UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark one)
| | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended
| | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ___________ to ______________.
Commission file number:

DIFFUSION PHARMACEUTICALS INC.
(Exact name of registrant as specified in its charter)
| | |
| (State of other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification Number) |
(Address of principal executive offices, including zip code)
(
(Registrant’s telephone number including area code)
| Title of Each Class | Trading Symbol(s) | Name of Each Exchange on Which Registered |
| | | |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ |
| | Smaller reporting company |
| Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act).Yes
The number of shares of common stock outstanding at November 8, 2021 was
DIFFUSION PHARMACEUTICALS INC.
FORM 10-Q
SEPTEMBER 30, 2021
INDEX
| Page |
|
| PART I – FINANCIAL INFORMATION |
1 |
| ITEM 1. FINANCIAL STATEMENTS |
1 |
| ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
14 |
| ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
22 |
| ITEM 4. CONTROLS AND PROCEDURES |
22 |
| PART II – OTHER INFORMATION |
23 |
| ITEM 1. LEGAL PROCEEDINGS |
23 |
| ITEM 1A. RISK FACTORS |
23 |
| ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS |
24 |
| ITEM 3. DEFAULTS UPON SENIOR SECURITIES |
24 |
| ITEM 4. MINE SAFETY DISCLOSURES |
24 |
| ITEM 5. OTHER INFORMATION |
24 |
| ITEM 6. EXHIBITS |
24 |
Note Regarding Company References and Other Defined Terms
Unless the context otherwise requires, in this Quarterly Report, (i) references to the “Company,” “we,” “our” or “us” refer to Diffusion Pharmaceuticals Inc. and its subsidiaries and (ii) references to “common stock” refer to the common stock, par value $0.001 per share, of the Company. We have also used several other defined terms in this Quarterly Report, many of which are explained or defined below:
| Term |
Definition |
| 2015 Equity Plan |
Diffusion Pharmaceuticals Inc. 2015 Equity Incentive Plan, as amended |
| 2017 Tax Act |
Tax Cuts and Jobs Act of 2017 |
| 401(k) Plan |
Diffusion Pharmaceuticals Inc. 401(k) Defined Contribution Plan |
| Altitude Trial |
our planned Phase 1b clinical trial evaluating the effects of TSC on V02 and PaO2 in normal healthy volunteers exposed to conditions that induce hypoxia |
| Annual Report |
our Annual Report on Form 10-K for the year ended December 31, 2020, filed with the SEC on March 16, 2021 |
| ASC |
Accounting Standard Codification of the FASB |
| ASUs |
Accounting Standards Updates of the FASB |
| Carlton Landlord | One Carlton, LLC |
| Carlton Lease | the Lease Agreement, dated March 31, 2017, related to our prior corporate headquarters located at 1317 Carlton Avenue in Charlotteville, Virginia |
| COVID Trial |
our Phase 1b clinical trial evaluating TSC in hospitalized COVID-19 patients, completed in February 2021 |
| COVID-19 |
Corona Virus Disease 2019, the novel coronavirus disease known as COVID-19, caused by SARS-CoV-2 infection |
| CMO |
contract manufacturing organization |
| CRO |
contract research organization |
| December 2019 Offering |
our registered direct public offering and sale of 6,266,787 shares of common stock and concurrent private placement of warrants to purchase up to 6,266,787 shares of common stock completed in December 2019 |
| DLCO |
diffusion capacity of lung for carbon monoxide |
| Exchange Act |
Securities Exchange Act of 1934, as amended |
| FASB |
Financial Accounting Standards Board |
| FDA |
U.S. Food and Drug Administration |
| February 2021 Offering |
our public offering and sale of 33,658,538 shares of common stock completed in February 2021 |
| G&A |
general and administrative |
| GAAP |
U.S. generally accepted accounting principles |
| GBM | glioblastoma multiforme brain cancer |
| Hypoxic Solid Tumor Program | our program of studies designed to evaluate the efficacy of TSC as a treatment for patients with hypoxic solid tumors |
| ILD-DLCO Trial |
our planned Phase 2a clinical trial evaluating the effects of TSC through the measure of DLCO through the lungs as a surrogate measure of oxygen transfer efficiency in patients with previously diagnosed interstitial lung disease who have a baseline DLCO test that is abnormal |
| IND |
investigational new drug application |
| January 2018 Offering |
our public offering and sale of 1,131,375 shares of common stock and warrants to purchase up to 1,131,375 shares of common stock completed in January 2018 |
| May 2019 Offering |
our registered direct public offering and sale of 1,317,060 shares of common stock and concurrent private placement of warrants to purchase up to 1,317,060 shares of common stock completed in May 2019 |
| May 2020 Investor Warrant Exercise |
the exercise of a previously outstanding warrant to purchase up to 5,000,000 shares of common stock at an exercise price of $0.35 per share in May 2020 pursuant to a warrant exercise agreement |
| May 2020 Offering |
our registered direct public offering and sale of 11,428,572 shares of common stock completed in May 2020 |
| Nasdaq |
Nasdaq Stock Market, LLC |
| NOL |
net operating loss |
| November 2019 Offering |
our public offering and sale of 5,104,429 shares of common stock, pre-funded warrants to purchase up to 6,324,143 shares of common stock, and warrants to purchase up to 22,857,144 shares of common stock completed in November 2019 |
| Oxygenation Trials |
collectively, the TCOM Trial, the Altitude Trial, and the ILD-DLCO Trial |
| PaO2 |
partial pressure of blood oxygen |
| Quarterly Report |
this Quarterly Report on Form 10-Q |
| R&D |
research and development |
| Regulation S-K |
Regulation S-K promulgated under the Securities Act |
| SEC |
U.S. Securities and Exchange Commission |
| Securities Act |
Securities Act of 1933, as amended |
| TCOM Trial |
our Phase 1b clinical trial evaluating the effects of TSC on peripheral tissue oxygenation in healthy normal volunteers using a TCOM device, completed in March 2021 |
| TCOM |
transcutaneous oxygen measurement |
| TSC |
trans sodium crocetinate |
| U.S. |
United States |
| VO2 |
maximal oxygen consumption |
Note Regarding Forward-Looking Statements
This Quarterly Report (including, for purposes of this Note Regarding Forward-Looking Statements, any information or documents incorporated herein by reference) includes express and implied forward-looking statements. By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics and industry change, and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward-looking statement contained in this Quarterly Report, we caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition, liquidity, and prospects may differ materially from the forward-looking statements contained in this Quarterly Report. In addition, even if our results of operations, financial condition, liquidity, and prospects are consistent with the forward-looking statements contained in this Quarterly Report, they may not be predictive of actual results or reflect unanticipated developments in future periods.
