UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported): November 18, 2013
STRATUS MEDIA GROUP, INC.
| NEVADA | 000-24477 | 86-0776876 |
| (State or other jurisdiction of incorporation) | (Commission File Number) | (IRS Employer Identification No.) |
1800 Century Park East, 6th Floor
Los Angeles, California 90067
(Address of principal executive offices)
(805) 884-9977
(Registrant’s telephone number, including area code)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (See General Instruction A.2. below):
| o | Written communications pursuant to Rule 425 under the Securities Act of 1933 (17 CFR 230.425) |
| o | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| o | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| o | Pre-commencement communications pursuant to Rule 13e-4(e) under the Exchange Act (17 CFR 240.13e-4(c)) |
| Item 1.01 | Entry into a Material Definitive Agreement |
In connection with the closing of the Mergers described in Item 1.02, below, Stratus Media Group, Inc. (the “Company”) entered into employment agreements with (a) Yael Schwartz, Ph.D., pursuant to which Dr. Schwartz was appointed President of Canterbury Laboratories LLC, and Hygeia Therapeutics, Inc. (the new subsidiaries of the Company acquired pursuant to the Mergers); and (b) Craig Abolin, Ph.D. pursuant to which Dr. Abolin was appointed Vice President of Research and Development of the new subsidiaries.
Under the Employment Agreement with Dr. Schwartz, she is to be employed for an initial period of three years. During the initial year of her employment term, she is to receive a base salary of $330,000. Thereafter, her base salary will be subject to mutually agreed upon increases. The Company’s board of directors (the “Board”) or Compensation Committee may grant Dr. Schwartz bonuses in its sole discretion. Dr. Schwartz is also eligible for grants of awards under the Company’s Incentive Compensation Plan.
Under the employment agreement with Dr. Abolin, he is to be employed for an initial period of three years. During the initial year, he is to receive a base salary of $241,000. Thereafter his base salary will be subject to mutually agreed upon increases. The Company’s Board or Compensation Committee may grant Dr. Abolin bonuses in its sole discretion. Dr. Abolin is also eligible for grants of awards under the Company’s Incentive Compensation Plan.
| Item 1.02 | Completion of Acquisition on Disposition of Assets |
Effective September 30, 2013, the Company entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Canterbury Acquisition LLC, a wholly owned subsidiary of the Company (“Canterbury Merger Sub”), Hygeia Acquisition, Inc., a wholly owned subsidiary of the Company (“Hygeia Merger Sub”), Canterbury Laboratories, LLC (“Canterbury”), Hygeia Therapeutics, Inc. (“Hygeia”) and Yael Schwartz, Ph.D., as Holder Representative, pursuant to which the Company agreed to acquire all of the capital stock of Canterbury and Hygeia (the “Mergers”) with Canterbury and Hygeia becoming wholly owned subsidiaries of the Company. The consideration for the Mergers is the issuance by the Company of an aggregate of 115,011,563 restricted shares of the Company’s common stock issued to the stakeholders of Canterbury and Hygeia. Effective November 18, 2013 (the “Effective Date”), the Mergers were completed, and Canterbury and Hygeia became wholly owned subsidiaries of the Company.
The foregoing description of the Mergers and related transactions does not purport to be complete and is qualified in its entirety by reference to the complete text of the Merger Agreement which was filed with the Securities and Exchange Commission (“SEC”) as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on October 10, 2013.
The shares of the Company’s common stock issued to the holders of the capital stock of Canterbury and Hygeia in connection with the Mergers were not registered under the Securities Act of 1933, as amended (the “Securities Act”), in reliance upon the exemption from registration provided by Section 4(2) of the Securities Act and Regulation D promulgated under that section, which exempts transactions by an issuer not involving any public offering. These securities may not be offered or sold in the U.S. absent registration or an applicable exemption from the registration requirements. Certificates representing these shares will contain a legend stating the restrictions applicable to such shares.
Changes to the Business. As of the Effective Date, the businesses of Canterbury and Hygeia (the “Canterbury Group”) constitute our sole line of business.
| 2 |
Changes to the Board of Directors and Executive Officers. Upon the closing of the Mergers, pursuant to the Merger Agreement Yael Schwartz, Ph.D. and Nelson Stacks were appointed by the Company’s board of directors (the “Board”) as directors of the Company. In addition, upon the closing of the Mergers, Dr. Schwartz was appointed as President of the Canterbury and Hygeia subsidiaries and Dr. Craig Abolin was appointed as Vice President of Research and Development of the subsidiaries.
All directors hold office for one-year terms until the election and qualification of their successors.
Accounting Treatment. The Mergers are being accounted for as the acquisition of a business. Canterbury and Hygeia are the acquired companies for financial reporting purposes as subsidiaries of the Company, with the former shareholders of Canterbury and Hygeia owning 20.6% of outstanding shares of the Company following the Mergers and the shareholders of the Company prior to the Mergers retaining 79.4% ownership of the outstanding shares of the Company following the Mergers. Consequently, the assets and liabilities and the operations that will be reflected in the historical financial statements prior to the Mergers are those of the Company and the consolidated financial statements after the Mergers will include the assets and liabilities of the Company, Canterbury and Hygeia, operations of Canterbury and Hygeia, and operations of the Company from the closing date of the Mergers.
Tax Treatment; Small Business Issuer. The Merger of Hygeia is intended to constitute a reorganization or other tax-deferred transaction within the meaning of the Internal Revenue Code of 1986, as amended (the “Code”). The Merger of Canterbury will be taxable to the holders of the equity of Canterbury.
Following the Mergers, we will continue to be a “smaller reporting company,” as defined in Item 10(f)(1) of Regulation S-K, as promulgated by the SEC.
As used in this Current Report on Form 8-K, all references to “we,” “our” and “us” in the following description of the business of Canterbury and Hygeia for periods prior to the closing of the Exchange refer to Canterbury and Hygeia, as a privately owned companies, and for periods subsequent to the closing of the Mergers refer to the Company and its subsidiaries consisting of Canterbury and Hygeia.
CORPORATE INFORMATION
Company History – Stratus Media Group, Inc.
On March 14, 2008, pursuant to an Agreement and Plan of Merger dated August 20, 2007 between Feris International, Inc. (“Feris”) and Pro Sports & Entertainment, Inc. (“PSEI”), a company engaged in the sports and entertainment business, Feris issued 49,500,000 shares of its common stock for all issued and outstanding shares of PSEI, resulting in PSEI becoming a wholly-owned subsidiary of Feris and the surviving entity for accounting purposes (“Reverse Merger”). In July 2008, Feris’ corporate name was changed to Stratus Media Group, Inc. PSEI, a California corporation, was organized on November 23, 1998. In August 2005, PSEI acquired the business of Stratus White, LLC, a company engaged in developing a loyalty reward program for credit cards.
In June 2011, the Company acquired Series A Convertible Preferred Stock of ProElite, Inc. (“ProElite”), that organized and promoted mixed martial arts (“MMA”) matches. These holdings of Series A Convertible Preferred Stock provide the Company voting rights on an as-converted basis equivalent to a 95% ownership in ProElite.
As a result of, among other factors, a lack of working capital, the Company suspended development of its businesses other than Pro Elite as of December 31, 2012 and suspended development of its MMA business effective June 30, 2013. The Company’s Board authorized management to pursue acquisition opportunities in the life sciences area in view of the experience and expertise in that area of its largest stockholders, Sol. J. Barer and Isaac Blech.
| 3 |
Description of the Businesses of Canterbury and Hygeia
Background
Hygeia was organized to develop and commercialize new classes of estrogens and anti-androgens developed in the laboratory of Dr. Richard Hochberg at Yale University (“Yale”). Yale patented these compounds and they were then exclusively licensed to Hygeia. To fund the research and development, Hygeia raised $1,000,000 through the sale of its Series A Preferred Stock in 2010. By 2011, and given the state of the economy, Hygeia found itself unable to raise additional capital and suspended further research and development efforts. During this period, management considered the possibility of developing several of the patent protected assets for various cosmeceutical applications because of the strong activity on skin cells discovered during several of the tests and trials conducted by Hygeia. To further these efforts, on March 28, 2011, Hygeia entered into an Exclusive Development Collaboration Agreement with Ferndale Pharma Group, Inc. (“Ferndale”), an experienced developer, manufacturer and formulator of cosmeceutical products. The relationship with the Ferndale focused on the development of one of Hygeia’s estrogenic compounds for topical skin use as a remedy for aging skin. Hygeia identified the compound as “CL-214”.
As a result of that work, and in an effort to better position itself for a financing, Hygeia reorganized and separated into two companies. Effective as of October 20, 2011, Hygeia and Canterbury entered into an Agreement and Plan of Reorganization and Separation (the “Reorganization Agreement”). In accordance with the Reorganization Agreement, Hygeia spun out Canterbury creating two (2) side-by-side companies with than identical equity ownership. In connection with the reorganization, the exclusive license that Hygeia had entered into with Yale University was separated and all of the patent rights and other intellectual property rights related to the development of non-prescription, non-Food and Drug Administration (“FDA”) regulated products were transferred to Canterbury. All of the patent rights and other intellectual property rights related to the development of prescription, FDA approved products remained with Hygeia.
The Businesses’ Overview
Canterbury is engaged in the premium cosmeceutical business. Cosmeceuticals are sometimes described as cosmetic products with “drug-like benefits”. Generally, cosmeceuticals are products sold over-the-counter, without the regulatory requirement of FDA approval. Since the products are not FDA approved, medical benefits cannot be either claimed or discussed. The development of cosmeceuticals is short and typically ranges from twelve (12) to eighteen (18) months.
With the rise of a more knowledgeable, wealthy and beauty-conscious class of urban consumers, management believes that cosmeceuticals have become one of the fastest growing cosmetic options and include products for skin care, hair care, sun care, lip care, foot care, tooth and gum care. Through an analysis of the developments taking place globally, management believes that the market is presently dominated by skin care and hair care cosmeceuticals.
Hygeia is engaged in the prescription dermatology and prescription women’s health business. All prescription drugs must gain FDA approval before commercialization. Typically, the development of prescription drugs can take anywhere from five to nine years. The Hygeia pipeline consists of two lead compounds. One compound is under development for vulvar and vaginal atrophy and skin fragility, conditions affecting menopausal women through senescence due to the absence of estrogen. The other lead compound is under development for the treatment conditions of androgen excess e.g. acne, male-pattern baldness (androgenic alopecia) and hirsutism (unwanted excess hair).
| 4 |
Hormonal aging radically affects the mucous membranes, skin and hair of women in menopause due to loss of estrogen which affects how women look and feel and their sexual activity.
| · | Management believes it is an urgent problem for women. As a result, many women are purchasing anti-aging products at a high rate into their seventies, which management believes makes anti-aging products the fastest growing segment in all personal care categories. | |
| · | Management believes competitive products are unworkable: currently available prescription hormone replacement comes with risks and current Over-The-Counter (“OTC”) products are ineffective because they do not address the root problem. | |
| · | Management believes that the markets are underserved: products that ‘speak’ to the urogenital, skin and hair changes that women experience in the menopausal years to senescence are few. Marketing portrays 45+ women as old and these women, who feel dynamic and spirited, don’t relate. |
There is a scientific reason for women’s concerns. Aging accelerates for women when they go through menopause. When women enter menopause, the effects of aging accelerate in estrogen-dependent tissues such as the urogenital region, skin and hair. After the age of forty (40), estrogen production declines and the action of male hormone that women make (androgen) is unopposed. This change of control of both male and female hormones has profound effects on skin and hair as shown below:
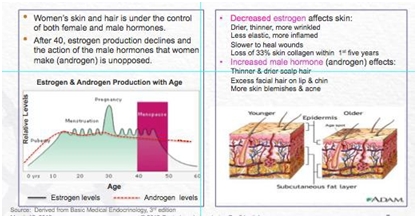
Management believes that more women are experiencing the effects of pre- and post-menopause; there are 64.5 million U.S. women over 45 years of age and this group will grow +5.4% by 2015.
| 1. | The Solution |
Management believes that the Canterbury and Hygeia compounds are unique, safe and effective. Our technology creates so-called “soft” modulators of estrogen and androgen hormone receptors. In scientific terminology, “soft” means that the active ingredient has a predictable route of metabolism. Designed to convert rapidly to inactive metabolites, these novel and patent-protected molecules deliver strong topical effects on skin and mucous membranes without the potential for undesirable systemic side effects, irritation or toxicity seen with some currently marketed prescription hormone replacement drugs and cosmeceuticals. Management believes that this characteristic will allow for use of our products safely over body surface areas for the long term and at concentrations that will be highly effective.
| 5 |
Why management believes the compounds are safe. Chemically engineered as esters in the laboratory of Dr. Richard Hochberg at the Yale Medical School, all of our principal performance ingredients, estrogens and anti-androgens, undergo predictable metabolism to single inactive metabolites. After topical application they are absorbed and broken down by enzymes below the skin surface. The hydrolytic enzymes (esterases) below the skin and mucous membrane surfaces in body fat and blood breakdown our products into single inactive acid metabolites and, as acids, are rapidly excreted from the body.
Why management believes the compounds are effective: Hormonal aging affects all women. Science has long established that estrogen plays a vital role in support of total body collagen. Collagen is the main connective tissue that holds us together. It is a vital component of most structures in the body and plays a very important role in the support of skin and other tissues. Collagen support begins to decline at age forty (40) during peri-menopause, and accelerates with the onset of menopause. Within five (5) years after the onset of menopause, a woman will lose approximately 33% of her skin collagen. Estrogen supports collagen synthesis through its receptors, tiny “locks” that exist all over the body which are activated by “keys”, the estrogen molecule. These receptors are known to exist on skin. In fact, there are more estrogen receptors in facial skin than the skin of many other parts of the body. The decline in estrogen also makes skin drier and causes inflammation (redness). When circulating androgen is unopposed by sufficient levels of estrogen, the skin becomes more prone to blemishes, unwanted thick hairs appear on the chin, upper lip and sideburn areas and scalp hair thins. In fact, 50% of menopausal women will notice excessive, permanent hair loss (androgenic alopecia).
Management believes that the solution for aging skin deprived of estrogen and being exposed to unopposed circulating androgen is to offer another set of “keys” with our unique proprietary ingredients as shown below:
| 2. | The Regulatory Environment. |
The FDA defines the differences between a cosmetic/cosmeceutic and a drug as follows:
Cosmetic: “articles intended to be rubbed, poured, sprinkled or sprayed on, introduced into, or otherwise applied to the human body….for cleansing, beautifying, promoting attractiveness, or altering the appearance” [FD&C Act, sec. 201(i)].
Drug: “intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease” and “intended to affect the structure of any function of the body” [FD&C Act, sec. 210(g) (1)].
| 3. | Intellectual Property |
We have the exclusive license to three (3) patents (together “Yale Patents”):
U.S. Patent No. 7,015,211. 15.alpha.-Substituted Estradiol Carboxylic Acid Esters as Locally Active Estrogens Richard Hochberg, Inventor. Submitted March 9, 2004; granted March 21, 2006; expires March 9, 2024.
U.S. Patent No. 6,476,012. Estradiol-16.alpha Carboxylic Acid Esters as Locally Active Estrogens Richard Hochberg, Inventor. Submitted January 23, 2002; granted November 5, 2002; expires January 23, 2022.
U.S. Patent No. 8,552,061 Locally Active “Soft” Anti-Androgens Submitted May 2, 2008; granted October 8, 2013; expires May 2, 2028.
For all patents, strong freedom-to-operate opinions have been rendered and patent strategies developed.
| 6 |
| 4. | Scientific Summary |
Our scientific studies have shown that our “soft estrogenic” compounds for vulvar and vaginal atrophy, skin fragility and skin aging are both safe and effective.
| · | All of our principal performance ingredients exhibit varying degrees of attraction to the estrogen receptor and all have varying degrees of ability to activate the receptor. |
| · | All of our principal performance ingredients are capable of stimulating cellular repair and increasing the synthesis of skin cells. |
| · | All of our principal performance ingredients are susceptible to hydrolysis (rapid breakdown occurs in less than 20 minutes) indicating that all are safe and do not have systemic effects. |
Our data indicates that our estrogenic compounds will help the skin in two (2) different ways: genomically (DNA-related) and non-genomically (non-DNA related). The estrogenic compounds are safe, and devoid of systemic exposure. Genomically, they act on DNA to maintain skin structure and thickness by maintaining elastin and collagen and maintain blood flow to the skin by increasing the production of new blood vessels. Non-genomically, our proprietary estrogenic compounds will reduce inflammation and redness, inhibit the breakdown, increase moisture and decrease the incidence of facial hair and blemishes.
