, share based compensation expense recognized during the years ended December 31, 2022 and December 31, 2021 were $70.5 million and $133.8 million, respectively. Share based compensation expenses are part of personnel related expenditure in direct costs and selling, general and administrative expenses.
Depreciation and amortization
| | | | | | | | | | | |
| Year Ended
December 31, | |
| (dollars in thousands) | 2022 | 2021 | Change |
| Depreciation | $ | 106,426 | | $ | 75,484 | | $ | 30,942 | |
| % of revenue | 1.4 | % | 1.4 | % | 41.0 | % |
| Amortization | $ | 463,087 | | $ | 239,503 | | $ | 223,584 | |
| % of revenue | 6.0 | % | 4.4 | % | 93.4 | % |
Depreciation expense for the year increased by $30.9 million or 41.0%, to $106.4 million, compared to $75.5 million for the year ended December 31, 2021. The depreciation charge reflects the impact of the acquisition and investments in facilities, information systems and equipment supporting the Company’s continued growth. As a percentage of revenue, the depreciation expense remained consistent at 1.4% of revenues, for the year ended December 31, 2022 and December 31, 2021. The increase in the depreciation charge for the years ended December 31, 2022 is due to the increase in our depreciable asset base acquired through the Merger as well as additional spend related to computer hardware and software.
Amortization expense for the year ended December 31, 2022 increased by $223.6 million or 93.4%, to $463.1 million, compared to $239.5 million for the year ended December 31, 2021. The amortization expense represents the amortization of intangible assets acquired during business combinations. The increase in amortization expense for the year reflects the amortization of newly acquired intangibles resulting from the Merger. As a percentage of revenue, the amortization expense increased to 6.0% for the year ended December 31, 2022, compared to 4.4% of revenue for the year ended December 31, 2021.
Restructuring, transaction and integration-related expenses associated with the Merger
| | | | | | | | | | | |
| Year Ended
December 31, | |
| (dollars in thousands) | 2022 | 2021 | Change |
| Transaction and integration-related expenses | $ | 39,695 | | $ | 198,263 | | $ | (158,568) | |
| % of revenue | 0.5 | % | 3.6 | % | (80.0) | % |
| Restructuring costs | $ | 31,143 | | $ | 31,105 | | $ | 38 | |
| % of revenue | 0.4 | % | 0.5 | % | 0.1 | % |
During the year ended December 31, 2022, the Company incurred $70.8 million for restructuring, transaction and integration-related expenses primarily associated with the Merger. The charge includes transaction and integration costs of $39.7 million associated with advisory costs, severance arrangements, retention agreements and ongoing integration activities.
The Company has also undertaken a restructuring program following the announcement of the Merger to review its global office footprint, optimize its locations to best fit the requirements of the Company and reorganize its workforce to drive future growth. This program has resulted in a charge of $31.1 million in the year ended December 31, 2022. In the year ended December 31, 2021, a restructuring charge of $31.1 million was recognized.
We expect to incur some additional expenses associated with the Merger; however, the timing and the amount of these expenses depends on various factors such as, but not limited to, the execution of integration activities and the aggregate amount of synergies we achieve from these activities.
Income from operations
| | | | | | | | | | | |
| Year Ended
December 31, | |
| (dollars in thousands) | 2022 | 2021 | Change |
| Income from operations | $ | 795,237 | | $ | 378,529 | | $ | 416,708 | |
| % of revenue | 10.3 | % | 6.9 | % | 110.1 | % |
Income from operations increased by $416.7 million, or 110.1%, to $795.2 million, compared to $378.5 million for the year ended December 31, 2021. As a percentage of revenue, income from operations increased to 10.3% of revenues compared to 6.9% of revenues for year ended December 31, 2021.
Income from operations in Ireland increased to $218.1 million compared to $132.0 million for the year ended December 31, 2021.
In the Rest of Europe region, income from operations increased to $253.8 million compared to $177.9 million for the year ended December 31, 2021. As a percentage of revenues, income from operations in the Rest of Europe region increased to 15.7% compared to 15.1% for the year ended December 31, 2021.
In the U.S. region, income from operations increased by $215.7 million, to $254.8 million, compared to $39.1 million for the year ended December 31, 2021. As a percentage of revenues, income from operations in the U.S. region increased to 7.1% compared to 1.5% for the year ended December 31, 2021.
In other regions, income from operations increased by $38.9 million to $68.5 million compared to $29.6 million for the year ended December 31, 2021. As percentage of revenues, income from operations in the other regions increased to 12.1% compared to 8.3% for the year ended December 31, 2021.
Interest income and expense
| | | | | | | | | | | | | | |
| Year Ended
December 31, | Change |
| (dollars in thousands) | 2022 | 2021 | $ | % |
| Interest income | $ | 2,345 | | $ | 574 | | $ | 1,771 | | 308.5 | % |
| Interest expense | $ | (229,731) | | $ | (182,423) | | $ | (47,308) | | 25.9 | % |
Interest expense increased to $229.7 million compared to $182.4 million for the year ended December 31, 2021. The increase in the year reflects the impact of financing costs associated with the Merger and the average gross amount of outstanding borrowing and the impact of rising interest rates during the year. Interest income for the year ended December 31, 2022 increased to $2.3 million, compared to $0.6 million for the year ended December 31, 2021.
Income tax expense
| | | | | | | | | | | | | | |
| Year Ended
December 31, | Change |
| (dollars in thousands) | 2022 | 2021 | $ | % |
| Income tax expense | $ | 59,411 | | $ | 41,334 | | $ | 18,077 | | 43.7 | % |
| Effective income tax rate | 10.5 | % | 21.0 | % | | |
| | | | |
| | | | |
Provision for income taxes for the year increased to $59.4 million compared to $41.3 million for the year ended December 31, 2021. The Company’s effective tax rate for the year ended December 31, 2022 was 10.5% compared to 21.0% for the year ended December 31, 2021 primarily due to the full year amortization impact of acquired intangible assets and non-deductive expenditures incurred in 2021 related to the Merger. The Company’s effective tax rate remains principally a function of the distribution of pre-tax profits amongst the territories in which it operates.
B. Liquidity and Capital Resources
The CRO industry is generally not capital intensive. The Group’s principal operating cash needs are payment of salaries, office rents, travel expenditures and payments to investigators. Investing activities primarily reflect capital expenditures for facilities and information systems enhancements, the purchase and sale of short term investments and acquisitions. Financing activities primarily reflect the servicing of the Company's external debt.
Our clinical research and development contracts are generally fixed price with some variable components and range in duration from a few weeks to several years. Revenue from contracts is generally recognized as income on the basis of the relationship between costs incurred and the total estimated contract costs. The cash flow from contracts typically consists of a small down payment at the time the contract is entered into, with the balance paid in installments over the contract duration, in some cases on the achievement of certain milestones. Therefore, cash receipts do not correspond to costs incurred and revenue recognized on contracts.
Cash and cash equivalents and net borrowings
| | | | | | | | | | | | | | | | | | | | |
| Balance December 31, 2021 | (Drawn
down)/
repaid | Net cash inflow/ (outflow) | Other non-cash adjustments | Effect of exchange rates | Balance December 31, 2022 |
| dollars in thousands |
| Cash and equivalents | | | | | | |
| Cash and cash equivalents | 752,213 | | — | | (446,725) | | — | | (16,720) | | 288,768 | |
| Available for sale investments | 1,712 | | — | | 1 | | — | | — | | 1,713 | |
| | | | | | |
| | | | | | |
| | | | | | |
| |
| | | | | | |
| | | | | | |
| Senior Secured Credit Facilities & Senior Secured Notes | (5,436,312) | | 800,000 | | — | | (17,875) | | — | | (4,654,187) | |
| | | | | | |
| | | | | | |
| Net cash and cash equivalents and borrowings | (4,682,387) | | 800,000 | | (446,724) | | (17,875) | | (16,720) | | (4,363,706) | |
The Company’s cash and cash equivalents and available for sale investments at December 31, 2022 amounted to $290.5 million compared with cash and available for sale investments of $753.9 million at December 31, 2021. The Company’s
cash and available for sale investments at December 31, 2022 comprised cash and cash equivalents of $288.8 million and available for sale investments of $1.7 million. The Company’s cash and available for sale investments at December 31, 2021 comprised cash and cash equivalents of $752.2 million and available for sale investments of $1.7 million.
In conjunction with the completion of the Merger Agreement, on July 1, 2021, ICON entered into a Senior Secured Credit Facilities providing for a senior secured term loan facility of $5,515.0 million and a senior secured revolving loan facility in an initial aggregate principal amount of $300.0 million. The senior secured term loan facility will mature in July 2028 and the revolving loan facility will mature in July 2026. At December 31, 2022 and December 31, 2021, no amounts were outstanding under the revolving loan facility with the exception of $4.5 million letters of credit given to landlords to guarantee lease arrangements.
In addition to the Senior Secured Credit Facilities, on July 1, 2021, the Company, issued $500.0 million in aggregate principal amount of 2.875% senior secured notes in a private offering. The senior secured notes will mature on July 15, 2026.
On March 31, 2022 the Company repaid $300.0 million of the senior secured term loan facility and made a quarterly interest payment of $35.1 million. On June 30, 2022 the Company repaid $100.0 million of the senior secured term loan facility and made a quarterly interest payment of $39.4 million. On September 30, 2022 the Company repaid $200.0 million of the senior secured term loan facility and made a quarterly interest payment of $53.6 million. On December 30, 2022 the Company repaid $200.0 million of the senior secured term loan facility and made a quarterly interest payment of $66.1 million.
The Company has contractual liabilities for lease arrangements of $189.7 million which will be predominantly settled over the next five year period through cash payments.
Cash flows
Net cash from operating activities
Net cash provided by operating activities decreased by $265.8 million to $563.3 million for the year ended December 31, 2022 as compared to net cash provided by operating activities of $829.1 million for the year ended December 31, 2021. The decrease in net cash provided by operating activities of $265.8 million is due to changes in working capital of $685.2 million, offset by increases in cash inflows from net income of $419.4 million.
The change in working capital is primarily attributable to increases in Accounts Receivable of $534.2 million and Unbilled Revenue of $314.9 million, offset by increases in Unearned Revenue of $262.1 million. These changes result from differences in timing of revenue recognition and billing on clinical trials. The days’ revenue outstanding at December 31, 2022 was 54 days compared to 31 days at December 31, 2021. Cash generated from working capital and days’ revenue outstanding may be positively or negatively impacted by, amongst others, the scheduling of contractual milestones over a study or trial duration, the achievement of a particular milestone during the period, the timing of receipt of invoices from third parties for reimbursable costs and the timing of cash receipts from customers. Contract fees are generally payable in installments based on the achievement of certain performance targets or “milestones” (e.g. target patient enrollment rates, clinical testing sites initiated or case report forms completed), such milestones being specific to the terms of each individual contract, while revenues on contracts are recognized as contractual obligations are performed. A decrease in the number of days’ revenue outstanding during a period will result in cash inflows to the Company while an increase in days revenue outstanding will lead to cash outflows.
Net cash used in investing activities
Net cash used in investing activities was $145.9 million for the year ended December 31, 2022 compared to net cash used in investing activities of $6,024.2 million for the year ended December 31, 2021. Net cash used in investing activities for the year ended December 31, 2022 were primarily related to cash outflows of $142.2 million for capital expenditures made mainly relating to investment in facilities and IT infrastructure and $5.6 million in relation to investments in long-term equity.
In the year ended December 31, 2021, net cash used in investing activities of $6,024.2 million was largely attributable to the cash element of the Merger consideration of $5,914.5 million (net of cash acquired). During the year, capital expenditure of $93.8 million was made mainly related to the investment in facilities and IT infrastructure. Further cash outflow of $2.5 million was made in respect of the Company's investment in Oncacare, a loan of $10.0 million was provided to Oncacare and $4.1 million in relation to investments in long-term equity.
Net cash used in financing activities
Net cash used in financing activities amounted to $864.2 million for the year ended December 31, 2022 compared with net cash provided by financing activities of $5,114.7 million for the year ended December 31, 2021. In the year ended December 31, 2022, the Company repaid external financing of $800.0 million and repurchased shares to the value of $100.0 million. This was offset by $35.8 million that was received by the Company from the exercise of share options.
In the year ended December 31, 2021, the Company drew down external financing of $6,015.0 million to fund the completion of the Merger. This was offset by debt discount and certain debt issue costs of $109.9 million. As part of the external
financing, the Company paid financing professional fees of $30.3 million and made payments of principal on the external debt of $513.8 million. The Company also repaid the 2020 Senior Notes on July 1, 2021, including early repayment charges, totaling $364.0 million. During the year ended December 31, 2021, $118.6 million was received by the Company from the exercise of share options.
Net cash outflow
As a result of these cash flows, cash and cash equivalents decreased by $463.4 million for the year ended December 31, 2022 compared to a decrease of $88.1 million for the year ended December 31, 2021.
C. Research and development, patents and licenses
ICON plays a critical role in new drug development by undertaking activities in each of the different stages of the drug development process. Clinical trials result in an advancement in the field of medical science as they establish the safety and efficacy of new drugs, thus resolving scientific uncertainty. As one of a number of world leaders in clinical research and commercialization, ICON is a trusted partner for pharmaceutical and medical device companies in helping them to accelerate the development of drugs and devices that save lives and improve the quality of life. ICON's role in ensuring that the trial design is scientifically valid is a crucial part of the design and involves scientists, medical doctors and biostatisticians. ICON works with the sponsors in designing the conduct of the clinical research trial. ICON's role of conducting clinical trials is an integral part of the research and development process leading ultimately to a decision as to whether or not each drug is safe for human consumption, has the desired effect on targeted diseases and the best means of delivering that drug to the patient.
D. Trend information
Other than as disclosed elsewhere in this annual report, we are not aware of any trends, uncertainties, demands, or commitments or events since December 31, 2022 that are reasonably likely to have a material adverse effect on our revenues, income, profitability, liquidity or capital resources, or that would cause the reported financial information in this annual report to be not necessarily indicative of future operating results or financial conditions.
E. Critical Accounting Estimates
Note 2 to the audited consolidated financial statements provided elsewhere in this Form 20-F describes the significant accounting policies used in the preparation of the consolidated financial statements. The preparation of our consolidated financial statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported amounts of revenues and expenses during the period. We base our estimates and judgments on historical experience and on the other factors that we believe are reasonable under current circumstances. Actual results may differ from these estimates if these assumptions prove to be incorrect or if conditions develop other than as assumed for the purposes of such estimates. The following is a discussion of the critical accounting estimates and judgments used by management. The application of these critical accounting estimates is discussed with the Audit Committee of the Board of Directors.
Revenue Recognition - Clinical Trial Services
Clinical trial services are a single performance obligation satisfied over time i.e. the full-service obligation in respect of a clinical trial (including those services performed by investigators and other parties) is considered a single performance obligation. Promises offered to the customer are not distinct within the context of the contract. We have concluded that ICON is the contract principal in respect of both direct services and in the use of third parties (principally investigator services) that support the clinical research project. The transaction price is determined by reference to the contract or change order value (total service revenue and pass-through/ reimbursable expenses) adjusted to reflect a realizable contract value. An assessment of the realizable contract value is judgmental in nature. The realizable value assessment is updated at each reporting period, having regard to (i) contract terms and (ii) customer experience.
Revenue is recognized on a percentage completion basis as the single performance obligation is satisfied. The progress towards completion for clinical service contracts is measured therefore based on an input measure being total project costs (inclusive of third party costs) at each reporting period. Measurement of the progress towards completion involves judgment and estimation. Assessment of completion requires an evaluation of labor and related time cost incurred at the reporting date and third party costs incurred at the reporting date. The assessment of third party costs incurred (principally investigator costs) requires a review of activity performed and recorded by the third party services providers. The timing of payments to third parties in respect of cost incurred reflect invoicing by third parties. The timing difference between the activity performed and receipt of invoices from third parties may result in significant accrued amounts at reporting periods.
The assessment of progress towards completion also requires an up to date evaluation of the forecast costs to complete in respect of these projects. Given the long-term nature of the clinical trials, and the complex nature of those trials, the forecast costs to complete (being internal direct costs and costs that will be incurred by third parties (principally investigators)) is judgmental. Forecast time (and related costs) is determined by reference to (i) contract terms and (ii) past experience. Forecast third party costs to complete are determined by project by reference to (i) contract terms and (ii) past experience.
Business Combinations
We use the acquisition method to account for business combinations, and accordingly, the identifiable assets acquired, the liabilities assumed and any non-controlling interest in the acquiree are recorded at their estimated fair values on the date of the acquisition. We use significant judgments, estimates and assumptions in determining the estimated fair value of assets acquired, liabilities assumed and non-controlling interest. Estimated fair values were based on various valuation methodologies, including an income approach using primarily discounted cash flow techniques for the customer relationships intangible assets. The aforementioned income methods utilize management's estimates of future operating results and cash flows discounted using a weighted-average cost of capital that reflects market participant assumptions. We have recorded and allocated to our reporting units the excess of the purchase price over the fair value of the net assets acquired, known as goodwill.
Recoverability of Goodwill and Long-Lived Assets
Goodwill
The Company assesses its goodwill for impairment annually or when events or circumstances indicate a possible impairment. The annual impairment test for goodwill includes an option to perform a qualitative assessment of whether it is more likely than not that a reporting unit's fair value is less than its carrying value. Reporting units are businesses with discrete financial information that is available and reviewed by management. If the Company determines that it is more likely than not that the fair value of a reporting unit is less than its carrying value, then the Company performs the quantitative goodwill impairment test. The Company may also choose to bypass the qualitative assessment for any reporting unit in its goodwill assessment and proceed directly to performing the quantitative assessment.
In performing a qualitative assessment, the Company considers relevant events and circumstances for each reporting unit, including (i) current year results, (ii) financial performance versus management’s annual and multi-year strategic plans, (iii) changes in the reporting unit carrying value since prior year, (iv) industry and market conditions in which the reporting unit operates, (v) macroeconomic conditions, including discount rate changes, and (vi) changes in products or services offered by the reporting unit. Based on the results of the qualitative assessment, if the Company concludes that it is not more likely than not that the fair value of the reporting unit is less than its carrying values of the reporting unit, then no quantitative assessment is performed.
A quantitative assessment includes the estimation of the fair value of each reporting unit as compared to the carrying value of the reporting unit. The Company estimates the fair value of a reporting unit using both income-based and market-based valuation methods. The income-based approach is based on the reporting unit's forecasted future cash flows that are discounted to the present value using the reporting unit's weighted average cost of capital. For the market-based approach, the Company utilizes a number of factors such as publicly available information regarding the market capitalization of the Company as well as operating results, business plans, market multiples, and present value techniques. Based upon the range of estimated values developed from the income and market-based methods, the Company determines the estimated fair value for the reporting unit. If the estimated fair value of the reporting unit exceeds the carrying value, the goodwill is not impaired and no further review is required.
The income-based fair value methodology requires management's assumptions and judgments regarding economic conditions in the markets in which the Company operates and conditions in the capital markets, many of which are outside of management's control. At the reporting unit level, fair value estimation requires management's assumptions and judgments regarding the effects of overall economic conditions on the specific reporting unit, along with assessment of the reporting unit's strategies and forecasts of future cash flows, terminal growth rates and discount rates.
Under the market-based fair value methodology, judgment is required in evaluating market multiples and recent transactions.
The Company completed its most recent annual goodwill impairment testing as of September 30, 2022, a date which is consistent with the prior year. For the years ended December 31, 2022 and 2021, the Company determined that there was no impairment of goodwill.
Other long lived assets
The Company assesses long-lived assets (such as intangible assets) for impairment whenever events or changes in circumstances indicate that the carrying amount of the asset group might not be recoverable. If indicators of impairment are present, we evaluate the carrying value of long-lived asset in relation to estimates of future undiscounted cash flows. Any future impairment could have a material adverse effect on our financial condition or results of operations.
Taxation
Given the global nature of our business and the multiple taxing jurisdictions in which we operate, the determination of the Company’s provision for income taxes requires significant judgments and estimates, the ultimate tax outcome of which may not be certain. Although we believe our estimates are reasonable, the final outcome of these matters may be different than those reflected in our historical income tax provisions and accruals.
The provision for income taxes includes federal, state, local and foreign taxes. We apply the asset and liability method of accounting for income taxes. Under the asset and liability method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amount of existing assets and liabilities and their respective tax bases and for operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which these temporary differences are expected to be recovered or settled. We account for the impact of GILTI (“global intangible low-taxed income”) in the period it arises and therefore have not provided for deferred taxes in respect of this item. Recognition of deferred income tax assets is based on management’s belief that it is more likely than not that the income tax benefit associated with certain temporary differences, income tax operating loss, capital loss carryforwards, and income tax credits, would be realized. Deferred tax assets are reduced by a valuation allowance to the amount that is more likely than not to be realized. We recognize the effect of income tax positions only if those positions will more likely than not be sustained. We determined the amount of the valuation allowance based, in part, on our assessment of future taxable income and in light of our ongoing income tax strategies. If our estimate of future taxable income or tax strategies changes at any time in the future, we would record an adjustment to our valuation allowance. Recording such an adjustment could have a material effect on our financial condition or results of operations.
Item 6. Directors, Senior Management and Employees.
A.Directors and Senior Management
The following table and accompanying biographies set forth certain information concerning each of ICON plc’s Directors, officers and other key employees as of February 24, 2023.
| | | | | | | | |
| Name | Age | Position |
| Ciaran Murray | 60 | Chair and Director |
| Dr. Steve Cutler (1)(5) | 62 | Chief Executive Officer and Director |
| Brendan Brennan (1)(5) | 44 | Chief Financial Officer |
| Rónán Murphy (2)(3)(5) | 65 | Lead Independent Director |
| Dr. John Climax | 70 | Director |
| Joan Garahy (2)(4) | 60 | Director |
| Eugene McCague (3)(4) | 64 | Director |
| Julie O'Neill (3)(4) | 56 | Director |
| Dr. Linda Grais (2) | 66 | Director |
| Diarmaid Cunningham | 48 | Chief Administrative Officer, General Counsel & Company Secretary |
(1)Named Executive Officer of the Company.
(2)Member of Compensation and Organization Committee.
(3)Member of Audit Committee.
(4)Member of Nominating, Sustainability and Governance Committee.
(5)Member of Execution Committee.
Ciaran Murray
Mr. Ciaran Murray graduated with a Bachelor of Commerce degree from University College Dublin in 1982. Mr. Murray subsequently qualified as a chartered accountant with PwC. Following qualification, Mr. Murray gained extensive global experience working as an executive in the fast moving consumer goods and technology sectors in Ireland, Italy, the UK and the US. Mr. Murray has been the Chair of ICON plc since March 2017 and an outside Director since May 2018. Mr. Murray served as Chief Executive Officer from October 2011 until March 2017 and was Chief Financial Officer from joining ICON plc in 2005 until his appointment as Chief Executive Officer in 2011. During his time with ICON plc, Mr. Murray was recognized for his leadership of ICON and the CRO industry. Mr. Murray served as Chair of the Association of Clinical Research Organizations (ACRO) which represents the CRO industry globally. In addition, Mr. Murray was named as a leader in CRO Innovation by PharmaVOICE100, a listing of the most influential people in the bio pharma industry. University College Dublin awarded Mr. Murray an honorary degree of Doctor of Laws in 2013 for his support of third level research and innovation in Ireland. In 2018, the Royal Dublin Society awarded Mr. Murray the RDS Gold Medal for Enterprise for making an exceptional impact on Irish industry and commerce. Mr. Murray is also a member of the advisory Board of UCD Smurfit Business School.
Dr. Steve Cutler
Dr. Steve Cutler was appointed Chief Executive Officer of ICON plc in March 2017, having previously served as Chief Operating Officer from January 2014. Dr. Cutler served as Group President of Clinical Research Services since November 2011 until his appointment as Chief Operating Officer. Dr. Cutler was appointed to the Board of ICON plc in November 2015. Prior to joining the Company, Dr. Cutler held the position of Chief Executive Officer of Kendle, having previously served as Chief Operating Officer. Prior to Kendle, Dr. Cutler spent 14 years with Quintiles where he served as Senior Vice President, Global Project Management; Senior Vice President, Clinical, Medical and Regulatory; Senior Vice President, Project Management - Europe; and Vice President, Oncology - Europe, as well as regional leadership positions in South Africa and Australia. Prior to joining Quintiles, Dr. Cutler held positions with Sandoz (now Novartis) in Australia and Europe. Dr. Cutler holds a B.Sc. and a Ph.D. from the University of Sydney and a Masters of Business Administration from the University of Birmingham (UK).
Brendan Brennan
Mr. Brendan Brennan has served as Chief Financial Officer since February 2012. Mr. Brennan has developed his career over the last 20+ years from experience in various industries. Mr. Brennan joined ICON in 2006 and he has served in a number of senior finance roles in the Company including the role of Senior Vice President of Corporate Finance. Prior to this he developed his broad financial experience in Cement Roadstone Holdings, a major Irish building materials organization. Mr. Brennan also spent a number of years in public accounting with PwC. Mr. Brennan is a Fellow of the Institute of Chartered Accountants in Ireland and holds a bachelor’s degree in Accounting and Finance from Dublin City University. Over his years in the CRO industry, Mr. Brennan has been involved in many industry organizations and developments including ACRO (Association of Clinical Research Organizations) where he was the founding Chairman of the industry CFO round table group, a group formed to aid CROs dealing with the various industry challenges. Mr. Brennan held the position of Chairman of the round table from its foundation in 2017 to December 31, 2019.
Rónán Murphy
Mr. Rónán Murphy has served as an outside Director of the Company since October 2016. He was appointed as Lead Independent Director in January 2019. Mr. Murphy is the former Senior Partner of PwC Ireland. He was elected Senior Partner in 2007 and was re-elected for a further four year term in 2011. Following completion of the maximum two terms, Mr. Murphy retired from the firm in 2015. Mr. Murphy was also a member of the PwC EMEA Leadership Board for a five year period from 2010 to 2015. Mr. Murphy joined PwC in 1980 and was admitted to the Partnership in 1992. Mr. Murphy is presently Chairman of Greencoat Renewables PLC and a non-executive Director of Davy Stockbrokers. Mr. Murphy currently serves as a council member of the ESRI and as Chair of Business in the Community Ireland. He is also a founding Board Member of the British Irish Chamber of Commerce. Mr. Murphy completed a Bachelor of Commerce and Masters in Business Studies at University College Dublin before qualifying as a chartered accountant in 1982.
Dr. John Climax
Dr. John Climax, one of the Company’s co-founders, served as Chairman of the Board of the Company from November 2002 to December 2009 and as Chief Executive Officer from June 1990 to October 2002. Since January 2010 he has held a position as an outside Director of the Company. Dr. Climax has over 30 years of experience in the clinical research industry. Dr. Climax received his primary degree in pharmacy in 1977 from the University of Singapore, his masters in applied pharmacology in 1979 from the University of Wales and his Ph.D. in pharmacology from the National University of Ireland in 1982. He has authored a significant number of papers and presentations, and holds adjunct professorship at the Royal College of Surgeons of Ireland and an honorary professorship at the National University of Singapore. He is currently Executive Chairman of DS Biopharma and CEO of Afimmune, both of which are private companies.
Joan Garahy
Ms. Joan Garahy was appointed as an outside Director of ICON plc in November 2017. Ms. Garahy is a Non-Executive Director of UNICEF Ireland, Irish Residential Properties REIT plc, and IPB Insurance CLG. Ms. Garahy’s previous executive roles include founder and CEO of ClearView Investment & Pensions Limited, a financial advisory company, Managing Director of HBCL Investment & Pensions Ltd, Director of Investments at HC Financial Services Group, Head of Research at the Irish National Pension Reserve Fund, Head of Research at Hibernian Investment Managers and her equity analyst roles with Goodbody Stockbrokers and NCB Group. Ms. Garahy was also previously Senior Independent non-executive Director of Kerry Group plc and a non executive director at Galway University Foundation and she is currently a member of the board of The Irish Chamber Orchestra. Ms. Garahy is a Qualified Financial Advisor, she holds a Bachelor of Science degree from University College Galway, a Master of Science from University College Dublin and a Diploma in Accounting & Finance from ACCA.
Eugene McCague
Mr. Eugene McCague was appointed as an outside Director of the Company in October 2017. Mr. McCague was a corporate partner of Arthur Cox, one of Ireland’s premier law firms, from 1988 until June 2017. During his time with Arthur Cox, Mr. McCague served as both managing partner and chairman of Arthur Cox and also advised a wide range of public and private companies on mainstream corporate work, mergers and acquisitions, corporate restructurings and corporate governance. In addition to his distinguished legal career, Mr. McCague also has extensive board experience with commercial, government and educational organizations. Mr. McCague currently serves on the board of the Irish branch of AON Insurance and he also serves as chairman of Ibec, Ireland’s leading business representative association. Mr. McCague’s previous board roles include the Health Service Executive, the Irish state body which administers public health service in Ireland, chairman of the governing body of the Dublin Institute of Technology, chairman of the Dublin Institute of Technology Foundation and chairman of the governing authority of University College Dublin and director of Fly Leasing Limited. Mr. McCague was also president of the Dublin Chamber of Commerce in 2006. Mr. McCague holds a Bachelor of Civil Law degree and a diploma in European Law from University College Dublin.
Julie O’Neill
Ms. Julie O’Neill has served as an outside Director of ICON plc since July 2019. Ms. O’Neill was formerly Executive Vice President, Global Operations of Alexion Pharmaceuticals, Inc., where she was responsible for global manufacturing operations and expanding and improving supply chain and quality operations in the US, Europe, and Asia. Before joining Alexion, Ms. O’Neill was Vice President of Operations and General Manager for Ireland at Gilead Sciences and earlier in her career, Ms. O'Neill held leadership positions in operations, manufacturing and quality functions at Burnil Pharmacies and Helsinn Birex Pharmaceuticals. Ms. O’Neill serves as a Board Member of DBV Technologies, Hookipa Pharma Inc.,ILC Dover, Achilles Therapeutics plc and Angus Chemical Company. She also chairs the board of Ireland’s National Institute for Bioprocessing Research and Training. Ms. O’Neill holds a Bachelor of Science in Pharmacy from Trinity College Dublin, a Masters of Business Administration from University College Dublin and is a Chartered Director of The Institute of Directors in Ireland. Ms. O'Neill is also a member of the Strategy Committee of the State Claims Agency.
Dr. Linda Grais
Dr. Linda Grais has served as an outside Director of ICON plc since July 2021 having previously served as a member of the PRA Health Sciences board from October 2015 to July 2021. Dr. Grais served as a member of the board of directors of Ocera Therapeutics, Inc. from January 2008 through December 2017 and as President and Chief Executive Officer of Ocera Therapeutics, Inc. from June 2012 to December 2017. Prior to her employment by Ocera, Dr. Grais served as a managing member at InterWest Partners, a venture capital firm from May 2005 until February 2011. From July 1998 to July 2003, Dr. Grais was a founder and executive vice president of SGX Pharmaceuticals Inc., a drug discovery company focusing on new treatments for cancer. Prior to that, she was a corporate attorney at Wilson Sonsini Goodrich & Rosati, where she practiced in such areas as venture financings, public offerings and strategic partnerships. Before practicing law, Dr. Grais worked as an assistant clinical professor of Internal Medicine and Critical Care at the University of California, San Francisco. She currently serves on the boards of directors of Corvus Pharmaceuticals and Arca Biopharma, Inc. and sits on the compensation and audit committees of Arca Biopharma, Inc. Dr. Grais received a B.A. from Yale University, an M.D. from Yale Medical School and a J.D. from Stanford Law School.
Diarmaid Cunningham
Mr. Diarmaid Cunningham is Chief Administrative Officer, General Counsel and Company Secretary. Mr. Cunningham joined the Company as General Counsel in November 2009. From 2009 until 2013, Mr. Cunningham was based in the Company’s global headquarters in Dublin. In 2013, Mr. Cunningham was seconded to the Company’s US headquarters in Pennsylvania and that secondment ended in 2018 when Mr. Cunningham returned to Dublin. In July 2016, Mr. Cunningham’s role expanded to include Chief Administrative Officer in addition to General Counsel. This expansion of his role means Mr. Cunningham has responsibility to the Company’s Quality Assurance, Client Contracts Services, Facilities and Procurement groups in addition to his responsibility for the Company’s Legal group. Mr. Cunningham graduated with a Bachelor of Business and Legal Studies from University College Dublin in 1997, qualified as a lawyer in 2001 and completed the Stanford Executive Program at Stanford University in California in 2015. Mr. Cunningham served as Secretary to the Board of the Association of Clinical Research Organizations (ACRO) in 2013, 2014, 2020 and 2021. ACRO represents the CRO industry globally to key stakeholders including pharmaceutical, biotech and medical device companies, regulators, legislators and patient groups. Prior to joining the Company, Mr. Cunningham spent 10 years with A&L Goodbody, one of Ireland's premier corporate law firms. In January 2021, Mr. Cunningham was appointed as a non-executive director of the Irish charity The Jack & Jill Foundation.