Forward-looking statements appear in a number of places throughout this Quarterly Report . We may, in some cases, use terms such as “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately,” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Forward-looking statements also include statements regarding our intentions, beliefs, projections, outlook, analyses or expectations concerning, among other things:
| • |
the success and timing of our clinical and preclinical studies, including our ability to enroll subjects in our ongoing and planned clinical studies at anticipated rates; |
| • |
our ability to obtain and maintain regulatory approval of our product candidates and, if approved, our products, including the labeling under any approval we may obtain; |
| • |
our plans and ability to develop and commercialize our product candidates and the outcomes of our research and development activities; |
| • |
the accuracy of our estimates of the size and characteristics of the potential markets for our product candidates, the rate and degree of market acceptance of any of our product candidates that may be approved in the future, and our ability to serve those markets; |
| • |
the success of products that are or may become available which also target the potential markets for our product candidates; |
| • |
obtaining and maintaining intellectual property protection for our product candidates and our proprietary technology; |
| • |
our ability to operate our business without infringing the intellectual property rights of others and the potential for others to infringe upon our intellectual property rights; |
| • |
our failure to recruit or retain key scientific or management personnel or to retain our executive officers; |
| • |
the performance of third parties, including contract research organizations, manufacturers, suppliers, and outside consultants, to whom we outsource certain operational, staff and other functions; |
| • |
our ability to obtain additional financing in the future and continue as a going concern; |
| • |
our estimates regarding expenses, future revenues, capital requirements, and needs for additional financing; |
| • |
regulatory developments in the U.S., E.U., and other foreign jurisdictions; |
| • |
recently enacted and future legislation related to the healthcare system, including trends towards managed care and healthcare cost containment, the impact of any significant spending reductions or cost controls affecting publicly funded or subsidized healthcare programs, or any replacement, repeal, modification, or invalidation of some or all of the provisions of the Affordable Care Act; |
| • |
any significant breakdown, infiltration, or interruption of our information technology systems and infrastructure; |
| • |
our ability to satisfy the continued listing requirements of the NASDAQ Capital Market or any other exchange on which our securities may trade in the future; |
| • |
uncertainties related to general economic, political, business, industry, and market conditions, including the ongoing COVID-19 pandemic; and |
| • |
other risks and uncertainties, including those discussed under the heading "Risk Factors" in this Annual Report and elsewhere in our other public filings. |
As a result of these and other factors, known and unknown, actual results could differ materially from our intentions, beliefs, projections, outlook, analyses, or expectations expressed in any forward-looking statements in this Quarterly Report. Accordingly, we cannot assure you that the forward-looking statements contained or incorporated by reference in this Quarterly Report will prove to be accurate or that any such inaccuracy will not be material. You should also understand that it is not possible to predict or identify all such factors, and you should not consider any such list to be a complete set of all potential risks or uncertainties. In light of the foregoing and the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. For all forward-looking statements, we claim the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995.
Any forward-looking statements that we make in this Quarterly Report speak only as of the date of such statement, and, except as required by applicable law or by the rules and regulations of the SEC, we undertake no obligation to update such statements to reflect events or circumstances after the date of this Quarterly Report or to reflect the occurrence of unanticipated events. Comparisons of current and any prior period results are not intended to express any ongoing or future trends or indications of future performance, unless explicitly expressed as such, and should only be viewed as historical data.
Note Regarding Trademarks, Trade Names and Service Marks
This Quarterly Report contains certain trademarks, trade names, and service marks of ours, including “DIFFUSIO2N.” All other trade names, trademarks, and service marks appearing in this Quarterly Report are, to the knowledge of Diffusion, the property of their respective owners. To the extent any such terms appear without the trade name, trademark, or service mark notice, such presentation is for convenience only and should not be construed as being used in a descriptive or generic sense.
PART I – FINANCIAL INFORMATION
| ITEM 1. |
FINANCIAL STATEMENTS |
Diffusion Pharmaceuticals Inc.
Consolidated Balance Sheets
(unaudited)
| September 30, 2021 | December 31, 2020 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | $ | ||||||
| Prepaid expenses, deposits and other current assets | ||||||||
| Total current assets | ||||||||
| Property and equipment, net | ||||||||
| Intangible asset | ||||||||
| Right of use asset | ||||||||
| Other assets | ||||||||
| Total assets | $ | $ | ||||||
| Liabilities and Stockholders’ Equity | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | $ | ||||||
| Accrued expenses and other current liabilities | ||||||||
| Current operating lease liability | ||||||||
| Total current liabilities | ||||||||
| Deferred income taxes | ||||||||
| Noncurrent operating lease liability | ||||||||
| Total liabilities | ||||||||
| Commitments and Contingencies (Note 7) | ||||||||
| Stockholders’ Equity: | ||||||||
| Common stock, $0.001 par value: | ||||||||
| Common stock, $ par value: shares authorized; and shares issued and outstanding at September 30, 2021 and December 31, 2020, respectively | ||||||||
| Additional paid-in capital | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Total stockholders' equity | ||||||||
| Total liabilities and stockholders' equity | $ | $ | ||||||
See accompanying notes to unaudited interim consolidated financial statements.
Diffusion Pharmaceuticals Inc.
Consolidated Statements of Operations
(unaudited)
| Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||
| 2021 | 2020 | 2021 | 2020 | |||||||||||||
| Operating expenses: | ||||||||||||||||
| Research and development | $ | $ | $ | $ | ||||||||||||
| Intangible asset impairment charge | ||||||||||||||||
| General and administrative | ||||||||||||||||
| Depreciation | ||||||||||||||||
| Loss from operations | ||||||||||||||||
| Other income: | ||||||||||||||||
| Interest income | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| Loss from operations before income tax benefit | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| Income tax benefit | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||
| Deemed dividend arising from warrant exchange | ( | ) | ||||||||||||||
| Net loss attributable to common stockholders | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||
| Per share information: | ||||||||||||||||
| Net loss per share of common stock, basic and diluted | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||
| Weighted average shares outstanding, basic and diluted | ||||||||||||||||
See accompanying notes to unaudited interim consolidated financial statements.
Diffusion Pharmaceuticals Inc.
Consolidated Statement of Changes in Stockholders' Equity
Three and Nine Months Ended September 30, 2020
(unaudited)
| Stockholders' Equity |
||||||||||||||||||||
| Common Stock |
Additional |
|
Total |
|||||||||||||||||
| Shares |
Amount |
Paid-in Capital |
Accumulated Deficit |
Stockholders' Equity |
||||||||||||||||
| Balance at July 1, 2020 |
$ | $ | $ | ( |
) | $ | ||||||||||||||
| Issuance of common stock upon exercise of warrants |
||||||||||||||||||||
| Stock-based compensation expense |
— | |||||||||||||||||||
| Net loss |
— | ( |
) | ( |
) | |||||||||||||||
| Balance at September 30, 2020 |
$ | $ | $ | ( |
) | $ | ||||||||||||||
| Stockholders' Equity |
||||||||||||||||||||
| Common Stock |
Additional |
|
Total |
|||||||||||||||||
| Shares |
Amount |
Paid-in Capital |
Accumulated Deficit |
Stockholders' Equity |
||||||||||||||||
| Balance at January 1, 2020 |
$ | $ | $ | ( |
) | $ | ||||||||||||||
| Sale of common stock and warrants, net of issuance costs |
||||||||||||||||||||
| Issuance of common stock upon exercise of warrants |
||||||||||||||||||||
| Stock-based compensation expense |
— | |||||||||||||||||||
| Net loss |
— | ( |
) | ( |
) | |||||||||||||||
| September 30, 2020 |
$ | $ | $ | ( |
) | $ | ||||||||||||||
See accompanying notes to unaudited interim consolidated financial statements.
Diffusion Pharmaceuticals Inc.
Consolidated Statement of Changes in Stockholders' Equity
Three and Nine Months Ended September 30, 2021
(unaudited)
| Stockholders' Equity |
||||||||||||||||||||
| Common Stock |
Additional |
|
Total |
|||||||||||||||||
| Shares |
Amount |
Paid-in Capital |
Accumulated Deficit |
Stockholders' Equity |
||||||||||||||||
| Balance at July 1, 2021 |
$ | $ | $ | ( |
) | $ | ||||||||||||||
| Stock-based compensation expense |
— | |||||||||||||||||||
| Net loss |
— | ( |
) | ( |
) | |||||||||||||||
| Balance at September 30, 2021 |
$ | $ | $ | ( |
) | $ | ||||||||||||||
| Stockholders' Equity |
||||||||||||||||||||
| Common Stock |
Additional |
|
Total |
|||||||||||||||||
| Shares |
Amount |
Paid-in Capital |
Accumulated Deficit |
Stockholders' Equity |
||||||||||||||||
| Balance at January 1, 2021 |
$ | $ | $ | ( |
) | $ | ||||||||||||||
| Sale of common stock and warrants, net of issuance costs |
||||||||||||||||||||
| Issuance of common stock upon exercise of warrants |
||||||||||||||||||||
| Stock-based compensation expense |
— | |||||||||||||||||||
| Net loss |
— | ( |
) | ( |
) | |||||||||||||||
| Balance at September 30, 2021 |
$ | $ | $ | ( |
) | $ | ||||||||||||||
See accompanying notes to unaudited interim consolidated financial statements.
Diffusion Pharmaceuticals Inc.
