UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
Current Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 17, 2017
GENEREX BIOTECHNOLOGY CORPORATION
(Exact name of registrant as specified in its charter)
| Delaware | 000-29169 | 98-0178636 | ||
(State or other jurisdiction of Incorporation) |
(Commission File Number) |
(I.R.S Employer Identification No.) |
10102 USA Today Way Miramar, Florida |
33025 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (416) 364-2551
4145 North Service Rd, Suite 200, Burlington, Ontario Canada L7L 6A3
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Current Report contains forward-looking statements. These statements are based on the Company’s (as hereinafter defined) current beliefs, expectations and assumptions about future events, conditions and results and on information currently available to them. All statements, other than statements of historical fact, included herein regarding the Company’s strategy, future operations, financial position, future revenues, projected costs, plans, prospects and objectives are forward-looking statements. Words such as “expect,” “may,” “anticipate,” “intend,” “would,” “plan,” “believe,” “estimate,” “should,” and similar words and expressions are intended to identify forward-looking statements, but are not the exclusive means of identifying forward-looking statements. Forward-looking statements in this Current Report include express or implied statements concerning the Company’s future revenues, expenditures, capital or other funding requirements, the adequacy of the Company’s current cash and working capital to fund present and planned operations and financing needs, the growth of the Company’s business, the timing of our expansion plans, the cost of raw materials and labor, consumer preferences, the effect of government regulations on the Company’s business, the Company’s ability to compete in its industry, as well as future economic and other conditions both generally and in the Company’s specific geographic markets. These statements are based on currently available operating, financial and competitive information and are subject to various risks, uncertainties and assumptions that could cause actual results to differ materially from those anticipated or implied in the forward-looking statements due to a number of factors including, but not limited to, those set forth below in the section entitled “Risk Factors” in this Current Report. Given those risks, uncertainties and other factors, many of which are beyond the Company’s control, you should not place undue reliance on these forward-looking statements.
Before purchasing any securities of the Company, you should carefully read and consider the risks described under the section entitled “Risk Factors.” You should be prepared to accept any and all of the risks associated with purchasing the securities, including a loss of all of your investment.
The forward-looking statements relate only to events as of the date on which the statements are made. Neither the Company nor Hema (as hereinafter defined) undertakes any obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, even if experience or future changes make it clear that any projected results or events expressed or implied therein will not be realized. You are advised, however, to consult any further disclosures the Company makes in future public filings, statements and press releases.
EXPLANATORY NOTE
On January 17, 2016, we entered into and closed Acquisition Agreement (the “Acquisition Agreement”) with the equity owners of Hema Diagnostic Systems, LLC (“Hema”) pursuant to which we acquired a majority of the equity interests in Hema in exchange for our Stock and our obligation to issue Common Stock Purchase Warrants (the “Acquisition”). We have the right to acquire the remainder of the Hema equity interests for nominal consideration provided that the stock and warrants have a specified value and we have registered for resale the Company’s shares issued to the Hema equity owners. The Acquisition is described in detail in Item 2.01 below. We intend to focus Hema’s business going forward, but do not intend to discontinue our pre-Acquisition activities.
Reference’s to Hema include its two wholly owned subsidiaries, Rapid Medical Diagnostics Corp. and Hema Diagnostic Systems Panama, S.A.. Rapid Medical Diagnostics was established to develop products and hold patents used by Hema Diagnostic Systems, LLC. Hema Diagnostic Systems Panama, S.A. was established to distribute Hema Diagnostic Systems, LLC’s products in Central and South America. Prior to the Acquisition, equity interest in Hema Diagnostic Systems Panama, S.A. and Rapid Diagnostic Systems were separately held by the equity owners of Hema, and financial statements of the three companies were prepared on a combined basis, as they were under common control and management. Immediately prior to Closing of the Acquisition, the equity owners contributed to Hema the equity of the other two companies, making them wholly owned subsidiaries of Hema.
| i |
This Current Report contains summaries of the material terms of various agreements executed in connection with the transactions described herein. The summaries of these agreements are subject to, and are qualified in their entirety by, reference to these agreements, which are filed as exhibits hereto and incorporated herein by reference.
| ii |
TABLE OF CONTENTS
| CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS | ii | ||||
| EXPLANATORY NOTE | |||||
| TABLE OF CONTENTS | iii | ||||
| Item 1.01. | Entry into a Material Definitive Agreement | 1 | |||
| Item 2.01. | Completion of Acquisition or Disposition of Assets | 1 | |||
| Item 3.02. | Unregistered Sales of Equity Securities | 28 | |||
| Item 5.01. | Changes in Control of Registrant | 28 | |||
| Item 5.02. | Departure of Directors or Principal Officers; Election of Directors; Appointment of Principal Officers; Compensatory Arrangements of Certain Officers | 28 | |||
| Item 9.01. | Financial Statements and Exhibits | 347 | |||
| iii |
Item 1.01. Entry into a Material Definitive Agreement.
Acquisition Agreement
On January 17, 2017, Generex Biotechnology, Inc., entered into the Acquisition Agreement with the equity owners of Hema Diagnostic Systems, LLC. The disclosures set forth in Item 2.01 below relating to the Acquisition Agreement transactions affected thereby are hereby incorporated by reference into this Item 1.01.
Item 2.01. Completion of Acquisition or Disposition of Assets.
ACQUISITION AND RELATED TRANSACTIONS
Acquisition Agreement
On January 17, 2017, we entered into and an Acquisition Agreement (the “Acquisition Agreement”) among Generex Biotechnology Corporation, Hema Diagnostic Systems, LLC, a Florida Limited Liability Company (“Hema”) and the equity owners of Hema pursuant to which we acquired a majority of the equity interests in Hema in exchange for our Stock and commitment to issue Common Stock Purchase Warrants (the “Acquisition”). Closing under the Acquisition Agreement occurred January 18.
At Closing, we acquired 4,950 of Hema’s 10,000 previously outstanding limited liability company units in exchange for 53,191,000 shares of Generex common stock, par value $.0001 per share, which had a value of $250,000, based on the closing bid price for our Common Stock on the OTCPINK marketplace on the trading day immediately preceding the Closing Date. Immediately following closing, we contributed 20,000 shares of Generex common stock to Hema, in exchange for 300 newly limited liability company units. Following these two actions, Generex holds 5,250 of Hema’s 10,300 outstanding units, or approximately 51% of Hema’s equity. The remainder of Hema’s outstanding equity will then be held by Stephen Berkman. Prior to closing, Mr. Berkman was Hema’s majority owner.
Following Closing, we intend to engage in a reverse split of our common stock. Pursuant to the Acquisition Agreement, within two trading days following the effectiveness of a planned reverse stock split, we will issue to Mr. Berkman 230,000 shares of common stock and warrants exercisable for 15,000,000 shares of our common stock at an exercise price equal to the volume weighted average price of the Company’s common stock a ten day period beginning on the day after the reverse stock split is effective.
We will have the right to purchase all of the remaining Hema equity interests form Mr. Berkman for an aggregate price of $1.00 if, at any time within three years after Closing
| • | All of the common stock issued to the Hema equity owners, as well as the common stock for which the warrants may be exercised, have been registered for resale; and |
| • | The aggregate value of all such shares, including the share underlying the Warrants, is at least $15,000,000 |
In the event the above conditions are not me, Mr. Berkman would retain his approximately 49% interest in Hema unless we negotiated a further arm’s length price with him. Pursuant to a Registration Rights Agreement entered into at Closing, we have agreed file a registration statement with respect to all of the shares, including the shares underlying the warrants, within sixty days after effectiveness of the reverse stock split.
Hema is currently indebted to Mr. Berkman in the amount of $13,260,462 for loans, advances and other consideration. This debt was secured by a security interest in Hema’s assets. At Closing. Berkman terminated his
| 1 |
security interest on the Company’s assets. At such time as the condition set forth in Section 1.1(e), above, is satisfied, the loan payable from Acquiree to Berkman shall be deemed satisfied in full.
The Acquisition Agreement contains customary representations, warranties, and covenants of the Generex, Hema and Hema’s equity. Breaches of representations and warranties are secured by customary indemnification provisions.
Hema operates both directly and through two subsidiaries. Prior to closing under the Acquisition Agreement, the two subsidiaries were separately owned by Hema’s equity owners. Immediately prior to Closing, the equity owners assigned the ownership of these two companies to Hema. One of these companies. Hema Diagnostic Systems Panama, S.A., which was organized to distributed Hema’s products in Central and South America, is organized under the laws of Panama. Record transfer of the ownership of this entity requires the consent of the Panamanian agency with authority over business organizations. We have applied for this consent and consider it to be a purely administrative issue.
DESCRIPTION OF BUSINESS
Description of Hema’s Business
As used in the remainder of this Item 2.01, “Company,” “we,” “us,” “our” and HDS refer to Hema.
OVERVIEW
Hema Diagnostic Systems (referred to as HDS or Hema) was established December 2000 as a Florida Limited Liability Company and is in the business of developing, manufacturing, and distributing of in-vitro medical diagnostics for infectious diseases administered at the point of care level with results as soon as 10-15 minutes. We manufacture and sell rapid diagnostic devices based upon our own proprietary EXPRESS technology as well as cassette devices based on customary designs used generally in the industry.
Hema’s mission is to deliver the highest standard of quality product and solutions that are accurate, reliable, and cost effective for worldwide distribution and deployment.
Since its founding, Hema has been developing and continues to develop an expanding line of Rapid Diagnostic Tests (RDTs) including those for the following infectious diseases such as Human Immunodeficiency Virus (HIV) – ½ w/p24Ab, tuberculosis-XT, malaria, hepatitis, syphilis, typhoid, dengue and other infectious diseases.
Today, we have developed a substantial line of RDT’s known as RAPID 1-2-3 HEMA® ready to “go-to-market.”
Due to the potential infectious character of the whole blood test sample, our Express series of RDTs are designed to perform and deliver test results while sealed within the Express housing, carefully controlling the potentially infectious test sample. This design helps to increase our ability to control the possibility of cross-contamination. Most of our competitors’ products, while inexpensive, are not as user-friendly allowing for increased user-error and requires substantially more training and have greater risk of cross-contamination.
We have been designing and engineering delivery systems that incorporate advanced technologies of rapid test strips for use in our Express series of devices and which yield a rapid response for point-of-care patient testing and treatment.
Each RDT incorporates an accurate test strip that has been striped with specific antigens or antibodies combined in a proprietary cocktail and then incorporated into an easy-to-use and user-friendly delivery system. The HDS delivery
| 2 |
systems include our standard “cassette” design, our patented “Express” housing device as well as our new “Express II”.
Each system delivers its own advantages which enhance the use, application and performance of each diagnostic. This ease of use in the Express delivery systems ensure that our RDTs perform efficiently and effectively providing the most accurate and repeatable test results available while, at the same time, minimizing the transference of a potentially infected blood sample.
The Company maintains a Federal Drug Administration (FDA) registered facility in Miramar, Florida and is certified under both ISO9001 and ISO13485 for the Design, Development, Production and Distribution of the in-vitro devices. Approval of our HIV rapid test has been issued by the United States Agency for International Development (USAID). Additionally, some of our products qualified for and carry the European Union “CE” Mark, which allows us to enter into CE Member countries subject to individual country requirements. Currently, we have two malaria rapid tests approved under World Health Organization (WHO) guidelines. We anticipate that a third malaria test will be approved by the end of 2016. Our HDS products have also received registrations and approvals issued by other foreign governments. HDS is currently in the planning phase for entering into the newly announced, WHO “Pre-Qualified Approval” process for other HDS tests. This process allow expedited approval of rapid tests, reducing the current 24-30 month process down to approximately 6-9 months. WHO approval is necessary for our products to be used in those countries which rely upon the expertise of the WHO, as well as for NGO funding for the purchase of diagnostic products.
We maintain current U.S. Certificates of Exportability that are issued by two FDA divisions-CBER and CDRH. CBER (Center for Biologicals Evaluation and Research) is the FDA regulatory division that oversees biological devices and which include our HIV, Hepatitis B and Hepatitis C. The other division, Center for Devices and Radiological Health (CDRH), is responsible for the oversight of other HDS devices which include Tuberculosis, Syphilis, and the remaining product line. Our HDS facility maintains FDA Establishment Registration status and is in accord with GMP (Good Manufacturing Practice) as confirmed by the FDA.
We do not currently have FDA approval to sell our products in the United States. We intend on submitting our devices to the FDA under a Pre-Market Approval Application (PMA) or through the 510K process. The 510K would require the appropriate regulatory administrative submissions as well as a limited scientific review by the FDA to determine completeness (acceptance and filing reviews); in-depth scientific, regulatory, and Quality System review by appropriate FDA personnel (substantive review); review and recommendation by the appropriate advisory committee (panel review); and final deliberations, documentation, and notification of the FDA decision. The PMA process is more extensive, requiring clinical trials to support the application. We expect to apply to FDA for approval of our first RDT for FDA 510K approval within the next 3 months. We anticipate the FDA process will be completed within 9 months after submission. During this timeline, we will be preparing documentation for additional rapid tests to undergo either the FDA PMA or 510k process including 510k de novo.
OUR PRODUCTS
While we sell “cassette” based diagnostic tests based on standard designs, we expect our success will be tied to development, manufacture and sale of products based on our proprietary Express device platform systems. Recent advances in our device platform technology can be directly applied to individual test strip which is disease specific. line. These technologies further increase the performance capabilities of each test and its’ ability to detect diseases in an efficient and cost-effective manner.
The Rapid 1-2-3 Hema® Express platform is designed to ensure ease of use, accuracy of performance, and cost-effectiveness of production. Test results of each Rapid 1-2-3 Hema Express test device are easy to read under all conditions even while conducting testing in the field. Additionally, the Rapid 1-2-3 Hema Express does not require the use of water or electricity. Testing can be conducted with the patient and test Our Malaria RDTs will be availale in our Express II platform.
| 3 |
The Express platforms are available in the following presentations:
| • | Rapid 1-2-3 Hema Express HIV 1/2 w/p24Ab |
| • | Rapid 1-2-3 Hema Express II HIV 1/2 w/p24Ab |
| • | Rapid 1-2-3 Hema Express Tuberculosis-XT |
| • | Rapid 1-2-3 Hema Express II Tuberculosis-XT |
| • | Rapid 1-2-3 Hema Express II Malaria pF |
| • | Rapid 1-2-3 Hema Express II Malaria pF/pV |
| • | Rapid 1-2-3 Hema Express II Malaria pF/Pan |
| • | Rapid 1-2-3 Hema Express Syphilis |
| • | Rapid 1-2-3 Hema Express Dengue NS1 |
| • | Rapid 1-2-3 Hema Express Dengue IgG/IgM |
HDS is also in the process of developing the platform for the qualitative testing for other infectious diseases including Typhoid, Chikungunya, Zika and other diseases. A new HDS housing, designated as the Rapid 1-2-3 Hema Express III Sepsis, is currently in the design evaluation process phase.
Our Solution
Due to the potential infectious character of the whole blood test sample, our Express series of RDTs are designed to perform and deliver test results while within the sealed Express housing. This increases our ability to control the possibility of cross-contamination.
The degree of difficulty in using a rapid test is generally determined by the delivery system/housing design itself. One of the most common reasons for rapid test failure is due to user-error which is most commonly attributed to the misuse of a rapid test or of the test sample. The greater the degree of difficulty in performing the RDT, the greater the chance for user error. The Express series of devices substantially reduces the difficulty factor through its user-friendly test process and careful controls the test sample. It should be clear that the easier and more user-friendly a rapid test is to use, the greater success in producing and delivering accurate and repeatable test results.
We believe the Express device has the potential to expand its use to include additional test samples such as urine, fecal matter and oral fluids. We need to perform additional testing to validate the use of our products with these other test media.
To expedite the training in the use of the HDS rapid tests, we have designed each Express series device to operate in the same manner thereby reducing the amount of training needed in the use of other Express series RDTs. Once trained in the use of one Express series RDT, the clinician will know and understand how to run each additional Express device regardless of the diseases being tested.
Over the past 30 years, the most common rapid test delivery systems, known as “cassettes,” has undergone very few changes in their design and operation. While the internal tests strips within the cassette have continued to evolve, the cassette design has remained static.
The popularity of the cassette is basically due to the cost of the cassette device and the fact that for years, test administrators have known of no other test platform. The cassette is considered by many, as not being user-friendly and as such, can be counter-productive to delivering an accurate and repeatable test result.
| 4 |
HDS has moved forward with the design of two patent-protected delivery systems. The Express delivery systems, which include the Express and Express II, are both user-friendly and do, to a very great degree, control the possibility of cross-contamination and loss of control of a potentially infected test sample.
The Express and the Express II both incorporate into their design, a sample take-up system that adsorbs the whole blood, serum or plasma sample directly onto the device test strip. When the test sample meets a predetermined line, sufficient sample size has been achieved. The Express or Express II is then inserted directly into a pre-filled diluent pod which contains the exact amount of diluent, creating a water tight seal. This process helps to eliminate cross-contamination from the point of sample acquisition to that of test processing. Once the test procedure is finished, the device remains sealed and can be disposed of through incineration.
Cassettes
Over the past 30 years, most administrators of RDTs have been trained on the use of cassettes. For this reason, we have maintained a line of rapid cassette tests for a number of diseases.
The cassette is a semi-complicated and low cost delivery system that has been used in the worldwide markets. The cassette is not user-friendly and substantial time is required to train the test administrator in its use.
The configuration and method of use of the cassette has not changed over time and it requires that a blood sample be taken from the fingerstick and then added into the cassette device. This method of sample transfer does not allow for control of the potentially infected test sample, nor does it deliver a consistent and reliably accurate test sample volume to run the cassette device effectively. Once the blood sample has been added into the cassette device, diluent drops are added into the cassette device using a hand held dropper bottle. The dropper bottle delivers an unreliable and a relatively inaccurate volume of diluent. The failure of having too little or too much blood sample and/or too little or too much diluent can deliver inaccurate test results know as a “false-positive” or a “false-negative” as well as in some cases a “non-flow”. Once the test process is completed, the test administrator must dispose of all of the multiple potentially infected components
The process of using a cassette device is prone to misuse -which is the core reason for most cassette test failures. Additionally, cassette test procedure offers a substantial opportunity for cross-contamination. While accepted out of tradition, the cassette is a design that requires substantial care while performing the testing procedure.
Due to the historical nature of the cassette design, we continue to offer our cassette presentation, two of which have already been approved under the World Health Organization (WHO) List of Approved Malaria Devices, and offered o those markets which require WHO approval.
HDS Express
We believe that the first major competitor to the cassette device is the HDS RAPID 1-2-3 HEMA EXPRESS system. This new technology addresses many of the problems that administrators of rapid test devices have encountered when using a cassette type of device- especially concerning ease-of-use and user-error.
The Express system is designed to substantially reduce, human error, cross-contamination and cross-infection which is achieved through its’ simplicity of design and ease of use. This contributes greatly to the delivery of more repeatable and accurate test results.
During the design phase of each new Express device, a test strip is stripped with carefully selected and specific antigens or antibodies specific to a particular diseases. It is then carefully tested and evaluated to determine its degree of “Specificity” (the ability of the test to correctly identify those without the disease) and “Sensitivity” (the ability of a test to correctly identify those with the disease). HDS strives to meet the highest possible sensitivity and specificity performance levels. Once approved, the test strip is then inserted into the previous validated Express and its performance, with each newly design strip, is evaluated. If the device meets design standards, it is validated.
We believe the EXPRESS represents a substantial improvement over the use of a CASSETTE.
| 5 |
The EXPRESS is very user-friendly. Diluent used to operate the device is premeasured and contained in a sealed plastic pod. This helps to prevent user error thereby increasing test accuracy.
The required amount of blood or serum/plasma needed to be taken-up and into the device is easily determined through the use of a visible line on the sample take-up pad. This also helps to prevent user error. The sample pad quickly and easily absorbs the test sample carefully controlling the flow of a potentially infected blood sample.
Unlike the CASSETTE, there is no external transfer of potentially infected blood/serum and unlike the cassette, there is no guess work when combining diluent with the test sample.
