As filed with the Securities and Exchange Commission on September 16, 2016
Registration No.
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
SUN BIOPHARMA, INC.
(Exact name of registrant as specified in its charter)
|
Delaware |
2834 |
87-0543922 |
712 Vista Blvd, Suite 305
Waconia, Minnesota 55387
(952) 479-1196
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
David B. Kaysen
President and Chief Executive Officer
Sun BioPharma, Inc.
712 Vista Blvd, Suite 305
Waconia, Minnesota 55387
(952) 479-1196
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
|
W. Morgan Burns, Esq. Fax: (612) 766-1600 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended, check the following box. ☐
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer ☐ |
|
Accelerated filer ☐ |
|
Non-accelerated filer ☐ |
|
Smaller reporting company ☑ |
CALCULATION OF REGISTRATION FEE
|
Title of Each Class of Securities to be Registered |
Amount to be Registered(1) |
Proposed Maximum Offering Price per Share(2) |
Proposed Maximum Aggregate Offering Price |
Amount of Registration Fee |
|
Common stock, par value $0.001 per share |
2,221,000 shares |
$2.505 |
$5,563,605 |
$560.26 |
|
Common stock, par value $0.001 per share (3) |
1,110,500 shares |
$2.505 |
$2,781,803 |
$280.13 |
|
Total |
3,331,500 shares |
$8,345,408 |
$840.39 |
|
(1) |
Consists of shares of common stock issued and outstanding and shares of common stock issued or issuable upon the exercise of warrants. Pursuant to Rule 416 under the Securities Act of 1933, as amended, this registration statement also registers shares of common stock that may be issued pursuant to the operation of certain-anti-dilution provisions of the warrants. |
|
(2) |
Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(c) of the Securities Act of 1933, as amended, based on the average bid and ask sales prices of the registrant’s common stock as reported by OTC Markets Group, Inc. on September 12, 2016. |
|
(3) |
Issuable upon exercise of warrants. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. The selling stockholders may not sell these securities until the registration statement filed with Securities and Exchange Commission is declared effective. This preliminary prospectus is not an offer to sell these securities, and the selling stockholders are not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
|
PRELIMINARY PROSPECTUS |
SUBJECT TO COMPLETION, DATED SEPTEMBER 16, 2016 |

Sun BioPharma, Inc.
3,331,500 Shares Common Stock
This prospectus relates to 3,331,500 shares of our common stock, which are issued and outstanding or may be issued upon exercise of warrants. These shares consist of shares of our common stock that we issued to the selling stockholders pursuant to private placements of our common stock and shares of our common stock issuable upon the exercise of warrants to purchase our common stock. Such common stock may be sold from time to time by the selling stockholders named herein.
We are not offering any shares of common stock for sale under this prospectus, and we will not receive any of the proceeds from the sale or other disposition of the shares offered hereby. The prices at which the selling stockholders may sell the shares will be determined by the prevailing market price for the shares or in negotiated transactions.
Our common stock is quoted on the OTCPink tier of the over-the-counter markets administered by the OTC Markets Group, Inc., under the symbol “SNBP.” On September 12, 2016, the last reported sale price of our common stock was $2.00 per share.
Investing in our common stock involves a high degree of risk. See “Risk Factors” beginning on page 6 of this prospectus for a discussion of information that should be considered in connection with an investment in our common stock.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is September 16, 2016
Table of Contents
Page
|
Prospectus Summary |
1 |
|
The Offering |
5 |
|
SECURITIES PURCHASE AGREEMENTS |
5 |
|
Risk Factors |
6 |
|
CAUTIONARY Note Regarding Forward-Looking Statements |
17 |
|
Use of Proceeds |
17 |
|
Price Range of Common Stock |
17 |
|
Dividend Policy |
19 |
|
PRINCIPAL AND SELLING STOCKHOLDERS |
19 |
|
DESCRIPTION OF Securities |
22 |
|
PLAN OF DISTRIBUTION |
26 |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
28 |
|
Business |
38 |
|
Management |
56 |
|
Executive Compensation |
61 |
|
Legal Matters |
66 |
|
Experts |
66 |
|
Where You Can Find More Information |
66 |
ABOUT THIS PROSPECTUS
You should rely only on the information contained in, or incorporated by reference into, this prospectus. We have not authorized anyone to provide you with any information that is different. Neither we, nor the selling stockholders, are making any offer to sell these securities in any jurisdiction where the offer is not permitted. The information in this prospectus is accurate only as of the applicable dates, regardless of the time of delivery of this prospectus or any sale of shares of our common stock. Our business, financial condition, results of operations and prospects may have changed since that date.
For investors outside the United States: Neither we nor the selling stockholder have taken any action to permit a public offering of the shares of our common stock or the possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
Prospectus Summary
This summary highlights information contained elsewhere in this prospectus and does not contain all of the information that you should consider in making your investment decision. Before investing in our securities, you should carefully read this entire prospectus, including our financial statements and the related notes and the information set forth under the headings “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in each case included elsewhere in this prospectus. Unless otherwise stated or the context requires otherwise, references in this prospectus to “Sun BioPharma”, the “Company”, “we”, “us”, “our” and similar references refer to Sun BioPharma, Inc.(“SBI”) and its wholly-owned subsidiary, Sun BioPharma Australia Pty Ltd. (“SBA”).
Business Overview
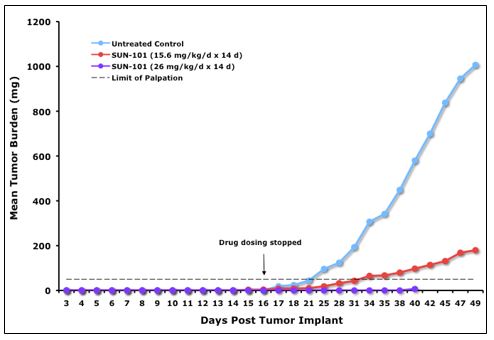
Sun BioPharma, Inc. is a clinical stage drug development company founded with technology licensed from the University of Florida. We have exclusively licensed the worldwide rights to a compound derived from this technology, which has been designated as SBP-101, from the University of Florida Research Foundation, Inc. (“UFRF”). SBP-101 exhibits extraordinary specificity for the exocrine pancreas, with therapeutic potential for both pancreatic cancer and pancreatitis indications. Studies in dogs revealed ablation, or “chemical resection,” of the exocrine pancreatic architecture, while leaving the islet cells functionally unchanged. We may refer to this effect as: “pharmaceutical pancreatectomy with islet auto-transplant” (“PP-IAT”). Xenograft studies of human pancreatic cancer cells transplanted into mice indicate that SBP-101 suppresses both primary and metastatic growth of these cells. To facilitate and accelerate the development of this compound in the pancreatic cancer indication, SBI has also acquired data and materials related to this technology from other researchers. We believe that SBP-101, if successfully developed, may represent a novel approach that effectively treats pancreatic cancer and pancreatitis, and could become the dominant product in these markets. Only three first-line treatment options for pancreatic cancer have been approved by the United States Food & Drug Administration (“FDA”) in the last 20 years, and no drugs have been approved for the treatment of patients with pancreatitis.
We estimate that completion of necessary preclinical development work, the completion of a Phase 1 clinical trial in pancreatic cancer and initiation of a Phase 1 clinical trial in pancreatitis, will require additional funding of at least $10 million to $20 million in addition to amounts we have previously raised. Additional clinical trials will be subsequently required for FDA approval if the results of the first clinical trials of SBP-101 are positive. We estimate that the additional time and cost to obtain FDA and European Medicines Agency (“EMA”) approval and to bring SBP-101 to market in these two indications will be 5 to 7 years with related costs up to $200 million.
With the approximately $13.5 million we have raised to-date, we have:
|
● |
organized the Company; |
|
● |
evaluated and secured the intellectual property for our core technology; |
|
● |
completed initial pre-clinical steps in the development plan for SBP-101; |
|
● |
secured an orphan drug designation from the FDA; |
|
● |
submitted an Investigative New Drug (“IND”) application to the FDA (May 18, 2015); |
|
● |
received an acceptance of the IND application from the FDA (August 21, 2015); |
|
● |
commenced a Phase 1 clinical trial in the pancreatic ductal adenocarcinoma; |
|
● |
completed enrollment and follow-up of two cohorts of patients; and |
|
● |
commenced further pre-clinical studies for the use of SBP-101 to treat pancreatitis. |
Recent Developments
On August 15, 2016 we reported additional progress in the Phase 1 Study of the Company’s candidate SBP101 for pancreatic cancer. The study’s Data Safety Monitoring Board has completed its independent safety review of the data from the dosing of the second cohort of patients. As a result of this review, the Company has implemented progression to the third patient cohort in the dose escalation phase of the study.
Risks Associated with Our Business
Our business is subject to many significant risks, as more fully described in the section entitled “Risk Factors” immediately following this prospectus summary. You should read and carefully consider these risks, together with the risks set forth under the section titled “Risk Factors” and all of the other information in this prospectus, including the financial statements and the related notes included elsewhere in this prospectus, before deciding whether to invest in our common stock. If any of the risks discussed in this prospectus actually occur, our business, financial condition or operating results could be materially and adversely affected. In particular, our risks include, but are not limited to, the following:
|
● |
We are an early stage company and it will take several years to have any of our proposed product candidates approved, assuming such approval can be obtained at all. We therefore do not expect to generate revenue from product sales for at least the next several years. |
|
● |
Notwithstanding the successful completion of a financing in June through August 2016 in which we received approximately $2.2 million in gross proceeds, as a result of the pre-revenue nature of our company and our then current lack of financial liquidity, the Report of Independent Registered Public Accounting Firm for our 2015 financial statements, which are included as part of this prospectus, contains a statement concerning our ability to continue as a “going concern”. |
|
● |
Our limited operating history makes it difficult for you to evaluate our business to date and to assess our future viability. |
|
● |
Our lack of diversification increases the risk of an investment in our Company and our future viability may be adversely impacted if we fail to diversify. |
|
● |
Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates. |
|
● |
The market for our product candidate is highly competitive and is subject to rapid scientific change, which could have a material adverse effect on our business, results of operations and financial condition. |
|
● |
Clinical trials required for our product candidate are expensive and time-consuming, and their outcome is highly uncertain. If any of our drug trials are delayed or yield unfavorable results, we will have to delay application for or may be unable to obtain regulatory approval for our product candidate. |
|
● |
We are subject to extensive regulation, and if we fail to obtain, or if there are delays in obtaining, required regulatory approvals, we will not be able to commercialize our product candidate, and our ability to generate revenue and the viability of our company will be materially impaired. |
|
● |
We face substantial competition, which may result in others discovering, developing or commercializing competing products before or more successfully than we do. |
|
● |
Our directors, executive officers and significant stockholders have substantial control over us and could limit stockholders’ ability to influence the outcome of key transactions, including changes of control. |
Implications of Being an Emerging Growth Company
We qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act, or JOBS Act, enacted in April 2012. An emerging growth company may take advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not limited to:
|
● |
Being required to provide only two years of audited financial statements in addition to any required unaudited interim financial statements, with correspondingly reduced disclosure in the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section of this prospectus; |
|
● |
Not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes- Oxley Act of 2002, or Sarbanes-Oxley Act; |
|
● |
Reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements; and |
|
● |
Exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
We may take advantage of these provisions for up to five years after the first sale of our common equity securities pursuant to an effective registration statement under the Securities Act of 1933, as amended (the “Securities Act”). However, if certain events occur prior to the end of such five year period, including if we become a “large accelerated filer,” our annual gross revenues exceed $1 billion, or we issue more than $1 billion of non-convertible debt in any three year period, we would cease to be an emerging growth company prior to the end of such five year period.
We may choose to take advantage of some but not all of these reduced requirements. We have taken advantage of certain of the reduced disclosure obligations, which include reduced executive compensation disclosure in this registration statement and may elect to take advantage of other reduced requirements in future filings. As a result, the information that we provide to our stockholders may be different than you might receive from other public reporting companies in which you hold equity interests.
Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards until such time as those standards apply to private companies. However, we have irrevocably elected not to avail ourselves of this extended transition period for complying with new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
We are also a “smaller reporting company” as defined in Rule 12b-2 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and have elected to take advantage of certain of the scaled disclosure available to smaller reporting companies.
Reverse Merger and Related Transactions
On September 4, 2015, we completed a reverse merger transaction in which SB Acquisition Corporation, Inc., a Delaware corporation and wholly-owned subsidiary of Cimarron Medical, Inc., or Merger Sub, merged with and into Sun BioPharma, Inc., a Delaware corporation (“SBI”), with SBI remaining as the surviving entity and a wholly-owned operating subsidiary of Cimarron Medical, Inc. This transaction is referred to throughout this prospectus as the “Merger.” In the Merger, each outstanding share of capital stock of SBI was automatically exchanged for 4 shares of Cimarron Medical common stock. As a result of the Merger, the former stockholders of SBI owned approximately 95% of the shares of the outstanding capital stock. In connection with the Merger, Sun BioPharma, Inc. changed its name to “Sun BioPharma Research, Inc.” and Cimarron Medical, Inc. changed its name to “Sun BioPharma, Inc.”
Concurrent with the Merger, two former directors and then-majority shareholders of Cimarron Medical, Inc., David Fuhrman and Robert Sargent (through his entity, Rare Principle, L.C.), sold (i) an aggregate of 571,266 shares of Cimarron Medical common stock, and (ii) a $250,000 portion of loan indebtedness they were owed by Cimarron Medical, Inc., to certain parties for total consideration of $250,000 (collectively, the “Stock and Debt Transactions”). The loan indebtedness was not modified from its existing terms and is not convertible into equity. The purchasers under the Stock and Debt Transactions consisted of the Ryan R. Gilbertson 2012 Irrevocable Family Trust, which acquired 471,093 shares and $125,000 principal amount of indebtedness; Douglas M. Polinsky, a then-former director of SBI, who acquired 216,705 shares and $100,000 principal amount of indebtedness; Providence Investments LLC, which acquired 170,532 shares and $25,000 principal amount of indebtedness; and Clearline Ventures, LLC, which acquired 218,000 shares. The Ryan R. Gilbertson 2012 Irrevocable Family Trust, Douglas M. Polinsky, Providence Investments LLC and Clearline Ventures, LLC (or their respective affiliates) held shares of SBI common stock at the time of the Merger. As a result, the former stockholders of SBI owned approximately 99% of the shares of the outstanding capital stock after giving effect to both the Merger and the Stock and Debt Transactions.
Reincorporation
On May 25, 2016, Sun BioPharma, Inc. completed a reincorporation into the State of Delaware from the State of Utah pursuant to an agreement and plan of merger between Sun BioPharma, Inc., a Utah corporation, and its wholly owned subsidiary, Sun BioPharma Research, Inc., a Delaware corporation. Upon the reincorporation, each outstanding certificate representing shares of the Utah corporation’s common stock was deemed, without any action by the holders thereof, to represent the same number and class of shares of our company’s common stock. As of May 25, 2016, the rights of our shareholders began to be governed by Delaware law and our current certificate of incorporation and bylaws.
Corporate Information
We were incorporated in Delaware in September 2011. Our corporate mailing address is 712 Vista Blvd, #305, Waconia, MN 55387. Our telephone number is (952) 479-1196, and our website is www.sunbiopharma.com. The information on our website is not part of this prospectus. We have included our website address as a factual reference and do not intend it to be an active link to our website. The information contained in or connected to our website is not incorporated by reference into, and should not be considered part of, this prospectus.
Sun BioPharma®, the Sun BioPharma logo, and other trademarks or service marks of Sun BioPharma, Inc. appearing in this prospectus are our property. Trade names, trademarks, and service marks of other companies appearing in this prospectus are the property of the respective holders.
The Offering
|
Common stock offered by the selling stockholders |
3,331,500 shares by the selling stockholders | |
|
|
||
|
Common stock outstanding before this offering |
32,151,306 Shares (see summary below) | |
|
Common stock outstanding after this offering |
32,151,306 Shares (see summary below) | |
|
|
||
|
Use of Proceeds |
The selling stockholders will receive all of the proceeds from the sale of the shares offered for sale by them under this prospectus. See “Use of Proceeds” on page 17 of this prospectus. | |
|
|
||
|
OTCPink Marketplace |
Our common stock is quoted on the OTCPink tier of the over-the-counter markets administered by the OTC Markets Group, Inc. under the symbol “SNBP.” | |
|
|
||
|
Risk Factors |
See “Risk Factors” beginning on page 6 of this prospectus and the other information included in this prospectus for a discussion of factors you should carefully consider before investing in our securities. |
The number of shares of our common stock outstanding before and after this offering is based on 32,151,306 shares of our common stock outstanding as of September 12, 2016, and excludes:
|
● |
3,163,600 shares of common stock issuable upon the exercise of outstanding stock options as of the date of this prospectus at a weighted average exercise price of $0.27 per share; |
|
● |
15,000,000 additional shares of common stock reserved and available for future issuances under our 2016 Stock Option Plan; |
|
● |
3,660,500 shares of common stock issuable upon exercise of stock purchase warrants at a weighted average exercise price of $0.59 per share, including 1,110,500 shares of common stock issuable pursuant to warrants held by the selling stockholders; and |
|
● |
an estimated 2,466,667 shares of common stock potentially issuable pursuant to convertible promissory notes. |
Unless otherwise indicated, all information in this prospectus assumes no exercise of the outstanding options or warrants or the conversion of shares issuable pursuant to convertible promissory notes.
SECURITIES PURCHASE AGREEMENTS
On each of June 10, 2016, June 24, 2016, August 11, 2016, and September 2, 2016, we entered into Securities Purchase Agreements with the selling stockholders named herein, pursuant to which we sold an aggregate of 2,221,000 shares of common stock and warrants to purchase an aggregate of 1,110,500 additional shares of common stock. The purchase price for each unit, consisting of one share of common stock and a warrant to purchase one-half share of common stock, was $1.00. The warrants are exercisable for a period of five years from their respective date of issuance at an exercise price of $1.50 per share.
Under the terms of the Securities Purchase Agreements, we agreed to file one or more registration statements, including the registration statement of which this prospectus is a part, as permissible and necessary to register under the Securities Act, the sale of the shares of our common stock that have been and may be issued to the selling stockholders under the Securities Purchase Agreements. See “Selling Stockholders” for more information.
Risk Factors
Any investment in our common stock involves a high degree of risk. Investors should carefully consider the risks described below and all of the information contained in this prospectus before deciding whether to purchase our common stock. Our business, financial condition or results of operations could be materially adversely affected by these risks if any of them actually occur. This prospectus also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including the risks we face as described below and elsewhere in this prospectus.
Risks Related to Our Business
We are a company with limited revenue history for you to evaluate our business.
Our Company has limited operating history for you to consider in evaluating our business and prospects. As such, it is difficult for potential investors to evaluate our business.
We have experienced negative cash flows for our operating activities since inception, primarily due to the investments required to commercialize our primary drug candidate, SBP-101. Our financing cash flows were positive due to the proceeds from equity and promissory notes issuances. Our net cash used in operating activities for 2015 was approximately $3.9 million and for the six months ended June 30, 2016 was approximately $631,000.
Our operations are subject to all of the risks, difficulties, complications and delays frequently encountered in connection with the formation of any new business, as well as those risks that are specific to the pharmaceutical and biotechnology industries in which we compete. Investors should evaluate us in light of the delays, expenses, problems and uncertainties frequently encountered by companies developing markets for new products, services and technologies. We may never overcome these obstacles.
As a result of our current lack of financial liquidity, we and our auditors have expressed substantial doubt regarding our ability to continue as a “going concern.”
As a result of our current lack of financial liquidity, our auditors’ report for our 2015 financial statements, which are incorporated by reference into this report, contains a statement concerning our ability to continue as a “going concern.” Our lack of sufficient liquidity could make it more difficult for us to secure additional financing or enter into strategic relationships on terms acceptable to us, if at all, and may materially and adversely affect the terms of any financing that we may obtain and our public stock price generally.
Our continuation as a “going concern” is dependent upon, among other things, achieving positive cash flow from operations and, if necessary, augmenting such cash flow using external resources to satisfy our cash needs. Our plans to achieve positive cash flow include engaging in offerings of securities, negotiating up-front and milestone payments on our current and potential future product candidates or royalties from sales of our products that secure regulatory approval and any milestone payments associated with such approved products. These cash sources could, potentially, be supplemented by financing or other strategic agreements. However, we may be unable to achieve these goals or obtain required funding on commercially reasonable terms or at all and therefore may be unable to continue as a going concern.
Our lack of diversification increases the risk of an investment in our Company and our financial condition and results of operations may deteriorate if we fail to diversify.
Our board of directors has centered our attention on our drug development activities, which are initially focused on the polyamine analogue compound we licensed from the UFRF. Our ability to diversify our investments will depend on our access to additional capital and financing sources and the availability and identification of suitable opportunities.
Larger companies have the ability to manage their risk by diversification. However, we lack and expect to continue to lack diversification, in terms of both the nature and geographic scope of our business. As a result, we will likely be impacted more acutely by factors affecting pharmaceutical and biotechnology industries in which we compete than we would if our business were more diversified, enhancing our risk profile. If we cannot diversify our operations, our financial condition and results of operations could deteriorate.
We may be unable to obtain the additional capital that is required to execute our business plan, which could restrict our ability to grow.
We expect that our current capital and our other existing resources will be sufficient only to provide a limited amount of working capital and may not be sufficient to fund our expected continuing opportunities. We likely will require additional capital to continue to operate our business.
Future acquisitions, research and development and capital expenditures, as well as our administrative requirements, such as clinical trial costs, salaries, insurance expenses and general overhead expenses, as well as legal compliance costs and accounting expenses, will require a substantial amount of additional capital and cash flow. There is no guarantee that we will be able to raise additional capital required to fund our ongoing business on commercially reasonable terms or at all.
We intend to pursue sources of additional capital through various financing transactions or arrangements, including collaboration arrangements, debt financing, equity financing or other means. We may not be successful in locating suitable financing transactions on commercially reasonable terms, in the time period required or at all, and we may not obtain the capital we require by other means. If we do not succeed in raising additional capital, our resources may not be sufficient to fund our operations going forward.
Any additional capital raised through the sale of equity may dilute the ownership percentage of our stockholders. This could also result in a decrease in the fair market value of our equity securities because our assets would be owned by a larger pool of outstanding equity. The terms of securities we issue in future capital transactions may be more favorable to our new investors, and may include preferences, superior voting rights and the issuance of warrants or other derivative securities which may have a further dilutive effect.
Our ability to obtain needed financing may be impaired by such factors as the capital markets, both generally and in the pharmaceutical and other drug development industries in particular, our status as a new enterprise without a significant demonstrated operating history, the limited diversity of our activities and/or the loss of key personnel. If the amount of capital we are able to raise from financing activities is not sufficient to satisfy our capital needs, even to the extent that we reduce our operations, we may be required to cease our operations.
We may incur substantial costs in pursuing future capital financing, including investment banking fees, legal fees, accounting fees, securities law compliance fees, printing and distribution expenses and other costs, which may adversely impact our financial condition.
We may not be able to effectively manage our growth, which may harm our profitability.
Our strategy envisions expanding our business. If we fail to effectively manage our growth, our financial results could be adversely affected. Growth may place a strain on our management systems and resources. We must continue to refine and expand our business development capabilities, our systems and processes and our access to financing sources. As we grow, we must continue to hire, train, supervise and manage new employees. We cannot assure you that we will be able to:
|
• |
expand our systems effectively or efficiently or in a timely manner; |
|
• |
allocate our human resources optimally; |
|
• |
identify and hire qualified employees or retain valued employees; or |
|
• |
incorporate effectively the components of any business that we may acquire in our effort to achieve growth. |
If we are unable to manage our growth, our operations and our financial results could be adversely affected by inefficiency, which could diminish our profitability.
Our business may suffer if we do not attract and retain talented personnel.
Our success will depend in large measure on the abilities, expertise, judgment, discretion, integrity and good faith of our management and other personnel in conducting our business. We have a small management team, and the loss of a key individual or inability to attract suitably qualified staff could materially adversely impact our business.
Our success depends on the ability of our management, employees, consultants and joint venture partners, if any, to interpret market data correctly and to interpret and respond to economic market and other conditions in order to locate and adopt appropriate investment opportunities, monitor such investments, and ultimately, if required, to successfully divest such investments. Further, no assurance can be given that our key personnel will continue their association or employment with us or that replacement personnel with comparable skills can be found. We will seek to ensure that management and any key employees are appropriately compensated; however, their services cannot be guaranteed. If we are unable to attract and retain key personnel, our business may be adversely affected.
We have only recently commenced operations and may never achieve profitability. If we continue to incur operating losses, we may be unable to continue our operations.
We commenced operations in 2011. If we continue to incur operating losses and fail to become a profitable company, we may be unable to continue our operations. In the absence of substantial revenue from the sale of products or other sources, the amount, timing, nature or source of which cannot be predicted, our losses will continue as we conduct our research and development activities.
The market for our product candidate is highly competitive and is subject to rapid scientific change, which could have a material adverse effect on our business, results of operations and financial condition.
The pharmaceutical and biotechnology industries in which we compete are highly competitive and characterized by rapid and significant technological change. We face intense competition from organizations such as pharmaceutical and biotechnology companies, as well as academic and research institutions and government agencies. Some of these organizations are pursuing products based on technologies similar to our technology. Other of these organizations have developed and are marketing products, or are pursuing other technological approaches designed to produce products that are competitive with our product candidates in the therapeutic effect these competitive products have on the disease targeted by our product candidate. Our competitors may discover, develop or commercialize products or other novel technologies that are more effective, safer or less costly than any that we may develop. Our competitors may also obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for our product candidate.
Many of our competitors are substantially larger than we are and have greater capital resources, research and development staffs and facilities than we have. In addition, many of our competitors are more experienced in drug discovery, development and commercialization, obtaining regulatory approvals, and drug manufacturing and marketing.
We anticipate that the competition with our product candidate and technology will be based on a number of factors including product efficacy, safety, availability and price. The timing of market introduction of our planned future product candidates and competitive products will also affect competition among products. We expect the relative speed with which we can develop our product candidates, complete the required clinical trials, establish a strategic partner and supply appropriate quantities of the product candidate for late stage trials, if required, to be important competitive factors. Our competitive position will also depend upon our ability to attract and retain qualified personnel, to obtain patent protection in non-US markets, which we currently do not have, or otherwise develop proprietary products or processes and to secure sufficient capital resources for the period between technological conception and commercial sales or out-license to a pharmaceutical partner. If we fail to develop and deploy our proposed product candidate in a successful and timely manner, we will in all likelihood not be competitive.
Our product candidate is based on new technology and, consequently, is inherently risky. Concerns about the safety and efficacy of our product candidate could limit our future success.
We are subject to the risks of failure inherent in the development of product candidates based on new technologies. These risks include the possibility that any product candidates we create will not be effective, that our current product candidate will be unsafe or otherwise fail to receive the necessary regulatory approvals or that our product candidate will be hard to manufacture on a large scale or will be uneconomical to market.
Many pharmaceutical products cause multiple potential complications and side effects, not all of which can be predicted with accuracy and many of which may vary from patient to patient. Long term follow-up data may reveal additional complications associated with our product candidate. The responses of potential physicians and others to information about complications could materially affect the market acceptance of our product candidate, which in turn would materially harm our business.
Clinical trials required for our product candidate are expensive and time-consuming, and their outcome is highly uncertain. If any of our drug trials are delayed or yield unfavorable results, we will have to delay application for or may be unable to obtain regulatory approval for our product candidate.
We must conduct extensive testing of our product candidate before we can obtain regulatory approval to market and sell it. We need to conduct both preclinical animal testing and human clinical trials. Conducting these trials is a lengthy, time-consuming, and expensive process. These tests and trials may not achieve favorable results for many reasons, including, among others, failure of the product candidate to demonstrate safety or efficacy, the development of serious or life-threatening adverse events (or side effects) caused by or connected with exposure to the product candidate, difficulty in enrolling and maintaining subjects in the clinical trial, lack of sufficient supplies of the product candidate or comparator drug, if required, and the failure of clinical investigators, trial monitors, contractors, consultants, or trial subjects to comply with the trial plan or protocol. A clinical trial may fail because it did not include a sufficient number of patients to detect the endpoint being measured or reach statistical significance. A clinical trial may also fail because the dose(s) of the investigational drug included in the trial were either too low or too high to determine the optimal effect of the investigational drug in the disease setting. Many clinical trials are conducted under the oversight of Independent Data Monitoring Committees (“IDMCs”). These independent oversight bodies are made up of external experts who review the progress of ongoing clinical trials, including available safety and efficacy data, and make recommendations concerning a trial’s continuation, modification, or termination based on interim, unblinded data. Any of our ongoing clinical trials may be discontinued or amended in response to recommendations made by responsible IDMCs based on their review of interim trial results.
We will need to reevaluate our product candidate if it does not test favorably and either conduct new trials, which are expensive and time consuming, or abandon our drug development program. Even if we obtain positive results from preclinical or clinical trials, we may not achieve the same success in future trials. Many companies in the biopharmaceutical industry have suffered significant setbacks in clinical trials, even after promising results have been obtained in earlier trials. The failure of clinical trials to demonstrate safety and effectiveness for the desired indication could harm the development of our product candidate, and our business, financial condition, and results of operations may be materially harmed.
Due to our reliance on third-parties to conduct our clinical trials, we are unable to directly control the timing, conduct, expense and quality of our clinical trials, which could adversely affect our clinical data and results and related regulatory approvals.
We extensively outsource our clinical trial activities and expect to directly perform only a small portion of the preparatory stages for planned trials. We rely on independent third-party contract research organizations (“CROs”) to perform most of our clinical trials, including document preparation, site identification, screening and preparation, pre-study visits, training, program management and bio-analytical analysis. Many important aspects of the services performed for us by the CROs are out of our direct control. If there is any dispute or disruption in our relationship with our CROs, our clinical trials may be delayed. Moreover, in our regulatory submissions, we rely on the quality and validity of the clinical work performed by third-party CROs. If a CRO’s processes, methodologies or results are determined to be invalid or inadequate, our own clinical data and results and related regulatory approvals could be adversely affected or invalidated.
Regulatory and legal uncertainties could result in significant costs or otherwise harm our business.
In order to manufacture and sell our product candidate, we must comply with extensive international and domestic regulations. In order to sell our product candidate in the United States, approval from the FDA is required. The FDA approval process is expensive and time-consuming. We cannot predict whether our product candidate will be approved by the FDA. Even if our product candidate is approved, we cannot predict the time frame for approval. Foreign regulatory requirements differ from jurisdiction to jurisdiction and may, in some cases, be more stringent or difficult to obtain than FDA approval. As with the FDA, we cannot predict if or when we may obtain these regulatory approvals. If we cannot demonstrate that our product candidate can be used safely and successfully in a broad segment of the indicated patient population on a long-term basis, our product candidate would likely be denied approval by the FDA and the regulatory agencies of foreign governments.
We may be unable to formulate or manufacture our product candidate in a way that is suitable for clinical or commercial use.
Changes in product formulations and manufacturing processes may be required as our product candidate progresses in clinical development and is ultimately commercialized. If we are unable to develop suitable product formulations or manufacturing processes to support large scale clinical testing of our product candidate, we may be unable to supply necessary materials for our clinical trials, which would delay the development of our product candidate. Similarly, if we are unable to supply sufficient quantities of our product candidate or develop product formulations suitable for commercial use, we will not be able to successfully commercialize our product candidate.
We lack sales, marketing and distribution capabilities and will rely on third parties to market and distribute our product candidate, which may harm or delay our product development and commercialization efforts.
We currently have no sales, marketing, or distribution capabilities and do not intend to develop such capabilities in the foreseeable future. If we are unable to establish sales, marketing or distribution capabilities either by developing our own sales, marketing, and distribution organization or by entering into agreements with others, we may be unable to successfully sell any products that we are able to begin to commercialize. If we, and our strategic partners, if any, are unable to effectively sell our products, our ability to generate revenues will be harmed. We may not be able to hire, in a timely manner, the qualified sales and marketing personnel for our needs, if at all. In addition, we may not be able to enter into any marketing or distribution agreements on acceptable terms, if at all. If we cannot establish sales, marketing and distribution capabilities as we intend, either by developing our own capabilities or entering into agreements with third parties, sales of future products, if any, will be harmed.
We may be required to defend lawsuits or pay damages for product liability claims.
Product liability is a major risk in testing and marketing biotechnology and pharmaceutical products. We may face substantial product liability exposure in human clinical trials and in the sale of products after regulatory approval. Product liability claims, regardless of their merits, could exceed policy limits, divert management’s attention, and adversely affect our reputation and the demand for our product. In any such event, your investment in our securities could be materially and adversely affected.
Federal and state pharmaceutical marketing compliance and reporting requirements may expose us to regulatory and legal action by state governments or other government authorities.
The Food and Drug Administration Modernization Act (the “FDMA”), established a public registry of open clinical trials involving drugs intended to treat serious or life-threatening diseases or conditions in order to promote public awareness of and access to these clinical trials. Under the FDMA, pharmaceutical manufacturers and other trial sponsors are required to post the general purpose of these trials, as well as the eligibility criteria, location and contact information of the trials. Failure to comply with any clinical trial posting requirements could expose us to negative publicity, fines and other penalties, all of which could materially harm our business.
In recent years, several states, including California, Vermont, Maine, Minnesota, New Mexico and West Virginia have enacted legislation requiring pharmaceutical companies to establish marketing compliance programs and file periodic reports on sales, marketing, pricing and other activities. Similar legislation is being considered in other states. Many of these requirements are new and uncertain, and available guidance is limited. Unless we are in full compliance with these laws, we could face enforcement actions and fines and other penalties and could receive adverse publicity, all of which could harm our business.
If the product candidate we develop becomes subject to unfavorable pricing regulations, third party reimbursement practices or healthcare reform initiatives, our ability to successfully commercialize our product candidate may be impaired.
Our future revenues, profitability and access to capital will be affected by the continuing efforts of governmental and private third party payors to contain or reduce the costs of health care through various means. We expect a number of federal, state and foreign proposals to control the cost of drugs through government regulation. We are unsure of the impact recent health care reform legislation may have on our business or what actions federal, state, foreign and private payors may take in response to the recent reforms. Therefore, it is difficult to predict the effect of any implemented reform on our business. Our ability to commercialize our product candidate successfully will depend, in part, on the extent to which reimbursement for the cost of such product candidate and related treatments will be available from government health administration authorities, such as Medicare and Medicaid in the United States, private health insurers and other organizations. Significant uncertainty exists as to the reimbursement status of newly approved health care products, particularly for indications for which there is no current effective treatment or for which medical care typically is not sought. Adequate third party coverage may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product research and development. If adequate coverage and reimbursement levels are not provided by government and third party payors for use of our product candidates, our product candidates may fail to achieve market acceptance and our results of operations will be harmed.
Healthcare legislative reform measures may have a material adverse effect on our business and results of operations.
In the United States, there have been and continue to be a number of legislative initiatives to contain healthcare costs. For example, in March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or PPACA, was passed, which substantially changed the way health care is financed by both governmental and private insurers, and has significantly impacted the US pharmaceutical industry. The PPACA, among other things, subjects biologic products to potential competition by lower-cost biosimilars, addresses a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected, increases the minimum Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program and extends the rebate program to individuals enrolled in Medicaid managed care organizations, establishes annual fees and taxes on manufacturers of certain branded prescription drugs, and subjects additional drugs to lower pricing under the 340B Drug Discount Program by adding new entities to the program.
Risks Related to Our Intellectual Property
UFRF, our sole licensor, may under certain circumstances terminate our license agreement, which is required for us to conduct our proposed business.
Our license agreement with UFRF provides it with the right to terminate our agreement upon written notice to us if we do not meet all of our requirements under the license agreement that requires us to file an IND application with the FDA, have a commercial sale of a licensed product within an agreed upon period of time and raise certain amounts of capital. If the license or any other agreement we enter into with UFRF is terminated for any reason, our business will be materially adversely affected, and our business will in all likelihood fail.
If we are unable to obtain, maintain and enforce our proprietary rights, we may not be able to compete effectively or operate profitably.
We have entered into a license agreement with UFRF. The patents underlying the licensed intellectual property and positions, and those of other biopharmaceutical companies, are generally uncertain and involve complex legal, scientific and factual questions.
Our ability to develop and commercialize drugs depends in significant part on our ability to: (i) obtain and/or develop broad, protectable intellectual property; (ii) obtain additional licenses to the proprietary rights of others on commercially reasonable terms; (iii) operate without infringing upon the proprietary rights of others; (iv) prevent others from infringing on our proprietary rights; and (v) protect our trade secrets.