Consolidated Statements of Cash Flows
(unaudited)
| Nine Months Ended September 30, |
||||||||
| 2021 |
2020 |
|||||||
| Operating activities: |
||||||||
| Net loss |
$ | ( |
) | $ | ( |
) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Depreciation |
||||||||
| Stock-based compensation expense |
||||||||
| Deferred income taxes |
( |
) | ( |
) | ||||
| Intangible asset impairment charge | ||||||||
| Changes in operating assets and liabilities: |
||||||||
| Prepaid expenses, deposits and other assets |
( |
) | ( |
) | ||||
| Accounts payable, accrued expenses and other liabilities |
||||||||
| Net cash used in operating activities |
( |
) | ( |
) | ||||
| Cash flows provided by financing activities: |
||||||||
| Proceeds from the sale of common stock and warrants, net of issuance costs |
||||||||
| Proceeds from the exercise of common stock warrants |
||||||||
| Payment of financing costs |
( |
) | ||||||
| Net cash provided by financing activities |
||||||||
| Net increase in cash and cash equivalents |
||||||||
| Cash and cash equivalents at beginning of period |
||||||||
| Cash and cash equivalents at end of period |
$ | $ | ||||||
See accompanying notes to unaudited interim consolidated financial statements.
| 1. |
Organization and Description of Business |
Diffusion Pharmaceuticals Inc., a Delaware corporation, is a biopharmaceutical company developing novel therapies that enhance the body’s ability to deliver oxygen to the areas where it is needed most. The Company’s lead product candidate, TSC, is being developed to enhance the diffusion of oxygen to tissues with low oxygen levels, also known as hypoxia, a serious complication of many of medicine’s most intractable and difficult-to-treat conditions.
| 2. |
Liquidity |
The Company has not generated any revenues from product sales and has funded operations primarily from the proceeds of public and private offerings of equity, convertible debt and convertible preferred stock. Substantial additional financing will be required by the Company to continue to fund its research and development activities. No assurance can be given that any such financing will be available when needed, or at all, or that the Company’s research and development efforts will be successful.
The Company regularly explores alternative means of financing its operations and seeks funding through various sources, including public and private securities offerings, collaborative arrangements with third parties and other strategic alliances and business transactions. The Company does not have any commitments to obtain additional funds and may be unable to obtain sufficient funding in the future on acceptable terms, if at all. If the Company cannot obtain the necessary funding, it will need to delay, scale back or eliminate some or all of its research and development programs or enter into collaborations with third parties to commercialize potential products or technologies that it might otherwise seek to develop or commercialize independently; consider other various strategic alternatives, including a merger or sale of the Company; or cease operations. If the Company engages in collaborations, it may receive lower consideration upon commercialization of such products than if it had not entered such arrangements or if it entered into such arrangements at later stages in the product development process.
Operations of the Company are subject to certain risks and uncertainties including various internal and external factors that will affect whether and when the Company’s product candidates become approved drugs and how significant their market share will be, some of which are outside of the Company’s control. The length of time and cost of developing and commercializing these product candidates and/or failure of them at any stage of the drug approval process will materially affect the Company’s financial condition and future operations. The Company expects that its existing cash and cash equivalents as of September 30, 2021 will enable it to fund its operating expenses and capital expenditure requirements through 2023.
| 3. | Basis of Presentation and Summary of Significant Accounting Policies |
The Summary of Significant Accounting Policies included in the Company's Annual Report for the year ended December 31, 2020 have not materially changed.
Basis of Presentation
The accompanying unaudited interim consolidated financial statements of the Company have been prepared in accordance with GAAP for interim financial information as found in the ASC and ASUs of the FASB, and with the instructions to Form 10-Q and Article 10 of Regulation S-X promulgated by the SEC. In the opinion of management, the accompanying unaudited interim consolidated financial statements of the Company include all normal and recurring adjustments (which consist primarily of accruals, estimates and assumptions that impact the unaudited interim consolidated financial statements) considered necessary to present fairly the Company’s financial position as of September 30, 2021, results of operations for the three and nine months ended September 30, 2021 and 2020 and cash flows for the nine months ended September 30, 2021 and 2020. Operating results for the nine months ended September 30, 2021 are not necessarily indicative of the results that may be expected for the year ending December 31, 2021. The unaudited interim consolidated financial statements presented herein do not contain the required disclosures under GAAP for annual financial statements. The accompanying unaudited consolidated interim financial statements should be read in conjunction with the annual audited financial statements and related notes as of and for the year ended December 31, 2020 filed with the SEC as part of the Company's Annual Report on Form 10-K on March 16, 2021.
Use of Estimates
The preparation of unaudited interim consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of assets and liabilities at the date the financial statements and reported amounts of expense during the reporting period. The COVID-19 pandemic had no material impact on the Company's estimates and assumptions used in the preparation of the unaudited interim consolidated financial statements for the three and nine months ended September 30, 2021. However, the full extent to which the ongoing COVID-19 pandemic will directly or indirectly impact the Company's business, results of operations and financial condition, including sales, expenses, reserves and allowances, clinical trials, research and development costs and employee-related amounts, will depend on future developments that are highly uncertain, including as a result of new information that may emerge concerning COVID-19, governmental and business responses to the pandemic, further actions taken to contain or treat COVID-19, the ongoing economic impact on local, regional, national and international markets, and the speed of the anticipated economic recovery. Due to the uncertainty of factors surrounding these estimates or judgments, actual results may materially vary from the Company’s estimates. Estimates and assumptions are periodically reviewed, and the effects of revisions are reflected in the unaudited interim consolidated financial statements in the period they are determined. The Company’s future assessment of the magnitude and duration of COVID-19, as well as other factors, could result in material impacts to the Company’s consolidated financial statements in future reporting periods.
Fair Value of Financial Instruments
The carrying amounts of the Company’s financial instruments, including cash equivalents and accounts payable approximate fair value due to the short-term nature of those instruments.
Intangible Asset
In the third quarter of 2021, the Board of Directors decided to no longer dedicate financial resources to the Company's DFN-529 (formerly RES-529) intangible asset and any future internal development efforts were abandoned. In connection with this decision, the Company concluded that DFN-529 was impaired in its entirety and as such, the Company recognized a non-cash impairment charge of $
Net Loss Per Common Share
Basic net loss per share is computed by dividing net loss attributable to common stockholders by the weighted average number of shares of common stock outstanding during each period. Diluted net loss per share includes the effect, if any, from the potential exercise or conversion of securities, such as convertible debt, convertible preferred stock, common stock warrants, stock options and unvested restricted stock that would result in the issuance of incremental shares of common stock. In computing the basic and diluted net loss per share applicable to common stockholders, the weighted average number of shares remains the same for both calculations due to the fact that when a net loss exists, dilutive shares are not included in the calculation as the impact is anti-dilutive.
The following potentially dilutive securities outstanding have been excluded from the computation of diluted weighted average shares outstanding, as they would be anti-dilutive as of the dates indicated below:
| As of September 30, | ||||||||
| 2021 | 2020 | |||||||
| Common stock warrants | ||||||||
| Stock options | ||||||||
| Unvested restricted stock awards | ||||||||
Recently Adopted Accounting Pronouncements
In December 2019, the FASB issued ASU No. 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes. This guidance applies to all entities and aims to reduce the complexity of tax accounting standards while enhancing reporting disclosures. This guidance was effective for fiscal years beginning after December 15, 2020 and interim periods therein. The Company adopted ASU No. 2019-12 in the first quarter of 2021 and the adoption did not have a material impact on the Company's consolidated financial statements.