A topical, safe anti-androgen can safely and effectively treat excess androgen stimulation of the pilosebaceous unit of the skin and scalp. Excess androgen stimulation results in blemishes, oiliness, hair loss/thinning and unwanted facial hair. Scientific studies conducted with our “soft” anti-androgen have shown a strong safety and efficacy profile:
| · | Significant blockade of topical androgen-dependent tissue in an animal model of anti- androgen sensitivity without effects on androgen dependent internal tissues. |
| · | Potent inhibition of the androgen receptor. |
| · | Rapid breakdown to an inactive metabolite in human plasma. |
| · | Melting point close to that of human skin making it very permeable to the pilosebaceous unit where the oil glands and the hair follicles reside. |
Canterbury Laboratories
Canterbury currently has a cosmeceutical product program based on the16α- carboxylic acid esters of estrogen. This program is currently called, Nextgen Estrogenics.
Ferndale and Canterbury Laboratories
To begin the process of understanding the licensed compounds for cosmeceutical application, Canterbury entered into an Exclusive Development Collaboration (“EDC”) with Ferndale to select a lead product from Canterbury portfolio of ten (10) topical assets. Pursuant to the EDC, Ferndale performed early development studies to identify a lead dermaceutical candidate suitable for aging skin. As a result of the studies, a lead product, which Canterbury refers to as “CL-214” was selected and will be developed and commercialized by Ferndale Pharma Group’s Biopelle Division for sale through the offices of physicians, healthcare providers and plastic surgeons.
Ferndale is a privately owned company located in Ferndale, Michigan. Established in 1897, Ferndale is a holding company operating through six (6) specialty healthcare companies all focused on offering high-value prescription and over-the-counter products treating a wide variety of medical disorders ranging from benign anorectal disorders to skin conditions. Ferndale has over thirty (30) years of experience manufacturing topical Rx and OTC drugs, medical devices and cosmeceuticals for both domestic and international distribution.
| 7 |
Following the completion of the EDC studies, Canterbury, on March 22, 2012, entered into a Sublicense Agreement (the “Sublicense”) with Ferndale for the formulation, manufacture, sale and marketing of CL-214 within Ferndale’s established marketing channel for cosmeceuticals, which are the offices of surgeons, physicians and other health care providers (the “Distribution Channel”). Ferndale is responsible for all costs and expenses associated with developing marketing products for sale through the Distribution Channel. The Territory is the world.
In consideration of Canterbury entering into the Sublicense, Ferndale has agreed to pay to Canterbury the following amounts on a country by country basis:
| A. | Royalties |
Ten (10%) percent of Net Sales of products sold within the Territory where the Yale Patent is valid and in force; Four and One-Half (4 ½ %) percent of Net Sales sold within the Territory when the Yale Patent has expired and Two (2%) percent of Net Sales when the Yale Patent has been held invalid by final judgment of a court of competent jurisdiction.
| B. | Use Fee |
i. One Hundred Thousand ($100,000) Dollars payable within thirty (30) days following the first commercial sale of a product in the United States and Canada;
ii. Twenty Thousand ($20,000) Dollars payable within thirty (30) days following the first commercial sale of a product in each of the following countries: (a) Germany, (b) France, (c) United Kingdom, (d) Japan and (e) Brazil; and
iii. Any fees received by Ferndale from a distributor or other comparable party during the Term shall be divided equally and paid by Ferndale to Canterbury when received.
| C. | Sales Milestone Payments |
i. One Hundred Thousand ($100,000) Dollars at such time as the trailing twelve (12) months of Net Sales in any country in the Territory first exceeds One Million ($1,000,000) Dollars;
ii. Two Hundred Thousand ($200,000) Dollars at such time as the trailing twelve (12) months of Net Sales in any country in the Territory first exceeds Five Million ($5,000,000) Dollars; and
iii. Four Hundred Thousand ($400,000) Dollars at such time as the trailing twelve (12) months of Net Sales in any country in the Territory first exceeds Ten Million ($10,000,000) Dollars.
For purposes of the Ferndale Sublicense Agreement, the United States and Canada are considered to be one (1) country. Net Sales has the customary definition with the usual and standard permitted deductions provided, however, that under no circumstances can the aggregate deductions from gross sales exceed Seven and One-Half (7 ½ %) percent of the gross amount actually received by Ferndale or an Affiliate. None of the amounts described above have yet been paid to Canterbury. Ferndale has, to date, neither developed nor begun marketing any product covered by the Sublicense.
In addition to the Sublicense, Canterbury and Ferndale have agreed to enter into a Supply Agreement on commercially reasonable terms pursuant to which Ferndale has committed to purchase all of its required supply of CL-214 from Canterbury at Canterbury’s cost of raw material and directly related costs and expenses. The Supply Agreement has not yet been executed and the terms have not been finalized.
| 8 |
The term of the Sublicense, which is subject to the terms and conditions of the Yale License, will continue in full force and effect until the last of the claims in the Yale Patents expire, lapse or are declared to be invalid by a non-appealable decision of a court of competent jurisdiction. Ferndale may voluntarily terminate the license upon ninety (90) days prior written notice to Canterbury. Further, either party, upon thirty (30) days prior written notice and the failure to correct within that time period, may terminate the Sublicense upon the occurrence of a material breach or a default by the other party. Finally, either party may immediately terminate the Agreement if the other party is adjudged a bankrupt, becomes insolvent or enters into a composition with its creditors or if a receiver is appointed.
The Sublicense with Ferndale is Canterbury’s first collaboration. Canterbury believes, but has not established, that there are multiple distribution and marketing channels available for its products, from direct retail sales to consumers to infomercials and the internet. With additional resources and qualified partners and collaborators, Canterbury intends to explore all of these options. To date, Canterbury has not negotiated any agreements other than the Sublicense with Ferndale.
The Cosmeceutical Market for Aging Skin
Management believes that skin care is one of the most important categories in the global beauty and personal care industry. Anti-aging products continue to be a significant market performer, showing consistently high increases in revenue over the last five (5) years. While spending has curbed since the economic decline in late 2008, skin care products are one area of consumption that has not generally been negatively affected. Growth in the cosmeceuticals market worldwide is primarily attributed to the aging population in the United States and across the globe. Market gains are driven by a highly receptive, fast-expanding group of middle-aged customers who want to prevent and redress visible damage to the skin caused by aging, sun damage and other environmental stressors. There is also an increase in disposable income in emerging markets like Asia and South America. (Euromonitor: 2011)
For women in their late 40’s and early 50’s, aging accelerates due to the hormonal changes of menopause. Management believes that women’s top fear of aging is losing attractiveness. Many women are experiencing these fears, with 51 million U.S. women between the ages of forty-five and seventy (45-70).
Anti-aging is no longer just about reducing fine lines and minimizing wrinkles but in having skin that is hydrated, evenly toned, textured and supple. Management believes that today’s consumer wants a product that addresses all seven (7) signs of aging: dehydration, fine lines, wrinkles, skin discoloration, large pores, loss of elasticity and fullness. The product(s) that can address all of these issues and is correctly priced will succeed. Anti-aging is fueling the fast-growing cosmeceutical market; these women are actively seeking solutions for aging skin and hair. Anti-aging is the fastest growing segment of the personal care and cosmeceutical industries. Cosmeceutical anti-aging skincare is the fastest growing segment, projected to grow to $3.7 Billion by 2016 with +8. 3% Compound Annual Growth Rate (“CAGR”) (2010-2016, Mintel).
Canterbury believes that the science and technology behind the development of CL-214, and other members of our product portfolio, have the potential to make Canterbury a market leader by focusing on a plan that maximizes the value of its unique portfolio of assets:
| · | The need: For women in their late 40’s and early 50’s, aging accelerates due to the hormonal changes of menopause. Women’s top fear of aging is losing attractiveness. Many women are experiencing these fears, with 51 million U.S. women between the ages of forty-five and seventy (45-70). |
| · | Canterbury’s Solution: Canterbury’s proprietary ingredients bring a new, differentiated benefit to the anti-aging market. Our ingredients safely halt and reverse age-related hormonal changes in women’s skin and hair. Unlike other anti-aging topical cosmeceuticals, Canterbury’s ingredients act only at the point of application, are non-irritating and spare internal organs from unnecessary systemic exposure. |
| 9 |
| · | Anti-aging is fueling the fast-growing cosmeceutical market: These women are actively seeking solutions for aging skin and hair. Management believes that anti-aging is the fastest growing segment of the personal care and cosmeceutical industries. Cosmeceutical anti-aging skincare is the fastest growing segment, projected to grow to $3.7 Billion by 2016 with +8. 3% CAGR (2010-2106, Mintel). |
| · | Competition: Despite the growth in cosmeceuticals, many of the current anti-aging topical products are either ineffective, unsafe or both. As is the case with the retinoids, their effectiveness is limited by constraints on how much can be applied to skin without causing photo-sensitivity to the sun’s rays and irritation. |
| · | Canterbury’s products can fit multiple product segments: Canterbury’s ingredients can be formulated for multiple cosmeceutical applications where the total addressable U.S. market is $5.5 billion. Canterbury is focused on the cosmeceutical skin and hair care segments where the addressable U.S. market is $2.3 billion and $550 million, respectively. |
| · | Canterbury’s business plan maximizes the value of the ingredients and creates a large and growing business in skin and hair care: Canterbury’s plan is sequenced to attack the largest cosmeceutical market quickly with a unique benefit of halting and reversing the effects of aging, then accelerating growth in other key segments while leveraging current brand and channel assets. |
Current Status of Canterbury’s Products
Canterbury’s first product for aging skin, CL-214, will initially be sold and marketed by Ferndale Pharma Group through physican offices and medi-spas world-wide. Key findings in the synthesis and scale-up manufacturing of CL-214 have opened up opportunities for new patents. Preparation for manufacturing is expected to be completed in the second quarter of 2014 and the first batch of CL-214 will then start formulation development. Management believes that the product will be ready for Ferndale’s launch following human skin assessment studies in the fourth quarter of 2014.
Hygeia Therapeutics
Development Programs
HYG-102, the lead estrogenic candidate, is a member of the 15 alpha-carboxylic acid esters of estrogen, HYG-102, is under development for the topical treatment of skin aging (thinning and fragility) and vulvar and vaginal atrophy. HYG-102 is the first estrogenic drug candidate engineered to be rapidly deactivated to non-estrogenic metabolites by hydrolytic enzymes and represents a new generation of effective estrogens. In animal models, HYG-102 has strong estrogenic effects at the site of application but no effect on the most estrogen-sensitive systemic tissues even at high multiples of the locally effective dose. These observations are consistent with rapid formation of inactive metabolites. The expected major metabolite, HYG-103, has no detectable estrogenic effects in vitro.
Key findings to date in estrogen-deficient animals demonstrate superior safety over estradiol. HYG-102 has no impact on uterine tissues even at supra-therapeutic doses administered intravaginally (50x therapeutic dose) or subcutaneously (300x therapeutic dose). These studies indicate a favorable therapeutic index relative to currently marketed estrogen containing products. Half-life following intravaginal dosing is 20 minutes. In human keratinocytes, which comprise 90% of epithelium, HYG-102 was significantly proliferative. A proprietary model of human skin thickness (Living Skin Equivalency Model) demonstrated that HYG-102 elicited a positive trend toward increased thickness. These studies bode well for efficacy in the treatment of age-related skin fragility.
| 10 |
HYG-440 is our lead anti-androgenic candidate for the topical treatment of acne, hirsutism and androgenic alopecia. HYG-440 has strong androgen-receptor affinity and can inhibit androgenic effects of co-administered androgens in rat cells transfected with androgen receptors. The expected major metabolite has no detectable androgen-receptor affinity or ability to interfere with the androgenic effects of endogenous androgens. In vivo proof-of-concept studies will start shortly in the Syrian hamster. Hygeia recently shortened the synthesis route of HYG-440 from 7 steps to 2 steps creating a potential for a new process patent.
HYG-102440 is a combination product of HYG-102 and HYG-440 and will be developed for the topical treatment of hair loss due to increased hair follicle sensitivity to androgens.
Product Rationale
HYG-102: Estrogens are known to support skin health by maintaining skin thickness, elasticity and moisture content in both men and women. Estrogen levels and skin thickness and elasticity decline with age. Skin loses half of its elasticity by age 60 and continues to decline with age. Clinically, thinning skin leads to delicate wrinkles, ease of bruising and tearing, poor wound healing and sensitivity to cold. Vaginal wall atrophy, a condition that significantly reduces quality of life, affects 47% of women within three years of menopause and approaches 100% over time. The profound effects of estrogens on skin were known long before it was discovered that estrogen receptors are present in all epithelial tissues but most abundant in skin and the uterus. Decades after estrogens were first used over-the-counter (OTC) in facial creams, shampoos and hair conditioners to restore and maintain skin and hair health, unwanted estrogenic side effects were linked to the use of those products. In 1994, safety concerns finally led to the removal of all estrogens and other hormone-containing OTC products in the U.S. Less than ten (10) years later, the use of estrogen-containing prescription products was associated with an increased risk of cancer and cardiovascular disease. These risks associated with estrogen use have made many doctors and patients hesitant to use estrogens to manage aging skin and vaginal and vulvar atrophy associated with low estrogen.
HYG-440: Excess testosterone-like hormones in the skin of both men and women can lead to the overproduction of sebum which can block skin pores and lead to localized infection and inflammation. An anti-androgen applied to the skin can block the actions of testosterone-like hormones and heal acne. In some women, excess skin androgen (testosterone-like hormones) can lead to unwanted localized hair growth (hirsutism). An anti-androgen applied to those skin areas can greatly minimize excess body hair growth caused by excess androgens in the skin. Paradoxically, excess androgen in the scalp can cause androgenic alopecia or baldness and is the most common cause of hair loss affecting both men and women. An anti-androgen applied to the scalp at the first signs of thinning can block the actions of testosterone-like hormones. Hair thinning in some women coincides with menopause when estrogen levels decrease and androgen levels increase. Therefore, a combination product of HYG-102 and HYG-440 (HYG-102440) would be expected (but has not yet been established), to be more effective in those women than an anti-androgen alone.
Market Potential
VVA: Vulvar and vaginal atrophy (VVA) is a urogenital disorder caused by a decrease in estrogen, typically occurring during menopause. When estrogen levels are low, the tissues of the vulvar vaginal region become less moist and the elastic and collagen fibers that give the vaginal wall stretch and stretchiness decreases in number. The skin of the opening becomes thinner and less protective. Thus, the vulvar and vaginal region becomes painful to intercourse and there is an increased incidence of urinary tract infections. In extreme cases, thinning of the tissue can lead to tiny abrasions that cause the sides of the vaginal opening to stick together and the opening may become fused closed. The VVA market is in need of a product with lower systemic activity since treatment guidelines issued by the FDA favor estrogenic products with lower systemic effects. Management believes that only 1 in 4 women with VVA symptoms are being treated because of safety concerns of currently marketed estrogen-containing products. Prevalence, severity and awareness of the condition is increasing as the population ages. Women spend 1/3 of their lives in menopause. Management believes that the post-menopausal VVA market in the US is currently approximately $1,022 billion. The CAGR has grown by 8.8% over the past 5 years and the world-wide market is expected to grow to $3 billion dollars by 2019.
| 11 |
Aging Skin (Skin Fragility): Currently, there are no preventative treatments for age-related skin fragility (thinning), bruising, and slow healing. Severe skin-thinning seen in nursing home patients often leads to skin tearing which can take 10-21 days to heal and increases nursing care costs for institutions, individuals and the community. Estrogen-containing hormone replacement therapy has been shown to improve skin thickness and elasticity, but is not approved for this purpose due to systemic side effects. HYG-102 has the potential to penetrate and expand the world-wide aging skin market in all adult age groups.
Acne, Alopecia and Hirsutism: The world-wide anti-acne market is $2.8 billion and constitutes the largest prescription market in dermatology. Currently available prescription anti-acne products are associated with undesirable side effects e.g., skin irritation, photosensitivity, hypopigmentation and GI-upset. There are no other known non-systemic “soft” topical anti-androgens in development. Hirsutism affects about 10% of the female population and Americans spend $1 billion dollars annually for the removal of unwanted hair. Androgenic baldness is a greatly underserved market and is primed for a safe, effective, non-invasive treatment. It affects both men and women and women constitute nearly half of the hair-loss market. In the U.S. alone, consumers spend $1.2 billion annually on topical treatments for thinning hair. This market is greatly underserved for safe and non-invasive remedies.