Board Diversity Matrix
| | | | | | | | |
| Board Diversity Matrix (As of February 24, 2023) |
| Total Number of Directors | 8 |
| Part I: Gender Identity |
| Female | Male |
| Directors | 3 | 5 |
| Part II: Demographic Background |
| Asian | | 1 |
| White | 3 | 4 |
| LGBTQ+ | 1 |
| Did Not Disclose Demographic Background | 0 |
| | | | | | | | |
| Board Diversity Matrix (As of July 27, 2022) |
| Total Number of Directors | 9 |
| Part I: Gender Identity |
| Female | Male |
| Directors | 3 | 6 |
| Part II: Demographic Background |
| Asian | | 1 |
| White | 3 | 5 |
| LGBTQ+ | 1 |
| Did Not Disclose Demographic Background | 0 |
B.Compensation
Compensation Discussion & Analysis
Remuneration policy
The Compensation and Organization Committee seeks to achieve the following goals with the Company’s executive compensation programs: to attract, motivate and retain key executives and to reward executives for value creation. The Committee seeks to foster a performance-oriented environment by ensuring that a significant portion of each executive’s cash and equity compensation is based on the achievement of performance targets that are important to the Company, its shareholders and other stakeholders.
The Company’s executive compensation program has three main elements: base salary, a bonus plan and equity incentives in the form of share related awards granted under the Company’s equity incentive plans. All elements of key executives’ compensation are determined by the Compensation and Organization Committee based on the achievement of the Group’s and individual performance objectives. Base salary, bonus awards and Directors’ fees were determined by the Compensation and Organization Committee in U.S. dollars or euro.
Outside Directors’ remuneration
Outside Directors are remunerated by way of Directors’ fees and are also eligible for participation in the share equity incentive schemes. During 2022, each outside Director (excluding the Board Chairman) was paid an annual retainer of $90,000 and additional fees for Board Committee service.
Mr. Murray’s Executive Chairman term expired on May 12, 2018 and he transitioned to the outside Director role of Chair. The current arrangement with the Chair provides for payment of €330,000 (translated at average rate for the year: $346,891) annually.
Mr. Rónán Murphy was appointed as Lead Independent Director with effect from January 1, 2019 and receives an additional annual fee of $25,000 for this role.
Outside Directors are not eligible for performance related bonuses and no pension contributions are made on their behalf. The Compensation and Organization Committee sets outside Directors' remuneration.
Executive Directors’ and Key Executive Officers’ remuneration
Total cash compensation is divided into a base salary portion and a bonus incentive portion. The Committee targets total cash compensation with regard to healthcare/biopharmaceutical companies of similar market capitalization and peer CRO companies, adjusted upward or downward based on individual performance and experience and level of responsibility. The Compensation and Organization Committee believes that the higher the executive’s level of responsibility within the Company, the greater the percentage of the executive’s compensation that should be tied to the Company’s performance. Target bonus incentive for executive officers range between 60% and 125% with actual pay outs for 2022 ranging from 48% to 100%, of salary, based on Group and individual performance.
A total bonus of $1.5 million was awarded to the following individuals; Dr. Steve Cutler Chief Executive Officer ($1.2 million) and Mr. Brendan Brennan Chief Financial Officer ($0.3 million) to reflect their contribution to the performance of the Company during 2022. These amounts were approved by the Compensation and Organization Committee and will be paid during the year ended December 31, 2023.
The Company’s executives are eligible to receive equity incentives, including stock options, Restricted Share Units and Performance Share Units, granted under the Company’s equity incentive plans. If executives receive equity incentive grants, they are normally approved annually at the first scheduled meeting of the Committee in the fiscal year. The grant date and value is determined by the Committee and the number of units granted is determined based on the closing price of the Company's shares on the day of grant. Newly hired executives may receive sign-on grants. In addition, the Committee may, at its discretion, issue additional equity incentive awards to executives if the Committee determines such awards are necessary to ensure appropriate incentives are in place. The equity awards granted to each participant are determined by the Committee at the start of each year based on peer group data, advice from independent compensation consultants, and Committee judgment.
All executive officers are eligible to participate in applicable pension plans. The Company’s contributions are generally a fixed percentage of their annual compensation, supplementing contributions by the executive. The Company has the discretion to make additional contributions if deemed appropriate by the Committee. The Company’s contributions are determined at the peer group median of comparable Irish companies and peer CRO companies. Contributions to this plan are recorded as an expense in the Consolidated Statement of Operations.
Third party Agreements and Arrangements
ICON has not identified any arrangements or agreements relating to compensation or other payments provided by a third party to ICON’s directors or director nominees in connection with their candidacy or board service as required to be disclosed pursuant to NASDAQ Rule 5250(b)(3).
Executive Compensation
Summary compensation table - Year ended December 31, 2022
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Name & principal
position | Year | Salary | Bonus | Pension
contribution | All other compensation | Subtotal | Share-based
compensation | Director’s Fees | Total
compensation |
| | $’000 | $’000 | $’000 | $’000 | $’000 | $’000 | $’000 | $’000 |
Dr. Steve Cutler
Chief Executive Officer | 2022 | 1,168 | | 1,173 | | 125 | | 31 | | 2,497 | | 7,487 | | 44 | | 10,028 | |
| | | | | | | | | |
Brendan Brennan,
Chief Financial Officer | 2022 | 556 | | 342 | | 70 | | 30 | | 998 | | 1,645 | | — | | 2,643 | |
| Total | 2022 | 1,724 | | 1,515 | | 195 | | 61 | | 3,495 | | 9,132 | | 44 | | 12,671 | |
Summary compensation table - Year ended December 31, 2021
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Name & principal
position | Year | Salary * | Bonus | Pension
contribution | All other compensation | Subtotal | Share-based
compensation | Director’s Fees | Total
compensation |
| | $’000 | $’000 | $’000 | $’000 | $’000 | $’000 | $’000 | $’000 |
Dr. Steve Cutler
Chief Executive Officer | 2021 | 1,146 | | 2,300 | | 121 | | 31 | | 3,598 | | 5,959 | | 44 | | 9,601 | |
| | | | | | | | | |
Brendan Brennan,
Chief Financial Officer | 2021 | 607 | | 914 | | 76 | | 35 | | 1,632 | | 1,341 | | — | | 2,973 | |
| Total | 2021 | 1,753 | | 3,214 | | 197 | | 66 | | 5,230 | | 7,300 | | 44 | | 12,574 | |
Director Compensation
Summary compensation table - Year ended December 31, 2022
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name | Year | Salary | Company
pension contribution | All other compensation | Subtotal | Share-based
compensation | Director’s fees | Total
Compensation |
| | $’000 | $’000 | $’000 | $’000 | $’000 | $’000 | $’000 |
| Ciaran Murray | 2022 | — | | — | | — | | — | | 267 | | 363 | | 630 | |
| Steve Cutler | 2022 | 1,168 | | 125 | | 1,204 | | 2,497 | | 7,487 | | 44 | | 10,028 | |
| Rónán Murphy | 2022 | — | | — | | — | | — | | 200 | | 150 | | 350 | |
| Hugh Brady* | 2022 | — | | — | | — | | — | | 80 | | 54 | | 134 | |
| John Climax | 2022 | — | | — | | — | | — | | 213 | | 90 | | 303 | |
| Joan Garahy | 2022 | — | | — | | — | | — | | 200 | | 123 | | 323 | |
| William Hall* | 2022 | — | | — | | — | | — | | 80 | | 58 | | 138 | |
| Eugene McCague | 2022 | — | | — | | — | | — | | 200 | | 125 | | 325 | |
| Julie O'Neill | 2022 | — | | — | | — | | — | | 200 | | 110 | | 310 | |
| Mary Pendergast* | 2022 | — | | — | | — | | — | | 80 | | 54 | | 134 | |
| Colin Shannon** | 2022 | — | | — | | — | | — | | — | | 85 | | 85 | |
| Linda Grais | 2022 | — | | — | | — | | — | | 133 | | 98 | | 231 | |
| Total | 2022 | 1,168 | | 125 | | 1,204 | | 2,497 | | 9,140 | | 1,354 | | 12,991 | |
* Professor Hugh Brady, Professor William Hall and Ms. Mary Pendergast resigned from the Board on July 26, 2022.
** Mr. Colin Shannon resigned from the Board on December 9, 2022.
Summary compensation table - Year ended December 31, 2021
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name | Year | Salary * | Company
pension contribution | All other compensation | Subtotal | Share-based
compensation | Director’s fees | Total
Compensation |
| | $’000 | $’000 | $’000 | $’000 | $’000 | $’000 | $’000 |
| Ciaran Murray | 2021 | — | | — | | — | | — | | 200 | | 372 | | 572 | |
| Steve Cutler | 2021 | 1,146 | | 121 | | 2,331 | | 3,598 | | 5,959 | | 44 | | 9,601 | |
| Rónán Murphy | 2021 | — | | — | | — | | — | | 240 | | 144 | | 384 | |
| Hugh Brady | 2021 | — | | — | | — | | — | | 253 | | 90 | | 343 | |
| John Climax | 2021 | — | | — | | — | | — | | 253 | | 78 | | 331 | |
| Joan Garahy | 2021 | — | | — | | — | | — | | 200 | | 110 | | 310 | |
| William Hall | 2021 | — | | — | | — | | — | | 253 | | 103 | | 356 | |
| Eugene McCague | 2021 | — | | — | | — | | — | | 200 | | 119 | | 319 | |
| Julie O'Neill | 2021 | 0 | 0 | 0 | — | | 200 | | 86 | | 286 | |
| Mary Pendergast | 2021 | — | | — | | — | | — | | 253 | | 90 | | 343 | |
| Colin Shannon* | 2021 | — | | — | | — | | — | | — | | 45 | | 45 | |
| Linda Grais* | 2021 | — | | — | | — | | — | | — | | 45 | | 45 | |
| Total | 2021 | 1,146 | | 121 | | 2,331 | | 3,598 | | 8,011 | | 1,326 | | 12,935 | |
* Mr. Colin Shannon and Dr. Linda Grais were appointed to the Board on July 1, 2021.
Disclosure of Compensation Agreements
Employment Contracts, Termination of Employment and Change in Control Arrangements
The Company does not have any termination or change of control agreements with its named executive officers other than as set out below and in the agreements relating to their equity incentives which provide for accelerated vesting on change of control.
Directors’ and Executive Officers’ service agreements and letters of engagement
The following information reflects the agreements in effect as of December 31, 2022.
Mr. Ciaran Murray
Mr. Ciaran Murray has served as Chair of the Board of Directors since May 2018 having served as Executive Chairman of the Board of Directors from March 2017 until May 2018. Mr. Murray served as Chief Executive Officer of the Company from October 2011 until March 2017. Mr. Murray has served as a Director of the Company since September 2011. He previously served as Chief Financial Officer of the Company from October 2005 until October 2011. Mr. Murray entered into an agreement with the Company in respect of his role as Executive Chairman which was effective from March 2017. Mr. Murray’s Executive Chairman term expired on May 12, 2018 and he transitioned to Chair. The current arrangement with Mr. Murray provides for the payment to him of fees of €330,000 (translated at average rate for the year: $346,891) per annum in respect of his position as Chairman. His previous service agreement as Executive Chairman included termination provisions and also includes certain post-termination clauses including non-disclosure, non-competition and non-solicitation provisions which still apply. As Chief Financial Officer, Chief Executive and Executive Chairman, Mr. Murray was granted and held ordinary share options, Restricted Share Units and Performance Share Units. The vesting of the ordinary share options and Restricted Share Units which were unvested on Mr. Murray ceasing to be an ICON plc employee on May 12, 2018 were accelerated and the outstanding ordinary share options and Restricted Share Units vested on that date. The unvested Performance Share Units with vesting dates between May 12, 2018 and March 2019 were forfeited on Mr. Murray ceasing to be an ICON plc employee on May 12, 2018. He was previously granted and held at February 24, 2023 58,646 ordinary share options at exercise prices ranging from $71.95 to $125.74 per share and 1,388 Restricted Share Units, which vest in May 2023.
Dr. Steve Cutler
Dr. Steve Cutler has served as Chief Executive Officer since March 2017 having served as Chief Operating Officer of the Company from January 2014 until March 2017. Prior to his appointment as Chief Operating Officer he served as Group President Clinical Research Services since November 2011. He has served as an Executive Director of the Company since November 2015. The Chief Executive Officer service agreement with Dr. Cutler is terminable on 12 months’ notice by either party. Under the terms of this agreement Dr. Cutler is entitled to receive an annual salary of $1,173,251 effective from April 1, 2022 and a bonus to be agreed by the Compensation and Organization Committee. He is also entitled to receive a pension contribution, a car allowance of $12,000 and medical insurance coverage for himself and his dependents. He was previously granted and held at February 24, 2023 208,885 ordinary share options at exercise prices ranging from $71.95 to $231.68 per share, 18,173 Restricted Share Units which vest on various dates between March 2023 and March 2025 and 32,121 (up to a maximum of 64,242) Performance Share Units which vest between March 2023 and March 2025 subject to the fulfillment of certain performance conditions. His Chief Executive Officer service agreement requires him to devote his full time and attention to his duties for the Company excepting certain outside director positions authorized by the Company. The agreement with Dr. Cutler includes termination and change of control provisions and also includes certain post-termination clauses including non-disclosure, non-competition and non-solicitation provisions. Dr. Cutler has a separate agreement with the Company in respect to his role as a director of ICON plc. Under the terms of this agreement he is entitled to receive an annual fee of $44,000.
Mr. Brendan Brennan
Mr. Brendan Brennan has served as Chief Financial Officer since February 2012 having previously served as acting Chief Financial Officer since October 2011. Prior to this appointment, he served in a number of senior finance roles in the Company including the role of Senior Vice President of Corporate Finance. The service agreement with Mr. Brennan is terminable on 12 months’ notice by either party. Under the terms of this agreement Mr. Brennan is entitled to receive an annual salary of $621,467 (€532,032) effective from April 1, 2022 and a bonus to be agreed by the Compensation and Organization Committee. He is also entitled to receive a pension contribution, a car allowance of €20,000 and medical insurance coverage for himself and his dependents. He was previously granted and held at February 24, 2023 53,105 ordinary share options at exercise prices ranging from $83.47 to $231.68 per share, 3,958 Restricted Share Units, which vest on various dates between March 2023 and March 2025, and 7,048 (up to a maximum of 14,096) Performance Share Units which vest between March 2023 and March 2025 subject to the fulfillment of certain performance conditions. His service agreement requires him to devote his full time and attention to his duties for the Company excepting certain outside Director positions authorized by the Board. The agreement with Mr. Brennan includes termination and change of control provisions and also includes certain post-termination clauses including non-disclosure, non-competition and non-solicitation provisions.
Mr. Rónán Murphy
Mr. Rónán Murphy has served as Lead Independent Director from January 2019 having served as an outside Director of the Company since October 2016. The current arrangements with Mr. Murphy provide for the payment to him of Directors fees of $147,500 per annum. He was previously granted and held at February 24, 2023 9,622 ordinary share options at an exercise prices ranging from $90.03 to $125.74 and 925 Restricted Share Units, which vest in May 2023.
Dr. John Climax
Dr. John Climax, one of the Company’s co-founders, served as Chairman of the Board of the Company from November 2002 to December 2009. He also served as Chief Executive Officer of the Company from June 1990 to October 2002 and is currently an outside Director of the Company. The current arrangements with Dr. Climax provide for the payment to him of Directors fees of $90,000 per annum. He was previously granted and held at February 24, 2023 33,255 ordinary share options at exercise prices ranging from $65.60 to $125.74 per share and 925 Restricted Share Units, which vest in May 2023.
Ms. Joan Garahy
Ms. Joan Garahy has served as an outside Director of the Company since November 2017. The current arrangements with Ms. Garahy provide for the payment to her of Directors fees of $122,500 per annum. She was previously granted and held at February 24, 2023 5,005 ordinary share options at an exercise price of $125.74 and 925 Restricted Share Units, which vest in May 2023.
Mr. Eugene McCague
Mr. Eugene McCague has served as an outside Director of the Company since October 2017. The current arrangements with Mr. McCague provide for the payment to him of Directors fees of $122,500 per annum. He was previously granted and held at February 24, 2023 5,005 ordinary share options at an exercise price of $125.74 and 925 Restricted Share Units, which vest in May 2023.
Ms. Julie O'Neill
Ms. Julie O'Neill has served as an outside Director of the Company since July 2019. The current arrangements with Ms. O'Neill provide for the payment to her of Directors fees of $115,000 per annum. She was previously granted and held at February 24, 2023 925 Restricted Share Units, which vest in May 2023.
Dr. Linda Grais
Dr. Linda Grais has served as an outside Director of the Company since July 2021 having served as a member of the PRA Health Sciences, Inc. board since October 2015. The current arrangements with Dr. Grais provide for the payment to her of Director fees of $102,500 per annum. She was previously granted and held at February 24, 2023 925 Restricted Share Units, which vest in May 2023.
C.Board Practices
Board of Directors
The business of the Company is managed by the Directors who may exercise all the powers of the Company which are not required by the Companies Act 2014 of Ireland or by the Constitution of the Company to be exercised by the Company in general meeting. A meeting of Directors, at which a quorum is present, may exercise all powers exercisable by the Directors. The Directors may delegate (with power to sub-delegate) to any Director holding any executive office and to any Committee consisting of one or more Directors, together with such other persons as may be appointed to such Committee by the Directors, provided that a majority of the members of each Committee appointed by the Directors shall at all times consist of Directors and that no resolution of any such Committee shall be effective unless two of the members of the Committee present at the meeting at which it was passed are Directors.
The Board comprises one executive and seven outside Directors at the date of this report. The outside Directors bring independent judgment to bear on issues of strategy, performance, resources, key appointments and standards. The Company considers all of its outside Directors to be of complementary skills, experience and knowledge and each outside Director has specific skills, experience and knowledge that are valuable to the Company. The Board members between them have strong financial, pharmaceutical, CRO, scientific, medical and other skills and knowledge which are harnessed to address the challenges facing the Group. The Board meets regularly throughout the year and all Directors have full and timely access to the information necessary for them to discharge their duties. The Directors have access to the advice and services of the Company Secretary and may seek external independent professional advice where required. The Board considers its current size (8 Directors) to be adequate but continues to look for suitable qualified potential candidates to join the Board.
As set out below, certain other matters are delegated to Board Committees and all Board Committees report to the Board. The Company maintains what it considers an appropriate level of insurance cover in respect of legal action against its Directors. The Board, through the Nominating, Sustainability and Governance Committee, engages in succession planning for the Board and in so doing considers the strength and depth of the Board and the levels of knowledge, skills and experience of the Directors necessary for the Company to achieve its objectives. The Board meets at least four times each year. During the year ended December 31, 2022 the Board held five board meetings. All Directors allocated sufficient time to the Company during the year ended December 31, 2022 to effectively discharge their responsibilities to the Company.
Directors’ retirement and re-election
The Company’s Constitution provides that, unless otherwise determined by the Company at a general meeting, the number of Directors shall not be more than 15 nor less than 3. The Constitution also provides that one third of the Directors who are subject to retirement by rotation, rounded down to the next whole number if it is a fractional number, shall retire from office at each annual general meeting.
The Board of Directors adopted a Non-Executive Director Policy for Service on April 24, 2018. The Policy was amended on April 21, 2020 and provides that, subject to individual waiver by the Board, an outside Director of ICON plc shall serve on the Board of the Directors for an initial term which expires at the fourth annual general meeting after their appointment. Each outside director may serve a further term of 3 years, subject to the Board’s approval. After the second 3 year term the Board may request that the outside Director serve up to 3 further terms of 1 year each. After a third 1 year term the Board may request that the outside director serve for further 1 year terms in the event that the Board has particular requirement or desire for the outside director’s skill, knowledge or experience. For an outside Director who previously served as an executive of the Company, the initial 3 year term referred to in this policy is deemed to commence on the date that he/she is determined to be independent as per the NASDAQ Rules. This policy does not apply to Dr. John Climax as he is a founder of the Company.
However in July 2022, the Board of Directors unanimously agreed that all of the Directors will retire and stand for re-election annually at the Annual General Meeting (AGM). Accordingly, at the AGM of the Company to be held in 2023, all directors will retire and offer themselves for re-election.
Lead Independent Director
The Board of Directors adopted a Lead Independent Director Charter on February 14, 2017 which provides that in circumstances where the Chairman of the Board is not independent, the independent members of the Board of Directors shall appoint, from among their number, a Lead Independent Director. The Lead Independent Director shall generally assist in optimizing the effectiveness and independence of the Board of Directors by performing such duties as described in the charter, on behalf of the Board of Directors, including coordinating the meetings of the other non-employee and independent directors, and such other duties as determined from time to time by the Board of Directors and/or its independent members. Mr. Rónán Murphy was appointed as Lead Independent Director with effect from January 1, 2019.
Board Committees
The Board has delegated some of its responsibilities to Board Committees. There are currently four Committees. These are the Audit Committee, the Compensation and Organization Committee, the Nominating, Sustainability and Governance Committee and the Execution Committee. The Integration Committee was retired by the Board on April 26, 2022. Each Committee has been charged with specific responsibilities and each has written terms of reference that are reviewed periodically. Minutes of Committee meetings are available to all members of the Board. The Company Secretary is available to act as secretary to each of the Board Committees if required. Appropriate key executives are regularly invited to attend meetings of the Board Committees. The Audit Committee, Compensation and Organization Committee and Nominating, Sustainability and Governance Committee each completed a self-evaluation of the performance of the Committee in respect to the year ended December 31, 2021 and each Committee was satisfied with their performance. The evaluations in respect to the year ended December 31, 2022 will be completed in 2023.
Audit Committee
The Audit Committee meets a minimum of four times a year. It reviews the quarterly and annual financial statements, the effectiveness of the system of internal control and recommends the appointment and removal of the external auditors. It monitors the adequacy of internal accounting practices and addresses all issues raised and recommendations made by the external auditors. The Audit Committee pre-approves all audit and non-audit services provided to the Company by its external auditors on a quarterly basis. The Audit Committee, on a case by case basis, may approve additional services not covered by the quarterly pre-approval, as the need for such services arises. The Audit Committee reviews all services which are provided by the external auditor to review the independence and objectivity of the external auditor, taking into consideration relevant professional and regulatory requirements. The Chief Financial Officer, the Head of Internal Audit, the Chief Administrative Officer and General Counsel and the external auditors normally attend all meetings of the Audit Committee and have direct access to the Committee Chairperson at all times. The Audit Committee is currently comprised of three independent Directors: Rónán Murphy (Chairperson), Eugene McCague and Julie O'Neill. On April 26, 2022, Hugh Brady stepped down as a member of the Committee and Julie O'Neill joined the Committee.
Compensation and Organization Committee
The Compensation and Organization Committee is responsible for senior executive remuneration. The Committee aims to ensure that remuneration packages are competitive so that individuals are appropriately rewarded relative to their responsibility, experience and value to the Company. Annual bonuses for the executive Directors and senior executive management are determined by the Committee based on the achievement of the Company’s objectives. The Committee also oversees succession planning for the Company’s senior management. The Compensation and Organization Committee is currently comprised of the following independent Directors: Joan Garahy (Chairperson), Rónán Murphy and Linda Grais. On April 26, 2022, William Hall and Mary Pendergast stepped down as members of the Committee and Linda Grais joined the Committee.
Nominating, Sustainability and Governance Committee
The Nominating, Sustainability and Governance Committee is responsible for Board succession, oversight of the Board and committee composition and performance and oversight of the Company's corporate governance and business ethics initiatives and strategies and activities in respect to environmental, social and governance (ESG) matters. The Committee reviews the membership of the Board of the Company and Board Committees on an ongoing basis. As part of this, it regularly evaluates the balance of skills, knowledge and experience on the Board and then, based on this evaluation, identifies and, if appropriate, recommends individuals to join the Board of the Company. The Committee uses external search consultants as needed to assist it in identifying potential new outside Directors. Once potential suitable candidates are identified either by the external search consultants or by members of the Nominating, Sustainability and Governance Committee, the Committee then discusses and considers the skills, knowledge and experience of the potential candidate. The Committee will assess if the Board of the Company requires and would benefit from the potential candidate’s skills, knowledge and experience and, if it decides the potential candidate is suitable, the Committee would recommend to the Board of the Company that the potential candidate be appointed. The Board of the Company then decides whether or not to appoint the candidate. The Committee considers diversity of the Board members when making recommendations to the Board of the Company. The Committee Charter was updated in February 2022 to include specific responsibilities in respect to the oversight of the Company's strategic plans, objectives and risks relating to ESG matters. The Committee changed its name to Nominating, Sustainability and Governance Committee in
April 2022. The Nominating, Sustainability and Governance Committee currently comprises the following independent Directors: Eugene McCague (Chairperson), Joan Garahy and Julie O'Neill. On April 26, 2022, William Hall stepped down as a member of the Committee and Julie O'Neill joined the Committee.
Integration Committee
The Integration Committee was set up in April 2021 to assist the Board with its oversight responsibilities in relation to the integration of PRA Health Sciences into the ICON Group. The Integration Committee was retired by the Board on April 26, 2022. Matters that were in the remit of the Integration Committee are now delivered by Management to the full Board. During 2022, up to its retirement in April, the Integration Committee was comprised of the following independent Directors: Ciaran Murray (Chairperson), Rónán Murphy, Eugene McCague and Julie O'Neill.
Execution Committee
The primary function of the Execution Committee is to exercise the powers and authority of the Board in intervals between meetings of the Board within the limits set out in the Charter of the Execution Committee. The Execution Committee exercises business judgment to act in what the Committee members reasonably believe to be in the best interest of the Company and its shareholders. All powers exercised by the Execution Committee are ratified at board meetings. This Committee convenes as often as it determines to be necessary or appropriate. The Execution Committee is currently comprised of the following Directors and Officers: Steve Cutler (Chairperson), Rónán Murphy and Brendan Brennan.
Attendance at Board and Committee meetings
Attendance at Board and Committee meetings by the Directors who held office during 2022 are set out as follows:
| | | | | | | | | | | | | | | | | |
| Directors’ Attendance Table | | | | | |
| | Board | Audit | Compensation
and
Organization | Nominating, Sustainability
and
Governance | Integration (3) |
| | |
| Director | Number of meetings attended / number of meetings eligible to attend as a Director |
| Ciaran Murray (1) | 5/5 | — | — | — | 2/2 |
| Dr. Steve Cutler | 5/5 | — | — | — | — |
| Rónán Murphy (1) | 5/5 | 5/5 | 3/3 | — | 2/2 |
| Prof. Hugh Brady (1) (4) | 3/3 | 2/2 | — | — | — |
| Dr. John Climax (1) | 5/5 | — | — | — | — |
| Joan Garahy (1) | 5/5 | — | 2/3 | 3/4 | — |
| Prof. William Hall (1) | 3/3 | | 2/2 | 2/2 | — |
| Eugene McCague (1) | 5/5 | 5/5 | — | 4/4 | 2/2 |
| Julie O'Neill (1) | 5/5 | 3/3 | — | 2/2 | 2/2 |
| Mary Pendergast (1) | 3/3 | — | 2/2 | — | — |
| Colin Shannon (5) | 5/5 | — | — | — | — |
| Dr. Linda Grais (1) | 5/5 | — | 1/1 | — | — |
(1)Independent Director as defined under NASDAQ Rule 5605(a)(2).
(2)All decisions by the Execution Committee were made by written resolution and therefore no meetings were held.
(3)The Integration Committee was retired on April 26, 2022.
(4)Professor Hugh Brady, Professor William Hall and Ms. Mary Pendergast resigned from the Board on July 26, 2022.
(5)Mr. Colin Shannon resigned from the Board on December 9, 2022.
D.Employees
At December 31, 2022, December 31, 2021 and December 31, 2020 we employed approximately 41,100, 38,330 and 15,730 people respectively. Our employees are not unionized and we believe we have a satisfactory relationship with our employees.
E.Share Ownership
Shares
The following table sets forth certain information as of February 24, 2023 regarding beneficial ownership of our ordinary shares by all of our current Directors and executive officers. Unless otherwise indicated below, to our knowledge, all persons listed below have sole voting and investment power with respect to their ordinary shares, except to the extent authority is shared by spouses under applicable law.
| | | | | | | | |
Name of Owner or
Identity of Group | No. of
Shares (1) | % of total
Shares |
| Mr. Ciaran Murray | 1,680 | | — | % |
| Dr. Steve Cutler | 42,377 | | 0.05 | % |
| Mr. Brendan Brennan | 23,547 | | 0.03 | % |
| Mr. Rónán Murphy | 1,680 | | — | % |
| Dr. John Climax | 509,297 | | 0.62 | % |
| Ms. Joan Garahy | 1,680 | | — | % |
| Mr. Eugene McCague | 1,680 | | — | % |
| Ms. Julie O'Neill | 1,490 | | — | % |
| Dr. Linda Grais | 3,994 | | — | % |
(1)As used in these tables, each person has the sole or shared power to vote or direct the voting of a security, or the sole or shared investment power with respect to a security (i.e. the power to dispose, or direct the disposition, of a security). A person is deemed as of any date to have "beneficial ownership" of any security if that such person has the right to acquire such security within 60 days after such date.
Restricted Share Units and Performance Share Units
The following table sets forth certain information as of February 24, 2023 regarding beneficial ownership of Restricted Share Units (“RSUs”) and Performance Share Units (“PSUs”) which have been issued to our current Directors and executive officers.
| | | | | | | | | | | | | | |
Name of Owner or
Identity of Group | No. of
RSUs | Vesting Date | No. of PSUs (1) | Vesting Date |
| Mr. Ciaran Murray | 1,388 | May 21, 2023 | | |
| Dr. Steve Cutler | 2,958 | | March 3, 2023 | 11,202 | | March 3, 2023 |
| 3,201 | | March 3, 2023 | 10,354 | | March 3, 2024 |
| 3,018 | | March 3, 2023 | 10,565 | | March 3, 2025 |
| 2,959 | | March 3, 2024 | | |
| 3,018 | | March 3, 2024 | | |
| 3,019 | | March 3, 2025 | | |
| Mr. Brendan Brennan | 694 | March 3, 2023 | 2,425 | | March 3, 2023 |
| 698 | March 3, 2023 | 2,444 | | March 3, 2024 |
| 622 | March 3, 2023 | 2,179 | | March 3, 2025 |
| 698 | March 3, 2024 | | |
| 622 | March 3, 2024 | | |
| 624 | March 3, 2025 | | |
| Mr. Rónán Murphy | 925 | May 21, 2023 | | |
| Dr. John Climax | 925 | May 21, 2023 | | |
| Ms. Joan Garahy | 925 | May 21, 2023 | | |
| Mr. Eugene McCague | 925 | May 21, 2023 | | |
| Ms. Julie O'Neill | 925 | May 21, 2023 | | |
| Dr. Linda Grais | 925 | May 21, 2023 | | |
(1)Of the issued PSUs, performance conditions will determine how many vest. If performance targets are exceeded, additional PSUs will be issued and will vest in accordance with the terms of the relevant PSU award. The PSUs vest based on service and specified EPS targets over the periods 2020 – 2022, 2021 – 2023 and 2022 - 2024. Depending on the actual amount of EPS from 2020 to 2024, up to a maximum of 39,169 additional PSUs may also be granted to Dr. Steve Cutler and Mr. Brendan Brennan.
Share Options
The following table sets forth certain information as of February 24, 2023 regarding options to acquire ordinary shares of the Company by all of our current Directors and executive officers.
| | | | | | | | | | | | | |
Name of Owner or
Identity of Group | No. of Options (1) | Exercise price | Expiration Date | | |
| Mr. Ciaran Murray | 45,948 | | $71.95 | | March 4, 2024 | | |
| 7,693 | | $90.03 | | May 19, 2025 | | |
| 5,005 | | $125.74 | | May 18, 2026 | | |
| Dr. Steve Cutler | 6,128 | | $71.95 | | March 4, 2024 | | |
| 25,156 | | $83.47 | | March 3, 2025 | | |
| 29,613 | | $115.11 | | March 3, 2026 | | |
| 32,272 | | $140.38 | | March 3, 2027 | | |
| 42,386 | | $159.33 | | March 3, 2028 | | |
| 37,461 | | $174.96 | | March 3, 2029 | | |
| 35,869 | | $231.68 | | March 3, 2030 | | |
| Mr. Brendan Brennan | 9,306 | | $83.47 | | March 3, 2025 | | |
| 9,584 | | $115.11 | | March 3, 2026 | | |
| 8,796 | | $140.38 | | March 3, 2027 | | |
| 9,176 | | $159.33 | | March 3, 2028 | | |
| 8,842 | | $174.96 | | March 3, 2029 | | |
| 7,401 | | $231.68 | | March 3, 2030 | | |
| Mr. Rónán Murphy | 4,617 | | $90.03 | | May 19, 2025 | | |
| 5,005 | | $125.74 | | May 18, 2026 | | |
| Dr. John Climax | 10,000 | | $68.39 | | March 18, 2023 | | |
| 10,557 | | $65.60 | | May 20, 2024 | | |
| 7,693 | | $90.03 | | May 19, 2025 | | |
| 5,005 | | $125.74 | | May 18, 2026 | | |
| Ms. Joan Garahy | 5,005 | | $125.74 | | May 18, 2026 | | |
| Mr. Eugene McCague | 5,005 | | $125.74 | | May 18, 2026 | | |
(1) The title of securities covered by all of the above options are non-qualified.