Patents that we may acquire and those that might be issued in the future, may be challenged, invalidated or circumvented, and the rights granted thereunder may not provide us with proprietary protection or competitive advantages against competitors with similar technology. Furthermore, our competitors may independently develop similar technologies or duplicate any technology we develop. Because of the extensive time required for development, testing and regulatory review of a potential product candidates, it is possible that, before any of our product candidates can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thus reducing any advantage of the patent.
Because patent applications in the United States and many foreign jurisdictions are typically not published until at least 12 months after filing, or in some cases not at all, and because publications of discoveries in the scientific literature often lag behind actual discoveries, neither we nor our licensors can be certain that either we or our licensors were the first to make the inventions claimed in issued patents or pending patent applications, or that we were the first to file for protection of the inventions set forth in these patent applications.
Additionally, UFRF previously elected to seek protection for certain elements of the licensed technology only in the United States, and the time to file for international patent protection has expired. This limits the strength of the Company’s intellectual property position in certain markets and could affect the overall value of the Company to a potential corporate partner.
We may be exposed to infringement or misappropriation claims by third parties, which, if determined adversely to us, could cause us to pay significant damage awards.
There has been substantial litigation and other proceedings regarding patent and other intellectual property rights in the pharmaceutical and biotechnology industries. We may become a party to various types of patent litigation or other proceedings regarding intellectual property rights from time to time even under circumstances where we are not using and do not intend to use any of the intellectual property involved in the proceedings.
The cost of any patent litigation or other proceeding, even if resolved in our favor, could be substantial. Some of our competitors may be able to sustain the cost of such litigation or proceedings more effectively than we will be able to because our competitors may have substantially greater financial resources. If any patent litigation or other proceeding is resolved against us, we or our collaborators may be enjoined from developing, manufacturing, selling or importing our drugs without a license from the other party and we may be held liable for significant damages. We may not be able to obtain any required license(s) on commercially acceptable terms or at all.
Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace. Patent litigation and other proceedings may also absorb significant management time.
Obtaining and maintaining our patent protection depends upon compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The United States Patent and Trademark Office and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent process. There are situations in which noncompliance can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case.
Confidentiality agreements with employees and others may not adequately prevent disclosure of our trade secrets and other proprietary information and may not adequately protect our intellectual property, which could impede our ability to compete.
Because we operate in the highly technical field of medical technology development, we rely in part on trade secret protection in order to protect our proprietary trade secrets and unpatented know-how. However, trade secrets are difficult to protect, and we cannot be certain that others will not develop the same or similar technologies on their own. We have taken steps, including entering into confidentiality agreements with all of our employees, consultants and corporate partners to protect our trade secrets and unpatented know-how. These agreements generally require that the other party keep confidential and not disclose to third parties any confidential information developed by the party or made known to the party by us during the course of the party’s relationship with us. We also typically obtain agreements from these parties which provide that inventions conceived by the party in the course of rendering services to us will be our exclusive property. However, these agreements may not be honored and may not effectively assign intellectual property rights to us. Enforcing a claim that a party illegally obtained and is using our trade secrets or know-how is difficult, expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets or know-how. The failure to obtain or maintain trade secret protection could adversely affect our competitive position.
We may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As is common in the biotechnology industry, we employ individuals who were previously employed at other biotechnology companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Risks Related to Our Common Stock and this Offering
Our directors, executive officers and significant stockholders have substantial control over us and could limit stockholders’ ability to influence the outcome of key transactions, including changes of control.
As of September 12, 2016 our directors and executive officers beneficially owned 30.4% of our outstanding common stock and together are able to influence significantly all matters requiring approval by our stockholders. In addition, three holders of greater than five percent of our outstanding common stock beneficially owned 33.0% and, acting together, would be able to influence significantly all matters requiring approval by our stockholders, including the election of directors and the approval of mergers or other significant corporate transactions. These stockholders may have interests that differ from other stockholders, and they may vote in a way with which other stockholders disagree and that may be adverse to the interests of other stockholders. The concentration of ownership of our common stock may have the effect of delaying, preventing or deterring a change of control of our company, could deprive our stockholders of an opportunity to receive a premium for their common stock as part of a sale of our company, and may affect the market price of our common stock. This concentration of ownership of our common stock may also have the effect of influencing the completion of a change in control that may not necessarily be in the best interests of all of our stockholders.
Our common stock is eligible for quotation on the over-the-counter-market but not listed on any national securities exchange.
Our shares of common stock are eligible for quotation on the over-the-counter markets under the symbol “SNBP.” Despite eligibility for quotation, no assurance can be given that any market for our common stock will develop or, if one develops, that it will be maintained for any period of time. Quotation on the over-the-counter markets is generally understood to be a less active, and therefore less liquid, trading market than other types of markets such as a national securities exchange. In comparison to a listing on a national securities exchange, quotation on the over-the-counter markets is expected to have an adverse effect on the liquidity of shares of our common stock, both in terms of the number of shares that can be bought and sold at a given price, but also through delays in the timing of transactions and reduction in analyst and media coverage. This may result in lower prices for our common stock than might otherwise be obtained and could also result in a larger spread between the bid and ask prices for our common stock.
Our common stock is a “penny stock,” which may make it difficult to sell shares of our common stock.
Our common stock is categorized as a “penny stock” as defined in Rule 3a51-1 of the Exchange Act and is subject to the requirements of Rule 15g-9 of the Exchange Act. Under this rule, broker-dealers who sell penny stocks must, among other things, provide purchasers of these stocks with a standardized risk-disclosure document prepared by the SEC. Under applicable regulations, our common stock will generally remain a “penny stock” until and for such time as its per-share price is $5.00 or more (as determined in accordance with SEC regulations), or until we meet certain net asset or revenue thresholds. These thresholds include the possession of net tangible assets (i.e., total assets less intangible assets and liabilities) in excess of $2 million or average revenues equal to at least $6 million for each of the last three years.
The penny-stock rules substantially limit the liquidity of securities in the secondary market, and many brokers choose not to participate in penny-stock transactions. As a result, there is generally less trading in penny stocks. If you become a holder of our common stock, you may not always be able to resell shares of our common stock in a public broker’s transaction, if at all, at the times and prices that you feel are fair or appropriate.
Trading in our stock has been minimal and investors may not be able to sell as much stock as they want at prevailing prices.
The average daily trading volume in our common stock has been minimal. If trading in our stock continues at that level, it may be difficult for investors to sell or buy substantial quantities of shares in the public market at any given time at prevailing prices. Moreover, the market price for shares of our common stock may be made more volatile because of the relatively low volume of trading in our common stock. When trading volume is low, significant price movement can be caused trading a relatively small number of shares, which increases stock price volatility.
Offers or availability for sale of a substantial number of shares of our common stock may cause the price of our common stock to decline and cause investors to lose part or all of their investment.
If our stockholders sell substantial amounts of our common stock in the public market or upon the expiration of any statutory holding period under Rule 144, or upon expiration of lock-up periods applicable to outstanding shares, or issued upon the exercise of outstanding options or warrants, it could create a circumstance commonly referred to as an “overhang” and in anticipation of which the market price of our common stock could fall. The existence of an overhang, whether or not sales have occurred or are occurring, also could make more difficult our ability to raise additional financing through the sale of equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate. As of September 12, 2016, we had outstanding stock options to purchase 3,163,600 shares of our common stock at a weighted-average exercise price of $0.27 per share, outstanding warrants to purchase 3,660,500 shares of common stock at a weighted-average exercise price of $0.59 per share, including 1,110,500 warrants issued pursuant to the Securities Purchase Agreements, and outstanding convertible notes payable convertible into an estimated 2,466,667 shares at a weighted-average conversion price of $1.13.
Securities analysts may not initiate coverage or continue to cover our common stock, and this may have a negative impact on the market price of our common stock.
Common stock prices are often significantly influenced by the research and reports that securities analysts publish about companies and their business. We do not have any control over these analysts. There is no guarantee that securities analysts will cover our common stock. If securities analysts do not cover our common stock, the lack of research coverage may adversely affect the market price of our common stock. If our common stock is covered by securities analysts and our stock is downgraded, our stock price will likely decline. If one or more of these analysts ceases to cover us or fails to publish regular reports on us, we can lose visibility in the financial markets, which can cause our stock price or trading volume to decline.
Raising additional capital may cause dilution to our stockholders or restrict our operations.
To the extent that we raise additional capital through the sale of equity or convertible debt securities, stockholders’ ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect their rights as stockholders. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring additional debt, making capital expenditures or declaring dividends. Any of these events could adversely affect our ability to achieve our product development and commercialization goals and harm our business. We do not anticipate any adverse effects stemming from the lack of available credit facilities at this time.
The protection provided by the federal securities laws relating to forward-looking statements does not apply to us. The lack of this protection could harm us in the event of an adverse outcome in a legal proceeding relating to forward-looking statements made by us.
Although federal securities laws provide a safe harbor for forward-looking statements made by a public company that files reports under the federal securities laws, this safe harbor is not available to certain issuers, including penny stock issuers. We believe we are not currently eligible for the statutory safe harbor included in the Exchange. As a result, we will not have the benefit of this statutory safe harbor protection in the event of certain legal actions based upon forward-looking statements. The lack of this protection in a contested proceeding could harm our financial condition and, ultimately, the value of our common stock.
Anti-takeover provisions could negatively impact our stockholders
Provisions of Delaware law and provisions of our Certificate of Incorporation could make it more difficult for a third party to acquire control of us or have the effect of discouraging a third party from attempting to acquire control of us. We are subject to certain anti-takeover provisions under the Delaware General Corporation Law. Additionally, our Certificate of Incorporation authorizes our board of directors to issue one or more classes or series of preferred stock without stockholder approval and such preferred stock could be issued as a defensive measure in response to a takeover proposal. These provisions could make it more difficult for a third party to acquire us even if an acquisition might be in the best interest of our stockholders.
If we issue preferred stock, the rights of holders of our common stock and the value of such common stock could be adversely affected.
Our board of directors is authorized to issue classes or series of preferred stock, without any action on the part of the stockholders. The board of directors also has the power, without stockholder approval, to set the terms of any such classes or series of preferred stock, including voting rights, dividend rights and preferences over the common stock with respect to dividends or upon the liquidation, dissolution or winding-up of our business and other terms. If we issue preferred stock in the future that has a preference over the common stock with respect to the payment of dividends or upon liquidation, dissolution or winding-up, or if we issue preferred stock with voting rights that dilute the voting power of the common stock, the rights of holders of the common stock or the value of the common stock would be adversely affected.
We have identified a significant deficiency in internal control over financial reporting, if we fail to maintain effective internal controls over financial reporting, the price of our common stock may be adversely affected.
We are required to establish and maintain appropriate internal controls over financial reporting. Failure to establish those controls, or any failure of those controls once established, could adversely impact our public disclosures regarding our business, financial condition or results of operations. Any failure of these controls could also prevent us from maintaining accurate accounting records and discovering accounting errors and financial fraud.
In the course of completing its assessment of internal control over financial reporting as of December 31, 2015, management did not identify any material weaknesses but did identify a significant deficiency in the number of personnel available to serve the Company’s accounting function, specifically management believes that we may not be able to adequately segregate responsibility over financial transaction processing and reporting. A significant deficiency is a deficiency, or a combination of deficiencies, in internal control over financial reporting, that is less severe than a material weakness yet important enough to merit attention by those responsible for oversight of the Company’s financial reporting. Although we are unable to remediate the significant deficiency with current personnel, we are mitigating its potential impact, primarily through greater involvement of senior management in the review and monitoring of financial transaction processing and reporting.
In addition, management’s assessment of internal controls over financial reporting may identify additional weaknesses and conditions that need to be addressed or other potential matters that may raise concerns for investors. Any actual or perceived weaknesses and conditions that need to be addressed in our internal control over financial reporting, disclosure of management’s assessment of our internal controls over financial reporting, or disclosure of our public accounting firm’s attestation to or report on management’s assessment of our internal controls over financial reporting may have an adverse impact on the price of our common stock.
We are an emerging growth company and we cannot be certain if reduced disclosure requirements applicable to emerging growth companies will make our Common Stock less attractive to investors.
We are an emerging growth company under the JOBS Act. For as long as we continue to be an emerging growth company, we intend to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies including, but not limited to, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, exemptions from the requirements of holding a nonbinding advisory stockholder vote on executive compensation and any golden parachute payments not previously approved, exemption from the requirement of auditor attestation in the assessment of our internal control over financial reporting and exemption from any requirement that may be adopted by the Public Company Accounting Oversight Board. If we do, the information that we provide stockholders may be different than what is available with respect to other public companies. We cannot predict if investors will find our common stock less attractive because we will rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
CAUTIONARY Note Regarding Forward-Looking Statements
This prospectus contains forward-looking statements within the meaning of Section 27A of the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended. In some cases, you can identify forward-looking statements by the following words: “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. Forward-looking statements are not a guarantee of future performance or results, and will not necessarily be accurate indications of the times at, or by, which such performance or results will be achieved. Forward-looking statements are based on information available at the time the statements are made and involve known and unknown risks, uncertainties and other factors that may cause our results, levels of activity, performance or achievements to be materially different from the information expressed or implied by the forward-looking statements in this report. These factors include:
|
● |
the fact that we are a company with limited operating history for you to evaluate our business; |
|
● |
our lack of diversification and the corresponding risk of an investment in our Company; |
|
● |
potential deterioration of our financial condition and results due to failure to diversify; |
|
● |
our ability to obtain additional capital required to implement our business plan; |
|
● |
our ability to effectively manage growth and the corresponding impact on our profitability; and |
|
● |
other risk factors included under the caption “Risk Factors” beginning on page 6 of this report. |
You should read the matters described in “Risk Factors” and the other cautionary statements made in this report as being applicable to all related forward-looking statements wherever they appear in this report. We cannot assure you that the forward-looking statements in this report will prove to be accurate and therefore prospective investors are encouraged not to place undue reliance on forward-looking statements. You should read this report completely. Other than as required by law, we undertake no obligation to update or revise these forward-looking statements, even though our situation may change in the future.
Use of Proceeds
The selling stockholders will receive all of the proceeds from the sale of the shares offered for sale by it under this prospectus. We will not receive proceeds from the sale of the shares by the selling stockholder. We will bear all reasonable expenses incident to this registration statement. Any transfer taxes payable on these shares and any commissions and discounts payable to underwriters, agents, brokers or dealers will be paid by the selling stockholders.
Price Range of Common Stock
There is no “established trading market” for our shares of common stock. Commencing July 29, 2014, our shares of common stock became generally eligible for quotation on the over-the-counter markets under the symbol “CRSO”. Effective as of September 9, 2015, our common stock became quoted on the OTCPink tier of the over-the-counter markets administered by OTC Markets Group, Inc. under the new symbol “SNBP.” Despite eligibility for quotation, no assurance can be given that any market for our common stock will develop or be maintained. If an “established trading market” ever develops in the future, the sale of shares of our common stock that are deemed to be “restricted securities” pursuant to Rule 144 of the SEC by members of management or others may have a substantial adverse impact on any such market.
Set forth below are the high and low bid prices for our common stock for each quarter of 2014, 2015 and 2016 for which data is available. These bid prices were obtained from OTC Markets Group Inc. from which data is available only after July 28, 2014. All prices listed below reflect inter-dealer prices, without retail mark-up, mark-down or commissions and may not represent actual transactions.
|
Fiscal 2016 |
High |
Low |
||||||
|
Third Quarter (through September 12, 2016) |
$ | 2.01 | $ | 2.01 | ||||
|
Second Quarter |
$ | 4.50 | $ | 3.90 | ||||
|
First Quarter |
$ | 1.80 | $ | 1.19 | ||||
|
Fiscal 2015 |
High |
Low |
||||||
|
Fourth Quarter |
$ | 7.09 | $ | 3.00 | ||||
|
Third Quarter |
$ | 1.00 | $ | 1.00 | ||||
|
Second Quarter |
$ | 1.00 | $ | 0.60 | ||||
|
First Quarter |
$ | 0.60 | $ | 0.60 | ||||
|
Fiscal 2014 |
High |
Low |
||||||
|
Fourth Quarter |
$ | 0.60 | $ | 0.60 | ||||
|
Third Quarter |
None |
None |
||||||
The closing price of our common stock as quoted on the OTCPink tier of the over-the-counter markets administered by the OTC Markets Group, Inc. on September 12, 2016 was $2.00 per share. As of September 12, 2016, we had 193 stockholders of record of our common stock.
Penny Stock Rules
The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00 (other than securities registered on certain national securities exchanges, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system).
A purchaser is purchasing penny stock which limits the ability to sell the stock. The shares offered by this prospectus constitute penny stock under the Exchange Act. The shares will remain penny stocks for the foreseeable future. The classification of penny stock makes it more difficult for a broker-dealer to sell the stock into a secondary market, which makes it more difficult for a purchaser to liquidate his/her investment. Any broker-dealer engaged by the purchaser for the purpose of selling his or her shares in us will be subject to Rules 15g-1 through 15g-10 of the Exchange Act. Rather than creating a need to comply with those rules, some broker-dealers will refuse to attempt to sell penny stock.
The penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from those rules, to deliver a standardized risk disclosure document, which:
|
● |
contains a description of the nature and level of risk in the market for penny stock in both public offerings and secondary trading; |
|
● |
contains a description of the broker’s or dealer’s duties to the customer and of the rights and remedies available to the customer with respect to a violation of such duties or other requirements of the Securities Act of 1934, as amended; |
|
● |
contains a brief, clear, narrative description of a dealer market, including “bid” and “ask” price for the penny stock and the significance of the spread between the bid and ask price; |
|
● |
contains a toll-free telephone number for inquiries on disciplinary actions; |
|
● |
defines significant terms in the disclosure document or in the conduct of trading penny stocks; and |
|
● |
contains such other information and is in such form (including language, type, size and format) as the Securities and Exchange Commission shall require by rule or regulation; |
The broker-dealer also must provide, prior to effecting any transaction in a penny stock, to the customer:
|
● |
the bid and offer quotations for the penny stock; |
|
● |
the compensation of the broker-dealer and its salesperson in the transaction; |
|
● |
the number of shares to which such bid and ask prices apply, or other comparable information relating to the depth and liquidity of the market for such stock; and |
|
● |
monthly account statements showing the market value of each penny stock held in the customer’s account. |
In addition, the penny stock rules require that prior to a transaction in a penny stock not otherwise exempt from those rules; the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written acknowledgment of the receipt of a risk disclosure statement, a written agreement to transactions involving penny stocks, and a signed and dated copy of a written suitability statement. These disclosure requirements will have the effect of reducing the trading activity in the secondary market for our stock because it will be subject to these penny stock rules. Therefore, stockholders may have difficulty selling their securities.
Dividend Policy
We have never declared or paid cash dividends on our capital stock. Following the completion of this offering, we intend to retain our future earnings, if any, to finance the further development and expansion of our business and do not expect to pay cash dividends in the foreseeable future. Payment of future cash dividends, if any, will be at the discretion of our board of directors after taking into account various factors, including our financial condition, operating results, current and anticipated cash needs, outstanding indebtedness, plans for expansion and restrictions imposed by lenders, if any.
PRINCIPAL AND SELLING STOCKHOLDERS
2016 Financing
On each of June 10, 2016, June 24, 2016, August 11, 2016, and September 2, 2016, we entered into Securities Purchase Agreements (the “Purchase Agreements”) with the selling stockholders named herein, pursuant to which we sold an aggregate of 2,221,000 shares of common stock (the “Shares”) and warrants to purchase an aggregate of 1,110,500 additional shares of common stock (the “Warrant Shares”). The purchase price for each unit, consisting of one share of common stock and a warrant to purchase one-half share of common stock, was $1.00. The warrants are exercisable for a period of five years from their respective date of issuance at an exercise price of $1.50 per share.
Under the terms of the Purchase Agreements we agreed to file one or more registration statements, including the registration statement of which this prospectus is a part, as permissible and necessary to register under the Securities Act, the sale of the shares of our common stock that have been and may be issued to the selling stockholders under the Purchase Agreement.
The selling stockholders may from time to time offer and sell any or all of the shares of our common stock set forth below pursuant to this prospectus. When we refer to the “selling stockholder” in this prospectus, we mean the entity listed in the table below, and its respective pledgees, donees, permitted transferees, assignees, successors and others who later come to hold any of the selling stockholder’s interests in shares of our common stock other than through a public sale.
Security Ownership of Certain Beneficial Owners, Management and Other Selling Stockholders
The following table sets forth for each person known to us to beneficially own more than five percent of our outstanding shares of common stock, each of our directors, each of our executive officers, all of our directors and executive officers as a group and each selling stockholder, the name, the number and percentage of shares of common stock beneficially owned as of September 12, 2016, the maximum number of Shares and Warrant Shares that may be offered pursuant to this prospectus and the number and percentage of shares of common stock that would be beneficially owned after the sale of the maximum number of Shares and Warrant Shares, and is based upon information provided to us by each selling stockholder for use in this prospectus. The information presented in the table is based on 32,151,306 shares of our common stock outstanding on September 12, 2016.
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Unless otherwise indicated below, to our knowledge, the persons and entities named in the table have sole voting and investment power with respect to all shares beneficially owned, subject to community property laws where applicable. For purposes of the table below, shares of common stock issuable pursuant to options and warrants held by a selling stockholder that can be acquired within 60 days of September 12, 2016, are deemed to be outstanding and to be beneficially owned by the selling stockholder holding the securities but are not treated as outstanding for the purpose of computing the percentage ownership of any other selling stockholder. Except as otherwise noted below, the address for each director, officer or selling stockholder listed in the table is c/o Sun BioPharma, Inc., 712 Vista Blvd #305, Waconia, Minnesota 55387.
|
Shares Beneficially Owned |
Maximum Number of Shares to Be Sold |
Shares Beneficially Owned After the Sale of the Maximum Number of Shares |
||||||||||||||||||
| Name |
Number |
Percentage |
Hereunder(1) |
Number |
Percentage |
|||||||||||||||
|
Executive Officers and Directors: |
||||||||||||||||||||
|
Michael T. Cullen |
4,340,764 | (2) | 13.2 | % | 75,000 | 4,265,764 | 12.9 | % | ||||||||||||
|
David B. Kaysen |
75,000 | (3) | * | 75,000 | – | – | ||||||||||||||
|
Scott Kellen |
52,500 | (4) | * | 52,500 | – | – | ||||||||||||||
|
Suzanne Gagnon |
855,000 | (5) | 2.6 | % | 45,000 | 810,000 | 2.5 | % | ||||||||||||
|
Dalvir S. Gill |
– | – | – | – | – | |||||||||||||||
|
Jeffrey S. Mathiesen |
– | – | – | – | – | |||||||||||||||
|
J. Robert Paulson, Jr. |
– | – | – | – | – | |||||||||||||||
|
Paul W. Schaffer |
1,133,296 | (6) | 3.5 | % | 150,000 | 983,296 | 3.1 | % | ||||||||||||
|
D. Robert Schemel |
3,727,836 | (7) | 11.6 | % | 3,727,836 | 11.6 | % | |||||||||||||
|
All directors and current executive officers as a group (9 persons) |
10,184,396 | (8) | 30.5 | % | 397,500 | 9,786,896 | 29.4 | % | ||||||||||||
|
5% Stockholders: |
||||||||||||||||||||
|
Ryan R. Gilbertson |
5,592,730 | (9) | 16.7 | % | – | 5,592,730 | 16.7 | % | ||||||||||||
|
Paul M. Herron |
2,454,860 | (10) | 7.6 | % | – | 2,454,860 | 7.6 | % | ||||||||||||
|
Clifford F. McCurdy III |
1,840,000 | (11) | 5.7 | % | – | 1,840,000 | 5.7 | % | ||||||||||||
|
Ryan R. Gilbertson 2012 |
1,671,093 | (12) | 5.0 | % | – | 1,671,093 | 5.0 | % | ||||||||||||
| Shares Beneficially Owned |
Maximum Number of Shares to Be Sold |
Shares Beneficially Owned After the Sale of the Maximum Number of shares |
||||||||||||||||||
| Name | Number | Percentage | Hereunder(1) | Number | Percentage | |||||||||||||||
|
Other Selling Stockholders: |
||||||||||||||||||||
|
John Avena |
15,000 | * | 15,000 | – | – | |||||||||||||||
|
Richard M. & Evelyn M. Bott |
150,000 | * | 150,000 | – | – | |||||||||||||||
|
Scott R. Burnett |
75,000 | * | 75,000 | – | – | |||||||||||||||
|
Grant J. Christianson |
788,004 | (13) | 2.4 | % | 90,000 | 698,004 | 2.2 | % | ||||||||||||
|
John Dammermann |
750,000 | 2.3 | % | 750,000 | – | – | ||||||||||||||
|
Robert J. Dollfus, Jr. |
37,500 | * | 37,500 | – | – | |||||||||||||||
|
Randy Dooley |
75,000 | * | 75,000 | – | – | |||||||||||||||
|
James C. Duffy |
37,500 | * | 37,500 | – | – | |||||||||||||||
|
David R. Eckes |
37,500 | * | 37,500 | – | – | |||||||||||||||
|
H & H Food Events, Inc. |
15,000 | * | 15,000 | – | – | |||||||||||||||
|
David Harle |
75,000 | * | 75,000 | – | – | |||||||||||||||
|
Matthew & Stacy Hilman |
15,000 | * | 15,000 | – | – | |||||||||||||||
|
Chad Kompelien |
15,000 | * | 15,000 | – | – | |||||||||||||||
|
Karen Kompelien |
15,000 | * | 15,000 | – | – | |||||||||||||||
|
Chris & Carrie Larson |
15,000 | * | 15,000 | – | – | |||||||||||||||
|
Loel & Linda Larson |
401,416 | (14) | 1.2 | % | 37,500 | 363,916 | 1.1 | % | ||||||||||||
|
Thomas X. Neenan |
424,000 | (15) | 1.3 | % | 24,000 | 400,000 | 1.2 | % | ||||||||||||
|
Terrance O. Noble Trust Dtd 12/16/2009 |
300,000 | * | 300,000 | – | – | |||||||||||||||
|
Diane M. Norgaard |
144,372 | (16) | * | 30,000 | 114,372 | * | ||||||||||||||
|
Adepero Okulaja, MD |
15,000 | * | 15,000 | – | – | |||||||||||||||
|
Walter Palmer |
225,000 | (17) | * | 150,000 | 75,000 | * | ||||||||||||||
|
Thomas L. Riordan |
75,000 | * | 75,000 | – | – | |||||||||||||||
|
Doug Sherk |
37,500 | * | 37,500 | – | – | |||||||||||||||
|
Tom Torgerson |
750,000 | 2.3 | % | 750,000 | – | – | ||||||||||||||
|
Stuart H. Walker |
75,000 | * | 75,000 | – | – | |||||||||||||||
|
Michael Walker |
22,500 | * | 22,500 | – | – | |||||||||||||||
| * | Less than one percent. |
|
(1) |
Unless otherwise indicated, one-third of the shares of common stock identified are subject to warrants. |
|
(2) |
Includes 1,895,764 shares held by the Cullen Living Trust, 800,000 shares subject to stock options and 25,000 shares subject to warrants. |
|
(3) |
Includes 25,000 shares subject to warrants. |
|
(4) |
Includes 17,500 shares subject to warrants. |
|
(5) |
Includes 10,000 shares held by the Gagnon Family Trust and 400,000 shares subject to stock options. |
|
(6) |
Includes 189,092 shares and 50,000 shares subject to warrants held by the Paul Shaffer Trust. |
|
(7) |
Includes 2,833,548 shares held by spouse. |
|
(8) |
Includes 1,200,000 shares subject to stock options and 132,500 shares subject to warrants. |
|
(9) |
Based on Schedule 13D filed with the SEC on September 14, 2015, reflecting the stockholder’s beneficial ownership as of September 4, 2015. Includes 800,000 shares subject to warrants, and an estimated 444,444 potentially issuable pursuant to convertible promissory notes. Also includes an estimated 177,776 shares potentially issuable pursuant to convertible promissory notes held by Total Depth Foundation. |
|
(10) |
Based on Schedule 13G filed with the SEC on September 14, 2015, reflecting the stockholder’s beneficial ownership as of September 4, 2015. Includes 414,860 shares held jointly with spouse and 200,000 shares subject to warrants. |
|
(11) |
Based on Schedule 13G filed with the SEC on September 14, 2015, reflecting the stockholder’s beneficial ownership as of September 4, 2015. Includes 800,000 shares subject to warrants, and an estimated 444,444 potentially issuable pursuant to convertible promissory notes. Also includes an estimated 177,776 shares potentially issuable pursuant to convertible promissory notes held by Total Depth Foundation. |
|
(12) |
Based on Schedule 13G filed with the SEC on September 14, 2015, reflecting the stockholder’s beneficial ownership as of September 4, 2015. Includes 1,200,000 shares subject to warrants. |
|
(13) |
Includes 30,000 shares subject to warrants. |
|
(14) |
Includes 12,500 shares subject to warrants. |
|
(15) |
Includes 8,000 shares subject to warrants. |
|
(16) |
Includes 10,000 shares subject to warrants. |
| (17) | Includes 50,000 shares subject to warrants. |
DESCRIPTION OF Securities
The following is a summary description of our capital stock. It does not purport to be complete and is subject to, and is qualified in its entirety by, the provisions of our certificate of incorporation and bylaws, copies of which are attached hereto and incorporated herein by reference.
Authorized Capital
Our authorized capital stock consists of: (1) 200,000,000 shares of common stock, $0.001 par value per share, and (2) 20,000,000 shares of preferred stock, $0.001 par value per share. As of September 12, 2016, there were approximately 193 holders of record of our common stock and no holders of preferred stock. As of the same date, we had 32,151,306 shares of common stock outstanding and no shares of preferred stock outstanding.
Common Stock
Voting Rights
Each share of common stock entitles the holder to one vote for all purposes and cumulative voting is not permitted in the election of directors. Significant corporate transactions, such as amendments to the certificate of incorporation, mergers, sales of assets and dissolution or liquidation, require approval by the affirmative vote of the majority of the outstanding shares of common stock. Other matters to be voted upon by the holders of common stock normally require the affirmative vote of a majority of the shares present at the particular stockholders meeting.
Dividend Rights
Holders of common stock are entitled to receive such dividends as may be declared by the board of directors out of assets legally available therefore, and to share ratably in the assets of our Company available upon liquidation. However, we do not anticipate payment of any dividends in the foreseeable future. See “Dividend Policy.”
Liquidation and Preemptive Rights
In the event of liquidation, dissolution or winding-up, holders of our common stock are entitled to share ratably in the assets of our Company available upon liquidation. There are no preemptive, subscription, conversion or redemption rights pertaining to our common stock. The absence of preemptive rights could result in a dilution of the interest of investors should additional shares of common stock be issued.
Preferred Stock
Our board of directors has the authority, without first obtaining the approval of our stockholders, to establish one or more series of preferred stock and to fix:
|
● |
the number of shares of such series; |
|
● |
the designations, preferences and relative rights, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences; and |
|
● |
any qualifications, limitations or restrictions. |
We believe that the ability of the board to issue one or more series of preferred stock provides flexibility in structuring possible future financings and acquisitions, and in meeting other corporate needs that may arise. The authorized shares of preferred stock, as well as authorized and unissued shares of common stock, are available for issuance without action by the holders of common stock, unless such action is required by applicable law or the rules of any stock exchange or automated quotation system on which our securities may be listed or traded.
The board may authorize, without stockholder approval, the issuance of preferred stock with voting and conversion rights that could adversely affect the voting power and other rights of holders of common stock. Although the board has no current intention of doing so, it could issue a series of preferred stock that could, depending on the terms of such series, impede the completion of a merger, tender offer or other takeover attempt of our Company. The board could also issue preferred stock having terms that could discourage an acquisition attempt through which an acquirer may be able to change the composition of the board, including a tender offer or other transaction that some, or a majority, of the stockholders might believe to be in their best interests or in which stockholders might receive a premium for their stock over the then-current market price. Any issuance of preferred stock therefore could have the effect of decreasing the market price of our common stock.
The board will make any determination to issue such shares based on its judgment as to the best interests of our Company and stockholders. We have no current plans to issue any preferred stock.
Options
As of September 12, 2016, we had issued and outstanding options to purchase 3,163,600 shares of common stock with a weighted average exercise price of $0.27 per share, all of which were issued under the Sun BioPharma, Inc. 2011 Stock Option Plan (the “2011 Plan”).
On March 3, 2016, our Board of Directors, upon recommendation of its compensation committee, approved the Sun BioPharma, Inc. 2016 Omnibus Incentive Plan (the “2016 Plan”), which was approved by shareholders at a meeting held May 17, 2016. The purpose of the 2016 Plan is to provide long-term incentives to persons with responsibility for success and growth at our Company. The 2016 Plan authorizes the issuance of up to 15,000,000 shares of our common stock pursuant to awards granted thereunder, all of which were available for grant as of September 12, 2016. We ceased making awards under the 2011 Plan upon shareholder approval of the 2016 Plan.
Warrants
As of September 12, 2016, we had issued and outstanding warrants to purchase 3,660,500 shares of common stock and no warrants to purchase shares of preferred stock outstanding. Of the outstanding warrants, warrants to purchase 2,550,000 shares of common stock are exercisable at a price of $0.1875 per share for up to ten (10) years after the date of issuance (or earlier upon a change of control or an initial public offering, each as defined in the warrants). The remaining warrants to purchase 1,110,500 shares of common stock were issued pursuant to the Purchase Agreements and are exercisable for a period of five years from the date of issuance at an exercise price of $1.50 per share.
Long-term debt
As of June 30, 2016 our Company had an outstanding term note of $300,000 at a fixed interest rate of 4.125%. No principal or interest payments are due until the maturity date, October 26, 2017, unless a mandatory repayment event occurs. A mandatory repayment event includes, (i) a liquidity event defined as a sale of all or substantially all of our assets; a merger, consolidation, share exchange or similar transaction as a result of which the persons holding our equity constituting a majority of the outstanding equity by voting power or economic participation immediately prior to the transaction hold less than a majority of such voting power or economic participation immediately after such transaction; or a sale or transfer of outstanding equity in a transaction as a result of which the persons holding our equity constituting a majority of the outstanding equity by voting power or economic participation immediately prior to the transaction hold less than a majority of such voting power or economic participation immediately after such transaction, (ii) an event of default, (iii) a failure to maintain a Florida base of operations for more than 6 months, (iv) a sale or transfer of licensed technology, (v) any false representation to the Institute, (vi) a violation of law by us or one of our principal officers, or (vii) an achievement of aggregate revenues during any fiscal year of more than $4,000,000 from sales of products and/or services.
Demand notes payable
As of June 30, 2016 our Company had an outstanding unsecured, demand notes payable of $250,000. These demand notes have no stated interest rate or maturity date.
Convertible Notes
As of June 30, 2016, our Company had outstanding $2,775,000 aggregate principal amount of Convertible Promissory Notes (collectively, the “Notes”). Each such Note bears 5.0% simple interest per annum on its unpaid principal balance. All unpaid principal and unpaid and accrued interest on each Note are due and payable on the earlier to occur of (i) written demand of the holder after December 27, 2018 (the “Maturity Date”), (ii) the initial public offering of our Common Stock, (iii) a Change of Control (as defined in the Notes), or (iv) the continuance of an Event of Default that is not cured within the cure period (as defined in the Notes). Prior to the Maturity Date, any or all of the outstanding principal amount and accrued and unpaid interest of each Note may, upon written election of the holder, be converted into fully paid and nonassessable shares of our commons stock at a rate of $1.125 per share. If all of the outstanding Notes had converted into shares of common stock as of June 30, 2016, the holders would have received an estimated total of 2,466,667 shares of common stock, excluding accrued interest, which we have historically paid in cash on a quarterly basis.
Registration Rights
Pursuant to the Purchase Agreements, the Company has agreed to prepare and file a registration statement with the SEC within 60 days after the closing of the sales of securities under the Purchase Agreements for purposes of registering the resale of the Shares and the Warrant Shares. The Company has also agreed, among other things, to indemnify the selling holders under the registration statements from certain liabilities and to pay all fees and expenses (excluding underwriting discounts and selling commissions and legal fees) incident to the Company’s obligations under the Purchase Agreements.