The EXPRESS design incorporates the sample take-up pad which absorbs the blood directly up and into the device.
This unique means of blood acquisition substantially decreases the potential transfer of disease to an uninfected person.
The Express® is a patented delivery system which integrates any HDS test strip into a single, self-contained delivery system. It is very easy to use. Each Express device is individually packaged in a foil pouch validated to withstand damaging humidity.
The process for use is very simple:
| 1) | Read the instructions on the back of the pouch. |
| 2) | Open pouch and remove the Express device, the lancet and the pre-filled diluent pod. |
| 3) | Clean the finger with alcohol and then, prick the finger with a safety lancet and allow a bead of blood to appear on the fingertip. |
| 4) | Touch the tip of the Express sample take-up pad to the drop of blood. |
| 5) | Immediately the blood sample will flow onto and up the sample take-up pad. |
| 6) | Once the blood sample reached the “sufficient sample” line. Open the pre-filled diluent pod and insert the Express into the pre-filled diluent pod, creating a water-tight seal. The diluent will immediately mix with the blood sample and flow up and onto the test strip. |
| 7) | Over the next few minutes the “Control” line will appear confirming the test is operational. If the patient is positive for the disease being tested, a second “Test” line. |
| 8) | Once the test is completed, dispose in an appropriate manner. |
HDS Express II®
The newly designed and developed RAPID 1-2-3 HEMA EXPRESS II establishes a common ground between the EXPRESS and the cassette.
This new hybrid design combines the same basic simple, easy to use system of the EXPRESS with the lower production costs of a cassette - all while maintaining same performance standards and repeatability of the EXPRESS.
With fewer components in the EXPRESS II configuration, the cost of assembly was reduced by up to 30% without any loss in performance.
Automated assembly further decreases production costs through the elimination of additional labor and the associated overhead.
| 6 |
MARKET GROWTH
In a recently released report entitled “GLOBAL MARKETS FOR RAPID MEDICAL DIAGNOSTIC KITS” from BCC Research, it stated that the global market for rapid medical diagnostic kits (RDTs) was valued at nearly $18.4 billion in 2012. BCC Research expects the market to reach more than $24.2 billion by 2017 and register a five-year compound annual growth rate (CAGR) of 5.7% for the period 2012 to 2017.
Frost and Sullivan, a multi-national research and consulting organization reported in a June 23, 2016, that the U.S. Point of Care testing market is expected to reach $4.6 billion by 2020, with the largest growth segments in Infectious Diseases, Cardiac and Coagulation PT/NR. Their report further explained that growth is driven, in part, by the following:
| • | Point of Care Testing (POC/RDT): |
“POC tests are expanding into the retail space with large consumers (CVS, Walgreens and Target) building their footprint.” This also translates into patients getting tested at their doctor's office or medical clinic and getting results for immediate diagnosis and treatment. Additionally, “new business models such as expanding into retail clinics, mobile clinics and patient self-testing -testing opens up a wide range of opportunities..."
| • | Need for Speed in Test Results: |
In-vitro diagnostics delivers results faster with minimal invasive diagnostic tools. Additionally, POCs “…reduce turn-around-time from days to minutes”.
| • | Affordable Pricing: |
“Use of cheaper disposable consumables eliminates expensive reagent costs…” The cost in providing accurate test results is always a factor. POCs when combined with accuracy, simplicity of use and a lower cost will create a greater demand, further feeding the expansion of the POC market.
The demand for affordable POCs/RDTs continues to increase driven by cost, reliability and performance. As a POC test can be administered and evaluated in a pharmacy, a clinic or a doctors’ office, the need to incur laboratory costs, including sample transport, are avoided. Cost savings when using a POC/RDT versus a laboratory process is substantial.
While we do not yet have any products approved for sale in the United States, HDS products are also designed to address the need for testing in the expanding U.S. Point of Care. As such, it is our intention to enter into the appropriate regulatory processes to achieve the sale and use of multiple HDS Express devices in the U.S. some of which are anticipated to enter into the Over-the-Counter (OTC) market.
Internationally, many countries are seeing an expanding use of POC RTDs as new tests and technologies arise to address the detection of new infectious diseases. This is especially true where laboratory testing is difficult to access or non-existent.
Additionally, POCs/RDTs can be developed and validated more easily than lab based tests, allowing for a quicker response to address new and emerging diseases.
Market growth on an international basis targets three primary diseases, Malaria, HIV and Tuberculosis. In the World Malaria Day Report issued by the WHO in April 2016, it was reported that there were 214 million new cases of malaria worldwide in 2015 (range 149–303 million). The African Region accounted for most global cases of malaria (88%), followed by the South-East Asia Region (10%) and the Eastern Mediterranean Region (2%). Excluded were other parts of the world including the key markets of South America, the Caribbean and now, Western Europe. RDT sales in the WHO noted markets was approximately 314 million.
| 7 |
Human Immunodeficiency Virus (HIV) Testing Market
In 2015, the Grand View Research stated that the Global HIV Diagnostics Market is expected to reach $4.48 billion by 2022, growing at an estimated compound annual growth rate of 9.5% from 2015 to 2022.
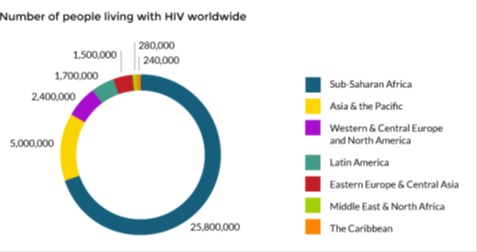
According to AVERT, there are over 36.9 million people living with HIV worldwide. It is estimated that over 2 million new cases are diagnosed each year.
The illustration below shows that the majority of the people living with HIV are in the Sub-Saharan Africa with 25.8 million people followed by Asia & the Pacific with 5.0 million and Western & Central Europe and North America with 2.4 million people.
By 2018, it is reported that the HIV rapid diagnostic test market is projected to be 162 million tests per year which is an increase from 81 million tests sold or deployed.
Our RTDs would be the first line of defense in diagnosing that would lead to mitigating the spread of the HIV disease.
Tuberculosis (TB) Testing Market
According to the US CDC, Tuberculosis (TB) is the leading infectious cause of death worldwide. TB is an airborne disease and spread by coughing or sneezing, and the most vulnerable are women, children, and those living with HIV/AIDS. It is highly contagious in closed or confined locations.
According to the WHO report reviewed in October 2016. In 2015, the WHO reported that 10.4 million were infected with TB and 1.8 million died from TB including 400,000 who were co-infected with HIV. Additionally, over 95% of TB deaths occur in low and middle-income countries, and it is among the top 5 causes of death for women between the ages of 15 to 44.
The WHO repost also estimated that 1 million children become infected and 170,000 die from TB. This number excludes those with an HIV co-infection. TB is also a leading cause of death of people living with HIV. In 2015, it is reported that 35% of HIV/TB deaths are due to TB.
The World Health Organization (WHO) estimates that two billion people—one third of the world's population—are infected with Mycobacterium tuberculosis (M.tb), the bacteria that causes TB. This includes the three tiered infections of TB: 1) M. Tuberculosis only; 2) M.TB and HIV coinfection; 3) M.TB MDR (multiple drug resistant)
| 8 |
M.TB MDR refers to the growing resistance of TB to available drugs, which means the disease is becoming more deadly and difficult to treat. It is reported that 480,000 new cases of people who are resistant to existing drugs for TB each year. A contributor to the growth of the drug resistant form of TB is the unnecessary TB treatment given to those patients who were improperly tested with an inaccurate TB test- being deemed to be positive when really being negative.
It is our hope that early detection and identification would lead to a faster treatment and care.
Malaria Testing Market
According to the World Malaria Report 2015 issued by the WHO, malaria transmission occurs in five WHO regions with 214 million cases of malaria globally.
In that same report, it is reported that approximately 3.2 billion people, which is nearly half of the world's population, are at risk of malaria with 88% of malaria cases and 90% of malaria deaths occurring in Sub-Saharan Africa. Children aged under 5 years account for more than two thirds of all deaths. Additionally, in 2015, an estimated 214 million cases were reported.
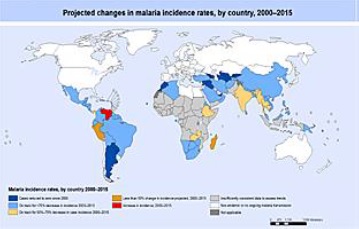
Countries most affected by malaria in Africa
In the March 14, 2016 report issued by the WHO, it stated that the sale of RTDs in the endemic countries has increased from 46 million units in 2008 to 319 million units in 2013. It is projected that this volume will grow to 400 million units in 2016 and beyond.
It is our desire to be the more cost effective alternative solution.
Other Infectious Disease Test Markets
| 9 |
The Company is developing and engineering in-vitro diagnostics for the following infectious diseases. Our goal is to deliver a diagnostic that is specific for each disease, circumventing the difficulty of cross-reactivity which is especially for Vector Diseases such as Chikungunya and Zika:
| • | Typhoid | |
| • | Chikungunya | |
| • | Zika | |
| • | Anthrax | |
| • | Sepsis, among others |
Sepsis
The Company intends to devote resources to develop and engineer a new and novel rapid diagnostic test for sepsis. Sepsis is a systemic infection of the body primarily found in hospital environments and a major cause of disease and death in the United States and worldwide.
As recently reported by the Global Sepsis Alliance (GSA), it stated “In the U.S., sepsis accounts for far more deaths than the number of deaths from prostate cancer, breast cancer and AIDS combined.” “GSA is a nonprofit organization that support the efforts of more than 1 million caregivers in than 70 countries as they seek to better understand and combat what many experts believe to be the leading cause of death worldwide: Sepsis.
In
a recent PR Newswire release dated Sept. 28, 2016, it was reported that “the sepsis diagnostics market is expected to reach
USD 564.1 million by 2021, at a CAGR of 8.8% from 2016 to 2021.
The sepsis diagnostics market is primarily driven by the rising prevalence of sepsis in the neonate and adult population across
the globe. In addition, rising geriatric population, growing number of surgical procedures, high incidence of hospital-acquired
infections, and increasing number of product approvals are supporting the growth of this market. On the other hand, lack of standard
protocols and awareness as well as shortage of skilled staff are the major challenges in this market.
The Company is developing a quantitative, multiplex, rapid point-of-care diagnostic assay for direct bedside as well as for E.R. use by physicians and medical personnel to either rule in infectious sepsis or rule it out. The assay is to be configured for use with a simple volume of blood and the results will be available in 15-20 minutes.
The multiplex assay will be a rapid triage or screening tool but it will also allow the physician to monitor the progress of the patient after a definitive diagnosis has been made. As such, it is a multi-purpose diagnostic and monitoring assay.
This assay will be based on the detection of biomarkers, usually proteins, which are normally produced in the body under sepsis conditions. The detection of multiple biomarkers in sepsis patients and their quantitation will also allow the physician to closely monitor the development of the sepsis syndrome in real-time and to aid in determining the overall effects of treatment choices and to alter treatment, if necessary
PATENTS AND INTELLECTUAL PROPERTY
We hold a U.S. Patent for our sample delivery system which expires in 2026. This is the basis for our Express system platforms, as follows:
| U.S. Patent No. | Issued | Expires | Nature | Type | Description |
| 7,749,771 | 7/6/2010 | / /2026 | test device | utility | Device and methods for detecting analyte in a sample |
| 10 |
We also have received or applied for patent protection in Brazil.
We two US registered trademarks for the names Rapid 1-2-3 Hema Express® and Rapid 1-2-3®.
We believe our long-term success will substantially depend upon our ability to obtain patent protection for our technology and our ability to protect our technology from infringement, misappropriation, discovery and duplication. We cannot be sure that any future patents will be granted, or that any patents which we now own or obtain in the future will fully protect our position. Our patent rights and the patent rights of medical device companies in general, are uncertain and con include complex legal and factual issues. We believe that our existing technology and the patents which we hold or for which we have applied do not infringe anyone else's patent rights. We believe our patent rights will provide meaningful protection against others duplicating our proprietary technologies. We cannot be sure of this, however, because of the complexity of the legal and scientific issues that could arise in litigation over these issues.
REGULATORY PROCESS AND APPROVAL
Governmental Regulation
The manufacturing and marketing of the our existing and proposed diagnostic products are regulated by the United States Food and Drug Administration ( “FDA" ) and comparable regulatory bodies in other countries. Our products are also regulated by, subject to approval by, or must meet standards set by, of certain non-governmental organizations involved in the purchase and distribution of products like ours. These regulations and standards govern almost all aspects of development, production and marketing, including product testing, authorizations to market, labeling, promotion, manufacturing and record keeping.
The Company's FDA regulated products require some form of action by that agency before they can be marketed in the United States, and, after approval or clearance, the Company must continue to comply with other FDA requirements applicable to marketed products, e.g. Quality Systems (for medical devices). Failure to comply with the FDA ' s requirements can lead to significant penalties, both before and after approval or clearance.
There are two review procedures by which medical devices can receive FDA clearance or approval. Some products may qualify for clearance under Section 510(k) of the Federal Food, Drug and Cosmetic Act, in which the manufacturer provides a pre-market notification that it intends to begin marketing the product, and shows that the product is substantially equivalent to another legally marketed product (i.e., that it has the same intended use and is as safe and effective as a legally marketed device and does not raise different questions of safety and effectiveness). In some cases, the submission must include data from human clinical studies. Marketing may commence when the FDA issues a clearance letter finding such substantial equivalence.
If the medical device does not qualify for the 510(k) procedure (either because it is not substantially equivalent to a legally marketed device or because it is required by statute and the FDA ' s implementing regulations have an approved application), the FDA must approve a Pre-Marketing Application ( " PMA " ) before marketing can begin. PMA ' s must demonstrate, among other matters, that the medical device provides a reasonable assurance of safety and effectiveness. A PMA application is typically a complex submission, including the results of non-clinical and clinical studies. Preparing a PMA application is a much more expensive, detailed and time-consuming process as compared with a 510(K) pre-market notification.
In addition, the FDA regulates the export of medical devices that have not been approved for marketing in the United States. The Federal Food, Drug and Cosmetic Act contains general requirements for any medical device that may not be sold in the United States and is intended for export. Specifically, a medical device intended for export is not deemed to be adulterated or misbranded if the product: (1) complies with the specifications of the foreign purchaser; (2) is not in conflict with the laws of the country to which it is intended for export; (3) is prominently labeled on the outside of the shipping package that it is intended for export; and (4) is not sold or offered for sale in the United States. However, the Federal Food, Drug and Cosmetic Act does permit the export of devices to any country in the world, if the device complies with the laws of the importing country and has valid marketing authorization in one of several " listed " countries under the theory that these listed countries have sophisticated mechanisms for the review of medical devices for safety and effectiveness.
| 11 |
The Company is also subject to regulations in foreign countries governing products, human clinical trials and marketing, and may need to obtain approval or evaluations by international public health agencies, such as the World Health Organization, in order to sell diagnostic products in certain countries. Approval processes vary from country to country, and the length of time required for approval or to obtain other clearances may in some cases be longer than that required for United States governmental approvals. On the other hand, the fact that our HIV diagnostic tests are of value in the AIDS epidemic may lead to some government process being expedited. The extent of potentially adverse governmental regulation affecting HDS that might arise from future legislative or administrative action cannot be predicted.
Our products rely on international regulatory approvals for sale into markets outside of the USA, and, domestically, our devices would require US FDA approval and in some cases, WHO approvals. These approvals allow for passage to the Global Fund funding process.
It is our intent to focus on both the domestic and international regulatory approvals.
Domestically, we intend on submitting our devices to the FDA under a Pre-Market Approval Application (PMA) or through the 510K process. The 510K would require the appropriate regulatory administrative submissions as well as a limited scientific review by the FDA to determine completeness (acceptance and filing reviews); in-depth scientific, regulatory, and Quality System review by appropriate FDA personnel (substantive review); review and recommendation by the appropriate advisory committee (panel review); and final deliberations, documentation, and notification of the FDA decision. The PMA process is more extensive, requiring clinical trials to support the application. We expect to apply to FDA for approval of our first RDT to be submitted to the FDA for 510K approval within the next 3 months. We anticipate the FDA process will be completed within 9-12 months after submission. During this timeline we will be preparing documentation for additional rapid tests to undergo either the FDA PMA or 510k process. We have not yet competed an assessment of whether our products will qualify for approval under the 5010K process or we will be required to engage in the more cumbersome Pre-Market Approval Application.
Internationally, we intend on submitting our Express devices and cassettes to the World Health Organization (WHO) process which requires a full regulatory and quality documentation dossier, produced and compiled by the Company. WHO process requires laboratory testing and evaluation and then clinical trials for public deployment and documentation throughout the whole process.
Once the WHO process is complete and documented, there is a submission into the Global Fund, which is a partnership between governments, civil society, the private sector and people affected by infectious diseases specifically HIV/AIDs, tuberculosis, and malaria.
The Global Fund raises and invests nearly $4 billion a year to support programs run by local experts in countries that are most in need.
It is our intent to submit all of our RTDs and cassettes and Express to the FDA, WHO and the Global Fund for regulatory review and approval for HIV, TB and malaria.
Currently, both our cassette malaria pF and malaria pF/pV have been approved under the WHO process. The cassette malaria pF/Pan was submitted to the WHO in February 2015, and we anticipate final review and approval before the end of the year.
PRODUCT STRATEGY
Our product strategy is to offer RTDs and other medical devices and tests that are consistent with simplicity in design; that are cost effective; that are easy to use; deliver fast and accurate results with a substantially reduced possibility of user error.
| 12 |
All product components required to operate the RDT are contained in each individual RDT foil pouch based upon options selected by the buyer. Each pouch has written and pictorial instructions clearly illustrating product use on the back. Each is color coded for each specific diagnostic thereby making test administering easy. This minimizes cross-contamination.
Our product mix was achieved by incorporating our Express or Express II housing/delivery system with a validated test strip. This allows us to accommodate our current and future test strips into either delivery system.
COMPETITIVE ADVANTAGE
We believe our unique and simple EXPRESS product design delivers significant advantages over our competition.
Due to the potential infectious character of the whole blood test sample, our Express series of RDTs are designed to perform and deliver test results while sealed within the Express housing, carefully controlling the potentially infectious test sample. This design helps to increase our ability to control the possibility of cross-contamination. Most of our competitors’ products, while inexpensive, are not as user-friendly, require substantially more training and have greater risk of cross- contamination.
Our products are more intuitive and self-explanatory than our competitors making it easier and safer to use. Our products require less training and education. Each Express is configured to operate in the same way regardless of the type of disease being tested.
With ease of use, simple design and faster results, our products allow for more tests administered at the patient point of care level.
We will compete on the basis these advantages. Most of our competitors’ products, while inexpensive, are not as user-friendly, require substantially more training and have greater risk of cross- contamination.
COMPETITION
The diagnostics industry is a multi-billion dollar international industry and is intensely competitive. Many of our competitors are substantially larger and have greater financial, research, manufacturing and marketing resources. Industry competition in general is based on the following:
| • | Scientific and technological capability; |
| • | Proprietary know-how; |
| • | The ability to develop and market products and processes; |
| • | The ability to obtain FDA or other required regulatory approvals; |
•
|
The ability to manufacture products that meet applicable governmental and NGO requirements; |
| • | The ability to manufacture products cost-effectively; |
| • | Access to adequate capital; |
| • | The ability to attract and retain qualified personnel; and |
| • | The availability of patent protection. |
| 13 |
We believe our scientific and technological capabilities as well as our proprietary technology and know-how relating to our rapid tests, particularly for the development and manufacture of tests for the detection of antibodies to infectious diseases, are very strong.
Alere Inc.
Alere is our main competitor and one of the major player in RTDs for infectious diseases. Alere markets the Alere HIV Combo Ag/Ab test, which uses the lateral flow technology patent. Alere acquired the patent from Abbott over a decade ago. Alere subsequently acquired Standard Diagnostics of Korea and Accon of China.
In early 2016, Abbot Laboratories agreed to acquire Alere for $5.8 billion. However, Abbot recently started litigation to terminate the agreement. In the event Abbot does acquire Alere, Alere would lose the strength of Abbott, becoming as formidable competitor as it currently is.