Current Status of Hygeia Products
Hygeia’s “soft estrogen” and “soft anti-androgen” have completed in vitro and in vivo proof-of-concept studies in widely accepted tissue and animal models. Chemical synthesis process development for both HYG-102, the “soft” analog of 15 alpha-carboxylic acid esters of estrogen and HYG-440, the “soft anti-androgen, have opened up new patent strategies. Both development programs will run in parallel. In the first quarter of 2014, we intend to scale up manufacturing and start formulation development for HYG-102 and HYG-440. This will be largely completed by the third quarter of 2016. Drug metabolism, pharmacokinetic testing (DMPK), toxicology studies and IND (“Investigative New Drug” application) will extend from the second quarter 2014 to the third quarter of 2014. It is anticipated that successful completion of DMPK and toxicology studies for HYG-102, will open up a Physician IND for the treatment of age-related skin fragility. Similarly, successful completion of the same for HYG-440 for acne will enable a Physician IND for the treatment of hirsuitism and alopecia. Management believes clinical studies for the treatment of VVA and acne will commence during the first quarter of 2016 and an NDA (new drug application) for HYG-102 and HYG-440 will be filed by 2018.
Employees
As of the Effective Date, the Canterbury Group had two employees, both of whom are full time employees. None of our employees is represented by a collective bargaining agreement. We consider our relations with our employees to be good.
Facilities
The Canterbury Group currently has no permanent executive offices and no lease obligations.
Legal Proceedings
We are not involved in any pending or threatened legal proceedings.
Forward-Looking Statements
This Current Report on Form 8-K and other written and oral statements made from time to time by us may contain so-called “forward-looking statements,” all of which are subject to risks and uncertainties. Forward-looking statements can be identified by the use of words such as “expects,” “plans,” “will,” “forecasts,” “projects,” “intends,” “estimates,” and other words of similar meaning. One can identify them by the fact that they do not relate strictly to historical or current facts. These statements are likely to address our growth strategy, financial results and product and development programs. One must carefully consider any such statement and should understand that many factors could cause actual results to differ from our forward looking statements. These factors may include inaccurate assumptions and a broad variety of other risks and uncertainties, including some that are known and some that are not. No forward looking statement can be guaranteed and actual future results may vary materially.
| 12 |
Information regarding market and industry statistics contained in this Current Report on Form 8-K is included based on information available to us that we believe is accurate. It is generally based on industry and other publications that are not produced for purposes of securities offerings or economic analysis. We have not reviewed or included data from all sources, and cannot assure investors of the accuracy or completeness of the data included in this Current Report. Forecasts and other forward-looking information obtained from these sources are subject to the same qualifications and the additional uncertainties accompanying any estimates of future market size, revenue and market acceptance of products and services. We do not assume any obligation to update any forward-looking statement. As a result, investors should not place undue reliance on these forward-looking statements.
Management’s Discussion and Analysis of Financial Condition and Results of Operations
This discussion should be read in conjunction with the other sections of this Current Report on Form 8-K, including “Risk Factors,” “Description of the Businesses of Canterbury and Hygeia” and the Financial Statements attached hereto as Item 9.01 and the related exhibits. The various sections of this discussion contain a number of forward-looking statements, all of which are based on our current expectations and could be affected by the uncertainties and risk factors described throughout this Current Report on Form 8-K as well as other matters over which we have no control. See “Forward-Looking Statements.” Our actual results may differ materially.
Recent Events
On November 18, 2013, we completed the Mergers pursuant to which we acquired all of the capital stock of Canterbury and Hygeia, which became our wholly owned subsidiaries. In connection with the Mergers, we succeeded to the businesses of Canterbury and Hygeia as our sole lines of business. The Mergers are being accounted for as a recapitalization. Canterbury and Hygeia are the acquirers for accounting purposes and we are the acquired company. Accordingly, Canterbury’s and Hygeia’s historical financial statements for periods prior to the acquisition have become those of the registrant retroactively restated for, and giving effect to, the number of shares received in the Mergers. Operations reported for periods prior to the share exchange are those of Canterbury and Hygeia.
The following discussion relates to the operations of Canterbury and Hygeia and should be read in conjunction with the Notes to Financial Statements.
History of Business
Hygeia Therapeutics, Inc. (“Hygeia”), a Delaware Corporation, based in Holden, Massachusetts was formerly known as Orcas Therapeutics, Inc. It was incorporated on November 14, 2005 to acquire and develop biodegradable hormone receptor modulators for topical indications. Hygeia is focused on developing topical therapies for conditions where localized treatments offer advantages over systemic therapies. It also conducts testing on drugs including topical synthetic estrogen and anti-androgen.
Hygeia has signed an Exclusive License Agreement (the “Yale License”) with Yale University (“Yale”) under U.S. Patent 7,015,211“15.alpha.-Substituted Estradiol Carboxylic Acid Esters as Locally Active Estrogens,” U.S. Patent 6,476,012 “Estradiol-16.alpha Carboxylic Acid Esters as Locally Active Estrogens” and U.S. Patent 8,552,061 “Locally active "soft" antiandrogens” (“Yale Patents”). Hygeia agreed to pay royalty fees to Yale quarterly beginning in the first calendar quarter in which net sales occur.
| 13 |
Canterbury Laboratories, LLC (the “Canterbury”), is a Delaware Limited Liability Company that was formed on October 14, 2011 and began operations on February 22, 2012. Initially, the Company was a wholly owned subsidiary of Hygeia Therapeutics, Inc. (“Hygeia”). Canterbury is engaged in the premium cosmeceutical business. Cosmeceuticals are the latest addition to the health industry and are sometimes described as cosmetic products with “drug-like benefits”. Generally, cosmeceuticals are products sold over-the-counter, without the regulatory requirement of FDA approval.
A reorganization and separation agreement was signed on October 14, 2011 between Canterbury and Hygeia under which Hygeia received 100% of all issued and outstanding units of all classes of limited liability company membership interests of Canterbury. Hygeia distributed these profit units to holders of its common and preferred stock, with each holder of one share of common or preferred stock in Hygeia given one profit unit in Canterbury. Further, 720,821 shares were issued to the Hygeia’s non-qualifying stock option (“NSO”) holders to liquidate the 720,821 shares of outstanding NSO’s. Holders of Hygeia stock purchase warrants for 1,782,901 shares were issued in exchange an equal number of units of Canterbury stock purchase warrants. Pursuant to the license agreement 1,606,035 shares of Series A convertible preferred stock was issued to Yale University for the Yale License. In February 2012, Hygeia assigned its rights and obligations related to non-prescription products under the Yale License to Canterbury.
As of July 31, 2013, equity holders of Hygeia held 94% of the membership units of Canterbury. Accordingly, the financial results of Hygeia and Canterbury are presented herein on a combined basis and the combination of Hygeia and Canterbury will be referred to herein as the “Company” or “Canterbury Group.”
OPERATIONS
Overview
Canterbury is engaged in the premium cosmeceutical business. Cosmeceuticals are the latest addition to the health industry and are sometimes described as cosmetic products with “drug-like benefits.” Generally, cosmeceuticals are products sold over-the-counter, without the regulatory requirement of FDA approval. Since the products are not FDA approved, medical benefits cannot be either claimed or discussed.
With the rise of a more knowledgeable, wealthy, and beauty-conscious class of urban consumers, management believes that cosmeceuticals have become one of the fastest growing cosmetic options and include products for skin care, hair care, sun care, lip care, foot care, tooth and gum care. Through an analysis of the developments taking place globally, management believes that the market is presently dominated by skin care and hair care cosmeceuticals.
Hygeia is engaged in the prescription dermatology and prescription women’s health business. All prescription drugs must gain FDA approval before commercialization. Typically, development of prescription drugs can take anywhere from five to nine years. The Hygeia pipeline consists of a two lead compounds. One compound is under development for vulvar and vaginal atrophy and skin fragility, conditions affecting menopausal women through senescence due to the absence of estrogen. The other lead compound is under development for the treatment conditions of androgen excess e.g. acne, male-pattern baldness and hirsutism.
| 14 |
Market
Women will spend approximately one-third of their life in menopause. Hormonal aging radically affects their mucous membranes skin and hair due to loss of estrogen which affects how women look and feel and their sexual activity.
Management believes it is an urgent problem for women. As a result, many women are purchasing anti-aging products at a high rate into their seventies, which management believes makes anti-aging the fastest growing segment in all personal care categories. Management believes competitive products are unworkable: Currently available prescription hormone replacement comes with risks and current OTC products are ineffective by not addressing the root problem. Management believes that the markets are underserved: Products that ‘speak’ to the urogenital, skin and hair changes that women experience in the menopausal years to senescence are few. Marketing portrays 45+ women as old and these women, who feel dynamic and spirited, don’t relate.
There is a scientific reason for women’s concerns. Aging accelerates for women when they go through menopause. When women enter menopause, the effects of aging accelerate in estrogen-dependent tissues such as the urogenital region, skin and hair. After the age of forty (40), estrogen production declines and the action of male hormone that women make (androgen) is unopposed. Management believes that more women are experiencing the effects of pre- and post-menopause; there are 64.5 million U.S. women over 45 years of age and this group will grow +5.4% by 2015.
Products
Management believes that the Canterbury and Hygeia compounds are unique, safe and effective. Our technology creates so-called “soft” modulators of estrogen and androgen hormone receptors. In scientific terminology, “soft” means that the active ingredient has a predictable route of metabolism. Designed to convert rapidly to inactive metabolites, these novel and patent-protected molecules deliver strong topical effects on skin and mucous membranes without the potential for undesirable systemic side effects, irritation or toxicity seen with some currently marketed prescription hormone replacement drugs and cosmeceuticals. Management believes that this characteristic will allow for use of our products safely over body surface areas for the long term and at concentrations that will be highly effective.
Chemically engineered as esters in the laboratory of Dr. Richard Hochberg at Yale Medical School, all of our principal performance ingredients undergo predictable metabolism to single inactive metabolites. After topical application they are absorbed and broken down by enzymes below the skin surface. The hydrolytic enzymes (esterases) below the skin and mucous membrane surfaces in body fat and blood breakdown our products into single inactive acid metabolites and, as acids, are rapidly excreted from the body.
Hormonal aging affects all women. Science has long established that estrogen plays a vital role in support of total body collagen. Collagen is the main connective tissue that holds us together. It is a vital component of most structures in the body and plays a very important role in the support of skin and other tissues. Collagen support begins to decline at age forty (40) during peri-menopause, and accelerates with the onset of menopause. Within five (5) years after the onset of menopause, a woman will lose 33% of her skin collagen. Estrogen supports collagen synthesis through its receptors, tiny “locks” that exist all over the body which are activated by “keys,” the estrogen molecule. These receptors are known to exist on skin. In fact, there are more estrogen receptors in facial skin than the skin of many other parts of the body. The decline in estrogen also makes skin drier and causes inflammation (redness). When circulating androgen is unopposed by sufficient levels of estrogen, the skin becomes more prone to blemishes, unwanted thick hairs appear on the chin, upper lip and sideburn areas and scalp hair thins. In fact, 50% of menopausal women will notice excessive, permanent hair loss (androgenic alopecia). Management believes that the solution for aging skin deprived of estrogen and being exposed to unopposed circulating androgen is to offer another set of “keys” with our unique proprietary ingredients.
| 15 |
Description of our Revenues, Costs and Expenses
Revenues
Past revenues were derived from fees received under an Economic Development Collaboration (“EDC”) done with Ferndale Pharmaceuticals. Future revenues are expected to consist of development fees, licensing fees and product sales.
Gross Profit (loss)
Our gross profit represents revenues less the Cost of Sales (“COS”). Past COS were for development and testing expenses paid to third parties for work performed under the EDC. Future COS are expected to be costs related to licensing fees and product costs.
Operating Expenses
Our selling, general and administrative expenses include personnel, travel, office and other costs for development and administrative functions of the Company. Legal and professional services are paid to outside attorneys, auditors and consultants are broken out separately given the size of these expenses relative to selling, general and administrative expenses. Operating expenses also include research and development expenses
Interest Expense
Our interest expense results from accruing interest on loans payable and notes payable.
Critical Accounting Policies
Goodwill and Intangible Assets
The intangible asset is the value of the $175,000 the Company paid for Yale License in 2011, net of amortization. The Company reviewed the value of the intangible asset as part of its annual reporting process. To review the value of the intangible asset and December 31, 2012 and 2011, the Company followed ASC Topic 350 “Intangibles- Goodwill and Other Intangible Assets” and first examined the facts and circumstances for the asset to determine if it was more likely than not that an impairment had occurred. The Company then compares discounted cash flow forecasts related to the asset with the stated value of the asset on the balance sheet. The objective is to determine the value of the intangible asset to an industry participant who is a willing buyer not under compulsion to buy and the Company is a willing seller not under compulsion to sell. Based on this process, the Company determined that the value of the intangible asset was not impaired as of December 31, 2012 and 2011 or September 30, 2013.
The forecast for the revenue streams associated with the intangible asset were discounted at a range of discount rates determined by taking the risk-free interest rate at the time of valuation, plus premiums for equity risk to small companies in general, for factors specific to the Company and the business for a total discount rate of 24%. Terminal values were determined by taking cash flows in year five of the forecast, then applying an annual growth of 2% and discounting that stream of cash flows by the discount rate used for that section of the business.
Revenue Recognition
The Company performs research to develop prescription pharmaceuticals and also develops the compound for incorporation into a non-prescription, cosmeceutical product formulation ("Product") under EDC. The EDC party agreed to pay the Company the costs and expenses associated with the contract and fees for management services provided by the Company. Revenue is recognized when each sub-project of the product research is completed and delivered. Royalty revenue would be paid pursuant to the EDC agreement for ultimate product sales.
| 16 |
Income Taxes
The Company utilizes ASC Topic 740 “Accounting for Income Taxes,” which requires recognition of deferred tax assets and liabilities for the expected future tax consequences of events that were included in the financial statements or tax returns. Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each year-end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized. The provision for income taxes represents the tax payable for the period and the change during the period in deferred tax assets and liabilities.
Canterbury is a limited liability partnership for tax purposes and income and losses are distributed to the members. Hygeia is a C Corporation for tax purposes and Hygeia utilizes ASC Topic 740 “Accounting for Income Taxes,” which requires recognition of deferred tax assets and liabilities for the expected future tax consequences of events included in the financial statements or tax returns. Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each year-end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized. As of December 31, 2012, the Company had a deferred tax asset of $723,901 that was fully reserved and a net operating loss carryforward of $1,809,752 for Federal and state tax purposes. As of September 30, 2013, the Company had a deferred tax asset of approximately $775,000 that was fully reserved and a net operating loss carryforward of approximately $1,940,000 for Federal and state tax purposes.
The ability of Hygeia to utilize these net operating loss carryforwards is subject to a number of conditions and significant changes in the ownership structure of Hygeia will significantly impact the availability of these net operating loss carryforwards to reduce taxable income in future periods.
Results of Operations for the Year Ended December 31, 2012
Revenues
Revenues for 2012 were $246,731, a decrease of $71,415 from $318,146 in 2011, when most of the work was performed for the Ferndale EDC. Future revenues are expected to increase with the addition of additional development work and the initiation of licensing fees from Ferndale as they establish and expand revenues from products licensed from the Company.
Gross Profit
Gross profit for 2012 was $123,357, a decrease of $169,464 from $292,821 in 2011 from cost of revenues in 2012 of $123,374 versus $25,325 in 2011. Future gross profits are expected to increase with the addition of licensing fees from Ferndale as they establish and expand revenues from products licensed from the Company.
Operating Expenses
G&A expenses in 2012 of $324,261 decreased by $30,055 from $354,316 in 2011, primarily related to a $12,500 reduction in travel expenses and a $8,700 reduction in supply expense. As the Company expands operations to develop its business, general and administrative expenses are expected to increase significantly.
| 17 |
Legal and professional services were $77,965 in 2012, a decrease of $17,140 from $95,105 in 2011 from reduced levels of contract and licensing work. As the Company expands operations to develop its business, legal and professional expenses are expected to increase significantly.
Research and Development (“R&D”) expense declined from $83,000 in 2011 to $0 in 2012 as the research work to establish the Ferndale EDC was completed in 2011. The Company intends to increase its R&D efforts in the future to continue development of its existing compounds and to develop new compounds.