Anti-Takeover Provisions
Provisions of our certificate of incorporation and amended and restated bylaws may delay or discourage transactions involving an actual or potential change in our control or change in our management, including transactions in which stockholders might otherwise receive a premium for their shares, or transactions that our stockholders might otherwise deem to be in their best interests. Therefore, these provisions could adversely affect the price of our common stock. Among other things, our certificate of incorporation and amended and restated bylaws:
|
● |
provide that all vacancies, including newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum; |
|
● |
authorizes our board of directors to issue one or more classes or series of preferred stock without stockholder approval; |
|
● |
require that any action to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders and not be taken by written consent; |
|
● |
provide for a classified board of directors, which means that our directors are divided into three classes that are elected to three-year terms on a staggered basis and the entire board of directors cannot be replaced in any one year; |
|
● |
provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide notice in writing in a timely manner, and also specify requirements as to the form and content of a stockholder’s notice; |
|
● |
do not provide for cumulative voting rights (therefore allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election, if they should so choose); |
|
● |
provide that special meetings of our stockholders may be called only by the Chairman of the Board, our Chief Executive Officer or by the board of directors pursuant to a resolution adopted by a majority of the total number of authorized directors; and |
|
● |
provide that stockholders will be permitted to amend our amended and restated bylaws only upon receiving at least 66.67% of the votes entitled to be cast by holders of all outstanding shares then entitled to vote generally in the election of directors, voting together as a single class. |
Limitation on Liability of Directors and Indemnification
Our certificate of incorporation limits the liability of the directors to the fullest extent permitted by Delaware law. Delaware law provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except for liability for any:
|
● |
breach of their duty of loyalty to us or our stockholders; |
|
● |
act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
|
● |
unlawful payment of dividends or redemption of shares as provided in Section 174 of the Delaware General Corporation Law; or |
|
● |
transaction from which the directors derived an improper personal benefit. |
These limitations of liability do not apply to liabilities arising under federal securities laws and do not affect the availability of equitable remedies such as injunctive relief or rescission.
Our bylaws provide that we will indemnify our directors and executive officers, and may indemnify other officers, employees and other agents, to the fullest extent permitted by law. Our amended and restated bylaws also permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in connection with their services to us, regardless of whether our amended and restated bylaws permit indemnification. We have obtained a directors’ and officers’ liability insurance policy.
We have entered, and intend to continue to enter, into separate indemnification agreements with our executive officers, in addition to the indemnification provided for in our certificate of incorporation and bylaws. These agreements, among other things, require us to indemnify our executive officers for certain expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by an executive officer in any action or proceeding arising out of their services as one of our executive officers, or any of our subsidiaries or any other company or enterprise to which the person provides services at our request.
Except as described above under “Certain Relationships and Related Party Transactions,” at present there is no pending litigation or proceeding involving any of the current or former directors or officers as to which indemnification is required or permitted, and we are not aware of any threatened litigation or proceeding that may result in a claim for indemnification.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC this indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is VStock Transfer, which can be contacted at 18 Lafayette Place, Woodmere, New York, 11598, info@vstocktransfer.com, or +1 (212) 828-8436.
PLAN OF DISTRIBUTION
The selling stockholders and any of their pledgees, donees, transferees, assignees and successors-in-interest may, from time to time, sell any or all of their shares of common stock on any stock exchange, market or trading facility on which the shares are traded or quoted or in private transactions. These sales may be at fixed or negotiated prices. The selling stockholders may use any one or more of the following methods when selling shares:
|
● |
ordinary brokerage transactions and transactions in which the broker-dealer solicits investors; |
|
● |
block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
|
● |
purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
|
● |
an exchange distribution in accordance with the rules of the applicable exchange; |
|
● |
privately negotiated transactions; |
|
● |
through the writing of options on the shares; |
|
● |
to cover short sales made after the date that the registration statement of which this prospectus is a part is declared effective by the SEC; |
|
● |
broker-dealers may agree with the selling stockholders to sell a specified number of such shares at a stipulated price per share; and |
|
● |
a combination of any such methods of sale. |
The selling stockholders may also sell shares under Rule 144 of the Securities Act, if available, rather than under this prospectus. The selling stockholders shall have the sole and absolute discretion not to accept any purchase offer or make any sale of shares if it deems the purchase price to be unsatisfactory at any particular time.
The selling stockholders or their respective pledgees, donees, transferees or other successors in interest, may also sell the shares directly to market makers acting as principals and/or broker-dealers acting as agents for themselves or their customers. Such broker-dealers may receive compensation in the form of discounts, concessions or commissions from the selling stockholders and/or the purchasers of shares for whom such broker-dealers may act as agents or to whom they sell as principal or both, which compensation as to a particular broker-dealer might be in excess of customary commissions. Market makers and block purchasers purchasing the shares will do so for their own account and at their own risk. It is possible that a selling stockholder will attempt to sell shares of common stock in block transactions to market makers or other purchasers at a price per share which may be below the then existing market price. We cannot assure that all or any of the shares offered in this prospectus will be issued to, or sold by, the selling stockholders. The selling stockholders and any brokers, dealers or agents, upon effecting the sale of any of the shares offered in this prospectus, may be deemed to be “underwriters” as that term is defined under the Securities Act, the Exchange Act and the rules and regulations of such acts. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act.
We are required to pay all fees and expenses incident to the registration of the shares, including fees and disbursements of counsel to the selling stockholders, but excluding brokerage commissions or underwriter discounts.
The selling stockholders, alternatively, may sell all or any part of the shares offered in this prospectus through an underwriter. The selling stockholders have not entered into any agreement with a prospective underwriter and there is no assurance that any such agreement will be entered into.
The selling stockholders may pledge their shares to their brokers under the margin provisions of customer agreements. If a selling stockholder defaults on a margin loan, the broker may, from time to time, offer and sell the pledged shares. The selling stockholders and any other persons participating in the sale or distribution of the shares will be subject to applicable provisions of the Exchange Act, and the rules and regulations under such act, including, without limitation, Regulation M. These provisions may restrict certain activities of, and limit the timing of purchases and sales of any of the shares by, the selling stockholders or any other such person. In the event that any of the selling stockholders are deemed an affiliated purchaser or distribution participant within the meaning of Regulation M, then the selling stockholders will not be permitted to engage in short sales of common stock. Furthermore, under Regulation M, persons engaged in a distribution of securities are prohibited from simultaneously engaging in market making and certain other activities with respect to such securities for a specified period of time prior to the commencement of such distributions, subject to specified exceptions or exemptions. In addition, if a short sale is deemed to be a stabilizing activity, then the selling stockholders will not be permitted to engage in a short sale of our common stock. All of these limitations may affect the marketability of the shares.
If a selling stockholder notifies us that it has a material arrangement with a broker-dealer for the resale of the common stock, then we would be required to amend the registration statement of which this prospectus is a part, and file a prospectus supplement to describe the agreements between the selling stockholder and the broker-dealer.
Management’s Discussion and Analysis of
Financial Condition and Results of Operations
The following discussion of our financial condition and results of operations should be read in conjunction with our financial statements and the notes to those financial statements included elsewhere in this prospectus. This discussion contains forward-looking statements, which are based on our assumptions about the future of our business. Our actual results could differ materially from those contained in the forward-looking statements. Please read “Cautionary Note Regarding Forward-Looking Statements” included elsewhere in this prospectus for additional information regarding forward-looking statements used in this prospectus.
Overview
Sun BioPharma, Inc. and its wholly-owned subsidiary, Sun BioPharma Australia Pty Ltd. (“SBA”) (collectively “we,” “us,” “our,” and the “Company”) exist for the primary purpose of advancing the commercial development of a proprietary polyamine analogue for pancreatic cancer and for a second indication in chronic pancreatitis. We have exclusively licensed the worldwide rights to this compound, which has been designated as SBP-101, from UFRF.
In June 2015, we changed our name to Sun BioPharma Research, Inc. (“SBR”) as part of a plan to merge with Cimarron Medical, Inc., (“Cimarron”). We entered into an Agreement and Plan of Merger with Cimarron and SB Acquisition Corporation, a wholly owned subsidiary of Cimarron, on June 12, 2015. The merger of SB Acquisition Corporation with and into SBR on September 4, 2015 (the “Merger”) resulted in all of the issued and outstanding common stock of SBR being converted into the right to receive an aggregate of 28,442,484 shares of Cimarron’s common stock, representing four shares of Cimarron common stock for every one share of SBR common stock cancelled in the Merger. As a result of this transaction, former SBR shareholders received approximately 95% of the outstanding capital stock of Cimarron. Concurrent with the completion of the Merger, Cimarron’s name was changed to “Sun BioPharma, Inc.”
Under GAAP, our predecessor entity. SBR, was deemed to be the acquirer for accounting purposes because its former stockholders owned a substantial majority of the issued and outstanding shares of our common stock after the Merger. Further, as Cimarron’s business operations and net assets, at the time of the Merger, were nominal relative to SBR’s business operations and net assets, the Merger was accounted for as a capital transaction and the activity presented in these financial statements represents the current and historical operations of Sun BioPharma, Inc.
During the year ended December 31, 2015, we incurred approximately $325,000 of costs associated with the Merger and as of December 31, 2015, assumed $250,000 of demand notes payable, net, after giving effect to the disposition of the legacy business operations of Cimarron. The transaction costs for the Merger are included in general and administrative expenses in our Consolidated Statements of Operations and Comprehensive Loss. See Note 8 to the Consolidated Financial Statements appearing elsewhere in this prospectus for additional information regarding the Merger.
On May 17, 2016, our shareholders approved a proposal to change the corporate domicile of Sun BioPharma, Inc., formerly known as Cimarron, from the State of Utah to the State of Delaware through a merger (the “Reincorporation”). The Reincorporation, in which Sun BioPharma, Inc. was merged with and into Sun BioPharma Research Inc., with Sun BioPharma, Inc. being the surviving entity, effected only a change in the legal domicile of the Company and other changes of a legal nature. The Reincorporation did not result in any change in the name, business, management, fiscal year, accounting, location of the principal executive offices, assets or liabilities of the Company. The current directors of the Company continued as directors of the surviving corporation.
In August 2015, the FDA granted an Investigational New Drug (“IND”) approval for our SBP-101 product candidate and we enrolled our first patient in our Phase 1 clinical trial on January 4, 2016. We estimate that completion of necessary preclinical development work, the completion of a Phase 1 clinical trial in pancreatic cancer and initiation of a Phase 1 clinical trial in pancreatitis, will require additional funding of $10 million to $20 million. Additional clinical trials will likely be required for FDA or other similar approvals if the results of the first clinical trial of our SBP-101 product candidate is positive. We estimate that the additional time and cost to obtain FDA and European Medicines Agency (“EMA”) approval and to bring our SBP-101 product candidate to market in these two indications will be 5 to 7 years with related costs up to $200 million.
Financial Overview
We have incurred losses of $15.5 million since inception. For the six months ended June 30, 2016 and 2015, we incurred net losses of $1.8 million and $3.4 million, respectively, and negative cash flows from operating activities of $631,000 and $2.1 million, respectively. For the year ended December 31, 2015 and 2014, we incurred net losses of $4.9 million and $3.5 million, respectively, and negative cash flows from operating activities of $3.9 million and $3.3 million, respectively. We expect to incur substantial losses, which will continue to generate negative net cash flows from operating activities, as we continue to pursue research and development activities and commercialize our SBP-101 product candidate.
Our cash and cash equivalents were $1.9 million as of June 30, 2016, compared to $925,000 as of December 31, 2015. We believe our cash and cash equivalents as of June 30, 2015, will be sufficient to fund our planned operations through the end of 2016.
We will need to obtain additional funds to continue our operations and execute our current business plans, including completing our current Phase 1 clinical trial, planning for required future regulatory submissions and trials and pursuing regulatory approvals in the United States, the European Union and other international markets. We historically have financed our operations principally from the sale of convertible debt and equity securities. While we have been successful in the past in obtaining the necessary capital to support our operations, and have similar future plans to obtain additional financing, there is no assurance that we will be able to obtain additional financing under commercially reasonable terms and conditions, or at all. This risk would increase if our clinical data is inconclusive or not positive or economic conditions worsen in the market as a whole or in the pharmaceutical or biotechnology markets individually.
On March 1, 2016 we instituted substantial salary deferrals for all senior employees in order to conserve cash.
In May 5, 2016, SBA, our subsidiary, received a $772,000 tax refund related to 2015 research and development activities.
In June 2016, pursuant to a Securities Purchase Agreement, the Company sold units (the “Units”) with each Unit consisting of a share of common stock and a warrant to purchase one-half of a share of common stock. A total of 1,951,000 Units were purchased by the Purchasers consisting of an aggregate of 1,951,000 shares of the Company’s common stock and warrants to purchase an aggregate of 975,000 shares of the Company’s common stock. The purchase price for each Unit was $1.00 and the Warrants will be exercisable for a period of five years from the date of issuance at an exercise price of $1.50 per share. The Company received aggregate gross proceeds of $1,755,000 from June closings under this private placement transaction and an additional $196,000 was invested by management through the conversion of previously deferred compensation. In August 2016, the Company sold an additional 220,000 Units for aggregate gross proceeds of $220,000. In September, the Company sold an additional 50,000 Units for aggregate gross proceeds of $50,000.
If we are unable to obtain additional financing when needed, we will need to reduce our operations by taking actions that may include, among other things, reducing use of outside professional service providers, reducing staff or further reducing staff compensation, significantly modifying or delaying the development of our SBP-101 product candidate, licensing rights to third parties, including the right to commercialize our SBP-101 product candidate for pancreatic cancer, acute pancreatitis or other applications that we would otherwise seek to pursue, or discontinue operations entirely.
Key Components of Our Results of Operations
General and Administrative Expenses
Our selling, general and administrative expenses consist primarily of salaries, benefits and other costs, including stock-based compensation, for our executive and administrative personnel; legal and other professional fees; travel, insurance and other corporate costs. We expect to incur a significant increase in general and administrative expenses as a result of becoming a public company in September 2015. These increases are anticipated to include higher costs for insurance, costs related to quarterly, annual and other periodic filings with the SEC and payments to outside consultants, lawyers and accountants. We also expect to incur significant costs to comply with the corporate governance, internal controls and similar requirements applicable to public companies.
Research and Development Expenses
Since its inception, we have focused our activities on the development of its SBP-101 product candidate. We expense both internal and external research and development costs as incurred. Research and development costs include expenses incurred to design, develop, test, seek approval for and enhance our SBP-101 product candidate and production processes. Expenses related to research and development consist primarily of third-party service providers monitoring and accumulating data related to our preclinical studies; sponsored research agreements; developing and scaling the manufacturing process necessary to produce sufficient amounts of our SBP-101 product candidate for use in our pre-clinical studies and expected future clinical trials; consulting resources with specialized expertise related to execution of our development plan for our SBP-101 product candidate; personnel costs, including salaries, benefits and share-based compensation and costs to license and maintain our licensed intellectual property.
We expense costs associated with obtaining licenses for patented technologies when it is determined there is no alternative future use of the intellectual property subject to the license.
While our research and development expenses to date have been focused on the development of our SBP-101 product candidate, we expect that a large percentage of our research and development expenses in the future will be incurred in support of our current Phase 1 clinical trial and anticipated future clinical trials. We cannot determine with certainty the timing of initiation, the duration or the completion costs of current or future preclinical studies and clinical trials of our product candidates. At this time, due to the inherently unpredictable nature of preclinical and clinical development, we are unable to estimate with any certainty the costs we will incur and the timelines we will require in the continued development of our product candidates and our other pipeline programs. Clinical and preclinical development timelines, the probability of success and development costs can differ materially from expectations. Our future research and development expenses will depend on the preclinical and clinical success of each product candidate that we develop, as well as ongoing assessments of the commercial potential of such product candidates. In addition, we cannot forecast which product candidates may be subject to future collaborations, when such arrangements will be secured, if at all, and to what degree such arrangements would affect our development plans and capital requirements.
Completion of clinical trials may take several years or more, and the length of time generally varies according to the type, complexity, novelty and intended use of a product candidate. The cost of clinical trials may vary significantly over the life of a project as a result of differences arising during clinical development, including, among others:
|
● |
per patient trial costs; |
|
● |
the number of trials required for approval; |
|
● |
the number of sites included in the trials; |
|
● |
the length of time required to enroll suitable patients; |
|
● |
the number of doses that patients receive; |
|
● |
the number of patients that participate in the trials; |
|
● |
the drop-out or discontinuation rates of patients; |
|
● |
the duration of patient follow-up; |
|
● |
potential additional safety monitoring or other studies requested by regulatory agencies; |
|
● |
the number and complexity of analyses and tests performed during the trial; |
|
● |
the phase of development of the product candidate; and |
|
● |
the efficacy and safety profile of the product candidate. |
Our expenses related to clinical trials are based on estimates of the services received and efforts expended pursuant to contracts with multiple clinical trial sites and, for certain trials, contract research organizations, (“CROs”), which administer clinical trials on our behalf. The financial terms of these agreements are subject to negotiation and vary from contract to contract and may result in uneven payment flows. Generally, these agreements set forth the scope of work to be performed at a fixed fee or unit price. Payments under the contracts depend on factors such as the successful enrollment of patients and the completion of clinical trial milestones. Expenses related to clinical trials generally are accrued based on contracted amounts and the achievement of milestones, such as number of patients enrolled. If timelines or contracts are modified based upon changes to the clinical trial design or scope of work to be performed, we modify our estimates of accrued expenses accordingly.
Other Income (Expense)
Other income (expense) consists of interest income, cash and non-cash interest expense and transaction gains and losses resulting from transactions denominated in other than our functional currency.
Critical Accounting Policies and Estimates
Our management’s discussion and analysis of financial condition and results of operations is based on our financial statements, which have been prepared in accordance with GAAP. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses. On an ongoing basis, we evaluate these estimates and judgments, including those described below. We base our estimates on our historical experience and on various other assumptions that we believe to be reasonable under the circumstances. These estimates and assumptions form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results and experiences may differ materially from these estimates.
While our significant accounting policies are more fully described in Note 4 to our Consolidated Financial Statements appearing elsewhere in this prospectus, we believe that the following accounting policies are the most critical to aid you in fully understanding and evaluating our reported financial results and affect the more significant judgments and estimates that we use in the preparation of our financial statements.
Fair Value Estimates of Common Stock
Prior to becoming eligible for quotation on the over-the-counter markets, determining the fair value per share or our common stock for use in estimating the fair values of share based payments required making complex and subjective judgments. The Company used the implied valuations based upon the terms from our sales of convertible notes payable to estimate our enterprise value for the dates on which these transactions occurred. The estimated enterprise values considered certain discounts related to control and lack of marketability.
Our board of directors also considered the estimated fair value of our common stock in relation to a number of objective and subjective factors, including external market conditions affecting the biotechnology industry sector. Our board of directors also retained an independent financial valuation firm to provide independent estimates of our enterprise value. Until an active trading market develops for our common stock, estimating the fair value per share of our common stock will continue to be highly subjective. There is inherent uncertainty in these estimates.
Share-Based Compensation
We recognize compensation expense in an amount equal to the estimated grant date fair value of each option grant, or stock award over the estimated period of service and vesting. This estimation of the fair value of each stock-based grant or issuance on the date of grant involves numerous assumptions by management. Although we calculate the fair value under the Black Scholes option pricing model, which is a standard option pricing model, this model still requires the use of numerous assumptions, including, among others, the expected life, volatility of the underlying equity security, a risk free interest rate and expected dividends. The model and assumptions also attempt to account for changing employee behavior as the stock price changes and capture the observed pattern of increasing rates of exercise as the stock price increases. The use of different assumptions by management in connection with these assumptions in the Black Scholes option pricing model can produce substantially different results.
Research and development costs
We charge research and development costs, including clinical trial costs, to expense when incurred. Our human clinical trials are, and will be, performed at clinical trial sites and are administered jointly by us with assistance from contract research organizations (“CROs”). Costs of setting up clinical trial sites are accrued upon execution of the study agreement. Expenses related to the performance of clinical trials generally are accrued based on contracted amounts and the achievement of agreed upon milestones, such as patient enrollment, patient follow-up, etc. We monitor levels of performance under each significant contract, including the extent of patient enrollment and other activities through communications with the clinical trial sites and CROs, and adjust the estimates, if required, on a quarterly basis so that clinical expenses reflect the actual effort expended at each clinical trial site and by each CRO.
Results of Operations
Comparison of the three and six-months ended June 30, 2016 to the three and six-months ended June 30, 2015 (in thousands):
|
Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||||||||||
|
2016 |
2015 |
Percent Change |
2016 |
2015 |
Percent Change |
|||||||||||||||||||
|
Operating expenses: |
||||||||||||||||||||||||
|
General and administrative |
$ | 419 | $ | 585 | (28.4 | )% | $ | 900 | $ | 1,788 | (49.7 | )% | ||||||||||||
|
Research and development |
530 | 608 | (12.8 | ) | 1,024 | 1,596 | (35.8 | ) | ||||||||||||||||
|
Total operating expenses |
949 | 1,193 | (20.5 | ) | 1,924 | 3,384 | (43.1 | ) | ||||||||||||||||
|
Other expense, net |
(119 | ) | (60 | ) | 98.3 | (81 | ) | (118 | ) | (31.4 | ) | |||||||||||||
|
Income tax benefit |
90 | 38 | 136.8 | 206 | 95 | 116.8 | ||||||||||||||||||
|
Net loss |
$ | (978 | ) | $ | (1,215 | ) | (20.1 | )% | $ | (1,799 | ) | $ | (3,407 | ) | (47.2 | )% | ||||||||
General and administrative expense
Our general and administrative (“G&A”) expenses decreased 28.4% to $419,000 in the second quarter of 2016 down from $585,000 in the second quarter of 2015. G&A expenses decreased 49.7% to $900,000 in the six months ended June 30, 2016 from $1.8 million in comparable period of 2015. These decreases were due primarily to a reduction in stock-based compensation. Decreased legal and accounting fees associated related to pursuing the merger with Cimarron Medical, Inc. during 2015 also contributed to the year-over-year declines. These decreases were partially offset by increased salaries and increased reporting and compliance costs associated with becoming a public company.
Research and development expense
Our research and development (“R&D”) expenses decreased 12.8% to $530,000 in the second quarter of 2016 down from $608,000 in the second quarter of 2015. R&D expenses decreased 35.8% to $1.0 million in the six months ended June 30, 2016 down from $1.6 million in the six months ended June 30, 2015. These decreases in R&D expenses were due primarily to decreased costs of preclinical studies, other product development costs and stock-based compensation. These reductions were partially offset by increased expenses associated with the conduct of our Phase 1 clinical trial of SBP-101, our initial product candidate.
Other expense, net
Other expense, net, increased 98.3% to $119,000 in the current quarter and decreased 31.4% to $81,000 for the six months ended June 30, 2016 as compared with the same periods in the prior year. Other expense, net, consists primarily of interest expense on convertible promissory notes and long-term debt and foreign currency transaction gains and losses. The current year fluctuations resulted primarily from the impact of fluctuations in the foreign currency exchange rates between the United States and Australian dollars on the translation of the SBA financial statements.
Income tax benefit
Income tax benefit increased to $90,000 and $206,000 for the three and six months ended June 30, 2016, respectively. Our income tax benefit is derived primarily from refundable tax credits associated with our R&D activities conducted in Australia.
Comparison of the Year Ended December 31, 2015 to the Year Ended December 31, 2014 (in thousands)
|
Year Ended December 31, |
||||||||||||
|
2015 |
2014 |
Percent Change | ||||||||||
|
Operating expenses: |
||||||||||||
|
General and administrative |
$ | 2,592 | $ | 1,079 | 140.2 | % | ||||||
|
Research and development |
2,852 | 2,366 | 20.5 | |||||||||
|
Total operating expenses |
5,444 | 3,445 | 58.0 | |||||||||
|
Other expense, net |
(239 | ) | (194 | ) | 23.2 | |||||||
|
Income tax benefit |
756 | 108 | 700.0 | |||||||||
|
Net loss |
$ | (4,927 | ) | $ | (3,531 | ) | 42.8 | % | ||||
General and administrative and research and development expenses include non-cash stock-based compensation expense as a result of our issuance of stock options and stock grants. We expense the fair value of equity awards over their vesting periods. The terms and vesting schedules for share-based awards vary by type of grant and the employment status of the grantee. The awards granted through December 31, 2015 vest primarily upon time-based conditions. We expect to record additional non-cash compensation expense in the future, which may be significant. The following table summarizes the stock-based compensation expense in our statement of Consolidated Statements of Operations and Comprehensive Loss for the years ended December 31, 2015 and 2014 (in thousands):
|
Year Ended December 31, |
||||||||
|
2015 |
2014 |
|||||||
|
General and administrative |
$ | 759 | $ | 74 | ||||
|
Research and development |
217 | 122 | ||||||
|
Total stock-based compensation |
$ | 976 | $ | 196 | ||||
General and administrative expense
Our general and administrative (“G&A”) expenses increased 140.2% to $2.6 million in 2015 up from $1.1 million in 2014. These increases were due primarily to increased legal and accounting fees associated with completing the Merger, the reporting, compliance requirements and other costs associated with being a public company, increased salaries related to the changes in management in the second half of 2015 and increases in share-based compensation.
Research and product development expense
Our research and development (“R&D”) expenses increased 20.5% to $2.9 million in 2015 up from $2.4 million in 2014. The overall increase in R&D expenses results primarily from the combination of increased costs associated with pursuing our investigational new drug application with the FDA, costs of preparing for and initiating our Phase 1 clinical trial and increased costs from expanding our clinical and research personnel partially offset by reductions in the costs of required preclinical testing in the current year. An increase in share-based compensation also contributed to the current year increase in research and development expenses.
Other expense, net
Other expense, net, increased 23.2% to $239,000 in the current year up from $194,000 in the prior year. Other expense, net, consists primarily of interest expense on convertible promissory notes and long-term debt and foreign currency transaction losses. The current year increases are primarily due to increases in losses associated with transactions in foreign currencies.
Income tax benefit
Income tax benefit increased to $756,000 in 2015 up from $108,000 in 2014. Our income tax benefit is derived primarily from refundable tax credits associated with our R&D activities conducted in Australia. The current year increase reflects an increase in the costs eligible for the Australian R&D tax credit.
Liquidity and Capital Resources
The following table summarizes our liquidity and capital resources as of June 30, 2016 and December 31, 2015 and our cash flow data for the six months ended June 30, 2016 and 2015 and is intended to supplement the more detailed discussion that follows (in thousands):
|
Liquidity and Capital Resources |
June 30, 2016 |
December 31, 2015 |
||||||
|
Cash and cash equivalents |
$ | 1,898 | $ | 925 | ||||
|
Working capital (deficit) |
$ | 419 | $ | 357 | ||||
|
Six Months Ended June 30, |
||||||||
|
Cash Flow Data |
2016 |
2015 |
||||||
|
Cash provided by (used in): |
||||||||
|
Operating activities |
$ | (631 | ) | $ | (2,141 | ) | ||
|
Financing activities |
1,603 | 1,512 | ||||||
|
Effect of exchange rate changes on cash and cash equivalents |
1 | (19 | ) | |||||
|
Net decrease in cash and cash equivalents |
$ | 973 | $ | (648 | ) | |||
Working Capital
Our total cash resources, including short-term investments, totaled $1.9 million as of June 30, 2016, compared to $925,000 as of December 31, 2015. We had $1.7 million in current liabilities, and working capital of $419,000 as of June 30, 2016, compared to $1.4 million in current liabilities and $357,000 in working capital as of December 31, 2015. In May 2016, we received $772,000 of the $904,000 income tax receivable reported as of June 30, 2016. This receivable related to refundable tax credits for the 2015 research and development activities of our subsidiary Sun BioPharma Australia Pty Ltd. In June 2016, pursuant to a Securities Purchase Agreement, the Company sold units (the “Units”) with each Unit consisting of a share of common stock and a warrant to purchase one-half of a share of common stock. A total of 1,951,000 Units were purchased by the Purchasers consisting of an aggregate of 1,951,000 shares of the Company’s common stock and warrants to purchase an aggregate of 975,500 shares of the Company’s common stock. The purchase price for each Unit was $1.00 and the Warrants will be exercisable for a period of five years from the date of issuance at an exercise price of $1.50 per share. The Company received aggregate gross proceeds of $1,755,000 from June closings under this private placement transaction and an additional $196,000 was invested by management through the conversion of previously deferred compensation. In August 2016, the Company sold an additional 220,000 Units for aggregate gross proceeds of $220,000. In September, the Company sold an additional 50,000 Units for aggregate gross proceeds of $50,000.
Cash Flows
Net Cash Used in Operating Activities
Net cash used in operating activities was $631,000 in the six-months ended June 30, 2016 compared to $2.1 million in the six-months ended June 30, 2015. The net cash used in each of these periods primarily reflects the net loss for these periods, and is the partially offset by the effects of changes in operating assets and liabilities and, in the six-months ended June 30, 2015, by non-cash charges recorded for stock-based compensation.
Net Cash Provided by Financing Activities
Net cash provided by financing activities was $1.6 million for the six-months ended June 30, 2016, compared to $1.5 million in the six-months ended June 30, 2015. Net cash provided by financing activities was comprised of net proceeds from the sale of common stock and warrants and from the exercise of stock options and stock purchase warrants.
Capital Requirements
As we continue to pursue our operations and execute our business plan, including completing our current Phase 1 clinical trial for our product candidate SBP-101 in pancreatic cancer, planning for required future regulatory submissions and trials, and pursuing regulatory approvals in the United States, the European Union and other international markets, we expect to continue to incur substantial and increasing losses, which will continue to generate negative net cash flows from operating activities.
Our future capital uses and requirements depend on numerous current and future factors. These factors include, but are not limited to, the following:
|
● |
the progress of clinical trials required to support our applications for regulatory approvals, including our Phase 1 clinical trial, a human clinical trial in Australia and the United States; |
|
● |
our ability to demonstrate the safety and effectiveness of our SBP-101 product candidate; |
|
● |
our ability to obtain regulatory approval of our SBP-101 product candidate in the United States, the European Union or other international markets; |
|
● |
the market acceptance and level of future sales of our SBP-101 product candidate; |
|
● |
the rate of progress in establishing reimbursement arrangements with third-party payors; |
|
● |
the effect of competing technological and market developments; |
|
● |
the cost and delays in product development that may result from changes in regulatory oversight applicable to our SBP-101 product candidate; and |
|
● |
the costs involved in filing and prosecuting patent applications and enforcing or defending patent claims. |
To date, we have used primarily convertible debt and equity financings to fund our ongoing business operations and short-term liquidity needs, and we expect to continue this practice for the foreseeable future.
Our cash and cash equivalents were $1.9 million as of June 30, 2016, compared to $925,000 as of December 31, 2015. Although we do not have any current capital commitments, we expect that we will increase our projected expenditures once we have additional capital on hand in order to continue our efforts to grow our business, complete our Phase 1 clinical trial for our SBP-101 product candidate and prepare to submit an Investigational New Drug application for the use of SBP-101 to treat acute pancreatitis. Accordingly, we expect to make additional expenditures in product development to continue enhancing our current products. However, we do not have any definitive plans as to the exact amounts or particular uses at this time, and the exact amounts and timing of any expenditure may vary significantly from our current intentions.
As of June 30, 2016, we did not have any existing credit facilities under which we could borrow funds. We historically have financed our operations principally from the sale of convertible debt and equity securities. While we have been successful in the past in obtaining the necessary capital to support our operations, and have similar future plans to obtain additional financing, there is no assurance that we will be able to obtain additional financing under commercially reasonable terms and conditions, or at all.
We will need to obtain additional funds to continue our operations and execute our business plans, including completing our current Phase 1 clinical, planning for required future regulatory submissions and trials and pursuing regulatory approvals in the United States, the European Union and other international markets. We historically have financed our operations principally from the sale of convertible debt and equity securities. While we have been successful in the past in obtaining the necessary capital to support our operations, and have similar future plans to obtain additional financing, there is no assurance that we will be able to obtain additional financing under commercially reasonable terms and conditions, or at all. This risk would increase if our clinical data is inconclusive or not positive or economic conditions worsen in the market as a whole or in the pharmaceutical or biotechnology markets individually.
On March 1, 2016 we instituted substantial salary deferrals for all senior employees in order to conserve cash.
In May 5, 2016, SBA, our subsidiary, received a $772,000 tax refund related to 2015 research and development activities.
In June, 2016, pursuant to a Securities Purchase Agreement, the Company sold units (the Units”) with each Unit consisting of a share of common stock and a warrant to purchase one-half of a share of common stock. A total of 1,951 Units were purchased by the Purchasers consisting of an aggregate of 1,951,000 shares of the Company’s common stock and warrants to purchase an aggregate of 975,500 shares of the Company’s common stock. The purchase price for each Unit was $1.00 and the Warrants will be exercisable for a period of five years from the date of issuance at an exercise price of $1.50 per share. The Company received aggregate gross proceeds of $1,755,000 from June closings under this private placement transaction and an additional $196,000 was invested by management through the conversion of previously deferred compensation. In August 2016, the Company sold an additional 220,000 Units for aggregate gross proceeds of $220,000. In September, the Company sold an additional 50,000 Units for aggregate gross proceeds of $50,000.
To the extent that we raise additional capital through the sale of equity or convertible debt securities, the interests of our current stockholders would be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of our current stockholders. If we issue preferred stock, it could affect the rights of our stockholders or reduce the value of our common stock. In particular, specific rights granted to future holders of preferred stock may include voting rights, preferences as to dividends and liquidation, conversion and redemption rights, sinking fund provisions, and restrictions on our ability to merge with or sell our assets to a third party. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. Any of these events could adversely affect our ability to achieve our regulatory approvals and commercialization goals and harm our business.
Our future success is dependent upon our ability to obtain additional financing, the success of our current Phase 1 clinical trial and required future trials and our ability to obtain marketing approval for our SBP-101 product candidate in the United States, the European Union and other international markets. If we are unable to obtain additional financing when needed, if our Phase 1 clinical trial is not successful, if we do not receive regulatory approval required to conduct future trials or if once these studies are concluded, we do not receive marketing approval for our SBP-101 product candidate, we would not be able to continue as a going concern and would be forced to cease operations. The interim financial statements included in this report have been prepared assuming that we will continue as a going concern and do not include any adjustments relating to the recoverability or classification of assets or the amounts of liabilities that might result from the outcome of these uncertainties.
Indebtedness
We currently have $2,775,000 outstanding in convertible promissory notes that accrue annual interest of 5%, payable quarterly, and are convertible into common stock at $1.125 per share. These notes mature in December 2018. We have $300,000 outstanding in an unsecured loan that accrues annual interest of 4.125%. All principal and accrued interest on this loan are payable in October 2017. We also have $250,000 of unsecured demand notes which we assumed in connection with the Merger. These demand notes have no stated interest rate or maturity date.
License Agreement
On December 22, 2011, we entered into an exclusive license agreement with UFRF, which was acquired in exchange for $15,000 in cash and the issuance of 10% of its common stock. Upon executing the license agreement, 800,000 shares of common stock were issued to UFRF which was determined to have a fair value of $20,000 based upon an estimated fair value of our predecessor’s common stock of $0.025 per share. The license agreement also contained an anti-dilution provision which required us to issue additional shares to UFRF sufficient for UFRF to maintain its 10% ownership interest in our predecessor entity until it secured an addition $2.0 million external investment. This investment was received during 2012.
The license agreement requires the Company to pay royalties to UFRF ranging from 2.5% to 5% of net sales of licensed products developed from the licensed technology. Minimum annual royalties are required after the initial occurrence of a commercial sale of a marketed product. Royalties are payable for the longer of (i) the last to expire of the claims in the licensed patents or (ii) ten (10) years from the first commercial sale of a licensed product in each country in which licensed product is sold. The minimum annual royalties are as follows:
|
● |
$50,000 is due 270 days after occurrence of first commercial sale; |
|
● |
$100,000 is due on the first anniversary date of the first payment; |
|
● |
$100,000 is due on the second anniversary date of the first payment; and |
|
● |
$300,000 is due on the third anniversary date of the first payment and subsequent anniversary dates thereafter, continuing for the life of the license agreement. |
The Company is subject to six different milestone payments under the license agreement.
|
● |
$50,000 is due upon enrollment of the first subject in a Phase I clinical trial; |
|
● |
$300,000 is due upon enrollment of the first subject in a Phase II clinical trial; |
|
● |
$3,000,000 is due upon approval of a New Drug Application; |
|
● |
$2,000,000 is due upon approval to manufacture and market in either the European Union or Japan (one time only); |
|
● |
$1,000,000 is due upon the first time annual net sales of licensed product or licensed process by the Company reaches $100,000,000; and |
|
● |
$3,000,000 is due upon the first time annual net sales of licensed product or licensed process by the Company reaches $500,000,000. |
As of June 30, 2016, we have incurred $50,000 of milestone payment obligations to UFRF. The Company is also committed to pay an annual license maintenance fee of $10,000.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements.