Standard Diagnostics
Standard Diagnostics was a state funded entity in South Korea established to build and expand into the international markets under its own brand until it was acquired by Inverness, the predecessor to Alere, in 2006.
With funding from Inverness for regulatory registrations and a previously established cassette product line, Standard was able to capture a strong market share of purchased for use in Africa with funding from WHO and the Global Fund. Currently, Standard is the strongest competitor on an international basis, incorporating a cassette design into each of their products.
Chembio Diagnostic Systems, Inc.
Chembio Diagnostic Systems is a publicly traded diagnostic company that develops, manufactures and commercializes diagnostic solutions. Chembio uses its patented Next Generation DPP (Dual Path Platform) technology that makes claims of significant advantages over the Alere’s lateral-flow technology.
It has continued building its product line and entered into US FDA approval for a rapid HIV test approved for professional use only in the United States.
Other Competition
As infectious diseases are epidemic and in the minds of the public, there will be more competitors coming into the market place. However, competition will be based upon the implementation of a cassette or a “dipstick” format.
FACILITIES
The Company’s corporate office for product development and regulatory affairs is an FDA Registered Facility with a fully staffed laboratory and assembly facility in Miramar, Florida.
Based on order size, delivery requirements and current orders in process, our Miramar facility can handle up to 4 million RTD devices, all of which are currently hand assembled. We have long-standing relationships with subcontractors to handle additional production requirements. Currently, shipments have been made to agencies for regulatory approvals and for initial market entry and we are in process to apply for a US. FDA, and newly announced WHO approvals which will reduce the WHO process form 24-30 months down to approximately 6-9 months.
Cassette production is conducted through subcontractors in India and China. Each site operates under GMP (Good Manufacturing Practice) as well as being compliant with ISO 9001 and ISO13485. All HDS cassettes are included in our U.S. Certificate of Exportability and European Union CE Mark registrations. Additionally, two of our cassette malaria tests are approved by the WHO with a third approval due to be announced before the end of 2016.
| 14 |
We have established Quality and Assembly Agreements as well as Confidentiality Agreements with our subcontractors. All are subject to our inspection at a moment’s notice.
The quality of final assembly of each of our products is maintained under the strict guidelines of our internal Quality System, which forms the basis for the Company’s ISO13485 rating.
Full quality oversight is mandatory and final batch release testing is conducted on each lot of products assembled prior to shipment release.
With full automation, the Company anticipates to produce up to 10 million Express devices annually. Expanded production would allow for additional expansion beyond this volume. Additionally, subcontractors would provide approximately 60 million cassette tests per year.
SALES DEVELOPMENT
Our sales will be dependent on regulatory approvals issued by such agencies as the WHO, FDA and registration with the Global Fund. These approvals are a key element in the sales and marketing effort on an international basis.
WHO Approved
Following the successful fulfillment of previous PFSCM (Partnership for Supply Chain Management) and WHO shipments, HDS continues to participate in requests for proposals from PFSCM for our currently WHO-approved HDS Malaria test.
The Company is now awaiting the approval of an additional Malaria RDT which the WHO has stated will be announced by December 15, 2016 pending test results. This will then be the third HDS Malaria test that is approved under the WHO program.
The Company will also participate in the newly designed and recently announced WHO Pre-Qualification Program for Malaria RDTs. It is our intention to present the new Malaria Express II devices for Pf, Pf/Pv and Pf/Pan for this Pre-Qualification Program. The WHO will also extend the expedited approval process to include other diseases including HIV.
Long Term WHO Agreement
In February 2016, HDS signed a Long Term Two Year Agreement with the WHO for the supply of the first HDS Malaria tests.
Offering a highly competitive rate in close cooperation with our subcontractor, we expect to see increased sales for these products during the life of that agreement. However, the agreement with WHO allows us to compete for WHO funded projects, but does not guarantee any specific sales.
USAID
USAID has submitted to the Company a request for participation in a Long Term Agreement for the HDS Malaria Tests. The prerequisite will be the same as those requirements satisfied during the WHO evaluation process and which qualified our tests for purchase by the WHO. In FY2105, USAID purchased $24.5 million in malaria tests. USAID is the lead U.S. Government agency that works to end extreme global poverty and enable resilient, democratic societies to realize their potential and which supports the sale of RDTs to those same countries.
European Union
The European Union has also initiated the preliminary purchase process of our Express devices for Typhoid, Hepatitis-C, Hepatitis-B, Tuberculosis, Syphilis, Malaria and Dengue.
| 15 |
The requirement was made by established NGOs in Greece in cooperation with other international funding agencies.
Purchases of EXPRESS devices for Malaria and Tuberculosis ordered are to be financed by the Internal Security Fund of the European Union and details in the RFQ specifies the advanced performance specifications of the “HEMA RAPID” device.
The current requirement is for 300,000 total tests at an average of $2.10/test and is pending confirmation of funds and a renegotiation on ship schedule. Currently, due to difficulties within Greece, this requirement has been held up pending resolution.
Concurrent to this requirement is a pending order for approximately 250,000 HDS Express HIV tests. This order is being coordinated by a Netherlands NGO that has and continues to act as supply-chain management. This organization has committed to including HDS products in their current inventories and has additional requirements that span beyond the initial 250,000 devices. This includes several other RFP opportunities for other HDS testing approved products.
We have received a commitment from an NGO in Paris with sustaining ties to the country of Nigeria. The commitment signals a storing intent on the part of this NGO, but is not a binding order for products. The issuance of the order depends greatly upon the exchange rate of the target country and the US dollar. As of this date, the rate of exchange has delayed the issuance of a purchase order.
LEGAL PROCEEDINGS
Hema is not a party to any pending legal proceedings. Pursuant to the terms of the Acquisition Agreement, responsibility for any Hema liability emerging from Hema’s business prior to closing relies wholly with the pre-transaction Hema shareholders but there is no assurance they would have the assets available to pay any liability resulting from litigation.
RISK
FACTORS RELATING TO HEMA’S BUSINESS
In addition to the other information included in this Current Report on Form 8-K, you should carefully review and consider the factors discussed in Part I, Item 1A - Risk Factors of our Annual Report on Form 10-K for the year ended July 31, 2016 and our subsequent Quarterly Reports on Form 10-Q. These factors materially affect our business, financial condition or future results of operations. The risks, uncertainties and other factors described in our Annual Report on Form 10-K, our Reports on Form 10-Q and below are not the only ones facing our company. Additional risks, uncertainties and other factors not presently known to us or that we currently deem immaterial may also impair our business operations, financial condition or operating results. Any of the risks, uncertainties and other factors could cause the trading price of our common stock to decline substantially.
Risks related to our industry, business and strategy
Because we may not be able to obtain or maintain the necessary regulatory approvals for some of our products, we may not generate revenues in the amounts we expect, or in the amounts necessary to continue our business. Our existing products as well as our manufacturing facility must meet quality standards and are subject to inspection by a number of domestic regulatory and other governmental and non-governmental agencies.
All of Hema’s proposed and existing products are subject to regulation in the U.S. by the U.S. Food and Drug Administration, the U.S. Department of Agriculture and/or other domestic and international governmental, public health agencies, regulatory bodies or non-governmental organizations. In particular, we are subject to strict
| 16 |
governmental controls on the development, manufacturing, labeling, distribution and marketing of our products. The process of obtaining required approvals or clearances varies according to the nature of, and uses for, a specific product. These processes can involve lengthy and detailed laboratory testing, human or animal clinical trials, sampling activities, and other costly, time-consuming procedures. The submission of an application to a regulatory authority does not guarantee that the authority will grant an approval or clearance for that product. Each authority may impose its own requirements and can delay or refuse to grant approval or clearance, even though a product has been approved in another country.
The time taken to obtain approval or clearance varies depending on the nature of the application and may result in the passage of a significant period of time from the date of submission of the application. Delays in the approval or clearance processes increase the risk that we will not succeed in introducing or selling the subject products, and we may determine to devote our resources to different products.
Changes in government regulations could increase our costs and could require us to undergo additional trials or procedures, or could make it impractical or impossible for us to market our products for certain uses, in certain markets, or at all.
Changes in government regulations may adversely affect our financial condition and results of operations because we may have to incur additional expenses if we are required to change or implement new testing, manufacturing and control procedures. If we are required to devote resources to develop such new procedures, we may not have sufficient resources to devote to research and development, marketing, or other activities that are critical to our business.
We can manufacture and sell our products only if we comply with regulations and quality standards established by government agencies such as the FDA and the U.S. Department of Agriculture (“USDA”) as well as by non-governmental organizations such as the International Organization for Standardization (“ISO”) and WHO. We have implemented a quality control system that is intended to comply with applicable regulations. Although FDA approval is not required for the export of our products, there are export regulations promulgated by the FDA that specifically relate to the export of our products that require compliance with FDA quality system regulation and that also require meeting certain documentary requirements regarding the approval of the product in export markets. Although we believe that we meet the regulatory standards required for the export of our products, these regulations could change in a manner that could adversely impact our ability to export our products.
Our products may not be able to compete with new diagnostic products or existing products developed by well-established competitors, which would negatively affect our business.
The diagnostic industry is focused on the testing of biological specimens in a laboratory or at the point-of-care and is highly competitive and rapidly changing. Some of our principal competitors may have considerably greater financial, technical and marketing resources than we do. Several companies produce diagnostic tests that compete directly with our testing product line, including but not limited to, Chembio Diagnostics and Abbot Laboratories. Furthermore these and/or other companies have or may have products incorporating molecular and/or other advanced technologies that over time could directly compete with our testing product line. As new products incorporating new technologies enter the market, our products may become obsolete or a competitor's products may be more effective or more effectively marketed and sold.
There are competing products that could significantly reduce our U.S. sales of rapid HIV tests.
In 2006 Alere, Inc. acquired a division from Abbott Diagnostic located in Japan that manufactured and marketed a rapid HIV test product line called Determine®. The Determine® format was developed for the developing world and remote settings and, central to the needs of that market. The format is essentially a test strip that is integrated into a thin foil wrapper. When opened, the underside of the wrapper serves as the test surface for applying the blood sample and performing the test. This design reduces costs and shipping weights and volumes and provides an advantage for the developing world markets it serves. Some of the disadvantages of the platform are the amount of blood sample that is needed (50 microliters versus 2.5, 5 and 10 for our lateral flow barrel, lateral flow cassette, and DPP® products respectively), the open nature of the test surface, and the absence of a true control that differentiates biological from other kinds of samples.
| 17 |
The so-called "3rd generation" version of this product has been marketed for many years and is the leading rapid HIV test that is used in a large majority of the national algorithms of countries funded by PEPFAR and the Global Fund, as well as many other countries in the world. That product is not FDA-approved though it is CE marked. The newest Determine® HIV version, which was developed and manufactured by Alere's subsidiary in Israel, Orgenics, is the so-called "4th Generation" version Determine® test. According to its claims, this product detects HIV antibodies and P24 HIV antigens. Because the P24 antigen is known to occur in HIV-positive individuals' blood samples before antibodies do, the 4th generation Determine® test is designed to detect HIV infection earlier than tests that solely rely on antibody detection. HDS’ tests, as well as all of the other currently FDA-approved rapid HIV tests, only detect antibodies.
The initial "4th generation" Alere Determine® rapid test product that was also CE marked and that Alere launched internationally some years ago has not been successfully commercialized to the best of our knowledge and at least certain published studies were not favorable for this product. However the 4th generation product that is now FDA-approved was apparently modified as compared to the initial international version, and it may perform more satisfactorily. Alere received FDA approval of this modified product in August 2013 and CLIA waiver for it in December 2014. Alere is also aggressively pursuing development of the market for this product. Moreover there is support by a number of key opinion leaders for the public health value of such 4th generation tests, and this product represents a significant competitive threat to Chembio as well as to each of the other rapid HIV test manufacturers (OraSure and Trinity primarily).
During 2011, Biolytical, Inc. of Vancouver, Canada received FDA approval and in 2012 received CLIA waiver of a flow-through rapid HIV test called "INSTI". The flow-through technology used in the INSTI test is older than lateral flow, and requires handling of multiple components (3 vials of solution) to perform the test in multiple steps. However, these steps can be accomplished in less than ten minutes, and the actual test results occur in only one minute after those steps are completed. Therefore sample-to-result time is shorter than any of the competitive products. The product also has good performance claims. There are settings where that reduced total test time, despite the multiple steps required, may be a distinct advantage, and we believe Biolytical has made some progress in penetrating certain public health markets.
Therefore, even though our lateral flow products currently enjoy a substantial market share in the U.S. rapid HIV test market, and we have an additional rapid HIV test, the DPP® HIV 1/2 Assay, there a number of risks and uncertainties concerning current and anticipated developments in this market. Although we have no specific knowledge of any other new product that is a significant competitive threat to our products, or that will render our products obsolete, if we fail to maintain and enhance our competitive position or fail to introduce new products and product features, our customers may decide to use products developed by our competitors, which could result in a loss of revenues and cash flow.
More generally, the point-of-care diagnostics industry is undergoing rapid technological changes, with frequent introductions of new technology-driven products and services. As new technologies become introduced into the point-of-care diagnostic testing market, we may be required to commit considerable additional efforts, time and resources to enhance our current product portfolio or develop new products. We may not have the available time and resources to accomplish this, and many of our competitors have substantially greater financial and other resources to invest in technological improvements. We may not be able to effectively implement new technology-driven products and services or be successful in marketing these products and services to our customers, which would materially harm our operating results.
Our use of third-party suppliers, some of which may constitute our sole supply source, for certain important product components presents a risk that could have negative consequences for other business.
A number of our components and critical raw materials are provided by third-party suppliers, some of which may be sole-source suppliers, which impacts our ability to manufacture or sell product if our suppliers cannot or will not deliver those materials in a timely fashion, or at all, due to an interruption in their supply, quality or technical issues, or any other reason. If this occurs, we could incur substantial expense and time to be able to reestablish the appropriate quality, cost, regulatory and market-acceptance circumstances needed for commercial success. Even with the needed expense and time, we may not be able to reestablish any or all of these factors. The absence of any one or more of these factors could prevent us from being able to commercially produce and market the affected product or products.
| 18 |
New developments in health treatments or new non-diagnostic products may reduce or eliminate the demand for our products.
The development and commercialization of products outside of the diagnostics industry could adversely affect sales of our products. For example, the development of a safe and effective vaccine to HIV or treatments for other diseases or conditions that our products are designed to detect, could reduce or eventually eliminate the demand for our HIV or other diagnostic products and result in a loss of revenues.
We may not have sufficient resources to effectively introduce and market our products, which could materially harm our operating results.
Introducing and achieving market acceptance for our products will require substantial marketing efforts and will require us and/or our contract partners, sales agents, and/or distributors to make significant expenditures of time and money. In some instances we will be significantly or totally reliant on the marketing efforts and expenditures of our contract partners, sales agents, and/or distributors. If they do not have or commit the expertise and resources to effectively market the products that we manufacture, our operating results will be materially harmed.
The success of our business depends on, in addition to the market success of our products, our ability to raise additional capital through the sale of debt or equity or through borrowing, and we may not be able to raise capital or borrow funds on attractive terms and/or in amounts necessary to continue our business, or at all.
Our liquidity and cash requirements will depend on several factors. These factors include, among others, (1) the level of revenues; (2) the extent to which, if any, that revenue level improves operating cash flows; (3) our investments in research and development, facilities, marketing, regulatory approvals, and other investments we may determine to make; and (4) our investment in capital equipment and the extent to which it improves cash flow through operating efficiencies. We do not expect to generate positive cash flow in next twelve months, and we cannot be sure that we will be successful in raising sufficient capital to fund our needs. If we are not able to raise additional capital from another source, we will be required to substantially reduce our operating costs, including the possibilities of suspending our unfunded research and development activities, and quickly curtailing any cash flow negative product initiatives.
Our near term sales are difficult to predict in the uncertain status of pending orders and certain regulatory approvals, and the uncertain time until we have approval to sell in the US. We believe that underlying demand for HIV rapid testing in the United States remains strong, and that the restoration of some of the funding cutbacks from sequestration and the implementation of the Affordable Care Act and of the United States Preventive Services Task Force recommendations will have a positive impact on the development of the market.
However, development of new customers with this product is costly and time-consuming.
Currently, we are dependent on international sales of our products, since we have no products approved by the FDA for US sales. The nature of international business is such that it can be volatile from period to period, depending on ordering patterns of donor-funded programs.
A number of factors can slow or prevent international sales increases or cause sales decreases, or substantially increase the cost of achieving sales assuming they are achieved. These factors include:
| • | economic conditions and the absence of or reduction in available funding sources; |
| • | regulatory requirements and customs regulations; |
| • | cultural and political differences; |
| • | foreign exchange rates, currency fluctuations and tariffs; | |
| • | dependence on and difficulties in managing international distributors or representatives; |
| • | the creditworthiness of foreign entities; |
| • | difficulties in foreign accounts receivable collection; |
| 19 |
| • | competition; |
| • | pricing; and |
| • | any inability we may have in maintaining or increasing revenues. |
If we are unable to increase our revenues from domestic and/or international customers, our operating results will be materially harmed.
Although we have an ethics and anti-corruption policy in place, and have no knowledge or reason to know of any practices by our employees, agents or distributors that could be construed as in violation of such policies, our business includes sales of products to countries where there is or may be widespread corruption.
HDS has a policy in place prohibiting its employees, distributors and agents from engaging in corrupt business practices, including activities prohibited by the United States Foreign Corrupt Practices Act (the “FCPA”). Nevertheless, because we work through independent sales agents and distributors outside the United States, we do not have control over the day-to-day activities of such independent agents and distributors. In addition, in the donor-funded markets in Africa where we sell our products, there is significant oversight from PEPFAR, the Global Fund, and advisory committees comprised of technical experts concerning the development and establishment of national testing protocols. This is a process that includes an overall assessment of a product which includes extensive product performance evaluations including five active collaborations and manufacturer’s quality systems, as well as price and delivery. In Brazil, where we have had a total of six product collaborations with FIOCRUZ, the programs through which our products may be deployed are all funded by the Brazilian Ministry of Health. Although FIOCRUZ is affiliated with the Brazilian Ministry of Health, and is its sole customer. We have no knowledge or reason to know of any activities by our employees, distributors or sales agents of any actions which could be in violation of the FCPA, although there can be no assurance of this.
To the extent that we are unable to collect our outstanding accounts receivable, our operating results could be materially harmed.
There may be circumstances and timing that require us to accept payment terms, including delayed payment terms, from distributors or customers, which, if not satisfied, could cause financial losses.
We generally accept payment terms which require us to ship product before the contract price has been paid fully, and there also are circumstances pursuant to which we may accept further delayed payment terms pursuant to which we may continue to deliver product. To the extent that these circumstances result in significant accounts receivables and those accounts receivables are not paid on a timely basis, or are not paid at all, especially if concentrated in one or two customers, we could suffer financial losses.
Item 3 Properties.
Hema’s corporate offices, product development facilities, regulatory affairs offices, and laboratory and assembly facilities are contained in a 5,627 square foot facility in Mirama, Florida. The facility is leased through June 30, 2017, with a current monthly rent of $4,134. Our facility is an FDA Registered Facility. Based on order size, delivery requirements and current orders in process, our Miramar facility can handle up to 4 million RTD devices, all of which are currently hand assembled. We have relationships with subcontractors to handle additional production requirements.
| 20 |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS OF HEMA.
The following discussion and analysis by management provides information with respect to Hema’s financial condition and results of operations for the fiscal years ended December 31, 2015 and 2014, as well as the nine month periods ended September 30, 2016 and 2015. This discussion should be read in conjunction with the information in the consolidated financial statements and the notes pertaining thereto contained in Item 9.01 - Financial Statements of Business Acquired of this Current Report on Form 8-K and the information discussed in , Item 1A - Risk Factors. This discussion includes forward-looking statements that involve risk and uncertainties. As a result of many factors, such as those set forth above under “Risk Factors,” actual results may differ materially from those anticipated in these forward-looking statements.