| 4. | Accrued Expenses and Other Current Liabilities |
Accrued expenses and other current liabilities consisted of the following as of the dates indicated below:
| September 30, 2021 | December 31, 2020 | |||||||
| Accrued payroll and payroll related expenses | ||||||||
| Accrued professional fees | ||||||||
| Accrued clinical studies expenses | ||||||||
| Other accrued expenses | ||||||||
| Total | $ | $ | ||||||
| 5. |
Stockholders' Equity and Common Stock Warrants |
February 2021 Common Stock Offering
In February 2021, the Company completed the February 2021 Offering in which it offered and sold
Common Stock Warrants
As of September 30, 2021, the Company had the following warrants outstanding to acquire shares of its common stock:
| Outstanding |
Range of exercise price per share |
Expiration dates |
|||||||||
| Common stock warrants issued in 2017 related to Series A convertible preferred stock offering |
$ |
March 2022 |
|||||||||
| Common stock warrants issued in 2018 related to the January 2018 Offering |
$ |
- | $ |
January 2023 |
|||||||
| Common stock warrants issued related to the May 2019 Offering |
$ |
- | $ |
May and December 2024 |
|||||||
| Common stock warrants issued related to the November 2019 Offering |
$ |
November 2024 |
|||||||||
| Common stock warrants issued related to the December 2019 Offering |
$ |
- | $ |
December 2024 and June 2025 |
|||||||
| Common stock warrants issued related to the May 2020 Offering |
$ |
May 2025 |
|||||||||
| Common stock warrants issued related to May 2020 Investor Warrant Exercise |
$ |
November 2025 |
|||||||||
| Common stock warrants issued related to the February 2021 Offering |
$ |
February 2026 |
|||||||||
During the nine months ended September 30, 2021,
| 6. | Stock-Based Compensation |
2015 Equity Plan
The 2015 Equity Plan provides for increases to the number of shares reserved for issuance thereunder each January 1 equal to
The Company recorded stock-based compensation expense in the following expense categories of its unaudited interim consolidated statements of operations for the periods indicated:
| Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||
| 2021 | 2020 | 2021 | 2020 | |||||||||||||
| Research and development | $ | $ | $ | $ | ||||||||||||
| General and administrative | ||||||||||||||||
| Total stock-based compensation expense | $ | $ | $ | $ | ||||||||||||
The following table summarizes the activity related to all stock option grants for the nine months ended September 30, 2021:
| Number of Options | Weighted average exercise price per share | Weighted average remaining contractual life (in years) | Aggregate intrinsic value | |||||||||||||
| Balance at January 1, 2021 | $ | |||||||||||||||
| Granted | ||||||||||||||||
| Forfeited | ( | ) | ||||||||||||||
| Outstanding at September 30, 2021 | $ | $ | ||||||||||||||
| Exercisable at September 30, 2021 | $ | $ | ||||||||||||||
| Vested and expected to vest at September 30, 2021 | $ | $ | ||||||||||||||
The weighted average grant date fair value of stock option awards granted during the nine months ended September 30, 2021 was $
Options granted were valued using the Black-Scholes option-pricing model and the weighted average assumptions used to value the options granted during the nine months ended September 30, 2021 and 2020 were as follows:
| 2021 | 2020 | |||||||
| Expected term (in years) | ||||||||
| Risk-free interest rate | % | % | ||||||
| Expected volatility | % | % | ||||||
| Dividend yield | % | % | ||||||
Restricted Stock Unit Awards
The Company issues restricted stocks ("RSU") to newly elected, non-executive members of the board of directors that vest in six, tri-monthly installments beginning
The following table summarizes activity related to RSU stock-based payment awards:
| Number of shares | Weighted-average grant date fair value | |||||||
| Unvested balance at January 1, 2021 | $ | |||||||
| Granted | $ | |||||||
| Unvested balance at September 30, 2021 | $ | |||||||
The Company recognized approximately $
| 7. | Commitments and Contingencies |
Office Space Rental
The Company has a non-cancelable operating lease for office and laboratory space in Charlottesville, Virginia, which began in April 2017, and as of September 30, 2021, has a remaining lease term of approximately
Rent expense related to the Company's operating leases for the three months ended September 30, 2021 and 2020 was approximately $
| Rental Commitments | ||||
| 2021 | $ | |||
| 2022 | ||||
| Total | ||||
| Less: imputed interest | ( | ) | ||
| $ | ||||
Research and Development Arrangements
In the course of normal business operations, the Company enters into agreements with universities and CROs to assist in the performance of research and development activities and contract manufacturers to assist with chemistry, manufacturing, and controls related expenses. Expenditures to CROs represent a significant cost in clinical development for the Company. The Company could also enter into additional collaborative research, contract research, manufacturing, and supplier agreements in the future, which may require upfront payments and long-term commitments of cash.
Defined Contribution Retirement Plan
The Company has established a 401(k) defined contribution plan that covers all employees who qualify under the terms of the plan. Eligible employees may elect to contribute to the 401(k) Plan up to
Legal Proceedings
On August 7, 2014, a complaint was filed in the Superior Court of Los Angeles County, California by Paul Feller, the former Chief Executive Officer of the Company’s legal predecessor under the caption Paul Feller v. RestorGenex Corporation, Pro Sports & Entertainment, Inc., ProElite, Inc. and Stratus Media Group, GmbH (Case No. BC553996). The complaint asserts various causes of action, including, among other things, promissory fraud, negligent misrepresentation, breach of contract, breach of employment agreement, breach of the covenant of good faith and fair dealing, violations of the California Labor Code and common counts. The plaintiff is seeking, among other things, compensatory damages in an undetermined amount, punitive damages, accrued interest and an award of attorneys’ fees and costs. On December 30, 2014, the Company filed a petition to compel arbitration and a motion to stay the action. On April 1, 2015, the plaintiff filed a petition in opposition to the Company’s petition to compel arbitration and a motion to stay the action. After a related hearing on April 14, 2015, the court granted the Company’s petition to compel arbitration and a motion to stay the action. On January 8, 2016, the plaintiff filed an arbitration demand with the American Arbitration Association. On November 19, 2018 at an Order to Show Cause Re Dismissal Hearing, the court found sufficient grounds not to dismiss the case and an arbitration hearing was scheduled, originally for November 2020 but later postponed due to the COVID-19 pandemic and related restrictions on gatherings in the State of California. In addition, following the November 2018 hearing, an automatic stay was placed on the arbitration in connection with the plaintiff filing for personal bankruptcy protection. On October 22, 2021, following a determination by the bankruptcy trustee not to pursue the claims and release them back to the plaintiff, the parties entered into a stipulation to abandon arbitration and return the matter to state court. A case management conference has been scheduled by the court for February 23, 2022 to set a trial date.
The Company believes the claims in this matter are without merit and intends to defend itself vigorously. However, at this stage, the Company is unable to predict the outcome and possible loss or range of loss, if any, associated with its resolution or any potential effect the matter may have on the Company’s financial position. Depending on the outcome or resolution of this matter, it could have a material effect on the Company’s financial position, results of operations and cash flows.
| 8. | Subsequent Events |
Lease Termination
On November 8, 2021, we entered into a Deed of Lease Termination Agreement with the Carlton Landlord providing for the early termination of the Carlton Lease related to our prior corporate headquarters. The Carlton Lease was previously scheduled to expire on April 30, 2022 and, as of September 30, 2021, our aggregate amount of future minimum rental payments thereunder was approximately $
In lieu of the fixed office and laboratory space previously available to us under the Carlton Lease, we have recently entered into short term agreements to utilize membership-based co-working space in both Charlottesville, Virginia and Philadelphia, Pennsylvania.
Nasdaq Bid Price Deficiency Notice
As previously disclosed, on May 6, 2021, the Company received a written notice from the staff of the Listing Qualifications Department of Nasdaq relating to the minimum bid price requirement contained in Nasdaq Listing Rule 5550(a)(2). This notice indicated that the Company was not in compliance with such rule because the bid price for the Company’s common stock had closed below $1.00 per share for the previous 30 consecutive business days. In accordance with Nasdaq Listing Rule 5810(c)(3)(A), the Company was provided 180 calendar days, or until November 2, 2021, to regain compliance with the Rule.
On November 3, 2021, the Company received an additional notice from the staff providing that, although the Company had not regained compliance with the bid price rule by November 2, 2021, in accordance with Nasdaq Listing Rule 5810(c)(3)(A), the Staff has determined that the Company is eligible for an additional 180 calendar days from the date of such notice, or until May 2, 2022, to regain compliance. To regain compliance, the bid price for the Company’s common stock must close at $1.00 per share or more for a minimum of 10 consecutive business days.