In February 2018, the Board approved the appointment of Mr. Murray as Chair of the Board of Directors with effect from May 12, 2018. Mr. Murray ceased to be an employee of the Company as of this date. Mr. Murray was granted and held ordinary share options, Restricted Share Units and Performance Share Units as Chief Financial Officer, Chief Executive Officer and Executive Chairman. The vesting of the ordinary share options and Restricted Share Units which were unvested on Mr. Murray ceasing to be an ICON plc employee (May 12, 2018) were accelerated and the outstanding ordinary share options and Restricted Share Units vested on that date. The unvested Performance Share Units with vesting dates between May 12, 2018 and March 31. 2019 were forfeited on Mr. Murray ceasing to be an ICON plc employee on May 12, 2018.
Equity Incentive Plans
On April 30 2019, the Company approved the 2019 Consultants and Directors Restricted Share Unit Plan (the “2019 Consultants RSU Plan”), which was effective as of May 16, 2019, pursuant to which the Compensation and Organization Committee of the Company’s Board of Directors may select any consultant, adviser or Non-Executive Director retained by the Company, or a Subsidiary to receive an award under the plan. 250,000 ordinary shares have been reserved for issuance under the 2019 Consultants RSU Plan. The awards are at par value and vest over a service period. Awards granted to Non-Executive Directors vest over twelve months.
On April 23, 2013 the Company adopted the 2013 Employees Restricted Share Unit and Performance Share Unit Plan (the “2013 RSU Plan”) pursuant to which the Compensation and Organization Committee of the Company’s Board of Directors may select any employee, or any Director holding a salaried office or employment with the Company, or a Subsidiary to receive an award under the plan. On May 11, 2015, the 2013 RSU Plan was amended and restated in order to increase the number of ordinary shares that can be issued under the RSU Plan by 2.5 million shares. Accordingly, an aggregate of 4.1 million ordinary shares have been reserved for issuance under the 2013 RSU Plan. The shares are awarded at par value and vest over a service period. Awards under the 2013 RSU Plan may be settled in cash or shares at the option of the Company. No awards may be granted under the 2013 RSU Plan after May 11, 2025.
On July 21, 2008 the Company adopted the Employee Share Option Plan 2008 (the “2008 Employee Plan”) pursuant to which the Compensation and Organization Committee of the Company’s Board of Directors may grant options to any employee, or any Director holding a salaried office or employment with the Company or a Subsidiary for the purchase of ordinary shares. On the same date, the Company also adopted the Consultants Share Option Plan 2008 (the “2008 Consultants Plan”), pursuant to which the Compensation and Organization Committee of the Company’s Board of Directors may grant options to any consultant, adviser or Non-Executive Director retained by the Company or any Subsidiary for the purchase of ordinary shares.
On February 14, 2017 both the 2008 Employee Plan and the 2008 Consultants Plan (together the “2008 Option Plans”) were amended and restated in order to increase the number of options that can be issued under the 2008 Consultants Plan from 0.4 million to 1.0 million and to extend the date for options to be granted under the 2008 Option Plans. An aggregate of 6.0 million ordinary shares have been reserved under the 2008 Employee Plan as reduced by any shares issued or to be issued pursuant to options granted under the 2008 Consultants Plan under which a limit of 1.0 million shares applies. Further, the maximum number of ordinary shares with respect to which options may be granted under the 2008 Employee Option Plan, during any calendar year to any employee shall be 0.4 million ordinary shares. There is no individual limit under the 2008 Consultants Plan. No options may be granted under the 2008 Option Plans after February 14, 2027.
Each option granted under the 2008 Option Plans will be a nonqualified stock option, or NSO and not an incentive stock option as described in Section 422 of the Internal Revenue Code. Each grant of an option under the 2008 Options Plans will be evidenced by a Stock Option Agreement between the optionee and the Company. The exercise price will be specified in each Stock Option Agreement, however, option prices will not be less than 100% of the fair market value of an ordinary share on the date the option is granted.
Share option awards are granted with an exercise price equal to the market price of the Company’s shares at date of grant. Share options typically vest over a period of five years from date of grant and expire eight years from date of grant. Share options granted to Non-Executive Directors during 2018 vest over 12 months and expire eight years from the date of grant.
Legacy PRA Equity Incentive Plans
The following represent the legacy PRA equity incentive plans, which still have equity outstanding but have been terminated as of July 1, 2021 as to grants of future awards.
Pursuant to the Merger Agreement, effective on July 1, 2021, each outstanding stock option and restricted stock unit under the PRA Plans was assumed by the Company and converted into a stock option or Restricted Share Unit exercisable for or payable in Ordinary Shares based on the ratio of the average trading price per Ordinary Share for the ten days prior to July 1, 2021, and the corresponding value of the merger consideration for each PRA Share. Accordingly, the plans as detailed below were assumed by the Company.
PRA Health Sciences, Inc. 2020 Stock Incentive Plan was amended and restated and assumed by the Company effective as of July 1, 2021. The 2020 Stock Incentive Plan (“the 2020 Plan”), was approved by the PRA stockholders at their annual meeting on May 18, 2020. The 2020 Plan allowed for the issuance of stock options, stock appreciation rights, restricted shares and restricted stock units, other stock-based awards, and performance compensation awards as permitted by applicable laws. The 2020 Plan authorized the issuance of 2.5 million shares of common stock plus all shares that remained available under the prior plan on May 18, 2020.
The PRA Health Sciences, Inc. 2018 Stock Incentive Plan was amended and restated and assumed by the Company effective as of July 1, 2021. The 2018 Stock Incentive Plan (the “2018 Plan”), was approved by the PRA stockholders at their annual meeting on May 31, 2018. The 2018 Plan allowed for the issuance of stock options, stock appreciation rights, restricted shares and restricted stock units, other stock-based awards, and performance compensation awards as permitted by applicable laws. The 2018 Plan authorized the issuance of 2 million shares of common stock plus all shares that remained available under the 2014 Plan on May 31, 2018 (which included shares carried over from the 2013 Plan).
The PRA Health Sciences, Inc. 2014 Omnibus Incentive Plan was amended and restated and assumed by the Company effective as of July 1, 2021 (the “2014 Plan”). On November 23, 2014, the PRA Health Sciences, Inc. Board of Directors approved the formation of the 2014 Plan for Key PRA Employees. The 2014 Plan allowed for the issuance of stock options, stock appreciation rights, restricted shares and restricted stock units, other stock-based awards, and performance compensation awards as permitted by applicable laws.
The 2013 Stock Incentive Plan for Key Employees of PRA Health Sciences and its Subsidiaries was amended and restated and assumed by the Registrant effective as of July 1, 2021 (the “2013 Plan”). On September 23, 2013, the PRA Health Sciences, Inc. Board of Directors approved the formation of the 2013 Plan for Key Employees of Pinnacle Holdco Parent, Inc. and its subsidiaries. The 2013 Plan allowed for the issuance of stock options and other stock-based awards as permitted by applicable laws. The number of shares available for grant under the 2013 Plan was 12.5% of the outstanding shares at closing on a fully diluted basis. The 2013 Plan authorized the issuance of 2,052,909 shares of common stock.
Item 7. Major Shareholders and Related Party Transactions.
A.Major Shareholders
The following table sets forth certain information regarding beneficial ownership of ICON's ordinary shares as of February 24, 2023 (i) by each person that beneficially owns more than 5% of the outstanding ordinary shares, based upon information known to us and publicly available information; and (ii) by all of our current Directors, officers and other key employees as a group. Unless otherwise indicated below, to our knowledge, all persons listed below have sole voting and investment power with respect to their ordinary shares, except to the extent authority is shared by spouses under applicable law. None of the persons listed below have voting rights that differ from any other person listed below.
| | | | | | | | | | | | | | | | | | | | |
| 2022 (4) | 2021 (5) | 2020 (6) |
| Name of Owner or Identity of Group | No. of Shares (1) | Percent of Class | No. of Shares (1) | Percent of Class | No. of Shares (1) | Percent of Class |
| | | | | | |
Massachusetts Financial Services Company (2) | 8,119,214 | | 9.9 | % | 7,228,317 | | 8.9 | % | 2,268,437 | | 3.9 | % |
| WCM Investment Management (2) | 5,885,414 | | 7.2 | % | 7,179,979 | | 8.8 | % | 3,976,550 | | 7.5 | % |
| | | | | | |
| All Directors, officers and other key employees as a group (3) | 1,116,311 | | 1.4 | % | 1,129,726 | | 1.4 | % | 1,152,168 | | 2.2 | % |
(1)As used in this table, each person has the sole or shared power to vote or direct the voting of a security, or the sole or shared investment power with respect to a security (i.e., the power to dispose, or direct the disposition, of a security). A person is deemed as of any date to have "beneficial ownership" of any security if that such person has the right to acquire such security within 60 days after such date.
(2)Neither the Company nor any of its officers, Directors or affiliates holds any voting power in this entity.
(3)Includes 401,416 ordinary shares issuable upon the exercise of stock options granted by the Company, 31,940 RSUs awarded by the Company to Directors, officers and other key employees and 88,074 PSUs awarded by the Company to Directors, officers and other key employees. Of the PSUs, performance conditions determine how many of them will vest and, if performance targets are exceeded, additional PSUs will be issued and vest in accordance with the terms of the relevant PSU award, the figure included is the maximum amount of PSUs that may be issued.
(4)This information is based on the Schedule 13G, Amendment No. 2, filed with the SEC by Massachusetts Financial Services Company on February 8, 2023 for shares held on December 30, 2022; and the Schedule 13G/ Amendment No. 8 filed with the SEC by WCM Investment Management on February 10, 2023 for shares held on December 31, 2022, respectively.
(5)This information is based on the Schedule 13G, Amendment No. 1, filed with the SEC by Massachusetts Financial Services Co on February 2, 2022 for shares held on December 31, 2021; and the Schedule 13F filed with the SEC by WCM Investment Management on January 26, 2022 for shares held on December 31, 2021; respectively.
(6)This information is based on the Schedule 13F/ Amendment No. 1 filed with the SEC by Massachusetts Financial Services Company on May 12, 2021 for shares held on December 31, 2020; and the Schedule 13F, Amendment No. 1, filed with the SEC by WCM Investment Management on February 23, 2021 for shares held on December 31, 2020; respectively.
ICON plc, is not directly or indirectly, owned or controlled by another corporation or by any government.
B.Related Party Transactions
Subsidiaries of the Company earned revenue of $2,000 (December 31, 2021: $30,000) from DS Biopharma Limited (formerly Dignity Sciences Limited) during the year ended December 31, 2022. Dr. John Climax is Executive Chairman and a Director and shareholder of DS Biopharma Limited. $12,000 was recorded as due from DS Biopharma Limited at December 31, 2022 (December 31, 2021: $12,000).
Subsidiaries of the Company earned revenue of $235,000 (December 31, 2021: $551,000) from Afimmune Limited during the year ended December 31, 2022. Dr. John Climax is Chief Executive Officer and a Director and shareholder of Afimmune Limited. $263,000 was recorded as due from Afimmune Limited at December 31, 2022 (December 31, 2021: $197,000).
Subsidiaries of the Company earned revenue of $428,000 from Corvus Pharmaceuticals during the year ended December 31, 2022. Dr. Linda Grais serves as a Director and shareholder of Corvus Pharmaceuticals. $231,000 was recorded as due from Corvus Pharmaceuticals at December 31, 2022.
On July 24, 2020, a subsidiary of the Company, ICON Clinical Research Limited, entered into an agreement to jointly establish a new company, Oncacare, with a third party. The Company has invested $4.9 million to obtain a 49% interest in the voting share capital of Oncacare. The Company provided corporate support services to Oncacare to the value of $451,000 during the year ended December 31, 2022 (December 31, 2021: $465,000). $715,000 was recorded as due from Oncacare at December 31, 2022 (December 31, 2021: $264,000). During the year ended December 31, 2021, the Company provided a loan of $10 million to Oncacare in order to fund the continued start up of the business' operations. The loan accrues annual interest at 1.6% and the loan is repayable on June 30, 2025. The Company has recorded losses of $3.1 million and $2.2 million representing its pro rata share of the losses in Oncacare during the year ended December 31, 2022 and December 31, 2021, respectively. The carrying value of the Company's investment in Oncacare was reduced by $2.3 million of pro rata losses. The remaining $0.8 million in pro rata losses served to reduce the carrying value of the Company's loan receivable from Oncacare to $9.2 million. At December 31, 2022 accrued interest of $183,000 remained outstanding.
The majority investor in Oncacare has the right to sell the 51% majority voting share capital exclusively to the Company in an eighteen month period, commencing January 1, 2023 and ICON also has the right to acquire the 51% majority voting share capital from August 1, 2025.
C. Interests of experts and counsel
Not applicable
Item 8. Financial Information.
A.Consolidated Statements and Other Financial Information
See Item 18.
Legal Proceedings
We do not expect any current litigation to have a materially adverse effect on our financial condition or results of operations. However, from time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. Litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business.
Dividend Policy
We have not paid cash dividends on our ordinary shares and do not currently intend to pay cash dividends on our ordinary shares in the foreseeable future.
B.Significant Changes
There have been no significant changes to our business that we believe could reasonably be expected to have a material adverse effect on our business, results of operations and financial condition.
Item 9. The Offer and Listing.
A.Offer and listing details
ICON’s ordinary shares are traded on the NASDAQ Global Select Market under the symbol “ICLR”. ICON plc’s American Depositary Receipt ("ADR") program was terminated on January 31, 2013 and ICON plc’s ordinary shares began directly trading on NASDAQ on February 4, 2013. Prior to that date, ICON plc’s American Depositary Shares ("ADSs") were traded on NASDAQ and ICON plc’s Depository for the ADSs was The Bank of New York Mellon.
B.Plan of distribution
Not applicable.
C.Markets
NASDAQ.
D.Selling shareholders
Not applicable.
E.Dilution
Not applicable.
F.Expenses of the issue
Not applicable.
Item 10. Additional Information.
A.Share Capital
Not applicable.
B.Memorandum and articles of association
Constitution
We hereby incorporate by reference our Constitution, as amended, located under the heading “Constitution of the Company” in Exhibit 3.1.
The following is a summary of certain provisions of the current Constitution of the Company. This summary does not purport to be complete and is qualified in its entirety by reference to the complete text of the Constitution of the Company, which are included as an exhibit to this annual report.
Objects
The Company is incorporated under the name ICON plc, and is registered in Ireland under registered number 145835. The Company's objects, which are detailed in the Constitution of the Company, are broad and include, but are not limited to the carrying on the business of an investment holding company.
Directors
Subject to certain exceptions, Directors may not vote on matters in which they have a material interest. Any Director who holds any executive office, serves on any Committee or otherwise performs services, which, in the opinion of the Directors, are outside the scope of the ordinary duties of a Director, may be paid such extra remuneration as the Directors may determine. The Directors may exercise all the powers of the Company to borrow money. These powers may be amended by special resolution of the shareholders. There is no requirement for Directors to hold shares set out in the Constitution. The Directors are not required to retire at any particular age. The Constitution provides that one-third of the Directors must retire and offer themselves for re-election at each Annual General Meeting ("AGM") of the Company and that the Directors to retire by rotation are those who have been longest in office since their last appointment or reappointment. However in July 2022, the Board of Directors unanimously agreed that all of the Directors will retire and stand for re-election annually at each AGM. All of the shareholders entitled to attend and vote at the AGM may vote on the re-election of Directors.
Rights, Preferences and Dividends Attaching to Shares
The Company has only one class of shares, Ordinary Shares with a par value of €0.06 per share. All such Ordinary Shares rank equally with respect to voting, payment of dividends and on any winding-up of the Company. Any dividend, interest or other sum payable to a shareholder that remains unclaimed for one year after having been declared may be invested by the Directors for the benefit of the Company until claimed. If the Directors so resolve, any dividend which has remained unclaimed for 12 years from the date of its declaration shall be forfeited and cease to remain owing by the Company. In the event of the Company being wound up, if the assets available for distribution among the Members shall be more than sufficient to repay the whole of the share capital paid up or credited as paid up at the commencement of the winding up, the excess shall be distributed among the Members in proportion to the capital at the commencement of the winding up paid up or credited as paid up on the said Ordinary Shares held by them respectively. An Ordinary Share shall be deemed to be a redeemable share in certain circumstances. The liability of shareholders to invest additional capital is limited to the amounts remaining unpaid on the shares held by them.
Action Necessary to Change the Rights of Shareholders
The rights attaching to shares in the Company may be varied by special resolutions passed at class meetings of that class of shareholders of the Company.
Annual and General Meetings
The AGM shall be held in such place and at such time as shall be determined by the board, but no more than 15 months shall pass between the dates of consecutive AGMs. Directors may call an Extraordinary General Meeting (“EGM”) at any time. The members, in accordance with the Constitution of the Company and Irish Company law, may also requisition EGMs. Notice of the AGM or an EGM passing any special resolution must be given at least 21 clear days prior to the scheduled date and, in the case of any other general meeting, not less than 14 clear days’ notice. All holders of Ordinary Shares are entitled to attend, speak at and vote at general meetings of the Company.
Limitations on the Right to Own Shares
There are no limitations on the right to own shares in the Constitution of the Company.
Disclosure of Share Ownership
Under Irish law, the Company can require parties to disclose their interests in shares. The Constitution of the Company entitle the Directors to require parties to provide details regarding their identity and the nature and extent of any interest which such parties hold in Ordinary Shares. Under Irish law, if a party acquires or disposes of Ordinary Shares so as to bring their interest above or below 3% of the total issued share capital of the Company, they must notify the Company of that. The Company would also need to be notified of the acquisition by an existing substantial (i.e. 3% plus) shareholder, of every movement of one whole percentage integer (e.g. 3.9% to 4.1% but not 4.1% to 4.9%) or more.
Other Provisions of the Constitution
There are no provisions in the Constitution of the Company:
(i) delaying or prohibiting a change in the control of the Company, but which operate only with respect to a merger, acquisition or corporate restructuring;
(ii) discriminating against any existing or prospective holder of shares as a result of such shareholder owning a substantial number of shares; or
(iii) governing changes in capital, in each case, where such provisions are more stringent than those required by law.
C.Material Contracts
The following is a summary of each contract (not being a contract entered into in the ordinary course of business) that has been entered into: (a) within the two years immediately preceding the date of this Form 20-F which are, or may be, material to us; or (b) at any time which contain obligations or entitlements which is, or may be, material to us as at the date of this Form 20-F:
Agreement and Plan of Merger
On February 24, 2021, we entered into an Agreement and Plan of Merger (the “Merger Agreement”) with PRA Health Sciences, Inc. (“PRA”), ICON US Holdings Inc., a Delaware corporation and subsidiary of ICON (“US HoldCo”), and Indigo Merger Sub, Inc., a Delaware corporation and subsidiary of ICON and US HoldCo (“Merger Subsidiary”). On July 1, 2021 (the
“Closing Date”), pursuant to the terms and subject to the conditions of the Merger Agreement, Merger Sub was merged with and into PRA, with PRA surviving as a subsidiary of ICON and US HoldCo.
As a result of the Merger, each share of PRA common stock issued and outstanding immediately prior to the completion of the Merger (other than shares held by any shareholder who properly demands and perfects his, her or its appraisal rights with respect to such shares and treasury shares held by PRA) was canceled and converted into the right to receive: (i) from ICON, 0.4125 of one ICON ordinary share and (ii) from US Holdco and the surviving corporation $80.00 in cash, without interest.
Equity awards of PRA that are outstanding prior to the effective time of the Merger were generally treated as follows (subject to the terms and conditions set forth in the Merger Agreement):
•Each outstanding PRA stock option and restricted stock unit was assumed by ICON on the same terms and conditions (including vesting conditions) and converted to a stock option or restricted stock unit based on ICON ordinary shares with the number of ICON ordinary shares and exercise price in the case of stock options determined at a conversion ratio as set forth under the Merger Agreement; and
•Each outstanding share of PRA restricted stock was vested at the Closing and was canceled and converted into the right to receive the per share merger consideration.
The foregoing description of the Merger and the Merger Agreement, and the related transactions contemplated thereby, does not purport to be complete and is subject to, and qualified in its entirety by reference to, the full text of the Merger Agreement which is attached as Exhibit 2.1 to ICON’s Current Report on Form 6-K filed with the Securities and Exchange Commission (the “SEC”) on February 24, 2021 and incorporated herein by reference herein.
Senior Secured Credit Facilities
In conjunction with the completion of the Merger Agreement, on July 1, 2021, ICON entered into a credit agreement providing for a senior secured term loan facility of $5,515 million and a senior secured revolving loan facility in an initial aggregate principal amount of $300 million (the "Senior Secured Credit Facilities"). The proceeds of the senior secured term loan facility were used to repay the outstanding amount of (i) PRA’s existing credit facilities and (ii) the Company's senior secured notes and fund, in part, the Merger. The senior secured term loan facility will mature in July 2028 and the revolving loan facility will mature in July 2026.
Borrowings under the senior secured term loan facility amortize in equal quarterly installments in an amount equal to 1.00% per annum of the principal amount, with the remaining balance due at final maturity. The interest rate margin applicable to borrowings under the senior secured term loan facility is USD Term SOFR and a Term SOFR Adjustment depending on the interest period chosen plus an applicable margin of 2.25% (with a step up of 0.25% if the first lien net leverage ratio exceeds 4.00 to 1.00). The senior secured term loan facility is subject to a floor of 0.50%. The interest rate margin applicable to borrowings under the revolving loan facility will be, at the option of the borrower, either (i) the applicable base rate plus an applicable margin of 1.00%, 0.60% or 0.25% based on ICON’s current corporate family rating assigned by S&P of BB- (or lower), BB or BB+ (or higher), respectively, or (ii) Term SOFR plus a Term SOFR Adjustment on the interest period chosen plus an applicable margin of 2.00%, 1.60% or 1.25% based on ICON’s current corporate family rating assigned by S&P of BB- (or lower), BB or BB+ (or higher), respectively.
The Borrowers’ (as defined in the Senior Secured Credit Facilities) obligations under the Senior Secured Credit Facilities are guaranteed by ICON and the subsidiary guarantors. The Senior Secured Credit Facilities are secured by a lien on substantially all of ICON’s, the Borrowers’ and each of the subsidiary guarantor’s assets (subject to certain exceptions), and the Senior Secured Credit Facilities will have a first-priority lien on such assets, which will rank pari passu with the lien securing the Senior Secured Notes (see below), subject to other permitted liens.
The Senior Secured Credit Facilities contain customary negative covenants, including, but not limited to, restrictions on the ability of ICON and its subsidiaries to merge and consolidate with other companies, incur indebtedness, grant liens or security interests on assets, pay dividends or make other restricted payments, sell or otherwise transfer assets or enter into transactions with affiliates. In addition, the revolving credit loan facility contains a financial covenant that requires ICON to maintain a Total Net Leverage Ratio (as defined in the Senior Secured Credit Facilities) of 5.75:1.00 prior to June 30, 2023 and 4.50:1.00 on and after June 30, 2023, subject to a step-down of 0.50:1.00 following a Material Acquisition (as defined in the Senior Secured Credit Facilities), which will be tested at the end of any fiscal quarter only if amounts are drawn under the revolving credit loan facility (excluding cash collateralized and backstopped letters of credit) in excess of 30% of the revolving commitments.
The Senior Secured Credit Facilities provide that, upon the occurrence of certain events of default, the obligations thereunder may be accelerated. Such events of default will include payment defaults to the lenders thereunder, material inaccuracies of representations and warranties, covenant defaults, cross-defaults to other material indebtedness, voluntary and involuntary bankruptcy proceedings, material money judgments, material pension-plan events, change of control and other customary events of default.
Senior Secured Notes
In addition to the Senior Secured Credit Facilities, on July 1, 2021, Indigo Merger Sub, Inc. (which was merged with and into PRA Health Sciences, Inc.) (the “Issuer”), a wholly-owned subsidiary of the Company, issued $500.0 million in aggregate principal amount of 2.875% senior secured notes due July 2026 (the “Senior Secured Notes”) in a private offering (the “Offering”). The Senior Secured Notes will mature on July 15, 2026. The Issuer will pay interest on the Senior Secured Notes on January 15 and July 15 of each year. Interest on the Senior Secured Notes will accrue at a rate of 2.875% per annum.
The proceeds from the Offering and borrowings made under the Senior Secured Credit Facilities, together with cash on hand, were used to (i) fund the cash consideration payable by ICON for the Merger, (ii) repay existing indebtedness of ICON and PRA and (iii) pay fees and expenses related to the Merger, the Offering and the Senior Secured Credit Facilities.
The Senior Secured Notes are guaranteed on a senior secured basis by ICON and its direct and indirect subsidiaries that guarantee the Senior Secured Credit Facilities. The Senior Secured Notes are secured by a lien on substantially all of ICON’s, the Issuer’s and each of the subsidiary guarantor’s assets (subject to certain exceptions), and the Senior Secured Notes have a first-priority lien on such assets, which rank pari passu with the liens securing the Senior Secured Credit Facilities, subject to other permitted liens.
At any time prior to July 15, 2023, the Issuer may redeem all or part of the Senior Secured Notes at a redemption price equal to 100% of the principal amount of the Notes plus an applicable make whole premium and accrued and unpaid interest to, but not including the redemption date. At any time prior to July 15, 2023, the Issuer may, with the proceeds of certain equity offerings, redeem up to 40% of the aggregate principal amount of the Senior Secured Notes at a redemption price of 102.875% of the principal amount plus accrued and unpaid interest to, but not including, the date of redemption. In addition, at any time and from time to time prior to July 15, 2023, the Issuer may redeem up to 10% per annum of the aggregate principal amount of the Senior Secured Notes at a redemption price of 103.000% of the aggregate principal amount thereof, plus accrued and unpaid interest, if any, to, but excluding, the date of redemption.
The Indenture contains contain customary negative covenants, including, but not limited to, restrictions on the ability of ICON and its subsidiaries to merge and consolidate with other companies, incur indebtedness, grant liens or security interests on assets, pay dividends or make other restricted payments, sell or otherwise transfer assets or enter into transactions with affiliates. The Indenture contains customary events of default and remedies.
D.Exchange Controls and Other Limitations Affecting Security Holders
Irish exchange control regulations ceased to apply from and after December 31, 1992. Except as indicated below, there are no restrictions on non-residents of Ireland dealing in domestic securities, which includes shares or depository receipts of Irish companies. Except as indicated below, dividends and redemption proceeds also continue to be freely transferable to non-resident holders of such securities.
The Financial Transfers Act, 1992 gives power to the Minister for Finance of Ireland to make provision for the restriction of financial transfers between Ireland and other countries and persons. Financial transfers are broadly defined, and include all transfers which would be movements of capital or payments within the meaning of the treaties governing the European Communities. The acquisition or disposal of shares issued by an Irish incorporated company and associated payments may fall within this definition. In addition, dividends or payments on redemption or purchase of shares and payments on a liquidation of an Irish incorporated company would fall within this definition.
The Financial Transfers Act, 1992 prohibits financial transfers involving a number of persons, entities and bodies, which is subject to amendment on an ongoing, regular basis and currently includes, but is not limited to: certain persons and activities in Belarus, Bosnia & Herzegovina, Burundi, Sudan, South Sudan, the Central African Republic, Libya, Lebanon, Mali, the Democratic People's Republic of Korea, Myanmar/Burma, Tunisia, Zimbabwe, Venezuela, certain persons, entities and bodies in the Syrian Arab Republic, the Republic of Guinea-Bissau, Nicaragua, Democratic Republic of Congo, Iran, Ukraine and Russia; persons associated with the Taliban in Afghanistan, associated with ISIL (Da’esh) and Al-Qaeda, associated with Turkey’s unauthorized drilling activities in the Eastern Mediterranean and certain known terrorists and terrorist groups and countries that harbor certain terrorist groups, without the prior permission of the Central Bank of Ireland.
There are no restrictions under the Company’s Constitution or under Irish Law that limit the right of non-residents or foreign owners to hold the Company’s ordinary shares or vote at general meetings of the Company.
E.Taxation
General
The following discussion is based on existing Irish tax law, Irish court decisions and the practice of the Revenue Commissioners of Ireland, and the convention between the United States and Ireland for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with respect to taxes on income and capital gains (the "Treaty"). This discussion does not purport to deal with the tax consequences of owning the ordinary shares for all categories of investors, some of which may be subject to special rules. Prospective purchasers of ordinary shares are advised to consult their own tax advisors concerning the overall tax consequences arising in their own particular situations under Irish law. Each prospective investor should understand that future legislative, administrative and judicial changes could modify the tax consequences described below, possibly with retroactive effect.
As used herein, the term "U.S. Holder" means a beneficial owner of ordinary shares that (i) owns the ordinary shares as capital assets; (ii) is a U.S. citizen or resident, a U.S. corporation, an estate the income of which is subject to U.S. federal income taxation regardless of its source or a trust that meets the following two tests: (A) a U.S. court is able to exercise primary supervision over the administration of the trust, and (B) one or more U.S. persons have the authority to control all substantial decisions of the trust; and for the purpose of the discussion under Irish Taxation of U.S. Holders (A) is not a resident of, or ordinarily resident in, Ireland for the purposes of Irish tax; and (B) is not engaged in trade or business in Ireland through a permanent establishment.
AS USED HEREIN, REFERENCES TO THE ORDINARY SHARES SHALL INCLUDE SHARES HELD IN THE ACCOUNTS OF PARTICIPANTS THROUGH THE DEPOSITARY TRUST COMPANY (“THE DTC”).
Irish Taxation
Irish corporation tax on income
ICON is a public limited company incorporated and resident for tax purposes in Ireland by virtue of its place of central management and control being in Ireland.
Companies which are resident in the Republic of Ireland ("Ireland") are subject to Irish corporation tax on their total profits (wherever arising and, generally, whether or not remitted to Ireland). The question of residence, by virtue of management and control, is essentially one of fact. It is the present intention of the Company's management to continue to manage and control the Company from Ireland, so that the Company will continue to be resident in Ireland.
The standard rate of Irish corporation tax on trading income (with certain exceptions) is currently 12.5%.
A research and development tax credit is available in Ireland where an Irish resident company incurs qualifying expenditure on research and development activities. Qualifying expenditure incurred in a particular accounting period results in a tax credit of 25% of that expenditure.
Corporation tax is charged at the rate of 25% on a company's non-trading income and certain types of trading income not eligible for the lower rate of 12.5% referred to above.
Capital gains arising to an Irish resident company are liable to tax at 33%. However, a capital gains tax exemption is available in Ireland for qualifying Irish resident companies in respect of disposals of certain qualifying shareholdings.
The exemption from capital gains tax on the disposal of shares by an Irish resident company will apply where certain conditions are met. These conditions principally are:
•The company claiming the exemption must hold (directly or indirectly) at least 5% of the ordinary share capital of the company in which the interest is being disposed of, throughout a continuous period of at least 12 months, in the two-year period prior to disposal;
•The shares being disposed of must be in a company, which at the date of disposal, is resident in a Member State of the European Communities or in a country with which Ireland has signed or made specific arrangements to sign a double tax agreement (together a “Relevant Territory”);
•The shares must be in a company which is primarily a trading company or the company making the disposal together with its “5% plus subsidiaries” should be primarily a trading group; and
•The shares must not derive the greater part of their value from land or mineral rights in Ireland.
Irish withholding tax on dividends
Unless specifically exempted, all dividends paid by the Company, will be subject to Irish withholding tax. The current rate for dividend withholding tax is 25%.
An individual shareholder who is neither resident nor ordinarily resident for tax purposes in Ireland but is resident in a country with which Ireland has a double tax treaty (which includes the U.S.), or in a member state of the European Communities, other than Ireland (together, a Relevant Territory), will be exempt from withholding tax provided he or she makes the requisite declaration.
Irish resident corporate shareholders will be exempt from withholding tax. Where the shareholding held by the recipient company, in the company paying the dividend is not 51% or greater a declaration must be made to avail of the exemption.