Depreciation and amortization of $17,196 in 2012 increased by $12,747 from $4,449 in 2011 related to increased amortization of the amount paid for the Yale License.
Interest Expense
There was no debt outstanding during 2012 and 2011so there was no interest expense for these years.
Results of Operations for the Nine Months Ended September 30, 2013
Revenues
Revenues for the nine months ended September 30, 2013 (“2013 Interim Period”) were $127,167 compared with $80,245 of revenues for the nine months ended September 30, 2012 (“2012 Interim Period”) from increased activity for the Ferndale EDC. Future revenues are expected to increase with additional development work and the addition of licensing fees from Ferndale as they establish and expand revenues from products licensed from the Company.
Gross Profit
Gross profit for the 2013 Interim Period was $37,780 compared to $41,740 in gross profit for the 2012 Interim Period. Future gross profits are expected to increase with the additional development work and the addition of licensing fees from Ferndale as they establish and expand revenues from products licensed from the Company.
Operating Expenses
General and Administrative (“G&A”) expenses were $210,359 in the 2013 Interim Period, an increase of $62,937 from $147,422 in the 2012 Interim Period, primarily related to increased travel expenses of $25,750 and other expenses incurred to secure funding and develop business opportunities. As the Company expands operations to develop its business, G&A expenses are expected to increase significantly.
Legal and professional services were $329,224 in the 2013 Interim Period, an increase of $289,304 from $39,920 in the 2012 Interim Period primarily from an increase in legal expenses of $144,000 from expanded financing and contract efforts, consulting expenses of $92,500 for the development of a marketing and branding plan and $52,000 for outside accountants to prepare financial statements for 2011, 2012 and the nine months ended September 30, 2013. As the Company expands operations to develop its business, legal and professional expenses are expected to increase significantly.
Depreciation and amortization of $24,566 in the 2013 Interim Period increased by $15,838 from $8,728 in the 2012 Interim Period from increased amortization of the amount paid for the Yale License.
| 18 |
Interest Expense
Interest expense was $20,267 for the 2013 Interim Period as the Company incurred $715,000 of debt during this period. There was no debt or interest expense during the 2012 Interim Period.
Liquidity and Capital Resources
The report of our independent registered public accounting firm on the financial statements as of and for the years ended December 31, 2012 and 2011 contains an explanatory paragraph expressing substantial doubt about our ability to continue as a going concern as a result of recurring losses, a working capital deficiency, and negative cash flows. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that would be necessary if we are unable to continue as a going concern.
As of September 30, 2013, our principal sources of liquidity consisted of accounts payable, accrued expenses and the issuance of debt and equity securities. In addition to funding operations, our principal short-term and long-term liquidity needs have been, and are expected to be, the settling of obligations to our creditors, the funding of operating losses until we achieve profitability, and general corporate purposes. In addition, commensurate with our level of sales, we will require working capital for sales and marketing costs to market our products and technologies, production expenses and inventory. At September 30, 2013, we had $129,672 of cash on hand and we had negative working capital of $322,277. At December 31, 2012 we had $6,673 of cash on hand and negative working capital of $470,515. At December 31, 2011 we had $48,090 of cash on hand and negative working capital of $369,999.
Cash Flows
The following table sets forth our cash flows for 2012 and 2011 and for the nine months ended September 30, 2013 and 2012:
| Year Ended December 31, | Nine Months Ended September 30, | |||||||||||||||
| 2012 | 2011 | 2013 | 2012 | |||||||||||||
| Operating activities | $ | (217,728 | ) | $ | 21,430 | $ | (567,977 | ) | $ | (118,500 | ) | |||||
| Investing activities | (1,349 | ) | (2,418 | ) | (3,650 | ) | (922 | ) | ||||||||
| Financing activities | 177,660 | 5,912 | 694,626 | 143,102 | ||||||||||||
| Net change in cash | $ | (41,417 | ) | $ | 24,924 | $ | 122,999 | $ | 23,680 | |||||||
Operating Activities
Operating cash flows for 2012 reflect our net loss of $296,065, offset by adjustments for non-cash items totaling $78,337 for $53,886 of accrued expenses, $17,196 for depreciation and amortization and $7,255 for prepaid expenses. Operating cash flows for 2011 reflect our net loss of $244,049, offset by adjustments for non-cash items totaling $265,479 for $217,115 of accrued expenses, $4,449 for depreciation and amortization and $43,915 for prepaid expenses.
Operating cash flows for the nine months ended September 30, 2013 reflect our net loss of $576,546, increased by a reduction for non-cash items of $673, consisting a use of $1,584 for accrued expenses and $24,566 for depreciation and amortization, offset by a use of cash of $23,655 for accounts receivable. Operating cash flows the nine months ended September 30, 2012 reflect our net loss of $154,330, offset by adjustments for non-cash items of $27,102 for accrued expenses and $8,728 for depreciation and amortization.
| 19 |
Investing Activities
Capital constraints limited cash used in investing activities of $1,349, $2,418, $3,650 and $922 for 2012, 2011, nine months ended September 30, 2013 and 2012, respectively.
Summary of Contractual Obligations
Set forth below is information concerning our known contractual obligations as of September 30, 2013 that are fixed and determinable by year.
| Beyond | ||||||||||||||||||||
| Total | 2013 | 2014 | 2015 | 2015 | ||||||||||||||||
| Convertible notes payable | $ | 715,000 | $ | – | $ | 715,000 | $ | – | $ | – | ||||||||||
| Accrued interest | 85,800 | 28,600 | 57,200 | – | – | |||||||||||||||
| Total | $ | 800,800 | $ | 28,600 | $ | 772,200 | $ | – | $ | – | ||||||||||
Financing Activities
During 2012, we received net cash proceeds of $179,702 from Canterbury Series A convertible preferred stock and used $2,042 to repay advances from a shareholder/member. During 2011, we received cash proceeds of $912 from a related party and $5,000 from advances from a shareholder/member. In the nine months ended September 30, 2013, we received $715,000 from convertible notes and used $20,374 for offering costs of ownership units for Canterbury. In the nine months ended September 30, 2012, we received $171,425 in cash proceeds from Canterbury Series A convertible preferred stock and used $16,600 to repay a related party and $12,181 for offering costs for ownership units.
Off Balance Sheet Arrangements
We have no off balance sheet arrangements.
RISK FACTORS
Risks Related to Our Business (As it Pertains to Canterbury and Hygeia)
We have a limited operating history, a history of losses and expect to incur losses for the foreseeable future.
Because of our focus on research and development, we have not yet established many of the necessary functions and systems that will be central to conducting business, including an administrative structure, sales and marketing activities and personnel recruitment. The likelihood of our success must be considered in light of the problems, expenses, difficulties, complications, competition and delays frequently encountered in connection with the development of a new business and new products. There can be no assurance that we will be able to generate sufficient funds from operations or be able to raise sufficient capital to enable us to continue with our business plan and develop new products or, if developed, that the products will be commercially successful. Any factor adversely affecting the sale of future products, including delays in product development, flaws or lack of acceptance of the products would have a material adverse effect on our business, financial condition and results of operations.
We are subject to all of the risks inherent in both the creation of a new business and the development of new products, including the absence of a history of significant operations and the absence of established products that have been produced and sold.
| 20 |
Our success will depend on achieving brand recognition within our targeted markets.
Establishing our brand is critical to our ability to establish and expand our customer base and revenues. We believe that the importance of brand recognition will increase as the number of competitors grows. To attract and retain customers and partners, we intend to increase expenditures to support sales and marketing of our products. We will spend additional funds to secure and maintain protection for our trademarks. Our inability to establish brand recognition will have a materially adverse effect on our business, financial condition and results of operations.
The high level of competition in our industry could harm our business, financial performance, market share and profitability. Many of our competitors have substantially greater resources than we do.
The business of developing prescription drugs for dermatology indications and selling cosmeceuticals for aging skin is highly competitive. These markets include numerous manufacturers, distributors, marketers and retailers that actively compete for consumers both in the United States and abroad. In particular, the cosmeceutical market is highly sensitive to the introduction of new products, which may rapidly capture a significant share of the market. In addition, our products may be, or are at the risk of becoming, obsolete due to new product introductions or new technologies. Our competitors may foresee the course of market development more accurately than we do, develop products and technologies that are superior to ours, produce similar products at a lower cost than we can or adapt more quickly to consumer preferences. Any of these developments would harm our operating results.
We plan to compete in select product categories against a number of multinational manufacturers and pharmaceutical companies, some of which are larger and have substantially greater resources than we do. Therefore, these larger competitors have the ability to spend more aggressively on advertising, marketing and research and to grow more quickly through acquisitions.
Our competitors may attempt to gain market share by offering products at prices at or below the prices at which our products may be offered. Competitive pricing may require us to reduce our prices, which would decrease our profitability or result in lost sales. Our competitors, many of whom have greater resources than ours, may be better able to withstand these price reductions and lost sales. We cannot assure you that future price or product changes by our competitors will not adversely affect our net sales or that we will be able to react with price or product changes of our own to maintain our current market position.
If our products do not appeal to a broad range of consumers, our sales and our business would be harmed.
Our success will depend on our products’ (as and when available for sale) appeal to a broad range of consumers whose preferences cannot be predicted with certainty and are subject to change. If our products do not meet consumer demands, our sales will suffer. In addition, our growth depends upon our ability to develop new products through new product lines, product line extensions and product improvements, which involve numerous risks. New product launches are essential to our growth. As we grow, our reliance on new products will increase. We may not be able to accurately identify consumer preferences, translate our knowledge into consumer-accepted products or successfully integrate new products with our existing product platform or operations. We may also experience increased expenses incurred in connection with product development or marketing and advertising that are not subsequently supported by a sufficient level of sales, which would negatively affect our margins. Furthermore, product development may divert management’s attention from other business concerns, which could cause sales of our existing products to suffer. We may not be able to successfully develop new products in the future, and our newly developed products may not contribute favorably to our operating results.
| 21 |
We are a small company that relies on a few key employees to ensure that our business operates efficiently. If we were to lose the services of any of these key employees, we would experience difficulty in replacing them, which would affect our business operations and harm our business and results of operations.
Our success depends to a significant degree upon the business expertise and continued contributions of our senior management team, any one of whom would be difficult to replace. As a result, our future results will depend significantly upon the efforts and retention of key employees, such as Yael Schwartz, Ph.D., the President of our Canterbury subsidiary, and Craig R. Abolin, Ph.D., Vice President of Research and Development for our Canterbury subsidiary. We rely on these individuals for managing our Canterbury subsidiary, developing our business strategy and maintaining strategic relationships. Any of these employees could, with little or no prior notice, voluntarily terminate their employment with us at any time. The loss of service of any of these key employees would harm our business and results of operations.
In addition, our senior management team may not be able to successfully manage our company as it grows larger. If they are unable to handle these increased responsibilities and we are unable to identify, hire and integrate new personnel, our business, results of operations and financial condition would suffer. Even if we are able to identify new personnel, the integration of new personnel into our business will inevitably occur over an extended period of time. During that time, the lack of sufficient senior management personnel would cause our results of operations to suffer.
Our initiatives to expand into product categories may not be successful and any failure to expand into new product categories would harm our business, results of operations, financial condition and future growth potential.
In order to expand our business, we plan to further develop additional products. We may not be successful in our expansion efforts in these areas. Each of these product initiatives involves significant risks, as well as the possibility of unexpected consequences, including:
| · | Our prescription based dermatology products may fail at any stage of development and/or not obtain FDA approval for commercialization |
| · | Sales of the new products to retailer customers may not be as high as we anticipate; |
| · | The rate of purchases by consumers may not be as high as we or our retailer customers anticipate; |
| · | Returns of new products by retailer customers may exceed our expectations; |
| · | Our marketing strategies and merchandising efforts may be ineffective and fail to reach the targeted consumer base or engender the desired consumption; |
| · | We may incur unexpected costs as a result of the continued development and launch of new products; |
| · | Our pricing strategies may not be accepted by retailer customers and/or their consumers; |
| · | We may experience a decrease in sales of our existing products as a result of introducing new products; |
| · | There may be delays or other difficulties impacting our ability, or the ability of our third-party manufacturers and suppliers, to timely manufacture, distribute and ship products in connection with launching new products; and |
| · | Attempting to accomplish all of the elements of expansion in multiple product categories simultaneously may prove to be an operational and financial burden on us and we may be unable to successfully accomplish all of the elements of the expansion simultaneously, if at all. |
| 22 |
Each of the risks referred to above could delay or impede our ability to successfully expand into new product categories, which would harm our business, results of operations, financial condition and future growth potential.
We may be unable to increase our sales through new and existing distribution channels which would limit our growth and harm our business, results of operations and financial condition.
Products similar to ours are sold in department stores, door-to-door, on the Internet, through home shopping television shows, by mail-order and through telemarketing by representatives of direct sales companies. Our growth strategy includes entering new distribution channels such as home shopping television. Any failure to successfully enter new distribution channels could limit our growth. In addition, consumers could choose to purchase cosmetics through distribution channels in which we do not participate. Our ability to continue to grow and achieve similar profit margins is dependent on our continued expansion through multiple distribution channels.
Consumers may reduce discretionary purchases of our products as a result of a general economic downturn or sudden disruption in the economy, which would negatively affect our net sales.
We believe that consumer spending on cosmetics products is influenced by a number of factors, including general economic conditions, inflation, interest rates, energy costs and the availability of discretionary income, all of which are beyond our control. Consumer purchases of discretionary items tend to decline during recessionary periods, when disposable income is lower. Any resulting material reduction in our sales would negatively affect our business, financial condition and results of operations. In addition, sudden disruptions in the economy or adverse weather conditions can have a short or long-term impact on consumer spending. A downturn in the economy or a sudden disruption of business conditions would negatively affect our net sales.
If we are unable to successfully execute any material part of our growth strategy, our future growth and ability to make profitable investments in our business would be harmed.
Our ability to succeed depends on our ability to grow our business while maintaining profitability. We may not be able to sustain our growth or profitability on a quarterly or annual basis in future periods. Our future growth and profitability will depend upon a number of factors, including, without limitation:
| · | The level of competition in both the cosmeceutical and prescription dermatology industry; |
| · | Our ability to continue to successfully execute our growth strategy; |
| · | Our ability to continuously offer new products; |
| · | Our ability to maintain efficient, timely and cost-effective research, development, production and delivery of our products; |
| · | Our ability to obtain sufficient production capacity for our products; |
| · | The efficiency and effectiveness of our sales and marketing efforts in building product and brand awareness; |
| · | Our ability to identify and respond successfully to emerging trends in the dermatology and skincare beauty industry; |
| · | The level of physician and consumer acceptance of our products; and |
| · | General economic conditions and consumer confidence. |
| 23 |
We may not be successful in executing our growth strategy, and even if we achieve targeted growth, we may not be able to sustain profitability. Failure to successfully execute any material part of our growth strategy would significantly impair our future growth and our ability to make profitable investments in our business.
If we are unable to protect our intellectual property our ability to compete would be negatively impacted.
We attempt to protect our intellectual property under the patent and trademark laws. The market for our products depends to a significant extent upon the goodwill associated with our trademarks and trade names. We own the material trademarks and trade name rights used in connection with the packaging, marketing and sale of our products. Therefore, trademark and trade name protection is important to our business. Although we have registered or applied to register many of our trademarks in the United States and in certain foreign countries, we cannot assure you that all of our trademark applications will be approved.
We also own design patents that relate to some of our products. The design patents we own could be challenged, invalidated or circumvented by others and may not be of sufficient scope or strength to provide us with any meaningful protection or commercial advantage. Although we have registered or applied to register additional design patents in the United States and in certain foreign countries, we cannot assure you that any of our design patent applications will be approved. In addition, we do not own any formula patents. Our suppliers or other third parties hold certain formula patents for the manufacture of our products. If our relationships with our suppliers were interrupted or terminated, or if we are unable to use formulas covered by third-party patents, our business could be harmed and it would negatively impact our results of operations.
Third parties may also oppose our trademark and design patent applications, or otherwise challenge our use of our trademarks or design patents. We cannot assure you that competitors will not infringe our trademarks or our design patents, or that we will have adequate time and resources to enforce our trademarks and design patents and to protect our rights through litigation or otherwise, or that we will be successful in doing so.