The Notice has no effect on the listing or trading of the Company’s common stock at this time, and the Company is currently evaluating its alternatives to resolve this listing deficiency, including, if necessary and subject to the approval of its board of directors and stockholders, implementing a reverse stock split.
| ITEM 2. |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
You should read the following discussion of our financial condition and results of operations together with the unaudited interim consolidated financial statements and the notes thereto included elsewhere in this report and other financial information included in this report. The following discussion may contain predictions, estimates and other forward-looking statements that involve a number of risks and uncertainties, including those discussed under “Part I — Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations—Special Note Regarding Forward-Looking Statements” in this report and under “Part I — Item 1A. Risk Factors” in our Annual Report and under “Part II—Other Information – Item 1A. Risk Factors” in our Quarterly Reports on Form 10-Q for the quarterly periods ended March 31, 2021 and June 30, 2021. These risks could cause our actual results to differ materially from any future performance suggested below.
Diffusion Pharmaceuticals: Enhancing Oxygen, Fueling Life
We are a biopharmaceutical company developing novel therapies that enhance the body’s ability to deliver oxygen to the areas where it is needed most. Our lead product candidate, TSC, is being developed to enhance the diffusion of oxygen to tissues with low oxygen levels, also known as hypoxia, a serious complication of many of medicine’s most intractable and difficult-to-treat conditions.
TSC Development Update
Our Lead Indication: Using TSC to Treat Hypoxic Solid Tumors
Solid tumors comprise approximately 90% of all adult cancers and, according to the American Cancer Society, roughly 1.9 million new cancer cases will be diagnosed in the U.S. during 2021. It is also well-documented in the scientific literature that the prevalence of hypoxic regions across numerous primary solid tumor types directly contributes to treatment resistance whether it be radiotherapy, chemotherapy, and immunotherapy, increasing the metastatic potential of these tumors and the probability of unsuccessful treatment. We believe TSC’s novel mechanism of action lends itself to treating this unmet medical need, so we are designing our Hypoxic Solid Tumor Program to support the use of TSC as a treatment of hypoxic solid tumors.
This program is being designed based on previously obtained data that we believe support the potential benefits of TSC as a treatment for hypoxic solid tumors, including preclinical evidence supporting TSC’s enhancement of radiotherapy and clinical evidence of TSC's effects in unresectable glioblastoma multiforme tumors from our previous Phase 2 study evaluating TSC in patients with GBM treated with standard of care radiation and chemotherapy. Additionally, with the outcome of the TCOM Trial, originally reported in June 2021, we believe we now have direct evidence of TSC's ability to enhance oxygenation in humans, including additional dosing information that will be integral to designing a clinical study to treat hypoxic solid tumors.
Our plan is to use the information obtained to date on TSC to submit a briefing document related to our development plan for discussion with the FDA during the first quarter of 2022. In addition to questions about clinical trial design and chemistry, manufacturing, and controls, we anticipate the submission will include the information required to obtain FDA feedback on potential accelerated pathways to approval for TSC. In addition, the results of the Altitude Trial and ILD-DLCO Trial, which we anticipate reporting during the first half of 2022 as further described below under the heading, “Our Oxygenation Trials,” will also be used to further guide the design of the program. We believe receiving FDA feedback on the proposed regulatory pathway underlying our Hypoxic Solid Tumor Program will enhance the probability of successfully developing and, if approved, commercializing TSC for use as a treatment for hypoxic solid tumors.
Accordingly, the start of the first study in our Hypoxic Solid Tumor Program, which we currently expect to be a Phase 2 clinical trial, will be subject to the timing of FDA feedback and the availability of clinical drug supply.
Our Oxygenation Trials
TCOM Trial: TSC's Effects on Peripheral Tissue Oxygenation
In June 2021, we reported a positive trend in oxygenation from our TCOM Trial, which evaluated the effect of TSC versus placebo on peripheral tissue oxygenation in healthy normal volunteers using transcutaneous oxygen monitoring. Analysis of the primary endpoint data indicated a positive dose-response trend in TCOM readings with TSC as compared to placebo that persisted through the measurement period, and the trends observed in the primary endpoint data indicated an improvement in peripheral oxygenation with TSC with no evidence of hyperoxygenation.
In September 2021, we issued a letter to our stockholders including a further discussion of the results of the TCOM Trial, as well as certain additional facts and figures from our supplemental analysis of the study data.
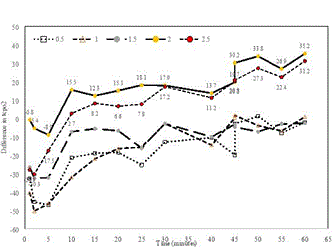
Figure 1. Effects of TSC on transcutaneous oxygen pressure (tcpO2). The graph was created by subtracting the median placebo response from the dose and time matched median TSC response.
For example, the chart above was created by subtracting the median response observed in the TCOM Trial's placebo group from the median response observed in each TSC dosage group at each of the measurement times during the one-hour period following dosing. As you can see in the chart, these data indicate peripheral tissue oxygenation increased relative to the placebo group following TSC administration and persisted through the one-hour measurement period, particularly at the two highest doses tested (2.0 mg/kg and 2.5 mg/kg).
We believe the results of the TCOM Trial provide clinical evidence that TSC facilitates the passive diffusion of oxygen from areas of high concentration to areas of low concentration without causing hyperoxygenation and, accordingly, represent a positive and meaningful step towards the accomplishment of the overall strategic objectives of our Oxygenation Trials set forth in November 2020. Moreover, the doses at which the largest effects of TSC were observed in the TCOM study are higher than the doses tested in any of the recent clinical trials of TSC, and these data obtained from the TCOM Trial is being used to guide the design of our remaining Oxygenation Trials – the Altitude Trial and the ILD-DLCO Trial.
We believe the two remaining Oxygenation Trials, the Altitude Trial and the ILD-DLCO Trial, remain integral to our overall development plan for TSC, as they are intended to provide further information regarding TSC’s mechanism of action and dose response characteristics, as well as support for what we view as the broad therapeutic potential of TSC to treat a variety of conditions complicated by hypoxia. As described below, the Altitude Trial is designed to provide specific information on TSC’s effects on oxygen consumption and the ILD-DLCO Trial is designed to evaluate the effects of TSC on the uptake of oxygen through the lungs. Accordingly, we intend to continue to execute the Altitude Trial and the ILD-DLCO Trial to develop this additional data in parallel with designing our Hypoxic Solid Tumor Program.
Altitude Trial: TSC’s Effects Under Induced Hypoxic Conditions
This trial will be a double-blind, randomized, placebo-controlled study which will evaluate the effects of TSC on maximal oxygen consumption, or VO2, and partial pressure of blood oxygen, or PaO2, in normal healthy volunteers subjected to incremental levels of physical exertion while exposed to hypoxic and hypobaric conditions (i.e., simulated altitude). The trial will evaluate the difference in effect of TSC on oxygen availability and consumption compared to placebo. We expect to dose the first patient in the Altitude Trial in the coming days, to complete dosing of patients in the study in late December 2021 or early January 2022, and anticipate reporting topline results from the study within two months of completion.
ILD-DLCO Trial: TSC's Effects on Oxygen Transfer Efficiency
This trial will be a double-blind, randomized, placebo-controlled study which will evaluate the effects of TSC on the diffusion of carbon monoxide through the lungs, or DLCO, in patients with previously diagnosed interstitial lung disease who have an abnormal baseline DLCO test result. DLCO will act as a surrogate measure of oxygen transfer efficiency, or uptake, from the alveoli of the lungs, through the plasma, and onto hemoglobin within red blood cells. The study will evaluate the difference in effect of TSC on improvement in DLCO, as well as distance covered in a standard six-minute walk test. We anticipate initiating the ILD-DLCO Trial in the late fourth quarter of 2021 and completing the study in the first quarter of 2022, with topline results reported within two months of study completion.