Recent Accounting Pronouncements
See Note 4 to the Consolidated Financial Statements contained in Item 8 below for a discussion of recent accounting pronouncements.
Business
Overview
We are a clinical stage drug development company founded with technology licensed from The University of Florida. The polyamine analogue compound we have licensed from UFRF, which we refer to as “SBP-101,” exhibits extraordinary specificity for the exocrine pancreas, with therapeutic potential for both pancreatic cancer and pancreatitis indications. Studies in dogs revealed ablation, or “chemical resection,” of the exocrine pancreatic architecture, while leaving the islet cells functionally unchanged. We may refer to this effect as: “pharmaceutical pancreatectomy with islet auto-transplant” (“PP-IAT”). Xenograft studies of human pancreatic cancer cells transplanted into mice indicate that SBP-101 suppresses both primary and metastatic growth of these cells. To facilitate and accelerate the development of this compound in the pancreatic cancer indication, we have also acquired data and materials related to this technology from other researchers. We believe that SBP-101, if successfully developed, may represent a novel approach that effectively treats pancreatic cancer and pancreatitis, and could become the dominant product in these markets. Only three first-line treatment options for pancreatic cancer have been approved by the FDA in the last 20 years, and no drugs have been approved for the treatment of patients with pancreatitis.
We estimate that completion of necessary preclinical development work, the completion of a Phase 1 clinical trial in pancreatic cancer and initiation of a Phase 1 clinical trial in pancreatitis, will require additional funding of at least $10 million to $20 million in addition to amounts we have previously raised. Additional clinical trials will be subsequently required for FDA approval if the results of the first clinical trials of SBP-101 are positive. We estimate that the additional time and cost to obtain FDA and European Medicines Agency (“EMA”) approval and to bring SBP-101 to market in these two indications will be 5 to 7 years with related costs up to $200 million.
With the approximately $13.5 million raised to date, we have:
|
● |
organized the Company; |
|
● |
evaluated and secured the intellectual property for our core technology; |
|
● |
completed initial pre-clinical steps in the development plan for SBP-101; |
|
● |
secured an orphan drug designation from the US FDA; |
|
● |
submitted an IND application to the US FDA (May 18, 2016); |
|
● |
received an acceptance of an IND application from the FDA (August 21, 2015); |
|
● |
commenced a Phase 1 clinical trial for pancreatic ductal adenocarcinoma; |
|
● |
completed enrollment and follow-up of two cohorts of patients; and |
|
● |
commenced further pre-clinical studies for the use of SBP-101 to treat pancreatitis. |
Our Investigative New Drug (“IND”) application for the Phase I clinical trial was submitted to the FDA on May 18, 2015. The application was accepted by the FDA in August 2015, and we began enrolling patients in our Phase 1 clinical trial for the pancreatic cancer indication in January 2016.
Introduction
An effective treatment for pancreatic cancer remains a major unmet medical need. Adenocarcinoma of the pancreas, which accounts for 95% of all cases of pancreatic cancer, has a median overall survival rate of 8 to 11 months in patients with favorable prognostic signs and optimal chemotherapy. In 2016, more than 53,000 Americans, and over 300,000 persons worldwide, are estimated to receive a new diagnosis of this disease. Pancreatic cancer is now the third most common cause of cancer death in the United States. A recent report from the Pancreatic Cancer Action Network states that pancreatic cancer deaths in the United States have surpassed those from breast cancer and will soon surpass deaths from colorectal cancer, where earlier detection and modestly successful drug interventions have been developed, to rank number two in deaths, behind only lung cancer in 2020. The five-year survival rate for metastatic pancreatic cancer remains less than three percent (3%), and there has been little significant improvement in survival since gemcitabine was approved in the United States in 1996.
Pancreatic cancer is generally not diagnosed early because the initial clinical signs and symptoms are vague and non-specific. By the time of diagnosis, the cancer is most often locally advanced or metastatic, meaning spread to regional lymph nodes, liver, lung and/or peritoneum, and is seldom amenable to surgical resection with curative intent.
Currently, surgical resection offers the only potentially curative therapy, but most patients have disease that is unresectable at the time of diagnosis. The prognosis for these patients is poor and most die from complications related to progression of the disease. The mainstay of treatment for metastatic disease is chemotherapy. Current first-line chemotherapy treatment regimens vary from single agent gemcitabine and various gemcitabine combinations to the multi-chemotherapy drug combination, FOLFIRINOX (Conroy NEJM 2011), frequently supplemented with white blood cell (“WBC”) growth factors. These combination therapies deliver median survival benefits ranging from 7 weeks (Von Hoff NEJM 2013) to 4 months (Conroy NEJM 2011) for selected patients with good performance status, meaning good physical health, when compared with gemcitabine alone.
University laboratory studies have demonstrated that SBP-101 induces programmed cell death, or “apoptosis,” in the acinar and ductal cells of the pancreas by activation of caspase 3 and poly adenosine diphosphate-ribose polymerase (“PARP”). In animal models at two independent laboratories, SBP-101, alone or in combination, has demonstrated nearly complete suppression of transplanted human pancreatic cancer tumor models, including metastases. We intend to develop and commercialize SBP-101as a unique and novel targeted approach to treating pancreatic cancer. We may develop SBP-101 as either a stand-alone therapy (monotherapy) or in combination with standard chemotherapy agents. We also intend to continue evaluation of the potential value of SBP-101 in the treatment of patients with pancreatitis.
Pancreatic Cancer
Adenocarcinoma of the pancreas afflicts approximately 61,000 people in the European Union (Eurostat 2014), over 51,000 people in the United States annually, and 337,000 people worldwide (World Health Organization 2014, NIH/NCI). It is the seventh leading cause of death from cancer in Europe (GLOBOCAN 2012) and the third leading cause of death from cancer in the United States (SEER Cancer Statistics Factsheets 2016). Pancreatic ductal adenocarcinoma (“PDA”) represents approximately 95% of all pancreatic cancers. Considering that the median overall survival for previously untreated patients with good performance status is between 8.5 months (Von Hoff 2013) and 11.1 months (Conroy 2011) with the best available treatment regimens, effective treatment for PDA remains a major unmet medical need.
Pancreatic cancer is generally not diagnosed early because the initial clinical signs and symptoms are vague and non-specific. The most common presenting symptoms include weight loss, epigastric (upper central region of the abdomen) and/or back pain, and jaundice. The back pain is typically dull, constant, and of visceral origin radiating to the back, in contrast to the epigastric pain which is vague and intermittent. Less common symptoms include nausea, vomiting, diarrhea, anorexia, and new onset diabetes or glucose intolerance (Hidalgo 2010). By the time the diagnosis is made, the cancer often is locally advanced or metastatic and is seldom amenable to surgical resection with curative intent.
For the minority of patients who present with resectable disease, surgery is the treatment of choice. Depending on the location of the tumor the operative procedures may involve cephalic pancreatoduodenectomy, referred to as a “Whipple procedure”, distal pancreatectomy or total pancreatectomy. Pancreatic enzyme deficiency and diabetes are frequent complications of these procedures. Up to 70% of patients with pancreatic cancer present with biliary obstruction, that can be relieved by percutaneous or endoscopic stent placement. However, even if the tumor is fully resected, the outcome in patients with pancreatic cancer is disappointing (Hidalgo 2010, Seufferlein 2012). Post-operative administration of chemotherapy improved progression-free and overall survival in three large, randomized clinical trials (Hidalgo 2010), but median post-surgical survival in patients treated in all three trials was similar: only 20-22 months.
For the majority of patients who present with unresectable locally advanced or metastatic disease, management options range from chemotherapy alone to combined forms of treatment with radiation therapy and chemotherapy. However, due to the increased toxicity of combined treatment, randomized trials of such combined regimens have had low enrollment, precluding a firm conclusion as to any advantage of adding radiation to chemotherapy (Hidalgo 2010).
Gemcitabine was the first chemotherapeutic agent approved for the treatment of PDA, providing a median survival duration of 5.65 months (Burris 1997). Gemcitabine monotherapy was the standard of care for patients with metastatic pancreatic cancer until combination therapy with gemcitabine plus erlotinib (Tarceva®) was shown to increase median survival by 2 weeks. This modest benefit was tempered by a significant side effect profile and high cost, limiting its adoption as a standard treatment regimen. More recently, the multidrug chemotherapy combination of leucovorin, fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) was shown to provide a median survival benefit of 4.3 months (OS = 11.1 months) over gemcitabine alone (6.8 months), but its side effect profile limits the regimen to select patients with a good performance status and often requires supplementation with WBC growth factor therapy. Nab-paclitaxel (Abraxane®) received marketing authorization for use in combination with gemcitabine after showing an increase in overall survival of 7 weeks compared to gemcitabine alone (Von Hoff 2013). Thus, combination therapies have demonstrated a modest survival benefit compared to gemcitabine alone as summarized in the table below (Thota 2014).
Current Treatment Approaches: Survival & Toxicity Profiles Across Three Major Positive Clinical Trials
|
Gemcitabine vs. Gemcitabine/Erlotinib Phase 3 trial |
ACCORD 11 Trial |
Metastatic Pancreatic Adenocarcinoma Clinical Trial (MPACT) |
||||||||||||||||||||||
|
Gemcitabine |
Gemcitabine/ Erlotinib |
Gemcitabine |
FOLFIRINOX 1 |
Gemcitabine |
Gemcitabine/ Nab- Pacilataxel |
|||||||||||||||||||
|
One-Year survival |
17% | 23% | 20.6% | 48.4% | 22% | 35% | ||||||||||||||||||
|
Median Overall Survival |
5.91 mo | 6.24 mo | 6.8 mo | 11.1 mo | 6.7 mo | 8.5 mo | ||||||||||||||||||
|
Median Progression-Free Survival |
3.55 mo | 3.75 mo | 3.3 mo | 6.4 mo | 3.7 mo | 5.5 mo | ||||||||||||||||||
|
Overall Response Rate |
8% | 8.6% | 9.4% | 31.6% | 23% | |||||||||||||||||||
|
Toxicity |
||||||||||||||||||||||||
|
Neutropenia |
– | – | 21% | 45.7% | 27% | 38% | ||||||||||||||||||
|
Febrile neutropenia |
– | – | 1.2% | 5.4% | 1% | 3% | ||||||||||||||||||
|
Thrombocytopenia |
– | – | 3.6% | 9.1% | 9% | 13% | ||||||||||||||||||
|
Diarrhea |
2% | 6% | 1.8% | 12.7% | 1% | 6% | ||||||||||||||||||
|
Sensory neuropathy |
– | – | 0% | 9% | 1% | 17% | ||||||||||||||||||
|
Fatigue |
15% | 15% | 17.8% | 23.6% | 7% | 17% | ||||||||||||||||||
|
Rash |
6% | 1% | – | – | – | – | ||||||||||||||||||
|
Stomatitis |
<1% | 0% | – | – | – | – | ||||||||||||||||||
|
Infection |
17% | 16% | – | – | – | – | ||||||||||||||||||
Source: Thota R et al., Oncology 2014; Jan 28(1):70–74
Other drugs are currently under investigation, but none have received marketing authorization for the treatment of PDA.
Pancreatitis
Two additional potential indications for SBP-101 are the treatment of patients with the serious and potentially life-threatening conditions of acute/recurrent acute and chronic pancreatitis. In the United States, acute pancreatitis occurs in approximately 300,000 patients per year, 10% of whom progress to chronic pancreatitis.
Patients with chronic pancreatitis endure repeated episodes of abdominal pain, often with progression to narcotic dependency and to pancreatic enzyme deficiency, as well as insulin dependent diabetes mellitus as a consequence of the ultimate destruction of pancreatic function. Once a patient has suffered from repeated painful bouts of chronic pancreatitis and become narcotic and pancreatic enzyme-dependent, they may be offered a total pancreatectomy. A total pancreatectomy is a surgical procedure resulting in the resection, or removal, of the pancreas (guaranteeing both pancreatic enzyme deficiency as well as insulin-dependent diabetes mellitus), and often includes the spleen, gall bladder and appendix. The operation is both extensive, requiring 8+ hours in the operating room, and expensive. While the goal of a total pancreatectomy in patients with chronic pancreatitis is pain relief, as many as 60% remain narcotic dependent, and even with islet auto transplantation, meaning the isolation and reintroduction of any of the patient’s remaining functional insulin producing islet cells, over 70% remain insulin dependent. The combination of a total pancreatectomy and islet auto transplant (“TP & IAT”) represents a small subset of the surgical approaches to patients with chronic pancreatitis. Thus, a patient with chronic pancreatitis may face months of abdominal pain, narcotic dependence, the onset of diabetes mellitus, the requirement for both insulin and pancreatic enzyme replacement, and finally, an extensive and expensive surgical procedure which may not materially improve any of their symptoms.
1FOLFIRINOX represents leucovirin, fluorouracil, irinotecan, and oxaliplatin.
Patients with acute pancreatitis experience abdominal pain, which can be severe and even life threatening. Acute pancreatitis occurs most often in adults aged 30-40 years, and is associated in some cases with increased consumption of alcohol and tobacco, and in other cases, with the presence of stones in the bile or pancreatic duct system. In a small minority of cases the disease may be hereditary, but many cases have no clear precipitating etiology, or cause. There are no specific agents approved for treatment of acute or chronic pancreatitis, as such, current treatment is limited to supportive care with intravenous fluids, narcotics and the avoidance of oral intake.
SBP-101, which has demonstrated the specificity to target the acinar and ductal cells of the pancreas, may represent an opportunity for up to 30,000 US patients annually with chronic pancreatitis to receive an early, non-surgical intervention into the natural history of their disease, with the potential to avoid narcotic dependency, insulin dependency and months of painful bouts of chronic pancreatitis. Patients would still require pancreatic enzyme replacement, but may be able to avoid surgery, diabetes, insulin and narcotic dependency. We believe that our consultations with pancreatitis experts at Harvard University, the Ohio State University, the University of Minnesota, Cedars Sinai Medical Center and the National Institute of Health (“NIH”) has resulted in enthusiastic endorsement of the study of SBP-101 in the treatment of patients with pancreatitis.
Clinical development of SBP-101 for the treatment of patients with pancreatitis is expected to proceed following the pancreatic cancer indication, with FDA consultation in a pre-IND meeting, completion of a series of IND-enabling nonclinical toxicology and pharmacology studies, and submission of an IND package to the FDA.
Proprietary Technology
Function and Characteristics of Polyamines
Polyamines are metabolically distinct entities within human cells that bind to and facilitate DNA replication, RNA transcription and processing, and protein (such as pancreatic enzymes) synthesis. Human cells contain three essential and naturally occurring polyamines “putrescine, spermidine, and spermine” that, in contrast to cell building blocks such as amino acids and sugars, remain as metabolically distinct entities inside the cell. Polyamines perform many functions necessary for cellular proliferation and protein synthesis. The critical balance of polyamines within cells is maintained by several enzymes such as ornithine decarboxylase (“ODC”) and spermidine/spermine N1 acetyl transferase (“SSAT”). All of these homeostatic enzymes are short-lived, rapidly inducible intracellular proteins that serve to tightly and continuously regulate native polyamine pools. These enzymes constantly maintain polyamines within a very narrow range of concentration inside the cell.
Polyamine Analogues
Polyamine analogues such as SBP-101 are structurally similar to naturally occurring polyamines, and are recognized by the cell’s polyamine uptake system, allowing these compounds to gain rapid entrance to the cell. Evidence suggests that pancreatic acinar cells, because of their extraordinary protein synthesis capacity, exhibit enhanced uptake of polyamines and polyamine analogues such as SBP-101. Because of preferential uptake by pancreatic acinar cells, polyamine analogies such as SBP-101 disrupt the cell’s polyamine balance and biosynthetic network, and induce programmed cell death, or apoptosis, via caspase 3 activation and PARP cleavage. Proof of concept has been demonstrated in multiple human pancreatic cancer models, both in vivo and in vitro, that pancreatic ductal adenocarcinoma exhibits sensitivity to SBP-101. Many tumors, including pancreatic cancer, display an increased uptake rate of polyamines and polyamine analogues.
SBP-101
SBP-101 is a proprietary polyamine analogue, which accumulates in the acinar cells of the beagle pancreas causing a complete pharmaceutical resection of the exocrine pancreas, without producing an inflammatory response. Due to a physiologically high intracellular concentration, SBP-101 induces disruption in acinar and ductal cells and pancreatic adenocarcinoma cells, which exhibit similar characteristics. Pancreatic islet cells, which secrete insulin, are structurally and functionally dissimilar to acinar cells and are not impacted by SBP-101.
The primary mechanism of action for SBP-101 has been demonstrated to include the enhanced uptake of the compound in the exocrine pancreas. This effect leads to corresponding depressed levels of native polyamines, with caspase 3 activation, PARP cleavage and apoptotic destruction (programmed cell death) of the exocrine pancreatic architecture without an inflammatory response. In animal models at two independent laboratories, SBP-101 has demonstrated significant suppression of transplanted human pancreatic cancer cells, including metastatic pancreatic cancer growth. See “Proof of Principle” below.
We believe that SBP-101 will have a distinct advantage over current pancreatic cancer therapies in that it specifically targets the exocrine pancreas and may cause ablation, or pharmaceutical resection, of the acinar cells, as well as the primary and metastatic pancreatic cancer, while leaving the insulin-producing islet cells and most non-pancreatic tissue unharmed. Most current cancer therapies (including chemotherapy, radiation or surgery) are associated with significant side effects that further reduce the patient’s quality of life. However, we believe that the adverse effects of SBP-101 will be principally limited to the gastrointestinal tract. It is expected that SBP-101 will produce exocrine pancreatic insufficiency and other GI adverse events, which are often present as a common complication of advanced pancreatic cancer and part of the natural history of the disease. Exocrine pancreatic insufficiency is treatable with currently marketed digestive enzyme replacement capsules, such as Creon® (AbbVie). As the endocrine pancreas is expected to be unaffected by SBP-101, no new requirement for insulin is expected.
Proof of Principle
SBP-101 has been tested and found effective in several in-vivo models of human pancreatic cancer. SBP-101 was used to treat mice subcutaneously transplanted with the human pancreatic cancer cell line PANC-1. A dose-response for efficacy was demonstrated with a 26 mg/kg daily injection resulting in near complete suppression of the transplanted tumor, as shown in Figure 1.
|
Figure 1. Impact of SBP-101 on PANC-1 Tumor Burden in a Murine Xenograft Model |
|
|
|
Source: Study BERG20100R1a(MIR1581) |
A separate orthotopic xenograft study (direct transplant of tumor into the pancreas) employed a particularly aggressive human pancreatic cancer cell line, L3.6pl, that is known to metastasize from the pancreas to the liver and the peritoneum in mice. Mice implanted with L3.6pl were treated with SBP-101 that had been sourced with a different synthetic process from that of the PANC-1 study, and the results were compared with untreated control mice and with mice treated with gemcitabine (then current “gold standard” treatment). SBP-101 (at 25 mg/kg) was demonstrably more effective than the comparator gemcitabine therapy in suppressing the tumor, as shown in Figure 2.
|
Figure 2. L3.6pl Orthotopic Xenograft Dose-response Study - Mean (±SD) Tumor Volume after Treatment with SBP-101, Gemcitabine (Gemzar) or Both |
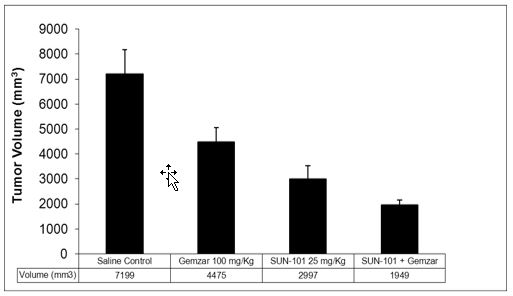 |
|
Source: Study101-Biol-101-001 |
The potential for SBP-101 as an effective therapy for pancreatic cancer has therefore been demonstrated in vivo by separate investigators, employing different human pancreatic cancer cell lines in different animal models, using SBP-101 synthesized by two different routes, while arriving at nearly equal, and remarkably effective, doses of 25 and 26 mg/kg, respectively. Additionally, when compared in vitro to existing therapies, SBP-101 produced superior results in suppressing growth of pancreatic cancer cells.
Development Plan for SBP-101
Development of SBP-101 for the pancreatic cancer indication includes a pre-clinical and a clinical phase. The pre-clinical phase consists of four primary components: chemistry, manufacturing and controls (“CMC”), preclinical (laboratory and animal) pharmacology studies, preclinical toxicology studies, and regulatory submissions in Australia and the United States. Pursuant to a potentially earlier start of human clinical trials in Australia, a Human Research Ethics Committee (“HREC”) application was submitted with subsequent Clinical Trial Notification (“CTN”) to the Therapeutic Goods Administration (“TGA”) . Complementing the Australian initiative, a similar, but considerably more extensive, preclinical package has been submitted to the US FDA in support of an Investigational New Drug (“IND”) application, enabling the same clinical trial to open at sites in the United States. The initial clinical trial in previously treated patients with locally advanced or metastatic pancreatic cancer is a Phase 1 First-in-Human study with a dose-escalation phase, with a possible expansion phase at the anticipated recommended treatment dose, conducted at clinical sites in both Australia and the United States. We have engaged expert clinicians who treat pancreatic cancer at major cancer treatment centers in Melbourne and Adelaide, Australia as well as the Mayo Clinic Scottsdale and HonorHealth in Scottsdale, Arizona. These Key Opinion Leaders (“KOLs”), with demonstrated, proven performance in pancreatic cancer studies, have enthusiastically agreed to participate as investigators for our Phase 1 First-in-Human study.
In January 2016 we initiated a Phase 1 clinical trial of SBP-101 in previously treated pancreatic cancer patients in Australia. We currently have three sites in Australia and two sites in the United States participating in our study and our first US patient was enrolled in July 2016. We estimate that additional funding of $8.0 to $10 million will be required to complete our Phase 1 First-in-Human study. Once human data have been acquired with SBP-101 in a Phase 1 trial, we will evaluate the safety data and estimated tumor response rate and determine whether this novel approach to pancreatic cancer could be safe and effective. A response rate of at least 30% with a reasonable safety profile will justify continued development of SBP-101 for patients with pancreatic adenocarcinoma.
Cancer therapeutics typically require a successful randomized Phase 3 trial that shows a survival advantage, with costs often exceeding $250-350 million before efficacy is established. We believe that the unique specificity of SBP-101 to the pancreas and pancreatic ductal adenocarcinoma will permit a potential safety and efficacy demonstration and decision point to be reached with a randomized Phase 2 study following a successful Phase 1 demonstration of safety and tolerability with cancer project spending of less than $20 million for completion of the Phase 1 study.
Given the laboratory evidence of comparative efficacy, we believe that SBP-101 has the potential to change the standard of care for patients with pancreatic cancer, either as monotherapy, or more likely, in combination with existing therapy.
Preclinical Development
To enable IND and HREC/CTN submission and as part of our pharmacology work, we have conducted plasma and urine assay development and validation in animals, in vitro metabolism studies in liver microsomes and hepatocytes, in vitro interaction studies with hepatic and renal transporters, a protein binding study, animal pharmacokinetic and metabolism/mass balance studies, and human plasma and urine assay development and validation. As a part of the pharmacology evaluation, we have conducted in vitro pharmacology screen profiling assay, a study in six human pancreatic cell lines, and studies in tumor xenograft models in mice using human pancreatic cancer PANC-1 tumor fragments, human pancreatic cancer BxPC-3 tumor fragments and human pancreatic cancer cells (L3.6pl) injected orthotopically in the tail of the pancreas of nude mice.
To meet regulatory requirements and to establish the safety profile of SBP-101, we have conducted, in rodents and non-rodents, toxicology dose-ranging studies, IND-enabling general toxicology studies, and genetic toxicology studies, including an Ames test. Exploratory studies in mice and rats and a Good Laboratory Practice (“GLP”)-compliant dog toxicology study have been completed. The relationship between dose and exposure (pharmacokinetics) has been described for all three species. We have also completed a preclinical hERG assay to detect any electrocardiographic QTc interval effects (IKrpotassium ion channel testing). Additionally, although not required prior to conducting our Phase 1 trial, we may also conduct reproductive toxicity, immunotoxicity as well as phototoxicity testing if we determine these tests to be necessary. As we anticipate the possibility of using SBP-101 in combination therapy with gemcitabine and/or Abraxane®, we expect to conduct appropriate nonclinical studies to evaluate the use of these or other combinations, including assessing the comparative efficacy of SBP-101, gemcitabine and nab- paclitaxel in various combinations.
Although epidemiology of pancreatic cancer indicates that this is a disease of the older patient and is seen only rarely in the pediatric population, preliminary discussions with pediatric oncologists have nonetheless suggested that SBP-101 be considered for exploratory studies in children with pancreatic cancer.
We have met FDA-mandated Chemistry, Manufacturing and Control (“CMC”) requirements with a combination of in-house expertise and contractual arrangements. To date, preparation of anticipated metabolites and an internal standard as a prerequisite for analytical studies have been completed through a Sponsored Research Agreement with the University of Florida and a contract manufacturer. We have Service Agreements with Syngene International Ltd. for the manufacture and supply of specific quantities of Good Manufacturing Practice (“GMP”) SBP-101 active pharmaceutical ingredient (“API”) and for the development of synthetic process improvements. Investigational product (IP or clinical trial supply) has been made and tested at Albany Molecular Research Inc. (“AMRI”) in Burlington, MA. Initial lots of GMP compliant API have been prepared by Syngene International Ltd and released for conversion into supply dosage form. A first clinical trial supply lot has been successfully prepared and released by AMRI. In addition, efforts continue to refine both the synthetic process at Syngene and to prepare improved formulations of the clinical supply.
Pancreatic Cancer Investigational New Drug (IND) – Form 1571 Submission.
The preclinical work to support the IND submission has been completed. An IND application package containing an Investigator’s Brochure; a statement of general investigative plans; the proposed Phase 1 pancreatic cancer study protocol, a data management and statistical plan; the CMC data; and the pharmacology, ADME (absorption, distribution, metabolism, excretion), and toxicology data was submitted and accepted for filing by the US FDA. Preparation of the SBP-101 IND for pancreatic cancer required collaboration by our manufacturing, preclinical toxicology, pharmacokinetic and metabolism experts, our regulatory affairs project management, and our in-house clinical expertise. Acceptance for filing of our pancreatic cancer IND, and allowance thereof by the FDA, has enabled Sun BioPharma to proceed in the United States with its Phase 1 clinical trial, a safety and tolerability study in previously treated patients with metastatic pancreatic ductal adenocarcinoma. This is further discussed in “Clinical Development” below.
Clinical Development – Pancreatic Cancer
Given the unique effects of SBP-101 on the mammalian pancreas, special factors have been considered in the design of the first-in-man study.
Phase 1 Clinical Trial Design
A Phase 1 study in patients with pancreatic cancer, with a projected duration of approximately 24 months, commenced the enrollment of patients in January 2016. This study is a dose-escalation study with 8-week cycles of treatment at each dose level. At least two cycles of therapy at each dose level are anticipated for patients enrolled in this trial, with up to five treatment cycles permitted for patients with clinical responses or stable disease.
The absence of non-target organ adverse events suggests non-overlapping toxicity in the case of subsequent combination of SBP-101 with conventional chemotherapeutic agents, such as gemcitabine or nab-paclitaxel, or even FOLFIRINOX.
The specific nature of the pancreatic effect initially suggested a single cancer indication (pancreatic ductal adenocarcinoma) for the compound. Accordingly, the first human study of SBP-101 is designed to be a dose-escalation study, i.e., a Phase 1 study producing Phase 2 results, or a Phase 1a/1b study.
An unexpected but favorable characteristic of the pancreatic action of SBP-101 was the lack of an effect on the normal insulin-producing islet cells in animals. Preservation of the islet cells implies the absence of diabetes as a complication of SBP-101 therapy, although the necessity of supplementary oral pancreatic enzymes is expected to be unavoidable. It is important to note that diabetes is a common co-morbidity in patients with pancreatic cancer, but it is not expected to be an adverse effect of treatment with SBP-101. The anticipated adverse effect of exocrine pancreatic insufficiency is mitigated by our recognition that many patients with pancreatic ductal adenocarcinoma require pancreatic enzyme replacement as a feature of their underlying disease, a complication so common that pancreatic enzyme replacement with one of several commercially available products is typically covered by United States and Australian health care plans. Patients with cystic fibrosis, chronic pancreatitis and pancreatic cancer are the populations most often treated with pancreatic enzyme replacement.
Our current clinical evaluation of SBP- 101 employs a careful dose-finding strategy with intervals between cycles of therapy. Correlation between systemic drug exposure, pharmacologic and toxic effects will facilitate appropriate dose and schedule determination for an optimal treatment regimen.
Patients in our current Phase 1 trial undergo regular pancreatic and hepatic enzyme assays, and periodic abdominal CT follow-up. Patients are also carefully monitored for clinical signs of GI adverse events.
Given the life-threatening nature of pancreatic ductal adenocarcinoma, the limited efficacy of current treatment options, and the long history of failures in pancreatic ductal adenocarcinoma developmental therapeutics, we will attempt to evaluate SBP-101 expeditiously as noted below.
Phase 2 Pivotal Clinical Trial
Unlike most early-stage cancer drugs, SBP-101’s specificity of anticipated effects enables our first in human trial to be a dose-escalation study in the target pancreatic cancer population. This rare opportunity results in a simplified path to determine the success or failure of SBP-101 in the treatment of this disease and may result in an expedited development pathway.
If the results of the Phase 1 clinical trial demonstrate sufficiently successful efficacy results, we intend to meet with the US FDA to obtain advice on potential breakthrough therapy designation and an accelerated approval strategy. We will actively seek potential commercial partners and the opportunity to evaluate combination therapy alternatives.
If we are able to successfully complete FDA recommended clinical studies, we intend to seek marketing authorization from the FDA, the EMA (European Union), Ministry of Health and Welfare (Japan) and TGA (Australia). The submission fees may be waived when SBP-101 has been designated an orphan drug in each geographic region, as described under “Orphan Drug Status.”
Total Development Costs
The development and commercialization of SBP-101 involves a preclinical and a clinical development phase. We believe that we have completed our preclinical development work and we estimate that completing the Phase 1a/1b clinical trial in pancreatic cancer will require additional funding of $10 million to $20 million, in addition to what we have already raised, and take through the end of 2017.. Additional clinical trials will be subsequently required if the results of the Phase 1 pancreatic cancer trial are positive. We estimate the total time and cost to obtain FDA and EU approval and bring SBP-101 to market is 5 to 7 years and up to two hundred million dollars ($200 million), although this process could be accelerated and less funds would be needed if SBP-101 qualifies for Breakthrough Status. A breakthrough therapy designation conveys fast track program features, more intensive FDA guidance on an efficient drug development program, an organizational commitment involving senior managers, and eligibility for rolling review and priority review.
Orphan Drug Status
The Orphan Drug Act (“ODA”) provides special status to drugs which are intended for the safe and effective treatment, diagnosis or prevention of rare diseases that affect fewer than 200,000 people in the United States, or that affect more than 200,000 persons but for which a manufacturer is not expected to recover the costs of developing and marketing such a drug. Orphan drug designation has the advantage of reducing drug development costs by: (i) streamlining the FDA’s approval process, (ii) providing tax breaks for expenses related to the drug development, (iii) allowing the orphan drug manufacturer to receive assistance from the FDA in funding the clinical testing necessary for approval of an orphan drug, and (iv) facilitating drug development efforts. More significantly, the orphan drug manufacturer’s ability to recover its investment in developing the drug is also greatly enhanced by the FDA granting the manufacturer seven years of exclusive US marketing rights upon approval. Designation of a drug candidate as an orphan drug therefore provides its sponsor with the opportunity to adopt a faster and less expensive pathway to commercializing its product. Given the prevalence of pancreatic cancer in the United States, we have obtained US Orphan Drug Status in 2014 and we intend to submit an application for Orphan Drug Status in Europe, Japan and Australia when we have further clinical experience.
Intellectual Property Status
Intellectual property licensed by us from the University of Florida includes Patent No.6,160,022 covering the method of use, which expires in 2019. In addition, we have filed US Patent Application No. 62/238,916 which is a method application covering the use of SBP-101 to treat pancreatitis.
In 2014, the US FDA granted SBP-101 Orphan Drug Status for pancreatic cancer which may provide seven years of market exclusivity if SBP-101 is approved for this indication.
Development Project Managers
Project managers have been hired or contracted to coordinate all the functions identified in our Development Plan for SBP-101. The personnel responsible for overseeing critical functions of the Development Plan are as follows:
Our CMC program is under the direction of Dr. Thomas Neenan, Ph.D., a founding member of Sun BioPharma, Inc. and our Chief Scientific Officer, and an experienced pharmaceutical industry synthetic chemist. Dr. Neenan has commissioned Contract Manufacturing Organizations (“CMOs”), who have improved the process for synthesis of SBP-101, and who have produced high- quality compound, chemically identical to that synthesized by Dr. Bergeron at the University of Florida. Dr. Neenan’s completed work includes development, confirmation and documentation of the synthetic chemistry process, analytical purity, reproducibility, stability (shelf-life), degradation products and pharmaceutical formulation and packaging. This work has culminated in a supply of drug to support preclinical work and human clinical trials.
Dr. Ajit Shah, Ph.D., is our Vice President of Clinical Pharmacology. Dr. Shah has extensive prior experience with numerous other compounds at both large and mid-size sponsoring companies, including Pfizer and MGI Pharma. His completed work includes development of analytical methods to quantify levels of drug and characterization of metabolites in plasma, urine and tissues, plus distribution of the compound in living tissues, metabolic pathways and products, anticipated drug blood levels, half- life in the organism, and excretion pathways. Dr. Shah’s work has enabled informed dose and schedule planning for human clinical trials.
Dr. Anthony Kiorpes, Ph.D., D.V.M., has responsibility for our toxicology program, a role he has assumed previously for many preclinical projects at other companies. His studies have determined single- and multiple-dose safety profiles in rodent and non-rodent species, enabling improved safety monitoring in the design of clinical trials for SBP-101. Dr. Kiorpes’ results have helped management to predict and prevent potential side effects in humans.
Dr. Michael Cullen, M.D., M.B.A, our founder and Executive Chairman, an experienced drug development specialist with 10 prior NDA approvals, has led our overall Clinical and Regulatory Affairs & Project Management effort, including timeline and budget management, critical path timeline synchronization, IND/HREC/CTN package submissions, management of industry partner collaborative efforts, initial EU Regulatory Affairs planning, and collaboration on oversight of outsourced CMC efforts. Dr. Cullen has recruited additional experienced and talented staff in the positions of statistical analyses, manufacturing operations, clinical operations, clinical research and non-clinical studies.
Dr. Suzanne Gagnon, M.D., our Chief Medical Officer and a director, an experienced CMO, having served in that capacity for several private and public companies, including BioPharm/IBAH/Omnicare, ICON, Idis, NuPathe, Luitpold and Rhone-Poulenc Rorer where she helped develop docetaxel, still an important chemotherapy agent, will join Michael Cullen, M.D. in leading the design of our clinical trials, recruiting investigators, monitoring the safety of the patients and reporting the findings to the FDA, EMA and TGA, and in the medical literature. We have engaged Courante Oncology, an experienced clinical CRO, to manage clinical operations in the United States, and have engaged a CRO for our Australian operations. These two CROs will provide regulatory documentation for HREC/CTN and Investigational Review Board (“IRB”) submissions, FDA 1571 regulation compliance, and informed consents, as well as clinical study site qualification, contracting and payment, study conduct monitoring, data collection, analysis and reporting.
Competition
The development and commercialization of new products to treat cancer is intensely competitive and subject to rapid and significant technological change. While we believe that our knowledge, experience and scientific resources provide us with competitive advantages, we face substantial competition from major pharmaceutical companies, specialty pharmaceutical companies, and biotechnology companies worldwide. Many of our competitors have significantly greater financial, technical, and human resources. Smaller and early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. As a result, our competitors may discover, develop, license or commercialize products before or more successfully than we do.
We face competition with respect to our current product candidates, and will face competition with respect to future product candidates, from segments of the pharmaceutical, biotechnology and other related markets that pursue approaches to targeting molecular alterations and signaling pathways associated with cancer. Our competitors may obtain regulatory approval of their products more rapidly than we do or may obtain patent protection or other intellectual property rights that limit our ability to develop or commercialize our product candidates. Our competitors may also develop drugs that are more effective, more convenient, less costly, or possessing better safety profiles than our products, and these competitors may be more successful than us in manufacturing and marketing their products.