We also face the risk of claims that we have infringed third parties’ intellectual property rights. Any claims of intellectual property infringement, even those without merit, could expose us to the following risks, among others:
| · | we may be required to defend against infringement claims which are expensive and time-consuming; |
| · | we may be required to cease making, licensing or using products that incorporate the challenged intellectual property; |
| · | We may be required to re-design, re-engineer or re-brand our products or packaging; or |
| · | We may be required to enter into royalty or licensing agreements in order to obtain the right to use a third party’s intellectual property. |
Any of these outcomes would negatively impact our business, results of operations and financial condition.
| 24 |
We will require a significant amount of cash, and any failure to generate and raise sufficient cash would impair our ability to support our future growth or operating requirements, which would harm our business.
Our ability to fund working capital needs and planned capital expenditures depends on our ability to generate cash flow in the future. Our ability to generate future cash flow is subject to general economic, financial, competitive, legislative, regulatory and other factors that are beyond our control. We cannot assure you that our business will generate cash flow from operations or that our cash needs will not increase beyond what we currently anticipate our cash needs to be. If we had to raise additional capital, equity or debt financing may not be available at all or may be available only on terms that are not favorable to us.
Any variation in the quality of our products or delay in our ability to fill orders would harm our relationships with potential retailer customers.
Our success will depend upon our quality control and on our ability to deliver products in a timely manner. If our products are not delivered according to retailer customers’ delivery deadlines or are found to be defective or not to specification, our relationships with our customers would suffer, our brands’ reputation would be harmed and we could lose market share. We could also experience increased return rates or become subject to liability claims. These negative results would have a harmful effect on our business, results of operations and financial condition.
We plan to purchase semi-finished goods and components from a limited number of third party suppliers, which reduces our control over the manufacturing process and may cause variations in quality or delays in our ability to fill orders.
We plan to purchase semi-finished goods and components from foreign and U.S. suppliers. We will depend on these suppliers to deliver products that are free from defects, that comply with our specifications, that meet our delivery requirements and that are competitive in cost. If our suppliers deliver products that are defective or that otherwise do not meet our specifications, our product failure and return rates may increase, and the reputation of our products and our brand may suffer. In addition, if our suppliers do not meet our delivery requirements or cease doing business with us for any reason, we might miss our retailer customers’ delivery deadlines, which could in turn cause our customers to cancel or reduce orders, refuse to accept deliveries or demand reduced prices. Even if acceptable alternative suppliers are found, the process of locating and securing such alternatives is likely to disrupt our business and we cannot assure you that we will be able to secure alternative suppliers on acceptable terms that provide the same quality product or comply with all applicable laws. Extended unavailability of necessary components or finished goods would cause us to be unable to market one or more of our products for a period of time. Any of these events would cause our business, results of operations and financial condition to suffer.
Catastrophic loss, delays in deliveries or other disruptions at any of our facilities would negatively impact our business.
Significant unscheduled downtime at any facility where our products are manufactured or assembled due to equipment breakdowns, fires, power failures, earthquakes and other natural disasters, severe weather conditions or other disruptions would adversely affect our ability to provide products in a timely manner. Although we anticipate maintaining insurance coverage for our facilities, we cannot assure you that our insurance coverage will be adequate to cover all of our losses in the event of a catastrophic loss. Insurance could also become more expensive and difficult to maintain and may not be available on commercially reasonable terms or at all.
| 25 |
Regulations governing our industry could have a significant negative effect on our business, results of operations and financial condition.
Our business is subject to numerous laws and regulations. The formulation, manufacturing, packaging, labeling, registration, advertising, distribution, importation, storage and sale of our cosmetic products are subject to extensive regulation by various Federal agencies, including the U.S. Food and Drug Administration, or the “FDA,” the U.S. Federal Trade Commission, or the “FTC,” the U.S. Environmental Protection Agency, or the “EPA,” and by various agencies of the states, localities and foreign countries in which our products are manufactured, distributed and sold. Failure by us or our manufacturers to comply with those laws and regulations could lead to enforcement action and the imposition of significant penalties or claims, resulting in significant loss of sales, and could have a negative effect on our business, results of operations and financial condition. If we fail to comply with Federal, state or foreign laws and regulations, we could be required to suspend manufacturing operations, change product formulations, suspend the sale of certain products, initiate product recalls, change product labeling, packaging or advertising or take other corrective actions. Any of these actions could harm our business, financial condition and results of operations. In addition, the adoption of new laws or regulations or changes in the interpretations of existing laws or regulations may result in significant compliance costs or discontinuation of products. Our failure to comply with FDA, FTC, EPA or state laws and regulations, or with laws and regulations in foreign markets, that cover our advertising, including direct claims and advertising by us, may result in enforcement actions and imposition of penalties or otherwise materially adversely affect the distribution and sale of our products and our business.
Under the Federal Food, Drug, and Cosmetic Act, or “FDCA”, cosmetics/cosmeceuticals are defined as articles or components of articles that are applied to the human body and intended to cleanse, beautify or alter its appearance, with the exception of soap. Cosmeceuticals, unlike prescription drugs, are not subject to pre-market approval by the FDA but the product and ingredients must be tested to assure safety. If safety has not been adequately substantiated, a specific label warning is required. The FDA monitors compliance of cosmeceutical products through random inspection of cosmeceutical manufacturers and distributors to ensure that the products neither contain false or misleading labeling nor are manufactured under unsanitary conditions. Inspections also may occur from consumer or competitor complaints filed with the FDA. In the event the FDA does find false or misleading labeling or unsanitary conditions or otherwise a failure to comply with FDA requirements, our distribution channel may be affected by a possible product recall or insufficient product in the marketplace resulting in reduced product sales and revenue to us and increased costs to our operations.
We may also, at some point in the future, be subject to a variety of other laws and regulations. Our failure to comply, or assertions that we have failed to comply, with these laws and regulations could have a material adverse effect on our business in a particular market or in general. To the extent we decide to commence or expand operations in additional countries, laws and regulations in those countries, or the cost of complying with such laws and regulations, may prevent or delay entry into or expansion of operations in those markets or could have a negative effect on our operating margins for products sold in those countries. Regulatory requirements can vary widely from country to country and could further delay the introduction of our products into those countries. We may not be able to enter into acceptable agreements to market and commercialize our products in international markets.
Our ability to sustain satisfactory levels of sales in our markets is dependent in significant part on our ability to introduce additional products into those markets. Government laws and regulations in both our domestic and international markets can delay or prevent the introduction, or require the reformulation or withdrawal, of our products.
| 26 |
The regulatory status of our cosmeceutical products could change, and we may be required to conduct clinical trials to establish efficacy and safety or cease to market some or all of our products, which would require significant time and resources.
The FDA does not have a pre-market approval system for cosmetics/cosmeceuticals, and we believe we are permitted to market our cosmeceuticals and have them manufactured without submitting safety or efficacy data to the FDA. However, the FDA may in the future determine to regulate our cosmetics or the ingredients included in our cosmetics as drugs or biologics. If certain of our products are deemed to be drugs or biologics, rather than cosmetics, we would be required to conduct clinical trials to demonstrate the safety and efficacy of these products in order to continue to market and sell them. In such event, we may not have sufficient resources to conduct the required clinical trials, we may not be able to establish sufficient efficacy or safety to resume the sale of these products, we may not gain regulatory approval of the trial design, the clinical trials may be subject to unanticipated delays due to their time-consuming nature and the outcome of any clinical trial is uncertain. Any inquiries by the FDA or any foreign regulatory authorities into the regulatory status of our cosmetics and any related interruption in the marketing and sale of these products could severely damage our brand reputation and image in the marketplace, as well as our relationships with retailer customers, which would harm our business, results of operations and financial condition.
Some of our cosmeceuticals may considered over-the-counter, or “OTC,” drug products by the FDA. The FDA regulates the formulation, manufacturing, packaging, labeling and distribution of OTC drug products pursuant to a monograph system that specifies active drug ingredients and acceptable product claims that are generally recognized as safe and effective for particular uses. If any of these products that are OTC drugs are not in compliance with the applicable FDA monograph, we would be required to (i) reformulate such product, (ii) cease to make certain use claims relating to such product or (iii) cease to sell such product until we receive further FDA approval. If more stringent regulations are promulgated, we may not be able to comply with such statutes or regulations without incurring substantial expense. In addition, OTC drug products must be manufactured in accordance with pharmaceutical good manufacturing practice regulations. Our OTC drug manufacturers are subject to ongoing periodic unannounced inspection by the FDA as well as regular and ongoing inspections. In addition, inspections may be commenced as a result of consumer or competitor complaints related to our products. Corresponding state agencies may also inspect our facility to ensure strict compliance with drug good manufacturing practices and other government regulations and corresponding foreign standards. We have minimal control over third-party manufacturers’ compliance with these regulations and standards. If the FDA finds a violation of drug good manufacturing practices, it may enjoin the manufacturer’s operations, seize products, or criminally prosecute the manufacturer, any of which could require us to find alternative manufacturers, resulting in additional time and expense.
Our products, both prescription and cosmeceutical, may cause unexpected and undesirable side effects that would limit their use, require their removal from the market or prevent their further development. Product liability claims resulting from these undesirable side effects would hurt our business. In addition, we are vulnerable to claims that our products are not as effective as we claim them to be.
Unexpected and undesirable side effects caused by our products for which we have not provided sufficient label warnings could result in the recall or discontinuance of sales of some or all of our products. Unexpected and undesirable side effects could prevent us from achieving or maintaining market acceptance of the affected products or could substantially increase the costs and expenses in marketing new products. We have been, and may in the future be, subject to various product liability claims resulting from those undesirable side effects caused by our products. Product liability claims may result in negative publicity regarding our company, brand or products that may harm our reputation and sales. In addition, if one of our products is found to be defective we may be required to recall it, which may result in substantial expense, adverse publicity and loss of sales, which would substantially harm our brand. Although we maintain product liability insurance coverage, potential product liability claims may exceed the amount of our insurance coverage or potential product liability claims may be excluded under the terms of our policy, which would cause our financial condition to suffer. In addition, we may be required to pay higher premiums and accept higher deductibles in order to secure adequate insurance coverage in the future.
| 27 |
In addition, consumer or industry analysts may assert claims that our products are not as effective as we claim them to be. We are particularly susceptible to these risks because our marketing heavily relies on the assertions that our products adhere to our founder’s commitment to product purity and quality, are hypoallergenic and are ideal for women who have skin conditions that can be exacerbated by traditional cosmetics. Unexpected and undesirable side effects associated with our products or assertions that our products are not as effective as we claim them to be also could cause negative publicity regarding our company, brand or products, which could in turn harm our reputation and our business.
Significant increases in energy prices would adversely affect our financial results.
Our freight cost will be impacted by changes in fuel prices through surcharges and price increases. Fuel prices and surcharges affect freight cost both on inbound shipments from our suppliers to our assembly and distribution facilities and on outbound freight from our distribution center to our retailer customers. Increases in fuel prices and surcharges and other factors may increase freight costs and thereby increase our cost of sales and selling, general and administrative expenses. We may also be negatively affected by increases in utility costs at our assembly and distribution facilities.
Risks Relating to Our Strategy
Our strategy includes plans for expansion by acquisition, which will require additional capital.
We need to raise additional capital to complete any acquisitions, and there can be no assurances we will be able to do so, or will choose to do so. While we intend that the value added by acquisitions will more than offset the dilution created by the issuance of shares for acquisitions, there can be no assurance that this offset will occur. Additional financing for future acquisitions may be unavailable and, depending on the terms of the proposed acquisitions, financings may be restricted by the terms of credit agreements and privately placed debt securities contained in the financing. Any debt financing would require payments of principal and interest and would adversely impact our cash flow. Furthermore, future acquisitions may result in charges to operations relating to losses to the acquired events, interest expense, or the write down of goodwill, thereby increasing our losses or reducing or eliminating our earnings, if any.
We are investigating potential acquisitions, which have inherent risks.
Although management continues to investigate potential acquisitions, any acquisitions consummated by the Company involve substantial expenditures and risks on our part. There can be no assurance that acquisitions will be identified or completed successfully or, if completed, will yield the expected benefits to us, or will not materially and adversely affect our business, financial condition or results of operations. There can be no assurance that the value attributed by the market to acquisitions will offset the dilution created by the issuance of any additional shares issued in connection with an acquisition. Furthermore, consummation of the intended acquisitions could result in charges to operations relating to losses from the acquired events, interest expense, or the write down of goodwill, which would increase our losses or reduce or eliminate our earnings, if any. As a result of the foregoing, there can be no assurance as to when the intended acquisitions will be consummated or that they will be consummated. Furthermore, the results of the intended acquisitions may fail to conform to the assumptions of management. Therefore, in analyzing the information in this document, stockholders should consider that the intended acquisitions may not be consummated at all.
Future acquisitions by us could result in (a) potentially dilutive issuances of equity securities, (b) the incurrence of substantial additional indebtedness and/or (c) incurrence of expenses for interest, operating losses and the write down of goodwill and other intangible assets, any or all of which could materially and adversely affect our business, financial condition and results of operations. Acquisitions involve numerous risks, including difficulties in the assimilation of the operations, technologies, services and products of the acquired companies and the diversion of management’s attention from other business concerns. In the event that such acquisitions were to occur, there can be no assurance that our business, financial condition and results of operations would not be materially and adversely affected.
| 28 |
Stockholders may suffer substantial dilution as the result of subsequent financings or if we issue additional securities.
We require substantial additional funds to complete our research and development and operate our businesses. However, there can be no assurance that any financing will occur, or, if it does, that it will occur in a timely fashion or that it will result in raising sufficient additional funds. If we are unable to raise funds on terms favorable to existing stockholders, our stockholders’ position and the value of their investment may be materially adversely affected, significantly diminished, and possibly liquidated.
Risks Relating to our Organization and our Common Stock
The price of our common stock has fluctuated in the past and the stock is thinly traded. If trading volume increases in the future, the fluctuations in price could be greater than those experienced in the past.
From January 1, 2011 to September 30, 2013, the average price of our common stock was $0.42 per share, with a low of $0.06 and a high of $1.00, on an average trading volume per day of 33,708 shares. The closing price of our common stock on November 20, 2013 was $0.04 per share. It is possible that trading volumes could increase significantly and such increased volume could lead to significant fluctuations in the price of our stock.
The Company is the result of a “reverse merger” with a shell entity in 2008, resulting in a limitation on shareholder’s use of Rule 144 exemptions for resale.
Since the Company had a “reverse merger” with a shell entity in 2008, resale of the Company’s shares under Rule 144 may be limited. The use of Rule 144 is the most common method of selling restricted shares. Rule 144(i) pertains to shares issued by a former shell company that executed a reverse merger. Under Rule 144(i), sales of shares may only be made under certain conditions, including a sale or intended sale of the stock and if we have filed all Annual and Quarterly reports required under the securities laws. Therefore permission may be granted to remove the restrictive legend on stock certificates only for a specified sale of securities and not as a “blanket” removal of the restrictive legend.
Our management will be able to exert significant influence over us to the detriment of minority stockholders.
Two of our directors beneficially own almost 41% of our outstanding common stock. These stockholders, if they act together, will continue to be able to exert significant influence on our management and affairs and all matters requiring stockholder approval, including significant corporate transactions. This concentration of ownership may have the effect of delaying or preventing our change in control and might affect the market price of our common stock.
Exercise of options and warrants will dilute your percentage of ownership.
We have outstanding options and warrants to purchase 90,950,435 shares of our common stock. In the future, we may grant additional stock options, warrants and convertible securities. The exercise or conversion of stock options, warrants or convertible securities will dilute the percentage ownership of our other stockholders. The dilutive effect of the exercise or conversion of these securities may adversely affect our ability to obtain additional capital. The holders of these securities may be expected to exercise or convert them when we would be able to obtain additional equity capital on terms more favorable than these securities.
| 29 |
Our stock price may be volatile.
The stock market in general, and the stock prices of life sciences companies in particular, have experienced volatility that often has been unrelated to the operating performance of any specific public company. The market price of our common stock is likely to be highly volatile and could fluctuate widely in price in response to various factors, many of which are beyond our control, including the following:
| · | changes in our industry; |
| · | competitive pricing pressures; |
| · | our ability to obtain working capital financing; |
| · | additions or departures of key personnel; |
| · | sales of our common stock; |
| · | our ability to execute our business plan; |
| · | operating results that fall below expectations; |
| · | loss of any strategic relationship; |
| · | regulatory developments; |
| · | economic and other external factors; and |
| · | period-to-period fluctuations in our financial results. |
In addition, the securities markets have from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of our common stock.
We have not paid dividends in the past and do not expect to pay dividends in the future. Any return on investment may be limited to the value of our common stock.