The Hema financial statements included in this Report are were prepared on a combined basis with Rapid Medical Diagnostics Corp. and Hema Diagnostic Systems, S.A.. During the periods represented by the financial statements, these two companies were separately owned by Hema’s equity owners. Immediately prior to the Acquisition, these two companies became wholly owned subsidiaries of Hema, and our financial statements for periods after the Acquisition will be presented on a consolidated basis. Since all transactions and accounts between and among the three entities have been eliminated in the combined financial statements, we do not believe the combined financial statements differ materially from financial statements presented on a consolidated basis.
Overview of Business
Hema Diagnostic Systems was established in December, 2000 to market and distribute rapid test devices for infectious diseases. Since 2002, we have been developing an expanding line of rapid diagnostic tests (RDTs) including such diseases as Human Immunodeficiency Virus (HIV) – 1/2, tuberculosis, malaria, hepatitis, syphilis, typhoid, dengue as well as other infectious diseases. We distribute our own products and manufacture our devices in-house as well as through contract manufacturers. Some sub-components, made to our specifications, are produced in China, India and Germany. Our products are rapid immunochromatographic medical diagnostics that are administered at the point of care (POC) level and which can produce results as in as little as 10-15 minutes.
Due to the potential infectious character of the whole blood test sample, our Express series of RDTs are designed to perform and deliver test results while within the sealed Express housing, carefully controlling the potentially infectious test sample. This design helps to increase our ability to control the possibility of cross-contamination. Most of our competitors’ products, while inexpensive, are not as user-friendly, require substantially more training and have greater risk of cross- contamination.
We have two subsidiaries which are integral parts of our primary business. Rapid Medical Diagnostics Corp. has historically been the reserve for our research and development and holds the patent(s) on our technology. Hema Diagnostic Systems, S.A. was established to distribute our products in Central and South America.
Our products are subject to extensive regulatory oversight by government and other organizations and rely on international regulatory approvals for sale into markets outside of the USA. Domestically, our devices would require US FDA approval and in some cases, international sales require World Health Organization (WHO) approval.
We maintain a Federal Drug Administration (FDA) registered facility in Miramar, Florida and are certified under both ISO9001 and ISO13485 for the “Design, Development, Production and Distribution” of the in-vitro devices. Approval of our HIV rapid test has been issued by the United States Agency for International Development (USAID). USAID approval allows us to offer our product to those countries where USAID provides such funding. Some of our products have qualified for and use the European Union issued “CE” Mark, which allows us to enter into CE Member countries subject to individual country documentation and approval. Currently, two malaria rapid tests are approved under World Health Organization (WHO) guidelines with a third awaiting approval in December 2016 pending test results. WHO approval is necessary for those countries who rely upon the expertise of the WHO, as well as for NGO funding. HDS products have also received registrations and approvals issued by other foreign governments. HDS is currently in the planning phase for entering into the newly announced, WHO “Pre-Qualified Approval” process for other HDS tests. This process allows expedited approval of rapid tests, reducing the current 24-30 month process time down to approximately 6-9 months. HDS products are also listed and offered internationally through the UNICEF and UNDP. On February 2016, we entered into a Long Term Agreement with the WHO for the approved rapid tests. While receiving small orders resulting from this Agreement, we anticipate larger orders from the WHO as our relationship expands.
| 21 |
We maintain current U.S. Certificates of Exportability that are issued by two FDA divisions-CBER and CDRH. CBER (Center for Biologicals Evaluation and Research) is the FDA regulatory division that oversees biological devices and which include our HIV, Hepatitis B and Hepatitis C. The other division, Center for Devices and Radiological Health (CDRH), is responsible for the oversight of other HDS devices which include Tuberculosis, Syphilis, and the remaining product line. Certificates of Exportability are issued to Hema Diagnostic Systems. Our HDS facility maintains FDA Establishment Registration status and is in accord with GMP (Good Manufacturing Practice) as confirmed by the FDA.
We do not currently have FDA approval to sell any of our products in the United States. We intend on submitting our devices to the FDA under a Pre-Market Approval Application (PMA) or through the 510K process. The 510K would require the appropriate regulatory administrative submissions as well as a limited scientific review by the FDA to determine completeness (acceptance and filing reviews); in-depth scientific, regulatory, and Quality System review by appropriate FDA personnel (substantive review); review and recommendation by the appropriate advisory committee (panel review); and final deliberations, documentation, and notification of the FDA decision. The PMA process is more extensive, requiring clinical trials to support the application. We expect to apply to FDA for approval of our first RDT to be submitted to the FDA for 510K approval within the next 3 months. We anticipate the FDA process will be completed within 9 months after submission. During this timeline we will be preparing documentation for additional rapid tests to undergo either the FDA PMA or 510k process.
Our target markets include domestic sales within the USA as well as internationally. The US FDA approval will serve to expedite international approvals as well as allow us to enter into the domestic market.
We have a limited history of operations, and our revenues have been insignificant compared to our expenses. We have not been profitable and our owners deficit was $13,788,014 at September 30, 2016. As of September 30, 2016, our current cash position is not sufficient to meet our working capital needs for the next twelve months. To continue operations, we will require additional funds to support our working capital requirements and any development activities, or will need to suspend operations. Our past activities have been primarily financed by loans and capital contributions from our former primary. We entered into the Acquisition with the expectation that the existence of a public market for Generex’s stock would enable us to access financing from various sources. We cannot provide any assurance that we will obtain the required funding. Our inability to obtain required funding in the near future or our inability to obtain funding on favorable terms will have a material adverse effect on our operations and our strategic development plan for future growth. If we cannot successfully raise additional capital and implement our strategic development plan, our liquidity, financial condition and business prospects will be materially and adversely affected and we may have to cease operations.
Hema operates in only one segment: in-vitro medical diagnostic devices (RDTs) administered at the point of care level.
Accounting for Research and Development Projects
In the fiscal years ended December 31. 2015 and 2014, research and development expenses were more than 50% of our total expense. In the nine months ended September 30, 2015, research and development continued to be our largest expense category. Most of research and development activities to date have involved developing the platform technologies for our in-vitro point of care level rapid response diagnostics. As a result, we have not made significant distinctions in the accounting for research and development expenses among products, as a significant portion of all research has involved improvements to the platform technologies which benefits all of our products.
Because of various uncertainties, including the requirements for governmental regulatory approvals, WHO approvals and the issuance of purchase orders by governmental and non-governmental agencies who are the primary purchasers of our products, we cannot predict when any products may begin to produce net cash inflows.
| 22 |
Critical Accounting Policies
Our discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements which have been prepared in conformity with accounting principles generally accepted in the United States of America. It requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
We consider certain accounting policies related to impairment of long-lived assets, intangible assets and accrued liabilities to be critical to our business operations and the understanding of our results of operations:
Going Concern. As shown in the accompanying financial statements, we have not been profitable and have reported recurring losses from operations. These factors raise substantial doubt about our ability to continue to operate in the normal course of business. The accompanying consolidated financial statements do not include any adjustments that might be necessary should we be unable to continue as a going concern. Estimates which are particularly significant to our financial statements include estimates relating to the determination of impairment of assets, the useful life of property and equipment and the recoverability of advances.
Inventory. Our inventory is stated at the lower of cost or net realizable value. Cost is determined using the Weighted Average method. We periodically evaluates our inventory for any obsolete or slow moving items based on production lots and advances in production design or technology. Any inventory determined to be obsolete or slow moving is removed from inventory and disposed or a provision is made to reduce slow moving inventory to its net realizable value. At December 31, 2015 and 2014, we recorded a reserve for obsolescence of $7,750 and $0, respectively.
Revenue Recognition. We have yet to fully commercialize its products and secure appropriate permits, approvals and licenses necessary to begin full worldwide distribution. We have had minimal sales to date. The revenue we have received primarily are related to trial samples and evaluation of the efficacy and suitability of the products to specific target markets. Sales and the related cost of sales are recognized primarily upon shipment of products (normally when title passes). Our revenue recognition policies are in compliance with ASC Topic 605, which establishes criteria that must be satisfied before revenue is realized or realizable and earned.
We recognize revenue when all of the following four criteria are met:
| • | persuasive evidence of a sales arrangement exists, | |
| • | delivery has occurred, | |
| • | the sales price is fixed or determinable and | |
| • | collectability is probable. |
Impairment of Long-Lived Assets. We evaluate long-lived assets for impairment, including property, plant and equipment and intangible assets, when events or changes in circumstances indicate that the carrying value of such assets may not be recoverable or the assets are being held for sale. Upon the occurrence of a triggering event, the asset is reviewed to assess whether the estimated undiscounted cash flows expected from the use of the asset plus the residual value from the ultimate disposal exceeds the carrying value of the asset. If the carrying value exceeds the estimated recoverable amounts, the asset is written down to the estimated fair value. Any resulting impairment loss is reflected on the Combined Statements of Operations..
Intangible Assets. Our intangible assets consist of patent patented technology and trademarks. The determination of the related estimated useful lives and whether or not these assets are impaired involves significant judgments. Amortization is computed by applying the straight line method based on the remaining patent life. Our primary patent expires in 2026.
Income Taxes. Hema Diagnostic Systems, LLC is a limited liability corporation. Prior to the Acquisition, Rapid Medical Diagnostic Corp. was a Subchapter S corporation. Federal and state income tax regulations do not require a limited liability corporation or a Subchapter S corporation to pay income taxes. Rather each member’s allocable share of the profit or loss is reported in each member’s individual income tax return. Hema Diagnostics Systems Panama, S.A. is a Panamanian company. Due to its operational losses, no taxes are required. Accordingly, no provision or liability for income taxes is reflected for this reporting entity in the financial statements filed with tis Current Report. Following the Acquisition, all of these entities will be wholly owned subsidiary’s consolidated in Generex’s US federal income tax returns.
| 23 |
Results of Operations
Year ended December 31, 2015 Compared to Year ended December 31, 2014
We had a net loss for the fiscal year ended December 31, 2015 $1,041,889 versus a net loss of $999,529 in the prior fiscal year. The net loss in both years resulted from operating losses in of $985,939 in 2015 and $982,150 in 2014. Our major categories of expenses were materially consistent over both years Our revenues decreased from $18,023 in 2014 to $5,703 in 2015, primarily due to a decrease in available funds to purchase our products.
Our accrued interest expense, net of interest income in fiscal 2015 was $24,778 compared to the previous year’s fiscal period at $23,616. Interest expense arose primarily from loans payable to Stephen Berkman, Hema’s founder and former primary shareholder
Nine Months ended September 30, 2016 Compared to Nine Months ended September 30, 2015
Our net loss for the nine months ended September 30, 2016 was $959,273, an increase from our net loss of $724,175 for the comparable nine month period in 2015. The increase in net loss is attributable to an increase in operating expenses, which were $889,348 in the 2016 period and $724,173 in the 2015 period. An increase in interest expense net of interest income, which was $79,572 in the nine months ended September 30, 2016, an increase from $24,874 in the comparable 2015 period, also contributed to the increased net loss. The increase in operating expenses is primarily attributable to an increase in professional fees, which were $147,787 in the 2016 period versus $5,428 in the 2015 period. The increase in professional fees is primarily attributable to audit fees, accounting and increase in as well as an increase in fund raising and business development fees. The variation in interest expense is attributable to an increase in the interest rate from .21% to .75%.
Our revenues increased from $4,828 in the none months ended September 30, 2015 to $16,953 in the comparable 2016 period primarily due to expanded purchases from Supply Chain Management organizations. As additional products are approved by such agencies as the FDA and the WHO, we expect to see increased revenue. These approvals will allow easier access to international acceptance and registrations on a country by country basis allowing an increase in revenue.
Financial Condition, Liquidity and Resources
Sources of Liquidity
To date we have financed our development stage activities primarily from capital contributions and loans from Hema’s previous primary owner.
In the 9 months ended September 30, 2016, the former primary owner contributed $884,500 to the capital of the Company. Additional
cash was generated in this period by the repayment in full of an $897,000 loan to Lawrence Salvo, Hema’s President and CEO
and now a member of our board of directors. During the year ended December 31, 2015, Hema’s former principal owner made
capital contributions of $1,111,000.
While Hema did not receive any loans from it former principal owner in 2015 or 2016, as of the date of this Current Report, Hema owed this individual $13,260,472 for previous loans. The loans bear interest at .75% per annum and by their terms are payable on demand. In connection with the acquisition, the former principal owner has agreed to forgive these loans if the value of our stock owned by him achieves a specific level and the stock is registered for resale. See Item 1 – Acquisition, above.
| 24 |
As of September 31, 2016 and the date of this Current report, our current cash position is not sufficient to meet our working capital needs for the next twelve months. Therefore, we will require additional funds to support our working capital requirements and any development or other activities, or will need to curtail our research and development and other planned activities or suspend operations.
We will no longer be able to rely on Hema’s former primary owner for necessary financing. Going forward, we expect to finance our activities date primarily through private placements of Generex common stock, or preferred stock or debt securities convertible into common stock. These transactions will likely require us to register the privately sold securities for resale. We have no commitments for any such financing, however, and we do not know if we will be able to obtain the required financing.
Unforeseen problems with the conduct or results of Phase III clinical trials for Oral-lyn™ or further negative developments in general economic conditions could interfere with our ability to raise additional capital as needed, or materially adversely affect the terms upon which such capital is available. We cannot provide any assurance that we will obtain the required funding. Our inability to obtain required funding in the near future or our inability to obtain funding on favorable terms will have a material adverse effect on our operations and our strategic development plan for future growth. If we cannot successfully raise additional capital and implement our strategic development plan, our liquidity, financial condition and business prospects will be materially and adversely affected and we may have to cease operations.
Funding Requirements and Commitments
If we obtain necessary financing, we expect to devote substantial resources to obtaining WHO approval for additional products, obtaining FDA approval to sell our products in the United States, further developing our existing products and developing applications of our products for new diseases and conditions, as well as producing the necessary molds for the production of our delivery platforms and in the purchase of automated assembly equipment necessary to establish and maintain a competitive price in the world markets.
Our future funding requirements and commitments and our ability to raise additional capital will depend on factors that include:
| • | the timing and amount of expense incurred to complete research and development; |
| • | the costs and timing of the regulatory process as we seek approval of our existing and new products in development; |
| • | our ability to generate new relationships with industry partners throughout the world that will provide us with long-term commercialization opportunities; |
| • | the timing, receipt and amount of sales, if any, from our products which are currently approved by WHO; |
| • | the cost of manufacturing of our products as well as component costs from our qualified sub-contractors, and the cost of marketing and sales activities of those products; |
| • | the costs of prosecuting, maintaining, and enforcing patent claims, if any claims are made; |
| • | our ability to obtain the necessary financing to fund our operations and effect our strategic development plan; and |
| • | the receptivity of the financial market to medical device and diagnostics companies. |
| 25 |
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenue or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors, and we do not have any non-consolidated special purpose entities.
Tabular Disclosure of Contractual Obligations
Hema is, and both before and after the Acquisition, Generex is, a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and is not required to provide the information required under this item.
Recently Adopted Accounting Pronouncements
In February 2015, the FASB issued ASU 2015-02, Consolidation (Topic 810)—Amendments to the Consolidation Analysis (“ASU 2015-02”), which provides guidance on evaluating whether a reporting entity should consolidate certain legal entities. Specifically, the amendments modify the evaluation of whether limited partnerships and similar legal entities are variable interest entities (“VIEs”) or voting interest entities. Further, the amendments eliminate the presumption that a general partner should consolidate a limited partnership, as well as affect the consolidation analysis of reporting entities that are involved with VIEs, particularly those that have fee arrangements and related party relationships. ASU 2015-02 is effective for interim and annual reporting periods beginning after December 15, 2016, with early adoption permitted. A reporting entity may apply the amendments using a modified retrospective approach or a full retrospective application. We are currently evaluating the impact, if any, that adopting ASU 2015-02 will have on Hema’s financial statements.
In February 2016, the FASB issued ASU 2016-02, Leases, which will amend current lease accounting to require lessees to recognize (i) a lease liability, which is a lessee’s obligation to make lease payments arising from a lease, measured on a discounted basis, and (ii) a right-of-use asset, which is an asset that represents the lessee’s right to use, or control the use of, a specified asset for the lease term. ASU 2016-02 does not significantly change lease accounting requirements applicable to lessors; however, certain changes were made to align, where necessary, lessor accounting with the lessee accounting model. This standard will be effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. We are currently reviewing the provisions of this ASU to determine if there will be any impact on our results of operations, cash flows or financial condition.
In March 2016, the FASB issued ASU 2016-09, Compensation – Stock Compensation: Improvements to Employee Share-Based Payment Accounting, which relates to the accounting for employee share-based payments. This standard addresses several aspects of the accounting for share-based payment award transactions, including: (a) income tax consequences; (b) classification of awards as either equity or liabilities; and (c) classification on the statement of cash flows. This standard will be effective for fiscal years beginning after December 15, 2016, including interim periods within those fiscal years. We are currently reviewing the provisions of this ASU to determine if there will be any impact on our results of operations, cash flows or financial condition.
| 26 |
Meetings of Our Board of Directors
CERTAIN TRANSACTIONS
Changes in Control
We know of no arrangements, including any pledge by any person of our securities, the operation of which may at a subsequent date result in the change in control of Generex.
Review of Related Party Transactions
We presently have a policy requiring approval by stockholders or by a majority of disinterested directors of transactions in which one of our directors has a material interest apart from such director's interest in Generex. We also have a policy requiring the approval by the Audit Committee for any transactions in which a director or an executive officer has a material interest apart from such director's or officer’s interest in Generex.
Related Transactions
Prior to January 1, 2016, Hema advanced an aggregate of $893,372 to the Lawrence Salvo, Hema’s President and CEO, and since the closing of the Acquisition an officer and director Generex. These advances bore interest at .21% during 2015 and until paid in September, 2016. During September, 2016, the advances were repaid in full.
Hema is currently indebted to Stephen Berkman, the former principal equity owner of HDS, in the amount of $13,260,462 for loans, advances and other consideration. This debt was secured by a security interest in Hema’s assets. At Closing. Berkman terminated his security interest on the Company’s assets. At such time as certain conditions relating to the value of Generex securities owned by Mr. Berkman are satisfied, the loan payable from to Mr. Berkman will be deemed satisfied in full. See “Acquisition and Related Transactions,” above.
The debt to Mr. Berkman accrued prior to 2015. HDS did not receive any loans from Mr. Berkman in 2015 or 2016. The loans bear interest at .75% per annum and by their terms are payable on demand.
Hema historically made advances to Luis Agudelo, Hema’s Director of Latin American Sales and board member. As of December 31, 2015, net advances were $20,635. As of September 30, 206, net advances werer$16,013. During October 2016, an additional $14,735 was repaid. Mr. Agudelo is not an officer or director of Generex.
HDS has historically engaged in commercial and financial transactions with companies owned by one or more of its equity owners. From inception through 2007, China World Wide, a company owned by Mr. Salvo and Mr. Berkman provided supplemental funding to HDS on a non-interest basis. At September 30, 2016, HDS had an unpaid balance to China World Wide of $83,554. China World Wide has also assisted HDS in the development of its distribution and sourcing in China.
International Diagnostics and Medical Supply Corp. (IDMS) was established to create hemodialysis facilities on a global scale. IDMS is owned by Mr. Salvo, Mr. Agudelo and Mr. Berkman. Due to the high initial cost of establishing a hemodialysis facility, IDMS has not yet secured satisfactory funding to execute its business plan. Consequently, it has only engaged in minimal organizational activities. At September 30, 2016 HDS had an unpaid balance due from IDMS of $2,500 advanced to IDMS for legal fees incurred.
| 27 |
Item 3.02. Unregistered Sales of Equity Securities.
The disclosures set forth in Item 2.01 above are hereby incorporated by reference into this Item 3.02. The securities issued in connection with the Acquisition Agreement are restricted securities issued in reliance on the exemption provided in Section 4(a)(2) of the Securities Act of 1933.
Item 5.01. Changes in Control of Registrant.
The disclosures set forth in Item 2.01 above are hereby incorporated by reference into this Item 5.01.
Item 5.02. Departure of Directors or Principal Officers; Election of Directors; Appointment of Principal Officers; Compensatory Arrangements of Certain Officers.