In addition, we may need to develop our product candidates in collaboration with diagnostic companies, and we will face competition from other companies in establishing these collaborations. Our competitors will also compete with us in recruiting and retaining qualified scientific, management and commercial personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
Furthermore, we also face competition more broadly across the market for cost-effective and reimbursable cancer treatments. The most common methods of treating patients with cancer are surgery, radiation and drug therapy, including chemotherapy, hormone therapy and targeted drug therapy or a combination of such methods. There are a variety of available drug therapies marketed for cancer. In many cases, these drugs are administered in combination to enhance efficacy. While our product candidates, if any are approved, may compete with these existing drug and other therapies, to the extent they are ultimately used in combination with or as an adjunct to these therapies, our product candidates may be approved as companion treatments and not be competitive with current therapies. Some of these drugs are branded and subject to patent protection, and others are available on a generic basis. Insurers and other third-party payors may also encourage the use of generic products or specific branded products. We expect that if our product candidates are approved, they will be priced at a premium over competitive generic, including branded generic, products. As a result, obtaining market acceptance of, and gaining significant share of the market for, any of our product candidates that we successfully introduce to the market will pose challenges. In addition, many companies are developing new therapeutics, and we cannot predict what the standard of care will be as our product candidates progress through clinical development.
SBP-101
Commercialization
We have not established a sales, marketing or product distribution infrastructure nor have we devoted significant management resources to planning such an infrastructure because our lead product candidate is still in early clinical development. We currently anticipate that we will partner with a larger pharmaceutical organization having the expertise and capacity to perform these functions.
Manufacturing and Suppliers
We do not own or operate, and currently have no plans to establish, any manufacturing facilities. We currently rely, and expect to continue to rely, on third parties for the manufacture of our product candidates for preclinical and clinical testing as well as for commercial manufacture of any products that we may commercialize. If needed, we aim to engage, by entering into a supply agreement or through another arrangement, third party manufacturers to provide us with additional SBP-101 clinical supply. For all of our product candidates, we aim to identify and qualify manufacturers to provide the active pharmaceutical ingredient and fill-and-finish services prior to submission of an NDA to the FDA.
Employees
As of September 1, 2016, we had 9 employees, six of whom were full-time employees and three of whom were part-time employees. We may hire additional employees to support the growth of our businesses. We believe that operational responsibilities can be handled by our current employees and independent consultants. We have historically used, and expect to continue to use, the services of independent consultants and contractors to perform various professional services. We believe that this use of third-party service providers enhances our ability to minimize general and administrative expenses. None of our employees is represented by a labor union, and we consider our relationship with our employees to be good.
Properties
Our primary business functions, including research and development, are conducted by our employees and independent contractors on a distributed basis. Accordingly, we do not currently own or lease any real property. Our corporate mailing address is 712 Vista Blvd. #305, Waconia, Minnesota 55387.
Material Agreements
Standard Exclusive License Agreement dated December 22, 2011, between us and UFRF. This agreement grants us an exclusive license to the proprietary technology covered by issued United States Patents Nos. US 5,962,533, which expired in February 2016, and US 6,160,022, with reservations by UFRF for academic or government uses. Under this agreement, we agree to pay various royalties, expenses and milestone payments to UFRF. Additionally, pursuant to this agreement we then issued to UFRF 800,000 shares of common stock. Anti-dilution protection for UFRF pursuant to this agreement required us to issue additional shares to UFRF in order for UFRF to maintain its ownership stake at ten percent (10%) of the total number of issued and outstanding shares of our common stock, calculated on a fully diluted basis, until such time as we had received a total of two million dollars ($2,000,000) in exchange for our issuance of equity securities. This requirement was met in 2012, and UFRF is therefore afforded no further anti-dilution protection. Pursuant to this anti-dilution provision, we issued an additional 344,232 shares of common stock to UFRF increasing the total shares of common stock issued to UFRF to 1,144,232 shares.
Under the License Agreement, We have a number of performance related milestones we must meet in order to retain our rights to the technology. Included in such milestones is the commitment to have our first commercial sale of a product incorporating the technology by the end of 2020. Also, in the event that we are not actively pursuing commercialization of the technology in any country or territory other than the United States and certain other countries by the end of 2014, UFRF may terminate the license as to that country or territory under certain circumstances. UFRF may also terminate this license for standard and similar causes such as material breach of the agreement, bankruptcy, failure to pay royalties and other customary conditions.
The foregoing description of the material terms of the License Agreement is qualified by the full text of the License Agreement, a copy of which was filed as Exhibit 10.5 to our current report on Form 8-K filed on September 11, 2015 and is incorporated herein by reference.
Government Regulation
FDA Approval Process
In the United States, pharmaceutical products are subject to extensive regulation by FDA. The Federal Food, Drug and Cosmetic Act and other federal and state statutes and regulations govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling and import and export of pharmaceutical products. Failure to comply with applicable US requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending NDAs, warning or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties and criminal prosecution.
Pharmaceutical product development for a new product or certain changes to an approved product in the United States typically involves preclinical laboratory and animal tests, the submission to FDA of an IND which must become effective before clinical testing may commence, and adequate and well-controlled clinical trials to establish the safety and effectiveness of the drug for each indication for which FDA approval is sought. Satisfaction of FDA pre-market approval requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity and novelty of the product or disease.
Preclinical tests include laboratory evaluation of product chemistry, formulation and toxicity, as well as animal trials to assess the characteristics and potential safety and efficacy of the product. The conduct of the preclinical tests must comply with federal regulations and requirements, including good laboratory practices. The results of preclinical testing are submitted to FDA as part of an IND along with other information, including information about product chemistry, manufacturing and controls, and a proposed clinical trial protocol. Long-term preclinical tests, such as animal tests of reproductive toxicity and carcinogenicity, may continue after the IND is submitted.
A 30-day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If FDA has neither commented on nor questioned the IND within this 30-day period, the clinical trial proposed in the IND may begin.
Clinical trials involve the administration of the investigational new drug to healthy volunteers or patients under the supervision of a qualified investigator. Clinical trials must be conducted: (i) in compliance with federal regulations; (ii) in compliance with good clinical practice, or GCP, an international standard meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, administrators and monitors; as well as (iii) under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol involving testing on US patients and subsequent protocol amendments must be submitted to FDA as part of the IND.
FDA may order the temporary, or permanent, discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial either is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. The study protocol and informed consent information for patients in clinical trials must also be submitted to an institutional review board, or IRB, for approval. An IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB’s requirements, or may impose other conditions.
Clinical trials to support NDAs for marketing approval are typically conducted in three sequential phases, but the phases may overlap. In Phase 1, the initial introduction of the drug into healthy human subjects/patients, the drug is tested to assess metabolism, pharmacokinetics, pharmacological actions, side effects associated with increasing doses, and, if possible, early evidence of effectiveness. Phase 2 usually involves trials in a limited patient population to determine the effectiveness of the drug for a particular indication, dosage tolerance and optimum dosage, and to identify common adverse effects and safety risks. If a compound demonstrates evidence of effectiveness and an acceptable safety profile in Phase 2 evaluations, Phase 3 trials are undertaken to obtain the additional information about clinical efficacy and safety in a larger number of patients, typically at geographically dispersed clinical trial sites, to permit FDA to evaluate the overall benefit-risk relationship of the drug and to provide adequate information for the labeling of the drug. In many cases FDA requires two adequate and well-controlled Phase 3 clinical trials to demonstrate the efficacy of the drug. A single Phase 3 trial with other confirmatory evidence may be sufficient in instances where the study is a large multicenter trial demonstrating internal consistency and a statistically very persuasive finding of a clinically meaningful effect on mortality, irreversible morbidity or prevention of a disease with a potentially serious outcome and confirmation of the result in a second trial would be practically or ethically impossible.
After completion of the required clinical testing, an NDA is prepared and submitted to FDA. FDA approval of the NDA is required before marketing of the product may begin in the United States. The NDA must include the results of all preclinical, clinical and other testing and a compilation of data relating to the product’s pharmacology, chemistry, manufacture and controls. The cost of preparing and submitting an NDA is substantial, and the fees are typically increased annually.
FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. Once the submission is accepted for filing, FDA begins an in-depth review. FDA has agreed to certain performance goals in the review of new drug applications to encourage timeliness. Most applications for standard review drug products are reviewed within twelve months from submission; most applications for priority review drugs are reviewed within eight months from submission. Priority review can be applied to drugs that FDA determines offer major advances in treatment, or provide a treatment where no adequate therapy exists. The review process for both standard and priority review may be extended by FDA for three additional months to consider certain late-submitted information, or information intended to clarify information already provided in the submission.
FDA may also refer applications for novel drug products, or drug products that present difficult questions of safety or efficacy, to an outside advisory committee—typically a panel that includes clinicians and other experts—for review, evaluation and a recommendation as to whether the application should be approved. FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations.
Before approving an NDA, FDA will typically inspect one or more clinical sites to assure compliance with GCP. Additionally, FDA will inspect the facility or the facilities at which the drug is manufactured. FDA will not approve the product unless compliance with current good manufacturing practice, or GMP—a quality system regulating manufacturing—is satisfactory and the NDA contains data that provide substantial evidence that the drug is safe and effective in the indication studied.
After FDA evaluates the NDA and the manufacturing facilities, it issues either an approval letter or a complete response letter. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional testing, or information, in order for FDA to reconsider the application. If, or when, those deficiencies have been addressed to FDA’s satisfaction in a resubmission of the NDA, FDA will issue an approval letter. FDA has committed to reviewing such resubmissions in two or six months depending on the type of information included.
An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. As a condition of NDA approval, FDA may require a risk evaluation and mitigation strategy, or REMS, to help ensure that the benefits of the drug outweigh the potential risks. REMS can include medication guides, communication plans for healthcare professionals, and elements to assure safe use, or ETASU. ETASU can include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring and the use of patient registries. The requirement for a REMS can materially affect the potential market and profitability of the drug. Moreover, product approval may require substantial post-approval testing and surveillance to monitor the drug’s safety or efficacy. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA or NDA supplement before the change can be implemented. An NDA supplement for a new indication typically requires clinical data similar to that in the original application, and FDA uses the same procedures and actions in reviewing NDA supplements as it does in reviewing NDAs.
Fast Track Designation and Accelerated Approval
FDA is required to facilitate the development, and expedite the review, of drugs that are intended for the treatment of a serious or life-threatening disease or condition for which there is no effective treatment and which demonstrate the potential to address unmet medical needs for the condition. Under the Fast Track program, the sponsor of a new product candidate may request that FDA designate the product candidate for a specific indication as a Fast Track drug concurrent with, or after, the filing of the IND for the product candidate. FDA must determine if the product candidate qualifies for Fast Track Designation within 60 days of receipt of the sponsor’s request.
Under the Fast Track program and FDA’s accelerated approval regulations, FDA may approve a drug for a serious or life-threatening illness that provides meaningful therapeutic benefit to patients over existing treatments based upon a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments.
In clinical trials, a surrogate endpoint is a measurement of laboratory or clinical signs of a disease or condition that substitutes for a direct measurement of how a patient feels, functions, or survives. Surrogate endpoints can often be measured more easily or more rapidly than clinical endpoints. A product candidate approved on this basis is subject to rigorous post-marketing compliance requirements, including the completion of Phase 4 or post-approval clinical trials to confirm the effect on the clinical endpoint. Failure to conduct required post-approval studies, or confirm a clinical benefit during post-marketing studies, will allow FDA to withdraw the drug from the market on an expedited basis. All promotional materials for product candidates approved under accelerated regulations are subject to priority review by FDA.
If a submission is granted Fast Track Designation, the sponsor may engage in more frequent interactions with FDA, and FDA may review sections of the NDA before the application is complete. This rolling review is available if the applicant provides, and FDA approves, a schedule for the submission of the remaining information and the applicant pays applicable user fees. However, FDA’s time period goal for reviewing an application does not begin until the last section of the NDA is submitted. Additionally, Fast Track Designation may be withdrawn by FDA if FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
Breakthrough Therapy Designation
FDA is also required to expedite the development and review of the application for approval of drugs that are intended to treat a serious or life-threatening disease or condition where preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. Under the Breakthrough Therapy program, the sponsor of a new product candidate may request that FDA designate the product candidate for a specific indication as a breakthrough therapy concurrent with, or after, the filing of the IND for the product candidate. FDA must determine if the product candidate qualifies for Breakthrough Therapy designation within 60 days of receipt of the sponsor’s request.
Orphan Drug Designation and Exclusivity
The Orphan Drug Act provides incentives for the development of products intended to treat rare diseases or conditions. Under the Orphan Drug Act, the FDA may grant orphan designation to a drug intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making a drug available in the United States for this type of disease or condition will be recovered from sales of the product. If a sponsor demonstrates that a drug is intended to treat a rare disease or condition, the FDA will grant orphan designation for that product for the orphan disease indication, assuming that the same drug has not already been approved for the indication for which the sponsor is seeking orphan designation. If the same drug has already been approved for the indication for which the sponsor is seeking orphan designation, the sponsor must present a plausible hypothesis of clinical superiority in order to obtain orphan designation. Orphan designation must be requested before submitting an NDA. After the FDA grants orphan designation, the FDA discloses the identity of the therapeutic agent and its potential orphan use.
Orphan designation may provide manufacturers with benefits such as research grants, tax credits, PDUFA application fee waivers, and eligibility for orphan drug exclusivity. If a product that has orphan designation subsequently receives the first FDA approval of the active moiety for that disease or condition for which it has such designation, the product is entitled to orphan drug exclusivity, which for seven years prohibits the FDA from approving another product with the same active ingredient for the same indication, except in limited circumstances. Orphan drug exclusivity will not bar approval of another product under certain circumstances, including if a subsequent product with the same active ingredient for the same indication is shown to be clinically superior to the approved product on the basis of greater efficacy or safety, or providing a major contribution to patient care, or if the company with orphan drug exclusivity is not able to meet market demand. Further, the FDA may approve more than one product for the same orphan indication or disease as long as the products contain different active ingredients. Moreover, competitors may receive approval of different products for the indication for which the orphan drug has exclusivity or obtain approval for the same product but for a different indication for which the orphan drug has exclusivity.
In the European Union, orphan drug designation also entitles a party to financial incentives such as reduction of fees or fee waivers and 10 years of market exclusivity is granted following drug or biological product approval. This period may be reduced to 6 years if the orphan drug designation criteria are no longer met, including where it is shown that the product is sufficiently profitable not to justify maintenance of market exclusivity.
Orphan drug designation must be requested before submitting an application for marketing approval. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
Post-Approval Requirements
Once an NDA is approved, a product will be subject to certain post-approval requirements. For instance, the FDA closely regulates the post-approval marketing and promotion of drugs, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the internet. Drugs may be marketed only for the approved indications and in accordance with the provisions of the approved labeling.
Adverse event reporting and submission of periodic reports are required following FDA approval of an NDA. FDA also may require post-marketing testing, known as Phase 4 testing, risk evaluation and mitigation strategies, or REMS, and surveillance to monitor the effects of an approved product, or FDA may place conditions on an approval that could restrict the distribution or use of the product. In addition, quality control, drug manufacture, packaging and labeling procedures must continue to conform to current good manufacturing practices, or cGMPs, after approval. Drug manufacturers and certain of their subcontractors are required to register their establishments with FDA and certain state agencies. Registration with FDA subjects entities to periodic unannounced inspections by FDA, during which the Agency inspects manufacturing facilities to assess compliance with cGMPs. Accordingly, manufacturers must continue to expend time, money and effort in the areas of production and quality-control to maintain compliance with cGMPs. Regulatory authorities may withdraw product approvals or request product recalls if a company fails to comply with regulatory standards, if it encounters problems following initial marketing, or if previously unrecognized problems are subsequently discovered.
Additional Regulations and Environmental Matters
In addition to FDA restrictions on marketing of pharmaceutical products, we are subject to additional healthcare regulation and enforcement by the federal government and by authorities in the states and foreign jurisdictions in which we conduct our business. These laws, which generally will not be applicable to us or our product candidates unless and until we obtain FDA marketing approval for any of our product candidates, include transparency laws, anti-kickback statutes, false claims statutes and regulation regarding providing drug samples, among others.
The federal Anti-Kickback Statute prohibits, among other things, individuals and entities from knowingly and willfully offering, paying, soliciting or receiving remuneration to induce or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. Violations of the federal Anti-Kickback Statute are punishable by imprisonment, criminal fines, civil monetary penalties and exclusion from participation in federal healthcare programs.
Federal false claims laws and civil monetary penalties, including the False Claims Act, prohibit, among other things, any person or entity from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to have a false claim paid. Recently, several pharmaceutical companies have been prosecuted under these laws for allegedly inflating drug prices they report to pricing services, which in turn were used by the government to set Medicare and Medicaid reimbursement rates, and for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. In addition, certain marketing practices, including off-label promotion, may also violate false claims laws.
HIPAA imposes criminal and civil liability for, among other things, executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters.
HIPAA, as amended by the HITECH Act and its implementing regulations, also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information. Many states and foreign jurisdictions also have laws and regulations that govern the privacy and security of individually identifiable health information, and such laws often vary from one another and from HIPAA.
The federal Physician Payment Sunshine Act requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to report annually to CMS, information related to payments or other transfers of value made to physicians and teaching hospitals, and ownership and investment interests held by the physicians and their immediate family members.
The majority of states also have statutes or regulations similar to the federal Anti-Kickback Law and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Our activities may also be certain state laws regarding the privacy and security of health information that may not be preempted by HIPAA, as well as additional tracking and reporting obligations regarding payments to healthcare providers and marketing expenditures.
In addition to regulatory schemes that apply, or may in the future apply, to our business, we are or may become subject to various environmental, health and safety laws and regulations governing, among other things, laboratory procedures and any use and disposal by us of hazardous or potentially hazardous substances in connection with our research and development activities. We do not presently expect such environmental, health and safety laws or regulations to materially impact our present or planned future activities.
Coverage and Reimbursement
Sales of any of our product candidates that may be approved will depend, in part, on the extent to which the cost of the product will be covered by third party payors. Third party payors may limit coverage to an approved list of products, or formulary, which might not include all drug products approved by the FDA for an indication. A payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Further, one payor’s determination to provide coverage for a drug product does not assure that other payors will also provide coverage for the drug product. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
Any product candidates for which we obtain marketing approval may not be considered medically necessary or cost-effective by third party payors, and we may need to conduct expensive pharmacoeconomic studies in the future to demonstrate the medical necessity and/or cost effectiveness of any such product. Nonetheless, our product candidates may not be considered medically necessary or cost effective. The US government, state legislatures and foreign governments have shown increased interest in implementing cost containment programs to limit government-paid health care costs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Continued interest in and adoption of such controls and measures, and tightening of restrictive policies in jurisdictions with existing controls and measures, could limit payments for pharmaceuticals such as the product candidates we are developing.
Health Reform
The United States and some foreign jurisdictions are considering or have enacted a number of legislative and regulatory proposals to change the healthcare system in ways that could affect our ability to sell our products profitably. Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and expanding access. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives. By way of example, in March 2010, the ACA was signed into law, which intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add transparency requirements for the healthcare and health insurance industries, impose taxes and fees on the health industry and impose additional health policy reforms. With regard to pharmaceutical products, among other things, the ACA expanded and increased industry rebates for drugs covered under Medicaid programs and made changes to the coverage requirements under the Medicare prescription drug benefit. We continue to evaluate the effect that the ACA has on our business.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. These changes included aggregate reductions to Medicare payments to providers of up to 2% per fiscal year effective April 1, 2013 and, due to subsequent legislative amendments to the statute, will stay in effect through 2024 unless additional Congressional action is taken. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, reduced Medicare payments to several providers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on customers for our drugs, if approved, and, accordingly, our financial operations.
In the coming years, additional legislative and regulatory changes could be made to governmental health programs that could significantly impact pharmaceutical companies and the success of our product candidates.
Legal Proceedings
We are not currently party to any material legal proceedings. From time to time, we may be named as a defendant in legal actions arising from our normal business activities. We believe that we have obtained adequate insurance coverage or rights to indemnification in connection with potential legal proceedings that may arise.
Accounting matters
Cherry Bekaert LLP has been engaged by the audit committee of our board of directors to serve as our independent registered public accounting firm for fiscal 2016, and our stockholders ratified such engagement at the annual meeting held on May 17, 2016. Notwithstanding the ratification by our stockholders, the audit committee, in its discretion, may appoint another independent registered public accounting firm at any time during the year if the committee believes that such a change would be in the best interests of our company and its stockholders.
As a result of the Merger, our Company was deemed to have changed its independent registered public accounting firm. Accordingly, on September 4, 2015, the Company’s Board of Directors effectively discharged Mantyla McReynolds LLP (“MMR”) as its independent registered public accounting firm. With the exception of a “going concern” modification, the report of MMR on the financial statements of the Company for its two most recent fiscal years prior to the Merger contained no adverse opinion or disclaimer of opinion, and was not qualified or modified as to uncertainty, audit scope or accounting principle. In connection with MMR’s audit for the fiscal years ended December 31, 2013 and 2014, and through the date of dismissal, there were no disagreements with MMR on any matter of accounting principles or practices, financial statement disclosure or auditing scope or procedure, which disagreements if not resolved to the satisfaction of MMR would have caused them to make reference thereto in its report on the financial statements for such years.
During the two most recent completed fiscal years prior to the Merger and through the date of dismissal, none of the events specified in Item 304(a)(1)(iv) of Regulation S-K had occurred, with the exception of material weaknesses identified in the Company’s internal control over financial reporting prior to the Merger.
On September 4, 2015, the Company retained Cherry Bekaert LLP to serve as its principal independent registered public accounting firm. During the two most recent fiscal years and to the date of this report, the Company has not consulted with Cherry Bekaert LLP regarding either: (i) the application of accounting principles to a specified transaction, either completed or proposed; or the type of audit opinion that might be rendered on the Company’s financial statements, and either a written report was provided to the Company or oral advice was provided that Cherry Bekaert LLP concluded was an important factor considered by the Company in reaching a decision as to the accounting, auditing or financial reporting issue; or (ii) any matter that was the subject of a disagreement and required to be reported under Item 304(a)(1)(iv) of Regulation S-K and the related instructions thereto.
We previously provided MMR with a copy of the foregoing disclosure and requested that it furnish us with a letter addressed to the SEC stating whether it agrees with the above statements. A copy of the letter from MMR was filed with the SEC as Exhibit 16.1 to a current report on Form 8-K filed September 11, 2015 (File No. 000-55242).
Management
The name, age and position of each of our directors and executive officers as of the date of this Prospectus, and the names of certain persons who have agreed to serve as directors on or before the closing of the offering are as follows:
Executive Officers and Directors
|
Name |
Age |
Position | ||
|
Michael T. Cullen |
70 |
Executive Chairman of the Board and Director | ||
|
Suzanne Gagnon |
60 |
Chief Medical Officer and Director | ||
|
Dalvir S. Gill |
58 |
Director | ||
|
David B. Kaysen |
67 |
President, Chief Executive Officer and Director | ||
|
Scott B. Kellen |
51 |
Chief Financial Officer and Vice President of Finance | ||
|
Jeffrey S. Mathiesen |
55 |
Director | ||
|
J. Robert Paulson, Jr. |
60 |
Director | ||
|
Paul W. Schaffer |
73 |
Director | ||
|
D. Robert Schemel |
61 |
Director |
Executive Officers
Michael T. Cullen, M.D., M.B.A., co-founded Sun BioPharma, Inc. in November 2011 and had continuously served as Chairman its board of directors since that date. He previously served as its Chief Executive Officer and President from November 2011 to June 2015. Dr. Cullen brings 25 years of pharmaceutical experience to our Company, including expertise in working with development-stage companies in planning, designing and advancing drug candidates from preclinical through clinical development. Dr. Cullen provided due diligence consulting to the pharmaceutical industry from 2009 to 2011, after one year in transition consulting to Eisai Co., Ltd. He developed several oncology drugs as Chief Medical Officer for MGI Pharma Inc. from 2000 to 2008, and previously at G.D. Searle, SunPharm Corporation, and as Vice President for Clinical Consulting at IBAH Inc., the world’s fifth largest contract research organization, where he provided consulting services on business strategy, creating development plans, regulatory matters and designing clinical trials for several development stage companies in the pharmaceutical industry. Dr. Cullen was also a co-founder and Chief Executive Officer of IDD Medical, a pharmaceutical start-up company. Dr. Cullen joined 3M Pharmaceuticals in 1988 and contributed to the development of cardiovascular, pulmonary and immune-response modification drugs. Over the course of his career Dr. Cullen has been instrumental in obtaining the approval of ten drugs, including three (3) since 2004: Aloxi®, Dacogen® and Lusedra®. Board-certified in Internal Medicine, Dr. Cullen practiced from 1977 to 1988 at Owatonna Clinic, Owatonna, MN, where he served as president. Dr. Cullen earned his MD and BS degrees from the University of Minnesota and his MBA from the University of St. Thomas and completed his residency and Board certification in Internal Medicine through the University of North Carolina in Chapel Hill and Wilmington, NC.
David B. Kaysen has served as our President since August 2015 and as Chief Executive Officer and as a director of our Company since July 2015. Prior to joining the Company, Mr. Kaysen was a self-employed medical technology consultant since April 2013. Mr. Kaysen previously was the President, Chief Executive Officer and a board member of Uroplasty, Inc. (now Cogentix Medical, Inc.), a publically traded medical device company, from May 2006 through April 2013. Prior to that, Mr. Kaysen served as President and CEO and as a director of Diametrics Medical, a publicly traded diagnostics company, and Rehabilicare Inc. (now Compex Technologies), a publicly traded neuromodulation medical device company. Mr. Kaysen holds a Bachelor of Science in Business Administration from the University of Minnesota.
Scott Kellen has served as Vice President and Chief Financial Officer since October 1, 2015. Prior to joining Sun BioPharma, Inc., Mr. Kellen was the Chief Financial Officer of Kips Bay Medical, Inc. from 2010 through 2015 originally joining to help lead them through their initial public offering and multiple follow-on offerings. In March 2012, Scott also became the Chief Operating Officer. From 2007 to 2009, Scott served as Director of Finance for Transoma Medical, Inc., during which time Transoma prepared for its proposed initial public offering, which was withdrawn in February 2008 due to deteriorated market conditions. From 2005 to 2007, Scott served as the Corporate Controller for ev3 Inc. during that company’s initial public offering and during additional follow-on offerings. From 2003 to 2005, Scott served as Senior Audit Manager of Deloitte & Touche, LLP (now Deloitte LLP), providing auditing and consulting services to mid-size public companies adjusting to the requirements of the Sarbanes-Oxley Act of 2002. Altogether, Scott has spent more than 20 years in the medical device industry, serving early stage and growth companies that produced Class II and III devices. Scott has a Bachelor of Science degree in Business Administration from the University of South Dakota and is a Certified Public Accountant (inactive).
Other Directors
Suzanne Gagnon, M.D., has served as our Chief Medical Officer since January 2015 and as a director of our Company since June 2015. Previously, Dr. Gagnon served as the Lead Clinical Consultant to the Company. Prior to working for the Company, Dr. Gagnon was the President of Gagnon Consulting LLC from July 2014 through December 2014 consulting on medical, safety and regulatory matters. From December 2001 through July 2014, Dr. Gagnon had acted as the Chief Medical Officer for three companies, ICON Clinical Research, Nupathe, Inc. and Idis, Inc.
Dalvir S. Gill, Ph.D. has served as a director of our Company since March 2016. Mr. Gill has served as the Chief Executive Officer and a director of TransCelerate BioPharma, Inc., a nonprofit organization focused on improving the health of people around the world by simplifying and enhancing the research and development of innovative new therapies since January 2013. Previously, he was the President of Phase II-IV Drug Development at PharmaNet-i3, an international contract research organization, from July to December 2012. Dr. Gill earned his B.Sc. in Applied Biology from the University of Hertfordshire and his Ph.D. in Pathobiology from the Royal Free Hospital School of Medicine, University of London. He also holds a diploma in the health economics of pharmaceuticals from the executive program of the Stockholm School of Economics. Dr. Gill has more than 25 years of drug development experience. We believe that Dr. Gill brings strategic insight and leadership and a wealth of experience in the pharmaceutical industry to the Board of Directors, as well as knowledge of the regulatory and clinical requirements associated with the development of new drug compounds.
Jeffrey S. Mathiesen has served as a director of our Company since September 2015. He has served as Chief Financial Officer of Gemphire Therapeutics Inc., a publically held biopharmaceutical company since January 2015. Previously, he served as Chief Financial Officer of Sunshine Heart, Inc., a publicly traded medical device company, from March 2011 to January 2015. From December 2005 to April 2010, Mr. Mathiesen served as Vice President and Chief Financial Officer of Zareba Systems, Inc., a manufacturer and marketer of medical products, perimeter fencing and security systems that was purchased by Woodstream Corporation in April 2010. Mr. Mathiesen has held executive positions with publicly traded companies dating back to 1993, including vice president and chief financial officer positions. Mr. Mathiesen holds a B.S. in Accounting from the University of South Dakota and is also a Certified Public Accountant. We believe that Mr. Mathiesen brings financial insight and leadership and a wealth of experience in capital markets to the Board of Directors, as well as knowledge of public company accounting and financial reporting requirements.
J. Robert Paulson, Jr., M.B.A. has served as a director of our Company since September 2015. Mr. Paulson has served as President, CEO, and a director of NxThera, Inc., a venture-funded medical device company developing a novel convective water vapor energy system to treat a variety of endourological conditions, including benign prostatic hyperplasia and prostate cancer since 2009. Previously, he was President, CEO and a director of Restore Medical Inc. from 2005 until its acquisition by Medtronic in July 2008. He was CFO and VP of Global Marketing for Endocardial Solutions, which was acquired by St. Jude Medical in 2005. Before that, he was the Sr. VP/General Manager of Advanced Bionics, and held several executive positions with Medtronic, including VP/General Manager of the Surgical Navigation Technologies business, VP Corporate Strategy, and Director of Corporate Development. Mr. Paulson has held senior positions in marketing, corporate development, legal and finance at General Mills, and practiced corporate, M&A and securities law with the Minneapolis law firm of Lindquist & Vennum. He has served as a director of Veran Medical since 2008, and is a former director of Ablation Frontiers, Vascular Solutions and Medical CV. We believe that Mr. Paulson brings strategic insight and leadership and a wealth of experience in healthcare to the Board of Directors, as well as knowledge of capital markets and early stage companies.
Paul W. Schaffer has served as a director since January 2014. Mr. Schaffer graduated from Minnesota Pharmacy School in 1966. He owned and operated a compounding pharmacy, Bloomington Drug, for 42 years. Mr. Schaffer is an experienced biotech investor. We believe that Mr. Schaffer brings a wealth of experience in pharmaceutical development and manufacturing to the Board of Directors, as well as knowledge of regulations and issues facing pharmaceutical companies.
D. Robert Schemel has served as a director since March 2012. Mr. Schemel has over 39 years’ experience in the agriculture industry. From 1973-2005, Mr. Schemel owned and operated a farming operation in Kandiyohi County, Minnesota, building a 9000-acre operation producing corn, soybeans and sugar beets. Mr. Schemel has extensive experience in serving on boards of directors. From 1992-1996 he served as a board member for ValAdCo and then from 1996-2003 he served as the Chairman of the Board for Phenix Biocomposites. He is currently a member of the Southern Minnesota Beet Sugar Co-op which oversees the operation of the largest US sugar processing facility and a molasses desugarization facility in Renville, Minnesota, which has a total economic benefit currently exceeding $180 million annually We believe that Mr. Schemel brings business insight and leadership as well as significant experience in the development and growth of early stage companies.
Director Independence; Structure of the Board of Directors
Our board of directors consists of eight directors. Five of our directors are independent directors, as defined under the applicable rules of The NASDAQ Stock Market, which we have voluntarily adopted as our standard for director independence. These independent directors are Messrs. Gill, Mathiesen, Paulson, Schaffer and Schemel. There is no familial relationship among any of our directors or executive officers.
Committees of the Board of Directors
Our Board of Directors has established three standing committees: Audit, Compensation, and Nominating and Governance. The membership of each committee is as follows:
|
|
Committees |
|||||||
| Director |
Audit |
Compensation |
Nominating and Governance |
Independent Directors | ||||
|
Michael T. Cullen |
– |
– |
– |
|||||
|
Suzanne Gagnon |
– |
– |
– |
|||||
|
Dalvir S. Gill |
– |
– |
– |
☑ | ||||
|
David B. Kaysen |
– |
– |
– |
|||||
|
Jeffrey S. Mathiesen |
Chair |
– |
Member |
☑ | ||||
|
J. Robert Paulson, Jr. |
– |
Member |
Chair |
☑ | ||||
|
Paul W. Schaffer |
Member |
Member |
– |
☑ | ||||
|
D. Robert Schemel |
Member |
Chair |
Member |
☑ | ||||
Audit Committee
The Audit Committee’s primary functions, among others, are to: (a) assist the Board of Directors in discharging its statutory and fiduciary responsibilities with regard to audits of the books and records of our Company and the monitoring of its accounting and financial reporting practices; (b) carry on appropriate oversight to determine that our Company and its subsidiaries have adequate administrative and internal accounting controls and that they are operating in accordance with prescribed procedures and codes of conduct; and (c) independently review our Company’s financial information that is distributed to shareholders and the general public. The Audit Committee held three meetings during 2015. The Audit Committee has a charter, which is available on our website at www.sunbiopharma.com.
All of the members of the Audit Committee meet the requirements for financial literacy under the applicable rules and regulations of the SEC. Our Board of Directors has determined that Jeffrey S. Mathiesen is qualified to serve as an audit committee financial expert, as that term is defined under the applicable rules of the SEC. Each member of the Audit Committee satisfies the independence requirements of Rule 10A-3(b)(1) of the Securities Exchange Act.
Compensation Committee
The Compensation Committee reviews and recommends to our Board of Directors on an annual basis the goals and objectives relevant to the annual compensation of our executive officers in light of their respective performance evaluations. Our Compensation Committee is responsible for administering our 2011 Equity Incentive Plan, as amended, including approval of individual grants of stock options and other awards. The Compensation Committee held three meetings during 2015. The Compensation Committee has a charter, which is available on our website at www.sunbiopharma.com.
Nominating and Governance Committee
The Nominating and Governance Committee is primarily responsible for identifying individuals qualified to serve as members of our Board of Directors, recommending individuals to our Board of Directors for nomination as directors and committee membership, reviewing the compensation paid to our non-employee directors and recommending adjustments in director compensation, as necessary, in addition to overseeing the annual evaluation of our Board of Directors. The Nominating and Governance Committee held two meetings during 2015. The Nominating and Governance Committee has a charter that is available on our website at www.sunbiopharma.com.
Code of Business Conduct and Ethics
We have adopted a code of business conduct and ethics that applies to all of our employees, officers and directors, including those officers responsible for financial reporting. The code of business conduct and ethics is available on our website at www.sunbiopharma.com. We expect that any amendments to the code, or any waivers of its requirements, will be disclosed on our website.
Role of the Board in Risk Oversight
One of the key functions of our Board of Directors is informed oversight of our risk management process. The Board of Directors does not have a standing risk management committee, but rather administers this oversight function directly through the Board of Directors as a whole, as well as through various standing committees of our Board of Directors that address risks inherent in their respective areas of oversight. In particular, our Board of Directors is responsible for monitoring and assessing strategic risk exposure and our Audit Committee has the responsibility to consider and discuss our major financial risk exposures and the steps our management has taken to monitor and control these exposures, including guidelines and policies to govern the process by which risk assessment and management is undertaken. The Audit Committee also monitors compliance with legal and regulatory requirements. Our Nominating and Governance Committee monitors the effectiveness of our corporate governance practices, including whether they are successful in preventing illegal or improper liability-creating conduct. Our Compensation Committee assesses and monitors whether any of our compensation policies and programs has the potential to encourage excessive risk-taking.
Compensation Committee Interlocks and Insider Participation
None of the members of the compensation committee nor any director nominee proposed to become a member of the compensation committee is or has at any time during the last completed fiscal year been an officer or employee of our Company. None of our executive officers has served as a member of the board of directors or as a member of the compensation or similar committee, of any entity that has one or more executive officers who served on our board of directors during the last completed fiscal year.