Non-Irish resident corporate shareholders will be exempt from withholding tax on the production of the appropriate certificates and declarations where they:
•are resident in a Relevant Territory and are not controlled (directly or indirectly) by Irish residents;
•are ultimately controlled (directly or indirectly) by residents of a Relevant Territory;
•have the principal class of their shares, or shares of a 75% parent, substantially and regularly traded on one or more recognized stock exchanges in a Relevant Territory (including Ireland) or Territories; or
•are wholly owned by two or more companies, each of whose principal class of shares is substantially and regularly traded on one or more recognized stock exchanges in a Relevant Territory (including Ireland) or Territories.
U.S. holders of ordinary shares should note, however, that detailed documentation requirements may need to be complied with. Special arrangements are available in the case of an interest in shares held in Irish companies through a depositary or in accounts of participants through the DTC. In certain cases, the depositary or the DTC can receive and pass on a dividend from an Irish company without deducting withholding tax, provided the depositary or the DTC is a qualifying intermediary, and provided the person beneficially entitled to the distribution would meet the same conditions outlined above for the withholding tax exemption to apply and has provided the qualifying intermediary with the appropriate declarations. The depositary or the DTC shall be regarded as a qualifying intermediary provided the following conditions are met:
•the depositary or the DTC is resident in a Relevant Territory;
•the depositary or the DTC have entered into a qualifying intermediary agreement with the Irish tax authorities (Irish Revenue Commissioners); and
•the depositary or the DTC have been authorized by the Irish Revenue Commissioners as a qualifying intermediary and such authorization has not expired or been revoked.
Irish income tax on dividends
Irish resident or ordinarily resident shareholders will generally be liable to Irish income tax on dividend income at their marginal rate of income tax. This income may also be liable to Pay Related Social Insurance (“PRSI”) of up to 4% and the Universal Social Charge (“USC”) of up to 11% (up to 15% in total).
Under certain circumstances, non-Irish resident shareholders will be subject to Irish income tax on dividend income. Where withholding tax of 25% has been deducted, this will fully satisfy the non-Irish resident shareholder’s tax liability. No PRSI or USC should apply in these circumstances.
However, a non-Irish resident shareholder will not have an Irish income tax liability on dividends from the Company if the holder is neither resident nor ordinarily resident in Ireland and the holder is:
•an individual resident in a Relevant Territory;
•a corporation that is ultimately controlled by person(s) resident in a Relevant Territory;
•a corporation whose principal class of shares (or its 75% or greater parent’s principal class of shares) is substantially and regularly traded on a recognized stock exchange in an EU country or in a Relevant Territory;
•a corporation resident in another EU member state or in a Relevant Territory, which is not controlled directly or indirectly by Irish residents; or
•a corporation that is wholly owned by two or more corporations each of whose principal class of shares is substantially and regularly traded on a recognized stock exchange in an EU country or in a Relevant Territory.
U.S. Holders who do not qualify for the above income tax exemption may be able to obtain treaty benefits under the Treaty.
Irish domicile levy
Certain non-Irish resident individuals that are domiciled in Ireland will be subject to an annual levy of €200,000 if the market value of their Irish-located property on 31 December exceeds €5,000,000, their worldwide annual income exceeds €1,000,000 and their liability to Irish income tax in that year is less than €200,000.
Irish capital gains tax on disposal of shares
Irish resident or ordinarily resident shareholders will be liable to capital gains tax at 33% on gains arising from the disposal or part disposal of their shareholding.
A person who is not resident or ordinarily resident in Ireland, who has not been an Irish resident within the past five years and who does not carry on a trade in Ireland through a branch or agency will not be subject to Irish capital gains tax on the disposal of ordinary shares or shares held in accounts of participants through the DTC, so long as the shares do not derive the greater part of their value from Irish land or mineral rights.
There are provisions to subject a person who disposes of an interest in a company while temporarily being non-Irish resident, to Irish capital gains tax. This treatment will apply to Irish domiciled individuals:
•who cease to be Irish resident;
•who beneficially own the relevant assets when they cease to be resident;
•if there are not more than 5 years of assessment between the last year of Irish tax residence prior to becoming temporarily non-resident and the tax year that he/she resumes Irish tax residency;
•who dispose of the relevant assets during this temporary non-residence; and
•the interest disposed of represents 5% or greater of the issued share capital of the company or is worth at least €500,000.
In these circumstances the person will be deemed, for Irish capital gains tax purposes, to have sold and immediately reacquired the interest in the company on the date of his or her departure and will be subject to tax at 33% of the taxable gain.
Irish capital acquisitions tax
Irish capital acquisitions tax (referred to as CAT) applies to gifts and inheritances. Subject to certain tax-free thresholds, gifts and inheritances are liable to tax at 33%.
Where a gift or inheritance is taken under a disposition made after December 1, 1999 it will be within the charge to CAT:
•to the extent that the property of which the gift or inheritance consists is situated in Ireland at the date of the gift or inheritance;
•where the person making the gift or inheritance is or was resident or ordinarily resident in Ireland at the date of the disposition under which the gift or inheritance is taken;
•in the case of a gift taken under a discretionary trust where the person from whom the gift is taken was resident or ordinarily resident in Ireland at the date he/she made the settlement, or at the date of the gift or, if he/she is dead at the date of the gift, at the date of his/her death; or
•where the person receiving the gift or inheritance is resident or ordinarily resident in Ireland at the date of the gift or inheritance.
For these purposes a non-Irish domiciled individual will not be regarded as resident or ordinarily resident in the Republic of Ireland on a particular date unless they are resident or ordinarily resident in Ireland on that date and have been resident for the 5 consecutive tax years immediately preceding the year of assessment in which the date falls.
The person who receives the gift or inheritance (“the beneficiary”) is primarily liable for CAT. In the case of an inheritance, where a beneficiary and personal representative of the deceased are both non-residents, a solicitor must be appointed to be responsible for paying inheritance tax. Taxable gifts or inheritances received by an individual since December 5, 1991 from donors in the same threshold class are aggregated and only the excess over a specified tax-free threshold is taxed. The tax-free threshold is dependent on the relationship between the donor and the beneficiary and the aggregation since December 5, 1991 of all previous gifts and inheritances, within the same tax threshold.
The tax-free threshold amounts that apply are:
•€16,250 in the case of persons who are not related to one another;
•€32,500 in the case of gifts or inheritances received from inter alia a brother or sister or from a brother or sister of a parent or from a grandparent; and
•€335,000 in the case of gifts and inheritances received from a parent (or from a grandparent by a minor child of a deceased child) and specified inheritances received by a parent from a child for gifts or inheritances taken on or after October 9, 2019.
Gifts and inheritances passing between spouses are exempt from CAT.
A gift or inheritance of the ordinary shares or American Depositary Shares (ADSs) in the Company will be within the charge to CAT, notwithstanding that the person from whom or by whom the gift or inheritance is received is domiciled or resident outside Ireland.
The Estate Tax Convention between Ireland and the United States generally provides for CAT paid on inheritances in Ireland to be credited against U.S. Federal Estate tax payable in the U.S. and for tax paid in the U.S. to be credited against tax payable in Ireland, based on priority rules set forth in the Estate Tax Convention. The Estate Tax Convention does not apply to CAT paid on gifts.
Irish stamp duty
Irish stamp duty, which is a tax on certain documents, is payable on all transfers of ordinary shares (other than between spouses) whenever a document of transfer is executed. Where the transfer is attributable to a sale of shares, stamp duty will be charged at a rate of 1%, rounded to the nearest euro. The stamp duty is calculated on the amount or value of the consideration (i.e. purchase price) or, if the transfer is by way of a gift (subject to certain exceptions) or for consideration less than the market value, on the market value of the shares. Where the consideration for the sale is expressed in a currency other than euro, the duty will be charged on the euro equivalent calculated at the rate of exchange prevailing on the date of the transfer.
Transfers through the DTC of book entry interests in shares are not subject to Irish stamp duty.
A transfer of ordinary shares by a shareholder to a depositary or custodian for deposit and a transfer of ordinary shares from the depositary or the custodian for the purposes of the withdrawal of the underlying ordinary shares in accordance with the terms of a deposit agreement will be subject to stamp duty at the 1% rate if the transfer relates to a sale, a contemplated sale, a gift or any other change in the beneficial ownership of such ordinary shares. However, transfers of ordinary shares into or out of the DTC are not subject to Irish stamp duty where no change in beneficial ownership of the shares has occurred and provided a contract for sale in respect of the transferring shares is not in place.
The person accountable for payment of stamp duty is normally the transferee or, in the case of a transfer by way of gift, or for a consideration less than the market value, all parties to the transfer.
Transfers of ordinary shares between associated companies (broadly, companies within a 90% group relationship and subject to the satisfaction of certain conditions) are exempt from stamp duty in Ireland. In the case of transfers of ordinary shares where no beneficial interest passes (e.g. a transfer of shares from a beneficial owner to his nominee), no stamp duty arises.
No stamp duty shall arise on the transfer of ordinary shares where the consideration for the transfer does not exceed €1,000, provided the instrument contains a statement certifying that the transaction does not form part of a larger transaction or a series of larger transactions, in respect of which the amount of the total consideration attributable to the shares would exceed €1,000.
F.Dividends and paying agents
Not applicable.
G.Statement by experts
Not applicable.
H.Documents on Display
We are subject to the informational requirements of the Securities Exchange Act of 1934, as amended, (the “Exchange Act”) and file reports and other information with the SEC. The SEC maintains a website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC at http://www.sec.gov.
We “incorporate by reference” information that we file with the SEC, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is an important part of this
report and more recent information automatically updates and supersedes more dated information contained or incorporated by reference in this report. Our SEC file number for Exchange Act reports is 333-08704.
As a foreign private issuer, we are exempt from certain rules under the Exchange Act, including prescribing the furnishing and content of proxy statements to shareholders.
We will provide without charge to each person, including any beneficial owner, on the written or oral request of such person, a copy of any or all documents referred to above which have been or may be incorporated by reference in this report (not including exhibits to such incorporated information that are not specifically incorporated by reference into such information). Requests for such copies should be directed to us at the following address: ICON plc, South County Business Park, Leopardstown, Dublin 18, Ireland, D18 X5R3 Attention: Corporate Governance, email: corporate.governance@iconplc.com.
I.Subsidiary Information
Not applicable.
Exemptions From Corporate Governance Listing Requirements Under the NASDAQ Marketplace Rules
NASDAQ may provide exemptions from certain NASDAQ corporate governance standards to a foreign private issuer if, among other reasons those standards are contrary to a law, rule or regulation of a public authority exercising jurisdiction over such issuer or contrary to generally accepted business practices in the issuer’s home country of domicile, provided, that, the foreign private issuer properly notifies NASDAQ and makes the required disclosure except to the extent that such exemptions would be contrary to United States federal securities laws.
The exemptions that the Company relies on, and the practices the Company adheres to, are as follows:
•The Company is exempt from provisions set forth in NASDAQ Rule 5620(c), which requires each issuer (other than limited partnerships) to provide for a quorum in its by-laws for any meeting of the holders of common stock, which shall in no case be less than 33.33% of the outstanding shares of the issuer’s common voting stock. The Company’s Constitution requires that only 3 members be present, in person or by proxy, at a shareholder meeting to constitute a quorum. This quorum requirement is in accordance with Irish law and generally accepted business practices in Ireland.
•The Company is exempt from provisions set forth in NASDAQ Rule 5635(c) which requires (other than for certain specified exceptions) shareholder approval prior to the establishment or material amendment of a stock option or purchase plan or other equity compensation arrangement made or materially amended, pursuant to which stock may be acquired by officers, Directors, employees or consultants. Irish law does not require shareholder approval with respect to equity compensation arrangements. Accordingly, the 2019 Consultants and Directors Restricted Share Unit Plan, the 2013 Employees Restricted Share Unit Plan and the amendments to the Employee Share Option Plan 2008 and Consultants Share Option Plan 2008 were adopted by the Board of Directors without shareholder approval.
•The Company is exempt from provisions set forth in NASDAQ Rule 5605(b)(2), which requires independent Directors to hold regularly scheduled meetings at which only independent Directors are present. Irish law does not require independent Directors to hold regularly scheduled meetings at which only independent Directors are present. The Company holds regularly scheduled meetings which all of the Directors may attend and the Lead Independent Director may call meetings of the independent Directors and non-employee Directors of the Board, as appropriate, in accordance with the Lead Independent Director Charter.
Item 11. Quantitative and Qualitative Disclosures about Market Risk.
The principal market risks (i.e. risk of loss arising from adverse changes in market rates and prices) to which we are exposed include foreign currency risk and interest rate risk.
Foreign Currency Exchange Risk
We are subject to a number of foreign currency risks given the global nature of our operations. The principal foreign currency risks to which the business is subject to includes both foreign currency translation risk and foreign currency transaction risk.
Although domiciled in Ireland, we report our results in U.S. dollars. As a consequence, the results of our non-U.S. based operations, when translated into U.S. dollars, could be affected by fluctuations in exchange rates between the U.S. dollar and the currencies of those operations.
We are also subject to foreign currency transaction exposures as the currency in which our contracts are priced can be different from the currencies in which costs relating to those contracts are incurred. Our operations in the United States are not materially exposed to such currency differences as the majority of revenues and costs are in U.S. dollars. However, outside the United States the multinational nature of our activities means that contracts may be priced in a single currency, most often U.S. dollars, or euro, while costs arise in a number of currencies, depending, among other things, on which of our offices provide staff for the contract and the location of investigator sites. Although many such contracts benefit from some degree of natural hedging due to the matching of contract revenues and costs in the same currency, where costs are incurred in currencies other than those in which contracts are priced, fluctuations in the relative value of those currencies could have a material effect on our results of operations. We regularly review our foreign currency exposures and enter into forward currency contracts to manage our exposure. We had no open foreign currency contracts at December 31, 2022.
The following significant exchange rates applied during the year:
| | | | | | | | | | | | | | |
| Average Rate | Closing Rate |
| 2022 | 2021 | 2022 | 2021 |
| Euro:USD | 1.0512 | 1.1886 | | 1.0705 | 1.1370 | |
| Pound Sterling:USD | 1.2347 | 1.3788 | | 1.2083 | 1.3532 | |
Interest Rate Risk
We are exposed to interest rate risk in respect of our cash and cash equivalents and available for sale investments. Our treasury function actively manages our available cash resources and invests surplus cash balances to ensure optimum returns for the Company. Financial instruments are classified either as cash and cash equivalents or available for sale investments depending upon the maturity of the related investment. Funds may be invested in the form of floating rate notes and medium term minimum “A-” rated corporate securities. We may be subject to interest rate risk in respect of interest rate changes on amounts invested. Interest rate risk is managed by monitoring the composition of the Company’s investment portfolio on an ongoing basis having regard to current market interest rates and future trends.
Because the Company has variable rate debt, fluctuations in interest rates affect our business. We attempt to minimize interest rate risk and lower our overall borrowing costs through the utilization of interest rate cap and interest rate swap derivative agreements. We have entered into certain interest rate cap and interest rate swap agreements with three financial institutions with respect to a portion of our outstanding debt. Accordingly, any change in market value associated with these agreements is offset by the opposite market impact on the portion of the debt covered by such agreements. As December 31, 2022, we have interest rate cap agreements with a notional value of $2.1 billion to minimize interest rate risk on approximately $4.7 billion of variable rate indebtedness. Because we do not attempt to hedge all of our variable rate debt, we may incur higher interest costs for the portions of our unhedged debt.
We regularly evaluate our debt arrangements, as well as market conditions, and we will explore the opportunity to modify our existing arrangements or pursue additional financing arrangements that may result in the issuance of new debt securities by us or our affiliates.
The sensitivity analysis below represents the hypothetical change in the net interest payable of a 1% movement in market interest rates.
| | | | | | | | | | | |
| Interest for the year ended December 31, 2022 (in thousands) | Interest
Change 1% increase in
market interest rate
(in thousands) | Interest
Change 1% decrease in
market interest rate
(in thousands) |
| Interest income | $2,345 | | $8,322 | | $1 | |
| Interest expense* | ($229,731) | | ($277,546) | | ($181,982) | |
| | ($227,386) | | ($269,224) | | ($181,981) | |
*8% of the interest costs fixed due to high yield bond issuance. $20.5 million financing fees have been allocated to interest cost which are not impacted by a change in interest rate.
Item 12. Description of Securities Other than Equity Securities.
Not applicable.
Part II
Item 13. Defaults, Dividend Arrearages and Delinquencies.
None.
Item 14. Material Modifications to the Rights of Security Holders and Use of Proceeds.
None.
Item 15. Controls and Procedures.
A.Disclosure controls and procedures
An evaluation was carried out under the supervision and with the participation of the Company's management, including the Chief Executive Officer (CEO) and the Chief Financial Officer (CFO), of the effectiveness of our disclosure controls and procedures as at December 31, 2022. Based on that evaluation, the CEO and CFO have concluded that the Company's disclosure controls and procedures are effective to ensure that information required to be disclosed by the Company in reports that it files or submits under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified in Securities and Exchange Commission rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by the Company in the reports that it files or submits under the Securities Exchange Act of 1934 is accumulated and communicated to the Company’s management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
B.Management's Annual Report on Internal Accounting Control over Financial Reporting
Reference is made to page 95 of this Form 20-F.
C.Attestation Report of Independent Registered Public Accounting Firm
Reference is made to page 98 of this Form 20-F.
D.Changes in Internal Controls over Financial Reporting
There were no changes in our internal controls over financial reporting during the period covered by this Form 20-F that have materially affected or are reasonably likely to materially affect our internal controls over financial reporting.
Item 16. Reserved.
Item 16A. Audit Committee Financial Expert
Mr. Rónán Murphy acts as the Audit Committee financial expert serving on our Audit Committee and Board of Directors. The Board has determined that Mr. Murphy is independent.
Item 16B. Code of Ethical Conduct
Our Global Code of Ethical Conduct applies to all officers, directors (including a non-executive director when carrying out their duties as a director of ICON plc), and employees of ICON plc, its subsidiaries and branches. There are no waivers from the provisions of the Code of Ethical Conduct that are required to be disclosed. This Code of Ethical Conduct is available on our website at: https://investor.iconplc.com/corporate-governance/governance-documents.
Item 16C. Principal Accountant Fees and Services
Our principal accountants for the years ended December 31, 2022 and December 31, 2021 were KPMG, Dublin, Ireland (Audit firm ID: 1116). The table below summarizes the fees for professional services rendered by KPMG for the audit of our annual financial statements for the years ended December 31, 2022 and December 31, 2021 and fees billed for other services rendered by KPMG.
| | | | | | | | | | | | | | |
| Year ended
December 31, 2022
(in thousands) | Year ended
December 31, 2021
(in thousands) |
| Audit fees (1) | $ | 4,680 | | 47.1 | % | $ | 2,906 | | 32.0 | % |
| Audit related fees (2) | 34 | | 0.4 | % | 2,113 | | 23.2 | % |
| Tax fees (3) | 5,219 | | 52.5 | % | 4,066 | | 44.8 | % |
| All other fees (4) | — | | — | % | — | | — | % |
Total | $ | 9,933 | | 100 | % | $ | 9,085 | | 100 | % |
(1) Audit fees include annual audit fees for the Company and its subsidiaries.
(2) Audit related fees principally consist of fees for financial due diligence services, fees for the audit of employee benefit plans and fees for pension reviews.
(3) Tax fees are fees for tax compliance and tax consultation services.
(4) All other fees include the aggregate fees billed for products and services other than the services reported in items (1) through (3) above. There were no other fees amounts billed to the company in the years ended December 31, 2022 and December 31, 2021.
The Audit Committee pre-approves all audit and non-audit services provided to the Company by its auditors.
Item 16D. Exemptions from the Listing Standards for Audit Committees
Not applicable.
Item 16E. Purchases of Equity Securities by the Issuer and Affiliated Purchasers
In the year ended December 31, 2022, 420,530 ordinary shares were redeemed by the Company for a total consideration of $100.0 million. All ordinary shares that are redeemed under the buyback program were canceled in accordance with the constitutional documents of the Company and the nominal value of these shares transferred to an undenominated capital fund as required under Irish Company law.
| | | | | | | | | | | | | | |
| | Total Number
of Shares Purchased | Average Price Paid per Share | Total Number
of Shares Purchased | Total Price
Paid for Shares Purchased (in thousands) |
| | |
| March 03/01/2022 - 03/31/2022 | 420,530 | | $237.75 | | 420,530 | | $99,983 | |
| | 420,530 | | $237.75 | | 420,530 | | $99,983 | |
No ordinary shares were redeemed by the Company during the year ended December 31, 2021.
Under the repurchase programs, a broker purchased the Company's shares from time to time on the open market or in privately negotiated transactions in accordance with agreed terms and limitations. The programs are designed to allow share repurchases during periods when the Company would ordinarily not be permitted to do so because it may be in possession of material non-public or price-sensitive information, applicable insider trading laws or self-imposed trading blackout periods. The Company's instructions to the broker were irrevocable and the trading decisions in respect of the repurchase programs were made independently of and uninfluenced by the Company. The Company confirms that on entering the share repurchase plans it had no material non-public, price-sensitive or inside information regarding the Company or its securities. Furthermore, the Company will not enter into additional plans whilst in possession of such information. The timing and actual number of shares acquired by way of the redemption will be dependent on market conditions, legal and regulatory requirements and the other terms and limitations contained in the programs. In addition, acquisitions under the programs may be suspended or discontinued in certain circumstances in accordance with the agreed terms. Therefore, there can be no assurance as to the timing or number of shares that may be acquired under the programs.
Item 16F. Changes in Registrant's Certifying Accountant
Not applicable.
Item 16G. Corporate Governance
See Item 10 "Exemptions from Corporate Governance Listing Requirements under the NASDAQ Marketplace Rules".
Item 16H. Mine Safety Disclosure
Not applicable.
Item 16I. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
Part III
Item 17. Financial Statements.
See item 18.
Item 18. Financial Statements.
The following consolidated financial statements of ICON plc and its subsidiaries, and the independent registered public accounting firm's report thereon, are included in Part III, Item 18 of this Annual Report:
Management's Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934.
The Company's internal control over financial reporting is a process designed by, or under the supervision of, the Company's executive and financial officers and effected by the Company's board of Directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external reporting purposes in accordance with generally accepted accounting principles.
A company's internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorization of management and Directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of the inherent limitation due to, for example, the potential for human error or circumvention of control, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of the Company's internal control over financial reporting as of December 31, 2022. In making this assessment, we used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control – Integrated Framework 2013. Based upon the assessment performed, we determined that, as of December 31, 2022 the Company's internal control over financial reporting was effective. There have been no changes in the Company's internal control over financial reporting during 2022 that have materially affected, or are reasonably likely to affect materially, the Group's internal control over financial reporting.
KPMG, an independent registered public accounting firm, has audited the consolidated financial statements of ICON plc and subsidiaries as of and for the year ended December 31, 2022, included herein, and has issued an audit report on the effectiveness of our internal control over financial reporting, which is included below.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors
ICON plc:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of ICON plc and subsidiaries (the Company) as of December 31, 2022 and 2021, the related consolidated statements of operation, comprehensive income, shareholders’ equity, and cash flows for each of the years in the three‑year period ended December 31, 2022, and the related notes (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the results of its operations and its cash flows for each of the years in the three‑year period ended December 31, 2022, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2022, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission, and our report dated February 24, 2023 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of a critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Revenue recognition for clinical trial service contracts.
As discussed in Note 3 to the consolidated financial statements, the Company recognized revenue of US$7,741,386 thousand for the year ended 31 December 2022, a portion of which relates to clinical trial service revenue. As discussed in Note 2 to the consolidated financial statements, clinical trial service revenue is recognized over time, using an input measure, being total project costs (inclusive of third-party costs, principally pass-through/ reimbursable expenses) incurred at each reporting period as a percentage of forecasted total project costs, to measure progress towards satisfying the Company’s performance obligation. The transaction price is based on the contract or latest change order value, adjusted to reflect the estimated realizable contract value.
We identified the evaluation of revenue recognition for clinical trial service revenue as a critical audit matter. Complex and subjective auditor judgment was required to evaluate the Company’s estimate of total forecast project costs and the estimated realizable contract values.
The following are the primary procedures we performed to address this critical audit matter:
We evaluated the design and tested the operating effectiveness of certain internal controls related to the revenue process, including controls over total forecast project costs and estimated realizable contract values.
We tested the total forecast project costs and the realizable contract values for a selection of clinical trial service contracts, by evaluating:
–direct costs incurred, both during the year and cumulative over the life of the contracts. We tested the accuracy and completeness of the direct costs by comparing the amounts to source data
–third-party costs incurred, both during the year and cumulative over the life of the contracts. We tested the accuracy and completeness of the third-party costs incurred by comparing the costs to invoices received
–findings from interviews with operational personnel of the Company to assess progress to date, the estimate of remaining costs to be incurred and factors impacting the amount of time and costs to complete the selected contracts, including an understanding of the nature and complexity of the work to be performed
–correspondence of amendments to the scope or contract value, if any, between the Company and the customer for the selected contracts as part of our evaluation of contract progress
–quarterly movements in forecast project costs and project margins and investigating the reasons for those movements, and
–the reasonableness of the Company’s adjustments from total contract value to arrive at realizable contract value. We confirmed total contract value with customers and compared the assumptions used to derive the adjustments from total contract value to realizable contract value to underlying records.
We also evaluated the Company’s methods, assumptions and data used to accurately estimate total forecast project costs and realizable contract values, by comparing historical estimates developed at contract inception to actual results for a selection of clinical trial service contracts.
(signed) KPMG
We have served as the Company’s auditor since 1990.
Dublin, Ireland
February 24, 2023
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors
ICON plc:
Opinion on Internal Control Over Financial Reporting
We have audited ICON plc and subsidiaries’ (the Company) internal control over financial reporting as of December 31, 2022, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2022, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of December 31, 2022 and 2021, the related consolidated statements of operations, comprehensive income, shareholders’ equity, and cash flows for each of the years in the three-year period ended December 31, 2022, and the related notes (collectively, the consolidated financial statements), and our report dated February 24, 2023, expressed an unqualified opinion on those consolidated financial statements.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
(signed) KPMG
Dublin, Ireland
February 24, 2023
ICON plc
CONSOLIDATED BALANCE SHEETS
| | | | | | | | | |
| | December 31, 2022 | December 31, 2021 | |
| ASSETS | (in thousands) | |
| Current Assets: | | | |
| Cash and cash equivalents | $ | 288,768 | | $ | 752,213 | | |
| Available for sale investments (Note 8a) | 1,713 | | 1,712 | | |
| Accounts receivable, net of allowance for credit losses (Note 4) | 1,731,388 | | 1,342,770 | | |
| Unbilled revenue (Note 4) | 957,655 | | 623,121 | | |
| Other receivables | 63,658 | | 56,760 | | |
| Prepayments and other current assets | 137,094 | | 114,323 | | |
| Income taxes receivable | 48,790 | | 50,299 | | |
| Total current assets | 3,229,066 | | 2,941,198 | | |
| | | |
| Non-current Assets: | | | |
| Property, plant and equipment, net (Note 9) | 350,320 | | 336,444 | | |
| Goodwill (Note 10) | 8,971,670 | | 9,037,931 | | |
| Intangible assets, net (Note 11) | 4,278,659 | | 4,710,843 | | |
| Operating right-of-use assets (Note 15) | 153,832 | | 198,123 | | |
| Other receivables | 70,790 | | 70,557 | | |
| Income taxes receivable | 21,380 | | 18,637 | | |
| Deferred tax asset (Note 20) | 76,930 | | 48,392 | | |
| Equity method investments (Note 8c) | — | | 2,373 | | |
| Investments in equity-long term (Note 8b) | 32,631 | | 22,592 | | |
| Total Assets | $ | 17,185,278 | | $ | 17,387,090 | | |
| LIABILITIES AND SHAREHOLDERS’ EQUITY | | | |
| Current Liabilities: | | | |
| Accounts payable | $ | 81,194 | | $ | 90,764 | | |
| Unearned revenue (Note 4) | 1,507,449 | | 1,323,961 | | |
| Other liabilities (Note 12) | 1,005,025 | | 949,629 | | |
| Income taxes payable | 41,783 | | 59,433 | | |
| Current bank credit lines and loan facilities (Note 13) | 55,150 | | 55,150 | | |
| Total current liabilities | 2,690,601 | | 2,478,937 | | |
| Non-current Liabilities: | | | |
| Non-current bank credit lines and loan facilities (Note 13) | 4,599,037 | | 5,381,162 | | |
| Lease liabilities (Note 15) | 131,644 | | 159,483 | | |
| Non-current other liabilities (Note 16) | 38,260 | | 42,596 | | |
| Non-current income taxes payable | 239,188 | | 172,109 | | |
| Deferred tax liability (Note 20) | 988,585 | | 1,085,976 | | |
| Commitments and contingencies (Note 17) | — | | — | | |
| Total Liabilities | 8,687,315 | | 9,320,263 | | |
| | | |
| Shareholders' Equity: | | | |
Ordinary shares par value 6 euro cents per share; 100,000,000 shares authorized (Note 18) | | | |
81,723,555 shares issued and outstanding at December 31, 2022 and
81,554,683 shares issued and outstanding at December 31, 2021. | 6,649 | | 6,640 | | |
| Additional paid-in capital | 6,840,306 | | 6,733,910 | | |
| Other undenominated capital (Note 18b) | 1,162 | | 1,134 | | |
| Accumulated other comprehensive income (Note 25) | (171,538) | | (90,937) | | |
| Retained earnings | 1,821,384 | | 1,416,080 | | |
| Total Shareholders' Equity | 8,497,963 | | 8,066,827 | | |
| | | |
| | | |
| Total Liabilities and Shareholders’ Equity | $ | 17,185,278 | | $ | 17,387,090 | | |
The accompanying notes are an integral part of these consolidated financial statements.
ICON plc
CONSOLIDATED STATEMENTS OF OPERATIONS
| | | | | | | | | | | |
| | Year Ended December 31, |
| | 2022 | 2021 | 2020 |
| | (in thousands, except share and per share data) |
| | | |
| | | |
| | | |
| Revenue | $ | 7,741,386 | | $ | 5,480,826 | | $ | 2,797,288 | |
| | | |
Costs and expenses: | | | |
| | | |
| Direct costs (excluding depreciation and amortization) | 5,527,045 | | 3,972,612 | | 1,979,883 | |
| Selling, general and administrative | 778,753 | | 585,330 | | 342,449 | |
| Depreciation and amortization | 569,513 | | 314,987 | | 66,126 | |
| Transaction and integration related (Note 6) | 39,695 | | 198,263 | | (759) | |
| Restructuring (Note 19) | 31,143 | | 31,105 | | 18,089 | |
| Total costs and expenses | 6,946,149 | | 5,102,297 | | 2,405,788 | |
| | | |
| Income from operations | 795,237 | | 378,529 | | 391,500 | |
| Interest income | 2,345 | | 574 | | 2,724 | |
| Interest expense (Note 13) | (229,731) | | (182,423) | | (13,019) | |
| | | |
| Income before income tax expense | 567,851 | | 196,680 | | 381,205 | |
| Income tax expense (Note 20) | (59,411) | | (41,334) | | (47,875) | |
| | | |
| Income before share of earnings from equity method investments | 508,440 | | 155,346 | | 333,330 | |
| Share of equity method investments | (3,136) | | (2,161) | | (366) | |
| | | |
| Net Income | 505,304 | | 153,185 | | 332,964 | |
| Net income attributable to noncontrolling interest | — | | — | | (633) | |
| Net income attributable to the Group | $ | 505,304 | | $ | 153,185 | | $ | 332,331 | |
| | | |
| Net income per Ordinary Share attributable to the Group (Note 24): | | | |
| | | |
| Basic | $ | 6.20 | | $ | 2.28 | | $ | 6.20 | |
| Diluted | $ | 6.13 | | $ | 2.25 | | $ | 6.15 | |
| | | |
| Weighted average number of ordinary shares outstanding: | | | |
| Basic (Note 2) | 81,532,320 | | 67,110,186 | | 52,859,911 | |
| Diluted (Note 2) | 82,468,363 | | 68,068,311 | | 53,283,585 | |
The accompanying notes are an integral part of these consolidated financial statements.