At the Closing of the Acquisition, our board of directors was reconstituted by
| • | the appointment of Joseph Mosacato, serving as Chairman of the Board, Andrew Greene, Esq., Andrew Ro, Jason Terrell, MD, Gary H. Lyman, MD, Craig Eagle, MD and Jacob Dagan, Ph.D, and |
| • | the resignation of Mark Fletcher as a director . |
At the Effective Time, our executive management team was also reconstituted by
| • | the resignations of Mark Fletcher as President and Chief Executive Officer (Mr. Fletcher remains Executive Vice President, Secretary and General Counsel) and David Brusegard, as Secretary and Chief Operating Officer, and |
| • | The appointment of the officers listed in the table below. |
DIRECTORS AND EXECUTIVE OFFICERS
The following table sets forth information regarding the members of our board of directors and our executive officers and other significant employees. All of our officers and directors were appointed on the effective date of the Merger. All of our directors hold office until the next annual meeting of stockholders and their successors are duly elected and qualify. Executive officers serve at the request of the board of directors.
| Name | Age | Office(s) held |
| Joseph Moscato | 53 | Chief Executive Officer, President, Chairman of the Board |
| Andrew Greene, Esq. | 53 | Chief Operating Officer, Director |
| Andrew Ro | 46 | Chief Investment Officer, Senior VP of Investments, Director |
| Mark Corrao | 59 | Chief Financial Officer, Treasurer |
| Jason Terrell, MD | 36 | Chief Scientific Officer, Chief Medical Officer |
| Mark Fletcher, Esq. | 51 | Executive Vice President & General Counsel, Secretary |
| Richard Purcell | 56 | Senior VP of Research & Drug Development |
| Lawrence Salvo, | 64 | Senior VP of Diagnostic, CEO and President of HDS, Director |
| Gary H. Lyman, MD | 60 | Director |
| Craig Eagle, MD | 54 | Director |
| Jacob Dagan, Ph.D | 72 | Executive Vice President Business Development |
| James M. Anderson, Jr. | 68 | Director |
| Brian T. McGee | 54 | Director |
| 28 |
Set forth below is a brief description of the background and business experience of each of our current executive officers and directors.
Joseph Moscato. Mr. Moscato serves as the Chief Executive Officer, President and Chairman of the Board.
Mr. Moscato has over 30 years of experience in healthcare, sales and marketing, distribution management and finance. Mr. Moscato brings his marketing and advertising acumen to drug discovery, diagnostic and treatment development and commercialization. Since 2009, Mr. Moscato has been working as an exclusive consultant to Generex Biotechnology Corp. Mr. Moscato has originated and negotiated several licensing deals with the top biopharmaceutical companies; has advised in the equity financing totaling over $300 million; and has implemented the broad strategic vision for the company. Mr. Moscato has worked and consulted for Pfizer in several capacities from sales and marketing to new drug discovery & development for licensing. He has worked with other biopharmaceutical companies such as GlaxoSmithKline, Johnson & Johnson, Parke-Davis, Amgen and others. Mr. Moscato has consulted for several healthcare focused private equity, hedge funds and family offices.
Mr. Moscato also owned several advertising and marketing agencies focused on media, entertainment and healthcare with clients ranging from Motorola, Chadmoore Wireless, Nextel, Cannon, Sharp, GlaxoSmithKline, Pfizer, and other biopharmaceutical companies. Mr. Moscato’s agency was acquired by William Douglas McAdams, one of the largest independent healthcare advertising and marketing agencies.
Andrew Greene, Esq. Mr. Greene serves as the Chief Operating Officer, Director.
Mr. Greene has over 20 years of experience in all aspects of corporate management, board of director development, operations, marketing, financing and legal. He has owned and operated his own management and corporate consulting and legal advisory firm focused on early stage companies regarding their business plan development, roll out of operations and management execution as well as implementing cost savings initiatives. Mr. Greene has conducted due diligence for all phases of corporate execution; has developed expertise in operational plans and strategies including growth, marketing, technology, and expansion. Mr. Greene graduated with a law degree from California Western School of Law and English Legal Jurisprudence from Cambridge University as a part of the Cambridge England International Exchange Program. Mr. Greene is an active member of the New York State Bar Association.
Andrew Ro. Mr. Ro serves as the Chief Investment Officer, Senior VP of Investments, Director.
Mr. Ro has over 20 years of experience in the financial markets ranging from trading global futures and equity markets, senior secured debt, convertible securities, private investments in public equities (PIPEs) and investing. Mr. Ro was a Consultant and Registered Representative with boutique investment and merchant banks where he consulted and advised US and international companies on capital markets, operational, and regulatory issues as well as involved in capital raising, mergers & acquisitions, and strategic implementation. Mr. Ro was a Partner with an active Investment Fund where he was involved in originating, structuring, negotiating and closing financing transactions providing growth capital, acquisition financing, recapitalization, restructuring and general working capital to late-stage venture, distressed and middle market companies across all industries and sectors. Mr. Ro originated and structured over $2 billion in total commitments and managed a portfolio of over $650 million in investments. Mr. Ro graduated from George Mason University with a Bachelor in Science in Economics. He also held a Series 3, Series 7, and Series 63 licenses.
| 29 |
Mark Corrao. Mr. Corrao serves as the Chief Financial Officer, Treasurer.
Mr. Corrao has experience in financial management with a proven track record of raising capital and extraordinary bottom line management. He has been involved in the initial registration of numerous public companies and subsequent SEC quarterly and annual reporting and has developed, authored and presented numerous business plans and models inclusive of budgets, forecasts, cash flow, cash management and investment strategies. From 2012 to present he has been a Managing Director and CFO of The Mariner Group LLC, which has merged with the CFO Squad, creating a much larger and diverse multi-talented organization. The CFO Squad is a financial and business advisory firm providing outsourced and part-time CFO services for emerging to midsized companies (both private and public) in a wide range of businesses and industries. He has been the Chief Financial Officer and a director for a pharmaceutical company specializing in the research and development of novel and new therapeutic agents designed to reduce oxidative stress and act as immune modulators and Neuroprotectants. From 2010-12, he served as Chief Financial Officer of New York Business Efficiency Experts, Inc. which provides professional services in the financial areas of accounting, taxation, auditing, venture capital and SEC registrations (reporting). He served as a Director and Chief Financial Officer for a manufacturer of proprietary software for the prevention of identity theft and the protection of computer systems from unauthorized access. Additionally, Mr. Corrao is currently the CFO for a software company specializing in internet applications; a medical device company; and is member of the Board of Directors and Chairman of the Audit Committee of an international self-improvement and consumer company.
Jason B. Terrell, MD. Dr. Terrell is the Chief Scientific Officer, Chief Medical Officer
Dr. Terrell has extensive expertise in the pharmaceutical and medical diagnostic device industry in the areas of business development, clinical trial organization, regulatory affairs and commercialization strategies. Dr. Terrell has served as chief medical and scientific officers for several public and private companies like VolitionRx, a NYSE traded international medical diagnostic company. Dr. Terrell previously served as a Corporate Medical Director for Any Lab Test Now, the nation’s largest direct to consumer medical testing franchise, where he supervised clinical operations for over 70 locations throughout the United States. Dr. Terrell is a summa cum laude graduate from Hardin-Simmons University with a degree in Biochemistry. He graduated as recipient of the Holland Medal of Honor for the top graduate in the School of Science and Mathematics. Dr. Terrell was honored with the Hardin-Simmons University Outstanding Young Alumni Award and currently serves on the University’s Board of Development. Dr. Terrell attended The University of Texas School of Medicine in Houston and received General Medicine Internship and Pathology Residency training at the Texas Tech University Health Sciences Center.
Richard Purcell. Mr. Purcell serves as the Executive Vice President Research & New Drug Development
Mr. Purcell has over 30 years of experience in consulting and advising emerging biopharmaceutical and technology companies on new business strategy, operations management, clinical development of novel compounds, data solutions for clinical and medical applications, patient engagement & communication, medical education for professionals and consumers, and data analytics for outcomes research. Mr. Purcell oversaw strategic planning, clinical operations, data management, regulatory filings, and R&D and was involved with business development and out-licensing activities for the company’s technology platform.
He started his career as a molecular biologist, where he developed and patented a second generation TPA with increased half-life. He also worked at a major pharmaceutical company where he conducted primary research and published several manuscripts on the topics of AIDS and immunomodulators. Mr. Purcell also headed the Life Sciences Consulting Group for Kline and Company, where he conducted market, technology and business analysis for the commercial development of pharmaceutical and biotechnology products for therapeutic and diagnostic applications.
Rich graduated with a degree in Biochemical Sciences from Princeton University, and attended Rutgers Graduate School of Management majoring in marketing and finance. He is a member of NJTC, HIMSS, the Patient-Centered Primary Care Collaborative, the Drug Information Association and the Licensing Executives Society. He is also an Adjunct Professor of Biology at Monmouth University where he developed and teaches The Business of Biotechnology.
Mark A. Fletcher, Esq. Mr. Fletcher serves as the Executive Vice President & General Counsel,Secretary.
Mr. Fletcher has served as our President and Chief Executive Officer of Generex Biotechnology Corp, the predecessor company, since March 2011. Mr. Fletcher has served as a member of the Board of Directors at our annual meeting of stockholders. He has served as Executive Vice President and General Counsel and continues in his role as General Counsel. Prior to joining, Mr. Fletcher was engaged in the private practice of law as a partner at Goodman and Carr LLP, a leading Toronto law firm. Mr. Fletcher was a partner at Brans, Lehun, Baldwin LLP, a law firm in Toronto. Mr. Fletcher received his LL.B. from the University of Western Ontario in 1989 and was admitted to the Ontario Bar in 1991.
| 30 |
Lawrence Salvo. Mr. Salvo serves as the Executive VP of Diagnostics, CEO and President of HDS, Director
Mr. Salvo has over 20 years of experience in the design and development of rapid medical diagnostic tests for infectious diseases and over 30 years has successfully been directly involved in the management of international distribution and sales including high technology areas in electro-optics and other technical design applications. During that time, Mr. Salvo has developed and maintained substantial and long-term relationships within the People’s Republic of China as well as throughout South and Central America and into the African Continent. His experience includes direct interaction with such international organizations as the WHO, the Global Fund, USAID, the European Union and with multiple supply-chain management groups and multiple NGO’s. Mr. Salvo has been the primary force behind the concept of the design and development of the patented delivery systems of Hema Diagnostic Systems, which are incorporated into many of the current and future RDT’s for various infectious diseases as well as detection applications. These delivery systems continue to evolve and expand the application of rapid testing and are configured for human and veterinary uses.
Mr. Salvo is the founder of Hema Diagnostic Systems which grew out of the predecessor company, International Diagnostics and Medical Supply and has also been directly responsible for all international negotiations. Mr. Salvo brings the full weight of his international experience, development and design expertise along with his substantial knowledge of the international RDT markets acquired from throughout the world. He is a graduate from St. Vincent de Paul Major Seminary, Boynton Beach, Florida
Gary H. Lyman, MD, MPH, FASCO, FRCP (Edin). Dr. Lyman serves as a Director.
Dr. Lyman is Professor of Medicine and Director of Comparative Effectiveness and Outcomes Research – Oncology at Duke University and the Duke Cancer Institute. Dr. Lyman is also a Senior Fellow at the Duke Center for Clinical Health Policy Research. Dr. Lyman previously served as Professor of Medicine, Director of Medical Oncology and Chief of Medicine at the H Lee Moffitt Cancer Center and Research Institute.
Dr. Lyman is active with the American Society of Clinical Oncology, serving as Chair-Elect of the ASCO Clinical Practice Guideline Committee having chaired the Methodology Subcommittee for several years. Dr. Lyman also Chairs several ASCO guideline panels including those related to Prevention and Treatment of Venous Thromboembolism in Cancer, Sentinel Node Biopsy in Early-Stage Breast Cancer and Melanoma, Use of Antiemetics in Patients Receiving Cancer Chemotherapy and Weight-Based Chemotherapy Dosing. Dr. Lyman is also a member of the ASCO Biomarkers Guideline Working Group, the Comparative Effectiveness Research Task Force and the Cost of Care Task Force and in 2010, Dr. Lyman received the prestigious ASCO Statesman Award and was recently elected to the ASCO Board of Directors. Dr. Lyman was past Vice Chairman and is now an advisor to the US Food and Drug Administration and the Oncology Drug Advisory Committee (ODAC). He is Editor-In-Chief of Cancer Investigation and the Peer Review Editor for ASCO’s Journal of Oncology Practice and on the Editorial Board of the Journal of Clinical Oncology and several other subspecialty journals. In addition to serving as a Fellow of ASCO, Dr. Lyman is a Fellow of the Royal College of Physicians (Edinburgh), the American College of Physicians, the American College of Preventive Medicine and the American College of Clinical Pharmacology.
Dr. Lyman received his undergraduate and medical degree from the State University of New York in Buffalo and completed internal medicine residency at the University of North Carolina in Chapel Hill. He subsequently completed a Clinical Hematology/Oncology Fellowship at the Roswell Park Memorial Institute and a Postdoctoral Fellowship in Biostatistics at the Harvard School of Public Health and the Dana Farber Cancer Center.
| 31 |
Craig Eagle, MD. Dr. Eagle serves as a Director.
Dr. Eagle serves as Vice President of Strategic Alliances and Partnerships for the Oncology business unit at Pfizer Inc. In 2003, Dr. Eagle led the worldwide development of Celecoxib in oncology to oversee the global research program. He was responsible for the global research plans and teams for Irinotecan and Dalteparin. Since 2007, he served as Head of the Oncology Therapeutic Area Global Medical Group for Pfizer, including the US oncology business. Dr. Eagle led, or been directly involved with, teams that resulted in eight new products or indications. He has led integration of the Pfizer/Wyeth oncology businesses and portfolio.
Dr. Eagle has a wealth of oncology experience. He joined Pfizer Australia in 2001 as part of the medical group. In Australia, his role involved leading and participating in scientific research, regulatory and pricing & re-imbursement negotiations for compounds in therapeutic areas including oncology, anti-infectives, respiratory, arthritis and pain management.
Dr. Eagle has been a Member of Scientific Advisory Board at Generex Biotechnology Corp. since August 2010. He has been a Member of Strategic Advisory Board at Provectus Pharmaceuticals, Inc. since August 2011. He has been a Director of Regenicin, Inc. since September 7, 2010. He has been a Director at Assured Pharmacy Inc. since June 2009.
Dr. Eagle attended Medical School at the University of New South Wales, Sydney, Australia and received his general internist training at Royal North Shore Hospital in Sydney. He completed his hemato-oncology and laboratory hematology training at Royal Prince Alfred Hospital in Sydney. He was granted Fellowship in the Royal Australasian College of Physicians (FRACP) and the Royal College of Pathologists Australasia (FRCPA). After his training, Dr. Eagle performed basic research at the Royal Prince of Wales hospital to develop a new monoclonal antibody to inhibit platelets.
Jacob Dagan, Ph.D. Dr. Dagan serves as Executive Vice President Business Development
Dr. Dagan is a senior executive, with more than 25 years of proven success, in: healthcare management, corporate and product development, in the US. Combining extensive experience, in operations, marketing, sales and hospital management, with strong bio medical scientific skills. In 2005 founded with partners ProMed Capital, LLC, an investment organization investing in Israeli medical device startups. Since 2006 invest in eight companies, and six more are in the pipe line. In each company, hands on involvement as active manager, board member or chairman of the board. Continues to own and operate Medical Service Options (MSO-Israel). Member on the Board of Governors of the Technion-Israel Institute of Technology.
In 2013 founded three startups and applied for patent protection for each, covering the fields of New Laser printer, Orthodental brace for bone stimulation and an oral drug delivery device. In 2015 moved back to the USA and started MSO-USA, involved as CEO of two Israeli startups, Meditemp and AlfaRhythm. In 2016 started a corporation for contract development and manufacturing of medical and health related lasers. Currently acts as CEO of a multi-specialty surgical corporation in the City of New York.
Past
Director of BioMedical Engineering at Sheeba Medical Center and Associate Professor of Bio-Medical Engineering at Tel Aviv University.
Held senior management positions with Sharplan Lasers, developing the field of applications of Lasers in Medicine. Has been involved
in fund raising and strategy consulting to Bio-Medical, Bio-Technology companies and on the Board or in operating responsibilities
of several start-ups in Israel and the USA.
Received Doctorate from Columbia University focusing on Nuclear Medicine applications in Cardiology B.Sc in Mechanical and Nuclear
Engineering and an MSc in Nuclear Sciences from the Technion, Israel Inst. of Technology.
Dr. James H. Anderson, Jr. serves as a Director. Dr. Anderson has previously served as Chairman of the Corporate Governance and Nominating Committee and a member of the Generex Compensation Committee, and has served on the Generex Scientific Advisory Board since October 2010. Dr. Anderson is a diabetologist and endocrinologist who has been in the pharmaceutical industry for over 25 years. He is currently CEO and President of Symcopeia, a private drug discovery and development company focused on new mechanisms of action for the treatment of diabetes mellitus, and diabetes related obesity and cardiovascular diseases. Dr. Anderson also serves as medical director of PTS Diagnostics, a cardiometabolic medical device company. From 2005 to 2009, Dr. Anderson served as Senior Medical Director for Diabetes and Cardiometabolic Medicine with Eli Lilly and Company and had medical responsibility for diabetes and cardiometabolic drug development, and drove the clinical development, registration and launch of two families of diabetes care products, Humulin® and Humalog. At Eli Lilly, Dr. Anderson contributed to the inventions of the first recombinant DNA produced human insulin analog products, led multiple clinical drug development projects, was responsible for 6 US NDAs and had clinical responsibility for all insulin products worldwide. Dr. Anderson is an elected Fellow of the Faculty of Pharmaceutical Medicine of the Royal Colleges of Physicians of the UK, was a founding board member of the American Association of Pharmaceutical Physicians and is a Fellow of the American College of Endocrinology. Dr. Anderson has been active in the American Diabetes Association and is a member of the International Diabetes Federation, the European Association for the Study of Diabetes, and the Endocrine Society. Dr. Anderson is a founding editorial board member of two journals for diabetes, and serves on the editorial boards or as a reviewer for 5 other diabetes/endocrine journals. Dr. Anderson is a Clinical Associate Professor of Medicine for the Division of Endocrinology and Metabolism at the Indiana University School of Medicine and was awarded an M.D. from the LSU School of Medicine. Dr. Anderson attained the rank of Lieutenant Colonel in the US Army Medical Corps and during his military career, he served as the Chairman, Department of Clinical Investigation at the Army’s largest healthcare center, and Chief of the Medical Division of the US Army Medical Research Institute for Infectious Diseases. The Board believes that Dr. Anderson’s extensive experience in the pharmaceutical industry, his experience in the diabetes and endocrinology fields, combined with his business experience and judgment, provide our Board with valuable scientific and operational expertise.
| 32 |
Brian T. McGee serves as a Director. Mr. McGee has previously served as Chairman of the Generex Audit Committee and a member of the Generex Compensation Committee and the Generex Corporate Governance and Nominating Committee. Mr. McGee has been a partner of Zeifmans LLP ("Zeifmans") since 1995. Mr. McGee began working at Zeifmans shortly after receiving a B.A. degree in Commerce from the University of Toronto in 1985. Zeifmans is a Chartered Accounting firm based in Toronto, Ontario. A significant element of Zeifmans’ business is public corporation accounting and auditing. Mr. McGee is a Chartered Accountant. Throughout his career, Mr. McGee has focused on, among other areas, public corporation accounting and auditing. In 1992, Mr. McGee completed courses focused on International Taxation and Corporation Reorganizations at the Canadian Institute of Chartered Accountants and in 2003, Mr. McGee completed corporate governance courses on compensation and audit committees at Harvard Business School. In April 2004 Mr. McGee received his CPA designation from The American Institute of Certified Public Accountants. Mr. McGee has received a certificate in International Financial Reporting Standards issued by The Institute of Chartered Accountants in England and Wales in 2010. The Board believes that Mr. McGee’s knowledge and understanding of accounting and finance, his education and training in accounting and corporate governance, and his extensive experience in the accounting industry.