None of the members of the compensation committee is or has at any time during the last completed fiscal year been an officer or employee of our Company. None of our executive officers has served as a member of the board of directors, or as a member of the compensation or similar committee, of any entity that has one or more executive officers who served on our board of directors or compensation committee during the last completed fiscal year.
Director Compensation
Directors who are also our employees receive no additional compensation for serving on our Board of Directors. During 2015, our Company reimbursed non-employee directors for out-of-pocket expenses incurred in connection with attending meetings of our Board of Directors and its committees.
Non-Employee Director Compensation for 2015
The following table sets forth information concerning annual compensation for our non-employee directors during the year ended December 31, 2015:
|
Name |
Option Awards(a) ($) |
Total ($) |
||||||
|
Jeffrey S. Mathiesen |
– | – | ||||||
|
J. Robert Paulson, Jr. |
– | – | ||||||
|
Paul W. Schaffer |
13,600(b) | 13,600 | ||||||
|
D. Robert Schemel |
80,200(c) | 80,200 | ||||||
| (a) | Amounts shown in the “Option Awards” column represent the aggregate grant date fair value of these awards computed in accordance with FASB ASC Topic 718. For additional information regarding the calculation of grant date fair value of options granted during 2015, see Note 9 to the consolidated financial statements appearing in our annual report on Form 10-K for the fiscal year ended December 31, 2015. |
|
(b) |
Represents, a non-qualified stock option to purchase 80,000 shares of common stock granted in January 2015. |
| (c) | Represents non-qualified stock options to purchase 60,000 shares and 400,000 shares granted in January and March 2015, respectively. |
Limitation of Directors’ and Officers’ Liability and Indemnification
The Delaware General Corporation Law authorizes corporations to limit or eliminate, subject to specified conditions, the personal liability of directors to corporations and their stockholders for monetary damages for breach of their fiduciary duties. Our certificate of incorporation and amended and restated bylaws limit the liability of our directors to the fullest extent permitted by Delaware law.
We have obtained director and officer liability insurance to cover liabilities our directors and officers may incur in connection with their services to us. Our certificate of incorporation and amended and restated bylaws also provide that we will indemnify and advance expenses to any of our directors and officers who, by reason of the fact that he or she is one of our officers or directors, is involved in a legal proceeding of any nature. We will repay certain expenses incurred by a director or officer in connection with any civil, criminal, administrative or investigative action or proceeding, including actions by us or in our name. Such indemnifiable expenses include, to the maximum extent permitted by law, attorney’s fees, judgments, fines, settlement amounts and other expenses reasonably incurred in connection with legal proceedings. A director or officer will not receive indemnification if he or she is found not to have acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interest.
We have entered into agreements to indemnify our directors and officers and intend to enter into these agreements in the future. These agreements provide that we will, among other things, indemnify and advance expenses to our directors and officers for certain expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by any such person in any action or proceeding, including any action by us arising out of such person’s services as our director or officer, or any other company or enterprise to which the person provides services at our request. We believe that these provisions and agreements are necessary to attract and retain qualified persons as directors and officers.
Such limitation of liability and indemnification does not affect the availability of equitable remedies. In addition, we have been advised that in the opinion of the Securities and Exchange Commission, indemnification for liabilities arising under the Securities Act, is against public policy as expressed in the Securities Act and is therefore unenforceable.
There is no pending litigation or proceeding involving any of our directors, officers, employees or agents in which indemnification will be required or permitted. We are not aware of any threatened litigation or proceeding that may result in a claim for such indemnification.
Executive Compensation
Base salaries for each of our named executive officers were initially established based on arm’s-length negotiations with the applicable executive. Our Compensation Committee reviews our executive officers’ salaries annually. When negotiating or reviewing base salaries, the Compensation Committee expects to consider market competitiveness based on their market experience, the executive’s expected future contribution to our success and the relative salaries and responsibilities of our other executives. None of our Company’s continuing executive officers were employed by the Company during the most recent completed fiscal year.
Summary Compensation Table
The following table provides information regarding the compensation earned during fiscal 2015 and fiscal 2014 by our named executive officers:
|
Name and principal position |
Fiscal Year |
Salary |
Option awards |
Total |
||||||||||
|
Michael T. Cullen |
2015 |
90,000 | 140,000 | 230,000 | ||||||||||
| Executive Chairman and Former President and Chief Executive Officer(b) |
2014 |
70,000 | – | 70,000 | ||||||||||
|
David B. Kaysen |
2015 |
77,955 | – | 78,000 | ||||||||||
| President and Chief Executive Officer(c) |
||||||||||||||
|
Scott Kellen |
2015 |
50,000 | – | 50,000 | ||||||||||
| Chief Financial Officer(d) | ||||||||||||||
|
Clifford F. McCurdy, III |
2015 |
46,667 | 140,000 | 187,000 | ||||||||||
| Former Chief Operating Officer and Interim Chief Executive Officer(e) |
2014 |
105,000 | – | 105,000 | ||||||||||
|
(a) |
The values of option awards in this table represent the fair value of such awards granted during the fiscal year, as computed in accordance with FASB ASC 718. The assumptions used to determine the valuation of the awards are discussed in Note 9 to our consolidated financial statements, included in our annual report on Form 10-K for the fiscal year ended December 31, 2015. |
| (b) | Mr. Cullen resigned as President and Chief Executive Officer in June 2015. |
| (c) | Mr. Kaysen commenced employment in July 2015. |
| (d) | Mr. Kellen commenced employment with our Company’s in October 2015. |
| (e) | Mr. McCurdy resigned all positions in August 2015. |
Outstanding Equity Awards as of December 31, 2015
|
Option Awards |
||||||||||||||||
| Name |
Number of securities underlying unexercised options (#) exercisable |
Number of securities underlying unexercised options (#) unexercisable |
Option exercise price ($) |
Option expiration Date |
||||||||||||
|
Michael T. Cullen |
800,000 | – | $0.32 | 3/5/2026 | ||||||||||||
|
David B. Kaysen |
– | – | – | – | ||||||||||||
|
Scott Kellen |
– | – | – | – | ||||||||||||
|
Clifford F. McCurdy, III(a) |
– | – | – | – | ||||||||||||
|
(a) |
Mr. McCurdy resigned all positions in August 2015, as a result of which all outstanding equity awards expired on their terms in November 2015. |
Employment Agreements
We are party to employment agreements with our Executive Chairman, President and Chief Executive Officer, and Chief Financial Officer (collectively, the “Executives”). In addition to the specific terms summarized below, each of the Executives is eligible to participate in the other compensation and benefit programs generally available to our employees, including our other executive officers. Each employment agreement also includes customary confidentiality, non-competition and non-solicitation covenants.
In accordance with the employment agreements, the base salaries of each Executive is reviewed annually by the Board. The Board may authorize an increase for the applicable year, but may not reduce an Executive’s base salary below its then-current level other than with the Executive’s consent or pursuant to a general wage reduction in respect of substantially all of the Company’s executive officers.
Executive Chairman
Under his employment agreement, as amended, Dr. Cullen is entitled to receive an initial annualized base salary equal to $384,000. From October 2015 until June 15, 2016, Dr. Cullen received $7,500 per month in cash and the remaining $24,000 was accrued and will not become payable until the earlier of (i) the completion of any transaction or series of related transactions involving the issuance of equity securities (including any securities that are convertible into or exercisable for equity securities) resulting in gross cash proceeds of $10,000,000 or more (a “Qualified Financing”) and (ii) a change of control (as defined in the employment agreement and, collectively with a Qualified Financing, a “Payment Triggering Event”). From June 16, 2016 until a Payment Triggering Event or the Payment Cutoff Date (defined below), 50% of the cash compensation due to Dr. Cullen will be accrued and will not become payable until a Payment Triggering Event occurs. If a Payment Triggering Event has not occurred prior to June 30, 2018 (the “Payment Cutoff Date”), all compensation then accrued under Dr. Cullen’s employment agreement will be forfeited. Starting with the fiscal year ending December 31, 2016, Dr. Cullen is eligible for an annual performance-based cash bonus with a target amount equal to no less than 45% of his base salary. Payment of the bonus amount will be subject to achievement of metrics to be established by the Board of Directors and Dr. Cullen’s continued employment with the Company through the end of the applicable cash bonus period.
President and Chief Executive Officer
Under his employment agreement, as amended, Mr. Kaysen is entitled to receive an initial annualized base salary equal to $420,000. From the commencement of his employment in July 2015 through June 15, 2016, Mr. Kaysen served as a part-time employee, during which time he was entitled to receive a pro-rata amount. However, from March 1, 2016 until a Payment Triggering Event, 50% of the cash compensation due to Mr. Kaysen will be accrued and will not become payable until a Payment Triggering Event. If a Payment Triggering Event does not occur prior to the Payment Cutoff Date, all compensation then accrued under Mr. Kaysen’s employment agreement will be forfeited.
Starting with the fiscal year ending December 31, 2016, Mr. Kaysen is eligible for an annual performance-based cash bonus with a target amount equal to no less than 60% of his base salary. Payment of the bonus amount will be subject to achievement of metrics to be established by the Board of Directors and Mr. Kaysen’s continued employment with the Company through the end of the applicable cash bonus period. Mr. Kaysen is also eligible to receive cash bonuses of (i) $260,000 upon the completion of a Qualified Financing and (ii) $36,000 upon the completion of certain other objectives specified in his employment agreement.
Upon the completion of a Qualified Financing, Mr. Kaysen will also receive options to purchase an aggregate of 800,000 shares of our common stock at an exercise price to be established at the time of grant. Such options, when issued, are expected to be immediately vested and exercisable with respect to at least 500,000 shares, with the remainder vesting in increments of 100,000 additional shares on each of the six-, twelve-, and eighteen-month anniversaries of the grant date.
Chief Financial Officer
Under his employment agreement, as amended, Mr. Kellen is entitled to receive an initial annualized base salary equal to $240,000. Mr. Kellen received a pro rata amount for October 2015, during which he served as a part-time employee. However, from March 1, 2016 through June 15, 2016, 50% of the cash compensation due to Mr. Kellen was accrued and will not become payable until a Payment Triggering Event. From June 16, 2016 forward, one-third of the cash compensation due to Mr. Kaysen will be accrued and will not become payable until a Payment Triggering Event. If a Payment Triggering Event does not occur prior to the Payment Cutoff Date, all cash compensation then accrued under Mr. Kellen’s employment agreement will be forfeited.
Starting with the fiscal year ending December 31, 2016, Mr. Kellen is eligible for an annual performance-based cash bonus with a target amount equal to no less than 40% of his base salary. Payment of the bonus amount will be subject to achievement of metrics to be established by the Board of Directors and Mr. Kellen’s continued employment with the Company through the end of the applicable cash bonus period.
Upon the completion of a Qualified Financing, Mr. Kellen will also receive options to purchase an aggregate of 300,000 shares of our common stock at an exercise price to be established at the time of grant. Such options, when issued, are expected to be immediately vested and exercisable with respect to at least 75,000 shares, with the remainder vesting in increments of 75,000 additional shares on each of the six-, twelve-, and eighteen-month anniversaries of the grant date.
Potential Payments Upon Termination or Change-in-Control
Under their respective employment agreements, if an Executives’ employment is terminated by us for any reason other than for “cause” (as defined in the applicable employment agreement) or by the Executive for “good reason” (as defined in the applicable employment agreement), then the Executive will be eligible to receive an amount equal to his respective annualized salary plus an amount equal to a prorated portion of his cash bonus target for the year in which the termination occurred, in addition to other amounts accrued on or before the date of termination. If any such termination occurs within six months prior or two year after a “change of control” (as defined in the applicable employment agreement), then Dr. Cullen and Mr. Kellen would instead receive an amount equal to his respective annualized salary, plus an amount equal to his full cash bonus target for the year in which the termination occurred. Upon a similar termination, Mr. Kaysen would receive an amount equal to 1.5 times his annualized salary, plus an amount equal to his full cash bonus target.
Certain Relationships and Related Party Transactions
The following is a summary of transactions since January 1, 2014 to which our company has been a party and in which the amount involved exceeded $21,000, which is approximately 1% of the average of our total assets as of December 31, 2015, and in which any of our directors, executive officers, or beneficial holders of more than 5% of our capital stock had or will have a direct or indirect material interest, other than compensation arrangements that are described under the heading “Executive Compensation: Employment Agreements” and “Director Compensation” above.
During 2015, Ryan R. Gilbertson received $34,417 in cash compensation for his service as SBI’s Executive Vice President of Business Development. As additional compensation for his service, on March 6, 2015, Mr. Gilbertson received options to purchase an aggregate of 400,000 shares of SBI’s common stock at $0.3175 per share. Mr. Gilbertson is a beneficial holder of more than 5% of our capital stock.
During 2014, Christopher R. Johnson received options to purchase 40,000 shares of our common stock for $0.2275 per share for his service as SBI’s Executive Vice President of Finance. During 2015, Mr. Johnson received $35,400 in cash compensation for his service and on March 6, 2015, he received options to purchase an aggregate of 400,000 shares of SBI’s common stock at $0.3175 per share. At the time, Mr. Johnson was a beneficial holder of more than 5% of our capital stock.
Recent Equity Issuances to Directors, Executive Officers and 5% Stockholders
The following table shows all issuances of common stock since the beginning of January 1, 2014 to directors, executive officers and holders of more than 5% of our capital stock.
|
Director Executive Officer or Stockholder |
Date |
Shares Issued |
Consideration | ||||
|
Michael T. Cullen |
4/28/2015 |
600,000 |
$112,500 in connection with exercise of warrants | ||||
|
Michael T. Cullen |
6/24/2016 |
50,000 |
$50,000 pursuant to Securities Purchase Agreement | ||||
|
Michael T. Cullen |
12/31/2014 |
20,000 |
$1,750 in connection with exercise of options | ||||
|
Suzanne Gagnon |
5/6/2014 |
400,000 |
Services rendered as an employee. | ||||
|
Suzanne Gagnon |
6/24/2016 |
30,000 |
$30,000 pursuant to Securities Purchase Agreement | ||||
|
Ryan R. Gilbertson |
12/31/2014 |
363,600 |
$99,990 in connection with option exercise | ||||
|
Ryan R. Gilbertson |
2/19/2015 |
363,600 |
$99,990 in connection with option exercise | ||||
|
David B. Kaysen |
6/24/2016 |
50,000 |
$50,000 pursuant to Securities Purchase Agreement | ||||
|
Scott Kellen |
6/24/2016 |
$35,000 |
$35,000 pursuant to Securities Purchase Agreement | ||||
|
Douglas Polinsky |
12/31/2014 |
400,000 |
$100,000 in connection with option exercise | ||||
|
Douglas Polinsky |
2/19/2015 |
400,000 |
$100,000 in connection with option exercise | ||||
|
Paul W. Schaffer |
12/31/2014 |
80,000 |
$18,200 in connection with option exercise | ||||
|
Paul W. Schaffer |
4/18/2015 |
200,000 |
$37,500 in connection with warrant exercise | ||||
|
Paul W. Schaffer |
5/22/2015 |
80,000 |
$25,400 in connection with option exercise | ||||
|
Paul W. Schaffer |
6/24/2016 |
100,000 |
$100,000 pursuant to Securities Purchase Agreement | ||||
|
Paul W. Schaffer Trust |
4/17/2015 |
89,092 |
$100,000 principal amount of converted indebtedness | ||||
|
D. Robert Schemel |
5/20/2015 |
1,000,000 |
$187,500 in connection with warrant exercise | ||||
|
D. Robert Schemel |
12/31/2014 |
320,000 |
$51,200 in connection with option exercise | ||||
|
D. Robert Schemel |
5/20/2015 |
60,000 |
$19,050 in connection with option exercise | ||||
|
D. Robert Schemel |
5/20/2015 |
400,000 |
$127,000 in connection with option exercise | ||||
Promoters and Certain Control Persons
Michael T. Cullen, a founder of SBI, received an option to purchase 20,000 shares of SBI’s common stock at $0.0875 per share on December 29, 2011, as compensation for his service as SBI’s Chief Medical Officer. During 2012 and 2013, Dr. Cullen received cash compensation for his service as SBI’s Chief Medical Officer in the amounts of $22,500 and $90,000, respectively. See the disclosure in the “Summary Compensation Table” above for Dr. Cullen’s compensation during 2014 and 2015 and under the heading “Employment Agreements” above for additional information regarding the terms of his employment.
Paul M. Herron, a founder of SBI, received an option to purchase 20,000 shares of SBI’s common stock at $0.0875 per share on December 29, 2011, as compensation for his service as SBI’s President and Chief Executive Officer. During 2012, 2013 and 2014, Mr. Herron received cash compensation for his service as SBI’s Chief Executive Officer in the amounts of $22,500, $90,000 and $105,000, respectively.
Clifford F. McCurdy, III, a founder of SBI, received an option to purchase 20,000 shares of SBI’s common stock at $0.0875 per share on December 29, 2011, as compensation for his service as SBI’s Chief Operating Officer. During 2012 and 2013, Mr. McCurdy received cash compensation for his service as SBI’s Chief Operating Officer in the amounts of $11,250 and $90,000, respectively. See the disclosure in the “Summary Compensation Table” above for Mr. McCurdy’s compensation during 2014 and 2015.
Thomas X. Neenan, a founder of SBI, received cash compensation for service as a consultant to SBI in the amounts of $15,000; $60,000; and $84,000 during 2012, 2013 and 2014, respectively. As additional compensation for his service as a consultant to SBI, Dr. Neenan received an option to purchase 40,000 shares of SBI’s common stock at $0.1100 per share on June 13, 2013, an option to purchase 40,000 shares of SBI’s common stock at $0.2500 per share on January 22, 2014 and an option to purchase 60,000 shares of SBI’s common stock at $0.2275 per share on July 31, 2014. During 2015, Dr. Neenan received cash compensation in the amount of 93,000 and an option to purchase 300,000 shares of SBI’s common stock at $0.3175 per share granted on March 5, 2015 as compensation for his service as SBI’s Chief Scientific Officer.
Legal Matters
The validity of the shares of common stock being offered by this prospectus has been passed upon for us by Faegre Baker Daniels LLP, Minneapolis, Minnesota.
Experts
The financial statements as of December 31, 2015 and 2014 and for the two years in the period ended December 31, 2015 included in this prospectus have been so included in reliance on the report of Cherry Bekaert LLP, an independent registered public accounting firm, appearing elsewhere herein, given on the authority of said firm as experts in auditing and accounting.
Where You Can Find More Information
We have filed with the SEC a registration statement on Form S-1 under the Securities Act that registers the distribution of the securities offered under this prospectus. The registration statement, including the attached exhibits and schedules and the information incorporated by reference into this prospectus, contains additional relevant information about us and the securities. The rules and regulations of the SEC allow us to omit from this prospectus certain information included in the registration statement.
We are subject to the informational requirements of the Securities Exchange Act and are required to file annual, quarterly and current reports, proxy statements and other information with the SEC. You may read and copy this information and the registration statement at the SEC public reference room located at 100 F Street, N.E., Room 1580, Washington D.C. 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. Any information we file with the SEC, including the documents incorporated by reference into this prospectus, is also available on the SEC’s website at http://www.sec.gov. We also make these documents publicly available, free of charge, on our website at www.sunbiopharma.com as soon as reasonably practicable after filing such documents with the SEC. The information contained in, or that can be accessed through, our website is not part of this prospectus.
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of
Sun BioPharma, Inc.
We have audited the accompanying consolidated balance sheets of Sun BioPharma, Inc. (the “Company”) as of December 31, 2015 and 2014 and the related consolidated statements of operations and comprehensive loss, stockholders’ deficit and cash flows for the years then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with standards of the Public Company Accounting Oversight Board (United States of America). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis of designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Sun BioPharma, Inc. at December 31, 2015 and 2014 and the results of its operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has recurring losses and negative cash flows from operations that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are described in Note 3 to the financial statements. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Cherry Bekaert
Tampa, Florida
March 8, 2016
Sun BioPharma, Inc.
Consolidated Balance Sheets
(In thousands, except share amounts)
|
December 31, |
||||||||
|
2015 |
2014 |
|||||||
|
ASSETS |
||||||||
|
Current assets: |
||||||||
|
Cash and cash equivalents |
$ | 925 | $ | 1,654 | ||||
|
Short-term investments, net |
— | 499 | ||||||
|
Stock subscription receivable |
— | 94 | ||||||
|
Prepaid expenses and other current assets |
74 | 18 | ||||||
|
Income tax receivable |
733 | 108 | ||||||
|
Total current assets |
1,732 | 2,373 | ||||||
|
Other assets, net |
76 | 105 | ||||||
|
Total assets |
$ | 1,808 | $ | 2,478 | ||||
|
LIABILITIES AND SHAREHOLDERS’ DEFICIT |
||||||||
|
Current liabilities: |
||||||||
|
Accounts payable |
$ | 585 | $ | 297 | ||||
|
Accrued expenses |
505 | 132 | ||||||
|
Demand notes payable |
250 | — | ||||||
|
Accrued interest |
35 | 38 | ||||||
|
Total current liabilities |
1,375 | 467 | ||||||
|
Long-term liabilities: |
||||||||
|
Convertible notes payable |
2,775 | 3,000 | ||||||
|
Long-term debt |
300 | 300 | ||||||
|
Accrued interest |
39 | 27 | ||||||
|
Total long-term liabilities |
3,114 | 3,327 | ||||||
|
Commitments and contingencies (Note 7) |
||||||||
|
Shareholders’ deficit: |
||||||||
|
Preferred stock, $0.001 par value; 10,000,000 and 5,000,000 authorized as of December 31, 2015 and 2014, respectively; no shares issued or outstanding as of December 31, 2015 and 2014 |
— | — | ||||||
|
Common stock, $0.001 par value; 100,000,000 and 20,000,000 authorized; 29,892,806 and 5,688,927 shares issued and outstanding, as of December 31, 2015 and 2014, respectively |
30 | 6 | ||||||
|
Additional paid-in capital |
10,943 | 7,264 | ||||||
|
Accumulated deficit |
(13,667 | ) | (8,569 | ) | ||||
|
Accumulated other comprehensive gain (loss), net |
13 | (17 | ) | |||||
|
Total shareholders’ deficit |
(2,681 | ) | (1,316 | ) | ||||
|
Total liabilities and shareholders’ deficit |
$ | 1,808 | $ | 2,478 | ||||
See accompanying notes to consolidated financial statements.
Sun BioPharma, Inc.
Consolidated Statements of Operations and Comprehensive Loss
(In thousands, except share and per share amounts)
|
Year Ended December 31, |
||||||||
|
2015 |
2014 |
|||||||
|
Operating expenses |
||||||||
|
General and administrative |
$ | 2,592 | $ | 1,079 | ||||
|
Research and development |
2,852 | 2,366 | ||||||
|
Operating loss |
(5,444 | ) | (3,445 | ) | ||||
|
Other income (expense): |
||||||||
|
Interest income |
8 | 6 | ||||||
|
Interest expense |
(183 | ) | (184 | ) | ||||
|
Other expense |
(64 | ) | (16 | ) | ||||
|
Change in fair value of derivatives |
— | — | ||||||
|
Total other income (expense) |
(239 | ) | (194 | ) | ||||
|
Loss before income tax benefit |
(5,683 | ) | (3,639 | ) | ||||
|
Income tax benefit |
756 | 108 | ||||||
|
Net loss |
(4,927 | ) | (3,531 | ) | ||||
|
Foreign currency translation adjustment gain (loss) |
30 | (13 | ) | |||||
|
Comprehensive loss |
$ | (4,897 | ) | $ | (3,544 | ) | ||
|
Basic and diluted net loss per share |
$ | (0.35 | ) | $ | (0.69 | ) | ||
|
Weighted average shares outstanding – basic and diluted |
14,073,174 | 5,109,644 | ||||||
See accompanying notes to consolidated financial statements.
Sun BioPharma, Inc.
Consolidated Statements of Shareholders’ Deficit
(In thousands except share and per share amounts)
|
Accumulated |
||||||||||||||||||||||||
|
Additional |
Other |
Total |
||||||||||||||||||||||
|
Common Stock |
Paid-In |
Accumulated |
Comprehensive |
Shareholders’ |
||||||||||||||||||||
|
Shares |
Amount |
Capital |
Deficit |
Gain (Loss) |
Deficit |
|||||||||||||||||||
|
Balances at December 31, 2013 |
5,005,522 | 5 | 6,450 | (5,038 | ) | (4 | ) | 1,413 | ||||||||||||||||
|
Issuance of common stock for services |
100,000 | — | 91 | — | — | 91 | ||||||||||||||||||
|
Conversion of convertible notes payable and accrued interest into common stock |
22,505 | — | 101 | — | — | 101 | ||||||||||||||||||
|
Exercise of stock options |
535,900 | 1 | 492 | — | — | 493 | ||||||||||||||||||
|
Exercise of stock warrants |
25,000 | — | 25 | — | — | 25 | ||||||||||||||||||
|
Share-based compensation expense |
— | — | 105 | — | — | 105 | ||||||||||||||||||
|
Net loss |
— | — | — | (3,531 | ) | — | (3,531 | ) | ||||||||||||||||
|
Foreign currency translation adjustment, net of taxes of $0 |
— | — | — | — | (13 | ) | (13 | ) | ||||||||||||||||
|
Balances at December 31, 2014 |
5,688,927 | $ | 6 | $ | 7,264 | $ | (8,569 | ) | $ | (17 | ) | $ | (1,316 | ) | ||||||||||
|
Exercise of stock options |
647,634 | 1 | 692 | — | — | 693 | ||||||||||||||||||
|
Exercise of stock warrants |
500,000 | — | 375 | — | — | 375 | ||||||||||||||||||
|
Conversion of convertible notes payable and accrued interest into common stock |
50,194 | — | 226 | — | — | 226 | ||||||||||||||||||
|
Issuance of common stock in a private offering, net of issuance costs of $12 |
190,625 | — | 1,513 | — | — | 1,513 | ||||||||||||||||||
|
Issuance of common stock for services |
33,241 | — | 42 | — | — | 42 | ||||||||||||||||||
|
Share-based compensation expense |
— | — | 933 | — | — | 933 | ||||||||||||||||||
|
Exercise price modification of common stock warrants |
— | — | 171 | (171 | ) | — | — | |||||||||||||||||
|
Merger transaction – See Note 8 |
22,782,185 | 23 | (273 | ) | — | — | (250 | ) | ||||||||||||||||
|
Net loss |
— | — | — | (4,927 | ) | — | (4,927 | ) | ||||||||||||||||
|
Foreign currency translation adjustment, net of taxes of $0 |
— | — | — | — | 30 | 30 | ||||||||||||||||||
|
Balances at December 31, 2015 |
29,892,806 | $ | 30 | $ | 10,943 | $ | (13,667 | ) | $ | 13 | $ | (2,681 | ) | |||||||||||
See accompanying notes to consolidated financial statements.
Sun BioPharma, Inc.
Consolidated Statements of Cash Flows
(In thousands)
| Year Ended December 31, | |||||||||
|
2015 |
2014 |
||||||||
|
Cash flows from operating activities: |
|||||||||
|
Net loss |
$ | (4,927 | ) | $ | (3,531 | ) | |||
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|||||||||
|
Amortization of debt issuance costs |
28 | 28 | |||||||
|
Non-cash interest expense |
— | 50 | |||||||
|
Unrealized loss on investment |
— | 2 | |||||||
|
Share-based compensation |
976 | 196 | |||||||
|
Changes in operating assets and liabilities: |
|||||||||
|
Income and other tax receivables |
(610 | ) | (9 | ) | |||||
|
Rebate receivable |
— | 47 | |||||||
|
Prepaid expenses and other assets |
(45 | ) | 5 | ||||||
|
Accounts payable and accrued liabilities |
681 | (131 | ) | ||||||
|
Net cash used in operating activities |
(3,897 | ) | (3,343 | ) | |||||
|
Cash flows from investing activities: |
|||||||||
|
Proceeds from sales and maturities of short-term investments |
500 | — | |||||||
|
Purchases of short-term investments |
— | (501 | ) | ||||||
|
Net cash provided (used in) by investing activities |
500 | (501 | ) | ||||||
|
Cash flows from financing activities: |
|||||||||
|
Proceeds from issuance of common stock, net of selling costs of $12 |
1,513 | — | |||||||
|
Proceeds from issuance of debt, net of debt issuance costs of $9k |
— | 2,391 | |||||||
|
Proceeds from the exercise of stock options |
762 | 424 | |||||||
|
Proceeds from the exercise of stock purchase warrants |
400 | — | |||||||
|
Net cash provided by financing activities |
2,675 | 2,815 | |||||||
|
Effect of exchange rate changes on cash and cash equivalents |
(7 | ) | (11 | ) | |||||
|
Net decrease in cash and cash equivalents |
(729 | ) | (1,040 | ) | |||||
|
Cash and cash equivalents at beginning of period |
1,654 | 2,694 | |||||||
|
Cash and cash equivalents at end of period |
$ | 925 | $ | 1,654 | |||||
|
Supplemental disclosure of cash flow information: |
|||||||||
|
Cash paid during period for interest |
$ | 145 | $ | 106 | |||||
|
Supplemental disclosure of non-cash transactions: |
|||||||||
|
Conversion of notes payable and accrued interest into common stock |
$ | 226 | $ | 101 | |||||
|
Notes payable assumed in merger (Note 6) |
$ | 250 | $ | — | |||||
See accompanying notes to consolidated financial statements.
Sun BioPharma, Inc.
Notes to Consolidated Financial Statements
1. Business
Sun BioPharma, Inc., formerly known as Cimarron Medical, Inc., (“Cimarron”) and its wholly-owned subsidiaries, Sun BioPharma Research, Inc. (“SBR”) and Sun BioPharma Australia Pty Ltd. (“SBA” and collectively with Cimarron and SBR, “we,” “us,” “our,” and the “Company”) exist for the primary purpose of advancing the commercial development of a proprietary polyamine analogue for pancreatic cancer and for a second indication in chronic pancreatitis. We have exclusively licensed the worldwide rights to this compound, which has been designated as SBP-101, from the University of Florida Research Foundation, Inc. (“UFRF”). SBR was incorporated under the laws of the State of Delaware on September 21, 2011. Sun BioPharma Australia Pty Ltd was established on May 24, 2013, and incorporated under the laws of Australian Securities and Investments Commission.
SBR entered into an Agreement and Plan of Merger with Cimarron and SB Acquisition Corporation, a wholly owned subsidiary of Cimarron, on June 12, 2015. The merger of SB Acquisition Corporation with and into SBR on September 4, 2015 (the “Merger”) resulted in all of the issued and outstanding common stock of SBR being converted into the right to receive an aggregate of 28,442,484 shares of Cimarron’s common stock, representing four shares of Cimarron common stock for every one share of SBR common stock cancelled in the Merger. As a result of this transaction, former SBR shareholders owned approximately 98.8% of the outstanding capital stock of Cimarron. Concurrent with the completion of the Merger, Cimarron’s name was changed to “Sun BioPharma, Inc.”
Under accounting principles generally accepted in the United States (“GAAP”), SBR was deemed to be the acquirer for accounting purposes because its legacy shareholders owned a substantial majority of the issued and outstanding shares of Cimarron’s common stock after the Merger. Further, as Cimarron’s business operations and net assets, at the time of the Merger, were nominal relative to SBR’s business operations and net assets, we have accounted for the Merger as a capital transaction and the activity presented in these financial statements represents the current and historical operations of SBR. All share and per share amounts included in these Notes are presented on an as converted basis, which gives effect to the exchange of four shares of Cimarron common stock for every one share of SBR common stock.
See Note 8 for additional information regarding the Merger.
2. Risks and Uncertainties
The Company operates in a highly regulated and competitive environment. The development, manufacturing and marketing of pharmaceutical products require approval from, and are subject to ongoing oversight by, the Food and Drug Administration (“FDA”) in the United States, the Therapeutic Goods Administration (“TGA”) in Australia, the European Medicines Agency (“EMA”) in the European Union, and comparable agencies in other countries. Obtaining approval for a new pharmaceutical product is never certain, may take many years, and is normally expected to involve substantial expenditures.
We have incurred losses of $13.7 million since SBR’s inception in 2011. For the year ended December 31, 2015, we incurred a net loss and negative cash flows from operating activities of $4.9 million and $3.9 million, respectively. We expect to incur substantial losses for the foreseeable future, which will continue to generate negative net cash flows from operating activities, as we continue to pursue research and development activities and seek to commercialize our primary product candidate, SBP-101. As of December 31, 2015, we had cash and cash equivalents of $925,000, working capital of $357,000 and shareholders’ deficit of $2.7 million. We believe our cash and cash equivalents as of December 31, 2015, will be sufficient to fund our planned operations through the first quarter of 2016. The Company’s principal sources of cash have included the issuance of convertible debt and equity securities.
The accompanying consolidated financial statements have been prepared assuming that we will continue as a going concern which contemplates the realization of assets and liquidation of liabilities in the normal course of business and do not include any adjustments relating to the recoverability or classification of assets or the amounts of liabilities that might result from the outcome of these uncertainties. Our ability to continue as a going concern, realize the carrying value of our assets and discharge our liabilities in the ordinary course of business is dependent upon a number of factors, including our ability to obtain additional financing, the success of our development efforts, our ability to obtain marketing approval for our SBP-101 product candidate in the United States, Australia, the European Union or other markets and ultimately our ability to market and sell our SBP-101 product candidate. These factors, among others, raise substantial doubt about our ability to continue operations as a going concern. See Note 3 entitled “Liquidity and Management’s Plans.”
3. Liquidity and Management Plans
We will need to seek additional sources of funds to support our current business plans. We may seek to raise additional funds through various sources, such as equity and debt financings, or through strategic collaborations and license agreements. We can give no assurances that we will be able to secure additional sources of funds to support our operations, or if such funds are available to us, that such additional financing will be sufficient to meet our needs or on terms acceptable to us. This risk would increase if our clinical data is not positive or economic and market conditions deteriorate.
If we are unable to obtain additional financing when needed, we would need to scale back our operations taking actions that may include, among other things, reducing use of outside professional service providers, reducing staff or staff compensation, significantly modify or delay the development of our SBP-101 product candidate, license to third parties the rights to commercialize our SBP-101 product candidate for pancreatic cancer, acute pancreatitis or other applications that we would otherwise seek to pursue, or cease operations.
Our future success is dependent upon our ability to obtain additional financing, the success of our development efforts, our ability to obtain marketing approval for our SBP-101 product candidate in the United States or other markets and ultimately our ability to market and sell our SBP-101 product candidate. If we are unable to obtain additional financing when needed, if our clinical trials are not successful, if we are unable to obtain marketing approval, we would not be able to continue as a going concern and would be forced to cease operations and liquidate our company.
There can be no assurances that we will be able to obtain additional financing on commercially reasonable terms, or at all. The sale of additional convertible debt or equity securities would likely result in dilution to our current shareholders.
4. Summary of Significant Accounting Policies
Basis of Presentation
We have prepared the accompanying consolidated financial statements in conformity with accounting principles generally accepted in the United States of America (“GAAP”). Our fiscal year ends on December 31.
Use of estimates
The preparation of consolidated financial statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amount of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Principles of consolidation
The accompanying consolidated financial statements include the assets, liabilities and expenses of Sun BioPharma, Inc. and our wholly-owned subsidiaries. All significant intercompany transactions and balances have been eliminated in consolidation.
Cash and Cash Equivalents
Cash and cash equivalents consist of cash and money market funds with original maturities of three months or less. The carrying value of these instruments approximates fair value. We have not experienced any losses in our cash and cash equivalents.
Concentration of Credit Risk
Financial instruments that potentially subject the company to significant concentrations of credit risk consist primarily of cash and cash equivalents. Cash and cash equivalents are primarily deposited in demand and money market accounts. At times, such deposits may be in excess of insured limits. Investments in money market funds are not considered to be bank deposits and are not insured or guaranteed by the federal deposit insurance company or other government agencies. These money market funds seek to preserve the value of the investment at $1.00 per share; however, it is possible to lose money investing in these funds. The Company has not experienced any losses on its deposits of cash and cash equivalents.
Short-term investments
We consider all investments with maturities greater than three months and less than one year at the time of purchase as short-term investments. At December 31, 2014, short-term investments consisted of a mutual fund investment reported at fair value. We seek to manage our investments to achieve our goal of preserving principal and maintaining adequate liquidity at all times. Short-term investments are considered trading securities by the company. As such, unrealized gains and losses are included in earnings and recorded as interest income in the accompanying Consolidated Statements of Operations and Comprehensive Loss.