ICON plc
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
| | | | | | | | | | | |
| | Year Ended December 31, |
| | 2022 | 2021 | 2020 |
| | (in thousands) |
| Net income | $ | 505,304 | | $ | 153,185 | | $ | 332,964 | |
| Other comprehensive income, net of tax | | | |
| Currency translation adjustment | (89,530) | | (60,617) | | 46,526 | |
| | | |
| Unrealized capital loss - investments | — | | — | | (231) | |
| Actuarial gain/(loss) on defined benefit pension plan | 12,657 | | 4,266 | | (4,138) | |
| Amortization of cash flow hedge | — | | 113 | | (910) | |
| (Loss)/gain on cash flow hedge | (3,728) | | 778 | | (905) | |
| Total comprehensive income | 424,703 | | 97,725 | | 373,306 | |
| Less net income attributable to noncontrolling interest | — | | — | | (633) | |
| Total comprehensive income attributable to the Group | $ | 424,703 | | $ | 97,725 | | $ | 372,673 | |
The accompanying notes are an integral part of these consolidated financial statements.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| ICON plc |
| CONSOLIDATED STATEMENT OF SHAREHOLDERS' EQUITY AND COMPREHENSIVE INCOME |
| (in thousands, except share and per share data) |
| | Group | |
| Shares | Amount | Additional
Paid-in
Capital | Other
Undenominated
Capital | Accumulated
Other
Comprehensive
Income | Retained
Earnings | Total | Redeemable Noncontrolling Interest |
| Balance at January 1, 2020 | 53,622,206 | | $ | 4,635 | | $ | 577,961 | | $ | 1,052 | | $ | (75,819) | | $ | 1,110,226 | | $ | 1,618,055 | | $ | 39,510 | |
| | | | | | | | |
| Comprehensive Income (net of tax): | | | | | | | | — | |
| Net income | — | | — | | — | | — | | — | | 332,331 | | 332,331 | | 633 | |
| Currency translation adjustment | — | | — | | — | | — | | 46,526 | | — | | 46,526 | | — | |
| | | | | | | | |
| Unrealized capital loss - investments | — | | — | | — | | — | | (231) | | — | | (231) | | — | |
| Actuarial loss on defined benefit pension plan | — | | — | | — | | — | | (4,138) | | — | | (4,138) | | — | |
| Amortization of cash flow hedge | — | | — | | — | | — | | (910) | | — | | (910) | | — | |
| Loss on cash flow hedge | — | | — | | — | | — | | (905) | | — | | (905) | | — | |
| Total comprehensive income | | | | | | | 372,673 | | |
| Exercise of share options | 193,417 | | 13 | | 13,176 | | — | | — | | — | | 13,189 | | — | |
| Issue of restricted share units / performance share units | 207,688 | | 14 | | — | | — | | — | | — | | 14 | | — | |
| Share based compensation expense | — | | — | | 25,981 | | — | | — | | — | | 25,981 | | — | |
| Share issue costs | — | | — | | (14) | | — | | — | | — | | (14) | | — | |
| Repurchase of ordinary shares | (1,235,218) | | (82) | | — | | 82 | | — | | (175,000) | | (175,000) | | — | |
| Share repurchase costs | — | | — | | — | | — | | — | | (140) | | (140) | | — | |
| Noncontrolling interest adjustment to redemption amount | — | | — | | — | | — | | — | | (4,522) | | (4,522) | | 4,522 | |
| Exercise of call option on noncontrolling interest shares | — | | — | | — | | — | | — | | — | | — | | (44,665) | |
| Balance at December 31, 2020 | 52,788,093 | | $ | 4,580 | | $ | 617,104 | | $ | 1,134 | | $ | (35,477) | | $ | 1,262,895 | | $ | 1,850,236 | | — | |
The accompanying notes are an integral part of these consolidated financial statements.
| | | | | | | | | | | | | | | | | | | | | | | |
| ICON plc |
| CONSOLIDATED STATEMENT OF SHAREHOLDERS' EQUITY AND COMPREHENSIVE INCOME |
| (in thousands, except share and per share data) |
| | Group |
| Shares | Amount | Additional
Paid-in
Capital | Other
Undenominated
Capital | Accumulated
Other
Comprehensive
Income | Retained
Earnings | Total |
| Balance at December 31, 2020 | 52,788,093 | | $ | 4,580 | | $ | 617,104 | | $ | 1,134 | | $ | (35,477) | | $ | 1,262,895 | | $ | 1,850,236 | |
| Comprehensive income (net of tax): | | | | | | | |
| Net income | — | | — | | — | | — | | — | | 153,185 | | 153,185 | |
| Currency translation adjustment | — | | — | | — | | — | | (60,617) | | — | | (60,617) | |
| | | | | | | |
| Actuarial gain on defined benefit pension plan | — | | — | | — | | — | | 4,266 | | — | | 4,266 | |
| Amortization of cash flow hedge | — | | — | | — | | — | | 113 | | — | | 113 | |
| Settlement of cash flow hedge | — | | — | | — | | — | | 778 | | — | | 778 | |
| Total comprehensive income | | | | | | | 97,725 | |
| | | | | | | |
| Exercise of share options | 1,065,529 | | 77 | | 118,512 | | — | | — | | — | | 118,589 | |
| Issue of restricted share units / performance share units | 328,634 | | 23 | | — | | — | | — | | — | | 23 | |
| Share based compensation expense | — | | — | | 133,553 | | — | | — | | — | | 133,553 | |
| Share issue costs | — | | — | | (853) | | — | | — | | — | | (853) | |
| Issue of shares associated with a business combination | 27,372,427 | | 1,960 | | 5,656,195 | | — | | — | | — | | 5,658,155 | |
| Replacement share-based awards issued to acquiree employees | — | | — | | 209,399 | | — | | — | | — | | 209,399 | |
| Balance at December 31, 2021 | 81,554,683 | | $ | 6,640 | | $ | 6,733,910 | | $ | 1,134 | | $ | (90,937) | | $ | 1,416,080 | | $ | 8,066,827 | |
The accompanying notes are an integral part of these consolidated financial statements.
| | | | | | | | | | | | | | | | | | | | | | | |
| ICON plc |
| CONSOLIDATED STATEMENT OF SHAREHOLDERS' EQUITY AND COMPREHENSIVE INCOME |
| (in thousands, except share and per share data) |
| | Group |
| Shares | Amount | Additional
Paid-in
Capital | Other
Undenominated
Capital | Accumulated
Other
Comprehensive
Income | Retained
Earnings | Total |
| Balance at December 31, 2021 | 81,554,683 | | $ | 6,640 | | $ | 6,733,910 | | $ | 1,134 | | $ | (90,937) | | $ | 1,416,080 | | $ | 8,066,827 | |
| Comprehensive income (net of tax): | | | | | | | |
| Net income | — | | — | | — | | — | | — | | 505,304 | | 505,304 | |
| Currency translation adjustment | — | | — | | — | | — | | (89,530) | | — | | (89,530) | |
| | | | | | | |
| Actuarial gain on defined benefit pension plan | — | | — | | — | | — | | 12,657 | | — | | 12,657 | |
| Loss on cash flow hedge | — | | — | | — | | — | | (3,728) | | — | | (3,728) | |
| Total comprehensive income | | | | | | | 424,703 | |
| Exercise of share options | 348,286 | | 21 | | 35,807 | | — | | — | | — | | 35,828 | |
| Issue of restricted share units / performance share units | 241,116 | | 16 | | — | | — | | — | | — | | 16 | |
| Share based compensation expense | — | | — | | 70,606 | | — | | — | | — | | 70,606 | |
| Share issuance costs | — | | — | | (17) | | — | | — | | — | | (17) | |
| Repurchase of ordinary shares | (420,530) | | (28) | | — | | 28 | | — | | (99,983) | | (99,983) | |
| Share repurchase costs | — | | — | | — | | — | | — | | (17) | | (17) | |
| Balance at December 31, 2022 | 81,723,555 | | $ | 6,649 | | $ | 6,840,306 | | $ | 1,162 | | $ | (171,538) | | $ | 1,821,384 | | $ | 8,497,963 | |
The accompanying notes are an integral part of these consolidated financial statements.
ICON plc
CONSOLIDATED STATEMENTS OF CASH FLOWS
| | | | | | | | | | | |
| Year Ended December 31, |
| 2022 | 2021 | 2020 |
| Cash flows from operating activities: | (in thousands) |
| Net income | $ | 505,304 | | $ | 153,185 | | $ | 332,964 | |
| Adjustments to reconcile net income to net cash provided by operating activities: | | | |
| Depreciation and amortization expense | 569,513 | | 314,987 | | 66,126 | |
| Impairment of long lived assets | 28,767 | | 20,037 | | 5,411 | |
| Reduction in carrying value of operating right-of-use assets | 45,215 | | 45,339 | | 28,480 | |
| Loss on equity method investments | 3,136 | | 2,161 | | 366 | |
| Amortization of financing costs and debt discount | 17,749 | | 12,890 | | 523 | |
| Stock compensation expense | 70,523 | | 133,844 | | 26,271 | |
| Loss on extinguishment of debt | — | | 14,434 | | — | |
| Loss on issuance of debt | — | | 59,460 | | — | |
| Deferred tax (benefit)/expense | (124,985) | | (60,616) | | 927 | |
| Unrealized foreign exchange (gain)/loss | (13,009) | | (6,054) | | 5,979 | |
| Other non-cash items | 11,324 | | 4,480 | | (7,859) | |
| Changes in operating assets and liabilities: | | |
| Accounts receivable | (420,695) | | 113,513 | | (175,040) | |
| Unbilled revenue | (332,592) | | (17,656) | | (5,748) | |
| Unearned revenue | 192,944 | | (69,121) | | 291,844 | |
| Other net assets | 10,121 | | 108,259 | | (2,209) | |
| Net cash provided by operating activities | 563,315 | | 829,142 | | 568,035 | |
| Cash flows from investing activities: | | | |
| Purchase of property, plant and equipment | (142,160) | | (93,750) | | (40,885) | |
| Purchase of subsidiary undertakings (net of cash acquired) | — | | (5,914,475) | | (47,931) | |
| Investment in equity method investments | — | | (2,450) | | (2,450) | |
| Loan to equity method investment | — | | (10,000) | | — | |
| Sale of available for sale investments | 481 | | 497 | | 47,902 | |
| Purchase of available for sale investments | (482) | | (480) | | — | |
| Proceeds from investments in equity - long term | 1,906 | | 500 | | 87 | |
| Purchase of investments in equity - long term | (5,612) | | (4,077) | | (3,299) | |
| Net cash used in investing activities | (145,867) | | (6,024,235) | | (46,576) | |
| Cash flows from financing activities: | | | |
| Financing costs | — | | (30,328) | | (1,554) | |
| Drawdown of credit lines and facilities | 75,000 | | 5,905,100 | | 350,000 | |
| Repayment of credit lines and facilities | (875,000) | | (877,780) | | (350,000) | |
| Purchase of noncontrolling interest | — | | — | | (43,923) | |
| Proceeds from the exercise of equity compensation | 35,844 | | 118,589 | | 13,203 | |
| Share issue costs | (17) | | (853) | | (14) | |
| Repurchase of ordinary shares | (99,983) | | — | | (175,000) | |
| Share repurchase costs | (17) | | — | | (140) | |
| Settlement of cash flow hedge | — | | — | | (905) | |
| Net cash (used in)/provided by financing activities | (864,173) | | 5,114,728 | | (208,333) | |
| Effect of exchange rate movements on cash | (16,720) | | (7,727) | | 6,870 | |
| Net (decrease)/increase in cash and cash equivalents | (463,445) | | (88,092) | | 319,996 | |
| Cash and cash equivalents at beginning of year | 752,213 | | 840,305 | | 520,309 | |
| Cash and cash equivalents at end of year | 288,768 | | 752,213 | | 840,305 | |
The accompanying notes are an integral part of these consolidated financial statements.
ICON plc
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
1. Description of Business
ICON plc and its subsidiaries ("the Company" or "ICON") is a clinical research organization ("CRO"), providing outsourced development services on a global basis to the pharmaceutical, biotechnology and medical device industries. We specialize in the strategic development, management and analysis of programs that support all stages of the clinical development process from compound selection to Phase I-IV clinical studies. Our mission is to improve the lives of patients by accelerating the development of our customers' drugs and devices through innovative solutions.
We believe that we are one of a select group of CROs with the expertise and capability to conduct clinical trials in most major therapeutic areas on a global basis and have the operational flexibility to provide development services on a stand-alone basis or as part of an integrated "full-service" solution. At December 31, 2022 we had approximately 41,100 employees, in 111 locations in 53 countries. During the year ended December 31, 2022, we derived approximately 46.2%, 46.5% and 7.3% of our revenue in the United States, Europe and Rest of World, respectively.
ICON’s ordinary shares are traded on the NASDAQ Global Select Market under the symbol “ICLR”.
We began operations in 1990 and have expanded our business through internal growth, together with a number of strategic acquisitions to enhance our capabilities and expertise in certain areas of the clinical development process. We are incorporated in Ireland and our principal executive office is located at: South County Business Park, Leopardstown, Dublin 18, Republic of Ireland. The contact telephone number of this office is +353 1 2912000.
2. Summary of Significant Accounting Policies
The accounting policies noted below were applied in the preparation of the accompanying financial statements of the Company and are in conformity with accounting principles generally accepted in the United States.
Basis of consolidation
The consolidated financial statements include the financial statements of the Company and all of its subsidiaries. All significant intercompany profits, transactions and account balances have been eliminated. The results of subsidiary undertakings acquired in the period are included in the Consolidated Statement of Operations from the date of acquisition.
Use of estimates
The preparation of financial statements in conformity with generally accepted accounting principles in the United States requires management to make estimates and judgments that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reported period. Actual results could differ from those estimates. The principal management estimates and judgments used in preparing the financial statements relate to revenue recognition and intangible assets acquired in a business combination.
Disclosure of fair value of financial instruments
Cash, cash equivalents, other receivables, available for sale investments, accounts receivable, accounts payable, investigator payments and income taxes payable have carrying amounts that approximate fair value due to the short term maturities of these instruments. Other liabilities' carrying amounts approximate fair value based on net present value of estimated future cash flows. Debt is measured at historical cost.
Financial instruments are measured in the Consolidated Balance Sheet at amortized cost or fair value using a fair value hierarchy of valuation inputs. The fair value hierarchy prioritizes the inputs into three levels based on the extent to which inputs used in measuring fair value are observable in the market. Each fair value measurement is reported in one of three levels, which is determined by the lowest level input that is significant to the fair value measurement in its entirety. These levels are:
•Level 1: Inputs are based upon unadjusted quoted prices for identical instruments traded in active markets.
•Level 2: Inputs are based upon quoted prices for similar instruments in active markets, quoted prices for identical or similar instruments in markets that are not active and model-based valuation techniques for which all significant assumptions are observable in the market or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
•Level 3: Inputs are generally unobservable and typically reflect management’s estimates of assumptions that market participants would use in pricing the asset or liability.
The Company classifies its investments in short term debt or equity investments as available for sale, as it does not actively trade such securities nor does it intend to hold them to maturity. The fair value of short term investments are represented by level 1 fair value measurements – quoted prices in active markets for identical assets. The unrealized movements in fair value are recognized in equity until disposal or sale, at which time, those unrealized movements from prior periods are recognized in the Consolidated Statement of Operations. Losses other than temporary, which reduce the carrying amount below cost are recognized in Consolidated Statement of Operations.
Business combinations
The cost of a business combination is measured as the aggregate of the fair value of assets received, liabilities assumed and equity instruments issued in exchange for control. The Company records and allocates to its reporting units the excess of the cost over the fair value of the net assets acquired, known as goodwill. Where a business combination agreement provides for an adjustment to the cost of the acquisition which is contingent upon future events, the amount of the estimated adjustment is recognized at the acquisition date at the fair value of the contingent consideration. Any changes to this estimate outside the measurement period will depend on the classification of the contingent consideration. If the contingent consideration is classified as equity it shall not be re-measured and the settlement shall be accounted for within equity. If the contingent consideration is classified as a liability any adjustments will be accounted for through the Consolidated Statement of Operations or Other Comprehensive Income depending on whether the liability is considered a financial instrument.
The assets, liabilities and contingent liabilities of businesses acquired are measured at their fair values at the date of acquisition. In the case of a business combination which is completed in stages, the fair values of the identifiable assets, liabilities and contingent liabilities are determined at the date of each exchange transaction. When the initial accounting for a business combination is determined provisionally, any subsequent adjustments to the provisional values allocated to the identifiable assets, liabilities and contingent liabilities are made within twelve months of the acquisition date and presented as adjustments to goodwill in the reporting period in which the adjustments are determined.
The Company allocates a share of net income to the noncontrolling interest holders based on percentage ownership.
Foreign currencies and translation of subsidiaries
The Company's financial statements are prepared in United States dollars. The financial statements of subsidiaries with other functional currencies are translated at period end rates for the Consolidated Balance Sheets and average rates for the Consolidated Statements of Operations. Translation gains and losses arising are reported as a movement on accumulated other comprehensive income.
Transactions in currencies other than the functional currency of the subsidiaries of the Company are recorded at the rate ruling at the date of the transaction. Monetary assets and liabilities denominated in currencies other than the functional currency of the subsidiaries of the Company are translated into the functional currency of that entity at exchange rates prevailing at the Balance Sheet date. Adjustments resulting from these translations are charged or credited to income. Foreign currency gains and losses on intercompany transactions classified as long-term investments are reported in other comprehensive income as currency translation adjustments. Amounts charged or credited to the Consolidated Statements of Operations for the years ended December 31, 2022, December 31, 2021 and December 31, 2020 were as follows:
| | | | | | | | | | | |
| Year ended
December 31, |
| | 2022 | 2021 | 2020 |
| (in thousands) |
Amounts (credited)/charged | $ | (25,997) | | $ | (14,316) | | $ | 5,979 | |
Revenue recognition
The Company earns revenues by providing a number of different services to its customers. These services, which are integral elements of the clinical development process, include clinical trials management, consulting, contract staffing, data services and laboratory services. These services, which are described below, can be purchased collectively or individually as part of a clinical trial contract. There is not significant variability in how economic factors affect these services. Contracts range in duration from a number of months to several years.
ASC 606 requires application of five steps: (1) identify the contract(s) with a customer; (2) identify the performance obligation in the contract; (3) determine the transaction price; (4) allocate the transaction price to the performance obligations in the contract; and (5) recognize revenue when (or as) the entity satisfies the performance obligation(s), which have been applied to revenue recognized from each service described below.
Clinical trial service revenue
A clinical trial service is a single performance obligation satisfied over time, i.e. the full-service obligation in respect of a clinical trial (including those services performed by investigators and other parties) is considered a single performance obligation. Promises offered to the customer are not distinct within the context of the contract. ICON is the contract principal in respect of both direct services and in the use of third parties (principally investigator services) that support the clinical research projects. The transaction price is determined by reference to the contract or change order value (total service revenue and pass-through/ reimbursable expenses) adjusted to reflect a realizable contract value. Revenue is recognized over time as the single performance obligation is satisfied. The progress towards completion for clinical service contracts is measured based on an input measure being total project costs incurred (inclusive of pass-through/ reimbursable expenses) at each reporting period as a percentage of forecasted total project costs.
Laboratory services revenue
Revenue is recognized when, or as, obligations under the terms of a contract are satisfied, which occurs when control of the products or services are transferred to the customer. Revenue for laboratory services is measured as the amount of consideration we expect to receive in exchange for transferring products or services. Where contracts with customers contain multiple performance obligations, the transaction price is allocated to each performance obligation based on the estimated relative selling price of the promised good or service. Service revenue is recognized over time as the services are delivered to the customer based on the extent of progress towards completion of the performance obligation. The determination of the methodology to measure progress requires judgment and is based on the nature of services provided. This requires an assessment of the transfer of value to the customer. The right to invoice measure of progress is generally related to rate per unit contracts, as the extent of progress towards completion is measured based on discrete service or time-based increments, such as samples tested or labor hours incurred. Revenue is recorded in the amount invoiced since that amount corresponds to the value of the Company's performance and the transfer of value to the customer.
Contracting services revenue
The Company has availed of the practical expedient which results in recognition of revenue on a right to invoice basis. Application of the practical expedient reflects the right to consideration from the customer in an amount that corresponds directly with the value to the customer of the performance completion to date. This reflects hours performed by contract staff.
Consulting services revenue
Our consulting services contracts represent a single performance obligation satisfied over time. The transaction price is determined by reference to contract or change order value. Revenue is recognized over time as the performance obligation is satisfied. The progress towards completion for consulting contracts is measured based on total project inputs (time) at each reporting period as a percentage of forecasted total project inputs.
Data services revenue
The Company provides data reports and analytics to customers based on agreed-upon specifications, including the timing of delivery, which is typically either weekly, monthly, or quarterly. If a customer requests more than one type of data report or series of data reports within a contract, each distinct type of data report is a separate performance obligation. The contracts provide for the Company to be compensated for the value of each deliverable. The transaction price is determined using list prices, discount agreements, if any, and negotiations with the customers, and generally includes any out-of-pocket expenses. Typically, the Company bills in advance of services being provided with the amount being recorded as unearned revenue.
When multiple performance obligations exist, the transaction price is allocated to performance obligations on a relative standalone selling price basis. In cases where the Company contracts to provide a series of data reports, or in some cases data, the Company recognizes revenue over time using the “units delivered” output method as the data or reports are delivered. Expense reimbursements are recorded to revenue as the expenses are incurred as they relate directly to the services performed.
Certain arrangements include upfront customization or consultative services for customers. These arrangements often include payments based on the achievement of certain contractual milestones. Under these arrangements, the Company contracts with a customer to carry out a specific study, ultimately resulting in delivery of a custom report or data product. These arrangements are a single performance obligation given the integrated nature of the service being provided. The Company typically recognizes revenue under these contracts over time, using an output-based measure, generally time elapsed, to measure progress and transfer of control of the performance obligation to the customer. Expense reimbursements are recorded to revenue as the expenses are incurred as they relate directly to the service performed.
The Company enters into contracts with some of its larger data suppliers that involve non-monetary terms. The Company issues purchase credits to be used toward the data supplier's purchase of the Company's services based on the fair value of the data obtained. In exchange, the Company receives monetary discounts on the data received from the data suppliers. The fair value of the revenue earned from the customer purchases is recognized as services are delivered as described above.
At the end of the contract year, any unused customer purchase credits may be forfeited or carried over to the next contract year based on the terms of the data supplier contract.
Commissions
Incremental costs of obtaining a contract are recognized as an asset on the Consolidated Balance Sheet in respect of those contracts that exceed one year. Where commission costs relate to contracts that are less than one year, the practical expedient is applied as the amortization period of the asset which would arise on deferral would be one year or less.
Reimbursable expenses
Reimbursable expenses comprise investigator payments and certain other costs which are reimbursed by clients under terms specific to each contract to the investigators. The Company includes reimbursed expenses in revenue and direct costs as the Company is primarily responsible for fulfilling the promise to provide the specified service, including integration of the related services into a combined output to the customer.
Direct costs
Direct costs consist of compensation, associated employee benefits and share-based payments for project-related employees and other direct project-related costs.
Reimbursable expenses are presented within direct costs. This presentation is to align the presentation of costs with our assessment that our clinical trial service is a single performance obligation satisfied over time. Reimbursable expenses are recorded once the activity which forms the basis for the cost has occurred. Payments are made based on predetermined contractual arrangements. Timing of payments may differ from the timing of the expense.
Cash and cash equivalents
Cash and cash equivalents include cash and highly liquid investments with initial maturities of three months or less and are stated at cost, which approximates market value.
Investments in debt, equity and other
Available for sale investments
The Company classifies short-term investments as available for sale. The investments are reported at fair value, with unrealized gains or losses reported in a separate component of shareholders' equity. Any differences between the cost and fair value of the investments are represented by accrued interest and unrealized gains/losses. Realized gains and losses are determined using specific identification
Long term investments
The Company classifies its interests in funds having considered the nature of its investment, the extent of influence over operating and financial decisions and the availability of readily determinable fair values. The Company determined that the interests in funds at December 31, 2022 meet the definition of equity securities without readily determinable fair values. The Company concluded that the interests held at December 31, 2021 and December 31, 2022 qualify for the NAV practical expedient in ASC 820 'Fair value measurements and disclosures'. Any increases or decreases in fair value are recognized in net income in the period. These are therefore measured at Level 3 of the fair value hierarchy.
Equity method investments
The Company’s investments that are not consolidated are accounted for under the equity method if the Company exercises significant influence that is considered to be greater than minor. These investments are classified as equity method investments on the accompanying Consolidated Balance Sheet. The Company records its pro rata share of the earnings/losses of these investments in Share of equity method investments in the Consolidated Statement of Operations. The Company reviews these for impairment whenever events or changes in circumstances indicate that the carrying amounts may not be recoverable.
Accounts receivable, net and unbilled revenue
Accounts receivable and unbilled revenue are recorded at fair value less an estimate of the credit losses expected to be incurred on the Company's accounts receivable portfolio. The Company's estimate of expected credit losses considers historical credit loss information that is adjusted, where necessary, for current conditions and reasonable and supportable forecasts. Historical credit loss experience provides the basis for the estimation of expected credit losses. The Company's receivables and unbilled services are predominantly due from large and mid-tier pharmaceutical and biotechnology companies that share similar risk characteristics. The Company monitors their portfolio of receivables and unbilled services for any deterioration in current or expected credit quality (for example, expected delinquency level), and adjusts the allowance for credit losses as required.
Changes in the allowance for credit losses are recorded as a provision for (or reversal of) credit loss expense in the Consolidated Statement of Operations. Losses are charged against the allowance when management believes the uncollectibility of a previously provisioned amount is confirmed.
Accounts receivable early payment discounting
Where the Company enters into an agreement to sell certain portfolios of its accounts receivable balances, the sale is accounted for in accordance with ASC Topic 860 'Transfers and Servicing' (ASC 860). Agreements which result in true sales of the transferred receivables, as defined in ASC 860, which occur when receivables are transferred without recourse to ICON, are excluded from amounts reported in the Consolidated Balance Sheet. Cash proceeds received from such sales are included in operating cash flows.
Property, plant and equipment
Property, plant and equipment is stated at cost less accumulated depreciation. Depreciation of property, plant and equipment is computed using the straight line method based on the estimated useful lives of the assets as listed below:
| | | | | |
| | Years |
| Building | 40 |
| Computer equipment and software | 2-8 |
| Office furniture and fixtures | 8 |
| Laboratory equipment | 5 |
| Motor vehicles | 5 |
Leasehold improvements are amortized using the straight line method over the estimated useful life of the asset or the lease term, whichever is shorter.
Leases
The Company determines if an arrangement is a lease at inception and reassess if there are changes in terms and conditions of the contract. Finance leases, if any, are depreciated on the same basis as property, plant and equipment. At December 31, 2022 and December 31, 2021, the Company did not account for any leases as finance leases.
Operating leases are included in operating right-of-use assets, other liabilities and non-current operating lease liabilities on our Consolidated Balance Sheet with the lease charge recognized on a straight-line basis over the lease term. ROU assets and lease liabilities are recognized based on the present value of future minimum lease payments over the lease term at commencement date or date of transition. Our lease terms may also include options to extend or terminate. The Company actively reviews options to extend or terminate leases and adjusts the ROU asset and lease liability when it is reasonably certain the option will be exercised. The ROU asset is adjusted for any prepayments made at the date of commencement and any initial direct costs incurred. As most of the Company's leases do not provide an implicit rate, the discount rate used is based on the rate of traded corporate bonds available at the commencement date adjusted for country risk, liquidity and lease term.
The Company accounts for lease and non-lease components separately with lease components flowing through the Consolidated Balance Sheet and non-lease components expensed directly to the Consolidated Statements of Operations.
Leasehold improvements are amortized over the shorter of the depreciable lives of the corresponding fixed assets or the lease term including any applicable renewals. Certain property leases include variable lease payments resulting from periodic rent increases based on an index which cannot be reasonably estimated at the lease commencement date. These costs are expensed as incurred on the Consolidated Statements of Operations.
In some cases, the Company enters into sublease agreements and becomes both a lessee and a lessor for the same underlying asset. Subleases are accounted for as operating leases separately from the lease they relate to, but similar in manner as all other leases.
ROU assets for operating leases are occasionally reduced by impairment losses. The Company uses the long-lived assets impairment guidance in Subtopic 360-10, Property, Plant, and Equipment – Overall, to determine whether an ROU asset is impaired, and if so, the amount of the impairment loss to recognize.
Intangible assets
Intangible assets are measured at their fair value when acquired and amortized on the straight line basis over their respective useful lives. The Company has no indefinite life intangibles other than goodwill. The Company evaluates its intangibles for impairment when indicators of impairment exist.
Intangible assets are amortized on a straight-line basis over the expected useful life, as set forth in the table below:
| | | | | |
| Estimated Useful Life |
| Customer relationships | 23 years |
| Order backlog | 3 years |
| Trade names | 3 years |
| Patient database | 7 years |
| Technology assets | 5 years |
The Company periodically assesses the useful lives of intangible assets to evaluate whether what was established at acquisition continues to be appropriate.
Impairment of goodwill and long-lived assets
Goodwill is tested for impairment annually or more frequently if an event or circumstance indicates that an impairment loss may have been incurred. The annual impairment test for goodwill includes an option to perform a qualitative assessment of whether it is more likely than not that a reporting unit's fair value is less than its carrying value. Reporting units are businesses with discrete financial information that is available and reviewed by management. If the Company determines that it is more likely than not that the fair value of a reporting unit is less than its carrying value, then the Company performs the quantitative goodwill impairment test. The Company may also chose to bypass the qualitative assessment for any reporting unit in its goodwill assessment and proceed directly to performing the quantitative assessment. The Company recognizes an impairment charge for the amount by which the reporting unit's carrying amount exceeds its fair value.
Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Fair value is determined through various valuation techniques including discounted cash flow models and third-party independent appraisals, as considered necessary. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of the asset to future undiscounted net cash flows expected to be generated by the asset. If such assets are considered to be impaired, the impairment to be recognized is measured at the amount by which the carrying amount of the asset exceeds the fair value of the asset. Assets to be disposed of are reported at the lower of the carrying amount of the asset or fair value less selling costs.
Debt issuance costs
Debt issuance costs relating to the Company’s long-term debt are recorded as a direct reduction of long-term debt; these costs are deferred and amortized to interest expense using the effective interest method, over the respective terms of the related debt. Debt issuance costs relating to the Company’s revolving credit facilities are recorded as an asset; these costs are deferred and amortized to interest expense using the straight-line method. Early repayment of debt facilities can result in modification of the debt and the acceleration of the amortization of debt issuance costs.
Derivative financial instruments
The Company uses derivative financial instruments to reduce exposures to interest rates and foreign currencies. Derivatives are recorded on the balance sheet at fair value at each balance sheet date utilizing pricing models for non-exchange-traded contracts.
Our accounting policies for derivative financial instruments are based on whether they meet the criteria for designation as cash flow or fair value hedges. A designated hedge of the exposure to variability in the future cash flows of an asset or a liability, or of a forecast transaction, is referred to as a cash flow hedge. A designated hedge of the exposure to changes in fair value of an asset or a liability is referred to as a fair value hedge. The criterion for designating a derivative as a hedge includes the assessment of the instrument's effectiveness in risk reduction, matching of the derivative instrument to its underlying transaction and the probability that the underlying transaction will occur. For derivatives with cash flow hedge accounting designation, we report the gain or loss from the effective portion of the hedge as a component of Other Comprehensive Income
and reclassify it into earnings in the same period or periods in which the hedged transaction affects earnings and within the same Consolidated Statement of Operations line item as the impact of the hedged transaction. For derivatives with fair value hedge accounting designation, we recognize gains or losses from the change in fair value of these derivatives, as well as the offsetting change in the fair value of the underlying hedged item, in earnings. Fair value gains and losses arising on derivative financial instruments not qualifying for hedge accounting are reported in our Consolidated Statement of Operations.
The company has entered into certain put and call arrangements to purchase equity in unconsolidated entities at a future date. These arrangements are accounted for at fair value at the balance sheet date.
Income taxes
The Company applies the asset and liability method of accounting for income taxes. Under the asset and liability method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amount of existing assets and liabilities and their respective tax bases and for operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which these temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. Deferred tax assets are reduced by a valuation allowance to the amount that is more likely than not to be realized. The Company recognizes the effect of income tax positions only if those positions will more likely than not be sustained. Recognized income tax positions are measured at the largest amount of tax benefit that is greater than 50 percent likely of being realized upon settlement. Interest and penalties related to income taxes are included in income tax expense and classified with the related liability on the Consolidated Balance Sheet. The Company accounts for the impact of GILTI (“global intangible low-taxed income”) in the period it arises and has therefore not provided for deferred taxes in respect of this item.
Government grants
Government grants received relating to capital expenditures are shown by deducting the grant from the asset's carrying amount and crediting them to income on a basis consistent with the depreciation policy of the relevant assets. Grants relating to categories of operating expenditures are shown as deferred income and credited to income in the period in which the expenditure to which they relate is charged.
Under the grant agreements amounts received may become repayable in full should certain circumstances specified within the grant agreements occur, including downsizing by the Company, disposing of the related assets, ceasing to carry on its business or the appointment of a receiver over any of its assets. The Company has not recognized any loss contingency having assessed as remote the likelihood of these events arising.