Directors
Our bylaws authorize no less than one (1) and no more than twelve (12) directors. We currently have nine directors.
Pursuant to the terms of the Acquisition Agreement, Joseph Moscato, Chairman, together with Andrew Greene, Andrew Ro, Jason Terrell, Richard Purcell, Dr. Gary Lyman, and Dr. Craig Eagle were appointed as our directors.
All directors hold office for one-year terms until the election and qualification of their successors. Officers are elected by the board of directors and serve at the discretion of the board.
There are no family relationships between or among the directors, executive officers or persons nominated or chosen by the Company to become directors or executive officers.
| 33 |
Item 9.01. Financial Statements and Exhibits.
(a) Financial Statements of Business Acquired.
In accordance with Item 9.01(a), the Combined Financial Statements of Hema Diagnostic Systems and Associates as of and for the years ended December 31, 2015 and 2016, and as of and for the nine months ended September 30, 2016 and 2015, are included in this Report following the signature page
(c) Pro forma financial information.
Pro Froma financial information required by Item 9.01(c) will be filed by amendment to this Current Report within 71 days after the date of this Current Report.
(d) Exhibits.
The following exhibits are being filed herewith this Current Report:
| Exhibit No. | Description of Exhibit | |
| 10.1 | Acquisition Agreement among Generex Biotechnology Corporation, Hema Diagnostic Systems, LLC, Stephen L. Berkman and the other Equity Owners of Hema Diagnostic Systems, LLC |
| 34 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
GENEREX BIOTECHNOLOGY CORPORATION. | |
| Date: January 18, 2017 | /s/ Joseph Moscato |
| Joseph Moscato | |
| President and Chief Executive Officer |
| 35 |
HEMA DIAGNOSTIC SYSYTEMS, LLC and ASSOCIATES
COMBINED Financial Statements
TABLE OF CONTENTS
| Page | |||||
Audited Financial Statements as of and for the Years Ended December 31, 2015 and 2014 |
|||||
Report of Independent Registered Public Accounting Firm |
F-1 | ||||
Combined Balance Sheets as of December 31, 2015 and 2014 |
F-2 | ||||
| Combined Statements of Operations for the years ended December 31, 2015 and 2014 | F-3 | ||||
| Combined Statements of Changes in Owners’ Equity for the period from January 1, 2014 through December 31, 2015 | F-4 | ||||
| Combined Statements of Cash Flows for the years ended December 31, 2015 and 2014 | F-5 | ||||
| Notes to Combined Financial Statements | F-7 | ||||
| 36 |
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders
Hema Diagnostics Systems, LLC
Miramar, Florida
We have audited the accompanying combined balance sheets of Hema Diagnostics Systems, LLC, Hema Diagnostics Systems Panama, PTY and Rapid Medical Diagnostics, Corp. as of December 31, 2015 and 2014, and the related combined statements of operations, owners’ deficit, and cash flows for the two years then ended. These combined financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these combined financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform an audit to obtain reasonable assurance about whether the combined financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the combined financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall combined financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the combined financial position of Hema Diagnostics Systems, LLC, Hema Diagnostics Systems Panama, PTY and Rapid Medical Diagnostics, Corp. as of December 31, 2015 and 2014, and the results of its combined operations and cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has incurred negative working capital and cash flows; and has suffered recurring losses from operations; which raises substantial doubt about its ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Liggett & Webb, P.A. |
| Boynton Beach, Florida |
| November 7, 2016 |
| F-1 |
HEMA DIAGNOSTIC SYSTEMS, LLC and ASSOCIATES
Combined Balance Sheets
| December 31, 2015 | December 31, 2014 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash | 29,928 | 40,499 | ||||||
| Accounts receivable, net | 5,604 | 91 | ||||||
| Inventory, net | 29,303 | 41,575 | ||||||
| Advances and loans receivable | 928,174 | 782,723 | ||||||
| TOTAL CURRENT ASSETS | 993,009 | 864,888 | ||||||
| PROPERTY AND EQUIPMENT, NET | 12,936 | 22,767 | ||||||
| OTHER ASSETS, NET | 38,265 | 40,829 | ||||||
| TOTAL ASSETS | 1,044,210 | 928,484 | ||||||
| LIABILITIES AND OWNERS' DEFICIT | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts payable | 368,618 | 367,176 | ||||||
| Accrued interest | 8,924 | 54,669 | ||||||
| Customer deposits | 59,775 | 59,775 | ||||||
| Loan payables - shareholder | 14,144,391 | 14,144,391 | ||||||
| Due to affiliates, net | 81,054 | 83,225 | ||||||
| Other current liabilities | 21,689 | 29,040 | ||||||
| TOTAL CURRENT LIABILITIES | 14,757,451 | 14,738,276 | ||||||
| COMMITMENTS AND CONTINGENCIES (See Note 12) | ||||||||
| OWNERS' DEFICIT | ||||||||
| Owners equity | 5,856,329 | 4,745,329 | ||||||
| Accumulated deficit | (19,569,570 | ) | (18,555,121 | ) | ||||
| TOTAL OWNERS' DEFICIT | (13,713,241 | ) | (13,809,792 | ) | ||||
| TOTAL LIABILITIES AND OWNERS' DEFICIT | 1,044,210 | 928,484 |
See accompanying notes to the combined financial statements
| F-2 |
HEMA DIAGNOSTIC SYSTEMS, LLC and ASSOCIATES
Combined Statements of Operations
| For the Years Ended December 31, | ||||||||
| 2015 | 2014 | |||||||
| REVENUES,NET | 5,703 | 18,025 | ||||||
| COST OF REVENUES | 990 | 9,788 | ||||||
| GROSS PROFIT | 4,713 | 8,237 | ||||||
| OPERATING EXPENSES | ||||||||
| Selling and Marketing Expenses | 55,751 | 58,202 | ||||||
| Research and Development | 534,809 | 527,257 | ||||||
| General and Administrative Expenses: | ||||||||
| Personnel expense | 257,998 | 256,185 | ||||||
| Professional fees | 16,216 | 28,965 | ||||||
| Facilities | 70,617 | 64,085 | ||||||
| Other general and administrative expenses | 55,261 | 47,456 | ||||||
| TOTAL OPERATING EXPENSES | 990,662 | 982,150 | ||||||
| LOSS FROM OPERATIONS | (985,939 | ) | (973,913 | ) | ||||
| OTHER INCOME (EXPENSE) | ||||||||
| Interest income (expense), net | (24,778 | ) | (25,616 | ) | ||||
| Other income (expense), net | (3,732 | ) | — | |||||
| TOTAL OTHER INCOME (EXPENSE) | (28,510 | ) | (25,616 | ) | ||||
| NET LOSS | (1,014,449 | ) | (999,529 | ) | ||||
See accompanying notes to the combined financial statements
| F-3 |
HEMA DIAGNOSTIC SYSTEMS, LLC and ASSOCIATES
Combined Statements of Owners’ Deficit
| For the Years ended December 31, 2015 and 2014 | ||||||||||||
| Owners' Equity | Accumulated Deficit | Total Owners' Deficit | ||||||||||
| BALANCE AT JANUARY 1, 2014 | 4,745,329 | (17,555,592 | ) | (12,810,263 | ) | |||||||
| Net Loss | — | (999,529 | ) | (999,529 | ) | |||||||
| BALANCE AT DECEMBER 31, 2014 | 4,745,329 | (18,555,121 | ) | (13,809,792 | ) | |||||||
| Capital contributions | 1,111,000 | — | 1,111,000 | |||||||||
| Net Loss | — | (1,014,449 | ) | (1,014,449 | ) | |||||||
| BALANCE AT DECEMBER 31, 2015 | 5,856,329 | (19,569,570 | ) | (13,713,241 | ) | |||||||
See accompanying notes to the combined financial statements
| F-4 |
HEMA DIAGNOSTIC SYSTEMS, LLC and ASSOCIATES
Combined Statements of Cash Flows
| For the Years ended December 31, | ||||||||
| 2015 | 2014 | |||||||
| Loss on disposal of fixed asset | 620 | — | ||||||
| Changes in operating assets and liabilities: | ||||||||
| (Increase) decrease in accounts receivable, net | (551 | ) | 1,184 | |||||
| (Increase) decrease in inventory | 4,522 | 6,697 | ||||||
| Increase in accounts payable | (2,828 | ) | 2,932 | |||||
| Accrued interest | 27,254 | 28,930 | ||||||
| Increase in customer deposits | — | (2,025 | ) | |||||
| Net cash used in operating activities | (970,869 | ) | (952,527 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Employee loans | (145,451 | ) | (129,013 | ) | ||||
| Purchase of fixed assets | — | (1,659 | ) | |||||
| Due to affiliates | 3,773 | (329 | ) | |||||
| Net cash used in investing activities | (141,678 | ) | (131,001 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Proceeds from shareholder loans | — | 1,117,873 | ||||||
| Repayment of line of credit | (3,080 | ) | (5,329 | ) | ||||
| Shareholder loan repayment | (5,944 | ) | (5,944 | ) | ||||
| Capital contributions | 1,111,000 | — | ||||||
| Net cash provided by financing activities | 1,101,976 | 1,106,600 | ||||||
| NET CHANGE IN CASH | (10,571 | ) | 23,072 | |||||
| CASH AT THE BEGINNING OF THE PERIOD | 40,499 | 17,427 | ||||||
| CASH AT THE END OF THE PERIOD | 29,928 | 40,499 | ||||||
| SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION | ||||||||
| Cash paid during the period for: | ||||||||
| Income taxes | — | — | ||||||
| Interest | 30,687 | 30,903 | ||||||
Non Cash Investing and Financing
During 2015, $296,369 of amounts due from Hema Diagnostics Systems Panama was transferred to our founder and shareholder as partial satisfaction of a balance due him by Hema Diagnostics.
See accompanying notes to the combined financial statements
| F-5 |
HEMA DIAGNOSTIC SYSTEMS, LLC and ASSOCIATES
Notes to Combined Financial Statements
NOTE 1. ORGANIZATION AND NATURE OF OPERATIONS
The Combined Financial Statements of Hema Diagnostic Systems, LLC and Associates (collectively the “Company”) include the accounts of Hema Diagnostic Systems, LLC; Hema Diagnostics Systems Panama, PTY and Rapid Medical Diagnostics, Corp as the entities are under common control and management. All transactions and accounts between and among the entities have been eliminated. The Company has evaluated subsequent events through November 7, 2016, which is the date the Combined Financial Statements were available to be issued.
HEMA DIAGNOSTIC SYSTEMS, LLC (“HDS”), was founded to market and distribute certain third party medical testing device technology. When new and innovative medical device testing technology became available that was both proprietary from and competitive to the previous third party technology, the principals decided to pursue the commercialization of the new technologies. HDS, a Florida limited liability corporation founded December 14, 2000 and began operations in 2002 to perform product research and development, create distribution channels and sales and marking and administration functions and is currently commercializing the new proprietary medical testing device patents and technology. HDS has not yet begun to generate significant revenues and is still in the process of perfecting production techniques and obtaining the appropriate certifications for a series of medical devices that will be able to detect certain diseases quickly and cost effectively.
HEMA DIAGNOSTICS SYSTEMS PANAMA, PTY (“HDP”) was established to distribute HDS products in Central and South America. HDS operates as the administration and disbursing arm for HDP. HDS is affiliated with HDP through common ownership and operates under a Management Services Agreements (“MSA”) that provides for the reimbursement of expenses incurred by HDS on behalf of its affiliates. HDS receives a service fee for performing these administration services as specified in the MSA agreements. The affiliate’s ability to repay HDS for funds advanced on their behalf, is entirely dependent on the successful commercialization of Rapid Medical Diagnostics technology and the resulting royalty payments generated there from. In late 2015, HDP’s balance due to HDS of $295,564 was transferred to a founder and shareholder as partial satisfaction in the balance due to him by HDS.
RAPID MEDICAL DIAGNOSTICS, CORP. (“RMD”) was established to develop products and hold patents for HDS and is affiliated with HDS through common ownership and management. HDS operates as the administration and disbursing arm of RMD in accordance with a Management Services Agreements (“MSA”) between the parties that provides HDS a service fee for performing these administrative and disbursing services. Consequently, HDS records substantial amounts due from RMD as a result of transactions disbursed by HDS on RMD’s behalf. RMD’s ability to repay HDS for funds advanced on its behalf, is entirely dependent on the successful commercialization of RMD patents and technologies by HDS and the resulting royalty payments generated there from. RMD has licensed its patents and technologies exclusively to HDS, which will entitle RMD to receive royalties from HDS once those technologies achieve commercial viability (see Note 8). To date there has been no royalty paid to or earned by RMD. Correspondingly, HDS has waived its right to receive service fees under the MSA until HDS successfully commercializes RMD’s licensed technologies. In late 2013, RMD’s balance due to HDS of approximately $632,000 was transferred to a founder and shareholder as partial satisfaction of the balance due to him by HDS.
| F-6 |
NOTE 2. GOING CONCERN
The accompanying combined financial statements have been prepared assuming that the Company will continue as a going concern. The Company generated net losses of approximately $1,014,000 and $1,000,000 for the years ended December 31, 2015 and 2014, respectively. The net loss incurred in 2015 has resulted in an accumulated deficit of approximately $19,570,000 and a total Owners’ deficit of approximately $13,713,000 at December 31, 2015. Financing activities provided approximately $1,111,000 during 2015, which was primarily due to additional capital contribution from a shareholder. During 2016, the Company continues to incur losses and require cash advances.
In response to the losses incurred in 2015, the Company continues to constantly evaluate and monitor its cash needs and existing cash burn rate, in order to make adjustments to its operating expenses. Cash on hand was approximately $30,000 at December 31, 2015.
No assurances can be given that the Company will achieve success in obtaining sufficient levels of end user sell-through necessary to fully sustain its operations, without seeking additional financing. There also can be no assurances that additional financing, if required, can be obtained, or obtained on reasonable terms acceptable to the Company.
NOTE 3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Use of estimates
The preparation of combined financial statements in accordance with accounting principles generally accepted in the United States of America (“US-GAAP) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting periods. Actual results could differ from those estimates. Estimates which are particularly significant to the financial statements include estimates relating to the determination of impairment of assets, the useful life of property and equipment and the recoverability of advances.
| F-7 |
NOTE 3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Cash and cash equivalents
The Company considers short-term interest bearing investments with initial maturities of three months or less to be cash equivalents. The Company has no cash equivalents at December 31, 2015 and 2014.
Inventory
Inventory is stated at the lower of cost or net realizable value. Cost is determined using the Weighted Average method. The Company periodically evaluates its inventory for any obsolete or slow moving items based on production lot# and advances in production design or technology. Any inventory determined to be obsolete or slow moving is removed from inventory and disposed or a provision is made to reduce slow moving inventory to its net realizable value. At December 31, 2015 and 2014, the Company recorded a reserve for obsolescence of $7,750 and $0, respectively.
Property and equipment
Property and equipment consists of furniture and office equipment, and is stated at cost less accumulated depreciation. Depreciation is determined by using the 200% double declining method for equipment and the straight- line method for leasehold improvements, over the estimated useful lives of the related assets, generally five to fifteen years.
Expenditures for repairs and maintenance of equipment are charged to expense as incurred. Major replacements and betterments are capitalized and depreciated over the remaining useful lives of the related assets.
Intangible assets, net
The Company’s intangible assets consist of patent patented technology. Amortization is computed by applying the straight line method based on the remaining patent life. The primary patent expires in 2026.
Impairment of Long-Lived Assets
The Company evaluates long-lived assets for impairment, including property, plant and equipment and intangible assets, when events or changes in circumstances indicate that the carrying value of such assets may not be recoverable or the assets are being held for sale. Upon the occurrence of a triggering event, the asset is reviewed to assess whether the estimated undiscounted cash flows expected from the use of the asset plus the residual value from the ultimate disposal exceeds the carrying value of the asset. If the carrying value exceeds the estimated recoverable amounts, the asset is written down to the estimated fair value. Any resulting impairment loss is reflected on the Combined Statements of Operations.
| F-8 |
NOTE 3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Revenue recognition
The Company has yet to fully commercialize its products and secure appropriate permits and licenses necessary to begin full worldwide distribution. The Company has had negligible sales to date, primarily related to trial samples and evaluation of the efficacy and suitability of the products to specific target markets.
Sales and the related cost of sales are recognized primarily upon shipment of products (normally when title passes). The Company’s revenue recognition policies are in compliance with ASC Topic 605, which establishes criteria that must be satisfied before revenue is realized or realizable and earned.
The Company recognizes revenue when all of the following four criteria are met:
• persuasive evidence of a sales arrangement exists,
• delivery has occurred,
• the sales price is fixed or determinable and
• collectability is probable.
Income taxes
Hema Diagnostic Systems, LLC is a limited liability corporation. Rapid Medical Diagnostic Corp. is a Subchapter S corporation. Federal and state income tax regulations do not require a limited liability corporation or a Subchapter S corporation to pay income taxes. Rather each member’s allocable share of the profit or loss is reported in each member’s individual income tax return. Hema Diagnostics Systems Panama, PTY is a Panamanian company. Due to its operational losses, no taxes are required. Accordingly, no provision or liability for income taxes is reflected for this reporting entity in the accompanying financial statements. The Company’s 2012 – 2015 tax returns remain subject to examination by federal, state or foreign tax authorities.
Risks and uncertainties
The Company’s business could be impacted by continuing price pressure on its product manufacturing, acceptance of its products in the market place, new competitors, changing federal and/or state legislation, new technologies and other factors. Adverse changes in these areas could negatively impact the Company’s financial position, results of operations and cash flows.
| F-9 |
NOTE 4. RECENTLY ISSUED ACCOUNTING STANDARDS AND DEVELOPMENTS
Accounting standards promulgated by the FASB are subject to change. Changes in such standards may have an impact on the Company’s future consolidated financial statements. The following are a summary of recent accounting developments.
In February 2015, the FASB issued ASU 2015-02, Consolidation (Topic 810)—Amendments to the Consolidation Analysis (“ASU 2015-02”), which provides guidance on evaluating whether a reporting entity should consolidate certain legal entities. Specifically, the amendments modify the evaluation of whether limited partnerships and similar legal entities are variable interest entities (“VIEs”) or voting interest entities. Further, the amendments eliminate the presumption that a general partner should consolidate a limited partnership, as well as affect the consolidation analysis of reporting entities that are involved with VIEs, particularly those that have fee arrangements and related party relationships. ASU 2015-02 is effective for interim and annual reporting periods beginning after December 15, 2016, with early adoption permitted. A reporting entity may apply the amendments using a modified retrospective approach or a full retrospective application. The Company is currently evaluating the impact, if any, that adopting ASU 2015-02 will have on its combined financial statements.
In February 2016, the FASB issued ASU 2016-02, Leases, which will amend current lease accounting to require lessees to recognize (i) a lease liability, which is a lessee’s obligation to make lease payments arising from a lease, measured on a discounted basis, and (ii) a right-of-use asset, which is an asset that represents the lessee’s right to use, or control the use of, a specified asset for the lease term. ASU 2016-02 does not significantly change lease accounting requirements applicable to lessors; however, certain changes were made to align, where necessary, lessor accounting with the lessee accounting model. This standard will be effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. We are currently reviewing the provisions of this ASU to determine if there will be any impact on our results of operations, cash flows or financial condition.
In March 2016, the FASB issued ASU 2016-09, Compensation – Stock Compensation: Improvements to Employee Share-Based Payment Accounting, which relates to the accounting for employee share-based payments. This standard addresses several aspects of the accounting for share-based payment award transactions, including: (a) income tax consequences; (b) classification of awards as either equity or liabilities; and (c) classification on the statement of cash flows. This standard will be effective for fiscal years beginning after December 15, 2016, including interim periods within those fiscal years. We are currently reviewing the provisions of this ASU to determine if there will be any impact on our results of operations, cash flows or financial condition.