Income taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the consolidated financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carry-forwards. Deferred tax assets and liabilities are measured using enacted rates, for each of the jurisdictions in which the Company operates, expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in operations in the period that includes the enactment date. Valuation allowances are established when necessary to reduce deferred tax assets to the amount that is more likely than not to be realized. The Company has provided a full valuation allowance against the gross deferred tax assets as of December 31, 2015 and 2014. See Note 10 for additional information. The Company’s policy is to classify interest and penalties related to income taxes as income tax expense in the Consolidated Statements of Operations and Comprehensive Loss.
Debt issuance costs
Costs associated with the issuance of debt instruments are capitalized. These costs are amortized on a straight-line basis, which approximates the effective interest method, over the term of the debt agreements and are included in interest expense.
Research and development costs
Research and development costs to date have consisted primarily of expenses incurred for third-party service providers monitoring and accumulating data related to our preclinical studies; sponsored research agreements; developing and scaling the manufacturing process necessary to produce sufficient amounts of SBP-101 for use in our pre-clinical studies and human clinical trials; consulting resources with specialized expertise related to execution of our development plan for our SBP-101 product candidate; and costs to license and maintain our licensed intellectual property. Moving forward, research and development expenditures will shift to focus on costs related to the execution of human clinical trials and related efforts to obtain regulatory approval for SBP-101.
We charge research and development costs, including clinical trial costs, to expense when incurred. Our human clinical trials are, and will be, performed at clinical trial sites and are administered jointly by us with assistance from contract research organizations (“CROs”). Costs of setting up clinical trial sites are accrued upon execution of the study agreement. Expenses related to the performance of clinical trials generally are accrued based on contracted amounts and the achievement of agreed upon milestones, such as patient enrollment, patient follow-up, etc. We monitor levels of performance under each significant contract, including the extent of patient enrollment and other activities through communications with the clinical trial sites and CROs, and adjust the estimates, if required, on a quarterly basis so that clinical expenses reflect the actual effort expended at each clinical trial site and by each CRO.
We expense costs associated with obtaining licenses for patented technologies when it is determined there is no alternative future use of the intellectual property subject to the license.
Fair value determination of the company’s common stock
Prior to becoming a public company, determining the fair value per share or our common stock for use in estimating the fair values of share based payments required making complex and subjective judgments. The Company used the implied valuations based upon the terms from our sales of convertible notes payable to estimate our enterprise value for the dates on which these transactions occurred. The estimated enterprise values considered certain discounts related to control and lack of marketability.
Our Board of Directors also considered the estimated fair value of our common stock in relation to a number of objective and subjective factors, including external market conditions affecting the biotechnology industry sector. Our board of directors also retained an independent financial valuation firm to provide independent estimates of our enterprise value. Until an active trading market develops for our common stock, estimating the fair value per share of our common stock will continue to be highly subjective. There is inherent uncertainty in these estimates.
Share-based compensation
In accounting for share-based incentive awards we measure and recognize the cost of employee and non-employee services received in exchange for awards of equity instruments based on the grant date fair value of those awards. Compensation cost is recognized ratably using the straight-line attribution method over the expected vesting period, which is considered to be the requisite service period.
The fair value of share-based awards is estimated at the date of grant using the Black-Scholes option pricing model. Risk free interest rates are based upon U.S. Treasury rates appropriate for the expected term of each award. Expected volatility and forfeiture rates are based primarily on the volatility rates of a set of guideline companies, which consist of public and recently public biotechnology companies. The assumed dividend yield is zero, as we do not expect to declare any dividends in the foreseeable future. The expected term of options granted is determined using the “simplified” method. Under this approach, the expected term is presumed to be the mid-point between the average vesting date and the end of the contractual term.
Foreign Currency Translation
The functional currency of Sun BioPharma Australia Pty Ltd is the Australian Dollar (“AUD”). Accordingly, assets and liabilities, and equity transactions of Sun BioPharma Australia Pty Ltd are translated into U.S. dollars at period-end exchange rates. Expenses are translated at the average exchange rate in effect for the period. The resulting translation gains and losses are recorded as a component of accumulated comprehensive loss in the Consolidated Statements of Operations and Comprehensive Loss. During the years ended December 31, 2015 and 2014, any reclassification adjustments from accumulated other comprehensive loss to operations were inconsequential.
Comprehensive Loss
Comprehensive loss consists of our net loss and the effect of foreign currency translation.
Net Loss per Share
We compute net loss per share by dividing our net loss (the numerator) by the weighted-average number of common shares outstanding (the denominator) during the period. Shares issued during the period and shares reacquired during the period are weighted for the portion of the period that they were outstanding. The computation of diluted earnings per share, or EPS, is similar to the computation of basic EPS except that the denominator is increased to include the number of additional common shares that would have been outstanding if the dilutive potential common shares had been issued. Diluted EPS is the same as basic EPS due to common equivalent shares being excluded from the calculation, as their effect is anti-dilutive.
The following table summarizes our calculation of net loss per common share for the periods (in thousands, except share and per share data):
| December 31, | ||||||||
|
2015 |
2014 |
|||||||
|
Net loss |
$ | (4,927 | ) | $ | (3,531 | ) | ||
|
Weighted average shares outstanding—basic and diluted |
14,073,174 | 5,109,644 | ||||||
|
Basic and diluted net loss per share |
$ | (0.35 | ) | $ | (0.69 | ) | ||
The following outstanding potential common shares were not included in the diluted net loss per share calculations as their effects were not dilutive:
| Year Ended December 31, | ||||||||
|
2015 |
2014 |
|||||||
|
Employee and non-employee stock options |
3,463,600 | 5,487,752 | ||||||
|
Common shares issuable upon conversion of notes payable |
2,466,667 | 2,666,668 | ||||||
|
Common shares issuable under common stock purchase warrants |
2,550,000 | 4,550,000 | ||||||
| 8,480,267 | 12,704,420 | |||||||
Recently Issued Accounting Pronouncement
In April 2015, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2015-03, Interest - Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs. Entities that have historically presented debt issuance costs as an asset, related to a recognized debt liability, will be required to present those costs as a direct deduction from the carrying amount of that debt liability. This presentation will result is debt issuance cost being presented the same way debt discounts have historically been handled. The ASU does not change the recognition, measurement, or subsequent measurement guidance for debt issuance costs. This guidance will be effective for interim and annual reporting periods beginning after December 15, 2015 (our fiscal 2016). We plan to adopt this guidance in our fiscal year beginning on January 1, 2016 and do not expect the adoption of ASU 2015-03 to have a material impact on our consolidated financial statements.
In February 2016, the FASB issued ASU 2016-02, Leases. The guidance in ASU 2016-02 supersedes the lease recognition requirements in ASC Topic 840, Leases. ASU 2016-02 requires an entity to recognize assets and liabilities arising from a lease for both financing and operating leases, along with additional qualitative and quantitative disclosures. ASU 2016-02 is effective for fiscal years beginning after December 15, 2018, with early adoption permitted. The Company is currently evaluating the effect this standard will have on its Consolidated Financial Statements
5. Fair Value of Financial Instruments
We apply the provisions of FASB ASC Topic 820, Fair Value Measurement, which defines fair value, establishes a framework for measuring fair value under GAAP, and enhances disclosures about fair value measurements.
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between knowledgeable and willing market participants. Valuation techniques used to measure fair value, as required by ASC Topic 820, must maximize the use of observable inputs and minimize the use of unobservable inputs.
The standard describes a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value. Our assessment of the significance of a particular input to the fair value measurements requires judgment, and may affect the valuation of the assets and liabilities being measured and their placement within the fair value hierarchy. The three levels of input are:
|
|
Level 1—Quotedprices in active markets for identical assets or liabilities. |
| Level 2—Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. | |
| Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
Our cash and equivalents and short-term investments consist of bank deposits and, at December 31, 2014, money market funds. Our money market funds are traded in active markets and are recorded at fair value based upon quoted market prices.
Other financial instruments, including accounts payable and accrued liabilities, are carried at cost, which we believe approximates fair value because of the short-term maturity of these instruments.
There were no financial assets measured at fair value at December 31, 2015.
A summary of financial assets (in thousands) measured at fair value on a recurring basis at December 31, 2014 is as follows:
|
December 31, 2014 |
||||||||||||||||
|
Total |
Quoted Prices In Active Markets (Level 1) |
Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
|||||||||||||
|
Money market funds |
$ | 500 | $ | 500 | $ | — | $ | — | ||||||||
6. Indebtedness
Long-term debt
On October 26, 2012, SBR entered into an unsecured loan agreement (the ”Agreement”) with the Institute for Commercialization of Public Research, Inc. (the "Institute"). Under the terms of the agreement, SBR borrowed $300,000 at a fixed interest rate of 4.125%. No principal or interest payments are due until the maturity date, October 26, 2017, unless a mandatory repayment event occurs. A mandatory repayment event includes, (i) a liquidity event defined as a sale of all or substantially all of the assets of SBR; a merger, consolidation, share exchange or similar transaction as a result of which the persons holding SBR equity constituting a majority of the outstanding equity by voting power or economic participation immediately prior to the transaction hold less than a majority of such voting power or economic participation immediately after such transaction; or a sale or transfer of outstanding equity of SBR in a transaction as a result of which the persons holding SBR equity constituting a majority of the outstanding equity by voting power or economic participation immediately prior to the transaction hold less than a majority of such voting power or economic participation immediately after such transaction, (ii) an event of default, (iii) a failure to maintain a Florida base of operations for more than 6 months, (iv) a sale or transfer of licensed technology, (v) any false representation to the Institute, (vi) a violation of law by SBR or one of its principal officers, or (vii) an achievement of aggregate revenues during any fiscal year of more than $4,000,000 from sales of products and/or services. The Long-term debt was assumed by Cimarron in connection with the Merger.
Demand notes payable
In conjunction with the Merger, and after giving effect to the disposition of the nominal business operations of Cimarron on September 28, 2015, we assumed $250,000 of unsecured demand notes that were previously issued by Cimarron. These demand notes have no stated interest rate or maturity date and accordingly are reported as current liabilities in our consolidated balance sheet. See Note 9 below for additional information regarding the Merger.
Convertible notes payable
In the fourth quarter of 2013, SBR initiated an offering of convertible promissory notes (the “Convertible Notes” or “New Notes”). In total, gross proceeds raised were $3.1 million of which $700,000 and $2.4 million were raised in December 2013 and January 2014, respectively. The Convertible Notes accrue interest at 5% per year, payable quarterly, are convertible into shares of common stock at $1.125 per share at the option of the holder and mature in December 2018.
Sale of the New Notes was contingent upon (i) the conversion of $2.3 million of then outstanding convertible notes (the “Old Notes”) into common stock at $0.25 per share, (ii) fixing the number of warrants issuable to the holders of the Old Notes at 50% of the then then outstanding convertible notes, with those warrants exercisable at $0.25 per share, (iii) the issuance of employment agreements to the four individuals leading the new financing round, providing compensation in the form of option grants, and (iv) raising a minimum of $3,000,000 from the sale of the New Notes. These conditions were satisfied in January 2014, however management, having received verbal commitments for $3.0 million of New Note subscriptions on December 27, 2013, concluded that the satisfaction of the conditions for the sale of the New Notes was probable of being achieved, recorded the conversion of the Old Notes, and accrued but unpaid interest, into common stock on that date, in accordance with accounting principles generally accepted in the United States of America. Conversion of the Old Notes and related accrued interest resulted in the issuance of 9,639,116 shares of SBR common stock, and warrants to purchase a total of 4,650,000 shares of SBR common stock at $0.25 per share. These stock purchase warrants expire on December 27, 2023.
In 2015 and 2014, holders of the Convertible Notes converted $225,000 and $100,000, respectively, plus accrued interest, into 200,776 and 90,020 shares, respectively, of SBR common stock. In addition, in 2015 and 2014, 2,000,000 and 100,000, respectively, warrants were exercised. See Note 8 for additional information.
The Convertible Notes were assumed by Cimarron in connection with the Merger. See Note 8 for more information regarding treatment of the Convertible Notes in the Merger
7. Commitments and Contingencies
License agreement
On December 22, 2011, SBR entered into an exclusive license agreement with the university of Florida research foundation (“UFRF”), which was acquired in exchange for $15,000 in cash and the issuance of 10% of its common stock. Upon executing the license agreement, 800,000 shares of common stock were issued to UFRF which was determined to have a fair value of $20,000 based upon an estimated fair value of SBR’s common stock of $0.025 per share. The license agreement also contained an anti-dilution provision which required SBR to issue additional shares to UFRF sufficient for UFRF to maintain its 10% ownership interest in SBR until SBR secured an addition $2.0 million external investment in SBR. This investment was received during 2012.
The license agreement requires the company to pay royalties to UFRF ranging from 2.5% to 5% of net sales of licensed products developed from the licensed technology. Minimum annual royalties are required after the initial occurrence of a commercial sale of a marketed product. Royalties are payable for the longer of (i) the last to expire of the claims in the licensed patents or (ii) ten (10) years from the first commercial sale of a licensed product in each country in which licensed product is sold. The minimum annual royalties are as follows:
|
● |
$50,000 is due 270 days after occurrence of first commercial sale; |
|
● |
$100,000 is due on the first anniversary date of the first payment; |
|
● |
$100,000 is due on the second anniversary date of the first payment; and |
|
● |
$300,000 is due on the third anniversary date of the first payment and subsequent anniversary dates thereafter, continuing for the life of the license agreement. |
In addition, the company is subject to six different milestone payments under the license agreement.
|
● |
$50,000 is due upon enrollment of the first subject in a phase 1 clinical trial; |
|
● |
$300,000 is due upon enrollment of the first subject in a phase ii clinical trial; |
|
● |
$3,000,000 is due upon approval of a new drug application; |
|
● |
$2,000,000 is due upon approval to manufacture and market in either the European union or japan (one time only); |
|
● |
$1,000,000 is due upon the first time annual net sales of licensed product or licensed process by the company reaches $100,000,000; and |
|
● |
$3,000,000 is due upon the first time annual net sales of licensed product or licensed process by the company reaches $500,000,000. |
The license agreement is subject to customary and usual termination provisions. The license agreement was assumed by Cimarron in connection with the Merger. As of December 31, 2015 and 2014, no royalty or milestone payments were due. The Company is also committed to pay an annual license maintenance fee of $10,000.
Clinical Trials
We are currently conducting a Phase 1 study in patients with pancreatic cancer, for a duration of approximately 24 months. The first patient was enrolled in January 2016. This study is expected to include a dose-escalation phase with 8-week cycles of treatment at each dose level. At least two cycles of therapy at each dose level are anticipated in this trial, with continued treatment permitted for patients with clinical responses or stable disease. The projected safety profile suggests that repeat cycles would be well tolerated. Additional clinical trials will be subsequently required if the results of the Phase 1 pancreatic cancer trial are positive. We estimate the total time and cost to obtain FDA and EU approval and bring SBP-101 to market is 6 to 7 years and up to two-hundred million dollars ($200 million). Clinical trial costs are expensed as incurred.
Indemnification of Directors and Officers
The bylaws of the Company provide that it will indemnify and advance expenses to its directors and officers to the fullest extent permitted by law or, if applicable, pursuant to indemnification agreements. They further provide that we may choose to indemnify other employees or agents of our Company from time to time. Section 16-10a-908 of the Utah Revised Business Corporation Act and the Company’s bylaws permit it to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in connection with their services to the Company, regardless of whether the bylaws permit indemnification. We maintain a directors’ and officers’ liability insurance policy for that purpose. As of December 31, 2015 there was no pending litigation or proceeding involving any director or officer of the Company as to which indemnification is required or permitted, and we are not aware of any threatened litigation or proceeding that may result in a claim for indemnification. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Company, the Company has been advised that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
The Company believes the fair value of these indemnification agreements is minimal. Accordingly, the Company had not recorded any liabilities for these obligations as of December 31, 2015 or 2014.
8. Shareholders’ Equity
Cimarron Medical, Inc. Merger Transaction
On June 12, 2015, SBR entered into an Agreement and Plan of Merger (the “Merger”) with Cimarron and SB Acquisition Corporation, a wholly owned subsidiary of Cimarron. The resulting merger of SB Acquisition Corporation with and into SBR on September 4, 2015, resulted in all of the issued and outstanding common stock of SBR being converted into the right to receive an aggregate of 28,442,484 shares of Cimarron’s common stock, representing four shares of Cimarron common stock for every one share of SBR common stock cancelled in the Merger. All of the shares of common stock issued pursuant to the Merger are “restricted securities” under Rule 144. As a result of this transaction, former SBR shareholders owned approximately 98.8% of the outstanding capital stock, giving SBR’s former shareholders substantial control of Cimarron. In connection with the Merger, Cimarron’s Board of Directors and management team were replaced by members of SBR’s Board of Directors and management team and Cimarron’s name was changed to “Sun BioPharma, Inc.”
In addition, outstanding options and warrants to purchase SBR common stock before the Merger were converted into options and warrants to purchase an aggregate of 5,043,600 shares and 2,550,000 shares, respectively, of Cimarron’s common stock. Approximately $2.8 million aggregate principal amount of SBR outstanding convertible promissory notes were converted into convertible promissory notes payable by Cimarron and convertible into shares of Cimarron common stock at a rate of $1.125 per share. Immediately prior to the Merger, Cimarron had 1,450,322 shares of common stock outstanding with no other capital stock or rights to acquire additional shares outstanding.
Under GAAP, SBR was deemed to be the acquirer for accounting purposes because its former shareholders owned a substantial majority of the issued and outstanding shares of Cimarron’s common stock after the Merger. Further, as Cimarron’s business operations and net assets, at the time of the Merger, were nominal relative to SBR’s business operations and net assets, we have accounted for the Merger as a capital transaction.
SBR incurred approximately $325,000 of costs associated with the Merger and assumed $250,000 of demand notes payable, net, after giving effect to the disposition of the legacy business operations of Cimarron, discussed below. The transaction costs for the Merger are included in general and administrative expenses in our Consolidated Statements of Operations and Comprehensive Loss.
Sale of Legacy Cimarron Medical Business Operations
On September 28, 2015, we sold all of our ownership interest in the legacy business operations of Cimarron, which previously had been contributed to our then wholly owned subsidiary, Cimarron Medical Software, Inc., to Sampleminded, Inc. In exchange, Sampleminded, Inc. agreed to assume our payment obligations under approximately $305,000 of aggregate principal amount of outstanding promissory notes.
Authorized Capital Stock
Our Amended and Restated Articles of Incorporation, as amended authorize our Company to issue up to 110,000,000 shares of capital stock, with 100,000,000 shares designated as common stock, $.001 par value per share, and the remaining 10,000,000 shares available for designation and issuance as shares of preferred stock, $.001 par value per share.
Private Placement
Pursuant to the June 12, 2015 Agreement and Plan of Merger, SBR was obligated to undertake efforts to engage in a private placement of its common stock. On September 4, 2015, immediately prior to the closing of the Merger, SBR sold shares of its common stock for total proceeds of $1,513,000, net of offering costs, which shares ultimately resulted in the issuance of an incremental 762,500 shares of Cimarron common stock in the Merger.
Warrants
In April 2015, the Board of Directors of SBR agreed to reduce the exercise price of outstanding warrants issued in connection with certain notes payable from $0.25 per share to $0.1875 per share. This exercise price modification resulted in the recognition of a deemed dividend of $170,625, which was charged to accumulated deficit and credited to additional paid-in-capital. In 2015, SBR received $375,000 from warrant holders who exercised warrants at the reduced price. In 2014, SBR received $25,000 from warrant exercises. These exercises ultimately resulted in the issuance of an incremental 2,100,000 shares of Cimarron common stock in the Merger. As of December 31, 2015, warrants exercisable for 2,550,000 shares remain outstanding.
Shares Reserved
Shares of common stock reserved for future issuance are as follows:
|
December 31, 2015 |
||||
|
Stock options outstanding |
3,463,600 | |||
|
Shares available for grant under equity incentive plan |
5,722,264 | |||
|
Common shares issuable upon conversion of notes payable |
2,466,667 | |||
|
Common shares issuable under common stock purchase warrants |
2,550,000 | |||
|
Total |
14,202,531 | |||
9. Share-Based Compensation
The Sun BioPharma, Inc. 2011 Stock Option Plan (the “Plan”) was adopted by the SBR Board of Directors in September, 2011 and approved by SBR shareholders in January, 2012. We assumed the Plan as part of the Merger. The Plan permits the granting of incentive and non-statutory stock options, restricted stock, stock appreciation rights, performance units, performance shares and other stock awards to eligible employees, directors and consultants. We grant options to purchase shares of common stock under the Plan at no less than the fair market value of the underlying common stock as of the date of grant. Options granted under the Plan have a maximum term of ten years and generally vest over zero to two years for employees. Under the Plan, a total of 14,000,000 shares of common stock were originally reserved for issuance. As of December 31, 2015, 5,722,264 shares remained available for the issuance of future grants under the Plan and options to purchase 3,463,600 shares of common stock were outstanding under the Plan.
We recognize share-based compensation based on the value of the portion of awards that are ultimately expected to vest. Guidance requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The term “forfeitures” is distinct from “cancellations” or “expirations” and represents only the unvested portion of a surrendered option. We will re-evaluate this estimate periodically and adjust the forfeiture rate on a prospective basis as necessary. Ultimately, the actual expense recognized over the vesting period will only be for those shares that actually vest.
A summary of option activity is as follows:
|
Shares Underlying Options |
Weighted Average Exercise Price Per Share |
|||||||
|
Options outstanding at December 31, 2013 |
6,851,352 | $ | 0.24 | |||||
|
Granted |
780,000 | 0.24 | ||||||
|
Exercised |
(2,143,600 | ) | 0.23 | |||||
|
Cancelled |
— | — | ||||||
|
Forfeitures |
— | — | ||||||
|
Options outstanding at December 31, 2014 |
5,487,752 | $ | 0.24 | |||||
|
Granted |
5,340,000 | 0.32 | ||||||
|
Exercised |
(2,590,536 | ) | 0.20 | |||||
|
Cancelled |
(4,773,616 | ) | 0.22 | |||||
|
Forfeitures |
— | — | ||||||
|
Options outstanding at December 31, 2015 |
3,463,600 | $ | 0.27 | |||||
|
Options exercisable at December 31, 2015 |
3,463,600 | $ | 0.27 | |||||
A summary of the status of our unvested shares during the year ended and as of December 31, 2015 is as follows:
|
Shares Under Option |
Weighted Average Grant-Date Fair Value |
|||||||
|
Unvested at December 31, 2014 |
2,224 | $ | 0.06 | |||||
|
Granted |
5,340,000 | 0.18 | ||||||
|
Vested |
(5,342,224 | ) | 0.18 | |||||
|
Forfeitures |
— | — | ||||||
|
Unvested at December 31, 2015 |
— | $ | — | |||||
Information about stock options outstanding, vested and expected to vest as of December 31, 2015, is as follows:
|
Outstanding, Vested and Expected to Vest |
Options Vested |
|||||||||||||||||||||
|
Weighted Average |
Weighted Average |
|||||||||||||||||||||
|
Remaining |
Weighted |
Remaining |
||||||||||||||||||||
|
Per Share |
Contractual |
Average |
Options |
Contractual |
||||||||||||||||||
|
Exercise Price |
Shares |
Life (Years) |
Exercise Price |
Exercisable |
Life (Years) |
|||||||||||||||||
| $ 0.09 | – |
0.11 |
563,600 | 6.85 | $ | 0.10 | 563,600 | 6.85 | ||||||||||||||
| 0.23 | – |
0.25 |
460,000 | 8.11 | 0.25 | 460,000 | 8.11 | |||||||||||||||
| 0.32 |
|
2,440,000 | 9.18 | 0.32 | 2,440,000 | 9.18 | ||||||||||||||||
| 3,463,600 | 8.66 | $ | 0.27 | 3,463,600 | 8.66 | |||||||||||||||||
The cumulative grant date fair value of employee options vested during the years ended December 31, 2015 and 2014 was $933,000 and $105,000, respectively. Total proceeds received for options exercised during the years ended December 31, 2015 and 2014 were $693,000 and $493,000, respectively.
The assumptions used in calculating the fair value under the Black-Scholes option valuation model are set forth in the following table for options issued by the Company for the years ended December 31, 2015, 2014 and 2013:
|
2015 |
2014 |
|||||||||||
|
Common stock fair value |
$0.32 | $0.11 | - | $0.23 | ||||||||
|
Risk-free interest rate |
1.57% | - | 1.61% | 0.75% | - | 1.76% | ||||||
|
Expected dividend yield |
0% | 0% | ||||||||||
|
Expected option life (years) |
5.0 | 5.0 | ||||||||||
|
Expected stock price volatility |
62.60% | - | 64.59% | 69.37% | - | 70.93% | ||||||
Nonemployee Stock-Based Compensation
We account for stock options granted to nonemployees in accordance with FASB ASC 505. In connection with stock options granted to nonemployees, which were fully vested upon issuance, we recorded $70,000 and $26,000 for nonemployee stock-based compensation during the years ended December 31, 2015 and 2014, respectively.
Stock-Based Payments
In the first quarter of 2015, our Board of Directors authorized the issuance of 132,964 shares of our common stock to two vendors who agreed to provide services to the Company upon terms that provided for a portion of their consideration to be paid in shares of our common stock. The fair value of each share of common stock was determined by our Board of Directors, and accordingly, we recorded an expense of $42,000.
In the first quarter of 2014, we engaged an outside consultant to provide certain services to us upon terms that provided for a portion of the consideration to be paid in shares of our common stock. In conjunction with this agreement, our Board of Directors authorized the issuance of 400,000 shares of our common stock. The fair value of each share of common stock was determined by our Board of Directors, and accordingly, we recorded an expense of $91,000.
10. Income Taxes
We have incurred net operating losses since inception. We have not reflected the benefit of net operating loss carryforwards in the accompanying financial statements and have established a full valuation allowance against our deferred tax assets.
At December 31, 2015 and 2014, the Company had an income tax receivable of $733,000 and $108,000, respectively, comprised of refundable tax credits related to research and development activities of our subsidiary Sun BioPharma Australia Pty Ltd.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes, and operating losses and tax credit carryforwards.
The significant components of our deferred tax assets and liabilities are as follows (in thousands):
| December 31, | ||||||||
|
2015 |
2014 |
|||||||
|
Deferred tax assets: |
||||||||
|
Net operating loss carryforwards |
$ | 3,395 | $ | 2,395 | ||||
|
Research credit carryforwards |
236 | 152 | ||||||
|
Accrued expenses |
— | 35 | ||||||
|
Share-based compensation |
148 | 38 | ||||||
|
Other |
32 | — | ||||||
|
Total deferred tax assets |
3,811 | 2,620 | ||||||
|
Valuation allowance |
(3,811 | ) | (2,620 | ) | ||||
|
Net deferred tax asset |
$ | — | $ | — | ||||
Realization of the future tax benefits is dependent on our ability to generate sufficient taxable income within the carry-forward period. Because of our history of operating losses, management believes that the deferred tax assets arising from the above-mentioned future tax benefits are currently not likely to be realized and, accordingly, we have provided a full valuation allowance. The net valuation allowance increased by $1.2 million and $1.3 million for the years ended December 31, 2015 and 2014, respectively.
A reconciliation of the statutory tax rates and the effective tax rates is as follows:
|
Year Ended December 31, |
||||||||
|
2015 |
2014 |
|||||||
|
Statutory rate |
34.0% | 34.0% | ||||||
|
Permanent differences |
(10.3) | (1.6) | ||||||
|
State and local income taxes |
0.1 | — | ||||||
|
Credits and other |
(0.1) | 6.1 | ||||||
|
State tax rate true-up |
5.3 | — | ||||||
|
Valuation allowance |
(29.0) | (38.5) | ||||||
|
Effective rate |
0.0% | 0.0% | ||||||
Net operating losses and tax credit carryforwards as of December 31, 2015, are as follows:
|
Amount (In thousands) |
Expiration Years |
|||||||
|
Net operating losses—federal |
$ | 9,935 | Beginning 2031 | |||||
|
Tax credits—federal |
236 | Beginning 2041 | ||||||
Utilization of the net operating loss carryforwards and credits may be subject to a substantial annual limitation due to the ownership change limitations provided by the Internal Revenue Code of 1986, as amended (the “IRC”), and similar state provisions. We have not performed a detailed analysis to determine whether an ownership change under Section 382 of the IRC has occurred. The effect of an ownership change would be the imposition of an annual limitation on the use of net operating loss carryforwards attributable to periods before the change.
The Company is subject to taxation in the United States and Australia. Tax returns, since the inception of Sun BioPharma, Inc. in 2011 and thereafter, are subject to examinations by federal and state tax authorities and may change upon examination. Tax returns of Sun BioPharma Australia Pty Ltd. for the year ended December 31, 2013 and thereafter are subject to examination by the Australian tax authorities.
11. Subsequent Event
On January 4, 2016, we enrolled the first patient in our Phase 1 clinical trial of SBP-101 in patients with previously treated pancreatic cancer. Under the terms of our License Agreement with the University of Florida Research Foundation, a milestone obligation of $50,000 is due and payable based upon this first enrollment and accordingly we recorded this obligation as a license expense as of this date. Due to our current financial condition, we have requested, and UFRF has agreed, to provide us 90 day payment terms for this obligation.
PART I – FINANCIAL INFORMATION
Item 1. Financial Statements.
Sun BioPharma, Inc.
Condensed Consolidated Balance Sheets
(In thousands, except share amounts)
|
June 30, 2016 |
December 31, 2015 |
|||||||
| (Unaudited) | ||||||||
|
ASSETS |
|
|||||||
|
Current assets: |
||||||||
|
Cash and cash equivalents |
$ | 1,898 | $ | 925 | ||||
|
Prepaid expenses and other current assets |
53 | 74 | ||||||
|
Income tax receivable |
193 | 733 | ||||||
|
Total current assets |
2,144 | 1,732 | ||||||
|
Total assets |
$ | 2,144 | $ | 1,732 | ||||
|
LIABILITIES AND STOCKHOLDERS’ DEFICIT |
||||||||
|
Current liabilities: |
||||||||
|
Accounts payable |
$ | 797 | $ | 585 | ||||
|
Accrued liabilities |
643 | 505 | ||||||
|
Demand notes payable |
250 | 250 | ||||||
|
Accrued interest |
35 | 35 | ||||||
|
Total current liabilities |
1,725 | 1,375 | ||||||
|
Long-term liabilities: |
||||||||
|
Convertible notes payable, net |
2,722 | 2,712 | ||||||
|
Long-term debt, net |
291 | 287 | ||||||
|
Accrued interest |
45 | 39 | ||||||
|
Total long-term liabilities |
3,058 | 3,038 | ||||||
|
Commitments and contingencies (Note 6) |
||||||||
|
Stockholders’ deficit: |
||||||||
|
Preferred stock, $0.001 par value; 20,000,000 and 10,000,000 authorized as of June 30, 2016 and December 31, 2015, respectively; no shares issued or outstanding as of June 30, 2016 and December 31, 2015 |
— | — | ||||||
|
Common stock, $0.001 par value; 200,000,000 and 100,000,000 authorized as of June 30, 2016 and December 31, 2015, respectively; 31,881,306 and 29,892,806 shares issued and outstanding, as of June 30, 2016 and December 31, 2015, respectively |
32 | 30 | ||||||
|
Additional paid-in capital |
12,815 | 10,943 | ||||||
|
Accumulated deficit |
(15,466 | ) | (13,667 | ) | ||||
|
Accumulated comprehensive (loss) gain, net |
(20 | ) | 13 | |||||
|
Total stockholders’ deficit |
(2,639 | ) | (2,681 | ) | ||||
|
Total liabilities and stockholders’ deficit |
$ | 2,144 | $ | 1,732 | ||||
See accompanying notes to condensed consolidated financial statements.
Sun BioPharma, Inc.
Condensed Consolidated Statements of Operations and Comprehensive Loss
(In thousands, except share and per share amounts)
(Unaudited)
|
Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
|
2016 |
2015 |
2016 |
2015 |
|||||||||||||
|
Operating expenses |
||||||||||||||||
|
General and administrative |
$ | 419 | $ | 585 | $ | 900 | $ | 1,788 | ||||||||
|
Research and development |
530 | 608 | 1,024 | 1,596 | ||||||||||||
|
Operating loss |
(949 | ) | (1,193 | ) | (1,924 | ) | (3,384 | ) | ||||||||
|
Other income (expense): |
||||||||||||||||
|
Interest income |
1 | 3 | 2 | 5 | ||||||||||||
|
Interest expense |
(45 | ) | (46 | ) | (90 | ) | (86 | ) | ||||||||
|
Other income (expense) |
(75 | ) | (17 | ) | 7 | (37 | ) | |||||||||
|
Total other income (expense) |
(119 | ) | (60 | ) | (81 | ) | (118 | ) | ||||||||
|
Loss before income tax benefit |
$ | (1,068 | ) | $ | (1,253 | ) | $ | (2,005 | ) | $ | (3,502 | ) | ||||
|
Income tax benefit |
90 | 38 | 206 | 95 | ||||||||||||
|
Net loss |
(978 | ) | (1,215 | ) | (1,799 | ) | (3,407 | ) | ||||||||
|
Foreign currency translation adjustment gain (loss) |
42 | (9 | ) | (33 | ) | (13 | ) | |||||||||
|
Comprehensive loss |
$ | (932 | ) | $ | (1,224 | ) | $ | (1,831 | ) | $ | (3,420 | ) | ||||
|
Basic and diluted net loss per share |
$ | (0.03 | ) | $ | (0.18 | ) | $ | (0.06 | ) | $ | (0.55 | ) | ||||
|
Weighted average shares outstanding – basic and diluted |
30,126,755 | 6,585,533 | 30,058,942 | 6,225,722 | ||||||||||||
See accompanying notes to condensed consolidated financial statements.
Sun BioPharma, Inc.
Condensed Consolidated Statements of Stockholders’ Deficit
(In thousands except share and per share amounts)
(Unaudited)
|
Accumulated |
||||||||||||||||||||||||
|
Additional |
Other |
Total |
||||||||||||||||||||||
|
Common Stock |
Paid-In |
Accumulated |
Comprehensive |
Stockholders’ |
||||||||||||||||||||
|
Shares |
Amount |
Capital |
Deficit |
Loss |
Deficit |
|||||||||||||||||||
|
Balances at December 31, 2015 |
29,892,806 | $ | 30 | $ | 10,943 | $ | (13,667 | ) | $ | 13 | $ | (2,681 | ) | |||||||||||
|
Net loss |
— | — | — | (1,799 | ) | — | (1,799 | ) | ||||||||||||||||
|
Foreign currency translation adjustment, net of taxes of $0 |
— | — | — | — | (33 | ) | (33 | ) | ||||||||||||||||
|
Issuance of common stock and warrants, net of offering costs of $152 |
1,951,000 | 2 | 1,797 | — | — | 1,799 | ||||||||||||||||||
|
Issuance of common stock for services |
37,500 | — | 75 | — | — | 75 | ||||||||||||||||||
|
Balances at June 30, 2016 |
31,881,306 | $ | 32 | $ | 12,815 | $ | (15,466 | ) | $ | (20 | ) | $ | (2,639 | ) | ||||||||||
See accompanying notes to consolidated financial statements.
Sun BioPharma, Inc.
Condensed Consolidated Statements of Cash Flows
(In thousands)
(Unaudited)
|
Six Months Ended June 30, |
||||||||
| 2016 | 2015 | |||||||
|
Cash flows from operating activities: |
||||||||
|
Net loss |
$ | (1,799 | ) | $ | (3,407 | ) | ||
|
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
|
Amortization of debt issuance costs |
14 | 14 | ||||||
|
Non-cash interest expense |
6 | 7 | ||||||
|
Stock-based compensation |
— | 976 | ||||||
|
Unrealized gain on investment |
— | (3 | ) | |||||
|
Changes in operating assets and liabilities: |
||||||||
|
Income and other tax receivables |
563 | 10 | ||||||
|
Prepaid expenses and other current assets |
85 | (4 | ) | |||||
|
Accounts payable and accrued liabilities |
500 | 266 | ||||||
|
Net cash used in operating activities |
(631 | ) | (2,141 | ) | ||||
|
Cash flows from financing activities: |
||||||||
|
Proceeds from issuance of common stock and warrants, net of offering costs of $152 |
1,603 | — | ||||||
|
Proceeds from issuance of common stock |
— | 350 | ||||||
|
Proceeds from the exercise of stock options |
— | 762 | ||||||
|
Proceeds from the exercise of stock purchase warrants |
— | 400 | ||||||
|
Net cash provided by financing activities |
1,603 | 1,512 | ||||||
|
Effect of exchange rate changes on cash and cash equivalents |
1 | (19 | ) | |||||
|
Net increase (decrease) in cash and cash equivalents |
973 | (648 | ) | |||||
|
Cash and cash equivalents at beginning of period |
925 | 1,653 | ||||||
|
Cash and cash equivalents at end of period |
$ | 1,898 | $ | 1,005 | ||||
|
Supplemental disclosure of cash flow information: |
||||||||
|
Cash paid during period for interest |
$ | 70 | $ | 69 | ||||
|
Supplemental disclosure of non-cash transactions: |
||||||||
|
Deferred compensation exchanged for common stock and warrants |
$ | 196 | — | |||||
|
Issuance of common stock for services |
$ | 75 | — | |||||
See accompanying notes to condensed consolidated financial statements.