Research and development credits
Research and development credits are available to the Company under the tax laws in certain jurisdictions, based on qualifying research and development spend as defined under those tax laws. Research and development credits may be recognized as a reduction of income tax expense. However, certain tax jurisdictions provide refundable credits that are not wholly dependent on the Company's ongoing income tax status or income tax position. In these circumstances the benefit of these credits is not recorded as a reduction to income tax expense, but rather as a reduction of operating expenditure.
Pension costs
The Company contributes to defined contribution plans covering all eligible employees. The Company contributes to these plans based upon various fixed percentages of employee compensation and such contributions are expensed as incurred.
The Company operates, through certain subsidiaries, a defined benefit plan for certain employees located in the United Kingdom and Switzerland. The Company accounts for the costs of these plans in accordance with ASC 715-30 'Defined Benefit Plans – Pension'. These plans are presented in accordance with the requirements of ASC 715-60 'Defined Benefit Plans – Other Postretirement'. The Company also maintains various retirement plans across the Group, many of which are required by local employment laws.
Share-based compensation
The Company accounts for its share options, Restricted Share Units ("RSUs") and Performance Share Units ("PSUs") in accordance with the provisions of ASC 718 'Compensation – Stock Compensation'. Share-based compensation expense for equity-settled awards made to employees and directors is measured and recognized based on estimated grant date fair values. These equity-settled awards include employee share options, RSUs and PSUs.
Share-based compensation expense for share options awarded to employees and directors is estimated at the grant date based on each option's fair value as calculated using the Black-Scholes option-pricing model. Share-based compensation for RSUs and PSUs awarded to employees and directors is calculated based on the market value of the Company's shares on
the date of award of the RSUs and PSUs. The value of awards expected to vest is recognized as an expense over the requisite service periods. Forfeitures are estimated on the date of grant and revised if actual or expected forfeiture activity differs materially from original estimates.
Estimating the grant date fair value of share options as of the grant date using an option-pricing model, such as the Black-Scholes model, is affected by the Company's share price as well as assumptions regarding a number of complex variables. These variables include, but are not limited to, the expected share price volatility over the term of the awards, risk-free interest rates and the expected term of the awards.
Liability classified awards are measured at the fair value of the award on the grant date and remeasured at each reporting period at fair value until the award is settled.
Replacement awards
In connection with the completion of the Merger, the company issued replacement awards to the holders of PRA equity awards on July 1, 2021. An exchange of share-based compensation awards in a business combination is treated as a modification under ASC 718. The replacement awards and the original acquiree awards are measured at fair value at the acquisition date and calculated using the fair-value-based measurement principles in ASC 718. Amounts attributable to pre-combination vesting are accounted for as part of the consideration transferred for the acquiree. Amounts attributable to post-combination vesting are accounted for separate from the business combination and are recognized as compensation cost in the post-combination period.
Transaction and integration-related expenses
Transaction and integration-related expenses are the incremental costs directly attributable to the completion and integration activities associated with the Company’s recent acquisitions. The costs consist of investment banking fees, advisory costs, retention agreements with employees, accelerated share compensation charges, contingent consideration valuation adjustments and ongoing integration activities. The Company accounts for these transaction and integration-related costs as expenses in the period in which the costs are incurred and the services are received.
Restructuring
Restructuring charges reflect certain one-time costs arising from reorganization programs announced by Company management. These programs generally result in asset impairments and workforce reductions in order to optimize the Company’s structure and facilitate improved long-term performance. Impairment charges are taken when the value-in-use of the asset is less than the asset’s carrying value. Workforce related charges are taken when an approved reorganization program is communicated to the relevant employee groups.
Redeemable noncontrolling interests and equity
The Company acquired a majority ownership interest in MeDiNova during the year ended December 31, 2019. Included in the purchase agreement were put and call option arrangements with the noncontrolling interest holders that required (put option) or enabled (call option) the Company to purchase the remaining minority ownership at a future date. The option was accounted for as temporary equity, which is presented separately as redeemable noncontrolling interest on the Consolidated Balance Sheet. This classification reflects the assessment that the instruments are contingently redeemable in accordance with ASC 480-10-S99 'Distinguishing Liabilities from Equity'. On March 9, 2020, ICON exercised its option to call the remaining shares and took 100% ownership of MeDiNova.
Redeemable noncontrolling interests are accreted to their redemption value over the period from the date of issuance to the first date on which the option is exercisable. The change in the option's redemption value is recorded against retained earnings. In a computation of earnings per share, the accretion of redeemable noncontrolling interests to their redemption value is a reduction of net income attributable to the Group. Basic and diluted net income per ordinary share attributable to the Group includes the adjustment to reflect the accretion of the noncontrolling interest to its redemption value until the redemption of the noncontrolling interest on March 9, 2020.
Net income per ordinary share
Basic net income per ordinary share attributable to the Company has been computed by dividing net income available to ordinary shareholders by the weighted average number of ordinary shares outstanding during the period. Diluted net income per ordinary share is computed by adjusting the weighted average number of ordinary shares outstanding during the period for all potentially dilutive ordinary shares outstanding during the period and adjusting net income for any changes in income or loss that would result from the conversion of such potential ordinary shares. There is no difference in net income used for basic and diluted net income per ordinary share. Basic and diluted net income per ordinary share attributable to the Company includes the adjustment to reflect the accretion of the noncontrolling interest in MeDiNova to its redemption value.
Reclassifications
Certain prior period amounts in the consolidated financial statements and accompanying notes have been reclassified to conform to the current period’s presentation. Most notably, the Company has presented transaction and integration-related expenses as a separate line in the Consolidated Statement of Operations and reclassified certain costs incurred in the year ended December 31, 2020 within this line. These costs consist of transaction and integration-related expenses and contingent consideration valuation adjustments related to ICON's prior period acquisitions. These costs were previously presented in the selling, general and administrative expenses but have been reclassified to transaction and integration-related expenses to conform to the current period’s presentation.
Impact of Recently Issued or Adopted Accounting Standards
No new accounting pronouncement issued or effective has had, or is expected to have, a significant impact on the Company’s consolidated financial statements.
3. Disaggregation of Revenue
Revenue disaggregated by customer profile is as follows:
| | | | | | | | | | | | | | |
| Year ended | | |
| December 31, 2022 | December 31, 2021 | December 31, 2020 | | | |
| (in thousands) | | |
| Top client | $ | 683,546 | | $ | 441,173 | | $ | 337,904 | | | | |
| Clients 2-5 | 1,506,087 | | 1,291,946 | | 754,906 | | | | |
| Clients 6-10 | 1,112,636 | | 752,325 | | 350,865 | | | | |
| Clients 11-25 | 1,585,739 | | 1,077,073 | | 501,643 | | | | |
| Other | 2,853,378 | | 1,918,309 | | 851,970 | | | | |
| Total | $7,741,386 | $5,480,826 | $2,797,288 | | | |
Our customers have similar profiles and economic characteristics, and therefore have similar degrees of risk and growth opportunities.
4. Contract Balances
Accounts receivable and unbilled revenue are as follows:
| | | | | | | | | | | |
| December 31, 2022 | | December 31, 2021 |
| (in thousands) |
| Billed services (accounts receivable) | $ | 1,751,950 | | | $ | 1,349,851 | |
| Allowance for credit losses (note 5) | (20,562) | | | (7,081) | |
| Accounts receivable (net) | 1,731,388 | | | 1,342,770 | |
| Unbilled services (unbilled revenue) | $ | 957,655 | | | $ | 623,121 | |
| Accounts receivable and unbilled revenue, net | $ | 2,689,043 | | | $ | 1,965,891 | |
Unbilled services and unearned revenue or payments on account (contract assets and liabilities) were as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| (in thousands, except percentages) | December 31, 2022 | | December 31, 2021 | | $ Change | | % Change |
| | | | | |
| Unbilled services (unbilled revenue) | $ | 957,655 | | | $ | 623,121 | | | $ | 334,534 | | | 53.7 | % |
| Unearned revenue (payments on account) | (1,507,449) | | | (1,323,961) | | | (183,488) | | | 13.9 | % |
| Net balance | $ | (549,794) | | | $ | (700,840) | | | $ | 151,046 | | | 21.6 | % |
Timing may differ between the satisfaction of performance obligations and the invoicing and collection of amounts related to our contracts with customers. We record assets for amounts related to performance obligations that are satisfied but not yet billed and/or collected. These assets are recorded as unbilled revenue and therefore contract assets rather than accounts receivable when receipt of the consideration is conditional on something other than the passage of time. Liabilities are recorded for amounts that are collected in advance of the satisfaction of performance obligations or billed in advance of the revenue being earned.
Unbilled services/revenue balances arise where invoicing or billing is based on the timing of agreed milestones related to service contracts for clinical research. Contractual billing arrangements in respect of certain reimbursable expenses (principally investigators) require billing by the investigator to the Company prior to billing by the Company to the customer. As there is no contractual right of set-off between unbilled services (contract assets) and unearned revenue (contract liabilities), each are separately presented gross on the Consolidated Balance Sheet.
The Company is the contract principal in respect of both direct services and in the use of third parties (principally investigator services) that support a clinical trial. The progress towards completion for clinical service contracts is measured based on total project costs (including reimbursable costs). Amounts owed to investigators and others in respect of reimbursable expenses at December 31, 2022 and December 31, 2021 were $406.3 million and $323.6 million (see note 12 - Other liabilities).
Unbilled services as at December 31, 2022 increased by $334.5 million as compared to December 31, 2021. Unearned revenue increased by $183.5 million over the same period resulting in a decrease of $151.0 million in the net balance of unbilled services and unearned revenue or payments on account between December 31, 2021 and December 31, 2022. These fluctuations are primarily due to the completion of the Merger on July 1, 2021 but are also partially due to timing of payments and invoicing related to the Group's clinical trial management contracts. Billings and payments are established by contractual provisions including predetermined payment schedules which may or may not correspond to the timing of the transfer of control of the Company's services under the contract. Unbilled services arise from long-term contracts when a cost-based input method of revenue recognition is applied and revenue recognized exceeds the amount billed to the customer.
The credit loss expense recognized on the Group's receivables and unbilled services was $17.8 million and $0.9 million for the twelve months ended December 31, 2022 and 2021, respectively.
As of December 31, 2022 approximately $13.7 billion of revenue is expected to be recognized in the future in respect of unsatisfied performance obligations compared to $13.3 billion as of December 31, 2021. As of December 31, 2022 the Company expects to recognize revenue on approximately 52% of the unrealized performance obligation over the next twelve months compared to 48% as of December 31, 2021, with the remainder recognized thereafter over the duration of the customer contracts.
5. Allowance for Credit Losses
The Company does business with most major international pharmaceutical companies. Provision for credit losses at December 31, 2022 and December 31, 2021 comprises:
| | | | | | | | |
| December 31, 2022 | December 31, 2021 |
| | (in thousands) |
| Opening provision | $ | 7,081 | | $ | 7,149 | |
| Amounts used during the year | (3,913) | | (116) | |
| Amounts provided during the year | 17,800 | | 705 | |
| Amounts released during the year | — | | (544) | |
| Foreign exchange | (406) | | (113) | |
Closing provision | $ | 20,562 | | $ | 7,081 | |
6. Business Combinations
PRA Health Sciences, Inc. Acquisition
On July 1, 2021 (the "Merger Date"), the Company completed the Acquisition of PRA by means of a merger whereby Indigo Merger Sub, Inc., a Delaware corporation and subsidiary of ICON, merged with and into PRA Health Sciences, Inc., the parent of the PRA Health Sciences ("the Acquisition" and "the Merger"). The combined Group has retained the name ICON and brought together approximately 38,000 (as at the Merger date) employees across the globe, creating the world's largest clinical research organization with a singular focus on clinical research and commercialization. The Merger was accounted for as a business combination using the acquisition method of accounting in accordance with ASC Topic 805, Business Combinations.
The combined Company leverages its enhanced operations to transform clinical trials and accelerate biopharma customers’ commercial success through the development of much needed medicines and medical devices. The new ICON has a renewed focus on leveraging data, applying technology and accessing diverse patient populations to speed up drug development.
Upon completion of the Merger, pursuant to the terms of the Merger Agreement, PRA became a wholly owned subsidiary of the ICON Group. Under the terms of the Merger, PRA shareholders received per share $80 in cash and 0.4125 shares of ICON stock. The trading of PRA common stock on NASDAQ was suspended prior to market open on July 1, 2021.
In the years ended December 31, 2022 and 2021, the Company incurred Merger-related expenses of $39.7 million and $198.3 million, respectively, which were accounted for separately from the business combination and expensed as incurred within the “Transaction and integration related” line item of the Consolidated Statement of Operations. These costs consist primarily of investment banker fees, advisory fees, legal costs, accounting and consulting fees, share-based compensation expense, and employee retention bonuses. Included in transaction and integration costs in the years ended December 31, 2022 and 2021 are acquisition related costs (as defined by ASC 805) of $0.8 million and $57.1 million, respectively.
The Company also incurred approximately $86.7 million of Merger-related financing fees which are included in the “Interest expense” line item in the Consolidated Statement of Operations for the year ended December 31, 2021. During the year ended December 31, 2021, the Company deferred $76.2 million of financing costs incurred as a result of the Senior Secured Credit Facility and Senior Secured Notes. These costs will be amortized over the term of the related debt.
The Merger Date fair value of the consideration transferred consisted of the following:
| | | | | |
| (in thousands) |
| Fair value of cash consideration | 5,308,646 | |
| Fair value of ordinary shares issued to acquiree stockholders | 5,658,126 | |
| Fair value of replacement share-based awards issued to acquiree employees | 209,399 | |
| Repayment of term loan obligations and accrued interest * | 865,800 | |
| 12,041,971 | |
* This represents the portion of PRA debt paid by ICON. PRA also paid $401.6 million from available cash to settle debt obligations that existed at the Merger Date.
The following table summarizes the allocation of the consideration transferred based on the Merger Date fair values of assets acquired and liabilities assumed, with the excess of the purchase price over the estimated fair values of the identifiable net assets acquired recorded as goodwill:
| | | | | |
| July 1, |
| 2021 |
| (in thousands) |
| Cash and cash equivalents | $ | 259,971 | |
| Accounts receivable and unbilled revenue | 934,308 | |
| Other current assets | 125,156 | |
| Fixed assets | 156,851 | |
| Operating lease right-of-use assets | 180,601 | |
| Goodwill * | 8,084,314 | |
| Intangible assets | 4,919,000 | |
| Deferred tax assets | 25,190 | |
| Other assets | 33,928 | |
| Accounts payable | (50,259) | |
| Accrued expenses and other current liabilities | (380,048) | |
| Current portion of operating lease liabilities | (36,506) | |
| Unearned revenue | (739,278) | |
| Non-current portion of operating lease liabilities | (147,204) | |
| Deferred tax liabilities | (1,119,762) | |
| Other non-current liabilities | (204,291) | |
| |
| Net assets acquired | $ | 12,041,971 | |
| |
| |
| |
| |
| |
* The goodwill in connection with the Merger is primarily attributable to the assembled workforce of PRA and the expected synergies of the Merger. None of the goodwill recognized is deductible for income tax purposes.
The following table summarizes the fair value of identified intangible assets and their respective useful lives as of the Merger Date (in thousands, except for estimated useful lives):
| | | | | | | | |
| Estimated Fair Value | Estimated Useful Life |
| Customer relationships | 3,938,000 | | 23 years |
| Order backlog | 500,000 | | 3 years |
| Trade names | 202,000 | | 3 years |
| Patient database | 168,000 | | 7 years |
| Technology assets | 111,000 | | 5 years |
| 4,919,000 | | |
At June 30, 2022, the Company completed its review of the July 1, 2021 acquisition balance sheet of PRA and completed the final valuation associated with certain assets acquired and liabilities assumed. In the period since the Merger Date, the Company recognized certain measurement period adjustments as shown in the table below:
| | | | | |
| Measurement period adjustments |
| |
| (in thousands) |
| Cash and cash equivalents | $ | — | |
| Accounts receivable and unbilled revenue | — | |
| Other current assets | 14,465 | |
| Fixed assets | (6,137) | |
| Operating lease right-of-use assets | (11,744) | |
| Goodwill | 70,436 | |
| Intangible assets * | 44,000 | |
| Deferred tax assets | (147,039) | |
| Other assets | (1,166) | |
| Accounts payable | — | |
| Accrued expenses and other current liabilities | (37,496) | |
| Current portion of operating lease liabilities | 1,865 | |
| Unearned revenue ** | 19,623 | |
| Non-current portion of operating lease liabilities | 10,454 | |
| Non-current deferred tax liabilities | 193,837 | |
| Other non-current liabilities | (151,098) | |
* In the year ended December 31, 2022, the Company incurred $2.2 million amortization which related to the year ended December 31, 2021 due to the timing of when the measurement period adjustment was identified.
** The unearned revenue measurement period adjustment also includes $16.0 million as a result of the early adoption of ASU 2021-08 'Business Combinations (Topic 805) - Accounting for Contract Assets and Contract Liabilities from Contracts with Customers' in Quarter 4 2021.
7. Fair Value
The Company records certain assets and liabilities at fair value. Fair value is defined as the price that would be received to sell an asset, or paid to transfer a liability in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants at the measurement date. A three-level fair value hierarchy that prioritizes the inputs used to measure fair value is described below. This hierarchy requires entities to maximize the use of observable inputs and minimize the use of unobservable inputs. The three levels of inputs used to measure fair value are as follows:
•Level 1 — Quoted prices in active markets for identical assets or liabilities.
•Level 2 — Observable inputs other than quoted prices included in Level 1, such as quoted prices for similar assets and liabilities in active markets; quoted prices for identical or similar assets and liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data.
•Level 3 — Unobservable inputs that are supported by little or no market activity. This includes certain pricing models, discounted cash flow methodologies, and similar techniques that use significant unobservable inputs.
The carrying amounts of financial instruments, including cash and cash equivalents, accounts receivable, unbilled services, contract assets, accounts payable, and unearned revenue approximate fair value due to the short maturities of these instruments.
As of December 31, 2022, the fair value of the major classes of the Company's assets and liabilities measured at fair value on a recurring basis were as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| Level 1 | Level 2 | Level 3 | Investments Measured at Net Asset Value | Total |
| Assets: | | | | | |
| Available for sale securities (short-term) (a) | 1,713 | | — | | — | | — | | 1,713 | |
| Available for sale investments (long-term) (b) | — | | — | | — | | 32,663 | | 32,663 | |
| Derivative instruments (c) | — | | 12 | | — | | — | | 12 | |
| Total assets | $ | 1,713 | | $ | 12 | | $ | — | | $ | 32,663 | | $ | 34,388 | |
| | | | | |
| Liabilities: | | | | | |
| Derivative instruments (c) | — | | 3,670 | | — | | — | | 3,670 |
| Total Liabilities | $ | — | | $ | 3,670 | | $ | — | | $ | — | | 3,670 |
As of December 31, 2021, the fair value of the major classes of the Company's assets measured at fair value on a recurring basis were as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| Level 1 | Level 2 | Level 3 | Investments Measured at Net Asset Value | Total |
| Assets: | | | | | |
| Available for sale securities (short-term) (a) | 1,712 | $ | — | | $ | — | | — | | 1,712 |
| Available for sale investments (long-term) (b) | — | | $ | — | | $ | — | | 20,579 | 20,579 |
| Total assets | $ | 1,712 | | $ | — | | $ | — | | $ | 20,579 | | $ | 22,291 | |
(a) Represents the fair value of investments in highly liquid investments with maturities of greater than three months and a minimum "A-" rated fixed term deposits and are based on quoted market prices.
(b) To determine the classification of its interests in long-term investments, the Company considered the nature of its investment, the extent of influence over operating and financial decisions and the availability of readily determinable fair values. The Company determined that the interests in funds at December 31, 2022 and December 31, 2021 meet the definition of equity securities without readily determinable fair values. The Company concluded that the interests held at December 31, 2022 and December 31, 2021 qualify for the Net Asset Value (NAV) practical expedient in ASC 820 'Fair value measurements and disclosures'. Any increases or decreases in fair value are recognized in net income in the period.
(c) Represents the fair value of interest rate cap and interest rate swap agreements. The fair value of the agreements are the estimated amount that the Company would receive or pay to terminate such agreements, taking into account market interest rates and the remaining time to maturities or using market inputs with mid-market pricing as a practical expedient for bid-ask spread. The Company held no derivative instruments at December 31, 2021.
Non-recurring Fair Value Measurements
Certain assets and liabilities are carried on the accompanying Consolidated Balance Sheet at cost and are not re-measured to fair value on a recurring basis. These assets include finite-lived intangible assets that are tested for impairment when a triggering event occurs and goodwill that is tested for impairment annually or when a triggering event occurs. As of December 31, 2022, assets carried on the balance sheet and not re-measured to fair value on a recurring basis totaled approximately $13,250.4 million and are identified as Level 3 assets. These assets are comprised of goodwill of $8,971.7 million and identifiable intangible assets, net of $4,278.7 million.
As of December 31, 2022 and 2021, the fair value of total debt approximated $4,650.3 million and $5,507.2 million, respectively, as determined under Level 2 measurement for these financial instruments.
8. Investments
(a) Available for sale investments (short-term)
The Company held $1.7 million of available for sale investments at both December 31, 2022 and December 31, 2021. The Company classifies its investment in short term investments as available for sale. Short term investments comprise highly liquid investments with maturities of greater than three months and minimum "A-" rated fixed term deposits.
Short term investments at December 31, 2022 have an average maturity of 2.8 years compared to 2.7 years at December 31, 2021. The contractual maturity of certain investments in the portfolio is greater than 12 months; however, classification as short-term investments reflects the Company practice and intention in respect of these investments. The Company recognizes the unrealized losses at fair value in equity as these unrealized losses on short term investments have been considered as temporary.
(b) Available for sale investments (long-term)
The Company entered into subscription agreements with a number of funds. Capital totaling $20.6 million had been advanced under the terms of the subscription agreements at December 31, 2022 (December 31, 2021: $16.9 million). The Company determined that the interests in the funds meet the definition of equity securities without readily determinable fair values. The Company concluded that the interests held at December 31, 2022 qualify for the NAV practical expedient in ASC 820 'Fair value measurements and disclosures'. These are therefore measured at Level 3 of the fair value hierarchy. There was an increase in fair value of $6.3 million (December 31, 2021: $3.2 million) recognized in net income during the year bringing the carrying value of the subscriptions to $32.6 million at December 31, 2022 (December 31, 2021: $22.6 million). At December 31, 2022, the Company had committed to future investments of $23.3 million in respect of these funds.
(c) Equity method investments
The Company has invested $4.9 million to obtain a 49% interest in the voting share capital of Oncacare. The Company’s investment in Oncacare is accounted for under the equity method due to the Company's ability to exercise significant influence over Oncacare that is considered to be greater than minor. The Company records its pro rata share of the earnings/losses of this investment in 'Share of equity method investments' in the Consolidated Statement of Operations.
The majority investor has the right to sell the 51% majority voting share capital exclusively to the Company in an eighteen month period, commencing January 1, 2023 and ICON also has the right to acquire the 51% majority voting share capital from August 1, 2025.
The following table represents our equity method investments at December 31, 2022:
| | | | | | | | | | | |
| Ownership Percentage | Carrying Value | Carrying Value |
| | | |
| December 31, 2022 | December 31, 2022 | December 31, 2021 |
| | (in thousands) |
| Oncacare Limited | 49 | % | $ | — | | $ | 2,373 | |
| | | |
During the year ended December 31, 2021, the Company provided a loan of $10 million to Oncacare in order to fund the continued development of the business operations. The loan accrues annual interest at 1.6% and the loan is repayable on June 30, 2025.
The Company has recorded losses of $3.1 million and $2.2 million representing its pro rata share of the losses in Oncacare during the year ended December 31, 2022 and December 31, 2021, respectively. The carrying value of the Company's investment in Oncacare was reduced by $2.3 million of pro rata losses. The remaining $0.8 million in pro rata losses served to reduce the carrying value of the Company's loan receivable from Oncacare. The outstanding balance of the Company's loan receivable with Oncacare at December 31, 2022 is $9.2 million.
9. Property, Plant and Equipment, net
The carrying amount of Property, Plant and Equipment for the years ended December 31, 2022 and 2021 is as follows:
| | | | | | | | |
| December 31, 2022 | December 31, 2021 |
| | (in thousands) |
| Cost | | |
| Land | $ | 3,724 | | $ | 3,724 | |
| Building | 70,880 | | 82,017 | |
| Computer equipment and software | 500,135 | | 506,322 | |
| Office furniture and fixtures | 50,600 | | 107,507 | |
| Laboratory equipment | 59,946 | | 29,210 | |
| Leasehold improvements | 65,167 | | 70,123 | |
| Motor vehicles | 41 | | 65 | |
| | |
| | 750,493 | | 798,968 | |
| Less accumulated depreciation and asset write offs | (400,173) | | (462,524) | |
| | |
| Property, plant and equipment (net) | $ | 350,320 | | $ | 336,444 | |
Property, Plant and Equipment depreciation expense is as follows:
| | | | | | | | |
| December 31, 2022 | December 31, 2021 |
| | (in thousands) |
| Depreciation expense | $ | 106,426 | | $ | 75,484 | |
The Company regularly updates its register of property, plant and equipment and during the year ended December 31, 2022 and the year ended December 31, 2021, certain fully depreciated assets were written off as they were no longer used in the Company.
10. Goodwill
The change in the carrying amount of Goodwill for the years ended December 31, 2022 and 2021 is as follows:
| | | | | | | | |
| December 31, 2022 | December 31, 2021 |
| | (in thousands) |
| Opening goodwill | $ | 9,037,931 | | $ | 936,257 | |
| Current year acquisitions (note 6) | — | | 8,120,006 | |
| Prior period acquisition (note 6) | (35,692) | | — | |
| Foreign exchange movement | (30,569) | | (18,332) | |
| Closing goodwill | $ | 8,971,670 | | $ | 9,037,931 | |
There were no goodwill impairment losses for the years ended December 31, 2022 and 2021.
11. Intangible Assets
The carrying amount of Intangible Assets for the years ended December 31, 2022 and 2021 is as follows:
| | | | | | | | |
| December 31, 2022 | December 31, 2021 |
| Cost | (in thousands) |
| Customer relationships | $ | 4,076,435 | | $ | 4,056,642 | |
| Order backlog | 536,934 | | 528,022 | |
| Trade names & brands | 204,621 | | 204,685 | |
| Patient database | 170,238 | | 170,525 | |
| Technology assets | 120,984 | | 121,507 | |
| Total cost | 5,109,212 | | 5,081,381 | |
| | |
| Accumulated amortization | (830,553) | | (370,538) | |
Net book value | $ | 4,278,659 | | $ | 4,710,843 | |
On July 1, 2021, ICON plc announced the completion of its Merger with PRA Health Sciences, Inc. The Merger resulted in the recognition of Customer relationships of $3,938.0 million, Order backlog of $500.0 million, Trade names of $202.0 million, Patient database of $168.0 million and Technology assets of $111.0 million. These assets will be amortized over their expected useful lives of between 3 and 23 years. The valuation of these assets were finalized in June 30, 2022. In total, $677.1 million has been amortized in the period since the date of acquisition, $453.6 million has been amortized in the year ended December 31, 2022.
Future intangible asset amortization expense for the years ended December 31, 2023 to December 31, 2027 is as follows:
| | | | | |
| | Year Ended
December 31, 2022 |
| (in thousands) |
| 2023 | $ | 460,711 | |
| 2024 | 339,940 | |
| 2025 | 223,674 | |
| 2026 | 209,681 | |
| 2027 | 198,300 | |
| | $ | 1,432,306 | |
12. Other Liabilities
The carrying amount of Other Liabilities for the years ended December 31, 2022 and 2021 is as follows:
| | | | | | | | |
| December 31, 2022 | December 31, 2021 |
| | (in thousands) |
| General trade and overhead liabilities* | $ | 530,204 | | $ | 459,814 | |
| Personnel related liabilities | 395,862 | | 413,185 | |
| Operating lease liabilities | 43,657 | | 49,949 | |
| Facility related liabilities | 16,896 | | 12,055 | |
| Other liabilities | 12,852 | | 7,204 | |
| Restructuring liabilities | 5,512 | | 7,377 | |
| Short term government grants | 42 | | 45 | |
| | $ | 1,005,025 | | $ | 949,629 | |
*includes amounts due to third parties in respect of accrued reimbursable investigator expenses of $406.3 million at December 31, 2022 and $323.6 million at December 31, 2021.
13. Non-current Bank Credit Lines and Loan Facilities
The Company had the following debt outstanding as of December 31, 2022 and 2021:
| | | | | | | | | | | | | | | | | |
| | | Principal amount | |
| Interest rate as of | Interest rate as of | December 31, | December 31, | |
| (in thousands) | December 31, 2022 | December 31, 2021 | 2022 | 2021 | Maturity Date |
| Credit Facilities: | | | | | |
| Senior Secured Term Loan | 7.092 | % | 2.750 | % | $ | 4,201,213 | | $ | 5,001,213 | | July 2028 |
| | | | | |
Senior Secured Notes | 2.875 | % | 2.875 | % | 500,000 | | 500,000 | | July 2026 |
| | | | | |
| | | | | |
| | | | | |
| Total debt | | | 4,701,213 | | 5,501,213 | | |
| | | | | |
| Less current portion of long-term debt and debt issuance costs | | | (55,150) | | (55,150) | | |
| Total long-term debt | | | 4,646,063 | | 5,446,063 | | |
Less long-term portion of debt issuance costs and debt discount | | | (47,026) | | (64,901) | | |
| Total long-term debt, net | | | $ | 4,599,037 | | $ | 5,381,162 | | |
The Company paid a $27.6 million debt discount in connection with the Senior Secured Credit Facility and Senior Secured Notes on July 1, 2021.
The Company incurred interest costs from various financing arrangements during the years ended December 31, 2022, December 31, 2021 and December 31, 2020 as set out in the table below. These costs have been charged in the interest expense line of the Consolidated Statement of Operations. In the years ending December 31, 2022 and December 31, 2021, the Company expensed $17.7 million and $86.7 million of transaction related financing costs (inclusive of the amortization of financing fees which were previously capitalized) associated with the debt facilities used to finance the Merger.
| | | | | | | | | | | |
| Year ended |
| December 31, 2022 | December 31, 2021 | December 31, 2020 |
| (in thousands) |
| | | |
| Interest expense on drawn facilities | $ | 209,189 | | $ | 93,809 | | $ | 13,406 | |
| Amortization of financing costs | 17,749 | | 12,890 | | 523 | |
| Transaction and one time financing costs | — | | 75,391 | | — | |
| Other financing costs/(credits) | 2,793 | | 333 | | (910) | |
| Total financing costs | $ | 229,731 | | $ | 182,423 | | $ | 13,019 | |
As of December 31, 2022, the contractual maturities of the Company's debt obligations were as follows:
| | | | | | | | | | | | | | |
| Current maturities of long-term debt: | | | (in thousands) |
| 2023 | | | | 55,150 | |
| 2024 | | | | 55,150 | |
| 2025 | | | | 55,150 | |
| 2026 | | | | 55,150 | |
| 2027 and thereafter | | | | 4,480,613 | |
| Total | | | | $ | 4,701,213 | |
| | | | |
| | | | |
| | | | |
The Company's primary financing arrangements are its senior secured credit facilities (the "Senior Secured Credit Facilities"), which consists of a senior secured term loan and a revolving credit facility, and the senior secured notes (the "Senior Secured Notes").
Senior Secured Credit Facilities
In conjunction with the completion of the Merger Agreement, on July 1, 2021, ICON entered into a credit agreement providing for a senior secured term loan facility of $5,515 million and a senior secured revolving loan facility in an initial aggregate principal amount of $300 million (the "Senior Secured Credit Facilities"). The proceeds of the senior secured term loan facility were used to repay in full (i) PRA’s existing credit facilities and (ii) the Company's notes and fund, in part, the Merger. The senior secured term loan facility will mature in July 2028 and the senior secured revolving loan facility will mature in July 2026. The credit agreement governing the Senior Secured Credit Facilities provides that borrowings denominated in U.S. Dollars will bear interest based on LIBOR or the base rate (as elected by the borrower), plus an applicable margin. On the 29th of November 2022, the Company agreed with its lenders to the early adoption of Term SOFR as the reference rate within the Credit Agreement ahead of the June 2023 USD LIBOR cessation date. LIBOR is no longer an applicable reference rate available to the Company under the terms of the Credit Agreement.
Borrowings under the senior secured term loan facility amortize in equal quarterly installments in an amount equal to 1.00% per annum of the principal amount, with the remaining balance due at final maturity. The interest rate margin applicable to borrowings under the senior secured term loan facility is USD Term SOFR and a Term SOFR Adjustment depending on the interest period chosen plus an applicable margin of 2.25%. The senior secured term loan facility is subject to a floor of 0.50%.