Further, during the third quarter of 2021, we received clearance of our IND for TSC from the Pulmonary, Allergy, and Critical Care division of the FDA, an important step as we prepare to initiate the ILD-DLCO Trial. This also represents the fourth division with which we have an open IND for TSC – joining the Cardiology and Nephrology, Neurology, and Oncology divisions – which we believe underscores TSC’s broad therapeutic potential to treat a variety of conditions complicated by hypoxia.
The information we expect to obtain from the Altitude Trial and ILD-DLCO Trial during the first half of 2022 — particularly when combined with the information we have acquired over the last year from the TCOM Trial and our COVID-19 Trial — will continue to refine and enhance our understanding of TSC’s mechanism of action, dose-response effects, and, more specifically, effects on oxygen consumption and uptake of oxygen through the lungs. In addition to providing insights regarding additional, non-oncology indications in which the TSC platform may have potential, the results of the Altitude Trial and ILD-DLCO Trial will also inform our design of our Hypoxic Solid Tumor Program studies.
TSC Supply Chain Update
As broadly reported by companies across industrial sectors, supply chain disruptions, shortages, and other issues have been widespread during 2021, and we have also recently been subject to delays and interruptions in our TSC drug supply. As previously reported in our Annual Report , TSC is currently manufactured by our primary CMO partner at a facility that now manufactures COVID-19 vaccines under the Defense Production Act. In response these delays and interruptions, we are in the process of evaluating opportunities to increase the robustness of our drug supply and overall supply chain to be more responsive to our needs.
We are actively managing this process and believe we are taking the necessary steps to ensure the availability of high-quality drug supply to support the next steps in TSC’s development. However, TSC is a freeze-dried, injectable product requiring a sophisticated manufacturing process. As a result, any change to all or a portion of our manufacturing process, together with uncertainty regarding the general availability of manufacturing materials as a result of more general global supply chain shortages and disruptions, could affect the cost and timing of the available clinical study drug supply of TSC.
Organizational Update
Enhancing Our Organization, Embracing Remote Work
During the third quarter, we continued to enhance our operating team with the addition of new employees in a variety of functions across our organization, including clinical operations, CMC, finance, and, more recently, medical writing. As part of our growth strategy, we have embraced the remote work culture that became prevalent at many organizations during the COVID-19 pandemic. We believe our people are critical to our ability to efficiently and successfully execute our stated strategy, and this remote operating paradigm allows us to enhance our available pool of talented human resources while also conserving financial resources. For example, in November 2021, we negotiated and entered into an agreement providing for the early termination of our lease of the Carlton Avenue facility in Charlottesville, Virginia. We also believe in the power of collaboration. In turn, and in lieu of the fixed office and laboratory space previously available to us under that agreement, we have recently entered into short term agreements to utilize membership-based co-working space in both Charlottesville, Virginia and Philadelphia, Pennsylvania. We believe these arrangements will permit our team combine the advantages of remote and in-person work in environments intended to foster creativity and innovation.
Financial Summary
As of September 30, 2021, we had cash and cash equivalents of $40.3 million. We have incurred operating losses since inception, have not generated any product revenue and have not achieved profitable operations. We incurred net losses of $12.2 million and $20.6 million for the three and nine months ended September 30, 2021, respectively. Our accumulated deficit as of September 30, 2021 was $126.5 million, and we expect to continue to incur substantial losses in future periods. We anticipate that our operating expenses will increase substantially as we continue to advance the development of TSC, including any costs related to:
| • |
our ongoing and planned clinical trials, including the remaining Oxygenation Trials and our Hypoxic Solid Tumor Program; |
| • |
any additional studies we may undertake, including other preclinical and clinical studies to support the filing of any NDA with the FDA; |
| • | near-term investments intended to improve the quality and robustness of our supplier relationships and overall supply chain; |
| • |
other research, development, and manufacturing activities designed to develop and optimize formulation, manufacturing processes, dosage, dose forms, and other characteristics prior to regulatory approval; |
| • |
the maintenance, expansion, and protection our global intellectual property portfolio; |
| • |
the hiring of additional clinical, manufacturing, scientific, sales, or other personnel; |
| • | research and development related to other product candidates that may be complementary or synergistic with TSC, including DFN-529 or any other product candidates we may acquire or in-license in the future; and |
| • |
investments in operational, financial, and management information systems. |
We intend to use our existing cash and cash equivalents for working capital and to fund the research and development of TSC. We expect that our cash and cash equivalents as of September 30, 2021 will enable us to fund our operating expenses and capital expenditure requirements through 2023.
Financial Operations Overview
Revenues
We have not yet generated any revenue from product sales. We do not expect to generate revenue from product sales for the foreseeable future.
Research and Development Expense
R&D expenses include, but are not limited to, third-party CRO arrangements and employee-related expenses, including salaries, benefits, stock-based compensation and travel expense reimbursement. R&D activities are central to our business model. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical studies. As we advance our product candidates, we expect the amount of R&D costs will continue to increase for the foreseeable future. R&D costs are charged to expense as incurred.
Intangible Asset Impairment Charge
In the third quarter of 2021, the Company made a determination to no longer dedicate resources to the Company’s DFN-529 intangible asset and any future development efforts were abandoned. In connection with this decision, the Company concluded that DFN-529 was impaired in its entirety.
General and Administrative Expense
G&A expenses consist principally of salaries and related costs for executive and other personnel, including stock-based compensation, other employee benefit costs, expenses associated with investment bank and other financial advisory services, and travel expenses. Other G&A expenses include, facility-related costs, communication expenses and professional fees for legal, patent prosecution and maintenance, consulting, accounting, and other professional services.
Interest Income
Interest income is interest earned from our cash and cash equivalents.
Income Tax Benefit
The Company recorded an income tax benefit of $0.4 million during the three and nine months ended September 30, 2021, respectively. The income tax benefit was due to the tax effect of the reduction in the deferred tax liability associated with the basis difference from the IPR&D indefinite lived intangible asset. The Company maintains a full valuation allowance against its deferred tax assets due to the Company’s history of losses as of September 30, 2021.
Our NOLs and tax credit carryforwards are subject to review and possible adjustment by the Internal Revenue Service and state tax authorities. NOL and tax credit carryforwards may become subject to an annual limitation in the event of a greater than 50.0% cumulative change in the ownership interest of significant stockholders over a three year period, as defined under Sections 382 and 383 of the Internal Revenue Code as well as similar state provisions. The amount of the annual limitation is determined based on the Company’s value immediately prior to the ownership change, and subsequent ownership changes may further affect the limitation in future years. In 2019, due to the significant changes to our stockholder base as a result of the equity financing we completed during that year, we performed an analysis under Section 382 of the Internal Revenue Code and, as a result, reduced the magnitude of our NOL carryforwards to account for the ownership changes. In addition, the cumulative benefit of our NOLs was remeasured, resulting in tax expense recognized during the year ended December 31, 2019. We have not yet performed an analysis to determine whether or not ownership changes that have occurred in the year ended December 31, 2020 or during the three or nine months ended September 30, 2021 give rise to any further limitations.
Results of Operations for Three Months Ended September 30, 2021 Compared to Three Months Ended September 30, 2020
The following table sets forth our results of operations for the three months ended September 30, 2021 and 2020.
| Three Months Ended September 30, |
||||||||||||
| 2021 |
2020 |
Change |
||||||||||
| Operating expenses: |
||||||||||||
| Research and development |
$ | 2,105,815 | $ | 3,137,553 | $ | (1,031,738 | ) | |||||
| Intangible asset impairment charge | 8,639,000 | — | 8,639,000 | |||||||||
| General and administrative |
1,930,082 | 2,112,375 | (182,293 | ) | ||||||||
| Depreciation |
19,100 | 24,192 | (5,092 | ) | ||||||||
| Loss from operations |
12,693,997 | 5,274,120 | 7,419,877 | |||||||||
| Other income: |
||||||||||||
| Interest income |
(50,710 | ) | (29,233 | ) | (21,477 | ) | ||||||
| Loss from operations before income tax benefit |
(12,643,287 | ) | (5,244,887 | ) | (7,398,400 | ) | ||||||
| Income tax benefit |
(443,893 | ) | (805,676 | ) | 361,783 | |||||||
| Net loss |
$ | (12,199,394 | ) | $ | (4,439,211 | ) | $ | (7,760,183 | ) | |||
We recognized $2.1 million in R&D expenses during the three months ended September 30, 2021 compared to $3.1 million during the three months ended September 30, 2020. This decrease was due to lower project spending due to the completion and/or wind-down of our clinical studies evaluating TSC in Covid-19, GBM, and stroke.