Sun BioPharma, Inc.
Notes to Condensed Consolidated Financial Statements
1. Business
Sun BioPharma, Inc. and its wholly-owned subsidiary Sun BioPharma Australia Pty Ltd. (collectively “we,” “us,” “our,” and the “Company”) exist for the primary purpose of advancing the commercial development of a proprietary polyamine analogue for pancreatic cancer and for a second indication in chronic pancreatitis. We have exclusively licensed the worldwide rights to this compound, which has been designated as SBP-101, from the University of Florida Research Foundation, Inc. (“UFRF”).
2. Risks and Uncertainties
We operate in a highly regulated and competitive environment. The development, manufacturing and marketing of pharmaceutical products require approval from, and are subject to ongoing oversight by, the Food and Drug Administration (“FDA”) in the United States, the Therapeutic Goods Administration (“TGA”) in Australia, the European Medicines Agency (“EMA”) in the European Union, and comparable agencies in other countries. Obtaining approval for a new pharmaceutical product is never certain, may take many years, and is normally expected to involve substantial expenditures.
We have incurred losses of $15.5 million since our inception in 2011. For the six months ended June 30, 2016, we incurred a net loss and negative cash flows from operating activities of $1.8 million and $631,000, respectively. We expect to incur substantial losses for the foreseeable future, which will continue to generate negative cash flows from operating activities, as we continue to pursue research and development activities and seek to commercialize our initial product candidate, SBP-101. As of June 30, 2016, we had cash and cash equivalents of $1.9 million, working capital of $419,000 and stockholders’ deficit of $2.6 million. The Company’s principal sources of cash have historically included the issuance of convertible debt and equity securities.
The accompanying condensed consolidated financial statements have been prepared assuming that we will continue as a going concern which contemplates the realization of assets and liquidation of liabilities in the normal course of business. The condensed consolidated financial statements do not include any adjustments relating to the recoverability or classification of assets or the amounts of liabilities that might result from the outcome of these uncertainties. Our current independent registered public accounting firm, included a paragraph emphasizing this going concern uncertainty in their audit report on our 2015 financial statements dated March 8, 2016. Our ability to continue as a going concern, realize the carrying value of our assets and discharge our liabilities in the ordinary course of business is dependent upon a number of factors, including our ability to obtain additional financing, the success of our development efforts, our ability to obtain marketing approval for our SBP-101 product candidate in the United States, Australia, the European Union or other markets and ultimately our ability to market and sell our SBP-101 product candidate. These factors, among others, raise substantial doubt about our ability to continue operations as a going concern. See note 4 entitled “Liquidity and Management’s Plans.”
3. Basis of Presentation
We have prepared the accompanying interim condensed consolidated financial statements in accordance with US GAAP for interim financial information and with the instructions to Form 10-Q and Regulation S-X of the Securities and Exchange Commission (“SEC”). Accordingly, they do not include all of the information and footnotes required by US GAAP for complete financial statements. These interim condensed consolidated financial statements reflect all adjustments consisting of normal recurring accruals, which, in the opinion of management, are necessary to present fairly our consolidated financial position, consolidated results of operations and consolidated cash flows for the periods and as of the dates presented. Our fiscal year ends on December 31. The condensed consolidated balance sheet as of December 31, 2015 was derived from audited consolidated financial statements, but does not include all disclosures required by U.S. GAAP. These interim condensed consolidated financial statements should be read in conjunction with the annual consolidated financial statements and the notes thereto included in our Annual Report on Form 10-K filed with the SEC on March 8, 2016 and our other filings with the SEC. The nature of our business is such that the results of any interim period may not be indicative of the results to be expected for future interim periods or for the entire year.
Recently Adopted Accounting Pronouncement
In April 2015, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2015-03, Interest - Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs. This ASU requires debt issuance costs to be presented as a direct deduction from the carrying amount of the related debt rather than as an asset. In 2016, the company retrospectively adopted this update, as required, and the amounts reclassified from other assets to long-term debt on the condensed consolidated balance sheets. These reclassifications did not impact net income.
Recently Issued Accounting Pronouncement
In February 2016, the FASB issued ASU No. 2016-02, Leases. The guidance in ASU 2016-02 supersedes the lease recognition requirements in the Accounting Standards Codification Topic 840, Leases. ASU 2016-02 requires an entity to recognize assets and liabilities arising from a lease for both financing and operating leases, along with additional qualitative and quantitative disclosures. ASU 2016-02 is effective for fiscal years beginning after December 15, 2018, with early adoption permitted. The Company does not expect the adoption of ASU 2016-02 to have a material impact on its Consolidated Financial Statements.
In March 2016, the FASB issued ASU No. 2016-09, Improvements to Employee Stock-Based Payment Accounting. The guidance in ASU 2016-09 is intended to simplify certain aspects of the accounting for employee stock-based payments, including the accounting for income taxes, forfeitures, statutory withholding requirements, and classification on the statement of cash flows. The standard is effective for interim and annual periods beginning after December 15, 2016, with early adoption permitted. We are currently assessing the impact of this standard on our financial condition and results of operations.
4. Liquidity and Management Plans
We will need to obtain additional funds to continue our operations and execute our current business plans. We may seek to raise additional funds through various sources, such as equity and debt financings, or through strategic collaborations and license agreements. We can give no assurances that we will be able to secure additional sources of funds to support our operations, or if such funds are available to us, that such additional financing will be sufficient to meet our needs or on terms acceptable to us. This risk would increase if our clinical data is inconclusive or not positive or economic conditions worsen in the market as a whole or in the pharmaceutical or biotechnology markets individually.
On March 1, 2016 we instituted substantial salary deferrals for all senior employees in order to conserve cash. If we are unable to obtain additional financing when needed, we will need to reduce our operations by taking actions that may include, among other things, reducing use of outside professional service providers, reducing staff or further reducing staff compensation, significantly modifying or delaying the development of our SBP-101 product candidate, licensing rights to third parties, including the right to commercialize our SBP-101 product candidate for pancreatic cancer, acute pancreatitis or other applications that we would otherwise seek to pursue, or discontinuing operations entirely.
On May 5, 2016, we received a $772,000 tax rebate under the Australian R&D Incentive Rebate program related to 2015 research and development activities.
In June 2016, pursuant to Securities Purchase Agreements, we received aggregate gross proceeds of $1,755,000 from two June closings under these private placement transactions. See “Private Placement” in note 9 for additional information.
Our future success is dependent upon our ability to obtain additional financing, the success of our development efforts, our ability to obtain marketing approval for our SBP-101 product candidate in the United States or other markets and ultimately our ability to market and sell our SBP-101 product candidate. If we are unable to obtain additional financing when needed, if our clinical trials are not successful or if we are unable to obtain marketing approval, we would not be able to continue as a going concern and would be forced to cease operations and liquidate our company.
There can be no assurances that we will be able to obtain additional financing on commercially reasonable terms, or at all. The sale of additional convertible debt or equity securities would likely result in dilution to our current stockholders.
5. Summary of Significant Accounting Policies
Principles of consolidation
The accompanying condensed consolidated financial statements include the assets, liabilities and expenses of Sun BioPharma, Inc. and our wholly-owned subsidiaries. All significant intercompany transactions and balances have been eliminated in consolidation.
Use of estimates
The preparation of condensed consolidated financial statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the condensed consolidated financial statements and the reported amount of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Debt issuance costs
Costs associated with the issuance of debt instruments are capitalized and presented as a direct deduction from the carrying amount of the related debt liability. These costs are amortized on a straight-line basis, which approximates the effective interest method, over the term of the debt agreements and are included in interest expense.
Research and development costs
Research and development costs to date have consisted primarily of expenses incurred for third-party service providers monitoring and accumulating data related to our preclinical and clinical studies; sponsored research agreements; developing and scaling the manufacturing process necessary to produce sufficient amounts of SBP-101 for use in our pre-clinical studies and human clinical trials; consulting resources with specialized expertise related to execution of our development plan for our SBP-101 product candidate; and costs to license and maintain our licensed intellectual property. Moving forward, research and development expenditures will shift to focus on costs related to the execution of human clinical trials and related efforts to obtain regulatory approval for SBP-101.
We charge research and development costs, including clinical trial costs, to expense when incurred. Our human clinical trials are, and will be, performed at clinical trial sites and are administered jointly by us with assistance from contract research organizations (“CROs”). Costs of setting up clinical trial sites are accrued upon execution of the study agreement. Expenses related to the performance of clinical trials generally are accrued based on contracted amounts and the achievement of agreed upon milestones, such as patient enrollment, patient follow-up, etc. We monitor levels of performance under each significant contract, including the extent of patient enrollment and other activities through communications with the clinical trial sites and CROs, and adjust the estimates, if required, on a quarterly basis so that clinical expenses reflect the actual effort expended at each clinical trial site and by each CRO.
All material CRO contracts are terminable by us upon written notice and we are generally only liable for actual effort expended by the CROs and certain non-cancelable expenses incurred at any point of termination
We expense costs associated with obtaining licenses for patented technologies when it is determined there is no alternative future use of the intellectual property subject to the license.
Fair Value Determination of the Company’s Common Stock
Prior to becoming a public company in September 2015, determining the fair value per share or our common stock for use in estimating the fair values of stock-based payments required complex and subjective judgments. The Company used the implied valuations based upon the terms from our sales of convertible notes payable to estimate our enterprise value for the dates on which these transactions occurred. The estimated enterprise values considered certain discounts related to control and lack of marketability.
Our board of directors also considered the estimated fair value of our common stock in relation to a number of objective and subjective factors, including external market conditions affecting the biotechnology industry sector. Our board of directors also retained an independent financial valuation firm to provide independent estimates of our enterprise value. Until an active trading market develops for our common stock, estimating the fair value per share of our common stock will continue to be highly subjective. There is inherent uncertainty in these estimates.
Stock-based compensation
Stock-based incentive awards are accounted for under the provisions of FASB ASC 718, Compensation — Stock Compensation, which requires companies to measure and recognize the cost of employee and non-employee services received in exchange for awards of equity instruments based on the grant date fair value of those awards. Compensation cost is recognized ratably using the straight-line attribution method over the expected vesting period, which is considered to be the requisite service period.
The fair value of stock-based awards is estimated at the date of grant using the Black-Scholes option pricing model. Risk free interest rates are based upon U.S. Treasury rates appropriate for the expected term of each award. Expected volatility is based primarily on the volatility rates of a set of guideline companies, which consist of public and recently public biotechnology companies. The assumed dividend yield is zero, as we do not expect to declare any dividends in the foreseeable future. The expected term of options granted is determined using the “simplified” method. Under this approach, the expected term is presumed to be the mid-point between the average vesting date and the end of the contractual term.
Foreign currency translation adjustments
The functional currency of Sun BioPharma Australia Pty Ltd is the Australian Dollar (“AUD”). Accordingly, assets and liabilities, and equity transactions of Sun BioPharma Australia Pty Ltd are translated into U.S. dollars at period-end exchange rates. Revenues and expenses are translated at the average exchange rate in effect for the period. The resulting translation gains and losses are recorded as a component of accumulated comprehensive loss presented within the stockholders’ deficit. During the six-month periods ended June 30, 2016 and 2015, any reclassification adjustments from accumulated other comprehensive loss to operations was inconsequential.
6. Accrued Liabilities
Accrued liabilities consist of the following (in thousands):
|
June 30, 2016 |
December 31, 2015 |
|||||||
|
Deferred payroll and related expenses |
$ | 325 | $ | 169 | ||||
|
Product and process development expenses |
231 | 259 | ||||||
|
Professional services |
42 | 75 | ||||||
|
Clinical trial related expense |
42 | — | ||||||
|
Other |
3 | 2 | ||||||
|
Total accrued liabilities |
$ | 643 | $ | 505 | ||||
7. Debt issuance costs
The following table summarizes the deferred financing costs which are presented as a direct deduction from the carrying amount of their related debt liabilities (in thousands):
|
June 30, 2016 |
December 31, 2015 |
|||||||||||||||
|
Convertible Notes Payable |
Long-Term Debt |
Convertible Notes Payable |
Long-Term Debt |
|||||||||||||
|
Loan principal amount |
$ | 2,775 | $ | 300 | $ | 2,775 | $ | 300 | ||||||||
|
Deferred financing costs |
105 | 37 | 105 | 37 | ||||||||||||
|
Accumulated Amortization |
(52 | ) | (28 | ) | (40 | ) | (26 | ) | ||||||||
|
Unamortized balance |
53 | 9 | 65 | 11 | ||||||||||||
|
Loan amount, net |
$ | 2,722 | $ | 291 | $ | 2,712 | $ | 287 | ||||||||
We recorded amortization of debt issuance costs of $14,000 for both of the six month periods ended June 30, 2016 and 2015.
8. Net Loss Per Share
The following table summarizes our calculation of net loss per common share for each of the periods presented (in thousands, except share and per share data):
|
Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
|
2016 |
2015 |
2016 |
2015 |
|||||||||||||
|
Net loss |
$ | (978 | ) | $ | (1,215 | ) | $ | (1,799 | ) | $ | (3,407 | ) | ||||
|
Weighted average shares outstanding—basic and diluted |
30,126,755 | 6,585,533 | 30,058,942 | 6,225,722 | ||||||||||||
|
Basic and diluted net loss per share |
$ | (0.03 | ) | $ | (0.18 | ) | $ | (0.06 | ) | $ | (0.55 | ) | ||||
The following outstanding potential common shares are not included in diluted net loss per share calculations as their effects would have been anti-dilutive:
|
Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
|
2016 |
2015 |
2016 |
2015 |
|||||||||||||
|
Employee and non-employee stock options |
3,163,600 | 5,287,736 | 3,163,600 | 5,287,736 | ||||||||||||
|
Estimated common shares issuable upon conversion of notes payable |
2,466,667 | 2,667,443 | 2,466,667 | 2,667,443 | ||||||||||||
|
Common shares issuable under common stock purchase warrants |
3,525,500 | 4,650,000 | 3,525,500 | 4,650,000 | ||||||||||||
9. Stockholders’ Equity
Private Placement
In June 2016, we entered into Securities Purchase Agreements (the “Purchase Agreements”) with the purchasers named therein (the “Purchasers”) and closed the transaction governed thereby. Pursuant to the Purchase Agreements, we sold units consisting of a share of common stock and a warrant to purchase one-half of a share of common stock (the “Units”) . A total of 1,951,000 Units were purchased by the Purchasers consisting of an aggregate of 1,951,000 shares of the Company’s common stock (the “Shares”) and warrants (the “Warrants”) to purchase an aggregate of 975,500 shares of the Company’s common stock (the “Warrant Shares”). The purchase price for each Unit was $1.00 and the Warrants are exercisable for a period of five years from the dates of issuance at an exercise price of $1.50 per share. The Company received aggregate gross proceeds of $1,755,000 from two June closings under these private placement transactions and an additional $196,000 was invested by management through the conversion of previously deferred compensation.
Pursuant to the Purchase Agreements, we have agreed to use commercial best efforts to prepare and file a registration statement with the Securities and Exchange Commission (the “SEC”) within 60 days after the first closing of the private placement transactions for purposes of registering the resale of the Shares and the Warrant Shares. We have also agreed, among other things, to indemnify the selling holders under the registration statements from certain liabilities and to pay all fees and expenses (excluding underwriting discounts and selling commissions and legal fees) incident to our obligations under the Purchase Agreements.
Stock-Based Payments
In the first quarter of 2016, our Board of Directors authorized the issuance of 37,500 shares of our common stock to two vendors who agreed to provide services to the Company upon terms that provided for a portion of their consideration to be paid in shares of our common stock. The fair value of each share of common stock was determined by our Board of Directors, and accordingly, we recorded a charge of $75,000.
10. Stock-Based Compensation
2016 Omnibus Incentive Plan
The Sun BioPharma, Inc. 2016 Omnibus Incentive Plan (the “2016 Plan”) was adopted by our Board of Directors in March 2016 and approved by our stockholders at our annual meeting of stockholders on May 17, 2016. The 2016 Plan permits the granting of incentive and non-statutory stock options, restricted stock, stock appreciation rights, performance units, performance shares and other stock awards to eligible employees, directors and consultants. We grant options to purchase shares of common stock under the 2016 Plan at no less than the fair market value of the underlying common stock as of the date of grant. Options granted under the Plan have a maximum term of ten years. Under the Plan, a total of 15,000,000 shares of common stock are reserved for issuance. As of June 30, 2016, no awards were outstanding under the 2016 Plan.
2011 Stock Option Plan
The Sun BioPharma, Inc. 2011 Stock Option Plan (the “2011 Plan”) was adopted by the our Board of Directors in September, 2011 and approved by our stockholders in January, 2012. In conjunction with stockholder approval of the 2016 Plan, the Board terminated the 2011 Plan, although awards outstanding under the 2011 Plan will remain outstanding in accordance with and pursuant to the terms thereof. Options granted under the 2011 Plan have a maximum term of ten years and generally vest over zero to two years for employees. As of June 30, 2016, options to purchase 3,163,600 shares of common stock remained outstanding under the 2011 Plan.
We recognize stock-based compensation based on the value of the portion of awards that are ultimately expected to vest. Guidance requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The term “forfeitures” is distinct from “cancellations” or “expirations” and represents only the unvested portion of a surrendered option. We will re-evaluate this estimate periodically and adjust the forfeiture rate on a prospective basis as necessary. Ultimately, the actual expense recognized over the vesting period will only be for those shares that actually vest.
There were no options granted during the six months ended June 30, 2016.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the costs and expenses, other than the underwriting discounts and commissions and non-accountable expense allowance, payable by us in connection with the sale of common stock being registered. All amounts shown are estimates, except the SEC registration fee and the Financial Industry Regulatory Authority, or FINRA, filing fee.
|
SEC registration fee |
$ | 841 | ||
|
Legal fees and expenses |
$ | 70,000 | ||
|
Accounting fees and expenses |
$ | 8,000 | ||
|
Total |
$ | 78,841 |
Item 14. Indemnification of Directors and Officers.
Section 145 of the Delaware General Corporation Law provides that a corporation may indemnify any person made a party to an action by reason of the fact that he or she was a director, executive officer, employee or agent of the corporation or is or was serving at the request of the corporation against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by him or her in connection with such action if he or she acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful, except that, in the case of an action by or in right of the corporation, no indemnification may generally be made in respect of any claim as to which such person is adjudged to be liable to the corporation.
Our certificate of incorporation and amended and restated bylaws limit the liability of our directors to the fullest extent permitted by Delaware law. Delaware law provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except for liability for any:
|
● |
breach of their duty of loyalty to us or our stockholders; |
|
● |
act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
|
● |
unlawful payment of dividends or redemption of shares as provided in Section 174 of the Delaware General Corporation Law; or |
|
● |
transaction from which the directors derived an improper personal benefit. |
These limitations of liability do not apply to liabilities arising under federal securities laws and do not affect the availability of equitable remedies such as injunctive relief or rescission. Our amended and restated bylaws provide that we will indemnify our directors and executive officers, and may indemnify other officers, employees and other agents, to the fullest extent permitted by law.
As permitted by the Delaware General Corporation Law, we have entered into indemnification agreements with each of our directors and executive officers that require us to indemnify such persons against expenses, judgments, penalties, fines, settlements and other amounts actually and reasonably incurred, including expenses of a derivative action, in connection with an actual or threatened proceeding if any of them may be made a party because he or she is or was one of our directors. We will be obligated to pay these amounts only if the director acted in good faith and in a manner that he or she reasonably believed to be in or not opposed to our best interests. With respect to any criminal proceeding, we will be obligated to pay these amounts only if the director had no reasonable cause to believe his or her conduct was unlawful. The indemnification agreements also set forth certain procedures that will apply in the event of a claim for indemnification.
The Purchase Agreements (which are substantially in the form provided as Exhibit 10.15 hereto) provide for indemnification by the selling stockholders of us and our executive officers and directors, and by us of the selling stockholders for certain liabilities, including liabilities arising under the Securities Act, in connection with matters specifically provided in writing by the selling stockholders for inclusion in the registration statement.
Section 145(g) of the Delaware General Corporation Law permits a corporation to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee, or agent of the corporation arising out of his or her actions in connection with their services to us, regardless of whether our amended and restated bylaws permit indemnification. We have purchased and intend to maintain insurance on behalf of any person who is or was a director or officer against any loss arising from any claim asserted against him or her and incurred by him or her in any such capacity, subject to certain exclusions.
Item 15. Recent Sales of Unregistered Securities.
All share and per share amounts included in this Item 15 with respect to transactions on or before September 4, 2015, have been adjusted to give effect to the exchange of four shares of Company common stock for every one share of SBI common stock in the Merger.
Common Stock
In 2012, we sold an aggregate of 2,634,560 shares of common stock to certain “accredited investors” as defined in Regulation D promulgated under the Securities Act of 1933, as amended, for aggregate cash proceeds of approximately $2,305,000 which were used primarily to fund company operations.
In December 2013, we issued 9,639,116 shares of common stock in connection with the conversion of $2,325,000 aggregate principal amount and approximately $85,000 of accrued, but unpaid interest of then outstanding convertible promissory notes.
In June 2014, we issued 400,000 shares of its common stock in exchange for services rendered and to be rendered by an employee.
Also during 2014, we issued and aggregate of 2,143,600 shares of common stock in connection with the exercise of stock options for aggregate proceeds of approximately $493,000. During the same period, we issued 100,000 shares of common stock in connection with the exercise of warrants for aggregate proceeds of $25,000. The proceeds for the foregoing exercises were used primarily to fund company operations.
In March of 2015, we issued 40,000 shares of its common stock in exchange for services rendered and to be rendered by an employee.
In April 2015, we sold an aggregate of 175,000 shares of common stock to certain “accredited investors” as defined in Regulation D promulgated under the Securities Act of 1933, as amended, for aggregate proceeds of $350,000. The proceeds were used primarily to fund company operations.
In September 2015, we sold an aggregate of 587,500 shares of common stock to certain “accredited investors” as defined in Regulation D promulgated under the Securities Act of 1933, as amended, for aggregate proceeds of $1,175,000. The proceeds were used primarily to fund company operations.
Also during 2015, but prior to the Merger, we issued 2,590,536 shares of common stock in connection with the exercise of stock options for aggregate proceeds of approximately $693,000. During the same period, we issued 2,000,000 shares of common stock in connection with the exercise of warrants for aggregate proceeds of $375,000. The proceeds from the foregoing exercises were used primarily to fund company operations. We also issued 200,776 shares of common stock during 2015 in connection with the conversion of $225,000 aggregate principal amount of convertible promissory notes.
In June through September 2016 we sold an aggregate of 2,221,000 Shares pursuant to the Purchase Agreements.
Stock Options
All of the Stock Options granted by we prior to the Merger were awarded under the 2011 Plan and include a ten year term from the date of their respective grant dates.
In December of 2011, we granted options to purchase an aggregate of 320,000 shares of its common stock with an exercise price of $0.0875 per share to its employees and consultants in exchange for services rendered.
During 2012, we granted options to purchase an aggregate of 183,600 shares of its common stock with an exercise price of $0.0875 per share to its employees, consultants and directors in exchange for services rendered.
In May and June of 2013, we granted options to purchase an aggregate of 480,000 shares of its common stock with an exercise price of $0.11 per share to its employees, consultants and directors in exchange for services rendered.
In December of 2013, we granted options to purchase an aggregate of 3,617,344 shares of its common stock with an exercise price of $0.25 per share and 2,250,408 shares of its common stock with an exercise price of $0.275 per share to its employees and consultants in exchange for services rendered.
In January of 2014, we granted options to purchase an aggregate of 400,000 shares of its common stock with an exercise price of $0.25 per share to its employees, consultants and directors in exchange for services rendered.
In July of 2014, we granted options to purchase an aggregate of 380,000 shares of its common stock with an exercise price of $0.2275 per share to it employees, consultants and directors in exchange for services rendered.
In January of 2015, we granted options to purchase an aggregate of 220,000 shares of its common stock with an exercise price of $0.3175 per share to its directors in exchange for services rendered.
In March of 2015, we granted options to purchase an aggregate of 5,120,000 shares of its common stock with an exercise price of $0.3175 per share to its employees, consultants and directors in exchange for services rendered.
Warrants
In May and June of 2013, in connection with and as an inducement for the sale of convertible notes, we issued warrants to purchase an aggregate of 4,650,000 shares of our common stock, exercisable for ten years after the date of issuance at price equal to $0.1875 per share.
In June through September 2016, in conjunction with the sale of equity securities pursuant to the Purchase Agreements, we issued warrants to purchase the Warrant Shares, consisting of an aggregate of 1,110,500 additional shares of common stock, exercisable for five years after the date of issuance, at an exercise price of $1.50 per share.
Convertible Promissory Notes
In May and June of 2013, we issued an aggregate of $2,325,000 in principal amount of convertible promissory notes, which had a conversion price equal to an equivalent of $0.25 per share of our common stock.
In December 2013 and January 2014, we issued convertible promissory notes in the aggregate principal amount of $3,100,000 to investors and its current stockholders, which had a conversion price equal to $1.125 per share of common stock. See “Promissory Notes” under “Description of Securities” below for a description of the material terms of these convertible promissory notes.
None of the foregoing transactions involved any underwriters, underwriting discounts or commissions, or any public offering, and we believe the transactions were exempt from the registration requirements of the Securities Act in reliance on Section 4(2) thereof, and the rules and regulations promulgated thereunder, or Rule 701 thereunder, as transactions by an issuer not involving a public offering or transactions pursuant to compensatory benefit plans and agreements relating to compensation as provided under such Rule 701. The recipients of securities in such transactions represented their intention to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof, and appropriate legends were affixed to the share certificates and instruments issued in such transactions.
Exemptions from Registration for Sales of Restricted Securities
We issued all of the foregoing securities to a limited number of persons who were “accredited investors” or “sophisticated investors,” as those terms are defined in Rule 501 of Regulation D of the SEC, without the use of any general solicitations or advertising to market or otherwise offer the securities for sale. In addition, each such person had prior access to all material information about our Company and represented to us in writing (i) that it was an accredited investor or sophisticated investor investing with the assistance of a purchaser representative, (ii) that it was acquiring the common stock, warrants or promissory notes, each as applicable, for its own account and not with a view to distribute them and (iii) that the common stock, warrants or promissory notes each investor acquired were restricted securities. The Company and its predecessor entities, as applicable, also caused the filing of notices on Form D with the SEC with respect to each transaction for which such filing is required under Regulation D. Based on the foregoing, we believe that the offer and sale of these securities were exempt from the registration requirements of the Securities Act, pursuant to Sections 4(2) and 4(6) thereof. Registration of sales to “accredited investors” is preempted from state regulation by Section 18 of the Securities Act, though states may require the filing of notices, a fee and other administrative documentation.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits.
We have filed the exhibits listed on the accompanying Exhibit Index appearing immediately after the signature page hereof.
(b) Financial Statement Schedules.
All schedules are omitted as the required information is inapplicable or the information is presented in the financial statements or related notes.
Schedule II. Valuation and Qualifying Accounts
All other schedules are omitted as the required information is inapplicable or the information is presented in the financial statements or related notes.
Item 17. Undertakings.
|
(1) |
File, during any period in which offers or sells are being made, a post-effective amendment to this registration statement: |
|
(i) |
To include any prospectus required by section 10(a)(3) of the Securities Act of 1933; |
|
(ii) |
To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement. |
|
(iii) |
To include material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; |
provided, however, that paragraphs (1)(i), (1)(ii) and (1)(iii) above do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the Registrant pursuant to Section 13 and Section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement.
|
(2) |
That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
|
(3) |
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
|
(4) |
That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this registration statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Minneapolis, State of Minnesota on this 16th day of September, 2016.
|
|
SUN BIOPHARMA, INC.
By: /s/ David B. Kaysen |
Power Of Attorney
Each person whose signature appears below hereby constitutes and appoints David B. Kaysen and Scott Kellen, and each of them acting individually, as his true and lawful attorneys-in-fact and agents, with full power of each to act alone, with full powers of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities, to sign the Registration Statement filed herewith and any and all amendments to said Registration Statement (including post-effective amendments and any related registration statements thereto filed pursuant to Rule 461 and otherwise), and file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, with full power of each to act alone, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully for all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or his or their substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
|
Signatures |
|
Title |
|
Date |
|
/s/ David B. Kaysen |
President and Chief Executive Officer |
September 16, 2016 | ||
|
David B. Kaysen |
(principal executive officer) | |||
|
/s/ Scott Kellen |
Chief Financial Officer |
September 16, 2016 | ||
|
Scott Kellen |
(principal financial and accounting officer) | |||
|
/s/ Michael T. Cullen |
Executive Chairman of the Board and |
September 16, 2016 | ||
|
Michael T. Cullen |
Director | |||
|
/s/ Suzanne Gagnon |
Director |
September 16, 2016 | ||
|
Suzanne Gagnon |
||||
|
/s/ Dalvir Gill |
Director |
September 16, 2016 | ||
|
Dalvir Gill |
||||
|
/s/ Jeffrey S. Mathiesen |
Director |
September 16, 2016 | ||
|
Jeffrey S. Mathiesen |
||||
|
/s/ J. Robert Paulson, Jr. |
Director |
September 16, 2016 | ||
|
J. Robert Paulson, Jr. |
||||
|
/s/ Paul W. Schaffer |
Director |
September 16, 2016 | ||
|
Paul W. Schaffer |
||||
|
/s/ D. Robert Schemel |
Director |
September 16, 2016 | ||
|
D. Robert Schemel |
SUN BIOPHARMA, INC.
Registration Statement on Form S-1
Exhibit Index
Unless otherwise indicated, all documents incorporated into this registration statement by reference to a document filed with the SEC pursuant to the Exchange Act are located under SEC file number 000-55242.
|
Exhibit No. |
Description | ||
|
2.1 |
Agreement and Plan of Merger, dated June 12, 2015, by and among Sun BioPharma, Inc. (f/k/a Cimarron Medical, Inc.), Sun BioPharma Research, Inc. (f/k/a Sun BioPharma, Inc.), and SB Acquisition Corporation (incorporated by reference to Exhibit 2.1 to current report on Form 8-K filed June 18, 2015) | ||
|
2.2 |
Amendment No. 1 to Agreement and Plan of Merger, dated August 3, 2015 (incorporated by reference to Exhibit 2.1 to current report on Form 8-K filed August 4, 2015) | ||
|
2.3 |
Agreement and Plan of Merger dated May 25, 2016 (incorporated by reference to Exhibit 2.1 to quarterly report on Form 10-Q for the quarter ended June 30, 2016) | ||
|
3.1 |
Certificate of Incorporation (incorporated by reference to Exhibit 3.1 to quarterly report on Form 10-Q for the quarter ended June 30, 2016) | ||
|
3.2 |
Bylaws (incorporated by reference to Exhibit 3.2 to quarterly report on Form 10-Q for the quarter ended June 30, 2016) | ||
|
5.1 |
+ |
Opinion of Faegre Baker Daniels LLP | |
|
10.1 |
Form of Warrant to Purchase Shares of Stock (incorporated by reference to Exhibit 4.3 to current report on Form 8-K filed September 11, 2015) | ||
|
10.2 |
Form of Convertible Promissory Note (incorporated by reference to Exhibit 4.2 to current report on Form 8-K filed September 11, 2015) | ||
|
10.3 |
* |
Sun BioPharma, Inc. 2011 Stock Option Plan, as amended through January 1, 2015 (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed September 11, 2015) | |
|
10.4 |
* |
Form of Incentive Stock Option Agreement for awards under 2011 Stock Option Plan, as amended (incorporated by reference to Exhibit 10.2 to current report on Form 8-K filed September 11, 2015) | |
|
10.5 |
* |
Form of Non-Qualified Stock Option Agreement for awards under 2011 Stock Option Plan, as amended (incorporated by reference to Exhibit 10.3 to current report on Form 8-K filed September 11, 2015) | |
|
10.6 |
* |
Indemnification Agreement, dated September 4, 2015 (incorporated by reference to Exhibit 10.4 to current report on Form 8-K filed September 11, 2015) | |
|
10.7 |
** |
Standard Exclusive License Agreement by and between the University of Florida Research Foundation, Inc. and Sun BioPharma, Inc., dated December 22, 2011 (incorporated by reference to Exhibit 10.5 to current report on Form 8-K filed September 11, 2015) | |
|
10.8 |
* |
Sun BioPharma, Inc. 2016 Omnibus Incentive Plan (incorporated by reference to Appendix E to definitive proxy statement on Schedule 14A filed April 11, 2016) | |
|
10.9 |
* |
Form of Incentive Stock Option Agreement for awards under 2016 Omnibus Incentive Plan (incorporated by reference to Exhibit 10.4 to quarterly report on Form 10-Q for the quarter ended June 30, 2016) | |
|
10.10 |
* |
Form of Non-Qualified Stock Option Agreement for awards under 2016 Omnibus Incentive Plan (incorporated by reference to Exhibit 10.4 to quarterly report on Form 10-Q for the quarter ended June 30, 2016) | |
|
10.11 |
* |
Employment Agreement with Michael T. Cullen, dated December 2, 2015 (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed December 4, 2015) | |
|
10.12 |
* |
Employment Agreement with David B. Kaysen, dated December 2, 2015 (incorporated by reference to Exhibit 10.2 to current report on Form 8-K filed December 4, 2015) | |
|
10.13 |
* |
Employment Agreement with Scott Kellen, dated December 2, 2015 (incorporated by reference to Exhibit 10.3 to current report on Form 8-K filed December 4, 2015) | |
|
10.14 |
* |
Employment Agreement with Suzanne Gagnon, dated December 2, 2015 (incorporated by reference to Exhibit 10.9 to annual report on Form 10-K for the year ended December 31, 2015) | |
|
10.15 |
Form of Securities Purchase Agreement (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed June 14, 2016) | ||
|
Exhibit No. |
Description | ||
|
10.16 |
Form of Warrant to Purchase Shares of Stock (incorporated by reference to Exhibit 10.2 to current report on Form 8-K filed June 14, 2016) | ||
|
10.17 |
+ |
Amendment No. 1 to Employment Agreement with Michael T. Cullen, dated September 12, 2016 | |
|
10.18 |
+ |
Amendment No. 1 to Employment Agreement with David B. Kaysen, dated September 12, 2016 | |
|
10.19 |
+ |
Amendment No. 1 to Employment Agreement with Scott Kellen, dated September 12, 2016 | |
|
10.20 |
+ |
Amendment No. 1 to Employment Agreement with Suzanne Gagnon, dated September 12, 2016 | |
|
21.1 |
+ |
List of Subsidiaries | |
|
23.1 |
+ |
Consent of Independent Registered Public Accounting Firm. | |
|
23.2 |
+ |
Consent of Faegre Baker Daniels LLP (included in Exhibit 5.1). | |
|
24.1 |
+ |
Powers of Attorney (see signature page). | |
|
101.INS*** |
XBRL Instance |
|
101.SCH*** |
XBRL Taxonomy Extension Schema |
|
101.CAL*** |
XBRL Taxonomy Extension Calculation |
|
101.DEF*** |
XBRL Taxonomy Extension Definition |
|
101.LAB*** |
XBRL Taxonomy Extension Labels |
|
101.PRE*** |
XBRL Taxonomy Extension Presentation |
|
+ |
Filed herewith |
|
++ |
Furnished herewith |
|
* |
Management compensatory plan or arrangement required to be filed as an exhibit to this report. |
|
** |
Application has been made to the Securities and Exchange Commission to seek confidential treatment of certain provisions of this exhibit. Omitted material for which confidential treatment has been requested has been filed separately with the Securities and Exchange Commission. |
*** XBRL information is furnished and not filed or a part of a registration statement or prospectus for purposes of sections 11 or 12 of the Securities Act of 1933, as amended, is deemed not filed for purposes of section 18 of the Securities Exchange Act of 1934, as amended, and otherwise is not subject to liability under these sections.