There were various other accounting standards and interpretations issued in 2015, none of which are expected to have a material impact on the Company’s financial position, operations or cash flows.
| F-10 |
NOTE 5. INVENTORY
Inventory at December 31, 2015 and 2014 consisted of the following:
| 2015 | 2014 | |||||||
| Production materials | 30,281 | 37,175 | ||||||
| Sub-assemblies | 5,001 | 1,900 | ||||||
| Finished goods | 1,771 | 2,500 | ||||||
| 37,053 | 41,575 | |||||||
| Less: Obsolesence reserve | (7,750 | ) | — | |||||
| 29,303 | 41,575 |
Production materials - Consists of certain subcomponents fabricated by outside third parties as well as other components purchased in bulk quantities. The carrying value of these units reflects their direct acquisition cost along with associated transportation costs to the Company’s assembly and packaging facilities.
Sub-assemblies - Consists of units partially completed on the assembly line or by outside third parties. The carrying value of these units includes all direct material and labor costs involved to complete the associated assembly.
Finished goods - Consists of completed units in market ready packaging. The carrying value of these units include the cost of components and materials, labor necessary to assemble them.
Obsolescence reserve - Management evaluated the inventory at December 31, 2015 and 2014 and provided an allowance for obsolescence of $7,750 and $0, respectively, primarily associated with production materials and sub-assemblies. Management’s evaluation for obsolescence includes identifying materials and components that are not currently or likely to be used in production in the near future as well as tracking certain components by lot number and expiry date.
NOTE 6. ADVANCES and LOANS RECEIVABLE
Advances and Loans Receivable as of December 31, 2015 and 2014 consisted of the following:
| 2015 | 2014 | |||||||
| Advances to officer | 893,372 | 749,580 | ||||||
| Advances to management | 20,635 | 17,253 | ||||||
| Loans to employees | 14,167 | 15,890 | ||||||
| 928,174 | 782,723 |
Advance to officer - Consists of funds advanced to the Company’s President and CEO, board member and shareholder. During September 2016, the advance to officer of $893,372 was repaid in full.
| F-11 |
NOTE 6. ADVANCES and LOANS RECEIVABLE (continued)
Advances to management - Consists of funds advanced to the Director of Latin American Sales, board member and shareholder. During October 2016, $14,735 was repaid.
Loans to employees - Consists of funds loaned primarily to one employee. That employee has arranged a payback schedule and commencing in August 2016 began making agreed payments of approximately $950 per month.
NOTE 7. PROPERTY AND EQUIPMENT
Property and equipment as of December 31, 2015 and 2014 consisted of the following:
| Estimated Useful Lives | 2015 | 2014 | ||||||||
| Computer Equipment & Software | 5 yrs 200DDB | 10,244 | 15,360 | |||||||
| Equipment | 5 yrs 200DDB | 47,148 | 44,364 | |||||||
| Furniture | 7 yrs 200DDB | 1,402 | 1,402 | |||||||
| Lab Equipment | 5 yrs 200DDB | 23,220 | 70,166 | |||||||
| Leasehold Improvements | 15 yrs SL | 40,445 | 40,445 | |||||||
| Office Equipment | 5 yrs 200DDB | 1,990 | 4,206 | |||||||
| Tools | 5 yrs 200DDB | 12,191 | 183,495 | |||||||
| Total | 136,640 | 359,438 | ||||||||
| Less Accumulated Depreciation | (123,704 | ) | (336,671 | ) | ||||||
| Property and Equipment, net | 12,936 | 22,767 |
Depreciation expense for the years ended December 31, 2015 and 2014 totaled $9,212 and $5,840 respectively. Equipment additions during the year ended December 31, 2015 and 2014 were $0 and $1,659, respectively.
NOTE 8. OTHER ASSETS
Other assets as of December 31, 2015 and 2014 consisted of the following:
| 2015 | 2014 | |||||||
| Deposits | 8,362 | 8,362 | ||||||
| Patents | 51,274 | 51,274 | ||||||
| Less Amortization | (21,371 | ) | (18,807 | ) | ||||
| 38,265 | 40,829 |
Amortization expense for the years ended December 31, 2015 and 2014 totaled $2,563 and $3,444 respectively. The Company has several patents most of which are dormant as the Company lacks the funds to properly commercialize them. The current products and planned future products rely on the “Express” patent, the costs of which are reflected above. This patent expires in 2026.
| F-12 |
NOTE 9. LOAN PAYABLE - SHAREHOLDER
The Company received substantially all of its funding from its primary shareholder, who owns 98.9% of the Company as of December 31, 2015 and 2014. The Company borrowed $0 and $1,117,873 during the year ended December 31, 2015 and 2014, respectively. The loan is unsecured, payable on demand and earns interest at 0.21%. Accordingly, the Company recorded interest expense of $27,255 and $28,083 during the year ended December 31, 2015 and 2014, respectively.
NOTE 10. TRANSACTIONS with AFFILIATES
Transactions with affiliates as of December 31, 2015 and 2014 consisted of the following:
| 2015 | 2014 | |||||||
| Due to CWW | 83,554 | 83,554 | ||||||
| Due from IDMS | (2,500 | ) | — | |||||
| Due from PROVET LABS | — | (329 | ) | |||||
| 81,054 | 83,225 |
China World Wide (CWW) - was established 25 years ago to distribute certain third party products that are not competing with HDS products. HDS is affiliated with CWW through common ownership. From inception through 2007, CWW provided supplemental funding to HDS on a non-interest basis. At December 31, 2015 and 2014, HDS had an unpaid balance to CWW of $83,554. CWW has also assisted HDS in the development of its distribution and sourcing in China.
International Diagnostics and Medical Supply Corp. (IDMS) - was established 2 years ago to create hemodialysis facilities on a global scale. These services will not compete with HDS products. HDS is affiliated with IDMS through common ownership. Do to the high initial cost of establishing a hemodialysis facility, IDMS has not yet secured satisfactory funding to execute its business plan. Consequently, it has only engaged in minimal organizational activities. At December 31, 2015 HDS had an unpaid balance due from IDMS of $2,500 advanced to IDMS for legal fees incurred.
ProVet Labs (PROVET) - was established 7 years ago to provide rapid dialysis products for veterinary use. These services will not compete with HDS products. HDS is affiliated with PROVET through common ownership. PROVET has not yet secured satisfactory funding to execute its business plan. Consequently, it has only engaged in minimal organizational activities. At December 31, 2014 HDS had an unpaid balance due from PROVET of $329 advanced to PROVET for business licenses.
| F-13 |
NOTE 11. LICENSES and PATENTS
All patents and licenses are held by Rapid Medical Diagnostics Corporation, an affiliate company through common owners. HDS licenses the rights to certain technologies used in the development, manufacture and commercialization of its products from RMD. Agreements covering these license arrangements were entered into on January 1, 2005 for a term of five years and may be automatically renewed annually, unless either party gives notice 60 days prior to the renewal date. To date the agreements have been renewed annually and are currently in effect. These agreements provide for royalties between the parties, once a commercialized product is marketed and begins distribution.
NOTE 12. COMMITMENTS and CONTINGENCIES
Legal contingencies
From time to time, the Company may be a defendant in pending or threatened legal proceeding arising in the normal course of its business. Management is not aware of any pending, threatened or asserted claims.
Lease commitments
| 2016 | 2017 | 2018 | 2019 | 2020 and beyond | Total | |||||||||||||||||||
| HDS Office Lease | 13,696 | 6,979 | — | — | — | 20,675 | ||||||||||||||||||
| Auto Lease | 12,259 | 11,789 | 11,789 | 3,930 | 39,767 | |||||||||||||||||||
| Computer Equipment | ||||||||||||||||||||||||
| Lease #1 | 3,291 | 823 | 4,114 | |||||||||||||||||||||
| Lease #2 | 1,663 | 1,663 | 139 | 3,465 | ||||||||||||||||||||
| 30,909 | 21,254 | 11,928 | 3,930 | — | 68,021 | |||||||||||||||||||
HDS Office Lease – Business lease for 5,627 square feet of office and storage space located at 10102 USA Today Way, Miramar FL 33025. The lease term is from May 12, 2012 through June 30, 2017. Having previously been extended, the lease provides no further renewal option. The above payment commitments reflect base rent, estimated common are maintenance costs and applicable sales tax.
Auto Lease – Closed end vehicle lease which called for 39 monthly payments of $1,099.85 for our CEO and President which expires in April of 2016 was replaced with a similar closed end vehicle lease which calls for 36 monthly payments of $982.39 expires April 7, 2019. The above payment commitments reflect both vehicle leases.
Computer Lease – Two leases were originated with the same supplier to provide office computer equipment. Lease #1 calls for 36 monthly payments of $274.21 commencing April 1, 2014 and expiring on March 31, 2017. Lease #2 calls for 36 monthly payments of $138.61 commencing February 19, 2015 and expiring on February 18, 2018.
| F-14 |
NOTE 13. CUSTOMER and SUPPLIER CONCENTRATION
Customer concentration – The Company is currently in the process of commercializing its products and as of December 31, 2015 has not begun any substantial sales or marketing efforts. Accordingly, the Company has recorded only marginal sales to date, which were not of sufficient size to classify the customer as significant to the Company’s revenue. Those marginal sales primarily reflect instances where samples and trial products were shipped at a billable value.
Supplier concentration – As the Company is in the process of commercializing its products, the bulk of its purchasing activities are focused on regulatory, legal and consulting services rather than production or branding activities. As to production activities, alternate suppliers of components and raw materials are readily available should the need arise. Legal and consulting services generally relate to intellectual property, regulatory matters and financial consulting. There are a range of alternate consultants available to the Company should the need arise.
NOTE 14. SUBSEQUENT EVENTS
Advances to Shareholder/CEO - As of December 31, 2015, the Company had advanced $893,372 to its Shareholder/CEO. In September 2016, the Shareholder/CEO repaid the advances in full.
Loans from shareholder - During the period from January 1, 2016 through November 7, 2016, the Company has received an additional capital contribution of $884,500 from its principal shareholder. The Company also repaid $898,664 of the outstanding debt, bringing the total balance outstanding to $13,245,737. The loan bears interest at 0.21% in 2015, which was raised to 0.75% for 2016 and is payable on demand.
Letter of intent to be acquired (LOI) - On August 26, 2016 the Company received a Letter of Intent (“LOI”) from Generex Biotechnology Corporation (“Generex”) to acquire the Company. Generex is a registrant under the Securities and Exchange Act of 1934. Its common stock is traded over the counter under the symbol “GNBT”. The terms of the agreement provide that:
| • | HDS will – |
| o | issue a dedicated class of first ranking voting preferred securities representing 51% of the outstanding equity of the Company to Generex |
| o | deliver Stephen Berkman’s full and final release of all indebtedness owed by HDS and its affiliates to Generex |
| • | Generex will – |
| o | issue $250,000 worth of Generex restricted common stock plus 250,000 of restricted common shares to Stephen Berkman |
| o | also issue a warrant to acquire up to 15,000,000 shares of Generex common stock to Stephen Berkman |
| o | grant Stephen Berkman one seat on the Generex Board of Directors |
| o | If at any time during 36 months following the closing date, the aggregate value of the stock consideration is equal to or exceeds $15,000,000, be entitled to acquire 100% of the outstanding equity securities of HDS for $1.00. |
This LOI expires on November 30, 2016.
| F-15 |
HEMA DIAGNOSTIC SYSYTEMS, LLC and ASSOCIATES
CONDENSED COMBINED Unaudited Financial Statements
TABLE OF CONTENTS
| Page | ||||
Unaudited Financial Statements as of and for the Nine Months Ended September 30, 2016 and 2015 |
||||
|
||||
Condensed Combined Balance Sheets as of September 30, 2016 (unaudited) and December 31, 2015 |
F-16 | |||
| Unaudited Condensed Combined Statements of Operations for the nine months ended September 30, 2016 and 2015 | F-17 | |||
| Unaudited Condensed Combined Statements of Changes in Owners’ Equity for the period from January 1, 2016 through September 30, 2016 | F-18 | |||
| Unaudited Condensed Combined Statements of Cash Flows for the nine months ended September 30, 2016 and 2015 | F-19 | |||
| Notes to Condensed Combined Unaudited Financial Statements | F-20 | |||
| F-16 |
HEMA DIAGNOSTIC SYSTEMS, LLC and ASSOCIATES
Condensed Combined Balance Sheets
| September
30, 2016 | December
31, 2015 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash | 25,441 | 29,928 | ||||||
| Accounts receivable, net | 7,571 | 5604 | ||||||
| Inventory, net | 33,524 | 29,303 | ||||||
| Advances and loans receivable | 113,128 | 928,174 | ||||||
| TOTAL CURRENT ASSETS | 179,664 | 993,009 | ||||||
| PROPERTY AND EQUIPMENT, NET | 6,640 | 12,936 | ||||||
| OTHER ASSETS, NET | 36,343 | 38,265 | ||||||
| TOTAL ASSETS | 222,647 | 1,044,210 | ||||||
| LIABILITIES AND OWNERS' DEFICIT | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts payable | 425,128 | 368,618 | ||||||
| Accrued interest | 158,788 | 81,924 | ||||||
| Customer deposits | 59,775 | 59,775 | ||||||
| Loan payables - shareholder | 13,260,472 | 14,144,391 | ||||||
| Due to affiliates, net | 80,504 | 81,054 | ||||||
| Other current liabilities | 25,994 | 21,689 | ||||||
| TOTAL CURRENT LIABILITIES | 14,010,661 | 14,757,451 | ||||||
| COMMITMENTS AND CONTINGENCIES | ||||||||
| OWNERS' DEFICIT | ||||||||
| Owners equity | 6,740,829 | 5,856,329 | ||||||
| Accumulated deficit | (20,528,843 | ) | (19,569,570 | ) | ||||
| TOTAL OWNERS' DEFICIT | (13,788,014 | ) | (13,713,241 | ) | ||||
| TOTAL LIABILITIES AND OWNERS' DEFICIT | 22,647 | 1,044,210 | ||||||
See accompanying notes to the condensed combined unaudited financial statements
| F-17 |
HEMA DIAGNOSTIC SYSTEMS, LLC and ASSOCIATES
Condensed Combined Statements of Operations
(Unaudited)
| For the
Nine Months Ended September 30, | ||||||||
| 2016 | 2015 | |||||||
| REVENUES,NET | 16,963 | 4,828 | ||||||
| COST OF REVENUES | 12,414 | 2,788 | ||||||
| GROSS PROFIT | 4,549 | 2,040 | ||||||
| OPERATING EXPENSES | ||||||||
| Selling and Marketing Expenses | 32,197 | 57,029 | ||||||
| Research and Development | 407,609 | 384,100 | ||||||
| General and Administrative Expenses: | ||||||||
| Personnel expense | 212,920 | 193,220 | ||||||
| Professional fees | 147,787 | 6,428 | ||||||
| Facilities | 52,222 | 46,257 | ||||||
| Other general and administrative expenses | 36,613 | 39,181 | ||||||
| TOTAL OPERATING EXPENSES | 889,348 | 726,215 | ||||||
| LOSS FROM OPERATIONS | (884,799 | ) | (724,175 | ) | ||||
| OTHER INCOME (EXPENSE) | ||||||||
| Interest income (expense), net | (79,572 | ) | (24,874 | ) | ||||
| Other income (expense), net | 5,098 | 2,445 | ||||||
| TOTAL OTHER INCOME (EXPENSE) | (74,474 | ) | (22,428 | ) | ||||
| NET LOSS | (959,273 | ) | (746,603 | ) | ||||
See accompanying notes to the condensed combined unaudited financial statements
| F-18 |
HEMA DIAGNOSTIC SYSTEMS, LLC and ASSOCIATES
Condensed Combined Statements of Owners’ Deficit
(Unaudited)
| Owners' Equity | Accumulated Deficit | Total Owners' Deficit | ||||||||||
| BALANCE AT JANUARY 1, 2016 | 5,856,329 | (19,569,570 | ) | (13,713,241 | ) | |||||||
| Capital contributions | 884,500 | — | 884,500 | |||||||||
| Net Loss | — | (959,273 | ) | (959,273 | ) | |||||||
| BALANCE AT SEPTEMBER 30, 2016 | 6,740,829 | (20,528,843 | ) | (13,788,014 | ) |
See accompanying notes to the condensed combined unaudited financial statements
| F-19 |
HEMA DIAGNOSTIC SYSTEMS, LLC and ASSOCIATES
Condensed Combined Statements of Cash Flows
(Unaudited)
For the Nine Months Ended September 30, | ||||||||
| 2016 | 2015 | |||||||
| CASH FLOW FROM OPERATING ACTIVITIES: | ||||||||
| NET LOSS | (959,273 | ) | (746,603 | ) | ||||
| ADJUSTMENTS TO RECONCILE NET LOSS TO NET CASH USED IN OPERATING ACTIVITIES: | ||||||||
| Depreciation and amortization | 8,218 | 8,832 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| (Increase) decrease in accounts receivable, net | (1,967 | ) | (4,017 | ) | ||||
| (Increase) decrease in inventory | (4,221 | ) | (3,469 | ) | ||||
| Increase in accounts payable | 56,511 | 6,927 | ||||||
| Increase in accrued interest | 76,863 | 24,520 | ||||||
| Decrease in other current liabilities | 4,305 | 3,199 | ||||||
| Net cash used in operating activities | (819,564 | ) | (710,611 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Employee advances | 815,046 | (105,592 | ) | |||||
| Decrease (increase) in other assets | — | (5,151 | ) | |||||
| Due to affiliates | (550 | ) | — | |||||
| Net cash provided by (used in) investing activities | 814,496 | (110,743 | ) | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Capital contribution | 884,500 | 793,400 | ||||||
| Proceeds (repayments) from shareholders loans | (883,919 | ) | 236 | |||||
| Net cash provided by financing activities | 581 | 793,636 | ||||||
| NET DECREASE IN CASH | (4,487 | ) | (27,718 | ) | ||||
| CASH AT THE BEGINNING OF THE PERIOD | 29,928 | 40,499 | ||||||
| CASH AT THE END OF THE PERIOD | 25,441 | 12,781 | ||||||
| SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION: | ||||||||
| Cash paid during the period for: | — | — | ||||||
| Income taxes | — | — | ||||||
| Interest | ||||||||
See accompanying notes to the condensed combined unaudited financial statements
| F-20 |
HEMA DIAGNOSTIC SYSTEMS, LLC and ASSOCIATES
Notes to Condensed Combined Unaudited Financial Statements
For the Nine months ended September 30, 2016
NOTE 1. ORGANIZATION AND NATURE OF OPERATIONS
The Combined Financial Statements of Hema Diagnostic Systems, LLC and Associates (collectively the “Company”) include the accounts of Hema Diagnostic Systems, LLC; Hema Diagnostics Systems Panama, PTY and Rapid Medical Diagnostics, Corp. as the entities are under common control and management. All transactions and accounts between and among the entities have been eliminated. The Company has evaluated subsequent events through November 17, 2016, which is the date the Combined Financial Statements were available to be issued.
HEMA DIAGNOSTIC SYSTEMS, LLC (“HDS”), was founded to market and distribute certain third party medical testing device technology. When new and innovative medical device testing technology became available that was both proprietary from and competitive to the previous third party technology, the principals decided to pursue the commercialization of the new technologies. HDS, a Florida limited liability corporation founded December 14, 2000 and began operations in 2002 to perform product research and development, create distribution channels and sales and marketing and administration functions and is currently commercializing the new proprietary medical testing device patents and technology. HDS has not yet begun to generate significant revenues and is still in the process of perfecting production techniques and obtaining the appropriate certifications for a series of medical devices that will be able to detect certain diseases quickly and cost effectively.
HEMA DIAGNOSTICS SYSTEMS PANAMA, PTY (“HDP”) was established to distribute HDS products in Central and South America. HDS operates as the administration and disbursing arm for HDP. HDS is affiliated with HDP through common ownership and operates under a Management Services Agreements (“MSA”) that provides for the reimbursement of expenses incurred by HDS on behalf of its affiliates. HDS receives a service fee for performing these administration services as specified in the MSA agreements. The affiliate’s ability to repay HDS for funds advanced on their behalf, is entirely dependent on the successful commercialization of Rapid Medical Diagnostics technology and the resulting royalty payments generated there from.