The interest rate margin applicable to borrowings under the revolving loan facility will be, at the option of the borrower, either (i) the applicable base rate plus an applicable margin of 1.00%, 0.60% or 0.25% based on ICON’s current corporate family rating assigned by S&P of BB- (or lower), BB or BB+ (or higher), respectively, or (ii) Term SOFR plus a Term SOFR Adjustment on the interest period chosen plus an applicable margin of 2.00%, 1.60% or 1.25% based on ICON’s current corporate family rating assigned by S&P of BB- (or lower), BB or BB+ (or higher), respectively. In addition, lenders under the revolving loan facility are entitled to commitment fees as a percentage of the applicable margin at the time of drawing and utilization fees dependent on the proportion of the facility drawn. At December 31, 2022, $300.0 million remained undrawn under the senior secured revolving loan facility.
The Borrowers’ (as defined in the Senior Secured Credit Facility) obligations under the Senior Secured Credit Facilities are guaranteed by ICON and the subsidiary guarantors. The Senior Secured Credit Facilities are secured by a lien on substantially all of ICON’s, the Borrowers’ and each of the subsidiary guarantor’s assets (subject to certain exceptions), and the Senior Secured Credit Facilities will have a first-priority lien on such assets, which will rank pari passu with the lien securing the Senior Secured Notes, subject to other permitted liens. The Company is permitted to make prepayments on the senior secured term loan without penalty.
On December 30, 2022, the Company repaid $200.0 million of the senior secured term loan facility and made a quarterly interest payment of $66.1 million. This repayment resulted in an additional charge associated with previously capitalized fees of $1.8 million.
On September 30, 2022, the Company repaid $200.0 million of the senior secured term loan facility and made a quarterly interest payment of $53.6 million. This repayment resulted in an additional charge associated with previously capitalized fees of $1.9 million.
On June 30, 2022, the Company repaid $100.0 million of the senior secured term loan facility and made a quarterly interest payment of $39.4 million. This repayment resulted in an additional charge associated with previously capitalized fees of $0.9 million.
On March 31, 2022, the Company repaid $300.0 million of the senior secured term loan facility and made a quarterly interest payment of $35.1 million. This repayment resulted in an additional charge associated with previously capitalized fees of $3.2 million.
On December 29, 2021, the Company repaid $500.0 million of the senior secured term loan facility and made a quarterly interest payment of $40.8 million. This repayment resulted in an additional charge associated with previously capitalized fees of $5.6 million.
On September 27, 2021, the Company repaid $13.8 million of the senior secured term loan facility and made a quarterly interest payment of $40.4 million.
Senior Secured Notes
In addition to the Senior Secured Credit Facilities, on July 1, 2021, a subsidiary of the Company issued $500 million in aggregate principal amount of 2.875% senior secured notes due 2026 in a private offering (the “Offering”). The Senior Secured Notes will mature on July 15, 2026.
Fair Value of Debt
The estimated fair value of the Company’s debt was $4,650.3 million and $5,507.2 million at December 31, 2022 and December 31, 2021, respectively. The fair values of the Senior Secured Credit Facilities and Senior Secured Notes were determined based on Level 2 inputs, which are based on rates at which the debt is traded among financial institutions.
14. Derivatives
The Company has entered into interest rate cap and swap agreements for purposes of managing its exposure to interest rate fluctuations. These financial derivative agreements are designated as Cash Flow Hedges.
On November 29, 2022, the Company entered into two interest rate cap agreements ("2022 Caps") with an initial total notional value of $2,101 million to limit its exposure to changes in the variable interest rate on its Senior Secured Credit Facilities. Interest on the 2022 Caps began accruing on December 30, 2022 and the interest rate cap expires on December 31, 2024. The Company pays a fixed rate of 0.42% and receives a variable rate equal to the amount that the three-month SOFR rate exceeds 4.75%.
On November 29, 2022, the Company entered into an interest rate swap agreement ("2022 Swap") with an initial notional value of $1,101 million to limit its exposure to changes in the variable interest rate on its Senior Secured Credit Facilities. Interest on the 2022 Swaps begins accruing on December 31, 2024 and the interest rate swap expires on December 31, 2026. The Company pays a fixed rate of 3.4% and receives a variable rate of interest equal to the three-month SOFR on the 2022 Swap.
The critical terms of the caps and swap are substantially the same as the underlying borrowings. The interest rate caps and swap are accounted for as cash flow hedges as these transactions were executed to hedge the Company's interest payments and for accounting purposes are considered highly effective. As such, the effective portion of the hedges is recorded as unrealized gains (losses) on derivatives in Accumulated Other Comprehensive Income.
The fair value of these cash flow hedges represents the present value of the anticipated net payments the Company will make to the counterparty, which, when they occur, are reflected as interest expense on the consolidate statement of income.
The fair value of the Company’s derivative financial instruments, on a gross basis, and the line items on the accompanying consolidate balance sheets to which they were recorded are summarized in the following table:
| | | | | | | | | | | | | | |
| | December 31, 2022 |
| | Asset | Liability | Notional |
| | (in thousands) |
| Derivatives designated as hedging instruments: |
| | | | |
| Interest Rate Caps | Other Assets and Liabilities | $12 | $3,363 | 2,100,606 |
| Interest Rate Swap | Other Assets and Liabilities | $— | $307 | 1,100,606 |
| | | | |
| Total derivatives designated as hedging instruments | $12 | $3,670 | 3,201,213 |
During 2023, the Company estimates that an additional $3.3 million will be reflected as interest expense in the consolidated statements.
At December 31, 2021 the fair value of the Company's derivative financial instruments was $nil as the Company had no outstanding derivative financial instruments.
The Company recognized $0.1 million of gain within OCI and reclassified $0.1 million of gain from OCI to the income statement for the year end December 31, 2022.
15. Operating Leases
Lease costs recorded under operating leases as of December 31, 2022 and 2021 were as follows:
| | | | | | | | |
| Year ended |
| December 31, 2022 | December 31, 2021 |
| (in thousands) |
| | |
| Operating lease costs | $ | 53,880 | | $ | 51,200 | |
| Income from sub-leases | (1,165) | | (1,338) | |
| Net operating lease costs | $ | 52,715 | | $ | 49,862 | |
Of the total cost of $52.7 million incurred in the year ended December 31, 2022, $48.3 million is recorded within selling, general and administration costs and $4.4 million is recorded within direct costs. Of the total cost of $49.9 million incurred in the year ended December 31, 2021, $47.5 million is recorded within selling, general and administration costs and $2.4 million is recorded within direct costs.
Right-of-use assets obtained, in exchange for lease obligations, net of early termination options now reasonably certain to be exercised, during the years ended December 31, 2022 and December 31, 2021 totaled $28.7 million and $10.2 million, respectively.
During the years ended December 31, 2022 and December 31, 2021, impairments of operating right-of-use assets were recognized within restructuring charges for $24.8 million and $15.4 million, respectively, as part of an office consolidation program (see note 19 - Restructuring Charges).
The weighted average remaining lease term and weighted-average discount rate at December 31, 2022 were 6.90 years and 2.45%, respectively. The weighted average remaining lease term and weighted-average discount rate at December 31, 2021 were 6.91 years and 2.51%, respectively.
Future minimum lease payments under non-cancelable leases as of December 31, 2022 were as follows:
| | | | | |
| Minimum rental |
| payments |
| (in thousands) |
| 2023 | $ | 47,479 | |
| 2024 | 34,400 | |
| 2025 | 25,045 | |
| 2026 | 20,430 | |
| 2027 | 17,249 | |
| Thereafter | 45,065 | |
| Total future minimum lease payments | 189,668 | |
| Lease imputed interest | (14,367) | |
| Total | $ | 175,301 | |
Operating lease liabilities are presented as current and non-current. Operating lease liabilities of $43.7 million and $49.9 million have been included in other liabilities as at December 31, 2022 and December 31, 2021, respectively.
16. Non-current other liabilities
The carrying amount of Non-current other liabilities for the years ended December 31, 2022 and 2021 is as follows:
| | | | | | | | |
| December 31, 2022 | December 31, 2021 |
| | (in thousands) |
| Defined benefit pension obligations, net (note 21) | $ | 13,033 | | $ | 16,262 | |
| Other non-current liabilities | 25,227 | | 26,334 | |
| | $ | 38,260 | | $ | 42,596 | |
17. Commitments and Contingencies
Litigation
We do not expect any litigation to have a materially adverse effect on our financial condition or results of operations. However, from time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. Litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business.
Operating Leases
The Company has several non-cancelable operating leases, primarily for facilities, that expire over the next twelve years. These leases generally contain renewal options and require the Company to pay all executory costs such as maintenance and insurance. See note 15 - Operating leases for rental expense pursuant to ASC 842 for the years ended December 31, 2022 and December 31, 2021 and future minimum rental commitments as of December 31, 2022.
18. Share Capital
Holders of ordinary shares are entitled to receive such dividends as may be recommended by the Board of Directors of the Company and approved by the shareholders and/or such interim dividends as the Board of Directors of the Company may decide. On liquidation or a winding up of the Company, the par value of the ordinary shares are repaid out of the assets available for distribution among the holders of the ordinary shares of the Company. Holders of ordinary shares have no conversion or redemption rights. On a show of hands, every holder of an ordinary share present in person or proxy at a general meeting of shareholders shall have one vote, for each ordinary share held with no individual having more than one vote.
(a)Employee share based payments
During the year ended December 31, 2022, 348,286 options were exercised by employees at an average exercise price of $102.87 per share for total proceeds of $35.8 million. During the year ended December 31, 2022, 195,029 ordinary shares were issued in respect of certain RSUs and 46,087 ordinary shares were issued in respect of PSUs previously awarded by the Company.
During the year ended December 31, 2021, 1,065,529 options were exercised by employees at an average exercise price of $111.29 per share for total proceeds of $118.6 million. During the year ended December 31, 2021, 446,404 ordinary shares were issued in respect of certain RSUs and 44,132 ordinary shares were issued in respect of PSUs previously awarded by the Company.
On July 1, 2021, the Company completed the Acquisition of PRA. In accordance with the terms of the Merger Agreement, the Company issued 27,372,427 shares of the Company’s ordinary share capital at par value in exchange for all outstanding PRA shares of common stock.
During the year ended December 31, 2020, 193,417 options were exercised by employees at an average exercise price of $68.19 per share for total proceeds of $13.2 million. During the year ended December 31, 2020, 144,172 ordinary shares were issued in respect of certain RSUs and 63,516 ordinary shares were issued in respect of PSUs previously awarded by the Company.
(b)Share Repurchase Program
A resolution was passed at the Company’s Annual General Meeting (“AGM”) on July 22, 2016, which authorized the Directors to purchase (buyback) up to 10% of the outstanding shares in the Company. This authorization was renewed at the Company's AGM on each of July 25, 2017, July 24, 2018, July 23, 2019, July 21, 2020, July 20, 2021 and July 26, 2022. On October 3, 2016, the Company commenced a share buyback program of up to $400 million. The share buyback program was completed during the year ended December 31, 2018 with a total of 4,026,576 ordinary shares redeemed for a total consideration of $372.1 million. On January 8, 2019, the Company commenced a further share buyback program of up to 1.0 million ordinary shares which was completed during the year ended December 31, 2019. These shares were redeemed by the Company for a total consideration of $141.6 million. On October 22, 2019, the Company commenced a further share buyback program. At December 31, 2019, 35,100 ordinary shares were redeemed by the Company for a total consideration of $5.3 million. During the year ended December 31, 2020, 1,235,218 ordinary shares were redeemed by the Company under this buyback program for a total consideration of $175.0 million. On February 18, 2022, the Company commenced a share buyback program which was fully complete at March 31, 2022. Under this buyback program, 420,530 ordinary shares were redeemed by the Company for total consideration of $100.0 million.
All ordinary shares that were redeemed under the buyback program were canceled in accordance with the Constitution of the Company and the nominal value of these shares transferred to other undenominated capital as required under Irish Company law.
Under the repurchase program, a broker purchased or may purchase the Company's shares from time to time on the open market or in privately negotiated transactions in accordance with agreed terms and limitations. The program was and may be in the future designed to allow share repurchases during periods when the Company would ordinarily not be permitted to do so because it may be in possession of material non-public or price-sensitive information or due to applicable insider trading laws or self-imposed trading blackout periods. The Company's instructions to the broker in such cases were or may in the future be irrevocable and the trading decisions in respect of the repurchase program were made or will be made independently of and uninfluenced by the Company. The Company confirms that on entering the share repurchase plans it had no material non-public, price-sensitive or inside information regarding the Company or its securities. Furthermore, the Company will not enter into additional plans whilst in possession of such information. The timing and actual number of shares acquired by way of the redemption will be dependent on market conditions, legal and regulatory requirements and the other terms and limitations contained in the program. In addition, acquisitions under the program may be suspended or discontinued in certain circumstances in accordance with the agreed terms. Therefore, there can be no assurance as to the timing or number of shares that may be acquired under the program.
19. Restructuring Charges
A restructuring charge of $31.1 million was recognized during the year ended December 31, 2022 under a restructuring plan adopted following a review of operations. The restructuring plan reflected resource rationalization across the business to improve employee utilization and an office consolidation program to optimize the Company's office footprint. The restructuring plan resulted in a charge of $2.7 million relating to workforce reductions, an impairment of right-of-use assets and associated unavoidable costs totaling $24.5 million and fixed asset impairment of $4.0 million. It is company policy to disclose operating right-of-use asset impairment as a restructuring charge when it is part of a coordinated program to decommission space.
| | | | | | | | | | | |
| | Year Ended |
| December 31, 2022 | December 31, 2021 | December 31, 2020 |
| | (in thousands) |
| Restructuring charges | $ | 31,143 | | $ | 31,105 | | $ | 18,089 | |
Net charge | $ | 31,143 | | $ | 31,105 | | $ | 18,089 | |
At December 31, 2022, a total liability of $6.0 million was recorded on the Consolidated Balance Sheet relating to restructuring activities. The total liability included $1.4 million of facilities related liabilities of which $0.9 million is included within other liabilities and $0.5 million is included within non-current other liabilities. The remaining provision of $4.6 million relates to workforce reduction and is included within other liabilities.
| | | | | | | | | |
| Year Ended |
| December 31, 2022 | December 31, 2021 | |
| (in thousands) |
| | | |
| Opening provision | $ | 10,311 | | $ | 4,675 | | |
| Additional provision in the year | 4,364 | | 11,273 | | |
| Utilization | (8,653) | | (5,637) | | |
| Ending provision | $ | 6,022 | | $ | 10,311 | | |
20. Income Taxes
The Company's United States and Irish based subsidiaries file income tax returns in the United States and Ireland respectively. Other foreign subsidiaries are taxed separately under the laws of their respective countries.
The components of income before income tax expense are as follows:
| | | | | | | | | | | |
| | Year ended |
| December 31, 2022 | December 31, 2021 | December 31, 2020 |
| | (in thousands) |
| Ireland | $ | 432,963 | | $ | 231,893 | | $ | 280,310 | |
| United States | (270,440) | | (278,413) | | 41,950 | |
| Other | 405,328 | | 243,200 | | 58,945 | |
Income before income tax expense | $ | 567,851 | | $ | 196,680 | | $ | 381,205 | |
The components of income tax expense are as follows:
| | | | | | | | | | | |
| | Year ended |
| December 31, 2022 | December 31, 2021 | December 31, 2020 |
| | (in thousands) |
| Income tax expense: | | | |
| Current tax expense: | | | |
| Ireland | $ | 53,248 | | $ | 18,469 | | $ | 28,963 | |
| United States | 60,753 | | 35,478 | | 3,022 | |
| Other | 70,395 | | 48,003 | | 14,963 | |
Total current tax expense | 184,396 | | 101,950 | | 46,948 | |
| | | |
| Deferred tax (benefit)/expense: | | | |
| Ireland | (6,166) | | 553 | | 1,654 | |
| United States | (118,475) | | (52,717) | | 4,577 | |
| Other | (344) | | (8,452) | | (5,304) | |
Total deferred tax (benefit)/expense | (124,985) | | (60,616) | | 927 | |
| | | |
| Income tax expense allocated to continuing operations | 59,411 | | 41,334 | | 47,875 | |
| | | |
| Income tax expense was allocated to the following components of other comprehensive income: | | | |
| | | |
| Currency impact on long term funding | 7,211 | | 1,776 | | 68 | |
| | | |
| | | |
| Total | $ | 66,622 | | $ | 43,110 | | $ | 47,943 | |
Ireland's statutory income tax rate is 12.5%. The Company's consolidated reported income tax expense differed from the amount that would result from applying the Irish statutory rate as set forth below:
| | | | | | | | | | | |
| | Year ended |
| December 31, 2022 | December 31, 2021 | December 31, 2020 |
| | (in thousands) |
| Taxes at Irish statutory rate of 12.5% (2021:12.5%; 2020:12.5%) | $ | 70,980 | | $ | 24,586 | | $ | 47,651 | |
| Rate differential from amortization of intangible assets | (59,330) | | (31,228) | | (2,298) | |
| Foreign and other income taxed at higher rates | 52,464 | | 51,273 | | 10,241 | |
| Research & development tax incentives | (2,608) | | (3,120) | | (1,243) | |
| Movement in valuation allowance | (777) | | 3,101 | | 3,581 | |
| Effects of change in tax rates | (300) | | (128) | | 108 | |
| Change in unrecognized tax benefits | 8,392 | | 5,246 | | (1,672) | |
| Impact of stock compensation | (8,756) | | (9,083) | | (5,150) | |
| | | |
| Other | (654) | | 687 | | (3,343) | |
Income tax expense | $ | 59,411 | | $ | 41,334 | | $ | 47,875 | |
The tax effects of temporary differences that give rise to significant portions of deferred tax assets and deferred tax liabilities are presented below:
| | | | | | | | |
| December 31, 2022 | December 31, 2021 |
| | (in thousands) |
| Deferred tax liabilities: | | |
| Property, plant and equipment | $ | 10,927 | | $ | 19,606 | |
| Operating right-of-use-assets | 23,260 | | 33,449 | |
| Goodwill | 37,150 | | 33,354 | |
| Intangible assets | 1,078,302 | | 1,201,086 | |
| Other | 9,054 | | 1,761 | |
Total deferred tax liabilities recognized | 1,158,693 | | 1,289,256 | |
| | |
| Deferred tax assets: | | |
| Operating loss and tax credits carryforwards | 88,697 | | 86,893 | |
| Property, plant and equipment | 6,010 | | 5,846 | |
| Operating lease liabilities | 27,593 | | 36,106 | |
| Intangible assets | 3,602 | | 4,596 | |
| Accrued expenses and unbilled revenue | 64,016 | | 69,198 | |
| Stock compensation | 21,862 | | 25,557 | |
| Deferred compensation | 2,917 | | 3,445 | |
| Unearned revenue | 66,565 | | 64,924 | |
| Other | 9,155 | | 602 | |
| Total deferred tax assets | 290,417 | | 297,167 | |
| Valuation allowance for deferred tax assets | (43,379) | | (45,495) | |
| Deferred tax assets recognized | 247,038 | | 251,672 | |
Overall net deferred tax asset/(liability) | $ | (911,655) | | $ | (1,037,584) | |
At December 31, 2022 Ireland subsidiaries had tax credit carryforwards for income tax purposes that may be carried forward indefinitely, available for offset against future tax liabilities, if any, of $16.6 million.
At December 31, 2022 U.S. subsidiaries had U.S. federal and state net operating loss ("NOL") carryforwards of approximately $5.4 million and $401.9 million, respectively. These NOLs are available for offset against future taxable income and the expiry dates are shown in the table below. Of the $5.4 million U.S. federal NOLs, approximately $3.5 million is available for offset against future U.S. federal taxable income in 2023. The subsidiaries' ability to use the remaining U.S. federal and state NOL carryforwards is limited on an annual basis due to change of ownership in 2014, 2017, and 2019, as defined by Section 382 of the Internal Revenue Code of 1986, as amended. Of the U.S. federal NOLs, $5.3 million are limited by Section 382 for the years 2023 - 2036. As of December 31, 2022, U.S subsidiaries also had disallowed interest carryforwards of $93.0 million that can be carried forward indefinitely. These carryforwards are available for offset against future taxable income in the event that the U.S subsidiaries have excess capacity for interest deductions in future years.
The expected expiry dates of the US NOLs are as follows:
| | | | | | | | |
| Federal
NOL's | State
NOL's |
| | (in thousands) |
| 2023-2036 | $ | 5,268 | | $ | 249,530 | |
| 2033-2041 | — | | 145,632 | |
| Indefinite | 95 | | 6,736 | |
| | |
| | $ | 5,363 | | $ | 401,898 | |
In addition, we also have general business tax credit carryforwards of approximately $0.8 million that are available to reduce future U.S. federal and state income taxes. The general business tax credits are non-refundable and are due to expire between the years 2026-2038.
At December 31, 2022 other than those in the U.S. and Ireland, we had operating loss carryforwards for income tax purposes that may be carried forward indefinitely, available to offset against future taxable income, if any, of approximately $37.9 million. At December 31, 2022 those subsidiaries also had additional operating loss carryforwards of $14.8 million which are due to expire between 2023 and 2029 and operating loss carryforwards of $18.2 million which are due to expire between 2030 and 2039. In addition, at December 31, 2022 those subsidiaries had tax credit carryforwards for income tax purposes that may be carried forward indefinitely, available to offset against future tax liabilities, if any, of $4.7 million.
The valuation allowance at December 31, 2022 was approximately $43.4 million. The valuation allowance for deferred tax assets as of December 31, 2021 and December 31, 2020 was $45.5 million and $32.8 million respectively. The net change in the total valuation allowance was a decrease of $2.1 million during 2022 and an increase of $12.8 million during 2021. Of the total decrease of $2.1 million in 2022, $0.8 million was recognized within income tax expense and a decrease of $1.3 million was recognized in Other Comprehensive Income. Of the total increase of $12.8 million in 2021, $9.3 million was in respect of an acquired entity, $4.4 million was recognized within income tax expenses, and $0.9 million was recognized in Other Comprehensive Income.
The valuation allowances at December 31, 2022 and December 31, 2021 were primarily related to operating losses and tax credits carried forward that, in the judgment of management, are not more likely than not to be realized. In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities, loss utilization, projected future taxable income and mitigation strategies in making this assessment. In respect of deferred tax assets not subject to a valuation allowance, management considers that it is more likely than not that these deferred tax assets will be realized on the basis that there will be sufficient reversals of deferred tax liabilities and taxable income in future periods.
The Company has recognized a deferred tax liability of $1.6 million (2021: $0.8 million) for investments in foreign subsidiaries where the Company does not consider the earnings to be indefinitely reinvested. For the deferred tax liability not recognized in respect of temporary differences related to investments in foreign subsidiaries which are considered to be indefinitely reinvested, it is not practicable to calculate the exact unrecognized deferred tax liability, however it is not expected to be material as Ireland allows a tax credit in respect of distributions from foreign subsidiaries at the statutory tax rate in the jurisdiction of the subsidiary so that no material tax liability would be expected to arise in Ireland in the event these earnings were ever remitted. In addition, withholding taxes applicable to remittances from foreign subsidiaries would not be expected to be material given Ireland’s tax treaty network and the EU parent subsidiary directive.
A reconciliation of the beginning and ending amount of total unrecognized tax benefits is as follows:
| | | | | | | | | | | |
| December 31, 2022 | December 31, 2021 | December 31, 2020 |
| | (in thousands) |
| Unrecognized tax benefits at start of year | $ | 202,065 | | $ | 19,078 | | $ | 20,156 | |
| Increase related to acquired tax positions | | 170,047 | | — | |
| Increase related to prior year tax positions | 16,098 | | 204 | | 401 | |
| Decrease related to prior year tax positions | (5,442) | | (1,695) | | (1,271) | |
| Increase related to current year tax positions | 5,701 | | 18,613 | | 2,931 | |
| Settlements | — | | (844) | | (369) | |
| Lapse of statute of limitations | (838) | | (3,338) | | (2,770) | |
Unrecognized tax benefits at end of year | $ | 217,584 | | $ | 202,065 | | $ | 19,078 | |
The relevant statute of limitations for unrecognized tax benefits totaling $37.9 million could potentially expire during 2023.
Included in the balance of total unrecognized tax benefits at December 31, 2022 were potential benefits of $217.6 million, which if recognized, would affect the effective rate on income tax from continuing operations. The balance of total unrecognized tax benefits at December 31, 2021 and December 31, 2020 included potential benefits which, if recognized, would affect the effective rate of income tax from continuing operations of $202.1 million and $19.1 million respectively.
Interest and penalties recognized during the year ended December 31, 2022 amounted to a net charge of $7.1 million (2021: $1.9 million, 2020: ($0.6 million)) and are included within the income tax expense. Total accrued interest and penalties as of December 31, 2022 and December 31, 2021 were $22.6 million and $15.5 million respectively and are included in closing income taxes payable at those dates.
Our major tax jurisdictions are Ireland and the United States. We may potentially be subjected to tax audits in both our major jurisdictions. In Ireland, tax periods open to audit include the years ended December 31, 2018, December 31, 2019, December 31, 2020, December 31, 2021 and December 31, 2022. In the United States, tax periods open to audit include the years ended December 31, 2016, December 31, 2017, December 31, 2018, December 31, 2019, December 31, 2020, December 31, 2021 and December 31, 2022. During such audits, local tax authorities may challenge the positions taken by us in our tax returns.
21. Employee Benefits
Defined Contribution Plans
Defined contribution or profit sharing style plans ("the Plans") are offered in a number of countries. In some cases, these plans are required by local laws or regulations. Certain employees are eligible to participate in the Plans and participants in the Plans may elect to defer a portion of their pre-tax earnings into a pension plan, which is run by an independent party. The Company matches participant's contributions up to certain levels of the participant's annual compensation.
The Company's United States operations maintain retirement plans (the "U.S. Plans") that qualify as a deferred salary arrangement under Section 401(k) of the Internal Revenue Code. Participants in the U.S. Plans may elect to defer a portion of their earnings, up to the Internal Revenue Service annual contribution limit. The Company matches participant's contributions at varying amounts, subject to a maximum of 4.5% of the participant's annual compensation. Contributions to this U.S. Plan are recorded, in the year contributed, as an expense in the Consolidated Statement of Operations. Contributions for the years ended December 31, 2022, December 31, 2021 and December 31, 2020 were $30.2 million, $23.7 million and $17.0 million respectively.
Pension and Postretirement Benefit Plans
The Company maintains various retirement plans across the Group, many of which are required by local employment laws. The balances recorded to the balance sheet are as follows:
| | | | | | | | |
| December 31, 2022 | December 31, 2021 |
| Other receivables | $ | 6,492 | | $ | — | |
| Non-current other liabilities (note 16) | (13,033) | | (16,262) | |
In addition to the specific defined-benefit schemes shown separately below, the Company maintains several other retirement plans which have a cumulative total net obligation of $11.6 million and $8.0 million recorded to non-current liabilities as of December 31, 2022 and December 31, 2021, respectively.
ICON Development Solutions Limited pension plan
One of the Company's subsidiaries, ICON Development Solutions Limited, operates a defined benefit pension plan in the United Kingdom for its employees. The plan is managed externally and the related pension costs and liabilities are assessed in accordance with the advice of a professionally qualified actuary. Plan assets at December 31, 2022, December 31, 2021 and December 31, 2020, consist of units held in independently administered funds. The pension costs of this plan are presented in the following tables in accordance with the requirements of ASC 715-60 'Defined Benefit Plans – Other Postretirement'. The plan has been closed to new entrants with effect from July 1, 2003.
| | | | | | | | |
Funded status | December 31, 2022 | December 31, 2021 |
| | (in thousands) |
| Projected benefit obligation | $ | (19,558) | | $ | (41,813) | |
| Fair value of plan assets | 26,050 | | 36,198 | |
| Funded status | $ | 6,492 | | $ | (5,615) | |
The funded status as at December 31, 2022 is included in other long-term receivables on the Consolidated Balance Sheet. The funded status as at December 31, 2021 is included in non-current other liabilities on the Consolidated Balance Sheet.
| | | | | | | | |
Change in benefit obligation
| December 31, 2022 | December 31, 2021 |
| | (in thousands) |
| Benefit obligation at beginning of year | $ | 41,813 | | $ | 43,988 | |
| Service cost | 117 | | 134 | |
| Interest cost | 672 | | 665 | |
| Plan participants' contributions | 19 | | 23 | |
| | |
| Benefits paid | (514) | | (489) | |
| Actuarial gain | (18,636) | | (2,097) | |
| Foreign currency exchange rate changes | (3,913) | | (411) | |
Benefit obligation at end of year | $ | 19,558 | | $ | 41,813 | |
| | | | | | | | |
Change in plan assets | December 31, 2022 | December 31, 2021 |
| | (in thousands) |
| Fair value of plan assets at beginning of year | $ | 36,198 | | $ | 34,612 | |
| Expected return on plan assets | 1,258 | | 1,171 | |
| Actual return on plan assets | (7,305) | | 1,176 | |
| Employer contributions | 70 | | 91 | |
| Plan participants' contributions | 19 | | 23 | |
| Benefits paid | (514) | | (489) | |
| Foreign currency exchange rate changes | (3,676) | | (386) | |
| Fair value of plan assets at end of year | $ | 26,050 | | $ | 36,198 | |
The fair values of the assets above do not include any of the Company's own financial instruments, property occupied by, or other assets used by, the Company.
The following amounts were recorded in the Consolidated Statement of Operations as components of the net periodic benefit cost:
| | | | | | | | | | | |
| December 31, 2022 | December 31, 2021 | December 31, 2020 |
| | (in thousands) |
| Service cost | $ | 117 | | $ | 134 | | $ | 100 | |
| Interest cost | 672 | | 665 | | 746 | |
| Expected return on plan assets | (1,258) | | (1,171) | | (1,214) | |
| Amortization of net loss | 228 | | 625 | | 160 | |
| | | |
| Net periodic benefit cost | $ | (241) | | $ | 253 | | $ | (208) | |
The following assumptions were used at the commencement of the year in determining the net periodic pension benefit cost for the years ended December 31, 2022, December 31, 2021 and December 31, 2020:
| | | | | | | | | | | |
| December 31, 2022 | December 31, 2021 | December 31, 2020 |
| Discount rate | 1.8 | % | 1.5 | % | 2.1 | % |
| Rate of compensation increase | 3.7 | % | 3.4 | % | 3.3 | % |
| Expected rate of return on plan assets | 3.8 | % | 3.4 | % | 4.0 | % |
| | | | | | | | | | | |
Other comprehensive income | December 31, 2022 | December 31, 2021 | December 31, 2020 |
| | (in thousands) |
| Actuarial (gain)/loss - benefit obligation | $ | (18,636) | | $ | (2,097) | | $ | 5,294 | |
| Actuarial loss/(gain) – plan assets | 7,305 | | (1,176) | | (878) | |
| Actuarial loss recognized in net periodic benefit cost | (228) | | (625) | | (160) | |
| Total | $ | (11,559) | | $ | (3,898) | | $ | 4,256 | |
The estimated net loss and prior service cost for the defined benefit pension plan that will be amortized from accumulated other comprehensive income into net periodic benefit cost over the next year are $0.3 million and $Nil respectively.
Benefit Obligation
The following assumptions were used in determining the benefit obligation at December 31, 2022 and December 31, 2021:
| | | | | | | | |
| December 31, 2022 | December 31, 2021 |
| Discount rate | 4.9 | % | 1.8 | % |
| Rate of compensation increase | 3.6 | % | 3.7 | % |
A single discount rate is used which, when used to discount the projected benefit cash flows underlying a pension scheme with a 19 year duration, gives the same result as a full AA corporate bond yield curve.
Actuarial gains on the benefit obligation during 2022 resulted from changes in the assumptions compared to those adopted at December 2021. Changes in the assumptions reflect the changes in market conditions from December 2021 to December 2022 and the actuarial gain is primarily due to the change in the discount rate.
Plan Assets
The Company's pension plan asset allocation is as follows:
| | | | | | | | |
Asset Category | December 31, 2022 | December 31, 2021 |
| Government Bonds | 88 | % | — | % |
| Diversified Bonds | 12 | % | — | % |
| Equities | — | % | 24 | % |
| Corporate Bonds (including 50% high yield bonds) | — | % | 37 | % |
| Secured Loans and Multi Asset Credit | — | % | 39 | % |
| | |
| | |
| | 100 | % | 100 | % |
During 2022, the scheme's asset strategy changed to align the plan assets more closely with government and diversified bonds. There is no self-investment in employer related assets. The Company’s assumption for the expected return on plan assets was determined by the weighted average of the long-term expected rate of return on each of the asset classes invested as of the balance sheet date. The expected long-term rate of return on assets is 3.8% at December 31, 2022 and December 31, 2021, respectively.