We have never paid cash dividends on our common stock and do not anticipate doing so in the foreseeable future. The payment of dividends on our common stock will depend on earnings, financial condition and other business and economic factors affecting us at such time as our board of directors may consider relevant. If we do not pay dividends, our common stock may be less valuable because a return on your investment will only occur if our stock price appreciates.
| 30 |
There is currently a limited trading market for our common stock and we cannot ensure that one will ever develop or be sustained.
To date, there has been a limited trading market for our common stock. We cannot predict how liquid the market for our common stock might become. Our common stock is quoted for trading on the OTCQB under the symbol SMDI, and as soon as is practicable, we intend to apply for listing of our common stock on either the NYSE Amex, The Nasdaq Capital Market or other national securities exchanges, assuming that we can satisfy the initial listing standards for such exchanges. We currently do not satisfy the initial listing standards, and cannot ensure that we will be able to satisfy such listing standards or that our common stock will be accepted for listing on any such exchanges. Additionally, because we may be considered a shell company, we may be subject to the “seasoning” rules adopted by NASDAQ and NYSE which could further delay any listing. Should we fail to satisfy the initial listing standards of such Mergers, or our common stock is otherwise rejected for listing and remain listed on the OTCQB, the trading price of our common stock could suffer and the trading market for our common stock may be less liquid and our common stock price may be subject to increased volatility. Furthermore, for companies whose securities are traded in the OTCQB, it is more difficult (1) to obtain accurate quotations, (2) to obtain coverage for significant news events because major wire services generally do not publish press releases about such companies, and (3) to obtain needed capital.
Our common stock is currently deemed a “penny stock,” which makes it more difficult for our investors to sell their shares.
Our common stock is subject to the “penny stock” rules adopted under Section 15(g) of the Exchange Act. The penny stock rules generally apply to companies whose common stock is not listed on The Nasdaq Stock Market or other national securities exchange and trades at less than $5.00 per share, other than companies that have had average revenue of at least $6,000,000 for the last three years or that have tangible net worth of at least $5,000,000 ($2,000,000 if the company has been operating for three or more years). These rules require, among other things, that brokers who trade penny stock to persons other than “established customers” complete certain documentation, make suitability inquiries of investors and provide investors with certain information concerning trading in the security, including a risk disclosure document and quote information under certain circumstances. Many brokers have decided not to trade penny stocks because of the requirements of the penny stock rules and, as a result, the number of broker-dealers willing to act as market makers in such securities is limited. If we remain subject to the penny stock rules for any significant period, it could have an adverse effect on the market, if any, for our securities. If our securities are subject to the penny stock rules, investors will find it more difficult to dispose of our securities.
Offers or availability for sale of a substantial number of shares of our common stock may cause the price of our common stock to decline.
If our stockholders sell substantial amounts of our common stock in the public market upon the expiration of any statutory holding period, under Rule 144, or issued upon the exercise of outstanding options or warrants, it could create a circumstance commonly referred to as an “overhang” and in anticipation of which the market price of our common stock could fall. Additionally, the former equity holders of Canterbury and Hygeia who received shares of our Common Stock are subject to a lock-up on the sale of their shares for one year, but thereafter may sell their shares under Rule 144. The existence of an overhang, whether or not sales have occurred or are occurring, also could make more difficult our ability to raise additional financing through the sale of equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate.
In January 2013, the Company signed a term sheet (“Term Sheet”) with an outside financial firm (“Financial Firm”) to have that firm acquire certain portions of the Company’s liabilities to creditors, employees and former employees (“Creditors”). The Financial Firm entered into agreements in July 2013 with such Creditors to acquire $1,865,386 in liabilities (“Liability Settlement”) of the Company and filed a complaint on July 29, 2013 with the Second Judicial Circuit, Leon County, Florida seeking a judgment against the Company for the Liability Settlement. A court order based on this complaint was issued on October 7, 2013. Based on conditions agreed to in the Term Sheet, the Company will settle that judgment by issuing common stock to the Financial Firm. Under an exemption from registration in the SEC regulations, common stock issued pursuant to this court order is tradable without restrictions. This common stock will be issued in tranches such that the Financial Firm will not own more than 9.99% of outstanding shares at any time and will be priced at 80% of average closing bids during such period of time in which the dollar trading volume of the stock is three times the Liability Settlement (“Settlement Period”). The Financial Firm will sell the shares to generate proceeds to pay the Creditors.
If the court order had been issued on September 30, 2013 and using the stock price of $0.108 as of that date, the Financial Firm would have received approximately 20,500,000 shares of common stock for the Liability Settlement and the Company would record a loss of approximately $373,000 based on the excess of the value of the shares issued over the value of the liabilities acquired by the Financial Firm. If the bid price drops during the Settlement Period, the Company is obligated to issue additional shares to ensure the Financial Firm has sufficient stock to cover the Liability Settlement. Until the Financial Firm repays all the creditors, the Company will have a liability on its balance sheet for the value of the number of shares of stock owed to the Financial Firm. This liability will be adjusted on a quarterly basis for changes in the market price of the Company’s stock, which will produce gains and losses in the period that such adjustment is made. For example, if the current bid price fell during the Settlement Period to an price of $0.05, the Company would issue approximately 26,000,000 additional shares to the Financial Firm for total shares issued of 46,500,000 and the Company would record an additional loss of $1,295,000 for these shares. The selling activities of the Financial Firm could put downward pressure on the stock price. The Financial Firm held a promissory note for $50,000 that was converted into 833,333 shares of common stock on October 3, 2013 and received a fee of 150,000 shares of common stock on October 7, 2013. An initial tranche of 20,000,000 shares was issued to the Financial Firm on November 15, 2013.
STOCKHOLDINGS OF CERTAIN BENEFICIAL OWNERS, DIRECTORS AND EXECUTIVE OFFICERS
The following table sets forth, as of November 18, 2013, the number and percentage of shares of Common Stock beneficially owned, directly or indirectly, taking into account the consummation of the Mergers, by each of our directors, and executive officers, beneficial owners known by the Company of more than five percent of the outstanding shares of our Common Stock and by our directors and executive officers as a group. Beneficial ownership is determined in accordance with the Rule 13d-3(a) of the Securities Exchange Act of 1934, as amended, and does not necessarily indicate ownership for any other purpose, and generally includes voting or investment power with respect to the shares and shares which such person has the right to acquire within 60 days of November 18, 2013.
| 31 |
| Beneficial Owner (a) | Amount and Nature of Beneficial Ownership (b) | Percent of Class(c) | ||||||||
| 5% Stockholders: | ||||||||||
| River Charitable Remainder Unitrust, West Charitable Remainder Unitrust, Liberty Charitable Remainder Trust, Isaac Blech, Vice Chairman of the Board | 173,095,238 | (d) | 32.2% | |||||||
| Sol J. Barer, Chairman of the Board | 45,833,333 | (e) | 8.5% | |||||||
| Paul Feller | 24,255,000 | (f) | 4.5% | |||||||
| Other Directors and Executive Officers: | ||||||||||
| Jerold Rubinstein, Chief Executive Officer | 27,975,000 | (g) | 4.9% | |||||||
| Yael Schwartz, President of its Canterbury and Hygeia subsidiaries, director | 2,397,673 | -- | – | |||||||
| Craig Abolin, Vice President of Research and Development of Canterbury and Hygeia | 2,283,720 | -- | – | |||||||
| Nelson Stacks, Director | – | -- | – | |||||||
| John Moynahan, Chief Financial Officer | 1,860,000 | (h) | – | |||||||
| Timothy Boris, General Counsel | 6,300,000 | (i) | 1.1% | |||||||
| Randall Cross, Director | 510,417 | (j) | – | |||||||
| Michael Dunleavy, Sr., Director | 483,333 | (k) | – | |||||||
| Glenn Golenberg, Director | 956,944 | (l) | – | |||||||
| Seymour Siegel, Director | 212,500 | (m) | – | |||||||
| John Schneider, Director | 662,500 | (n) | – | |||||||
| All Current Directors and Executive Officers as a Group (11 Persons) | 286,825,658 | 51.2% | ||||||||
____________
| (a) | The address for each Beneficial Owner is c/o Stratus Media Group, Inc., 1800 Century Park East, 6th Floor, Los Angeles, CA 90067 |
| (b) | The persons named in this table have sole voting and investment power with respect to all shares of common stock shown as beneficially owned by them, subject to applicable community property laws. |
| (c) | Based on 537,327,815 shares deemed outstanding as of November 8, 2013 after giving effect to the Mergers. |
| (d) | This amount consists of (i) 71,428,571 shares of Common Stock held by Liberty Charitable Remainder Unitrust; (ii) 71,428,571 shares of Common Stock held by West Charitable Remainder Unitrust; (iii) 11,904,762 shares of Common Stock held by River Charitable Remainder Unitrust; and (iv) 18,333,334 shares of Common Stock held by Isaac Blech. Mr. Blech is the sole trustee of each of the Trusts and has the sole voting and dispositive power of each of the Trusts. Mr. Blech disclaims beneficial ownership of the Common Stock owned by each of the Trusts except to the extent of his pecuniary interest therein. This amount does not include 11,904,762 shares held by Miriam Wimpfheimer Blech, Mr. Blech’s wife. Mr. Blech disclaims beneficial ownership of the shares owned by Ms. Blech and Ms. Blech disclaims beneficial ownership of the shares owned by Mr. Blech and the Trusts. |
| (e) | Does not include a presently indeterminable amount of shares which may be issued pursuant to a Secured Convertible Promissory Note issued to Dr. Barer. |
| (f) | The record owner of 20,057,921 shares is Bateman & Company (“Bateman”). Mr. Feller has advised the Company that he had transferred the shares to Bateman as security for a loan. |
| (g) | Includes 675,000 vested shares of a restricted stock grant related to board service; 2,300,000 vested shares of a stock option granted in connection with employment, and 25,000,000 shares of a stock option grant on March 27, 2013 that vested immediately upon issuance. |
| (h) | Consists of 1,560,000 vested options and restricted stock of 300,000 granted in connection with an employment agreement. |
| (i) | Includes 200,000 vested options related to an employment agreement, 100,000 vested options made August 20, 2012 and 6,000,000 shares of a stock option grant on March 27, 2013 that vested immediately upon issuance. |
| (j) | Includes vested shares of a restricted stock grant related to board service prior to July 1, 2011 and vested shares of a restricted stock grant related to board service after July 1, 2011. |
| (k) | Includes vested shares of a restricted stock grant related to board service prior to July 1, 2011 and vested shares related to board service after July 1, 2011. |
| (l) | Includes vested shares of a restricted stock grant related to board service prior to July 1, 2011 and vested shares of a restricted stock grant related to board service after July 1, 2011. |
| (m) | Includes vested shares of a stock option related to board service after August 20, 2012. |
| (n) | Represents vested shares of a restricted stock grant related to advisory board service prior to August 20, 2012 and the vested shares of a restricted stock grant related to board service after August 20, 2012. |
| 32 |
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Directors
The following table sets forth, as of the Effective Date, the names of, and certain information concerning, our directors:
| Director | End of | |||||||
| Name | Age | Position | Since | Term | ||||
| Jerold Rubinstein | 74 | Chief Executive Officer | 2011 | 2013 | ||||
| Isaac Blech | 63 | Vice Chairman of the Board | 2013 | 2014 | ||||
| Sol J. Barer | 65 | Chairman of the Board | 2013 | 2014 | ||||
| Yael Schwartz | 65 | Director and President of Canterbury and Hygeia subsidiaries | 2013 | 2014 | ||||
| Nelson Stacks | 43 | Director | 2013 | 2014 | ||||
| Glenn Golenberg | 72 | Director | 2009 | 2013 | ||||
| Seymour Siegel (Sy) | 71 | Director | 2012 | 2013 | ||||
| Michael Dunleavy, Sr. | 59 | Director | 2009 | 2013 | ||||
| Randall Cross | 58 | Director | 2009 | 2013 | ||||
| John A. Schneider, Jr. (Jack) | 74 | Director | 2012 | 2013 |
Jerold Rubinstein – is a Director and Chief Executive Officer of the Company. Mr. Rubinstein is the chairman of the audit committee of CKE Restaurants, the parent company of Carl’s jr. Restaurants and Hardees Restaurants. Also he serves as the non-executive chairman of US Global investors Inc., a mutual fund advisory company. Mr. Rubinstein has started and sold many companies over the years, including Bel Air Savings and Loan and DMX, a cable and satellite music distribution company. Mr. Rubinstein started and sold XTRA Music Ltd., a satellite and cable music distribution company in Europe. Most recently Mr. Rubinstein consults with and serves on 3 early stage development companies. Mr. Rubinstein is both a CPA and attorney. Mr. Rubinstein joined the board in April 2011.
Yael Schwartz – Yael Schwartz, Ph.D. has more than 25 years’ experience in drug discovery and product development. From 1998 to 2007 Dr. Schwartz had positions of increasing responsibility at Sepracor, Inc. (now Sunovion) where she played key leadership roles on teams that launched 3 drugs that are currently in clinical practice for the treatment of asthma (Xopenex), insomnia (Lunesta) and chronic obstructive pulmonary disease (Brovana). Prior to that she contributed to the development of drugs for the treatment of urinary bladder cancer (Valstar) and hypertension (Carvedilol). Since 2007, Dr. Schwartz has been the Founder, President, CEO and Director of Hygeia. Dr. Schwartz adapted and streamlined development strategies and budgets to ensure effective achievement of scientific and business objectives. In 2011, Dr. Schwartz founded Canterbury. Dr. Schwartz received her doctorate degree in Endocrine Physiology from a joint program at the University of Massachusetts Medical School and Worcester Polytechnic Institute.
Isaac Blech – Isaac Blech became a director and Vice Chairman of the Board of the Company on November 1, 2013. Mr. Blech has established some of the leading biotechnology companies in the world during the past 30 years. These include Celgene Corporation, ICOS Corporation, Nova Pharmaceutical Corporation, Pathogenesis Corporation, and Genetics Systems Corporation. Collectively, these companies have produced major advances in a broad array of diseases including the diagnosis and/or treatment of cancer, chlamydia, sexual dysfunction, cystic fibrosis, and AIDS. Their combined value is well in excess of $40 billion. Celgene Corporation introduced two major cancer drugs, and has a current valuation of over $35 billion. ICOS Corporation discovered the drug Cialis, and was acquired by Eli Lilly for over $2 billion. Nova Pharmaceutical Corporation developed a new treatment for brain cancer, and after merging with Scios Corporation, was purchased for $2 billion. Pathogeneses Corporation created TOBI® for cystic fibrosis, the first inhaled antibiotic approved by the FDA, and was acquired by Chiron Corp for $660 million. Genetics Systems developed the first inexpensive and accurate test to diagnosis chlamydia, allowing thousands of babies to be born to women who otherwise would have become sterile from pelvic inflammatory disease. Genetics Systems was acquired for 3% of Bristol Myers’ stock. Mr. Blech is currently a major shareholder and board member of ContraFect Corporation, which is creating new therapies for infectious diseases, is a director and major shareholder of Medgenics, Inc., and is the Vice Chairman of Premier Alliance Group, Inc., and is the Vice Chairman of BillMyParents, Inc. Mr. Blech is also a director of Edge Therapeutics, Inc., a biopharmaceutics company. Mr. Blech is also the Founder, Vice Chairman and a major shareholder of Cerecor, Inc., a neuroscience company developing new treatments for cough and other medical implications. Mr. Blech received a Bachelor of Arts degree from City University of New York, Baruch College.
| 33 |
Sol J. Barer Ph.D. – Dr. Barer became a director and Chairman of the Board of the Company on November 1, 2013. He is currently the Managing Partner of SJBarer Consulting LLC. He previously served in various positions at Celgene Corporation (a biopharmaceutical company focused on the treatment of cancer and inflammatory diseases), including Chairman and Chief Executive Officer from May 2006 until June 2010, Executive Chairman from June 2010 until December 2010 and Non-Executive Chairman from January 2011 until June 2011. Prior to that, he held several other positions within Celgene, including President and Chief Operating Officer. Dr. Barer joined the Celanese Research Company in 1974 and formed the biotechnology group that was subsequently spun out to form Celgene. Dr. Barer currently serves on the Boards of Directors of Amicus Therapeutics (a biopharmaceutical company focused on the development of novel small molecule drugs for the treatment of genetic diseases), InspireMD, Inc. (a medical device company focused on the development and commercialization of stent system technology), Medgenics (a gene therapy company) and Aegerian Pharmaceuticals, Inc. (a company focused on the development of novel, life-altering therapies for patients with debilitating, often fatal diseases) and several privately held biotechnology companies including Edge Therapeutics, Inc., a biopharmaceutics company. Dr. Barer holds a B.S. degree from Brooklyn College and a Ph.D. degree in Organic Chemistry from Rutgers University.