We recognized a non-recurring $8.6 million non-cash impairment charge related to the write down of our DFN-529 IPR&D asset.
G&A expenses were $1.9 million during the three months ended September 30, 2021 compared to $2.1 million during the three months ended September 30, 2020. The decrease in G&A expenses was primarily due to a $0.5 million decrease in salaries related to executive separation payments recorded in the prior year period as well as a $0.2 million decrease in outside legal and professional fees. This decrease was partially offset by an increase in salaries and other costs associated with the hiring of new employees to support our R&D efforts as well as an increase in insurance costs.
We recognized an income tax benefit of $0.4 million due to the tax effect of the reduction in the deferred tax liability associated with the basis differences from the DFN-529 IPR&D intangible asset that was written down in the third quarter of 2021. We recognized an income tax benefit of $0.8 million during the three months ended September 30, 2020 to reflect the utilization of indefinite deferred tax liabilities as a source of income against indefinite lived portions of our deferred tax assets. Prior to 2021, we recognized the full income tax benefit allowed by the 2017 Tax Act to utilize indefinite deferred tax liabilities as a source of income against indefinite lived portions of our deferred tax assets.
Results of Operations for Nine Months Ended September 30, 2021 Compared to Nine Months Ended September 30, 2020
The following table sets forth our results of operations for the nine months ended September 30, 2021 and 2020.
| Nine Months Ended September 30, |
||||||||||||
| 2021 |
2020 |
Change |
||||||||||
| Operating expenses: |
||||||||||||
| Research and development |
$ | 6,994,866 | $ | 6,845,203 | $ | 149,663 | ||||||
| Intangible asset impairment charge | 8,639,000 | — | 8,639,000 | |||||||||
| General and administrative |
5,510,365 | 4,964,440 | 545,925 | |||||||||
| Depreciation |
67,302 | 78,233 | (10,931 | ) | ||||||||
| Loss from operations |
21,211,533 | 11,887,876 | 9,323,657 | |||||||||
| Other income: |
||||||||||||
| Interest income |
(146,354 | ) | (89,246 | ) | (57,108 | ) | ||||||
| Loss from operations before income tax benefit |
(21,065,179 | ) | (11,798,630 | ) | (9,266,549 | ) | ||||||
| Income tax benefit |
(443,893 | ) | (1,675,381 | ) | 1,231,488 | |||||||
| Net loss |
$ | (20,621,286 | ) | $ | (10,123,249 | ) | $ | (10,498,037 | ) | |||
We recognized $7.0 million in R&D expenses during the nine months ended September 30, 2021 compared to $6.8 million during the nine months ended September 30, 2020. This increase was primarily attributable to a $1.2 million increase in expense related to drug manufacturing as described in part above under the heading “TSC Supply Chain Update.” and a $0.6 million increase in salary and related expenses due to increases in headcount. These increases were offset by lower project spending due to the completion and/or wind-down of our clinical studies evaluating TSC in Covid-19, GBM, and stroke.
We recognized a nonrecurring $8.6 million non-cash impairment charge related to the write down of our DFN-529 IPR&D asset.
G&A expenses were $5.5 million during the nine months ended September 30, 2021 compared to $5.0 million during the nine months ended September 30, 2020. The increase in G&A expense was primarily due to a $1.0 million increase in salaries and other costs associated with the hiring of new employees. These amounts were slightly offset by $0.5 million in salaries related to executive separation payments recorded in the prior year period.
We recognized an income tax benefit of $0.4 million due to the tax effect of the reduction in the deferred tax liability associated with the basis differences from the DFN 529 IPR&D intangible asset that was written down in the third quarter of 2021. We recognized an income tax benefit of $1.7 million during the nine months ended 2020 to reflect the utilization of indefinite deferred tax liabilities as a source of income against indefinite lived portions of our deferred tax assets. Prior to 2021, we recognized the full income tax benefit allowed by the 2017 Tax Act to utilize indefinite deferred tax liabilities as a source of income against indefinite lived portions of our deferred tax assets.
Liquidity and Capital Resources
Working Capital
To date, we have funded our operations primarily through the sale and issuance of preferred stock, common stock and convertible promissory notes. As of September 30, 2021, we had $40.3 million in cash and cash equivalents, working capital of $38.0 million and an accumulated deficit of $126.5 million. We expect to continue to incur net losses for the foreseeable future. We intend to use our existing cash and cash equivalents to fund our working capital and research and development of our product candidates.
Cash Flows
The following table sets forth our cash flows for the nine months ended September 30, 2021 and 2020:
| Nine Months Ended September 30, |
||||||||
| Net cash (used in) provided by: |
2021 |
2020 |
||||||
| Operating activities |
$ | (11,476,302 | ) | $ | (10,158,041 | ) | ||
| Financing activities |
33,295,752 | 17,890,875 | ||||||
| Net increase in cash and cash equivalents |
$ | 21,819,450 | $ | 7,732,834 | ||||
Operating Activities
Net cash used in operating activities of $11.5 million during the nine months ended September 30, 2021 was primarily attributable to our net loss of $20.6 million and a change in our deferred income taxes of $0.4 million. These amounts were partially offset by a $8.6 million non cash impairment charge in connection with the write down of our DFN-529 IPR&D asset, $0.6 million in stock-based compensation expense, and a net change in our operating assets and liabilities of $0.2 million.
Net cash used in operating activities of $10.2 million during the nine months ended September 30, 2020 was primarily attributable to our net loss of $10.1 million and our change in deferred income taxes of $1.7 million. These amounts were partially offset by a net change in operating assets and liabilities of $1.4 million, $0.6 million in stock-based compensation expense, and $0.1 million in depreciation expense. The net change in our operating assets and liabilities was primarily attributable to an increase in accounts payable and accrued expenses and was slightly offset by an increase in our prepaid expenses, deposits and other current assets.
Financing Activities
Net cash provided by financing activities was $33.3 million during the nine months ended September 30, 2021, which was attributable to net proceeds of $31.1 million received from the sale of our common stock in connection with the February 2021 Offering and $2.2 million in proceeds received from the exercise of previously issued common stock warrants.
Net cash provided by financing activities of $17.9 million during the nine months ended September 30, 2020, was primarily attributable to $10.8 million in gross proceeds received in connection with the May 2020 Offering and $8.0 million in gross proceeds received in connection with the exercise of previously outstanding warrants to purchase common stock. These cash inflows were offset in part by the payment of $1.0 million in financing costs.
Capital Requirements
We expect to continue to incur substantial expenses and generate significant operating losses as we continue to pursue our business strategy of developing TSC. Our operations have consumed substantial amounts of cash since inception and we expect to continue to spend substantial amounts of cash to advance the clinical development of the TSC platform, as well as associated investments into our manufacturing, regulatory, and other related capabilities. As of the date of this Quarterly Report, most our cash resources for clinical development are dedicated to our remaining Oxygenation Trials and our Hypoxic Solid Tumor Program, near-term investments intended to improve the quality and robustness of our supplier relationships and overall supply chain, and general operating expenses. While we believe we have adequate cash resources to continue operations through 2023, we anticipate that we will need additional funding in order to complete development of TSC which, if available, could be obtained through additional capital raising transactions, entry into strategic partnerships or collaborations, or alternative financing arrangements.
As of September 30, 2021, we did not have any credit facilities in place under which we could borrow funds or any other sources of committed capital. In the future, we may seek to raise additional funds through various sources. However, we can give no assurances that we will be able to secure additional sources of funds to support our operations, or if such funds are available to us, that such additional financing will be sufficient to meet our needs or be on terms acceptable to us. This risk may increase if economic and market conditions deteriorate, including as a result of unanticipated market shocks, such as the COVID-19 pandemic and ongoing global supply chain disruptions, or inflationary pressure. If we are unable to obtain additional financing when needed, we may need to terminate, significantly modify, or delay the development of TSC or our product candidates, or we may need to obtain funds through collaborations or otherwise on terms that may require us to relinquish rights to our technologies or product candidates that we might otherwise seek to develop or commercialize independently. If we are unable to raise adequate additional capital as and when required in the future, we could be forced to cease development activities and terminate our operations, and you could experience a complete loss of your investment.