RAPID MEDICAL DIAGNOSTICS, CORP. (“RMD”) was established to develop products and hold patents for HDS and is affiliated with HDS through common ownership and management. HDS operates as the administration and disbursing arm of RMD in accordance with a Management Services Agreements (“MSA”) between the parties that provides HDS a service fee for performing these administrative and disbursing services. Consequently, HDS records substantial amounts due from RMD as a result of transactions disbursed by HDS on RMD’s behalf. RMD’s ability to repay HDS for funds advanced on its behalf, is entirely dependent on the successful commercialization of RMD patents and technologies by HDS and the resulting royalty payments generated there from. RMD has licensed its patents and technologies exclusively to HDS, which will entitle RMD to receive royalties from HDS once those technologies achieve commercial viability. To date there has been no royalty paid to or earned by RMD. Correspondingly, HDS has waived its right to receive service fees under the MSA until HDS successfully commercializes RMD’s licensed technologies.
| F-21 |
NOTE 2. GOING CONCERN
The accompanying combined financial statements have been prepared assuming that the Company will continue as a going concern. The Company generated net losses of approximately $959,000 for the nine months ended September 30, 2016. The net loss incurred in 2016 has resulted in an accumulated deficit of approximately $20,529,000 and a total Owners’ deficit of approximately $13,788,000 at September 30, 2016.
In response to the losses incurred in 2016, the Company continues to constantly evaluate and monitor its cash needs and existing cash burn rate, in order to make adjustments to its operating expenses. Cash on hand was approximately $25,000 at September 30, 2016.
No assurances can be given that the Company will achieve success in obtaining sufficient levels of end user sell-through necessary to fully sustain its operations, without seeking additional financing. The Company intends to pursue the equity transaction discussed in Note 14, seek additional lending, equity or joint venture partners. However, there can be no assurances that additional financing, if required, can be obtained, or obtained on reasonable terms acceptable to the Company.
NOTE 3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying unaudited condensed combined financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) for interim financial information. Accordingly, they do not include all of the information and footnotes required by U.S. GAAP for complete financial statements. In the opinion of management, such statements include all adjustments (consisting only of normal recurring items) which are considered necessary for a fair presentation of the unaudited condensed combined financial position of Hema Diagnostic Systems, LLC and Associates as of September 30, 2016 and the unaudited condensed combined results of its operations and cash flows for the nine months ended September 30, 2016. The unaudited condensed combined results of operations for the nine months ended September 30, 2016 are not necessarily indicative of the operating results for the full year. It is recommended that these unaudited condensed combined financial statements be read in conjunction with the audited financial statements and related disclosures of the Company for the years ended December 31, 2015 and 2014.
Use of estimates
The preparation of combined financial statements in accordance with accounting principles generally accepted in the United States of America (“US-GAAP) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting periods. Actual results could differ from those estimates. Estimates which are particularly significant to the financial statements include estimates relating to the determination of impairment of assets, the useful life of property and equipment and the recoverability of advances.
| F-22 |
NOTE 3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Cash and cash equivalents
The Company considers short-term interest bearing investments with initial maturities of three months or less to be cash equivalents. The Company has no cash equivalents at September 30, 2016 and December 31, 2015.
Inventory
Inventory is stated at the lower of cost or net realizable value. Cost is determined using the Weighted Average method. The Company periodically evaluates its inventory for any obsolete or slow moving items based on production lot# and advances in production design or technology. Any inventory determined to be obsolete or slow moving is removed from inventory and disposed or a provision is made to reduce slow moving inventory to its net realizable value. At September 30, 2016 and December 31, 2015, the Company recorded a reserve for obsolescence of $7,750 and $7,750, respectively.
Property and equipment
Property and equipment consists of furniture and office equipment, and is stated at cost less accumulated depreciation. Depreciation is determined by using the 200% double declining method for equipment and the straight- line method for leasehold improvements, over the estimated useful lives of the related assets, generally five to fifteen years.
Expenditures for repairs and maintenance of equipment are charged to expense as incurred. Major replacements and betterments are capitalized and depreciated over the remaining useful lives of the related assets.
Intangible assets, net
The Company’s intangible assets consist of patent patented technology. Amortization is computed by applying the straight line method based on the remaining patent life. The primary patent expires in 2024.
Impairment of Long-Lived Assets
The Company evaluates long-lived assets for impairment, including property, plant and equipment and intangible assets, when events or changes in circumstances indicate that the carrying value of such assets may not be recoverable or the assets are being held for sale. Upon the occurrence of a triggering event, the asset is reviewed to assess whether the estimated undiscounted cash flows expected from the use of the asset plus the residual value from the ultimate disposal exceeds the carrying value of the asset. If the carrying value exceeds the estimated recoverable amounts, the asset is written down to the estimated fair value. Any resulting impairment loss is reflected on the Combined Statements of Operations.
| F-23 |
NOTE 3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Revenue recognition
The Company has yet to fully commercialize its products and secure appropriate permits and licenses necessary to begin full worldwide distribution. The Company has had negligible sales to date, primarily related to trial samples and evaluation of the efficacy and suitability of the products to specific target markets.
Sales and the related cost of sales are recognized primarily upon shipment of products (normally when title passes). The Company’s revenue recognition policies are in compliance with ASC Topic 605, which establishes criteria that must be satisfied before revenue is realized or realizable and earned.
The Company recognizes revenue when all of the following four criteria are met:
• persuasive evidence of a sales arrangement exists,
• delivery has occurred,
• the sales price is fixed or determinable and
• collectability is probable.
Income taxes
Hema Diagnostic Systems, LLC is a limited liability corporation. Rapid Medical Diagnostic Corp. is a Subchapter S corporation. Federal and state income tax regulations do not require a limited liability corporation or a Subchapter S corporation to pay income taxes. Rather each member’s allocable share of the profit or loss is reported in each member’s individual income tax return. Hema Diagnostics Systems Panama, PTY is a Panamanian company. Due to its operational losses, no taxes are required. Accordingly, no provision or liability for income taxes is reflected for this reporting entity in the accompanying financial statements. The Company’s 2012 – 2015 tax returns remain subject to examination by federal, state or foreign tax authorities.
Stock-Based payments
The Company accounts for transactions in which services are received in exchange for stock based on the fair value of such services received from non-employees, in accordance with ASC 505-50, "Equity Based Payments to Non-employees."
The Company follows ASC 718, "Compensation — Stock Compensation", in accounting for its stock based payments. This standard states that compensation cost or the value of stock issued for services are measured at the grant date based on the value of the stock granted and is recognized over the vesting or service period.
Risks and uncertainties
The Company’s business could be impacted by continuing price pressure on its product manufacturing, acceptance of its products in the market place, new competitors, changing federal and/or state legislation, new technologies and other factors. Adverse changes in these areas could negatively impact the Company’s financial position, results of operations and cash flows.
| F-24 |
NOTE 4. RECENTLY ISSUED ACCOUNTING STANDARDS AND DEVELOPMENTS
Accounting standards promulgated by the FASB are subject to change. Changes in such standards may have an impact on the Company’s future consolidated financial statements. The following are a summary of recent accounting developments.
In February 2015, the FASB issued ASU 2015-02, Consolidation (Topic 810)—Amendments to the Consolidation Analysis (“ASU 2015-02”), which provides guidance on evaluating whether a reporting entity should consolidate certain legal entities. Specifically, the amendments modify the evaluation of whether limited partnerships and similar legal entities are variable interest entities (“VIEs”) or voting interest entities. Further, the amendments eliminate the presumption that a general partner should consolidate a limited partnership, as well as affect the consolidation analysis of reporting entities that are involved with VIEs, particularly those that have fee arrangements and related party relationships. ASU 2015-02 is effective for interim and annual reporting periods beginning after December 15, 2016, with early adoption permitted. A reporting entity may apply the amendments using a modified retrospective approach or a full retrospective application. The Company is currently evaluating the impact, if any, that adopting ASU 2015-02 will have on its combined financial statements.
In February 2016, the FASB issued ASU 2016-02, Leases, which will amend current lease accounting to require lessees to recognize (i) a lease liability, which is a lessee’s obligation to make lease payments arising from a lease, measured on a discounted basis, and (ii) a right-of-use asset, which is an asset that represents the lessee’s right to use, or control the use of, a specified asset for the lease term. ASU 2016-02 does not significantly change lease accounting requirements applicable to lessors; however, certain changes were made to align, where necessary, lessor accounting with the lessee accounting model. This standard will be effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. We are currently reviewing the provisions of this ASU to determine if there will be any impact on our results of operations, cash flows or financial condition.
In March 2016, the FASB issued ASU 2016-09, Compensation – Stock Compensation: Improvements to Employee Share-Based Payment Accounting, which relates to the accounting for employee share-based payments. This standard addresses several aspects of the accounting for share-based payment award transactions, including: (a) income tax consequences; (b) classification of awards as either equity or liabilities; and (c) classification on the statement of cash flows. This standard will be effective for fiscal years beginning after December 15, 2016, including interim periods within those fiscal years. We are currently reviewing the provisions of this ASU to determine if there will be any impact on our results of operations, cash flows or financial condition.
There were various other accounting standards and interpretations issued in 2015 and 2016, none of which are expected to have a material impact on the Company’s financial position, operations or cash flows.
| F-25 |
NOTE 5. INVENTORY
Inventory at September 30, 2016 and December 31, 2015 consisted of the following:
| 2016 | 2015 | |||||||
| Production materials | 33,964 | 30,281 | ||||||
| Sub-assemblies | 4,031 | 5,001 | ||||||
| Finished goods | 3,279 | 1,771 | ||||||
| 41,274 | 37,053 | |||||||
| Less: Obsolescence reserve | (7,750 | ) | (7,750 | ) | ||||
| 33,524 | 29,303 |
Production materials - Consists of certain subcomponents fabricated by outside third parties as well as other components purchased in bulk quantities. The carrying value of these units reflects their direct acquisition cost along with associated transportation costs to the Company’s assembly and packaging facilities.
Sub-assemblies - Consists of units partially completed on the assembly line or by outside third parties. The carrying value of these units includes all direct material and labor costs involved to complete the associated assembly.
Finished goods - Consists of completed units in market ready packaging. The carrying value of these units include the cost of components, materials and labor necessary to assemble them.
Obsolescence reserve - Management evaluated the inventory at September 30, 2016 and December 31, 2015 and provided an allowance for obsolescence of $7,750 and $7,750, respectively, primarily associated with production materials and sub-assemblies. Management’s evaluation for obsolescence includes identifying materials and components that are not currently or likely to be used in production in the near future as well as tracking certain components by lot number and expiry date.
NOTE 6. ADVANCES and LOANS RECEIVABLE
Advances and Loans Receivable as of September 30, 2016 and December 31, 2015 consisted of the following:
| 2016 | 2015 | |||||||
| Loan to officer | 78,331 | 893,372 | ||||||
| Employee advances | 16,013 | 20,635 | ||||||
| Loans to employees | 18,784 | 14,167 | ||||||
| 113,128 | 928,174 | |||||||
| F-26 |
NOTE 6. ADVANCES and LOANS RECEIVABLE (continued)
Advance to officer - Consists of funds advanced to the Company’s President and CEO, board member and shareholder. During September 2016, the CEO repaid $897,000.
Advances to management - Consists of funds advanced to the Director of Latin American Sales, board member and shareholder. During October 2016, $14,735 was repaid.
Loans to employees - Consists of funds loaned primarily to one employee. That employee has arranged a payback schedule and commencing in August 2016 began making agreed payments of approximately $950 per month.
NOTE 7. PROPERTY AND EQUIPMENT
Property and equipment as of September 30, 2016 and December 31, 2015 consisted of the following:
| Estimated Useful Lives | 2016 | 2015 | ||||||||
| Computer Equipment & Software | 5 yrs 200DDB | 10,244 | 10,244 | |||||||
| Equipment | 5 yrs 200DDB | 47,148 | 47,147 | |||||||
| Furniture | 7 yrs 200DDB | 1,402 | 1,402 | |||||||
| Lab Equipment | 5 yrs 200DDB | 23,220 | 23,220 | |||||||
| Leasehold Improvements | 15 yrs SL | 40,445 | 40,445 | |||||||
| Office Equipment | 5 yrs 200DDB | 1,990 | 1,990 | |||||||
| Tools | 5 yrs 200DDB | 12,191 | 12,191 | |||||||
| Total | 136,640 | 136,640 | ||||||||
| Less Accumulated Depreciation | (130,000 | ) | (123,704 | ) | ||||||
| Property and Equipment, net | 6,640 | 12,936 | ||||||||
Depreciation expense for the nine months ended September 30, 2016 and 2015 totaled $6,296 and $6,910, respectively.
NOTE 8. OTHER ASSETS
Other assets as of September 30, 2016 and December 31, 2015 consisted of the following:
| 2016 | 2014 | |||||||
| Deposits | 8,362 | 8,362 | ||||||
| Patents | 51,274 | 51,274 | ||||||
| Less Amortization | (23,293 | ) | (21,371 | ) | ||||
| 36,343 | 38,265 | |||||||
| F-27 |
NOTE 8. OTHER ASSETS (continued)
Amortization expense for the nine months ended September 30, 2016 and 2015 totaled $1,922 and $1,922, respectively. The Company has several patents most of which are dormant as the Company lacks the funds to properly commercialize them. The current products and planned future products rely on the “Express” patent, the costs of which are reflected above. This patent expires in 2027.
NOTE 9. LOAN PAYABLE - SHAREHOLDER
The Company received substantially all of its funding from its primary shareholder, who owns 98.9% of the Company as of September 30, 2016. The Company borrowed no funds during the period from January 1, 2016 through September 30, 2016. However, the Company repaid $883,919 in September 2016. The loan is unsecured, payable on demand and earns interest for 2016 at 0.75%, which was increased from 0.21% for 2015. Accordingly, the Company recorded interest expense of $76,863 and $24,874 during the nine months ended September 30, 2016 and 2015, respectively..
NOTE 10. TRANSACTIONS with AFFILIATES
Transactions with affiliates as of September 30, 2016 and December 31, 2015 consisted of the following:
| 2016 | 2015 | |||||||
| Due from IDMS | (2,500 | ) | (2,500 | ) | ||||
| Other | (550 | ) | — | |||||
| Loan CWW | 83,544 | 83,554 | ||||||
| 50,504 | 81,054 | |||||||
China World Wide (CWW) - was established 25 years ago to distribute certain third party products that are not competing with HDS products. HDS is affiliated with CWW through common ownership. From inception through 2007, CWW provided supplemental funding to HDS on a non-interest basis. At December 31, 2015 HDS had an unpaid balance to CWW of $83,554. CWW has also assisted HDS in the development of its distribution and sourcing in China.
International Diagnostics and Medical Supply Corp. (IDMS) - was established 2 years ago to create hemodialysis facilities on a global scale. These services will not compete with HDS products. HDS is affiliated with IDMS through common ownership. Do to the high initial cost of establishing a hemodialysis facility, IDMS has not yet secured satisfactory funding to execute its business plan. Consequently, it has only engaged in minimal organizational activities. At September 30, 2016 HDS had an unpaid balance due from IDMS of $2,500 advanced to IDMS for legal fees incurred.
| F-28 |
NOTE 11. LICENSES and PATENTS
All patents and licenses are held by Rapid Medical Diagnostics Corporation, an affiliate company through common owners. HDS licenses the rights to certain technologies used in the development, manufacture and commercialization of its products from RMD. Agreements covering these license arrangements were entered into on January 1, 2005 for a term of five years and may be automatically renewed annually, unless either party gives notice 60 days prior to the renewal date. To date the agreements have been renewed annually and are currently in effect. These agreements provide for royalties between the parties, once a commercialized product is marketed and begins distribution.
NOTE 12. COMMITMENTS and CONTINGENCIES
Legal contingencies
From time to time, the Company may be a defendant in pending or threatened legal proceeding arising in the normal course of its business. Management is not aware of any pending, threatened or asserted claims.
Operating lease commitments
| 2016 | 2017 | 2018 | 2019 | 2020 and beyond | Total | |||||||||||||||||||
| HDS Office Lease | 20,938 | 41,876 | — | — | — | 62,814 | ||||||||||||||||||
| Auto Lease | 2,947 | 11,788 | 11,789 | 5,892 | 32,415 | |||||||||||||||||||
| Computer Equipment | ||||||||||||||||||||||||
| Lease #1 | 823 | 823 | 1,645 | |||||||||||||||||||||
| Lease #2 | 416 | 1,663 | 139 | 2,218 | ||||||||||||||||||||
| Office Copier | 1,469 | 4,406 | 4,406 | 4,406 | 4,682 | 19,369 | ||||||||||||||||||
| 26,592 | 60,556 | 16,333 | 10,298 | 4,682 | 118,461 | |||||||||||||||||||
HDS Office Lease – Business lease for 5,627 square feet of office and storage space located at 10102 USA Today Way, Miramar FL 33025. The lease term is from May 12, 2012 through June 30, 2017. Having previously been extended, the lease provides no further renewal option. The above payment commitments reflect base rent, estimated common are maintenance costs and applicable sales tax.
Auto Lease – Closed end vehicle lease which called for 39 monthly payments of $1,099 for our CEO and President which expires in April of 2016 was replaced with a similar closed end vehicle lease which calls for 36 monthly payments of $982 expires April 7, 2019. The above payment commitments reflect both vehicle leases.
| F-29 |
NOTE 12. COMMITMENTS and CONTINGENCIES (continued)
Computer Lease – Two leases were originated with the same supplier to provide office computer equipment. Lease #1 calls for 36 monthly payments of $274 commencing April 1, 2014 and expiring on March 31, 2017. Lease #2 calls for 36 monthly payments of $139 commencing February 19, 2015 and expiring on February 18, 2018.
Copier Lease – The lease was originated to provide office copier equipment. The lease calls for 60 monthly payments of $367 commencing June 3, 2016 and expiring on May 3, 2021.
NOTE 13. CUSTOMER and SUPPLIER CONCENTRATION
Customer concentration – The Company is currently in the process of commercializing its products and as of September 30, 2016 has not begun any substantial sales or marketing efforts. Accordingly, the Company has recorded only marginal sales to date, which were not of sufficient size to classify the customer as significant to the Company’s revenue. Those marginal sales primarily reflect instances where samples and trial products were shipped at a billable value.
Supplier concentration – As the Company is in the process of commercializing its products, the bulk of its purchasing activities are focused on regulatory, legal and consulting services rather than production or branding activities. As to production activities, alternate suppliers of components and raw materials are readily available should the need arise. Legal and consulting services generally relate to intellectual property, regulatory matters and financial consulting. There are a range of alternate consultants available to the Company should the need arise.
NOTE 14. SUBSEQUENT EVENTS
Advances from shareholder - During the period from October 1, 2016 through November 17, 2016, the Company has received an additional capital contribution of $192,500 from its principal shareholder. The Company repaid $14,735 of its outstanding loan balance due to its principal shareholder, bringing the total balance outstanding to $13,245,737. The loan bears interest at 0.75% during 2016 and is payable on demand.
Letter of intent to be acquired (LOI) - On August 26, 2016 the Company received a Letter of Intent (“LOI”) from Generex Biotechnology Corporation (“Generex”) to acquire the Company. Generex is a registrant under the Securities and Exchange Act of 1934. Its common stock is traded over the counter under the symbol “GNBT”. The terms of the agreement provide that:
| • | HDS will – |
| o | issue a dedicated class of first ranking voting preferred securities representing 51% of the outstanding equity of the Company to Generex |
| o | deliver Stephen Berkman’s full and final release of all indebtedness owed by HDS and its affiliates to Generex |
| F-30 |
NOTE 14. SUBSEQUENT EVENTS (continued)
| • | Generex will – |
| o | issue $250,000 worth of Generex restricted common stock plus 250,000 of restricted common shares to Stephen Berkman |
| o | also issue a warrant to acquire up to 15,000,000 shares of Generex common stock to Stephen Berkman |
| o | grant Stephen Berkman one seat on the Generex Board of Directors |
| o | If at any time during 36 months following the closing date, the aggregate value of the stock consideration is equal to or exceeds $15,000,000, be entitled to acquire 100% of the outstanding equity securities of HDS for $1.00. |
This LOI expires on November 30, 2016.
| F-31 |