Nelson K. Stacks – Mr. Stacks served as Chairman of the Board of Canterbury prior to the Mergers. From December 2011 to present, Mr. Stacks has been the CEO and Director of WaveGuide Technology, the world’s smallest and most sensitive handheld NMR for detection of cancer, infectious diseases, oil and gas exploration and industrial anti-counterfeiting. From December 2011 to January 2013, Mr. Stacks was CEO and Director of Molecular Insight Pharmaceuticals, a biotechnology company focused on cancer diagnostics and therapeutic treatments as well as orphan neuroendocrine cancers. From July 2009 to August 2011, Mr. Stacks served as the, CEO and Director of Vascular Pathways Incorporated where he raised 14M from venture capitalists and brought a revolutionary peripheral IV catheter to the market and sold products to the US Military and various US and international hospitals. Prior to this position, from March 2006 to July 2009, he served as a venture partner and turnaround CEO for various portfolio companies with Queensland Inocutal Corporation, Queensland Biocapital Funds, a $70B superannuation and venture fund. Over his career, Mr. Stacks has been a venture capitalist, in the United States as the General Partner at 3i Ventures and earlier at Oak Investment Partners. Mr. Stacks is a member of the fourth class of Kauffman Fellows and has invested in all areas of healthcare and information technology. He also previously served as the Chairman of Xbio Systems, a clinical trial software management system, and as CEO, and Executive Director of Xenome Limited, a venom peptide company focused on cancer pain therapy. Mr. Stacks received an MBA from the F.W. Olin Graduate School of Business at Babson College and a BA from The University of Rochester.
Glenn Golenberg – has been engaged in the financial services industry since 1966, Mr. Golenberg has arranged financings well in excess of $1.5 billion and has served as a financial advisor in more than two hundred transactions. Mr. Golenberg is presently a co-founder and Managing Director of Golenberg & Company, a merchant banking firm that invests in and offers financial advisory services to a wide variety of businesses. Founded in 1978, the Los Angeles merchant banking firm of Golenberg & Company also had offices in Cleveland and New York. Mr. Golenberg is also Managing Director of The Bellwether Group, a merchant bank which provides strategic and financial advisory services to expansion-stage emerging technology and life-science related companies. He is actively involved with a number of companies, and is a Director of Stratus Media Group, ProElite, Virtual Media and the ASTRUM fund. In addition, Glenn recently joined the Board of Governors of JLTV, “America’s Chosen Network.” He was co-vice chairman of Skyview Capital and is Senior Advisor to Outsource Partners International, Inc. In addition, he has been chairman of numerous operating companies in which he and partners held a controlling interest. In the past, Mr. Golenberg has served as a director or advisor of numerous publicly and privately held companies. He is a member of the Business Advisory Council at his alma mater, Miami University in Oxford, Ohio, and served on the Graduate Executive Board of the Wharton Graduate School of the University of Pennsylvania, where he received his MBA degree. Mr. Golenberg has been appointed to the Board of Governors, the Executive Committee, the Finance Committee and the Investment Committee of Cedars-Sinai Hospital, the Investment and Finance Committees of Wilshire Boulevard Temple, and the board, the Executive and the Finance Committees of the Jewish Community Foundation. Mr. Golenberg also served on the Executive and Finance Committees of the Jewish Federation and has served as Chairman of the Pension Investment Committee as well as Vice General Chairman of the United Jewish Fund. He was a member of Kennedy Center’s National Committee for the Performing Arts and was Co-Chairman of the Special Gifts Committee of the Music Center in Los Angeles, and was currently Vice Chairman of the International Advisory Board of the Tel Aviv University Recanati Business School, and is a member of the executive committee of the American friends of Tel Aviv university. Mr. Golenberg also was a member of the executive committee of the America-Israel Friendship League. Mr. Golenberg joined the board in April 2009.
| 34 |
Seymour (Sy) Siegel – Mr. Siegel is a CPA, inactive, and a principal emeritus at Rothstein Kass, a national firm of accountants and consultants, where he is a trusted advisor to business owners and responsible for business introductions. Mr. Siegel was a founder of Siegel Rich & Co. CPA’s, which eventually merged with WeiserMazars LLP, a large regional firm. He was a senior partner there until selling his interest and co-founding a business advisory firm, which later became a part of Rothstein Kass. He has been a director and officer of numerous business, philanthropic and civic organizations. As a professional director, he has served on the boards of approximately 10 public companies over the last 20 years, generally as audit committee chairman. Mr. Siegel has been chairman of the audit committee for Emerging Vision, Inc. and Global Aircraft Solutions, Inc. He is currently a director and chairman of the audit committees of Hauppauge Digital, Inc., Air Industries Group, Inc., and Premier Alliance Group, Inc.
Randall Cross – played football for the University of California, Los Angeles, where he received All Conference honors, All American honors and played an important role in the UCLA victory at the Rose Bowl in 1976. After being drafted in the second round of the 1976 NFL draft by the San Francisco 49ers, Mr. Cross played professional football with the San Francisco 49ers from 1976 to 1988, where he received six All Pro selections, three Pro Bowl selections and was a key player in the 49ers’ Superbowl championships in 1982, 1985 and 1989. Since 1989, he has been a broadcaster and analyst of the NFL for CBS and NBC, working on both network’s coverage of NFL regular season, playoff and Superbowl games. He currently co-hosts “The Opening Drive” show on FM 92.9 WZGM “The Game” in Atlanta. Off the field, Mr. Cross has been involved in marketing and promotions in several areas, including insurance, commodities and local and national retail sales. Mr. Cross joined the board in April 2009.
Michael Dunleavy, Sr. – From 2003 until 2010 Mr. Dunleavy was the head coach and from 2008 to 2010 also the general manager of the Los Angeles Clippers. From 2011 to 2012 he was part of a financing group that tried to buy the New Orleans Hornets. From 2012 to present he has been broadcasting for a number of media firms and serves as a Principal and board member for Remedy Analytics, where he owns 20% of that company. Selected in the sixth round (99th pick overall) by Philadelphia in 1976, Mr. Dunleavy played 11 seasons in the NBA with career averages of 8.0 points and 3.9 assists in 438 games for Philadelphia (1976-78), Houston (1978-82), San Antonio (1982-83) and Milwaukee (1983-85, 1988-1990). He began his coaching career as head coach for the Los Angeles Lakers is 1990. He then went on to coaching the Portland Trail Blazers and under his guidance the team matched its second best victory total in Blazers history. Mr. Dunleavy earned the 1999 NBA Coach of the Year award. In 2010, he coached his 1,000th career game and won his 500th career game prior to leaving the Los Angeles Clippers. Mr. Dunleavy joined the board in October 2009.
John A. (Jack) Schneider, Jr. – served for over 30 years as Managing Director of Allen & Co., an international investor, underwriter, and broker to some of the biggest names in entertainment, technology, and information. Mr. Schneider also pioneered The Allen & Company Sun Valley Conference, widely considered one of the most influential gatherings of international business leaders, annually. Mr. Schneider is Chairman of the Buoniconti Fund to Cure Paralysis, a role he has held for 25 years. He has contributed for over a decade as a board member of the National Mentoring Partnership and has extensive contributions with the St. Vincent’s Services to kids. Since 2012, Mr. Schneider has been a managing director at Dominick & Dominick, a Wall Street investment firm founded in 1870.
| 35 |
Executive Officers
The following table sets forth, as of the Effective Date, the name of, and certain information concerning, our executive officers other than Mr. Rubinstein and Dr. Schwartz:
| Name | Age | Position | ||
| John Moynahan | 56 | Senior Vice President and Chief Financial Officer | ||
| Timothy Boris | 44 | General Counsel and Vice President of Legal Affairs | ||
| Craig Abolin | 65 | Vice President of Research and Development of Canterbury and Hygeia |
John Moynahan – With over 37 years of business experience, Mr. Moynahan has been a treasurer for four years and CFO for 18 years of publicly-traded companies ranging from development stage to a billion dollars in annual revenues. During this span, Mr. Moynahan has been responsible for SEC reporting and compliance, successfully executing an IPO, completing over $500 million in debt financings, over $120 million in equity financings, and investigating and closing acquisitions with companies such as Fisher Scientific Group, Card Systems Solutions, Inc., Innovative Technology Applications, Inc., and Xybernaut Corporation. Mr. Moynahan joined the Company in 2007 as a consultant and became an employee in 2009. Mr. Moynahan began his career in the New York City office of Ernst & Young in 1979. He received a B.A. from Colgate University, where he was elected to the Phi Beta Kappa honor society, an M.B.A from New York University and a C.P.A. from New York State. Mr. Moynahan is a co-inventor on five issued U.S. patents and over 100 corresponding international patents involving wearable computing technology.
Timothy Boris – Mr. Boris joined Stratus Media Group in August 2011. He has been practicing law for more than sixteen years. From 2005 to 2011, he was in private practice representing corporate and entertainment clients. He is a former partner at the firm of Hager & Dowling. His areas of practice have included litigation, entertainment and corporate law. He received a Bachelor’s of Business Administration from the University of Michigan and a juris doctorate from the University of San Diego School of Law.
Craig Abolin – From November 1979 to September 1981, Dr. Abolin was a Senior Scientist, Drug Metabolism for Astra Pharmaceutical Products, Inc., a company engaged in drug discovery, development and marketing; From October 1981 to June 1997, Dr. Abolin was Bioanalytics Unit Head and Group Leader for Sandoz Research Institute/Novartis, a company engaged in drug discovery, development and marketing; From July 1997 to April, 2000 Dr. Abolin was a Pharmacokineticist for Hurley Consulting Associates, Ltd., a company engaged in providing technical clinical contract services for the pharmaceutical industry; From May, 2000 until July 2007, Dr. Abolin was Director of Drug Metabolism for Sepracor Inc., a company engaged in drug discovery, in-licensing, development and marketing; From November 2007 to present, Dr. Abolin was cofounder and Chief Scientific Officer for Hygeia. From March 2012 to present, Dr. Abolin was cofounder and Chief Scientific Officer for Canterbury. He received a B.S. in Pharmacy from West Virginia University, a M.S. in Pharmaceutics from West Virginia University and a PhD in Pharmceutical Chemistry from the University of California San Francisco.
| 36 |
Family Relationships
There are no family relationships among the directors and officers.
Term of Office
Our directors and officers hold office until the earlier of their death, resignation, removal or the end of their stated term.
The Board of Directors and Committees
The Board of Directors is responsible for the supervision of the overall affairs of the Company. The Board met 6 times during the year ended December 31, 2012. As of the Effective Date, the Audit Committee will be chaired by Jerold Rubinstein and includes Sol J. Barer, Yael Schwartz and Isaac Blech. The Compensation Committee is chaired by Isaac Blech and includes Sol J. Barer, Yael Schwartz and Jerold Rubinstein.
Term of Office
Our directors and officers hold office until the earlier of their death, resignation, removal or the end of their stated term.
Code of Ethics
We have not adopted a Code of Ethics that is applicable to our directors and our employees, but intend to do so after the Effective Date.
Audit Committee
The Audit Committee’s responsibilities include, but are not limited to, the following:
| · | appointing, evaluating and retaining the independent registered public accounting firm, |
| · | reviewing and discussing with management and the independent registered public accounting firm our annual and quarterly financial statements and disclosures, |
| · | discussing our systems of internal control over financial reporting, and |
| · | meeting separately with the independent registered public accounting firm. |
The audit committee was reestablished during 2009 and met 5 times during 2012. The committee currently consists of Jerold Rubinstein, who is the Chairman and who the Company believes qualifies as a financial expert; Isaac Blech, Yael Schwartz and Sol J. Barer.
Compensation Committee
The compensation committee was established during 2011. The Compensation Committee will administer the Company’s compensation and benefit plans, in particular, the incentive compensation and equity-based plans, and will approve salaries, bonuses, and other compensation arrangements and policies for the Company’s officers, including the Chief Executive Officer. The Compensation Committee did not meet during 2012.
| 37 |
EXECUTIVE COMPENSATION
Overview of Executive Compensation Program
Until the Compensation Committee is established, the Board has responsibility for establishing, implementing and monitoring our executive compensation program philosophy and practices. The Board seeks to ensure that the total compensation paid to our executive officers is fair, reasonable and competitive.
Executive Compensation
The following table sets forth information concerning the compensation earned by our Executive Officers during fiscal 2012 and 2011:
| Name and Principal Position | Year | Salary | Bonus | Stock Award Shares | Non-equity Incentive Plan Compensation | All Other Compensation | Total | |||||||||||||||||||
| Jerold Rubinstein, Chief Executive Officer and Chairman of the Board | 2012 | $ | 125,000 | (a) | $ | – | 2,300,000 | $ | – | $ | 223,900 | (b) | $ | 348,900 | ||||||||||||
| 2011 | $ | – | (a) | $ | – | – | $ | – | $ | 133,333 | (c) | $ | 133,333 | |||||||||||||
| John Moynahan, Chief Financial Officer | 2012 | $ | 220,000 | $ | – | – | $ | – | $ | 20,466 | (d) | $ | 240,466 | |||||||||||||
| 2011 | $ | 220,000 | $ | – | – | $ | – | $ | 10,387 | (d) | $ | 230,387 | ||||||||||||||
| Timothy Boris, General Counsel and Vice President of Legal Affairs | 2012 | $ | 180,000 | $ | – | 300,000 | $ | – | $ | – | $ | 180,000 | ||||||||||||||
| 2011 | $ | 180,000 | $ | – | 300,000 | $ | – | $ | – | $ | 180,000 | |||||||||||||||
| Paul Feller, former Chief Executive Officer and former Chairman of the Board | 2012 | $ | 125,000 | (e) | $ | – | – | $ | – | $ | – | $ | 125,000 | |||||||||||||
| 2011 | $ | 240,000 | $ | – | – | $ | – | $ | – | $ | 240,000 | |||||||||||||||
| William Kelly, former Chief Operating Officer | 2012 | $ | 240,000 | (f) | $ | – | – | $ | – | $ | – | $ | 240,000 | |||||||||||||
| 2011 | $ | 240,000 | $ | – | – | $ | – | $ | – | $ | 240,000 | |||||||||||||||
____________
| (a) | Mr. Rubinstein started as C.E.O. on June 28, 2012. |
| (b) | Represents $100,000 as chairman of the audit committee up to June 28, 2012, $100,000 as chairman of the board following that date, $50,000 as member of the board of directors, six months of an auto allowance of $650 per month and $20,000 as consulting fee for May and June 2012. |
| (c) | Represents $100,000 as chairman of the audit committee from April 2011 to the end of the year and $33,000 as a board member from April 2011 to the end of the year. |
| (d) | Represents cost of living increases earned in these years but not paid. |
| (e) | Mr. Feller's employment ended June 28, 2012. |
| (f) | Mr. Kelly's employment ended in March 2013. |
| 38 |
Effective June 28, 2012, Jerold Rubinstein was elected by the Company’s board of directors as Chairman of the Board, CEO and a director of the Company’s subsidiaries. The Board of Directors of PEI also elected him as Chairman of the Board and CEO. Under the terms of an employment agreement dated June 28, 2012, Mr. Rubinstein will receive an annual salary of $250,000 per year and will continue to serve on the Company’s board of directors and as Chairman of the Company’s Audit Committee and shall continue to receive his compensation for such services. The term of this agreement is six months with an automatic six month extension unless the Company provides written notice of non-renewal 30 days prior to the end of the initial six-month term. This executive has been granted options to purchase 2,300,000 shares of the Company’s common stock at $0.35 per share, which was the closing price of the Company’s common stock on the day of option grant. These options vest monthly over a twelve-month period. In the event the Company does not renew the second six month period, the executive resigns or the Company terminates the executive’s employment without cause, all options will immediately vest and the executive will receive all unpaid salary for the full twelve month period.
On August 8, 2011, the Company entered into any employment contract with Timothy Boris as the Company’s General Counsel and Vice President of Legal Affairs at an annual salary of $180,000. In December 2011, he received options to purchase 300,000 shares of common stock at $0.54 that had 100,000 shares vest upon grant, 100,000 shares vest at the end of year one and 100,000 shares vest at the end of year two. This contract expired on August 8, 2012 and was renewed under the same terms until August 8, 2013. In August 2012 Mr. Boris received options to purchase 300,000 shares of common stock at $0.38 that had 100,000 shares vest upon grant, 100,000 shares vest at the end of year one and 100,000 shares vest at the end of year two. Both of these option grants have a five-year life.