To the extent that we raise additional capital in the future through the sale of our common stock or securities convertible or exchangeable for common stock such as common stock warrants, convertible preferred stock, or convertible debt instruments, the interests of our current stockholders may be diluted or otherwise impacted. In particular, specific rights granted to future holders of preferred stock or convertible debt securities may include voting rights, preferences as to dividends and liquidation, conversion and redemption rights, sinking fund provisions, and restrictions on our ability to merge with or sell our assets to a third party. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures, or declaring dividends.
Critical Accounting Policies
The Critical Accounting Policies included in our Form 10-K for the year ended December 31, 2020, filed with the SEC pursuant to Section 13 or 15(d) under the Securities Act on March 16, 2021 have not changed.
| ITEM 3. |
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
As a “smaller reporting company” as defined by Item 10 of Regulation S-K, promulgated by the SEC under the U.S. Securities Act of 1933, as amended, we are not required to provide the information required by this Item 3.
| ITEM 4. |
CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) that are designed to provide reasonable assurance that information required to be disclosed by us in the reports we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms and that such information is accumulated and communicated to our management, including our principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure. In designing and evaluating our disclosure controls and procedures, we recognize that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and we are required to apply our judgment in evaluating the cost-benefit relationship of possible internal controls. Our management evaluated, with the participation of our principal executive officer and principal financial officer, the effectiveness of the design and operation of our disclosure controls and procedures as of the end of the period covered in this report. Based on that evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective as of the end of such period to provide reasonable assurance that information required to be disclosed in our Exchange Act reports is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding disclosure.
Change in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting (as such term is defined in Exchange Act Rule 13a-15(f) that occurred during the quarter ended September 30, 2021 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
PART II – OTHER INFORMATION
| ITEM 1. |
LEGAL PROCEEDINGS |
Please refer to Note 7, Commitments and Contingencies in the notes accompanying the unaudited interim consolidated financial statements included in Part I, Item 1 of this Quarterly Report, which is incorporated herein by reference.
| ITEM 1A. |
RISK FACTORS |
As of the date of this Quarterly Report, there have been no material changes to our risk factors previously disclosed in our Annual Report and our subsequent quarterly reports on Form 10-Q, other than as set forth below.
If we cannot regain compliance with the Nasdaq Capital Market continued listing standards , our common stock could be delisted, which would harm our business, the trading price of our common stock, our ability to raise additional capital and the liquidity of the market for our common stock.
Our common stock is currently listed on the Nasdaq Capital Market. To maintain the listing of our common stock on the Nasdaq Capital Market, we are required to meet certain listing requirements, including, among others, either: (i) a minimum closing bid price of $1.00 per share, a market value of publicly held shares (excluding shares held by our executive officers, directors and 10% or more stockholders) of at least $1 million and stockholders’ equity of at least $2.5 million; or (ii) a minimum closing bid price of $1.00 per share, a market value of publicly held shares (excluding shares held by our executive officers, directors and 10% or more stockholders) of at least $1 million and a total market value of listed securities of at least $35 million. There is no assurance that we will continue to meet the minimum closing price requirement and other listing requirements.
For example, on November 3, 2021, we received a written notice from the staff of the Listing Qualifications Department of Nasdaq relating to the minimum bid price requirement contained in Nasdaq Listing Rule 5550(a)(2). As previously disclosed, on May 6, 2021 the Company received a written notice from the staff indicating that the Company was not in compliance with the minimum bid price rule because the bid price for the Company’s common stock had closed below $1.00 per share for the previous 30 consecutive business days. In accordance with Nasdaq Listing Rule 5810(c)(3)(A), the Company was provided 180 calendar days, or until November 2, 2021, to regain compliance. The November 3 notice stated that, although the Company had not regained compliance prior to November 2, 2021, in accordance with Nasdaq Listing Rule 5810(c)(3)(F), the staff has determined that the Company is eligible for an additional 180 calendar days from the date of the second notice, or until May 2, 2022, to regain compliance.
To regain compliance, the bid price for our common stock must close at $1.00 per share or more for a minimum of 10 consecutive business days. Nasdaq’s written notice has no effect on the listing or trading of our common stock at this time, and we are currently evaluating our alternatives to resolve this listing deficiency. If necessary to regain compliance with Nasdaq listing standards, we intend, subject to approval of our board of directors and stockholders, to implement a reverse stock split. However, there can be no assurance that the reverse stock split will be approved or will result in a sustained higher stock price that will allow us to meet the Nasdaq stock price listing requirements, and there is no guarantee we will continue to satisfy the other Nasdaq Capital Market continued listing standards.
| ITEM 2. |
UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS |
Unregistered Sales of Equity Securities
None.
Issuer Purchases of Equity Securities
None.
| ITEM 3. |
DEFAULTS UPON SENIOR SECURITIES |
None.
| ITEM 4. |
MINE SAFETY DISCLOSURES |
Not applicable.
| ITEM 5. |
OTHER INFORMATION |
On November 8, 2021, we entered into a Deed of Lease Termination Agreement with the Carlton Landlord providing for the early termination of the Carlton Lease related to our prior corporate headquarters. The Carlton Lease was previously scheduled to expire on April 30, 2022 and, as of September 30, 2021, our aggregate amount of future minimum rental payments thereunder was approximately $69,536. In connection with the termination, we will make a one-time payment to the Carlton Landlord of approximately $14,000, after deducting anticipated returns of deposits in accordance with the Carlton Lease.
In addition to the economic savings, our decision to terminate the Carlton Lease early was also driven by our more general embrace of the remote work culture that became prevalent at many organizations during the COVID-19 pandemic. We believe our people are critical to our ability to efficiently and successfully execute our stated strategy, and that a remote operating paradigm allows us to enhance our available pool of talented human resources while also conserving financial resources.
We also believe in the value of collaboration. In lieu of the fixed office and laboratory space previously available to us under the Carlton Lease, we have recently entered into short term agreements to utilize membership-based co-working space in both Charlottesville, Virginia and Philadelphia, Pennsylvania. We believe these arrangements will permit our team to combine the advantages of remote and in-person work in environments intended to foster creativity and innovation.
| ITEM 6. |
EXHIBITS |
See attached Exhibit Index.
DIFFUSION PHARMACEUTICALS INC.
QUARTERLY REPORT ON FORM 10-Q
EXHIBIT INDEX
| Exhibit No. |
Description |
Method of Filing |
| 31.1 |
Filed herewith |
|
| 31.2 |
Filed herewith |
|
| 32.1 |
Furnished herewith |
|
| 32.2 |
Furnished herewith |
|
| 101 |
The following materials from Diffusion’s quarterly report on Form 10-Q for the quarter ended September 30, 2021 formatted in Inline XBRL (Extensible Business Reporting Language): (i) the Unaudited Interim Consolidated Balance Sheets, (ii) the Unaudited Interim Consolidated Statements of Operations, (iii) the Unaudited Interim Consolidated Statement of Changes in Stockholders’ Equity, (iv) the Unaudited Interim Consolidated Statements of Cash Flows, and (v) Notes to Unaudited Interim Consolidated Financial Statements |
Filed herewith |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL and contained in Exhibit 101) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Date: November 10, 2021
| DIFFUSION PHARMACEUTICALS INC. |
|||
| By: |
/s/ Robert J. Cobuzzi, Jr. |
||
| Robert J. Cobuzzi, Jr. |
|||
| President and Chief Executive Officer |
|||
| (Principal Executive Officer) |
|||
| By: |
/s/ William Hornung |
||
| William Hornung |
|||
| Chief Financial Officer |
|||
| (Principal Financial Officer and Principal Accounting Officer) |
|||