Plan Asset Fair Value Measurements
| | | | | | | | | |
| | December 31, 2022 | December 31, 2021 | |
| (in thousands) | |
| Government Bonds | $ | 22,887 | | $ | — | | |
| Diversified Bonds | $ | 3,163 | | $ | — | | |
| Equities | $ | — | | $ | 8,782 | | |
| Corporate Bonds (including 50% high yield bonds) | $ | — | | $ | 13,434 | | |
| Secured Loans and Multi Asset Credit | $ | — | | $ | 13,982 | | |
| | | |
| | $ | 26,050 | | $ | 36,198 | | |
The value of assets held by the plan are represented by quoted prices in active markets for identical assets and are therefore classified as level 1 investments.
Cash Flows
The Company expects to contribute $0.1 million to the pension fund in the year ending December 31, 2023.
The following annual benefit payments, which reflect expected future service as appropriate, are expected to be paid.
| | | | | |
| (in thousands) |
| 2023 | $ | 302 | |
| 2024 | 371 | |
| 2025 | 402 | |
| 2026 | 703 | |
| 2027 | 643 | |
| Years 2028 - 2032 | 3,614 | |
The expected cash flows are estimated figures based on the members expected to retire over the next 10 years assuming no early retirements, withdrawals or commutation of pension for cash. At the present time it is not clear whether annuities will be purchased when members reach retirement or whether pensions will be paid each month out of scheme assets. The cash flows above have been estimated on the assumption that pensions will be paid monthly out of scheme assets. If annuities are purchased, then the expected benefit payments will be significantly different from those shown above.
Aptiv Solutions pension plan
On May 7, 2014 the Company acquired 100% of the common stock of Aptiv Solutions ("Aptiv"). The Company has a defined benefit plan covering its employees in Switzerland as mandated by the Swiss government. Benefits are based on the employee's years of service and compensation. Benefits are paid directly by the Company when they become due, in conformity with the funding requirements of applicable government regulations. The plan is managed externally and the related pension costs and liabilities are assessed in accordance with the advice of a professionally qualified actuary. Plan assets at December 31, 2022 and December 31, 2021 consist of units held in independently administered funds. The pension costs of this plan are presented in the following tables in accordance with the requirements of ASC 715-60 'Defined Benefit Plans – Other Postretirement'.
| | | | | | | | |
Funded surplus/(deficit) | December 31, 2022 | December 31, 2021 |
| | (in thousands) |
| Projected benefit obligation | $ | (5,806) | | $ | (7,643) | |
| Fair value of plan assets | 5,681 | | 6,964 | |
| Funded deficit | $ | (125) | | $ | (679) | |
The funded deficit at December 31, 2022 and December 31, 2021 are included in non-current other liabilities on the Consolidated Balance Sheet.
The change in benefit obligation is presented in the following table. The discount rates used in calculating the benefit obligation in years ended December 31, 2022 and December 31, 2021 were 2.3% and 0.4%, respectively.
| | | | | | | | |
Change in benefit obligation
| December 31, 2022 | December 31, 2021 |
| | (in thousands) |
| Benefit obligation at beginning of year | $ | 7,643 | | $ | 8,620 | |
| Service cost | 146 | | 150 | |
| Interest cost | 30 | | 12 | |
| Plan participants' contributions | 82 | | 95 | |
| Settlement | (218) | | (483) | |
| Prior service cost | (23) | | (82) | |
| Benefits paid and transferred balances | (182) | | 76 | |
| Actuarial gain | (1,527) | | (484) | |
| Foreign currency exchange rate changes | (145) | | (261) | |
| Benefit obligation at end of year | $ | 5,806 | | $ | 7,643 | |
| | | | | | | | |
| Change in plan assets | December 31, | December 31, |
| 2022 | 2021 |
| (in thousands) |
| Fair value of plan assets at beginning of year | $ | 6,964 | | $ | 7,601 | |
| Expected return on plan assets | 29 | | 15 | |
| Actual return on plan assets | (987) | | (238) | |
| Scheme contributions | 114 | | 128 | |
| Plan participants' contributions | 82 | | 95 | |
| Benefits paid and transferred balances | (182) | | 76 | |
| Settlement | (218) | | (483) | |
| Foreign currency exchange rate changes | (121) | | (230) | |
| Fair value of plan assets at end of year | $ | 5,681 | | $ | 6,964 | |
The fair values of the assets above do not include any of the Company's own financial instruments, property occupied by, or other assets used by, the Company.
PRA Switzerland AG pension plan
On July 1, 2021, the Company completed the Acquisition of PRA. PRA Switzerland AG, a subsidiary of the Company has a defined benefit plan covering its employees in Switzerland as mandated by the Swiss government. Benefits are based on the employee's years of service and compensation. Benefits are paid directly by the Company when they become due, in conformity with the funding requirements of applicable government regulations. The plan is managed externally and the related pension costs and liabilities are assessed in accordance with the advice of a professionally qualified actuary. Plan assets at December 31, 2022 consist of units held in independently administered funds. The pension costs of this plan are presented in the following tables in accordance with the requirements of ASC 715-60 'Defined Benefit Plans – Other Postretirement'.
| | | | | | | | | | | | |
Funded surplus/(deficit) | December 31, 2022 | December 31, 2021 | | | | |
| | (in thousands) | | | |
| Projected benefit obligation | $ | (5,345) | | $ | (4,990) | | | | | |
| Fair value of plan assets | 4,059 | | 3,017 | | | | | |
| Funded deficit | $ | (1,286) | | $ | (1,973) | | | | | |
| | | | | | |
| | | | | | |
The funded deficit at December 31, 2022 and December 31, 2021 are included in non-current other liabilities on the Consolidated Balance Sheet.
The change in benefit obligation is presented in the following table. The discount rate used in calculating the benefit obligation in years ended December 31, 2022 and December 31, 2021 was 2.3% and 0.4%, respectively.
| | | | | | | | | |
Change in benefit obligation
| December 31, 2022 | December 31, 2021 | |
| | (in thousands) |
| Benefit obligation at beginning of period | $ | 4,990 | | $ | 4,890 | | |
| Service cost | 404 | | 207 | | |
| Interest cost | 20 | | 19 | | |
| Plan participants’ contributions | 2,396 | | 135 | | |
| | | |
| | | |
| Settlement | (844) | | — | | |
| Benefits paid | (946) | | (113) | | |
| Actuarial (gain)/loss | (627) | | 1 | | |
| Foreign currency exchange rate changes | (48) | | (149) | | |
| Benefit obligation at end of year | $ | 5,345 | | $ | 4,990 | | |
| | | | | | | | | |
Change in plan assets | December 31, 2022 | December 31, 2021 | |
| (in thousands) |
| Fair value of plan assets at beginning of period | $ | 3,017 | | $ | 2,849 | | |
| Expected return on plan assets | 29 | | 15 | | |
| | | |
| Actual return on plan assets | 87 | | — | | |
| Scheme contributions | 325 | | 135 | | |
| Plan participants’ contributions | 2,396 | | 135 | | |
| Benefits paid | (946) | | (113) | | |
| | | |
| Settlement | (844) | | — | | |
| Foreign currency exchange rate changes | (5) | | (4) | | |
| Fair value of plan assets at end of year | $ | 4,059 | | $ | 3,017 | | |
The fair values of the assets above do not include any of the Company's own financial instruments, property occupied by, or other assets used by, the Company.
22. Equity Incentive Schemes and Stock Compensation Charges
Share Options
On July 21, 2008 the Company adopted the Employee Share Option Plan 2008 (the "2008 Employee Plan") pursuant to which the Compensation and Organization Committee of the Company's Board of Directors may grant options to any employee, or any Director holding a salaried office or employment with the Company or a Subsidiary for the purchase of ordinary shares. On the same date, the Company also adopted the Consultants Share Option Plan 2008 (the "2008 Consultants Plan"), pursuant to which the Compensation and Organization Committee of the Company's Board of Directors may grant options to any consultant, adviser or non-executive Director retained by the Company or any Subsidiary for the purchase of ordinary shares.
On February 14, 2017 both the 2008 Employee Plan and the 2008 Consultants Plan (together the "2008 Option Plans") were amended and restated in order to increase the number of options that can be issued under the 2008 Consultants Plan from 0.4 million to 1.0 million and to extend the date for options to be granted under the 2008 Option Plans.
An aggregate of 6.0 million ordinary shares have been reserved under the 2008 Employee Plan, as reduced by any shares issued or to be issued pursuant to options granted under the 2008 Consultants Plan, under which a limit of 1.0 million shares applies. Further, the maximum number of ordinary shares with respect to which options may be granted under the 2008 Employee Option Plan, during any calendar year to any employee shall be 0.4 million ordinary shares. There is no individual limit under the 2008 Consultants Plan. No options may be granted under the 2008 Option Plans after February 14, 2027.
Each option granted under the 2008 Option Plans is an employee stock option, or Nonqualified Stock Option ("NSO"), as described in Section 422 or 423 of the Internal Revenue Code. Each grant of an option under the 2008 Options Plans is evidenced by a Stock Option Agreement between the optionee and the Company. The exercise price is specified in each Stock Option Agreement, however option prices are not less than 100% of the fair market value of an ordinary share on the date the option is granted. Share option awards are granted with an exercise price equal to the market price of the Company's shares at date of grant. Share options typically vest over a period of five years from date of grant and expire eight years from date of grant.
PRA Equity Incentive Plans
The following represent the PRA equity incentive plans, that have been terminated as of July 1, 2021, as to grants of future awards.
Pursuant to the Merger Agreement, effective on July 1, 2021, each stock option and restricted stock unit under the PRA Plans was assumed by the Company and converted into a stock option or Restricted Share Unit exercisable for or payable in Ordinary Shares based on the ratio of the average trading price per Ordinary Share for the ten days prior to July 1, 2021, and the corresponding value of the Merger consideration for each PRA Share. Accordingly, the plans as detailed below were assumed by the Company.
PRA Health Sciences, Inc. 2020 Stock Incentive Plan (the "2020 Plan”), 2018 Stock Incentive Plan (the "2018 Plan"), 2014 Omnibus Incentive Plan (the "2014 Plan"), and 2013 Stock Incentive Plan (the "2013 Plan") were amended and restated and assumed by the Registrant effective as of July 1, 2021.
The 2020 Stock Incentive Plan, was approved by the PRA stockholders at their annual meeting on May 18, 2020. The 2020 Plan allowed for the issuance of stock options, stock appreciation rights, restricted shares and restricted stock units, other stock-based awards, and performance compensation awards as permitted by applicable laws. The 2020 Plan authorized the issuance of 2.5 million shares of common stock plus all shares that remained available under the prior plan on May 18, 2020.
The 2018 Stock Incentive Plan (the “2018 Plan”), was approved by the PRA stockholders at their annual meeting on May 31, 2018. The 2018 Plan allowed for the issuance of stock options, stock appreciation rights, restricted shares and restricted stock units, other stock-based awards, and performance compensation awards as permitted by applicable laws. The 2018 Plan authorized the issuance of 2 million shares of common stock plus all shares that remained available under the 2014 Plan on May 31, 2018 (which included shares carried over from the 2013 Plan).
On November 23, 2014, the PRA Health Sciences, Inc. Board of Directors approved the formation of the 2014 Plan for Key PRA Employees. The 2014 Plan allowed for the issuance of stock options, stock appreciation rights, restricted shares and restricted stock units, other stock-based awards, and performance compensation awards as permitted by applicable laws.
On September 23, 2013, the PRA Health Sciences, Inc. Board of Directors approved the formation of the 2013 Plan for Key Employees of Pinnacle Holdco Parent, Inc. and its subsidiaries. The 2013 Plan allowed for the issuance of stock options and other stock-based awards as permitted by applicable laws. The number of shares available for grant under the 2013 Plan was 12.5% of the outstanding shares at closing on a fully diluted basis. The 2013 Plan authorized the issuance of 2,052,909 shares of common stock.
The following table summarizes the transactions for the Company's share option plans for the years ended December 31, 2022, December 31, 2021 and December 31, 2020:
| | | | | | | | | | |
| | Options Granted
Under Plans | | Weighted Average Exercise Price | |
| Outstanding at December 31, 2019 | 656,107 | | | $ | 87.80 | | |
| Granted | 107,737 | | | $ | 159.83 | | |
| Exercised | (193,417) | | | $ | 68.19 | | |
| Canceled | (16,681) | | | $ | 92.21 | | |
| Outstanding at December 31, 2020 | 553,746 | | | $ | 108.53 | | |
| Assumed through business combinations * | 2,177,130 | | | $ | 108.78 | | |
| Granted | 100,299 | | | $ | 177.76 | | |
| Exercised | (1,065,529) | | | $ | 111.29 | | |
| Canceled/expired | (70,186) | | | $ | 128.46 | | |
| Outstanding at December 31, 2021 | 1,695,460 | | | $ | 110.38 | | |
| Granted | 108,643 | | | $ | 229.94 | | |
| Exercised | (348,286) | | | $ | 102.87 | | |
| Canceled/expired | (77,698) | | | $ | 143.08 | | |
| Outstanding at December 31, 2022 | 1,378,119 | | | $ | 119.86 | | |
| Vested and exercisable at December 31, 2022 | 1,047,803 | | | $ | 102.29 | | |
*Represents stock options issued as replacement awards in connection with the Merger.
The weighted average remaining contractual life of options outstanding and options exercisable at December 31, 2022, was 4.69 years and 4.25 years respectively (2021: 5.39 years and 4.55 years respectively).
Outstanding and exercisable share options:
The following table summarizes information concerning outstanding and exercisable share options as of December 31, 2022:
| | | | | | | | | | | | | | | | | | | | | |
| Options Outstanding | | Options Exercisable | |
Range Exercise
Price | Number of
Shares | Weighted
Average
Remaining
Contractual Life | Weighted Average Exercise Price | | Number of
Shares | Weighted Average Exercise Price | |
| | | | | | | |
14.88 - 96.15 | 494,565 | | 2.70 | — | | | 494,565 | | — | | |
103.81 - 124.00 | 226,489 | | 5.58 | — | | | 203,605 | | — | | |
125.74 - 147.26 | 386,518 | | 5.65 | — | | | 303,054 | | — | | |
159.33 - 231.68 | 270,547 | | 6.24 | — | | | 46,579 | | — | | |
| | | | | | | |
14.88 - 231.68 | 1,378,119 | | 4.69 | $ | 119.86 | | | 1,047,803 | | $ | 102.29 | | |
Options outstanding include both vested and unvested options as at December 31, 2022. Options exercisable represent options which have vested at December 31, 2022. From the date of grant, substantially all options vest over a five year period.
Fair value of Stock Options Assumptions
The weighted average fair value of options granted during the years ended December 31, 2022, December 31, 2021 and December 31, 2020 was calculated using the Black-Scholes option pricing model. The weighted average fair values and assumptions were as follows:
| | | | | | | | | | | |
| | Year Ended |
| December 31, 2022 | December 31, 2021 | December 31, 2020 |
| Weighted average fair value | $ | 68.42 | | $ | 49.15 | | $ | 42.43 | |
| | | |
| Assumptions: | | | |
| Expected volatility | 31 | % | 30 | % | 30 | % |
| Dividend yield | — | % | — | % | — | % |
| Risk-free interest rate | 1.86 | % | 0.78 | % | 0.57 | % |
| Expected life | 5.0 years | 5.0 years | 5.0 years |
The weighted average fair value of options assumed on the date of the Merger was calculated using the Black-Scholes option pricing model. The weighted average fair values on the date of the Merger and assumptions used were as follows:
| | | | | |
| July 1, 2021 |
| Weighted average grant date fair value | $ | 107.21 | |
| Assumptions: | |
| Expected volatility | 30 | % |
| Dividend yield | — | % |
| Risk-free interest rate | 0.56 | % |
| Expected life | 3.5 years |
Expected volatility is based on the historical volatility of our common stock over a period equal to the expected term of the options; the expected life represents the weighted average period of time that options granted are expected to be outstanding given consideration to vesting schedules and our historical experience of past vesting and termination patterns. The risk-free rate is based on the U.S. government zero-coupon bonds yield curve in effect at time of the grant for periods corresponding with the expected life of the option.
Restricted Share Units and Performance Share Units
On April 23, 2013 the Company adopted the 2013 Employees Restricted Share Unit and Performance Share Unit Plan (the "2013 RSU Plan") pursuant to which the Compensation and Organization Committee of the Company's Board of Directors may select any employee, or any Director holding a salaried office or employment with the Company, or a Subsidiary to receive an award under the plan. On May 11, 2015 the 2013 RSU Plan was amended and restated in order to increase the number of shares that can be issued under the RSU Plan by 2.5 million shares. Accordingly, an aggregate of 4.1 million ordinary shares have been reserved for issuance under the 2013 RSU Plan. The shares are awarded at par value and vest over a service period. Awards under the 2013 RSU Plan may be settled in cash or shares at the option of the Company. No awards may be granted under the 2013 RSU Plan after May 11, 2025.
On April 30 2019, the Company approved the 2019 Consultants and Directors Restricted Share Unit Plan (the “2019 Consultants RSU Plan”), which was effective as of May 16, 2019, pursuant to which the Compensation and Organization Committee of the Company’s Board of Directors may select any consultant, adviser or non-executive Director retained by the Company, or a Subsidiary to receive an award under the plan. 250,000 ordinary shares have been reserved for issuance under the 2019 Consultants RSU Plan. The awards are at par value and vest over a service period. Awards granted to non-executive directors during 2020 and 2021 vest over twelve months.
The Company has awarded RSUs and PSUs to certain key individuals of the Group. The following table summarizes RSU and PSU activity for the year ended December 31, 2022:
| | | | | | | | | | | | | | | | |
| PSU Outstanding
Number of Shares | PSU
Weighted Average
Grant Date
Fair Value | | RSU Outstanding
Number
of Shares | RSU
Weighted Average
Grant Date Fair Value | |
| Outstanding at December 31, 2021 | 154,190 | | $ | 160.23 | | | 572,785 | | $ | 191.20 | | |
| | | | | | |
| Granted | 64,682 | | $ | 229.79 | | | 302,307 | | $ | 216.85 | | |
| Shares vested | (46,087) | | $ | 140.48 | | | (195,029) | | $ | 174.35 | | |
| Forfeited | (20,365) | | $ | 185.90 | | | (97,451) | | $ | 205.25 | | |
| | | | | | |
| Outstanding at December 31, 2022 | 152,420 | | $ | 192.29 | | | 582,612 | | $ | 207.73 | | |
The fair value of RSUs vested for the year ended December 31, 2022 totaled $34.1 million (2021: $83.5 million). The share price range for the year was $137.47 - $265.96 (2021: $115.11 - $206.71).
The fair value of PSUs vested for the year ended December 31, 2022 totaled $6.5 million (2021: $5.1 million). The share price range for the year was $137.47 - $166.51 (2021: $115.11 - $125.74).
The PSUs vest based on service and specified EPS targets over the period 2021 – 2023 and 2022 – 2024. Depending on the actual amount of EPS from 2021 to 2024, up to an additional 76,210 PSUs may also be granted.
Non-cash stock compensation expense
Income from operations for the year ended December 31, 2022 is stated after charging $70.5 million in respect of non-cash stock compensation expense. Non-cash stock compensation expense has been allocated as follows:
| | | | | | | | | | | |
| | Year ended |
| December 31, 2022 | December 31, 2021 | December 31, 2020 |
| | (in thousands) |
| Direct costs | $ | 22,854 | | $ | 18,551 | | $ | 8,557 | |
| Selling, general and administrative | 47,669 | | 41,457 | | 17,714 | |
| Transaction and integration related * | — | | 73,836 | | — | |
| Total compensation costs | $ | 70,523 | | $ | 133,844 | | $ | 26,271 | |
* Represents the post combination portion of the accelerated vesting of awards following the completion of the Merger
The income tax expense for the year ended December 31, 2022 reflects a net income tax benefit of $12.9 million in connection with stock compensation (including excess tax benefits) and the total tax benefit in connection with stock options exercised during 2022 was $7.7 million. The income tax expense for the year ended December 31, 2021 reflects a net income tax benefit of $22.7 million in connection with stock compensation (including excess tax benefits) and the total tax benefit in connection with stock options exercised during 2021 was $23.9 million. The income tax expense for the year ended December 31, 2020 reflects a net income tax benefit of $6.9 million in connection with stock compensation (including excess tax benefits) and the total tax benefit realized in connection with stock options exercised during 2020 was $2.5 million.
23. Business Segment and Geographical Information
The Company has the expertise and capability to conduct clinical trials in most major therapeutic areas on a global basis and has the operational flexibility to provide development services on a stand-alone basis or as part of an integrated "full-service" solution. The Company has expanded through internal growth, together with a number of strategic acquisitions to enhance its expertise and capabilities in certain areas of the clinical development process.
The Company determines and presents operating segments based on the information that is internally provided to the chief operating decision maker, the (‘CODM’) in accordance with ASC 280 'Segment Reporting'. The Company determined that the CODM was comprised of the Chief Executive Officer and the Chief Financial Officer.
The Company operates as one reporting segment, which is the provision of outsourced development services on a global basis to the pharmaceutical, biotechnology and medical devices industries.
Revenues are allocated to individual entities based on where the work is performed in accordance with the Company's global transfer pricing model. Revenues and income from operations in Ireland are a function of our global contracting model and the Group’s transfer pricing model.
ICON Ireland acts as the Group entrepreneur under the Company’s global transfer pricing model given its role in the development and management of the Group, its ownership of key intellectual property and customer relationships, its key role in the mitigation of risks faced by the Group and its responsibility for maintaining the Company’s global network. ICON Ireland enters into the majority of the Company’s customer contracts.
ICON Ireland remunerates other operating entities in the ICON Group on the basis of a guaranteed cost plus mark-up for the services they perform in each of their local territories. The cost plus mark-up for each ICON entity is established to ensure that each of ICON Ireland and the ICON entities that are involved in the conduct of services for customers, earn an appropriate arms-length return having regard to the assets owned, risks borne, and functions performed by each entity from these intercompany transactions. The cost plus mark-up policy is reviewed annually to ensure that it is market appropriate. The integration of entities acquired through the Merger into this global network and global transfer pricing model remains ongoing.
The geographic split of revenue disclosed for each region outside Ireland is the cost plus revenue attributable to these entities. The residual revenues of the Group, once each ICON entity has been paid its respective intercompany service fee, generally fall to be retained by ICON Ireland. As such, revenues and income from operations in Ireland are a function of this global transfer pricing model and comprise revenues of the Group after deducting the cost plus revenues attributable to the activities performed outside Ireland.
There have been no changes to the basis of segmentation or the measurement basis for the segment results since the prior year.
Reportable geographic information at December 31, 2022 and December 31, 2021 and for the years ended December 31, 2022, December 31, 2021 and December 31, 2020 is as follows:
a) The distribution of revenue by geographical area was as follows:
| | | | | | | | | | | |
| | Year ended |
| December 31, 2022 | December 31, 2021 | December 31, 2020 |
| | | |
| | (in thousands) |
| Ireland | $ | 1,984,567 | | $ | 1,365,909 | | $ | 1,181,292 | |
| Rest of Europe | 1,618,350 | | 1,175,515 | | 416,884 | |
| U.S. | 3,574,610 | | 2,581,007 | | 925,563 | |
| Other | 563,859 | | 358,395 | | 273,549 | |
Total | $ | 7,741,386 | | $ | 5,480,826 | | $ | 2,797,288 | |
b) The distribution of income from operations (including Restructuring) by geographical area was as follows:
| | | | | | | | | | | |
| | Year ended |
| December 31, 2022 | December 31, 2021 | December 31, 2020 |
| | (in thousands) |
| Ireland * | $ | 218,088 | | $ | 131,961 | | $ | 276,478 | |
| Rest of Europe | 253,799 | | 177,863 | | 35,765 | |
| U.S. | 254,849 | | 39,132 | | 58,018 | |
| Other | 68,501 | | 29,573 | | 21,239 | |
Total | $ | 795,237 | | $ | 378,529 | | $ | 391,500 | |
* Includes the full amount of the amortization charge associated with the intangible asset acquired in the Merger.
c) The distribution of long-lived assets (property, plant and equipment and operating right-of-use assets), net, by geographical area was as follows:
| | | | | | | | | |
| December 31, 2022 | December 31, 2021 | |
| | (in thousands) |
| Ireland | $ | 143,025 | | $ | 118,253 | | |
| Rest of Europe | 99,721 | | 121,174 | | |
| U.S. | 213,311 | | 239,828 | | |
| Other | 48,095 | | 55,312 | | |
Total | $ | 504,152 | | $ | 534,567 | | |
24. Net Income Per Ordinary Share
Basic net income per ordinary share attributable to the Group has been computed by dividing net income available to ordinary shareholders by the weighted average number of ordinary shares outstanding during the period.
Diluted net income per ordinary share is computed by adjusting the weighted average number of ordinary shares outstanding during the period for all potentially dilutive ordinary shares outstanding during the period and adjusting net income for any changes in income or loss that would result from the conversion of such potential ordinary shares.
There is no difference in net income used for basic and diluted net income per ordinary share.
Basic and diluted net income per ordinary share attributable to the Group for the year ended December 31, 2020 includes an adjustment to reflect the accretion of the noncontrolling interest in MeDiNova to its redemption value. The noncontrolling interest was acquired in the year ended December 31, 2020 and therefore no adjustment has been required in the years ended December 31, 2022 and December 31, 2021.
The reconciliation of the number of shares used in the computation of basic and diluted net income per ordinary share is as follows:
| | | | | | | | | | | |
| December 31, 2022 | December 31, 2021 | December 31, 2020 |
| Weighted average number of ordinary shares outstanding for basic net income per ordinary share | 81,532,320 | | 67,110,186 | | 52,859,911 | |
| Effect of dilutive share options outstanding | 936,043 | | 958,125 | | 423,674 | |
| Weighted average number of ordinary shares outstanding for diluted net income per ordinary share | 82,468,363 | | 68,068,311 | | 53,283,585 | |
The reconciliation between net income attributable to the Group per the Consolidated Statement of Operations and the net income used to calculate net income per ordinary share attributable to the Group is as follows:
| | | | | | | | | | | |
| December 31, 2022 | December 31, 2021 | December 31, 2020 |
| (in thousands) |
| Net income attributable to the Group | $ | 505,304 | | 153,185 | | $ | 332,331 | |
| Noncontrolling interest adjustment to redemption amount | — | | — | | (4,522) | |
| Net income attributable to the Group (including NCI redemption adjustment) | 505,304 | | 153,185 | | 327,809 | |
| | | |
| December 31, 2022 | December 31, 2021 | December 31, 2020 |
| Net income per Ordinary Share attributable to the Group (including NCI redemption adjustment): | | | |
| Basic | $ | 6.20 | | $ | 2.28 | | $ | 6.20 | |
| Diluted | $ | 6.13 | | $ | 2.25 | | $ | 6.15 | |
| | | |
25. Accumulated Other Comprehensive Income
| | | | | | | | |
| Year ended |
| December 31, 2022 | December 31, 2021 |
| | (in thousands) |
| Currency translation adjustments | $ | (175,369) | | $ | (85,840) | |
| | |
| Actuarial gain/(loss) on defined benefit pension plan (note 21) | 7,559 | | (5,098) | |
| Loss on cash flow hedge | (3,728) | | — | |
| Amortization of loss on cash flow hedge | — | | 1 | |
| Total | $ | (171,538) | | $ | (90,937) | |
26. Supplemental Disclosure of Cash Flow Information
| | | | | | | | | | | |
| | Year ended |
| December 31, 2022 | December 31, 2021 | December 31, 2020 |
| | (in thousands) |
| Cash paid for interest | $ | 210,918 | | $ | 106,205 | | $ | 13,062 | |
| Cash paid for income taxes (net of refunds) | $ | 116,322 | | $ | 55,105 | | $ | 27,604 | |
27. Related Parties
Subsidiaries of the Company earned revenue of $2,000 (December 31, 2021: $30,000) from DS Biopharma Limited (formerly Dignity Sciences Limited) during the year. Dr. John Climax is Executive Chairman and a Director and shareholder of DS Biopharma Limited. $12,000 was recorded as due from DS Biopharma Limited at December 31, 2022 (December 31, 2021: $12,000).
Subsidiaries of the Company earned revenue of $235,000 (December 31, 2021: $551,000) from Afimmune Limited during the year. Dr. John Climax is Chief Executive Officer and a Director and shareholder of Afimmune Limited. $263,000 was recorded as due from Afimmune Limited at December 31, 2022 (December 31, 2021: $197,000).
Subsidiaries of the Company earned revenue of $428,000 from Corvus Pharmaceuticals during the year. Dr. Linda Grais serves as a Director and shareholder of Corvus Pharmaceuticals. $231,000 was recorded as due from Corvus Pharmaceuticals at December 31, 2022.
On July 24, 2020, a subsidiary of the Company, ICON Clinical Research Limited, entered into an agreement to jointly establish a new company, Oncacare, with a third party. The Company has invested $4.9 million to obtain a 49% interest in the voting share capital of Oncacare. The Company provided corporate support services to Oncacare to the value of $451,000 during the year ended December 31, 2022 (December 31, 2021: $465,000). $715,000 was recorded as due from Oncacare at December 31, 2022 (December 31, 2021: $264,000). During the year ended December 31, 2021, the Company provided a loan of $10 million to Oncacare in order to fund the continued start up of the business' operations. The loan accrues annual interest at 1.6% and the loan is repayable on June 30, 2025. The Company has recorded losses of $3.1 million and $2.2 million representing its pro rata share of the losses in Oncacare during the year ended December 31, 2022 and December 31, 2021, respectively. The carrying value of the Company's investment in Oncacare was reduced by $2.3 million of pro rata losses. The remaining $0.8 million in pro rata losses served to reduce the carrying value of the Company's loan receivable from Oncacare. At December 31, 2022 accrued interest of $183,000 remained outstanding.
The majority investor in Oncacare has the right to sell the 51% majority voting share capital exclusively to the Company in an eighteen month period, commencing January 1, 2023 and ICON also has the right to acquire the 51% majority voting share capital from August 1, 2025.
28. Subsequent Events
The Company has evaluated subsequent events from the Balance Sheet date through February 24, 2023, the date at which the consolidated financial statements were available to be issued. The Company has determined that there are no items to disclose.
Item 19. Exhibits.
Exhibits of ICON plc and subsidiaries
| | | | | | | | |
Exhibit
Number | | Title |
| | |
| | Agreement and Plan of Merger, dated as of February 24, 2021, by and among ICON plc, ICON US Holdings Inc., Indigo Merger Sub, Inc and PRA Health Sciences, Inc. (incorporated by reference to exhibit 2.1 to the Form 6K (file No. 333-08704) filed on February 24, 2021. |
| | |
| | Description of Securities Registered Under Section 12 of the Exchange Act. |
| | |
| | Credit Agreement, dated as of July 1, 2021, by and among ICON Luxembourg, S.À R.L., ICON Clinical Investments, LLC, Indigo Merger Sub, Inc. (which, after giving effect to the Merger on the Closing Date was succeeded by PRA Health Sciences, Inc.), ICON Public Limited Company, the other borrowers party thereto from time to time, the subsidiary guarantors party thereto from time to time, lenders party thereto Citibank, N.A., as administrative agent, and Citibank, N.A., London Branch, as collateral agent (incorporated by reference to exhibit 99.1 to the Form 6K (File No. 333-08704) filed on July 1, 2021). |
| | |
| | Indenture, dated as of July 1, 2021, by and among Indigo Merger Sub, Inc., PRA Health Sciences, Inc., the guarantors party thereto and Citibank, N.A., London Branch as trustee, notes collateral agent, paying agent, transfer agent and registrar (incorporated by reference to exhibit 99.2 to the Form 6K (File No. 333-08704) filed on July 1, 2021). |
| | |
| | Amendment No. 1 to the Credit Agreement referred to at Exhibit 2.3, dated as of November 29, 2022, by and among ICON Public Limited Company and Citibank, N.A., as administrative agent. |
| | |
| | Description of the Constitution of the Company (incorporated by reference to exhibit 99.2 to the Form 6K (File No. 333-08704) filed on July 25, 2016). |
| | |
| | Section 302 certifications. |
| | |
| | Section 906 certifications. |
| | |
| | List of Subsidiaries (incorporated by reference to Item 4 of Form 20-F filed herewith). |
| | |
| | Consent of KPMG, Independent Registered Public Accounting Firm. |
| | |
| 101.1* | | Interactive Data Files (Inline XBRL – Related Documents). |
| | |
| 104 | | Cover Page Interactive Data File (embedded within the Inline XBRL document and included in Exhibit 101) |
* Filed herewith
SIGNATURES
The Registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| | | | | | | | |
| | ICON plc | |
| | | |
| | | |
| | | |
| | /s/ Brendan Brennan | |
| | Brendan Brennan | |
| | Chief Financial Officer | |
Date: February 24, 2023