On November 1, 2010, the Company entered into an employment agreement with John Moynahan, who had been providing accounting and financial services to the Company as a consultant pursuant to a consulting agreement dated November 14, 2007. This agreement expired on August 1, 2012. Under the agreement, Mr. Moynahan was to receive an annual salary of $220,000 for the first year of the contract, subject to an annual increase of the Consumer Price Index plus 2%, and will be eligible for a $50,000 bonus in the first year of this contract, with bonuses thereafter based on objectives established by the Company’s board of directors and Mr. Moynahan’s performance against those objectives. Under this agreement, Mr. Moynahan received a grant of 300,000 shares and a five-year stock option grant to purchase 1,560,000 shares of common stock at $2.00 per share, with 1,040,000 shares that vested upon the signing of the agreement and 520,000 shares that will vest on September 1, 2011. Such options shall terminate forty-five (45) days after the Executive’s employment with the Company is terminated if such termination is for Cause or is the result of a resignation by Executive for reasons other than Good Reason. Such options shall not be assignable by Executive. Each option described above shall be subject to customary anti-dilution provision with respect to any stock splits, mergers, reorganizations or other such events.
As set forth in Item 1.01 of this Current Report on Form 8-K, effective as of the Effective Date of the Mergers, the Company entered into employment agreements with Yael Schwartz and Craig Abolin as follows:
Under the Employment Agreement with Dr. Schwartz, she is to be employed for an initial period of three years. During the initial year of her employment term, she is to receive a base salary of $330,000. Thereafter, her base salary will be subject to mutually agreed upon increases. The Company’s board of directors (the “Board”) or Compensation Committee may grant Dr. Schwartz bonuses in its sole discretion. Dr. Schwartz is also eligible for grants of awards under the Company’s Incentive Compensation Plan.
Under the employment agreement with Dr. Abolin, he is to be employed for an initial period of three years. During the initial year, he is to receive a base salary of $241,000. Thereafter his base salary will be subject to mutually agreed upon increases. The Company’s Board or Compensation Committee may grant Dr. Abolin bonuses in its sole discretion. Dr. Abolin is also eligible for grants of awards under the Company’s Incentive Compensation Plan.
| 39 |
OUTSTANDING EQUITY AWARDS AT APRIL 15, 2013
The following table sets forth certain information relating to unexercised and outstanding options for each named executive officer as of April 15, 2013. No other equity awards otherwise reportable in this table had been granted to any of our executive officers as of that date.
| Unexercised | ||||||||
| Options | Option | Option | ||||||
| Outstanding | that are | Exercise | Expiration | |||||
| Name | Options | Exercisable | Price | Date | ||||
| Jerold Rubinstein | 25,000,000 | 25,000,000 | $0.03 | 3/27/18 | ||||
| Jerold Rubinstein | 2,300,000 | 2,300,000 | $0.35 | 6/28/2017 | ||||
| John Moynahan | 1,540,000 | 1,540,000 | $0.54 | 11/1/2015 | ||||
| Timothy Boris | 6,000,000 | 6,000,000 | $0.03 | 3/27/18 | ||||
| Timothy Boris | 300,000 | 200,000 | $0.54 | 12/29/2016 | ||||
| Timothy Boris | 300,000 | 100,000 | $0.38 | 8/20/2017 |
Employment Agreements
As of September 30, 2013, there were no future minimum payments under the employment agreements.
Option Plans
The Company is intending to adopt, but has not yet completed, its Stock Compensation Program (the “Stock Compensation Program”). This program is intended to provide key employees, vendors, directors, consultants and other key contributors to Company growth an opportunity to participate in the Company’s success. It is estimated that 15% of total shares outstanding will be authorized in options and reserved for this program. Awards under the program may be made in the form of incentive stock options, nonqualified stock options, restricted shares, rights to purchase shares under an employee stock plan, grants of options to non-employee directors, and or other specified stock rights as defined under the plan. Subject to Shareholder approval, the Company plans to adopt a new stock option plan in 2014.
EQUITY COMPENSATION PLAN INFORMATION
The following table provides information as of October 15, 2013 regarding compensation plans (including individual compensation arrangements) under which our securities are authorized for issuance. Information is included for both equity compensation plans approved by our stockholders and equity compensation plans not approved by our stockholders.
| Plan Category | Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights | Weighted-average Exercise Price of Outstanding Options, Warrants and Rights | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding securities in the first column) | |||||||||
| Equity compensation plans approved by stockholders | – | $ | – | – | ||||||||
| Equity compensation plans not approved by stockholders | 38,943,523 | $ | 0.12 | – | ||||||||
| Total | 38,943,523 | $ | 0.12 | – | ||||||||
The above-referenced stock option grants were issued without registration in reliance upon the exemption afforded by Section 4(2) of the Securities Act of 1933, as amended, based on certain representations made to us by the recipients.
| 40 |
Director Compensation
Board of Directors Compensation:
Outside Board members have received a grant of 450,000 options upon joining the Board that vest monthly over a 36-month period. Each board member is entitled to an annual payment of $50,000. Jerold Rubinstein received an additional $150,000 per annum as the chairman of the board. Board members received $179,167 cash compensation in 2012. As of December 31, 2012, $216,010 in board cash compensation is still outstanding.
Upon joining the board in 2011, Mr. Rubinstein received an additional grant of 450,000 shares of restricted common stock as chairman of the audit committee that vest over a 36 month period. Mr. Golenberg received an additional grant of 450,000 shares of restricted common stock that vests over a 36 month period as chairman of the compensation committee. As of July 1, 2011, the Board of Directors elected to cancel a total of 1,550,000 options granted to Messrs. Cross and Dunleavy and Golenberg in 2009 for board service and to Mr. Golenberg in 2009 and 2010 as chairman of the audit committee, and replace those options with grants of 540,833 shares of restricted stock equal to 50% of the number of vested options. These grants vest one-third on January 1, 2012, one-third on January 1, 2013 and one-third on January 1, 2014. Pursuant to these grants, Mr. Cross received a grant of 162,500 shares of restricted stock, of which 54,167 shares vested on January 1, 2012; Mr. Dunleavy received a grant of 130,000 shares of restricted stock, of which 43,333 shares vested on January 1, 2012; and Mr. Golenberg received a grant of 248,333 shares of restricted stock, of which 82,778 shares vested on January 1, 2012.
The Board is currently reviewing the future compensation to be paid to Board members.
DESCRIPTION OF CAPITAL STOCK
The following description of our capital stock summarizes the material terms and provisions of the indicated securities. For the complete terms of our Common Stock please refer to our articles of incorporation, and bylaws that we have filed with the SEC.
We are authorized to issue 1,000,000,000 shares of Common Stock, $0.001 par value per share, and 5,000,000 shares of Preferred Stock.
Common Stock
Voting. Each holder of Common Stock shall have one vote in respect of each share of stock held of record on the books of the corporation for the election of directors and on all matters submitted to a vote of our stockholders.
Dividends. The holders of shares of Common Stock shall be entitled to receive, when and if declared by the board of directors, out of our assets which are by law available for dividends, dividends payable in cash, property or shares of capital stock.
Dissolution, Liquidation or Winding Up. In the event of any dissolution, liquidation or winding up of our affairs, holders of Common Stock shall be entitled, unless otherwise provided by law or our articles of incorporation, including any certificate of designations for a series of preferred stock, to receive all of our remaining assets of whatever kind available for distribution to stockholders ratably in proportion to the number of shares of Common Stock held by them respectively.
Other Rights and Restrictions. Holders of our Common Stock do not have preemptive rights, and they have no right to convert their Common Stock into any other securities. Our Common Stock is not subject to redemption by us. The rights, preferences and privileges of common stockholders are subject to the rights of the stockholders of any series of preferred stock that are issued and outstanding or that we may issue in the future.
| 41 |
Preferred Stock
The preferred stock may be issued in one or more series and our Board of Directors, without further approval from our stockholders, is authorized to fix the dividend rights and terms, conversion rights, voting rights, redemption rights, liquidation preferences and other rights and restrictions relating to any series. Issuances of preferred stock, while providing flexibility in connection with possible financings, acquisitions and other corporate purposes, could, among other things, adversely affect the voting power of the holders of our common stock.
MARKET FOR COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASE OF EQUITY SECURITIES
Price Range of Common Stock
The following table sets forth the high and low prices of our common stock during the past two years, for each period indicated, as reported by the OTCBB or OTCQB the dates indicated. Such quotations reflect prices between dealers in securities and do not include any retail mark-up, markdowns or commissions and may not necessarily represent actual transactions.
Fiscal Period
| High | Low | |||
| 2013 | ||||
| First quarter | $0.20 | $0.08 | ||
| Second quarter | $0.24 | $0.14 | ||
| Third quarter | $0.19 | $0.06 | ||
| 2012 | ||||
| First quarter | $0.54 | $0.40 | ||
| Second quarter | $0.55 | $0.31 | ||
| Third quarter | $0.50 | $0.30 | ||
| Fourth quarter | $0.44 | $0.12 | ||
| 2011 | ||||
| First quarter | $0.65 | $0.27 | ||
| Second quarter | $1.02 | $0.33 | ||
| Third quarter | $0.89 | $0.48 | ||
| Fourth quarter | $0.81 | $0.40 |
As of December 31, 2012, the Company believes there were approximately 1,300 stockholders of record of our common stock.
Dividend Policy
Since our inception, we have never declared or paid any cash dividends. We currently expect to retain earnings for use in the operation and expansion of our business, and therefore do not anticipate paying any cash dividends in the foreseeable future.
Item 3.02 |
Unregistered Shares of Equity Securities |
Pursuant to the Merger Agreement, the Company issued to the stakeholders of Canterbury and Hygeia an aggregate of 115,011,563 restricted shares of the Company’s Common Stock. The shares of the Company’s common stock issued to the holders of the capital stock of Canterbury and Hygeia in connection with the Mergers were not registered under the Securities Act of 1933, as amended (the “Securities Act”), in reliance upon the exemption from registration provided by Section 4(2) of the Securities Act and Regulation D promulgated under that section, which exempts transactions by an issuer not involving any public offering. These securities may not be offered or sold in the U.S. absent registration or an applicable exemption from the registration requirements. Certificates representing these shares will contain a legend stating the restrictions applicable to such shares.
| 42 |
Item 5.02 |
Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers |
In connection with the closing of the Mergers described in Item 1.02, below, the Company entered into employment agreements with (a) Yael Schwartz, Ph.D., pursuant to which Dr. Schwartz was appointed President of Canterbury and (the new subsidiaries of the Company acquired pursuant to the Mergers; and (b) Craig Abolin, Ph.D. pursuant to which Dr. Abolin was appointed Vice President of Research and Development of the new subsidiaries.
Under the Employment Agreement with Dr. Schwartz, she is to be employed for an initial period of three years. During the initial year of her employment term, she is to receive a base salary of $330,000. Thereafter, her base salary will be subject to mutually agreed upon increases. The Board or the Compensation Committee may grant Dr. Schwartz bonuses in its sole discretion. Dr. Schwartz is also eligible for grants of awards under the Company’s Incentive Compensation Plan.
Under the employment agreement with Dr. Abolin, he is to be employed for an initial period of three years. During the initial year, he is to receive a base salary of $241,000. Thereafter his base salary will be subject to mutually agreed upon increases. The Board or the Compensation Committee may grant Dr. Abolin bonuses in its sole discretion. Dr. Abolin is also eligible for grants of awards under the Company’s Incentive Compensation Plan.
Changes to the Board of Directors and Executive Officers. Upon the closing of the Mergers, pursuant to the Merger Agreement, Yael Schwartz, Ph.D. and Nelson Stacks were appointed by the Company’s board of directors (the “Board”) as directors of the Company. In addition, upon the closing of the Mergers, Dr. Schwartz was appointed as President of Canterbury and Hygeia and Dr. Craig Abolin was appointed as Vice President of Research and Development of Canterbury and Hygeia. The terms of their employment are set forth in Item 1.01 and their background is set forth in Item 1.02 under “Directors, Executive Officers and Corporate Governance.”
| Item 5.06 | Change in Shell Company Status |
While the Company believes that immediately prior to the Effective Date it may not be deemed to have been a shell company under Rule 12b-2 under the Exchange Act, the Company believes that, even if the Company may be deemed to have been a shell company, the Mergers have the effect of the Company ceasing to be a shell company.
| Item 9.01 | Financial Statements and Exhibits |
(a) Financial Statements of Businesses Acquired. In accordance with Item 9.01(a), Canterbury’s audited financial statements for the fiscal years ended December 31, 2011 and 2012 are filed in this Current Report on Form 8-K as Exhibit 99.1 and Canterbury’s unaudited financial statements for the nine months ended September 30, 2012 are filed as Exhibit 99.2.
(b) Pro Forma Financial Information. In accordance with Item 9.01(b), our pro forma financial statements are filed as Exhibit 99.3.
| 43 |
(d) Exhibits.
| Exhibit Number | Description | |
| 10.1 | Agreement and Plan of Merger among the Company, Canterbury Acquisition LLC, Hygeia Acquisition, Inc., Canterbury, Hygeia and Yael Schwartz.(1) | |
| 10.2 | Employment Agreement between the Company and Yael Schwartz | |
| 10.3 | Employment Agreement between Company and Craig Abolin | |
| 10.4 | Registration Rights Agreement | |
| 10.5 | Exclusive License Agreement with Yale University effective as of October 22, 2007, as amended | |
| 10.6 | Sublicense Agreement with Ferndale Pharma Group, Inc. dated as of March 22, 2012 | |
| 10.7 | Collaboration Agreement with Ferndale Pharma Group, Inc. dated as of July 25, 2013 | |
| 10.8 | Master Services Agreement with MicroConstants dated March 22, 2012. | |
| 10.9 | Master Contract and Services Agreement with GL Synthesis effective as of March 25, 2012 | |
| 10.10 | Service Agreement with CEREP dated October 18, 2011 | |
| 99.1 | Canterbury audited financial statements for the fiscal years ended December 31, 2012 and 2011 and unaudited financial statements for the nine months ended September 30, 2013 and 2012 | |
| 99.2 | Pro forma unaudited statements of operations for the fiscal year ended December 31, 2012, pro forma unaudited statement of operations for the nine months ended September 30, 2013 and pro forma unaudited statement of financial position for September 30, 2013 |
____________
| (1) | Incorporated by reference to Exhibit 10.01 to the Company’s Current Report on Form 8-K filed on October 2, 2013. |
| 44 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| STRATUS MEDIA GROUP, INC. | ||
| Dated: November 22, 2012 | By: | /s/ Jerold Rubinstein |
|
Jerold
Rubinstein, Chief Executive Officer | ||
| 45 |
Exhibit Index
| Exhibit Number | Description | |
| 10.1 | Agreement and Plan of Merger among the Company, Canterbury Acquisition LLC, Hygeia Acquisition, Inc., Canterbury, Hygeia and Yael Schwartz.(1) | |
| 10.2 | Employment Agreement between the Company and Yael Schwartz | |
| 10.3 | Employment Agreement between Company and Craig Abolin | |
| 10.4 | Registration Rights Agreement | |
| 10.5 | Exclusive License Agreement with Yale University effective as of October 22, 2007, as amended | |
| 10.6 | Sublicense Agreement with Ferndale Pharma Group, Inc. dated as of March 22, 2012 | |
| 10.7 | Collaboration Agreement with Ferndale Pharma Group, Inc. dated as of July 25, 2013 | |
| 10.8 | Master Services Agreement with MicroConstants dated March 22, 2012. | |
| 10.9 | Master Contract and Services Agreement with GL Synthesis effective as of March 25, 2012 | |
| 10.10 | Service Agreement with CEREP dated October 18, 2011 | |
| 99.1 | Canterbury audited financial statements for the fiscal years ended December 31, 2012 and 2011 and unaudited financial statements for the nine months ended September 30, 2013 and 2012 | |
| 99.2 | Pro forma unaudited statements of operations for the fiscal year ended December 31, 2012, pro forma unaudited statement of operations for the nine months ended September 30, 2013 and pro forma unaudited statement of financial position for September 30, 2013 |
____________
(1) Incorporated by reference to Exhibit 10.01 to the Company’s Current Report on Form 8-K filed on October 2, 2013.
| 46 |

